Abstract
Background
Up to 80% of hospitalised patients receive intravenous therapy at some point during their admission. About 20% to 70% of patients receiving intravenous therapy develop phlebitis. Infusion phlebitis has become one of the most common complications in patients with intravenous therapy. However, the effects of routine treatments such as external application of 75% alcohol or 50% to 75% magnesium sulphate (MgSO4) are unsatisfactory. Therefore, there is an urgent need to develop new methods to prevent and alleviate infusion phlebitis.
Objectives
To systematically assess the effects of external application of Aloe vera for the prevention and treatment of infusion phlebitis associated with the presence of an intravenous access device.
Search methods
The Cochrane Peripheral Vascular Diseases Group Trials Search Co‐ordinator (TSC) searched the Specialised Register (last searched February 2014) and CENTRAL (2014, Issue 1). In addition the TSC searched MEDLINE to week 5 January 2014, EMBASE to Week 6 2014 and AMED to February 2014. The authors searched the following Chinese databases until 28 February 2014: Chinese BioMedical Database; Traditional Chinese Medical Database System; China National Knowledge Infrastructure; Chinese VIP information; Chinese Medical Current Contents; Chinese Academic Conference Papers Database and Chinese Dissertation Database; and China Medical Academic Conference. Bibliographies of retrieved and relevant publications were searched. There were no restrictions on the basis of date or language of publication.
Selection criteria
Randomised controlled trials (RCTs) and quasi‐randomised controlled trials (qRCTs) were included if they involved participants receiving topical Aloe vera or Aloe vera‐derived products at the site of punctured skin, with or without routine treatment at the same site.
Data collection and analysis
Two review authors independently extracted the data on the study characteristics, description of methodology and outcomes of the eligible trials, and assessed study quality. Data were analysed using RevMan 5.1. For dichotomous outcomes, the effects were estimated by using risk ratio (RR) with its 95% confidence interval (CI). For continuous outcomes, mean differences (MD) with 95% CIs were used to estimate their effects.
Main results
A total of 43 trials (35 RCTs and eight qRCTs) with 7465 participants were identified. Twenty‐two trials with 5546 participants were involved in prevention of Aloe vera for phlebitis, and a further 21 trials with 1919 participants were involved in the treatment of phlebitis. The included studies compared external application of Aloe vera alone or plus non‐Aloe vera interventions with no treatment or the same non‐Aloe vera interventions. The duration of the intervention lasted from one day to 15 days. Most of the included studies were of low methodological quality with concerns for selection bias, attrition bias, reporting bias and publication bias.
The effects of external application of fresh Aloe vera on preventing total incidence of phlebitis varied across the studies and we did not combine the data. Aloe vera reduced the occurrence of third degree phlebitis (RR 0.06, 95% CI 0.03 to 0.11, P < 0.00001) and second degree phlebitis (RR 0.18, 95% CI 0.10 to 0.31, P < 0.00001) compared with no treatment. Compared with external application of 75% alcohol, or 33% MgSO4 alone, Aloe vera reduced the total incidence of phlebitis (RR 0.02, 95% CI 0.00 to 0.28, P = 0.004 and RR 0.43, 95% CI 0.24 to 0.78, P = 0.005 respectively) but there was no clear evidence of an effect when compared with 50% or 75% MgSO4 (total incidence of phlebitis RR 0.41, 95% CI 0.16 to 1.07, P = 0.07 and RR 1.10 95% CI 0.54 to 2.25, P = 0.79 respectively; third degree phlebitis (RR 0.28, 95% CI 0.07 to 1.02, P = 0.051 and RR 1.19, 95% CI 0.08 to 18.73, P = 0.9 respectively; second degree phlebitis RR 0.68, 95% CI 0.21 to 2.23, P = 0.53 compared to 75% MgSO4) except for a reduction in second degree phlebitis when Aloe vera was compared with 50% MgSO4 (RR 0.26, 95% CI 0.14 to 0.50, P < 0.0001).
For the treatment of phlebitis, Aloe vera was more effective than 33% or 50% MgSO4 in terms of both any improvement (RR 1.16, 95% CI 1.09 to 1.24, P < 0.0001 and RR 1.22, 95% CI 1.16 to 1.28, P < 0.0001 respectively) and marked improvement of phlebitis (RR 1.97, 95% CI 1.44 to 2.70, P < 0.001 and RR 1.56, 95% CI 1.29 to 1.87, P = 0.0002 respectively). Compared with 50% MgSO4, Aloe vera also improved recovery rates from phlebitis (RR 1.42, 95% CI 1.24 to 1.61, P < 0.0001). Compared with routine treatments such as external application of hirudoid, sulphonic acid mucopolysaccharide and dexamethasone used alone, addition of Aloe vera improved recovery from phlebitis (RR 1.75, 95% CI 1.24 to 2.46, P = 0.001) and had a positive effect on overall improvement (marked improvement RR 1.26, 95% CI 1.09 to 1.47, P = 0.0003; any improvement RR 1.23, 95% CI 1.13 to 1.35, P < 0.0001). Aloe vera, either alone or in combination with routine treatment, was more effective than routine treatment alone for improving the symptoms of phlebitis including shortening the time of elimination of red swelling symptoms, time of pain relief at the location of the infusion vein and time of resolution of phlebitis. Other secondary outcomes including health‐related quality of life and adverse effects were not reported in the included studies.
Authors' conclusions
There is no strong evidence for preventing or treating infusion phlebitis with external application of Aloe vera. The current available evidence is limited by the poor methodological quality and risk of selective outcome reporting of the included studies, and by variation in the size of effect across the studies. The positive effects observed with external application of Aloe vera in preventing or treating infusion phlebitis compared with no intervention or external application of 33% or 50% MgSO4 should therefore be viewed with caution.
Keywords: Humans; Aloe; Administration, Topical; Central Venous Catheters; Central Venous Catheters/adverse effects; Infusions, Intravenous; Infusions, Intravenous/adverse effects; Phlebitis; Phlebitis/drug therapy; Phlebitis/etiology; Phlebitis/prevention & control; Phytotherapy; Phytotherapy/methods; Randomized Controlled Trials as Topic
Plain language summary
Aloe vera for prevention and treatment of infusion phlebitis
Infusion phlebitis is acute inflammation of a vein in the presence of intravenous therapy. In modern medical practice, more than 80% of inpatients will receive intravenous therapy during their admission, and about 20% to 70% of them may develop infusion phlebitis. Therefore infusion phlebitis is the most common complication of intravenous therapy. However, routine treatments for prevention or treatment of phlebitis such as external application of 75% alcohol or 50% to 75% magnesium sulphate (MgSO4) are unsatisfactory.
This review examined 35 randomised controlled trials and eight quasi‐randomised controlled trials with 7465 participants. Twenty‐two trials with 5546 participants were involved in looking at prevention of phlebitis with Aloe vera, and a further 21 trials with 1919 participants were involved in looking at Aloe vera for the treatment of phlebitis. The included trials mainly compared external application of fresh Aloe vera alone or with another non‐Aloe vera treatment such as a wet compress of 75% alcohol or 33%, 50% or 75% MgSO4 with no treatment or the same non‐Aloe vera treatment. The duration of intervention lasted from one day to 15 days. Most of the included studies were of low methodological quality with concerns for selection bias, attrition bias, reporting bias and publication bias. The incidence of phlebitis at varying degrees of severity as well as the resolution rate and level of improvement of phlebitis were investigated.
The available evidence suggests that external application of fresh Aloe vera alone or combined with other non‐Aloe vera treatment may be effective for the prevention and treatment of infusion phlebitis resulting from the intravenous therapy. The conclusions should be cautiously interpreted due to the low methodological quality of the included trials.
Background
Description of the condition
Intravenous therapy is the giving of substances directly into a vein. Intravenous therapy may be used to correct electrolyte imbalances, deliver medications, for blood transfusion, or as fluid replacement. In modern medical practice up to 80% of hospitalised patients receive intravenous therapy at some point during their admission (Tager 1983; Tjon 2000). Compared with other routes of administration, the intravenous route is the fastest way to deliver fluids and medications throughout the body. The devices for intravenous therapy usually include a hypodermic needle, peripheral venous cannula or intravenous catheter and this is the most common invasive procedure among patients admitted to hospital (Waitt 2004). It is estimated that 200 million peripheral intravenous devices are placed each year in America alone (Maki 2008; Mermel 2001), while one in three inpatients have at least one peripheral venous catheter in situ (Boyd 2011). Medication, fluids, nutrition, and blood products can all be given via the peripheral intravenous route, and the plastic catheter may stay in situ long term. Although common, these practices are not devoid of complications, which may increase duration of hospital stay and admission cost (Waitt 2004). The complications that occur frequently in patients with peripheral intravenous therapy include infusion phlebitis, infection, infiltration or extravasation, fluid overload, hypothermia, electrolyte imbalance and embolism. Infusion phlebitis is in almost all cases a biochemical reaction to the mechanical irritation caused by the presence of the intravenous catheter (Maki 1991). The remaining cases may be chemical or infectious. Studies have shown that 20% to 70% of patients receiving peripheral intravenous therapy develop phlebitis (Hershey 1984; Monreal 1999; Tully 1981). In China, according to statistics, about 80% of the patients with intravenous therapy develop varying degrees of infusion phlebitis (Tang 2001).
Description of the intervention
Aloe or Aloe vera is a genus containing many species of flowering succulent plants with a rosette of large, thick, fleshy leaves (Klein 1988). Aloe vera species are frequently cultivated as ornamental plants both in gardens and in pots. Aloe has been known and used for centuries for its health, beauty and skin care properties. Two thousand years ago, the Greek scientists regarded Aloe vera as the universal panacea and the Egyptians called Aloe the plant of immortality (Surjushe 2008). It has a long history of use as an anti‐inflammatory herbal application for burns and for a variety of conditions in traditional medicine (Hutter 1996). Today, Aloe vera is still used as a herbal medicine. Aloe vera is used either internally or externally in humans and has some medicinal effects which have been supported by scientific and medical research (Vogler 1999). As a herbal medicine, Aloe vera juice is commonly used internally to relieve digestive discomfort (Langmead 2004) and externally to relieve skin discomforts such as minor burns, wounds and various skin conditions such as eczema and scabies (Feily 2009; Oyelami 2009; Visuthikosol 1995). The topical or external applications usually use aloe‐derived products, which include aloe vera cream, aloe vera mucilage, aloe vera gel etc. (Vogler 1999). However, crude aloe vera components such as fresh aloe vera leaf, fresh aloe vera stem, and aloe vera juice are also used as adjuvant treatment to treat skin disorders (Peng 2009; Zhang 2010).
How the intervention might work
Aloe vera contains 75 potentially active constituents including vitamins, enzymes, minerals, sugars, lignin, saponins, salicylic acids and amino acids (Shelton 1991), some of which have several pharmacological actions. These include the carboxypeptidase that inactives bradykinin, salicylates and substances that inhibit local vasoconstriction (Klein 1988). The anti‐inflammatory compound called C‐glucosyl chromone has been isolated from gel extracts (Hutter 1996). It has been proven that fresh Aloe vera can promote the attachment and growth of normal human cells in vitro and enhance the healing of wounded monolayers of cells (Winters 1981) whereby Aloe vera gel not only increased the collagen content of the wound but also changed the collagen composition and increased the degree of collagen cross linking (Chithra 1998). Some studies have shown that pure Aloe vera is effective in preserving skin circulation following frostbite injury (McCauley 1990) and in accelerating wound healing (Fulton 1990) and increasing the breaking strength of resulting scar tissue in patients (Heggers 1996). In addition, Aloe vera can stimulate fibroblasts, which produce the collagen and elastin fibres, making the skin more elastic and less wrinkled; and Aloe vera has cohesive effects on the superficial flaking epidermal cells by sticking them together, to soften the skin (West 2003). To summarise, the mechanism of action of Aloe vera includes healing properties, anti‐inflammatory activity, effects on the immune system, moisturising and anti‐aging effects and antiseptic effects (Surjushe 2008). Aloe may therefore be beneficial for the prevention and treatment of infusion phlebitis.
Why it is important to do this review
Infusion phlebitis is acute inflammation of a vein in the presence of and directly connected to an intravenous access device. It may be influenced by the pH and osmotic pressure of the solution, the size of the vein used, the size and material of the catheter, and the infusion period (Kuwahara 1998); and may be the most common complication of intravenous therapy. Especially during cancer chemotherapy, the toxicity of and irritation caused by the chemotherapy drugs can directly lead to phlebitis and even tissue necrosis. Yoh et al reported that the incidence of phlebitis after a six‐minute infusion of vinorelbine was 16% to 33% (Yoh 2007). Phlebitis not only brings great physiological and psychological suffering to the patients but also reduces the treatment effect and prolongs the process of treatment. The infusion phlebitis may initially manifest itself in the form of pain, erythema and swelling, warmth, hardening and thickening of the skin in the injection area (Schmid 2000). However, it can quickly develop into more serious symptoms such as severe local tissue ulceration and necrosis, even leading to serious limb dysfunction, and could ultimately cause thrombus formation and pyrexia. This is most likely to be caused by the pH and osmotic pressure of the solution, the mechanical irritation of the catheter or chemical irritation of the drugs (Emiko 2009). Therefore, infusion phlebitis does not only increase the psychological burden of the patient it also affects the quality of care. The routine or traditional methods for prevention and treatment of infusion phlebitis are varied, for example self care, anti‐inflammatory medication, cortisone preparations, rubbing or flushing the site with 75% alcohol or 0.9% saline solution, and compresses with 50% to 75% magnesium sulphate, but none are completely effective (Curran 1990; Nakayama 2002; O'Grady 2002). There is an urgent need to develop new methods to prevent and alleviate infusion phlebitis.
In clinical settings in China, it is common for Aloe vera to be externally applied to prevent and treat infusion phlebitis (Cao 2008; Hu 2006; Lu 2008; Yang 2008). Studies on its effectiveness have small sample sizes and the real effect is difficult to confirm. This systematic review of randomised and quasi‐randomised trials was conducted to clarify its efficacy.
Objectives
To systematically assess the effects of external application of Aloe vera for the prevention and treatment of infusion phlebitis associated with the presence of an intravenous access device.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) and quasi‐randomised controlled trials (qRCTs) which evaluated Aloe vera used alone or in combination with other treatments for infusion phlebitis were included irrespective of blinding, publication status and language. We excluded non‐randomised studies. Randomised cross‐over trials and cluster‐RCTs were eligible.
Types of participants
We included participants, regardless of gender, age or ethnic origin, who suffered from phlebitis of a peripheral limb vein that was associated with the presence of an intravenous access device, or who were at risk of developing phlebitis because of insertion of an intravenous access device. Peripheral limb veins are frequently used for siting intravenous access devices, including the dorsal venous network of the hand, veins of the forearm, veins of the cubital fossa, and dorsalis pedis veins.
Types of interventions
We included trials that compared topical external application of any type of Aloe vera (fresh Aloe vera slice or fresh Aloe vera juice) or aloe‐derived products, or Aloe vera plus non‐Aloe vera treatments, at the site of punctured skin with no treatment, routine treatment or the same non‐Aloe vera treatment at the same site. We excluded trials without a definite duration of treatment. In addition, we excluded those trials in which Aloe vera plus other non‐Aloe vera treatments were used in the treatment group and the same non‐Aloe vera treatments were not used in the control group.
Types of outcome measures
Primary outcomes
1. The incidence of phlebitis (for preventive effect) as assessed by the total incidence of phlebitis. We also assessed the incidence of third degree and second degree phlebitis.
2. The rate of resolution of phlebitis (for treatment effect) as assessed by the total improvement rate of phlebitis. We also assessed the recovery rate and marked improvement rate of phlebitis.
Secondary outcomes
1. The duration of successful placement of a venous catheter, defined as the length of time which the venous catheter, such as the needle, was retained in the vein after infusion.
2. Symptom improvement of phlebitis.
3. Health‐related quality of life, using quality of life measures as reported by the study authors.
4. Adverse effects: any adverse events as a result of the use of Aloe vera for treatment or prevention, such as toxic response, anaphylaxis, etc.
Search methods for identification of studies
There were no restrictions on the basis of date or language of publication.
Electronic searches
The Cochrane Peripheral Vascular Diseases Group Trials Search Co‐ordinator (TSC) searched the Specialised Register (last searched February 2014) and the Cochrane Central Register of Controlled Trials (CENTRAL) (2014, Issue 1), part of The Cochrane Library (www.thecochranelibrary.com). See Appendix 1 for details of the search strategy used to search CENTRAL. The Specialised Register is maintained by the TSC and is constructed from weekly electronic searches of MEDLINE, EMBASE, CINAHL, AMED, and through handsearching relevant journals. The full list of the databases, journals and conference proceedings which have been searched, as well as the search strategies used, are described in the Specialised Register section of the Cochrane Peripheral Vascular Diseases Group module in The Cochrane Library (www.thecochranelibrary.com).
In addition, the TSC searched MEDLINE (Appendix 2), EMBASE (Appendix 3) and Allied and Complementary Medicine (AMED) (Appendix 4).
The following trial databases were searched by the TSC for details of ongoing and unpublished studies using the term "aloe vera":
World Health Organization International Clinical Trials Registry Platform (http://apps.who.int/trialsearch/);
ClinicalTrials.gov (http://clinicaltrials.gov/);
Current Controlled Trials (http://www.controlled‐trials.com/).
Author searches
The review authors searched the following Chinese databases for published and unpublished studies:
Chinese BioMedical Database (1980 to 28 February 2014) (http://www.imicams.ac.cn/);
Traditional Chinese Medical Database System (1984 to 28 February 2014) (http://cowork.cintcm.com/engine/windex.jsp);
China National Knowledge Infrastructure (1994 to 28 February 2014) (http://acad.cnki.net/kns55/default.aspx);
Chinese VIP Information (1989 to 28 February 2014) (http://cstj.cqvip.com/);
Chinese Medical Current Contents (1994 to 28 February 2014) (http://210.34.66.109/esource/showdb.jsp?ID=6);
Chinese Academic Conference Papers Database and Chinese Dissertation Database (1994 to February 2014) (http://acad.cnki.net/kns55/brief/result.aspx?dbPrefix=CMFD);
China Medical Academic Conference (1994 to 28 February 2014) (http://acad.cnki.net/kns55/Navigator.aspx?ID=CPFD);
WangFang database (1987 to 28 February 2014) (http://www.wanfangdata.com.cn/).
The search strategies which were used are listed in Appendix 5 to Appendix 12.
Searching other resources
We searched the bibliographies of all relevant publications for further studies. One review author (Zheng GH) contacted authors and experts in the field of surgical nursing (Guan FG, Zhao RH) for any information about unpublished studies.
Data collection and analysis
Selection of studies
Two review authors (Chen HY and Chu JF) independently screened the abstract, title, or both sections of every record retrieved according to the inclusion criteria. The full text of literature with any unclear information in the title or abstract was retrieved for clarification. We investigated all potentially relevant articles as full text. We selected the most complete publication or pooled the data if there were two or more publications relating to one trial. Any disagreements were resolved by discussion.
Data extraction and management
Mei LJ and Yang L independently extracted data using a data extraction form and the results were checked for accuracy by a third author (Zheng GH). Disagreements were resolved by discussion. In the case of duplicate publications and companion papers of a primary paper, we maximised the yield of information by simultaneous evaluation of all available data. The information that was extracted from each included report included the following.
1. Basic information: study design, title, authors, publication status.
2. Participant characteristics: mean age, proportion of each gender, sample number.
3. Methodological description: method of randomisation, method of concealment of allocation, exclusions post‐randomisation, blinding, losses to follow up.
4. Intervention characteristics: type of intervention (prevention or treatment), routes of administration, period of treatment, duration of follow up.
5. Outcomes: primary and secondary outcomes, safety outcomes.
6. Other: infusion given, site of vein, type of catheter, risk factors for phlebitis.
Assessment of risk of bias in included studies
Two review authors (Zheng GH, Yang L) independently assessed the risk of bias of each trial by using the tool developed by The Cochrane Collaboration (Higgins 2011) and the following criteria were used. Disagreements were resolved by consensus.
1. Was the allocation sequence randomly generated?
2. Was the treatment allocation adequately concealed?
3. Was knowledge of the allocated interventions adequately prevented during the study?
4. Were incomplete outcome data adequately addressed?
5. Are reports of the study free of suggestion of selective outcome reporting?
6. Was the study apparently free of other problems that could put it at a high risk of bias?
Measures of treatment effect
Dichotomous outcomes
We expressed dichotomous data (for example the rate of resolution of phlebitis, incidence of phlebitis) as the risk ratio (RR) with its 95% confidence interval (CI). We pooled data using the fixed‐effect model or the random‐effects model when heterogeneity among studies was obvious. We did not calculate the risk difference (RD) or include the number needed to treat (NNT) in the analysis because this was not feasible with the data available. We did not perform the evaluation of adverse events because the data were unavailable in the included studies. If adverse events data are available in future updates we will calculate the number needed to harm (NNH).
Continuous data
We analysed continuous outcomes by using mean differences (MD) with 95% CIs.
Unit of analysis issues
The primary analysis was based on per individual randomised. Cross‐over trials and cluster‐randomised trials were not found for this review. For trials with more than two intervention groups, we combined groups to create a single pair‐wise comparison (Higgins 2011a).
Dealing with missing data
We attempted to obtain relevant missing data from the authors of the included trials, if feasible, and carefully performed evaluations of important numerical data such as the number of screened and randomised participants as well as the intention‐to‐treat (ITT) and per protocol (PP) populations.
Assessment of heterogeneity
We tested the heterogeneity between the included trials by examining the I2 statistic, quantifying inconsistency across studies, to assess the impact of heterogeneity on the meta‐analysis (Higgins 2003). We analysed the study components such as the participants, diseases, interventions, comparisons and outcomes if obvious heterogeneity (I2 > 75%) was found, and explained the source of heterogeneity by subgroup analysis and sensitivity analysis. We used a random‐effects model meta‐analysis as an overall summary if the heterogeneity among trials was considered acceptable (85% > I2 > 50%). If heterogeneity was substantial (I2 > 85%) we did not pool the data and produced a narrative qualitative summary.
Assessment of reporting biases
We used funnel plots to assess publication bias when a sufficient number of studies were included (at least 10 studies) as recommended by the Cochrane Handbook for Systematic Reviews of Interventions 5.1.0 (Higgins 2011; Sterne 2001).
Data synthesis
For the predefined outcomes which were available, measured similarly, and of sufficient quality we statistically summarised the pooled effects and performed statistical analyses according to the statistical guidelines referenced in the current version of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a).
We performed a meta‐analysis of the data using a fixed‐effect model prior to a random‐effects model only if the test of heterogeneity was not significant. However, if heterogeneity was substantial (I2 > 85%), we did not perform a meta‐analysis at all and a narrative, qualitative summary was carried out.
Subgroup analysis and investigation of heterogeneity
We carried out the following subgroup analyses to explore possible sources of heterogeneity, for primary outcomes only.
1. Duration of treatment (such as Aloe vera used for three, five or seven days, etc).
2. Types of Aloe vera products (such as a thin slice or the juice of Aloe vera and Aloe‐derived products).
Sensitivity analysis
Where possible, we performed the following sensitivity analyses to explore the effect of potential biases.
1. Repeating the analysis excluding unpublished studies.
2. Repeating the analysis taking account of study quality.
3. Reanalysing the data using different statistical methods.
4. Repeating the analysis excluding qRCTs.
Results
Description of studies
For a detailed description of the studies, see Characteristics of included studies; Characteristics of excluded studies.
Results of the search
See Figure 1.
1.

Study flow diagram.
Included studies
A total of 43 studies (35 RCTs and eight qRCTs) with 7465 participants were included in the review (Cao 2008; Chen 2009; Chen 2010; Chen 2012; Deng 2010; Deng 2012; Dong 2001; Dong 2008; Gao 2007; Gao 2012; Hou 2010; Hu 2009; Ji 2007; Jin 2010; Li 2003; Li 2006; Li 2009; Li 2011; Liang 2004; Liu 2003; Liu 2006; Liu 2012; Liu 2012b; Lu 2004; Pan 2008; Peng 2009; Ren 2008; Tan 2002; Tang 2011; Wang 2006; Wang 2008; Wang 2010; Wang 2010b; Wu 2009; Xiao 2008; Yang 2008; Yao 2009; Yu 2006; Zhang 2010; Zhang 2013; Zheng 2010; Zhong 2011; Zhou 2006).
The number of participants in each trial ranged from 40 (Chen 2009) to 3000 (Hu 2009). The age of participants ranged from 18 to 75 years old except for one study where the age of participants ranged from one month to three years old (Gao 2007). All included studies were conducted in China, and were performed in the hospital setting.
Various formulations of Aloe vera or aloe‐derived products were used in the included studies. A slice of fresh aloe 5 cm to 15 cm long and 3 cm wide was most commonly used and reported on in 31 included studies. Six studies used fresh aloe leaf divided into pieces 15 cm to 20 cm long and 5 cm to 10 cm wide (Hou 2010; Hu 2009; Pan 2008; Wang 2010b; Xiao 2008; Zhang 2010). Other aloe vera products used were the juice of fresh aloe leaf in three included studies (Deng 2010; Jin 2010; Ren 2008), aloe glue in two studies (Chen 2010; Li 2011), and dry aloe linimentum in one study (Lu 2004).
Twenty‐two studies with 5546 participants involved the use of Aloe vera for prevention of phlebitis (Cao 2008; Chen 2012; Dong 2008; Hou 2010; Hu 2009; Ji 2007; Li 2011; Liang 2004; Liu 2003; Liu 2006; Liu 2012b; Pan 2008; Peng 2009; Ren 2008; Tan 2002; Wang 2006; Wu 2009; Xiao 2008; Yao 2009; Yu 2006; Zhang 2010; Zheng 2010). Of these 22 studies, 13 trials with 4272 participants compared Aloe vera alone in the treatment group with no treatment in the control group (Cao 2008; Dong 2008; Hu 2009; Li 2011; Liu 2006; Liu 2012b; Pan 2008; Peng 2009; Tan 2002; Xiao 2008; Yao 2009; Yu 2006; Zheng 2010), and two trials with 248 participants compared Aloe vera alone with 50% MgSO4 (Ren 2008; Zhang 2010). Two trials with 200 participants compared Aloe vera versus 33% MgSO4 (Chen 2012; Hou 2010). One trial with 66 participants compared Aloe vera alone with potato slices (Wu 2009). One trial with 150 participants compared Aloe vera alone with 75% alcohol (Liang 2004). One trial with 147 participants compared aloe plus an ice bag compress with an ice bag compress plus 75% MgSO4 (Liu 2003). One study was a three‐arm trial with 320 participants comparing Aloe vera versus potato slice versus no treatment (Wang 2006). The remaining trial with 142 participants compared Aloe vera plus saline (NaCl2) plus dexamethasone with NaCl2 plus dexamethasone in the control group (Ji 2007).
The therapeutic effect of Aloe vera for phlebitis was evaluated in 21 studies involving 1919 participants (Chen 2009; Chen 2010; Deng 2010; Deng 2012; Dong 2001; Gao 2007; Gao 2012; Jin 2010; Li 2003; Li 2006; Li 2009; Liu 2012; Lu 2004; Tang 2011; Wang 2008; Wang 2010; Wang 2010b; Yang 2008; Zhang 2013; Zhong 2011; Zhou 2006). Of these, 10 studies with 880 participants compared aloe alone in the treatment group with 50% MgSO4 in the control group (Chen 2010; Deng 2010; Deng 2012; Gao 2007; Li 2003; Li 2009; Liu 2012; Tang 2011; Wang 2010; Yang 2008), and three studies with 422 participants compared Aloe vera alone with 33% MgSO4 (Dong 2001; Gao 2012; Li 2006). The remaining eight studies compared Aloe vera alone or Aloe vera plus other treatment, such as sulphanilamide pyrimidine, potato, sulphonic acid mucopolysaccharide cream, in the treatment group versus the same 'other treatment' in the control group (Chen 2009; Jin 2010; Lu 2004; Wang 2008; Wang 2010b; Zhang 2013; Zhong 2011; Zhou 2006).
Excluded studies
In total 60 studies were excluded on the basis of their full text versions. Reasons for exclusion were as follows: self‐controlled clinical trial (Zou 2004), an incorrect control used (the non‐Aloe vera treatment used in the treatment group but not used in the control group) in 50 studies (Cao 2007; Dai 2007; Deng 2001; Deng 2009; Gong 2004; Gu 2013; Guo 2009; Hu 2011; Huang 2005, Huang 2009; Jiang 2007; Li 2003b; Li 2006b; Li 2010; Li 2011b; Liang 2011; Lin 2009; Lu 2004b; Lu 2007; Lu 2008; Lu 2010; Lu 2010b; Lu 2011; Ma 2008; Ma 2009; Mo 2008; Peng 2002; Shao 2008; Shen 2013; Shi 2013; Su 2011; Tang 2008; Tang 2008b; Wang 2004;Wang 2010c;Wang 2011;Wang 2012; Wang 2013; Wei 2012; Wu 2010;Xu 2007;Xu 2008;Yan 2012; Yang 2010;Ye 2008; Ye 2011;Zhang 2004; Zhang 2008; Zhu 2010; Zhuang 2008), and an unclear intervention duration in nine studies (Chen 2007; Huang 2013; Lei 2012; Liu 2009; Zhang 2003; Zhang 2005; Zhang 2006; Zhang 2011; Zhou 2001). Although we were able to contact one of the authors, we did not obtain more information to judge the duration of the intervention.
