Abstract
Background
Anxiety in relation to surgery is a well‐known problem. Melatonin offers an atoxic alternative to benzodiazepines in ameliorating this condition in the pre‐ and postoperative period.
Objectives
To assess the effect of melatonin on pre‐ and postoperative anxiety in adults when comparing melatonin with placebo or when comparing melatonin with benzodiazepines.
Search methods
The following databases were searched on 19 April 2013: CENTRAL, MEDLINE, EMBASE, CINAHL and Web of Science. For ongoing trials and protocols we searched clinicaltrials.gov, Current Controlled Trials and the World Health Organization (WHO) International Clinical Trials Registry Platform. We reran the search in October 2014. We will deal with any studies of interest when we update the review.
Selection criteria
Randomized, placebo‐controlled or standard treatment‐controlled, or both, studies that evaluated the effect of preoperatively administered melatonin on preoperative or postoperative anxiety. We included adult patients of both genders (15 to 90 years of age) undergoing any kind of surgical procedure in which it was necessary to use general, regional or topical anaesthesia.
Data collection and analysis
Data were extracted independently by two review authors. Data extracted included information about study design, country of origin, number of participants and demographic details, type of surgery, type of anaesthesia, intervention and dosing regimen, preoperative anxiety outcome measures and postoperative anxiety outcome measures.
Main results
This systematic review identified 12 randomized controlled trials (RCTs) including 774 patients that assessed melatonin for treating preoperative anxiety, postoperative anxiety or both. Four of the 12 studies compared melatonin, placebo and midazolam, whereas the remaining eight studies compared melatonin and placebo only.
The quality of the evidence for our primary outcome (melatonin versus placebo for preoperative anxiety) was high. More than half of the included studies had a low risk of selection bias and at least 75% of the included studies had a low risk of attrition, performance and detection bias. Most of the included studies had an unclear risk of reporting bias.
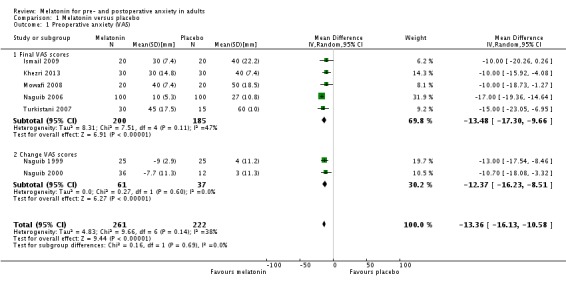
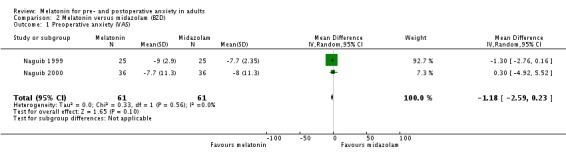
Eight out the 10 studies that assessed the effect of melatonin on preoperative anxiety using a visual analogue scale (VAS) (ranging from 0 to 100 mm, higher scores indicate greater anxiety) showed a reduction compared to placebo. The reported estimate of effect (relative effect ‐13.36, 95% confidence interval (CI) ‐16.13 to ‐10.58; high quality evidence) was based on a meta‐analysis of seven studies. Two studies did not show any difference between melatonin and placebo. Two studies comparing melatonin with midazolam using a VAS found no evidence of a difference in preoperative anxiety between the two groups (relative effect ‐1.18, 95% CI ‐2.59 to 0.23; low quality evidence).
Eight studies assessed the effect of melatonin on postoperative anxiety. Four of these studies measuring postoperative anxiety 90 minutes postoperatively using a VAS did not find any evidence of a difference between melatonin and placebo (relative effect ‐3.71, 95% CI ‐9.26 to 1.84). Conversely, two studies showed a reduction of postoperative anxiety measured six hours after surgery using the State‐Trait Anxiety Inventory (STAI) when comparing melatonin with placebo (relative effect ‐5.31, 95% CI ‐8.78 to ‐1.84; moderate quality evidence). Two studies comparing melatonin with midazolam using a VAS did not find any evidence of a difference between the two groups in postoperative anxiety (relative effect ‐2.02, 95% CI ‐5.82 to 1.78).
Authors' conclusions
When compared to placebo, melatonin given as premedication (tablets or sublingually) can reduce preoperative anxiety in adults (measured 50 to 100 minutes after administration). Melatonin may be equally as effective as standard treatment with midazolam in reducing preoperative anxiety in adults (measured 50 to 100 minutes after administration). The effect of melatonin on postoperative anxiety (measured 90 minutes and 6 hours after surgery) in adults is mixed but suggests an overall attenuation of the effect compared to preoperatively.
Keywords: Adult; Humans; Anti‐Anxiety Agents; Anti‐Anxiety Agents/therapeutic use; Anxiety; Anxiety/drug therapy; Clonidine; Clonidine/therapeutic use; Drug Administration Schedule; Melatonin; Melatonin/therapeutic use; Midazolam; Midazolam/therapeutic use; Postoperative Care; Preoperative Care; Publication Bias; Randomized Controlled Trials as Topic; Surgical Procedures, Operative; Surgical Procedures, Operative/psychology
Melatonin for pre‐ and postoperative anxiety in adults
Review question
We reviewed the evidence about the effect of melatonin compared to placebo or benzodiazepines ('Valium'‐like drugs that reduce anxiety) on pre‐ and postoperative anxiety in adults undergoing surgery.
Background
Anxiety occurs both before and after surgery in up to 80% of patients. Patients' anxiety before and after surgery can lead to unwanted events and effects. Melatonin is a hormone produced in the pineal gland in the brain that regulates circadian rhythm. Studies have shown that melatonin can reduce anxiety. In comparison to the widely used benzodiazepines in treating anxiety, melatonin produces no 'hang‐over effects' and has no known serious side effects and could therefore be a worthy alternative.
Study characteristics
The evidence was current to April 2013. We found 12 studies involving 774 patients. The age of the participants, in the studies, ranged from 19 to 80 years. Types of surgery and anaesthesia varied. The melatonin doses varied from 3 to14 mg and were administered 50 to 100 minutes before surgery. Midazolam (a benzodiazepine) doses ranged from 3.5 to 15 mg.
We reran the search in October 2014. We will deal with any studies of interest when we update the review.
Key results
Four studies compared melatonin, placebo and midazolam; eight studies compared melatonin and placebo only.
Comparing the effect of melatonin with placebo, melatonin may reduce preoperative anxiety. It may also reduce postoperative anxiety compared with placebo, measured six hours after surgery.
When comparing the effect of melatonin with midazolam preoperatively, there was no difference in anxiety. Postoperatively, there was no difference when comparing the effect of melatonin with placebo on anxiety measured 90 minutes after surgery or when comparing the effect of melatonin with midazolam.
Quality of the evidence
The quality of the evidence varied by outcome. We are confident that melatonin reduces anxiety preoperatively from the short term data in the review. We are less certain of this effect six hours postoperatively.
Whether the anxiety reducing effect of melatonin can be applied to all surgical patients remains unclear, as many factors influence the risk of anxiety; among these are age, gender, type of surgery, type of anaesthesia, and cultural and religious differences. Younger age and female gender are independent risk factors for anxiety and this may be a limitation as four studies only included women and three only included patients older than 60 years. Eight studies were carried out in Middle‐East countries; this might be a limitation with regard to generalizability.
Conclusions
Melatonin compared to placebo, given as premedication (tablets or under the tongue (sublingually)) reduced preoperative anxiety (measured 50 to 100 minutes after administration). Melatonin may be equally as effective as standard treatment with midazolam in reducing preoperative anxiety (measured 50 to 100 minutes after administration). When compared to placebo, melatonin may reduce postoperative anxiety (six hours after surgery).
Summary of findings
Summary of findings for the main comparison.
| Melatonin compared with placebo for preoperative anxiety | |||
|
Patient or population: Patients undergoing elective surgery Settings: Hospital Intervention: Melatonin Comparison: Placebo | |||
| Outcomes | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) |
| VAS (0 to 100 mm) measured approximately 90 min after premedication 0: no anxiety 100: maximum anxiety possible |
‐13.36 (‐16.13, ‐10.58) | 483 (7) |
⊕⊕⊕⊕ high |
| CI: Confidence interval; VAS: Visual Analogue Scale | |||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||
Summary of findings 2.
| Melatonin compared with midazolam (BZD) for preoperative anxiety | |||
|
Patient or population: Patients undergoing elective surgery Settings: Hospital Intervention: Melatonin Comparison: Midazolam | |||
| Outcomes | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) |
| VAS (0 to 100 mm) measured approximately 90 min after premedication 0: no anxiety 100: maximum anxiety possible |
‐1.18 (‐2.59, 0.23) | 122 (2) |
⊕⊕⊝⊝ low |
| CI: Confidence interval; VAS: Visual Analogue Scale | |||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||
The quality of the evidence is downgraded two levels due to the risk of bias in the included studies, the wide confidence interval of one study and the relatively low overall number of participants.
Summary of findings 3.
| Melatonin compared with placebo for postoperative anxiety | |||
|
Patient or population: Patients undergoing elective surgery Settings: Hospital Intervention: Melatonin Comparison: Placebo | |||
| Outcomes | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) |
|
Postoperative anxiety (STAI) Measured 6 hours postoperatively. The range of scores is 20 to 80, the higher scores indicating greater anxiety |
‐5.31 (‐8.78, ‐1.84) | 73 (2) |
⊕⊕⊕⊝ moderate |
| CI: Confidence interval; STAI: State‐Trait Anxiety Inventory | |||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||
The quality of the evidence is downgraded one level due to the low overall number of participants.
Background
Description of the condition
Anxiety is a human reaction to any unknown situation and is defined as a state of uneasiness and apprehension (Jellish 2012). Anxiety frequently occurs in patients throughout the perioperative period (Jellish 2012; Johnston 1980).
Preoperative anxiety is described as an unpleasant state of tension that is secondary to a patient being concerned about a disease, hospitalization, incapacitation, anaesthesia, surgery, or his or her anticipation of postoperative pain and the unknown (Caumo 2001a; Ramsay 1972). In clinical studies, the prevalence of preoperative anxiety has varied widely, from 11% to 80%, depending on the methods used to assess it (Corman 1958; Johnston 1980; Norris 1967; Wallace 1984). Surprisingly, in only a minority of patients does the anxiety peak on the day of surgery. In fact, high levels of anxiety occur for at least five or six days prior to admission to hospital and, for some patients, anxiety remains high for several days after surgery (Johnston 1980). Risk factors for preoperative anxiety include female gender, high trait anxiety, negative future perception, history of cancer and smoking, previous psychiatric disorders, moderate to intense depressive symptoms and higher educational level (> 12 years) (Caumo 2001a). Previous surgery reduces the risk for preoperative anxiety (Caumo 2001a). Furthermore, preoperative anxiety has been found to correlate with high postoperative anxiety (Caumo 2001).
Historically postoperative anxiety has received less attention than preoperative anxiety, however recent evidence suggests that postoperative anxiety may have adverse effects on postoperative outcome (Jellish 2012). Risk factors shown to be associated with postoperative anxiety are moderate to intense postoperative pain, preoperative state anxiety, history of smoking, negative future perception and minor psychiatric disorders (Jellish 2012). Systemic multimodal analgesia has been shown to be a protective factor for postoperative anxiety (Jellish 2012).
According to the current literature, medical interventions with the most widely used anxiolytic‐sedatives (benzodiazepines), effective communication strategies in the perioperative period, perioperative education and music therapy can be used to successfully reduce surgical patients' anxiety (Bailey 2010; Jellish 2012).
Description of the intervention
Melatonin (N‐acetyl‐5‐methoxytryptamine) is synthesized from tryptophan and secreted principally by the pineal gland. It has an endogenous circadian rhythm of secretion induced by the suprachiasmatic nuclei of the hypothalamus that is entrained to the light and dark cycle (Claustrat 2005). Melatonin has several putative functions including the regulation of circadian rhythm, and sedative, analgesic, anxiolytic, anti‐inflammatory, antioxidative and oncostatic effects (Brzezinski 1997; Ebadi 1998; Maestroni 1993; Reiter 1995).
Exogenous melatonin is produced synthetically from reacting chemical compounds (Jarratt 2011). Synthetic (pharmaceutical grade) melatonin is produced from pharmacy‐grade ingredients under strict laboratory conditions. It is presented as tablets, capsules, liquids or powder.
Although synthetic melatonin is molecularly identical to endogenous melatonin its bioavailability varies widely. Oral doses (1 to 5 mg) result in serum melatonin concentrations that are 10 to 100 times higher than the usual night‐time peak within one hour after ingestion, followed by a decline to baseline values in four to eight hours. Very low oral doses (0.1 to 0.3 mg) given in the daytime result in peak serum concentrations that are within the normal night‐time range (Brzezinski 1997).
How the intervention might work
Anxiety is considered to be a multifactorial phenomenon in which genetic, biochemical, humoral, neurophysiological and psychological factors are integrated.
Autoradiographic studies and receptor assays in humans have demonstrated the presence of melatonin receptors in various regions of the central nervous system (CNS) and in other tissues (Stankov 1991). In addition, both experimental (Tian 2010) and clinical studies (Acil 2004; Caumo 2007; Caumo 2009; Ionescu 2008; Ismail 2009; Khezri 2013; Mowafi 2008; Naguib 1999; Naguib 2000; Naguib 2006; Turkistani 2007) have shown an anxiolytic effect of melatonin. Exogenous administration of melatonin has been found to facilitate the onset of sleep and improve its quality (Wurtman 1995). In comparison to the widely used benzodiazepines as premedication, melatonin produces no residual effects or suppression of rapid eye movement sleep (Zhdanova 1995) and could therefore be a worthy alternative.
