Abstract
Background
System change interventions for smoking cessation are policies and practices designed by organizations to integrate the identification of smokers and the subsequent offering of evidence‐based nicotine dependence treatments into usual care. Such strategies have the potential to improve the provision of smoking cessation support in healthcare settings, and cessation outcomes among those who use them.
Objectives
To assess the effectiveness of system change interventions within healthcare settings, for increasing smoking cessation or the provision of smoking cessation care, or both.
Search methods
We searched databases including the Cochrane Tobacco Addiction Group Specialized Register, CENTRAL, MEDLINE, Embase, CINAHL, and PsycINFO in February 2016. We also searched clinical trial registries: WHO clinical trial registry, US National Institute of Health (NIH) clinical trial registry. We checked ‘grey' literature, and handsearched bibliographies of relevant papers and publications.
Selection criteria
Randomized controlled trials (RCTs), cluster‐RCTs, quasi‐RCTs and interrupted time series studies that evaluated a system change intervention, which included identification of all smokers and subsequent offering of evidence‐based nicotine dependence treatment.
Data collection and analysis
Using a standardized form, we extracted data from eligible studies on study settings, participants, interventions and outcomes of interest (both cessation and system‐level outcomes). For cessation outcomes, we used the strictest available criteria to define abstinence. System‐level outcomes included assessment and documentation of smoking status, provision of advice to quit or cessation counselling, referral and enrolment in quitline services, and prescribing of cessation medications. We assessed risks of bias according to the Cochrane Handbook and categorized each study as being at high, low or unclear risk of bias. We used a narrative synthesis to describe the effectiveness of the interventions on various outcomes, because of significant heterogeneity among studies.
Main results
We included seven cluster‐randomized controlled studies in this review. We rated the quality of evidence as very low or low, depending on the outcome, according to the GRADE standard. Evidence of efficacy was equivocal for abstinence from smoking at the longest follow‐up (four studies), and for the secondary outcome ‘prescribing of smoking cessation medications' (two studies). Four studies evaluated changes in provision of smoking cessation counselling and three favoured the intervention. There were significant improvements in documentation of smoking status (one study), quitline referral (two studies) and quitline enrolment (two studies). Other secondary endpoints, such as asking about tobacco use (three studies) and advising to quit (three studies), also indicated some positive effects.
Authors' conclusions
The available evidence suggests that system change interventions for smoking cessation may not be effective in achieving increased cessation rates, but have been shown to improve process outcomes, such as documentation of smoking status, provision of cessation counselling and referral to smoking cessation services. However, as the available research is limited we are not able to draw strong conclusions. There is a need for additional high‐quality research to explore the impact of system change interventions on both cessation and system‐level outcomes.
Keywords: Humans, Organizational Policy, Counseling, Counseling/statistics & numerical data, Health Facilities, Hotlines, Hotlines/statistics & numerical data, Organizational Innovation, Randomized Controlled Trials as Topic, Smoking, Smoking/therapy, Smoking Cessation, Smoking Cessation/methods, Smoking Cessation/statistics & numerical data, Tobacco Use Cessation Devices, Tobacco Use Disorder, Tobacco Use Disorder/diagnosis, Tobacco Use Disorder/therapy
Plain language summary
Do organizational changes to support people quitting smoking improve services and help more people to quit?
Background
Smoking is a cause of many health problems, including cancers, heart and lung diseases. Health professionals (e.g. doctors, nurses, pharmacists, dentists, etc.) may be able to reduce this harm by helping smokers to quit during a clinic visit. However, it may be difficult for health care providers to recognize smokers. They may also feel they cannot deliver good support as they do not have enough time, skills, training, budget or resources. A change within health professionals' wider organization may help to improve their involvement in care to help people to stop smoking, and in turn improve the chances of them quitting smoking. These changes may include introducing a system for asking patients if they smoke and recording smoking status on the patient health records; providing health care providers with training, budget or resources to help them deliver more effective quitting support; identifying a dedicated staff member to provide quitting support; introducing new rules to restrict smoking or support quitting activities; introducing advice to quit smoking into routine care; and paying health workers for delivering cessation support. This review aimed to find out whether implementing these organizational changes improves health professionals’ involvement in quit smoking activities, and whether it helps smokers stop smoking. We assessed the following activities by health professionals to evaluate their involvement in quit smoking activities: 1) asking about tobacco use; 2) documenting smoking status on patient health records; 3) advising smokers to quit; 4) counselling to quit; 5) providing medicines to quit smoking; and 6) referring to other centers such as quitlines (a telephone support service for smokers to quit) where smokers can obtain further help.
Study characteristics
We searched for studies published up to February 2016.
Our search identified seven studies which investigated changes made to the way organizations offered stop‐smoking support in healthcare settings; six studies were conducted in the USA and one in Spain. Two studies evaluated the changes implemented in primary care clinics and another two studies evaluated changes in dental clinics. One study each evaluated changes in community pharmacy, Veterans Affairs Medical Center and pediatric practice. All included studies were supported or funded by government agencies.
None of the studies implemented all the recommended changes or activities listed above. Five studies implemented four types of changes and two studies implemented three types of changes. Identifying all smokers, training health professionals and advising smokers to quit were part of all included studies.
Key results
Of the seven studies identified, only four evaluated the effect of organizational changes on study participants' quitting status. Of these, two found that organizational changes helped people to quit smoking, but the other two studies reported that they did not, and hence no conclusion could be drawn on this factor. Activities such as counselling to quit, recording smoking status in patient health records, and referring smokers to an outside stop‐smoking clinic improved after those changes were made. Of the three studies which evaluated factors such as asking about tobacco use and advising to quit, two reported that organizational changes could improve both of those factors.
Quality of the evidence
The overall quality of the evidence was judged to be low because of the small number of available studies and inadequate study designs. More well‐conducted studies are needed to fill this knowledge gap. Some studies are already underway but need to be evaluated in detail to include in the review.
Summary of findings
Summary of findings for the main comparison. System change interventions for tobacco control.
| System change interventions for tobacco control (primary cessation outcome) | |||
| Patient or population: Smokers Settings: Any healthcare delivery setting Intervention: System change | |||
| Outcomes* | No of Participants (studies) | Quality of the evidence (GRADE) | Comments |
| Cessation outcome Self‐reported/verified abstinence Follow‐up: 6 to 24 months | 7142 (4 studies) | ⊕⊝⊝⊝ very low1,2 | 2 of the 4 studies significantly favoured the intervention. Mixed effect and low quality evidence preclude drawing conclusions |
| NB. Illustrative comparative risks and relative effects columns have been removed, as only narrative syntheses were conducted due to the presence of significant heterogeneity among studies. | |||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||
1Self‐reported abstinence was verified only in one study, and one study reported higher dropout rate in one group. 2High clinical heterogeneity among included studies; outcomes are measured at different time points, and in different settings.
Summary of findings 2. System change interventions for tobacco control.
| System change interventions for tobacco control (secondary outcomes) | |||
| Patient or population: Smokers Settings: Any healthcare delivery setting Intervention: System change | |||
| Outcomes* | No of Participants (studies) | Quality of the evidence (GRADE) | Comments |
| Provision of cessation counselling Proportion of smokers counselled to quit | 10,949 (4 studies) | ⊕⊕⊝⊝ low1,2,3 | 3 of the 4 studies significantly favoured the intervention. The low quality of the evidence precludes drawing conclusions |
| Asking about tobacco use Proportion of smokers asked about tobacco use | 2615 (3 studies)* | ⊕⊕⊝⊝ low1,3 | 2 of the 3 studies significantly favoured the intervention. The low quality of the evidence precludes drawing conclusions |
| Provision of cessation advice Proportion of smokers advised to quit | 3003 (3 studies)* | ⊕⊕⊝⊝ low1,3 | 2 of the 3 studies significantly favoured the intervention. The low quality of the evidence precludes drawing conclusions |
| Quitline referral Proportion of smokers referred to quitline | 3006 (3 studies)* | ⊕⊝⊝⊝ very low1,3 | All 3 studies significantly favoured the intervention. However, the low quality of the evidence precludes drawing conclusions |
| Quitline enrolment Proportion of smokers enrolled in quitline | 1191 (2 studies)* | ⊕⊝⊝⊝ very low1,3 | Both studies significantly favoured the intervention. However, the low quality of the evidence precludes drawing conclusions |
| Prescription of NRT or other pharmacotherapy Proportion of smokers received NRT prescription | 2615 (2 studies) | ⊕⊕⊝⊝ low1,3 | Of the 2 studies, 1 significantly favoured the intervention. Mixed effect and low‐quality evidence preclude drawing conclusions |
| NB. Illustrative comparative risks and relative effects columns have been removed, as only narrative syntheses were conducted due to the presence of significant heterogeneity among studies *We have not included data from one study as the data were collected as counts (no denominator) | |||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||
1Included studies had high risk of detection bias. 2Large difference in effect size between studies. 3High clinical heterogeneity among included studies; different settings, providers and intervention.
Background
Description of the condition
The consequences of tobacco use are well recognized and understood, as are the benefits of smoking cessation (Critchley 2012; Ebbert 2005; Peto 2000; Taylor 2002). Smoking cessation not only leads to significant and immediate health benefits, but also decreases most of the related risks within a few years of quitting (WHO 2011). Even people who quit later in life gain benefit. For example, among smokers who quit at the age of 65 years, men gain two years of life on average and women gain three (Taylor 2002). Quitting smoking is associated with a 36% reduction in risk of all‐cause mortality among people with coronary heart disease, which is significant when compared with other secondary preventive therapies such as lowering cholesterol (Critchley 2012). Given the high prevalence of smoking, even a modest improvement in smoking cessation rates could translate to major health and economic benefits.
Addressing tobacco use within a healthcare setting requires clinical, programme, and system‐level changes. According to clinical practice guidelines for treating tobacco use and dependence, all healthcare organizations should develop plans to support consistent and effective identification, documentation and treatment of tobacco smokers (Fiore 2008). As a minimum requirement, all healthcare providers should ask the tobacco use status of the people who consult them, should briefly advise all smokers to quit, and refer them to quitline or other smoking cessation services (Revell 2005).
Even though there are guidelines and evidence to provide smoking cessation services at every clinical encounter, reports suggest that healthcare providers are not delivering the recommended levels of support to those who smoke (Braun 2004). Previous studies have reported suboptimal rates of smoking cessation services, delivered by different types of healthcare professionals, in various healthcare settings (Aquilino 2003; Braun 2004; Thorndike 1998). The levels of smoking cessation support in hospitals are also low (Freund 2005; Freund 2008). It is evident that even in high‐income countries, current healthcare systems are not well organized to address the issue of smoking.
Description of the intervention
“System change smoking cessation interventions describe specific strategies that healthcare administrators, managed care organisations, and purchasers of health plans can implement to treat tobacco dependence” (AHRQ 2012).They involve systematic identification of smokers and subsequent offering of evidence‐based cessation treatments (Fiore 2007). Fiore 2007 has suggested six system‐level strategies to facilitate treatment of tobacco dependence:
Implement a system for identifying smokers and documenting tobacco‐use status in every clinic and hospital;
Provide education, resources and feedback to promote provider interventions;
Dedicate staff to provide smoking cessation treatment and assess its delivery in staff performance evaluations;
Promote hospital policies that support and provide smoking cessation services;
Provide evidence‐based tobacco dependence treatments (both counselling and pharmacotherapy);
Reimburse providers for the delivery of effective tobacco dependence treatments and include these services among their defined duties.
