Abstract
Background
Poor adherence to antiepileptic medication is associated with increased mortality, morbidity and healthcare costs. In this review, we focus on interventions designed and tested in randomised controlled trials and quasi‐randomised controlled trials to assist people with adherence to antiepileptic medication. This is an updated version of the original Cochrane review published in the Cochrane Library, Issue 1, 2010.
Objectives
To determine the effectiveness of interventions aimed at improving adherence to antiepileptic medication in adults and children with epilepsy.
Search methods
For the latest update, on 4 February 2016 we searched the Cochrane Epilepsy Group Specialized Register, the Cochrane Central Register of Controlled Trials (CENTRAL) via the Cochrane Register of Studies Online (CRSO), MEDLINE (Ovid 1946 to 4 February 2016), CINAHL Plus (EBSCOhost 1937 to 4 February 2016), PsycINFO (EBSCOhost 1887 to 4 February 2016), ClinicalTrials.gov, and the WHO International Clinical Trials Registry Platform. We also searched the reference lists of relevant articles.
Selection criteria
Randomised and quasi‐randomised controlled trials of adherence‐enhancing interventions aimed at people with a clinical diagnosis of epilepsy (as defined in individual studies), of any age and treated with antiepileptic drugs in a primary care, outpatient or other community setting.
Data collection and analysis
All review authors independently assessed lists of potentially relevant citations and abstracts. At least two review authors independently extracted data and performed quality assessment of each study according to the Cochrane tool for assessing risk of bias. We graded the level of evidence for each outcome according to the GRADE working group scale.The studies differed widely according to the type of intervention and measures of adherence; therefore combining data was not appropriate.
Main results
We included 12 studies reporting data on 1642 participants (intervention = 833, control = 809). Eight studies targeted adults with epilepsy, one study included participants of all ages, one study included participants older than two years, one study targeted caregivers of children with epilepsy, and one study targeted families of children with epilepsy. We identified six ongoing trials. Follow‐up time was generally short in most trials, ranging from one to 12 months. The trials examined three main types of interventions: educational interventions, behavioural interventions and mixed interventions. All studies compared treatment versus usual care or 'no intervention', except for two studies. Due to heterogeneity between studies in terms of interventions, methods used to measure adherence and the way the studies were reported, we did not pool the results and these findings were inappropriate to be included in a meta‐analysis. Education and counselling of participants with epilepsy resulted in mixed success (moderate‐quality evidence). Behavioural interventions such as use of intensive reminders provided more favourable effects on adherence (moderate‐quality evidence). The effect on adherence to antiepileptic drugs described by studies of mixed interventions showed improved adherence in the intervention groups compared to the control groups (high‐quality evidence).
Authors' conclusions
Behavioural interventions such as intensive reminders and the use of mixed interventions demonstrate some positive results; however, we need more reliable evidence on their efficacy, derived from carefully‐designed randomised controlled trials before we can draw a firm conclusion. Since the last version of this review, none of the new relevant studies have provided additional information that would lead to significant changes in our conclusions. This current update includes 12 studies, of which six came from the latest searches.
Plain language summary
Strategies for improving how people with epilepsy take their medication
Background
People with epilepsy can find it difficult to take their medicines as prescribed, and this is thought to be a reason for poor control of seizures. This review of trials reports on ways of improving how they take their antiepileptic medication.
Study characteristics
We searched scientific databases for clinical trials looking at ways of improving adherence to drug treatment in people with epilepsy. We limited our search to randomised controlled trials involving people with a clinical diagnosis of epilepsy of any age and treated with antiepileptic drugs in a primary care, outpatient or other community setting. The results are up‐to‐date to February 2016.
Key results
We identified twelve trials (1642 participants). The trials were conducted in different countries with the majority from the United States. The trials examined three main types of interventions: i) education and counselling of participants about topics such as epilepsy and medication used to control epilepsy, ii) behavioural interventions such as asking epileptic patients to link the intention of taking their medication with a particular time, place and other routine activity and iii) the use of more than one intervention (mixed interventions). Behavioural interventions and mixed interventions resulted in an improved adherence in the intervention groups compared to the control groups. Four trials showed that when adherence improved in the intervention groups, seizure frequency or seizure severity was decreased.
Many of the included trials are of moderate quality and have limitations in the design. Therefore, it is difficult to draw firm conclusions. We need carefully‐designed randomised controlled trials involving more people with longer follow‐up periods to identify the best intervention to improve adherence to antiepileptic medication.
Summary of findings
Summary of findings for the main comparison. Summary of findings table.
| Strategies for improving adherence to antiepileptic drug treatment in people with epilepsy | ||||||
| Patient or population: adults and children with epilepsy Setting: all settings Intervention: adherence‐enhancing intervention Comparison: no intervention or other intervention | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with no intervention or other intervention | Risk with adherence‐enhancing intervention | |||||
| Effects on adherence (behavioural interventions) assessed with: MEMS caps and self‐reported Antiretroviral General Adherence Scale (AGAS) Follow‐up: range 1 month to 3 months | Not estimable See comments | Not estimable See comments | ‐ | 89 (2 RCTs) | ⊕⊕⊕⊝2 MODERATE | Only 1 study showed significant improvement in adherence (see Summary of results for each included study Table 2). Due to different interventions and assessment methods no further conclusions can be drawn. |
| Effects on adherence (educational interventions) assessed with: Serum or plasma concentration and Medication Adherence Scale (MAS) Follow‐up: range 4 weeks to 13 months | Not estimable See comments | Not estimable See comments | ‐ | 1153 (8 RCTs) | ⊕⊕⊕⊝1, 2 MODERATE | Only 3 trials presented significant results of improved adherence. Due to different interventions and assessment methods, no further conclusions can be drawn (see Summary of results for each included study Table 2). |
| Effects on adherence (mixed interventions) assessed with: Serum or plasma concentration and Medication Adherence scale (MAS) Follow‐up: range 6 months to 12 months | Not estimable See comments | Not estimable See comments | ‐ | 522 (3 RCTs) | ⊕⊕⊕⊕1 HIGH | Only 2 studies reported significant improvement in adherence. Due to heterogeneity of interventions and assessment methods, no further conclusions can be drawn (see Summary of results for each included study Table 2). |
| Seizure frequency and seizure severity Follow‐up: range 4 months to 12 months | Not estimable See comments | Not estimable See comments | ‐ | 1293 (6 RCTs) | ⊕⊕⊕⊝1, 2 MODERATE | Decreased seizure frequency and/or seizure severity related to improved adherence with AED was described in 4 out of 6 trials presenting this secondary outcome (see Summary of results for each included study Table 2) |
| Self‐efficacy assessed with: the Epilepsy Self‐Efficacy Scale (ESES) and Sherer`s Self‐Efficacy Scale Follow‐up: range 3 months to 6 months | Not estimable See comments | Not estimable See comments | ‐ | 358 (4 RCTs) | ⊕⊕⊝⊝2, 3 LOW | Only 1 study presented significantly important results supporting improvement in self‐efficacy skills. Other studies reporting positive effects as a result of an intervention with mixed reliability (see Summary of results for each included study Table 2) |
| Quality of life assessed with: Quality of Life in Epilepsy Scale (QOLIE‐10) Follow‐up: range 4 months to 6 months | Not estimable See comments | Not estimable See comments | ‐ | 117 (2 RCTs) | ⊕⊕⊕⊕1 HIGH | 1 study reported significant benefit in intervention group, another study failed to present results supporting the added value of an intervention (see Summary of results for each included study Table 2) |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1The majority of studies measuring this outcome were not at high risk of bias. 2The quality of the evidence of the studies measuring this outcome was downgraded due to the lack of precision or lack of consistency, or both. 3The quality of the evidence of the studies measuring this outcome was downgraded due to the lack directness
Background
This review is an update of a previously published review in the Cochrane Database of Systematic Reviews (2010, Issue 1) (Al‐aqeel 2011).
Description of the condition
The International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy define epilepsy as "a disorder of the brain characterised by an enduring predisposition to generate epileptic seizures and by the neurobiological, cognitive, psychological and social consequences of this condition" (Fisher 2005). The definition of epilepsy requires the occurrence of at least one epileptic seizure. Epilepsy is one of the most common neurological disorders worldwide, with a prevalence estimated to be between 4 and 10 per 1000 people (Sander 2003). The systematic review by Ngugi 2011 presented the pooled incidence of epilepsy and included 33 relevant studies. The median incidence of epilepsy was 50.4 per 100,000 people per year and ranged from 30.3 to 66.7 per 100,000 people per year (median 45.0) in industrialised countries, and from 28.0 to 239.5 cases per 100,000 people per year (median 81.7) generally quoted for middle‐income countries.
The term 'adherence' describes the extent to which a person takes their medication as prescribed with respect to dosage and dosing intervals (Cramer 2008). Adherence is not the same as 'concordance', which includes a consensual agreement about treatment‐taking that is established between patient and practitioner (Eatock 2007). However, both terms are quantifiable parameters and both describe the dose quantity and the medication intake in general (Vrijens 2012). Adherence, as a process, includes three stages according to Vrijens et al: initiation, implementation and discontinuation.
Non‐adherence can be intentional, with patients acting in a certain way according to their own expectations of treatment, side effects and lifestyle choice; or non‐intentional, when patients do not adhere through forgetfulness, misunderstanding or uncertainty about clinicians' recommendations, which might result from a more passive behaviour. Non‐adherence to medication is a prevalent and persistent healthcare problem, particularly for people with a chronic disorder (Lehane 2006).
A few older studies (Helgeson 1990; Peterson 1984; Pryse‐Phillips 1982; Shope 1980) and one newer study (Li 2013) included in this review used the term “medication compliance”, although the description of the outcome by the authors is comparable with the term “adherence” according to the new taxonomy (Vrijens 2012).
Description of the intervention
Interventions designed to enhance medication adherence include a simplified dosage regimen, combinations of more thorough patient instruction and counselling, (intensive) reminders, close follow‐up, supervised self‐monitoring, rewards for success, family therapy, psychological therapy and telephone follow‐up.
Why it is important to do this review
Of those people diagnosed with epilepsy, the vast majority are treated with antiepileptic drugs, and approximately 70% can become seizure‐free once the most effective regimen is followed (Eatock 2007). Unfortunately, evidence suggests that adherence to medication among people with epilepsy is sub optimal (Briesacher 2008; Davis 2008; Ettinger 2009a).
Poor adherence to antiepileptic drugs is associated with increased mortality, emergency department visits, hospitalisations, fractures and head injuries (Davis 2008; Ettinger 2009b; Faught 2008). Seizure risk is 21% higher among non‐adherers than adherers (Manjunath 2009). Increased frequency of seizures can have serious repercussions on an individual's perceived quality of life (Baker 1997; Hovinga 2008). It appears also to be associated with increased utilisation and costs of inpatient and emergency services (Davis 2008; Ettinger 2009b; Faught 2008).
To tackle the problem of non‐adherence, we need to identify the most effective adherence‐enhancing interventions and find out how well they improve adherence in people with epilepsy. Several systematic reviews published in the Cochrane Library have looked at adherence‐enhancing interventions. For instance, the Nieuwlaat 2014 review included unconfounded randomised controlled trials (RCTs) of interventions to improve adherence with prescribed medication, measuring both medication adherence and clinical outcome (such as seizure frequency), with at least 80% follow‐up of each group studied and, for long‐term treatments, at least six months follow‐up for studies with positive findings at earlier time points. Of all 182 RCTs identified, only 17 had the lowest risk of bias for study design features and their primary clinical outcome. Only five out of the 17 RCTs reported improvements in both adherence and clinical outcomes. The review identified one study looking at antiepileptic drugs, which reported improved medication adherence by combining a number of interventions such as counselling, a special medication container, self‐recording of medication intake and seizures, and mailed reminders to collect prescription refills and attend clinic appointments (Peterson 1984).
Considering the burden of poor adherence to antiepileptic drugs, substantial efforts in adherence research and assessing whether these efforts have led to more effective interventions for epilepsy, an updated review will be highly relevant. These gaps can be addressed by summarising new high‐quality evidence from RCTs to date. We have therefore updated our comprehensive systematic review, published in 2011, by searching for recent studies published up to February 2016.
Objectives
To determine the effectiveness of interventions aimed at improving adherence to antiepileptic medication in adults and children with epilepsy.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials and quasi‐randomised controlled trials comparing adherence‐enhancing interventions versus no intervention or other intervention.
Types of participants
The target population consisted of people with a clinical diagnosis of epilepsy (as defined in individual studies), of any age and of either gender, treated with antiepileptic drugs in a primary care, outpatient or other community setting. We examined interventions targeting all types of epilepsy. We excluded studies that examined people with epilepsy with neurological comorbidities, such as mental retardation and behavioural problems.
Types of interventions
Interventions of any type intended to increase adherence to antiepileptic medication. We considered interventions that were aimed at patients as well as at parents and caregivers, including but not exclusive to the following:
Simplification of drug regimen.
Patient education and information.
Intensified patient care (increasing follow‐up, sending out reminders, etc.).
Complex behavioural approach (increasing motivation by arranging group sessions, giving out rewards, etc.).
Control groups should have received no intervention, another intervention or 'usual care'.
Types of outcome measures
Primary outcomes
Improved adherence to medication (including any definition of adherence and noting how this was defined and measured in each study)
Secondary outcomes
Seizure frequency or seizure severity, as measured by the Liverpool Seizure Severity Scale or similar measure
Treatment side effects
Serious adverse events
Costs or cost effectiveness of adherence‐modifying interventions
Self‐efficacy
Quality of life
Search methods for identification of studies
Electronic searches
We ran the original search in June 2010. We ran subsequent searches in July 2012, February 2013, September 2014, September 2015, and February 2016.
For the latest update, we searched the following electronic databases:
Cochrane Epilepsy Group Specialized Register, 4 February 2016, using the search strategy outlined in Appendix 1.
Cochrane Central Register of Controlled Trials (CENTRAL) via the Cochrane Register of Studies Online (CRSO), 4 February 2016, using the search strategy outlined in Appendix 2.
MEDLINE (Ovid 1946 to 4 February 2016), using the search strategy outlined in Appendix 3.