Due to the various effects of melatonin (regulation of circadian rhythm, and sedative, analgesic, anti‐inflammatory, antioxidative and oncostatic effects (Brzezinski 1997; Ebadi 1998; Maestroni 1993; Reiter 1995)) it is not possible to distinguish the direct anxiolytic effect since it is possibly an interaction of several of these mechanisms.
Melatonin is considered a drug of low toxicity. A safety study done with very high oral doses of melatonin (Nickkholgh 2011) (50 mg/kg body weight orally) showed no serious adverse events and a systematic review from 2006 (Buscemi 2006) reported headache, dizziness, nausea and sleepiness as the most common side effects but with frequencies comparable to placebo.
Why it is important to do this review
Patients' preoperative anxiety influences their postoperative anxiety (Caumo 2001), pain (Kain 2000; Thomas 1995), analgesic requirements (Thomas 1995), length of hospital stay (Caumo 2001) and satisfaction with their perioperative care and treatment (Caumo 2001a; Jamison 1993). Perioperative anxiety can lead to aggressive reactions that result in an increase in the distress experienced by the patient and make the management and control of postoperative pain more difficult (Caumo 2001a). In addition, psychological distress, including pre‐ and postoperative anxiety, may lead to more frequent demands for analgesics in patient‐controlled analgesia as well as increased intraoperative analgesic requirements (Ip 2009; Pan 2006). Overall, it appears that patients with a high state and trait anxiety level or a high level of distress (depression, anxiety, stress) preoperatively may experience higher rates of postoperative complications and have impaired wound healing (Mavros 2011). Furthermore, preoperative anxiety has been shown to be a predictor of mortality and major morbidity in older patients (> 70 years) undergoing cardiac surgery (Williams 2013). Overall, treating anxiety in the perioperative period can improve the perioperative experience of the patient (Jellish 2012).
It is common practice in many day case surgical units to use benzodiazepines, opioids or beta‐blockers as anxiolytic premedication when needed (Walker 2009). Safe use of these drugs is limited by their known adverse effects. In particular, benzodiazepines are known for psychomotor impairment, cognitive impairment, daytime sleepiness and sedation ('hang‐over effect') even after single‐dose administration (Ashton 1994; Edwards 1981; Gudex 1991; Woods 1992).
The potential clinical benefits of new therapeutic options in this setting have only been sparsely investigated. Several studies have investigated the perioperative anxiolytic effect of melatonin (Capuzzo 2006), where some have found positive results (Acil 2004; Caumo 2007; Caumo 2009; Ionescu 2008; Ismail 2009; Khezri 2013; Mowafi 2008; Naguib 1999; Naguib 2000; Naguib 2006; Turkistani 2007). Furthermore, melatonin is a non‐toxic drug with no reports of serious adverse events with short term use (less than three months) (Buscemi 2006; Nordlund 1977; Seabra 2000).
The hypnotic, antinociceptive and anticonvulsant properties of melatonin endow this neurohormone with the profile of a novel hypnotic‐anaesthetic agent (Naguib 2007). Melatonin administration is also associated with a tendency toward faster recovery and a lower incidence of postoperative excitement than with midazolam (Naguib 2007). Therefore, we found it important and relevant to investigate whether melatonin can provide the premedication anxiolytic effect and postoperative anxiolytic effect sometimes needed in day case and in‐patient surgery.
Objectives
To assess the effect of melatonin on pre‐ and postoperative anxiety in adults when comparing melatonin with placebo or when comparing melatonin with benzodiazepines.
Methods
Criteria for considering studies for this review
Types of studies
We included randomized placebo‐controlled or standard treatment‐controlled, or both, studies that evaluated the effect of melatonin on preoperative or postoperative anxiety.
We included studies irrespective of language and publications status. We excluded quasi‐randomized studies.
Types of participants
We included adult patients of both genders (15 to 90 years of age) undergoing any kind of surgical procedure in which it was necessary to use general, regional or topical anaesthesia.
Types of interventions
In order to be included, patients had to receive melatonin, placebo or a benzodiazepine administered on the day before surgery or immediately before surgery.
The intervention group (melatonin) was compared to a group receiving placebo or compared to a group receiving benzodiazepines.
Types of outcome measures
Primary outcomes
Preoperative anxiety measured by a visual analogue scale (VAS), State‐Trait Anxiety Inventory (STAI), or any other validated assessment tool.
The STAI is an administered analysis of reported anxiety symptoms. Its subscales measure state anxiety and trait anxiety. It is used in health research to differentiate anxiety from depression. The range of scores is 20 to 80, the higher scores indicating greater anxiety.
VAS is a 100 mm scale, ranging from 0 to 100, where the extremes are marked 'no anxiety' and 'worst anxiety ever'.
Both the simple VAS and the STAI have proved to be useful and valid measures of preoperative anxiety, and are equivalent in terms of the assessment of preoperative anxiety (Kindler 2000; Millar 1995).
Secondary outcomes
Postoperative anxiety measured by VAS or STAI.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library (April 2013), MEDLINE (OvidSP) (1966 to 19 April 2013), EMBASE (OvidSP) (1980 to 19 April 2013); CINAHL (EBSCOhost) (1982 to 19 April 2013) and ISI Web of Science (1945 to 19 April 2013). We reran the search in October 2014. We will deal with any studies of interest when we update the review.
We did not apply any language restrictions.
We combined our subject search terms with the sensitive search strategies described in Section 6.4 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) to search for randomized controlled trials (RCTs) in MEDLINE and EMBASE.
We searched CENTRAL using the terms found in Appendix 1. We adapted our MEDLINE search strategy (Appendix 2) to reflect the subject headings found in the thesauri used by EMBASE (Appendix 3), CINAHL (Appendix 4) and Web of Science (Appendix 5).
For ongoing trials, we searched the following databases:
Clinicaltrials.gov;
Current Controlled Trials;
World Health Organization (WHO) International Clinical Trials Registry Platform (WHO's Trial Search ).
Searching other resources
We screened the reference lists of all eligible trials and reviews. We contacted authors of published trials (MVH) when necessary.
Data collection and analysis
Selection of studies
Using the results of the above searches, we screened all titles and abstracts for eligibility and excluded the ones that clearly did not meet the inclusion criteria. Two authors (MVH and NLH) independently performed this screening. For the remaining studies, we read the full manuscript in order to assess if it should be included. Each of the two authors independently documented the reason for a trial's exclusion and combined the results in one document (see Excluded studies).
In the case of insufficient published information in order to make a decision about inclusion, we contacted the corresponding author of the relevant trial (MVH).
Details on the included studies can be seen in Characteristics of included studies.
Data extraction and management
Two authors (MVH and NLH) independently extracted data using a standard form and agreed on the data before entry into RevMan. Any discrepancies in the extracted data were resolved by discussion (MVH and NLH).
In the case of additional information being required, MVH or NLH contacted the corresponding author of the relevant trial.
Data extracted included information about: study design, country of origin, number of participants and demographic details, type of surgery and anaesthesia, intervention and dosing regimen, preoperative anxiety outcome measures, and postoperative anxiety outcome measures.
Assessment of risk of bias in included studies
Two authors (MVH and NH) independently assessed the methodological quality of the included trials.
We performed the assessment as suggested in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), see the 'Risk of bias' table in Characteristics of included studies.
The authors assessed the risk of bias for the following domains.
Random sequence generation.
Allocation concealment.
Incomplete outcome data.
Selective reporting.
Blinding of participants and personnel.
Blinding of outcome assessment.
The authors judged each of the above domains to have a low (adequate), high (inadequate) or unclear risk of bias and resolved any disagreement by discussion.
1. Random sequence generation (checking for possible selection bias)
We considered random sequence generation adequate if it was generated by a computer or random number table algorithm. We judged other processes, such as tossing a coin, adequate if the whole sequence was generated prior to the start of the trial and if it was performed by a person not otherwise involved in patient recruitment.
We considered random sequence generation unclear if there was insufficient information about the sequence generation process to permit judgement.
We considered random sequence generation inadequate if a non‐random system, such as dates, names or identification numbers, was used.
2. Allocation concealment (checking for possible selection bias)
We considered concealment adequate if the process that was used prevented patient recruiters, investigators and participants from knowing the intervention allocation of the next participant to be enrolled in the study. Acceptable systems included: a central allocation system, sealed opaque envelopes or an on‐site locked computer.
We considered allocation concealment unclear if the method of concealment was not described.
We considered concealment inadequate if the allocation method that was used allowed the patient recruiters, investigators or participants to know the treatment allocation of the next participant to be enrolled in the study; for example, alternate medical record numbers, reference to case record numbers or date of birth, an open allocation sequence or unsealed envelopes.
3. Incomplete outcome data (checking for possible attrition bias)
We considered dropout or missing data reported as adequate if studies had no dropouts or missing data. We also considered the domain adequate if studies described reasons for dropouts and there were balanced numbers of participants dropping out across intervention groups.
4. Selective reporting (checking for possible reporting bias)
We considered selective reporting adequate if the study protocol was available and all of the study's pre‐specified outcomes were reported in the article.
We considered selective reporting unclear if a study protocol was referred to but not obtainable, or if no study protocol was available.
We considered selective reporting inadequate if one or more outcomes reported in the article were not pre‐specified in the study protocol.
5. Blinding of participants and personnel (checking for possible performance bias)
We considered blinding adequate if the participants and personnel were each blinded to the intervention. With regards to the intervention, blinding is considered adequate if the melatonin, placebo or benzodiazepines have an identical appearance.
We considered blinding unclear if there was insufficient information to permit judgement.
We considered blinding inadequate if the participants and personnel were not blinded to the intervention.
6. Blinding of outcome assessment (checking for possible detection bias)
We considered blinding of outcome assessors adequate if the blinding was sufficiently described.
We considered blinding of outcome assessors unclear if there was insufficient information to permit judgement.
We considered blinding of outcome assessors inadequate if the outcome assessors were not blinded.
Measures of treatment effect
We extracted VAS data for our primary outcome as the mean (SD) or median (interquartile range (IQR), range). We chose to analyse VAS data as continuous data and presented these as the mean difference when outcome measures were on the same scale. We expressed the overall results for our primary outcome as effect size with 95% confidence intervals.
Unit of analysis issues
We only included randomized placebo‐controlled, standard treatment controlled, single or double‐blind trials. The unit of randomization was the individual patient; hence there were no unit of analysis issues.
Dealing with missing data
Whenever possible, we contacted the original investigators to request missing data.
Assessment of heterogeneity
We assessed the clinical heterogeneity of included studies, assessed as clinical diversity (for example different types of anaesthesia (regional, general, topical), differences in patient characteristics, variable melatonin doses, differences in analgesics etc.) and as methodological diversity (variability in study design and risk of bias).
We assessed statistical heterogeneity with the I2 statistic, thereby estimating the percentage of total variance across studies that was due to heterogeneity rather than chance (Higgins 2011).
The authors interpreted the values of the I2 statistic as follows (Higgins 2011):
0% to 40%, might not be important;
30% to 60%, may represent moderate heterogeneity;
50% to 90%, may represent substantial heterogeneity;
75% to 100%, considerable heterogeneity.
Assessment of reporting biases
We were not able to assess publication bias or small study effects in a qualitative manner using a funnel plot as there were less than 10 studies included in the meta‐analysis. If sufficient numbers of included studies are available in future updates of the review, the authors will assess publication bias by using funnel plots in Review Manager 5.2 (RevMan 5.2).
Data synthesis
We performed the data synthesis and statistical analysis using Review Manager software (RevMan 5.2). Because the population was varied, we included all types of: anaesthesia, surgery, age groups, dosing regimens and study sizes. Due to this variation a random‐effects model was suitable for the meta‐analysis.
Due to some studies using several different doses of melatonin or benzodiazepines, we chose to pool these groups.
Studies reported our primary outcome as the mean (SD) or median (IQR, range). One study used a numerical rating scale (NRS) (Capuzzo 2006) and we assumed that this was comparable to the VAS (Hjermstad 2011). If the studies did not present data in a tabular fashion, we read the values directly from the graphs. If the studies reported changes from baseline (VAS change scores), corresponding negative or positive values were used. Both studies reporting VAS and VAS change scores were entered in the same meta‐analysis as subgroups and the results of both subgroups were pooled.
In two studies (Naguib 1999; Naguib 2000) data were only presented graphically, and in one of the studies (Naguib 2000) the graph for melatonin and midazolam was difficult to read and the authors were contacted, but we received no answer. From the graph, the mean and SD had to be measured with a ruler and the VAS score was thus read. In the study by Naguib 1999 it was straightforward to measure mean and SD for the both the placebo and the melatonin arms. In the study by Naguib 2000 the mean of the placebo arm could be read and we assumed that the bar with the highest value indicated the SD of the placebo group. For the melatonin and the midazolam arms three doses were used and the means of the doses were pooled as they had an equal number of patients. We did not find it possible to read the SD of the six arms as we could not distinguish the error bars. Therefore, we chose to impute the SD for both the melatonin and the midazolam arms from the SD of the placebo arm. One study (Acil 2004) did not report SD for pre‐ or postoperative anxiety (we contacted the author but received no answer), so this study was not included in the meta‐analyses as the conversion from P value to SD was nonsensical. Two studies (Naguib 2006; Turkistani 2007) reported preoperative anxiety as the median (range) and we converted these to mean (SD) using the method in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). However, since this method was not robust, we performed sensitivity analysis excluding these two studies. For all other studies, we converted median (IQR) to mean (SD) using the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). However, for one study (Capuzzo 2006), regarding the data on preoperative anxiety, it was not possible to estimate a SD from the IQR as the outcome distribution was skewed; so this study was not included in the meta‐analyses for preoperative anxiety. For all studies reporting median, we assumed a symmetrical distribution of data and used the median value directly in the meta‐analyses as the mean. However, two studies (Capuzzo 2006; Khezri 2013) reported a median of 0, thereby violating the assumption of symmetry, and we therefore performed a sensitivity analysis without these two studies.
