Abstract
Background
Alcohol‐related liver disease is due to excessive alcohol consumption. It includes a spectrum of liver diseases such as alcohol‐related fatty liver, alcoholic hepatitis, and alcoholic cirrhosis. Mortality associated with alcoholic hepatitis is high. The optimal pharmacological treatment of alcoholic hepatitis and other alcohol‐related liver disease remains controversial.
Objectives
To assess the comparative benefits and harms of different pharmacological interventions in the management of alcohol‐related liver disease through a network meta‐analysis and to generate rankings of the available pharmacological interventions according to their safety and efficacy in order to identify potential treatments. However, even in the subgroup of participants when the potential effect modifiers appeared reasonably similar across comparisons, there was evidence of inconsistency by one or more methods of assessment of inconsistency. Therefore, we did not report the results of the network meta‐analysis and reported the comparative benefits and harms of different interventions using standard Cochrane methodology.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, Science Citation Index Expanded, World Health Organization International Clinical Trials Registry Platform and randomised controlled trials registers until February 2017 to identify randomised clinical trials on pharmacological treatments for alcohol‐related liver diseases.
Selection criteria
Randomised clinical trials (irrespective of language, blinding, or publication status) including participants with alcohol‐related liver disease. We excluded trials that included participants who had previously undergone liver transplantation and those with co‐existing chronic viral diseases. We considered any of the various pharmacological interventions compared with each other or with placebo or no intervention.
Data collection and analysis
Two review authors independently identified trials and independently extracted data. We calculated the odds ratio (OR) and rate ratio with 95% confidence intervals (CIs) using both fixed‐effect and random‐effects models based on available‐participant analysis with Review Manager. We assessed risk of bias according to Cochrane, controlled risk of random errors with Trial Sequential Analysis, and assessed the quality of the evidence using GRADE.
Main results
We identified a total of 81 randomised clinical trials. All the trials were at high risk of bias, and the overall quality of the evidence was low or very low for all outcomes.
Alcoholic hepatitis
Fifty randomised clinical trials included 4484 participants with alcoholic hepatitis. The period of follow‐up ranged from one to 12 months. Because of concerns about transitivity assumption, we did not perform the network meta‐analysis. None of the active interventions showed any improvement in any of the clinical outcomes reported in the trials, which includes mortality (at various time points), cirrhosis, decompensated cirrhosis, liver transplantation. None of the trials reported health‐related quality of life or incidence of hepatocellular carcinoma.
Severe alcoholic hepatitis
Of the trials on alcoholic hepatitis, 19 trials (2545 participants) included exclusively participants with severe alcoholic hepatitis (Maddrey Discriminat Function > 32). The period of follow‐up ranged from one to 12 months. There was no alteration in the conclusions when only people with severe alcoholic hepatitis were included in the analysis.
Source of funding: Eleven trials were funded by parties with vested interest in the results. Sixteen trials were funded by parties without vested interest in the results. The source of funding was not reported in 23 trials.
Other alcohol‐related liver diseases
Thirty‐one randomised clinical trials included 3695 participants with other alcohol‐related liver diseases (with a wide spectrum of alcohol‐related liver diseases). The period of follow‐up ranged from one to 48 months. The mortality at maximal follow‐up was lower in the propylthiouracil group versus the no intervention group (OR 0.45, 95% CI 0.26 to 0.78; 423 participants; 2 trials; low‐quality evidence). However, this result is based on two small trials at high risk of bias and further confirmation in larger trials of low risk of bias is necessary to recommend propylthiouracil routinely in people with other alcohol‐related liver diseases. The mortality at maximal follow‐up was higher in the ursodeoxycholic acid group versus the no intervention group (OR 2.09, 95% CI 1.12 to 3.90; 226 participants; 1 trial; low‐quality evidence).
Source of funding: Twelve trials were funded by parties with vested interest in the results. Three trials were funded by parties without vested interest in the results. The source of funding was not reported in 16 trials.
Authors' conclusions
Because of very low‐quality evidence, there is uncertainty in the effectiveness of any pharmacological intervention versus no intervention in people with alcoholic hepatitis or severe alcoholic hepatitis. Based on low‐quality evidence, propylthiouracil may decrease mortality in people with other alcohol‐related liver diseases. However, these results must be confirmed by adequately powered trials with low risk of bias before propylthiouracil can be considered effective.
Future randomised clinical trials should be conducted with approximately 200 participants in each group and follow‐up of one to two years in order to compare the benefits and harms of different treatments in people with alcoholic hepatitis. Randomised clinical trials should include health‐related quality of life and report serious adverse events separately from adverse events. Future randomised clinical trials should have a low risk of bias and low risk of random errors.
Plain language summary
Medical treatment of alcohol‐related liver disease
Background
Alcohol‐related liver disease or alcoholic liver disease is liver disease related to excessive alcohol consumption. It includes a spectrum of liver diseases that includes alcoholic steatosis (simple fatty liver or simple steatosis or accumulation of fat in liver cells), alcoholic hepatitis (inflammation of liver cells), and alcoholic cirrhosis (destruction of liver cells and replacement with scar tissue). This can cause major health problems such as excessive tiredness, and liver failure leading to vomiting blood, confusion, and death. A number of medical treatments have been used to treat alcohol‐related liver disease. The best way to treat alcohol‐related liver disease is not clear. We sought to resolve this issue by searching for existing studies on the topic. We included all randomised clinical trials whose results were reported until February 2017. We included only studies in which participants had not undergone liver transplantation previously and those who did not have liver disease due to other causes such as viral infections. Apart from using standard Cochrane methods which allow comparison of only two treatments at a time (direct comparison), we planned to use an advanced method which allows comparison of the many different treatments that are individually compared in the trials (network meta‐analysis). However, because of the nature of the information available, we could not determine whether the network meta‐analysis results were reliable. Therefore, we used standard Cochrane methodology.
Study characteristics
We identified 81 trials which were eligible for our review. We have presented the results for people with differing spectrum of alcohol‐related liver disease separately.
Key results
Alcoholic hepatitis
Fifty randomised clinical trials included 4484 participants with alcoholic hepatitis. The period of follow‐up ranged from one to 12 months. Because of the nature of the information available, we used methods similar to Cochrane methodology. None of the active interventions showed any improvement in any of the clinical outcomes reported in the trials, which includes deaths (at various time points), cirrhosis, liver failure or liver transplantation. None of the trials reported health‐related quality of life or incidence of primary liver cancer.
Severe alcoholic hepatitis
Of the trials on alcoholic hepatitis, 19 trials (2545 participants) included exclusively participants with severe alcoholic hepatitis. The period of follow‐up ranged from one to 12 months. There was no alteration in the conclusions when only people with severe alcoholic hepatitis were included in the analysis.
Source of funding: Eleven trials were funded by parties with vested interest in the results. Sixteen trials were funded by parties without vested interest in the results. The source of funding was not reported in 23 trials.
Other alcohol‐related liver diseases
Thirty‐one randomised clinical trials included 3695 participants with other alcohol‐related liver diseases (with a wide spectrum of alcohol‐related liver diseases). The period of follow‐up ranged from one to 48 months. The risk of deaths was lower in the propylthiouracil group than in the no intervention group and higher in the ursodeoxycholic acid group than in the no intervention group. However, these results are based on trials with methodological deficiencies that make the results unreliable. As a result, trials of low risk of bias of sufficient sample size are required before propylthiouracil can be recommended routinely. There was no evidence of improvement in any of the remaining clinical outcomes by any of the interventions compared with no intervention.
Source of funding: Twelve trials were funded by parties with vested interest in the results. Three trials were funded by parties without vested interest in the results. The source of funding was not reported in 16 trials.
Quality of the evidence
The overall quality of the evidence was very low and all the trials were at unclear or high risk of bias, which means that there is possibility of making wrong conclusions overestimating benefits or underestimating harms of one treatment or the other because of the way that the studies were conducted. Further high‐quality randomised clinical trials are necessary.
Summary of findings
Summary of findings for the main comparison. Glucocorticosteroids compared with no intervention for alcoholic hepatitis (all severity).
| Glucocorticosteroids compared with no intervention for alcoholic hepatitis (all severity) | |||||
|
Patient or population: participants with alcoholic hepatitis (all severity) Settings: secondary or tertiary care Intervention: glucocorticosteroids Comparison: no intervention | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (trials) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| No intervention | Glucocorticosteroids | ||||
|
Mortality at maximal follow‐up Follow‐up: 1 to 12 months |
348 per 1000 | 353 per 1000 (301 to 409) | OR 0.85 (0.67 to 1.08) | 1147 (12 trials) | ⊕⊝⊝⊝ very low1,2,3 |
| Early mortality (mortality up to 90 days) | 217 per 1000 | 391 per 1000 (313 to 472) | OR 1.00 (0.71 to 1.39) | 672 (4 trials) | ⊕⊝⊝⊝ very low1,2,3 |
|
Serious adverse events (proportion) Follow‐up: 12 months |
390 per 1000 | 467 per 1000 (385 to 552) | OR 1.37 (0.98 to 1.93) |
546 (1 trial) |
⊕⊝⊝⊝ very low1,2,3 |
| Serious adverse events (number) | None of the trials reported this outcome. | ||||
| Health‐related quality of life | None of the trials reported this outcome. | ||||
| *The basis for the assumed risk is the mean control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio. | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1Risk of bias: trial(s) were at high risk of bias (downgraded by one level)
2Imprecision: small sample size (downgraded by one level)
3Imprecision: Confidence intervals overlapped a clinically significant increase or reduction and clinically insignificant increase or reduction (downgraded by one level).
Summary of findings 2. Pentoxifylline compared with no intervention for alcoholic hepatitis (all severity).
| Pentoxifylline compared with no intervention for alcoholic hepatitis (all severity) | |||||
|
Patient or population: participants with alcoholic hepatitis (all severity) Settings: secondary or tertiary care Intervention: pentoxifylline Comparison: no intervention | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (trials) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| No intervention | Pentoxifylline | ||||
|
Mortality at maximal follow‐up Follow‐up: 1 to 12 months |
348 per 1000 | 331 per 1000 (271 to 396) | OR 0.77 (0.58 to 1.02) | 881 (6 trials) | ⊕⊝⊝⊝ very low1,2,3 |
| Early mortality (mortality up to 90 days) | 217 per 1000 | 431 per 1000 (342 to 528) | OR 1.18 (0.81 to 1.74) | 545 (1 trial) | ⊕⊝⊝⊝ very low1,2,3 |
|
Serious adverse events (proportion) Follow‐up: 12 months |
390 per 1000 | 406 per 1000 (327 to 491) | OR 1.07 (0.76 to 1.51) | 545 (1 trial) | ⊕⊝⊝⊝ very low1,2,3 |
| Serious adverse events (number) | None of the trials reported this outcome. | ||||
| Health‐related quality of life | None of the trials reported this outcome. | ||||
| *The basis for the assumed risk is the mean control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio. | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1Risk of bias: trial(s) were at high risk of bias (downgraded one level)
2Imprecision: small sample size (downgraded one level)
3Imprecision: Confidence intervals overlapped a clinically significant increase or reduction and clinically insignificant increase or reduction (downgraded one level).
Summary of findings 3. Colchicine compared with no intervention for alcoholic hepatitis (all severity).
| Colchicine compared with no intervention for alcoholic hepatitis (all severity) | |||||
|
Patient or population: participants with alcoholic hepatitis (all severity) Settings: secondary or tertiary care Intervention: colchicine Comparison: no intervention | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (trials) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| No intervention | Colchicine | ||||
|
Mortality at maximal follow‐up Follow‐up: 3 to 4 months |
348 per 1000 | 86 per 1000 (32 to 206) | OR 1.31 (0.47 to 3.64) | 139 (2 trials) | ⊕⊝⊝⊝ very low1,2,3 |
| Early mortality (mortality up to 90 days) | 217 per 1000 | 185 per 1000 (9 to 853) | OR 3.18 (0.13 to 81.01) | 69 (1 trial) | ⊕⊝⊝⊝ very low1,2,3 |
| Serious adverse events (proportion) | None of the trials reported this outcome. | ||||
| Serious adverse events (number) | None of the trials reported this outcome. | ||||
| Health‐related quality of life | None of the trials reported this outcome. | ||||
| *The basis for the assumed risk is the mean control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio. | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1Risk of bias: trial(s) were at high risk of bias (downgraded one level)
2Imprecision: small sample size (downgraded one level)
3Imprecision: Confidence intervals overlapped a clinically significant increase or reduction and clinically insignificant increase or reduction (downgraded one level).
Summary of findings 4. Insulin plus glucagon compared with no intervention for alcoholic hepatitis (all severity).
| Insulin plus glucagon compared with no intervention for alcoholic hepatitis (all severity) | |||||
|
Patient or population: participants with alcoholic hepatitis (all severity) Settings: secondary or tertiary care Intervention: insulin plus glucagon Comparison: no intervention | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (trials) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| No intervention | Insulin plus glucagon | ||||
|
Mortality at maximal follow‐up Follow‐up: 1 to 6 months |
348 per 1000 | 423 per 1000 (307 to 550) | OR 1.14 (0.69 to 1.90) | 265 (5 trials) | ⊕⊝⊝⊝ very low1,2,3 |
| Early mortality (mortality up to 90 days) | 217 per 1000 | 623 per 1000 (371 to 821) | OR 2.57 (0.92 to 7.15) | 72 (1 trial) | ⊕⊝⊝⊝ very low1,2,3 |
| Serious adverse events (proportion) | None of the trials reported this outcome. | ||||
| Serious adverse events (number) | None of the trials reported this outcome. | ||||
| Health‐related quality of life | None of the trials reported this outcome. | ||||
| *The basis for the assumed risk is the mean control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio. | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1Risk of bias: trial(s) were at high risk of bias (downgraded one level)
2Imprecision: small sample size (downgraded one level)
3Imprecision: Confidence intervals overlapped a clinically significant increase or reduction and clinically insignificant increase or reduction (downgraded one level).
Summary of findings 5. Propylthiouracil compared with no intervention for alcoholic hepatitis (all severity).
| Propylthiouracil compared with no intervention for alcoholic hepatitis (all severity) | |||||
|
Patient or population: participants with alcoholic hepatitis (all severity) Settings: secondary or tertiary care Intervention: propylthiouracil Comparison: no intervention | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (trials) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| No intervention | Propylthiouracil | ||||
|
Mortality at maximal follow‐up Follow‐up: 1 to 2 months |
348 per 1000 | 427 per 1000 (243 to 636) | OR 1.16 (0.50 to 2.72) | 108 (2 trials) | ⊕⊝⊝⊝ very low1,2,3 |
| Early mortality (mortality up to 90 days) | None of the trials reported this outcome. | ||||
| Serious adverse events (proportion) | None of the trials reported this outcome. | ||||
| Serious adverse events (number) | None of the trials reported this outcome. | ||||
| Health‐related quality of life | None of the trials reported this outcome. | ||||
| *The basis for the assumed risk is the mean control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio. | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1Risk of bias: trial(s) were at high risk of bias (downgraded one level)
2Imprecision: small sample size (downgraded one level)
3Imprecision: Confidence intervals overlapped a clinically significant increase or reduction and clinically insignificant increase or reduction (downgraded one level).
Summary of findings 6. Glucocorticosteroids compared with no intervention for severe alcoholic hepatitis.
| Glucocorticosteroids compared with no intervention for severe alcoholic hepatitis | |||||
|
Patient or population: participants with severe alcoholic hepatitis Settings: secondary or tertiary care Intervention: glucocorticosteroids Comparison: no intervention | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (trials) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| No intervention | Glucocorticosteroids | ||||
|
Mortality at maximal follow‐up Follow‐up: 2 to 12 months |
409 per 1000 | 378 per 1000 (304 to 456) | OR 0.88 (0.63 to 1.21) | 607 (2 trials) | ⊕⊝⊝⊝ very low1,2,3 |
| Early mortality (mortality up to 90 days) | 272 per 1000 | 274 per 1000 (210 to 348) | OR 1.01 (0.71 to 1.43) | 607 (2 trials) | ⊕⊝⊝⊝ very low1,2,3 |
|
Serious adverse events (proportion) Follow‐up: 12 months |
390 per 1000 | 467 per 1000 (385 to 552) | OR 1.37 (0.98 to 1.93) |
546 (1 trial) |
⊕⊝⊝⊝ very low1,2,3 |
| Serious adverse events (number) | None of the trials reported this outcome. | ||||
| Health‐related quality of life | None of the trials reported this outcome. | ||||
| *The basis for the assumed risk is the mean control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio. | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1Risk of bias: trial(s) were at high risk of bias (downgraded one level)
2Imprecision: small sample size (downgraded one level)
3Imprecision: Confidence intervals overlapped a clinically significant increase or reduction and clinically insignificant increase or reduction (downgraded one level).
Summary of findings 7. Pentoxifylline compared with no intervention for severe alcoholic hepatitis.
| Pentoxifylline compared with no intervention for severe alcoholic hepatitis | |||||
|
Patient or population: participants with severe alcoholic hepatitis Settings: secondary or tertiary care Intervention: pentoxifylline Comparison: no intervention | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (trials) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| No intervention | Pentoxifylline | ||||
|
Mortality at maximal follow‐up Follow‐up: 1 to 12 months |
409 per 1000 | 356 per 1000 (290 to 425) | OR 0.80 (0.59 to 1.07) | 726 (4 trials) | ⊕⊝⊝⊝ very low1,2,3 |
| Early mortality (mortality up to 90 days) | 272 per 1000 | 306 per 1000 (232 to 394) | OR 1.18 (0.81 to 1.74) | 545 (1 trial) | ⊕⊝⊝⊝ very low1,2,3 |
|
Serious adverse events (proportion) Follow‐up: 12 months |
390 per 1000 | 406 per 1000 (327 to 491) | OR 1.07 (0.76 to 1.51) | 545 (1 trial) | ⊕⊝⊝⊝ very low1,2,3 |
| Serious adverse events (number) | None of the trials reported this outcome. | ||||
| Health‐related quality of life | None of the trials reported this outcome. | ||||
| *The basis for the assumed risk is the mean control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio. | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1Risk of bias: trial(s) were at high risk of bias (downgraded one level)
2Imprecision: small sample size (downgraded one level)
3Imprecision: Confidence intervals overlapped a clinically significant increase or reduction and clinically insignificant increase or reduction (downgraded one level).
Summary of findings 8. Anabolic steroids compared with no intervention for alcohol‐related disorders (others).
| Anabolic steroids compared with no intervention for alcohol‐related disorders (others) | |||||
|
Patient or population: alcohol‐related disorders (others) Settings: secondary or tertiary care Intervention: anabolic steroids Comparison: no intervention | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (trials) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| No intervention | Anabolic steroids | ||||
|
Mortality at maximal follow‐up Follow‐up: 6 to 28 months |
252 per 1000 | 303 per 1000 (191 to 446) | OR 1.29 (0.70 to 2.39) | 248 (2 trials) | ⊕⊝⊝⊝ very low1,2,3 |
| Early mortality (mortality up to 90 days) | None of the trials reported this outcome. | ||||
| Serious adverse events (proportion) | None of the trials reported this outcome. | ||||
| Serious adverse events (number) | None of the trials reported this outcome. | ||||
| Health‐related quality of life | None of the trials reported this outcome. | ||||
| *The basis for the assumed risk is the mean control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio. | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1Risk of bias: trial(s) were at high risk of bias (downgraded one level)
2Imprecision: small sample size (downgraded one level)
3Imprecision: Confidence intervals overlapped a clinically significant increase or reduction and clinically insignificant increase or reduction (downgraded one level).
Summary of findings 9. Antioxidants compared with no intervention for alcohol‐related disorders (others).
| Antioxidants compared with no intervention for alcohol‐related disorders (others) | |||||
|
Patient or population: alcohol‐related disorders (others) Settings: secondary or tertiary care Intervention: antioxidants Comparison: no intervention | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (trials) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| No intervention | Antioxidants | ||||
|
Mortality at maximal follow‐up Follow‐up: 12 to 24 months |
252 per 1000 | 398 per 1000 (250 to 567) | OR 1.96 (0.99 to 3.89) | 255 (2 trials) | ⊕⊝⊝⊝ very low1,2,3,4 |
| Early mortality (mortality up to 90 days) | None of the trials reported this outcome. | ||||
| Serious adverse events (proportion) | None of the trials reported this outcome. | ||||
| Serious adverse events (number) | None of the trials reported this outcome. | ||||
| Health‐related quality of life | None of the trials reported this outcome. | ||||
| *The basis for the assumed risk is the mean control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio. | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1Risk of bias: trial(s) were at high risk of bias (downgraded one level)
2Imprecision: small sample size (downgraded one level)
3Imprecision: Confidence intervals overlapped a clinically significant increase or reduction and clinically insignificant increase or reduction (downgraded one level).
Summary of findings 10. Colchicine compared with no intervention for alcohol‐related disorders (others).
| Colchicine compared with no intervention for alcohol‐related disorders (others) | |||||
|
Patient or population: alcohol‐related disorders (others) Settings: secondary or tertiary care Intervention: colchicine Comparison: no intervention | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (trials) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| No intervention | Colchicine | ||||
|
Mortality at maximal follow‐up Follow‐up: 41 to 48 months |
252 per 1000 | 272 per 1000 (212 to 340) | OR 1.11 (0.80 to 1.53) | 604 (2 trials) | ⊕⊝⊝⊝ very low1,2,3 |
| Early mortality (mortality up to 90 days) | None of the trials reported this outcome. | ||||
| Serious adverse events (proportion) | None of the trials reported this outcome. | ||||
| Serious adverse events (number) | None of the trials reported this outcome. | ||||
| Health‐related quality of life | None of the trials reported this outcome. | ||||
| *The basis for the assumed risk is the mean control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio. | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1Risk of bias: trial(s) were at high risk of bias (downgraded one level)
2Imprecision: small sample size (downgraded one level)
3Imprecision: Confidence intervals overlapped a clinically significant increase or reduction and clinically insignificant increase or reduction (downgraded one level).
Summary of findings 11. Propylthiouracil compared with no intervention for alcohol‐related disorders (others).
| Propylthiouracil compared with no intervention for alcohol‐related disorders (others) | |||||
|
Patient or population: alcohol‐related disorders (others) Settings: secondary or tertiary care Intervention: propylthiouracil Comparison: no intervention | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (trials) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| No intervention | Propylthiouracil | ||||
|
Mortality at maximal follow‐up Follow‐up: 1 to 24 months |
252 per 1000 | 132 per 1000 (81 to 208) | OR 0.45 (0.26 to 0.78) | 423 (2 trials) | ⊕⊕⊝⊝ low1,2 |
| Early mortality (mortality up to 90 days) | None of the trials reported this outcome. | ||||
| Serious adverse events (proportion) | None of the trials reported this outcome. | ||||
| Serious adverse events (number) | None of the trials reported this outcome. | ||||
| Health‐related quality of life | None of the trials reported this outcome. | ||||
| *The basis for the assumed risk is the mean control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio. | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1Risk of bias: trial(s) were at high risk of bias (downgraded one level)
2Imprecision: small sample size (downgraded one level)
3Imprecision: Confidence intervals overlapped a clinically significant increase or reduction and clinically insignificant increase or reduction (downgraded one level).
Background
Description of the condition
Alcohol‐related liver disease or alcoholic liver disease is liver disease related to excessive alcohol consumption (BLT 2014; NCBI 2014a; NHS 2014). It includes a spectrum of liver diseases that includes alcoholic steatosis (simple fatty liver (simple steatosis)), overt alcoholic hepatitis, and alcoholic cirrhosis (Gao 2011; NCBI 2014a). Fatty liver, which indicates accumulation of fat in the liver parenchymal cells (NCBI 2014b), is usually the earliest manifestation of alcohol‐related liver disease and develops in about 90% to 95% of people who consume large quantities of alcohol (O'Shea 2010; Gao 2011). About 10% to 40% of people with fatty liver develop liver fibrosis (O'Shea 2010; Gao 2011). One fifth of people who develop liver fibrosis develop alcoholic liver cirrhosis (advanced liver fibrosis) (Gao 2011). About 10% to 35% of people who consume large quantities of alcohol may also develop alcoholic hepatitis (Gao 2011). Alcoholic hepatitis is characterised by liver parenchymal necrosis and inflammation (Gao 2011; NCBI 2014c), and presents clinically as jaundice and liver failure that usually occur after several decades of consumption of large quantities of alcohol (Lucey 2009). About 18% of people with alcoholic hepatitis progress to cirrhosis (O'Shea 2010). A significant proportion of people may develop alcoholic cirrhosis without developing alcoholic hepatitis (O'Shea 2010).
Alcohol increases the fats that reach the liver from the intestine, increases fatty acid synthesis (lipogenesis), and decreases the breakdown of fatty acids (by decreasing beta‐oxidation of fatty acids) resulting in accumulation of fat in liver cells (Lucey 2009; Gao 2011). The process of alcohol metabolism results in generation of reactive oxygen species, lipid peroxidation, mitochondrial glutathione depletion, and S‐adenosylmethionine depletion (Lucey 2009; Gao 2011). This sensitises liver cells to injury (Gao 2011). Acetate, a breakdown product of alcohol, causes inflammation (Gao 2011). In addition, alcohol increases gut permeability and translocation of bacteria from the bowel resulting in increased bacterial lipopolysaccharides, which in turn cause inflammation by activation of Kupffer cells (Lucey 2009; Gao 2011). Thus, alcohol can cause alcoholic hepatitis (Lucey 2009; Gao 2011). Alcohol may also inhibit proliferation of liver cells, thereby decreasing liver regeneration in response to injury (Gao 2011). Acetaldehyde, another breakdown product of alcohol, activates hepatic stellate cells (Gao 2011). Activation of Kupffer cells also promotes fibrogenesis by activating the hepatic stellate cells (Gao 2011). In addition to activation of Kupffer cells, increased lipopolysaccharides due to translocation of bacteria from the bowel also promotes fibrogenesis directly by activating hepatic stellate cells (Gao 2011). Natural killer cells destroy activated hepatic stellate cells and produce interferon‐gamma (Gao 2011). Alcohol inhibits natural killer cells and so promotes fibrosis (Gao 2011).
The true prevalence of alcohol‐related liver disease is difficult to estimate. Overall, alcohol causes 38 deaths per 100,000 men and 28 deaths per 100,000 women every year in Europe due to liver cirrhosis, alcoholic hepatitis, alcohol‐related injuries, and cancers attributable to alcohol consumption (WHO 2013). Worldwide, of every 100 deaths, alcohol causes 5.9 deaths (7.6 deaths per 100 deaths in men and four deaths per 100 deaths in women) (WHO 2014). About 30% of people with alcoholic hepatitis die within three months of hospital admission (Whitfield 2009). Alcohol is the most common cause of liver cirrhosis in Europe (Blachier 2013). Cirrhosis has two phases ‐ an asymptomatic 'compensated cirrhosis' phase and a symptomatic 'decompensated cirrhosis' phase characterised by clinical symptoms such as upper gastrointestinal bleeding from varices, ascites, encephalopathy, jaundice, or renal failure (D'Amico 2006). The median survival after compensated liver disease can be more than 10 years, while that of decompensated liver disease is less than two years (D'Amico 2006). The only definitive treatment for decompensated liver cirrhosis is liver transplantation. Alcoholic cirrhosis is the second most common cause of liver transplantation in the USA (SRTR 2012). The median survival after liver transplantation is in excess of 10 years (Duffy 2010; SRTR 2012; Schoening 2013). There is also improvement in the health‐related quality of life of people with chronic liver disease after liver transplantation (Yang 2014).
Description of the intervention
Alcohol abstinence is the main form of preventing and limiting the damage due to alcohol‐related liver disease (O'Shea 2010; Gao 2011). Disulfiram, frequently prescribed to promote alcohol abstinence, is not recommended in people with alcohol‐related liver disease because of its liver toxicity (Gao 2011). Various interventions have been used for the treatment of alcohol‐related liver disease. These include pharmacological interventions such as glucocorticosteroids, anabolic‐androgenic steroids, pentoxifylline, anti‐tumour necrosis factor (infliximab and etanercept), colchicine, S‐adenosylmethionine, N‐acetyl cysteine, propylthiouracil, vitamin E and other antioxidants, and milk thistle (silymarin or Silybum marianum extract); nutritional supplement, for example, protein and vitamin supplementation; and psychotherapy for alcohol abstinence (Ferri 2006; Rambaldi 2006; Rambaldi 2015; Stewart 2007; Lucey 2009; Whitfield 2009; O'Shea 2010; Fede 2011; Gao 2011; Nguyen‐Khac 2011; Mathurin 2013). Glucocorticoids may be combined with other treatments (Stewart 2007; O'Shea 2010; Nguyen‐Khac 2011; Mathurin 2013).
How the intervention might work
Glucocorticosteroids, pentoxifylline, and anti‐tumour necrosis factor are aimed at decreasing the inflammation (Lucey 2009; Gao 2011; Mathurin 2013). In addition to its action on tumour necrosis factor (TNF) (and hence its effect on inflammation), pentoxifylline might prevent hepatorenal syndrome by maintaining kidney function (Lucey 2009; Mathurin 2013). S‐adenosylmethionine (SAMe), N‐acetyl cysteine (NAC), propylthiouracil, Vitamin E and other antioxidants, and milk thistle (silymarin) are aimed at decreasing the oxidative damage to liver cells (Hicks 1992; Stewart 2007; Lucey 2009; Abenavoli 2010; Gao 2011; Nguyen‐Khac 2011; Anstee 2012). Anabolic‐androgenic steroids have been evaluated in alcohol‐related liver disease because of their anabolic (muscle building) effect (Lucey 2009), and direct effects on liver metabolism (Gluud 1988). Colchicine has been evaluated in alcohol‐related liver disease because of its anti‐inflammatory and anti‐fibrogenic properties (O'Shea 2010).
Why it is important to do this review
We included only pharmacological therapies used in the treatment of alcoholic liver disease with the aim of reducing liver injury. The current guidelines on management of alcohol‐related liver disease by the European Association for the Study of Liver (EASL) and American Association for the Study of Liver Diseases (AASLD) are similar in terms of recommending glucocorticoids for severe alcoholic hepatitis unless there are contraindications, in which case pentoxifylline is recommended (O'Shea 2010; EASL 2012). EASL guidelines also suggest that N‐acetyl cysteine may be useful in people with severe alcoholic fatty liver disease receiving corticosteroids (EASL 2012). The role of the other pharmacological agents, the benefit of combination therapies, and the relative ranking of these different interventions is not clear from these guidelines. Network meta‐analyses allow combination of the direct evidence and indirect evidence, and allow ranking of different interventions in terms of the different outcomes (Salanti 2011; Salanti 2012). This systematic review and attempted network meta‐analysis aimed to provide the best level of evidence for the role of different pharmacological interventions in the treatment of people with alcohol‐related liver disease.
Objectives
To assess the comparative benefits and harms of different pharmacological interventions in the management of alcohol‐related liver disease through a network meta‐analysis and to generate rankings of the available pharmacological interventions according to their safety and efficacy in order to identify potential treatments. However, even in the subgroup of participants when the potential effect modifiers appeared reasonably similar across comparisons, there was evidence of inconsistency by one or more methods of assessment of inconsistency. Therefore, we assessed the comparative benefits and harms of different interventions using standard Cochrane methodology. However, we have presented the methods of the network meta‐analysis for one of the subgroups where the potential effect modifiers appeared reasonably similar across comparisons; the data used for network meta‐analysis and the results in Appendix 1; Appendix 2; Appendix 3; Appendix 4; Appendix 5; Appendix 6. Once new data appear allowing the conduct of a network meta‐analysis which is reliable, we will perform a revised analysis, and then, we will move it back into the Methods section and Results section of the Cochrane review.
Methods
Criteria for considering studies for this review
Types of studies
We considered only randomised clinical trials for this meta‐analysis irrespective of language, publication status, or date of publication. We excluded studies of other design because of the risk of bias in such studies. We are all aware that such exclusions make us focus much more on potential benefits and not fully assess the risks of serious adverse events as well as risks of adverse events.
Types of participants
We included randomised clinical trials involving participants with alcohol‐related liver disease irrespective of the method of diagnosis of the disease and severity of alcohol‐related liver disease. We excluded randomised clinical trials in which participants had undergone liver transplantation previously. We also excluded randomised clinical trials in which participants had other causes of chronic liver disease such as hepatitis C virus, hepatitis B virus, and hepatitis delta virus infections along with alcohol‐related liver disease, or non‐alcohol‐related liver disease.
Types of interventions
We included any of the following pharmacological interventions that are possible treatments for alcohol‐related liver disease (i.e. alcoholic fatty liver, alcoholic hepatitis, alcoholic cirrhosis), and which can be compared with each other either alone or in combination or with placebo or no intervention.
The interventions that we considered a priori included:
glucocorticosteroids;
anabolic‐androgenic steroids;
pentoxifylline;
anti‐tumour necrosis factor (infliximab and etanercept);
colchicine;
S‐adenosylmethionine;
N‐acetyl cysteine;
propylthiouracil;
antioxidants (including vitamin E);
milk thistle (silymarin).
We included anti‐tumour necrosis factor (anti‐TNF), granulocyte stimulation factor (GSF), rifaximin, and ursodeoxycholic acid (UDCA), used either alone or in a combination of the above interventions after searching the references. We have reported the findings for these interventions in the Results and Discussion sections of the review.
In this systematic review, we included only pharmacological interventions where the target of treatment is the liver, i.e. we did not include nutritional, psychotherapy, and other supportive therapy required to manage complications such as infections, portal hypertension, or alcohol withdrawal syndrome, or therapies targeted at promoting alcohol abstinence or decreasing alcohol dependence.
Types of outcome measures
We assessed the comparative benefits and harms (and report the relative ranking) of available pharmacological interventions aimed at reducing liver injury with alcohol‐related liver disease for the following outcomes.
Primary outcomes
Mortality at maximal follow‐up.
Early mortality (up to three months).
-
Adverse events. Depending on the availability of data, we planned to classify adverse events as serious or non‐serious. We defined a non‐serious adverse event as any untoward medical occurrence not necessarily having a causal relationship with the intervention but resulting in a dose reduction or discontinuation of treatment (any time after commencement of treatment) (ICH‐GCP 1997). We defined a serious adverse event as any event that would increase mortality; is life‐threatening; requires hospitalisation; results in persistent or significant disability; is a congenital anomaly/birth defect; or any important medical event that might jeopardise the person or require intervention to prevent it (ICH‐GCP 1997). We used the definition used by study authors for non‐serious and serious adverse events:
proportion of participants with serious adverse events;
number of serious adverse events.
-
Health‐related quality of life as defined in the included trials using a validated scale such as EQ‐5D or 36‐item Short Form (SF‐36) (EuroQol 2014; Ware 2014):
short term (up to one year);
medium term (one to five years);
long term (beyond five years).
We planned to consider long‐term health‐related quality of life more important than short‐term or medium‐term health‐related quality of life, although short‐term and medium‐term health‐related quality of life are also important primary outcomes.
Secondary outcomes
-
Liver transplantation (maximal follow‐up):
proportion of participants with liver transplantation;
time to liver transplantation.
-
Decompensated liver disease (maximal follow‐up):
proportion of participants with decompensated liver disease;
time to liver decompensation.
We had to calculate the decompensated liver disease rate rather than proportion since it was not always clear whether these episodes of decompensation occurred in the same participant or different participants.
-
Cirrhosis (maximal follow‐up) (in participants without cirrhosis):
proportion of participants with cirrhosis;
time to cirrhosis.
Proportion of participants with hepatocellular carcinoma (maximal follow‐up).
-
Any adverse events:
proportion of participants with any type of adverse event;
number of any type of adverse event.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, and Science Citation Index Expanded (Royle 2003) from inception to February 2017 for randomised clinical trials comparing two or more of the above interventions (including placebo or no intervention) without applying any language restrictions. We searched for all possible comparisons formed by the interventions of interest. To identify further ongoing or completed trials, we also searched the World Health Organization International Clinical Trials Registry Platform search portal (www.who.int/ictrp/en/), which searches various trial registers, including ISRCTN and ClinicalTrials.gov. Appendix 7 shows the search strategies that we used.
Searching other resources
We searched the references of the identified trials and the existing Cochrane reviews on alcohol‐related liver disease to identify additional trials for inclusion.
Data collection and analysis
Selection of studies
Two review authors (EB and MK) independently identified trials for inclusion by screening the titles and abstracts. We sought full‐text articles for any references that at least one of the review authors identified for potential inclusion. We selected trials for inclusion based on the full‐text articles, or, if no full text existed, then the abstracts of the trial. We listed the excluded full‐text references with reasons for their exclusion in the 'Characteristics of excluded trials' table. We also listed any ongoing trials identified primarily through the search of the clinical trial registers for further follow‐up. We resolved discrepancies through discussion and by arbitration with KG, DT, and ET.
Data extraction and management
Two review authors (EB and MK) independently extracted the following data.
-
Outcome data (for each outcome and for each intervention group whenever applicable):
number of participants randomised;
number of participants included for the analysis;
number of participants with events for binary outcome, mean and standard deviation for continuous outcomes, number of events for count outcomes, and the number of participants with events and the mean follow‐up period for time‐to‐event outcomes;
definition of outcomes or scale used if appropriate.
-
Data on potential effect modifiers:
participant characteristics such as age, sex, co‐morbidities, and proportion of participants with severe alcoholic hepatitis;
details of the intervention and control (including dose, frequency, and duration);
risk of bias (assessment of risk of bias in included trials).
-
Other data:
year and language of publication;
country in which the participants were recruited;
year(s) in which the trial was conducted;
inclusion and exclusion criteria;
follow‐up time points of the outcome.
Data were not available separately for different severities of alcoholic hepatitis. Therefore, the subgroup analysis of severe alcoholic hepatitis was based on a study level. We sought unclear or missing information by contacting the trial authors. If there was any doubt whether trials shared the same participants, completely or partially (by identifying common authors and centres), we attempted to contact the trial authors to clarify whether the trial report was duplicated. We resolved any differences in opinion through discussion.
Assessment of risk of bias in included studies
We followed the guidance given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) and described in the Cochrane Hepato‐Biliary Group Module (Gluud 2016) to assess the risk of bias in included studies. Specifically, we assessed the risk of bias in included trials for the following domains using the methods below (Schulz 1995; Moher 1998; Kjaergard 2001; Wood 2008; Savović 2012a; Savović 2012b; Lundh 2017).
Allocation sequence generation
Low risk of bias: the study authors performed sequence generation using computer random number generation or a random number table. Drawing lots, tossing a coin, shuffling cards, and throwing dice were adequate if an independent person not otherwise involved in the study performed them.
Unclear risk of bias: the study authors did not specify the method of sequence generation.
High risk of bias: the sequence generation method was not random. We planned to only include such studies for assessment of harms.
Allocation concealment
Low risk of bias: the participant allocations could not have been foreseen in advance of, or during, enrolment. A central and independent randomisation unit controlled allocation. The investigators were unaware of the allocation sequence (e.g. if the allocation sequence was hidden in sequentially numbered, opaque, and sealed envelopes).
Unclear risk of bias: the study authors did not describe the method used to conceal the allocation so the intervention allocations may have been foreseen before, or during, enrolment.
High risk of bias: it is likely that the investigators who assigned the participants knew the allocation sequence. We planned to only include such studies for assessment of harms.
Blinding of participants and personnel
Low risk of bias: any of the following: no blinding or incomplete blinding, but the review authors judged that the outcome was not likely to be influenced by lack of blinding; or blinding of participants and key study personnel ensured, and it was unlikely that the blinding could have been broken.
Unclear risk of bias: any of the following: insufficient information to permit judgement of 'low risk' or 'high risk'; or the trial did not address this outcome.
High risk of bias: any of the following: no blinding or incomplete blinding, and the outcome was likely to be influenced by lack of blinding; or blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome was likely to be influenced by lack of blinding.
Blinded outcome assessment
Low risk of bias: any of the following: no blinding of outcome assessment, but the review authors judged that the outcome measurement was not likely to be influenced by lack of blinding; or blinding of outcome assessment ensured, and unlikely that the blinding could have been broken.
Unclear risk of bias: any of the following: insufficient information to permit judgement of 'low risk' or 'high risk'; or the trial did not address this outcome.
High risk of bias: any of the following: no blinding of outcome assessment, and the outcome measurement was likely to be influenced by lack of blinding; or blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement was likely to be influenced by lack of blinding.
Incomplete outcome data
Low risk of bias: missing data were unlikely to make treatment effects depart from plausible values. The study used sufficient methods, such as multiple imputation, to handle missing data.
Unclear risk of bias: there was insufficient information to assess whether missing data in combination with the method used to handle missing data were likely to induce bias on the results.
High risk of bias: the results were likely to be biased due to missing data.
Selective outcome reporting
Low risk of bias: the trial reported the following predefined outcomes: (e.g. at least one of the long‐term outcomes related to the disease process, namely, mortality, decompensated liver disease, or requirement for transplantation along with treatment‐related adverse events). If the original trial protocol was available, the outcomes should have been those called for in that protocol. If the trial protocol was obtained from a trial registry (e.g. www.clinicaltrials.gov), the outcomes sought should have been those enumerated in the original protocol if the trial protocol was registered before or at the time that the trial was begun. If the trial protocol was registered after the trial was begun, those outcomes were not considered to be reliable.
Unclear risk of bias: not all predefined, or clinically relevant and reasonably expected, outcomes were reported fully, or it was unclear whether data on these outcomes were recorded or not.
High risk of bias: one or more predefined or clinically relevant and reasonably expected outcomes were not reported, despite the fact that data on these outcomes should have been available and even recorded.
For‐profit bias
Low risk of bias: the trial appeared to be free of industry sponsorship or other type of for‐profit support that could manipulate the trial design, conductance, or results of the trial.
Uncertain risk of bias: the trial may or may not have been free of for‐profit bias as no information on clinical trial support or sponsorship was provided.
High risk of bias: the trial was sponsored by industry or received other type of for‐profit support.
Other bias
Low risk of bias: the trial appeared to be free of other components (e.g. inappropriate control or dose or administration of control, baseline differences, early stopping) that could put it at risk of bias.
Uncertain risk of bias: the trial may or may not have been free of other components that could put it at risk of bias.
High risk of bias: there were other factors in the trial that could put it at risk of bias (e.g. baseline differences, early stopping).
We considered a trial at low risk of bias if we assessed the trial to be at low risk of bias across all domains. Otherwise, we considered trials to be at high risk of bias.
Measures of treatment effect
For dichotomous variables (e.g. short‐term and medium‐term mortality or liver transplantation, proportion of participants with adverse events, decompensated liver disease, cirrhosis, or hepatocellular carcinoma), we calculated the odds ratio (OR) with 95% confidence intervals (CIs). For continuous variables (e.g. health‐related quality of life reported on the same scale), we planned to calculate the mean difference (MD) with 95% CI. We planned to use standardised mean difference (SMD) values with 95% CI for health‐related quality of life if included trials used different scales. For count outcomes (e.g. number of adverse events), we calculated the rate ratio with 95% CI. For time‐to‐event data (e.g. mortality at maximal follow‐up or requirement for liver transplantation, time to liver decompensation, and time to cirrhosis), we planned to use the hazard ratio (HR) with 95% confidence intervals. However, because of the nature of the data available, we calculated the odds ratio with 95% CI for these outcomes also. We also calculated Trial Sequential Analysis‐adjusted CI to control random errors (Thorlund 2011).
Unit of analysis issues
The unit of analysis were people with alcohol‐related liver disease according to the intervention group to which they were randomly assigned.
Cluster‐randomised clinical trials
As expected, we did find cluster‐randomised clinical trials. However, had we found any such trials, we planned to include them provided that the effect estimate adjusted for cluster correlation is available.
Cross‐over randomised clinical trials
We included the outcomes after the period of first intervention since alcohol‐related liver disease is a chronic unstable disease and the treatments could potentially have a residual effect. On the other hand, alcoholic hepatitis is an acute illness and the intervention given initially can influence the outcome of the patients with alcoholic disease.
Trials with multiple intervention groups
We collected data for all trial intervention groups that met the inclusion criteria. The codes for analysis, that we used, accounts for the correlation between the effect sizes from studies with more than two groups.
Dealing with missing data
We performed an intention‐to‐treat analysis whenever possible (Newell 1992). Otherwise, we used the data that were available to us (e.g. a trial might have reported only per‐protocol analysis results). As such per‐protocol analyses may be biased, we planned to conduct best‐worst case scenario analyses (good outcome in intervention group and bad outcome in control group) and worst‐best case scenario analyses (bad outcome in intervention group and good outcome in control group) as sensitivity analyses whenever possible. However, we did not perform these analyses because of lack of sufficient information.
For continuous outcomes, we planned to impute the standard deviation from P values according to guidance given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). If the data were likely to be normally distributed, we planned to use the median for meta‐analysis when the mean was not available. If it was not possible to calculate the standard deviation from the P value or the confidence intervals, we planned to impute the standard deviation using the largest standard deviation in other trials for that outcome. This form of imputation may decrease the weight of the study for calculation of mean differences and may bias the effect estimate to no effect for calculation of standardised mean differences (Higgins 2011).
Assessment of heterogeneity
We assessed clinical and methodological heterogeneity by carefully examining the characteristics and design of included trials. Different study designs and risk of bias may contribute to methodological heterogeneity. We used the I2 test and Chi2 test for heterogeneity, and overlapping of CIs to assess heterogeneity.
Assessment of reporting biases
We used visual asymmetry on a funnel plot to explore reporting bias in the presence of at least 10 trials that could be included for a comparison (Egger 1997; Macaskill 2001). In the presence of heterogeneity that could be explained by subgroup analysis, we planned to produce a funnel plot for each subgroup in the presence of an adequate number of trials (at least 10 trials). We used the linear regression approach described by Egger 1997 to determine funnel plot asymmetry.
We also considered selective reporting as evidence of reporting bias.
Data synthesis
We performed the meta‐analyses according to the recommendations of Cochrane (Higgins 2011), using the software package Review Manager 5 (RevMan 2014). We used a random‐effects model (DerSimonian 1986) and a fixed‐effect model (DeMets 1987). In the case of a discrepancy between the two models, we reported the more conservative results; otherwise, we reported only the results from the fixed‐effect model.
We performed the direct comparisons using the same codes and the same technical details.
Calculation of required information size and Trial Sequential Analysis
For calculation of the required information size, see Appendix 8. We performed Trial Sequential Analysis to control the risk of random errors (Wetterslev 2008; Thorlund 2011; TSA 2011; Wetterslev 2017) when there were at least two trials included for the comparisons involving an active intervention versus no intervention for the following outcomes: mortality at maximal follow‐up, serious adverse events (proportion), and health‐related quality of life. These are outcomes that determine whether an intervention should be used. We used an alpha error as per guidance of Jakobsen 2014, power of 90% (beta error of 10%), a relative risk reduction of 20%, a control group proportion observed in the trials, and the diversity observed in the meta‐analysis.
Subgroup analysis and investigation of heterogeneity
We planned to assess the differences in the effect estimates between the following subgroups.
Trials with low risk of bias compared to trials with high risk of bias.
Biopsy‐confirmed alcohol‐related liver disease.
Different severity of alcoholic hepatitis.
Different regimens of pharmacological interventions. For example, different doses and different durations.
We planned to use the Chi2 test for subgroup differences to identify subgroup differences.
Sensitivity analysis
If a trial reported only per‐protocol analysis results, we planned to re‐analyse the results using the best‐worst scenario and worst‐best case scenario analyses as sensitivity analyses whenever possible.
Presentation of results
We presented the results in a 'Summary of findings' table format, downgrading the quality of the evidence for risk of bias, inconsistency, indirectness, imprecision, and publication bias using GRADE (Guyatt 2011). We presented the 'Summary of findings' tables for primary outcomes comparing active interventions versus no interventions when there were at least two trials for at least one of the primary outcomes.
Results
Description of studies
Results of the search
We identified 11,178 references through electronic searches of CENTRAL (Wiley) (n = 1406), MEDLINE (OvidSP) (n = 5901), Embase (OvidSP) (n = 1830), Science Citation Index expanded (n = 1942), ClinicalTrials.gov (n = 51) and WHO Trials register (n = 48). After removing duplicate references, there were 8021 references. We excluded 7866 clearly irrelevant references through reading titles and abstracts. We identified two references by reference searching. We retrieved a total of 157 full text references for further assessment in detail. We excluded 29 references (27 trials) for the reasons stated in the Characteristics of excluded studies. Thus, we included a total of 81 trials described in 128 references (Characteristics of included studies). The reference flow is shown in Figure 1.
1.
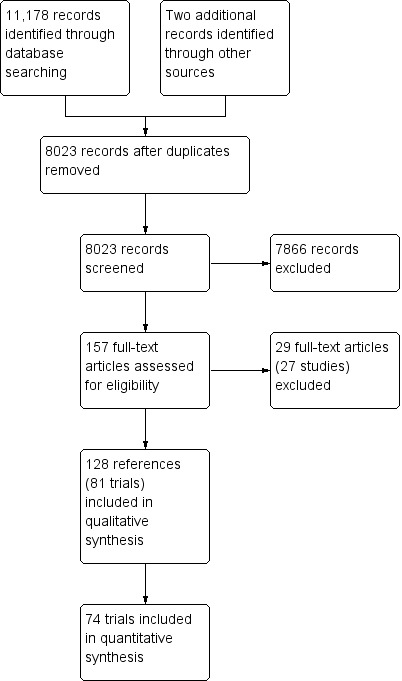
Study flow diagram.
Included studies
Of the 81 trials included in the review, 50 trials were conducted exclusively in people with acute alcoholic hepatitis (Helman 1971; Porter 1971; Campra 1973; Resnick 1974; Blitzer 1977; Maddrey 1978; Shumaker 1978; Depew 1980; Baker 1981; Serrano‐Cancino 1981; Halle 1982; Mirouze 1982; Theodossi 1982; Radvan 1983; Mendenhall 1984; Carithers 1989; Trinchet 1989a; Trinchet 1989b; Akriviadis 1990; Bird 1991; McHutchinson 1991; Ramond 1992; Trinchet 1992; Richardet 1993; Bird 1998; Akriviadis 2000; Spahr 2002; Mezey 2004; Naveau 2004; Stigliano 2005; Paladugu 2006; Phillips 2006; Stewart 2007; Boetticher 2008; De 2009; Popescu 2009; Lebrec 2010; Moreno 2010; Nguyen‐Khac 2011; Garrido Garcia 2012; Sidhu 2012a; Sidhu 2012b; Mathurin 2013; De 2014; Park 2014; Singh 2014; Basu 2015; Higuera de la Tijera 2015; Thursz 2015; Tkachenko 2016), while 31 trials were conducted in people with different alcoholic disorders (fatty liver, alcoholic hepatitis, and cirrhosis) (Fenster 1966; Geminiani 1979; Orrego 1979; Pierri 1985; Salvagnini 1985; Gluud 1986; Bories 1987; Orrego 1987; Feher 1989; Takase 1989; Plevris 1991; Sainz 1992; Keiding 1994; De la Maza 1995; Lotterer 1995; Diaz Belmont 1996; Fleig 1997; Velussi 1997; Caballeria 1998; Colman 1998; Pares 1998; Mato 1999; Stenner 2000; Cortez‐Pinto 2002; Lucena 2002; De Silva 2003; Pelletier 2003; Morgan 2005; Fernandez‐Rodriguez 2008; Medici 2011; Kim 2012).
Acute alcoholic hepatitis
We included a total of 4484 participants in 50 trials on acute alcoholic hepatitis (Helman 1971; Porter 1971; Campra 1973; Resnick 1974; Blitzer 1977; Maddrey 1978; Shumaker 1978; Depew 1980; Baker 1981; Serrano‐Cancino 1981; Halle 1982; Mirouze 1982; Theodossi 1982; Radvan 1983; Mendenhall 1984; Carithers 1989; Trinchet 1989a; Trinchet 1989b; Akriviadis 1990; Bird 1991; McHutchinson 1991; Ramond 1992; Trinchet 1992; Richardet 1993; Bird 1998; Akriviadis 2000; Spahr 2002; Mezey 2004; Naveau 2004; Stigliano 2005; Paladugu 2006; Phillips 2006; Stewart 2007; Boetticher 2008; De 2009; Popescu 2009; Lebrec 2010; Moreno 2010; Nguyen‐Khac 2011; Garrido Garcia 2012; Sidhu 2012a; Sidhu 2012b; Mathurin 2013; De 2014; Park 2014; Singh 2014; Basu 2015; Higuera de la Tijera 2015; Thursz 2015; Tkachenko 2016). A total of 26 interventions (25 active and one inactive interventions) were evaluated in the 50 trials. One trial was a cross‐over randomised clinical trial (Richardet 1993); and the remaining trials were parallel‐group design trials. Two trials had three interventions (Mendenhall 1984; Basu 2015); two trials had four interventions (Higuera de la Tijera 2015; Thursz 2015); and the remaining trials had two interventions. A total of 48 trials (4335 participants) contributed to one or more outcomes.
A total of 2545 participants included in 19 trials had severe acute alcoholic hepatitis, defined as Maddrey Discriminant Function (DF) > 32 (Ramond 1992; Akriviadis 2000; Spahr 2002; Naveau 2004; Paladugu 2006; De 2009; Moreno 2010; Nguyen‐Khac 2011; Garrido Garcia 2012; Sidhu 2012a; Sidhu 2012b; Mathurin 2013; De 2014; Park 2014; Singh 2014; Basu 2015; Higuera de la Tijera 2015; Thursz 2015; Tkachenko 2016). A total of 13 interventions (12 active and one inactive interventions) were evaluated in the 19 trials. All the trials were parallel‐group design trials. One trial had three interventions (Basu 2015); two trials had four interventions (Higuera de la Tijera 2015; Thursz 2015); and the remaining trials had two interventions. A total of 18 trials (2477 participants contributed to one or more outcomes).
Further details about the mean age in the participants, proportion of females, inclusion and exclusion criteria, details of the intervention, and the outcomes reported are available in the Characteristics of included studies.
Source of funding: Eleven trials were funded by parties with vested interest in the results (Resnick 1974; Blitzer 1977; Shumaker 1978; Baker 1981; Trinchet 1989a; Trinchet 1989b; Bird 1991; Akriviadis 2000; Spahr 2002; Naveau 2004; Mathurin 2013). Sixteen trials were funded by parties without vested interest in the results (Helman 1971; Porter 1971; Maddrey 1978; Halle 1982; Carithers 1989; Akriviadis 1990; Ramond 1992; Trinchet 1992; Bird 1998; Mezey 2004; Stewart 2007; Boetticher 2008; Sidhu 2012a; Park 2014; Higuera de la Tijera 2015; Thursz 2015). The source of funding was not reported in the remaining trials.
Alcohol‐related liver diseases (others)
We included a total of 3695 participants in 31 trials on alcohol‐related liver disease (Fenster 1966; Geminiani 1979; Orrego 1979; Pierri 1985; Salvagnini 1985; Gluud 1986; Bories 1987; Orrego 1987; Feher 1989; Takase 1989; Plevris 1991; Sainz 1992; Keiding 1994; De la Maza 1995; Lotterer 1995; Diaz Belmont 1996; Fleig 1997; Velussi 1997; Caballeria 1998; Colman 1998; Pares 1998; Mato 1999; Stenner 2000; Cortez‐Pinto 2002; Lucena 2002; De Silva 2003; Pelletier 2003; Morgan 2005; Fernandez‐Rodriguez 2008; Medici 2011; Kim 2012). A total of 15 interventions (14 active and one inactive interventions) were evaluated in the 31 trials. One trial was a cross‐over randomised clinical trial (Plevris 1991); the remaining trials were parallel‐group design trials. All the trials had only two interventions. A total of 26 trials (3212 participants contributed to one or more outcomes).
Further details about the mean age in the participants, proportion of females, inclusion and exclusion criteria, details of the intervention, and the outcomes reported are available in the Characteristics of included studies.
Source of funding: Twelve trials were funded by parties with vested interest in the results (Fenster 1966; Orrego 1979; Orrego 1987; Keiding 1994; De la Maza 1995; Pares 1998; Mato 1999; Lucena 2002; De Silva 2003; Pelletier 2003; Fernandez‐Rodriguez 2008; Medici 2011). Three trials were funded by parties without vested interest in the results (Gluud 1986; Cortez‐Pinto 2002; Kim 2012). The source of funding was not reported in the remaining trials.
Excluded studies
Twenty‐seven studies were excluded because of the reasons listed in the Characteristics of excluded studies. While the reasons for exclusion of 24 studies are clear and do not warrant further discussion, exclusion of three studies warrants further discussion (Mathurin 1996; Spahr 2008; Kolasani 2016). Mathurin 1996 reported the long‐term follow‐up of participants included in Ramond 1992; however, at the end of the study period, all the participants were administered the intervention, and the randomisation was lost. Therefore, we did not include this study in our analysis (Ramond 1992). In Spahr 2008 and Kolasani 2016, the intervention and control groups were allowed to take other drugs targeted at treatment of alcoholic liver disease as per clinicians' discretion. We accepted only when it was possible to know that the co‐interventions were administered equally in the intervention and control groups of the trial; therefore, we excluded these two studies (Spahr 2008; Kolasani 2016).
Risk of bias in included studies
The risk of bias in trials is summarised in Figure 2 and Figure 3. As shown in these figures, none of the trials were at low risk of bias in all the domains and were considered to be at high risk of bias.
2.
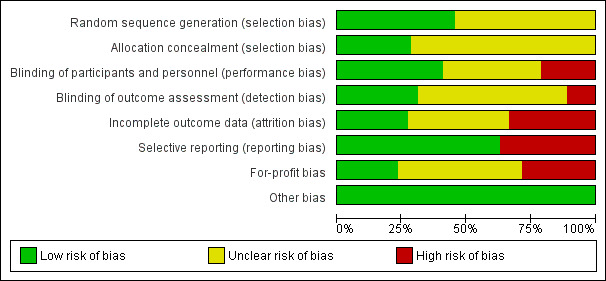
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included trials.
3.
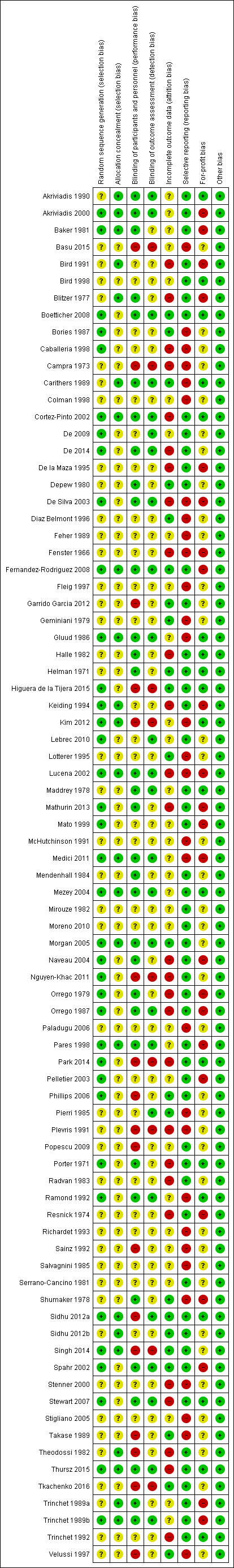
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Alcoholic hepatitis
Twenty‐one trials were at low risk of bias due to random sequence generation (Porter 1971; Baker 1981; Trinchet 1989b; Ramond 1992; Akriviadis 2000; Spahr 2002; Mezey 2004; Naveau 2004; Phillips 2006; Stewart 2007; Boetticher 2008; De 2009; Lebrec 2010; Nguyen‐Khac 2011; Sidhu 2012a; Mathurin 2013; De 2014; Park 2014; Singh 2014; Higuera de la Tijera 2015; Thursz 2015). The remaining trials were at unclear risk of bias.
Fourteen trials were at low risk of bias due allocation concealment (Blitzer 1977; Baker 1981; Theodossi 1982; Carithers 1989; Trinchet 1989a; Trinchet 1989b; Akriviadis 1990; Bird 1991; Akriviadis 2000; Mezey 2004; Sidhu 2012a; Sidhu 2012b; Singh 2014; Thursz 2015).The remaining trials were at unclear risk of bias.
Overall, seven trials were at low risk of bias due to both random sequence generation and allocation concealment and were considered to be at low risk of selection bias (Baker 1981; Trinchet 1989b; Akriviadis 2000; Mezey 2004; Sidhu 2012a; Singh 2014; Thursz 2015).
Alcohol‐related liver disease (others)
Sixteen trials were at low risk of bias due to random sequence generation (Orrego 1979; Gluud 1986; Bories 1987; Orrego 1987; Keiding 1994; Caballeria 1998; Pares 1998; Mato 1999; Cortez‐Pinto 2002; Lucena 2002; De Silva 2003; Pelletier 2003; Morgan 2005; Fernandez‐Rodriguez 2008; Medici 2011; Kim 2012). The remaining trials were at unclear risk of bias.
Nine trials were at low risk of bias due allocation concealment (Gluud 1986; Keiding 1994; Pares 1998; Cortez‐Pinto 2002; Lucena 2002; Morgan 2005; Fernandez‐Rodriguez 2008; Medici 2011; Kim 2012). The remaining trials were at unclear risk of bias.
Overall, nine trials were at low risk of bias due to both random sequence generation and allocation concealment, and were considered to be at low risk of selection bias (Gluud 1986; Keiding 1994; Pares 1998; Cortez‐Pinto 2002; Lucena 2002; Morgan 2005; Fernandez‐Rodriguez 2008; Medici 2011; Kim 2012)
Blinding
Alcoholic hepatitis
Twenty‐three trials were at low risk of performance bias (Helman 1971; Porter 1971; Blitzer 1977; Maddrey 1978; Shumaker 1978; Depew 1980; Baker 1981; Halle 1982; Mendenhall 1984; Carithers 1989; Trinchet 1989a; Trinchet 1989b; Akriviadis 1990; Ramond 1992; Akriviadis 2000; Spahr 2002; Mezey 2004; Naveau 2004; Stewart 2007; Boetticher 2008; Mathurin 2013; De 2014; Thursz 2015). Twelve trials were at high risk of performance bias (Campra 1973; Theodossi 1982; Phillips 2006; Popescu 2009; Nguyen‐Khac 2011; Garrido Garcia 2012; Sidhu 2012a; Park 2014; Singh 2014; Basu 2015; Higuera de la Tijera 2015; Tkachenko 2016). The remaining trials were at unclear risk of performance bias.