CINAHL Plus (EBSCOhost 1937 to 4 February 2016), using the search strategy outlined in Appendix 4.
PsycINFO (EBSCOhost 1887 to 4 February 2016), using the search strategy outlined in Appendix 5.
ClinicalTrials.gov, 4 February 2016, using the search strategy outlined in Appendix 6.
WHO International Clinical Trials Registry Platform (ICTRP), 4 February 2016, using the search strategy outlined in Appendix 7.
Previously, review authors searched Embase (Ovid 1980 to June 2012) using the search strategy outlined in Appendix 8; however, randomised and quasi‐randomised controlled trials published in Embase are now included in CENTRAL, so there was no longer any need to search separately in Embase.
Searching other resources
We screened the reference lists of all retrieved articles to identify additional publications.
Data collection and analysis
Selection of studies
All review authors independently assessed lists of potentially relevant citations and abstracts. Each review author indicated whether a citation was:
relevant (meeting all prespecified inclusion criteria);
possibly relevant (meeting some, but not all, inclusion criteria); or
rejected (not relevant to the review; did not meet any of the inclusion criteria).
We obtained articles classified in categories 1 and 2 in full, and at least two of the review authors reviewed them independently. The review authors reached their final decision by consensus, with disagreements resolved by discussion.
We used a reference management system (EndNote) to identify and extract duplicate studies.
Data extraction and management
At least two review authors independently extracted data from the full papers, with disagreements handled in the same way as for study selection. Extracted information included details of randomisation methods, demographics and clinical characteristics of each group, entry and exclusion criteria, number of participants excluded or lost to follow‐up, details of the intervention, baseline and postintervention results and methods of analysis.
We kept records in the form of a 'Quality of reporting of meta‐analyses', or QUOROM, statement (Moher 1999).
Assessment of risk of bias in included studies
We assessed the methodological quality of trials using the Cochrane 'Risk of bias' guidelines (Higgins 2011). We examined the following sources of bias.
Selection bias: systematic differences between baseline characteristics of the groups compared.
Performance bias: systematic differences between groups in the care provided, or in exposure to factors other than the interventions of interest.
Attrition bias: systematic differences between groups in withdrawal from a study.
Detection bias: systematic differences between groups in how outcomes are determined.
Reporting bias: systematic differences between reported and unreported findings.
In addition to rating the quality of individual studies, we evaluated the overall strength of evidence for outcomes identified as critical or important for clinical decision‐making using the GRADE approach. These outcomes included: effects on adherence (behavioural interventions), effects on adherence (educational interventions), effects on adherence (mixed interventions), seizure frequency and severity, self‐efficacy, and quality of life. The GRADE approach considers evidence from randomised controlled trials as high quality, which may be downgraded based on consideration of any of five areas: design (risk of bias), consistency across studies, directness of the evidence, precision of estimates and presence of publication bias.
Measures of treatment effect
We analysed interventions for adults independently from those aimed at children. We grouped trials according to types of interventions and compared outcomes independently of each other.
For dichotomous outcomes (proportions of participants with improved adherence per group), we used the risk ratio (RR) as the summary statistic. For continuous data, we used the mean difference (MD) (when all trials reported the outcome using the same scale) or the standardised mean difference (SMD) (when trials used different scales). For all data, we computed 95% confidence intervals (CIs).
If in the original reports participants were not analysed within the group to which they were randomly assigned, but information in the trial report was sufficient, we attempted to restore participants to their correct group to allow an intention‐to‐treat analysis.
Dealing with missing data
We contacted trial authors to ask for missing information and data.
Assessment of heterogeneity
We assessed clinical (age, gender, epilepsy type and duration of epilepsy) and methodological (randomisation concealment, losses to follow‐up, adherence measurement and reporting) differences between studies. If a group of studies seemed to be similar enough to be pooled in meta‐analysis, we planned to assess statistical heterogeneity of pooled results by using the I2 statistic (Higgins 2011). However, due to clinical and methodological heterogeneity between identified studies, we did not perform statistical heterogeneity tests.
Data synthesis
We undertook a quantitative analysis of all included studies. We summarised data statistically if they were available, were of sufficient quality and were sufficiently similar, and if we observed no important clinical and methodological heterogeneity. If no significant heterogeneity was present, we had planned to synthesise the data using a fixed‐effect model; otherwise we used a random‐effects model. We performed statistical analysis using Review Manager (RevMan 2014).
Included trials were heterogeneous in terms of types of adherence‐enhancing interventions and methods used to measure and report adherence. This did not allow pooling of data.
Subgroup analysis and investigation of heterogeneity
We planned to analyse interventions and results in children and adults as separate subgroups throughout the review (results, analysis, discussion, implications for practice and research sections).
We planned to conduct subgroup analyses of the primary outcomes, classifying the trials by interventions used, numbers of interventions, types of adherence measurement used, duration of follow‐up and epilepsy type, if the data permitted.
Sensitivity analysis
We planned to undertake sensitivity analyses to explore the influence of factors such as the quality of included studies on the results.
Summary of findings Tables
We created a Summary of findings table using the browser‐based GRADEpro software, available to Cochrane review authors (GRADEpro 2014) for the primary outcome (improved adherence to medication) and secondary outcomes (seizure frequency or seizure severity, self‐efficacy, and quality of life). For each outcome we summarised the following information.
Risk in the intervention group and its 95% confidence interval based on the assumed risk in the comparison group and the relative effect of the intervention.
Relative magnitude of effect and its 95% confidence interval.
Numbers of participants and studies addressing these outcomes.
A grade of the overall quality of the body of evidence for each outcome as described in Assessment of risk of bias in included studies.
Any relevant comments.
Results
Description of studies
Results of the search
The search of the seven databases resulted in 812 'hits'. Of those, 372 articles were obtained from MEDLINE, 317 from CENTRAL, 62 from PsycINFO, 25 from the Cochrane Epilepsy Group Specialised Register, 13 from ClinicalTrials.gov, 12 from CINAHL, and 11 from ICTRP (see Figure 1). In previous versions of this review, searching Embase (Ovid 1980 to June 2012) contributed 2913 citations. However, the citations did not yield any new studies other than those identified in other databases.
1.
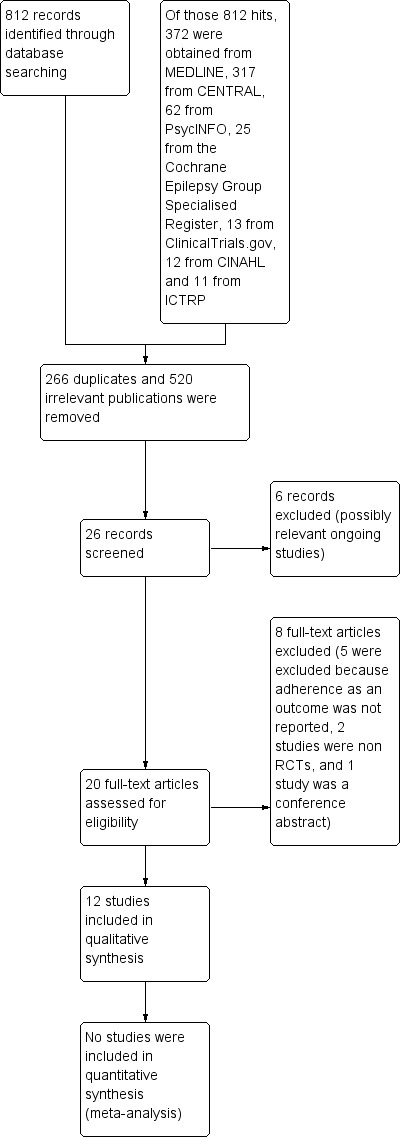
Study flow diagram (PRISMA Template)
We screened titles and abstracts and excluded duplicate (n = 266) and irrelevant publications (n = 520). The most common reason for exclusion at this stage was that no adherence‐enhancing intervention was performed, and/or no changes in adherence to medication were measured. The other common reason for exclusion was that the study population did not consist of participants with a clinical diagnosis of epilepsy; this was expected, as antiepileptic medications have many other clinical uses. The total number of citations after irrelevant and duplicate references were removed was 26. Two review authors reviewed these independently. Six trials are possibly relevant ongoing studies (see Characteristics of ongoing studies table). We excluded eight trials (see Characteristics of excluded studies table). The reasons for exclusion are as follows: five because adherence as an outcome was not reported, two were not designed as RCTs, and one where we were unable to obtain sufficient information to make a sound decision. We therefore included 12 studies in the current update, reporting data on 1642 participants (intervention = 833, control = 809), of which 1369 were new for this update. Six trials from the original review (Al‐aqeel 2011) reported on 273 participants. Eight of the 12 targeted adults with epilepsy (Brown 2009; Dash 2015; DiIorio 2009; Dilorio 2011; Helgeson 1990; Peterson 1984; Pryse‐Phillips 1982; Tang 2014), one included participants of all ages (Ibinda 2014), one included participants older than two years (Li 2013), one targeted caregivers of children with epilepsy (Shope 1980), and one targeted families of children with epilepsy (Modi 2013) (see Characteristics of included studies table).
Included studies
See: Characteristics of included studies table.
Five studies were conducted in the USA (DiIorio 2009; Dilorio 2011; Helgeson 1990; Modi 2013; Shope 1980), one in Australia (Peterson 1984), one in Canada (Pryse‐Phillips 1982), one in the UK (Brown 2009), two in China (Li 2013; Tang 2014), one in India (Dash 2015), and one in Kenya (Ibinda 2014). All studies compared treatment versus 'usual care' or 'no intervention', except for two studies. Tang 2014 compared educational versus behavioural interventions, and Pryse‐Phillips 1982 compared an educational intervention presented in different formats: oral form, oral and written, and by telephone contact only. Follow‐up times ranged from four weeks to one year. All included studies were randomised controlled trials (RCTs), or described the randomisation procedure if 'RCT' was not specifically mentioned.
Type of interventions
Interventions examined by trials could be grouped into behavioural, educational and mixed interventions.
Behavioural interventions
Implementation intention interventions, which involved the completion of a simple worksheet by participants and linking of the intention of taking medication with a particular time, place and other routine activity (Brown 2009). For instance, the participant could write, "If it is 8 am and I am in the bathroom and have finished brushing my teeth, then I will take my first dose".
Face‐to‐face introductory motivational interviews followed by four telephone‐based motivational interviews over 12 weeks. This intervention was provided by a specially‐trained nurse and was aimed at enhancing self‐management practices in the following areas: medication, information, seizures, safety and lifestyle (DiIorio 2009).
Educational interventions
One on‐one teaching in a structured format, covering aspects such as treatment modalities was administered by an epilepsy nurse in four sessions lasting at least 30 minutes; also pamphlets were provided, mostly with animations, to explain the different aspects of the disease (Dash 2015).
A one‐day educational programme providing epilepsy‐related information such as types of seizures, causes of epilepsy, effects of epilepsy on child development, treatment of epilepsy, side effects of drugs and what to do during a seizure. A brochure detailing all of the topics discussed was given to each participant (Ibinda 2014).
The first component of the intervention (session 1) provided education on epilepsy treatment, antiepileptic drug (AED) adherence and the family’s specific epilepsy treatment regimen (i.e. dosing schedule). Sessions 2 through 4 aimed to teach families a problem‐solving approach for their identified AED adherence barriers (Modi 2013).
An online epilepsy self‐management programme, Web Epilepsy Awareness, Support, and Education (WebEase), that assists people with taking medication, managing stress and improving sleep quality (Dilorio 2011).
A two‐day Seizures and Epilepsy Education programme designed to provide medical education and psychosocial therapy to participants and families (Helgeson 1990).
Three groups were given oral information about the name of the drugs; its colour, shape and strength; the therapeutic effect; and dosage, precautions and possible unwanted effects; the same information supplemented by its presentation in written form; and the same information by telephone contact only (Pryse‐Phillips 1982).
Two mothers' discussion group meetings, each lasting 1½ hours. The aim of these meetings was to provide mothers with information that would enable them to know what they should do for their children and why, and would allow them to increase their sense of responsibility while making their commitment (Shope 1980).
Medication education in the form of oral education and written materials, reinforced by monthly calls from the pharmacist over the next six months (Tang 2014).
Mixed interventions
A programme with four components: (1) intensive education, (2) consultation services to ensure that clinical providers and telephone support were available for participants at any time, (3) reminders provided by keeping a simple record with specifically‐designed cards, and (4) repeated participant reminders about medical adherence sent every month (Li 2013).
Patient counselling on the goals of AEDs and the importance of sufficient adherence and intensive reminders: diary of medication use and seizures, Dosett medication container (pill organiser), and prescription refill and appointment‐keeping reminders (Peterson 1984).
Medication education (see description above) was combined with a behavioural intervention: a modified medication schedule which was presented in the form of a table that illustrated the daily medication therapy of participants with pictures of AEDs, and providing them with cues to take their medication (Tang 2014).
Adherence assessment and reporting
Adherence to AEDs was measured both directly and indirectly. Five trials used serum or plasma concentration of the AED (Helgeson 1990; Ibinda 2014; Peterson 1984; Pryse‐Phillips 1982; Shope 1980). Indirect measurement techniques included use of the Medication Event Monitoring System (MEMS), an electronic monitoring cap that recorded the number and timing of bottle openings (Brown 2009; DiIorio 2009; Modi 2013); assessment of participant‐reported adherence using the Antiretroviral General Adherence Scale (AGAS) (DiIorio 2009) or the Medication Adherence Scale (MAS or Morisky MAS) (Dash 2015; Dilorio 2011; Li 2013; Ibinda 2014;Tang 2014); and tracking of prescription refill frequency and appointment keeping (Peterson 1984).
Adherence was reported as mean score, percentage change in adherence score from baseline to post‐intervention, percentage of doses taken, percentage of days correct doses were taken, percentage of doses taken on schedule, percentage of mean change from baseline to post‐intervention and percentage of change from the initial level towards the mean of the accepted therapeutic range (see Summary of results for each included study, Table 2).