We analysed continuous data using an inverse variance method. We performed the analysis using Review Manager software (RevMan 5.2).
We chose to perform three meta‐analyses: primary outcome melatonin versus placebo (VAS) preoperatively, primary outcome melatonin versus midazolam (VAS) preoperatively, secondary outcome melatonin versus placebo (STAI) postoperatively.
Subgroup analysis and investigation of heterogeneity
We planned to perform the following subgroup analyses.
Participants' age (≤ 60 or > 60 years).
Anaesthetic modality (regional or general).
Melatonin dose (anticipated range 1 to 20 mg).
However, the clinical diversity of the studies was too large to be able to show a specific effect of the chosen variable (age, anaesthetic modality, melatonin dose) and therefore no subgroup analyses were performed.
Sensitivity analysis
We performed sensitivity analysis where we repeated the meta‐analysis for preoperative anxiety (VAS) after exclusion of two studies (Naguib 2006; Turkistani 2007). This was due to the fact that these two studies only reported median (range) for the VAS data on preoperative anxiety and this resulted in very large standard errors (we contacted the corresponding author of these studies to retrieve more detailed data but there was no response and an e‐mail delivery failure, respectively). We also performed sensitivity analysis for postoperative anxiety (VAS) after exclusion of two studies (Capuzzo 2006; Khezri 2013) due to the violation of the assumption of symmetry.
Summary of findings tables
We used the principles of the GRADE system (Guyatt 2008) to assess the quality of the body of evidence associated with our specific primary outcome (preoperative anxiety) in our review and constructed a 'Summary of findings' (SoF) table using Review Manager 5.2 (RevMan 5.2). The GRADE approach appraises the quality of a body of evidence based on the extent to which one can be confident that an estimate of effect or association reflects the item being assessed. The quality of a body of evidence considers study limitations, inconsistent results, indirectness of evidence, imprecision and publication bias.
Results
Description of studies
See Characteristics of included studies, Characteristics of excluded studies, Characteristics of studies awaiting classification and Characteristics of ongoing studies.
Twelve studies, published between 1999 and 2013, met the inclusion criteria. All studies compared melatonin with placebo, four studies also compared melatonin with the benzodiazepine midazolam (Acil 2004; Ionescu 2008; Naguib 1999; Naguib 2000) and one study compared melatonin with clonidine (Caumo 2009).
Results of the search
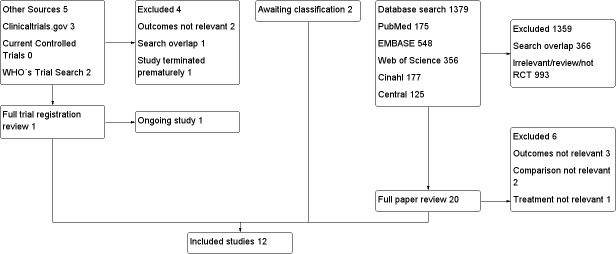
We identified 1036 references in primary electronic databases in April 2013 from our search strategy. We reran the search in October 2014. In this search we found 343 citations and 2 of these were of interest and are now awaiting classification. We will deal with these studies of interest when we update the review. Five additional references were identified through clinical trial registration databases.
Out of the total of 1379 references, 1359 were excluded: 366 duplicates and 993 after detailed reading of the title and abstract. Out of the five additional references we excluded four: one duplicate, one trial terminated prematurely and two after detailed reading of the trial description (Figure 1).
Figure 1.
Flow diagram.
We obtained the full text report for 20 studies to check if they strictly fulfilled all the inclusion criteria and excluded 6 due to the outcomes not being relevant, the study missing the right comparison group or the treatment not being relevant (Characteristics of excluded studies). Two studies are awaiting classification when we update the review (Characteristics of studies awaiting classification). Furthermore, one ongoing study (Characteristics of ongoing studies) was excluded from the additional references, leaving 12 studies which completely fulfilled the inclusion criteria for this review (Acil 2004; Capuzzo 2006; Caumo 2007; Caumo 2009; Ionescu 2008; Ismail 2009; Khezri 2013; Mowafi 2008; Naguib 1999; Naguib 2000; Naguib 2006; Turkistani 2007).
Included studies
See Characteristics of included studies.
There were 774 patients randomized in classically designed RCTs, of whom 701 patients (10 studies) (Acil 2004; Capuzzo 2006; Ionescu 2008; Ismail 2009; Khezri 2013; Mowafi 2008; Naguib 1999; Naguib 2000; Naguib 2006; Turkistani 2007) had data concerning preoperative anxiety and 568 patients (8 studies) (Acil 2004; Capuzzo 2006; Caumo 2007; Caumo 2009; Ionescu 2008; Khezri 2013; Naguib 1999; Naguib 2000) had data concerning postoperative anxiety. The age of the included patients ranged from 19 to 80 years.
The number of participants in the studies varied from 33 to 200. Of the 12 studies, four studies only included women (Caumo 2007; Caumo 2009; Naguib 1999; Naguib 2000). Eight out of the 12 studies were carried out in Middle‐East countries (Saudi Arabia, Turkey and Iran), one in Italy, one in Romania and two in Brazil.
Type of surgery and anaesthesia
Two studies were performed in patients undergoing abdominal hysterectomy (Caumo 2007; Caumo 2009), two studies in patients undergoing cataract surgery (Ismail 2009; Khezri 2013), two studies in patients undergoing laparoscopic cholecystectomy (Acil 2004; Ionescu 2008), two studies in patients undergoing gynaecological laparoscopic procedures (Naguib 1999; Naguib 2000), one study in patients undergoing elective hand surgery (Mowafi 2008), two studies in patients undergoing different surgical procedures (not specified) (Capuzzo 2006; Turkistani 2007) and one study did not specify the type of surgery (Naguib 2006).
Eight studies used general anaesthesia (Acil 2004; Caumo 2007; Caumo 2009; Ionescu 2008; Naguib 1999; Naguib 2000; Naguib 2006; Turkistani 2007), two studies used topical anaesthesia (Ismail 2009; Khezri 2013), one study used regional anaesthesia (Mowafi 2008) and one study did not specify the type of anaesthesia (Capuzzo 2006).
Interventions
The melatonin doses varied from 3 to 14 mg and were administered sublingually or orally approximately 60 to 90 minutes before surgery. Three studies also administered one dose of melatonin the evening before surgery (Caumo 2007; Caumo 2009; Ionescu 2008) and one of these studies (Caumo 2009) compared melatonin with placebo and clonidine.
Midazolam was administered in doses ranging from 3.5 to 15 mg.
Missing information and unspecified issues
In the case of any missing information or unspecified issues, we contacted the authors to clarify these issues. Details can be seen in the 'notes' in the Characteristics of included studies.
Excluded studies
We excluded nine studies; for detailed reasons see Characteristics of excluded studies.
Awaiting classification
Two studies (Khezri 2013a; Pokharel 2014) are awaiting classification; see Characteristics of studies awaiting classification.
Ongoing studies
One study (Hosseini 2014) is ongoing; see Characteristics of ongoing studies.
Risk of bias in included studies
We assessed each study using the Cochrane risk of bias tool. Overall findings are presented in the 'Risk of bias' graph (Figure 2), which shows the authors' judgements about each risk of bias item presented as percentages across all included studies; and the 'Risk of bias' summary (Figure 3), which shows the authors' judgements about each risk of bias item for each included study.
Figure 2.
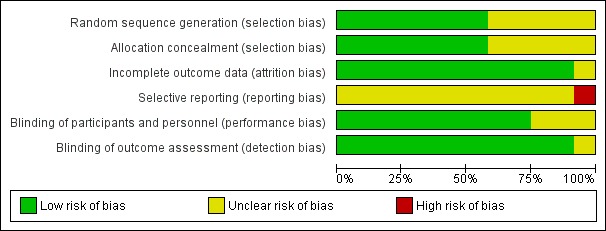
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Figure 3.
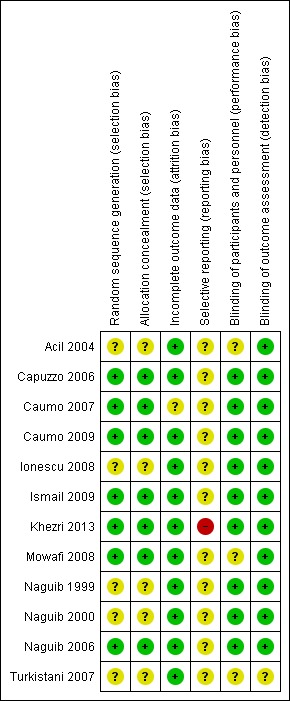
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Seven studies adequately described the method used to generate the random sequence and conceal the allocation (Capuzzo 2006; Caumo 2007; Caumo 2009; Ismail 2009; Khezri 2013; Mowafi 2008; Naguib 2006).
Blinding
Blinding of participants and personnel was adequately described in nine studies (Capuzzo 2006; Caumo 2007; Caumo 2009; Ionescu 2008; Ismail 2009; Khezri 2013; Naguib 1999; Naguib 2000; Naguib 2006) and blinding of outcome assessors was adequately described in 11 studies (Acil 2004; Capuzzo 2006; Caumo 2007; Caumo 2009; Ionescu 2008; Ismail 2009; Khezri 2013; Mowafi 2008; Naguib 1999; Naguib 2000; Naguib 2006).
Incomplete outcome data
All studies included in this review, except one (Caumo 2007), had a low risk of attrition bias. The studies adequately accounted for their dropouts and reported reasons for attrition and exclusions.
Selective reporting
There was an unclear risk of reporting bias as there was no study protocol available for 11 studies. One study (Khezri 2013) had an available protocol but the outcomes were not in accordance with what was reported in the article, as two outcomes (analgesic consumption by fentanyl requirements and intraoperative conditions assessed by scale) were not mentioned in the protocol.
Effects of interventions
See: Table 1; Table 2; Table 3
See 'Summary of findings' tables (Table 1; Table 2; Table 3), 'Additional' table (Table 6) and 'Data and analyses' tables (Data and analyses).
Table 1.
Primary and secondary outcomes
| Author, year | Preoperative VAS | Preoperative STAI | Postoperative VAS | Postoperative STAI |
| Acil 2004 | ↓ (90 min after premed.) compared to placebo → (90 min after premed.) compared to midazolam |
NM | ↓ (90 min postop) compared to placebo ↓ (90 min postop) compared to midazolam |
NM |
| Capuzzo 2006 | → (90 min after premed.) compared to placebo | NM | → (in recovery room) compared to placebo | NM |
| Caumo 2007 | NM | NM | NM | ↓ (6 h postop) compared to placebo |
| Caumo 2009 | NM | NM | NM | ↓ (6 h postop) compared to placebo → (6 h postop) compared to clonidine |
| Ionescu 2008 | NM | → (90 min after premed.) compared to placebo → (90 min after premed.) compared to midazolam |
NM | ↓ (6 h postop) compared to placebo → (6 h postop) compared to midazolam |
| Ismail 2009 | ↓ (90 min after premed.) compared to placebo | NM | NM | NM |
| Khezri 2013 | ↓ (60 min after premed.) compared to placebo | NM | ↓ (before discharge from recovery room) compared to placebo | NM |
| Mowafi 2008 | ↓ (90 min after premed.) compared to placebo | NM | NM | NM |
| Naguib 1999 | ↓ (90 min after premed.) compared to placebo → (90 min after premed.) compared to midazolam |
NM | → (90 min postop) compared to placebo → (90 min postop) compared to midazolam |
NM |
| Naguib 2000 | ↓ (90 min after premed.) compared to placebo → (90 min after premed.) compared to midazolam |
NM | → (90 min postop) compared to placebo → (90 min postop) compared to midazolam |
NM |
| Naguib 2006 | ↓ (50 min after premed.) compared to placebo | NM | NM | NM |
| Turkistani 2007 | ↓ (approximately 100 min after premed.) compared to placebo | NM | NM | NM |
Year: year of publication
Postop.: postoperative
Premed.: premedication
VAS: Visual analogue scale
STAI: State‐Trait Anxiety Inventory
→: no significant difference between groups
↓ : lower, significant difference compared to placebo or midazolam
NM: not measured
min: minutes
h: hours
Preoperative anxiety
We assessed preoperative anxiety between 50 to 100 minutes after premedication to be able to extract data from all studies. If the studies applied different doses of melatonin or midazolam we pooled the reported results.
Melatonin versus placebo
Eight studies (Acil 2004; Ismail 2009; Khezri 2013; Mowafi 2008; Naguib 1999; Naguib 2000; Naguib 2006; Turkistani 2007) reported a statistically significant reduction of preoperative anxiety measured by VAS when comparing melatonin with placebo. When extracting and converting results (median (IQR) to median (SD)) from one of these studies (Ismail 2009) the statistically significant effect of this study was lost, however the overall effect of the meta‐analysis with seven studies was significant (relative effect ‐13.36, 95% confidence interval (CI) ‐16.13 to ‐10.58; Figure 4). One study (Acil 2004) was not included in this meta‐analysis because the study did not report SD, and another (Capuzzo 2006) due to skewed outcome distribution. When performing a sensitivity analysis we showed a statistically significant reduction of preoperative anxiety (relative effect ‐11.24, 95% CI ‐14.04 to ‐8.44; Analysis 1.2).
Figure 4.
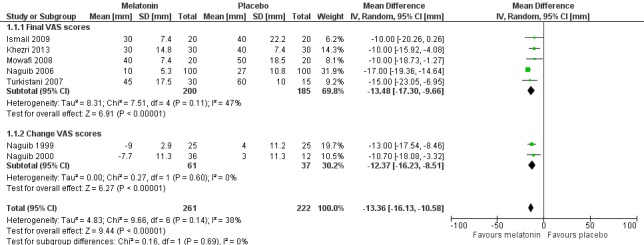
Forest plot of comparison: 1 Melatonin versus placebo, outcome: 1.1 Preoperative anxiety (VAS) (mm) with subgroup 1.1.1 Final VAS scores and subgroup 1.1.2 Change VAS scores.
