Abstract
Background
The optimal rhythm management strategy for people with non‐paroxysmal (persistent or long‐standing persistent) atrial fibrilation is currently not well defined. Antiarrhythmic drugs have been the mainstay of therapy. But recently, in people who have not responded to antiarrhythmic drugs, the use of ablation (catheter and surgical) has emerged as an alternative to maintain sinus rhythm to avoid long‐term atrial fibrillation complications. However, evidence from randomised trials about the efficacy and safety of ablation in non‐paroxysmal atrial fibrillation is limited.
Objectives
To determine the efficacy and safety of ablation (catheter and surgical) in people with non‐paroxysmal (persistent or long‐standing persistent) atrial fibrillation compared to antiarrhythmic drugs.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE Ovid, Embase Ovid, conference abstracts, clinical trial registries, and Health Technology Assessment Database. We searched these databases from their inception to 1 April 2016. We used no language restrictions.
Selection criteria
We included randomised trials evaluating the effect of radiofrequency catheter ablation (RFCA) or surgical ablation compared with antiarrhythmic drugs in adults with non‐paroxysmal atrial fibrillation, regardless of any concomitant underlying heart disease, with at least 12 months of follow‐up.
Data collection and analysis
Two review authors independently selected studies and extracted data. We evaluated risk of bias using the Cochrane 'Risk of bias' tool. We calculated risk ratios (RRs) for dichotomous data with 95% confidence intervals (CIs) a using fixed‐effect model when heterogeneity was low (I² <= 40%) and a random‐effects model when heterogeneity was moderate or substantial (I² > 40%). Using the GRADE approach, we evaluated the quality of the evidence and used the GRADE profiler (GRADEpro) to import data from Review Manager 5 to create 'Summary of findings' tables.
Main results
We included three randomised trials with 261 participants (mean age: 60 years) comparing RFCA (159 participants) to antiarrhythmic drugs (102) for non‐paroxysmal atrial fibrillation. We generally assessed the included studies as having low or unclear risk of bias across multiple domains, with reported outcomes generally lacking precision due to low event rates. Evidence showed that RFCA was superior to antiarrhythmic drugs in achieving freedom from atrial arrhythmias (RR 1.84, 95% CI 1.17 to 2.88; 3 studies, 261 participants; low‐quality evidence), reducing the need for cardioversion (RR 0.62, 95% CI 0.47 to 0.82; 3 studies, 261 participants; moderate‐quality evidence), and reducing cardiac‐related hospitalisation (RR 0.27, 95% CI 0.10 to 0.72; 2 studies, 216 participants; low‐quality evidence) at 12 months follow‐up. There was substantial uncertainty surrounding the effect of RFCA regarding significant bradycardia (or need for a pacemaker) (RR 0.20, 95% CI 0.02 to 1.63; 3 studies, 261 participants; low‐quality evidence), periprocedural complications, and other safety outcomes (RR 0.94, 95% CI 0.16 to 5.68; 3 studies, 261 participants; very low‐quality evidence).
Authors' conclusions
In people with non‐paroxysmal atrial fibrillation, evidence suggests a superiority of RFCA to antiarrhythmic drugs in achieving freedom from atrial arrhythmias, reducing the need for cardioversion, and reducing cardiac‐related hospitalisations. There was uncertainty surrounding the effect of RFCA with significant bradycardia (or need for a pacemaker), periprocedural complications, and other safety outcomes. Evidence should be interpreted with caution, as event rates were low and quality of evidence ranged from moderate to very low.
Plain language summary
Benefits and harms of ablation for people with non‐paroxysmal atrial fibrillation
Background
Atrial fibrillation is a heart condition that causes an irregular and often abnormally fast heart rate (tachycardia). A normal heart rate should be regular and between 60 and 100 beats a minute when resting. In atrial fibrillation, the heart rate is irregular and can sometimes be very fast. In some cases, it can be considerably higher than 100 beats a minute. This can cause symptoms such as dizziness, shortness of breath, and tiredness that affect quality of life, but more importantly, atrial fibrillation increases the risk of suffering a stroke.
In the majority of people, atrial fibrillation is recurrent and progresses from self‐terminating short episodes (paroxysmal), to longer episodes (persistent) with the need for cardioversion into normal heart rhythm, or it can progress into permanent forms. Management of atrial fibrillation includes control of symptoms, and reducing the risk of stroke. One strategy to achieve this is to restore the normal heart rhythm by using medications. However, not all people respond well to heart rhythm drugs and therefore a new medical procedure, called ablation, using either a catheter or through surgery, has been developed to overcome this problem. The number of randomised trials comparing heart rhythm drugs versus ablation is limited.
The aim of this systematic review is to compare the benefits and harms of ablation (using either catheter or surgery) to heart rhythm drugs in people with persistent or long‐standing persistent (non‐paroxysmal) atrial fibrillation.
Study characteristics
We searched scientific databases from their inception to 1 April 2016 and found three studies where people are randomly allocated into one of two or more treatment groups (known as randomised trials). The three trials included 261 adults (mean age: 60 years) comparing catheter ablation (159 participants) to heart rhythm drugs (102) for non‐paroxysmal atrial fibrillation at 12 months follow‐up.
Key results
When compared to participants receiving heart rhythm drugs, those participants receiving catheter ablation were more likely to be free from atrial fibrillation, had reduced risk of being hospitalised due to cardiac causes, and had a reduced risk of needing cardioversion after 12 months. There was uncertainty surrounding the effect of catheter ablation with significant bradycardia (or need for a pacemaker), periprocedural complications, and other safety outcomes.
Quality of evidence
Evidence should be interpreted with caution as evidence quality ranged from moderate to very low across the different outcomes due to the limitations of the original studies. It is likely that further high‐quality and adequately powered trials may affect the confidence in reported results.
Summary of findings
Summary of findings for the main comparison. Ablation compared to antiarrhythmic drug for participants with non‐paroxysmal atrial fibrillation.
| Ablation compared to antiarrhythmic drugs for participants with non‐paroxysmal atrial fibrillation | |||||
| Population: people with non‐paroxysmal atrial fibrillation Settings: hospital Intervention: ablation Comparison: antiarrhythmic drugs | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Antiarrhythmic drugs | Ablation | ||||
| Freedom from atrial arrhythmia Follow‐up: 12 months | Study population | RR 1.84 (1.17 to 2.88) | 261 (3 studies) | ⊕⊕⊝⊝ low1,2 | |
| 353 per 1000 | 649 per 1000 (413 to 1000) | ||||
| Moderate population | |||||
| 429 per 1000 | 789 per 1000 (502 to 1000) | ||||
| Participants needing cardioversion Follow‐up: 12 months | Study population | RR 0.62 (0.47 to 0.82) | 261 (3 studies) | ⊕⊕⊕⊝ moderate2 | |
| 422 per 1000 | 261 per 1000 (198 to 346) | ||||
| Moderate population | |||||
| 500 per 1000 | 310 per 1000 (235 to 410) | ||||
| Cardiac hospitalisation Hospitalisations directly related to ablation or antiarrhythmic drugs Follow‐up: 12 months | Study population | RR 0.27 (0.10 to 0.72) | 216 (2 studies) | ⊕⊕⊝⊝ low3 | |
| 181 per 1000 | 49 per 1000 (18 to 130) | ||||
| Moderate population | |||||
| 203 per 1000 | 55 per 1000 (20 to 146) | ||||
| Significant bradycardia or need for a pacemaker Follow‐up: 12 months | Study population | RR 0.20 (0.02 to 1.63) | 261 (3 studies) | ⊕⊕⊝⊝ low4 | |
| 49 per 1000 | 10 per 1000 (1 to 80) | ||||
| Moderate population | |||||
| 0 per 1000 | 0 per 1000 (0 to 0) | ||||
| Periprocedural complications and other safety outcomes Follow‐up: 12 months | Study population | RR 0.94 (0.16 to 5.68) | 261 (3 studies) | ⊕⊝⊝⊝ very low1,4 | |
| 78 per 1000 | 74 per 1000 (13 to 445) | ||||
| Moderate population | |||||
| 42 per 1000 | 39 per 1000 (7 to 239) | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | |||||
| GRADE Working Group grades of evidence High‐quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate‐quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low‐quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low‐quality: We are very uncertain about the estimate. | |||||
1 Unexplained heterogeneity; downgraded one level of evidence. 2 Serious imprecision due to low event rates compared to total participants; downgraded one level of evidence. 3 Very serious imprecision due to very low event rates compared to total participants; downgraded two levels of evidence. 4 Very serious imprecision due to very low event rates compared to total participants, with confidence interval crossing line of no effect; downgraded two levels of evidence.
Background
Description of the condition
Atrial fibrillation is currently the most common serious arrhythmia, with a prevalence of 1% to 2% in the general population, and the incidence increasing with age (Rahman 2014). In the majority of people, the disease is recurrent and progresses from being paroxysmal (self‐terminating short episodes) to a persistent (longer episodes, need for cardioversion into normal sinus rhythm), or permanent form (Kerr 2005). People with atrial fibrillation have poorer outcomes and significantly poorer quality of life compared with healthy controls, people with coronary heart disease (Dorian 2000), or the general population (Thrall 2006). Management of atrial fibrillation includes reduction of stroke risk, control of symptoms, and prevention of tachycardia‐induced cardiomyopathy. To achieve the latter two, controlling the heart rate can be the preferred way to manage atrial fibrillation in some people (Wyse 2002), while others may require therapy to maintain normal sinus rhythm and prevent atrial fibrillation recurrence in order to control their symptoms. Furthermore, restoration of sinus rhythm improves both quality of life and exercise capacity (Singh 2006). Therapy to maintain sinus rhythm includes antiarrhythmic drugs or ablation procedures.
Description of the intervention
The use of catheter ablation for treatment of atrial fibrillation based on electrical isolation of triggers from the pulmonary veins has grown rapidly over the last decade (Jaïs 2008). Evidence from randomised trials (mainly in people where antiarrhythmic drugs have failed) indicates clear benefit for paroxysmal atrial fibrillation (Hakalahti 2015; Khan 2014; Morillo 2014; Nair 2009). However, ablation success is reduced in people with persistent or long‐standing persistent (from now on referred to as 'non‐paroxysmal') atrial fibrillation, where it is associated with longer procedure duration and lower long‐term success rates compared to paroxysmal atrial fibrillation (Calkins 2012). Although guidelines have suggested that operators should consider more aggressive ablation strategies (including linear lesions and targeting of complex fractionated electrocardiograms) for non‐paroxysmal atrial fibrillation (Andrade 2012; Pokushalov 2013), recent evidence from the STAR AF II trial has challenged this view (Verma 2015).
Current reported success rates for persistent atrial fibrillation vary significantly between studies and the evidence is primarily derived from non‐randomised studies. Single‐centre cohort studies have reported a single procedure one‐year atrial fibrilation‐free survival rate of less than 30% (Brooks 2010). Randomised trials comparing different ablation techniques have shown that pulmonary vein isolation as a single procedure has a one‐year atrial fibrilation‐free survival rate of around 40% (Elayi 2008; Oral 2005). Adding linear ablation or targeting people with complex fractionated atrial electrocardiograms (CFAEs) (or both) might increase the reported success rate. However, the evidence for the efficacy and safety of catheter ablation in non‐paroxysmal atrial fibrillation comes primarily from analysis of case series. The largest and longest case series (80 participants) reported a single procedure success rate of around 50% using an aggressive ablation protocol (Rivard 2012). The recent European Survey on Methodology and Results of Catheter Ablation for Atrial Fibrillation, conducted in 72 medium‐ to high‐volume centres (i.e. 50 or more atrial fibrillation ablations per year) from 10 European countries, reported a 29.5% overall success rate of ablation at one year for persistent atrial fibrillation (Arbelo 2014).
Endocardial catheter‐based techniques for atrial fibrillation ablation initially used radiofrequency energy sources. Newer energy sources have now evolved, which include cryoenergy, laser, and high frequency ultrasound (Cappato 2010). Surgical techniques, such as the epicardial approach, as well as hybrid surgical and endocardial techniques previously involved the Cox maze procedure but now increasingly utilise radiofrequency energy or cryoablation, either intraoperatively during open surgery or via an epicardial approach. Some of these techniques have been assessed in either observational studies or randomised trials (Calkins 2012).
