Abstract
Background
There is significant uncertainty in the treatment of intermediate‐stage hepatocellular carcinoma which is defined by the Barcelona Clinic Liver Cancer (BCLC) as hepatocellular carcinoma stage B with large, multi‐nodular, Child‐Pugh status A to B, performance status 0 to 2, and without vascular occlusion or extrahepatic disease.
Objectives
To assess the comparative benefits and harms of different interventions used in the treatment of intermediate‐stage hepatocellular carcinoma (BCLC stage B) through a network meta‐analysis and to generate rankings of the available interventions according to their safety and efficacy. However, we found only one comparison. Therefore, we did not perform the network meta‐analysis, and we assessed the comparative benefits and harms of different interventions versus each other, or versus placebo, sham, or no intervention (supportive treatment only) using standard Cochrane methodology.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, Science Citation Index Expanded, World Health Organization International Clinical Trials Registry Platform, and randomised clinical trials registers to September 2016 to identify randomised clinical trials on hepatocellular carcinoma.
Selection criteria
We included only randomised clinical trials, irrespective of language, blinding, or publication status, in participants with intermediate‐stage hepatocellular carcinoma, irrespective of the presence of cirrhosis, size, or number of the tumours (provided they met the criteria of intermediate‐stage hepatocellular carcinoma), of presence or absence of portal hypertension, of aetiology of hepatocellular carcinoma, and of the future remnant liver volume. We excluded trials which included participants who had previously undergone liver transplantation. We considered any of the various interventions compared with each other or with no active intervention (supportive treatment only). We excluded trials which compared variations of the same intervention: for example, different methods of performing transarterial chemoembolisation.
Data collection and analysis
We used standard methodological procedures expected by Cochrane. We calculated the hazard ratio (HR) with 95% confidence intervals (CI) using both fixed‐effect and random‐effects models based on available‐participant analysis with Review Manager. We assessed risk of bias according to Cochrane, controlled risk of random errors with Trial Sequential Analysis using Stata, and assessed the quality of the evidence using GRADE.
Main results
Three randomised clinical trials, including 430 participants, met the inclusion criteria for this review; however, data from two trials with 412 participants could be included in only one primary outcome (i.e. mortality). All three trials were at high risk of bias. All three trials included supportive care as cointervention. The comparisons included in the two trials reporting on mortality were: systemic chemotherapy with sorafenib versus no active intervention; and transarterial chemoembolisation plus systemic chemotherapy with sorafenib versus transarterial chemoembolisation alone. The trials did not report the duration of follow‐up; however, it appeared that the participants were followed up for a period of about 18 to 30 months. The majority of the participants in the trials had cirrhotic livers. The trials included participants with intermediate‐stage hepatocellular carcinoma arising from viral and non‐viral aetiologies. The trials did not report the portal hypertension status of the participants. The mortality was 50% to 70% over a median follow‐up period of 18 to 30 months. There was no evidence of difference in mortality at maximal follow‐up between systemic chemotherapy versus no chemotherapy (hazard ratio 0.85, 95% CI 0.60 to 1.18; participants = 412; studies = 2; I2 = 0%; very low quality evidence). A subgroup analysis performed by stratifying the analysis by the presence or absence of transarterial chemoembolisation as cointervention did not alter the results. None of the trials reported on serious adverse events other than mortality, health‐related quality of life, recurrence of hepatocellular carcinoma, or length of hospital stay. One of the trials providing data was funded by the pharmaceutical industry, the other did not report the source of funding, and the trial with no data for the review was also funded by the pharmaceutical industry. We found two ongoing trials.
Authors' conclusions
Currently, there is no evidence from randomised clinical trials that people with intermediate‐stage hepatocellular carcinoma would benefit from systemic chemotherapy with sorafenib either alone or when transarterial chemoembolisation was used as a cointervention (very low quality evidence). We need high‐quality randomised clinical trials designed to measure differences in clinically important outcomes (e.g. all‐cause mortality or health‐related quality of life).
Plain language summary
Treatment of intermediate‐stage primary liver cancer (hepatocellular carcinoma)
Background
Hepatocellular carcinoma (primary liver cancer) arises from the liver cells and is distinct from secondary liver cancer, arising from other parts of the body and spreading to the liver. Hepatocellular carcinoma can be classified in many ways. One classification is by Barcelona Clinic Liver Cancer (BCLC) group stage which classifies the cancer based on how long the person is expected to live (life expectancy). This classification is broadly based on the size of the cancer, number of cancers in the liver, how well the liver works, and whether one's activities are affected by the cancer. People with intermediate‐stage hepatocellular carcinoma have large, multiple cancers, but they do not have full‐blown liver failure. Cancer is confined to the liver, and there is no restriction of daily activities. There is significant uncertainty in the treatment of people with intermediate‐stage hepatocellular carcinoma. We sought to resolve this uncertainty by searching for existing studies on the topic. We included all randomised clinical trials (well‐designed clinical trials where people are randomly put into one of two or more treatment groups) whose results were reported to September 2016. We included only trials in which participants with intermediate‐stage hepatocellular carcinoma had not undergone liver transplantation previously. Apart from using standard Cochrane methods which allow comparison of only two treatments at a time (direct comparison), we planned to use an advanced method which allows comparison of the many different treatments that are individually compared in the trials (network meta‐analysis). However, because there was only one comparison, we could only use standard Cochrane methodology.
Study characteristics
Only three trials with 430 participants met our inclusion criteria; however, two of the trials (412 participants) only reported death and no other measures of how well the treatments worked. All three trials included supportive care (treatment to prevent, control, or relieve complications and side effects and improve comfort and quality of life) as a co‐intervention. The trials assessed transarterial chemoembolisation (where anti‐cancer drugs block the blood supply and treat the cancer through the vessels supplying the cancer), chemotherapy using sorafenib (a drug which blocks cancer growth), or a combination of transarterial chemoembolisation and sorafenib. It appeared that the trials followed participants for about 18 to 30 months from the initiation of treatment.
Two trials were funded by the pharmaceutical industry; one trial did not report the source of funding.
Key results
Over 18 to 30 months, 50% to 75% of participants died. There was no evidence of any difference between the people who received chemotherapy and those who did not receive chemotherapy. None of the trials reported complications, health‐related quality of life (a measure of a person's satisfaction with their life and health), cancer recurrence, or length of hospital stay. Overall, there is currently no evidence for benefit of any active treatment in addition to supportive treatment for intermediate‐stage hepatocellular carcinoma. There is significant uncertainty on this and further high‐quality randomised clinical trials are required.
Quality of evidence
The overall quality of evidence was low or very low and all the trials were at high risk of bias, which means that there is possibility of making the wrong conclusions, overestimating benefits, or underestimating harms of one treatment or the other because of the way that the trials were conducted.
Summary of findings
Summary of findings 1. Chemotherapy versus no chemotherapy for intermediate‐stage hepatocellular carcinoma.
| Chemotherapy versus no chemotherapy for intermediate‐stage hepatocellular carcinoma | |||||
|
Patient or population: people with intermediate‐stage hepatocellular carcinoma Settings: secondary or tertiary care Intervention: systemic chemotherapy Control: no systemic chemotherapy a Cointervention: transarterial chemoembolisation in both groups in 1 trial | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| No systemic chemotherapya | Systemic chemotherapya | ||||
| Mortality (at maximal follow‐up) ‐ chemotherapy versus no chemotherapy Median follow‐up in trials: 12 to 24 months | 500 per 1000 | 445 per 1000 (340 to 559) | HR 0.85 (0.60 to 1.18) | 412 (2 trials) | ⊕⊕⊝⊝ Very low1,2,3 |
| Short‐term and medium‐term mortality | None of the trials reported short‐term or medium‐term mortality. | ||||
| Adverse events | None of the trials reported adverse events. | ||||
| Quality of life | None of the trials reported quality of life at any time point. | ||||
| Disease recurrence | None of the trials reported disease recurrence. | ||||
| Length of hospital stay | None of the trials reported length of hospital stay. | ||||
| *The basis for the assumed risk was the control group proportion in the studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; HR: hazard ratio. | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1 Downgraded one level because of within‐study risk of bias: the trials were at high risk of bias.
2 Downgraded one level because of imprecision: the sample size was small.
3 Downgraded one level because of imprecision: the confidence intervals overlapped no effect and clinically significant effect.
Background
Description of the condition
Hepatocellular carcinoma is the major form of primary liver cancer (Bosetti 2014; NCBI 2014). An estimated 782,000 people develop hepatocellular carcinoma and 746,000 people die because of primary liver cancer each year worldwide (IARC 2014a). It is the sixth most common cancer overall with an age standardised incidence rate of 10.1 per 100,000 population per year (IARC 2014b). It is the second most common cause of death from cancer worldwide (IARC 2014a). It is more common in men than women (IARC 2014a). There is global variation in the incidence and mortality related to primary liver cancer. Approximately half of all primary liver cancers occur in China (395,000 people per year). Northern Europe has the lowest incidence of primary liver cancer (IARC 2014a). There is an increase in the incidence of hepatocellular carcinoma in many countries (Davila 2004; Jepsen 2007; Pocobelli 2008; Taura 2009; Von Hahn 2011; Witjes 2012; Bosetti 2014; Ladep 2014). This increase is attributed to hepatitis C virus infection (Davila 2004; Taura 2009). Alcohol‐related liver disease, hepatitis B virus infection, and hepatitis C virus infection are major risk factors for hepatocellular carcinoma (Davila 2004; Bosetti 2014). Other risk factors include aflatoxin in foods (toxins produced by Aspergillus fungus), smoking, being overweight, diabetes, and non‐alcohol related steatohepatitis (Lee 2009; Polesel 2009; Starley 2010; Chen 2012; Liu 2012; Bosetti 2014; Turati 2014). The incidence of hepatocellular carcinoma is higher in people with a family history of hepatocellular carcinoma, and lower in people with high intake of vegetables and coffee (Turati 2012; Sang 2013; Bosetti 2014; Yang 2014). The association between oral contraceptives and hepatocellular carcinoma is unclear and there is currently no evidence of an increased risk between women using oral contraceptives and women who do not use oral contraceptive based on one meta‐analysis of observational studies (Maheshwari 2007). Hepatocellular carcinoma usually develops in cirrhotic livers although it may also develop in non‐cirrhotic livers (Arnaoutakis 2014; Gaddikeri 2014). Hepatocellular carcinomas that develop in non‐cirrhotic livers are usually solitary but larger compared to hepatocellular carcinomas that develop in cirrhotic livers (Gaddikeri 2014). The role of routine screening for hepatocellular carcinomas in people with chronic liver disease is controversial with one systematic review concluding that there was no evidence of benefit of routine screening for people with hepatocellular carcinoma (Kansagara 2014).
Description of the intervention
Several classifications of hepatocellular carcinoma have been proposed. This includes clinical staging classifications, histopathological classifications, and molecular classifications (Wu 1996; Henderson 2003; Van Deusen 2005; Cillo 2006; Nanashima 2006; Van Malenstein 2011). Of these, the Barcelona Clinic Liver Cancer (BCLC) staging system (Llovet 1999; Llovet 2003), and the Milan criteria (Mazzaferro 1996), are important classification systems that determine the management of hepatocellular carcinoma. Appendix 1 and Appendix 2 show these classification systems in detail. Stage 0 (very early hepatocellular carcinoma) and stage A (early hepatocellular carcinoma) of BCLC staging correspond approximately to tumours falling within Milan criteria although Stage A of the BCLC staging system includes a single tumour of any size while a single tumour should be less than 5 cm to fall within Milan criteria. This review examines the treatment options for people with intermediate‐stage hepatocellular carcinoma (large multi‐nodular tumours with no evidence of vascular invasion or extrahepatic spread and performance status 0). A separate review covers the treatment options for people with very‐early hepatocellular carcinoma (single nodule less than 2 cm in diameter, Child‐Pugh A cirrhosis, and performance status 0) and early hepatocellular carcinoma (single tumour or two or three lesions less than 3 cm in maximum diameter with no evidence of vascular invasion or extrahepatic spread, Child‐Pugh A or B cirrhosis, and performance status 0) (Majumdar 2017).
Various treatments are aimed at intermediate‐stage hepatocellular carcinoma. These can be broadly classified as surgical, ablative techniques, radiotherapy, transcatheter arterial embolisation (TAE), and transcatheter arterial chemoembolisation (TACE).
The surgical management of hepatocellular carcinoma is in the form of liver resection and liver transplantation (Bruix 2011; EASL 2012; Asham 2013). Liver resection is performed to ensure that all the tumours are removed with adequate remnant liver to carry out the normal functions of the liver (Asham 2013). Liver resection is usually performed by open technique although laparoscopic (key hole) liver resection can be performed in a selection of people (Nguyen 2009). Complications related to liver resection include mortality, liver failure, bile leak, bleeding, liver abscess, abdominal abscess, wound infection, and general complications such as heart failure and renal failure (Nguyen 2009; Xiong 2012). Liver transplantation involves removal of the diseased liver and transplanting a liver graft from another donor (usually a cadaveric donor) (SRTR 2012; NHSBT 2014). Living donor liver transplantation is associated with increased complications and increased incidence of retransplantation and constitutes only a small proportion of the global liver transplantation (Wan 2014). The complications of liver transplantation include mortality, graft failure, graft rejection, biliary stricture, hepatic artery thrombosis, and wound infections (Gurusamy 2014; Wan 2014).
Ablation is usually in the form of radiofrequency ablation (Bruix 2011; EASL 2012; Asham 2013), but other modalities exist, such as chemical ablation using percutaneous alcohol or acetic acid injections, ablations such as microwave ablation, laser (light amplification by stimulated emission of radiation) ablation, cryoablation, high‐intensity focused ultrasound (HIFU), and irreversible electroporation (Head 2004; Germani 2010; Sindram 2010; Chan 2013a). Complications related to ablation include mortality, liver failure, bleeding, liver abscess, bile duct injuries, and tumour dissemination through the needle tract ('seeding') or into the peritoneum (Chan 2013a; McDermott 2013).
Radiotherapy is usually in the form of stereotactic body radiotherapy and radioembolisation. Stereotactic body radiotherapy involves delivering external radiotherapy in large divided doses (usually five or fewer doses) with the radiation focused on the lesions (Kollar 2014). Radioembolisation involves intra‐arterial injection of small microspheres (25 mm to 35 mm) containing the radionuclide Yttrium (Forner 2014). Major complications of radiotherapy include worsening of cirrhosis and liver toxicity, which may manifest as liver failure (Forner 2014; Kollar 2014).
TAE involves embolisation of the hepatic artery without using any chemotherapeutic agents, while TACE involves injection of a chemotherapeutic agent prior to embolisation of the hepatic artery (Pleguezuelo 2008). TACE can also be performed using drug‐eluting beads (Forner 2014; Hoffmann 2014). Both TAE and TACE are unstandardised procedures, with varying chemotherapeutic and embolising agents used and different protocols of retreatment following the index embolisation (Tsochatzis 2014). Major complications of TAE and TACE include mortality, liver failure, liver and splenic abscesses, acute cholecystitis, damage to the bile ducts, renal failure, and severe upper gastrointestinal bleeding (Pleguezuelo 2008).
How the intervention might work
Liver resection and liver transplantation work by removing the cancer. Chemical ablations using alcohol injections and acetic acid injections destroy the cancer tissue (Sindram 2010). Thermal ablations cause destruction of cancer tissue by heat or cold (Sindram 2010). TAE and TACE cause ischaemia to the tumour thereby causing tumour necrosis (Pleguezuelo 2008). TACE combines the effect of chemotherapy agents, which inhibit the tumour in addition to the effect of ischaemia on the tumour, although the main effect of TACE may be due to the ischaemia rather than the chemotherapy delivered via the artery (Pleguezuelo 2008).
