Abstract
Background
Venous thromboembolism (VTE) is a prevalent and serious condition. Its medical treatment requires anticoagulation, usually with either unfractionated or low molecular weight heparin (LMWH). Administration of unfractionated heparin (UFH) is usually intravenous (IV) but can be subcutaneous as well. This is an update of a review first published in 2009.
Objectives
To assess the effects of subcutaneous UFH versus intravenous UFH, subcutaneous LMWH or any other anticoagulant drug for the initial treatment of venous thromboembolism.
Search methods
For this update, the Cochrane Vascular Information Specialist searched the Specialised Register (last searched 30 November 2016) and CENTRAL (2016, Issue 10). The Cochrane Vascular Information Specialist also searched trials registries for details of ongoing or unpublished studies.
Selection criteria
Randomised controlled trials comparing subcutaneous UFH to control, such as subcutaneous LMWH, continuous intravenous UFH or other anticoagulant drugs in participants with acute venous thromboembolism.
Data collection and analysis
Two review authors (JS and LR) independently extracted data and assessed the risk of bias in the trials. We used meta‐analyses when we considered heterogeneity low. The primary outcomes were symptomatic recurrent venous thromboembolism (deep vein thrombosis and/or pulmonary embolism), VTE‐related mortality, adverse effects of treatment including major bleeding, and all‐cause mortality. We calculated all outcomes using an odds ratio (OR) with a 95% confidence interval (CI).
Main results
We included one additional study in this update, bringing the total number of studies in the review to 16 randomised controlled trials, with a total of 3593 participants (1745 participants in the intervention group and 1848 participants in the control group). Eight trials used intravenous UFH as the control treatment, seven trials used LMWH, and one trial had three arms with both drugs as the controls. We did not identify trials comparing subcutaneous UFH with other anticoagulant drugs. We downgraded the quality of the evidence to low due to lack of blinding in studies, which led to a risk of performance bias, and also for imprecision, as reflected by the wide confidence intervals.
When comparing subcutaneous versus IV UFH, there was no difference in the incidence of symptomatic recurrent VTE at three months (odds ratio (OR) 1.66, 95% confidence interval (CI) 0.89 to 3.10; 8 studies; N = 965; low‐quality evidence), symptomatic recurrent deep vein thrombosis (DVT) at three months (OR 3.29, 95% CI 0.64 to 17.06; 1 study; N = 115; low‐quality evidence), pulmonary embolism (PE) at three months (OR 1.44, 95% CI 0.73 to 2.84; 9 studies; N = 1161; low‐quality evidence), VTE‐related mortality at three months (OR 0.98, 95% CI 0.20 to 4.88; 9 studies; N = 1168; low‐quality evidence), major bleeding (OR 0.91, 95% CI 0.42 to 1.97; 4 studies; N = 583; low‐quality evidence) or all‐cause mortality (OR 1.74, 95% CI 0.67 to 4.51; 8 studies; N = 972; low‐quality evidence). There were no episodes of asymptomatic VTE occurring within three months of the commencement of treatment.
When comparing subcutaneous UFH versus LMWH, there was no difference in the incidence of recurrent VTE at three months (OR 1.01, 95% CI 0.63 to 1.63; 5 studies; N = 2156; low‐quality evidence), recurrent DVT at three months (OR 1.38, 95% CI 0.73 to 2.63; 3 studies; N = 1566; low‐quality evidence), PE (OR 0.84, 95% CI 0.36 to 1.96; 5 studies, N = 1819; low‐quality evidence), VTE‐related mortality (OR 0.53, 95% CI 0.17 to 1.67; 8 studies; N = 2469; low‐quality evidence), major bleeding (OR 0.72, 95% CI 0.43 to 1.20; 5 studies; N = 2300; low‐quality evidence) or all‐cause mortality (OR 0.73, 95% CI 0.50 to 1.07; 7 studies; N = 2272; low‐quality evidence). There were no episodes of asymptomatic VTE occurring within three months of the commencement of treatment.
Authors' conclusions
There is no evidence of a difference between subcutaneous versus intravenous UFH for preventing VTE recurrence, VTE‐related or all‐cause mortality, and major bleeding. According to GRADE criteria, the quality of the evidence was low. There is also no evidence of a difference between subcutaneous UFH and LMWH for preventing VTE recurrence, VTE‐related or all‐cause mortality or major bleeding.
Plain language summary
Subcutaneous unfractionated heparin for the initial treatment of venous thromboembolism
Background Venous thromboembolism (VTE) is a condition where a blood clot forms in the deep veins (most commonly of the leg) and can travel up to block the arteries in the lungs (a life‐threatening condition known as pulmonary embolism). Treating VTE requires injections of a drug called heparin, which stops further clots forming. Heparin comes in two forms: unfractionated heparin (UFH) and low molecular weight heparin (LMWH). UFH can be administered as a continuous intravenous (IV) infusion or intermittently as an injection under the skin (subcutaneous), while LMWH is injected subcutaneously. This review measures the effects of subcutaneous UFH versus IV UFH and LMWH for preventing recurrent clots, mortality and major bleeding. This is an update of a review published in 2009.
Key results After searching for relevant studies up to November 2016, we found one study to add to this update. In total, we included 16 randomised controlled trials in 3593 participants in this review. This update showed that there was no evidence of a difference between subcutaneous UFH versus intravenous UFH or subcutaneous LMWH for preventing recurrent clots, death or major bleeding.
Quality of the evidence The quality of the evidence was low due to lack of blinding in the included studies and imprecision of the results due to the small number of reported events.
Summary of findings
Background
Description of the condition
Venous thromboembolism (VTE) describes the formation of thrombus in the deep veins, most commonly in the legs (deep vein thrombosis, or DVT). VTE may also refer to the subsequent embolisation of all or part of the thrombus to the pulmonary circulation (pulmonary embolism, or PE). DVT of the lower limbs may be associated with localised pain, swelling and erythema as well as the development of pulmonary emboli and the later occurrence of post‐thrombotic syndrome (persistent swelling, erythema and ulceration). PE presents acutely with shortness of breath, pain on inspiration, tachycardia and right heart overload, and if untreated, it can lead to chronic thromboembolic pulmonary hypertension, acute circulatory collapse and death. Increasingly, in the era of more liberal central venous catheterisation, DVT may involve the upper extremities. Rarely, it may also affect other venous circulation (cerebral veins, portal and mesenteric veins, etc.).
In addition to DVT and PE, thrombus can also form in the superficial veins, where it is associated with local pain and inflammation (superficial venous thrombosis). This tends to be associated with lower mortality and morbidity rates than DVT, although some patients may be at a higher risk of DVT formation depending on the location of the clot (Chengelis 1996; Nasr 2015).
Venous thromboembolism (VTE) is comprised of DVT and PE and can occur spontaneously. However, there are many risk factors for VTE, including periods of inactivity, dehydration, hospitalisation, trauma, clotting disorders and previous thrombosis, varicose veins with phlebitis, pregnancy, oral combined hormonal contraceptives, malignancy, obesity, smoking, and age (Anderson 2003; NICE 2010).
The incidence of VTE in mostly white populations is between 100 and 200 per 100,000 person‐years (Heit 2015; White 2003). Of these, it is estimated that 45 to 117 cases per 100,000 person‐years are due to DVT (without PE), and 29 to 78 are due to PE (with or without DVT) (Heit 2015). Recurrent VTE occurs in approximately 7.4% of patients at 1 year and up to 30.4% of patients by 10 years (Cushman 2007; Heit 2015; White 2003).
Description of the intervention
Heparin is a heterogeneous mixture of branched glycosaminoglycans (GAG), discovered in 1916 (McLean 1916).
The anticoagulant action of heparin requires the binding of antithrombin (AT). Heparin binds to AT through a unique glucosamine unit that is contained within a pentasaccharide sequence present in a fraction of the GAG molecules. Currently, three therapeutic heparin preparations are available for clinical use: unfractionated heparin (UFH) with a molecular weight of approximately 15,000 daltons; its derivative low molecular weight heparin (LMWH), with an average molecular weight of 4000 to 5000 daltons; and the significantly more expensive pentasaccharide. Although LMWH has largely replaced UFH in the setting of acute VTE treatment, many people do not benefit from its use due to increased risk of complications, specifically bleeding in patients with severe renal failure.
How the intervention might work
Complications of heparin use may include bleeding; heparin‐induced thrombocytopenia (HIT); and in the long term, heparin‐induced osteoporosis. Consequently, it is important to monitor coagulation factors, specifically the activated partial thromboplastin time (aPTT), when using UFH. There are two preferred modes of administering this treatment: a continuous intravenous (IV) mode and an intermittent subcutaneous mode. Depending on the method chosen, pharmacokinetic analyses demonstrate differences in heparin bioavailability and early achievement of a therapeutic aPTT goal, favouring the intravenous route (Hull 1986).
Nevertheless, investigators have evaluated the subcutaneous route of administration for VTE due to its ease of application, early mobilisation and hospital discharge, and presumably less line‐related complications. People have received the treatment either in weight‐adjusted or aPTT‐adjusted doses, and investigators have compared results with other available treatment modalities.
Why it is important to do this review
Two meta‐analyses comparing LMWH versus intravenous UFH have shown LMWH to be non‐inferior to UFH with regards to recurrent DVT, PE, bleeding and thrombocytopenia (reduction in the number of platelets) (Dolovich 2000; Quinlan 2004). However, there were no trials utilising subcutaneous UFH for this indication in these analyses. The present review was originally completed in 2009 (Vardi 2009), and an update is necessary to incorporate evidence from any new studies completed since then. Additionally, Cochrane has developed new methodology during that time that should be incorporated in the updated review.
Objectives
To assess the effects of subcutaneous UFH versus intravenous UFH, subcutaneous LMWH or any other anticoagulant drug for the initial treatment of venous thromboembolism.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials comparing the effects of subcutaneous UFH versus intravenous UFH, LMWH or any other anticoagulant drug for the initial treatment of venous thromboembolism. We included trials with more than two treatment groups and analysed them accordingly. We did not expect to find any cross‐over trials in the setting of VTE. We included trials with interventions and follow‐up periods of any duration.
We excluded randomised controlled trials without truly random allocation to the treatment or control group or without allocation concealment, in view of the fact that prior knowledge of treatment allocation may have led to biased participant allocation, treatment or reporting. After allocation, further concealment of treatment may be impossible due to the differences between preparations and routes of administration. Thus, despite recognising that this may lead to biased treatment or reporting, post‐allocation blinding was not a prerequisite, and we addressed it in a sensitivity analysis.
We acknowledge that non‐randomised studies or studies using other randomisation methods (for example cluster randomisation) may provide useful information about this problem. However, for this review, we did not consider such studies.
Types of participants
Adults (aged 18 years or older) with a diagnosis of new or recurrent VTE. Ideally, the diagnosis of DVT of the leg was made with the use of compression ultrasonography, colour‐coded duplex ultrasonography or contrast venography, and the diagnosis of PE with high probability ventilation‐perfusion scan or pulmonary arterial filling defects on computed tomography or invasive angiography.
Types of interventions
Initial treatment with subcutaneous UFH for individuals with VTE, administered at any regimen, in trials of any duration.
-
Subcutaneous UFH:
fixed weight‐adjusted dose;
aPTT‐adjusted dose.
-
Other treatment modalities:
intravenous UFH;
subcutaneous LMWH;
other.
We expected studies to administer supplementary treatment of VTE with an oral anticoagulant titration. We considered its use in a subgroup analysis.
Types of outcome measures
Primary outcomes
Incidence of symptomatic recurrent VTE at three months
Incidence of symptomatic recurrent DVT at three months
PE at three months
VTE‐related mortality at three months
Major bleeding (as defined by the International Society on Thrombosis and Haemostasis (ISTH) (Schulman 2005): fatal bleeding; symptomatic bleeding in a critical area or organ, such as intracranial, intraspinal, intraocular, retroperitoneal, intra‐articular or pericardial, or intramuscular with compartment syndrome; bleeding causing a fall in haemoglobin level of 20 g/L (1.24 mmol/L) or more, or leading to transfusion of two or more units of whole blood or red cells; any combination of the above)
All‐cause mortality
Secondary outcomes
Incidence of asymptomatic VTE at three months
Treatment‐related morbidity: minor bleeding (bleeding that is clinically overt but not meeting the definition of serious bleeding provided by the ISTH) and heparin‐induced thrombocytopenia
Length of hospital stay
Quality of life
Search methods for identification of studies
We did not restrict the search for eligible studies by language.
Electronic searches
For this update, the Cochrane Vascular Information Specialist (CIS) searched the following databases for relevant trials.
Cochrane Vascular Specialised Register (30 November 2016).
Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 10) via the Cochrane Register of Studies Online.
See Appendix 1 for details of the search strategy used for CENTRAL.
The Cochrane Vascular Specialised Register is maintained by the CIS and is constructed from weekly electronic searches of MEDLINE Ovid, Embase Ovid, CINAHL, AMED, and through handsearches of relevant journals. The full list of the databases, journals and conference proceedings included in these searches, as well as the search strategies used, are described in the Specialised Register section of the Cochrane Vascular module in the Cochrane Library (www.cochranelibrary.com).
The CIS searched the following trial registries for details of ongoing and unpublished studies.
ClinicalTrials.gov (www.clinicaltrials.gov).
World Health Organization International Clinical Trials Registry Platform (www.who.int/trialsearch).
ISRCTN Register (www.isrctn.com/).
See Appendix 2 for details of the search strategies.
Searching other resources
We handsearched the reference lists of relevant trials and reviews identified for additional studies.
Data collection and analysis
Selection of studies
For this update, two review authors (JS, LR) independently scanned the titles, abstracts and keywords of every record retrieved. We retrieved full articles for further assessment if the information given suggested that the study fulfilled the inclusion criteria and did not meet the exclusion criteria. If there was any doubt regarding these criteria based on the title and abstract, we retrieved the full article for clarification.
Data extraction and management
For studies that fulfilled the inclusion criteria, we abstracted relevant population and intervention characteristics using standard data extraction templates. For details, see Characteristics of included studies and Appendix 3 (Additional study information). We resolved disagreements by discussion.
For this update, two review authors (JS, LR) extracted the following data.
General information: author, title, publication (published/unpublished; duplicate/multiple publication), language of publication, year of publication, country, complete reference or source, contact details, rural or urban setting, single centre versus multicentre, setting, stated aim of the study, sponsor, ethics committee approval and description of conflict of interests.
Trial design: prospective study, control group, parallel study, placebo controlled, active‐medication controlled, use of cross‐over design (and if so, description of run‐in period, wash‐out period and carry‐over effect described), description of period effect, sampling method and power calculation, selection bias (randomisation, unit of randomisation and allocation concealment adequacy), performance bias (blinding of participants and caregivers, method of blinding, check of blinding, check of blinding method), attrition bias (intention‐to‐treat analysis, description of withdrawals, drop‐outs description and losses to follow‐up, change of groups (if cross‐overs), number of dropouts and withdrawals and loss to follow‐up, reasons and description for dropouts, withdrawals or losses to follow‐up), and detection bias (blinding of outcome assessors), overall quality assessment, definition of inclusion criteria, definition of exclusion criteria,, and specified subgroups (predefined and defined post hoc).
Participants: venous thromboembolism (VTE) diagnostic criteria description, VTE diagnostic criteria validity,, baseline characteristics (i.e. number of participants, age, sex, race, body mass index, comorbidities, concomitant medications, identical treatment of groups (apart from intervention)).
Intervention: dose adjustment for subcutaneous UFH (weight‐adjusted or aPTT‐adjusted), bolus intravenous heparin in subcutaneous arm, number of daily subcutaneous doses, daily heparin cumulative dose, duration of heparin therapy (days), warfarin dose, length of follow‐up, compliance.
Outcomes assessed for short, intermediate and long term as defined above: incidence of symptomatic recurrent deep vein thrombosis (DVT) or pulmonary embolism (PE), mortality related to propagation of VTE, treatment‐related mortality during heparin treatment, incidence of asymptomatic propagation of VTE, treatment‐related morbidity during heparin treatment (major bleeding, minor bleeding, heparin‐induced thrombocytopenia (HIT), other), length of hospital stay, quality of life.
Effect modifiers: compliance, change of concomitant medication, warfarin therapy.
We sought any relevant missing information on the trials from the original author(s) of the article, if required.
Assessment of risk of bias in included studies
Two review authors (JS, LR) independently used the Cochrane 'Risk of bias' tool to assess the risk of bias for each of the included studies (Higgins 2011). The tool provides a protocol for judgements on sequence generation, allocation methods, blinding of participants, investigators and outcome assessors, incomplete outcome data, selective outcome reporting and any other relevant biases. We judged each of these domains as being at either high, low or unclear risk of bias according to Higgins 2011 and provided support for each judgement, resolving any disagreements by discussion. We present the conclusions in a 'Risk of bias' table.
Measures of treatment effect
We based the analysis on intention‐to‐treat data from the individual clinical trials. For the primary and secondary outcomes, which are binary measures, we computed odds ratios (ORs) using a fixed‐effect model and calculated the 95% confidence intervals (CI) of the effect sizes. For the continuous outcomes such as length of hospital stay and quality of life, we planned to use mean differences (MDs) with 95% CIs where the scales were the same, and where scales were different but the outcome was the same, we planned to use the standardised mean difference (SMD) with 95% CIs.
Unit of analysis issues
The unit of analysis was the individual participant.
Dealing with missing data
We sought relevant missing data from authors where necessary and feasible. We carefully evaluated important numerical data such as screened, eligible and randomised participants as well as intention‐to‐treat and per‐protocol population. We investigated dropouts, losses to follow‐up and withdrawn study participants.
Assessment of heterogeneity
We assessed heterogeneity between the trials by visual examination of the forest plot to check for overlapping CIs, the Chi2 test for homogeneity with a 10% level of significance and the I2 statistic to measure the degree of inconsistency between the studies. An I2 result of greater than 50% may represent moderate to substantial heterogeneity (Deeks 2011).
Assessment of reporting biases
We planned to assess publication bias by funnel plots if a sufficient number of studies (10 or more) were available in the meta‐analyses. There are many reasons for funnel plot asymmetry, and we planned to consult the Cochrane Handbook for Systematic Reviews of Interventions to aid the interpretation of the results (Sterne 2011).
Data synthesis
The review authors independently extracted the data. One review author (LR) entered the data into Review Manager 5 (RevMan 2014), and the second review author (JS) cross‐checked data entry. We resolved any discrepancies by consulting the source publication.
If data were available, sufficiently similar and of sufficient quality, we provided a statistical summary. We used a fixed‐effect model to meta‐analyse the data. If the I2 statistic indicated heterogeneity greater than 50%, we performed a random‐effects model analysis instead.
Subgroup analysis and investigation of heterogeneity
We planned to perform subgroup analyses, according to the following clinically logical pre‐defined groups.
-
Participants.
VTE at randomisation: DVT with/without PE versus DVT without PE versus PE without DVT.
VTE: first versus recurrent.
Severity: haemodynamically stable versus unstable, respiratory stable versus unstable.
Age.
Renal function.
Underlying pathology (e.g. orthopaedic patients).
-
Intervention.
Number of daily subcutaneous heparin injections.
Type of dose adjustment; weight‐adjusted versus aPTT‐adjusted.
Initial intravenous bolus heparin given versus not given.
Concomitant oral anticoagulant use.
Timing of oral anticoagulant initiation.
We performed neither a dose‐response analysis nor any indirect comparisons between groups not directly evaluated head‐to‐head in a clinical trial.