How the intervention might work
Ablation to prevent atrial fibrillation is primarily based on electrical isolation of triggers, mainly premature atrial beats and atrial tachycardia arising from the pulmonary veins at the venous ostium or around the antral area of the veins (Haïssaguerre 1998). Pulmonary vein isolation is therefore the mainstay of therapy. While pulmonary vein isolation is effective in people with paroxysmal atrial fibrillation, it is less effective in people with non‐paroxysmal atrial fibrillation, and therefore a variety of complementary ablation targets have been investigated including lines, CFAE mapping, and rotors to increase the success of catheter ablation of atrial fibrillation (Andrade 2012; Narayan 2012). These ablation strategies are thought to either compartmentalise the atria or reduce the critical mass of tissue required for maintenance of atrial fibrillation (lines), or they are thought to represent sites of atrial fibrillation rotors (CFAE). However, there is no robust evidence that adding other targets to pulmonary vein isolation is beneficial. Recently there have been developments in signals processing and mapping techniques to target rotors thought to be the extra‐pulmonary vein sources of atrial fibrillation maintenance (Narayan 2012). Other approaches have been reported in a few trials, including targeting of the cardiac autonomic system (ganglionated plexi ablation) and ablation of the ganglionic plexi alone or in conjunction with pulmonary vein isolation (Kottkamp 2015).
Why it is important to do this review
The best rhythm management strategy for people with non‐paroxysmal atrial fibrillation is currently not well defined. Antiarrhythmic drugs have been the mainstay of therapy, however a meta‐analysis of non‐randomised and randomised studies of all antiarrhythmic drugs showed an average success rate for prevention of atrial fibrillation recurrence of 52% over one year (Calkins 2009). In addition, antiarrhythmic drugs have serious side effects including ventricular arrhythmias and lung disease (Singh 2005). Non‐pharmacological interventions (catheter and surgical ablation techniques) have been developed as alternatives to maintain sinus rhythm in people with atrial fibrillation. Several international society practice guidelines recommend both antiarrhythmic drugs as well as radiofrequency catheter ablation (RFCA) as acceptable options for rhythm control in people with atrial fibrillation (Calkins 2012; Camm 2012). However, there has been a tremendous upsurge in the use of RFCA, driven by the idea that it is a better therapy and that it might change the natural history of the disease. This has the potential to have a significant impact on health systems worldwide (Kneeland 2009; Kumar 2013).
Antiarrythmic drugs are perceived to be a less acceptable therapeutic option, despite being more readily available and cheaper, and possibly being more effective in particular groups of people with atrial fibrillation (Kumar 2013). With the diversification of atrial fibrillation ablation techniques, an analysis of efficacy outcomes and safety is critical to inform the field and help identify optimal treatment strategies. Several systematic reviews have been conducted over recent years, but these have concentrated mainly on paroxysmal atrial fibrillation (Cheng 2014; Khan 2014; Nault 2010). When non‐paroxysmal atrial fibrillation has been the focus of attention, reviews have included non‐randomised studies and case series, with largely inconclusive results (Calkins 2009). In addition, none of the previous reviews have used state‐of‐the‐art systematic review methods, such as those implemented by Cochrane. Therefore, there is a need for a de novo systematic review using Cochrane recommended methods to evaluate the efficacy and safety of ablation (catheter and surgical) versus antiarrhythmic drugs in non‐paroxysmal atrial fibrillation. This will help to inform the adoption of an optimal treatment strategy.
Objectives
To determine the efficacy and safety of ablation (catheter and surgical) in people with non‐paroxysmal (persistent or long‐standing persistent) atrial fibrillation compared to antiarrhythmic drugs.
Methods
Criteria for considering studies for this review
Types of studies
We included only randomised trials of parallel‐group design with the individual as the unit of randomisation. All studies had at least 12 months of follow‐up.
Types of participants
We included three studies with adults aged 18 years and over with persistent atrial fibrillation (defined as lasting more than seven days or requiring termination by cardioversion either with drugs or by direct current cardioversion) or long‐standing persistent atrial fibrillation (defined as lasting more than one year when it is decided to adopt a rhythm control intervention), regardless of any concomitant underlying heart disease. Where studies had a mixed population, at least 50% of participants should have had either persistent or long‐standing persistent atrial fibrillation (Forleo 2009). If studies had 50% or more participants with paroxysmal atrial fibrillation, we contacted the authors to obtain information on the participants with only non‐paroxysmal atrial fibrillation (Stabile 2006).
Types of interventions
We included trials using the radiofrequency catheter ablation (RFCA) technique. The comparison was approved antiarrhythmic drugs, which includes any of the following: flecainide, propafenone, quinidine, amiodarone, sotalol, dofetilide, or dronedarone.
We excluded all studies where the comparator was rate control and excluded concomitant surgical ablation studies (that is, surgical atrial fibrillation ablation done during open heart surgery for another indication or condition).
Types of outcome measures
We defined outcome measures according to a recent consensus statement regarding randomised trials in atrial fibrillation. Where atrial fibrillation was defined as a common supraventricular arrhythmia that is characterised by chaotic contraction of the atrium, needing an electrocardiogram (ECG) recording for its diagnosis (Calkins 2012). We evaluated the following outcomes at 12 months and for the longest term available.
Primary outcomes
Freedom from atrial arrhythmias (i.e. atrial fibrillation, atrial flutter, or atrial tachycardia) or recurrence of any atrial arrhythmias
Participants needing cardioversion
Cardiac hospitalisation
Secondary outcomes
All‐cause mortality
Fatal or non‐fatal stroke
Any embolic complication
Combined endpoint of any major adverse cardiac event (MACE)
Significant bradycardia or need for a pacemaker
Health‐related quality of life measured by a validated scale
Cost
Periprocedural complications and other safety outcomes
Periprocedural complications and other safety outcomes here refers to adverse events and/or complications arising from ablation e.g. pericarditis, pericardial effusion, minor vascular access complications.
Search methods for identification of studies
Electronic searches
We searched the following sources from their inception. Which also includes the year the first ablation procedure was performed to the specified date, and placed no restrictions on language of publication.
CENTRAL; Issue 2 of 12, March 2016 (the Cochrane Library)
MEDLINE (OVID 1946 to February week 4 2016)
EMBASE (OVID, 1980 to 2016 week 09)
Health Technology Assessment Database; Issue 1 of 4, January 2016
Conference Proceedings Citation Index‐Science (CPCI‐S); 1990 to present (Web of Science)
ClinicalTrials.gov (clinicaltrials.gov; searched 1 April 2016)
World Health Organization International Clinical Trials Registry Platform (who.int/ictrp/en; searched 3 March 2016)
We adapted the preliminary search strategy for MEDLINE (Ovid) for use in the other databases. We applied the Cochrane sensitivity‐maximising RCT filter to MEDLINE (Ovid) and adapted it to the other databases (Lefebvre 2011), except CENTRAL. For details of terms used in search strategies please see Appendix 1.
Searching other resources
We identified other potentially eligible trials or ancillary publications by handsearching the reference lists of retrieved included trials, systematic reviews, meta‐analyses, and health technology assessment reports. We also contacted study authors of included or registered trials to identify any further studies we may have missed.
Data collection and analysis
Selection of studies
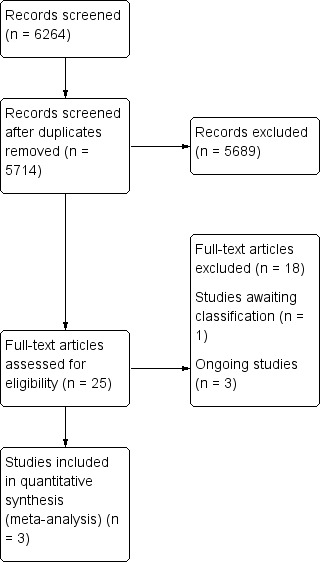
Two review authors (JN, OO) independently screened titles and abstracts for inclusion of all the potential studies. We retrieved the full‐text study reports/publication and three authors (JN, OO and AJA) independently screened the full‐text and identified studies for inclusion; any disagreement was resolved with consultation between the other review authors (GA, CAM and JPC). We have presented a PRISMA flow diagram showing the process of study selection (Figure 1).
1.
Study flow diagram.
Data extraction and management
We extracted the following study characteristics.
Methods: study design, study duration, length of follow‐up, details of any 'run in' period, number of study centres and location, study setting, withdrawals, and date of study.
Participants: number, mean age, age range and standard deviation (SD), gender, severity of condition, diagnostic criteria, smoking history, underlying heart disease conditions, left atrial size (mean and SD) proportion of normal/abnormal, duration of atrial fibrillation (mean and SD), inclusion criteria, and exclusion criteria.
Interventions: type of ablation and technique used, comparisons, concomitant medications, and excluded medications.
Outcomes: primary and secondary outcomes specified and collected, and time points reported. We extracted both numbers of events and means as well as estimated effect sizes and 95% confidence intervals (CIs).
Notes: funding for trial, and notable conflicts of interest of trial authors.
For studies that met our inclusion criteria, two review authors (JN, OO) independently extracted data from the trials and transferred the data into a pro forma with any disagreements resolved by discussion, by consultation with a third review author (GA), or, when required, by contacting authors of included studies. We tried to find the protocol of each included study and report primary, secondary, and other outcomes in comparison with data in publications.
Assessment of risk of bias in included studies
Two review authors (JN, OO) independently assessed risk of bias for each included study. We resolved disagreements by consultation with a third review author (GA or JPC) or by general consensus. We applied the Cochrane 'Risk of bias' tool to the following domains.
Random sequence generation.
Allocation concealment.
Blinding of participants and personnel.
Blinding of outcome assessment.
Incomplete outcome data.
Selective outcome reporting.
Other bias (e.g. industry funding).
We judged each potential source of bias as 'high', 'low' or 'unclear' and provided quote(s) from the study report together with justification(s) for our judgement in the 'Risk of bias' table. We summarised the 'Risk of bias' judgements across different studies for each of the domains listed. When considering the treatment effects, we took into account the risk of bias for the studies that contributed to that outcome. We considered the implications of missing outcome data from individual participants per outcome, such as high dropout rates (for example, above 15%) or disparate attrition rates (for example, a difference of 10% or more between study arms).
Measures of treatment effect
We expressed dichotomous outcome data as risk ratios (RRs) with 95% CIs. We analysed all included studies using intention‐to‐treat analyses.
Unit of analysis issues
All included trials were randomised at the individual participant level.
Dealing with missing data
We contacted authors of included studies to obtain missing numerical outcome data and to verify key study characteristics, where possible. Where this was not possible, and the missing data were thought to introduce serious bias, we considered exploring the impact of including such studies in the overall assessment of results by conducting sensitivity analyses. We also obtained information from trial registries. For trials where more than 50% of participants had paroxsymal atrial fibrillation, we contacted the trial authors to obtain data on non‐paroxsymal participants. We then included the data obtained in our analyses.
Assessment of heterogeneity
We identified heterogeneity through visual inspection of the forest plots and by using a standard Chi² test with a significance level of α = 0.1. We also use the I² statistic to quantify the heterogeneity across trials. We attempted to determine possible reasons for heterogeneity by examining individual studies and subgroup characteristics.
Assessment of reporting biases
We were unable to assess small‐study bias as the number of included studies was not sufficient for an informative funnel plot (Higgins 2011).
Data synthesis
We undertook meta‐analyses if the participants, interventions, and the comparisons were similar enough for pooling to be appropriate (Wood 2008). If I² is less than or equal to 40%, we used a fixed‐effect model, whereas if the I² statistic was greater than 40%, we used both the fixed‐effect and random‐effects model (Higgins 2011), but reported results from the random‐effects model.
