Abstract
Background
Individual counselling from a smoking cessation specialist may help smokers to make a successful attempt to stop smoking.
Objectives
The review addresses the following hypotheses:
1. Individual counselling is more effective than no treatment or brief advice in promoting smoking cessation. 2. Individual counselling is more effective than self‐help materials in promoting smoking cessation. 3. A more intensive counselling intervention is more effective than a less intensive intervention.
Search methods
We searched the Cochrane Tobacco Addiction Group Specialized Register for studies with counsel* in any field in May 2016.
Selection criteria
Randomized or quasi‐randomized trials with at least one treatment arm consisting of face‐to‐face individual counselling from a healthcare worker not involved in routine clinical care. The outcome was smoking cessation at follow‐up at least six months after the start of counselling.
Data collection and analysis
Both authors extracted data in duplicate. We recorded characteristics of the intervention and the target population, method of randomization and completeness of follow‐up. We used the most rigorous definition of abstinence in each trial, and biochemically‐validated rates where available. In analysis, we assumed that participants lost to follow‐up continued to smoke. We expressed effects as a risk ratio (RR) for cessation. Where possible, we performed meta‐analysis using a fixed‐effect (Mantel‐Haenszel) model. We assessed the quality of evidence within each study using the Cochrane 'Risk of bias' tool and the GRADE approach.
Main results
We identified 49 trials with around 19,000 participants. Thirty‐three trials compared individual counselling to a minimal behavioural intervention. There was high‐quality evidence that individual counselling was more effective than a minimal contact control (brief advice, usual care, or provision of self‐help materials) when pharmacotherapy was not offered to any participants (RR 1.57, 95% confidence interval (CI) 1.40 to 1.77; 27 studies, 11,100 participants; I2 = 50%). There was moderate‐quality evidence (downgraded due to imprecision) of a benefit of counselling when all participants received pharmacotherapy (nicotine replacement therapy) (RR 1.24, 95% CI 1.01 to 1.51; 6 studies, 2662 participants; I2 = 0%). There was moderate‐quality evidence (downgraded due to imprecision) for a small benefit of more intensive counselling compared to brief counselling (RR 1.29, 95% CI 1.09 to 1.53; 11 studies, 2920 participants; I2 = 48%). None of the five other trials that compared different counselling models of similar intensity detected significant differences.
Authors' conclusions
There is high‐quality evidence that individually‐delivered smoking cessation counselling can assist smokers to quit. There is moderate‐quality evidence of a smaller relative benefit when counselling is used in addition to pharmacotherapy, and of more intensive counselling compared to a brief counselling intervention.
Plain language summary
Does individually‐delivered counselling help people to stop smoking?
Background
Individual counselling is commonly used to help people who are trying to quit smoking. The review looked at trials of counselling by a trained therapist providing one or more face‐to‐face sessions, separate from medical care. The outcome was being a non smoker at least six months later.
Study characteristics
We searched for trials in May 2016 and identified 49 trials Inlcuding around 19,000 participants. All the trials involved one or more face‐to‐face counselling sessions lasting at least 10 minutes, but most were much longer. Many also included further telephone contact for additional support. Thirty‐three of the trials compared individual counselling to a control group that only had minimal support, which could be usual care, brief advice about stopping smoking, or written materials. Of these, 27 did not offer any medication such as nicotine replacement therapy (NRT), which also helps people stop. Six of the 33 provided NRT or other medication to everyone in the trial. Twelve studies compared more intensive to less intensive counselling, and five compared different types of counselling.
Results and quality of evidence
Combining the results of the studies showed that having individual counselling could increase the chance of quitting by between 40% and 80%, compared to minimal support. This means that if seven out of 100 smokers managed to quit for at least six months using the sort of brief support given to the control groups, then between 10 and 12 in 100 would be expected to be successful after having counselling. We judged the quality of this evidence to be high. If everyone also had NRT or other medication, and 11 in 100 could quit in the control group, between 11 and 16 in 100 would be expected to be successful with the addition of counselling. We assessed this evidence as being of moderate quality, because the size of benefit was less certain. Having more intensive counselling support, for example more sessions, probably helps more, but the additional benefit is likely to be small, and again was of moderate quality because the size of benefit was uncertain. The few studies that compared different types of counselling did not show any differences between them.
Summary of findings
Background
Psychological interventions to aid smoking cessation include self‐help materials, brief therapist‐delivered interventions such as advice from a physician or nurse, intensive counselling delivered on an individual basis or in a group, and combinations of these approaches. Previous reviews have shown a small but consistent effect of brief, therapist‐delivered interventions (Stead 2013a). The effect of self‐help interventions is less clear (Hartmann‐Boyce 2014). More intensive intervention in a group setting increases quit rates (Stead 2017).
In this review, we assess the effectiveness of more intensive counselling delivered by a smoking cessation counsellor to a person on a one‐to‐one basis. One problem in assessing the value of individual counselling is that of confounding with other interventions. For example, counselling delivered by a physician in the context of a clinical encounter may have different effects from that provided by a non‐clinical counsellor. One approach to this problem is to employ statistical modelling (logistic regression) to control for possible confounders, an approach used by the US Public Health Service in preparing clinical practice guidelines (AHCPR 1996; Fiore 2000; Fiore 2008). An alternative approach is to review only unconfounded interventions. This is the approach we have adopted in the Cochrane Tobacco Addiction Review Group. We therefore specifically exclude from this review counselling provided by doctors or nurses during the routine clinical care of the patient, and focus on smoking cessation counselling delivered by specialist counsellors. We define counselling broadly, based only on a minimum time spent in contact with the smoker, not according to the use of any specific behavioural approach.
Objectives
The review addresses the following hypotheses:
1. Individual counselling is more effective than no treatment or brief advice in promoting smoking cessation. 2. Individual counselling is more effective than self‐help materials in promoting smoking cessation. 3. A more intensive counselling intervention is more effective than a less intensive intervention.
Methods
Criteria for considering studies for this review
Types of studies
Randomized or quasi‐randomized controlled trials (RCTs) with a minimum follow‐up of six months, where at least one treatment arm consisted of an unconfounded intervention from a counsellor. Studies in which the treatment arm combined counselling and pharmacotherapy, and the control condition had neither, are covered in a separate review (Stead 2016).
Types of participants
Any smokers, except pregnant women (smoking cessation interventions in pregnancy are addressed by a separate review, Chamberlain 2013). We also exclude trials recruiting only children and adolescents.
Types of interventions
We defined individual counselling as a face‐to‐face encounter between a smoker and a counsellor trained in assisting smoking cessation. This review specifically excludes studies of counselling delivered by doctors and nurses as part of clinical care, which are covered in separate reviews (Rice 2013; Stead 2013a). It also excludes studies of interventions that combined counselling with provision of pharmacotherapy, compared to brief support (Stead 2016), studies of motivational interviewing (Lindson‐Hawley 2015) and interventions which address multiple risk factors in addition to smoking. We include studies that evaluate the effect of counselling as an addition to pharmacotherapy.
We include studies comparing different counselling approaches if they are not covered by other Cochrane Reviews of specific interventions. Comparisons between individual counselling and behavioural therapy conducted in groups are covered in the Cochrane Review of group behavioural therapy (Stead 2017).
Types of outcome measures
The outcome was smoking cessation at the longest reported follow‐up. We used sustained abstinence where available, or multiple point prevalence. We included studies using self‐report with or without biochemically‐validated cessation, and performed sensitivity analyses to determine whether the estimates differed significantly in studies without verification.
Search methods for identification of studies
We searched the Cochrane Tobacco Addiction Group Specialized Register for studies with counsel* in title, abstract or keyword fields. At the time of the search the Register included the results of searches of the Cochrane Central Register of Controlled trials (CENTRAL), issue 4, 2016; MEDLINE (via OVID) to update 20160513; EMBASE (via OVID) to week 201621; PsycINFO (via OVID) to update 20160516. See the Tobacco Addiction Group Module in the Cochrane Library for full search strategies and list of other resources searched. We also checked previous reviews and meta‐analyses for relevant studies, including all studies in the previous US guidelines (AHCPR 1996; Fiore 2000; Fiore 2008). The most recent search was conducted in May 2016.
Data collection and analysis
One author (LS, who is also the Cochrane Information Specialist for the Tobacco Addiction Group) prescreened results of the searches. Both authors checked reports of studies of potentially relevant interventions.
Both authors extracted data independently.
Information extracted included descriptive details on the setting of the study, the population, and details of intervention(s) and control conditions, including number and duration of planned sessions.
Assessment of risk of bias in included studies
We assessed risk of selection, detection and attrition bias, based on the reported methods of randomization and allocation concealment (selection bias), use of biochemical validation of self‐reported abstinence (detection bias) and numbers lost to follow‐up (attrition bias).
Measures of treatment effect & data synthesis
We summarized individual study results as a risk ratio (RR), calculated as: (number of quitters in intervention group/number randomized to intervention group) / (number of quitters in control group/number randomized to control group). We assumed that participants lost to follow‐up continued to smoke and included them as such in denominators. Where appropriate we performed meta‐analysis using a Mantel‐Haenszel fixed‐effect method to estimate a pooled risk ratio with a 95% confidence interval (CI) (Greenland 1985). We estimated the amount of statistical heterogeneity between trials using the I2 statistic (Higgins 2003). Values over 50% can be regarded as moderate heterogeneity, and values over 75% as high.
We made the following comparisons:
Individual counselling versus no treatment, brief advice or self‐help materials
More intensive versus less intensive individual counselling
Comparisons between counselling methods matched for contact time
Results
Description of studies
We include 49 studies in this updated review, with around 19,000 participants. Thirty‐three studies (11 new for this update) contribute to the primary analysis comparing individual counselling to a minimal contact behavioural intervention. Eleven studies (six new) compared different intensities of counselling and five (two new) compared different counselling approaches which were similar in intensity of contact.
In a few cases we resolved difficulties in applying the inclusion criteria by discussion. In two cases (Wiggers 2006; Aveyard 2007) we were uncertain whether the providers were acting as specialist counsellors or were providing interventions as part of usual care in other healthcare roles. We included both after discussion about this aspect of their designs. We included one study that had only five months follow‐up (Kim 2005).
Study populations
Nineteen of the 49 studies recruited medical or surgical hospital inpatients (Pederson 1991; Ockene 1992; Stevens 1993; Rigotti 1997; Simon 1997; Dornelas 2000; Molyneux 2003; Simon 2003; Hennrikus 2005; Pedersen 2005; Brunner 2012), or outpatients (Weissfeld 1991; Kim 2005; Tonnesen 2006; Hennrikus 2010; Chan 2012; Ramon 2013; Thankappan 2013; Chen 2014). One recruited some inpatients (Schmitz 1999). Four other studies recruited drug‐ and alcohol‐dependent veterans attending residential rehabilitation (Bobo 1998; Burling 1991; Burling 2001; Mueller 2012). One study recruited new mothers in maternity wards (Hannover 2009); we considered the subgroup of trial participants who were smoking at this point. Other studies recruited smokers in primary care clinics (Fiore 2004; Aveyard 2007; Marley 2014; Ramos 2010), dental clinics (Nohlert 2009), primary care and local community (Aleixandre 1998), local community and university (Alterman 2001), communities and worksites (Nakamura 2004), at a periodic healthcare examination (Bronson 1989), at a Planned Parenthood clinic (Glasgow 2000), employees volunteering for a company smoking cessation programme (Windsor 1988), participants in a lung cancer screening study (Marshall 2016), and community volunteers (Jorenby 1995; Lifrak 1997; Ahluwalia 2006; Killen 2008; McCarthy 2008, Wu 2009; Garvey 2012; Kim 2015). Lack of interest in quitting was not an explicit exclusion criterion in any study, but the level of motivation to quit smoking was sometimes difficult to assess. One trial enrolled all smokers admitted to hospital (Stevens 1993), whilst one enrolled 90% of smokers approached (Rigotti 1997). In one large study in primary care 68% of smokers agreed to participate and 52% met the inclusion criteria and were recruited (Fiore 2004). In other studies a larger proportion of eligible smokers may have declined randomization because of lack of interest in quitting.
Special populations included Australian Aboriginal people (Marley 2014), homeless people (Okuyemi 2013), people under community corrections supervision (Cropsey 2015) and people with schizophrenia (Williams 2010). Two studies recruited Asian minority populations in the US; Kim 2015 (Koreans) and Wu 2009 (Chinese), and one recruited African Americans (Ahluwalia 2006).
Two studies recruited only women: Schmitz 1999 recruited 53 women hospitalized with coronary artery disease (CAD) and 107 volunteers with CAD risk factors. Glasgow 2000 recruited women attending Planned Parenthood clinics, who were not selected for motivation to quit. Weissfeld 1991 recruited only men, while Simon 2003 and Nakamura 2004 recruited predominantly men.
Thirty studies were conducted in the USA, three in Spain (Aleixandre 1998; Ramos 2010; Ramon 2013), three in Denmark (Pedersen 2005; Tonnesen 2006; Brunner 2012), two in the UK (Molyneux 2003; Aveyard 2007), two in Australia (Marley 2014; Marshall 2016), and one each in Germany (Hannover 2009), Switzerland (Mueller 2012), Sweden (Nohlert 2009), Netherlands (Wiggers 2006), Hong Kong (Chan 2012), China (Chen 2014), Japan (Nakamura 2004), Korea (Kim 2005), and India (Thankappan 2013).
Intervention components
The counselling interventions typically included the following components: review of a participant's smoking history and motivation to quit, help in the identification of high‐risk situations, and the generation of problem‐solving strategies to deal with such situations. Counsellors may also have provided non‐specific support and encouragement. Some studies provided additional components such as written materials, video or audiotapes. The main components used in each study are shown in the Characteristics of included studies tables.
Intervention providers
The therapists who provided the counselling were generally described as smoking cessation counsellors. Their professional backgrounds included social work, psychology, psychiatry, health education and nursing. In one study, the therapist for some of the sessions was a nurse practitioner (Alterman 2001), and in two others the therapists were research doctors or nurses trained in counselling (Molyneux 2003; Hennrikus 2005). In Aveyard 2007 all the support was from primary care nurses who were not full‐time counsellors. We included this study because the nurses were trained to provide counselling support as part of the National Health Service Stop Smoking Services and were not offering it as part of usual care. In Tonnesen 2006 the counselling was provided by nurses employed in a lung clinic, and in Wiggers 2006 it was provided by nurse practitioners in a cardiology outpatient clinic.
Studies with minimal contact controls
In the 33 studies with a minimal contact control the treatments offered to the control comparison group ranged from usual care to up to 15 minutes of advice, with or without the provision of self‐help materials. To be classified as individual counselling the trials had to involve at least one session with face‐to‐face contact lasting more than 10 minutes, although the duration was typically much longer. The face‐to‐face counselling in Kim 2005 was the shortest, at only 11 minutes on average. Three tested a single face‐to‐face session without further support by telephone (Weissfeld 1991 (low‐intensity arm); Molyneux 2003; Marshall 2016). Nine others offered a single face‐to‐face session with further support by telephone (Windsor 1988; Weissfeld 1991 (high‐intensity arm); Stevens 1993; Rigotti 1997; Simon 1997; Dornelas 2000; Glasgow 2000; Hennrikus 2005; Kim 2005). All the other studies planned multiple sessions of face‐to‐face support, and sometimes also telephone contacts.
In the meta‐analysis we have not distinguished between brief advice, usual care or provision of self‐help materials as the control intervention with which counselling is compared. Provision of written materials was generally accompanied by brief advice; no trials directly addressed the effect of providing counselling as an addition to a structured self‐help programme. One trial offered 15 minutes of counselling on a healthy diet to controls (Chan 2012), and one offered autogenic training, a relaxation‐based programme not shown to aid cessation (Mueller 2012).
Within this group of studies, pharmacotherapy was systematically provided to participants in all trial arms in six trials. Nicotine patch was provided to all participants in Jorenby 1995; Simon 2003; Fiore 2004; Okuyemi 2013. Cropsey 2015 provided bupropion to all participants. Wiggers 2006 provided nicotine patches to participants ready to quit in either trial arm. Since the use of pharmacotherapy might change the relative effect of additional counselling, we include these studies in a subgroup analysis. In one trial (Simon 1997) smokers randomized to receive counselling were given a prescription for nicotine gum if there were no contraindications. Although 65% in the counselling condition used gum compared to 17% of the control group, its use was not significantly associated with quitting.
Studies of counselling intensity
Eleven studies compared intensive counselling to less intensive interventions that also met our definition of counselling by involving more than 10 minutes of face‐to‐face contact. We considered these studies separately from those using a minimal‐contact control. Eight of these studies provided pharmacotherapy to all participants and we included subgroups for studies with and without pharmacotherapy. Tonnesen 2006 contributed to both subgroups.
Weissfeld 1991 compared two intensities of counselling with a control; both intensities are combined versus control in the first analysis but compared in this analysis.
Lifrak 1997 compared two intensities of counselling as an adjunct to nicotine patch therapy. The lower‐intensity one was a four‐session advice and education intervention from a nurse practitioner who reviewed self‐help materials and monitored patch use. The higher‐intensity intervention added 16 weekly sessions of cognitive behavioural relapse prevention therapy.
Alterman 2001 used similar interventions to Lifrak 1997, but added a lower‐intensity control of a single 30‐minute session with a nurse practitioner.
Tonnesen 2006 compared seven visits and five phone calls with a contact time of 4½ hours to four visits and six calls taking 2½ hours. This trial had a factorial design, also comparing a nicotine sublingual tablet and placebo; we entered the arms with and without NRT in separate subgroups.
Aveyard 2007 compared seven weekly contacts with four contacts for people receiving cessation support with nicotine patches.
Killen 2008 provided six counselling session and combined NRT and bupropion, and compared different schedules of extended contact.
Nohlert 2009 compared eight 40‐minute sessions over four months with a single 30‐minute session introducing a self‐help programme.
Wu 2009 compared four 60‐minute culturally‐tailored counselling sessions to four 60‐minute health education sessions covering general health, nutrition, exercise and tobacco. All sessions were in Chinese, and all participants were offered nicotine patch.
Williams 2010 compared 24 weekly 45‐minute counselling sessions to nine 20‐minute sessions that focused on medication management. All participants were given nicotine patches.
Brunner 2012 provided a 30‐minute counselling session and offer of nicotine patch during a hospital stay and tested the effect of an additional five outpatient sessions including free samples of NRT; we included this in the non‐pharmacotherapy subgroup, as it was not provided as standard to all participants.
Kim 2015 compared eight weekly 40‐minute sessions of culturally‐tailored counselling to eight 10‐minute sessions focusing on medication management. All participants received nicotine patches.