Fifteen trials were at low risk of detection bias (Maddrey 1978; Carithers 1989; Trinchet 1989b; Akriviadis 1990; Ramond 1992; Akriviadis 2000; Spahr 2002; Mezey 2004; Stewart 2007; Boetticher 2008; De 2009; Lebrec 2010; Sidhu 2012a; De 2014; Thursz 2015). Seven trials were at high risk of detection bias (Campra 1973; Nguyen‐Khac 2011; Park 2014; Singh 2014; Basu 2015; Higuera de la Tijera 2015; Tkachenko 2016). The remaining trials were at unclear risk of detection bias.
Overall, 12 trials were at low risk of performance bias and detection bias (Maddrey 1978; Carithers 1989; Trinchet 1989b; Akriviadis 1990; Ramond 1992; Akriviadis 2000; Spahr 2002; Mezey 2004; Stewart 2007; Boetticher 2008; De 2014; Thursz 2015).
Alcohol‐related liver disease (others)
Ten trials were at low risk of performance bias (Orrego 1979; Gluud 1986; Orrego 1987; Pares 1998; Cortez‐Pinto 2002; Lucena 2002; De Silva 2003; Morgan 2005; Fernandez‐Rodriguez 2008; Medici 2011). Five trials were at high risk of performance bias (Takase 1989; Plevris 1991; Sainz 1992; Velussi 1997; Kim 2012). The remaining trials were at unclear risk of performance bias.
Ten trials were at low risk of detection bias (Pierri 1985; Gluud 1986; Orrego 1987; Pares 1998; Cortez‐Pinto 2002; Lucena 2002; De Silva 2003; Morgan 2005; Fernandez‐Rodriguez 2008; Medici 2011). Two trials were at high risk of detection bias (Plevris 1991; Kim 2012). The remaining trials were at unclear risk of detection bias.
Overall, nine trials were at low risk of performance bias and detection bias (Gluud 1986; Orrego 1987; Pares 1998; Cortez‐Pinto 2002; Lucena 2002; De Silva 2003; Morgan 2005; Fernandez‐Rodriguez 2008; Medici 2011).
Incomplete outcome data
Alcoholic hepatitis
Thirteen trials were at low risk of attrition bias (Helman 1971; Shumaker 1978; Depew 1980; Carithers 1989; Spahr 2002; Phillips 2006; Boetticher 2008; Garrido Garcia 2012; Sidhu 2012a; Sidhu 2012b; Singh 2014; Higuera de la Tijera 2015; Tkachenko 2016). Sixteen trials were at high risk of attrition bias (Porter 1971; Campra 1973; Resnick 1974; Blitzer 1977; Halle 1982; Theodossi 1982; Radvan 1983; Bird 1991; Trinchet 1992; Naveau 2004; Stewart 2007; Nguyen‐Khac 2011; Mathurin 2013; De 2014; Park 2014; Thursz 2015). The remaining trials were at unclear risk of attrition bias.
Alcohol‐related liver disease (others)
Nine trials were at low risk of attrition bias (Geminiani 1979; Pierri 1985; Bories 1987; Takase 1989; Lotterer 1995; Diaz Belmont 1996; Velussi 1997; Morgan 2005; Fernandez‐Rodriguez 2008). Eleven trials were at high risk of attrition bias (Fenster 1966; Orrego 1979; Orrego 1987; Plevris 1991; Keiding 1994; De la Maza 1995; Caballeria 1998; Stenner 2000; Cortez‐Pinto 2002; Lucena 2002; De Silva 2003). The remaining trials were at unclear risk of attrition bias.
Selective reporting
Alcoholic hepatitis
We could find a published protocol for only one trial (Thursz 2015). Forty‐one trials were at low risk of selecting outcome reporting bias as they reported the important clinical outcomes (mortality and adverse events) (Helman 1971; Porter 1971; Resnick 1974; Blitzer 1977; Maddrey 1978; Depew 1980; Baker 1981; Serrano‐Cancino 1981; Halle 1982; Mirouze 1982; Theodossi 1982; Radvan 1983; Mendenhall 1984; Trinchet 1989a; Trinchet 1989b; Akriviadis 1990; Bird 1991; Trinchet 1992; Bird 1998; Akriviadis 2000; Spahr 2002; Mezey 2004; Naveau 2004; Phillips 2006; Stewart 2007; Boetticher 2008; De 2009; Popescu 2009; Lebrec 2010; Moreno 2010; Nguyen‐Khac 2011; Garrido Garcia 2012; Sidhu 2012a; Sidhu 2012b; Mathurin 2013; De 2014; Park 2014; Singh 2014; Higuera de la Tijera 2015; Thursz 2015; Tkachenko 2016). The remaining trials were considered to be at high risk of selective outcome reporting bias because they did not report either mortality or adverse events or both, outcomes expected to be measured in such clinical outcomes.
Alcohol‐related liver disease (others)
We were unable to find a published protocol for any trial. Ten trials were at low risk of due to selecting outcome reporting bias as they reported the important clinical outcomes (mortality and adverse events) (Orrego 1979; Orrego 1987; Keiding 1994; De la Maza 1995; Pares 1998; Mato 1999; Cortez‐Pinto 2002; Pelletier 2003; Morgan 2005; Fernandez‐Rodriguez 2008). The remaining trials were considered to be at high risk of selective outcome reporting bias because they reported neither mortality nor adverse events nor both and these are outcomes expected otherwise to be measured in such clinical outcomes.
Other potential sources of bias
Alcoholic hepatitis
Sixteen trials were at low risk of for‐profit bias (Helman 1971; Porter 1971; Maddrey 1978; Halle 1982; Carithers 1989; Akriviadis 1990; Ramond 1992; Trinchet 1992; Bird 1998; Mezey 2004; Stewart 2007; Boetticher 2008; Sidhu 2012a; Park 2014; Higuera de la Tijera 2015; Thursz 2015). Eleven trials funded by pharmaceutical industry were considered to be at high risk of for‐profit bias (Resnick 1974; Blitzer 1977; Shumaker 1978; Baker 1981; Trinchet 1989a; Trinchet 1989b; Bird 1991; Akriviadis 2000; Spahr 2002; Naveau 2004; Mathurin 2013). The remaining trials were at unclear risk of for‐profit bias.
All the trials were at low of other bias.
Alcohol‐related liver disease (others)
Three trials were at low risk of for‐profit bias (Gluud 1986; Cortez‐Pinto 2002; Kim 2012). Twelve trials funded by pharmaceutical industry were considered to be at high risk of for‐profit bias (Fenster 1966; Orrego 1979; Orrego 1987; Keiding 1994; De la Maza 1995; Pares 1998; Mato 1999; Lucena 2002; De Silva 2003; Pelletier 2003; Fernandez‐Rodriguez 2008; Medici 2011). The remaining trials were at unclear risk of for‐profit bias.
All the trials were at low risk of other bias.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7; Table 8; Table 9; Table 10; Table 11
Alcoholic hepatitis
Mortality at maximal follow‐up
A total of 48 trials including 4337 participants reported mortality at maximal follow‐up (Helman 1971; Porter 1971; Campra 1973; Resnick 1974; Blitzer 1977; Maddrey 1978; Shumaker 1978; Depew 1980; Baker 1981; Serrano‐Cancino 1981; Halle 1982; Mirouze 1982; Theodossi 1982; Radvan 1983; Mendenhall 1984; Carithers 1989; Trinchet 1989a; Trinchet 1989b; Akriviadis 1990; Bird 1991; McHutchinson 1991; Ramond 1992; Trinchet 1992; Bird 1998; Akriviadis 2000; Spahr 2002; Mezey 2004; Naveau 2004; Stigliano 2005; Paladugu 2006; Phillips 2006; Stewart 2007; Boetticher 2008; De 2009; Popescu 2009; Lebrec 2010; Moreno 2010; Nguyen‐Khac 2011; Garrido Garcia 2012; Sidhu 2012a; Sidhu 2012b; Mathurin 2013; De 2014; Park 2014; Singh 2014; Higuera de la Tijera 2015; Thursz 2015; Tkachenko 2016) (Analysis 1.1). The period of follow‐up ranged from one month to 12 months. The risk of mortality at maximal follow‐up was higher in the anti‐tumour necrosis factor (anti‐TNF) group versus the no intervention group (odds ratio (OR) 4.64, 95% confidence interval (CI) 1.31 to 16.42; 48 participants; 1 trial). There was no evidence of differences in any of the remaining comparisons of active interventions versus no intervention. The results did not change using the random‐effects model.
1.1. Analysis.
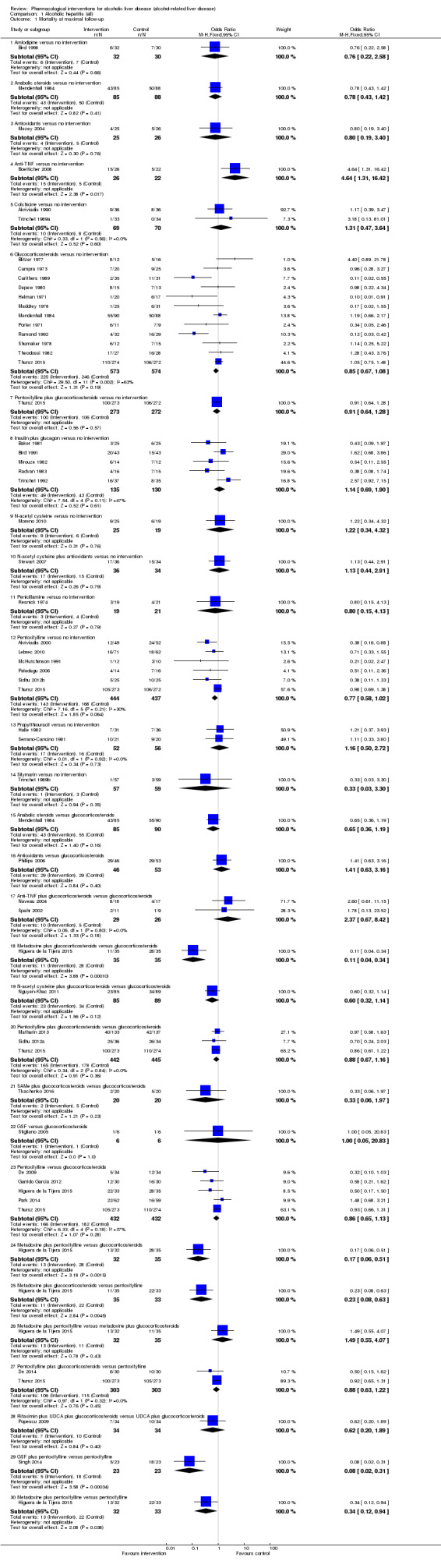
Comparison 1 Alcoholic hepatitis (all), Outcome 1 Mortality at maximal follow‐up.
Early mortality (up to three months)
Thirty‐days mortality
A total of 38 trials including 3433 participants reported 30‐days mortality (Porter 1971; Blitzer 1977; Maddrey 1978; Shumaker 1978; Depew 1980; Baker 1981; Serrano‐Cancino 1981; Halle 1982; Mirouze 1982; Theodossi 1982; Radvan 1983; Carithers 1989; Trinchet 1989a; Akriviadis 1990; Bird 1991; McHutchinson 1991; Ramond 1992; Trinchet 1992; Bird 1998; Spahr 2002; Naveau 2004; Stigliano 2005; Paladugu 2006; Phillips 2006; Boetticher 2008; De 2009; Popescu 2009; Moreno 2010; Nguyen‐Khac 2011; Garrido Garcia 2012; Sidhu 2012a; Sidhu 2012b; Mathurin 2013; De 2014; Park 2014; Higuera de la Tijera 2015; Thursz 2015; Tkachenko 2016) (Analysis 1.2). The more conservative random‐effects model was used. There was no evidence of any differences in 30‐days mortality in any of the comparisons comparing active intervention versus no intervention.
1.2. Analysis.
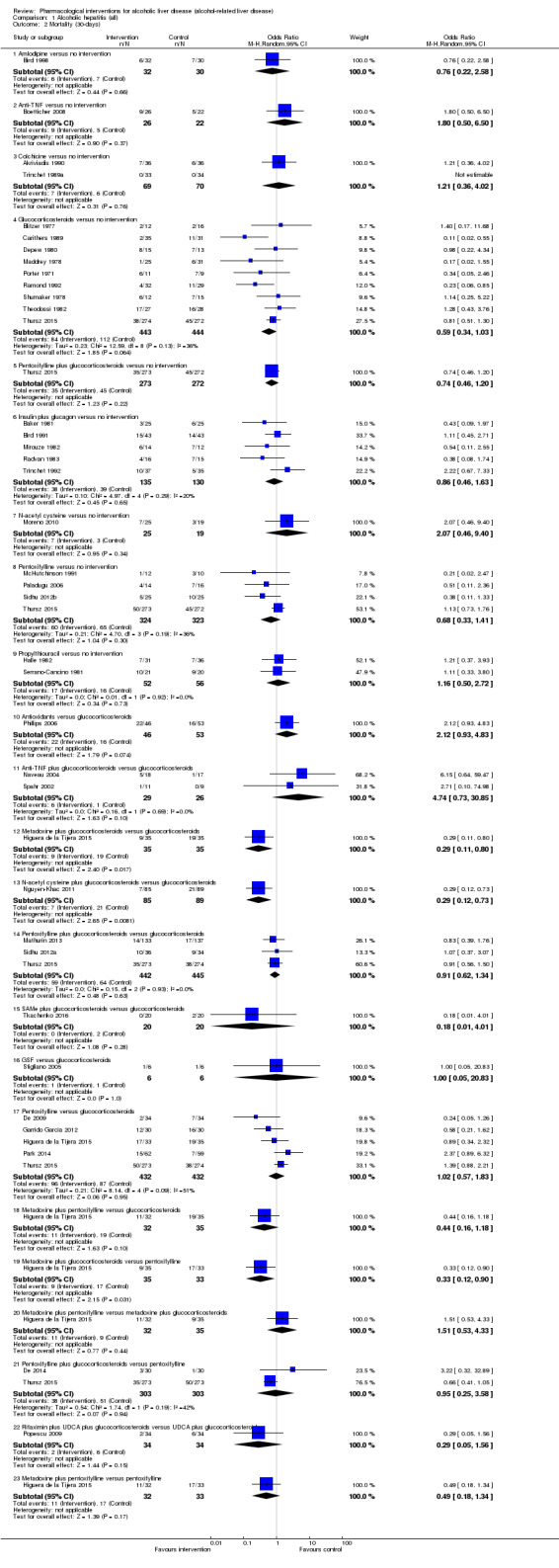
Comparison 1 Alcoholic hepatitis (all), Outcome 2 Mortality (30‐days).
Ninety‐days mortality
A total of 14 trials including 2027 participants reported 90‐days mortality (Helman 1971; Blitzer 1977; Trinchet 1989a; Trinchet 1989b; Ramond 1992; Trinchet 1992; Spahr 2002; Mezey 2004; De 2009; Nguyen‐Khac 2011; De 2014; Singh 2014; Higuera de la Tijera 2015; Thursz 2015) (Analysis 1.3). There was no evidence of any differences in 90‐days mortality in any of the comparisons comparing active intervention versus no intervention. The results did not change using the random‐effects model.
1.3. Analysis.
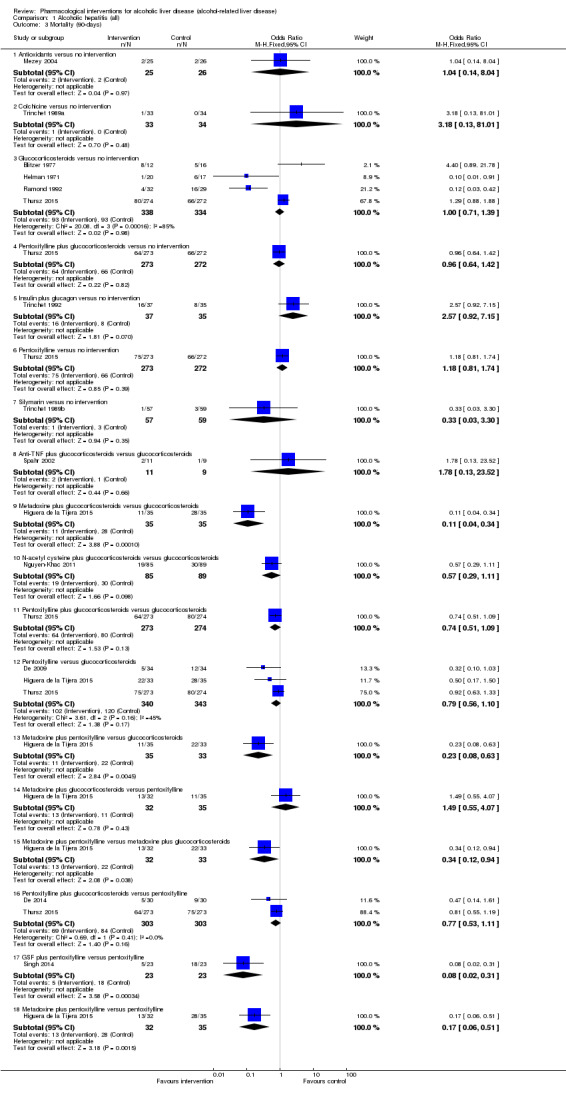
Comparison 1 Alcoholic hepatitis (all), Outcome 3 Mortality (90‐days).
Serious adverse events
Two trials (1140 participants) reported serious adverse events (proportion) (Boetticher 2008; Thursz 2015) (Analysis 1.4). There was no evidence of any differences in serious adverse events (proportion) in any of the comparisons. There was only one trial included in each comparison. Therefore, random‐effects model is not applicable.
1.4. Analysis.
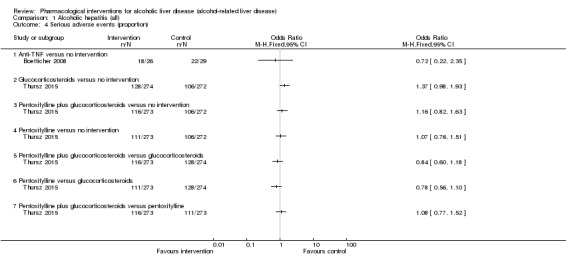
Comparison 1 Alcoholic hepatitis (all), Outcome 4 Serious adverse events (proportion).
Two trials (73 participants) reported serious adverse events (number) (Boetticher 2008; Naveau 2004) (Analysis 1.5). The number of serious adverse events was higher in the anti‐TNF group versus the no intervention group (rate ratio 1.86; 95% CI 1.01 to 3.43). The number of serious adverse events was higher in the glucocorticosteroids plus anti‐TNF group versus the glucocorticosteroids group (rate ratio 4.72; 95% CrI 1.37 to 16.31). There was only one trial included in each comparison. Therefore, random‐effects model is not applicable.
1.5. Analysis.

Comparison 1 Alcoholic hepatitis (all), Outcome 5 Serious adverse events (number).
Health‐related quality of life
None of the trials reported health‐related quality of life at any time point.
Liver transplantation
A total of three trials including 530 participants reported liver transplantation (Bird 1991; Nguyen‐Khac 2011; Mathurin 2013) (Analysis 1.6). There was no evidence of differences in any of the comparisons. There was only one trial included in each comparison. Therefore, random‐effects model is not applicable.
1.6. Analysis.
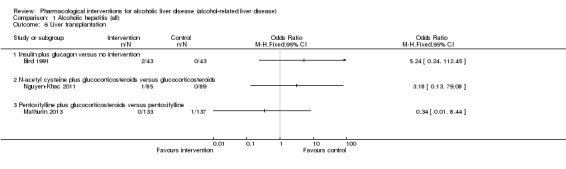
Comparison 1 Alcoholic hepatitis (all), Outcome 6 Liver transplantation.
Decompensated cirrhosis
A total of 17 trials including 2405 participants reported decompensated cirrhosis (Shumaker 1978; Depew 1980; Halle 1982; Trinchet 1989a; Akriviadis 1990; Akriviadis 2000; Naveau 2004; Paladugu 2006; Boetticher 2008; De 2009; Nguyen‐Khac 2011; Garrido Garcia 2012; Sidhu 2012a; Mathurin 2013; De 2014; Higuera de la Tijera 2015; Thursz 2015) (Analysis 1.7). There was no evidence of any differences in decompensated cirrhosis in any of the comparisons comparing active intervention versus no intervention. The results did not change using the random‐effects model.
1.7. Analysis.
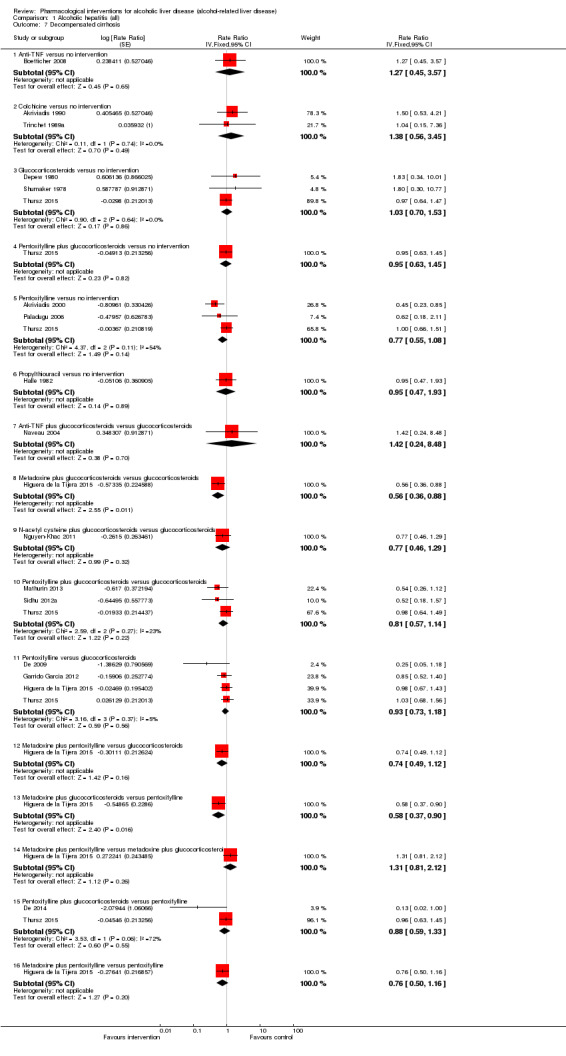
Comparison 1 Alcoholic hepatitis (all), Outcome 7 Decompensated cirrhosis.
Cirrhosis
One trial including 37 participants reported new onset cirrhosis (Helman 1971) (Analysis 1.8). There was no evidence of difference in the proportion of people who developed cirrhosis between glucocorticosteroids and no intervention groups (OR 1.32, 95% CI 0.19 to 9.02).
1.8. Analysis.
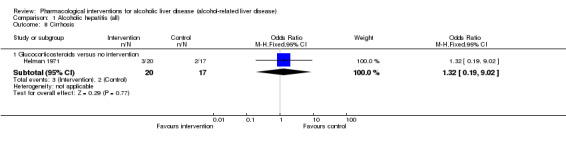
Comparison 1 Alcoholic hepatitis (all), Outcome 8 Cirrhosis.
Hepatocellular carcinoma
None of the trials reported hepatocellular carcinoma.
Any adverse events
A total of 21 trials including 1458 participants reported adverse events (proportion) (Porter 1971; Blitzer 1977; Maddrey 1978; Baker 1981; Halle 1982; Theodossi 1982; Trinchet 1989a; Trinchet 1989b; Bird 1991; Bird 1998; Akriviadis 2000; Spahr 2002; Naveau 2004; Phillips 2006; Stewart 2007; Boetticher 2008; Popescu 2009; Moreno 2010; Sidhu 2012b; Mathurin 2013; Singh 2014) (Analysis 1.9). The proportion of people with any adverse events was higher in the glucocorticosteroids group (OR 4.00, 95% CI 1.80 to 8.88; 159 participants; 4 trials) and in the pentoxifylline group (OR 2.21, 95% CI 1.10 to 4.46; 151 participants = 151; 2 trials) versus the no intervention group. There was no evidence of any differences in any adverse events (proportion) in any of the remaining comparisons comparing active intervention versus no intervention. The results did not change using the random‐effects model.
1.9. Analysis.
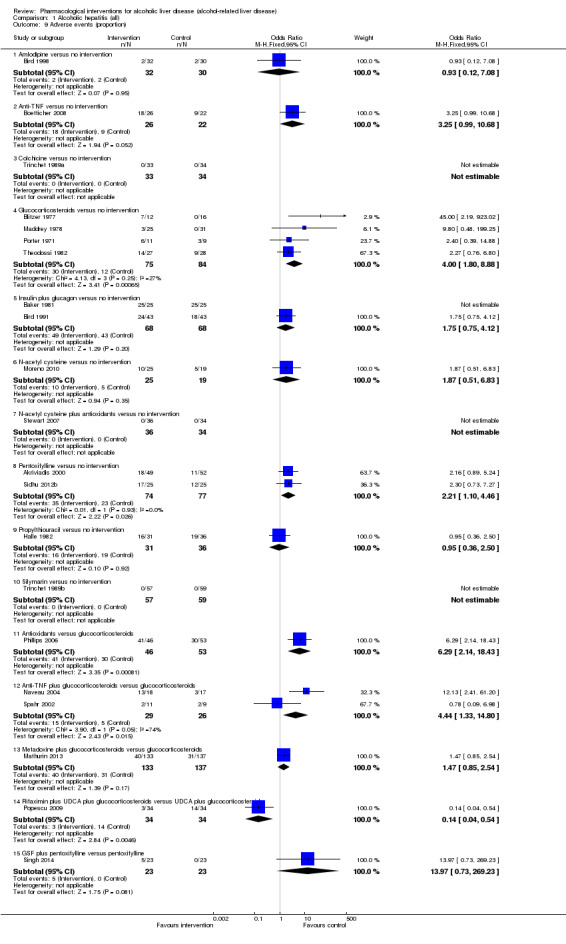
Comparison 1 Alcoholic hepatitis (all), Outcome 9 Adverse events (proportion).
A total of 32 trials including 3221 participants reported adverse events (number) (Porter 1971; Blitzer 1977; Maddrey 1978; Shumaker 1978; Depew 1980; Baker 1981; Halle 1982; Theodossi 1982; Trinchet 1989a; Akriviadis 1990; Bird 1991; Bird 1998; Akriviadis 2000; Spahr 2002; Naveau 2004; Phillips 2006; Stewart 2007; Boetticher 2008; De 2009; Popescu 2009; Moreno 2010; Nguyen‐Khac 2011; Garrido Garcia 2012; Sidhu 2012a; Sidhu 2012b; Mathurin 2013; De 2014; Park 2014; Singh 2014; Higuera de la Tijera 2015; Thursz 2015; Tkachenko 2016) (Analysis 1.10). The more conservative random‐effects model was used. The number of any adverse events was higher in the anti‐TNF group versus the no intervention group (rate ratio 2.26, 95% CI 1.05 to 4.85; 48 participants; 1 trial) and in the glucocorticosteroids group (rate ratio 1.43, 95% CI 1.10 to 1.87; 760 participants; 7 trials) versus the no intervention group (rate ratio 1.34; 95% CrI 1.02 to 1.76; 201 participants; 2 trials). There was no evidence of any differences in any adverse events (number) in any of the remaining comparisons comparing active intervention versus no intervention.
1.10. Analysis.
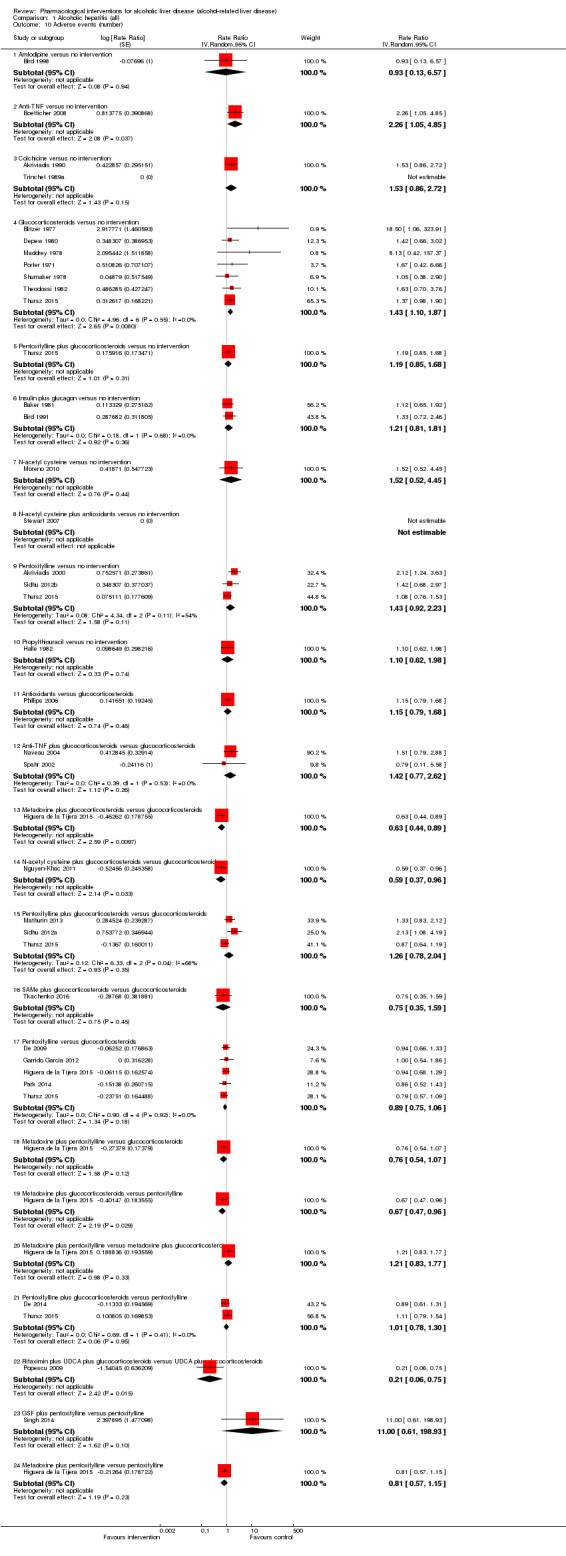
Comparison 1 Alcoholic hepatitis (all), Outcome 10 Adverse events (number).
Subgroup analysis and sensitivity analysis
We did not perform any subgroup analysis other than performing an analysis of a subset of trials including severe alcoholic hepatitis exclusively, which we have presented below. We did not perform the other subgroup analyses because all the trials were at high risk of bias; separate data were not available in biopsy‐confirmed alcohol‐related liver disease in many trials, and because of the few trials included in each comparison. We did not perform a sensitivity analysis since the reasons for dropouts were not adequately described in each intervention group to perform a meaningful sensitivity analysis.
Reporting bias
As there was only comparison with more than 10 trials (glucocorticosteroids versus no intervention), we explored reporting bias via funnel plot for this comparison only. Although the Egger's test did not reveal any evidence of reporting bias (P = 0.11), visualisation appears to suggest that there was reporting bias favouring glucocorticoids.
Sample size calculations and Trial Sequential Analysis
As shown in Figure 4, Figure 5, and Figure 6, the accrued sample sizes were only small fractions of the diversity‐adjusted required information size (DARIS). The Z‐curve did not cross any of the trial sequential boundaries indicating that there was a high risk of random errors. The Trial Sequential Analysis‐adjusted CIs were as follows.
4.
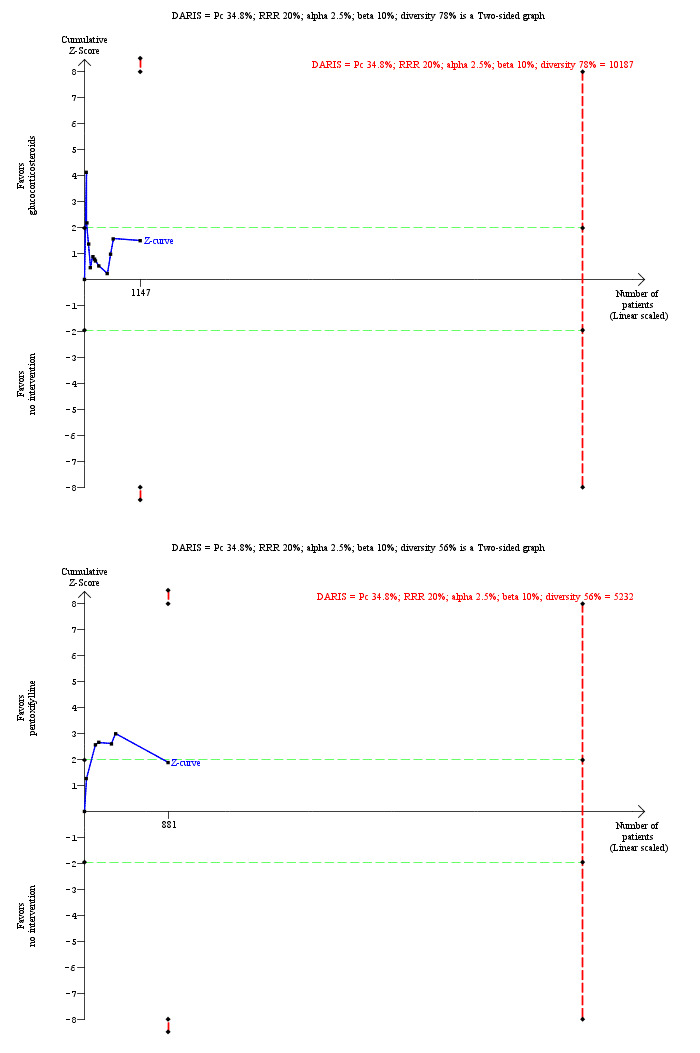
Trial Sequential Analysis of mortality at maximal follow‐up (alcoholic hepatitis): glucocorticosteroids versus no intervention and pentoxifylline versus no intervention.
Based on an alpha error of 2.5%, power of 90% (beta error of 10%), a relative risk reduction (RRR) of 20%, a control group proportion observed in the trials (Pc = 34.8%), and diversity observed in the analyses (78% and 56%), the accrued sample size (1147 participants for glucocorticosteroids versus no intervention and 881 participants for pentoxifylline versus no intervention) was lower than the diversity‐adjusted required information size (DARIS) (10,187 participants for glucocorticosteroids versus no intervention and 5232 participants for pentoxifylline versus no intervention). The Z‐curve (blue line) crosses the conventional boundaries (dotted green lines) favouring glucocorticosteroids and pentoxifylline for the two comparisons, but does not cross the conventional boundaries after the large trial (Thursz 2015). The Z‐curve does not cross any of trial sequential monitoring boundaries (dotted red lines). This indicates that there is a high risk of random errors in both these comparisons.
5.
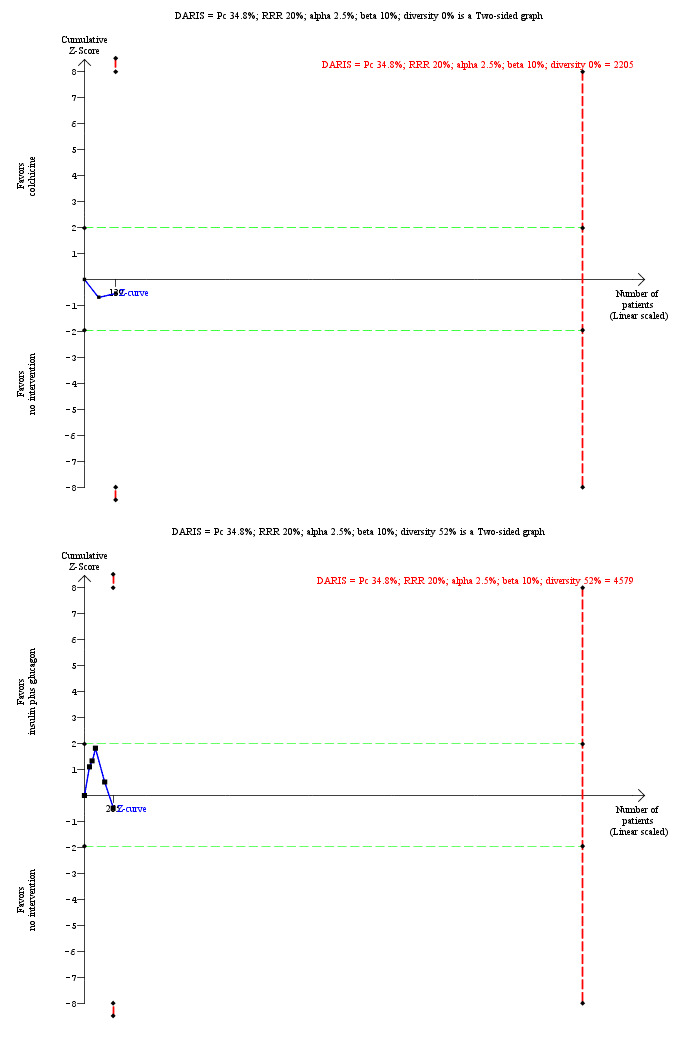
Trial Sequential Analysis of mortality at maximal follow‐up (alcoholic hepatitis): colchicine versus no intervention and insulin plus glucagon versus no intervention.
Based on an alpha error of 2.5%, power of 90% (beta error of 10%), a relative risk reduction (RRR) of 20%, a control group proportion observed in the trials (Pc = 34.8%), and diversity observed in the analyses (0% and 52%), the accrued sample size (139 participants for colchicine versus no intervention and 265 participants for insulin plus glucagon versus no intervention) was lower than the diversity‐adjusted required information size (DARIS) (2205 participants for colchicine versus no intervention and 4579 participants for insulin plus glucagon versus no intervention). The Z‐curve (blue line) does not cross the conventional boundaries (dotted green lines) or the trial sequential monitoring boundaries (dotted red lines). This indicates that there is a high risk of random errors in both these comparisons.
6.
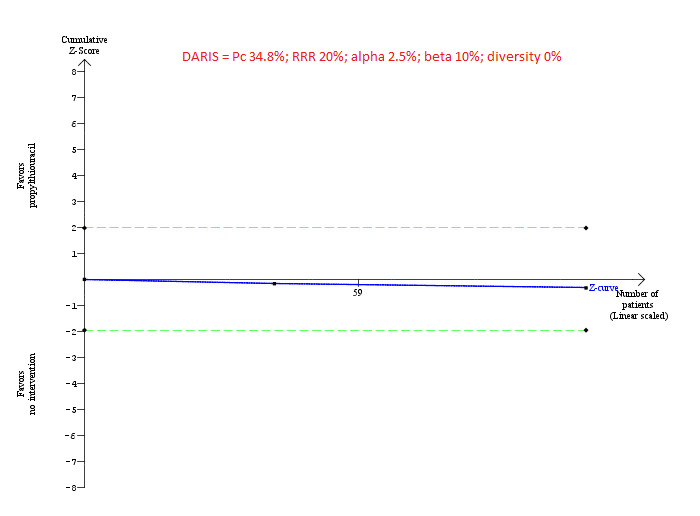
Trial Sequential Analysis of mortality at maximal follow‐up (alcoholic hepatitis): propylthiouracil versus no intervention.
Based on an alpha error of 2.5%, power of 90% (beta error of 10%), a relative risk reduction (RRR) of 20%, a control group proportion observed in the trials (Pc = 34.8%), and diversity observed in the analyses (0%), the accrued sample size (108 participants) was only a small proportion of the diversity‐adjusted required information size (DARIS) (2205 participants); therefore, the trial sequential monitoring boundaries were not drawn.The Z‐curve (blue line) does not cross the conventional boundaries (dotted green lines). This indicates that there is a high risk of random errors in this comparison.
Glucocorticosteroids versus no intervention: 0.66 (95% CI 0.07 to 6.05).
Pentoxifylline versus no intervention: 0.77 (95% CI 0.25 to 2.39).
Colchicine versus no intervention: 1.31 (95% CI 0.02 to 84.61).
Insulin plus glucagon versus no intervention: 1.14 (95% CI 0.14 to 9.14).
Propylthiouracil versus no intervention: not estimable (as the accrued sample size was very small).
These wide confidence intervals indicate that there is high risk of random error.
Quality of the evidence
The quality of the evidence was low or very low for all the outcomes (Table 1; Table 2; Table 3; Table 4; Table 5). The reasons for downgrading include: high risk of bias in trials (downgraded by one level), heterogeneity in some comparisons as evidenced by differences in the effect estimates obtained by the fixed‐effect model and random‐effects model (downgraded by one level), imprecision (small sample size; downgraded by one level), and imprecision (confidence intervals overlapped a clinically significant increase or reduction and clinically insignificant increase or reduction; downgraded by one level).
Subset of trials including severe alcoholic hepatitis exclusively
Mortality at maximal follow‐up
A total of 18 trials including 2477 participants reported mortality at maximal follow‐up (Ramond 1992; Akriviadis 2000; Spahr 2002; Naveau 2004; Paladugu 2006; De 2009; Moreno 2010; Nguyen‐Khac 2011; Garrido Garcia 2012; Sidhu 2012a; Sidhu 2012b; Mathurin 2013; De 2014; Park 2014; Singh 2014; Higuera de la Tijera 2015; Thursz 2015; Tkachenko 2016) (Analysis 2.1). The follow‐up varied between one month and 12 months. There was no evidence of any differences in mortality at maximal follow‐up in any of the comparisons comparing active intervention versus no intervention. The results did not change using the random‐effects model.
2.1. Analysis.
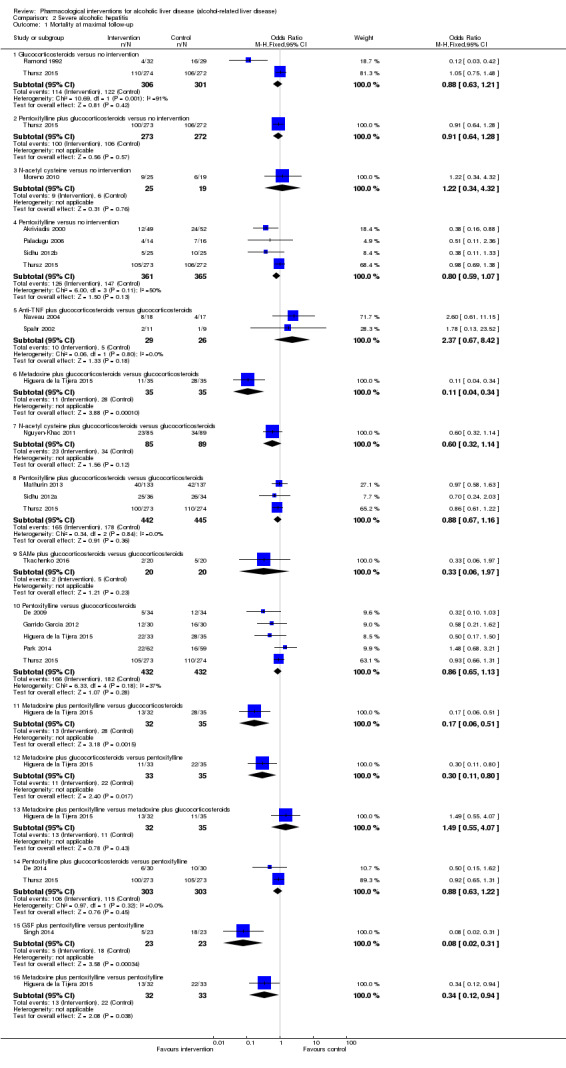
Comparison 2 Severe alcoholic hepatitis, Outcome 1 Mortality at maximal follow‐up.
Early mortality (up to three months)
Thirty‐days mortality
A total of 16 trials including 2330 participants reported 30‐days mortality (Ramond 1992; Spahr 2002; Naveau 2004; Paladugu 2006; De 2009; Moreno 2010; Nguyen‐Khac 2011; Garrido Garcia 2012; Sidhu 2012a; Sidhu 2012b; Mathurin 2013; De 2014; Park 2014; Higuera de la Tijera 2015; Thursz 2015; Tkachenko 2016) (Analysis 2.2). There was no evidence of any differences in 30‐days mortality in any of the comparisons comparing active intervention versus no intervention. The results did not change using the random‐effects model.
2.2. Analysis.
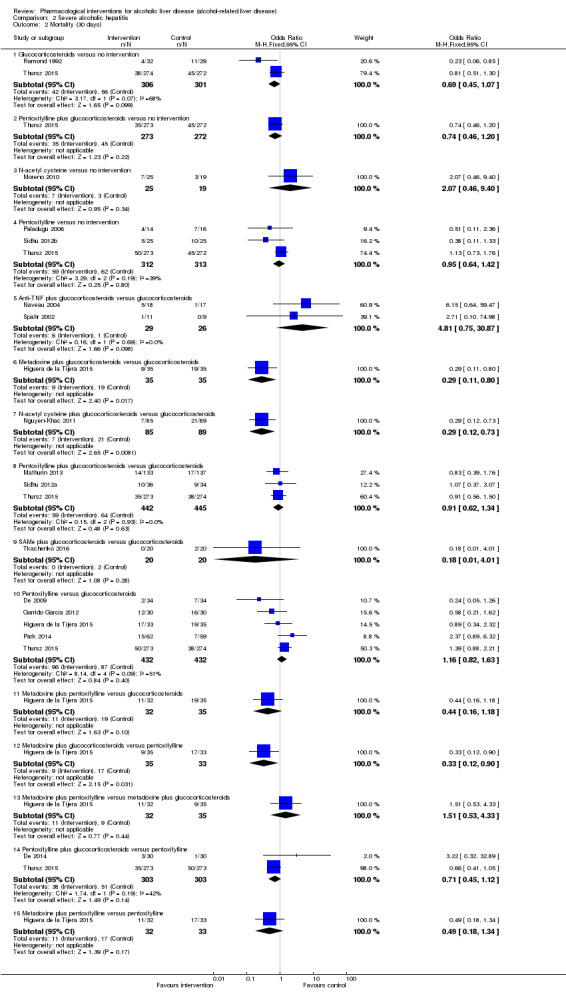
Comparison 2 Severe alcoholic hepatitis, Outcome 2 Mortality (30 days).
Ninety‐days mortality
A total of eight trials including 1656 participants reported 90‐days mortality (Ramond 1992; Spahr 2002; De 2009; Nguyen‐Khac 2011; De 2014; Singh 2014; Higuera de la Tijera 2015; Thursz 2015) (Analysis 2.3). There was no evidence of any differences in 90‐days mortality in any of the comparisons comparing active intervention versus no intervention. The results did not change using the random‐effects model.
2.3. Analysis.
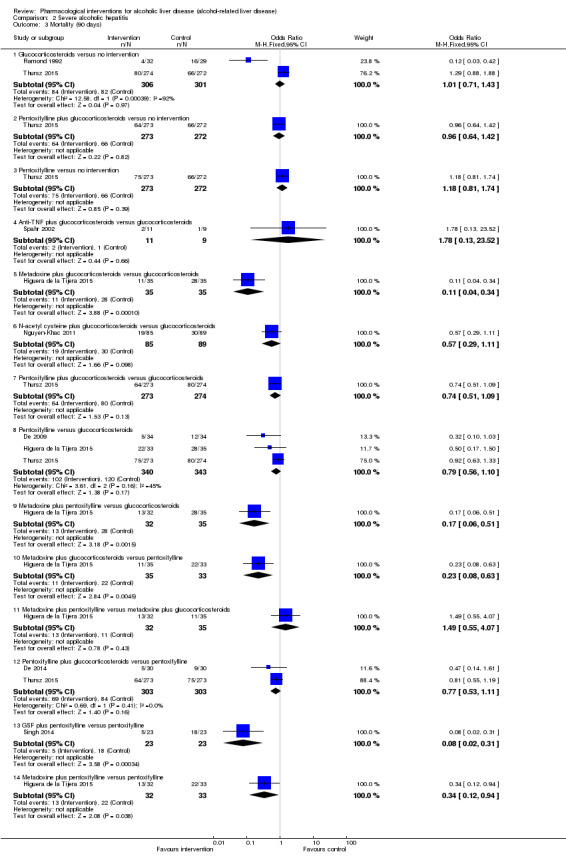
Comparison 2 Severe alcoholic hepatitis, Outcome 3 Mortality (90 days).
Serious adverse events
Only one trial (1092 participants; four interventions) reported serious adverse events (proportion) (Thursz 2015) (Analysis 2.4). There was no evidence of differences in any of the comparisons. Only one trial (35 participants) reported serious adverse events (number) (Naveau 2004) (Analysis 2.6). The number of serious adverse events was higher in the glucocorticosteroids plus anti‐TNF group versus the glucocorticosteroids group (rate ratio 4.72; 95% CI 1.37 to 16.31). As there was only one trial in each comparison, the issue of random‐effects model did not arise.
2.4. Analysis.
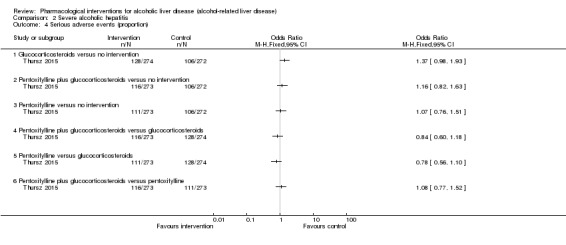
Comparison 2 Severe alcoholic hepatitis, Outcome 4 Serious adverse events (proportion).
2.6. Analysis.
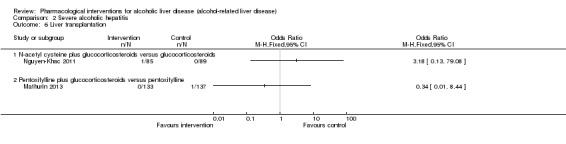
Comparison 2 Severe alcoholic hepatitis, Outcome 6 Liver transplantation.
Health‐related quality of life
None of the trials reported health‐related quality of life at any time point.
Liver transplantation
A total of two trials including 444 participants reported liver transplantation (Nguyen‐Khac 2011; Mathurin 2013) (Analysis 2.6). There was no evidence of differences in any of the comparisons. As there was only one trial in each comparison, the issue of random‐effects model did not arise.
Decompensated cirrhosis
A total of 11 trials including 2095 participants reported decompensated cirrhosis (Akriviadis 2000; Naveau 2004; Paladugu 2006; De 2009; Nguyen‐Khac 2011; Garrido Garcia 2012; Sidhu 2012a; Mathurin 2013; De 2014; Higuera de la Tijera 2015; Thursz 2015) (Analysis 2.7). There was no evidence of any differences in decompensated cirrhosis in any of the comparisons comparing active intervention versus no intervention. The results did not change using the random‐effects model.
2.7. Analysis.

Comparison 2 Severe alcoholic hepatitis, Outcome 7 Decompensated cirrhosis.
Cirrhosis
None of the trials reported the proportion of people who developed cirrhosis.
Hepatocellular carcinoma
None of the trials reported the proportion of people who developed hepatocellular carcinoma.
Any adverse events
A total of four trials including 241 participants reported adverse events (proportion) (Akriviadis 2000; Moreno 2010; Sidhu 2012b; Singh 2014) (Analysis 2.8). The proportion of people with any adverse events was higher in the pentoxifylline group versus the no intervention group (OR 2.21, 95% CI 1.10 to 4.46; 151 participants = 151; 2 trials). There was no evidence of any differences in any adverse events (proportion) in any of the remaining comparisons comparing active intervention versus no intervention. The results did not change using the random‐effects model.
2.8. Analysis.
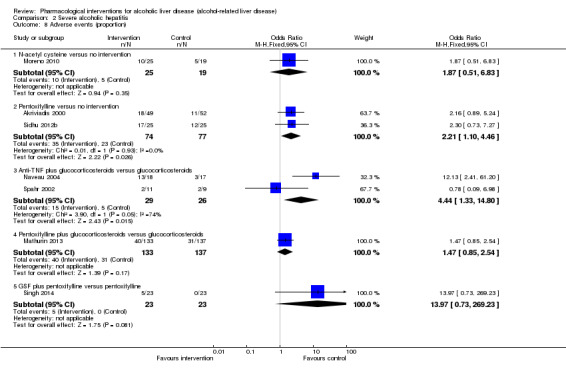
Comparison 2 Severe alcoholic hepatitis, Outcome 8 Adverse events (proportion).
A total of 16 trials including 2386 participants reported adverse events (number) (Akriviadis 2000; Spahr 2002; Naveau 2004; De 2009; Moreno 2010; Nguyen‐Khac 2011; Garrido Garcia 2012; Sidhu 2012a; Sidhu 2012b; Mathurin 2013; De 2014; Park 2014; Singh 2014; Higuera de la Tijera 2015; Thursz 2015; Tkachenko 2016) (Analysis 2.9). There was no evidence of any differences in any adverse events (number) in any of the comparisons comparing active intervention versus no intervention. The results did not change using the random‐effects model.
2.9. Analysis.
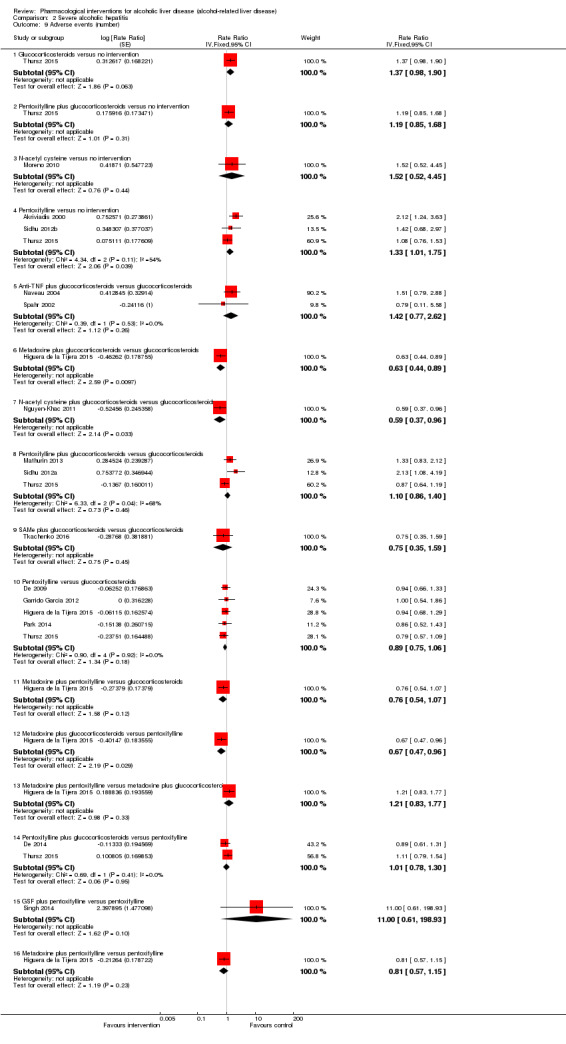
Comparison 2 Severe alcoholic hepatitis, Outcome 9 Adverse events (number).
Subgroup analysis and sensitivity analysis
We did not perform any subgroup analysis because all the trials were at high risk of bias; separate data were not available in biopsy‐confirmed alcohol‐related liver disease in many trials, and because of the few trials included in each comparison. We did not perform a sensitivity analysis since the reasons for dropouts were not adequately described in each intervention group to perform a meaningful sensitivity analysis.
Reporting bias
As there was no comparison including more than 10 trials, we did not explore reporting bias.
Sample size calculations and Trial Sequential Analysis
As shown in Figure 7, the accrued sample sizes were only small fractions of the diversity‐adjusted required information size (DARIS).The Z‐curve did not cross any of the trial sequential boundaries indicating that there was a high risk of random errors. The Trial Sequential Analysis‐adjusted CIs were as follows.
7.
Trial Sequential Analysis of mortality at maximal follow‐up (severe alcoholic hepatitis): glucocorticosteroids versus no intervention and pentoxifylline versus no intervention.
Based on an alpha error of 2.5%, power of 90% (beta error of 10%), a relative risk reduction (RRR) of 20%, a control group proportion observed in the trials (Pc = 40.9%), and diversity observed in the analyses (98% and 76%), the accrued sample size (607 participants for glucocorticosteroids versus no intervention and 726 participants for pentoxifylline versus no intervention) was lower than the diversity‐adjusted required information size (DARIS) (72,755 participants for glucocorticosteroids versus no intervention and 7148 participants for pentoxifylline versus no intervention). For glucocorticosteroids versus no intervention, the accrued sample size was so small that the trial sequential monitoring boundaries were not drawn. The Z‐curve (blue line) crosses the conventional boundaries (dotted green lines) favouring glucocorticosteroids and pentoxifylline for the two comparisons, but does not cross the conventional boundaries after the large trial (Thursz 2015). The Z‐curve does not cross any of trial sequential monitoring boundaries (dotted red lines) for pentoxifylline versus no intervention. This indicates that there is a high risk of random errors in both these comparisons.
Glucocorticosteroids versus no intervention: not estimable (as the accrued sample size was only a small fraction of required information size).
Pentoxifylline versus no intervention: 0.60 (95% CI 0.05 to 7.48).
These wide confidence intervals indicate that there is high risk of random error.
Quality of the evidence
The quality of the evidence was low or very low for all the outcomes (Table 6; Table 7). The reasons for downgrading include: high risk of bias in trials (downgraded by one level), imprecision (small sample size; downgraded by one level), and imprecision (confidence intervals overlapped a clinically significant increase or reduction and clinically insignificant increase or reduction; downgraded by one level).
Alcohol‐related liver diseases (others)
Mortality at maximal follow‐up
A total of 16 trials including 2727 participants reported mortality at maximal follow‐up (Fenster 1966; Geminiani 1979; Orrego 1979; Pierri 1985; Gluud 1986; Bories 1987; Orrego 1987; Keiding 1994; De la Maza 1995; Fleig 1997; Caballeria 1998; Pares 1998; Mato 1999; Cortez‐Pinto 2002; Pelletier 2003; Morgan 2005) (Analysis 3.1). The period of follow‐up ranged from one month to 48 months. The risk of mortality at maximal follow‐up was lower in the propylthiouracil group versus the no intervention group (OR 0.45, 95% CI 0.26 to 0.78; 423 participants; 2 trials). The risk of mortality at maximal follow‐up was higher in the ursodeoxycholic acid group versus the no intervention group (OR 2.09, 95% CI 1.12 to 3.90; 226 participants; 1 trial). There was no evidence of difference in any of the remaining comparisons.
3.1. Analysis.
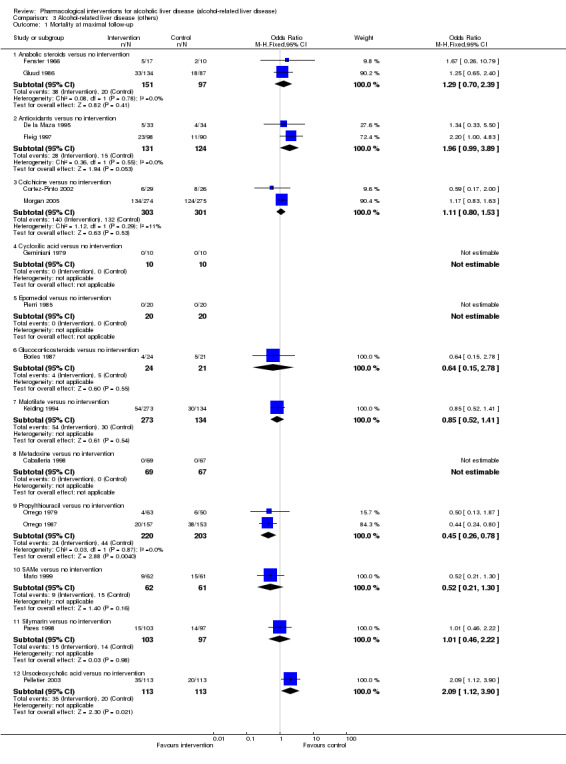
Comparison 3 Alcohol‐related liver disease (others), Outcome 1 Mortality at maximal follow‐up.
Early mortality (up to three months)
Thirty‐days mortality
A total of six trials including 580 participants reported 30‐days mortality (Geminiani 1979; Orrego 1979; Pierri 1985; Bories 1987; Caballeria 1998; Pelletier 2003) (Analysis 3.2). There was no evidence of difference in any of the comparisons. There was only one trial included in each comparison. Therefore, random‐effects model was not applicable.
3.2. Analysis.
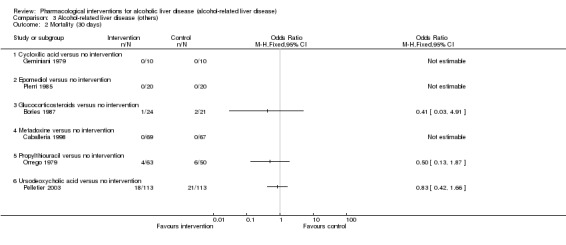
Comparison 3 Alcohol‐related liver disease (others), Outcome 2 Mortality (30 days).
Ninety‐days mortality
A total of three trials including 208 participants reported 90‐days mortality (Pierri 1985; Bories 1987; Mato 1999) (Analysis 3.3). There was no evidence of difference in any of the comparisons. There was only one trial included in each comparison. Therefore, random‐effects model was not applicable.
3.3. Analysis.
Comparison 3 Alcohol‐related liver disease (others), Outcome 3 Mortality (90 days).
Serious adverse events
Only one trial (37 participants) reported serious adverse events (proportion) (Medici 2011) (Analysis 3.4). There were no serious adverse events in either SAMe or no intervention groups. None of the trials reported serious adverse events (number).
3.4. Analysis.
Comparison 3 Alcohol‐related liver disease (others), Outcome 4 Serious adverse events (proportion).
Health‐related quality of life
None of the trials reported health‐related quality of life at any time point.
Liver transplantation
Only one trial including 123 participants reported liver transplantation (Mato 1999) (Analysis 3.5). There was no evidence of difference between SAMe and no intervention (OR 0.32, 95% CI 0.03 to 3.13; 123 participants; 1 trial).