The quality of evidence was evaluated using the GRADE approach (Higgins 2011) and the GRADE profiler (GRADEPRO) 3.6 (GRADEpro GDT) was employed to import data from Review Manager 5.3 (RevMan 2014) to create 'Summary of findings' table (Table 1). Outcomes reported in the summary of findings table include:
Freedom from atrial arrhythmia
Participants needing cardioversion
Cardiac hospitalisation
significant bradycardia or need for a pacemaker
Periprocedural complications and other safety outcomes
Subgroup analysis and investigation of heterogeneity
We planned on conducting a subgroup analysis, however due to the small number of included studies we were unable to do so.
Sensitivity analysis
We planned on conducting a sensitivity analysis, however due to the small number of included studies we were unable to do so.
Results
Description of studies
Results of the search
Appendix 1 outlines the search strategies, and Figure 1 includes the PRISMA flow chart depicting numbers of included and excluded studies. After de‐duplication, the search resulted in 5714 results, of which we excluded 5689 records as they were not relevant to our review question. We assessed 25 full‐text articles for eligibility. Five out of these 25 studies had a mix of atrial fibrillation participants, with more than 50% having paroxysmal atrial fibrillation. We contacted authors for data on non‐paroxysmal atrial fibrillation cases. We only received information from the Stabile 2006 trial. We excluded 18 studies and reasons for full‐text exclusion are shown in Characteristics of excluded studies.
We also identified one study awaiting classification (NCT00821353), and three ongoing studies (NCT00196209; NCT00911508; NCT01420393). The study awaiting classification compares radiofrequency catheter ablation with rhythm control in participants with hypertrophic cardiomyopathy and paroxymal or chronic atrial fibrillation (NCT00821353). This trial was yet to be published when this review was developed, with no study result posted. Details are outlined in the Characteristics of studies awaiting classification section. Although we identified three ongoing studies, the comparison arm used in NCT00196209 and NCT01420393 was not antiarrhythmic drugs. NCT01420393 compared ablation versus rate control, while NCT00196209 compared catheter ablation versus external electric cardioversion. NCT00911508 compared rate control or rhythm control drug therapy for atrial fibrillation to catheter ablation. Details are outlined in the Characteristics of ongoing studies section.
A total of three studies were suitable for inclusion.
Included studies
A summary description of studies included is reported in Characteristics of included studies. Studies were published between 2006 and 2014. Of the three randomised trials included (Forleo 2009; Mont 2014; Stabile 2006), one was conducted in Spain (Mont 2014), and two in Italy (Stabile 2006; Forleo 2009). With the exception of Forleo 2009, all studies were prospectively registered (ClinicalTrials.gov identifier: NCT00227344 and NCT00863213). The total number of participants included was 261 (mean age: 60 years) comparing radiofrequency catheter ablation (RFCA) (159 participants) with antiarrhythmic drugs (102). Though we set out to include trials with outcomes evaluated at 12 months or for the longest term available, most trials reported a follow‐up of 12 months, except Stabile 2006 that reported a median of 18 months of follow‐up. The majority of participants recruited were male, with the percentage of women ranging from 11.6% to 40.9%. All trials included participants that have not responded to antiarrhythmic drug therapy. Further details of included studies and the characteristics of participants included in the studies are described in Table 2 and Table 3, respectively.
1. Further details of included studies.
| Study Name | Forleo 2009 | Mont 2014 | Stabile 20061 |
| Study period | January 2005‐September 2006 | May 2009‐November 2011 | February 2002‐June 2003 |
|
No. participants per arm (Intervention/comparator) |
35 / 35 | 98 / 48 | 26 / 19 |
| Average follow‐up (months) | 12 | 12 | 18 |
|
No. participants lost‐to‐follow up (intervention/comparator) |
0 / 0 | 3 / 0 | This information was not available for the sub‐group with persistent AF. |
|
% participants with paroxysmal atrial fibrillation (intervention/comparator) |
37 / 46 | 0 / 0 | 0 / 0 Though the original trial included paroxysmal atrial fibrillation, this reported only analysed persistent atrial fibrillation |
| Interventions postrandomisation and before Ablation | AADs were not suspended before the ablation. | AAD were discontinued ≥5 half‐life periods (or ≥1 week for amiodarone) before ablation. | Not described. |
|
Type of ablation (Surgical vs radiofrequency catheter) |
Radiofrequency Catheter | Radiofrequency Catheter | Radiofrequency Catheter |
| Ablation technique | Pulmonary vein isolation, segmental ostial + left atrial linear lesion (roof line, mitral isthmus) + CFAE ablation. | Pulmonary vein isolation, circumferential ablation ± cavo‐tricuspid isthmus ± left atrial linear lesion ± CFAE ablation | Pulmonary vein isolation, circumferential ablation, plus left atrial linear lesion ± cavo‐tricuspid isthmus. |
| Use of AADs posterior to ablation | Participants were discharged on AAD. Discontinuation of AADs was complete within 1 month in participants without structural heart disease and up to 3 months in the remaining participants. |
AADs for 3 months (blanking period) | AADs were given for the whole duration of the study. Participants were preferentially on amiodarone. In participants with history of intolerance to amiodarone, a class IC antiarrhythmic was administered. The final decision was left to the physician. |
| Comparator arm | ADT at maximum tolerable dose either as single or combination. The recommended regimen was: oral flecainide 100 mg e/12 hours, oral propafenone (150–300 mg) 3 times daily, oral sotalol at an initial dose of 80 mg three times daily, and oral amiodarone 600 mg/day for 2 weeks, 400 mg/day for the next 2 weeks, and 200 mg daily thereafter. In participants with persistent atrial fibrillation, cardioversion was performed under a new ADT to maintain the sinus rhythm. |
Discontinuation of the AADs was not required before inclusion in the ADT group. Participants were treated depending on physician’s choice and according to current guidelines. There was not predefined protocol on the use of ADT during the blanking period. |
The antiarrhythmic drug preferentially administered was amiodarone. In participants with history of intolerance to amiodarone, a class IC antiarrhythmic was administered. The final decision was left to the physician. |
AAD: antiarrhythmic drugs; ADT: antiarrhythmic drug therapy; CFAE: complex fractionated atrial electrograms
1 Stabile 2006: only participants with persistent atrial fibrillation were included in the analysis.
2. Characteristics of participants included in the studies.
| Study Name | Forleo 2009 | Mont 2014 | Stabile 2006 |
|
Mean Age (years) (intervention/comparison) |
63.2 / 64.8 | 55 / 55 | 62.2 / 62.3 |
| % of women | 38.6 | 22.6 | 40.9 |
| Selection criteria atrial fibrilation‐related | Symptomatic paroxysmal or persistent atrial fibrillation for ≥6 months | Symptomatic persistent atrial fibrillation: >7 or <7 days requiring electrical or pharmacological cardioversion. Participants with long‐standing persistent atrial fibrillation were excluded. | Persistent atrial fibrillation: occurrence in the previous 12 months of ≥2 episodes of atrial fibrillation, each lasting > 7 days before being terminated, or lasting less than 7 days but necessitating early cardioversion. In all participants, the first diagnosis of atrial fibrillation had been made at least 6 months before enrolment |
| History of AADs | Participants had to be refractory to ≥1 class 1–3 AADs. | Participants had to be refractory to at least one class I or class III AADs. | Participants had to be intolerant to AADs or in whom two or more AADs regimens had failed. |
|
Atrial fibrillation History (years) (intervention/comparison) |
3.4 / 3 | N.R | 5.1 / 7.1 |
|
Mean LA size (mm) (intervention/comparison) |
44.3 / 45.2 | 41.3 / 42.7 | 46 / 45.4 |
|
Mean LVEF (%) (intervention/comparison) |
54.6 / 52.6 | 61.1 / 60.8 | 59.1 / 57.9 |
|
% any CV co‐morbidity[1] (intervention/comparison) |
45.7 / 54.3 | 10 / 8 | 63.2 / 62.3 |
% CV co‐morbidities: Oral refers to Nonischemic cardiomyopathy, coronary artery disease, valvular heart disease and congenital heart disease. Forleo refers to structural heart disease (CHD, dilated cardiomyopathy, valve disease and previous embolic episodes). Mont refers to TIA, Stroke, peripheral embolism and ischaemic cardiomyopathy. Stabile refers to heart disease. NR, not reported
In all three trials, ablation was through radiofrequency to isolate the pulmonary veins and details of the specific technique used in the three trials are described in Table 2. The need for a second ablation (due to recurrent atrial fibrillation or flutter within the blanking period) was reported in 8.2% of the participants in Mont 2014. In all trials, the ablation group also received antiarrhythmic drugs mainly during the blanking period (range: 1 to 3 months after ablation) with the exception of Stabile 2006, where the antiarrhythmic drugs were used throughout the duration of the study (Personal communication from Study authors Bertaglia 2015 [pers comm]). In the antiarrhythmic drug arm (comparison), the decision on the specific antiarrhythmic drug was based on recommended guidelines or physician preference with amiodarone (Stabile 2006: 66%; Forleo 2009: 63%; and Mont 2014: 46%) being the most commonly used antiarrhythmic drug. Full details of interventions can be found in Table 2.
Excluded studies
We excluded 18 studies on second pass and 5711 studies in total (Figure 1). Reasons for exclusion were mainly due to studies not addressing prespecified population, intervention, and comparison characteristics. Excluded studies were either on paroxysmal atrial fibrillation, rate control, or on concomitant surgical ablation studies. For studies with a mixed population of atrial fibrillation, with more than 50% having paroxysmal atrial fibrillation, we contacted authors for data on only non‐paroxysmal atrial fibrillation cases. If data were not provided as requested, we excluded these studies. Full details of 18 studies excluded on second pass can be found in Characteristics of excluded studies.
Risk of bias in included studies
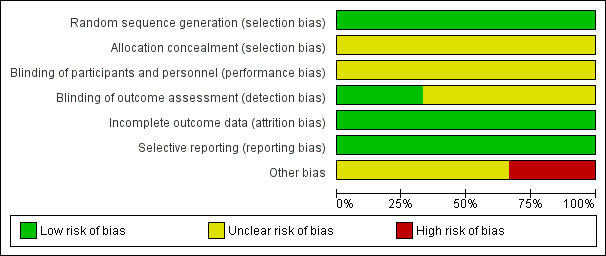
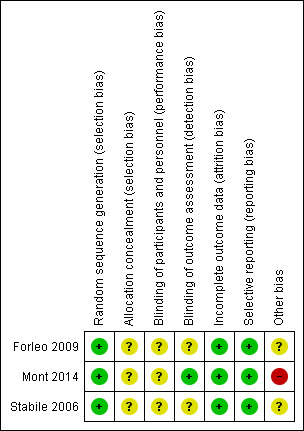
Figure 2 and Figure 3 demonstrate the overall and trial specific information on risk of bias.
2.
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
The risk of bias was low for sequence generation, and unclear for allocation concealment in all studies. We judged allocation to be unclear because information from study authors was not available to clarify the allocation.
Blinding
Given the nature of the intervention, blinding of participants and personnel was not possible. This makes it difficult to judge the direction of effect due to blinding. Therefore, we considered the risk of performance bias to be unclear. However, blinding of outcome assessment was unclear for two of the included studies ( Forleo 2009 and Stabile 2006) and low for Mont 2014.
Incomplete outcome data
Regarding, Intention‐to‐treat used, attrition bias, and losses to follow‐up, we judged all trials to be at low risk.
Selective reporting
We judged all three trials to be at low risk of outcome reporting bias for our primary outcomes. We judged two studies at low risk for all secondary outcomes (Mont 2014; Stabile 2006).
Other potential sources of bias
Regarding other potential biases, Mont 2014 was terminated before reaching the planned sample size due to a lower than expected recruitment rate, resulting in a loss of statistical power. However, the study authors claim that the difference between groups in the primary endpoint was higher than assumed in the sample size calculation, which likely compensated for the loss of statistical power in the sample size. Apart from the Mont 2014 trial, no other study was sponsored by industry (Medtronic and Biosense Webster). One of the investigators from Forleo 2009 reported to have received lecture fees from Industry.
Effects of interventions
See: Table 1
The main result findings are reported in Table 1. Analysis 1.1, Analysis 1.2, Analysis 1.3, Analysis 1.4, Analysis 1.5, Analysis 1.6, and Analysis 1.7 describe the forest plots for the efficacy and safety of ablation for people with non‐paroxysmal atrial fibrillation for various outcomes.
