Abstract
Background
Atherosclerotic cardiovascular disease (ASCVD) is the leading cause of death and disability worldwide, yet ASCVD risk factor control and secondary prevention rates remain low. A fixed‐dose combination of blood pressure‐ and cholesterol‐lowering and antiplatelet treatments into a single pill, or polypill, has been proposed as one strategy to reduce the global burden of ASCVD.
Objectives
To determine the effect of fixed‐dose combination therapy on all‐cause mortality, fatal and non‐fatal ASCVD events, and adverse events. We also sought to determine the effect of fixed‐dose combination therapy on blood pressure, lipids, adherence, discontinuation rates, health‐related quality of life, and costs.
Search methods
We updated our previous searches in September 2016 of CENTRAL, MEDLINE, Embase, ISI Web of Science, and DARE, HTA, and HEED. We also searched two clinical trials registers in September 2016. We used no language restrictions.
Selection criteria
We included randomised controlled trials of a fixed‐dose combination therapy including at least one blood pressure‐lowering and one lipid‐lowering component versus usual care, placebo, or an active drug comparator for any treatment duration in adults 18 years old or older, with no restrictions on presence or absence of pre‐existing ASCVD.
Data collection and analysis
Three review authors independently selected studies for inclusion and extracted the data for this update. We evaluated risk of bias using the Cochrane 'Risk of bias' assessment tool. We calculated risk ratios (RR) for dichotomous data and mean differences (MD) for continuous data with 95% confidence intervals (CI) using fixed‐effect models when heterogeneity was low (I2 < 50%) and random‐effects models when heterogeneity was high (I2 ≥ 50%). We used the GRADE approach to evaluate the quality of evidence.
Main results
In the initial review, we identified nine randomised controlled trials with a total of 7047 participants and four additional trials (n = 2012 participants; mean age range 62 to 63 years; 30% to 37% women) were included in this update. Eight of the 13 trials evaluated the effects of fixed‐dose combination (FDC) therapy in populations without prevalent ASCVD, and the median follow‐up ranged from six weeks to 23 months. More recent trials were generally larger with longer follow‐up and lower risk of bias. The main risk of bias was related to lack of blinding of participants and personnel, which was inherent to the intervention. Compared with the comparator groups (placebo, usual care, or active drug comparator), the effects of the fixed‐dose combination treatment on mortality (FDC = 1.0% versus control = 1.0%, RR 1.10, 95% CI 0.64 to 1.89, I2 = 0%, 5 studies, N = 5300) and fatal and non‐fatal ASCVD events (FDC = 4.7% versus control = 3.7%, RR 1.26, 95% CI 0.95 to 1.66, I2 = 0%, 6 studies, N = 4517) were uncertain (low‐quality evidence). The low event rates for these outcomes and indirectness of evidence for comparing fixed‐dose combination to usual care versus individual drugs suggest that these results should be viewed with caution. Adverse events were common in both the intervention (32%) and comparator (27%) groups, with participants randomised to fixed‐dose combination therapy being 16% (RR 1.16, 95% CI 1.09 to 1.25, 11 studies, 6906 participants, moderate‐quality evidence) more likely to report an adverse event . The mean differences in systolic blood pressure between the intervention and control arms was ‐6.34 mmHg (95% CI ‐9.03 to ‐3.64, 13 trials, 7638 participants, moderate‐quality evidence). The mean differences (95% CI) in total and LDL cholesterol between the intervention and control arms were ‐0.61 mmol/L (95% CI ‐0.88 to ‐0.35, 11 trials, 6565 participants, low‐quality evidence) and ‐0.70 mmol/L (95% CI ‐0.98 to ‐0.41, 12 trials, 7153 participants, moderate‐quality evidence), respectively. There was a high degree of statistical heterogeneity in comparisons of blood pressure and lipids (I2 ≥ 80% for all) that could not be explained, so these results should be viewed with caution. Fixed‐dose combination therapy improved adherence to a multidrug strategy by 44% (26% to 65%) compared with usual care (4 trials, 3835 participants, moderate‐quality evidence).
Authors' conclusions
The effects of fixed‐dose combination therapy on all‐cause mortality or ASCVD events are uncertain. A limited number of trials reported these outcomes, and the included trials were primarily designed to observe changes in ASCVD risk factor levels rather than clinical events, which may partially explain the observed differences in risk factors that were not translated into differences in clinical outcomes among the included trials. Fixed‐dose combination therapy is associated with modest increases in adverse events compared with placebo, active comparator, or usual care but may be associated with improved adherence to a multidrug regimen. Ongoing, longer‐term trials of fixed‐dose combination therapy will help demonstrate whether short‐term changes in risk factors might be maintained and lead to expected differences in clinical events based on these changes.
Plain language summary
Fixed‐dose combination drug therapy for the prevention of heart disease and stroke
Review question: We reviewed the evidence about the effect of fixed‐dose combination drug therapy on the prevention of heart attacks and strokes. We found 13 studies including 9059 participants.
Background: We wanted to discover whether using fixed‐dose combination therapy was better or worse than other alternatives, such as usual care, placebo, or giving drugs separately, for the prevention of heart attacks and strokes. This report represents an update from a previous review published in 2014.
Study characteristics: The evidence is current to September 2016. Four studies included individuals with a prior heart attack or stroke or with a high predicted risk for having an initial heart attack and five studies had long‐term (12 months or more) follow‐up. The main risk of bias was related to lack of blinding of participants and personnel, which was inherent to the intervention. Most study participants were middle‐aged men with moderate elevations in blood pressure or cholesterol. Two studies specifically included ethnic Aboriginal or Maori minorities in half of the study participants. The fixed‐dose combinations ranged from two to five drugs; all studies included at least one blood pressure‐lowering and one cholesterol‐lowering drug.
Key results: The effects of fixed‐dose combination drug therapy on all‐cause mortality and fatal and non‐fatal heart attacks and strokes are uncertain, primarily due to the low number of participants experiencing these events in these studies (fewer than 5% for both) and comparisons with usual care (low‐quality evidence). Fixed‐dose combination drug therapy leads to more adverse events than control (32% versus 27%), including placebo (moderate‐quality evidence). This information is not surprising since aspirin, blood pressure‐lowering drugs and cholesterol drugs are known to increase the risk for side effects compared with placebo. Fixed‐dose combination therapy may modestly lower blood pressure (˜6 mmHg) and cholesterol (‐0.6 mmol/L in LDL cholesterol), but these effects were not consistent (moderate‐quality evidence for blood pressure and LDL cholesterol but low‐quality evidence of total cholesterol). Fixed‐dose combination therapy appears to improve adherence to medications to prevent ASCVD (moderate‐quality evidence).
Quality of the evidence: The quality of evidence from these studies generally ranged from moderate to low. Ongoing trials of fixed‐dose combination drug therapy will likely inform clinical endpoints to guide decision‐making.
Summary of findings
Summary of findings for the main comparison. Fixed‐dose combination therapy for the prevention of atherosclerotic cardiovascular diseases (ASCVD).
| Fixed‐dose combination therapy for the prevention of atherosclerotic cardiovascular diseases (ASCVD) | ||||||
|
Patient or population: adults older than 18 years, with no restriction regarding presence of ASCVD; participants generally had elevated risk of ASCVD (as estimated by the presence of at least one abnormal cardiovascular risk factor) without prevalent CVD (two studies included > 10% of participants with prior ASCVD) Settings: outpatient Intervention: fixed‐dose combination therapy of varying drug combinations ranging from two to five drugs Comparison: usual care, placebo, or active drug therapy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk based on event rates or mean changes from baseline in the comparator group | Corresponding risk | |||||
| Comparator group, including placebo, usual care, or active drug comparator | Fixed‐dose combination therapy | |||||
|
All‐cause mortality Median follow‐up range: 9 to 23 months |
Total | RR = 1.10 (0.64 to 1.89) |
5300 (5 studies) |
⊕⊕⊝⊝ Lowa,b | Low event rates among trials that were not designed nor powered to detect differences in clinical outcomes. Four of the five trials included had high‐quality usual care as the comparator group | |
| 10 per 1000 |
11 per 1000 (6 to 19) |
|||||
|
ASCVD event, such as fatal or non‐fatal myocardial infarction or stroke. Median follow‐up range: 8 weeks to 23 months |
Total | RR = 1.26 (0.95 to 1.66) | 4517 (6 studies) |
⊕⊕⊝⊝ Lowa,b | Low event rates among trials that were not designed nor powered to detect differences in clinical outcomes. Four of the five trials included had high‐quality usual care as the comparator group | |
| 37 per 1000 |
46 per 1000 (35 to 61) |
|||||
|
Any investigator‐defined adverse event Median follow‐up range: 6 weeks to 23 months |
271 per 1000 |
314 per 1000 (295 to 339) |
RR = 1.16 (1.09 to 1.25) |
6906 (11 studies |
⊕⊕⊕⊝ Moderatec | We would expect the rate of adverse events to be higher with fixed‐dose combination compared with placebo, and the difference between fixed‐dose combination and usual care depends on what care is provided |
|
Systolic blood pressure, mmHg Median follow‐up range: 6 weeks to 12 months |
The mean change in systolic blood pressure ranged across control groups from ‐17.9 mmHg to 0.9 mmHg | The mean difference in change in systolic blood pressure between the intervention and comparator groups was ‐6.34 mmHg (95% CI ‐9.03 to ‐3.64) | 7638 (13 studies) |
⊕⊕⊕⊝ Moderated | ||
|
Total cholesterol, mmol/L Median follow‐up range: 6 weeks to 23 months |
The mean change in total cholesterol ranged across control groups from ‐1.6 mmol/L to 0.2 mmol/L. | The mean difference in change in total cholesterol between the intervention and comparator groups was ‐0.61 mmol/L (‐0.88 to ‐0.35) | 6565 (11 studies) |
⊕⊕⊝⊝ Lowd,e | ||
|
LDL cholesterol, mmol/L Median follow‐up range: 6 weeks to 23 months |
The mean change in LDL cholesterol ranged across control groups from ‐1.4 mmol/L to 0.1 mmol/L | The mean difference in change in LDL cholesterol between the intervention and comparator groups was ‐0.70 mmol/L (95% CI ‐0.98 to ‐0.41) | 7153 (12 studies) |
⊕⊕⊕⊝ Moderated | ||
|
Adherence, variable definitions Median follow‐up range: 9 to 23 months |
534 per 1000 |
769 per 1000 (673 to 882) |
RR = 1.44 (1.26 to 1.65) | 3835 (4 studies) |
⊕⊕⊕⊝ Moderateb | All four trials included had high‐quality comparator care as the comparator group either as usual care or provision of individual drug components |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is the outcomes of the study control arms. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ASCVD = atherosclerotic cardiovascular disease; CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
aDowngraded by one level due to imprecision due to low event rates. bDowngraded one level due to indirectness of evidence, including high quality 'usual care' as comparator group in four of five trials study limitations, which may not be comparable to settings where fixed‐dose combination therapy might be deployed, including low‐ and middle‐income country settings with low treatment rates. cDowngraded one level due to indirectness of evidence, including different comparators that could be usual care, placebo, or active comparator. dDowngraded one level due to heterogeneity likely due to different participants, fixed‐dose combinations, and comparator groups. eDowngraded one level due to reporting bias demonstrated through funnel plot asymmetry.
Background
Description of the condition
Atherosclerotic cardiovascular disease (ASCVD) is a principal cause of death worldwide. In 2013, more than 17 million deaths globally were attributed to ASCVD, over 80% of which occurred in low‐ and middle‐income countries (Roth 2015). Furthermore, the situation is not expected to improve, with global ASCVD mortality estimated to increase, largely because of population growth and aging (Roth 2015). These trends are largely driven by atherosclerotic cardiovascular diseases, principally ischaemic heart disease and cerebrovascular disease. Therefore, preventing deaths and disease due to ASCVD is a priority for global public health (WHO 2013).
Optimising modifiable risk factors reduces long‐term ASCVD mortality and morbidity (Berry 2012). Individuals with both hypertension and dyslipidaemia have a greater risk of ASCVD than those with either hypertension or dyslipidaemia alone (Neaton 1992; Thomas 2002), highlighting the importance of considering overall ASCVD risk as opposed to individual risk factors (Perk 2012). Therefore, adopting a multifactorial approach to ASCVD risk management, where multiple risk factors are modified simultaneously, is a more effective way of reducing ASCVD events than focusing on single risk factors in isolation (Gaede 2003).
Current national and international approaches to ASCVD prevention incorporate both primary and secondary prevention (Perk 2012; NICE 2010). Primary prevention aims to prevent ASCVD events in those who have no clinical evidence of ASCVD who are considered to be at elevated risk for an ASCVD event. To achieve this, guidelines recommend intervening usually when five‐ or 10‐year predicted risk levels exceed thresholds where benefits outweigh risks (NICE 2008; NICE 2010; Perk 2012; Stone 2013). ASCVD incidence and mortality are reduced by antihypertensives (Collins 1990) and statins, which improve the lipid profile (Taylor 2013). Secondary prevention requires blood pressure control, cholesterol lowering, and use of antiplatelet drugs to prevent further ASCVD events, which is known to be effective (ATT‐Collaboration 2002; Baigent 2005; Karmali 2016; Rashid 2003).
The same ASCVD risk factors operate globally (O'Donnell 2010; Yusuf 2004) making multifactorial prevention strategies relevant, but conventional approaches targeting high risk individuals, conducting investigations, prescribing various medications, regular monitoring, and drug dose titration to optimise ASCVD risk factors are difficult to implement. In fact, access, availability, and adherence to medications for the prevention and control of ASCVD are generally low (Yusuf 2011). In response to this treatment gap, the World Health Organization has set an 80% availability target for essential medicines in public and private pharmacies for the prevention and control of ASCVD and other noncommunicable diseases and a 50% treatment target for eligible individuals to reduce the risk of premature mortality from noncommunicable disease by 25% by 2025 (WHO 2013). In collaboration with the Centers for Disease Control and Prevention, World Heart Federation, and other organisations, the World Health Organization's Global Hearts technical package has also recommended fixed‐dose combination therapy for improving adherence to multidrug therapy (WHO 2016).
Description of the intervention
A fixed‐dose combination pill was proposed in 2001 by a World Health Organization (WHO) and Wellcome Trust expert group (WHO 2001) and was subsequently specified as a combination of four drugs (beta‐blocker, angiotensin‐converting enzyme (ACE)‐inhibitor, aspirin, and statin), which was estimated to reduce ASCVD events by 75% in people with clinical evidence of ASCVD (Yusuf 2002). This concept was followed in 2003 by a proposed Polypill® (a combination of folic acid, aspirin, three low‐dose antihypertensives, and a low‐dose statin), which was intended for both secondary prevention and primary prevention in all people aged 55 years and over and was estimated to reduce ASCVD events by about 80% (Wald 2003). More contemporary evidence has indicated that the effects of fixed‐dose combination treatment may be less than was initially proposed, but that this strategy may improve the blood pressure and lipid profile to near expected levels (PILL‐collaborative 2011; TIPS 2009). The controversial aspect of the polypill was that it was intended to be used at a population level without screening of blood cholesterol or blood pressure (Wald 2011) because an age threshold of 55 years and above would be used to determine eligibility for treatment (Lonn 2010; Wald 2003).
While aspirin is indicated for secondary prevention of ASCVD (Baigent 2009), the use of aspirin for primary prevention of ASCVD is generally indicated when the absolute risk of cardiovascular disease outweighs the risk of severe bleeding (Karmali 2016). Also, doubt exists regarding folic acid since recent large randomised trials have indicated no ASCVD benefit (Armitage 2010; Holmes 2011). On the other hand, statins and antihypertensives as single treatments are known to be relatively safe and individually beneficial in terms of reducing ASCVD risk and thereby cardiovascular events for both secondary prevention and primary prevention (ALLHAT‐investigators 2002; Colhoun 2004; CTT 2012; HPSCG 2002; Julius 2004; Kearney 2008; LaRosa 2005; Ostergren 2008; Papademetriou 2003; Sever 2003; Taylor 2013; Turnbull 2003). Therefore, although uncertainty exists regarding possible components, the consensus is that the minimal fixed‐dose combination for primary and secondary ASCVD prevention should include at least one antihypertensive and one statin.
There is widespread evidence regarding the efficacy and safety of antihypertensives and statins when administered concomitantly (Messerli 2006; Preston 2007), and of multiple antihypertensives when administered as a single tablet (Bangalore 2007; Gupta 2010). Clinicians may be wary of combination therapy due to the potential restrictions on individualised management (Viera 2011); that is, the ability to amend standard therapy because of medical history or adverse events, such as avoiding a beta‐blocker in a person with asthma or changing from an ACE‐inhibitor due to cough, and because of the inability to titrate each drug prescribed according to clinical response (Lonn 2010). It is also unclear if there are unique adverse events associated with fixed‐dose combination therapy beyond the individual components.
How the intervention might work
The effectiveness of the drugs comprising a fixed‐dose combination is generally well understood, and the principles behind using pharmacotherapy at a population level are that the drugs themselves are inexpensive, simple to administer for easier clinical decision making, might not require a medically trained practitioner, and may provide a more effective option than the promotion of lifestyle changes for multiple risk factor control. Yet convincing evidence of the benefits of such interventions has not been achieved (Beaglehole 2011; Ebrahim 2011; Lonn 2010). Although modifying national health policy has been successful in some high‐income countries, such as in Scandinavia (Vartiainen 2010), population‐level pharmacotherapy can be politically challenging in both high‐ and low‐ to middle‐income countries (Lonn 2010; Yusuf 2011) and may not meet with patient approval. However, patient adherence to the fixed‐dose combination therapy is expected to be better than with multiple tablets, but it has been argued that they will likely have a greater potential for adverse effects than behavioural or lifestyle changes and that a purely biological approach is too narrow to allow the social, economic, and behavioural complexities of ASCVD prevention to be appreciated and confronted (Franco 2004).
However, fixed‐dose combination therapy still has several unknowns. These include (i) the best constituents, whether two or three or four or five drugs are required; (ii) evidence of safety, effectiveness, and cost‐effectiveness; and (iii) whether increasing the number of constituents will produce a favourable risk‐benefit profile. In particular, the evidence is limited concerning benefits and risks of fixed‐dose combination therapy for primary prevention in those people with low or intermediate ASCVD risk (event rates at or below 1% per year).
Why it is important to do this review
Various fixed‐dose combination pills are now being manufactured, and there is evidence that physicians are aware of this option and are potentially willing to prescribe it, though perhaps not without some reservations (Viera 2011). There is an emerging literature of randomised controlled trials comparing fixed‐dose combination therapy with placebo or standard practice in both the primary and secondary prevention of ASCVD, as well as in assessing safety and tolerability (de Cates 2014; Elley 2012). Since the publication of these reviews (de Cates 2014; Elley 2012), additional fixed‐dose combination trial data have been published, which provide the rationale for this update. Also, in 2016, the Sixth Joint Task Force of the European Society of Cardiology and Other Societies identified fixed‐dose combination therapy as a IIb, level of evidence B recommendation for improving adherence in the European Guidelines on Cardiovascular Disease Prevention in Clinical Practice (ESC 2016), and the World Health Organization has identified fixed‐dose combination therapy as a strategy to improve adherence (WHO 2016).
Objectives
To determine the effect of fixed‐dose combination therapy on all‐cause mortality, fatal and non‐fatal ASCVD events, and adverse events. We also sought to determine the effect of fixed‐dose combination therapy on blood pressure, lipids, adherence, discontinuation rates, health‐related quality of life, and costs.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCT).
Types of participants
Adults 18 years and older with no restriction regarding presence of ASCVD.
Types of interventions
A fixed‐dose combination therapy, a combination of several active components into a single pill with the aim being to optimise ASCVD risk and reduce ASCVD fatal and non‐fatal events. At least one statin and one antihypertensive agent should be included. We examined different combinations and doses in stratified analyses, where possible.
Trials were considered where the comparison group was usual care, placebo, or an active drug comparator.
Types of outcome measures
Primary outcomes
Clinical outcomes including mortality (cardiovascular and all‐cause); non‐fatal ASCVD endpoints such as myocardial infarction, coronary artery bypass grafting (CABG), percutaneous transluminal coronary angioplasty (PTCA), angina or angiographically‐defined ischaemic heart disease, stroke, transient ischaemic attack (TIA), carotid endarterectomy, or peripheral arterial disease (PAD). The previous version of the review included the broader outcome of CVD, but we have narrowed this definition for this update to include only ASCVD.
Investigator‐defined adverse events including the proportion of participants experiencing specific symptoms including: myalgias, cough, elevated liver enzymes, gastric irritation or dyspepsia.
Secondary outcomes
Systolic and diastolic blood pressure
Total and LDL cholesterol
Adherence
Discontinuation rates
Health‐related quality of life, measured according to any well validated and adjusted scale concerning quality of life
Costs of fixed‐dose combination therapy
Search methods for identification of studies
Electronic searches
We searched the following electronic databases:
Cochrane Central Register of Controlled Trials (CENTRAL, Issue 8, 2016) in the Cochrane Library;
MEDLINE (Ovid) (1946 to 19 September 2016);
Embase (Ovid) (1980 to Week 38, September 2016);
ISI Web of Science (1970 to 19 September 2016);
Database of Abstracts of Reviews of Effects (DARE), Health Technology Assessment Database (HTA), and Health Economics Evaluations Database (HEED) in the Cochrane Library (2016, Issue 8).
The searches were limited to records published since 2000. The fixed‐dose combination therapy was conceptualised in 2001, so relevant trials will only appear after this date. The searches were initially run in January 2012 (Appendix 1) and updated in July 2013 (Appendix 2), January 2015, February 2016, and September 2016 (Appendix 3). We used the Cochrane sensitivity‐maximising RCT filter (Lefebvre 2011) for MEDLINE and adaptations of it were used for Embase and Web of Science.
Searching other resources
We searched the metaRegister of controlled trials (mRCT) (www.controlled‐trials.com/mrct), clinicaltrials.gov (www.clinicaltrials.gov), and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (apps.who.int/trialsearch/) for ongoing trials on 24 December 2011 and the latter two registers on 27 September 2016 for this update to review existing ongoing studies that had been identified and to find any recent registrations. In addition, we checked reference lists of reviews and retrieved articles for additional studies and performed citation searches on key articles. We contacted experts in the field for unpublished and ongoing trials and study authors where necessary for additional information.
Data collection and analysis
Selection of studies
From the searches, three review authors (EB, MP, MH) reviewed the title and abstract of each paper for this update and retrieved potentially relevant references. Following this initial screening, we obtained the full‐text reports of potentially relevant studies, and three authors (EB, MP, MH) independently selected studies to be included in the review using predetermined inclusion criteria. In all cases we resolved disagreements about any study inclusions by consensus.
Data extraction and management
Two review authors (EB, MH) independently extracted data using a proforma, and contacted principal investigators to provide additional relevant information where necessary. EB and MH extracted details of the study design, participant characteristics, study setting, intervention and comparator, and outcome data including details of outcome assessment, adverse effects, and methodological quality (randomisation, blinding, attrition) from each of the included studies. We resolved disagreements about extracted data by consensus.
Assessment of risk of bias in included studies
We assessed risk of bias according to the Cochrane 'Risk of bias' assessment tool, including examining the quality of the random sequence generation and allocation concealment, description of dropouts and withdrawals (including intention‐to‐treat analysis), blinding (participants, personnel, and outcome assessment), and selective outcome reporting (Higgins 2011a). For cluster‐randomised trials, we have followed the Cochrane Handbook for Systematic Reviews of Interventions' recommendations for assessing risk of bias, with particular attention across the domains of: recruitment; baseline imbalances; loss of clusters; incorrect analyses; and comparability with individually randomised trials (Higgins 2011b). Two review authors (EB, MH) independently assessed the risk of bias in the included studies.
One author (MDH) evaluated the quality of evidence using the GRADE approach for this update using the checklist outlined by Meader 2014. We have reported the rationale for downgrading the quality of evidence for each of our included outcomes: imprecision due to low event rates; indirectness of evidence; including high quality 'usual care' as comparator group, which may not be comparable to settings where fixed‐dose combination therapy might be deployed (including low‐ and middle‐income country settings with low treatment rates), as well as different comparators that could be usual care, placebo or active comparator. Additional reasons for downgrading the overall quality of evidence include heterogeneity likely due to different participants, fixed‐dose combinations, and comparator groups and reporting bias. We have reported the absolute and relative effects, quality of evidence, and specific reason(s) applied for downgrading the overall quality of evidence for each listed outcome in our Table 1.
Measures of treatment effect
We processed data in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011). We expressed dichotomous outcomes as risk ratios (RR), and calculated 95% confidence intervals (CI) for each study. For continuous variables, we compared net changes (that is intervention group minus control group differences) and calculated mean difference (MD) and 95% CI for each study. For TIPS 2009, we compared the effects of fixed‐dose combination therapy on mean (standard deviation (SD)) levels of blood pressure and cholesterol against the study arms without active components as reported by the study authors. Where SDs were not reported in the outcomes of interest (TIPS 2009), we used baseline SDs per Elley 2012 and Furukawa 2006.
Unit of analysis issues
One trial was a cross‐over trial (Wald 2012), and the fixed‐dose combination was unlikely to have a cross‐over effect on the measured risk factors. Thus, we analysed the treatment effect as a parallel‐group trial (Deeks 2011). No trials were cluster‐randomised trials.
Dealing with missing data
We sought missing data from investigators to obtain key information or missing numerical outcome data where possible. We obtained updated data from two trials (Malekzadeh 2010; Soliman 2009) in the initial version of this review and none for this update. We investigated attrition rates, losses to follow‐up, withdrawals, and critically appraised methods for handling missing data and imputation methods. If SDs for outcomes were not reported and were not provided by study authors, then we imputed these values from data within the trial using methods outlined in the Cochrane Handbook for Systematic Reviews of Interventions, Chapter 16.1.3 (Higgins 2011b).
Assessment of heterogeneity
For each outcome, we carried out tests of heterogeneity using the Chi2 test of heterogeneity and the I2 statistic (Higgins 2003). Where no or minimal heterogeneity was present, we performed fixed‐effect model meta‐analyses. Where substantial heterogeneity was detected (I2 ≥ 50%), we evaluated the results for possible explanations (for example participants and interventions) and performed random‐effects model meta‐analyses with cautious interpretation.
Assessment of reporting biases
We evaluated reporting bias by creating funnel plots for outcomes with at least 10 trials to evaluate for asymmetry which could represent true heterogeneity, poor methodological design leading to small study bias, publication bias or a combination thereof.
Data synthesis
We synthesised our results through fixed‐effect or random‐effects meta‐analyses based on heterogeneity identified for each outcome. We have reported RRs or MDs with corresponding 95% CIs. To evaluate the quality of evidence for each outcome, we used the GRADE approach (GRADE 2013) and the 'Checklist to aid consistency and reproducibility of GRADE assessments' (Meader 2014) for these assessments, which we included in the 'Summary of findings' table.
Subgroup analysis and investigation of heterogeneity
If there were sufficient studies, we aimed to conduct the following subgroup analyses.
Age
Sex
Primary prevention (populations where 10% or less had pre‐existing ASCVD) versus secondary prevention (population where > 10% had pre‐existing ASCVD)
Two‐drug versus three‐drug or more fixed‐dose combination therapies
Comparator group as usual care versus placebo or inactive control
Data were available to perform subgroup analyses on the latter three analyses.
Sensitivity analysis
We performed sensitivity analyses by excluding studies at high risk of bias. We created funnel plots and performed tests of asymmetry (Egger 1997) according to the available outcomes of systolic blood pressure and total cholesterol to assess possible publication bias through funnel plot asymmetry.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies; Characteristics of ongoing studies; Characteristics of studies awaiting classification.
Results of the search
We have presented the PRISMA flowchart in Figure 1 (Moher 2009). The 2014 review included 14 reports of nine trials (CRUCIAL 2011; CUSP 2009; Malekzadeh 2010; PILL 2011; Soliman 2009; TIPS 2009; TOGETHER 2010; UMPIRE 2013; Wald 2012). Our updated search identified 5629 reports, and we identified five reports through handsearching and 16 trials through trials register searches. After de‐duplication, we screened 4376 records and excluded 4236 records based on review of the title or abstract. After full‐text review of the remaining 140 reports, we excluded 96 records and included 22 reports of eight trials. This included eight additional reports of four trials included in the 2014 systematic review and 14 reports of four new trials.
1.