How the intervention might work
The barriers to providing effective smoking cessation support include: lack of support from the organization, perceived objections from patients, lack of systems for identifying smokers, lack of staff time and skill, perceived inability to change practices, perceived lack of efficacy of tobacco dependence treatments, and the cost of providing care (Wolfenden 2009). A strategic system change approach may be effective in addressing these multidimensional factors associated with low smoking care provision and may improve the number of people achieving abstinence. Outcomes for chronically‐ill people will improve only when healthcare systems reconfigure themselves to address the needs and concerns of their patients (Wagner 1998). Tobacco smoking is a chronic relapsing condition that often requires ongoing medical and behavioural interventions, and is consequently considered a chronic health condition (Hudson 2010). A system‐level change may therefore be essential to deal with the issue.
Why it is important to do this review
System change interventions are multicomponent, and may vary significantly in their intensity, content and delivery. It is not clear which types of approach are more effective than others. A summary of this evidence is critical as, to our knowledge, no systematic review assessing the effectiveness of such interventions has yet been published. This review is intended to identify various system change interventions for smoking cessation, across various healthcare settings, and to evaluate the effectiveness of such approaches at both the participant and organizational levels.
Objectives
To assess the effectiveness of system change interventions within healthcare settings, for increasing smoking cessation or the provision of smoking cessation care, or both.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials (RCTs), cluster‐RCTs with at least two intervention sites and two comparator sites, and quasi‐RCTs. Interrupted time series studies (ITS) are also eligible for inclusion if they have a clearly‐defined point in time when the intervention occurred and at least three data points before and three after the intervention.
Types of participants
People who smoke and are receiving care in a healthcare setting; and the staff working in these healthcare settings.
Types of interventions
System change interventions for smoking cessation are policies and practices designed by organizations to integrate the identification of all smokers and the subsequent offering of evidence‐based smoking cessation treatments (pharmacological or non‐pharmacological, or both) into the routine delivery of health care (Fiore 2007). Thus we include interventions which have been developed for identifying people who smoke, documenting smoking status and providing tobacco dependence treatment in various healthcare settings (primary, secondary or tertiary care).
We considered studies using the components of the system change model described in Fiore 2007, detailed in the Description of the intervention section of this review.
We did not consider studies focusing only on training health professionals, identification of smokers (electronic health records) or providing smoking cessation counselling, without a system change approach. As identification of all smokers and providing treatment to them are the crucial components of system change smoking cessation interventions, we considered only studies which aimed to identify all smokers accessing the system and to provide treatment. We excluded studies of interventions with research personnel involvement in intervention delivery. We also excluded studies targeting a single type of health professional within the health service (unless the organization included only one health profession, e.g. a pharmacy employing only pharmacists without any pharmacy technicians or pharmacy assistants), as organizational‐level interventions should target most health professionals in the system in order to deliver tobacco cessation interventions at every clinical encounter. Hence the system change intervention should be designed to integrate the provision of smoking cessation services within the routine delivery of health care.
Types of outcome measures
Primary outcomes
Following the standard methodology of the Cochrane Tobacco Addiction Group, the primary outcome was abstinence from smoking at longest follow‐up, assessed as point prevalence (defined as prevalence of abstinence during a time window immediately preceding the follow‐up) or continued or prolonged abstinence (defined as abstinence between quit day or predetermined grace period and a follow‐up time). We used the strictest available criteria to define abstinence, preferring continuous or prolonged abstinence over point prevalence abstinence, and biochemically‐validated abstinence over self‐reported abstinence.
Studies that did not assess smoking cessation were also eligible for inclusion if they reported any secondary outcome and met the other inclusion criteria. We examined and reported the classification of primary and secondary outcomes in each included study.
Secondary outcomes
Secondary outcomes include process outcomes measured as organizational‐level outcomes or outcomes of care:
Organizational‐level outcome measures, including the number of smokers identified with smoking status documented and number of health professionals trained or dedicated to providing cessation support.
Outcomes of care, including the number of smokers counselled, given self‐help materials, offered nicotine replacement therapy (NRT) or other pharmacotherapy, nomination of a quit date, referral to specialist smoking cessation services (such as telephone quitline support) or other health professionals, and provision of a follow‐up appointment.
Search methods for identification of studies
Electronic searches
We searched the following databases:
Cochrane Tobacco Addiction Group Specialized Register, 1st February 2016;
Cochrane Central Register of Controlled Trials (CENTRAL), 2016 Issue 1;
MEDLINE/MEDLINE In Process (via OVID) 1946 to 15th February 2016;
Embase (via OVID), 1947 to 15th February 2016;
PsycINFO (via OVID), 1806 to 15th February 2016;
CINAHL (via EBSCO), 1938 to 15th February 2016.
Search strategies included both key words and MeSH/Emtree. We did not apply any language restrictions. The search strategy for MEDLINE is presented in Appendix 1.
Searching other resources
We also searched clinical trial registries (the WHO clinical trial registry and the US National Institute of Health (NIH) clinical trial registry) for ongoing studies. We sought additional studies by screening the references of relevant reviews and identified studies (citation tracking). We also used personal bibliographies and communication with experts in the field to identify any other studies.
Data collection and analysis
Selection of studies
DT implemented the search strategy and merged search results using reference management software (EndNote, Thomson Reuters). We reviewed the titles and abstracts of studies for possible inclusion, and subjected those selected to full‐text assessment. We linked together multiple reports of the same study. Two authors (DT and JG) independently assessed all the full‐text articles retrieved, and included those studies meeting the inclusion criteria in the review. We resolved any discrepancies by discussion with the third author (BB), a content area expert who acted as an arbiter for disagreement about the intervention or content of the study. Another arbiter (MJA), who is an expert in clinical trials and meta‐analysis, checked any methodological discrepancies. We noted characteristics of the studies excluded (after full‐text assessment), including the reason for exclusion.
Data extraction and management
Two authors (DT and JG) independently extracted data, using a pilot‐tested standardized data collection form. We entered the collected data into Review Manager 5 (RevMan). We contacted authors of the studies by email, where data were not available or unclear.
We extracted the following information from each of the selected studies:
lead and corresponding authors’ information;
date of publication;
location and setting;
methods of recruitment and inclusion criteria;
methods of randomization, allocation concealment and blinding;
study design, duration and follow‐up details;
characteristics of participants (e.g. age, sex and smoking status);
specific details of the intervention (type, duration, content, format and delivery of intervention, use of pharmacotherapy, adherence to therapy and information about the providers);
control group component;
number of participants in each arm;
outcome measures and definitions, including any biochemical validation, and time point at which they were measured and reported;
Results: estimate of effect with confidence interval and subgroup analysis (summary data of intervention and control group were entered separately into RevMan) and missing data;
funding and declarations of interest for the primary investigators;
authors’ conclusions;
additional comments and information.
If studies were reported in more than one publication (e.g. different time points of the study), we extracted the data from all publications onto separate data collection forms and combined them. If there was one full journal article and multiple conference abstracts available, we considered only the journal article. We resolved any disagreements in the data collection process by discussion with a third author (MJA).
Assessment of risk of bias in included studies
Two authors (DT and JG) independently assessed the risk of bias in included studies, with any disagreements resolved by discussion and consensus, and by consulting a third author (MJA) where necessary.
We assessed studies with a separate control group (RCTs, cluster‐RCTs and quasi‐RCTs) using the seven standard Cochrane criteria:
sequence generation;
allocation concealment;
blinding of participants and personnel;
blinding of outcome assessment;
incomplete outcome data;
selective outcome reporting;
other potential bias.
We judged each criterion for bias on a three‐point scale ‘low risk’, ‘high risk’ and ‘unclear risk’ (Higgins 2011), and constructed a 'Risk of bias' table.
‘Low risk’, when there was a low risk of bias across all key domains.
‘Unclear risk’, when there was an unclear risk of bias in one or more of the key domains.
‘High risk’, when there was a high risk of bias in one or more of the key domains.
For each included study, we provide a summary assessment of risk of bias.
Measures of treatment effect
We present the intervention effect for each outcome descriptively, summmarizing nominal variables using numbers and proportions. Wherever possible, we have produced a risk ratio (quitters in treatment group/total randomised to the treatment group) / (quitters in control group/total randomised to the control group) for the outcome of each individual trial.
Unit of analysis issues
In the case of trials with repeated observations, we considered the longest follow‐up for the analysis (Higgins 2011). We assessed all reported secondary outcomes only at a single time point.
Although heterogeneity precluded us from performing a meta‐analysis for this review, we had considered using adjusted estimates of effect measures for analyses of cluster‐randomized trials. Had such data been unavailable, we would have conducted an approximate analysis where the required information could be obtained (Higgins 2011).
Dealing with missing data
We report the number of participants lost to follow‐up by group, where available. For the primary outcome, we used an intention‐to‐treat analysis approach where possible. This assumes that people lost to follow‐up continue smoking (West 2005). However, we also extracted and reported the strategies used in each study to deal with missing data for the primary outcome measure, and where adjustments have been made as part of the study analyses we also report the summary statistics directly from the study reports.
Assessment of heterogeneity
We explored heterogeneity visually using tables and forest plots, by comparing the effect sizes of studies grouped according to potential effect modifiers. These included:
Type of intervention (e.g. identification of smokers, documentation of smoking status, treatment, training of health professionals, feedback of services, etc.);
Intensity of intervention (e.g. counselling, pharmacotherapy, both counselling and pharmacotherapy, duration of intervention, etc.);
Type of health professional involved;
Setting (primary, secondary and tertiary);
Study design (RCTs, cluster‐RCTs, quasi‐RCTs or ITS studies);
Quality of studies.
We assessed statistical heterogeneity using the Chi2 test for homogeneity (with significance defined at the alpha‐level of 10%) and quantified using the I2 statistic (Higgins 2011). We considered pooling the data using a meta‐analysis where heterogeneity was less than 50% (Higgins 2011).
Assessment of reporting biases
We did not systematically assess publication bias, because of the limited number of studies included, in accordance with the Cochrane Handbook (Higgins 2011). If sufficient studies are available in future updates, we will assess publication bias using funnel plots.
Data synthesis
We conducted a meta‐analysis for the primary outcome measure using a random‐effects model. However, due to the presence of significant heterogeneity (I2 = 78%), we deemed it inappropriate to present pooled effects, and we therefore present a narrative synthesis of the included studies. We report major characteristics and results for each trial.
Subgroup analysis and investigation of heterogeneity
We did not conduct any subgroup analyses, due to the limited number of included studies and the decision not to pool them.
Sensitivity analysis
We did not conduct any sensitivity analyses, due to the limited number of included studies and the decision not to pool them.
