Abstract
Background
Skin‐to‐skin care (SSC), often referred to as 'kangaroo care' (KC) due to its similarity with marsupial behaviour of ventral maternal‐infant contact, is one non‐pharmacological intervention for pain control in infants.
Objectives
The primary objectives were to determine the effect of SSC alone on pain from medical or nursing procedures in neonates compared to no intervention, sucrose or other analgesics, or additions to simple SSC such as rocking; and to determine the effects of the amount of SSC (duration in minutes), method of administration (e.g. who provided the SSC) of SSC in reducing pain from medical or nursing procedures in neonates
The secondary objectives were to determine the safety of SSC care for relieving procedural pain in infants; and to compare the SSC effect in different postmenstrual age subgroups of infants.
Search methods
For this update, we used the standard search strategy of the Cochrane Neonatal Review group to search the Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 1); MEDLINE via PubMed (1966 to 25 February 2016); Embase (1980 to 25 February 2016); and CINAHL (1982 to 25 February 2016). We also searched clinical trials' databases, conference proceedings, and the reference lists of retrieved articles for randomized controlled trials and quasi‐randomized trials.
Selection criteria
Studies with randomisation or quasi‐randomisation, double‐ or single‐blinded, involving term infants (≥ 37 completed weeks' postmenstrual age (PMA) to a maximum of 44 weeks' PMA and preterm infants (< 37 completed weeks PMA) receiving SSC for painful procedures conducted by healthcare professionals.
Data collection and analysis
The main outcome measures were physiological or behavioural pain indicators and composite pain scores. A mean difference (MD) with 95% confidence interval (CI) using a fixed‐effect model was reported for continuous outcome measures. We included variations on type of tissue‐damaging procedure, provider of care, and duration of SSC.
Main results
Twenty‐five studies (n = 2001 infants) were included. Nineteen studies (n = 1065) used heel lance as the painful procedure, one study combined venepuncture and heel stick (n = 50), three used intramuscular injection (n = 776), one used 'vaccination' (n = 60), and one used tape removal (n = 50). The studies were generally strong and had low or uncertain risk of bias. Blinding of the intervention was not possible, making them subject to high risk, depending on the method of scoring outcomes.
Seventeen studies (n = 810) compared SSC to a no‐treatment control. Although 15 studies measured heart rate during painful procedures, data from only five studies (n = 161) could be combined for a mean difference (MD) of −10.78 beats per minute (95% CI −13.63 to −7.93) favouring SSC. Meta‐analysis of four studies (n = 120) showed no difference in heart rate following the painful procedure (MD 0.08, 95% CI −4.39 to 4.55). Two studies (n = 38) reported heart rate variability with no significant differences. Two studies (n = 101) in a meta‐analysis on oxygen saturation at 30 and 60 seconds following the painful procedure did not show a difference. Duration of crying meta‐analysis was performed on four studies (n = 133): two (n = 33) investigated response to heel lance (MD = −34.16, 95% CI −42.86 to −25.45), and two (n = 100) following IM injection (MD = −8.83, 95% CI −14.63 to −3.02), favouring SSC. Five studies, one consisting of two substudies (n = 267), used the Premature Infant Pain Profile (PIPP) as a primary outcome, which favoured SCC at 30 seconds (MD −3.21, 95% CI −3.94 to −2.47), at 60 seconds (3 studies; n = 156) (MD −1.64, 95% CI −2.86 to −0.43), and at 90 seconds (n = 156) (MD −1.28, 95% CI −2.53 to −0.04); but at 120 seconds there was no difference (n = 156) (MD 0.07, 95% CI −1.11 to 1.25). No studies on return of heart rate to baseline level, cortisol levels, and facial actions could be combined for meta‐analysis findings.
Eight studies compared SSC to another intervention with or without a no‐treatment control. Two cross‐over studies (n = 80) compared mother versus other provider (father, another female) on PIPP scores at 30, 60, 90, and 120 seconds with no significant difference. When SSC was compared to other interventions, there were not enough similar studies to pool results in an analysis. One study compared SSC (n = 640) with and without dextrose and found that the combination was most effective and that SSC alone was more effective than dextrose alone. Similarly, in another study SSC was more effective than oral glucose for heart rate (n = 95). SSC either in combination with breastfeeding or alone was favoured over a no‐treatment control, but not different to breastfeeding. One study compared SSC alone and in combination with both sucrose and breastfeeding on heart rate (HR), NIPS scores, and crying time (n = 127). The combinations were more effective than SSC alone for NIPS and crying. Expressed breast milk was compared to SSC in one study (n = 50) and found both equally effective on PIPP scores. There were not enough participants with similar outcomes and painful procedures to compare age groups or duration of SSC. No adverse events were reported in any of the studies.
Authors' conclusions
SSC appears to be effective as measured by composite pain indicators with both physiological and behavioural indicators and, independently, using heart rate and crying time; and safe for a single painful procedure. Purely behavioural indicators tended to favour SSC but with facial actions there is greater possibility of observers not being blinded. Physiological indicators were mixed although the common measure of heart rate favoured SSC. Two studies compared mother‐providers to others, with non‐significant results. There was more heterogeneity in the studies with behavioural or composite outcomes. There is a need for replication studies that use similar, clearly defined outcomes. Studies examining optimal duration of SSC, gestational age groups, repeated use, and long‐term effects of SSC are needed. Of interest would be to study synergistic effects of SSC with other interventions.
Keywords: Humans; Infant, Newborn; Breast Feeding; Heart Rate; Heart Rate/physiology; Hydrocortisone; Hydrocortisone/analysis; Infant, Premature; Injections, Intramuscular; Injections, Intramuscular/adverse effects; Kangaroo‐Mother Care Method; Kangaroo‐Mother Care Method/methods; Oxygen Consumption; Oxygen Consumption/physiology; Pain Management; Pain Management/methods; Phlebotomy; Phlebotomy/adverse effects; Punctures; Punctures/adverse effects; Randomized Controlled Trials as Topic; Saliva; Saliva/chemistry; Term Birth
Plain language summary
Skin‐to‐skin care with newborns cuts down procedural pain
Review question: Is skin‐to‐skin care effective in cutting down pain from procedures in newborns? Are there any safety issues?
Background: Newborns wearing only a diaper being held next to their mother's bare chest is referred to as skin‐to‐skin contact and is also sometimes called 'kangaroo care' because of its similarity to the way kangaroo mothers care for their young. Newborns, especially those who must spend time in a Neonatal Intensive Care Unit, must have various tests and procedures as part of their care, for example heel stick, venous puncture, and injections. Giving analgesic drugs for these procedures can often pose problems so that alternatives to drugs must be found.
Study characteristics: Twenty‐eight studies in which newborn babies who were by chance in the kangaroo care group or condition were included from an extensive search of the literature. Skin‐to‐skin care was clearly defined and could have been compared to no pain‐reducing strategies or other pain‐reducing strategies such as sweet taste. Studies were examined which examined well‐established signs of pain, both physiological and behavioural, as well as a combination of physiological and behavioural signs. Different providers of skin‐to‐skin care other than the mother were included.
Key results: Kangaroo care appears to reduce the pain response to, and recovery from, these frequent procedures, although few studies could be combined to provide strong evidence. As far as it has been reported, skin‐to‐skin care is safe. Although it appears that skin‐to‐skin care is effective, the size of the benefit remains uncertain.
Quality of evidence: The quality of evidence in these studies was generally low for the response to the actual procedure but was moderate for recovery from the procedure.
Summary of findings
Summary of findings for the main comparison. Skin‐to‐Skin care for procedural pain in neonates ‐ summary of findings.
| Studies examining Skin‐to‐skin care vs no treatment control | ||||||
| Patient or population: procedural pain in neonates Setting: Multiple Intervention: Skin‐to‐skin care Comparison: control | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with control | Risk with Skin‐to‐skin care | |||||
| Heart rate during painful procedure | The mean heart rate during painful procedure in the intervention group was 10.78 fewer (13.63 fewer to 7.93 fewer) | ‐ | 161 (5 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 2 3 | ||
| Heart rate following painful procedure | The mean heart rate following painful procedure in the intervention group was 0.08 more (4.39 fewer to 4.55 more) | ‐ | 120 (4 RCTs) | ⊕⊕⊕⊕ HIGH | ||
| PIPP Score 30 seconds after painful procedure | The mean PIPP Score 30 seconds after painful procedure in the intervention group was 3.2 fewer (3.94 fewer to 2.47 fewer) | ‐ | 268 (5 RCTs) | ⊕⊕⊕⊝ MODERATE 4 | ||
| NIPS ‐ Proportion of infants in low or no pain during procedure | Study population | not estimable | 480 (3 RCTs) | ⊕⊝⊝⊝ VERY LOW 2 3 5 | ||
| 46 per 1,000 | 0 per 1,000 (0 to 0) | |||||
| Moderate | ||||||
| 20 per 1,000 | 0 per 1,000 (0 to 0) | |||||
| NIPS ‐ Infants in no pain during recovery | Study population | not estimable | 380 (2 RCTs) | ⊕⊕⊕⊝ MODERATE 5 | ||
| 316 per 1,000 | 0 per 1,000 (0 to 0) | |||||
| Moderate | ||||||
| 485 per 1,000 | 0 per 1,000 (0 to 0) | |||||
| Duration of cry (seconds) following heel lance | The mean duration of cry (seconds) following heel lance in the intervention group was 34.16 fewer (42.86 fewer to 25.45 fewer) | ‐ | 33 (2 RCTs) | ⊕⊕⊕⊝ MODERATE 5 | ||
| Duration of cry (seconds) following IM injection | The mean duration of cry (seconds) following IM injection in the intervention group was 8.83 fewer (14.63 fewer to 3.02 fewer) | ‐ | 100 (2 RCTs) | ⊕⊕⊕⊝ MODERATE 3 | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
1 Pooled effect significantly influenced by a single high RoB study
2 Large heterogeneity statistics
3 Confidence interval crosses MID threshold
4 Intervention is less effective against active control
5 All studies in analysis were assessed as having high RoB
Background
Description of the condition
The preterm neonate frequently spends the first days or weeks of life in a Neonatal Intensive Care Unit (NICU), where numerous painful procedures are part of routine care (Johnston 1997; Simons 2003; Stevens 2003; Johnston 2008; Johnston 2011b; Cruz 2016). There is substantial evidence that long‐term blunting of behavioural, autonomic, and hormonal responses — and even brain and cognitive development — result from early untreated exposure to pain in preterm neonates (Johnston 1996; Oberlander 2000; Grunau 2007a; Grunau 2007b; Brummelte 2012; Vinall 2014). The most common painful procedures are heel lance and intravenous line insertions. Topical anaesthetics have not been found to be effective in this population (Larsson 1996; Stevens 1999a). Sucrose has been shown to be effective (Stevens 2013); but frequently repeated doses of sucrose in the very preterm neonate may not be safe (Johnston 2002; Lefrak 2006; Johnston 2007a). Parenteral analgesics either have negative sequelae (Marsh 1997; Anand 2004; Carbajal 2005); or have not been tested for pain in this population (Cuzzolin 2001). Behavioural methods of pain control such as non‐nutritive sucking, simulated rocking, facilitated tucking and positioning have been tested (Pillai Riddell 2015), with non‐nutritive sucking having a significant effect even in very preterm neonates (Campos 1994; Corff 1995; Stevens 1999b; Akman 2002; Carbajal 2002; Boyle 2004; Cignacco 2007). There is a large volume of literature on pain in neonates, including a review of over 40 measures of pain (Stevens 2007). Several studies have reported important age differences in response, with more preterm neonates having less robust and less sustained responses (Craig 1984; Johnston 1993; Stevens 2007; Gibbins 2008).
Description of the intervention
Recently there has been growing interest in how mothers of preterm neonates can contribute to the promotion of growth and comfort in the NICU setting. This has been based on two premises: (1) the loss of comfort‐providing roles of parents in critical care settings; and (2) the effect of maternal touch specifically in the skin‐to‐skin care (SSC) paradigm, on various parameters of neonatal stability and state regulation. In studies of parents of critically ill children and infants, parents were concerned about pain management and found their child’s suffering a primary source of stress (Miles 1992; Youngblut 1992; Moehn 1996; Wereszczak 1997). Even in situations where the staff believed that they were handling the child’s pain well and that the parents were not distressed, this was not the case from the parents’ perspective (Simons 2001). In a US and UK study of 11 NICUs, with 200 parents, almost all parents reported that their infant had experienced moderate to severe pain that was worse than they had expected (Franck 2001). Concerns about pain predicted the most important variance of parental stress. Another major concern of parents is the loss of their parental role, including to provide comfort (Miles 1989; Shields‐Poë 1997; Ko 1998). In the above study of NICU parents, 87% stated that they would wish to participate in managing their infant’s pain (Franck 2002). In a study of mothers engaged in kangaroo care (KC) while their infants underwent routine heel lance in the NICU, 80% of the mothers reported positive feelings and 90% said they would do it again (Campbell‐Yeo 2008). A recent study examining NICU staff nurse beliefs surrounding the use of SSC for pain management found that while neonatal staff nurses also positively viewed SSC as an effective pain‐relieving intervention, they noted contextual challenges such as heavy nurse‐workload and lack of maternal presence prevented its utilization (Benoit 2016).
Skin‐to‐skin care, referred to as kangaroo care because of its similarity to marsupial behaviour, was first developed as a method of providing warmth for low birth weight infants in Bogota, Columbia in 1979 (Whitelaw 1985). During SSC, a diaper‐clad infant is held upright between the mother's breasts, at an angle of approximately 60°, providing maximal skin‐to‐skin contact between the baby and parent. A survey on the holding policy in 215 NICUs in the US indicated that almost three‐quarters of the units allowed parents to hold their extubated infant in SSC (Franck 2002). There is extensive literature on KC in developing countries that is not reviewed here (Charpak 2005).
How the intervention might work
Several recent reviews have been published that report on the positive outcomes associated with SSC (Campbell‐Yeo 2015; Boundy 2016). A review of clinical trials of SSC on targeted infant outcomes of breastfeeding, behaviour, and physiological adaption in healthy neonates found 30 studies that met the inclusion criteria, four being with late preterm infants (Moore 2012). They reported evidence supporting SSC for success and duration of breastfeeding (Carfoot 2003; Johnson 2006; Moore 2012). Physiological stability and temperature control have been consistently reported as improved during SSC (de Leeuw 1991; Christensson 1992; Bauer 1998; Ludington‐Hoe 1999; Gazzolo 2000; Bohnhorst 2001; Chwo 2002; Ibe 2004; Ludington‐Hoe 2004; McCain 2005; Hunt 2008). For a newborn, behaviour is primarily based on the sleep and wake state dimension of neurobehavioural organization involving the ability to make smooth transitions between sleep, quiet, and awake phases; and to maintain the most desirable state of quiet sleep (Ludington‐Hoe 1996). Several studies have shown that one to three hours spent in SSC resulted in increased frequency of quiet sleep, longer duration of quiet sleep, and decreased crying (de Leeuw 1991; Ludington‐Hoe 1992; Michelsson 1996; Feldman 2002; Erlandsson 2007; Kostandy 2008). For example, a randomized controlled trial (RCT) of healthy newborns randomly assigned to receive KC for one hour starting within 15 minutes after birth found that at the four‐hour observation time KC infants slept longer, were mostly in a quiet sleep state, exhibited more flexor movements and postures, and showed fewer extensor movements (Ferber 2004). Feldman and colleagues have reported sustained neurobehavioural regulation from 30 to 37 weeks' gestational age as a result of early KC in the NICU (Feldman 2003). A Cochrane review by Conde‐Agudelo reported three studies on mortality and morbidity and did not address pain response (Conde‐Agudelo 2014). Given that SSC promotes autonomic stability and state regulation as well as bonding between the mother and the infant, it is logical that it would be tested as an intervention for pain where the response to painful stimuli includes autonomic arousal and crying, in addition to its advantage of giving mothers back their comforting role.
Why it is important to do this review
The American Pediatric Society and Canadian Paediatric Society's Fetus and Newborn Committee incorporated SSC as a recommended intervention. However, no systematic review with the rigour of The Cochrane Collaboration had been conducted until the first review (Johnston, 2014). There could, for example, be a publication bias that would favour positive outcomes. There has been a Cochrane review of SSC for mortality and morbidity, which did favour SSC over usual care controls for infections and weight gain (Conde‐Agudelo 2014).
Objectives
Primary objectives
To determine the effect of SSC alone on pain from medical or nursing procedures in neonates undergoing painful procedures compared to no intervention, sucrose or other analgesics, or additions to simple SSC such as rocking.
To determine the effects of the amount of SSC (duration in minutes), method of administration (who provided the SSC, positioning of caregiver and neonate pair) of SSC in reducing pain from medical or nursing procedures in neonates.
Secondary objectives
-
To determine the safety of SSC care for relieving procedural pain in infants, specifically reports of:
bradycardia (heart rate less than 100 for 15 seconds);
desaturation (transcutaneous oxygen saturation readings of less than 80% for 15 seconds); or
apnoea (absence of spontaneous respiration for 20 seconds, or 10 seconds if accompanied by bradycardia or desaturation (Lagercrantz 1992)).
To compare the SSC effect in different postmenstrual age subgroups of infants: less than 32 weeks, 32 to 36 weeks, full term (37 to 42 weeks).
Methods
Criteria for considering studies for this review
Types of studies
Studies with randomisation or quasi‐randomisation, and blinded (for example, coding video tapes of infant faces only or using physiological data from monitors) or not‐blinded assessors for pain response were considered for inclusion. This included different designs such as classic randomized controlled trials, randomized cross‐over trials, and cluster as well as quasi‐experimental designs.
Types of participants
Term infants (≥ 37 completed weeks postmenstrual age (PMA)) and preterm infants (< 37 completed weeks PMA) to a maximum of 44 weeks' PMA receiving SSC for painful procedures conducted by doctors, nurses, or other healthcare professionals. The painful procedures that were included are those that are tissue damaging or considered painful, such as endotracheal suctioning or tape removal (Carbajal 2008).
Types of interventions
The infant, wearing no more than a diaper, in ventral skin contact with another person during a painful procedure. We were interested in any comparisons of dosage (duration of time in SSC), any adjuvant therapies (sucrose or other sweet tastes, pacifier, topical anaesthetics, systemic analgesics), provider of SSC (mother, father, nurse, other), and variations of SSC such as the addition of rocking or music.
Types of outcome measures
Primary outcomes
Pain response to an invasive procedure, or recovery from an invasive procedure, or both, as measured by at least one of the following.
Behavioural indicators (audible cry duration in seconds or milliseconds; audible crying time as a proportion of total procedure time; proportion of time of total procedure that had predefined facial actions reflecting grimace e.g. brow bulge, eye squeeze, nasolabial furrow; proportion of time that had predefined body movements e.g. limb thrashing, fisting, finger splaying, limb and torso flexion).
Physiological indicator changes from baseline or between groups in heart rate (HR), respiratory rate, oxygen (O₂) saturation/transcutaneous oxygen tension (tcpO₂), and near‐infrared spectroscopy (NIRS). These measures should be reported before the tissue‐damaging part of the procedure, during the procedure, and in the time to recovery following the procedure.
Hormonal indicators (salivary cortisol, serum beta‐endorphins) obtained from body fluids (saliva, serum) with description of analyses e.g. radio‐immune assay techniques.
-
Validated composite pain scores (including a combination of behavioural, physiological, and contextual indicators). There are over 50 measures of pain in neonates in the literature. The ones that we assessed as being valid for neonates undergoing procedural pain include:
Premature Infant Pain Profile (PIPP) (Stevens 1996; Stevens 2010). The PIPP includes gestational age, behavioural state, heart rate, oxygen saturation, and three facial reactions (brow bulge, eye squeeze, and nasolabial furrow). The range is 0 to 21 with a score of 6 indicating pain.
COMFORT scale (van Dijk 2000). This scale measures alertness, calmness, respiratory response or crying, physical movement, muscle tone and facial tension, and separate latent variables for heart rate (HR) baseline and mean arterial blood pressure baseline (MAP).
Behavioral Indicators of Infant Pain (BIIP) (Holsti 2007). The BIIP combines sleep and wake states, five facial actions and two hand actions.
Neonatal Infant Pain Scale (NIPS) (Lawrence 1993). The NIPS includes facial expression, cry, breathing pattern, arms, legs, state of arousal.
Neonatal Pain, Agitation, and Sedation Scale (N‐PASS) (Hummel 2008; Hummel 2010). N‐PASS was originally developed to measure ongoing pain but has recently been validated as a measure of acute pain. It includes crying and irritability, behaviour and state, facial expression, extremities and tone, and vital signs (heart rate, respiratory rate, blood pressure, oxygen saturation). It also has scores that rate sedation as well as pain and agitation.
Douleur Aiguë du Nouveau‐né (DAN) (Carbajal 1997). The scale scores pain with a range from 0 to 10 with three items: facial expression, limb movements, and vocal expression with ratings per item ranging from 0 to 4, 0 to 3 and 0 to 3, respectively.
All of these indicators yield continuous data, although the NIPS was reported as proportion of infants in low, moderate, or severe pain and was analyzed as risk ratio, not mean difference.
There are repeated measures across time and conditions within participants. For the cross‐over design studies, the first condition was analyzed.
These indicators were taken immediately prior to, during, and immediately following the painful procedure. The differences between the changes from baseline between groups were used.
Secondary outcomes
Response of SSC provider, including self‐report, cortisol, and physiological indicators.
Adverse events including (Lagercrantz 1992):
bradycardia (heart rate less than 100 for 15 seconds);
desaturation (transcutaneous oxygen saturation levels less than 80 for 15 seconds);
apnoea (absence of spontaneous respirations for more than 20 seconds or for 10 seconds if accompanied by bradycardia or desaturation).
These indicators are binary and were categorized as 'yes' or 'no'.
Search methods for identification of studies
Electronic searches
For the 2016 update we conducted a comprehensive search including: Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 1) in the Cochrane Library; MEDLINE via PubMed (1966 to 25 February 2016); Embase (1980 to 25 February 2016); and CINAHL (1982 to 25 February 2016) using the following search terms: ((painful procedure OR invasive procedure OR heel lance OR heel stick OR blood procurement OR venipuncture OR intravenous start OR arterial line insertion OR injection OR immunization AND analgesia OR pain OR comfort) AND (skin‐to‐skin OR kangaroo care OR kangaroo mother care)), plus database‐specific limiters for RCTs and neonates (see Appendix 1 for the full search strategies for each database). We did not apply language restrictions.
We searched clinical trials' registries for ongoing or recently completed trials (clinicaltrials.gov; the World Health Organization’s International Trials Registry and Platform www.whoint/ictrp/search/en/; and the ISRCTN Registry).
For the previous review, these databases were searched in August 2013: Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library; Evidence‐Based Medicine Reviews; MEDLINE (1950 onwards); PubMed (1975 onwards); Embase (1974 onwards); CINAHL (1982 onwards); Web of Science (1980 onwards); LILACS database (1982 onwards); SCIELO database (1982 onwards); PsycInfo (1980 onwards); AMED (1985 onwards); Dissertation‐Abstracts International (1980 onwards).
Searching other resources
In addition to the electronic searches noted above, we searched the following sources: Canadian Agency for Drugs and Technologies in Health (CADTH), University of British Columbia (UBC) Library, EAGLE, National Technical Information Service (NTIS), PsycEXTRA, Wikipedia, and the Web of Knowledge. We manually searched bibliographies of the most recent relevant paediatric, neonatal, and pain journals and recent major paediatric pain conference proceedings. We did not include unpublished studies in our search, except with author response. We listed abstracts under excluded studies. We did not impose language restrictions.
We made efforts to seek unpublished studies using Paediatric Pain and Neonatology Listservs, requesting readers to reply.
Data collection and analysis
We developed a data‐extraction Excel file that allowed us to make decisions about whether or not to include a study for initial selection. We selected studies that addressed the efficacy and safety of SSC compared to another condition for relieving pain in infants. Four review authors (MCY, AF, TD, BB) independently screened the titles and abstracts of all the references retrieved by the search strategy. At this stage, efforts were made to aim more for sensitivity than specificity: that is, we wished to be more inclusive than exclusive.
We resolved any differences by discussion among the screening review authors as well as a fifth review author (CJ). We used Review Manager (RevMan) 5 software to collate the data.
Selection of studies
Using the studies selected from the above steps, we independently assessed the full texts of relevant papers to determine whether or not they met the inclusion criteria. We evaluated studies for methodological quality and appropriateness for inclusion according to the selection criteria. We resolved disagreements by discussion with two review authors (CJ and MCY).
We listed rejected studies in the 'Characteristics of excluded studies' table, and we recorded the reasons for exclusion. Review authors were not blinded to author, institution, journal, or results of a study during the selection process.
All studies meeting the inclusion criteria underwent quality assessment and data extraction.
Data extraction and management
The following data were extracted.
Study designs: methods of randomization, intervention, cross‐over design, single centre or multi‐centric.
Participants: PMA, sex, postnatal age at time of intervention, setting.
Interventions: position duration, provider, adjuvant therapies (pharmacological and non‐pharmacological).
Outcomes: pain indicator (behavioural, physiological, and composite), recovery times.
Side effects, provider response, study refusals, withdrawals and dropouts, if reported.
We made attempts to contact the study authors if data were missing or needed to be clarified.
Assessment of risk of bias in included studies
We used the Cochrane tool for assessing risk of bias, Cochrane Handbook for Systematic Reviews of Interventions, Table 8.5.a (Higgins 2011). We examined:
sequence generation;
allocation concealment;
blinding of participants, personnel, assessors;
incomplete outcome data;
selective outcome reporting;
other possible sources of bias.
There were three possible answers: low risk, high risk, and unclear risk.
Funnel plots were not performed given the small number of papers that could be combined for analysis.
Four review authors (MCY, AF, TD, BB) independently scored each study for quality, with verification by CJ .
Measures of treatment effect
In studies with continuous data, mean differences (MD) and standard deviations (SD) in each group and effect size (ES) for the total were used.
Unit of analysis issues
The unit of analysis was the neonates receiving SSC. There were instances in which there were repeated measures, for example scores taken every 30 seconds within a condition (SSC or comparison). There were no cluster randomized trials. For cross‐over trials, the first condition data were used and the study was treated as an RCT (Elbourne 2002).
Dealing with missing data
We contacted all authors of studies for missing data, or if clarification was required. When the contact was not reciprocated, or the author was unable to provide the requested data, the study was excluded from the data synthesis.
Assessment of heterogeneity
The decision to perform a fixed‐effect meta‐analysis was based on the clinical decision regarding the appropriateness of combining trials and outcomes (Erez 1996; Hedges 1998; Overton 1998; Field 2003). Heterogeneity was explored using the I² statistic.
The statistical analysis was performed using RevMan 5 software, which is provided by Cochrane. We applied the Chi² test (Q test) and the I² statistic to assess between‐study heterogeneity. With continuous data, we expressed the effect as mean difference (MD) and 95% confidence interval (CI).