Studies of counselling methods or timing
Five studies compared different counselling approaches that had similar contact times. We considered these separately from the groups above.
Schmitz 1999 involved six one‐hour sessions. One intervention used a coping skills relapse prevention model. It was compared with a health belief model that focused on smoking‐related health information, the relationship with coronary disease and the benefits of quitting.
Ahluwalia 2006 provided three face‐to‐face visits and three phone contacts extending over six weeks, and 2 mg nicotine gum for eight weeks. One intervention used motivational interviewing and the other a health education focus.
McCarthy 2008 provided eight 10‐minute counselling sessions during assessment visits in a trial that also compared bupropion to placebo. The counselling was consistent with US practice guidelines. The control focused on medication use and adherence, and general support and encouragement.
Garvey 2012 compared two different schedules of 14 counselling sessions, either front‐loaded with six sessions in the first two weeks after quit date, or just two in that period. All participants received nicotine patches.
Ramon 2013 directly compared delivery of counselling either entirely face‐to‐face or with a combination of face‐to‐face and telephone to a control group where all contact after the pre‐quit session was by telephone.
Excluded studies
We excluded one study that provided motivational interviewing as part of an intervention to reduce passive smoke exposure in households with young children (Emmons 2001). Cessation was a secondary outcome and there was no significant difference in quit rates, which were not reported separately by group. A sensitivity analysis including this study assuming equal quit rates did not alter the review results.
We list 48 other studies identified as potentially relevant but which did not meet the full inclusion criteria, with their reasons for exclusion in the table Characteristics of excluded studies. We note where studies were included in other Cochrane Reviews.
Risk of bias in included studies
We assessed the risks of selection bias, detection bias and attrition bias.
Twenty‐seven studies reported the method for generating the randomization sequence in sufficient detail to be classified as having a low risk of bias, but only 14 also described a method of allocation likely to ensure that the assignment was concealed until after allocation, and thus being at low risk of selection bias (Simon 1997; Weissfeld 1991; Windsor 1988; Kim 2005; Ahluwalia 2006; Wiggers 2006; Aveyard 2007; Killen 2008; McCarthy 2008; Williams 2010; Chan 2012; Ramon 2013; Marley 2014; Marshall 2016). In most other trials, neither the method of randomization nor the use of allocation concealment was described. We judged five trials to be at high risk of selection bias, due to the method of randomization or concealment, or both (Stevens 1993; Bobo 1998; Dornelas 2000; Hannover 2009; Brunner 2012).
We judged the risk of detection bias to be low if self‐reported abstinence was confirmed biochemically. Eight studies were at high risk of bias because no validation was attempted and trial arms had different amounts of contact with study staff, making differential misreporting of abstinence more likely (Bronson 1989; Stevens 1993; Aleixandre 1998; Pedersen 2005; Nohlert 2009; Thankappan 2013; Kim 2005; Ahluwalia 2006). We rated three studies as unclear; one study tested for cotinine but did not report validated rates (Bobo 1998), and in two others validation was incomplete and results were based on self‐report (Pederson 1991; Marshall 2016).
We judged the risk of attrition bias to be low if loss to follow‐up was reported by group, was no greater than 50% and not substantially different between groups. Most studies reported the number of participants who dropped out or were lost to follow‐up, and included these people as smokers in analysis denominators. We judged most studies to be at low risk of bias, because the percentage lost was small and similar across conditions. We classified two studies as being at high risk (Ramos 2010; Mueller 2012), and one as unclear (Burling 1991). One study (Fiore 2004) excluded randomized participants who failed to collect their free supply of nicotine patches, and as a consequence also did not receive any additional behavioural components to which they were allocated. The proportions excluded were similar in all the intervention groups, so we have used the denominators as given.
Overall we classified 11 of the 49 included studies (22%) as being at low risk of bias on all the domains we considered. A summary is displayed in Figure 1.
1.
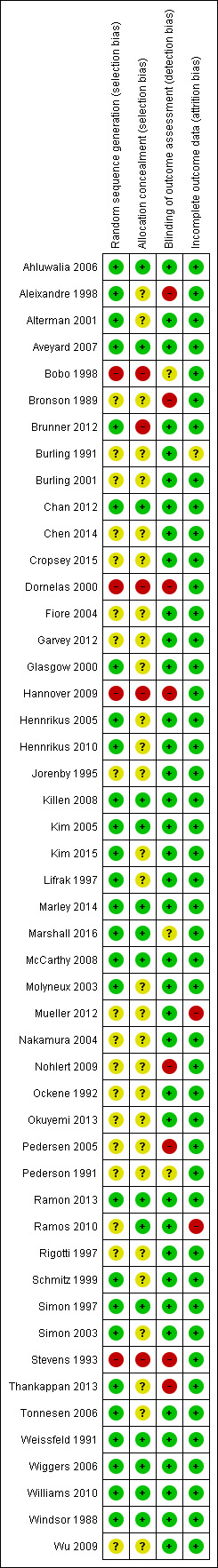
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
We did not formally assess the risk of performance bias. There was little information about blinding of participants or staff during treatment. Whilst the therapists delivering counselling could not have been blinded, in some cases other care providers were noted to be unaware of intervention status. It was unclear what information participants were given, but almost all trials included an active control group that received some information about stopping smoking. Because of this, we do not consider that the risk of bias from this aspect of design for this group of studies is high.
Effects of interventions
Summary of findings for the main comparison. Individual counselling compared to minimal contact control for smoking cessation.
| Patient or population: People who smoke Setting: Healthcare and community settings Intervention: Individual counselling from a smoking cessation counsellor including at least one face‐to‐face session lasting 10 minutes or more Comparison: Minimal‐contact control (usual care, brief advice or self‐help materials) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Numbers quit in control condition | Numbers quit after individual counselling | |||||
| Smoking cessation at longest follow‐up ‐ 6 months or more No systematic pharmacotherapy |
Study population | RR 1.57 (1.40 to 1.77) | 11,100 (27 RCTs) | ⊕⊕⊕⊕ HIGH | Limiting to studies at low risk of bias on all assessed domains marginally increases estimate of effect | |
| 7 per 100 | 11 per 100 (10 to 12) | |||||
| Smoking cessation at longest follow‐up ‐ 6 months or more Pharmacotherapy offered to all participants |
Study population | RR 1.24 (1.01 to 1.51) | 2662 (6 RCTs) | ⊕⊕⊕⊝ MODERATE 1 | Higher control group quit rate reflecting use of pharmacotherapy | |
| 11 per 100 | 13 per 100 (11 to 16) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1Downgraded due to wide confidence intervals.
Summary of findings 2. More intensive compared to less intensive counselling for smoking cessation.
| More intensive compared to less intensive counselling for smoking cessation | ||||||
| Patient or population: People who smoke Setting: Healthcare and community settings Intervention: More intensive individual counselling (± pharmacotherapy) Comparison: Individual counselling (± pharmacotherapy) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Numbers quit with less intensive counselling | Numbers quit with more intensive counselling | |||||
| Smoking cessation at longest follow‐up | Without pharmacotherapy | RR 1.29 (1.09 to 1.53) | 2920 (11 RCTs) | ⊕⊕⊕⊕ HIGH | Effect estimates for subgroups of studies with and without pharmacotherapy for all participants overlapped, so the overall pooled estimate is used with alternative control group estimates from subgroups | |
| 9 per 100 1 | 12 per 100 (10 to 14) | |||||
| With pharmacotherapy | ||||||
| 14 per 100 2 | 18 per 100 (15 to 21) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1Based on average in studies without pharmacotherapy.
2Based on average in studies with pharmacotherapy.
Counselling versus minimal contact control
We estimated a pooled effect size based on 33 studies of counselling, including one (Burling 1991) where there were no quitters and which therefore did not contribute to the meta‐analysis. The risk ratio (RR) was 1.48 (95% confidence interval (CI) 1.34 to 1.64, n = 13,762; Analysis 1.1), with some evidence of heterogeneity (I2 = 46%). Restricting the analysis to seven studies at low risk of bias on all domains (Windsor 1988; Weissfeld 1991; Simon 1997; Kim 2005; Wiggers 2006; Chan 2012; Marley 2014) did not alter the conclusions; the point estimate increased slightly (RR 1.65, 95% CI 1.32 to 2.06). The estimate was higher in the subgroup of 27 studies where pharmacotherapy was not provided (RR 1.57, 95% CI 1.40 to 1.77; n = 11,100; I2 = 50%) than in the six testing the additional effect of counselling when participants had access to pharmacotherapy (RR 1.24, 95% CI 1.01 to 1.51; n = 2662; I2 = 0%) and a test for subgroup difference detected a difference between subgroups with and without pharmacotherapy. We base the estimates of absolute effect in Table 1 on the subgroup estimates.
1.1. Analysis.
Comparison 1 Individual counselling compared to minimal contact control, Outcome 1 Smoking cessation at longest follow‐up.
2.
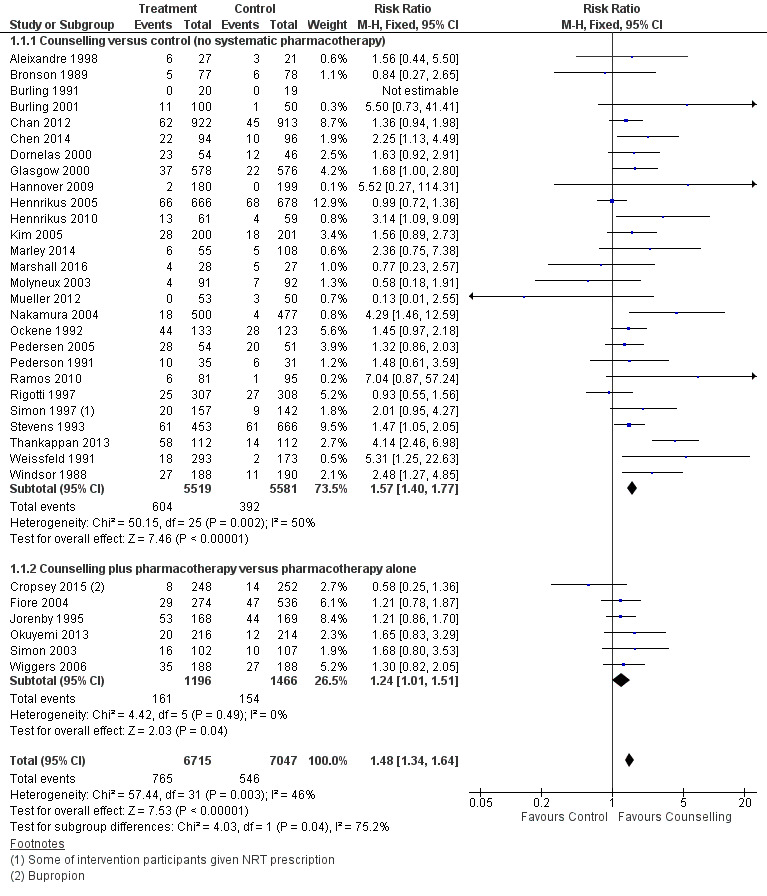
Forest plot of comparison: 1 Individual counselling compared to minimal contact control, outcome: 1.1 Smoking cessation at longest follow‐up.
More intensive versus less intensive counselling
Eight of the studies compared different levels of counselling as adjuncts to pharmacotherapy, and four did not offer medication (Tonnesen 2006 contributes different arms to each subgroup). The estimates in the two subgroups overlapped. Pooling all 11 studies, there was evidence of a small benefit from more intensive compared to brief counselling (RR 1.29, 95% CI 1.09 to 1.53; n = 2920; I2 = 48%; Analysis 2.1), a change from the previous version of the review in which pooling five studies did not detect evidence of benefit. The moderate heterogeneity was attributable to two new studies with large effects. The control groups in these were distinct, with Wu 2009 offering general health education and Kim 2015 focusing on medication management. A sensitivity analysis excluding these two studies no longer detected evidence of a dose response to counselling intensity. Limiting the analysis to four studies at low risk of bias also failed to suggest evidence of benefit.
2.1. Analysis.
Comparison 2 More intensive versus less intensive counselling, Outcome 1 Smoking cessation at longest follow‐up.
Comparisons between counselling approaches
We did not pool these clinically heterogeneous five studies. Only one of them detected a significant difference between different types of counselling, where number of contacts and general intensity were similar. Schmitz 1999, comparing a relapse prevention approach to a health belief model, showed no significant difference, but with wide confidence intervals (RR 0.94, 95% CI 0.45 to 1.98; n = 160; analysis 3.1.1). Ahluwalia 2006 compared a motivational interviewing to a health education approach and the point estimate favoured the latter (RR 0.51, 95% CI 0.34 to 0.76; n = 755; analysis 3.1.2). Participants were making quit attempts and using nicotine gum or placebo and therefore the motivational aspect may have been less relevant. McCarthy 2008 was also a pharmacotherapy trial with a factorial design and the specific behavioural components did not increase quitting over instructions about medication and general support (RR 0.93, 95% CI 0.62 to 1.39; n = 463; analysis 3.1.3). There was no evidence of an interaction between medication and counselling in either of the factorial trials. Garvey 2012 did not show that front‐loading the schedule of sessions was associated with greater quit success, but CIs did not exclude no effect (RR 1.81, 95% CI 0.79 to 4.15; n = 242; analysis 3.1.4). Ramon 2013 did not detect a difference between face‐to‐face and telephone counselling (RR 1.39, 95% CI 0.89 to 2.19; n = 301; analysis 3.1.5), or combined contact (face‐to‐face plus telephone) versus telephone only (RR 1.44, 95% CI 0.92 to 2.25; n = 299), but confidence intervals were again wide.
Discussion
There is consistent evidence that individual counselling increases the likelihood of cessation compared to less intensive support. Individual counselling, used independently of pharmacotherapy, was estimated to increase cessation by 40% to 80% after at least six months, based on pooling 27 trials with over 11,000 participants. Assuming a control group quit rate of 7% from a brief intervention, the provision of counselling would be expected to result in 10% to 12% quit, an absolute increase of 3% to 5%. We rated the quality of this evidence as high, using the GRADE approach (Table 1). This estimate was based on using counselling without any pharmacotherapy. The six trials that offered pharmacotherapy (typically nicotine replacement therapy) to all participants had a smaller and less certain effect. Assuming a control quit rate of 11% reflecting the benefit of medication, the addition of counselling could result in an absolute increase of 0% to 5%. We rated this as moderate quality using GRADE, because of the imprecision of the estimate. It is possible that the relative additional benefit is smaller when the quit rates in the control group are already increased by the use of an effective pharmacotherapy, but the absolute benefit of counselling could be similar, whether or not pharmacotherapy is used.
Almost half the trials recruited people in hospital settings, but there was no evidence of heterogeneity of results in different settings.
These results are consistent with the US Public Health Service practice guideline (Fiore 2008), which supports the use of intensive counselling. The guideline evidence in this area is based on meta‐analyses conducted for the previous update of the guideline (Fiore 2000), and includes indirect comparisons. These included an analysis of 58 trials where treatment conditions differed in format (self‐help, individual counselling with person‐to‐person contact, proactive telephone counselling or group counselling) and estimated an odds ratio (OR) for successful cessation with individual counselling compared to no intervention of 1.7 (95% confidence interval (CI) 1.4 to 2.0) (Fiore 2008 Table 6.13). Individual counselling in their categorization would have also included counselling from a physician. When they separately analysed the effect of different providers of care the estimates suggest that non‐physician clinicians (a category including psychologists, social workers and counsellors) are similarly effective compared to a no‐provider reference group (OR 1.7, 95% CI 1.3 to 2.1) as physicians (OR 2.2, 95% CI 1.5 to 3.2) (Fiore 2008 Table 6.11).
In our review there was no evidence of significant heterogeneity between relative quit rates in the different trials. Absolute quit rates varied across studies but this is likely to be related to the motivation of the smokers to attempt to quit and the way in which cessation was defined. Cessation rates were generally higher in trials where nicotine replacement therapy (NRT) was also used (Alterman 2001; Jorenby 1995; Lifrak 1997; Simon 2003), although there were exceptions (Ahluwalia 2006; Aveyard 2007). Rates were also higher amongst people with cardiovascular disease (Ockene 1992 ; Dornelas 2000; Pedersen 2005). Quit rates tended to be lower in studies recruiting hospitalized patients unselected for their readiness to quit (Stevens 1993; Rigotti 1997; Molyneux 2003). All these features of a trial are likely to affect absolute quit rates, confounding a possible effect of the exact content of the intervention.
Whilst we took account of the broad nature of the support offered to the control group when pooling studies, variation in the components used as part of, for example, a usual care control, may still give rise to heterogeneity. Treatment effects could be underestimated if those studies using effective interventions tended to provide relatively helpful usual care or brief advice. An ongoing systematic review is conducting a detailed analysis of behavioural intervention and control elements, and is expected to provide more evidence about this (de Bruin 2016).
The following description of the intervention used in the Coronary Artery Smoking Intervention Study (CASIS) (Ockene 1992) is broadly typical of the interventions used: "The telephone and individual counseling sessions were based on a behavioral multicomponent approach in which counselors used a series of open‐ended questions to assess motivation for cessation, areas of concern regarding smoking cessation, anticipated problems and possible solutions. Cognitive and behavioral self‐management strategies, presented in the self help materials, were discussed and reinforced". Although we cannot exclude the possibility that small differences in components, and in the therapists' training or skills, have an effect on the outcome, it is not possible to detect such differences in the meta‐analysis.
Most of the counselling interventions in this review included repeated contact, but differed according to whether face‐to‐face or telephone contact was used after an initial meeting. There are too few trials to draw conclusions from indirect comparisons about the relative efficacy of the various contact strategies. Again, the homogeneity of the results suggests that the way in which contact is maintained may not be important. A separate Cochrane Review of telephone counselling suggests that telephone support aids quitting (Stead 2013b).
The 11 trials that directly compared different intensities of individual support detected only weak evidence of a dose‐response effect which was sensitive to exclusion of outlying trials, and restriction to trials judged to be at low risk of bias. In some of the trials in this comparison the difference between the counselling protocols may be too small to affect long‐term quitting. The intended difference may also be eroded if the more intensive support cannot be consistently delivered. Eight of the trials provided pharmacotherapy to all participants, so were testing the additional benefit of more intensive individual counselling. As seen in the trials offering pharmacotherapy in the primary analysis, the relative effect of the additional support may be smaller in relation to the higher rates of cessation in the control arm receiving combined behavioural and pharmacological support. A separate Cochrane Review (Stead 2015) has assessed the effect of increasing the amount of any type of behavioural support when used alongside pharmacotherapy. It analysed 47 studies including relevant studies from this review, and concluded that "increasing the amount of behavioural support is likely to increase the chance of success by about 10% to 25%". The estimates in this review are consistent with that range.