1.1. Analysis.
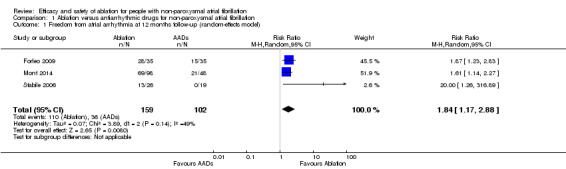
Comparison 1 Ablation versus antiarrhythmic drugs for non‐paroxysmal atrial fibrillation, Outcome 1 Freedom from atrial arrhythmia at 12 months follow‐up (random‐effects model).
1.2. Analysis.
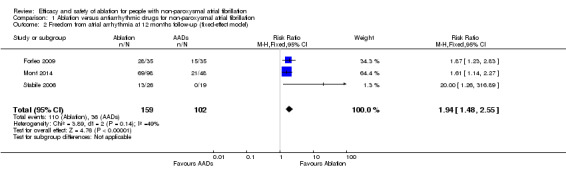
Comparison 1 Ablation versus antiarrhythmic drugs for non‐paroxysmal atrial fibrillation, Outcome 2 Freedom from atrial arrhythmia at 12 months follow‐up (fixed‐effect model).
1.3. Analysis.
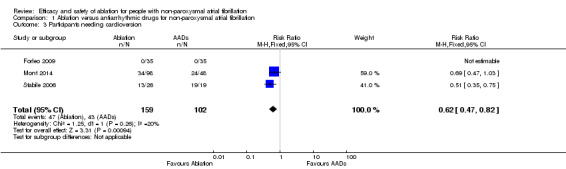
Comparison 1 Ablation versus antiarrhythmic drugs for non‐paroxysmal atrial fibrillation, Outcome 3 Participants needing cardioversion.
1.4. Analysis.
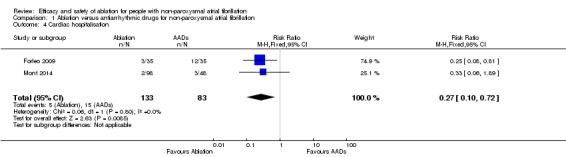
Comparison 1 Ablation versus antiarrhythmic drugs for non‐paroxysmal atrial fibrillation, Outcome 4 Cardiac hospitalisation.
1.5. Analysis.
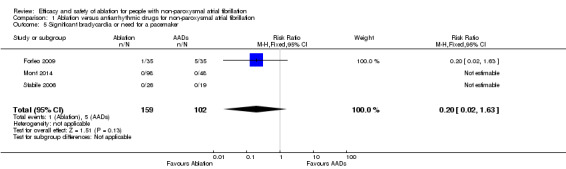
Comparison 1 Ablation versus antiarrhythmic drugs for non‐paroxysmal atrial fibrillation, Outcome 5 Significant bradycardia or need for a pacemaker.
1.6. Analysis.
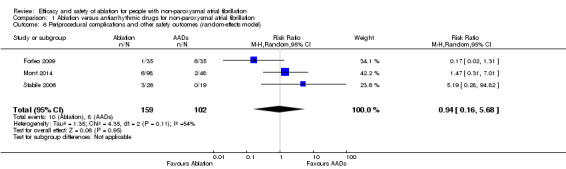
Comparison 1 Ablation versus antiarrhythmic drugs for non‐paroxysmal atrial fibrillation, Outcome 6 Periprocedural complications and other safety outcomes (random‐effects model).
1.7. Analysis.
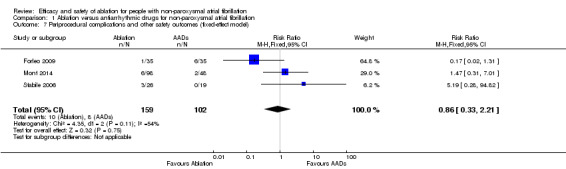
Comparison 1 Ablation versus antiarrhythmic drugs for non‐paroxysmal atrial fibrillation, Outcome 7 Periprocedural complications and other safety outcomes (fixed‐effect model).
Primary outcomes
Freedom from atrial arrhythmias or recurrence of any atrial arrhythmias
All three trials reported information on this outcome. All studies included a blanking period and any atrial fibrillation or flutter detected during this period was not included in the analysis. The definition of atrial arrhythmias, mode of ascertainment, and frequency of evaluation to detect an atrial arrhythmia varied considerably by study (details are reported in Table 4). After pooling data, radiofrequency catheter ablation (RFCA) increased freedom from atrial arrhythmias at 12 months compared with antiarrhythmic drugs (risk ratio (RR) 1.84, 95% confidence interval (CI) 1.17 to 2.88; 3 studies, 261 participants; low‐quality evidence) (Analysis 1.1). We judged the quality of evidence as low as a result of unexplained heterogeneity and imprecision due to small event rates compared to total participants (Table 1).
3. Study characteristics regarding the ascertainment of their primary outcome ‐ freedom from atrial arrhythmias.
| Study name | Forleo 2009 | Mont 2014 | Stabile 2006 |
| Outcome definition | Time to the first atrial fibrillation (or atypical flutter) recurrence after 5 weeks and within 12 months after randomisation. | Any episode of atrial fibrillation or flutter lasting > 24 hours or requiring cardioversion after a 3‐month blanking period. | Absence of any recurrence of atrial arrhythmias (atrial fibrillation or flutter) lasting > 30 seconds in the 1‐year follow‐up, after the 1‐month blanking period. |
| Censoring | Participants were censored after first atrial fibrillation recurrence. | Participants were censored after first atrial fibrillation recurrence. | Participants were censored after first occurrence of atrial arrhythmias (atrial fibrillation or flutter). |
| Definition of atrial arrhythmias (primary outcome) | Electrocardiographically‐confirmed episode of atrial fibrillation or atypical flutter had to last "> 30 seconds". | Atrial fibrillation or flutter lasting > 24 hours or requiring cardioversion. In cases where the Holter recorded atrial fibrillation < 24 hours, symptoms were taken into consideration. |
Atrial arrhythmias lasting > 30 seconds. |
| Blanking period | 5 weeks. | 3 months. | 1 month. |
| Mode of ascertainment | Pulse evaluation confirmed by ECG when any arrhythmia was suspected and Holter monitoring. | A 24‐hour Holter monitor. | Transtelephonic ECG recording (Life watch monitor) and Holter monitoring. |
| Frequency of ascertainment | Pulse: regularly. Holter: during visits a 1, 3, 6, 9 and 12 months. |
Holter: 6 and 12 months. | Transtelephonic ECG: daily for 3 months and whenever they had palpitations. Holter: 1, 4, 7, 10, and 13 months. |
ECG: electrocardiogram
Participants needing cardioversion
All three studies reported information on this outcome. However, Forleo 2009 reported zero participants needing cardioversion after the blanking period in both arms. Only the event data from Mont 2014 and Stabile 2006 contributed to the meta‐analysis. After pooling data from these studies, participants randomised to RFCA had a reduced risk of needing cardioversion (RR 0.62, 95% CI 0.47 to 0.82); I2 = 20%, 3 studies, 261 participants; moderate‐quality evidence) (Analysis 1.3). As a result of imprecision due to small event rates compared to total participants, we judged the quality of evidence to be of moderate‐quality (Table 1) .
Cardiac hospitalisation
Forleo 2009 and Mont 2014 provided the event data for cardiac hospitalisation and result findings showed evidence of catheter ablation reducing the risk of cardiac hospitalisation (RR 0.27, 95% CI 0.10 to 0.72; I2 = 0%, 2 studies, 216 participants; low‐quality evidence) (Analysis 1.4). However, Mont 2014 only reported on atrial fibrilation‐related hospitalisations. Stabile 2006, when contacted (Bertaglia 2015 [pers comm]), reported no data specifically on cardiac hospitalisations, but only data on all hospitalisations as follows: ablation median of 1 (interquartile range (IQR): 1, 2) and antiarrhythmic drug arm median of 2 (IQR: 1, 2), this includes the hospitalisations required for ablation. Thus, we judged data from Stabile 2006 not suitable for meta‐analysis. As a result of significant imprecision due to small event rates compared to total participants, we judged the quality of evidence to be low (Table 1).
Secondary outcomes
Significant bradycardia or need for a pacemaker
All three studies reported on this outcome. However studies by Mont 2014 and Stabile 2006 reported zero events in either arm. Only the event data from Forleo 2009 contributed to the meta‐analysis. Result findings showed substantial uncertainty surrounding the summary estimate (RR 0.20, 95% CI 0.02 to 1.63; 3 studies, 261 participants; low‐quality evidence) (Analysis 1.5). As a result of imprecision with confidence intervals crossing the 'no effect' line, we judged the quality of evidence to be low (Table 1).
Periprocedural complications and other safety outcomes
All three studies reported event data on this outcome. Periprocedural complications and other safety outcomes reported were adverse events and/or complications arising from ablation, e.g. pericarditis, pericardial effusion, and minor vascular access complications. Result findings showed that RFCA showed no effect with periprocedural complications and other safety outcomes compared with antiarrhythmic drugs (RR 0.94, 95% CI 0.16 to 5.68; 3 studies, 261 participants; very low‐quality evidence) (Analysis 1.6). As a result of imprecision due to small event rates with confidence intervals crossing the 'no effect' line, and unexplained heterogeneity, we judged the quality of evidence to be very low (Table 1).
All‐cause mortality
Two trials reported all‐cause mortality. In the Mont 2014 study, authors reported that no death was observed in either arm after 12 months of follow‐up, while the cause of death in Stabile 2006 was gastrointestinal haemorrhage (information provided by the authors). Given the extremely low number of events (N = 1 for Stabile 2006) and the absence of events in the comparison arm, we decided not to pool the results for this outcome.
Fatal or non‐fatal stroke
Information on stroke was reported in two studies (Mont 2014; Stabile 2006), and they both reported zero stroke events in either arm.
Any embolic complication
All three included studies reported zero embolic complications in either arm (Forleo 2009; Mont 2014; Stabile 2006).
Combined endpoint of any major adverse cardiac event (MACE)
None of the included studies reported on the combined endpoint of MACE.
Health‐related quality of life
All studies, except Stabile 2006 reported this outcome, but used different tools. In Forleo 2009, the information reported was not suitable for meta‐analysis and not available from the study authors. Forleo 2009 used the Medical Outcomes Study 36‐item short‐form health survey (SF‐36) to evaluate quality of life and reported improvements in the mean change in quality of life scores in the ablation arm compared with the mean change in quality of life scores in the antiarrhythmic drug arm for five out of eight SF‐36 subscales. However, this was only reported as "P < 0.05, PVI versus ADT group" which is insufficient for meta‐analysis. Mont 2014 used an atrial fibrillation‐quality of life questionnaire and authors reported no difference in the global score of quality of life between the two arms at six months (5.5, 95% CI ‐2.3 to 13.4) or 12 months (3.8, 95% CI ‐5.2 to 12.8). Likewise, no differences were observed for the physical, psychological, and sexual domains.
Cost
None of the three included trials reported on cost. However, screening identified one cost‐effectiveness study from the perspective of the UK National Health Service (NHS) (McKenna 2008). This study examined the cost‐effectiveness of RFCA compared with antiarrhythmic drugs in adults with paroxysmal atrial fibrillation predominantly refractory to at least one previous antiarrhythmic drug. The antiarrhythmic drug considered was amiodarone. The efficacy of the intervention included in the cost‐effectiveness models was derived from trials where the majority of the participants had paroxysmal atrial fibrillation, therefore the findings from this analysis are not applicable to people with non‐paroxysmal atrial fibrillation.