Analysis 1.2.
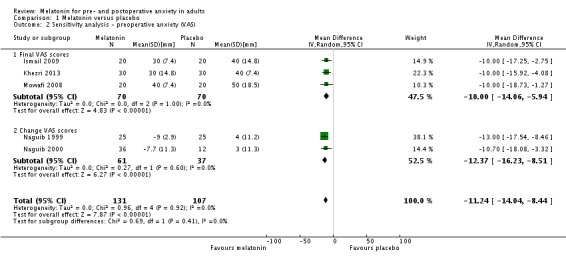
Comparison 1 Melatonin versus placebo, Outcome 2 Sensitivity analysis ‐ preoperative anxiety (VAS).
One study (Capuzzo 2006) showed no significant difference between melatonin and placebo in preoperative anxiety measured by NRS.
One study (Ionescu 2008) showed no significant difference between melatonin and placebo in preoperative anxiety measured by STAI.
Melatonin versus midazolam
Three studies (Acil 2004; Naguib 1999; Naguib 2000) compared melatonin with midazolam, using VAS. The meta‐analysis excluding one of the studies (Acil 2004) showed no significant difference in preoperative anxiety between the two groups (relative effect ‐1.18, 95% CI ‐2.59 to 0.23; Figure 5).
Figure 5.
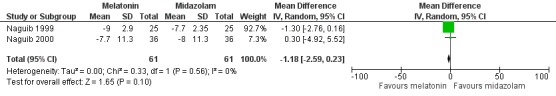
Forest plot of comparison: 2 Melatonin versus midazolam (BZD), outcome: 2.1 Preoperative anxiety (VAS).
One study (Ionescu 2008) measured preoperative anxiety using the STAI and compared melatonin with midazolam, and showed no significant difference between the two groups.
Postoperative anxiety
We assessed postoperative anxiety at two different times. We chose to group results from in the recovery room, at recovery room discharge and 90 minutes postoperatively as one group, and six hours postoperatively as another, in order to explore immediate and delayed anxiety.
Melatonin versus placebo
Five studies (Acil 2004; Capuzzo 2006; Khezri 2013; Naguib 1999; Naguib 2000) compared melatonin with placebo approximately 90 minutes postoperatively, using VAS or NRS. The meta‐analysis excluding one study (Acil 2004) showed no significant difference in postoperative anxiety between the two groups (relative effect ‐3.71, 95% CI ‐9.26 to 1.84; Analysis 1.3). When performing a sensitivity analysis there was still no significant difference (relative effect ‐1.20, 95% CI ‐4.75 to 2.35; Analysis 1.5).
Analysis 1.3.

Comparison 1 Melatonin versus placebo, Outcome 3 Postoperative anxiety (VAS).
Analysis 1.5.
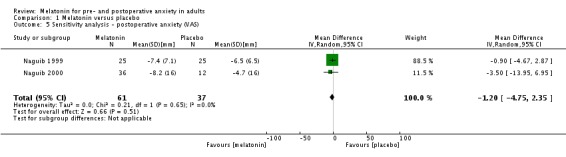
Comparison 1 Melatonin versus placebo, Outcome 5 Sensitivity analysis ‐ postoperative anxiety (VAS).
Three studies (Caumo 2007; Caumo 2009; Ionescu 2008) compared melatonin with placebo six hours postoperatively and measured postoperative anxiety using the STAI, however one of these studies only looked at the actual state anxiety (STAI‐S) and was therefore not included in the meta‐analysis. The two remaining studies (Caumo 2007; Caumo 2009) showed a statistically significant reduction in postoperative anxiety (relative effect ‐5.31, 95% CI ‐8.78 to ‐1.84; Figure 6). The study not included in the meta‐analysis (Ionescu 2008) showed a statistically significant reduction in postoperative anxiety measured six hours postoperatively.
Figure 6.

Forest plot of comparison: 1 Melatonin versus placebo, outcome: 1.4 Postoperative anxiety (STAI).
Melatonin versus midazolam
Three studies (Acil 2004; Naguib 1999; Naguib 2000) compared melatonin with midazolam approximately 90 minutes postoperatively, using VAS. The meta‐analysis excluding one study (Acil 2004) showed no significant difference in postoperative anxiety between the two groups (relative effect ‐2.02, 95%CI ‐5.82 to 1.78; Analysis 2.2).
Analysis 2.2.
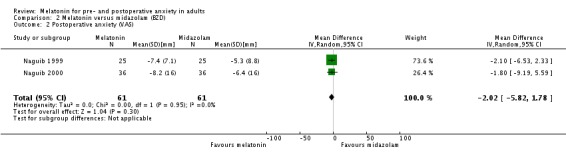
Comparison 2 Melatonin versus midazolam (BZD), Outcome 2 Postoperative anxiety (VAS).
One study (Ionescu 2008) measured postoperative anxiety using the STAI‐S six hours postoperatively and showed no significant difference between the two groups.
Discussion
Summary of main results
This systematic review identified 12 randomized controlled trials (RCTs) assessing melatonin for treating preoperative anxiety, postoperative anxiety, or both. Four of the 12 studies compared melatonin, placebo and midazolam, whereas the remaining eight studies compared melatonin and placebo only.
When considering the individual studies, 8 out the 10 studies that assessed the effect of melatonin on preoperative anxiety showed a significant reduction compared to placebo. A meta analysis including seven of these studies still showed a significant reduction compared to placebo. Two studies did not show any difference between melatonin and placebo.
Four studies comparing melatonin with midazolam showed no difference in preoperative anxiety between the two groups.
Eight studies assessed the effect of melatonin on postoperative anxiety. Three out of five of these individual studies measuring postoperative anxiety 90 minutes postoperatively did not show any difference between melatonin and placebo. A meta‐analysis including four of these studies did not find any evidence of a difference between melatonin and placebo either. Conversely, three studies showed a significant reduction of postoperative anxiety measured six hours after surgery when comparing melatonin with placebo. The four studies comparing melatonin with midazolam did not show any difference between the two groups in postoperative anxiety.
Overall completeness and applicability of evidence
The minimal clinically significant difference in pre‐ and postoperative anxiety VAS score has not been investigated previously. However, with regard to acute pain VAS scores it has been estimated that 9 to 14 mm on a 0 to 100 mm VAS is the minimal clinically significant difference (Kelly 1998; Kelly 2001). Thus, the main results from the meta‐analyses (13.36 mm and 11.24 mm) could possibly be considered clinically relevant.
Whether the anxiolytic effect of melatonin can be applied to all surgical patients remains unclear, as many factors have an influence on the risk of preoperative anxiety; among these are age, gender, type of surgery, type of anaesthesia, and cultural and religious differences (Caumo 2001a; Domar 1989; Kindler 2000; Lovering 2006). Younger age and female gender have been shown to be independent risk factors for preoperative anxiety (Caumo 2001a; Domar 1989; Kindler 2000). This may influence the external validity of our results as four of the studies in this review (Caumo 2007; Caumo 2009; Naguib 1999; Naguib 2000) only included women, and three of the studies (Capuzzo 2006; Ismail 2009; Khezri 2013) only included patients older than 60 years.
With regard to preoperative anxiety and the type of surgery, there are conflicting opinions in the literature, with one study (Caumo 2001a) showing that medium or major surgery leads to higher preoperative anxiety, and another study (Domar 1989) showing no difference regarding types of surgery and preoperative anxiety. The type of anaesthesia used, regional versus general, can also influence anxiety levels in different directions (Haugen 2009; Mitchell 2008; Mitchell 2010; Mitchell 2012). As far as general anaesthesia is concerned, many patients fear waking up during surgery or not waking up after surgery (Mitchell 2010; Ramsay 1972). Furthermore, general surgery is for the most part used for major surgery, which in itself may influence the risk of anxiety (Caumo 2001a). As far as regional anaesthesia is concerned, patients experience the anxiety of being awake during the procedure, involving all the noises, lights and pain associated with this (Mitchell 2008). The studies included in this review vary from minor to major surgery, performed with general, regional or topical anaesthesia, and there were not enough studies to be able to complete a subgroup analysis regarding type of surgery or anaesthesia.
Cultural and religious differences have been shown to influence the actual perception of anxiety (Lovering 2006). Eight out of the 12 studies were carried out in Middle‐East countries (Saudi Arabia, Turkey and Iran), one in Italy, one in Romania and two in Brazil. This could lead to an imbalance and influence the external validity as some cultures are over represented and others under represented.
In nine out of the 12 included studies the method of measuring preoperative anxiety has been the use of the VAS (one study NRS (Capuzzo 2006)) and only three studies have used the STAI. The VAS and STAI as anxiety measuring techniques were used to measure pre‐ and postoperative anxiety and have been validated in a surgical population (Kindler 2000). To date the gold standard for anxiety evaluation is the STAI, but its architecture of 20 multiple choice questions for anxiety alone limits its use as a bedside instrument, whereas the VAS scale allows patients to easily indicate their degree of pre‐ or postoperative anxiety by simply marking a point on a horizontal line. The simple VAS is a very easily applied method for both doctor and patient and has proven to be both useful and a valid measure of preoperative anxiety (Kindler 2000; Millar 1995).
Of the 12 studies in our review, six studies administered melatonin sublingually and six studies administered it orally as tablets. With sublingual administration (comparable to intravenous administration) first pass metabolism is surpassed compared to oral administration and this leads to variation in bioavailability (Brzezinski 1997). Due to the heterogeneity of the studies using sublingual or oral melatonin, additional studies are required in order to perform a relevant subgroup analysis.
Of the eight studies assessing postoperative anxiety, only three studies measured anxiety six hours postoperatively, whereas the remaining studies mainly assessed anxiety in the very near postoperative period (60 to 90 min). The only studies that showed an effect of melatonin were those measuring anxiety several hours after surgery. Hence, more studies are warranted to clearly determine the effect of melatonin on postoperative anxiety in the delayed postoperative period.
Quality of the evidence
More than half of the included studies have a low risk of selection bias and at least 75% of the included studies have a low risk of attrition, performance and detection bias. Most of the included studies have an unclear risk of reporting bias.
The estimate of effect for the primary outcome (preoperative anxiety) was judged as having a high and low quality of evidence, for the comparison of melatonin versus placebo and melatonin versus midazolam, respectively. The estimate of effect for the secondary outcome (postoperative anxiety) was judged as having a moderate quality of evidence for both comparisons. The reasons for the downgrading were an overall low number of participants, imprecision due to the wide confidence interval, and the risk of bias in each of the included studies.
Potential biases in the review process
To obtain additional information we contacted the authors of 10 of the included studies. One author answered sufficiently (Capuzzo 2006) whereas information regarding the remaining nine studies was not obtained as the authors did not reply, despite repeated attempts. This might introduce a potential source of bias.
Another potential source of bias in our estimate of effect could be that in studies only presenting data in a graphical fashion, we read the graphs. This could introduce uncertainty to the exact results.
The intention of this review was not to collect data on adverse events as the toxicity profile of melatonin is sufficiently understood from previous research. Hence, by omitting this outcome measure, this might introduce a potential source of bias. However, retrospectively, when viewing all the included studies in this review, we found that half of the studies did not report on side effects (Acil 2004; Capuzzo 2006; Caumo 2007; Caumo 2009; Naguib 2006; Turkistani 2007), three studies specifically reported that no side effects were noted (Ionescu 2008; Naguib 1999; Naguib 2000) and three studies reported side effects of melatonin comparable to placebo (Ismail 2009; Khezri 2013; Mowafi 2008).
Agreements and disagreements with other studies or reviews
Only one other systematic review has looked at the effect of melatonin as an anxiolytic in the perioperative period (Yousaf 2010). Their review identified 10 studies concerning perioperative anxiety. These studies are also included in our review, together with two others (Khezri 2013; Turkistani 2007). The study by Khezri et al was published after the search date of the review by Yousaf et al. The study by Turkistani et al was not included due to lack of a pre‐intervention anxiety score (exclusion criterion). In our review, the remaining 11 included studies all assessed pre‐intervention anxiety levels. However, due to the randomized design of all the included studies we did not believe that the lack of this specific assessment should be considered a potential confounder, hence this was not an exclusion criterion in our review.
There was agreement with our findings and the above mentioned review (Yousaf 2010) which reported, in accordance with our review, that melatonin premedication is effective in ameliorating perioperative anxiety. Yet, no quantitative analyses were undertaken (Yousaf 2010), mainly explained by the retrieved data being presented in a graphical fashion or as median and range. Furthermore, the authors (Yousaf 2010) found the heterogeneity of the studies too extensive to synthesize the data quantitatively. When exploring heterogeneity in our review, we found an I2 of 54% for our main analysis (Figure 4). We considered this moderate and not a substantial issue, hence we only performed random‐effects model meta‐analyses. In the process of analysing the data, we also performed a sensitivity analysis (data not shown) by excluding two studies which only reported median (range) for the VAS data on preoperative anxiety, resulting in very large standard errors. The I2 value for this sensitivity analysis was 10%. Due to the authors' (Yousaf 2010) assessment of heterogeneity, they conclude that future studies should focus on investigating the effect on more varied surgical populations and the optimal dosing regimen.
Authors' conclusions
When compared to placebo, melatonin given as premedication (tablets or sublingually) can reduce preoperative anxiety in adults (measured 50 to 100 minutes after administration). The significance of a 13‐point reduction in anxiety could be considered clinically relevant, and the reduction seems comparable to the reduction seen with midazolam. Melatonin may be equally as effective as the standard treatment with midazolam in reducing preoperative anxiety in adults (measured 50 to 100 minutes after administration), but results should be interpreted with caution as they are based on very low overall participant numbers. The effect of melatonin compared to placebo on postoperative anxiety in adults was mixed.