Risk of bias in included studies
Of the 43 included studies, all included studies were judged as at high risk of bias because one or more main aspects of the bias assessment was labelled high or unclear risk of bias. We tried to contact the original study authors but we received no response from most of them. See Figure 2 and Figure 3.
2.
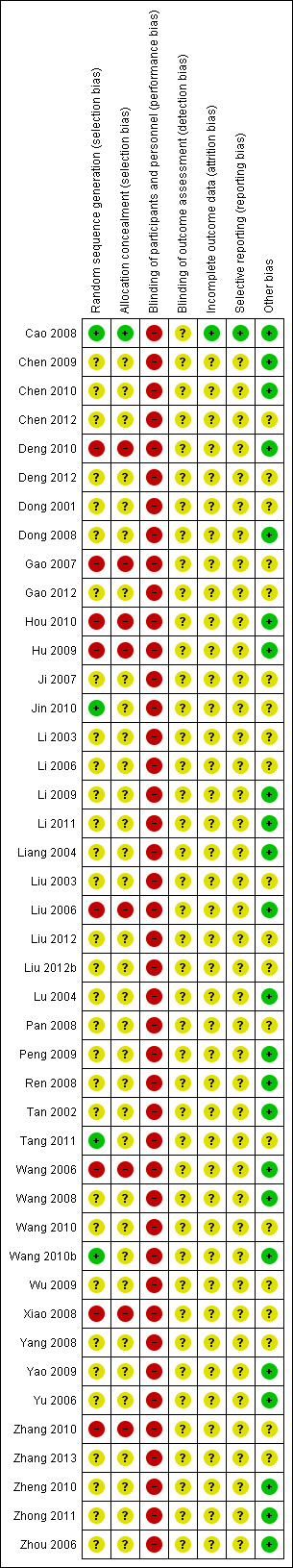
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.
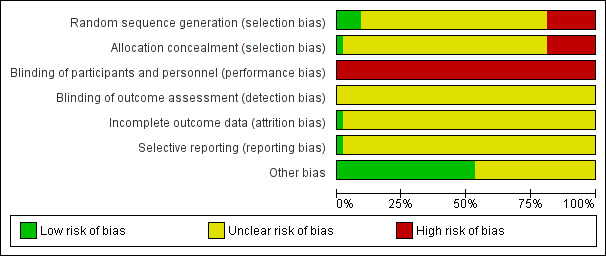
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Four studies with adequate sequence generation used random digit tables or computer random digit generators (Cao 2008; Jin 2010; Tang 2011; Wang 2010b). Eight studies were of qRCT design and judged as at high risk of bias because of alternate allocation using the participant' birthdays or admission sequences (Deng 2010; Gao 2007; Hou 2010; Hu 2009; Liu 2006; Wang 2006; Xiao 2008; Zhang 2010). In the other studies the method was unclear because of a lack of useful information reported in the study papers.
Adequate allocation concealment, by sealed opaque envelopes, was used in one study (Cao 2008). The eight qRCTs were judged to be at high risk of bias for allocation concealment as described above (Deng 2010; Gao 2007; Hou 2010; Hu 2009; Liu 2006; Wang 2006; Xiao 2008; Zhang 2010). The remainder of the studies were judged to be at unclear risk of bias because of insufficient reporting.
Blinding
Blinding of participants and nurses was not possible due to the external application of Aloe vera. Therefore, we assessed blinding as at high risk of bias in all included studies. No included studies reported the blinding of outcome assessors, and we judged this risk of bias to be unclear because of a lack of sufficient information reported in the papers.
Incomplete outcome data
The flow of participants was not reported in any of the included studies so we were uncertain whether or not the reported outcome data were appropriate. One study reported that the withdrawal and attrition rates between the treatment and control groups were similar (Cao 2008) and this study was therefore judged to be at low risk of bias. For the remaining 42 studies it was not clear how many were randomised and how many withdrawn following randomisation.
Selective reporting
No study protocols were available for any of the included studies. Therefore, it was difficult to judge whether selective reporting bias existed in the studies. Only for Cao 2008 it was clear that the expected outcomes were reported. We judged the remaining studies to be at unclear risk of bias.
Other potential sources of bias
The baseline information were considered to be similar between the comparison groups in 23 (Cao 2008; Chen 2009; Chen 2010; Deng 2010; Dong 2008; Hou 2010; Hu 2009; Li 2009; Li 2011; Liang 2004; Liu 2006; Lu 2004; Peng 2009; Ren 2008; Tan 2002; Wang 2006; Wang 2008; Wang 2010b; Yao 2009; Yu 2006; Zheng 2010; Zhong 2011; Zhou 2006) of the included studies. The remaining 20 studies (Chen 2012; Deng 2012; Dong 2001; Gao 2007; Gao 2012; Ji 2007; Jin 2010; Li 2003; Li 2006; Liu 2003; Liu 2012; Liu 2012b; Pan 2008; Tang 2011; Wang 2010; Wu 2009; Xiao 2008; Yang 2008; Zhang 2010; Zhang 2013) were judged as at unclear risk of bias because they lacked a detailed description of the baseline information.
Effects of interventions
Primary outcomes
Of the 43 studies that met the selection criteria, 22 studies reported the prophylactic effect of Aloe vera (Cao 2008; Chen 2012; Dong 2008; Hou 2010; Hu 2009; Ji 2007; Li 2011; Liang 2004; Liu 2003; Liu 2006; Liu 2012b; Pan 2008; Peng 2009; Ren 2008; Tan 2002; Wang 2006; Wu 2009; Xiao 2008; Yao 2009; Yu 2006; Zhang 2010; Zheng 2010) by measuring the total incidence of phlebitis, which was divided into first degree, second degree and third degree phlebitis according to the severity of the symptoms.
In the pooled analysis of the prophylactic effect we included the numbers of patients with first degree and second degree phlebitis in the outcome 'incidence of second degree phlebitis', and the numbers of patients with third, second and first degree phlebitis in the outcome total incidence of phlebitis.
Twenty‐one studies reported the treatment effect of Aloe vera (Chen 2009; Chen 2010; Deng 2010; Deng 2012; Dong 2001; Gao 2007; Gao 2012; Jin 2010; Li 2003; Li 2006; Li 2009; Liu 2012; Lu 2004; Tang 2011; Wang 2008; Wang 2010; Wang 2010b; Yang 2008; Zhang 2013; Zhong 2011; Zhou 2006) by using the resolution rate of phlebitis (or total improvement rate of phlebitis). Patients were divided into those with recovery, marked improvement and improvement as the outcomes.
In this review we did not select the improvement outcome alone for analysis because we considered that the outcomes recovery, marked improvement and improvement were correlative. For example, a study showing a high number of participants with the recovery outcome will accordingly show a low number of participants with the outcomes marked improvement or improvement. Therefore, we assumed a cumulative effect of the marked improvement and improvement outcomes, and ruled that the outcome marked improvement contained the patients with recovery plus those with a marked improvement; and the outcome of total improvement contained the numbers showing recovery plus those with marked improvement plus improvement in the pooled analysis of the treatment effect.
Prevention of infusion phlebitis
Total incidence of phlebitis
The total incidence of phlebitis was directly reported in four studies (Chen 2012; Hou 2010; Liang 2004; Peng 2009), and calculated by the review authors by using the formula: all grades of phlebitis = first degree phlebitis plus second degree phlebitis plus third degree phlebitis, in the other studies (Cao 2008; Dong 2008; Hu 2009; Ji 2007; Li 2011; Liu 2003; Liu 2006; Liu 2012b; Pan 2008; Ren 2008; Tan 2002; Wang 2006; Wu 2009; Xiao 2008; Yao 2009; Yu 2006; Zhang 2010; Zheng 2010). The results showed that Aloe vera decreased the total incidence of phlebitis when compared with no other intervention at different intervention periods; the risk ratio (RR) with three and seven days intervention duration was 0.44 (95% CI 0.29 to 0.66) and 0.28 (95% CI 0.11 to 0.68) respectively. The total incidence at five days of intervention showed an I2 of 93%, which was higher than the pre‐planned cut‐off for pooling data. However, due to limitations of the software we were unable to switch off the totals for individual subgroups (Analysis 1.1). An overall pooled analysis was not performed because of the obvious heterogeneity among the included studies (Analysis 1.1). Compared with external application of a potato slice (Wang 2006; Wu 2009), Aloe vera had no obvious advantage for reducing the total incidence of phlebitis with the pooled RR being 0.90 (95% CI 0.58 to 1.40, P = 0.65, I2 = 0%, Analysis 2.1). However, significant differences were observed with Aloe vera for preventing all grades of phlebitis compared with external application of 75% alcohol (Liang 2004) and 33% MgSO4 (Chen 2012; Hou 2010) with a RR of 0.02 (95% CI 0.0 to 0.28, P = 0.004, Analysis 6.1) and 0.43 (95% CI 0.24 to 0.78, P = 0.005, Analysis 3.1) respectively. Compared with 50% MgSO4 (Ren 2008; Zhang 2010), external application of Aloe vera had no obvious advantage for the total incidence of phlebitis and the pooled RR was 0.41 (95% CI 0.16 to 1.07, P = 0.07, I2 = 76%, Analysis 4.1). One trial (Liu 2006) compared Aloe vera plus an ice bag with an ice bag plus 75% MgSO4 and the effect on the prevention of all grades of phlebitis was not significant (RR 1.10, 95% CI 0.54 to 2.25, P = 0.79, Analysis 5.1).
1.1. Analysis.
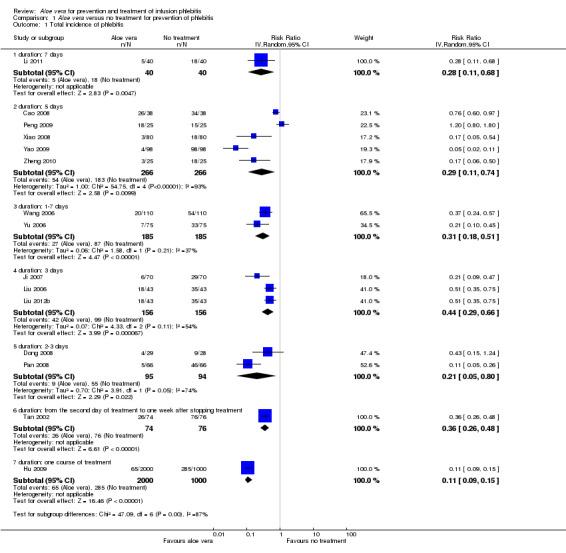
Comparison 1 Aloe vera versus no treatment for prevention of phlebitis, Outcome 1 Total incidence of phlebitis.
2.1. Analysis.
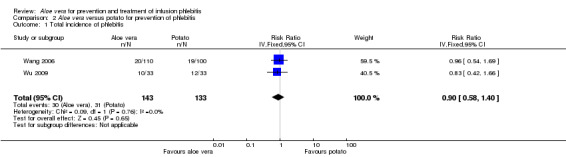
Comparison 2 Aloe vera versus potato for prevention of phlebitis, Outcome 1 Total incidence of phlebitis.
6.1. Analysis.
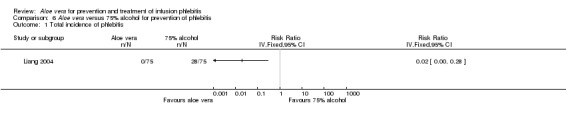
Comparison 6 Aloe vera versus 75% alcohol for prevention of phlebitis, Outcome 1 Total incidence of phlebitis.
3.1. Analysis.
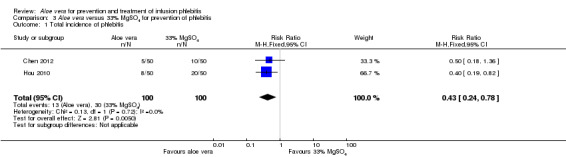
Comparison 3 Aloe vera versus 33% MgSO4 for prevention of phlebitis, Outcome 1 Total incidence of phlebitis.
4.1. Analysis.
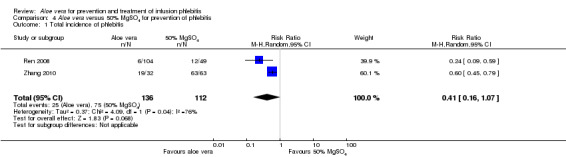
Comparison 4 Aloe vera versus 50% MgSO4 for prevention of phlebitis, Outcome 1 Total incidence of phlebitis.
5.1. Analysis.
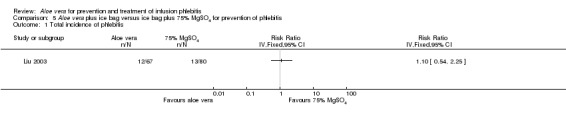
Comparison 5 Aloe vera plus ice bag versus ice bag plus 75% MgSO4 for prevention of phlebitis, Outcome 1 Total incidence of phlebitis.
Incidence of second degree phlebitis
Fourteen studies (Cao 2008; Dong 2008; Hu 2009; Ji 2007; Li 2011; Liu 2006; Liu 2012b; Pan 2008; Tan 2002; Wang 2006; Xiao 2008; Yao 2009; Yu 2006; Zheng 2010) with a combined total of 4585 participants compared Aloe vera with no other intervention and reported the numbers of participants with second degree phlebitis. The application of Aloe vera alone had a significant effect on preventing second degree phlebitis (including first degree phlebitis) with a pooled RR of 0.18 (95% CI 0.10 to 0.31, P < 0.0001, I2 = 70%, Analysis 1.2). There was no significant difference between Aloe vera and a potato slice on second degree phlebitis (Wang 2006; Wu 2009) (RR 1.14, 95% CI 0.49 to 2.67, P = 0.76, Analysis 2.2). A significant difference was observed between Aloe vera and 50% MgSO4 (Ren 2008; Zhang 2010) (RR 0.26, 95% CI 0.14 to 0.50, P < 0.0001, Analysis 4.2). The comparison of Aloe vera plus an ice bag with external application of 75% MgSO4 plus an ice bag (Liu 2003) showed no significant advantage in preventing second degree phlebitis (RR 0.68, 95% CI 0.21 to 2.23, P = 0.53, Analysis 5.2).
1.2. Analysis.
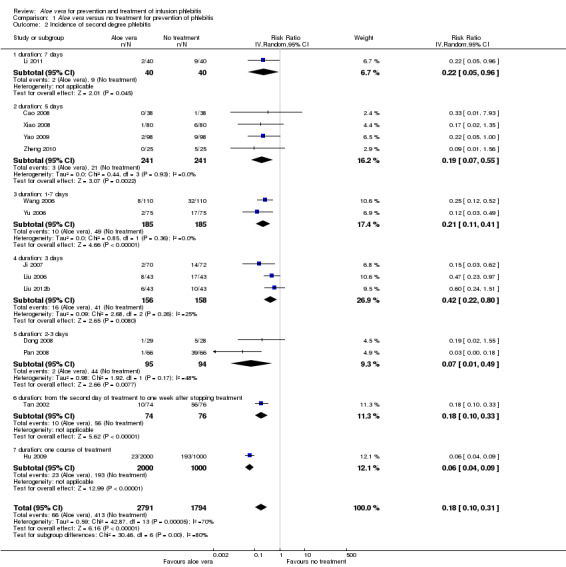
Comparison 1 Aloe vera versus no treatment for prevention of phlebitis, Outcome 2 Incidence of second degree phlebitis.
2.2. Analysis.
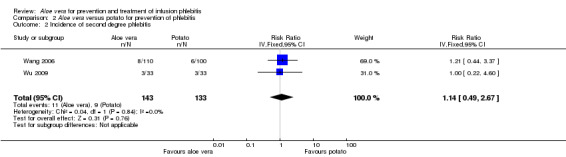
Comparison 2 Aloe vera versus potato for prevention of phlebitis, Outcome 2 Incidence of second degree phlebitis.
4.2. Analysis.

Comparison 4 Aloe vera versus 50% MgSO4 for prevention of phlebitis, Outcome 2 Incidence of second degree phlebitis.
5.2. Analysis.
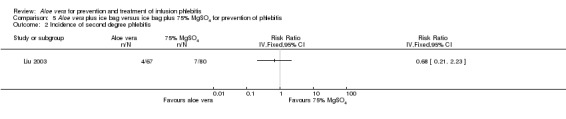
Comparison 5 Aloe vera plus ice bag versus ice bag plus 75% MgSO4 for prevention of phlebitis, Outcome 2 Incidence of second degree phlebitis.
Incidence of third degree phlebitis
Comparing Aloe vera alone with no prophylactic intervention (14 studies) (Cao 2008; Dong 2008; Hu 2009; Ji 2007; Li 2011; Liu 2006; Liu 2012b; Pan 2008; Tan 2002; Wang 2006; Xiao 2008; Yao 2009; Yu 2006; Zheng 2010) we found a lower incidence of third degree phlebitis using Aloe vera alone, with a significant difference in the incidence pf phlebitis (RR 0.06, 95% CI 0.03 to 0.11, P < 0.00001, I2 = 44%, Analysis 1.3). Two studies (Wang 2006; Wu 2009) compared Aloe vera with potato slice for preventing phlebitis and no significant difference was found in the incidence of third degree phlebitis (RR 0.45, 95% CI 0.04 to 4.94, P = 0.52, Analysis 2.3). In the two studies (Ren 2008; Zhang 2010) comparing Aloe vera with 50% MgSO4 external compresses, a prophylactic effect on third degree phlebitis was not obvious (RR 0.28, 95% CI 0.07 to 1.02, P = 0.05, I2 = 0%, Analysis 4.3). One study reported the effect of Aloe vera combined with other interventions. Liu 2003 compared Aloe vera plus ice bag compresses with ice bag plus 75% MgSO4. No significant difference between the comparison groups was found in the incidence of third degree phlebitis (RR 1.19, 95% CI 0.08 to 18.73, P = 0.9, Analysis 5.3).
1.3. Analysis.
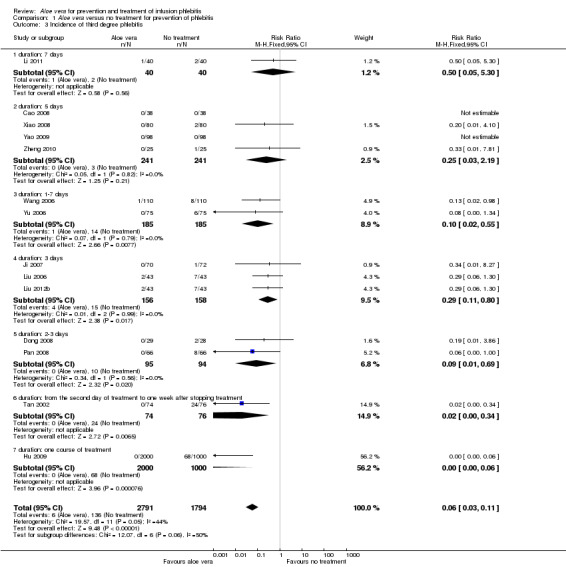
Comparison 1 Aloe vera versus no treatment for prevention of phlebitis, Outcome 3 Incidence of third degree phlebitis.
2.3. Analysis.
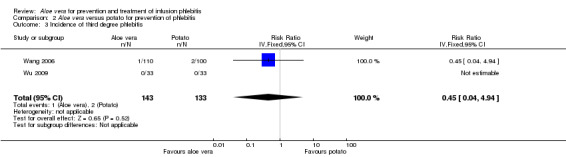
Comparison 2 Aloe vera versus potato for prevention of phlebitis, Outcome 3 Incidence of third degree phlebitis.
4.3. Analysis.
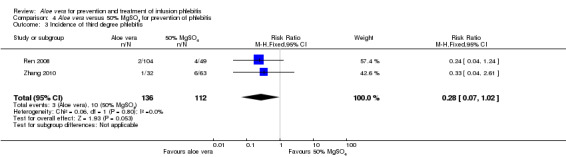
Comparison 4 Aloe vera versus 50% MgSO4 for prevention of phlebitis, Outcome 3 Incidence of third degree phlebitis.
5.3. Analysis.
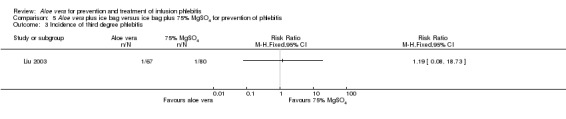
Comparison 5 Aloe vera plus ice bag versus ice bag plus 75% MgSO4 for prevention of phlebitis, Outcome 3 Incidence of third degree phlebitis.
Treatment of infusion phlebitis
The rate of resolution of phlebitis
Recovery rate of phlebitis
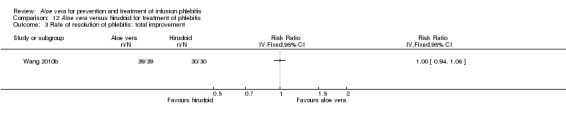
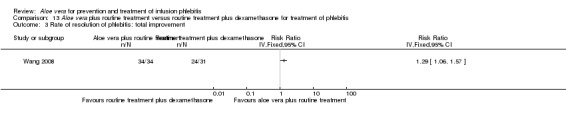
In the studies of Aloe vera alone versus other treatments, seven trials (Deng 2010; Deng 2012; Gao 2007; Li 2003; Liu 2012; Tang 2011; Yang 2008) compared Aloe vera with 50% MgSO4. The treatment effect was statistically significant for the recovery rate of phlebitis (RR 1.42, 95% CI 1.24 to 1.61, P < 0.0001, I2 = 41%, Analysis 8.1). Compared with non‐Aloe vera treatment, Aloe vera plus the same non‐Aloe vera treatment (Chen 2009; Jin 2010; Zhang 2013; Zhong 2011) showed a significant difference for recovery rate of phlebitis (RR 1.75, 95% CI 1.24 to 2.46, P = 0.001, I2 = 57%, Analysis 9.1). One trial (Zhou 2006) compared Aloe vera with potato slice and the RR for the recovery rate of phlebitis was 2.77 (95% CI 1.52 to 5.04, P = 0.0009, Analysis 11.1). One trial (Wang 2010b) reported the result of application of Aloe vera versus hirudoid on the recovery rate of phlebitis and the RR value was 0.36 (95% CI 0.15 to 0.82, P = 0.02, Analysis 12.1). One trial compared Aloe vera plus routine treatment with the same routine treatment plus dexamethasone, the RR was 1.95 (95% CI 1.30 to 2.93, P = 0.001, Analysis 13.1).
8.1. Analysis.
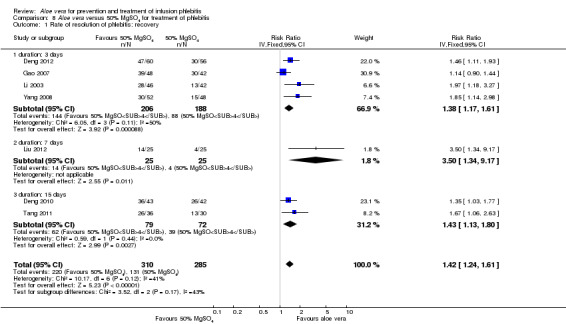
Comparison 8 Aloe vera versus 50% MgSO4 for treatment of phlebitis, Outcome 1 Rate of resolution of phlebitis: recovery.
9.1. Analysis.
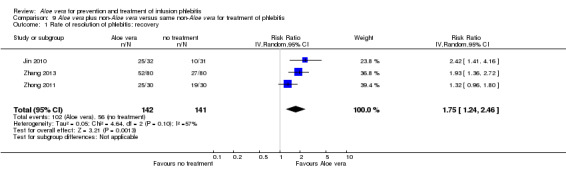
Comparison 9 Aloe vera plus non‐Aloe vera versus same non‐Aloe vera for treatment of phlebitis, Outcome 1 Rate of resolution of phlebitis: recovery.
11.1. Analysis.
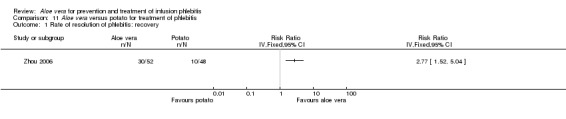
Comparison 11 Aloe vera versus potato for treatment of phlebitis, Outcome 1 Rate of resolution of phlebitis: recovery.
12.1. Analysis.
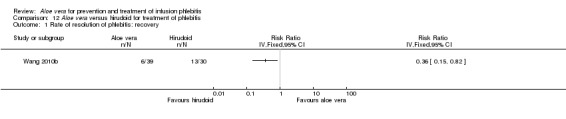
Comparison 12 Aloe vera versus hirudoid for treatment of phlebitis, Outcome 1 Rate of resolution of phlebitis: recovery.
13.1. Analysis.
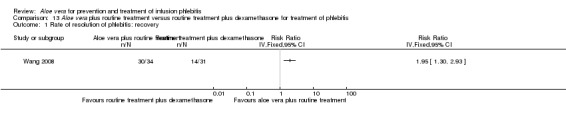
Comparison 13 Aloe vera plus routine treatment versus routine treatment plus dexamethasone for treatment of phlebitis, Outcome 1 Rate of resolution of phlebitis: recovery.
Marked improvement rate of phlebitis
Two studies (Dong 2001; Li 2006) compared Aloe vera with 33% MgSO4 for marked improvement of phlebitis, showing a statistically significant difference (RR 1.97, 95% CI 1.44 to 2.70, P < 0.001, I2 = 33%, Analysis 7.2). Nine trials with 814 participants compared external application of Aloe vera versus 50% MgSO4 and the pooled RR for marked improvement (including recovery) of phlebitis for treatment with Aloe vera compared to 50% MgSO4 was 1.56 (95% CI 1.29 to 1.87, P = 0.0002, I2 = 73%, Analysis 8.2). In the studies of Aloe vera plus non‐Aloe vera treatment versus the same non‐Aloe vera treatment (Chen 2009; Jin 2010; Zhong 2011) the results showed that the application of Aloe vera combined with other treatments had a significant effect on the marked improvement rate of phlebitis (RR 1.26, 95% CI 1.09 to 1.47, P = 0.003, I2 = 0%, Analysis 9.2). One trial (Lu 2004) compared Aloe vera with 75% alcohol and the RR of marked improvement of phlebitis was 3.00 (95% CI 1.09 to 8.25, P = 0.03, Analysis 10.1). One trial (Zhou 2006) comparing Aloe vera with a potato slice in the treatment of phlebitis showed a statistically significant difference in the outcome of marked improvement (RR 1.89, 95% CI 1.36 to 2.62, P = 0.0001, Analysis 11.2). A single study (Wang 2010b) showed that Aloe vera was more effective at increasing the rate of marked improvement of phlebitis than hirudoid alone (RR 0.58, 95% CI 0.44 to 0.78, P = 0.0002, Analysis 12.2). One trial comparing Aloe vera plus routine treatment with the same routine treatment plus dexamethasone (Wang 2008) showed a statistically significant difference on the marked improvement rate of phlebitis (RR 1.95, 95% CI 1.30 to 2.93, P = 0.0001, Analysis 13.2).
7.2. Analysis.
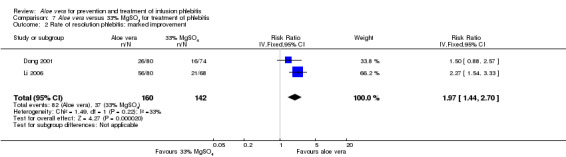
Comparison 7 Aloe vera versus 33% MgSO4 for treatment of phlebitis, Outcome 2 Rate of resolution phlebitis: marked improvement.
8.2. Analysis.
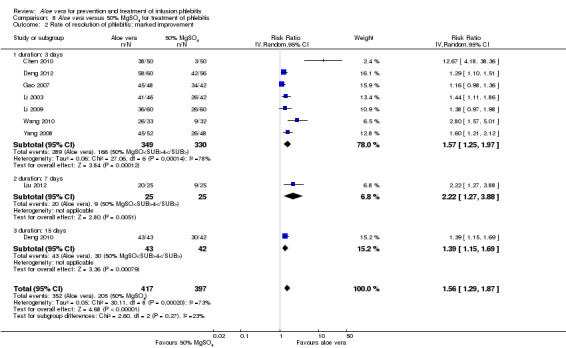
Comparison 8 Aloe vera versus 50% MgSO4 for treatment of phlebitis, Outcome 2 Rate of resolution of phlebitis: marked improvement.