Discussion
Summary of main results
The main findings of this systematic review and meta‐analysis in people with non‐paroxysmal atrial fibrillation who have not responded to antiarrhythmic drug therapy suggest that radiofrequency catheter ablation (RFCA) is superior to antiarrhythmic drugs in achieving freedom from atrial arrhythmias, reducing the need for cardioversion, and reducing cardiac hospitalisation at 12 months. There was substantial uncertainty surrounding the effect of RFCA on significant bradycardia (or need for a pacemaker) and no effect on total mortality, stroke, embolic complications, or any major adverse cardiac event. Result findings should be interpreted with caution, as the quality of the evidence was at the very best moderate, mainly due to extremely low numbers of outcomes in the pooled analysis together with substantial heterogeneity within included studies (Table 1).
Overall completeness and applicability of evidence
Despite the widespread use of RFCA as treatment for non‐paroxysmal atrial fibrillation, only three trials with 261 participants that fulfilled the inclusion criteria were eligible. Most of the studies were performed before the definition of 'long‐standing persistent' was introduced. The Mont 2014 study excluded participants with long‐standing persistent atrial fibrillation, while the other two included studies had a mixed population of persistent and long‐standing persistent atrial fibrillation (Forleo 2009; Stabile 2006), without the ability to differentiate the two. All studies were conducted in high‐income countries, and due to strict selection criteria of participants included in these studies, the applicability of this evidence to certain groups is limited. These groups are women, elderly (> 70 years), people with comorbidities, and people naive to antiarrhythmic drugs. Likewise, it is important to note that (with the exception of Mont 2014) included studies were designed and started recruitment more than 10 years ago, and all use a single source of energy, namely RFCA. Not a single surgical ablation trial was eligible, and therefore, although aiming to broaden the evidence, this systematic review and meta‐analysis only compared RFCA with antiarrhythmic drugs. Furthermore, novel technologies such as contact force catheters were not included. Evidence should be interpreted with caution, as event rates were low across reported outcomes with the quality of evidence ranging from moderate to very low.
Quality of the evidence
The quality of evidence, using the GRADE approach and GRADEpro (GRADEpro GDT), for efficacy and safety of ablation for people with non‐paroxysmal atrial fibrillation versus antiarrhythmic drugs is reported in Table 1. The quality ranged from moderate to very low across the different outcomes. This was mainly due to moderate to substantial heterogeneity and imprecise results due to extremely low numbers of events for outcomes analysed.
It is important to highlight certain limitations in terms of design of the included trials. The definitions of their primary outcome (freedom from atrial arrhythmias) including the frequencies and monitoring strategies to detect atrial fibrillation or flutter varied for the three included trials (Table 4). Two of the included trials (Forleo 2009; Stabile 2006), predated the current monitoring and atrial fibrillation recurrence recommendations suggested when conducting randomised trials evaluating the efficacy of RFCA (Calkins 2012). This could have led to under‐reporting of arrhythmia recurrence, though unlikely to introduce systematic bias (due to randomised design) this might have reduced statistical power in included studies. Moreover, there was no information on the recurrence of atrial fibrillation according to whether or not these were symptomatic. These issues highlight the need for adherence to internationally agreed definitions of atrial fibrillation ablation success. A further limitation of these studies is that efficacy of persistent ablation remains suboptimal for a single procedure, averaging about 50% maintenance of sinus rhythm. This limits the ability of many studies to fully establish the procedures' efficacy due to the fact that at least 30% require a repeat intervention to maintain sinus rhythm, with the cumulative risk of complications and additional hospitalisations, plus the lag in ensuing follow‐up. Most studies have only objectively evaluated the participants in the one‐year window postprocedure, when further intervention means that very often they may be in the first three‐ to six‐month follow‐up phase of the second procedure. This makes evaluating the full balanced comparisons of outcomes challenging.
Potential biases in the review process
We followed the methods as outlined in the published Cochrane protocol (Amit 2016). The methodological and search strategies were rigorous and comprehensive and we consider it unlikely that we missed substantial trials. In order to minimise the consequences of reporting bias, we contacted study authors, asking for necessary information when this was, either, not available or inadequately reported. By applying this strategy we obtained unpublished data from the Stabile 2006 study relevant only to non‐paroxsymal atrial fibrillation participants for both primary and secondary outcomes. With the exception of the outcomes not reported in the Table 1, all analysed primary and secondary outcomes in this review were adequately reported by all identified trials.
Agreements and disagreements with other studies or reviews
Current practice guidelines vary on their recommendations for RFCA in people with non‐paroxsymal atrial fibrillation; the European Society of Cardiology (ESC) guideline recommendation class for persistent symptomatic atrial fibrillation that is refractory to antiarrhythmic drugs is IIa; this was not updated in the 2012 ESC guidelines for the management of atrial fibrillation (Camm 2012). The recent Wynn 2014 systematic review and meta‐analysis that included both randomised and non‐randomised trials, reported a benefit for RFCA of reducing atrial fibrillation recurrence (odds ratio (OR) 0.32, 95% confidence interval (CI) 0.20 to 0.53). Our findings are in agreement with previous reviews, although reporting a more modest effect in favour of RFCA regarding atrial fibrillation recurrence. In contrast to the Wynn 2014 review, we expanded the coverage of clinical outcomes and observed a significant reduction in the need for cardioversion and hospitalisation, which may be relevant in terms of reducing health resource utilisation. However, the importance of these findings is weakened by the overall quality of the evidence; moderate‐quality for participants needing cardioversion and low‐quality for hospitalisation. Additional outcomes included in this review, not covered by previous systematic reviews, were total mortality, stroke, embolic complications, and any major adverse cardiac event (MACE). Unfortunately the number of events in included trials was too low to preclude any reliable conclusion on the effects that RFCA may have on these outcomes.
Authors' conclusions
Implications for practice.
The available evidence suggests that radiofrequency catheter ablation (RFCA) is effective in restoring and maintaining sinus rhythm as well as reducing both cardioversion and cardiac hospital admissions in younger people (mean age 60 years) with primarily non‐paroxysmal atrial fibrillation who have not responded to antiarrhythmic drug therapy with 12 months follow‐up. However, quality of the evidence was moderate at the very best. Current practice suggests that RFCA is being recommended in this younger population, despite lack of strong evidence (Cappato 2010). Personal choice, benefit and risk, supported by an atrial fibrillation heart team should be considered, also bearing in mind the stated limitations with included studies and the quality of reported outcomes. Further high‐quality research is needed to improve the selection of people that will benefit the most from RFCA.
Implications for research.
Based on the quality of the evidence reported, moderate‐quality at the very best, it is very likely that further adequately powered and high‐quality randomised trials will have an impact on our confidence in the current estimates of effect that RFCA has on people with non‐paroxsymal atrial fibrillation. Key characteristics of these high‐quality randomised trials should include standardised methods for monitoring rhythm, longer follow‐up, broader selection of participants (in particular at high risk of hard endpoints), larger sample size, use of stricter endpoints in terms of success of ablation, and use of validated quality of life instruments more suited to participants with atrial fibrillation. The impact of RFCA on health resource utilisation (cost‐effectiveness) also needs to be consistently captured in future randomised trials; we expect the ongoing CABANA trial to provide some information in this regard (NCT00911508).
Acknowledgements
The authors would like to thank Nicole Martin, Charlene Bridges, and Joanne Abbott for assistance with searches and obtaining articles. We are also extremely grateful to Dr Emanuele Bertaglia and Giuseppe Stabile for responding to our requests for data.
Appendices
Appendix 1. Search strategies
CENTRAL #1 MeSH descriptor: [Atrial Fibrillation] this term only #2 (atrial near/3 fibrillat*):ti,ab,kw (Word variations have been searched) #3 (auricular* near/3 fibrillat*):ti,ab,kw (Word variations have been searched) #4 (atrium near/3 fibrillat*):ti,ab,kw (Word variations have been searched) #5 atrial arrhythmi*:ti,ab,kw (Word variations have been searched) #6 #1 or #2 or #3 or #4 or #5 #7 MeSH descriptor: [Catheter Ablation] this term only #8 (catheter near/6 (ablat* or isolat*)):ti,ab,kw (Word variations have been searched) #9 (transcatheter and (ablat* or isolat*)):ti,ab,kw (Word variations have been searched) #10 ((surgical near/3 ablat*) or MAZE procedure):ti,ab,kw (Word variations have been searched) #11 #7 or #8 or #9 or #10 #12 #6 and #11
MEDLINE OVID 1. Atrial Fibrillation/ 2. (atrial adj3 fibrillat*).tw. 3. (auricular* adj3 fibrillat*).tw. 4. (atrium adj3 fibrillat*).tw. 5. atrial arrhythmi*.tw. 6. or/1‐5 7. Catheter Ablation/ 8. (catheter adj6 (ablat$ or isolat$)).tw. 9. (transcatheter and (ablat$ or isolat$)).tw. 10. ((surgical adj3 ablat$) or MAZE procedure).tw. 11. or/7‐10 12. 6 and 11 13. adverse effects.fs. 14. contraindications.fs. 15. poisoning.fs. 16. toxicity.fs. 17. drug effects.fs. 18. (toxi* adj2 (effect or effects or reaction* or event or events or outcome*)).tw. 19. (adverse* adj2 (effect or effects or reaction* or event or events or outcome*)).tw. 20. (side adj3 (effect or effects)).tw. 21. (adr or adrs).tw. 22. or/13‐21 23. exp animals/ not humans.sh. 24. 22 not 23 25. 12 and 24 26. randomized controlled trial.pt. 27. controlled clinical trial.pt. 28. randomized.ab. 29. placebo.ab. 30. drug therapy.fs. 31. randomly.ab. 32. trial.ab. 33. groups.ab. 34. 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 35. exp animals/ not humans.sh. 36. 34 not 35 37. 12 and 36
EMBASE OVID 1. heart atrium fibrillation/ 2. (atrial adj3 fibrillat*).tw. 3. (auricular* adj3 fibrillat*).tw. 4. (atrium adj3 fibrillat*).tw. 5. atrial arrhythmi*.tw. 6. or/1‐5 7. Catheter Ablation/ 8. (catheter adj6 (ablat$ or isolat$)).tw. 9. (transcatheter and (ablat$ or isolat$)).tw. 10. ((surgical adj3 ablat$) or MAZE procedure).tw. 11. or/7‐10 12. ae.fs. 13. to.fs. 14. co.fs. 15. si.fs. 16. (toxi* adj2 (effect or effects or reaction* or event or events or outcome*)).tw. 17. (adverse* adj2 (effect or effects or reaction* or event or events or outcome*)).tw. 18. (side adj3 (effect or effects)).tw. 19. (adr or adrs).tw. 20. adverse drug reaction/ 21. or/12‐20 22. (animal/ or nonhuman/) not human/ 23. 21 not 22 24. 6 and 11 and 23 25. random$.tw. 26. factorial$.tw. 27. crossover$.tw. 28. cross over$.tw. 29. cross‐over$.tw. 30. placebo$.tw. 31. (doubl$ adj blind$).tw. 32. (singl$ adj blind$).tw. 33. assign$.tw. 34. allocat$.tw. 35. volunteer$.tw. 36. crossover procedure/ 37. double blind procedure/ 38. randomized controlled trial/ 39. single blind procedure/ 40. 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 41. (animal/ or nonhuman/) not human/ 42. 40 not 41 43. 6 and 11 and 42 44. 24 or 43
Data and analyses
Comparison 1. Ablation versus antiarrhythmic drugs for non‐paroxysmal atrial fibrillation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Freedom from atrial arrhythmia at 12 months follow‐up (random‐effects model) | 3 | 261 | Risk Ratio (M‐H, Random, 95% CI) | 1.84 [1.17, 2.88] |
| 2 Freedom from atrial arrhythmia at 12 months follow‐up (fixed‐effect model) | 3 | 261 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.94 [1.48, 2.55] |
| 3 Participants needing cardioversion | 3 | 261 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.47, 0.82] |
| 4 Cardiac hospitalisation | 2 | 216 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.10, 0.72] |
| 5 Significant bradycardia or need for a pacemaker | 3 | 261 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.02, 1.63] |
| 6 Periprocedural complications and other safety outcomes (random‐effects model) | 3 | 261 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.16, 5.68] |
| 7 Periprocedural complications and other safety outcomes (fixed‐effect model) | 3 | 261 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.33, 2.21] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Forleo 2009.