Future studies should be conducted with more patients of both gender and all ages. Studies should be conducted in several more countries, especially in Europe and America as these groups have not been studied yet. To clarify the effect in different surgical populations, more homogenous studies are needed with regard to type of anaesthesia and type of surgery. Even though a significant effect was found in this review, studies could be conducted with larger doses of melatonin to explore the possibility of even larger effects. In order to explore the prophylactic effect of melatonin on perioperative anxiety, future studies could also investigate the effect of treatment given approximately one week preoperatively and continuing until one week postoperatively. When conducting future studies the adverse effect profile of melatonin should be investigated more systematically and consistently.
Acknowledgements
We thank Jane Cracknell for her knowledge and practical engagement in the process and Karen Hovhannisyan for help in constructing our search strategies.
We also thank Tobias Wirenfeldt Klausen for his statistical knowledge and support.
We would like to thank Mark Neuman (content editor), Marialena Trivella (statistical editor), Ewan McNicol, Rita Katznelson (peer reviewers) and Durhane Wong‐Rieger (consumer representative) for their help and editorial advice during the preparation of the protocol for this systematic review.
We would like to thank Mark Neuman (content editor), Nathan Pace (statistical editor), Farhanah Yousaf, Ewan D McNicol and Patricia Tong (peer reviewers) for their help and editorial advice during the preparation of this systematic review.
Appendices
Appendix 1. Search strategy for CENTRAL, the Cochrane Library
#1 MeSH descriptor Melatonin explode all trees #2 Melatonin or N‐acetyl‐5‐methoxytryptamine #3 (#1 OR #2) #4 MeSH descriptor Anxiety explode all trees #5 MeSH descriptor Preoperative Care explode all trees #6 preoperativ* or anxiety* or pain:ti,ab or (analges* near treatment):ti,ab or (postoperative near period):ti,ab #7 (#4 OR #5 OR #6) #8 (#3 AND #7)
Appendix 2. Search strategy for MEDLINE (OvidSP)
1. exp Melatonin/ or Melatonin.af. or N‐acetyl‐5‐methoxytryptamine.mp. 2. exp Anxiety/ or Preoperative Period/ or Preoperative Care/ or exp Anesthesia Recovery Period/ or (preoperativ* or anxiety*).af. or pain.ti,ab. or (analges* adj3 treatment).mp. or postoperative period.ti,ab. 3. 1 and 2 4. ((randomized controlled trial or controlled clinical trial).pt. or randomized.ab. or placebo.ab. or drug therapy.fs. or randomly.ab. or trial.ab. or groups.ab.) not (animals not (humans and animals)).sh. 5. 3 and 4
Appendix 3. Search strategy for EMBASE (OvidSP)
1. exp melatonin/ or Melatonin.af. or N‐acetyl‐5‐methoxytryptamine.mp. 2. exp anxiety/ or preoperative treatment/ or preoperative period/ or preoperative care/ or postanesthesia care/ or postoperative analgesia/ or postoperative care/ or (preoperativ* or anxiety*).af. or pain.ti,ab. or (analges* adj3 treatment).mp. or postoperative period.ti,ab. 3. 1 and 2 4. (randomized‐controlled‐trial/ or randomization/ or controlled‐study/ or multicenter‐study/ or phase‐3‐clinical‐trial/ or phase‐4‐clinical‐trial/ or double‐blind‐procedure/ or single‐blind‐procedure/ or (random* or cross?over* or multicenter* or factorial* or placebo* or volunteer*).mp. or ((singl* or doubl* or trebl* or tripl*) adj3 (blind* or mask*)).ti,ab. or (latin adj square).mp.) not (animals not (humans and animals)).sh. 5. 3 and 4
Appendix 4. Search strategy for CINAHL (EBSCO host)
S1. (MM "Melatonin") or TX Melatonin or N‐acetyl‐5‐methoxytryptamine S2. ( (MM "Anxiety+") OR (MH "Preoperative Care") OR (MH "Preoperative Period") OR (MH "Postoperative Care") ) or TX ( preoperativ* or anxiety* ) or AB pain or AB ( analges* and treatment ) or postoperative period S3. S1 and S2
Appendix 5. Search strategy for ISI Web of Science
#1 TS=(Melatonin or N‐acetyl‐5‐methoxytryptamine) #2 TS=(preoperativ* or anxiety*) or TS=(analges* SAME treatment) or TS=(postoperative SAME period) or TI=pain #3 #2 AND #1
Data and analyses
Comparison 1.
Melatonin versus placebo
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Preoperative anxiety (VAS) | 7 | 483 | Mean Difference (IV, Random, 95% CI) | ‐13.36 [‐16.13, ‐10.58] |
| 1.1 Final VAS scores | 5 | 385 | Mean Difference (IV, Random, 95% CI) | ‐13.48 [‐17.30, ‐9.66] |
| 1.2 Change VAS scores | 2 | 98 | Mean Difference (IV, Random, 95% CI) | ‐12.37 [‐16.23, ‐8.51] |
| 2 Sensitivity analysis ‐ preoperative anxiety (VAS) | 5 | 238 | Mean Difference (IV, Random, 95% CI) | ‐11.24 [‐14.04, ‐8.44] |
| 2.1 Final VAS scores | 3 | 140 | Mean Difference (IV, Random, 95% CI) | ‐10.0 [‐14.06, ‐5.94] |
| 2.2 Change VAS scores | 2 | 98 | Mean Difference (IV, Random, 95% CI) | ‐12.37 [‐16.23, ‐8.51] |
| 3 Postoperative anxiety (VAS) | 4 | 296 | Mean Difference (IV, Random, 95% CI) | ‐3.71 [‐9.26, 1.84] |
| 3.1 Final VAS scores | 2 | 198 | Mean Difference (IV, Random, 95% CI) | ‐5.14 [‐14.93, 4.66] |
| 3.2 Change VAS scores | 2 | 98 | Mean Difference (IV, Random, 95% CI) | ‐1.20 [‐4.75, 2.35] |
| 4 Postoperative anxiety (STAI) | 2 | 73 | Mean Difference (IV, Random, 95% CI) | ‐5.31 [‐8.78, ‐1.84] |
| 5 Sensitivity analysis ‐ postoperative anxiety (VAS) | 2 | 98 | Mean Difference (IV, Random, 95% CI) | ‐1.20 [‐4.75, 2.35] |
Analysis 1.1.
Comparison 1 Melatonin versus placebo, Outcome 1 Preoperative anxiety (VAS).
Analysis 1.4.

Comparison 1 Melatonin versus placebo, Outcome 4 Postoperative anxiety (STAI).
Comparison 2.
Melatonin versus midazolam (BZD)
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Preoperative anxiety (VAS) | 2 | 122 | Mean Difference (IV, Random, 95% CI) | ‐1.18 [‐2.59, 0.23] |
| 2 Postoperative anxiety (VAS) | 2 | 122 | Mean Difference (IV, Random, 95% CI) | ‐2.02 [‐5.82, 1.78] |
Analysis 2.1.
Comparison 2 Melatonin versus midazolam (BZD), Outcome 1 Preoperative anxiety (VAS).
What's new
Last assessed as up‐to‐date: 19 April 2013.
| Date | Event | Description |
|---|---|---|
| 9 February 2017 | Amended | Plain language summary: we clarified that the age range referred to the age of the participants in the studies |
Differences between protocol and review
Our original intention (Hansen 2012) with this review was to clarify whether melatonin could be a worthy alternative and potentially substitute the use of the standard, anxiolytic premedication treatment with benzodiazepines, with all its known disadvantages. During the phase from development of the protocol to writing the review, we realised that pain and anxiety are related but the exact pathophysiological mechanisms are not entirely clear and treatment strategies for the two entities are different. We therefore believe that the topic of the analgesic effect of melatonin and the subsequent analgesic requirements deserves its own Cochrane Review, hence why we have chosen in this review to focus only on melatonin's anxiolytic effect in the perioperative period, explaining why we have removed two secondary outcomes (pain and analgesic treatment). We were concerned that the overall picture of melatonin's anxiolytic effect would be confused by including studies focusing on antinociception and the associated treatment.
In our review we therefore believe that we have successfully fulfilled the predetermined aim and even improved the clarity of the objective with regard to the anxiolytic effect of melatonin.
Specific changes.
TITLE:
We have changed the title to 'Melatonin for pre‐ and postoperative anxiety in adults' thereby covering the objectives.
BACKGROUND:
We have added two paragraphs about postoperative anxiety in 'Description of the condition' and also added some sentences in 'Why it is important to do this review'.
OBJECTIVES:
We have added postoperative anxiety to cover the perioperative period.
METHODS:
We have added postoperative anxiety to the paragraph in 'Types of studies'.
We have added topical anaesthesia to the 'Types of participants'.
In 'Types of outcome measures' the secondary outcomes pain and analgesic treatment have been omitted.
In 'Measures of treatment effect' ‐ as we did not have categorical data the sentence "we will present categorical data..." was omitted.
In 'Measures of treatment effect' ‐ regarding NNTB and NNTH, it was not possible to calculate these, therefore the sentence was deleted.
We changed the wording in 'Assessment of heterogeneity' to suit the heterogeneity we found in the included studies.
As we did not have dichotomous data the wording in 'Data synthesis' was changed accordingly. We added a new reference comparing NRS with VAS. We also added detailed information on the data synthesis according to the included studies.
As we have omitted two of the secondary outcomes, the 'Summary of findings tables' text was adapted to the relevant outcomes.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Randomized, double‐blind, placebo‐controlled study Location: Turkey Study design: parallel, 3‐armed (melatonin, midazolam, placebo) |
|
| Participants | A total of 66 patients, 22 patients in each arm Age: melatonin 39.9 ± 7.5, midazolam 37.3 ± 7.8, placebo 39.2 ± 6.8 Gender: not described ASA class: I‐II Type of surgery: laparoscopic cholecystectomy Type of anaesthesia: general Baseline (anxiety, pain) described: yes, no |
|
| Interventions | Melatonin: 5 mg Midazolam: 15 mg Placebo Administration route: sublingual Time of administration: 90 min preoperatively |
|
| Outcomes | 1. Anxiety measured by visual analogue scale (pre‐ and postoperative) 2. Sedation score 1 to 4 3. Orientation score 0 to 2 4. Psychomotor performance measured with Trail Making A and B tests and the Word Fluency test All outcomes were evaluated before (baseline), and 10, 30, 60 and 90 min after premedication had been given, and after the operation at 15, 30, 60 and 90 min in the recovery room 5. Pain measured by visual analogue scale 6. Satisfaction score (yes or no) |
|
| Notes | Sample size calculation: not described Author (Karagöz) contacted by e‐mail on 4 July 2013 to clarify unspecified issues: no answer Author (Acil) contacted by telephone on 9, 14, 17 October 2013 to clarify unspecified issues: no answer |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided. Described as randomized |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No reported dropouts or missing data |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Information not provided for taste of study drug |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "a doctor blinded to the group assignment performed all tests" (page 554) (Acil 2004) |
| Methods | Randomized, double‐blind, placebo‐controlled study Location: Italy Study design: parallel, 2‐armed |
|
| Participants | A total of 150 patients randomized, 12 did not complete 138 patients completed: 67 in melatonin group and 71 in placebo group Age: melatonin 73.2 ± 5.9, placebo 72.1 ± 5.4 Gender (M/F) in %: melatonin (48/52), placebo (52/48) ASA class: I‐III Type of surgery: elective surgery Type of anaesthesia: general or spinal Baseline (anxiety, pain) described: yes, yes |
|
| Interventions | Melatonin: 10 mg Placebo Administration route: oral Time of administration: 90 min preoperatively |
|
| Outcomes | 1. Anxiety measured by numerical rating scale (0 to 10) (pre‐ and postoperative) 2. Depression measured by numerical rating scale (0 to 10) 3. Pain measured by numerical rating scale (0 to 10) 4. Satisfaction with anaesthesia (0 to 100) 5. Cognitive function (Frontal Assessment Battery and Babcock Story Recall Test) |
|
| Notes | Sample size calculation: described Author (Capuzzo M) contacted by e‐mail 4 July 2013: investigators and assessors blinded. Mistake in the dropout numbers in the 2 groups; should be 4 in the placebo group and 8 in the melatonin group |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The pharmacist prepared, by computer‐generated randomization, 150 sealed envelopes, each reporting a code number and containing 2 capsules. Each indistinguishable capsule contained either 5 mg melatonin or placebo." (page 121) (Capuzzo 2006) |
| Allocation concealment (selection bias) | Low risk | Quote: "The pharmacist prepared, by computer‐generated randomization, 150 sealed envelopes, each reporting a code number and containing 2 capsules. Each indistinguishable capsule contained either 5 mg melatonin or placebo." (page 121) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Patients not completing the study were almost balanced in numbers across intervention groups and with similar reasons for lack of completion |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Patients and personnel blinded. Quote: "indistinguishable capsules" (page 121) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors and investigator blinded (confirmed by e‐mail contact with the author) |
| Methods | Randomized, double‐blind, placebo‐controlled study Location: Brazil Study design: parallel, 2‐armed |
|
| Participants | A total of 35 patients randomized, 2 did not complete 33 patients completed: 17 in melatonin group and 16 in placebo group Age: melatonin 44.82 ± 4.58, placebo 43.88 ± 4.09 Gender (M/F) in %: melatonin (0/100), placebo (0/100) ASA class: I‐III Type of surgery: abdominal hysterectomy Type af anaesthesia: general and epidural Baseline (anxiety, pain) described: yes, yes |
|
| Interventions | Melatonin: 5 mg Placebo Administration route: oral Time of administration: 10:00 pm the night before surgery and 1 hour preoperatively |
|