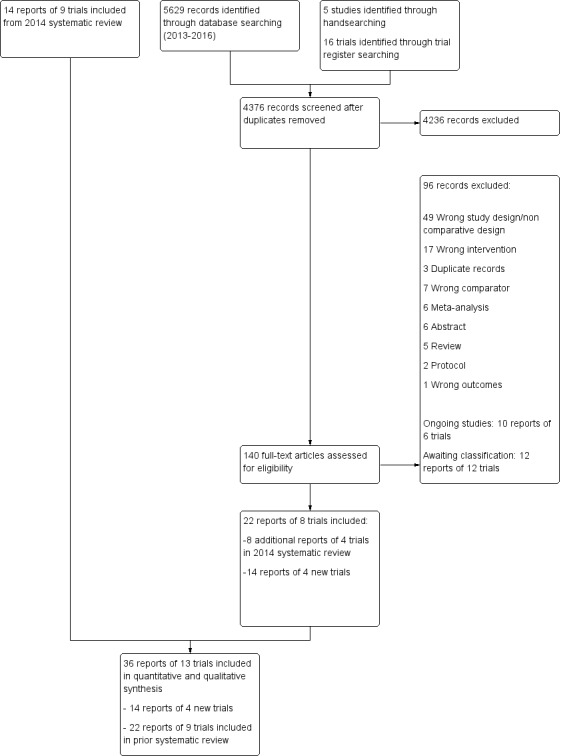
Flow diagram
Overall, we have included 36 reports of 13 trials in this update (CRUCIAL 2011; CUSP 2009; FOCUS 2014; IMPACT 2014; Kanyini GAP 2014; Malekzadeh 2010; OLSTA 2016; PILL 2011; Soliman 2009; TIPS 2009; TOGETHER 2010; UMPIRE 2013; Wald 2012), as well as 10 reports of six ongoing trials (NCT01826019; INTEGRATE; PolyIran; NCT02278471; NCT02596126; NCT01646437) and 12 reports of 12 trials awaiting classification (Fommei 2015; NCT00530946; NCT01004705; NCT01005290; NCT01362218; NCT01406431; NCT01764178; NCT02075619; NCT02569814; NCT02662894; NCT02791958; NCT02842359).
Included studies
Details of the methods, participants, intervention, comparison group and outcome measures for each of the studies included in the review are shown in the Characteristics of included studies table. We included nine trials with 7047 participants randomised in the initial review, with four additional trials (FOCUS 2014; IMPACT 2014; Kanyini GAP 2014; OLSTA 2016; n = 2012 participants) in this update. The six largest trials (CRUCIAL 2011; FOCUS 2014; IMPACT 2014; Kanyini GAP 2014; TIPS 2009; UMPIRE 2013) randomized 7349 (81%) of all participants. The duration of the intervention and follow‐up periods was generally short‐term (six weeks in one study (TOGETHER 2010), eight weeks in two studies (CUSP 2009, OLSTA 2016), 12 weeks in four studies (PILL 2011; Soliman 2009; TIPS 2009; Wald 2012)) or medium‐term (nine months in one study (FOCUS 2014)); however, five studies had a median follow‐up period of 12 months or more (CRUCIAL 2011; IMPACT 2014; Kanyini GAP 2014; Malekzadeh 2010; UMPIRE 2013). All trials reported changes in blood pressure and cholesterol, whereas mortality was only reported in five trials (CRUCIAL 2011; FOCUS 2014; IMPACT 2014; Kanyini GAP 2014; UMPIRE 2013). Five trials (CRUCIAL 2011; IMPACT 2014; Kanyini GAP 2014; Soliman 2009; UMPIRE 2013) compared fixed‐dose combination therapy against usual care, whereas the other trials compared combination therapy against either active control or placebo. One trial (TIPS 2009) included nine arms with different drug combinations, which led to restricting our analyses to comparisons between fixed‐dose combination therapy and groups without either blood pressure‐ or cholesterol‐lowering drugs (depending upon the analysis) and lowered the sample sizes in these analyses.
The included studies frequently had complex inclusion and exclusion criteria that were generally based upon freedom from prior cardiovascular disease, an age threshold ranging from older than 21 years to older than 55 years in women, a composite measure of short‐term (10‐year) risk (5‐year predicted Framingham ASCVD risk ≥ 7.5% in PILL 2011), or one to three elevated cardiovascular disease risk factors. FOCUS 2014, IMPACT 2014, Kanyini GAP 2014 and UMPIRE 2013 specifically enrolled participants with established ASCVD or an elevated risk of ASCVD (≥ 15% predicted risk over five years), while CRUCIAL 2011 included more than 18% of participants with peripheral artery disease (PAD) and more than 14% with prior transient ischaemic attack (TIA) or stroke. The participants were generally middle‐aged with a mean (SD) age ranging from 52.6 (9.6) years (CUSP 2009) to 63.7 (12.7) years (Kanyini GAP 2014). The majority of trials enrolled predominantly men, with two trials randomising more than 80% men (PILL 2011; UMPIRE 2013) compared with one trial that enrolled only 27% men (Soliman 2009). Two trials enrolled 50% ethnic Aboriginal/Torres Strait Islander (Kanyini GAP 2014) or Maori (IMPACT 2014) individuals by design. Baseline systolic blood pressure ranged from 125 mmHg to 166 mmHg, and baseline total cholesterol ranged from 4.2 mmol/L to 6.1 mmol/L.
The drugs included in the various fixed‐dose combination pills varied (Table 2), with four studies including two drugs (CRUCIAL 2011; CUSP 2009; OLSTA 2016; TOGETHER 2010), one study including three drugs (FOCUS 2014), seven studies including four drugs (IMPACT 2014; Kanyini GAP 2014; Malekzadeh 2010; PILL 2011; Soliman 2009; UMPIRE 2013; Wald 2012), and one study including five drugs (TIPS 2009). Eight studies included aspirin (FOCUS 2014; IMPACT 2014; Kanyini GAP 2014; Malekzadeh 2010; PILL 2011; Soliman 2009; TIPS 2009; UMPIRE 2013), and blood pressure‐ and cholesterol‐lowering drugs were included, by definition, in all 13 studies. The blood pressure components included either a calcium channel blocker, thiazide diuretic, beta‐blocker, ACE‐inhibitor, or angiotensin receptor blocker (ARB), or a combination thereof. In terms of lipid‐lowering drugs, simvastatin was used in eight trials (FOCUS 2014; IMPACT 2014; Kanyini GAP 2014; PILL 2011; Soliman 2009; TIPS 2009; UMPIRE 2013; Wald 2012), atorvastatin was used in four trials (CRUCIAL 2011; CUSP 2009; Malekzadeh 2010; TOGETHER 2010), and rosuvastatin was used in one trial (OLSTA 2016).
1. Polypill content by trial.
| Study | Polypill contents (dose) | Comparator |
| CRUCIAL 2011 | Amlodipine 5 mg to 10 mg Atorvastatin 10 mga | Usual care |
| CUSP 2009 | Amlodipine 5 mg Atorvastatin 20 mg |
Placebo |
| FOCUS 2014 | Aspirin 100 mg Ramipril 2.5 mg, 5 mg, or 10 mg Simvastatin 40 mg |
Individual components: Aspirin 100 mg Ramipril 2.5 mg, 5 mg, or 10 mg Simvastatin 40 mg |
| IMPACT 2014 | Aspirin 75 mg Atenolol 50 mg Lisinopril 10 mg Simvastatin 40 mg or Aspirin 75 mg Hydrochlorothiazide 12.5 mg Lisinopril 10 mg Simvastatin 4 0mg |
Usual care |
| Kanyini GAP 2014 | Aspirin 75 mg Atenolol 50 mg Lisinopril 10 mg Simvastatin 40 mg or Aspirin 75 mg Hydrochlorothiazide 12.5 mg Lisinopril 10 mg Simvastatin 40 mg |
Usual care |
| Malekzadeh 2010 | Aspirin 81 mg Atorvastatin 20 mg Enalapril 2.5 mg Hydrochlorothiazide 12.5 mg |
Placebo |
| OLSTA 2016 | Olmesartan 40 mg Rosuvastatin 20 mg |
|
| PILL 2011 | Aspirin 75 mg Hydrochlorothiazide 12.5 mg Lisinopril 10 mg Simvastatin 20 mg |
Placebo |
| Soliman 2009 | Aspirin 75 mg Hydrochlorothiazide 12.5 mg Lisinopril 10 mg Simvastatin 20 mg |
Usual care |
| TIPS 2009 | Aspirin 100 mg Atenolol 50 mg Hydrochlorothiazide 12.5 mg Ramipril 5 mg Simvastatin 20 mg |
8 other drug/drug combination groups:
|
| TOGETHER 2010 | Amlodipine 5 mg to 10 mg Atorvastatin 10 mg |
Amlodipine 5 mg, 10 mg |
| UMPIRE 2013 | Aspirin 75 mg Atenolol 50 mg Lisinopril 10 mg Simvastatin 40 mg or Aspirin 75 mg Hydrochlorothiazide 12.5 mg Lisinopril 10 mg Simvastatin 40 mg |
Usual care |
| Wald 2012 | Amlodipine 2.5 mg Hydrochlorothiazide 12.5 mg Losartan 25 mg Simvastatin 40 mg |
Placebo |
aSite investigators could request dosages of amlodipine and atorvastatin 5/20 mg and 10/20 mg.
Excluded studies
Details and reasons for exclusion for the studies that underwent full‐text review are presented in the Characteristics of excluded studies table. The majority of excluded studies were not RCTs.
Risk of bias in included studies
Details are provided for each of the included studies in the risk of bias tables in Characteristics of included studies and in Figure 2 and Figure 3.
2.
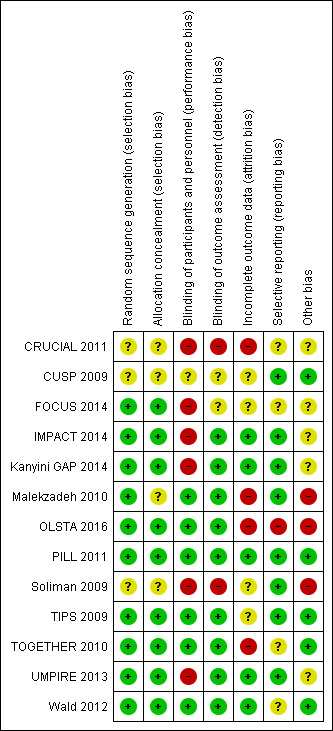
Risk of bias summary: review authors' judgements about each risk of bias item for each included study
3.
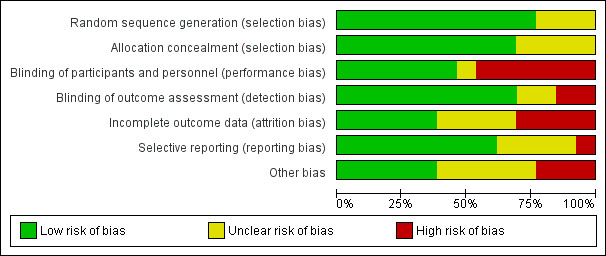
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
Allocation
The methods of random sequence generation or allocation concealment were unclear in four of the included studies (CRUCIAL 2011; CUSP 2009; Malekzadeh 2010; Soliman 2009). In the nine studies where randomisation and allocation concealment were clear, we judged the methods used to have a low risk of bias (FOCUS 2014; IMPACT 2014; Kanyini GAP 2014; OLSTA 2016; PILL 2011; TIPS 2009; TOGETHER 2010; UMPIRE 2013; Wald 2012).
Blinding
Five of the 13 included studies had a high risk for performance bias because the comparator group was usual care (CRUCIAL 2011; IMPACT 2014; Kanyini GAP 2014; Soliman 2009; UMPIRE 2013). However, three of these studies included blinded outcome assessment (IMPACT 2014; Kanyini GAP 2014; UMPIRE 2013) and had low risk of detection bias except for self‐reported outcomes (e.g. self‐reported adherence). One trial did not report whether or not the outcome assessment committee was blinded for adjudicating clinical events (FOCUS 2014), but the participants and personnel were not blinded to group allocation. The remaining seven trials stated that they were double‐blinded (participants and study personnel, including outcome assessors, were blinded to treatment allocation) and were regarded as having low risk of bias in this domain.
Incomplete outcome data
Most studies reported losses to follow‐up, but there were generally minimal differences in the proportion of losses to follow‐up between the intervention and control arms. Four studies had a high risk of attrition bias (CRUCIAL 2011; Malekzadeh 2010; OLSTA 2016; TOGETHER 2010), including use of last observation carried forward for missing continuous variables. Four studies had an unclear risk of attrition bias (CUSP 2009; FOCUS 2014; Soliman 2009; TIPS 2009), and five studies had low risk of attrition bias (IMPACT 2014; Kanyini GAP 2014; PILL 2011; UMPIRE 2013; Wald 2012).
Selective reporting
The risk of bias associated with selective reporting was low in eight studies (CUSP 2009; IMPACT 2014; Kanyini GAP 2014; Malekzadeh 2010; PILL 2011; Soliman 2009; TIPS 2009; UMPIRE 2013), unclear in four studies (CRUCIAL 2011; FOCUS 2014; TOGETHER 2010; Wald 2012), and high in one study (OLSTA 2016).
Other potential sources of bias
Malekzadeh 2010 used a run‐in period to exclude potential participants who had adherence rates less than 70%. In Soliman 2009, participants had varying degrees of background blood pressure and lipid‐lowering therapies between groups. In other cases there was insufficient information to judge the risk of bias in other sources of bias not covered above, and we categorised them all as unclear. In UMPIRE 2013, participants randomised to the intervention arm received fixed‐dose combination therapy at no cost compared with participants randomised to usual care who were responsible for their drug costs, which may have led to increased adherence in the intervention arm. In FOCUS 2014, the threshold of adherence using the Morisky‐Green Questionnaire was changed from 16 or more to 20 during the study, which has uncertain effects on this outcome. OLSTA 2016 was funded, executed, and monitored by the manufacturing company of the fixed‐dose combination that was studied.
Effects of interventions
See: Table 1
Primary outcomes
All‐cause mortality
Five secondary prevention trials, including 5300 participants, reported all‐cause mortality rates at the end of the study period with median follow‐up ranging from 9 to 23 months (CRUCIAL 2011; FOCUS 2014; IMPACT 2014; Kanyini GAP 2014; UMPIRE 2013). Mortality rates were low in both groups (1% in the intervention group compared with 1% in the comparator group; only 53 total deaths), and participants randomised to the intervention had no evidence of increased mortality compared with the comparator group (RR 1.10, 95% CI 0.64 to 1.89, I2 = 0%, Analysis 1.1) in the context of relatively few events. There were no differences among subgroups related to type of comparator (Analysis 1.2; Analysis 1.3) or number of drugs in the intervention (Analysis 1.4; Analysis 1.5).
1.1. Analysis.
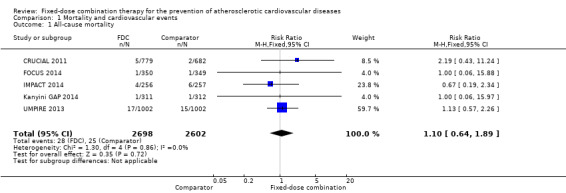
Comparison 1 Mortality and cardiovascular events, Outcome 1 All‐cause mortality.
1.2. Analysis.
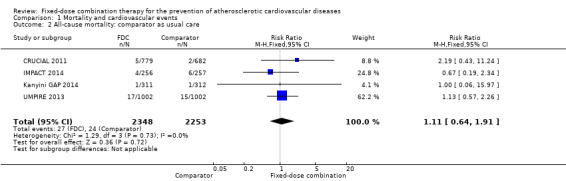
Comparison 1 Mortality and cardiovascular events, Outcome 2 All‐cause mortality: comparator as usual care.
1.3. Analysis.
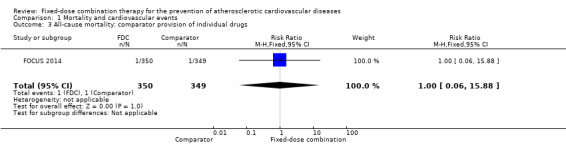
Comparison 1 Mortality and cardiovascular events, Outcome 3 All‐cause mortality: comparator provision of individual drugs.
1.4. Analysis.
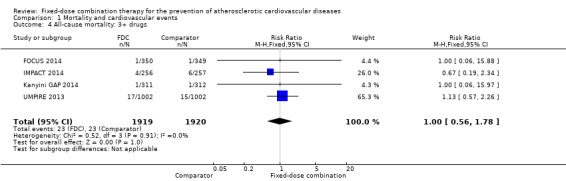
Comparison 1 Mortality and cardiovascular events, Outcome 4 All‐cause mortality: 3+ drugs.
1.5. Analysis.
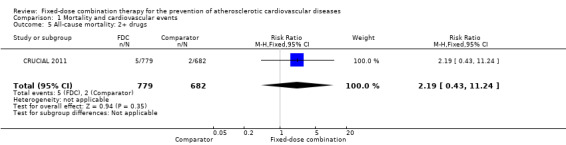
Comparison 1 Mortality and cardiovascular events, Outcome 5 All‐cause mortality: 2+ drugs.
Major ASCVD events
Only six out of 13 studies, including 4517 participants, reported rates of ASCVD events (FOCUS 2014; IMPACT 2014; Kanyini GAP 2014; Malekzadeh 2010; OLSTA 2016; UMPIRE 2013). ASCVD events were uncommon in both groups (4.7% rate in the intervention group compared with 3.7% in the comparator group; only 188 total ASCVD events), resulting in uncertainty of the effect of fixed‐dose combination therapy on this outcome (RR 1.26, 95% CI 0.95 to 1.66, I2 = 0%, Analysis 1.6). This uncertainty remained when evaluating subgroups of primary or secondary prevention trials (Analysis 1.7; Analysis 1.8), type of comparator (Analysis 1.9; Analysis 1.10), or number of drugs in the intervention (Analysis 1.11; Analysis 1.12).
1.6. Analysis.
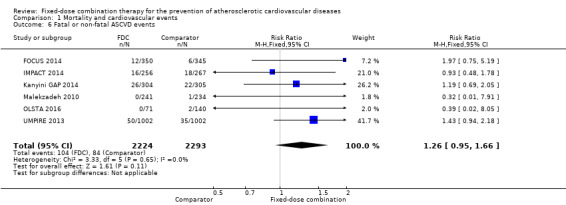
Comparison 1 Mortality and cardiovascular events, Outcome 6 Fatal or non‐fatal ASCVD events.
1.7. Analysis.
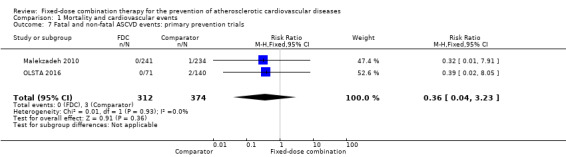
Comparison 1 Mortality and cardiovascular events, Outcome 7 Fatal and non‐fatal ASCVD events: primary prevention trials.
1.8. Analysis.
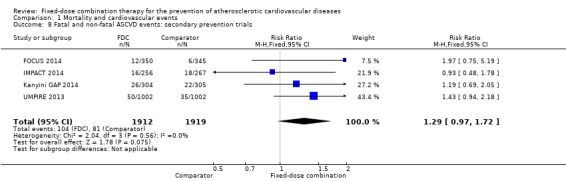
Comparison 1 Mortality and cardiovascular events, Outcome 8 Fatal and non‐fatal ASCVD events: secondary prevention trials.
1.9. Analysis.
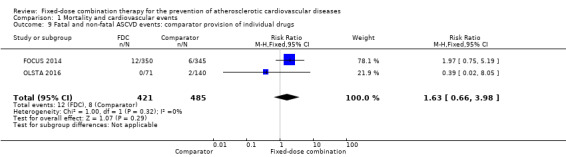
Comparison 1 Mortality and cardiovascular events, Outcome 9 Fatal and non‐fatal ASCVD events: comparator provision of individual drugs.
1.10. Analysis.
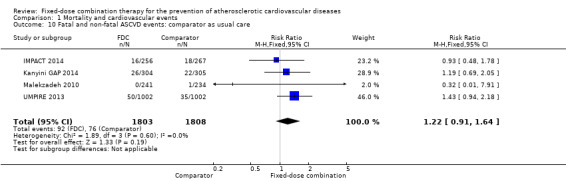
Comparison 1 Mortality and cardiovascular events, Outcome 10 Fatal and non‐fatal ASCVD events: comparator as usual care.
1.11. Analysis.
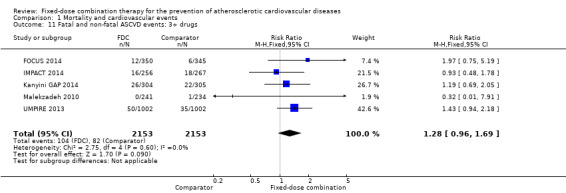
Comparison 1 Mortality and cardiovascular events, Outcome 11 Fatal and non‐fatal ASCVD events: 3+ drugs.
1.12. Analysis.
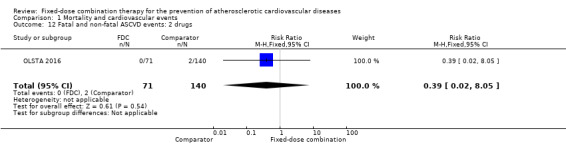
Comparison 1 Mortality and cardiovascular events, Outcome 12 Fatal and non‐fatal ASCVD events: 2 drugs.
Adverse events
We included 11 trials including 6906 participants reporting aggregated rates of adverse events in both groups in the meta‐analysis. The risk for adverse events was higher in participants in the intervention arm compared with participants in the control arm (32% versus 27%, RR 1.16, 95% CI 1.09 to 1.25, I2 = 0%, Analysis 2.1). There was a trend toward higher rate of adverse events in primary prevention trials (RR 1.37, 95% CI 1.17 to 1.60, Analysis 2.2) compared with secondary prevention trials (RR 1.11, 95% CI 1.03 to 1.20, Analysis 2.3) but there were no differences among other subgroups. Specific side effects that were evaluated included myalgias (8 studies, 4% versus 3%, RR 1.11, 95% CI 0.84 to 1.48, Analysis 2.8), increased liver enzymes (4 studies, 7% versus 6%, RR 1.04, 95% CI 0.74 to 1.47, I2 = 0%, Analysis 2.9), cough (5 studies, 5% versus 3%, RR 1.86, 95% CI 0.75 to 4.59, I2 = 76%, Analysis 2.10), gastric irritation and dyspepsia (4 studies, 3% versus 2%, RR 1.33, 95% CI 0.64 to 2.74, I2 = 67%, Analysis 2.11), and bleeding (2 studies, 2% versus 0.2%, RR 5.68, 95% CI 1.01 to 32.03, I2 = 0%, Analysis 2.12).
2.1. Analysis.
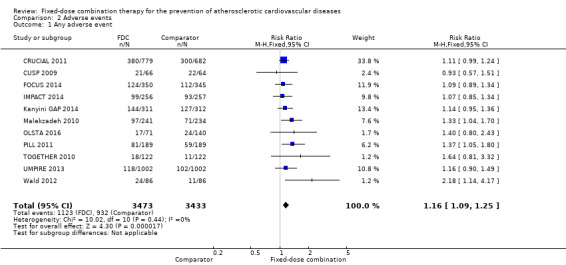
Comparison 2 Adverse events, Outcome 1 Any adverse event.
2.2. Analysis.
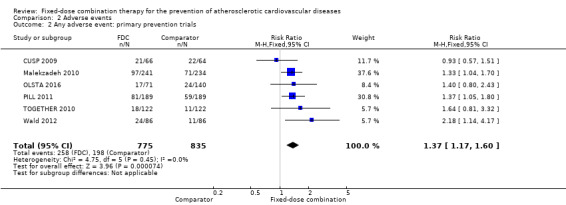
Comparison 2 Adverse events, Outcome 2 Any adverse event: primary prevention trials.
2.3. Analysis.
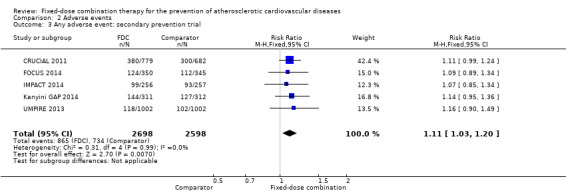
Comparison 2 Adverse events, Outcome 3 Any adverse event: secondary prevention trial.
2.8. Analysis.
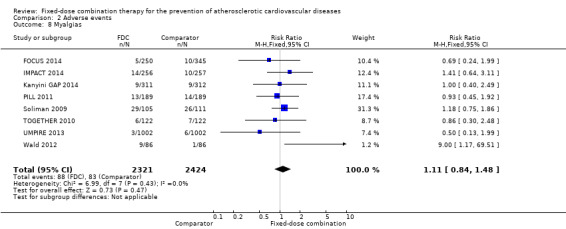
Comparison 2 Adverse events, Outcome 8 Myalgias.
2.9. Analysis.
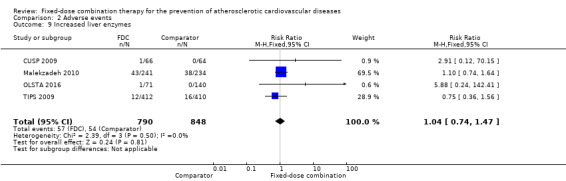
Comparison 2 Adverse events, Outcome 9 Increased liver enzymes.
2.10. Analysis.
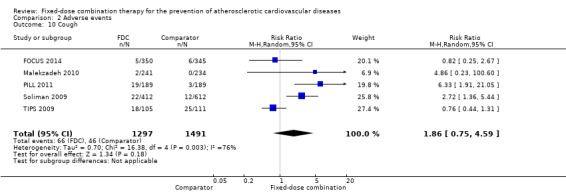
Comparison 2 Adverse events, Outcome 10 Cough.
2.11. Analysis.
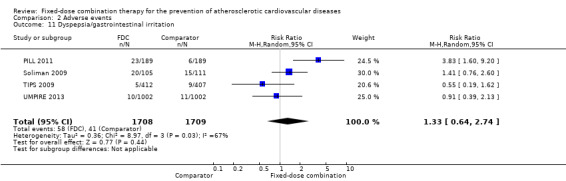
Comparison 2 Adverse events, Outcome 11 Dyspepsia/gastrointestinal irritation.
2.12. Analysis.
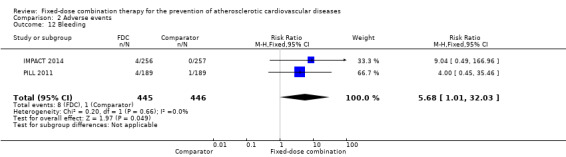
Comparison 2 Adverse events, Outcome 12 Bleeding.
Secondary outcomes
Blood pressure
All 13 trials reported changes in systolic and diastolic blood pressure in 7638 participants. There was a large degree of heterogeneity among the trials for both systolic blood pressure (I2 = 92%) and diastolic blood pressure (I2 = 91%). No single trial explained this heterogeneity, nor was it explained by primary versus secondary prevention trials nor two‐drug versus three or more drug combinations. Using a random‐effects model, the MD in systolic blood pressure between the intervention and control arms was ‐6.34 mmHg (95% CI ‐9.03 to ‐3.64, Analysis 3.1), and the MD in diastolic blood pressure between the intervention and control arms was ‐3.33 mmHg (95% CI ‐4.86 to ‐1.79, Analysis 3.2). Trials that included usual care in the comparator group (CRUCIAL 2011; CUSP 2009; IMPACT 2014; Kanyini GAP 2014; UMPIRE 2013) did not have as large reductions in systolic blood pressure (MD ‐3.44 mmHg, 95% CI ‐7.61 to 0.74) compared with other trials (Analysis 3.5), but the direction of effect was similar. These results should be interpreted with caution given the degree of heterogeneity. There was no evidence of funnel plot asymmetry for systolic blood pressure. There were no differences in subgroup analyses evaluating the effect on systolic blood pressure by primary or secondary prevention trials (Analysis 3.3; Analysis 3.4). The effects were lower in trials that included usual care as the comparator (MD ‐3.44 mmHg, 95% CI ‐7.61 to 0.74, Analysis 3.5) compared with trials that used a placebo as the comparator (MD ‐10.77 mmHg, 95% CI ‐12.72 to ‐8.81, Analysis 3.6). There were no differences between trials with 3+ drugs or 2 drugs (Analysis 3.7; Analysis 3.8).
3.1. Analysis.
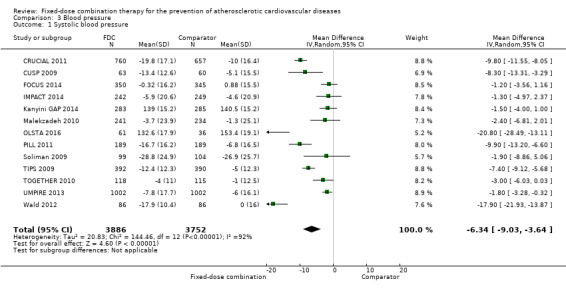
Comparison 3 Blood pressure, Outcome 1 Systolic blood pressure.
3.2. Analysis.
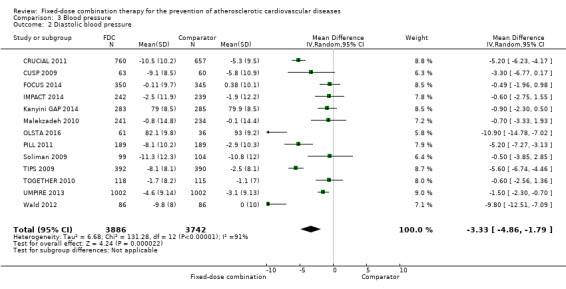
Comparison 3 Blood pressure, Outcome 2 Diastolic blood pressure.