| Methods | RCT | |
| Participants |
Country: Italy Inclusion criteria: Diabetes mellitus 2 participants with symptomatic paroxysmal or persistent AF for ≥ 6 months refractory to ≥ 1 class 1‐3 antiarrhythmic drugs. Exclusion criteria:
Randomised: Control: 35, Intervention: 35 Age (mean in years): Control: 64.8, Intervention: 63.2 % Male gender: Control: 23, Intervention: 20 |
|
| Interventions |
Control: New ADT. In participants with persistent AF, cardioversion was performed under a new ADT to maintain the sinus rhythm. 5‐week blanking period. Intervention: Pulmonary vein isolation. Participants were discharged on antiarrhythmic drugs. |
|
| Outcomes | Analysis was by intention‐to‐treat. Primary outcomes 1. Freedom from atrial arrhythmias Control: 15/35, Intervention: 28/35 2. % Needing cardioversion after blanking period Control: 0/35, Intervention: 0/35 |
|
| Notes | Persistent AF was not self‐terminating within 7 days and permanent AF if cardioversion had failed or had not been attempted. Pilot study and not adequately powered. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Eligible participants were randomised to receive either pulmonary vein isolation or a new ADT according to a computer‐generated study list. |
| Allocation concealment (selection bias) | Unclear risk | Method for allocation concealment not specified by authors. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Open RCT. Not possible to blind participants receiving ablation due to the nature of the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | At each visit, participants were asked whether medical events or symptoms suggestive of cardiac arrhythmias occurred and an ECG Holter Monitoring was performed to detect the presence of asymptomatic arrhythmias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. Pre‐specified outcomes reported |
| Other bias | Unclear risk | Pilot study and not adequately powered. |
Mont 2014.
| Methods | RCT | |
| Participants |
Country: Spain Inclusion criteria: Participants with symptomatic persistent atrial fibrillation (> 7 or < 7 days requiring electrical or pharmacological cardioversion) refractory to at least one class I or class III antiarrhythmic drug. Exclusion criteria:
Randomised: Control: 48, Intervention: 98 Age (mean in years): Control: 55, Intervention: 55 % Male gender: Control: 77, Intervention: 77.5 |
|
| Interventions |
Control: Participants were treated depending on physician’s choice and according to current guidelines. Discontinuation of the antiarrhythmic treatment was not required before inclusion in the ADT group. There was no predefined protocol on the use of ADT during the blanking period. Intervention: Pulmonary vein ablation. Antiarrhythmic drugs were discontinued ≥ 5 half‐life periods (or ≥ 1 week for amiodarone) before ablation; antiarrhythmics were reinitiated immediately after CA for the blanking period. 3‐month blanking period. |
|
| Outcomes | Analysis was by intention‐to‐treat Primary outcomes 1. Freedom from atrial arrhythmias Control: 43.7%, Intervention: 70.4% 2. % Needing cardioversion after blanking period Control: 50, Intervention: 34.7 |
|
| Notes | Possible loss of statistical power as study was terminated before reaching planned sample size due to lower than expected recruitment rate. The study was supported by an unrestricted grant from Medtronic and Biosense Webster. FB was supported by a grant from Hospital Clinic (premi de Fi de Residencia Emili Letang). No other potential conflict of interest relevant to this study was reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Recruited participants were randomly assigned to either ablation (CA group) or medical therapy (ADT group) according to a 2:1 blocked randomisation list stratified by centre. |
| Allocation concealment (selection bias) | Unclear risk | Method for allocation concealment not specified by authors. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Open RCT. Not possible to blind participants receiving ablation due to the nature of the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were blinded. The primary endpoint was assessed by an independent endpoint committee, which evaluated the episodes based on the information received. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 83 participants (84%) in intervention group provided outcome data compared to all of the control group. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. Pre‐specified outcomes were reported |
| Other bias | High risk | Loss of statistical power. Study was terminated before reaching the planned sample size due to a lower than expected recruitment rate (study limitations) though authors claim that "the difference between groups in the primary endpoint was higher than assumed in the sample size calculation, which likely compensated for the loss of statistical power." |
Stabile 2006.
| Methods | RCT | |
| Participants |
Country: Italy Inclusion criteria: Participants with paroxysmal or persistent AF who were intolerant of antiarrhythmic drugs or in whom two or more antiarrhythmic drug regimens had failed. Exclusion criteria:
Randomised: Control: 19, Intervention: 26 Age (mean in years): Control: 62.3, Intervention: 62.2 %Male gender: Control: 64, Intervention: 54 |
|
| Interventions |
Control: The antiarrhythmic drug preferentially administered was amiodarone. In participants with history of intolerance to amiodarone, a class IC antiarrhythmic was administered. The final decision was left to the physician. Intervention: Pulmonary vein isolation, circumferential ablation, plus left atrial linear lesion ± cavo‐tricuspid isthmus. |
|
| Outcomes | Analysis was by intention‐to‐treat Primary outcomes 1. Absence of any recurrence of atrial arrhythmias Control: 6/69, Intervention: 38/68 2. % Needing cardioversion after blanking period Control: 0/69, Intervention: 0/77 |
|
| Notes | Only participants that had persistent AF were included in the analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated. |
| Allocation concealment (selection bias) | Unclear risk | Method for allocation concealment not specified by authors. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not possible to blind participants receiving ablation due to the nature of the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. Pre‐specified outcomes were reported |
| Other bias | Unclear risk | Our analysis is restricted to the subsample of participants with persistent AF. |
ADT: antiarrhythmic drug treatment AF: atrial fibrillation CA: cardiac ablation ECG: electrocardiogram RCT: randomised controlled trial
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Andrade 2014 | Mixed population with > 75% paroxysmal atrial fibrillation. |
| Bladino 2013 | Not RCT. |
| Cosedis 2012 | Not addressing prespecified population; participants with paroxysmal atrial fibrillation. |
| Gaita 2008 | Not addressing prespecified intervention; study comparing two different ablation strategies. |
| Hunter 2014 | Not addressing prespecified comparison; ablation compared to rate control and follow‐up not up to 12 months. |
| Jais 2008 | Not addressing prespecified population; participants with paroxysmal atrial fibrillation. |
| Jones 2013 | Not addressing prespecified comparison; study on rate control. |
| Krittayaphong 2003 | Mixed population with > 90% paroxysmal atrial fibrillation. |
| MacDonald 2011 | Not addressing prespecified comparison; study on rate control. |
| Morillo 2014 | Not addressing prespecified intervention; on participants with paroxysmal atrial fibrillation. |
| Oral 2006 | Study did not directly compare antiarrhythmic drug therapy to circumferential pulmonary vein ablation. Also, 77% of the participants in the AAD group crossed over to undergo circumferential pulmonary vein ablation in addition to antiarrhythmic drug therapy. |
| Packer 2013 | Mixed population with > 75% paroxysmal atrial fibrillation. |
| Pappone 2006 | Not addressing prespecified population; participants with paroxysmal atrial fibrillation. |
| Raatikainen 2015 | Not addressing prespecified population; participants with paroxysmal atrial fibrillation. |
| Schneider 2015 | Not addressing prespecified population; participants with atrial flutter. |
| Tang 2006 | Not RCT. |
| Wazni 2005 | Mixed population with > 90% paroxysmal atrial fibrillation. |
| Wilber 2010 | Not addressing prespecified population; participants with paroxysmal atrial fibrillation. |
AAD: antiarrhythmic drug RCT: randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
NCT00821353.
| Methods | RCT |
| Participants |
Country: Poland Eligibility:
Inclusion criteria: Participants with hypertrophic cardiomyopathy and paroxysmal or chronic atrial fibrillation Exclusion criteria:
Estimated enrolment: 90 Follow‐up: 12 months |
| Interventions |
Control: Antiarrhythmic drugs (preferably amiodarone) and cardioversion in cases of chronic AF Intervention: Radiofrequency catheter ablation |
| Outcomes |
Primary Outcome 1. Freedom from atrial fibrillation and atrial flutter (> 1 min) on or off antiarrhythmic medications Secondary outcomes 1. Changes in total symptomatic and asymptomatic AF burden 2. Incidence of complications 3. Changes in left atrial diameter and left ventricular function 4. Changes in level of Nt‐pro‐BNP 5. Changes in symptom severity and quality of life 6. Changes in exercise capacity assessed by cardiopulmonary exercise testing |
| Notes | Please refer to this study by its ClinicalTrials.gov identifier: NCT00821353. Phase three completed but not published as of when this review was developed. |
AF: atrial fibrillation RCT: randomised controlled trial CT: computerized tomography GFR: glomular filtration rate LVOT: left ventricular outflow tract Nt‐pro‐BNP: N‐terminal pro‐brain natriuretic peptide NYHA: New York Heart Association RCT: randomised controlled trial TEE: transesophageal echocardiography
Characteristics of ongoing studies [ordered by study ID]
NCT00196209.
| Trial name or title | Randomized study comparing cardioversion vs. catheter ablation in patients with persistent atrial fibrillation |
| Methods | RCT |
| Participants |
Country: Germany Eligibility:
Inclusion criteria:
Exclusion criteria:
Estimated enrolment: 130 Follow‐up: 6 months |
| Interventions |
Control: Cardioversion and drug prophylaxis to treat persistent atrial fibrillation. Intervention: Catheter ablation to treat persistent atrial fibrillation. |
| Outcomes |
Primary outcome: Event‐free survival after 6 months (i.e. freedom of atrial tachyarrhythmias ‐ as evaluated in a 7‐day‐Holter, stroke, pulmonary vein stenosis ‐ as evaluated in a CT‐/MRT scan 6 months after the initial procedure ‐ and death). Secondary outcomes:
|
| Starting date | August 2005 |
| Contact information | Heidi L Estner, MD; 0049 89 1218 2020; estner@dhm.mhn.de |
| Notes | Please refer to this study by its ClinicalTrials.gov identifier: NCT00196209. The recruitment status of this study is unknown. |
NCT00911508.
| Trial name or title | Catheter ablation vs anti‐arrhythmic drug therapy for atrial fibrillation trial |
| Methods | RCT |
| Participants |
Country: USA Eligibility:
Inclusion criteria: Over the preceding 6 months have:
Exclusion criteria:
Estimated enrolment: 2204 Follow‐up: until date of event |
| Interventions |
Control: Current state‐of‐the‐art drug therapy for atrial fibrillation (rate control or rhythm control). Treating physicians will be encouraged to follow the American College of Cardiology/American Heart Association/European Society of Cardiology Atrial Fibrillation Guidelines with regard to drug therapy for atrial fibrillation. Intervention: Pulmonary vein isolation using a circumferential ablative approach in the left atrium. Ablation may be performed using circular mapping catheter‐guided ablation, antral isolation using a circular guided approach, or wide area circumferential ablation. |
| Outcomes |
Primary outcome: LA catheter ablation is superior to rate or rhythm control drug therapy for decreasing the incidence of the composite endpoint of total mortality, disabling stroke, serious bleeding, or cardiac arrest in participants warranting therapy for AF. Secondary outcomes: LA catheter ablation is superior to rate or rhythm control drug therapy for reducing total mortality.
|
| Starting date | August 2009 |
| Contact information | Douglas L Packer, MD, Mayo Clinic |
| Notes | Please refer to this study by its ClinicalTrials.gov identifier: NCT00911508. This study is ongoing, but not recruiting participants. |
NCT01420393.
| Trial name or title | A randomized ablation‐based atrial fibrillation rhythm control versus rate control trial in patients with heart failure and high burden atrial fibrillation |
| Methods | RCT |
| Participants | Country: Canada Eligibility:
Inclusion criteria: Participants with one of the following AF categories and at least one ECG documentation of AF.