3.5. Analysis.
Comparison 3 Alcohol‐related liver disease (others), Outcome 5 Liver transplantation.
Decompensated cirrhosis
A total of three trials including 1182 participants reported decompensated cirrhosis (Keiding 1994; Pelletier 2003; Morgan 2005) (Analysis 3.6). There was no evidence of difference in any of the comparisons. There was only one trial included in each comparison. Therefore, random‐effects model was not applicable.
3.6. Analysis.
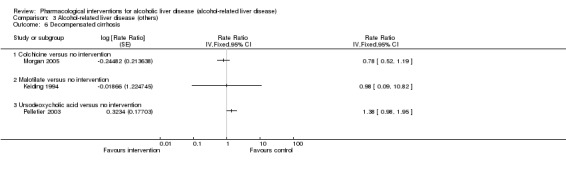
Comparison 3 Alcohol‐related liver disease (others), Outcome 6 Decompensated cirrhosis.
Cirrhosis
None of the trials reported the proportion of people who developed cirrhosis.
Hepatocellular carcinoma
A total of two trials including 344 participants reported hepatocellular carcinoma (Gluud 1986; Mato 1999) (Analysis 3.7). There was no evidence of difference in any of the comparisons. There was only one trial included in each comparison. Therefore, random‐effects model was not applicable.
3.7. Analysis.
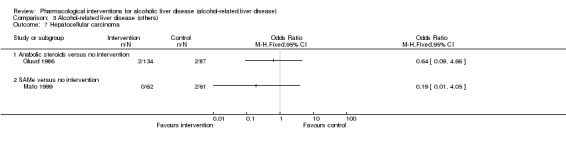
Comparison 3 Alcohol‐related liver disease (others), Outcome 7 Hepatocellular carcinoma.
Any adverse events
A total of 18 trials including 2342 participants reported adverse events (proportion) (Orrego 1979; Orrego 1987; Feher 1989; Takase 1989; Plevris 1991; Keiding 1994; Lotterer 1995; Diaz Belmont 1996; Velussi 1997; Caballeria 1998; Pares 1998; Mato 1999; Cortez‐Pinto 2002; De Silva 2003; Morgan 2005; Fernandez‐Rodriguez 2008; Medici 2011; Kim 2012) (Analysis 3.8). There was no evidence of difference in any of the comparisons. Using the random‐effects model, there was no alteration in the results. A total of 11 trials including 1965 participants reported adverse events (number) (Caballeria 1998; Cortez‐Pinto 2002; Fernandez‐Rodriguez 2008; Keiding 1994; Mato 1999; Medici 2011; Morgan 2005; Orrego 1979; Orrego 1987; Pares 1998; Plevris 1991) (Analysis 3.9). There was no evidence of difference in any of the comparisons. Changing the model from fixed‐effect model to random‐effects model did not alter the conclusions.
3.8. Analysis.
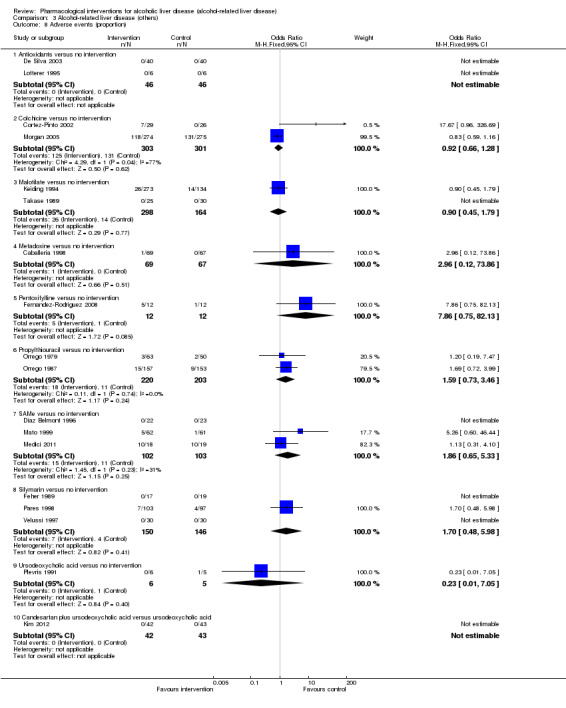
Comparison 3 Alcohol‐related liver disease (others), Outcome 8 Adverse events (proportion).
3.9. Analysis.
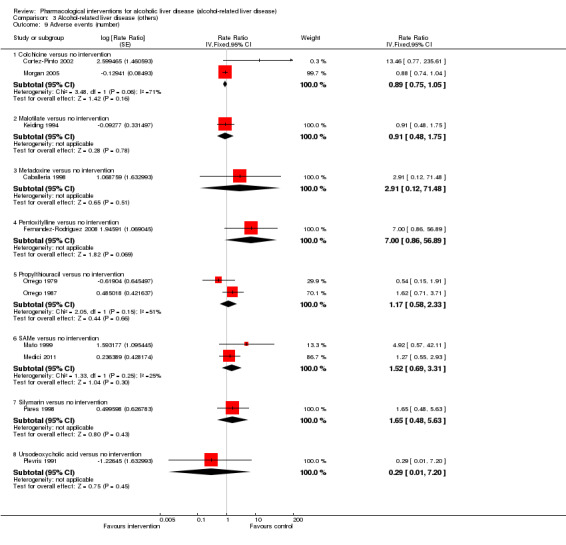
Comparison 3 Alcohol‐related liver disease (others), Outcome 9 Adverse events (number).
Subgroup analysis and sensitivity analysis
We did not perform any subgroup analysis because all the trials were at high risk of bias; separate data were not available in biopsy‐confirmed alcohol‐related liver disease in many trials, and because of the few trials included in each comparison. We did not perform a sensitivity analysis because the reasons for dropouts were not adequately described in each intervention group in order to perform a meaningful sensitivity analysis.
Reporting bias
Since there was no comparison with more than 10 trials, we did not explore reporting bias.
As shown in Figure 8 and Figure 9, the accrued sample sizes were only small fractions of the diversity‐adjusted required information size (DARIS).The Z‐curve did not cross any of the trial sequential boundaries indicating that there was a high risk of random errors. The Trial Sequential Analysis‐adjusted CIs were as follows.
8.
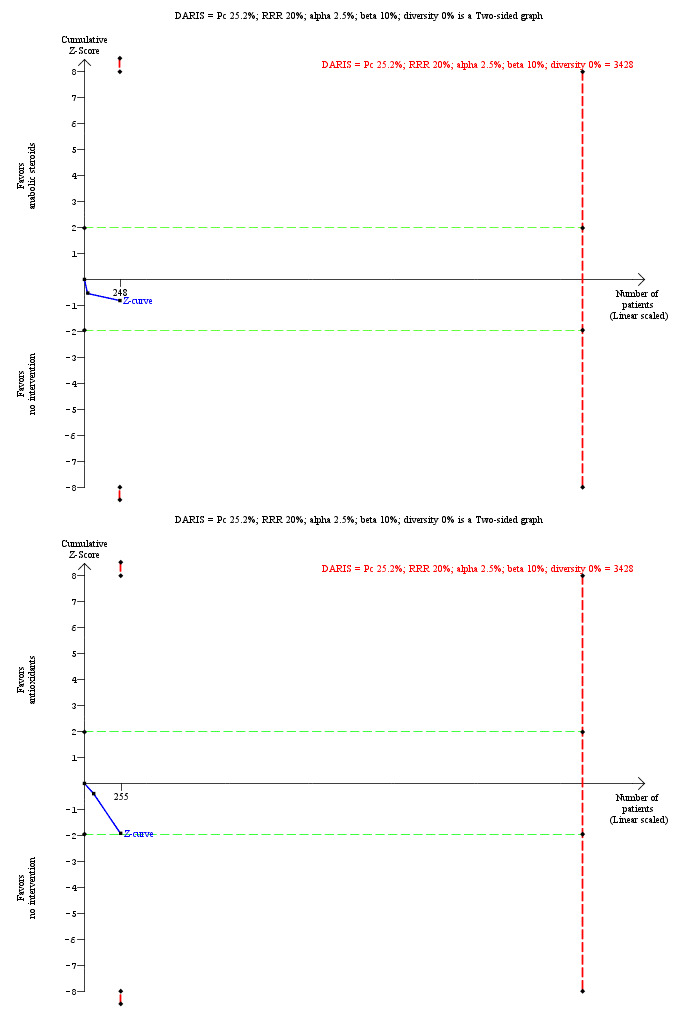
Trial Sequential Analysis of mortality at maximal follow‐up (alcohol‐related disorders (others)): anabolic steroids versus no intervention and antioxidants versus no intervention.
Based on an alpha error of 2.5%, power of 90% (beta error of 10%), a relative risk reduction (RRR) of 20%, a control group proportion observed in the trials (Pc = 25.2%), and diversity observed in the analyses (0% in the both the comparisons), the accrued sample size (248 participants for anabolic steroids versus no intervention and 255 participants for antioxidants versus no intervention) was lower than the diversity‐adjusted required information size (DARIS) (3428 participants for both comparisons). The Z‐curve (blue line) does not cross the conventional boundaries (dotted green lines) or the trial sequential monitoring boundaries (dotted red lines). This indicates that there is a high risk of random errors in both these comparisons.
9.
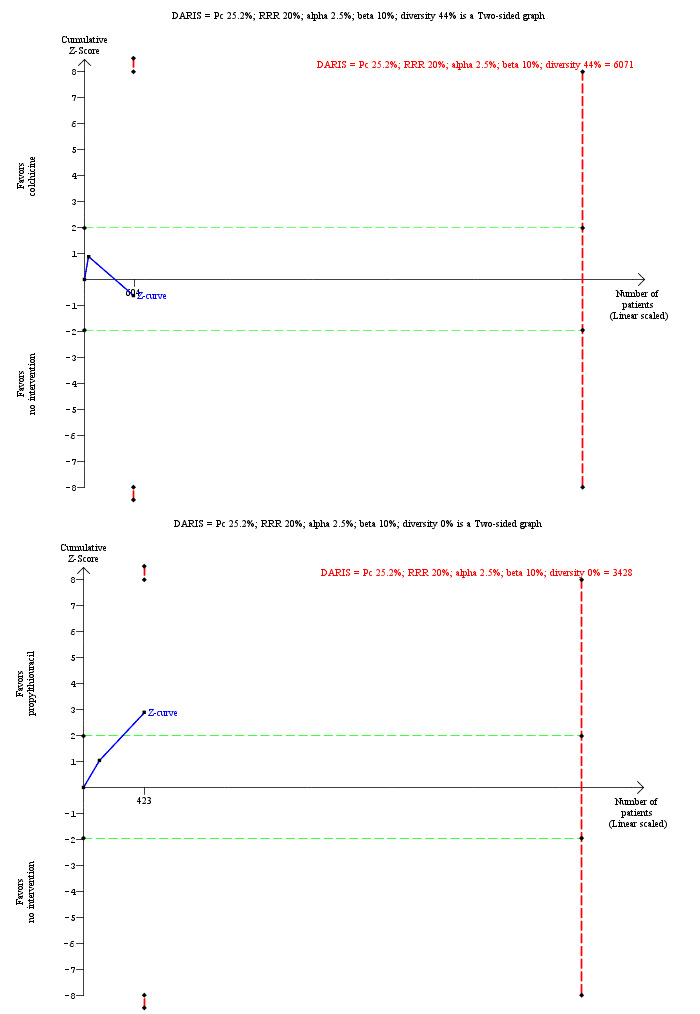
Trial Sequential Analysis of mortality at maximal follow‐up (alcohol‐related disorders (others)): colchicine versus no intervention and propylthiouracil versus no intervention.
Based on an alpha error of 2.5%, power of 90% (beta error of 10%), a relative risk reduction (RRR) of 20%, a control group proportion observed in the trials (Pc = 25.2%), and diversity observed in the analyses (44% and 0%), the accrued sample size (604 participants for colchicine versus no intervention and 423 participants for propylthiouracil versus no intervention) was lower than the diversity‐adjusted required information size (DARIS) (6071 participants for colchicine versus no intervention and 3428 participants for propylthiouracil versus no intervention). For the comparison of colchicine versus no intervention, the Z‐curve (blue line) does not cross the conventional boundaries (dotted green lines) or the trial sequential monitoring boundaries (dotted red lines). For the comparison, propylthiouracil versus no intervention, the Z‐curve crosses the conventional boundaries while it does not cross the trial sequential monitoring boundaries. This indicates that there is a high risk of random errors in both these comparisons.
Anabolic steroids versus no intervention: 1.29 (95% CI 0.11 to 15.86).
Antioxidants versus no intervention: 1.96 (95% CI 0.12 to 31.91).
Colchicine versus no intervention: 1.11 (95% CI 0.30 to 4.15).
Propylthiouracil versus no intervention: 0.45 (95% CI 0.05 to 4.13).
These wide confidence intervals indicate that there is high risk of random error.
Quality of the evidence
The quality of the evidence was low or very low for all the outcomes (Table 8; Table 9; Table 10; Table 11). The reasons for downgrading include: high risk of bias in trials (downgraded by one level), heterogeneity in some comparisons as evidenced by differences in the effect estimates obtained by fixed‐effect model and random‐effects model (downgraded by one level), imprecision (small sample size; downgraded by one level), and imprecision (confidence intervals overlapped a clinically significant increase or reduction and clinically insignificant increase or reduction; downgraded by one level).
Discussion
Summary of main results
Alcoholic hepatitis
A total of 50 trials (4484 participants with alcoholic hepatitis) were included under this comparison. A total of 48 trials (4335 participants) contributed to one or more outcomes. The type of participants included in the trials appeared to have different severity. Therefore, network meta‐analysis was inappropriate because of the possible violation of transitivity assumption. None of the active interventions reduced mortality at any time point versus no intervention. On the other hand, anti‐tumour necrosis factor (anti‐TNF) may increase the complications and mortality. None of the trials reported health‐related quality of life. There was no improvement in any of the clinical outcomes such as cirrhosis, decompensated cirrhosis, liver transplantation by any active interventions compared with no intervention. None of the trials reported the proportion of participants who developed hepatocellular carcinoma. Overall, there does not appear to be any benefit with any active intervention in participants with alcoholic hepatitis. However, it should be noted that the trials included were at high risk of bias and quality of the evidence was low, as described below. So, there is significant uncertainty in this.
Severe acute alcoholic hepatitis
A total of 19 trials (2545 participants with severe alcoholic hepatitis) were included under this comparison. A total of 18 trials (2477 participants) contributed to one or more outcomes. Although the type of participants included in the trials appeared to be similar across comparisons (Appendix 4), there was evidence of inconsistency by one or more methods in all the outcomes in which it was possible to assess inconsistency. Therefore, we have presented the results of network meta‐analysis in the appendix and have presented only the main comparisons. As for the participants with alcoholic hepatitis of any severity, there was no evidence of differences indicating any clinical benefit of any active interventions versus no intervention. On the other hand, anti‐TNF may increase the complications.
Based on the network meta‐analysis (which we have presented in the appendix because of the uncertainty about the reliability of the results), it appears that granulocyte stimulation factor (GSF) plus pentoxifylline, glucocorticosteroids plus metadoxine, and pentoxifylline plus metadoxine may decrease mortality at maximal follow‐up compared with no intervention. However, it should be noted that these estimates are based on indirect comparisons and were based on single small trials. While these interventions warrant further investigation by direct comparisons in new trials, it is not appropriate to recommend these interventions based on the network meta‐analysis results.
Alcohol‐related liver disease (others)
A total of 31 trials (3695 participants with different phases of alcohol‐related liver disease) were included under this comparison. A total of 26 trials (3212 participants) contributed to one or more outcomes. The risk of mortality at maximal follow‐up was lower in the propylthiouracil group versus the no intervention group and higher in the ursodeoxycholic acid group versus the no intervention group. However, the trials which were included in the comparison between propylthiouracil and no intervention were reported in 1979 and 1987. These were small trials of high risk of bias. In addition, the risk of random errors was high as demonstrated by Trial Sequential Analysis. As a result, trials of low risk of bias of sufficient sample size are required before propylthiouracil can be recommended routinely.
Health‐related quality of life and the incidence of cirrhosis was not reported in any of the trials. There was no evidence of any clinical benefit in terms of decompensated cirrhosis or liver transplantation. Therefore, there is nothing to suggest that any active intervention improves clinical outcomes in people with any type of alcohol‐related liver disease.
Overall completeness and applicability of evidence
In this review, we have included all the randomised clinical trials on people with alcohol‐related liver disease with and without evidence of alcoholic hepatitis. However, the majority of the trials excluded participants with infection or gastrointestinal bleeding. This is likely to be because of the harmful effect of glucocorticosteroids, which were used in many of the trials either alone or in combination. Therefore, the findings of the review are only applicable in people with alcoholic hepatitis or other forms of alcoholic liver disease who do not have infections or gastrointestinal bleeding.
Quality of the evidence
The overall quality of the evidence was low or very low for all the comparisons. The quality of the evidence was low or very low for all the outcomes. The reasons for downgrading include: high risk of bias in trials (downgraded by one level), imprecision (small sample size; downgraded by one level), and imprecision (confidence intervals overlapped a clinically significant increase or reduction and clinically insignificant increase or reduction; downgraded by one level).
Potential biases in the review process
We selected a range of databases without any language restrictions. We did not rely on the network meta‐analysis results when we suspected the transitivity assumption may not be true or when the evidence was based on small trials of high risk of bias. In addition, we have performed the analysis using the fixed‐effect model and the random‐effects model, and used the more conservative model. We have also assessed the risk of random errors using Trial Sequential Analysis. These are the strengths of the review process.
We have excluded studies which compared variations in duration or dose in the different treatments. Hence this review does not provide information on whether one variation is better than other. Another major limitation of the review was the paucity of data. There were few trials included under each comparison. In many comparisons, there was only one trial included under the comparison. This makes it difficult to assess whether the effect estimates are reproducible. This paucity of data decreases the confidence in the results.
We only included randomised clinical trials which are known to focus mostly on benefits and do not collect and report harms in a detailed manner. According to our choice of studies (i.e. only randomised clinical trials), we might have missed a large number of studies that address reporting of harms. Accordingly, this review is biased towards benefits ignoring harms. We did not search for interventions and trials registered at regulatory authorities (e.g. FDA (US Food and Drug Administration); EMA (European Medicines Agency), etc). This may have overlooked trials and as such trials usually are unpublished, the lack of inclusion of such trials may make our comparisons look more advantageous than they really are.
Agreements and disagreements with other studies or reviews
We identified one other network meta‐analysis on severe alcoholic hepatitis (Singh 2015). The authors concluded that in patients with severe alcoholic hepatitis, pentoxifylline and corticosteroids (alone and in combination with pentoxifylline or N‐acetyl cysteine) can reduce 30‐day mortality. No intervention decreases risk of mortality between three months and 12 months. Our conclusions differ because we did not rely on the results of the network meta‐analysis of uncertain reliability.
Our conclusions also differ from the Cochrane review on propylthiouracil (Fede 2011). This was because we performed a separate analysis of alcoholic hepatitis and other alcoholic liver disease. Our review agrees with the findings of the Cochrane review on pentoxifylline, which concluded that the evidence on the effect of pentoxifylline was inconclusive (Whitfield 2009). The findings of our review do not agree with the findings of a systematic review on glucocorticosteroids which concluded that glucocorticosteroids might be effective in people with severe alcoholic hepatitis and that further randomised clinical trials were necessary (Rambaldi 2008). A large randomised clinical trial demonstrating no evidence of effect of glucocorticosteroids or pentoxifylline, or their combination on people with severe alcoholic disease was published by Thursz 2015, and whether this trial will change the conclusions of the meta‐analysis by Rambaldi 2008 or not may become evident when an update of this review is published by Pavlov and colleagues later this year (Pavlov 2016).
We also disagree with EASL (European Association for the Study of Liver) and AASLD (American Association for the Study of Liver Diseases), which recommend glucocorticosteroids in people with severe alcoholic hepatitis and pentoxifylline in people with contraindications to glucocorticosteroids (O'Shea 2010; EASL 2012). This again may be due to the recent publication of a large trial (of more than 1000 participants) with at least 500 participants included for each pairwise comparison between glucocorticosteroids, pentoxifylline, a combination of the above, and placebo. In this trial, there was no evidence of any benefit of treatment, as opposed to previous small trials, some of which showed benefits and some of which did not show any evidence of benefit of these drugs (Thursz 2015). This may also be because of improvement in general medical care for these patients, which means that the treatment effect might have reduced.
Authors' conclusions
Implications for practice.
Because of very low‐quality evidence, there is uncertainty in the effectiveness of any pharmacological intervention versus no intervention in people with alcoholic hepatitis or severe alcoholic hepatitis. Based on low‐quality evidence, propylthiouracil may decrease mortality in people with other alcohol‐related liver diseases. However, these have to be confirmed by adequately powered trials with low risk of bias before propylthiouracil can be considered effective.
Implications for research.
Large randomised clinical trials should be conducted with approximately 200 participants in each group to compare the benefits and harms of different interventions in people with alcoholic hepatitis. The interventions compared should include no intervention (placebo) as the one of the trial groups. They may also include glucocorticosteroids, pentoxifylline, and combinations involving these drugs such as granulocyte stimulation factor (GSF) plus pentoxifylline, pentoxifylline plus metadoxine, or glucocorticosteroids as one of the intervention groups as these combinations appear to decrease mortality based on network meta‐analysis. Such trials should follow up participants for at least one to two years and should include health‐related quality of life and report serious adverse events separately from adverse events. The trials should be designed and reported using guidance from SPIRIT statement (Chan 2013) and CONSORT statements (Schulz 2010).
What's new
| Date | Event | Description |
|---|---|---|
| 12 April 2017 | Amended | The Cochrane Central Editorial Unit requested removal of the 'attempted network meta‐analysis' phrase from the end of the review title, as this further description of the review might create confusion in the reader. Although we followed the planned methodology for network meta‐analysis and we performed network meta‐analyses, we decided not to report the obtained results because there was evidence of inconsistency by one or more methods of assessment of inconsistency. Therefore, we did not report the results of the network meta‐analysis and reported the comparative benefits and harms of different interventions using standard Cochrane methodology. |
Notes
Considerable overlap is evident in the methods sections of this protocol and those of several other reviews written by the same group of authors.
Acknowledgements
We thank the Cochrane Comparing of Multiple Interventions Methods Group and the Cochrane Hepato‐Biliary Group for their support and advice. We thank the copy‐editors for their support.
Peer reviewers of the review: Theis Lange, Denmark; Adriani Nikolakopolou, Greece. Contact Editor: Christian Gluud, Denmark. Sign‐off Editor: Christian Gluud, Denmark.
Cochrane Review Group funding acknowledgement: The Danish State is the largest single funder of the Cochrane Hepato‐Biliary Group through its investment in The Copenhagen Trial Unit, Centre for Clinical Intervention Research, Rigshospitalet, Copenhagen University Hospital, Denmark. Disclaimer: The views and opinions expressed in this review are those of the authors and do not necessarily reflect those of the Danish State or The Copenhagen Trial Unit.
Appendices
Appendix 1. Methods of the network meta‐analysis
Measures of treatment effect
Relative treatment effects
For dichotomous variables ( e.g. proportion of participants with serious adverse events or any adverse events), we calculated the odds ratio with 95% credible interval (or Bayesian confidence interval) (Severini 1993). For continuous variables (e.g. health‐related quality of life reported on the same scale), we planned to calculate the mean difference with 95% credible interval. We planned to use standardised mean difference values with 95% credible interval for health‐related quality of life if included trials used different scales. For count outcomes (e.g. number of adverse events and serious adverse events), we calculated the rate ratio with 95% credible interval. For time‐to‐event data ( e.g. mortality at maximal follow‐up), we used the hazard ratio with 95% credible interval.
Relative ranking
We estimated the ranking probabilities for all interventions of being at each possible rank for each intervention. Then, we obtained the surface under the cumulative ranking curve (SUCRA) (cumulative probability) and rankogram (Salanti 2011; Chaimani 2013).
Unit of analysis issues
The codes for analysis, that we used, accounts for the correlation between the effect sizes from trials with more than two groups.
Assessment of heterogeneity
We assessed clinical and methodological heterogeneity by carefully examining the characteristics and design of included trials. We assessed the presence of clinical heterogeneity by comparing effect estimates under different categories of potential effect modifiers. Different study designs and risk of bias may contribute to methodological heterogeneity.We assessed the statistical heterogeneity by comparing the results of the fixed‐effect model meta‐analysis and the random‐effects model meta‐analysis, between‐study standard deviation (Tau2 and comparing this with values reported in study of the distribution of between‐study heterogeneity (Turner 2012)), and by calculating the I2 (using Stata/SE 14.2). If we identified substantial heterogeneity, clinical, methodological, or statistical, we planned to explore and address heterogeneity in a subgroup analysis (see Subgroup analysis and investigation of heterogeneity section).
Assessment of transitivity across intervention comparisons
We assessed the assumption of transitivity by comparing the distribution of the potential effect modifiers (clinical: associated features of decompensated cirrhosis such as presence of infection, hepatorenal syndrome, gastrointestinal bleeding, ascites; methodological: risk of bias, year of randomisation, duration of follow‐up) across the different pair‐wise comparisons.
Assessment of reporting biases
We judged the reporting bias in the network meta‐analysis by the completeness of the search (i.e. searching various databases and including conference abstracts), as we could find no meaningful order to perform a comparison‐adjusted funnel plot (Chaimani 2012) (i.e. there was no specific change in the risk of bias in the trials, sample size, or the control group used over time).
Data synthesis
Methods for indirect and mixed comparisons
We conducted network meta‐analyses to compare multiple interventions simultaneously for each of the primary and secondary outcomes. Network meta‐analysis combines direct evidence within trials and indirect evidence across trials (Mills 2012). We obtained a network plot to ensure that the trials were connected by treatments using Stata/SE 14.2 (Chaimani 2013). We excluded any trials that were not connected to the network. We conducted a Bayesian network meta‐analysis using the Markov chain Monte Carlo method in OpenBUGS 3.2.3 as per the guidance from the National Institute for Health and Care Excellence (NICE) Decision Support Unit (DSU) documents (Dias 2014a). We modelled the treatment contrast (i.e. log odds ratio for binary outcomes, mean difference or standardised mean difference for continuous outcomes, log rate ratio for count outcomes, and log hazard ratio for time‐to‐event outcomes) for any two interventions ('functional parameters') as a function of comparisons between each individual intervention and an arbitrarily selected reference group ('basic parameters') (Lu 2006) using appropriate likelihood functions and links. We used binomial likelihood and logit link for binary outcomes, Poisson likelihood and log link for count outcomes, binomial likelihood and complementary log‐log link for time‐to‐event outcomes, and planned to use normal likelihood and identity link for continuous outcomes. We used no intervention as the reference group. We performed a fixed‐effect model and random‐effects model for the network meta‐analysis. We have reported both models for comparison with the reference group in a forest plot. For each pairwise comparison in a table, we have reported the fixed‐effect model if the two models reported similar results; otherwise, we have reported the more conservative model.
We used a hierarchical Bayesian model using three different initial values, using codes provided by NICE DSU (Dias 2014a). We used a normal distribution with large variance (10,000) for treatment effect priors (vague or flat priors). For the random‐effects model, we used a prior distributed uniformly (limits: 0 to 5) for between‐trial standard deviation but assumed similar between‐trial standard deviation across treatment comparisons (Dias 2014a). We used a 'burn‐in' of 5000 simulations, checked for convergence visually, and ran the models for another 10,000 simulations to obtain effect estimates. If we did not obtain convergence, we planned to increase the number of simulations for 'burn‐in'. If we did not obtain convergence still, we planned to use alternate initial values and priors using methods suggested by van Valkenhoef 2012. We also estimated the probability that each intervention ranks at one of the possible positions using the NICE DSU codes (Dias 2014a).
Assessment of inconsistency
We assessed inconsistency (statistical evidence of the violation of transitivity assumption) by fitting both an inconsistency model and a consistency model. We used the inconsistency models used in the NICE DSU manual as we used common between‐study standard deviation (Dias 2014b). In addition, we used design‐by‐treatment full interaction model (Higgins 2012) and IF (inconsistency factor) plots (Chaimani 2013) to assess inconsistency. In the presence of inconsistency, we planned to assess whether the inconsistency is because of clinical or methodological heterogeneity by performing separate analyses for each of the different subgroups mentioned in the Subgroup analysis and investigation of heterogeneity section.
We identified areas in the network where substantial inconsistency might be present in terms of clinical and methodological diversities between trials and limited network meta‐analysis to a subset of trials with a Maddrey Discriminant Factor of 32 or above.
Direct comparison
We performed the direct comparisons (for the comparisons in which network meta‐analysis was performed) using the same codes and the same technical details.
Subgroup analysis and investigation of heterogeneity for network meta‐analysis
We planned to assess the differences in the effect estimates between the subgroups listed in 'Subgroup analysis and investigation of heterogeneity' using meta‐regression with the help of the OpenBUGS code (Dias 2012a) if we included a sufficient number of trials. We planned to use the potential modifiers as study level co‐variates for meta‐regression. We planned to calculate a single common interaction term (Dias 2012a). If the 95% credible intervals of the interaction term did not overlap zero, we planned to consider this as evidence of difference in subgroups.
Presentation of results
We have presented the effect estimates with 95% credible interval (CrI) for each pairwise comparisons calculated from the direct comparisons and network meta‐analysis. We also presented the cumulative probability of the treatment ranks (i.e. the probability that the treatment is within the top two, the probability that the treatment is within the top three, etc.) in graphs (surface under the cumulative ranking curve or SUCRA) (Salanti 2011). We have also plotted the probability that each treatment is best, second best, third best etc for each of the different outcomes (rankograms), which are generally considered more informative (Salanti 2011; Dias 2012b).
We planned to follow the approach suggested by Puhan and colleagues (Puhan 2014). First, we planned to calculate the direct and indirect effect estimates and 95% credible intervals using the node‐splitting approach (Dias 2010), i.e. calculate the direct estimate for each comparison by including only trials in which there was direct comparison of treatments and the indirect estimate for each comparison by excluding the trials in which there was direct comparison of treatments. Then we planned to rate the quality of direct and indirect effect estimates using GRADE methodology, which takes into account the risk of bias, inconsistency, directness of evidence, imprecision, and publication bias (Guyatt 2011). Then, we planned to present the estimates of the network meta‐analysis and rate the quality of network meta‐analysis effect estimates as the best quality of the evidence between the direct and indirect estimates (Puhan 2014). In addition, in the same table, we planned to present illustrations and information on the number of trials and participants as per the standard 'Summary of findings' table.
Appendix 2. Severe alcoholic hepatitis: data
| #Mort_Max; intervention codes: 1 = No active intervention; 2 = Glucocorticosteroids; 3 = Glucocorticosteroids plus anti‐TNF; 4 = Glucocorticosteroids plus metadoxine; 5 = Glucocorticosteroids plus N‐acetyl cysteine; 6 = Glucocorticosteroids plus pentoxifylline; 7 = Glucocorticosteroids plus SAMe; 8 = GSF plus pentoxifylline; 9 = NAC;10 = Pentoxifylline; 11 = Pentoxifylline plus metadoxine. | ||||||||||||||
| list(ns=18,nt=11) | ||||||||||||||
| r[,1] | n[,1] | r[,2] | n[,2] | r[,3] | n[,3] | r[,4] | n[,4] | t[,1] | t[,2] | t[,3] | t[,4] | na[] | time[] | #study |
| 106 | 272 | 110 | 274 | 100 | 273 | 105 | 273 | 1 | 2 | 6 | 10 | 4 | 12 | #Thursz 2015 |
| 16 | 29 | 4 | 32 | NA | NA | NA | NA | 1 | 2 | NA | NA | 2 | 2 | #Ramond 1992 |
| 6 | 19 | 9 | 25 | NA | NA | NA | NA | 1 | 9 | NA | NA | 2 | 6 | #Moreno 2010 |
| 24 | 52 | 12 | 49 | NA | NA | NA | NA | 1 | 10 | NA | NA | 2 | 6 | #Akriviadis 2000 |
| 7 | 16 | 4 | 14 | NA | NA | NA | NA | 1 | 10 | NA | NA | 2 | 1 | #Paladugu 2006 |
| 10 | 25 | 5 | 25 | NA | NA | NA | NA | 1 | 10 | NA | NA | 2 | 1 | #Sidhu 2012b |
| 4 | 17 | 8 | 18 | NA | NA | NA | NA | 2 | 3 | NA | NA | 2 | 12 | #Naveau 2004 |
| 1 | 9 | 2 | 11 | NA | NA | NA | NA | 2 | 3 | NA | NA | 2 | 3 | #Spahr 2002 |
| 28 | 35 | 11 | 35 | 22 | 33 | 13 | 32 | 2 | 4 | 10 | 11 | 4 | 3 | #Higuera de la Tijera 2015 |
| 34 | 89 | 23 | 85 | NA | NA | NA | NA | 2 | 5 | NA | NA | 2 | 6 | #Nguyen‐Khac 2011 |
| 42 | 137 | 40 | 133 | NA | NA | NA | NA | 2 | 6 | NA | NA | 2 | 6 | #Mathurin 2013 |
| 26 | 34 | 25 | 36 | NA | NA | NA | NA | 2 | 6 | NA | NA | 2 | 6 | #Sidhu 2012a |
| 5 | 20 | 2 | 20 | NA | NA | NA | NA | 2 | 7 | NA | NA | 2 | 6 | #Tkachenko 2016 |
| 12 | 34 | 5 | 34 | NA | NA | NA | NA | 2 | 10 | NA | NA | 2 | 12 | #De 2009 |
| 16 | 30 | 12 | 30 | NA | NA | NA | NA | 2 | 10 | NA | NA | 2 | 1 | #Garrido Garcia 2012 |
| 16 | 59 | 22 | 62 | NA | NA | NA | NA | 2 | 10 | NA | NA | 2 | 6 | #Park 2014 |
| 10 | 30 | 6 | 30 | NA | NA | NA | NA | 6 | 10 | NA | NA | 2 | 12 | #De 2014 |
| 5 | 23 | 18 | 23 | NA | NA | NA | NA | 8 | 10 | NA | NA | 2 | 3 | #Singh 2014 |
| END | ||||||||||||||
| #Mort_30; intervention codes: 1 = No active intervention; 2 = Glucocorticosteroids; 3 = Glucocorticosteroids plus anti‐TNF; 4 = Glucocorticosteroids plus metadoxine; 5 = Glucocorticosteroids plus N‐acetyl cysteine; 6 = Glucocorticosteroids plus pentoxifylline; 7 = Glucocorticosteroids plus SAMe; 8 = NAC; 9 = Pentoxifylline; 10 = Pentoxifylline plus metadoxine. | ||||||||||||||
| list(ns=16,nt=10) | ||||||||||||||
| r[,1] | n[,1] | r[,2] | n[,2] | r[,3] | n[,3] | r[,4] | n[,4] | t[,1] | t[,2] | t[,3] | t[,4] | na[] | #study | |
| 45 | 272 | 38 | 274 | 35 | 273 | 50 | 273 | 1 | 2 | 6 | 9 | 4 | #Thursz 2015 | |
| 11 | 29 | 4 | 32 | NA | NA | NA | NA | 1 | 2 | NA | NA | 2 | #Ramond 1992 | |
| 3 | 19 | 7 | 25 | NA | NA | NA | NA | 1 | 8 | NA | NA | 2 | #Moreno 2010 | |
| 7 | 16 | 4 | 14 | NA | NA | NA | NA | 1 | 9 | NA | NA | 2 | #Paladugu 2006 | |
| 10 | 25 | 5 | 25 | NA | NA | NA | NA | 1 | 9 | NA | NA | 2 | #Sidhu 2012b | |
| 1 | 17 | 5 | 18 | NA | NA | NA | NA | 2 | 3 | NA | NA | 2 | #Naveau 2004 | |
| 0.5 | 10 | 1.5 | 12 | NA | NA | NA | NA | 2 | 3 | NA | NA | 2 | #Spahr 2002 | |
| 19 | 35 | 9 | 35 | 17 | 33 | 11 | 32 | 2 | 4 | 9 | 10 | 4 | #Higuera de la Tijera 2015 | |
| 21 | 89 | 7 | 85 | NA | NA | NA | NA | 2 | 5 | NA | NA | 2 | #Nguyen‐Khac 2011 | |
| 17 | 137 | 14 | 133 | NA | NA | NA | NA | 2 | 6 | NA | NA | 2 | #Mathurin 2013 | |
| 9 | 34 | 10 | 36 | NA | NA | NA | NA | 2 | 6 | NA | NA | 2 | #Sidhu 2012a | |
| 2.5 | 21 | 0.5 | 21 | NA | NA | NA | NA | 2 | 7 | NA | NA | 2 | #Tkachenko 2016 | |
| 7 | 34 | 2 | 34 | NA | NA | NA | NA | 2 | 9 | NA | NA | 2 | #De 2009 | |
| 16 | 30 | 12 | 30 | NA | NA | NA | NA | 2 | 9 | NA | NA | 2 | #Garrido Garcia 2012 | |
| 7 | 59 | 15 | 62 | NA | NA | NA | NA | 2 | 9 | NA | NA | 2 | #Park 2014 | |
| 1 | 30 | 3 | 30 | NA | NA | NA | NA | 6 | 9 | NA | NA | 2 | #De 2014 | |
| END | ||||||||||||||
| #Mort_90; intervention codes: 1 = No active intervention; 2 = Glucocorticosteroids; 3 = Glucocorticosteroids plus anti‐TNF; 4 = Glucocorticosteroids plus metadoxine; 5 = Glucocorticosteroids plus N‐acetyl cysteine; 6 = Glucocorticosteroids plus pentoxifylline; 7 = GSF plus pentoxifylline; 8 = Pentoxifylline; 9 = Pentoxifylline plus metadoxine. | ||||||||||||||
| list(ns=8,nt=9) | ||||||||||||||
| r[,1] | n[,1] | r[,2] | n[,2] | r[,3] | n[,3] | r[,4] | n[,4] | t[,1] | t[,2] | t[,3] | t[,4] | na[] | #study | |
| 66 | 272 | 80 | 274 | 64 | 273 | 75 | 273 | 1 | 2 | 6 | 8 | 4 | #Thursz 2015 | |
| 16 | 29 | 4 | 32 | NA | NA | NA | NA | 1 | 2 | NA | NA | 2 | #Ramond 1992 | |
| 1 | 9 | 2 | 11 | NA | NA | NA | NA | 2 | 3 | NA | NA | 2 | #Spahr 2002 | |
| 28 | 35 | 11 | 35 | 22 | 33 | 13 | 32 | 2 | 4 | 8 | 9 | 4 | #Higuera de la Tijera 2015 | |
| 30 | 89 | 19 | 85 | NA | NA | NA | NA | 2 | 5 | NA | NA | 2 | #Nguyen‐Khac 2011 | |
| 12 | 34 | 5 | 34 | NA | NA | NA | NA | 2 | 8 | NA | NA | 2 | #De 2009 | |
| 9 | 30 | 5 | 30 | NA | NA | NA | NA | 6 | 8 | NA | NA | 2 | #De 2014 | |
| 5 | 23 | 18 | 23 | NA | NA | NA | NA | 7 | 8 | NA | NA | 2 | #Singh 2014 | |
| END | ||||||||||||||
| #SAE_Pro; intervention codes: 1 = No active intervention; 2 = Glucocorticosteroids; 3 = Glucocorticosteroids plus pentoxifylline; 4 = Pentoxifylline. | ||||||||||||||
| list(ns=1,nt=4) | ||||||||||||||
| r[,1] | n[,1] | r[,2] | n[,2] | r[,3] | n[,3] | r[,4] | n[,4] | t[,1] | t[,2] | t[,3] | t[,4] | na[] | #study | |
| 106 | 272 | 128 | 274 | 116 | 273 | 111 | 273 | 1 | 2 | 3 | 4 | 4 | #Thursz 2015 | |
| END | ||||||||||||||
| #SAE_Num; intervention codes: 1 = Glucocorticosteroids; 2 = Glucocorticosteroids plus anti‐TNF. | ||||||||||||||
| list(ns=1,nt=2) | ||||||||||||||
| r[,1] | E[,1] | r[,2] | E[,2] | r[,3] | E[,3] | t[,1] | t[,2] | t[,3] | na[] | #study | ||||
| 3 | 17 | 15 | 18 | NA | NA | 1 | 2 | NA | 2 | #Naveau 2004 | ||||
| END | ||||||||||||||
| #LT; intervention codes: 1 = Glucocorticosteroids; 2 = Glucocorticosteroids plus N‐acetyl cysteine; 3 = Glucocorticosteroids plus pentoxifylline. | ||||||||||||||
| list(ns=2,nt=3) | ||||||||||||||
| r[,1] | n[,1] | r[,2] | n[,2] | r[,3] | n[,3] | t[,1] | t[,2] | t[,3] | na[] | time[] | #study | |||
| 0.5 | 90 | 1.5 | 86 | NA | NA | 1 | 2 | NA | 2 | 6 | #Nguyen‐Khac 2011 | |||
| 1.5 | 138 | 0.5 | 134 | NA | NA | 1 | 3 | NA | 2 | 6 | #Mathurin 2013 | |||
| END | ||||||||||||||
| #Decom_Cirr; intervention codes: 1 = No active intervention; 2 = Glucocorticosteroids; 3 = Glucocorticosteroids plus anti‐TNF; 4 = Glucocorticosteroids plus metadoxine; 5 = Glucocorticosteroids plus N‐acetyl cysteine; 6 = Glucocorticosteroids plus pentoxifylline; 7 = Pentoxifylline; 8 = Pentoxifylline plus metadoxine. | ||||||||||||||
| list(ns=11,nt=8) | ||||||||||||||
| r[,1] | E[,1] | r[,2] | E[,2] | r[,3] | E[,3] | r[,4] | E[,4] | t[,1] | t[,2] | t[,3] | t[,4] | na[] | #study | |
| 45 | 272 | 44 | 274 | 43 | 273 | 45 | 273 | 1 | 2 | 6 | 7 | 4 | #Thursz 2015 | |
| 31 | 26 | 13 | 24.5 | NA | NA | NA | NA | 1 | 7 | NA | NA | 2 | #Akriviadis 2000 | |
| 7 | 1.3 | 4 | 1.2 | NA | NA | NA | NA | 1 | 7 | NA | NA | 2 | #Paladugu 2006 | |
| 2 | 17 | 3 | 18 | NA | NA | NA | NA | 2 | 3 | NA | NA | 2 | #Naveau 2004 | |
| 55 | 8.8 | 31 | 8.8 | 50 | 8.2 | 37 | 8 | 2 | 4 | 7 | 8 | 4 | #Higuera de la Tijera 2015 | |
| 34 | 44.5 | 25 | 42.5 | NA | NA | NA | NA | 2 | 5 | NA | NA | 2 | #Nguyen‐Khac 2011 | |
| 21 | 68.5 | 11 | 66.5 | NA | NA | NA | NA | 2 | 6 | NA | NA | 2 | #Mathurin 2013 | |
| 9 | 17 | 5 | 18 | NA | NA | NA | NA | 2 | 6 | NA | NA | 2 | #Sidhu 2012a | |
| 8 | 34 | 2 | 34 | NA | NA | NA | NA | 2 | 7 | NA | NA | 2 | #De 2009 | |
| 34 | 2.5 | 29 | 2.5 | NA | NA | NA | NA | 2 | 7 | NA | NA | 2 | #Garrido Garcia 2012 | |
| 8 | 30 | 1 | 30 | NA | NA | NA | NA | 6 | 7 | NA | NA | 2 | #De 2014 | |
| END | ||||||||||||||
| #AE_Pro; intervention codes: 1 = No active intervention; 2 = GSF plus pentoxifylline; 3 = NAC; 4 = Pentoxifylline. | ||||||||||||||
| list(ns=4,nt=4) | ||||||||||||||
| r[,1] | n[,1] | r[,2] | n[,2] | r[,3] | n[,3] | t[,1] | t[,2] | t[,3] | na[] | #study | ||||
| 5 | 19 | 10 | 25 | NA | NA | 1 | 3 | NA | 2 | #Moreno 2010 | ||||
| 11 | 52 | 18 | 49 | NA | NA | 1 | 4 | NA | 2 | #Akriviadis 2000 | ||||
| 12 | 25 | 17 | 25 | NA | NA | 1 | 4 | NA | 2 | #Sidhu 2012b | ||||
| 5.5 | 24 | 0.5 | 24 | NA | NA | 2 | 4 | NA | 2 | #Singh 2014 | ||||
| END | ||||||||||||||
| #AE_Num; intervention codes: 1 = No active intervention; 2 = Glucocorticosteroids; 3 = Glucocorticosteroids plus anti‐TNF; 4 = Glucocorticosteroids plus metadoxine; 5 = Glucocorticosteroids plus N‐acetyl cysteine; 6 = Glucocorticosteroids plus pentoxifylline; 7 = Glucocorticosteroids plus SAMe; 8 = GSF plus pentoxifylline; 9 = NAC; 10 = Pentoxifylline; 11 = Pentoxifylline plus metadoxine. | ||||||||||||||
| list(ns=16,nt=11) | ||||||||||||||
| r[,1] | E[,1] | r[,2] | E[,2] | r[,3] | E[,3] | r[,4] | E[,4] | t[,1] | t[,2] | t[,3] | t[,4] | na[] | #study | |
| 61 | 272 | 84 | 274 | 73 | 273 | 66 | 273 | 1 | 2 | 6 | 10 | 4 | #Thursz 2015 | |
| 5 | 9.5 | 10 | 12.5 | NA | NA | NA | NA | 1 | 9 | NA | NA | 2 | #Moreno 2010 | |
| 20 | 26 | 40 | 24.5 | NA | NA | NA | NA | 1 | 10 | NA | NA | 2 | #Akriviadis 2000 | |
| 12 | 2.1 | 17 | 2.1 | NA | NA | NA | NA | 1 | 10 | NA | NA | 2 | #Sidhu 2012b | |
| 15 | 17 | 24 | 18 | NA | NA | NA | NA | 2 | 3 | NA | NA | 2 | #Naveau 2004 | |
| 2 | 2.2 | 2 | 2.8 | NA | NA | NA | NA | 2 | 3 | NA | NA | 2 | #Spahr 2002 | |
| 81 | 8.8 | 51 | 8.8 | 71 | 8.2 | 56 | 8 | 2 | 4 | 10 | 11 | 4 | #Higuera de la Tijera 2015 | |
| 46 | 44.5 | 26 | 42.5 | NA | NA | NA | NA | 2 | 5 | NA | NA | 2 | #Nguyen‐Khac 2011 | |
| 31 | 68.5 | 40 | 66.5 | NA | NA | NA | NA | 2 | 6 | NA | NA | 2 | #Mathurin 2013 | |
| 12 | 17 | 27 | 18 | NA | NA | NA | NA | 2 | 6 | NA | NA | 2 | #Sidhu 2012a | |
| 16 | 10 | 12 | 10 | NA | NA | NA | NA | 2 | 7 | NA | NA | 2 | #Tkachenko 2016 | |
| 66 | 34 | 62 | 34 | NA | NA | NA | NA | 2 | 10 | NA | NA | 2 | #De 2009 | |
| 20 | 2.5 | 20 | 2.5 | NA | NA | NA | NA | 2 | 10 | NA | NA | 2 | #Garrido Garcia 2012 | |
| 31 | 29.5 | 28 | 31 | NA | NA | NA | NA | 2 | 10 | NA | NA | 2 | #Park 2014 | |
| 56 | 30 | 50 | 30 | NA | NA | NA | NA | 6 | 10 | NA | NA | 2 | #De 2014 | |
| 5.5 | 6.8 | 0.5 | 7 | NA | NA | NA | NA | 8 | 10 | NA | NA | 2 | #Singh 2014 | |
| END | ||||||||||||||
| Abbreviations: GSF = granulocyte stimulating factor; TNF = tumour necrosis factor; SAMe = S‐adenosyl methionine. | ||||||||||||||
Appendix 3. Severe alcoholic hepatitis: results (text)
The data used in the analysis is available in Appendix 2. A summary of potential effect modifiers and the overall risk of bias in the comparisons are listed in Appendix 4. There does not appear to be any concerns about transitivity assumption. The network plot for all outcomes with more than one trial are shown in Figure 38. As shown in Figure 38, Five outcomes (mortality at maximal follow‐up, 30‐days mortality, 90‐days mortality, decompensated cirrhosis, and adverse events (number) have treatment comparisons in which direct and indirect estimates were available ('closed loops'). The ranking probabilities of different treatments for different outcomes in which network meta‐analysis was performed is shown in Appendix 5. These ranking probabilities are also presented as figures which show the cumulative probability of being best, second best, etc (SUCRA) and rankogram which show the ranking probability of each treatment at each different rank (best treatment, second best, etc). These ranking probabilities should be interpreted with extreme caution because of the sparse networks with high risk of bias trials and evidence of inconsistency noted in many outcomes.
10.
The network plots showing the comparisons in there were at least two trials. The size of the node (circle) provides a measure of the number of trials in which the particular intervention was included as one of the arms. The thickness of the line provides a measure of the number of direct comparisons between two nodes (interventions). Five outcomes (mortality at maximal follow‐up, 30‐days mortality, 90‐days mortality, decompensated cirrhosis, and adverse events (number) have intervention comparisons in which direct and indirect estimates were available ('closed loops'). Legend: _ = plus; AntiTNF = anti‐TNF (tumour necrosis factor); Control = No active intervention; GSF = granulocyte stimulating factor; NAC = N‐acetyl cysteine; SAMe = S‐adenosyl methionine.
Mortality at maximal follow‐up
A total of 18 trials including 2477 participants reported mortality at maximal follow‐up (Ramond 1992; Akriviadis 2000; Spahr 2002; Naveau 2004; Paladugu 2006; De 2009; Moreno 2010; Nguyen‐Khac 2011; Garrido Garcia 2012; Sidhu 2012a; Sidhu 2012b; Mathurin 2013; De 2014; Park 2014; Singh 2014; Higuera de la Tijera 2015; Thursz 2015; Tkachenko 2016). The follow‐up varied between one month and 12 months. In the network meta‐analysis, the between‐study standard deviation (τ) was 0.4098 (τ2 = 0.1679; lies within the 95% range for all‐cause mortality in pharmacological comparisons). The I2 could not be estimated. Although there was no evidence of inconsistency as per model fit, there was evidence of inconsistency as evidenced by the treatment‐by‐design model (P = 0.0154), and inconsistency factor. The inconsistency plot is shown in Figure 39. The confidence intervals of the inconsistency factor for some of the loops do not overlap one suggesting inconsistency.
11.
IF (Inconsistency Factor) plots of mortality at maximal follow‐up in which direct and indirect estimates were available. There appears to be inconsistency as the confidence intervals do not overlap 1 for the following loops: no intervention ‐ glucocorticosteroids ‐ pentoxifylline plus glucocorticosteroids (confidence intervals: 0.00 to 0.14) and glucocorticosteroids ‐ pentoxifylline plus glucocorticosteroids ‐ pentoxifylline (0.00 to 0.78).
Forest plots of mortality at maximal follow‐up (network meta‐analysis estimates and direct comparisons when available) are shown in Figure 40. Both fixed‐effect model and random‐effects model for other interventions compared to no intervention are provided in Figure 40. As shown in the figure, the direct estimates and network meta‐analysis estimates of different models were similar. Several comparisons in which there was evidence of difference in fixed‐effect model did not have any evidence of difference based on random‐effects model. The more conservative random‐effects model was used to arrive at conclusions. The pairwise meta‐analysis estimates of the random‐effects model are shown in Appendix 6. It appears that GSF plus pentoxifylline, glucocorticosteroids plus metadoxine, pentoxifylline, and pentoxifylline plus metadoxine decrease mortality at maximal follow‐up compared with no intervention. The surface area under the curve for each treatment being best, second best, third best, and so on and the ranking probabilities of each treatment being best, second best, third best, and so on are show in Figure 41. None of the treatments seems to be clearly better than others, although GSF plus pentoxifylline appears to be one of the top‐ranking interventions.
12.
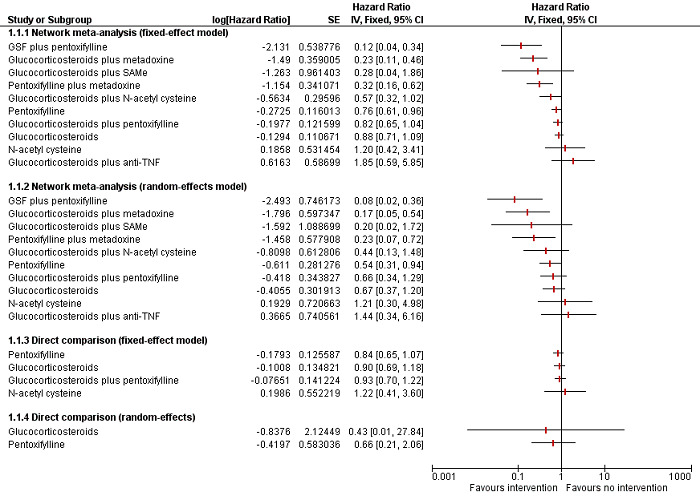
Forest plot of mortality at maximal follow‐up (network meta‐analysis estimates and direct comparisons when available): Both fixed‐effect model and random‐effects model for other interventions compared to no intervention are provided. The direct estimates and network meta‐analysis estimates are similar. Based on the network meta‐analysis, it appears that GSF plus pentoxifylline, glucocorticosteroids plus metadoxine, and pentoxifylline plus metadoxine decrease mortality at maximal follow‐up compared with no intervention.
Abbreviations: GSF = granulocyte stimulating factor; TNF = tumour necrosis factor; SAMe = S‐adenosyl methionine.
13.
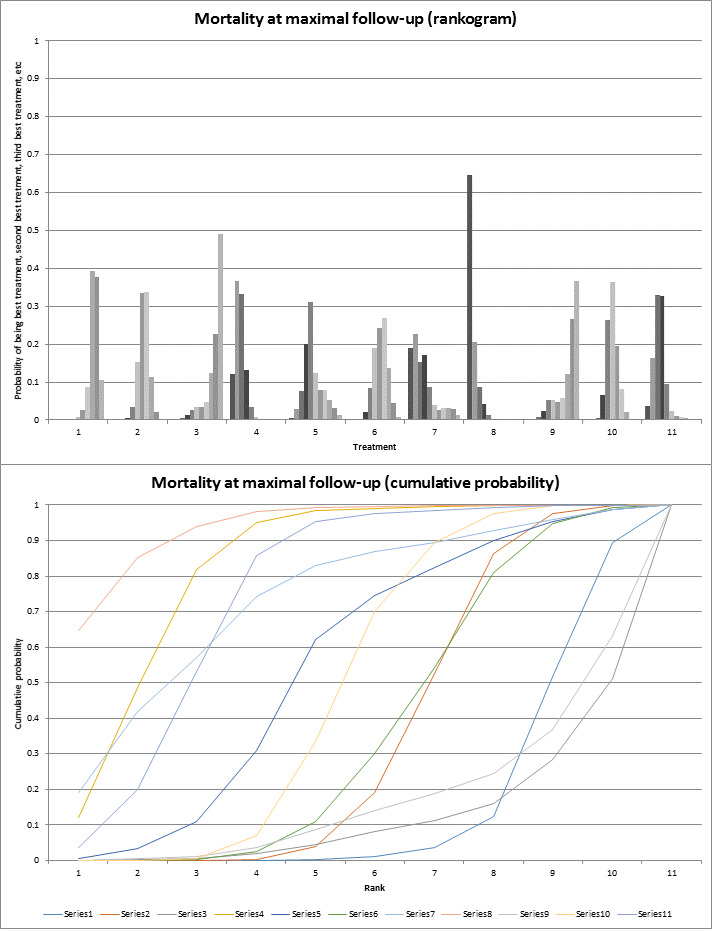
The top figure shows the surface area under the curve constructed on the basis of the ranking probabilities. The bottom figure shows the probability of each intervention being best, second best, third best, and so on. None of the interventions seems to be clearly better than others, although GSF plus pentoxifylline appears to be one of the top‐ranking interventions. Legend: 1: No active intervention; 2: Glucocorticosteroids; 3: Glucocorticosteroids plus anti‐TNF; 4: Glucocorticosteroids plus metadoxine; 5: Glucocorticosteroids plus N‐acetyl cysteine; 6: Glucocorticosteroids plus pentoxifylline; 7: Glucocorticosteroids plus SAMe; 8: GSF plus pentoxifylline; 9: N‐acetyl cysteine; 10: Pentoxifylline; 11: Pentoxifylline plus metadoxine.
Abbreviations: GSF = granulocyte stimulating factor; TNF = tumour necrosis factor; SAMe = S‐adenosyl methionine.
Early mortality (up to three months)
30‐days mortality
A total of 16 trials including 2330 participants reported 30‐days mortality (Ramond 1992; Spahr 2002; Naveau 2004; Paladugu 2006; De 2009; Moreno 2010; Nguyen‐Khac 2011; Garrido Garcia 2012; Sidhu 2012a; Sidhu 2012b; Mathurin 2013; De 2014; Park 2014; Higuera de la Tijera 2015; Thursz 2015; Tkachenko 2016). In the network meta‐analysis, the between‐study standard deviation (τ) was 0.4295 (τ2 = 0.1845; lies within the 95% range for all‐cause mortality in pharmacological comparisons). The I2 could not be estimated. Although there was no evidence of inconsistency as per model fit and treatment‐by‐design model (P = 0.6931), the inconsistency plot shows that the confidence intervals of the inconsistency factor for one of the loops do not overlap one suggesting inconsistency (Figure 42). Forest plots of 30‐days mortality (network meta‐analysis estimates and direct comparisons when available) are shown in Figure 43. Both fixed‐effect model and random‐effects model for other interventions compared to no intervention are provided in Figure 43. As shown in the figure, the direct estimates and network meta‐analysis estimates of different models were similar. It appears that glucocorticosteroids plus N‐acetyl cysteine and glucocorticosteroids plus metadoxine decrease 30‐days compared with no intervention. Several comparisons in which there was evidence of difference in fixed‐effect model did not have any evidence of difference based on random‐effects model. The more conservative random‐effects model was used to arrive at conclusions. The pairwise meta‐analysis estimates of the random‐effects model are shown in Appendix 6. The surface area under the curve for each treatment being best, second best, third best, and so on and the ranking probabilities of each treatment being best, second best, third best, and so on are show in Figure 44. None of the treatments seems to be clearly better than others, although glucocorticosteroids plus SAMe, glucocorticosteroids plus metadoxine, and glucocorticosteroids plus N‐acetyl cysteine appear to be the top‐ranking interventions.
14.
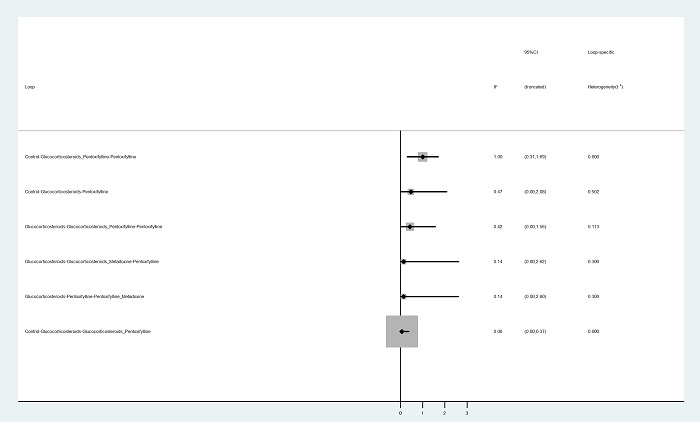
IF (Inconsistency Factor) plots of 30‐days mortality in which direct and indirect estimates were available. There appears to be inconsistency for the loop 'no intervention ‐ glucocorticosteroids ‐ pentoxifylline plus glucocorticosteroids' as the confidence intervals do not overlap 1 (confidence intervals: 0.00 to 0.37).
15.
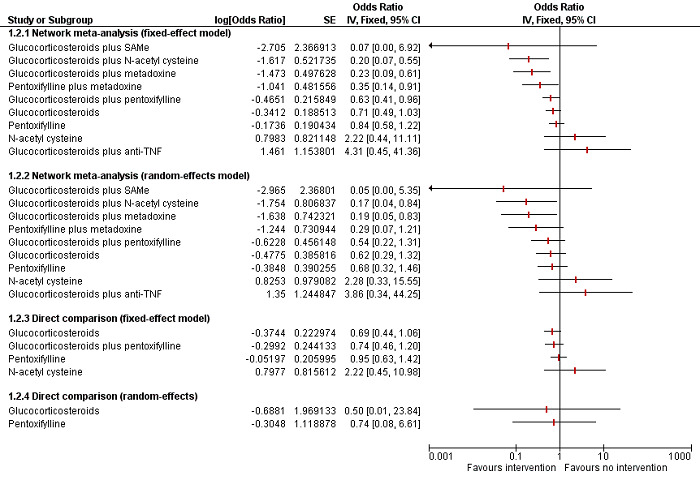
Forest plot of 30‐days mortality (network meta‐analysis estimates and direct comparisons when available): Both fixed‐effect model and random‐effects model for other interventions compared to no intervention are provided. The direct estimates and network meta‐analysis estimates are similar. Based on the network meta‐analysis, it appears that glucocorticosteroids plus N‐acetyl cysteine and glucocorticosteroids plus metadoxine decrease 30‐days compared with no intervention.
Abbreviations: TNF = tumour necrosis factor; SAMe = S‐adenosyl methionine.
16.