9.2. Analysis.
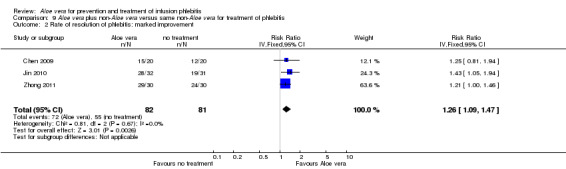
Comparison 9 Aloe vera plus non‐Aloe vera versus same non‐Aloe vera for treatment of phlebitis, Outcome 2 Rate of resolution of phlebitis: marked improvement.
10.1. Analysis.
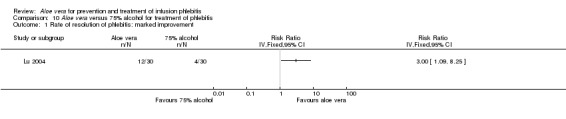
Comparison 10 Aloe vera versus 75% alcohol for treatment of phlebitis, Outcome 1 Rate of resolution of phlebitis: marked improvement.
11.2. Analysis.
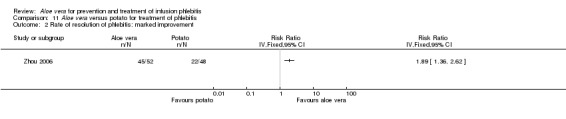
Comparison 11 Aloe vera versus potato for treatment of phlebitis, Outcome 2 Rate of resolution of phlebitis: marked improvement.
12.2. Analysis.
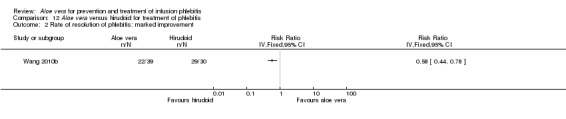
Comparison 12 Aloe vera versus hirudoid for treatment of phlebitis, Outcome 2 Rate of resolution of phlebitis: marked improvement.
13.2. Analysis.
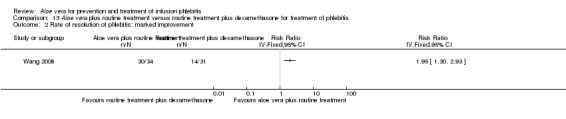
Comparison 13 Aloe vera plus routine treatment versus routine treatment plus dexamethasone for treatment of phlebitis, Outcome 2 Rate of resolution of phlebitis: marked improvement.
Total improvement rate of phlebitis
Three trials (Dong 2001; Gao 2012; Li 2006) reported the effect of Aloe vera versus external application of 33% MgSO4 in the treatment of phlebitis showing a statistically significant difference (RR 1.16, 95% CI 1.09 to 1.24, P < 0.0001, I2 = 26%, Analysis 7.3). Comparing external application of Aloe vera with external application of 50% MgSO4, data for improvement (including recovery and marked improvement) of phlebitis were available in 10 studies (Chen 2010; Deng 2010; Deng 2012; Gao 2007; Li 2003; Li 2009; Liu 2012; Tang 2011; Wang 2010; Yang 2008) and showed a significant difference in the total improvement of phlebitis (RR 1.22, 95% CI 1.16 to 1.28, P < 0.0001, I2 = 0%, Analysis 8.3). In studies of Aloe vera combined with non‐Aloe vera treatment versus non‐Aloe vera treatment, one trial (Jin 2010) compared Aloe vera plus hirudoid with hirudoid, one trial compared Aloe vera plus sulphonic acid mucopolysaccharide cream with sulphonic acid mucopolysaccharide cream (Chen 2009), and one compared Aloe vera plus sulphanilamide pyrimidine with sulphanilamide pyrimidine (Zhong 2011). A significant difference between the comparison groups was observed (RR 1.23, 95% CI 1.13 to 1.35, P < 0.00001, I2 = 37%, Analysis 9.3). One trial (Lu 2004) compared Aloe vera versus 75% alcohol, another (Zhou 2006) compared Aloe vera versus potato slice, with their results showing that application of Aloe vera had a significant effect on the improvement of phlebitis (RR 2.0, 95% CI 1.14 to 3.52, P = 0.02, Analysis 10.2; RR 1.25, 95% CI 1.06 to 1.47, P = 0.008, Analysis 11.3 respectively). However, one trial (Wang 2010b) showed no difference between Aloe vera and hirudoid alone, with the same rate of improvement of phlebitis (39/39, 100% in the Aloe vera group versus 30/30, 100% in the hirudoid group, Analysis 12.3). One trial (Wang 2008) compared Aloe vera plus routine treatment versus the same routine treatment plus dexamethasone; a significant difference between the comparison groups could be observed (RR 1.29, 95% CI 1.06 to 1.57, P = 0.01, Analysis 13.3).
7.3. Analysis.
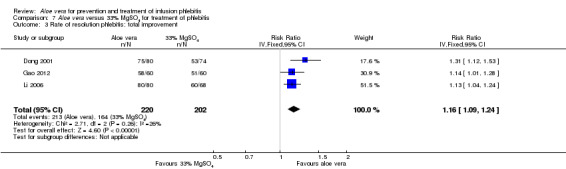
Comparison 7 Aloe vera versus 33% MgSO4 for treatment of phlebitis, Outcome 3 Rate of resolution phlebitis: total improvement.
8.3. Analysis.
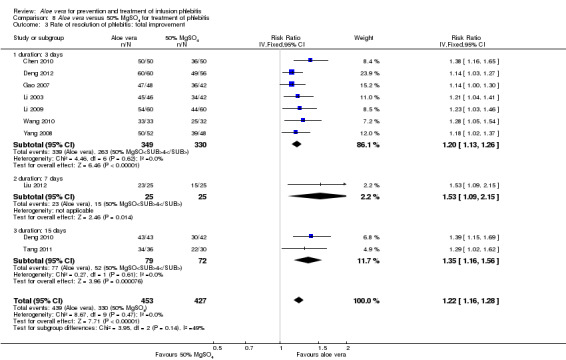
Comparison 8 Aloe vera versus 50% MgSO4 for treatment of phlebitis, Outcome 3 Rate of resolution of phlebitis: total improvement.
9.3. Analysis.
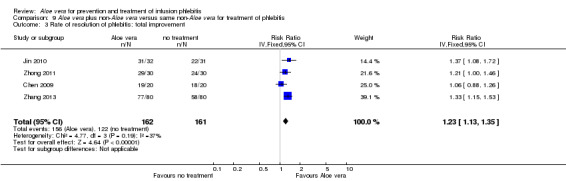
Comparison 9 Aloe vera plus non‐Aloe vera versus same non‐Aloe vera for treatment of phlebitis, Outcome 3 Rate of resolution of phlebitis: total improvement.
10.2. Analysis.
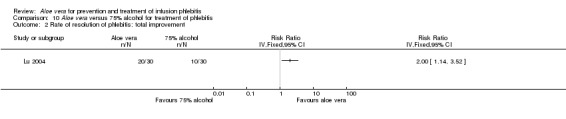
Comparison 10 Aloe vera versus 75% alcohol for treatment of phlebitis, Outcome 2 Rate of resolution of phlebitis: total improvement.
11.3. Analysis.
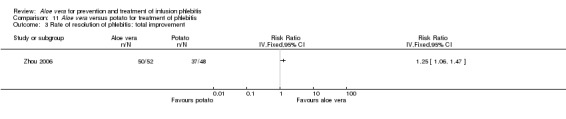
Comparison 11 Aloe vera versus potato for treatment of phlebitis, Outcome 3 Rate of resolution of phlebitis: total improvement.
12.3. Analysis.
Comparison 12 Aloe vera versus hirudoid for treatment of phlebitis, Outcome 3 Rate of resolution of phlebitis: total improvement.
13.3. Analysis.
Comparison 13 Aloe vera plus routine treatment versus routine treatment plus dexamethasone for treatment of phlebitis, Outcome 3 Rate of resolution of phlebitis: total improvement.
Secondary outcomes
Prevention of infusion phlebitis
Duration of successful placement of a venous catheter
The duration of successful placement of a venous catheter is defined as the length of time which the venous catheter, such as the needle, is successfully retained in the vein after infusion.
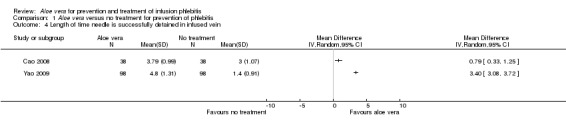
Two trials (Cao 2008; Yao 2009) compared Aloe vera with no treatment for the time the needle was successfully retained in the infused vein. The application of Aloe vera had a significant effect on the length of time the needle was successfully retained in the vein compared with no treatment. The MD was 0.79 days (95% CI 0.33 to 1.25) in Cao 2008 and 3.40 days (95% CI 3.08 to 3.72) in Yao 2009. A pooled analysis was not performed because of the heterogeneity between the two trials (I2 = 99%) (Analysis 1.4).
1.4. Analysis.
Comparison 1 Aloe vera versus no treatment for prevention of phlebitis, Outcome 4 Length of time needle is successfully detained in infused vein.
Other secondary outcomes
Other secondary outcomes such as health‐related quality of life and adverse effects, predefined in the protocol, were not reported in the included studies on prevention of infusion phlebitis.
Treatment of infusion phlebitis
Symptom improvement of phlebitis
Time of elimination of red swelling symptoms in phlebitis
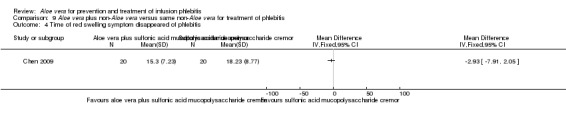
The time for elimination of red swelling symptoms in the infusion vein was measured in one trial (Chen 2009) comparing Aloe vera plus sulphonic acid mucopolysaccharide cream versus sulphonic acid mucopolysaccharide cream. The analysis showed that application of Aloe vera combined with sulphonic acid mucopolysaccharide cream was not significantly better than the application of sulphonic acid mucopolysaccharide cream alone in shortening the time of red swelling symptom elimination in the infusion vein (MD ‐2.93 hours, 95% CI ‐7.91 to 2.05, P = 0.25, Analysis 9.4).
9.4. Analysis.
Comparison 9 Aloe vera plus non‐Aloe vera versus same non‐Aloe vera for treatment of phlebitis, Outcome 4 Time of red swelling symptom disappeared of phlebitis.
Time of pain relief at the location of the infusion vein
Data for time of pain relief at the location of the infusion vein could be obtained from a single trial (Chen 2009). Compared with the application of sulphonic acid mucopolysaccharide cream alone, Aloe vera combined with sulphonic acid mucopolysaccharide cream shortened the time for pain relief at the location of the infusion vein (MD ‐0.97 hours, 95% CI ‐1.58 to ‐0.36, P = 0.002, Analysis 9.5).
9.5. Analysis.
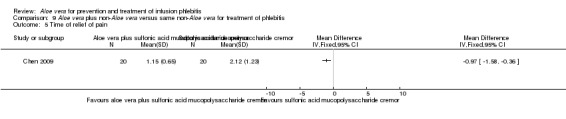
Comparison 9 Aloe vera plus non‐Aloe vera versus same non‐Aloe vera for treatment of phlebitis, Outcome 5 Time of relief of pain.
Time of resolution of phlebitis
One trial (Wang 2010) of Aloe vera versus external application of 50% MgSO4 reported the time of marked improvement and time of improvement of phlebitis symptoms. Compared with the external application of 50% MgSO4, application of Aloe vera had a significant effect in shortening the time for marked improvement and improvement of phlebitis symptom (MD ‐20.0 hours, 95% CI ‐25.23 to ‐14.77, P < 0.0001, Analysis 8.4; MD ‐13.0 hours, 95% CI: ‐16.39 to ‐9.61, P < 0.0001, Analysis 8.5 respectively).
8.4. Analysis.
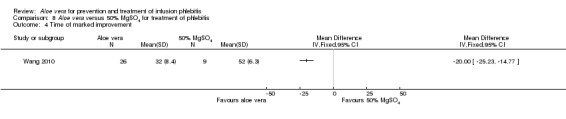
Comparison 8 Aloe vera versus 50% MgSO4 for treatment of phlebitis, Outcome 4 Time of marked improvement.
8.5. Analysis.
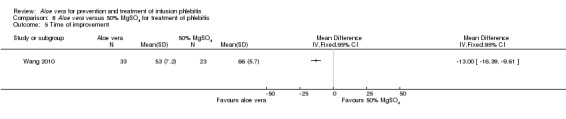
Comparison 8 Aloe vera versus 50% MgSO4 for treatment of phlebitis, Outcome 5 Time of improvement.
Other secondary outcomes
Other secondary outcomes such as health‐related quality of life and adverse effects, predefined in the protocol, were not reported in the included studies on treatment of infusion phlebitis.
Subgroup analysis
Analysis of data for 14 trials (Cao 2008; Dong 2008; Hu 2009; Ji 2007; Li 2011; Liu 2006; Liu 2012b; Pan 2008; Tan 2002; Wang 2006; Xiao 2008; Yao 2009; Yu 2006; Zheng 2010) with 4585 participants suggested that external application of fresh Aloe vera resulted in an overall benefit on prevention of third degree phlebitis compared with no treatment in the control group (RR 0.06, 95% CI 0.03 to 0.11, I2 = 44%, Analysis 1.3). We did not find definitive evidence that study duration explained the observed variation of effect. The test for subgroup differences by duration of treatment did not reach statistical significance overall (P = 0.06, Analysis 1.3).
Results of the subgroup analysis based on the incidence of second degree phlebitis were similar and an obvious advantage could be found comparing external application of Aloe vera for varied durations of treatment with no treatment (Analysis 1.2). The difference between the subgroups was significant (Chi2 = 29.81, df = 6, P < 0.0001) and showed that the varied duration of treatment might explain some of the overall heterogeneity (Analysis 1.2). For the incidence of total phlebitis, the results of the subgroup analysis according to the varied durations showed that external application of Aloe vera was effective for prevention of phlebitis compared with no treatment but the overall heterogeneity was not substantially decreased between subgroups (Analysis 1.1).
Comparing Aloe vera and 50% MgSO4, nine trials (Chen 2010; Deng 2010; Deng 2012; Gao 2007; Li 2003; Li 2009; Liu 2012; Wang 2010; Yang 2008) with 814 participants reported marked improvement and total improvement outcomes; and seven trials (Deng 2010; Deng 2012; Gao 2007; Li 2003; Liu 2012; Tang 2011; Yang 2008) with 595 participants reported recovery as an outcome. All of the pooled results showed that external application of Aloe vera improved symptoms of phlebitis compared with the application of 50% MgSO4. Heterogeneity was observed among the included trials. We undertook subgroup analysis by duration of treatment (three, seven or 15 days). Based on the test for subgroup differences across the three outcomes, we did not have evidence of a statistically significant association between study duration and effect size (Analysis 8.1; Analysis 8.2; Analysis 8.3).
The pre‐planned subgroup analysis for types of Aloe vera products (such as a thin slice or the juice of Aloe vera and Aloe derived products)' was not conducted because of insufficient data.
Sensitivity analysis
When the meta‐analysis for incidence of third degree phlebitis (I2 = 44%) with the comparison Aloe vera versus no treatment was based on a fixed‐effect model (RR 0.06, 95% CI 0.03 to 0.11, P < 0.00001, Analysis 1.3) the RR was lower and the 95% CI was wider compared with when calculated with the random‐effects model (RR 0.13, 95% CI 0.05 to 0.34, P < 0.0001, Analysis 14.1), but the selection of the statistical model did not have a substantial impact on the significance of effects.
14.1. Analysis.
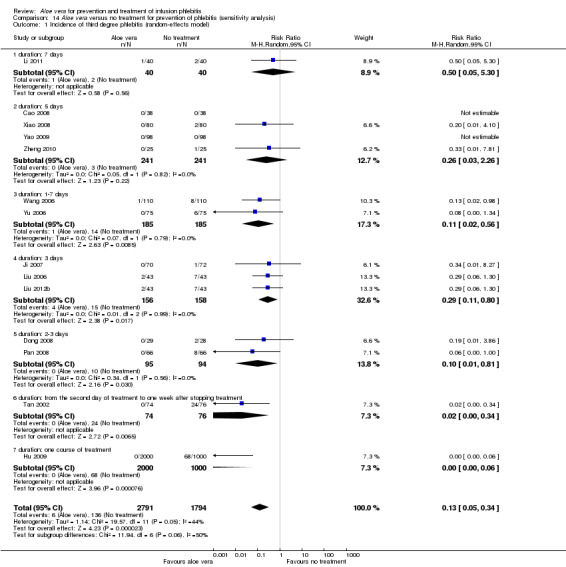
Comparison 14 Aloe vera versus no treatment for prevention of phlebitis (sensitivity analysis), Outcome 1 Incidence of third degree phlebitis (random‐effects model).
Where possible, we performed sensitivity analyses by excluding qRCTs. The decrease in heterogeneity was very obvious in the analysis of external explication of Aloe vera versus no treatment for incidence of third degree phlebitis and incidence of second degree phlebitis on excluding the four qRCTs (Hu 2009; Liu 2006; Wang 2006; Xiao 2008). The RR with the inclusion of qRCTs was 0.06 (95% CI 0.03 to 0.11, P < 0.00001, I2 = 44%, Analysis 1.3) and 0.18 (95% CI 0.10 to 0.31, P < 0.00001, I2 = 70%, Analysis 1.2) respectively. But the observed effect was RR 0.11 (95% CI 0.05 to 0.26, P < 0.00001, I 2= 0%, Analysis 14.2) and 0.16 (95% CI 0.11 to 0.24, P < 0.00001, I2 = 26%, Analysis 14.3) when qRCTs were excluded. However, qRCTs did not substantially reduce the overall heterogeneity for the total incidence of phlebitis in the comparison external application of Aloe vera versus no treatment (Analysis 14.4).
14.2. Analysis.
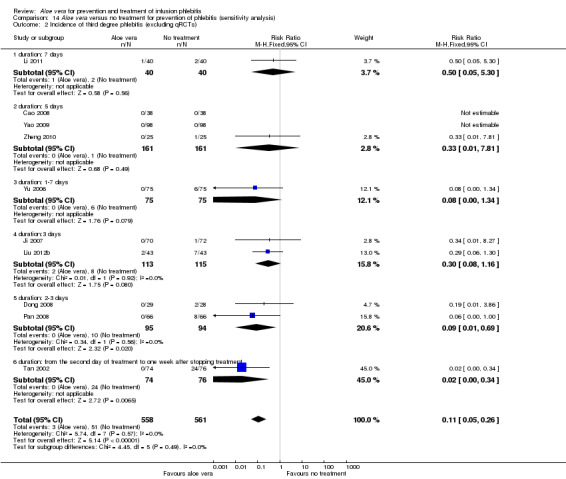
Comparison 14 Aloe vera versus no treatment for prevention of phlebitis (sensitivity analysis), Outcome 2 Incidence of third degree phlebitis (excluding qRCTs).
14.3. Analysis.
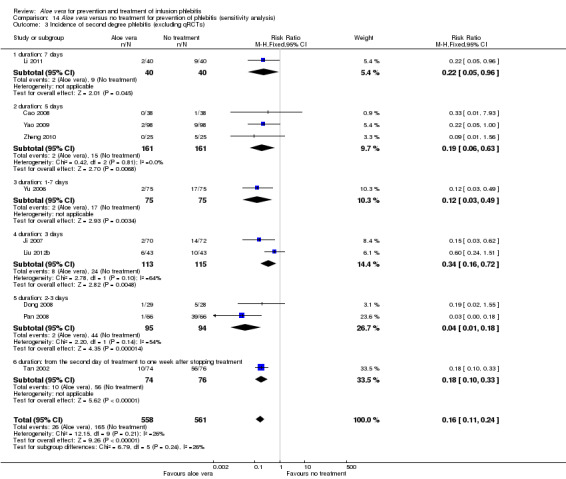
Comparison 14 Aloe vera versus no treatment for prevention of phlebitis (sensitivity analysis), Outcome 3 Incidence of second degree phlebitis (excluding qRCTs).
14.4. Analysis.
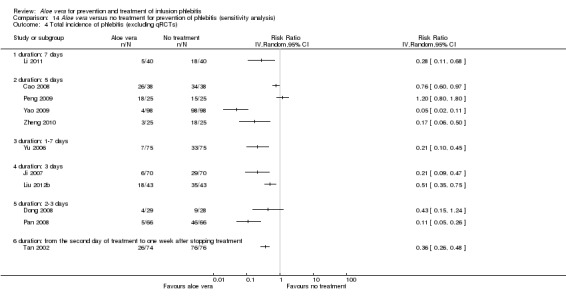
Comparison 14 Aloe vera versus no treatment for prevention of phlebitis (sensitivity analysis), Outcome 4 Total incidence of phlebitis (excluding qRCTs).
We did not perform the pre‐planned sensitivity analyses for study quality and unpublished studies because the study quality of the included studies was similar and there were no unpublished trials included in the review.
Publication bias
We identified possible publication bias in the assessment of the total incidence of phlebitis, incidence of second degree phlebitis and the incidence of third degree phlebitis, with 10 or more trials available for meta‐analysis. The funnel plots (Figure 4; Figure 5; Figure 6) showed obvious asymmetry suggesting evidence of publication bias.
4.
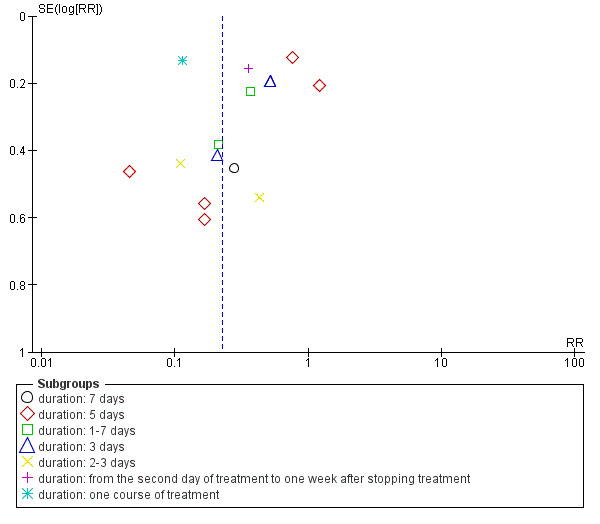
Funnel plot of comparison: 1 Aloe vera versus no treatment for prevention of phlebitis, outcome: 1.1 Total incidence of phlebitis.
5.
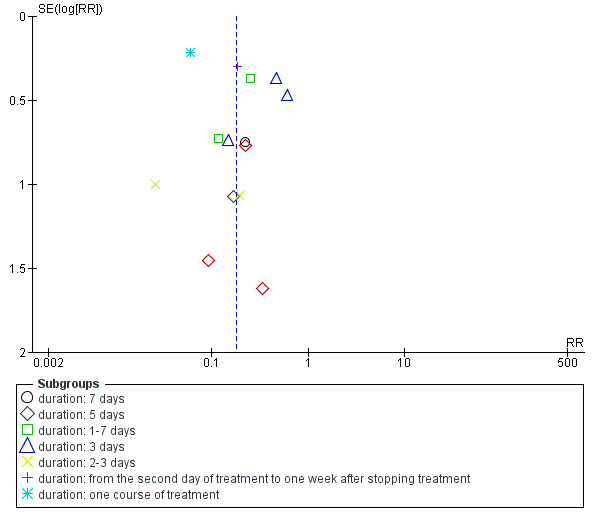
Funnel plot of comparison: 1 Aloe vera versus no treatment for prevention of phlebitis, outcome: 1.2 Incidence of second degree phlebitis.
6.
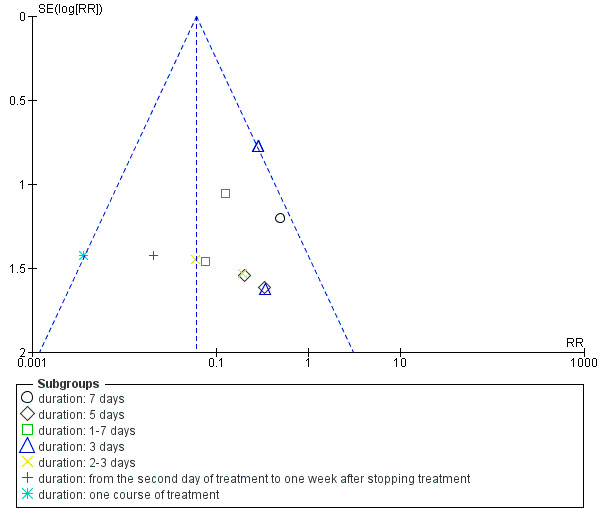
Funnel plot of comparison: 1 Aloe vera versus no treatment for prevention of phlebitis, outcome: 1.3 Incidence of third degree phlebitis.
Discussion
Summary of main results
In this review, external application of Aloe vera was associated with a statistically significant lower incidence of phlebitis compared with no prophylactic treatment; the RR was 0.28 (95% CI 0.11 to 0.68) for seven days of the intervention, and 0.29 (95% CI 0.11 to 0.74) for five days duration. In addition, Aloe vera reduced the degree of severity of phlebitis, with the RR for the incidence of third degree or second degree phlebitis significantly lower in participants receiving applications of Aloe vera than in participants with no prophylactic treatment (RR 0.06, 95% CI 0.03 to 0.11 and 0.18, 95% CI 0.10 to 0.31 respectively). When compared with external application of a potato slice, the incidence of phlebitis appeared lower with the application of Aloe vera (RR 0.90, 95% CI 0.58 to 1.40), but this difference did not reach statistical significance. Compared with external application of 75% alcohol, there was a statistically significant difference in the incidence of phlebitis (RR 0.02, 95% CI 0.00 to 0.28). Compared with 50% MgSO4, there was no statistically significant difference with the external application of Aloe vera in third degree phlebitis and all grades of phlebitis (RR 0.28, 95% CI 0.07 to 1.02; RR 0.41, 95% CI 0.16 to 1.07), but the second degree phlebitis did show a statistically significant improvement (RR 0.26, 95% CI 0.14 to 0.49). Compared with 33% MgSO4, there was an obvious reduction in the risk of phlebitis (RR 0.43, 95% CI 0.24 to 0.78). In the comparison of Aloe vera combined with non‐Aloe vera treatment versus the non‐Aloe vera treatment, there was greater improvement in the prophylaxis of phlebitis with Aloe vera plus 0.9% NaCI2 plus dexamethasone versus 0.9% NaCI2 plus dexamethasone than with Aloe vera plus an ice bag versus an ice bag plus 75% MgSO4.
Recovery, marked improvement and total improvement of phlebitis were significantly increased in trial participants treated with the external application of Aloe vera compared with the external application of 50% MgSO4 (RR 1.42, 95% CI 1.24 to 1.61; RR 1.56, 95% CI 1.29 to 1.87; RR 1.22, 95% CI 1.16 to 1.28 respectively). Furthermore, the same effects were observed in comparisons with 33% MgSO4 on marked improvement (RR 1.97 95% CI 1.44 to 2.70) and total improvement (RR 1.16 95% CI 1.09 to 1.24). Compared with external application of 75% alcohol or a potato slice, the treatment effects (including recovery, marked improvement and total improvement) of external application of Aloe vera for phlebitis were statistically significant. Compared with external application of hirudoid, the treatment effects, including recovery, marked improvement and total improvement, were less in trial participants treated with external application of Aloe vera. In the application of Aloe vera combined with non‐Aloe vera treatment, the treatment effects including the recovery, marked improvement and total improvement outcomes were better in participants with Aloe vera plus routine treatment or hirudoid than the same routine treatment plus dexamethasone or hirudoid alone; but the same beneficial effect was not evident in the trial of Aloe vera plus sulphanilamide pyrimidine versus the application of sulphanilamide pyrimidine alone. In addition, compared with external application of 50% MgSO4, the time to symptom improvement of phlebitis was obviously shortened in participants treated with Aloe vera. But compared with sulphonic acid mucopolysaccharide cream, application of Aloe vera plus sulphonic acid mucopolysaccharide cream did not obviously shorten the time for symptom improvement of phlebitis. When compared with no treatment, the time the infusion needle was retained in the vein was lengthened in participants receiving application of Aloe vera.
Overall completeness and applicability of evidence
The participants of the included studies were mainly the varied cancer patients who would be or had been part of a program of chemotherapy, and some of the participants were infused with 20% mannitol (Cao 2008; Jin 2010; Li 2006; Li 2009; Liang 2004; Tan 2002; Zheng 2010). Although some evidence indicated that the external application of Aloe vera was beneficial to treat or prevent phlebitis associated with the infusion of chemotherapy drugs or 20% mannitol, the included participants were not typical and showed a lack of clinical representation. The age of the included participants in all but one study ranged from 18 years to 75 years old. Only 90 participants in one study (Gao 2007) were children (aged from one month to three years old). The evaluated interventions in this review mainly involved fresh aloe leaf, usually cut into a thin slice (2 to 6 cm wide by 5 to 10 cm long) or squeezed into aloe juice. No other Aloe vera‐derived products were used in the included studies. In the evaluation of the treatment effect, composite outcomes, for example marked improvement or improvement of phlebitis symptoms, were used in many of the included studies. The use of these composite outcomes can reduce the ability to understand the precise impact of Aloe vera use in improving the clinical symptoms of phlebitis. All included studies were performed in China, and no studies from other countries were identified. The use of the raw plant products of Aloe vera may be impossible to implement in countries other than China but the review's conclusion may be helpful for the development of research on Aloe vera products. Perhaps authorised products of aloe vera will be applied in countries other than China in the future.