'Summary of findings' table
Following standard Cochrane methodology, we created 'Summary of findings' tables. We created one for the primary cessation outcome, and a second for the secondary outcomes. We considered it important to summarize both the primary cessation outcome and the secondary outcomes, because of the two‐phase nature of system change interventions, with the intervention potentially influencing health professional behaviour, which can go on to potentially affect the patient‐level outcome. Focusing on both sets of outcomes could help to identify why the intervention is successful or not. Also following standard Cochrane methodology, we used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome, and to draw conclusions about the quality of evidence within the text of the review. As it was impossible to carry out statistical analyses, we provide a narrative summary of outcomes in both 'Summary of findings' tables.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies
Results of the search
The database search yielded 9278 titles. We found three additional studies through other sources (handsearching and trial registry searching). After removing duplicates, we screened 7004 titles and abstracts, reviewed 159 full‐text articles, and shortlisted 48 studies. Of those, we excluded 39 after thoroughly reviewing the full text, with reasons recorded in the Characteristics of excluded studies table. We found two ongoing studies through the searches (Bonevski 2016; Ostroff 2014); we will perform a full eligibility check once the studies are complete. See Figure 1 for an illustration of eligibility decisions.
1.
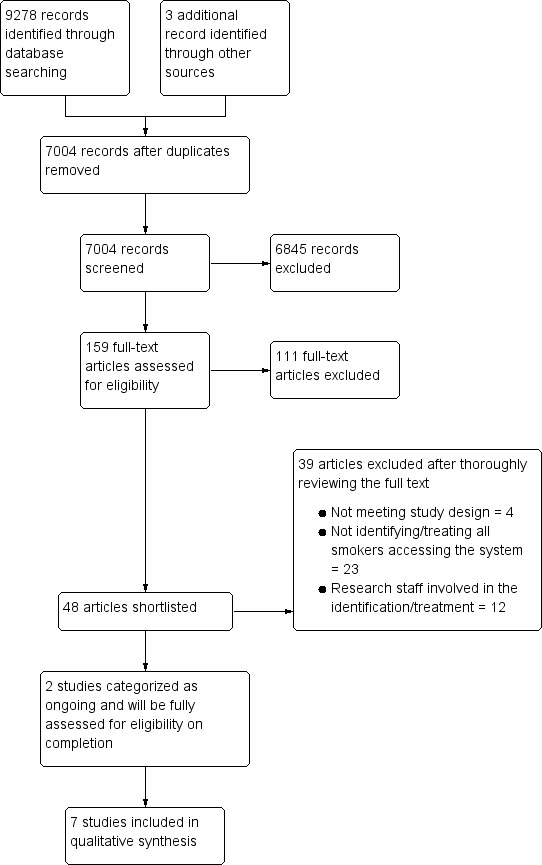
Study flow diagram.
Included studies
We included studies if the intervention was designed to integrate the identification of smokers and the subsequent offering of evidence‐based tobacco dependence treatment into routine care. This review includes seven cluster‐RCTs. All the studies except one (Cabezas 2011 in Spain) were conducted in the USA. The settings of the included studies were: two in primary care clinics (Cabezas 2011; Rothemich 2010), two in dental clinics (Gordon 2010; Little 2009), and one each in a community pharmacy (Patwardhan 2012), a Veterans Affairs medical centre (Joseph 2004) and a paediatric practice (Winickoff 2014). Winickoff 2014 focused on parents attending an outpatient paediatric practice. None evaluated a system change intervention for inpatient smokers. One study had two reports (Winickoff 2013 and Winickoff 2014) which we collated and included in this review as Winickoff 2014.
Intervention
We provide a summary of intervention components implemented by each study as an additional table (Table 3).
1. Components of interventions in included studies.
| Identification/ documentation of smoking status | Smoking cessation Training/ resources/feedback to providers | Dedicated staff to support cessation activities | Policies to improve access to cessation interventions | Free smoking cessation treatment from the organization | Reimburse clinicians for providing smoking cessation support | |
| Cabezas 2011 | Yes | Yes | No | No | Yes | No |
| Gordon 2010 | Yes | Yes | No | No | Yes | No |
| Joseph 2004 | Yes | Yes | No | Yes | Yes | No |
| Little 2009 | Yes | Yes | No | Yes | Yes | No |
| Patwardhan 2012 | Yes | Yes | No | Yes | Yes | No |
| Rothemich 2010 | Yes | Yes | No | Yes | Yes | No |
| Winickoff 2013 | Yes | Yes | No | Yes | Yes | No |
All included studies used the services of existing staff to provide the intervention. None of the studies incorporated all six system change strategies. Five studies implemented four system‐level strategies (Joseph 2004; Little 2009; Patwardhan 2012; Winickoff 2014) and two studies implemented three strategies (Cabezas 2011; Gordon 2010). Identifying all smokers, training staff and providing evidence‐based treatment were components of all seven studies.
Four studies (Little 2009; Patwardhan 2012; Rothemich 2010; Winickoff 2014) implemented a system of identifying smokers. Rothemich 2010 used a vital sign stamp to mark paper patient records; Little 2009 used a new field in the electronic health record; and Winickoff 2014 used a specific action sheet attached to medical records to identify smokers. In Patwardhan 2012, dental technicians identified smokers by asking about their tobacco use and documented the status on a form that was then attached to their prescription to notify pharmacists. In Joseph 2004, various strategies were recommended for implementation across intervention sites to improve identification of smokers and documentation of smoking status by health professionals, including the ‘smoking as a vital sign’ approach, use of an electronic clinical reminders system and adaptation of a note template to include smoking status. Finally, two studies (Cabezas 2011; Gordon 2010) identified smokers either by office staff or by clinicians, who asked about tobacco use, but without reporting the methods of documentation.
All included studies provided clinicians with training. The duration of training ranged from 30 minutes to 20 hours. Two studies (Little 2009; Rothemich 2010) provided feedback to the clinicians and practices. Little 2009 derived data from electronic health records on rates of tobacco use assessment, advice, counselling, referral offers and referral acceptances, which were used to deliver feedback, and provided monthly performance feedback at provider, clinic and cross‐clinic levels. In Rothemich 2010, feedback was provided by the quitline service at both patient and practice levels. Patient‐level feedback included the number of counselling sessions completed by the participant, smoking status at last contact, difficulties in contacting participants and reasons for any unsuccessful enrolment or early termination. Practice‐level feedback was provided quarterly and included the volume of referrals and summary data such as readiness to quit, quit attempts and smoking status.
None of the studies reported an intervention which involved a 'champion' co‐ordinating tobacco dependence services. However, a core implementation group, which included study staff, professional leaders and administrators of each of the intervention clinics, was present in Little 2009 to facilitate the implementation of intervention components.
All the studies included provision of cessation advice by clinicians to all identified smokers, except one (Patwardhan 2012) that did not provide cessation advice to those who had already decided to quit smoking. Smokers were instead referred to a specialist quitline service. Five studies (Gordon 2010; Little 2009; Patwardhan 2012; Rothemich 2010; Winickoff 2014) referred smokers to smoking cessation services external to the organization. All seven studies included pharmacotherapy (NRT or prescription medications, or both) in the intervention.
In four studies (Cabezas 2011; Gordon 2010; Joseph 2004; Winickoff 2014) pharmacotherapy was provided within the organization. In the other studies, the provision of pharmacotherapy was co‐ordinated from smoking cessation services external to the organization.
None of the included studies reported reimbursement to the providers for delivery of smoking cessation care.
Control group
In four studies (Cabezas 2011; Gordon 2010; Little 2009; Winickoff 2014) usual care was the control condition. In Joseph 2004, each control clinic received five copies of the AHCPR smoking cessation guideline (AHCPR 1996). In Patwardhan 2012, control group pharmacies received an informal presentation on quitline services, quitline cards and enrolment in Fax to Quit (FTQ) services (a free service). In Rothemich 2010, a traditional tobacco use vital sign stamp (only smoking status recorded) was used in control clinics.
Outcomes
Primary outcomes
Four studies (Cabezas 2011; Gordon 2010; Joseph 2004; Winickoff 2014) reported cessation outcomes. All these studies used self‐reported cessation, except for Winickoff 2014, which used cotinine‐validated abstinence.
Secondary outcomes
All the studies except Cabezas 2011 reported system‐level outcomes (process outcomes). One study (Gordon 2010) assessed system‐level outcomes only for the intervention participants, and another (Patwardhan 2012) collected system‐level outcome data from healthcare providers. Reported system‐level outcomes included the number of people asked about tobacco use, number of smokers advised to quit, number of smokers counselled to quit, number of smokers referred to a specialized smoking cessation clinic, and provision of pharmacotherapy.
Excluded studies
We excluded studies if the study design did not meet the criteria for inclusion, if there was substantial involvement of research personnel in the provision of smoking cessation care, or if the intervention targeted a single health professional (or profession in the case of multidisciplinary health services) within the service. We also excluded studies if the intervention targeted a specific population instead of providing support to all smokers attending the clinic. Specific reasons for exclusion can be found in the Characteristics of excluded studies table.
Risk of bias in included studies
We present rhe 'Risk of bias' assessment for included studies in the 'Risk of bias' table, under each individual Characteristics of included studies table. We also present these results in graphical form in Figure 2 and Figure 3.
2.
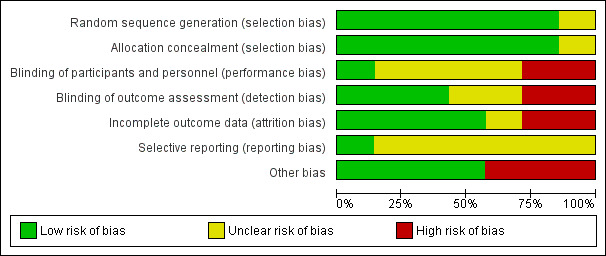
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
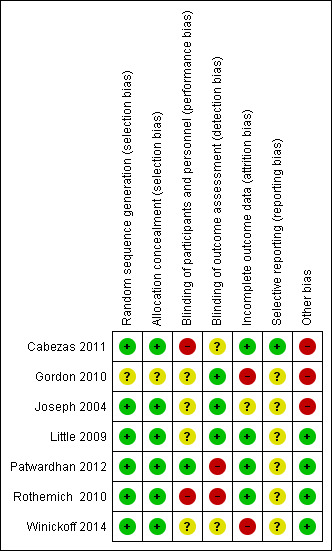
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Five of the seven studies (71%) (Cabezas 2011; Joseph 2004; Patwardhan 2012; Rothemich 2010; Winickoff 2014) adequately described the random sequence generation and were considered to have low risk of bias for this domain.
Blinding
Blinding of both participants and personnel (to avoid performance bias) was not possible due to the study design in any of the included studies, except for Patwardhan 2012, where the study personnel were not aware of the existence of two groups and therefore were considered to be effectively blinded. We rated three studies (Gordon 2010; Joseph 2004; Little 2009) at low risk of detection bias. Joseph 2004 assessed outcomes using a blinded outcome assessor; Little 2009 collected data from electronic health records; and Gordon 2010 collected outcome data using a postal survey.
Incomplete outcome data
Four studies (Cabezas 2011; Gordon 2010; Joseph 2004; Winickoff 2014) reported cessation outcomes and included follow‐up data. In Cabezas 2011, although the dropout rate was high, it was similar in both groups (43.3% in intervention and 44.8% in control), and hence we rated it at low risk of bias. Gordon 2010 reported moderate dropout rates, but the rate was higher in the intervention group (30.7% versus 26.1%; P < 0.01), so we judged this study to be at high risk of bias for this domain. Although not statistically significant, the follow‐up rate in Winickoff 2014 was marginally higher in the control group (64.5% versus 72.4%; P = 0.11) and we therefore rated it at high risk of bias. Joseph 2004 did not report dropout rates by group and we therefore considered this to be at unclear risk of bias.