3.5. Analysis.
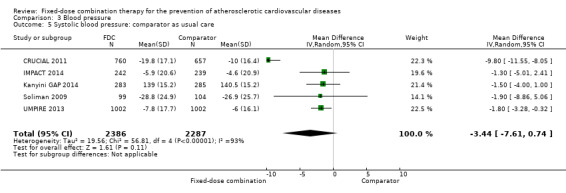
Comparison 3 Blood pressure, Outcome 5 Systolic blood pressure: comparator as usual care.
3.3. Analysis.
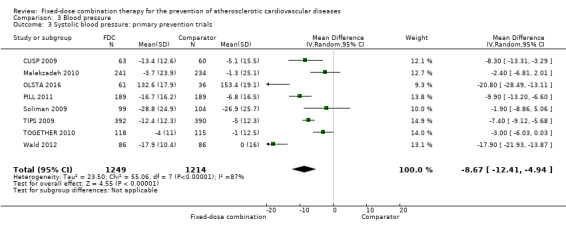
Comparison 3 Blood pressure, Outcome 3 Systolic blood pressure: primary prevention trials.
3.4. Analysis.
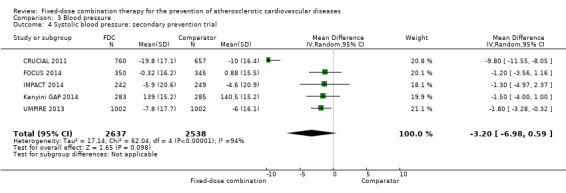
Comparison 3 Blood pressure, Outcome 4 Systolic blood pressure: secondary prevention trial.
3.6. Analysis.
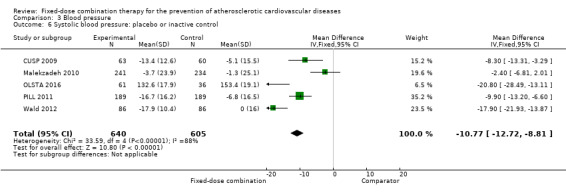
Comparison 3 Blood pressure, Outcome 6 Systolic blood pressure: placebo or inactive control.
3.7. Analysis.
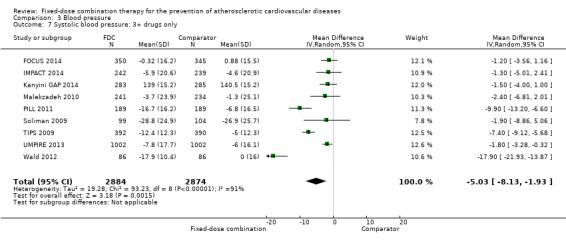
Comparison 3 Blood pressure, Outcome 7 Systolic blood pressure: 3+ drugs only.
3.8. Analysis.
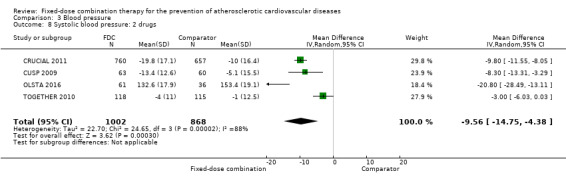
Comparison 3 Blood pressure, Outcome 8 Systolic blood pressure: 2 drugs.
Lipids
Eleven trials reported changes in total cholesterol in 6565 participants, and 12 trials reported changes in LDL cholesterol in 7153 participants. There was a large degree of heterogeneity among the trials for both total cholesterol (I2 = 98%) and LDL cholesterol (I2 = 98%). No single trial explained this heterogeneity. Using a random‐effects model, the MD in total cholesterol between the intervention and control arm was ‐0.61 mmol/L (95% CI ‐0.88 to ‐0.35, Analysis 4.1). Using a random‐effects model, MD in LDL cholesterol between the intervention and control arms was ‐0.70 mmol/L (95% CI ‐0.98 to ‐0.41, Analysis 4.2). Trials that included usual care in the comparator group (CRUCIAL 2011; CUSP 2009; IMPACT 2014; Kanyini GAP 2014; UMPIRE 2013) did not have as large reductions in total cholesterol (MD ‐0.16 mmol/L, 95% CI ‐0.44 to 0.12) compared with other trials (Analysis 4.5), but the direction of effect was similar. These results should be interpreted with caution given the degree of heterogeneity. There was evidence of funnel plot asymmetry for total cholesterol (Figure 4). The effects of fixed‐dose combination therapy on total cholesterol were greater in the seven primary prevention trials (MD ‐0.92 mmol/L, 95% CI ‐1.18 to 0.65, Analysis 4.3) compared with the four secondary prevention trials (MD ‐0.16 mmol/L, 95% CI ‐0.49 to 0.17, Analysis 4.4), which may have been due to the higher use of placebo control in primary prevention trials. The effects were lower in trials that included usual care as the comparator (MD ‐0.16 mmol/L, 95% CI ‐0.44 to 0.12, Analysis 4.5) compared with trials that used a placebo as the comparator (MD ‐0.83 mmol/L, 95% CI ‐0.99 to ‐0.67, Analysis 4.6). There were no differences in the effect among trials that included 3+ drugs (MD ‐0.48 mmol/L, 95% CI ‐0.80 to ‐0.16, Analysis 4.7) compared with 2 drugs (MD ‐0.94 mmol/L, 95% CI ‐1.50 to ‐0.38, Analysis 4.8), which is expected because of the use of statin therapy in all fixed‐dose combinations.
4.1. Analysis.
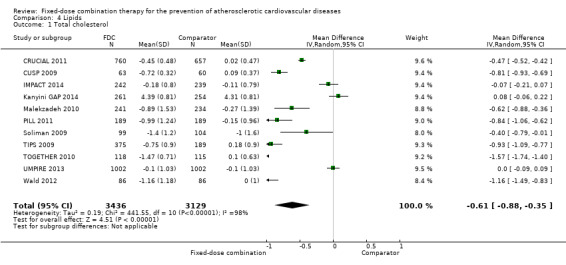
Comparison 4 Lipids, Outcome 1 Total cholesterol.
4.2. Analysis.
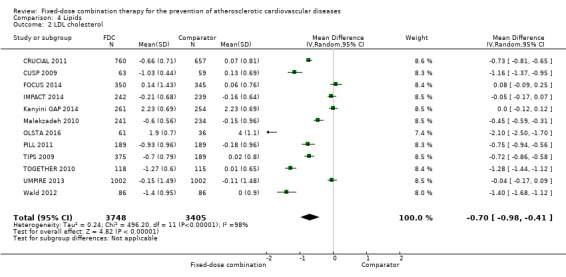
Comparison 4 Lipids, Outcome 2 LDL cholesterol.
4.5. Analysis.
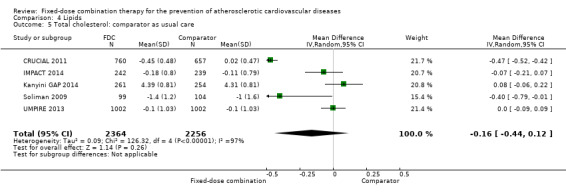
Comparison 4 Lipids, Outcome 5 Total cholesterol: comparator as usual care.
4.
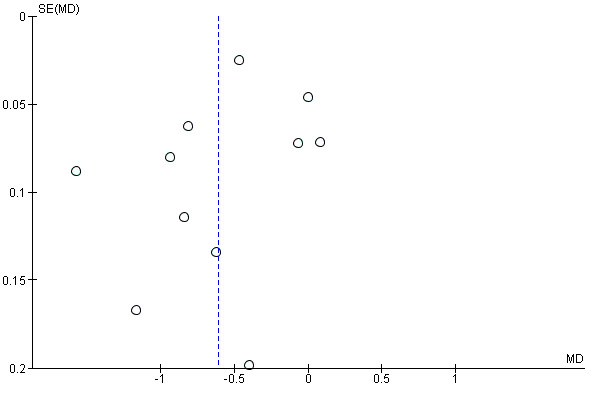
Funnel plot of comparison: 3 Cholesterol, outcome: 3.1 Total cholesterol.
4.3. Analysis.
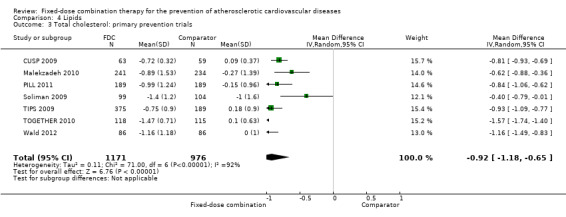
Comparison 4 Lipids, Outcome 3 Total cholesterol: primary prevention trials.
4.4. Analysis.
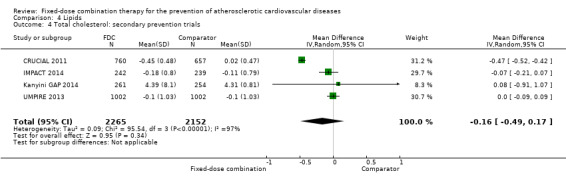
Comparison 4 Lipids, Outcome 4 Total cholesterol: secondary prevention trials.
4.6. Analysis.
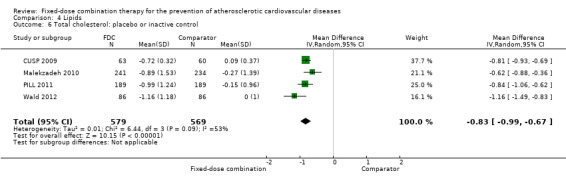
Comparison 4 Lipids, Outcome 6 Total cholesterol: placebo or inactive control.
4.7. Analysis.
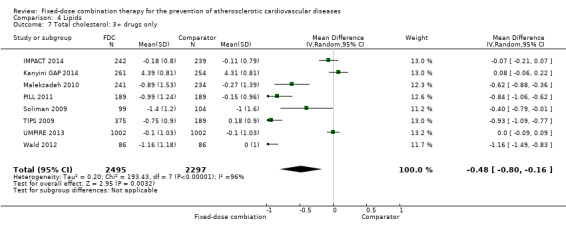
Comparison 4 Lipids, Outcome 7 Total cholesterol: 3+ drugs only.
4.8. Analysis.
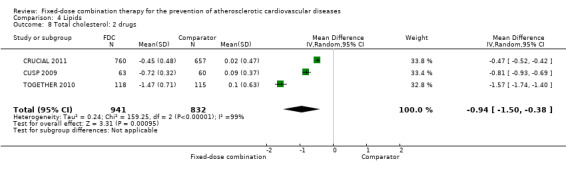
Comparison 4 Lipids, Outcome 8 Total cholesterol: 2 drugs.
Adherence
Four trials reported adherence in 3835 participants (FOCUS 2014, IMPACT 2014, Kanyini GAP 2014, UMPIRE 2013; all secondary prevention trials and all combinations included 3+ drugs), and in three of these trials (IMPACT 2014, Kanyini GAP 2014, UMPIRE 2013) adherence was defined as taking aspirin, statin, and two or more blood pressure‐lowering drugs. Adherence was assessed through self‐report (FOCUS 2014, IMPACT 2014, Kanyini GAP 2014, UMPIRE 2013), pill count (FOCUS 2014), and linkage to pharmacy data (IMPACT 2014). Adherence was higher in the intervention group compared with the control groups (74% versus 53%, RR 1.44, 95% CI 1.26 to 1.65, I2 = 80%, moderate‐quality evidence, Analysis 5.1). The heterogeneity of effect was largely explained by IMPACT 2014, but the magnitude and direction of effect was similar after excluding this trial (post‐hoc analysis: RR 1.35 95% CI 1.25 to 1.46, I2 = 34%). The effect of fixed‐dose combination therapy was similar in the three trials that used usual care as the comparator (Analysis 5.2) compared with the one trial with the comparator of providing individual drugs (Analysis 5.3).
5.1. Analysis.
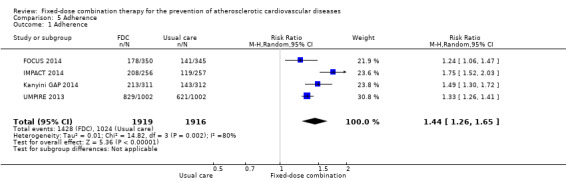
Comparison 5 Adherence, Outcome 1 Adherence.
5.2. Analysis.
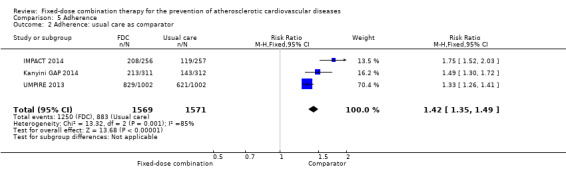
Comparison 5 Adherence, Outcome 2 Adherence: usual care as comparator.
5.3. Analysis.
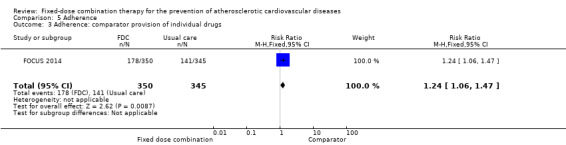
Comparison 5 Adherence, Outcome 3 Adherence: comparator provision of individual drugs.
Discontinuation
Rates of discontinuation were reported in both groups in seven trials including 3118 participants with active control or placebo as the comparator (CUSP 2009; FOCUS 2014; Malekzadeh 2010; PILL 2011; TIPS 2009; TOGETHER 2010; Wald 2012). Discontinuation rates were higher in individuals randomized to fixed‐dose combination therapy (12% versus 10%, RR 1.24, 95% CI 1.01 to 1.51, I2 = 0%, Analysis 6.1).
6.1. Analysis.
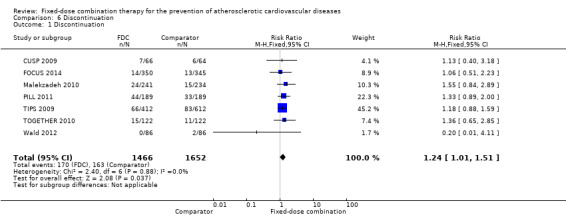
Comparison 6 Discontinuation, Outcome 1 Discontinuation.
Health‐related quality of life
Three trials including 3009 participants (IMPACT 2014, Kanyini GAP 2014, UMPIRE 2013) reported health‐related quality‐of‐life measures at the end of the study period using the EQ‐5D instrument. Mean (SD) summary index scores demonstrated no effect of fixed‐dose combination on EQ‐5D scores compared with usual care (MD 0.22, 95% CI ‐1.02 to 1.46, I2 = 0%, Analysis 7.1).
7.1. Analysis.
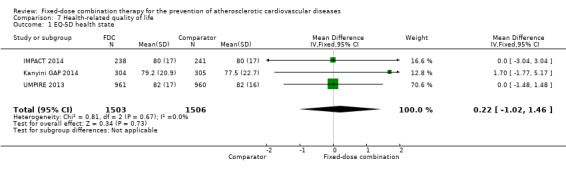
Comparison 7 Health‐related quality of life, Outcome 1 EQ‐5D health state.
Costs
One study (Kanyini GAP 2014) reported direct Medicare benefit costs (n = 551 participants) and pharmacy benefit costs (n = 458 participants) among a sub‐sample of individuals randomised to fixed‐dose combination therapy or usual care who agreed to have their records linked to Medicare benefits. As part of the trial design, individuals randomised to the fixed‐dose combination therapy arm incurred out‐of‐pocket costs typical for the Pharmaceutical Benefits Scheme, ranging from AUS 0 to AUS 35 per month. Unadjusted Medicare costs were similar (MD AUS 12, 95% CI ‐259 to 235) but unadjusted pharmacy costs appeared lowered in participants randomised to fixed‐dose combination therapy (MD AUS 995, 95% CI ‐1366 to ‐624).
Discussion
Summary of main results
This systematic review demonstrates that the effects of fixed‐dose combination therapy on all‐cause mortality or ASCVD events are uncertain. However, the event rates for these outcomes were very low, only five (all‐cause mortality) and six (ASCVD) events out of 13 trials reported these outcomes, respectively, and these trials used usual care as their comparator. The uncertainty from this update suggests that future research will likely change this estimate. The trend toward greater number of ASCVD events in the group randomised to fixed‐dose combination may be due to chance, performance bias due to lack blinding of the study personnel and participants, or the effects of switching or initiating the fixed‐dose combination, but merits further investigation. Adverse events were common in both the intervention (30%) and comparator (24%) groups, with participants randomised to fixed‐dose combination therapy being 20% more likely to report an adverse event. Notably, no serious adverse events were reported. The trials reported reductions in systolic and diastolic blood pressure and total and LDL cholesterol. These risk factor changes would have been expected to result in a reduction in ASCVD events if sustained, but the trials reporting changes in risk factors were generally too short to detect a potential difference by their design. There was also substantial heterogeneity in these estimates, so these effects on risk factors should be interpreted with caution.
The trials demonstrated a 26% (95% CI 2% to 55%) increased risk of discontinuing the study medication (discontinuation rate range 10% to 23%) compared with either usual care, placebo, or an active drug (aspirin, statin, or thiazide in the case of TIPS 2009). We were unable to explain the heterogeneity of effects on blood pressure or lipids in terms of primary versus secondary prevention trials, the number of drugs in the fixed‐dose combination pills, or the comparator group being active control, placebo or usual care. It is possible that the heterogeneity is due to the characteristics of the participants studied, differences in the potency of the antihypertensives and statins used, and the differences in treatments used in the comparison groups. The apparent paradox of the intervention leading to higher discontinuation rates and higher adherence is largely dependent on the comparator group. For example, in trials that included usual care as the comparator, the trials were not able to measure and thus report discontinuation rates.
Overall completeness and applicability of evidence
The included trials used five different polypills: three of the studies (CRUCIAL 2011; CUSP 2009; TOGETHER 2010) included polypills with only two drugs (one blood pressure‐lowering drug (amlodipine) and one statin (atorvastatin)); three studies (PILL 2011; Soliman 2009; UMPIRE 2013) used the Dr Reddy's Lab Red Heart Pill that includes four drugs (aspirin, lisinopril, simvastatin, and hydrochlorothiazide), and the remaining studies included different four‐drug (Malekzadeh 2010; Wald 2012) or five‐drug combinations (TIPS 2009). These trials were performed in 32 countries, including 19 low‐ and middle‐income countries, where the burden of ASCVD is greater than in high‐income countries (Roth 2015). However, the provision of usual care in trials led to far higher adherence rates than have been reported in community‐based studies evaluating multidrug adherence in low‐ and middle‐income countries (Yusuf 2011).
The decision to combine the estimates of these different drug combinations and different comparators was made, and meta‐analysis for this review was performed to evaluate the estimated effect size of fixed‐dose combination therapy. A rationale for fixed‐dose combination therapy is that it is more likely to be taken than multiple dose regimens. However, we found a higher likelihood of discontinuation for fixed‐dose treatment than for placebo. Comparisons of adherence across trials are hampered by differing definitions, which should be standardised in future reporting of these trials. Trials using 'usual care' comparison groups reported reasonably high levels of adherence and low levels of discontinuation, but these may be misleading as there is no relevant comparison.
There are six ongoing trials (NCT01826019; INTEGRATE; PolyIran; NCT02278471; NCT02596126; NCT01646437), and 12 trials that await classification (Fommei 2015; NCT00530946; NCT01004705; NCT01005290; NCT01362218; NCT01406431; NCT01764178; NCT02075619; NCT02569814; NCT02662894; NCT02791958; NCT02842359). The results of these trials are likely to have an important impact on our confidence in the estimates of effect and may change the estimates given the number of trials, number of participants, length of follow‐up, and estimated number of events relative to the current evidence base. These trials evaluate the effects of combinations in various settings, including among older individuals (NCT02596126) and within complex health system interventions that incorporate clinician decision support (INTEGRATE) and non‐physician health workers (NCT01826019).
Quality of the evidence
The main risk of bias was related to lack of blinding of participants and personnel, which was inherent to the intervention. Using other GRADE domains, we judged the quality of evidence of fixed‐dose combination therapy for all‐cause mortality and ASCVD events to be low, which was driven by imprecision (low event rates) and indirectness of evidence. The comparator of usual care was of a higher standard than might be expected outside of the research setting and particularly higher than has been reported in low‐ and middle‐income countries based on previous research (Yusuf 2011). This observation is further supported by the SPACE collaboration demonstrating a differential effect of the intervention on adherence among individuals with low baseline treatment, suggesting that individuals who have low treatment rates at baseline are more likely to benefit (Webster 2016a). We judged the quality of evidence for fixed‐dose combination therapy on adverse events to be moderate, due to indirectness of evidence, because the comparator group included individuals receiving usual care, which included drug prescription rates that were higher than those seen in non‐research settings, as well as placebo, which would not be an expected comparator for fixed‐dose combinations in clinical settings. We judged the quality of evidence for the effect of fixed‐dose combination therapy on systolic blood pressure and LDL cholesterol to be moderate, due to unexplained heterogeneity that was likely driven by differences in populations, fixed‐dose combinations, and comparator groups. We judged the quality of evidence for fixed‐dose combination on total cholesterol as low because of unexplained heterogeneity as outlined for systolic blood pressure and LDL cholesterol; we further downgraded the quality of evidence for total cholesterol for reporting bias due to funnel plot asymmetry. We judged the quality of evidence for fixed‐dose combination therapy on adherence to be moderate due to indirectness of evidence based on the high quality care provided in the comparator of usual care (IMPACT 2014; Kanyini GAP 2014; UMPIRE 2013) or active drug comparator provided to these participants (FOCUS 2014).
Potential biases in the review process
For the TIPS 2009 and Wald 2012 studies, we relied upon the point estimates and standard deviations extracted by Elley 2012, since these data points were not specifically provided in the text of the manuscripts. Elley and colleagues estimated the outcome standard deviations using baseline standard deviations as reported by Furukawa and colleagues (Furukawa 2006).
Agreements and disagreements with other studies or reviews
Our results demonstrated modestly lower reductions in systolic (‐6.34 mmHg versus ‐9.20 mmHg) and diastolic blood pressure (‐3.33 mmHg versus ‐5.00 mmHg) and lower total (‐0.61 mmol/L versus ‐1.22 mmol/L) and LDL cholesterol (‐0.70 mmol/L versus ‐1.02 mmol/L) compared with an earlier systematic review (Elley 2012). The absolute and relative adverse event rates were similar to those reported by Elley 2012, but the absolute and relative discontinuation rates were lower in our review. These differences are accounted for by our inclusion of seven additional studies (CRUCIAL 2011; FOCUS 2014; IMPACT 2014; Kanyini GAP 2014; OLSTA 2016; Soliman 2009; UMPIRE 2013).
The changes in blood pressure were lower than those predicted by Wald and Law (diastolic blood pressure: ‐3.33 mmHg versus ‐11 mmHg, Wald 2003), which may be due to the number of blood pressure‐lowering drugs, baseline blood pressures, or comparison to usual care groups that received very high‐quality care demonstrated by adherence rates in the comparator groups, which would not be typical in most communities (Yusuf 2011). The changes in LDL cholesterol were also lower than those predicted by Wald and Law (‐0.70 mmol/L versus 1.8 mmol/L) for similar reasons to those outlined above.
We have reported a similar direction and magnitude of effects that were reported in the individual participant data meta‐analysis performed by the Single Pill to Avert Cardiovascular Events (SPACE) collaboration (Webster 2016a), which included data from IMPACT 2014; Kanyini GAP 2014; UMPIRE 2013. In the SPACE collaboration meta‐analysis, the relative effect on adherence was larger (80% versus 50%, RR 1.58, 95% CI 1.32 to 1.90) but the effect on systolic blood pressure (SBP) (−2.5 mmHg; 95% CI −4.5 to −0.4) and LDL cholesterol (−0.1 mmol/L; 95% CI −0.2 to 0.0) were lower but with greater precision. These investigators evaluated the interaction between baseline treatment and adherence and SBP and demonstrated a greater effect of fixed‐dose combination therapy on adherence and SBP among individuals with low baseline treatment compared with individuals with high baseline treatment.
Bangalore 2007 have previously performed a systematic review and meta‐analysis of the effect of fixed‐dose combination therapy on adherence for chronic conditions including hypertension, diabetes, and HIV and reported a 24% (95% CI 19% to 29%) lower rate of discontinuation compared with control. These results were similar to those reported by Gupta 2010, who reported an increased odds of adherence with fixed‐dose combination therapy for blood pressure compared with usual care (OR 1.21, 95% CI 1.03 to 1.43). Gupta and colleagues demonstrated trends toward improved blood pressure control and side effects (Gupta 2010). The differences in discontinuation rates and adherence between these studies and our study may be due to the fact that participants in the Bangalore and Gupta meta‐analyses received active drug in either arm compared with our meta‐analysis where comparator group participants received either usual care (and possibly no drugs), placebo, or alternative drugs with potentially lower rates of side effects (TIPS 2009).
Virdee 2013 interviewed 11 primary care physicians and five practice nurses in nine Birmingham, UK practices about their knowledge and attitudes toward fixed‐dose combination therapy. The majority of respondents were uncertain about how they would incorporate fixed‐dose combination therapy in their practice and whether it was designed for primary or secondary ASCVD prevention. Most felt reluctant about using a specific age cut‐off to initiate therapy, despite acknowledging potential advantages to this approach. Most respondents felt unease at the concept of minimal or no monitoring of patients taking a fixed‐dose combination therapy, despite the proposal by Wald and Law (Wald 2003). In March 2010, Viera and colleagues surveyed US physicians about their willingness to prescribe fixed‐dose combination therapy. Nearly two out of every three physicians reported that they would prescribe fixed‐dose combination therapy for people at moderate risk for ASCVD and more than four out of every five physicians reported that they would prescribe fixed‐dose combination therapy for people at high risk for ASCVD. These disparate data using different methods of data collection suggest varying potential for uptake among physicians.
Authors' conclusions
Implications for practice.
The effects of fixed‐dose combination therapy on all‐cause mortality or atherosclerotic cardiovascular disease (ASCVD) events are uncertain. A limited number of trials reported these outcomes, and the included trials were primarily designed to observe changes in ASCVD risk factor levels rather than clinical events, which may partially explain the observed differences in risk factors that were not translated into differences in clinical outcomes among the included trials. Fixed‐dose combination therapy is associated with modest increases in adverse events compared with placebo, active comparators, or usual care which may result from improved adherence to a multidrug regimen. Ongoing, longer‐term trials of fixed‐dose combination therapy will help demonstrate whether short‐term changes in risk factors might be maintained and lead to expected differences in clinical events based on these changes.
Implications for research.
High‐quality randomised controlled trials are needed to evaluate if the effect of fixed‐dose combination therapies on risk factor levels translates into improvements in fatal and non‐fatal events in both primary and secondary ASCVD‐prevention settings. Ongoing trials will be informative; studies awaiting classification may be as well. The certainty of effect following the inclusion of these trials relies, at least in part, on their conduct and event rates. Some of these trials will also help demonstrate the effectiveness of fixed‐dose combination therapy in conjunction with other health system interventions. Larger studies are also needed to evaluate the risk of serious adverse events in varied populations.
What's new
| Date | Event | Description |
|---|---|---|
| 12 January 2017 | New search has been performed | The searches were re‐run on 19 September 2016. Differences between 2014 review and 2017 update: Title changed from cardiovascular disease to atherosclerotic cardiovascular disease for greater clarity in the target disease of combinations with at least one blood pressure‐lowering drug and one lipid‐lowering drug. |
| 6 January 2017 | New citation required but conclusions have not changed | Four additional trials reported in this update compared with 2014 review. No change in the overall direction and magnitude of effects with the addition of these additional trials. More ongoing trials identified. |
Acknowledgements
We are grateful for the assistance from Dr Curt Furberg who provided additional information on data reported in Soliman 2009, for the assistance from Dr Tom Marshall and colleagues for extra data for Malekzadeh 2010, for the assistance of Henry Lishi Li for translation of one excluded manuscript, and for the assistance of Ms Amy Rogers for her editorial assistance with the review. We are grateful for the assistance of Ms. Nicola Wright for her work on the previous version of this review.