1. Summary of results for each included study.
| Behavioral interventions | |||
| References | Assessment methods | Statistical analysis | Study results |
| Brown 2009 | All participants completed a 14‐page packet of self‐report measures. Adherence was measured with MEMS cap. To assess the equivalence of control and intervention groups, and to identify factors that could moderate the impact of the intervention, a collection of self‐report measures was applied (methods such as a single‐item self‐estimate of the number of missed doses during the preceding month, the Brief Illness Perception Questionnaire (BIPQ), Theory of Planed Behaviour (TPB), Multiple Ability Self Report Questionnaire (MASQ), Hospital Anxiety and Depression Scale (HADS), Prospective and Retrospective Memory Questionnaire (PRMQ), the Liverpool Seizure Severity Scale (LSSS) were administered at baseline and at follow‐up. | Analysis of variance (ANOVA) and Chi2 test. | Intervention participants showed improved adherence relative to controls on all 3 outcomes: doses taken in total (93.4% vs 79.1%), days on which correct dose was taken (88.7% vs 65.3%), and doses taken on schedule (78.8% vs 55.3%) (P < 0.01) |
| DiIorio 2009 | Adherence was measured using MEMS cap and self‐reported medication adherence via Antiretrovial General Adherence Scale (AGAS) (at baseline and follow‐up assessment). The following scales were also used: Epilepsy Self‐Management Scale (follow‐up assessment only), Epilepsy Self‐Efficacy scale and knowledge about the epilepsy measured by Epilepsy Knowledge Questionnaire (EKQ) | Independent t‐test used to compare treatment and control group on variables assessed at follow‐up | The results on adherence were following: prescribed doses taken overall in the intervention group was 81.29% (SD 13.48) and doses taken on schedule 53.27% SD (17.74). The results for adherence and self‐efficacy were in the correct direction and statistically significant only at the 0.10 level, suggesting that the intervention may also improve confidence in self‐management |
| Educational interventions | |||
| References | Assessment methods | Statistical analysis | Study results |
| Dash 2015 | Drug adherence and self‐care were measured respectively using the modified Morisky Medication Adherence Scale (MMAS) and the Epilepsy Self‐Efficacy Scale (ESES) | Statistical analysis was carried out using SPSS (version 16 for Windows), a paired t‐test was applied |
In the intervention group, the pre‐test mean MMAS score was 6.58, whereas the post‐test mean MMAS score was 7.53; the difference was significant (P = 0.001). The mean MMAS scores for the control group's pre‐test and post‐test were 6.46 and 6.58 respectively, which were not significantly different (P = 0.224) |
| Dilorio 2011 | Medication adherence was measured using the Medication Adherence scale (MAS ‐ 8‐item measurement of self‐reported medication‐taking behaviours); perceived stress was measured by Perceived Stress Scale (PSS) and the Revised Epilepsy Stressor Inventory (ESI‐R). Pittsburgh Sleep Quality Index (PSQI), Epilepsy Self‐Management Scale (ESMS), Epilepsy Self‐Efficacy Scale (ESES), Epilepsy Knowledge Profile (EKP) and the Quality of Life in Epilepsy Scale‐10 (QOLIE‐10) measurements were also assessed | Repeated measures analyses of variance (ANOVA) were conducted using SPSS Version 18 | Trends toward statistical significance were noted for medication adherence (P = 0.118), stress (P = 0.098), self‐management (P = 0.098), and knowledge (P = 0.077). Participants who completed WebEase modules (intervention group) reported an increase in self‐efficacy (P = 0.013), meaning that they were more positive about their ability to manage medication, stress, or sleep issues |
| Helgeson 1990 | Blood test measuring serum drug level was used to assess adherence with medication. The following measurements were also performed: level of anxiety was assessed by the State‐Trait Anxiety Inventory, State Anxiety Scale (STAI), Washington Psychosocial Seizure Inventory (WPSI), the Acceptance of Disability (AD) scale, Sherer’s Self‐Efficacy Scale and Epilepsy knowledge and medical management 50‐item true‐false questionnaire | Repeated measures analyses of variance (ANOVA) and a series of paired t‐tests | Percentage change scores in blood AED levels (adherence) in the intervention group increased significantly F (1,24) = 4.18, P < 0.05 The treatment group showed a significant decrease in level of fear of death and brain damage due to seizures, F( 1,36) = 7.49 (P = 0.009) and a significant decrease in hazardous medical self‐management practices, F(1,36) = 29.67 (P = 0.0001) |
| Ibinda 2014 | Improvement in adherence to AEDs was assessed by self‐report using the 4‐item Morisky Medication Adherence Scale. Plasma drug concentrations were measured using a fluorescence polarisation immunoassay analyser (TDxFLx Abbott Laboratories) Epilepsy beliefs were measured using KEBAS | Pearson’s Chi2 test, modified Poisson regression t‐tests and logistic regression. All statistical analyses were performed using STATA (version 12) | No significant difference in adherence to AEDs was noted between the 2 groups based on self‐reports (OR 1.00, 95% CI 0.71 to 1.40; P = 1.00) or in detectable drug levels (OR 1.46, 95% CI 0.74 to 2.90; P = 0.28). No difference in seizure frequency was found between groups |
| Modi 2013 | Caregivers completed baseline questionnaires and all families were provided with MEMS‐6 Track‐Cap to monitor adherence. Caregivers (intervention group) also completed several questionnaires: psychosocial (e.g. quality of life), epilepsy knowledge, social problem‐solving skills, epilepsy medication management, feasibility‐acceptability questionnaire, Medical Chart Review and background Information Form | Means, SDs and frequencies were measured using IBM SPSS statistics software (version 20) | Mean percentage change in adherence from baseline to post‐intervention was 31.5 (SD 52.9) for the intervention group and 9.3 (SD 8.7) for the control group (no significance levels were reported). The impact on quality of life due to the implementation of the intervention reported a significant benefit (mean 6.75 (SD 0.6)). Other outcomes measures included assessment of feasibility and acceptability of the adherence intervention |
| Pryse‐Phillips 1982 | Serum drug levels of phenobarbitone, phenytoin, carbamazepine, sodium valproate, and ethosuximide were performed using a gas liquid chromatograph or by the EMITm method on each occasion where relevant | Comparisons of means in paired samples Student’s t‐test, correlation coefficients, and linear regressions were performed using an IBM computer | The results show whether information was given in oral form alone or both orally and in written form; adherence to drug treatment as measured by serum levels was not improved |
| Shope 1980 | Adherence was assessed by measurement of serum drug levels using blood tests | Analyses of variance (ANOVA) analyses of co‐variance (ANCOVA) and Chi2 tests were performed | The mean score of the intervention group on the combined adherence score was 2.9, which is significantly higher than the mean score in the control group 2.2 (F(1,48) = 6.36, P = 0.015) |
| Mixed interventions | |||
| References | Assessment methods | Statistical analysis | Study results |
| Li 2013 | To assess drug adherence, 6‐response‐options rating scales were applied. With regard to lifestyle or habits, 6 similar ratings were used to measure frequency of seizure‐provoking events. The subsequent seizure assessment for intervention group was obtained from the epilepsy tracking card. In control group medical adherence ratings were derived from self‐reported data and calculated AED adherence by counting the remaining pills to count the number of missed doses |
Chi2 test, or correlated Chi2 test or Fisher’s exact test and one‐way ANOVA were used to conduct statistical analyses with SPSS (version 17.0) | Adherence improved in the intervention group, as most members (142 (77.6%) compared to 17 (9.6%)) rated their adherence as excellent or very good, but it remained nearly unchanged in the control group. A moderate correlation was found between the changes in AED adherence and seizure control (r = 0.4, P < 0.05), and a weaker correlation was found between lifestyle and seizure control (r = 0.328, P < 0.05). The percentage of participants reported a reduction in seizures in at least 50% (including those who were seizure‐free) rose to 79.8% in the intervention group, compared to 61.0% in the control group (P < 0.05). |
| Peterson 1984 | Adherence was assessed by changes in plasma anticonvulsant levels (provided that the participant's medication regimen had not been altered in the preceding 2 weeks), a check of the participant's prescription record book to determine prescription refill frequency, medication seizure diary (to record Dosett container check) and participant appointment‐keeping frequency (those who had attended all scheduled appointments in the previous 6 months were considered compliant) | McNemar tests for related samples, Wilcoxon matched‐pair tests, Stuart‐Maxwell tests, and Student’s paired t‐tests, Chi2 tests, Mann‐Whitney tests, and Student’s unpaired t‐tests | Study shows that adherence (mean plasma levels) can be improved and seizure frequency lessened by compliance‐improving intervention. Although the differences between the 2 groups in mean anticonvulsant dosages were not statistically significant, they might be clinically important |
|
Tang 2014* (*The study is presented in this review as both types of interventions: educational and mixed) |
Adherence was measured using the 4‐item Morisky Medication Adherence Scale (MMAS‐4); seizure control was reported according to the participants’ records and telephone follow‐ups by the pharmacist; a questionnaire was developed to evaluate the level of each participant's knowledge of AEDs; the 31‐item Quality of Life in Epilepsy Inventory (QOLIE‐31) was used to measure the quality of life. Adherence, the AEDs knowledge, the number of seizures and other measures were evaluated at the beginning and at the end of follow‐up. The quality of life was only measured after intervention |
All analyses were performed using the IBM SPSS statistics (version 19). Tests such as Pearson's Chi2 tests, student's t‐tests and Mann–Whitney U test were performed | The adherence and knowledge of AEDs increased greatly after intervention in all participants, the number of seizures and missed dosages also decreased. However, no significant differences were observed between 2 groups: increased adherence (62.3% vs 64.3%, P = 0.827); increased knowledge of AEDs (88.7% vs 80.4%, P = 0.231) and improved seizure control (64.2% vs 64.3%, P = 0.988) |
SD: standard deviation
Excluded studies
See the Characteristics of excluded studies table.
We defined a list of the most common characteristics for the exclusion criteria.
1. Inappropriateness of the study design: not a RCT or no randomisation procedure performed, or both. 2. Adherence as an outcome is not reported or no adherence‐enhancing intervention, or both. 3. Not an original publication. 4. The study was not performed in the field of epilepsy.
Risk of bias in included studies
We applied the full version of Cochrane’s tool for assessing risk of bias. Descriptions by domain are provided below (see 'Risk of bias' summary for each included study, Figure 2).
2.
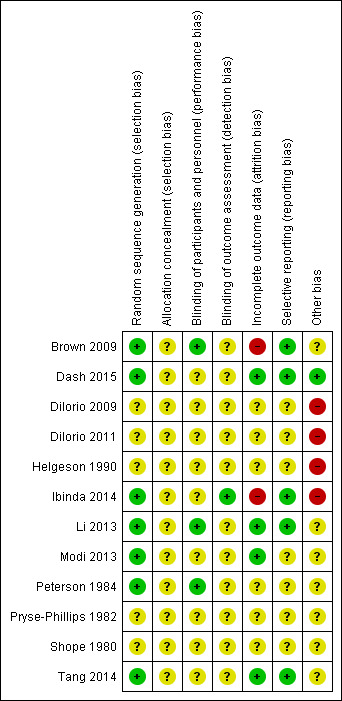
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Random sequence generation (selection bias)
Four trials used computer‐generated randomisation, which we considered to be an adequate randomisation procedure (Brown 2009; Dash 2015; Ibinda 2014; Tang 2014). One study reported the use of permuted block randomisation with a block size of two (Modi 2013). Another study (Li 2013) stated that a simple randomisation method was used but did not describe it further. One trial used the toss of a coin (Peterson 1984). Data on the method of randomisation were missing from the other trial reports, so we cannot properly judge the adequacy of randomisation.
Allocation
This domain was not properly reported in any of 12 trials and thus cannot be evaluated.
Eight studies provided comparative baseline information on the intervention and control groups (Brown 2009; Dilorio 2011; Dash 2015; Helgeson 1990; Ibinda 2014; Li 2013; Peterson 1984; Tang 2014). DiIorio 2009 and Shope 1980 provided demographic characteristics for the whole study sample but did not present the characteristics of each group. In Modi 2011 the authors provided the characteristics for the two groups and stated that the statistical comparison was not conducted owing to small sample sizes. Six studies provided baseline adherence levels for both groups (Dilorio 2011; Dash 2015; Ibinda 2014; Li 2013; Modi 2011; Tang 2014).
Blinding
None of the trials reported blinding of participants to the intervention they were receiving, as it was not possible in this particular setting. Blinding of healthcare providers or outcome assessors or both was reported in only four trials. Laboratory technicians determining drug levels in the blood assays were blinded to randomisation in Ibinda 2014, although the blinding procedure is incomplete and we therefore judge the study to have a high risk of bias. Li 2013 reported that study designers, local physicians and the data analyst were blinded to the intervention. Brown 2009 blinded the neurologist and clinic and pharmacy staff to group participation. Peterson 1984 blinded physicians treating study participants. Although blinding of healthcare providers should avoid systematic differences in the care provided (performance bias), this approach is vulnerable to disclosure by participants. In all trials it was unclear whether blinding of outcome assessors was maintained, and we therefore cannot determine the risk of detection bias.
Incomplete outcome data
Eight trials reported losses to follow‐up (Brown 2009; Dash 2015; DiIorio 2009; Helgeson 1990; Ibinda 2014; Li 2013; Peterson 1984; Tang 2014). However, it was apparent that participants lost to follow‐up were excluded from the analysis in only four trials (Dash 2015; Li 2013; Modi 2013; Tang 2014). Missing outcome data detected in Brown 2009 and Ibinda 2014 were likely to be related to true outcome, and to cause high risk of bias. The number of participants lost to follow‐up ranged from 2 to 157.
Selective reporting
Selective outcome reporting bias could occur, for instance, if seizure frequency was measured and analysed but was not reported in the study results. Five studies (Brown 2009; Dash 2015; Ibinda 2014; Li 2013; Tang 2014) published all expected outcomes, even though the study protocol was available only for Ibinda 2014. As no other study protocols were available to us and we were unable to contact all study authors, we cannot confirm or exclude this type of bias in the other seven studies.