Sensitivity analysis
We planned to perform sensitivity analyses in order to explore the influence of the following factors on effect size, repeating the analysis by:
excluding data from unpublished studies;
taking account of study quality, as specified above;
excluding any very long or large studies to establish how much they dominated the results;
excluding studies using the following filters: diagnostic criteria, language of publication, source of funding (industry versus other), country.
Summary of findings table
We presented the main findings of the review results in a 'Summary of findings' table, reporting the quality of evidence (according to Atkins 2004), the magnitude of effect of the interventions examined, and the sum of available data on symptomatic recurrent VTE at three months, symptomatic recurrent DVT at three months, PE at three months, VTE‐related mortality at three months, major bleeding, all‐cause mortality and asymptomatic VTE at three months, . We used the GRADEpro software to assist in the preparation of the 'Summary of findings' table (GRADEpro GDT).
Results
Description of studies
Results of the search
See Figure 1.
1.
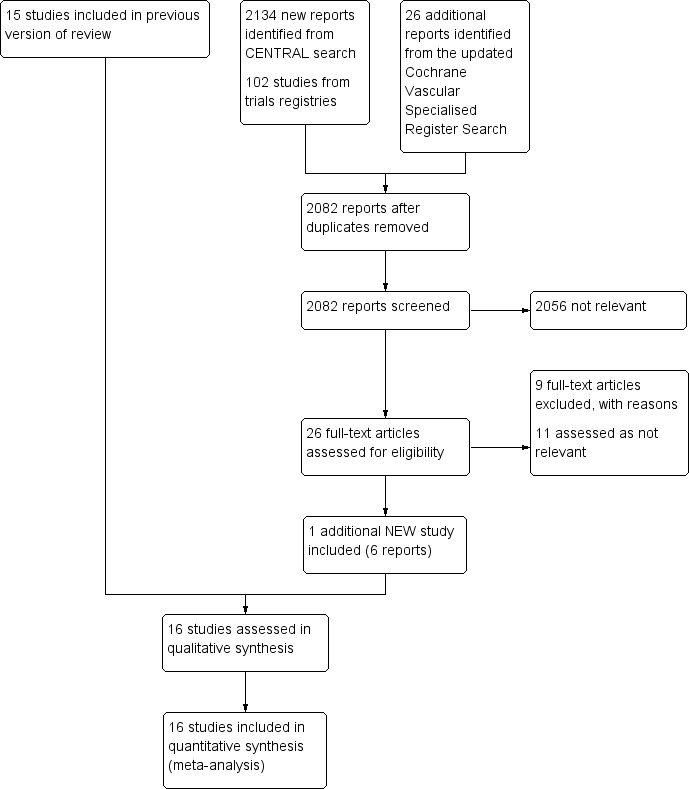
Study flow diagram.
Included studies
For this update, we identified one additional study that met the inclusion criteria for this review (Leizorovicz 2011), bringing the total number of included studies to 16 randomised controlled trials, involving 3593 participants (Andersson 1982; Belcaro 1999; Bentley 1980; Doyle 1987; Faivre 1987; Holm 1986; Hull 1986; Kearon 2006; Krähenbühl 1979; Leizorovicz 2011; Lopaciuk 1990; Lopaciuk 1992; Peternel 2002; Pini 1990; Prandoni 2004; Walker 1987). For detailed descriptions see Characteristics of included studies and Appendix 3.
Eight studies compared subcutaneous UFH versus intravenous UFH (Andersson 1982; Bentley 1980; Doyle 1987; Hull 1986; Krähenbühl 1979; Lopaciuk 1990; Pini 1990; Walker 1987), seven studies compared subcutaneous UFH versus LMWH (Faivre 1987; Holm 1986; Kearon 2006; Leizorovicz 2011; Lopaciuk 1992; Peternel 2002; Prandoni 2004), and one study compared subcutaneous UFH to both intravenous UFH and subcutaneous LMWH (Belcaro 1999).. For the long‐term treatment, nine studies utilised warfarin, three used acenocoumarol, and one used subcutaneous UFH. In three studies, the long‐term management was not clear. Thirteen trials monitored the subcutaneous heparin dose through aPTT measurements and one through anti‐factor Xa (anti‐Xa) measurements, while in two studies the subcutaneous heparin dose was fixed or based solely on weight.
Fourteen studies took place in an inpatient setting (Andersson 1982; Bentley 1980; Doyle 1987; Faivre 1987; Holm 1986; Hull 1986; Krähenbühl 1979; Leizorovicz 2011; Lopaciuk 1990; Lopaciuk 1992; Peternel 2002; Pini 1990; Prandoni 2004; Walker 1987), and two in both inpatient and outpatient settings (Belcaro 1999; Kearon 2006). All trials included participants with DVT. Four trials allowed for participants with PE in their inclusion criteria (Faivre 1987; Kearon 2006; Leizorovicz 2011; Prandoni 2004). Four trials excluded people with PE (Doyle 1987; Holm 1986; Peternel 2002; Walker 1987), and an additional two trials excluded people with massive PE (Faivre 1987; Lopaciuk 1990). The remaining trials did not clearly describe PE inclusion. We did not identify any trials that included only participants with PE. Studies recruited participants upon diagnosis of VTE and randomised them to treatment groups. Eight of the included studies administered an initial intravenous heparin bolus prior to initiating subcutaneous heparin treatment (Andersson 1982; Hull 1986; Krähenbühl 1979; Leizorovicz 2011; Lopaciuk 1990; Lopaciuk 1992; Peternel 2002; Prandoni 2004). One study maintained the infusion for 24 hours before the first subcutaneous administration (Holm 1986). The duration of the intervention ranged from a minimum of seven days to achievement of international normalised ratio (INR) target level for oral anticoagulation in all included trials apart from one, which administered subcutaneous heparin for three months (Belcaro 1999). Diagnostic modalities for DVT included venous occlusion plethysmography, thermography, phlebography, venography, and colour‐duplex sonography; as well as lung scan or CT‐angiography for PE. Follow‐up length was as long as the intervention duration in eight studies and three months in seven studies (Belcaro 1999; Doyle 1987; Hull 1986; Kearon 2006; Lopaciuk 1990; Lopaciuk 1992; Prandoni 2004). One study reported death rate at 12 months (Doyle 1987). One study was terminated early, as an interim safety analysis revealed an excess mortality rate in the subcutaneous heparin group (Leizorovicz 2011).
Excluded studies
After careful evaluation of the full publications, we excluded nine additional studies from this update (Nakamura 2010; NCT01956955; Quiros 2001; Riess 2014; Rodgers 1999; Romera 2009; Ucar 2015; Van Doormaal 2009; Van Doormaal 2010), for a total number of 16 excluded studies. The main reasons for exclusion were the method of administration of heparin and involvement of thrombolysis or VTE prophylaxis. For further details see Characteristics of excluded studies.
Risk of bias in included studies
For details on methodological quality of included studies, see Figure 2 and Figure 3.
2.
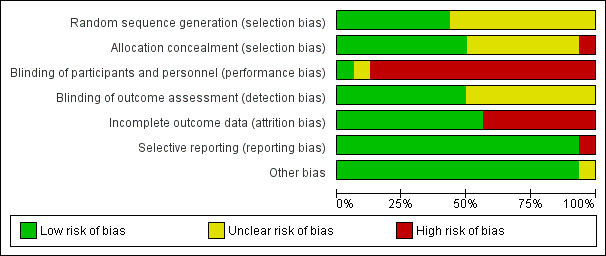
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
3.
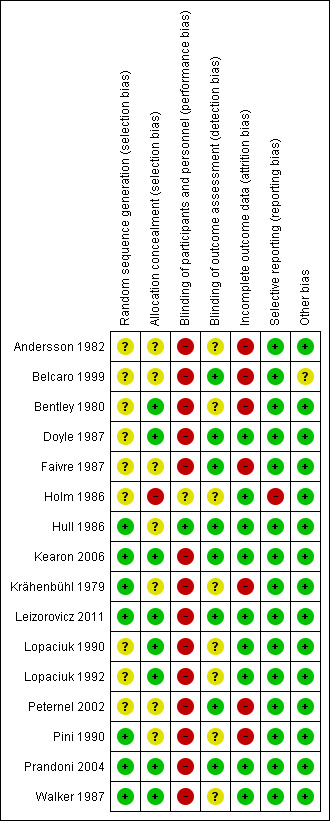
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Allocation
Five studies described the use of computer‐generated random sequences (Hull 1986; Kearon 2006; Leizorovicz 2011; Pini 1990; Prandoni 2004), one study described 'drawing of lots' (Krähenbühl 1979), and another study described the use of a random number table to allocate participants to treatment groups (Walker 1987). We therefore deemed these seven studies to be at low risk of selection bias. All other studies stated that they randomised participants but did not provide a clear description of random sequence generation, so we considered them to be at unclear risk of selection bias (Andersson 1982; Belcaro 1999; Bentley 1980; Doyle 1987; Faivre 1987; Holm 1986; Lopaciuk 1990; Lopaciuk 1992; Peternel 2002).
We judged eight studies to be at low risk of selection bias due to allocation concealment (Bentley 1980; Doyle 1987; Kearon 2006; Leizorovicz 2011; Lopaciuk 1990; Lopaciuk 1992; Prandoni 2004; Walker 1987). Five of these studies described the use of 'sealed envelopes' to maintain allocation concealment (Bentley 1980; Doyle 1987; Lopaciuk 1990; Lopaciuk 1992; Walker 1987). The Cochrane Handbook for Systematic Reviews of Interventions states that allocation concealment should be achieved through sequentially numbered, opaque, sealed envelopes, opened only after irreversible assignment to a participant. However, due to the age of the studies included in this review, we decided that studies describing the use of envelopes to maintain allocation concealment would be at lower risk of selection bias than those that did not and that we would deem them to be at low risk. Three studies described the use of central telephone randomisation (Kearon 2006; Leizorovicz 2011; Prandoni 2004). Leizorovicz 2011 specifically stated that "no allocation concealment mechanism was attempted as the study was open"; however, we still considered the study to be at low risk of selection bias, as this statement appeared to contradict the description of "central telephone randomisation". We therefore assumed that the authors were referring to the blinding of participants and personnel as "allocation concealment". Furthermore, authors also stated that "care was taken to ensure that outcome assessors and data analysts were kept blinded to the allocation".
We judged Holm 1986 to be at high risk of selection bias due to allocation concealment. Authors stated that participants' allocations to treatment groups depended on the order of participant admission: "the vials [of low molecular weight or unfractionated heparin] had been randomised in advance and numbered consecutively, the number of patient admission determining the number of vial used". As personnel potentially had knowledge of the order of the vials – allowing them to control the composition of the treatment groups by manipulating the order of participant admission – we deemed this study to be at high risk of selection bias.
No other studies provided descriptions of allocation concealment, so we deemed them to be at unclear risk for allocation concealment (Andersson 1982; Belcaro 1999; Faivre 1987; Hull 1986; Krähenbühl 1979; Peternel 2002; Pini 1990).
Blinding
Only one study adequately reported the blinding of participants and personnel, so we considered it as being at low risk of performance bias (Hull 1986). One study reported that it was "double‐blind" but did not provide any further information, so we assessed it as being at unclear risk (Holm 1986). The remaining fourteen studies were not blinded, so we considered them to be at high risk of performance bias (Andersson 1982; Belcaro 1999; Bentley 1980; Doyle 1987; Faivre 1987; Kearon 2006; Krähenbühl 1979; Leizorovicz 2011; Lopaciuk 1990; Lopaciuk 1992; Peternel 2002; Pini 1990; Prandoni 2004; Walker 1987).
For measuring the risk of detection bias, we decided that due to the subjective nature of certain criteria, we would rate studies as being at high risk of detection bias if they did not adequately blind for the following outcomes: recurrent VTE at three months; recurrent DVT at three months; PE – excluding PE found at autopsy; incidence of asymptomatic VTE at three months; quality of life; and incidence of HIT. However, we thought that VTE‐related mortality at three months, all‐cause mortality and major and minor bleeding (if they followed the definition provided by the International Society on Thrombosis and Haemostasis) were objective enough to not require blinding.
In total, we judged eight studies to be at low risk of detection bias (Belcaro 1999; Doyle 1987; Faivre 1987; Hull 1986; Kearon 2006; Leizorovicz 2011; Peternel 2002; Prandoni 2004). Two studies were only included in the analysis of VTE‐related mortality at three months and all‐cause mortality, so we automatically deemed them to be at low risk of detection bias (Faivre 1987; Peternel 2002), while six studies adequately blinded for all six subjective outcomes (Belcaro 1999; Doyle 1987; Hull 1986; Kearon 2006; Leizorovicz 2011; Prandoni 2004). The remaining eight studies did not state whether personnel assessing suspected PE were adequately blinded, so we deemed them to be at unclear risk of detection bias (Andersson 1982; Bentley 1980; Holm 1986; Krähenbühl 1979; Lopaciuk 1990; Lopaciuk 1992; Pini 1990; Walker 1987).
Incomplete outcome data
Nine studies adequately accounted for all missing data, and we judged them to be at low risk of attrition bias (Doyle 1987; Holm 1986; Hull 1986; Kearon 2006; Leizorovicz 2011; Lopaciuk 1990; Lopaciuk 1992; Prandoni 2004; Walker 1987). The remaining seven studies did not adequately deal with missing data, so we deemed them to be at high risk of attrition bias (Andersson 1982; Belcaro 1999; Bentley 1980; Faivre 1987; Krähenbühl 1979; Peternel 2002; Pini 1990).
Selective reporting
Due to the age of the studies included in the review, there was only one available protocol for an included study (Kearon 2006). We therefore based our judgements of selective reporting solely on the reporting of pre‐specified outcomes in the Methods sections. Fifteen papers reported on all pre‐specified outcomes, and we deemed them to be at low risk of reporting bias (Andersson 1982; Belcaro 1999; Bentley 1980; Doyle 1987; Faivre 1987; Hull 1986; Kearon 2006; Krähenbühl 1979; Leizorovicz 2011; Lopaciuk 1990; Lopaciuk 1992; Peternel 2002; Pini 1990; Prandoni 2004; Walker 1987). We considered one study to be at high risk of reporting bias, as authors presented results for leg pain but did not present the method of measuring pain in the Methods section (Holm 1986).
Other potential sources of bias
We rated 15 studies as being at low risk of other bias (Andersson 1982; Bentley 1980; Doyle 1987; Faivre 1987; Holm 1986; Hull 1986; Kearon 2006; Krähenbühl 1979; Leizorovicz 2011; Lopaciuk 1990; Lopaciuk 1992; Peternel 2002; Pini 1990; Prandoni 2004; Walker 1987). We considered the risk of other bias to be unclear in one study, as different groups received treatment in different locations, with groups 1 and 2 receiving different treatments in hospital and group 3 receiving treatment at home (Belcaro 1999).
Effects of interventions
Summary of findings for the main comparison. Subcutaneous unfractionated heparin compared to intravenous unfractionated heparin for the initial treatment of venous thromboembolism.
| Subcutaneous unfractionated heparin compared to intravenous unfractionated heparin for the initial treatment of venous thromboembolism | |||||
| Patient or population: people aged ≥ 18 years with a diagnosis of new or recurrent VTE Setting: inpatient and outpatient Intervention: subcutaneous unfractionated heparin Comparison: intravenous unfractionated heparin | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Risk with intravenous unfractionated heparin | Risk with subcutaneous unfractionated heparin | ||||
| Symptomatic recurrent VTE at 3 months | Study population | OR 1.66 (0.89 to 3.10) | 965 (8 RCTs) | ⊕⊕⊝⊝ Lowa | |
| 35 per 1000 | 57 per 1000 (32 to 102) | ||||
| Symptomatic recurrent DVT at 3 months | Study population | OR 3.29 (0.64 to 17.06) | 115 (1 RCT) | ⊕⊕⊝⊝ Lowb | |
| 34 per 1000 | 105 per 1000 (22 to 379) | ||||
| PE at 3 months | Study population | OR 1.44 (0.73 to 2.84) | 1161 (9 RCTs) | ⊕⊕⊝⊝ Lowc | |
| 26 per 1000 | 37 per 1000 (19 to 70) | ||||
| VTE‐related mortality at 3 months | Study population | OR 0.98 (0.20 to 4.88) | 1168 (9 RCTs) | ⊕⊕⊝⊝ Lowc | |
| 3 per 1000 | 3 per 1000 (1 to 17) | ||||
| Major bleedingd (7 days ‐ 12 months) |
Study population | OR 0.91 (0.42 to 1.97) | 583 (4 RCTs) | ⊕⊕⊝⊝ Lowe | |
| 48 per 1000 | 44 per 1000 (21 to 91) | ||||
| All‐cause mortality (5 days to 12 months) |
Study population | OR 1.74 (0.67 to 4.51) | 972 (8 RCTs) | ⊕⊕⊝⊝ Lowa | |
| 12 per 1000 | 21 per 1000 (8 to 54) | ||||
| Asymptomatic VTE at 3 months | No study measured this outcome | ||||
| *The basis for the assumed risk was the average risk in the intravenous unfractionated heparin group (i.e. the number of participants with events divided by total number of participants of the intravenous heparin group included in the meta‐analysis). The risk in the subcutaneous unfractionated heparin group (and its 95% confidence interval) is based on the assumed risk in the intravenous unfractionated heparin group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; DVT: deep vein thrombosis; PE: pulmonary embolism; RCT: randomised controlled trial; OR: odds ratio; VTE: venous thromboembolism | |||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
aWe downgraded the quality of the evidence to low due to a high risk of performance bias in eight studies (Andersson 1982; Belcaro 1999; Bentley 1980; Doyle 1987; Krähenbühl 1979; Lopaciuk 1990; Pini 1990; Walker 1987), plus a high risk of attrition bias in five studies (Andersson 1982; Belcaro 1999; Bentley 1980; Krähenbühl 1979; Pini 1990). We also downgraded for imprecision, as reflected by the wide confidence intervals. bWe downgraded the quality of the evidence to low for imprecision as only one study with a small number of participants was included, leading to a wide confidence interval around the effect estimate (Hull 1986). cWe downgraded the quality of the evidence to low due to a high risk of performance bias in seven studies (Andersson 1982; Bentley 1980; Doyle 1987; Krähenbühl 1979; Lopaciuk 1990; Pini 1990; Walker 1987), plus a high risk of attrition bias in four studies (Andersson 1982; Bentley 1980; Krähenbühl 1979; Pini 1990). We also downgraded for imprecision reflected by the wide confidence intervals. d Major bleeding as defined by the International Society on Thrombosis and Haemostasis (ISTH) (Schulman 2005); fatal bleeding; symptomatic bleeding in a critical area or organ, such as intracranial, intraspinal, intraocular, retroperitoneal, intra‐articular or pericardial, or intramuscular with compartment syndrome; bleeding causing a fall in haemoglobin level of 20 g/L (1.24 mmol/L) or more, or leading to transfusion of two or more units of whole blood or red cells; any combination of the above. eWe downgraded the quality of the evidence to low due to a high risk of performance bias in three studies (Doyle 1987; Lopaciuk 1990; Pini 1990), plus a high risk of attrition bias in one study (Pini 1990). We also downgraded for imprecision, as reflected by the wide confidence intervals.