Assessment of reporting biases
We sought protocols in trial registries and compared the reports to the protocols in order to determine if there might be selective reporting. We would have attempted to contact the corresponding authors if there had been discrepancies, but there were none.
In examining the studies for duplication bias, we closely examined articles from repeated authors or sites and compared sample size, characteristic, and details of the studies. When there appeared to be overlap, we attempted to contact the corresponding author, or when everything was similar we assumed it was a duplicate and included only one of the articles.
When we were not successful in contacting authors, the possible sources of reporting bias were included in our conclusions.
We had planned to do an analysis of publication bias to determine if negative results were less likely to be published in peer‐reviewed journals. However, we found no examples of significant negative results, other than for one of several outcomes in one study, including in trial registries and in the grey literature. Therefore, this analysis was not conducted.
We examined the range of languages, locations, and citation sources to examine potential bias. Only English language reports were found, although some were from non‐anglophone countries.
Data synthesis
For studies using similar outcomes, both in terms of the pain indicator and the time frame examined, we pooled data and analyzed it together. We computed mean differences. Data were entered into RevMan via the table of means and standard deviations per group in order to develop a forest plot.
Quality of evidence
We used the GRADE approach, as outlined in the GRADE Handbook (Schünemann 2013), to assess the quality of evidence for the following (clinically relevant) outcomes: heart rate during painful procedure, heart rate following painful procedure, PIPP score 30 seconds after painful procedure, NIPS ‒ proportion of infants in low or no pain during procedure, NIPS – infants in no pain during recovery, duration of cry following heel lance, duration of cry following intramuscular (IM) injection. Two authors independently assessed the quality of the evidence for each of the outcomes above. We considered evidence from randomized controlled trials as high quality but downgraded the evidence one level for serious (or two levels for very serious) limitations based upon the following: design (risk of bias); consistency across studies; directness of the evidence; precision of estimates; and presence of publication bias. We used the GRADEpro 2014 Guideline Development Tool to create a Table 1 to report the quality of the evidence. The GRADE approach results in an assessment of the quality of a body of evidence in one of four grades: 1. High: we are very confident that the true effect lies close to that of the estimate of the effect. 2. Moderate: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. 3. Low: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. 4. Very low: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
Subgroup analysis and investigation of heterogeneity
We were unable to form group analyses as we had intended for the following categories: gestational age less than 32 weeks, between 32 to 36 weeks, and full term (37 to 42 weeks); or duration or 'dose' of SSC. There were not sufficient studies with similar outcomes to compare the effect of SSC on these factors.
As above, we performed heterogeneity tests using the Chi² test and I² statistic.
Sensitivity analysis
We were not able to conduct a sensitivity analysis as there were not enough studies examining similar outcomes with similar age groups or procedures.
Results
Description of studies
See Characteristics of included studies and Characteristics of excluded studies below.
Results of the search
Two authors identified 16 additional studies for possible inclusion in this 2016 update. Of these 16 additional studies, ten were excluded leaving a total of 6 new studies for inclusion in the updated review for a total of 25 unique studies (Figure 1). Two reports were of the same study, so that only one was included and it counted as one of the 25 unique studies (Sajedi 2007; Kashaninia 2008).
1.
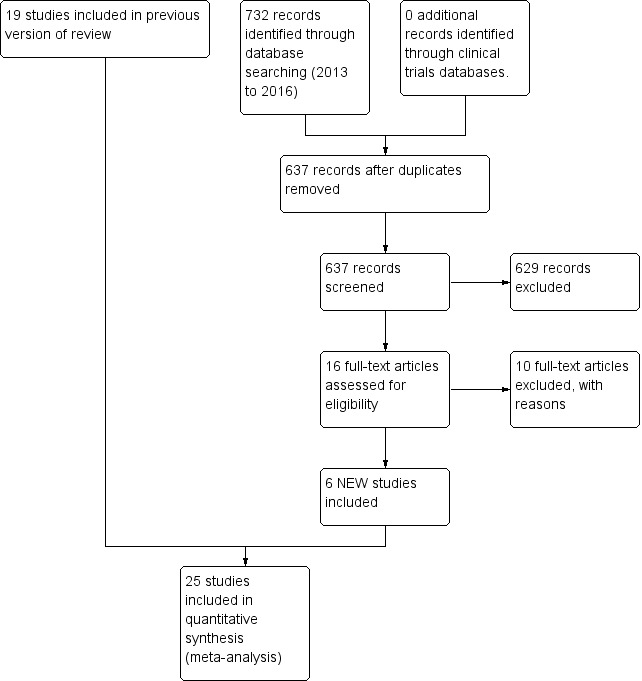
Study flow diagram: review update
Included studies
The 25 included studies reported on a total of 2001 infants. Among the included studies, seven were with full‐term neonates (Gray 2000; Sajedi 2007; Chermont 2009; Saeidi 2011; Gabriel 2013; Kostandy 2013; Liu 2015); and the remaining 18 were with preterm neonates (Johnston 2003; Ludington‐Hoe 2005; Castral 2008; Freire 2008; Johnston 2008; Kostandy 2008; Akcan 2009; Cong 2009; Johnston 2009; Okan 2010; Cong 2011; Johnston 2011; Cong 2012; Johnston 2012; Nanavati 2013; Nimbalkar 2013; Mosayebi 2014; Gao 2015). Details of each study are outlined in the tables under Characteristics of included studies.
Most (19) of the included studies examined responses to the painful procedure of heel lance (Gray 2000; Johnston 2003; Ludington‐Hoe 2005; Castral 2008; Freire 2008; Johnston 2008; Kostandy 2008; Cong 2009; Johnston 2009; Okan 2010; Cong 2011; Johnston 2011; Cong 2012; Johnston 2012; Gabriel 2013; Nimbalkar 2013; Mosayebi 2014; Gao 2015; Liu 2015) and are shown in Table 2. Four studies examined the response to intramuscular injection (Table 3) (Sajedi 2007; Chermont 2009; Saeidi 2011; Kostandy 2013); one study included both venipuncture and heel lance (Table 4) (Akcan 2009); three studies examined different providers (Table 5); and one study examined response to tape removal (Table 6) (Nanavati 2013).
1. Trials assessing pain during heel lance.
| Study | Design | Participants | Intervention | Outcome | Metrics Used | Results |
| Castral 2008 | Randomized controlled trial |
59 infants (31 intervention, 28 control) Postmenstrual age, mean, days: 248.3 (intervention), 254.4 (control) Birth weight, mean, grams: 1748.8 (intervention), 1846.2 (control) |
Intervention: 15 minutes of skin‐to‐skin care before, during and following heel lance Control: standard care during heel lance Provider: mother |
Neonatal Facial Coding System (NFCS) and heart rate at baseline, treatment, heel cleaning, heel lance, heel squeezing, wound compression, and recovery | Mean, mean difference (Treatment ‐control) Std. error, P value, 95% confidence intervals | Statistically significant differences between treatment and control groups during puncture, heel squeeze and post phases of heel lance. Infants receiving skin‐to‐skin contact more likely than infant controls to have significantly lower NFCS scores for heel lance (P = 0.023) and for heel squeeze. Both groups showed increased heart rate during puncture and heel squeeze although changes in these measures were less for treated infants (average increase of 19 bpm from baseline to heel puncture and squeezing in treatment group compared to average increase value of 23 bpm during puncture and 34 bpm during heel squeezing in control group) Means and standard deviations for NFCS scores and cry duration were obtained from the author |
| Cong 2009 | Randomized cross‐over | 14 infants (13 intervention, 10 control) Postnatal age, mean ± SD, days: 6 ± 1 (total) Postmenstural age, range, weeks:30‐32 Birth weight, mean ± SD, grams: 1775 ± 292 (total) Weight on day of study, mean ± SD, grams: 1706 ± 293 (total) |
Intervention: 60 minutes of skin‐to‐skin care before, during and following heel lance Control: standard care during heel lance Provider: mother |
Heart rate, low frequency (LF) and high frequency (HF) power, LF/HF power, and state at baseline, heel warming, heel lance, and recovery | Not reported | HR significantly lower in the KC condition (146 ± 9 bpm) than in IC (152 ± 13 bpm) during BL period (P < 0.05) and HS period (KC 159 ± bpm versus IC 165 ± 14 bpm, P < 0.05). HR increased significantly during HS from the BL and HW periods in both KC (P < 0.05) and IC conditions (P < 0.001), and returned to BL values during RC in both conditions. LF was higher in KC at BL (P < 0.01) and HS (P < 0.001) and HF was higher in KC at BL than in IC condition (P < 0.05). LF/HF ratio fluctuated less across periods in KC than in IC condition and was significantly lower during RC in KC than in IC (P < 0.001). LF and HF increased during HS from BL and HW, and dropped in the RC period in both KC (LF, P < 0.05 and HF, P < 0.01) and IC (LF, P < 0.01 and HF, P < 0.001) conditions. The LF/HF ratio was lower during HS than during BL, HW, and RC in both KC (P < 0.01) and IC (P < 0.001) conditions |
| Cong 2011 | Prospective randomized cross‐over | 28 infants: 14 infants ‐ 80 min SSC (Study 1); 10 infants ‐ 30 min SSC (Study 2) Postnatal age, mean ± SD, days: 5 ± 1 (Study 1); 6 ± 2 (Study 2) Postmenstrual age, range, weeks: 30‐32 Birth weight, mean ± SD, grams: 1779 ± 277 (Study 1); 1577 ± 327 (Study 2) |
Intervention: (a) Study 1: 60 minutes of skin‐to‐skin care before heel lance, with continued SSC during procedure, and followed by 20 minutes SSC post‐procedure; (b) Study 2: 10 minutes of skin‐to‐skin care before heel lance, with continued SSC during procedure, and followed by 20 minutes SSC post‐heel lance Control: standard care during heel lance Provider: mother |
PIPP score, salivary and serum cortisol at baseline, heel warming, heel lance and recovery | Mean, standard deviations | Study 2 showed lower PIPP scores at four time points during recovery (P < 0.05 to P < 0.001), lower salivary cortisol at the end of recovery (P < 0.05) and lower serum cortisol during heel lance for the kangaroo care heel lance condition (KCH) (P < 0.05) as swell as clinically lower PIPP scores in the KCH condition during heel lance |
| Cong 2012 | Randomized cross‐over | 26 preterm infants (PMA 28 0/7 to 32 6/7 weeks): 22 infants ‐ 30 min SSC (Study a); 25 infants ‐ 15 min SSC (Study b); 23 infants control Postnatal age, mean ± SD, days: 14.5 ± 6.3 (Study a); 13.8 ± 5.6 (Study b); 13.5 ± 5.6 (control) Birth weight, mean ± SD, grams: 1444.6 ± 379.0 |
Intervention: (a) Study a: 30 minutes of skin‐to‐skin care before and throughout heel lance (b) Study b: 15 minutes of skin‐to‐skin care before and throughout heel lance Control: standard care during heel lance Provider: mother |
Heart rate, heart rate variability (low frequency and high frequency power), LF/HF ratio, Infant behavioural state | Mean, standard deviations | HR changes from baseline to heel stick were significantly less in KC30 and KC15 than in IC, and more infants had HR decrease in IC than in 2 KC conditions. In IC, LF and HF significantly increased from baseline to heel stick and dropped from heel stick to recovery; in 2 KC conditions, no changes across study phases were found. During heel stick, LF and HF were significantly higher in IC than in KC30. In all 3 conditions, LF/HF ratio decreased from baseline to heel stick and increased to recovery; no differences were found between IC and two KC conditions. Both longer and shorter KC before and throughout heel stick can stabilize HR response in preterm infants, and longer KC significantly affected infants’ sympathetic and parasympathetic responses during heel stick compared with incubator care |
| Freire 2008 | Randomized controlled trial | 95 infants (31 intervention, 33 control, 31 comparison) Postmenstrual age, range, weeks: 28‐36 |
Intervention: 10 minutes of skin‐to‐skin care before, during heel lance Control: standard care during heel lance Comparison: Sweet taste 2 minutes before heel lance Provider: mother |
PIPP score | Mean, standard deviations | Heart rate variation and oxygen saturation significantly lower in kangaroo group compared to incubator and glucose groups (P = 0.0001 and P = 0.0012, respectively). Shorter duration of facial activity (brow bulge, eye squeeze and nasolabial furrowing) (P = 0.0001) and significantly lower PIPP score (P = 0.0001) observed in the kangaroo care method group Means and standard deviations for PIPP scores were obtained from the author |
| Gabriel 2013 | Randomized controlled trial | 136 infants (127 in analysis)(33/31 skin‐to‐skin, 33/32 sucrose, 35/35 sucrose + ssc, 35/29 skin‐to‐skin + breastfeeding) Gestational age, range, median, weeks: skin‐to‐skin: 37 to 41, 39; sucrose: 37 to 41, 39; sucrose + skin‐to‐skin: 37 to 41, 40; sucrose + breastfeeding: 37 to 42, 40 Birth weight, range, mean, grams: skin‐to‐skin: 2832 to 3900, 3359; sucrose: 1945 to 4176, 3215; sucrose + skin‐to‐skin: 2340 to 4108, 3349; sucrose + breastfeeding: 2266 to 4338, 3289 |
Group 1: 5 minutes of skin‐to‐skin care before, during heel lance. Group 2: 2 mL of 24% sucrose 2 minutes before heel lance Group 3: 5 minutes of skin‐to‐skin care before, during heel lance + 2 mL of 24% sucrose 2 minutes before heel lance Group 4: 5 minutes of breastfeeding with skin‐to‐skin before, during heel lance. Provider: skin‐to‐skin care and breastfeeding provided by the mother. Sucrose administered by a nurse. |
Crying time, % of crying in blood sampling, heart rate, NIPS | median and IQR (crying time, % of crying during blood sample, NIPS), mean and standard deviations (heart rate). | Breastfeeding + skin‐to‐skin group lower median NIPS score during heel stick (P < 0.01). NIPS scores in sucrose + skin‐to‐skin group lower than sucrose alone group 2 minutes after procedure (P = 0.02). Percentage of neonates with moderate‐to‐severe pain lowest in breastfeeding + skin‐to‐skin group, breastfeeding + skin‐to‐skin and sucrose + skin‐to‐skin had lower percentage of crying time compared to skin‐to‐skin alone. |
| Gao 2015 | Randomized controlled trial | 80 infants (75 in analysis) (40/38 in skin‐to‐skin, 40/37 in control) Gestational age, range, weeks: 27 to 37 Postnatal age, mean ± SD, days (heel stick 1,2,3,4): Skin‐to‐skin: 3.5 ± 0.3,4.3 ± 0.8,5.3 ± 0.6,6.7 ± 0.8 Control: 3.6 ± 0.4,4.2 ± 0.8,5.2 ± 0.7,6.8 ± 0.8 Birth weight, mean ± SD, grams: Intervention: 2017.8 ± 154.7; Control: 2030 ± 135.6 |
Intervention: Prone in incubator for 1 heel lance, Skin‐to‐skin 30 minutes pre‐heel lance for three consecutive procedures. Control: prone in incubator × 4 heel lances Provider: mother |
Crying time(seconds), grimacing time (seconds), heart rate | mean, standard deviations | Between group: Crying (P < 0.001), Grimacing (P < 0.001), HR (P < 0.001) significantly lower in skin‐to‐skin group. Within group: No loss in efficacy of skin‐to‐skin over time |
| Gray 2000 | Randomized controlled trial | 30 infants (15 control, 15 intervention) Postnatal age, range, hours: 33 to 55 Postmenstrual age, weeks: ≥ 37 Birth weight, mean (range), grams: 3300 (2600 to 3700) |
Intervention: 10 to 15 minutes of skin‐to‐skin care before heel lance Control: standard care during heel lance Provider: mother |
Heart rate during blood collection, cry duration and grimacing during recovery period | Mean | Infants held by mother in skin‐to‐skin contact, cried and grimaced for an average of 1 and 2 seconds, respectively, for entire recovery period. Control infants cried for a mean of 32 seconds and grimaced for a mean of 30 seconds of the 3‐minute recovery period (P < 0.001). Heart rate of skin‐to‐skin infants increased by about 8 to 10 bpm during blood collection whereas control infants heart rate rose by 36 to 38 bpm to an asymptote of 160bpm |
| Johnston 2003 | Randomized cross‐over | 74 infants Postnatal age, range, days: 0 to 10 Postmenstrual age, mean ± SD (range), weeks: 33.7 ± 1.1 (32.0 to 36.0) Birth weight, mean ± SD (range), grams: 2054 ± 406 (1320 to 3125) |
Intervention: 30 minutes of skin‐to‐skin care before and during heel lance Control: standard care during heel lance Provider: mother |
PIPP score at 30, 60, 90, and 120 minutes | Mean, 95% confidence interval |
Significantly lower PIPP scores in KC condition at 30 seconds (difference, 1.5 points; P = 0.04), 60 seconds (difference, 2.2 points; P = 0.002), and 90 seconds (difference, 0.6 point; P = 0.37) after heel‐lancing procedure. Heart rate and oxygen saturation similar in both conditions. Facial actions contributed significantly to total pain score (0.0 < P < 0.005), with facial actions averaging 20% greater in control versus KC condition Means and standard deviations for heart rate were obtained from the author |
| Johnston 2008 | Randomized cross‐over | 61 infants Postmenstrual age, mean ± SD, weeks: 30.5 ± 1 Birth weight, mean ± SD, grams: 1421 ± 490 |
Intervention: 15 minutes of skin‐to‐skin care before and during heel lance Comparison: swaddling in incubator 15 minutes before heel lance Provider: mother |
PIPP score at 30, 60, 90, and 120 minutes Time to return to baseline Heart rate |
Mean | Mean PIPP scores not significantly lower in KMC condition 30 and 60 seconds post‐heel lance Significant difference by 90 seconds post‐heel lance (KMC 8.871 (95% CI 7.85 to 9.89) versus Incubator 10.677 (95% CI 9.56to 11.79) P < 0.001). Insignificant difference continued to 120 seconds (8.86 (95% CI 7.48 to 10.26) versus 10.21 (95% CI 9.03to 11.39) P = 0.145). Significant difference in time returning to baseline heart rate at end of blood sampling (123 seconds (95% CI 103 to 142) for the KMC and 193 seconds for incubator (95% CI 158 to 227) (F (61,1) = 13.6, P < 0.0000). Facial actions significantly lower in KMC than incubator throughout phases. Maximum heart rate significantly lower at 30, 60 and 90 seconds. Minimum oxygen saturation levels significantly higher at 60 and 90 seconds Means and standard deviations for PIPP scores were obtained from the author |
| Johnston 2009 | Randomized cross‐over | 90 infants Postnatal age, range, days: 1 to 14 Postmenstrual age, mean ± SD, weeks: 33.4 ± 1.1 Birth weight, mean ± SD, grams: 1968 ± 388 |
Intervention: 30 minutes of skin‐to‐skin care before and during heel lance Comparison: 30 minutes of enhanced skin‐to‐skin care (rocking, singing/talking to baby, offering finger/pacifier for baby to suck Provider: mother |
PIPP score at 30, 60, 90, and 120 minutes | Mean | Mean PIPP scores not significantly different between conditions for any of the 30 s blocks of time. No difference in condition for examining time for heart rate to return to baseline Means and standard deviations for PIPP scores were obtained from the author |
| Johnston 2011 | Randomized cross‐over | 62 preterm infants (PMA 28 to 36 weeks) Postnatal age, mean, days: 5 to 10 Birth weight, mean ± SD, grams: 1565 ± 469 (father KC/mother KC); 1610 ± 494 (mother KC/father KC) |
Intervention: 30 minutes of skin‐to‐skin care before and during heel lance provided by mother Comparison: 30 minutes of skin‐to‐skin care before and during heel lance provided by father Provider: mother or father |
PIPP score at 30, 60, 90, and 120 minutes, time for HR to return to baseline | Mean difference, 95% confidence interval | Infants in maternal KC displayed significantly lower scores on the PIPP at 30 and 60 seconds after the heel lance than when in paternal KC (30 seconds mean difference 1.44 (95% CI 0.23 to 2.63); 60 seconds mean difference 1.55 (95% CI, 0.07 to 3.03). No differences at 90 and 120 seconds. The difference in time to return to KC heart rate before the heel lance was significant, with the time in maternal KC being 204 seconds and in paternal KC, 246 seconds (mean difference, 42 seconds (95% CI 5.16 to 81.06 seconds). |
| Johnston 2012 | Randomized cross‐over | 18 preterm infants (PMA 28 to 36 completed weeks) Postnatal age, range, days: within 10 days Birth weight, mean, grams: 2200 |
Intervention: 30 minutes of skin‐to‐skin care before and during heel stick provided by the mother Comparison: 30 minutes of skin‐to‐skin care before and during heel lance provided by an unrelated woman Provider: mother or an unrelated woman |
PIPP score at 30, 60, 90, and 120 minutes | Estimate of effect size (based Cohen’s formula, based on mean differences divided by the standard deviation) | The effect sizes on the pain scores (PIPP) were small, ranging from 1.1 to 1.7. The effect size at 30 sec was 0.23, at 60 sec was 0.24, at 90 sec was 0.43 and at 120 sec was 0.37 There was a 48% participation rate, with only 40 of 82 eligible cases having maternal consent. The main reason for refusal was discomfort with another woman providing kangaroo care |
| Kostandy 2008 | Randomized cross‐over | 10 infants Postmenstrual age, range, weeks: 30 to 32 Birth weight, mean ± SD, grams: 1577 ± 327.00 |
Intervention: 30 minutes of skin‐to‐skin care before and during heel lance Control: standard care during heel lance Provider: mother |
Cry duration at baseline, warming, heel lance, and recovery | Mean, standard deviation | Significant difference in crying time between study phases on both days (F (1,8) = 10.25, P < 0.001). When in KC as compared to the incubator, crying time was less during the heel lance (P = 0.001) and recovery (P = 0.01) phases |
| Liu 2015 | Randomized controlled trial | 40 infants Gestational age, mean ± SD, weeks: Intervention: 39.3 ± 0.94; Control: 39.36 ± 0.63 Birth weight, mean ± SD, grams: Intervention: 3337g ± 409.1; Control: 3740g ± 298.9 |
Intervention: Skin‐to‐skin care 15 minutes pre, during, and one minute after heel lance Control: Post bath, swaddled during and 1 min after heel lance. Provider: mother |
DAN score, crying time, pain facial expression duration, SpO2, HR | Mean, standard deviations | Decreased heart rate (P < 0.01), pain facial expression time (P = 0.041), crying time (P = 0.033), and DAN score (P < 0.01); increased oxygen saturation (P < 0.05) in skin‐to‐skin group. |
| Ludington‐Hoe 2005 | Randomized cross‐over | 23 preterm infants (< 37 weeks PMA) Postnatal age, mean ± SD, days: 22 ± 11.4 Postmenstrual age, mean ± SD, weeks: 31.4 ± 2.7 |
Intervention: 3 hours of skin‐to‐skin care before and during heel lance Control: standard care during heel lance Provider: mother |
Heart rate, respiratory rate, oxygen saturation, cry duration, behavioural state | Mean, standard deviation | Heart rate and length of crying in response to pain significantly reduced during KC and the KC heel lance as compared to when infants were in the warmer and had a heel lance in the warmer. Significant main effects were found for heart rate (F(1,32) = 3.54, P = 0.042) and cry length (F(1,32) = 5.20; P = 0.01). Mean rise in heart rate from baseline to heel lance was less in the KC condition than in the warmer condition (F(1, 32) = 3.01, P = 0.047). Crying length during KC heel lance significantly less than during warmer heel lance (F(1,32) = 7.38, P = 0.003) and post‐lance period (P = 0.02) |
| Mosayebi 2014 | Randomized Crossover | 64 preterm infants (GA range, mean, weeks ± SD = 30 to 36, 33 ± 1.95) Postnatal age, range, mean ± SD, days: 3 to 14, 7.28 ± 3.65 Birth weight range, mean ± SD: 1000 to 3500, 2095.85 ± 672.27 |
Intervention: 15 minutes of skin‐to‐skin care before, during, and two‐minutes post heel lance Control: 15 minutes prone and swaddled in an incubator before heel lance Provider: mother |
PIPP | mean, standard deviations | Mean score during and two minutes after intervention lower in skin‐to‐skin condition (P < 0.0) |
| Nimbalkar 2013 | Randomized cross‐over | 47 preterm infants (PMA 32 0/7 to 36 6/7 weeks) Postnatal age, mean, days: within 10 days Birth weight, mean, grams: 1730 (intervention), unclear (control) |
Intervention: 15 minutes of skin‐to‐skin care before, during, and 15 minutes after heel lance Control: standard care during heel lance Provider: mother |
PIPP score | Mean, standard deviation | Heart rate, behaviour and facial scores were statistically significant and lower in KMC group. But there was no statistically significant difference in oxygen saturation (SpO₂). The difference (4.85) in PIPP score was clinically and statistically significant (P < 0.001) |
| Okan 2010 | Prospective randomized controlled trial |
107 infants (35 treatment, 36 control, 36 comparison) Postnatal age, mean ± SD, days: 33.1± 5 Postmenstrual age, mean ± SD, days: 39.5 ± 0.6 |
Intervention: 15 min of skin‐to‐skin care before and during heel lance Control: standard care during heel lance Comparison: skin‐to‐skin care and breastfeeding before and during heel lance Provider: mother |
Crying time after painful stimulus Change in heart rate Change in SaO₂ NFCS |
Median, 25% to 75% IQR | Heart rate, oxygen saturation changes and length of crying were significantly reduced in treatment and comparison groups compared with control (P < 0.001). No difference found between treatment and comparison group Length of crying ‐ Intervention: 65 (50 to 133); Control: 184 (107 to 281); Comparison: 48 (40 to 98) Means and standard deviations for NFCS scores, heart rate and oxygen saturation were obtained from the author |
units: heart rate – beats/minute (bpm); crying time – seconds; postmensrutal age (PMA); Douleur Aigue Neonatal (DAN);Neonatal Facial Coding Scale (NFCS); Premature Infant Pain Profile (PIPP)
