Abstract
Background
Clara cell secretory protein (CCSP) is an immune‐modulating and anti‐inflammatory agent. CCSP is available synthetically as recombinant human Clara cell protein (rhCC10). It has been shown in animal models to reduce lung injury, improve pulmonary compliance and oxygenation, decrease systemic inflammation and up‐regulate surfactant protein and vascular endothelial growth factor expression. These properties makes intratracheally administered CCSP a potential agent in prevention of chronic lung disease (CLD).
Objectives
To determine the effect of intratracheal CCSP administration compared to placebo or no treatment on morbidity and mortality in preterm infants with or at risk of respiratory distress syndrome (RDS).
Search methods
We searched CENTRAL (The Cochrane Library, October 2010), MEDLINE and PREMEDLINE (1950 to October 2010), EMBASE (1980 to October 2010) and CINAHL (1982 to October 2010). We searched proceedings of scientific meetings, Google Scholar and reference lists of identified studies, and contacted expert informants and surfactant manufacturers.
Selection criteria
Published, unpublished and ongoing randomised controlled, cluster‐randomised or quasi‐randomised trials of intratracheal CCSP administration, compared to placebo or no treatment on morbidity and mortality in preterm infants at risk of RDS.
Data collection and analysis
Two authors independently assessed studies for eligibility and quality, and extracted data.
Main results
One pilot study was identified and included. This study enrolled 22 preterm infants 700 to 1300g with established RDS who required ventilation for surfactant administration. Infants received one intratracheal dose of placebo (n = 7), 1.5 mg/kg (n = 8) or 5 mg/kg (n = 7) rhCC10 within four hours of surfactant treatment. At either dose of rhCC10, no significant difference was reported in CLD (36 weeks postmenstrual age or 28 days), mortality, intraventricular haemorrhage, periventricular leukomalacia, patent ductus arteriosus, necrotising enterocolitis, sepsis or days supplemental oxygen compared to placebo. A significant increase in days mechanical ventilation was reported for infants receiving rhCC10 5mg/kg (mean difference 12.00, 95% confidence interval 0.39 to 23.61) but not at the lower dose. The study reported that a single intratracheal dose of rhCC10 was well tolerated and resulted in a significant reduction in tracheal aspirate neutrophil and total cell count, and lung protein concentration. There was no significant difference reported in tracheal aspirate cytokine levels between groups.
Authors' conclusions
There are insufficient data to determine the role of rhCC10 in clinical practice. Further studies are required to determine if rhCC10 reduces lung inflammation in infants at risk of CLD, and to determine dose and dosing strategy.
Plain language summary
Intratracheal Clara cell secretory protein (CCSP) administration in preterm infants with or at risk of respiratory distress syndrome
There is insufficient evidence from randomised controlled trials to guide the use of CCSP administration in preterm infants at risk of respiratory distress syndrome. Respiratory distress syndrome caused by a deficiency of the naturally occurring lining chemicals of the lung (surfactant) occurs mainly in infants born before term. The usual treatment includes instilling artificial surfactant directly into the newborn infant's trachea followed by mechanical ventilation. However, this process can lead to lung injury, which can affect the infant's long‐term health. A potential preventative strategy is to administer CCSP. This protein has the potential to reduce the lung damage caused by mechanical ventilation and thus may prevent further complications secondary to this damage known as chronic lung disease (CLD). This review found a small randomised controlled trial of intratracheal CCSP administration in preterm infants with respiratory distress syndrome that reported CCSP is well tolerated but this study did not have sufficient subjects to detect important clinical effects. In view of the encouraging results from this trial and other animal studies, high‐quality trials of intratracheal CCSP administration in preterm infants with or at risk of respiratory distress syndrome are justified.
Background
Description of the condition
Respiratory distress syndrome (RDS) is one of most important causes of morbidity and mortality in preterm infants. Use of antenatal corticosteroids (Roberts 2006; Crowther 2007) and prophylaxis or treatment with exogenous surfactant (Soll 1998; Soll 1999; Soll 2001; Soll 2002; Stevens 2007; Soll 2009) have resulted in a substantial decrease in morbidity and mortality from prematurity and RDS. However, despite the benefits of prenatal steroid and surfactant, many infants develop bronchopulmonary dysplasia (BPD) and chronic lung disease (CLD). Histopathologically, BPD is characterised by arrest of lung development, impaired alveolarisation, altered pulmonary microvasculature and pulmonary fibrosis (Bancalari 2001). CLD has been defined as a requirement for supplemental oxygen and/or respiratory support for at least 28 days after birth and abnormal respiratory examination (Allen 2003).
The aetiology of CLD in preterm infants is multifactorial. Implicated factors include lung immaturity, intrauterine growth restriction (Bardin 1997), infection (Hannaford 1999), oxidant stress (Warner 1998), ventilator‐induced lung injury (VILI) (Coalson 1999; Clark 2000) and in‐utero inflammation (Watterberg 1996). Researchers have shown that inflammation has a central role in the pathogenesis of CLD. Exposure of the immature lung to hyperoxia, mechanical ventilation, and infection initiate a cascade of proinflammatory cytokines that leads to lung inflammation and chronic lung injury (Merritt 1983; Ogden 1984). These inflammatory changes are characterised by marked neutrophil and macrophage infiltrate, necrosis and release of inflammatory mediators (Merritt 1983; Oei 2002; Wang 2002; Su 2005; Kakkera 2005). Infants with CLD also have reduced anti‐inflammatory capacity (Merritt 1983) including reduced production of Clara cell secretory protein (CCSP) (Ramsay 2001; Loughran‐Fowlds 2006) and an inability to produce an apoptotic neutrophil response (programmed cell death without release of intracellular inflammatory mediators) in the first week after birth (Oei 2003; Kotecha 2003). The basis of this inflammatory dysregulation is still uncertain.
CCSP levels in tracheal aspirates of preterm babies have been shown to be two to four fold lower than in the mature newborn lung (Lassus 2000). Furthermore, decreased levels of CCSP in tracheal aspirates of ventilated, preterm infants were shown to correlate with the development of BPD (Ramsay 2001; Loughran‐Fowlds 2006).
Description of the intervention
CCSP is available synthetically as recombinant human Clara cell protein (rhCC10). It is produced in Escherichia coli bacteria (Mantile 2000) and purified by a proprietary process (Claragen, Inc., College Park, MD). In newborn piglets, intratracheal rhCC10, in 1, 5, or 25 mg/kg was given immediately after surfactant replacement therapy (Chandra 2003). Pulmonary compliance and oxygenation were significantly improved in animals receiving 5 mg/kg intratracheal rhCC10. In human infants the dosing, safety and pharmacokinetic data of rhCC10 has been reported only in a placebo‐controlled, randomised trial in ventilated human preterm infants with RDS eligible for inclusion in this review (Levine 2005). The dose was 1.5 or 5 mg/kg formulated in 2 ml/kg sterile, unbuffered saline. Intratracheally administered rhCC10 was taken up into the circulation and cleared from the blood within 48 hrs. A substantial portion of the circulating rhCC10 is excreted via the kidneys. Only atelectasis has been reported as a post‐administration side effect although the potential exists for hypoxia and bradycardia during administration (Chandra 2003).