Summary of findings 2. Subcutaneous unfractionated heparin compared to low molecular weight heparin for the initial treatment of venous thromboembolism.
| Subcutaneous unfractionated heparin compared to low molecular weight heparin for the initial treatment of venous thromboembolism | |||||
| Patient or population: people aged ≥ 18 years with a diagnosis of new or recurrent VTE Setting: inpatient and outpatient Intervention: subcutaneous unfractionated heparin Comparison: low molecular weight heparin | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Risk with low molecular weight heparin | Risk with subcutaneous unfractionated heparin | ||||
| Symptomatic recurrent VTE at 3 months | Study population | OR 1.01 (0.63 to 1.63) | 2156 (5 RCTs) | ⊕⊕⊝⊝ Lowa | |
| 31 per 1000 | 32 per 1000 (20 to 50) | ||||
| Symptomatic recurrent DVT at 3 months | Study population | OR 1.38 (0.73 to 2.63) | 1566 (3 RCTs) | ⊕⊕⊝⊝ Lowb | |
| 20 per 1000 | 28 per 1000 (15 to 52) | ||||
| PE at 3 months | Study population | OR 0.84 (0.36 to 1.96) | 1819 (5 RCTs) | ⊕⊕⊝⊝ Lowa | |
| 12 per 1000 | 10 per 1000 (4 to 23) | ||||
| VTE‐related mortality at 3 months | Study population | OR 0.53 (0.17 to 1.67) | 2469 (8 RCTs) | ⊕⊕⊝⊝ Lowc | |
| 6 per 1000 | 3 per 1000 (1 to 11) | ||||
| Major bleedingd (3 months) | Study population | OR 0.72 (0.43 to 1.20) | 2300 (5 RCTs) | ⊕⊕⊝⊝ Lowa | |
| 31 per 1000 | 23 per 1000 (14 to 37) | ||||
| All‐cause mortality (7 days ‐ 3 months) | Study population | OR 0.73 (0.50 to 1.07) | 2272 (7 RCTs) | ⊕⊕⊝⊝ Lowe | |
| 58 per 1000 | 43 per 1000 (30 to 62) | ||||
| Asymptomatic VTE at 3 months | No study measured this outcome | ||||
| *The basis for the assumed risk was the average risk in the low molecular weight heparin group (i.e. the number of participants with events divided by total number of participants of the low molecular weight heparin group included in the meta‐analysis). The risk in the subcutaneous unfractionated heparin group (and its 95% confidence interval) is based on the assumed risk in the low molecular weight heparin group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; DVT: deep vein thrombosis; PE: pulmonary embolism; RCT: randomised controlled trial; OR: odds ratio; VTE: venous thromboembolism | |||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
aWe downgraded the quality of the evidence to low due to high risk of selection and reporting bias in one study (Holm 1986), plus a high risk of performance bias in four studies (Kearon 2006; Leizorovicz 2011; Lopaciuk 1992; Prandoni 2004). We also downgraded for imprecision, as reflected by the wide confidence intervals. bWe downgraded the quality of the evidence to low due to a high risk of performance bias in three studies (Kearon 2006; Lopaciuk 1992; Prandoni 2004). We also downgraded for imprecision, as reflected by the wide confidence intervals. cWe downgraded the quality of the evidence to low due to a high risk of performance bias in seven studies (Belcaro 1999; Faivre 1987; Kearon 2006; Leizorovicz 2011; Lopaciuk 1992; Peternel 2002; Prandoni 2004), a high risk of attrition bias in three studies (Belcaro 1999; Faivre 1987; Peternel 2002), and a high risk of selection and reporting bias in one study (Holm 1986). We also downgraded for imprecision, as reflected by the wide confidence intervals. d Major bleeding as defined by the International Society on Thrombosis and Haemostasis (ISTH) (Schulman 2005); fatal bleeding; symptomatic bleeding in a critical area or organ, such as intracranial, intraspinal, intraocular, retroperitoneal, intra‐articular or pericardial, or intramuscular with compartment syndrome; bleeding causing a fall in haemoglobin level of 20 g/L (1.24 mmol/L) or more, or leading to transfusion of two or more units of whole blood or red cells; any combination of the above. eWe downgraded the quality of the evidence to low due to a high risk of performance bias in six studies (Faivre 1987; Kearon 2006; Leizorovicz 2011; Lopaciuk 1992; Peternel 2002; Prandoni 2004), a high risk of attrition bias in two studies (Faivre 1987; Peternel 2002), and a high risk of selection and reporting bias in one study (Holm 1986). We also downgraded for imprecision reflected by the wide confidence intervals.
For a summary of outcomes see Table 1; Table 2. For details of outcomes see Data and analyses.
Subcutaneous UFH versus intravenous UFH
Symptomatic recurrent VTE at three months
Eight studies with a combined total of 965 participants measured recurrent VTE at three months (Andersson 1982; Bentley 1980; Doyle 1987; Hull 1986; Krähenbühl 1979; Lopaciuk 1990; Pini 1990; Walker 1987). The rate of recurrence was similar between participants treated with subcutaneous (27 events/485 participants) versus IV UFH (17 events/480 participants), leading to an odds ratio (OR) of 1.66 (95% CI 0.89 to 3.10; N = 965; 8 studies; I2 = 0%; low‐quality evidence; Analysis 1.1). All eight studies excluded participants with PE, so we could not perform subgroup analysis based on VTE at randomisation.
1.1. Analysis.
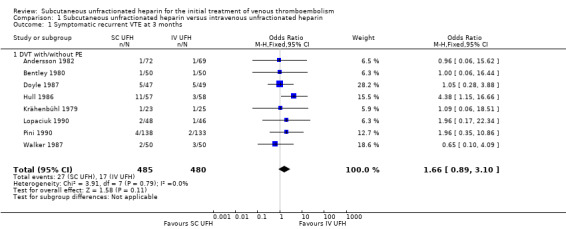
Comparison 1 Subcutaneous unfractionated heparin versus intravenous unfractionated heparin, Outcome 1 Symptomatic recurrent VTE at 3 months.
Symptomatic recurrent DVT at three months
One study with 115 participants measured recurrent DVT at three months (Hull 1986), finding a similar rate between participants treated with subcutaneous (6 events/57 participants) versus IV UFH (2 events/58 participants), leading to an OR of 3.29 (95% CI 0.64 to 17.06; N = 115; 1 study; low‐quality evidence; Analysis 1.2). This study included only DVT participants, so we could not perform subgroup analysis based on VTE at randomisation.
1.2. Analysis.
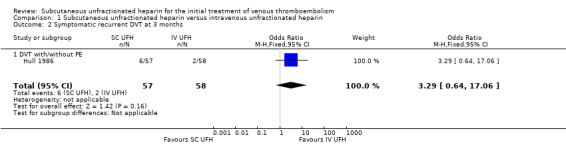
Comparison 1 Subcutaneous unfractionated heparin versus intravenous unfractionated heparin, Outcome 2 Symptomatic recurrent DVT at 3 months.
PE at three months
Nine studies with a combined total of 1161 participants measured incidence of PE at three months (Andersson 1982; Belcaro 1999; Bentley 1980; Doyle 1987; Hull 1986; Krähenbühl 1979; Lopaciuk 1990; Pini 1990; Walker 1987). Incidence was similar between participants treated with subcutaneous (21 events/584 participants) versus IV UFH (15 events/577 participants), leading to an OR of 1.44 (95% CI 0.73 to 2.84; N = 1161; 9 studies; I2 = 0%; low‐quality evidence; Analysis 1.3). All nine studies excluded participants with PE, so we could not perform subgroup analysis based on VTE at randomisation.
1.3. Analysis.
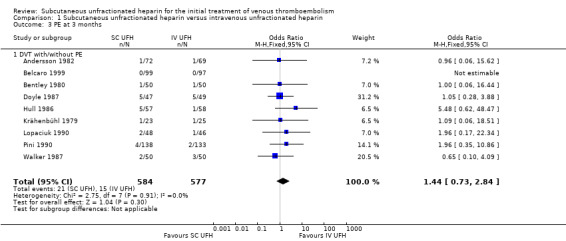
Comparison 1 Subcutaneous unfractionated heparin versus intravenous unfractionated heparin, Outcome 3 PE at 3 months.
VTE‐related mortality at three months
Nine studies with a combined total of 1168 participants measured VTE‐related mortality at three months (Andersson 1982; Belcaro 1999; Bentley 1980; Doyle 1987; Hull 1986; Krähenbühl 1979; Lopaciuk 1990; Pini 1990; Walker 1987). However, only three studies reported any cases of this outcome (Hull 1986; Lopaciuk 1990; Pini 1990), which was similar for participants treated with subcutaneous (2 events/588 participants) versus IV UFH (2 events/580 participants), leading to an OR of 0.98 (95% CI 0.20 to 4.88; N = 1168; 9 studies; I2 = 0%; low‐quality evidence; Analysis 1.4). All nine studies excluded participants with PE, so we could not perform subgroup analysis based on VTE at randomisation.
1.4. Analysis.
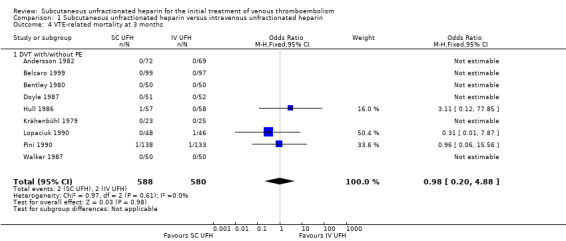
Comparison 1 Subcutaneous unfractionated heparin versus intravenous unfractionated heparin, Outcome 4 VTE‐related mortality at 3 months.
Major bleeding
Four studies with a combined total of 583 participants measured incidence of major bleeding during the study period (Doyle 1987; Hull 1986; Lopaciuk 1990; Pini 1990). The incidence of major bleeding was similar between participants treated with subcutaneous (13 events/294 participants) versus IV UFH (14 events/289 participants), leading to an OR of 0.91 (95% CI 0.42 to 1.97; N = 583; 4 studies; I2 = 0%; low‐quality evidence; Analysis 1.5). All four studies excluded participants with PE, so we could not perform subgroup analysis based on VTE at randomisation.
1.5. Analysis.
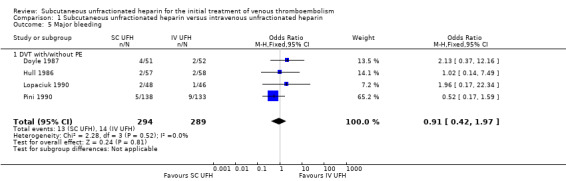
Comparison 1 Subcutaneous unfractionated heparin versus intravenous unfractionated heparin, Outcome 5 Major bleeding.
All‐cause mortality
Eight studies with a combined total of 972 participants measured all‐cause mortality (Andersson 1982; Bentley 1980; Doyle 1987; Hull 1986; Krähenbühl 1979; Lopaciuk 1990; Pini 1990; Walker 1987). This outcome was similar for participants treated with subcutaneous (11 events/489 participants) versus IV UFH (6 events/483 participants), leading to an OR of 1.74 (95% CI 0.67 to 4.51; N = 972; 8 studies; I2 = 0%; low‐quality evidence; Analysis 1.6). All eight studies excluded participants with PE, so we could not perform subgroup analysis based on VTE at randomisation.
1.6. Analysis.
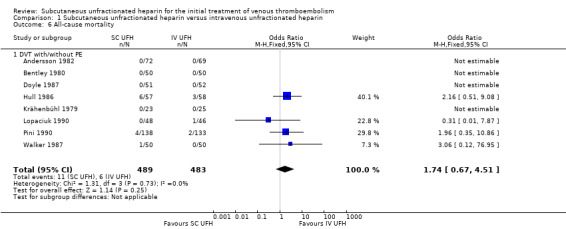
Comparison 1 Subcutaneous unfractionated heparin versus intravenous unfractionated heparin, Outcome 6 All‐cause mortality.
Asymptomatic VTE at three months
No studies comparing subcutaneous UFH with IV UFH reported any episodes of asymptomatic VTE occurring within three months of the commencement of treatment.
Treatment‐related morbidity
Minor bleeding
Five studies with a combined total of 779 participants measured incidence of minor bleeding during the study period (Belcaro 1999; Doyle 1987; Hull 1986; Lopaciuk 1990; Pini 1990). Incidence was similar for participants treated with subcutaneous (18 events/393 participants) versus IV UFH (26 events/386 participants), leading to an OR of 0.63 (95% CI 0.33 to 1.20; N = 779; 5 studies; I2 = 0%; low‐quality evidence; Analysis 1.7). All five studies excluded participants with PE, so we could not perform subgroup analysis based on VTE at randomisation.
1.7. Analysis.
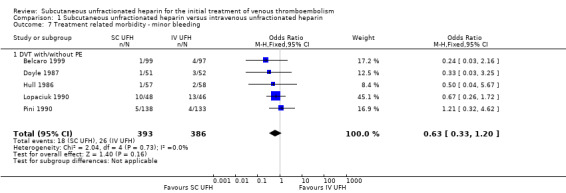
Comparison 1 Subcutaneous unfractionated heparin versus intravenous unfractionated heparin, Outcome 7 Treatment related morbidity ‐ minor bleeding.
Heparin‐induced thrombocytopenia
None of the studies comparing subcutaneous UFH with IV UFH reported episodes of HIT.
Length of hospital stay
The study by Belcaro 1999 measured days in hospital, but the subcutaneous UFH group were treated at home, so we could not make a comparison. The mean (± standard deviation) length of hospital stay in the IV UFH group was 5.4 ± 1.4 days.
Quality of life
None of the included studies measured quality of life as an outcome.
Subcutaneous UFH versus LMWH
Symptomatic recurrent VTE at three months
Five studies with a combined total of 2156 participants measured recurrent VTE at three months (Holm 1986; Kearon 2006; Leizorovicz 2011; Lopaciuk 1992; Prandoni 2004). The rate of recurrent VTE at three months was similar for participants treated with subcutaneous UFH (34 events/1071 participants) versus LMWH (34 events/1085 participants), leading to an OR of 1.01 (95% CI 0.63 to 1.63; N = 2156; 5 studies; I2 = 0%; low‐quality evidence; Analysis 2.1). We observed no differences between the VTE at randomisation subgroups 'DVT with/without PE' versus 'DVT without PE' (P = 0.38).
2.1. Analysis.
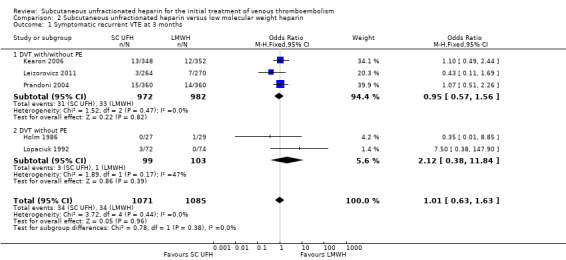
Comparison 2 Subcutaneous unfractionated heparin versus low molecular weight heparin, Outcome 1 Symptomatic recurrent VTE at 3 months.
Symptomatic recurrent DVT at three months
Three studies with a combined total of 1566 participants measured recurrent DVT at three months (Kearon 2006; Lopaciuk 1992; Prandoni 2004), finding similar rates for participants treated with subcutaneous UFH (22 events/780 participants) versus LMWH (16 events/786 participants), leading to an OR of 1.38 (95% CI 0.73 to 2.63; N = 1566; 3 studies; I2 = 0%; low‐quality evidence; Analysis 2.2). We observed no differences between the VTE at randomisation subgroups 'DVT with/without PE' versus 'DVT without PE' (P = 0.37).
2.2. Analysis.
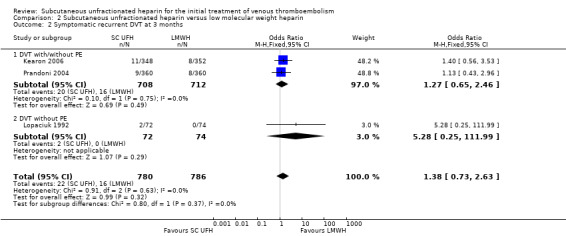
Comparison 2 Subcutaneous unfractionated heparin versus low molecular weight heparin, Outcome 2 Symptomatic recurrent DVT at 3 months.
PE at three months
Five studies with a combined total of 1819 participants measured incidence of PE at three months (Belcaro 1999; Holm 1986; Kearon 2006; Lopaciuk 1992; Prandoni 2004). Incidence was similar for participants treated with subcutaneous UFH (9 events/906 participants) versus LMWH (11 events/913 participants), leading to an OR of 0.84 (95% CI 0.36 to 1.96; N = 1819; 5 studies; I2 = 0%; low‐quality evidence) (Analysis 2.3). We observed no differences between the VTE at randomisation subgroups 'DVTwith/without PE' versus 'DVT without PE' (P = 0.81).
2.3. Analysis.
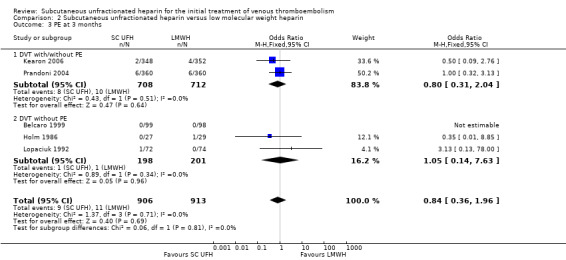
Comparison 2 Subcutaneous unfractionated heparin versus low molecular weight heparin, Outcome 3 PE at 3 months.
VTE‐related mortality at three months
Eight studies with a combined total of 2469 participants measured VTE‐related mortality at three months (Belcaro 1999; Faivre 1987; Holm 1986; Kearon 2006; Leizorovicz 2011; Lopaciuk 1992; Peternel 2002; Prandoni 2004). The outcome was similar for participants treated with subcutaneous UFH (4 events/1230 participants) versus LMWH (8 events/1239 participants), leading to an OR of 0.53 (95% CI 0.17 to 1.67; N = 2469; 8 studies; I2 = 0%; low‐quality evidence; Analysis 2.4). There were no cases of VTE‐related mortality in the four studies incorporating participants with DVT but without PE.
2.4. Analysis.
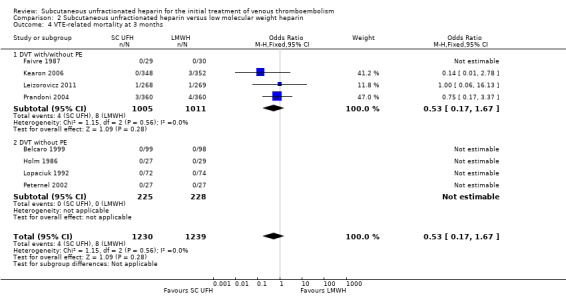
Comparison 2 Subcutaneous unfractionated heparin versus low molecular weight heparin, Outcome 4 VTE‐related mortality at 3 months.
Major bleeding
Five studies with a combined total of 2300 participants measured incidence of major bleeding during the study period (Belcaro 1999; Kearon 2006; Leizorovicz 2011; Lopaciuk 1992; Prandoni 2004). The incidence of major bleeding was similar for participants treated with subcutaneous UFH (26 events/1147 participants) versus LMWH (36 events/1153 participants), leading to an OR of 0.72 (95% CI 0.43 to 1.20; N = 2300; 5 studies; I2 = 0%; low‐quality evidence; Analysis 2.5). We observed no differences between the VTE at randomisation subgroups DVT regardless of PE status versus DVT without PE(P = 0.36).
2.5. Analysis.
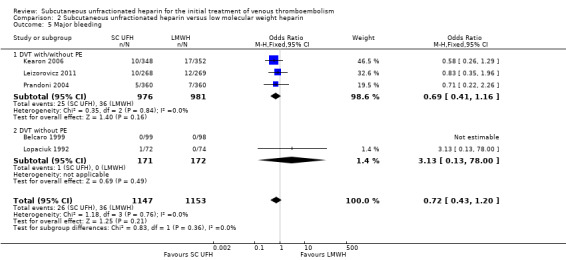
Comparison 2 Subcutaneous unfractionated heparin versus low molecular weight heparin, Outcome 5 Major bleeding.