Appendices
Appendix 1. Search strategies 2012
The Cochrane Library
#1 MeSH descriptor Cardiovascular Diseases explode all trees #2 cardio* #3 cardia* #4 heart* #5 coronary* #6 angina* #7 ventric* #8 myocard* #9 pericard* #10 isch?em* #11 emboli* #12 arrhythmi* #13 thrombo* #14 atrial fibrillat* #15 tachycardi* #16 endocardi* #17 (sick next sinus) #18 MeSH descriptor Stroke explode all trees #19 (stroke or stokes) #20 cerebrovasc* #21 cerebral vascular #22 apoplexy #23 (brain near/2 accident) #24 ((brain* or cerebral or lacunar) near/2 infarct*) #25 MeSH descriptor Hypertension explode all trees #26 hypertensi* #27 peripheral next arter* next disease* #28 ((high or increased or elevated) near/2 (blood next pressure)) #29 MeSH descriptor Hyperlipidemias explode all trees #30 hyperlipid* #31 hyperlip?emia* #32 hypercholesterol* #33 hypercholester?emia* #34 hyperlipoprotein?emia* #35 hypertriglycerid?emia* #36 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35) #37 MeSH descriptor Drug Combinations, this term only #38 polypill* #39 (drug near/2 combin*) #40 ((multi* or several) near/2 (ingredient* or component)) #41 policap #42 quintapill #43 (single near/2 pill* near/2 comb*) #44 single‐pill #45 Red Heart pill* #46 (#37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44 OR #45) #47 36 and 46, from 2000 to 2012
MEDLINE Ovid
1 exp Cardiovascular Diseases/ 2 cardio*.tw. 3 cardia*.tw. 4 heart*.tw. 5 coronary*.tw. 6 angina*.tw. 7 ventric*.tw. 8 myocard*.tw. 9 pericard*.tw. 10 isch?em*.tw. 11 emboli*.tw. 12 arrhythmi*.tw. 13 thrombo*.tw. 14 atrial fibrillat*.tw. 15 tachycardi*.tw. 16 endocardi*.tw. 17 (sick adj sinus).tw. 18 exp Stroke/ 19 (stroke or stokes).tw. 20 cerebrovasc*.tw. 21 cerebral vascular.tw. 22 apoplexy.tw. 23 (brain adj2 accident*).tw. 24 ((brain* or cerebral or lacunar) adj2 infarct*).tw. 25 exp Hypertension/ 26 hypertensi*.tw. 27 peripheral arter* disease*.tw. 28 ((high or increased or elevated) adj2 blood pressure).tw. 29 exp Hyperlipidemias/ 30 hyperlipid*.tw. 31 hyperlip?emia*.tw. 32 hypercholesterol*.tw. 33 hypercholester?emia*.tw. 34 hyperlipoprotein?emia*.tw. 35 hypertriglycerid?emia*.tw. 36 or/1‐35 37 Drug Combinations/ 38 polypill*.tw. 39 (drug adj2 combin*).tw. 40 ((multi* or several) adj2 (ingredient* or component*)).tw. 41 policap.tw. 42 quintapill.tw. 43 (single adj2 pill* adj2 comb*).tw. 44 single‐pill.tw. 45 Red Heart pill*.tw. 46 or/37‐45 47 randomised controlled trial.pt. 48 controlled clinical trial.pt. 49 randomised.ab. 50 placebo.ab. 51 drug therapy.fs. 52 randomly.ab. 53 trial.ab. 54 groups.ab. 55 47 or 48 or 49 or 50 or 51 or 52 or 53 or 54 56 exp animals/ not humans.sh. 57 55 not 56 58 36 and 46 59 58 and 57 60 limit 59 to yr="2000 ‐Current"
Embase Ovid
1 exp Cardiovascular Diseases/ 2 cardio*.tw. 3 cardia*.tw. 4 heart*.tw. 5 coronary*.tw. 6 angina*.tw. 7 ventric*.tw. 8 myocard*.tw. 9 pericard*.tw. 10 isch?em*.tw. 11 emboli*.tw. 12 arrhythmi*.tw. 13 thrombo*.tw. 14 atrial fibrillat*.tw. 15 tachycardi*.tw. 16 endocardi*.tw. 17 (sick adj sinus).tw. 18 exp cerebrovascular disease/ 19 (stroke or stokes).tw. 20 cerebrovasc*.tw. 21 cerebral vascular.tw. 22 apoplexy.tw. 23 (brain adj2 accident*).tw. 24 ((brain* or cerebral or lacunar) adj2 infarct*).tw. 25 exp Hypertension/ 26 hypertensi*.tw. 27 peripheral arter* disease*.tw. 28 ((high or increased or elevated) adj2 blood pressure).tw. 29 exp Hyperlipidemias/ 30 hyperlipid*.tw. 31 hyperlip?emia*.tw. 32 hypercholesterol*.tw. 33 hypercholester?emia*.tw. 34 hyperlipoprotein?emia*.tw. 35 hypertriglycerid?emia*.tw. 36 or/1‐35 37 Drug Combinations/ 38 polypill*.tw. 39 (drug adj2 combin*).tw. 40 ((multi* or several) adj2 (ingredient* or component*)).tw. 41 policap.tw. 42 quintapill.tw. 43 (single adj2 pill* adj2 comb*).tw. 44 single‐pill.tw. 45 Red Heart pill*.tw. 46 or/37‐45 47 36 and 46 48 random$.tw. 49 factorial$.tw. 50 crossover$.tw. 51 cross over$.tw. 52 cross‐over$.tw. 53 placebo$.tw. 54 (doubl$ adj blind$).tw. 55 (singl$ adj blind$).tw. 56 assign$.tw. 57 allocat$.tw. 58 volunteer$.tw. 59 crossover procedure/ 60 double blind procedure/ 61 randomised controlled trial/ 62 single blind procedure/ 63 48 or 49 or 50 or 51 or 52 or 53 or 54 or 55 or 56 or 57 or 58 or 59 or 60 or 61 or 62 64 (animal/ or nonhuman/) not human/ 65 63 not 64 66 47 and 65 67 limit 66 to yr="2000 ‐Current"
ISI Web of Science
25 #24 AND #23 24 TS=(random* or blind* or allocat* or assign* or trial* or placebo* or crossover* or cross‐over*) 23 #22 AND #14 22 #21 OR #20 OR #19 OR #18 OR #17 OR #16 OR #15 21 TS=(single‐pill or "red heart pill") 20 TS=(single near/2 pill* near/2 comb*) 19 TS=(policap or quintapill) 18 TS=(several near/2 ingredient* or several near/2 component) 17 TS=(multi* near/2 ingredient* or multi* near/2 component) 16 TS=(drug near/2 combin*) 15 TS=polypill* 14 #13 OR #12 OR #11 OR #10 OR #9 OR #8 OR #7 OR #6 OR #5 OR #4 OR #3 OR #2 OR #1 13 TS=(hyperlipid* or hyperlip?emia* or hyperchlosterol* or hypercholester?emia* or hyperlipoprotein?emia* or hypertriglycerid?emia*) 12 TS=(high near/2 "blood pressure" or increased near/2 "blood pressure" or elevated near/2 "blood pressure") 11 TS=(hypertensi* or "peripheral arter* disease*") 10 TS=(brain* near/2 infarct* OR cerebral near/2 infarct* OR lacunar near/2 infarct*) 9 TS=(brain near/2 accident) 8 TS=apoplexy 7 TS=(stroke or strokes or cerebrovasc* or "cerebral vascular") 6 TS=("sick sinus") 5 TS=(tachycardi* or endocardi*) 4 TS="atrial fibrillat*" 3 TS=(pericard* or isch?em* or emboli* or arrhythmi* or thromo*) 2 TS=(cardia* or heart* or coronary* or angina* or ventric* or myocard*) 1 TS=(cardio)
Appendix 2. Search strategies 2013
The Cochrane Library
#1 MeSH descriptor Cardiovascular Diseases explode all trees #2 cardio* #3 cardia* #4 heart* #5 coronary* #6 angina* #7 ventric* #8 myocard* #9 pericard* #10 isch?em* #11 emboli* #12 arrhythmi* #13 thrombo* #14 atrial fibrillat* #15 tachycardi* #16 endocardi* #17 (sick next sinus) #18 MeSH descriptor Stroke explode all trees #19 (stroke or stokes) #20 cerebrovasc* #21 cerebral vascular #22 apoplexy #23 (brain near/2 accident) #24 ((brain* or cerebral or lacunar) near/2 infarct*) #25 MeSH descriptor Hypertension explode all trees #26 hypertensi* #27 peripheral next arter* next disease* #28 ((high or increased or elevated) near/2 (blood next pressure)) #29 MeSH descriptor Hyperlipidemias explode all trees #30 hyperlipid* #31 hyperlip?emia* #32 hypercholesterol* #33 hypercholester?emia* #34 hyperlipoprotein?emia* #35 hypertriglycerid?emia* #36 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35) #37 MeSH descriptor Drug Combinations, this term only #38 polypill* #39 (drug near/2 combin*) #40 ((multi* or several) near/2 (ingredient* or component)) #41 policap #42 quintapill #43 (single near/2 pill* near/2 comb*) #44 single‐pill #45 Red Heart pill* #46 (#37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44 OR #45) #47 36 and 46, from 2000 to 2013
MEDLINE Ovid
1 exp Cardiovascular Diseases/ 2 cardio*.tw. 3 cardia*.tw. 4 heart*.tw. 5 coronary*.tw. 6 angina*.tw. 7 ventric*.tw. 8 myocard*.tw. 9 pericard*.tw. 10 isch?em*.tw. 11 emboli*.tw. 12 arrhythmi*.tw. 13 thrombo*.tw. 14 atrial fibrillat*.tw. 15 tachycardi*.tw. 16 endocardi*.tw. 17 (sick adj sinus).tw. 18 exp Stroke/ 19 (stroke or stokes).tw. 20 cerebrovasc*.tw. 21 cerebral vascular.tw. 22 apoplexy.tw. 23 (brain adj2 accident*).tw. 24 ((brain* or cerebral or lacunar) adj2 infarct*).tw. 25 exp Hypertension/ 26 hypertensi*.tw. 27 peripheral arter* disease*.tw. 28 ((high or increased or elevated) adj2 blood pressure).tw. 29 exp Hyperlipidemias/ 30 hyperlipid*.tw. 31 hyperlip?emia*.tw. 32 hypercholesterol*.tw. 33 hypercholester?emia*.tw. 34 hyperlipoprotein?emia*.tw. 35 hypertriglycerid?emia*.tw. 36 or/1‐35 37 Drug Combinations/ 38 polypill*.tw. 39 (drug adj2 combin*).tw. 40 ((multi* or several) adj2 (ingredient* or component*)).tw. 41 policap.tw. 42 quintapill.tw. 43 (single adj2 pill* adj2 comb*).tw. 44 single‐pill.tw. 45 Red Heart pill*.tw. 46 or/37‐45 47 randomized controlled trial.pt. 48 controlled clinical trial.pt. 49 randomized.ab. 50 placebo.ab. 51 drug therapy.fs. 52 randomly.ab. 53 trial.ab. 54 groups.ab. 55 47 or 48 or 49 or 50 or 51 or 52 or 53 or 54 56 exp animals/ not humans.sh. 57 55 not 56 58 36 and 46 59 58 and 57 60 limit 59 to yr="2000 ‐Current" 61 (2012* or 2013*).ed. 62 60 and 61 63 limit 62 to “core clinical journals (aim)”
Embase Ovid
1 exp Cardiovascular Diseases/ 2 cardio*.tw. 3 cardia*.tw. 4 heart*.tw. 5 coronary*.tw. 6 angina*.tw. 7 ventric*.tw. 8 myocard*.tw. 9 pericard*.tw. 10 isch?em*.tw. 11 emboli*.tw. 12 arrhythmi*.tw. 13 thrombo*.tw. 14 atrial fibrillat*.tw. 15 tachycardi*.tw. 16 endocardi*.tw. 17 (sick adj sinus).tw. 18 exp cerebrovascular disease/ 19 (stroke or stokes).tw. 20 cerebrovasc*.tw. 21 cerebral vascular.tw. 22 apoplexy.tw. 23 (brain adj2 accident*).tw. 24 ((brain* or cerebral or lacunar) adj2 infarct*).tw. 25 exp Hypertension/ 26 hypertensi*.tw. 27 peripheral arter* disease*.tw. 28 ((high or increased or elevated) adj2 blood pressure).tw. 29 exp Hyperlipidemias/ 30 hyperlipid*.tw. 31 hyperlip?emia*.tw. 32 hypercholesterol*.tw. 33 hypercholester?emia*.tw. 34 hyperlipoprotein?emia*.tw. 35 hypertriglycerid?emia*.tw. 36 or/1‐35 37 Drug Combinations/ 38 polypill*.tw. 39 (drug adj2 combin*).tw. 40 ((multi* or several) adj2 (ingredient* or component*)).tw. 41 policap.tw. 42 quintapill.tw. 43 (single adj2 pill* adj2 comb*).tw. 44 single‐pill.tw. 45 Red Heart pill*.tw. 46 or/37‐45 47 36 and 46 48 random$.tw. 49 factorial$.tw. 50 crossover$.tw. 51 cross over$.tw. 52 cross‐over$.tw. 53 placebo$.tw. 54 (doubl$ adj blind$).tw. 55 (singl$ adj blind$).tw. 56 assign$.tw. 57 allocat$.tw. 58 volunteer$.tw. 59 crossover procedure/ 60 double blind procedure/ 61 randomized controlled trial/ 62 single blind procedure/ 63 48 or 49 or 50 or 51 or 52 or 53 or 54 or 55 or 56 or 57 or 58 or 59 or 60 or 61 or 62 64 (animal/ or nonhuman/) not human/ 65 63 not 64 66 47 and 65 67 limit 66 to yr="2000 ‐Current" 68 (2012* or 2013*).em. 69 67 and 68 70 limit 69 to priority journals
ISI Web of Science
25 #24 AND #23 24 TS=(random* or blind* or allocat* or assign* or trial* or placebo* or crossover* or cross‐over*) 23 #22 AND #14 22 #21 OR #20 OR #19 OR #18 OR #17 OR #16 OR #15 21 TS=(single‐pill or "red heart pill") 20 TS=(single near/2 pill* near/2 comb*) 19 TS=(policap or quintapill) 18 TS=(several near/2 ingredient* or several near/2 component) 17 TS=(multi* near/2 ingredient* or multi* near/2 component) 16 TS=(drug near/2 combin*) 15 TS=polypill* 14 #13 OR #12 OR #11 OR #10 OR #9 OR #8 OR #7 OR #6 OR #5 OR #4 OR #3 OR #2 OR #1 13 TS=(hyperlipid* or hyperlip?emia* or hyperchlosterol* or hypercholester?emia* or hyperlipoprotein?emia* or hypertriglycerid?emia*) 12 TS=(high near/2 "blood pressure" or increased near/2 "blood pressure" or elevated near/2 "blood pressure") 11 TS=(hypertensi* or "peripheral arter* disease*") 10 TS=(brain* near/2 infarct* OR cerebral near/2 infarct* OR lacunar near/2 infarct*) 9 TS=(brain near/2 accident) 8 TS=apoplexy 7 TS=(stroke or strokes or cerebrovasc* or "cerebral vascular") 6 TS=("sick sinus") 5 TS=(tachycardi* or endocardi*) 4 TS="atrial fibrillat*" 3 TS=(pericard* or isch?em* or emboli* or arrhythmi* or thromo*) 2 TS=(cardia* or heart* or coronary* or angina* or ventric* or myocard*) 1 TS=(cardio)
Appendix 3. Search strategies 2016
CENTRAL/DARE/HTA/NHS EDD
#1 MeSH descriptor Cardiovascular Diseases explode all trees
#2 cardio*
#3 cardia*
#4 heart*
#5 coronary*
#6 angina*
#7 ventric*
#8 myocard*
#9 pericard*
#10 isch?em*
#11 emboli*
#12 arrhythmi*
#13 thrombo*
#14 atrial fibrillat*
#15 tachycardi*
#16 endocardi*
#17 (sick next sinus)
#18 MeSH descriptor Stroke explode all trees
#19 (stroke or stokes)
#20 cerebrovasc*
#21 cerebral vascular
#22 apoplexy
#23 (brain near/2 accident)
#24 ((brain* or cerebral or lacunar) near/2 infarct*)
#25 MeSH descriptor Hypertension explode all trees
#26 hypertensi*
#27 peripheral next arter* next disease*
#28 ((high or increased or elevated) near/2 (blood next pressure))
#29 MeSH descriptor Hyperlipidemias explode all trees
#30 hyperlipid*
#31 hyperlip?emia*
#32 hypercholesterol*
#33 hypercholester?emia*
#34 hyperlipoprotein?emia*
#35 hypertriglycerid?emia*
#36 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35)
#37 MeSH descriptor Drug Combinations, this term only
#38 polypill*
#39 (drug near/2 combin*)
#40 ((multi* or several) near/2 (ingredient* or component))
#41 policap
#42 quintapill
#43 (single near/2 pill* near/2 comb*)
#44 single‐pill
#45 Red Heart pill*
#46 (#37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44 OR #45)
#47 36 and 46, from 2013 to 2016
MEDLINE OVID
Cochrane sensitivity‐maximising RCT filter applied (Handbook 2011)
1 exp Cardiovascular Diseases/
2 cardio*.tw.
3 cardia*.tw.
4 heart*.tw.
5 coronary*.tw.
6 angina*.tw.
7 ventric*.tw.
8 myocard*.tw.
9 pericard*.tw.
10 isch?em*.tw.
11 emboli*.tw.
12 arrhythmi*.tw.
13 thrombo*.tw.
14 atrial fibrillat*.tw.
15 tachycardi*.tw.
16 endocardi*.tw.
17 (sick adj sinus).tw.
18 exp Stroke/
19 (stroke or stokes).tw.
20 cerebrovasc*.tw.
21 cerebral vascular.tw.
22 apoplexy.tw.
23 (brain adj2 accident*).tw.
24 ((brain* or cerebral or lacunar) adj2 infarct*).tw.
25 exp Hypertension/
26 hypertensi*.tw.
27 peripheral arter* disease*.tw.
28 ((high or increased or elevated) adj2 blood pressure).tw.
29 exp Hyperlipidemias/
30 hyperlipid*.tw.
31 hyperlip?emia*.tw.
32 hypercholesterol*.tw.
33 hypercholester?emia*.tw.
34 hyperlipoprotein?emia*.tw.
35 hypertriglycerid?emia*.tw.
36 or/1‐35
37 Drug Combinations/
38 polypill*.tw.
39 (drug adj2 combin*).tw.
40 ((multi* or several) adj2 (ingredient* or component*)).tw.
41 policap.tw.
42 quintapill.tw.
43 (single adj2 pill* adj2 comb*).tw.
44 single‐pill.tw.
45 Red Heart pill*.tw.
46 or/37‐45
47 randomized controlled trial.pt.
48 controlled clinical trial.pt.
49 randomized.ab.
50 placebo.ab.
51 drug therapy.fs.
52 randomly.ab.
53 trial.ab.
54 groups.ab.
55 47 or 48 or 49 or 50 or 51 or 52 or 53 or 54
56 exp animals/ not humans.sh.
57 55 not 56
58 36 and 46
59 58 and 57
60 limit 59 to yr="2000 ‐Current"
61 (2013* or 2014* or 2015* or 2016*).ed.
62 60 and 61
Embase OVID
Cochrane RCT filter (Handbook 2011)
1 exp Cardiovascular Diseases/
2 cardio*.tw.
3 cardia*.tw.
4 heart*.tw.
5 coronary*.tw.
6 angina*.tw.
7 ventric*.tw.
8 myocard*.tw.
9 pericard*.tw.
10 isch?em*.tw.
11 emboli*.tw.
12 arrhythmi*.tw.
13 thrombo*.tw.
14 atrial fibrillat*.tw.
15 tachycardi*.tw.
16 endocardi*.tw.
17 (sick adj sinus).tw.
18 exp cerebrovascular disease/
19 (stroke or stokes).tw.
20 cerebrovasc*.tw.
21 cerebral vascular.tw.
22 apoplexy.tw.
23 (brain adj2 accident*).tw.
24 ((brain* or cerebral or lacunar) adj2 infarct*).tw.
25 exp Hypertension/
26 hypertensi*.tw.
27 peripheral arter* disease*.tw.
28 ((high or increased or elevated) adj2 blood pressure).tw.
29 exp Hyperlipidemias/
30 hyperlipid*.tw.
31 hyperlip?emia*.tw.
32 hypercholesterol*.tw.
33 hypercholester?emia*.tw.
34 hyperlipoprotein?emia*.tw.
35 hypertriglycerid?emia*.tw.
36 or/1‐35
37 Drug Combinations/
38 polypill*.tw.
39 (drug adj2 combin*).tw.
40 ((multi* or several) adj2 (ingredient* or component*)).tw.
41 policap.tw.
42 quintapill.tw.
43 (single adj2 pill* adj2 comb*).tw.
44 single‐pill.tw.
45 Red Heart pill*.tw.
46 or/37‐45
47 36 and 46
48 random$.tw.
49 factorial$.tw.
50 crossover$.tw.
51 cross over$.tw.
52 cross‐over$.tw.
53 placebo$.tw.
54 (doubl$ adj blind$).tw.
55 (singl$ adj blind$).tw.
56 assign$.tw.
57 allocat$.tw.
58 volunteer$.tw.
59 crossover procedure/
60 double blind procedure/
61 randomized controlled trial/
62 single blind procedure/
63 48 or 49 or 50 or 51 or 52 or 53 or 54 or 55 or 56 or 57 or 58 or 59 or 60 or 61 or 62
64 (animal/ or nonhuman/) not human/
65 63 not 64
66 47 and 65
67 limit 66 to yr="2000 ‐Current"
68 (2013* or 2014* or 2015* or 2016*).em.
69 67 and 68
ISI Web of Science
RCT filter adapted from Cochrane RCT filter.
25 #24 AND #23, from 2013 to 2016
24 TS=(random* or blind* or allocat* or assign* or trial* or placebo* or crossover* or cross‐over*)
23 #22 AND #14
22 #21 OR #20 OR #19 OR #18 OR #17 OR #16 OR #15
21 TS=(single‐pill or "red heart pill")
20 TS=(single near/2 pill* near/2 comb*)
19 TS=(policap or quintapill)
18 TS=(several near/2 ingredient* or several near/2 component)
17 TS=(multi* near/2 ingredient* or multi* near/2 component)
16 TS=(drug near/2 combin*)
15 TS=polypill*
14 #13 OR #12 OR #11 OR #10 OR #9 OR #8 OR #7 OR #6 OR #5 OR #4 OR #3 OR #2 OR #1
13 TS=(hyperlipid* or hyperlip?emia* or hyperchlosterol* or hypercholester?emia* or hyperlipoprotein?emia* or hypertriglycerid?emia*)
12 TS=(high near/2 "blood pressure" or increased near/2 "blood pressure" or elevated near/2 "blood pressure")
11 TS=(hypertensi* or "peripheral arter* disease*")
10 TS=(brain* near/2 infarct* OR cerebral near/2 infarct* OR lacunar near/2 infarct*)
9 TS=(brain near/2 accident)
8 TS=apoplexy
7 TS=(stroke or strokes or cerebrovasc* or "cerebral vascular")
6 TS=("sick sinus")
5 TS=(tachycardi* or endocardi*)
4 TS="atrial fibrillat*"
3 TS=(pericard* or isch?em* or emboli* or arrhythmi* or thromo*)
2 TS=(cardia* or heart* or coronary* or angina* or ventric* or myocard*)
1 TS=(cardio)
Clinical Trials Register Searches
clinicaltrials.gov clinicaltrials.gov/ct2/home
Advanced Search
Search Terms: polypill OR "fixed dose" OR "drug combination" OR "drug combinations"
Study Type: Interventional Studies
Conditions: cardiovascular OR hypertension OR dyslipidemia OR hyperlipidemia OR hypercholesterolemia
WHO ICTRP apps.who.int/trialsearch/
polypill AND cardiovascular OR polypill AND hypertension OR polypill AND dyslipidemia OR polypill AND hyperlipidemia OR polypill AND hypercholesterolemia OR fixed dose AND cardiovascular OR fixed dose AND hypertension OR fixed dose AND dyslipidemia OR fixed dose AND hyperlipidemia OR fixed dose AND hypercholesterolemia OR drug combination AND cardiovascular OR drug combination AND hypertension OR drug combination AND dyslipidemia OR drug combination AND hyperlipidemia OR drug combination AND hypercholesterolemia OR drug combinations AND cardiovascular OR drug combinations AND hypertension OR drug combinations AND dyslipidemia OR drug combinations AND hyperlipidemia OR drug combinations AND hypercholesterolemia
Data and analyses
Comparison 1. Mortality and cardiovascular events.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality | 5 | 5300 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.64, 1.89] |
| 2 All‐cause mortality: comparator as usual care | 4 | 4601 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.64, 1.91] |
| 3 All‐cause mortality: comparator provision of individual drugs | 1 | 699 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.06, 15.88] |
| 4 All‐cause mortality: 3+ drugs | 4 | 3839 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.56, 1.78] |
| 5 All‐cause mortality: 2+ drugs | 1 | 1461 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.19 [0.43, 11.24] |
| 6 Fatal or non‐fatal ASCVD events | 6 | 4517 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.95, 1.66] |
| 7 Fatal and non‐fatal ASCVD events: primary prevention trials | 2 | 686 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.04, 3.23] |
| 8 Fatal and non‐fatal ASCVD events: secondary prevention trials | 4 | 3831 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.97, 1.72] |
| 9 Fatal and non‐fatal ASCVD events: comparator provision of individual drugs | 2 | 906 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.63 [0.66, 3.98] |
| 10 Fatal and non‐fatal ASCVD events: comparator as usual care | 4 | 3611 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.91, 1.64] |
| 11 Fatal and non‐fatal ASCVD events: 3+ drugs | 5 | 4306 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.96, 1.69] |
| 12 Fatal and non‐fatal ASCVD events: 2 drugs | 1 | 211 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.02, 8.05] |
Comparison 2. Adverse events.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Any adverse event | 11 | 6906 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [1.09, 1.25] |
| 2 Any adverse event: primary prevention trials | 6 | 1610 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [1.17, 1.60] |
| 3 Any adverse event: secondary prevention trial | 5 | 5296 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [1.03, 1.20] |
| 4 Any adverse event: comparator as usual care | 4 | 4601 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [1.03, 1.21] |
| 5 Adverse event: comparator as placebo or inactive control | 7 | 2305 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [1.12, 1.43] |
| 6 Adverse event: 3+ drugs only | 7 | 4860 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [1.08, 1.30] |
| 7 Adverse events: 2 drugs | 4 | 2046 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [1.01, 1.25] |
| 8 Myalgias | 8 | 4745 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.84, 1.48] |
| 9 Increased liver enzymes | 4 | 1638 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.74, 1.47] |
| 10 Cough | 5 | 2788 | Risk Ratio (M‐H, Random, 95% CI) | 1.86 [0.75, 4.59] |
| 11 Dyspepsia/gastrointestinal irritation | 4 | 3417 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.64, 2.74] |
| 12 Bleeding | 2 | 891 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.68 [1.01, 32.03] |
2.4. Analysis.
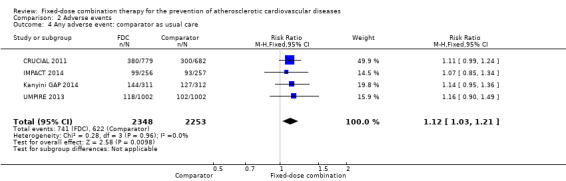
Comparison 2 Adverse events, Outcome 4 Any adverse event: comparator as usual care.
2.5. Analysis.
Comparison 2 Adverse events, Outcome 5 Adverse event: comparator as placebo or inactive control.
2.6. Analysis.
Comparison 2 Adverse events, Outcome 6 Adverse event: 3+ drugs only.
2.7. Analysis.
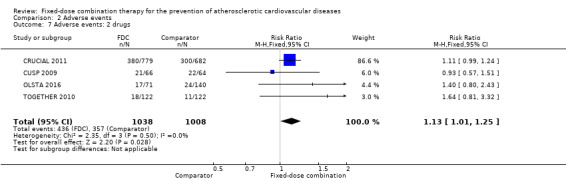
Comparison 2 Adverse events, Outcome 7 Adverse events: 2 drugs.