Why it is important to do this review
The current guidelines on the management of hepatocellular carcinoma by the European Association for the Study of the Liver and the American Association for the Study of Liver Diseases recommend TACE for people with intermediate‐stage hepatocellular carcinoma (Bruix 2011; EASL 2012). In this situation, TACE is considered palliative (Bruix 2011; EASL 2012) and there is no evidence that TACE increases survival or health‐related quality of life (Oliveri 2011). Some researchers advocate liver transplantation for selected people with intermediate‐stage hepatocellular carcinoma, while other researchers do not (Germani 2011; Prasad 2011). However, it must be noted that people with hepatocellular carcinoma have to compete with other people waiting for liver transplantation. In 2012, pretransplant deaths occurred at the rate of 5.8 deaths per 100 waiting‐list years in the US (SRTR 2012), and in the year to the end of March 2014, 12% of people on the liver transplantation waiting list in the UK died or became too unwell to have a transplant (NHSBT 2014). This indicates an organ shortage necessitating an organ allocation policy. The Milan criteria are now used for organ transplant allocation in many countries. In the US, eligible people with hepatocellular carcinoma are given exceptional status so that they do not remain on the waiting list too long, as delay in transplantation will increase the chance of tumour progression or dissemination (OPTN 2014). People with hepatocellular carcinoma must meet the Milan criteria but, in addition, need to have a minimum tumour size of 2 cm if they have a single tumour and a minimum tumour size of 1 cm each if they have two or three lesions to be considered eligible for exceptional status (OPTN 2014). However, expanding the existing criteria for liver transplantation for people with intermediate‐stage hepatocellular carcinoma should be carefully assessed and should be evidence‐based because of the impact that this might have on other people requiring liver transplantation. There have also been calls to recommend liver resection for people with intermediate‐stage hepatocellular carcinoma (Guglielmi 2014). Thus, the optimal management of people with intermediate‐stage hepatocellular carcinoma is not known. Network meta‐analysis allows combination of the direct evidence and indirect evidence and allows ranking of different interventions in terms of the different outcomes (Salanti 2011; Salanti 2012). There has been no network meta‐analysis on the different interventions for intermediate‐stage hepatocellular carcinoma. This systematic review and attempted network meta‐analysis attempts to provide the best level of evidence for the role of different treatment options for people with intermediate‐stage hepatocellular carcinoma.
Objectives
To assess the comparative benefits and harms of different interventions used in the treatment of intermediate‐stage hepatocellular carcinoma (BCLC stage B) through a network meta‐analysis and to generate rankings of the available interventions according to their safety and efficacy. However, there was only one comparison included for this review. Therefore, we did not perform the network meta‐analysis, and we assessed the comparative benefits and harms of different interventions using standard Cochrane methodology. When more trials become available, we will attempt to conduct network meta‐analysis in order to generate rankings of the available interventions according to their safety and efficacy. This is why we retain the planned methodology for network meta‐analysis in our Appendix 3. Once data appear allowing for the conduct of network meta‐analysis, this Appendix 3 will be moved back into the Methods section.
Methods
Criteria for considering studies for this review
Types of studies
We considered only randomised clinical trials, irrespective of language, publication status, or date of publication. We excluded studies of other design because of the risk of bias in such studies. We are all aware that such exclusions make us focus much more on potential benefits and not fully assess the risks of serious adverse events as well as risks of adverse events.
Types of participants
We included participants with intermediate‐stage hepatocellular carcinoma (BCLC stage B) irrespective of the presence of cirrhosis, size and number of the tumours (provided they met the criteria of intermediate‐stage hepatocellular carcinoma), presence or absence of portal hypertension, aetiology of hepatocellular carcinoma, and the future remnant liver volume. We excluded randomised clinical trials in which participants had undergone liver transplantation previously.
Types of interventions
We planned to include any of the following interventions that are possible treatments for intermediate‐stage hepatocellular carcinoma either alone or in combination tested versus each other, or versus placebo or sham, or no intervention (supportive care):
liver resection;
liver transplantation;
radiofrequency ablation;
microwave ablation;
other ablations (laser ablation, cryoablation, HIFU, irreversible electroporation);
alcohol injection;
acetic acid injection;
radiotherapy (stereotactic body radiotherapy or radioembolisation);
systemic chemotherapy;
TAE;
TACE;
supportive care.
The above list is not exhaustive. If we identified interventions that we were not aware of, we would have considered them as eligible and would have included them in the review, if they were used primarily for the treatment of hepatocellular carcinoma. If liver resection or liver transplantation were combined with ablation, TAE, or TACE, we planned to categorise the intervention as liver resection or liver transplantation. This is because liver resection and liver transplantation are the major components in such interventions, with ablation, TAE, or TACE playing exclusively a supportive role to liver resection or liver transplantation. However, we planned to exclude such interventions from a sensitivity analysis (see Sensitivity analysis). If we found a sufficient number of trials on one or more of the other methods of ablation (laser ablation, cryoablation, HIFU, irreversible electroporation), we planned to consider the specific method of ablation with sufficient trials as a separate intervention.
Types of outcome measures
We planned to assess the comparative benefits and harms of available interventions aimed at treating people with intermediate‐stage hepatocellular carcinoma for the following outcomes.
Primary outcomes
Mortality at maximal follow‐up (time to death).
-
Mortality:
short‐term mortality (up to one year);
medium‐term mortality (one to five years).
-
Adverse events (within three months of cessation of treatment). Depending on the availability of data, we planned to attempt to classify adverse events as serious and non‐serious. We defined a non‐serious adverse event as any untoward medical occurrence not necessarily having a causal relationship with the treatment but resulting in a dose reduction or discontinuation of treatment (any time after commencement of treatment) (ICH‐GCP 1997). We defined a serious adverse event as any event that would increase mortality; was life threatening; required hospitalisation; resulted in persistent or significant disability; was a congenital anomaly/birth defect; or any important medical event that might jeopardise the person or require intervention to prevent it. We used the definition used by study authors for non‐serious and serious adverse events:
proportion of participants with serious adverse events;
number of serious adverse events;
proportion of participants with any type of adverse event;
number of any type of adverse event.
-
Quality of life as defined in the included trials using a validated scale such as EQ‐5D or 36‐item Short Form (SF‐36) (EuroQol 2014; Ware 2014):
short‐term (up to one year);
medium‐term (one to five years);
long‐term (beyond five years).
We considered long‐term quality of life more important than short‐term or medium‐term quality of life, although short‐term or medium‐term quality of life are also important primary outcomes.
Secondary outcomes
-
Disease recurrence (maximum follow‐up):
proportion of participants with hepatocellular carcinoma recurrence (includes recurrence in the liver and metastatic disease);
proportion of participants with local recurrence (recurrence in the liver);
time to hepatocellular carcinoma recurrence;
time to local recurrence.
Length of hospital stay for the intervention and intervention‐related complications. If intervention was performed in two or more sessions, we planned to calculate the total length of hospital stay for all the sessions. Similarly, we planned to include length of hospital stay for readmissions within 30 days of intervention because of intervention‐related complications in the length of hospital stay.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 8), MEDLINE (OvidSP), Embase (OvidSP), and Science Citation Index Expanded (Web of Knowledge) (Royle 2003) from inception to 30 September 2016 for randomised clinical trials comparing two or more of the above interventions. We searched for all possible comparisons formed by the interventions of interest. To identify further ongoing or completed trials, we also searched the World Health Organization International Clinical Trials Registry Platform search portal (apps.who.int/trialsearch/), which searches various trial registers, including ISRCTN and ClinicalTrials.gov. Appendix 4 shows the search strategies that we used and the time spans of the searches.
Searching other resources
We searched the references of the identified trials and the existing Cochrane Reviews on hepatocellular carcinoma to identify additional trials for inclusion.
Data collection and analysis
Selection of studies
Review authors (KG, AM, or DR between them) independently identified the trials for inclusion by screening the titles and abstracts. We sought full‐text articles for any references that at least one of the review authors identified for potential inclusion. We selected trials for inclusion based on the full‐text articles. We have listed the excluded full‐text references with reasons for their exclusion in the Characteristics of excluded studies table. We listed any ongoing trials identified primarily through the search of the clinical trial registers for further follow‐up (Characteristics of ongoing studies table). We resolve discrepancies through discussion.
Data extraction and management
Two review authors (KG and AM or DR) independently extract the following data.
-
Outcome data (for each outcome and for each intervention arm whenever applicable):
number of participants randomised;
number of participants included for the analysis;
number of participants with events for binary outcomes, mean and standard deviation for continuous outcomes, number of events for count outcomes, and the number of participants with events and the mean follow‐up period for time‐to‐event outcomes;
definition of outcomes or scale used if appropriate.
-
Data on potential effect modifiers:
participant characteristics such as age, sex, comorbidities, proportion of people with or without cirrhosis, tumour size, number of tumours, presence of portal hypertension, aetiology of hepatocellular carcinoma, and adjuvant treatments such as immunotherapy;
details of the intervention and control (including dose, frequency, and duration);
risk of bias (assessment of risk of bias in included studies).
-
Other data:
year and language of publication;
country in which the participants were recruited;
year(s) in which the trial was conducted;
inclusion and exclusion criteria;
follow‐up time points of the outcome.
If available, we planned to obtain the data separately for people with and without cirrhosis, presence compared to absence of portal hypertension, and viral compared to non‐viral aetiology. We sought unclear or missing information by attempting to contacting the trial authors, but we did not obtain any additional information. If there was any doubt whether trials shared the same participants, completely or partially (by identifying common authors and centres), we planned to contact the trial authors to clarify whether the trial report was duplicated. We resolved any differences in opinion through discussion.
Assessment of risk of bias in included studies
We followed the guidance given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) and described in the Cochrane Hepato‐Biliary Group Module (Gluud 2016) to assess the risk of bias in included studies. Specifically, we assessed the risk of bias in included trials for the following domains using the methods below (Schulz 1995; Moher 1998; Kjaergard 2001; Wood 2008; Savović 2012a; Savović 2012b; Lundh 2017).
Allocation sequence generation
Low risk of bias: the study authors performed sequence generation using computer random number generation or a random number table. Drawing lots, tossing a coin, shuffling cards, and throwing dice were adequate if an independent person not otherwise involved in the study performed them.
Unclear risk of bias: the study authors did not specify the method of sequence generation.
High risk of bias: the sequence generation method was not random. We only included such studies for assessment of harms.
Allocation concealment
Low risk of bias: the participant allocations could not have been foreseen in advance of, or during, enrolment. A central and independent randomisation unit controlled allocation. The investigators were unaware of the allocation sequence (e.g. if the allocation sequence was hidden in sequentially numbered, opaque, and sealed envelopes).
Unclear risk of bias: the study authors did not describe the method used to conceal the allocation so the intervention allocations may have been foreseen before, or during, enrolment.
High risk of bias: it is likely that the investigators who assigned the participants knew the allocation sequence. We only included such studies for assessment of harms.
Blinding of participants and personnel
Low risk of bias: any of the following: no blinding or incomplete blinding, but the review authors judged that the outcome was not likely to be influenced by lack of blinding; or blinding of participants and key study personnel ensured, and it was unlikely that the blinding could have been broken.
Unclear risk of bias: any of the following: insufficient information to permit judgement of 'low risk' or 'high risk'; or the trial did not address this outcome.
High risk of bias: any of the following: no blinding or incomplete blinding, and the outcome was likely to be influenced by lack of blinding; or blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome was likely to be influenced by lack of blinding.
Blinding of outcome assessors
Low risk of bias: any of the following: no blinding of outcome assessment, but the review authors judged that the outcome measurement was not likely to be influenced by lack of blinding; or blinding of outcome assessment ensured, and unlikely that the blinding could have been broken.
Unclear risk of bias: any of the following: insufficient information to permit judgement of 'low risk' or 'high risk'; or the trial did not address this outcome.
High risk of bias: any of the following: no blinding of outcome assessment, and the outcome measurement was likely to be influenced by lack of blinding; or blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement was likely to be influenced by lack of blinding.
Incomplete outcome data
Low risk of bias: missing data were unlikely to make treatment effects depart from plausible values. The study used sufficient methods, such as multiple imputation, to handle missing data.
Unclear risk of bias: there was insufficient information to assess whether missing data in combination with the method used to handle missing data were likely to induce bias on the results.
High risk of bias: the results were likely to be biased due to missing data.
Selective outcome reporting
Low risk of bias: the trial reported the following predefined outcomes: at least medium‐term or long‐term mortality and treatment‐related adverse events. If the original trial protocol was available, the outcomes should have been those called for in that protocol. If the trial protocol was obtained from a trial registry (e.g. www.clinicaltrials.gov), the outcomes sought should have been those enumerated in the original protocol if the trial protocol was registered before or at the time that the trial was begun. If the trial protocol was registered after the trial was begun, those outcomes were not considered to be reliable.
Unclear risk of bias: not all predefined, or clinically relevant and reasonably expected, outcomes were reported fully, or it was unclear whether data on these outcomes were recorded or not.
High risk of bias: one or more predefined or clinically relevant and reasonably expected outcomes were not reported, despite the fact that data on these outcomes should have been available and even recorded.
For‐profit bias
Low risk of bias: the trial appeared to be free of industry sponsorship or other type of for‐profit support that could manipulate the trial design, conductance, or results of the trial.
Unclear risk of bias: the trial may or may not be free of for‐profit bias as no information on clinical trial support or sponsorship was provided.
High risk of bias: the trial was sponsored by industry or received other type of for‐profit support.
Other bias
Low risk of bias: the trial appeared to be free of other components (e.g. inappropriate control or dose or administration of control) that could put it at risk of bias.
Unclear risk of bias: the trial may or may not be free of other components that could put it at risk of bias.
High risk of bias: there were other factors in the trial that could put it at risk of bias (e.g. inappropriate control or dose or administration of control).
We considered a trial to be at low risk of bias if we assessed the trial as at low risk of bias across all domains. Otherwise, we considered trials at unclear risk of bias or at high risk of bias regarding one or more domains as at high risk of bias. Since blinding of healthcare providers is impossible for all the comparisons and blinding of the participants is unlikely for comparisons involving surgery, we planned to assess the potential influence of lack of blinding on the outcomes carefully. We planned to classify the trials to be at high risk of bias for all the outcomes other than mortality because of the potential influence of lack of blinding on the other outcomes.
Measures of treatment effect
For dichotomous variables (e.g. short‐term mortality, medium‐term mortality, and proportion of participants with adverse events), we planned to calculate the odds ratio with 95% confidence intervals (CI). For continuous variables (e.g. hospital stay and quality of life reported on the same scale), we planned to calculate the mean difference with 95% CI. We planned to use standardised mean difference values with 95% CI for quality of life if included trials used different scales. For count outcomes (e.g. number of adverse events), we planned to calculate the rate ratio with 95% CI. For time‐to‐event data (e.g. mortality at maximal follow‐up), we calculated hazard ratio (HR) with 95% CI.
Unit of analysis issues
The unit of analysis was the person with intermediate‐stage hepatocellular carcinoma according to the intervention group to which they were randomly assigned.
Cluster randomised clinical trials
We found no cluster randomised clinical trials. However, if we found them, we planned to include them provided that the effect estimate adjusted for cluster correlation was available.
Cross‐over randomised clinical trials
We found no cross‐over randomised clinical trials. If we identified any, we planned to only include the outcome results after the period of first intervention since the first intervention may have a permanent impact on the outcome (i.e. have a residual effect).
Trials with multiple treatment groups
We planned to collect data for all trial treatment groups that met the inclusion criteria.
Dealing with missing data
We performed an intention‐to‐treat analysis whenever possible (Newell 1992). Otherwise, we used the data available to us (e.g. a trial may have reported only per‐protocol analysis results). As such 'per‐protocol' analyses may be biased, we planned to conduct best‐worst case scenario analyses (good outcome in intervention group and bad outcome in control group) and worst‐best case scenario analyses (bad outcome in intervention group and good outcome in control group) as sensitivity analyses whenever possible.
For continuous outcomes, we planned to impute the standard deviation from P values according to guidance given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). If the data were likely to be normally distributed, we planned to use the median for meta‐analysis when the mean was not available. If it was not possible to calculate the standard deviation from the P value or the confidence intervals, we planned to impute the standard deviation using the largest standard deviation in other trials for that outcome. This form of imputation may decrease the weight of the study for calculation of mean differences and may bias the effect estimate to no effect for calculation of standardised mean differences (Higgins 2011).
Assessment of heterogeneity
We planned to assess clinical and methodological heterogeneity by carefully examining the characteristics and design of included trials. We planned to assess the presence of clinical heterogeneity by comparing effect estimates in people with and without cirrhosis, presence or absence of portal hypertension, aetiology of hepatocellular carcinoma, and adjuvant treatment with immunotherapy. Different study designs and risk of bias may contribute to methodological heterogeneity.
We used the I2 test and Chi2 test for heterogeneity, and overlapping of CIs to assess heterogeneity. If we identified substantial heterogeneity, clinical, methodological, or statistical, we explored and address heterogeneity in a subgroup analysis (see Subgroup analysis and investigation of heterogeneity).
Assessment of reporting biases
We planned to use visual asymmetry on a funnel plot to explore reporting bias in the presence of at least 10 trials that could be included for a direct comparison (Egger 1997; Macaskill 2001). In the presence of heterogeneity that could be explained by subgroup analysis, we planned to produce a funnel plot for each subgroup in the presence of the adequate number of trials. We planned to use the linear regression approach described by Egger 1997 to determine funnel plot asymmetry.