2. Trials assessing pain during intramuscular injection.
| Study | Design | Participants | Intervention | Outcome | Metrics Used | Results |
| Chermont 2009 | RCT | 640 infants (160 skin‐to‐skin care, 160 control, 160 comparison1, 160 comparison2) Postnatal age, mean ± SD, hrs: 293 ± 13 (skin‐to‐skin care), 29 ± 15 (control), 29 ± 13 (comparison1), 27 ± 13 (comparison2) postmenstrual age, mean ± SD, wk: 39 ± 1 (for all groups) Birth weight, mean ± SD, g: 3164 ± 371 (intervention); 3163 ± 418 (control); 3252 ± 389 (comparison1); 3240 ± 418 (comparison2) |
Intervention: skin‐to‐skin contact, initiated 2 minutes before injection and persisting throughout procedure Control: standard care during injection Comparison1: oral 25% dextrose treatment (1 mL), given 2 minutes before injection Comparison2: combination of oral dextrose treatment and skin‐to‐skin contact strategies. Provider: mother provided skin‐to‐skin; oral dextrose provided by nurse or neonatologist. |
Neonatal Facial Coding System (NFCS), Neonatal Infant Pain Scale (NIPS), and Premature Infant Pain Profile (PIPP) scores at baseline, cleansing, injection, and recovery | Mean, standard error | NFCS and NIPS scores for the 4 groups at the 4 study times showed that main effect of time and analgesic procedures were statistically significant (P < 0.001), as was interaction between time and procedure (P < 0.001). Either skin‐to‐skin contact or 25% dextrose treatment alone did not significantly affect pain scores during injection, but the combination of both significantly decreased these scores during the invasive procedure. Mean PIPP scores showed significant differences among groups (P < 0.001). PIPP scores were lower when IM vaccine injections were given to healthy neonates during skin‐to‐skin contact with their mothers, regardless of whether oral 25% dextrose treatment was administered. Isolated use of the sweetener did not decrease PIPP scores, compared with standard care. Heart rate and oxygen saturation variability (not defined) were reported significantly to favour of SSC over both control and sucrose. |
| Kostandy 2013 | Randomized controlled trial | 36 term infants (Gestational Age, mean, weeks = 39.6) Postnatal age, mean, hours = Intervention: 24.29; Control: 28.35 Birth weight mean ± SD, grams: Intervention: 3389.7 ± 333.3; Control: 3326.8 ± 324.08 |
Intervention: skin‐to‐skin for 10‐15 minutes before, and during IM injection. Control: supine in bassinet 10 to 15 minutes before IM injection. Provider: mother |
Cry time, behavioural state, heart rate | means, standard deviations | neonates in skin‐to‐skin group had shorter cry time during recovery (16 vs 72 seconds, P = 0.007), calmer behavioural state (2.82 vs 6.47 time points to reach non‐crying state P = 0.005) |
| Saeidi 2010 | RCT | 60 full‐term infants (80% of case group and 73.3% of control group had 40 weeks GA) Birth weight, mean ± SD, grams: 3242 ± 306.6 (intervention), 3151 ± 331.5 (control) |
Intervention: 30 minutes skin to skin contact Control: standard care during injection Provider: mother provided skin‐to‐skin care |
Behavioural changes using the Neonatal/Infant Pain Scale (NIPS) 2 minutes before, during, and 3 minutes after intervention heart rate oxygen saturation |
NIPS: number (%) O₂ saturation: mean, SD HR and crying interval: P values |
Mean pain intensity during the intervention was significantly lower in the case group (P < 0.006). Mean pain intensity 3 minutes after intervention was also significantly lower in the case group (P < 0.021). Mean duration of crying was significantly lower in the case group as well (P < 0.001) |
| Sajedi 2007 | RCT | 100 infants (50 intervention, 50 control) Postmenstrual age, mean ± SD, weeks: 39.36 ± 1.45 (intervention), 39.12 ± 1.42 (control) Birth weight, mean ± SD, grams: 3083.2 ± 258.33 (intervention), 3142.2 ± 242.3 (control) |
Intervention: 10 minutes of skin‐to‐skin care before and during painful procedure, and 3 minutes after injection Control: standard care during injection Provider: mother provided skin‐to‐skin care |
Neonatal Infant Pain Scale (NIPS), Behavioural responses (facial expression, breathing pattern, state of arousal, arm and leg movements, and cry), heart rate and oxygen saturation before, during and after injection | Mean, standard deviations, Chi², degrees of freedom | Significantly more severe behavioural responses immediately after injection in control than intervention group (P < 0.001). NIPS scores immediately after injection significantly higher in control than intervention group (P < 0.001). Duration of crying post‐injection significantly longer in control than intervention group (P = 0.001). No significant difference in mean heart rate before injection (P = 0.4) but during (P < 0.001), and after (P < 0.001) injection, favouring the KC group. No significant difference in the blood oxygen saturation before (P = 0.7) but during (P < 0.001) and after (P < 0.001) injection between the 2 groups, favouring the KC group |
3. Trials assessing pain during heel lance and venepuncture.
| Study | Design | Participants | Intervention | Outcome | Metrics Used | Results |
| Akcan 2009 | RCT | 50 preterm infants (25 SSC, 25 control), PMA 31.6 ± 2.0 weeks, Birth weight 1669 ± 530 (total) |
Intervention: 45 minutes of uninterrupted skin‐to‐skin every day for 5 days, with the painful procedure carried out on the 5th day Control: standard care during painful procedure Provider: mother |
Premature Infant Pain Profile (PIPP) scores at baseline, the 1st, 2nd, and 3rd minute of the painful procedure, and the 1st and 2nd minute after the painful procedure | Means, 95% CI, Chi² | KC was found to be effective in decreasing pain during and after invasive procedure in premature infants. PIPP scores at the first, second, and third minute of the procedure were 7, 4 and 4 in the KC group and 15, 15.5 and 15 in control (P < 0.001, P = 0.001, P = 0.047, respectively). PIPP scores at the 1st and 2nd minute after painful procedure were 4 and 4 in infants in KC and 12.5 and 7 in infants in the control group, respectively. PIPP scores soon after the invasive procedure were significantly lower in infants in the KC group compared to the control group (P < 0.001, P = 0.023, respectively) |
4. Trials assessing pain with different skin‐to‐skin providers.
| Study | Design | Participants | Intervention | Outcome | Metrics Used | Results |
| Johnston 2011 | Randomized cross‐over | 62 preterm infants (PMA 28 to 36 weeks) Postnatal age, mean, days: 5 to 10 Birth weight, mean ± SD, grams: 1565 ± 469 (father KC/mother KC); 1610 ± 494 (mother KC/father KC) |
Intervention: 30 minutes of skin‐to‐skin care before and during heel lance provided by mother Comparison: 30 minutes of skin‐to‐skin care before and during heel lance provided by father Provider: mother or father |
PIPP score at 30, 60, 90, and 120 minutes, time for HR to return to baseline | Mean difference, 95% CI | Infants in maternal KC displayed significantly lower scores on the PIPP at 30 and 60 seconds after the heel lance than when in paternal KC (30 seconds mean difference 1.435 (95% confidence interval 0.23 to 2.63); 60 seconds mean difference, 1.548 (95% CI 0.07 to 3.03). No differences at 90 and 120 seconds The difference in time to return to KC heart rate before the heel lance was significant, with the time in maternal KC being 204 seconds and in paternal KC, 246 seconds (mean difference 42 seconds (95% CI 5.16 to 81.06 seconds) |
| Johnston 2012 | Randomized cross‐over | 18 preterm infants (PMA 28 to 36 completed weeks) Postnatal age, range, days: within 10 days Birth weight, mean, grams: 2200 |
Intervention: 30 minutes of skin‐to‐skin care before and during heel stick provided by the mother Comparison: 30 minutes of skin‐to‐skin care before and during heel lance provided by an unrelated woman Provider: mother or an unrelated woman |
PIPP score at 30, 60, 90, and 120 minutes | Estimate of effect size (based on Cohen’s formula, based on mean differences divided by the standard deviation) | The effect sizes on the pain scores (PIPP) were small, ranging from 1.1 to 1.7. The effect size at 30 sec was 0.23, at 60 sec was 0.24, at 90 sec was 0.43 and at 120 sec was 0.37 There was a 48% participation rate, with only 40 of 82 eligible cases having maternal consent. The main reason for refusal was discomfort with another woman providing kangaroo care |
5. Trials assessing pain during tape removal.
| Study | Design | Participants | Intervention | Outcome | Metrics Used | Results |
| Nanavati 2013 | Randomized controlled trial | 50 preterm neonates (Gestational age, mean ± SD weeks: Intervention: 32.72 ± 2.03; Control: 32.4 ± 2.16) Postnatal age, mean ± SD, days: Intervention: 7.12 ± 6.64; Control: 5.4 ± 3.65) Birth weight, mean ± SD, grams: Intervention: 1352.76 ± 150.12, Control: 1235.48 ± 169.12 |
Intervention: skin‐to‐skin care 15 minutes before, and during tape removal. Control: swab soaked with expressed breast milk inserted in infants mouth 2 minutes before, and during tape removal. Provider: mother |
PIPP | mean, standard deviation | Post intervention PIPP score was no significantly different between groups (P = 0.62) |
Outcome measures were varied among studies, with many including more than one. Physiological measures included heart rate during the painful procedure (Gray 2000; Johnston 2003; Ludington‐Hoe 2005; Sajedi 2007; Castral 2008; Freire 2008; Johnston 2008; Cong 2009; Okan 2010; Saeidi 2011; Cong 2012; Gabriel 2013; Kostandy 2013; Nimbalkar 2013; Gao 2015; Liu 2015) and after the painful procedure (Gray 2000; Ludington‐Hoe 2005; Sajedi 2007; Castral 2008; Johnston 2008; Cong 2009; Cong 2012; Gabriel 2013; Liu 2015); heart rate recovery (time to return to baseline levels post‐procedure (Johnston 2008; Johnston 2009; Johnston 2011; Johnston 2012; Kostandy 2013; Gao 2015); spectral analysis of electrocardiogram (ECG) signals of low frequency spectrum, high frequency spectrum, and low‐to‐high frequency ratio (Cong 2009; Cong 2012); transcutaneous oxygen saturation levels (Johnston 2003; Ludington‐Hoe 2005; Sajedi 2007; Johnston 2008; Okan 2010; Saeidi 2011; Liu 2015); respiratory rate (Ludington‐Hoe 2005); and salivary cortisol levels (Cong 2011). Behavioural state was used in two studies (Ludington‐Hoe 2005; Cong 2009). Cry duration was an outcome for seven studies (Gray 2000; Ludington‐Hoe 2005; Kostandy 2008; Okan 2010; Saeidi 2011; Gabriel 2013; Kostandy 2013). Facial grimacing, not according to a validated measure, was used in three studies (Gray 2000; Okan 2010; Gao 2015), while in three others the validated Neonatal Facial Coding Scale (NFCS) was used (Castral 2008; Chermont 2009; Okan 2010). Validated composite pain measures that included both physiological and behavioural indicators were used in 17 studies. The Premature Infant Pain Profile (PIPP) was used in 12 studies (Johnston 2003; Freire 2008; Johnston 2008; Akcan 2009; Chermont 2009; Johnston 2009; Cong 2011; Johnston 2011; Johnston 2012; Nanavati 2013; Nimbalkar 2013; Mosayebi 2014), the Neonatal Infant Pain Scale (NIPS) was used in four studies (Sajedi 2007; Chermont 2009; Saeidi 2011; Gabriel 2013) and the Douleur Aiguë du Nouveau‐né (DAN) for one Liu 2015.
Excluded studies
Of the 29 studies that were excluded, three focused on breastfeeding (Uga 2008; Abdel‐Razek 2009; Obeidat 2015), four did not have SSC as defined in this review (Bellieni 2002; Arditi 2006; Bellieni 2007; Vivancos 2010), and three did not have ventral skin contact as a part of their SSC intervention (Reis 2003; Axelin 2009; Campbell‐Yeo 2012). One had skin‐to‐skin contact but not during the procedure (Mitchell 2013). Maternal interview was the focus of one study (Silva 2004); and maternal mood and stress the focus of another with no comparison group (Castral 2015). Three studies did not randomize (Chidambaram 2014; Choudhary 2015; Olsson 2015). One study used maternal voice without actual contact (Johnston 2007b); while another used neurobehavioural scores (NIDCAP) associated with pain, which are not validated as a pain measure (Ferber 2008). One study, Kashaninia 2008, was a duplicate of another (Sajedi 2007). One was a protocol of an ongoing study (Campbell‐Yeo 2013). One study used diaper change as procedure, which is not within our definition of being tissue damaging or painful (Lyngstad 2014). Finally, seven studies reported on SSC alone without implementation of a painful procedure (Mooncey 1997; Gazzolo 2000; Mörelius 2005; Miles 2006; Erlandsson 2007; Gabriel 2010; Schlez 2011).
Risk of bias in included studies
The risk of bias for each study may be seen under Characteristics of included studies and as percentages across all included studies in Figure 2.
2.
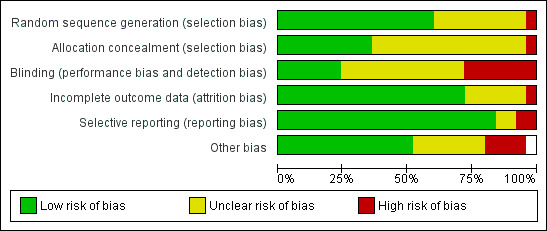
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Random sequence generation, a procedure to avoid selection bias, was adequate in 16 studies. Allocation concealment, another source of selection bias, was deemed adequate for 10 studies.
Blinding
Blinding to avoid performance or detection bias by definition is impossible to achieve given the nature of the intervention. Clearly reported blinding of assessors was adequate in only nine of the studies. Although 12 of the remaining trials did institute measures to overcome detection bias, there was some uncertainty that the video recordings were made in such a way that the SSC provider could not be identified. Only two studies specifically mentioned how they dealt with blinding the observers.
Incomplete outcome data
Incomplete outcome data were rated above low risk in 19 studies.
Selective reporting
Reporting was adequate in all but three studies. Two were unclear and one study's parameters as reported in the trial registry were not included in the report.
Other potential sources of bias
Fifteen studies had low risk of other biases. There were three individual cases of high bias: there was a combination of two data sets; different times for the painful procedure by group were not included in the regression analysis; and consent was obtained after randomization. Seven other studies had unclear potential bias. In one, there was some potential for inconsistency among sites regarding sucrose use in the usual care group. A power calculation was not reported in two studies, and the washout period was not described in the others.
Heterogeneity results
There were many outcomes for which heterogeneity could not be measured via the I² statistic, that is, where only one study reported an outcome such as change in heart rate or change in oxygen saturation. When the I² statistic could be calculated the results showed a wide range, with the physiological outcomes having I² values of 0% and some composite measures having values over 50%, for example, PIPP at 30 seconds following heel lance versus control, I² = 94%.
Effects of interventions
See: Table 1
Inconsistencies in the outcomes prevented all studies from being included in meta‐analyses. Each study is thus reported separately and appears in Table 2 and Table 3 below, grouped according to the painful intervention.
1. Effectiveness of skin‐to‐skin care (SSC) compared to control (Comparison 1)
1.1 Heart rate response
Fifteen studies examined heart rate during the heel lance procedure (Gray 2000; Johnston 2003; Ludington‐Hoe 2005; Sajedi 2007; Castral 2008; Freire 2008; Johnston 2008; Cong 2009; Okan 2010; Cong 2012; Gabriel 2013; Nimbalkar 2013; Gao 2015; Liu 2015) or IM injection (Kostandy 2013). Only five studies could be combined in an analysis (Ludington‐Hoe 2005; Castral 2008; Cong 2009; Cong 2012; Liu 2015) with all but Liu 2015 (who compared SSC to swaddled in incubator) comparing SSC to incubator. Since results of the first condition only were not reported in cross‐over designs, and not all authors responded to requests for the data for the first condition separately, not all crossover studies could be included in the meta‐analysis. One study — Gao 2015 — reported mean scores over three heel lances, but we were not able to get data for only one. The duration of time for which the heart rate was collected either varied between studies or was not reported. Finally, when authors did respond, the calculations were conducted differently (that is maximum, not mean heart rate, was acquired). Cong 2012 reported two studies in the same manuscript, one of SSC for 30 minutes and one of SSC for 15 minutes. One other study, Sajedi 2007, examined heart rate averaged over two minutes during intramuscular injection and reported lower scores, that is, in favour of SSC. Johnston 2008 provided unpublished data for the first condition on maximum heart rate with significant differences in favour of SSC. Okan 2010 reported the median heart rate for SSC plus breast feeding, SSC alone, and control, and found significantly higher heart rate in the control but similar levels in the two intervention groups. Johnston 2003 reported no overall differences; nor did Gao 2015, who compared heart rate over three heel lances. Kostandy 2013 did not find any differences between groups.
The meta‐analysis across the five studies of heel lance versus incubator control (one with additional swaddling) showed differences between the experimental and control groups ranging from 0.57 to 13.53 beats per minute with a significant MD of −10.78 (95% CI −13.63 to −7.93) beats per minute (Analysis 1.1). One study, with additional swaddling (Liu 2015), made the difference of whether effect was significant. This effect was based on very low level evidence.
1.1. Analysis.
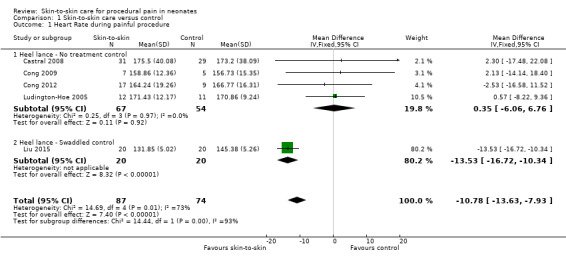
Comparison 1 Skin‐to‐skin care versus control, Outcome 1 Heart Rate during painful procedure.
1.2 Heart rate recovery
Heart rate following the painful procedure was reported in seven articles, but only four could be entered into the analysis (Ludington‐Hoe 2005; Castral 2008; Cong 2009; Cong 2012). The MD was not statistically significant with MD 0.08 (95% CI −4.39 to 4.55) (Analysis 1.2). The level of evidence for this effect was high. Findings from Gray 2000 favoured SSC, but Kostandy 2013 found no difference. Johnston 2008 reported that the time to return to baseline heart rate following the application of the adhesive bandage (signifying the end of blood sampling) was significantly faster at 123 seconds (95% CI 103 to 142) for the KC condition and 193 seconds for the incubator condition (95% CI 158 to 227; F (61, 1) = 13.6, P < 0.001).
1.2. Analysis.
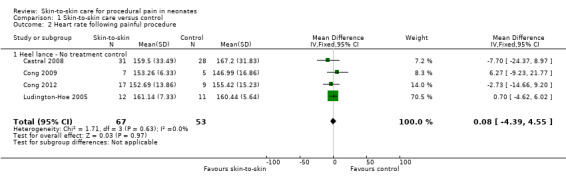
Comparison 1 Skin‐to‐skin care versus control, Outcome 2 Heart rate following painful procedure.
1.3 Heart rate variability
Two studies reported heart rate variability as an outcome (Cong 2009; Cong 2012). Both studies had a cross‐over design and the first condition was separated out for this review. Low frequency was not significant (MD −2.11, 95% CI −17.69 to 13.47) (Analysis 1.3); nor was high frequency (MD −5.11, CI −23.36 to 13.14) (Analysis 1.4); nor was the low frequency/high frequency (LF/HF) ratio significantly different (MD −3.77, −13.69 to 6.14) (Analysis 1.8). In Cong 2012, infants were randomly ordered into 15 minutes of SSC, 30 minutes of SSC, and incubator control. The heart rate variability results were not significantly different among the conditions. The level of evidence for the effect of SSC on heart rate variability (LF, HF, and LF/HF ratio) during painful procedure was low, whereas the level of evidence for the effect of SSC on heart rate variability LF and HF following painful procedure was high. The effect of SSC on heart rate variability LF/HF ratio after painful procedure is supported by low‐level evidence.
1.3. Analysis.
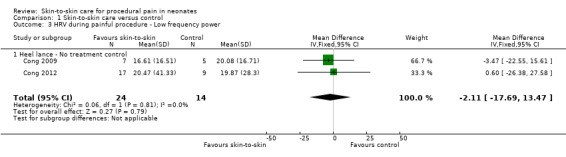
Comparison 1 Skin‐to‐skin care versus control, Outcome 3 HRV during painful procedure ‐ Low frequency power.
1.4. Analysis.
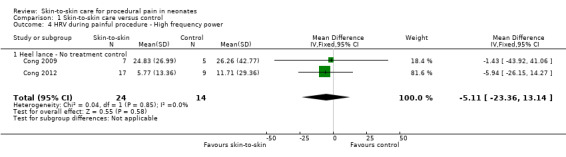
Comparison 1 Skin‐to‐skin care versus control, Outcome 4 HRV during painful procedure ‐ High frequency power.
1.8. Analysis.
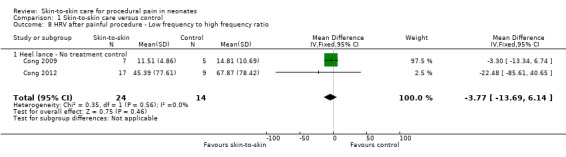
Comparison 1 Skin‐to‐skin care versus control, Outcome 8 HRV after painful procedure ‐ Low frequency to high frequency ratio.
1.4 Oxygen saturation during painful procedure
Four studies used oxygen saturation as an outcome (Ludington‐Hoe 2005; Sajedi 2007; Johnston 2008; Liu 2015), however only the latter two could be combined for analysis as they specified 30‐second intervals. The MD at both 30 and 60 seconds was not significant: at 30 seconds, MD was 1.73 (95% CI −0.53 to 3.99) (Analysis 1.9); and at 60 seconds MD was 2.17 (95% CI −0.12 to 4.46) (Analysis 1.10). Sajedi 2007 examined full‐term neonates receiving intramuscular injection. Sajedi 2007 reported an almost 4% lower oxygen saturation (P < 0.001) in the control group, favouring SSC. Ludington‐Hoe 2005 did not find significant differences between SSC and incubator care. The effect of SSC on oxygen saturation at 30 seconds is supported by low‐level evidence and the effect at 60 seconds is supported by very low level evidence.
1.9. Analysis.
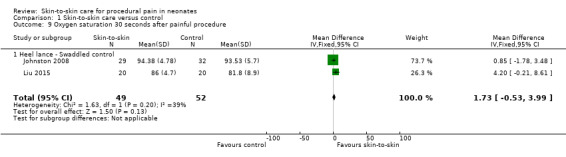
Comparison 1 Skin‐to‐skin care versus control, Outcome 9 Oxygen saturation 30 seconds after painful procedure.
1.10. Analysis.
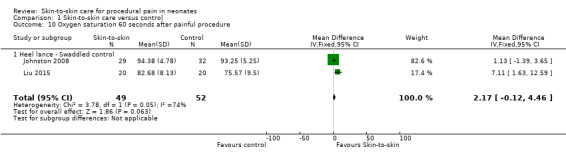
Comparison 1 Skin‐to‐skin care versus control, Outcome 10 Oxygen saturation 60 seconds after painful procedure.
1.5 Oxygen saturation after painful procedure
Two of the same studies as above (Ludington‐Hoe 2005; Sajedi 2007) as well as Saeidi 2011 also reported oxygen saturation at the end of the painful procedure. Sajedi 2007 reported a significant 2.8% higher oxygen saturation in the SSC group, but the Ludington‐Hoe 2005 study showed wide variance with a similar magnitude of difference which was not significant. Saeidi 2011 reported non‐significant differences in oxygen saturation.
1.6 Change in oxygen saturation
Only one study examined change in oxygen saturation (Freire 2008), in which the difference was not significant between SSC and standard care control (Table 2). Nimbalkar 2013's raw data showed a difference in oxygen saturation but following the Bonferroni correction this was not significant.
1.7 Salivary cortisol
Only one study used salivary cortisol levels as an outcome (Cong 2011 (Study 1 and 2)). There were two subsamples in that study, one receiving SSC for 80 minutes and the other for 30 minutes. There were significantly higher salivary cortisol levels in the 80‐minute SSC group (mean ± SD: 0.19 ± 0.10) than the standard care control group (mean ± SD: 0.15 ± 0.06), P < 0.05. Conversely, salivary cortisol levels were lower in the 30‐minute SSC group (mean ± SD: 0.21 ± 0.12) than the standard care control group (mean ± SD: 0.57 ± 0.61), P < 0.05 (Table 2).
1.8 Premature Infant Pain Profile (PIPP) at 30 seconds
Six studies used the PIPP as the outcome for heel lance (Johnston 2003; Freire 2008; Johnston 2008; Cong 2011 (Study 1 and 2) Mosayebi 2014) or IM injection (Chermont 2009). Cong 2011 was analyzed for the two amounts of time of SSC so that the first study of 80‐minute SSC was entered first and the second study of SSC for 30 minutes was entered second, although both are listed as Cong 2011. The PIPP was reported in 30‐second blocks from the time of the heel lance. At 30 seconds, based on analyses of four of the studies (Johnston 2003; Freire 2008; Johnston 2008; Cong 2011), there was moderate‐level evidence of a significant effect in favour of SSC (MD −3.21, 95% CI −3.94 to −2.47), although Cong 2011 (study 1) and Johnston 2008 did not find a significant difference (Analysis 1.11). Mosayebi 2014 used the PIPP but changed the scoring to an ordinal scale which could not be entered into the analysis, although the results favoured SSC. The means and standard deviations are reported in the text, but not separated between first and second condition and the authors did not forward us those separated data.
1.11. Analysis.
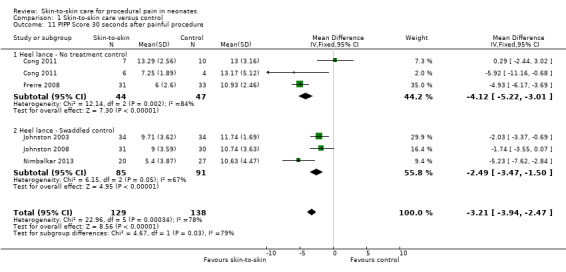
Comparison 1 Skin‐to‐skin care versus control, Outcome 11 PIPP Score 30 seconds after painful procedure.
1.9 PIPP scores at 60 seconds
Three studies used the PIPP as an outcome for heel lance (Johnston 2003; Johnston 2008; Cong 2011 (Study 1 and 2)); and one used it as an outcome for heel lance or venipuncture (Akcan 2009). There was a significant difference in favour of SSC in the analysis of heel lance (MD −1.64, 95% CI −2.86 to −0.43). This effect is supported by low‐level evidence. Johnston 2008 and Cong 2011 (Study 1) did not find a significant difference (Analysis 1.12); while Akcan 2009 did (SSC = 7.0, Control = 15.0, P < 0.001).
1.12. Analysis.
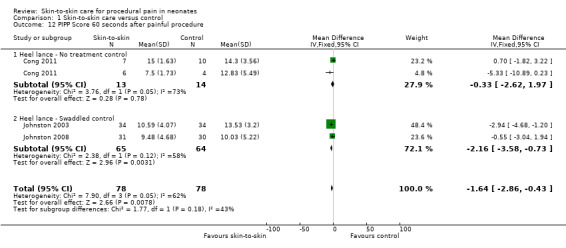
Comparison 1 Skin‐to‐skin care versus control, Outcome 12 PIPP Score 60 seconds after painful procedure.
1.10 PIPP at 90 seconds
There was a significant difference in favour of SSC with three studies for the PIPP score at 90 seconds (MD −1.28, 95% CI −2.53 to −0.04) (Analysis 1.13) (Johnston 2003; Johnston 2008; Cong 2011 (Study 2)). This effect is supported by moderate level evidence.