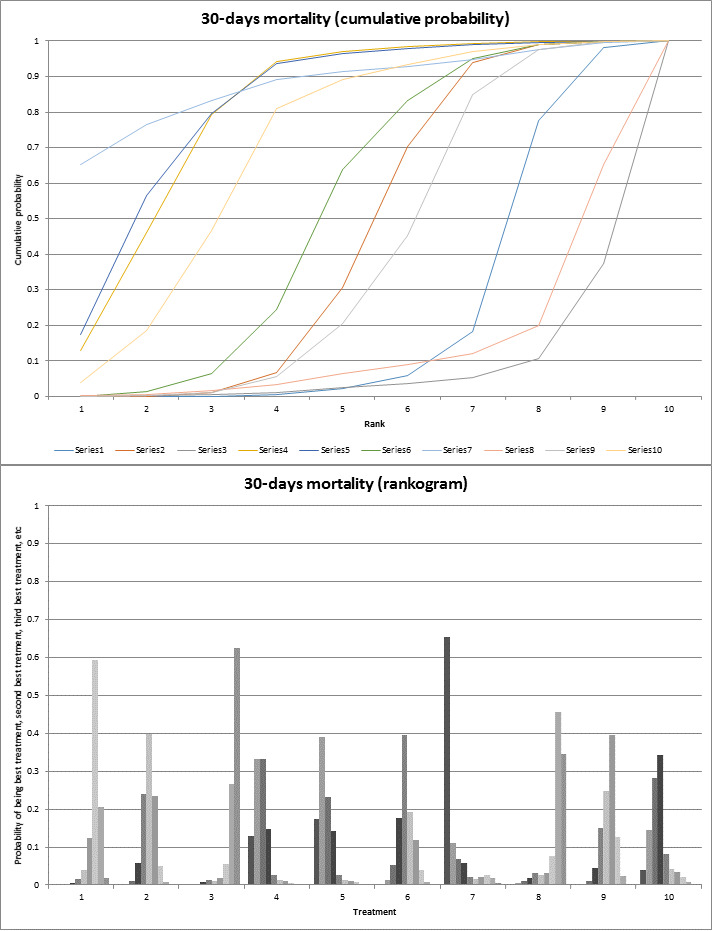
The top figure shows the surface area under the curve constructed on the basis of the ranking probabilities. The bottom figure shows the probability of each intervention being best, second best, third best, and so on. None of the interventions seems to be clearly better than others, although glucocorticosteroids plus SAMe, glucocorticosteroids plus metadoxine, and glucocorticosteroids plus N‐acetyl cysteine appear to be the top‐ranking interventions. Legend: 1: No active intervention; 2: Glucocorticosteroids; 3: Glucocorticosteroids plus anti‐TNF; 4: Glucocorticosteroids plus metadoxine; 5: Glucocorticosteroids plus N‐acetyl cysteine; 6: Glucocorticosteroids plus pentoxifylline; 7: Glucocorticosteroids plus SAMe; 8: N‐acetyl cysteine; 9: Pentoxifylline; 10: Pentoxifylline plus metadoxine.
Abbreviations: TNF = tumour necrosis factor; SAMe = S‐adenosyl methionine.
90‐days mortality
A total of eight trials including 1656 participants reported 90‐days mortality (Ramond 1992; Spahr 2002; De 2009; Nguyen‐Khac 2011; De 2014; Singh 2014; Higuera de la Tijera 2015; Thursz 2015). In the network meta‐analysis, the between‐study standard deviation (τ) was 1.524 (τ2 = 2.32; lies beyond the 95% range for all‐cause mortality in pharmacological comparisons suggesting heterogeneity). The I2 could not be estimated. Although there was no evidence of inconsistency as per model fit and inconsistency factor (inconsistency plot: Figure 45), there was evidence of inconsistency as evidenced by the treatment‐by‐design model (P = 0.0023). Forest plots of 90‐days mortality (network meta‐analysis estimates and direct comparisons when available) are shown in Figure 46. Both fixed‐effect model and random‐effects model for other interventions compared to no intervention are provided in Figure 46. As shown in the figure, the estimates from the fixed‐effect model appear to be different from those of random‐effects model. The more conservative random‐effects model was used to arrive at conclusions. There is no evidence of differences between any of the comparisons. The pairwise meta‐analysis estimates of the random‐effects model are shown in Appendix 6. The surface area under the curve for each treatment being best, second best, third best, and so on and the ranking probabilities of each treatment being best, second best, third best, and so on are show in Figure 47. GSF plus pentoxifylline appears to be the top‐ranking intervention.
17.
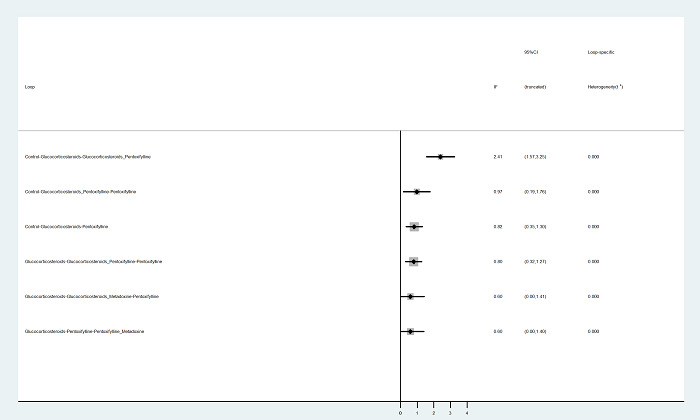
IF (Inconsistency Factor) plots of 90‐days mortality in which direct and indirect estimates were available. There appears to be no evidence of inconsistency in any of the loops as the confidence intervals of all the loops overlap 1.
18.
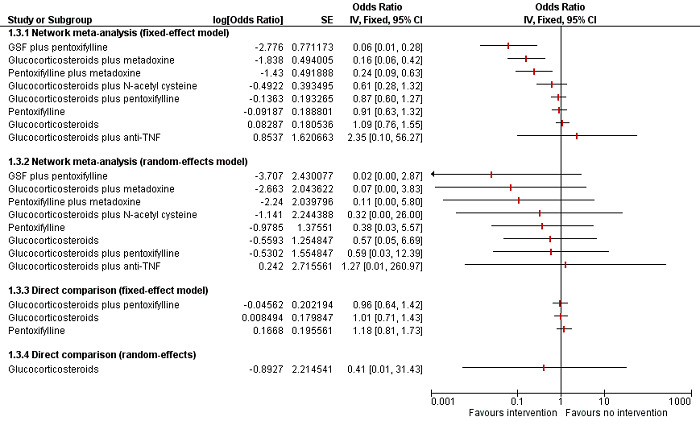
Forest plot of 90‐days mortality (network meta‐analysis estimates and direct comparisons when available): Both fixed‐effect model and random‐effects model for other interventions compared to no intervention are provided. The direct estimates and network meta‐analysis estimates appear different. Based on the random‐effects network meta‐analysis and direct comparison, there is no evidence of differences between any of the comparisons.
Abbreviations: GSF = granulocyte stimulating factor; TNF = tumour necrosis factor.
19.
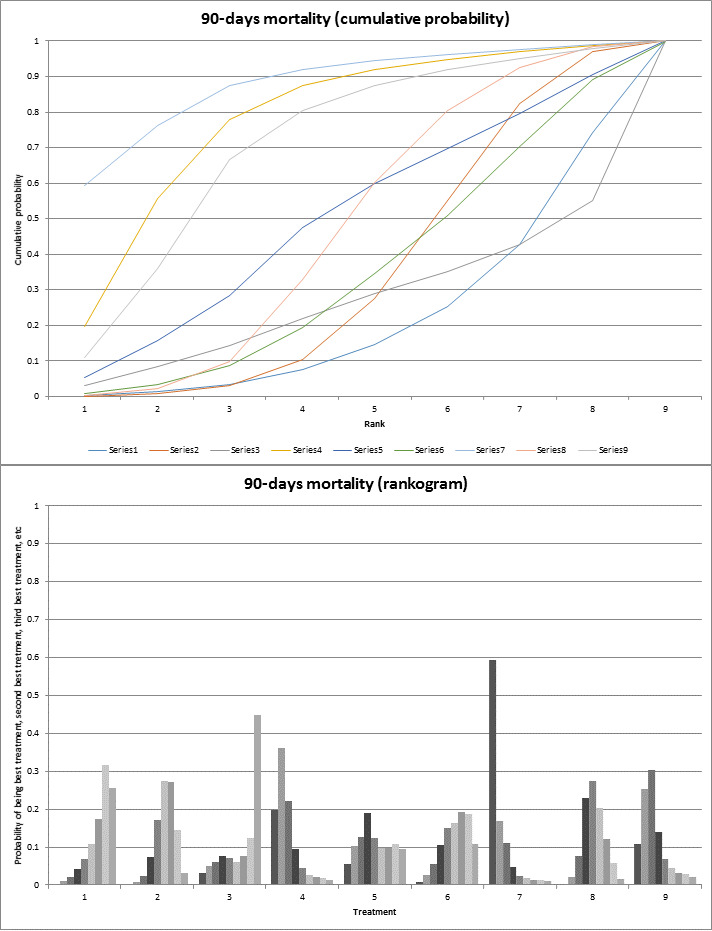
The top figure shows the surface area under the curve constructed on the basis of the ranking probabilities. The bottom figure shows the probability of each intervention being best, second best, third best, and so on. GSF plus pentoxifylline appears to be the top‐ranking intervention.
Legend: 1: No active intervention; 2: Glucocorticosteroids; 3: Glucocorticosteroids plus anti‐TNF; 4: Glucocorticosteroids plus metadoxine; 5: Glucocorticosteroids plus N‐acetyl cysteine; 6: Glucocorticosteroids plus pentoxifylline; 7: GSF plus pentoxifylline; 8: Pentoxifylline; 9: Pentoxifylline plus metadoxine.
Abbreviations: GSF = granulocyte stimulating factor; TNF = tumour necrosis factor.
Serious adverse events
Only one trial (1092 participants; four interventions) reported serious adverse events (proportion) (Thursz 2015). As shown in Appendix 6, there was no evidence of differences in any of the comparisons. Only one trial (35 participants) reported serious adverse events (number) (Naveau 2004). The number of serious adverse events was more in the glucocorticosteroids plus anti‐TNF group versus the glucocorticosteroids group (rate ratio 5.14; 95% CrI 1.62 to 23.50).
Health‐related quality of life
None of the trials reported health‐related quality of life at any time point.
Liver transplantation
A total of two trials including 448 participants reported liver transplantation (Nguyen‐Khac 2011; Mathurin 2013). In the network meta‐analysis, the between‐study standard deviation (τ) was so small that it was determined by the prior used. The I2 could not be estimated. Since there was no closed loop, we did not assess inconsistency. Forest plots of liver transplantation (network meta‐analysis estimates and direct comparisons when available) are shown in Figure 48. Both fixed‐effect model and random‐effects model for other interventions compared to glucocorticosteroids are provided in Figure 48. As shown in the figure, the estimates from the different comparisons are similar. The pairwise meta‐analysis estimates of the fixed‐effect model are shown in Appendix 6. There was no evidence of differences in any of the comparisons. The surface area under the curve for each treatment being best, second best, third best, and so on and the ranking probabilities of each treatment being best, second best, third best, and so on are show in Figure 49. Glucocorticosteroids plus pentoxifylline appears to be the top‐ranking intervention.
20.
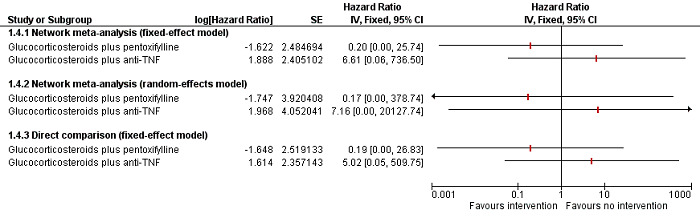
Forest plot of liver transplantation (network meta‐analysis estimates and direct comparisons when available): Both fixed‐effect model and random‐effects model for other interventions compared to glucocorticosteroids are provided. Since there was only one trial for each comparison, random‐effects model is not applicable for direct comparisons. The direct estimates and network meta‐analysis estimates appear similar. There is no evidence of differences between any of the comparisons.
Abbreviations: TNF = tumour necrosis factor.
21.
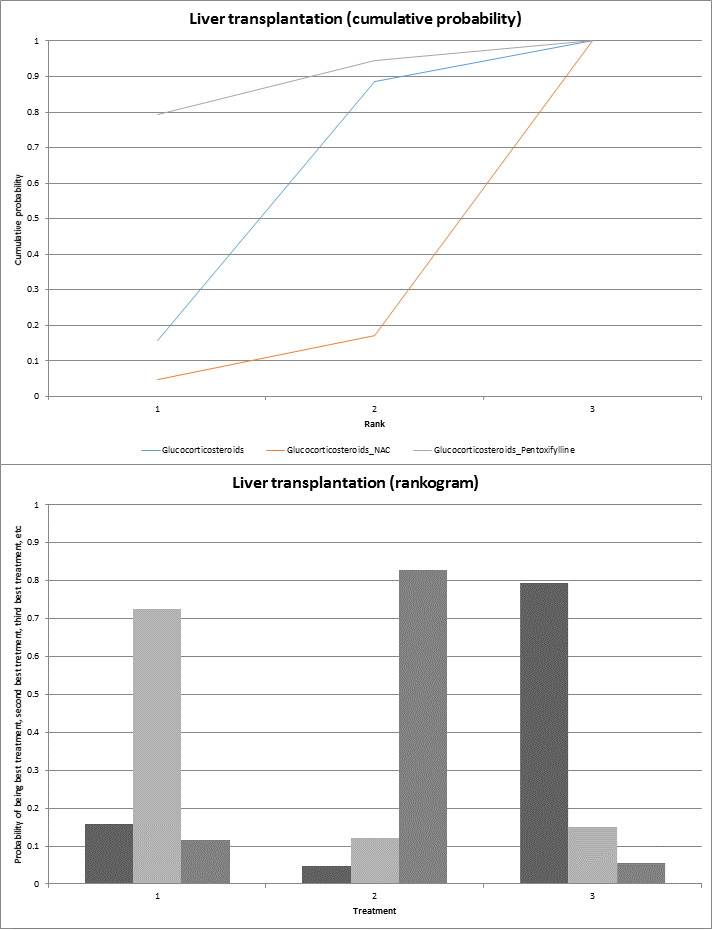
The top figure shows the surface area under the curve constructed on the basis of the ranking probabilities. The bottom figure shows the probability of each intervention being best, second best, third best, and so on. Glucocorticosteroids plus pentoxifylline appears to be the top‐ranking intervention.
Legend: 1: Glucocorticosteroids; 2: Glucocorticosteroids plus N‐acetyl cysteine; 3: Glucocorticosteroids plus pentoxifylline
Decompensated cirrhosis
A total of 11 trials including 2095 participants reported decompensated cirrhosis (Akriviadis 2000; Naveau 2004; Paladugu 2006; De 2009; Nguyen‐Khac 2011; Garrido Garcia 2012; Sidhu 2012a; Mathurin 2013; De 2014; Higuera de la Tijera 2015; Thursz 2015). In the network meta‐analysis, the between‐study standard deviation (τ) was so small that it was determined by the prior used. The I2 could not be estimated. Although there was no evidence of inconsistency as per model fit, there was evidence of inconsistency as evidenced by the treatment‐by‐design model (P = 0.0445), and inconsistency factor. The inconsistency plot is shown in Figure 50. The confidence intervals of the inconsistency factor for some of the loops do not overlap one suggesting inconsistency. Forest plots of decompensated cirrhosis (network meta‐analysis estimates and direct comparisons when available) are shown in Figure 51. Both fixed‐effect model and random‐effects model for other interventions compared to no intervention are provided in Figure 51. As shown in the figure, the direct estimates and network meta‐analysis estimates of different models were similar. Several comparisons in which there was evidence of difference in fixed‐effect model did not have any evidence of difference based on random‐effects model. The more conservative random‐effects model was used to arrive at conclusions. The pairwise meta‐analysis estimates of the random‐effects model are shown in Appendix 6. It appears that glucocorticosteroids plus metadoxine decreases decompensated cirrhosis compared with no intervention. The surface area under the curve for each treatment being best, second best, third best, and so on and the ranking probabilities of each treatment being best, second best, third best, and so on are show in Figure 52. Glucocorticosteroids plus metadoxine appears to be the top‐ranking intervention.
22.
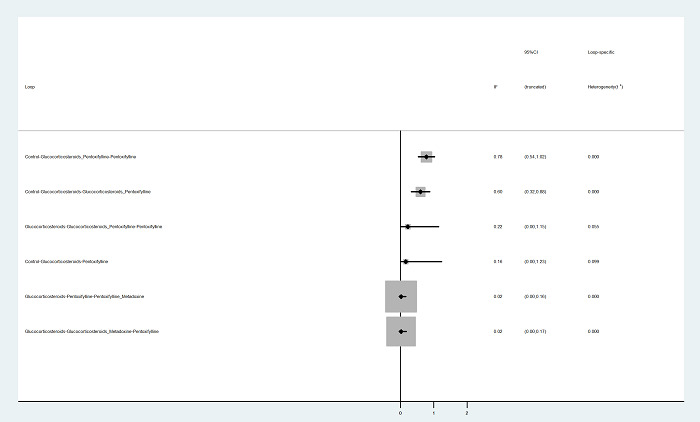
IF (Inconsistency Factor) plots of decompensated cirrhosis in which direct and indirect estimates were available. There appears to be inconsistency in several loops as the confidence intervals do not overlap 1. The loops in which the confidence intervals do not overlap 1 are: no intervention ‐ glucocorticosteroids ‐ pentoxifylline plus glucocorticosteroids (confidence intervals: 0.32 to 0.88), glucocorticosteroids ‐ pentoxifylline ‐ metadoxine plus pentoxifylline (0.00 to 0.16), and glucocorticosteroids ‐ metadoxine plus glucocorticosteroids ‐ pentoxifylline (0.00 to 0.17).
23.
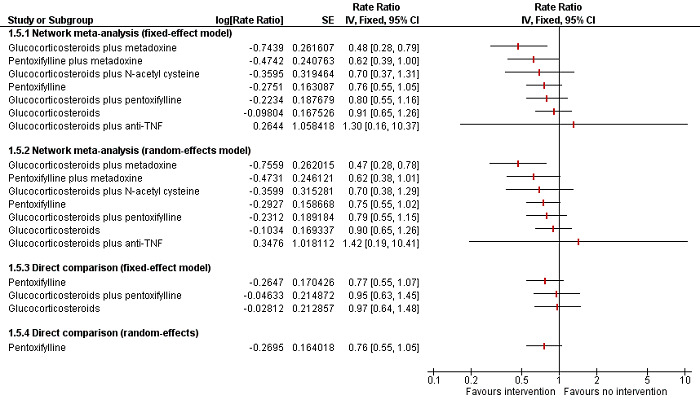
Forest plot of decompensated cirrhosis (network meta‐analysis estimates and direct comparisons when available): Both fixed‐effect model and random‐effects model for other interventions compared to no intervention are provided. The direct estimates and network meta‐analysis estimates appear similar. Glucocorticosteroids plus metadoxine appears to have a lower decompensated cirrhosis rate than no intervention. There was no evidence of any differences in any other interventions versus no intervention in the random‐effects model.
Abbreviations: TNF = tumour necrosis factor.
24.
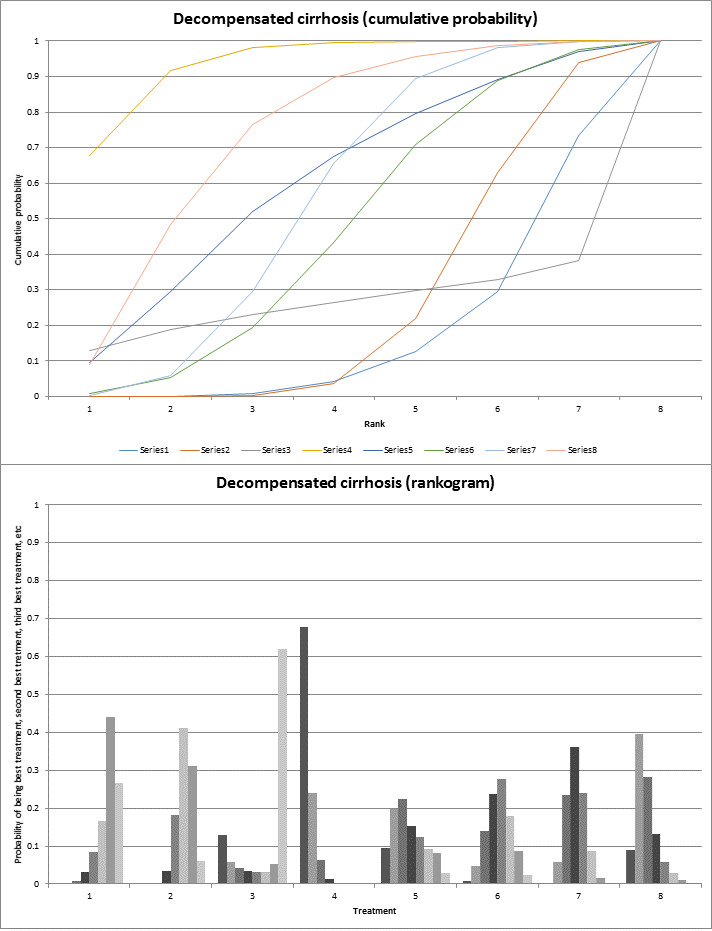
The top figure shows the surface area under the curve constructed on the basis of the ranking probabilities. The bottom figure shows the probability of each intervention being best, second best, third best, and so on. Glucocorticosteroids plus metadoxine appears to be the top‐ranking intervention.
Legend: 1: No active intervention; 2: Glucocorticosteroids; 3: Glucocorticosteroids plus anti‐TNF; 4: Glucocorticosteroids plus metadoxine; 5: Glucocorticosteroids plus N‐acetyl cysteine; 6: Glucocorticosteroids plus pentoxifylline; 7: Pentoxifylline; 8: Pentoxifylline plus metadoxine.
Abbreviations: TNF = tumour necrosis factor.
Cirrhosis
None of the trials reported the proportion of people who developed cirrhosis.
Hepatocellular carcinoma
None of the trials reported the proportion of people who developed hepatocellular carcinoma.
Any adverse events
A total of four trials including 241 participants reported adverse events (proportion) (Akriviadis 2000; Moreno 2010; Sidhu 2012b; Singh 2014). In the network meta‐analysis, the between‐study standard deviation (τ) was 1.474 (τ2 = 2.1727; lies beyond the 95% range for adverse events in pharmacological comparisons suggesting heterogeneity). The I2 could not be estimated. Since there was no direct and indirect estimates for any of the comparisons, we did not assess inconsistency. Forest plots of any adverse events (proportion) (network meta‐analysis estimates and direct comparisons when available) are shown in Figure 53. Both fixed‐effect model and random‐effects model for other interventions compared to no intervention are provided in Figure 53. As shown in the figure, the direct estimates and network meta‐analysis estimates of different models were similar. Several comparisons in which there was evidence of difference in fixed‐effect model did not have any evidence of difference based on random‐effects model. The more conservative random‐effects model was used to arrive at conclusions. The pairwise meta‐analysis estimates of the random‐effects model are shown in Appendix 6. There was no evidence of any difference between any intervention and no intervention. The surface area under the curve for each treatment being best, second best, third best, and so on and the ranking probabilities of each treatment being best, second best, third best, and so on are show in Figure 54. No active intervention appears to be the top‐ranking intervention, while GSF plus pentoxifylline appears to the worst intervention with regards to adverse events (proportion).
25.
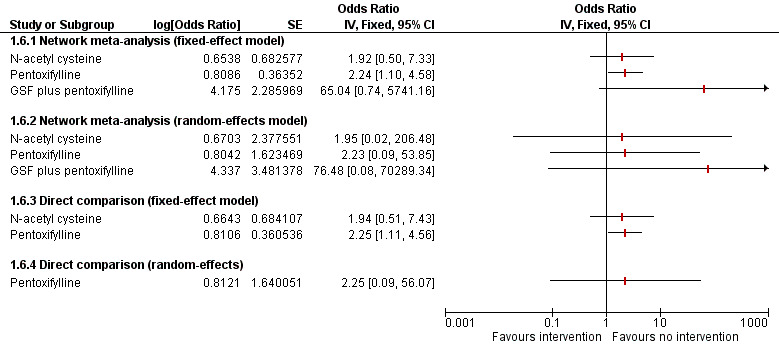
Forest plot of adverse events (proportion) (network meta‐analysis estimates and direct comparisons when available): Both fixed‐effect model and random‐effects model for other interventions compared to no intervention are provided. The direct estimates and network meta‐analysis estimates appear similar. There was no evidence of any differences in any other interventions versus no intervention using the random‐effects model.
Abbreviations: GSF = granulocyte stimulating factor.
26.
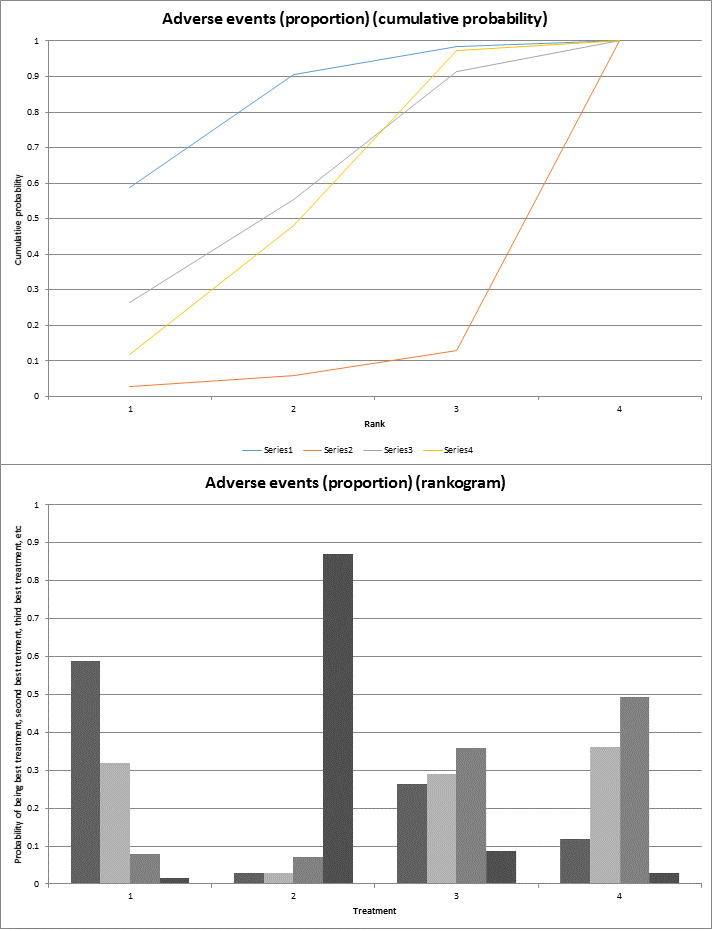
The top figure shows the surface area under the curve constructed on the basis of the ranking probabilities. The bottom figure shows the probability of each intervention being best, second best, third best, and so on. No active intervention appears to be the top‐ranking intervention, while GSF plus pentoxifylline appears to be the worst‐ranking intervention.
Legend: 1: No active intervention; 2: GSF plus pentoxifylline; 3: N‐acetyl cysteine 4: Pentoxifylline.
Abbreviations: GSF = granulocyte stimulating factor.
A total of 16 trials including 2386 participants reported adverse events (number) (Akriviadis 2000; Spahr 2002; Naveau 2004; De 2009; Moreno 2010; Nguyen‐Khac 2011; Garrido Garcia 2012; Sidhu 2012a; Sidhu 2012b; Mathurin 2013; De 2014; Park 2014; Singh 2014; Higuera de la Tijera 2015; Thursz 2015; Tkachenko 2016). In the network meta‐analysis, the between‐study standard deviation (τ) was so small that it was determined by the prior used. The I2 could not be estimated. Although there was no evidence of inconsistency as per model fit and treatment‐by‐design model (P = 0.1901), the inconsistency plot shows that the confidence intervals of the inconsistency factor for one of the loops do not overlap one suggesting inconsistency (Figure 55). Forest plots of decompensated cirrhosis (network meta‐analysis estimates and direct comparisons when available) are shown in Figure 56. Both fixed‐effect model and random‐effects model for other interventions compared to no intervention are provided in Figure 56. As shown in the figure, the direct estimates and network meta‐analysis estimates of different models were similar. Several comparisons in which there was evidence of difference in fixed‐effect model did not have any evidence of difference based on random‐effects model. The more conservative random‐effects model was used to arrive at conclusions. The pairwise meta‐analysis estimates of the random‐effects model are shown in Appendix 6. The number of adverse events were higher in the glucocorticosteroids, pentoxifylline, and pentoxifylline plus glucocorticosteroids groups versus the no intervention group. The surface area under the curve for each treatment being best, second best, third best, and so on and the ranking probabilities of each treatment being best, second best, third best, and so on are show in Figure 57. No active intervention appears to be the top‐ranking intervention, while GSF plus pentoxifylline appears to the worst intervention with regards to adverse events (number).
27.
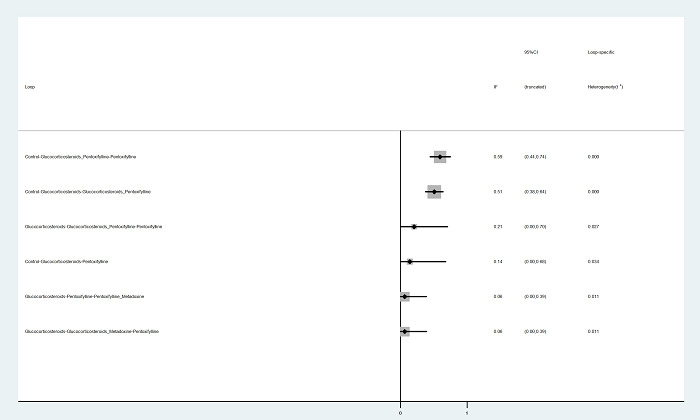
IF (Inconsistency Factor) plots of adverse events (number) in which direct and indirect estimates were available. There appears to be inconsistency in several loops as the confidence intervals do not overlap 1. The loops in which the confidence intervals did not overlap 1 are: no intervention ‐ pentoxifylline ‐ pentoxifylline plus glucocorticosteroids (confidence intervals: 0.44 to 0.74), no intervention ‐ glucocorticosteroids ‐ pentoxifylline plus glucocorticosteroids (confidence intervals: 0.38 to 0.64), no intervention ‐ glucocorticosteroids ‐ pentoxifylline (0.00 to 0.68), glucocorticosteroids ‐ pentoxifylline‐ metadoxine plus pentoxifylline (0.00 to 0.39), and glucocorticosteroids ‐ metadoxine plus glucocorticosteroids ‐ pentoxifylline (0.00 to 0.39).
28.
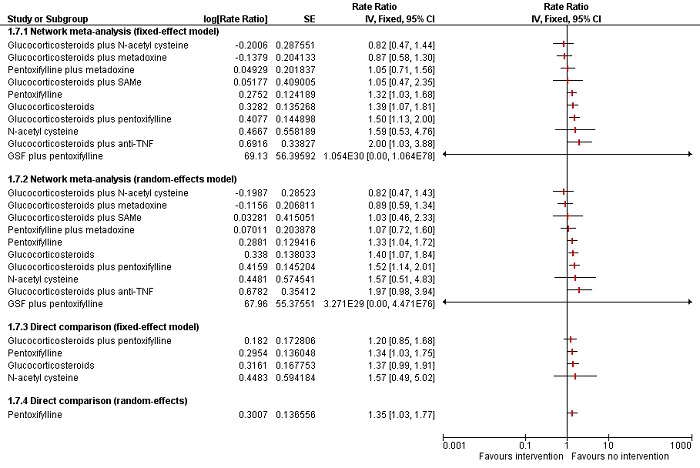
Forest plot of adverse events (number) (network meta‐analysis estimates and direct comparisons when available): Both fixed‐effect model and random‐effects model for other interventions compared to no intervention are provided. The direct estimates and network meta‐analysis estimates are similar. Based on the network meta‐analysis, it appears that glucocorticosteroids, pentoxifylline, and pentoxifylline plus glucocorticosteroids have more adverse events compared with no intervention.
Abbreviations: GSF = granulocyte stimulating factor; TNF = tumour necrosis factor; SAMe = S‐adenosyl methionine.
29.
The top figure shows the surface area under the curve constructed on the basis of the ranking probabilities. The bottom figure shows the probability of each intervention being best, second best, third best, and so on. None of the interventions seems to be clearly better than others, although GSF plus pentoxifylline appears to be the worst‐ranking intervention. Legend: 1: No active intervention; 2: Glucocorticosteroids; 3: Glucocorticosteroids plus anti‐TNF; 4: Glucocorticosteroids plus metadoxine; 5: Glucocorticosteroids plus N‐acetyl cysteine; 6: Glucocorticosteroids plus pentoxifylline; 7: Glucocorticosteroids plus SAMe; 8: GSF plus pentoxifylline; 9: N‐acetyl cysteine; 10: Pentoxifylline; 11: Pentoxifylline plus metadoxine.
Abbreviations: GSF = granulocyte stimulating factor; TNF = tumour necrosis factor; SAMe = S‐adenosyl methionine.
Appendix 4. Severe alcoholic hepatitis: potential effect modifiers
| Study name | Intervention 1 | Intervention 2 | Intervention 3 | Intervention 4 | Hepatic encephalopathy | Recent uncontrolled bacterial infection or spontaneous bacterial peritonitis | Recent gastrointestinal bleeding | Hepatorenal syndrome | Ascites | Year of study | Follow‐up | Risk of bias |
| Naveau 2004 | Glucocorticosteroids plus anti‐TNF | Glucocorticosteroids | Not stated | No | No | No | Not stated | 2001 to 2002 | 12 | High | ||
| Spahr 2002 | Glucocorticosteroids plus anti‐TNF | Glucocorticosteroids | Not stated | No | No | Severe renal failure were excluded | Not stated | Not stated | 3 | High | ||
| Higuera de la Tijera 2015 | Glucocorticosteroids plus metadoxine | Glucocorticosteroids | Pentoxifylline plus metadoxine | Pentoxifylline | Not stated | Not stated | Not stated | Not stated | Yes | 2010 to 2013 | 3 | High |
| Nguyen‐Khac 2011 | Glucocorticosteroids plus N‐acetyl cysteine | Glucocorticosteroids | Not stated | No | No | No | Not stated | 2004 to 2009 | 6 | High | ||
| Mathurin 2013 | Glucocorticosteroids plus pentoxifylline | Glucocorticosteroids | Not stated | No | Not stated | No | Not stated | 2007 to 2010 | 6 | High | ||
| Sidhu 2012a | Glucocorticosteroids plus pentoxifylline | Glucocorticosteroids | Not stated | Not stated | Not stated | No | Not stated | 2007 to 2009 | 6 | High | ||
| Tkachenko 2016 | Glucocorticosteroids plus SAMe | Glucocorticosteroids | Not stated | No | No | Not stated | Not stated | 2013 to 2015 | 6 | High | ||
| De 2009 | Pentoxifylline | Glucocorticosteroids | Not stated | No | No | No | Not stated | 2006 to 2008 | 12 | High | ||
| Garrido Garcia 2012 | Pentoxifylline | Glucocorticosteroids | Not stated | No | Not stated | Not stated | Not stated | 2008 to 2011 | 1 | High | ||
| Park 2014 | Pentoxifylline | Glucocorticosteroids | Yes | No | No | No | Yes | 2009 to 2012 | 6 | High | ||
| Basu 2015 | Mycophenolate plus pentoxifylline | Glucocorticosteroids plus pentoxifylline | Mycophenolate plus pentoxifylline plus glucocorticosteroids | No | No | No | Not stated | Not stated | Not stated | 3 | High | |
| Ramond 1992 | Glucocorticosteroids | No active intervention | Yes | No | No | Not stated | Not stated | 1987 to 1990 | 2 | High | ||
| Thursz 2015 | Glucocorticosteroids | No active intervention | Glucocorticosteroids plus pentoxifylline | Pentoxifylline | Not stated | Not stated | Not stated | Not stated | Not stated | 2010 to 2012 | 12 | High |
| Moreno 2010 | N‐acetyl cysteine | No active intervention | Not stated | Not stated | Not stated | Not stated | Not stated | 2000 to 2005 | 6 | High | ||
| Akriviadis 2000 | Pentoxifylline | No active intervention | Not stated | No | No | Not stated | Not stated | 1992 to 1997 | 6 | High | ||
| Paladugu 2006 | Pentoxifylline | No active intervention | Yes | Not stated | Not stated | Not stated | Not stated | Not stated | 1 | High | ||
| Sidhu 2012b | Pentoxifylline | No active intervention | Not stated | Not stated | No | Not stated | Not stated | Not stated | 1 | High | ||
| De 2014 | Glucocorticosteroids plus pentoxifylline | Pentoxifylline | Not stated | No | Not stated | No | Not stated | 2010 to 2012 | 12 | High | ||
| Singh 2014 | GSF plus pentoxifylline | Pentoxifylline | Grade 3 or 4 were excluded | No | No | No | Not stated | 2010 to 2012 | 3 | High |
Appendix 5. Severe alcoholic hepatitis: results (probability table)
| Mortality at maximal follow‐up | |||||||||||
| Rank | No active intervention | Glucocorticosteroids | Glucocorticosteroids plus anti‐TNF | Glucocorticosteroids plus metadoxine | Glucocorticosteroids plus N‐acetyl cysteine | Glucocorticosteroids plus pentoxifylline | Glucocorticosteroids plus SAMe | GSF plus pentoxifylline | N‐acetyl cysteine | Pentoxifylline | Pentoxifylline plus metadoxine |
| 1 | 0 | 0 | 0.000167 | 0.1202 | 0.0056 | 0 | 0.1908 | 0.6467 | 0.000667 | 0 | 0.0359 |
| 2 | 0 | 0 | 0.001867 | 0.3661 | 0.0287 | 0.000233 | 0.2275 | 0.2069 | 0.003667 | 0.000267 | 0.1648 |
| 3 | 0 | 0.0003 | 0.004167 | 0.3335 | 0.07563 | 0.003633 | 0.1529 | 0.08597 | 0.008067 | 0.005667 | 0.3302 |
| 4 | 0.000567 | 0.004 | 0.01263 | 0.1311 | 0.1998 | 0.0203 | 0.1724 | 0.04233 | 0.02317 | 0.06577 | 0.3279 |
| 5 | 0.0013 | 0.0338 | 0.027 | 0.0338 | 0.3115 | 0.08537 | 0.08577 | 0.01203 | 0.0521 | 0.2637 | 0.09363 |
| 6 | 0.008567 | 0.1536 | 0.0345 | 0.0067 | 0.1241 | 0.1903 | 0.0386 | 0.0031 | 0.0535 | 0.3645 | 0.02247 |
| 7 | 0.02563 | 0.3343 | 0.03363 | 0.0038 | 0.07777 | 0.2431 | 0.02763 | 0.0011 | 0.0468 | 0.1959 | 0.01033 |
| 8 | 0.08817 | 0.3372 | 0.04663 | 0.002267 | 0.0781 | 0.2682 | 0.03223 | 0.000767 | 0.05787 | 0.08063 | 0.007933 |
| 9 | 0.3932 | 0.1138 | 0.124 | 0.001567 | 0.0527 | 0.1361 | 0.03067 | 0.000633 | 0.1219 | 0.0209 | 0.004567 |
| 10 | 0.3764 | 0.02127 | 0.226 | 0.000833 | 0.0324 | 0.04457 | 0.02813 | 0.000267 | 0.266 | 0.002467 | 0.0016 |
| 11 | 0.1062 | 0.001633 | 0.4894 | 0.000167 | 0.01367 | 0.008233 | 0.01343 | 0.0002 | 0.3663 | 0.0002 | 0.000633 |
| 30‐days mortality | |||||||||||
| Rank | No active intervention | Glucocorticosteroids | Glucocorticosteroids plus anti‐TNF | Glucocorticosteroids plus metadoxine | Glucocorticosteroids plus N‐acetyl cysteine | Glucocorticosteroids plus pentoxifylline | Glucocorticosteroids plus SAMe | N‐acetyl cysteine | Pentoxifylline | Pentoxifylline plus metadoxine | |
| 1 | 0 | 3.33E‐05 | 0.0003 | 0.1292 | 0.1735 | 0.001467 | 0.6535 | 0.0018 | 0.0001 | 0.04013 | |
| 2 | 0.000267 | 0.000633 | 0.001333 | 0.3319 | 0.3907 | 0.01303 | 0.1107 | 0.0044 | 0.001733 | 0.1454 | |
| 3 | 0.000967 | 0.009633 | 0.0036 | 0.3323 | 0.2308 | 0.05153 | 0.06967 | 0.009467 | 0.009233 | 0.2828 | |
| 4 | 0.004433 | 0.05683 | 0.006933 | 0.149 | 0.1422 | 0.178 | 0.05847 | 0.01717 | 0.04497 | 0.3421 | |
| 5 | 0.01553 | 0.2396 | 0.01203 | 0.02667 | 0.02703 | 0.3949 | 0.02063 | 0.03153 | 0.1504 | 0.08167 | |
| 6 | 0.03893 | 0.3974 | 0.0115 | 0.01423 | 0.01423 | 0.1929 | 0.01633 | 0.0253 | 0.2475 | 0.04163 | |
| 7 | 0.1228 | 0.2359 | 0.01793 | 0.01 | 0.01093 | 0.1196 | 0.0199 | 0.03197 | 0.3957 | 0.0353 | |
| 8 | 0.5931 | 0.05133 | 0.05427 | 0.0045 | 0.006533 | 0.0397 | 0.0266 | 0.0769 | 0.1263 | 0.0208 | |
| 9 | 0.2059 | 0.0084 | 0.2666 | 0.001733 | 0.003533 | 0.0081 | 0.01893 | 0.4551 | 0.0226 | 0.0091 | |
| 10 | 0.0181 | 0.0002 | 0.6255 | 0.000533 | 0.000633 | 0.000867 | 0.005233 | 0.3463 | 0.001433 | 0.001133 | |
| 90‐days mortality | |||||||||||
| Rank | No active intervention | Glucocorticosteroids | Glucocorticosteroids plus anti‐TNF | Glucocorticosteroids plus metadoxine | Glucocorticosteroids plus N‐acetyl cysteine | Glucocorticosteroids plus pentoxifylline | GSF plus pentoxifylline | Pentoxifylline | Pentoxifylline plus metadoxine | ||
| 1 | 0.0028 | 0.0009 | 0.03237 | 0.1969 | 0.05467 | 0.0081 | 0.5935 | 0.001667 | 0.1092 | ||
| 2 | 0.01017 | 0.007267 | 0.05103 | 0.3604 | 0.1029 | 0.02517 | 0.1698 | 0.02117 | 0.2522 | ||
| 3 | 0.02173 | 0.02393 | 0.05987 | 0.2224 | 0.1268 | 0.0547 | 0.1103 | 0.07563 | 0.3046 | ||
| 4 | 0.04257 | 0.07313 | 0.0769 | 0.09473 | 0.1901 | 0.1064 | 0.04713 | 0.2297 | 0.1394 | ||
| 5 | 0.06963 | 0.1718 | 0.07023 | 0.04523 | 0.1251 | 0.1514 | 0.02373 | 0.2742 | 0.0686 | ||
| 6 | 0.1069 | 0.275 | 0.0617 | 0.0274 | 0.09883 | 0.1647 | 0.0173 | 0.2027 | 0.0454 | ||
| 7 | 0.1742 | 0.2713 | 0.07567 | 0.02207 | 0.09767 | 0.1916 | 0.01383 | 0.121 | 0.03263 | ||
| 8 | 0.3156 | 0.1458 | 0.1233 | 0.01837 | 0.1085 | 0.1884 | 0.01363 | 0.05867 | 0.02777 | ||
| 9 | 0.2565 | 0.03083 | 0.4489 | 0.01253 | 0.0955 | 0.1094 | 0.01083 | 0.0152 | 0.0203 | ||
| Liver transplantation | |||||||||||
| Rank | Glucocorticosteroids | Glucocorticosteroids plus N‐acetyl cysteine | Glucocorticosteroids plus pentoxifylline | ||||||||
| 1 | 0.2082 | 0.1372 | 0.6546 | ||||||||
| 2 | 0.6107 | 0.1842 | 0.2051 | ||||||||
| 3 | 0.1811 | 0.6786 | 0.1403 | ||||||||
| Decompensated cirrhosis | |||||||||||
| Rank | No active intervention | Glucocorticosteroids | Glucocorticosteroids plus anti‐TNF | Glucocorticosteroids plus metadoxine | Glucocorticosteroids plus N‐acetyl cysteine | Glucocorticosteroids plus pentoxifylline | Pentoxifylline | Pentoxifylline plus metadoxine | |||
| 1 | 0 | 6.67E‐05 | 0.1293 | 0.6768 | 0.09567 | 0.007133 | 0.0022 | 0.08877 | |||
| 2 | 0.001367 | 0.000333 | 0.05803 | 0.2411 | 0.1991 | 0.04733 | 0.05793 | 0.3948 | |||
| 3 | 0.008167 | 0.002333 | 0.04287 | 0.0629 | 0.2254 | 0.141 | 0.2345 | 0.2828 | |||
| 4 | 0.03253 | 0.03383 | 0.03517 | 0.0144 | 0.1539 | 0.238 | 0.3603 | 0.1319 | |||
| 5 | 0.0856 | 0.1822 | 0.03153 | 0.003767 | 0.1232 | 0.2761 | 0.239 | 0.05857 | |||
| 6 | 0.167 | 0.4109 | 0.0313 | 0.000767 | 0.09313 | 0.1787 | 0.08803 | 0.03017 | |||
| 7 | 0.4395 | 0.3101 | 0.053 | 0.0002 | 0.08133 | 0.08747 | 0.01707 | 0.01137 | |||
| 8 | 0.2659 | 0.06027 | 0.6188 | 0 | 0.0282 | 0.02427 | 0.000867 | 0.001733 | |||
| Adverse events (proportion) | |||||||||||
| Rank | No active intervention | GSF plus pentoxifylline | N‐acetyl cysteine | Pentoxifylline | |||||||
| 1 | 0.587 | 0.0292 | 0.2649 | 0.1189 | |||||||
| 2 | 0.319 | 0.02962 | 0.2902 | 0.3612 | |||||||
| 3 | 0.07953 | 0.0703 | 0.358 | 0.4922 | |||||||
| 4 | 0.01445 | 0.8709 | 0.08696 | 0.02772 | |||||||
| Adverse events (number) | |||||||||||
| Rank | No active intervention | Glucocorticosteroids | Glucocorticosteroids plus anti‐TNF | Glucocorticosteroids plus metadoxine | Glucocorticosteroids plus N‐acetyl cysteine | Glucocorticosteroids plus pentoxifylline | Glucocorticosteroids plus SAMe | GSF plus pentoxifylline | N‐acetyl cysteine | Pentoxifylline | Pentoxifylline plus metadoxine |
| 1 | 0.0518 | 0 | 0.001167 | 0.2058 | 0.4203 | 0 | 0.2057 | 3.33E‐05 | 0.0877 | 0 | 0.02753 |
| 2 | 0.1617 | 0 | 0.003933 | 0.3168 | 0.2313 | 0.000133 | 0.1413 | 0 | 0.05437 | 3.33E‐05 | 0.09043 |
| 3 | 0.2702 | 0.000467 | 0.0075 | 0.2515 | 0.1365 | 0.000333 | 0.1052 | 0 | 0.05173 | 0.002133 | 0.1744 |
| 4 | 0.2874 | 0.004333 | 0.0142 | 0.1436 | 0.092 | 0.004267 | 0.1096 | 0 | 0.05487 | 0.02543 | 0.2644 |
| 5 | 0.1782 | 0.0427 | 0.02753 | 0.061 | 0.07057 | 0.0183 | 0.1234 | 6.67E‐05 | 0.06967 | 0.1478 | 0.2607 |
| 6 | 0.04603 | 0.1844 | 0.0438 | 0.01757 | 0.02797 | 0.0695 | 0.07893 | 0 | 0.07047 | 0.3447 | 0.1167 |
| 7 | 0.004 | 0.353 | 0.0424 | 0.002833 | 0.0101 | 0.1517 | 0.0523 | 0 | 0.04183 | 0.303 | 0.03887 |
| 8 | 0.000633 | 0.2978 | 0.0674 | 0.000833 | 0.0075 | 0.3452 | 0.06877 | 3.33E‐05 | 0.0624 | 0.1316 | 0.01783 |
| 9 | 0 | 0.1066 | 0.2669 | 0.0001 | 0.003433 | 0.3216 | 0.0774 | 3.33E‐05 | 0.175 | 0.0413 | 0.007633 |
| 10 | 0 | 0.01077 | 0.525 | 3.33E‐05 | 0.000367 | 0.08893 | 0.0373 | 0.000367 | 0.3317 | 0.003967 | 0.001567 |
| 11 | 0 | 0 | 0.000167 | 0 | 0 | 0 | 6.67E‐05 | 0.9995 | 0.0003 | 0 | 0 |
| Abbreviations: GSF = granulocyte stimulating factor; TNF = tumour necrosis factor; SAMe = S‐adenosyl methionine. | |||||||||||
Appendix 6. Severe alcoholic hepatitis: results (effect estimates table)
| Mortality at maximal follow‐up | |||||||||||
| No active intervention | Glucocorticosteroids | Glucocorticosteroids plus anti‐TNF | Glucocorticosteroids plus metadoxine | Glucocorticosteroids plus N‐acetyl cysteine | Glucocorticosteroids plus pentoxifylline | Glucocorticosteroids plus SAMe | GSF plus pentoxifylline | N‐acetyl cysteine | Pentoxifylline | Pentoxifylline plus metadoxine | |
| No active intervention | ‐ | 0.43[0.01,26.31] | ‐ | ‐ | ‐ | 0.93[0.70,1.22] | ‐ | ‐ | 1.22[0.43,3.78] | 0.66[0.18,1.76] | ‐ |
| Glucocorticosteroids | 0.67[0.34,1.11] | ‐ | 2.15[0.04,115.47] | 0.23[0.11,0.46] | 0.65[0.37,1.10] | 0.91[0.34,2.44] | 0.33[0.04,1.68] | ‐ | ‐ | 0.84[0.41,1.43] | 0.32[0.15,0.62] |
| Glucocorticosteroids plus anti‐TNF | 1.44[0.32,5.89] | 2.19[0.58,8.19] | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Glucocorticosteroids plus metadoxine | 0.17[0.05,0.48] | 0.25[0.08,0.70] | 0.11[0.02,0.61] | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | 2.99[1.41,6.43] | 1.40[0.61,3.24] |
| Glucocorticosteroids plus N‐acetyl cysteine | 0.44[0.12,1.35] | 0.67[0.23,1.89] | 0.30[0.06,1.65] | 2.65[0.61,12.28] | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Glucocorticosteroids plus pentoxifylline | 0.66[0.32,1.23] | 0.99[0.60,1.71] | 0.45[0.11,1.93] | 3.95[1.30,13.52] | 1.48[0.48,4.99] | ‐ | ‐ | ‐ | ‐ | 0.88[0.02,28.56] | ‐ |
| Glucocorticosteroids plus SAMe | 0.20[0.02,1.43] | 0.31[0.04,1.97] | 0.14[0.01,1.31] | 1.22[0.11,10.99] | 0.46[0.04,4.01] | 0.32[0.03,2.15] | ‐ | ‐ | ‐ | ‐ | ‐ |
| GSF plus pentoxifylline | 0.08[0.02,0.33] | 0.12[0.03,0.51] | 0.06[0.01,0.40] | 0.48[0.09,2.85] | 0.19[0.03,1.09] | 0.13[0.03,0.53] | 0.40[0.04,5.84] | ‐ | ‐ | 6.79[2.49,23.52] | ‐ |
| N‐acetyl cysteine | 1.21[0.30,5.12] | 1.84[0.44,9.04] | 0.85[0.11,6.54] | 7.34[1.33,51.06] | 2.76[0.47,18.63] | 1.86[0.41,9.29] | 6.01[0.57,86.66] | 15.09[2.06,124.34] | ‐ | ‐ | ‐ |
| Pentoxifylline | 0.54[0.29,0.87] | 0.82[0.51,1.26] | 0.37[0.09,1.52] | 3.26[1.14,9.65] | 1.23[0.39,3.82] | 0.83[0.44,1.42] | 2.63[0.39,24.12] | 6.55[1.68,26.15] | 0.45[0.09,1.82] | ‐ | 0.47[0.22,0.94] |
| Pentoxifylline plus metadoxine | 0.23[0.07,0.66] | 0.35[0.12,0.93] | 0.16[0.03,0.84] | 1.41[0.41,4.86] | 0.53[0.12,2.22] | 0.35[0.11,1.03] | 1.16[0.13,12.10] | 2.88[0.50,15.44] | 0.19[0.03,1.02] | 0.43[0.15,1.18] | ‐ |
| 30‐days mortality | |||||||||||
| No active intervention | Glucocorticosteroids | Glucocorticosteroids plus anti‐TNF | Glucocorticosteroids plus metadoxine | Glucocorticosteroids plus N‐acetyl cysteine | Glucocorticosteroids plus pentoxifylline | Glucocorticosteroids plus SAMe | N‐acetyl cysteine | Pentoxifylline | Pentoxifylline plus metadoxine | ‐ | |
| No active intervention | ‐ | 0.50[0.01,20.51] | ‐ | ‐ | ‐ | 0.74[0.46,1.19] | ‐ | 2.22[0.50,12.32] | 0.74[0.07,5.25] | ‐ | ‐ |
| Glucocorticosteroids | 0.62[0.25,1.16] | ‐ | 6.25[0.09,721.26] | 0.28[0.10,0.77] | 0.28[0.10,0.68] | 0.91[0.26,3.45] | 0.10[0.00,1.91] | ‐ | 0.99[0.28,2.68] | 0.43[0.16,1.16] | ‐ |
| Glucocorticosteroids plus anti‐TNF | 3.86[0.45,59.56] | 6.27[0.90,89.30] | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Glucocorticosteroids plus metadoxine | 0.19[0.04,0.71] | 0.32[0.08,1.10] | 0.05[0.00,0.50] | ‐ | ‐ | ‐ | ‐ | ‐ | 3.18[1.17,9.12] | 1.53[0.53,4.56] | ‐ |
| Glucocorticosteroids plus N‐acetyl cysteine | 0.17[0.03,0.72] | 0.28[0.07,1.10] | 0.04[0.00,0.48] | 0.88[0.13,6.04] | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Glucocorticosteroids plus pentoxifylline | 0.54[0.19,1.11] | 0.86[0.43,1.67] | 0.14[0.01,1.07] | 2.71[0.68,11.92] | 3.10[0.66,14.86] | ‐ | ‐ | ‐ | 1.86[0.05,131.63] | ‐ | ‐ |
| Glucocorticosteroids plus SAMe | 0.05[0.00,1.70] | 0.09[0.00,2.53] | 0.01[0.00,0.63] | 0.28[0.00,10.41] | 0.32[0.00,12.04] | 0.10[0.00,3.17] | ‐ | ‐ | ‐ | ‐ | ‐ |
| N‐acetyl cysteine | 2.28[0.36,16.64] | 3.75[0.53,34.33] | 0.59[0.02,11.40] | 12.00[1.26,147.82] | 13.50[1.29,186.23] | 4.40[0.60,42.35] | 46.02[0.86,20050.31] | ‐ | ‐ | ‐ | ‐ |
| Pentoxifylline | 0.68[0.27,1.25] | 1.10[0.59,1.87] | 0.17[0.01,1.31] | 3.46[0.97,13.01] | 3.95[0.86,18.07] | 1.27[0.58,2.67] | 12.21[0.40,4031.93] | 0.29[0.03,2.02] | ‐ | 0.49[0.17,1.31] | ‐ |
| Pentoxifylline plus metadoxine | 0.29[0.06,1.09] | 0.47[0.13,1.67] | 0.07[0.00,0.74] | 1.48[0.36,6.78] | 1.67[0.26,11.57] | 0.54[0.13,2.26] | 5.44[0.14,1863.11] | 0.13[0.01,1.19] | 0.43[0.12,1.58] | ‐ | ‐ |
| 90‐days mortality | |||||||||||
| No active intervention | Glucocorticosteroids | Glucocorticosteroids plus anti‐TNF | Glucocorticosteroids plus metadoxine | Glucocorticosteroids plus N‐acetyl cysteine | Glucocorticosteroids plus pentoxifylline | GSF plus pentoxifylline | Pentoxifylline | Pentoxifylline plus metadoxine | ‐ | ‐ | |
| No active intervention | ‐ | 0.41[0.01,29.67] | ‐ | ‐ | ‐ | 0.96[0.64,1.42] | ‐ | 1.18[0.81,1.74] | ‐ | ‐ | ‐ |
| Glucocorticosteroids | 0.57[0.04,5.69] | ‐ | 2.16[0.14,72.24] | 0.11[0.03,0.31] | 0.56[0.28,1.09] | 0.74[0.50,1.09] | ‐ | 0.63[0.06,4.39] | 0.16[0.05,0.47] | ‐ | ‐ |
| Glucocorticosteroids plus anti‐TNF | 1.27[0.01,293.83] | 2.31[0.02,343.78] | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Glucocorticosteroids plus metadoxine | 0.07[0.00,3.09] | 0.12[0.00,3.89] | 0.05[0.00,14.01] | ‐ | ‐ | ‐ | ‐ | 4.51[1.67,13.41] | 1.50[0.54,4.27] | ‐ | ‐ |
| Glucocorticosteroids plus N‐acetyl cysteine | 0.32[0.00,22.20] | 0.56[0.01,19.91] | 0.24[0.00,78.41] | 4.55[0.03,734.36] | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Glucocorticosteroids plus pentoxifylline | 0.59[0.02,10.96] | 1.02[0.07,16.15] | 0.45[0.00,94.16] | 8.31[0.16,520.61] | 1.83[0.02,175.21] | ‐ | ‐ | 0.89[0.02,31.34] | ‐ | ‐ | ‐ |
| GSF plus pentoxifylline | 0.02[0.00,2.47] | 0.04[0.00,3.51] | 0.02[0.00,9.96] | 0.35[0.00,63.12] | 0.08[0.00,23.08] | 0.04[0.00,3.96] | ‐ | 14.67[3.82,69.90] | ‐ | ‐ | ‐ |
| Pentoxifylline | 0.38[0.02,4.47] | 0.66[0.08,4.74] | 0.29[0.00,39.10] | 5.32[0.17,158.54] | 1.17[0.02,74.89] | 0.64[0.05,6.73] | 14.95[0.33,743.23] | ‐ | 0.33[0.11,0.91] | ‐ | ‐ |
| Pentoxifylline plus metadoxine | 0.11[0.00,4.92] | 0.19[0.01,5.30] | 0.08[0.00,21.03] | 1.52[0.04,66.35] | 0.34[0.00,43.55] | 0.18[0.00,9.39] | 4.31[0.03,733.63] | 0.29[0.01,8.21] | ‐ | ‐ | ‐ |
| Serious adverse events (proportion) | |||||||||||
| Glucocorticosteroids | Glucocorticosteroids plus pentoxifylline | Pentoxifylline | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| No active intervention | 1.38[0.98,1.93] | 1.16[0.83,1.62] | 1.07[0.76,1.51] | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Glucocorticosteroids | ‐ | 0.84[0.60,1.17] | 0.78[0.56,1.09] | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Glucocorticosteroids plus pentoxifylline | ‐ | ‐ | 0.93[0.66,1.30] | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Liver transplantation | |||||||||||
| Glucocorticosteroids | Glucocorticosteroids plus N‐acetyl cysteine | Glucocorticosteroids plus pentoxifylline | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Glucocorticosteroids | ‐ | 5.02[0.16,1608.41] | 0.19[0.00,6.94] | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Glucocorticosteroids plus N‐acetyl cysteine | 6.61[0.22,2697.28] | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Glucocorticosteroids plus pentoxifylline | 0.20[0.00,6.23] | 0.02[0.00,3.97] | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Decompensated liver disease | |||||||||||
| No active intervention | Glucocorticosteroids | Glucocorticosteroids plus anti‐TNF | Glucocorticosteroids plus metadoxine | Glucocorticosteroids plus N‐acetyl cysteine | Glucocorticosteroids plus pentoxifylline | Pentoxifylline | Pentoxifylline plus metadoxine | ‐ | ‐ | ‐ | |
| No active intervention | ‐ | 0.97[0.64,1.48] | ‐ | ‐ | ‐ | 0.95[0.62,1.45] | 0.76[0.55,1.05] | ‐ | ‐ | ‐ | ‐ |
| Glucocorticosteroids | 0.90[0.65,1.27] | ‐ | 1.50[0.24,14.34] | 0.56[0.36,0.87] | 0.76[0.45,1.26] | 0.79[0.56,1.10] | 0.93[0.72,1.18] | 0.73[0.48,1.11] | ‐ | ‐ | ‐ |
| Glucocorticosteroids plus anti‐TNF | 1.42[0.20,11.03] | 1.56[0.22,12.10] | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Glucocorticosteroids plus metadoxine | 0.47[0.28,0.78] | 0.52[0.34,0.78] | 0.33[0.04,2.45] | ‐ | ‐ | ‐ | 1.75[1.13,2.82] | 1.32[0.82,2.14] | ‐ | ‐ | ‐ |
| Glucocorticosteroids plus N‐acetyl cysteine | 0.70[0.37,1.27] | 0.77[0.45,1.28] | 0.48[0.06,3.64] | 1.48[0.75,2.85] | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Glucocorticosteroids plus pentoxifylline | 0.79[0.55,1.15] | 0.88[0.65,1.20] | 0.56[0.07,3.88] | 1.68[1.03,2.80] | 1.14[0.63,2.14] | ‐ | 0.90[0.61,1.33] | ‐ | ‐ | ‐ | ‐ |
| Pentoxifylline | 0.75[0.55,1.02] | 0.83[0.65,1.04] | 0.53[0.07,3.70] | 1.58[1.05,2.46] | 1.07[0.62,1.93] | 0.94[0.67,1.31] | ‐ | ‐ | ‐ | ‐ | ‐ |
| Pentoxifylline plus metadoxine | 0.62[0.38,0.99] | 0.69[0.45,0.99] | 0.44[0.05,3.20] | 1.32[0.81,2.15] | 0.89[0.47,1.71] | 0.78[0.48,1.24] | 0.83[0.55,1.22] | ‐ | ‐ | ‐ | ‐ |
| Adverse events (proportion) | |||||||||||
| No active intervention | GSF plus pentoxifylline | N‐acetyl cysteine | Pentoxifylline | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| No active intervention | ‐ | ‐ | 1.94[0.53,7.80] | 2.25[0.09,57.34] | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| GSF plus pentoxifylline | 76.48[0.19,162754.79] | ‐ | ‐ | 0.03[0.00,0.53] | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| N‐acetyl cysteine | 1.95[0.02,204.38] | 0.02[0.00,48.67] | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Pentoxifylline | 2.23[0.10,58.03] | 0.03[0.00,5.01] | 1.15[0.00,334.96] | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Adverse events (number) | |||||||||||
| No active intervention | Glucocorticosteroids | Glucocorticosteroids plus anti‐TNF | Glucocorticosteroids plus metadoxine | Glucocorticosteroids plus N‐acetyl cysteine | Glucocorticosteroids plus pentoxifylline | Glucocorticosteroids plus SAMe | GSF plus pentoxifylline | N‐acetyl cysteine | Pentoxifylline | Pentoxifylline plus metadoxine | |
| No active intervention | ‐ | 1.37[0.98,1.90] | ‐ | ‐ | ‐ | 1.20[0.85,1.68] | ‐ | ‐ | 1.57[0.55,5.64] | 1.35[1.03,1.75] | ‐ |
| Glucocorticosteroids | 1.40[1.07,1.85] | ‐ | 1.43[0.78,2.68] | 0.63[0.44,0.89] | 0.59[0.35,0.94] | 1.10[0.87,1.41] | 0.74[0.33,1.57] | ‐ | ‐ | 0.90[0.75,1.06] | 0.76[0.54,1.06] |
| Glucocorticosteroids plus anti‐TNF | 1.97[1.03,4.12] | 1.42[0.76,2.71] | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Glucocorticosteroids plus metadoxine | 0.89[0.59,1.33] | 0.63[0.45,0.88] | 0.45[0.21,0.90] | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | 1.50[1.06,2.17] | 1.21[0.82,1.77] |
| Glucocorticosteroids plus N‐acetyl cysteine | 0.82[0.47,1.43] | 0.58[0.35,0.95] | 0.41[0.18,0.92] | 0.92[0.52,1.66] | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Glucocorticosteroids plus pentoxifylline | 1.52[1.15,2.03] | 1.08[0.87,1.33] | 0.77[0.38,1.48] | 1.70[1.18,2.51] | 1.85[1.09,3.18] | ‐ | ‐ | ‐ | ‐ | 0.90[0.70,1.16] | ‐ |
| Glucocorticosteroids plus SAMe | 1.03[0.45,2.29] | 0.74[0.34,1.56] | 0.52[0.19,1.37] | 1.16[0.51,2.58] | 1.26[0.51,3.01] | 0.69[0.31,1.48] | ‐ | ‐ | ‐ | ‐ | ‐ |
| GSF plus pentoxifylline | infinity[227.69,infinity] | infinity[155.87,infinity] | infinity[100.69,infinity] | infinity[244.69,infinity] | infinity[270.16,infinity] | infinity[144.32,infinity] | infinity[226.11,infinity] | ‐ | ‐ | 0.00[0.00,0.00] | ‐ |
| N‐acetyl cysteine | 1.57[0.55,5.23] | 1.12[0.37,3.92] | 0.79[0.22,3.26] | 1.76[0.57,6.28] | 1.93[0.57,7.30] | 1.03[0.35,3.56] | 1.54[0.39,6.50] | 0.00[0.00,0.01] | ‐ | ‐ | ‐ |
| Pentoxifylline | 1.33[1.04,1.73] | 0.95[0.81,1.13] | 0.67[0.34,1.27] | 1.50[1.07,2.11] | 1.63[0.98,2.72] | 0.88[0.71,1.09] | 1.29[0.60,2.88] | 0.00[0.00,0.01] | 0.85[0.24,2.52] | ‐ | 0.81[0.57,1.14] |
| Pentoxifylline plus metadoxine | 1.07[0.72,1.59] | 0.77[0.56,1.05] | 0.54[0.26,1.07] | 1.21[0.83,1.76] | 1.31[0.74,2.33] | 0.71[0.49,1.01] | 1.03[0.47,2.39] | 0.00[0.00,0.00] | 0.69[0.19,2.09] | 0.81[0.58,1.10] | ‐ |
| The table provides the effect estimate (hazard ratio for mortality at maximal follow‐up and liver transplantation; odds ratio for 30‐days mortality, 90‐days mortality, serious adverse events (proportion), and adverse events (proportion); and rate ratio for serious adverse events (number), decompensated cirrhosis; adverse events (number)) of each pairwise comparison for mortality at maximal follow‐up. The top half of the table indicates the effect estimates from the direct comparisons.The bottom half of the table indicates the effect estimates from the network meta‐analysis. For network meta‐analysis, to identify the effect estimate of a comparison, say A versus B, look at the cell that occupies the row corresponding to intervention A and the column corresponding to intervention B. This gives the information directly. If that cell is empty (indicated by a '‐', you have to look at row corresponding to Intervention B and column corresponding to intervention A. You will have to take the inverse of this number (i.e. 1/number) to get the intervention effect of A versus B. For direct comparisons, this is exactly opposite, i.e. look at the cell that occupies the column corresponding to intervention A and the row corresponding to intervention B to get the effect estimate directly. If that cell is empty (indicated by a '‐', you have to look at column corresponding to intervention B and row corresponding to intervention A. You will have to take the inverse of this number (i.e. 1/number) to get the intervention effect of A versus B. If the cell corresponding to B versus A is also missing in direct comparisons, this means that there was no direct comparison. Treatment effects with evidence of difference are shown by italics. Abbreviations: GSF = granulocyte stimulating factor; TNF = tumour necrosis factor; SAMe = S‐adenosyl methionine. | |||||||||||
Appendix 7. Search strategies
| Database | Time span | Search strategy |
| The Central Register of Controlled Trials (CENTRAL) (Wiley) | Issue 2, 2017 | #1 MeSH descriptor: [Liver Diseases, Alcoholic] explode all trees #2 (alcohol* and (liver disease* or steatosis or fibrosis or hepatitis or cirrhosis)) #3 #1 or #2 |
| MEDLINE (OvidSP) | January 1947 to Feb 2017 | 1. exp Liver Diseases, Alcoholic/ 2. (alcohol* and (liver disease* or steatosis or fibrosis or hepatitis or cirrhosis)).mp. 3. 1 or 2 4. randomized controlled trial.pt. 5. controlled clinical trial.pt. 6. randomized.ab. 7. placebo.ab. 8. drug therapy.fs. 9. randomly.ab. 10. trial.ab. 11. groups.ab. 12. 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 13. exp animals/ not humans.sh. 14. 12 not 13 15. 3 and 14 |
| EMBASE (OvidSP) | January 1974 to Feb 2017 | 1. exp alcohol liver disease/ 2. (alcohol* and (liver disease* or steatosis or fibrosis or hepatitis or cirrhosis)).mp. 3. 1 or 2 4. exp crossover‐procedure/ or exp double‐blind procedure/ or exp randomized controlled trial/ or single‐blind procedure/ 5. (((((random* or factorial* or crossover* or cross over* or cross‐over* or placebo* or double*) adj blind*) or single*) adj blind*) or assign* or allocat* or volunteer*).af. 6. 4 or 5 7. 3 and 6 |
| Science Citation Index Expanded (Web of Knowledge) | January 1945 to Feb 2017 | #1 TS=(alcohol* and (liver disease* or steatosis or fibrosis or hepatitis or cirrhosis)) #2 TS=(random* OR rct* OR crossover OR masked OR blind* OR placebo* OR meta‐analysis OR systematic review* OR meta‐analys*) |
| World Health Organization International Clinical Trials Registry Platform Search Portal (apps.who.int/trialsearch/Default.aspx) | Feb 2017 | alcoholic liver disease or alcoholic hepatitis or alcoholic cirrhosis |
| ClinicalTrials.gov | Feb 2017 | Interventional trials | "alcoholic liver disease" OR "alcoholic hepatitis" OR "alcoholic cirrhosis" | Phase 2, 3, 4 |
Appendix 8. Sample size calculation
The short‐term mortality (90‐days mortality) in people with alcoholic hepatitis is about 27.1% based on the data from the studies. The required information size based on a control group proportion of 27.7%, a relative risk reduction of 20% in the experimental group, type I error of 5%, and type II error of 20% is 1,912 participants. Network analyses are more prone to the risk of random errors than direct comparisons (Del Re 2013). Accordingly, a greater sample size is required in indirect comparisons than direct comparisons (Thorlund 2012). The power and precision in indirect comparisons depends upon various factors, such as the number of participants included under each comparison and the heterogeneity between the trials (Thorlund 2012). If there is no heterogeneity across the trials, the sample size in indirect comparisons would be equivalent to the sample size in direct comparisons. The effective indirect sample size can be calculated using the number of participants included in each direct comparison (Thorlund 2012). For example, a sample size of 2500 participants in the direct comparison A versus C (nAC) and a sample size of 7500 participants in the direct comparison B versus C (nBC) results in an effective indirect sample size of 1876 participants. However, in the presence of heterogeneity within the comparisons, the sample size required is higher. In the above scenario, for an I2 statistic for each of the comparisons A versus C (IAC2) and B versus C (IBC2) of 25%, the effective indirect sample size is 1407 participants. For an I2 statistic for each of the comparisons A versus C and B versus C of 50%, the effective indirect sample size is 938 participants (Thorlund 2012). If there were only three groups and the sample size in the trials is more than the required information size, one can calculate the effective indirect sample size using the following generic formula (Thorlund 2012):
((nAC x (1 ‐ IAC2)) x (nBC x (1 ‐ IBC2))/((nAC x (1 ‐ IAC2)) + (nBC x (1 ‐ IBC2)).