We judged three studies (Little 2009; Patwardhan 2012; Rothemich 2010) which reported only system‐level outcomes to be at low risk of bias, as there were no follow‐ups involved.
Selective reporting
Only Cabezas 2011 had a published protocol and was therefore rated at low risk of bias for this domain. We considered all other studies to be at unclear risk of bias, as there was no means of checking whether selective reporting had taken place.
Other potential sources of bias
Three studies which reported cessation outcomes used unverified self‐report data.
Effects of interventions
See: Table 1 for a summary of the main comparisons in this review.
Primary outcome
Four studies (Cabezas 2011; Gordon 2010; Joseph 2004; Winickoff 2014) evaluated the effect of a system change intervention on smoking cessation. Of these, two studies (Cabezas 2011; Gordon 2010) found that the quit rate was higher in the system change intervention group than in the control group. In Cabezas 2011, the primary end point, one‐year self‐reported continuous abstinence at two‐year follow‐up, was significantly higher in the intervention group than in the control group, at 8.1% (120/1482) versus 5.8% (78/1345). The adjusted odds ratio (OR) reported by the study was 1.5 (95% confidence interval (CI) 1.05 to 2.14). We calculated the unadjusted intention‐to‐treat risk ratio (RR) to be 1.41 (95% CI 1.07 to 1.86). Gordon 2010 reported significant improvement in both point prevalence abstinence (11.3% [158/1394] versus 6.8% [79/1155]; unadjusted RR 1.66, 95% CI 1.28 to 2.15) and prolonged abstinence (5.3% [74/1394] versus 1.9% [22/1155]; unadjusted RR 2.79, 95% CI 1.74 to 4.46) at six‐month follow‐up. In Joseph 2004 and Winickoff 2014, the intervention did not result in better cessation rates at one‐year follow‐up (11.4% [32/280] versus 13.2% [39/295]; unadjusted RR 0.86, 95% CI 0.56 to 1.34; and 4.3% [24/556] versus 4.1% [26/635]; adjusted OR 1.07, 95% CI 0.64 to 1.78, respectively). The design effect (clustering effect) was taken into account in the analyses conducted by Cabezas 2011 and Winickoff 2014.
In the original analyses, Gordon 2010 used multiple imputation to handle missing data, Cabezas 2011 and Winickoff 2014 considered all lost to follow‐up as smokers, and Joseph 2004 evaluated smoking status based only on available data (complete‐case analysis).
Secondary outcomes
We provide a summary of secondary outcomes in an additional table (Table 4).
2. Summary of secondary outcomes.
| Study | Asking about tobacco use | Documentation of smoking status | Advice to quit | Counselling to quit | Initiation of NRT or other pharmacotherapy | Quitline referral | Quitline enrolment |
| Cabezas 2011 | Not assessed | Not assessed | Not assessed | Not assessed | Not assessed | Not assessed | Not assessed |
| Gordon 2010 | Not assessed | Not assessed | Not assessed | Not assessed | Not assessed | Not assessed | Not assessed |
| Joseph 2004 | No difference between groups (76.0% vs 74.3%; P = 0.71) | Favoured intervention (60.7% vs 67.0%; P < 0.001) | Not assessed | No difference between groups (73.9% vs 71.8%; P = 0.60) | No difference between groups (14.7% vs 18.0%; P = 0.38) | Not assessed | Not assessed |
| Little 2009 | Not assessed | Not assessed | Not assessed | Favoured intervention (69% vs 3%; P < 0.01) | Not assessed | Not assessed | Not assessed |
| Patwardhan 2012* | Favoured intervention (636 vs 5; P < 0.001) | Not assessed | Favoured intervention (25 vs 3; P < 0.01) | Not assessed | Not assessed | Favoured intervention (240 vs 85; P = 0.02) | Favoured intervention (81 vs 8; P < 0.001) |
| Rothemich 2010 | Not assessed | Not assessed | No difference between groups (58.2% vs 55.3%; P = 0.39). | Favoured intervention (34.4% vs 27.7%; P = 0.001) | Not assessed | Favoured intervention (21.4% vs 8.7%; P < 0.001) | Not assessed |
| Winickoff 2013 | Favoured intervention (59.4% vs 32.6%; P < 0.001) | Not assessed | Favoured intervention (50.5% vs 26.9%; P < 0.001). | Favoured intervention (54.7% vs 19.2%; P < 0.001) | Favoured intervention (18.5% vs 2.4%; P < 0.001) | Favoured intervention (37.2% vs 9.3%; P < 0.001) | Favoured intervention (4.1% vs 1.1%; P < 0.01) |
*data collected as counts (no denominator)
Asking about tobacco use
Of the three studies (Joseph 2004; Patwardhan 2012; Winickoff 2014) that evaluated the effect of a system change intervention on identification of smokers, two reported significant improvements in the intervention group. In Patwardhan 2012, 636 people (measured as counts; no denominator) were screened for tobacco use in all experimental‐group pharmacies, compared with five in all control‐group pharmacies (P < 0.001). Winickoff 2014 also favoured intervention (59.4% versus 32.6% screened; P < 0.001). However, in Joseph 2004, the intervention did not improve the identification of smokers (76.0% versus 74.3% screened; P = 0.71).
Tobacco screening was part of standard care for both groups in two studies (Little 2009; Rothemich 2010), with similar screening rates across the intervention and control groups prior to the start of the studies.
Documentation of smoking status
One study (Joseph 2004) reported the effect of the system change intervention on documentation of smoking status. Before intervention, control sites were significantly more likely to document smoking status than intervention sites (63.1% versus 55.7% respectively; P < 0.001). However, the direction of this difference was reversed after the intervention was implemented (60.7% versus 67.0% respectively; P < 0.001).
Advice to quit
Of the three studies (Patwardhan 2012; Rothemich 2010; Winickoff 2014) that evaluated the effect of a system change intervention on the number of smokers advised to quit, two reported significant improvements in the intervention group. In Patwardhan 2012, 25 smokers (measured as counts; no denominator) were advised to quit in the experimental‐group pharmacies compared with three in the control‐group pharmacies (P < 0.01). Winickoff 2014 also favoured intervention (50.5% versus 26.9%; P < 0.001). However, in Rothemich 2010 the intervention did not improve the rate of advice, with no significant difference in quitting advice across groups (58.2% intervention versus 55.3% control; P = 0.39).
In one study (Little 2009), the provision of advice to quit was standard practice for both groups prior to the start of the study and pre‐study rates were similar across groups.
Counselling to quit
Four studies (Joseph 2004; Little 2009; Rothemich 2010; Winickoff 2014) evaluated the effect of an intervention on the number of smokers subsequently counselled to quit. One study (Little 2009) reported the combined effect on both counselling and referral to quitline services. Rothemich 2010 reported the effect on the discussion of methods to quit. Three of these studies reported significant improvements in the rate of counselling in the intervention group; (Little 2009: 69% intervention versus 3% control; P < 0.01), (Rothemich 2010: 34.4% intervention versus 27.7% control; P = 0.001), and (Winickoff 2014: 54.7% intervention versus 19.2% control; P < 0.001). However, in Joseph 2004 the intervention did not improve counselling rates (73.9% intervention versus 71.8% control; P = 0.60).
Initiation of NRT or other pharmacotherapy
Two studies (Joseph 2004; Winickoff 2014) evaluated the effect of a system change intervention on the prescription of NRT. Winickoff 2014 reported a significant improvement in the intervention group versus control group (18.5% versus 2.4% respectively; P < 0.001). However, Joseph 2004 reported no significant difference between groups (14.7% intervention versus 18.0% control; P = 0.38).
Quitline referral and enrolment
Rates of quitline referral were assessed by three studies (Patwardhan 2012; Rothemich 2010; Winickoff 2014), and all reported significantly higher rates in the intervention arm. In Rothemich 2010 (21.4% intervention versus 8.7% control; P < 0.001) and in Winickoff 2014 (37.2% intervention versus 9.3% control; P < 0.001), a higher proportions of intervention participants were referred to a quitline. In Patwardhan 2012, 240 intervention participants received a quitline card compared to 85 control participants (P = 0.02; measured as counts; no denominator).
Two studies which evaluated quitline enrolment also favoured the intervention. In Winickoff 2014, a higher proportion of intervention participants were enrolled in a quitline programme following intervention (4.1% intervention versus 1.1% control; P < 0.01). In Patwardhan 2012, 81 intervention participants were enrolled in a quitline compared to eight in the control group (P < 0.001; measured as counts; no denominator).
Provided self‐help materials
One study (Gordon 2010) assessed the rate of receipt of reading materials among intervention participants (66.5% received reading materials), but this was not measured for the control participants, so we can report no between‐group comparisons.
Discussion
Summary of main results
This review includes seven cluster‐RCTs evaluating the effect of system change interventions on cessation or system‐level outcomes, or both. The seven studies were heterogeneous for types of settings, interventions, providers and outcome measures. When we attempted meta‐analysis this was corroborated by significant statistical heterogeneity, and we therefore do not report pooled effect estimates for any outcomes.
On the basis of the available evidence, it is difficult to draw any firm conclusions about the success of system change interventions in changing cessation practice or enhancing quit rates. The evidence was equivocal for the primary outcome of smoking cessation. However, all studies which evaluated our secondary outcomes, such as documentation of smoking status, quitline referrals and enrolment favoured the intervention. Three of the four studies which evaluated the provision of cessation counselling also supported the intervention. Of the three studies which evaluated outcomes such as asking about tobacco use and advising to quit, two favoured the intervention in both cases. The evidence for using NRT as part of a system change intervention was also uncertain (of the two studies, one favoured the intervention and the other did not show any difference between groups).
When interpreting the findings of this review, it is important to consider the two‐phase nature of the system change intervention: system change interventions directly promote the delivery of smoking cessation services (i.e. process outcomes), which could subsequently lead to improvement in smoking cessation outcomes among smokers. Hence improvement in smoking cessation is the second phase of the intervention effect. This might be one of the reasons that most of the evaluated process outcomes (secondary outcomes) were improved; however, the effect on the cessation outcome remains inconclusive.
Overall completeness and applicability of evidence
The results should be interpreted with caution, for the following reasons:
We could include only seven studies in this review, of which only four evaluated the primary outcome and only a few evaluated each of the secondary outcomes;
Clinical practice guidelines for treating tobacco use and dependence (AHRQ 2012) recommend implementing all components of the system change approach; however, none of the included studies implemented all the components of the system change intervention;
Although all studies included some components of a system change intervention, such as assessment of smoking status, training clinicians and assisting smokers, the intensity and extent varied widely among studies;
None reported reimbursing clinicians or dedicating a staff member to the provision of smoking cessation care;
Although guidelines (AHRQ 2012) recommend regular education and training of all staff on providing smoking cessation support, none of the included studies provided ongoing education, and the duration of smoking cessation training also varied widely across studies;
Only four studies provided pharmacotherapy from the clinic.
The majority of studies were conducted in the USA and none was conducted in low‐ or middle‐income countries. Hence, the generalizability of the findings to low‐ and middle‐income countries is unknown.