Comparison 3. Blood pressure.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Systolic blood pressure | 13 | 7638 | Mean Difference (IV, Random, 95% CI) | ‐6.34 [‐9.03, ‐3.64] |
| 2 Diastolic blood pressure | 13 | 7628 | Mean Difference (IV, Random, 95% CI) | ‐3.33 [‐4.86, ‐1.79] |
| 3 Systolic blood pressure: primary prevention trials | 8 | 2463 | Mean Difference (IV, Random, 95% CI) | ‐8.67 [‐12.41, ‐4.94] |
| 4 Systolic blood pressure: secondary prevention trial | 5 | 5175 | Mean Difference (IV, Random, 95% CI) | ‐3.20 [‐6.98, 0.59] |
| 5 Systolic blood pressure: comparator as usual care | 5 | 4673 | Mean Difference (IV, Random, 95% CI) | ‐3.44 [‐7.61, 0.74] |
| 6 Systolic blood pressure: placebo or inactive control | 5 | 1245 | Mean Difference (IV, Fixed, 95% CI) | ‐10.77 [‐12.72, ‐8.81] |
| 7 Systolic blood pressure: 3+ drugs only | 9 | 5758 | Mean Difference (IV, Random, 95% CI) | ‐5.03 [‐8.13, ‐1.93] |
| 8 Systolic blood pressure: 2 drugs | 4 | 1870 | Mean Difference (IV, Random, 95% CI) | ‐9.56 [‐14.75, ‐4.38] |
Comparison 4. Lipids.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total cholesterol | 11 | 6565 | Mean Difference (IV, Random, 95% CI) | ‐0.61 [‐0.88, ‐0.35] |
| 2 LDL cholesterol | 12 | 7153 | Mean Difference (IV, Random, 95% CI) | ‐0.70 [‐0.98, ‐0.41] |
| 3 Total cholesterol: primary prevention trials | 7 | 2147 | Mean Difference (IV, Random, 95% CI) | ‐0.92 [‐1.18, ‐0.65] |
| 4 Total cholesterol: secondary prevention trials | 4 | 4417 | Mean Difference (IV, Random, 95% CI) | ‐0.16 [‐0.49, 0.17] |
| 5 Total cholesterol: comparator as usual care | 5 | 4620 | Mean Difference (IV, Random, 95% CI) | ‐0.16 [‐0.44, 0.12] |
| 6 Total cholesterol: placebo or inactive control | 4 | 1148 | Mean Difference (IV, Random, 95% CI) | ‐0.83 [‐0.99, ‐0.67] |
| 7 Total cholesterol: 3+ drugs only | 8 | 4792 | Mean Difference (IV, Random, 95% CI) | ‐0.48 [‐0.80, ‐0.16] |
| 8 Total cholesterol: 2 drugs | 3 | 1773 | Mean Difference (IV, Random, 95% CI) | ‐0.94 [‐1.50, ‐0.38] |
Comparison 5. Adherence.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Adherence | 4 | 3835 | Risk Ratio (M‐H, Random, 95% CI) | 1.44 [1.26, 1.65] |
| 2 Adherence: usual care as comparator | 3 | 3140 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.42 [1.35, 1.49] |
| 3 Adherence: comparator provision of individual drugs | 1 | 695 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [1.06, 1.47] |
Comparison 6. Discontinuation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Discontinuation | 7 | 3118 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [1.01, 1.51] |
Comparison 7. Health‐related quality of life.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 EQ‐5D health state | 3 | 3009 | Mean Difference (IV, Fixed, 95% CI) | 0.22 [‐1.02, 1.46] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
CRUCIAL 2011.
| Methods | Open label, cluster‐randomised trial | |
| Participants | 136 clusters; 1461 total participants (779 intervention; 682 comparator participants) from 19 countries (Costa Rica, Croatia, Czech Republic, Dominican Republic, Indonesia, Jordan, Kuwait, Lebanon, Malaysia, Mexico, Panama, Philippines, South Korea, Russia, Taiwan, Thailand, Turkey, United Arab Emirates, Venezuela) Men and women aged 35‐79 years with hypertension and total cholesterol < 250 mg/dL plus 3 or more risk factors (current smoker, peripheral artery disease, type 2 diabetes, family history of early CHD before aged 55 years in first‐degree relative; left ventricular hypertrophy on ECG; history of transient ischaemic attack or stroke three or more months prior to screening; ECG abnormalities; age > 55 years (men) or > 65 years (women), total cholesterol > 250mg/dL, or HDL < 40mg/dL) |
|
| Interventions | Intervention: single pill amlodipine/atorvastatin (5 mg/10 mg‐10 mg/10 mg; site investigators could request dosages of 5/20 mg and 10/20 mg) in addition to other hypertensive/lipid‐lowering therapy as required, as well as therapeutic lifestyle counselling change Comparator: usual care, including therapeutic lifestyle counselling change |
|
| Outcomes | SBP, DBP, LDL‐C, total cholesterol; all‐cause mortality reported | |
| Notes | Comparator: inactive/usual care | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Investigators ‐ randomly assigned", "randomisation was stratified", "investigator as unit of randomisation" |
| Allocation concealment (selection bias) | Unclear risk | Due to cluster randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open label |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open label |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 93/779 (11.9%) discontinued intervention; 44/682 (6.5%) discontinued in usual care arm |
| Selective reporting (reporting bias) | Unclear risk | Not all outcomes available for meta‐analysis |
| Other bias | Unclear risk | Differences between two arms in terms of baseline blood pressure, ECG abnormalities, PVD |
CUSP 2009.
| Methods | Individual‐level RCT | |
| Participants | 130 participants (66 intervention; 64 comparator) from the USA with coexisting, untreated hypertension (SBP = 140 mmHg‐169 mmHg or DBP = 90 mmHg‐105 mmHg) and dyslipidaemia (LDL‐C = 110 mg/dL‐160 mg/dL) but without a history of cardiovascular disease; age > 21 years | |
| Interventions | Intervention: single pill amlodipine/atorvastatin (5 mg/20 mg) + therapeutic lifestyle changes Comparator: therapeutic lifestyle changes |
|
| Outcomes | Target for BP < 140/90 mm Hg and LDL‐C < 100 mg/dL (2.59 mmol ⁄L) at week 4 and week 8: the percentage of participants in whom the single LDL‐C goal was reached at weeks 4 and 8; mean changes from baseline in SBP and DBP at weeks 4 and 8; mean changes from baseline in LDL‐C at weeks 4 and 8; 10‐year Framingham risk of CHD at weeks 4 and 8 | |
| Notes | Comparator: inactive/usual care | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specifically stated: "Patients were randomised in a double‐blind manner" |
| Allocation concealment (selection bias) | Unclear risk | Not specifically stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not specifically stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specifically stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear how data from participants lost to follow‐up were handled |
| Selective reporting (reporting bias) | Low risk | Primary outcomes reported (week 4 blood pressure and LDL targets) |
| Other bias | Low risk | No other sources of bias are identifiable |
FOCUS 2014.
| Methods | "randomized, open‐label, active‐controlled, piggyback, 2‐group parallel trial" | |
| Participants | 695 participants (350 polypill; 345 comparator) across 63 sites in 4 countries (Argentina, Italy, Paraguay, Spain) Details about Phase 2 participants (age, sex) not provided in the primary manuscript. Inclusion criteria: “The study population included men and women age > 40 years with a history of acute MI within the last 2 years...Due to slow recruitment, after the initial 591 participants had been included, an amendment to the initial protocol was approved to allow for the inclusion of patients with any past history of an acute MI, regardless of duration from enrollment.” Exclusion criteria: "secondary dyslipidemia, contraindication to any of the components of the polypill, participation in another trial, previous percutaneous transluminal coronary angioplasty with a drug‐eluting stent within the previous year, severe congestive heart failure (New York Heart Association functional class III to IV), serum creatinine > 2 mg/dL, any condition limiting life expectancy < 2 years, and pregnancy or pre‐menopause.” |
|
| Interventions | Intervention: "aspirin (100 mg), simvastatin (40 mg), and ramipril at 3 different doses (2.5 mg, 5 mg, or 10 mg, which allowed for up‐titration at the discretion of the physician)” in hard‐shell gelatin capsule Comparator: aspirin, simvastatin, and ramipril provided separately Drugs were provided free of cost for both arms |
|
| Outcomes | Primary
Secondary
Outcomes measured at 1, 4, and 9 months Follow‐up: 9 months |
|
| Notes | Comparator: individual drugs (aspirin, simvastatin, ramipril) provided separately | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "a central electronic randomization service assigned participants to 1 of 2 arms” |
| Allocation concealment (selection bias) | Low risk | “a central electronic randomization service assigned participants to 1 of 2 arms” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open label trial |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Open label trial; no evidence of blinded outcome assessment committee |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Similar loss to follow‐up between groups (intervention 12.3%; comparator 10.1%, Table 2) but could influence primary outcome |
| Selective reporting (reporting bias) | Unclear risk | Primary outcome was reported, but the threshold for defining adherence was changed from 16‐20 during the trial. The effects of this change are uncertain. Data on 4‐month outcomes not reported but not likely different than longer term trends |
| Other bias | Unclear risk | Relatively small study to detect any differences in clinical outcomes; could be considered low risk of small study bias for adherence |
IMPACT 2014.
| Methods | "Open label randomised control trial" | |
| Participants | 513 participants (from 91 General Practitioners); target = 600 participants in New Zealand 256 polypill; 257 comparator Mean (SD) age: 62 (8) years for both arms Maori ethnicity: 50% for both arms Women: 39% intervention; 34% comparator CAD: 35% intervention; 38% comparator DM: 44% intervention; 41% comparator Employed: 46% intervention; 44% comparator “Given the available funding resources, the recruitment target was revised down to 500, which provided 89‐93% power to detect the same differences between risk factors and 92% power to detect a 30% relative improvement in adherence.” Inclusion criteria: "Adults aged 18‐79 years at high risk of cardiovascular disease (based on either established disease (coronary, cerebrovascular, or peripheral vascular) or ≥15% five year risk of a cardiovascular event); patient’s general practitioner considered all the drugs in at least one of the two versions of the fixed‐dose combination treatment available were recommended and was uncertain if treatment was best provided as fixed‐dose combination based treatment or as usual care" Exclusion criteria: "contraindications to any of the components of the fixed dose combination, congestive heart failure, haemorrhagic stroke, active stomach or duodenal ulcer, receipt of an oral anticoagulant, concerns by the general practitioner about the risk to a patient of changing his or her cardiovascular disease drugs, impending alteration of a drug regimen for an important length of time (for example, planned coronary bypass graft operation), or the participant was unlikely to complete the trial or trial procedures" |
|
| Interventions | Intervention:
Comparator: "The control is usual management. Physicians in both groups are encouraged to prescribe in line with New Zealand CVD risk assessment and management guidelines.” "both trial drugs and usual drugs were dispensed through community pharmacies." "Participants were required to pay what they would normally pay to receive a single government subsidised drug" "Standard patient co‐payments of NZ$5 (£2.6; €3.1; $4.3) for each item every three months" |
|
| Outcomes | Primary:
Secondary:
Outcomes measured: baseline, 1, 6, 12 months, end of trial Follow‐up: median of 23 months in both arms |
|
| Notes | Comparator: usual care | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “A central randomisation service randomly assigned (1:1) participants to fixed dose combination based treatment or usual care.” |
| Allocation concealment (selection bias) | Low risk | “A central randomisation service randomly assigned (1:1) participants to fixed dose combination based treatment or usual care.” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open label trial |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Adherence: self‐report but corroborated by pharmacy claims data but definition favours intervention (requiring second BP lowering drug, though sensitivity analyses showed similar direction of effect) LDL/SBP objectively measured and not likely too susceptible to bias SAE/CV events self‐reported but objective and reviewed by endpoints committee |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up rates low and balanced |
| Selective reporting (reporting bias) | Low risk | Outcome reporting largely matches protocol; 6‐month data may not have been reported but not likely different than longer term outcome trends |
| Other bias | Unclear risk | Small study bias to evaluate differences in clinical outcomes |
Kanyini GAP 2014.
| Methods | "randomized, open‐label trial" | |
| Participants | 623 participants (311 polypill, 312 comparator) from 33 centres (12 Aboriginal Medical Services); target = 1000 participants in Australia Mean (SD) age: 63.4 (12.5) years intervention; 63.7 (12.7) years comparator Women: 37% intervention; 37% comparator Indigenous: 51% overall (not reported by group) CVD: 59% intervention; 63% comparator CHD: 52% intervention; 54% comparator CM: 60% intervention; 55% comparator Inclusion criteria: "18 years or over and able to give informed consent, have a history of coronary heart disease (myocardial infarction, stable or unstable angina pectoris, or coronary revascularization procedure), and/ or ischaemic cerebrovascular disease, and/or peripheral vascular disease; or a calculated 5‐year CVD risk of 15% or greater*...Each participant had to have, in their doctor’s view, indications for all and no contraindications to any component of at least one of two polypills" *including a 5% increment for Aboriginal or Torres Strait Islander identification Exclusion criteria: “Participants were excluded if it was felt clinically inappropriate to alter medications.” |
|
| Interventions | Intervention
Comparator: "usual care" "Out‐of‐pocket expenses for the polypill were incurred identically to those for any other drug listed in the Pharmaceutical Benefits Scheme, which is the government subsidy programme through which most drugs are obtained in Australia." |
|
| Outcomes | Primary
Secondary
Time points measured: baseline, 1 month, and q6 month through 24 months Follow‐up: intervention: median 20.7 months, comparator: median 18.1 months |
|
| Notes | Comparator: usual care | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Central, computer‐based randomization to polypill‐based strategy or usual care was stratified by primary healthcare centre, type of indication (established CVD versus high risk), Indigenous identification and level of preventive treatment at baseline.” |
| Allocation concealment (selection bias) | Low risk | “Central, computer‐based randomization to polypill‐based strategy or usual care was stratified by primary healthcare centre, type of indication (established CVD versus high risk), Indigenous identification and level of preventive treatment at baseline.” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open label trial |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Adherence: high risk of bias because it was self reported SBP/TC/events: low risk of bias because these are objective measures, and the latter was adjudicated by a blinded outcome assessment committee |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low rates of losses to follow‐up and missingness, with rates balanced between the groups |
| Selective reporting (reporting bias) | Low risk | No differences between primary reports and protocol |
| Other bias | Unclear risk | Small study bias for events but low risk of bias for adherence and change in risk factors |
Malekzadeh 2010.
| Methods | Individual‐level, blocked RCT | |
| Participants | 475 participants (241 polypill; 234 control) from Golestan, Iran without CVD, hypertension, or hyperlipidaemia aged 50‐79 years (men) and 55‐79 years (women) | |
| Interventions | Intervention: polypill (aspirin 81 mg, enalapril 2.5 mg, atorvastatin 20 mg and hydrochlorothiazide 12.5 mg) Comparator: placebo |
|
| Outcomes | Hospital admissions/major cardiovascular events/seated and standing BP, LDL‐C, total cholesterol, triglycerides, HDL‐C and fasting glucose | |
| Notes | Comparator: inactive/placebo | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated block randomisation |
| Allocation concealment (selection bias) | Unclear risk | Computer generation allocation to numbered list of blister packs manufactured by Alborz Darou, but differences between intervention and comparator groups for baseline gender (38% versus 28%), systolic (125 mmHg vs 130 mmHg) and diastolic blood pressure (78 mmHg vs 81 mmHg) were seen |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical blister packs used for participant blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors (clinicians) blinded to allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High rate of loss to follow‐up at 12 months (experimental 32%; control 22%) |
| Selective reporting (reporting bias) | Low risk | Primary outcome reported (changes in blood pressure and LDL cholesterol) |
| Other bias | High risk | Run‐in period excluded participants with low (< 70%) adherence; large differences in baseline characteristics between intervention and control groups |
OLSTA 2016.
| Methods | Individual‐level RCT, 2:1:1:1 design with triple dummy | |
| Participants | 181 “Korean patients with mild to moderate hypertension and dyslipidemia” defined by JNC VII and ATP III. Participants underwent 4 week run‐in period and were recruited from 25 centres in Korea Exclusion criteria:
FDC: 71 participants, mean (SD) age 61.9 (8.1) years; 44% women; 44% diabetes; 0% CHD Olmesartan: 38 participants, mean (SD) age 59.5 (6.9) years; 33% women; 39% diabetes; 0% CHD Rosuvastatin: 38 participants, mean (SD) age 61.8 (8.0) years; 31% women; 22% diabetes; 0% CHD Placebo: 34 participants, mean (SD) age 62.5 (8.2) years; 28% women; 31% DM; 0% CHD |
|
| Interventions | Intervention: fixed‐dose combination of olmesartan medoxomil 40 mg + rosuvastatin 20 mg Comparator 1: Olmesartan medoxomil 40 mg Comparator 2: Rosuvastatin 20 mg Comparator 3: Placebo |
|
| Outcomes | Primary
Secondary
|
|
| Notes | Reported differences in baseline characteristics, which may or may not be due to chance: 3.3 mm SBP difference between rosuvastatin and placebo arms (but only those who completed follow‐up had baseline data reported); 3.3‐year difference in age between olmesartan and placebo group; 16% difference in women between FDC and placebo; 22% difference in DM between FDC and rosuvastatin |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centralized, computer generator random sequence |
| Allocation concealment (selection bias) | Low risk | Centralized, computer generator random sequence |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, triple dummy |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded study staff |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High loss to follow‐up rate in intervention group with complete case analysis only (no imputation) FDC: 10/71 (14%) Olmesartan: 2/38 (5%) Rosuvastatin: 2/38 (5%) Placebo: 5/34 (15%) |
| Selective reporting (reporting bias) | High risk | Protocol (NCT01764295) published in January 2013, after trial initiation |
| Other bias | High risk | Small study bias with short follow‐up; sponsored by Daewoong Pharmaceutical, which also performed trial execution and monitoring |
PILL 2011.
| Methods | Individual‐level RCT | |
| Participants | 378 participants (189 intervention; 189 comparator) from 7 countries (Australia, Brazil, India, Netherlands, New Zealand, UK, USA) with 5‐year Framingham coronary heart disease risk ≥ 7.5% or if Framingham risk was between 5% and 7.5%, two or more additional untreated risk factors were needed (body mass index > 30 kg/m2, waist circumference > 102 cm in men or > 88 cm in women; heart rate > 80 bpm; fasting glucose 5.6 mmol/L‐7 mmol/L, triglycerides > 1.7 mmol/L; family history of first degree relative with premature ischaemic heart disease or stroke (men < 55 years; women: < 65 years), or glomerular filtration rate < 60mL/min | |
| Interventions | Intervention: Red heart pill (aspirin 75 mg, lisinopril 10 mg, hydrochlorothiazide 12.5 mg and simvastatin 20 mg) Comparator: placebo |
|
| Outcomes | Change in SBP; change in LDL‐C; tolerability; secondary outcomes included discontinuation, DBP, total cholesterol, HDL‐C, total cholesterol:HDL cholesterol ratio, non‐HDL cholesterol, triglycerides, frequency of switching/adding open‐label treatment, estimated effects on CVD risk | |
| Notes | Comparator: inactive/placebo | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central computer‐based randomisation |
| Allocation concealment (selection bias) | Low risk | Central computer‐based randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Specifically reported and use of placebo control |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors and study staff all blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low rates of loss to follow‐up (experimental 2%; control 1%); however, last observation carried forward method used for missing continuous data at week 12 |
| Selective reporting (reporting bias) | Low risk | Outcomes outlined in methods paper were reported in the primary manuscript |
| Other bias | Low risk | No other sources of bias are identifiable |
Soliman 2009.
| Methods | Open label, parallel‐group RCT | |
| Participants | 216 participants (105 polypill; 111 comparator); ≥ 40 years for men and ≥ 50 years for women; estimated 10‐year World Health Organization total cardiovascular risk score ≥ 20% without established cardiovascular disease | |
| Interventions | Intervention: Red Heart pill 2b (75 mg aspirin, 20 mg simvastatin, 10 mg lisinopril and 12.5 mg hydrochlorothiazide) Comparator: standard practice defined by the study investigators |
|
| Outcomes | SBP, total cholesterol, 10‐year cardiovascular disease risk, adherence, fasting glucose, creatinine, potassium, and liver enzymes | |
| Notes | Comparator: inactive/usual care | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No method of randomisation stated |
| Allocation concealment (selection bias) | Unclear risk | No method of randomisation stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open label |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open label |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear how missing data were handled |
| Selective reporting (reporting bias) | Low risk | Primary outcomes (blood pressure, cholesterol, ten year CVD risk) all reported |
| Other bias | High risk | Use of non‐study antihypertensives and statins very different between centres |
TIPS 2009.
| Methods | Individual‐level RCT | |
| Participants | 2053 participants (205 aspirin; 205 thiazide; 209 thiazide + ramipril; 207 thiazide + atenolol; 205 ramipril + atenolol; 204 thiazde + ramipril + atenolol; 204 thiazide + ramipril + atenolol + aspirin; 202 simvastatin; 412 Polycap [thiazide + ramipril + atenolol + simvastatin + aspirin); 45‐80 years old without prior cardiovascular disease but with at least one risk factor: type 2 diabetes; blood pressure > 140/90 mmHg but < 160/100 mmHg; smoker within the past five years; waist‐to‐hip ratio > 0.85 for women and 0.90 for men; LDL cholesterol > 3.1 mmol/L but less 4.5 mmol/L or HDL cholesterol < 1.04 mmol/L | |
| Interventions | Intervention: Polycap (thiazide 12.5 mg, atenolol 50 mg, ramipril 5 mg, simvastatin 20 mg, aspirin 100 mg) Comparator: 8 other drug/drug combination groups listed above |
|
| Outcomes | LDL for the effect of lipid‐lowering drugs, BP for antihypertensive drugs, heart rate for the effects of atenolol, urinary 11‐dehydrothromboxane B2 for the antiplatelet effects of aspirin, rates of discontinuation of drugs for safety | |
| Notes | Comparator: active | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central computer randomisation |
| Allocation concealment (selection bias) | Low risk | Central computer randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo control using identical capsule |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blinding reported; probably occurred given research team's prior studies |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear how missing SBP and LDL‐C data at week 12 follow‐up were handled |
| Selective reporting (reporting bias) | Low risk | Primary outcomes reported |
| Other bias | Low risk | No other sources of bias are identifiable |
TOGETHER 2010.
| Methods | Individual‐level randomised, double dummy controlled trial | |
| Participants | 244 participants (122 intervention; 122 control) from the USA with history of hypertension but no history of CVD or diabetes with ≥ 2 risk factors: age ≥ 45 years for men; ≥ 55 years for women; current smoker; family history of premature coronary heart disease in first degree relative; HDL cholesterol < 40 mg/dl; waist circumference > 102 cm in men and > 88 cm in women | |
| Interventions | Intervention: single pill amlodipine (5/10 mg) plus atorvastatin 20 mg + therapeutic lifestyle changes Comparator: amlodipine (5/10 mg) + therapeutic lifestyle changes |
|
| Outcomes | Proportion achieving a BP goal < 140/90 mmHg and LDL‐C < 100 mg/dl at week 6; BP and LDL‐C goal at week 4; BP goal at weeks 4 and 6; change in SBP, DBP, LDL‐C, total cholesterol, HDL‐C, triglycerides at weeks 4 and 6; predicted 10‐year Framingham coronary heart disease risk score, adverse events | |
| Notes | Comparator: active | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central, computer‐based telerandomisation |
| Allocation concealment (selection bias) | Low risk | Central, computer‐based telerandomisation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind labelled bottles and double dummy |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Reportedly double blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Last observation carried forward used for non‐completers for final analysis |
| Selective reporting (reporting bias) | Unclear risk | Primary outcomes reported |
| Other bias | Low risk | No other sources of bias are identifiable |
UMPIRE 2013.
| Methods | Randomised, open label, blinded endpoint clinical trial of an FDC‐based treatment strategy compared with usual care | |
| Participants | ≥18 years old and established CVD or an estimated 5‐year CVD risk of 15% or greater in India and 3 European countries (England, Ireland, and the Netherlands) | |
| Interventions | Intervention: one of two versions of the fixed‐dose combination ((1) aspirin 75 mg, simvastatin 40 mg, lisinopril 10 mg, atenolol 50 mg or (2) aspirin 75 mg, simvastatin 40 mg, lisinopril 10 mg, hydrochlorothiazide 12.5 mg) Comparator: usual care |
|
| Outcomes | Primary: adherence to indicated medications (self‐reported current use of antiplatelet, statin, and ≥ 2 BP‐lowering therapies, defined as taking the medication for at least 4 days during the week preceding the visit) at baseline and at the end of the trial and changes in SBP and LDL‐C from baseline to the end of the trial. Secondary: adherence at 12 months, reasons for stopping cardiovascular medications, quality of life, serious adverse events, and changes in total cholesterol, HDL‐C, triglycerides, and creatinine from baseline to 12 months and end of study and cardiovascular events (including coronary heart disease, heart failure leading to death or hospital admission, and cerebrovascular or peripheral arterial disease events) |
|
| Notes | Comparator: inactive/usual care | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation occurred through web‐based clinical data management system |
| Allocation concealment (selection bias) | Low risk | Randomisation occurred through web‐based clinical data management system |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel were unblinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | At the end of the study, data on self‐reported adherence, systolic BP, and LDL‐C were available for 1921 (96%), 1849 (92%), and 1807 (90%) randomized participants, respectively |
| Selective reporting (reporting bias) | Low risk | All primary and secondary outcomes reported |
| Other bias | Unclear risk | Participants randomized to the intervention arm received fixed‐dose combination therapy at no cost compared with participants randomized to usual care who were responsible for their drug costs |
Wald 2012.
| Methods | Individual‐level randomised double‐blind placebo‐controlled cross‐over trial | |
| Participants | 86 individuals (43 Polypill then placebo; 43 placebo then Polypill) aged 50 years or over without history of cardiovascular disease who were previously taking simvastatin and blood pressure‐lowering drugs; limited to participants living in London or could travel easily to London | |
| Interventions | Intervention: fixed‐dose combination (amlodipine 2.5mg, losartan 25mg, hydrochlorothiazide 12.5mg, simvastatin 40mg) daily for 12 weeks Comparator: placebo |
|
| Outcomes | SBP, DBP, total cholesterol, LDL‐C, HDL‐C, triglycerides, apoB, adherence (pill counts of fixed‐dose combination compared with placebo), adverse events | |
| Notes | Comparator: inactive/placebo | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated block randomisation |
| Allocation concealment (selection bias) | Low risk | Computer‐generated block randomisation with sequential identical blister packs |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo controlled |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors reported as being blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Primary outcomes reported |
| Selective reporting (reporting bias) | Unclear risk | Adverse event data not clearly described; only proportion of individuals with "symptom", which was assumed to be an adverse event |
| Other bias | Low risk | No need for intention‐to‐treat analysis as cross‐over design. Any losses to follow‐up clear |
apoB: apolipoprotein B CHD: coronary heart disease CVD: cardiovascular disease DBP: diastolic blood pressure ECG: electrocardiogram HDL‐C: high‐density lipoprotein cholesterol LDL‐C: low‐density lipoprotein cholesterol PVD: peripheral vascular disease RCT: randomised controlled trial SBP: systolic blood pressure
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abdellatif 2012 | Wrong intervention |
| Agabiti Rosei 2014 | Wrong intervention |
| Agarwal 2013 | Wrong intervention |
| Anonymous 2010 | Wrong study design |
| Anonymous 2011 | Wrong study design |
| Anonymous 2012a | Wrong study design |
| Anonymous 2012b | Wrong intervention |
| Anonymous 2013a | Review |
| Anonymous 2013b | Wrong intervention |
| Athyros 2013 | Wrong study design |
| Athyros 2014 | Wrong study design |
| Bashir 2011 | Wrong study design |
| Becerra 2015 | Wrong study design |
| Bittencourt 2013 | Wrong study design |
| Bittencourt 2014 | Wrong study design |
| Blank 2007 | Review |
| Briasoulis 2013 | Wrong intervention |
| Bryant 2013 | Wrong study design |
| Carey 2012 | Review |
| Cass 2013 | Duplicate |
| Castellano 2014a | Wrong study design |
| Castellano 2014b | Wrong study design |
| Castellano 2015 | Wrong study design |
| Chae 2015 | Wrong comparator |
| ChineseExpert 2013 | Wrong study design |
| Chrysant 2014 | Wrong study design |
| Crunkhorn 2012 | Wrong intervention |
| Dabhadkar 2013 | Wrong study design |
| deCates 2014 | Meta‐analysis |
| Delgado Montero 2012 | Wrong study design |
| Dimitrov 2012 | Wrong intervention |
| Dresser 2012 | Wrong intervention |
| Dresser 2013 | Wrong comparator |
| Elley 2012 | Meta‐analysis |
| Fedacko 2013 | noncomparative design |
| Feldman 2012 | Wrong study design |
| Feldman 2014 | Wrong study design |
| Feng 2012 | Wrong intervention |
| Galindo Ocana 2012 | Wrong study design |
| Gaziano 2013 | Wrong study design |
| Holzgreve 2014 | Review |
| Huang 2016 | Wrong study design |
| Huffman 2012 | Wrong study design |
| Huffman 2014 | Wrong study design |
| Ito 2012 | Wrong study design |
| Ivanovic 2013 | Wrong study design |
| Jadhav 2014 | Wrong intervention |
| Jang 2015 | Wrong intervention |
| Jaques 2011 | Wrong study design |
| Kawashiri 2015 | Wrong intervention |
| Kereiakes 2012 | Wrong intervention |
| Khaled 2015 | Wrong study design |
| Laba 2014a | Wrong intervention |
| Laba 2014b | Abstract |
| Lafeber 2011 | Wrong study design |
| Lafeber 2012 | Wrong study design |
| Lafeber 2013a | Wrong study design |
| Lafeber 2013b | Wrong study design |
| Lafeber 2014a | Wrong study design |
| Lafeber 2014b | Abstract |
| Lafeber 2014c | Wrong outcomes |
| Lafeber 2014d | Wrong comparator |
| Lafeber 2015 | Wrong comparator |
| Lafeber 2016 | Wrong study design |
| Law 2006 | Wrong study design |
| Liu 2014 | Abstract |
| Liu 2015 | Duplicate |
| Marazzi 2016 | Wrong intervention |
| Mishchenko 2014 | Abstract |
| Mossello 2015 | Wrong study design |
| Neutel 2009 | Duplicate |
| Nguyen 2013 | Wrong study design |
| OliverasVila 2014 | Wrong study design |
| Reiner 2013 | Review |
| Selak 2013 | Wrong comparator |
| Selak 2016 | Meta‐analysis |
| Sepanlou 2012 | Wrong intervention |
| Sigamani 2012 | Wrong comparator |
| Simonyi 2016 | Wrong study design |
| Son 2013 | Wrong comparator |
| Tanaka 2014 | Noncomparative design |
| Truelove 2014 | Abstract |
| Wald 2016 | Wrong study design |
| Wang 2012 | Abstract |
| Webster 2013 | Protocol |
| Webster 2014 | Wrong study design |
| Webster 2015a | Wrong study design |
| Webster 2015b | Meta‐analysis |
| Webster 2016a | Meta‐analysis |
| Webster 2016b | Wrong study design |
| Wei 2013 | Protocol |
| Wijns 2014 | Wrong study design |
| Wiley 2014 | Wrong study design |
| Xing 2013 | Meta‐analysis |
| Zeng 2016 | Wrong study design |
| Zomer 2013 | Wrong study design |
Characteristics of studies awaiting assessment [ordered by study ID]
Fommei 2015.