There is currently no method to calculate the effective indirect sample size for a network analysis involving more than three intervention groups. So, we are unable to estimate the random errors for the whole network.
Data and analyses
Comparison 1. Alcoholic hepatitis (all).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality at maximal follow‐up | 48 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Amlodipine versus no intervention | 1 | 62 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.22, 2.58] |
| 1.2 Anabolic steroids versus no intervention | 1 | 173 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.43, 1.42] |
| 1.3 Antioxidants versus no intervention | 1 | 51 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.8 [0.19, 3.40] |
| 1.4 Anti‐TNF versus no intervention | 1 | 48 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.64 [1.31, 16.42] |
| 1.5 Colchicine versus no intervention | 2 | 139 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.47, 3.64] |
| 1.6 Glucocorticosteroids versus no intervention | 12 | 1147 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.67, 1.08] |
| 1.7 Pentoxifylline plus glucocorticosteroids versus no intervention | 1 | 545 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.64, 1.28] |
| 1.8 Insulin plus glucagon versus no intervention | 5 | 265 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.69, 1.90] |
| 1.9 N‐acetyl cysteine versus no intervention | 1 | 44 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.34, 4.32] |
| 1.10 N‐acetyl cysteine plus antioxidants versus no intervention | 1 | 70 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.44, 2.91] |
| 1.11 Penicillamine versus no intervention | 1 | 40 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.15, 4.13] |
| 1.12 Pentoxifylline versus no intervention | 6 | 881 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.58, 1.02] |
| 1.13 Propylthiouracil versus no intervention | 2 | 108 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.50, 2.72] |
| 1.14 Silymarin versus no intervention | 1 | 116 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.03, 3.30] |
| 1.15 Anabolic steroids versus glucocorticosteroids | 1 | 175 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.36, 1.19] |
| 1.16 Antioxidants versus glucocorticosteroids | 1 | 99 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.63, 3.16] |
| 1.17 Anti‐TNF plus glucocorticosteroids versus glucocorticosteroids | 2 | 55 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.37 [0.67, 8.42] |
| 1.18 Metadoxine plus glucocorticosteroids versus glucocorticosteroids | 1 | 70 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.04, 0.34] |
| 1.19 N‐acetyl cysteine plus glucocorticosteroids versus glucocorticosteroids | 1 | 174 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.32, 1.14] |
| 1.20 Pentoxifylline plus glucocorticosteroids versus glucocorticosteroids | 3 | 887 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.67, 1.16] |
| 1.21 SAMe plus glucocorticosteroids versus glucocorticosteroids | 1 | 40 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.06, 1.97] |
| 1.22 GSF versus glucocorticosteroids | 1 | 12 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.05, 20.83] |
| 1.23 Pentoxifylline versus glucocorticosteroids | 5 | 864 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.65, 1.13] |
| 1.24 Metadoxine plus pentoxifylline versus glucocorticosteroids | 1 | 67 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.06, 0.51] |
| 1.25 Metadoxine plus glucocorticosteroids versus pentoxifylline | 1 | 68 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.08, 0.63] |
| 1.26 Metadoxine plus pentoxifylline versus metadoxine plus glucocorticosteroids | 1 | 67 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.49 [0.55, 4.07] |
| 1.27 Pentoxifylline plus glucocorticosteroids versus pentoxifylline | 2 | 606 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.63, 1.22] |
| 1.28 Rifaximin plus UDCA plus glucocorticosteroids versus UDCA plus glucocorticosteroids | 1 | 68 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.20, 1.89] |
| 1.29 GSF plus pentoxifylline versus pentoxifylline | 1 | 46 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.08 [0.02, 0.31] |
| 1.30 Metadoxine plus pentoxifylline versus pentoxifylline | 1 | 65 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.12, 0.94] |
| 2 Mortality (30‐days) | 38 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Amlodipine versus no intervention | 1 | 62 | Odds Ratio (M‐H, Random, 95% CI) | 0.76 [0.22, 2.58] |
| 2.2 Anti‐TNF versus no intervention | 1 | 48 | Odds Ratio (M‐H, Random, 95% CI) | 1.8 [0.50, 6.50] |
| 2.3 Colchicine versus no intervention | 2 | 139 | Odds Ratio (M‐H, Random, 95% CI) | 1.21 [0.36, 4.02] |
| 2.4 Glucocorticosteroids versus no intervention | 9 | 887 | Odds Ratio (M‐H, Random, 95% CI) | 0.59 [0.34, 1.03] |
| 2.5 Pentoxifylline plus glucocorticosteroids versus no intervention | 1 | 545 | Odds Ratio (M‐H, Random, 95% CI) | 0.74 [0.46, 1.20] |
| 2.6 Insulin plus glucagon versus no intervention | 5 | 265 | Odds Ratio (M‐H, Random, 95% CI) | 0.86 [0.46, 1.63] |
| 2.7 N‐acetyl cysteine versus no intervention | 1 | 44 | Odds Ratio (M‐H, Random, 95% CI) | 2.07 [0.46, 9.40] |
| 2.8 Pentoxifylline versus no intervention | 4 | 647 | Odds Ratio (M‐H, Random, 95% CI) | 0.68 [0.33, 1.41] |
| 2.9 Propylthiouracil versus no intervention | 2 | 108 | Odds Ratio (M‐H, Random, 95% CI) | 1.16 [0.50, 2.72] |
| 2.10 Antioxidants versus glucocorticosteroids | 1 | 99 | Odds Ratio (M‐H, Random, 95% CI) | 2.12 [0.93, 4.83] |
| 2.11 Anti‐TNF plus glucocorticosteroids versus glucocorticosteroids | 2 | 55 | Odds Ratio (M‐H, Random, 95% CI) | 4.74 [0.73, 30.85] |
| 2.12 Metadoxine plus glucocorticosteroids versus glucocorticosteroids | 1 | 70 | Odds Ratio (M‐H, Random, 95% CI) | 0.29 [0.11, 0.80] |
| 2.13 N‐acetyl cysteine plus glucocorticosteroids versus glucocorticosteroids | 1 | 174 | Odds Ratio (M‐H, Random, 95% CI) | 0.29 [0.12, 0.73] |
| 2.14 Pentoxifylline plus glucocorticosteroids versus glucocorticosteroids | 3 | 887 | Odds Ratio (M‐H, Random, 95% CI) | 0.91 [0.62, 1.34] |
| 2.15 SAMe plus glucocorticosteroids versus glucocorticosteroids | 1 | 40 | Odds Ratio (M‐H, Random, 95% CI) | 0.18 [0.01, 4.01] |
| 2.16 GSF versus glucocorticosteroids | 1 | 12 | Odds Ratio (M‐H, Random, 95% CI) | 1.0 [0.05, 20.83] |
| 2.17 Pentoxifylline versus glucocorticosteroids | 5 | 864 | Odds Ratio (M‐H, Random, 95% CI) | 1.02 [0.57, 1.83] |
| 2.18 Metadoxine plus pentoxifylline versus glucocorticosteroids | 1 | 67 | Odds Ratio (M‐H, Random, 95% CI) | 0.44 [0.16, 1.18] |
| 2.19 Metadoxine plus glucocorticosteroids versus pentoxifylline | 1 | 68 | Odds Ratio (M‐H, Random, 95% CI) | 0.33 [0.12, 0.90] |
| 2.20 Metadoxine plus pentoxifylline versus metadoxine plus glucocorticosteroids | 1 | 67 | Odds Ratio (M‐H, Random, 95% CI) | 1.51 [0.53, 4.33] |
| 2.21 Pentoxifylline plus glucocorticosteroids versus pentoxifylline | 2 | 606 | Odds Ratio (M‐H, Random, 95% CI) | 0.95 [0.25, 3.58] |
| 2.22 Rifaximin plus UDCA plus glucocorticosteroids versus UDCA plus glucocorticosteroids | 1 | 68 | Odds Ratio (M‐H, Random, 95% CI) | 0.29 [0.05, 1.56] |
| 2.23 Metadoxine plus pentoxifylline versus pentoxifylline | 1 | 65 | Odds Ratio (M‐H, Random, 95% CI) | 0.49 [0.18, 1.34] |
| 3 Mortality (90‐days) | 14 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Antioxidants versus no intervention | 1 | 51 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.14, 8.04] |
| 3.2 Colchicine versus no intervention | 1 | 67 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.18 [0.13, 81.01] |
| 3.3 Glucocorticosteroids versus no intervention | 4 | 672 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.71, 1.39] |
| 3.4 Pentoxifylline plus glucocorticosteroids versus no intervention | 1 | 545 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.64, 1.42] |
| 3.5 Insulin plus glucagon versus no intervention | 1 | 72 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.57 [0.92, 7.15] |
| 3.6 Pentoxifylline versus no intervention | 1 | 545 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.81, 1.74] |
| 3.7 Silymarin versus no intervention | 1 | 116 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.03, 3.30] |
| 3.8 Anti‐TNF plus glucocorticosteroids versus glucocorticosteroids | 1 | 20 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.78 [0.13, 23.52] |
| 3.9 Metadoxine plus glucocorticosteroids versus glucocorticosteroids | 1 | 70 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.04, 0.34] |
| 3.10 N‐acetyl cysteine plus glucocorticosteroids versus glucocorticosteroids | 1 | 174 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.29, 1.11] |
| 3.11 Pentoxifylline plus glucocorticosteroids versus glucocorticosteroids | 1 | 547 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.51, 1.09] |
| 3.12 Pentoxifylline versus glucocorticosteroids | 3 | 683 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.56, 1.10] |
| 3.13 Metadoxine plus pentoxifylline versus glucocorticosteroids | 1 | 68 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.08, 0.63] |
| 3.14 Metadoxine plus glucocorticosteroids versus pentoxifylline | 1 | 67 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.49 [0.55, 4.07] |
| 3.15 Metadoxine plus pentoxifylline versus metadoxine plus glucocorticosteroids | 1 | 65 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.12, 0.94] |
| 3.16 Pentoxifylline plus glucocorticosteroids versus pentoxifylline | 2 | 606 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.53, 1.11] |
| 3.17 GSF plus pentoxifylline versus pentoxifylline | 1 | 46 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.08 [0.02, 0.31] |
| 3.18 Metadoxine plus pentoxifylline versus pentoxifylline | 1 | 67 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.06, 0.51] |
| 4 Serious adverse events (proportion) | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 Anti‐TNF versus no intervention | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Glucocorticosteroids versus no intervention | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Pentoxifylline plus glucocorticosteroids versus no intervention | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.4 Pentoxifylline versus no intervention | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.5 Pentoxifylline plus glucocorticosteroids versus glucocorticosteroids | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.6 Pentoxifylline versus glucocorticosteroids | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.7 Pentoxifylline plus glucocorticosteroids versus pentoxifylline | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Serious adverse events (number) | 2 | Rate Ratio (Fixed, 95% CI) | Totals not selected | |
| 5.1 Anti‐TNF versus no intervention | 1 | Rate Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Anti‐TNF plus glucocorticosteroids versus glucocorticosteroids | 1 | Rate Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Liver transplantation | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.1 Insulin plus glucagon versus no intervention | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 N‐acetyl cysteine plus glucocorticosteroids versus glucocorticosteroids | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.3 Pentoxifylline plus glucocorticosteroids versus pentoxifylline | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Decompensated cirrhosis | 17 | Rate Ratio (Fixed, 95% CI) | Subtotals only | |
| 7.1 Anti‐TNF versus no intervention | 1 | Rate Ratio (Fixed, 95% CI) | 1.27 [0.45, 3.57] | |
| 7.2 Colchicine versus no intervention | 2 | Rate Ratio (Fixed, 95% CI) | 1.38 [0.56, 3.45] | |
| 7.3 Glucocorticosteroids versus no intervention | 3 | Rate Ratio (Fixed, 95% CI) | 1.03 [0.70, 1.53] | |
| 7.4 Pentoxifylline plus glucocorticosteroids versus no intervention | 1 | Rate Ratio (Fixed, 95% CI) | 0.95 [0.63, 1.45] | |
| 7.5 Pentoxifylline versus no intervention | 3 | Rate Ratio (Fixed, 95% CI) | 0.77 [0.55, 1.08] | |
| 7.6 Propylthiouracil versus no intervention | 1 | Rate Ratio (Fixed, 95% CI) | 0.95 [0.47, 1.93] | |
| 7.7 Anti‐TNF plus glucocorticosteroids versus glucocorticosteroids | 1 | Rate Ratio (Fixed, 95% CI) | 1.42 [0.24, 8.48] | |
| 7.8 Metadoxine plus glucocorticosteroids versus glucocorticosteroids | 1 | Rate Ratio (Fixed, 95% CI) | 0.56 [0.36, 0.88] | |
| 7.9 N‐acetyl cysteine plus glucocorticosteroids versus glucocorticosteroids | 1 | Rate Ratio (Fixed, 95% CI) | 0.77 [0.46, 1.29] | |
| 7.10 Pentoxifylline plus glucocorticosteroids versus glucocorticosteroids | 3 | Rate Ratio (Fixed, 95% CI) | 0.81 [0.57, 1.14] | |
| 7.11 Pentoxifylline versus glucocorticosteroids | 4 | Rate Ratio (Fixed, 95% CI) | 0.93 [0.73, 1.18] | |
| 7.12 Metadoxine plus pentoxifylline versus glucocorticosteroids | 1 | Rate Ratio (Fixed, 95% CI) | 0.74 [0.49, 1.12] | |
| 7.13 Metadoxine plus glucocorticosteroids versus pentoxifylline | 1 | Rate Ratio (Fixed, 95% CI) | 0.58 [0.37, 0.90] | |
| 7.14 Metadoxine plus pentoxifylline versus metadoxine plus glucocorticosteroids | 1 | Rate Ratio (Fixed, 95% CI) | 1.31 [0.81, 2.12] | |
| 7.15 Pentoxifylline plus glucocorticosteroids versus pentoxifylline | 2 | Rate Ratio (Fixed, 95% CI) | 0.88 [0.59, 1.33] | |
| 7.16 Metadoxine plus pentoxifylline versus pentoxifylline | 1 | Rate Ratio (Fixed, 95% CI) | 0.76 [0.50, 1.16] | |
| 8 Cirrhosis | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 Glucocorticosteroids versus no intervention | 1 | 37 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.19, 9.02] |
| 9 Adverse events (proportion) | 21 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 Amlodipine versus no intervention | 1 | 62 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.12, 7.08] |
| 9.2 Anti‐TNF versus no intervention | 1 | 48 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.25 [0.99, 10.68] |
| 9.3 Colchicine versus no intervention | 1 | 67 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.4 Glucocorticosteroids versus no intervention | 4 | 159 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.00 [1.80, 8.88] |
| 9.5 Insulin plus glucagon versus no intervention | 2 | 136 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.75 [0.75, 4.12] |
| 9.6 N‐acetyl cysteine versus no intervention | 1 | 44 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.87 [0.51, 6.83] |
| 9.7 N‐acetyl cysteine plus antioxidants versus no intervention | 1 | 70 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.8 Pentoxifylline versus no intervention | 2 | 151 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.21 [1.10, 4.46] |
| 9.9 Propylthiouracil versus no intervention | 1 | 67 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.36, 2.50] |
| 9.10 Silymarin versus no intervention | 1 | 116 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.11 Antioxidants versus glucocorticosteroids | 1 | 99 | Odds Ratio (M‐H, Fixed, 95% CI) | 6.29 [2.14, 18.43] |
| 9.12 Anti‐TNF plus glucocorticosteroids versus glucocorticosteroids | 2 | 55 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.44 [1.33, 14.80] |
| 9.13 Metadoxine plus glucocorticosteroids versus glucocorticosteroids | 1 | 270 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.47 [0.85, 2.54] |
| 9.14 Rifaximin plus UDCA plus glucocorticosteroids versus UDCA plus glucocorticosteroids | 1 | 68 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.04, 0.54] |
| 9.15 GSF plus pentoxifylline versus pentoxifylline | 1 | 46 | Odds Ratio (M‐H, Fixed, 95% CI) | 13.97 [0.73, 269.23] |
| 10 Adverse events (number) | 32 | Rate Ratio (Random, 95% CI) | Subtotals only | |
| 10.1 Amlodipine versus no intervention | 1 | Rate Ratio (Random, 95% CI) | 0.93 [0.13, 6.57] | |
| 10.2 Anti‐TNF versus no intervention | 1 | Rate Ratio (Random, 95% CI) | 2.26 [1.05, 4.85] | |
| 10.3 Colchicine versus no intervention | 2 | Rate Ratio (Random, 95% CI) | 1.53 [0.86, 2.72] | |
| 10.4 Glucocorticosteroids versus no intervention | 7 | Rate Ratio (Random, 95% CI) | 1.43 [1.10, 1.87] | |
| 10.5 Pentoxifylline plus glucocorticosteroids versus no intervention | 1 | Rate Ratio (Random, 95% CI) | 1.19 [0.85, 1.68] | |
| 10.6 Insulin plus glucagon versus no intervention | 2 | Rate Ratio (Random, 95% CI) | 1.21 [0.81, 1.81] | |
| 10.7 N‐acetyl cysteine versus no intervention | 1 | Rate Ratio (Random, 95% CI) | 1.52 [0.52, 4.45] | |
| 10.8 N‐acetyl cysteine plus antioxidants versus no intervention | 1 | Rate Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.9 Pentoxifylline versus no intervention | 3 | Rate Ratio (Random, 95% CI) | 1.43 [0.92, 2.23] | |
| 10.10 Propylthiouracil versus no intervention | 1 | Rate Ratio (Random, 95% CI) | 1.10 [0.62, 1.98] | |
| 10.11 Antioxidants versus glucocorticosteroids | 1 | Rate Ratio (Random, 95% CI) | 1.15 [0.79, 1.68] | |
| 10.12 Anti‐TNF plus glucocorticosteroids versus glucocorticosteroids | 2 | Rate Ratio (Random, 95% CI) | 1.42 [0.77, 2.62] | |
| 10.13 Metadoxine plus glucocorticosteroids versus glucocorticosteroids | 1 | Rate Ratio (Random, 95% CI) | 0.63 [0.44, 0.89] | |
| 10.14 N‐acetyl cysteine plus glucocorticosteroids versus glucocorticosteroids | 1 | Rate Ratio (Random, 95% CI) | 0.59 [0.37, 0.96] | |
| 10.15 Pentoxifylline plus glucocorticosteroids versus glucocorticosteroids | 3 | Rate Ratio (Random, 95% CI) | 1.26 [0.78, 2.04] | |
| 10.16 SAMe plus glucocorticosteroids versus glucocorticosteroids | 1 | Rate Ratio (Random, 95% CI) | 0.75 [0.35, 1.59] | |
| 10.17 Pentoxifylline versus glucocorticosteroids | 5 | Rate Ratio (Random, 95% CI) | 0.89 [0.75, 1.06] | |
| 10.18 Metadoxine plus pentoxifylline versus glucocorticosteroids | 1 | Rate Ratio (Random, 95% CI) | 0.76 [0.54, 1.07] | |
| 10.19 Metadoxine plus glucocorticosteroids versus pentoxifylline | 1 | Rate Ratio (Random, 95% CI) | 0.67 [0.47, 0.96] | |
| 10.20 Metadoxine plus pentoxifylline versus metadoxine plus glucocorticosteroids | 1 | Rate Ratio (Random, 95% CI) | 1.21 [0.83, 1.77] | |
| 10.21 Pentoxifylline plus glucocorticosteroids versus pentoxifylline | 2 | Rate Ratio (Random, 95% CI) | 1.01 [0.78, 1.30] | |
| 10.22 Rifaximin plus UDCA plus glucocorticosteroids versus UDCA plus glucocorticosteroids | 1 | Rate Ratio (Random, 95% CI) | 0.21 [0.06, 0.75] | |
| 10.23 GSF plus pentoxifylline versus pentoxifylline | 1 | Rate Ratio (Random, 95% CI) | 11.00 [0.61, 198.93] | |
| 10.24 Metadoxine plus pentoxifylline versus pentoxifylline | 1 | Rate Ratio (Random, 95% CI) | 0.81 [0.57, 1.15] |
Comparison 2. Severe alcoholic hepatitis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality at maximal follow‐up | 18 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Glucocorticosteroids versus no intervention | 2 | 607 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.63, 1.21] |
| 1.2 Pentoxifylline plus glucocorticosteroids versus no intervention | 1 | 545 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.64, 1.28] |
| 1.3 N‐acetyl cysteine versus no intervention | 1 | 44 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.34, 4.32] |
| 1.4 Pentoxifylline versus no intervention | 4 | 726 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.59, 1.07] |
| 1.5 Anti‐TNF plus glucocorticosteroids versus glucocorticosteroids | 2 | 55 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.37 [0.67, 8.42] |
| 1.6 Metadoxine plus glucocorticosteroids versus glucocorticosteroids | 1 | 70 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.04, 0.34] |
| 1.7 N‐acetyl cysteine plus glucocorticosteroids versus glucocorticosteroids | 1 | 174 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.32, 1.14] |
| 1.8 Pentoxifylline plus glucocorticosteroids versus glucocorticosteroids | 3 | 887 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.67, 1.16] |
| 1.9 SAMe plus glucocorticosteroids versus glucocorticosteroids | 1 | 40 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.06, 1.97] |
| 1.10 Pentoxifylline versus glucocorticosteroids | 5 | 864 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.65, 1.13] |
| 1.11 Metadoxine plus pentoxifylline versus glucocorticosteroids | 1 | 67 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.06, 0.51] |
| 1.12 Metadoxine plus glucocorticosteroids versus pentoxifylline | 1 | 68 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.11, 0.80] |
| 1.13 Metadoxine plus pentoxifylline versus metadoxine plus glucocorticosteroids | 1 | 67 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.49 [0.55, 4.07] |
| 1.14 Pentoxifylline plus glucocorticosteroids versus pentoxifylline | 2 | 606 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.63, 1.22] |
| 1.15 GSF plus pentoxifylline versus pentoxifylline | 1 | 46 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.08 [0.02, 0.31] |
| 1.16 Metadoxine plus pentoxifylline versus pentoxifylline | 1 | 65 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.12, 0.94] |
| 2 Mortality (30 days) | 16 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Glucocorticosteroids versus no intervention | 2 | 607 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.45, 1.07] |
| 2.2 Pentoxifylline plus glucocorticosteroids versus no intervention | 1 | 545 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.46, 1.20] |
| 2.3 N‐acetyl cysteine versus no intervention | 1 | 44 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.07 [0.46, 9.40] |
| 2.4 Pentoxifylline versus no intervention | 3 | 625 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.64, 1.42] |
| 2.5 Anti‐TNF plus glucocorticosteroids versus glucocorticosteroids | 2 | 55 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.81 [0.75, 30.87] |
| 2.6 Metadoxine plus glucocorticosteroids versus glucocorticosteroids | 1 | 70 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.11, 0.80] |
| 2.7 N‐acetyl cysteine plus glucocorticosteroids versus glucocorticosteroids | 1 | 174 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.12, 0.73] |
| 2.8 Pentoxifylline plus glucocorticosteroids versus glucocorticosteroids | 3 | 887 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.62, 1.34] |
| 2.9 SAMe plus glucocorticosteroids versus glucocorticosteroids | 1 | 40 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.01, 4.01] |
| 2.10 Pentoxifylline versus glucocorticosteroids | 5 | 864 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.82, 1.63] |
| 2.11 Metadoxine plus pentoxifylline versus glucocorticosteroids | 1 | 67 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.16, 1.18] |
| 2.12 Metadoxine plus glucocorticosteroids versus pentoxifylline | 1 | 68 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.12, 0.90] |
| 2.13 Metadoxine plus pentoxifylline versus metadoxine plus glucocorticosteroids | 1 | 67 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.51 [0.53, 4.33] |
| 2.14 Pentoxifylline plus glucocorticosteroids versus pentoxifylline | 2 | 606 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.45, 1.12] |
| 2.15 Metadoxine plus pentoxifylline versus pentoxifylline | 1 | 65 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.18, 1.34] |
| 3 Mortality (90 days) | 8 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Glucocorticosteroids versus no intervention | 2 | 607 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.71, 1.43] |
| 3.2 Pentoxifylline plus glucocorticosteroids versus no intervention | 1 | 545 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.64, 1.42] |
| 3.3 Pentoxifylline versus no intervention | 1 | 545 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.81, 1.74] |
| 3.4 Anti‐TNF plus glucocorticosteroids versus glucocorticosteroids | 1 | 20 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.78 [0.13, 23.52] |
| 3.5 Metadoxine plus glucocorticosteroids versus glucocorticosteroids | 1 | 70 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.04, 0.34] |
| 3.6 N‐acetyl cysteine plus glucocorticosteroids versus glucocorticosteroids | 1 | 174 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.29, 1.11] |
| 3.7 Pentoxifylline plus glucocorticosteroids versus glucocorticosteroids | 1 | 547 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.51, 1.09] |
| 3.8 Pentoxifylline versus glucocorticosteroids | 3 | 683 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.56, 1.10] |
| 3.9 Metadoxine plus pentoxifylline versus glucocorticosteroids | 1 | 67 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.06, 0.51] |
| 3.10 Metadoxine plus glucocorticosteroids versus pentoxifylline | 1 | 68 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.08, 0.63] |
| 3.11 Metadoxine plus pentoxifylline versus metadoxine plus glucocorticosteroids | 1 | 67 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.49 [0.55, 4.07] |
| 3.12 Pentoxifylline plus glucocorticosteroids versus pentoxifylline | 2 | 606 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.53, 1.11] |
| 3.13 GSF plus pentoxifylline versus pentoxifylline | 1 | 46 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.08 [0.02, 0.31] |
| 3.14 Metadoxine plus pentoxifylline versus pentoxifylline | 1 | 65 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.12, 0.94] |
| 4 Serious adverse events (proportion) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 Glucocorticosteroids versus no intervention | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Pentoxifylline plus glucocorticosteroids versus no intervention | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Pentoxifylline versus no intervention | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.4 Pentoxifylline plus glucocorticosteroids versus glucocorticosteroids | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.5 Pentoxifylline versus glucocorticosteroids | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.6 Pentoxifylline plus glucocorticosteroids versus pentoxifylline | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Serious adverse events (number) | 1 | Rate Ratio (Fixed, 95% CI) | Totals not selected | |
| 5.1 Anti‐TNF plus glucocorticosteroids versus glucocorticosteroids | 1 | Rate Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Liver transplantation | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.1 N‐acetyl cysteine plus glucocorticosteroids versus glucocorticosteroids | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Pentoxifylline plus glucocorticosteroids versus pentoxifylline | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Decompensated cirrhosis | 11 | Rate Ratio (Fixed, 95% CI) | Subtotals only | |
| 7.1 Glucocorticosteroids versus no intervention | 1 | Rate Ratio (Fixed, 95% CI) | 0.97 [0.64, 1.47] | |
| 7.2 Pentoxifylline plus glucocorticosteroids versus no intervention | 1 | Rate Ratio (Fixed, 95% CI) | 0.95 [0.63, 1.45] | |
| 7.3 Pentoxifylline versus no intervention | 3 | Rate Ratio (Fixed, 95% CI) | 0.77 [0.55, 1.08] | |
| 7.4 Anti‐TNF plus glucocorticosteroids versus glucocorticosteroids | 1 | Rate Ratio (Fixed, 95% CI) | 1.42 [0.24, 8.48] | |
| 7.5 Metadoxine plus glucocorticosteroids versus glucocorticosteroids | 1 | Rate Ratio (Fixed, 95% CI) | 0.56 [0.36, 0.88] | |
| 7.6 N‐acetyl cysteine plus glucocorticosteroids versus glucocorticosteroids | 1 | Rate Ratio (Fixed, 95% CI) | 0.77 [0.46, 1.29] | |
| 7.7 Pentoxifylline plus glucocorticosteroids versus glucocorticosteroids | 3 | Rate Ratio (Fixed, 95% CI) | 0.81 [0.57, 1.14] | |
| 7.8 Pentoxifylline versus glucocorticosteroids | 4 | Rate Ratio (Fixed, 95% CI) | 0.93 [0.73, 1.18] | |
| 7.9 Metadoxine plus pentoxifylline versus glucocorticosteroids | 1 | Rate Ratio (Fixed, 95% CI) | 0.74 [0.49, 1.12] | |
| 7.10 Metadoxine plus glucocorticosteroids versus pentoxifylline | 1 | Rate Ratio (Fixed, 95% CI) | 0.58 [0.37, 0.90] | |
| 7.11 Metadoxine plus pentoxifylline versus metadoxine plus glucocorticosteroids | 1 | Rate Ratio (Fixed, 95% CI) | 1.31 [0.81, 2.12] | |
| 7.12 Pentoxifylline plus glucocorticosteroids versus pentoxifylline | 2 | Rate Ratio (Fixed, 95% CI) | 0.88 [0.59, 1.33] | |
| 7.13 Metadoxine plus pentoxifylline versus pentoxifylline | 1 | Rate Ratio (Fixed, 95% CI) | 0.76 [0.50, 1.16] | |
| 8 Adverse events (proportion) | 7 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 N‐acetyl cysteine versus no intervention | 1 | 44 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.87 [0.51, 6.83] |
| 8.2 Pentoxifylline versus no intervention | 2 | 151 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.21 [1.10, 4.46] |
| 8.3 Anti‐TNF plus glucocorticosteroids versus glucocorticosteroids | 2 | 55 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.44 [1.33, 14.80] |
| 8.4 Pentoxifylline plus glucocorticosteroids versus glucocorticosteroids | 1 | 270 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.47 [0.85, 2.54] |
| 8.5 GSF plus pentoxifylline versus pentoxifylline | 1 | 46 | Odds Ratio (M‐H, Fixed, 95% CI) | 13.97 [0.73, 269.23] |
| 9 Adverse events (number) | 16 | Rate Ratio (Fixed, 95% CI) | Subtotals only | |
| 9.1 Glucocorticosteroids versus no intervention | 1 | Rate Ratio (Fixed, 95% CI) | 1.37 [0.98, 1.90] | |
| 9.2 Pentoxifylline plus glucocorticosteroids versus no intervention | 1 | Rate Ratio (Fixed, 95% CI) | 1.19 [0.85, 1.68] | |
| 9.3 N‐acetyl cysteine versus no intervention | 1 | Rate Ratio (Fixed, 95% CI) | 1.52 [0.52, 4.45] | |
| 9.4 Pentoxifylline versus no intervention | 3 | Rate Ratio (Fixed, 95% CI) | 1.33 [1.01, 1.75] | |
| 9.5 Anti‐TNF plus glucocorticosteroids versus glucocorticosteroids | 2 | Rate Ratio (Fixed, 95% CI) | 1.42 [0.77, 2.62] | |
| 9.6 Metadoxine plus glucocorticosteroids versus glucocorticosteroids | 1 | Rate Ratio (Fixed, 95% CI) | 0.63 [0.44, 0.89] | |
| 9.7 N‐acetyl cysteine plus glucocorticosteroids versus glucocorticosteroids | 1 | Rate Ratio (Fixed, 95% CI) | 0.59 [0.37, 0.96] | |
| 9.8 Pentoxifylline plus glucocorticosteroids versus glucocorticosteroids | 3 | Rate Ratio (Fixed, 95% CI) | 1.10 [0.86, 1.40] | |
| 9.9 SAMe plus glucocorticosteroids versus glucocorticosteroids | 1 | Rate Ratio (Fixed, 95% CI) | 0.75 [0.35, 1.59] | |
| 9.10 Pentoxifylline versus glucocorticosteroids | 5 | Rate Ratio (Fixed, 95% CI) | 0.89 [0.75, 1.06] | |
| 9.11 Metadoxine plus pentoxifylline versus glucocorticosteroids | 1 | Rate Ratio (Fixed, 95% CI) | 0.76 [0.54, 1.07] | |
| 9.12 Metadoxine plus glucocorticosteroids versus pentoxifylline | 1 | Rate Ratio (Fixed, 95% CI) | 0.67 [0.47, 0.96] | |
| 9.13 Metadoxine plus pentoxifylline versus metadoxine plus glucocorticosteroids | 1 | Rate Ratio (Fixed, 95% CI) | 1.21 [0.83, 1.77] | |
| 9.14 Pentoxifylline plus glucocorticosteroids versus pentoxifylline | 2 | Rate Ratio (Fixed, 95% CI) | 1.01 [0.78, 1.30] | |
| 9.15 GSF plus pentoxifylline versus pentoxifylline | 1 | Rate Ratio (Fixed, 95% CI) | 11.00 [0.61, 198.93] | |
| 9.16 Metadoxine plus pentoxifylline versus pentoxifylline | 1 | Rate Ratio (Fixed, 95% CI) | 0.81 [0.57, 1.15] |
2.5. Analysis.
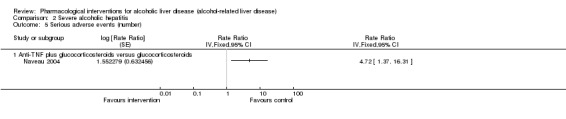
Comparison 2 Severe alcoholic hepatitis, Outcome 5 Serious adverse events (number).
Comparison 3. Alcohol‐related liver disease (others).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality at maximal follow‐up | 16 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Anabolic steroids versus no intervention | 2 | 248 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.70, 2.39] |
| 1.2 Antioxidants versus no intervention | 2 | 255 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.96 [0.99, 3.89] |
| 1.3 Colchicine versus no intervention | 2 | 604 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.80, 1.53] |
| 1.4 Cycloxilic acid versus no intervention | 1 | 20 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.5 Epomediol versus no intervention | 1 | 40 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.6 Glucocorticosteroids versus no intervention | 1 | 45 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.15, 2.78] |
| 1.7 Malotilate versus no intervention | 1 | 407 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.52, 1.41] |
| 1.8 Metadoxine versus no intervention | 1 | 136 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.9 Propylthiouracil versus no intervention | 2 | 423 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.26, 0.78] |
| 1.10 SAMe versus no intervention | 1 | 123 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.21, 1.30] |
| 1.11 Silymarin versus no intervention | 1 | 200 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.46, 2.22] |
| 1.12 Ursodeoxycholic acid versus no intervention | 1 | 226 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.09 [1.12, 3.90] |
| 2 Mortality (30 days) | 6 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Cycloxilic acid versus no intervention | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Epomediol versus no intervention | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Glucocorticosteroids versus no intervention | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 Metadoxine versus no intervention | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.5 Propylthiouracil versus no intervention | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.6 Ursodeoxycholic acid versus no intervention | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Mortality (90 days) | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Epomediol versus no intervention | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Glucocorticosteroids versus no intervention | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 SAMe versus no intervention | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Serious adverse events (proportion) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 SAMe versus no intervention | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Liver transplantation | 1 | 123 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.03, 3.13] |
| 5.1 SAMe versus no intervention | 1 | 123 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.03, 3.13] |
| 6 Decompensated cirrhosis | 3 | Rate Ratio (Fixed, 95% CI) | Totals not selected | |
| 6.1 Colchicine versus no intervention | 1 | Rate Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Malotilate versus no intervention | 1 | Rate Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.3 Ursodeoxycholic acid versus no intervention | 1 | Rate Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Hepatocellular carcinoma | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7.1 Anabolic steroids versus no intervention | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 SAMe versus no intervention | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Adverse events (proportion) | 18 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 Antioxidants versus no intervention | 2 | 92 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 Colchicine versus no intervention | 2 | 604 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.66, 1.28] |
| 8.3 Malotilate versus no intervention | 2 | 462 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.45, 1.79] |
| 8.4 Metadoxine versus no intervention | 1 | 136 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.96 [0.12, 73.86] |
| 8.5 Pentoxifylline versus no intervention | 1 | 24 | Odds Ratio (M‐H, Fixed, 95% CI) | 7.86 [0.75, 82.13] |
| 8.6 Propylthiouracil versus no intervention | 2 | 423 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.59 [0.73, 3.46] |
| 8.7 SAMe versus no intervention | 3 | 205 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.86 [0.65, 5.33] |
| 8.8 Silymarin versus no intervention | 3 | 296 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.70 [0.48, 5.98] |
| 8.9 Ursodeoxycholic acid versus no intervention | 1 | 11 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.01, 7.05] |
| 8.10 Candesartan plus ursodeoxycholic acid versus ursodeoxycholic acid | 1 | 85 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Adverse events (number) | 11 | Rate Ratio (Fixed, 95% CI) | Subtotals only | |
| 9.1 Colchicine versus no intervention | 2 | Rate Ratio (Fixed, 95% CI) | 0.89 [0.75, 1.05] | |
| 9.2 Malotilate versus no intervention | 1 | Rate Ratio (Fixed, 95% CI) | 0.91 [0.48, 1.75] | |
| 9.3 Metadoxine versus no intervention | 1 | Rate Ratio (Fixed, 95% CI) | 2.91 [0.12, 71.48] | |
| 9.4 Pentoxifylline versus no intervention | 1 | Rate Ratio (Fixed, 95% CI) | 7.00 [0.86, 56.89] | |
| 9.5 Propylthiouracil versus no intervention | 2 | Rate Ratio (Fixed, 95% CI) | 1.17 [0.58, 2.33] | |
| 9.6 SAMe versus no intervention | 2 | Rate Ratio (Fixed, 95% CI) | 1.52 [0.69, 3.31] | |
| 9.7 Silymarin versus no intervention | 1 | Rate Ratio (Fixed, 95% CI) | 1.65 [0.48, 5.63] | |
| 9.8 Ursodeoxycholic acid versus no intervention | 1 | Rate Ratio (Fixed, 95% CI) | 0.29 [0.01, 7.20] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Akriviadis 1990.
| Methods | Randomised clinical trial | |
| Participants | Country: USA. Number randomised: 74. Post‐randomisation dropouts: 2 (2.7%). Revised sample size: 72. Average age: 41 years. Females: 23 (31.9%). Inclusion criteria: 1. Palpable hepatomegaly. 2. Serum bilirubin of 5 mg/dL or more. 3. One or more of the followings: hepatic tenderness, fever above 100 degrees F, leucocytosis above 12,000/mm3. Exclusion criteria: 1. Life‐threatening bacterial infection. 2. Massive gastrointestinal haemorrhage. 3. Renal insufficiency (serum creatinine greater than 2.5 mg/dL). 4. Serum positivity for hepatitis B surface antigen. 5. Rapidly improving liver tests. 6. Clinical evidence of advanced alcoholic cirrhosis. | |
| Interventions | Participants were randomly assigned to two groups. Group 1 (n = 36): colchicine (1 mg once daily). Group 2 (n = 36): placebo. Duration: 30 days | |
| Outcomes | mortality, adverse events, decompensated cirrhosis. | |
| Notes | Reasons for post‐randomisation dropouts: 1 withdrew consent and 1 left the hospital, not considered for analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "all patients who fulfilled the above criteria were randomly placed into two treatment groups". Comment: Further details were not available. |
| Allocation concealment (selection bias) | Low risk | Quote: "a coordinator that was not an investigator randomly selected sealed envelopes for treatment decision, drugs were coded and distributed by the hospital pharmacy". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The study was double blinded…one group received identical placebo". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "An ombudsman who was not an investigator was appointed to resolve issues arising from possible life‐threatening complications of therapy". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "There were two drop‐outs: one patient withdrew his consent 1 day after randomization, and a second patient left the hospital against medical advice on the third day of hospitalization". Comment: There were post‐randomisation dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: all important outcomes were reported. |
| For‐profit bias | Low risk | Quote: "funded by the Division of Research Resources of the National Resources of the National Institutes of Health, Grant No. MOl‐RR‐43". |
| Other bias | Low risk | Comment: there was no other bias. |
Akriviadis 2000.
| Methods | Randomised clinical trial | |
| Participants | Country: USA. Number randomised: 102. Post‐randomisation dropouts: 1 (1%). Revised sample size: 101. Average age: 42 years. Females: 26 (25.7%). Inclusion criteria: 1. History of heavy ethanol abuse. 2. Admission diagnosis of acute alcoholic hepatitis. 3. Jaundice 4. Discriminant Fraction higher or equal to 32. 5. One or more of: palpable tender hepatomegaly, fever, leukocytosis (white blood cell count higher than 12,000/mm3 with predominantly neutrophil differentiation), hepatic encephalopathy, hepatic systolic bruit hepatomegaly. Exclusion criteria: 1. Concomitant bacterial infections. 2. Active gastrointestinal haemorrhage. 3. Severe cardiovascular or pulmonary disease. 4. decreasing serum bilirubin values or rapid improvement of other liver test results over the first post admission days. 5. Clinical evidence of advanced alcoholic cirrhosis. | |
| Interventions | Participants were randomly assigned to two groups. Group 1 (n = 49): pentoxifylline (400 mg thrice daily). Group 2 (n = 52): placebo. Duration: 4 weeks | |
| Outcomes | mortality, adverse events, decompensated cirrhosis. | |
| Notes | Gastrointestinal bleeding included in the adverse events because not specified if upper, variceal or lower. Reasons for post‐randomisation dropouts: there was 1 dropout: a patient from the PTX group left the hospital against medical advice 1 day after randomisation. He received only 3 capsules of PTX and therefore was excluded from analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A coordinator who was not an investigator randomly selected sealed envelopes for treatment decisions, and drugs were coded and distributed by the hospital pharmacy". |
| Allocation concealment (selection bias) | Low risk | Quote: "Sealed envelopes for treatment decisions, drugs were coded and distributed by the hospital pharmacy". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Vitamin B12 tablets were chosen as placebo because their size and appearance are similar to those of PTX tablets, thus facilitating identical filling of the capsules. The study was double‐blinded. Drugs were coded and distributed by the hospital pharmacy". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The study was double‐blinded. An ombudsman who was not an investigator was appointed to resolve potential issues arising from possible life‐threatening complications of therapy". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "There was 1 dropout: a patient from the PTX group left the hospital against medical advice 1 day after randomisation. He received only 3 capsules of PTX and therefore was excluded from analysis. The 5 patients from both groups who missed follow‐up appointments were later contacted by phone or were seen at a later date in the clinic, and their survival status was ascertained. Survival status was also assessed after discharge from the hospital, over a 6‐month follow‐up period, for all patients from both groups who survived the index hospitalisation". Comment: only one missing of 102. |
| Selective reporting (reporting bias) | Low risk | Comment: all important outcomes were reported. |
| For‐profit bias | High risk | Quote: "Hoeschst‐Roussel Pharmaceuticals Inc for TNF measurements". |
| Other bias | Low risk | Comment: there was no other bias. |
Baker 1981.
| Methods | Randomised clinical trial | |
| Participants | Country: USA. Number randomised: 51. Post‐randomisation dropouts: 1 (2%). Revised sample size: 50. Average age: 42 years. Females: 21 (42%). Inclusion criteria: 1. History of excessive alcohol consumption, 120 mL or more per day for more than 1 year, a history of multiple repetitive alcoholic binges for years, or family substantiation of excessive alcohol consumption for years without specific quantitation. 2. A liver biopsy demonstrating the lesion of alcoholic hepatitis, or when prolongation of the prothrombin time refractory to parenteral vitamin K precluded biopsy an abnormal SGOT which was greater than the SGPT. Exclusion criteria: 1. Presence of active infections. 2. Gastrointestinal haemorrhage. 3. Pancreatitis. | |
| Interventions | Participants were randomly assigned to two groups. Group 1 (n = 25): regular insulin 2 U/hour plus glucagon 200 μg/hour in 200 mL 5 % dextrose over 12 hours daily. Group 2 (n = 25): placebo. Duration: 3 weeks | |
| Outcomes | mortality, adverse events. | |
| Notes | Reasons for post‐randomisation dropouts: one patient left the study against medical advice on second day infusion therapy and was not included in the data analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "…using computer‐generated random numbers". |
| Allocation concealment (selection bias) | Low risk | Quote: "Patients were assigned to insulin and glucagon or control groups by sealed envelopes". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Patients in the control group received 5 % dextrose in an identical fashion. Two grams of human serum albumin were added to the bottles containing insulin and glucagon to prevent absorption of the hormones to the infusion apparatus and to the control bottles to maintain identical appearance of the solutions". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "One patient left the hospital against medical advice on the 2nd day of the infusion therapy and is not included in the data analysis". Comment: there were post‐randomisation dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: all important outcomes were reported. |
| For‐profit bias | High risk | Quote: "Novo Research Institute, Copenhagen, Denmark, and by Clinical Research Center Grant ". |
| Other bias | Low risk | Comment: there was no other bias. |
Basu 2015.
| Methods | Randomised clinical trial | |
| Participants | Country: USA.
Number randomised: 30.
Post‐randomisation dropouts: not stated.
Revised sample size: 30.
Average age: not stated.
Females: not stated.