We also considered selective reporting as evidence of reporting bias.
Data synthesis
We performed the meta‐analyses according to the recommendations of The Cochrane Collaboration (Higgins 2011), using the software package Review Manager 5 (RevMan 2014). We used a random‐effects model (DerSimonian 1986) and a fixed‐effect model (DeMets 1987). In the case of a discrepancy between the two models, we planned to report both results. However, since there was no discrepancy, we have reported only the results from the fixed‐effect model.
Calculation of required information size and Trial Sequential Analysis
For calculation of the required information size, see Appendix 5. We performed Trial Sequential Analysis to control the risks of random errors (Wetterslev 2008; Thorlund 2011; TSA 2011) when there were at least two trials included in the outcome. We used an alpha error as per guidance of Jakobsen 2014, power of 90% (beta error of 10%), a relative risk reduction of 20%, a control group proportion observed in the trials, and the heterogeneity observed in the meta‐analysis. Since the only outcome was mortality at maximal follow‐up, a time‐to‐event outcome, we performed the Trial Sequential Analysis using Stata/SE 14.2 using methods suggested by Miladinovic 2013.
Subgroup analysis and investigation of heterogeneity
We planned to assess the differences in the effect estimates between the following subgroups.
Trials at low risk of bias compared to trials at high risk of bias.
People with and without cirrhosis.
Presence compared to absence of portal hypertension.
Viral compared to non‐viral aetiology.
Use of immunotherapy or antiviral therapy or other treatments as adjuvant therapy compared to no use.
We planned to use the chi2 test for subgroup differences to identify subgroup differences.
Sensitivity analysis
If a trial reported only per‐protocol analysis results, we planned to reanalyse the results using the best‐worst case scenario and worst‐best case scenario analyses as sensitivity analyses whenever possible. In addition, we planned to exclude trials in which liver resection or liver transplantation were combined with ablation, TAE, or TACE.
Presentation of results and GRADE assessments
If trials reported on all our predefined outcomes, we planned to report all of them in a 'Summary of findings' table format, downgrading the quality of evidence for risk of bias, inconsistency, indirectness, imprecision, and publication bias using GRADE (Guyatt 2011).
Results
Description of studies
Results of the search
We identified 4048 references through electronic searches of CENTRAL (N = 264), MEDLINE (N = 1723), Embase (N = 451), Science Citation Index Expanded (N = 1443), World Health Organization International Clinical Trials Registry Platform (N = 137), and ClinicalTrials.gov (N = 30). After the removal of 680 duplicates, we obtained 3368 references. We then excluded 3294 clearly irrelevant references through screening titles and reading abstracts. We retrieved 74 references for further assessment. No references were identified through scanning reference lists of the identified randomised trials. We excluded 63 references (62 studies) for the reasons listed in the Characteristics of excluded studies table; three references (two trials) were ongoing trials with no interim data (Seinstra 2012; NCT02854839; Characteristics of ongoing studies table). In total, eight references (three trials) met the inclusion criteria (Bruix 2012; de Stefano 2015; Lencioni 2016). The study flow diagram summarises the reference flow (Figure 1).
1.
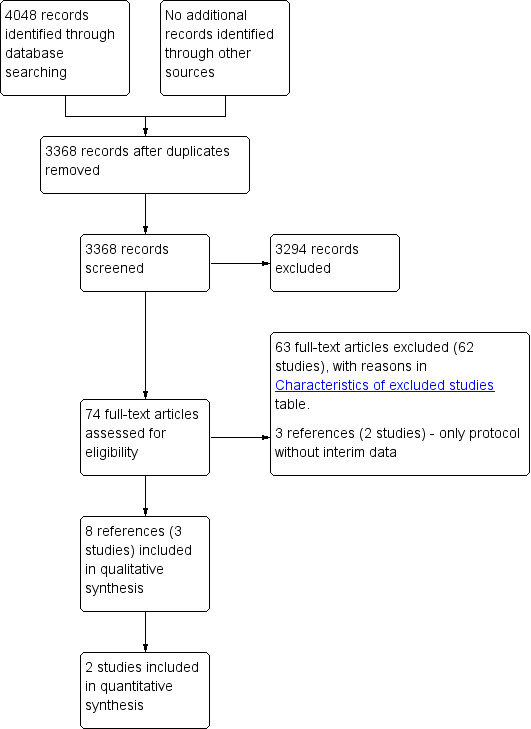
Study flow diagram.
Included studies
We included three trials with 430 participants in this review (Bruix 2012; de Stefano 2015; Lencioni 2016). Two of the trials (412 participants) provided data for only one outcome of this review, that is, mortality (Bruix 2012; Lencioni 2016). All trials included supportive care as a cointervention. The comparisons included in the trials were:
systemic chemotherapy with sorafenib versus no active intervention (one trial with 105 participants; Bruix 2012);
radiofrequency ablation plus systemic chemotherapy with sorafenib versus radiofrequency ablation (one trial with 18 participants; de Stefano 2015 ‐ an ongoing trial and the interim report did not contain any outcomes of interest);
transarterial chemoembolisation plus systemic chemotherapy with sorafenib versus transarterial chemoembolisation (one trial with 307 participants; Lencioni 2016).
The mean age in the trials that reported this information was 64 years (Lencioni 2016) and 69 years (Bruix 2012). The proportion of females in the trials that reported this information was 15% (46/307 participants) (Lencioni 2016) and 15.2% (16/105 participants) (Bruix 2012). The proportion of participants with cirrhosis in the trial that reported this information was 87.9% (270/307 participants) (Lencioni 2016). None of the trials reported whether participants had portal hypertension. The proportion of participants with viral aetiology in the trials that reported this information was 37.1% (39/105 participants) (Bruix 2012) and 64.2% (197/307 participants) (Lencioni 2016). None of the trials reported use of immunotherapy or antiviral therapies as adjuvant therapy.
Follow‐up period: the three trials did not report the duration of follow‐up. However, the Kaplan‐Meier curves from the two trials that provided data for this review suggested that participants were followed up for a period of about 18 to 30 months (Bruix 2012; Lencioni 2016).
Source of funding: two trials were funded by the pharmaceutical industry (Bruix 2012; de Stefano 2015); one trial did not report the source of funding (Lencioni 2016).
Excluded studies
We excluded 63 references for the reasons given in the Characteristics of excluded studies table.
Risk of bias in included studies
The risk of bias is summarised in Figure 2 and Figure 3. None of the trials were at low risk of bias for all domains; hence, all trials were at high risk of bias.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
One trial reported the sequence generation adequately and was at low risk of bias in this domain (Bruix 2012). The remaining trials were at unclear risk of bias in this domain (de Stefano 2015; Lencioni 2016). One trial reported allocation concealment adequately, and was at low risk of bias in this domain (Bruix 2012). The remaining trials were at unclear risk of bias in this domain (de Stefano 2015; Lencioni 2016). Overall, one trial was at low risk of selection bias (Bruix 2012); the remaining trials were at unclear risk of bias (de Stefano 2015; Lencioni 2016).
Blinding
Two trials achieved blinding of participants, healthcare providers, and outcome assessors (Bruix 2012; Lencioni 2016); these two trials were at low risk of performance and detection biases. In one trial, there was no blinding of participants, healthcare providers, or outcome assessors (de Stefano 2015); this trial was at high risk of performance and detection biases.
Incomplete outcome data
There were no post‐randomisation dropouts in two trials (Bruix 2012; Lencioni 2016); these two trials were at low risk of attrition bias. The remaining trial did not report whether there were any post‐randomisation dropouts (de Stefano 2015); this trial was at unclear risk of attrition bias.
Selective reporting
Published protocols of the trials were not available for any of the trials. The trials did not report either mortality or adverse events, or both; therefore, all three trials were at high risk of reporting bias (Bruix 2012; de Stefano 2015; Lencioni 2016).
Other potential sources of bias
Two trials were funded by the pharmaceutical industry and were at high risk of 'for‐profit bias' (Bruix 2012; de Stefano 2015). One trial did not report the source of funding and was at unclear risk of 'for‐profit bias' (Lencioni 2016). There was no other bias in any of the trials.
Effects of interventions
See: Table 1
Mortality (at maximal follow‐up)
Two trials (412 participants) reported mortality at maximal follow‐up (Bruix 2012; Lencioni 2016). Mortality was 50% to 75% over a median follow‐up period of 12 to 24 months in the two trials (Bruix 2012; Lencioni 2016). We performed a meta‐analysis assuming that the presence or absence of transarterial embolisation did not influence the effect of systemic chemotherapy. There was no evidence of difference in mortality at maximal follow‐up between systemic chemotherapy versus no chemotherapy (hazard ratio 0.85, 95% CI 0.60 to 1.18; participants = 412; studies = 2; I2 = 0; very low quality evidence; Analysis 1.1). The interpretation of results did not change by using a random‐effects model. A subgroup analysis stratified by the presence or absence of transarterial chemoembolisation as cointervention did not alter the results.
1.1. Analysis.

Comparison 1: Interventions for hepatocellular carcinoma, Outcome 1: Mortality at maximal follow‐up
Trial Sequential Analysis: based on an alpha error of 5%, power of 90% (beta error of 10%), a relative risk reduction of 20%, the control group proportion of 50%, and observed heterogeneity of 0%, the required information size was 2011. As shown in Figure 4, the z‐curve (blue line) did not cross any of the boundaries indicating a high risk of random error.
4.
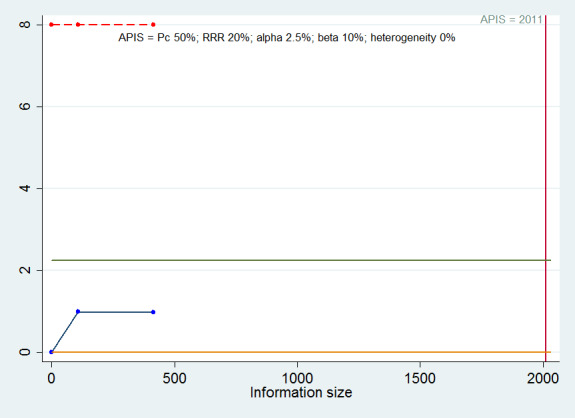
Trial Sequential Analysis of mortality at maximal follow‐up: based on an alpha error of 2.5%, power of 90% (beta error of 10%), a relative risk reduction (RRR) of 20%, the control group proportion (Pc) of 50%, and observed heterogeneity (0%), the a priori information size (APIS) was 2011. As shown in the figure, the cumulative Z‐curve (blue line) do not cross any of trial sequential monitoring boundaries (red lines). They do not cross the conventional alpha boundary of 2.5% (green line) either.
Short‐term mortality (up to one year)
None of the trials reported proportion of people dead in the short‐term (up to one year).
Medium‐term mortality (one to five years)
None of the trials reported proportion of people dead in the medium‐term (one to five years).
Serious adverse events
None of the trials reported serious adverse events.
Adverse events
None of the trials reported the proportion or number of adverse events.
Quality of life
None of the trials reported quality of life at any time point.
Disease recurrence
None of the trials reported recurrence of hepatocellular carcinoma.
Length of hospital stay
None of the trials reported length of hospital stay.
Reporting bias
We did not explore reporting bias because of the sparse data.
Subgroup analysis
We did not perform any subgroup analyses other than stratification by presence or absence of transarterial chemoembolisation as a cointervention because of the sparse data.
Sensitivity analysis
Since none of the trials reported any binary outcomes, we did not perform any sensitivity analyses. Since none of the trials reported length of hospital stay, the issue of imputing standard deviation did not arise.
Quality of evidence
The overall quality of the evidence was very low for the reported outcome of mortality. All the trials were at high risk of bias (resulting in downgrading it one level). There was no evidence of inconsistency in the only outcome. There was no issue of indirectness, since the outcome reported was a clinical outcome and only direct comparisons were used. The sample size was small (downgraded by one level) and the confidence intervals overlapped no effect and clinically significant effect (downgraded one level). We did not explore publication bias because of the too few trials included in this review (Table 1).
Discussion
Summary of main results
We included two trials (412 participants) in one outcome for this review (Bruix 2012; Lencioni 2016). Because of the sparse data, we did not perform a network meta‐analysis (as there were no comparisons in which it was possible to obtain direct and indirect estimates, which would have allowed the assessment of inconsistency) and we used Frequentist methods for performing the direct comparisons. Of the two trials which provided data for this review, one trial compared active interventions (systemic chemotherapy; Bruix 2012) in addition to supportive treatments versus supportive treatments only. One trial compared two active interventions (transarterial chemoembolisation with and without systemic chemotherapy; Lencioni 2016). The only outcome reported in the trials was mortality at maximal follow‐up. The trials did not report the mean or median follow‐up, but it appeared that the participants were followed up for 18 to 30 months (Bruix 2012; Lencioni 2016). Thus, even the mortality at maximal follow‐up appeared to refer to medium‐term mortality only. There was no evidence of differences in mortality between the groups in any of the comparisons. None of the trials reported the proportion of people with serious adverse events or number of serious adverse events, adverse events (proportion), adverse events (number), quality of life, disease recurrence, or length of hospital stay. In one trial, more than 70% of people died during the follow‐up period (Bruix 2012); in another trial, approximately 50% of people died during the follow‐up period (Lencioni 2016). Therefore, the follow‐up period appears to be sufficiently long to detect any survival benefits of the active intervention. However, the sample size was small in all the comparisons and significant benefits or harms of intervention could not be ruled out.
Overall completeness and applicability of evidence
This review included only trial participants with intermediate‐stage hepatocellular carcinoma (i.e. BCLC B stage; i.e. large, multi‐nodular, Child‐Pugh status A to B, and performance status 0). Therefore, this review is applicable only to people with intermediate‐stage hepatocellular carcinoma. It included a mixture of viral and non‐viral aetiologies and people with cirrhotic and non‐cirrhotic livers. Hence, the review is applicable to viral or non‐viral aetiologies and people with cirrhotic and non‐cirrhotic livers. None of the trials reported the proportion of people with portal hypertension. Therefore, it is not clear whether the findings of the review are applicable in people with portal hypertension.
Quality of the evidence
The overall quality of the evidence was very low. All the trials were at high risk of bias resulting in downgrading by one level. Since there was only one trial included under each comparison, it was not possible to assess inconsistency. There was no issue of indirectness, since mortality at maximal follow‐up is a clinical outcome and only direct comparisons were used. The sample size was small (all comparisons downgraded by one level) and the confidence intervals overlapped no effect and clinically significant effect for all comparisons (downgraded by one level). Within‐study risk of bias and imprecision were the major reasons for downgrading the quality of evidence. We did not explore publication bias because of the too few trials included in this review.
Potential biases in the review process
The strengths of our review process are that we selected a range of databases without any language restrictions. Three review authors independently selected the trials and extracted the data, minimising the errors. We conducted the systematic review according to the guidance provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We included only randomised clinical trials as they provide the best estimates of intervention effect.
The quality of evidence was very low. This is mainly because of the sparse data. This is the major limitation of this review. The BCLC classification is widely used in the Western hemisphere. However, a number of the excluded studies predated BCLC criteria. Furthermore, BCLC is not used in the Eastern hemisphere, where a number of the excluded trials originated from. As a result, the majority of studies were excluded on the basis of not meeting BCLC B criteria. Using an alternative classification for people with the hepatocellular carcinoma, such as the CLIP or Okuda classifications (Okuda 1985; CLIP 1998), might have resulted in the inclusion of more trials in the analysis. However, this would not have met our objectives.
We only included randomised clinical trials which are known to focus mostly on benefits and do not collect and report harms in a detailed manner. According to our choice of studies (i.e. only randomised clinical trials), we might have missed a large number of studies that address reporting of harms. Accordingly, this review is biased towards benefits ignoring harms. We did not search for interventions and trials registered at regulatory authorities (e.g., FDA (US Food and Drug Administration); EMA (European Medicines Agency), etc). This may have overlooked trials and as such trials usually are unpublished, the lack of inclusion of such trials may make our comparisons look more advantageous than they really are.
Agreements and disagreements with other studies or reviews
This is the first systematic review on this topic in people with intermediate‐stage hepatocellular carcinoma. We agreed with Lencioni 2016 that systemic therapy with sorafenib in addition to transarterial chemoembolisation did not offer any clinical benefit. We disagreed with Bruix 2012 that systemic chemotherapy with sorafenib in addition to supportive treatment is beneficial in people with intermediate‐stage hepatocellular carcinoma. While there has been no systematic review of the effect of sorafenib in people with intermediate‐stage hepatocellular carcinoma, one network meta‐analysis on advanced hepatocellular carcinoma showed that sorafenib may have survival benefit (Niu 2016).