1.13. Analysis.
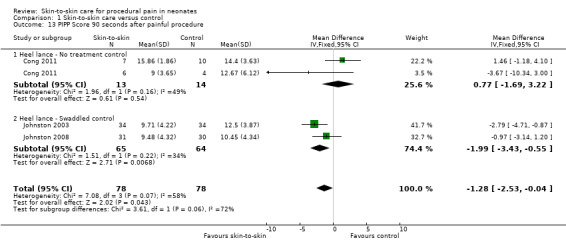
Comparison 1 Skin‐to‐skin care versus control, Outcome 13 PIPP Score 90 seconds after painful procedure.
1.11 PIPP at 120 seconds
Three studies used the PIPP at 120 seconds as an outcome for heel lance (Johnston 2003; Johnston 2008; Cong 2011); and one used it as an outcome for heel lance or venipuncture (Akcan 2009). There was an MD of 0.07 (95% CI −1.11 to 1.25), reflecting no significant difference (Analysis 1.14). This effect is supported by moderate level evidence. Akcan 2009 reported PIPP of 4 in the SSC group compared to 15.5 in control group (P = 0.001).
1.14. Analysis.
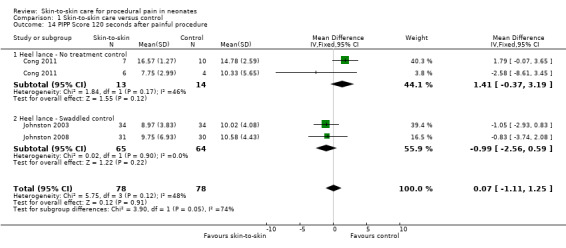
Comparison 1 Skin‐to‐skin care versus control, Outcome 14 PIPP Score 120 seconds after painful procedure.
1.12 PIPP following end of procedure
Two studies, Cong 2011 (two studies of 80 and 30 minutes SSC) and Akcan 2009, followed PIPP scores beyond the time of the procedure. In both Cong 2011 studies the PIPP scores favoured SSC, measured in 30‐second blocks for two minutes following the procedure, by between 8.12 and 0.4 points on the PIPP. The closer to the end of the procedure the greater the difference was in scores. In the first and second minute following end of procedure, Akcan 2009 reported PIPPs of 4 and 4 in infants in KC and 12.5 and 7 in infants in the control group (P < 0.001, P = 0.023, respectively).
1.13 Neonatal Facial Coding System (NFCS) during painful procedure
One study used the NFCS as an outcome for heel lance on preterm neonates (Castral 2008), and another used it as an outcome in full‐term neonates for intramuscular injection (Chermont 2009). In Castral 2008 there was a mean difference of 1.87 in favour of SSC (P < 0.001). There was no difference between skin‐to‐skin alone and the standard care control in Chermont 2009.
1.14 NFCS at recovery
Similarly, at recovery one study used the NFCS as an outcome for heel lance in preterm neonates (Castral 2008); and another for intramuscular injection in full‐term neonates (Chermont 2009). Both studies favoured SSC.
1.15 Duration of crying after painful procedure
Six studies included cry duration as an outcome. Meta‐analysis was conducted including two studies investigating response to heel lance (MD −34.16, 95% CI −42.86 to −25.45) (Analysis 1.19) (Ludington‐Hoe 2005; Kostandy 2008); and two following IM injection (MD −8.83, 95% CI −14.63 to −3.02) with results favouring SSC (Analysis 1.20) (Kostandy 2013; Sajedi 2007). The level of evidence for the effect of SSC on duration of cry was moderate. The other two studies did not provide enough information to include their results in the analysis (Gray 2000; Okan 2010). Gray 2000 reported that infants held by mother in skin‐to‐skin contact cried an average of 1 second while control infants cried for a mean of 32 seconds of the 3‐minute recovery period (P < 0.001). Similarly Okan 2010 found reduced length of crying in SSC (mean 65 seconds vs 184 seconds in control, P < 0.001).
1.19. Analysis.
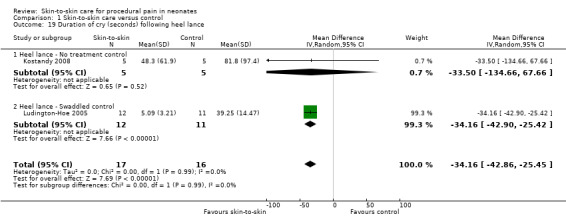
Comparison 1 Skin‐to‐skin care versus control, Outcome 19 Duration of cry (seconds) following heel lance.
1.20. Analysis.
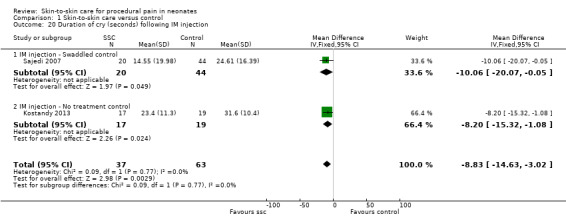
Comparison 1 Skin‐to‐skin care versus control, Outcome 20 Duration of cry (seconds) following IM injection.
1.16 Neonatal Infant Pain Scale (NIPS)
Chermont 2009, Saeidi 2011, Sajedi 2007 used the NIPS as an ordinal outcome and these were able to be entered into an analysis. There was a significant difference in proportion of infants in no pain (Risk Difference (RD) 0.10, 95% CI 0.06 to 0.15) versus severe pain (RD −0.16, 95% CI −0.22 to −0.10) in favour of SSC during the painful procedure. The level of evidence for this effect was very low. Similarly during recovery, there was a significant relative risk in favour of SSC of those with no pain (RD 0.35, 95% CI 0.26 to 0.44) and those with severe pain (RD −0.23, 95% CI −0.31 to −0.15). The level of evidence in favour of SSC for the proportion of infants in no pain during recovery was moderate. Gabriel 2013 used NIPS and reported results comparing interventions in median scores, but had only active comparisons with no control group.
1.17 Serum cortisol
Only one study examined serum cortisol level, comparing 80 minutes and 30 minutes of SSC with a standard care control (Cong 2011). The study showed similar serum cortisol levels in the 80‐minute SSC group (mean ± SD: 5.73 ± 1.97) than the standard care control group (mean ± SD: 5.32 ± 1.72). Conversely, serum cortisol levels were lower in the 30‐minute SSC group (mean ± SD: 5.63 ± 2.30) than the standard care control group (mean ± SD: 9.15 ± 6.59), P < 0.05 (Table 2).
1.18 Douleur Aiguë du Nouveau‐né (DAN) Scale
One study used the DAN as an outcome measure and found significant differences in favour of the SSC group, although the report does not include specific values, only significance levels (Liu 2015).
1.19 Sleep and wake state
Five studies reported on sleep and wake state (Ludington‐Hoe 2005; Sajedi 2007; Kashaninia 2008; Cong 2009; Cong 2012). Since this is a categorical or ordinal outcome, no analysis was performed. One study, Sajedi 2007, was conducted with full term neonates while the others were with preterm neonates. That study reported state as a dichotomous outcome, 'fussy' or 'any other state', and reported a higher proportion of infants to be in a 'fussy' state in the control condition. There were no differences in sleep and wake state at the time of the invasive procedure, although Cong 2012 reported more infants in the SSC group in quiet sleep during recovery following the procedure, as did Ludington‐Hoe 2005 who reported that infants in SSC were more likely to be in deep sleep during baseline and heel warming. Kashaninia 2008 reported state as a dichotomous outcome, 'fussy' or 'any other state', and reported a higher proportion of infants to be in a 'fussy’ state in the control condition.
2. Effectiveness of skin‐to‐skin care (SSC) with different providers (Comparison 2)
Two studies compared different providers of SSC (Johnston 2011; Johnston 2012), although Johnston 2012 was reported only as a pilot study aimed at examining feasibility and effect size (Table 5). Since both studies examined preterm neonates undergoing heel lance and used PIPP scores at 30‐second intervals over two minutes following the heel lance as well as heart rate recovery (defined as time for the heart rate to return to baseline levels) they were entered into a comparison. Differences in heart rate recovery were not significant (MD −32.58, 95% CI −94.52 to 28.59) in spite of the large mean difference in favour of the mother, due mostly to high variation. The level of evidence for the effect on heart rate recovery was low. PIPP scores similarly had large mean differences favouring the mother but the variance was also large so that any difference was non‐significant (Analysis 2.2; Analysis 2.3; Analysis 2.4; Analysis 2.5). The level of evidence for the effect on PIPP 30 at 30, 60, and 90 seconds following painful procedure was moderate, and at 120 seconds following painful procedure was high.
2.2. Analysis.
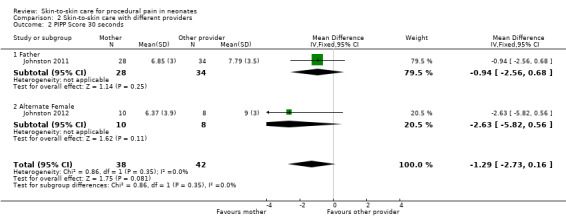
Comparison 2 Skin‐to‐skin care with different providers, Outcome 2 PIPP Score 30 seconds.
2.3. Analysis.
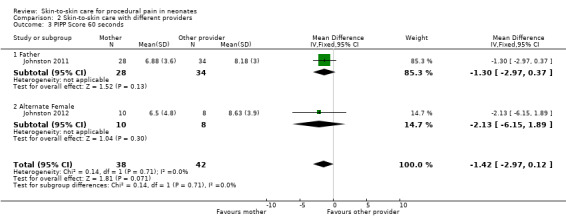
Comparison 2 Skin‐to‐skin care with different providers, Outcome 3 PIPP Score 60 seconds.
2.4. Analysis.
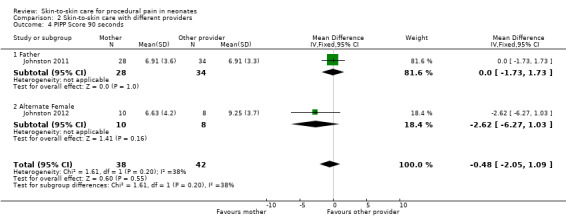
Comparison 2 Skin‐to‐skin care with different providers, Outcome 4 PIPP Score 90 seconds.
2.5. Analysis.
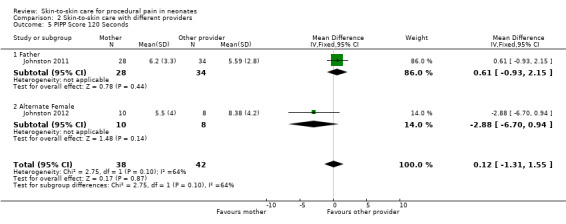
Comparison 2 Skin‐to‐skin care with different providers, Outcome 5 PIPP Score 120 Seconds.
3. Effectiveness of skin‐to‐skin care (SSC): analysis by duration of SSC
Cong 2011 and Cong 2012 reported results from different durations of SSC, the first comparing 80‐ to 30‐minutes SSC and the second comparing 30 to 15 minutes. Both studies examined preterm neonates undergoing heel lance and some physiological outcomes were the same so that an analysis was able to be performed in that report, favouring 30 minutes. No comparisons could be made between other reports.
4. Effectiveness of skin‐to‐skin care (SSC) compared to alternative treatments
There were no studies that could be combined for analysis. There were some interesting comparisons that are described below.
4.1 SSC versus sweet taste
The study by Chermont 2009 on full‐term newborns receiving an intramuscular injection compared SSC alone or in combination with dextrose to incubator controls. On the PIPP outcome, SSC was most effective with or without the addition of dextrose. On the NFCS and NIPS, SSC was favoured over dextrose or control, although the combination was most effective. Freire 2008 also compared SSC with sweet taste (glucose) to control in preterm neonates undergoing heel lance, with the PIPP score. Heart rate and oxygen saturation variability (not defined) were reported to significantly favour SSC over both control and glucose. This was also reported for the composite measure of these variables, the PIPP. All outcomes favoured SSC.
Gabriel 2013 compared SSC alone and in combination with both sucrose and breastfeeding on heart rate, NIPS scores, and crying time. The combinations were both more effective than SSC alone for NIPS and crying.
4.2 SSC versus breastfeeding
Okan 2010 compared SSC to breastfeeding or swaddled control in full‐term neonates undergoing heel lance. In all outcomes (heart rate, oxygen saturation, NFCS, and duration of crying) there were no differences between SSC or breastfeeding, but both were better than the swaddled control group. Gabriel 2013, as above, compared SSC alone and in combination with both sucrose and breastfeeding on heart rate, NIPS scores, and crying time . The combinations were both more effective than SSC alone for NIPS and crying.
4.3 SSC versus enhanced SSC
One study, Johnston 2009, examined PIPP scores in preterm neonates undergoing heel lance for differences between SSC and SSC enhanced by the mother rocking, singing and offering the infant a finger or pacifier for sucking. There were no differences between the conditions.
4.4 SSC versus Expressed Breastmilk
One study, Nanavati 2013, comparing SSC with expressed breast milk for adhesive tape removal, found both interventions equally effective in diminishing pain.
5. Effectiveness of skin‐to‐skin care (SSC): analysis by dose or duration of SSC
The range of time for SSC prior to the intervention was two minutes (Saeidi 2011) to three hours (Ludington‐Hoe 2005). The only studies that compared times were Cong 2011 and Cong 2012. However, these studies used different outcomes and thus no analyses could be conducted. In Cong 2011, 80‐minute SSC and 30‐minute SSC were independently compared to control for the physiological variables of heart rate and heart rate variability. SSC was favoured only in the 30‐minute condition. In Cong 2012, the PIPP was used as an outcome for SSC for 15 minutes, SSC for 30 minutes, or control. The 30‐minute SSC was favoured over control and 15‐minute SSC. Although these two studies, not directly compared, seemed to favour 30 minutes to either longer or shorter doses, other studies using different outcomes favoured (or did not favour) SSC for times longer and shorter than 30 minutes so no conclusion could be made.
6. Effectiveness of skin‐to‐skin care (SSC): analysis by postmenstrual age (PMA)
Outcomes of studies reporting different PMAs were different so that comparisons could not be made. Studies examined different times and some used the same outcomes (for example, PIPP) but the comparisons and painful procedures were different so that an effect size could not be estimated.
Discussion
Summary of main results
In this revision of the first review of skin‐to‐skin care (SSC) for procedural pain in neonates, 25 studies were found that met the selection criteria of using SSC as an intervention to reduce pain. Most of the studies used the most common painful event of heel lance as the painful procedure, although venipuncture and intramuscular injections were also among the painful procedures. A small proportion of studies could be compared due to variations in design or outcomes. Even if the outcome measures were the same, they were often used differently enough that they could not be combined.
The most detailed information was found in studies with preterm neonates undergoing heel lance for SSC versus control with either heart rate, heart rate variability, or the composite measure PIPP and NIPS as outcomes. With the addition of six studies from the first review to this revision, meta‐analyses of heart rate, crying time, and composite measures of pain PIPP and NIPS were significantly favouring SSC. Oxygen saturation, and heart rate variability differences were not significant.
Two studies examined different providers of SSC and were able to be entered into an analysis for the PIPP and heart rate recovery. The differences between mother provider and other provider were not significant.
No analyses could be conducted on the effect on outcomes of duration of SSC or different age groups of infants.
Overall completeness and applicability of evidence
Although most of the studies used heel lance as the painful procedure, several used injections, including ones found for this newer review, such that we included both procedures in some analyses. The data analyses tables separate out the procedures, but we report the overall values which include both sessions.
Although it would be of interest to know if there was a dose‐response relationship, that is, did the number of minutes in SSC increase the effectiveness, we were not able to conduct that analysis. We were not even able to make a direct comparison of differences with 30 minutes as a cut‐off point. Ludington‐Hoe 2005 reported the longest duration of SSC prior to the painful event of three hours, and Saeidi 2011 reported the shortest duration of two minutes. Both of these studies reported results favouring SSC but no comparisons could be made. One study, Cong 2012, reported on two samples, one receiving 30 minutes of SSC and the other only 15 minutes, and both were compared to standard care. There were positive results only for the group receiving 30 minutes of SSC, reported as lower serum and salivary cortisol levels.
The providers of SSC were compared in two studies, which showed mean differences in favour of the mother, but the variance was very large so there were no significant differences. Only three studies included full‐term neonates and one was for heel lance, for which standard deviations were not available (Gray 2000). The other two studies used intramuscular injection rather than heel lance so that a comparison between full‐term neonates and preterm neonates was not possible (Sajedi 2007; Chermont 2009).
No studies reported any adverse events.
Quality of the evidence
The studies that were included were generally strongly designed and free from bias. More than half (15/25) of the studies reported using adequate random allocation, and most reported low risk of bias related to incomplete data (19/25), selective reporting (20/25), and other forms of possible bias (13/25). Under half the studies (10/25) reported adequate allocation concealment and just under a quarter reported adequate blinding (6/25). Sixteen of the studies did report measures such as blinded assessors using objective outcome measures and/or 'close up video recording of infant faces', but few addressed the issue of whether the presence of the mother may have been detectable to the assessor. Little information was provided regarding the 'usual care' control, so in the first comparison of SSC versus no‐treatment control we were uncertain precisely what the control condition was.
The quality of the evidence varied. The quality of the evidence for heart rate in response to the painful procedure was low, but in recovery from the procedure, it was high. It is important to note that the significant effect size found in heart rate during procedure was due to a single trial (Liu 2015), with the remaining four studies finding no effect. While this was the only trial to compare SSC to a swaddled control (vs no treatment), it is unlikely that this is the source of the variation as swaddling would be expected to support regulation of heart rate (Pillai Riddell 2015) and reduce the comparative effectiveness of SSC. The quality of evidence regarding heart rate variability during the procedure was low. Following the procedure for HF and LF, quality of evidence was moderate, but for LF/HF it was low. Oxygen saturation studies had low or very low quality of evidence for SSC. The PIPP at 30 and 90 seconds for SSC was supported by moderate quality of evidence but at 60 seconds this was low. Cry duration following the tissue‐damaging part of the procedure as an indicator of effectiveness of SSC was of moderate quality. The NIPS studies for SSC had very low quality of evidence during the procedure; but after the procedure, the quality was moderate. In summary, in spite of the range of quality, it seems as though there is better evidence for SSC in recovery from a painful procedure than during it.
The degree of heterogeneity of the studies varied a great deal, but interestingly the more heterogeneous outcomes were physiological, even though there is a greater potential for bias in behavioural outcomes that require human judgement. Only a few studies reported how video recordings avoided identification of the condition. The conflicting results between physiological outcomes, mostly showing no differences, and composite or behavioural outcomes generally favouring SSC would suggest caution in interpretation. Further research is needed in order to solve the long‐standing confusion and controversy about which indicators are most appropriate (Pillai Riddell 2016). The numerical analyses are very limited, i.e. of the 25 included studies (N = 2001), only up to six provided data for the primary outcomes. This is a potential source of bias as it may be that authors chose not to report statistically insignificant findings.
Potential biases in the review process
Two of the authors of this review (CJ, MCY) authored studies that were reviewed (Johnston 2003; Johnston 2008; Johnston 2009; Johnston 2011; Johnston 2012). Another author (AF) studied SSC for her doctoral dissertation and a manuscript is under review. DI is the manager of the Neonatal Intensive Care Unit of the IWK Health Centre and champions SSC as a practice of family‐centred care as well as for procedural pain management.
Agreements and disagreements with other studies or reviews
There have been a few reviews of non‐pharmacological interventions for procedural pain relief in neonates and all support the practice of SSC (Cignacco 2007; Yamanda 2008; Warnock 2010; Pillai Riddell 2015). No studies or reviews were found that disagreed.
Authors' conclusions
Implications for practice.
Only a maximum of six data sets could be pooled, which did not enable us to determine an effect size on all outcomes. Nevertheless studies comparing skin‐to‐skin care to standard care, which was rarely defined, favoured skin‐to‐skin care or were non‐significant. No studies favoured standard care. In the three studies comparing skin‐to‐skin care to sweet solution, skin‐to‐skin care was more effective in two studies and a possible synergistic effect was reported in the other. The addition of breastfeeding did not appear to increase the effectiveness of SSC in one study, but did in another. When skin‐to‐skin care was enhanced by the addition of the mother's voice or rocking, it had no additional benefit. There were no adverse events reported in any of the studies. Therefore, it would seem that for neonates who are able to be held in the skin‐to‐skin care paradigm, using it for the painful procedures of heel lance, venipuncture, and intramuscular injection is potentially beneficial and not harmful. However, the degree of benefit, although not estimable, may not be large.
Implications for research.
There are numerous areas in this topic that require further research before definitive statements can be made. First of all, more studies are needed that use outcomes that are the same as the ones in this review so that an effect size can be estimated. Secondly, studies need to be more rigorous about randomization, allocation concealment, and blinding. Thirdly, when wide age ranges are used, particularly when full‐term and preterm neonates are in the same study, results for each group should be reported separately. Although it seems as though a 'dose' as low as 10 minutes was effective, more studies testing different durations of the provision of skin‐to‐skin care might allow for a dose response analysis to be conducted. All the studies were conducted using skin‐to‐skin care for a single procedure. It would be interesting to determine if the effect changed with repeated use. Once more studies meet these criteria, studies on dose — that is, duration of skin‐to‐skin care — and other providers would be of interest. More fundamentally, it would be of interest to explore the underlying mechanism of the comforting effect of skin‐to‐skin care and its long‐term impact. One cross‐over study that was excluded due to lack of randomisation found that SSC reduced haemodynamic brain response to venepuncture measured using near‐infrared spectroscopy (Olsson 2015); however, more rigorous studies are needed to elucidate such neurologic mechanisms.
What's new
| Date | Event | Description |
|---|---|---|
| 26 February 2016 | New citation required and conclusions have changed | It was found that the physiological indicator of heart rate also favours Skin‐to‐skin contact, as well as in earlier review, behavioural and composite indicators of pain, although only one study contributed to this change. |
| 13 January 2016 | New search has been performed | Six more trials were found in the updated searching and included in the meta‐analysis. |
Acknowledgements
The Québec Interuniversity Nursing Intervention Research Group (GRISIIQ) provided direct funding for this review. Indirect funding was from Fonds de la recherche en santé du Québec (FRSQ), Canadian Institutes of Health Research (CIHR), Nova Scotia Health Research Foundation (NSHRF).
Appendices
Appendix 1. Standard search methodology
PubMed: ((infant, newborn[MeSH] OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or infan* or neonat*) AND (randomized controlled trial [pt] OR controlled clinical trial [pt] OR Clinical Trial[ptyp] OR randomized [tiab] OR placebo [tiab] OR clinical trials as topic [mesh: noexp] OR randomly [tiab] OR trial [ti]) NOT (animals [mh] NOT humans [mh]))
Embase: (infant, newborn or newborn or neonate or neonatal or premature or very low birth weight or low birth weight or VLBW or LBW or Newborn or infan* or neonat*) AND (human not animal) AND (randomized controlled trial or controlled clinical trial or randomized or placebo or clinical trials as topic or randomly or trial or clinical trial)
CINAHL: (infant, newborn OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or Newborn or infan* or neonat*) AND (randomized controlled trial OR controlled clinical trial OR randomized OR placebo OR clinical trials as topic OR randomly OR trial OR PT clinical trial)
Cochrane Library: (infant or newborn or neonate or neonatal or premature or very low birth weight or low birth weight or VLBW or LBW)
Data and analyses
Comparison 1. Skin‐to‐skin care versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Heart Rate during painful procedure | 5 | 161 | Mean Difference (IV, Fixed, 95% CI) | ‐10.78 [‐13.63, ‐7.93] |
| 1.1 Heel lance ‐ No treatment control | 4 | 121 | Mean Difference (IV, Fixed, 95% CI) | 0.35 [‐6.06, 6.76] |
| 1.2 Heel lance ‐ Swaddled control | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | ‐13.53 [‐16.72, ‐10.34] |
| 2 Heart rate following painful procedure | 4 | 120 | Mean Difference (IV, Fixed, 95% CI) | 0.08 [‐4.39, 4.55] |
| 2.1 Heel lance ‐ No treatment control | 4 | 120 | Mean Difference (IV, Fixed, 95% CI) | 0.08 [‐4.39, 4.55] |
| 3 HRV during painful procedure ‐ Low frequency power | 2 | 38 | Mean Difference (IV, Fixed, 95% CI) | ‐2.11 [‐17.69, 13.47] |
| 3.1 Heel lance ‐ No treatment control | 2 | 38 | Mean Difference (IV, Fixed, 95% CI) | ‐2.11 [‐17.69, 13.47] |
| 4 HRV during painful procedure ‐ High frequency power | 2 | 38 | Mean Difference (IV, Fixed, 95% CI) | ‐5.11 [‐23.36, 13.14] |
| 4.1 Heel lance ‐ No treatment control | 2 | 38 | Mean Difference (IV, Fixed, 95% CI) | ‐5.11 [‐23.36, 13.14] |
| 5 HRV during painful procedure ‐ Low frequency to high frequency ratio | 2 | 38 | Mean Difference (IV, Fixed, 95% CI) | 2.33 [‐2.94, 7.59] |
| 5.1 Heel lance ‐ No treatment control | 2 | 38 | Mean Difference (IV, Fixed, 95% CI) | 2.33 [‐2.94, 7.59] |
| 6 HRV after painful procedure ‐ Low frequency power | 2 | 38 | Mean Difference (IV, Fixed, 95% CI) | 0.58 [‐0.92, 2.07] |
| 6.1 Heel lance ‐ No treatment control | 2 | 38 | Mean Difference (IV, Fixed, 95% CI) | 0.58 [‐0.92, 2.07] |
| 7 HRV after painful procedure ‐ High frequency power | 2 | 38 | Mean Difference (IV, Fixed, 95% CI) | 0.05 [‐0.18, 0.29] |
| 7.1 Heel lance ‐ No treatment control | 2 | 38 | Mean Difference (IV, Fixed, 95% CI) | 0.05 [‐0.18, 0.29] |
| 8 HRV after painful procedure ‐ Low frequency to high frequency ratio | 2 | 38 | Mean Difference (IV, Fixed, 95% CI) | ‐3.77 [‐13.69, 6.14] |
| 8.1 Heel lance ‐ No treatment control | 2 | 38 | Mean Difference (IV, Fixed, 95% CI) | ‐3.77 [‐13.69, 6.14] |
| 9 Oxygen saturation 30 seconds after painful procedure | 2 | 101 | Mean Difference (IV, Fixed, 95% CI) | 1.73 [‐0.53, 3.99] |
| 9.1 Heel lance ‐ Swaddled control | 2 | 101 | Mean Difference (IV, Fixed, 95% CI) | 1.73 [‐0.53, 3.99] |
| 10 Oxygen saturation 60 seconds after painful procedure | 2 | 101 | Mean Difference (IV, Fixed, 95% CI) | 2.17 [‐0.12, 4.46] |
| 10.1 Heel lance ‐ Swaddled control | 2 | 101 | Mean Difference (IV, Fixed, 95% CI) | 2.17 [‐0.12, 4.46] |
| 11 PIPP Score 30 seconds after painful procedure | 5 | 267 | Mean Difference (IV, Fixed, 95% CI) | ‐3.21 [‐3.94, ‐2.47] |
| 11.1 Heel lance ‐ No treatment control | 2 | 91 | Mean Difference (IV, Fixed, 95% CI) | ‐4.12 [‐5.22, ‐3.01] |
| 11.2 Heel lance ‐ Swaddled control | 3 | 176 | Mean Difference (IV, Fixed, 95% CI) | ‐2.49 [‐3.47, ‐1.50] |
| 12 PIPP Score 60 seconds after painful procedure | 3 | 156 | Mean Difference (IV, Fixed, 95% CI) | ‐1.64 [‐2.86, ‐0.43] |
| 12.1 Heel lance ‐ No treatment control | 1 | 27 | Mean Difference (IV, Fixed, 95% CI) | ‐0.33 [‐2.62, 1.97] |
| 12.2 Heel lance ‐ Swaddled control | 2 | 129 | Mean Difference (IV, Fixed, 95% CI) | ‐2.16 [‐3.58, ‐0.73] |
| 13 PIPP Score 90 seconds after painful procedure | 3 | 156 | Mean Difference (IV, Fixed, 95% CI) | ‐1.28 [‐2.53, ‐0.04] |
| 13.1 Heel lance ‐ No treatment control | 1 | 27 | Mean Difference (IV, Fixed, 95% CI) | 0.77 [‐1.69, 3.22] |
| 13.2 Heel lance ‐ Swaddled control | 2 | 129 | Mean Difference (IV, Fixed, 95% CI) | ‐1.99 [‐3.43, ‐0.55] |
| 14 PIPP Score 120 seconds after painful procedure | 3 | 156 | Mean Difference (IV, Fixed, 95% CI) | 0.07 [‐1.11, 1.25] |
| 14.1 Heel lance ‐ No treatment control | 1 | 27 | Mean Difference (IV, Fixed, 95% CI) | 1.41 [‐0.37, 3.19] |
| 14.2 Heel lance ‐ Swaddled control | 2 | 129 | Mean Difference (IV, Fixed, 95% CI) | ‐0.99 [‐2.56, 0.59] |
| 15 NIPS ‐ Proportion of infants in low or no pain during procedure | 3 | 480 | Risk Difference (M‐H, Fixed, 95% CI) | 0.10 [0.06, 0.15] |
| 15.1 IM Injection ‐ No treatment control | 1 | 320 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.03 [‐0.08, 0.01] |
| 15.2 IM Injection ‐ Swaddled control | 2 | 160 | Risk Difference (M‐H, Fixed, 95% CI) | 0.38 [0.28, 0.47] |
| 16 NIPS ‐ Infants in severe pain following procedure | 3 | 480 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.16 [‐0.22, ‐0.10] |
| 16.1 IM Injection ‐ No treatment control | 1 | 320 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.07 [‐0.14, 0.01] |
| 16.2 IM Injection ‐ Swaddled control | 2 | 160 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.35 [‐0.44, ‐0.26] |
| 17 NIPS ‐ Infants in no pain during recovery | 2 | 380 | Risk Difference (M‐H, Fixed, 95% CI) | 0.35 [0.26, 0.44] |
| 17.1 IM Injection ‐ No treatment control | 1 | 320 | Risk Difference (M‐H, Fixed, 95% CI) | 0.38 [0.27, 0.48] |
| 17.2 IM Injection ‐ Swaddled control | 1 | 60 | Risk Difference (M‐H, Fixed, 95% CI) | 0.20 [0.02, 0.38] |
| 18 NIPS ‐ Infants in severe pain during recovery | 2 | 380 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.23 [‐0.31, ‐0.15] |
| 18.1 IM Injection ‐ No treatment control | 1 | 320 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.23 [‐0.32, ‐0.14] |
| 18.2 IM Injection ‐ Swaddled control | 1 | 60 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.2 [‐0.38, ‐0.02] |
| 19 Duration of cry (seconds) following heel lance | 2 | 33 | Mean Difference (IV, Random, 95% CI) | ‐34.16 [‐42.86, ‐25.45] |
| 19.1 Heel lance ‐ No treatment control | 1 | 10 | Mean Difference (IV, Random, 95% CI) | ‐33.5 [‐134.66, 67.66] |
| 19.2 Heel lance ‐ Swaddled control | 1 | 23 | Mean Difference (IV, Random, 95% CI) | ‐34.16 [‐42.90, ‐25.42] |
| 20 Duration of cry (seconds) following IM injection | 2 | 100 | Mean Difference (IV, Fixed, 95% CI) | ‐8.83 [‐14.63, ‐3.02] |
| 20.1 IM injection ‐ Swaddled control | 1 | 64 | Mean Difference (IV, Fixed, 95% CI) | ‐10.06 [‐20.07, ‐0.05] |
| 20.2 IM injection ‐ No treatment control | 1 | 36 | Mean Difference (IV, Fixed, 95% CI) | ‐8.20 [‐15.32, ‐1.08] |