Authors' conclusions
Implications for practice.
Counselling interventions given outside routine clinical care, by smoking cessation counsellors including health educators and psychologists, assist smokers to quit.
Implications for research.
Individual counselling is an established treatment for smoking cessation. Identifying the most effective and cost‐effective intensity and duration of treatment for different populations of smokers is still an area for research. However, differences in relative effect are likely to be small, especially when counselling is used alongside pharmacotherapy. Small trials are unlikely to provide clear evidence of long‐term efficacy.
What's new
| Date | Event | Description |
|---|---|---|
| 12 March 2018 | Amended | Correction to plain language summary to say that individual counselling could increase the chance of quitting by between 40% and 80%, so there is consistency with risk ratio (the bracket was previously, erroneously given as 40%‐60%). |
History
Protocol first published: Issue 4, 1998 Review first published: Issue 2, 1999
| Date | Event | Description |
|---|---|---|
| 23 November 2016 | New citation required but conclusions have not changed | No change to main conclusions. |
| 23 November 2016 | New search has been performed | Searches updated, 19 new studies included. 'Summary of findings' table added. |
| 16 July 2008 | New search has been performed | Updated for 2008 issue 4 with nine new studies. No changes to conclusions |
| 21 May 2008 | Amended | Converted to new review format. |
| 8 February 2005 | New citation required but conclusions have not changed | Updated for 2005 Issue 2 with three new studies. No changes to conclusions. |
| 7 April 2002 | New citation required but conclusions have not changed | Updated for 2002 Issue 3 with six new studies. No changes to conclusions. |
Acknowledgements
Our thanks to Peter Hajek and the late Roger Secker‐Walker for their helpful comments on the first version of this review. Nete Villebro translated a Danish paper
Data and analyses
Comparison 1. Individual counselling compared to minimal contact control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Smoking cessation at longest follow‐up | 33 | 13762 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.48 [1.34, 1.64] |
| 1.1 Counselling versus control (no systematic pharmacotherapy) | 27 | 11100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.57 [1.40, 1.77] |
| 1.2 Counselling plus pharmacotherapy versus pharmacotherapy alone | 6 | 2662 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [1.01, 1.51] |
Comparison 2. More intensive versus less intensive counselling.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Smoking cessation at longest follow‐up | 11 | 2920 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [1.09, 1.53] |
| 1.1 No pharmacotherapy | 4 | 872 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.42 [0.98, 2.06] |
| 1.2 Adjunct to pharmacotherapy | 8 | 2048 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [1.04, 1.52] |
Comparison 3. Comparisons between counselling approaches of similar intensity.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Smoking cessation at longest follow‐up | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Relapse prevention versus health belief model | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Motivational interviewing versus health education | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Counselling versus equal sessions of psychoeducation | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Front‐loaded versus weekly counselling | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 Face‐to‐face versus telephone counselling | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
3.1. Analysis.
Comparison 3 Comparisons between counselling approaches of similar intensity, Outcome 1 Smoking cessation at longest follow‐up.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ahluwalia 2006.
| Methods | Study design: Randomized controlled trial Setting: Community health centre, USA Recruitment: community volunteers interested in quitting |
|
| Participants | 755 African‐American light smokers (≤ 10 cpd) 67% women, av. age 45, av. cpd 8 | |
| Interventions | Therapists: trained counsellors Factorial trial, 2 mg nicotine gum/placebo arms collapsed for this review 1. Counselling using motivational interviewing (MI) approach. 3 in‐person visits at randomization, wk 1, wk 8, and phone contact at wk 3, wk 6, wk 16, S‐H materials 2. Counselling using health education (HE) approach. Same schedule and materials as 1 |
|
| Outcomes | PP abstinence at 6m (7‐day PP) Validation: cotinine ≤ 20 ng/ml | |
| Notes | Not in main analysis; compares 2 counselling styles. No significant effect of gum, no evidence of interaction. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centrally generated blocked scheme, block size 36 |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes opened sequentially |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation of abstinence planned, but low level of cotinine validation. All participants received same level of contact so risk of differential misreporting judged to be low |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 118 (15.6%) lost to follow‐up included in ITT analysis. HE participants less likely to be lost. Alternative assumptions about losses did not alter conclusions |
Aleixandre 1998.
| Methods | Study design: Randomized controlled trial Setting: Primary care clinic, Spain Recruitment: clinic and community volunteers |
|
| Participants | 48 smokers (excludes 6 dropouts) 65% women, av. age 36, av. cpd 24 ‐ 27 | |
| Interventions | Therapist: unclear, primary care clinic staff 1. 'Advanced', 4 x 30‐min over 4 wks, video, cognitive therapy, social influences, relapse prevention 2. 'Minimal' 3‐min advice immediately after randomization |
|
| Outcomes | Abstinence at 12 m Validation: no biochemical validation | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified on cigarette consumption and age, block size 4. |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No validation of abstinence and different levels of contact |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 6 post‐randomization dropouts excluded from ITT analyses. Their inclusion would marginally increase effect size |
Alterman 2001.
| Methods | Study design: Randomized controlled trial Setting: cessation clinic, USA Recruitment: community volunteers |
|
| Participants | 240 smokers of > 1 pack/day 45% ‐ 54% women, av. age 40, av. cpd 27 | |
| Interventions | Therapists: Nurse practitioners (NP) and trained counsellors All interventions included 8 wks nicotine patch (21 mg with weaning) 1. Low‐intensity. Single session with NP 2. Moderate intensity. as 1 plus additional 3 sessions at wks 3, 6, 9 with NP 3. High‐intensity. As 2. + 12 sessions cognitive behavioural therapy with trained therapist within 15 wks |
|
| Outcomes | Abstinence at 1 yr Validation: urine cotinine < 50 ng/ml, CO ≤ 9ppm | |
| Notes | Only contributes to intensive versus minimal intervention, using 3 vs 2+1. Quit rates significantly lower in 2 than 1 or 3. Using 3 vs 1; 26/80 vs 20/80; RR 1.30 [0.79, 2.13]. Using 3 vs 2; 26/80 vs 9/80; RR 2.89 [1.45, 5.77]. Overall estimate in 2016 no longer sensitive to choice of arms | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'Urn technique' |
| Allocation concealment (selection bias) | Unclear risk | No details given. Allocation took place after baseline session common to all conditions |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation of abstinence |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 30 (12.5%) lost to follow‐up included in ITT analysis |
Aveyard 2007.
| Methods | Study design: Randomized controlled trial Setting: 26 general practices (primary care clinics), UK Recruitment: 92% volunteers in response to mailings |
|
| Participants | 925 smokers 51% women, av. age 43, 50% smoked 11 ‐ 20 cpd | |
| Interventions | Therapists: Practice nurses trained to provide cessation support and manage NRT Both interventions included 8 wks of 16 mg nicotine patch 1. Basic support; 1 visit (20 ‐ 40 mins) before quit attempt, phone call on TQD, visits/phone calls at 7 ‐ 14 days and at 21 ‐ 28 days (10 ‐ 20 mins) 2. Weekly support; as 1. plus additional call at 10 days and visits at 14 and 21 days |
|
| Outcomes | Abstinence at 12 m (sustained at 1, 4, 12, 26 wks) Validation: CO < 10 ppm at treatment visits, saliva cotinine < 15 ng/ml at follow‐ups | |
| Notes | Not in main analysis; compares higher and lower intensity counselling. Therapists were not full‐time specialist counsellors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random‐number generator |
| Allocation concealment (selection bias) | Low risk | Numbered sealed envelopes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation of abstinence; staff making follow‐up calls were blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 288 (31%) lost to follow‐up, similar across groups, included in ITT analysis |
Bobo 1998.
| Methods | Study design: Cluster‐randomized controlled trial Setting: 12 residential centres for alcohol/drug treatment, USA Recruitment: inpatient volunteers |
|
| Participants | (50 participants in each of 12 sites) 67% men, av. age 33 50% smoked > 1 pack/day | |
| Interventions | Therapists: centre staff for 1st session, trained counsellors for telephone sessions 1. 4 x 10 ‐ 15 min sessions. 1st during inpatient stay. 3 by telephone, 8, 12, 16 wks post‐discharge 2. No intervention | |
| Outcomes | Abstinence at 12 m post‐discharge (7 day PP) Validation: saliva cotinine, but validated quit rates not reported (A primary outcome for the study was alcohol abstinence) | |
| Notes | Cluster‐randomized, so individual data not used in primary meta‐analysis. Adjusted OR 1.02 (CI 0.50 to 2.49). Inclusion would not materially change results of analysis 1.1. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Matched pairs of centres allocated by coin toss, 2 centres declined participation after allocation |
| Allocation concealment (selection bias) | High risk | Cluster‐randomized with participant recruitment (by research team) after centre allocation so potential for selection bias |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Biochemical validation of abstinence but validated results not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 22% lost to follow‐up. Including them as smokers made little difference to estimates |
Bronson 1989.
| Methods | Study design: Randomized controlled trial Setting: internal medicine practice, USA Recruitment: attenders for periodic health examinations |
|
| Participants | 155 smokers 38% men, av. age 42, av. cpd 25 | |
| Interventions | Therapist: smoking cessation counsellor 1. 2 x 20‐min counselling sessions during a periodic health examination (benefits of quitting, assessment of motivation, quit plan, high risk/problem solving) 2. Control: completed smoking behaviour questionnaire |
|
| Outcomes | Abstinence at 18 m (sustained from 6 ‐ 18 m) Validation: no biochemical validation at 18 m, limited sample for saliva cotinine at 6 m | |
| Notes | 18 m data reported in Secker‐Walker 1990 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized, method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Physicians carrying out health examinations were blind to group assignment and would have given similar advice to all participants Long‐term abstinence not validated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 20 (13%) not contacted at 6 m and 18 m, included in ITT analysis |
Brunner 2012.
| Methods | Study design: Randomized controlled trial Setting: Single hospital, Denmark Recruitment: Inpatients with acute ischaemic stroke or TIA invited to participate |
|
| Participants | 94 inpatients | |
| Interventions | Therapists: Single study nurse provided initial session for all participants, and 5 telephone and 1 outpatient session. Main counselling by"'authorized smoking cessation instructor" 1. Minimal intervention: 1 x 30‐min session, offer of nicotine patch during hospital stay 2. Intensive intervention; additional 5 outpatient sessions from counsellor, duration NS. Study nurse also offered 30‐min session at 6 wks and 5 telephone sessions at 2 days, 1 wk, 3 wks, 3 m, 4 m. Free samples of NRT |
|
| Outcomes | Abstinence 6m after discharge Validation: CO < 8 ppm |
|
| Notes | New for 2016 update Contributes to comparison of more versus less intensive Analysis 2.1 (no pharma subgroup) only. 8 minimal and 29 intensive intervention participants used NRT at some time |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomized using a computer‐generated list of odd and even numbers. These numbers, representing minimal and intensive smoking cessation intervention, respectively, were used to create consecutive numbered sealed envelopes." |
| Allocation concealment (selection bias) | High risk | "After having obtained informed consent, the study nurse opened the randomization envelope and the patients were informed to which intervention they had been assigned." No mention that envelopes were opaque. Intensive intervention participants more likely to be younger, male, heavier smokers, suggesting possibility of selection bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation of abstinence |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up small and similar between conditions |
Burling 1991.
| Methods | Study design: Randomized controlled trial Setting: Inpatient substance abuse treatment centre, USA Recruitment: inpatient volunteers |
|
| Participants | 39 male veteran inpatients | |
| Interventions | Therapist: paraprofessional counsellor (Social Work Master's candidate) 1. Smoking cessation programme; daily 15‐min counselling session and computer‐guided nicotine fading with contingency contract 2. Wait‐list control |
|
| Outcomes | Abstinence 6 m after discharge Validation ‐ none; no self‐reported quitters at 6 m | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized, method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No validation, but no self‐reported quitters |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Loss to follow‐up not reported |
Burling 2001.
| Methods | Study design: Randomized controlled trial Setting: Inpatient veterans rehabilitation centre, USA Recruitment: inpatient volunteers |
|
| Participants | 150 veteran drug‐ and alcohol‐dependent smokers 95% men, av. age 40, av. cpd 17 | |
| Interventions | Therapists: Masters/Doctoral level counsellors All participants were receiving standard substance abuse treatment, smoking banned in building. 1. Multicomponent. 9‐wk programme; 7 wks daily counselling, 2 wks bi‐weekly. TQD wk 5. Nicotine fading, contingency contracting, relapse prevention, coping skills practice. Nicotine patch (14 mg) 4 wks 2. As 1, but skills generalized to drug and alcohol relapse prevention 3. Usual care. Other programmes and NRT available |
|
| Outcomes | Abstinence at 12m (sustained at 1, 3, 6 m follow‐ups) Continuous abstinence rates taken from graph and abstract. PP rates also reported Validation: CO and cotinine | |
| Notes | 1+2 vs 3 Using PP rates would give lower estimate of treatment effect. No significant difference between 1 & 2, but favoured 1. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized, method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation of abstinence |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 12 (8%) lost to follow‐up included in ITT analysis |
Chan 2012.
| Methods | Study design: Randomized controlled trial Country: Hong Kong Recruitment: Cardiac outpatient clinics at 10 major hospitals |
|
| Participants | 1860 Chinese cardiac patients smoking ≥ 1 cig in past week. 91% men, av. age 58, av. cpd 12. Excluded from study if "too clinically ill." | |
| Interventions | Therapist: nurse counsellors 1. Intervention: At baseline, 30‐min individual face‐to‐face counselling matched to stage of readiness to quit. At 1 wk and 1 m: telephone calls from nurse counsellor, re‐assessment of stage and counselling to suit that stage, av. phone call length 15 mins 2. Control: 15‐min, individual face‐to‐face counselling on healthy diet from nurse counsellor at baseline Pharmacotherapy: No smoking cessation drugs provided, but stage‐matched medication counselling on NRT was discussed with intervention participants "if deemed appropriate". |
|
| Outcomes | 7‐day PP at 12 m (30‐day PP at 12 m and 3 m and 6 m outcomes also reported) Validation: CO ≤ 8 ppm, urinary cotinine < 100 ng/ml |
|
| Notes | New for 2016 update Validated rates used in MA; only about 25% of people self‐reporting abstinence were validated. Participants in intervention group had higher stage of readiness to quit smoking than in the control group. Adjusted OR provided in text (unadjusted OR 1.35, 95% CI 0.91 to 2.00; adjusted OR 1.26, 95% CI 0.85 to 1.87); numbers used in MA are unadjusted. 54% intervention received all counselling. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The allocation sequence was generated sequentially by the project co‐ordinator based on simple random sampling procedure using MS Excel." |
| Allocation concealment (selection bias) | Low risk | "serially numbered sealed and opaque envelope" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation of abstinence |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Similar rates of follow‐up in both groups at 12 m (85.5% intervention and 84.3% control) "No statistically significant difference was found between the two groups." ITT analysis conducted, 25 who died during study removed from denominators |
Chen 2014.
| Methods | Study design: Randomied controlled trial Setting: Hospital, China Recruitment: community volunteers and referrals from outpatient clinics |
|
| Participants | 190 smokers, > 1 cpd, 97% men, av.age 50, av. cpd 20. All had lung function tests; 85 had COPD and 105 were asymptomatic | |
| Interventions | Therapist: "Interventions provided by 2 doctors with experience of professional smoking cessation treatment." 1. Cognitive counselling, 20 mins at baseline and 9 calls > 10 mins at 1 ‐ 4 wks, 6 wks, 8 wks, 3 ‐ 5 m. S‐H materials 2. Brief advice |
|
| Outcomes | Abstinence at 6 m sustained from week 4 Validation: CO < 10 ppm |
|
| Notes | New for 2016 update | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "assigned to the intervention or control group according to the randomized digital table" stratified by motivation to quit |
| Allocation concealment (selection bias) | Unclear risk | No further details |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation of abstinence |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 12 withdrawals included as smokers |
Cropsey 2015.
| Methods | Study design: Randomized controlled trial Setting: Community corrections facility, USA Recruitment: smokers under community corrections supervision |
|
| Participants | 500 smokers; 33% women; av. age 37.4; av. cpd 17.9 | |
| Interventions | Therapist: Clincal psychologist 1. Control. Brief physician advice to set TQD 1 ‐ 2 wks after starting bupropion, stressed adherence 2. Intervention. As 1. plus 4 x 20 ‐ 30‐min counselling sessions; cognitive and behavioural strategies Pharmacotherapy: All participants received bupropion for 12 wks |
|
| Outcomes | Abstinence at 12 m (PP) Validation: CO ≤ 3 ppm at all visits |
|
| Notes | New for 2016 update Paper reports differential abstinence by race. Author confirmed quit rates in Fig 2, used to calculate numbers quit |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized, blocked on race, no further details |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation of abstinence |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 23% I, 26% C lost to follow‐up |
Dornelas 2000.
| Methods | Study design: Randomized controlled trial Setting: Hospital inpatients, USA Recruitment: Acute MI patients (not selected for motivation to quit) |
|
| Participants | 100 MI patients (98% smoked in previous wk) 23% women, aged 27 ‐ 83, av. cpd 29 | |
| Interventions | Therapist: Psychologist 1. 8 x 20‐min sessions, 1st during hospitalization, 7 by phone (< 1, 4, 8, 12, 20 and 26 wks post‐discharge). Stage‐of‐change model, motivational interviewing, relapse prevention 2. Minimal care. Recommended to watch online patient education video, referral to local resources |
|
| Outcomes | Sustained abstinence at 1 yr (no smoking since discharge) Validation: household member confirmation for 70%. 1 discrepancy found | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "drawing random numbers from an envelope" |
| Allocation concealment (selection bias) | High risk | as above |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No biochemical validation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 20 (20%) lost to follow‐up included in ITT analysis |
Fiore 2004.
| Methods | Study design: Randomized controlled trial Setting: Primary care patients, 16 clinics, USA Recruitment: Clinic attenders willing to accept treatment |
|
| Participants | 961 smokers of 10+ cpd. (A further 908 were allowed to select treatment. Demographic details based on 1869) 58% women, av. age 40, av. cpd 22 | |
| Interventions | Therapists: Trained cessation counsellors (Self‐selected group of factorial trial not included in meta‐analysis) 1. Nicotine patch, 22 mg, 8 wks incl tapering 2. As 1 plus Committed Quitters programme, single telephone session and tailored S‐H 3. As 2 plus individual counselling, 4 x 15 ‐ 25‐min sessions, pre‐quit, ˜TQD, next 2 wks |
|
| Outcomes | Continuous abstinence at 1 yr (no relapse lasting 7 days), also PP Validation: CO, cut‐off not specified. 2 discordant | |
| Notes | 3 versus 1 and 2 used in meta‐analysis. More conservative than 3 versus 2. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized, method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation of abstinence |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Denominators in meta‐analysis based on numbers who collected patches (85%, similar across arms) |
Garvey 2012.