| Methods | Randomised cross‐over trial |
| Participants | Well‐controlled non‐complicated hypertensive outpatients under multiple therapy with at least one hypertensive drug and/or a statin and/or aspirin |
| Interventions | Single once‐a‐day administration (mono‐administration) with at least one hypertensive drug and/or a statin and/or aspirin Comparator: usual care (multiple administration with at least one hypertensive drug and/or a statin and/or aspirin |
| Outcomes | Adherence to treatment, adverse events, ambulatory blood pressure monitoring and lipid profile |
| Notes |
NCT00530946.
| Methods | Randomised open‐label, parallel trial |
| Participants | The outpatient with concurrent hypertension and hyper‐LDL‐cholesterolemia is a male or female >= 20 to < 80 years of age at Visit 1.The SBP at Visit 4 (Week ‐1) and Visit 5 (Week 0) is continuously SBP >= 140 mmHg and < 180 mmHg, LDL‐C >= 140 mg/dL and < 250 mg/dL at Visit 3 (Week ‐2) and 4 (Week ‐1) |
| Interventions | Drug: amlodipine 2.5 mg/atorvastatin 5 mg (single pill combination, dosed once daily for 8 weeks) Drug: amlodipine 2.5mg/atorvastatin 10mg (single pill combination, dosed once daily for 8 weeks) Drug: amlodipine 5 mg/atorvastatin 5 mg (single pill combination, dosed once daily for 8 weeks) Drug: amlodipine 5 mg/atorvastatin 10 mg (single pill combination, dosed once daily for 8 weeks) Comparator: active comparator: CI‐1038 2.5 mg/5 mg (intervention: drug: amlodipine 2.5 mg/atorvastatin 5 mg) active comparator: CI‐1038 2.5 mg/10 mg (intervention: drug: amlodipine 2.5 mg/atorvastatin 10 mg) active comparator: CI‐1038 5 mg/5 mg (intervention: drug: amlodipine 5 mg/atorvastatin 5 mg) active comparator: CI‐1038 5 mg/10 mg (intervention: drug: amlodipine 5 mg/atorvastatin 10 mg) |
| Outcomes |
Primary outcomes:
Secondary outcomes:
|
| Notes |
NCT01004705.
| Methods | Randomised open‐label cross‐over trial |
| Participants | Male or female participants ≥18 years of age Previously untreated LDL cholesterol ≥ 100 mg/dL and ≤ 180 mg/dL |
| Interventions | Once‐daily oral dose of combination of acetylsalicylic acid, simvastatin, and ramipril (containing 100 mg acetylsalicylic acid, 40 mg simvastatin, and 5 or 10 mg ramipril) Comparator: once‐daily oral dose of Simvastatin 40 mg |
| Outcomes |
Primary outcomes
Secondary outcomes
|
| Notes |
NCT01005290.
| Methods | Randomised open‐label, cross‐over trial |
| Participants | Participants will be ≥ 18 years old. Previously untreated systolic pressure result of ≥ 120 < 160 mmHg and diastolic pressure result of ≥ 80 < 100 mmHg |
| Interventions | A once‐daily oral dose of the cardiovascular fixed‐dose combination pill (containing 100 mg acetylsalicylic acid, 40 mg simvastatin, and 5 mg ramipril) for 1 week followed by a once‐daily oral dose of the cardiovascular fixed‐dose combination pill (containing 100 mg acetylsalicylic acid, 40 mg simvastatin, and 10 mg ramipril) for 4 weeks Comparator: a once‐daily oral dose of 5 mg ramipril for 1 week followed by a once‐daily oral dose of 10 mg ramipril for 4 weeks |
| Outcomes |
Primary outcomes
Secondary outcomes
|
| Notes |
NCT01362218.
| Methods | Randomised open‐label, parallel assignment |
| Participants | Male or female participants aged ≥ 18 and < 75 years Previously untreated or not treated with fibrates during the last 6 weeks or with any other lipid‐lowering drug for the last 4 weeks LDL‐cholesterol ≥ 130 and ≤ 220 mg/dL Systolic blood pressure ≥ 120 and < 160 mmHg and diastolic blood pressure ≥ 70 and < 100 mmHg |
| Interventions | Drug: cardiovascular fixed‐dose combination pill (acetylsalicylic acid, simvastatin and ramipril) Comparator: simvastatin given together with the reference drugs ramipril and acetylsalicylic acid |
| Outcomes |
Primary outcomes
Secondary outcomes
|
| Notes |
NCT01406431.
| Methods | Randomised, open‐label, cross‐over assignment |
| Participants | Healthy male volunteers Age 20‐55 years at the time of screening BMI 19‐26 kg/m2 at the time of screening |
| Interventions | Pitavastatin 4 mg (2 tablets), valsartan 160 mg (1 tablet). Other name: Livalo, Diovan Comparator drug: pitavastatin, valsartan |
| Outcomes |
Primary Outcomes: ‐Cmax of study drugs after single oral administration ‐AUClast of study drugs after single oral administration Secondary Outcomes ‐AUCinf, Tmax and t1/2β of study drugs after single oral administration |
| Notes |
NCT01764178.
| Methods | Randomised, open‐label, cross‐over trial |
| Participants | Healthy male volunteers Age 20‐55 years at the time of screening BMI 19‐26 kg/m2 at the time of screening |
| Interventions | Livalo fixed combination drug (pitavastatin + valsartan) Comparator: pitavastatin, valsartan |
| Outcomes |
Primary outcomes:
Secondary outcomes:
|
| Notes |
NCT02075619.
| Methods | Open‐label, single‐centre, randomised, single‐dose, three‐way cross‐over, six‐sequence study |
| Participants |
|
| Interventions |
Experimental: Sequence 1 Four participants (2 Chinese and 2 white) will receive 1 amlodipine 10 mg tablet and 1 rosuvastatin 20 mg tablet in Period 1; 1 GSK3074477 fixed‐dose combination (FDC) formulation‐1 tablet in Period 2 and 1 GSK3074477 FDC formulation‐2 tablet in Period 3; all treatments will be administered orally in fasted state. The 3 treatment periods will be separated by a washout period of between 12‐17 days Experimental: Sequence 2 Four participants (2 Chinese and 2 white) will receive 1 amlodipine 10 mg tablet and 1 rosuvastatin 20 mg tablet in Period 1; 1 GSK3074477 FDC formulation‐2 tablet in Period 2 and 1 GSK3074477 FDC formulation‐1 tablet in Period 3; all treatments will be administered orally in fasted state. The 3 treatment periods will be separated by a washout period of between 12‐17 days Experimental: Sequence 3 Four participants (2 Chinese and 2 white) will receive 1 GSK3074477 FDC formulation‐1 tablet in Period 1, 1 amlodipine 10 mg tablet and 1 rosuvastatin 20 mg tablet in Period 2; and 1 GSK3074477 FDC formulation‐2 tablet in Period 3; all treatments will be administered orally in fasted state. The 3 treatment periods will be separated by a washout period of between 12‐17 days Experimental: Sequence 4 Four participants (2 Chinese and 2 white) will receive 1 GSK3074477 FDC formulation‐1 tablet in Period 1; 1 GSK3074477 FDC formulation‐2 tablet in Period 2; and 1 amlodipine 10 mg tablet and 1 rosuvastatin 20 mg tablet in Period 3; all treatments will be administered orally in fasted state. The 3 treatment periods will be separated by a washout period of between 12‐17 days Experimental: Sequence 5 Four participants (2 Chinese and 2 white) will receive 1 GSK3074477 FDC formulation‐2 tablet in Period 1; 1 amlodipine 10 mg tablet and 1 rosuvastatin 20 mg tablet in Period 2; and 1 GSK3074477 FDC formulation‐1 tablet in Period 3; all treatments will be administered orally in fasted state. The 3 treatment periods will be separated by a washout period of between 12‐17 days Experimental: Sequence 6 Four participants (2 Chinese and 2 white) will receive 1 GSK3074477 FDC formulation‐2 tablet in Period 1; 1 GSK3074477 FDC formulation‐1 tablet in Period 2; and 1 amlodipine 10 mg tablet and 1 rosuvastatin 20 mg tablet in Period 3; all treatments will be administered orally in fasted state. The 3 treatment periods will be separated by a washout period of between 12‐17 days |
| Outcomes |
Primary outcomes:
Secondary outcomes:
|
| Notes |
NCT02569814.
| Methods | Randomised, open‐label, cross‐over assignment trial |
| Participants | Healthy men, aged 19‐50 years |
| Interventions | Group1 Fimasartan/amlodipine combination tablet and rosuvastatin individual tablets at 1st day as period I. And then, after wash out for 2 weeks, as period II, Group 1 participants take a fimasartan/amlodipine/rosuvastatin combination tablet at 15th day Group 2 A fimasartan/amlodipine/rosuvastatin combination tablet at 1st day as period I. And then, after wash out for 2 weeks, as period II, Group 2 participants take fimasartan/amlodipine combination tablet and rosuvastatin individual tablets at 15th day |
| Outcomes |
Primary outcome ‐Cmax of fimasartan, amlodipine and rosuvastatin Secondary outcome ‐AUCt (Area Under the Curve) of fimasartan, amlodipine and rosuvastatin |
| Notes |
NCT02662894.
| Methods | Randomised parallel‐assignment, open‐label trial |
| Participants | Participants of both sexes aged 18‐65 years Participants diagnosed with uncontrolled hypertension Participants with intermediate and high risk dyslipidaemia, according to the V Brazilian Guidelines on Dyslipidemia and Prevention of Atherosclerosis Ability to understand and consent to participate in this clinical study, manifested by signing the Informed Consent |
| Interventions | Valsartan + rosuvastatin FDC Fixed‐dose combination of valsartan (160 mg or 320 mg) + rosuvastatin (20 mg), once daily for 4 weeks Comparator: separate tablets of valsartan (160 mg or 320 mg) + rosuvastatin (20 mg), once daily for 4 weeks |
| Outcomes |
Primary outcomes:
Secondary outcomes:
|
| Notes |
NCT02791958.
| Methods | Randomised, open‐label, parallel‐assignment trial |
| Participants | Men or women aged ≥ 18 and < 75 years People with Stage 1 (SBP/DBP: 140‐159/90‐99 mmHg) or Stage 2 (SBP/DBP: ≥ 160/≥ 100 mmHg) hypertension, either untreated or after a wash out period LDL cholesterol level of ≥ 100 mg/dL and, either untreated or after the wash out period Untreated with BP‐lowering and/or lipid‐lowering medication Treated with BP‐lowering and/or lipid‐lowering medication can be included if the medication can be safely withdrawn as per physician's judgment |
| Interventions | A once‐daily oral dose of the Cardiovascular Fixed Dose Combination Pill AAR (acetylsalicylic acid 100 mg, atorvastatin 40 mg and ramipril 10 mg) for 4 weeks Comparator
|
| Outcomes |
Primary outcomes
Secondary outcome measures
|
| Notes |
NCT02842359.
| Methods | Randomised, open‐label, parallel‐assignment trial |
| Participants | Aged ≥ 19 years to < 75 years No medication history of hyperlipidaemia and hypertension within 3 months following registration, among people with type 2 diabetes diagnosed with hyperlipidaemia and stage I hypertension (systolic blood pressure: ≥ 140 mmHg, ≤ 159 mmHg or diastolic blood pressure: ≥ 90 mmHg, ≤ 99 mmHg), with adequately controlled haemoglobin levels Diagnosis of diabetes: haemoglobin A1c ≥ 6.5% or; fasting plasma glucose level above 8 hour ≥ 126 mg/dL or plasma glucose ≥ 200 mg/dL ( 11.1 mmol/l) 2 h after a 75 g glucose load or symptoms (such as polyuria, polydipsia, unexplained weight loss) and a random plasma glucose ≥ 200 mg/dL (11.1 mmol/L) |
| Interventions | Irbesartan/atorvastatin fixed‐dose combination: pharmaceutical form: tablet; route of administration: oral; other name: Rovelito Comparators Irbesartan SR47436: pharmaceutical form: tablet; route of administration: oral; other name: Aprovel Atorvastatin: pharmaceutical form: tablet; route of administration: oral; other name: Newvast |
| Outcomes |
Primary outcomes: (time frame: 4 weeks‐maximum 5 weeks)
Secondary outcomes: (Time frame: 4 weeks up to maximum 5 weeks)
|
| Notes |
Characteristics of ongoing studies [ordered by study ID]
INTEGRATE.
| Trial name or title | INTEGRATE Study: A pragmatic cluster randomised controlled trial of an integrated general practice and pharmacy‐based intervention to promote the prescription and use of appropriate preventive medications among individuals at high cardiovascular risk |
| Methods | Cluster‐randomized control, open‐label, parallel‐assignment |
| Participants | All adult patients (18 years) attending the GP will be potentially be eligible to receive the HealthTracker intervention. All adult patients who are recommended for the component medications according to current guidelines are eligible to be prescribed the polypill therapy. All adult patients attending the paired pharmacy with a new prescription for a CVD prevention medication will be eligible to receive the pharmacy intervention |
| Interventions | The integrated intervention comprises the following three elements: (1) HealthTracker, (2) availability of the Polypills and (3) Pharmacy Adherence Support Service (PASS) ** Eight CVD polypills will be available and they are:
|
| Outcomes |
Primary Outcomes
Secondary Outcomes
|
| Starting date | 1 March 2016 |
| Contact information | Prof Anushka Patel, apatel@georgeinstitute.org |
| Notes |
NCT01646437.
| Trial name or title | The International Polycap Study‐3 |
| Methods | 2 x 2 x 2 randomised controlled trial, factorial design (3 arms: Polycap, aspirin, vitamin D) |
| Participants | 5000 participants (women 60 years or older and men 55 years or older) without known heart disease or prior stroke and without a clear indication or contraindication to any of the study medications and INTERHEART risk score of 10 or greater |
| Interventions | Polycap vs. placebo; embedded in trial comparing enteric coated aspirin vs. placebo and vitamin D vs. placebo |
| Outcomes | Composite of major CVD (CV death, non‐fatal stroke, non‐fatal MI), plus heart failure, resuscitated cardiac arrest, or revascularisation with evidence of ischaemia in participants taking Polycap versus placebo |
| Starting date | June 2012; protocol updated on clinicaltrials.gov on May 2015 (ClinicalTrials.gov Identifier: NCT01646437) |
| Contact information | Dr. Salim Yusuf, Population Health Research Institute |
| Notes |
NCT01826019.
| Trial name or title | Heart Outcomes Prevention and Evaluation 4 (HOPE‐4) |
| Methods | Open‐label, parallel, cluster‐randomised controlled trial design |
| Participants | HT Phase: at least 50 urban and rural communities in Canada, Colombia and Malaysia will be randomised to participate in an intensive CV risk detection and control program by NPHW or to care as usual for 12 months CVD Phase: continuation and expansion of HT Phase to include at least 190 urban and rural communities in countries within Asia, South America, Sub‐Saharan Africa, and Canada that will be allocated to participate in an intensive CV risk detection and control programme supported by NPHWs or to care as usual for up to 6 years Inclusion criteria Individuals (≥ 50 years) with at least ONE of the following criteria:
|
| Interventions | Intensive CV risk detection, counselling and follow‐up programme by NPHW; recommended CV medications will include combinations of anti‐hypertensive medications (both low and high doses) and a lipid‐lowering agent (e.g. statin) in accordance with treatment algorithm (precise formulations used may differ in each country); use of treatment supporters to reinforce adherence. Comparator: usual care. Participants in control communities will be referred to usual care |
| Outcomes |
Primary outcomes
Secondary outcomes
|
| Starting date | August 2014 |
| Contact information | Contact: Patricio Lopez‐Jaramillo, MD jplopezj@gmail.com |
| Notes |
NCT02278471.
| Trial name or title | The SCCS Polypill Pilot Trial |
| Methods | Randomised, parallel‐assignment, open‐label trial |
| Participants | Enrolled at the SCCS site in Mobile, Alabama, obtain care at Franklin Primary Health Center, or live in the surrounding area Aged 45‐75 years Baseline systolic blood pressure ≥ 120 mm Hg |
| Interventions | The study medication will be a fixed‐dose combination pill (polypill) containing: atorvastatin 10 mg, amlodipine 2.5 mg, losartan 25 mg, and hydrochlorothiazide 12.5 mg. Once daily medication. Comparator: usual care: they will remain on the same care that they are used to receiving |
| Outcomes |
Primary outcomes
Secondary outcome measures
|
| Starting date | December 2015 |
| Contact information | Judy P. Mitchell 251‐436‐7631 judy.mitchell@franklinprimary.org |
| Notes |
NCT02596126.
| Trial name or title | Secondary Prevention of Cardiovascular Disease in the Elderly Trial (SECURE) |
| Methods | Randomised, open‐label, parallel‐assignment |
| Participants | A total number of 3206 participants will be randomized (1:1) to treatment arms. Participants will be recruited across seven countries in Europe (Spain, Italy, Germany, France, Hungary, Poland, and Czech Republic)
Inclusion criteria
Documented diabetes mellitus or previous treatment with oral hypoglycemic drugs or insulin. Mild to moderate renal dysfunction: creatinine clearance 60‐30 mL/min/1.73 m2. Prior myocardial infarction: defined as an AMI occurring before the index event documented in a medical report. Prior coronary revascularization: coronary artery bypass grafting (CABG) or percutaneous coronary intervention (PCI). Prior stroke: history of a documented stroke, defined as an acute episode of focal cerebral, spinal, or retinal dysfunction caused by infarction of central nervous system tissue, not resulting in death. Age ≥ 75 years. |
| Interventions | (A) aspirin 100 mg, atorvastatin 40 mg, and ramipril (2.5 mg, or 5 mg, or 10mg) or (B) aspirin 100 mg, atorvastatin 20 mg, and ramipril (2.5 mg, or 5 mg, or 10mg) Other name: Polypill Comparator: participants allocated to the usual care arm will receive standard of care therapies for secondary prevention according to the ESC guidelines. Drugs and doses will be left at the discretion of the treating physicians |
| Outcomes |
Primary outcome measures
Secondary Outcomes
|
| Starting date | January 2016 |
| Contact information | Jose Maria Castellano Vazquez, MD, PhD, josemaria.castellano@cnic.es |
| Notes |
PolyIran.
| Trial name or title | PolyIran |
| Methods | Zelen design, randomised controlled trial nested within the Golestan cohort study (110:90 ratio) |
| Participants | 7000 (2400 in related PolyIran Liver trial) cohort participants over 50 years in Iran followed for 5 years |
| Interventions | Fixed‐combination therapy (aspirin 80 mg, hydrochlorthiazide 12.5 mg, valsartan 40 mg, and atorvastatin 20 mg (PolyPill 4–2, Alborz‐Darou, Ghazvin, Iran),) + usual care versus usual care alone |
| Outcomes |
Primary outcome
Measured at 2.5 years and 5 years |
| Starting date | October 2011 |
| Contact information | Reza Malekzadeh MD, Digestive Disease Research Institute, Tehran University of Medical Sciences, Shariati Hospital, 1411713135, Tehran, Iran. Tel: +98 (21) 8241‐5000, Fax: +98 (21) 8241‐5400, E‐mail: malek@tums.ac.ir Tom Marshall MD, School of Health and Population Sciences, University of Birmingham, Edgbaston, Birmingham B15 2TT, UK. Tel: 44 (0)121 414 7832, Fax: 44 (0)121 414 7878, E‐mail: T.P.Marshall@bham.ac.uk. |
| Notes |
PolyIran protocol: Eur J Prev Cardiol. 2015; 22(12) 1609–1617. PolyIran Liver protocol: Arch Iran Med. 2015; 18(8): 515 – 523. Registriations: NCT00603590, NCT01245608, NCT01271985 |
Differences between protocol and review
The background section has been shortened. Previous inclusion of HDL cholesterol and triglycerides as outcomes were excluded, and subgroup analysis evaluating the comparator group as usual care versus placebo or inactive control added.
Differences between 2014 review and 2017 update
In the 2017 update, cardiovascular disease has been changed to atherosclerotic cardiovascular disease for greater clarity in the target disease of combinations with at least one blood pressure‐lowering drug and one lipid‐lowering drug. We also moved discontinuation rates from the primary outcome section, where it was reported under adverse events, to an individual secondary outcome. The rationale for this change was two‐fold: 1) investigator‐defined adverse event rates did not necessarily include discontinuation rates, and 2) discontinuation rates could not be reported when the comparator group was usual care. We included trials with active single drug comparators but not trials comparing different fixed‐dose combinations. We have also removed the dose subgroup analysis because most fixed‐dose combinations included moderate doses of either blood pressure‐lowering drugs, lipid‐lowering drugs, or both.
Contributions of authors
All authors contributed to the development or update of the protocol. For this update, Ehete Bahiru screened titles and abstracts, assessed studies for inclusion and exclusion, extracted data, and edited the update. Angharad de Cates screened titles and abstracts, assessed studies for inclusion and exclusion, extracted data, contacted authors, and drafted the original review. Matthew Farr and Morag Jarvis screened titles and abstracts, assessed studies for inclusion and exclusion, and extracted data for the original review. Mohan Palla screened titles and abstracts and assessed studies for inclusion and exclusion for the update. Karen Rees supervised the title screening and data extraction for the initial review and contributed to writing the original review and to editing of the update. Shah Ebrahim assisted in analyses and interpretation and contributed to writing of the review. Mark Huffman contacted study authors, screened titles and abstracts, assessed studies for inclusion and exclusion, extracted data, performed the analyses, and drafted the review and update.
Sources of support
Internal sources
Warwick Medical School, University of Warwick, UK.
Department of Non‐Communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, UK.
External sources
NIHR Cochrane Programme Grant, UK.
Karen Rees is also funded by the National Institute for Health Research (NIHR) Collaboration for Leadership in Applied Health Research and Care West Midlands at University Hospitals Birmingham NHS Foundation Trust, UK.
Declarations of interest
Mark Huffman has received grant support from Cochrane to support the production of this update. Dr. Huffman also receives grant support from World Heart Federation to serve as senior program advisor for its Emerging Leaders program, which is supported by Boehringer Ingelheim and Novartis and has been supported by AstraZeneca and Bupa. Dr. Huffman has also received travel support from the World Heart Federation for its polypill satellite meeting at the World Congress of Cardiology and Cardiovascular Health in 2016.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
CRUCIAL 2011 {published data only}
- Cho EJ, Kim JH, Sutradhar S, Yunis C, Westergaard M. Proactive multifactorial intervention strategy reduces the risk of cardiovascular disease estimated with region‐specific risk assessment models in Pacific Asian patients participating in the CRUCIAL trial. Journal of Korean Medical Science 2013;28:1741‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho EJ, Kim JH, Sutradhar S, Yunis C, Westergaard M. Reduction in cardiovascular risk using a proactive multifactorial intervention is consistent among patients residing in Pacific Asian and non‐Pacific Asian regions: a CRUCIAL trial subanalysis. Vascular Health and Risk Management 2014;10:145‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hradec J, Zamorano J, Sutradhar S. Post hoc analysis of the Cluster Randomized Usual Care versus Caduet Investigation Assessing Long‐term risk (CRUCIAL) trial. Current Medical Research and Opinion 2013;29:589‐96. [DOI] [PubMed] [Google Scholar]
- Kim JH, Zamorano J, Erdine S, Pavia A, Al‐Khadra A, Sutradhar S, Yunis C. Reduction in cardiovascular risk using proactive multifactorial intervention versus usual care in younger (< 65 years) and older (≥ 65 years) patients in the CRUCIAL trial. Current Medical Research and Opinion 2013;29:453‐63. [DOI] [PubMed] [Google Scholar]
- Pavia A, Zamorano J, Sutradhar S, Yunis C. Changes in calculated coronary heart disease risk using proactive multifactorial intervention versus continued usual care in Latin‐American and non‐Latin‐American patients enrolled in the CRUCIAL trial. Current Medical Research and Opinion 2012;28:1667‐76. [DOI] [PubMed] [Google Scholar]
- Pavia LA, Erdine S, Zamorano J, Kim JH, Al KA, Westergaard M, et al. Cardiovascular risk factor management: single‐pill amlodipine/atorvastatin versus usual care in patients with hypertension and additional risk factors ‐ the CRUCIAL trial. Journal of Hypertension 2010;Conference:e278‐9. [Google Scholar]
- Pavia LA, Zamorano J, Kim JH, Erdine S, Al KA, Westergaard M, et al. Treatment strategies for cardiovascular risk factor management in patients with hypertension and additional risk factors‐experiences from the usual care arm of the CRUCIAL trial. Journal of Hypertension 2010;Conference:e276‐7. [Google Scholar]
- Zamorano J, Erdine S, Pavia A, Kim JH, Al‐Khadra A, Westergaard M, et al. Proactive multiple cardiovascular risk factor management compared with usual care in patients with hypertension and additional risk factors: The CRUCIAL trial. Current Medical Research and Opinion 2011;27(4):821‐33. [DOI] [PubMed] [Google Scholar]
CUSP 2009 {published data only}
- Neutel JM, Bestermann WH, Dyess EM, Graff A, Kursun A, Sutradhar S, et al. The use of a single‐pill calcium channel blocker/statin combination in the management of hypertension and dyslipidemia: a randomized, placebo‐controlled, multicenter study. Journal of Clinical Hypertension 2009;11(1):22‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
FOCUS 2014 {published data only}
- Castellano JM, Sanz G, Peñalvo JL, Bansilal S, Fernández‐Ortiz A, Alvarez L, et al. A polypill strategy to improve adherence: results from the FOCUS project. Journal of the American College of Cardiology 2014;64:2071‐82. [DOI: 10.1016/j.jacc.2014.08.021] [DOI] [PubMed] [Google Scholar]
- Sanz G, Fuster V, Guzmán L, Guglietta A, Arnáiz JA, Martínez F, et al. The fixed‐dose combination drug for secondary cardiovascular prevention project: improving equitable access and adherence to secondary cardiovascular prevention with a fixed‐dose combination drug. Study design and objectives. American Heart Journal 2011;165:811‐7. [DOI] [PubMed] [Google Scholar]
IMPACT 2014 {published data only}
- Selak V, Elley C, Crengle S, Wadham A, Rafter N, Bullen C. Polypills for high risk patients: results of a New Zealand randomised controlled trial. Heart Lung and Circulation. 2014; Vol. 23:e13.