How the intervention might work
CCSP, also known as Clara cell protein and CC‐10, is a 10‐kD protein secreted by nonciliated bronchiolar cells, located mainly in the airways (Peri 1993). CCSP has numerous anti‐inflammatory properties and a role in modulating innate immunity (Chandra 2003). These immune‐modulating and anti‐inflammatory properties of CCSP make it a potential candidate for use in the prevention of CLD (Ramsay 2001; Chandra 2003). It has been reported to inhibit the action of interferon‐ϒ, affects numerous cytokines and tumour necrosis factor production, inhibits neutrophil and phagocytic chemotaxis (Ramsay 2001; Chandra 2003). It also inhibits phospholipase‐A2‐mediated inhibition of fibroblast migration in vitro which may help prevent surfactant degradation (Dierynck 1996; Mango 1998). Animal models of acute lung injury showed that intratracheal rhCC10 reduces lung injury, improves pulmonary compliance and oxygenation without any adverse effects in adult mice (Johnston 1997), newborn piglets (Chandra 2003) and preterm lambs (Shashikant 2005).
CCSP and surfactant act synergistically to decrease lung injury secondary to hyperoxia and mechanical ventilation. In a premature lamb model of RDS, CCSP combined with surfactant, compared to surfactant alone, decreased lung injury and systemic inflammation (Shashikant 2005). CCSP has also been reported to up‐regulate surfactant protein and vascular endothelial growth factor expression in a premature lamb model of RDS (Wolfson 2008).
Why it is important to do this review
Despite significant advances in neonatal intensive care, CLD results in a significant health burden to preterm infants born at less than 32 weeks' gestation who received mechanical ventilation. CLD results in substantial neonatal and infant morbidities and health resource utilisation (Allen 2003). CLD is associated with chronic respiratory difficulties (Kilbride 2003; Doyle 2006), prolonged and recurrent hospitalisations (Chye 1995), neurodevelopmental disabilities including cerebral palsy, neurosensory and motor disabilities (Skidmore 1990; Hughes 1999; Majnemer 2000) and poor cognitive outcome (Hughes 1999). CLD has a major impact on the daily life of families that persists beyond the neonatal period (Korhonen 1999). As CCSP has anti‐inflammatory and innate immunity modulating properties, intratracheal CCSP may reduce CLD.
Objectives
To determine the effect of intratracheal CCSP administration compared to placebo or no treatment on morbidity and mortality in preterm infants with or at risk of respiratory distress syndrome (RDS).
Methods
Criteria for considering studies for this review
Types of studies
Trials using randomisation or quasi‐randomisation of patients regardless of unit of allocation (individual or cluster) were eligible for inclusion. Published or unpublished studies were eligible for inclusion.
Types of participants
Preterm infants at risk of RDS (less than 32 completed weeks' gestation) treated at or shortly after birth or with suspected RDS (less than 37 completed weeks' gestation) treated within the first seven days of life.
Types of interventions
Intratracheal CCSP instillation at any dose compared with either placebo or no treatment.
We defined prophylactic therapy as all treatment strategies in which the intent was to treat a preterm infant based on the risk of RDS within the first hour of life. We defined risk of RDS as gestational age less than 32 weeks' or birthweight less than 1250 grams.
We defined treatment of established disease ("rescue therapy") as treatment of a preterm infant less than 37 weeks' gestational age requiring respiratory support and having signs and symptoms of RDS.
Types of outcome measures
Primary outcomes
Chronic lung disease (CLD) defined as need for oxygen or respiratory support at 36 weeks' postmenstrual age;
mortality prior to hospital discharge;
neurodevelopmental disability at least 18 months postnatal age (defined as neurological abnormality including cerebral palsy on clinical examination, developmental delay more than two standard deviations below population mean on a standardised test of development, blindness (visual acuity less than 6/60), or deafness (any hearing impairment requiring amplification) at any time after term corrected);
adverse effects of CCSP administration including hypoxia and bradycardia during administration, or other adverse effects attributed to CCSP by study authors.
Secondary outcomes
Secondary outcome measures reported after enrolment and intervention include:
doses of surfactant received;
doses of surfactant per infant;
days of mechanical ventilation;
days of continuous positive airway pressure (CPAP);
days of high‐flow nasal cannula;
days of low‐flow nasal cannula;
days of supplemental oxygen administration;
incidence of pulmonary interstitial emphysema (PIE);
incidence of pneumothorax;
use of high frequency oscillatory ventilation (HFOV) as a rescue treatment for respiratory distress;
use of jet ventilation as a rescue treatment for respiratory distress;
use of extracorporeal membrane oxygenation (ECMO) as a rescue treatment for respiratory distress;
use of postnatal corticosteroids as rescue treatment for respiratory distress;
CLD defined as need for oxygen or respiratory support at 28 days of age;
use of diuretic as a prophylaxis or rescue treatment for CLD;
use of postnatal corticosteroid as a prophylaxis or rescue treatment for CLD;
use of home oxygen;
asthma diagnosed by physician or challenge test;
rehospitalisation for asthma;
rehospitalisation for hyperactive airway disease;
rehospitalisation for pneumonia;
neonatal mortality (mortality less than 28 days of age);
intraventricular haemorrhage (IVH) (any and severe ‐ Papile grade 3 ‐ 4);
periventricular leukomalacia (PVL);
patent ductus arteriosus (PDA) ‐ symptomatic or treated with cyclo‐oxygenase inhibitors or surgical ligation;
necrotising enterocolitis (NEC) (proven = Bell stage equal to or greater than 2);
retinopathy of prematurity (ROP) (any and severe = stage equal to or greater than 3);
apnoea treated with methylxanthines or respiratory support;
time to regain birth weight (days);
systemic infection in first 48 hours of life;
postnatal growth failure (weight less than 10th percentile at discharge);
duration of hospitalisation (days).
Search methods for identification of studies
See: Cochrane Neonatal Group methods used in reviews.
We used the standard search strategy of the Cochrane Neonatal Review Group as outlined in The Cochrane Library. Unpublished studies were eligible for review. The search of MEDLINE and PREMEDLINE (via OVID interface) included the following MeSH terms and text‐words: “infant, premature, preterm, newborn, neonate”, “Clara cell protein, Clara cell secretory protein, uteroglobin". Searches will be limited to “clinical trials ‐ all”.
The search strategy for MEDLINE and PREMEDLINE was as follows:
#1 exp infant premature
#2 exp infant newborn
#3 exp obstetric labor premature
#4 exp premature birth
#5 prematur*.mp OR neonat*.mp OR preterm.mp OR infant*.mp or newborn.mp or preterm.mp
#6 #1 or #2 or #3 or #4 or #5
#7 Clara cell protein.mp
#8 Clara cell secretory protein.mp or (clara.mp adj cell*.mp) adj protein.mp
#9 uteroglobin.mp or exp uteroglobin
#10 #7 or #8 or #9
#11 #6 and #10
We did not apply language restrictions.