| Methods | Study design: Randomized controlled trial Setting: Smoking cessation research clinic, Boston USA Recruitment: Community volunteers, motivated to quit |
|
| Participants | 278 smokers of ≥ 5 cpd. 53% women, av. age 47, av. cpd 18 | |
| Interventions | Therapist: MA or BA in psychology, 3 full days training Both group received nicotine patches for 12 weeks, dose tailored to baseline smoking 1. Front‐loaded CBT‐based counselling; 2 pre‐quit and 12 post‐quit, 6 post‐quit sessions received in first 2 weeks. Pre‐quit sessions approx. 45 mins each, post‐quit 20 ‐ 30 mins. Last 3 sessions at 6 m, 9 m, 12 m 2. Weekly counselling. Same number and duration of sessions, but weekly to 12 wks |
|
| Outcomes | Continuous abstinence from quit date at 12 m, (never smoking for 7+ consecutive days nor for 7+ consecutive episodes and PP also reported) Validation: CO < 8 ppm |
|
| Notes | New for 2016 update Analysis 3, not pooled with other studies. Authors report significantly lower likelihood of relapse, using hazard ratio and continuous abstinence to define relapse. Risk ratio based on 11.7% versus 6.3% abstinent at 12 m is not significant |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Block randomization, method of sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | "randomization occurred at the end of the baseline visit following the consenting process and administration of baseline measures" but no additional information |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation of abstinence |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 20 (14%) front‐loaded and 16 (11.5%) weekly did not start or dropped out before quit date. Not included in denominators for MA. Later losses treated as smokers |
Glasgow 2000.
| Methods | Study design: Randomized controlled trial Setting: 4 Planned Parenthood clinics, USA Recruitment: Clinic attenders, unselected for motivation |
|
| Participants | 1154 female smokers Av. age 24, av. cpd 12 | |
| Interventions | Therapists: 4 hours training Both groups received 20‐sec provider advice. 1. Video (9 mins) targeted at young women. 12 ‐ 15 min counselling session, personalized strategies, stage‐targeted S‐H materials. Offered telephone support call 2. Generic S‐H materials |
|
| Outcomes | Abstinence at 6 m (for 30 days) Validation: saliva cotinine ≤ 10 ng/ml | |
| Notes | 26% did not want telephone component, 31% of remainder not reached | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomized, block size 4, fixed schedule |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation of abstinence |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 10% loss to follow‐up included in ITT analysis |
Hannover 2009.
| Methods | Study design: Randomized controlled trial Setting: Maternity wards in 6 hospitals, Germany Recruitment: Women in hospital post partum |
|
| Participants | 379 women who were smoking postpartum (subgroup of trial participants). av. age for all participants 26, av. cpd 14 | |
| Interventions | Therapist: 4 counsellors trained in motivational interviewing 1. Counselling; face‐to‐face session in mothers' homes, duration NS, 2 phone boosters at 4 and 12 wks 2. Usual care and S‐H materials at screening |
|
| Outcomes | Sustained abstinence at 24 m (PP also reported, followed up at 6, 12, 18 m) Validation: none |
|
| Notes | New for 2016 update Using earlier or PP outcome would not affect meta‐analysis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "simple randomization .... allocating women to either intervention or control group alternating in the order on the screening forms". Whether the allocation sequence would begin with treatment or control condition was decided ad hoc. |
| Allocation concealment (selection bias) | High risk | No possibility of concealment |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No biochemical validation of abstinence |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 16% intervention and 6% control lost or withdrew |
Hennrikus 2005.
| Methods | Study design: Randomized controlled trial Setting: 4 hospitals, USA Recruitment: Newly‐admitted inpatients invited to participate, not selected by motivation |
|
| Participants | 2095 current smokers 53% women, av. age 47, cpd NS, 15 ‐ 20% precontemplators | |
| Interventions | Therapists: research nurses with 12 hours training 1. Control: modified usual care: smoking cessation booklet in hospital (not used in meta‐analysis) 2. Brief advice (A): as control, plus labels in records to prompt advice from nurses and physicians 3. Brief advice and counselling (A+C): As 2, plus 1 bedside (or phone) session using motivational interviewing and relapse prevention approaches and 3 to 6 calls (2 ‐ 3 days, 1 wk, 2 ‐ 3 wks, 1 m, 6 m) |
|
| Outcomes | Abstinence at 12 m (7‐day PP) Validation: saliva cotinine < 15 ng/ml | |
| Notes | Brief advice + counselling compared to brief advice. Including Usual Care in control as well would marginally increase relative effect but not change conclusion of no effect. Authors reported relatively high and differential levels of refusal to provide samples, and samples that failed to confirm abstinence | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomly ordered within blocks of 30 assignments" |
| Allocation concealment (selection bias) | Unclear risk | Allocation by research assistant, concealment not described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation of abstinence |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 78 (3.7%) excluded from ITT analysis due to death or too ill for follow‐up. 426 (20%) lost to follow‐up included in ITT analysis; higher loss from treatment than control |
Hennrikus 2010.
| Methods | Study design: Randomized controlled trial Setting: 2 medical centres; USA Recruitment: probable smokers with lower extremity PAD |
|
| Participants | 687 current smokers with PAD; 15% women, av. age 60, av. cpd 18 | |
| Interventions | Therapists: smoking cessation counsellor 1. Verbal advice to quit from vascular provider 2. Letter from vascular provider + intensive counselling, at least 6 sessions over 5 m, first in person then phone. Information about pharmacotherapies but not provided |
|
| Outcomes | Abstinence at 6 m (PP) Validation: saliva cotinine < 10 ng/ml, or CO < 8 ppm for people using NRT |
|
| Notes | New for 2016 update High use of pharmacotherapies in both groups; 87% in I, 67% in C |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "predetermined block randomization schedule stratified by medical center" |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation of abstinence |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 25% I, 17% C lost at 6 m. 4 deaths (3I, 1C) excluded from MA denominators |
Jorenby 1995.
| Methods | Study design: Randomized controlled trial Setting: Clinical research centres, USA (2 sites) Recruitment: community volunteers |
|
| Participants | 504 smokers 15+ cpd av. age 44, av. cpd 26 ‐ 29 | |
| Interventions | Therapists: Trained smoking cessation counsellors Factorial trial; compared 22 mg/day vs 44 mg/day nicotine patch and 3 types of adjuvant treatment. All participants had 8 weekly assessments by research staff 1. Minimal ‐ S‐H materials from physician at screening visit for trial entry, instructed not to smoke whilst wearing patch. No further contact with counsellors 2. Individual ‐ S‐H at screening visit + motivational message. Met nurse counsellor x 3 after TQD Counsellor helped generate problem‐solving strategies and provided praise and encouragement 3. Group ‐ S‐H + motivational message. 8 x 1‐hr weekly group sessions. Skills training, problem‐solving skills |
|
| Outcomes | 7‐day PP abstinence at 26 wks Validation; CO < 10 ppm | |
| Notes | No significant difference in dose‐related outcome and no dose‐counselling interaction at 26 wks reported, so patch arm collapsed in analysis. 2 vs 1, counselling vs NRT alone, comparison with group counselling covered in Cochrane group therapy review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized, method not stated |
| Allocation concealment (selection bias) | Unclear risk | "In a double blind manner" for NRT, but not specified for counselling |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation of abstinence |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 16.3% lost to follow‐up included in ITT analysis, no difference across conditions |
Killen 2008.
| Methods | Study design: Randomized controlled trial Setting: Community cessation clinic, USA Recruitment: Community volunteers |
|
| Participants | 301 smokers (≥ 10 cpd or 3.5 packs/wk) (excludes 3 participants who received wrong treatment); 40% women, av. age ˜46, av. cpd ˜20 | |
| Interventions | Therapists: 'one of three staff interventionists trained and supervised by the study psychologist and psychiatrist who had previous training in behavioral therapy' All participants received 6 x 30‐min individual CBT sessions at baseline, TQD, 1, 2, 4, 6 wks, and combination pharmacotherapy (Bupropion (300 mg, 9 wks) and NRT (21 mg patch, 8 wks incl tapering)) 1. Extended therapy: 4 x 30‐min sessions at 8, 12, 16, 20 wks, and weekly check in calls to automated system; report of relapse or craving prompted proactive calls 2. Standard therapy: 5‐min general support calls at 8, 12, 16, 20 wks |
|
| Outcomes | Abstinence at 52 wks (7‐day abstinence at both 20 and 52 wks) (Continuous abstinence also reported but not used in MA as could underestimate any effect on recycling) Validation: CO < 10 ppm (11 self‐reported quitters no longer living in study area accepted as quitters without validation) |
|
| Notes | New for 2016 update. Tested extended duration therapy, contributes only to comparison of counselling intensity (Analysis 2.1) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomized using a permuted block method (block size = 4), stratified on gender |
| Allocation concealment (selection bias) | Low risk | Participants assigned to next available ID number in corresponding gender. Researchers and participants were blinded to extended treatment assignment to the end of the open‐label phase |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation of abstinence |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 89% followed up in standard‐care group, 90% followed up intervention group |
Kim 2005.
| Methods | Study design: Randomized controlled trial Setting: Outpatient clinic, South Korea Recruitment: outpatients, not selected on motivation |
|
| Participants | 401 daily smokers, 65% willing to quit within 1 m 92% men, av. age 52 | |
| Interventions | Therapists: Retired nurses trained in cessation Test of 5As approach. All participants had first been Asked about smoking status and Advised to quit by physicians and told to go to onsite counsellors, who Assessed willingness to quit, and enrolled and randomized them 1. Intervention: Counsellors provided Assist and Arrange components to participants willing to quit within 1 m; set quit date, provided S‐H materials, supplied cigarette substitute (˜11 min average). Culturally specific for Koreans. Other participants given 4Rs. Follow‐up calls at 1 wk and 1 m (˜7 mins) 2. Control: Counsellors told participants to quit without further assistance |
|
| Outcomes | Abstinence at 5 m Validation: CO ≤ 7 ppm | |
| Notes | Marginal to include because 5 m follow‐up and counselling was very brief | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "random list determined by fixed randomization with an allocation ratio of 1:1, a block size of 6 and 12 allocation strata" |
| Allocation concealment (selection bias) | Low risk | "sealed opaque envelopes which the counselors opened at the formal enrollment of the study participants" (judged low based on level of detail provided) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation of abstinence |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 7 lost to follow‐up included in ITT analysis |
Kim 2015.
| Methods | Study design: Randomized controlled trial Setting: Korean community, USA Recruitment: Korean smokers wanting to quit |
|
| Participants | 109 Korean smokers; 16% women, av. age 50, av. cpd 17 | |
| Interventions | Therapist: 1 of 2 Korean bilingual clinicians 1. Culturally‐tailored counselling; 8 x 40‐min weekly sessions, TQD between 2nd and 4th 2. Minimal counselling; 8 x 10‐min weekly sessions focusing on medication management Pharmacotherapy: all participants received 8‐week supply of nicotine patch |
|
| Outcomes | Abstinence at 6 m Validation: cotinine (Nicalert 1 < 10 ‐ 30 ng/ml), CO < 6 ppm |
|
| Notes | New for 2016 update Contributes to comparison 2.1.2 more intensive vs less intensive counselling with pharmacotherapy Kim 2012 assumed to report a subset of these participants but unable to confirm with author |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated, stratified by gender |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation of abstinence |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 24% (13/55) I, 31% (17/54) C lost, included as smokers in analyses |
Lifrak 1997.
| Methods | Study design: Randomized controlled trial Setting: substance abuse outpatient facility, USA Recruitment: community volunteers |
|
| Participants | 69 smokers av. age 39, av. cpd 25 | |
| Interventions | Therapists: nurse practitioner for 1 and 2, clinical social worker or psychiatrist experienced in addiction treatment for 2. Both interventions included use of nicotine patch (24‐hr, 10 wks tapered dose) 1. Moderate intensity ‐ 4 meetings with nurse who reviewed S‐H materials and instructed in patch use 2. High intensity. As 1 plus 16 weekly 45‐min cognitive behavioural relapse‐prevention therapy |
|
| Outcomes | Abstinence at 12 m, 1 wk PP Validation: urine cotinine for some participants, but no corrections made for misreporting | |
| Notes | Both interventions regarded as counselling, used in comparison of intensity. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomization (block size 10) |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Some biochemical validation of abstinence, all participants had active therapy |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 12 administrative dropouts/exclusions not included, treatment group not specified. All others included |
Marley 2014.
| Methods | Study design: Randomized controlled trial Setting: 2 Aboriginal Community Controlled Health Services (ACCHS) centres; Australia Recruitment: Active and passive ‐ Aboriginal and Torres Strait Islander smokers (current or who had quit within 2 weeks of enrolling) wishing to quit smoking or cut down on the amount of cigarettes they smoked |
|
| Participants | 163 smokers; 54% women; av. cpd 15 | |
| Interventions | Therapists: smoking cessation counsellors 1. Usual care: routine care relating to smoking cessation at local primary healthcare service, including advice on quitting, pharmacotherapy, and self‐initiated follow‐up 2. Usual care plus smoking cessation counselling at face‐to‐face visits, weekly for the first 4 wks, monthly to 6 m and 2‐monthly to 12 m (12 sessions) |
|
| Outcomes | Abstinence at 12 m (7‐day PP) Validation: urine cotinine < 50 ng/mL |
|
| Notes | New for 2016 update | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated, held centrally |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes held centrally. Allocation via telephone after enrolment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation of abstinence (and staff doing assay blinded) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 12% lost overall, 4 deaths (3I , 1C) and 1C withdrew consent not included in MA denominators |
Marshall 2016.
| Methods | Study design: Randomized controlled trial Setting: Lung cancer screening study, Australia Recruitment: Smokers participating in a lung cancer screening study, volunteering for substudy |
|
| Participants | 55 smokers, 36% women, av. age 63, av. cpd 25 | |
| Interventions | Therapist: single thoracic physician 1. Single counselling session; tailored motivational approach inducing discussion of lung function results and lung cancer risk (but not scan results). Planned duration not reported; mean duration 26.5 mins. Same materials and referral as control 2. Standard S‐H materials and Quitline referral |
|
| Outcomes | Abstinence at 12 m (PP) Validation: CO < 10 ppm but only 4 tested |
|
| Notes | New for 2016 update Pilot study. Treated as counselling because physician not providing intervention as part of usual care |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "random number generator" |
| Allocation concealment (selection bias) | Low risk | "concealed randomization" ‐ judged low risk |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Incomplete validation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5 losses coded as smokers |
McCarthy 2008.
| Methods | Study design: Randomized controlled trial Setting: Clinic, USA Recruitment: community volunteers |
|
| Participants | 463 smokers 50% women, av. age 36 ‐ 41 across arms, av. cpd 22 | |
| Interventions | Therapists: trained college‐aged or bachelor's level staff, supervised by experienced counsellor Factorial trial. Bupropion/placebo pharmacotherapy arms collapsed 1. Counselling; 8 x 10‐min sessions, 2 prequit, TQD, 5 over 4 wks 2. Psychoeducation about medication, support and encouragement. Same no. of sessions, 80 mins less contact time |
|
| Outcomes | 7‐day PP abstinence at 12 m Validation: CO ≤ 10 ppm | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Low risk | Staff who screened and enrolled participants were unaware of the experimental condition to be assigned |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation of abstinence |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 171 (37%) failed to attend quit date visit or lost to follow‐up, included in ITT analysis |
Molyneux 2003.
| Methods | Study design: Randomized controlled trial Setting: Hospital, UK Recruitment: Hospital inpatients |
|
| Participants | 274 smokers (183 in relevant arms) admitted to medical and surgical wards, smoked in last 28 days 60% men, av. age 60, median cpd 17, 81% had previous quit attempt | |
| Interventions | Therapists: research doctor or nurse trained in cessation counselling 1. Usual Care, no smoking advice 2. Brief (20‐min) bedside counselling + advice leaflet + advice on NRT 3. As 2, plus choice of NRT product (not relevant to this review) |
|
| Outcomes | Continuous abstinence at 12 m Validation: CO < 10 ppm | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "List generated for each centre allocating equally in random permuted blocks of nine." |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation of abstinence |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 72 (39%) lost to follow‐up included in ITT analysis |
Mueller 2012.
| Methods | Study design: Randomized controlled trial Country: Switzerland Recruitment: Individuals enrolled in a 21‐day inpatient alcohol detoxification treatment programme |
|
| Participants | 103 alcohol‐dependent smokers with stay long enough to complete 10‐day treatment programme; 29% women, av. age 44; av. cpd 25.5 I/30.5 C | |
| Interventions | Therapists: 2 psychologists 1 Intervention: 5 x 30‐min cognitive behavioral therapy sessions focused on smoking cessation 2 Control: Autogenic training (relaxation) Participants intending to quit offered nicotine patch during inpatient phase |
|
| Outcomes | Abstinence at 6 m (PP) Verification: CO < 10 ppm, cotinine |
|
| Notes | New for 2016 update. Some participants were treated individually and some in small groups | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized, method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation of abstinence |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High and differential loss to follow‐up; 53% I, 34% C. (All participants lost to follow‐up classified as non‐abstinent in analyses) |
Nakamura 2004.
| Methods | Study design: Randomized controlled trial Setting: communities and worksites, Japan Recruitment: Smokers with hypertension and/or hypercholesterolaemia having health check‐ups |
|
| Participants | 977 smokers 98% men, av. age 45, av. cpd 25, ˜20% in preparation/ contemplation | |
| Interventions | Therapists: mostly public health nurses 1. Intervention: Stage‐base counselling, 1 x 40‐min, 4 x 20 ‐ 30‐min at 1, 2, 4, 6 m. + phone call if TQD set 2. Control: Matched contact intervention for hypertension (161) or hypercholesterolaemia (318) |
|
| Outcomes | Abstinence at 6 m, sustained 4 PP at 1, 2, 4, 6 m Validation: CO ≤ 8 ppm | |
| Notes | Recruited a largely unmotivated population | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not stated |
| Allocation concealment (selection bias) | Unclear risk | No information given |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation of abstinence |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 54 (5.5%) lost to follow‐up included in ITT analysis |
Nohlert 2009.