| Outcomes | 1. Postoperative pain assessed by pain scores (100 mm visual analogue scale) 2. Postoperative pain assessed by analgesic consumption (morphine in patient controlled analgesia) 3. Rest‐activity cycles measured by actigraphy 4. Anxiety assessed by State‐trait Anxiety Inventory (postoperative) |
|
| Notes | Sample size calculation: described Author contacted by e‐mail on 5 July 2013 to clarify unspecified issues: no answer |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "... using a random number table..." (page 1264) (Caumo 2007) |
| Allocation concealment (selection bias) | Low risk | Quote: " Blinding and randomization were performed by two investigators not involved in the patients evaluations." (page 1264) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "Two patients, however, were excluded for major protocol violations." (page 1266) |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Adequately described in the article |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Adequately described in the article |
| Methods | Randomized, double‐blind, placebo‐controlled study Location: Brazil Study design: parallel, 3‐armed |
|
| Participants | A total of 63 patients randomized, 4 did not complete 59 patients completed, 20 in melatonin group, 19 in clonidine group and 20 in placebo group Age: melatonin 43.40 ± 5.48, clonidine 45.26 ± 3.40, placebo 45.35 ± 5.67 Gender (M/F) in %: melatonin: (0/100), clonidine (0/100), placebo (0/100) ASA class: I‐III Type of surgery: abdominal hysterectomy Type af anaesthesia: general and epidural |
|
| Interventions | Melatonin: 5 mg Clonidine: 100 μg Placebo Administration route: oral Time of administration: 10:00 pm the night before surgery and 1 hour preoperatively for melatonin and placebo. For clonidine an extra dose was given 36 hours postoperatively and the melatonin and placebo group both received placebo at this time |
|
| Outcomes | 1. Postoperative pain assessed by pain scores (100 mm visual analogue scale) 2. Postoperative pain assessed by analgesic consumption (morphine in patient controlled analgesia) 3. Anxiety assessed by State‐Trait Anxiety Inventory (postoperative) |
|
| Notes | Sample size calculation: described Author contacted by e‐mail on 5 July 2013 to clarify unspecified issues: no answer |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequately described in the article |
| Allocation concealment (selection bias) | Low risk | Quote: "During the entire protocol timeline, blinding and randomization were undertaken by 2 investigators who were not involved in the patient's evaluation" (page 101) (Caumo 2009) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Flow diagram (figure 1) shows the 4 patients who did not complete and reasons. Quote: "...were excluded from analysis..." (page 103) |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "During the entire protocol timeline, blinding and randomization were undertaken by 2 investigators who were not involved in the patient's evaluation. Other individuals involved in the patient's care were unaware of the treatment group to which the patient belonged" (page 101) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "To ensure blinding, postoperative assessment was performed by a different physician from the 1 who carried out the preoperative evaluation" (page 101) |
| Methods | Randomized, double‐blind, placebo‐controlled study Location: Romania Study design: parallel, 3‐armed |
|
| Participants | A total of 53 patients randomized 53 patients completed: 18 in melatonin group, 17 in midazolam group, and 18 in placebo group Age: melatonin 43.05 ± 11.40, midazolam 48.76 ± 12.61, placebo 48.38 ± 10.11 Gender (M/F) in %: not adequately described ASA class: I‐II Type of surgery: laparoscopic cholecystectomy Type of anaesthesia: general Baseline (anxiety, pain) described: no, no |
|
| Interventions | Melatonin: 3 mg Midazolam: 3.75 mg Placebo Administration route: sublingual Time of administration: the night before surgery and 90 min preoperatively |
|
| Outcomes | 1. Sedation assessed by score 1 to 4 2. Anxiety assessed by CD Spielberger's questionnaire, State‐Trait Anxiety Inventory (STAI‐S) (pre‐ and postoperative) 3. Quality of postoperative sleep (good sleep, insomnia, nightmares) 4. Amnesia after recovery from anaesthesia (5 pictures) 5. Postoperative pain assessed by a visual analogue scale ‐ verbal rating (1 to 5) 6. Intraoperative fentanyl requirements |
|
| Notes | Sample size calculation: described but not adequately Author contacted by e‐mail on 5 July 2013 to clarify unspecified issues: no answer |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "...randomly allocated..." (page 9) (Ionescu 2008) |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No reported dropouts or missing data |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "To maintain the double‐blind nature of the study, the syringes were unmarked." (page 9) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "A registrar blinded to the group assignment performed all the tests" (page 9) |
| Methods | Randomized, double‐blind, placebo‐controlled study Location: Saudi Arabia Study design: parallel, 2‐armed |
|
| Participants | A total of 40 patients randomized 40 patients completed: 20 in melatonin group, 20 in placebo group Age: melatonin 72.8 ± 8.1, placebo 68.5 ± 7.9 Gender (M/F) in %: melatonin (55/45), placebo (50/50) ASA class: I‐III Type of surgery: cataract surgery Type of anaesthesia: topical Baseline (anxiety, pain) described: yes, no |
|
| Interventions | Melatonin: 10 mg Placebo Administration route: oral Time of administration: 90 min preoperatively |
|
| Outcomes | 1. Anxiety assessed by Verbal Anxiety Score (0 to 10) (preoperative) 2. Pain assessed by Verbal Pain Score (0 to 10) 3. Analgesic consumption by fentanyl requirements 4. Intraocular pressure (IOP) measured by Shioetz tonometer 5. Haemodynamcis (heart rate and mean arterial pressure) |
|
| Notes | Sample size calculation: described | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly allocated using an online research randomizer..." (page 1147) (Ismail 2009) |
| Allocation concealment (selection bias) | Low risk | Quote: "...randomly allocated using an online research randomizer..." (page 1147) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No reported dropouts or missing data |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "an ophthalmologist who was blinded..." and "...the operating surgeon, who was blinded to patient allocation..." and "The attending anaesthesiologist who was unaware of patient group assignment..." (page 1147) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The attending anaesthesiologist who was unaware of patient group assignment managed the patients and recorded all data" (page 1147) |
| Methods | Randomized, double‐blind, placebo‐controlled study Location: Iran Study design: parallel, 2‐armed |
|
| Participants | A total of 60 patients randomized 60 patients completed: 30 in melatonin group, 30 in placebo group Age: melatonin 63.5 ± 15.28, placebo 70.38 ± 13.48 Gender (M/F) in %: melatonin (40/60), placebo (57/43) ASA class: I‐III Type of surgery: cataract surgery Type of anaesthesia: topical Baseline (anxiety, pain) described: yes, yes |
|
| Interventions | Melatonin: 3 mg Placebo Administration route: sublingual Time of administration: 60 min preoperatively |
|
| Outcomes | 1. Anxiety assessed by Verbal Anxiety Score (0 to 10) (pre‐ and postoperative) 2. Pain assessed by Verbal Pain Score (0 to 10) 3. Intraoperative conditions assessed by scale 4. Intraocular pressure (IOP) measured by Shioetz tonometer 5. Haemodynamcis (heart rate, systolic blood pressure, diastolic blood pressure) 6. Analgesic consumption by fentanyl requirements |
|
| Notes | Sample size calculation: described | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Using a computer‐generated randomization schedule..." (page 320) (Khezri 2013) |
| Allocation concealment (selection bias) | Low risk | Quote: "Patients were given the study drugs by a nurse who was unaware of the study" (page 320) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No reported dropouts or missing data |
| Selective reporting (reporting bias) | High risk | The outcomes listed in the Iranian Registry of Clinical Trials are reported in the article. In addition 2 other outcomes are reported in the article (analgesic consumption and intraoperative conditions) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "...a nurse who was unaware of the study" (page 320). "The ophthalmologist, who was blinded to the group..." (page 320). "...in which the patients, investigators, anaesthesiologist, and the surgeon were blinded to the given treatment..." (page 320) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The ophthalmologist, who was blinded to the group..." (page 320). "...in which the patients, investigators, anaesthesiologist, and the surgeon were blinded to the given treatment..." (page 320) |
| Methods | Randomized, double‐blind, placebo‐controlled study Location: Saudi Arabia Study design: parallel, 2‐armed |
|
| Participants | A total of 40 patients randomized 40 patients completed: 20 in melatonin group, 20 in placebo group Age: melatonin 44.6 ± 11.4, placebo 42.8 ± 12.1 Gender (M/F) in %: melatonin (60/40), placebo (50/50) ASA class: I‐II Type of surgery: hand surgery Type of anaesthesia: regional Baseline (anxiety, pain) described: yes, yes |
|
| Interventions | Melatonin: 10 mg Placebo Administration route: oral Time of administration: 90 min preoperatively |
|
| Outcomes | 1. Tourniquet‐related pain by Verbal Pain Score (0 to 10) 2. Analgesic consumption by fentanyl requirements and diclofenac consumption 3. Anxiety assessed by Verbal Anxiety Score (0 to 10) (preoperative) 4. Haemodynamics by mean arterial pressure and heart rate 5. Onset and recovery of sensory and motor blockade (minutes) |
|
| Notes | Sample size calculation: described Author contacted by e‐mail on 9 August 2013 to clarify unspecified issues: no answer |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly allocated using an online research randomizer..." (page 1422) (Mowafi 2008) |
| Allocation concealment (selection bias) | Low risk | Quote:"Patients were randomly allocated using an online research randomizer..." (page 1422) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No reported dropouts or missing data |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of patients, surgeons or personnel not described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All the evaluations were performed by a blinded observer" (page 1423) |
| Methods | Randomized, double‐blind, placebo‐controlled study Location: Saudi Arabia Study design: parallel, 3‐armed |
|
| Participants | A total of 75 patients randomized 75 patients completed: 25 in melatonin group, 25 in placebo group, 25 in midazolam group Age: melatonin 29.6 (22 to 43), placebo 30.1 (22 to 40), midazolam 29.5 (19 to 44) Gender (M/F) in %: melatonin (0/100), placebo (0/100), midazolam (0/100) ASA class: I Type of surgery: gynaecological laparoscopic procedures Type of anaesthesia: general Baseline (anxiety, pain) described: yes, no |
|
| Interventions | Melatonin: 5 mg Placebo: saline Midazolam: 15 mg Administration route: sublingual Time of administration: approximately 100 min preoperatively |
|
| Outcomes | 1. Anxiety assessed by Visual Analogue Scale (0 to 100) (pre‐ and postoperative) 2. Orientation score (0 to 2) 3. Sedation score (0 to 4) 4. Psychomotor activity measured by The Digit‐symbol Substitution Test and Trieger dot test 5. Amnesia by showing line diagrams 6. Postoperative pain assessed by Visual Analogue Scale (0 to 100) and morphine consumption (mg) |
|
| Notes | Sample size calculation: described Author contacted by e‐mail on 9 August 2013 to clarify unspecified issues ‐ e‐mail returned = delivery failure |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "... allocated randomly..." (page 876) (Naguib 1999) |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No reported dropouts or missing data |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "... marked only with a coded label to maintain the double‐blind nature of the study" (page 876) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The same psychologist blinded to group assignment performed all test scoring and calculations" (page 876) |
| Methods | Randomized, double‐blind, placebo‐controlled study Location: Saudi Arabia Study design: parallel, 3‐armed, comparative, dose‐response study |
|
| Participants | A total of 84 patients randomized 84 patients completed: 36 (12, 12, 12) in melatonin group, 12 in placebo group, 36 (12, 12, 12) in midazolam group Age: melatonin 0.05 mg/kg (30.3 ± 5.6), 0.1 mg/kg (28.4 ± 6.1), 0.2 mg/kg (28.2 ± 6.1) Midazolam: 0.05 mg/kg (23.4 ± 3.9), 0.1 mg/kg (26.2 ± 6.6), 0.2 mg/kg (28.9 ± 6.0) Placebo 29.8 ± 6.1 Gender (M/F) in %: melatonin (0/100), placebo (0/100), midazolam (0/100) ASA class: I Type of surgery: gynaecological laparoscopic procedures Type of anaesthesia: general Baseline (anxiety, pain) described: yes, no |
|
| Interventions | Melatonin: 0.05 mg/kg, 0.1 mg/kg, 0.2 mg/kg Placebo: saline Midazolam: 0.05 mg/kg, 0.1 mg/kg, 0.2 mg/kg Administration route: sublingual Time of administration: approximately 100 min preoperatively |
|
| Outcomes | 1. Anxiety assessed by Visual Analogue Scale (0 to 100) (pre‐ and postoperative) 2. Orientation score (0 to 2) 3. Sedation score (0 to 4) 4. Psychomotor activity measured by The Digit‐symbol Substitution Test and Trieger dot test 5. Amnesia by showing line diagrams 6. Postoperative pain assessed by Visual Analogue Scale (0 to 100) and morphine consumption (mg) |