- Selak V, Elley CR, Bullen C, Crengle S, Wadham A, Rafter N, et al. Effect of fixed dose combination treatment on adherence and risk factor control among patients at high risk of cardiovascular disease: randomised controlled trial in primary care. BMJ 2014;348:g3318. [DOI] [PubMed] [Google Scholar]
- Selak V, Elley CR, Crengle S, Harwood M, Doughty R, Arroll B, et al. IMProving Adherence using Combination Therapy (IMPACT): design and protocol of a randomised controlled trial in primary care. Contemporary Clinical Trials 2011;32:909‐15. [DOI] [PubMed] [Google Scholar]
- Selak V, Harwood M, Raina EC, Bullen C, Wadham A, Parag V, et al. Polypill‐based therapy likely to reduce ethnic inequities in use of cardiovascular preventive medications: findings from a pragmatic randomised controlled trial. European Journal of Preventive Cardiology 2016;23:1537‐45. [DOI] [PubMed] [Google Scholar]
Kanyini GAP 2014 {published data only}
- Laba TL, Hayes A, Lo S, Peiris DP, Usherwood T, Hillis GS, et al. An economic case for a cardiovascular polypill? A cost analysis of the Kanyini GAP trial. Medical Journal of Australia 2014;201:671‐3. [DOI] [PubMed] [Google Scholar]
- Laba TL, Howard K, Rose J, Peiris D, Redfern J, Usherwood T, et al. Patient preferences for a polypill for the prevention of cardiovascular diseases. Annals of Pharmacotherapy 2015;49:528‐39. [DOI] [PubMed] [Google Scholar]
- Liu H, Laba TL, Massi L, Jan S, Usherwood T, Patel A, et al. Facilitators and barriers to implementation of a pragmatic clinical trial in Aboriginal health services. Medical Journal of Australia 2015;203:24‐7. [DOI] [PubMed] [Google Scholar]
- Liu H, Massi L, Laba TL, Peiris D, Usherwood T, Patel A, et al. Patients' and providers' perspectives of a polypill strategy to improve cardiovascular prevention in Australian primary health care: a qualitative study set within a pragmatic randomized, controlled trial. Circulation: Cardiovascular Quality and Outcomes 2015;8:301‐8. [DOI] [PubMed] [Google Scholar]
- Liu H, Patel A, Brown A, Eades S, Hayman N, Jan S, et al. Rationale and design of the Kanyini guidelines adherence with the polypill (Kanyini‐GAP) study: a randomised controlled trial of a polypill‐based strategy amongst indigenous and non indigenous people at high cardiovascular risk. BMC Public Health 2010;10:458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel A, Cass A, Peiris D, Usherwood T, Brown A, Jan S, et al. A pragmatic randomized trial of a polypill‐based strategy to improve use of indicated preventive treatments in people at high cardiovascular disease risk. European Journal of Preventive Cardiology 2015;22:920‐30. [DOI] [PubMed] [Google Scholar]
- Truelove M, Patel A, Bompoint S, Brown A, Cass A, Hillis GS, et al. The effect of a cardiovascular polypill strategy on pill burden. Cardiovascular Therapy 2015;33:347‐52. [DOI] [PubMed] [Google Scholar]
Malekzadeh 2010 {published data only}
- Malekzadeh F, Marshall T, Pourshams A, Gharravi M, Aslani A, Nateghi A, et al. A pilot double‐blind randomised placebo‐controlled trial of the effects of fixed‐dose combination therapy ('polypill') on cardiovascular risk factors. International Journal of Clinical Practice 2010;64(9):1220‐7. [DOI] [PubMed] [Google Scholar]
OLSTA 2016 {published data only}
- Park JS, Shin JH, Hong TJ, Seo HS, Shim WJ, Baek SH, et al. Efficacy and safety of fixed‐dose combination therapy with olmesartan medoxomil and rosuvastatin in Korean patients with mild to moderate hypertension and dyslipidemia: an 8‐week, multicenter, randomized, double‐blind, factorial‐design study (OLSTA‐D RCT: OLmesartan rosuvaSTAtin from Daewoong). Drug Design, Development and Therapy 2016;10:2599‐609. [DOI] [PMC free article] [PubMed] [Google Scholar]
PILL 2011 {published and unpublished data}
- Lafeber M, Webster R, Visseren FLj, Bots ML, Grobbee DE, Spiering W, et al. Estimated cardiovascular relative risk reduction from fixed‐dose combination pill (polypill) treatment in a wide range of patients with a moderate risk of cardiovascular disease. European Journal of Preventive Cardiology 2016;23:1289‐97. [DOI] [PubMed] [Google Scholar]
- PILL CG. An international randomised placebo‐controlled trial of a four‐component combination pill ("polypill") in people with raised cardiovascular risk. PLoS One 2011;6(5):e19857. [DOI] [PMC free article] [PubMed] [Google Scholar]
Soliman 2009 {published data only}
- Soliman EZ, Mendis S, Dissanayake WP, Somasundaram NP, Gunaratne PS, Jayasingne IK, et al. A Polypill for primary prevention of cardiovascular disease: a feasibility study of the World Health Organization. Trials 2011;12:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soliman EZ, Mendis S, Dissanayake WP, Somasundaram NP, Gunaratne PS, Jayasingne IK, et al. A Polypill for primary prevention of cardiovascular disease: a feasibility study of the World Health Organization. Trials 2011;5:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
TIPS 2009 {published data only}
- Indian Polycap Study, Yusuf S, Pais P, Afzal R, Xavier D, Teo K, et al. Effects of a polypill (Polycap) on risk factors in middle‐aged individuals without cardiovascular disease (TIPS): a phase II, double‐blind, randomised trial. Lancet 2009;373(9672):1341‐51. [DOI] [PubMed] [Google Scholar]
TOGETHER 2010 {published data only}
- Grimm R, Malik M, Yunis C, Sutradhar S, Kursun A, TOGETHER Investigators. Simultaneous treatment to attain blood pressure and lipid goals and reduced CV risk burden using amlodipine/atorvastatin single‐pill therapy in treated hypertensive participants in a randomized controlled trial. Vascular Health & Risk Management 2010;6:261‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm RH, Malik M, Yunis C, Sutradhar S, Kursun A. The critical importance of treating absolute cardiovascular risk as opposed to individual cardiovascular risk factors in isolation. Journal of Clinical Hypertension 2009;Conference:A120‐1. [Google Scholar]
UMPIRE 2013 {published data only}
- Salam A, Stewart F, Singh K, Thom S, Williams HJ, Patel A, et al. INterpreting the Processes of the UMPIRE Trial (INPUT): protocol for a qualitative process evaluation study of a fixed‐dose combination (FDC) strategy to improve adherence to cardiovascular medications. BMJ Open 2013;3:e002313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thom S, Field J, Poulter N, Patel A, Prabhakaran D, Stanton A, et al. Use of a Multidrug Pill In Reducing cardiovascular Events (UMPIRE): rationale and design of a randomised controlled trial of a cardiovascular preventive polypill‐based strategy in India and Europe. European Journal of Preventive Cardiology 2014;21(2):252‐61. [DOI: ] [DOI] [PubMed] [Google Scholar]
- Thom S, Poulter N, Field J, Patel A, Prabhakaran D, Stanton A, et al. Effects of a fixed‐dose combination strategy on adherence and risk factors in patients with or at high risk of CVD: the UMPIRE randomized clinical trial. JAMA 2013;310:918‐29. [DOI: 10.1001/jama.2013.277064] [DOI] [PubMed] [Google Scholar]
- Wood F, Salam A, Singh K, Day S, Jan S, Prabhakaran D, et al. Process evaluation of the impact and acceptability of a polypill for prevention of cardiovascular disease. BMJ Open 2015;5:e008018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wald 2012 {published data only}
- Wald D, Morris J, Wald N. Randomised polypill crossover trial in people aged 50 and over. PLoS One 2012;7(7):e41297. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Abdellatif 2012 {published data only}
- Abdellatif AA. Role of single‐pill combination therapy in optimizing blood pressure control in high‐risk hypertension patients and management of treatment‐related adverse events. Journal of Clinical Hypertension (Greenwich) 2012;14:718‐26. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Agabiti Rosei 2014 {published data only}
- Agabiti‐Rosei E, Manolis A, Zava D, Omboni S, Zodiac Study Group. Zofenopril plus hydrochlorothiazide and irbesartan plus hydrochlorothiazide in previously treated and uncontrolled diabetic and non‐diabetic essential hypertensive patients. Advances in Therapy 2014;31(2):217‐33. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Agarwal 2013 {published data only}
- Agarwal R, Weir MR. Blood pressure response with fixed‐dose combination therapy: comparing hydrochlorothiazide with amlodipine through individual‐level meta‐analysis. Journal of Hypertension 2013;31(8):1692‐701. [DOI: ] [DOI] [PubMed] [Google Scholar]
Anonymous 2010 {published data only}
- Anonymous. I take medications for blood pressure, cholesterol and other conditions. I have a two‐part question: are there any long‐term problems from taking a dozen or so pills a day, and has there been much progress in developing a "polypill" that combines several drugs into a single pill?. Heart Adviser 2010;13:8. [PubMed] [Google Scholar]
Anonymous 2011 {published data only}
- Anonymous. Doubts surround study results touting polypill's benefits. The experimental pill contains four heart medications. Heart Adviser 2011;14:4. [PubMed] [Google Scholar]
Anonymous 2012a {published data only}
- Anonymous. Treating many conditions‐‐with just one pill. The polypill could make your medicines much easier to take. Harvard Women's Health Watch 2012;20:5. [PubMed] [Google Scholar]
Anonymous 2012b {published data only}
- Anonymous. Hypertension: improved therapy adherence after switch to a fixed drug combinations. MMW Fortschritte der Medizin 2012;154:78‐9. [PubMed] [Google Scholar]
Anonymous 2013a {published data only}
- Anonymous. Coming soon: many drugs in one pill. Multidrug combinations may make medicines easier to swallow. Harvard Heart Letter 2013;23(7):5. [PubMed] [Google Scholar]
Anonymous 2013b {published data only}
- Anonymous. Combined heart protection for the hypertensive patient. MMW Fortschritte der Medizin 2013;155(1):66‐7. [PubMed] [Google Scholar]
Athyros 2013 {published data only}
- Athyros VG, Katsiki N, Karagiannis A. Cardiovascular risk reduction with combination of anti‐atherosclerotic medications in younger and older patients. Current Medical Research and Opinion 2013;29(7):791‐2. [DOI: ] [DOI] [PubMed] [Google Scholar]
Athyros 2014 {published data only}
- Athyros VG, Katsiki N, Karagiannis A, Mikhailidi DP. Combination of statin plus renin angiotensin system inhibition for the prevention or the treatment of atherosclerotic cardiovascular disease. Current Pharmaceutical Design 2014;20(40):6299‐305. [DOI] [PubMed] [Google Scholar]
Bashir 2011 {published data only}
- Bashir S, Sherwani MU, Shabbir I, Batool A. Efficacy of fix dose combination (atorvastatin and amlodipine) in treatment of uncontrolled hypertension and dyslipidemia. Journal of Ayub Medical College Abbottabad 2011;23:97‐100. [PubMed] [Google Scholar]
Becerra 2015 {published data only}
- Becerra V, Gracia A, Desai K, Abogunrin S, Brand S, Chapman R, et al. Cost‐effectiveness and public health benefit of secondary cardiovascular disease prevention from improved adherence using a polypill in the UK. BMJ Open 2015;5(5):e007111. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bittencourt 2013 {published data only}
- Bittencourt MS, Blaha M, Blankstein R, Vargas J, Budoff M, Agatston A, et al. Eligibility for polypill therapy, subclinical atherosclerosis, and cardiovascular events‐national implications for the appropriate use of preventive pharmacotherapy: multi‐ethnic study of atherosclerosis (MESA). Journal of the American College of Cardiology 2013;1:E813. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bittencourt 2014 {published data only}
- Bittencourt MS, Blaha MJ, Blankstein R, Budoff M, Vargas JD, Blumenthal RS, et al. Polypill therapy, subclinical atherosclerosis, and cardiovascular events ‐ implications for the use of preventive pharmacotherapy. Journal of the American College of Cardiology 2014;63(5):434‐43. [DOI: 10.1016/j.jacc.2013.08.1640] [DOI] [PMC free article] [PubMed] [Google Scholar]
Blank 2007 {published data only}
- Blank R, Hobbs FDR, Zamorano J, Girerd X. A single‐pill combination of amlodipine besylate and atorvastatin calcium (update). Drugs of Today 2007;43:157‐77. [DOI: ] [DOI] [PubMed] [Google Scholar]
Briasoulis 2013 {published data only}
- Briasoulis A, Bakris G. Initial single‐pill combination therapy for cardiovascular risk factor management: it is not just convenience. Journal of Hypertension 2013;31(8):1537‐8. [DOI: ] [DOI] [PubMed] [Google Scholar]
Bryant 2013 {published data only}
- Bryant L, Martini N, Chan J, Chang L, Marmoush A, Robinson B, et al. Could the polypill improve adherence? The patient perspective. Journal of Primary Health Care 2013;5(1):28‐35. [PubMed] [Google Scholar]
Carey 2012 {published data only}
- Carey KM, Comee MR, Donovan JL, Kanaan AO. A polypill for all? Critical review of the polypill literature for primary prevention of cardiovascular disease and stroke. Annals of Pharmacotherapy 2012;46:688‐95. [DOI: ] [DOI] [PubMed] [Google Scholar]
Cass 2013 {published data only}
- Cass A, Patel A, Rodgers A. A pragmatic trial of a polypill‐based strategy to improve adherence to indicated preventive treatments among people at high cardiovascular disease risk. Nephrology 2013;18:33. [DOI: ] [DOI] [PubMed] [Google Scholar]
Castellano 2014a {published data only}
- Castellano JM, Sanz G, Fuster V. Evolution of the polypill concept and ongoing clinical trials. Canadian Journal of Cardiology 2014;30(5):520‐6. [DOI: ] [DOI] [PubMed] [Google Scholar]
Castellano 2014b {published data only}
- Castellano JM, Sanz G, Ortiz AF, Garrido E, Bansilal S, Fuster V. A polypill strategy to improve global secondary cardiovascular prevention from concept to reality. Journal of the American College of Cardiology 2014;64(6):613‐21. [DOI: 10.1016/j.jacc.2014.06.009] [DOI] [PubMed] [Google Scholar]
Castellano 2015 {published data only}
- Castellano JM, Bueno H, Fuster V. The cardiovascular polypill: clinical data and ongoing studies. International Journal of Cardiology 2015;201:S8‐14. [DOI: ] [DOI] [PubMed] [Google Scholar]
Chae 2015 {published data only}
- Chae DW, Son M, Kim Y, Son H, Jang SB, Seo JM, et al. Pharmacokinetics of a telmisartan/rosuvastatin fixed‐dose combination: a single‐dose, randomized, open‐label, 2‐period crossover study in healthy Korean subjects. International Journal of Clinical Pharmacology and Therapeutics 2015;53(10):883‐9. [DOI: ] [DOI] [PubMed] [Google Scholar]
ChineseExpert 2013 {published data only}
- Chinese Expert, Consensus, Recommendation Group on the Development of Cardiovascular Disease Polypill in China. Consensus recommendation on the development of cardiovascular disease polypill in China. Chung Hua Hsin Hsueh Kuan Ping Tsa Chih 2013;41(2):91‐3. [PubMed] [Google Scholar]
Chrysant 2014 {published data only}
- Chrysant SG, Chrysant GS. Future of polypill use for the prevention of cardiovascular disease and strokes. The American Journal of Cardiology 2014;114(4):641‐5. [DOI: 10.1016/j.amjcard.2014.05.049] [DOI] [PubMed] [Google Scholar]
Crunkhorn 2012 {published data only}
- Crunkhorn S. Trial watch: dual‐acting combination meets heart failure end point. Nature Reviews Drug Discovery 2012;11:740. [DOI: ] [DOI] [PubMed] [Google Scholar]
Dabhadkar 2013 {published data only}
- Dabhadkar KC, Bellam N. Polypill strategy for primary prevention of cardiovascular disorders. Drugs Today 2013;49:317‐24. [DOI: 10.1358/dot.2013.49.5.1950148] [DOI] [PubMed] [Google Scholar]
deCates 2014 {published data only}
- Cates AN, Farr MRB, Wright N, Jarvis MC, Rees K, Ebrahim S, et al. Fixed‐dose combination therapy for the prevention of cardiovascular disease. Cochrane Database of Systematic Reviews 2014, Issue 4. [DOI: 10.1002/14651858.CD009868.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Delgado Montero 2012 {published data only}
- Delgado‐Montero A, Zamorano JL. Atorvastatin calcium plus amlodipine for the treatment of hypertension. Expert Opinion on Pharmacotherapy 2012;13(18):2673‐85. [DOI: 10.1517/14656566.2012.742064] [DOI] [PubMed] [Google Scholar]
Dimitrov 2012 {published data only}
- Dimitrov Y, Baguet JP, Hottelart C, Marboeuf P, Tartiere JM, Ducher M, et al. Is there a BP benefit of changing the time of aspirin administration in treated hypertensive patients?. European Journal of Preventive Cardiology 2012;19:706‐11. [DOI: ] [DOI] [PubMed] [Google Scholar]
Dresser 2012 {published data only}
- Dresser G, Nelson S, Mahon J, Zou G, Vandervoort M, Wong C, et al. Single pill combination therapy for treatment of hypertension in the combined stitch and STITCH2 study populations. Journal of Clinical Hypertension 2012; Vol. 14.
Dresser 2013 {published data only}
- Dresser GK, Nelson SA, Mahon JL, Zou G, Vandervoort MK, Wong CJ, et al. Simplified therapeutic intervention to control hypertension and hypercholesterolemia: a cluster randomized controlled trial (STITCH2). Journal of Hypertension 2013;31(8):1702‐13. [DOI: ] [DOI] [PubMed] [Google Scholar]
Elley 2012 {published data only}
- Elley CR, Gupta AK, Webster R, Selak V, Jun M, Patel A, et al. The efficacy and tolerability of 'polypills': meta‐analysis of randomised controlled trials. PLoS ONE 2012;7:e52145. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fedacko 2013 {published data only}
- Fedacko J, Pella D, Jarcuska P, Sabol F, Kmec J, Lopuchovsky T, et al. Slovak trial on cardiovascular risk reduction following national guidelines with CaDUET (the STRONG DUET study). Advances in Therapy 2013;30(1):60‐70. [DOI: ] [DOI] [PubMed] [Google Scholar]
Feldman 2012 {published data only}
- Feldman RD, Flack J, Howes L, Jenssen T, Reeves R, Shi H, et al. Impact of age and gender on blood pressure and low‐density lipoprotein cholesterol reduction: results of a pooled analysis. Current Medical Research and Opinion 2012;28:1421‐33. [DOI] [PubMed] [Google Scholar]
Feldman 2014 {published data only}
- Feldman RD, Nattel S. Increasing appreciation for the role of single‐pill combinations for the prevention of atherosclerotic disease: a pro‐polypill polemic. Canadian Journal of Cardiology 2014;30(5):517‐9. [DOI: ] [DOI] [PubMed] [Google Scholar]
Feng 2012 {published data only}
- Feng Y, Xu H, Chen K. Natural polypill Xuezhikang: its clinical benefit and potential multicomponent synergistic mechanisms of action in cardiovascular disease and other chronic conditions. Journal of Alternative and Complementary Medicine 2012;18:318‐28. [DOI: ] [DOI] [PubMed] [Google Scholar]
Galindo Ocana 2012 {published data only}
- Galindo‐Ocana J, Bernabeu‐Wittel M, Formiga F, Fuertes‐Martin A, Baron‐Franco B, Murcia‐Zaragoza JM, et al. Effects of renin‐angiotensin blockers/inhibitors and statins on mortality and functional impairment in polypathological patients. European Journal of Internal Medicine 2012;23:179‐84. [DOI: 10.1016/j.ejim.2011.06.004] [DOI] [PubMed] [Google Scholar]
Gaziano 2013 {published data only}
- Gaziano JM. Progress with the polypill?. JAMA 2013;310(9):910‐1. [DOI: ] [DOI] [PubMed] [Google Scholar]
Holzgreve 2014 {published data only}
- Holzgreve H. Health and longevity by one tablet daily. Fiction and hard facts on the polypill. MMW Fortschritte der Medizin 2014;156(1):55‐7. [PubMed] [Google Scholar]
Huang 2016 {published data only}
- Huang Z, Chen C, Li S, Kong F, Shan P, Huang W. Combined treatment with amlodipine and atorvastatin calcium reduces circulating levels of intercellular adhesion molecule‐1 and tumor necrosis factor‐alpha in hypertensive patients with prediabetes. Frontiers in Aging Neuroscience 2016;8:206. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Huffman 2012 {published data only}
- Huffman MD, Bhatnagar D. Novel treatments for cardiovascular disease prevention. Cardiovascular Therapeutics 2012;30(5):257‐63. [DOI: 10.1111/j.1755-5922.2011.00280.x] [DOI] [PMC free article] [PubMed] [Google Scholar]
Huffman 2014 {published data only}
- Huffman MD, Yusuf S. Polypills: essential medicines for cardiovascular disease secondary prevention?. Journal of the American College of Cardiology 2014;63(14):1368‐70. [DOI: ] [DOI] [PubMed] [Google Scholar]
Ito 2012 {published data only}
- Ito K, Shrank WH, Avorn J, Patrick AR, Brennan TA, Antman EM, et al. Comparative cost‐effectiveness of interventions to improve medication adherence after myocardial infarction. Health Services Research 2012;47:2097‐117. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ivanovic 2013 {published data only}
- Ivanovic B, Tadic M. Fixed combination of amlodipine/atorvastatin: from mechanisms to trials. Journal of Cardiovascular Pharmacology and Therapeutics 2013;18(6):544‐9. [DOI: ] [DOI] [PubMed] [Google Scholar]
Jadhav 2014 {published data only}
- Jadhav U, Hiremath J, Namjoshi DJ, Gujral VK, Tripathi KK, Siraj M, et al. Blood pressure control with a single‐pill combination of indapamide sustained‐release and amlodipine in patients with hypertension: The EFFICIENT Study. PLoS One 2014;9(4):6. [DOI: 10.1371/journal.pone.0092955] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jang 2015 {published data only}
- Jang JY, Lee SH, Kim BS, Seo HS, Kim WS, Ahn Y, et al. Additive beneficial effects of valsartan combined with rosuvastatin in the treatment of hypercholesterolemic hypertensive patients. Korean Circulation Journal 2015;45(3):225‐33. [DOI: 10.4070/kcj.2015.45.3.225] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jaques 2011 {published data only}
- Jaques H. The polypill concept: the story so far. [Erratum appears in European Heart Journal 2012 Mar; 33(5):551]. European Heart Journal 2011;32:2471‐2. [PubMed] [Google Scholar]
Kawashiri 2015 {published data only}
- Kawashiri MA, Sakata K, Gamou T, Kanaya H, Miwa K, Ueda K, et al. Impact of combined lipid lowering with blood pressure control on coronary plaque regression: rationale and design of MILLION study. Heart Vessels 2015;30(5):580‐6. [DOI: ] [DOI] [PubMed] [Google Scholar]
Kereiakes 2012 {published data only}
- Kereiakes DJ, Chrysant SG, Izzo JL, Littlejohn T, Melino M, Lee J, et al. Olmesartan/amlodipine/hydrochlorothiazide in participants with hypertension and diabetes, chronic kidney disease, or chronic cardiovascular disease: a subanalysis of the multicenter, randomized, double‐blind, parallel‐group TRINITY study. Cardiovascular Diabetology 2012;11:13. [DOI: 10.1186/1475-2840-11-134] [DOI] [PMC free article] [PubMed] [Google Scholar]
Khaled 2015 {published data only}
- Khaled SA, Burley JC, Alexander MR, Yang J, Roberts CJ. 3D printing of five‐in‐one dose combination polypill with defined immediate and sustained release profiles. Journal of Controlled Release 2015;217:308‐14. [DOI: 10.1016/j.jconrel.2015.09.028] [DOI] [PubMed] [Google Scholar]
Laba 2014a {published data only}
- Laba TL, Howard K, Rose J, Jan S. Using DCE to assess adherence and treatment preferences for combination therapies for cardiovascular disease. Global Heart 2014;1:e44. [DOI: ] [Google Scholar]
Laba 2014b {published data only}
- Laba TL, Hayes A, Jan S, Rodgers A, Patel A, Cass A, et al. Can a CVD polypill save money in the 'real world'?. Value in Health 2014;17(7):A482. [DOI: ] [DOI] [PubMed] [Google Scholar]
Lafeber 2011 {published data only}
- Lafeber M, Spiering W, Bots ML, Valk V, Visseren FL, Grobbee DE. Cardiovascular polypill in high risk patients. Nederlands Tijdschrift Voor Geneeskunde 2011;155:A3070. [PubMed] [Google Scholar]
Lafeber 2012 {published data only}
- Lafeber M, Spiering W, Singh K, Guggilla RK, Patil V, Webster R. The cardiovascular polypill in high‐risk patients. European Journal of Preventive Cardiology 2012;19:1234‐42. [DOI: ] [DOI] [PubMed] [Google Scholar]
Lafeber 2013a {published data only}
- Lafeber M, Grobbee DE, Spiering W, Graaf Y, Bots ML, Visseren FL. The combined use of aspirin, a statin, and blood pressure‐lowering agents (polypill components) in clinical practice in patients with vascular diseases or type 2 diabetes mellitus. European Journal of Preventive Cardiology 2013;20(5):771‐8. [DOI: ] [DOI] [PubMed] [Google Scholar]
Lafeber 2013b {published data only}
- Lafeber M, Spiering W, Graaf Y, Nathoe H, Bots ML, Grobbee DE, et al. The combined use of aspirin, a statin, and blood pressure‐lowering agents (polypill components) and the risk of vascular morbidity and mortality in patients with coronary artery disease. American Heart Journal 2013;166(2):282‐9.e1. [DOI: ] [DOI] [PubMed] [Google Scholar]
Lafeber 2014a {published data only}
- Lafeber M, Grobbee DE, Bots ML, Thom S, Webster R, Rodgers A, et al. The Evening versus Morning Polypill Utilization Study: The TEMPUS rationale and design. European Journal of Preventive Cardiology 2014;21(4):425‐33. [DOI: ] [DOI] [PubMed] [Google Scholar]
Lafeber 2014b {published data only}
- Lafeber M, Spiering W, Grobbee DE, Bots ML, Webster R, Thom S, et al. Impact of switching from different treatment regimens to a fixed‐dose combination pill (polypill) in patients with cardiovascular disease or similarly high risk. Circulation 2014;129:AP381. [DOI] [PubMed] [Google Scholar]
Lafeber 2014c {published data only}
- Lafeber M, Stanton A, Thom S, Hughes A, Grobbee DE, Rodgers A, et al. A randomized controlled trial of a cardiovascular fixed‐dose combination pill (polypill) treatment strategy compared with usual care on carotid intima media thickness progression in individuals at high risk of cardiovascular disease. Circulation 2014;129:AP445. [Google Scholar]
Lafeber 2014d {published data only}
- Lafeber M, Grobbee DE, Schrover IM, Thom S, Webster R, Rodgers A, et al. The effect of morning or evening use of a fixed‐dose combination pill (polypill) on LDL‐cholesterol, ambulatory blood pressure and adherence in patients with cardiovascular disease. European Journal of Preventive Cardiology 2014;1:S132. [DOI: ] [Google Scholar]
Lafeber 2015 {published data only}
- Lafeber M, Grobbee DE, Schrover IM, Thom S, Webster R, Rodgers A, et al. Comparison of a morning polypill, evening polypill and individual pills on LDL‐cholesterol, ambulatory blood pressure and adherence in high‐risk patients; a randomized crossover trial. International Journal of Cardiology 2015;181:193‐9. [DOI: ] [DOI] [PubMed] [Google Scholar]
Lafeber 2016 {published data only}
- Lafeber M, Spiering W, Visseren FLJ, Grobbee DE. Multifactorial prevention of cardiovascular disease in patients with hypertension: the cardiovascular polypill. Current Hypertension Reports 2016;18(5):40. [DOI: 10.1007/s11906-016-0648-3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Law 2006 {published data only}
- Law MG. Cardiovascular complications of HIV: an overview of risk and a novel approach to prevention ‐ the HIV polypill study. Current Opinion in HIV and AIDS 2006;1(6):482‐7. [DOI: 10.1097/01.coh.0000247389.08485.87] [DOI] [PubMed] [Google Scholar]
Liu 2014 {published data only}
- Liu H, Massi L, Laba T, Jan S. Understanding adherence to a cardiovascular polypill strategy‐a process evaluation of a pragmatic clinical trial. Global Heart 2014;1:e118. [DOI: ] [Google Scholar]
Liu 2015 {published data only}
- Liu H, Massi L, Laba TL, Peiris D, Usherwood T, Patel A, et al. Patients' and providers' perspectives of a polypill strategy to improve cardiovascular prevention in Australian Primary Health Care. Circulation: Cardiovascular Quality and Outcomes 2015;8(3):301‐8. [DOI: ] [DOI] [PubMed] [Google Scholar]
Marazzi 2016 {published data only}
- Marazzi G, Pelliccia F, Campolongo G, Cacciotti L, Poggi S, Tanzilli A, et al. Greater cardiovascular risk reduction with once‐daily fixed combination of three antihypertensive agents and statin versus free‐drug combination: The ALL‐IN‐ONE trial. International Journal of Cardiology 2016;222:885‐7. [DOI: ] [DOI] [PubMed] [Google Scholar]
Mishchenko 2014 {published data only}
- Mishchenko O, Bezditko N, Adonkina V, Tkachova O. Pharmacoeconomic grounding of using polypill amlodipine with atorvastatin versus monodrugs in patients with hypertension and dyslipidemia in Ukraine. Value in Health 2014;17(7):A475. [DOI: ] [DOI] [PubMed] [Google Scholar]
Mossello 2015 {published data only}
- Mossello E. Targeting vascular risk factors in older adults: from polypill to personalized prevention. JAMA Internal Medicine 2015;175(12):1949‐50. [DOI: ] [DOI] [PubMed] [Google Scholar]
Neutel 2009 {published data only}
- Neutel JM, Bestermann WH, Dyess EM, Graff A, Kursun A, Sutradhar S, et al. The use of a single‐pill calcium channel blocker/statin combination in the management of hypertension and dyslipidemia: a randomized, placebo‐controlled, multicenter study. Journal of Clinical Hypertension 2009;11(1):22‐30. [DOI: 10.1111/j.1751-7176.2008.00058.x] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nguyen 2013 {published data only}
- Nguyen C, Cheng‐Lai A. The polypill a potential global solution to cardiovascular disease. Cardiology in Review 2013;21:49‐54. [DOI: 10.1097/CRD.0b013e3182755429] [DOI] [PubMed] [Google Scholar]
OliverasVila 2014 {published data only}
- Oliveras Vila T, Ferrer Massot M, Curos Abadal A, Rueda Sobella F, Serra Flores J, Carrillo Suarez, et al. Real‐life use of the polypill components (ASA+ACEI+statins) after an acute coronary syndrome and long‐term mortality. International Journal of Cardiology 2014;177(1):209‐10. [DOI: ] [DOI] [PubMed] [Google Scholar]
Reiner 2013 {published data only}
- Reiner Z. Polypill is not a 'vaccine‐like' solution for primary cardiovascular disease prevention in all parts of the world. Journal of Epidemiology and Community Health 2013;67(12):981‐2. [DOI: 10.1136/jech-2013-203220] [DOI] [PubMed] [Google Scholar]
Selak 2013 {published data only}
- Selak V, Crengle S, Elley CR, Wadham A, Harwood M, Rafter N, et al. Recruiting equal numbers of indigenous and non‐indigenous participants to a 'polypill' randomized trial. International Journal for Equity in Health 2013;12:44. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Selak 2016 {published data only}
- Selak V, Bullen C, Stepien S, Arroll B, Bots M, Bramley D, et al. Do polypills lead to neglect of lifestyle risk factors? Findings from an individual participant data meta‐analysis among 3140 patients at high risk of cardiovascular disease. European Journal of Preventive Cardiology 2016;23(13):1393‐400. [DOI: ] [DOI] [PubMed] [Google Scholar]
Sepanlou 2012 {published data only}
- Sepanlou SG, Farzadfar F, Jafari E, Danaei G. Cardiovascular disease prevention using fixed dose pharmacotherapy in Iran: updated meta‐analyses and mortality estimation. Archives of Iranian Medicine 2012;15(9):531‐7. [DOI: ] [PubMed] [Google Scholar]
Sigamani 2012 {published data only}
- Sigamani A, Pais P, Xavier D, Koon T, Xavier F, Girish P, et al. Comparison of risk factor reduction and tolerability of a full dose versus low dose of a polypill (polycap) in individuals at high risk of cardiovascular diseases: a phase II, double blind randomized trial. Circulation 2012;125(19):e803. [DOI: ] [DOI] [PubMed] [Google Scholar]
Simonyi 2016 {published data only}
- Simonyi G, Ferenci T. One year persistence of atorvastatin and amlodipine fixed dose combination versus atorvastatin therapy. Orvosi Hetilap 2016;157(11):425‐9. [DOI: ] [DOI] [PubMed] [Google Scholar]
Son 2013 {published data only}
- Son H, Roh H, Lee D, Chang H, Kim J, Yun C, et al. Pharmacokinetics of rosuvastatin/olmesartan fixed‐dose combination: a single‐dose, randomized, open‐label, 2‐period crossover study in healthy Korean subjects. Clinical Therapeutics 2013;35(7):915‐22. [DOI: ] [DOI] [PubMed] [Google Scholar]
Tanaka 2014 {published data only}
- Tanaka M, Nishimura R, Nishimura T, Kawai T, Meguro S, Irie J, et al. Effect of single tablet of fixed‐dose amlodipine and atorvastatin on blood pressure/lipid control, oxidative stress, and medication adherence in type 2 diabetic patients. Diabetology and Metabolic Syndrome 2014;6:8. [DOI: 10.1186/1758-5996-6-56] [DOI] [PMC free article] [PubMed] [Google Scholar]
Truelove 2014 {published data only}
- Truelove M, Webster R, Bompoint S, Patel A. Impact of pill burden on the effects of a polypill‐based strategy on use of indicated medications in people with or at high risk of cardiovascular disease. Global Heart 2014;1:e298. [DOI: ] [Google Scholar]
Wald 2016 {published data only}
- Wald NJ, Luteijn JM, Morris JK, Taylor D, Oppenheimer P. Cost‐benefit analysis of the polypill in the primary prevention of myocardial infarction and stroke. European Journal of Epidemiology 2016;31(4):415‐26. [DOI: 10.1007/s10654-016-0122-1] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2012 {published data only}
- Wang YQ, Hu Z, Yang Y, Gao PJ. Effect of amlodipine and amlodipine plus atorvastatin on impaired vascular function in patients with hypertension. Journal of Hypertension 2012;30:e163. [DOI: ] [Google Scholar]
Webster 2013 {published data only}
- Webster R, Patel A, Billot L, Cass, A, Burch C, Neal B, et al. Prospective meta‐analysis of trials comparing fixed dose combination based care with usual care in individuals at high cardiovascular risk: the SPACE Collaboration. International Journal of Cardiology 2013;170(1):30‐5. [DOI: ] [DOI] [PubMed] [Google Scholar]
Webster 2014 {published data only}
- Webster R, Rodgers A. PREVENTION coronary artery calcium and polypill therapy. Nature Reviews Cardiology 2014;11(1):7. [DOI: 10.1038/nrcardio.2013.185] [DOI] [PubMed] [Google Scholar]
Webster 2015a {published data only}
- Webster R, Rodgers A. Polypill: progress and challenges to global use: update on the trials and policy implementation. Current Cardiology Reports 2015;17(12):121. [DOI: ] [DOI] [PubMed] [Google Scholar]
Webster 2015b {published data only}
- Webster R, Bullen C, Patel A, Rodgers A, Selak V, Thom S. Impact of cardiovascular polypill based therapy on healthy lifestyle behavior. European Journal of Preventive Cardiology 2015;1:S150. [DOI: ] [Google Scholar]
Webster 2016a {published data only}
- Webster R, Patel A, Selak V, Billot L, Bots ML, Brown A, et al. Effectiveness of fixed dose combination medication ('polypills') compared with usual care in patients with cardiovascular disease or at high risk: a prospective, individual patient data meta‐analysis of 3140 patients in six countries. International Journal of Cardiology 2016;205:147‐56. [DOI: ] [DOI] [PubMed] [Google Scholar]
Webster 2016b {published data only}
- Webster R, Rodgers A. Polypill treatments for cardiovascular diseases. Expert Opinion on Drug Delivery 2016;13(1):1‐6. [DOI: ] [DOI] [PubMed] [Google Scholar]
Wei 2013 {published data only}
- Wei XL, Zou GY, Gong WW, Yin J, Yu YX, Walley J, et al. Cardiovascular disease risk reduction in rural China: a clustered randomized controlled trial in Zhejiang. Trials 2013;14:10. [DOI: 10.1186/1745-6215-14-354] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wijns 2014 {published data only}
- Wijns W, Rusinaru D. "De‐risking" risk reduction should coronary artery calcium scoring be the gatekeeper to preventive pharmacotherapy with the polypill?. Journal of the American College of Cardiology 2014;63(5):444‐6. [DOI: 10.1016/j.jacc.2013.09.044] [DOI] [PubMed] [Google Scholar]
Wiley 2014 {published data only}
- Wiley B, Fuster V. The concept of the polypill in the prevention of cardiovascular disease. Annals of Global Health 2014;80(1):24‐34. [DOI: 10.1016/j.aogh.2013.12.008] [DOI] [PubMed] [Google Scholar]
Xing 2013 {published data only}
- Xing DM, Zhang JH, Li L, Zhu MJ, Shang HC. Intervention effects and safety of cardiovascular polypill for the relevant risk factors of coronary heart disease: a systematic review. Chinese Journal of Evidence‐Based Medicine 2013;13:446‐51. [DOI: ] [Google Scholar]
Zeng 2016 {published data only}
- Zeng R, Wang M, Zhang L. Is time an important problem in management of hypertension and hypercholesterolemia by using an amlodipine‐atorvastatin single pill combination?. Medical Science Monitor 2016;22:2648‐55. [DOI: 10.12659/msm.896843] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zomer 2013 {published data only}
- Zomer E, Owen A, Magliano DJ, Ademi Z, Reid CM, Liew D. Predicting the impact of polypill use in a metabolic syndrome population: an effectiveness and cost‐effectiveness analysis. American Journal of Cardiovascular Drugs 2013;13(2):121‐8. [DOI: ] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Fommei 2015 {published data only}
- Fommei E, Ghione S, Biagini S, Corrao G, Mancia G. A proposal for the idea of a flexible‐combination polypill in arterial hypertension. Journal of Hypertension. 2015; Vol. 33:e258.