| Methods | Study design: Randomized controlled trial Setting: Dental clinics, Sweden Recruitment: smokers identified by dental and healthcare personnel screening, accepting support |
|
| Participants | 300 smokers, 80% women, av. cpd 15 | |
| Interventions | Therapists: 3 trained dental hygienists 1. High‐intensity counselling; 8 x 40‐mins over 4 m 2. Low‐intensity counselling; 1 x 30‐min session explaining an 8 wk S‐H programme Both conditions given information on NRT but no recommendation on whether to use |
|
| Outcomes | Continuous abstinence at 1 yr, PP also reported (Nohlert 2009). (6‐yr follow‐up in Nohlert 2013) Validation: none |
|
| Notes | New for 2016 update Half the participants had used NRT, no difference in use between conditions |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | randomized "... independent person using an envelope technique in blocks of four" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No biochemical validation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | < 10% lost in each arm at 1 yr. 6 baseline dropouts not included in MA denominator |
Ockene 1992.
| Methods | Study design: Randomized controlled trial Setting: Cardiac catheterization labs at 3 hospitals, USA Recruitment: inpatient smokers or recent quitters with coronary artery stenosis, following arteriography |
|
| Participants | 267 smokers (256 surviving at 12 m follow‐up) av. age 53, av. cpd 25 | |
| Interventions | Therapists: Masters‐level health educators 1. Minimal intervention ‐ 10‐min advice and review of an information sheet 2. Inpatient counselling session, 30 mins, outpatient visits and telephone calls. Opportunity to attend group programme |
|
| Outcomes | Abstinence at 12 m (sustained for 6 m) Validation: saliva cotinine < 20 ng/ml | |
| Notes | Average length of contact for intervention was 1.22 hrs (20 mins to > 5 hrs) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not stated |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation of abstinence |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No mention of losses to follow‐up and all survivors included in denominators. |
Okuyemi 2013.
| Methods | Study design: Randomized controlled trial Setting: 8 emergency homeless shelters and transitional housing units in Minneapolis/St Paul, Minnesota, USA Recruitment: through health fairs, staff informational sessions, fliers at homeless shelters and word of mouth |
|
| Participants | 430 homeless adult smokers; 75% men; av. age 44; cpd 19; motivated to quit | |
| Interventions | Therapists: Counsellors 1. Control: Brief advice 10 ‐ 15 mins 2. Intervention: 6 x 15 ‐ 20‐min MI counselling sessions, baseline and wks 1, 2, 4, 6 and 8 Pharmacotherapy: All participants in both groups received a 2‐wk supply of 21 mg nicotine patches, every 2 wks over the 8‐wk treatment period All participants received a health educational resource called The Power to Quit: A Quit Smoking Guide, developed by the project investigators |
|
| Outcomes | Abstinence at 6 m (7‐day PP) Validation: CO ≤ 10 ppm. Salivary cotinine ≤ 20 ng/ml if CO > 10 ppm for those who self‐reported abstinence |
|
| Notes | New for 2016 update | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization schedule prepared by study statistician, but no detail given on how |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation of abstinence |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up, 22% (48/216) I 29% (63/214) C, all treated as smokers in MA |
Pedersen 2005.
| Methods | Study design: Randomized controlled trial Setting: hospital, Denmark Recruitment: Inpatients with cardiac disease |
|
| Participants | 105 smokers 36% women, ˜70% aged > 50 | |
| Interventions | Therapists: counsellors 1. Usual‐care control: in‐hospital advice to quit + information about NRT + NRT available 2. Intervention: As 1, plus 5 x 30‐min post‐discharge contacts |
|
| Outcomes | Abstinence at 12 m (PP) Validation: none | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized, method not described |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes, but not stated to be numbered |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No validation of abstinence |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 10 (9.5%) lost to follow‐up, included in ITT analysis |
Pederson 1991.
| Methods | Study design: Randomized controlled trial Setting: Chest unit, USA Recruitment: Inpatients with COPD |
|
| Participants | 74 cigarette smokers av. age 53, 75% smoked 20+ cpd | |
| Interventions | Therapist: Non‐specialist trained in counselling 1. Advice to quit 2. Individual counselling; between 3 and 8 15 ‐ 20‐min sessions on alternate days during hospitalizations S‐H manual, support and encouragement |
|
| Outcomes | Abstinence at 6 m Sample validated by COHb | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Only sample validated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 8 lost to follow‐up were re‐included in ITT analysis by review authors. 8 deaths excluded |
Ramon 2013.
| Methods | Study design: Randomized controlled trial Setting: 6 smoking cessation clinics, Spain Recruitment: smokers of ≥ 10 cpd, motivated to quit |
|
| Participants | 600 smokers (400 in relevant arms), 49% women, av. age 47, av. cpd ˜25 | |
| Interventions | Therapists: 'physician or psychologist specialized in smoking cessation' 1. Individual counselling; 8 x 15 ‐ 20 mins, pre‐quit then 3, 5, 7, 10, 12 , 24 and 52 wks 2. Telephone counselling; 3, 5, 7, 10, 12, 24 wks , and at the clinic at wk 52 3. Combination; 4 face‐to‐face and 3 telephone sessions All participants offered pharmacotherapy; 6% refused, of remainder 47% varenicline, 33% nicotine patches, 14% combination patches and gum/lozenges, 6% bupropion |
|
| Outcomes | Abstinence at 52 wks, sustained from wk 2 (PP also reported) Validation: CO < 10 ppm at wk 52 (8 misreports, evenly distributed) |
|
| Notes | New for 2016 update Comparison 3.1.5 1 and 3 vs 2, face‐to‐face or face‐to‐face and telephone‐to‐telephone only. Telephone condition split to avoid double counting |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generated randomization system based on a permuted block randomization list where each block was used by one centre." |
| Allocation concealment (selection bias) | Low risk | "An independent researcher in the coordination centre generated a random sequence, and centres were informed about smoker allocation after consent to participation" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation of abstinence |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up 12% Individual, 18% telephone |
Ramos 2010.
| Methods | Study design: Randomized controlled trial Setting: Primary care clinics, Mallorca, Spain Recruitment; smokers "prepared and able to fix a date to quit smoking" |
|
| Participants | 287 smokers, 54% women, av. age ˜44, av. cpd 20 | |
| Interventions | Therapists: “microteam,” composed of 1 physician and 1 nurse. They distributed the visits among themselves as they saw fit; all they were instructed to do was to conduct some of the visits together. 1. Intensive individual intervention, 6 sessions (duration and timing not described) 2. Intensive group‐based intervention (duration and timing not described but stated to be longer than individual option) (not used in this review) 3. Minimal intervention |
|
| Outcomes | Abstinence at 12 m, continuous (PP also reported) Validation: CO, cut‐off not described |
|
| Notes | New for 2016 update 1 vs 3 in comparison 1.1; comparison with group therapy covered in Cochrane review of group therapy |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized, method not described |
| Allocation concealment (selection bias) | Low risk | "An allocation concealment method based on the use of sequentially‐numbered, opaque, sealed envelopes was used ... A block of 60 envelopes (20 for III, 20 for IGI and 20 for MI) was prepared in the central research unit for each participating health centre and subsequently sent out." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation of abstinence |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High loss to follow‐up in all conditions; 69% Individual, 76% Minimal |
Rigotti 1997.
| Methods | Study design: Randomized controlled trial Setting: Hospital, USA Recruitment: Inpatients in medical or surgical services, smoking > 1 cig in month before admission |
|
| Participants | 615 smokers or recent quitters (excluding 35 deaths). 37% of intervention and 32% of controls had a current smoking‐related health problem | |
| Interventions | Therapist: research assistant supervised by a nurse 1. Usual care 2. Single bedside counselling session (motivational interviewing, cognitive behavioural and relapse prevention techniques), av. 15 mins, S‐H materials, chart prompts, 1 ‐ 3 telephone calls post‐discharge |
|
| Outcomes | Abstinence at 6 m (PP, sustained abstinence reported based on self‐report) Validation: saliva cotinine for people living in Mass (85% of quitters) | |
| Notes | Use of validated PP rather than sustained abstinence gives more conservative treatment effect | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Each day's list of eligible smokers put in random order and participants recruited consecutively in this order. Randomized by research assistant |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation of abstinence for majority |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 73 (22.4%) lost to follow‐up included in ITT analysis, no evidence of differential loss. 35 (5.4%) deaths excluded |
Schmitz 1999.
| Methods | Study design: Randomized controlled trial Setting: Hospital, USA Recruitment: women with or at risk of CAD |
|
| Participants | 2 separate samples recruited: 53 inpatients with CAD who stopped smoking during hospitalization and wanted to stay quit 107 women volunteering for cessation treatment who had > 1 CAD risk factor | |
| Interventions | Therapists: 2 smoking counsellors + 2 clinical psychology interns 1. Coping skills, relapse prevention, 6 x 1‐hr including stress management, homework 2. Health Belief model, 6 x 1‐hr, smoking‐related health information about disease state or CAD profile Focus on benefits of stopping |
|
| Outcomes | Abstinence at 6 m (PP) Validation: CO < 9 ppm, urine cotinine < 10 ng/ml Not all quitters tested, confirmation rates not reported | |
| Notes | Post‐randomization dropouts who did not complete baseline and begin treatment were not included in any data Quit rates were lower in the CAD sample than in the at‐risk group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomly assigned", stratified on smoking rate and myocardial infarction status |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Some validation of abstinence, arms had similar intensities of treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Pretreatment dropouts were excluded, all others included in ITT analysis |
Simon 1997.
| Methods | Study design: Randomized controlled trial Setting: Veterans Administration hospital, USA Recruitment: smokers undergoing non‐cardiac surgery |
|
| Participants | 299 smokers (smoked within 2 wks of admission) (excl 25 deaths) 98% men, av. age 54, av. cpd 20 | |
| Interventions | Therapist: public health educator 1. Multicomponent: single counselling session (30 ‐ 60 mins) prior to discharge (based on social learning theory and stages of change). Video, prescription for nicotine gum if no contraindications. 5 follow‐up counselling calls over 3 m 2. Brief counselling (10 mins) and S‐H materials |
|
| Outcomes | Abstinence at 12 m Validation: serum or saliva cotinine < 15 ng/ml. 6 self‐reports confirmed only by "significant other". | |
| Notes | 65% of Group 1 and 17% of Group 2 reported using NRT, but use of NRT was not significantly associated with quitting in either group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Random list of assignments" |
| Allocation concealment (selection bias) | Low risk | "Sealed opaque envelopes opened on formal enrollment" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation of abstinence |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 25 (8%) lost to follow‐up included in ITT analysis, 25 (8%) died, excluded from denominator |
Simon 2003.
| Methods | Study design: Randomized controlled trial Setting: Veterans Affairs hospital, USA Recruitment: Hospitalized smokers in contemplation or preparation stage of change |
|
| Participants | 209 smokers, 20+ cigs in total in wk before hospitalization, excludes 14 deaths during follow‐up 97% men, av. age 55, av. cpd 23 | |
| Interventions | Therapists: trained nurse or public health educator 1. Intensive counselling: single counselling session (30 ‐ 60 mins) prior to discharge (based on social learning theory and stages of change), 5 telephone counselling calls < 30 mins, 1 and 3 wks, monthly for 3 m + S‐H. Recycling encouraged. Nicotine patches begun in hospital, dose‐based on pre‐hospitalization smoking rates. 2 m supply at discharge 2. Nicotine patches as 1. ˜10‐min session on risks and benefits, S‐H. |
|
| Outcomes | Abstinence at 12 m (7‐day PP) Validation: saliva cotinine < 15 ng/ml | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomly assigned using computerized algorithm" |
| Allocation concealment (selection bias) | Unclear risk | No details provided |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation of abstinence |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 7 (3%) lost to follow‐up included in ITT analysis, 14 (6%) died and excluded from denominator |
Stevens 1993.
| Methods | Study design: Controlled trial Setting: 2 Health Maintenance Organization hospitals, USA Recruitment: All hospitalized smokers or recent ex‐smokers with stay > 36 hrs |
|
| Participants | 1119 smokers or recent quitters (5%) av. age 44, av. cpd 20 | |
| Interventions | Therapists: Masters level cessation counsellors 1. 20‐min counselling session, 12‐min video, quit kit, choice of S‐H materials, 1 ‐ 2 follow‐up telephone calls, access to hotline, bimonthly newsletter mailings 2. Usual care |
|
| Outcomes | Abstinence at 12 m (2 PP, 3 and 12m) Validation: due to low success in obtaining samples for cotinine analysis, data are based on self‐report only | |
| Notes | We report a sensitivity analysis on the effect of exclusion of this non‐random study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not random, intervention alternated between hospitals on a monthly basis in order to avoid contamination |
| Allocation concealment (selection bias) | High risk | Intervention or control status of hospital known when participants recruited |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Self‐reported quitting only |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 6% loss to follow‐up, no difference by group, included in ITT analysis |
Thankappan 2013.
| Methods | Study design: Randomized controlled trial Setting: 2 diabetes clinics, India Recruitment: diabetic smokers attending clinic, not selected for readiness to quit |
|
| Participants | 224 male diabetic smokers, av. age 53 | |
| Interventions | Therapist: trained non‐physician counsellor 1. Physician advice 2. As 1, and counselling at each visit for 6 m; 4 x 30‐min, baseline, 1, 3, 6 m, based on 5 As/5Rs |
|
| Outcomes | Abstinence at 6 m Validation: none at 6 m, samples collected for cotinine later |
|
| Notes | New for 2016 update 2014 paper gives longer‐term outcome but not absolute numbers quit. Report on later validation using cotinine found reasonable accuracy with some misreporting likely due to environmental tobacco smoke |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Sequentially, every four patients enrolled were randomized into the two intervention groups using a computer generated random sequence to achieve a block size of four" |
| Allocation concealment (selection bias) | Unclear risk | Their medical records were then flagged with different coloured stickers by the counsellor in order to identify group assignment ‐ unclear whether allocation concealed |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No validation of abstinence |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 12.5% loss to follow‐up at 6 m |
Tonnesen 2006.
| Methods | Study design: Randomized controlled trial Setting: 7 chest clinics, Denmark Recruitment: outpatient attenders |
|
| Participants | 370 smokers of > 1 cpd with COPD 52% women, av. age 61, av. cpd 20 | |
| Interventions | Therapists: 20 nurses with cessation experience, trained to support medication use and provide standardized counselling Factorial trial. Nicotine sublingual tablet and placebo arms collapsed in MA 1. High support: 7 x 20 ‐ 30‐min clinic visits (0, 2, 4, 8, 12 wks, 6 m, 12 m) & 5 x 10‐min phone calls (1, 6, 10 wks , 4½ m, 9 m), total contact time 4½ hrs 2. Low support: 4 clinic visits (0, 2 wks, 6 m, 12 m) and 6 phone calls (1, 4, 6, 9, 12 wks, 9 m), total time 2½ hrs |
|
| Outcomes | Sustained abstinence at 12 m (validated at all visits from wk 2, PP also reported) Validation: CO < 10 ppm | |
| Notes | Compares higher‐ and lower‐intensity counselling. Therapists were not full‐time specialist counsellors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomization list at each centre |
| Allocation concealment (selection bias) | Unclear risk | Allocation process not described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation of abstinence |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 82 (22%) lost to follow‐up, included in ITT analysis |
Weissfeld 1991.
| Methods | Study design: Randomized controlled trial Setting: Veterans Administration outpatient clinics, USA Recruitment: veterans attending walk‐in and general medicine clinics invited to attend quit smoking programme |
|
| Participants | 466 male smokers av. age 55 years, av. cpd 26 | |
| Interventions | Therapists: smoking cessation counsellors 1. Control: Pamphlet on hazards of smoking 2. Low‐Intensity counselling: Single session 20 ‐ 30 mins and S‐H booklet 3. High‐intensity counselling: Same initial session, with sustained contact of 3 m. 1 further face‐to‐face session, telephone calls and mailings, behavioural S‐H manual. Prescription and sample of nicotine gum and instructions for use |
|
| Outcomes | Abstinence for 1 m at 6 m (9 m for high‐intensity group, 6 m after last contact) Validation: nicotine metabolites in urine | |
| Notes | Using validated quit rates there was no difference between 2 and 3, although self‐reported quitting was greater in 3. Main analysis uses 2 and 3 vs 1 with sensitivity analysis of 2 vs 1. Comparison of intensity uses 3 vs 2 39% of group 3 used nicotine gum vs 8% and 7% in 2 and 1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization: 2 stages; initially in 1:2 to control or intervention, then 1:1 to high or low intensity occurred after delivery of low‐intensity session. Random number table |
| Allocation concealment (selection bias) | Low risk | Consecutively‐numbered envelopes containing treatment assignment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation of abstinence |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 34 (7.3%) died or lost to follow‐up included in ITT analysis. More lost in high‐intensity group |
Wiggers 2006.
| Methods | Study design: Randomized controlled trial Setting: Cardiovascular outpatient department, Netherlands Recruitment: People attending regular consultation; those consenting referred to nurse practitioner |
|
| Participants | 385 smokers (8 deaths excluded from outcomes) 37% women, av. age 59, av. cpd 21 | |
| Interventions | Therapist: nurse practitioner In both groups, participants planning to quit received 8 wks nicotine patch with instruction from nurse. 1. Minimal Intervention Strategy for cardiology patients (C‐MIS). 15 ‐ 30 mins at baseline, 1 phone call at 2 wks, additional session on request. Assessment of dependency and motivation, barriers; TQD set for motivated participants 2. Usual care without motivational counselling. |
|
| Outcomes | Abstinence for 7 days at 12 m Validation: Urine or saliva nicotine/cotinine/thiocyanate. Self‐reported smokers also tested; validated rates include smokers with negative biochemical results, so self‐reported non‐smoking used in MA | |
| Notes | Included on grounds that participants were referred to nurse practitioner for counselling; not part of usual care | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A computerized balanced randomization programme taking prognostic factors (e.g. clinic attendance, age and gender) into account." |
| Allocation concealment (selection bias) | Low risk | "While patients completed their baseline questionnaire (and signed a written informed consent) nurses randomly assigned ..." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation of abstinence |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 withdrawal due to cognitive problems and 8 deaths during follow‐up not included in analyses. At 12 m 45 not reached by mail or phone, included in ITT. More unmarried participants lost |
Williams 2010.