|
| Notes | Sample size calculation: described Author contacted by e‐mail on 9 August 2013 to clarify unspecified issues ‐ e‐mail returned = delivery failure |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "...randomly allocated..." (page 473) (Naguib 2000) |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No reported dropouts or missing data |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "... marked only with a coded label to maintain the double‐blind nature of the study" (page 474) "The contents of the syringe was given sublingually...by a resident not involved in the management of the patient or in the data collection" (page 474) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The same psychologist blinded to group assignment performed all test scoring and calculations" (page 474) |
| Methods | Randomized, double‐blind, placebo‐controlled study Location: Saudi Arabia Study design: parallel, 2‐armed |
|
| Participants | A total of 200 patients randomized 200 patients completed: melatonin + propofol (MP) (50), melatonin + thiopental (MT) (50), placebo + propofol (PP) (50), placebo + thiopental (PT) (50) Age: MP (32.4 ± 19.9), MT (34.9 ± 8.9), PP (34.4 ± 8.9), PT (31.6 ± 10.9) Gender (M/F) in %: MP (52/48), MT (46/54), PP (30/70), PT (38/62) ASA class: I Type of surgery: not reported Type of anaesthesia: general Baseline (anxiety, pain) described: yes, no |
|
| Interventions | Melatonin (0.2 mg/kg) + propofol Melatonin (0.2 mg/kg) + thiopental Placebo + propofol Placebo + thiopental Placebo: saline Administration route: sublingual Time of administration: approximately 50 min preoperatively |
|
| Outcomes | 1. Anxiety assessed by Visual Analogue Scale (0 to 100) (preoperative) 2. Orientation score (0 to 2) 3. Sedation score (0 to 4) 4. Induction of anaesthesia assessed by response to verbal command and eye‐lash reflex |
|
| Notes | Sample size calculation: described Author contacted by e‐mail on 9 August 2013 to clarify unspecified issues ‐ e‐mail returned = delivery failure |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...according to a computer‐generated list" (page 1448) (Naguib 2006) |
| Allocation concealment (selection bias) | Low risk | Quote: "...according to a computer‐generated list" (page 1448) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No reported dropouts or missing data |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote:"...marked only with a coded label to maintain the double‐blind nature of the study" (page 1449) "The contents of the syringe was given sublingually...by a resident not involved in the management of the patient or in the data collection" (page 1449) "The attending anaesthesiologist was unaware of the premedication or induction medication used" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "One investigator blinded to group assignment performed all test scoring in the perioperative period." (page 1449) |
| Methods | Randomized, double‐blind, placebo‐controlled study Location: Saudi Arabia Study design: parallel, 3‐armed |
|
| Participants | A total of 45 patients randomized 45 patients completed: melatonin 3 mg ‐ M3 (15), melatonin 5 mg ‐ M5 (15), no premedication ‐ P (15) Age: M3 32.4 (18 to 47), M5 27.1 (15 to 45), P 30.2 (19 to 41) Gender (M/F) in %: M3 (47/53), M5 (67/33), P (53/47) ASA class: I‐II Type of surgery: different surgical procedures Type of anaesthesia: general Baseline (anxiety, pain) described: yes, no |
|
| Interventions | Melatonin 3 mg, 5 mg, or no premedication Administration route: oral Time of administration: approximately 100 min preoperatively |
|
| Outcomes | 1. Anxiety measured by VAS from 0 to 100 (preoperative) 2. BIS score (Bispectral Index) 3. Induction of anaesthesia assessed by response to verbal command and eye‐lash reflex 4. Time to be fit for recovery room discharge (minutes) 5. Heart rate and mean arterial pressure 6. Induction dose of propofol |
|
| Notes | Sample size calculation: described Author contacted by e‐mail on 9 August 2013 and by telephone on 9, 14, 17 October 2013 to clarify unspecified issues: no answer |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | Quote: "...using a sealed‐envelope technique" (page 400) (Turkistani 2007) Missing more information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No reported dropouts or missing data |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "... an anaesthesiologist, who was blinded to the premedication, injected propofol..." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Borazan 2010 | Intervention was not aimed at treating pre‐ or postoperative anxiety |
| Evagelidis 2009 | Intervention was not aimed at treating pre‐ or postoperative anxiety |
| Hansen 2014 | Too long treatment with melatonin preoperatively |
| Johns 2012 | Intervention was not aimed at treating pre‐ or postoperative anxiety |
| Nasr 2014 | There was neither a placebo nor a benzodiazepine group |
| Peng 2010 | Study was terminated prematurely and data will not be published (according to first author ‐ contacted by e‐mail July 2013) |
| Radwan 2010 | Intervention was not aimed at treating pre‐ or postoperative anxiety |
| Wawrzyniak 2014 | There was neither a placebo nor a benzodiazepine group |
| Yoo 2013 | Intervention was not aimed at treating pre‐ or postoperative anxiety |
ASA: American Society of Anaesthesiologists physical status classification
M: Male
F: Female
min: minutes
mg: milligram
μg: microgram
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Randomized, double‐blind, placebo‐controlled study ‐ 3 armed |
| Participants | A total of 120 patients, M/F, 35 to 85 years of age, ASA I‐III scheduled for retrobulbar eye block for cataract surgery |
| Interventions | 1) 6 mg melatonin 2) 600 mg gabapentin 3) placebo orally 90 min before arrival in the operating room |
| Outcomes | Verbal pain score (VPS), Verbal anxiety score (VAS), sedation score, mean arterial blood pressure, heart rate, satisfaction of surgeon |
| Notes |
| Methods | Randomized, double‐blind, placebo‐controlled 4 armed study |
| Participants | A total of 80 patients, M/F, ASA 1 and 2, age 18 to 65 years, having anxiety VAS score of 3 or more and planned for general anaesthesia for laparoscopic cholecystectomy |
| Interventions | 1) 0.5 mg alprazolam 2) 3 mg melatonin 3) combination 0.5 mg alprazolam and 3 mg melatonin 4) placebo 90 min before surgery |
| Outcomes | VAS anxiety score (baseline, 15 min, 30 min, 60 min after premedication), sedation score, orientation score, number of patients with intact memory, number of patients with loss of memory for being transferred to operating room |
| Notes |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | The effect of melatonin, clonidine and gabapentin on reducing the preoperative anxiety and postoperative pain in patients undergoing laparoscopic cholecystectomy |
| Methods | Randomized, double‐blind, placebo‐controlled trial |
| Participants | 88 patients (4 groups, 22 in each group), M/F, age 18 to 60 years, scheduled for elective laparoscopic cholecystectomy |
| Interventions | Placebo, melatonin 6 mg, clonidine 0.2 mg or gabapentin 600 mg 120 min before surgery |
| Outcomes | Anxiety: the night before surgery and when entering the operating room ‐ measured by STAI Pain: 2 h before and 1, 2, 6, 12 and 24 h after surgery ‐ measured by VAS and PCA |
| Starting date | 01‐06‐2014 |
| Contact information | Vahideh Sadat Hosseini. Shariati Hospital, North Kargar St., Tehran Iran |
| Notes | Study found at: WHO's Trial Search |
Contributions of authors
Conceiving the review: Ismail Gögenur (IG), Jacob Rosenberg (JR), Ann Merete Møller (AMM)
Co‐ordinating the review: Melissa Voigt Hansen (MVH), Natalie Løvland Halladin (NLH)
Undertaking manual searches: MVH, NLH
Screening search results: MVH, NLH
Organizing retrieval of papers: MVH, NLH
Screening retrieved papers against inclusion criteria: MVH, NLH
Appraising quality of papers: MVH, NLH
Abstracting data from papers: Tobias Wirenfeldt Klausen (TWK), MVH, NLH
Writing/calling to authors of papers for additional information: MVH, NLH, IG
Providing additional data about papers: MVH, NLH
Obtaining and screening data on unpublished studies: MVH, NLH
Data management for the review: TWK, MVH, NLH
Entering data into Review Manager (RevMan 5.2): TWK, MVH, NLH
RevMan statistical data: TWK
Other statistical analysis not using RevMan: TWK
Interpretation of data: MVH, NLH, AMM
Statistical inferences: TWK
Writing the review: MVH, NLH, JR, IG, AMM, TWK
Securing funding for the review: JR, IG
Performing previous work that was the foundation of the present study: IG, JR, MVH, NLH
Guarantor for the review (one author): MVH
Person responsible for reading and checking review before submission: MVH
Sources of support
Internal sources
-
No support provided, Other.
None
External sources
-
University of Copenhagen, Denmark.
The first and second author (MVH and NLH) have both received 3‐year scholarships (salary) to cover a 3‐year phD.
Declarations of interest
Melissa V Hansen: has undertaken research projects for a PhD. One of the studies involved in her PhD was a randomized, placebo‐controlled trial called "The effect of melatonin on depression, anxiety, cognitive function and sleep disturbances in breast cancer patients". Dr Hansen has an agreement with Pharma Nord, who provided the melatonin and placebo tablets for the RCT. Pharma Nord has no influence or rights whatsoever on this RCT study regarding design, data analysis or reporting of data. Pharma Nord has had nothing to do with the Cochrane review and has not in any way been able to influence this process. Dr Hansen has not received any funding in cash from Pharma Nord and will not in the future receive any funding. She has only received melatonin and placebo tablets for her RCT study.
Natalie Halladin: no conflicts of interest.
Jacob Rosenberg: no conflicts of interest.
Ismail Gögenur: no conflicts of interest.
Ann Merete Møller: no conflicts of interest.
Edited (no change to conclusions)
References
References to studies included in this review
- Acil M, Basgul E, Celiker V, Karagoz AH, Demir B, Aypar U. Perioperative effects of melatonin and midazolam premedication on sedation, orientation, anxiety scores and psychomotor performance. European Journal of Anaesthesiology 2004;21(7):553‐7. [; PUBMED: 15318468] [DOI] [PubMed] [Google Scholar]; 3045691
- Capuzzo M, Zanardi B, Schiffino E, Buccoliero C, Gragnaniello D, Bianchi S, et al. Melatonin does not reduce anxiety more than placebo in the elderly undergoing surgery. Anesthesia and Analgesia 2006;103(1):121‐3, table of contents. [; PUBMED: 16790638] [DOI] [PubMed] [Google Scholar]; 3045693
- Caumo W, Torres F, Moreira NL Jr, Auzani JA, Monteiro CA, Londero G, et al. The clinical impact of preoperative melatonin on postoperative outcomes in patients undergoing abdominal hysterectomy. Anesthesia and Analgesia 2007;105(5):1263‐71. [; PUBMED: 17959953] [DOI] [PubMed] [Google Scholar]; 3045695
- Caumo W, Levandovski R, Hidalgo MP. Preoperative anxiolytic effect of melatonin and clonidine on postoperative pain and morphine consumption in patients undergoing abdominal hysterectomy: a double‐blind, randomized, placebo‐controlled study. The Journal of Pain 2009;10(1):100‐8. [; PUBMED: 19010741] [DOI] [PubMed] [Google Scholar]; 3045697
- Ionescu D, Bãdescu C, Ilie A, Miclutia I, Iancu C, Ion D, et al. Melatonin as premedication for laparoscopic cholecystectomy: a double‐blind, placebo‐controlled study. South African Journal of Anaesthesiology and Analgesia 2008;14(4):8‐11. [] [Google Scholar]; 3045699
- Ismail SA, Mowafi HA. Melatonin provides anxiolysis, enhances analgesia, decreases intraocular pressure, and promotes better operating conditions during cataract surgery under topical anesthesia. Anesthesia and Analgesia 2009;108(4):1146‐51. [; PUBMED: 19299777] [DOI] [PubMed] [Google Scholar]; 3045701
- Khezri MB, Merate H. The effects of melatonin on anxiety and pain scores of patients, intraocular pressure, and operating conditions during cataract surgery under topical anesthesia. Indian Journal of Ophthalmology 2013;61(7):319‐24. [; PUBMED: 23552356] [DOI] [PMC free article] [PubMed] [Google Scholar]; 3045703
- Mowafi HA, Ismail SA. Melatonin improves tourniquet tolerance and enhances postoperative analgesia in patients receiving intravenous regional anesthesia. Anesthesia and Analgesia 2008;107(4):1422‐6. [; PUBMED: 18806063] [DOI] [PubMed] [Google Scholar]; 3045705
- Naguib M, Samarkandi AH. Premedication with melatonin: a double‐blind, placebo‐controlled comparison with midazolam. British Journal of Anaesthesia 1999;82(6):875‐80. [; PUBMED: 10562782] [DOI] [PubMed] [Google Scholar]; 3045707
- Naguib M, Samarkandi AH. The comparative dose‐response effects of melatonin and midazolam for premedication of adult patients: a double‐blinded, placebo‐controlled study. Anesthesia and Analgesia 2000;91(2):473‐9. [; PUBMED: 10910871] [DOI] [PubMed] [Google Scholar]; 3045709
- Naguib M, Samarkandi AH, Moniem MA, Mansour Eel‐D, Alshaer AA, Al‐Ayyaf HA, et al. The effects of melatonin premedication on propofol and thiopental induction dose‐response curves: a prospective, randomized, double‐blind study. Anesthesia and Analgesia 2006;103(6):1448‐52. [; PUBMED: 17122221] [DOI] [PubMed] [Google Scholar]; 3045711
- Turkistani A, Abdullah KM, Al‐Shaer AA, Mazen KF, Alkatheri K. Melatonin premedication and the induction dose of propofol. European Journal of Anaesthesiology 2007;24(5):399‐402. [; PUBMED: 17094871] [DOI] [PubMed] [Google Scholar]; 3045713