We found no evidence from randomised clinical trials to support the American Association for the Study of Liver Diseases recommend TACE for people with intermediate‐stage hepatocellular carcinoma (Bruix 2011; EASL 2012). The possible reason for disagreements is that we have used evidence from randomised clinical trials only, which are generally considered the best quality of evidence; non‐randomised studies are likely to provide a biased estimate of the effects in this situation as TACE is generally performed only when there is sufficient remnant liver volume and when there is no vascular spread (Lencioni 2013), and palliative treatment alone is considered appropriate for people with insufficient remnant liver volume or when there is vascular spread. Any differences in survival or quality of life could be due to the extent of disease rather than the intervention itself. Therefore, only evidence from randomised clinical trials can provide a reasonable estimate of the effects of TACE compared to symptomatic treatment only.
Authors' conclusions
Implications for practice.
Currently, there is no evidence from randomised clinical trials that people with intermediate‐stage hepatocellular carcinoma would benefit from systemic chemotherapy with sorafenib either alone or with transarterial chemoembolisation as a cointervention (very low quality evidence).
Implications for research.
We need high‐quality randomised clinical trials designed to measure clinically important differences (e.g. all‐cause mortality or health‐related quality of life) and following the SPIRIT (Standard Protocol Items: Recommendations for Interventional Trials; Chan 2013b) and CONSORT guidelines (Schulz 2010). Future trials on hepatocellular carcinoma should report the outcomes separately by stage of hepatocellular carcinoma, so that it is possible to determine whether interventions are beneficial in people with intermediate‐stage hepatocellular carcinoma.
What's new
| Date | Event | Description |
|---|---|---|
| 15 June 2020 | Amended | A typo in the word 'carcinoma', used as free text in the Search strategy, was spotted. There are no differences in the number of references retrieved when the typos are corrected because of the nature of the error (i.e. the term adds nothing to existing terms). |
History
Protocol first published: Issue 4, 2015 Review first published: Issue 3, 2017
| Date | Event | Description |
|---|---|---|
| 12 April 2017 | Amended | The Cochrane Central Editorial Unit requested removal of the 'attempted network meta‐analysis' phrase from the end of the review title, as this further description of the review might create confusion in the reader. Although we followed the planned methodology for network meta‐analysis, we found only one comparison. Therefore, we did not perform the network meta‐analysis, and we assessed the comparative benefits and harms of different interventions versus each other, or versus placebo, sham, or no intervention (supportive treatment only) using standard Cochrane methodology. |
Notes
Considerable overlap is evident in the 'Methods' sections of this review and those of several other reviews written by the same group of authors.
Acknowledgements
We thank the Cochrane Comparing of Multiple Interventions Methods Group and the Cochrane Hepato‐Biliary for their support and advice.
Cochrane Review Group funding acknowledgement: the Danish State is the largest single funder of The Cochrane Hepato‐Biliary Group through its investment in The Copenhagen Trial Unit, Centre for Clinical Intervention Research, Rigshospitalet, Copenhagen University Hospital, Denmark.
Disclaimer: the views and opinions expressed in this review are those of the review authors and do not necessarily reflect those of the Danish State or The Copenhagen Trial Unit.
Peer reviewers of review: Chet Hamill, USA; Paul Kwo, USA; Robert J Lewandowski, USA. Contact editor: Christian Gluud, Denmark. Sign‐off editor: Christian Gluud, Denmark.
Appendices
Appendix 1. Barcelona Clinic Liver Cancer (BCLC) staging classification
Stage 0: very early‐stage hepatocellular carcinoma (single tumour less than 2 cm). Stage A: early‐stage hepatocellular carcinoma (single tumour or three tumours less than 3 cm in maximum diameter). Stage B: intermediate‐stage hepatocellular carcinoma (large multiple tumours). Stage C: advanced‐stage hepatocellular carcinoma (vascular invasion or extrahepatic spread or restriction in activities). Stage D: end‐stage hepatocellular carcinoma (poor performance status or Child‐Pugh C liver functional status (based on bilirubin levels, albumin levels, prothrombin time or international normalised ratio (INR), presence of ascites, and presence hepatic encephalopathy).
Simplified from sources: Llovet 1999; Llovet 2003.
Appendix 2. Milan criteria
1. Single lesion less than 5 cm in diameter. 2. Two or three lesions less than 3 cm in maximum diameter. 3. No preoperative evidence or suspicion of invasion of blood vessels or lymph nodes by tumour. 4. No preoperative evidence of extrahepatic metastases.
People meet the Milan criteria if they meet either criteria numbers 1, 3, and 4 or criteria numbers 2, 3, and 4.
Simplified from source: Mazzaferro 1996.
Appendix 3. Methods for network meta‐analysis if we find this is possible in the future
Measures of treatment effect
Relative treatment effects
For dichotomous variables (e.g. proportion of participants with serious adverse events or any adverse events), we will calculate the odds ratio with 95% credible interval (or Bayesian confidence interval) (Severini 1993). For continuous variables (e.g. quality of life reported on the same scale), we will calculate the mean difference with 95% credible interval. We will use standardised mean difference values with 95% credible interval for quality of life if included trials use different scales. For count outcomes (e.g. number of adverse events and serious adverse events), we will calculate the rate ratio with 95% credible interval. For time‐to‐event data (e.g. mortality at maximal follow‐up), we will calculate hazard ratio with 95% credible interval.
Relative ranking
We will estimate the ranking probabilities for all treatments of being at each possible rank for each intervention. Then, we will obtain the surface under the cumulative ranking curve (SUCRA) (cumulative probability) and rankogram (Salanti 2011; Chaimani 2013).
Unit of analysis issues
We will collect data for all trial treatment groups that meet the inclusion criteria. The codes for analysis, that we will use, accounts for the correlation between the effect sizes from from trials with more than two groups.
Assessment of heterogeneity
We will assess clinical and methodological heterogeneity by carefully examining the characteristics and design of included trials. We will assess the presence of clinical heterogeneity by comparing effect estimates under different categories of potential effect modifiers. Different study designs and risk of bias may contribute to methodological heterogeneity.
We will assess the statistical heterogeneity by comparing the results of the fixed‐effect model meta‐analysis and the random‐effects model meta‐analysis, between‐study standard deviation (tau2 and comparing this with values reported in the study of the distribution of between‐study heterogeneity (Turner 2012)), and by calculating I2 (using Stata/SE 14.2). If we identify substantial heterogeneity, clinical, methodological, or statistical, we will explore and address heterogeneity in a subgroup analysis (see ‘Subgroup analysis and investigation of heterogeneity for network meta‐analysis’ section).
Assessment of transitivity across treatment comparisons
We will evaluate the plausibility of transitivity assumption (the assumption that the participants included in the different studies with different immunosuppressive regimens can be considered to be a part of a multi‐arm randomised clinical trial and could potentially have been randomised to any of the treatments) (Salanti 2012). In other words, any participant that meets the inclusion criteria is, in principle, equally likely to be randomised to any of the above eligible interventions. If there is any concern that the clinical safety and effectiveness are dependent upon the effect modifiers, we will continue to do traditional Cochrane pair‐wise comparisons and we will not perform a network meta‐analysis on all participant subgroups.
Assessment of reporting biases
For the network meta‐analysis, we will judge the reporting bias by the completeness of the search (i.e. searching various databases and including conference abstracts), as we do not currently find any meaningful order to perform a comparison‐adjusted funnel plot as suggested by Chaimani 2012. However, if we find any meaningful order, for example, the control group used depended upon the year of conduct of the trial, we will use comparison‐adjusted funnel plot as suggested by Chaimani 2012.
Data synthesis
Methods for indirect and mixed comparisons
We will conduct network meta‐analyses to compare multiple interventions simultaneously for each of the primary and secondary outcomes. Network meta‐analysis combines direct evidence within trials and indirect evidence across trials (Mills 2012). We will obtain a network plot to ensure that the trials were connected by treatments using Stata/SE 14.2 (Chaimani 2013). We will exclude any trials that were not connected to the network. We will conduct a Bayesian network meta‐analysis using the Markov chain Monte Carlo method in OpenBUGS 3.2.3 as per the guidance from the National Institute for Health and Care Excellence (NICE) Decision Support Unit (DSU) documents (Dias 2014a). We will model the treatment contrast (i.e. log odds ratio for binary outcomes, mean difference or standardised mean difference for continuous outcomes, log rate ratio for count outcomes, and log hazard ratio for time‐to‐event outcomes) for any two interventions ('functional parameters') as a function of comparisons between each individual intervention and an arbitrarily selected reference group ('basic parameters') (Lu 2006) using appropriate likelihood functions and links. We will use binomial likelihood and logit link for binary outcomes, Poisson likelihood and log link for count outcomes, binomial likelihood and complementary log‐log link for time‐to‐event outcomes, and normal likelihood and identity link for continuous outcomes. We will perform a fixed‐effect model and random‐effects model for the network meta‐analysis. We will report both models for comparison with the reference group in a forest plot. For pairwise comparison, we will report the fixed‐effect model if the two models reported similar results; otherwise, we will report the more conservative model.
We will use a hierarchical Bayesian model using three different initial values using codes provided by NICE DSU (Dias 2014a). We will use a normal distribution with large variance (10,000) for treatment effect priors (vague or flat priors). For the random‐effects model, we will use a prior distributed uniformly (limits: 0 to 5) for between‐trial standard deviation but assumed similar between‐trial standard deviation across treatment comparisons (Dias 2014a). We will use a 'burn‐in' of 5000 simulations, check for convergence visually, and run the models for another 10,000 simulations to obtain effect estimates. If we did not obtain convergence, we will increase the number of simulations for 'burn‐in'. If we do not obtain convergence still, we will use alternate initial values and priors using methods suggested by van Valkenhoef 2012. We will also estimate the probability that each intervention ranks at one of the possible positions using the NICE DSU codes (Dias 2014a).
Assessment of inconsistency
We will assess inconsistency (statistical evidence of the violation of transitivity assumption) by fitting both an inconsistency model and a consistency model. We will use the inconsistency models used in the NICE DSU manual, as we plan to use a common between‐study deviation for the comparisons (Dias 2014b). In addition, we will use the design‐by‐treatment full interaction model (Higgins 2012) and IF (inconsistency factor) plots (Chaimani 2013) to assess inconsistency. In the presence of inconsistency, we will assess whether the inconsistency is because of clinical or methodological heterogeneity by performing separate analyses for each of the different subgroups mentioned in the ‘Subgroup analysis and investigation of heterogeneity for network meta‐analysis’ section below.
If there is evidence of inconsistency, we will identify areas in the network where substantial inconsistency might be present in terms of clinical and methodological diversities between trials and, when appropriate, limit network meta‐analysis to a more compatible subset of trials.
Direct comparison
We will perform the direct comparisons using the same codes and the same technical details.
Sample size calculations
To control for the risk of random errors, we will interpret the information with caution when the accrued sample size in the network meta‐analysis (i.e. across all treatment comparisons) was less than the required sample size (required information size). For calculation of the required information size, see Appendix 5.
Subgroup analysis and investigation of heterogeneity for network meta‐analysis
We will assess the differences in the effect estimates between the subgroups listed in Subgroup analysis and investigation of heterogeneity using meta‐regression with the help of the OpenBUGS code (Dias 2012a) if we include a sufficient number of trials. We will use the potential modifiers as study level co‐variates for meta‐regression. We will calculate a single common interaction term (Dias 2012a). If the 95% credible intervals of the interaction term do not overlap zero, we will consider this as evidence of difference in subgroups.
Presentation of results
We will present the effect estimates with 95% CrI for each pairwise comparisons calculated from the direct comparisons and network meta‐analysis. We will also present the cumulative probability of the treatment ranks (i.e. the probability that the treatment is within the top two, the probability that the treatment is within the top three, etc.) in graphs (surface under the cumulative ranking curve or SUCRA) (Salanti 2011). We will also plot the probability that each treatment is best, second best, third best etc for each of the different outcomes (rankograms), which are generally considered more informative (Salanti 2011; Dias 2012b).
We will present the 'Summary of findings' tables for mortality. In the 'Table 1', we will follow the approach suggested by Puhan et al. (Puhan 2014). First, we will calculate the direct and indirect effect estimates and 95% credible intervals using the node‐splitting approach (Dias 2010), i.e. calculate the direct estimate for each comparison by including only trials in which there was direct comparison of treatments and the indirect estimate for each comparison by excluding the trials in which there was direct comparison of treatments. Then we will rate the quality of direct and indirect effect estimates using GRADE which takes into account the risk of bias, inconsistency, directness of evidence, imprecision, and publication bias (Guyatt 2011). Then, we will present the estimates of the network meta‐analysis and rate the quality of network meta‐analysis effect estimates as the best quality of evidence between the direct and indirect estimates (Puhan 2014). In addition, in the same table, we will present illustrations and information on the number of trials and participants as per the standard 'Summary of Findings' Table.
Appendix 4. Search strategies
| Database | Time span | Search strategy |
| The Central Register of Controlled Trials (CENTRAL) | Issue 8, 2016. | #1 MeSH descriptor: [Carcinoma, Hepatocellular] explode all trees #2 (((hepat* or liver) and carcinoma*) or hepatocellular carcinoma or hepatocarcinoma or hepatoma or HCC or "primary liver cancer") #3 #1 or #2 #4 (intermediate or large or multinodular) #5 #3 and #4 |
| MEDLINE (OvidSP) | January 1947 to September 2016. | 1. exp Carcinoma, Hepatocellular/ 2. (((hepat* or liver) and carcinoma*) or hepatocellular carcinoma or hepatocarcinoma or hepatoma or HCC or "primary liver cancer").ti,ab. 3. 1 or 2 4. (intermediate or large or multinodular).ti,ab. 5. 3 and 4 6. randomized controlled trial.pt. 7. controlled clinical trial.pt. 8. randomized.ab. 9. placebo.ab. 10. drug therapy.fs. 11. randomly.ab. 12. trial.ab. 13. groups.ab. 14. 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 15. exp animals/ not humans.sh. 16. 14 not 15 17. 5 and 16 |
| Embase (OvidSP) | January 1974 to September 2016. | 1. exp liver cell carcinoma/ 2. (((hepat* or liver) and carcinoma*) or hepatocellular carcinoma or hepatocarcinoma or hepatoma or HCC or "primary liver cancer").ti,ab. 3. 1 or 2 4. (intermediate or large or multinodular).ti,ab. 5. 3 and 4 6. exp crossover‐procedure/ or exp double‐blind procedure/ or exp randomized controlled trial/ or single‐blind procedure/ 7. (((((random* or factorial* or crossover* or cross over* or cross‐over* or placebo* or double*) adj blind*) or single*) adj blind*) or assign* or allocat* or volunteer*).af. 8. 6 or 7 9. 5 and 8 |
| Science Citation Index Expanded (Web of Knowledge) | January 1945 to September 2016. | #1 TS=(((hepat* or liver) and carcinoma*) or hepatocellular carcinoma or hepatocarcinoma or hepatoma or HCC or "primary liver cancer") #2 TS=(intermediate or large or multinodular) #3 TS=(random* OR rct* OR crossover OR masked OR blind* OR placebo* OR meta‐analysis OR systematic review* OR meta‐analys*) #4 #1 AND #2 AND #3 |
| World Health Organization International Clinical Trials Registry Platform Search Portal (apps.who.int/trialsearch/Default.aspx) | September 2016. | Title: (intermediate or large or multinodular) Condition: "hepatocellular carcinoma" or "primary liver cancer" or "liver cell cancer" or hepatoma |
| ClinicalTrials.gov | September 2016. | intermediate OR large OR multinodular | Interventional Studies | "hepatocellular carcinoma" OR "primary liver cancer" OR "liver cell cancer" OR hepatoma | Phase 2, 3, 4 |
Appendix 5. Sample size calculation
On average, 75% of people with intermediate‐stage hepatocellular carcinoma are alive at 24 months (Bruix 1998). The required information size based on a control group proportion of 75%, a relative risk reduction of 20% in the intervention group, type I error of 5%, and type II error of 50% is 304 participants. Network analyses are more prone to the risk of random errors than direct comparisons (Del Re 2013). Accordingly, a greater sample size is required in indirect comparisons than direct comparisons (Thorlund 2012). The power and precision in indirect comparisons depends upon various factors, such as the number of participants included under each comparison and the heterogeneity between the trials (Thorlund 2012). If there is no heterogeneity across the trials, the sample size in indirect comparisons would be equivalent to the sample size in direct comparisons. The effective indirect sample size can be calculated using the number of participants included in each direct comparison (Thorlund 2012). For example, a sample size of 2500 participants in the direct comparison A versus C (nAC) and a sample size of 7500 participants in the direct comparison B versus C (nBC) results in an effective indirect sample size of 1876 participants. However, in the presence of heterogeneity within the comparisons, the sample size required is higher. In the above scenario, for an I2 statistic for each of the comparisons A versus C (IAC2) and B versus C (IBC2) of 25%, the effective indirect sample size is 1407 participants. For an I2 statistic for each of the comparisons A versus C and B versus C of 50%, the effective indirect sample size is 938 participants (Thorlund 2012). If there were only three groups and the sample size in the trials is more than the required information size, we planned to calculate the effective indirect sample size using the following generic formula (Thorlund 2012):
((nAC x (1 ‐ IAC2)) x (nBC x (1 ‐ IBC2))/((nAC x (1 ‐ IAC2)) + (nBC x (1 ‐ IBC2)).