Quality of the evidence
The quality of evidence for all the outcomes, including the primary outcome, was low or very low, as reported in Table 1, so that we can draw no robust conclusions about how useful system change interventions are. There was a high risk of bias in many studies. Four studies evaluated cessation outcomes, but only one had biochemical verification of the self‐reported data. There was inconsistency in most of the outcomes evaluated, which is likely to be due to differences in settings, populations, providers and interventions. Effect sizes also differed greatly between studies. We therefore judge that the overall quality of evidence of the included studies was low.
Potential biases in the review process
The search strategy used in this review was carefully developed, and reviewed by experts in the field, including the review group Information Specialist. We conducted a comprehensive search of a large number of databases. One author went through all references identified by the electronic searches, excluding papers that clearly were not eligible, and two authors independently assessed all potentially eligible titles and abstracts against the eligibility criteria to ensure that no important references were missed. We also searched reference lists of included studies. Despite all of this, we cannot rule out the possibility of missing some important studies. There is always a potential risk of publication bias. Unfortunately, because we found few studies for inclusion in this review, we could not systematically assess publication bias.
Agreements and disagreements with other studies or reviews
Various components of the system change approach, such as training health professionals, using electronic health records for identifying smokers, advising and counselling smokers to quit and providing pharmacotherapy have been evaluated separately in other Cochrane Reviews. However, none of them evaluated the effectiveness of a system change approach. Levy 2004 estimated that such strategies could reduce smoking prevalence by 2% to 3.5% at a population level. AHRQ 2012 guidelines also promote the use of a system change approach to address tobacco smoking.
Authors' conclusions
Implications for practice.
Our review could not draw any firm conclusions, as only a handful of relatively low‐quality studies were available. However, there was some evidence for the effect of system change interventions on secondary outcomes, such as asking about tobacco use, documentation of smoking status, advising to quit, provision of cessation counselling, referral to and enrolment in quitlines.
Implications for research.
Despite the potential of a system change approach to address tobacco use, only limited evidence of relatively low quality is currently available. Well‐powered cluster‐RCTs are essential to fill in this knowledge gap. Future studies should include all the components of a system change approach, as recommended by Fiore 2007, to make up the intervention. As clinicians frequently cite lack of reimbursement as a barrier to providing smoking cessation support (Wolfenden 2009), it is important to include such components in the intervention. It is also important to include both biochemically‐validated cessation and system‐level outcomes in every study. As yet there is also no evidence for hospital‐based system change interventions for inpatient smokers, which is a deficit to be addressed by future research. Controlled trials from low‐ and middle‐income countries are also required to fill the knowledge gap.
Acknowledgements
We thank Lindsay Stead and Nicola Lindson‐Hawley of the Cochrane Tobacco Addiction Group for their assistance throughout the review process.
Appendices
Appendix 1. MEDLINE search strategy
Database: Ovid MEDLINE(R) 1946 to Present with Daily Update
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 RANDOMIZED‐CONTROLLED‐TRIAL.pt. (406684)
2 CONTROLLED‐CLINICAL‐TRIAL.pt. (90068)
3 PRAGMATIC‐CLINICAL‐TRIAL.pt. (243)
4 CLINICAL‐TRIAL.pt. (496740)
5 Meta analysis.pt. (61477)
6 exp Clinical Trial/ (723676)
7 Random‐Allocation/ (85490)
8 randomized‐controlled trials/ (100862)
9 double‐blind‐method/ (133088)
10 single‐blind‐method/ (21336)
11 placebos/ (33028)
12 Research‐Design/ (86981)
13 ((clin$ adj5 trial$) or placebo$ or random$).ti,ab. (932222)
14 ((singl$ or doubl$ or trebl$ or tripl$) adj5 (blind$ or mask$)).ti,ab. (131807)
15 (volunteer$ or prospectiv$).ti,ab. (597284)
16 exp Follow‐Up‐Studies/ (534630)
17 exp Retrospective‐Studies/ (563374)
18 exp Prospective‐Studies/ (404707)
19 exp Evaluation‐Studies/ or Program‐Evaluation.mp. (258074)
20 Comparative study/ (1723981)
21 smoking cessation.mp. or exp Smoking Cessation/ (27521)
22 "Tobacco‐Use‐Cessation"/ (831)
23 "Tobacco‐Use‐Disorder"/ (8920)
24 Tobacco‐Smokeless/ (3056)
25 exp Tobacco‐/ (25853)
26 ((quit$ or stop$ or ceas$ or giv$) adj5 smoking).ti,ab. (11154)
27 exp Smoking/pc, th [Prevention & Control, Therapy] (17140)
28 (animals not humans).sh. (4157323)
29 (educat* adj5 (smok* or tobacco)).mp. (6922)
30 (dedicat* adj2 staff*).mp. (287)
31 (hospital adj2 policy).mp. (777)
32 organizational policy/ (12876)
33 "Delivery of Health Care, Integrated"/ (9336)
34 Health Care Reform/ (29384)
35 Health Services Accessibility/ (56182)
36 Patient Care Team/ (55495)
37 Patient‐Centered Care/ (12691)
38 health system chang*.mp. (344)
39 (system* adj2 chang*).mp. (12033)
40 (system* adj2 intervention*).mp. (2627)
41 (integrat* adj6 (smok* or tobacco)).ti,ab. (529)
42 (Organi?ation* adj2 intervention*).mp. (482)
43 Organi?ation* structure*.mp. (2802)
44 (organi?ation* adj2 chang*).mp. (3192)
45 (system* adj2 approach*).mp. (16490)
46 ((system* adj2 reform) or (Organi?ation* adj2 reform*)).mp. (1118)
47 Decision Making, Organizational/ (10649)
48 Organizational Innovation/ (21745)
49 Patient Identification Systems/ (1920)
50 inservice training/ (18138)
51 ("environmental change" or "environmental changes").mp. (7253)
52 ("environmental intervention" or "environmental interventions").mp. (682)
53 "re?engineering".mp. (814)
54 exp Hospital Restructuring/ (7364)
55 "Practice change".mp. (731)
56 ((Identif* adj3 (smok* or tobacco*)) or (Document* adj3 (smok* or tobacco*))).mp. (3017)
57 Patient Education as Topic/ (73925)
58 "Referral and Consultation"/ (55276)
59 Guideline Adherence/ or Guideline/ or Practice Guideline/ (50669)
60 Health Services Research/ (32145)
61 ((system* adj2 modif*) or (Organi?ation* adj2 modif*)).mp. (5533)
62 or/1‐20 (4169369)
63 or/21‐27 (71503)
64 62 and 63 (18983)
65 or/29‐61 (459365)
66 64 and 65 (2685)
67 66 not 28 (2682)
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Cabezas 2011.
| Methods | Design: Cluster‐randomized clinical trial Setting: 176 Primary Care Centres (PCCs) in Spain Intervention providers: Physicians and nurses Data collection: Baseline survey at the clinic and then telephone follow‐up interviews Study duration: 24 months follow‐up (study conducted between 2003 and 2005) |
|
| Participants | 2827 participants, age (mean ± SD) 42.8 ± 13.6 years, 50.0% men, cigarettes per day (mean ± SD) 20.4 ± 10.8 Intervention group: 1345 people from 82 PCCs; control group: 1482 people from 94 PCCs |
|
| Interventions | 1) Intervention
2) Control: Usual care that included brief quitting advice for disease related to smoking. Some control group smokers used cessation medications |
|
| Outcomes | 1‐year continuous abstinence at 2‐year follow‐up 6 months continuous abstinence at 2‐year follow‐up 6 months continuous abstinence at 1‐year follow‐up Point prevalence abstinence at 2‐year follow‐up Point prevalence abstinence at 1‐year follow‐up |
|
| Notes | The study was conducted with financial help from the Spanish Preventive Services Network granted by the Carlos III Health Institute. The authors state that they have no conflict of interest to declare. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Independent statistician blinded to the sites' identities generated random sequence using a computer programme |
| Allocation concealment (selection bias) | Low risk | Centralized randomization, PCCs were informed about their allocation only after giving final consent |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not feasible with the study design |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The clinical part of the questionnaire was administered by the clinicians involved in the study and non‐clinical part was administered by a blinded interviewer |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 43.3% in the intervention and 44.8% in the control group lost to follow‐up, included as smokers. Similar dropout rate in both groups |
| Selective reporting (reporting bias) | Low risk | The study protocol is available and all of the prespecified outcomes that are of interest in the review have been reported in the prespecified way |
| Other bias | High risk | Only 37.3% ex‐smokers confirmed their smoking status biochemically |
Gordon 2010.
| Methods | Design: Cluster‐randomized clinical trial Setting: 14 community health centre dental clinics in 3 states in the USA Intervention providers: Dentists, dental hygienists and dental assistants Data collection: Baseline survey at the clinic and then mailed follow‐up survey Study duration: 7.5 months follow‐up (study conducted between 2005 and 2008) |
|
| Participants | 2549 participants, aged (mean ± SD) 40.5 ± 12.6 years, 42.8% men, average cigarettes per day (mean ± SD) 16.1 ± 10.4. Intervention group: 1394 people; control group: 1155 people | |
| Interventions | 1) Intervention (based on 5As)
2) Control: Practitioners in the control group continued to provide usual care |
|
| Outcomes | 7‐day point prevalence abstinence and prolonged abstinence at 6 weeks and 7½ months follow‐up | |
| Notes | The study was supported by the National Institutes of Health, National Cancer Institute. No conflict of interest declaration was included in the manuscript. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomization not specified |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No blinding of outcome assessment, but the authors have judged that the outcome assessment is not likely to be biased, as a mail survey was used to collect follow‐up data |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Higher dropout rate in intervention group (30.7% vs 26.1%; P < 0.01). Multiple imputation to replace missing data |
| Selective reporting (reporting bias) | Unclear risk | No published protocol |
| Other bias | High risk | No biochemical verification of smoking status |
Joseph 2004.
| Methods | Design: Cluster‐randomized clinical trial Setting: 20 Veterans Affairs medical centres (VAMCs) in the USA Intervention providers: Physicians, nurses, psychologists and pharmacists Data collection: Telephone survey among 3 cohorts of participants; 1) baseline cross‐sectional survey; 2) a second cross‐sectional survey 1 year after initiation of the intervention; and 3) follow‐up survey of smokers identified in the baseline survey; data were also collected from medical records Study duration: 12 months follow‐up (the study dates are not reported in the manuscript) |
|
| Participants |
|
|
| Interventions | 1) Intervention
2) Control: provided 5 copies of AHCPR smoking cessation guideline to each control clinic |
|
| Outcomes | Cessation outcomes: Self‐reported smoking status at 1‐year follow‐up Process outcomes: Improvement in documentation of tobacco use, delivery of treatment to all smokers, and use of pharmacotherapy collected by participant surveys and from medical records |
|
| Notes | This study was supported by a grant from the Veterans Administration Health Services Research and Development Service. No conflict of interest declaration was included in the manuscript. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The study sites (n=20) were randomly assigned to the intervention or control group using simple (not stratified or block) randomisation" |
| Allocation concealment (selection bias) | Low risk | "The remaining 20 sites were randomised." Presumably randomized all clusters at once |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded interviewer |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropout rate by group not given |
| Selective reporting (reporting bias) | Unclear risk | No published protocol |
| Other bias | High risk | No biochemical verification of smoking status |
Little 2009.