Quality of the evidence
In general the included trials were small, except for one study (Hu 2009), and had diverse designs. Eight quasi‐randomised controlled trials qRCTs) were included (Deng 2010; Gao 2007; Hou 2010; Hu 2009; Liu 2006, Wang 2006; Xiao 2008; Zhang 2010). The uncertain duration of treatment, for example two to three days and one to seven days, was noted in the included trials. It is important to note that the methodological shortcomings were obvious in the assessment of the studies. Firstly, only very few studies described detailed information on random sequence generation (Cao 2008; Jin 2010; Wang 2006), and almost all of the included studies did not mention the method of allocation concealment, which is considered to be one of the most important causes of bias in a randomised controlled trials (Altman 2001). Secondly, blinding was not used in all included trials. While it is impossible to blind the participants and personnel in the studies involved in the external application of therapies, and not blinding may not influence the judgement of outcomes, not blinding the outcome assessors may result in an overestimation of the intervention effect. Thirdly, the variation between the results of the studies was substantive and we were unable to explain this variation despite undertaking subgroup analyses by intervention duration. In addition, due to unavailable protocols for all included studies, selective reporting and attrition biases were unclear. We also identified possible publication bias in the assessment of the total incidence of phlebitis, incidence of second degree phlebitis and the incidence of third degree phlebitis, where funnel plots showed obvious asymmetry suggesting evidence of publication bias.
Potential biases in the review process
We attempted to minimise bias in the review process. Firstly, we performed a comprehensive literature search without language limits to identify all relevant studies. Secondly, two review authors independently assessed the studies for inclusion and carried out data extraction to ensure all relevant data were obtained. Two review authors independently assessed the risk of bias of the included studies according to the rules of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), and disagreements were resolved by consensus. In addition, we strictly followed the protocol outline to perform the review. Nevertheless, potential biases still exist. For example, the process of assessing risk of bias involves some degree of subjective judgements from the assessors. The publication bias was not assessed using funnel plots for most comparisons in this review because the numbers of studies included in each comparison was less than 10. Publication bias is possible because we found that all included studies were published studies and the majority of their outcomes were positive. Many of the outcomes predefined in the protocol of this review (Zheng 2011), such as health‐related quality of life, adverse effects etc, were not measured in the included studies, therefore we could not review their data to weigh up the effect. In addition, several comparisons, for example Aloe vera plus non‐Aloe vera intervention versus non‐Aloe vera intervention, only involved either a single study or a few studies and could cause spuriously significant evidence.
Agreements and disagreements with other studies or reviews
We did not identify any other systematic reviews comparing external application of Aloe vera, with or without another intervention, with no treatment or the other intervention in preventing or treating infusion phlebitis.
Authors' conclusions
Implications for practice.
There is no strong evidence for the prevention or treatment of infusion phlebitis with external application of Aloe vera. The current available evidence is limited by the poor methodological quality and risk of selective outcome reporting of the included studies, and by variations in the direction and size of effect across the studies. The positive effects observed with external application of Aloe vera in preventing or treating infusion phlebitis compared with no intervention or external application of 33% or 50% MgSO4 should therefore be viewed with caution.
Implications for research.
Further research should consider the following points.
1. The methods of random sequence generation and allocation concealment should be adequately described.
2. Blinding of outcome assessors should be applied to reduce detection bias.
3. Clinical trial registers should be encouraged to provide the available protocols.
4. Participants who were withdrawals or dropouts during the trial should be clearly described.
5. Adverse events should be measured as a priority outcome.
What's new
| Date | Event | Description |
|---|---|---|
| 31 March 2017 | Amended | Minor copyediting oversight amended in the 'Implications for research' section |
Acknowledgements
We would like to thank Dr Marlene Stewart, Dr Karen Welch and the editors of the Cochrane Peripheral Vascular Disease Review Group.
Appendices
Appendix 1. CENTRAL search strategy
#1MeSH descriptor: [Aloe] explode all trees 51 #2aloe*:ti,ab,kw 131 #3#1 or #2 in Trials 117
Appendix 2. MEDLINE search strategy
Database: Ovid MEDLINE(R) <1946 to January Week 5 2014>
Search Strategy:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 Aloe/ (873)
2 aloe.ti,ab. (1235)
3 1 or 2 (1377)
4 Phlebitis/ (3177)
5 Infusions, Intravenous/ (46945)
6 phlebit$.ti,ab. (3073)
7 (infus$ or intravenous or catheter or cannul$).ti,ab. (479891)
8 or/4‐7 (495023)
9 3 and 8 (24)
Appendix 3. EMBASE search strategy
Database: Embase <1980 to 2014 Week 06>
Search Strategy:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 exp Aloe vera extract/ (1048)
2 aloe.ti,ab. (2179)
3 1 or 2 (2718)
4 exp phlebitis/ (24578)
5 phlebit$.ti,ab. (3751)
6 (infus$ or intravenous or catheter or cannul$).ti,ab. (621691)
7 intravenous drug administration/ (314008)
8 or/4‐7 (853151)
9 3 and 8 (52)
Appendix 4. AMED search strategy
Database: AMED (Allied and Complementary Medicine) <1985 to February 2014>
Search Strategy:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 exp Aloe/ (90)
2 aloe.ti,ab. (168)
3 1 or 2 (172)
4 Phlebit$.ti,ab. (4)
5 injections/ (432)
6 (infus$ or intravenous or catheter or cannul$).ti,ab. (1379)
7 4 or 5 or 6 (1790)
8 3 and 7 (1)
Appendix 5. CBM search strategy
(from 1980 to 28 February 2014)
1# 主题词 芦荟 1666
2# 主题词 静脉炎 10779
3# 缺省 临床试验 217892
4# 缺省 临床疗效 257752
5# 缺省 临床观察 217678
6# 缺省 疗效观察 265887
7# 缺省 预防 548917
8# 缺省 预防效果 2623
9# 缺省 随机 632259
10# 缺省 对照 989828
11# 缺省 3# OR 4# OR 5# OR 6# OR 7# OR 8# 1261645
12# 缺省 1# AND 2# AND 9# AND 10# AND 11# 49
The English translation
1# subject Aloe vera 1666
2# subject phlebitis 10779
3# default clinical trial 217892
4# default clinical effect 257752
5# default clinical observation 217678
6# default effect observation 265887
7# default prophylaxis 548917
8# default prophylaxis effect 2623
9# default random 632259
10# default control 989828
11# default 3# OR 4# OR 5# OR 6# OR 7# OR 8# 1261645
12# default 1# AND 2# AND 9# AND 10# AND 11# 49
Appendix 6. Chinese VIP Information search strategy
(from 1989 to 28 February 2014)
#1关键词:芦荟 n=3999
#2任意字段:静脉炎 n=12123
#3任意字段:随机 n=819209
#4任意字段:临床观察 n=218885
#5任意字段:疗效 n=1202848
#6任意字段:临床试验 n=27396
#7任意字段:预防 n=890314
#8任意字段:or #4‐7 n=2155723
#9任意字段: # 1 AND #2 AND #3 AND #8 n=52
The English translation
#1 key word: aloe n=3999
#2 all fields: phlebitis n=12123
#3 all fields: random n=819209
#4 all fields: clinical observation n=218885
#5 all fields: therapeutic effect n=1202848
6# all fields: clinical trial n=27396
7# all fields: prophylaxis n=890314
8# all fields: or #4‐7 n=2155723
9# all fields: #1 AND #2 AND #3 AND #8 n=52
Appendix 7. Traditional Chinese Medical Database System search strategy
from 1984 to 28 February 2014
芦荟 AND 静脉炎 AND (临床试验 OR 疗效 OR 临床观察 OR 预防 OR 预防效果) AND 随机 AND 对照
n=16
The English translation
Aloe AND phlebitis AND (clinical trial OR therapeutic effect OR clinical observation OR prophylaxis OR prophylaxis effect) AND random AND control
n=16
Appendix 8. WangFang database search strategy
(from 1987 to February 2014)
(临床试验 + 疗效 + 临床观察 + 预防) * 芦荟 * 静脉炎 * 随机 n=63
The English translation
(clinical trial +therapeutic effect+clinical observation+prophylaxis) *aloe*phlebitis*random
n=63
Appendix 9. CMCC database search strategy
(from 1994 to 28 February 2014)
芦荟/fld=关键词 AND 静脉炎/fld=摘要 AND (临床试验/fld=摘要 OR 疗效/fld=摘要 OR 临床观察/fld=摘要 OR 预防/fld=摘要) AND (随机/fld=摘要) n=36
The English translation
Aloe/fld=key word AND phlebitis/fld=abstract AND (clinical trial/fld=abstract OR therapeutic effect/fld=abstract OR clinical observation/fld=abstract OR prophylaxis/fld=abstract) AND (random/fld=abstract)
n=36
Appendix 10. ChinaMedical Academic Conference search strategy
(from 1994 to February 2014)
((全文=临床试验) OR (全文=疗效) OR (全文=临床观察) OR (全文=预防)) AND (关键词=芦荟) AND (全文=静脉炎) AND (摘要=随机) n=5
The English translation
((full text=clinical trial) OR (full text=therapeutic effect) OR (full text=clinical observation) OR (full text= prophylaxis)) AND (key word=aloe) AND (full text=phlebitis) AND (abstract=random)
n=5
Appendix 11. Chinese Academic Conference Papers Database and Chinese Dissertation Database search strategy
(from 1994 to 28 February 2014)
((全文=临床试验) OR (全文=疗效) OR (全文=临床观察) OR (全文=预防)) AND (关键词=芦荟) AND (全文=静脉炎) AND (摘要=随机) n=0
The English translation
((full text=clinical trial) OR (full text=therapeutic effect) OR (full text=clinical observation) OR (full text=prophylaxis) AND (abstract=random)
n=0
Appendix 12. ChinaNational Knowledge Infrastructure (CNKI) search strategy
(from 1994 to 28 February 2014)
((全文=临床试验) OR (全文=疗效) OR (全文=临床观察) OR (全文=预防)) AND (关键词=芦荟) AND (全文=静脉炎) AND (摘要=随机) n=79
The English translation
((full text=clinical trial) OR (full text=therapeutic effect) OR (full text=clinical observation) OR (full text=prophylaxis)) AND (key word=aloe) AND (full text=phlebitis) AND (abstract=random)
n=79
Data and analyses
Comparison 1. Aloe vera versus no treatment for prevention of phlebitis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total incidence of phlebitis | 15 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 1.1 duration: 7 days | 1 | 80 | Risk Ratio (IV, Random, 95% CI) | 0.28 [0.11, 0.68] |
| 1.2 duration: 5 days | 5 | 532 | Risk Ratio (IV, Random, 95% CI) | 0.29 [0.11, 0.74] |
| 1.3 duration: 1‐7 days | 2 | 370 | Risk Ratio (IV, Random, 95% CI) | 0.31 [0.18, 0.51] |
| 1.4 duration: 3 days | 3 | 312 | Risk Ratio (IV, Random, 95% CI) | 0.44 [0.29, 0.66] |
| 1.5 duration: 2‐3 days | 2 | 189 | Risk Ratio (IV, Random, 95% CI) | 0.21 [0.05, 0.80] |
| 1.6 duration: from the second day of treatment to one week after stopping treatment | 1 | 150 | Risk Ratio (IV, Random, 95% CI) | 0.36 [0.26, 0.48] |
| 1.7 duration: one course of treatment | 1 | 3000 | Risk Ratio (IV, Random, 95% CI) | 0.11 [0.09, 0.15] |
| 2 Incidence of second degree phlebitis | 14 | 4585 | Risk Ratio (IV, Random, 95% CI) | 0.18 [0.10, 0.31] |
| 2.1 duration: 7 days | 1 | 80 | Risk Ratio (IV, Random, 95% CI) | 0.22 [0.05, 0.96] |
| 2.2 duration: 5 days | 4 | 482 | Risk Ratio (IV, Random, 95% CI) | 0.19 [0.07, 0.55] |
| 2.3 duration: 1‐7 days | 2 | 370 | Risk Ratio (IV, Random, 95% CI) | 0.21 [0.11, 0.41] |
| 2.4 duration: 3 days | 3 | 314 | Risk Ratio (IV, Random, 95% CI) | 0.42 [0.22, 0.80] |
| 2.5 duration: 2‐3 days | 2 | 189 | Risk Ratio (IV, Random, 95% CI) | 0.07 [0.01, 0.49] |
| 2.6 duration: from the second day of treatment to one week after stopping treatment | 1 | 150 | Risk Ratio (IV, Random, 95% CI) | 0.18 [0.10, 0.33] |
| 2.7 duration: one course of treatment | 1 | 3000 | Risk Ratio (IV, Random, 95% CI) | 0.06 [0.04, 0.09] |
| 3 Incidence of third degree phlebitis | 14 | 4585 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.06 [0.03, 0.11] |
| 3.1 duration: 7 days | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.05, 5.30] |
| 3.2 duration: 5 days | 4 | 482 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.03, 2.19] |
| 3.3 duration: 1‐7 days | 2 | 370 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.02, 0.55] |
| 3.4 duration: 3 days | 3 | 314 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.11, 0.80] |
| 3.5 duration: 2‐3 days | 2 | 189 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.01, 0.69] |
| 3.6 duration: from the second day of treatment to one week after stopping treatment | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.02 [0.00, 0.34] |
| 3.7 duration: one course of treatment | 1 | 3000 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.00 [0.00, 0.06] |
| 4 Length of time needle is successfully detained in infused vein | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 2. Aloe vera versus potato for prevention of phlebitis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total incidence of phlebitis | 2 | 276 | Risk Ratio (IV, Fixed, 95% CI) | 0.90 [0.58, 1.40] |
| 2 Incidence of second degree phlebitis | 2 | 276 | Risk Ratio (IV, Fixed, 95% CI) | 1.14 [0.49, 2.67] |
| 3 Incidence of third degree phlebitis | 2 | 276 | Risk Ratio (IV, Fixed, 95% CI) | 0.45 [0.04, 4.94] |
Comparison 3. Aloe vera versus 33% MgSO4 for prevention of phlebitis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total incidence of phlebitis | 2 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.24, 0.78] |
Comparison 4. Aloe vera versus 50% MgSO4 for prevention of phlebitis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total incidence of phlebitis | 2 | 248 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.16, 1.07] |
| 2 Incidence of second degree phlebitis | 2 | 248 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.14, 0.50] |
| 3 Incidence of third degree phlebitis | 2 | 248 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.07, 1.02] |
Comparison 5. Aloe vera plus ice bag versus ice bag plus 75% MgSO4 for prevention of phlebitis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total incidence of phlebitis | 1 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Incidence of second degree phlebitis | 1 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Incidence of third degree phlebitis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 6. Aloe vera versus 75% alcohol for prevention of phlebitis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total incidence of phlebitis | 1 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected |
Comparison 7. Aloe vera versus 33% MgSO4 for treatment of phlebitis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Rate of resolution phlebitis: recovery | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.92, 1.43] |
| 2 Rate of resolution phlebitis: marked improvement | 2 | 302 | Risk Ratio (IV, Fixed, 95% CI) | 1.97 [1.44, 2.70] |
| 3 Rate of resolution phlebitis: total improvement | 3 | 422 | Risk Ratio (IV, Fixed, 95% CI) | 1.16 [1.09, 1.24] |
7.1. Analysis.
Comparison 7 Aloe vera versus 33% MgSO4 for treatment of phlebitis, Outcome 1 Rate of resolution phlebitis: recovery.
Comparison 8. Aloe vera versus 50% MgSO4 for treatment of phlebitis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Rate of resolution of phlebitis: recovery | 7 | 595 | Risk Ratio (IV, Fixed, 95% CI) | 1.42 [1.24, 1.61] |
| 1.1 duration: 3 days | 4 | 394 | Risk Ratio (IV, Fixed, 95% CI) | 1.38 [1.17, 1.61] |
| 1.2 duration: 7 days | 1 | 50 | Risk Ratio (IV, Fixed, 95% CI) | 3.5 [1.34, 9.17] |
| 1.3 duration: 15 days | 2 | 151 | Risk Ratio (IV, Fixed, 95% CI) | 1.43 [1.13, 1.80] |
| 2 Rate of resolution of phlebitis: marked improvement | 9 | 814 | Risk Ratio (IV, Random, 95% CI) | 1.56 [1.29, 1.87] |
| 2.1 duration: 3 days | 7 | 679 | Risk Ratio (IV, Random, 95% CI) | 1.57 [1.25, 1.97] |
| 2.2 duration: 7 days | 1 | 50 | Risk Ratio (IV, Random, 95% CI) | 2.22 [1.27, 3.88] |
| 2.3 duration: 15 days | 1 | 85 | Risk Ratio (IV, Random, 95% CI) | 1.39 [1.15, 1.69] |
| 3 Rate of resolution of phlebitis: total improvement | 10 | 880 | Risk Ratio (IV, Fixed, 95% CI) | 1.22 [1.16, 1.28] |
| 3.1 duration: 3 days | 7 | 679 | Risk Ratio (IV, Fixed, 95% CI) | 1.20 [1.13, 1.26] |
| 3.2 duration: 7 days | 1 | 50 | Risk Ratio (IV, Fixed, 95% CI) | 1.53 [1.09, 2.15] |
| 3.3 duration: 15 days | 2 | 151 | Risk Ratio (IV, Fixed, 95% CI) | 1.35 [1.16, 1.56] |
| 4 Time of marked improvement | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Time of improvement | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 9. Aloe vera plus non‐Aloe vera versus same non‐Aloe vera for treatment of phlebitis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Rate of resolution of phlebitis: recovery | 3 | 283 | Risk Ratio (IV, Random, 95% CI) | 1.75 [1.24, 2.46] |
| 2 Rate of resolution of phlebitis: marked improvement | 3 | 163 | Risk Ratio (IV, Fixed, 95% CI) | 1.26 [1.09, 1.47] |
| 3 Rate of resolution of phlebitis: total improvement | 4 | 323 | Risk Ratio (IV, Fixed, 95% CI) | 1.23 [1.13, 1.35] |
| 4 Time of red swelling symptom disappeared of phlebitis | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Time of relief of pain | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 10. Aloe vera versus 75% alcohol for treatment of phlebitis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Rate of resolution of phlebitis: marked improvement | 1 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Rate of resolution of phlebitis: total improvement | 1 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected |
Comparison 11. Aloe vera versus potato for treatment of phlebitis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Rate of resolution of phlebitis: recovery | 1 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Rate of resolution of phlebitis: marked improvement | 1 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Rate of resolution of phlebitis: total improvement | 1 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected |
Comparison 12. Aloe vera versus hirudoid for treatment of phlebitis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Rate of resolution of phlebitis: recovery | 1 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Rate of resolution of phlebitis: marked improvement | 1 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Rate of resolution of phlebitis: total improvement | 1 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected |
Comparison 13. Aloe vera plus routine treatment versus routine treatment plus dexamethasone for treatment of phlebitis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Rate of resolution of phlebitis: recovery | 1 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Rate of resolution of phlebitis: marked improvement | 1 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Rate of resolution of phlebitis: total improvement | 1 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected |
Comparison 14. Aloe vera versus no treatment for prevention of phlebitis (sensitivity analysis).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence of third degree phlebitis (random‐effects model) | 14 | 4585 | Risk Ratio (M‐H, Random, 95% CI) | 0.13 [0.05, 0.34] |
| 1.1 duration: 7 days | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.5 [0.05, 5.30] |
| 1.2 duration: 5 days | 4 | 482 | Risk Ratio (M‐H, Random, 95% CI) | 0.26 [0.03, 2.26] |
| 1.3 duration: 1‐7 days | 2 | 370 | Risk Ratio (M‐H, Random, 95% CI) | 0.11 [0.02, 0.56] |
| 1.4 duration: 3 days | 3 | 314 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.11, 0.80] |
| 1.5 duration: 2‐3 days | 2 | 189 | Risk Ratio (M‐H, Random, 95% CI) | 0.10 [0.01, 0.81] |
| 1.6 duration: from the second day of treatment to one week after stopping treatment | 1 | 150 | Risk Ratio (M‐H, Random, 95% CI) | 0.02 [0.00, 0.34] |
| 1.7 duration: one course of treatment | 1 | 3000 | Risk Ratio (M‐H, Random, 95% CI) | 0.00 [0.00, 0.06] |
| 2 Incidence of third degree phlebitis (excluding qRCTs) | 10 | 1119 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.05, 0.26] |
| 2.1 duration: 7 days | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.05, 5.30] |
| 2.2 duration: 5 days | 3 | 322 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.81] |
| 2.3 duration: 1‐7 days | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.08 [0.00, 1.34] |
| 2.4 duration:3 days | 2 | 228 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.08, 1.16] |
| 2.5 duration: 2‐3 days | 2 | 189 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.01, 0.69] |
| 2.6 duration: from the second day of treatment to one week after stopping treatment | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.02 [0.00, 0.34] |
| 3 Incidence of second degree phlebitis (excluding qRCTs) | 10 | 1119 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.11, 0.24] |
| 3.1 duration: 7 days | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.05, 0.96] |
| 3.2 duration: 5 days | 3 | 322 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.06, 0.63] |
| 3.3 duration: 1‐7 days | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.12 [0.03, 0.49] |
| 3.4 duration: 3 days | 2 | 228 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.16, 0.72] |
| 3.5 duration: 2‐3 days | 2 | 189 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.04 [0.01, 0.18] |
| 3.6 duration: from the second day of treatment to one week after stopping treatment | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.10, 0.33] |
| 4 Total incidence of phlebitis (excluding qRCTs) | 11 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 4.1 duration: 7 days | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 duration: 5 days | 4 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 duration: 1‐7 days | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.4 duration: 3 days | 2 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.5 duration: 2‐3 days | 2 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.6 duration: from the second day of treatment to one week after stopping treatment | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Cao 2008.
| Methods | Study design: Randomised controlled trial Method of randomisation: Computer program Concealment of allocation: Sealed envelope Exclusions post‐randomisation: Treatment group: 3 subjects dropped out because of infusion drugs leakage; control group: 1 subject dropped out because of the infusion drug leakage Losses to follow up: None Intention‐to‐treat analysis: Yes |
|
| Participants | Country: China Setting: Hospital Number: 76 participants (38 treatment, 38 control) Age: Mean 60.23 years, range from 50 ‐ 70 years Sex: Male/female 43/33 Inclusion criteria: Conscious, cooperation, age (50 ‐ 70 year), first undergo treatment with 20% mannitol (125 ml Q8h) Exclusion criteria: Confused state of mind, no cooperation, infusing other drugs which impair the vein (except for 20% mannitol), hypersensitive to aloe. |
|
| Interventions | Treatment: Fresh aloe slice (5 cm long, 3 cm wide) external application. Wet compress at punctured skin once a day Control: No treatment Duration: 5 days |
|
| Outcomes | Primary: The incidence of phlebitis (a) First degree: pain, red swelling appear on the punctured vein. Strip‐shaped changes in veins are not observed, and no palpable venous callosity Treatment group: 26/38. Control group: 33/38 (b) Second degree: pain, red swelling appear on the punctured vein. Strip‐shaped changes in vein are observed, and no palpable venous callosity. Treatment group: 0/38. Control group: 1/38 (c) Third degree: pain, red swelling appear on the punctured vein. Strip‐shaped changes in vein are observed, with palpable venous callosity. Treatment group: 0/38. Control group:0/38 Secondary: The length of time the needle is detained in the vein |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence was generated by using computer program |
| Allocation concealment (selection bias) | Low risk | Random sequence was concealed by using the sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. The outcome assessment is likely to be influenced by the lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Treatment group: 3 subjects dropped out because of infusion drugs leakage Control group: 1 subject dropped out because of the infusion drug leakage ITT analysis was performed in this study |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available, but it is clear that the published reports included all expected outcomes |
| Other bias | Low risk | There are no statistical differences in age, sex, illness, classification of phlebitis and nursing among the comparisons. The study appears to be free other sources of bias |
Chen 2009.
| Methods | Study design: Randomised controlled trial Method of randomisation: Not described Concealment of allocation: Not reported Exclusions post‐randomisation: Not reported Losses to follow up: None Intention‐to‐treat analysis: Not reported |
|
| Participants | Country: China Setting: Hospital Number: 60 participants (20 treatment, 20 control 1, 20 control 2) Age: Mean 48.2 years, range from 28 to 62 years Sex: Male/female 26/34 Inclusion criteria: Phlebitis met criteria of the Infusion Nursing Society of America Exclusion criteria: Not stated |
|
| Interventions | Treatment: Sulphonic acid mucopolysaccharide cream smeared at punctured skin for 4 ‐ 6 hours, once a day, then fresh aloe slice wet compress, at least twice a day Control 1: Sulphonic acid mucopolysaccharide cream smeared at puncture skin for 4 ‐ 6 hours, once a day Control 2: 33% MgSO4 wet compress at punctured skin, change when dried Duration: 14 days |
|
| Outcomes | Primary: The rate of resolution of phlebitis (a) Recovery: disappearance of swelling, callosity and strip‐shaped change with index of effective 100% (index of effective = (scores before treatment ‐ scores after treatment)/score before treatment Treatment group: 0/20, Control group 1: 0/20, Control group 2: 0/20 (b) Marked improvement: regression of swelling, callosity and strip‐shaped change with index of effective over 60% Treatment group: 15/20, Control group 1: 12/20, Control group 2: 7/20 (c) Improvement: regression of swelling with index of effective over 20%, no obvious regression on callosity and strip‐shaped change Treatment group: 4/20, Control group 1: 6/20, Control group 2: 6/20 (d) Not effective: index of effective less than 20%, no change of symptoms of phlebitis Treatment group: 1/20, Control group 1: 2/20, Control group 2: 7/20 Secondary: The time of red swelling eliminated, the time of relief of pain |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, but the detailed information of randomisation method was not described |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. The outcome assessment is likely to be influenced by the lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number randomised not stated |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available, and it is not clear if the published reports included all expected outcomes |
| Other bias | Low risk | There are no statistical differences in age, sex, illness, classification of phlebitis and program of nursing among the comparisons. The study appears to be free other sources of bias |
Chen 2010.
| Methods | Study design: Randomised controlled trial Method of randomisation: Not described Concealment of allocation: Not reported Exclusions post‐randomisation: Not reported Losses to follow up: None Intention‐to‐treat analysis: Not reported |
|
| Participants | Country: China Setting: Hospital Number: 100 participants (50 treatment, 50 control) Age: Mean 41.9 years, range from 18 to 76 years Sex: Male/female 64/36 Inclusion criteria: Phlebitis met criteria of the Infusion Nursing Society of America Exclusion criteria: Not stated |
|
| Interventions | Treatment: Aloe glue smeared at punctured skin, bid Control: 50% MgSO4 wet compress at punctured skin, bid Duration: 3 days |
|
| Outcomes | Primary: The rate of resolution of phlebitis (a) Marked improvement: no local red swelling, disappearance of pain Treatment group: 38/50. Control group: 3/50 (b) Improvement: regression of local red swelling, alleviation of pain Treatment group: 12/50. Control group: 33/50 (c) Not effective: no change of local red swelling and pain after treatment for 3 days Treatment group: 0/50. Control group: 14/50 Secondary: None |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, but the detailed information of randomisation method was not described |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. The outcome assessment is likely to be influenced by the lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number randomised not stated |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available, and it is not clear if the published reports included all expected outcomes |
| Other bias | Low risk | There are no statistical differences in age, sex, illness, classification of phlebitis and program of nursing among the comparisons. The study appears to be free other sources of bias |
Chen 2012.
| Methods | Study design: Randomised controlled trial Method of randomisation: Not described Concealment of allocation: Not reported Exclusions post‐randomisation: Not reported Losses to follow up: None Intention‐to‐treat analysis: Not reported |
|
| Participants | Country: China Setting: Hospital Number: 100 participants (50 treatment, 50 control) Age: Mean 42.26 years, range from 34 ‐ 56 years Sex: Male/female 0/100 Inclusion criteria: Breast cancer patients with CMF (cyclophosphamide, methotrexate and 5‐fluorouracil) chemotherapy Exclusion criteria: Not stated |
|
| Interventions | Treatment: Fresh aloe slice external application (wet compress at punctured skin), 3 ‐ 4 times a day Control: 33% MgSO4 wet compress at punctured skin, 3 ‐ 4 times a day Duration: 6 months |
|
| Outcomes | Primary: The incidence of phlebitis Treatment group: 5/50, Control group: 10/50 Secondary: None |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, but the detailed information of randomisation method was not described |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. The outcome assessment is likely to be influenced by the lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Flow of participants randomised was not available |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available, and it is not clear if the published reports included all expected outcomes |
| Other bias | Unclear risk | The baseline information between comparison groups were not described |
Deng 2010.