1.5. Analysis.
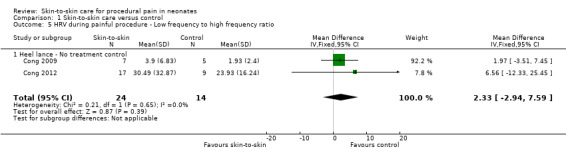
Comparison 1 Skin‐to‐skin care versus control, Outcome 5 HRV during painful procedure ‐ Low frequency to high frequency ratio.
1.6. Analysis.
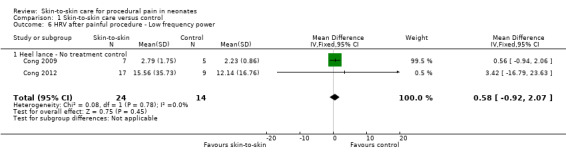
Comparison 1 Skin‐to‐skin care versus control, Outcome 6 HRV after painful procedure ‐ Low frequency power.
1.7. Analysis.
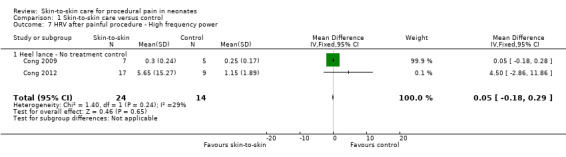
Comparison 1 Skin‐to‐skin care versus control, Outcome 7 HRV after painful procedure ‐ High frequency power.
1.15. Analysis.
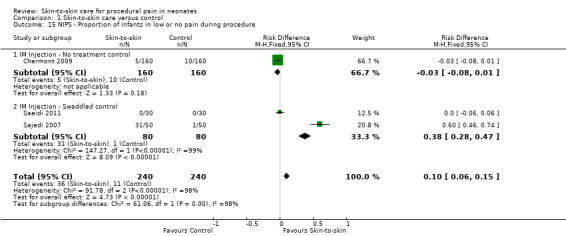
Comparison 1 Skin‐to‐skin care versus control, Outcome 15 NIPS ‐ Proportion of infants in low or no pain during procedure.
1.16. Analysis.
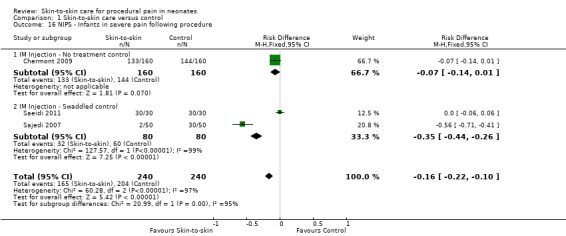
Comparison 1 Skin‐to‐skin care versus control, Outcome 16 NIPS ‐ Infants in severe pain following procedure.
1.17. Analysis.
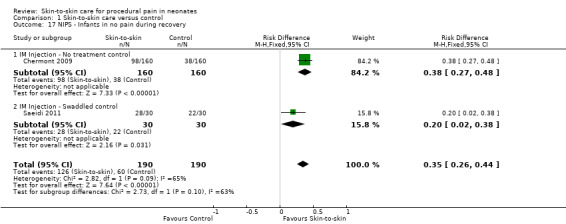
Comparison 1 Skin‐to‐skin care versus control, Outcome 17 NIPS ‐ Infants in no pain during recovery.
1.18. Analysis.
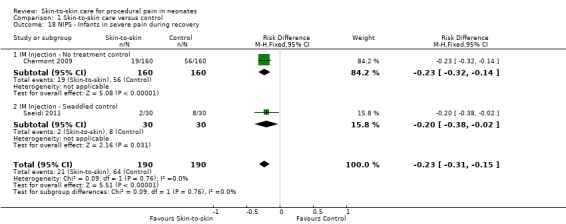
Comparison 1 Skin‐to‐skin care versus control, Outcome 18 NIPS ‐ Infants in severe pain during recovery.
Comparison 2. Skin‐to‐skin care with different providers.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Heart rate recovery | 2 | 78 | Mean Difference (IV, Random, 95% CI) | ‐32.97 [‐94.52, 28.59] |
| 1.1 Father | 1 | 62 | Mean Difference (IV, Random, 95% CI) | ‐26.0 [‐91.34, 39.34] |
| 1.2 Alternate Female | 1 | 16 | Mean Difference (IV, Random, 95% CI) | ‐88.0 [‐271.66, 95.66] |
| 2 PIPP Score 30 seconds | 2 | 80 | Mean Difference (IV, Fixed, 95% CI) | ‐1.29 [‐2.73, 0.16] |
| 2.1 Father | 1 | 62 | Mean Difference (IV, Fixed, 95% CI) | ‐0.94 [‐2.56, 0.68] |
| 2.2 Alternate Female | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | ‐2.63 [‐5.82, 0.56] |
| 3 PIPP Score 60 seconds | 2 | 80 | Mean Difference (IV, Fixed, 95% CI) | ‐1.42 [‐2.97, 0.12] |
| 3.1 Father | 1 | 62 | Mean Difference (IV, Fixed, 95% CI) | ‐1.30 [‐2.97, 0.37] |
| 3.2 Alternate Female | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | ‐2.13 [‐6.15, 1.89] |
| 4 PIPP Score 90 seconds | 2 | 80 | Mean Difference (IV, Fixed, 95% CI) | ‐0.48 [‐2.05, 1.09] |
| 4.1 Father | 1 | 62 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐1.73, 1.73] |
| 4.2 Alternate Female | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | ‐2.62 [‐6.27, 1.03] |
| 5 PIPP Score 120 Seconds | 2 | 80 | Mean Difference (IV, Fixed, 95% CI) | 0.12 [‐1.31, 1.55] |
| 5.1 Father | 1 | 62 | Mean Difference (IV, Fixed, 95% CI) | 0.61 [‐0.93, 2.15] |
| 5.2 Alternate Female | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | ‐2.88 [‐6.70, 0.94] |
2.1. Analysis.
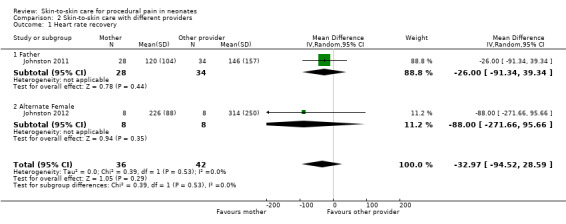
Comparison 2 Skin‐to‐skin care with different providers, Outcome 1 Heart rate recovery.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Akcan 2009.
| Methods | Randomized controlled trial | |
| Participants | 50 preterm infants (PMA 31.6 ± 2.0 weeks) Postnatal age, mean ± SD, days: 4.7 ± 4.4 (total), 4.9 ± 4.3 (intervention), 4.6 ± 4.5 (control) Birth weight, mean ± SD, grams: 1669 ± 530 (total), 1577± 491 (intervention), 1762 ± 561 (control) Painful procedure: heel lance or venepuncture Study period: February 2006 to December 2006 |
|
| Interventions | Intervention: 45 minutes of uninterrupted skin‐to‐skin every day for 5 days, with the painful procedure carried out on the 5th day Control: standard care during painful procedure Provider: mother |
|
| Outcomes | PIPP score at 1st, 2nd, and 3rd minute of painful procedure PIPP score 1st and 2nd minute after painful procedure |
|
| Notes | Country: Turkey Power calculation: no |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The infants were chosen for the groups using a random method by drawing out of a thick, non‐transparent envelope." Random method was not clearly described |
| Allocation concealment (selection bias) | Unclear risk | "The infants were chosen for the groups using a random method by drawing out of a thick, non‐transparent envelope." It is unclear whether the envelopes were sealed or numbered sequentially and if each individual participant was given an envelope or if group assignment was drawn from one envelope |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | "The mothers put on a gown leaving the chest area open and the infant was placed between the mother’s breasts with head upright to provide the greatest surface area for skin contact." "The video recordings and monitor records of the infants in both groups were analyzed by three experts (neonatology nurse, neonatologist and anaesthesiologist) who were totally blind to the study." Not clear if camera recording focused only on infants' faces or if mothers' skin/breasts could be noted by researchers |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | "A total of 100 infants dropped out of the study (91 discharged within 5 days of admission, mothers of 6 infants could not come to the unit regularly, and 3 mothers did not agree to implement KC). As a result, 50 infants comprised the sample, with 25 allocated to the KC group and the other 25 to the control group." Data were presented for all participants assigned to intervention and control groups. There is variability in the length of procedure (mean 1 minute for heel lance, 2.5 minutes for venepuncture) and authors do not report how this is addressed in analysis (e.g. number of infants in each time point, adjustment for procedure duration). |
| Selective reporting (reporting bias) | Low risk | Methods section reported that infants' behavioural responses to pain and physiologic variables, such as heart rate and oxygen saturation, were monitored and recorded. Although these outcomes compose the PIPP score, they were not individually reported or mentioned in the discussion |
| Other bias | Low risk | "Method of delivery, sex, postmenstrual age, birth weight, receipt of oxygen support prior to the procedure, or PIPP scores before or after the invasive procedure were similar (P > 0.05) in both groups." Study was apparently free from other sources of bias |
Castral 2008.
| Methods | Randomized controlled trial | |
| Participants | 59 preterm infants (PMA 248 days (intervention), 254 days (control)) Birthweight, mean, grams: 1749 (intervention), 1846 (control) Painful procedure: heel lance Study period: September 2005 to May 2006 |
|
| Interventions | Intervention: 15 minutes of skin‐to‐skin care before, during and following heel prick Control: standard care during painful procedure Provider: mother |
|
| Outcomes | Neonatal Facial Coding System (NFCS) and heart rate at heel prick, heel squeezing, wound compression, and recovery | |
| Notes | Country: Brazil Power calculation: no |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was achieved using a sequence of random numbers from a computer generated sequence." |
| Allocation concealment (selection bias) | Unclear risk | Methods of allocation concealment were not specified |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | "Two trained coders, who were blinded to the purpose of the study, coded for change in facial action following protocols established by Grunau and Craig (1987)." "The faces of all of the infants were continuously video‐recorded throughout the seven study phases to capture change in facial action." Not clear if camera recording focused only on infants' faces or if mothers' skin/breasts could be noted by researchers |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Of the 62 mothers initially approached, three infants were not entered into the study because two mothers declined participation and another mother was under treatment for tuberculosis. The remaining 59 infants who met the inclusion criteria and whose parent agreed to their infant's or to their infant's and their own participation were randomly assigned into two study groups: skin‐to‐skin (n = 31) or to the regular crib/incubator care (n = 28)." Data were presented for all participants assigned to intervention and control groups |
| Selective reporting (reporting bias) | Low risk | Outcomes listed in methods (facial action, behavioral state, crying and heart rate) were clearly presented in Tables 3, 4 and Figures 1, 2 |
| Other bias | High risk | "The duration of heel puncture (heel cleaning to wound compression) was significantly shorter for the treatment group than it was for the control group (P = 0.014)." The article stated that "measures were taken to minimize factors that could have led to group differences in duration. The same two trained nurses conducted all of the heel pricks using a standardized protocol". Study researchers, however, did not apparently control for differences in duration of heel puncture for the regression analysis |
Chermont 2009.
| Methods | Randomized controlled trial | |
| Participants | 640 term infants (mean PMA 39 ± 1 weeks, for all groups); 4 groups – standard care, skin‐to‐skin, 25% dextrose, skin‐to‐skin + 25% dextrose Postnatal age, mean ± SD, hrs: 293 ± 13 (skin‐to‐skin care), 29 ± 15 (control), 29 ± 13 (25% dextrose), 27 ± 13 (skin‐to‐skin + 25% dextrose) Birth weight, mean ± SD, g: 3164 ± 371 (intervention); 3163 ± 418 (control); 3252 ± 389 (25% dextrose); 3240 ± 418 (skin‐to‐skin + 25% dextrose) Painful procedure: intramuscular injection Study period: March 2006 to October 2007 |
|
| Interventions | Intervention: skin‐to‐skin contact, initiated 2 minutes before injection and persisting throughout procedure Control: standard care during painful procedure Comparison 1: oral 25% dextrose treatment (1 mL), given 2 minutes before injection Comparison 2: combination of oral dextrose treatment and skin‐to‐skin contact strategies Provider: mother provided skin‐to‐skin; oral dextrose provided by nurse or neonatologist |
|
| Outcomes | Neonatal Facial Coding System (NFCS) and Neonatal Infant Pain Scale (NIPS) score at baseline, cleansing, injection, and recovery; HR, O₂ saturation | |
| Notes | Country: Brazil Power calculation: yes |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomization was performed by using 2 boxes, 1 for male infants and 1 for female infants." |
| Allocation concealment (selection bias) | Unclear risk | "Each box was filled with 320 opaque sealed envelopes, corresponding to 80 envelopes for each analgesic procedure to be performed during immunization." Envelopes should ideally be opaque, sealed and sequentially numbered |
| Blinding (performance bias and detection bias) All outcomes | High risk | "Pain evaluators were aware of skin‐to‐skin contact but were blinded to whether the infant received water or dextrose." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "All randomly assigned patients completed the study, with no losses." |
| Selective reporting (reporting bias) | High risk | Data for the primary outcomes (NFCS, NIPS and PIPP scores) were presented in Tables 2 & 3 and Figure 2. The trial registry lists heart rate and oxygen saturation as secondary outcomes. Although these outcomes were covered within the pain scales, no discussion of the results were found |
| Other bias | Unclear risk | The authors failed to report the number of patients assessed for eligibility |
Cong 2009.
| Methods | Randomized cross‐over trial | |
| Participants | 14 preterm infants (PMA 30‐32 weeks); 13 intervention, 10 control Postnatal age, mean ± SD, days: 6 ± 1 Birth weight, mean ± SD, grams: 1775 ± 292 Weight on day of study, mean ± SD, grams: 1706 ± 293 Painful procedure: heel lance Study period: unclear |
|
| Interventions | Intervention: 60 minutes of skin‐to‐skin care before, during and following heel stick Control: standard care during painful procedure Provider: mother |
|
| Outcomes | Heart rate, low frequency (LF) and high frequency (HF) power, LF/HF power, and state at baseline, heel warming, heel stick, and recovery | |
| Notes | Country: United States Power calculation: yes |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A prospective cross‐over with random assignment by permuted block design was used. A statistician helped the investigator generate a list of randomization codes using the SAS® procedure PLAN. The list of random codes consisted of the subject's number and the treatment assignment. According to random codes, infants were assigned to two groups..." |
| Allocation concealment (selection bias) | Low risk | "A prospective cross‐over with random assignment by permuted block design was used. A statistician helped the investigator generate a list of randomization codes using the SAS® procedure PLAN. The list of random codes consisted of the subject's number and the treatment assignment. According to random codes, infants were assigned to two groups..." |
| Blinding (performance bias and detection bias) All outcomes | High risk | "Movement and artefact were eliminated by comparing amplitude (height) of the R‐wave to be included with the amplitude for the last acceptable R‐wave. Waves of more or less than 38% deviance from the previous wave were automatically eliminated. The researcher or research assistant who extracted the HRV data was not blinded from the study conditions. Although the bias was likely minimal, still it is important. A proper blinded data extraction process would be necessary to guard against bias pertaining to knowledge of study conditions in the future study." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Heart rate and HRV data were available for all days except one KC and four IC days due to equipment problems. The pair wise deletion was used for missing data; therefore, the final data were from 13 observations in KC and 10 observations in IC." |
| Selective reporting (reporting bias) | Low risk | Heart rate and variability (low frequency, high frequency, and low/high frequency power frequency power ratio) were presented in Table 3 and Figure 2. The Anderson Behavioral State Scoring System (ABSS) were used to measure infant state. The outcomes are discussed but are not presented in a table or figure |
| Other bias | Low risk | "The heel stick and subsequent blood draw were standardized and performed in accordance with the guidelines and step‐by‐step procedure developed by National Association of Neonatal Nurses." "One consistent person, the neonatal unit phlebotomist, did all the heel sticks and blood draws." "A 24‐hour routine IC washout period was incorporated into the design for both groups. Twenty‐four‐hours was sufficient to allow any lingering effects of KC to dissipate." |
Cong 2011.
| Methods | Randomized cross‐over trial | |
| Participants | 28 preterm infants (PMA 30‐32 weeks): 18 infants — 80 min SSC (Study a); 10 infants — 30 min SSC (Study b) Postnatal age, mean ± SD, days: 5 ± 1 (Study 1); 6 ± 2 (Study 2) Birth weight, mean ± SD, grams: 1779 ± 277 (Study 1); 1577 ± 327 (Study 2) Painful procedure: heel lance Study period: unclear |
|
| Interventions | Intervention: (a) Study a: 60 minutes of skin‐to‐skin care before heel stick, with continued SSC during procedure, and followed by 20 minutes SSC post‐procedure; (b) Study b: 10 minutes of skin‐to‐skin care before heel lance, with continued SSC during procedure, and followed by 20 minutes SSC post‐procedure Control: standard care during painful procedure Provider: mother |
|
| Outcomes | PIPP score, salivary and serum cortisol at baseline, heel warming, heel stick and recovery. | |
| Notes | Country: United States Power calculation: yes |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “permuted block randomization to determine the order of condition (KCH or IH first) was used. A list of randomization codes with four subjects in each randomization block using the SAS(R) procedure PLAN was developed by an independent statistician." |
| Allocation concealment (selection bias) | Low risk | “The list of random codes consisted of the subject’s number and assignment to groups; assignments were kept in sealed envelopes and opened in front of the mother after consent was obtained." |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | “A video camera recorder was set up and focused on the infants’ faces to record facial actions. The videotapes were independently scored by the researcher and one other certified PIPP scorer (trained to reliability by the PIPP creator), who was blind to the purpose of the study.” "Another limitation is that the PIPP scorers could not be blind to KCH because maternal respiratory movements moved the infant’s face up and down in the video, as previously reported and acknowledged by other KC pain researchers." Potential for bias as researcher coded videos and it is unknown if data were collected by the same individual |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were provided for all infants recruited in the study |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported in Tables 1‐2 and Figures 1‐2 |
| Other bias | Low risk | “Standard incubator care for 24 hr was considered a sufficient “wash out” period because physiological and behavioural state effects of KC disappear within 3 hr of KC cessation.” |
Cong 2012.
| Methods | Randomized cross‐over trial | |
| Participants | 26 preterm infants (PMA 28 0/7 to 32 6/7 weeks): 22 infants — 30 min SSC (Study a); 25 infants — 15 min SSC (Study b); 23 infants control Postnatal age, mean ± SD, days: 14.5 ± 6.3 (Study a); 13.8 ± 5.6 (Study b); 13.5 ± 5.6 (control) Birth weight, mean ± SD, grams: 1444.6 ± 379.0 Painful procedure: heel lance Study period: unclear |
|
| Interventions | Intervention: (a) Study a: 30 minutes of skin‐to‐skin care before and throughout heel lance (b) Study b: 15 minutes of skin‐to‐skin care before and throughout heel lance Control: standard care during painful procedure Provider: mother |
|
| Outcomes | Heart rate, heart rate variability (low frequency and high frequency power), LF/HF ratio, infant behavioural state | |
| Notes | Country: United States Power calculation: yes |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “A list of randomization codes with 4 subjects in each randomization block was developed by the statistician (the third author). The list of random codes consisted of the subject's number and assignment to sequence." |
| Allocation concealment (selection bias) | Low risk | “assignments were kept in sealed envelopes and opened in front of the mother after consent was obtained." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "A video camera was mounted on a tripod and focused on the infant's face to record facial actions, and the videotapes were later reviewed and scored." "In order to minimize bias, a research assistant who was blind to the purpose of the study helped analyse the data." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were provided for all infants recruited in the study; dropout rates described |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported in the Results section and Table 3 |
| Other bias | Low risk | “A 24‐ to 72‐hour washout period was applied between each study condition.” |
Freire 2008.
| Methods | Randomized controlled trial with three groups (routine care; skin‐to‐skin; routine care + oral glucose) | |
| Participants | 95 preterm infants (PMA 28 to 36 weeks) Painful procedure: heel lance Study period: unclear |
|
| Interventions | Intervention: 10 minutes of skin‐to‐skin care before, during heel stick Control: standard care during painful procedure Comparison: sweet taste 2 minutes before painful procedure Provider: mother |
|
| Outcomes | PIPP score | |
| Notes | Country: Brazil Power calculation: yes |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The groups were selected at random by a nurse on duty using closed envelopes" Not clear how the sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | "The groups were selected at random by a nurse on duty using closed envelopes" Not clear whether the envelopes were opaque, sealed and sequentially numbered |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "The examiner was blinded and trained to record any grimacing..." "Only the newborn's face was filmed in close‐up with little surrounding area and minimal colour to reduce the possibility of unblinding by the research assistants who recorded the tapes". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were 10 neonates excluded due to errors in the blinding of the video (30/Fig 1). Data were provided only for all other neonates |
| Selective reporting (reporting bias) | Low risk | All outcomes were clearly presented in Table 3 |
| Other bias | Low risk | Study was apparently free of other sources of bias |
Gabriel 2013.