There is currently no method to calculate the effective indirect sample size for a network analysis involving more than three intervention groups.
Data and analyses
Comparison 1. Interventions for hepatocellular carcinoma.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Mortality at maximal follow‐up | 2 | 412 | Hazard Ratio (IV, Fixed, 95% CI) | 0.85 [0.60, 1.18] |
| 1.1.1 Chemotherapy versus no chemotherapy | 1 | 105 | Hazard Ratio (IV, Fixed, 95% CI) | 0.72 [0.38, 1.37] |
| 1.1.2 Transarterial chemoembolisation with systemic chemotherapy versus transarterial chemoembolisation without systemic chemotherapy | 1 | 307 | Hazard Ratio (IV, Fixed, 95% CI) | 0.90 [0.61, 1.33] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bruix 2012.
| Study characteristics | ||
| Methods | Randomised clinical trial. | |
| Participants | Country: multi‐centric, international. Number randomised: 105. Post‐randomisation dropouts: 0 (0%). Revised sample size: 105. Mean age: 69 years. Females: 16 (15.2%). Cirrhosis: not stated. Portal hypertension: not stated. Viral aetiology: 39 (37.1%). Immunotherapy/antiviral adjuvant therapy: not stated. Mean follow‐up period (for all groups): not stated. Criteria for intermediate‐stage HCC and other inclusion criteria:
|
|
| Interventions | Participants were randomly assigned to 2 groups. Group 1: sorafenib (n = 54). Further details: chemotherapy: sorafenib 400 mg twice daily until radiological or symptomatic progression. Group 2: placebo (n = 51). |
|
| Outcomes | Mortality. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Study randomization was centralized, and assignment to study groups was conducted by computer to achieve a balance between the two groups". |
| Allocation concealment (selection bias) | Low risk | Quote: "Study randomization was centralized, and assignment to study groups was conducted by computer to achieve a balance between the two groups". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "phase 3, double‐blind, placebo‐controlled trial…All eligible patients were randomly assigned in a 1:1 ratio to receive continuous oral treatment with either 400 mg of sorafenib (consisting of two 200‐mg tablets) twice daily or matching placebo". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "phase 3, double‐blind, placebo‐controlled trial…All eligible patients were randomly assigned in a 1:1 ratio to receive continuous oral treatment with either 400 mg of sorafenib (consisting of two 200‐mg tablets) twice daily or matching placebo". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no post‐randomisation dropouts. |
| Selective reporting (reporting bias) | High risk | Comment: morbidity was not reported. |
| For‐profit bias | High risk | Quote: "The study was designed by Bayer HealthCare Pharmaceuticals in conjunction with the principal academic investigators". |
| Other bias | Low risk | Comment: no other bias noted. |
de Stefano 2015.
| Study characteristics | ||
| Methods | Randomised clinical trial. | |
| Participants | Country: Italy. Number randomised: 18. Post‐randomisation dropouts: not stated. Revised sample size: 18. Mean age: not stated. Females: not stated. Cirrhosis: not stated. Portal hypertension: not stated. Viral aetiology: not stated. Immunotherapy/antiviral adjuvant therapy: not stated. Mean follow‐up period (for all groups): not stated. Criteria for intermediate‐stage HCC and other inclusion criteria:
|
|
| Interventions | Participants were randomly assigned to 2 groups. Group 1: sorafenib (n = not stated). Further details: chemotherapy: sorafenib 400 mg twice daily until radiological or symptomatic progression. Group 2: sorafenib plus RFA (n = not stated). Further details: chemotherapy: sorafenib 400 mg twice daily until radiological or symptomatic progression. RFA: details not stated in abstract. |
|
| Outcomes | None of the outcomes of interest to our review were reported. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Randomization will be performed with a list of a priori sample prepared in sealed envelopes opaque". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "randomized open study". |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "randomized open study". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: neither mortality nor morbidity was not reported. |
| For‐profit bias | High risk | Quote: "Financial support Bayer HealthCare, Onyx Pharmaceuticals and Biocompatibles UK, Ltd". |
| Other bias | Low risk | Comment: no other bias noted. |
Lencioni 2016.
| Study characteristics | ||
| Methods | Randomised clinical trial. | |
| Participants | Country: multi‐centric, international. Number randomised: 307. Post‐randomisation dropouts: not stated. Revised sample size: 307. Mean age: 64 years. Females: 46 (15%). Cirrhosis: 270 (87.9%). Portal hypertension: not stated. Viral aetiology: 197 (64.2%). Immunotherapy/antiviral adjuvant therapy: not stated. Average follow‐up period in months (for all groups): not stated. Criteria for intermediate‐stage HCC and other inclusion criteria:
|
|
| Interventions | Participants were randomly assigned to 2 groups. Group 1: TACE plus sorafenib (n = 154). Further details: TACE: drug‐eluting beads containing doxorubicin 150 mg ‐ multiple cycles ‐ duration not stated. Chemotherapy: sorafenib 400 mg twice daily in 4 week cycles ‐ duration not stated. Group 2: TACE plus placebo (n = 153). Further details: TACE: drug‐eluting beads containing doxorubicin 150 mg ‐ multiple cycles ‐ duration not stated. |
|
| Outcomes | Mortality. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Randomization will be performed with a list of a priori sample prepared in sealed envelopes opaque". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "phase II randomized, double‐blind, placebo‐controlled study… Patients were randomized 1:1 to DEB‐TACE [doxorubicin‐eluting bead transarterial chemoembolization] (300‐500 µm beads; 150 mg doxorubicin) plus sorafenib (400 mg twice daily, continuously) or matching placebo". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "phase II randomized, double‐blind, placebo‐controlled study… Patients were randomized 1:1 to DEB‐TACE (300‐500 µm beads; 150 mg doxorubicin) plus sorafenib (400 mg twice daily, continuously) or matching placebo". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no post‐randomisation dropouts. |
| Selective reporting (reporting bias) | High risk | Comment: morbidity was not reported. |
| For‐profit bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other bias noted. |
BCLC: Barcelona Clinic Liver Cancer; HCC: hepatocellular carcinoma; RFA: radiofrequency ablation; TACE: transarterial chemoembolisation.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abdelaziz 2015 | Not intermediate‐stage HCC (people with performance status 0 or 1). |
| Anonymous 1995 | Not intermediate‐stage HCC (people with single tumour with BCLC A). |
| Arai 2010 | Not intermediate‐stage HCC (insufficient information in abstract to confirm BCLC stage). |
| Bartolozzi 1995 | Variations in transarterial chemoembolisation. |
| Becker 2005 | Not intermediate‐stage HCC (people with portal vein thrombosis and Okuda stage III). |
| Bruix 1998 | Not intermediate‐stage HCC (people with single tumour and performance status > 1). |
| Chen 2002 | Variations in transarterial chemoembolisation. |
| Chen 2007 | Not a randomised clinical trial. |
| Cheng 2004 | Not intermediate‐stage HCC (people with single tumour with BCLC A). |
| Cheng 2008 | Not intermediate‐stage HCC (people with single tumour with BCLC A. Retracted in DeAngelis 2009). |
| Choi 2014 | Not intermediate‐stage HCC (people with BCLC C). |
| Chow 2002 | Not intermediate‐stage HCC (people with performance status 0 to 3 and Child‐Pugh C). |
| DeAngelis 2009 | Retraction of an excluded trial (Cheng 2008). |
| Doffoel 2008 | Not intermediate‐stage HCC (people with portal vein thrombosis and performance status > 2). |
| El‐Kady 2013 | Not intermediate‐stage HCC (people with BCLC A. |
| Ferrari 2004 | Not intermediate‐stage HCC (people with single tumour with BCLC A). |
| Fischman 2014 | Not intermediate‐stage HCC (people with BCLC C). |
| Gallo 2006 | Not intermediate‐stage HCC (people with Child‐Pugh C). |
| Gish 2009 | Not intermediate‐stage HCC (people with performance status 1). |
| Golfieri 2014 | Variations in transarterial chemoembolisation. |
| Hebbar 2015 | Not intermediate‐stage HCC (insufficient information in abstract to confirm BCLC stage). |
| Hilgard 2008 | Not intermediate‐stage HCC (people with performance status > 1). |
| Hou 2009 | Not intermediate‐stage HCC (people with T4 N0 M0 stage, extrahepatic invasion). |
| Huo 2003 | Quasi‐randomised study. |
| Iezzi 2013 | Not a randomised clinical trial. |
| Inaba 2013 | Not intermediate‐stage HCC (people with performance status 0 to 2 and Child Pugh C). |
| Kolligs 2015 | Not intermediate‐stage HCC (people with BCLC C). |
| Kudo 2002 | Not intermediate‐stage HCC (people with BCLC A). |
| Kudo 2014 | Not intermediate‐stage HCC (people with BCLC C/D and performance status 1). |
| Lammer 2010 | Not intermediate‐stage HCC (people with performance status 1). |
| Li 2009 | Not intermediate‐stage HCC (people with single tumour). |
| Li 2010 | Not intermediate‐stage HCC (people with portal vein tumour thrombus). |
| Liu 2010 | Variations in microwave ablation. |
| Livraghi 2005 | Not intermediate‐stage HCC (people with BCLC C). |
| Llovet 2002 | Not intermediate‐stage HCC (people with BCLC C). |
| Llovet 2007 | Not intermediate‐stage HCC (people with performance status 1 or 2). |
| Lo 2002 | Not intermediate‐stage HCC (people with portal vein thrombosis and performance status > 2). |
| Malagari 2010 | Not intermediate‐stage HCC (people with BCLC A). |
| Meyer 2016 | Not intermediate‐stage HCC (people with performance status 1). |
| Mohnike 2013 | Not intermediate‐stage HCC (people with BCLC C). |
| Morimoto 2010 | Not intermediate‐stage HCC (people with single tumours and performance status > 1). |
| Morimoto 2011 | Not intermediate‐stage HCC (people with single tumours and performance status > 1). |
| Okusaka 2012 | Not intermediate‐stage HCC (people with single tumour and performance status 0 to 2). |
| Padia 2013 | Not a randomised clinical trial. |
| Pelletier 1998 | Not intermediate‐stage HCC (people with Okuda III stage and therefore Child‐Pugh C). |
| Sansonno 2012 | Not intermediate‐stage HCC (people with performance status 1). |
| Sarin 1994 | Not intermediate‐stage HCC (people withBCLC A). |
| Shi 2009 | Variations in transarterial chemoembolisation. |
| Tanaka 2014 | Not a randomised clinical trial. |
| Tang 2009 | Not intermediate‐stage HCC (people with portal vein tumour thrombus). |
| Ulbrich 2010a | Not intermediate‐stage HCC (people with BCLC A). |
| Ulbrich 2010b | Not intermediate‐stage HCC (people with BCLC A). |
| Wang 2007 | Not intermediate‐stage HCC (people with BCLC A). |
| Wang 2014 | Not intermediate‐stage HCC (people with BCLC C). |
| Wu 1995 | Not intermediate‐stage HCC (people with BCLC A). |
| Wu 1998 | Not intermediate‐stage HCC (people with Child‐Pugh C). |
| Xie 2015 | Not a randomised clinical trial. |
| Xu 2009 | Not a randomised clinical trial. |
| Yang 2008 | Not intermediate‐stage HCC (people with Child‐Pugh C). |
| Yin 2013 | Not intermediate‐stage HCC (people with BCLC A). |
| Zhang 2011 | Not intermediate‐stage HCC (people with recurrence after liver transplantation). |
| Zhou 2009 | Not intermediate‐stage HCC (people with portal vein thrombosis). |
BCLC: Barcelona Clinic Liver Cancer; HCC: hepatocellular carcinoma.
Characteristics of ongoing studies [ordered by study ID]
NCT02854839.
| Study name | NCT02854839. |
| Methods | Randomised clinical trial. |
| Participants | People with intermediate‐stage hepatocellular carcinoma. |
| Interventions | Intervention: MG4101. Control: placebo. |
| Outcomes | Overall survival, adverse events. |
| Starting date | September 2016. |
| Contact information | Kyung Gue Lee (kglee@greencross.com). |
| Notes |
Seinstra 2012.
| Study name | TRACE. |
| Methods | Randomised clinical trial. |
| Participants | Barcelona Clinic Liver Cancer staging system intermediate stage. |
| Interventions | Transarterial radioembolisation versus transarterial chemoembolisation. |
| Outcomes | Overall survival, adverse events, quality of life, costs, cost effectiveness. |
| Starting date | Not stated, but the publication stated that they have already started including participants. |
| Contact information | Maurice AAJ van den Bosch (mbosch@umcutrecht.nl). |
| Notes |
Differences between protocol and review
There was only one comparison. So we did not perform the network meta‐analysis, and assessed the comparative benefits and harms of different interventions using standard Cochrane methodology. The methodology that we plan to use if we conduct a network meta‐analysis in future is available in Appendix 3.
We performed Trial Sequential Analysis in addition to conventional method of assessing the risk of random errors using P‐value.
Contributions of authors
AM, DR, and KG selected the studies and extracted data. AM completed the characteristics of included and excluded studies tables. KG wrote the review. AM, DR, ET, BD, and DT critically commented on the review. All review authors approved this version before publication.
Sources of support
Internal sources
University College London, UK
External sources
National Institute for Health Research, UK
Declarations of interest
This report is independent research funded by the National Institute for Health Research (NIHR Cochrane Programme Grants, 13/89/03 ‐ Evidence‐based diagnosis and management of upper digestive, hepato‐biliary, and pancreatic disorders). The views expressed in this publication are those of the authors and not necessarily those of the National Health Service (NHS), the NIHR, or the Department of Health.
DR has no financial disclosures. AM has no financial disclosures. DT received funding from Astellas for his attendance at the International Liver Transplantation Society meeting in 2014. He received GBP 25,000 from Boston Scientific to fund a clinical research fellow in 2013. There are no other financial disclosures to report. BD has no financial disclosures. ET has participated in advisory boards for AstraZeneca and ViiV Healthcare. KG has no financial disclosures.