| Methods | Study design: Quasi‐randomised controlled trial Method of randomisation: Alternate allocation Concealment of allocation: None Exclusions post‐randomisation: Not reported Losses to follow up: None Intention‐to‐treat analysis: Not reported |
|
| Participants | Country: China Setting: Hospital Number: 85 participants (43 treatment, 42 control) Age: Mean 42.9 years, range from 21 to 75 years Sex: Male/female 46/39 Inclusion criteria: Phlebitis met the criteria of the Infusion Nursing Society of America Exclusion criteria: Not stated |
|
| Interventions | Treatment: The juice of fresh aloe leaf, rubbed on the punctured skin, once every 3 hours Control: 50% MgSO4 rubbed on the punctured skin, 3 ‐ 4 times per day Duration: 15 days |
|
| Outcomes | Primary: The rate of resolution of phlebitis (a) Recovery: disappearance of local pain, red swelling, scorching hot and strip‐shaped change in veins within 4 days Treatment group: 36/43, Control group: 4/42 (b) Improvement: disappearance degree of local pain, red swelling, scorching hot and strip‐shaped change in veins within 7 days over 80% Treatment group: 7/43, Control group: 26/42 (c) No improvement: no obvious improvement of symptoms within 15 days after treatment Treatment group: 0/43, Control group: 12/42 Secondary: None |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Sequence generated by the admission sequence of participants |
| Allocation concealment (selection bias) | High risk | Alternate allocation according to admission sequence |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. The outcome assessment is likely to be influenced by the lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number randomised not stated |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available, and it is not clear if the published reports included all expected outcomes |
| Other bias | Low risk | Sex, age, illness, the course of disease, program of chemotherapy and the severe degree of phlebitis between two groups are not statistically significant (P > 0.05). The study appears to be free other sources of bias |
Deng 2012.
| Methods | Study design: Randomised controlled trial Method of randomisation: Not described Concealment of allocation: Not reported Exclusions post‐randomisation: Not reported Losses to follow up: None Intention‐to‐treat analysis: Not reported |
|
| Participants | Country: China Setting: Hospital Number: 116 participants (60 treatment, 56 control) Age: Mean ± SD: treatment 67.62 ± 12.96 years; control 71.10 ± 11.06 years Sex: Male/female: treatment 40/20; control 34/22 Inclusion criteria: Phlebitis met the criteria of the Infusion Nursing Society of America Exclusion criteria: Complicated diabetes |
|
| Interventions | Treatment: Fresh aloe leaf wet compress, tid Control: 50% MgSO4, tid Duration: 3 days |
|
| Outcomes | Primary: The rate of resolution of phlebitis (a) Recovery: disappearance of local pain, red swelling, scorching hot and strip‐shaped change in veins Treatment group: 47/60, Control group: 30/56 (b) Marked improvement: disappearance degree of local pain, red swelling, scorching hot and strip‐shaped change in veins over 80% Treatment group: 11/60, Control group: 12/56 (c)Improvement: disappearance of pain, scorching hot at local skin. the shortened area of red swelling was less than 80% Treatment group: 2/60, Control group: 7/56 (c) No effect: no obvious change of symptoms after treatment Treatment group: 0/60, Control group: 7/56 Secondary: None |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, but the detailed information of randomisation method was not described |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. The outcome assessment is likely to be influenced by the lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Flow of participants randomised was not available |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available, and it is not clear if the published reports included all expected outcomes |
| Other bias | Unclear risk | The baseline information between comparison groups were not described |
Dong 2001.
| Methods | Study design: Randomised controlled trial Method of randomisation: Not described Concealment of allocation: Not reported Exclusions post‐randomisation: Not reported Losses to follow up: None Intention‐to‐treat analysis: Not reported |
|
| Participants | Country: China Setting: Hospital Number: 154 participants (80 treatment, 74 control) Age: mean 49.8 years, range from 23 to 76 years Sex: male/female 91/63 Inclusion criteria: Phlebitis met the criteria of the nursing conference in Hangzhou city 1990 Exclusion criteria: Not stated |
|
| Interventions | Treatment: Fresh aloe slice external application, once a day Control : 33% MgSO4 external application, tid Duration: 7 days |
|
| Outcomes | Primary: The rate of resolution of phlebitis (a) Marked improvement: disappearance of red swelling in the local vein, softening of callosity vein vessel, re‐open the block vein vessel within one treatment course; disappearance of pain within two hours. Treatment group: 26/80; Control group: 16/74 (b) Improvement: disappearance of phlebitis symptom over one treatment course and disappearance of pain over two hours Treatment group: 49/80; Control group: 37/74 (c) No improvement: symptoms of phlebitis and pain were unchanged after treatment Treatment group: 5/80; Control group: 21/74 Secondary: None |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, but the detailed information of randomisation method was not described |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. The outcome assessment is likely to be influenced by the lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Flow of participants randomised was not available |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available, and it is not clear if the published reports included all expected outcomes |
| Other bias | Unclear risk | The baseline information between comparison groups were not described |
Dong 2008.
| Methods | Study design: Randomised controlled trial Method of randomisation: Not described Concealment of allocation: Not reported Exclusions post‐randomisation: Not reported Losses to follow up: None Intention‐to‐treat analysis: Not reported |
|
| Participants | Country: China Setting: Hospital Number: 57 participants (29 treatment, 28 control) Age: Mean 57.8 years Sex: Male/female 2/55 Inclusion criteria: Breast cancer patients due to receive chemotherapy Exclusion criteria: Not stated |
|
| Interventions | Treatment: 2 cm thick fresh aloe slice external application. Wet compress at punctured skin for 48 ‐ 72h Control : No treatment Duration: 2 ‐ 3 days |
|
| Outcomes | Primary: The incidence of phlebitis (a) First degree: local slight pain with red skin Treatment group 3/25, Control group: 4/28 (b) Second degree: local moderate pain with slight swelling, scorching hot Treatment group 1/25, Control group: 3/28 (c) Third degree: local moderate pain with moderate swelling and edema forming with diameter over 1 cm Treatment group 0/25, Control group: 2/28 Secondary: None |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, but the detailed information of randomisation method was not described |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. The outcome assessment is likely to be influenced by the lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number randomised not stated |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available, and it is not clear if the published reports included all expected outcomes |
| Other bias | Low risk | There are no statistical differences in age, sex, course of disease and drugs of chemotherapy between comparison groups. The study appears to be free other sources of bias |
Gao 2007.
| Methods | Study design: Quasi randomised controlled trial Method of randomisation: Alternate allocation according to admission sequence Concealment of allocation: None Exclusions post‐randomisation: Not reported Losses to follow up: None Intention‐to‐treat analysis: Not reported |
|
| Participants | Country: China Setting: Hospital Number: 90 participants (48 treatment, 42 control) Age: Treatment: range from one month to 3 years; control: not described Sex: Treatment: male/female 23/25. control: male/female 22/20 Inclusion criteria: Not described Exclusion criteria: Not stated |
|
| Interventions | Treatment: Fresh aloe slice external application (wet compress), twice a day for phlebitis with red glow type, 4‐5 times a day for phlebitis with sclerosis type Control: 50% MgSO4 external application (wet compress) Duration: 3 days |
|
| Outcomes | Primary: The rate of resolution of phlebitis (a) Recovery: disappearance of pain, red swelling and scorching hot at local skin Treatment group: 39 /48, Control group: 30 /42 (b) Marked improvement: disappearance of pain, scorching hot at local skin. the shortened area of red swelling exceeded 80% Treatment group: 6/48, Control group: 4/42 (c) Improvement: disappearance of pain, scorching hot at local skin. the shortened area of red swelling was less than 80% Treatment group: 3/48, Control group: 2/42 (d) Not effective: symptoms of phlebitis had not obviously changed after treatment. Treatment group: 0/48, Control group: 6/42 Secondary: None |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Sequence generated by the admission sequence of participants |
| Allocation concealment (selection bias) | High risk | Alternate allocation according to admission sequence |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. The outcome assessment is likely to be influenced by the lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported blinding for outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number randomised not stated |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available, and it is not clear if the published reports included all expected outcomes |
| Other bias | Unclear risk | The baseline information between comparison groups were not described |
Gao 2012.
| Methods | Study design: Randomised controlled trial Method of randomisation: Not described Concealment of allocation: Not reported Exclusions post‐randomisation: Not reported Losses to follow up: None Intention‐to‐treat analysis: Not reported |
|
| Participants | Country: China Setting: Hospital Number: 120 participants (60 treatment, 60 control) Age: Mean ± SD: Treatment 63 ± 11.2 years; control 65 ± 9.7 years Sex: Treatment: male/female 37/23. control: male/female 35/25 Inclusion criteria: Not described Exclusion criteria: Not stated |
|
| Interventions | Treatment: Aloe glue external application, bid Control: 33% MgSO4 external application, bid Duration: 5 days |
|
| Outcomes | Primary: The rate of resolution of phlebitis (a) Recovery: disappearance of pain, red swelling and scorching hot at local skin Treatment group: 47 /60, Control group: 41 /60 (b) Marked improvement: disappearance of pain, scorching hot at local skin. the shortened area of red swelling exceeded 80% Treatment group: 11/60, Control group: 10/60 (c) Not effective: symptoms of phlebitis had not obviously changed after treatment Treatment group: 2/60, Control group: 9/60 Secondary: None |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, but the detailed information of randomisation method was not described |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. The outcome assessment is likely to be influenced by the lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported blinding for outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number randomised not stated |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available, and it is not clear if the published reports included all expected outcomes |
| Other bias | Unclear risk | The baseline information except for age, sex between comparison groups were not described |
Hou 2010.
| Methods | Study design: Quasi‐randomised controlled trial Method of randomisation: Sequence generated by odd or even of the bed number Concealment of allocation: None Exclusions post‐randomisation: Not reported Losses to follow up: None Intention‐to‐treat analysis: Not reported |
|
| Participants | Country: China Setting: Hospital Number: 100 participants (50 treatment, 50 control) Age: Mean 29 years, range from 21 to 42 years Sex: None reported Inclusion criteria: Invasive hydatidiform mole patients with treatment of chemotherapy Exclusion criteria: Not stated |
|
| Interventions | Treatment: Piece of fresh aloe leaf with about 10 cm long wet compress at punctured skin, 3 ‐ 4 times per day Control: External application of 33% MgSO4 (wet compress) at punctured skin, 3 ‐ 4 times per day Duration: one course of chemotherapy |
|
| Outcomes | Primary: The incidence of phlebitis Treatment group: 8/50, Control group: 20/50 Secondary: None |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Sequence generated by odd or even bed number |
| Allocation concealment (selection bias) | High risk | No allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. The outcome assessment is likely to be influenced by the lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number randomised not stated |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available, and it is not clear if the published reports included all expected outcomes |
| Other bias | Low risk | Sex, age, illness, the course of disease, program of chemotherapy and the severe degree of phlebitis between two group are not statistically significant (P > 0.05). The study appears to be free other sources of bias |
Hu 2009.
| Methods | Study design: Quasi‐randomised controlled trial Method of randomisation: Sequence generated by odd or even month of the participants' birthday Concealment of allocation: None Exclusions post‐randomisation: Not reported Losses to follow up: None Intention‐to‐treat analysis: Not reported |
|
| Participants | Country: China Setting: Hospital Number: 3000 participants (2000 treatment, 1000 control) Age: Mean 55 years, range from 10 to 84 years Sex: Male/female 2044/956 Inclusion criteria: Cancer patients with treatment of chemotherapy Exclusion criteria: Not stated |
|
| Interventions | Treatment: External application of a piece of fresh aloe leaf (15 cm long and 5 cm wide), wet compress at punctured skin, changes every two hours Control: No treatment Duration: One course of chemotherapy |
|
| Outcomes | Primary: The incidence of phlebitis (a) First degree: local pain, red swelling or edema, no strip‐shaped changes in veins, no palpable venous cord Treatment group: 42/2000, Control group: 92/1000 (b) Second degree: local pain, red swelling or edema, with strip‐shaped changes in veins, no palpable venous cord Treatment group: 232000, Control group: 125/1000 (c) Third degree: local pain, red swelling or edema, with strip‐shaped changes in veins, palpable venous cord Treatment group: 0/2000, Control group: 68/1000 Secondary: None |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Sequence generated by odd or even month of the participant's birthday |
| Allocation concealment (selection bias) | High risk | No allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. The outcome assessment is likely to be influenced by the lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number randomised not stated |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available, and it is not clear if the published reports included all expected outcomes |
| Other bias | Low risk | Sex, age, illness, the course of disease, program of chemotherapy and conditions of punctured veins between two group are not statistically significant (P > 0.05). The study appears to be free other sources of bias |
Ji 2007.
| Methods | Study design: Randomised controlled trial Method of randomisation: Not described Concealment of allocation: Not reported Exclusions post‐randomisation: Not reported Losses to follow up: None Intention‐to‐treat analysis: Not reported |
|
| Participants | Country: China Setting: Hospital Number: 42 participants Age: Mean 53 years, range from 36 ‐ 74 years Sex: Male/female 24/18 Inclusion criteria: Cancer patients with vinorelbine tartrate injection Exclusion criteria: Not stated |
|
| Interventions | Treatment : Fresh aloe slice external application (wet compress at punctured skin), bid Control: No treatment Duration: 3 days |
|
| Outcomes | Primary: The incidence of phlebitis (a) First degree: slightly pain, red swelling or hydrops were shown at the punctured vein. No change as trabs, no callosity was felt Treatment group: 4/70, Control group: 15/72 (b) Second degree: pain, red swelling or hydrops become more severe at the punctured vein. No change as trabs, no callosity was felt Treatment group: 2/70, Control group: 13/72 (c) Acute pain, severe red swelling and hydrops were shown at the punctured vein. Change as trabs appeared, callosity could be felt Treatment group: 0/70, Control group: 1/72 Secondary: None |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, but the detailed information of randomisation method was not described |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. The outcome assessment is likely to be influenced by the lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number randomised not stated |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available, and it is not clear if the published reports included all expected outcomes |
| Other bias | Unclear risk | The baseline information between comparison groups were not described |
Jin 2010.
| Methods | Study design: Randomised controlled trial Method of randomisation: Random digits table Concealment of allocation: Not reported Exclusions post‐randomisation: Not reported Losses to follow up: None Intention‐to‐treat analysis: Not reported |
|
| Participants | Country: China Setting: Hospital Number: 63 participants (32 treatment, 31 control) Age: Range from 19 to 88 years Sex: Male/female 35/28 Inclusion criteria: Phlebitis met criteria of the Infusion Nursing Society of America Exclusion criteria: Not stated |
|
| Interventions | Treatment: Fresh aloe juice smeared at punctured skin, then Hirudoid with 3 cm thick smeared, tid Control : Hirudoid smeared at punctured skin. tid Duration: 3 ‐ 7 days |
|
| Outcomes | Primary: The rate of resolution of phlebitis (a) Recovery: disappearance of red swelling, pain and scorching hot. elasticity of vein restored Treatment group: 25/32, Control group: 10/31 (b) Marked improvement: the disappeared area of red swelling, pain and scorching hot with slight pressing pain on the punctured veins over 70% Treatment group: 3/32, Control group: 9/31 (c) Improment: the disappeared area of red swelling, pain and scorching hot with obvious pressing pain on the punctured veins less than 70% Treatment group: 3/32, Control group: 4/31 (d) Not effective: no changes of symptoms of phlebitis after treatment Treatment group: 1/32, Control group: 8/31 Secondary: None |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence was generated by using random digits table |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. The outcome assessment is likely to be influenced by the lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number randomised not stated |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available, and it is not clear if the published reports included all expected outcomes |
| Other bias | Unclear risk | The baseline information between comparison groups were not described |
Li 2003.
| Methods | Study design: Randomised controlled trial Method of randomisation: Not described Concealment of allocation: Not reported Exclusions post‐randomisation: Not reported Losses to follow up: None Intention‐to‐treat analysis: Not reported |
|
| Participants | Country: China Setting: Hospital Number: 88 participants (46 treatment, 42 control) Age: Mean 56 years, range from 28 ‐ 85 years Sex: Male/female 47/41 Inclusion criteria: Phlebitis met the criteria of the "Fundamentals of Nursing" (Yin L 2002) Exclusion criteria: Not stated |
|
| Interventions | Treatment: Fresh aloe slice external application, 3 ‐ 4 times a day, for one hour Control: 50% MgSO4 external application, 3 ‐ 4 times a day, for one hour Duration: 3 days |
|
| Outcomes | Primary: The rate of resolution of phlebitis (a) Recovery: disappearance of red swelling, scorching hot and pain along the punctured vein, and no pressing pain at the punctured vein Treatment group: 28/46, Control group: 13/42 (b) Marked improvement: disappearance of red swelling, scorching hot. pain alleviated, slight pressing pain at the punctured vein Treatment group: 13/46, Control group: 10/42 (c) Improvement: alleviation of red swelling, scorching hot and pain, pressing pain existing at the punctured vein Treatment group: 4/46, Control group: 11/42 (d) Not effective: no change or more severe of phlebitis symptom after treatment Treatment group: 1/46, Control group: 0/42 Secondary: None |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, but the detailed information of randomisation method was not described |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. The outcome assessment is likely to be influenced by the lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number randomised not stated |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available, and it is not clear if the published reports included all expected outcomes |
| Other bias | Unclear risk | The baseline information between comparison groups were not described |
Li 2006.
| Methods | Study design: Randomised controlled trial Method of randomisation: Not described Concealment of allocation: Not reported Exclusions post‐randomisation: Not reported Losses to follow up: None Intention‐to‐treat analysis: Not reported |
|
| Participants | Country: China Setting: Hospital Number: 148 participants (80 treatment, 68 control) Age: Mean 46.7 years, range from 17 ‐ 72 years Sex: Male/female 98/50 Inclusion criteria: Phlebitis met criteria of the Infusion Nursing Society of America Exclusion criteria: Not stated |
|
| Interventions | Treatment: Fresh aloe slice external application (wet compress), tid, for one hour Control: 33% MgSO4 external application, tid Duration: 7 days |
|
| Outcomes | Primary: The rate of resolution of phlebitis (a) Marked improvement: disappearance of red swelling in the local vein, softening of callosity vein vessel, elasticity of vein vessel recover within one treatment course; disappearance of pain within two hour Treatment group: 56/80; Control group: 21/68 (b) Improvement: disappearance of phlebitis symptom over one treatment course and disappearance of pain over two hour Treatment group: 24/80; Control group: 39/68 (c) No improvement: symptoms of phlebitis and pain were unchanged after treatment Treatment group: 0/80; Control group: 8/68 Secondary: None |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, but the detailed information of randomisation method was not described |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. The outcome assessment is likely to be influenced by the lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number randomised not stated. Flow of participants randomised was not available |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available, and it is not clear if the published reports included all expected outcomes |
| Other bias | Unclear risk | The baseline information between comparison groups was not described |
Li 2009.
| Methods | Study design: Randomised controlled trial Method of randomisation: Not described Concealment of allocation: Not reported Exclusions post‐randomisation: Not reported Losses to follow up: None Intention‐to‐treat analysis: Not reported |
|
| Participants | Country: China Setting: Hospital Number: 180 participants (60 treatment 1, 60 treatment 2, 60 control) Age: Range from 16 to 82 years Sex: Male/female 98/82 Inclusion criteria: Phlebitis met criteria of the Infusion Nursing Society of America Exclusion criteria: Not stated |
|
| Interventions | Treatment 1: Fresh aloe slice wet compress at punctured skin for 20 minutes, twice a day Treatment 2: Fresh potato slice (0.1 ‐ 0.2 cm thick) wet compress at punctured skin for 30 ‐ 60 minutes, twice a day Control: 50% MgSO4 wet compress at punctured skin, changed every 4 ‐ 6 hours Duration: 3 days |
|
| Outcomes | Primary: The rate of resolution of phlebitis (a) Marked improvement: disappearance of local pain and red swelling, no pressing pain, elasticity of vein restored Treatment group 1: 34/60. Treatment group 2: 36/60, Control group: 26/60 (b) Improvement: obvious alleviation of local pain and red swelling, no pressing pain, elasticity of vein partially restored Treatment group 1: 18/60. Treatment group 2: 20/60, Control group: 18/60 (c) Not effective: no obvious improvement of phlebitis symptom after treatment Treatment group 1: 6/60. Treatment group 2: 6/60, Control group: 16/60 Secondary: None |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, but the detailed information of randomisation method was not described |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. The outcome assessment is likely to be influenced by the lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number randomised not stated |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available, and it is not clear if the published reports included all expected outcomes |
| Other bias | Low risk | There are no statistical differences in age, sex, the time and location of needles detained in the infused vein among the comparison groups. The study appears to be free other sources of bias |
Li 2011.
| Methods | Study design: Randomised controlled trial Method of randomisation: Not described Concealment of allocation: Not reported Exclusions post‐randomisation: Not reported Losses to follow up: None Intention‐to‐treat analysis: Not reported |
|
| Participants | Country: China Setting: Hospital Number: 80 participants (40 treatment, 40 control) Age: Treatment group: mean 60 years, range from 42 to 78 years; Control group: mean 61 years, range from 40 to 75 years Sex: Treatment group: male/female 32/8; Control group: male/ female 33/7 Inclusion criteria: Malignant tumour patients with chemotherapeutics Exclusion criteria: Not stated |
|
| Interventions | Treatment: Aloe glue was smeared at punctured skin, tid Control: No treatment Duration: 7 days |
|
| Outcomes | Primary: The incidence of phlebitis (a) First degree: local slight pain, red, no strip‐shaped changes in veins, no palpable venous cord Treatment group 3/40, Control group: 9/40 (b) Second degree: local severe pain, scorching hot, slight red swelling and edema, with strip‐shaped changes in veins, palpable venous cord Treatment group 1/40, Control group: 7/40 (c) Third degree: local pain, red swelling and edema over 1 cm, with strip‐shaped changes in veins, palpable venous cord Treatment group 1/40, Control group: 2/40 Secondary: None |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, but the detailed information of randomisation method was not described |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. The outcome assessment is likely to be influenced by the lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number randomised not stated |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available, and it is not clear if the published reports included all expected outcomes |
| Other bias | Low risk | There are no statistical differences in age, sex, illness, chemotherapy drugs, veins between treatment and control groups. The study appears to be free other sources of bias |
Liang 2004.
| Methods | Study design: Randomised controlled trial Method of randomisation: Not described Concealment of allocation: Not reported Exclusions post‐randomisation: Not reported Losses to follow up: None Intention‐to‐treat analysis: Not reported |
|
| Participants | Country: China Setting: Hospital Number: 150 participants (75 treatment, 75 control) Age: Mean 58 years Sex: Male/ female 86/64 Inclusion criteria: Inpatients due to receive therapy of 20% mannitol Exclusion criteria: Not stated |
|
| Interventions | Treatment: Fresh aloe piece external application, consecutively rubbing 10 minutes at the punctured local skin after infusion Control: 75% alcohol rubbing 10 minutes at the punctured local skin after infusion Duration: 7 ‐ 15 days |
|
| Outcomes | Primary: The incidence of phlebitis Treatment group: 0/75, Control group: 28/75 Secondary: None |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, but the detailed information of randomisation method was not described |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. The outcome assessment is likely to be influenced by the lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number randomised not stated |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available, and it is not clear if the published reports included all expected outcomes |
| Other bias | Low risk | There are not the statistical difference on age, sex, illness, dose of the infused mannitol between treatment and control groups. The study appears to be free other sources of bias |
Liu 2003.
| Methods | Study design: Randomised controlled trial Method of randomisation: Not described Concealment of allocation: Not reported Exclusions post‐randomisation: Not reported Losses to follow up: None Intention‐to‐treat analysis: Not reported |
|
| Participants | Country: China Setting: Hospital Number: 147 participants (67 treatment, 80 control) Age: Mean 62.4 years, from 45 to 76 years Sex: Male/female 59/88 Inclusion criteria: Cancer patients due to be administered drugs of chemotherapy Exclusion criteria: Not stated |
|
| Interventions | Treatment: Fresh aloe slice plus ice bag external application (wet compress at punctured skin) Control: 75% MgSO4 external application (wet compress at punctured skin) Duration: One course of chemotherapy |
|
| Outcomes | Primary: The incidence of phlebitis (a) First degree phlebitis: pain, red swelling in local of punctured vein, vein has no the change as trabs and callosity Treatment group: 8/67; Control group: 6/80 (b) Second degree phlebitis: red swelling in local of vein, vein has changed as trabs and no callosity is touched Treatment group: 3/67; Control group: 6/80 (c) Third degree phlebitis: red swelling in local of vein, vein has changed as trabs and callosity is touched Treatment group: 1/67; Control group: 1/80 Secondary: None |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, but the detailed information of randomisation method was not described |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. The outcome assessment is likely to be influenced by the lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Flow of participants randomised was not available |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available, and it is not clear if the published reports included all expected outcomes |
| Other bias | Unclear risk | The comparisons of baseline information between comparison groups were not described |
Liu 2006.
| Methods | Study design: Quasi randomised controlled trial Method of randomisation: Random sequence was generated by admission of inpatient Concealment of allocation: None Exclusions post‐randomisation: Not reported Losses to follow up: None Intention‐to‐treat analysis: Not reported |
|
| Participants | Country: China Setting: Hospital Number: 86 participants (43 treatment, 43 control) Age: Mean 56.3 years, range from 30 to 72 years Sex: Male/female 32/54 Inclusion criteria: Cancer patients due to receive treatment of vinorelbine chemotherapy Exclusion criteria: Not stated |
|
| Interventions | Treatment: Fresh aloe slice external application, fresh aloe slice of 4 ‐ 5 cm wide and 7 ‐ 8 cm long compressed at punctured skin, 4‐5 times per day Control: No treatment Duration: 3 days |
|
| Outcomes | Primary: The incidence of phlebitis (a) First degree: red swelling appear on the punctured vein, pressing pain is not obvious, elasticity of vein is moderate Treatment group: 10/43, Control group: 18/43 (b) Second degree: red swelling appear on the punctured vein, pressing pain and chromatosis is obvious, elasticity of vein is badly. lumens of vein becomes narrow Treatment group: 6/43, Control group: 10/43 (c) Third degree: above symptoms become severe, and the punctured vein appear the change as trabs Treatment group: 2/43, Control group: 7/43 Secondary: None |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Sequence generated by odd or even of the bed number |
| Allocation concealment (selection bias) | High risk | No allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. The outcome assessment is likely to be influenced by the lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number randomised not stated |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available, and it is not clear if the published reports included all expected outcomes |
| Other bias | Low risk | The baseline information sex, age, state of illness and dose of chemotherapy drug between comparison groups are not statistically significant (P > 0.05). The study appears to be free other sources of bias |
Liu 2012.
| Methods | Study design: Randomised controlled trial Method of randomisation: Not described Concealment of allocation: Not reported Exclusions post‐randomisation: Not reported Losses to follow up: None Intention‐to‐treat analysis: Not reported |
|
| Participants | Country: China Setting: Hospital Number: 50 participants (25 treatment, 25 control) Age: Mean 48 years, range from 19 to 78 years Sex: Male/female 34/16 Inclusion criteria: Phlebitis met criteria of the Infusion Nursing Society of America Exclusion criteria: Not stated |
|
| Interventions | Treatment : The fresh aloe slice of 4 ‐ 5 cm wide and 7 ‐ 8 cm long compressed at punctured skin, bid Control: 50% MgSO4 wet compress at punctured skin, bid Duration: 7 days |
|
| Outcomes | Primary: The rate of resolution of phlebitis (a) Recovery: disappearance of red swelling, scorching hot and pain along the punctured vein, and no pressing pain at the punctured vein Treatment group: 14/25, Control group: 4/25 (b) Marked improvement: disappearance of red swelling, pain, and changes like trabs in the local vein. no callosity on the vein is touched Treatment group one: 6/25; Treatment group two: 12/30, Control group: 5/25 (c) Improvement: disappearance of red swelling, pain in the local vein; partial disappearance of vein changes like trabs; the callosity on the vein can be touched Treatment group one: 3/25; Treatment group two: 8/30, Control group: 6/25 (d) No improvement: symptoms of phlebitis and pain were not obviously changed after treatment Treatment group one: 2/25; Treatment group two: 10/30, Control group: 10/25 Secondary: None |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, but the detailed information of randomisation method was not described |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. The outcome assessment is likely to be influenced by the lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Flow of participants randomised was not available |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available, and it is not clear if the published reports included all expected outcomes |
| Other bias | Unclear risk | The comparisons of baseline information between comparison groups were not described |
Liu 2012b.