| Methods | Study design: Randomized controlled trial Setting: Mental health outpatient clinics, USA Recruitment: People with schizophrenia or schizoaffective disorder, willing to use NRT |
|
| Participants | 100 smokers (> 10 cpd) using an atypical antipsychotic; 16% women, av. age ˜46, av. cpd 23 | |
| Interventions | Therapists: trained mental health clinicians provided both conditions Pharmacotherapy: nicotine patch (21 mg for 16 wks incl tapering) 1. Treatment of Addiction to Nicotine in Schizophrenia (TANS); 24 x 45‐min individual counselling sessions over 26 wks 2. Medical Management (MM); 9 x 20 mins over 26 wks |
|
| Outcomes | Continuous abstinence at 12 m Validation: CO < 10 ppm |
|
| Notes | New for 2016 update Contributes to comparison 2.1.2, more versus less intensive counselling as adjunct to pharmacotherapy |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "adaptive urn randomization procedure that accounts for motivation, gender, ethnicity, and heavy versus light smoking status" |
| Allocation concealment (selection bias) | Low risk | Judged that process for randomization prevented prior knowledge of condition |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation of abstinence |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 75% followed up at 12 m, authors report "not different between groups" |
Windsor 1988.
| Methods | Study design: Randomized controlled trial Setting: University worksite, USA Recruitment: Employees volunteering for a quit‐smoking programme |
|
| Participants | 378 smokers av. age 37, av. cpd 23 ‐ 27 | |
| Interventions | Therapist: health educator All groups received a 10‐min session of brief advice 1. + S‐H manuals 2. + S‐H and another session of counselling (20 ‐ 30 mins) with skills training, buddy selection and a contract 3. as 1, with monetary rewards for cessation 4. as 2, with monetary rewards for cessation |
|
| Outcomes | Abstinence at 1 yr (sustained at 6 wks, 6 m, 1 yr, no more than 2 cigs in period) Validation: saliva thiocyanate < 100μg/ml at all follow‐ups | |
| Notes | There was no apparent effect of monetary incentives so this arm is collapsed. 4 and 2 vs 3 and 1. Number of quitters estimated from graphs | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated assignment |
| Allocation concealment (selection bias) | Low risk | Sealed numbered envelopes opened after informed consent and baseline questionnaire |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation of abstinence |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 37 lost to follow‐up, included in ITT analysis |
Wu 2009.
| Methods | Study design: Randomized controlled trial Setting: Research unit for Asian health, NYC, USA Recruitment: via Asian Community Health Coalition member organizations |
|
| Participants | 139 Chinese smokers (any smoking in previous wk); 12% women, av. age 44, av. cpd NS, 25% smoked < 10 cpd, 49% had never attempted to quit | |
| Interventions | Therapist: Chinese speaking counsellor Pharmacotherapy: NRT. Patch for 8 wks (could start at any time in 6 m period) 1. Culturally‐tailored counselling in Chinese, 4 x 60 mins and S‐H 2. Health education in Chinese: 4 x 60 mins, including general health, nutrition, exercise and tobacco |
|
| Outcomes | Abstinence at 6 m (PP) Validation: CO < 6 ppm |
|
| Notes | New for 2016 update Conditions had same contact time, but control did not focus on smoking |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized, method not stated |
| Allocation concealment (selection bias) | Unclear risk | Details not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation of abstinence |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 10% intervention and 14% control lost to follow‐up at 6 m and counted as smokers in ITT analysis |
av ‐ average (mean) CAD: coronary artery disease CI ‐ confidence interval CO ‐ carbon monoxide COHb ‐ carboxyhaemoglobin COPD ‐ chronic obstructive pulmonary disease cpd ‐ cigarettes per day ITT ‐ intention‐to‐treat m ‐ month MA ‐ meta‐analysis MI ‐ myocardial infarction min ‐ minute NRT ‐ Nicotine replacement therapy OR ‐ odds ratio PP ‐ point prevalence (abstinent at defined period) PAD ‐ peripheral artery disease ppm ‐ parts per million S‐H ‐ Self help materials TIA ‐ transient ischaemic attack TQD ‐ Target Quit Date wk ‐ week yr ‐ year
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Alonso‐Pérez 2007 | Allocation to behavioural treatment was by clinic attended; each of 3 primary care clinics provided different treatment. Differences between outcomes could have been due to differences between patients in different clinics; no way to estimate effect of clustering |
| Berndt 2014 | Face‐to‐face counselling confounded with provision of NRT, compared to usual care control. Comparison with telephone counselling confounded by different duration of treatment and therapists |
| Bock 2014 | Main intervention component was motivational interviewing, see Lindson‐Hawley 2015 |
| Bolman 2002 | Intervention provided by a nurse as part of usual care, included in Cochrane Review of nursing interventions (Rice 2013) |
| Borrelli 2005 | Intervention provided by a nurse during normal duties, included in Cochrane Review of nursing interventions (Rice 2013) |
| Calabro 2012 | Multicomponent intervention included access to internet‐based resources and health feedback in addition to 2 counselling sessions, as adjunct to offer of NRT (see Stead 2015) |
| Camarelles 2002 | Compares individual to group counselling, see Cochrane Review of group‐based interventions (Stead 2017) |
| Canga 2000 | Intervention provided by a nurse, included in Cochrane Review of nursing interventions (Rice 2013) |
| Catley 2016 | Participants were smokers not planning to quit. Currently listed as an ongoing study in Lindson‐Hawley 2015 'Motivational interviewing for smoking cessation' and will be included there |
| Clarke 2013 | Short follow‐up (˜4.5 months from start of intervention, 3 months from prison discharge). Participants were abstinent whilst in prison |
| Colby 1998 | Short follow‐up (3 months) |
| Dezee 2013 | No brief‐advice control, comparison was with internet support |
| Emmons 2001 | Data not available for intervention and control groups separately. No significant difference reported. Cessation was a secondary outcome in this trial using motivational interviewing to reduce passive smoke exposure. Participants were not selected by motivation to quit |
| Froelicher 2004 | Intervention provided by a nurse; included in Cochrane Review of nursing interventions (Rice 2013) |
| Gariti 2009 | Control group had multiple sessions for 'medication management'. Included in Stead 2015 |
| Ghanem 2014 | Unpublished study. Insufficient detail in either abstract to include or to enable contact with author for further information |
| Gifford 2004 | Trial of an acceptance and commitment‐based treatment intervention that included multiple group sessions in addition to individual counselling. Comparator was nicotine patch therapy |
| Gifford 2011 | Trial of an acceptance and commitment‐based treatment intervention that included multiple group sessions in addition to individual counselling as adjunct to bupropion |
| Gorini 2012 | Counselling intervention was brief advice provided by midwives conducting PAP test, not by dedicated cessation counsellors |
| Harris 2010 | Participants included smokers not planning to quit. See Lindson‐Hawley 2015 |
| Hilberink 2005 | Intervention provided by physicians and nurses in usual care setting, not specialist counselling |
| Hokanson 2006 | Participants included smokers not planning to quit, and recent quitters. See Lindson‐Hawley 2015 |
| Hyman 2007 | Multiple risk factor intervention |
| Kadowaki 2000 | Intervention was multicomponent and included advice/counselling from a physician, nurse and a group programme. Follow‐up only 5 months |
| Lando 1992 | There was no face‐to‐face contact with counsellors. Contact was by proactive telephone calls |
| Lloyd‐Richardson 2009 | Compared motivational interviewing to less intensive counselling, as adjuncts to nicotine patch, see Lindson‐Hawley 2015 |
| Lopez 2007 | Multiple risk factor intervention enrolling smokers and nonsmokers |
| Malchodi 2003 | Intervention specifically for pregnant women, see Cochrane Review of smoking cessation interventions in pregnancy (Chamberlain 2013) |
| Marks 2002 | Intervention was provided in a self‐help format |
| McCarthy 2016 | All participants received counselling, intervention was a 'practice quitting' programme |
| Mildestvedt 2007 | Multiple risk factor lifestyle intervention |
| Mooney 2007 | Short follow‐up (6 wks). Study added a pharmacotherapy compliance‐enhancing component to individual counselling using CBT |
| Niaura 1999 | All participants received individual counselling; Included in Cochrane NRT review (Stead 2008) |
| Okuyemi 2006 | Intervention combined group and individual counselling with pharmacotherapy |
| Rabkin 1984 | The health education arm of the trial included a group meeting with didactic lecture, film and discussion, followed by a single individual session with a therapist. We decided that this did not meet the criteria for individual counselling |
| Raja 2014 | Short‐term study, outcome was nicotine dependence |
| Rodriguez 2003 | Intervention combined the systematic use of NRT with counselling; covered in Cochrane Review of worksite interventions (Cahill 2014) |
| Sanz‐Pozo 2006 | Intervention provided by nurses in a primary care clinic, included in Cochrane Review of nursing interventions (Rice 2013) |
| Savant 2013 | Not restricted to smokers; more than half of participants used chewing tobacco. Cessation rates not given by type of tobacco use |
| Schnoll 2005 | Short follow‐up (3 months). Compared 2 counselling approaches, no difference detected |
| Schwartz 1967 | Success was defined as reduction in smoking of over 85%, not complete abstinence |
| Secades‐Villa 2009 | Cluster‐randomised by primary care centre with 1 centre per condition; no way to allow for intra‐cluster correlation |
| Sherman 2007 | Primary outcome was not cessation; assessed rates of receiving counselling, referral and treatment |
| Soria 2006 | Motivational interviewing intervention by primary care physician during routine care |
| Stein 2006 | Test of motivational interviewing; not all participants attempted to quit |
| Stevens 2000 | Intervention providers were respiratory therapists, not counsellors. Included in Cochrane Review of interventions in hospital inpatients, (Rigotti 2012) |
| Williams 2006 | Study targeted multiple risk factors |
| Wittchen 2011 | Counselling was delivered by non‐specialist physicians |
| Woodruff 2002 | Short follow‐up (3 months) |
Characteristics of ongoing studies [ordered by study ID]
Bonevski 2011.
| Trial name or title | Call it Quits |
| Methods | Block‐randomized controlled trial |
| Participants | Socially disadvantaged population, target n = 400 |
| Interventions | The smoking cessation (intervention) group will receive an intensive participant‐centred smoking cessation intervention offered by the caseworker over a minimum of 3 face‐to‐face visits (each 2 weeks apart) which will begin immediately following baseline survey completion, followed by at least 2 phone contacts (1 week apart). This intervention will constitute an add‐on to participants' usual regular counselling visits, reducing additional costs to the Centre and to participants. If a participant requires further contact, staff will provide further quitting assistance and record what they delivered on their checklist. The control group will receive minimum ethical care. |
| Outcomes | 2 primary outcome measures obtained at 1‐, 6‐, and 12‐month follow‐up: 1) 24‐hour expired air CO‐validated self‐reported smoking cessation; and 2) 7‐day self‐reported smoking cessation. Continuous abstinence will also be measured at 6‐ and 12‐month follow‐up |
| Starting date | 01/02/2010 |
| Contact information | billie.bonevski@newcastle.edu.au; Faculty of Health and Medicine, Centre for Translational Neuroscience and Mental Health, School of Medicine and Public Health, University of Newcastle, Callaghan, New South Wales, Australia |
| Notes |
Garvey 2012a.
| Trial name or title | Duration of Behavioral Counseling Treatment Needed to Optimize Smoking Abstinence |
| Methods | Randomized trial |
| Participants | 450 daily smokers |
| Interventions | Participants are randomized to 1 of 3 behavioural treatments: (1) Brief Duration (3‐month) smoking‐cessation counselling; (2) Moderate Duration (6‐month) counselling; or (3) Extended Duration (12 month) counselling |
| Outcomes | Primary: abstinence at 1 year. Secondary; abstinence at 2 years |
| Starting date | 2008 |
| Contact information | Arthur J. Garvey, Ph.D., Harvard School of Dental Medicine |
| Notes |
Differences between protocol and review
For the 2017 update, we include 'Summary of findings' tables for the main comparisons.
Contributions of authors
TL and LS jointly conceived the review, developed the protocol, extracted data, wrote the text and are guarantors. LS conducted the searches and preliminary screening of studies.
Sources of support
Internal sources
Oxford University Department of Primary Health Care, UK.
National Institute for Health Research School for Primary Care Research, UK.
External sources
NHS Research and Development Programme, UK.
Declarations of interest
Tim Lancaster: None known. Lindsay Stead: None known.
Edited (no change to conclusions)
References
References to studies included in this review
Ahluwalia 2006 {published data only}
- Ahluwalia JS, Okuyemi K, Nollen N, Choi WS, Kaur H, Pulvers K, et al. The effects of nicotine gum and counseling among African American light smokers: A 2 x 2 factorial design. Addiction 2006;101(6):883‐91. [DOI] [PubMed] [Google Scholar]
- Ho MK, Mwenifumbo JC, Al Koudsi N, Okuyemi KS, Ahluwalia JS, Benowitz NL, et al. Association of nicotine metabolite ratio and CYP2A6 genotype with smoking cessation treatment in African‐American light smokers. Clinical Pharmacology and Therapeutics 2009;85(6):635‐43. [CENTRAL: 701215; CRS: 9400123000013799; 28553; PUBMED: 19279561] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nollen NL, Mayo MS, Sanderson CL, Okuyemi KS, Choi WS, Kaur H, et al. Predictors of quitting among African American light smokers enrolled in a randomized, placebo‐controlled trial. Journal of General Internal Medicine 2006;21:590‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuyemi KS, Cox LS, Nollen NL, Snow TM, Kaur H, Choi W, et al. Baseline characteristics and recruitment strategies in a randomized clinical trial of African‐American light smokers. American Journal of Health Promotion 2007;21:183‐91. [DOI] [PubMed] [Google Scholar]
- Okuyemi KS, Faseru B, Sanderson CL, Bronars CA, Ahluwalia JS. Relationship between menthol cigarettes and smoking cessation among African American light smokers. Addiction 2007;102:1979‐86. [DOI] [PubMed] [Google Scholar]
- Okuyemi KS, Pulvers KM, Cox LS, Thomas JL, Kaur H, Mayo MS, et al. Nicotine dependence among African American light smokers: a comparison of three scales. Addictive Behaviors 2007;32(10):1989‐2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Aleixandre 1998 {published data only}
- Aleixandre ME, Mitjans JF, Casanova MA, Sanchez J, Belloch A. Clinical trial of two interventions of tobacco cessation in primary care. Atencion Primaria 1996;18(Suppl 1):212. [Google Scholar]
- Aleixandre i Marti E, Casanova Matutano MA, Mitjans Lafont J, Sanchez Monfort J, Sanmartin Almenar A. Clinical trial of two tobacco use cessation interventions in primary care [Ensayo clinico de dos intervenciones de deshabituacion tabaquica en atencion primaria]. Atencion Primaria 1998;22(7):424‐8. [PubMed] [Google Scholar]
Alterman 2001 {published data only}
- Alterman AI, Gariti P, Mulvaney F. Short‐ and long‐term smoking cessation for three levels of intensity of behavioral treatment. Psychology of Addictive Behaviors 2001;15(3):261‐4. [PubMed] [Google Scholar]
Aveyard 2007 {published data only}
- Aveyard P, Brown K, Saunders C, Alexander A, Johnstone E, Munafò MR, et al. Weekly versus basic smoking cessation support in primary care: a randomised controlled trial. Thorax 2007;62(10):898‐903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaves C. Smoking cessation trial may be missing the point.[comment]. Thorax 2008;63(3):291‐2. [PubMed] [Google Scholar]
Bobo 1998 {published data only}
- Bobo JK, McIlvain HE, Lando HA, Walker RD, Leed Kelly A. Effect of smoking cessation counseling on recovery from alcoholism: findings from a randomized community intervention trial. Addiction 1998;93(6):877‐87. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Leed Kelly A, Russell KS, Bobo JK, McIlvain H. Feasibility of smoking cessation counseling by phone with alcohol treatment center graduates. Journal of Substance Abuse Treatment 1996;13(3):203‐10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bronson 1989 {published data only}
- Bronson DL, Flynn BS, Solomon LJ, Vacek PM, Secker‐Walker RH. Smoking cessation counselling during periodic health examinations. Archives of Internal Medicine 1989;149:1653‐6. [PubMed] [Google Scholar]
- Secker‐Walker RH, Lynn BS, Solomon LJ, Vacek PM, Bronson DL. Predictors of smoking behavior change 6 and 18 months after individual counseling during periodic health examinations. Preventive Medicine 1990;19(6):675‐85. [DOI] [PubMed] [Google Scholar]
Brunner 2012 {published data only}
- Brunner Frandsen N, Sorensen M, Hyldahl TK, Henriksen RM, Bak S. Smoking cessation intervention after ischemic stroke or transient ischemic attack. A randomized controlled pilot trial. Nicotine & Tobacco Research 2012;14(4):443‐7. [CENTRAL: 814359; CRS: 9400123000012563; EMBASE: 2012192968; PUBMED: 22193575] [DOI] [PubMed] [Google Scholar]
Burling 1991 {published data only}
- Burling TA, Marshall GD, Seidner AL. Smoking cessation for substance abuse inpatients. Journal of Substance Abuse 1991;3(3):269‐76. [DOI] [PubMed] [Google Scholar]
Burling 2001 {published data only}
- Burling TA, Burling AS, Latini D. A controlled smoking cessation trial for substance‐dependent inpatients. Journal of Consulting and Clinical Psychology 2001;69(2):295‐304. [DOI] [PubMed] [Google Scholar]
Chan 2012 {published data only}
- Chan SS, Leung DY, Lau C, Wong V, Lam T. Cost‐effectiveness analysis of a low intensity nurse‐led stage‐matched smoking cessation intervention to cardiac patients in Hong Kong. Circulation 2010;122(2):E87. [CENTRAL: 792456; CRS: 9400123000006051] [Google Scholar]
- Chan SS, Leung DY, Wong DC, Lau CP, Wong VT, Lam TH. A randomized controlled trial of stage‐matched intervention for smoking cessation in cardiac out‐patients. Addiction (Abingdon, England) 2012;107(4):829‐37. [CENTRAL: 831052; CRS: 9400123000012976; EMBASE: 22118418; PUBMED: 22118418] [DOI] [PubMed] [Google Scholar]
- Chan SSC, Lam TH, Lau C‐P. The effectiveness of a nurse‐delivered smoking cessation intervention for cardiac patients: a randomised controlled trial. Nicotine & Tobacco Research 2005;7(4):692. [CENTRAL: 527413; CRS: 9400123000003748] [Google Scholar]
Chen 2014 {published data only}
- Chen J, Chen P. The individualized smoking counselling for smoking cessation in COPD patients and general smokers. Respirology (Carlton, Vic.) 2011;16:116. [CENTRAL: 814367; CRS: 9400123000012446; 7010] [Google Scholar]
- Chen J, Chen Y, Chen P, Liu Z, Luo H, Cai S. Effectiveness of individual counseling for smoking cessation in smokers with chronic obstructive pulmonary disease and asymptomatic smokers. Experimental and Therapeutic Medicine 2014;7(3):716‐20. [CENTRAL: 977728; CRS: 9400129000000799; EMBASE: 2014068598; PUBMED: 24520273] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cropsey 2015 {published data only}
- Cropsey KL, Clark CB, Zhang X, Hendricks PS, Jardin BF, Lahti AC. Race and medication adherence moderate cessation outcomes in criminal justice smokers. American Journal of Preventive Medicine 2015;49(3):335‐44. [PUBMED: 26091924 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dornelas 2000 {published data only}
- Dornelas EA, Sampson RA, Gray JF, Waters D, Thompson PD. A randomized controlled trial of smoking cessation counseling after myocardial infarction. Preventive Medicine 2000;30(4):261‐8. [DOI] [PubMed] [Google Scholar]
Fiore 2004 {published data only}
- Fiore MC, McCarthy DE, Jackson TC, Zehner ME, Jorenby DE, Mielke M, et al. Integrating smoking cessation treatment into primary care: An effectiveness study. Preventive Medicine 2004;38(4):412‐20. [DOI] [PubMed] [Google Scholar]
Garvey 2012 {published data only}
- Garvey AJ, Hoskinson RA, Wadler BA, Wood EE, Kinnunen T, Kalman D, et al. Effects of front‐loaded vs. standard weekly counseling on 6‐month abstinence rates (POS1‐66). Society for Research on Nicotine and Tobacco 13th Annual Meeting February 21‐24, Austin, Texas. 2007:54. [CENTRAL: 616128; CRS: 9400123000004541; 14531]
- Garvey AJ, Kalman D, Hoskinson RAJ, Kinnunen T, Wadler BM, Thomson CC, et al. Front‐loaded versus weekly counseling for treatment of tobacco addiction. Nicotine & Tobacco Research 2012;14(5):578‐85. [CENTRAL: 832525; CRS: 9400123000014483; EMBASE: 2012254939; PUBMED: 22058190] [DOI] [PMC free article] [PubMed] [Google Scholar]
Glasgow 2000 {published data only}
- Glasgow RE, Whitlock EP, Eakin EG, Lichtenstein E. A brief smoking cessation intervention for women in low‐income planned parenthood clinics. American Journal of Public Health 2000;90:786‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hannover 2009 {published data only}
- Hannöver W, Thyrian JR, Röske K, Grempler J, Rumpf HJ, John U, et al. Smoking cessation and relapse prevention for postpartum women: results from a randomized controlled trial at 6, 12, 18 and 24 months. Addictive Behaviors 2009;34(1):1‐8. [CENTRAL: 702834; CRS: 9400123000005273; EMBASE: 2008517114; PUBMED: 18804331] [DOI] [PubMed] [Google Scholar]
Hennrikus 2005 {published data only}
- Hennrikus D, Lando HA, McCarty MC, Vessey JT. The effectiveness of a systems approach to smoking cessation in hospital inpatients. Society for Research on Nicotine and Tobacco 7th Annual Meeting March 23‐23 Seattle Washington. 2001:47.