All‐cause mortality
Seven studies with a combined total of 2272 participants measured all‐cause mortality (Faivre 1987; Holm 1986; Kearon 2006; Leizorovicz 2011; Lopaciuk 1992; Peternel 2002; Prandoni 2004). This outcome was similar for participants treated with subcutaneous UFH (49 events/1131 participants) versus LMWH (66 events/1141 participants), leading to an OR of 0.73 (95% CI 0.50 to 1.07; N = 2272; 7 studies; I2 = 0%; low‐quality evidence; Analysis 2.6). We observed no differences between the VTE at randomisation subgroups 'DVT with/without PE' versus 'DVT without PE' (P = 0.41).
2.6. Analysis.
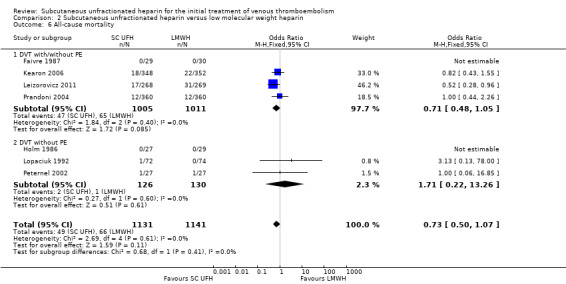
Comparison 2 Subcutaneous unfractionated heparin versus low molecular weight heparin, Outcome 6 All‐cause mortality.
Asymptomatic VTE at three months
There were no episodes of asymptomatic VTE occurring within three months of the commencement of treatment reported by any studies comparing subcutaneous UFH versus LMWH.
Treatment‐related morbidity
Minor bleeding
Five studies with a combined total of 2300 participants measured incidence of minor bleeding within the study period (Belcaro 1999; Kearon 2006; Leizorovicz 2011; Lopaciuk 1992; Prandoni 2004). The incidence of minor bleeding was similar for participants treated with subcutaneous UFH (81 events/1147 participants) versus LMWH (83 events/1153 participants), leading to an OR of 0.98 (95% CI 0.71 to 1.37; N = 2300; 5 studies; I2 = 0%;; Analysis 2.7). We observed no differences between the VTE at randomisation subgroups 'DVTwith/without PE' versus 'DVT without PE' (P = 0.93).
2.7. Analysis.
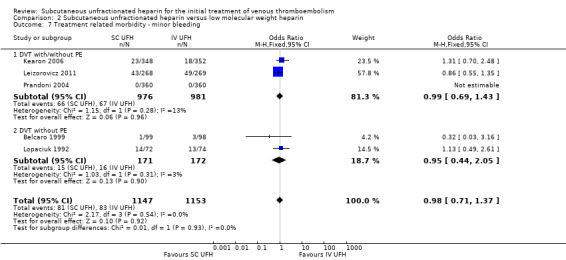
Comparison 2 Subcutaneous unfractionated heparin versus low molecular weight heparin, Outcome 7 Treatment related morbidity ‐ minor bleeding.
Heparin‐induced thrombocytopenia
Three studies with a combined total of 1954 participants measured the incidence of HIT (Kearon 2006; Leizorovicz 2011; Prandoni 2004). The outcome was similar for participants treated with subcutaneous UFH (3 events/972 participants) versus LMWH (2 events/982 participants), leading to an OR of 1.52 (95% CI 0.25 to 9.14; N = 1954; 3 studies; I2 = 0%; Analysis 2.8). All three studies included participants with PE, so we could not perform subgroup analysis based on VTE at randomisation.
2.8. Analysis.
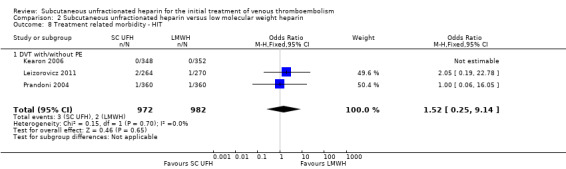
Comparison 2 Subcutaneous unfractionated heparin versus low molecular weight heparin, Outcome 8 Treatment related morbidity ‐ HIT.
Length of hospital stay
Belcaro 1999 measured days in hospital, but the subcutaneous UFH group received treatment at home, so we could not make a comparison. The mean length of hospital stay in the LMWH group was 5.1 ± 1.0 days.
Quality of life
None of the included studies measured quality of life as an outcome
Subgroup analysis
Data were not available for subgroup analysis by first or recurrent VTE, severity of VTE, age of participants, renal function or underlying pathology of VTE. Additionally, data were not available for subgroup analysis by number of daily subcutaneous heparin injections, type of dose adjustment, initial intravenous bolus heparin given versus not given, concomitant oral anticoagulant use or timing of oral anticoagulant initiation. We report results of subgroup analyses by VTE at randomisation above.
Sensitivity analysis
We performed sensitivity analyses in order to explore the influence of certain factors on effect size. We considered two studies large compared with others (Kearon 2006; Prandoni 2004). Both compared subcutaneous UFH versus LMWH. Exclusion of these trials from the analysis of outcomes did not influence the results (Analysis 3.1; Analysis 3.2; Analysis 3.3; Analysis 3.4; Analysis 3.5; Analysis 3.6; Analysis 3.7). We did not analyse the effect of published versus unpublished trials, as no unpublished data were available. Sensitivity of the results to the quality of trials was not feasible, as we judged all but one trial to be at a high risk of bias. Furthermore, we could not perform sensitivity analyses by diagnostic criteria, language of publication or source of funding due to insufficient data.
3.1. Analysis.
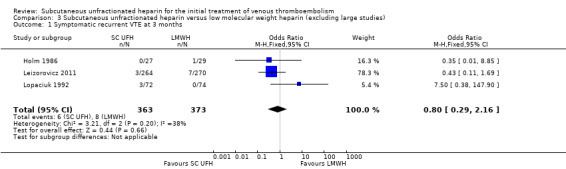
Comparison 3 Subcutaneous unfractionated heparin versus low molecular weight heparin (excluding large studies), Outcome 1 Symptomatic recurrent VTE at 3 months.
3.2. Analysis.
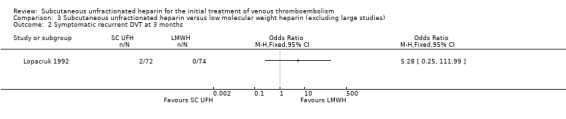
Comparison 3 Subcutaneous unfractionated heparin versus low molecular weight heparin (excluding large studies), Outcome 2 Symptomatic recurrent DVT at 3 months.
3.3. Analysis.
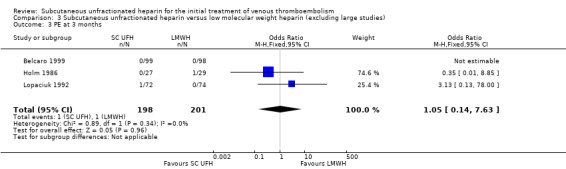
Comparison 3 Subcutaneous unfractionated heparin versus low molecular weight heparin (excluding large studies), Outcome 3 PE at 3 months.
3.4. Analysis.
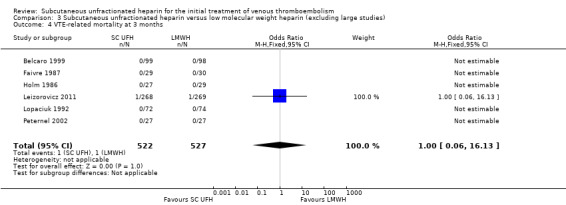
Comparison 3 Subcutaneous unfractionated heparin versus low molecular weight heparin (excluding large studies), Outcome 4 VTE‐related mortality at 3 months.
3.5. Analysis.
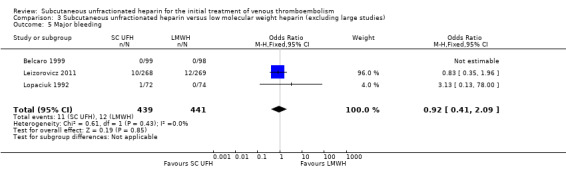
Comparison 3 Subcutaneous unfractionated heparin versus low molecular weight heparin (excluding large studies), Outcome 5 Major bleeding.
3.6. Analysis.
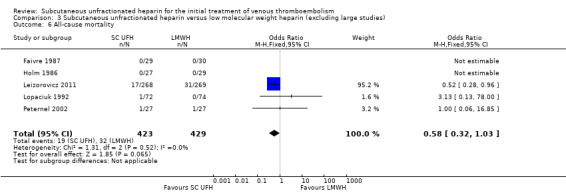
Comparison 3 Subcutaneous unfractionated heparin versus low molecular weight heparin (excluding large studies), Outcome 6 All‐cause mortality.
3.7. Analysis.
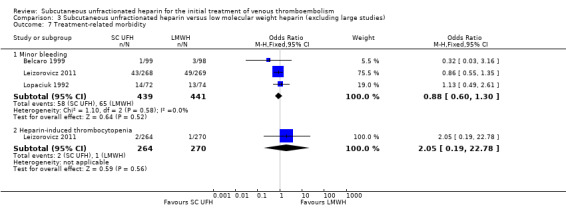
Comparison 3 Subcutaneous unfractionated heparin versus low molecular weight heparin (excluding large studies), Outcome 7 Treatment‐related morbidity.
Discussion
Summary of main results
Symptomatic recurrent VTE at three months
Meta‐analyses showed no difference in the rate of symptomatic recurrent VTE at three months between subcutaneous UFH versus IV UFH or LMWH. Our analyses showed little or no statistical heterogeneity between the included studies. When comparing subcutaneous UFH versus LMWH, the subgroup analysis by VTE at randomisation showed no difference between participants that had DVT without PE and those that had DVT with/without PE. Furthermore, we observed no difference when excluding two large studies from the analysis.
Symptomatic recurrent DVT at three months
Meta‐analyses showed no difference in the rate of symptomatic recurrent DVT at three months between subcutaneous UFH versus IV UFH or LMWH.
PE at three months
Meta‐analyses showed no difference in the rate of PE between subcutaneous UFH and IV UFH nor LMWH. When comparing subcutaneous UFH versus LMWH, subgroup analysis by VTE at randomisation showed no difference between participants that had DVT without PE and those that had DVT with/without PE.
VTE‐related mortality at three months
Meta‐analyses showed no difference in the rate of VTE‐related mortality at three months between subcutaneous UFH versus IV UFH or LMWH. There were no cases of VTE‐related mortality in the four studies incorporating participants that had DVT without PE. Furthermore, we observed no difference when excluding two large studies from the analysis.
Major bleeding
Meta‐analyses showed no difference in the rate of major bleeding between subcutaneous UFH versus IV UFH or LMWH. When comparing subcutaneous UFH versus LMWH, subgroup analysis by VTE at randomisation showed no difference between participants that had DVT without PE and those that had DVT with/without PE. Furthermore, we observed no difference when excluding two large studies from the analysis.
All‐cause mortality
Meta‐analyses showed no difference in the rate of all‐cause mortality between subcutaneous UFH versus IV UFH or LMWH. When comparing subcutaneous UFH versus LMWH, subgroup analysis by VTE at randomisation showed no difference between participants that had DVT without PE and those that had DVT with/without PE.
Asymptomatic VTE at three months
None of the included studies reported any episodes of asymptomatic VTE occurring within three months of the commencement of treatment.
Treatment‐related morbidity
Minor bleeding
Meta‐analyses showed no difference in the incidence of minor bleeding between subcutaneous UFH and IV UFH or LMWH. When comparing subcutaneous UFH versus LMWH, subgroup analysis by VTE at randomisation showed no difference between participants that had DVT without PE and those that had DVT with/without PE. Furthermore, we observed no difference when excluding two large studies from the analysis.
Heparin‐induced thrombocytopenia
None of the studies comparing subcutaneous UFH versus IV UFH reported any episodes of HIT. Meta‐analyses showed no difference in the incidence of HIT between participants treated with subcutaneous UFH versus LMWH. When comparing subcutaneous UFH versus LMWH, subgroup analysis by VTE at randomisation showed no difference between participants that had DVT without PE and those that had DVT with/without PE. Furthermore, we observed no difference when excluding two large studies from the analysis.
Length of hospital stay
One three‐armed study, comparing subcutaneous UFH versus IV UFH versus LMWH, measured length of hospital stay associated with each treatment. However, the subcutaneous UFH group received treatment at home, so we could not make a comparison. The mean length of hospital stay was 5.4 ± 1.4 days in the IV UFH and 5.1 ± 1.0 days in the LWMH groups, respectively.
Quality of life
None of the included studies measured quality of life as an outcome.
Overall completeness and applicability of evidence
This review assessed whether subcutaneous UFH reduced the rate of recurrent VTE, VTE‐related mortality, major bleeding and all‐cause mortality in participants with VTE. Eight studies used IV UFH as the comparator and seven studies used LMWH, while one three‐armed trial compared all three of those treatment possibilities. We did not identify trials comparing subcutaneous UFH with other anticoagulant drugs. All trials included participants with deep vein thrombosis. Seven trials excluded people with a PE, four trials included PE participants, and the remaining trials did not clearly describe PE inclusion. With the exception of asymptomatic VTE at three months and health‐related quality of life, the included studies measured and reported all of the addressed outcomes. As all the trials had strict inclusion criteria, resulting in an overall participant population with almost identical conditions, statistical heterogeneity was logically low for all outcomes. Furthermore, studies used similar concentrations for each particular drug.
We planned subgroup analyses by first or recurrent VTE, severity, age, renal function, underlying pathology, number of daily subcutaneous heparin injections, type of dose adjustment, initial intravenous bolus heparin given versus not given, concomitant oral anticoagulant use, and timing of oral anticoagulant initiation. However, we could not perform these subgroup analyses because of the lack of participant‐level data.
Although many researchers consider DVT and PE to be manifestations of the same disorder, we elected to present them in the form of subgroups, as there is evidence of clinically significant differences between them. Most recurrent events occur at the same site as the original thrombosis (in other words, in a person presenting with a PE, a recurrent event after treatment is much more likely to be another PE). For comparisons and outcomes where subgroup analyses were possible, we did not observe any differences between studies recruiting participants that had DVT without PE and participants that had DVT with/without PE.
The American College of Chest Physicians (ACCP) clinical practice guidelines for the treatment of VTE suggest UFH as the treatment of choice for patients with severe renal failure (Kearon 2012). This is a grade 2C recommendation, based on low‐quality evidence that LMWH is associated with increased bleeding in patients with impaired renal function. Only one trial included in our review studied participants with impaired renal function (Leizorovicz 2011), comparing subcutaneous UFH versus LMWH tinzaparin in the treatment of acute DVT. The trial was terminated early due to a difference in mortality that favoured the group treated with UFH. However, rates of major bleeding and recurrent VTE were similar between the two groups.
Quality of the evidence
The risk of bias was high in 15 out of the 16 included studies, reflecting low methodological quality (Figure 2; Figure 3). This was largely due to the lack of blinding in 14 studies, which led to a high risk of performance bias. The risk of detection bias was lower: 8 of the 16 included studies reported that outcomes assessors were blinded to the treatment and adjudicated by a central independent committee. We judged seven studies to be at high risk of attrition bias for failing to account for missing data, one study to be at high risk of selection bias because of insufficient reporting of the methods used to conceal treatment allocation, and another study to be at high risk of reporting bias because it reported a significant result on an outcome that was not pre‐specified. We could not investigate publication bias because we could not assess asymmetry in a funnel plot with the limited number of studies included in the meta‐analysis.
For all outcomes in both comparisons, we downgraded the quality of the evidence to low due to the high risk of bias within each included study and also due to imprecision stemming from the small number of outcome events, as reflected by the wide confidence intervals.
Potential biases in the review process
The search was as comprehensive as possible, and we are confident that we have included all relevant studies. However, the possibility remains that we missed some relevant trials, particularly in the grey literature (for example conference proceedings). Two review authors independently performed study selection and data extraction in order to minimise bias in the review process. We performed data collection according to the process suggested by Cochrane. We also followed Cochrane processes as described by Higgins 2011 for assessing the risk of bias.
Agreements and disagreements with other studies or reviews
A meta‐analysis comparing subcutaneous heparin with intravenous heparin published in 1992 concluded that the subcutaneous mode of administration was more efficacious and less toxic than the intravenous mode of administration (Hommes 1992). Another more recent review of the literature comparing subcutaneous UFH versus subcutaneous LMWH concluded that subcutaneous UFH was an attractive alternative to LWMH for VTE, being "cheap, effective and safe" (Munro 2008).
Since the introduction of LMWH, there has been a shift away from the older and less easy‐to‐use UFH. Several other meta‐analyses of the medical literature have been published over the years, suggesting enhanced efficacy and safety profile for LMWH (Erkens 2010; Gould 1999).
Authors' conclusions
Implications for practice.
Low‐quality evidence suggests there is no difference in effectiveness between subcutaneous UFH, IV UFH and LMWH for preventing recurrent VTE at three months, VTE‐related mortality, major bleeding and all‐cause mortality. Therefore, for people with difficult venous access or people who could be treated at home, subcutaneous UFH appears to be an acceptable alternative to IV UFH. Futhermore, in patients with severe renal impairment, subcutaneous UFH can be used instead of LMWH.
Implications for research.
Further research is required to consolidate non‐monitored subcutaneous administration of UFH in the setting of VTE. Future research should target specific patient groups, e.g. patients with chronic kidney disease and elderly patients, and specific VTE states (e.g. DVT versus PE), and researchers should analyse data separately for their response to the proposed intervention. Finally, studies should evaluate cost‐effectiveness, comparing continuous infusions of UFH versus subcutaneous administration of LMWH.
What's new
| Date | Event | Description |
|---|---|---|
| 9 November 2018 | Review declared as stable | This Cochrane review has been marked stable and will only be updated should new studies be identified. |
History
Protocol first published: Issue 4, 2007 Review first published: Issue 4, 2009
| Date | Event | Description |
|---|---|---|
| 30 November 2016 | New search has been performed | Searches rerun. One new study included and nine new studies excluded. |
| 30 November 2016 | New citation required but conclusions have not changed | Searches rerun. One new study included and nine new studies excluded. Review updated using current Cochrane standards. New authors have taken over this review. |
Notes
This Cochrane review has been marked stable and will only be updated if new studies are identified as people are moving towards LMWH and newer drugs in favour of UFH.
Acknowledgements
We would like to thank Dr M Vardi, Dr E Zittan, and Dr H Bitterman for their contributions to previous versions of this review.
We would like to thank Dr K Welch for searching the Cochrane Vascular Specialised Register and the Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library (www.thecochranelibrary.com). We would also like to thank Dr M Stewart, Managing Editor of Cochrane Vascular for her assistance and advice in completing this review.