Electronic searches
We adapted the search strategy above to search the following electronic databases:
1. Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library, October 2010);
2. MEDLINE and PREMEDLINE (1950 to October 2010) via OVID interface;
3. EMBASE (1980 to October 2010) via OVID interface;
4. CINAHL (1982 to October 2010) via EBSCO interface;
5. GoogleScholar.
Searching other resources
We carried out additional searches as follows:
1. Ongoing trials in the following trial registries (searched October 2010):
ClinicalTrials.gov (U.S. National Institutes of Health),
Current Controlled Trials,
Australian New Zealand Clinical Trials Registry,
International Clinical Trials Registry Platform (ICTRP).
2. Abstracts of conferences including proceedings of the:
Pediatric Academic Societies (American Pediatric Society, Society for Pediatric Research and European Society for Pediatric Research) from 2000 to 2010 from the journal Pediatric Research and Abstracts Online;
European Academy of Paediatric Societies (EAPS) (The European Society for Paediatric Research (ESPR), the European Academy of Paediatrics (EAP) and the European Society of Paediatric and Neonatal Intensive Care (ESPNIC)) from 2003 to 2010 from Abstracts Online;
Perinatal Society of Australia and New Zealand (PSANZ) from 1996 to 2010 (handsearch);
3. Reference lists of included studies and published reviews.
We supplemented these searches by contacting:
content experts and authors of published trials;
pharmaceutical companies that developed rhCC10 for possible unpublished studies using their product.
Data collection and analysis
We used the standardised review method of the Cochrane Neonatal Review Group (CNRG) for conducting a systematic review (http://neonatal.cochrane.org/en/index.html). We entered and cross‐checked data using Review Manager 5 (RevMan 5) software (RevMan 2008).
Selection of studies
Both review authors independently assessed study eligibility for inclusion in this review according to prespecified selection criteria.
Data extraction and management
Both review authors independently extracted data from the full‐text articles using a specifically designed spreadsheet to manage the information. We used these forms to decide trial inclusion/exclusion, extract data from eligible trials and for requesting additional published information from authors of the original report. We entered and cross‐checked data using RevMan 5 software (RevMan 2008). We then compared the extracted data for any differences. If noted, we then resolved differences by discussion and consensus.
Assessment of risk of bias in included studies
We used the standardised review methods of the CNRG (http://neonatal.cochrane.org/en/index.html) to assess the methodological quality of included studies. Review authors independently assessed study quality and risk of bias using the following criteria documented in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008):
1) adequate sequence generation;
2) allocation concealment;
3) blinding of participants, personnel and outcome assessors;
4) incomplete outcome data;
5) selective outcome reporting;
6) other sources of bias.
When necessary, we requested additional information and clarification of published data from the authors of individual trials. We assessed each trial for risk of bias based on the criteria listed above and marked as:
a) low risk of bias;
b) unclear risk of bias;
c) high risk of bias.
We resolved any discrepancies by discussion and consensus. We planned to provide information on levels of agreement between review authors and/or details of resolution of differences.
Measures of treatment effect
We analysed treatment effects in the individual trials using RevMan 5 (RevMan 2008).
Dichotomous data
We reported dichotomous data using relative risk (RR) and risk difference (RD), each with 95% confidence interval (CI). For statistically significant reduction in RD we calculated the number needed to treat (NNT) or number needed to harm (NNH) and associated 95% CI.
Continuous data
We reported continuous data using mean difference (MD) with 95% CI.
Unit of analysis issues
The unit of randomisation was the intended unit of analysis and we expected this to be individual infants. Cluster randomised controlled trials were planned to be included.
Cluster‐randomised trials
We planned to include cluster randomised trials in the analyses along with individually randomised trials. We intended to analyse them using the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008) using an estimate of the intra‐cluster correlation coefficient (ICC) derived from the trial (if possible), or from another source. If ICCs from other sources were used, we intended to report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identified both cluster randomised trials and individually randomised trials, we planed to synthesise the relevant information. We planned to consider it reasonable to combine the results from both if there was little heterogeneity between the study designs and we planned to consider interaction between the effect of intervention and the choice of randomisation unit to be unlikely.
Dealing with missing data
We obtained missing data from the authors when possible. If this was not possible, then we planned to conduct analyses on available data (i.e. ignoring the missing data). In addition, we planned to conduct another analysis by using imputation method (both best‐ and worst‐case scenarios) and last observation carried forward to the final assessment (LOCF) method for dichotomous and continuous outcome data respectively.
For dichotomous outcomes we planned to conduct both best‐ and worst‐case scenarios and intention‐to‐treat (ITT) analysis with imputation. We planned to compare results obtained from two analysis options to have a better understanding of the robustness of results relative to the different analytic approaches. We planned to consider an imputation approach of best‐case scenarios (i.e. all missing participants in the intervention group did not experience poor outcomes (e.g. death, BPD) and all missing participants in the control group experienced poor outcomes) and worst‐case scenarios (i.e. all missing participants in the intervention group experienced the event and all missing participants in the control condition did not). We planned to conduct sensitivity analysis to compare results based on different imputation assumptions (i.e. using uncertainty method calculated from best‐ versus worst‐case scenarios (Gamble 2005)).
We planned to analyse missing continuous data on an endpoint basis, including only participants with a final assessment, or analysed using LOCF if the trial authors report any LOCF data.
Assessment of heterogeneity
We used RevMan 5 software (RevMan 2008) to assess the heterogeneity of treatment effects between trials. We used the following two formal statistics described below.
1) The Chi2 test, to assess whether observed variability in effect sizes between studies is greater than would be expected by chance. Since this test has low power when the number of studies included in the meta‐analysis is small, we planned to set the probability at the 10% level of significance.
2) The I2 statistic to ensure that pooling of data is valid. We planned to grade the degree of heterogeneity as: 0% to 30%: might not be important; 31% to 50%: moderate heterogeneity; 51% to 75%: substantial heterogeneity; 76% to 100%: considerable heterogeneity.
Where there was evidence of apparent or statistical heterogeneity, we planned to assess the source of the heterogeneity using sensitivity and subgroup analysis looking for evidence of bias or methodological differences between trials.
Assessment of reporting biases
We planned to assess reporting and publication bias by examining degree of asymmetry of a funnel plot in RevMan 5 (RevMan 2008).
Data synthesis
We planned to perform statistical analyses according to the recommendations of CNRG (http://neonatal.cochrane.org/en/index.html). We planned to analyse all infants randomised on an ITT basis. We planned to analyse treatment effects in the individual trials. We planned to use a fixed‐effect model in the first instance to combine the data. For any meta‐analyses, for categorical outcomes we planned to calculate typical estimates of RR and RD, each with 95% CI; for continuous outcomes we planed to calculate the mean difference (MD) if outcomes are measured in the same way between trials, and standardised mean difference (SMD) to combine trials that measure the same outcome, but use different scales. When we judged meta‐analysis to be inappropriate, we planned to analyse and interpret individual trials separately.
Subgroup analysis and investigation of heterogeneity
We planned to explore potential sources of clinical heterogeneity through the following a priori subgroup analyses:
dose of CCSP equal to or greater than 5.0 mg/kg;
timing of CCSP administration (prophylactic, early rescue (within the first two hours of birth), late rescue (within the first week of life), very late rescue (after the first week of life));
co‐administration with surfactant (none, prophylaxis surfactant, rescue surfactant);
type of CCSP e.g. synthetic recombinant human Clara cell protein (rhCC10);
gestational age (less than 28, 28 to 31, 32 to 34 and 35 or more completed weeks' gestation); and
ventilation strategy (intubation, treatment with rapid extubation; intubation, treatment with continued ventilation).