Other potential sources of bias
Seven studies (Brown 2009; Li 2013; Modi 2013; Peterson 1984; Pryse‐Phillips 1982; Shope 1980; Tang 2014) reported insufficient information to judge whether or not other risks of bias might have been introduced. Three studies discussed possible threats to validity: Dilorio 2011 argued that self‐reported responses might be affected by social desirability biases, including the tendency to overemphasise behaviour in favour of the desired outcomes; Helgeson 1990 reported many statistically non‐significant results; and Ibinda 2014 may have been affected by traditional religious and cultural beliefs. Only two studies (Ibinda 2014; Li 2013) performed appropriate sample size calculations.
Only two studies (Dash 2015; Li 2013) met four quality criteria (low risk of bias), two studies (Brown 2009; Tang 2014) met three quality criteria, three studies (Ibinda 2014; Modi 2013; Peterson 1984) met two quality criteria for risks of bias.
Effects of interventions
See: Table 1
The effects of interventions on identified outcomes can be found in Table 1, and the results by study are described in Table 2.
Effects on adherence
Behavioural interventions
Two studies examined behavioural interventions. Only one of them (Brown 2009), with 69 participants, reported statistically significant results. The implementation intention intervention participants showed improved adherence relative to control. The percentage of doses taken in the intervention group was 93.4% (SD 12.3%), versus 79.1% (SD 28.1%) in the control group (P = 0.01). The percentage of days on which correct doses were taken in the intervention group was 88.7% (SD 15.1%), versus 65.3% (SD 35.6%) in the control group (P = 0.01). The percentage of doses taken on schedule in the intervention group was 78.8% (SD 23.5%), versus 55.3% (SD 34.8%) in the control group (P = 0.001). The overall adherence scores were generated by standardising and then averaging the three percentage measures. The mean overall adherence score in the intervention group was 0.35 (SD 0.55), versus 0.40 (SD 1.15) in the control group (P < 0.01).
Use of motivational interviewing to enhance self‐management practices had no effect on adherence, in another study (DiIorio 2009), with 20 participants. The percentage of doses taken in the intervention group was 81.29% (SD 13.48%), versus 82.19% (SD 21.76%) in the control group (P = 0.912). The percentage of doses taken on schedule in the intervention group was 53.27% (SD 17.74%) versus 66.01% (SD 29.61%) in the control group (P = 0.258). The mean AGAS score in the intervention group was 4.28 (SD 0.74), versus 4.46 (SD 0.58) in the control group (P = 0.523).
Educational interventions
Eight studies assessed the added value of educational interventions, but only three studies presented statistically significant results.
In the epilepsy health education group, the pretest mean adherence score was 6.58, whereas the post‐test mean score was 7.53; the difference was statistically significant (P = 0.001). The mean adherence scores for the control group's pretest and post‐test were 6.46 and 6.58 respectively, which were not statistically significantly different (P = 0.224) (Dash 2015).
One year after an educational intervention was provided, there was no statistically significant difference in adherence to AEDs based on detectable drug levels (odds ratio (OR) 1.46, 95% CI 0.74 to 2.90, P = 0.28) or by self‐reports (OR 1.00, 95% CI 0.71 to 1.40, P = 1.00) between the intervention and nonintervention groups (Ibinda 2014).
In the group of children and their caregivers who received educational interventions the mean percentage change in adherence from baseline to postintervention was 31.5 (SD 52.9), versus 9.3 (SD 8.7) in the no‐intervention group (Modi 2013). The statistical comparison was not conducted, owing to small sample sizes.
Use of the online epilepsy self‐management programme WebEase was found to be an effective means of enhancing adherence in Dilorio 2011, with 148 participants. The mean adherence score after 12 weeks was 7.33 in the intervention group (SD 1.833), versus 6.90 (SD 2.33) in the control group (P = 0.049), with a mean difference of 0.43 (95% CI ‐0.24 to1.10).
In Helgeson 1990, the intervention group showed a significant and sustained increase in blood serum concentrations of antiepileptic medication from baseline to four‐month follow‐up (a mean increase of 70%). Over the same period, the control group showed a mean decline in blood serum levels of 18% (P < 0.05).
Pryse‐Phillips 1982 reported that whether information was given in oral form alone or both orally and in written form, it produced no significant rise or fall in the mean serum level of prescribed antiepileptic medication.
Shope 1980 reported that the mean adherence score derived from serum level for children of parents who received the intervention was 2.9 versus 2.2 in the control group (P = 0.015).
Tang 2014 reported that adherence improved in both the medication education group (62.3%) and the medication education with behavioural intervention group (64.3%), but the difference between the two groups was not statistically significant (P = 0.827).
Mixed interventions
Three studies focused on mixed interventions, with one study (Tang 2014) comparing an educational intervention plus a behavioural component to a single educational intervention (described in the 'Educational intervention' section)
Li 2013 reported no statistically significant differences at baseline between the numbers of participants in intervention and non‐intervention groups who rated their adherence as excellent or very good (12.6% versus 9.1% respectively; P = 0.579). One year after the intervention was provided, 77.6% of intervention group members rated their adherence as excellent or very good, versus 9.6% in the non‐intervention group (P < 0.001).
Use of patient prompts, such as mailed reminders for prescription refills and appointments, together with a counselling leaflet, produced positive effects on adherence (Peterson 1984). At follow‐up, mean serum levels of phenytoin, carbamazepine and sodium valproate were higher in the intervention group than in the control group, and this was accompanied by a greater shift from sub therapeutic to therapeutic plasma levels in the intervention group than in the control group (P < 0.005). The high serum level can be explained by participants taking more medication rather than higher doses, as no significant changes in AED dosages were reported within treatment groups. The proportion of compliant participants, as judged by prescription refill frequencies, was higher in the intervention group than in the control group (88% versus 50%; P > 0.01). There was no difference between the intervention and control groups for appointment‐keeping (59% versus 65%; P > 0.5).
After intervention, the adherence increased greatly in all participants (Tang 2014) and the number who missed AEDs decreased to 45.0% from 64.3% (P = 0.988). However,we found no statistically significant differences between the two groups: increased adherence (62.3% in education group versus 64.3% in education/behavioural group, P = 0.827).
Effects on seizure frequency or seizure severity
Seizure frequency or seizure severity or both as important secondary outcome were described in six studies, with four of them (Dash 2015; Li 2013; Peterson 1984; Tang 2014) presenting improved adherence and decreased seizure frequency in the intervention groups.
Dash 2015 reported a higher proportion of participants with decreased seizure frequency in the intervention compared to the control group (34.1% versus 18.6%; P = 0.043 ) six months after the intervention. The rest of the participants either had increased seizure frequency (12.3% versus 14.3%; P = 0.811), or unchanged (53.6% versus 67.1%; P = 0.099).
Tang 2014 reported improved seizure control in the medication education group by 64.2% and in the behavioural intervention group by 64.3% from baseline, but there was no statistically significant difference between the two interventions (P = 0.988).
Ibinda 2014 reported no difference in seizure frequency between the groups (P = 0.58).
Li 2013 reported that before the intervention, baseline numbers of participants with more than a 50% seizure reduction were similar in the two groups (50.9% versus 45.8%; P = 0.337). After the intervention, the proportion of participants with more than a 50% seizure reduction rose to 79.8% in the intervention group, compared with 61.0% in the non‐intervention group (P < 0.05).
Peterson 1984 compared the number of seizures between intervention and control groups at follow‐up and found no statistically significant differences (median 2.5 versus 3.5; P > 0.5). However, the reduction in seizure frequency from baseline was statistically significant for the intervention group (median from 6 to 2.5; P < 0.01) but was not statistically significant for the control group (median from 4 to 3.5; P > 0.1).
Seizure frequencies were reported not to have significantly changed from baseline to follow‐up in either intervention or control groups in Helgeson 1990.
Effects on side effects, serious adverse events or costs
No trial examined the effects of interventions on treatment side effects and serious adverse events. Dilorio 2011 argues that the intervention will be more cost‐effective, mainly because it is an online product that will save working hours and require less administration in comparison with usual care. However no further information on cost effectiveness was presented. No further reports described costs associated with adherence‐modifying interventions.
Effects on self‐efficacy
Four studies (Dash 2015; DiIorio 2009; Dilorio 2011; Helgeson 1990) reported self‐efficacy effects.
Three of the four studies used the Epilepsy Self‐Efficacy Scale (ESES), which measures different aspects of efficacy in people with epilepsy, rating the items on an 11‐point (Likert) rating scale covering personal levels of confidence regarding the ability to manage epilepsy. The fourth study (Helgeson 1990) presented self‐efficacy using the Sherer’s Self‐Efficacy Scale.
Dash 2015 used continuous variables to represent a total score and the assessment was administered by a specialised epilepsy nurse. The intervention, however, did not improved the overall self‐efficacy score in participants with epilepsy.
DiIorio 2009 reports a positive effect of self‐efficacy on the understanding ability for self‐management practices. Much higher levels of self‐efficacy (mean intervention group 8.63 (SD 1.23) compared to the mean in the control group of 7.51 (SD 1.53)) were shown in the intervention group, resulting in better seizure management and epilepsy knowledge. However the results were not statistically significant at the P < 0.05 level (t = 1.757, P = 0.097).
The later study by Dilorio 2011 showed higher levels of self‐efficacy at postintervention measurement in participants receiving the intervention compared to the control group. The trend testing was significant, with a postintervention mean of 188.02 (SD 32.88) versus 171.17 (SD 40.21) in the intervention group versus the control group respectively (F = 6.49, P = 0.0130).
Helgeson 1990 reported general and social self‐efficacy using Sherer's Self‐Efficacy scale. Both mean scores, however, were higher in the control group compared to the intervention group at pre‐assessment and at four months follow‐up.
Effects on quality of life
Two studies (Modi 2013; Tang 2014) included the quality of life as a secondary outcome.
Modi 2013 measured the impact on quality of life using a feasibility and acceptability questionnaire. The questionnaire included one item “Treatment helped improve my child’s quality of life” rated on a 7‐point Likert scales by 4 families who received the intervention. A mean 6.75 (SD 0.6) was reported.
Tang 2014 presented the overall quality of life using the Quality of Life in Epilepsy Scale‐10 (QOLIE‐10), with each of the 10 items rated on a five‐point scale. The difference in scores between the two groups was not statistically significant (P = 0.9475).
Discussion
Summary of main results
We identified 12 studies examining the effects on adherence to antiepileptic drugs using different interventions. Among these, two studies used behavioural interventions, eight studies used educational interventions and three studies used mixed interventions. One study (Tang 2014) compared an educational intervention plus a behavioural component to a single educational intervention, and we have therefore presented the study results in both the educational intervention and in the mixed intervention categories.
The aim of this review was to assess the effectiveness of interventions aimed at improving adherence to antiepileptic medication. Education and counselling of people with epilepsy have shown mixed success. Behavioural interventions such as the use of intensive reminders and implementation intention interventions have demonstrated more favourable effects on adherence. Mixed interventions were shown to improve adherence in the intervention groups compared to the control groups.
However, because of the very limited number of trials and the small sample sizes, further studies are needed to confirm the initial indications that adherence to antiepileptic drugs can be improved by these means.
Overall completeness and applicability of evidence
Applicability of the findings to everyday practice is uncertain for many reasons. Firstly, translation of education, counselling and motivation from the trial setting to everyday practice is not necessarily feasible. Secondly, improving medication adherence will not necessarily translate into clinical benefits for the patient (Nieuwlaat 2014; Roter 1998). The effects of adherence‐enhancing interventions must therefore be judged by their clinical outcomes. Outcomes such as reduced seizure frequency and side effects were not always reported by the included trials. Out of six trials that reported seizure frequency, four showed a statistically significant decrease in seizure frequency when adherence was also improved. Thirdly, the value of adherence research to clinical practice is enriched by studying the relationship between adherence and factors known to influence adherence (DiMatteo 2004). Only two trials examined the relationship between adherence and patient‐related factors (Brown 2009; Ibinda 2014), and found no statistically significant relationship between them. Fourthly, short‐term follow‐up makes it difficult to ascertain whether interventions with promising adherence‐improving effects can maintain their effects over time. Finally, adherence‐enhancing interventions require utilisation of healthcare resources, meaning that cost‐effectiveness information is required for informed decision‐making about their implementation. None of the identified trials discussed the cost implications of these interventions.
Quality of the evidence
Twelve trials met our inclusion criteria. Insufficient reporting of what happened in these trials has hindered our ability to ascertain all risks of bias. For instance, we were unable to establish for all the trials the adequacy of the generation of the allocation sequence and of allocation concealment, and differences between baseline characteristics of the groups that were compared. Information on calculation of the statistical power of the sample size was provided in only two studies (Ibinda 2014; Li 2013). Inadequate sample size could increase the likelihood of a type II error, and other biases.
In adherence‐enhancing intervention trials, the validity and reliability of adherence measurement and reporting are central. Among the different measures of adherence, no single intervention can be regarded as a gold standard, and use of multiple measures of adherence is recommended (DiMatteo 2004; Eatock 2007; Nichol 1999; Paschal 2008; Vermeire 2001). Only three trials used more than one adherence measure (DiIorio 2009; Ibinda 2014; Peterson 1984).
We examined the overall strength of evidence for selected outcomes. This was high for effects on adherence with mixed interventions and quality of life; moderate for effects on adherence with behavioural interventions, effects on adherence with educational interventions, and seizure frequency and severity; and low for self‐efficacy.
Potential biases in the review process
We did not contact authors of excluded trials to enquire whether adherence was measured but not reported, and therefore cannot exclude outcome reporting bias from our review (Kirkham 2010).
Agreements and disagreements with other studies or reviews
Several systematic reviews have examined the issues of adherence‐enhancing interventions in general (Nieuwlaat 2014; Peterson 2003; Roter 1998); others have focused on adherence in older people (Higgins 2004), adherence to lipid‐lowering medication (Schedlbauer 2010), to type 2 diabetes treatment recommendations (Vermeire 2005), and to antihypertensive medication (Schroeder 2004). Remarks on internal and external validity of the adherence literature in previous reviews are concordant with those in our review. Reviews looking at the methodological rigour of the literature on patient compliance with medication have similarly emphasised the importance of reliability and validity of adherence measurement (Cramer 2008; DiMatteo 2004; Nichol 1999; Vermeire 2001).