Appendices
Appendix 1. CENTRAL search strategy
| #1 | MESH DESCRIPTOR Thrombosis | 1238 |
| #2 | MESH DESCRIPTOR Thromboembolism | 899 |
| #3 | MESH DESCRIPTOR Venous Thromboembolism | 242 |
| #4 | MESH DESCRIPTOR Venous Thrombosis EXPLODE ALL TREES | 2005 |
| #5 | (thrombus* or thrombopro* or thrombotic* or thrombolic* or thromboemboli* or thrombos* or embol* or microembol*):TI,AB,KY | 17662 |
| #6 | MESH DESCRIPTOR Pulmonary Embolism EXPLODE ALL TREES | 735 |
| #7 | (PE or DVT or VTE):TI,AB,KY | 4611 |
| #8 | ((vein* or ven*) near thromb*):TI,AB,KY | 6276 |
| #9 | (blood near3 clot*):TI,AB,KY | 2696 |
| #10 | (pulmonary near3 clot*):TI,AB,KY | 5 |
| #11 | (lung near3 clot*):TI,AB,KY | 4 |
| #12 | #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 | 22923 |
| #13 | MESH DESCRIPTOR Heparin | 2794 |
| #14 | (unfractionated or UFH):TI,AB,KY | 1235 |
| #15 | *heparin*:TI,AB,KY | 8806 |
| #16 | (calciparin* or eparin* or liquaemin or panheprin or multiparin* or hepalean or CY216):TI,AB,KY | 39 |
| #17 | heparinic:TI,AB,KY | 1 |
| #18 | #13 OR #14 OR #15 OR #16 OR #17 | 8855 |
| #19 | #12 AND #18 | 4164 |
Appendix 2. Trial registries searches
Clinicaltrials.gov
97 studies for (thrombosis OR embolism) AND heparin AND randomized AND (subcutaneous OR sc OR s.c)
WHO
32 records for 9 trials found
subcutaneous OR sc OR s.c in title
and
thrombosis OR embolism in condition
and
heparin in intervention
ISRCTN
No results found for Condition: thrombosis OR embolism AND Interventions: heparin AND subcutaneous
Appendix 3. Additional study information
| Study ID | Setting (in or out patient) of SC administration | Control | Initial IV heparin bolus before SC administration? | Vitamin K antagonist | Vitamin K antagonist timing | Dose adjustment |
| Andersson 1982 | Inpatient | IV heparin | Yes | Warfarin | 1‐2 days | aPTT |
| Belcaro 1999 | Inpatient and outpatient | IV heparin + LMWH | No | No (SC extended period) | NA | Fixed dose |
| Bentley 1980 | Inpatient | IV heparin | No | Warfarin | 3 days | aPTT |
| Doyle 1987 | Inpatient | IV heparin | Yes | Warfarin | 7 days | aPTT |
| Faivre 1987 | Inpatient | LMWH | No | Not stated | NA | aPTT |
| Holm 1986 | Inpatient | LMWH | Yes (first 24 hours continuous) | Warfarin | 1 day | Anti Xa inhibitor |
| Hull 1986 | Inpatient | IV heparin | Yes | Warfarin | 6‐7 days | aPTT |
| Kearon 2006 | Inpatient and outpatient | LMWH | No | Warfarin | 1 day | Weight adjusted |
| Krähenbühl 1979 | Inpatient | IV heparin | Yes | Unclear | NA | aPTT |
| Leizorovicz 2011 | Inpatient | LMWH | Unclear | Unclear | 1‐3 days | aPTT |
| Lopaciuk 1990 | Inpatient | IV heparin | Yes | Sintron | Unclear | aPTT |
| Lopaciuk 1992 | Inpatient | LMWH | Yes | Sintron | 7 days | aPTT |
| Peternel 2002 | Inpatient | LMWH | Yes | Warfarin | 2 days | aPTT |
| Pini 1990 | Inpatient | IV heparin | No | Sintron | 3 days | Unclear |
| Prandoni 2004 | Inpatient | LMWH | Yes | Warfarin | 2 days | aPTT |
| Walker 1987 | Inpatient | IV heparin | No | Warfarin | 7 days | aPTT |
| aPTT: activated partial thromboplastin time; IV: intravenous; LMWH: low molecular weight heparin; NA: not applicable; SC: subcutaneous. | ||||||
Data and analyses
Comparison 1. Subcutaneous unfractionated heparin versus intravenous unfractionated heparin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Symptomatic recurrent VTE at 3 months | 8 | 965 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.66 [0.89, 3.10] |
| 1.1 DVT with/without PE | 8 | 965 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.66 [0.89, 3.10] |
| 2 Symptomatic recurrent DVT at 3 months | 1 | 115 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.29 [0.64, 17.06] |
| 2.1 DVT with/without PE | 1 | 115 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.29 [0.64, 17.06] |
| 3 PE at 3 months | 9 | 1161 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.73, 2.84] |
| 3.1 DVT with/without PE | 9 | 1161 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.73, 2.84] |
| 4 VTE‐related mortality at 3 months | 9 | 1168 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.20, 4.88] |
| 4.1 DVT with/without PE | 9 | 1168 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.20, 4.88] |
| 5 Major bleeding | 4 | 583 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.42, 1.97] |
| 5.1 DVT with/without PE | 4 | 583 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.42, 1.97] |
| 6 All‐cause mortality | 8 | 972 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.74 [0.67, 4.51] |
| 6.1 DVT with/without PE | 8 | 972 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.74 [0.67, 4.51] |
| 7 Treatment related morbidity ‐ minor bleeding | 5 | 779 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.33, 1.20] |
| 7.1 DVT with/without PE | 5 | 779 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.33, 1.20] |
Comparison 2. Subcutaneous unfractionated heparin versus low molecular weight heparin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Symptomatic recurrent VTE at 3 months | 5 | 2156 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.63, 1.63] |
| 1.1 DVT with/without PE | 3 | 1954 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.57, 1.56] |
| 1.2 DVT without PE | 2 | 202 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.12 [0.38, 11.84] |
| 2 Symptomatic recurrent DVT at 3 months | 3 | 1566 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.38 [0.73, 2.63] |
| 2.1 DVT with/without PE | 2 | 1420 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.65, 2.46] |
| 2.2 DVT without PE | 1 | 146 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.28 [0.25, 111.99] |
| 3 PE at 3 months | 5 | 1819 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.36, 1.96] |
| 3.1 DVT with/without PE | 2 | 1420 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.31, 2.04] |
| 3.2 DVT without PE | 3 | 399 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.14, 7.63] |
| 4 VTE‐related mortality at 3 months | 8 | 2469 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.17, 1.67] |
| 4.1 DVT with/without PE | 4 | 2016 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.17, 1.67] |
| 4.2 DVT without PE | 4 | 453 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Major bleeding | 5 | 2300 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.43, 1.20] |
| 5.1 DVT with/without PE | 3 | 1957 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.41, 1.16] |
| 5.2 DVT without PE | 2 | 343 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.13 [0.13, 78.00] |
| 6 All‐cause mortality | 7 | 2272 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.50, 1.07] |
| 6.1 DVT with/without PE | 4 | 2016 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.48, 1.05] |
| 6.2 DVT without PE | 3 | 256 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.71 [0.22, 13.26] |
| 7 Treatment related morbidity ‐ minor bleeding | 5 | 2300 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.71, 1.37] |
| 7.1 DVT with/without PE | 3 | 1957 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.69, 1.43] |
| 7.2 DVT without PE | 2 | 343 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.44, 2.05] |
| 8 Treatment related morbidity ‐ HIT | 3 | 1954 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.52 [0.25, 9.14] |
| 8.1 DVT with/without PE | 3 | 1954 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.52 [0.25, 9.14] |
Comparison 3. Subcutaneous unfractionated heparin versus low molecular weight heparin (excluding large studies).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Symptomatic recurrent VTE at 3 months | 3 | 736 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.29, 2.16] |
| 2 Symptomatic recurrent DVT at 3 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 PE at 3 months | 3 | 399 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.14, 7.63] |
| 4 VTE‐related mortality at 3 months | 6 | 1049 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.06, 16.13] |
| 5 Major bleeding | 3 | 880 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.41, 2.09] |
| 6 All‐cause mortality | 5 | 852 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.32, 1.03] |
| 7 Treatment‐related morbidity | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Minor bleeding | 3 | 880 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.60, 1.30] |
| 7.2 Heparin‐induced thrombocytopenia | 1 | 534 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.05 [0.19, 22.78] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Andersson 1982.
| Methods |
Study design: open randomised controlled trial Duration of intervention: at least 5 days to INR target Duration of follow‐up: acute phase only Run‐in period: NA Intention‐to‐treat analysis: no Language of publication: English |
|
| Participants |
Who participated: people with acute DVT Country: Sweden Number of study centres: 3 Setting: hospital Number: 141 (SC UFH group 72; IV UFH group 69) Age mean (range): SC UFH group 64 years (23 to 88); IV UFH group 64 years (20 to 88) Sex (M/F): SC UFH group 47/25; IV UFH group 41/28 Inclusion criteria: clinical signs of acute DVT Exclusion criteria: not stated Diagnostic criteria: phlebography, venous occlusion plethysmography, thermography |
|
| Interventions |
Intervention (route, total dose/day, frequency): IV UFH bolus dose (sodium heparin) (5000 IU/mL) followed by SC UFH (25000 IU/mL) twice daily aPTT adjusted + warfarin Control (route, total dose/day, frequency): IV UFH bolus dose (sodium heparin) (5000 IU/mL) followed by continuous IV UFH aPTT adjusted + warfarin Treatment before study: NA Titration period: NA |
|
| Outcomes |
Primary outcome: therapeutic efficacy with repeat imaging Secondary outcomes: bleeding, pulmonary emboli, aPTT, heparin dose |
|
| Notes | Stated aim of the study: assess therapeutic effect and number of complications in the two groups | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States random but no description of randomisation method provided |
| Allocation concealment (selection bias) | Unclear risk | No description of allocation concealment provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No description of blinding provided
Different methods of administration meant adequate blinding was most likely not achieved
"Intravenous infusions were administered by mobile infusion pumps" "Subcutaneous injections were given into the anterior abdominal wall using a 23 gauge needle" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk |
Outcomes requiring blinding Recurrent VTE at 3 months: data used – no description of blinding outcome assessors Recurrent DVT at 3 months: NA PE – excluding PE found at autopsy: data used – no description of blinding outcome assessors Incidence of heparin‐induced thrombocytopenia: NA Incidence of asymptomatic recurrent VTE at 3 months: NA Quality of life: NA Outcomes not requiring blinding Major bleeding: data not used ‐ not meeting ISTH definition Minor bleeding: data not used ‐ not meeting definition of minor bleeding VTE‐related mortality: data used All‐cause mortality: data used |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 40 participants (out of 141) withdrawn from the study "due to inabilities to achieve these investigations during weekends and holidays, technical reasons or because some patients refused further investigations" 19 participants withdrawn from the subcutaneous group and 21 participants withdrawn from the intravenous group. However, the number of participants withdrawn for each reason is not presented. No deaths were reported as occurring during the course of the study |
| Selective reporting (reporting bias) | Low risk | No evidence of selective outcome reporting |
| Other bias | Low risk | No evidence of other biases |
Belcaro 1999.
| Methods |
Study design: open randomised aPTT‐controlled trial Duration of intervention: 3 months for SC heparin; until INR target in LMWH and IV heparin Duration of follow‐up: 3 months Run‐in period: NA Intention‐to‐treat analysis: no Language of publication: English |
|
| Participants |
Who participated: people with acute DVT Country: Italy (Chieti and Pescara), UK Number of study centres: 3 Setting: SC UFH ‐ outpatient; LMWH ‐ out/inpatient; IV UFH ‐ inpatient Number: 325 randomised, 294 completed the study (SC UFH 99; LMWH 98; IV UFH 97) Age (mean ± SD): SC UFH 54 ± 9 years; LMWH 54 ± 11 years; IV UFH 53 ± 10 years Sex (M/F): SC UFH 52/47; LMWH 54/44; IV UFH 57/40 Inclusion criteria: acute proximal DVT diagnosed by colour duplex ultrasonography Exclusion criteria: 2 or more previous episodes of DVT or PE, current active bleeding, active ulcers, bleeding or coagulation disorder, concurrent PE, treatment for DVT with standard heparin > 48 h, home treatment not possible, neoplasia requiring surgery or chemotherapy in three months, likelihood of low compliance, pregnancy, platelets < 100,000 × 109/L Diagnostic criteria: colour duplex |
|
| Interventions |
Intervention (route, total dose/day, frequency): SC heparin (12,500 IU twice daily), fixed dose (no oral anticoagulation) administered exclusively at home Control (route, total dose/day, frequency): group 1: LMWH (100 Axa IU/kg twice daily) administered primarily at home + warfarin; group 2: IV bolus (5000 IU) followed by continuous IV UFH aPTT adjusted + warfarin Treatment before study: NA |
|
| Outcomes | Outcomes not specified as primary or secondary Outcomes: symptomatic or asymptomatic recurrent DVT or DVT extension at 3 months, bleeding during the administration of the study drug, PE, length of stay in hospital, number of participants treated directly at home without admission |
|
| Notes | Stated aim of the study: to compare intravenous standard heparin (in hospital) with oral anticoagulant treatment to LMWH and oral anticoagulant treatment administrated primarily at home, to SC heparin administered at home | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States random but no description of randomisation method provided |
| Allocation concealment (selection bias) | Unclear risk | No description of allocation concealment provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Use of open study design |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk |
Outcomes requiring blinding Recurrent VTE at 3 months: data not used – results for symptomatic, asymptomatic and extended VTE not presented separately Recurrent DVT at 3 months: data not used – see recurrent VTE at 3 months PE – excluding PE found at autopsy: data used – "All reported outcome events were reviewed by a central panel including all monitors and, by form evaluation, by five external reviewers unaware of the treatments assigned and the patient's identity" Incidence of heparin‐induced thrombocytopenia: NA Incidence of asymptomatic recurrent VTE at 3 months: data not used – see recurrent VTE at 3 months Quality of life: NA Outcomes not requiring blinding Major bleeding: data used Minor bleeding: data used VTE‐related mortality: data used All‐cause mortality: data not used – unclear how many deaths occurred in each group |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 31 (out of 325) participants were withdrawn from the study Although the paper states that six participants died during the course of the study – all other withdrawals are unaccounted for |
| Selective reporting (reporting bias) | Low risk | No evidence of selective outcome reporting |
| Other bias | Unclear risk | Different groups were treated in different locations with groups 1 and 2 receiving different treatments in hospital and group 3 receiving treatment at home |
Bentley 1980.
| Methods |
Study design: open randomised controlled trial Duration of intervention: 7 days to INR target Duration of follow‐up: 7 days Run‐in period: NA Intention‐to‐treat analysis: no Language of publication: English |
|
| Participants |
Who participated: people with acute DVT Country: UK Number of study centres: 1 Setting: inpatient Age (mean ± SD): SC UFH group 60.49 ± 14.32 years; IV UFH group 58.18 ± 12.66 years Sex (M/F): not specified but describes "well matched for age, sex ..." Inclusion criteria: acute calf DVT diagnosed by venography Exclusion criteria: contra‐indication to heparin, thrombus extension < 5 cm Diagnostic criteria: venography |
|
| Interventions |
Intervention (route, total dose/day, frequency): SC UFH (calcium heparin), initial dose 40,000 IU/day followed by aPTT‐adjusted dose twice daily + warfarin Control (route, total dose/day, frequency): IV UFH (sodium heparin), initial dose 40,000 IU/day followed by aPTT‐adjusted continuous dose + warfarin Treatment before study: NA |
|
| Outcomes | Outcomes not specified as primary or secondary Outcomes: cutaneous haematoma, macroscopic haematuria, major bleeding, DVT extension, new or extended PE, aPTT, heparin level |
|
| Notes | Stated aim of the study: to compare the safety and efficacy of IV and SC heparin | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States random but no description of randomisation method provided |
| Allocation concealment (selection bias) | Low risk | "Patients were randomised using sealed envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No description of blinding provided Different methods of heparin administration – intravenous compared to subcutaneous – probably prevented adequate blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk |
Outcomes requiring blinding Recurrent VTE at 3 months: data used – no description of blinding outcome assessors Recurrent DVT at 3 months: NA PE – excluding PE found at autopsy: data used – no description of blinding outcome assessors Incidence of heparin‐induced thrombocytopenia: NA Incidence of asymptomatic recurrent VTE at 3 months: NA Quality of life: NA Outcomes not requiring blinding Major bleeding: data not used ‐ not meeting ISTH definition Minor bleeding: data not used ‐ not meeting definition of minor bleeding VTE‐related mortality: data used All‐cause mortality: data used Description of blinding outcome assessors for PE |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The study states 3 participants (out of 100) were withdrawn from the study – but from which groups is unclear Later in the paper it states that the heparin treatment of 6 participants was halted (2 in SC group and 4 in IV group) However, all participants are included in the final analysis of venographic results without further explanation No deaths were reported as occurring during the course of the study |
| Selective reporting (reporting bias) | Low risk | Study states estimations of platelet count; haemoglobin and hematocrit were made at the beginning, middle and end of the trial period – however – results are only presented for participants with minor bleeds. Nevertheless these were not outcomes of our review and therefore the study was judged to be at low risk of reporting bias. |
| Other bias | Low risk | No evidence of other biases |
Doyle 1987.
| Methods |
Study design: open randomised controlled trial Duration of intervention: 10 days Duration of follow‐up: 12 months Run‐in period: NA Intention‐to‐treat analysis: no Language of publication: English |
|
| Participants |
Who participated: people with acute DVT Country: Canada Number of study centres: 1 Setting: inpatients Number: 103 SC UFH 51; IV UFH 52 Age mean (range): SC UFH 66.6 years (31 to 96); IV UFH 64.6 (25 to 94) years Sex (M/F): SC UFH 23/28; IV UFH 32/20 Inclusion criteria: acute proximal or calf DVT diagnosed by venography Exclusion criteria: clinically suspected PE, active peptic ulceration, bleeding disorder, no informed consent Diagnostic criteria: venography |
|
| Interventions |
Intervention (route, total dose/day, frequency): SC UFH (calcium heparin), initial dose 15,000 IU, then twice daily, aPTT adjusted + warfarin Control (route, total dose/day, frequency): IV UFH (calcium heparin), initial dose 5,000 IU, then continuous, aPTT adjusted + warfarin Treatment before study: NA |
|
| Outcomes |
Primary outcome: PE Secondary outcomes: other lung scan abnormalities, bleeding, leg symptoms, death |
|
| Notes | Stated aim of the study: to determine the efficacy and safety of adjusted SC calcium heparin compared with continuous IV calcium heparin as the initial treatment for acute DVT | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States random but no description of randomisation method provided |
| Allocation concealment (selection bias) | Low risk | use of "sealed envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Use of "open" trial design |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk |
Outcomes requiring blinding Recurrent VTE at 3 months: data used ‐ the scintigrams were interpreted in random order by 2 experienced experimental observers who were blinded to the method of treatment Recurrent DVT at 3 months: NA PE – excluding PE found at autopsy: data used – see recurrent VTE at 3 months Incidence of heparin‐induced thrombocytopenia: NA Incidence of asymptomatic recurrent VTE at 3 months: NA Quality of life: NA Outcomes not requiring blinding Major bleeding: data used Minor bleeding: data used VTE‐related mortality: data used All‐cause mortality: data used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 7 participants (out of 103) were withdrawn from the study – 4 in SC group; 3 in the IV group Reasons for withdrawal were clearly presented: "2 had major bleeding; 1 refused the scan; 1 required surgery and 3 could not have the scans for technical reasons" During follow‐up 10 participants died – none from PE |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No evidence of other biases |
Faivre 1987.