Sensitivity analysis
We planned to explore methodological heterogeneity through the use of sensitivity analyses. We planned to perform these through excluding trials of lower quality, based on a lack of any of the following: adequate randomisation, allocation concealment, blinding of treatment, greater than 10% loss to follow‐up.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies.
Results of the search
One randomised controlled trial (Levine 2005) met our inclusion criteria (see table 'Characteristics of included studies'). No ongoing trials were identified.
Included studies
Types of participants:Levine 2005 enrolled 22 preterm infants ≤ 24 hours age, birth weight 700 to 1300 grams, gestational age ≥ 24 weeks, diagnosis of RDS based on clinical and radiographic criteria, requirement for intubation and mechanical ventilation, and receipt of surfactant 100 mg/kg. Infants were excluded from the study if major congenital abnormalities; evidence of perinatal asphyxia; evidence of sepsis; enrolment in any other study involving administration of another investigational drug; any other condition that, in the judgment of the attending physician, might increase the risk for adverse events.
Types of interventions:Levine 2005 compared a single dose of intratracheal rhCC10 (either 1.5 or 5 mg/kg), formulated in a volume of 2 ml/kg sterile unbuffered saline, within 4 hours after surfactant replacement therapy versus placebo. rhCC10 or placebo was administered intratracheally in two equal aliquots via a pre‐measured feeding tube placed into the distal third of the endotracheal tube, with the patient in the right and then left lateral decubitus position and 30 degrees of Trendelenburg.
rhCC10 was produced in Escherichia coli bacteria and purified by a proprietary process (Claragen, Inc., College Park, MD). The protein for the study was provided as a 98% pure solution of the human CC10 homodimer.
Types of outcomes measures:Levine 2005 reported safety, pharmacokinetics and anti‐inflammatory activity of rhCC10. Clinical outcome measures included chronic lung diease (CLD), mortality prior to hospital discharge, days of intermittent positive pressure ventilation (IPPV), days of IMV and continuous positive airway pressure (CPAP), days of supplemental oxygen administration, intraventricular haemorrhage (IVH), periventricular leukomalacia (PVL), patent ductus arteriosus (PDA), necrotising enterocolitis (NEC), hospitalisation at 36 weeks' gestation.
Excluded studies
No other randomised or quasi‐randomised controlled trials were identified for exclusion from the review.
Risk of bias in included studies
The single included study (Levine 2005) was low‐moderate risk of bias. Although the study had adequate enrolment procedures and reported an intention to treat analysis, the study was stopped early due to slow enrolment, had unblinded interim analyses by the DMC and had analysis of multiple respiratory endpoints. Ratings of methodological quality are given in the table 'Characteristics of included studies'.
Allocation
Levine 2005 implemented central randomisation. Subjects were randomly assigned on 1:2 basis to receive placebo or one of the two rhCC10 doses (1.5 or 5 mg/kg). Method of creating randomisation sequence was not reported. Assignment was stratified by rhCC10 dose i.e. patients were enrolled in two cohorts, the first cohort comparing placebo to 1.5 mg/kg of the study drug and a second cohort comparing placebo to 5.0 mg/kg of study drug.
Blinding
Levine 2005 masked the investigators, outcome assessors and families to study group.
Incomplete outcome data
Levine 2005 reported all enrolled infants in an intention to treat analysis. There were two deaths in the study, one in each of the treatment groups, who could not be evaluated for the clinical outcomes.
Selective reporting
Levine 2005 reported prespecified outcomes.
Other potential sources of bias
Levine 2005 stopped the study early. Projected sample size was 24 infants but the study stopped early after enrolling 22 subjects due to slow enrolment. DMC was unblinded to allocation and the number of interim analyses performed was not reported.
Effects of interventions
Comparison 1. Prophylactic treatment of preterm infants with CCSP versus placebo
No studies were found that enrolled infants at risk of RDS irrespective of need for respiratory support or diagnosis of RDS.
Comparison 2. Treatment of RDS with CCSP versus placebo
One study compared treatment of RDS with rhCC10 versus placebo (Levine 2005). As data for the placebo group are combined into a single strata compared to both infants who received 1.5mg/kg and 5mg/kg rhCC10, the data for the two groups were not able to be combined in meta‐analysis and the placebo group is the same for both dose comparisons so these have not been combined. An attempt was made to obtain contemporaneous control group data for each dose level from the study authors.
Primary outcome measures (outcomes 2.1 and 2.2):
rhCC10 1.5mg/kg versus placebo:Levine 2005 reported no significant difference in CLD at 36 weeks PMA (RR 0.50, 95% CI 0.06 to 4.33) or hospital mortality (RR 2.67, 95% CI 0.13 to 56.63). Other primary outcome measures were not reported.
rhCC10 5mg/kg versus placebo:Levine 2005 reported no significant difference in CLD at 36 weeks PMA (RR 1.75, 95% CI 0.42 to 7.23) or hospital mortality (RR 3.00, 95% CI 0.14 to 63.15). Other primary outcome measures were not reported.
Secondary outcome measures (outcomes 2.3 to 2.10):
rhCC10 1.5mg/kg versus placebo:Levine 2005 reported no significant difference in CLD at 28 days (RR 1.00, 95% CI 0.78 to 1.29), NEC (RR 4.44, 95% CI 0.25 to 79.42), IVH (RR 0.18, 95% CI 0.01 to 3.18), PVL (RR 0.30, 95% CI 0.01 to 6.29), culture‐proven sepsis (RR 2.67, 95% CI 0.13 to 56.63), PDA (RR 0.53, 95% CI 0.19 to 1.44), days mechanical ventilation (MD ‐3.40, 95% CI ‐11.98 to 5.18) and days supplemental oxygen (MD ‐7.60, 95% CI ‐17.98 to 2.78). Other secondary outcome measures were not reported.
rhCC10 5mg/kg versus placebo:Levine 2005 reported no significant difference in CLD at 28 days (RR 0.84, 95% CI 0.55 to 1.28), NEC (RR 3.00, 95% CI 0.14 to 63.15), IVH (RR 0.50, 95% CI 0.06 to 4.33), PVL (RR 0.33, 95% CI 0.02 to 7.02), culture‐proven sepsis (RR 3.00, 95% CI 0.14 to 63.15) and PDA (RR 0.40, 95% CI 0.11 to 1.41). Levine 2005 reported a significant increase in days mechanical ventilation (MD 12.00, 95% CI 0.39 to 23.61). Levine 2005 reported no significant difference in days supplemental oxygen (MD ‐1.60, 95% CI ‐18.05 to 14.85). Other secondary outcome measures were not reported. No significant difference was reported for duration of positive pressure support (IPPV or CPAP) between groups. The study reported a significant reduction in tracheal aspirate neutrophil and total cell count, and lung protein concentration. There was no significant difference reported in tracheal aspirate cytokine levels between groups.
Subgoup analyses
We prespecified the following subgroup analyses. As only one pilot study reported data, the outcomes are as reported above.
Dose of CCSP equal to or greater than 5.0 mg/kg: Levine 2005 reported a subgroup receiving rhCC10 5m/kg ‐ see subgroup analysis above.