| Methods | Design: Cluster‐randomized clinical trial Setting: 14 dental clinics in the USA Intervention providers: Dentist, office staff and hygienist Data collection: From electronic health records Study duration: No follow‐up, 14 months data collection (study conducted between 2006 and 2007) |
|
| Participants | Electronic health record‐generated data (asking, advising and referral) included all people visiting the participating clinics which included 66,516 patients (32,802 intervention and 33,714 control) | |
| Interventions | 1) Intervention
2) Control: Standard care |
|
| Outcomes | Number of participants counselled or referred, or both | |
| Notes | Only secondary outcomes were evaluated. This study was supported by the National Institute of Drug Abuse. No conflict of interest declaration was included in the manuscript. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Matched pairs of facilities were then randomly assigned to intervention or control." Presumably did the randomization |
| Allocation concealment (selection bias) | Low risk | Presumably randomized all clusters at once |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not specified |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Relevant data were generated from electronic health records (objective assessment) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No follow‐up involved |
| Selective reporting (reporting bias) | Unclear risk | No published protocol |
| Other bias | Low risk | |
Patwardhan 2012.
| Methods | Design: Cluster‐randomized clinical trial Setting: 16 community chain pharmacies in the USA Intervention providers: Pharmacists and technicians Data collection: from pharmacy staff using feedback form. Staff were instructed to check off relevant items (asked, advised, provided quitline cards) for the activity performed. Referral data were obtained from quitline reports Study duration: No follow‐up; 1 month data collection (study conducted between 2008 and 2009) |
|
| Participants | 32 pharmacists and 48 technicians (No data collected from pharmacy clients; all data collected from pharmacy staff). | |
| Interventions | 1) Intervention
2) Control: Control group pharmacies received an informal presentation on quitline services, quitline cards and enrolment in Fax to Quit (FTQ) services, a free service |
|
| Outcomes | Number of participants asked about tobacco use Number of tobacco users advised to Quit Number of tobacco users enrolled for quitline service Number of quitline cards given |
|
| Notes | Data collected form the pharmacy staff. The study was supported by the Wisconsin department of Health Services; the Sonderegger Research Center, School of Pharmacy, University of Wisconsin–Madison; and Clinical and Translational Science Award program, National Center for Research Resources, National Institutes of Health. The authors state that they had no conflicts of interest to declare. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Sixteen pharmacies were randomly assigned to a control group or experimental group using block randomisation, which involved random assignment into groups after matching on a block covariate" |
| Allocation concealment (selection bias) | Low risk | "Random assignment was carried out by research assistants blinded to the study goal. The authors were not involved in the assignment process" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Pharmacy staff were not aware of the existence of 2 groups in the study. The primary author who conducted staff training was blinded to pharmacists’ self‐efficacy scores |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The outcome data were directly obtained from the providers using a self‐filled documentation form |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No follow‐up in the study |
| Selective reporting (reporting bias) | Unclear risk | No published protocol |
| Other bias | Low risk | |
Rothemich 2010.
| Methods | Design: Cluster‐randomized clinical trial Setting: 16 primary care practices in the USA Intervention providers: Physicians, nurses and medical assistants Data collection: Participant self‐report via an exit survey Study duration: No follow‐up; 9 months data collection (study conducted between 2005 and 2006) |
|
| Participants | All persons visiting the participating clinics which included 10,395 patients (5669 intervention and 4726 control). Tobacco user population: 1815 adult smokers (857 intervention and 958 control) | |
| Interventions | 1) Intervention
2) Control: Traditional tobacco use vital sign stamp (only smoking status recorded) |
|
| Outcomes | Number of participants asked about tobacco use Number of participants advised to quit Number of participants received in‐office cessation support (primary end point) Number of participants discussing ideas and plans to quit smoking Number of participants referred to quitline |
|
| Notes | 36% of potentially eligible people did not participate in the survey, but the participation proportion did not differ between study groups. This study was funded through a grant from the Agency for Healthcare Research and Quality. One author declares stock in a quitline service provider. No other disclosures were made. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A random number generator was used to randomise practices within the strata to intervention and control arms" |
| Allocation concealment (selection bias) | Low risk | "From the potential pool of 51 sites, 29 practices were targeted for recruitment and 16 were enrolled." Presumably randomized all clusters at once |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not feasible with study design |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinded outcome assessor |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No follow‐up in the study |
| Selective reporting (reporting bias) | Unclear risk | No published protocol |
| Other bias | Low risk | |
Winickoff 2014.
| Methods | Design: Cluster‐randomized clinical trial Setting: 20 paediatric practices in the USA Intervention providers: Paediatricians, nurses and medical assistants Data collection: Telephone survey Study duration: 12 months follow‐up (study conducted between 2010 and 2012) |
|
| Participants | 1980 parent smokers; Intervention: 999 smokers (average age 30 years ranged between 18 ‐ 78 years and 78.6% women) Control: 981 smokers (average age 30.6 years, ranged between 18 ‐ 65 years and 77.9% women) | |
| Interventions | 1) Intervention
2) Control: Usual care |
|
| Outcomes | Biochemically validated parental smoking cessation rates Number of parents asked about tobacco use Number of parents advised and counselled to quit Number of parents prescribed cessation medication Number of parents referred to the state quitline |
|
| Notes | This study was supported the National Institute of Health. One author declares work as an unpaid consultant for a pharmaceutical company. No other disclosures to report. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A random generator was used to generate a sequence of group assignments within each of the 6 blocks created by combining the 2 strata" |
| Allocation concealment (selection bias) | Low risk | "The first 22 practices that responded were enrolled and randomly assigned to intervention or control groups." Presumably randomised all clusters at once |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Although not statistically significant, the follow‐up rate was marginally higher in the control group (64.5% vs 72.4%; P = 0.11) and hence considered as high risk of bias |
| Selective reporting (reporting bias) | Unclear risk | No published protocol |
| Other bias | Low risk | |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Althabe 2013 | Only midwives are involved in the program |
| Amemori 2013 | 3‐arm study comparing 2 types of interventions (education only vs education plus fee‐for‐service) with a control group; No evidence of organization‐wide involvement, and no identification or treatment components. The remuneration was provided only during the 6‐month study period |
| Anders 2011 | Only non‐urgent emergency department patients were targeted |
| Bentz 2007 | Evaluating the effect of electronic health record‐generated provider feedback to improve 5As delivery (only feedback introduced; no other components). Only GPs are targeted |
| Campbell 2006 | Comparing 2 methods of disseminating a smoking cessation programme; Not a system initiated change; no effort to improve identification or treatment component |
| Coleman 2007 | Implemented only the reimbursement (pay‐for‐performance) component of the system change intervention and targeted only GPs |
| Cooke 2001 | Comparing 2 methods of disseminating a smoking cessation programme; Not a system initiated change; no effort to improve identification or treatment component |
| Cummings 1989 | Only training implemented |
| Davies 2005 | Included hospitalized African‐American smokers only |
| Fellows 2012 | Only smokers interested in remaining abstinent after discharge and living within 50 miles of the participating hospital were included in the study |
| Ferketich 2014 | Only Medicaid‐enrolled smokers were included in the study; research assistant identified participants, not clinic staff |
| Fisher 2005 | Controlled before‐and‐after study, not meeting study design requirements |
| Freund 2009 | Controlled before‐and‐after study, not meeting study design requirements |
| Hennrikus 2005 | Research assistants identified smokers |
| Katz 2004 | Telephone follow‐up counselling was done by a researcher |
| Kendrick 1995 | No evidence of effort to identify and treat all smokers accessing the system. Interventions were different at various sites |
| Kim 2005 | Only those randomized to the intervention arm received assistance and follow‐up support, which were provided by a researcher; tailored only for Korean participants |
| Mahabee‐Gittens 2008 | Research staff provided the intervention |
| Maizlish 2006 | Before‐and‐after study, not meeting study design requirements |
| Manfredi 1999 | Research staff provided adjunct interventions (motivational call and reminder letter); only brief advice provided by the clinic staff |
| Manfredi 2000 | Research staff provided adjunct interventions (motivational call and reminder letter); only brief advice provided by the clinic staff |
| Manfredi 2004 | Research staff provided adjunct interventions (motivational call and reminder letter); only brief advice provided by the clinic staff |
| Manfredi 2011 | Research staff provided telephone counselling service |
| McFall 2010 | Not all smokers included in the intervention; RCT design |
| Murray 2013 | Research team was involved in the delivery of the intervention. Not all smokers were identified and treated |
| Pbert 2004 | Not all smokers included in the study; women receiving prenatal care and WIC services and planning to receive paediatric care at 1 of the CHCs were eligible. Those at less than 2 months to the due date and non‐pregnant excluded |
| Rindal 2013 | The intervention was provided only during recall dental visits. No training was provided |
| Roski 1998 | Quasi‐experimental research design. No evidence that all smokers accessing the system were identified and treated |
| Sherman 2008 | Intervention mainly consisted of referring to an onsite counselling programme; clinicians were not involved in the intervention. |
| Szatkowski 2016 | Implemented only the reimbursement (pay‐for‐performance) component of the system change intervention and targeted only GPs |
| Szpunar 2006 | No random selection of sites (controlled before‐and‐after study), not meeting study design requirements |
| Taggar 2012 | Implemented only the reimbursement (pay‐for‐performance) component of the system change intervention and targeted only GPs |
| Thomas 2016 | Not all smokers received intervention; RCT design; smokers were identified by a researcher |
| Unrod 2007 | Research staff identified smokers. Not all smokers received intervention |
| Vidrine 2013a | Research team was involved in delivering the intervention |
| Vidrine 2013b | Research team was involved in delivering the intervention |
| Walsh 1997 | Not all smokers received intervention; RCT design |
| Wolfenden 2005 | Not all smokers received intervention; RCT design ; smokers were identified by a researcher |
| Yano 2008 | No evidence of effort to identify and treat all smokers accessing the system |
Characteristics of ongoing studies [ordered by study ID]
Bonevski 2016.
| Trial name or title | An organizational change intervention for increasing the delivery of smoking cessation support in addiction treatment centres: study protocol for a randomized controlled trial |
| Methods | Design: Cluster‐randomized clinical trial Setting: 33 drug and alcohol treatment services in Australia Intervention providers: all staff Data collection: Participant surveys |
| Participants | People attending drug and alcohol treatment services |
| Interventions | Intervention includes assistance in developing smoke‐free policies, identifying all smokers, nomination of champions, staff training and educational participant and service resources, and free nicotine replacement therapy in order to integrate smoking cessation support as part of usual participant care |
| Outcomes | Biochemically‐verified 7‐day point prevalence abstinence at 6 weeks will be the primary outcome measure. |
| Starting date | 02/02/15 |
| Contact information | A/Prof Billie Bonevski, Centre for Translational Neuroscience and Mental Health, University of Newcastle, NSW, Australia |
| Notes | This study is a potentially included study. |
Ostroff 2014.
| Trial name or title | Dentists United to Extinguish Tobacco (DUET): a study protocol for a cluster randomized, controlled trial for enhancing implementation of clinical practice guidelines for treating tobacco dependence in dental care settings. |
| Methods | Design: Cluster‐randomized clinical trial Setting: 18 dental care clinics in the USA Intervention providers: dentist, dental hygienist, and dental assistants Data collection: patient exit interviews, provider surveys, site observations, chart audits |
| Participants | All people attending the clinic |
| Interventions | Intervention 1: Staff training and current best practice (CBP) Intervention 2: CBP and provider performance training (PF) Intervention 3: CBP, PF and provider reimbursement |
| Outcomes | Provider adherence to PHS guidelines, 7‐day point prevalence abstinence, 24‐hour quit attempts, provider attitudes and cost per quit |
| Starting date | |
| Contact information | |
| Notes | This study is ongoing and has a published protocol. The arm 3 may be eligible to include in this review if they make sufficient effort to identify all smokers |
Differences between protocol and review
We used the current 'Risk of bias' criteria table embedded in RevMan, rather than the previous version specified in the protocol. We have changed the classification of secondary outcomes, by combining the health professional and patient‐level outcomes and reporting them as 'outcome of care'.