Inclusion criteria: 1. Severe alcoholic hepatitis (Maddrey discriminant function > 32). 2. Age 25 to 60 years. 3. MELD > 26. Exclusion criteria: 1. Hepatitis A, B, C. 2. Gastrointestinal bleed. 3. Hepatic encephalopathy. 4. Sepsis. |
|
| Interventions | Participants were randomly assigned to three groups. Group 1 (n = not stated): mycophenolate (500 mg twice daily) plus pentoxifylline (400 mg once daily). Group 2 (n = not stated): glucocorticosteroids (prednisolone 30 mg once daily) plus pentoxifylline (400 mg once daily). Group 3 (n = not stated): mycophenolate (500 mg twice daily) plus glucocorticosteroids (prednisolone 30 mg once daily) plus pentoxifylline (400 mg once daily). Duration: 30 days | |
| Outcomes | None of the outcomes of interest were reported. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "a randomised‐open label placebo control". |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "a randomised‐open label placebo control". |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "a randomised‐open label placebo control". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: none of the outcomes of interest were reported. |
| For‐profit bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: there was no other bias. |
Bird 1991.
| Methods | Randomised clinical trial | |
| Participants | Country: UK. Number randomised: 90. Post‐randomisation dropouts: 4 (4.4%). Revised sample size: 86. Average age: 46 years. Females: 37 (43%). Inclusion criteria: 1. History of alcohol intake greater than 40 mg/day in women or 60 mg/day in men. 2. Diagnosis of acute alcoholic hepatitis confirmed by liver biopsy (characteristic histological features) OR by the presence of two or more of: palpable hepatomegaly, leukocytosis of more than 11 x 10^9 cells/L and “wipe out” on liver and spleen isotope scanning in the absence of any other known or suspected aetiological factor Exclusion criteria: 1. Pancreatitis. 2. Severe gastrointestinal haemorrhage. 3. Malignant disease. 4. Seropositivity for HBsAg. 5. Established alcoholic cirrhotic primarily admitted for the control of complications arising directly from cirrhosis (bleeding oesophageal or gastric varices). 6. Previous admissions for the management of the complications of cirrhosis. | |
| Interventions | Participants were randomly assigned to two groups. Group 1 (n = 43): insulin 30 U plus glucagon 3 mg (in 250 mL of 5% dextrose plus 1% human albumin solution , infused in 12 hours/day). Group 2 (n = 43): placebo. Duration: max 3 weeks | |
| Outcomes | mortality, adverse events, liver transplantation. | |
| Notes | Reasons for post‐randomisation dropouts: 2 malignancy, 2 histology compatible to non‐A, non‐B hepatitis (post‐randomisation). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: " Patients were randomised using sealed envelopes to receive either insulin and glucagon treatment or placebo after stratification on the basis of severity of illness". |
| Allocation concealment (selection bias) | Low risk | Quote: "sealed envelopes". |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: " The control group received the dextrose‐and‐albumin solution in identical fashion. The nursing staff administering the infusions was blinded as to the contents of the bags". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Four patients were withdrawn after randomisation because of the subsequent additional diagnosis of malignant disease in two patients (one case of pancreatic carcinoma and one of HCC) and histological changes that were finally attributed by the pathologist to non‐A, non‐B hepatitis (although these were negative on serological testing for hepatitis C virus in two other patients. In the treatment group 1 patient left the hospital against medical advice and 4 withdrew consent for the trial before death or the completion of the 21‐day course. In the placebo group 2 patients left the hospital on their own and 5 withdrew consent. Respectively 3 and 2 patients were lost to 6 months follow up". Comment: there were post‐randomisation dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: all important outcomes were reported. |
| For‐profit bias | High risk | Quote: "Novo Nordisk Pharmaceutical (Crawley. UK) provided Glucagon Novo and funded the hormone assay". |
| Other bias | Low risk | Comment: there was no other bias. |
Bird 1998.
| Methods | Randomised clinical trial | |
| Participants | Country: UK. Number randomised: 64. Post‐randomisation dropouts: 2 (3.1%). Revised sample size: 62. Average age: 52 years. Females: 26 (41.9%). Inclusion criteria: 1. History of alcohol intake greater than 40 mg/day in women or 60 mg/day in men. 2. Diagnosis of acute alcoholic hepatitis confirmed by liver biopsy (characteristic histological features) OR by the presence of two or more of: palpable hepatomegaly, leukocytosis of more than 11 x 10^9 cells/L and “wipe out” on liver and spleen isotope scanning in the absence of any other known or suspected aetiological factor Exclusion criteria: 1. Pancreatitis. 2. Severe gastrointestinal haemorrhage. 3. Malignant disease. 4. Seropositivity for viral infections such as HBsAg, anti‐HCV or anti‐HIV. 5. Established alcoholic cirrhotic primarily admitted for the control of complications arising directly from cirrhosis (Bleeding oesophageal or gastric varices, recurrent or chronic hepatic encephalopathy, ascites or oedema). 6. Previous admissions for the complications of portal hypertension. | |
| Interventions | Participants were randomly assigned to two groups. Group 1 (n = 32): amlodipine 5 mg or 10 mg once daily Group 2 (n = 30): placebo. Duration: 4 weeks | |
| Outcomes | mortality, adverse events. | |
| Notes | Reasons for post‐randomisation dropouts: 2 patients were excluded from the analysis (1 malignant disease, 1 haemochromatosis) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Consecutive patients were randomised in a double‐blind fashion using a ‘sets of four’ procedure after stratification on the basis of the severity of illness". Comment: Further details were not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "Double‐blind fashion. The control group received placebo capsules, similar in appearance and taste to the amlodipine. The treated patients received amlodipine (Pfizer, Sandwich, UK) 10 mg (two capsules) daily, or 5 mg (one capsule) if the prothrombin time was more than 3 s prolonged.In subjects with a prolonged prothrombin time on entry to the study, the daily dose of the trial medication was increased to 10 mg (i.e. two capsules) when the prothrombin time improved to only 3 or fewer seconds prolonged". Comment: Were there patients treated with 2 capsules in the placebo group as well?. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "Two patients were subsequently excluded from the analysis: one in whom a diagnosis of genetic haemochromatosis was made after liver biopsy and a second patient in whom an adenocarcinoma of the large bowel was diagnosed 2 weeks after entering the study". |
| Selective reporting (reporting bias) | Low risk | Comment: all important outcomes were reported. |
| For‐profit bias | Low risk | Quote: "Financial assistance from Greater Glasgow Health Board". |
| Other bias | Low risk | Comment: there was no other bias. |
Blitzer 1977.
| Methods | Randomised clinical trial | |
| Participants | Country: USA. Number randomised: 33. Post‐randomisation dropouts: 5 (15.2%). Revised sample size: 28. Average age: 48 years. Females: 0 (0%). Inclusion criteria: 1. Recent history of heavy alcohol consumption (more than one pint of whisky per day or its alcoholic equivalent). 2. Hepatomegaly based on physical examination (palpable more than 5 cm below the costal margin) and/or liver scan. 3. Total serum bilirubin greater than 5 mg/dL. 4. At least two of the following abnormalities: SGOT greater than 100 Reitman‐Frankel units per mL, serum albumin concentration less than 3 g/dL, prothrombin time more than 2 seconds greater than control value. Exclusion criteria: 1. Adrenocorticosteroids in the six months prior to admission. 2. Evidence of psychotic behavior precluding their co‐operation during the investigation. | |
| Interventions | Participants were randomly assigned to two groups. Group 1 (n = 12): glucocorticosteroids (prednisolone 10 mg four times daily (total of 40 mg per day)). Group 2 (n = 16): placebo. Duration:14 days, then tapering (20 mg for 4 days , 10 mg for 4 days, 5 mg for 4 days) | |
| Outcomes | mortality, adverse events. | |
| Notes | Reasons for post‐randomisation dropouts: group 1: 3 withdrawal, 2 GI (received 5 days of treatment), excluded from the analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were assigned by random, sealed‐envelope technique to receive either placebo or steroid". Comment: Further details were not available. |
| Allocation concealment (selection bias) | Low risk | Quote: " Sealed‐envelopes". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Double‐blind study; only the pharmacist was aware of the type of therapy. The control group received placebo tablets according to the same dosage schedule of the steroid group". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Double‐blind study; only the pharmacist was aware of the type of therapy". Comment: Further details were not available. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Five patients, who had each received less than 5 days of therapy, were subsequently excluded from analysis. Of these, three had left the hospital against medical advice or withdrew from the study, and in two experimental therapy had been stopped following gastrointestinal haemorrhage". Comment: there were post‐randomisation dropouts and no results were reported about them. |
| Selective reporting (reporting bias) | Low risk | Comment: all important outcomes were reported. |
| For‐profit bias | High risk | Quote: "Both prednisolone and placebo tablets were supplied by the Upjohn Co., Kalamazoo, Michigan". |
| Other bias | Low risk | Comment: there was no other bias. |
Boetticher 2008.
| Methods | Randomised clinical trial | |
| Participants | Country: USA. Number randomised: 48. Post‐randomisation dropouts: 0 (0%). Revised sample size: 48. Average age: 51 years. Females: 13 (27.1%). Inclusion criteria: 1. Patients older than 18 years of age at entry. 2. Clinical evaluation and testing supporting a diagnosis of alcoholic hepatitis including jaundice, hepatomegaly, leukocytosis, fever, elevations in transaminase levels. 3. MELD higher than 15. 4. Exclusion of other causes of hepatitis including viral (negative hepatitis B surface antigen and antibody to hepatitis C virus), autoimmune (antinuclear antibody titre 1:40, negative antimitochondrial antibody, and smooth muscle antibody), drugs, or metabolic disorders (normal ceruloplasmin levels) in the setting of compatible alcohol consumption. 5. Significant alcohol consumption ( higher than 40 g per day for a minimum of 6 months and within the 3 months prior to study enrolment). 6. In women: negative pregnancy test or proof of surgical sterility or postmenopausal. Exclusion criteria: 1. Hypersensitivity to etanercept. 2. Presence of infection documented by chest x‐ray or blood, urine, or ascites cultures. 3. History of autoimmune disease. 4. Treatment with corticosteroids, pentoxifylline, propylthiouracil, or thalidomide in the preceding 4 weeks prior to evaluation. 5. Breast‐feeding or pregnancy in women. | |
| Interventions | Participants were randomly assigned to two groups. Group 1 (n = 26): anti‐TNF (etanercept 25 mg/day) Group 2 (n = 22): placebo. Duration: administration at days 1,4,8,11,15,18 | |
| Outcomes | mortality, adverse events, decompensated cirrhosis. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was conducted through the use of logbooks in the study pharmacy at each individual site, in which randomly generated numbers (blocks of 4) for each strata were recorded. Enrolled patients were entered sequentially to receive the assigned treatment. Randomization was conducted separately at each center in absence of a stratification scheme". |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Patient, coordinator, and physician were blinded to randomisation group. The placebo group received subcutaneous injections of placebo on days 1, 4, 8, 11, 15, and 18 ( 2 days for each dosing date), whereas the etanercept group received subcutaneous injections of etanercept (25 mg) at identical time points". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "An independent data safety board reviewed interim data and analyses, which were blinded to all co‐investigators". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were post‐randomisation dropouts, 2 for group and they never received trial treatment but all patients analysed. |
| Selective reporting (reporting bias) | Low risk | Comment: all important outcomes were reported. |
| For‐profit bias | Low risk | Quote: "Supported by NIH, the NIH funded Mayo Clinical Research Unit (CTSA), Amgen (to V.S.), for study drug and part of cytokine analyses". |
| Other bias | Low risk | Comment: there was no other bias. |
Bories 1987.
| Methods | Randomised clinical trial | |
| Participants | Country: France. Number randomised: 45. Post‐randomisation dropouts: 0 (0%). Revised sample size: 45. Average age: 43 years. Females: 18 (40%). Inclusion criteria 1. Patients with acute alcoholic steatosis, fibrosis, or cirrhosis. | |
| Interventions | Participants were randomly assigned to two groups. Group 1 (n = 24): glucocorticosteroids (prednisolone 40 mg/day). Group 2 (n = 21): placebo. Duration: 30 days duration | |
| Outcomes | mortality. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "by random number table ('une table de nombre de hasard')". |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: all participants were included for analysis. |
| Selective reporting (reporting bias) | High risk | Comment: adverse events were not reported. |
| For‐profit bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: there was no other bias. |
Caballeria 1998.
| Methods | Randomised clinical trial | |
| Participants | Country: Spain. Number randomised: 136. Post‐randomisation dropouts: not stated. Revised sample size: 136. Average age: not stated Females: not stated Inclusion criteria: 1. Age between 20 and 70 years; (2) A well‐documented history of an average daily alcohol consumption exceeding 80 g/day and active drinking at the time of the study. 3. Mild clinical and laboratory abnormalities suggestive of early‐stage alcoholic liver disease. 4. Ultrasonographic evidence of fatty liver. Exclusion criteria: 1. Advanced alcoholic liver disease. 2. Non‐alcoholic liver disease. 3. Allergy to pyridoxine or derivatives. 4. Regular treatments during the month prior to the study. 5. Renal failure. 6. Other severe associated disease. | |
| Interventions | Participants were randomly assigned to two groups. Group 1 (n = 69): metadoxine 1500 mg/day. Group 2 (n = 67): placebo. Duration: 3 months treatment | |
| Outcomes | mortality, adverse events. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A random code was prepared by computer for each participating centre". |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "The study was conducted in a randomised, double‐blind fashion". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "The study was conducted in a randomised, double‐blind fashion". Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Twelve patients in the metadoxine group and 13 in the placebo group were excluded prematurely from the trial, mainly because of patient refusal to continue and loss to follow‐up". Comment: there were post‐randomisation dropouts. |
| Selective reporting (reporting bias) | High risk | Comment: mortality was not reported. |
| For‐profit bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: there was no other bias. |
Campra 1973.
| Methods | Randomised clinical trial | |
| Participants | Country: USA. Number randomised: 50. Post‐randomisation dropouts: 5 (10%). Revised sample size: 45. Average age: 43 years. Females: 28 (62.2%). Inclusion criteria: 1. Patients with severe acute alcoholic hepatitis. 2. Randomisation within 10 days of hospital admission. 3. No previous history of liver disease. 4. No contraindication for steroid therapy. 5. Judged to be seriously ill. 6. Other known illness. | |
| Interventions | Participants were randomly assigned to two groups. Group 1 (n = 20): glucocorticosteroids (methylprednisolone 40 mg/day). Group 2 (n = 25): no intervention. Duration: 6 weeks treatment | |
| Outcomes | mortality. | |
| Notes | Reasons for post‐randomisation dropouts: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were randomised to one of the two groups". |
| Allocation concealment (selection bias) | Unclear risk | Quote: "By using previously prepared sealed envelopes, patients were randomly allocated". Comment: Further details were not available. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "The trial was not double‐blind". |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "The trial was not double‐blind". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: there were post‐randomisation dropouts. |
| Selective reporting (reporting bias) | High risk | Comment: adverse events were not reported. |
| For‐profit bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: there was no other bias. |
Carithers 1989.
| Methods | Randomised clinical trial | |
| Participants | Country: USA. Number randomised: 67. Post‐randomisation dropouts: 0 (0%). Revised sample size: 67. Average age: 43 years. Females: 26 (38.8%). Inclusion criteria: 1. Spontaneous hepatic encephalopathy. 2. Discriminant function of 32 or greater Exclusion criteria: not reported | |
| Interventions | Participants were randomly assigned to two groups. Group 1 (n = 36): glucocorticosteroids (methylprednisolone 32 mg/day). Group 2 (n = 31): placebo. Duration: 28 days | |
| Outcomes | mortality. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "A random code was prepared for each of the four participating institutions…". Comment: the method of preparing the random code was not reported. |
| Allocation concealment (selection bias) | Low risk | Quote: "The random code sequence was kept by an independent source.". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double‐blind, placebo‐controlled". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double‐blind, placebo‐controlled". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: All patients were included for analysis.. |
| Selective reporting (reporting bias) | High risk | Comment: Adverse events were not reported. |
| For‐profit bias | Low risk | Quote: "Supported by a research grant from the National Institute of Alcohol Abuse and Alcoholism". |
| Other bias | Low risk | Comment: There was no other bias. |
Colman 1998.
| Methods | Randomised clinical trial | |
| Participants | Country: Australia. Number randomised: 129. Post‐randomisation dropouts: 0 (0%). Revised sample size: 129. Average age: not stated Females: not stated Inclusion criteria: 1. Alcoholic liver cirrhosis Exclusion criteria: not reported | |
| Interventions | Participants were randomly assigned to two groups. Group 1 (n = 63): colchicine 1 mg once daily. Group 2 (n = 66): placebo. Duration: mean 45 months | |
| Outcomes | None of the outcomes of interest were reported. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients with alcoholic cirrhosis were entered into a randomised. double blind, placebo controlled trial…". |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "Patients with alcoholic cirrhosis were entered into a randomised, double blind, placebo controlled trial". Comment: Further details were not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "41 patients had to be withdrawn from the study during follow up: 26 for non compliance, 10 for adverse events and 5 for geographic reasons". Comment: there were post‐randomisation dropouts. |
| Selective reporting (reporting bias) | High risk | Comment: none of the outcomes of interest was not reported. |
| For‐profit bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: there was no other bias. |
Cortez‐Pinto 2002.
| Methods | Randomised clinical trial | |
| Participants | Country: Portugal. Number randomised: 62. Post‐randomisation dropouts: 7 (11.3%). Revised sample size: 55. Average age: 54 years. Females: 6 (10.9%). Inclusion criteria: 1. Age between 18 and 65 years. 2. Biopsy‐ proven liver cirrhosis. 3. A well‐documented history of previous daily alcohol intake exceeding 40 g of ethanol in women and 60 g in men for more than 5 years. 4. Exclusion of other causes of liver disease were excluded. Exclusion criteria: 1. Haemochromatosis. 2. Wilson's disease. 3. Alfa‐1‐antitrypsin deficiency. 4. Autoimmune hepatitis. 5. Primary biliary cirrhosis. 6. Viral hepatitis. 7. Child‐Pugh class C. 8. Serum bilirubin greater than 10 mg/dL. 9. Gastrointestinal bleeding in the previous 15 days. 10. Refractory ascites. 11. Rrenal failure (creatinine greater than 2.5 mg/dL). 12. Cardiac failure. 13. Neoplasia. 14. Other serious illness. | |
| Interventions | Participants were randomly assigned to two groups. Group 1 (n = 29): colchicine 1 mg once daily. Group 2 (n = 26): placebo. Duration: 5 days per week for 6 months | |
| Outcomes | mortality, adverse events. | |
| Notes | Reasons for post‐randomisation dropouts: post‐randomisation: 2 (groupA) and 5 (group B) lost to follow‐up at 6 months, at 3 years 9, at 5 years 14, at 10 years 33/ no information on the number of dropouts in each group of patients. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Computer‐generated randomisation list (blocks of four)". |
| Allocation concealment (selection bias) | Low risk | Quote: "Coded drugs, similar in appearance, concealed from the clinicians. Study drugs identical in appearance, prepared at the hospital pharmacy, coded and distributed to the patient by the hospital pharmacy". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Double blind; therapy prepared by the pharmacist; at no time were the treatment codes disclosed for any patient, attending physicians or investigators". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "At no time were the treatment codes disclosed for any patient, attending physicians or investigators". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "7 patients not considered in the analysis because did not present at the first follow up visit; other drop outs during the following years". Comment: there were post‐randomisation dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: all important outcomes were reported. |
| For‐profit bias | Low risk | Quote: "This study was supported in part by a grant from the Center of Nutrition and Metabolism". |
| Other bias | Low risk | Comment: there was no other bias. |
De 2009.
| Methods | Randomised clinical trial | |
| Participants | Country: India. Number randomised: 70. Post‐randomisation dropouts: 2 (2.9%). Revised sample size: 68. Average age: 47 years. Females: 1 (1.5%). Inclusion criteria: 1. History of chronic alcohol intake of more than 50 g/d. 2. Clinical and biochemical features of severe alcoholic hepatitis [Maddrey Discriminant Function of 32 or greater, aspartate aminotransferase‐ alanine aminotransferase ratio higher than 2 (with AST lower than 500 IU/L and ALT lower than 200 IU/L)]. Exclusion criteria: 1. Acute or chronic viral hepatitis. 2. Autoimmune liver disease. 3. Wilson’s disease. 4. History of abstinence from alcohol in the last month. 5. Seropositivity for HIV. 6. Infection, sepsis or spontaneous bacterial peritonitis. 7. Gastrointestinal bleeding. 8. Hepatorenal syndrome. 9. Acute pancreatitis. 10. Any other severe associated disease (uncontrolled diabetes, hypertension, heart failure, pulmonary disease or malignancy) at the time of inclusion or in the previous 3 months. | |
| Interventions | Participants were randomly assigned to two groups. Group 1 (n = 34): pentoxifylline (400 mg thrice daily ) plus placebo once daily. Group 2 (n = 34): glucocorticosteroids (prednisolone 40 mg once daily) plus placebo. Duration: 4 weeks | |
| Outcomes | mortality, adverse events, decompensated cirrhosis. | |
| Notes | Reasons for post‐randomisation dropouts: 2 withdrawal of consent after randomisation, excluded from the analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The included patients were divided into two groups by a computer‐generated randomisation table". |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "After the initial 4 weeks, the study was opened and the patients allocated to the different groups were revealed". Comment: Further details were not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The investigators who allocated the patients to the groups, administered the drugs and collected the clinical and laboratory data, as well the statisticians, were all blinded regarding the nature of the pharmacotherapy". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "Two patients in group Ⅱ withdrew voluntarily from the study and were excluded". |
| Selective reporting (reporting bias) | Low risk | Comment: all important outcomes were reported. |
| For‐profit bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: there was no other bias. |
De 2014.
| Methods | Randomised clinical trial | |
| Participants | Country: India. Number randomised: 62. Post‐randomisation dropouts: 2 (3.2%). Revised sample size: 60. Average age: 42 years. Females: 0 (0%). Inclusion criteria: 1. History of chronic alcohol intake of more than 50 g/day. 2. Maddrey Discriminant Function of 32 or more. 3. AST:ALT ratio higher than 2 (with absolute value of AST lower than 500 IU/L and ALT lower than 200 IU/L). Exclusion criteria: 1. Acute or chronic viral hepatitis. 2. Autoimmune liver disease. 3. Wilson's disease. 4. HIV‐ positivity. 5. History of abstinence from alcohol in the last month. 6. Infection. 7. Sepsis. 8. Spontaneous bacterial peritonitis. 9. Acute pancreatitis. 10. Gastro‐intestinal bleeding. 11. Hepatorenal syndrome. 12. Uncontrolled diabetes mellitus. 13. Systemic hypertension. 14. Heart failure. 15. Pulmonary disease. 16. Malignancy at the time of inclusion or in the previous 3 months. | |
| Interventions | Participants were randomly assigned to two groups. Group 1 (n = 30): pentoxifylline 400 mg thrice daily plus glucocorticosteroids (prednisolone 40 mg once daily). Group 2 (n = 30): pentoxifylline 400 mg thrice daily. Duration: 4 weeks | |
| Outcomes | mortality, adverse events, decompensated cirrhosis. | |
| Notes | Reasons for post‐randomisation dropouts: one patient in Group 1 developed severe vertigo within 7 days after starting PTX and one patient in Group 2 withdrew voluntarily from the study and hence they were excluded. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The recruited patients were then divided into 2 groups by a computer generated randomisation table". |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The investigator, who allocated the patients to the groups, administered the drugs and collected the clinical and laboratory data, as well as statisticians were all blinded regarding the nature of the pharmacotherapy". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The investigator, who allocated the patients to the groups, administered the drugs and collected the clinical and laboratory data, as well as statisticians were all blinded regarding the nature of the pharmacotherapy". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "One patient in Group 1 developed severe vertigo within 7 days after starting PTX and one patient in Group 2 withdrew voluntarily from the study and hence they were excluded". Comment: there were post‐randomisation dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: all important outcomes were reported. |
| For‐profit bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: there was no other bias. |
De la Maza 1995.
| Methods | Randomised clinical trial | |
| Participants | Country: Chile. Number randomised: 74. Post‐randomisation dropouts: 7 (9.5%). Revised sample size: 67. Average age: 50 years. Females: 11 (16.4%). Inclusion criteria: 1. Two or more of the following: jaundice, encephalopathy, ascites, oedema, spider naevi, marked collateral circulation, bleeding disorders, oesophageal varices on endoscopy. 2. A history of 5 years or more of heavy alcohol consumption (daily alcohol intake greater 150 g). 3. Absence of hepatitis B surface antigen. 4. Absence of significant renal, pulmonary or cardiac disease. 5. Absence of clinical diabetes or malignant tumours (including hepatoma). Exclusion criteria: not reported | |
| Interventions | Participants were randomly assigned to two groups. Group 1 (n = 33): antioxidants (vitamin E 500 mg once daily). Group 2 (n = 34): placebo. Duration: 1 years treatment | |
| Outcomes | mortality. | |
| Notes | Reasons for post‐randomisation dropouts: group A: 4 lack of compliance, Group B: 3 lack of compliance (post‐randomisation). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Eligible patients were randomly and blindly assigned to an experimental or control group. Patients were previously stratified according to their Clinical Combined Laboratory Index ". |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "double blind". Comment: Further details were not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "double blind". Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Four experimental subjects and three controls were removed from the study due to lack of compliance". Comment: there were post‐randomisation dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: all important outcomes were reported. |
| For‐profit bias | High risk | Quote: "Financing was provided by INTA‐University of Chile, Roche and Saval Laboratories". |
| Other bias | Low risk | Comment: there was no other bias. |
De Silva 2003.
| Methods | Randomised clinical trial | |
| Participants | Country: Sri Lanka. Number randomised: 80. Post‐randomisation dropouts: not stated. Revised sample size: 80. Average age: 47 years. Females: 0 (0%). Inclusion criteria: clinical, biochemical, and where possible, histological evidence of alcoholic liver disease. Exclusion criteria: 1. Evidence of oesophageal varices. 2. Hepatic encephalopathy. 3. Other chronic diseases requiring long‐term drug therapy such as diabetes, malignancy, hypertension and others. | |
| Interventions | Participants were randomly assigned to two groups. Group 1 (n = 40): Liv.52 (3 twice daily). Group 2 (n = 40): placebo. Duration: 6 months treatment | |
| Outcomes | adverse events. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization done in India by an individual not associated with the care or assessment of patients, by means of a table of random numbers". |
| Allocation concealment (selection bias) | Unclear risk | This information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double blind, Identical characteristics of drug and placebo". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The investigators who performed blood analysis and those who did clinical assessments were blind to the intervention". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "A significant number of subjects dropped out at various stages of the trial. Despite many attempts, none of the dropouts could be contacted". Comment: there were post‐randomisation dropouts. |
| Selective reporting (reporting bias) | High risk | Comment: some important outcomes which will generally be assessed were not reported. |
| For‐profit bias | High risk | Quote: "Liv.52 and placebo tablets were supplied by Himalaya Drug Co., Bombay, India". |
| Other bias | Low risk | Comment: there was no other bias. |
Depew 1980.
| Methods | Randomised clinical trial | |
| Participants | Country: USA. Number randomised: 28. Post‐randomisation dropouts: 0 (0%). Revised sample size: 28. Average age: 49 years. Females: 12 (42.9%). Inclusion criteria: 1. Alcohol abusers. 2. Clinical diagnosis of severe acute alcoholic hepatitis manifested by hepatomegaly, leucocytosis and a serum bilirubin greater than 5 mg/dL. 3. Spontaneous hepatic encephalopathy in the absence of gastrointestinal haemorrhage, sedation, diuretic usage, or major electrolyte disturbances. Exclusion criteria: 1. Severe diabetes. 2. Active tuberculosis. 3. Serious bacterial infection. | |
| Interventions | Participants were randomly assigned to two groups. Group 1 (n = 15): glucocorticosteroids (prednisolone 40 mg once daily). Group 2 (n = 13): placebo. Duration: 28 days followed by tapered withdrawal over the ensuing 14 days | |
| Outcomes | mortality, adverse events, decompensated cirrhosis. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "all patients fulfilling the criteria who gave informed consent were randomised into two treatment protocols". Comment: Further details were not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "neither the principal investigator nor the physicians attending the patients were aware of the identity of the coded drugs". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: Further details were not available. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no post‐randomisation dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: all important outcomes were reported. |
| For‐profit bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: there was no other bias. |
Diaz Belmont 1996.
| Methods | Randomised clinical trial | |
| Participants | Country: Mexico. Number randomised: 45. Post‐randomisation dropouts: not stated. Revised sample size: 45. Average age: 41 years. Females: 5 (11.1%). Inclusion criteria: 1. ALD. 2. At least 3 months of active drinking before the beginning of the study. 3. Signed consent form. 4. Negative viral hepatitis markers. 5. Maddrey discriminant function higher of equal to 32. Exclusion criteria: 1. Grade IV hepatic encephalopathy. 2. Other cause of liver disease. 3. Doubtful alcoholic aetiology. 4. Other chronic co‐morbidities (TB, diabetes, active gastrointestinal bleeding). | |
| Interventions | Participants were randomly assigned to two groups. Group 1 (n = 22): s‐adenosyl‐L‐methionine (200 mg). Group 2 (n = 23): placebo. Duration: 15 days | |
| Outcomes | adverse events. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomized". Comment: Further details were not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "Double‐blind". Comment: Further details were not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Double‐blind". Comment: Further details were not available. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no post‐randomisation dropouts. |
| Selective reporting (reporting bias) | High risk | Comment: mortality was not reported. |
| For‐profit bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: there was no other bias. |
Feher 1989.
| Methods | Randomised clinical trial | |
| Participants | Country: Hungary. Number randomised: 36. Post‐randomisation dropouts: not stated. Revised sample size: 36. Average age: 46 years. Females: 9 (25%). Inclusion criteria: ALD Exclusion criteria: other causes of liver disease | |
| Interventions | Participants were randomly assigned to two groups. Group 1 (n = 17): silymarin 140 mg thrice daily. Group 2 (n = 19): placebo. Duration: 6 months treatment | |
| Outcomes | adverse events. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Random code". Comment: Further details were not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "Double‐blind". Comment: Further details were not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Double‐blind". Comment: Further details were not available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: mortality was not reported. |
| For‐profit bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: there was no other bias. |
Fenster 1966.
| Methods | Randomised clinical trial | |
| Participants | Country: USA. Number randomised: 32. Post‐randomisation dropouts: 5 (15.6%) Revised sample size: 27. Average age: 49 years. Females: 15 (55.6%). Inclusion criteria: 1. Clinical and laboratory evidence of chronic alcoholic liver disease. 2. Evidence of liver parenchymal dysfunction (distinct from signs of portal hypertension). 3. No gross gastro‐intestinal bleeding. 4. No evidence of prostatic carcinoma. Exclusion criteria: not reported. | |
| Interventions | Participants were randomly assigned to two groups. Group 1 (n = 17): anabolic steroids (methenolone 100 mg once daily or testosterone 100 mg once daily given randomly) Group 2 (n = 10): placebo. Duration: 1 month treatment | |
| Outcomes | mortality. | |
| Notes | Reasons for post‐randomisation dropouts: although they initiated treatment, they were not included in the analysis because they did not complete treatment schedule. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomised by coded names". |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "Double blind". Comment: Further details were not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: there were post‐randomisation dropouts. |
| Selective reporting (reporting bias) | High risk | This information was not available. |
| For‐profit bias | High risk | Quote: "medications provided by Squibb & Sons". |
| Other bias | Low risk | Comment: there was no other bias. |
Fernandez‐Rodriguez 2008.
| Methods | Randomised clinical trial | |
| Participants | Country: Spain. Number randomised: 24. Post‐randomisation dropouts: 0 (0%). Revised sample size: 24. Average age: 53 years. Females: 4 (16.7%). Inclusion criteria: cirrhosis of alcoholic origin. Exclusion criteria: 1. Aetiology other than alcohol abuse (infection with B or C hepatitis viruses). 2. Active or recent gastrointestinal bleeding (one week prior to inclusion). 3. Grade III‐IV hepatic encephalopathy. 4. Primary renal or cardiopulmonary disease. 5. Insulin‐requiring diabetes mellitus. 6. Ongoing bacterial infection. 7. Complete portal vein thrombosis. 8. Receiving drugs with haemodynamic effects such as β‐blockers, nitrites or non‐steroidal anti‐inflammatory drugs. | |
| Interventions | Participants were randomly assigned to two groups. Group 1 (n = 12): pentoxifylline 400 mg thrice daily. Group 2 (n = 12): placebo. Duration: 4 weeks | |
| Outcomes | adverse events. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Computer‐generated random series performed by a member of the pharmacy". |
| Allocation concealment (selection bias) | Low risk | Quote: "The sequence was concealed until intervention was assigned. An allocation code was kept in sealed envelopes in the hospital’s pharmacy until data analysis". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Patients, as well as researchers, were blinded to treatment assignment". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Patients, as well as researchers, were blinded to treatment assignment". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no post‐randomisation dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: all important outcomes were reported. |
| For‐profit bias | High risk | Quote: "Ferrer Pharmaceutical Company supplied the placebo". |
| Other bias | Low risk | Comment: there was no other bias. |
Fleig 1997.
| Methods | Randomised clinical trial | |
| Participants | Country: Germany/UK. Number randomised: 188. Post‐randomisation dropouts: not stated. Revised sample size: 188. Average age: 52 years. Females: 73 (38.8%). Inclusion criteria: Biopsy‐proven alcoholic cirrhosis Exclusion criteria: not reported | |
| Interventions | Participants were randomly assigned to two groups. Group 1 (n = 98): Liv.52 300 mg thrice daily. Group 2 (n = 90): placebo. Duration: 2 years | |
| Outcomes | mortality. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomised after stratification for disease severity". |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "double‐blind". Comment: Further details were not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "double‐blind". Comment: Further details were not available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: not reported if loss to follow up or dropouts. |
| Selective reporting (reporting bias) | High risk | Comment: not clear adverse events. |
| For‐profit bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: there was no other bias. |
Garrido Garcia 2012.
| Methods | Randomised clinical trial | |
| Participants | Country: Mexico. Number randomised: 60. Post‐randomisation dropouts: 0 (0%). Revised sample size: 60. Average age: 43 years. Females: 4 (6.7%). Inclusion criteria: 1. Clinical and biochemical criteria for alcoholic hepatitis. 2. Maddrey DF higher or equal than 32. Exclusion criteria: 1. Pregnancy. 2. serious bacterial infections at inclusion. 3. Cancer. 4. Other chronic co‐morbidities such as diabetes mellitus, hypertension, heart disease. 4. HIV‐infection. 5. Use of potentially hepatotoxic drugs. 6. Previous use of steroids. | |
| Interventions | Participants were randomly assigned to two groups. Group 1 (n = 30): pentoxifylline 400 mg thrice daily (total 1200 mg/day). Group 2 (n = 30): glucocorticosteroids (prednisolone 40 mg once daily). Duration: 28 days | |
| Outcomes | mortality, adverse events, decompensated cirrhosis. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomized". |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Different treatments. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no post‐randomisation dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: all important outcomes were reported. |
| For‐profit bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: there was no other bias. |
Geminiani 1979.
| Methods | Randomised clinical trial | |
| Participants | Country: Italy. Number randomised: 20. Post‐randomisation dropouts: not stated. Revised sample size: 20. Average age: 42 years. Females: 5 (25%). Inclusion criteria: alcoholic for at least 2 years, relatively constant alcohol intake. Exclusion criteria: not reported. | |
| Interventions | Participants were randomly assigned to two groups. Group 1 (n = 10): cycloxilic acid 240 mg/day. Group 2 (n = 10): placebo. Duration: 1 month treatment | |
| Outcomes | mortality. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomized". Comment: Further details were not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "Double‐blind". Comment: Further details were not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Double‐blind". Comment: Further details were not available. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no post‐randomisation dropouts. |
| Selective reporting (reporting bias) | High risk | Comment: adverse events were not reported. |
| For‐profit bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: there was no other bias. |
Gluud 1986.
| Methods | Randomised clinical trial | |
| Participants | Country: Denmark. Number randomised: 221. Post‐randomisation dropouts: 0 (0%). Revised sample size: 221. Average age: 53 years. Females: 0 (0%). Inclusion criteria: 1. A daily ethanol consumption greater than 50 g for more than 2 years. 2. Cirrhosis diagnosed by biopsy within last 6 months. 3. Specific aetiology of cirrhosis other than ethanol could be excluded Exclusion criteria: 1. Patients unable to co‐operate or who refused to give consent. 2. HBsAg‐ positivity. 3. HCC or other malignancies. 4. Other significant diseases. 5. Other non‐disease‐related causes (e.g., immigration). 6. Previous treatment with anabolic‐androgenic steroids. | |
| Interventions | Participants were randomly assigned to two groups. Group 1 (n = 134): anabolic steroids (testosterone 100 mg once daily). Group 2 (n = 87): placebo. Duration: 8 to 62 months (median 28) | |
| Outcomes | mortality, hepatocellular carcinoma. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization by skewed randomisation 3:2 in each hospital (series of 10 serially numbered boxes with 6 of test and 4 of placebo); then each patient allocated at the lowest box number". |
| Allocation concealment (selection bias) | Low risk | Quote: "numbered boxes". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double‐blind; drugs and placebo of identical appearance, taste and smell". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Double‐blind; physicians not allowed to know the testosterone serum levels". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "There were drop‐outs, but criteria well‐explained at the beginning of the study and all included in the analysis". |
| Selective reporting (reporting bias) | High risk | Quote: "Adverse events not quantified or clearly reported". Comment: some important outcomes which will generally be assessed were not reported. |
| For‐profit bias | Low risk | Quote: " supported by medical research foundations". |
| Other bias | Low risk | Comment: there was no other bias. |
Halle 1982.
| Methods | Randomised clinical trial | |
| Participants | Country: USA. Number randomised: 71. Post‐randomisation dropouts: 4 (5.6%). Revised sample size: 67. Average age: 39 years. Females: 6 (9%). Inclusion criteria: 1. Recent, heavy alcohol ingestion. 2. Serum bilirubin of 5 mg/dL or more. 3. At least one of the following: hepatic tenderness, fever above 100°F, or leukocytosis above 12,000 mm3 Exclusion criteria: 1. Serious bacterial infection. 2. Massive gastrointestinal bleeding. 3. Pre‐existing renal failure (serum creatinine greater than 2.5 mg/dL). 4. A previous or current thyroid disease. | |
| Interventions | Participants were randomly assigned to two groups. Group 1 (n = 31): propylthiouracil 75 mg four times daily. Group 2 (n = 36): placebo. Duration: 6 weeks | |
| Outcomes | mortality, adverse events, decompensated cirrhosis. | |
| Notes | Reasons for post‐randomisation dropouts: 2 refused treatment, 2 had bilirubin lower than 5 g/dL. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "all patients fulfilling the criteria who gave informed consent were randomised into two treatment groups". Comment: Further details were not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: ".identical placebo". "The investigators were not aware of which regimen the patient was receiving until the study ended". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: Further details were not available. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Two patients refused therapy on the first day; in 2 other patients treatment was discontinued as bilirubin was <5 mg/dl at randomisation". Comment: there were post‐randomisation dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: all important outcomes were reported. |
| For‐profit bias | Low risk | Quote: "This work was supported by the Hastings Foundation, Los Angeles, California. Dr. Halle received a Fellowship from the Canadian Liver Foundation and Dr. Pare from the Medical Research Council (Canada)". |
| Other bias | Low risk | Comment: there was no other bias. |
Helman 1971.
| Methods | Randomised clinical trial | |
| Participants | Country: USA. Number randomised: 37. Post‐randomisation dropouts: 0 (0%). Revised sample size: 37. Average age: 48 years. Females: 25 (67.6%). Inclusion criteria: 1. Alcoholic hepatitis confirmed by percutaneous needle biopsy before inclusion in the study. Exclusion criteria: 1. If a biopsy could not be obtained within the first week of hospitalisation. 2. Gastrointestinal bleeding requiring transfusion. 3. The purified protein derivative (PPD) test was positive | |
| Interventions | Participants were randomly assigned to two groups. Group 1 (n = 20): glucocorticosteroids (prednisolone 40 mg once daily). Group 2 (n = 17): placebo. Duration: 4 weeks | |
| Outcomes | mortality, cirrhosis. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were selected by a random, double‐blind technique". Comment: Further details were not available. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "drug treatment was randomly determined by the hospital pharmacist". Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "..without informing physicians, nurses, or patients until completion of the study". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no post‐randomisation dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: all important outcomes were reported. |
| For‐profit bias | Low risk | Quote: "supported in part by grants ES00129 AM05503, and AM090C0, U. S. Public Health Service". Comment: this information was not available. |
| Other bias | Low risk | Comment: there was no other bias. |
Higuera de la Tijera 2015.
| Methods | Randomised clinical trial | |
| Participants | Country: Mexico. Number randomised: 135. Post‐randomisation dropouts: 0 (0%). Revised sample size: 135. Average age: 43 years. Females: 11 (8.1%). Inclusion criteria: 1. Age between 18 and 65 years old. 2. History of heavy and chronic alcohol intake (more than 80 g per day within the previous 5 years). 3. Rapid onset of jaundice in the absence of biliary tract obstruction by ultrasound. 4. Painful hepatomegaly. 5. Ascites. 6. Transaminases increase more than two times above the normal value. 7. An aspartate aminotransferase/alanine aminotransferase ratio greater than 2 times normal. 8. Leucocytosis with a predominance of neutrophils. 9. Total bilirubin of more than 5 mg/dL. 10. Maddrey discriminant factor greater than 32. Exclusion criteria: 1. Acquired immunodeficiency syndrome. 2. Neoplasms. 3. Autoimmune diseases. 4. Psychiatric disorders different from alcoholism. 5. History of atopy or asthma. 6. Diabetes. 7. Pregnancy. 8. Hepatits B virus. 9. Hepatitis C virus. 10. Tuberculosis. 11. Intake of illicit drugs, herbal products, antioxidant supplements, or previous treatment with steroids or pentoxifylline within the previous 2 years. 12. Patients without family support or without access to telephone communication. | |
| Interventions | Participants were randomly assigned to four groups.
Group 1 (n = 35): glucocorticosteroids (prednisolone 40 mg once daily) plus metadoxine (500 mg thrice daily).
Group 2 (n = 35): glucocorticosteroids (prednisolone 40 mg once daily). Group 3 (n = 32): pentoxifylline (400 mg thrice daily) plus metadoxine (500 mg thrice daily). Group 4 (n = 33): pentoxifylline (400 mg thrice daily). Duration: 30 days |
|
| Outcomes | mortality, adverse events, decompensated cirrhosis. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "For the randomization, we used the Epidat 3.1 statistical program to construct a table of random numbers considering groups of equal size. For each patient, once verified if he or she met with the selection criteria, we assigned to a group of treatment according to the table of random numbers (author's reply)". |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "The present study was a randomized, open‐label clinical study". |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "The present study was a randomized, open‐label clinical study". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: all patients were included for analysis. |
| Selective reporting (reporting bias) | Low risk | Comment: all important outcomes were reported. |
| For‐profit bias | Low risk | Quote: "This work was partially sypported by funds granted to Higuera de la Tijera through the stimulus "Angeles Espinosa Yglesias 2010" granting FUNSALUD AC, AMPARO Foundation and FUNDHEPA AC, Mexico. Eurodrug laboratories de mexico s.a. donated metadoxine to Mexico' General Hospital for this study". |
| Other bias | Low risk | Comment: there was no other bias. |
Keiding 1994.
| Methods | Randomised clinical trial | |
| Participants | Country: UK/Denmark/Spain. Number randomised: 407. Post‐randomisation dropouts: 0 (0%). Revised sample size: 407. Average age: 49 years. Females: not stated Inclusion criteria: 1. A daily estimated alcohol intake of 80 g or more for the preceding 4 years. 2. A liver biopsy compatible with alcoholic liver disease taken within 6 months before the entry to the study, unless biopsy was impossible because of bleeding tendency, or the patient refused liver biopsy and the physicians of the liver unit had other evidence confirming the diagnosis. 3. The patient gave informed consent. 4. Age between 20 and 80 years of old. Exclusion criteria: 1. Advanced encephalopathy. 2. Other aetiology for chronic liver disease. 3. Other drug with intended effects similar to those of malotilate had been given during the preceding 6 months. 4. Other disease with expected survival less than 6 months was detectable. 5. Pregnancy. | |
| Interventions | Participants were randomly assigned to two groups. Group 1 (n = 270): malotilate (750 mg/d or 1500 mg/d given randomly) thrice daily. Group 2 (n = 134): placebo. Duration: 3 years | |
| Outcomes | mortality, adverse events, decompensated cirrhosis. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were allocated randomly to the three treatment groups within each centre in blocks of six patients ". |
| Allocation concealment (selection bias) | Low risk | Quote: "…using the sealed envelopes method". |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "In the present study the effect of malotilate on the survival of patients with alcoholic liver disease was examined in a multicenter, international, double‐blind, randomised, placebo‐controlled clinical trial. The tablets had identical appearance and taste (Zyma 15057)". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Sixty‐eight patients were lost to follow‐up (26, 23, and 19 patients in the three above‐mentioned treatment groups, respectively)". Comment: there were post‐randomisation dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: all important outcomes were reported. |
| For‐profit bias | High risk | Quote: "supported by Zyma SA, Nyon, Switzerland, and Nihon Nohyaku, Tokyo". |
| Other bias | Low risk | Comment: there was no other bias. |
Kim 2012.
| Methods | Randomised clinical trial | |
| Participants | Country: South Korea. Number randomised: 95. Post‐randomisation dropouts: not stated. Revised sample size: 95. Average age: 51 years. Females: 15 (15.8%). Inclusion criteria: 1. Age between 18 and 70 years. 2. Chronic alcoholic liver diseases. 3. Discontinuation of alcohol intake for at least 6 months before starting the study. Exclusion criteria: 1. Decompensated liver disease/ cirrhosis. 2. Hepatocellular carcinoma or other malignancies. 3. Hepatitis B, hepatitis C infection. 4. Autoimmune liver disease. 5. Previous alcohol intake lower than 140 g/week. | |
| Interventions | Participants were randomly assigned to two groups. Group 1 (n = 42): candesartan 8 mg plus ursodeoxycholic acid 600 mg/day. Group 2 (n = 43): ursodeoxycholic acid 600 mg/day. Duration: 6 months | |
| Outcomes | adverse events. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were chronologically randomised into two groups by the pharmacy at Wonju Christian Hospital using serially numbered sealed envelopes in batches of 90 that designated a patient to one of two treatments". |
| Allocation concealment (selection bias) | Low risk | Quote: "sealed envelopes". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Open to patients and investigators". |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Open to patients and investigators". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "12 patients were withdrawn from the study during the 6 months due to loss to follow up, low medical compliance and ingestion of alcohol". Comment: ITT analysis for fibrosis stage and scores, not for serum markers of fibrosis. |
| Selective reporting (reporting bias) | High risk | Comment: mortality was not reported. |
| For‐profit bias | Low risk | Quote: "This work was supported by Yonsei University Wonju College of Medicine Research Fund of 2008 and also by a grant from the Korea Healthcare technology R&D Project, Ministry of Health and Welfare, Republic of Korea. (A102065). No financial support from any pharmaceutical companies producing Angiotensin II type 1 receptor blocking agent (ARB) related drugs". |
| Other bias | Low risk | Comment: there was no other bias. |
Lebrec 2010.
| Methods | Randomised clinical trial | |
| Participants | Country: France. Number randomised: 133. Post‐randomisation dropouts: 0 (0%). Revised sample size: 133. Average age: 55 years. Females: not stated Inclusion criteria: 1. Child‐Pugh class C biopsy proven cirrhosis (higher than 9 points) 2. Age higher than 18 years. Exclusion criteria: 1. Pregnancy. 2. Anticoagulant treatment. 3. Noncorticosteroid immunosuppressive drugs. 4. Treated arterial hypertension. 5. Severe coronary artery disease. 6. HIV infection. 7. Hypersensitivity to pentoxifylline. 8. Transplanted patients. 9. Pentoxifylline treatment in the 3 months before the study. 10. Advanced hepatocellular carcinoma. 11. Associated illnesses with a life expectancy of 1 month. 12. Patients who could not be regularly followed up. | |
| Interventions | Participants were randomly assigned to two groups. Group 1 (n = 71): pentoxifylline 400 mg thrice daily. Group 2 (n = 62): placebo. Duration: 6 months | |
| Outcomes | mortality. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was computer‐generated". |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "Double‐blind. Pentoxifylline in opaque capsule form or identical capsules containing the placebo". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Events were recorded without knowing the randomisation code". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: subanalysis on subgroup of patients with alcoholic hepatitis, there were dropouts, not clear if patients from this group. |
| Selective reporting (reporting bias) | Low risk | Comment: all important outcomes were reported. |
| For‐profit bias | Unclear risk | Quote: "Supported by a grant from the French Ministry of Health. Pentoxifylline and placebo were purchased from Sanofi‐Aventis, Sanofi‐Aventis did not participate in any part of the study, including study design, data analysis, and manuscript preparation". |
| Other bias | Low risk | Comment: there was no other bias. |
Lotterer 1995.
| Methods | Randomised clinical trial | |
| Participants | Country: Germany. Number randomised: 12. Post‐randomisation dropouts: 0 (0%). Revised sample size: 12. Average age: not stated. Females: 4 (33.3%). Inclusion criteria: 1. Biopsy proven alcoholic cirrhosis of the liver. 2. Consumption of more than 60 g of alcohol daily for more than 10 years. 3. Abstinence for more than 8 weeks prior to initiation of treatment. Exclusion criteria: 1. Other viral active hepatitis. 2. HCC. | |
| Interventions | Participants were randomly assigned to two groups. Group 1 (n = 6): Liv.52 four times daily. Group 2 (n = 6): placebo. Duration: 24 weeks | |
| Outcomes | adverse events. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "prospective, randomised, placebo‐controlled, double‐blind and cross‐over design ". Comment: Further details were not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "prospective, randomised, placebo‐controlled, double‐blind and cross‐over design ". Comment: Further details were not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "There were no dropouts ". Comment: there were no post‐randomisation dropouts. |
| Selective reporting (reporting bias) | High risk | Comment: mortality was not reported. |
| For‐profit bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: there was no other bias. |
Lucena 2002.
| Methods | Randomised clinical trial | |
| Participants | Country: Spain. Number randomised: 60. Post‐randomisation dropouts: 11 (18.3%). Revised sample size: 49. Average age: 49 years. Females: 1 (2%). Inclusion criteria: 1. Age between 20 and 70 years. 2. Diagnosis of alcoholic cirrhosis within 5 years previous to inclusion and based on clinical and laboratory data with evidence of portal hypertension and ultrasound or upper gastrointestinal endoscopy. 3. Daily alcohol intake of 60 g or more in men and 40 g or more in women over a period of more than 5 years. 4. Histological diagnosis of cirrhosis within 6 months before inclusion if applicable (patient's refusal or coagulation disorders). Exclusion criteria: other causes of liver disease, HCC, anticipated need for LT within 1 year, age < 20 > 70 years, immunosuppression | |
| Interventions | Participants were randomly assigned to two groups. Group 1 (n = 24): silymarin 150 mg thrice daily. Group 2 (n = 25): placebo. Duration: 6 months | |
| Outcomes | None of the outcomes of interest were reported. | |
| Notes | 11 patients were withdrawn for different reasons, of these five because of hepatic decompensation, one of whom died, and 1 because of an adverse event. The authors have therefore considered just 49 patients for the analysis. Of these patients none died or had adverse events. Reasons for post‐randomisation dropouts: 11 withdrawn although receiving treatment (6 vs 5), 3 + 2 for alcohol‐consumption, 2 + 3 for hospital admission due to decompensation (1 died), 1 adverse event (silymarin). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Computerized sequence generation. The patients were assigned according to a balanced, randomly‐generated allocation sequence". Comment: according to the author's reply. |
| Allocation concealment (selection bias) | Low risk | Quote: "Sealed envelopes kept at the pharmacy department". Comment: according to the author's reply. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "…a placebo of identical appearance". Comment: according to the author's reply. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: according to the author's reply. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Eleven patients, 6 in the silymarin groups and 5 in the placebo group did not complete the trial and were withdrawn". Comment: there were post‐randomisation dropouts. |
| Selective reporting (reporting bias) | High risk | Comment: none of the outcomes of interest were reported. |
| For‐profit bias | High risk | Quote: "Silymarin and placebo were kindly supplied by Madaus, Cerafarm, Barcelona, Spain". |
| Other bias | Low risk | Comment: there was no other bias. |
Maddrey 1978.
| Methods | Randomised clinical trial | |
| Participants | Country: USA. Number randomised: 57. Post‐randomisation dropouts: 1 (1.8%). Revised sample size: 56. Average age: 41 years. Females: 20 (35.7%). Inclusion criteria: 1. History of long‐standing and recent alcoholism. 2. Percutaneous liver biopsy if coagulation parameters permitted. Exclusion criteria: 1. Active gastrointestinal bleeding. 2. Pancreatitis. 3. History of peptic ulcer disease. 4. Active infection. 5. Presence of hepatitis B antigen. 6. History of previous viral hepatitis. | |
| Interventions | Participants were randomly assigned to two groups. Group 1 (n = 25): glucocorticosteroids (prednisolone 5 mg once daily). Group 2 (n = 31): placebo. Duration: 28‐32 days | |
| Outcomes | mortality, adverse events. | |
| Notes | Reasons for post‐randomisation dropouts: one patient who was randomised to the placebo group bled from oesophageal varices before receiving the study drug. He subsequently stopped bleeding and survived. Another patient had an episode of upper gastrointestinal haemorrhage presumably from oesophageal varices after receiving prednisolone for 9 days and the drug was stopped. This patient subsequently survived but has not been included in the analysis because the study drug was discontinued. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "the study was conducted in a randomised double blind fashion". Comment: Further details were not available. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Random drug sequences were arranged within each group". Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The study was conducted in a randomised double blind fashion". "The investigators were not aware of which regimen the patient was receiving until the completion of the study". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The investigators were not aware of which regimen the patient was receiving until the completion of the study". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "One patient who was randomised to the placebo group bled from oesophageal varices before receiving the study drug. He subsequently stopped bleeding and survived. Another patient had an episode of upper gastrointestinal haemorrhage after receiving prednisolone for 9 days and the drug was stopped. This patient subsequently survived but has not been included in the analysis". Comment: there were post‐randomisation dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: all important outcomes were reported. |
| For‐profit bias | Low risk | Quote: "This study was supported by Research Grant AA00201 from the National Institute of Alcohol Abuse and Alcoholism of the National Institutes of Health, and by Grant RR‐35 from the Clinical Research Centers Program, United States Public Health Service. Prednisolone (5 mg) and identical placebo tablets were provided by the Division of Steroid Research, The Upjohn Company, Kalamazoo, Mich.)". |
| Other bias | Low risk | Comment: there was no other bias. |
Mathurin 2013.
| Methods | Randomised clinical trial | |
| Participants | Country: Belgium and France. Number randomised: 278. Post‐randomisation dropouts: 8 (2.9%). Revised sample size: 270. Average age: 52 years. Females: 107 (39.6%). Inclusion criteria: 1. Age between 18 and 70. 2. Heavy drinkers (more than 40 g/d of alcohol for women and more than 50 g/d for men). 3. Biopsy‐proven alcoholic hepatitis. 4. Recent onset of jaundice within the past 3months. 5. A Maddrey score of at least 32. Exclusion criteria: 1. Presence of hepatitis B surface antigen, hepatitis C virus or HIV antibodies. 2. Pregnancy. 3. Breastfeeding. 4. Concomitant or previous history of hepatocellular carcinoma. 5. Evolutive neoplasia likely to threaten 1‐year outcome. 6. Uncontrolled bacterial infection within 7 days. 7. Concomitant or previous history of fungal, viral, or parasitic infection. 8. Severe associated disease (cardiac failure, severe pulmonary disease, neoplastic disease, severe psychiatric disorders). 9. Portal thrombosis. 10. Acute pancreatitis. 11. Type 1 hepatorenal syndrome. 12. Serum creatinine at randomisation of more than 2.5 mg/dL. | |
| Interventions | Participants were randomly assigned to two groups. Group 1 (n = 133): glucocorticosteroids (prednisolone 40 mg) plus pentoxifylline (400 mg thrice daily). Group 2: (n = 137): glucocorticosteroids (prednisolone 40 mg) plus placebo. Duration: 28 days | |
| Outcomes | mortality, adverse events, decompensated cirrhosis, liver transplantation. | |
| Notes | Reasons for post‐randomisation dropouts: 7 did not meet the criteria for severe AAH, 1 withdrawal (post‐randomisation). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Eligible patients were randomly assigned at a 1:1 ratio. Randomization was centralized and patients were assigned in blocks of 6 by a computerized procedure to achieve a balance between the 2 groups, with stratification according to center". |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Double‐blind. One patient was excluded for not blinded administration of pentoxifylline by the general practitioner during the treatment period". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Double‐blind". Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "missing data did not exceed the 10%, two patients with HRS were excluded from the analysis". Comment: there were post‐randomisation dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: all important outcomes were reported. |
| For‐profit bias | High risk | Quote: "Supported by the Hospital‐Based Clinical Research Program. PTX and placebo provided by Sanofi‐Aventis Pharmaceuticals". |
| Other bias | Low risk | Comment: there was no other bias. |
Mato 1999.
| Methods | Randomised clinical trial | |
| Participants | Country: Spain. Number randomised: 123. Post‐randomisation dropouts: 0 (0%). Revised sample size: 123. Average age: 52 years. Females: 17 (13.8%). Inclusion criteria: 1. Age over 18 years. 2. History of ethanol consumption over 80 g per day for women and over 120 g per day for men, for more that 5 years. 3. Physical examination and biochemical tests compatible to alcoholic cirrhosis. 4. Confirmation with liver biopsy when applicable. Exclusion criteria: 1. Total serum bilirubin equal to or higher than 3 mg/dL. 2. Refractory ascites or gastrointestinal bleeding or encephalopathy within 1 week before entry into the trial. 3. Hepatocellular carcinoma. 4. Other treatments such as colchicine, malotilate, silymarin, penicillamine or corticosteroids. 5. Other aetiologies for liver cirrhosis. | |
| Interventions | Participants were randomly assigned to two groups. Group 1 (n = 62): s‐Adenosylmethionin 400 mg thrice daily. Group 2 (n = 61): placebo. Duration: 2 years | |
| Outcomes | mortality, adverse events, liver transplantation, hepatocellular carcinoma. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "According to centralized randomisation by a number table…". |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "….or a placebo of identical appearance, smell and taste, with the same schedule". Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "There were pt lost to follow up, assumption not specified. Ten patients (8%) were lost to follow‐up (three patients in the placebo group and seven patients in the AdoMet group), and their survival status was unknown at the close of the study, although four of the ten patients (two in each group) were alive in the months 23‐24 of follow‐up". Comment: presumably ITT. |
| Selective reporting (reporting bias) | Low risk | Comment: all important outcomes were reported. |
| For‐profit bias | High risk | Quote: "Supported by the Laboratories Europharma, S.A. group Boehringer Ingelheim Espafia, S.A., Madrid, Spain; Laboratories Knoll Farmaceutici Spa, L&ate, Milan, Italy; grant from the Comision Asesora de Investigacion Cientifica y Tecnica (SAF 96/0108), and (SAF98/0 132), Ministerio de Education y Ciencia, Spain; grant from the Fondo de Investigaciones Sanitarias (FIS 94/0231), Spain". |
| Other bias | Low risk | Comment: there was no other bias. |
McHutchinson 1991.
| Methods | Randomised clinical trial | |
| Participants | Country: USA. Number randomised: 22. Post‐randomisation dropouts: 0 (0%). Revised sample size: 22. Average age: not stated Females: not stated Inclusion criteria: 1. Bilirubin of 10 mg/dL or greater. 2. Prothrombin ratio of 40% or lower. 3. White blood count of 12,000 mm^3 or greater. 4. Tender hepatomegaly. 5. Fever of 100°F or greater. Exclusion criteria: not reported | |
| Interventions | Participants were randomly assigned to two groups. Group 1 (n = 12): pentoxifylline 1200 mg. Group 2 (n = 10): standard treatment. Duration: 10 days | |
| Outcomes | mortality. | |
| Notes | Abstract | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomized". Comment: Further details were not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: some important outcomes which will generally be assessed were not reported. |
| For‐profit bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: there was no other bias. |
Medici 2011.
| Methods | Randomised clinical trial | |
| Participants | Country: USA. Number randomised: 37. Post‐randomisation dropouts: not stated. Revised sample size: 37. Average age: 45 years. Females: 12 (32.4%). Inclusion criteria: 1. A positive history for chronic alcohol abuse according to World Health Organization definition and the AUDIT screening test. 2. ALD Exclusion criteria: 1. Child‐Pugh score>10. 2. Positive laboratory tests for chronic hepatitis B or C. 3. Primary biliary cirrhosis. 4. Autoimmune hepatitis. 5. Wilson disease. 6. Haemochromatosis. 7. Hepatocellular carcinoma with alpha‐fetoprotein >10 times the upper limit of normal. 8. Cancer. 9. Congestive heart failure. 10. Renal insufficiency with serum creatinine >1.2 mg/mL. 11. Use of antifolate drugs or corticosteroids. 12. Infectious illness. | |
| Interventions | Participants were randomly assigned to two groups. Group 1 (n = 18): s‐adenosyl‐L‐methionine 1.2 g/day. Group 2 (n = 19): placebo. Duration: 24 weeks treatment | |
| Outcomes | adverse events. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Volunteer ALD patients were randomly assigned by an independent UC Davis Medical Center Investigational Drug Service pharmacist in a 1:1 ratio to receive SAM or matching placebo, using a computer‐generated allocation sequence". |
| Allocation concealment (selection bias) | Low risk | Quote: "A sealed, opaque envelope was used to conceal the randomisation scheme and was kept by the independent pharmacist". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The study subjects, care providers, study coordinator, and those assessing outcomes were all blinded to intervention". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The study subjects, care providers, study coordinator, and those assessing outcomes were all blinded to intervention". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "11 patients were withdrawn from the study for resumed active drinking". Comment: iTT analysis for serum parameters, not for scores. |
| Selective reporting (reporting bias) | High risk | Comment: Comment: mortality was not reported. |
| For‐profit bias | High risk | Quote: "Supported by R01AA14562 (CHH), R01AG09834 (S.P.S.), P50 011999 (SWF), R01DK072398 (JFG), and UL1 RR024146 from the National Center for ResearchResources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. S‐adenosyl‐L‐methionine was provided as a generous gift from Abbott Laboratories. The funding agencies had no role in the study design, data analysis and interpretation, nor in drafting the manuscript". |
| Other bias | Low risk | Comment: there was no other bias. |
Mendenhall 1984.
| Methods | Randomised clinical trial | |
| Participants | Country: USA. Number randomised: 178. Post‐randomisation dropouts: 0 (0%). Revised sample size: 178. Average age: 51 years. Females: 0 (0%). Inclusion criteria: Diagnosis of moderate or severe alcoholic hepatitis based on conventional clinical and laboratory changes. Exclusion criteria: 1. Positive test for HBsAg. 2. Clinical or historical evidence of recent parenteral drug abuse. 3. Intractable congestive heart failure. 4. Neoplasms that commonly metastasise to the liver. 5. NAFLD. 6. Severe infections. 7. Active peptic ulcer disease. 8. Insulin‐dependent diabetes mellitus. 9. Corticosteroids within the preceding 3 months. | |
| Interventions | Participants were randomly assigned to three groups.
Group 1 (n = 90): glucocorticosteroids (prednisolone 60 mg tapered to 5 mg in 1 month).