Timing of CCSP administration (prophylactic, early rescue (within the first two hours of birth), late rescue (within the first week of life), very late rescue (after the first week of life)): Levine 2005 reported late rescue treatment.
Co‐administration with surfactant (none, prophylaxis surfactant, rescue surfactant): Levine 2005 reported co‐administration of rhCC10 within four hours of rescue surfactant treatment.
Type of CCSP e.g. rhCC10: Levine 2005 reported use of rhCC10.
Gestational age (less than 28, 28 to 31, 32 to 34 and 35 or more completed weeks' gestation); Levine 2005 reported preterm infants ≥ 24 weeks gestation with birthweight 700 to 1300 g.
Ventilation strategy (intubation, treatment with rapid extubation; intubation, treatment with continued ventilation): Levine 2005 reported surfactant and rhCC10 treatment with continued ventilation.
Sensitivity analysis
We planned to perform a sensitivity analysis based on the following: inadequate randomisation, allocation concealment or blinding of treatment, or greater than 10% loss to follow‐up. Levine 2005 reported adequate randomisation and allocation procedures although method of sequence generation was not reported. Levine 2005 also reported blinding of treatment and no losses for clinical outcomes reported.
Discussion
Summary of main results
A single small pilot randomised trial was identified and found eligible for inclusion in this review. The study showed that rhCC10 appears to be relatively safe and well tolerated. No major complications from rhCC10 were encountered. However, the study is underpowered to detect important clinical benefits and harms of rhCC10 administration for prevention of CLD and other clinical parameters.
A larger randomised clinical trial will be required to determine the efficacy of rhCC10. However, certain issues need to be addressed before applying rhCC10 in a larger randomised trial and clinical practice:
1. Dose of rhCC10: the optimal dose of rhCC10 is still unclear.
2. Optimal timing of rhCC10 administration: should rhCC10 be administered as a prophylactic measure at birth or should it used as a rescue intervention as well?
3. Concommitant administration with surfactant: this will need to be addressed before embarking on a larger RCT. Its not clear whether using rhCC10‐surfactant mixture is feasible. This will have the advantage of single administration thus reducing multiple handling of ventilated infants.
It will assist future meta‐analyses if authors report contemporaneous control groups separately in dose escalation studies.
Overall completeness and applicability of evidence
The data from this study are largely applicable to treatment of established RDS. The study is underpowered to detect important clinical benefits and harms of rhCC10 administration for prevention of CLD and other clinical parameters.
Quality of the evidence
The single included study (Levine 2005) was of low to moderate risk of bias. Although the study had adequate enrolment procedures and reported an intention‐to‐treat analysis, the study was stopped early due to slow enrolment and was underpowered to detect important clinical effects.
Potential biases in the review process
We performed an extensive search for published and unpublished literature including searches of trial registries for ongoing studies. Two review authors independently assessed eligibility, study quality and extracted data. Agreement was reached through consensus.
Agreements and disagreements with other studies or reviews
This review supports conclusions from other studies and reviews. The single study included in this review concluded "a single intratracheal dose of rhCC10 was well tolerated and had significant anti‐inflammatory effects in the lung. Multiple doses of rhCC10 will be investigated for efficacy in reducing pulmonary inflammation and ameliorating bronchopulmonary dysplasia in future studies" (Levine 2005). A non‐systematic review of the literature concluded that "innovative strategies like Clara Cell 10 kD protein still have to be assessed in future trials" (Thomas 2007).
Authors' conclusions
Implications for practice.
There are insufficient data to determine the role of rhCC10 in clinical practice. rhCC10 administration should be limited to clinical trials.
Implications for research.
It is currently unclear whether rhCC10 administration at the doses used in the study reduces lung inflammation with the potential to prevent lung injury. Further studies are required to determine if rhCC10 reduces lung inflammation in infants at risk of CLD, and to determine dose and dosing strategy.
What's new
| Date | Event | Description |
|---|---|---|
| 6 March 2017 | Amended | Minor edit |
Acknowledgements
As part of the pre‐publication editorial process, this protocol has been commented on by three peers (an editor and two referees who are external to the editorial team) and the Group's Statistical Adviser.
The Cochrane Neonatal Review Group has been funded in part with Federal funds from the Eunice Kennedy Shriver National Institute of Child Health and Human Development National Institutes of Health, Department of Health and Human Services, USA, under Contract No. HHSN267200603418C.
Data and analyses
Comparison 2. Treatment of RDS with CCSP versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Chronic lung disease (need for oxygen or respiratory support at 36 weeks' postmenstrual age) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 rhCC10 dose = 1.5 mg/kg | 1 | 14 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.06, 4.33] |
| 1.2 rhCC10 dose = 5 mg/kg | 1 | 13 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.75 [0.42, 7.23] |
| 2 Mortality prior to hospital discharge | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 rhCC10 dose = 1.5 mg/kg | 1 | 15 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.67 [0.13, 56.63] |
| 2.2 rhCC10 dose = 5 mg/kg | 1 | 14 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.14, 63.15] |
| 3 Chronic lung disease (need for oxygen or respiratory support at 28 days of age) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 rhCC10 dose = 1.5 mg/kg | 1 | 14 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.78, 1.29] |
| 3.2 rhCC10 dose = 5 mg/kg | 1 | 13 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.55, 1.28] |
| 4 Necrotising enterocolitis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 rhCC10 dose = 1.5 mg/kg | 1 | 15 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.44 [0.25, 79.42] |
| 4.2 rhCC10 dose = 5 mg/kg | 1 | 14 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.14, 63.15] |
| 5 Intraventricular haemorrhage (any grade) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 rhCC10 dose = 1.5 mg/kg | 1 | 15 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.01, 3.18] |
| 5.2 rhCC10 dose = 5 mg/kg | 1 | 14 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.06, 4.33] |
| 6 Periventricular leukomalacia | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 rhCC10 dose = 1.5 mg/kg | 1 | 15 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.01, 6.29] |
| 6.2 rhCC10 dose = 5 mg/kg | 1 | 14 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.02, 7.02] |
| 7 Culture‐proven sepsis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 rhCC10 dose = 1.5 mg/kg | 1 | 15 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.67 [0.13, 56.63] |
| 7.2 rhCC10 dose = 5 mg/kg | 1 | 14 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.14, 63.15] |
| 8 Patent ductus arteriosus | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 rhCC10 dose = 1.5 mg/kg | 1 | 15 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.19, 1.44] |
| 8.2 rhCC10 dose = 5 mg/kg | 1 | 14 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.4 [0.11, 1.41] |
| 9 Days of mechanical ventilation | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 9.1 rhCC10 dose = 1.5 mg/kg | 1 | 15 | Mean Difference (IV, Fixed, 95% CI) | ‐3.40 [‐11.98, 5.18] |
| 9.2 rhCC10 dose = 5 mg/kg | 1 | 14 | Mean Difference (IV, Fixed, 95% CI) | 12.0 [0.39, 23.61] |
| 10 Days of supplemental oxygen administration | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 10.1 rhCC10 dose = 1.5 mg/kg | 1 | 15 | Mean Difference (IV, Fixed, 95% CI) | ‐7.60 [‐17.98, 2.78] |
| 10.2 rhCC10 dose = 5 mg/kg | 1 | 14 | Mean Difference (IV, Fixed, 95% CI) | ‐1.60 [‐18.05, 14.85] |
2.1. Analysis.
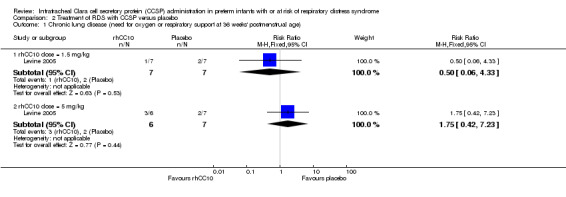
Comparison 2 Treatment of RDS with CCSP versus placebo, Outcome 1 Chronic lung disease (need for oxygen or respiratory support at 36 weeks' postmenstrual age).