Authors' conclusions
Implications for practice.
Despite the increasing number of trials indicating that poor adherence to antiepileptic medication is common and is associated with increased morbidity and mortality, the literature concerning interventions to improve adherence in epilepsy is still limited in quantity and quality. Behavioural interventions and mixed interventions demonstrate some positive results; however, no firm conclusions can be drawn regarding the long‐term effects of these interventions.
Implications for research.
The results of our review highlight gaps in research on the effectiveness of interventions aimed at improving adherence to antiepileptic medication. Our findings emphasise the need for further adequately‐powered randomised controlled trials that use a combination of adherence measurement techniques and that provide participant follow‐up for a longer period. The differences between subjective self‐reporting and objective blood tests are difficult to resolve in order to report firm conclusions. Trials should investigate the effects of interventions on adherence, as well as important clinical outcomes such as seizures. Researchers should minimise the risks of bias by using suitable randomisation techniques, concealment of allocation and blinding of both healthcare providers and outcome assessors. Differences in medical systems between countries are another implication, as low‐ and middle‐income countries face many difficulties and limited possibilities to apply hospital‐based results to the general population, even within the same country.
Patients' beliefs and preferences are prevalent influences on the medicine‐taking process (Rand 2000; Sieber 2000). One of the studies included in this review describes major limitations introduced by negative stereotypes about the medication treatment, and strong beliefs in traditional healing. Qualitative research involving people with epilepsy is of value in developing adherence‐enhancing interventions, in evaluating the validity of the content of these interventions, and in assessing their feasibility. This can be regarded as a foundational step before these interventions are tested in randomised controlled trials.
Finally, the impact of adherence‐enhancing interventions on resource utilisation and its cost effectiveness merit further research.
What's new
| Date | Event | Description |
|---|---|---|
| 10 May 2016 | New citation required but conclusions have not changed | Conclusions remain the same. |
| 4 February 2016 | New search has been performed | Searches updated on 4 February 2016. Six new studies have been included. |
History
Protocol first published: Issue 1, 2010 Review first published: Issue 1, 2011
| Date | Event | Description |
|---|---|---|
| 24 September 2015 | New search has been performed | searches updated |
| 4 September 2014 | New search has been performed | Searches updated on 4 September 2014 |
| 4 September 2014 | New citation required but conclusions have not changed | 4 new studies have been included (Dilorio 2011; Ibinda 2014; Li 2013; Modi 2013). Conclusions remain unchanged |
Appendices
Appendix 1. Cochrane Epilepsy Group Specialized Register search strategy
#1 MeSH DESCRIPTOR Patient Compliance Explode All
#2 MeSH DESCRIPTOR Medication Adherence Explode All
#3 MeSH DESCRIPTOR Health Behavior Explode All
#4 MeSH DESCRIPTOR Health Education Explode All
#5 MeSH DESCRIPTOR Patient Education as Topic Explode All
#6 MeSH DESCRIPTOR Behavior Therapy Explode All
#7 MeSH DESCRIPTOR Treatment Refusal Explode All
#8 MeSH DESCRIPTOR Patient Dropouts Explode All
#9 patient NEXT complian*
#10 patient NEXT adheren*
#11 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10
#12 INREGISTER AND >23/09/2015:CRSCREATED
#13 #11 AND #12
Appendix 2. CENTRAL via CRSO search strategy
#1 MESH DESCRIPTOR Patient Compliance EXPLODE ALL TREES
#2 MESH DESCRIPTOR Medication Adherence EXPLODE ALL TREES
#3 MESH DESCRIPTOR Health Behavior EXPLODE ALL TREES
#4 MESH DESCRIPTOR Health Education EXPLODE ALL TREES
#5 MESH DESCRIPTOR Patient Education as Topic EXPLODE ALL TREES
#6 MESH DESCRIPTOR Behavior Therapy EXPLODE ALL TREES
#7 MESH DESCRIPTOR Treatment Refusal EXPLODE ALL TREES
#8 MESH DESCRIPTOR Patient Dropouts EXPLODE ALL TREES
#9 (patient next complian*):TI,AB,KY
#10 (patient next adheren*):TI,AB,KY
#11 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10
#12 MESH DESCRIPTOR Epilepsy EXPLODE ALL TREES
#13 MESH DESCRIPTOR Seizures EXPLODE ALL TREES
#14 MESH DESCRIPTOR Anticonvulsants EXPLODE ALL TREES
#15 (epilep* OR seizure* OR convulsion* OR antiepilep*):TI,AB,KY
#16 #12 OR #13 OR #14 OR #15
#17 #11 AND #16
#18 31/08/2015 TO 29/02/2016:DL
#19 #17 AND #18
Appendix 3. MEDLINE search strategy
This strategy is based on the Cochrane Highly Sensitive Search Strategy for identifying randomised trials (Lefebvre 2011).
1. exp Patient Compliance/
2. (patient adj complian$).tw.
3. (patient adj adheren$).tw.
4. exp Medication Adherence/
5. exp Health Behavior/
6. exp Health Education/
7. exp Patient Education as Topic/
8. exp Behavior Therapy/
9. exp Treatment Refusal/
10. exp Patient Dropouts/
11. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10
12. (epilep$ or seizure$ or convulsion$).ti,ab.
13. exp Epilepsy/
14. exp Seizures/
15. exp Anticonvulsants/
16. antiepilep$.ti,ab.
17. 12 or 13 or 14 or 15 or 16
18. (randomized controlled trial or controlled clinical trial).pt. or (randomi?ed or placebo or randomly).ab.
19. clinical trials as topic.sh.
20. trial.ti.
21. 18 or 19 or 20
22. exp animals/ not humans.sh.
23. 21 not 22
24. 11 and 17 and 23
25. remove duplicates from 24
26. limit 25 to ed=20150924‐20160204
Appendix 4. CINAHL search strategy
S23 S8 and S14 and S21 Publication Year: 2014‐
S22 S8 and S14 and S21
S21 S15 or S16 or S17 or S18 or S19 or S20
S20 AB randomly
S19 AB placebo
S18 TI ( randomised OR randomized ) or AB ( randomised OR randomized )
S17 TI clin* N1 trial* or AB clin* N1 trial*
S16 (MM "Random Assignment")
S15 (MM "Clinical Trials")
S14 S9 or S10 or S11 or S12 or S13
S13 TI antiepilep* or AB antiepilep*
S12 TI ( epilep* OR seizure* OR convulsi* ) or AB ( epilep* OR seizure* OR convulsi* )
S11 (MM "Anticonvulsants")
S10 (MM "Seizures")
S9 (MM "Epilepsy")
S8 (S1 or S2 or S3 or S4 or S5 or S6 or S7)
S7 TI patient N1 adheren* or AB patient N1 adheren*
S6 TI patient N1 complian* or AB patient N1 complian*
S5 (MM "Patient Dropouts")
S4 (MM "Treatment Refusal")
S3 (MM "Patient Education")
S2 (MM "Health Behavior")
S1 (MM "Patient Compliance") or (MM "Medication Compliance")
Appendix 5. PsycINFO search strategy
S14 S4 AND S11 AND S12 Publication Year: 2014‐
S13 S4 AND S11 AND S12
S12 S9 OR S10
S11 S5 OR S6 OR S7 OR S8
S10 AB randomized OR AB placebo OR AB randomly
S9 DE "clinical trials" OR TI clin* trial* OR AB clin* trial* OR AB trial
S8 DE "carbamazepine" OR DE "chloral hydrate" OR DE "clonazepam" OR DE "diphenylhydantoin" OR DE "nitrazepam" OR DE "oxazepam" OR DE "pentobarbital" OR DE "phenobarbital" OR DE "primidone" OR DE "valproic acid"
S7 DE "epilepsy" OR DE "seizures" OR DE "anticonvulsive drugs"
S6 AB epilep* OR AB seizure* OR AB convulsi*
S5 TI epilep* OR TI seizure* OR TI convulsi*
S4 S1 OR S2 OR S3
S3 AB patient complian* OR AB patient adheren*
S2 TI patient complian* OR TI patient adheren*
S1 DE "treatment refusal" OR DE "treatment compliance" OR DE "treatment dropouts" OR DE "client education" OR DE "behavior therapy"
Appendix 6. ClinicalTrials.gov search strategy
adherence AND drug AND epilepsy | received from 09/23/2015 to 02/04/2016
Appendix 7. ICTRP search strategy
adherence AND drug AND epilepsy NOT NCT*
Date of registration on or after 23/09/2015 (results screened manually)
Appendix 8. EMBASE search strategy
1 patient compliance/
2 (patient adj complian$).ti,ab.
3 (patient adj adheren$).ti,ab.
4 exp health behavior/
5 patient education/
6 health education/
7 behavior therapy/
8 treatment refusal/
9 treatment withdrawal/
10 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9
11 (epilep$ or seizure$ or convulsion$).ti,ab.
12 exp epilepsy/
13 exp seizure/
14 exp anticonvulsive agent/
15 antiepilep$.ti,ab.
16 11 or 12 or 13 or 14 or 15
17 exp animal/
18 animal experiment/
19 nonhuman/
20 17 or 18 or 19
21 human/
22 human experiment/
23 21 or 22
24 23 not 20
25 Clinical trial/
26 Randomized controlled trial/
27 Randomization/
28 Single blind procedure/
29 Double blind procedure/
30 Crossover procedure/
31 Placebo/
32 Randomi?ed controlled trial$.tw.
33 RCT.tw.
34 Random allocation.tw.
35 Randomly allocated.tw.
36 Allocated randomly.tw.
37 (allocated adj2 random).tw.
38 Single blind$.tw.
39 Double blind$.tw.
40 ((treble or triple) adj blind$).tw.
41 Placebo$.tw.
42 Prospective study.tw.
43 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42
44 Case study/
45 Case report.tw.
46 Abstract report/ or letter/
47 44 or 45 or 46
48 43 not 47
49 24 and 48
50 10 and 16 and 49
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Brown 2009.
| Methods |
Study design: RCT Methods of randomisation: a computerised random‐number generator. Follow‐up : 4 weeks. Setting: 5 outpatient clinics at one hospital in the UK. Date it was conducted: participants were recruited between January and June 2007. Source of funding: Janssen Cilag, Epilepsy Action, and the University of Sheffield. Conflict of interest: not reported. |
|
| Participants |
Inclusion/exclusion criteria: people over 16 years of age. Patients were excluded if they were already using an adherence‐enhancing method that could be compromised if they took part in the study, if they were receiving a diagnosis of epilepsy for the first time or if they had learning difficulty. Sample size: 81 participants were recruited; 12 participants did not complete follow‐up measures, as they did not return their MEMS medication monitor bottles. Gender: 27 (40%) were men. Age: mean age was 41 years (SD 15.4) in the IG and 44 years (SD 16.4) in the CG. |
|
| Interventions |
Type of intervention: behavioural All participants completed a 14‐page packet of self‐report measures. The IG group participants were given an additional worksheet on which they specify the environmental cues for tablet taking, using the format of an "if/then" plan. This means participants would write when and where they intend to take their medication every day, and what they would be doing at the moment of taking them. |
|
| Outcomes |
Primary outcome measured: adherence It was measured via MEMS, an electronic monitoring cap that recorded the number and timing of bottle openings. From this information, the percentage of prescribed doses taken and the percentage of doses taken on time were calculated. Overall adherence scores were generated by standardising and then averaging the 3 percentage measures. Secondary outcome(s) measured: the number of missed doses during the preceding month, the Brief Illness Perception Questionnaire and the Liverpool Seizure Severity Scale were administered at baseline and at follow‐up. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised random‐number generator was reported |
| Allocation concealment (selection bias) | Unclear risk | No information on concealment was reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The doctors and clinic and pharmacy staff were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Information on blinding of other parties (e.g. outcome assessor) was not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Missing outcome data are reported and are likely to be related to true outcome |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but it is clear that the published reports include all expected outcomes |
| Other bias | Unclear risk | Insufficient rationale or evidence that an identified problem will introduce bias |
Dash 2015.
| Methods |
Study design: RCT Method of randomisation: computer‐generated table of random numbers. Setting: outpatient clinic of a referral teaching institute in India. Date it was conducted: June to December 2012. Follow up: 6 months. Source of funding: This study received no support in the form of grants, equipment, or drugs. Printing and publishing educational material funded by Center of Excellence Epilepsy, Department of Biotechnology, Ministry of Science and Technology, India. Conflict of interest:The authors declare no conflict of interest. |
|
| Participants |
Includion/exclusion criteria: People diagnosed with epilepsy at least 1 month prior to the date of the study, ≥ 15 years of age, ability to understand Hindi/English, and willingness to participate in the study. Sample size: 180 participants were recruited. After a follow‐up of 6 months, 82 patients in IG and 70 in the CG completed the questionnaires. Gender: Male 52 (63%) in IG and 44 (63%) in CG Age: mean age was 34 years (SD 10.65) in IG and 35 years (SD 11.61) in CG. |
|
| Interventions |
Type of intervention: Educational A one‐on‐one a structured format teaching administered by an epilepsy nurse in 4 sessions each lasting at least 30 mins.The teaching sessions covered the following domains: basic knowledge regarding epilepsy, myths and truths regarding epilepsy, diagnosis, treatment modalities (emphasis on compliance),living with epilepsy, and employment issues. Pamphlets written in Hindi and supplemented with illustrations and animations were also provided. The programme was developed by a group which included 3 epilepsy nurses, 2 epileptologist's and 2 social workers. |
|
| Outcomes |
Primary outcome(s) measured: Adherence and self‐care. Adherence was assessed using the modified Morisky Medication Adherence Scale (MMAS). Secondary outcome(s) measured: the change in seizure frequency. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was done using a computer‐generated table of random numbers |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information on blinding was reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information on blinding was reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data are reported |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but it is clear that the published reports include all expected outcomes |
| Other bias | Low risk | The study seems to be free of other sources of bias |
DiIorio 2009.