| Methods |
Study design: randomised controlled trial Duration of intervention: 10 days Duration of follow‐up: 10 days Run‐in period: NA Intention‐to‐treat analysis: no Language of publication: French |
|
| Participants |
Who participated: people with acute DVT and PE Country: France Number of study centres: 1 Setting: inpatient Number: 68 SC UFH 35; SC LMWH 33 (number evaluated: 59 SC UFH 29; SC LMWH 30) Age (mean ± SD) : SC UFH 63.6 ± 16.2 years; SC LMWH 65.6 ± 14.8 years Sex (M/F): 39/29 Inclusion criteria: acute DVT or PE diagnosed with phlebography or perfusion‐ventilation scan Exclusion criteria: over 2 weeks of symptoms, massive PE Diagnostic criteria: phlebography and lung scan |
|
| Interventions |
Intervention (route, total dose/day, frequency): SC UFH (calcium heparin) 500 IU/kg/day in form of twice daily injections, aPTT adjusted Control (route, total dose/day, frequency): SC LMWH 750 anti‐Xa/kg/day in form of twice daily injections Treatment before study: NA |
|
| Outcomes | Outcomes not specified as primary or secondary Outcomes: DVT extension, bleeding |
|
| Notes | Stated aim of the study: to assess the efficacy and safety of CY222 for the treatment of DVT compared with SC heparin | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States random but no description of randomisation method provided |
| Allocation concealment (selection bias) | Unclear risk | No description of allocation concealment provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No description of blinding provided and although both treatments were administered subcutaneously, the authors state that in the CY222 group participants received a fixed dose of (750 U anti‐Xa IC/kg/24 h) whist in the unfractionated heparin group dosage was adjusted to maintain partial thromboplastin time, making it unlikely participant and personnel were adequately blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk |
Outcomes requiring blinding Recurrent VTE at 3 months: NA Recurrent DVT at 3 months: NA PE – excluding PE found at autopsy: NA Incidence of heparin‐induced thrombocytopenia: NA Incidence of asymptomatic recurrent VTE at 3 months: NA Quality of life: NA Outcomes not requiring blinding Major bleeding: data not used ‐ not meeting ISTH definition Minor bleeding: NA VTE‐related mortality: data used All‐cause mortality: data used |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 9 participants (out of 68) were withdrawn from the study In the CY22 group 3 participants withdrew (cardiac insufficiency, migration of Greenfield filter) In the SC group 6 participants withdrew (3 retroperitoneal haematoma; 3 recurrent PE) |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No evidence of other biases |
Holm 1986.
| Methods |
Study design: double‐blind randomised controlled trial Duration of intervention: 7 days Duration of follow‐up: 7 days Run‐in period: NA Intention‐to‐treat analysis: no Language of publication: English |
|
| Participants |
Who participated: people with acute DVT Country: Norway Number of study centres: 1 Setting: inpatients Number: 56 (SC UFH 27; SC LMWH 29) Age (mean ± SD): SC UFH 60 ± 15.8 years; SC LMWH 61 ± 15.3 years Sex (M/F): 33/23 (SC UFH 17/10; SC LMWH 16/13) Inclusion criteria: acute DVT below the groin diagnosed by phlebography, with symptoms for fewer than 14 days Exclusion criteria: PE, pregnancy, history of cerebral haemorrhage, surgery in previous 6 days, diastolic BP > 115 mmHg, retinal haemorrhage, impaired renal function, impaired PT Diagnostic criteria: phlebography |
|
| Interventions |
Intervention (route, total dose/day, frequency): IV continuous infusion UFH for 24 hours, followed by SC UFH 10,000‐15,000 IU twice daily, anti‐Xa adjusted + warfarin Control (route, total dose/day, frequency): IV continuous infusion UFH for 24 hours, followed by SC LMWH 5000‐7500 IU twice daily, anti‐Xa adjusted + warfarin Treatment before study: NA |
|
| Outcomes | Outcomes not specified as primary or secondary Outcomes: DVT extension, new PE, bleeding, leg pain, death, haemoglobin, platelets |
|
| Notes | Stated aim of the study: to compare subcutaneous heparin and LMWH for the treatment of DVT | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States random but no description of randomisation method provided |
| Allocation concealment (selection bias) | High risk | "the vials [of low molecular weight or unfractionated heparin] had been randomised in advance and numbered consecutively, the number of patient admission determining the number of vial used" It is possible personnel had access to the order of the vials |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Paper states only "double blind" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk |
Outcomes requiring blinding Recurrent VTE at 3 months: data used – no description of blinding outcome assessors Recurrent DVT at 3 months: NA PE – excluding PE found at autopsy: data used – no description of blinding of outcome assessors Incidence of heparin‐induced thrombocytopenia: NA Incidence of asymptomatic recurrent VTE at 3 months: NA Quality of life: NA Outcomes not requiring blinding Major bleeding: data not used ‐ not meeting ISTH definition Minor bleeding: data not used ‐ not meeting definition of minor bleeding VTE‐related mortality: data used All‐cause mortality: data used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 participants (out of 56) were withdrawn from the trial – 2 from the LMWH group; 1 from UFH group Reasons for withdrawals are clearly presented: Reversal of DVT diagnosis; incorrect injection of ordinary heparin and suspected cerebral haemorrhage No deaths were reported as occurring during the course of the study |
| Selective reporting (reporting bias) | High risk | Study presents results for leg pain "leg pain disappeared somewhat quicker in patients receiving LH"; however, pain measures were not presented as an outcome in the Methods section In addition, the paper states that "there was no drop in platelet count or haemoglobin concentration"; however, how these parameters were measured is also unreported in the Methods section |
| Other bias | Low risk | No significant evidence of other biases; however, one patient was included twice (once in each group) and one patient transferred to the UFH group and so was not included in the final analysis This could potentially be considered an as‐treated analysis, and as such it may have potentially introduced selection bias; however, as only one patient was affected the potential risk of bias was considered small and was deemed unlikely to have significantly affected the results of the study |
Hull 1986.
| Methods |
Study design: double‐blind randomised controlled trial Duration of intervention: 10 days Duration of follow‐up: 3 months Run‐in period: NA Intention‐to‐treat analysis: no Language of publication: English |
|
| Participants |
Who participated: people with acute DVT Country: Canada Number of study centres: 1 Setting: inpatients Number: 115 Age (< 60 years / > 60 years): SC UFH 10/4; 7 IV UFH 11/47 Sex (M/F): SC UFH 27/30; IV UFH 28/30 Inclusion criteria: acute proximal (± calf) DVT diagnosed by venography Exclusion criteria: active bleeding, contraindication to heparin, already on heparin, no outpatient follow‐up available Diagnostic criteria: venography |
|
| Interventions |
Intervention (route, total dose/day, frequency): IV UFH 5000 IU bolus followed by SC UFH 15000 twice daily, aPTT adjusted + warfarin Control (route, total dose/day, frequency): IV UFH 5000 IU bolus followed by continuous IV UFH aPTT adjusted + warfarin Treatment before study: NA |
|
| Outcomes | Outcomes not specified as primary or secondary Outcomes: recurrent DVT, PE, bleeding, aPTT, death |
|
| Notes | Stated aim of the study: to compare continuous IV heparin to intermittent SC heparin for the initial treatment of proximal DVT | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A computer generated prescribed randomised arrangement was used to assign patients" |
| Allocation concealment (selection bias) | Unclear risk | No description of allocation concealment provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "those to receive continuous IV heparin were ... started on a continuous IV infusion … and placebo SC injections" "those to receive SC heparin were given SC heparin injections … and IV placebo infusions" "to prevent un‐blinding … masked pre‐labelled syringes and IV packs were used" "to prevent un‐blinding on the basis of knowledge of heparin clearance … all dose adjustments and anticoagulant monitoring … were [done at a] daily mid interval measurement" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk |
Outcomes requiring blinding Recurrent VTE at 3 months: data used ‐ "[d]iagnostic tests were interpreted independently and without knowledge of the results of the other tests or the patient's clinical state or the treatment group to which the patient had been assigned" Recurrent DVT at 3 months: data used – see recurrent VTE at 3 months PE – excluding PE found at autopsy: data used – See recurrent VTE at 3 months Incidence of heparin‐induced thrombocytopenia: NA Incidence of asymptomatic recurrent VTE at 3 months: NA Quality of life: NA Outcomes not requiring blinding Major bleeding: data used Minor bleeding: data used VTE‐related mortality: data used All‐cause mortality: data used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 0 participants (out of 115) were withdrawn from the study "[A]ll patients were followed during primary therapy and for three months during long term therapy and none were lost to follow up" 6 participants died in the subcutaneous group, 2 from VTE‐related causes; 3 participants died in the intravenous group, none from VTE‐related causes |
| Selective reporting (reporting bias) | Low risk | No evidence of selective outcome reporting |
| Other bias | Low risk | No evidence of other biases |
Kearon 2006.
| Methods |
Study design: open‐label, adjudicator‐blinded randomised controlled trial Duration of intervention: 5 days to INR target Duration of follow‐up: 3 months Run‐in period: NA Intention‐to‐treat analysis: no Language of publication: English |
|
| Participants |
Who participated: people with acute DVT or PE Country: Canada and New Zealand Number of study centres: 6 Setting: inpatients and outpatients Number: 708 (SC UFH 355; SC LMWH 353) Age (mean ± SD): SC UFH 60 ± 17 years; SC LMWH 60 ± 16 years Sex (M/F): SC UFH 182/173; SC LMWH 206/147 Inclusion criteria: 18 years or older with newly diagnosed DVT of the legs or PE diagnosed by compression ultrasonography or by venography, and by a high probability ventilation‐perfusion lung scan, by non diagnostic findings on lung scan accompanied by diagnostic findings for DVT, or by computed tomographic angiography Exclusion criteria: contraindication to subcutaneous therapy such as shock or major surgery in the past 48 hours, active bleeding, a life expectancy of less than 3 months, previous acute treatment for venous thromboembolism for more than 48 hours, receiving long‐term anticoagulant therapy, contraindication to heparin or to radiographic contrast, creatinine level of greater than 200 µmol/L (2.3 mg/dL), pregnant, enrolled in a competing study, unable to have follow‐up assessments because of geographic inaccessibility Diagnostic criteria: compression ultrasonography or venography, and high probability ventilation‐perfusion lung scan, non‐diagnostic findings on lung scan accompanied by diagnostic findings for deep vein thrombosis, or computed tomographic angiography Type of VTE: 571 DVT/174 PE |
|
| Interventions |
Intervention (route, total dose/day, frequency): unmonitored SC UFH, initial 333 IU/kg followed by 250 IU/kg twice daily + warfarin Control (route, total dose/day, frequency): SC LMWH 100 IU/kg twice daily + warfarin Treatment before study: NA |
|
| Outcomes |
Primary outcomes: the primary analysis for efficacy was the absolute difference in the proportion of eligible participants who had recurrent venous thromboembolism at 3 months. The primary analysis for safety was the absolute difference in the proportion of participants who received at least 1 dose of study drug who had an episode of major bleeding within 10 days of randomisation Secondary outcomes: recurrent VTE at 10 days, major or minor bleeding, death, aPTT |
|
| Notes | Stated aim of the study: to determine if fixed‐dose, weight‐adjusted, subcutaneous unfractionated heparin is as effective and safe as low molecular‐weight heparin for treatment of venous thromboembolism | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was computer generated with block sizes of 2 or 4" |
| Allocation concealment (selection bias) | Low risk | "[C]linical centres telephone an automated centralised system" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Use of "open‐label" study design |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk |
Outcomes requiring blinding Recurrent VTE at 3 months: data used ‐ all outcome events and deaths were classified by a central adjudication committee whose members were unaware of treatment assignment Recurrent DVT at 3 months: data used – see recurrent VTE at 3 months PE – excluding PE found at autopsy: data used – see recurrent VTE at 3 months Incidence of heparin‐induced thrombocytopenia: data used – see recurrent VTE at 3 months Incidence of asymptomatic recurrent VTE at 3 months: NA Quality of life: NA Outcomes not requiring blinding Major bleeding: data used Minor bleeding: data used VTE‐related mortality: data used All‐cause mortality: data used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 11 participants (out of 708) were withdrawn from the study – 10 in the UFH group; 1 in the LMWH group Reasons for withdrawals were clearly reported and the asymmetry in the withdrawals did not appear to be caused by the different treatment methods: UFH – 4 participants were receiving long‐term anticoagulant therapy; 3 diagnosis of VTE were reversed; 1 randomisation error; 1 withdrawal of consent and 1 withdrawal by physician LMWH – 1 withdrawal of consent During follow‐up there were 18 deaths in the UFH group (1 from bleeding) and 22 deaths in the LMWH group (3 from PE and 1 from bleeding) |
| Selective reporting (reporting bias) | Low risk | Protocol available ‐ no evidence of selective reporting |
| Other bias | Low risk | No significant evidence of other biases ‐ 5 participants who did not receive the study drug were not included in the final analysis of either safety or efficacy – something which could be considered an 'as‐treated' analysis that potentially introduced selection bias; however, the number of participants affected was considered too small to have had a significant impact on the results |
Krähenbühl 1979.
| Methods |
Study design: randomised controlled trial Duration of intervention: 7 days Duration of follow‐up: 6 weeks Run‐in period: NA Intention‐to‐treat analysis: no Language of publication: French |
|
| Participants |
Who participated: people with acute DVT of the lower limb Country: Switzerland Number of study centres: 1 Setting: inpatients Number: 48 (SC UFH 23; IV UFH 25) Age: not stated Sex (M/F): SC UFH 18/5; IV UFH 13/12) Inclusion criteria: DVT of lower limbs diagnosed by phlebography or colour duplex US, with symptoms < 1 week Exclusion criteria: none stated Diagnostic criteria: phlebography or colour duplex ultrasound |
|
| Interventions |
Intervention (route, total dose/day, frequency): IV bolus UFH (sodium heparin) 5000 IU, followed by SC UFH 15,000U/day twice daily (aPTT adjusted) Control (route, total dose/day, frequency): IV bolus UFH (sodium heparin) 5000 IU followed by IV continuous UFH (aPTT adjusted) Treatment before study: NA |
|
| Outcomes | Outcomes not specified as primary or secondary Primary Outcomes: symptoms duration, DVT extension, PE, aPTT |
|
| Notes | Stated aim of the study: to compare subcutaneous heparin and intravenous heparin for the treatment of deep vein thrombosis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Drawing of lots" |
| Allocation concealment (selection bias) | Unclear risk | No description of allocation concealment provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No description of blinding provided Different methods of heparin administration – intravenous compared to subcutaneous – probably prevented adequate blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk |
Outcomes requiring blinding Recurrent VTE at 3 months: data used – no description of blinding outcome assessors Recurrent DVT at 3 months: NA PE – excluding PE found at autopsy: data used – no description of blinding outcome assessors Incidence of heparin‐induced thrombocytopenia: NA Incidence of asymptomatic recurrent VTE at 3 months: NA Quality of life: NA Outcomes not requiring blinding Major bleeding: data not used ‐ not meeting ISTH definition Minor bleeding: data not used ‐ not meeting definition of minor bleeding VTE‐related mortality: data used All‐cause mortality: data used |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Only 24 participants (out of 48) received a second phlebograph: reasons for this loss are not clearly presented in the article |
| Selective reporting (reporting bias) | Low risk | No evidence of selective outcome reporting |
| Other bias | Low risk | No evidence of other biases |
Leizorovicz 2011.
| Methods |
Study design: international, multicentre, centrally randomised, open, parallel‐group study with blinded adjudication Duration of intervention: 90 ± 5 days Duration of follow‐up: NA Run‐in period: NA Intention‐to‐treat analysis: no Language of publication: English |
|
| Participants |
Who participated: people aged ≥ 75 years with creatinine clearance (CrCl) ≤ 60 mL/min or people aged ≥ 70 years with a CrCl of ≤ 30 mL/min (calculated using the Cockcroft–Gault formula) and with an acute, objectively confirmed (by compression ultrasonography or venography) lower limb DVT which required treatment Countries: Belgium; France; Germany; Spain; Serbia; Croatia; Romania and Poland Number of study centres: 8 Setting: inpatients at the time of randomisation; however, participants could be followed on a daily basis in or out of hospital after this point Number: 539 Age (< 60 years/ > 60 years): SC UFH 0/270; tinzaparin 0/269 Sex (M/F): SC UFH 102/168; tinzaparin 92/177 Inclusion criteria: objectively confirmed symptomatic proximal or distal DVT (or objectively confirmed asymptomatic DVT if proximal and associated with a PE) and provision of written informed consent Exclusion criteria: received treatment doses of heparins or thrombolytic agents within the previous 4 weeks (excluding the last 36 h) prior to randomisation; received oral anticoagulation within the preceding week; planned use of high doses of acetylsalicylic acid (ASA) (> 300 mg/day) or a non‐steroidal anti‐inflammatory drug (NSAID); requirement for thrombolytic therapy; end stage renal disease requiring dialysis; hepatic insufficiency (INR ≥ 1.5); bacterial endocarditis; planned epidural or spinal anaesthesia; planned surgery or recent surgery (within 2 weeks); thrombocytopenia (< 100 x 109/L); severe uncontrolled hypertension, overt bleeding and recent stroke Diagnostic criteria: compression ultrasonography or venography |
|
| Interventions |
Intervention (route, total dose/day, frequency): tinzaparin (SC, 175 IU/kg, once daily) Control (route, total dose/day, frequency): UFH (IV, 50 IU/kg bolus followed by SC, 400–600 IU/kg, twice daily which was then adjusted by APTT according to local practice) Treatment before study: NA |
|
| Outcomes |
Primary outcomes: clinically relevant bleedings (CRBs) by day 90 ± 5 Secondary outcomes: occurrence of symptomatic recurrent VTE prior to day 90 ± 5 and major and minor bleedings prior to day 90 ± 5 Tertiary outcomes: CRBs during the SC treatment phase, death from any cause prior to day 90 ± 5 and heparin‐induced thrombocytopenia |
|
| Notes | Stated aim of the study: to compare the safety profile of full weight‐based unadjusted‐dose tinzaparin (Innohep, LEO Pharma, Ballerup, Denmark) vs activated partial thromboplastin time (APTT)‐adjusted UFH as initial treatment of elderly participants with impaired renal function and acute DVT | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Treatment assignment was pre‐planned according to a computer generated randomisation sequence" |
| Allocation concealment (selection bias) | Low risk | "Central telephone randomisation" However, the paper also states: "No allocation concealment mechanism was attempted as the study was open. But care was taken to ensure that outcome assessors and data analysts were kept blinded to the allocation" This statement appears to be in contradiction with the description of central telephone randomisation and so it was assumed that in this context 'allocation concealment' referred to the blinding of participants and personnel, as an open study design does not preclude adequate allocation concealment ‐ this assumption was also more consistent with the reference to the blinding of outcome assessors |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Use of an "open" study design |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk |
Outcomes requiring blinding Recurrent VTE at 3 months: data used ‐ care was taken to ensure that outcome assessors and data analysts were kept blinded to the allocation Recurrent DVT at 3 months: NA PE – excluding PE found at autopsy: data used – see recurrent VTE at 3 months Incidence of heparin‐induced thrombocytopenia: data used – see recurrent VTE at 3 months Incidence of asymptomatic recurrent VTE at 3 months: NA Quality of life: NA Outcomes not requiring blinding Major bleeding: data used Minor bleeding: data used VTE‐related mortality: data used All‐cause mortality: data used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5 participants (out of 539) were withdrawn from the study for reasons that were clearly presented: 2 from the tinzaparin group as "no treatment [was] taken" 3 from the unfractionated heparin group 2 because of a withdrawal of consent and 1 because "no treatment [was] taken" During the course of the study 48 participants died: 31 participants from the tinzaparin group and 17 from the unfractionated heparin group The large imbalance in mortality between the treatment groups has been addressed by the authors and appears to have been caused by an increased prevalence of specific risk factors in the tinzaparin group including presence of infectious disease; ongoing malignancy; cardiac insufficiency; stratum of renal impairment and leg paralysis, which all correlated significantly with mortality Only 4 deaths could be directly attributed to the heparin treatment 3 in the tinzaparin group – 2 from bleeding and 1 from pulmonary embolism 1 in the unfractionated heparin group also from pulmonary embolism |
| Selective reporting (reporting bias) | Low risk | No evidence of selective outcome reporting |
| Other bias | Low risk | No significant evidence of other biases ‐ 3 participants transferred from the unfractionated heparin to the tinzaparin group and were included in the tinzaparin group for the analysis of adverse effects – something which constitutes an 'as treated' analysis and as such potentially introduced selection bias; however, the number of participants affected was considered too small to significantly affect the results |
Lopaciuk 1990.