Edited (no change to conclusions)
References
References to studies included in this review
Bruix 2012 {published data only}
- Bruix J, Raoul JL, Sherman M, Mazzaferro V, Bolondi L, Craxi A, et al. Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma: subanalyses of a phase III trial. Journal of hepatology 2012;57(4):821-9. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruix J, Raoul JL, Sherman M, Shan M, Lentini G, Nadel A. Efficacy and safety of sorafenib in patients with hepatocellular carcinoma (HCC): subanalysis of sharp trial based on Barcelona clinic liver cancer (BCLC) stage. Journal of Hepatology 2009;50(Suppl 1):S28. [Google Scholar]
- Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. New England Journal of Medicine 2008;359(4):378-90. [DOI] [PubMed] [Google Scholar]
de Stefano 2015 {published data only}
- Stefano G, Iodice V, Signoriello G, Scognamiglio U, Farella N. Efficacy and safety of combined sequential treatment with RFA and sorafenib in patients with HCC in intermediate stage ineligible for TACE: a prospective randomized open study. Journal of Hepatology 2015;62:S852-3. [Google Scholar]
- Stefano G, Scognamiglio U, Iodice V, Farella N, Simeone E, Montesarchio V. Efficacy and safety of combined sequential treatment with radiofrequency ablation and sorafenib in patients with hepatocellular carcinoma in intermediate stage ineligible for TACE: a prospective randomized open study. Annals of Oncology 2015;26:103-4. [Google Scholar]
Lencioni 2016 {published data only}
- Han G, Lencioni R, Llovet JM, Tak WY, Yang J, Guglielmi A, et al. Sorafenib plus transarterial chemoembolization with DC bead (DEB-TACE) for patients from Asian countries with intermediate-stage HCC: SPACE study results. Hepatology International 2013;7:S616. [Google Scholar]
- Lencioni R, Llovet JM, Han G, Tak WY, Yang J, Guglielmi A, et al. Sorafenib or placebo plus TACE with doxorubicin-eluting beads for intermediate stage HCC: the SPACE trial. Journal of Hepatology 2016;64(5):1090-8. [DOI] [PubMed] [Google Scholar]
- Lencioni R, Zou J, Leberre M, Meinhardt G, Voliotis D, Bruix J, et al. Sorafenib (SOR) or placebo (PL) in combination with transarterial chemoembolization (TACE) for intermediate-stage hepatocellular carcinoma (SPACE). Journal of Clinical Oncology 2010;28(Suppl 15):26. [Google Scholar]
References to studies excluded from this review
Abdelaziz 2015 {published data only}
- Abdelaziz AO, Nabeel MM, Elbaz TM, Shousha HI, Hassan EM, Mahmoud SH, et al. Microwave ablation versus transarterial chemoembolization in large hepatocellular carcinoma: prospective analysis. Scandinavian Journal of Gastroenterology 2015;50(4):479-84. [DOI] [PubMed] [Google Scholar]
Anonymous 1995 {published data only}
- Anonymous. A comparison of lipiodol chemoembolization and conservative treatment for unresectable hepatocellular carcinoma. Groupe d'etude et de traitement du carcinome hepatocellulaire. New England Journal of Medicine 1995;332(19):1256-61. [DOI] [PubMed] [Google Scholar]
Arai 2010 {published data only}
- Arai Y, Inaba Y, Yamamoto T, Kanai F, Aramaki T, Tanaka T, et al. A randomized phase II study of TSU-68 in patients (pts) with hepatocellular carcinoma (HCC) treated by transarterial chemoembolization (TACE). Journal of Clinical Oncology Conference 2010;28(15 Suppl 1):Abstract: 4030. [Google Scholar]
Bartolozzi 1995 {published data only}
- Bartolozzi C, Lencioni R, Caramella D, Vignali C, Cioni R, Mazzeo S, et al. Treatment of large HCC: transcatheter arterial chemoembolization combined with percutaneous ethanol injection versus repeated transcatheter arterial chemoembolization. Radiology 1995;197(3):812-8. [DOI] [PubMed] [Google Scholar]
Becker 2005 {published data only}
- Becker G, Soezgen T, Olschewski M, Laubenberger J, Blum HE, Allgaier HP. Combined TACE and PEI for palliative treatment of unresectable hepatocellular carcinoma. World Journal of Gastroenterology 2005;11(39):6104-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bruix 1998 {published data only}
- Bruix J, Llovet JM, Castells A, Montana X, Bru C, Ayuso MC, et al. Transarterial embolization versus symptomatic treatment in patients with advanced hepatocellular carcinoma: results of a randomized, controlled trial in a single institution. Hepatology (Baltimore, Md.) 1998;27(6):1578-83. [DOI] [PubMed] [Google Scholar]
Chen 2002 {published data only}
- Chen MS, Li JQ, Zhang YQ, Lu LX, Zhang WZ, Yuan YF, et al. High-dose iodized oil transcatheter arterial chemoembolization for patients with large hepatocellular carcinoma. World Journal of Gastroenterology 2002;8(1):74-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 2007 {published data only}
- Chen XP, Hu DY, Zhang ZW, Zhang BX, Chen YF, Zhang WG, et al. Role of mesohepatectomy with or without transcatheter arterial chemoembolization for large centrally located hepatocellular carcinoma. Digestive Surgery 2007;24(3):208-13. [DOI] [PubMed] [Google Scholar]
Cheng 2004 {published data only}
- Cheng SQ, Wu MC, Chen H, Shen F, Yang JH, Cong WM, et al. Transcatheter hepatic arterial chemoembolization and thymosin alpha1 in postoperative treatment of hepatocellular carcinoma. Zhonghua Zhong Liu Za Zhi 2004;26(5):305-7. [PubMed] [Google Scholar]
Cheng 2008 {published data only}
- Cheng BQ, Jia CQ, Liu CT, Fan W, Wang QL, Zhang ZL, et al. Chemoembolization combined with radiofrequency ablation for patients with hepatocellular carcinoma larger than 3 cm: a randomized controlled trial. (Retraction in: JAMA 2009; 301(18):1931). JAMA 2008;299(14):1669-77. [DOI] [PubMed] [Google Scholar]
Choi 2014 {published data only}
- Choi C, Koom WS, Kim TH, Yoon SM, Kim JH, Lee HS, et al. A prospective phase 2 multicenter study for the efficacy of radiation therapy following incomplete transarterial chemoembolization in unresectable hepatocellular carcinoma. International Journal of Radiation Oncology 2014;90(5):1051-60. [DOI] [PubMed] [Google Scholar]
Chow 2002 {published data only}
- Chow PK, Tai BC, Tan CK, Machin D, Win KM, Johnson PJ, et al. High-dose tamoxifen in the treatment of inoperable hepatocellular carcinoma: a multicenter randomized controlled trial. Hepatology (Baltimore, Md.) 2002;36(5):1221-6. [DOI] [PubMed] [Google Scholar]
DeAngelis 2009 {published data only}
- DeAngelis CD, Fontanarosa PB. Chemoembolization combined with radiofrequency ablation for patients with hepatocellular carcinoma larger than 3 cm: a randomized controlled trial. (Retraction of: JAMA 2008; 299(14):1669-77). JAMA 2009;301(18):1931. [DOI] [PubMed] [Google Scholar]
Doffoel 2008 {published data only}
- Doffoel M, Bonnetain F, Bouche O, Vetter D, Abergel A, Fratte S, et al. Multicentre randomised phase III trial comparing tamoxifen alone or with transarterial lipiodol chemoembolisation for unresectable hepatocellular carcinoma in cirrhotic patients. European Journal of Cancer 2008;44(4):528-38. [DOI] [PubMed] [Google Scholar]
El‐Kady 2013 {published data only}
- El-Kady NM, Esmat G, Mahmoud EH, Darweesh SK, Mahmoud SH, Elagawy WA. Hypertonic saline-enhanced radiofrequency versus chemoembolization sequential radiofrequency in the treatment of large hepatocellular carcinoma. European Journal of Gastroenterology & Hepatology 2013;25(5):628-33. [DOI] [PubMed] [Google Scholar]
Ferrari 2004 {published data only}
- Ferrari FS, Stella A, Gambacorta D, Magnolfi F, Fantozzi F, Pasquinucci P, et al. Treatment of large hepatocellular carcinoma: comparison between techniques and long term results. Radiologia Medica 2004;108(4):356-71. [PubMed] [Google Scholar]
Fischman 2014 {published data only}
- Fischman AM, Lewis SC, Patel R, Kim E, Nowakowski FS, Lookstein RA, et al. Prospective, randomized study of coiling vs surefire infusion system in Y-90: clinical outcomes in HCC patients-subgroup analysis of safety and efficacy from the COSY trial. Cardiovascular and Interventional Radiology 2014;37(2 Suppl 1):S252. [Google Scholar]
Gallo 2006 {published data only}
- Gallo C, De Maio E, Di Maio M, Signoriello G, Daniele B, Pignata S, et al. Tamoxifen is not effective in good prognosis patients with hepatocellular carcinoma. BMC Cancer 2006;6:196. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gish 2009 {published data only}
- Gish RG, Gordon SC, Nelson D, Rustgi V, Rios I. A randomized controlled trial of thymalfasin plus transarterial chemoembolization for unresectable hepatocellular carcinoma. Hepatology International 2009;3(3):480-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Golfieri 2014 {published data only}
- Golfieri R, Giampalma E, Renzulli M, Cioni R, Bargellini I, Bartolozzi C, et al. Randomised controlled trial of doxorubicin-eluting beads vs conventional chemoembolisation for hepatocellular carcinoma. British Journal of Cancer 2014;111(2):255-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hebbar 2015 {published data only}
- Hebbar M, Heurgue-Berlot A, Boige V, Malicot K, Bernard-Chabert B, Marcus C, et al. Randomized phase 2/3 trial of transcatheter arterial chemoembolization (TACE) plus sunitinib or placebo in patients with hepatocellular carcinoma (HCC) (PRODIGE 16/SATURNE study): results of the phase II part. European Journal of Cancer 2015;51:S434. [Google Scholar]
Hilgard 2008 {published data only}
- Hilgard P, Trojan J, Galle PR, Greten T, Erhardt A, Shan M, et al. Sorafenib improves survival in patients with hepatocellular carcinoma: results of a large multicenter, randomized, placebo-controlled phase III trial. Onkologie 2008;31(Suppl 1):78. [Google Scholar]
Hou 2009 {published data only}
- Hou YB, Chen MH, Yan K, Wu JY, Yang W. Adjuvant percutaneous radiofrequency ablation of feeding artery of hepatocellular carcinoma before treatment. World Journal of Gastroenterology 2009;15(21):2638-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Huo 2003 {published data only}
- Huo TI, Huang YH, Wu JC, Lee PC, Chang FY, Lee SD. Comparison of percutaneous acetic acid injection and percutaneous ethanol injection for hepatocellular carcinoma in cirrhotic patients: a prospective study. Scandinavian Journal of Gastroenterology 2003;38(7):770-8. [DOI] [PubMed] [Google Scholar]
Iezzi 2013 {published data only}
- Iezzi R, Cesario V, Siciliani L, Campanale M, De Gaetano AM, Siciliano M, et al. Single-step multimodal locoregional treatment for unresectable hepatocellular carcinoma: balloon-occluded percutaneous radiofrequency thermal ablation (BO-RFA) plus transcatheter arterial chemoembolization (TACE). Radiologia Medica 2013;118(4):555-69. [DOI] [PubMed] [Google Scholar]
Inaba 2013 {published data only}
- Inaba Y, Kanai F, Aramaki T, Yamamoto T, Tanaka T, Yamakado K, et al. A randomised phase II study of TSU-68 in patients with hepatocellular carcinoma treated by transarterial chemoembolisation. European Journal of Cancer 2013;49(13):2832-40. [DOI] [PubMed] [Google Scholar]
- Kaneko S, Inaba Y, Kanai F, Aramaki T, Yamamoto T, Tanaka T, et al. Final results of a randomized phase II study of TSU-68 after transarterial chemoembolisation in Japanese patients with unresectable hepatocellular carcinoma. Annals of Oncology 2012;23:ix243. [Google Scholar]
Kolligs 2015 {published data only}
- Kolligs FT, Bilbao JI, Jakobs T, Inarrairaegui M, Nagel JM, Rodriguez M, et al. Pilot randomized trial of selective internal radiation therapy vs. chemoembolization in unresectable hepatocellular carcinoma. Liver International 2015;35(6):1715-21. [DOI] [PubMed] [Google Scholar]
Kudo 2002 {published data only}
- Kudo M, Chung H, Ogawa C, Fukuda N, Suetomi Y, Minami Y, et al. Radiofrequency ablation therapy combined with or without subsegmental lipiodol TAE for large hepatocellular carcinoma: a randomized prospective study. Hepatology (Baltimore, Md.) 2002;36(4):693A-4A. [Google Scholar]
Kudo 2014 {published data only}
- Kudo M, Han G, Finn RS, Poon RT, Blanc JF, Yan L, et al. Brivanib as adjuvant therapy to transarterial chemoembolization in patients with hepatocellular carcinoma: a randomized phase III trial. Hepatology (Baltimore, Md.) 2014;60(5):1697-707. [DOI] [PubMed] [Google Scholar]
Lammer 2010 {published data only}
- Lammer J, Malagari K, Vogl T, Pilleul F, Denys A, Watkinson A, et al. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovascular and Interventional Radiology 2010;33(1):41-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Li 2009 {published data only}
- Li CX, Wu PH, Fan WJ, Huang JH, Zhang FJ, Zhang L, et al. Clinical effect of transcatheter arterial chemoembolization combined with high intensity focused ultrasound ablation in treatment of large hepatocellular carcinoma. National Medical Journal of China 2009;89(11):754-7. [PubMed] [Google Scholar]
Li 2010 {published data only}
- Li CX, Zhang WD, Zhang R, Zhang LA, Wu PH, Zhang FJ. Therapeutic effects and prognostic factors in high-intensity focused ultrasound combined with chemoembolisation for larger hepatocellular carcinoma. European Journal of Cancer 2010;46(13):2513-21. [DOI] [PubMed] [Google Scholar]
Liu 2010 {published data only}
- Liu FY, Yu XL, Liang P, Wang Y, Zhou P, Yu J. Comparison of percutaneous 915 MHz microwave ablation and 2450 MHz microwave ablation in large hepatocellular carcinoma. International Journal of Hyperthermia 2010;26(5):448-55. [DOI] [PubMed] [Google Scholar]
Livraghi 2005 {published data only}
- Livraghi T, Meloni F, Frosi A, Lazzaroni S, Bizzarri M, Frati L, et al. Treatment with stem cell differentiation stage factors of intermediate-advanced hepatocellular carcinoma: an open randomized clinical trial. Oncology Research 2005;15(7-8):399-408. [DOI] [PubMed] [Google Scholar]
Llovet 2002 {published data only}
- Llovet JM, Real MI, Montana X, Planas R, Coll S, Aponte J, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet 2002;359(9319):1734-9. [DOI] [PubMed] [Google Scholar]
Llovet 2007 {published data only}
- Llovet J, Mazzaferro V, Ricci S, Hilgard P, Raoul J, Zeuzem S, et al. Sorafenib improves survival in a large multi-center, randomized, placebo-controlled phase III trial in patients with hepatocellular carcinoma. European Journal of Cancer 2007;5(4 Suppl):261. [Google Scholar]
Lo 2002 {published data only}
- Lo CM, Ngan H, Tso WK, Liu CL, Lam CM, Poon RT, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology (Baltimore, Md.) 2002;35(5):1164-71. [DOI] [PubMed] [Google Scholar]
Malagari 2010 {published data only}
- Malagari K, Pomoni M, Kelekis A, Pomoni A, Dourakis S, Spyridopoulos T, et al. Prospective randomized comparison of chemoembolization with doxorubicin-eluting beads and bland embolization with beadblock for hepatocellular carcinoma. Cardiovascular and Interventional Radiology 2010;33(3):541-51. [DOI] [PubMed] [Google Scholar]
Meyer 2016 {published data only}
- Meyer T, Fox R, Ma YT, Ross PJ, James M, Strugess R, et al. TACE 2: a randomized placebo-controlled, double-blinded, phase III trial evaluating sorafenib in combination with transarterial chemoembolisation (TACE) in patients with unresectable hepatocellular carcinoma (HCC). Journal of Clinical Oncology Conference 2016;34:Abstract: 4018. [Google Scholar]
Mohnike 2013 {published data only}
- Mohnike K, Pech M, Seidensticker M, Seidensticker R, Bretschneider T, Schuette K, et al. A prospective randomized trial comparing HDR-brachytherapy and transarterial chemoembolization in hepatocellular carcinoma. Journal of Hepatology 2013;58:S50. [Google Scholar]
Morimoto 2010 {published data only}
- Morimoto M, Numata K, Kondou M, Nozaki A, Morita S, Tanaka K. Midterm outcomes in patients with intermediate-sized hepatocellular carcinoma: a randomized controlled trial for determining the efficacy of radiofrequency ablation combined with transcatheter arterial chemoembolization. Cancer 2010;116(23):5452-60. [DOI] [PubMed] [Google Scholar]
Morimoto 2011 {published data only}
- Morimoto M, Numata K, Kondo M, Nozaki A, Moriya S, Takizawa K. Long-term outcome in patients with intermediate-sized hepatocellular carcinoma: a randomized controlled trial to determine the efficacy of radiofrequency ablation combined with transcatheter arterial chemoembolization. Hepatology (Baltimore, Md.) 2011;54(4 Suppl):1366A-7A. [Google Scholar]