Exclusion criteria:
Estimated enrolment: 600 Follow‐up: 5 years |
| Interventions |
Control: Participants in the rate control group will receive optimal heart failure therapy and rate control measures to achieve a resting HR < 80 beats per minute (bpm) and 6‐minute walk HR < 110 bpm Intervention: Participants randomised to catheter ablation‐based AF rhythm control group will receive optimal heart failure therapy and one or more aggressive catheter ablation, which include PV antral ablation and LA substrate ablation with or without adjunctive antiarrhythmic drug. |
| Outcomes |
Primary outcome: Composite of all‐cause mortality and hospitalisation for heart failure defined as an admission to a health care facility for > 24 hours. Secondary outcomes:
|
| Starting date | September 2011 |
| Contact information | Anthony Tang, MD; anthonysltang@gmail.com |
| Notes | Please refer to this study by its ClinicalTrials.gov identifier: NCT01420393. This study is currently recruiting participants. |
AFEQT: atrial fibrillation effect on quality‐of‐life ASD: atrial septal defect AV: Atrioventricular CCS‐SAF: Canadian cardiovascular society severity of atrial fibrillation CHF: congestive heart failure EF: ejection fraction EQ5D: EuroQol five dimensions HR: heart rate HF: heart failure LA: left atrial LV: left ventricular LVEF: left ventricular ejection fraction MI: myocardial infarction MLWHF: Minnesota living with heart failure MRT: magnetic resonance tomography Nt‐pro‐BNP: N‐terminal pro‐brain natriuretic peptide PCI: percutaneous coronary intervention PTCA: percutaneous transluminal coronary angioplasty PV: pulmonary vein TIA: transient Ischaemic attack
Differences between protocol and review
In the protocol of the Review, we planned to address the 'percentage of participants needing cardioversion' as a primary outcome but addressed 'participants needing cardioversion' instead; participants needing cardioversion is easier to analyse and interpret.
We planned on searching relevant manufacturers' websites for trial information but did not do this because we searched other relevant websites, more suited to our Review questions instead.
We used a random‐effects model to incorporate unexplained moderate heterogeneity where the I² statistic was greater than 40%, as opposed to the I² statistic greater than 50%, as a more accurate conclusion would be drawn by investigating and using a random‐effects model to incorporate an I² statistic greater than 40%.
In the protocol of the Review our objective was to determine the effect of ablation to maintin sinus rhythm in patients with persistent or long‐standing persistent atrial fibrillation compared to anti‐arrhythmic drugs. In our Review we have modified to objective to determine the efficacy and safety of ablation (catheter and surgical) in people with non‐paroxysmal (persistent or long‐standing persistent) atrial fibrillation compared to antiarrhythmic drugs.
In the protocol of the Review we planned to include only randomised controlled trials (RCTs) of parallel‐group design with the individual or cluster as the unit of randomisation. In our Review we included only randomised trials of parallel‐group design with the individual as the unit of randomisation.
Contributions of authors
Jonathan Nyong: screening, statistical advice, and writing.
Guy Amit: design of study and expert advice on atrial fibrillation.
Alma J Adler: design of study, screening, and writing.
Onikepe O Owolabi: screening and writing.
David Prieto‐Merino: statistical advice.
Pablo Perel: design of study and writing.
Pier Lambiase: design of study and expert advice on atrial fibrillation.
Juan Pablo Casas: design of study and writing.
Carlos A Morillo: design of study, expert advice on atrial fibrillation, and writing.
Sources of support
Internal sources
No sources of support supplied
External sources
European Society of Cardiology, Other.
Declarations of interest
Jonathan Nyong: none known.
Guy Amit: receives funding from Johnson & Johnson for consultation for work unrelated to the content of this Cochrane review.
Alma J Adler: none known.
Onikepe O Owolabi: none known.
David Prieto‐Merino: none known.
Pablo Perel: none known.
Pier Lambiase: receives speaker fees and educational grant funding from Boston Scientific, and educational grant funding from Medtronic for work unrelated to the content of this Cochrane review.
Juan Pablo Casas: this systematic review was funded by the Atrial Fibrillation Task Force from the European Society of Cardiology. This grant is hold at University College London.
Carlos A Morillo: member of the boards of Merck Sharp & Dohme, Biotronik, receives funding from Boehringer Ingelheim and Sanofi Aventis for consultation, and receives speaker fees from Boehringer Ingelheim for work unrelated to the content of this Cochrane review.
New
References
References to studies included in this review
Forleo 2009 {published data only}
- Forleo G, Mantica M, Luca L, Leo R, Santini L, Panigada S, et al. Catheter ablation of atrial fibrillation in patients with diabetes mellitus type 2: results from a randomized study comparing pulmonary vein isolation versus antiarrhythmic drug therapy. Journal of Cardiovacsular Electrophysiology 2009;20(1):22‐8. [DOI] [PubMed] [Google Scholar]
Mont 2014 {published data only}
- Mont L, Bisbal F, Hernández‐Madrid A, Pérez‐Castellano N, Viñolas X, Arenal A, et al: SARA Investigators. Catheter ablation vs. antiarrhythmic drug treatment of persistent atrial fibrillation: a multicentre, randomized, controlled trial (SARA study). European Heart Journal 2014;35(8):501‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stabile 2006 {published data only}
- Stabile G, Bertaglia E, Senatore G, Simone A, Zoppo F, Donnici G, et al. Catheter ablation treatment in patients with drug‐refractory atrial fibrillation: a prospective, multi‐centre, randomized, controlled study (Catheter Ablation for the Cure of Atrial Fibrillation Study). European Heart Journal 2006;27(2):216‐21. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Andrade 2014 {published data only}
- Andrade JG, Khairy P, Macle L, Packer DL, Lehmann JW, Holcomb RG, et al. Incidence and significance of early recurrences of atrial fibrillation after cryoballoon ablation: insights from the multicenter Sustained Treatment of Paroxysmal Atrial Fibrillation (STOP AF) Trial. Circulation. Arrhythmia and Electrophysiology 2014;7(1):69‐75. [DOI] [PubMed] [Google Scholar]
Bladino 2013 {published data only}
- Blandino A, Toso E, Scaglione M, Anselmino M, Ferraris F, Sardi D, et al. Long‐term efficacy and safety of two different rhythm control strategies in elderly patients with symptomatic persistent atrial fibrillation. Journal of Cardiovascular Electrophysiology 2013;24(7):731‑8. [DOI] [PubMed] [Google Scholar]
Cosedis 2012 {published data only}
- Cosedis Nielsen J, Johannessen A, Raatikainen P, Hindricks G, Walfridsson H, Kongstad O, et al. Radiofrequency ablation as initial therapy in paroxysmal atrial fibrillation. New England Journal of Medicine 2012;367(17):1587‐95. [DOI] [PubMed] [Google Scholar]
Gaita 2008 {published data only}
- Gaita F, Caponi D, Scaglione M, Montefusco A, Corleto A, Monte F, et al. Long‐term clinical results of 2 different ablation strategies in patients with paroxysmal and persistent atrial fibrillation. Circulation. Arrhythmia and Electrophysiology 2008;1(4):269‐75. [DOI] [PubMed] [Google Scholar]
Hunter 2014 {published data only}
- Hunter RJ, Berriman TJ, Diab I, Kamdar R, Richmond L, Baker V, et al. A randomized controlled trial of catheter ablation versus medical treatment of atrial fibrillation in heart failure (the CAMTAF trial). Circulation. Arrhythmia and Electrophysiology 2014;7(1):31‐8. [DOI] [PubMed] [Google Scholar]
Jais 2008 {published data only}
- Jaïs P, Cauchemez B, Macle L, Daoud E, Khairy P, Subbiah R, et al. Catheter ablation versus antiarrhythmic drugs for atrial fibrillation: the A4 study. Circulation 2008;118(24):2498‐505. [DOI] [PubMed] [Google Scholar]
Jones 2013 {published data only}
- Jones DG, Haldar SK, Hussain W, Sharma R, Francis DP, Rahman‐Haley SL, et al. A randomized trial to assess catheter ablation versus rate control in the management of persistent atrial fibrillation in heart failure. Journal of the American College of Cardiology 2013;61(18):1894‐903. [DOI] [PubMed] [Google Scholar]
Krittayaphong 2003 {published data only}
- Krittayaphong R, Raungrattanaamporn O, Bhuripanyo K, Sriratanasathavorn C, Pooranawattanakul S, Punlee K, et al. A randomized clinical trial of the efficacy of radiofrequency catheter ablation and amiodarone in the treatment of symptomatic atrial fibrillation. Journal of the Medical Association of Thailand 2003;86 Suppl 1:S8‐16. [PubMed] [Google Scholar]
MacDonald 2011 {published data only}
- MacDonald MR, Connelly DT, Hawkins NM, Steedman T, Payne J, Shaw M, et al. Radiofrequency ablation for persistent atrial fibrillation in patients with advanced heart failure and severe left ventricular systolic dysfunction: a randomised controlled trial. Heart 2011;97(9):740‐7. [DOI] [PubMed] [Google Scholar]
Morillo 2014 {published data only}
- Morillo CA, Verma A, Connolly SJ, Kuck KH, Nair GM, Champagne J, et al. RAAFT‐2 Investigators. Radiofrequency ablation vs antiarrhythmic drugs as first‐line treatment of paroxysmal atrial fibrillation (RAAFT‐2): a randomized trial. JAMA 2014;311(7):692‐700. [DOI] [PubMed] [Google Scholar]
Oral 2006 {published data only}
- Oral H, Pappone C, Chugh A, Good E, Bogun F, Pelosi F Jr, et al. Circumferential pulmonary‐vein ablation for chronic atrial fibrillation. New England Journal of Medicine 2006;9(354):934‐41. [DOI] [PubMed] [Google Scholar]
Packer 2013 {published data only}
- Packer DL, Kowal RC, Wheelan KR, Irwin JM, Champagne J, Guerra PG, et al. Cryoballoon ablation of pulmonary veins for paroxysmal atrial fibrillation: first results of the North American Arctic (Front STOP‐AF) pivotal trial. Journal of the American College of Cardiology 2013;61(16):1713‐23. [DOI] [PubMed] [Google Scholar]
Pappone 2006 {published data only}
- Pappone C, Augello G, Sala S, Gugliotta F, Vicedomini G, Gulletta S, et al. A randomized trial of circumferential pulmonary vein ablation versus antiarrhythmic drug therapy in paroxysmal atrial fibrillation: the APAF Study. Journal of the American College of Cardiology 2006;48(11):2340‐7. [DOI] [PubMed] [Google Scholar]
Raatikainen 2015 {published data only}
- Raatikainen MJ, Hakalahti A, Uusimaa P, Nielsen JC, Johannessen A, Hindricks G, et al. MANTRA‐PAF investigators. Radiofrequency catheter ablation maintains its efficacy better than antiarrhythmic medication in patients with paroxysmal atrial fibrillation: On‐treatment analysis of the randomized controlled MANTRA‐PAF trial. International Journal of Cardiology 2015;198:108‐114. [DOI] [PubMed] [Google Scholar]
Schneider 2015 {published data only}
- Schneider R, Lauschke J, Tischer T, Schneider C, Voss W, Moehlenkamp F, et al. Pulmonary vein triggers play an important role in the initiation of atrial flutter: Initial results from the prospective randomized Atrial Fibrillation Ablation in Atrial Flutter (Triple A) trial. Heart Rhythm 2015;12(5):865‐71. [DOI] [PubMed] [Google Scholar]
Tang 2006 {published data only}
- Tang RB, Dong JZ, Liu XP, Fang DP, Long de Y, Liu XH, et al. Safety and efficacy of catheter ablation of atrial fibrillation in patients with diabetes mellitus ‐ single center experience. Journal of International Cardiac Electrophysiology 2006;17(1):41‐6. [DOI] [PubMed] [Google Scholar]
Wazni 2005 {published data only}
- Wazni OM, Marrouche NF, Martin DO, Verma A, Bhargava M, Saliba W, et al. Radiofrequency ablation vs antiarrhythmic drugs as first‐line treatment of symptomatic atrial fibrillation: a randomized trial. JAMA 2005;293(21):2634‐40. [DOI] [PubMed] [Google Scholar]
Wilber 2010 {published data only}
- Wilber DJ, Pappone C, Neuzil P, Paola A, Marchlinski F, Natale A, et al. ThermoCool AF Trial Investigators. Comparison of antiarrhythmic drug therapy and radiofrequency catheter ablation in patients with paroxysmal atrial fibrillation: a randomized controlled trial. JAMA 2010;303(4):333‐40. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
NCT00821353 {published data only}
- NCT00821353. Sinus rhythm maintenance in patients with hypertrophic cardiomyopathy and atrial fibrillation ‐ randomized comparison of antiarrhythmic therapy vs. radiofrequency catheter ablation (SHAARC). clinicaltrials.gov/ct2/show/NCT00821353.