| Methods | Study design: Randomised controlled trial Method of randomisation: Not described Concealment of allocation: Not reported Exclusions post‐randomisation: Not reported Losses to follow up: None Intention‐to‐treat analysis: Not reported |
|
| Participants | Country: China Setting: Hospital Number: 86 participants (43 treatment, 43 control) Age: From 25 to 75 years Sex: Male/female: treatment group 16/27; control group 23/20 Inclusion criteria: Patients with definite malignant tumour received treatment of chemotherapy; No drugs exosmose during treatment Exclusion criteria: Not stated |
|
| Interventions | Treatment: Fresh aloe leaf external application (wet compress at punctured skin after cleaned with distilled water) Control: No treatment Duration: One course of chemotherapy |
|
| Outcomes | Primary: The incidence of phlebitis. (a) First degree: red swelling appear on the punctured vein, pressing pain is not obvious, elasticity of vein is moderate Treatment group: 10/43, Control group: 18/43 (b) Second degree: red swelling appear on the punctured vein, pressing pain and chromatosis is obvious, elasticity of vein is badly. lumens of vein becomes narrow Treatment group: 6/43, Control group: 10/43 (c) Third degree: above symptoms become severe, and the punctured vein appear the change as trabs Treatment group: 2/43, Control group: 7/43 Secondary: None |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, but the detailed information of randomisation method was not described |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. The outcome assessment is likely to be influenced by the lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Flow of participants randomised was not available |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available, and it is not clear if the published reports included all expected outcomes |
| Other bias | Unclear risk | The comparisons of baseline information between comparison groups were not described |
Lu 2004.
| Methods | Study design: Randomised controlled trial Method of randomisation: Not described Concealment of allocation: Not reported Exclusions post‐randomisation: Not reported Losses to follow up: None Intention‐to‐treat analysis: Not reported |
|
| Participants | Country: China Setting: Hospital Number: 90 participants (30 treatment 1, 30 treatment 2, 30 control) Age: From 26 to 86 years Sex: Male/female 90/56 Inclusion criteria: Phlebitis met criteria of the Infusion Nursing Society of America Exclusion criteria: Not stated |
|
| Interventions | Treatment 1: Dry aloe linimentum 30 ml, bid. The dry aloe linimentum (dry aloe 250 g plus 100 ml 75% alcohol soaked for 10 days, then filtered out the juice to use) Treatment 2: Fresh aloe linimentum 30 ml, bid. The fresh aloe linimentum (fresh aloe 250 g plus 100 ml 75% alcohol soaked for 10 days, then filtered out the juice to use) Control: 75% alcohol, bid Duration: 6 days |
|
| Outcomes | Primary: The rate of resolution of phlebitis (a) Marked improvement: disappearance of red swelling, pain, and changes like trabs in the local vein. no callosity on the vein is touched Treatment group one: 20/30; Treatment group two: 12/30, Control group: 4/30 (b) Improvement: disappearance of red swelling, pain in the local vein. partial disappearance of vein changes like trabs. the callosity on the vein can be touched Treatment group one: 9/30; Treatment group two: 8/30, Control group: 6/30 (c) No improvement: symptoms of phlebitis and pain were not obviously changed after treatment Treatment group one: 1/30; Treatment group two: 10/30, Control group: 20/30 Secondary: None |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, but the detailed information of randomisation method was not described |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. The outcome assessment is likely to be influenced by the lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number randomised not stated |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available, and it is not clear if the published reports included all expected outcomes |
| Other bias | Low risk | There were not statistical difference in age, sex, the severe degree of phlebitis among comparison groups. The study appears to be free other sources of bias |
Pan 2008.
| Methods | Study design: Randomised controlled trial Method of randomisation: Not stated Concealment of allocation: Not reported Exclusions post‐randomisation: Not reported Losses to follow up: None Intention‐to‐treat analysis: Not reported |
|
| Participants | Country: China Setting: Hospital Number: 132 participants (66 treatment, 66 control) Age: Range from 26 to 88 years Sex: Male/female 72/60 Inclusion criteria: Cancer patients due to receive chemotherapy medicine fluorouracil injection Exclusion criteria: Not stated |
|
| Interventions | Treatment: Fresh aloe slice (15 ‐ 20 cm long, 6 ‐ 8 cm wide) wet compresses at the punctured site. changed when dried Control: No treatment Duration: 3‐5 days |
|
| Outcomes | Primary: The incidence of phlebitis (a) First degree: pain, red swelling appear on the punctured vein. Strip‐shaped changes in veins are not observed, and no palpable venous callosity Treatment group: 4/66; Control group: 7/66 (b) Second degree: pain, red swelling appear on the punctured vein. Strip‐shaped changes in vein are observed, and no palpable venous callosity Treatment group: 1/66. Control group: 31/66 (c) Third degree: pain, red swelling appear on the punctured vein. Strip‐shaped changes in vein are observed, with palpable venous callosity Treatment group: 0/66. Control group: 8/66 Secondary: None |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, but the detailed information of randomisation method was not described |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. The outcome assessment is likely to be influenced by the lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number randomised not stated |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available, and it is not clear if the published reports included all expected outcomes |
| Other bias | Unclear risk | The baseline information between comparisons groups were not described |
Peng 2009.
| Methods | Study design: Randomised controlled trial Method of randomisation: Not described Concealment of allocation: Not reported Exclusions post‐randomisation: Not reported Losses to follow up: None Intention‐to‐treat analysis: Not reported |
|
| Participants | Country: China Setting: Hospital Number: 50 participants (25 treatment, 25 control) Age: Range from 32 to 76 years Sex: Male/female 38/12 Inclusion criteria: Patients with chemotherapy medicine Chanchu injection Exclusion criteria: Not stated |
|
| Interventions | Treatment: Fresh aloe slice compress at punctured skin, changed when dried Control: No treatment Duration: 3 ‐ 5 days |
|
| Outcomes | Primary: The incidence of phlebitis Treatment group: 18/25, Control group: 15/25 Secondary: None |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, but the detailed information of randomisation method was not described |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. The outcome assessment is likely to be influenced by the lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number randomised not stated |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available, and it is not clear if the published reports included all expected outcomes |
| Other bias | Low risk | There are not the statistical difference in age, sex, illness, the program of chemotherapy between treatment and control groups. The study appears to be free other sources of bias |
Ren 2008.
| Methods | Study design: Randomised controlled trial Method of randomisation: Not described Concealment of allocation: Not reported Exclusions post‐randomisation: Not reported Losses to follow up: None Intention‐to‐treat analysis: Not reported |
|
| Participants | Country: China Setting: Hospital Number: 66 participants Age: Range from 32 to 76 years Sex: Male/female 46/20 Inclusion criteria: Cancer patients due to receive chemotherapy drug fluorouracil injection Exclusion criteria: Not stated |
|
| Interventions | Treatment 1: Fresh aloe juice rubbed repeatedly at punctured skin. 30 ‐ 60 min every time, once per 1 ‐ 2 hours Treatment 2: 3 cm thick fresh potato slice external compress at punctured skin, changed once per 1 ‐ 2 hours Control: 50% MgSO4 wet compress at punctured skin, changed once per 1 ‐ 2 hours Duration: 7 days |
|
| Outcomes | Primary: The incidence of phlebitis (a) Grade I: no local pain with red skin Treatment group 1: 3/104, Treatment group 2: 1/62, Control group: 4/49 (b) Grade II: local slight pain with red skin less than 2 days Treatment group 1: 3/104, Treatment group 2: 2/62, Control group: 4/49 (c) Grade III: local pain with swelling, scorching hot over 2 days Treatment group 1: 2/104, Treatment group 2: 1/62, Control group: 4/49 Secondary: None |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, but the detailed information of randomisation method was not described |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. The outcome assessment is likely to be influenced by the lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number randomised not stated |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available, and it is not clear if the published reports included all expected outcomes |
| Other bias | Low risk | There are not the statistical difference in age, sex, consist of diseases, condition of infusion vein among comparison groups. The study appears to be free other sources of bias |
Tan 2002.
| Methods | Study design: Randomised controlled trial Method of randomisation: Not described Concealment of allocation: Not reported Exclusions post‐randomisation: Not reported Losses to follow up: None Intention‐to‐treat analysis: Not reported |
|
| Participants | Country: China Setting: Hospital Number: 150 participants (76 treatment, 74 control) Age: Range from 17 to 78 years Sex: Male/female 99/51 Inclusion criteria: Patients with awake mind, first receiving a 20% mannitol injection, 250 ml per injection, 3 ‐ 4 times per day Exclusion criteria: Not stated |
|
| Interventions | Treatment: Fresh aloe slice external application (wet compresses at punctured skin) Control: No treatment Duration: 7 ‐ 15 days |
|
| Outcomes | Primary: The incidence of phlebitis (a) First degree phlebitis: pain, red swelling in local of punctured vein, vein has no the change as trabs and callosity Treatment group: 16/74; Control group: 20/76 (b) Second degree phlebitis: red swelling in local of vein, vein has changed as trabs and no callosity is touched Treatment group: 10/74; Control group: 32/76 (c) Third degree phlebitis: red swelling in local of vein, vein has changed as trabs and callosity is touched Treatment group: 0/74; Control group: 24/76 Secondary: None |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, but the detailed information of randomisation method was not described |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. The outcome assessment is likely to be influenced by the lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported blinding for the outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number randomised not stated |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available, and it is not clear if the published reports included all expected outcomes |
| Other bias | Low risk | There are not the statistical difference in age, sex, consist of diseases, the dose of infused mannitol between comparison groups. The study appears to be free other sources of bias |
Tang 2011.
| Methods | Study design: Randomised controlled trial Method of randomisation: Random number table Concealment of allocation: Not reported Exclusions post‐randomisation: Not reported Losses to follow up: None Intention‐to‐treat analysis: Not reported |
|
| Participants | Country: China Setting: Hospital Number: 66 participants (36 treatment, 30 control) Age: treatment: Mean 6.03 years, range from 3 ‐ 12 years. control: mean 6.11 years, range from 3 ‐ 12 years Sex: Male/female: treatment 23/13; control 18/12 Inclusion criteria: Not stated Exclusion criteria: Not stated |
|
| Interventions | Treatment: Fresh aloe vera juice external application, tid Control: 50% MgSO4 wet compress, tid Duration: 15 days |
|
| Outcomes | Primary: The rate of resolution of phlebitis (a) Recovery: disappearance of symptoms of phlebitis. function of vein is restored Treatment group: 26/36, Control group: 13/30 (b) Improvement: symptoms of phlebitis relieved obviously Treatment group: 8/36, Control group: 9/30 (c) Not effective: no change of symptoms of phlebitis after treatment Treatment group: 2/36, Control group: 8/30 Secondary: None |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised with random number table |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. The outcome assessment is likely to be influenced by the lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported blinding for the outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number randomised not stated |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available, and it is not clear if the published reports included all expected outcomes |
| Other bias | Unclear risk | The baseline information among comparison groups were not described |
Wang 2006.
| Methods | Study design: Quasi randomised controlled trial Method of randomisation: Random sequence was generated by admission number Concealment of allocation: None Exclusions post‐randomisation: Not reported Losses to follow up: None Intention‐to‐treat analysis: Not reported |
|
| Participants | Country: China Setting: Hospital Number: 320 participants (110 treatment 1, 100 treatment 2, 110 control) Age: Treatment 1: mean 41.2 years, range from 19 to 83 years. Treatment 2: mean 47.7 years, range from 11 to 75 years. Control group: mean 45.3 years, range from 16 to 79 years Sex: Treatment 1: male/female 61/49. Treatment 2: male/female 57/43. Control: male/female 62/48 Inclusion criteria: Cancer patients with treatment of chemotherapy Exclusion criteria: Not stated |
|
| Interventions | Treatment 1: Fresh aloe slice external application, about 10 cm long fresh aloe slice compressed at punctured skin, bid, for 4 ‐ 5 hours Treatment 2: Fresh potato slice external application. fresh potato slice with 2 cm thick compressed at punctured skin, bid, for 4 ‐ 5 hours Control: No treatment Duration: 1 ‐ 7 days |
|
| Outcomes | Primary: The incidence of phlebitis (a) First degree: pain, red swelling appear on the punctured vein. Change of vein as trabs is not observed, and no callosity touched Treatment group 1: 12/110, Treatment group 2: 13/100. Control group: 22/110 (b) Second degree:pain, red swelling appear on the punctured vein. Change of vein as trabs is observed, and no callosity touched Treatment group 1: 7/110, Treatment group 2: 4/100. Control group: 24/110 (c) Third degree: pain, red swelling appear on the punctured vein. Change of vein as trabs is observed, and callosity is touched Treatment group 1: 1/110, Treatment group 2: 2/100. Control group: 8/110 Secondary: None |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Random sequence was generated by admission number |
| Allocation concealment (selection bias) | High risk | Random sequence was generated by admission number |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. The outcome assessment is likely to be influenced by the lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number randomised not stated |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available, and it is not clear if the published reports included all expected outcomes |
| Other bias | Low risk | There are not the statistical difference in age, sex, illness, course of disease chemotherapy time and program of chemotherapy among comparison groups. The study appears to be free other sources of bias |
Wang 2008.
| Methods | Study design: Randomised controlled trial Method of randomisation: Not described Concealment of allocation: Not reported Exclusions post‐randomisation: Not reported Losses to follow up: None Intention‐to‐treat analysis: Not reported |
|
| Participants | Country: China Setting: Hospital Number: 65 participants (34 treatment, 31 control) Age: treatment: Mean 46.5 years, range from 35 ‐ 67 years; control: mean 45 years, range from 31 ‐ 62 years Sex: Not described Inclusion criteria: Gynaecologic neoplasms patient due to receive chemotherapy Exclusion criteria: Not stated |
|
| Interventions | Treatment: Fresh aloe slice external application; fresh aloe slice with 3 ‐ 4 cm wide and 7 ‐ 8 cm long wet compress at punctured skin for 4 hours per day plus 100 ml NaCI2 and 10 mg dexamethasone infused, once a day Control: 100 ml NaCI2 and 10 mg dexamethasone infused, once a day Duration: 3 ‐ 7 days |
|
| Outcomes | Primary: The rate of resolution of phlebitis (a) Recovery: disappearance of symptoms of phlebitis. function of vein is restored Treatment group: 30/34, Control group: 14/31 (b) Improvement: symptoms of phlebitis relieved obviously Treatment group: 4/34, Control group: 10/31 (c) Not effective: no change of symptoms of phlebitis after treatment Treatment group: 0/34, Control group: 7/31 Secondary: None |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, but the detailed information of randomisation method was not described |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. The outcome assessment is likely to be influenced by the lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number randomised not stated |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available, and it is not clear if the published reports included all expected outcomes |
| Other bias | Low risk | There are not the statistical difference in age, sex, illness, course of disease and dose of chemotherapy between treatment and control groups The study appears to be free other sources of bias |
Wang 2010.
| Methods | Study design: Randomised controlled trial Method of randomisation: Not described Concealment of allocation: Not reported Exclusions post‐randomisation: Not reported Losses to follow up: None Intention‐to‐treat analysis: Not reported |
|
| Participants | Country: China Setting: Hospital Number: 65 participants (33 treatment, 32 control) Age: Mean 63.5 years, range from 43 to 82 years Sex: Male/female 40/25 Inclusion criteria: Phlebitis met criteria of the nursing conference in Hangzhou city, 1990 Exclusion criteria: Not stated |
|
| Interventions | Treatment: Fresh aloe slice wet compress at punctured skin. tid, for 4 hours Control: 50% MgSO4 wet compress at punctured skin. 4 times a day, for 2 hours Duration: 3 days |
|
| Outcomes | Primary: The rate of resolution of phlebitis (a) Marked improvement: appearance of red swelling, pain and scorching hot on the punctured veins, with softening vein Treatment group: 26/33, Control group: 9/32 (b) Improment: alleviation of red swelling, pain and scorching hot with obvious pressing pain on the punctured veins Treatment group: 7/33, Control group: 16/32 (c) No effective: no changes of symptoms of phlebitis after treatment Treatment group: 0/33, Control group: 7/32 Secondary: None |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, but the detailed information of randomisation method was not described |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. The outcome assessment is likely to be influenced by the lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported blinding for the outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number randomised not stated |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available, and it is not clear if the published reports included all expected outcomes |
| Other bias | Unclear risk | The baseline information among comparison groups were not described |
Wang 2010b.
| Methods | Study design: Randomised controlled trial Method of randomisation: Using the random number table Concealment of allocation: Not reported Exclusions post‐randomisation: Not reported Losses to follow up: None Intention‐to‐treat analysis: Not reported |
|
| Participants | Country: China Setting: Hospital Number: 84 participants Age: Mean 58.86 years, range from 46 to 72 years Sex: Male/female 63/21 Inclusion criteria: Phlebitis met criteria of the Infusion Nursing Society of America Exclusion criteria: Not stated |
|
| Interventions | Treatment: at day: Fresh aloe leaf with 15 ‐ 20 cm long, 6 ‐ 8 cm wide and 0.5 cm thick wet compress at punctured skin, changed every 1 ‐ 2 hours; at night: Hirudoid painting, tid Control: Hirudoid painting, tid Duration: One course of treatment |
|
| Outcomes | Primary: The rate of resolution of phlebitis (a) Recovery: disappearance of local pain, red, swelling, scorching hot and strip‐shaped change in vein, callosity softened Treatment group: 13/30, Control group: 6/39 (b) Marked improvement: obvious alleviation of local pain, red, swelling, scorching hot and strip‐shaped change in vein, callosity softened Treatment group: 16/30, Control group: 16/39 (c) Improvement: slight alleviation of local pain, red, swelling and scorching hot. no regression of strip‐shaped change in vein, no softened callosity Treatment group: 1/30, Control group: 17/39 Secondary: None |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Using the random number table |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. The outcome assessment is likely to be influenced by the lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number randomised not stated |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available, and it is not clear if the published reports included all expected outcomes |
| Other bias | Low risk | There are no the statistical difference in age, sex, illness, the drugs,method and dose of chemotherapy, the condition of veins between treatment and control groups (P > 0.05). The study appears to be free other sources of bias |
Wu 2009.
| Methods | Study design: Randomised controlled trial Method of randomisation: Not described Concealment of allocation: Not reported Exclusions post‐randomisation: Not reported Losses to follow up: None Intention‐to‐treat analysis: Not reported |
|
| Participants | Country: China Setting: Hospital Number: 99 participants (33 treatment 1, 33 treatment 2, 33 treatment 3) Age: Mean 56 years, range from 23 ‐ 76 years Sex: Male/female 51/48 Inclusion criteria: Patients with chemotherapy Exclusion criteria: Not stated |
|
| Interventions | Treatment 1: Fresh aloe slice external application, fresh aloe slice with 10 cm long compress at punctured skin, for 4 ‐ 5 hours, tid Treatment 2: Fresh potato slice external application, fresh potato with 0.12 cm thick compress at punctured skin, for 4 ‐ 5 hours, 1 ‐ 2 times a day Treatment 3: Fresh aloe and potato alternating application Duration: 1 ‐ 7 days |
|
| Outcomes | Primary: The incidence of phlebitis (a) First degree: local pain, red swelling or edema, no strip‐shaped changes in veins, no palpable venous cord Treatment group 1: 7/33, Treatment group 2: 9/33, Treatment group 3: 1/33 (b) Second degree: local pain, red swelling or edema, with strip‐shaped changes in veins, no palpable venous cord Treatment group 1: 3/33, Treatment group 2: 3/33, Treatment group 3: 0/33 (c) Third degree: local pain, red swelling or edema, with strip‐shaped changes in veins, palpable venous cord Treatment group 1: 0/33, Treatment group 2: 0/33, Treatment group 3: 0/33 Secondary: None |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, but the detailed information of randomisation method was not described |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. The outcome assessment is likely to be influenced by the lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number randomised not stated |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available, and it is not clear if the published reports included all expected outcomes |
| Other bias | Unclear risk | The baseline information among comparison groups were not described |
Xiao 2008.
| Methods | Study design: Quasi‐randomised controlled trial Method of randomisation: Sequence generated by odd or even of the bed number Concealment of allocation: None Exclusions post‐randomisation: Not reported Losses to follow up: None Intention‐to‐treat analysis: Not reported |
|
| Participants | Country: China Setting: Hospital Number: 160 participants (80 treatment, 80 control) Age: Mean 41.3 years, range from 19 to 73 years Sex: Female 160 Inclusion criteria: Cancer patients due to receive treatment of chemotherapy Exclusion criteria: Not stated |
|
| Interventions | Treatment: Fresh aloe leaf piece sticking at punctured skin from 30 min before chemotherapy to 30 min after chemotherapy. Control: No treatment Duration: 5 days |
|
| Outcomes | Primary: The incidence of phlebitis (a) Grade 1. local pain, red swelling or edema, no strip‐shaped changes in veins, no palpable venous cord Treatment group: 2/80, Control group: 12/80 (b) Grade 2: local pain, red swelling or edema, with strip‐shaped changes in veins, no palpable venous cord Treatment group: 1/80, Control group: 4/80 (c) Grade 3. local pain, red swelling or edema, with strip‐shaped changes in veins, palpable venous cord Treatment group: 0/80, Control group: 2/80 Secondary: None |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Sequence generated by odd or even of the bed number |
| Allocation concealment (selection bias) | High risk | No allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. The outcome assessment is likely to be influenced by the lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number randomised not stated |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available, and it is not clear if the published reports included all expected outcomes |
| Other bias | Unclear risk | The baseline information between comparison groups were not described |
Yang 2008.
| Methods | Study design: Randomised controlled trial Method of randomisation: Not described Concealment of allocation: Not reported Exclusions post‐randomisation: Not reported Losses to follow up: None Intention‐to‐treat analysis: Not reported |
|
| Participants | Country: China Setting: Hospital Number: 100 participants (52 treatment, 48 control) Age: Mean 46 years, range from 18 to 69 years Sex: Male/female 46/54 Inclusion criteria: Phlebitis met criteria of the Infusion Nursing Society of America Exclusion criteria: Not stated |
|
| Interventions | Treatment: Fresh aloe slice external application, wet compress at punctured skin, changed every 30 min, for one hour, 3 ‐ 4 times a day Control: 50% MgSO4 external application, wet compress at punctured skin, changed every 30 min, for one hour, 3 ‐ 4 times a day Duration: 3 days |
|
| Outcomes | Primary: The rate of resolution of phlebitis (a) Recovery: disappearance of red swelling, pain and scorching hot Treatment group: 30/52, Control group: 15/48 (b) Marked improvement: alleviation of red swelling, pain and scorching hot with slight pressing pain on the punctured veins Treatment group: 15/52, Control group: 11/48 (c) Improvement: alleviation of red swelling, pain and scorching hot with obvious pressing pain on the punctured veins Treatment group: 5/52, Control group: 13/48 (d) Not effective: no changes of symptoms of phlebitis after treatment Treatment group: 2/52, Control group: 9/48 Secondary: None |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, but the detailed information of randomisation method was not described |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. The outcome assessment is likely to be influenced by the lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number randomised not stated |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available, and it is not clear if the published reports included all expected outcomes |
| Other bias | Unclear risk | The baseline information between comparison groups were not described |
Yao 2009.
| Methods | Study design: Randomised controlled trial Method of randomisation: No described Concealment of allocation: Not reported Exclusions post‐randomisation: Not reported Losses to follow up: None Intention‐to‐treat analysis: Not reported |
|
| Participants | Country: China Setting: Hospital Number: 196 participants (98 treatment, 98 control) Age: Treatment group: mean 51.01 years, range from 14 to 82 years, Control group: mean 48.51 years, range from 14 to 78 years Sex: Treatment group: male/female 60/38; Control group: male/ female 58/40 Inclusion criteria: Neurosurgery patients with the needle detained in the infused vein Exclusion criteria: Not stated |
|
| Interventions | Treatment: Fresh aloe slice wet compress at punctured skin, changed every 2 hours Control: No treatment Duration: 5 days |
|
| Outcomes | Primary: The incidence of phlebitis (a) First degree: local pain, red swelling or edema, no strip‐shaped changes in veins, no palpable venous cord Treatment group: 2/98, Control group: 89/98 (b) Second degree: local pain, red swelling or edema, with strip‐shaped changes in veins, no palpable venous cord Treatment group: 2/98, Control group: 9/98 (c) Third degree: local pain, red swelling or edema, with strip‐shaped changes in veins, palpable venous cord Treatment group: 0/98, Control group: 0/98 Secondary: detain time of needle |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, but the detailed information of randomizations method was not described |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. The outcome assessment is likely to be influenced by the lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number randomised not stated |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available, and it is not clear if the published reports included all expected outcomes |
| Other bias | Low risk | There are no the statistical difference in age, sex, illness, the infusion drugs between treatment and control groups. The study appears to be free other sources of bias |
Yu 2006.
| Methods | Study design: Randomised controlled trial Method of randomisation: Not described Concealment of allocation: Not reported Exclusions post‐randomisation: Not reported Losses to follow up: None Intention‐to‐treat analysis: Not reported |
|
| Participants | Country: China Setting: Hospital Number: 150 participants (75 treatment, 75 control) Age: Mean 57.6 years, range from 18 ‐ 86 years Sex: Male/female 64/86 Inclusion criteria: Cancer patients due to receive the chemotherapy Exclusion criteria: Not stated |
|
| Interventions | Treatment : Fresh aloe slice external application, fresh aloe slice 4 ‐ 6 cm long rubbed repeatedly near punctured skin for 6 ‐ 7 min before chemotherapy, then fresh aloe slice compress at punctured skin for 60 min after chemotherapy Control: No treatment Duration: 1 ‐ 7 days |
|
| Outcomes | Primary: The incidence of phlebitis (a) First degree: slightly discomfort at local vein. diameter range of pain, red swelling is less than 1 cm. elasticity of vein is normal Treatment group: 5/75, Control group: 16/75 (b) Second degree: diameter range of pain, red swelling exceed 5 cm. elasticity of vein become to weaken. speed of infusion become slowly Treatment group: 2/75, Control group: 11/75 (c) Third degree: diameter range of pain, red swelling exceed 10 cm. elasticity of vein disappear. speed of infusion become more slowly than second degree Treatment group: 0/75, Control group: 6/75 Secondary: None |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, but the detailed information of randomisation method was not described |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. The outcome assessment is likely to be influenced by the lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number randomised not stated |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available, and it is not clear if the published reports included all expected outcomes |
| Other bias | Low risk | There are no the statistical difference in age, sex, illness, the infusion drugs between treatment and control groups. The study appears to be free other sources of bias |
Zhang 2010.
| Methods | Study design: Quasi randomised controlled trial Method of randomisation: Random sequence was generated by the sequence of admission of inpatient Concealment of allocation: None Exclusions post‐randomisation: Not reported Losses to follow up: None Intention‐to‐treat analysis: Not reported |
|
| Participants | Country: China Setting: Hospital Number: 116 participants (32 treatment 1, 31 treatment 2, 63 control) Age: Range from 45 to 65 years Sex: Not reported Inclusion criteria: Cancer patients due to receive treatment of chemotherapy Exclusion criteria: Not stated |
|
| Interventions | Treatment 1: External application of piece of fresh aloe leaf, fresh aloe leaf with 10 cm long, 6 cm wide and 0.5 cm thick wet compress at punctured skin, changed every 2 hours Treatment 2: External application of sea tangle. sea tangle with 10 cm long, 6 cm wide and 0.5 cm thick wet compress at punctured skin, changed every 2 hours Control: 50% MgSO4 wet compress at punctured skin Duration: 4 days |
|
| Outcomes | Primary: The incidence of phlebitis (a) First degree: local pain, red swelling or edema, no strip‐shaped changes in veins, no palpable venous cord Treatment group1: 13/32, Treatment group 2: 12/31, Control group: 23/63 (b) Second degree: local pain, red swelling or edema, with strip‐shaped changes in veins, no palpable venous cord Treatment group1: 5/32, Treatment group 2: 7/31, Control group: 34/63 (c) Third degree: local pain, red swelling or edema, with strip‐shaped changes in veins, palpable venous cord Treatment group1: 1/32, Treatment group 2: 1/31, Control group: 6/63 Secondary: None |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Random sequence was generated by the sequence of admission, and there was not parallel between treatment and control groups |
| Allocation concealment (selection bias) | High risk | Alternate allocation and not parallel |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. The outcome assessment is likely to be influenced by the lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number randomised not stated |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available, and it is not clear if the published reports included all expected outcomes |
| Other bias | Unclear risk | The baseline information among comparison groups was not described |
Zhang 2013.
| Methods | Study design: Randomised controlled trial Method of randomisation: Not described Concealment of allocation: Not reported Exclusions post‐randomisation: Not reported Losses to follow up: None Intention‐to‐treat analysis: Not reported |
|
| Participants | Country: China Setting: Hospital Number: 160 participants (80 treatment, 80 control) Age: Mean 45 years, range from 30 to 75 years Sex: Male/female 86/74 Inclusion criteria: Phlebitis met criteria of the Infusion Nursing Society of America Exclusion criteria: Not stated |
|
| Interventions | Treatment: Hirudoid plus fresh aloe vera slice smeared at punctured skin, once a day Control: Hirudoid smeared at punctured skin alone, once a day Duration: 7 days |
|
| Outcomes | Primary: The rate of resolution of phlebitis (a) Recovery: disappearance of local pain, red swelling Treatment group: 52/80, Control group: 27/80 (b) Improvement: alleviation of local pain, red swelling Treatment group: 25/80, Control group: 31/80 (c) No effective: no change of local symptom after treatment Treatment group: 3/80, Control group: 22/80 Secondary: None |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, but randomisation method was not described |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. The outcome assessment is likely to be influenced by the lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number randomised not stated |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available, and it is not clear if the published reports included all expected outcomes |
| Other bias | Unclear risk | The baseline information among comparison groups was not described |
Zheng 2010.