| Methods |
Study Design: RCT Method of randomisation: not reported. Setting: 3 clinics in a large south‐eastern metropolitan area of the USA. Date it was conducted: not reported. Follow‐up: 12 weeks. Source of funding: Emory University Research Committee. Conflict of interest: not reported. |
|
| Participants |
Inclusion/exclusion criteria: 18 years or older, were able to understand and speak English, had telephone access and were mentally stable. Sample size: 22. Gender: 15 were men (68.2%). Age: mean age was 43 years (SD 13.51). |
|
| Interventions |
Type of intervention: Behavioural 5 motivational intervention sessions were conducted: 1 face‐to‐face and 4 telephone‐based. For each sessions, a specially trained nurse used a script that included key aspects of self‐management and discussed medication management with the participant. The nurse began by asking a general question about medication‐taking practices.Those who reported problems with medication were provided support. A goal and action plan of at least one strategy to improve adherence was developed. Participants were encouraged to develop their own solutions and to devise an action plan.Then, the nurse asked the participant to select 1 or 2 other self‐management components (information, seizure, safety and lifestyle issues) that were important to him or her. The rest of discussion aimed at identifying barriers and facilitators of desired behaviours, eliciting change strategies and building confidence. |
|
| Outcomes |
Primary outcome(s) measured: adherence It was measured via MEMS cap (presented as percentage of doses taken and percentage of doses taken on time) ,and self‐reported adherence by using AGAS. Secondary outcome(s) measured: outcome expectancy (a judgement of the likely consequences of practising self‐management strategies and epilepsy self‐management), self‐efficacy and knowledge of epilepsy (medical and social aspects). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | No information on concealment was reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information on blinding was reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information on blinding was reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient reporting of attrition/exclusions to permit judgement |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | High risk | Inadequate sample size could increase the likelihood of a type II error and other bias |
Dilorio 2011.
| Methods |
Study Design: RCT Method of randomisation: not reported. However, authors reported that after a random start for the first participant, participants were assigned alternatively to the intervention or control group. Date it was conducted: not reported. Setting: USA Follow up: 12 weeks. Source of funding: the CDC Epilepsy Program in the National Center for Chronic Disease Prevention and Health Promotion. Conflict of interest: not reported. |
|
| Participants |
Inclusion/exclusion criteria: 18 years of age or older, had been diagnosed with epilepsy, had been taking antiepileptic medication for at least 3 months, could speak and read English, had access to the Internet, were willing to participate in WebEase and had not participated in WebEase in the past. Sample size: 148 participants. Age: mean 41 years (SD 12.9) in IG and 40 years (SD 13.6) in CG. Gender: female 48 (68.6%) in IG and 61 (78.2%) in CG. |
|
| Interventions |
Type of intervention: educational In the WebEase (Web Epilepsy Awareness, Support, and Education) programme, participants spent 2 weeks in each of the 3 modules that constitute the core of WebEase: medication, stress and sleep management. After logging into the WebEase site, participants were first required to complete the MyLog section to record information about seizures, medication taking, stress and sleep quality ratings. Then, they were given access to the other components of WebEase, including the modules. Weekly reminders to continue working through the modules and exploring the site resources were sent to participants. At the end of 6 weeks, access to the programme was ended for participants |
|
| Outcomes |
Primary outcome(s) measured: adherence It was measured using the Medication Adherence Scale. Secondary outcome(s) measured: perceived stress, sleep quality, epilepsy self‐management, self‐efficacy, knowledge about epilepsy and quality of life. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | No information on concealment was reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information on blinding was reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information on blinding was reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient reporting of attrition/exclusions to permit judgement |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | High risk | Self‐reported responses can be affected by social desirability biases |
Helgeson 1990.
| Methods |
Study Design: RCT Method of randomisation: not reported. Setting: an outpatient clinic in the USA. Date it was conducted: not reported. Follow up: 4 months. Source of funding: partial financial support from the Epilepsy Foundation of America. Conflict of interest: not reported. |
|
| Participants |
Inclusion/exclusion criteria: mentally retarded, demented, or psychotic patients. Sample size: 120 people were recruited, and 100 agreed to participate. Of the 50 in the IG, only 23 completed a pre‐assessment questionnaire and 3 were excluded because they did not attend the whole 2‐day programme. Of the 50 in the CG, only 20 completed the pre‐assessment questionnaire and 18 returned the follow‐up questionnaire. Thus, the final sample included 38 participants: 20 in the intervention group and 18 in the control group. Gender: 14 (70%) in both groups were women. Age: mean age was 36.15 years (SD 12.81) in the intervention group and 38.56 years (SD 10.67) in the control group. Other characteristics: mean duration of seizure disorders was 17.40 years (SD 10.78) in the intervention group and 15.44 years (SD 11.14) in the control group. |
|
| Interventions |
Type of intervention: educational A 2‐day programme designed to provide medical education and psychosocial therapy for participants and families. No more information were given on the programme. |
|
| Outcomes |
Primary outcome(s) measured: adherence. It was measured using serum drug level and expressed as percentage of mean change from baseline to 4 months. Secondary outcome(s) measured: anxiety and depression, coping with epilepsy, self‐efficacy, psychosocial seizure inventory and epilepsy knowledge and medical management |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | No information on concealment was reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information on blinding was reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information on blinding was reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient reporting of attrition/exclusions to permit judgement |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | High risk | The risk may be explained by small sample size and limited follow‐up (4 months) |
Ibinda 2014.
| Methods |
Study Design: RCT Method of randomisation: via computer. Setting: Kenya Date it was conducted: recruitmentstarted August 2009 Follow up: 1 year Source of funding:Wellcome Trust Conflict of interest: the authors declare no conflict of interest. |
|
| Participants |
Inclusion/exclusion criteria: included people of all ages had active convulsive epilepsy, defined as at least two unprovoked convulsions, with one in the 12 months prior to being assessed. Sample size: 738 participants IG (n = 370); CG (n = 368) group. Analysis was done for 303 participants in the IG and for 278 participants in theCG who were observed at both the beginning and the end of the study. Assays of antiepileptic drugs were done on 105 in the IG and 86 in the CG who provided blood samples. Age: mean age in IG 19 years (SD 17.4) and 19.5 (SD 15.6) in CG Gender: female 47.2 % of IG and 49.6% in CG |
|
| Interventions |
Type of intervention: educational A 1‐day educational programme on epilepsy, types of seizures, causes of epilepsy, effects of epilepsy on child development, treatment of epilepsy, side effects of drugs, drug safety, what to do during a seizure, when to take a person with epilepsy to hospital, prevention of epilepsy, what a person with epilepsy can and cannot do and advice for families. in addition a brochure detailing all of the topics discussed was given to each participant. The intervention was designed and delivered by a team of epilepsy researchers and field staff. |
|
| Outcomes |
Primary outcome(s) measured: adherence It was assessed by plasma drug concentrations and self‐report using the 4‐item Morisky Medication Adherence Scale. Both measurements were compared between the baseline and the end of the study. Secondary outcome(s) measured: seizure frequency and Kilifi Epilepsy Beliefs and Attitudes Scores (KEBAS). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation was reported |
| Allocation concealment (selection bias) | Unclear risk | No information on concealment was reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit clear judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The laboratory technicians conducting the assays were blinded to the randomisation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Missing outcome data are reported and are likely to be related to true outcome |
| Selective reporting (reporting bias) | Low risk | The study protocol is available and all prespecified outcomes of interest have been reported |
| Other bias | High risk | Authors indicated that improved adherence in both groups could be explained by sharing of knowledge between groups |
Li 2013.
| Methods |
Study Design: RCT Method of randomisation: simple randomisation (random selection software). Setting: 2 rural communities of western China Date it was conducted: between September 2009 and December 2012. Follow up: 1 year Source of funding: not reported. Conflict of interest: The authors declare no conflict of interest. |
|
| Participants |
Inclusion exclusion criteria: (1) age ≥13 and <65 years and constant receipt of phenobarbital monotherapy. The exclusion criteria
included patients with severe mental retardation or neurologic diseases or psychosis; and patients receiving another one or two AEDs in addition to phenobarbital as additional therapy. Sample size: The study included a sample of 200 participants with epilepsy for each group (IG and CG). After a 12‐month follow‐up, 183 cases were retained in IG and 177 in CG. Gender: 105 male in IG and 99 male in CG. Age: mean age was 36.6 years (median 38) in the IG and 39.4 years (median 40) in the CG. Other Caracteristics: Mean duration since diagnosis was 12.3 years (median 14) in the IG and 10.6 years (median 12) in the CG. |
|
| Interventions |
Type of intervention: mixed A 4 components programme. First, intensive education that included explanation of epilepsy, emphasising the importance of receiving appropriate AED treatment and taking medication regularly. Second, consultation services where clinical providers and telephone support were available for participants at any time. Third, reminders in the form of keeping a simple record with a specifically‐designed card. Fourth, participants received repeated (> 3 times at each attending clinic) reminders about medical adherence every month. |
|
| Outcomes | Primary outcome(s) measured: adherence, seizure control and avoiding lifestyle‐precipitated seizures. Adherence and lifestyle each was graded on a 6‐point scale with possible scores and measured and compared between the groups before and after the intervention. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Simple randomisation was reported |
| Allocation concealment (selection bias) | Unclear risk | No information on concealment was reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The study designers, local physicians and data analyst were blinded to the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit clear judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data are reported |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but it is clear that the published reports include all expected outcomes |
| Other bias | Unclear risk | Insufficient rationale or evidence that an identified problem will introduce bias |
Modi 2013.
| Methods |
Study design: RCT Method of randomisation: via a permuted block randomisation method with block size of 2. Setting: a new‐onset seizure clinic at a pediatric children’s hospital in the USA. Date it was conducted: not reported. Follow up: 4 months. Source of funding: National Institute of Health Conflict of interest: the authors declare no conflict of interest. |
|
| Participants |
inclusion/exclusion criteria: diagnosis of a non epilepsy medical disorder requiring daily medication, a significant developmental disorder (e.g. autism), or the family living >90 miles away from the hospital. Families had to read/speak English. Sample size: 40 families were approached for study participation; 30 agreed to participate and 3 withdrew before randomisation or did not return. After a 1‐month run‐in period, participants with near perfect adherence (> 90%) were monitored (n = 19). Participants with adherence < 90% (n = 8) were randomly assigned to IG or to the CG group. Age: mean age was 8.0 years (SD 5.6) in the IG and 7.1 years (SD 2.3) in the CG. Gender: 50% male Other characteristics: mean duration since diagnosis was 3.2 months (SD 1.2) in the IG group and 1.5 months (SD 0.88) in the CG |
|
| Interventions |
Type of intervention: educational The IG received 4 sessions over > 2 months. The first component of the intervention (session 1) provided education on epilepsy treatment, AED adherence and the family’s specific epilepsy treatment regimen (i.e. dosing schedule). Sessions 2 through 4 aimed to teach families a problem‐solving approach for their identified AED‐adherence barriers. |
|
| Outcomes |
Primary outcome(s) measured: adherence It was measured by an electronic monitoring system that measures the time and date a pill bottle and cap were opened. Secondary outcome(s) measured: assessment of feasibility and acceptability of the adherence Intervention. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Permuted block randomisation with block size of 2 was described |
| Allocation concealment (selection bias) | Unclear risk | No information on concealment was reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information on blinding was reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information on blinding was reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced in numbers across groups with similar reasons for missing data |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient rationale or evidence that an identified problem will introduce bias |
Peterson 1984.
| Methods |
Study design: RCT Method of randomisation: coin‐toss randomisation. Setting: outpatient clinic at a hospital in Australia. Date it was conducted: not reported. Follow‐up: 4 weeks and 6 months. Source of funding: Astra Pharmaceuticals (pty) ltd supplied Dosetts Conflict of interest: not reported. |
|
| Participants |
Inclusion/exclusion criteria: people who were consecutive attenders at outpatient clinics during the study period, who were responsible for their own medication and who possessed a hospital pharmacy prescription book. Sample size: 53 participants were recruited. At follow‐up, 2 participants from the control group and 1 from the intervention group had not returned to the clinic and were excluded from the analysis. Gender: 15 (58%) men were included in the intervention group and 15 (56%) in the control group. Age: median age was 35 years (range 19 to 74) in the IG and 28 years (range 18 to 64) in the CG. Gender: female 44% in the IG and 42% in CG. |
|
| Interventions |
Type of intervention: mixed Intervention group participants were counselled on the goals of anticonvulsant therapy and the importance of good adherence in achieving these goals; a schedule of medication‐taking was devised that corresponded with participants' everyday habits; participants were given a copy of an educational leaflet; each participant was provided with a 'Dosett' medication container (pill organiser) and was counselled on its use; participants were instructed to use a medication/seizure diary; and participants were reminded by mail of upcoming appointments and of missed prescription refills. |
|
| Outcomes |
Primary outcome(s) measured: adherence It was assessed by (1) changes in plasma anticonvulsant levels (provided that the participant's medication regimen had not been altered in the preceding 2 weeks), (2) a check of the participant's prescription record book to determine prescription refill frequency (if refill frequency was 1 or more weeks later than expected at least once during the previous 6 months, the participant was considered non‐adherent) and (3) participant appointment‐keeping frequency (those who had attended all scheduled appointments in the previous 6 months were considered compliant). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Coin toss was reported |
| Allocation concealment (selection bias) | Unclear risk | No information on concealment was reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The physicians were blinded to the allocated interventions |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The information on blinding of other parties (e.g. outcome assessors) was not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient reporting of attrition/exclusions to permit judgement |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient rationale or evidence that an identified problem will introduce bias |
Pryse‐Phillips 1982.