- Hennrikus DJ, Lando HA, McCarty MC, Klevan D, Holtan N, Huebsch JA, et al. The TEAM project: the effectiveness of smoking cessation interventions with hospital patients. Preventive Medicine 2005;40(3):249‐58. [DOI] [PubMed] [Google Scholar]
- Lando H, Hennrikus D, McCarty M, Vessey J. Predictors of quitting in hospitalized smokers. Nicotine & Tobacco Research 2003;5(2):215‐22. [DOI] [PubMed] [Google Scholar]
Hennrikus 2010 {published data only}
- Hennrikus D, Joseph AM, Lando HA, Duval S, Ukestad L, Kodl M, et al. Effectiveness of a smoking cessation program for peripheral artery disease patients: a randomized controlled trial. Journal of the American College of Cardiology 2010;56(25):105‐12. [PUBMED: 21144971 ] [DOI] [PubMed] [Google Scholar]
Jorenby 1995 {published data only}
- Jorenby DE, Smith SS, Fiore MC, Hurt RD, Offord KP, Croghan IT, et al. Varying nicotine patch dose and type of smoking cessation counseling. JAMA 1995;274(17):1347‐52. [PubMed] [Google Scholar]
Killen 2008 {published data only}
- Bailey SR, Hammer SA, Bryson SW, Schatzberg AF, Killen JD. Using treatment process data to predict maintained smoking abstinence. American Journal of Health Behavior 2010;34(6):801‐10. [CENTRAL: 766985; CRS: 9400123000005814] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killen JD, Fortmann SP, Schatzberg AF, Arredondo C, Murphy G, Hayward C, et al. Extended cognitive behavior therapy for cigarette smoking cessation. Addiction 2008;103(8):1381‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2005 {published data only}
- Kim JR, Lee MS, Hwang JY, Lee JD. Efficacy of a smoking cessation intervention using the AHCPR guideline tailored for Koreans: a randomized controlled trial. Health Promotion International 2005;20(1):51‐9. [DOI] [PubMed] [Google Scholar]
Kim 2015 {published data only}
- Kim SS, Kim S‐H, Fang H, Kwon S, Shelley D, Ziedonis D. A culturally adapted smoking cessation intervention for Korean Americans: A mediating effect of perceived family norm toward quitting. Journal of Immigrant & Minority Health 2015;17(4):1120‐9. [CRS: 9400131000003387] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SS, Kim SH, Ziedonis D. Tobacco dependence treatment for Korean Americans: preliminary findings. Journal of Immigrant and Minority Health / Center for Minority Public Health 2012;14(3):395‐404. [CENTRAL: 833139; CRS: 9400123000015714; EMBASE: 21785963; PUBMED: 21785963] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lifrak 1997 {published data only}
- Lifrak P, Gariti P, Alterman AI, McKay J, Volpicelli J, Sparkman T, et al. Results of two levels of adjunctive treatment used with the nicotine patch. American Journal on Addictions 1997;6(2):93‐8. [PubMed] [Google Scholar]
Marley 2014 {published data only}
- Marley JV, Atkinson D, Kitaura T, Nelson C, Gray D, Metcalf S, et al. The Be Our Ally Beat Smoking (BOABS) study, a randomised controlled trial of an intensive smoking cessation intervention in a remote aboriginal Australian health care setting.. BMC Public Health 2014;14(32):1‐10. [PUBMED: 24418597] [DOI] [PMC free article] [PubMed] [Google Scholar]
Marshall 2016 {published data only}
- Marshall HM, Courtney DA, Passmore LH, McCaul EM, Yang IA, Bowman RV, et al. Brief tailored smoking cessation counseling in a lung cancer screening population is feasible: a pilot randomized controlled trial. Nicotine & Tobacco Research 2016;18(7):1665‐9. [CRS: 9400131000006073; PUBMED: 26834052] [DOI] [PubMed] [Google Scholar]
- Marshall HM, Yang IA, Passmore L, Mccaul EM, Bowman R, Fong KM. A randomized controlled trial of brief counselling intervention and audio materials for smoking cessation in a low‐dose CT screening study. Journal of Thoracic Oncology 2013;8(Supplement 2):S703. [CENTRAL: 996980; CRS: 9400129000001580; EMBASE: 71396835] [Google Scholar]
McCarthy 2008 {published data only}
- McCarthy DE. Mechanisms of tobacco cessation treatment: Self‐report mediators of counseling and bupropion sustained release treatment. Dissertation Abstracts International: Section B: The Sciences and Engineering 2007;67(9‐B):5414. [Google Scholar]
- McCarthy DE, Piasecki TM, Jorenby DE, Lawrence DL, Shiffman S, Baker TB. A multi‐level analysis of non‐significant counseling effects in a randomized smoking cessation trial. Addiction (Abingdon, England) 2010;105(12):2195‐208. [CENTRAL: 780680; CRS: 9400123000005902; EMBASE: 20840173; PUBMED: 20840173] [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy DE, Piasecki TM, Lawrence DL, Jorenby DE, Shiffman S, Fiore MC, et al. A randomized controlled clinical trial of bupropion SR and individual smoking cessation counseling. Nicotine & Tobacco Research 2008;10(4):717‐29. [DOI] [PubMed] [Google Scholar]
Molyneux 2003 {published data only}
- Molyneux A, Lewis S, Leivers U, Anderton A, Antoniak M, Brackenridge A, et al. Clinical trial comparing nicotine replacement therapy (NRT) plus brief counselling, brief counselling alone, and minimal intervention on smoking cessation in hospital inpatients. Thorax 2003;58(6):484‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mueller 2012 {published data only}
- Mueller SE, Petitjean SA, Wiesbeck GA. Cognitive behavioral smoking cessation during alcohol detoxification treatment: a randomized, controlled trial. Drug and Alcohol Dependence 2012;126(3):279‐85. [CENTRAL: 845210; CRS: 9400123000014751; PUBMED: 22726914] [DOI] [PubMed] [Google Scholar]
Nakamura 2004 {published data only}
- Nakamura M, Masui S, Oshima A, Okayama A, Ueshima H. Effects of stage‐matched repeated individual counseling on smoking cessation: A randomized controlled trial for the High‐risk Strategy by Lifestyle Modification (HISLIM) study. Environmental Health & Preventive Medicine 2004;9(4):152‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nohlert 2009 {published data only}
- Nohlert E, Helgason AR, Tillgren P, Tegelberg A, Johansson P. Comparison of the cost‐effectiveness of a high‐ and a low‐intensity smoking cessation intervention in Sweden: a randomized trial. Nicotine & Tobacco Research 2013;15(9):1519‐27. [CENTRAL: 871208; CRS: 9400107000001886; EMBASE: 2013526836; PUBMED: 23404735] [DOI] [PubMed] [Google Scholar]
- Nohlert E, Tegelberg A, Tillgren P, Johansson P, Rosenblad A, Helgason AR. Comparison of a high and a low intensity smoking cessation intervention in a dentistry setting in Sweden: a randomized trial. BMC Public Health 2009;9:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ockene 1992 {published data only}
- Ockene JK, Kristeller J, Goldberg R, Ockene IS, Merriam P, Barrett S, et al. Smoking cessation and severity of disease: The coronary artery smoking intervention study. Health Psychology 1992;11(2):119‐26. [DOI] [PubMed] [Google Scholar]
- Rosal MC, Ockene JK, Ma YS, Hebert JR, Ockene IS, Merriam P, et al. Coronary Artery Smoking Intervention Study (CASIS): 5‐year Follow‐up. Health Psychology 1998;17(5):476‐8. [DOI] [PubMed] [Google Scholar]
Okuyemi 2013 {published data only}
- Okuyemi KS, Goldade K, Whembolua GL, Thomas JL, Eischen S, Sewali B, et al. Motivational interviewing to enhance nicotine patch treatment for smoking cessation among homeless smokers: a randomized controlled trial. Addiction (Abingdon, England) 2013;108(6):1136‐44. [CENTRAL: 921008; CRS: 9400107000001568; PEER: Reviewed Journal: 2013‐16822‐021; PUBMED: 23510102] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pedersen 2005 {published data only}
- Pedersen L, Johansen S, Eksten L. Smoking cessation among patients with acute heart disease. A randomised intervention project. Ugeskrift for Laeger 2005;167(33):3044‐7. [PubMed] [Google Scholar]
Pederson 1991 {published data only}
- Pederson LL, Wanklin JM, Lefcoe NM. The effects of counseling on smoking cessation among patients hospitalized with chronic obstructive pulmonary disease: a randomized clinical trial. International Journal of the Addictions 1991;26(1):107‐19. [DOI] [PubMed] [Google Scholar]
Ramon 2013 {published data only}
- Ramon JM, Nerin I, Comino A, Pinet C, Abella F, Carreras JM, et al. A multicentre randomized trial of combined individual and telephone counselling for smoking cessation. Preventive Medicine 2013;57(3):183‐8. [CENTRAL: 871209; CRS: 9400107000001894; EMBASE: 2013515081; PUBMED: 23732247] [DOI] [PubMed] [Google Scholar]
Ramos 2010 {published data only}
- Ramos M, Ripoll J, Estrades T, Socias I, Fe A, Duro R, et al. Effectiveness of intensive group and individual interventions for smoking cessation in primary health care settings: a randomized trial. BMC Public Health 2010;10:89. [CENTRAL: 761411; CRS: 9400123000005754; PUBMED: 20178617] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rigotti 1997 {published data only}
- Rigotti NA, Arnsten JH, McKool KM, Wood‐Reid KM, Pasternak RC, Singer DE. Efficacy of a smoking cessation program for hospital patients. Archives of Internal Medicine 1997;157(22):2653‐60. [PubMed] [Google Scholar]
Schmitz 1999 {published data only}
- Schmitz JM, Spiga R, Rhoades HM, Fuentes F, Grabowski J. Smoking cessation in women with cardiac risk: a comparative study of two theoretically based therapies. Nicotine & Tobacco Research 1999;1(1):87‐94. [DOI] [PubMed] [Google Scholar]
Simon 1997 {published data only}
- Simon JA, Solkowitz SN, Carmody TP, Browner WS. Smoking cessation after surgery. A randomized trial. Archives of Internal Medicine 1997;157(12):1371‐6. [PubMed] [Google Scholar]
Simon 2003 {published data only}
- Simon JA, Carmody TP, Hudes ES, Snyder E, Murray J. Intensive smoking cessation counseling versus minimal counseling among hospitalized smokers treated with transdermal nicotine replacement: a randomized trial. American Journal of Medicine 2003;114(7):555‐62. [DOI] [PubMed] [Google Scholar]
Stevens 1993 {published data only}
- Meenan RT, Stevens VJ, Hornbrook MC, Chance PA, Glasgow RE, Hollis JF, et al. Cost‐effectiveness of a hospital‐based smoking cessation intervention. Medical Care 1998;36(5):670‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Stevens VJ, Glasgow RE, Hollis JF, Lichtenstein E, Vogt TM. A smoking‐cessation intervention for hospital patients. Medical Care 1993;31(1):65‐72. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Thankappan 2013 {published data only}
- Thankappan KR, Mini GK, Daivadanam M, Vijayakumar G, Sarma PS, Nichter M. Smoking cessation among diabetes patients: results of a pilot randomized controlled trial in Kerala, India. BMC Public Health 2013;13:47. [CENTRAL: 853596; CRS: 9400107000000056; EMBASE: 23331722; PUBMED: 23331722] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thankappan KR, Mini GK, Hariharan M, Vijayakumar G, Sarma PS, Nichter M. Smoking cessation among diabetic patients in Kerala, India: 1‐Year follow‐up results from a pilot randomized controlled trial. Diabetes Care 2014;37(12):e256‐7. [CENTRAL: 1036654; CRS: 9400050000000130; EMBASE: 2014925711; PUBMED: 25414396] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tonnesen 2006 {published data only}
- Tonnesen P, Mikkelsen K, Bremann L. Nurse‐conducted smoking cessation in patients with COPD using nicotine sublingual tablets and behavioral support. Chest 2006;130(2):334‐42. [DOI] [PubMed] [Google Scholar]
Weissfeld 1991 {published data only}
- Weissfeld JL, Holloway JL. Treatment for cigarette smoking in a Department of Veterans Affairs outpatient clinic. Archives of Internal Medicine 1991;151:973‐7. [MEDLINE: ] [PubMed] [Google Scholar]
Wiggers 2006 {published data only}
- Wiggers LC, Oort FJ, Dijkstra A, Haes JC, Legemate DA, Smets EM. Cognitive changes in cardiovascular patients following a tailored behavioral smoking cessation intervention. Preventive Medicine 2005;40(6):812‐21. [DOI] [PubMed] [Google Scholar]
- Wiggers LC, Smets EM, Oort FJ, Peters RJ, Storm‐Versloot MN, Vermeulen H, et al. The effect of a minimal intervention strategy in addition to nicotine replacement therapy to support smoking cessation in cardiovascular outpatients: a randomized clinical trial. European Journal of Cardiovascular Prevention and Rehabilitation 2006;13(6):931‐7. [DOI] [PubMed] [Google Scholar]
- Wiggers LC, Smets EM, Oort FJ, Storm‐Versloot MN, Vermeulen H, Loenen LB, et al. Adherence to nicotine replacement patch therapy in cardiovascular patients. International Journal of Behavioral Medicine 2006;13(1):79‐88. [DOI] [PubMed] [Google Scholar]
- Wiggers LCW, Oort FJ, Peters RJG, Legemate DA, Haes HCJM, Smets EMA. Smoking cessation may not improve quality of life in atherosclerotic patients. Nicotine & Tobacco Research 2006;8(4):581‐9. [DOI] [PubMed] [Google Scholar]
Williams 2010 {published data only}
- Williams JM, Steinberg ML, Zimmermann MH, Gandhi KK, Stipelman B, Budsock PD, et al. Comparison of two intensities of tobacco dependence counseling in schizophrenia and schizoaffective disorder. Journal of Substance Abuse Treatment 2010;38(4):384‐93. [CENTRAL: 750682; CRS: 9400123000005641; PUBMED: 20363089] [DOI] [PMC free article] [PubMed] [Google Scholar]
Windsor 1988 {published data only}
- Windsor RA, Lowe JB, Bartlett EE. The effectiveness of a worksite self‐help smoking cessation program: a randomized trial. Journal of Behavioral Medicine 1988;11(4):407‐21. [DOI] [PubMed] [Google Scholar]
Wu 2009 {published data only}
- Wu D, Ma GX, Zhou K, Zhou D, Liu A, Poon AN. The effect of a culturally tailored smoking cessation for Chinese American smokers. Nicotine & Tobacco Research 2009;11(12):1448‐57. [CENTRAL: 732128; CRS: 9400123000005485; PUBMED: 19915080] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Alonso‐Pérez 2007 {published data only}
- Alonso‐Pérez F, Secades‐Villa R, Duarte CG. Are psychological treatments effective to stop smoking? [Son eficientes los tratamientos psicológicos para dejar de fumar?]. Trastornos Adictivos 2007;9(1):21‐30. [Google Scholar]
Berndt 2014 {published data only}
- Berndt N, Bolman C, Froelicher ES, Mudde A, Candel M, Vries H, et al. Effectiveness of a telephone delivered and a face‐to‐face delivered counseling intervention for smoking cessation in patients with coronary heart disease: A 6‐month follow‐up. Journal of Behavioral Medicine 2014;37(4):709‐24. [CRS: 9400107000001638; PEER: Reviewed Journal: 2013‐21268‐001] [DOI] [PubMed] [Google Scholar]
- Berndt N, Bolman C, Lechner L, Mudde A, Verheugt FW, Vries H. Effectiveness of two intensive treatment methods for smoking cessation and relapse prevention in patients with coronary heart disease: study protocol and baseline description. BMC Cardiovascular Disorders 2012;12:33. [CENTRAL: 840357; CRS: 9400123000016156; EMBASE: 2012574240; PUBMED: 22587684] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bock 2014 {published data only}
- Bock BC, Papandonatos GD, Dios MA, Abrams DB, Azam MM, Fagan M, et al. Tobacco cessation among low‐income smokers: Motivational enhancement and nicotine patch treatment. Nicotine & Tobacco Research 2014;16(4):413‐22. [CENTRAL: 986341; CRS: 9400050000000050; EMBASE: 2014185069; PUBMED: 24174612] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bolman 2002 {published data only}
- Bolman C, Vries H, Breukelen G. A minimal‐contact intervention for cardiac inpatients: long‐term effects on smoking cessation. Preventive Medicine 2002;35(2):181‐92. [DOI] [PubMed] [Google Scholar]
Borrelli 2005 {published data only}
- Borrelli B, Novak S, Hecht J, Emmons K, Papandonatos G, Abrams D. Home health care nurses as a new channel for smoking cessation treatment: outcomes from project CARES (Community‐nurse Assisted Research and Education on Smoking). Preventive Medicine 2005;41(5‐6):815‐21. [DOI] [PubMed] [Google Scholar]
Calabro 2012 {published data only}