| Methods | Study design: Randomised controlled trial Method of randomisation: Not described Concealment of allocation: Not reported Exclusions post‐randomisation: Not reported Losses to follow up: None Intention‐to‐treat analysis: Not reported |
|
| Participants | Country: China Setting: Hospital Number: 50 participants (25 treatment, 25 control) Age: Mean 63.5 years, range from 35 to 78 years Sex: Male/female 31/19 Inclusion criteria: Cancer patients with treatment of chemotherapy Exclusion criteria: Not stated |
|
| Interventions | Treatment: Fresh aloe slice wet compress at punctured skin, changed every half hour Control: No treatment Duration: 5 days |
|
| Outcomes | Primary: The incidence of phlebitis (a) First degree: local pain, red swelling or edema, no strip‐shaped changes in veins, no palpable venous cord Treatment group: 3/25, Control group: 12/25 (b) Second degree: local pain, red swelling or edema, with strip‐shaped changes in veins, no palpable venous cord Treatment group: 0/25, Control group: 4/25 (c) Third degree: local pain, red swelling or edema, with strip‐shaped changes in veins, palpable venous cord Treatment group: 0/25, Control group: 1/25 Secondary: None |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, but the detailed information of randomisation method was not described |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. The outcome assessment is likely to be influenced by the lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number randomised not stated |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available, and it is not clear if the published reports included all expected outcomes |
| Other bias | Low risk | The baseline information such as age, sex, illness, amount of infused mannitol between treatment and control groups are not statistically significant (P > 0.05). The study appears to be free other sources of bias |
Zhong 2011.
| Methods | Study design: Randomised controlled trial Method of randomisation: Not described Concealment of allocation: Not reported Exclusions post‐randomisation: Not reported Losses to follow up: None Intention‐to‐treat analysis: Not reported |
|
| Participants | Country: China Setting: Hospital Number: 60 participants (30 treatment, 30 control) Age: Treatment group: mean 42 years, range from 18 to 72 years; Control group: mean 40 years, range from 17 to 70 years Sex: Treatment group: male/female 11/9; Control group: male/female 13/17 Inclusion criteria: Phlebitis met criteria of the Infusion Nursing Society of America Exclusion criteria: Not stated |
|
| Interventions | Treatment: Aloe plus sulphanilamide pyrimidine. Sulphanilamide pyrimidine was smeared at punctured skin, after one hour, flesh aloe leaf wet compressed at same site, once a day Control: Sulphanilamide pyrimidine was smeared at punctured skin, once a day Duration: 10 days |
|
| Outcomes | Primary: The rate of resolution of phlebitis (a) Recovery: disappearance of local pain, red swelling Treatment group: 25/30, Control group: 19/30 (b) Improvement: alleviation of local pain, red swelling Treatment group: 4/30, Control group: 5/30 (c) No effective: no change of local symptom after treatment Treatment group: 1/30, Control group: 6/30 Secondary: None |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, but the detailed information of randomisation method was not described |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. The outcome assessment is likely to be influenced by the lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number randomised not stated |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available, and it is not clear if the published reports included all expected outcomes |
| Other bias | Low risk | The baseline information such as age, sex, illness, the grade of phlebitis between treatment and control groups are not statistically significant (P > 0.05). The study appears to be free other sources of bias |
Zhou 2006.
| Methods | Study design: Randomised controlled trial Method of randomisation: Not described Concealment of allocation: Not reported Exclusions post‐randomisation: Not reported Losses to follow up: None Intention‐to‐treat analysis: Not reported |
|
| Participants | Country: China Setting: Hospital Number: 100 participants (52 treatment, 48 control) Age: Mean 48 years, range from 20 ‐ 76 years Sex: Male/female 58/42 Inclusion criteria: Phlebitis met the criteria of "Fundamentals of Nursing" (Yin L 2002) Exclusion criteria: Not stated |
|
| Interventions | Treatment: Fresh aloe slice external application, compressed at punctured skin for 10 hours a day, changed every 1 ‐ 2 hours Control: Fresh potato slice external application, compressed at punctured skin for 10 hours a day, changed every 1 ‐ 2 hours Duration: 3 days |
|
| Outcomes | Primary: The rate of resolution of phlebitis (a) Recovery: disappearance of red swelling, scorching hot and pain along the punctured vein, and no pressing pain at the punctured vein Treatment group: 30/52, Control group: 10/48 (b) Marked improvement: disappearance of red swelling, scorching hot, pain alleviated, slight pressing pain at the punctured vein Treatment group: 15/52, Control group: 12/48 (c) Improvement: alleviation of red swelling, scorching hot and pain, pressing pain existing at the punctured vein Treatment group: 5/52, Control group: 16/48 (d) Not effective: no change or more severe of phlebitis symptom after treatment Treatment group: 2/52, Control group: 10/48 Secondary: None |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, but the detailed information of randomisation method was not described |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. The outcome assessment is likely to be influenced by the lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number randomised not stated |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available, and it is not clear if the published reports included all expected outcomes |
| Other bias | Low risk | The baseline information such as age, sex, illness, the program of chemotherapy, the severe degree of phlebitis between treatment and control groups (P > 0.05). The study appears to be free other sources of bias |
bid: twice a day callosity: also called callus. A localised thickening and enlargement of the horny layer of the skin min: minutes tid: three times a day trabs: a thickening of the horny layer of the skin as a result of chronic pressure or friction
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Cao 2007 | Fresh aloe slice plus anisodamine in treatment group versus fresh aloe slice in control group |
| Chen 2007 | The duration of treatment was unclear in the study |
| Dai 2007 | Health education about phlebitis plus 0.2% novocaine 10 ml injection plus fresh aloe slice plus 0.9% NaCI2 100 ml once a day in treatment group versus no treatment in control group |
| Deng 2001 | The intervention was the mixed liquor of fresh aloe juice, 40% MgSO4 and vitamin B12 in treatment group versus only 40% MgSO4 in control group |
| Deng 2009 | Ice bag plus fresh Aloe vera plus bormeol flucinonide cream in treatment group versus 50% MgSO4 in control group |
| Gong 2004 | Self‐made compound aloe cream in treatment group, which included several Chinese medicines such as aloe, long gu, xue jie, huang bai, bormeol. Only 50% MgSO4 in control group |
| Gu 2013 | Sanyrene combined with aloe in treatment group versus usual nursing in control group |
| Guo 2009 | Fresh Aloe vera juice plus 40% MgSO4 plus glycerol plus vitamin B12 in treatment group versus 40% MgSO4 in control group |
| Hu 2011 | Fresh Aloe vera slice combination with fresh potato slice in the treatment group versus 50% MgSO4 in the control group |
| Huang 2005 | Crassulaceae power plus fresh aloe juice in treatment group versus 50% MgSO4 in control group |
| Huang 2009 | The aloe safflower extraction liquor (dry aloe 500 g, dry safflower 500 g and 75% alcohol 10,000 ml soaked for three days, then filtered out aloe and safflower) in treatment group versus 75% alcohol in control group |
| Huang 2013 | The duration of treatment was unclear in the study |
| Jiang 2007 | Shi run shao shang cream plus ice compress plus fresh aloe slice in treatment group versus 0.9% NaCI2 100 ml plus dexamethasone 5 ‐ 10 mg in control group |
| Lei 2012 | The duration of treatment was unclear in the study |
| Li 2003b | Self‐made aloe pigmentum in treatment group which was mainly made of menthol, camphor and Aloe vera. Only 50% MgSO4 in control group |
| Li 2006b | Ice compress plus fresh aloe slices in treatment group versus no treatment in control group |
| Li 2010 | The mixture of Aloe vera juice 5 ml and 40% MgSO4 5 ml and Vitamin B12 500 μg in treatment group versus 40% MgSO4 10 ml in control group |
| Li 2011b | Fresh Aloe vera slice plus hirudoid paste, once a day in treatment group. Mixed liquor of hypertonic glucose and vitamin B12 and dexamethasone in control group |
| Liang 2011 | Fresh Aloe vera slice plus honey in treatment group versus 50% MgSO4 in control group |
| Lin 2009 | Fresh Aloe vera slice plus Ruyijinhuang power in treatment group versus 50% MgSO4 in control group |
| Liu 2009 | The duration of treatment was not described in the original study |
| Lu 2004b | Aloe safflower alcohol extraction solution (made of dry aloe 500 g, dry safflower 500 g, 75% alcohol 5000 ml soaking for three days, and filter out aloe and safflower) in treatment group. Only 50% MgSO4 in control group |
| Lu 2007 | Self‐made liniment including fresh aloe 500 g, safflower 500 g and 75% alcohol 1000 ml) compress in experimental group versus 75% alcohol in control group |
| Lu 2008 | Aloe‐carthamus alcohol hydropathic distilled liquid in treatment group versus no treatment in control group |
| Lu 2010 | Moxibustion and compression of aloe‐safflower‐angelica alcohol hydropathical distilled liquid in treatment group versus 50% MgSO4 in control group |
| Lu 2010b | Treatment group 1: moxibustion, Treatment group 2: compress of aloe‐safflower‐angelica alcohol hydropathic distilled liquid. No control group |
| Lu 2011 | Mixed extraction liquor of Aloe vera, safflower, Chinese angelica and alcohol external application in treatment group. Moxibustion in control group |
| Ma 2008 | Yunnan white drug combination with fresh aloe slice in treatment group versus 33% MgSO4 in control group |
| Ma 2009 | Garlic spread combination with fresh Aloe vera slice in treatment group versus fresh Aloe vera slice in control group |
| Mo 2008 | Mixture of China wood oil and gypsum in treatment group versus fresh aloe slice in control group |
| Peng 2002 | Fresh aloe slices combination with irradiation of Zhoulin spectrum apparatus in treatment group versus only 50% MgSO4 in control group |
| Shao 2008 | Jinhuang power plus fresh aloe slice in treatment group versus fresh aloe slice in control group |
| Shen 2013 | The compound aloe suckling containing aloe, magnesium sulfate, dexamethasone and pentamul in treatment group versus 50% manesium sulfate in control group |
| Shi 2013 | The compound aloe suckling containing aloe, lidocaine, dexamethasone and vitamin B12 in treatment group versus usual nursing in control group |
| Su 2011 | Fresh Aloe vera slice plus anisodamine in treatment group versus 50% MgSO4 in control group |
| Tang 2008 | 50% MgSO4 combination with fresh Aloe vera slice in treatment group versus NaCI2 250 ml plus dexamethasone 5 mg injection in control group |
| Tang 2008b | POME irradiation plus fresh aloe slice in treatment group versus 50% MgSO4 in control group |
| Wang 2004 | Fresh aloe slices in treatment group, no control group |
| Wang 2010c | Fresh Aloe vera slice combination with heparin sodium in treatment group versus 50% MgSO4 in control group |
| Wang 2011 | Fresh aloe slice and hirudoid used alternately in treatment group versus 50% MgSO4 only in control group |
| Wang 2012 | Aloe plus hirudoid paste in treatment group versus 50% MgSO4 in control group |
| Wang 2013 | Aloe combined with honey dew in treatment group versus usual nursing in control group |
| Wei 2012 | Moxibustion combined Chinese medicine containing the exacting solution of Aloe vera, safflower and angelica in treatment group versus 50% MgSO4 in control group |
| Wu 2010 | Fresh Aloe vera slice plus irradiating with QK‐medioapparatus in treatment group versus 50% MgSO4 in control group |
| Xu 2007 | Fresh aloe slice plus potatoes slice in treatment group, and no treatment in control group |
| Xu 2008 | Mixture of 10 ml fresh aloe juice and 10 ml glycerol in treatment group versus 50% MgSO4 in control group |
| Yan 2012 | The duration of treatment was unclear in the study |
| Yang 2010 | Liquid mixture of fresh Aloe vera, 25% magnesium sulphate, 50% glucose injection, vitamin B12 injection in treatment group versus 50% magnesium sulphate in control group |
| Ye 2008 | Novocaine combination with fresh aloe slice in treatment group. The intervention method in control group was not described |
| Ye 2011 | Fresh Aloe vera slice plus hirudoid in treatment group versus 50% MgSO4 in control group |
| Zhang 2003 | The duration of treatment was unclear in the study |
| Zhang 2004 | Fresh aloe slice plus 50% MgSO4 in treatment group versus 2% procaine plus dexamethasone plus NaCI2 plus 50% MgSO4 in control group |
| Zhang 2005 | The duration of treatment was unclear in the study |
| Zhang 2006 | The duration of treatment was unclear in the study |
| Zhang 2008 | Fresh Aloe vera juice plus ultraviolet irradiation in treatment group versus 50% MgSO4 in control group |
| Zhang 2011 | The duration of treatment was unclear in the study |
| Zhou 2001 | The duration of treatment was unclear in the study |
| Zhu 2010 | Bactroban in treatment group versus fresh Aloe vera in control group |
| Zhuang 2008 | Dexamethasone 5 mg plus NaCI2 10 ml plus fresh aloe slice in treatment group versus 50% MgSO4 in control group |
| Zou 2004 | Self‐controlled clinical trial |
Differences between protocol and review
We have removed the grade 1 to 3 criteria for diagnosis and classification of phlebitis listed in the protocol. This is because the criteria for diagnosis and classification of phlebitis in each of the included studies varied slightly. Therefore we have listed them in the 'Characteristics of studies' table of each included study.
We have added a new exclusion item in the review. We have excluded the trials without definite duration of treatment. We believe that the treatment duration of Aloe vera for phlebitis has a very strong association with the effect of therapy or prophylaxis. The trials without definite duration of treatment would cause obvious clinical heterogeneity and lower the quality of the evidence if they were included.
We changed the planned subgroup analyses 'Types of intervention (such as Aloe vera and Aloe vera plus routine treatment)', 'The different uses of interventions (such as prevention and treatment)' and 'Types of control (such as 75% alcohol, 50% to 75% magnesium sulphate and no treatment)' in the protocol to the corresponding comparisons. We have added a new item for subgroup analysis in the review: duration of treatment (such as Aloe vera used for three, five or seven days, etc) because we believe it was an important factor in the effect of treatment.
We have added the WangFang database (Chinese database) to search for published studies.
We did not calculate the risk difference (RD) or include the number needed to treat (NNT) in the analyses because these were not feasible with the data available. We did not perform the evaluation of adverse events because of the unavailable data in the included studies. If adverse events data are available in future updates we will calculate the number needed to harm (NNH).
Contributions of authors
Guo Hua Zheng: planning and writing the protocol; designing the search strategies; undertaking searches; screening search results; analysis of data; interpretation of data; providing a clinical perspective; writing the review.
Liu Yang: appraising quality of papers; extracting data from papers; analysis of data; providing a clinical perspective; writing the review.
Jian Feng Chu: undertaking searches; screening search results; appraising quality of papers; entering data into RevMan; analysis of data; writing the protocol background; appraising quality of papers.
Hai Ying Chen: undertaking searches; screening search results; extracting data from papers; data collection for the review; providing general advice on the review.
Li Juan Mei: undertaking searches; screening search results; extracting data from papers; data collection for the review; providing general advice on the review.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Chief Scientist Office, Scottish Government Health Directorates, The Scottish Government, UK.
The PVD Group editorial base is supported by the Chief Scientist Office.
Declarations of interest
Guo Hua Zheng: none known Liu Yang: none known Hai Ying Chen: none known Jian Feng Chu: none known Lijuan Mei: none known
Edited (no change to conclusions)
References
References to studies included in this review
Cao 2008 {published data only}
- Cao H, Feng XQ, Yang R, Luo MZ. Aloe for the prevention of mannitol‐induced phlebitis [芦荟外敷预防甘露醇所致静脉炎的护理研究]. West China Medical Journal 2008;23(2):360‐1. [Google Scholar]
Chen 2009 {published data only}
- Chen YF, Chen LF, Tan HL, Jiao SY, Pan XL, Liang LJ. Clinical study of the treatment effect of the sulfonic acid mucopolysaccharide creamer added up by aloes on chemical phlebitis [多磺酸粘多糖乳膏加芦荟治疗化疗性静脉炎的效果观察]. Medicine and Health Care 2009;17(6):12‐3. [Google Scholar]
Chen 2010 {published data only}
- Chen SQ. Effect observation of aloe glue treating the infusion phlebitis [芦荟胶治疗静脉输液所致静脉炎的疗效观察]. Nursing and Clinic 2010;14 Suppl:137‐9. [Google Scholar]
Chen 2012 {published data only}
- Chen X. Therapeutic effects of wet compressing with fresh aloe to prevent chemotherapeutic phlebitis [新鲜芦荟片湿敷预防化疗性静脉炎疗效观察]. Journal of Chengde Medical College 2012;29(3):247‐9. [Google Scholar]
Deng 2010 {published data only}
- Deng JM. Effect observation of fresh aloe treating the chemotherapy phlebitis [新鲜芦荟汁治疗化疗性静脉炎疗效观察]. Today Nurse 2010;5:106‐8. [Google Scholar]
Deng 2012 {published data only}
- Deng YY. Effect analysis on aloe vera treating phlebitis caused by amiodarone [芦荟治疗胺碘酮所致静脉炎的效果分析]. Chinese General Practice Nursing 2012;10(7B):1858‐9. [Google Scholar]
Dong 2001 {published data only}
- Dong W, Lin J, Rao BQ. Aloes in the treatment of chemotherapeutic phlebitis: clinical observation in 80 cases [新鲜芦荟外敷治疗化疗性静脉炎80例疗效观察]. Nursing Journal of Chinese People’s Liberation Army 2001;18(1):13‐5. [Google Scholar]
Dong 2008 {published data only}
- Dong YL. Effect observation of aloe painting in prevention of chemotherapy induced phlebitis [新鲜芦荟外敷防治化疗性静脉炎的效果观察]. Modern Journal of Integrated and Traditional Chinese Western Medicine 2008;17(18):2839. [Google Scholar]
Gao 2007 {published data only}
- Gao JP. Effect observation of aloe treating mechanical phlebitis [鲜芦荟治疗小儿机械性静脉炎疗效观察]. Journal of Practical Medical Techniques 2007;14(28):3966‐7. [Google Scholar]
Gao 2012 {published data only}
- Gao AH, Yu Q. Clinical observation of external application of aloe vera glue treating phlebitis caused by mannitol [芦荟胶外敷治疗甘露醇所致静脉炎的临床观察]. Journal of Clinical Research 2012;29(12):2398‐9. [Google Scholar]
Hou 2010 {published data only}
- Hou X, Chen SL, Huang BP. Effect observation of preventing the chemotherapy phlebitis from patients with rodent hydatidiform mole by using the method of evidence based care [循证护理应用于侵蚀性葡萄胎患者预防性化疗性静脉炎的效果观察]. Chinese Journal Practice Nurse 2010;26(5A):69‐71. [Google Scholar]
Hu 2009 {published data only}
- Hu HL, Cao MS, Wang WJ, Shi MC. Clinical study on prophylaxis of phlebitis by applying aloe on patients undergoing chemotherapy [芦荟外敷预防化疗性静脉炎的临床研究]. Journal of Nursing Science 2006;19(9):1‐3. [Google Scholar]
- Hu HL, Wang WJ, Cao MS, Zhu DL, Li N, Shi MC. Effect observation of aloe painting in preventing chemotherapeutic phlebitis [芦荟外敷预防化疗病人静脉炎的疗效观察]. Zhejiang Journal of Traditional Chinese Medicine 2009;44(5):340‐1. [Google Scholar]
Ji 2007 {published data only}
- Ji XY, Zhang NY, Liu PJ. Clinical observation of preventing phlebitis caused by vinorelbine [芦荟预防盖诺致静脉炎的临床观察]. China Medical Herald 2007;4(16):41. [Google Scholar]
Jin 2010 {published data only}
- Jin WH. Effect observation of aloe combination with Hirudoid in treating phlebitis [鲜芦荟配合喜疗妥霜剂治疗静脉炎的效果观察]. Journal of Nursing 2010;17(12):54‐5. [Google Scholar]
Li 2003 {published data only}
- Li GM, Dai JY, Huang WP, Hong YF, Chen JQ. Evaluation of treating phlebitis with fresh aloe application [新鲜芦荟外敷治疗静脉炎的效果评价]. Modern Nursing 2003;9(2):92‐3. [Google Scholar]
Li 2006 {published data only}
- Li CP, Qi F. Observation of external application of aloe treating phlebitis caused by mannitol [新鲜芦荟外敷治疗甘露醇所致静脉炎效果观察]. Journal of Qilu Nursing 2006;12(9):836. [Google Scholar]
Li 2009 {published data only}
- Li JH. Observation the effect of aloe leaf therapy phlebitis by vein leaving alone needle with mannitol [新鲜芦荟叶贴敷治疗静脉留置针输注甘露醇所致静脉炎的疗效观察]. Attend to Practice and Research 2009;6(16):35‐7. [Google Scholar]
Li 2011 {published data only}
- Li SY, Li X. The clinical significance of aloe glue preventing the phlebitis from chemotherapy in the cancer patients [芦荟胶在化疗性静脉炎防治中的临床意义]. Zhongguo Yiyao Shijian Zazhi 2011;82(9):11‐2. [Google Scholar]
Liang 2004 {published data only}
- Liang QZ. 75 cases analysis of external application fresh aloes treating phlebitis caused by mannitol [鲜芦荟外用防护甘露醇所致静脉炎损伤75例分析]. Shandong Medical Journal 2004;44(11):29. [Google Scholar]
Liu 2003 {published data only}
- Liu YH, Li BQ. Effect observation of aloe plus ice compress treated chemotherapeutic phlebitis [芦荟加冰袋外敷预防化疗所致血栓性浅静脉炎的疗效观察]. Chinese Journal of Composite Clinical Medicine 2003;5(3):24‐5. [Google Scholar]
Liu 2006 {published data only}
- Liu HJ, Wu FZ, Chen HF. The effect observation of dexamethasone plus aloe vera applying to prevent the vein injury by venorebine chemotherapy [地塞米松加芦荟外敷预防盖诺化疗致静脉损伤的效果观察]. International Journal of Nursing 2006;25(1):54‐5. [Google Scholar]
Liu 2012 {published data only}
- Liu Q, Luo WL, Chen CM, Zhou RT, Chen XH. Effect observation and nursing on aloe vera painting treating phlebitis caused by PICC detaining [芦荟外敷治疗PICC置管后静脉炎的效果观察及护理]. Chinese Journal of Misdiagnostics 2012;12(4):812‐3. [Google Scholar]
Liu 2012b {published data only}
- Liu L. Effect observation of aloe painting treatment chemotherapeutic phlebitis [芦荟敷料治疗化疗性静脉炎的疗效观察]. Medical Information 2012;25(7):345‐6. [Google Scholar]
Lu 2004 {published data only}
- Lu Y. Study of dry aloe in treatment of phlebitis result in superficial vein catheter transfusion [干芦荟酒精搽剂治疗浅静脉留置针输液并发静脉炎的研究]. Chinese Journal of Current Traditional and Western Medicine 2004;2(5):472‐3. [Google Scholar]
Pan 2008 {published data only}
- Pan X, Feng YH, Xie M. Effect observation of aloe painting in prevention of phlebitis caused by fluorouracil [芦荟外敷预防化疗泵持续滴入氟尿嘧啶所致静脉炎的效果观察]. Family Nurse 2008;6(15):1350‐2. [Google Scholar]
Peng 2009 {published data only}
- Peng XL. The effect observation of aloe slices painting preventing chemotherapeutic phlebitis [新鲜芦荟薄片外敷预防化疗性静脉炎的疗效观察]. Journal of Military Surgeon in Southwest China. 2009;11(3):464‐5. [Google Scholar]
Ren 2008 {published data only}
- Ren GH, Ren QH, Lu XZ. Observation on effect of different ways to prevent continuously intravenous transfusion of fluoruracil induced phlebitis for patients [不同方法预防持续输注氟尿嘧啶致静脉炎效果观察]. Chinese Nursing Research 2008;22(23):2127‐8. [Google Scholar]
Tan 2002 {published data only}
- Tan YK, Tan LQ, Huang SM, Huang FX. Clinical observation of aloe in prevention of phlebitis caused by mannitol [芦荟预防甘露醇所致静脉炎的临床观察]. Journal of Mathematical Medicine 2002;15(5):413. [Google Scholar]
Tang 2011 {published data only}
- Tang H, Zhao SY, Liu G. Effect of natural aloe vera juice treating chemotherapeutic phlebitis in children [天然芦荟汁外涂治疗小儿化疗性静脉炎的效果]. Practical Clinical Medicine 2011;12(10):117‐8. [Google Scholar]
Wang 2006 {published data only}
- Wang JF, Yao HJ, Dong HM. 0bservation on two methods' curative effects of preventing chemotherapeutic phlebitis [两种方法预防化疗性静脉炎的疗效观察]. Nursing and Rehabilitation Journal 2006;5(1):8‐12. [Google Scholar]
Wang 2008 {published data only}
- Wang SS. Observation of treating chemotherapeutic phlebitis with sealed external application [外敷加封闭治疗化疗性静脉炎]. China’s Naturopathy 2008;16(1):25. [Google Scholar]
Wang 2010 {published data only}
- Wang XJ. Observation on therapeutic effects of Aloe in the treatment of phlebitis caused by sodium aescinate for injection [植物芦荟治疗注射用七叶皂苷钠所致静脉炎的疗效观察]. Clinical Medicine and Engineering 2010;17(12):119‐20. [Google Scholar]
Wang 2010b {published data only}
- Wang J, Hu M, Guo WL, Sui AX. Prophylaxis and treatment of vinorelbine‐induced phlebitis by external application of lidocaine, anisodamine, aloe and hirudoid [利多卡因山莨菪碱联合芦荟喜疗妥防治盖诺所致静脉炎]. Journal of Nursing Science 2010;25(3):44‐6. [Google Scholar]
Wu 2009 {published data only}