2.2. Analysis.

Comparison 2 Treatment of RDS with CCSP versus placebo, Outcome 2 Mortality prior to hospital discharge.
2.3. Analysis.
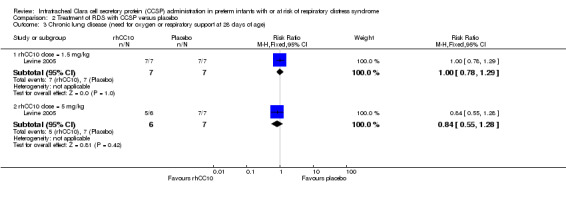
Comparison 2 Treatment of RDS with CCSP versus placebo, Outcome 3 Chronic lung disease (need for oxygen or respiratory support at 28 days of age).
2.4. Analysis.

Comparison 2 Treatment of RDS with CCSP versus placebo, Outcome 4 Necrotising enterocolitis.
2.5. Analysis.
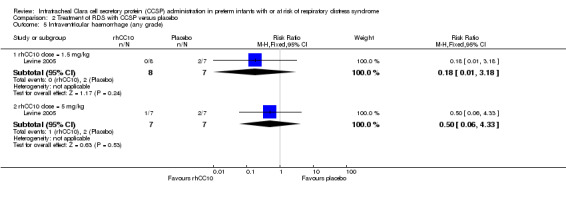
Comparison 2 Treatment of RDS with CCSP versus placebo, Outcome 5 Intraventricular haemorrhage (any grade).
2.6. Analysis.
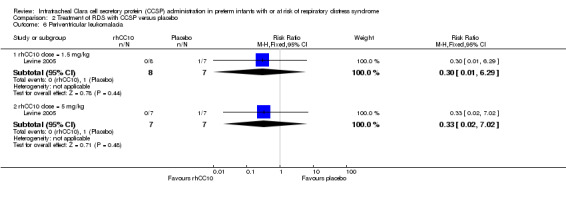
Comparison 2 Treatment of RDS with CCSP versus placebo, Outcome 6 Periventricular leukomalacia.
2.7. Analysis.
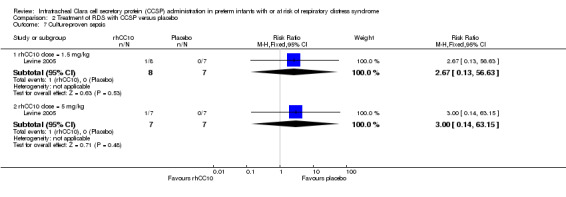
Comparison 2 Treatment of RDS with CCSP versus placebo, Outcome 7 Culture‐proven sepsis.
2.8. Analysis.
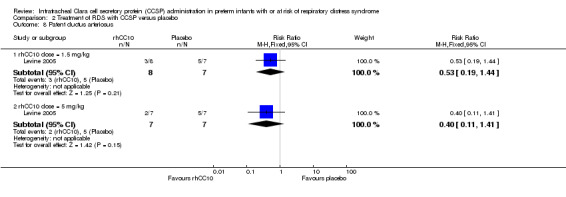
Comparison 2 Treatment of RDS with CCSP versus placebo, Outcome 8 Patent ductus arteriosus.
2.9. Analysis.
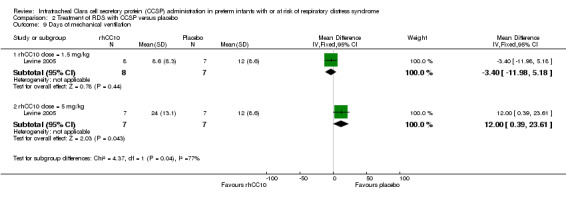
Comparison 2 Treatment of RDS with CCSP versus placebo, Outcome 9 Days of mechanical ventilation.
2.10. Analysis.

Comparison 2 Treatment of RDS with CCSP versus placebo, Outcome 10 Days of supplemental oxygen administration.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Levine 2005.
| Methods | Randomised, placebo‐controlled, double‐blinded, multicentre trial. | |
| Participants | 22 preterm infants who fulfilled the following criteria:
Infants who fulfilled one of the following criteria were excluded from the study:
|
|
| Interventions | rhCC10 1.5 or 5 mg/kg (n = 15): received a single dose of 1.5 mg/kg (n = 7) or 5 mg/kg (n = 8) rhCC10 within 4 hours after surfactant replacement therapy. Placebo group (n = 7): received a single dose of 2ml/kg sterile unbuffered saline placebo within 4 hours after surfactant replacement therapy. rhCC10 or placebo was administered intratracheally in two equal aliquots via a pre‐measured feeding tube placed into the distal third of the endotracheal tube, with the patient in the right and then left lateral decubitus position and 30 degrees of Trendelenburg. |
|
| Outcomes | Primary objective to determine safety and pharmacokinetics of rhCC10 in ventilated preterm infants with RDS. Clincal outcomes included: CLD at 36 weeks corrected gestational age, hospital mortality, CLD at 28 days, necrotising enterocolitis, intraventricular haemorrhage, periventricular leukomalacia, patent ductus arteriosus, culture‐proven sepsis, days of IPPV and days of supplemental oxygen. |
|
| Notes | The study was halted after enrolling 22 infants because of slow enrolment. The study is a phase 1 trial designed to assess the safety, pharmacokinetics, and anti‐inflammatory activity of rhCC10 and is underpowered to detect important clinical effects. Supported by National Heart, Lung, and Blood Institute Grant HL66965‐02 and sponsored by Claragen, Inc. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 24 randomisation envelopes labelled with the cohort (first or second) and the sequential number of the randomisation. 1:2 randomisation to placebo or rhCC10. Method of allocation of envelopes not reported. |
| Allocation concealment (selection bias) | Low risk | Envelopes kept in the pharmacy at Winthrop‐University Hospital. When a site enrolled a patient in the study, the pharmacist at the enrolling site contacted Winthrop‐University Hospital, where the next randomisation envelope was opened and the patient was assigned. |
| Blinding (performance bias and detection bias) Treatment | Low risk | Investigators and families were masked to the study group. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Investigators and families were masked to the study group. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All subjects accounted for. Intention‐to‐treat analysis performed ‐ two infants (one in each of 1.5 and 5 mg/kg) rhCC10 groups died before certain outcomes were ascertained (e.g. oxygen administration at 36 weeks) included in analysis. |
| Selective reporting (reporting bias) | High risk | Multiple respiratory outcomes reported including definitions and timing of reporting of CLD. |
| Other bias | High risk | The study was halted after enrolling 22 infants because of slow enrolment. The study was designed to assess the safety, pharmacokinetics and anti‐inflammatory activity of rhCC10 and is underpowered to detect important clinical effects. DSMC received randomisation code for ongoing, unblinded evaluation of information on patients enrolled in the study. Unclear as to how many interim analyses performed and whether these influenced the decision to stop. |
Differences between protocol and review
None.