References to ongoing studies
NCT00196209 {published data only}
- NCT00196209. Randomized study comparing cardioversion vs. catheter ablation in patients with persistent atrial fibrillation. clinicaltrials.gov/ct2/show/NCT00196209.
NCT00911508 {published data only}
- NCT00911508. Catheter ablation vs anti‐arrhythmic drug therapy for atrial fibrillation trial (CABANA). clinicaltrials.gov/ct2/show/NCT00911508.
NCT01420393 {published data only}
- NCT01420393. A randomized ablation‐based atrial fibrillation rhythm control versus rate control trial in patients with heart failure and high burden atrial fibrillation. clinicaltrials.gov/ct2/show/NCT01420393. [DOI] [PubMed]
Additional references
Andrade 2012
- Andrade JG, Macle L, Khairy P, Khaykin Y, Mantovan R, Martino G, et al. Incidence and significance of early recurrences associated with different ablation strategies for AF: a STAR‐AF substudy. Journal of Cardiovascular Electrophysiology 2012;23(12):1295‐301. [DOI] [PubMed] [Google Scholar]
Arbelo 2014
- Arbelo E, Brugada J, Hindricks G, Maggioni AP, Tavazzi L, Vardas P, et al. The atrial fibrillation ablation pilot study: a European Survey on Methodology and results of catheter ablation for atrial fibrillation conducted by the European Heart Rhythm Association. European Heart Journal 2014;35(22):1466‐78. [DOI] [PubMed] [Google Scholar]
Bertaglia 2015 [pers comm]
- Bertaglia E. Request for information about study for systematic review‐Catheter ablation for the cure of Atrial Fibrillation. Email to: Drs Stabile, Bertaglia and Senatore 09 July 2015.
Brooks 2010
- Brooks AG, Stiles MK, Laborderie J, Lau DH, Kuklik P, Shipp NJ, et al. Outcomes of long‐standing persistent atrial fibrillation ablation: a systematic review. Heart Rhythm 2010;7(6):835‐46. [DOI] [PubMed] [Google Scholar]
Calkins 2009
- Calkins H, Reynolds MR, Spector P, Sondhi M, Xu Y, Martin A, et al. Treatment of atrial fibrillation with antiarrhythmic drugs or radiofrequency ablation: two systematic literature reviews and meta‐analyses. Circulation. Arrhythmia and Electrophysiology 2009;2(4):349‐61. [DOI] [PubMed] [Google Scholar]
Calkins 2012
- Calkins H, Kuck KH, Cappato R, Brugada J, Camm AJ, Chen SA, et al. HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow‐up, definitions, endpoints, and research trial design. Europace 2012;14(4):528‐606. [DOI] [PubMed] [Google Scholar]
Camm 2012
- Camm AJ, Lip GY, Caterina R, Savelieva I, Atar D, Hohnloser SH, et al. ESC Committee for Practice Guidelines (CPG). 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. European Heart Journal 2012;33(21):2719‐47. [DOI] [PubMed] [Google Scholar]
Cappato 2010
- Cappato R, Calkins H, Chen SA, Davies W, Iesaka Y, Kalman J, et al. Updated worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circulation. Arrhythmia and Electrophysiology 2010;3:32‐8. [DOI] [PubMed] [Google Scholar]
Cheng 2014
- Cheng X, Li X, He Y, Liu X, Wang G, Cheng L, et al. Catheter ablation versus anti‐arrhythmic drug therapy for the management of a trial fibrillation: a meta‐analysis. Journal of Interventional Cardiac Electrophysiology 2014;41(3):267‐72. [DOI] [PubMed] [Google Scholar]
Dorian 2000
- Dorian P, Jung W, Newman D, Paquette M, Wood K, Ayers GM, et al. The impairment of health‐related quality of life in patients with intermittent atrial fibrillation: implications for the assessment of investigational therapy. Journal of the American College of Cardiology 2000;36(4):1303‐9. [DOI] [PubMed] [Google Scholar]
Elayi 2008
- Elayi CS, Verma A, Biase L, Ching CK, Patel D, Barrett C, et al. Ablation for longstanding permanent atrial fibrillation: results from a randomized study comparing three different strategies. Heart Rhythm 2008;5(12):1658‐64. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- Schünemann H, Brożek J, Guyatt G, Oxman A. The Grading of Recommendations Assessment, Development and Evaluation (GRADE) profile (pro) and Guideline development tool (GDT). Version 3.6. Hamilton (ON): GRADE Working Group, McMaster University, 2014.
Hakalahti 2015
- Hakalahti A, Biancari F, Nielsen JC, Raatikainen MJP. Radiofrequency ablation vs. antiarrhythmic drug therapy as first line treatment of symptomatic atrial fibrillation: systematic review and meta‐analysis. Europace 2015;17(3):370‐8. [DOI] [PubMed] [Google Scholar]
Haïssaguerre 1998
- Haïssaguerre M, Jaïs P, Shah DC, Takahashi A, Hocini M, Quiniou G, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. New England Journal of Medicine 1998;339(10):659‐66. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org. Wiley‐Blackwell.
Jaïs 2008
- Jaïs P, Cauchemez B, Macle L, Daoud E, Khairy P, Subbiah R, et al. Catheter ablation versus antiarrhythmic drugs for atrial fibrillation: the A4 study. Circulation 2008;118(24):2498‐505. [DOI] [PubMed] [Google Scholar]
Kerr 2005
- Kerr CR, Humphries KH, Talajic M, Klein GJ, Connolly SJ, Green M, et al. Progression to chronic atrial fibrillation after the initial diagnosis of paroxysmal atrial fibrillation: results from the Canadian Registry of Atrial Fibrillation. American Heart Journal 2005;149(3):489‐96. [DOI] [PubMed] [Google Scholar]
Khan 2014
- Khan AR, Khan S, Sheik MA, Khuder S, Grubb B, Moukarbel GV. Catheter ablation and antiarrhythmic drug therapy as first‐ or second‐line therapy in the management of atrial fibrillation: systematic review and meta‐analysis. Circulation. Arrhythmia and Electrophysiology 2014;7(5):853‐60. [DOI] [PubMed] [Google Scholar]
Kneeland 2009
- Kneeland PP, Fang MC. Trends in catheter ablation for atrial fibrillation in the United States. Hospital Medicine 2009;4:E1‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kottkamp 2015
- Kottkamp H, Bender R, Berg J. Catheter ablation of atrial fibrillation: how to modify the substrate?. Journal of the American College of Cardiology 2015;65(2):196‐201. [DOI] [PubMed] [Google Scholar]
Kumar 2013
- Kumar S, Walters TE, Halloran K, Morton JB, Hepworth G, Wong CX, et al. Ten‐year trends in the use of catheter ablation for treatment of atrial fibrillation vs. the use of coronary intervention for the treatment of ischaemic heart disease in Australia. Europace 2013;15:1702‐9. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
McKenna 2008
- C McKenna, S Palmer, M Rodgers, D Chambers, N Hawkins, S Golder, S Van Hout, C Pepper, D Todd, N Woolacott. Cost‐effectiveness of radiofrequency catheter ablation for the treatment of atrial fibrillation in the United Kingdom. Heart 2008;95:542–549. [DOI] [PubMed] [Google Scholar]
Nair 2009
- Nair GM, Nery PB, Diwakaramenon S, Healey JS, Connolly SJ, Morillo CA. A systematic review of randomized trials comparing radiofrequency ablation with antiarrhythmic medications in patients with atrial fibrillation. Journal of Cardiovascular Electrophysiology 2009;20:138‐44. [DOI] [PubMed] [Google Scholar]
Narayan 2012
- Narayan SM, Krummen DE, Shivkumar K, Clopton P, Rappel WJ, Miller JM. CONFIRM (conventional ablation for atrial fibrillation with or without focal impulse and rotor modulation) trial. Journal of the American College of Cardiology 2012;60(7):628‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nault 2010
- Nault I, Miyazaki S, Forclaz A, Wright M, Jadidi M, Jaïs P, et al. Drugs vs. ablation for the treatment of atrial fibrillation: the evidence supporting catheter ablation. European Heart Journal 2010;31(9):1046‐54. [DOI] [PubMed] [Google Scholar]
Oral 2005
- Oral H, Chugh A, Good E, Igic P, Elmouchi D, Tschopp DR, et al. Randomized comparison of encircling and nonencircling left atrial ablation for chronic atrial fibrillation. Heart Rhythm 2005;2(11):1165‐72. [DOI] [PubMed] [Google Scholar]
Pokushalov 2013
- Pokushalov E, Romanov A, Katritsis DG, Artyomenko S, Shirokova N, Karaskov A, et al. Ganglionated plexus ablation vs linear ablation in patients undergoing pulmonary vein isolation for persistent/long‐standing persistent atrial fibrillation: a randomized comparison. Heart Rhythm 2013;10(9):1280‐6. [DOI] [PubMed] [Google Scholar]
Rahman 2014
- Rahman F, Kwan GF, Benjamin EJ. Global epidemiology of atrial fibrillation. Nature Reviews. Cardiology 2014;11(11):639‐54. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rivard 2012
- Rivard L, Hocini M, Rostock T, Cauchemez B, Forclaz A, Jadidi AS, et al. Improved outcome following restoration of sinus rhythm prior to catheter ablation of persistent atrial fibrillation: a comparative multicenter study. Heart Rhythm 2012;9(7):1025‐30. [DOI] [PubMed] [Google Scholar]
Singh 2005
- Singh BN, Singh SN, Reda DJ, Tang XC, Lopez B, Harris CL, et al. Amiodarone versus sotalol for atrial fibrillation. New England Journal of Medicine 2005;352:1861‐72. [DOI] [PubMed] [Google Scholar]
Singh 2006
- Singh SN, Tang XC, Singh BN, Dorian P, Reda DJ, Harris CL, et al. Quality of life and exercise performance in patients in sinus rhythm versus persistent atrial fibrillation: a veterans affairs cooperative studies program substudy. Journal of the American College of Cardiology 2006;48(4):721‐30. [DOI] [PubMed] [Google Scholar]
Thrall 2006
- Thrall G, Lane D, Carroll D, Lip GY. Quality of life in patients with atrial fibrillation: a systematic review. American Journal of Medicine 2006;119(5):448.e1‐19. [DOI] [PubMed] [Google Scholar]
Verma 2015
- Verma A, Jiang CY, Betts TR, Chen J, Deisenhofer I, Mantovan R, et al. Approaches to catheter ablation for persistent atrial fibrillation. New England Journal of Medicine 2015;372(19):1812‐22. [DOI] [PubMed] [Google Scholar]
Wood 2008
- Wood L, Egger M, Gluud LL, Schulz KF, Juni P, Altman DG, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta‐epidemiological study. BMJ 2008;336(7644):601‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wynn 2014
- Wynn GJ, Das M, Bonnett LJ, Panikker S, Wong T, Gupta D. Efficacy of catheter ablation for persistent atrial fibrillation: a systematic review and meta‐analysis of evidence from randomized and nonrandomized controlled trials. Circulation. Arrhythmia and Electrophysiology 2014;7(5):841‐52. [DOI] [PubMed] [Google Scholar]
Wyse 2002
- Wyse DG, Waldo AL, DiMarco JP, Domanski MJ, Rosenberg Y, Schron EB, et al. A comparison of rate control and rhythm control in patients with atrial fibrillation. New England Journal of Medicine 2002;347(23):1825‐33. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Amit 2016
- Amit G, Adler AJ, Owolabi OO, Nyong J, Casas JP, Prieto‐Merino D, et al. Efficacy and safety of ablation for patients with non‐paroxysmal atrial fibrillation. Cochrane Database of Systematic Reviews 2016, Issue 2. [DOI: 10.1002/14651858.CD012088] [DOI] [PMC free article] [PubMed] [Google Scholar]