| Methods |
Study design: open randomised controlled trial Duration of intervention: 7 days Duration of follow‐up: 3 months Run‐in period: NA Intention‐to‐treat analysis: no Language of publication: Polish |
|
| Participants |
Who participated: people with acute proximal or calf DVT (with or without PE) Country: Poland Number of study centres: 5 Setting: inpatients Number: 94 (SC UFH 48; IV UFH 46) Age (mean ± SD): SC UFH 53.6 ± 13.1 years; IV UFH 50.5 ± 16.9 years Sex (M/F): SC UFH 23/25; IV UFH 24/22 Inclusion criteria: calf or proximal DVT diagnosed by phlebography, age 20 to 79 years Exclusion criteria: PE necessitating thrombolysis, gastric or duodenal ulcer Diagnostic criteria: phlebography Type of VTE: DVT |
|
| Interventions |
Intervention (route, total dose/day, frequency): bolus IV UFH (sodium heparin) 5000 IU, followed by SC UFH 500 IU/kg/day twice daily, aPTT adjusted + sintron (after 7 days) Control (route, total dose/day, frequency): bolus IV UFH (sodium heparin) 5000 IU, followed by continuous IV UFH aPTT adjusted + sintron (after 7 days) Treatment before study: NA |
|
| Outcomes | Outcomes not specified as primary or secondary Outcomes: DVT extension, aPTT, platelets, PE, bleeding, death |
|
| Notes | Stated aim of the study: to compare efficacy and safety of SC heparin versus IV heparin for DVT | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States random but no description of randomisation method provided |
| Allocation concealment (selection bias) | Low risk | Use of "sealed envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No description of blinding provided Different methods of heparin administration – intravenous compared to subcutaneous – probably prevented adequate blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk |
Outcomes requiring blinding Recurrent VTE at 3 months: data used – no description of blinding outcome assessors Recurrent DVT at 3 months: NA PE – excluding PE found at autopsy: data used – no description of blinding outcome assessors Incidence of heparin‐induced thrombocytopenia: NA Incidence of asymptomatic recurrent VTE at 3 months: NA Quality of life: NA Outcomes not requiring blinding Major bleeding: data used Minor bleeding: data used VTE‐related mortality: data used All‐cause mortality: data used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 participants (out of 94) were withdrawn from the study Reasons for withdrawals are clearly presented: Intravenous group – 1 patient died following a pulmonary embolism Subcutaneous group – 1 patient was withdrawn because of bleeding Inclusion of these participants into calculations does not change the results and they participants are correctly included in the analysis of bleeding and thrombotic complications |
| Selective reporting (reporting bias) | Low risk | No evidence of selective outcome reporting |
| Other bias | Low risk | No evidence of other biases |
Lopaciuk 1992.
| Methods |
Study design: open, stratified randomised controlled trial with blind evaluation of phlebographic results Duration of intervention: 10 days Duration of follow‐up: 3 months Run‐in period: NA Intention‐to‐treat analysis: no Language of publication: English |
|
| Participants |
Who participated: people with acute proximal or calf DVT Country: Poland Number of study centres: 6 Setting: inpatients Number: 149 (SC UFH 75 (3 excluded from analysis); SC LMWH 74) Age (mean ± SD): SC UFH 47.8 ±15.4 years; SC LMWH 49.1 ± 15.4 years Sex (M/F): SC UFH 42/30; SC LMWH 39/35 Inclusion criteria: calf or proximal DVT diagnosed by phlebography, symptoms shorter than 10 days Exclusion criteria: clinically suspected PE, phlegmasia caerulea dolens, treatment with anticoagulation prior to enrolment, VTE in previous 2 years, surgery or trauma in recent 3 days, contraindication to heparin, pregnancy, ATIII deficiency Diagnostic criteria: phlebography (blind evaluation of phlebographic results) |
|
| Interventions |
Intervention (route, total dose/day, frequency): bolus IV UFH 5000 IU, followed by SC UFH 250 IU/kg twice daily, aPTT adjusted + sintron Control (route, total dose/day, frequency): SC LMWH 225 IU/kg twice daily, fixed dose + sintron Treatment before study: NA |
|
| Outcomes | Outcomes not specified as primary or secondary Outcomes: DVT extension, recurrent DVT, PE, bleeding, death |
|
| Notes | Stated aim of the study: to determine the efficacy and safety of subcutaneous LMWH compared with SC UFH as the initial treatment of DVT of the lower limbs | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States random but no description of randomisation method provided |
| Allocation concealment (selection bias) | Low risk | Use of "sealed envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Use of "open" study design |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk |
Outcomes requiring blinding Recurrent VTE at 3 months: data used – Outcome assessors blinded for assessment of recurrent DVT "pre and post‐treatment phlebograms were assessed blindly" ‐ but no description of blinding of assessors for PE is provided Recurrent DVT at 3 months: data used – see recurrent VTE at 3 months PE – excluding PE found at autopsy: data used – see recurrent VTE at 3 months Incidence of heparin‐induced thrombocytopenia: NA Incidence of asymptomatic recurrent VTE at 3 months: NA Quality of life: NA Outcomes not requiring blinding Major bleeding: data used Minor bleeding: data used VTE‐related mortality: data used All‐cause mortality: data used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 participants (out of 149) were withdrawn from the trial Reasons for withdrawals are clearly presented: UFH group ‐ 1 patient had a recent history of DVT; 1 patient was diagnosed with antithrombin III deficiency and 1 patient developed major bleeding and was withdrawn from the study; however, their results did appear in the final analysis During follow‐up 1 patient from the UFH group died from renal failure |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No evidence of other biases |
Peternel 2002.
| Methods |
Study design: open, randomised controlled trial Duration of intervention: to INR target Duration of follow‐up: 7 days Run‐in period: NA Intention‐to‐treat analysis: no Language of publication: English |
|
| Participants |
Who participated: people with acute proximal DVT Country: Slovenia Number of study centres: 1 Setting: inpatients Number: 59 (SC UFH 28; SC LMWH 31) Age (mean ± SD): SC UFH 68 ± 13 years; SC LMWH 69 ±14 years Sex (M/F): SC UFH 15/13; SC LMWH 17/14 Inclusion criteria: proximal DVT diagnosed by ultrasound duplex Exclusion criteria: anticoagulant treatment with heparin or coumarins in the period of 10 days before admission, clinically significant pulmonary embolism or pregnancy Diagnostic criteria: ultrasound duplex |
|
| Interventions |
Intervention (route, total dose/day, frequency): bolus IV UFH, followed by SC UFH twice daily or TID, aPTT adjusted + warfarin Control (route, total dose/day, frequency): SC LMWH 200 IU/kg 4 times daily + warfarin Treatment before study: NA |
|
| Outcomes | Outcomes not specified as primary or secondary Outcomes: major bleeding, death, aPTT, haemostatic markers (F1+2, TAT, D‐dimer) |
|
| Notes | Stated aim of the study: to compare these markers in the acute phase of DVT during treatment either with subcutaneous aPTT‐adjusted UFH or with weight‐adjusted LMWH in order to estimate control of haemostatic system activation during both regimens | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States random but no description of randomisation method provided |
| Allocation concealment (selection bias) | Unclear risk | No description of allocation concealment provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No description of blinding provided Different numbers of injections at different times – probably prevented adequate blinding UFH – 1 bolus of heparin given intravenously followed by 2‐3 subcutaneous injections daily LWMH – 1 subcutaneous injection daily |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk |
Outcomes requiring blinding Recurrent VTE at 3 months: NA Recurrent DVT at 3 months: NA PE – excluding PE found at autopsy: NA Incidence of heparin‐induced thrombocytopenia: NA Incidence of asymptomatic recurrent VTE at 3 months: NA Quality of life: NA Outcomes not requiring blinding Major bleeding: data not used ‐ not meeting ISTH definition Minor bleeding: data not used ‐ not meeting definition of minor bleeding VTE‐related mortality: data used All‐cause mortality: data used |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Many of the 59 participants were withdrawn from the study; however, exact numbers withdrawn and from which group they were withdrawn are not presented in the paper Reasons for withdrawal are also not clearly identified – the paper does state that 2 participants died and other participants were withdrawn when INR > 2 for 2 days; however, if all participants were withdrawn for this reason is unclear |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No evidence of other biases |
Pini 1990.
| Methods |
Study design: open randomised controlled trial Duration of intervention: 7 days Duration of follow‐up: 7 days Run‐in period: NA Intention‐to‐treat analysis: no Language of publication: English |
|
| Participants |
Who participated: people with acute DVT Country: Italy Number of study centres: 1 Setting: inpatients Number: 271(SC UFH 138; IV UFH 133) Age mean (range): SC UFH 63.4 (16 to 87) years; IV UFH 60.9 (11 to 86) years Sex (M/F): SC UFH 83/55; IV UFH 72/61 Inclusion criteria: acute DVT diagnosed with strain‐gauge plethysmography or venography Exclusion criteria: bleeding disorder, abnormal results in haemostatic function screening tests, active peptic disease, on heparin treatment + acenocoumarol Diagnostic criteria: plethysmography or venography in diagnosis not concluded |
|
| Interventions |
Intervention (route, total dose/day, frequency): SC UFH (calcium heparin) 250 U/kg twice daily + acenocoumarol Control (route, total dose/day, frequency): IV UFH (sodium heparin bolus) followed by continuous IV UFH 500 U/Kg/day + acenocoumarol Treatment before study: NA |
|
| Outcomes | Outcomes not specified as primary or secondary Outcomes: DVT extension, PE, death, bleeding |
|
| Notes | Stated aim of the study: to compare IV and SC heparin for acute DVT in a large population study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were assigned by computer‐generated random numbers" |
| Allocation concealment (selection bias) | Unclear risk | No description of allocation concealment provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No description of blinding provided Different methods of heparin administration – intravenous compared to subcutaneous – probably prevented adequate blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk |
Outcomes requiring blinding Recurrent VTE at 3 months: data used – no description of blinding outcome assessors Recurrent DVT at 3 months: NA PE – excluding PE found at autopsy: data used – no description of blinding outcome assessors Incidence of heparin‐induced thrombocytopenia: NA Incidence of asymptomatic recurrent VTE at 3 months: NA Quality of life: NA Outcomes not requiring blinding Major bleeding: data used Minor bleeding: data used VTE‐related mortality: data used All‐cause mortality: data used |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Number of participants (out of 271) who were withdrawn from the study is not presented The study states that 23 participants were reported as not undergoing strain gauge plethysmography (SGP) but which group they came from is omitted as is weather any other participants were withdrawn – as only a subset of participants (251) underwent SGP – is unclear 4 participants in the SC group died (1 from PE and 1 from cerebral haemorrhage; 2 participants died in the intravenous group 1 from PE and 1 from pulmonary haemorrhage |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No evidence of other biases |
Prandoni 2004.
| Methods |
Study design: open randomised controlled trial Duration of intervention: 5 days to INR Duration of follow‐up: 3 months Run‐in period: NA Intention‐to‐treat analysis: yes Language of publication: English |
|
| Participants |
Who participated: people with acute VTE (DVT + PE) Number of study centres: 19 Setting: inpatients Number: 720 (SC UFH 360; SC LMWH 360) Age (mean ± SD): SC UFH 65.7 ± 15.6 years; SC LMWH 67.0 ± 14.8 years Sex M/F: SC UFH 158/202; SC LMWH 167/193 Inclusion criteria: people with DVT of the lower extremities and/or PE were eligible for the study, provided that the suspicion was objectively confirmed Exclusion criteria: age less than 18 years, pregnancy, contraindications to anticoagulant treatment, full‐dose anticoagulant treatment (either heparin or oral anticoagulants) for more than 24 h, haemodynamic instability, previous (less than 1 year earlier) episode of VTE, life expectancy less than 3 months, poor compliance, and geographic inaccessibility for follow‐up Diagnostic criteria: a positive result of at least 1 of the following tests was accepted for inclusion: ascending phlebography, compression ultrasound of the proximal vein system, echo colour Doppler scan of the calf vein system in the case of clinical suspicion of DVT, ventilation‐perfusion scanning, spiral computed tomographic scanning, and pulmonary angiography in the case of clinical suspicion of PE. In the presence of abnormal results of an ultrasound test of the lower extremities, the diagnosis of PE was also accepted if a perfusion lung scan was compatible with a high probability of PE when compared with the chest x‐ray Type of VTE: 601 DVT/119 PE |
|
| Interventions |
Intervention (route, total dose/day, frequency): IV bolus UFH (calcium heparin) 4000‐5000 IU followed by SC UFH twice daily, aPTT adjusted + warfarin Control (route, total dose/day, frequency): SC LMWH 85 U/kg twice daily + warfarin Treatment before study: NA |
|
| Outcomes |
Primary outcome: recurrent VTE at 3 month follow‐up Secondary outcomes: recurrent VTE during heparin treatment, bleeding during heparin treatment, death |
|
| Notes | Stated aim of the study: to assess the value of UFH or LMWH for treating the full spectrum of patients with VTE, including recurrent VTE and PE | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation … was performed with a computer algorithm" |
| Allocation concealment (selection bias) | Low risk | Use of a "24‐hour telephone service that recorded patient information before disclosure of the treatment assigned" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Use of an open study design |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk |
Outcomes requiring blinding Recurrent VTE at 3 months: data used – no description of blinding outcome assessors Recurrent DVT at 3 months: NA PE – excluding PE found at autopsy: data used – no description of blinding outcome assessors Incidence of heparin‐induced thrombocytopenia: NA Incidence of asymptomatic recurrent VTE at 3 months: NA Quality of life: NA Outcomes not requiring blinding Major bleeding: data used Minor bleeding: data used VTE‐related mortality: data used All‐cause mortality: data used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 0 participants (out of 720) were withdrawn from the study "[N]o patients were lost to follow up" "We … ensured follow up was complete for all randomised patients" During follow‐up 24 participants died: In the UFH group 12 participants died (3 from PE and 1 from haemorrhage); in the LMWH group 12 participants died (4 from PE) |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No evidence of other biases |
Walker 1987.
| Methods |
Study design: open randomised controlled trial Duration of intervention: 14 days Duration of follow‐up: 14 days Run‐in period: NA Intention‐to‐treat analysis: no Language of publication: English |
|
| Participants |
Who participated: people with acute lower limb DVT Country: UK Number of study centres: 5 Setting: inpatients Number: 100 (SC UFH 50; IV continuous UFH 50) Age (mean ± SD): SC UFH M 61 ± 11 years, F 63 ± 16 years; IV continuous UFH M 60 ± 14 years, F 63 ±15 years Sex (M/F): SC UFH 25/25; IV continuous UFH 28/22 Inclusion criteria: people with DVT of the legs (calf + proximal), phlebography proven, with a thrombus > 5 cm Exclusion criteria: PE or occlusive thrombus Diagnostic criteria: phlebography |
|
| Interventions |
Intervention (route, total dose/day, frequency): SC UFH (calcium heparin) 250 U/kg, aPTT adjusted + warfarin Control (route, total dose/day, frequency): IV continuous UFH (sodium heparin) aPTT adjusted + warfarin Treatment before study: NA |
|
| Outcomes | Outcomes not specified as primary or secondary Outcomes: DVT extension, injection site pain, PE, haemoglobin, platelets, aPTT |
|
| Notes | Stated aim of the study: to compare the efficacy and safety of SC versus IV heparin for leg DVT | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "[T]he randomisation code was drafted using a standard random number table" |
| Allocation concealment (selection bias) | Low risk | "[P]atient allocations were taken from sealed envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No description of blinding provided Different methods of heparin administration – intravenous compared to subcutaneous – probably prevented adequate blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk |
Outcomes requiring blinding Recurrent VTE at 3 months: data used – no description of blinding outcome assessors Recurrent DVT at 3 months: NA PE – excluding PE found at autopsy: data used – no description of blinding outcome assessors Incidence of heparin‐induced thrombocytopenia: NA Incidence of asymptomatic recurrent VTE at 3 months: NA Quality of life: NA Outcomes not requiring blinding Major bleeding: data not used ‐ not meeting ISTH definition Minor bleeding: data not used ‐ not meeting definition of minor bleeding VTE‐related mortality: data used All‐cause mortality: data used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4 participants (out of 100) were withdrawn from the study, reasons for withdrawals are clearly presented: Intravenous group ‐ 3 participants were excluded due to "technically unsatisfactory" phlebograms Subcutaneous group ‐ 1 patient died during the course of the study |
| Selective reporting (reporting bias) | Low risk | The paper states that haemoglobin concentration; packed red cell count and platelet count were estimated on days 1,7,14 but no results are presented for these measurements. Nevertheless these were not outcomes of our review and therefore the study was judged to be at low risk of reporting bias |
| Other bias | Low risk | No evidence of other biases |
aPTT: activated partial thromboplastin time;AT: antithrombin;BP: blood pressure; DVT: deep vein thrombosis;INR: international normalised ratio;ISTH: International Society on Thrombosis and Haemostasis; IU: international units; IV: intravenous; LMWH: low molecular weight heparin;NA: not applicable; PE: pulmonary embolism; SC: subcutaneous; UFH: unfractionated heparin;US: ultrasound; VTE: venous thromboembolism.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Fagher 1981 | RCT comparing continuous versus intermittent intravenous heparin administration in people diagnosed with DVT |
| Glazier 1976 | RCT comparing continuous versus intermittent Intravenous heparin administration in people with PE |
| Gruber 1979 | RCT comparing subcutaneous heparin and dextran for the prophylaxis of VTE |
| Horbach 1996 | RCT comparing subcutaneous LMWH versus subcutaneous UFH for the prophylaxis of VTE |
| Lockner 1986 | RCT comparing intravenous UFH versus intravenous LMWH in people diagnosed with DVT |
| Marchiori 2002 | RCT of people diagnosed with superficial vein thrombosis |
| Monreal 1994 | RCT comparing long‐term treatment of people with VTE |
| Nakamura 2010 | RCT comparing intravenous UFH versus LMWH in people diagnosed with PE |
| NCT01956955 | RCT comparing UFH versus LMWH plus thrombolytic treatment in people diagnosed with PE |
| Quiros 2001 | RCT comparing intravenous UFH versus intravenous LMWH in people diagnosed with DVT |
| Riess 2014 | RCT comparing intravenous UFH versus LMWH in people diagnosed with PE |
| Rodgers 1999 | RCT comparing intravenous UFH versus LMWH in people diagnosed with cancer‐associated DVT |
| Romera 2009 | RCT comparing LMWH versus VKA in people diagnosed with DVT |
| Ucar 2015 | RCT comparing UFH versus LMWH plus thrombolytic treatment in people diagnosed with PE |
| Van Doormaal 2009 | RCT comparing LMWH only in cancer‐related VTE |
| Van Doormaal 2010 | RCT comparing LMWH only in cancer‐related DVT |
DVT: deep vein thrombosis; LMWH: low molecular weight heparin; PE: pulmonary embolism; RCT: randomised controlled trial; UFH: unfractionated heparin; VKA: vitamin K antagonist; VTE: venous thromboembolism.