Contributions of authors
Both authors contributed to the protocol and review.
Declarations of interest
None.
Edited (no change to conclusions)
References
References to studies included in this review
Levine 2005 {published data only}
- Levine CR, Gewolb IH, Allen K, Welch R, Melby JM, Pilon A, et al. Safety, Pharmacokinetics and Anti‐Inflammatory Activity of rhCC10 in Premature Infants with Respiratory Distress Syndrome (RDS) (Abstract). PAS Annual Meeting. 2003. [DOI] [PubMed]
- Levine CR, Gewolb IH, Allen K, Welch RW, Melby JM, Pollack S, et al. The safety, pharmacokinetics, and anti‐inflammatory effects of intratracheal recombinant human Clara cell protein in premature infants with respiratory distress syndrome. Pediatric Research 2005;58(1):15‐21. [DOI] [PubMed] [Google Scholar]
Additional references
Allen 2003
- Allen J, Zwerdling R, Ehrenkranz R, Gaultier C, Geggel R, Greenough A, et al. Statement on the care of the child with chronic lung disease of infancy and childhood. American Journal of Respiratory and Critical Care Medicine 2003;168(3):356‐96. [DOI] [PubMed] [Google Scholar]
Bancalari 2001
- Bancalari E, Moral T. Bronchopulmonary dysplasia and surfactant. Biology of the Neonate 2001;80(Supp 1):7‐13. [DOI] [PubMed] [Google Scholar]
Bardin 1997
- Bardin C, Zelkowitz P, Papageorgiou A. Outcome of small‐for‐gestational age and appropriate‐for‐gestational age infants born before 27 weeks of gestation. Pediatrics 1997;100(2):E4. [DOI] [PubMed] [Google Scholar]
Chandra 2003
- Chandra S, Davis JM, Drexler S, Kowalewska J, Chester D, Koo HC. Safety and efficacy of intratracheal recombinant human Clara cell protein in a newborn piglet model of acute lung injury. Pediatric Research 2003;54(4):509‐15. [DOI] [PubMed] [Google Scholar]
Chye 1995
- Chye JK, Gray PH. Rehospitalization and growth of infants with bronchopulmonary dysplasia: a matched control study. Journal of Paediatrics and Child Health 1995;31(2):105‐11. [DOI] [PubMed] [Google Scholar]
Clark 2000
- Clark RH, Slutsky AS, Gerstmann DR. Lung protective strategies of ventilation in the neonate: what are they?. Pediatrics 2000;105(1 Pt 1):112‐4. [DOI] [PubMed] [Google Scholar]
Coalson 1999
- Coalson JJ, Winter VT, Siler‐Khodr T, Yoder BA. Neonatal chronic lung disease in extremely immature baboons. American Journal of Respiratory and Critical Care Medicine 1999;160(4):1333‐6. [DOI] [PubMed] [Google Scholar]
Crowther 2007
- Crowther CA, Harding JE. Repeat doses of prenatal corticosteroids for women at risk of preterm birth for preventing neonatal respiratory disease. Cochrane Database of Systematic Reviews 2007, Issue 3. [DOI: 10.1002/14651858.CD003935.pub2] [DOI] [PubMed] [Google Scholar]
Dierynck 1996
- Dierynck I, Bernard A, Roels H, Ley M. The human Clara cell protein: biochemical and biological characterisation of a natural immunosuppressor. Multiple Sclerosis 1996;1(6):385‐7. [DOI] [PubMed] [Google Scholar]
Doyle 2006
- Doyle LW, Victorian Infant Collaborative Study Group. Respiratory function at age 8‐9 years in extremely low birthweight/very preterm children born in Victoria in 1991‐1992. Pediatric Pulmonology 2006;41(6):570‐6. [DOI] [PubMed] [Google Scholar]
Gamble 2005
- Gamble C, Hollis S. Uncertainty method improved on best‐worst case analysis in a binary meta‐analysis. Journal of Clinical Epidemiology 2005;58:579‐88. [DOI] [PubMed] [Google Scholar]
Hannaford 1999
- Hannaford K, Todd DA, Jeffery H, John E, Byth K, Gilbert G. Role of Ureaplasma urealyticum in lung disease of prematurity. Archives of Disease in Childhood Fetal and Neonatal Edition 1999;81(3):F162‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2008
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 [updated September 2008]. The Cochrane Collaboration, 2008. Available from www.cochrane‐handbook.org.
Hughes 1999
- Hughes CA, OGorman LA, Shyr Y, Schork MA, Bozynski ME, McCormick MC. Cognitive performance at school age of very low birth weight infants with bronchopulmonary dysplasia. Journal of Developmental and Behavioral Pediatrics 1999;20(1):1‐8. [DOI] [PubMed] [Google Scholar]
Johnston 1997
- Johnston CJ, Mango GW, Finkelstein JN, Stripp BR. Altered pulmonary response to hyperoxia in Clara cell secretory protein deficient mice. American Journal of Respiratory Cell and Molecular Biology 1997;17(2):147‐55. [DOI] [PubMed] [Google Scholar]
Kakkera 2005
- Kakkera DK, Siddiq MM, Parton LA. Interleukin‐1 balance in the lungs of preterm infants who develop bronchopulmonary dysplasia. Biology of the Neonate 2005;87(2):82‐90. [DOI] [PubMed] [Google Scholar]
Kilbride 2003
- Kilbride HW, Gelatt MC, Sabath RJ. Pulmonary function and exercise capacity for ELBW survivors in preadolescence: effect of neonatal chronic lung disease. Journal of Pediatrics 2003;143(4):488‐93. [DOI] [PubMed] [Google Scholar]
Korhonen 1999
- Korhonen P, Koivisto AM, Ikonen S, Laippala P, Tammela O. Very low birthweight, bronchopulmonary dysplasia and health in early childhood. Acta Paediatrica 1999;88(12):1385‐91. [DOI] [PubMed] [Google Scholar]
Kotecha 2003
- Kotecha S, Mildner RJ, Prince LR, Vyas JR, Currie AE, Lawson RA, et al. The role of neutrophil apoptosis in the resolution of acute lung injury in newborn infants. Thorax 2003;58(11):961‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lassus 2000
- Lassus P, Nevalainen TJ, Eskola JU, Andersson S. Clara‐cell secretory protein in preterm infants’ tracheal aspirates correlates with maturity and increases in infection. Pediatric Pulmonology 2000;30(6):466–9. [DOI] [PubMed] [Google Scholar]
Loughran‐Fowlds 2006
- Loughran‐Fowlds A, Oei J, Wang H, Xu H, Wimalasundera N, Egan C, et al. The influence of gestation and mechanical ventilation on serum Clara cell secretory protein (CC10) concentrations in ventilated and nonventilated newborn infants. Pediatric Research 2006;60(1):103‐8. [DOI] [PubMed] [Google Scholar]
Majnemer 2000
- Majnemer A, Riley P, Shevell M, Birnbaum R, Greenstone H, Coates AL. Severe bronchopulmonary dysplasia increases risk for later neurological and motor sequelae in preterm survivors. Developmental Medicine and Child Neurology 2000;42(1):53‐60. [DOI] [PubMed] [Google Scholar]