| Methods | Randomized controlled trial | |
| Participants | 136 term infants (127 in analysis) (37 to 42 weeks GA); 4 groups: skin‐to‐skin, sucrose, sucrose + skin‐to‐skin, skin‐to‐skin + breastfeeding Postnatal age ‐ N/A Birth weight, mean (SD N/A), g: 3359 (skin‐to‐skin); 3215 (sucrose); 3349 (sucrose + skin‐to‐skin); 3289 (sucrose + breastfeeding) Painful procedure: heel lance Study period: unclear |
|
| Interventions | Group 1 ‐ Skin‐to‐skin five minutes before and during heel lance Group 2 ‐ 2 mL of 24% oral sucrose two minutes before heel lance Group 3 ‐ Skin‐to‐skin five minutes before and during heel lance + 2 mL; 24% sucrose two minutes before heel lance Group 4 ‐ Breastfeeding 5 minutes before heel lance |
|
| Outcomes | Crying time (seconds), % of crying during blood sampling, heart rate, NIPS | |
| Notes | Trial terminated after planned mid‐point analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | The authors indicate that they mixed together groups of envelopes that had the different intervention groups on them together and then allowed the parents to select an envelope. They thus did not use a method to generate the sequence that is approved in the Cochrane Handbook for Systematic Reviews of Interventions making this method at high risk of bias. |
| Allocation concealment (selection bias) | High risk | The authors indicate the envelopes utilized were opaque; however, they were not sequentially numbered and the parents who consented to take part in the study were the ones who selected the envelope. Additionally, there is no mention as to whether or not the participant information was written on the envelope prior to opening, which would be an additional requirement to make this method low risk of bias as per the Cochrane Handbook for Systematic Reviews of Interventions. |
| Blinding (performance bias and detection bias) All outcomes | High risk | The authors report that there were three outcome assessors for this study who all coded the NIPS. However, they report that they only reported on the data coded by a single outcome assessor for the study (as a result of good interclass correlation coefficient) out of the three individuals who were completing the coding. Therefore, as a single individual coded the outcome data across all of the intervention groups, there was no attempt to blind the outcome assessor to the interventions received. The authors provide no description of attempts to blind the outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The main reasons that the authors noted for missing outcome data in the BF group was non‐effective breastfeeding (n = 3), non‐correct SSC (n = 2) and technical problems (n = 1). While the authors followed an intention‐to‐treat protocol and noted that the missing data were greater in the breastfeeding group compared to the other groups, they have unexplained missing heart rate data, hence an assignment of high risk of bias. Authors report that "correct" heart rate was only obtained in a proportion of participants in each of the intervention conditions but do not specify the reason for inability to obtain correct heart rate in the remaining participants |
| Selective reporting (reporting bias) | Low risk | It appears that authors report on all outcomes for which, in the Methods section of the paper, they indicate they are collecting data . We looked for a study protocol on line; however, there does not appear to be one available. |
| Other bias | Unclear risk | The authors do not report on provision of any training of NIPS coders, simply report an ICC of > 0.6 between the three coders for the study and they go on to indicate that because of high ICC they only utilized the data coded by one of the observers. They also do not report any data on intra‐rater reliability. |
Gao 2015.
| Methods | Randomized Controlled Trial | |
| Participants | 80 preterm infants (75 in analysis) (27 to 37 weeks GA); 2 groups – skin‐to‐skin, incubator control Postnatal age ‐ N/A Birth weight, mean (SD N/A), g: 2017.8 (skin‐to‐skin); 2030 (incubator) Painful procedure: heel lance Study period: unclear |
|
| Interventions | First heel lance: both groups in incubator Next three heel lances: Treatment: Skin‐to‐skin: 30 minutes pre‐procedure Control: Prone in incubator: 30 minutes pre‐procedure |
|
| Outcomes | Crying time (seconds), grimacing time (seconds), heart rate | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The authors report that they randomly assigned the infants in the study to either the KMC or incubator condition using a "random table format". This statement does not provide enough information about the randomisation sequence and how it was generated and therefore has an unclear risk of bias. |
| Allocation concealment (selection bias) | Unclear risk | The authors report that they used a random table format to assign the infants in the study to the intervention conditions; however, they do not provide information regarding who had access to that table, who completed the randomisation, and any steps that were taken to conceal the allocation |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Recordings were assessed by blind reviewers. Camera was focused on face with little surrounding area with no sound, low colour. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The authors had some loss to follow‐up, but they provide rationale for the reasons that participants were lost, making this low risk of bias |
| Selective reporting (reporting bias) | Low risk | The authors report on all outcomes and provide complete within‐ and between‐group analyses to show differences between groups and across time. |
| Other bias | Low risk | There does not appear to be concerning issues related to other sources of bias. The authors provide detailed descriptions of how inter‐ and intra‐rater reliability were maintained for the behavioural outcomes reported. |
Gray 2000.
| Methods | Randomized controlled trial | |
| Participants | 30 term infants (≥ 37 weeks) Postnatal age, range, hours: 33 to 55 Birth weight, mean (range), grams: 3300 (2600 to 3700) Painful procedure: heel lance Study period: March 1998 to October 1998 |
|
| Interventions | Intervention: 10 to 15 minutes of skin‐to‐skin care before heel stick Control: standard care during painful procedure Provider: mother |
|
| Outcomes | Heart rate and cry duration in seconds during blood collection, and grimacing during recovery period | |
| Notes | Country: United States Power calculation: yes |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "They [healthy full‐term newborns] were assigned randomly the morning of the study." |
| Allocation concealment (selection bias) | Unclear risk | Methods of allocation concealment were not specified |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | "Their infant, wearing only a diaper, then was positioned on the mother so that skin‐to‐skin contact was maintained through her open gown. This arrangement left the infant’s face visible for filming from the side of the bed." "After a 2‐minute baseline period, during which the infant's face was filmed and heart rates were announced every 10 seconds from the monitor, the heel warmer was removed, and the heel was swabbed with alcohol." "videotape evaluations of infant pain reactions were conducted by research assistants who were not aware of either the purpose of the study or the number of different groups." "For grimacing, of course, knowledge of group assignment was unavoidable. The data obtained in these analyses were reliable among scorers." Not clear if mothers' skin/breasts could be noted by researchers |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were provided for all infants randomized except grimacing in 3 infants (all in the skin‐to‐skin contact group) where blood collection lasted > 3 minutes. (3/Figure 2) |
| Selective reporting (reporting bias) | Low risk | Data were provided for all outcome measures listed in the methods section (Figures 2‐4) |
| Other bias | Low risk | "Since L.G. conducted all heel sticks because of scheduling difficulties with the phlebotomists, a potential bias of differential treatment has been introduced. We are not concerned about this potential bias for a number of reasons. First, the duration of the procedure and apparent discomfort that it caused in control infants, expressed in crying, for example, was of the same order of magnitude as that caused by the phlebotomist in other studies conducted in our laboratory. Second, as indicated, mean blood collection times for both groups were not statistically different. Third, we went through a number of iterations...before a successful set of parameters was attained. It would seem to us that any systematic bias on the part of L.G. would have become manifest from the outset and not after a number of procedural changes." Study is apparently free of other sources of bias |
Johnston 2003.
| Methods | Randomized cross over trial | |
| Participants | 74 infants (32 to 36 weeks PMA) Postnatal age, range, days: 0 to 10 Birth weight, mean ± SD (range), grams: 2054 ± 406 (1320‐3125) Painful procedure: heel lance Study period: 9 April 2001 to 28 June 2002 |
|
| Interventions | Intervention: 30 minutes of skin‐to‐skin care before and during heel stick Control: standard care during painful procedure Provider: mother |
|
| Outcomes | PIPP score at 30, 60, 90, and 120 minutes Secondary outcomes: heart rate and oxygen saturation |
|
| Notes | Country: Canada Power calculation: yes |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Ordering of conditions was determined randomly by a computer‐generated program." |
| Allocation concealment (selection bias) | Low risk | Off site computer generated program (information obtained from authors) |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "The camera was in close‐up focus on the neonate's face, with little surrounding area, no sound, and minimal colour, and turned 60 degrees in the KC condition so as to decrease the possibility of unblinding by research assistants who scored the tapes. Research assistants, who were blinded to the purpose of the study by being told that the study was about neonatal facial actions, coded facial actions in the laboratory of the principal investigator." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were provided for all 74 neonates included in the study |
| Selective reporting (reporting bias) | Low risk | Data were provided for all outcome measures listed in the methods section. |
| Other bias | Low risk | "There was a minimum of 24 hours and a maximum of 7 days between conditions, because the frequency of blood sampling was determined by clinical considerations." Study is apparently free of other sources of bias |
Johnston 2008.
| Methods | Randomized cross‐over trial | |
| Participants | 61 preterm infants (PMA 30.5 ± 1 weeks) Postnatal age, range, days: 1 to 14 Birth weight, mean ± SD, grams: 1421 ± 490 Painful procedure: heel lance Study period: April 2003 to December 2005 |
|
| Interventions | Intervention: 15 minutes of skin‐to‐skin care before and during heel stick Comparison: swaddling in incubator 15 minutes before painful procedure Provider: mother |
|
| Outcomes | PIPP score at 30, 60, 90 and 120 minutes Time to return to baseline heart rate |
|
| Notes | Country: Canada Power calculation: yes |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Ordering of conditions was determined randomly by a computer‐generated program in the study centre and assignment was accessed on the web site by the site research nurse after consent was obtained." |
| Allocation concealment (selection bias) | Low risk | "...assignment was accessed on the web site by the site research nurse after consent was obtained." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "The camera was in close up focus on the infant's face with very little surrounding area, no sound, with minimal colour, and turned to an angle in the kangaroo condition as to mimic the prone position in order to decrease the possibility of unblinding by research assistants who scored the tapes. Research assistants, who were blinded to the purpose of the study by being told that the study was about infant facial actions, coded facial actions in the laboratory of the PI." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes were clearly presented for all infants included in the study. Dropout rates were detailed in Figure 2 |
| Selective reporting (reporting bias) | Low risk | Data were clearly reported for all outcome measures in Figures 2‐5 |
| Other bias | Low risk | Study was apparently free of other sources of bias |
Johnston 2009.
| Methods | Randomized cross‐over trial | |
| Participants | 90 preterm infants (PMA 32 0/7 to 36 0/7 weeks) Postnatal age, range, days: 1 to 14 Birth weight, mean ± SD, grams: 1968 ± 388 Painful procedure: heel lance Study period: April 2003 to December 2006 |
|
| Interventions | Intervention: 30 minutes of skin‐to‐skin care before and during heel stick Comparison: 30 minutes of enhanced skin‐to‐skin care (rocking, singing/talking to baby, offering finger/pacifier for baby to suck Provider: mother |
|
| Outcomes | PIPP score at 30, 60, 90, and 120 minutes | |
| Notes | Country: Canada Power calculation: yes |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Ordering of conditions was determined randomly by a computer‐generated program in the study center..." |
| Allocation concealment (selection bias) | Low risk | "...assignment was accessed on the web site by the site research nurse after consent was obtained." |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Authors were contacted via email and stated that "Camera [was] zoomed on face." Not clear if mothers' skin/breasts could be noted by researchers |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | "Of those 330 meeting the selection criteria, 187 were approached and 139 accepted to participate, giving a refusal rate of 26%." Not clear of why only 187 were approached "Not all infants had complete data at each time block, due to movement artefacts or hand obscuring the face, but there were no more than seven missing data at any point in time and it was not the same infants, so the analyses were conducted with some cases missing." |
| Selective reporting (reporting bias) | Low risk | Outcomes were clearly laid out in Figures 2 and 3 |
| Other bias | Unclear risk | "Two of the three committees approved the two KMC conditions without sucrose. The other site required usual use of sucrose but this was not consistent. To accommodate the study, if an infant received sucrose in the first session, then sucrose was administered for the second session and similarly, if sucrose was not administered in the first session, it was withheld in the second". This implies inconsistent use of sucrose Study was apparently free of other sources of bias |
Johnston 2011.
| Methods | Randomized cross‐over trial | |
| Participants | 62 preterm infants (PMA 28 to 36 weeks) Postnatal age, mean, days: 5 to 10 Birth weight, mean ± SD, grams: 1565 ± 469 (father KC/mother KC); 1610 ± 494 (mother KC/father KC) Painful procedure: heel lance Study period: 16 January 2008 to 24 March 2009 |
|
| Interventions | Intervention: 30 minutes of skin‐to‐skin care before and during heel lance provided by mother Comparison: 30 minutes of skin‐to‐skin care before and during heel lance provided by father Provider: mother or father |
|
| Outcomes | PIPP score at 30, 60, 90, and 120 minutes, time for HR to return to baseline | |
| Notes | Country: Canada Power calculation: yes |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "When clinical care required blood procurement, the research nurse went to the secure computer Web site for the order assignment that had been generated randomly in permutated blocks of 4 and 6." |
| Allocation concealment (selection bias) | Low risk | "When clinical care required blood procurement, the research nurse went to the secure computer Web site for the order assignment that had been generated randomly in permutated blocks of 4 and 6. The parents were then contacted by the research nurse, informing them of which one was to provide KC for that procedure." |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | "Close‐up video recordings of the infants' faces were made using a KS162 digital camera at 2 sites and a webcam at the third site." Not clear if mothers' skin/breasts could be noted by researchers |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | "...there were 185 infants who were determined to be eligible from 3 university‐affiliated level III neonatal intensive care units. A major reason for not being eligible was the unavailability of the father in the daytime. The refusal rate was 22%, mostly because one or the other parent did not want to do KC or particularly did not want to be videotaped, even though it was explained that the camera would be focused on the infant's face." Unclear of the exact number of participants |
| Selective reporting (reporting bias) | Unclear risk | Primary outcomes were clearly laid out in Table 3 and in the Results section |
| Other bias | Unclear risk | "Intrarater reliability was checked every 3 months, remaining more than 90%. When asked what they thought the study was about, the coders independently stated that it was about facial grimacing when infants were calm or crying." Washout period not described |
Johnston 2012.
| Methods | Randomized cross‐over trial | |
| Participants | 18 preterm infants (PMA 28 to 36 completed weeks) Postnatal age, range, days: within 10 days Birth weight, mean, grams: 2200 Painful procedure: heel lance Study period: October 2007 to January 2010 |
|
| Interventions | Intervention: 30 minutes of skin‐to‐skin care before and during heel stick provided by the mother Comparison: 30 minutes of skin‐to‐skin care before and during heel lance provided by an unrelated woman Provider: mother or an unrelated woman |
|
| Outcomes | PIPP score at 30, 60, 90, and 120 minutes | |
| Notes | Country: Canada Power calculation: no |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence generation not described in text. Author communication confirmed that an off‐site computer‐generated randomization and sequentially numbered allocation program was used |
| Allocation concealment (selection bias) | Low risk | Allocation concealment not described. Author communication confirmed that an off‐site computer‐generated randomization and sequentially numbered allocation program was used |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | "Close‐up video recordings of the infants' faces were made using a KS162 digital camera...or a webcam." Not clear if mothers' skin/breasts could be noted by researchers |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "...of the 82 infants meeting the selection criteria, 21 initially refused at the time of asking, and another refused after condition order had been randomized. The main reason for refusal was not wanting another woman to provide kangaroo care with her baby." Drop out rates clearly explained in Figure 2 and incomplete data accounted for |
| Selective reporting (reporting bias) | Low risk | Outcomes were clearly laid out in Figure 2 |
| Other bias | Unclear risk | "All data were coded and analyzed in the research laboratory at the off‐site university. Faces were coded second‐to‐ second on a stop frame system. Coders were trained on faces from similar studies, and inter‐rater reliability was over 90%. Coders were from outside the unit and did not know the purpose of the study, because the camera was focused on the infant’s face. Intrarater reliability was checked every 3 months and was maintained over 90%." Washout period not described |
Kostandy 2008.
| Methods | Randomized cross‐over trial | |
| Participants | Only 10 infants (30 to 32 weeks' PMA) enrolled Postnatal age, range, days: 2 to 9 Birth weight, mean ± SD, grams: 1577 ± 327.00 Painful procedure: heel lance Study period: unclear |
|
| Interventions | Intervention: 30 minutes of skin‐to‐skin care before and during painful procedure Control: standard care during painful procedure Provider: mother provided skin‐to‐skin care |
|
| Outcomes | Cry duration at baseline, warming, heel stick, and recovery | |
| Notes | Country: United States Power calculation: yes |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomisation was by permuted block design to ensure highest possible equivalence among infants." The system of randomization is not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | "Independent scorers of the videotapes were blind to the purpose and cross‐over design of the study." Not clear if mothers' skin/breasts could be noted by researchers |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "All mothers who were approached agreed to participate." |
| Selective reporting (reporting bias) | Low risk | Outcomes listed in methods match those reported |
| Other bias | Low risk | "26 subjects were needed to detect moderate difference in crying time; however, funding permitted recruitment of only 10 subjects." 24 hour between procedures ‐ carry‐over effect: "Heel sticks were done by a consistent neonatal phlebotomist who used the National Association of Neonatal Nursing's standardized heel stick procedure with a Tenderfoot(TM) spring‐loaded lancet." |
Kostandy 2013.
| Methods | Randomized Controlled Trial | |
| Participants | 36 term infants (GA 28 to 36 completed weeks) Birth weight, mean, ± SD, grams: 3358.25 (skin‐to‐skin: 3389.7 ± 333.3; Control: 3326.8 ± 324.08) Painful procedure: IM Injection Study period: July 2002 to December 2002 |
|
| Interventions | Skin‐to‐skin: 10 to 15 minutes prior to, and during, heel lance Control: infant supine in bassinet 10‐15 minutes prior to heel lance |
|
| Outcomes | Cry time (seconds), Anderson Behavioural State Scale, heart rate | |
| Notes | Country: United States Power calculation: yes |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Each mother‐neonate dyad was randomly assigned using the computerized minimization method. |
| Allocation concealment (selection bias) | Low risk | Randomization was completed for each participant, using a minimization method calculated by a computer program. Staff could not access this information prior to group assignment. |
| Blinding (performance bias and detection bias) All outcomes | High risk | The nature of the intervention makes blinding study staff and participants impossible, no blinding of outcomes assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All dyads completed the study |
| Selective reporting (reporting bias) | Low risk | All mothers and neonates enrolled in the study completed the protocol, all outcomes were reported. |
Liu 2015.
| Methods | Randomized Controlled Trial | |
| Participants | 40 term infants (mean = 39.3 weeks GA) Birth weight, mean, ± SD, grams: Skin‐to‐skin: 3337 ± 409.1; Control: 3740 ± 298.9) Painful procedure: heel lance Study period: April 2010 to December 2010 |
|
| Interventions | Intervention: skin‐to‐skin 15 mins pre heel lance, during, 1 minute after Control: post bath, swaddled during and 1 minute after |
|
| Outcomes | Douleur Aiguë du Nouveau‐né (DAN), cry time (seconds), pain facial expression (seconds), oxygen saturation, heart rate | |
| Notes | Power calculation: N/A Country: United States |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table method was used to generate the sequence. |
| Allocation concealment (selection bias) | Unclear risk | No information was provided regarding who had access to the number table used to allocate the intervention. It would be feasible to predict the nurse recruiting participants had access to this table; however, information as to who had access is not provided. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Impossible to blind personnel and participants to group assignment, no attempt to blind outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The authors indicate that they selected 40 infants (intervention n = 20; intervention n = 20), however, they do not provide numbers for each of the individual outcomes (only for the final number of infants included in the sample, which was 40). Therefore, it is unclear if there was incomplete data for any particular outcome. |
| Selective reporting (reporting bias) | High risk | The authors do not report if there was a statistically significant difference between the intervention and control group on the DAN ‐ they only report on the duration of facial expression and crying times. They do not report the DAN scores whatsoever in their results section. |
| Other bias | High risk | The authors do not discuss training of personal to complete facial coding for the scoring of the DAN. |
Ludington‐Hoe 2005.
| Methods | Randomized cross‐over trial | |
| Participants | 24 preterm infants (< 37 weeks PMA); results from 23 infants Postnatal age, mean ± SD, days: 22 ± 11.4 Painful procedure: heel lance Study period: unclear |
|
| Interventions | Intervention: 3 hours of skin‐to‐skin care before and during painful procedure Control: standard care during painful procedure Provider: mother |
|
| Outcomes | Heart rate, respiratory rate, oxygen saturation, cry duration, behavioural state | |
| Notes | Country: El Salvador and USA Power calculation: yes |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The cross‐over design controlled for all threats to internal validity except the interaction of selection and treatment, but assignment to group A or B independently and randomly by the Zellen technique insured balanced representation in both treatment sequences." |
| Allocation concealment (selection bias) | Unclear risk | "Consenting mother‐infant pairs were randomised by sealed envelope technique into 2 groups" It is not specified whether envelopes were sealed. opaque and sequentially numbered |
| Blinding (performance bias and detection bias) All outcomes | High risk | "...the observers were not blind to treatment and group, a condition that is being corrected by having all data videotaped and computer‐stored for scoring outside the clinical area in an ongoing study." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropout rates explained |
| Selective reporting (reporting bias) | Low risk | All outcomes are clearly presented in Tables 2, 3 |
| Other bias | High risk | "Each infant was tested on one day using this cross‐over design that controlled for intra‐ and inter‐subject variability and provided the highest possible equivalence among subjects exposed to both conditions. The cross‐over design controlled for all threats to internal validity except the interaction of selection and treatment, but assignment to group A or B independently and randomly by the Zellen technique insured balanced representation in both treatment sequences. Carry‐over effects from one condition to the next is a concern with any cross‐over design; previous KC research has shown that physiological and behavioral state effects of KC are not sustained long after KC is discontinued, making 3‐4 hours sufficient to minimize carry‐over effects." In Zellen's technique, patients are randomized before consent occurs; therefore in theory, consent can be sought conditionally |
Mosayebi 2014.
| Methods | Randomized Crossover Trial | |
| Participants | 64 preterm infants (mean ± SD: 33 ± 1.95 weeks) Postnatal age, mean ± SD, days: 7.28, ± 3.65 Birthweight, mean ± SD, grams: 2095.85 ± 672.27 Painful procedure: heel lance Study period: June 2012 to October 2012 |
|
| Interventions | Intervention: skin‐to‐skin 15 min pre, during, and 2 minutes post heel lance Control: Proned in incubator 15 min pre, during, and 2 minutes post heel lance |
|
| Outcomes | Premature Infant Pain Profile (PIPP) | |
| Notes | Country: Iran Power calculation: no |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization of neonates to give MC or incubator for the first heel lance was done by drawing out a thick, nontransparent envelope |
| Allocation concealment (selection bias) | Unclear risk | No mention of envelopes being sequentially numbered |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Impossible to blind bedside nurses or participants, no discussion of whether the mother's skin or breasts were visible, single assessor scored both conditions |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No attrition among consented participants. Complete data for all reported outcomes |
| Selective reporting (reporting bias) | Low risk | All outcomes outlined in trial registry reported in final publication |
| Other bias | Unclear risk | No report on the effect of treatment order |
Nanavati 2013.
| Methods | Randomized Controlled Trial | |
| Participants | 50 preterm infants (< 37 weeks GA) Gestational age, mean ± SD, weeks: skin‐to‐skin: 32.72 ± 2.03; control: 32.4 ± 2.16 Postnatal age, mean ± SD, days: skin‐to‐skin: 7.12 ± 6.64; control: 5.4 ± 3.65 Painful procedure: tape removal Study period: June 2012 to October 2012 |
|
| Interventions | Intervention: skin‐to‐skin 15 minutes before, and during tape removal Control: EBM soaked swab 2 minutes before, and during tape removal |
|
| Outcomes | Premature Infant Pain Profile | |
| Notes | Country: India Power Calculation: yes |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The babies were randomised to receive either KMC or EBM. A computer‐ generated randomisation sequence was used to assign infants to two treatment groups in 1:1 ratio." |
| Allocation concealment (selection bias) | Unclear risk | "Treatment allocations were inserted in sequentially numbered opaque envelopes and were sealed. Just prior to adhesive tape removal, a neonatal research nurse opened the sequentially numbered" |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding of participants and personnel impossible as a result of treatment characteristics, there did not appear to be any effort to blind outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Figure 2 provides an overview of all participants |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | |
Nimbalkar 2013.
| Methods | Randomized cross‐over trial | |
| Participants | 47 preterm infants (PMA 32 0/7 to 36 6/7 weeks) Postnatal age, mean, days: within 10 days Birth weight, mean, grams: 1730 (intervention), unclear (control) Painful procedure: heel lance Study period: 1 April 2009 to 30 September 2009 |
|
| Interventions | Intervention: 15 minutes of skin‐to‐skin care before, during, and 15 minutes after heel lance Control: standard care during painful procedure Provider: mother |
|
| Outcomes | PIPP score | |
| Notes | Country: India Power calculation: yes |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization of patients to give KMC or not for the first heel prick was done using graphpad.com (a web‐based program)..." |
| Allocation concealment (selection bias) | Unclear risk | "the random numbers were stored in opaque envelopes, which were opened once the patient entered study." Envelopes should be opaque, sealed, and sequentially numbered. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "The persons examining the video were unaware of the status of the neonate during analysis as the videography was done by focusing only on the baby's face and the surroundings were not visible, with the sound kept on mute." "Mothers were asked to keep their hands clasped behind the neonate's back throughout the procedure and refrain from touching the neonate's head with her face and from vocalizing to the neonate during filming (to keep observers blind)." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropout rates were clearly explained in Figure 2 |
| Selective reporting (reporting bias) | Low risk | Outcomes were clearly laid out in Figure 2 and Table 2 |
| Other bias | Low risk | "There was a minimum of 24 h and a maximum of 7 d gap between the conditions. The heels were assessed for any signs of inflammation so as to remove it as a confounding factor." "Only two staff nurses did the heel prick for these neonates to keep the procedure standardized and without bias." |
Okan 2010.
| Methods | Randomized controlled trial | |
| Participants | 107 full term infants (PMA 39.5 ± 0.6): Randomized to 3 groups: skin‐to‐skin + breast feeding; skin‐to‐skin; and standard care Postnatal age, mean ± SD, days: 33.1 ± 5 Painful procedure: heel lance Study period: unclear |
|
| Interventions | Intervention: held in mother's arms with skin‐to‐skin contact 15 minutes before and during painful procedure Comparison: breast fed with skin‐to‐skin contact for 15 minutes before and during painful procedure Control: wrapped in blankets and lying on table before, during and after painful stimulus Provider: mother provided skin‐to‐skin care and breastfeeding |
|
| Outcomes | Neonatal Facial Coding System Scores (NFCS), physiological responses (heart rate and oxygen saturation changes) and behavioural responses (duration of crying and grimacing) | |
| Notes | Country: Turkey Power calculation: yes |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Study infants were assigned to one of three groups using a random number digits table” |
| Allocation concealment (selection bias) | Unclear risk | “1200 (full‐term infants) met all inclusion criteria. Every day, at least six such infants were in the maternity wards, only one of whom was studied. In the morning, the name of an infant who met the inclusion criteria was drawn form a bag by a nurse not involved in the study.” |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | “this arrangement (skin‐to‐skin with mother) left the infants faces visible for recording from the side of the bed and simultaneously provided cover and comfort for the mothers.” “to minimise variability, the blood collection process was performed by the same nurse who was not aware of the purpose of the study, and the time spent squeezing the heel was recorded." “observers of facial actions recognized the groups while evaluating the recordings. In order to minimise any errors caused by prejudice, the video records were evaluated by two persons who were unaware of each other’s results.” Breastfeeding difficult to blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropout rates explained |
| Selective reporting (reporting bias) | Low risk | Outcomes listed in Methods match those reported |
| Other bias | Low risk | “All tests were performed...1‐2 hours after breastfeeding." “to minimise variability, the blood collection process was performed by the same nurse who was not aware of the purpose of the study, and the time spent squeezing the heel was recorded.” “there were no significant differences between the groups in the clinical characteristics, pretest behavioural state score, and blood collection time.” |
Saeidi 2011.