Contributions of authors
All authors contributed to conceptualising and designing the review.
DT was involved in developing search strategies, conducting searches, screening articles, extracting data and writing the first and subsequent drafts of the review.
JG was involved in developing search strategies, screening articles, extracting data, reviewing and revising drafts.
BB was involved in developing search strategies, reviewing and revising drafts. BB also acted as an arbiter to resolve any disagreements between DT and JG regarding study methodology.
MJA was involved in developing search strategies, reviewing and revising drafts. MJA also acted as an arbiter to resolve any disagreements between DT and JG regarding study methodology.
All authors reviewed and approved the final manuscript.
Declarations of interest
JG, MJA and BB have received an investigator‐initiated grant from Pfizer for the “Give Up For Good” study which aims to evaluate the effectiveness of a pharmacist‐driven system‐change smoking cessation programme at three Australian hospitals. They also hold an investigator‐initiated research grant from Boehringer‐Ingelheim for an unrelated study. MJA has also undertaken an unrelated consultancy for AstraZeneca. He received an honorarium for speaking at a Novartis Respiratory Symposium, assistance with attendance at the European Respiratory Society Congress from Boehringer‐Ingelheim and the World Health Summit from Sanofi. BB is also supported by an Australian National Health and Medical Research Council Career Development Fellowship (GNT1063206) and a Faculty of Health and Medicine, University of Newcastle Gladys M Brawn Career Development fellowship.
DT has no conflict of interest to declare.
New
References
References to studies included in this review
Cabezas 2011 {published data only}
- Cabezas C, Advani M, Puente D, Rodriguez‐Blanco T, Martin C: ISTAPS Study Group. Effectiveness of a stepped primary care smoking cessation intervention: Cluster randomized clinical trial (ISTAPS study). Addiction 2011;106(9):1696‐706. [DOI] [PubMed] [Google Scholar]
Gordon 2010 {published data only}
- Gordon JS, Andrews JA, Albert DA, Crews KM, Payne TJ, Severson HH. Tobacco cessation via public dental clinics: results of a randomized trial. American Journal of Public Health 2010;100(7):1307‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Joseph 2004 {published data only}
- Joseph MA, Arikian NJ, An LC, Nugent SM, Sloan RJ, Pieper CF. Results of a randomized controlled trial of intervention to implement smoking guidelines in Veterans Affairs medical centers: increased use of medications without cessation benefit. Medical Care 2004;42(11):1100‐10. [DOI] [PubMed] [Google Scholar]
Little 2009 {published data only}
- Little SJ, Hollis JF, Fellows JL, Snyder JJ, Dickerson JF. Implementing a tobacco assisted referral program in dental practices. Journal of Public Health Dentistry 2009;69(3):149‐55. [DOI] [PubMed] [Google Scholar]
Patwardhan 2012 {published data only}
- Patwardhan PD, Chewning BA. Effectiveness of intervention to implement tobacco cessation counseling in community chain pharmacies. Journal of the American Pharmacists Association 2012;52(4):507‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rothemich 2010 {published data only}
- Rothemich SF, Woolf SH, Johnson RE, Devers KJ, Flores SK, Villars P, et al. Promoting primary care smoking‐cessation support with quitlines: the QuitLink randomized controlled trial. American Journal of Preventive Medicine 2010;38(4):367‐74. [DOI] [PubMed] [Google Scholar]
Winickoff 2014 {published data only}
- Winickoff JP, Nabi‐Burza E, Chang Y, Regan S, Drehmer J, Finch S, et al. Implementation of a parental tobacco control intervention in pediatric practice. Pediatrics 2014;132(1):109‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Althabe 2013 {published data only}
- Althabe F, Aleman A, Mazzoni A, Berrueta M, Morello P, Colomar M, et al. Tobacco cessation intervention for pregnant women in Argentina and Uruguay: Study protocol. Reproductive Health 2013;10(1):44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Amemori 2013 {published data only}
- Amemori M, Virtanen J, Korhonen T, Kinnunen TH, Murtomaa H. Impact of educational intervention on implementation of tobacco counselling among oral health professionals: a cluster‐randomized community trial. Community Dentistry and Oral Epidemiology 2013;41(2):120‐9. [DOI] [PubMed] [Google Scholar]
Anders 2011 {published data only}
- Anders ME, Sheffer CE, Barone CP, Holmes TM, Simpson DD, Duncan AM. Emergency department‐initiated tobacco dependence treatment. American Journal of Health Behavior 2011;35(5):546‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bentz 2007 {published data only}
- Bentz CJ, Bayley KB, Bonin KE, Fleming L, Hollis JF, Hunt JS, et al. Provider feedback to improve 5A's tobacco cessation in primary care: a cluster randomized clinical trial. Nicotine & Tobacco Research 2007;9(3):341‐9. [DOI] [PubMed] [Google Scholar]
Campbell 2006 {published data only}
- Campbell E, Walsh RA, Sanson‐Fisher R, Burrows S, Stojanovski E. A group randomised trial of two methods for disseminating a smoking cessation programme to public antenatal clinics: effects on patient outcomes. Tobacco Control 2006;15(2):97‐102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Coleman 2007 {published data only}
- Coleman T, Lewis S, Hubbard R, Smith C. Impact of contractual financial incentives on the ascertainment and management of smoking in primary care. Addiction 2007;102(5):803‐8. [DOI] [PubMed] [Google Scholar]
Cooke 2001 {published data only}
- Cooke M, Mattick RP, Walsh RA. Differential uptake of a smoking cessation programme disseminated to doctors and midwives in antenatal clinics. Addiction 2001;96(3):495‐505. [DOI] [PubMed] [Google Scholar]
Cummings 1989 {published data only}
- Cummings SR, Coater TJ, Richard RJ, Hansen B, Zahnd EG, VanderMartin R, et al. Training physicians in counseling about smoking cessation. A randomized trial of the 'Quit for Life' program. Annals of Internal Medicine 1989;110(8):640‐7. [DOI] [PubMed] [Google Scholar]
Davies 2005 {published data only}
- Davies SL, Kohler CL, Fish L, Taylor BE, Foster GE, Annang L. Evaluation of an intervention for hospitalized African American smokers. American Journal of Health Behavior 2005;29(3):228‐39. [DOI] [PubMed] [Google Scholar]
Fellows 2012 {published data only}
- Fellows JL, Mularski R, Waiwaiole L, Funkhouser K, Mitchell J, Arnold K, et al. Health and economic effects from linking bedside and outpatient tobacco cessation services for hospitalized smokers in two large hospitals: study protocol for a randomized controlled trial. Trials 2012;13:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ferketich 2014 {published data only}
- Ferketich AK, Pennell M, Seiber EE, Wang L, Farietta T, Jin Y, et al. Provider‐delivered tobacco dependence treatment to medicaid smokers. Nicotine & Tobacco Research 2014;16(6):786‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fisher 2005 {published data only}
- Fisher E, Musick J, Scott C, Miller JP, Gram R, Richardson V, et al. Improving clinic‐ and neighborhood‐based smoking cessation services within federally qualified health centers serving low‐income, minority neighborhoods. Nicotine & Tobacco Research 2005;7 Suppl 1:S45‐56. [DOI] [PubMed] [Google Scholar]
Freund 2009 {published data only}
- Freund M, Campbell E, Paul C, Sakrouge R, Lecathelinais C, Knight J, et al. Increasing hospital‐wide delivery of smoking cessation care for nicotine‐dependent in‐patients: a multi‐strategic intervention trial. Addiction 2009;104(5):839‐49. [DOI] [PubMed] [Google Scholar]
Hennrikus 2005 {published data only}
- Hennrikus DJ, Lando HA, McCarty MC, Klevan D, Holtan N, Huebsch JA, et al. The TEAM project: the effectiveness of smoking cessation intervention with hospital patients. Preventive Medicine 2005;40(3):249‐58. [DOI] [PubMed] [Google Scholar]
Katz 2004 {published data only}
- Katz DA, Muehlenbruch DR, Brown RL, Fiore MC, Baker TB. Effectiveness of implementing the Agency for Healthcare Research and Quality Smoking Cessation Clinical Practice Guideline: A randomized, controlled trial. Journal of the National Cancer Institute 2004;96(8):594‐603. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kendrick 1995 {published data only}
- Kendrick JS, Zahniser SC, Miller N, Salas N, Stine J, Gargiullo PM, et al. Integrating smoking cessation into routine public prenatal care: the Smoking Cessation in Pregnancy project. American Journal of Public Health 1995;85(2):217‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2005 {published data only}
- Kim JR, Lee MS, Hwang JY, Lee JD. Efficacy of a smoking cessation intervention using the AHCPR guideline tailored for Koreans: A randomized controlled trial. Health Promotion International 2005;20(1):51‐9. [DOI] [PubMed] [Google Scholar]
Mahabee‐Gittens 2008 {published data only}
- Mahabee‐Gittens ME, Gordon JS, Krugh ME, Henry B, Leonard AC. A smoking cessation intervention plus proactive quitline referral in the pediatric emergency. Nicotine & Tobacco Research 2008;10(12):1745‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Maizlish 2006 {published data only}
- Maizlish NA, Ruland J, Rosinski ME, Hendry K. A systems‐based intervention to promote smoking as a vital sign in patients served by community health centers. American Journal of Medical Quality 2006;21(3):169. [DOI] [PubMed] [Google Scholar]
Manfredi 1999 {published data only}
- Manfredi C, Crittenden KS, Warnecke R, Engler J, Cho YI, Shaligram C. Evaluation of a motivational smoking cessation intervention for women in public health clinics. Preventive Medicine 1999;28(1):51‐60. [DOI] [PubMed] [Google Scholar]
Manfredi 2000 {published data only}
- Manfredi C, Crittenden KS, Cho YI, Engler J, Warnecke R. The effect of a structured smoking cessation program, independent of exposure to existing interventions. American Journal of Public Health 2000;90(5):751‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Manfredi 2004 {published data only}
- Manfredi C, Crittenden KS, Cho YI, Gao S. Long‐term effects (up to 18 months) of a smoking cessation program among women smokers in public health clinics. Preventive Medicine 2004;38(1):10‐9. [DOI] [PubMed] [Google Scholar]
Manfredi 2011 {published data only}
- Manfredi C, Cho YI, Warnecke R, Saunders S, Sullivan M. Dissemination strategies to improve implementation of the PHS smoking cessation guideline in MCH public health clinics: experimental evaluation results and contextual factors. Health Education Research 2011;26(2):348‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