Differences between protocol and review
For this update, we amended the outcomes of the review to reflect current terminology and practice. We redefined the outcome 'treatment‐related serious adverse effects, i.e. major bleeding; overall mortality' as two events, namely 'all‐cause mortality' and 'major bleeding'. In addition, we used a more comprehensive definition of bleeding.
Contributions of authors
JS: selected and assessed the quality of trials for inclusion in this update, extracted and entered data for analyses, and wrote the text of the review. LR: selected and assessed the quality of trials for inclusion in this update, extracted and entered data for analyses, and wrote the text of the review.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Chief Scientist Office, Scottish Government Health Directorates, The Scottish Government, UK.
The Cochrane Vascular editorial base is supported by the Chief Scientist Office.
-
National Institute for Health Research (NIHR), UK.
This project was supported by the NIHR, via Cochrane Programme Grant funding Cochrane Vascular (13/89/23). The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Declarations of interest
JS: none known. LR: none known.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Andersson 1982 {published data only}
- Andersson G, Fagrell B, Holmgren K, Johnsson H, Ljungberg B, Nilsson E, et al. Subcutaneous administration of heparin. A randomised comparison with intravenous administration of heparin to patients with deep‐vein thrombosis. Thrombosis Research 1982;27(6):631‐9. [DOI] [PubMed] [Google Scholar]
Belcaro 1999 {published data only}
- Belcaro G, Nicolaides AN, Cesarone MR, Laurora G, Sanctis MT, Incandela L, et al. Comparison of low‐molecular‐weight heparin, administered primarily at home, with unfractionated heparin, administered in hospital, and subcutaneous heparin, administered at home for deep‐vein thrombosis. Angiology 1999;50(10):781‐7. [DOI] [PubMed] [Google Scholar]
Bentley 1980 {published data only}
- Bentley PG, Kakkar VV, Scully MF, MacGregor IR, Webb P, Chan P, Jones N. An objective study of alternative methods of heparin administration. Thrombosis Research 1980;18(1‐2):177‐87. [DOI] [PubMed] [Google Scholar]
Doyle 1987 {published data only}
- Doyle DJ, Turpie AG, Hirsh J, Best C, Kinch D, Levine MN, et al. Adjusted subcutaneous heparin or continuous intravenous heparin in patients with acute deep vein thrombosis. A randomized trial. Annals of Internal Medicine 1987;107(4):441‐5. [DOI] [PubMed] [Google Scholar]
Faivre 1987 {published data only}
- Faivre R, Neuhart E, Kieffer Y, Bassand JP, Maurat JP. Efficacy of a very low molecular weight heparin fragment (CY 222) compared to standard heparin in patients with deep venous thrombosis. A randomized study [Efficacité d'un fragment d'héparine de très faible poids moléculaire (CY 222) comparée a l'héparine standard chez des patients porteurs d'une thrombose veineuse profonde. Étude randomisée]. Journal des Maladies Vasculaires 1987;12(Suppl. B):145‐6. [PubMed] [Google Scholar]
- Faivre R, Neuhart Y, Kieffer Y, Apfe lF, Magnin D, Didier D, et al. A new treatment of deep venous thrombosis: low molecular weight heparin fractions. Randomized study [Un nouveau traitement des thromboses veineuses profondes: les fractions d'héparine de bas poids ,olecuaire. Étude randomisée]. Presse Medicale 1988;17(5):197‐200. [PubMed] [Google Scholar]
Holm 1986 {published data only}
- Handeland GF, Abildgaard U, Holm HA, Arnesen KE. Dose adjusted heparin treatment of deep venous thrombosis: a comparison of unfractionated and low molecular weight heparin. European Journal of Clinical Pharmacology 1990;39(2):107‐12. [DOI] [PubMed] [Google Scholar]
- Holm HA, Ly B, Handeland GF, Abildgaard U, Arnesen KE, Gottschalk P, et al. Subcutaneous heparin treatment of deep venous thrombosis: a comparison of unfractionated and low molecular weight heparin. Haemostasis 1986;16(Suppl. 2):30‐7. [DOI] [PubMed] [Google Scholar]
Hull 1986 {published data only}
- Hull RD, Raskob GE, Hirsh J, Jay RM, Leclerc JR, Geerts WH, et al. Continuous intravenous heparin compared with intermittent subcutaneous heparin in the initial treatment of proximal‐vein thrombosis. New England Journal of Medicine 1986;315(18):1109‐14. [DOI] [PubMed] [Google Scholar]
Kearon 2006 {published data only}
- Kearon C, Ginsberg JS, Julian JA, Douketis J, Solymoss S, Ockelford P, et al. Comparison of fixed‐dose weight‐adjusted unfractionated heparin and low‐molecular‐weight heparin for acute treatment of venous thromboembolism. JAMA 2006;296(8):935. [DOI] [PubMed] [Google Scholar]
Krähenbühl 1979 {published data only}
- Krähenbühl B, Simon CA, Bouvier CA, Schinas P, Hopf MA, Cochet B. Heparin treatment. Comparison between intravenous and subcutaneous administration. Schweizerische Medizinische Wochenschrift 1979;109(36):1322‐5. [PubMed] [Google Scholar]
Leizorovicz 2011 {published data only}
- Leizorovicz A. IRIS an ongoing, international, multicentre, open, centrally randomised, parallel group study with tinzaparin or unfractionated heparin (ufh) administered subcutaneously (sc) to patients with deep vein thrombosis (DVT) or pulmonary embolism (PE). XXIst Congress of the International Society on Thrombosis and Haemostasis; 2007 Jul 6‐12; Geneva 2007:Abstract no: P‐M‐673.
- Leizorovicz A. Tinzaparin compared to unfractionated heparin for initial treatment of deep vein thrombosis in very elderly patients with renal insufficiency‐the IRIS Trial [Abstract No. 434]. Blood 2009;112:166. [Google Scholar]
- Leizorovicz A, Siguret V, Mottier D. Safety profile of tinzaparin versus subcutaneous unfractionated heparin in elderly patients with impaired renal function treated for acute deep vein thrombosis: The Innohep((R)) in Renal Insufficiency Study (IRIS). Thrombosis Research 2011;128(1):27‐34. [DOI] [PubMed] [Google Scholar]
- NCT00277394. Innohep© in elderly patients with impaired renal function treated for acute deep vein thrombosis [Safety profile of Innohep versus subcutaneous unfractionated heparin in elderly patients with impaired renal function treated for acute deep vein thrombosis]. clinicaltrials.gov/ct2/show/NCT00277394 (date first received 13 January 2006).
- Siguret V, Deudon C, Golmard J, Leizorovicz A, Pautas E, Gouin‐Thibault I. Pharmacodynamic response to unfractionated heparin used for initial treatment of acute deep vein thrombosis in elderly patients with renal impairment. A substudy of the IRIS clinical trial. Journal of Thrombosis and Haemostasis 2013;11(Suppl 2):805. [Google Scholar]
- Siguret V, Gouin‐Thibault I, Pautas E, Leizorovicz A. No accumulation of the peak anti‐Xa activity of tinzaparin in elderly patients with moderate‐to‐severe renal impairment: the IRIS substudy. Journal of Thrombosis and Haemostasis 2011;9(10):1966‐72. [DOI] [PubMed] [Google Scholar]
Lopaciuk 1990 {published data only}
- Lopaciuk S, Misiak A, Wisawski S, Ciesielski L, Korzycki J, Judkiewicz L, et al. Subcutaneous injections and intravenous infusion of sodium salt of heparin in the treatment of thrombosis of deep veins of the lower extremities [Podskórne wstrzyknięcia i dożylny wlew soli sodowej heparyny w leczeniu zakrzepicy żył głębokich kończyn dolnych]. Polski Tygodnik Lekarski 1990;45(47‐48):949‐52. [PubMed] [Google Scholar]
Lopaciuk 1992 {published data only}
- Lopaciuk S, Meissner AJ, Filipeckil S, Zawilska K, Sowier J, Ciesielski L, et al. Subcutaneous low molecular weight heparin versus subcutaneous unfractionated heparin in the treatment of deep vein thrombosis: a Polish multicenter trial. Thrombosis and Haemostasis 1992;68(1):14‐8. [PubMed] [Google Scholar]
Peternel 2002 {published data only}
- Peternel P, Terbizan M, Tratar G, Bozic M, Horvat D, Salobir B, et al. Markers of hemostatic system activation during treatment of deep vein thrombosis with subcutaneous unfractionated or low‐molecular weight heparin. Thrombosis Research 2002;105(3):241‐6. [DOI] [PubMed] [Google Scholar]
Pini 1990 {published data only}
- Pini M, Pattachini C, Quintavalla R, Poli T, Megha A, Tagliaferri A, et al. Subcutaneous vs intravenous heparin in the treatment of deep venous thrombosis? a randomized clinical trial. Thrombosis and Haemostasis 1990;64(2):222‐6. [PubMed] [Google Scholar]
Prandoni 2004 {published data only}
- Prandoni P, Carnovali M, Marchiori A, Galilei Investigators. Subcutaneous adjusted‐dose unfractionated heparin vs fixed‐dose low‐molecular‐weight heparin in the initial treatment of venous thromboembolism. Archives of Internal Medicine 2004;164(10):1077‐83. [DOI] [PubMed] [Google Scholar]
Walker 1987 {published data only}
- Walker MG, Shaw JW, Cumming JGR, Lea Thomas M. Subcutaneous calcium heparin versus intravenous sodium heparin in the treatment of established acute deep vein thrombosis of the legs: a multicentre prospective randomised trial. BMJ 1987;294(6581):1189‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Fagher 1981 {published data only}
- Fagher B, Lundh B. Heparin treatment of deep vein thrombosis. Effects and complications after continuous or intermittent heparin administration. Acta Medica Scandinavica 1981;210(5):357‐61. [PubMed] [Google Scholar]
Glazier 1976 {published data only}
- Glazier RL, Crowell EB. Randomized prospective trial of continuous vs intermittent heparin therapy. JAMA 1976;236(12):1365‐7. [PubMed] [Google Scholar]
Gruber 1979 {published data only}
- Gruber UF, Brun M, Brunner R, Gaugler U, Müller J, Schumacher S, et al. Subcutaneous heparin or intravenous dextran? [S.c. Heparin oder i.v. Dextran?]. Helvetica Chirurgica Acta 1979;46(1‐2):65‐8. [PubMed] [Google Scholar]
Horbach 1996 {published data only}
- Horbach T, Wolf H, Michaelis HC, Wagner W, Hoffmann A, Schmidt A, et al. A fixed‐dose combination of low molecular weight heparin with dihydroergotamine versus adjusted‐dose unfractionated heparin in the prevention of deep‐vein thrombosis after total hip replacement. Thrombosis and Haemostasis 1996;75(2):246‐50. [PubMed] [Google Scholar]
Lockner 1986 {published data only}
- Lockner D, Bratt G, Tornebohm E, Aberg W, Granqvist S. Intravenous and subcutaneous administration of Fragmin in deep venous thrombosis. Haemostasis 1986;16(Suppl. 2):25‐9. [DOI] [PubMed] [Google Scholar]
Marchiori 2002 {published data only}
- Marchiori A, Verlato F, Sabbion P, Camporese G, Rosso F, Mosena L, et al. High versus low doses of unfractionated heparin for the treatment of superficial thrombophlebitis of the leg. A prospective, controlled, randomized study. Haematologica 2002;87(5):523‐7. [PubMed] [Google Scholar]
Monreal 1994 {published data only}
- Monreal M, Lafoz E, Olive A, Rio LD, Vedia C. Comparison of subcutaneous unfractionated heparin with a low molecular weight heparin (Fragmin) in patients with venous thromboembolism and contraindications to coumarin. Thrombosis and Haemostasis 1994;71(1):7‐11. [PubMed] [Google Scholar]
Nakamura 2010 {published data only}
- Nakamura M, Okano Y, Minamigichi H, Tsujimoto H, Nakajima H, Kunieda T. Clinical assessment of fondaparinux for treatment of acute pulmonary embolism and acute deep vein thrombosis in Japanese patients. Chest 2010;138(4 MeetingAbstracts):410A. [DOI] [PubMed] [Google Scholar]
NCT01956955 {published data only}
- NCT01956955. Comparison of low‐molecular‐weight heparin (LMWH) and unfractionated heparin (UFH) in combination with thrombolytic treatment of acute massive pulmonary thromboembolism. clinicaltrials.gov/ct2/show/NCT01956955 (Date first received 27 September 2013).
Quiros 2001 {published data only}
- Quiros M. Use of heparin of low molecular weight in the ambulatory handling of patients with deep venous thrombosis. Comparative study: heparin non‐divided versus low molecular weight heparin [Uso de heparinas de bajo peso molecular en el manejo ambulatorio de pacientes con trombosis venosa profunda. Estudio comparativo: heparina no fraccionada vs heparina de bajo peso molecular]. Revista Costarricense de Cardiologia 2001;3(2):8‐13. [Google Scholar]
Riess 2014 {published data only}
- Riess H, Becker LK, Melzer N, Harenberg J. Treatment of deep vein thrombosis in patients with pulmonary embolism: subgroup analysis on the efficacy and safety of certoparin vs. unfractionated heparin. Blood Coagulation & Fibrinolysis 2014;25(8):838‐44. [DOI] [PubMed] [Google Scholar]
Rodgers 1999 {published data only}
- Rodgers GM. Treatment of cancer‐associated deep‐vein thrombosis with enoxaparin: comparison with unfractionated heparin therapy. Proceedings of the American Society of Clinical Oncology 1999; Vol. 18:193a.
Romera 2009 {published data only}
- Romera A, Cairols MA, Vila‐Coll R, Marti X, Colome E, Bonell A, et al. A randomised open‐label trial comparing long‐term sub‐cutaneous low‐molecular‐weight heparin compared with oral‐anticoagulant therapy in the treatment of deep venous thrombosis. European Journal of Vascular and Endovascular Surgery 2009;37(3):349‐56. [DOI] [PubMed] [Google Scholar]
Ucar 2015 {published data only}
- Ucar EY, Akgun M, Araz O, Tas H, Kerget B, Meral M, et al. Comparison of LMWH versus UFH for hemorrhage and hospital mortality in the treatment of acute massive pulmonary thromboembolism after thrombolytic treatment: randomized controlled parallel group study. Lung 2015; Vol. 193, issue 1:121‐7. [DOI] [PubMed]
Van Doormaal 2009 {published data only}
- Doormaal FF, Raskob GE, Davidson BL, Decousus H, Gallus A, Lensing AW, et al. Treatment of venous thromboembolism in patients with cancer: subgroup analysis of the Matisse clinical trials. Thrombosis and Haemostasis 2009;101(4):762‐9. [PubMed] [Google Scholar]
Van Doormaal 2010 {published data only}
- Doormaal FF, Cohen AT, Davidson BL, Decousus H, Gallus AS, Gent M, et al. Idraparinux versus standard therapy in the treatment of deep venous thrombosis in cancer patients: a subgroup analysis of the Van Gogh DVT trial. Thrombosis and Haemostasis 2010;104(1):86‐91. [DOI] [PubMed] [Google Scholar]
Additional references
Anderson 2003
- Anderson FA, Spencer FA. Risk factors for venous thromboembolism. Circulation 2003;107(23 Suppl 1):1‐9. [DOI] [PubMed] [Google Scholar]
Atkins 2004
- Atkins D, Best D, Briss PA, Eccles M, Falck‐Ytter Y, Flottorp S, et al. GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chengelis 1996
- Chengelis DL, Bendick PJ, Glover JL, Brown OW, Ranval TJ. Progression of superficial venous thrombosis to deep vein thrombosis. Journal of Vascular Surgery 1996;24(5):745‐9. [DOI] [PubMed] [Google Scholar]
Cushman 2007
- Cushman M. Epidemiology and risk factors for venous thrombosis. Seminars in Hematology 2007;44(2):62‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Dolovich 2000
- Dolovich LR, Ginsberg JS Douketis JD, Holbrook AM, Cheah G. A meta‐analysis comparing low‐molecular‐weight heparins with unfractionated heparin in the treatment of venous thromboembolism. Archives of Internal Medicine 2000;160(2):181‐8. [DOI] [PubMed] [Google Scholar]
Erkens 2010
- Erkens PMG, Prins MH. Fixed dose subcutaneous low molecular weight heparins versus adjusted dose unfractionated heparin for venous thromboembolism. Cochrane Database of Systematic Reviews 2010, Issue 9. [DOI: 10.1002/14651858.CD001100.pub3] [DOI] [PubMed] [Google Scholar]
Gould 1999
- Gould MK, Dembitzer AD, Doyle RL, Hastie TJ, Garber AM. Low‐molecular‐weight heparins compared with unfractionated heparin for treatment of acute deep venous thrombosis: a meta‐analysis of randomized controlled trials. Annals of Internal Medicine 1999;130(10):800‐9. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADE Working Group, McMaster University. GRADEpro GDT. Version accessed 2 November 2016. Hamilton (ON): GRADE Working Group, McMaster University, 2014.
Heit 2015
- Heit JA. Epidemiology of venous thromboembolism. Nature Reviews Cardiology 2015;12(8):464‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hommes 1992
- Hommes DW, Bura A, Mazzolai L, Büller HR, Cate JW. Subcutaneous heparin compared with continuous intravenous heparin administration in the initial treatment of deep vein thrombosis. A meta‐analysis. Annals of Internal Medicine 1992;116(4):279‐84. [DOI] [PubMed] [Google Scholar]
Kearon 2012
- Kearon C, Akl EA, Comerota AJ, Prandoni P, Bounameaux H, Goldhaber SZ, et al. Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest 2012;142(6):1698‐704. [DOI] [PMC free article] [PubMed] [Google Scholar]
McLean 1916
- McLean J. The thromboplastic action of cephalin. American Journal of Physiology 1916;41:250‐7. [Google Scholar]
Munro 2008
- Munro A, English C. Subcutaneous heparin is as good as low‐molecular weight heparin in the acute treatment of thromboembolic disease. Emergency Medicine Journal 2008;25(5):287‐9. [DOI] [PubMed] [Google Scholar]
Nasr 2015
- Nasr H, Scriven JM. Superficial thrombophlebitis (superficial venous thrombosis). BMJ 2015;350:h2039. [DOI] [PubMed] [Google Scholar]
NICE 2010
- National Clinical Guideline Centre. Venous thromboembolism: reducing the risk of venous thromboembolism (deep vein thrombosis and pulmonary embolism) in patients admitted to hospital. Methods evidence and guidance. www.nice.org.uk/guidance/cg92/evidence/cg92‐venous‐thromboembolism‐reducing‐the‐risk‐full‐guideline3 (accessed 30 September 2015).
Quinlan 2004
- Quinlan DJ, McQuillan A, Eikelboom JW. Low‐molecular‐weight heparin compared with intravenous unfractionated heparin for treatment of pulmonary embolism: a meta‐analysis of randomized, controlled trials. Annals of Internal Medicine 2004;140(3):175‐83. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schulman 2005
- Schulman S, Kearon C, Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non‐surgical patients. Journal of Thrombosis and Haemostasis 2005;3(4):692‐4. [DOI] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JAC, Egger M, Moher D. Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
White 2003
- White RH. The epidemiology of venous thromboembolism. Circulation 2003;107:I‐4‐I‐8. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Vardi 2007
- Vardi M, Bitterman H. Subcutaneous unfractionated heparin for the treatment of venous thromboembolism. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD006771] [DOI] [PubMed] [Google Scholar]
Vardi 2009
- Vardi M, Zittan E, Bitterman H. Subcutaneous unfractionated heparin for the initial treatment of venous thromboembolism. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD006771.pub2] [DOI] [PubMed] [Google Scholar]