| Methods | Randomized controlled trial | |
| Participants | 60 full term infants (80% of case group and 73.3% of control group had 40 weeks GA) Birth weight, mean ± SD, grams: 3242 ± 306.6 (intervention), 3151 ± 331.5 (control) Painful procedure: vaccination Study period: March to July 2006 |
|
| Interventions | Intervention: skin to skin contact 2 minutes before vaccination through 3 minutes after Control: standard care during painful procedure Provider: mother provided skin‐to‐skin care |
|
| Outcomes | Behavioural changes using the Neonatal/Infant Pain Scale (NIPS) 2 minutes before, during, and 3 minutes after intervention; heart rate and oxygen saturation | |
| Notes | Country: Iran Power calculation: unclear |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Samples were divided randomly into two groups." Unclear how the matching was done or how the sequence was generated. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information available to judge |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | "Neonatal reactions to pain were video recorded." Unclear whether video recording was focused on face of infant. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropout rates not reported |
| Selective reporting (reporting bias) | Unclear risk | NIPS outcomes were clearly presented in Tables 1‐3; O₂ saturation presented clearly under results but specific HR and crying interval data not reported |
| Other bias | Unclear risk | Power calculation not done |
Sajedi 2007.
| Methods | Randomized controlled trial | |
| Participants | 100 term neonates (PMA 39.4 ± 1.5 weeks (intervention), 39.1 ± 1.4 weeks (control) Birth weight, mean ± SD, grams: 3083 ± 258 (intervention), 3142 ± 242 (control) Painful procedure: intramuscular injection Study period: Unclear "2‐month observation period" |
|
| Interventions | Intervention: 10 minutes of skin‐to‐skin care before, during, and 3 minutes after painful procedure Control: standard care during painful procedure Provider: mother provided skin‐to‐skin care |
|
| Outcomes | Neonatal Infant Pain Scale (NIPS), behavioural outcomes (including facial expression, breathing pattern, state of arousal, arm movements, leg movements, and cry), heart rate and oxygen saturation before, during and after injection | |
| Notes | Country: Iran Power calculation: no |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The neonates were randomly assigned to intervention and control groups by using randomised permuted blocks. Randomization was done by a well‐trained nurse using a random numbers table." Unclear how the matching was done |
| Allocation concealment (selection bias) | Unclear risk | Methods of allocation concealment were not specified |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | "we filmed only the face of the neonate for evaluation of the duration of crying..." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Table 5 data for 20 infants in the intervention group were reported and for 44 in the control group. Stated that 30 infants in the intervention group and 6 in the control group did not cry at all |
| Selective reporting (reporting bias) | Low risk | Data of all outcomes were clearly presented in Tables 2‐4 (Kashaninia) and Tables 2‐3 (Sajedi) |
| Other bias | High risk | Study is a combination of 2 studies (Sajedi 2007 and Kashaninia 2008) |
BF = breastfeeding GA = gestational age HR = heart rate PMA = postmenstrual age
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abdel‐Razek 2009 | Inappropriate intervention (breastfeeding) |
| Arditi 2006 | Inappropriate intervention (no skin‐to‐skin care) |
| Axelin 2009 | Inappropriate intervention ("parental holding" which didn't include ventral skin contact) |
| Bellieni 2002 | Inappropriate intervention (no isolated skin‐to‐skin care) |
| Bellieni 2007 | Inappropriate intervention (no isolated skin‐to‐skin care) |
| Campbell‐Yeo 2012 | Inappropriate intervention (not consistently ventral contact) |
| Campbell‐Yeo 2013 | Protocol only. No results. |
| Castral 2015 | Inappropriate design (no comparison group). |
| Chidambaram 2014 | Design unclear. Author confirmed case‐control design, no random crossover. PIPP used at times outside guidelines for use. |
| Choudhary 2015 | No randomisation. |
| Erlandsson 2007 | Inappropriate participants (no painful procedure delivered by health care professionals) |
| Ferber 2008 | Inappropriate outcome (NIDCAP has some behaviours associated with pain, but is not a measure of pain) |
| Gabriel 2010 | Inappropriate participants (no painful procedure delivered by healthcare professionals) |
| Gazzolo 2000 | Inappropriate participants (no painful procedure delivered by healthcare professionals) |
| Johnston 2007 | Inappropriate intervention (no skin‐to‐skin care) |
| Kashaninia 2008 | Same data set as Sajedi 2007. |
| Lyngstad 2014 | Inappropriate design (diaper change not a painful procedure). |
| Miles 2006 | Inappropriate participants (skin‐to‐skin care did not take place during painful procedure) |
| Mitchell 2013 | Innapropriate intervention (no skin‐to‐skin during procedure). |
| Mooncey 1997 | Inappropriate participants (no painful procedure delivered by healthcare professionals) |
| Mörelius 2005 | Inappropriate participants (no painful procedure delivered by healthcare professional) |
| Obeidat 2015 | Innapropriate intervention (no skin‐to‐skin during procedure). |
| Olsson 2015 | Subjects not randomized |
| Reis 2003 | Inappropriate intervention (no ventral skin contact) |
| Schlez 2011 | Inappropriate participants (no painful procedure delivered by healthcare professionals) |
| Silva 2004 | Inappropriate intervention (study focuses on understanding maternal experiences during first contact with child; no painful procedure implemented) |
| Uga 2008 | Inappropriate intervention (breastfeeding) |
| Vivancos 2010 | Inappropriate intervention: (skin‐to‐skin care did not occur during the painful procedure) |
Characteristics of studies awaiting assessment [ordered by study ID]
Mahindre 2009.
| Methods | Cross‐over trial |
| Participants | 60 infants postmenstrual age: "30 infants between 28‐32 weeks and 30 between 32‐36 weeks" Painful procedure: "blood sampling" |
| Interventions | Intervention: "Kangaroo Mother Care" Control: "Conventional open care" Provider: Unclear |
| Outcomes | PIPP scores at 30, 60, 90 and 120 seconds; time required for heart rate and oxygen saturation to touch baseline |
| Notes | Country: India Power calculation: Unclear |
Characteristics of ongoing studies [ordered by study ID]
IRCT2014120217972N4.
| Trial name or title | Paternal vs Maternal Kangaroo and routine care for pain relief in preterm neonates |
| Methods | Randomized crossover trial |
| Participants | 240 infants 28 to 36 6/7 weeks GA |
| Interventions | Paternal Kangaroo Care, Maternal Kangaroo Care, Control (incubator) |
| Outcomes | Premature Infant Pain Profile, change in heart rate, change in O₂ saturation |
| Starting date | 23 September 2014 |
| Contact information | Zahra Godarzi School of Nursing and Midwifery, Tehran University of Medical Sciences Nusrat Sharghi street, Tohid square Tehran Tehran Iran, Islamic Republic Of Phone: 00982161054407 Fax: 00982166904252 godarziz@tums.ac.ir |
| Notes |
IRCT201505142639N16.
| Trial name or title | Efficacy of kangaroo mother care, breastfeeding and swaddling on BCG vaccine pain score in neonates |
| Methods | RCT |
| Participants | Inclusion criteria: Inclusion criteria: term neonates (gestation age of 37–42 weeks); birth weight of 2500 to 4000 g; product of normal vaginal delivery; without systemic illness and with healthy medical condition
Exclusion criteria: birth asphyxia; severe congenital malformation; receiving sedative hypnotic drugs within the past 24 hours Exclusion criteria: Age minimum: 0 Age maximum: 0 Gender: both male and female |
| Interventions | Intervention 1: breast feeding two minutes before vaccination until one minute after BCG vaccination . Intervention 2: kangaroo mother care of neonate with close contact of thorax and abdomen skin of mother ten minutes before vaccination until one minute after BCG vaccination. Intervention 3: swaddling of neonate ten minutes before vaccination until one minute after BCG vaccination . |
| Outcomes | Obtaining of pain score of less than four during BCG vaccine injection. Timepoint: during BCG vaccine injection. Method of measurement: Neonatal/Infant Pain Scale Pain score of BCG vaccine injection. Timepoint: before, during, one minute and two minutes after BCG vaccine injection. Method of measurement: Neonatal/Infant Pain Scale Duration of neonate crying during BCG vaccine injection. Timepoint: every 10 seconds after vaccine injection until stopping of crying. Method of measurement: By chronometer |
| Starting date | 24 April 2015 |
| Contact information | Name: Dr. Razieh Fallah Address: Pediatric Ward, Shahid Sadoughi Hospital, Ebn ‐ Sina BLVD, Shahid Ghandi BlVD, Yazd, I.R.Iran Yazd Iran, Islamic Republic Of Telephone: 00983538224000 Email: dr.raziehfallah@yahoo.com Affiliation: Shahid Sadoughi University of Medical Sciences, Yazd, Iran |
| Notes |
IRCT2015052914251N3.
| Trial name or title | Effect of neonate's massage and kangaroo mother care on neonates' pain |
| Methods | RCT |
| Participants | Inclusion criteria: lack of symptoms of severe disease or congenital abnormality that prevents neonates to exit from incubator; lack of any CNS abnormalities; lack of any obstetric or medical problems in mother; neonates who will be born in 32 to 37 weeks of age based on the age as recorded in the medical record; lack of intubation or connecting to ventilator in neonates; approve of haemodynamic stability and allow to neonates participate in the study and exit from incubator by physician.
Exclusion criteria: existence or occurrence of any kind of medical or obstetric problems for the mother; neonate dies during the study; any situation which does not allow neonates to exit the incubator. Exclusion criteria: Age minimum: 0 Age maximum: 0 Gender: both male and female |
| Interventions | Intervention 1: First intervention group: neonates will place on the mother's chest based on kangaroo mother method for 15 minutes before and 15 minutes after invasive procedures. Intervention 2: Second intervention group: neonates’ leg will be massaged by mother for 15 minutes before and 15 minutes after invasive procedures. Intervention 3: Control group: neonate, whilst connected to pulse oximetry in supine position, will be put in incubator without any touching and changes in position.. |
| Outcomes | Pain. Timepoint: before, during and after invasive procedures. Method of measurement: pain scale and pulse oximetry Heart rate. Timepoint: before, during and after invasive procedures. Method of measurement: pulse oximetry Oxygen saturation . Timepoint: before, during and after invasive procedures. Method of measurement: pulse oximetry |
| Starting date | 1 April 2015 |
| Contact information | Maryam Valimalayeri Faculty of Nursing and Midwifery, Hamadan University of Medical Sciences, Shaheed Fahmideh avenue Hamadan, Iran, Islamic Republic Of 00988138330641 valimalayeri@gmail.com Department of Pediatrics, Faculty of Nursing and Midwifery, Hamadan University of Medical Sciences |
| Notes |
Differences between protocol and review
We added the methodology and plan for 'Summary of findings' tables and GRADE recommendations, which were not included in the original protocol or in the previous review.
Contributions of authors
CJ oversaw the process and arbitrated disputes between other reviewers, and wrote narrative. MCY, TD, BB, and RZ contributed to content and editing of narrative. MCY, TD, BB, AF, RZ reviewed articles and rated them according to criteria. DS served as methodological expert. All authors reviewed and approved the final submission.
Sources of support
Internal sources
GRISIIQ, Canada.
External sources
-
Eunice Kennedy Shriver National Institute of Child Health and Human Development National Institutes of Health, Department of Health and Human Services, USA.
Editorial support of the Cochrane Neonatal Review Group has been funded with Federal funds from the Eunice Kennedy Shriver National Institute of Child Health and Human Development National Institutes of Health, Department of Health and Human Services, USA, under Contract No. HHSN275201100016C.
-
National Institute for Health Research, UK.
Editorial support for Cochrane Neonatal has been funded with funds from a UK National Institute of Health Research Grant (NIHR) Cochrane Programme Grant (13/89/12). The views expressed in this publication are those of the authors and not necessarily those of the NHS, the NIHR, or the UK Department of Health.
Declarations of interest
The authors have nothing to declare.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Akcan 2009 {published data only}
- Akcan E, Yiğit R, Atici A. The effect of kangaroo care on pain in premature infants during invasive procedures. Turkish Journal of Pediatrics 2009;51(1):14‐8. [PubMed] [Google Scholar]
Castral 2008 {published and unpublished data}
- Castral TC, Warnock F, Leite AM, Haas VJ, Scochi CG. The effects of skin‐to‐skin contact during acute pain in preterm newborns. European Journal of Pain 2008;12(4):464‐71. [DOI] [PubMed] [Google Scholar]
Chermont 2009 {published and unpublished data}
- Chermont AG, Falcão LF, Souza Silva EH, Cássia Xavier Balda R, Guinsburg R. Skin‐to‐skin contact and/or oral 25% dextrose for procedural pain relief for term newborn infants. Pediatrics 2009;124(6):e1101‐7. [DOI] [PubMed] [Google Scholar]
Cong 2009 {published and unpublished data}
- Cong X, Ludington‐Hoe SM, McCain G, Fu P. Kangaroo care modifies preterm infant heart rate variability in response to heel stick pain: Pilot study. Early Human Development 2009;85(9):561‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cong 2011 {published and unpublished data}
- Cong X, Ludington‐Hoe SM, Walsh S. Randomized crossover trial of kangaroo care to reduce biobehavioural pain responses in preterm infants: a pilot study. Biological Research for Nursing 2011;13(2):204‐16. [DOI] [PubMed] [Google Scholar]
Cong 2012 {published data only}
- Cong X, Cusson RM, Walsh S, Hussain N, Ludington‐Hoe SM, Zhang D. Effects of skin‐to‐skin contact on autonomic pain responses in preterm infants. The Journal of Pain 2012;13(7):636‐45. [DOI] [PubMed] [Google Scholar]
Freire 2008 {published and unpublished data}
- Freire NB, Garcia JB, Lamy ZC. Evaluation of analgesic effect of skin‐to‐skin contact compared to oral glucose in preterm neonates. Pain 2008;139(1):28‐33. [DOI] [PubMed] [Google Scholar]
Gabriel 2013 {published data only}
- Marin Gabriel MA, Rey Hurtado de Mendoza B, Jimenez Figueroa L, Medina V, Iglesias Fernández, B, Vazquez Rodríguez M, et al. Analgesia with breastfeeding in addition to skin‐to‐skin contact during heel prick. Archives of Diseases in Childhood. Fetal and Neonatal Edition 2013;98(6):F499‐503. [DOI: ] [DOI] [PubMed] [Google Scholar]
Gao 2015 {published data only}
- Gao H, Xu G, Gao H, Dong R, Fu H, Wang D, et al. Effect of repeated Kangaroo Mother Care on repeated procedural pain in preterm infants: A randomized controlled trial. International Journal of Nursing Studies 2015;Epub ahead of print(7):1157‐65. [DOI] [PubMed] [Google Scholar]
Gray 2000 {published data only}
- Gray L, Watt L, Blass EM. Skin‐to‐skin contact is analgesic in healthy newborns. Pediatrics 2000;105(1):e14. [DOI] [PubMed] [Google Scholar]
Johnston 2003 {published and unpublished data}
- Johnston CC, Stevens B, Pinelli J, Gibbins S, Filion F, Jack A, et al. Kangaroo care is effective in diminishing pain response in preterm neonates. Archives of Pediatrics & Adolescent Medicine 2003;157(11):1084‐8. [DOI] [PubMed] [Google Scholar]
Johnston 2008 {published and unpublished data}
- Johnston CC, Filion F, Campbell‐Yeo M, Goulet C, Bell L, McNaughton K, et al. Kangaroo mother care diminishes pain from heel lance in very preterm neonates: a crossover trial. BMC Pediatrics 2008;8:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Johnston 2009 {published and unpublished data}
- Johnston CC, Filion F, Campbell‐Yeo M, Goulet C, Bell L, McNaughton K, et al. Enhanced kangaroo mother care for heel lance in preterm neonates: a crossover trial. Journal of Perinatology 2009;29(1):51‐6. [DOI] [PubMed] [Google Scholar]
Johnston 2011 {published and unpublished data}
- Johnston CC, Campbell‐Yeo M, Filion F. Paternal vs maternal kangaroo care for procedural pain in preterm neonates: A randomized crossover trial. Archives of Pediatrics & Adolescent Medicine 2011;165(9):792‐6. [DOI] [PubMed] [Google Scholar]
Johnston 2012 {published and unpublished data}
- Johnston C, Byron J, Filion F, Campbell‐Yeo M, Gibbins S, Ng E. Alternative female kangaroo care for procedural pain in preterm neonates: A pilot study. Acta Paediatrica 2012;101(11):1147‐50. [DOI] [PubMed] [Google Scholar]
Kostandy 2008 {published data only}
- Kostandy RR, Ludington‐Hoe SM, Cong X, Abouelfettoh A, Bronson C, Stankus A, et al. Kangaroo Care (skin contact) reduces crying response to pain in preterm neonates: pilot results. Pain Management Nursing 2008;9(2):55‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kostandy 2013 {published and unpublished data}
- Kostandy R, Anderson GC, Good M. Skin‐to‐skin contact diminishes pain from hepatitis B vaccine injection in healthy full‐term neonates. Neonatal Network 2013;32(4):274‐80. [PUBMED: 23835546] [DOI] [PubMed] [Google Scholar]
Liu 2015 {published data only}
- Liu M, Zhao L, Li XF. Effect of skin contact between mother and child in pain relief of full‐term newborns during heel blood collection. Clinical and Experimental Obstetrics & Gynecology 2015;XLII(3):304‐8. [DOI: 10.12891/ccog1831.2015] [DOI] [PubMed] [Google Scholar]
Ludington‐Hoe 2005 {published data only}
- Ludington‐Hoe SM, Hosseini R, Torowicz DL. Skin‐to‐skin contact (kangaroo care) analgesia for preterm infant heel stick. AACN Clinical Issues: Advanced Practice in Acute and Critical Care 2005;16(3):373‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mosayebi 2014 {published data only}
- Mosayebi Z, Javidpour M, Rahmati M, Hagani H, Movahedia AH. The effect of kangaroo mother care on pain from heel lance in preterm newborns admitted to neonatal intensive care unit: A crossover randomized clinical trial. Journal of Comprehensive Pediatrics 2014;5(4):1‐6. [Google Scholar]
Nanavati 2013 {published data only}
- Nanavati RN, Balan R, Kabra NS. Effect of kangaroo mother care vs expressed breast milk administration on pain associated with removal of adhesive tape in very low birth weight neonates: a randomized controlled trial. Indian Pediatrics 2013;50(11):1011‐5. [PUBMED: 23798626] [DOI] [PubMed] [Google Scholar]
Nimbalkar 2013 {published and unpublished data}
- Nimbalkar SM, Chaudhary NS, Gadhavi KV, Phatak A. Kangaroo mother care in reducing pain in preterm neonates on heel prick. Indian Journal of Pediatrics 2013;80(1):6‐10. [DOI] [PubMed] [Google Scholar]
Okan 2010 {published data only (unpublished sought but not used)}
- Okan F, Ozdil A, Bulbul A, Yapici Z, Nuhoglu A. Analgesic effects of skin‐to‐skin contact and breastfeeding in procedural pain in healthy term neonates. Annals of Tropical Paediatrics 2010;30(2):119‐28. [DOI] [PubMed] [Google Scholar]
Saeidi 2011 {published data only}
- Saeidi R, Asnaashari Z, Amirnejad M, Esmaeili H, Robatsangi MG. Use of "Kangaroo Care" to alleviate the intensity of vaccination pain in newborns. Iranian Journal of Pediatrics March 2011;21(1):99‐102. [PMC free article] [PubMed] [Google Scholar]
Sajedi 2007 {published data only}
- Sajedi F, Kashaninia Z, Rahgozar M, Noghabi FA. The effect of Kangaroo Care on physiologic responses to pain of an intramuscular injection in neonates. Iranian Journal of Pediatrics 2007;17(4):339‐44. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abdel‐Razek 2009 {published data only}
- Abdel‐Razek A, Az El‐Dein N. Effect of breast‐feeding on pain relief during infant immunization injections. International Journal of Nursing Practice 2009;15(2):99‐104. [DOI] [PubMed] [Google Scholar]
Arditi 2006 {published data only}
- Arditi H, Feldman R, Eidelman AI. Effects of human contact and vagal regulation on pain reactivity and visual attention in newborns. Developmental Psychobiology 2006;48(7):561‐73. [DOI] [PubMed] [Google Scholar]
Axelin 2009 {published data only}
- Axelin A, Salanterä S, Kirjavainen J, Lehtonen L. Oral glucose and parental holding preferable to opioid in pain management in preterm infants. The Clinical Journal of Pain 2009;25(2):138‐45. [DOI] [PubMed] [Google Scholar]
Bellieni 2002 {published data only}
- Bellieni CV, Bagnoli F, Perrone S, Nenci A, Cordelli DM, Fusi M, et al. Effect of multisensory stimulation on analgesia in term neonates: a randomized controlled trial. Pediatric Research 2002;51(4):460‐3. [DOI] [PubMed] [Google Scholar]
Bellieni 2007 {published data only}
- Bellieni CV, Cordelli DM, Marchi S, Ceccarelli S, Perrone S, Maffei M, et al. Sensorial saturation for neonatal analgesia. The Clinical Journal of Pain 2007;23(3):219‐21. [DOI] [PubMed] [Google Scholar]
Campbell‐Yeo 2012 {published data only}
- Campbell‐Yeo ML, Johnston CC, Joseph KS, Feeley NL, Chambers CT, Barrington KJ. Cobedding and recovery time after heel lance in preterm twins: results of a randomized trial. Pediatrics 2012;130(3):500‐6. [DOI] [PubMed] [Google Scholar]
Campbell‐Yeo 2013 {published data only}
- Campbell‐Yeo M, Johnston C, Benoit B, Latimer M, Vincer M, Watker CD, et al. Trial of repeated analgesia with Kangaroo Mother Care (TRAKC Trial). BMC Pediatrics 2013;13:182. [PUBMED: 2428400] [DOI] [PMC free article] [PubMed] [Google Scholar]
Castral 2015 {published data only}
- Castral TC, Warnock F, Dos Santos CB, Dare MF, Moreira AC, Antonini SR, et al. Maternal mood and concordant maternal and infant salivary cortisol during heel lance while in kangaroo care. European Journal of Pain 2015;19(3):429‐38. [DOI] [PubMed] [Google Scholar]
Chidambaram 2014 {published data only}
- Chidambaram AG, Manjula S, Adhisivam B, Bhat BV. Effect of Kangaroo mother care in reducing pain due to heel prick among preterm neonates: a crossover trial. The Journal of Maternal Fetal & Neonatal Medicine 2014;27(5):488‐90. [PUBMED: 23796239] [DOI] [PubMed] [Google Scholar]
Choudhary 2015 {published data only}
- Choudhary M, Dogiyal H, Sharma D, Datt Gupta B, Madabhavi I, Choudhary JS, et al. To study the effect of kangaroo mother care on pain response in preterm neonates and to determine the behavioural and physiological responses to painful stimuli in preterm neonates: A study from western Rajasthan. The Journal of Maternal‐Fetal & Neonatal Medicine 2016;29(5):826‐31. [DOI] [PubMed] [Google Scholar]
Erlandsson 2007 {published data only}
- Erlandsson K, Dsilna A, Fagerberg I, Christensson K. Skin‐to‐skin care with the father after cesarean birth and its effect on newborn crying and prefeeding behavior. Birth: Issues in Perinatal Care 2007;34(2):105‐14. [DOI] [PubMed] [Google Scholar]
Ferber 2008 {published data only}
- Ferber SG, Makhoul IR. Neurobehavioural assessment of skin‐to‐skin effects on reaction to pain in preterm infants: a randomized, controlled within‐subject trial. Acta Pædiatrica 2008;97(2):171‐6. [DOI] [PubMed] [Google Scholar]
Gabriel 2010 {published data only}
- Marín Gabriel MA, Llana Martin I, López Escobar A, Fernández Villalba E, Romero Blanco I, Touza Pol P. Randomized controlled trial of early skin‐to‐skin contact; effects on the mother and the newborn. Acta Paediatrica 2010;99(11):1630‐4. [DOI] [PubMed] [Google Scholar]
Gazzolo 2000 {published data only}
- Gazzolo D, Masetti P, Meli M. Kangaroo care improves post‐extubation cardiorespiratory parameters in infants after open heart surgery. Acta Paediatrica 2000;89(6):728‐9. [DOI] [PubMed] [Google Scholar]
Johnston 2007 {published data only}
- Johnston CC, Filion F, Nuyt AM. Recorded maternal voice for preterm neonates undergoing heel lance. Advances in Neonatal Care 2007;7(5):258‐66. [DOI] [PubMed] [Google Scholar]
Kashaninia 2008 {published data only}
- Kashaninia Z, Sajedi F, Rahgozar M, Noghabi FA. The effect of Kangaroo Care on behavioral responses to pain of an intramuscular injection in neonates. Journal for Specialists in Pediatriac Nursing 2008;13(4):275‐80. [DOI] [PubMed] [Google Scholar]
Lyngstad 2014 {published data only}
- Lyngstad LT, Tandberg BS, Storm H, Ekeberg BL, Moen A. Does skin‐to‐skin contact reduce stress during diaper change in preterm infants?. Early Human Development 2014;90(4):169‐72. [PUBMED: 24548816] [DOI] [PubMed] [Google Scholar]
Miles 2006 {published data only}
- Miles R, Cowan F, Glover V, Stevenson J, Modi N. A controlled trial of skin‐to‐skin contact in extremely preterm infants. Early Human Development 2006;82(7):447‐55. [DOI] [PubMed] [Google Scholar]
Mitchell 2013 {published data only}
- Mitchell AJ, Yates CC, Williams DK, Chang JY, Hall RW. Does daily kangaroo care provide sustained pain and stress relief in preterm infants?. Journal of Neonatal‐Perinatal Medicine 2013;6(1):45‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mooncey 1997 {published data only}
- Mooncey S, Giannakoulopoulos X, Glover V, Acolet D, Modi N. The effect of mother‐infant skin‐to‐skin contact on plasma cortisol and beta‐endorphin concentrations in preterm newborns. Infant Behavior & Development 1997;20(4):553‐7. [Google Scholar]
Mörelius 2005 {published data only}
- Mörelius E, Theodorsson E, Nelson N. Salivary cortisol and mood and pain profiles during skin‐to‐skin care for an unselected group of mothers and infants in neonatal intensive care. Pediatrics 2005;116(5):1105‐13. [DOI] [PubMed] [Google Scholar]
Obeidat 2015 {published data only}
- Obeidat HM, Shuriquie MA. Effect of breast‐feeding and maternal holding in relieving painful responses in full‐term neonates. Journal of Perinatal & Neonatal Nursing 2015;29(3):248‐54. [DOI] [PubMed] [Google Scholar]
Olsson 2015 {published data only}
- Olsson E, Ahlsen G, Eriksson M. Skin‐to‐skin contact reduces near‐infrared spectroscopy pain responses in premature infants during blood sampling. Acta Paediatrica 2015;105(4):376‐80. [DOI] [PubMed] [Google Scholar]
Reis 2003 {published data only}
- Reis EC, Roth EK, Syphan JL, Tarbell SE, Holubkov R. Effective pain reduction for multiple immunization injections in young infants. Archives of Pediatrics & Adolescent Medicine 2003;157(11):1115‐20. [DOI] [PubMed] [Google Scholar]
Schlez 2011 {published data only}
- Schlez A, Litmanovitz I, Bauer S, Dolfin T, Regev R, Arnon S. Combining kangaroo care and live harp music therapy in the neonatal intensive care unit setting. Israel Medical Association Journal 2011;13(6):254‐8. [PubMed] [Google Scholar]
Silva 2004 {published data only}
- Silva LM, Clapis MJ. Understanding maternal experiences during her first contact with her child on the birth's room [Compreendendo a vivência materna no primeiro contato com seu filho na sala de parto]. Acta Paulista de Enfermagem 2004;17(3):286‐91. [Google Scholar]
Uga 2008 {published data only}
- Uga E, Candriella M, Perino A, Alloni V, Angilella G, Trada M, et al. Heel lance in newborn during breastfeeding: an evaluation of analgesic effect of this procedure. Italian Journal of Pediatrics 2008;34(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vivancos 2010 {published data only}
- Zavanella Vivancos RB, Moraes Leite A, Silvan Scochi CG, dos Santos CB. The skin to skin contact at birth and newborn crying during vaccination against Hepatitis B [O contato pele a pele ao nascimento e o choro de recém‐nascidos durante vacinação contra Hepatite B]. Acta Paulista de Enfermagem 2010;23(4):461‐5. [Google Scholar]
References to studies awaiting assessment
Mahindre 2009 {published data only}
- Mahindre A. Kangaroo Mother Care (KMC) diminishes pain in preterm Infants. Acta Paediatrica 2009;98(s460):181‐2. [Google Scholar]
References to ongoing studies
IRCT2014120217972N4 {published and unpublished data}
- IRCT2014120217972N4. Paternal vs Maternal Kangaroo and routine care for pain relief in preterm neonates after heel lancet procedure. http://apps.who.int/trialsearch/Trial2.aspx?TrialID=IRCT2014120217972N4 (accessed 26 February 2016).