| Methods |
Study design: RCT Method of randomisation: not reported. Setting: 2 outpatient clinics in the Canda. Date it was conducted: Follow‐up time: 4 weeks. Source of funding: Conflict of interest: |
|
| Participants |
Inclusion/exclusion criteria: people with psychotic and severe neurotic disorders were excluded. 50 participants were accepted into the study. No loss to follow‐up was reported. No further details on participants were provided |
|
| Interventions |
Type of intervention: educational Two structured interviews separated by 4 weeks were conducted in the clinic or by telephone. At each interview, the participant described his/her seizure, the medication given and general background information. An information pamphlet containing details on the name of the drugs; its colour, shape and strength; the therapeutic effect; and dosage, precautions and possible unwanted effects was read and explained or was read, explained and given to the participant to take home. |
|
| Outcomes |
Primary outcome(s) measured: adherence It was assessed by measurement of serum drug level and expressed as percentage of change from the initial level towards the mean of the accepted therapeutic range. Secondary outcome(s) measured: alteration in knowledge about epilepsy and alteration in insightful behaviour such as a request for advice from the physician due to loss of hair. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | No information on concealment was reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information on blinding was reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information on blinding was reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient reporting of attrition/exclusions to permit judgement |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient rationale or evidence that an identified problem will introduce bias |
Shope 1980.
| Methods |
Study design: RCT Method of randomisation: not reported. Setting: one paediatric seizure clinic in the USA. Date it was conducted: March 1977‐ April 1978 Follow up: 11 weeks following the intervention Source of funding: Epilepsy Foundation of America, Epilepsy Center of Michigan. Conflict of interest: not reported. |
|
| Participants |
inclusion/ exclusion criteria: children were eligible if they were prescribed a continuing dosage of phenobarbital and /or phenytoin; under 16 years of age; the only child of the family; accompanied by the person who took primary care of the child Sample size: 211 children were recruited. 70 children were judged non‐compliant because serum levels were below predicted levels for individual age and dosage. 3 children were dropped from the study because their physician discontinued their medication. Parents of the 67 children remaining in the study were allocated to intervention (28 parents) and control (37 parents).Of the 28 parents invited to the discussion meeting, only 14 attended the discussion Age: Mean age was 9 years and ranged from 1 to 15 years. Mean age of children in the CG was significantly higher than children assigned to the CG. Gender: Half of children were girls and 67% were black. Other characteristics: Half of the parents had less than 11th grade education and income under USD 8330. |
|
| Interventions |
Type of intervention: educational Two mothers' discussion group meetings, each lasting 1½ hours. The aim of these meetings was to provide mothers with information to enable them to know what to do for their children and why, increasing their sense of responsibility and obtaining their commitment. Follow‐up and interview and laboratory results were obtained at regularly‐scheduled visits a mean of 11 weeks following the discussion session. |
|
| Outcomes |
Primary outcome(s) measured: adherence It was assessed by measurement of serum drug levels and expressed as a score ranging from 1 to 4. One indicated zero level of medication in the serum, 2 indicated more than 30% less than predicted, 3 indicated within predicted mean and 4 indicated 30% more than predicted mean. Secondary outcome(s) measured: knowledge of seizure disorder, locus of control and dependency. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | No information on concealment was reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information on blinding was reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information on blinding was reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient reporting of attrition/exclusions to permit judgement |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient rationale or evidence that an identified problem will introduce bias |
Tang 2014.
| Methods |
Study design: RCT Method of randomisation: random‐number table. Setting: an outpatient clinic of a hospital in China Date it was conducted: September 2011‐ March 2013. Follow up: 1 year Souce of funding: not reported. Conflict of interest: the authors declare no conflict of interest |
|
| Participants |
Inclusion/exclusion criteria: people diagnosed with epilepsy, older than 16 years of age, took antiepileptic drugs for more than six months, and did not take their AEDs at least once over the past six months. Sample size: 124 were assigned to education intervention (n = 59) and education and behavioural intervention (n = 65). 56 and 53 patients completed the last assessment of all measures in education and behavioural intervention group and education only respectively. Age: mean age was 31 years (SD13.0) in education and behavioural intervention group and 30 years (SD 11.6) in education only group. Gender: men 49% in education and behavioural intervention group and 59% in education only group. |
|
| Interventions |
Type of intervention: mixed Study defines 2 intervention groups: the medication education group (group I) and the medication education with behavioural intervention group (group II). Group I was initially provided with medication education in the form of oral education and written materials, and this education was reinforced by monthly calls from the pharmacist over the next 6 months. The behavioural intervention provided to group II consisted of a modified medication schedule which was based on cue–dose training therapy |
|
| Outcomes |
Primary outcome(s) measured: adherence It was assessed by using the four‐item Morisky Medication Adherence Scale (MMAS‐4). Secondary outcome(s) measured: seizure control, knowledge of AEDs, quality of life and the number of participants who missed a dose of their AEDs. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed using a random‐number table |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information on blinding was reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information on blinding was reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data are reported |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but it is clear that the published reports include all expected outcomes |
| Other bias | Unclear risk | Insufficient rationale or evidence whether an important risk of bias exists |
AED: antiepileptic drug; AGAS: Antiretroviral General Adherence Scale; CG: control group; CI: confidence interval; IG: intervention group; MEMS: Medication Event Monitoring System; OR: odds ratio; SD: standard deviation; TAU: treatment as usual.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adamolekun 1999 | Not a RCT; the 24 health facilities were consecutively allocated to intervention or control. |
| Boggs 2007 | There is insufficient information to make a sound decision. |
| Cramer 1995 | A placebo‐controlled trial of vigabatrin; during the trial, the utility of an electronic monitoring device in evaluating what dose was actually received by participants was examined. However, randomisation was to receiving vigabatrin or placebo, not to any adherence‐enhancing intervention |
| Lewis 1990 | Effect of educational programme on parents' knowledge, dealing with anger and anxiety and decision‐making skills; adherence as an outcome is not reported. |
| May 2002 | Effect of educational programme on quality of life, knowledge and seizure frequency, but adherence as outcome is not reported. |
| McLaughlin 2011 | Examined the effectiveness of cognitive behavior therapy to manage seizures and improve psychosocial functioning in older adults with epilepsy. Adherence as an outcome is not reported. |
| Ridsdale 1997 | Effect of nurse intervention on knowledge, emotional state and seizure frequency, but no adherence outcome reported. |
| Ridsdale 1999 | Effect of nurse intervention on knowledge, emotional state and seizure frequency, but no adherence outcome reported. |
RCT: randomised controlled trial.
Characteristics of ongoing studies [ordered by study ID]
Kralj‐Hans 2014.
| Trial name or title | Self‐Management education for adults with poorly controlled epILEpsy (SMILE (UK)): a randomised controlled trial protocol |
| Methods | A multicentre, pragmatic, parallel‐group randomised controlled trial. The participants are followed up for 12 months |
| Participants | 428 adults who attended specialist epilepsy outpatient clinics at 15 NHS participating sites in the previous 12 months, and who fulfil other eligibility criteria will be randomised to receive the intervention (SMILE (UK) course with treatment as usual‐ TAU) or to have TAU only (control) |
| Interventions | The intervention is a group‐based, interactive course. It is delivered by 2 health professionals (educational facilitators, EFs) to groups of 8 – 12 participants |
| Outcomes | The primary outcome is the effect on participant‐reported quality of life (QoL). Secondary outcomes are seizure frequency and psychological distress (anxiety and depression), perceived impact of epilepsy, adherence to medication, management of adverse effects from medication, and improved self‐efficacy in management (mastery/control) of epilepsy |
| Starting date | |
| Contact information | Ines Kralj‐Hans. email: leone.ridsdale@kcl.ac.uk |
| Notes |
Leenen 2014.
| Trial name or title | (Cost)‐effectiveness of a multi‐component intervention for adults with epilepsy: study protocol of a Dutch randomised controlled trial (ZMILE study) |
| Methods | A parallel 2‐groups randomised trial |
| Participants | 100 eligible people with epilepsy will be included in the study. Eligible participants are adults aged 18 or over, living at home, diagnosed with epilepsy and using AEDs, who understand the Dutch language, and are able to use e‐Health devices |
| Interventions | A multi‐component intervention (MCI) which combines a self‐management/education programme of 6 meetings with e‐Health interventions. |
| Outcomes | The primary outcome will be self‐efficacy. Secondary outcomes include adherence, side effects, change in seizure severity and frequency, improved quality of life, proactive coping, and societal costs. |
| Starting date | |
| Contact information | Ben FM Wijnen. email: b.wijnen@maastrichtuniversity.nl |
| Notes |
NCT01851057.
| Trial name or title | Supporting Treatment Adherence Regimens in pediatric epilepsy: the STAR Trial |
| Methods | A parallel‐groups, single‐blind (Investigator), randomised trial |
| Participants | 200 children age 2 years to 12 years of both gender and their families |
| Interventions | A family‐tailored adherence intervention (STAR: Supporting Treatment Adherence Regimens) focuses on increasing epilepsy knowledge and problem‐solving skills around barriers to adherence for children with epilepsy and their families will be compared with education only |
| Outcomes | Primary outcome measures: adherence rate (short‐term). Secondary outcome measures: adherence rate (long‐term), health‐related quality of life, seizure absence/presence |
| Starting date | April 2013 |
| Contact information | Children's Hospital Medical Center, Cincinnati, USA |
| Notes |
NCT02015806.
| Trial name or title | Robust Evaluation to Measure Improvements in Nonadherence from Low‐cost Devices (REMIND) |
| Methods | A parallel‐groups, open‐label, randomised trial |
| Participants | Individuals age 18 ‐ 64 years of both gender have 1 ‐ 3 oral maintenance medication for chronic disease (including epilepsy) in the 12‐month period prior to study eligibility evaluation. |
| Interventions | The RxTimerCap (a pill bottle cap with a digital timer that shows the time elapsed since the medication was last taken ), the Take‐N‐Slide (a patented strip with toggles for each day of the week which are meant to be slid after taking a medication),and the standard pillbox (a plastic organisation box with 1 compartment for every day of the week). |
| Outcomes | Primary outcome measures: optimal medication adherence to all cardiovascular or non‐depression chronic disease medication |
| Starting date | March 2014 |
| Contact information | Niteesh K. Choudhry, MD, PhD, Brigham and Women's Hospital, USA |
| Notes |
NCT02165306.
| Trial name or title | Enhancing antiepileptic drug adherence |
| Methods | A parallel‐groups, double‐blind (investigator, outcomes assessor), randomised trial |
| Participants | Individuals 18 years and older of both genders, who are suffering from epilepsy |
| Interventions | Participants enrolled in the intervention arm will receive 2 educational sessions; each session involves face‐to‐face introductory motivational interviews (MI) to resolve participant ambivalence about change |
| Outcomes | Primary outcome measures: changes in participant‐reported medication adherence to antiepileptic drugs. Secondary outcome measures: changes in serum levels of antiepileptic drugs, changes in psychological predictors of medication adherence, changes in action planning, changes in coping planning, changes in quality of life, changes in habit strength, changes in seizure severity |
| Starting date | June 2014 |
| Contact information | Amir H Pakpour, Qazvin University Of Medical Sciences, Islamic Republic of Iran |
| Notes |
NCT02646631.
| Trial name or title | Behavioural and educational tools to improve epilepsy care |
| Methods | A parallel‐groups, single‐blind (participant), randomised trial. Participants will be followed for 3 months |
| Participants | Individuals 18 years and older of both genders, who are suffering from epilepsy |
| Interventions | Smartphone‐based self‐management intervention called Management of Risks in Epilepsy (MORE), or MORE + telephone‐based motivational interviewing (MI) |
| Outcomes | Primary outcome measures: percent adherence to anti‐epileptic drug schedule (pill counts) Secondary outcome measures: number of participants who complete the study, percentage of MI sessions completed, percentage of diary entries completed, adherence to antiepileptic drug schedule (self‐reported) as measured by the Morisky Medication Adherence Scale, seizure frequency, and quality of life |
| Starting date | January 2016 |
| Contact information | New York University School of Medicine, USA |
| Notes |
Differences between protocol and review
We created a summary of findings table for this update. See Methods for details.
We also added the effect of interventions on self efficacy and quality of life to our secondary outcomes.
Contributions of authors
2015 Update
Sinaa Al‐aqeel: involved in reviewing articles for eligibility, abstracting articles, preparation of tables, assessing risk of bias of all included studies and text preparation for 2015 update.
Olga Gershuni: involved in reviewing articles for eligibility, abstracting articles, preparation of tables assessing risk of bias of all included studies and text preparation for 2015 update.
Jawza Al‐sabhan: involved in reviewing articles for eligibility.
Mickael Hiligsmann: involved in reviewing articles for eligibility, abstracting articles, preparation of tables assessing risk of bias of all included studies, and text preparation for 2015 update.
2012 Update
Sinaa Al‐aqeel: involved in reviewing articles for eligibility, abstracting articles, and text preparation for 2012 update.
Jawza Al‐sabhan: involved in reviewing articles for eligibility.
2010 review
Sinaa Al‐aqeel developed the search strategy, performed the bibliographic searches and produced the first draft of the review.
Sinaa Al‐aqeel and Jawza Al‐sabhan reviewed articles for eligibility, assessed their methodological quality and extracted the data.
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Institute for Health Research, UK.
This review was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Epilepsy Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Declarations of interest
Alaqeel S: none known.
Olga Gershuni: none known.
Jawza Al‐sabhan; none known.