McFall 2010 {published data only}
- McFall M, Saxon AJ, Malte CA, Chow B, Bailey S, Baker DG, et al. Integrating tobacco cessation into mental health care for posttraumatic stress disorder: a randomized controlled trial. JAMA 2010;304(22):2485‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Murray 2013 {published data only}
- Murray R, Leonardi‐Bee J, Marsh J, Jayes L, Li J, Parrott S, et al. Systematic identification and treatment of smokers by hospital based cessation practitioners in a secondary care setting: cluster randomised controlled trial. BMJ 2013;347:f4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pbert 2004 {published data only}
- Pbert L, Ockene JK, Zapka J, Ma Y, Goins KV, Oncken C, et al. A community health center smoking‐cessation intervention for pregnant and postpartum women. American Journal of Preventive Medicine 2004;26(5):377‐85. [DOI] [PubMed] [Google Scholar]
Rindal 2013 {published data only}
- Rindal DB, Rush WA, Schleyer TKL, Kirshner M, Boyle RG, Thoele MJ, et al. Computer‐assisted guidance for dental office tobacco‐cessation counseling: a randomized controlled trial. American Journal of Preventive Medicine 2013;44(3 Suppl 3):260‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Roski 1998 {published data only}
- Roski J. Changing practice patterns as a result of implementing the Agency for Health Care Policy and Research guideline in 20 primary care clinics. Tobacco Control 1998;7 Suppl:S19‐20; discussion S4‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sherman 2008 {published data only}
- Sherman SE, Takahashi N, Kalra P, Gifford E, Finney JW, Canfield J, et al. Care coordination to increase referrals to smoking cessation telephone counseling: A demonstration project. American Journal of Managed Care 2008;14(3):141‐8. [PubMed] [Google Scholar]
Szatkowski 2016 {published data only}
- Szatkowski L, Aveyard P. Provision of smoking cessation support in UK primary care: impact of the 2012 QOF revision. British Journal of General Practice 2016;66(642):10‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Szpunar 2006 {published data only}
- Szpunar SM, Williams PD, Dagroso D, Enberg RN, Chesney JD. Effects of the tobacco use cessation automated clinical practice guideline. American Journal of Managed Care 2006;12(11):665‐73. [PubMed] [Google Scholar]
Taggar 2012 {published data only}
- Taggar JS, Coleman T, Lewis S, Szatkowski L. The impact of the Quality and Outcomes Framework (QOF) on the recording of smoking targets in primary care medical records: cross‐sectional analyses from The Health Improvement Network (THIN) database. BMC Public Health 2012;12:329. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thomas 2016 {published data only}
- Thomas D, Abramson MJ, Bonevski B, Taylor S, Poole SG, Paul E, et al. Integrating smoking cessation into routine care in hospitals ‐‐ a randomized controlled trial. Addiction 2016;111(4):14‐23. [DOI] [PubMed] [Google Scholar]
Unrod 2007 {published data only}
- Unrod M, Smith M, Spring B, DePue J, Redd W, Winkel G. Randomized controlled trial of a computer‐based, tailored intervention to increase smoking cessation counseling by primary care physicians. Journal of General Internal Medicine 2007;22(4):478‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vidrine 2013a {published data only}
- Vidrine JI, Shete S, Cao Y, Greisinger A, Harmonson P, Sharp B, et al. Ask‐Advise‐Connect: A new approach to smoking treatment delivery in health care settings. JAMA Internal Medicine 2013;173(6):458‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vidrine 2013b {published data only}
- Vidrine JI, Shete S, Li Y, Cao Y, Alford MH, Galindo‐Talton M, et al. The ask‐advise‐connect approach for smokers in a safety net healthcare system: A group‐randomized trial. American Journal of Preventive Medicine 2013;45(6):737‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Walsh 1997 {published data only}
- Walsh RA, Redman S, Brinsmead MW, Byrne JM, Melmeth A. A smoking cessation program at a public antenatal clinic. American Journal of Public Health 1997;87(7):1201‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wolfenden 2005 {published data only}
- Wolfenden L, Wiggers J, Knight J, Campbell E, Spigelman A, Kerridge R, et al. Increasing smoking cessation care in a preoperative clinic: a randomized controlled trial. Preventive Medicine 2005;41(1):284‐90. [DOI] [PubMed] [Google Scholar]
Yano 2008 {published data only}
- Yano EM, Rubenstein LV, Farmer MM, Chernof BA, Mittman BS, Lanto AB, et al. Targeting primary care referrals to smoking cessation clinics does not improve quit rates: implementing evidence‐based interventions into practice. Health Services Research 2008;43(5 P1):1637‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
Bonevski 2016 {published data only}
- Bonevski B, Guillaumier A, Shakeshaft A, Farrell M, Tzelepis F, Walsberger S, et al. An organisational change intervention for increasing the delivery of smoking cessation support in addiction treatment centres: study protocol for a randomized controlled trial. Trials 2016;17(1):290. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ostroff 2014 {published data only}
- Ostroff JS, Li Y, Shelley DR. Dentists United to Extinguish Tobacco (DUET): a study protocol for a cluster randomized, controlled trial for enhancing implementation of clinical practice guidelines for treating tobacco dependence in dental care settings. Implementation Science 2014;9:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
AHCPR 1996
- Fiore MC, Wetter DW, Bailey WC, Bennett G, Cohen SJ, Dorfman SF, Goldstein MG, Gritz ER, Hasselblad V, Henningfield JE. The Agency for Health Care Policy and Research Smoking Cessation Clinical Practice Guideline. JAMA 1996;275(16):1270‐80. [PubMed] [Google Scholar]
AHRQ 2012
- Agency for Healthcare Research and Quality. Systems Change: Treating Tobacco Use and Dependence: Based on the Public Health Service (PHS) Clinical Practice Guideline—2008 Update. www.ahrq.gov/professionals/clinicians‐providers/guidelines‐recommendations/tobacco/decisionmakers/systems/index.html December 2012 (Accessed 25 January 2017).
Aquilino 2003
- Aquilino ML, Farris KB, Zillich AJ, Lowe JB. Smoking‐cessation services in Iowa community pharmacies. Pharmacotherapy 2003;23(5):666‐73. [DOI] [PubMed] [Google Scholar]
Braun 2004
- Braun BL, Fowles JB, Solberg LI, Kind EA, Lando H, Pine D. Smoking‐related attitudes and clinical practices of medical personnel in Minnesota. American Journal of Preventive Medicine 2004;27(4):316‐22. [DOI] [PubMed] [Google Scholar]
Critchley 2012
- Critchley JA, Capewell S. Smoking cessation for the secondary prevention of coronary heart disease. Cochrane Database of Systematic Reviews 2012, Issue 2. [DOI: 10.1002/14651858.CD003041] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ebbert 2005
- Ebbert JO, Williams BA, Sun Z, Aubry MC, Wampfler JA, Garces YI, et al. Duration of smoking abstinence as a predictor for non‐small‐cell lung cancer survival in women. Lung Cancer 2005;47(2):165‐72. [DOI] [PubMed] [Google Scholar]
Fiore 2007
- Fiore MC, Keller PA, Curry SJ. Health system changes to facilitate the delivery of tobacco‐dependence treatment. American Journal of Preventive Medicine 2007;33(6 Suppl):S349‐56. [DOI] [PubMed] [Google Scholar]
Fiore 2008
- Fiore MC, Jaén CR, Baker TB, Bailey WC, Benowitz NL, Curry SJ, et al. Treating Tobacco Use and Dependence: 2008 Update. Clinical Practice Guideline. U.S. Department of Health and Human Services. Public Health Service May 2008.
Freund 2005
- Freund M, Campbell E, Paul C, Sakrouge R, Wiggers J. Smoking care provision in smoke‐free hospitals in Australia. Preventive Medicine 2005;41(1):151‐8. [DOI] [PubMed] [Google Scholar]
Freund 2008
- Freund M, Campbell E, Paul C, McElduff P, Walsh RA, Sakrouge R, et al. Smoking care provision in hospitals: a review of prevalence. Nicotine & Tobacco Research 2008;10(5):757‐74. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hudson 2010
- Hudson NL, Mannino DM. Smoking as a chronic disease. Journal of Community Health 2010;35(5):549‐53. [DOI] [PubMed] [Google Scholar]
Levy 2004
- Levy DT, Chaloupka F, Gitchell J. The effects of tobacco control policies on smoking rates: a tobacco control scorecard. Journal of Public Health Management and Practice 2004;10(4):338‐53. [DOI] [PubMed] [Google Scholar]
Peto 2000
- Peto R, Darby S, Deo H, Silcocks P, Whitley E, Doll R. Smoking, smoking cessation, and lung cancer in the UK since 1950: combination of national statistics with two case‐control studies. BMJ 2000;321(7257):323‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Revell 2005
- Revell CC, Schroeder SA. Simplicity matters: using system‐level changes to encourage clinician intervention in helping tobacco users quit. Nicotine & Tobacco Research 2005;7 Suppl 1:S67‐69. [DOI] [PubMed] [Google Scholar]
Taylor 2002
- Taylor DH Jr, Hasselblad V, Henley SJ, Thun MJ, Sloan FA. Benefits of smoking cessation for longevity. American Journal of Public Health 2002;92(6):990‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thorndike 1998
- Thorndike AN, Rigotti NA, Stafford RS, Singer DE. National patterns in the treatment of smokers by physicians. JAMA 1998;279(8):604‐8. [DOI] [PubMed] [Google Scholar]
Wagner 1998
- Wagner EH. Chronic disease management: what will it take to improve care for chronic illness?. Effective Clinical Practice 1998;1(1):2‐4. [PubMed] [Google Scholar]
West 2005
- West R, Hajek P, Stead L, Stapleton J. Outcome criteria in smoking cessation trials: proposal for a common standard. Addiction 2005;100(3):299‐303. [DOI] [PubMed] [Google Scholar]
WHO 2011
- Worl Health Organization. WHO Report on the Global Tobacco Epidemic: Warning about the Dangers of Tobacco. apps.who.int/iris/bitstream/10665/44616/1/9789240687813_eng.pdf. Geneva, 2011 (Accessed 25 January 2017).
Winickoff 2013
- Winickoff JP, Nabi‐Burza E, Chang Y, Finch S, Regan S, Wasserman R, et al. Implementation of a parental tobacco control intervention in pediatric practice. Pediatrics 2013;132(1):109‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wolfenden 2009
- Wolfenden L, Wiggers J, Campbell E, Knight J, Kerridge R, Spigelman A. Providing comprehensive smoking cessation care to surgical patients: the case for computers. Drug and Alcohol Review 2009;28(1):60‐5. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Thomas 2013
- Thomas D, Abramson MJ, Bonevski B, George J. System change interventions for smoking cessation. Cochrane Database of Systematic Reviews 2013, Issue 9. [DOI: 10.1002/14651858.CD010742] [DOI] [PMC free article] [PubMed] [Google Scholar]