Okusaka 2012 {published data only}
- Okusaka T, Kasugai H, Ishii H, Kudo M, Sata M, Tanaka K, et al. A randomized phase II trial of intra-arterial chemotherapy using SM-11355 (miriplatin) for hepatocellular carcinoma. Investigational New Drugs 2012;30(5):2015-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Padia 2013 {published data only}
- Padia SA, Shivaram G, Bastawrous S, Bhargava P, Vo NJ, Vaidya S, et al. Safety and efficacy of drug-eluting bead chemoembolization for hepatocellular carcinoma: comparison of small-versus medium-size particles. Journal of Vascular & Interventional Radiology 2013;24(3):301-6. [DOI] [PubMed] [Google Scholar]
Pelletier 1998 {published data only}
- Pelletier G, Ducreux M, Gay F, Luboinski M, Hagege H, Dao T, et al. Treatment of unresectable hepatocellular carcinoma with lipiodol chemoembolization: a multicenter randomized trial. Groupe CHC. Journal of Hepatology 1998;29(1):129-34. [DOI] [PubMed] [Google Scholar]
Sansonno 2012 {published data only}
- Sansonno D, Lauletta G, Russi S, Conteduca V, Sansonno L, Dammacco F. Transarterial chemoembolization plus sorafenib: a sequential therapeutic scheme for HCV-related intermediate-stage hepatocellular carcinoma: a randomized clinical trial. Oncologist 2012;17(3):359-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sarin 1994 {published data only}
- Sarin SK, Sreenivas DV, Saraya A, Mehta S, Lahoti D, Malhotra V. Improved survival with percutaneous ethanol injection in patients with large hepatocellular carcinoma. European Journal of Gastroenterology and Hepatology 1994;6(11):999-1003. [Google Scholar]
Shi 2009 {published data only}
- Shi M, Chen J, Lin X, Chen M, Guo R, Li S, et al. Prospective randomized controlled study of transarterial chemoembolization with doxorubicin versus doxorubicin/lobaplatin/mitomycin combination for unresectable hepatocellular carcinoma. Chinese Journal of Clinical Oncology 2009;36(1):9-12. [Google Scholar]
Tanaka 2014 {published data only}
- Tanaka M, Ando E, Simose S, Hori M, Kuraoka K, Ohno M, et al. Radiofrequency ablation combined with transarterial chemoembolization for intermediate hepatocellular carcinoma. Hepatology Research 2014;44(2):194-200. [DOI] [PubMed] [Google Scholar]
Tang 2009 {published data only}
- Tang QH, Zhou JP, Fu SY, Zhou WP. Effect of preoperative transcatheter arterial chemoembolization for treatment of resectable large hepatocellular carcinoma: a clinical randomized controlled trial. Academic Journal of Second Military Medical University 2009;30(12):1379-84. [Google Scholar]
Ulbrich 2010a {published data only}
- Ulbrich G, Kolblinger C, Pinter M, Reiberger T, Ferlitsch A, Sieghart W, et al. AVATACE-1 trial: bevacizumab as inhibitor of collateral tumor-vessel-growth during transarterial chemoembolisation (TACE) for hepatocellular carcinoma - a double-blind, randomized, placebo-controlled pilot-trial. Hepatology (Baltimore, Md.) 2010;52:1159A-60A. [Google Scholar]
Ulbrich 2010b {published data only}
- Ulbrich G, Pinter M, Reiberger T, Ferlitsch A, Sieghart W, Peck-Radosavljevic M. Interim safety-analysis of the AVATACE-1 trial (bevacizumab as inhibitor of collateral tumor-vessel-growth during transarterial chemoembolisation (TACE) for hepatocellular carcinoma - a double-blind randomized placebo-controlled pilot-trial. Journal of Hepatology 2010;52:S54. [Google Scholar]
Wang 2007 {published data only}
- Wang YB, Chen MH, Yan K, Yang W, Dai Y, Yin SS. Quality of life after radiofrequency ablation combined with transcatheter arterial chemoembolization for hepatocellular carcinoma: comparison with transcatheter arterial chemoembolization alone. Quality of Life Research 2007;16(3):389-97. [DOI] [PubMed] [Google Scholar]
Wang 2014 {published data only}
- Wang LJ, Bu WZ, Chen H, Li JP, Ma SS, Song JL. Clinical study of TACE with or without antiviral treatment for hepatitis B virus related unresectable hepatocellular carcinoma. Chinese Journal of Cancer Prevention and Treatment 2014;21(20):1617-22. [Google Scholar]
Wu 1995 {published data only}
- Wu CC, Ho YZ, Ho WL, Wu TC, Liu TJ, P'Eng FK. Preoperative transcatheter arterial chemoembolization for resectable large hepatocellular carcinoma: a reappraisal. British Journal of Surgery 1995;82(1):122-6. [DOI] [PubMed] [Google Scholar]
Wu 1998 {published data only}
- Wu P, Li L, Zhang Y. Transcatheter arterial chemo-embolization combined with CT-guided percutaneous intratumoral injection of lipiodol-ethanol for the treatment of primary hepatocellular carcinoma. Zhonghua Zhong Liu za Zhi [Chinese Journal of Oncology] 1998;20(5):391-3. [PubMed] [Google Scholar]
Xie 2015 {published data only}
- Xie ZR, Luo YL, Xiao FM, Liu Q, Ma Y. Health-related quality of life of patients with intermediate hepatocellular carcinoma after liver resection or transcatheter arterial chemoembolization. Asian Pacific Journal of Cancer Prevention 2015;16(10):4451-6. [DOI] [PubMed] [Google Scholar]
Xu 2009 {published data only}
- Xu KC, Niu LZ, Zhou Q, Hu YZ, Guo DH, Liu ZP, et al. Sequential use of transarterial chemoembolization and percutaneous cryosurgery for hepatocellular carcinoma. World Journal of Gastroenterology 2009;15(29):3664-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yang 2008 {published data only}
- Yang P, Liang M, Zhang Y, Shen B. Clinical application of a combination therapy of lentinan, multi-electrode RFA and TACE in HCC. Advances in Therapy 2008;25(8):787-94. [DOI] [PubMed] [Google Scholar]
Yin 2013 {published data only}
- Yin L. A prospective RCT comparing partial hepatectomy with TACE for multinodular HCC outside of Milan criteria. HPB : the Official Journal of the International Hepato Pancreato Biliary Association 2013;15:21-2. [Google Scholar]
Zhang 2011 {published data only}
- Zhang Q, Chen H, Li Q, Zang YJ, Chen XG, Zou WL, et al. Combination adjuvant chemotherapy with oxaliplatin, 5-fluorouracil and leucovorin after liver transplantation for hepatocellular carcinoma: a preliminary open-label study. Investigational New Drugs 2011;29(6):1360-9. [DOI] [PubMed] [Google Scholar]
Zhou 2009 {published data only}
- Zhou WP, Lai EC, Li AJ, Fu SY, Zhou JP, Pan ZY, et al. A prospective, randomized, controlled trial of preoperative transarterial chemoembolization for resectable large hepatocellular carcinoma. Annals of Surgery 2009;249(2):195-202. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
NCT02854839 {published data only}
- Lee KG, Kyung T. A study of MG4101 (allogeneic natural killer cell) for intermediate-stage of hepatocellular carcinoma. clinicaltrials.gov/ct2/show/NCT02854839 Date first received: 2016.
Seinstra 2012 {published data only}
- Seinstra BA, Defreyne L, Lambert B, Lam M, Verkooijen HM, Erpecum KJ, et al. Transarterial radioembolization versus chemoembolization for the treatment of hepatocellular carcinoma (trace): study protocol for a randomized controlled trial. Trials 2012;13:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seinstra BA, Defreyne L, Lambert B, Lam M, Verkooijen L, Van Erpecum KJ, et al. Transarterial radioembolization versus chemoembolization for the treatment of HCC: trace trial-an international multicenter randomized controlled trial. Journal of Vascular and Interventional Radiology 2012;23(3 Suppl):S150-1. [Google Scholar]
Additional references
Arnaoutakis 2014
- Arnaoutakis DJ, Mavros MN, Shen F, Alexandrescu S, Firoozmand A, Popescu I, et al. Recurrence patterns and prognostic factors in patients with hepatocellular carcinoma in noncirrhotic liver: a multi-institutional analysis. Annals of Surgical Oncology 2014;21(1):147-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Asham 2013
- Asham EH, Kaseb A, Ghobrial RM. Management of hepatocellular carcinoma. Surgical Clinics of North America 2013;93(6):1423-50. [DOI] [PubMed] [Google Scholar]
Bosetti 2014
- Bosetti C, Turati F, La Vecchia C. Hepatocellular carcinoma epidemiology. Best Practice & Research: Clinical Gastroenterology 2014;28(5):753-70. [DOI] [PubMed] [Google Scholar]
Bruix 2011
- Bruix J, Sherman M, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology (Baltimore, Md.) 2011;53(3):1020-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chaimani 2012
- Chaimani A, Salanti G. Using network meta-analysis to evaluate the existence of small-study effects in a network of interventions. Research Synthesis Methods 2012;3(2):161-76. [DOI] [PubMed] [Google Scholar]
Chaimani 2013
- Chaimani A, Higgins JP, Mavridis D, Spyridonos P, Salanti G. Graphical tools for network meta-analysis in STATA. PloS one 2013;8(10):e76654. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chan 2013a
- Chan AC, Cheung TT, Fan ST, Chok KS, Chan SC, Poon RT, et al. Survival analysis of high-intensity focused ultrasound therapy versus radiofrequency ablation in the treatment of recurrent hepatocellular carcinoma. Annals of Surgery 2013;257(4):686-92. [DOI] [PubMed] [Google Scholar]
Chan 2013b
- Chan A-W, Tetzlaff JM, Altman DG, Laupacis A, Gøtzsche PC, Krleža-Jerić K, et al. Spirit 2013 statement: defining standard protocol items for clinical trials. Annals of Internal Medicine 2013;158(3):200-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 2012
- Chen Y, Wang X, Wang J, Yan Z, Luo J. Excess body weight and the risk of primary liver cancer: an updated meta-analysis of prospective studies. European Journal of Cancer 2012;48(14):2137-45. [DOI] [PubMed] [Google Scholar]
Cillo 2006
- Cillo U, Vitale A, Grigoletto F, Farinati F, Brolese A, Zanus G, et al. Prospective validation of the Barcelona Clinic Liver Cancer staging system. Journal of Hepatology 2006;44(4):723-31. [DOI] [PubMed] [Google Scholar]
CLIP 1998
- The cancer of the liver Italian program (CLIP) investigators. A new prognostic system for hepatocellular carcinoma: a retrospective study of 435 patients: the Cancer of the Liver Italian Program (CLIP) investigators. Hepatology 1998;28(3):751-5. [DOI] [PubMed] [Google Scholar]
Davila 2004
- Davila JA, Morgan RO, Shaib Y, McGlynn KA, El-Serag HB. Hepatitis C infection and the increasing incidence of hepatocellular carcinoma: a population-based study. Gastroenterology 2004;127(5):1372-80. [DOI] [PubMed] [Google Scholar]
Del Re 2013
- Del Re AC, Spielmans GI, Flückiger C, Wampold BE. Efficacy of new generation antidepressants: differences seem illusory. PLoS One 2013;8(6):e63509. [DOI] [PMC free article] [PubMed] [Google Scholar]
DeMets 1987
- DeMets DL. Methods for combining randomized clinical trials: strengths and limitations. Statistics in Medicine 1987;6(3):341-50. [DOI] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177-88. [DOI] [PubMed] [Google Scholar]
Dias 2010
- Dias S, Welton NJ, Caldwell DM, Ades AE. Checking consistency in mixed treatment comparison meta-analysis. Statistics in Medicine 2010;29(7-8):932-44. [DOI] [PubMed] [Google Scholar]
Dias 2012a
- Dias S, Sutton AJ, Welton NJ, Ades AE. NICE DSU Technical Support Document 3: heterogeneity: subgroups, meta-regression, bias and bias-adjustment, September 2011 (last updated April 2012). www.nicedsu.org.uk/TSD3%20Heterogeneity.final%20report.08.05.12.pdf (accessed 27 March 2014).
Dias 2012b
- Dias S, Welton NJ, Sutton AJ, Ades AE. NICE DSU Technical Support Document 1: introduction to evidence synthesis for decision making, April 2011 (last updated April 2012). www.nicedsu.org.uk/TSD1%20Introduction.final.08.05.12.pdf (accessed 27 March 2014).
Dias 2014a
- Dias S, Welton NJ, Sutton AJ, Ades AE. NICE DSU Technical Support Document 2: a generalised linear modelling framework for pairwise and network meta-analysis of randomised controlled trials, August 2011 (last updated April 2014). www.nicedsu.org.uk/TSD2%20General%20meta%20analysis%20corrected%2015April2014.pdf (accessed 8 October 2014). [PubMed]
Dias 2014b
- Dias S, Welton NJ, Sutton AJ, Caldwell DM, Lu G, Ades AE. NICE DSU Technical Support Document 4: inconsistency in networks of evidence based on randomised controlled trials, May 2011 (last updated April 2014). www.nicedsu.org.uk/TSD4%20Inconsistency.final.15April2014.pdf (accessed 8 October 2014). [PubMed]
EASL 2012
- European Association for Study of Liver, European Organisation for Research and Treatment of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. Journal of Hepatology 2012;56(4):908-43. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ (Clinical Research Ed.) 1997;315(7109):629-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
EuroQol 2014
- EuroQol. About EQ-5D, 2014. www.euroqol.org/about-eq-5d.html (accessed 8 October 2014).
Forner 2014
- Forner A, Gilabert M, Bruix J, Raoul JL. Treatment of intermediate-stage hepatocellular carcinoma. Nature Reviews: Clinical Oncology 2014;11(9):525-35. [DOI] [PubMed] [Google Scholar]
Gaddikeri 2014
- Gaddikeri S, McNeeley MF, Wang CL, Bhargava P, Dighe MK, Yeh MMC, et al. Hepatocellular carcinoma in the noncirrhotic liver. American Journal of Roentgenology 2014;203(1):W34-47. [DOI] [PubMed] [Google Scholar]
Germani 2010
- Germani G, Pleguezuelo M, Gurusamy K, Meyer T, Isgro G, Burroughs AK. Clinical outcomes of radiofrequency ablation, percutaneous alcohol and acetic acid injection for hepatocellular carcinoma: a meta-analysis. Journal of Hepatology 2010;52(3):380-8. [DOI] [PubMed] [Google Scholar]
Germani 2011
- Germani G, Gurusamy K, Garcovich M, Toso C, Fede G, Hemming A, et al. Which matters most: number of tumors, size of the largest tumor, or total tumor volume? Liver Transplantation 2011;17(Suppl 2):S58-66. [DOI] [PubMed] [Google Scholar]
Gluud 2016
- Gluud C, Nikolova D, Klingenberg SL. Cochrane Hepato-Biliary Group. About Cochrane (Cochrane Review Groups (CRGs)) 2016, Issue 10. Art. No.: LIVER.
Guglielmi 2014
- Guglielmi A, Ruzzenente A, Conci S, Valdegamberi A, Vitali M, Bertuzzo F, et al. Hepatocellular carcinoma: surgical perspectives beyond the Barcelona Clinic Liver Cancer recommendations. World Journal of Gastroenterology 2014;20(24):7525-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gurusamy 2014
- Gurusamy KS, Nagendran M, Davidson BR. Methods of preventing bacterial sepsis and wound complications after liver transplantation. Cochrane Database of Systematic Reviews 2014, Issue 3. [DOI: 10.1002/14651858.CD006660.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction - GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383-94. [DOI] [PubMed] [Google Scholar]
Head 2004
- Head HW, Dodd GD, 3rd. Thermal ablation for hepatocellular carcinoma. Gastroenterology 2004;127(5 Suppl 1):S167-78. [DOI] [PubMed] [Google Scholar]
Henderson 2003
- Henderson JM, Sherman M, Tavill A, Abecassis M, Chejfec G, Gramlich T. AHPBA/AJCC consensus conference on staging of hepatocellular carcinoma: consensus statement. HPB : the Official Journal of the International Hepato Pancreato Biliary Association 2003;5(4):243-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2012
- Higgins JPT, Jackson D, Barrett JK, Lu G, Ades AE, White IR. Consistency and inconsistency in network meta-analysis: Concepts and models for multi-arm studies. Research Synthesis Methods 2012;3(2):98-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hoffmann 2014
- Hoffmann R, Rempp H, Syha R, Ketelsen D, Pereira PL, Claussen CD, et al. Transarterial chemoembolization using drug eluting beads and subsequent percutaneous MR-guided radiofrequency ablation in the therapy of intermediate sized hepatocellular carcinoma. European Journal of Radiology 2014;83(10):1793-8. [DOI] [PubMed] [Google Scholar]
IARC 2014a
- International Agency for Research on Cancer. GLOBOCAN 2012: estimated cancer incidence, mortality, and prevalence worldwide in 2012. Liver cancer estimated incidence, mortality and prevalence worldwide in 2012. globocan.iarc.fr/Pages/fact_sheets_cancer.aspx (accessed 17 October 2014).
IARC 2014b
- International Agency for Research on Cancer. World 2012 (estimated cancer incidence, all ages: both sexes). globocan.iarc.fr/old/summary_table_pop-html.asp?selection=224900&title=World&sex=0&type=0&window=1&sort=0&submit=%C2%A0Execute (accessed 17 October 2014).