NCT00530946 {published data only}
- NCT00530946. A randomized study to evaluate efficacy and safety of a fixed combination therapy of amlodipine and atorvastatin [A multi‐center, randomized study to evaluate efficacy and safety of a fixed combination therapy of amlodipine and atorvastatin in the treatment of concurrent hypertension and hyper‐LDL‐cholesterolemia]. clinicaltrials.gov/ct2/show/NCT00530946 (first received 13 September 2007).
NCT01004705 {published data only}
- NCT01004705. Cardiovascular fixed dose combination pill: a pharmacodynamic study of a fixed dose combination of acetylsalicylic acid, simvastatin, and ramipril in subjects with elevated LDL cholesterol. clinicaltrials.gov/ct2/show/NCT01004705 (first received 23 October 2009).
NCT01005290 {published data only}
- NCT01005290. Cardiovascular fixed dose combination pill: a pharmacodynamic interaction study to evaluate the effect of a fixed dose combination of acetylsalicylic acid, simvastatin and ramipril (cardiovascular fixed dose combination pill) on blood pressure. clinicaltrials.gov/ct2/show/NCT01005290 (first received 22 October 2009).
NCT01362218 {published data only}
- NCT01362218. Cardiovascular fixed combination pill ASR: pharmacodynamic clinical trial of a fixed dose combination of acetylsalicylic acid, simvastatin, and ramipril (cardiovascular polypill) on LDL cholesterol. clinicaltrials.gov/ct2/show/NCT01362218 (first received 26 May 2011).
NCT01406431 {published data only}
- NCT01406431. A single dose, sequence‐randomized, open‐label, 2x2 crossover study to compare pharmacokinetics between pitavastatin and valsartan co‐administration and Livalo® fixed combination drug in healthy male subjects. clinicaltrials.gov/ct2/show/NCT01406431 (first received 26 July 2011).
NCT01764178 {published data only}
- NCT01764178. A single dose, sequence‐randomized, open‐label, 2x2 crossover study to compare pharmacokinetics between pitavastatn and valsartan co‐administration and Livalo complex product in healthy male subjects. clinicaltrials.gov/ct2/show/NCT01764178 (first received 1 January 2013).
NCT02075619 {published data only}
- NCT02075619. An open‐label, randomized, single dose, three‐way crossover, six sequence pilot study to evaluate the relative bioavailability of one amlodipine 10mg tablet and rosuvastatin 20mg tablet to two fixed dose combination tablet formulations of amlodipine (10mg) and rosuvastatin (20mg) in healthy adult male and female subjects under fasting conditions. clinicaltrials.gov/ct2/show/NCT02075619 (first received 27 February 2014).
NCT02569814 {published data only}
- NCT02569814. A study to compare the pharmacokinetics and safety of a fixed dose combination of fimasartan/amlodipine/rosuvastatin. clinicaltrials.gov/ct2/show/NCT02569814 (first received 5 October 2015).
NCT02662894 {published data only}
- NCT02662894. Efficacy of fixed‐dose combination of valsartan + rosuvastatin versus their isolated components for hypertension and dyslipidemia. clinicaltrials.gov/ct2/show/NCT02662894 (first received 23 November 2015).
NCT02791958 {published data only}
- NCT02791958. Pharmacodynamic equivalence study of ramipril 10 mg and atorvastatin 40 mg administered as a cardiovascular fixed dose combination pill AAR as compared to monotherapy with the reference products Altace® 10 mg and Lipitor® 40 mg. clinicaltrials.gov/ct2/show/NCT02791958 (first received 15 February 2016).
NCT02842359 {published data only}
- NCT02842359. Efficacy evaluation of metabolic, anti‐inflammatory, and antioxidative factors of irbesartan/atorvastatin fixed‐dose combination in type 2 diabetic patients diagnosed with hyperlipidemia and hypertension, with adequately controlled blood glucose levels. clinicaltrials.gov/ct2/show/NCT02842359 (first received 20 July 2016).
References to ongoing studies
INTEGRATE {published data only}
- Hayek A, Joshi R, Usherwood T, Webster R, Kaur B, Saini B, et al. An integrated general practice and pharmacy‐based intervention to promote the use of appropriate preventive medications among individuals at high cardiovascular disease risk: protocol for a cluster randomized controlled trial. Implementation Science 2016;11:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi R, Patel A, Peiris D, Saini B, Usherwood T, Armor C, et al. INTegrated Electronic General practice support tool, phaRmacy led intervention And combination Therapy Evaluation trial (INTEGRATE). Heart Lung and Circulation. 2015:S385.
NCT01646437 {published data only}
- NCT01646437. The International Polycap Study‐3. clinicaltrials.gov/ct2/show/NCT01646437 (first received 10 July 2012).
NCT01826019 {published data only}
- NCT01826019. Heart Outcomes Prevention and Evaluation 4 (HOPE‐4). clinicaltrials.gov/ct2/show/NCT01826019 (first received 31 March 2013).
NCT02278471 {published data only}
- NCT02278471. The SCCS polypill pilot trial. clinicaltrials.gov/ct2/show/NCT02278471 (first received 13 October 2014).
NCT02596126 {published data only}
- NCT02596126. SEcondary prevention of Cardiovascular disease in the Elderly trial (SECURE). clinicaltrials.gov/ct2/show/NCT02596126 (first received 5 October 2015).
PolyIran {published data only}
- Merat S, Poustchi H, Hemming K, Jafari E, Radmard AR, Nateghi A, et al. PolyPill for prevention of cardiovascular disease in an urban Iranian population with special focus on nonalcoholic steatohepatitis: a pragmatic randomized controlled trial within a cohort (PolyIran ‐ Liver) ‐ study protocol. Archives of Iranian Medicine 2015;18:515‐23. [PubMed] [Google Scholar]
- NCT01271985. Prevention of cardiovascular disease in middle‐aged and elderly Iranians using a single polypill (PolyIran). clinicaltrials.gov/ct2/show/NCT01271985 (first received 14 December 2010).
- Ostovaneh MR, Poustchi H, Hemming K, Marjani H, Pourshams A, Nateghi A, et al. Polypill for the prevention of cardiovascular disease (PolyIran): study design and rationale for a pragmatic cluster randomized controlled trial. European Journal of Preventive Cardiology 2015;22:1609‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roshandel G, Ostovaneh MR, Poustchi H, Malekzadeh F, Sepanlou SG, Honarvar MR, et al. Reliability analysis of a newly developed questionnaire for quality control of follow‐up visits in PolyIran study. Archives of Iranian Medicine 2016;19:551‐5. [PubMed] [Google Scholar]
Additional references
ALLHAT‐investigators 2002
- ALLHAT‐investigators. Major outcomes in high‐risk hypertensive patients randomized to angiotensin‐converting enzyme inhibitor or calcium channel blocker vs diuretic: the Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA 2002;288(23):2981‐97. [DOI] [PubMed] [Google Scholar]
Armitage 2010
- Armitage JM, Bowman L, Clarke RJ, Wallendszus K, Bulbulia R, Rahimi K, et al. Effects of homocysteine‐lowering with folic acid plus vitamin B12 vs placebo on mortality and major morbidity in myocardial infarction survivors: a randomized trial. JAMA 2010;303(24):2486‐94. [DOI] [PubMed] [Google Scholar]
ATT‐Collaboration 2002
- ATT‐Collaboration. Collaborative meta‐analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ 2002;324(7329):71‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Baigent 2005
- Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, et al. Efficacy and safety of cholesterol‐lowering treatment: prospective meta‐analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2005;366(9493):1267‐78. [DOI] [PubMed] [Google Scholar]
Baigent 2009
- Baigent C, Blackwell L, Collins R, Emberson J, Godwin J, Peto R, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta‐analysis of individual participant data from randomised trials. Lancet 2009;373(9678):1849‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bangalore 2007
- Bangalore S, Kamalakkannan G, Parkar S, Messerli FH. Fixed‐dose combinations improve medication compliance: a meta‐analysis. American Journal of Medicine 2007;120:713‐8. [PUBMED: 17679131] [DOI] [PubMed] [Google Scholar]
Beaglehole 2011
- Beaglehole R, Bonita R, Horton R, Adams C, Alleyne G, Asaria P, et al. Priority actions for the non‐communicable disease crisis. Lancet 2011;377(9775):1438‐47. [DOI] [PubMed] [Google Scholar]
Berry 2012
- Berry JD, Dyer A, Cai X, Garside DB, Ning H, Thomas A, et al. Lifetime risks of cardiovascular disease. New England Journal of Medicine 2012;366(4):321‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Colhoun 2004
- Colhoun HM, Betteridge DJ, Durrington PN, Hitman GA, Neil HA, Livingstone SJ, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo‐controlled trial. Lancet 2004;364(9435):685‐96. [DOI] [PubMed] [Google Scholar]
Collins 1990
- Collins R, Peto R, MacMahon S, Hebert P, Fiebach NH, Eberlein KA, et al. Blood pressure, stroke, and coronary heart disease. Part 2, short‐term reductions in blood pressure: overview of randomised drug trials in their epidemiological context. Lancet 1990;335(8693):827‐38. [DOI] [PubMed] [Google Scholar]
CTT 2012
- Cholesterol Treatment Trialists' Collaboration. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta‐analysis of individual data from 27 randomised trials. Lancet 2012;380:581‐90. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
de Cates 2014
- Cates AN, Farr MR, Wright N, Jarvis MC, Rees K, Ebrahim S, et al. Fixed‐dose combination therapy for the prevention of cardiovascular disease. Cochrane Database of Systematic Reviews 2014, Issue 4. [DOI: 10.1002/14651858.CD009868.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Ebrahim 2011
- Ebrahim S, Taylor F, Ward K, Beswick A, Burke M, Davey Smith G. Multiple risk factor interventions for primary prevention of coronary heart disease. Cochrane Database of Systematic Reviews 2011, Issue 1. [DOI: 10.1002/14651858.CD001561.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
ESC 2016
- Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). European Heart Journal 2016;37(29):2315‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Franco 2004
- Franco OH, Bonneux L, Laet C, Peeters A, Steyerberg EW, Mackenbach JP. The polymeal: a more natural, safer, and probably tastier (than the polypill) strategy to reduce cardiovascular disease by more than 75%. BMJ 2004;329(7480):1447‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Furukawa 2006
- Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N. Imputing missing standard deviations in meta‐analyses can provide accurate results. Journal of Clinical Epidemiology 2006;59:7‐10. [DOI] [PubMed] [Google Scholar]
Gaede 2003
- Gaede P, Vedel P, Larsen N, Jensen GV, Parving HH, Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. New England Journal of Medicine 2003;348(5):383‐93. [DOI] [PubMed] [Google Scholar]
GRADE 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A (editors). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach. Updated October 2013. gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Gupta 2010
- Gupta AK, Arshad S, Poulter NR. Compliance, safety, and effectiveness of fixed‐dose combinations of antihypertensive agents: a meta‐analysis. Hypertension 2010;55(2):399‐407. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
- Higgins JPT, Deeks JJ, Altman DG (editors). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Holmes 2011
- Holmes MV, Newcombe P, Hubacek JA, Sofat R, Ricketts SL, Cooper J, et al. Effect modification by population dietary folate on the association between MTHFR genotype, homocysteine, and stroke risk: a meta‐analysis of genetic studies and randomised trials. Lancet 2011;378(9791):584‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
HPSCG 2002
- HPSCG. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high‐risk individuals: a randomised placebo‐controlled trial. Lancet 2002;360(9326):7‐22. [DOI] [PubMed] [Google Scholar]
Julius 2004
- Julius S, Kjeldsen SE, Weber M, Brunner HR, Ekman S, Hansson L, et al. Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the VALUE randomised trial. Lancet 2004;363(9426):2022‐31. [DOI] [PubMed] [Google Scholar]
Karmali 2016
- Karmali KN, Lloyd‐Jones DM, Berendsen MA, Goff DC Jr, Sanghavi DM, Brown NC, et al. Drugs for primary prevention of atherosclerotic cardiovascular disease: an overview of systematic reviews. JAMA Cardiology 2016;1(3):341‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kearney 2008
- Kearney PM, Blackwell L, Collins R, Keech A, Simes J, Peto R, et al. Efficacy of cholesterol‐lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta‐analysis. Lancet 2008;371(9607):117‐25. [DOI] [PubMed] [Google Scholar]
LaRosa 2005
- LaRosa JC, Grundy SM, Waters DD, Shear C, Barter P, Fruchart JC, et al. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. New England Journal of Medicine 2005;352(14):1425‐35. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of InterventionsVersion 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Lonn 2010
- Lonn E, Bosch J, Teo KK, Pais P, Xavier D, Yusuf S. The polypill in the prevention of cardiovascular diseases: key concepts, current status, challenges, and future directions. Circulation 2010;122(20):2078‐88. [DOI] [PubMed] [Google Scholar]
Meader 2014
- Meader N, King K, Llewellyn A, Norman G, Brown J, Rodgers M, et al. A checklist designed to aid consistency and reproducibility of GRADE assessments: development and pilot validation. Systematic Reviews 2014;3:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Messerli 2006
- Messerli FH, Bakris GL, Ferrera D, Houston MC, Petrella RJ, Flack JM, et al. Efficacy and safety of coadministered amlodipine and atorvastatin in patients with hypertension and dyslipidemia: results of the AVALON trial. Journal of Clinical Hypertension 2006;8(8):571‐81; quiz 82‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009). Preferred reporting items for systematic reviews and meta‐analyses: The PRISMA Statement. PLoS Medicine 2009;6(7):e1000097. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Neaton 1992
- Neaton JD, Wentworth D. Serum cholesterol, blood pressure, cigarette smoking, and death from coronary heart disease. Overall findings and differences by age for 316,099 white men. Multiple Risk Factor Intervention Trial Research Group. Archives of Internal Medicine 1992;152(1):56‐64. [PubMed] [Google Scholar]
NICE 2008
- NICE. Lipid Modification. Cardiovascular risk assessment and the modification of blood lipids for the primary and secondary prevention of cardiovascular disease. www.nice.org.uk/nicemedia/pdf/CG67NICEguideline.pdf 2008 (Accessed 17 January 2017).
NICE 2010
- NICE. Cardiovascular disease prevention. www.nice.org.uk/guidance/PH25 2010 (Accessed 17 January 2017).
O'Donnell 2010
- O'Donnell MJ, Xavier D, Liu L, Zhang H, Chin SL, Rao‐Melacini P, et al. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): a case‐control study. Lancet 2010;376(9735):112‐23. [DOI] [PubMed] [Google Scholar]
Ostergren 2008
- Ostergren J, Poulter NR, Sever PS, Dahlof B, Wedel H, Beevers G, et al. The Anglo‐Scandinavian Cardiac Outcomes Trial: blood pressure‐lowering limb: effects in patients with type II diabetes. Journal of Hypertension 2008;26(11):2103‐11. [DOI] [PubMed] [Google Scholar]
Papademetriou 2003
- Papademetriou V, Piller LB, Ford CE, Gordon D, Hartney TJ, Geraci TS, et al. Characteristics and lipid distribution of a large, high‐risk, hypertensive population: the lipid‐lowering component of the Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). Journal of Clinical Hypertension 2003;5(6):377‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Perk 2012
- Perk J, DeBacker G, Gohlke H, Graham I, Reiner Z, Verschuren M, et al. European Guidelines on cardiovascular disease prevention in clinical practice (version 2012). European Journal of Cardiology 2012;33:1635‐701. [PUBMED: 10.1093/eurheartj/ehs092] [DOI] [PubMed] [Google Scholar]
PILL‐collaborative 2011
- PILL‐collaborative, Rodgers A, Patel A, Berwanger O, Bots M, Grimm R, et al. An international randomised placebo‐controlled trial of a four‐component combination pill ("polypill") in people with raised cardiovascular risk. Plos One 2011;6(5):e19857. [DOI] [PMC free article] [PubMed] [Google Scholar]
Preston 2007
- Preston RA, Harvey P, Herfert O, Dykstra G, Jukema JW, Sun F, et al. A randomized, placebo‐controlled trial to evaluate the efficacy, safety, and pharmacodynamic interaction of coadministered amlodipine and atorvastatin in 1660 patients with concomitant hypertension and dyslipidemia: the respond trial. Journal of Clinical Pharmacology 2007;47(12):1555‐69. [DOI] [PubMed] [Google Scholar]
Rashid 2003
- Rashid P, Leonardi‐Bee J, Bath P. Blood pressure reduction and secondary prevention of stroke and other vascular events: a systematic review. Stroke 2003;34(11):2741‐8. [DOI] [PubMed] [Google Scholar]
Roth 2015
- Roth GA, Forouzanfar MH, Moran AE, Barber R, Nguyen G, Feigin VL, et al. Demographic and epidemiologic drivers of global cardiovascular mortality. New England Journal of Medicine 2015;372(14):1333‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sever 2003
- Sever PS, Dahlof B, Poulter NR, Wedel H, Beevers G, Caulfield M, et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower‐than‐average cholesterol concentrations, in the Anglo‐Scandinavian Cardiac Outcomes Trial ‐ Lipid Lowering Arm (ASCOT‐LLA): a multicentre randomised controlled trial. Lancet 2003;361(9364):1149‐58. [DOI] [PubMed] [Google Scholar]
Stone 2013
- Stone NJ, Robinson J, Lichtenstein AH, Merz CN, Blum CB, Eckel RH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2013;129(25 Suppl 2):S1‐45. [DOI: ] [DOI] [PubMed] [Google Scholar]
Taylor 2013
- Taylor F, Huffman MD, Macedo AF, Moore TH, Burke M, Davey Smith G, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database of Systematic Reviews 2013, Issue 1. [DOI: 10.1002/14651858.CD004816.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Thomas 2002
- Thomas F, Bean K, Guize L, Quentzel S, Argyriadis P, Benetos A. Combined effects of systolic blood pressure and serum cholesterol on cardiovascular mortality in young. European Heart Journal 2002;23(7):528‐35. [DOI] [PubMed] [Google Scholar]
Turnbull 2003
- Turnbull F. Effects of different blood‐pressure‐lowering regimens on major cardiovascular events: results of prospectively‐designed overviews of randomised trials. Lancet 2003;362(9395):1527‐35. [DOI] [PubMed] [Google Scholar]
Vartiainen 2010
- Vartiainen E, Laatikainen T, Peltonen M, Juolevi A, Mannisto S, Sundvall J, et al. Thirty‐five‐year trends in cardiovascular risk factors in Finland. International Journal of Epidemiology 2010;39(2):504‐18. [DOI] [PubMed] [Google Scholar]
Viera 2011
- Viera AJ, Sheridan SL, Edwards T, Soliman EZ, Harris R, Furberg CD. Acceptance of a polypill approach to prevent cardiovascular disease among a sample of U.S. physicians. Preventive Medicine 2011;52(1):10‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Virdee 2013
- Virdee SK, Greenfield SM, Fletcher K, McManus RJ, Hobbs FD, Mant J. Would primary healthcare professionals prescribe a polypill to manage cardiovascular risk? A qualitative interview study. BMJ Open 2013;3:e002498. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wald 2003
- Wald NJ, Law MR. A strategy to reduce cardiovascular disease by more than 80%. BMJ 2003;326(7404):1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wald 2011
- Wald DS, Wald NJ. The polypill in the prevention of cardiovascular disease. Preventive Medicine 2011;52(1):16‐7. [DOI] [PubMed] [Google Scholar]
WHO 2001
- World Health Organization. Secondary prevention of non‐communicable disease in low and middle income countries through community‐based and health service interventions. Report of WHO‐Wellcome Trust meeting of experts (1‐3 August 2001). apps.who.int/iris/bitstream/10665/42567/1/WHO_MPN_CVD_2002.01.pdf 2001.
WHO 2013
- World Health Organization. Global action plan for the prevention and control of noncommunicable diseases, 2013‐202. apps.who.int/iris/bitstream/10665/94384/1/9789241506236_eng.pdf 2013;1:1‐55. [Google Scholar]
WHO 2016
- World Health Organization. HEARTS Technical package for cardiovascular disease management in primary health care. www.who.int/cardiovascular_diseases/hearts/Hearts_package.pdf 2016;1:1‐76. [Google Scholar]
Yusuf 2002
- Yusuf S. Two decades of progress in preventing vascular disease. Lancet 2002;360(9326):2‐3. [DOI] [PubMed] [Google Scholar]
Yusuf 2004
- Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case‐control study. Lancet 2004;364(9438):937‐52. [DOI] [PubMed] [Google Scholar]
Yusuf 2011
- Yusuf S, Islam S, Chow CK, Rangarajan S, Dagenais G, Diaz R, et al. Use of secondary prevention drugs for cardiovascular disease in the community in high‐income, middle‐income, and low‐income countries (the PURE study): a prospective epidemiological survey. Lancet 2011;378(9798):1231‐43. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
deCates 2014
- Cates AN, Farr MR, Wright N, Jarvis MC, Rees K, Ebrahim S, et al. Fixed‐dose combination therapy for the prevention of cardiovascular disease. Cochrane Database of Systematic Reviews 2014, Issue 4. [DOI: 10.1002/14651858.CD009868.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