IRCT201505142639N16 {published and unpublished data}
- IRCT201505142639N16. Efficacy of kangaroo mother care, breastfeeding and swaddling on BCG vaccine pain score in neonates. http://apps.who.int/trialsearch/Trial2.aspx?TrialID=IRCT201505142639N16 (accessed 26 February 2016).
IRCT2015052914251N3 {published and unpublished data}
- IRCT2015052914251N3. Effect of neonate's massage and kangaroo mother care on neonates' pain. http://apps.who.int/trialsearch/Trial2.aspx?TrialID=IRCT2015052914251N3 (accessed 26 February 2016).
Additional references
Akman 2002
- Akman I, Ozek E, Bilgen H, Ozdogan T, Cebeci D. Sweet solutions and pacifiers for pain relief in newborn infants. The Journal of Pain 2002;3(3):199‐202. [DOI] [PubMed] [Google Scholar]
Anand 2004
- Anand KJ, Hall RW, Desai N, Shephard B, Bergqvist LL, Young TE, et al. NEOPAIN Trial Investigators Group. Effects of morphine analgesia in ventilated preterm neonates: primary outcomes from the NEOPAIN randomised trial. Lancet 2004;363(9422):1673‐82. [DOI] [PubMed] [Google Scholar]
Bauer 1998
- Bauer K, Pyper A, Sperling P, Uhrig C, Versmold H. Effects of gestational and postnatal age on body temperature, oxygen consumption, and activity during early skin‐to‐skin contact between preterm infants of 25‐30‐week gestation and their mothers. Pediatric Research 1998;44(2):247‐51. [DOI] [PubMed] [Google Scholar]
Benoit 2016
- Benoit B, Campbell‐Yeo M, Johnston C, Latimer M, Caddell K, Orr T. Staff nurse utilization of kangaroo care as an intervention for procedural pain in preterm infants. Advances in Neonatal Care 2016;16(3):229‐38. [In press] [DOI] [PubMed] [Google Scholar]
Bohnhorst 2001
- Bohnhorst B, Heyne T, Peter CS, Poets CF. Skin‐to‐skin (kangaroo) care, respiratory control, and thermoregulation. The Journal of Pediatrics 2001;138(2):193‐7. [DOI] [PubMed] [Google Scholar]
Boundy 2016
- Boundy EO, Dastjerdi R, Spiegelman D, Fawzi WW, Missmer SA, Lieberman E, et al. Kangaroo mother care and neonatal outcomes: a meta‐analysis. Pediatrics 2016;137(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
Boyle 2004
- Boyle EM, McIntosh N. Pain and compassion in the neonatal unit ‐‐ a neonatologist's view. Neuro Endocrinology Letters 2004;25 Suppl 1:49‐55. [PubMed] [Google Scholar]
Brummelte 2012
- Brummelte S, Grunau RE, Chau V, Poskitt KJ, Brant R, Vinall J, et al. Procedural pain and brain development in premature newborns. Annals of Neurology 2012;71(3):385‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Campbell‐Yeo 2008
- Campbell‐Yeo M, Johnston CC, Filion F, McNaughton K. A comparison of nurse and mother attitudes regarding maternal skin‐to‐skin care as a pain relieving strategy during heel lance for preterm neonates. 1st European Conference on the Kangaroo Mother Care Proceedings 2008.
Campbell‐Yeo 2015
- Campbell‐Yeo ML, Disher TC, Benoit BL, Johnston CC. Understanding kangaroo care and its benefits to preterm infants. Pediatric Health, Medicine and Therapeutics 2015;2015(6):15‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Campos 1994
- Campos RG. Rocking and pacifiers: two comforting interventions for heelstick pain. Research in Nursing and Health 1994;17(5):321‐31. [DOI] [PubMed] [Google Scholar]
Carbajal 1997
- Carbajal R, Paupe A, Hoenn E, Lenclen R, Olivier‐Martin M. APN: evaluation behavioral scale of acute pain in newborn infants [DAN:une échelle comportementale d’évaluation de la douleur aiguë du nouveau‐né]. Archives de Pediatrie 1997;4(7):623‐8. [DOI] [PubMed] [Google Scholar]
Carbajal 2002
- Carbajal R, Lenclen R, Gajdos V, Jugie M, Paupe A. Crossover trial of analgesic efficacy of glucose and pacifier in very preterm neonates during subcutaneous injections. Pediatrics 2002;110 (2 Pt 1):389‐93. [DOI] [PubMed] [Google Scholar]
Carbajal 2005
- Carbajal R, Lenclen R, Jugie M, Paupe A, Barton BA, Anand KJ. Morphine does not provide adequate analgesia for acute procedural pain among preterm neonates. Pediatrics 2005;115(6):1494‐500. [DOI] [PubMed] [Google Scholar]
Carbajal 2008
- Carbajal R, Rousset A, Danan C, Coquery S, Nolent P, Ducrocq S, et al. Epidemiology and treatment of painful procedures in neonates in intensive care units. JAMA 2008;300(1):60‐70. [DOI] [PubMed] [Google Scholar]
Carfoot 2003
- Carfoot S, Williamson PR, Dickson R. A systematic review of randomised controlled trials evaluating the effect of mother/baby skin‐to‐skin care on successful breast feeding. Midwifery 2003;19(2):148‐55. [DOI] [PubMed] [Google Scholar]
Charpak 2005
- Charpak N, Ruiz JG, Zupan J, Cattaneo A, Figueroa Z, Tessier R, et al. Kangaroo mother care: 25 years after. Acta Paediatrica 2005;94(5):514‐22. [DOI] [PubMed] [Google Scholar]
Christensson 1992
- Christensson K, Siles C, Moreno L, Belaustequi A, Fuente P, Lagercrantz H, et al. Temperature, metabolic adaptation and crying in healthy full‐term newborns cared for skin‐to‐skin or in a cot. Acta Paediatrica 1992;81(6‐7):488‐93. [DOI] [PubMed] [Google Scholar]
Chwo 2002
- Chwo MJ, Anderson GC, Good M, Dowling DA, Shiau SH, Chu DM. A randomized controlled trial of early kangaroo care for preterm infants: effects on temperature, weight, behavior, and acuity. Journal of Nursing Research 2002;10(2):129‐42. [DOI] [PubMed] [Google Scholar]
Cignacco 2007
- Cignacco E, Hamers JP, Stoffel L, Lingen RA, Gessler P, McDougall J, et al. The efficacy of non‐pharmacological interventions in the management of procedural pain in preterm and term neonates. A systematic literature review. European Journal of Pain 2007;11(2):139‐52. [DOI] [PubMed] [Google Scholar]
Conde‐Agudelo 2014
- Conde‐Agudelo A, Díaz‐Rossello JL. Kangaroo mother care to reduce morbidity and mortality in low birthweight infants. Cochrane Database of Systematic Reviews 2014, Issue 4. [DOI: 10.1002/14651858.CD002771.pub3] [DOI] [PubMed] [Google Scholar]
Corff 1995
- Corff KE, Seideman R, Venkataraman PS, Lutes L, Yates B. Facilitated tucking: a nonpharmacologic comfort measure for pain in preterm neonates. Journal of Obstetric, Gynecologic, and Neonatal Nursing 1995;24(2):143‐7. [DOI] [PubMed] [Google Scholar]
Craig 1984
- Craig KD, McMahon RJ, Morison JD, Zaskow C. Developmental changes in infant pain expression during immunization injections. Social Science and Medicine 1984;19(12):1331‐7. [DOI] [PubMed] [Google Scholar]
Cruz 2016
- Cruz MD, Fernandes AM, Oliveira CR. Epidemiology of painful procedures performed in neonates: a systematic review of observational studies. European Journal of Pain (London, England) 2016;20(4):489‐98. [DOI] [PubMed] [Google Scholar]
Cuzzolin 2001
- Cuzzolin L, Dal Cerè M, Fanos V. NSAID‐induced nephrotoxicity from the fetus to the child. Drug Safety 2001;24(1):9‐18. [DOI] [PubMed] [Google Scholar]
de Leeuw 1991
- Leeuw R, Collin EM, Dunnebier EA, Mirmiran M. Physiological effects of kangaroo care in very small preterm infants. Biology of the Neonate 1991;59(3):149‐55. [DOI] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. [DOI] [PubMed] [Google Scholar]
Erez 1996
- Erez A, Bloom MC, Wells MT. Using random rather than fixed effects models in meta‐analysis: implications for situational specificity and validity generalization. Personnel Psychology 1996;49(2):275‐306. [Google Scholar]
Feldman 2002
- Feldman R, Weller A, Sirota L, Eidelman AI. Skin‐to‐skin contact (Kangaroo care) promotes self‐regulation in premature infants: sleep‐wake cyclicity, arousal modulation, and sustained exploration. Developmental Psychology 2002;38(2):194‐207. [DOI] [PubMed] [Google Scholar]
Feldman 2003
- Feldman R, Eidelman AI. Skin‐to‐skin contact (Kangaroo care) accelerates autonomic and neurobehavioural maturation in preterm infants. Developmental Medicine and Child Neurology 2003;45(4):274‐81. [DOI] [PubMed] [Google Scholar]
Ferber 2004
- Ferber SG, Makhoul IR. The effect of skin‐to‐skin contact (kangaroo care) shortly after birth on the neurobehavioral responses of the term newborn: a randomized, controlled trial. Pediatrics 2004;113(4):858‐65. [DOI] [PubMed] [Google Scholar]
Field 2003
- Field AP. The problem in using fixed‐effects models of meta‐analysis on real‐world data. Understanding Statistics 2003; Vol. 2, issue 2:105‐24.
Franck 2001
- Franck LS, Scurr K, Couture S. Parent views of infant pain and pain management in the neonatal intensive care unit. Newborn and Infant Nursing Reviews 2001;1(2):106‐13. [Google Scholar]
Franck 2002
- Franck LS, Bernal H, Gale G. Infant holding policies and practices in neonatal units. Neonatal Network ‐ The Journal of Neonatal Nursing 2002;21(2):13‐20. [DOI] [PubMed] [Google Scholar]
Gibbins 2008
- Gibbins S, Stevens B, Beyene J, Chan PC, Bagg M, Asztalos E. Pain behaviours in extremely low gestational age infants. Early Human Development 2008;84(7):451‐8. [DOI] [PubMed] [Google Scholar]
GRADEpro 2014 [Computer program]
- McMaster University. GRADEpro. McMaster University, 2014.
Grunau 2007a
- Grunau RE, Haley DW, Whitfield MF, Weinberg J, Yu W, Thiessen P. Altered basal cortisol levels at 3, 6, 8 and 18 months in infants born at extremely low gestational age. The Journal of Pediatrics 2007;150(2):151‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Grunau 2007b
- Grunau RE, Tu MT. Long‐term consequences of pain in human neonates. In: Anand KJ, Stevens BJ, McGrath PJ editor(s). Pain in Neonates and Infants. 3rd Edition. Philadelphia: Elsevier, 2007:55‐76. [Google Scholar]
Hedges 1998
- Hedges LV, Vevea JL. Fixed‐ and random‐effects models in meta‐analysis. Psychological Methods 1998; Vol. 3, issue 4:486‐504.
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Holsti 2007
- Holsti L, Grunau RE. Initial validation of the behavioral indicators of infant pain (BIIP). Pain 2007;132(3):264‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hummel 2008
- Hummel P, Puchalski M, Creech SD, Weiss MG. Clinical reliability and validity of the N‐PASS: neonatal pain, agitation and sedation scale with prolonged pain. Journal of Perinatology 2008;28(1):55‐60. [DOI] [PubMed] [Google Scholar]
Hummel 2010
- Hummel P, Lawlor‐Klean P, Weiss MG. Validity and reliability of the N‐PASS assessment tool with acute pain. Journal of Perinatology 2010;30(7):474‐8. [DOI] [PubMed] [Google Scholar]
Hunt 2008
- Hunt F. The importance of kangaroo care on infant oxygen saturation levels and bonding. Journal of Neonatal Nursing 2008;14(2):47‐51. [Google Scholar]
Ibe 2004
- Ibe OE, Austin T, Sullivan K, Fabanwo O, Disu E, Costello AM. A comparison of kangaroo mother care and conventional incubator care for thermal regulation of infants < 2000 g in Nigeria using continuous ambulatory temperature monitoring. Annals of Tropical Paediatrics 2004;24(3):245‐51. [DOI] [PubMed] [Google Scholar]
Johnson 2006
- Johnson AN. The relationship of kangaroo holding to maternal breast milk. Journal of Pediatric Nursing 2006;21(2):137‐8. [Google Scholar]
Johnston 1993
- Johnston CC, Stevens B, Craig KD, Grunau RV. Developmental changes in pain expression in premature, full‐term, two‐ and four‐month‐old infants. Pain 1993;52(2):201‐8. [DOI] [PubMed] [Google Scholar]
Johnston 1996
- Johnston CC, Stevens BJ. Experience in a neonatal intensive care unit affects pain response. Pediatrics 1996;98(5):925‐30. [PubMed] [Google Scholar]
Johnston 1997
- Johnston CC, Collinge JM, Henderson SJ, Anand KJ. A cross‐sectional survey of pain and pharmacological analgesia in Canadian neonatal intensive care units. The Clinical Journal of Pain 1997;13(4):308‐12. [DOI] [PubMed] [Google Scholar]
Johnston 2002
- Johnston CC, Filion F, Snider L, Majnemer A, Limperopoulos C, Walker CD, et al. Routine sucrose analgesia during the first week of life in neonates younger than 31 weeks' postconceptional age. Pediatrics 2002;110(3):523‐8. [DOI] [PubMed] [Google Scholar]
Johnston 2007a
- Johnston CC, Filion F, Snider L, Limperopoulos C, Majnemer A, Pelausa E, et al. How much sucrose is too much sucrose?. Pediatrics 2007;119(1):226. [DOI] [PubMed] [Google Scholar]
Johnston 2007b
- Johnston CC, Filion F, Nuyt AM. Recorded maternal voice for preterm neonates undergoing heel lance. Advances in Neonatal Care 2007;7(5):258‐66. [DOI] [PubMed] [Google Scholar]
Johnston 2011b
- Johnston C, Barrington KJ, Taddio A, Carbajal R, Filion F. Pain in Canadian NICUs: have we improved over the past 12 years?. The Clinical Journal of Pain 2011;27(3):225‐32. [DOI] [PubMed] [Google Scholar]
Johnston, 2014
- Johnston C, Campbell‐Yeo M, Fernandes A, Inglis D, Streiner D, Zee R. Skin‐to‐skin care for procedural pain in neonates. Cochrane Database of Systematic Reviews 2014, Issue 1. [DOI: 10.1002/14651858.CD008435.pub2] [DOI] [PubMed] [Google Scholar]
Ko 1998
- Ko Y. Parental stress in the Neonatal Intensive Care Unit (NICU) [Chinese]. Nursing Research (China) 1998;6(5):427‐35. [Google Scholar]
Lagercrantz 1992
- Lagercrantz H. What does the preterm infant breathe for? Controversies on apnea of prematurity. Acta Paediatrica 1992;81(10):733‐6. [DOI] [PubMed] [Google Scholar]
Larsson 1996
- Larsson BA, Norman M, Bjerring P, Egekvist H, Lagercrantz H, Olsson GL. Regional variations in skin perfusion and skin thickness may contribute to varying efficacy of topical, local anaesthetics in neonates. Paediatric Anaesthesia 1996;6(2):107‐10. [DOI] [PubMed] [Google Scholar]
Lawrence 1993
- Lawrence J, Alcock D, McGrath P, Kay J, MacMurray SB, Dulberg C. The development of a tool to assess neonatal pain. Neonatal Network 1993;12(6):59‐66. [PubMed] [Google Scholar]
Lefrak 2006
- Lefrak L, Burch K, Caravantes R, Knoerlein K, DeNolf N, Duncan J, et al. Sucrose analgesia: identifying potentially better practices. Pediatrics 2006;118 Suppl 2:S197‐202. [DOI] [PubMed] [Google Scholar]
Ludington‐Hoe 1992
- Ludington‐Hoe SM, Hashemi MS, Argote LA, Medellin G, Rey H. Selected physiologic measures and behavior during paternal skin contact with Colombian preterm infants. Journal of Developmental Physiology 1992;18(5):223‐32. [PubMed] [Google Scholar]
Ludington‐Hoe 1996
- Ludington‐Hoe SM, Swinth JY. Developmental aspects of kangaroo care. Journal of Obstetric, Gynecologic, and Neonatal Nursing 1996;25(8):691‐703. [DOI] [PubMed] [Google Scholar]
Ludington‐Hoe 1999
- Ludington‐Hoe SM, Anderson GC, Simpson S, Hollingsead A, Argote LA, Rey H. Birth‐related fatigue in 34‐36‐week preterm neonates: rapid recovery with very early kangaroo (skin‐to‐skin) care. Journal of Obstetric, Gynecologic, and Neonatal Nursing 1999;28(1):94‐103. [DOI] [PubMed] [Google Scholar]
Ludington‐Hoe 2004
- Ludington‐Hoe SM, Anderson GC, Swinth JY, Thompson C, Hadeed AJ. Randomized controlled trial of kangaroo care: cardiorespiratory and thermal effects on healthy preterm infants. Neonatal Network ‐ The Journal of Neonatal Nursing 2004;23(3):39‐48. [DOI] [PubMed] [Google Scholar]
Marsh 1997
- Marsh DF, Hatch DJ, Fitzgerald M. Opioid systems and the newborn. British Journal of Anaesthesia 1997;79(6):787‐95. [DOI] [PubMed] [Google Scholar]
McCain 2005
- McCain GC, Ludington‐Hoe SM, Swinth JY, Hadeed AJ. Heart rate variability responses of a preterm infant to kangaroo care. Journal of Obstetric, Gynecologic, and Neonatal Nursing 2005;34(6):689‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Michelsson 1996
- Michelsson K, Christensson K, Rothganger H, Winberg J. Crying in separated and non‐separated newborns: sound spectrographic analysis. Acta Paediatrica 1996;85(4):471‐5. [DOI] [PubMed] [Google Scholar]
Miles 1989
- Miles MS, Carter MC, Riddle I, Hennessey J, Eberly TW. The pediatric intensive care unit environment as a source of stress for parents. Maternal‐Child Nursing Journal 1989;18(3):199‐206. [PubMed] [Google Scholar]
Miles 1992
- Miles MS, Funk SG, Kasper MA. The stress response of mothers and fathers of preterm infants. Research in Nursing and Health 1992;15(4):261‐9. [DOI] [PubMed] [Google Scholar]
Moehn 1996
- Moehn DG, Rossetti L. The effects of neonatal intensive care on parental emotions and attachment. Infant‐Toddler Intervention: The Transdisciplinary Journal 1996;6(3):229‐46. [Google Scholar]
Moore 2012
- Moore ER, Anderson GC, Bergman N, Dowswell T. Early skin‐to‐skin contact for mothers and their healthy newborn infants. Cochrane Database of Systematic Reviews 2012, Issue 5. [DOI: 10.1002/14651858.CD003519.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Oberlander 2000
- Oberlander TF, Grunau RE, Whitfield MF, Fitzgerald C, Pitfield S, Saul JP. Biobehavioral pain responses in former extremely low birth weight infants at four months' corrected age. Pediatrics 2000;105(1):e6. [DOI] [PubMed] [Google Scholar]
Overton 1998
- Overton RC. A comparison of fixed‐effects and mixed (random‐effects) models for meta‐analysis tests of moderator variable effects. Psychological Methods 1998;3(3):354‐79. [Google Scholar]
Pillai Riddell 2015
- Pillai Riddell RR, Racine NM, Gennis HG, Turcotte K, Uman LS, Horton RE. Non‐pharmacological management of infant and young child procedural pain. Cochrane Database of Systematic Reviews 2015, Issue 12. [DOI: 10.1002/14651858.CD006275.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pillai Riddell 2016
- Pillai Riddell R, Fitzgerald M, Slater R, Steens B, Johnston C, Campbell‐Yeo M. Using only behaviours to assess infant pain: a painful compromise?. Pain 2016;157(8):1579‐80. [DOI] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editors. GRADE Working Group. GRADE handbook for grading quality of evidence and strength of recommendations. Available from www.guidelinedevelopment.org/handbook Updated October 2013.
Shields‐Poë 1997
- Shields‐Poë D, Pinelli J. Variables associated with parental stress in neonatal intensive care units. Neonatal Network ‐ Journal of Neonatal Nursing 1997;16(1):29‐37. [PubMed] [Google Scholar]
Simons 2001
- Simons J, Franck L, Roberson E. Parent involvement in children's pain care: view of parents and nurses. Journal of Advanced Nursing 2001;36(4):591‐9. [DOI] [PubMed] [Google Scholar]
Simons 2003
- Simons SH, Dijk M, Anand KS, Roofthooft D, Lingen RA, Tibboel D. Do we still hurt newborn babies? A prospective study of procedural pain and analgesia in neonates. Archives of Pediatrics & Adolescent Medicine 2003;157(11):1058‐64. [DOI] [PubMed] [Google Scholar]
Stevens 1996
- Stevens BJ, Johnston CC, Petryshen P, Taddio A. Premature infant pain profile: Development and validation. The Clinical Journal of Pain 1996;12(1):13‐22. [DOI] [PubMed] [Google Scholar]
Stevens 1999a
- Stevens B, Johnston C, Taddio A, Jack A, Narciso J, Stremler R, et al. Management of pain from heel lance with lidocaine‐prilocaine (EMLA) cream: is it safe and efficacious in preterm infants?. Journal of Developmental & Behavioral Pediatrics 1999;20(4):216‐21. [DOI] [PubMed] [Google Scholar]
Stevens 1999b
- Stevens BJ, Johnston C, Franck L, Petryshen P, Jack A, Foster R. The efficacy of developmentally sensitive interventions and sucrose for relieving procedural pain in very low birth weight neonates. Nursing Research 1999;48(1):35‐43. [DOI] [PubMed] [Google Scholar]
Stevens 2003
- Stevens B, McGrath P, Gibbins S, Beyene J, Breau L, Camfield C, et al. Procedural pain in newborns at risk for neurologic impairment. Pain 2003;105(1‐2):27‐35. [DOI] [PubMed] [Google Scholar]
Stevens 2007
- Stevens BJ, Pillai Riddell RR, Oberlander TE, Gibbins S. Assessment of pain in neonates and infants. In: Anand KJ, Stevens BJ, McGrath PJ editor(s). Pain in Neonates and Infants. 3rd Edition. Philadelphia: Elsevier, 2007:67‐90. [Google Scholar]
Stevens 2010
- Stevens B, Johnston C, Taddio A, Gibbins S, Yamada J. The premature infant pain profile: evaluation 13 years after development. Clinical Journal of Pain 2010;26(9):813‐30. [DOI] [PubMed] [Google Scholar]
Stevens 2013
- Stevens B, Yamada J, Lee GY, Ohlsson A. Sucrose for analgesia in newborn infants undergoing painful procedures. Cochrane Database of Systematic Reviews 2013, Issue 1. [DOI: 10.1002/14651858.CD001069.pub4] [DOI] [PubMed] [Google Scholar]
van Dijk 2000
- Dijk M, Boer JB, Koot HM, Tibboel D, Passchier J, Duivenvoorden HJ. The reliability and validity of the COMFORT scale as a postoperative pain instrument in 0 to 3‐year‐old infants. Pain 2000;84(2‐3):367‐77. [DOI] [PubMed] [Google Scholar]
Vinall 2014
- Vinall J, Miller SP, Bjornson BH, Fitzpatrick KP, Poskitt KJ, Brant R, et al. Invasive procedures in preterm children: brain and cognitive development at school age. Pediatrics 2014;133(3):412‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Warnock 2010
- Warnock FF, Castral TC, Brant R, Sekilian M, Leite AM, Owens Sde L, et al. Brief Report: Maternal kangaroo care for neonatal pain relief: A systematic narrative review. Journal of Pediatric Psychology 2010;35(9):975‐84. [DOI] [PubMed] [Google Scholar]
Wereszczak 1997
- Wereszczak J, Miles MS, Holditch‐Davis D. Maternal recall of the neonatal intensive care unit. Neonatal Network ‐ Journal of Neonatal Nursing 1997;16(4):33‐40. [PubMed] [Google Scholar]
Whitelaw 1985
- Whitelaw A, Sleath K. Myth of the marsupial mother: Home care of the very low birth weight babies in Bogota, Colombia. Lancet 1985;1(8439):1206‐8. [DOI] [PubMed] [Google Scholar]
Yamanda 2008
- Yamanda J, Stinson J, Lamba J, Dickson A, McGrath PJ, Stevens B. A review of systematic reviews on pain interventions in hospitalized infants. Pain Research & Management 2008;13(5):413‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Youngblut 1992
- Youngblut JM, Shiao SY. Characteristics of a child's critical illness and parents' reactions: preliminary report of a pilot study. American Journal of Critical Care. 1992;1(3):80‐4. [PubMed] [Google Scholar]
References to other published versions of this review
Johnston 2014
- Johnston C, Campbell‐Yeo M, Fernandes A, Inglis D, Streiner D, Zee R. Skin‐to‐skin care for procedural pain in neonates. Cochrane Database of Systematic Reviews 2014, Issue 1. [DOI: 10.1002/14651858.CD008435.pub2] [DOI] [PubMed] [Google Scholar]