Group 2 (n = 88): placebo. Group 3 (n=85): anabolic steroids (oxandrolone 80 mg). Duration: 30 days treatment |
|
| Outcomes | mortality. | |
| Notes | 3 groups | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Treatment assignments were made by the Coordinating Center. The random assignment of tretment was balanced within each hospital, as well as according to disease severity". |
| Allocation concealment (selection bias) | Unclear risk | Comment: Further details were not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The patient, physician and the local hospital pharmacy had no knowledge of the specific medication in use". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: " Ten patients withdrew from the study before completing treatment, however they were included in the outcome analysis. If patients were lost to follow up after hospital‐discharge the VABIRL System was used to determine their status and the date of death". Comment: presumably ITT analysis. |
| Selective reporting (reporting bias) | Low risk | Quote: "Not precisely specified". Comment: all important outcomes were reported. |
| For‐profit bias | Unclear risk | Quote: "Supported by Cooperative Studies program of VAMRS. Matching placebos were prepared for each of these medications by Upjohn Company and G.D. Searle and Company". |
| Other bias | Low risk | Comment: there was no other bias. |
Mezey 2004.
| Methods | Randomised clinical trial | |
| Participants | Country: Spain and USA. Number randomised: 51. Post‐randomisation dropouts: 0 (0%). Revised sample size: 51. Average age: 48 years. Females: 17 (33.3%). Inclusion criteria: 1. Age between 18–70 years. 2. Recent history of heavy alcohol ingestion. 3. Moderate elevation of AST (lower than 10 times above normal. 4. AST/ALT ratio greater than 1. 5. No evidence of viral hepatitis, autoimmune liver disease, haemochromatosis, Wilson’s disease, drug‐induced hepatitis. Exclusion criteria: 1. Pregnancy. 2. Breast feeding. 3. Cardiovascular, pulmonary, kidney disease. 4. Pancreatitis. 5. Type I diabetes. 6. Recent (within 1 month) gastrointestinal bleeding. 7. Peptic ulcer disease. 8. Concurrent infection. 9. History of thrombophlebitis. 10. HIV positivity. 11. History of ingestion of more than 100 I.U. vitamin for the prior month. | |
| Interventions | Participants were randomly assigned to two groups. Group 1 (n = 25): antioxidants (vitamin E 1000 UI). Group 2 (n = 26): placebo. Duration: 3 months | |
| Outcomes | mortality. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Generation of the allocation sequence was done on a computer in blocks of 4". |
| Allocation concealment (selection bias) | Low risk | Quote: "For each patient entered into the trial the investigators opened a consecutive sealed container which had a 3 month supply of capsules". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The investigators and participants were blinded, The vitamin E capsules and placebo capsules, which were prepared and labeled by the hospital pharmacies, were identical in looks, smell, and taste". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The investigators were blinded ". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: there were patients lost to follow‐up: data considered?. |
| Selective reporting (reporting bias) | Low risk | Comment: all important outcomes were reported. |
| For‐profit bias | Low risk | This information was not available. |
| Other bias | Low risk | Comment: there was no other bias. |
Mirouze 1982.
| Methods | Randomised clinical trial | |
| Participants | Country: USA. Number randomised: 26. Post‐randomisation dropouts: 0 (0%). Revised sample size: 26. Average age: not stated Females: not stated Inclusion criteria: 1. Jaundice. 2. Fever. 3. Hepatomegaly. 4. Transaminases elevation. 5. At least 2 of the following: total bilirubin greater than 5 mg/dL, prothrombin time lower than 50 %, leukocytosis higher than 12,000/mm3. Exclusion criteria: not reported | |
| Interventions | Participants were randomly assigned to two groups. Group 1 (n = 14): insulin (36 U) plus 4 mg glucagon. Group 2 (n = 12): no active treatment. Duration: 2 weeks | |
| Outcomes | mortality. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The aim of this randomised clinical trial is ". Comment: Further details were not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | Low risk | Comment: all important outcomes were reported. |
| For‐profit bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: there was no other bias. |
Moreno 2010.
| Methods | Randomised clinical trial | |
| Participants | Country: Belgium. Number randomised: 44. Post‐randomisation dropouts: 0 (0%). Revised sample size: 44. Average age: 49 years. Females: 12 (27.3%). Inclusion criteria: 1. Biopsy proven AAH. 2. Maddrey Discriminant Function of 32 or more Exclusion criteria: not reported | |
| Interventions | Participants were randomly assigned to two groups. Group 1 (n = 25): N‐acetyl‐cysteine 300 mg/kg intravenously. Group 2 (n = 19): placebo. Duration: 14 days | |
| Outcomes | mortality, adverse events. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomised". Comment: Further details were not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | Low risk | Comment: all important outcomes were reported. |
| For‐profit bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: there was no other bias. |
Morgan 2005.
| Methods | Randomised clinical trial | |
| Participants | Country: USA. Number randomised: 549. Post‐randomisation dropouts: 0 (0%). Revised sample size: 549. Average age: 55 years. Females: 11 (2%). Inclusion criteria: 1. Clinical diagnosis of alcoholic cirrhosis (based on a long history of alcohol use and the exclusion of other causes of liver disease. 2. A modified Pugh score of 7 or greater. 3. Liver biopsy demonstrating cirrhosis unless contraindications to biopsy were present. Exclusion criteria: 1. Gastrointestinal bleeding within the prior 28 days requiring transfusion. 2. Illicit drug use in the prior 12 months. 3. HIV infection. 4. Cancer in the prior 10 years. 5. Serum creatinine greater than 1.5 mg/dL. 6. Total white blood cell count less than 3500/mL. 7. Age 70 years or greater. 8. Serious chronic disease interfering with adherence to the protocol follow‐up schedule. 9. No home telephone. 10. Refusal. | |
| Interventions | Participants were randomly assigned to two groups. Group 1 (n = 274): colchicine 0.6 mg twice daily. Group 2 (n = 275): placebo. Duration: 24 months to 72 months | |
| Outcomes | mortality, adverse events, decompensated cirrhosis. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patient enrollment and random assignment to treatment was by telephone call to the data‐coordinating centre (CSPCC, Perry Point, MD)". |
| Allocation concealment (selection bias) | Low risk | Quote: "Study medications were dispensed by each VA Pharmacy from prepackaged kits matched to the treatment ID number. The treatment randomisation scheme was based on permuted blocks of random length separately for each study centre". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Neither the patients, the nurses administering the treatment, nor the physicians assessing the outcomes were aware of the treatment group assignment until all data analysis was complete". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Neither the patients, the nurses administering the treatment, nor the physicians assessing the outcomes were aware of the treatment group assignment until all data analysis was complete". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "All analysed as assigned". Comment: there were no post‐randomisation dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: all important outcomes were reported. |
| For‐profit bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: there was no other bias. |
Naveau 2004.
| Methods | Randomised clinical trial | |
| Participants | Country: France. Number randomised: 36. Post‐randomisation dropouts: 1 (2.8%). Revised sample size: 35. Average age: 52 years. Females: 11 (31.4%). Inclusion criteria: 1. Age between 18 and 70 years old. 2. Chronic alcoholism (alcohol intake of more than 50 g per day over the previous year). 3. Severe alcoholic hepatitis (serum aspartate aminotransferase level of 1.5 N and a Maddrey score of 32). 4. Biopsy‐proven alcoholic hepatitis. Exclusion criteria: 1. Presence of hepatitis B surface antigen. 2. Hepatitis C virus or HIV antibodies. 3. Hepatocellular carcinoma. 4. Ethanol abstinence for more than 1 month. 5. Concomitant symptomatic or asymptomatic bacterial infection. 6. Severe bacterial infection within the previous 3 months (septicaemia, spontaneous bacterial peritonitis). 7. Concomitant or previous history of tuberculosis. 8. Severe associated disease (cardiac failure, severe pulmonary disease, neoplastic disease, severe psychiatric disorders). 9. Acute pancreatitis. 10. Gastrointestinal bleeding over the previous month. 11. Hepatorenal syndrome. 12. Acanthocytosis. 13. Patients in whom corticosteroids were contraindicated. 14. Patients who had taken infliximab or corticosteroids during the 3 months before inclusion. 15. Treatment with immunosuppressants, budesonide, and thalidomide during the 3 months before enrollment. 16. Suspected noncompliance. 17. Pregnancy. | |
| Interventions | Participants were randomly assigned to two groups. Group 1 (n = 18): glucocorticosteroids (prednisolone 40 mg) plus anit‐TNF (infliximab 10 mg/Kg). Group 2 (n = 17): glucocorticosteroids (prednisolone 40 mg) plus placebo. Duration: 28 days | |
| Outcomes | mortality, adverse events, decompensated cirrhosis. | |
| Notes | Reasons for post‐randomisation dropouts: one patient from group 2 was found not to have satisfied the inclusion criteria before treatment was started (bacterial urine infection) and did not receive the allocated treatment. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was carried out by computer‐generated allocation". |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "This study was a randomised, double blind, placebo‐controlled trial in two parallel groups. The placebo was identical in appearance to the infliximab solution". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: there were post‐randomisation dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: all important outcomes were reported. |
| For‐profit bias | High risk | Quote: "Infliximab and placebo treatments were donated by Schering‐Plough". Comment: the trial was funded by a party with vested interest in the results. |
| Other bias | Low risk | Comment: there was no other bias. |
Nguyen‐Khac 2011.
| Methods | Randomised clinical trial | |
| Participants | Country: France. Number randomised: 180. Post‐randomisation dropouts: 6 (3.3%). Revised sample size: 174. Average age: 52 years. Females: 69 (39.7%). Inclusion criteria: 1. Age of 18 years or older. 2. Average alcohol intake of more than 50 g per day during the 3 months before enrollment. 3. A Maddrey’s discriminant function of 32 or more. 4. Liver histologic findings consistent with alcoholic hepatitis. Exclusion criteria: 1. Hepatorenal syndrome. 2. Hepatocellular carcinoma. 3. Uncontrolled bacterial infection. 4. Gastrointestinal haemorrhage in the previous 4 day. 5. Infection with HCV, HBV, HIV. 6. Auto immune hepatitis. 7. Haemochromatosis. 8. Wilson’s disease. 9. Alpha1‐antitrypsin deficiency. 10. Acetaminophen‐induced hepatitis. 11. Cancer. 12. N‐acetyl‐cysteine allergy. 13. Serious cardiac, respiratory, neurologic disease. | |
| Interventions | Participants were randomly assigned to two groups. Group 1 (n = 85): glucocorticosteroids (prednisolone 40 mg) plus N‐acetylcysteine (150 first day then 100 mg/kg from days 2 to 5). Group 2 (n = 89): glucocorticosteroids (prednisolone 40 mg). Duration: steroids 28 days treatment, N‐acetylcysteine 5 days | |
| Outcomes | mortality, adverse events, decompensated cirrhosis, liver transplantation. | |
| Notes | Reasons for post‐randomisation dropouts: 3 were found to meet the exclusion criteria, 3 other reason. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed centrally in blocks of four by means of a computerized procedure, with stratification according to centre". |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "The treatment assignments were not concealed from the investigators or the patients". Comment: Further details were not available. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "The treatment assignments were not concealed from the investigators or the patients". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "see table, 6 post‐randomisation drop‐outs, not considered in the analysis". Comment: there were post‐randomisation dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: all important outcomes were reported. |
| For‐profit bias | Unclear risk | Quote: "Supported by Programme Hospitalier de Recherche Clinique plus Novartis?". |
| Other bias | Low risk | Comment: there was no other bias. |
Orrego 1979.
| Methods | Randomised clinical trial | |
| Participants | Country: Canada. Number randomised: 143. Post‐randomisation dropouts: 0 (0%). Revised sample size: 143. Average age: 49 years. Females: 18 (12.6%). Inclusion criteria: 1. One or more of the following clinical findings: hepatomegaly (liver palpable more than 3 cm below the costal margin), tender liver, jaundice, ascites, collateral circulation, spider nevi, and splenomegaly. 2. At least two of the following abnormal laboratory tests were required: SGOT more than 20 IU/L; serum gamma glutamyl transpeptidase more than 29 IU/L; serum alkaline phosphatase more than 36 IU/L; total serum bilirubin more than 1.5 mg/100 mL. 3. No more than 6 days of abstinence from alcohol. Exclusion criteria: 1. A known history of hypothyroidism. 2. A known history of diabetes or requirement of other therapies that contraindicated the use of Propylthiouracil. 3. Congestive heart failure. | |
| Interventions | Participants were randomly assigned to two groups. Group 1 (n = 63): propylthiouracil 300 mg once daily. Group 2 (n = 50): placebo. Duration: not clear. One or 2 months | |
| Outcomes | mortality, adverse events. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were assigned sequential numbers on admission to the different hospitals; the numbers had been previously distributed by a computerized random number generator between the lists of patients to receive PTU or placebo". |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Only the pharmacist at the ARF.CI had the information on the type of therapy (PTU or placebo) that any individual patient was receiving. Sixty‐three patients received identical placebo capsules according to the same dosage schedule". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Eight patients (5 PTU, 3 placebo) who discharged themselves from the hospital against medical advice within 3 days of admission were excluded from the study. One PTU patient was excluded when found to have HBsAg‐positive infectious hepatitis. One placebo patient was excluded because after 10 days of treatment he had an episode of severe acute pancreatitis". Comment: there were post‐randomisation dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: all important outcomes were reported. |
| For‐profit bias | High risk | Quote: "PTU was obtained from Charles E. Frosst and Co., Montreal, Quebec, Canada". Comment: this information was not available. |
| Other bias | Low risk | Comment: there was no other bias. |
Orrego 1987.
| Methods | Randomised clinical trial | |
| Participants | Country: USA. Number randomised: 360. Post‐randomisation dropouts: 50 (13.9%). Revised sample size: 310. Average age: 49 years. Females: 69 (22.3%). Inclusion criteria: 1. Alcoholism defined as excessive drinking resulting in several emergency room visits, hospitalisations, or important social problems related to drinking, a well‐documented history of an average daily consumption exceeding 80 g of ethanol, or spree drinking consisting of repeated prolonged inebriations. 2. Clinical or laboratory evidence of liver disease. Exclusion criteria: 1. Hepatoma. 2. The presence of hepatitis B surface antigen. 3. Contraindications to propylthiouracil therapy | |
| Interventions | Participants were randomly assigned to two groups. Group 1 (n = 157): propylthiouracil 300 mg twice daily. Group 2 (n = 153): placebo. Duration: 2 years | |
| Outcomes | mortality, adverse events. | |
| Notes | Reasons for post‐randomisation dropouts: non‐compliance: 25 Group A, 25 Group B, dropouts at 2 years: 104 Group A,. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Upon entering the study, patients were randomly assigned to receive either propylthiouracil or placebo, with use of a computerized random‐assignment method". |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Propylthiouracil was given in a double‐blind manner… Capsules contained 75 mg of propylthiouracil or placebo…The physician in the liver clinic when confronted with a possible side effect of propylthiouracil, referred the patient to the consulting physician, who had access to the drug or placebo code. In all cases in which a patient was seen by the consulting physician, the code numbers were changed and the drug adjustment was kept confidential from the staff conducting the trial". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The code was not broken until the analyses were completed". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: there were post‐randomisation dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: all important outcomes were reported. |
| For‐profit bias | High risk | Quote: ".and to W.D. Dorian of Merck Frosst Canada Inc for providing propylthiouracil". |
| Other bias | Low risk | Comment: there was no other bias. |
Paladugu 2006.
| Methods | Randomised clinical trial | |
| Participants | Country: India. Number randomised: 30. Post‐randomisation dropouts: 0 (0%). Revised sample size: 30. Average age: 47 years. Females: not stated Inclusion criteria: 1. Discriminant factor of 32 or greater. 2. Hepatic encephalopathy. Exclusion criteria: not reported | |
| Interventions | Participants were randomly assigned to two groups. Group 1 (n = 14): pentoxifylline 400 mg thrice daily. Group 2 (n = 16): placebo. Duration: 4 weeks | |
| Outcomes | mortality, decompensated cirrhosis. | |
| Notes | Abstract | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were included and randomised". Comment: Further details were not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: Further details were not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: some important outcomes which will generally be assessed were not reported. |
| For‐profit bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: there was no other bias. |
Pares 1998.
| Methods | Randomised clinical trial | |
| Participants | Country: Spain. Number randomised: 200. Post‐randomisation dropouts: 0 (0%). Revised sample size: 200. Average age: 50 years. Females: 42 (21%). Inclusion criteria: 1. A daily alcohol intake of more than 80 g in men and 60 g in women for a period longer than 5 years. 2. Liver cirrhosis supported by histology within the 3 months before inclusion in the trial or by laparoscopic examination in those with very low prothrombin index or platelet count in whom percutaneous liver biopsy could not be performed. Exclusion criteria: 1. Previous treatment with colchicine, malotilate, penicillamine or corticosteroids. 2. Life expectancy less than 6 months. 3. Drug‐addicted patients. 4. Pregnancy. 5. Other known etiologies for liver cirrhosis. | |
| Interventions | Participants were randomly assigned to two groups. Group 1 (n = 103): silymarin 150 mg thrice daily. Group 2 (n = 97): placebo. Duration: 2 years | |
| Outcomes | mortality, adverse events. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was carried out for all patients stratified by sex using a random‐number sequence table in the Hospital Clinic of Barcelona, which served as the coordinating centre". |
| Allocation concealment (selection bias) | Low risk | Quote: "When a patient was included in the study, the assigned treatment was obtained from the coordinating centre by telephone; the packs were coded with'x' or'y' by the coordinating centre; treatment and placebo drugs were identical in appearance". Comment: according to the author's reply. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Patients were randomly assigned to receive either 450 mg of silymarin (150 mg three times/day orally) or a placebo with identical appearance, smell and taste…". Comment: according to the author's reply. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: according to the author's reply. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "Fifteen patients (seven in the silymarin group and eight in the placebo group) did not reappear after their first visit, so that further information was not available". Comment: Not clear what kind of assumption if ITT analysis. There were post‐randomisation dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: all important outcomes were reported. |
| For‐profit bias | High risk | Quote: "Patients were randomly assigned to receive either 450 mg of silymarin (150 mg three times/day orally) or a placebo with identical appearance, smell and taste, kindly supplied by Madaus Cerafarm (Barcelona, Spain)". |
| Other bias | Low risk | Comment: there was no other bias. |
Park 2014.
| Methods | Randomised clinical trial | |
| Participants | Country: Korea. Number randomised: 124. Post‐randomisation dropouts: 3 (2.4%). Revised sample size: 121. Average age: 49 years. Females: 30 (24.8%). Inclusion criteria: 1. Age range of 20–75 years. 2. Average alcohol intake of more than 40 g per day during the 3 months before enrolment. 3. Recent onset of jaundice in the prior 3 months plus one or more of: hepatic encephalopathy, ascites, tender hepatomegaly, leukocytosis with predominantly neutrophil differentiation, fever, elevated liver enzymes. 4. Maddrey’s discriminant function of 32 or more. Exclusion criteria: 1. Bacterial infection. 2. Gastrointestinal bleeding. 3. Concomitant viral hepatitis. 4. Renal impairment. 5. Pancreatitis. 6. Hepatocellular carcinoma. 7. Autoimmune liver disease. 8. Wilson’s disease. 9. Haemochromatosis,. 10. Drug‐induced liver injury. | |
| Interventions | Participants were randomly assigned to two groups. Group 1 (n = 62): pentoxifylline 400 mg thrice daily. Group 2 (n = 59): glucocorticosteroids (prednisolone 40 mg once daily). Duration: 28 days | |
| Outcomes | mortality, adverse events. | |
| Notes | Reasons for post‐randomisation dropouts: 3 patients not included in the analysis, not meeting inclusion criteria. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomisation was centralised and balanced by centre. The allocation sequence was generated with a computer list of random set numbers stratified by centre". |
| Allocation concealment (selection bias) | Unclear risk | Quote: "open trial". Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "The study was a randomised, open label, parallel group clinical trial". |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "The study was a randomised, open label, parallel group clinical trial". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "3 patients were excluded from the analysis because did not receive the allocated intervention. 1 patients in pentoxifylline group and 3 patients in prednisolone group were lost to follow‐up, but we assessed the status (alive or dead) of patients lost to follow‐up by calling a family member". Comment: there were post‐randomisation dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: all important outcomes were reported. |
| For‐profit bias | Low risk | Quote: "Research Supporting Program of the Korean Association for the Study of the Liver". |
| Other bias | Low risk | Comment: there was no other bias. |
Pelletier 2003.
| Methods | Randomised clinical trial | |
| Participants | Country: France. Number randomised: 226. Post‐randomisation dropouts: 0 (0%). Revised sample size: 226. Average age: 50 years. Females: 87 (38.5%). Inclusion criteria: 1. Biopsy‐proven alcohol‐induced cirrhosis within 2 months before entry into the trial. 2. Serum bilirubin higher than 50 mol/L. Exclusion criteria: 1. A life‐threatening complication of cirrhosis within 1 week (bleeding, encephalopathy, sepsis, hepatorenal syndrome). 2. Evidence of hepatocellular carcinoma at sonography and serum a‐fetoprotein. 3. Associated hepatitis B or C viral infection. 4. Severe psychiatric disorders. 5. Alcoholic abstinence for more than 2 months. 6. Patients receiving ursodeoxycholic acid within 3 months from the study entry. 7. Patients with HIV antibodies. | |
| Interventions | Participants were randomly assigned to two groups. Group 1 (n = 113): ursodeoxycholic acid (13 to 15 mg/kg per day). Group 2 (n = 113): placebo. Duration: 6 months | |
| Outcomes | mortality, decompensated cirrhosis. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Random assignment to eitherUDCA or placebo was done in random blocks of 4". |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "Consecutive patients with alcohol‐induced cirrhosis and a serum bilirubin level higher than 50 mol/L and who agreed to participate were included in this multicenter, double‐blind trial comparing the effects of UDCA and a placebo". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "Twenty patients presented deviations to the protocol: 6 from the AUDC group and 14 from the placebo group". Comment: iTT analysis, assumption was not specified. |
| Selective reporting (reporting bias) | Low risk | Comment: all important outcomes were reported. |
| For‐profit bias | High risk | Quote: "Supported by the Synthelabo‐Recherche laboratory, France". |
| Other bias | Low risk | Comment: there was no other bias. |
Phillips 2006.
| Methods | Randomised clinical trial | |
| Participants | Country: UK. Number randomised: 101. Post‐randomisation dropouts: 2 (2%). Revised sample size: 99. Average age: 44 years. Females: 41 (41.4%). Inclusion criteria: 1. History of heavy alcohol consumption defined as greater than 80 g alcohol per day for men, or greater than 60 g alcohol per day for women, prior to the onset of illness of at least 1‐month duration. 2. Absence of alternative aetiology of liver disease. 3. Serum bilirubin greater than 100 micromol/L. 4. Serum AST lower than 300 IU/L. 5. Serum IgA greater than 5 g/L. 6. White cell count greater than 20x10^9/L. 7. Ultrasound evidence of hepatic fatty infiltration. 8. Hepatomegaly (clinical or radiological). Exclusion criteria: 1. Active sepsis (defined as positive microbiological culture, ascitic white cell count greater than 500 cells/mL or ascitic neutrophil count greater than 250 cells/mL, or radiological appearances consistent with pneumonia ), not treated with appropriate antibiotics for at least 48 h prior to randomisation. 2. Active significant gastrointestinal haemorrhage within the previous 48 h. 3. Shock necessitating inotropic support. 4. Evidence of co‐existing non‐alcoholic liver disease. 5. Pregnant or lactating women. 6. Patients with a history of allergy to any component of the regimen. | |
| Interventions | Participants were randomly assigned to two groups. Group 1 (n = 46): antioxidants plus intralipid solution. Group 2 (n = 53): glucocorticosteroids (oral prednisolone 30 mg once daily or methylprednisolone 24 mg intravenously). Duration: 28 days (+ 2 weeks tapering steroid). | |
| Outcomes | mortality, adverse events. | |
| Notes | Reasons for post‐randomisation dropouts: 2 from group 2 withdrew immediately post‐randomisation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients randomised using consecutive envelopes produced by computer‐generated randomisation". |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Not possible. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "ITT analysis". |
| Selective reporting (reporting bias) | Low risk | Comment: all important outcomes were reported. |
| For‐profit bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: there was no other bias. |
Pierri 1985.
| Methods | Randomised clinical trial | |
| Participants | Country: Italy. Number randomised: 40. Post‐randomisation dropouts: not stated. Revised sample size: 40. Average age: 48 years. Females: 8 (20%). Inclusion criteria: 1. A positive history for chronic alcohol for at least 2 years and consuming at least 80 g of alcohol daily. 2. Negative viral hepatitis markers. 3. Absence of other factors causing secondary steatosis. Exclusion criteria: not reported | |
| Interventions | Participants were randomly assigned to two groups. Group 1 (n = 20): epomediol 600 mg/day. Group 2 (n = 20): placebo. Duration: 90 days | |
| Outcomes | mortality. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "At random". Comment: Further details were not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no post‐randomisation dropouts. |
| Selective reporting (reporting bias) | High risk | Comment: Not reported data about safety. |
| For‐profit bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: there was no other bias. |
Plevris 1991.
| Methods | Cross‐over randomised clinical trial | |
| Participants | Country: UK. Number randomised: 12. Post‐randomisation dropouts: 1 (8.3%). Revised sample size: 11. Average age: 56 years. Females: 1 (9.1%). Inclusion criteria: ALD (alcoholic liver disease) Exclusion criteria: not reported | |
| Interventions | Participants were randomly assigned to two groups. Group 1 (n = 6): ursodeoxycholic acid (15 mg/kg/day). Group 2 (n = 5): placebo. Duration: 4 weeks | |
| Outcomes | adverse events. | |
| Notes | Reasons for post‐randomisation dropouts: 1 withdrawn for diarrhoea. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomised". Comment: Further details were not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Single‐blind study". |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Single‐blind". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "1 patient withdrew for collateral effect". |
| Selective reporting (reporting bias) | High risk | Comment: mortality was not reported. |
| For‐profit bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: there was no other bias. |
Popescu 2009.
| Methods | Randomised clinical trial | |
| Participants | Country: Romania. Number randomised: 68. Post‐randomisation dropouts: 0 (0%). Revised sample size: 68. Average age: not stated Females: not stated Inclusion criteria: acute alcoholic hepatitis Exclusion criteria: not reported | |
| Interventions | Participants were randomly assigned to two groups. Group 1 (n = 34): glucocorticosteroids (prednisolone 40 mg) plus ursodeoxycholic acid (600 mg) plus rifaximin (1200 mg/day for 28 days, then 800 mg/day for 10 days/month). Group 2 (n = 34): glucocorticosteroids (prednisolone 40 mg) plus ursodeoxycholic acid (600 mg). Duration: 28 days for prednisolone and ursodeoxycholic acid and then rifaximin alone in group 1 for 10 days per month for 1 year | |
| Outcomes | mortality, adverse events. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Equally randomised". Comment: Further details were not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | This information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | Low risk | Comment: all important outcomes were reported. |
| For‐profit bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: there was no other bias. |
Porter 1971.
| Methods | Randomised clinical trial | |
| Participants | Country: USA. Number randomised: 23. Post‐randomisation dropouts: 3 (13%). Revised sample size: 20. Average age: 47 years. Females: 7 (35%). Inclusion criteria: For admission to the study all three absolute criteria should be fulfilled. Also, two or more major criteria or one major and four or more minor criteria. Absolute criteria: 1. History of recent, heavy alcohol ingestion. 2. Serum total bilirubin of 5 mg per 100 mL or more. 3. Clinical and laboratory deterioration over the first 5 hospital days, a striking lack of improvement in the patient's clinical and biochemical status over this same period, or rapid, marked deterioration in less than 24 hours. Major criteria: 1. Liver biopsy showing alcoholic hepatitis. 2. Hepatic encephalopathy, persistent or progressive azotaemia unexplained by another process, with either a blood urea nitrogen over 20 mg or a creatinine over 1.5 mg per 100 mL (or both). 3. Total bilirubin over 20 mg per 100 mL. Minor criteria: 1. Fever not obviously secondary to another process. 2. Anorexia or nausea or vomiting. 3. Palpable hepatomegaly. 4. Palpable splenomegaly. 5. Oesophageal varices. 6. A prothrombin time prolonged three or more seconds over control. Exclusion criteria: 1. Active gastrointestinal bleeding. 2. Pancreatitis. 3. Radiologic evidence of peptic ulcer disease. 4. Active or questionably active pulmonary tuberculosis. 5. Life‐threatening bacterial infections | |
| Interventions | Participants were randomly assigned to two groups. Group 1 (n = 11): glucocorticosteroids (6‐methylprednisolone 40 mg thrice daily parenterally). Group 2 (n = 9): placebo. Duration: if clinical improvement: for 10 days, then given orally and the dose gradually tapered. If no clinical improvement: 40 mg parenterally till improvement or death | |
| Outcomes | mortality, adverse events. | |
| Notes | Reasons for post‐randomisation dropouts: 3 died. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomisation was achieved by a number drawn from a pool". |
| Allocation concealment (selection bias) | Unclear risk | Quote: "both the steroid and the placebo were packaged and coded by number in both parenteral and oral forms". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "neither patients nor physicians knew which form of treatment was used until the study had been completed". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "3 patients died within 36 hours of the start of therapy and were excluded from analysis before the code was broken". Comment: there were post‐randomisation dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: all important outcomes were reported. |
| For‐profit bias | Low risk | Quote: "gastroenterology‐research training grant from the National Institute of Arthritis and Metabolic Diseases and a grant from the National Institutes of Health". |
| Other bias | Low risk | Comment: there was no other bias. |
Radvan 1983.
| Methods | Randomised clinical trial | |
| Participants | Country: USA. Number randomised: 35. Post‐randomisation dropouts: 4 (11.4%). Revised sample size: 31. Average age: not stated Females: not stated Inclusion criteria: 1. Fever, jaundice and tender hepatomegaly following alcohol ingestion. 2. Serum bilirubin higher than 5 mg %, SGOT higher than SGPT, prothrombin time lower than 50 % and leucocytosis higher than 12,000 Exclusion criteria: not reported | |
| Interventions | Participants were randomly assigned to two groups. Group 1 (n = 16): insulin 2 U/hour glucagon 0.2 mg/hr at 40 mL/h. Group 2 (n = 15): no intervention. Duration: 2 weeks | |
| Outcomes | mortality. | |
| Notes | Reasons for post‐randomisation dropouts: two patients in each group failed to complete the protocol and their results were excluded from the analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomised clinical trial". Comment: Further details were not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Two patients in each group failed to complete the protocol and their results were excluded from the analysis". Comment: there were post‐randomisation dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: all important outcomes were reported. |
| For‐profit bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: there was no other bias. |
Ramond 1992.
| Methods | Randomised clinical trial | |
| Participants | Country: France. Number randomised: 65. Post‐randomisation dropouts: 4 (6.2%). Revised sample size: 61. Average age: 48 years. Females: 42 (68.9%). Inclusion criteria: 1. Biopsy‐proven alcoholic hepatitis. 2. Spontaneous hepatic encephalopathy or a discriminant function value higher than 32 (or both). Exclusion criteria: 1. Gastointestinal bleeding. 2. Bacterial infections. | |
| Interventions | Participants were randomly assigned to two groups. Group 1 (n = 32): glucocorticosteroids (prednisolone 40 mg once daily). Group 2 (n = 29): placebo. Duration: 28 days | |
| Outcomes | mortality. | |
| Notes | Reasons for post‐randomisation dropouts: 4 met exclusion criteria, infection. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "a random code was prepared by computer". |
| Allocation concealment (selection bias) | Unclear risk | Quote: "random sequences of drug or placebo were prepared by the pharmacist". Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The principal investigators and their associates were not aware of the randomisation procedure or of the medication that the patients were receiving throughout the trial. The pharmacist was the only person who knew which regimen the patient had received". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The principal investigators and their associates were not aware of the randomisation procedure or of the medication that the patients were receiving throughout the trial". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "Four patients were excluded from the analysis after randomisation. These four patients were alive at the end of the study". Comment: there were post‐randomisation dropouts. |
| Selective reporting (reporting bias) | High risk | Quote: "adverse events were not reported". Comment: some important outcomes which will generally be assessed were not reported. |
| For‐profit bias | Low risk | Quote: "Prednisolone and identical placebo tablets were provided by Laboratoire Houde (Paris)". |
| Other bias | Low risk | Comment: there was no other bias. |
Resnick 1974.
| Methods | Randomised clinical trial | |
| Participants | Country: USA. Number randomised: 43. Post‐randomisation dropouts: 3 (7%). Revised sample size: 40. Average age: 47 years. Females: 14 (35%). Inclusion criteria: 1. Hepatic decompensation accompanied by a history of heavy alcohol intake without other established aetiology of liver injury. 2. Serum bilirubin more than 2 mg %. 3. Albumin less than 3.5 mg %. 4. Prothrombin prolongation more than 2.5 seconds. 5. SGOT higher than SGPT (with SGOT lower than 500 U). Exclusion criteria: 1. Biopsy evidence of cirrhosis prior to the present hospitalisation. 2. Gastroesophageal varices. 3. Gastrointestinal bleeding. 4. Hepatic encephalopathy. 5. Renal insufficiency. 6. Penicillin hypersensitivity. | |
| Interventions | Participants were randomly assigned to two groups. Group 1 (n = 19): penicillamine 250 mg four times daily. Group 2 (n = 21): placebo. Duration: 8 weeks | |
| Outcomes | mortality. | |
| Notes | Reasons for post‐randomisation dropouts: 3 post‐randomisation due to carcinoma, lack of compliance (2 in placebo group, one in penicillamine). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "by random assignment". Comment: Further details were not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "a capsule physically identical to the active drug". Comment: Further details were not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "three additional randomised patients were subsequently excluded". Comment: there were post‐randomisation dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: all important outcomes were reported. |
| For‐profit bias | High risk | Quote: "MercK, Sharp and Dohme supplied penicillamine and a similarly appearing placebo". |
| Other bias | Low risk | Comment: there was no other bias. |
Richardet 1993.
| Methods | Cross‐over randomised clinical trial | |
| Participants | Country: France. Number randomised: 23. Post‐randomisation dropouts: not stated. Revised sample size: 23. Average age: not stated Females: not stated Inclusion criteria: 1. Patients with severe acute alcoholic hepatitis. | |
| Interventions | Participants were randomly assigned to two groups. Group 1 (n = 12): glucocorticosteroids (prednisolone 40 mg/day). Group 2 (n = 11): no intervention. Duration: 8 days | |
| Outcomes | None of the outcomes of interest were reported. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Results of a randomised study". |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: Neither mortality nor adverse events were reported. |
| For‐profit bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: there was no other bias. |
Sainz 1992.
| Methods | Randomised clinical trial | |
| Participants | Country: Spain. Number randomised: 54. Post‐randomisation dropouts: 0 (0%). Revised sample size: 54. Average age: not stated Females: not stated Inclusion criteria: 1. Alcoholic liver disease. Exclusion criteria: not reported | |
| Interventions | Participants were randomly assigned to two groups. Group 1 (n = 28): colchicine 1 mg once daily for 5 d/week. Group 2 (n = 26): no intervention. Duration: treatment duration not reported. | |
| Outcomes | None of the outcomes of interest were reported. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Cirrhotic and non‐cirrhotic patients were randomised in 2 groups". Comment: Further details were not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "….treatment (T) received colchicine 1 mg/d for 5 d a week. and control (C) without anti‐fibrotic treatment". Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "14 patients were lost during the follow‐up". Comment: Few details. |
| Selective reporting (reporting bias) | High risk | Comment: some important outcomes which will generally be assessed were not reported. |
| For‐profit bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: there was no other bias. |
Salvagnini 1985.
| Methods | Randomised clinical trial | |
| Participants | Country: Italy. Number randomised: 122. Post‐randomisation dropouts: not stated. Revised sample size: 122. Average age: not stated Females: not stated Inclusion criteria: 1. Alcohol abusers consecutively hospitalised in 5 clinical units because of liver disease. Exclusion criteria: 1. HBs Ag positive. 2. Decompensated cirrhosis. | |
| Interventions | Participants were randomly assigned to two groups. Group 1 (n = 60): silymarin 420 mg/day. Group 2 (n = 62): placebo. Duration: 45 days treatment | |
| Outcomes | None of the outcomes of interest were reported. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "RCT". Comment: Further details were not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "Double‐blind". Comment: Further details were not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Double‐blind". Comment: Further details were not available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: None of the outcomes of interest were reported. |
| For‐profit bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: there was no other bias. |
Serrano‐Cancino 1981.
| Methods | Randomised clinical trial | |
| Participants | Country: USA. Number randomised: 41. Post‐randomisation dropouts: 0 (0%). Revised sample size: 41. Average age: not stated Females: not stated Inclusion criteria: severe alcoholic hepatitis. Exclusion criteria: not reported | |
| Interventions | Participants were randomly assigned to two groups. Group 1 (n = 21): propylthiouracil 100 mg thrice daily. Group 2 (n = 20): placebo. Duration: 17 days (mean) | |
| Outcomes | mortality. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The patients were randomised in a double‐blinded manner". Comment: Further details were not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "The patients were randomised in a double‐blinded manner". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | Low risk | Comment: all important outcomes were reported. |
| For‐profit bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: there was no other bias. |
Shumaker 1978.
| Methods | Randomised clinical trial | |
| Participants | Country: USA. Number randomised: 27. Post‐randomisation dropouts: 0 (0%). Revised sample size: 27. Average age: 45 years. Females: 11 (40.7%). Inclusion criteria: minimal criteria: 1. A recent heavy alcoholic ingestion. 2. Bilirubin grater than 5 mg %. 3. Hospitalisation for at least 5 days without improvement in liver tests. 4. Rapid deterioration of the clinical condition during a 24‐hour period while under observation. Major criteria: 1. Liver biopsy showing alcoholic hepatitis. 2. Hepatic encephalopathy. 3. Azotemia unexplained by another process. 4. Hyperbilirubinemia. 5. Prothrombin time prolonged more than 4 seconds over control and unresponsive to parenteral administration of Vitamin K. Minor criteria: 1. Fever not obviously secondary to other conditions. 2. Leucocytes more than than 12,000. 3. Hepatomegaly. 4. Splenomegaly. 5. Liver stigmas. A patient should have all minimal criteria and a minimum of two major criteria or one major and two minor criteria. Exclusion criteria: 1. Active gastrointestinal bleeding. 2. Pancreatitis. 3. X‐ray evidence of peptic ulcer disease. 4. Active or questionably active tuberculosis. 5. Acute infection. 6. Sever psychiatric disorder. 7. SGOT greater than 500. | |
| Interventions | Participants were randomly assigned to two groups. Group 1 (n = 12): glucocorticosteroids (6‐methylprednisolone 80 mg). Group 2 (n = 15): placebo. Duration: 4 to 7 days; the medication was then tapered on a flexible schedule with cessation of therapy planned for 4 weeks | |
| Outcomes | mortality, adverse events, decompensated cirrhosis. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The patient was then randomised into a predetermined code provided by the drug manufacturer". |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: ""Clinical evaluation was carried out by junior staff physicians blinded to treatment status of the patients". "Following randomisation patients were placed on 80mg of 6‐methylprednisolone or equivalent number of placebo tablets"". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no post‐randomisation dropouts. |
| Selective reporting (reporting bias) | High risk | Comment: some important outcomes which will generally be assessed were not reported. |
| For‐profit bias | High risk | Quote: "Upjohn Co, Kalamazoo, prepared and supplied the medications and placebo". |
| Other bias | Low risk | Comment: there was no other bias. |
Sidhu 2012a.
| Methods | Randomised clinical trial | |
| Participants | Country: India. Number randomised: 70. Post‐randomisation dropouts: 0 (0%). Revised sample size: 70. Average age: 41 years. Females: 0 (0%). Inclusion criteria: 1. Clinical and laboratory results suggestive of alcoholic hepatitis. 2. Presence of severe alcoholic hepatitis defined by discriminant function of 32 or more. 3. Severe alcoholic hepatitis presenting as the first liver decompensating event. Exclusion criteria: 1. Chronic jaundice for over 3 months due to end‐stage liver disease. 2. Pre‐existing renal dysfunction including hepatorenal syndrome. 3. Acute pancreatitis. 4. Positive viral serology. 5. Severe cardiovascular or pulmonary diseases. 6. Neoplastic and other inflammatory conditions. 7. Spontaneous decline in discriminant function to less than 32 with symptomatic treatment after 5‐7 days of admission. | |
| Interventions | Participants were randomly assigned to two groups. Group 1 (n = 36): pentoxifylline 400 mg thrice daily plus glucocorticosteroids (prednisolone 40 mg once daily). Group 2 (n = 34): glucocorticosteroids (prednisolone 40 mg once daily). Duration: 28 days. Prednisolone was gradually tapered after the first 28 days, for 2 additional weeks | |
| Outcomes | mortality, adverse events, decompensated cirrhosis. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer‐generated random numbers". |
| Allocation concealment (selection bias) | Low risk | Quote: "sequentially numbered, sealed, opaque envelopes". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Group A received tablet PTX 400 mg thrice daily along with prednisolone 40 mg once a day per oral, and group B patients received prednisolone 40 mg once a day for 28 days". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "the randomisation sequence was concealed from the investigators until the intervention was assigned". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no post‐randomisation dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: all important outcomes were reported. |
| For‐profit bias | Low risk | Quote: "Department of Gastroenterology, Dayanand Medical College and Hospital, Ludhiana, Punjab". |
| Other bias | Low risk | Comment: there was no other bias. |
Sidhu 2012b.
| Methods | Randomised clinical trial | |
| Participants | Country: India. Number randomised: 50. Post‐randomisation dropouts: 0 (0%). Revised sample size: 50. Average age: 43 years. Females: 0 (0%). Inclusion criteria: 1. Clinical and laboratory features suggestive of alcoholic hepatitis. 2. Patients with discriminant function greater than 32. Exclusion criteria: 1. Positive viral serology. 2. Concomitant infections. 3. Active gastrointestinal bleeding. 4. Severe cardiovascular or pulmonary diseases. 5. A decline in discriminant function lower than 32 with symptomatic treatment for 5‐7 days. | |
| Interventions | Participants were randomly assigned to two groups. Group 1 (n = 25): pentoxifylline 400 mg thrice daily. Group 2 (n = 25): placebo. Duration: 28 days | |
| Outcomes | mortality, adverse events. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were randomised". |
| Allocation concealment (selection bias) | Low risk | Quote: "sequentially numbered (table of random numbers), sealed, opaque envelopes". |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no post‐randomisation dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: all important outcomes were reported. |
| For‐profit bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: there was no other bias. |
Singh 2014.
| Methods | Randomised clinical trial | |
| Participants | Country: India. Number randomised: 46. Post‐randomisation dropouts: 0 (0%). Revised sample size: 46. Average age: 43 years. Females: 0 (0%). Inclusion criteria: 1. Age 18‐75 years. 2. Average alcohol intake of more than 100 g/day during the 3 months before enrolment. 3. Modified discriminant factor higher than 32. Exclusion criteria: 1. Presence of hepatocellular carcinoma or portal vein thrombosis. 2. Refusal to participate in the study. 3. Prior treatment with steroids. 4. Any significant co‐morbidity including hepatorenal syndrome, grade 3 or 4 hepatic encephalopathy, upper gastrointestinal bleeding within the preceding. 5. Uncontrolled bacterial infection. 6. HIV infection. 7. Hepatitis B virus infection. 8. Hepatitis C virus seropositivity. 9. Autoimmune hepatitis. 10. Haemochromatosis. 11. Wilson’s disease. 12. Alpha‐1‐antitrypsin deficiency. 13. Pregnancy. 14. Any previous known hypersensitivity to G‐CSF. | |
| Interventions | Participants were randomly assigned to two groups. Group 1 (n = 23): G‐CSF (5 μg/Kg SC twice daily) plus pentoxifylline (400 mg thrice daily). Group 2 (n = 23): pentoxifylline (400 mg thrice daily). Duration: 5 days | |
| Outcomes | mortality, adverse events. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "This was an open‐label, randomised pilot study. A randomisation code was generated". |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization was performed with sequentially numbered envelopes". Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "This was an open‐label, randomised pilot study". |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "This was an open‐label, randomised pilot study". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no post‐randomisation dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: all important outcomes were reported. |
| For‐profit bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: there was no other bias. |
Spahr 2002.
| Methods | Randomised clinical trial | |
| Participants | Country: Switzerland. Number randomised: 20. Post‐randomisation dropouts: 0 (0%). Revised sample size: 20. Average age: 53 years. Females: 5 (25%). Inclusion criteria: 1. History of excessive alcohol intake (higher than 100‐150 g/day). 2. A liver chemistry profile suggestive of alcoholic hepatitis. 3. Maddrey's score between 32 and 55. 4. Written informed consent. Exclusion criteria: 1. Positive serology for hepatitis B, C and HIV. 2. Severe renal failure (serum creatinine less than 170 μmol/L). 3. Uncontrolled infection. 4. Recent (in the last 15 days) gastrointestinal bleeding. 5. Maddrey score greater than 55. | |
| Interventions | Participants were randomly assigned to two groups. Group 1 (n = 11): anti‐TNF (infliximab 5 mg/kg intravenous single dose) plus glucocorticosteroids (prednisolone (40 mg once daily). Group 2 (n = 9): placebo plus glucocorticosteroids (prednisolone 40 mg once daily). Duration: one day for infliximab or placebo, 28 days for prednisolone | |
| Outcomes | mortality, adverse events. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer‐generated list". |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "patients and heath care providers were blinded". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "the assessment of results was performed while blinded to treatment allocated". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no post‐randomisation dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: all important outcomes were reported. |
| For‐profit bias | High risk | Quote: "Essex Chemie AG". |
| Other bias | Low risk | Comment: there was no other bias. |
Stenner 2000.
| Methods | Randomised clinical trial | |
| Participants | Country: UK. Number randomised: 48. Post‐randomisation dropouts: 10 (20.8%). Revised sample size: 38. Average age: not stated Females: not stated Inclusion criteria: Biopsy confirmed ALD Exclusion criteria: not reported | |
| Interventions | Participants were randomly assigned to two groups. Group 1: ursodeoxycholic acid (I0 to 15 mg/kg/day). Group 2: placebo. Duration: 3 months | |
| Outcomes | None of the outcomes of interest were reported. | |
| Notes | Reasons for post‐randomisation dropouts: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomized". Comment: Further details were not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: there were post‐randomisation dropouts. |
| Selective reporting (reporting bias) | High risk | Comment: None of the outcomes of interest were reported. |
| For‐profit bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: there was no other bias. |
Stewart 2007.
| Methods | Randomised clinical trial | |
| Participants | Country: UK. Number randomised: 77. Post‐randomisation dropouts: 7 (9.1%). Revised sample size: 70. Average age: 44 years. Females: 32 (45.7%). Inclusion criteria: 1. Recent, heavy (higher than 40 g/day for women or 60 g/day for men) alcohol intake. 2. Age between 18 and 65. 3. A diagnostic liver biopsy or two of the following: hepatomegaly, leucocytosis higher than 11 x 109 cells/L, "white out" on liver and spleen isotope scanning. Exclusion criteria: 1. Evidence of malignancy. 2. Positive HBV or HCV serology. 3. Pregnant or lactating women. 4. Cirrhotic patients admitted primarily for control of the complications arising from portal hypertension. 5. Active infection. 6. Gastrointestinal bleeding. | |
| Interventions | Participants were randomly assigned to two groups. Group 1 (n = 36): N‐acetylcysteine (loading dose of 150 mg/kg followed by 100 mg/kg/day) for one week then other antioxidants for 6 months. Group 2 (n = 34): placebo. Duration: 1 week plus 6 months | |
| Outcomes | mortality, adverse events. | |
| Notes | Reasons for post‐randomisation dropouts: in total, 7 patients were lost to follow‐up; 3 from the active group and 4 from the placebo group, 7 patients were excluded from the analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer‐derived randomisation schedule". |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "the trial was blinded and control patients received identical placebo". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Our pharmacy provided identical active or placebo infusions and tablets to the patients. The outcomes were determined after the event (primary outcome was mortality) with no knowledge of the allocation". Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "In total, 7 patients were lost to follow‐up; 3 from the active group and 4 from the placebo group". Comment: there were post‐randomisation dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: all important outcomes were reported. |
| For‐profit bias | Low risk | Quote: "The authors who have taken part in this study declared that they have no relationship with the manufacturers of the drugs involved either in the past or present and did not receive funding from the manufacturers to carry out their research". |
| Other bias | Low risk | Comment: there was no other bias. |
Stigliano 2005.
| Methods | Randomised clinical trial | |
| Participants | Country: UK. Number randomised: 12. Post‐randomisation dropouts: 0 (0%). Revised sample size: 12. Average age: 49 years. Females: 4 (33.3%). Inclusion criteria: acute alcoholic hepatitis. Exclusion criteria: not reported | |
| Interventions | Participants were randomly assigned to two groups. Group 1 (n = 6): G‐CSF (2 cycles of 10 g/kg/day). Group 2 (n = 6): glucocorticosteroids (further details not available). Duration: 5 days | |
| Outcomes | mortality. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were randomised". Comment: Further details were not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: no comment on adverse events. |
| For‐profit bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: there was no other bias. |
Takase 1989.
| Methods | Randomised clinical trial | |
| Participants | Country: Japan.
Number randomised: 55.
Post‐randomisation dropouts: not stated.
Revised sample size: 55.
Average age: 64 years.
Females: 1 (1.8%).
Inclusion criteria: ALD. Exclusion criteria: not reported. |
|
| Interventions | Participants were randomly assigned to two groups. Group 1 (n = 25): malotilate 600 mg/day. Group 2 (n = 30): no intervention. Duration: 9 weeks treatment | |
| Outcomes | adverse events. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomly assigned". Comment: Further details were not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: "Controls, no placebo". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no post‐randomisation dropouts. |
| Selective reporting (reporting bias) | High risk | Comment: mortality was not reported. |
| For‐profit bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: there was no other bias. |
Theodossi 1982.
| Methods | Randomised clinical trial | |
| Participants | Country: UK. Number randomised: 60. Post‐randomisation dropouts: 5 (8.3%). Revised sample size: 55. Average age: not stated Females: 24 (43.6%). Inclusion criteria: 1. History of alcohol intake of 80 g or more daily for at least 5 years. 2. Serum bilirubin concentration greater than 80 μmol/L. 3. Serum aspartate transferase at least twice the upper limit of normal. 4. Prothrombin time prolonged by at least nine seconds. Exclusion criteria: 1. Hepatoma. 2. Recent myocardial infarction. 3. Accompanying cerebrovascular accident including evidence of subdural haematoma. 4. Active tuberculosis. | |
| Interventions | Participants were randomly assigned to two groups. Group 1 (n = 27): glucocorticosteroids (methylprednisolone 1 g intravenous daily). Group 2 (n = 28): no intervention. Duration: 3 days | |
| Outcomes | mortality, adverse events. | |
| Notes | Reasons for post‐randomisation dropouts: group 1: 1, Group 2: 4 (previous steroids, doubtful diagnosis). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were allocated by random sealed envelope". Comment: Further details were not available. |
| Allocation concealment (selection bias) | Low risk | Quote: "random sealed envelopes". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "patients were allocated to a control or treatment group". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "5 patients were not included in the analysis". Comment: there were post‐randomisation dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: all important outcomes were reported. |
| For‐profit bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: there was no other bias. |
Thursz 2015.
| Methods | Randomised clinical trial | |
| Participants | Country: UK. Number randomised: 1103. Post‐randomisation dropouts: 11 (0.99%). Revised sample size: 1092. Average age: 49 years. Females: 407 (37.2%). Inclusion criteria: 1. Clinical diagnosis of alcoholic hepatitis (history of recent excess alcohol consumption and the absence of other causes of liver disease). 2. Age of 18 years or older. 3. An average alcohol consumption of more than 80 g per day for men and more than 60 g per day for women. 4. A serum bilirubin level greater than 80 μmol per litre (4.7 mg per dL). 5. A discriminant function of 32 or higher. Exclusion criteria: 1. Jaundice for more than 3 months. 2. Cessation of alcohol consumption for more than 2 months before randomisation. 3. The presence of other causes of liver disease. 4. A serum aspartate aminotransferase level greater than 500 IU per litre or serum alanine transaminase level greater than 300 IU per litre. 5. Previous entry into the study within the preceding 6 months. | |
| Interventions | Participants were randomly assigned to four groups.
Group 1 (n = 273): glucocorticosteroids (prednisolone 40 mg once daily) plus pentoxifylline 400 mg thrice daily.
Group 2 (n = 272): placebo plus placebo. Group 3 (n = 274): glucocorticosteroids (prednisolone 40 mg one daily) plus placebo. Group 4 (n = 273): pentoxifylline 400 thrice daily plus placebo. Duration: 28 days |
|
| Outcomes | mortality, adverse events, decompensated cirrhosis. | |
| Notes | Reasons for post‐randomisation dropouts: group 1 (Combination group): 1 (incorrect randomisation). Group 2 (placebo‐placebo): 4 (incorrect randomisation). Group 3 (prednisolone/placebo): 3 (1 incorrect randomisation, 2 no usage of data). Group 4 (Pentoxifylline/placebo): 3 (1 incorrect randomisation, 2 no usage of data). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A Web‐based computer system (Tenalea, Forms‐ Vision) was used to enrol eligible patients and randomly assign them to study groups". |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization was performed with a block size of four, with stratification according to geographic area and risk category". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "STOPAH was a multicenter, randomised, double‐blind trial". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | This information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: there were post‐randomisation dropouts. 5 not considered for the analysis |
| Selective reporting (reporting bias) | Low risk | Comment: all important outcomes were reported. |
| For‐profit bias | Low risk | Quote: "Supported by a grant (08 14 44) from the National Institute for Health Research (NIHR) Health Technology Assessment program". |
| Other bias | Low risk | Comment: there was no other bias. |
Tkachenko 2016.
| Methods | Randomised clinical trial | |
| Participants | Country: Russia.
Number randomised: 40.
Post‐randomisation dropouts: 0 (0%).
Revised sample size: 40.
Average age: 46 years.
Females: 13 (32.5%).
Inclusion criteria: 1. 18 years or older. 2. Maddrey’s discriminant function of 32 or more. 3. Average alcohol intake of more than 50 g/day during the 3 months before enrolment. 4. Screening‐tests results. Exclusion criteria: 1. Cessation of alcohol consumption for more than 2 months before randomisation. 2. SAMe, UDCA, or pentoxifylline administration prior to hospitalisation. 3. Presence of other causes of liver disease. 4. Uncontrolled bacterial infection or gastrointestinal haemorrhage in the previous 4 days. 5. Cancer. 6. Psychiatric disease. 7. Drug abuse. 8. Serious cardiac, respiratory, or neurologic disease, and previous entry into the study within the preceding 6 months. |
|
| Interventions | Participants were randomly assigned to two groups. Group 1 (n = 20): glucocorticosteroids (prednisolone 40 mg/day for 28 days) plus SAMe (started as 800 mg intravenously daily converted to 1200 mg/day for 3 months). Group 2 (n = 20): glucocorticosteroids (prednisolone 40 mg/day for 28 days) Duration: 28 days (prednisolone) and 90 days (for SAMe) | |
| Outcomes | mortality, adverse events. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomization was performed with sequentially numbered envelopes". |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Randomization was performed with sequentially numbered envelopes". Comment: Further details were not available. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "a multicenter, open‐label, randomized controlled study". |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "a multicenter, open‐label, randomized controlled study". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no post‐randomisation dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: all important outcomes were reported. |
| For‐profit bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: there was no other bias. |
Trinchet 1989a.
| Methods | Randomised clinical trial | |
| Participants | Country: Belgium. Number randomised: 67. Post‐randomisation dropouts: 0 (0%). Revised sample size: 67. Average age: 52 years. Females: 29 (43.3%). Inclusion criteria: Histologically proven acute alcoholic hepatitis assessed by percutaneous liver biopsy. Exclusion criteria: 1. Hepatic encephalopathy. 2. Presence of ascites. 3. Prothrombin activity below 50%. 4. Platelet count below 100,000/μL. 5. Hepatocellular carcinoma. 6. Evident lack of compliance. 7. Refusal to participate in the study. | |
| Interventions | Participants were randomly assigned to two groups. Group 1 (n = 33): colchicine 1 mg once daily. Group 2 (n = 34): placebo. Duration: 6 months | |
| Outcomes | mortality, adverse events, decompensated cirrhosis. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly allocated into two groups". Comment: Further details were not available. |
| Allocation concealment (selection bias) | Low risk | Quote: "…using sealed envelopes with a stratification according to the presence or absence of cirrhosis". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Drugs ans placebo were administered in a double blind fashion. Control patients received placebo in a identical presentation". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: Not clear if ITT, there were patients who did not complete the trial, not clear if outcome assessed. |
| Selective reporting (reporting bias) | Low risk | Comment: all important outcomes were reported. |
| For‐profit bias | High risk | Quote: "Houde Pharmaceutical Laboratories, Paris, France supplied colchicine and placebo". |
| Other bias | Low risk | Comment: there was no other bias. |
Trinchet 1989b.
| Methods | Randomised clinical trial | |
| Participants | Country: France. Number randomised: 116. Post‐randomisation dropouts: not stated. Revised sample size: 116. Average age: not stated Females: not stated Inclusion criteria: 1. Histologically proven alcoholic hepatitis. Exclusion criteria: 1. Hepatic encephalopathy. 2. Contraindication to percutaneous liver biopsy. 3. Hepatocellular carcinoma. 4. Evident lack of discipline. 5. Refusal to enter the trial. | |
| Interventions | Participants were randomly assigned to two groups. Group 1 (n = 57): silymarin (420 mg/day oral). Group 2 (n = 59): placebo. Duration: 3 months treatment | |
| Outcomes | mortality, adverse events. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "random number table". |
| Allocation concealment (selection bias) | Low risk | Quote: "….using numbered sealed envelopes". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "a randomised double blind trial". Comment: identical placebo was used to achieve double‐blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "a randomised double blind trial". Comment: identical placebo was used to achieve double‐blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | Low risk | Comment: all important outcomes were reported. |
| For‐profit bias | High risk | Quote: "The authors thank Dr De Peufeilhoux and Dr Piquemal of Roger Bellon Laboratories for their assistance." |
| Other bias | Low risk | Comment: there was no other bias. |
Trinchet 1992.
| Methods | Randomised clinical trial | |
| Participants | Country: France. Number randomised: 82. Post‐randomisation dropouts: 10 (12.2%). Revised sample size: 72. Average age: 49 years. Females: 36 (50%). Inclusion criteria: 1. Age higher than 18 years. 2. Hospitalised chronic alcoholics (alcohol intake more than 100 g/day in men and 80 g/day in women). 3. Liver biopsy (at least two of the following three lesions of alcoholic hepatitis: polymorphonuclear inflammatory infiltration, Mallory bodies and hepatocellular damage). Exclusion criteria: 1. Hepatocellular carcinoma. 2. Insulin‐dependent diabetes. 3. An extrahepatic disorder involving poor short‐term prognosis. 4. any treatment that could potentially influence the course pf alcoholic hepatitis such as corticosteroids, anabolic steroids, propylthiouracil. | |
| Interventions | Participants were randomly assigned to two groups. Group 1 (n = 37): 30 IU of insulin plus 3 mg of glucagon. Group 2 (n = 35): placebo. Duration: 3 weeks | |
| Outcomes | mortality. | |
| Notes | Reasons for post‐randomisation dropouts: 10 not compatible biopsy (post‐randomisation). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "treatment was randomised by centre". Comment: Further details were not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: there were post‐randomisation dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: all important outcomes were reported. |
| For‐profit bias | Low risk | Quote: "Clinical Research Contract from the Assistance Publique, Hospitaux de Paris". |
| Other bias | Low risk | Comment: there was no other bias. |
Velussi 1997.
| Methods | Randomised clinical trial | |
| Participants | Country: Italy. Number randomised: 60. Post‐randomisation dropouts: 0 (0%). Revised sample size: 60. Average age: 63 years. Females: not stated Inclusion criteria: 1. Age 45 to 70 years. 2. Non‐insulin dependent diabetes mellitus with alcoholic liver cirrhosis. 3. Body mass index less than 29 kg/m2. 4. Ascertained diabetes for a period of at least 5 years and treated with insulin only. 5. Undergoing stable insulin therapy for a period of at least 2 years. 6. Presenting raised endogenous insulin secretion. 7. Fasting insulin levels and basal and stimulated Cpeptide levels above normal range (above 15 mU/ml for insulin; above 1 ng/mL for basal C‐peptide levels and 3 ng/mL stimulated C‐peptide levels). 8. Liver biopsy, performed no more than 4 years prior to enrolment, demonstrating liver cirrhosis. Exclusion criteria: 1. Negative for markers of hepatitis A, B and C. 2. No bleeding from oesophageal varices. 3. Not addicted to alcohol for a period of at least 2 years prior to the start of the study | |
| Interventions | Participants were randomly assigned to two groups. Group 1 (n = 30): silymarin 600 mg thrice daily. Group 2 (n = 30): no intervention. Duration: 12 months | |
| Outcomes | adverse events. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "On inclusion into the study, the patients were randomly assigned to one of two groups". Comment: Further details were not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "The control group (30 patients), were not treated with silymarin". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no post‐randomisation dropouts. |
| Selective reporting (reporting bias) | High risk | Comment: mortality was not reported. |
| For‐profit bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: there was no other bias. |
ALD = alcoholic liver disease ALT = alanine transaminase AST = aspartate transaminase G‐CSF = granulocyte‐colony stimulating factor HCC = hepatocellular carcinoma ITT = intention‐to‐treat IU = international unit LT = liver transplant MELD = model for end‐stage liver disease NAFLD = non‐alcoholic fatty liver disease PTX = pentoxifylline SAMe = s‐adenosyl‐L‐methionine SC = subcutaneous SGOT = serum glutamic oxaloacetic transaminase (currently called aspartate transaminase) SGPT = serum glutamic pyruvic transaminase (currently called alanine transaminase) TNF = tumour necrosis factor UDCA = ursodeoxycholic acid
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Achord 1987 | One group received nutritional supplements as treatment. |
| Bunout 1989 | One group received nutritional support as treatment. |
| Cabre 2000 | One group received nutritional support as treatment. |
| Calvey 1985 | Groups received different nutritional supports as treatment. |
| Carithers 1990 | Comment on Carithers 1989. |
| Conn 1978 | Editorial. |
| DiNubile 2015 | Comment on Thursz 2015. |
| Dupont 2012 | Groups received different nutritional supports as treatment. |
| Hendy 2016 | Comment on Thursz 2015. |
| Hirsch 1993 | One group received nutritional support as treatment. |
| Kearns 1992 | One group received nutritional support as treatment. |
| Kolasani 2016 | The intervention and control groups did not receive a standardised drug treatment regime. |
| Lesesne 1978 | One group received nutritional supplements as treatment. |
| Ma 2011 | One group received nutritional supplements as treatment. |
| Mathurin 1996 | Long‐term follow‐up of Ramond 1992, but patients in the placebo group received prednisolone routinely, breaking the randomisation. |
| Mezey 1991 | One group received nutritional supplements as treatment. |
| Moreno 2014 | Both groups received corticosteroids plus a different type of nutrition. |
| Nasrallah 1980 | One group received nutritional supplements as treatment. |
| Naveau 1986 | Groups received different nutritional supports as treatment. |
| Panos 1990 | One group received nutritional supplements as treatment. |
| Ramond 1992a | Comment on Ramond 1992. |
| Sas 2011 | One group received nutritional supplements as treatment. |
| Simon 1988 | One group received nutritional supplements as treatment. |
| Spahr 2008 | The intervention and control group did not have fixed drug regimens. |
| Thursz 2015a | Comment on Thursz 2015. |
| Verbeke 2015 | Comment on Thursz 2015. |
| Wenzel 1993 | Quasi‐randomised study (randomisation by date of birth). |
Differences between protocol and review
We used both the fixed‐effect model and random‐effects model, and used the more conservative model to arrive at conclusions, rather than using the model with the best fit as defined by deviance information criteria.
We also revised the network meta‐analysis extensively to ensure that these reflect recent developments in this field.
We analysed most outcomes as time‐to‐event outcomes since the length of follow‐up between the trials was very variable. Ignoring this difference in length of follow‐up in a network meta‐analysis means a major (and probably incorrect) assumption that the frequency of events was not dependent upon the length of follow‐up in the trials.
Contributions of authors
Elena Buzzetti, Maria Kalafateli identified trials and extracted the data. Elena Buzetti also wrote the first draft of the review.
Kurinchi Gurusamy wrote the protocol, performed the analysis, and wrote parts of the review. Douglas Thorburn, Emmanuel Tsochatzis, and Brian Davidson critically commented on the protocol and the review. Maja Thiele, Lise Lotte Gluud, Cinzia Del Giovane, Gro Askgaard, and Aleksander Krag critically commented on the review.
Sources of support
Internal sources
University College London, UK.
External sources
-
National Institute for Health Research, UK.
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure, Cochrane Programme Grant, or Cochrane Incentive funding to the Cochrane Hepato‐Biliary and Upper Gastrointestinal and Pancreatic Diseases Groups. The views and opinions expressed therein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, the National Health Service, or the Department of Health. The funding covered salary, equipment, and other resources to complete this review.
Declarations of interest
This report is independent research funded by the National Institute for Health Research (NIHR Cochrane Programme Grants, 13/89/03 ‐ Evidence‐based diagnosis and management of upper digestive, hepato‐biliary, and pancreatic disorders). The views expressed in this publication are those of the authors and not necessarily those of the National Health Service (NHS), the NIHR, or the Department of Health.
Kurinchi Gurusamy, Emmanuel Tsochatzis, and Brian Davidson have no financial disclosures.
Astellas funded Douglas Thorburn for his attendance at the International Liver Transplantation Society meeting in 2014. Douglas Thorburn has also received GBP 25,000 from Boston Scientific to fund a clinical research fellow in 2013. There are no other financial disclosures to report.