Mickael Hiligsmann; none known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Brown 2009 {published data only}
- Brown I, Sheeran P, Reuber M. Enhancing antiepileptic drug adherence: a randomized controlled trial. Epilepsy & Behavior 2009;16(4):634‐9. [DOI] [PubMed] [Google Scholar]
Dash 2015 {published data only}
- Dash D, Sebastian TM, Aggarwal M, Tripathi M. Impact of health education on drug adherence and self‐care in people with epilepsy with low education. Epilepsy & Behavior 2015;44:213–217. [DOI] [PubMed] [Google Scholar]
DiIorio 2009 {published data only}
- DiIorio C, Reisinger EL, Yeager KA, McCarty F. A telephone‐based self‐management program for people with epilepsy. Epilepsy & Behavior 2009;14(1):232‐6. [DOI] [PubMed] [Google Scholar]
Dilorio 2011 {published data only}
- DiIorio C, Bamps Y, Walker ER, Escoffery C. Results of a research study evaluating WebEase, an online epilepsy self‐management program. Epilepsy & Behavior 2011;22(3):469‐74. [DOI] [PubMed] [Google Scholar]
Helgeson 1990 {published data only}
- Helgeson DC, Mittan R, Tan SY, Chayasirisobhon S. Sepulveda Epilepsy Education: the efficacy of a psychoeducational treatment program in treating medical and psychosocial aspects of epilepsy. Epilepsia 1990;31(1):75‐82. [DOI] [PubMed] [Google Scholar]
Ibinda 2014 {published data only}
- Ibinda F, Mbuba CK, Kariuki SM, Chengo E, Ngugi AK, Odhiambo R, et al. Evaluation of Kilifi epilepsy education programme: a randomized controlled trial. Epilepsia 2014;55(2):344–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Li 2013 {published data only}
- Li J, Si Y, Hu J, Liu L, Deng Y, He J, et al. Enhancing medical compliance of patients with convulsive epilepsy in rural community: a randomized intervention trial. Epilepsia 2013;54(11):1988–96. [DOI] [PubMed] [Google Scholar]
Modi 2013 {published data only}
- Modi AC, Guilfoyle SM, Rausch J. Preliminary feasibility, acceptability, and efficacy of an innovative adherence intervention for children with newly diagnosed epilepsy. Journal of Pediatric Psychology 2013;38(6):605–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Peterson 1984 {published data only}
- Peterson GM, McLean S, Millingen KS. A randomised trial of strategies to improve patient compliance with anticonvulsant therapy. Epilepsia 1984;25(4):412‐7. [DOI] [PubMed] [Google Scholar]
Pryse‐Phillips 1982 {published data only}
- Pryse‐Phillips W, Jardine F, Bursey F. Compliance with drug therapy by epileptic patients. Epilepsia 1982;23(3):269‐74. [DOI] [PubMed] [Google Scholar]
Shope 1980 {published data only}
- Shope JT. Intervention to improve compliance with pediatric anticonvulsant therapy. Patient Counselling and Health Education 1980;2(3):135‐41. [DOI] [PubMed] [Google Scholar]
Tang 2014 {published data only}
- Tang F, Zhu G, Jiao Z, Ma C, Chen N, Wang B. The effects of medication education and behavioral intervention on Chinese patients with epilepsy. Epilepsy & Behavior 2014;37:157–64. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Adamolekun 1999 {published data only}
- Adamolekun B, Mielke JK, Ball DE. An evaluation of the impact of health worker and patient education on the care and compliance of patients with epilepsy in Zimbabwe. Epilepsia 1999;40(4):507‐11. [DOI] [PubMed] [Google Scholar]
Boggs 2007 {unpublished data only}
- Boggs J, DeToledo J. Compliance with once‐daily divalproex extended‐release tablets (Depakote‐ER) versus multiple‐daily dose valproic acid capsules (Depakene) in epilepsy. Epilepsia 2007;48 Suppl 6:340‐1. [Google Scholar]
Cramer 1995 {published data only}
- Cramer J, Vachon L, Desforges C, Sussman NM. Dose frequency and dose interval compliance with multiple antiepileptic medications during a controlled clinical trial. Epilepsia 1995;36(11):1111‐7. [DOI] [PubMed] [Google Scholar]
Lewis 1990 {published data only}
- Lewis MA, Salas I, Sota A, Chiofalo N, Leake B. Randomized trial of a program to enhance the competencies of children with epilepsy. Epilepsia 1990;31(1):101‐9. [DOI] [PubMed] [Google Scholar]
May 2002 {published data only}
- May TW, Pfafflin M. The efficacy of an educational treatment program for patients with epilepsy (MOSES): results of a controlled, randomized study. Modular Service Package Epilepsy. Epilepsia 2002;43(5):539‐49. [DOI] [PubMed] [Google Scholar]
McLaughlin 2011 {published data only}
- McLaughlin DP, McFarland K. A randomized trial of a group based cognitive behavior therapy program for older adults with epilepsy: the impact on seizure frequency, depression and psychosocial well‐being. Journal of Behavioral Medicine 2011;34(3):201‐7. [DOI] [PubMed] [Google Scholar]
Ridsdale 1997 {published data only}
- Ridsdale L, Robins D, Cryer C, Williams H. Feasibility and effects of nurse run clinics for patients with epilepsy in general practice: randomised controlled trial. BMJ 1997;314(7074):120‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ridsdale 1999 {published data only}
- Ridsdale L, Kwan I, Cryer C. The effect of a special nurse on patients' knowledge of epilepsy and their emotional state. Epilepsy Evaluation Care Group. British Journal of General Practice 1999;49(441):285‐9. [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
Kralj‐Hans 2014 {published data only}
- Self‐Management education for adults with poorly controlled epILEpsy (SMILE (UK)): a randomised controlled trial protocol. Ongoing study Starting date of trial not provided. Contact author for more information.
Leenen 2014 {published data only}
- (Cost)‐effectiveness of a multi‐component intervention for adults with epilepsy: study protocol of a Dutch randomised controlled trial (ZMILE study). Ongoing study Starting date of trial not provided. Contact author for more information.
NCT01851057 {published data only}
- Supporting Treatment Adherence Regimens in pediatric epilepsy: the STAR Trial. Ongoing study April 2013.
NCT02015806 {published data only}
- Robust Evaluation to Measure Improvements in Nonadherence from Low‐cost Devices (REMIND). Ongoing study March 2014.
NCT02165306 {published data only}
- Enhancing antiepileptic drug adherence. Ongoing study June 2014.
NCT02646631 {published data only}
- Behavioural and educational tools to improve epilepsy care. Ongoing study January 2016.
Additional references
Baker 1997
- Baker GA, Jacoby A, Buck D, Stalgis C, Monnet D. Quality of life of people with epilepsy: a European study. Epilepsia 1997;38(3):353‐62. [PUBMED: 9070599] [DOI] [PubMed] [Google Scholar]
Briesacher 2008
- Briesacher BA, Andrade SE, Fouayzi H, Chan KA. Comparison of drug adherence rates among patients with seven different medical conditions. Pharmacotherapy 2008;28(4):437‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cramer 2008
- Cramer JA, Roy A, Burrell A, Fairchild CJ, Fuldeore MJ, Ollendorf DA, et al. Medication compliance and persistence: terminology and definitions. Value in Health 2008;11(1):44‐7. [DOI] [PubMed] [Google Scholar]
Davis 2008
- Davis KL, Candrilli SD, Edin HM. Prevalence and cost of non adherence with antiepileptic drugs in an adult managed care population. Epilepsia 2008;49(3):446‐54. [PUBMED: 18031549] [DOI] [PubMed] [Google Scholar]
DiMatteo 2004
- DiMatteo MR. Variations in patients' adherence to medical recommendations: a quantitative review of 50 years of research. Medical Care 2004;42(3):200‐9. [DOI] [PubMed] [Google Scholar]
Eatock 2007
- Eatock J, Baker GA. Managing patient adherence and quality of life in epilepsy. Neuropsychiatric Disease and Treatment 2007;3(1):117‐31. [PUBMED: 19300542] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ettinger 2009a
- Ettinger AB, Baker GA. Best clinical and research practice in epilepsy of older people: focus on antiepileptic drug adherence. Epilepsy & Behavior 2009;15 Suppl 1:S60‐3. [PUBMED: 19303055] [DOI] [PubMed] [Google Scholar]
Ettinger 2009b
- Ettinger AB, Manjunath R, Candrilli SD, Davis KL. Prevalence and cost of non adherence to antiepileptic drugs in elderly patients with epilepsy. Epilepsy & Behavior 2009;14(2):324‐9. [PUBMED: 19028602] [DOI] [PubMed] [Google Scholar]
Faught 2008
- Faught E, Duh MS, Weiner JR, Guerin A, Cunnington MC. Nonadherence to antiepileptic drugs and increased mortality: findings from the RANSOM Study. Neurology 2008;71(20):1572‐8. [PUBMED: 18565827] [DOI] [PubMed] [Google Scholar]
Fisher 2005
- Fisher RS, Emde Boas W, Blume W, Elger C, Genton P, Lee P, et al. Epileptic seizures and epilepsy: definitions proposed by the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE). Epilepsia 2005;46(4):470‐2. [PUBMED: 15816939] [DOI] [PubMed] [Google Scholar]
GRADEpro 2014 [Computer program]
- McMaster University. The Grading of Recommendations Assessment, Development and Evaluation (GRADEpro) [Computer program on www.gradepro.org].. Version 3.6. McMaster University, 2014.
Higgins 2004
- Higgins N, Regan C. A systematic review of the effectiveness of interventions to help older people adhere to medication regimes. Age and Ageing 2004;33(3):224‐9. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hovinga 2008
- Hovinga CA, Asato MR, Manjunath R, Wheless JW, Phelps SJ, Sheth RD, et al. Association of non‐adherence to antiepileptic drugs and seizures, quality of life, and productivity: survey of patients with epilepsy and physicians. Epilepsy & Behavior 2008;13(2):316‐22. [DOI] [PubMed] [Google Scholar]
Kirkham 2010
- Kirkham JJ, Dwan KM, Altman DG, Gamble C, Dodd S, Smyth R, et al. The impact of outcome reporting bias in randomised controlled trials on a cohort of systematic reviews. BMJ 2010;340:c365. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Lehane 2006
- Lehane E, McCarthy G. Intentional and unintentional medication non‐adherence: A comprehensive framework for clinical research and practice? A discussion paper. International Journal of Nursing Studies accepted 13 July 2006;44 (2007):1468–1477. [DOI] [PubMed] [Google Scholar]
Manjunath 2009
- Manjunath R, Davis KL, Candrilli SD, Ettinger AB. Association of antiepileptic drug non adherence with risk of seizures in adults with epilepsy. Epilepsy & Behavior 2009;14(2):372‐8. [PUBMED: 19126436] [DOI] [PubMed] [Google Scholar]
Moher 1999
- Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF. Improving the quality of reports of meta‐analyses of randomised controlled trials: the QUOROM statement. Quality of Reporting of Meta‐analyses. Lancet 1999;354(9193):1896‐900. [PUBMED: 10584742] [DOI] [PubMed] [Google Scholar]
Ngugi 2011
- Ngugi AK, Kariuki SM, Bottomley C, Kleinschmidt I, Sander JW, Newton CR. Incidence of epilepsy. A systematic review and meta‐analysis. Neurology 2011;77(10):1005–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nichol 1999
- Nichol MB, Venturini F, Sung JC. A critical evaluation of the methodology of the literature on medication compliance. Annals of Pharmacotherapy 1999;33(5):531‐40. [DOI] [PubMed] [Google Scholar]
Nieuwlaat 2014
- Nieuwlaat R, Wilczynski N, Navarro T, Hobson N, Jeffery R, Keepanasseril A, et al. Interventions for enhancing medication adherence. Cochrane Database of Systematic Reviews 2014, Issue 11. [DOI: 10.1002/14651858.CD000011.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Paschal 2008
- Paschal AM, Hawley SR, Romain T, Ablah E. Measures of adherence to epilepsy treatment: review of present practices and recommendations for future directions. Epilepsia 2008;49(7):1115‐22. [DOI] [PubMed] [Google Scholar]
Peterson 2003
- Peterson AM, Takiya L, Finley R. Meta‐analysis of trials of interventions to improve medication adherence. American Journal of Health‐System Pharmacy 2003;60(7):657‐65. [DOI] [PubMed] [Google Scholar]
Rand 2000
- Rand CS, Sevick MA. Ethics in adherence promotion and monitoring. Controlled Clinical Trials 2000;21(5 Suppl 1):241S‐7S. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Roter 1998
- Roter DL, Hall JA, Merisca R, Nordstrom B, Cretin D, Svarstad B. Effectiveness of interventions to improve patient compliance: a meta‐analysis. Medical Care 1998;36(8):1138‐61. [DOI] [PubMed] [Google Scholar]
Sander 2003
- Sander JW. The epidemiology of epilepsy revisited. Current Opinion in Neurology 2003;16(2):165‐70. [PUBMED: 12644744] [DOI] [PubMed] [Google Scholar]
Schedlbauer 2010
- Schedlbauer A, Davies P, Fahey T. Interventions to improve adherence to lipid lowering medication. Cochrane Database of Systematic Reviews 2010, Issue 3. [DOI: 10.1002/14651858.CD004371.pub3] [DOI] [PubMed] [Google Scholar]
Schroeder 2004
- Schroeder K, Fahey T, Ebrahim S. Interventions for improving adherence to treatment in patients with high blood pressure in ambulatory settings. Cochrane Database of Systematic Reviews 2004, Issue 3. [DOI: 10.1002/14651858.CD004804] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sieber 2000
- Sieber WJ, Kaplan RM. Informed adherence: the need for shared medical decision making. Controlled Clinical Trials 2000;21(5 Suppl 1):S233‐40. [DOI] [PubMed] [Google Scholar]
Vermeire 2001
- Vermeire E, Hearnshaw H, Royen P, Denekens J. Patient adherence to treatment: three decades of research. A comprehensive review. Journal of Clinical Pharmacy and Therapeutics 2001;26(5):331‐42. [DOI] [PubMed] [Google Scholar]
Vermeire 2005
- Vermeire EIJJ, Wens J, Royen P, Biot Y, Hearnshaw H, Lindenmeyer A. Interventions for improving adherence to treatment recommendations in people with type 2 diabetes mellitus. Cochrane Database of Systematic Reviews 2005, Issue 2. [DOI: 10.1002/14651858.CD003638.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vrijens 2012
- Vrijens B, Geest S, Hughes DA, Przemyslaw K, Demonceau J, Ruppar T, et al. A new taxonomy for describing and defining adherence to medications. British Journal of Clinical Pharmacology 2012;73(5):691‐705. [DOI: 10.1111/j.1365-2125.2012.04167] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Al‐aqeel 2010
- Sinaa Al‐aqeel, Jawza Al‐sabhan. Strategies for improving adherence to antiepileptic drug treatment in patients with epilepsy. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD008312] [DOI] [PubMed] [Google Scholar]
Al‐aqeel 2011
- Al‐aqeel S, Al‐sabhan J. Strategies for improving adherence to antiepileptic drug treatment in patients with epilepsy. Cochrane Database of Systematic Reviews 2011, Issue 1. [DOI: 10.1002/14651858.CD008312.pub2] [DOI] [PubMed] [Google Scholar]