Mango 1998
- Mango GW, Johnston CJ, Reynolds SD, Finkelstein JN, Plopper CG, Stripp BR. Clara cell secretory protein deficiency increases oxidant stress response in conducting airways. American Journal of Physiology 1998;275(2 Pt 1):L348‐56. [DOI] [PubMed] [Google Scholar]
Mantile 2000
- Mantile G, Fuchs C, Cordella‐Miele E, Peri A, Mukherjee AB, Miele L. Stable, long‐term bacterial production of soluble, dimeric, disulfide‐bonded protein pharmaceuticals without antibiotic selection. Biotechnology Progress 2000;16(1):17‐25. [DOI] [PubMed] [Google Scholar]
Merritt 1983
- Merritt TA, Cochrane CG, Holcomb K, Bohl B, Hallman M, Strayer D, et al. Elastase and alpha 1‐proteinase inhibitor activity in tracheal aspirates during respiratory distress syndrome. Role of inflammation in the pathogenesis of bronchopulmonary dysplasia. Journal of Clinical Investigation 1983;72(2):656‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Oei 2002
- Oei J, Lui K, Wang H, Henry R. Decreased interleukin‐10 in tracheal aspirates from preterm infants developing chronic lung disease. Acta Paediatrica 2002;91(11):1194‐9. [DOI] [PubMed] [Google Scholar]
Oei 2003
- Oei J, Lui K, Wang H, Henry R. Decreased neutrophil apoptosis in tracheal fluids of preterm infants at risk of chronic lung disease. Archives of Disease in Childhood. Fetal and Neonatal Edition 2003;88(3):F245‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ogden 1984
- Ogden BE, Murphy SA, Saunders GC, Pathak D, Johnson JD. Neonatal lung neutrophils and elastase/proteinase inhibitor imbalance. American Review of Respiratory Disease 1984;130(5):817‐21. [DOI] [PubMed] [Google Scholar]
Peri 1993
- Peri A, Cordella‐Miele E, Miele L, Mukherjee AB. Tissue‐specific expression of the gene coding for human Clara cell 10‐kD protein, a phospholipase A2‐inhibitory protein. Journal of Clinical Investigation 1993;92(5):2099‐109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ramsay 2001
- Ramsay PL, DeMayo FJ, Hegemier SE, Wearden ME, Smith CV, Welty SE. Clara cell secretory protein oxidation and expression in premature infants who develop bronchopulmonary dysplasia. American Journal of Respiratory and Critical Care Medicine 2001;164(1):155‐61. [DOI] [PubMed] [Google Scholar]
RevMan 2008 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2008.
Roberts 2006
- Roberts D, Dalziel SR. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858.CD004454.pub2] [DOI] [PubMed] [Google Scholar]
Shashikant 2005
- Shashikant BN, Miller TL, Welch RW, Pilon A, Shaffer TH, Wolfson MR. Dose response to rhCC10‐augmented surfactant therapy in a lamb model of infant respiratory distress syndrome: physiological, inflammatory, and kinetic profiles. Journal of Applied Physiology 2005;99(6):2204‐11. [DOI] [PubMed] [Google Scholar]
Skidmore 1990
- Skidmore MD, Rivers A, Hack M. Increased risk of cerebral palsy among very low‐birthweight infants with chronic lung disease. Developmental Medicine and Child Neurology 1990;32(4):325‐32. [DOI] [PubMed] [Google Scholar]
Soll 1998
- Soll R. Prophylactic synthetic surfactant for preventing morbidity and mortality in preterm infants. Cochrane Database of Systematic Reviews 1998, Issue 2. [DOI: 10.1002/14651858.CD001079] [DOI] [PubMed] [Google Scholar]
Soll 1999
- Soll R. Early versus delayed selective surfactant treatment for neonatal respiratory distress syndrome. Cochrane Database of Systematic Reviews 1999, Issue 4. [DOI: 10.1002/14651858.CD001456] [DOI] [PMC free article] [PubMed] [Google Scholar]
Soll 2001
- Soll RF, Morley CJ. Prophylactic versus selective use of surfactant for preventing morbidity and mortality in preterm infants. Cochrane Database of Systematic Reviews 2001, Issue 2. [DOI: 10.1002/14651858.CD000510] [DOI] [PubMed] [Google Scholar]
Soll 2002
- Soll RF. Prophylactic natural surfactant extract for preventing morbidity and mortality in preterm infants. Cochrane Database of Systematic Reviews 2002, Issue 1. [DOI: 10.1002/14651858.CD000511] [DOI] [PMC free article] [PubMed] [Google Scholar]
Soll 2009
- Soll R, Özek E. Multiple versus single doses of exogenous surfactant for the prevention or treatment of neonatal respiratory distress syndrome. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD000141.pub2] [DOI] [PubMed] [Google Scholar]
Stevens 2007
- Stevens TP, Blennow M, Myers EW, Soll R. Early surfactant administration with brief ventilation vs. selective surfactant and continued mechanical ventilation for preterm infants with or at risk for respiratory distress syndrome. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD003063.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Su 2005
- Su BH, Chiu HY, Lin TW, Lin HC. Interleukin‐8 in bronchoalveolar lavage fluid of premature infants at risk of chronic lung disease. Journal of the Formosan Medical Association 2005;104(4):244‐8. [PubMed] [Google Scholar]
Thomas 2007
- Thomas W, Speer CP. Prevention and therapy of bronchopulmonary dysplasia ‐ Evidence and clinical practice. Chinese Journal of Contemporary Pediatrics 2007;9(3):264‐75. [PubMed] [Google Scholar]
Wang 2002
- Wang H, Oei J, Lui K, Henry R. Interleukin‐16 in tracheal aspirate fluids of newborn infants. Early Human Development 2002;67(1):79‐86. [DOI] [PubMed] [Google Scholar]
Warner 1998
- Warner BB, Stuart LA, Papes RA, Wispe JR. Functional and pathological effects of prolonged hyperoxia in neonatal mice. American Journal of Physiology 1998;275(1 Pt 1):L110‐7. [DOI] [PubMed] [Google Scholar]
Watterberg 1996
- Watterberg KL, Demers LM, Scott SM, Murphy S. Chorioamnionitis and early lung inflammation in infants in whom bronchopulmonary dysplasia develops. Pediatrics 1996;97(2):210‐5. [PubMed] [Google Scholar]
Wolfson 2008
- Wolfson MR, Funanage VL, Kirwin SM, Pilon AL, Shashikant BN, Miller TL, et al. Recombinant human Clara cell secretory protein treatment increases lung mRNA expression of surfactant proteins and vascular endothelial growth factor in a premature lamb model of respiratory distress syndrome. American Journal of Perinatology 2008;25(10):637‐45. [DOI] [PubMed] [Google Scholar]