Edited (no change to conclusions)
References
References to studies included in this review
Akriviadis 1990 {published data only}
- Akriviadis EA, Steindel H, Pinto PC, Fong TL, Kanel G, Reynolds TB, et al. Failure of colchicine to improve short‐term survival in patients with alcoholic hepatitis. Gastroenterology 1990;99:811‐8. [DOI] [PubMed] [Google Scholar]
Akriviadis 2000 {published data only}
- Akriviadis E, Botla R, Briggs W, Han S, Reynolds T, Shakil O. Improved short‐term survival with pentoxifylline treatment in severe acute alcoholic hepatitis. Hepatology 1997;26(4 (Pt 2)):250a. [DOI] [PubMed] [Google Scholar]
- Akriviadis E, Botla R, Briggs W, Han S, Reynolds T, Shakil O. Pentoxifylline improves short‐term survival in severe acute alcoholic hepatitis: a double‐blind, placebo‐controlled trial. Gastroenterology 2000;119:1637‐48. [DOI] [PubMed] [Google Scholar]
Baker 1981 {published data only}
- Baker AL, Jaspan JB, Haines NW. A randomized clinical trial of insulin and glucagon infusion for treatment of alcoholic hepatitis: progress report in 50 patients. Gastroenterology 1981;80:1410‐4. [PubMed] [Google Scholar]
- Hatfield G, Haines N, Schneider J. Randomized clinical trial of insulin and glucagon for alcoholic hepatitis. Gastroenterology 1979;76:1284. [PubMed] [Google Scholar]
Basu 2015 {published data only}
- Basu P, Shah NJ, Aloysius MM. Role of mycophenolate mofetil (mmf) in steroid non responsive severe acute alcoholic hepatitis: a randomized open label placebo control prospective clinical pilot trial. HPB 2015;17:44. [Google Scholar]
Bird 1991 {published data only}
- Bird G, Lau JY, Koskinas J, Wicks C, Williams R. Insulin and glucagon infusion in acute alcoholic hepatitis: a prospective randomized controlled trial. Hepatology 1991;14:1097‐101. [PubMed] [Google Scholar]
Bird 1998 {published data only}
- Bird GL, Prach AT, McMahon AD, Forrest JA, Mills PR, Danesh BJ. Randomised controlled double‐blind trial of the calcium channel antagonist amlodipine in the treatment of acute alcoholic hepatitis. Journal of Hepatology 1998;28:194‐8. [DOI] [PubMed] [Google Scholar]
Blitzer 1977 {published data only}
- Blitzer BL, Mutchnic MG, Joshi PH, Phillips MM, Fessel JM, Conn HO. Adrenocorticosteroid therapy in alcoholic hepatitis ‐ prospective, double‐blind, randomized study. Gastroenterology 1973;64(4):880. [DOI] [PubMed] [Google Scholar]
- Blitzer BL, Mutchnick MG, Joshi PH. Adrenocorticosteroid therapy in alcoholic hepatitis. A prospective, double blind randomized study. American Journal of Digestive Diseases 1977;22:477‐84. [DOI] [PubMed] [Google Scholar]
Boetticher 2008 {published data only}
- Boetticher NC, Peine CJ, Kwo P, Abrams GA, Patel T, Aqel B, et al. A randomized, double‐blinded, placebo‐controlled multicenter trial of etanercept in the treatment of alcoholic hepatitis. Gastroenterology 2008;135:1953‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bories 1987 {published data only}
- Bories P, Guedj JY, Mirouze D, Yousfi A, Michel H. Treatment of acute alcoholic hepatitis with prednisolone. 45 patients. Presse Medicale 1987;16(16):769‐72. [PubMed] [Google Scholar]
Caballeria 1998 {published data only}
- Caballeria J. Metadoxine in the treatment of alcoholic fatty liver a randomized multicenter trial. Hepatology 1996;24(4 (Pt 2)):444a. [Google Scholar]
- Caballeria J, Pares A, Bru C, Mercader J, Garcia Plaza A, Caballeria L, et al. Metadoxine accelerates fatty liver recovery in alcoholic patients: results of a randomized double‐blind, placebo‐control trial. Spanish Group for the Study of Alcoholic Fatty Liver. Journal of Hepatology 1998;28:54‐60. [DOI] [PubMed] [Google Scholar]
Campra 1973 {published data only}
- Campra JL, Hamlin EM Jr, Kirshbaum RJ, Olivier M, Redeker AG, Reynolds TB. Prednisone therapy of acute alcoholic hepatitis. Report of a controlled trial. Annals of Internal Medicine 1973;79(5):625‐31. [DOI] [PubMed] [Google Scholar]
Carithers 1989 {published data only}
- Carithers RL Jr, Herlong F, Diehl AM, Shaw EW, Combes B, Fallon HJ, et al. Methylprednisolone therapy in patients with severe alcoholic hepatitis. A randomized multicenter trial. Annals of Internal Medicine 1989;110(9):685‐90. [DOI] [PubMed] [Google Scholar]
- Maddrey WC, Carithers RL, Herlong HF, Combes B, Diehl AM, Shaw E, et al. Prednisolone therapy in patients with severe alcoholic hepatitis: results of a multicenter trial. Hepatology 1986;6:1202. [DOI] [PubMed] [Google Scholar]
Colman 1998 {published data only}
- Colman JC, Cromie SL, Cox JM, Roberts SK, Dudley E, Dudley FJ. The natural history of alcoholic cirrhosis: effect of colchicine. Hepatology 1998;28:510A. [Google Scholar]
Cortez‐Pinto 2002 {published data only}
- Cortez Pinto H, Alexandrino P, Camilo ME, Couveia‐Oliveira A, Santos PM, Alves MM, et al. Lack of effect of colchicine in alcoholic cirrhosis: Final results of a double‐blind randomized trial. Hepatology 2000;32(4 (Pt 2)):423a. [DOI] [PubMed] [Google Scholar]
- Cortez Pinto H, Alexandrino P, Santos PM, Alves MM, Camilo ME, Moura MC. Colchicine in alcoholic cirrhosis ‐ preliminary results of a double‐blind randomized trial. Hepatology 1992;16(2 (Pt 2)):229a. [DOI] [PubMed] [Google Scholar]
- Cortez‐Pinto H, Alexandrino P, Camilo ME, Gouveia‐Oliveira A, Santos PM, Alves MM, et al. Lack of effect of colchicine in alcoholic cirrhosis: final results of a double blind randomized trial. European Journal of Gastroenterology & Hepatology 2002;14:377‐81. [DOI] [PubMed] [Google Scholar]
De 2009 {published data only}
- De BK, Gangopadhyay S, Dutta D, Baksi SD, Pani A, Ghosh P. Pentoxifylline versus prednisolone for severe alcoholic hepatitis: a randomized controlled trial. World Journal of Gastroenterology 2009;15(13):1613‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
De 2014 {published data only}
- De B, Mandal S, Sau D, Mani S, Chatterjee S, Mondal S, et al. Pentoxifylline plus prednisolone versus pentoxifylline only for severe alcoholic hepatitis: a randomized controlled clinical trial. Annals of Medical and Health Sciences Research 2014;4(5):810‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
De la Maza 1995 {published data only}
- Maza MP, Petermann M, Bunout D, Hirsch S. Effects of long‐term vitamin E supplementation in alcoholic cirrhotics. Journal of the American College of Nutrition 1995;14:192‐6. [DOI] [PubMed] [Google Scholar]
Depew 1980 {published data only}
- Depew W, Boyer T, Omata M. Double‐blind controlled trial of prednisolone therapy in patients with severe acute alcoholic hepatitis and spontaneous encephalopathy. Gastroenterology 1980;78:524‐9. [PubMed] [Google Scholar]
- Depew WT, Boyer TD, Omata M, Redeker AG, Reynolds TB. Double‐blind controlled trial of corticosteroid‐therapy in severe alcoholic hepatitis with encephalopathy. Clinical Research 1978;26(2):A150. [Google Scholar]
De Silva 2003 {published data only}
- Silva HA, Saparamadu PA, Thabrew MI, Pathmeswaran A, Fonseka MM, Silva HJ. Liv.52 in alcoholic liver disease: a prospective, controlled trial. Journal of Ethnopharmacology 2003;84:47‐50. [DOI] [PubMed] [Google Scholar]
Diaz Belmont 1996 {published data only}
- Diaz Belmont A, Dominguez Henkel R, Uribe Ancira F. Parenteral S‐adenosylmethionine compared to placebos in the treatment of alcoholic liver diseases. Anales de Medicina Interna 1996;13:9‐15. [PubMed] [Google Scholar]
Feher 1989 {published data only}
- Feher J, Deak G, Muzes G, Lang I, Niederland V, Nekam K, et al. Liver‐protective action of silymarin therapy in chronic alcoholic liver diseases. Orvosi Hetilap 1989;130:2723‐7. [PubMed] [Google Scholar]
Fenster 1966 {published data only}
- Fenster LF. The non efficacy of short‐term anabolic steroid therapy in alcoholic liver disease. Annals of Internal Medicine 1966;65:738‐44. [Google Scholar]
Fernandez‐Rodriguez 2008 {published data only}
- Fernandez‐Rodriguez CM, Lledo JL, Lopez‐Serrano P, Gutierrez ML, Alonso S, Perez‐Fernandez MT, et al. Effect of pentoxifylline on survival, cardiac function and both portal and systemic hemodynamics in advanced alcoholic cirrhosis‐a randomized double‐blind placebo‐controlled trial. Revista Espanola de Enfermedades Digestivas 2008;100:481‐9. [DOI] [PubMed] [Google Scholar]
- Fernandez‐Rodriguez CM, Lledó JL, Lopez Serrano P, Alonso S, Cerro J, Sanz‐Mayordomo P. Pentoxifylline ameliorates vasodilation and left ventricular function in alcoholic cirrhosis. Journal of Hepatology 2005;42(Suppl 2):80. [Google Scholar]
Fleig 1997 {published data only}
- Fleig WE, Morgan MY, Holzer MA. The ayurvedic medicine LIV.52 in alcoholic cirrhosis of the liver: Results of a prospective, randomised, double‐blind, placebo‐controlled, clinical study. Zeitschrift fur Gastroenterologie 1998;36:100. [Google Scholar]
- Fleig WE, Morgan MY, Hölzer MA. The ayurvedic drug Liv52 in patients with alcoholic cirrhosis results of a prospective, randomized, doubleblind, placebo‐controlled clinical trial. Journal of Hepatology 1997;26(Suppl 1):S127. [Google Scholar]
Garrido Garcia 2012 {published data only}
- Garrido Garcia JR, Sánchez HG, Melchor LA, Elizalde BCI, Sánchez VL. Pentoxifylline versus steroid in short‐term survival in severe acute alcoholic hepatitis. Medicina Interna de México 2012;28(3):227‐33. [Google Scholar]
Geminiani 1979 {published data only}
- Geminiani S, Mazella G, Aldini R, Bazzoli F, Morselli A M, Rossi R, et al. Effect of cycloxilic acid administration on ethanol‐induced hepatic steatosis. Controlled clinical trial. Farmaco ‐ Edizione Pratica 1979;34:449‐55. [PubMed] [Google Scholar]
Gluud 1986 {published data only}
- Gluud C. Effect of testosterone treatment in men with alcoholic cirrhosis. Journal of Hepatology 1985;1(Suppl 1):S59. [Google Scholar]
- The Copenhagen Study Group for Liver Diseases. Testosterone treatment of men with alcoholic cirrhosis: a double‐blind study. The Copenhagen Study Group for Liver Diseases. Hepatology 1986;6(5):807‐13. [PubMed] [Google Scholar]
Halle 1982 {published data only}
- Halle P, Pare P, Kaptein E, Kanel G, Redeker AG, Reynolds TB. Double‐blind, controlled trial of propylthiouracil in patients with severe acute alcoholic hepatitis. Gastroenterology 1982;82:925‐31. [PubMed] [Google Scholar]
- Halle P, Pare P, Kaptein E, Kanel G, Redeker AG, Reynolds TB. Propylthiouracil therapy in severe acute alcoholic hepatitis. Gastroenterology 1980;79(5 (Pt 2)):1024. [PubMed] [Google Scholar]
Helman 1971 {published data only}
- Helman RA, Temko MH, Nye SW, Fallon HJ. Alcoholic hepatitis. Natural history and evaluation of prednisolone therapy. Annals of Internal Medicine 1971;74:311‐21. [DOI] [PubMed] [Google Scholar]
Higuera de la Tijera 2015 {published data only}
- Higuera‐de la Tijera F, Servin‐Caamano AI, Cruz‐Herrera J, Serralde‐Zuniga AE, Abdo‐Francis JM, Gutierrez‐Reyes G, et al. Treatment with metadoxine and its impact on early mortality in patients with severe alcoholic hepatitis. Annals of Hepatology 2014;13:343‐52. [PubMed] [Google Scholar]
- Higuera‐de la Tijera F, Servin‐Caamano AI, Serralde‐Zuniga AE, Cruz‐Herrera J, Perez‐Torres E, Abdo‐Francis JM, et al. Metadoxine improves the three‐ and six‐month survival rates in patients with severe alcoholic hepatitis. World Journal of Gastroenterology 2015;21(16):4975‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Keiding 1994 {published data only}
- Keiding S, Badsberg JH, Becker U, Bentsen KD, Bonnevie O, Caballeria J, et al. The prognosis of patients with alcoholic liver disease. An international randomized, placebo‐controlled trial on the effect of malotilate on survival. Journal of Hepatology 1994;20:454‐60. [DOI] [PubMed] [Google Scholar]
- Keiding S, Bendtsen K, Becker U, Bonnevie O, Hardt F, Juhl E, et al. Malotilate in alcoholic liver disease a randomized, double‐blind, international trial of the effect of long‐term treatment on survival. Hepatology 1990;12(2):419. [Google Scholar]
Kim 2012 {published data only}
- Kim MY, Baik SK, Jang YO, Yea CJ, Won CS, Byun JW, et al. The beneficial effect of angiotensin‐blocking agent on liver fibrosis in patients with alcoholic liver disease: a randomized controlled trial. Hepatology 2009;50:812A‐3A. [Google Scholar]
- Kim MY, Cho MY, Baik SK, Jeong PH, Suk KT, Jang YO, et al. Beneficial effects of candesartan, an angiotensin‐blocking agent, on compensated alcoholic liver fibrosis ‐ a randomized open‐label controlled study. Liver International 2012;32:977‐87. [DOI] [PubMed] [Google Scholar]
Lebrec 2010 {published data only}
- Lebrec D, Thabut D, Oberti F, Perarnau JM, Condat B, Barraud H, et al. Pentoxifylline (PTX) reduces the risk of complications in patients with advanced cirrhosis. Analysis of the results of a pivotal phase 3 trial. Journal of Hepatology 2009;50:S378. [Google Scholar]
- Lebrec D, Thabut D, Oberti F, Perarnau JM, Condat B, Barraud H, et al. Pentoxifylline does not decrease short‐term mortality but does reduce complications in patients with advanced cirrhosis. Gastroenterology 2010;138:1755‐U40. [DOI] [PubMed] [Google Scholar]
Lotterer 1995 {published data only}
- Lotterer E, Etzel R. LIV. 52 in patients with alcoholic liver cirrhosis ‐ pilot study of a controlled clinical trial. Forschende Komplementärmedizin 1995;2:12‐4. [Google Scholar]
Lucena 2002 {published data only}
- Lucena MI, Andrade RJ, Cruz JP, Rodriguez‐Mendizabal M, Blanco E, Sanchez de la Cuesta F. Effects of silymarin MZ‐80 on oxidative stress in patients with alcoholic cirrhosis. International Journal of Clinical Pharmacology & Therapeutics 2002;40:2‐8. [DOI] [PubMed] [Google Scholar]
Maddrey 1978 {published data only}
- Maddrey WC, Boitnott JK, Bedine MS, Weber FL Jr, Mezey E, White RI Jr. Corticosteroid therapy of alcoholic hepatitis. Gastroenterology 1978;75:193‐9. [PubMed] [Google Scholar]
Mathurin 2013 {published data only}
- Louvet A, Dao TM, Nahon P, Diaz E, Carbonell N, Boursier J, et al. Pentoxifylline does not improve short‐term survival in severe alcoholic hepatitis in combination with corticosteroids: results of a randomized controlled trial. Journal of Hepatology 2012;56:S533‐4. [Google Scholar]
- Mathurin P, Louvet A, Duhamel A, Nahon P, Carbonell N, Boursier J, et al. Prednisolone with vs without pentoxifylline and survival of patients with severe alcoholic hepatitis: a randomized clinical trial. JAMA 2013;310:1033‐41. [DOI] [PubMed] [Google Scholar]
Mato 1999 {published data only}
- Mato JM, Camara J, Fernandez de Paz J, Caballeria L, Coll S, Caballero A, et al. S‐adenosylmethionine in alcoholic liver cirrhosis: a randomized, placebo‐controlled, double‐blind, multicenter clinical trial. Journal of Hepatology 1999;30:1081‐9. [DOI] [PubMed] [Google Scholar]
- Mato JM, Camara J, Ortiz P, Rodes J. S‐adenosylmethionine in the treatment of alcoholic liver cirrhosis: Results from a multicentric placebo‐controlled, randomized, double‐blind clinical trial. Hepatology 1997;26(4 (Pt 2)):251a. [Google Scholar]
McHutchinson 1991 {published data only}
- McHutchinson JG, Runyon BA, Draguesku JQ, Cominelli F, Person JL, Castracane J. Pentoxyfylline may prevent renal impairment (hepatorenal syndrome) in severe acute alcoholic hepatitis. Hepatology 1991;14(Suppl S4):96A. [Google Scholar]
Medici 2011 {published data only}
- Medici V, Virata MC, Peerson JM, Stabler SP, French SW, Gregory JF 3rd, et al. S‐adenosyl‐L‐methionine treatment for alcoholic liver disease: a double‐blinded, randomized, placebo‐controlled trial. Alcoholism, Clinical and Experimental Research 2011;35:1960‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mendenhall 1984 {published data only}
- Mendenhall C. Survival after steroid treatment (T) for alcoholic hepatitis (AH). Hepatology 1983;3(5):850. [Google Scholar]
- Mendenhall C, Goldberg S. Risk factors and therapy in alcoholic hepatitis (AH). Gastroenterology 1977;72(5):A‐77. [Google Scholar]
- Mendenhall CL, Anderson S, Garcia‐Pont P. Short‐term and long‐term survival in patients with alcoholic hepatitis treated with oxandrolone and prednisolone. New England Journal of Medicine 1984;311:1464‐70. [DOI] [PubMed] [Google Scholar]
Mezey 2004 {published data only}
- Mezey E, Potter JJ, Rennie‐Tankersley L, Caballeria J, Pares A. A randomized placebo controlled trial of vitamin E for alcoholic hepatitis. Journal of Hepatology 2004;40:40‐6. [DOI] [PubMed] [Google Scholar]
- Mezey E, Potter JJ, Rennie‐Tankersley L, Caballeria J, Pares A. A randomized placebo controlled trial of vitamin E in alcoholic hepatitis. Hepatology 2003;38(4):264A. [DOI] [PubMed] [Google Scholar]
Mirouze 1982 {published data only}
- Mirouze D, Redeker A, Reynolds T, Michel H. A randomized clinical trial of insulin and glucagon infusion for treatment of severe acute alcoholic hepatitis: Report in 26 patients. Scandinavian Journal of Gastroenterology 1982;17:36. [Google Scholar]
Moreno 2010 {published data only}
- Moreno C, Langlet P, Hittelet A, Evrard S, Lasser L, Colle I. Enteral nutrition with or without N‐acetylcysteine in the treatment of severe acute alcoholic hepatitis: a randomized, multicenter, controlled trial. Journal of Hepatology 2009;50:S366. [DOI] [PubMed] [Google Scholar]
- Moreno C, Langlet P, Hittelet A, Lasser L, Degre D, Evrard S, et al. Enteral nutrition with or without N‐acetylcysteine in the treatment of severe acute alcoholic hepatitis: a randomized multicenter controlled trial. Journal of Hepatology 2010;53(6):1117‐22. [DOI] [PubMed] [Google Scholar]
Morgan 2005 {published data only}
- Morgan TR, Nemchausky B, Schiff E, Anand BS, Bloor J, Kidao J, et al. Colchicine does not prolong life in patients with advanced alcoholic cirrhosis: Results of a prospective, randomized, placebo‐controlled, multicenter VA trial (CSP 352). Gastroenterology 2002;122(4):A641. [Google Scholar]
- Morgan TR, Weiss DG, Nemchausky B, Schiff ER, Anand B, Simon F, et al. Colchicine treatment of alcoholic cirrhosis: a randomized, placebo‐controlled clinical trial of patient survival. Gastroenterology 2005;128:882‐90. [DOI] [PubMed] [Google Scholar]
Naveau 2004 {published data only}
- Naveau S, Chollet‐Martin S, Dharancy S, Mathurin P, Jouet P, Piquet MA, et al. A double blind randomized controlled trial of infliximab associated with prednisolone in acute alcoholic hepatitis. Gastroenterology 2004;126(4):A692. [DOI] [PubMed] [Google Scholar]
- Naveau S, Chollet‐Martin S, Dharancy S, Mathurin P, Jouet P, Piquet MA, et al. A double‐blind randomized controlled trial of infliximab associated with prednisolone in acute alcoholic hepatitis. Hepatology 2004;39(5):1390‐7. [DOI] [PubMed] [Google Scholar]
Nguyen‐Khac 2011 {published data only}
- Nguyen‐Khac E, Thevenot T, Piquet M, Benferhat S, Goria O, Chatelain D. Treatment of severe acute alcoholic hepatitis (AAH) with corticoids plus N‐acetyl cysteine (C+NAC) versus corticoids alone (C): a multicentre, randomized, controlled trial. Hepatology 2009;50(4 (Suppl)):346a‐7a. [Google Scholar]
- Nguyen‐Khac E, Thevenot T, Piquet MA, Benferhat S, Goria O, Chatelain D, et al. Glucocorticoids plus N‐acetylcysteine in severe alcoholic hepatitis. New England Journal of Medicine 2011;365:1781‐9. [DOI] [PubMed] [Google Scholar]
- Nguyen‐Khac E, Thevenot T, Piquet MA, Benferhat S, Goria O, Chatelain D, et al. Treatment of severe acute alcoholic hepatitis (AAH) with corticoids plus N‐acetyl cysteine (C+NAC) versus corticoids alone (C): a multicentre, randomized, controlled trial. Journal of Hepatology 2010;52:S38. [Google Scholar]
Orrego 1979 {published data only}
- Orrego H, Kalant H, Israel Y, Blake J, Medline A, Rankin JG, et al. Effect of short‐term therapy with propylthiouracil in patients with alcoholic liver disease. Gastroenterology 1979;76:105‐15. [PubMed] [Google Scholar]
Orrego 1987 {published data only}
- Orrego H, Blake JE, Blendis LM, Compton KV, Israel Y. Long‐term treatment of alcoholic liver disease with propylthiouracil. Lancet 1988;1(8590):892. [DOI] [PubMed] [Google Scholar]
- Orrego H, Blake JE, Blendis LM, Compton KV, Israel Y. Long‐term treatment of alcoholic liver disease with propylthiouracil. New England Journal of Medicine 1987;317:1421‐7. [DOI] [PubMed] [Google Scholar]
- Orrego H, Blake JE, Blendis LM, Compton KV, Volpe R, Israel Y. Long‐term treatment of alcoholic liver disease with propylthiouracil. Part 2: influence of drop‐out rates and of continued alcohol consumption in a clinical trial. Journal of Hepatology 1994;20(3):343‐9. [DOI] [PubMed] [Google Scholar]
Paladugu 2006 {published data only}
- Paladugu H, Sawant P, Dalvi L, Kudalkar J. Role of pentoxifylline in treatment of severe acute alcoholic hepatitis ‐ a randomized controlled trial. Journal of Gastroenterology and Hepatology 2006;21:A459. [Google Scholar]
Pares 1998 {published data only}
- Pares A, Planas R, Torres M, Caballeria J, Viver JM, Acero D, et al. Effects of silymarin in alcoholic patients with cirrhosis of the liver: results of a controlled, double‐blind, randomized and multicenter trial. Journal of Hepatology 1998;28:615‐21. [DOI] [PubMed] [Google Scholar]
Park 2014 {published data only}
- Kim DJ, Suk KT, Park SH, Tak WY, Yim HJ, Um SH, et al. Short‐term survival in patients with severe alcoholic hepatitis treated with pentoxifylline vs. corticosteroid: a non‐inferiority trial ‐ a preliminary report. Journal of Hepatology 2013;58:S219‐20. [Google Scholar]
- Park SH, Kim DJ, Kim YS, Yim HJ, Tak WY, Lee HJ, et al. Pentoxifylline vs. corticosteroid to treat severe alcoholic hepatitis: a randomised, non‐inferiority, open trial. Journal of Hepatology 2014;61:792‐8. [DOI] [PubMed] [Google Scholar]
Pelletier 2003 {published data only}
- Pelletier G, Roulot D, Davion T, Masliah C, Causse X, Oberti F, et al. A randomized controlled trial of ursodeoxycholic acid in patients with alcohol‐induced cirrhosis and jaundice. Hepatology 2003;37:887‐92. [DOI] [PubMed] [Google Scholar]
Phillips 2006 {published data only}
- Phillips M, Curtis H, Portmann B, Donaldson N, Bomford A, O'Grady J. Antioxidants versus corticosteroids in the treatment of severe alcoholic hepatitis‐a randomised clinical trial. Journal of Hepatology 2006;44:784‐90. [DOI] [PubMed] [Google Scholar]
- Phillips M, Curtis H, Portmann B, Donaldson N, Bomford A, O'Grady J. Antioxidants versus corticosteroids in the treatment of severe alcoholic hepatitis: a randomized trial. Hepatology 2001;34(4):250A. [DOI] [PubMed] [Google Scholar]
Pierri 1985 {published data only}
- Pierri G, Giorgio A, Amoroso P, Fico P, Finelli L. Epomediol treatment of fatty liver in alcohol abuse. A controlled clinical double‐blind study. Clinica Terapeutica 1985;112:527‐35. [PubMed] [Google Scholar]
Plevris 1991 {published data only}
- Plevris JN, Hayes PC, Bouchier IAD. Ursodeoxycholic acid in the treatment of alcoholic liver‐disease. European Journal of Gastroenterology & Hepatology 1991;3:653‐6. [Google Scholar]
- Plevris JN, Jones AL, Dawkes R, Howie AF, Bouchier IAD, Hayes PC. Ursodeoxycholic acid in the treatment of alcoholic liver disease. Gut 1993;34(4):S7. [Google Scholar]
Popescu 2009 {published data only}
- Popescu A, Voiculescu M. Improved survival in patients with advanced alcoholic hepatitis (AAH) with a combined treatment associating a non‐absorbable antibiotic rifaximin to corticoids plus ursodeoxicholic acid (UDCA). Journal of Hepatology 2009;50:S369. [Google Scholar]
Porter 1971 {published data only}
- Porter HP, Simon FR, Pope CE, Volwiler W, Fenster LF. Corticosteroid therapy in severe alcoholic hepatitis ‐ double‐blind drug trial. New England Journal of Medicine 1971;284:1350‐5. [DOI] [PubMed] [Google Scholar]
Radvan 1983 {published data only}
- Radvan G, Kanel G, Redeker A. Management of acute alcoholic hepatitis with insulin and glucagon infusion. Australian and New Zealand Journal of Medicine 1983;13:322. [Google Scholar]
Ramond 1992 {published data only}
- Ramond MJ, Poynard T, Rueff B, Mathurin P, Chaput JC, Benhamou JP. [corticotherapy improves short‐term survival in patients with severe acute alcoholic hepatitis. A bicenter double‐blind randomized study]. Gastroenterologie Clinique Et Biologique 1991;15(2 bis):A4. [Google Scholar]
- Ramond MJ, Poynard T, Rueff B, Mathurin P, Theodore C, Chaput JC, et al. A randomized trial of prednisolone in patients with severe alcoholic hepatitis. New England Journal of Medicine 1992;326:507‐12. [DOI] [PubMed] [Google Scholar]
- Ramond MJ, Poynard T, Rueff B, Theodore C, Matthurin P, Chaput JC, et al. Prednisolone markedly improves short‐term survival of patients with severe biopsy‐proven alcoholic hepatitis a double blind randomized bicenter trial. Journal of Hepatology 1991;13(Suppl 2):S63. [Google Scholar]
Resnick 1974 {published data only}
- Resnick RH, Boitnott J, Iber FL, Makopour H, Cerda JJ. Preliminary observations of d‐penicillamine therapy in acute alcoholic liver disease. Digestion 1974;11:257‐65. [DOI] [PubMed] [Google Scholar]
Richardet 1993 {published data only}
- Mal F, Pham Huu T, Richardet JP, Roulot D, Labadie H, Pol S, et al. Corticosteroids (Cs) does not influence plasma tumor necrosis factor‐alpha (pTNF) and interleukin 6 (pIl6) concentrations in severe alcoholic hepatitis (AH): a cross over study. Hepatology 1992;16(2 (Pt 2)):234a. [Google Scholar]
- Richardet JP, Dehoux M, Mal F, Roulot D, Labadie H, Pol S, et al. Influence of corticosteroids (Cs) on plasma cytokines concentrations in patients with severe alcoholic hepatitis (HA): Results of a randomized study. Journal of Hepatology 1993;18(Suppl 1):S75. [Google Scholar]
Sainz 1992 {published data only}
- Sainz S, Guarner C, Villanueva C, Ordonez J, Sancho EJ, Enriquez J. Colchicine treatment in alcoholic liver disease. A controlled and randomized study. Journal of Hepatology 1992;16(Suppl 1):S64. [Google Scholar]
Salvagnini 1985 {published data only}
- Salvaqnini M, Martines D, Piccoli A, Sciarrone R, Rizzo A, Campesato A, et al. Silymarin treatment in alcoholic liver disease: a double blind randomized trial. Journal of Hepatology 1985;1(Suppl 1):S124. [Google Scholar]
Serrano‐Cancino 1981 {published data only}
- Serrano‐Cancino H, Botero R, Jeffers L, Mariani A, Cowen G, Ravendhran N. Treatment of severe alcoholic hepatitis with propythiouracil (PTU). American Journal of Gastroenterology 1981;76:194. [Google Scholar]
Shumaker 1978 {published data only}
- Shumaker JB, Resnick RH, Galambos JT. A controlled trial of 6‐methylprednisolone in acute alcoholic hepatitis. With a note on published results in encephalopathic patients. American Journal of Gastroenterology 1978;69:443‐9. [PubMed] [Google Scholar]
Sidhu 2012a {published data only}
- Sidhu SS, Goyal O, Singla P, Gupta D, Chhina RS, Sood A. A prospective randomised trial to compare the efficacy of corticosteroids plus pentoxifylline versus corticosteroids alone in the treatment of severe alcoholic hepatitis. Journal of Hepatology 2010;52:S306‐S. [Google Scholar]
- Sidhu SS, Goyal O, Singla P, Gupta D, Sood A, Chhina RS, et al. Corticosteroid plus pentoxifylline is not better than corticosteroid alone for improving survival in severe alcoholic hepatitis (COPE trial). Digestive Diseases & Sciences 2012;57:1664‐71. [DOI] [PubMed] [Google Scholar]
Sidhu 2012b {published data only}
- Sidhu S, Singla M, Bhatia KL. Pentoxifylline reduces disease severity and prevents renal impairment in severe acute alcoholic hepatitis: a double blind, placebo controlled trial. Hepatology 2006;44(4 (Suppl 1)):373a‐4a. [Google Scholar]
- Sidhu SS, Goyal O, Singla M, Bhatia KL, Chhina RS, Sood A. Pentoxifylline in severe alcoholic hepatitis: a prospective, randomised trial. Journal of the Association of Physicians of India 2012;60:20‐2. [PubMed] [Google Scholar]
Singh 2014 {published data only}
- Singh V, Sharma AK, Narasimhan RL, Bhalla A, Sharma N, Sharma R. Granulocyte colony‐stimulating factor in severe alcoholic hepatitis: a randomized pilot study. American Journal of Gastroenterology 2014;109:1417‐23. [DOI] [PubMed] [Google Scholar]
- Singh V, Sharma AK, Narasimhan RL, Bhalla A, Sharma N, Sharma R. Granulocyte‐colony stimulating factor (G‐CSF) in alcoholic hepatitis: a randomized controlled trial. Gastroenterology 2013;144(5 suppl. 1):S1004. [DOI] [PubMed] [Google Scholar]
Spahr 2002 {published data only}
- Spahr L, Rubbia‐Brandt L, Frossard JL, Giostra E, Rougemont AL, Pugin J, et al. Combination of steroids with infliximab or placebo in severe alcoholic hepatitis: a randomized controlled pilot study. Journal of Hepatology 2002;37:448‐55. [DOI] [PubMed] [Google Scholar]
Stenner 2000 {published data only}
- Stenner J, Jazrawi R, Verma A, Ahmed H, Northfield T. Effect of UDCA on fibrosis markers in alcoholic liver disease. Clinical science 2000;98:17p. [Google Scholar]
Stewart 2007 {published data only}
- Stewart S, Prince M, Bassendine M, Hudson M, James O, Jones D, et al. A randomized trial of antioxidant therapy alone or with corticosteroids in acute alcoholic hepatitis. Journal of Hepatology 2007;47:277‐83. [DOI] [PubMed] [Google Scholar]
- Stewart S, Prince M, Bassendine M, Hudson M, James O, Jones D, et al. A trial of antioxidant therapy alone or with corticosteroids in acute alcoholic hepatitis. Journal of Hepatology 2002;36(Suppl 1):16. [DOI] [PubMed] [Google Scholar]
Stigliano 2005 {published data only}
- Stigliano R, Marelli L, Pang K, Lowdell M, Rolles K, Patch D, et al. G‐CSF mobilization of bone marrow‐derived hepatocyte and haematopoietic progenitors in acute alcoholic hepatitis ‐ a randomised controlled trial. Preliminary data. Hepatology 2005;42:741A. [Google Scholar]
Takase 1989 {published data only}
- Takase S, Matsuda Y, Yasuhara M, Takada A. Effects of malotilate treatment on alcoholic liver disease. Alcohol 1989;6(3):219‐22. [DOI] [PubMed] [Google Scholar]
- Takase S, Takada A, Yasuhara M, Sato H, Matsuda Y. Effects of malotilate treatment on the serum markers of hepatic fibrogenesis in liver cirrhosis. Gastroenterologia Japonica 1988 Dec;23(6):639‐45. [DOI] [PubMed] [Google Scholar]
Theodossi 1982 {published data only}
- Theodossi A, Eddleston A L, Williams R. Controlled trial of methylprednisolone therapy in severe acute alcoholic hepatitis. Gut 1982;23:75‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thursz 2015 {published data only}
- Forrest E, Mellor J, Stanton L, Bowers M, Ryder P, Austin A, et al. Steroids or pentoxifylline for alcoholic hepatitis (STOPAH): study protocol for a randomised controlled trial. Trials 2013;14:262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thursz M, Forrest E, Roderick P, Day C, Austin A, O'Grady J, et al. The clinical effectiveness and cost‐effectiveness of steroids or pentoxifylline for alcoholic hepatitis (STOPAH): a 2x2 factorial randomised controlled trial. Health Technology Assessment 2015;19(102):1‐104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thursz MR, Richardson P, Allison M, Austin A, Bowers M, Day CP, et al. Prednisolone or pentoxifylline for alcoholic hepatitis. New England Journal of Medicine 2015;372:1619‐28. [DOI] [PubMed] [Google Scholar]
Tkachenko 2016 {published data only}
- Tkachenko P, Komkova I, Maevskaya M, Ivashkin V. Prednisolone plus S‐adenosylmethionine in severe alcoholic hepatitis. Journal of Hepatology 2016;64(2 suppl. 1):S239. [DOI] [PubMed] [Google Scholar]
- Tkachenko P, Maevskaya M, Pavlov A, Komkova I, Pavlov C, Ivashkin V. Prednisolone plus S‐adenosil‐L‐methionine in severe alcoholic hepatitis. Hepatology International 2016;10(6):983‐7. [DOI] [PubMed] [Google Scholar]
Trinchet 1989a {published data only}
- Trinchet JC, Beaugrand M, Callard P, Hartmann DJ, Gotheil C, Nusgens BV, et al. Treatment of alcoholic hepatitis with colchicine. Results of a randomized double blind trial. Gastroenterologie Clinique et Biologique 1989;13:551‐5. [PubMed] [Google Scholar]
- Trinchet JC, Beaugrand M, Ferrier JP, Galet B, Callard P, Hartmann D, et al. Treatment of active alcoholic liver disease (AADL) by colchicine: Results of a 6 months double blind trial. Journal of Hepatology 1985;1(Suppl 1):S142. [Google Scholar]
- Trinchet JC, Galet B, Beaugrand M, Callard P, Ferrier JP. Treatment of alcoholic hepatitis with colchicine. Preliminary results of a double blind study in 46 patients. Gastroenterologie Clinique Et Biologique 1983;7(2):103a. [PubMed] [Google Scholar]
Trinchet 1989b {published data only}
- Trinchet JC, Coste T, Levy VG, Grippon P, Vivet F, Duchatelle V, et al. Treatment of alcoholic hepatitis by silymarine, a double blind multicenter study in 116 patients. Gastroenterologie Clinique Et Biologique 1987;11(2 bis):167a. [PubMed] [Google Scholar]
- Trinchet JC, Coste T, Levy VG, Vivet F, Duchatelle V, Legendre C, et al. A randomized double blind trial of silymarin in 116 patients with alcoholic hepatitis. Gastroenterologie Clinique Et Biologique 1989;13(2):120‐4. [PubMed] [Google Scholar]
Trinchet 1992 {published data only}
- Trinchet JC, Balkau B, Heintzmann F, Poupon RE, Callard P, Gotheil C, et al. Treatment of severe alcoholic hepatitis by infusion of insulin and glucagon a multicentre sequential trial. Journal of Hepatology 1990;11(Suppl 2):S63. [DOI] [PubMed] [Google Scholar]
- Trinchet JC, Balkau B, Poupon RE, Heintzmann F, Callard P, Gotheil C, et al. Treatment of severe alcoholic hepatitis by infusion of insulin and glucagon: a multicenter sequential trial. Hepatology 1992;15:76‐81. [DOI] [PubMed] [Google Scholar]
Velussi 1997 {published data only}
- Velussi M, Cernigoi AM, Monte A, Dapas F, Caffau C, Zilli M. Long‐term (12 months) treatment with an anti‐oxidant drug (silymarin) is effective on hyperinsulinemia, exogenous insulin need and malondialdehyde levels in cirrhotic diabetic patients. Journal of Hepatology 1997;26:871‐9. [DOI] [PubMed] [Google Scholar]
- Velussi M, Cernigoi AM, Viezzoli L, Dapas F, Caffau C, Zilli M. Silymarin reduces hyperinsulinemia, malondialdehyde levels, and daily insulin need in cirrhotic diabetic patients. Current Therapeutic Research ‐ Clinical and Experimental 1993;53(5):533‐45. [Google Scholar]
References to studies excluded from this review
Achord 1987 {published data only}
- Achord JL. A prospective randomized clinical trial of peripheral amino acid‐glucose supplementation in acute alcoholic hepatitis. American Journal of Gastroenterology 1987;82:871‐5. [PubMed] [Google Scholar]
Bunout 1989 {published data only}
- Bunout D, Aicardi V, Hirsch S, Petermann M, Kelly M, Silva G, et al. Nutritional support in hospitalized patients with alcoholic liver disease. European Journal of Clinical Nutrition 1989;43:615‐21. [PubMed] [Google Scholar]
Cabre 2000 {published data only}
- Cabre E, Rodriguez‐Iglesias P, Caballeria J, Quer JC, Sanchez‐Lombrana JL, Pares A, et al. Short‐ and long‐term outcome of severe alcohol‐induced hepatitis treated with steroids or enteral nutrition: a multicenter randomized trial. Hepatology 2000;32:36‐42. [DOI] [PubMed] [Google Scholar]
Calvey 1985 {published data only}
- Calvey H, Davis M, Williams R. Controlled trial of nutritional supplementation, with and without branched chain amino acid enrichment, in treatment of acute alcoholic hepatitis. Journal of Hepatology 1985;1:141‐51. [DOI] [PubMed] [Google Scholar]
Carithers 1990 {published data only}
- Carithers R, Herlong HF, Diehl AM, Shaw EW, Combes B, Fallon HJ, et al. Corticosteroid therapy of alcoholic hepatitis: How many studies will it take?. Hepatology 1990;12(3):619‐20. [DOI] [PubMed] [Google Scholar]
Conn 1978 {published data only}
- Conn HO. Steroid treatment of alcoholic hepatitis. The yeas and the nays. Gastroenterology 1978;74(2 Pt 1):319‐22. [PubMed] [Google Scholar]
DiNubile 2015 {published data only}
- DiNubile MJ. Prednisolone or pentoxifylline for alcoholic hepatitis. New England Journal of Medicine 2015;373(3):281‐2. [DOI] [PubMed] [Google Scholar]
Dupont 2012 {published data only}
- Dupont B, Dao T, Joubert C, Dupont‐Lucas C, Gloro R, Nguyen‐Khac E, et al. Randomised clinical trial: enteral nutrition does not improve the long‐term outcome of alcoholic cirrhotic patients with jaundice. Alimentary Pharmacology & Therapeutics 2012;35:1166‐74. [DOI] [PubMed] [Google Scholar]
Hendy 2016 {published data only}
- Hendy P. Pentoxifylline is ineffective in treating severe alcoholic hepatitis. Frontline Gastroenterology 2016;7(2):80‐1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hirsch 1993 {published data only}
- Hirsch S, Bunout D, Maza P, Iturriaga H, Petermann M, Icazar G, et al. Controlled trial on nutrition supplementation in outpatients with symptomatic alcoholic cirrhosis. JPEN. Journal of Parenteral and Enteral Nutrition 1993;17:119‐24. [DOI] [PubMed] [Google Scholar]
Kearns 1992 {published data only}
- Kearns PJ, Young H, Garcia G, Blaschke T, O'Hanlon G, Rinki M, et al. Accelerated improvement of alcoholic liver disease with enteral nutrition. Gastroenterology 1992;102:200‐5. [DOI] [PubMed] [Google Scholar]
Kolasani 2016 {published data only}
- Kolasani BP, Sasidharan P, Divyashanthi CM, Kumar A. Vitamin e improves quality of life in patients with alcoholic liver disease. National Journal of Physiology, Pharmacy and Pharmacology 2016;6(4):269‐75. [Google Scholar]
- Kolasani BP, Sasidharan P, Kumar A. Efficacy of vitamin e supplementation in patients with alcoholic liver disease: an open‐label, prospective, randomized comparative study. International Journal of Nutrition, Pharmacology, Neurological Diseases 2016;6(3):101‐10. [Google Scholar]
Lesesne 1978 {published data only}
- Lesesne HR, Bozymski EM, Fallon HJ. Treatment of alcoholic hepatitis with encephalopathy. Comparison of prednisolone with caloric supplements. Gastroenterology 1978;74:169‐73. [PubMed] [Google Scholar]
Ma 2011 {published data only}
- Ma AL, Guo XZ, Liu X, Xu Q, Wang TL. Efficacy comparison between bicyclol and polyene phosphatidylcholine treatments for alcoholic liver disease. Chung Hua Kan Tsang Ping Tsa Chih 2011;19:471‐2. [PubMed] [Google Scholar]
Mathurin 1996 {published data only}
- Mathurin P, Duchatelle V, Ramond MJ, Degott C, Bedossa P, Erlinger S, et al. Long term survival and prognostic factors in patients with severe biopsy‐proved alcoholic hepatitis (ah) treated by prednisolone: Randomized trial, new cohort and simulation. Hepatology 1994;20(4 (Pt 2)):319a. [Google Scholar]
- Mathurin P, Duchatelle V, Ramond MJ, Degott C, Bedossa P, Erlinger S, et al. Survival and prognostic factors in patients with severe alcoholic hepatitis treated with prednisolone. Gastroenterology 1996;110(6):1847‐53. [DOI] [PubMed] [Google Scholar]
Mezey 1991 {published data only}
- Mezey E, Caballeria J, Mitchell MC, Pares A, Herlong HF, Rodes J. Effect of parenteral amino acid supplementation on short‐term and long‐term outcomes in severe alcoholic hepatitis: a randomized controlled trial. Hepatology 1991;14:1090‐6. [PubMed] [Google Scholar]
Moreno 2014 {published data only}
- Moreno C, Trépo E, Louvet A, Degré D, Bastens B, Hittelet A, et al. Impact of intensive enteral nutrition in association with corticosteroids in the treatment of severe alcoholic hepatitis: a multicenter randomized controlled trial. Hepatology 2014;60(Suppl 1):269A‐70A. [Google Scholar]
Nasrallah 1980 {published data only}
- Nasrallah SM, Galambos JT. Aminoacid therapy of alcoholic hepatitis. Lancet 1980;2:1276‐7. [DOI] [PubMed] [Google Scholar]
Naveau 1986 {published data only}
- Naveau S, Pelletier G, Poynard T, Attali P, Poitrine A, Buffet C, et al. A randomized clinical trial of supplementary parenteral nutrition in jaundiced alcoholic cirrhotic patients. Hepatology 1986;6:270‐4. [DOI] [PubMed] [Google Scholar]
Panos 1990 {published data only}
- Panos MZ, Polson R, Johnson R, Portmann B, Williams R. Polyunsaturated phosphatidyl choline for acute alcoholic hepatitis: a double‐blind, randomized, placebo‐controlled trial. European Journal of Gastroenterology and Hepatology 1990;2:351‐5. [Google Scholar]
Ramond 1992a {published data only}
- Ramond MJ, Poynard T, Rueff B, Carey WD. Steroids in alcoholic hepatitis: another salvo of data. American Journal of Gastroenterology 1992;87(9):1219‐20. [PubMed] [Google Scholar]
Sas 2011 {published data only}
- Sas E, Grinevich V, Kravchuk U, Efimov O. Polyunsaturated phosphatidylcholine reduces insulin resistance and hepatic fibrosis in patients with alcoholic liver disease. results of randomized blinded prospective clinical study. Journal of Hepatology 2011;54:S207. [Google Scholar]
Simon 1988 {published data only}
- Simon D, Galambos JT. A randomized controlled study of peripheral parenteral nutrition in moderate and severe alcoholic hepatitis. Journal of Hepatology 1988;7:200‐7. [DOI] [PubMed] [Google Scholar]
Spahr 2008 {published data only}
- Spahr L, Lambert JF, Rubbia‐Brandt L, Chalandon Y, Frossard JL, Giostra E, et al. Granulocyte‐colony stimulating factor induces proliferation of hepatic progenitors in alcoholic steatohepatitis: a randomized trial. Hepatology 2008;48(1):221‐9. [DOI] [PubMed] [Google Scholar]
Thursz 2015a {published data only}
- Thursz MR, Forrest EH, Ryder S, STOPAH Investigators. Prednisolone or pentoxifylline for alcoholic hepatitis. New England Journal of Medicine 2015;373(3):282‐3. [DOI] [PubMed] [Google Scholar]
Verbeke 2015 {published data only}
- Verbeke L, Laleman W, Nevens F. Prednisolone or pentoxifylline for alcoholic hepatitis. New England Journal of Medicine 2015;373(3):281. [DOI] [PubMed] [Google Scholar]
Wenzel 1993 {published data only}
- Wenzel G, Kuklinski B, Ruhlmann C, Ehrhardt D. Alcohol‐induced toxic hepatitis‐a "free radical" associated disease. Lowering fatality by adjuvant antioxidant therapy. Zeitschrift für die gesamte Innere Medizin und ihre Grenzgebiete 1993;48:490‐6. [PubMed] [Google Scholar]
Additional references
Abenavoli 2010
- Abenavoli L, Capasso R, Milic N, Capasso F. Milk thistle in liver diseases: past, present, future. Phytotherapy Research 2010;24(10):1423‐32. [DOI] [PubMed] [Google Scholar]
Anstee 2012
- Anstee QM, Day CP. S‐adenosylmethionine (SAMe) therapy in liver disease: a review of current evidence and clinical utility. Journal of Hepatology 2012;57(5):1097‐109. [DOI] [PubMed] [Google Scholar]
Blachier 2013
BLT 2014
- British Liver Trust. Alcohol, 2014. www.britishlivertrust.org.uk/liver‐information/liver‐conditions/alcohol/ (accessed 23 October 2014).
Chaimani 2012
- Chaimani A, Salanti G. Using network meta‐analysis to evaluate the existence of small‐study effects in a network of interventions. Research Synthesis Methods 2012;3(2):161‐76. [DOI] [PubMed] [Google Scholar]
Chaimani 2013
- Chaimani A, Higgins JP, Mavridis D, Spyridonos P, Salanti G. Graphical tools for network meta‐analysis in STATA. PloS One 2013;8(10):e76654. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chan 2013
- Chan AW, Tetzlaff JM, Altman DG, Laupacis A, Gotzsche PC, Krleza‐Jeric K, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Annals of Internal Medicine 2013;158(3):200‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
D'Amico 2006
- D'Amico G, Garcia‐Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. Journal of Hepatology 2006;44(1):217‐31. [DOI] [PubMed] [Google Scholar]
Del Re 2013
- Re AC, Spielmans GI, Flückiger C, Wampold BE. Efficacy of new generation antidepressants: differences seem illusory. PLoS One 2013;8(6):e63509. [DOI] [PMC free article] [PubMed] [Google Scholar]
DeMets 1987
- DeMets DL. Methods for combining randomized clinical trials: strengths and limitations. Statistics in Medicine 1987;6(3):341‐50. [DOI] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. [DOI] [PubMed] [Google Scholar]
Dias 2010
- Dias S, Welton NJ, Caldwell DM, Ades AE. Checking consistency in mixed treatment comparison meta‐analysis. Statistics in Medicine 2010;29(7‐8):932‐44. [DOI] [PubMed] [Google Scholar]
Dias 2012a
- Dias S, Sutton AJ, Welton NJ, Ades AE. NICE DSU Technical Support Document 3: heterogeneity: subgroups, meta‐regression, bias and bias‐adjustment, September 2011 (last updated April 2012). www.nicedsu.org.uk/TSD3%20Heterogeneity.final%20report.08.05.12.pdf (accessed 27 March 2014).
Dias 2012b
- Dias S, Welton NJ, Sutton AJ, Ades AE. NICE DSU Technical Support Document 1: introduction to evidence synthesis for decision making, April 2011 (last updated April 2012). www.nicedsu.org.uk/TSD1%20Introduction.final.08.05.12.pdf (accessed 27 March 2014).
Dias 2014a
- Dias S, Welton NJ, Sutton AJ, Ades AE. NICE DSU technical support document 2: a generalised linear modelling framework for pair wise and network meta‐analysis of randomised controlled trials, August 2011 (last updated April 2014). www.nicedsu.org.uk/TSD2%20General%20meta%20analysis%20corrected%2015April2014.pdf (accessed 8 October 2014).
Dias 2014b
- Dias S, Welton NJ, Sutton AJ, Caldwell DM, Lu G, Ades AE. NICE DSU Technical Support Document 4: inconsistency in networks of evidence based on randomised controlled trials, May 2011 (last updated April 2014). www.nicedsu.org.uk/TSD4%20Inconsistency.final.15April2014.pdf (accessed 8 October 2014). [PubMed]
Duffy 2010
- Duffy JP, Kao K, Ko CY, Farmer DG, McDiarmid SV, Hong JC, et al. Long‐term patient outcome and quality of life after liver transplantation: analysis of 20‐year survivors. Annals of Surgery 2010;252(4):652‐61. [DOI] [PubMed] [Google Scholar]
EASL 2012
- European Association for Study of Liver. EASL clinical practical guidelines: management of alcoholic liver disease. Journal of Hepatology 2012;57(2):399‐420. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ (Clinical Research Ed.) 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
EuroQol 2014
- EuroQol. About EQ‐5D. www.euroqol.org/about‐eq‐5d.html (accessed 8 October 2014).
Fede 2011
- Fede G, Germani G, Gluud C, Gurusamy KS, Burroughs AK. Propylthiouracil for alcoholic liver disease. Cochrane Database of Systematic Reviews 2011, Issue 6. [DOI: 10.1002/14651858.CD002800.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ferri 2006
- Ferri M, Amato L, Davoli M. Alcoholics Anonymous and other 12‐step programmes for alcohol dependence. Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858.CD005032.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gao 2011
- Gao B, Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology 2011;141(5):1572‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gluud 1988
- Gluud C. Testosterone and alcoholic cirrhosis. Epidemiologic, pathophysiologic and therapeutic studies in men. Danish Medical Bulletin 1988;35(6):564‐75. [PubMed] [Google Scholar]
Gluud 2016
- Gluud C, Nikolova D, Klingenberg SL. Cochrane Hepato‐Biliary Group. About Cochrane (Cochrane Review Groups (CRGs)) 2016, Issue 10. Art. No.: LIVER.
Guyatt 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction ‐ GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [DOI] [PubMed] [Google Scholar]
Hicks 1992
- Hicks M, Wong LS, Day RO. Antioxidant activity of propylthiouracil. Biochemical Pharmacology 1992;43(3):439‐44. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. Cochrane, 2011. Available from www.cochrane‐handbook.org.
Higgins 2012
- Higgins JPT, Jackson D, Barrett JK, Lu G, Ades AE, White IR. Consistency and inconsistency in network meta‐analysis: Concepts and models for multi‐arm studies. Research Synthesis Methods 2012;3(2):98‐110. [DOI] [PMC free article] [PubMed] [Google Scholar]
ICH‐GCP 1997
- International Conference on Harmonisation Expert Working Group. International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use. ICH harmonised tripartite guideline. Guideline for good clinical practice CFR & ICH Guidelines. Vol. 1, Pennsylvania: Barnett International/PAREXEL, 1997. [Google Scholar]
Jakobsen 2014
- Jakobsen JC, Wetterslev J, Winkel P, Lange T, Gluud C. Thresholds for statistical and clinical significance in systematic reviews with meta‐analytic methods. BMC Medical Research Methodology 2014;14(1):120. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kjaergard 2001
- Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta‐analyses. Annals of Internal Medicine 2001;135(11):982‐9. [DOI] [PubMed] [Google Scholar]
Lu 2006
- Lu G, Ades AE. Assessing evidence inconsistency in mixed treatment comparisons. Journal of the American Statistical Association 2006;101(474):447‐59. [Google Scholar]
Lucey 2009
- Lucey MR, Mathurin P, Morgan TR. Alcoholic hepatitis. New England Journal of Medicine 2009;360(26):2758‐69. [DOI] [PubMed] [Google Scholar]
Lundh 2017
- Lundh A, Sismondo S, Lexchin J, Busuioc OA, Bero L. Industry sponsorship and research outcome. Cochrane Database of Systematic Reviews 2017, Issue 2. [DOI: 10.1002/14651858.MR000033.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Macaskill 2001
- Macaskill P, Walter SD, Irwig L. A comparison of methods to detect publication bias in meta‐analysis. Statistics in Medicine 2001;20(4):641‐54. [DOI] [PubMed] [Google Scholar]
Mills 2012
- Mills EJ, Ioannidis JP, Thorlund K, Schünemann HJ, Puhan MA, Guyatt GH. How to use an article reporting a multiple treatment comparison meta‐analysis. JAMA 2012;308(12):1246‐53. [DOI] [PubMed] [Google Scholar]
Moher 1998
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. Lancet 1998;352(9128):609‐13. [DOI] [PubMed] [Google Scholar]
NCBI 2014a
- NCBI. Liver diseases, alcoholic, 2014. www.ncbi.nlm.nih.gov/mesh/68008108 (accessed 23 October 2014).
NCBI 2014b
- NCBI. Fatty liver, 2014. www.ncbi.nlm.nih.gov/mesh/68005234 (accessed 23 October 2014).
NCBI 2014c
- NCBI. Hepatitis, alcoholic, 2014. www.ncbi.nlm.nih.gov/mesh/68006519 (accessed 23 October 2014).
Newell 1992
- Newell DJ. Intention‐to‐treat analysis: implications for quantitative and qualitative research. International Journal of Epidemiology 1992;21(5):837‐41. [DOI] [PubMed] [Google Scholar]
NHS 2014
- NHS Choices. Alcohol‐related liver disease. www.nhs.uk/conditions/liver_disease_(alcoholic)/Pages/Introduction.aspx (accessed 23 October 2014).
O'Shea 2010
- O'Shea RS, Dasarathy S, McCullough AJ, Practice Guideline Committee of the American Association for the Study of Liver Diseases, Practice Parameters Committee of the American College of Gastroenterology. Alcoholic liver disease. Hepatology 2010;51(1):307‐28. [DOI] [PubMed] [Google Scholar]
OpenBUGS 3.2.3 [Computer program]
- Members of OpenBUGS Project Management Group. OpenBUGS. Version 3.2.3. Members of OpenBUGS Project Management Group, 2014.
Pavlov 2016
- Pavlov CS, Tsochatzis E, Casazza G, Nikolova D, Volcek E, Gluud C. Glucocorticosteroids for people with alcoholic hepatitis. Cochrane Database of Systematic Reviews 2016, Issue 6. [DOI: 10.1002/14651858.CD001511.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Puhan 2014
- Puhan MA, Schünemann HJ, Murad MH, Li T, Brignardello‐Petersen R, Singh JA, et al. A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta‐analysis. BMJ (Clinical Research Ed.) 2014;349:g5630. [DOI] [PubMed] [Google Scholar]
Rambaldi 2006
- Rambaldi A, Gluud C. Anabolic‐androgenic steroids for alcoholic liver disease. Cochrane Database of Systematic Reviews 2006, Issue 4. [DOI: 10.1002/14651858.CD003045.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rambaldi 2008
- Rambaldi A, Saconato HH, Christensen E, Thorlund K, Wetterslev J, Gluud C. Systematic review: glucocorticosteroids for alcoholic hepatitis‐‐a Cochrane Hepato‐Biliary Group systematic review with meta‐analyses and trial sequential analyses of randomized clinical trials. Alimentary Pharmacology & Therapeutics 2008;27(12):1167‐78. [DOI] [PubMed] [Google Scholar]
Rambaldi 2015
- Rambaldi A, Gluud C. S‐adenosyl‐L‐methionine for alcoholic liver diseases. Cochrane Database of Systematic Reviews 2015, Issue 11. [DOI: 10.1002/14651858.CD002235.pub3] [DOI] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Royle 2003
- Royle P, Milne R. Literature searching for randomized controlled trials used in Cochrane reviews: rapid versus exhaustive searches. International Journal of Technology Assessment in Health Care 2003;19(4):591‐603. [DOI] [PubMed] [Google Scholar]
Salanti 2011
- Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple‐treatment meta‐analysis: an overview and tutorial. Journal of Clinical Epidemiology 2011;64(2):163‐71. [DOI] [PubMed] [Google Scholar]
Salanti 2012
- Salanti G. Indirect and mixed‐treatment comparison, network, or multiple‐treatments meta‐analysis: many names, many benefits, many concerns for the next generation evidence synthesis tool. Research Synthesis Methods 2012;3(2):80‐97. [DOI] [PubMed] [Google Scholar]
Savović 2012a
- Savović J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized controlled trials: combined analysis of meta‐epidemiological studies. Health Technology Assessment 2012;16(35):1‐82. [DOI] [PubMed] [Google Scholar]
Savović 2012b
- Savović J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized controlled trials. Annals of Internal Medicine 2012;157(6):429‐38. [DOI] [PubMed] [Google Scholar]
Schoening 2013
- Schoening WN, Buescher N, Rademacher S, Andreou A, Kuehn S, Neuhaus R, et al. Twenty‐year longitudinal follow‐up after orthotopic liver transplantation: a single‐center experience of 313 consecutive cases. American Journal of Transplantation 2013;13(9):2384‐94. [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. [DOI] [PubMed] [Google Scholar]
Schulz 2010
- Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ (Clinical Research Ed.) 2010;340:c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
Severini 1993
- Severini TA. Bayesian interval estimates which are also confidence intervals. Journal of the Royal Statistical Society. Series B (Methodological) 1993;55(2):533‐40. [Google Scholar]
Singh 2015
- Singh S, Murad MH, Chandar AK, Bongiorno CM, Singal AK, Atkinson SR, et al. Comparative effectiveness of pharmacological interventions for severe alcoholic hepatitis: a systematic review and network meta‐analysis. Gastroenterology 2015;149(4):958‐70.e12. [DOI] [PubMed] [Google Scholar]
SRTR 2012
- Scientific Registry of Transplant Recipients. OPTN/SRTR 2012 annual data report: liver, 2012. srtr.transplant.hrsa.gov/annual_reports/2012/pdf/03_liver_13.pdf (accessed 8 October 2014).
Stata/SE 14.2 [Computer program]
- StataCorp LP. Stata/SE 14.2 for Windows[64‐bit x86‐64]. Version 14. College Station: StataCorp LP, 2017.
Thorlund 2011
- Thorlund K, Engstrøm J, Wetterslev J, Brok J, Imberger G, Gluud C. User manual for Trial Sequential Analysis (TSA). Copenhagen Trial Unit, Centre for Clinical Intervention Research, Copenhagen, Denmark. Available from www.ctu.dk/tsa 2011:1‐115.
Thorlund 2012
- Thorlund K, Mills EJ. Sample size and power considerations in network meta‐analysis. Systematic Reviews 2012;1:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
TSA 2011 [Computer program]
- Copenhagen Trial Unit, Centre for Clinical Intervention Research, Copenhagen. TSA version 0.9. Copenhagen Trial Unit, Centre for Clinical Intervention Research, Copenhagen, 2011.
Turner 2012
- Turner RM, Davey J, Clarke MJ, Thompson SG, Higgins JP. Predicting the extent of heterogeneity in meta‐analysis, using empirical data from the Cochrane Database of Systematic Reviews. International Journal of Epidemiology 2012;41(3):818‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
van Valkenhoef 2012
- Valkenhoef G, Lu G, Brock B, Hillege H, Ades AE, Welton NJ. Automating network meta‐analysis. Research synthesis methods 2012;3(4):285‐99. [DOI] [PubMed] [Google Scholar]
Ware 2014
- Ware JE. SF‐36® health survey update, 2014. www.sf‐36.org/tools/sf36.shtml (accessed 8 October 2014).
Wetterslev 2008
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial Sequential Analysis may establish when firm evidence is reached in cumulative meta‐analysis. Journal of Clinical Epidemiology 2008;61(1):64‐75. [DOI] [PubMed] [Google Scholar]
Wetterslev 2017
- Wetterslev J, Jakobsen JC, Gluud C. Trial Sequential Analysis in systematic reviews with meta‐analysis. BMC Medical Research Methodology 2017;17(1):39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Whitfield 2009
- Whitfield K, Rambaldi A, Wetterslev J, Gluud C. Pentoxifylline for alcoholic hepatitis. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD007339.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2013
- World Health Organization ‐ Regional Office for Europe. Status report on alcohol and health in 35 European countries 2013. www.euro.who.int/__data/assets/pdf_file/0017/190430/Status‐Report‐on‐Alcohol‐and‐Health‐in‐35‐European‐Countries.pdf?ua=1 (accessed 23 October 2014).
WHO 2014
- World Health Organization. Global status report on alcohol and health 2014. http://apps.who.int/iris/bitstream/10665/112736/1/9789240692763_eng.pdf 2014.
Wood 2008
- Wood L, Egger M, Gluud LL, Schulz KF, Jüni P, Altman DG, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta‐epidemiological study. BMJ (Clinical Research Ed.) 2008;336(7644):601‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yang 2014
- Yang LS, Shan LL, Saxena A, Morris DL. Liver transplantation: a systematic review of long‐term quality of life. Liver International 2014;34(9):1298‐313. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Gurusamy 2015
- Gurusamy KS, Tsochatzis E, Thorburn D, Davidson BR. Pharmacological interventions for alcoholic liver disease (alcohol‐related liver disease): a network meta‐analysis. Cochrane Database of Systematic Reviews 2015, Issue 4. [DOI: 10.1002/14651858.CD011646] [DOI] [PMC free article] [PubMed] [Google Scholar]