- Wu LG, Lan JG, Wang HZ, Zhen GH. The application of evidence‐based nursing in the prevention of phlebitis [循证护理在预防化疗性静脉炎中的应用]. Journal of Fujian University of TCM 2009;19(2):15‐6. [Google Scholar]
Xiao 2008 {published data only}
- Xiao CL. Clinical observation of aloe painting in treating chemotherapy induced phlebitis [新鲜芦荟贴敷预防化疗后静脉炎的临床观察]. Jiangxi Medical Journal 2008;43(6):638‐9. [Google Scholar]
Yang 2008 {published data only}
- Yang YM, Yu SH, Tian LM, Zhang YP. Effect observation of external application of aloe in treating chemotherapeutic phlebitis [新鲜芦荟外敷治疗化疗性静脉炎的疗效观察]. Journal of Clinical Medicine in Practice 2008;4(10):24‐5. [Google Scholar]
Yao 2009 {published data only}
- Yao ML. Effect observation of aloe painting in prevention of phlebitis [鲜芦荟外敷预防营养液致静脉炎的疗效观察]. Chinese Journal of Clinical Rational Drug Use 2009;2(17):68. [Google Scholar]
Yu 2006 {published data only}
- Yu YF, Wang XX, Deng XL. A study on external application of fresh aloe to prevent patients from chemotherapeutic drugs induced venous injury [鲜芦荟外用预防化疗药物所致静脉损伤的研究]. Family Nurse 2006;23(411B):4‐6. [Google Scholar]
Zhang 2010 {published data only}
- Zhang GD. The study of aloe, sea tangle preventing the chemotherapy phlebitis by using external paving [芦荟外敷、海带外敷预防化疗性静脉炎的研究]. Lishizhen Medicine and Material Medical Research 2010;21(4):1011‐3. [Google Scholar]
Zhang 2013 {published data only}
- Zhang LY, Peng ZH, Zhang LL. Effect observation of Hirudoid combined with fresh aloe vera treating chemotherapeutic phlebitis [喜疗妥联合新鲜芦荟治疗化疗性静脉炎的疗效观察]. Yiayao Qianyan 2013;3(14):213‐4. [Google Scholar]
Zheng 2010 {published data only}
- Zheng HM, Lin KR, Wu XH, He YZ. The study of nursing measures to prevent phlebitis caused by Mannite in vein [预防甘露醇所致静脉炎的护理方法探讨]. Journal of Qilu Nursing 2010;16(7):56‐7. [Google Scholar]
Zhong 2011 {published data only}
- Zhong L. Application research of fresh aloe treating phlebitis [新鲜芦荟在静脉炎治疗中的应用研究]. Chinese Manipulation and Rehabilitation Medicine 2011;2(3):30‐2. [Google Scholar]
Zhou 2006 {published data only}
- Zhou LX, Yue LQ, Wang CM, Zeng MY, Yang XX. Study on therapeutic effect of fresh aloe and potato external applying to treat patients with phlebitis [新鲜芦荟与马铃薯外敷治疗化疗后静脉炎的效果比较]. Chinese Nursing Research 2006;20(15):1380‐1. [Google Scholar]
References to studies excluded from this review
Cao 2007 {published data only}
- Cao XP, Wu LX, Wang GH, Liu LH, Zhang CF. Effect of povidone iodine and aloe combined with anisodamine in preventing phlebitis [聚维酮碘与芦荟、山莨菪碱联合应用防治输液性静脉炎的效果]. Nursing Journal of Chinese People’s Liberation Army 2007;24(10):58‐9. [Google Scholar]
Chen 2007 {published data only}
- Chen JY, Liu WL, Zhao JY. Effect observation of prevention phlebitis caused by mannitol [甘露醇所致静脉炎预防效果观察]. Journal of Practical Traditional Chinese Medicine 2007;23(6):348‐9. [Google Scholar]
Dai 2007 {published data only}
- Dai Y, Zhang NZ, Wei YP. Nursing measures of preventing phlebitis due to chemotherapy with infusion 5‐fluorouraci [预防5‐氟脲嘧啶化疗所致静脉炎的护理措施]. Journal of Southeast China National Defence Medical Science 2007;9(1):24‐6. [Google Scholar]
Deng 2001 {published data only}
- Deng X, Jiang HL, Liang JQ, Zhou YZ, Li HP. Effect observation of aloe compound painting in treatment phlebitis [芦荟混合液外敷治疗静脉炎疗效观察]. Journal of Nursing Training 2001;16(9):711‐3. [Google Scholar]
Deng 2009 {published data only}
- Deng YQ. Effect observation of ice packs plus aloe and bormeol flucinonide cream in treating chemotherapeutic phlebitis [联合应用冰袋芦荟氟轻松冰片乳膏治疗化疗性静脉炎的效果观察]. Journal of Nursing. 2009;16(8A):58‐9. [Google Scholar]
Gong 2004 {published data only}
- Gong QZ. Observation of self‐made compound aloe cream treating phlebitis [自制复方芦荟膏外敷治疗输液所致静脉炎]. Chinese Medical Journal of Communications 2004;18(1):91‐2. [Google Scholar]
Gu 2013 {published data only}
- Gu XF. The effect observation of sayrene combined with aloe treating phlebitis caused by PICC [赛肤润联合芦荟冷敷治疗PICC所致静脉炎的效果观察]. Today Nurse 2013;9:133‐4. [Google Scholar]
Guo 2009 {published data only}
- Guo XL, Wang R. Effect observation of external application of aloe mixture in treating phlebitis [芦荟混合液外敷治疗静脉炎疗效观察]. Inner Mongol Journal of Traditional Chinese Medicine 2009;28(22):121‐2. [Google Scholar]
Hu 2011 {published data only}
- Hu LY. Clinical effect of aloe combination with potatoes treating the phlebitis from leakage of infusion [芦荟联合马铃薯治疗输液外漏所致静脉炎的临床疗效]. Clinical Nursing 2011;4:43‐4. [Google Scholar]
Huang 2005 {published data only}
- Huang CH, Yang YF, Huang SY, Huang YQ, Zhan SY. Clinical observation of chemotherapeutic phlebitis treated with gold theragran plus fresh aloe [红景天加鲜芦荟治疗化疗性静脉炎的临床观察]. Heilongjiang Nursing Journal 2005;11(11):829‐30. [Google Scholar]
Huang 2009 {published data only}
- Huang XX, Lu Y, Lu GM, Wang XL, Pei DH, Pan DH. Study on aloe safflower ethanol extract wet compressing for patients to prevent fluorouracil infusion induced phlebitis [芦荟红花乙醇提取液湿敷预防输注氟尿嘧啶并发静脉炎的研究]. Chinese Nursing Research 2009;23(11):3047‐9. [Google Scholar]
Huang 2013 {published data only}
- Huang WP. The effect observation of fresh aloe juice treating postchemotherapy phlebitis [鲜芦荟汁治疗化疗后静脉炎的疗效观察]. Journal of Chanzhou Practical Medicine 2013;29(4):245‐6. [Google Scholar]
Jiang 2007 {published data only}
- Jiang L, Yang XD. Effect observation of shi run shaoshang cream plus aloe treating phlebitis caused by vinorelbine tartrate [湿润烧伤膏加芦荟防治盖诺致静脉炎的疗效观察]. Today Nurse 2007;8:62‐3. [Google Scholar]
- Jiang L, Yang XD. Nursing measures of preventing phlebitis caused by vinorelbine [护理干预在防治盖诺致静脉炎中的应用]. International Medicine and Health Guidance News 2007;13(5):103‐4. [Google Scholar]
Lei 2012 {published data only}
- Lei R. Clinical study of fresh aloe juice preventing chemotherapy phlebitis [应用鲜芦荟汁预防化疗性静脉炎的临床研究]. Journal of International Nursing 2012;31(7):1323‐5. [Google Scholar]
Li 2003b {published data only}
- Li BJ. Observation of aloe pigmentum in treatment of phlebitis [芦荟搽剂治疗静脉炎的临床观察]. Nanfang Journal of Nursing 2003;3(3):55‐6. [Google Scholar]
Li 2006b {published data only}
- Li YP. Study of ice compress plus aloe preventing chemotherapeutic phlebitis [冰敷结合芦荟预防联合静脉化疗所致非外渗性静脉炎的探讨]. Journal of Guangxi Medical University 2006;23(S2):20‐1. [Google Scholar]
Li 2010 {published data only}
- Li JL. The mixed liquor of aloe treating 35 patients with phlebitis [芦荟混合液外敷治疗静脉炎35例]. Shanxi Chinese Medicine 2010;31(2):206‐7. [Google Scholar]
Li 2011b {published data only}
- LI ZF, Wang XL. Comfeel transparent dressing association with hirudoid and aloe treating phlebitis from dauorubicin [康惠尔透明贴联合喜疗妥芦荟防治柔红霉素所致静脉炎]. Journal of Clinical Nursing 2011;10(3):78‐80. [Google Scholar]
Liang 2011 {published data only}
- Liang FP. Effect observation of aloe combination with honey treating the phlebitis from infusion [芦荟汁加蜂蜜治疗输液引起静脉炎的疗效观察]. Today Nurse 2011;3:105‐7. [Google Scholar]
Lin 2009 {published data only}
- Lin HF, Wu X. 30 cases of aging phlebitis treated by the external application of ruyijinhuang powder plus aloe [如意金黄散加鲜芦荟外敷治疗老年性静脉炎30例]. Journal of Fujian University of TCM 2009;19(5):71‐3. [Google Scholar]
Liu 2009 {published data only}
- Liu X, Li CS. 16 case analysis of aloe external application treating phlebitis caused by vinorelbine for injection [鲜芦荟外用防护长春瑞滨静脉注射后所致静脉炎损伤16例分析]. World Health Digest 2009;6(22):125. [Google Scholar]
Lu 2004b {published data only}
- Lu Y. Aloe safflower alcohol extraction solution wet compressing for patients to prevent phlebitis induced by bufonin. [芦荟红花酒精提取液湿敷预防华蟾素引起的静脉炎]. Chinese Nursing Reseach 2004;17(9a):1555‐7. [Google Scholar]
Lu 2007 {published data only}
- Lu Y, Huang XX, Li XZ, Pan DH, Lu GM, Wang XL. Study of self‐made aloe and safflower liniment wet packing preventing phlebitis caused by stilamin [自制芦荟红花搽剂湿敷预防浅静脉留置针输注思他宁并发静脉炎的研究]. Guangxi Journal of Traditonal Chinese Medicine 2007;30(5):16‐7. [Google Scholar]
Lu 2008 {published data only}
- Lu Y, Huang XX, Li XZ, Wei HQ, Liu GM, Wang XL. Phlebitis caused by infusing huachansu in vein treated by aloe‐carthamus alcohol hydropathic distilled liquid [芦荟红花酒精提取液湿敷预防浅静脉留置针输液并发静脉炎的研究]. Liaoning Journal of Traditional Chinese Medicine 2008;10:1506‐7. [Google Scholar]
Lu 2010 {published data only}
- Lu Y, Wei HQ, Huang XX, Wang XL, Pi DH, Chen RQ. Study the effect by moxibustion and compression of aloe safflower angelica alcohol hydropathical distilled liquid in treated phlebitis resulting from vein remaining needle iv intravenous infusion [艾炙联合中药湿敷治疗输液并发静脉炎的疗效研究]. West China Medical Journal. 2010;25(1):29‐32. [Google Scholar]
Lu 2010b {published data only}
- Lu Y, Wei HQ, Li XZ, Huang XX, Ling YY, Liu GM, et al. Study the effect of homoisothermy moxibustion and Chinese medicine compression treating phlebitis resulting from vein remaining needle iv intravenous infusion [恒温炙及中药湿敷对留置针输入脂肪乳致静脉炎疗效的研究]. World Health Digest Medical Periodical 2010;7(36):109‐11. [Google Scholar]
Lu 2011 {published data only}
- Lu Y, Ling YY, Wei HQ, Li XZ, Huang XX, Liu GM. Study of large area homoisothermy moxibustion and Chinese medicine hydropathic compress treating phlebitis from the detained needle by used 3L infusion bag [大面积恒温炙及中药湿敷治疗留置针使用3L袋输液致静脉炎的研究]. Chinese General Nursing 2011;9(6c):1614‐5. [Google Scholar]
Ma 2008 {published data only}
- Ma QH. Effect Observation of Yunnanwhite drug plus aloe treating phlebitis [云南白药加鲜芦荟治疗静脉炎的效果观察]. Nursing Practice and Research 2008;5(9):31‐3. [Google Scholar]
Ma 2009 {published data only}
- Ma QH. Effect observation of aloe combination with garlic in treating phlebitis [大蒜泥联合鲜芦荟治疗静脉炎的效果观察]. Today Nurse 2009;5:64‐5. [Google Scholar]
Mo 2008 {published data only}
- Mo MJ, Zhu XJ. Effect of external application of aloes and gypsum mixture in treating phlebitis [桐油石膏与新鲜芦荟外敷治疗静脉炎的效果评价]. Nursing and Rehabilitation Journal 2008;7(11):855‐6. [Google Scholar]
Peng 2002 {published data only}
- Peng JL, Zhang XL, Zhan FQ. Effect observation of aloe combined with zhoulin spectrum analyzer in treatment of phlebitis [鲜芦荟联合周林频谱仪治疗化疗性静脉炎的疗效观察]. Modern Nursing 2002;8(6):457‐8. [Google Scholar]
Shao 2008 {published data only}
- Shao W, Mao WZ, Wang WY, Yang H. Effect study of Jinhuang power combination with fresh aloe treating the chemotherapy phlebitis [金黄散加新鲜芦荟外敷治疗化疗性静脉炎效果探讨]. Shanghai Nursing Journal 2008;8(4):48‐9. [Google Scholar]
Shen 2013 {published data only}
- Shen H, Yu XY. The observation and nursing of compound aloe treating the permeating phlebitis [复方芦荟乳治疗渗出型外周静脉炎的观察及护理]. Journal of Emergency in Traditional Chinese Medicine 2013;22(9):1640‐2. [Google Scholar]
Shi 2013 {published data only}
- Shi CH, Xiao Y. The clinical application observation of compound aloe sucking in the elderly patients with intravenous fluids [复方芦荟乳在老年患者静脉输液后护理中应用的效果观察]. Journal of Emergency in Traditional Chinese Medicine 2013;22(7):1259‐61. [Google Scholar]
Su 2011 {published data only}
- Su CL. Aloe to treatment with mountain drug scopolamine phlebitis research [芦荟配合山莨菪碱对治疗静脉炎的临床研究]. Practical Journal of Cardiac Cerebral Pneumal and Vascular Disease 2011;19(3):367‐9. [Google Scholar]
Tang 2008 {published data only}
- Tang Z, Cai HC, Zuo YM, Wang Z. Clinical observation of treatment of venorelbine‐induced chemotherapy phlebitis with MgSO4 plus aloe [硫酸镁联合芦荟外敷防治长春瑞滨致化疗性静脉炎的临床观察]. Medical Journal of National Defending Forces in Southwest China 2008;18(5):711‐2. [Google Scholar]
Tang 2008b {published data only}
- Tang JH, Liu J, Tian J, Gu XY. Clinical observation of two methods treating phlebitis caused by epirubicin [两种方法治疗表柔比星化疗引起静脉炎的临床观察]. Journal of Modern Oncology 2008;16(1):160. [Google Scholar]
Wang 2004 {published data only}
- Wang XH. Effect observation of aloe preventing embolism induced phlebitis [芦荟预防栓塞性静脉炎的效果观察]. Shenzhen Journal of integrated traditional Chinese and Western Medicine 2004;14(3):177‐8. [Google Scholar]
Wang 2010c {published data only}
- Wang F, Hu QF. Effect observation of aloe combination with heparin treating phlebitis from indwelling needle [芦荟加肝素钠封管液外用治疗留置针静脉炎的效果观察]. Today Nurse 2010;6(1):164‐6. [Google Scholar]
Wang 2011 {published data only}
- Wang YH. Effect observation of hirudoid and aloe alternately using to prevent the chemotherapy phlebitis [喜疗妥软膏与芦荟交替应用预防化疗性静脉炎的效果观察]. Medical Information 2011;24(7):4725‐6. [Google Scholar]
Wang 2012 {published data only}
- Wang J, Wang ZL, Zhao ZZ, Liao D. Effect observation and nursing of early use hirudoid plus aloe treating newborn transfusion exosmosis [新生儿早期使用喜辽妥加芦荟治疗输液外渗的疗效观察与护理]. Nursing Practice and Study 2012;9(13):125‐7. [Google Scholar]
Wang 2013 {published data only}
- Wang AQ. Nursing study of honey dew combined with aloe painting treating chemotherapy venous injury [蜜汁芦荟涂抹防治化疗静脉损伤的护理研究]. Guide of China Medicine 2013;11(2):667‐9. [Google Scholar]
Wei 2012 {published data only}
- Wei HQ, Li Y, Ning YY, Huang XX, Lu GM, Chen RQ, Wang XL. Clinical observation of moxibustion combined with Chinese medicine treating infusion phlebitis from remaining needle [艾炙联合中药湿敷治疗留置针输液并发静脉炎的临床观察]. Journal of Guangxi traditional Chinese Medical University 2012;15(2):115‐7. [Google Scholar]
Wu 2010 {published data only}
- Wu GQ. Aloe combination with the irradiating of QK‐medicoapparatus treating the mechanical phlebitis of PICC [芦荟外敷联合全科治疗仪照射治疗PICC置管后机械性静脉炎]. Modern Journal of Integrated Traditional Chinese and Western Medicine 2010;19(5):608‐10. [Google Scholar]
Xu 2007 {published data only}
- Xu YY, Liu WG. Clinical observation of venous protection on chemotherapy patients by aloe and potato slices [芦荟和马铃薯联合使用对化疗患者静脉保护的临床观察]. Journal of Qiqihar Medical College 2007;28(6):646‐8. [Google Scholar]
Xu 2008 {published data only}
- Xu SL, Zhou AJ. Observation of 40 cases with the infusion phlebitis treated by aloe juice [鲜芦荟汁治疗输液性静脉炎40例]. Medical Information Section of Operative Surgery 2008;21(12):1130‐2. [Google Scholar]
Yan 2012 {published data only}
- Yan S, Xu D, Yu Y, Liu YY, Liu XL. Clinical observation of aloe combined with magnesium sulfate treating infusion phlebitis [芦荟联合硫酸镁治疗药物所致静脉炎的临床观察]. Inner Mongol Journal of Traditional Chinese Medicine 2012;4:50. [Google Scholar]
Yang 2010 {published data only}
- Yang NL. The clinical study of fresh aloe vera with the mixture of magnesium sulphate in treating phlebitis [鲜芦荟配合硫酸镁混合液外敷治疗静脉炎的临床研究]. Journal of Qiqihar Medical College 2010;2:184‐6. [Google Scholar]
Ye 2008 {published data only}
- Ye XQ, Shao LT. Effect observation of novocaine combined with aloe preventing chemotherapeutic phlebitis [奴夫卡因联合芦荟外敷预防化疗性静脉炎的效果观察]. Jiangxi Medical Journal 2008;43(4):386‐7. [Google Scholar]
Ye 2011 {published data only}
- YE CY, Yang Y, He SL, Wu FW. The effect of early use hirudoid plus aloes treating newborn transfusion exosmosis and its nursing [新生儿输液外渗早期使用喜疗妥加芦荟的疗效及护理]. Modern Hospital 2011;11(6):99‐101. [Google Scholar]
Zhang 2003 {published data only}
- Zhang MH. Clinical observation of aloe in preventing phlebitis [芦荟预防浅静脉留置针输液并发静脉炎的临床观察]. Henan Traditional Chinese Medicine 2003;23(6):63‐4. [Google Scholar]
Zhang 2004 {published data only}
- Zhang LY, Ye MQ. Study of aloe in treatment of phlebitis [芦荟治疗静脉炎的探讨]. Sichuan Medical Journal 2004;25(3):285‐6. [Google Scholar]
Zhang 2005 {published data only}
- Zhang DY, Xu WH, Xie AZ. Clinical observation of aloe in treatment of chemotherapeutic phlebitis caused by vinorelbine. [芦荟外敷治疗盖诺化疗引起的静脉炎临床观察]. Modern Journal of Integrated Traditional Chinese and Western Medicine. 2005;14(16):2146. [Google Scholar]
Zhang 2006 {published data only}
- Zhang XQ, Song MM. Effect observation of aloe treating elderly phlebitis [芦荟治疗老年人静脉炎疗效观察]. Qingdao Medical Journal 2006;38(3):204‐5. [Google Scholar]
Zhang 2008 {published data only}
- Zhang YZ. Observation on effect of ultraviolet irradiation combining with fresh aloe juice local external applying to treat patients with chemotherapy induced phlebitis [紫外线照射配合鲜芦荟汁局部外敷治疗化疗性静脉炎效果观察]. Chinese Nursing Research 2008;23(23):2134. [Google Scholar]
Zhang 2011 {published data only}
- Zhang H, Liang JL. The effect observation of aloe leaf treating phlebitis caused by sodium aescinate [芦荟叶治疗七叶皂甙钠所致静脉炎的疗效观察]. The Chinese and Foreign Health Abstract 2011;8(48):175‐6. [Google Scholar]
Zhou 2001 {published data only}
- Zhou CX. Effect observation of aloe application preventing chemotherapeutic phlebitis [芦荟外敷预防化疗后静脉炎的效果观察]. Shanghai Nursing 2001;1(3):27. [Google Scholar]
Zhu 2010 {published data only}
- Zhu TE. Clinical study of bactroban treating phlebitis of drug [百多邦治疗药物性静脉炎的临床研究]. Strait Pharmaceutical Journal 2010;22(12):143‐5. [Google Scholar]
Zhuang 2008 {published data only}
- Zhuang YX, Shen JH. Observation of local blocking plus aloe painting in prevention of phlebitis caused by mannitol [局部封闭加芦荟外敷治疗甘露醇所致静脉炎的观察]. Today Nurse 2008;10:93‐4. [Google Scholar]
Zou 2004 {published data only}
- Zou LF, Yao HJ, Mei J, Wang FF, Wang AS. Observation of aloe preventing phlebitis caused by vinorelbine [新鲜芦荟汁预防盖诺药物所致静脉炎的观察]. Tianjin Journal of Nursing 2004;12(2):65. [Google Scholar]
Additional references
Altman 2001
- Altman DG, Schulz KF. Statistics notes: concealing treatment allocation in randomised trials. BMJ (Clinical Research Ed.) 2001;323(7310):446‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boyd 2011
- Boyd S, Aggarwal I, Davey P, Logan M, Nathwani D. Peripheral intravenous catheters: the road to quality improvement and safer patient care. Journal of Hospital Infection 2011;77(1):37‐41. [DOI] [PubMed] [Google Scholar]
Chithra 1998
- Chithra P, Sajithlal GB, Chandrakasan G. Influence of Aloe vera on collagen characteristics in healing dermal wounds in rats. Molecular and Cellular Biochemistry 1998;181(1‐2):71‐6. [DOI] [PubMed] [Google Scholar]
Curran 1990
- Curran CF, Luce JK, Page JA. Doxorubicin‐associated flare reactions. Oncology Nursing Forum 1990;17(3):387‐9. [PubMed] [Google Scholar]
Emiko 2009
- Kohno E, Murase S, Matsuyama K, Okamura N. Effect of corticosteroids on phlebitis induced by intravenous infusion of antineoplastic agents in rabbits. International Journal of Medical Sciences 2009;6(5):218‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Feily 2009
- Feily A, Namazi MR. Aloe vera in dermatology: a brief review. Giornale Italiano di Dermatologia e Venereologia 2009;144(1):85‐91. [PubMed] [Google Scholar]
Fulton 1990
- Fulton JE Jr. The stimulation of postdermabrasion wound healing with stabilized aloe vera gel‐polyethylene oxide dressing. The Journal of Dermatologic Surgery and Oncology 1990;16(5):460‐7. [DOI] [PubMed] [Google Scholar]
Heggers 1996
- Heggers JP, Kucukcelebi A, Listengarten D, Stabenau J, Ko F, Lyle D, et al. Beneficial effect of aloe on wound healing in an excisional wound model. Journal of Alternative and Complementary Medicine 1996;2(2):271‐7. [DOI] [PubMed] [Google Scholar]
Hershey 1984
- Hershey CO, Tomford JW, Mclaren CE, Porter DK, Cohen DI. The natural history of intravenous catheter‐associated phlebitis. Archives of Internal Medicine 1984;144(7):1373‐5. [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011a
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Review of Interventions version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hu 2006
- Hu HL, Cao MS, Wang WJ, Shi MC. Clinical study on prophylaxis of phlebitis by applying aloe on patients undergoing chemotherapy. Journal of Nursing Science 2006;21(10):1‐3. [Google Scholar]
Hutter 1996
- Hutter JA, Salmon M, Stavinoha WB, Satsangi N, Williams RF, Streeper RT, et al. Antiinflammatory C‐glucosyl chromone from Aloe barbadensis. Journal of Natural Products 1996;59(5):541‐3. [DOI] [PubMed] [Google Scholar]
Klein 1988
- Klein AD, Penneys NS. Aloe vera. Journal of the American Academy of Dermatology 1988;18(4 pt 1):714‐20. [DOI] [PubMed] [Google Scholar]
Kuwahara 1998
- Kuwahara T, Asanami S, Kubo S. Experimental infusion phlebitis: tolerance osmolality of peripheral venous endothelial cells. Nutrition 1998;14(6):496‐501. [DOI] [PubMed] [Google Scholar]
Langmead 2004
- Langmead L, Feakins RM, Goldthorpe S, Holt H, Tsironi E, Silva A, et al. Randomized, double‐blind, placebo‐controlled trial of oral aloe vera gel for active ulcerative colitis. Alimentary Pharmacology and Therapeutics 2004;19(7):739‐47. [DOI] [PubMed] [Google Scholar]
Maki 1991
- Maki DG, Ringer M. Risk factors for infusion‐related phlebitis with small peripheral venous catheters. Annals of Internal Medicine 1991;114(10):845‐54. [DOI] [PubMed] [Google Scholar]
Maki 2008
- Maki DG. Improving the safety of peripheral intravenous catheters. British Medical Journal 2008;337(7662):122‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
McCauley 1990
- McCauley RL, Heggers JP, Robson MC. Frostbite. Methods to minimize tissue loss. Postgraduate Medicine 1990;88(8):67‐8;73‐7. [DOI] [PubMed] [Google Scholar]
Mermel 2001
- Mermel LA, Farr BM, Sherertz RL, Raad II, O'Grady N, Harris JS, et al. Guidelines for the management of intravascular catheter‐related infections. Clinical Infectious Diseases 2001;32(9):1249‐72. [DOI] [PubMed] [Google Scholar]
Monreal 1999
- Monreal M, Quilez F, Rey‐Joly C, Rodriguez S, Sopena N, Neira C, et al. Infusion phlebitis in patients with acute pneumonia: a prospective study. Chest 1999;115(6):1576‐80. [DOI] [PubMed] [Google Scholar]
Nakayama 2002
- Nakayama S, Matsubara N, Sakai T, Aso N. The incidence of phlebitis in patients administered vinorelbine by intravenous bolus injection: a retrospective study. Gan To Kagaku Ryoho 2002;29(4):633‐5. [PubMed] [Google Scholar]
O'Grady 2002
- O'Grady NP, Alexander M, Dellinger EP, Gerberding JL, Heard SO, Maki DG, et al. Guidelines for the prevention of intravascular catheter‐related infections. Centers for Disease Control and Prevention. Morbidity and Mortality Weekly Report. Recommendations and Reports 2002;51(RR‐10):1‐29. [PubMed] [Google Scholar]
Oyelami 2009
- Oyelami OA, Onayemi A, Oyedeji OA, Adeyemi LA. Preliminary study of effectiveness of aloe vera in scabies treatment. Phytotherapy Research 2009;23(10):1482‐4. [DOI] [PubMed] [Google Scholar]
Schmid 2000
- Schmid MW. Risks and complications of peripherally and centrally inserted intravenous catheters. Critical Care Nursing Clinics of North America 2000;12(2):165‐74. [PubMed] [Google Scholar]
Shelton 1991
- Shelton M. Aloe vera, its chemical and therapeutic properties. International Journal of Dermatology 1991;30(10):679‐83. [DOI] [PubMed] [Google Scholar]
Sterne 2001
- Sterne JA, Egger M. Funnel plots for detecting bias in meta‐analysis: guidelines on choice of axis. Journal of Clinical Epidemiology 2001;54(10):1046‐55. [DOI] [PubMed] [Google Scholar]
Surjushe 2008
- Surjushe A, Vasani R, Saple DG. Aloe vera: a short review. Indian Journal of Dermatology 2008;53(4):163‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tager 1983
- Tager IB, Ginsberg MB, Ellis SE, Walsh NE, Dupont I, Simchen E, et al The Rhode Island Nosocomial Infection Consortium. An epidemiologic study of the risks associated with peripheral intravenous catheters. American Journal of Epidemiology 1983;118(6):839‐51. [DOI] [PubMed] [Google Scholar]
Tang 2001
- Tang YH, Gao LC, Liu GQ. Development in prevention and management of phlebitis: A Review. Nursing Journal of Chinese People's Liberation Army 2001;18(4):25‐6. [Google Scholar]
Tjon 2000
- Tjon JA, Ansani NT. Transdermal nitroglycerin for the prevention of intravenous infusion failure due to phlebitis and extravasation. Annals of Pharmacotherapy 2000;34(10):1189‐92. [DOI] [PubMed] [Google Scholar]
Tully 1981
- Tully JL, Friedland GH, Baldini LM, Goldmann DA. Complication of intravenous therapy with steel needles and Teflon catheters. A comparative study. American Journal of Medicine 1981;70(3):702‐6. [DOI] [PubMed] [Google Scholar]
Visuthikosol 1995
- Visuthikosol V, Chowchuen B, Sukwanarat Y, Sriurairatana S, Boonpucknavig V. Effect of aloe vera gel to healing of burn wound a clinical and histologic study. Journal of the Medical Association of Thailand 1995;78(8):403‐9. [PubMed] [Google Scholar]
Vogler 1999
- Vogler BK, Ernst E. Aloe vera: a systematic review of its clinical effectiveness. The British Journal of General Practice 1999;49(447):823‐8. [PMC free article] [PubMed] [Google Scholar]
Waitt 2004
- Waitt C, Waitt P, Pirmohamed M. Intravenous therapy. Postgraduate Medical Journal 2004;80(939):1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
West 2003
- West DP, Zhu YF. Evaluation of aloe vera gel gloves in the treatment of dry skin associated with occupational exposure. American Journal of Infection Control 2003;31(1):40‐2. [DOI] [PubMed] [Google Scholar]
Winters 1981
- Winters WD, Benavides R, Clouse WJ. Effects of aloe extracts on human normal and tumor cells in vitro. Economic Botany 1981;35(1):89‐95. [Google Scholar]
Yin L 2002
- Yin L. Fundamentals of Nursing. Third Edition. Beijing: People's Medical Publishing House, 2002. [Google Scholar]
Yoh 2007
- Yoh K, Niho S, Goto K, Ohmatsu H, Kubota K, Kakinuma R, et al. Randomized trial of drip infusion versus bolus injection of vinorelbine for the control of local venous toxicity. Lung Cancer 2007;55(3):337‐41. [DOI] [PubMed] [Google Scholar]
Zheng 2011
- Zheng GH, Yang L, Chu JF, Chen HY. Aloe Vera for prevention and treatment of infusion phlebitis. Cochrane Database of Systematic Reviews 2011, Issue 6. [DOI: 10.1002/14651858.CD009162] [DOI] [PMC free article] [PubMed] [Google Scholar]