ICH‐GCP 1997
- International Conference on Harmonisation Expert Working Group. International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use. ICH harmonised tripartite guideline. Guideline for good clinical practice CFR & ICH Guidelines. Vol. 1. Philadelphia (PA): Barnett International/PAREXEL, 1997. [Google Scholar]
Jakobsen 2014
- Jakobsen JC, Wetterslev J, Winkel P, Lange T, Gluud C. Thresholds for statistical and clinical significance in systematic reviews with meta-analytic methods. BMC Medical Research Methodology 2014;14(1):120. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jepsen 2007
- Jepsen P, Vilstrup H, Tarone RE, Friis S, Sorensen HT. Incidence rates of hepatocellular carcinoma in the U.S. and Denmark: recent trends. International Journal of Cancer 2007;121(7):1624-6. [DOI] [PubMed] [Google Scholar]
Kansagara 2014
- Kansagara D, Papak J, Pasha AS, O'Neil M, Freeman M, Relevo R, et al. Screening for hepatocellular carcinoma in chronic liver disease: a systematic review. Annals of Internal Medicine 2014;161(4):261-9. [DOI] [PubMed] [Google Scholar]
Kjaergard 2001
- Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta-analyses. Annals of Internal Medicine 2001;135(11):982-9. [DOI] [PubMed] [Google Scholar]
Kollar 2014
- Kollar L, Rengan R. Stereotactic body radiotherapy. Seminars in Oncology 2014;41(6):776-89. [DOI] [PubMed] [Google Scholar]
Ladep 2014
- Ladep NG, Khan SA, Crossey MM, Thillainayagam AV, Taylor-Robinson SD, Toledano MB. Incidence and mortality of primary liver cancer in England and Wales: changing patterns and ethnic variations. World Journal of Gastroenterology 2014;20(6):1544-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2009
- Lee YC, Cohet C, Yang YC, Stayner L, Hashibe M, Straif K. Meta-analysis of epidemiologic studies on cigarette smoking and liver cancer. International Journal of Epidemiology 2009;38(6):1497-511. [DOI] [PubMed] [Google Scholar]
Lencioni 2013
- Lencioni R, Petruzzi P, Crocetti L. Chemoembolization of hepatocellular carcinoma. Seminars in Interventional Radiology 2013;30(1):3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Liu 2012
- Liu Y, Chang CC, Marsh GM, Wu F. Population attributable risk of aflatoxin-related liver cancer: systematic review and meta-analysis. European Journal of Cancer 2012;48(14):2125-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Llovet 1999
- Llovet JM, Bru C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Seminars in Liver Disease 1999;19(3):329-38. [DOI] [PubMed] [Google Scholar]
Llovet 2003
- Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet 2003;362(9399):1907-17. [DOI] [PubMed] [Google Scholar]
Lu 2006
- Lu G, Ades AE. Assessing evidence inconsistency in mixed treatment comparisons. Journal of the American Statistical Association 2006;101(474):447-59. [Google Scholar]
Lundh 2017
- Lundh A, Sismondo S, Lexchin J, Busuioc OA, Bero L. Industry sponsorship and research outcome. Cochrane Database of Systematic Reviews 2017, Issue 2. [DOI: 10.1002/14651858.MR000033.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Macaskill 2001
- Macaskill P, Walter SD, Irwig L. A comparison of methods to detect publication bias in meta-analysis. Statistics in Medicine 2001;20(4):641-54. [DOI] [PubMed] [Google Scholar]
Maheshwari 2007
- Maheshwari S, Sarraj A, Kramer J, El-Serag HB. Oral contraception and the risk of hepatocellular carcinoma. Journal of Hepatology 2007;47(4):506-13. [DOI] [PubMed] [Google Scholar]
Majumdar 2017
- Majumdar A, Roccarina D, Thorburn D, Davidson BR, Tsochatzis E, Gurusamy KS. Management of people with early or very early stage hepatocellular carcinoma: a network meta-analysis. Cochrane Database of Systematic Reviews 2017, Issue 3. [DOI: 10.1002/14651858.CD011650.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mazzaferro 1996
- Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. New England Journal of Medicine 1996;334(11):693-9. [DOI] [PubMed] [Google Scholar]
McDermott 2013
- McDermott S, Gervais DA. Radiofrequency ablation of liver tumors. Seminars in Interventional Radiology 2013;30(1):49-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Miladinovic 2013
- Miladinovic J, Hozo I, Djulbegovic B. Trial sequential boundaries for cumulative meta-analyses. Stata Journal 2013;13(1):77-91. [Google Scholar]
Mills 2012
- Mills EJ, Ioannidis JP, Thorlund K, Schünemann HJ, Puhan MA, Guyatt GH. How to use an article reporting a multiple treatment comparison meta-analysis. JAMA 2012;308(12):1246-53. [DOI] [PubMed] [Google Scholar]
Moher 1998
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta-analyses? Lancet 1998;352(9128):609-13. [DOI] [PubMed] [Google Scholar]
Nanashima 2006
- Nanashima A, Sumida Y, Abo T, Shindou H, Fukuoka H, Takeshita H, et al. Modified Japan Integrated Staging is currently the best available staging system for hepatocellular carcinoma patients who have undergone hepatectomy. Journal of Gastroenterology 2006;41(3):250-6. [DOI] [PubMed] [Google Scholar]
NCBI 2014
- NCBI. Carcinoma, hepatocellular, 2014. www.ncbi.nlm.nih.gov/mesh/68006528 (accessed 17 October 2014).
Newell 1992
- Newell DJ. Intention-to-treat analysis: implications for quantitative and qualitative research. International Journal of Epidemiology 1992;21(5):837-41. [DOI] [PubMed] [Google Scholar]
Nguyen 2009
- Nguyen KT, Gamblin TC, Geller DA. World review of laparoscopic liver resection - 2,804 patients. Annals of Surgery 2009;250(5):831-41. [DOI] [PubMed] [Google Scholar]
NHSBT 2014
- NHS Blood and Transplant. Organ donation. Activity report 2013-2014. www.organdonation.nhs.uk/statistics/transplant_activity_report/ (accessed 8 October 2014).
Niu 2016
- Niu M, Hong D, Ma TC, Chen XW, Han JH, Sun J, et al. Short-term and long-term efficacy of 7 targeted therapies for the treatment of advanced hepatocellular carcinoma: a network meta-analysis: Efficacy of 7 targeted therapies for AHCC. Medicine 2016;95(49):e5591. [DOI] [PMC free article] [PubMed] [Google Scholar]
Okuda 1985
- Okuda K, Ohtsuki T, Obata H, Tomimatsu M, Okazaki N, Hasegawa H, et al. Natural history of hepatocellular carcinoma and prognosis in relation to treatment. Study of 850 patients. Cancer 1985;56(4):918-28. [PMID: ] [DOI] [PubMed] [Google Scholar]
Oliveri 2011
- Oliveri RS, Wetterslev J, Gluud C. Transarterial (chemo)embolisation for unresectable hepatocellular carcinoma. Cochrane Database of Systematic Reviews 2011, Issue 3. [DOI: 10.1002/14651858.CD004787.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
OpenBUGS 3.2.3 [Computer program]
- Members of OpenBUGS Project Management Group OpenBUGS. Version 3.2.3. Members of OpenBUGS Project Management Group, 2014.
OPTN 2014
- Organ Procurement and Transplantation Network. Policies, 2014. optn.transplant.hrsa.gov/ContentDocuments/OPTN_Policies.pdf (accessed on 17 October 2014).
Pleguezuelo 2008
- Pleguezuelo M, Marelli L, Misseri M, Germani G, Calvaruso V, Xiruochakis E, et al. TACE versus TAE as therapy for hepatocellular carcinoma. Expert Review of Anticancer Therapy 2008;8(10):1623-41. [DOI] [PubMed] [Google Scholar]
Pocobelli 2008
- Pocobelli G, Cook LS, Brant R, Lee SS. Hepatocellular carcinoma incidence trends in Canada: analysis by birth cohort and period of diagnosis. Liver International 2008;28(9):1272-9. [DOI] [PubMed] [Google Scholar]
Polesel 2009
- Polesel J, Zucchetto A, Montella M, Dal Maso L, Crispo A, La Vecchia C, et al. The impact of obesity and diabetes mellitus on the risk of hepatocellular carcinoma. Annals of Oncology 2009;20(2):353-7. [DOI] [PubMed] [Google Scholar]
Prasad 2011
- Prasad KR, Young RS, Burra P, Zheng SS, Mazzaferro V, Moon DB, et al. Summary of candidate selection and expanded criteria for liver transplantation for hepatocellular carcinoma: a review and consensus statement. Liver Transplantation 2011;17(Suppl 2):S81-9. [DOI] [PubMed] [Google Scholar]
Puhan 2014
- Puhan MA, Schünemann HJ, Murad MH, Li T, Brignardello-Petersen R, Singh JA, et al. A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta-analysis. BMJ (Clinical Research Ed.) 2014;349:g5630. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Royle 2003
- Royle P, Milne R. Literature searching for randomized controlled trials used in Cochrane reviews: rapid versus exhaustive searches. International Journal of Technology Assessment in Health Care 2003;19(4):591-603. [DOI] [PubMed] [Google Scholar]
Salanti 2011
- Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. Journal of Clinical Epidemiology 2011;64(2):163-71. [DOI] [PubMed] [Google Scholar]
Salanti 2012
- Salanti G. Indirect and mixed-treatment comparison, network, or multiple-treatments meta-analysis: many names, many benefits, many concerns for the next generation evidence synthesis tool. Research Synthesis Methods 2012;3(2):80-97. [DOI] [PubMed] [Google Scholar]
Sang 2013
- Sang LX, Chang B, Li XH, Jiang M. Consumption of coffee associated with reduced risk of liver cancer: a meta-analysis. BMC Gastroenterology 2013;13:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Savović 2012a
- Savović J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized controlled trials: combined analysis of meta-epidemiological studies. Health Technology Assessment 2012;16(35):1-82. [DOI] [PubMed] [Google Scholar]
Savović 2012b
- Savović J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized controlled trials. Annals of Internal Medicine 2012;157(6):429-38. [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408-12. [DOI] [PubMed] [Google Scholar]
Schulz 2010
- Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. PLoS Medicine 2010;7(3):e1000251. [DOI] [PMC free article] [PubMed] [Google Scholar]
Severini 1993
- Severini TA. Bayesian interval estimates which are also confidence intervals. Journal of the Royal Statistical Society. Series B (Methodological) 1993;55(2):533-40. [Google Scholar]
Sindram 2010
- Sindram D, Lau KN, Martinie JB, Iannitti DA. Hepatic tumor ablation. Surgical Clinics of North America 2010;90(4):863-76. [DOI] [PubMed] [Google Scholar]
SRTR 2012
- Scientific Registry of Transplant Recipients. OPTN/SRTR 2012 annual data report: liver. srtr.transplant.hrsa.gov/annual_reports/2012/pdf/03_liver_13.pdf (accessed 8 October 2014).
Starley 2010
- Starley BQ, Calcagno CJ, Harrison SA. Nonalcoholic fatty liver disease and hepatocellular carcinoma: a weighty connection. Hepatology (Baltimore, Md.) 2010;51(5):1820-32. [DOI] [PubMed] [Google Scholar]
Stata/SE 14.2 [Computer program]
- Stata/SE 14.2 for Windows[64-bit x86-64]. Version 14. College Station: StataCorp LP, 2017.
Taura 2009
- Taura N, Yatsuhashi H, Nakao K, Ichikawa T, Ishibashi H. Long-term trends of the incidence of hepatocellular carcinoma in the Nagasaki prefecture, Japan. Oncology Reports 2009;21(1):223-7. [PubMed] [Google Scholar]
Thorlund 2011
- Thorlund K, Engstrøm J, Wetterslev J, Brok J, Imberger G, Gluud C. User manual for Trial Sequential Analysis (TSA). ctu.dk/tsa/files/tsa_manual.pdf 2011 (accessed 29 November 2016).
Thorlund 2012
- Thorlund K, Mills EJ. Sample size and power considerations in network meta-analysis. Systematic Reviews 2012;1:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
TSA 2011 [Computer program]
- Copenhagen Trial Unit TSA - Trial Sequential Analysis. Version 0.9 Beta. Copenhagen: Copenhagen Trial Unit, 2011.www.ctu.dk/tsa/downloads.aspx.
Tsochatzis 2014
- Tsochatzis EA, Fatourou E, O'Beirne J, Meyer T, Burroughs AK. Transarterial chemoembolization and bland embolization for hepatocellular carcinoma. World Journal of Gastroenterology 2014;20(12):3069-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Turati 2012
- Turati F, Edefonti V, Talamini R, Ferraroni M, Malvezzi M, Bravi F, et al. Family history of liver cancer and hepatocellular carcinoma. Hepatology (Baltimore, Md.) 2012;55(5):1416-25. [DOI] [PubMed] [Google Scholar]
Turati 2014
- Turati F, Galeone C, Rota M, Pelucchi C, Negri E, Bagnardi V, et al. Alcohol and liver cancer: a systematic review and meta-analysis of prospective studies. Annals of Oncology 2014;25(8):1526-35. [DOI] [PubMed] [Google Scholar]
Turner 2012
- Turner RM, Davey J, Clarke MJ, Thompson SG, Higgins JP. Predicting the extent of heterogeneity in meta-analysis, using empirical data from the Cochrane Database of Systematic Reviews. International Journal of Epidemiology 2012;41(3):818-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Deusen 2005
- Van Deusen MA, Abdalla EK, Vauthey JN, Roh MS. Staging classifications for hepatocellular carcinoma. Expert Review of Molecular Diagnostics 2005;5(3):377-83. [DOI] [PubMed] [Google Scholar]
Van Malenstein 2011
- Van Malenstein H, Pelt J, Verslype C. Molecular classification of hepatocellular carcinoma anno 2011. European Journal of Cancer 2011;47(12):1789-97. [DOI] [PubMed] [Google Scholar]
van Valkenhoef 2012
- Valkenhoef G, Lu G, Brock B, Hillege H, Ades AE, Welton NJ. Automating network meta-analysis. Research Synthesis Methods 2012;3(4):285-99. [DOI] [PubMed] [Google Scholar]
Von Hahn 2011
- Von Hahn T, Ciesek S, Wegener G, Plentz RR, Weismuller TJ, Wedemeyer H, et al. Epidemiological trends in incidence and mortality of hepatobiliary cancers in Germany. Scandinavian Journal of Gastroenterology 2011;46(9):1092-8. [DOI] [PubMed] [Google Scholar]
Wan 2014
- Wan P, Yu X, Xia Q. Operative outcomes of adult living donor liver transplantation and deceased donor liver transplantation: a systematic review and meta-analysis. Liver Transplantation 2014;20(4):425-36. [DOI] [PubMed] [Google Scholar]
Ware 2014
- Ware JE. SF-36® health survey update, 2014. www.sf-36.org/tools/sf36.shtml (accessed on 8 October 2014).
Wetterslev 2008
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta-analysis. Journal of Clinical Epidemiology 2008;61(1):64-75. [DOI] [PubMed] [Google Scholar]
Witjes 2012
- Witjes CD, Karim-Kos HE, Visser O, den Akker SA, Vries E, Ijzermans JN, et al. Hepatocellular carcinoma in a low-endemic area: rising incidence and improved survival. European Journal of Gastroenterology and Hepatology 2012;24(4):450-7. [DOI] [PubMed] [Google Scholar]
Wood 2008
- Wood L, Egger M, Gluud LL, Schulz KF, Jüni P, Altman DG, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta-epidemiological study. BMJ (Clinical Research Ed.) 2008;336(7644):601-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wu 1996
- Wu PC, Fang JW, Lau VK, Lai CL, Lo CK, Lau JY. Classification of hepatocellular carcinoma according to hepatocellular and biliary differentiation markers. Clinical and biological implications. American Journal of Pathology 1996;149(4):1167-75. [PMC free article] [PubMed] [Google Scholar]
Xiong 2012
- Xiong JJ, Altaf K, Javed MA, Huang W, Mukherjee R, Mai G, et al. Meta-analysis of laparoscopic vs open liver resection for hepatocellular carcinoma. World Journal of Gastroenterology 2012;18(45):6657-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yang 2014
- Yang Y, Zhang D, Feng N, Chen G, Liu J, Chen G, et al. Increased intake of vegetables, but not fruit, reduces risk for hepatocellular carcinoma: a meta-analysis. Gastroenterology 2014;147(5):1031-42. [DOI] [PubMed] [Google Scholar]