References to studies excluded from this review
- Borazan H, Tuncer S, Yalcin N, Erol A, Otelcioglu S. Effects of preoperative oral melatonin medication on postoperative analgesia, sleep quality, and sedation in patients undergoing elective prostatectomy: a randomized clinical trial. Journal of Anesthesia 2010;24:155‐60. [] [DOI] [PubMed] [Google Scholar]; 3045715
- Evagelidis P, Paraskeva A, Petropoulos G, Staikou C, Fassoulaki A. Melatonin premedication does not enhance induction of anaesthesia with sevoflurane as assessed by bispectral index monitoring. Singapore Medical Journal. 2009;50:78‐81. [] [PubMed] [Google Scholar]; 3045717
- Hansen MV, Andersen LT, Madsen MT, Hageman I, Rasmussen LS, Bokmand S, et al. Effect of melatonin on depressive symptoms and anxiety in patients undergoing breast cancer surgery: a randomized, double‐blind, placebo‐controlled trial. Breast Cancer Research and Treatment 2014;145:683‐95. [] [DOI] [PubMed] [Google Scholar]; 3045719
- Johns NP, Johns JR. Melatonin as Adjuvant Therapy in Breast Cancer Patients (MIQOL‐B). Status in clinicaltrials.gov: currently recruiting. Data not published. [] [Google Scholar]; 3045721
- Nasr DA, Abdellatif AA. Efficacy of preoperative melatonin versus pregabalin on perioperative anxiety and postoperative pain in gynecological surgeries. Egyptian Journal of Anaesthesia 2014;30:89‐93. [] [Google Scholar]; 3045723
- Peng P. Perioperative melatonin in lumbar laminectomy. Status in clinicaltrials.gov: recruitment status unknown because the information has not been verified recently. Author contacted by email: study terminated prematurely, data will not be published. [] [Google Scholar]; 3045725
- Radwan K, Youssef M. El‐Tawdy A, Zeidan M, Kamal N. Melatonin versus gabapentin. A comparative study as preemptive medications. Internet Journal of Anesthesiology 2010;23:19. [] [Google Scholar]; 3045727
- Wawrzyniak K, Burduk PK, Cywinski JB, Kusza K, Kazmierczak W. Improved quality of surgical field during endoscopic sinus surgery after clonidine premedication ‐ a pilot study. International Forum of Allergy & Rhinology 2014;4:542‐7. [] [DOI] [PubMed] [Google Scholar]; 3045729
- Yoo D, Stundner O. Study of melatonin on sleep, pain and confusion after joint replacement surgery. Status in clinicaltrials.gov: currently recruiting. Data not published. [] [Google Scholar]; 3045731
References to studies awaiting assessment
- EMPTY; 3045733
- EMPTY; 3045734
References to ongoing studies
- The effect of melatonin, clonidine and gabapentin on reducing the preoperative anxiety and postoperative pain in patients undergoing laparoscopic cholecystectomy. Ongoing study 01‐06‐2014. ; 3045735
Additional references
- Ashton H. Guidelines for the rational use of benzodiazepines. When and what to use. Drugs 1994;48(1):25‐40. [PUBMED: 7525193] [DOI] [PubMed] [Google Scholar]
- Bailey L. Strategies for decreasing patient anxiety in the perioperative setting. AORN Journal 2010;92(4):445‐57. [PUBMED: 20888947] [DOI] [PubMed] [Google Scholar]
- Brzezinski A. Melatonin in humans. The New England Journal of Medicine 1997;336(3):186‐95. [PUBMED: 8988899] [DOI] [PubMed] [Google Scholar]
- Buscemi N, Vandermeer B, Hooton N, Pandya R, Tjosvold L, Hartling L, et al. Efficacy and safety of exogenous melatonin for secondary sleep disorders and sleep disorders accompanying sleep restriction: meta‐analysis. BMJ (Clinical research edition) 2006;332(7538):385‐93. [PUBMED: 16473858] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caumo W, Schmidt AP, Schneider CN, Bergmann J, Iwamoto CW, Adamatti LC, et al. Risk factors for postoperative anxiety in adults. Anaesthesia 2001;56(8):720‐8. [PUBMED: 11493233] [DOI] [PubMed] [Google Scholar]
- Caumo W, Schmidt AP, Schneider CN, Bergmann J, Iwamoto CW, Bandeira D, et al. Risk factors for preoperative anxiety in adults. Acta Anaesthesiologica Scandinavica 2001;45(3):298‐307. [PUBMED: 11207465] [DOI] [PubMed] [Google Scholar]
- Claustrat B, Brun J, Chazot G. The basic physiology and pathophysiology of melatonin. Sleep Medicine Reviews 2005;9(1):11‐24. [PUBMED: 15649735] [DOI] [PubMed] [Google Scholar]
- Corman HH, Hornick EJ, Kritchman M, Terestman N. Emotional reactions of surgical patients to hospitalization, anesthesia and surgery. American Journal of Surgery 1958;96(5):646‐53. [PUBMED: 13583329] [DOI] [PubMed] [Google Scholar]
- Domar AD, Everett LL, Keller MG. Preoperative anxiety: is it a predictable entity?. Anesthesia and Analgesia 1989;69(6):763‐7. [PUBMED: 2589657] [PubMed] [Google Scholar]
- Ebadi M, Govitrapong P, Phansuwan‐Pujito P, Nelson F, Reiter RJ. Pineal opioid receptors and analgesic action of melatonin. Journal of Pineal Research 1998;24(4):193‐200. [PUBMED: 9572527] [DOI] [PubMed] [Google Scholar]
- Edwards JG. Adverse effects of antianxiety drugs. Drugs 1981;22(6):495‐514. [PUBMED: 6119192] [DOI] [PubMed] [Google Scholar]
- Gudex C. Adverse effects of benzodiazepines. Social Science & Medicine (1982) 1991;33(5):587‐96. [PUBMED: 1962230] [DOI] [PubMed] [Google Scholar]
- Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck‐Ytter Y, Schunemann HJ. What is "quality of evidence" and why is it important to clinicians. BMJ 2008;336:995‐8. [PUBMED: 18456631] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haugen AS, Eide GE, Olsen MV, Haukeland B, Remme AR, Wahl AK. Anxiety in the operating theatre: a study of frequency and environmental impact in patients having local, plexus or regional anaesthesia. Journal of Clinical Nursing 2009;18(16):2301‐10. [PUBMED: 19583663] [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
- Hjermstad MJ, Fayers PM, Haugen DF, Caraceni A, Hanks GW, Loge JH, et al. Studies comparing Numerical Rating Scales, Verbal Rating Scales, and Visual Analogue Scales for assessment of pain intensity in adults: a systematic literature review. Journal of Pain and Symptom Management 2011;41(6):1073‐93. [PUBMED: 21621130] [DOI] [PubMed] [Google Scholar]
- Ip HY, Abrishami A, Peng PW, Wong J, Chung F. Predictors of postoperative pain and analgesic consumption: a qualitative systematic review. Anesthesiology 2009;111(3):657‐77. [PUBMED: 19672167] [DOI] [PubMed] [Google Scholar]
- Jamison RN, Taft K, O'Hara JP, Ferrante FM. Psychosocial and pharmacologic predictors of satisfaction with intravenous patient‐controlled analgesia. Anesthesia and Analgesia 1993;77(1):121‐5. [PUBMED: 8317718] [PubMed] [Google Scholar]
- Jarratt J. Perioperative melatonin use. Anaesthesia and Intensive Care 2011;39(2):171‐81. [PUBMED: 21485664] [DOI] [PubMed] [Google Scholar]
- Jellish WS, O'Rourke M. Anxiolytic use in the postoperative care unit. Anesthesiology Clinics 2012;30(3):467‐80. [PUBMED: 22989589] [DOI] [PubMed] [Google Scholar]
- Johnston M. Anxiety in surgical patients. Psychological Medicine 1980;10(1):145‐52. [PUBMED: 7384316] [DOI] [PubMed] [Google Scholar]
- Kain ZN, Sevarino F, Alexander GM, Pincus S, Mayes LC. Preoperative anxiety and postoperative pain in women undergoing hysterectomy. A repeated‐measures design. Journal of Psychosomatic Research 2000;49(6):417‐22. [PUBMED: 11182434] [DOI] [PubMed] [Google Scholar]
- Kelly AM. Does the clinically significant difference in visual analog scale pain scores vary with gender, age, or cause of pain?. Academic Emergency Medicine 1998;5(11):1086‐90. [PUBMED: 9835471] [DOI] [PubMed] [Google Scholar]
- Kelly AM. The minimum clinically significant difference in visual analogue scale pain score does not differ with severity of pain. Emergency Medicine Journal 2001;18(3):205‐7. [PUBMED: 11354213] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindler CH, Harms C, Amsler F, Ihde‐Scholl T, Scheidegger D. The visual analog scale allows effective measurement of preoperative anxiety and detection of patients' anesthetic concerns. Anesthesia and Analgesia 2000;90(3):706‐12. [PUBMED: 10702461] [DOI] [PubMed] [Google Scholar]
- Lovering S. Cultural attitudes and beliefs about pain. Journal of Transcultural Nursing 2006;17(4):389‐95. [PUBMED: 16946122] [DOI] [PubMed] [Google Scholar]
- Maestroni GJ. The immunoneuroendocrine role of melatonin. Journal of Pineal Research 1993;14(1):1‐10. [PUBMED: 8483103] [DOI] [PubMed] [Google Scholar]
- Mavros MN, Athanasiou S, Gkegkes ID, Polyzos KA, Peppas G, Falagas ME. Do psychological variables affect early surgical recovery?. PloS ONE 2011;6(5):e20306. [PUBMED: 21633506] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar K, Jelicic M, Bonke B, Asbury AJ. Assessment of preoperative anxiety: comparison of measures in patients awaiting surgery for breast cancer. British Journal of Anaesthesia 1995;74(2):180‐3. [PUBMED: 7696068] [DOI] [PubMed] [Google Scholar]
- Mitchell M. Conscious surgery: influence of the environment on patient anxiety. Journal of Advanced Nursing 2008;64(3):261‐71. [PUBMED: 18785887] [DOI] [PubMed] [Google Scholar]
- Mitchell M. General anaesthesia and day‐case patient anxiety. Journal of Advanced Nursing 2010;66(5):1059‐71. [PUBMED: 20337788] [DOI] [PubMed] [Google Scholar]
- Mitchell M. Influence of gender and anaesthesia type on day surgery anxiety. Journal of Advanced Nursing 2012;68(5):1014‐25. [PUBMED: 21806671] [DOI] [PubMed] [Google Scholar]
- Naguib M, Gottumukkala V, Goldstein PA. Melatonin and anesthesia: a clinical perspective. Journal of Pineal Research 2007;42(1):12‐21. [PUBMED: 17198534] [DOI] [PubMed] [Google Scholar]
- Nickkholgh A, Schneider H, Sobirey M, Venetz WP, Hinz U, Pelzl le H, et al. The use of high‐dose melatonin in liver resection is safe: first clinical experience. Journal of Pineal Research 2011;50(4):381‐8. [PUBMED: 21480979] [DOI] [PubMed] [Google Scholar]
- Nordlund JJ, Lerner AB. The effects of oral melatonin on skin color and on the release of pituitary hormones. The Journal of Clinical Endocrinology and Metabolism 1977;45(4):768‐74. [PUBMED: 914981] [DOI] [PubMed] [Google Scholar]
- Norris W, Baird WL. Pre‐operative anxiety: a study of the incidence and aetiology. British Journal of Anaesthesia 1967;39(6):503‐9. [PUBMED: 6027959] [DOI] [PubMed] [Google Scholar]
- Pan PH, Coghill R, Houle TT, Seid MH, Lindel WM, Parker RL, et al. Multifactorial preoperative predictors for postcesarean section pain and analgesic requirement. Anesthesiology 2006;104(3):417‐25. [PUBMED: 16508387] [DOI] [PubMed] [Google Scholar]
- Ramsay MA. A survey of pre‐operative fear. Anaesthesia 1972;27(4):396‐402. [PUBMED: 4634747] [DOI] [PubMed] [Google Scholar]
- Reiter RJ, Melchiorri D, Sewerynek E, Poeggeler B, Barlow‐Walden L, Chuang J, et al. A review of the evidence supporting melatonin's role as an antioxidant. Journal of Pineal Research 1995;18(1):1‐11. [PUBMED: 7776173] [DOI] [PubMed] [Google Scholar]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2013.
- Seabra ML, Bignotto M, Pinto LR Jr, Tufik S. Randomized, double‐blind clinical trial, controlled with placebo, of the toxicology of chronic melatonin treatment. Journal of Pineal Research 2000;29(4):193‐200. [PUBMED: 11068941] [DOI] [PubMed] [Google Scholar]
- Stankov B, Fraschini F, Reiter RJ. Melatonin binding sites in the central nervous system. Brain Research. Brain Research Reviews 1991;16(3):245‐56. [PUBMED: 1665096] [DOI] [PubMed] [Google Scholar]
- Thomas V, Heath M, Rose D, Flory P. Psychological characteristics and the effectiveness of patient‐controlled analgesia. British Journal of Anaesthesia 1995;74(3):271‐6. [PUBMED: 7718370] [DOI] [PubMed] [Google Scholar]
- Tian SW, Laudon M, Han L, Gao J, Huang FL, Yang YF, et al. Antidepressant‐ and anxiolytic effects of the novel melatonin agonist Neu‐P11 in rodent models. Acta Pharmacologica Sinica 2010;31(7):775‐83. [PUBMED: 20581849] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker KJ, Smith AF. Premedication for anxiety in adult day surgery. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD002192.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace G, Mindlin LJ. A controlled double‐blind comparison of intramuscular lorazepam and hydroxyzine as surgical premedicants. Anesthesia and Analgesia 1984;63(6):571‐6. [PUBMED: 6375464] [PubMed] [Google Scholar]
- Williams JB, Alexander KP, Morin JF, Langlois Y, Noiseux N, Perrault LP, et al. Preoperative anxiety as a predictor of mortality and major morbidity in patients aged >70 years undergoing cardiac surgery. The American Journal of Cardiology 2013;111(1):137‐42. [PUBMED: 23245838] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods JH, Katz JL, Winger G. Benzodiazepines: use, abuse, and consequences. Pharmacological Reviews 1992;44(2):151‐347. [PUBMED: 1356276] [PubMed] [Google Scholar]
- Wurtman RJ, Zhdanova I. Improvement of sleep quality by melatonin. Lancet1995; Vol. 346, issue 8988:1491. [PUBMED: 7491013] [DOI] [PubMed]
- Yousaf F, Seet E, Venkatraghavan L, Abrishami A, Chung F. Efficacy and safety of melatonin as an anxiolytic and analgesic in the perioperative period: a qualitative systematic review of randomized trials. Anesthesiology 2010;113(4):968‐76. [PUBMED: 20823763] [DOI] [PubMed] [Google Scholar]
- Zhdanova IV, Wurtman RJ, Lynch HJ, Ives JR, Dollins AB, Morabito C, et al. Sleep‐inducing effects of low doses of melatonin ingested in the evening. Clinical Pharmacology and Therapeutics 1995;57(5):552‐8. [PUBMED: 7768078] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
- Hansen MV, Halladin NL, Rosenberg J, Gögenur I, Møller AM. Melatonin for preoperative anxiety in adults. Cochrane Database of Systematic Reviews 2012, Issue 5. [DOI: 10.1002/14651858.CD009861] [DOI] [PMC free article] [PubMed] [Google Scholar]