- Calabro KS, Marani S, Yost T, Segura J, Mullin Jones M, Nelson S, et al. Project SUCCESS: results from a randomized controlled trial. ISRN Public Health 2012:Article ID 913713. [CENTRAL: 836719; CRS: 9400123000016091]
Camarelles 2002 {published data only}
- Camarelles F, Asensio A, Jimenez‐Ruiz C, Becerril B, Rodero D, Vidaller O. [Effectiveness of a group therapy intervention to quit smoking. Randomized clinical trial]. Medicina Clinica ‐ Barcelona 2002;119(2):53‐7. [DOI] [PubMed] [Google Scholar]
Canga 2000 {published data only}
- Canga N, Irala J, Vara E, Duaso MJ, Ferrer A, Martinez‐Gonzalez MA. Intervention study for smoking cessation in diabetic patients ‐ A randomized controlled trial in both clinical and primary care settings. Diabetes Care 2000;23(10):1455‐60. [DOI] [PubMed] [Google Scholar]
Catley 2016 {published data only}
- Catley D, Goggin K, Harris KJ, Richter KP, Williams K, Patten C, et al. A randomized trial of motivational interviewing: Cessation induction among smokers with low desire to quit. American Journal of Preventive Medicine 2016;50(5):573‐83. [CRS: 9400131000006612; EMBASE: 20160316060; PUBMED: 26711164] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catley D, Harris KJ, Goggin K, Richter K, Williams K, Patten C, et al. Motivational Interviewing for encouraging quit attempts among unmotivated smokers: study protocol of a randomized, controlled, efficacy trial. BMC Public Health 2012;12:456. [DOI] [PMC free article] [PubMed] [Google Scholar]
Clarke 2013 {published data only}
- Clarke JG, Stein LAR, Martin RA, Martin SA, Parker D, Lopes CE, et al. Forced smoking abstinence: Not enough for smoking cessation. JAMA Internal Medicine 2013;173(9):789‐94. [CENTRAL: 863880; CRS: 9400107000000372; EMBASE: 2013308469] [DOI] [PMC free article] [PubMed] [Google Scholar]
Colby 1998 {published data only}
- Colby SM, Monti PM, Barnett NP, Rohsenow DJ, Weissman K, Spirito A, et al. Brief motivational interviewing in a hospital setting for adolescent smoking: a preliminary study. Journal of Consulting and Clinical Psychology 1998;66(3):574‐8. [DOI] [PubMed] [Google Scholar]
Dezee 2013 {published data only}
- Dezee KJ, Wink JS, Cowan CM. Internet versus in‐person counseling for patients taking varenicline for smoking cessation. Military Medicine 2013;178(4):401‐5. [CENTRAL: 959429; CRS: 9400130000000072; PUBMED: 23707824] [DOI] [PubMed] [Google Scholar]
Emmons 2001 {published data only}
- Emmons KM, Hammond SK, Fava JL, Velicer WF, Evans JL, Monroe AD. A randomized trial to reduce passive smoke exposure in low‐income households with young children. Pediatrics 2001;108(1):18‐24. [DOI] [PubMed] [Google Scholar]
Froelicher 2004 {published data only}
- Froelicher ESS, Miller NH, Christopherson DJ, Martin K, Parker KM, Amonetti M, et al. High rates of sustained smoking cessation in women hospitalized with cardiovascular disease ‐ The Women's Initiative for Nonsmoking (WINS). Circulation 2004;109:587‐93. [DOI] [PubMed] [Google Scholar]
Gariti 2009 {published data only}
- Gariti P, Lynch K, Alterman A, Kampman K, Xie H, Varillo K. Comparing smoking treatment programs for lighter smokers with and without a history of heavier smoking. Journal of Substance Abuse Treatment 2009;37(3):247‐55. [CENTRAL: 720874; CRS: 9400123000005384; PUBMED: 19339135] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ghanem 2014 {unpublished data only}
- Ghanem M. Evaluation of intensive outpatient antismoking counselling program in a low‐income country. Chest 2014;145(3 Suppl):617A. [CRS: 9400129000001568; EMBASE: 71429168] [Google Scholar]
- Ghanem M, Agmy G, Metwally M. Outcome of out‐patient intensive anti‐smoking counselling program in a low‐income country [Abstract]. European Respiratory Society Annual Congress, Vienna, Austria, September 12‐16. 2009:4633. [CENTRAL: 765833; CRS: 9400123000005802]
Gifford 2004 {published data only}
- Gifford EV, Kohlenberg BS, Hayes SC, Antonuccio DO, Piasecki MM, Rasmussen Hall ML, et al. Acceptance‐based treatment for smoking cessation. Behavior Therapy 2004;35(4):689‐705. [Google Scholar]
Gifford 2011 {published data only}
- Gifford EV, Kohlenberg BS, Hayes SC, Pierson HM, Piasecki MP, Antonuccio DO, et al. Does acceptance and relationship focused behavior therapy contribute to bupropion outcomes? A randomized controlled trial of functional analytic psychotherapy and acceptance and commitment therapy for smoking cessation. Behavior Therapy 2011;42(4):700‐15. [CENTRAL: 814642; CRS: 9400123000012398; 6939; PUBMED: 22035998] [DOI] [PubMed] [Google Scholar]
Gorini 2012 {published data only}
- Chellini E, Gorini G, Carreras G, Giordano L, Anghinoni E, Iossa A, et al. The Pap smear screening as an occasion for smoking cessation and physical activity counselling: baseline characteristics of women involved in the SPRINT randomized controlled trial. BMC Public Health 2011;11:906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorini G, Carreras G, Giordano L, Anghinoni E, Iossa A, Coppo A, et al. The Pap smear screening as an occasion for smoking cessation and physical activity counselling: effectiveness of the SPRINT randomized controlled trial. BMC Public Health 2012;12:740. [CENTRAL: 864643; CRS: 9400107000000033; EMBASE: 22950883; PUBMED: 22950883] [DOI] [PMC free article] [PubMed] [Google Scholar]
Harris 2010 {published data only}
- Harris KJ, Catley D, Good GE, Cronk NJ, Harrar S, Williams KB. Motivational interviewing for smoking cessation in college students: a group randomized controlled trial. Preventive Medicine 2010;51(5):387‐93. [CENTRAL: 769285; CRS: 9400123000005834; EMBASE: 2010585366; PUBMED: 20828584] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hilberink 2005 {published data only}
- Hilberink SR, Jacobs JE, Bottema BJ, Vries H, Grol RP. Smoking cessation in patients with COPD in daily general practice (SMOCC): six months' results. Preventive Medicine 2005;41(5‐6):822‐7. [DOI] [PubMed] [Google Scholar]
Hokanson 2006 {published data only}
- Hokanson JM, Anderson RL, Hennrikus DJ, Lando HA, Kendall DM. Integrated tobacco cessation counseling in a diabetes self‐management training program: a randomized trial of diabetes and reduction of tobacco. Diabetes Educator 2006;32(4):562‐70. [DOI] [PubMed] [Google Scholar]
Hyman 2007 {published data only}
- Hyman DJ, Pavlik VN, Taylor WC, Goodrick GK, Moye L. Simultaneous vs sequential counseling for multiple behavior change. Archives of Internal Medicine 2007;167(11):1152‐8. [DOI] [PubMed] [Google Scholar]
Kadowaki 2000 {published data only}
- Kadowaki T, Watanabe M, Okayama A, Hishida K, Ueshima H. Effectiveness of smoking‐cessation intervention in all of the smokers at a worksite in Japan. Industrial Health 2000;38(4):396‐403. [DOI] [PubMed] [Google Scholar]
Lando 1992 {published data only}
- Lando HA, Hellerstedt WL, Pirie PL, McGovern PG. Brief supportive telephone outreach as a recruitment and intervention strategy for smoking cessation. American Journal of Public Health 1992;82(1):41‐6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lloyd‐Richardson 2009 {published data only}
- Lloyd‐Richardson EE, Stanton CA, GD Papandonatos, Shadel WG, Stein M, Tashima K, et al. Motivation and patch treatment for HIV+ smokers: a randomized controlled trial. Addiction 2009;104(11):1891‐900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niaura R, Richardson EE, Stanton C, Carton‐Lopez S, Morrow K, Shadel W. Motivation and patch treatment for HIV‐positive smokers: psychosocial barriers to cessation (POS1‐057). Society for Research on Nicotine and Tobacco 10th Annual Meeting February 18‐21, Phoenix, Arizona. 2004.
Lopez 2007 {published data only}
- Lopez ML, Iglesias JM, Valle MO, Comas A, Fernandez JM, Vries H, et al. Impact of a primary care intervention on smoking, drinking, diet, weight, sun exposure, and work risk in families with cancer experience. Cancer Causes & Control 2007;18(5):525‐35. [DOI] [PubMed] [Google Scholar]
Malchodi 2003 {published data only}
- Malchodi CS, Oncken C, Dornelas EA, Caramanica L, Gregonis E, Curry SL. The effects of peer counseling on smoking cessation and reduction. Obstetrics and Gynecology 2003;101(3):504‐10. [DOI] [PubMed] [Google Scholar]
Marks 2002 {published data only}
- Marks DF, Sykes CM. Randomized controlled trial of cognitive behavioural therapy for smokers living in a deprived area of London: Outcome at one‐year follow‐up. Psychology, Health and Medicine 2002;7:17‐24. [Google Scholar]
McCarthy 2016 {published data only}
- McCarthy DE, Bold KW, Minami H, Yeh VM. A randomized clinical trial of a tailored behavioral smoking cessation preparation program. Behaviour Research and Therapy 2016;78:19‐29. [CENTRAL: 1133297; CRS: 9400131000005793; EMBASE: 20160085215] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mildestvedt 2007 {published data only}
- Mildestvedt T, Meland E, Eide GE. No difference in lifestyle changes by adding individual counselling to group‐based rehabilitation RCT among coronary heart disease patients. Scandinavian Journal Of Public Health 2007;35(6):591‐8. [DOI] [PubMed] [Google Scholar]
Mooney 2007 {published data only}
- Mooney ME, Sayre SL, Hokanson PS, Stotts AL, Schmitz JM. Adding MEMS feedback to behavioral smoking cessation therapy increases compliance with bupropion: A replication and extension study. Addictive Behaviors 2007;32(4):875‐80. [DOI] [PubMed] [Google Scholar]
Niaura 1999 {published data only}
- Niaura R, Abrams DB, Shadel WG, Rohsenow DJ, Monti PM, Sirota AD. Cue exposure treatment for smoking relapse prevention: A controlled clinical trial. Addiction 1999;94(5):685‐96. [DOI] [PubMed] [Google Scholar]
Okuyemi 2006 {published data only}
- Okuyemi KS, Thomas JL, Hall S, Nollen NL, Richter KP, Jeffries SK, et al. Smoking cessation in homeless populations: A pilot clinical trial. Nicotine & Tobacco Research 2006;8(5):689‐99. [DOI] [PubMed] [Google Scholar]
Rabkin 1984 {published data only}
- Kaufert JM, Rabkin SW, Syrotuik J, Boyko E, Shane F. Health beliefs as predictors of success of alternate modalities of smoking cessation: results of a controlled trial. Journal of Behavioral Medicine 1986;9(5):475‐89. [DOI] [PubMed] [Google Scholar]
- Rabkin SW, Boyko E, Shane F, Kaufert J. A randomized trial comparing smoking cessation programs utilizing behaviour modification, health education or hypnosis. Addictive Behaviors 1984;9(2):157‐73. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Raja 2014 {published data only}
- Raja M, Saha S, Mohd S, Narang R, Reddy LK, Kumari M. Cognitive behavioural therapy versus basic health education for tobacco cessation among tobacco users: A randomized clinical trail. Journal of Clinical and Diagnostic Research 2014;8(4):ZC47‐9. [CENTRAL: 988479; CRS: 9400050000000056; EMBASE: 2014290928; PUBMED: 24959516] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rodriguez 2003 {published data only}
- Rodriguez‐Artalejo F, Lafuente‐Urdinguio P, Guallar‐Castillon P, Garteizaurrekoa‐Dublang P, Sainz‐Martinez O, Diez‐Azcarate JI, et al. One‐year effectiveness of an individualized smoking cessation intervention at the workplace: a randomized controlled trial.. Occupational Environmental Medicine 2003;60(5):358‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sanz‐Pozo 2006 {published data only}
- Sanz Pozo B, Miguel Díaz J, Aragón Blanco M, González González AI, Cortes Catalán M, Vázquez I. Effectiveness of non‐pharmacological primary care methods for giving up tobacco dependency [Efectividad de los métodos no farmacológicos para la deshabituación tabáquica en atención primaria]. Atencion Primaria 2003;32(6):366‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz‐Pozo B, Miguel‐Díez J, Anegon‐Blanco M, García‐Carballo M, Gómez‐Suárez E, Fernández‐Domínguez JF. Effectiveness of a programme of intensive tobacco counselling by nursing professionals [Efectividad de un programa de consejo antitabaco intensivo realizado por profesionales de enfermería]. Atencion Primaria 2006;37(5):266‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Savant 2013 {published data only}
- Savant SC, Hegde‐Shetiya S, Agarwal D, Shirhatti R, Shetty D. Effectiveness of individual and group counseling for cessation of tobacco habit amongst industrial workers in Pimpri, Pune‐‐an interventional study. Asian Pacific Journal of Cancer Prevention 2013;14(2):1133‐9. [CENTRAL: 996880; CRS: 9400129000002967; PUBMED: 23621201] [DOI] [PubMed] [Google Scholar]
Schnoll 2005 {published data only}
- Schnoll RA, Rothman RL, Wielt DB, Lerman C, Pedri H, Wang H, et al. A randomized pilot study of cognitive‐behavioral therapy versus basic health education for smoking cessation among cancer patients. Annals of Behavioral Medicine 2005;30(1):1‐11. [DOI] [PubMed] [Google Scholar]
Schwartz 1967 {published data only}
- Schwartz JL, Dubitzky M. Clinical reduction of smoking: a California study. Addiction 1967;14:35‐44. [Google Scholar]
Secades‐Villa 2009 {published data only}
- Secades‐Villa R, Alonso‐Pérez F, García‐Rodríguez O, Fernández‐Hermida JR. Effectiveness of three intensities of smoking cessation treatment in primary care. Psychological Reports 2009;105(3 Pt 1):747‐58. [CENTRAL: 729739; CRS: 9400123000005462; PUBMED: 20099536] [DOI] [PubMed] [Google Scholar]
Sherman 2007 {published data only}
- Sherman SE, Estrada M, Lanto AB, Farmer MM, Aldana I. Effectiveness of an on‐call counselor at increasing smoking treatment. Journal of General Internal Medicine 2007;22(8):1125‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Soria 2006 {published data only}
- Soria R, Legido A, Escolano C, Yeste AL, Montoya J. A randomised controlled trial of motivational interviewing for smoking cessation. British Journal of General Practice 2006;56(531):768‐74. [PMC free article] [PubMed] [Google Scholar]
Stein 2006 {published data only}
- Stein MD, Weinstock MC, Anderson BJ, Anthony JL. Relationship of depression to smoking outcomes in a methadone‐maintained population. Journal of Addictive Diseases 2007;26(1):35‐40. [DOI] [PubMed] [Google Scholar]
- Stein MD, Weinstock MC, Herman DS, Anderson BJ, Anthony JL, Niaura R. A smoking cessation intervention for the methadone‐maintained. Addiction 2006;101(4):599‐607. [DOI] [PubMed] [Google Scholar]
Stevens 2000 {published data only}
- Stevens VJ, Glasgow RE, Hollis JF, Mount K. Implementation and effectiveness of a brief smoking‐cessation intervention for hospital patients. Medical Care 2000;38(5):451‐9. [DOI] [PubMed] [Google Scholar]
Williams 2006 {published data only}
- Figueroa‐Moseley CD, Williams GC, Morrow GR, Jean‐Pierre P, Carroll J, Ryan J. Empowered to stop smoking: The impact of a SDT smoking cessation intervention on economically disadvantaged whites and blacks. Journal of Clinical Oncology 2006;24(18 Suppl):51S. [Google Scholar]
- Williams GC, McGregor H, Sharp D, Kouides RW, Levesque CS, Ryan RM, et al. A self‐determination multiple risk intervention trial to improve smokers' health. Journal of General Internal Medicine 2006;21(12):1288‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams GC, McGregor HA, Sharp D, Levesque C, Kouides RW, Ryan RM, et al. Testing a self‐determination theory intervention for motivating tobacco cessation: supporting autonomy and competence in a clinical trial. Health Psychology 2006;25:91‐101. [DOI] [PubMed] [Google Scholar]
- Williams GC, Minicucci DS, Kouides RW, Levesque CS, Chirkov VI, Ryan RM, et al. Self‐determination, smoking, diet and health. Health Education Research 2002;17(5):512‐21. [DOI] [PubMed] [Google Scholar]
Wittchen 2011 {published data only}
- Wittchen HU, Hoch E, Klotsche J, Muehlig S. Smoking cessation in primary care ‐ a randomized controlled trial of bupropione, nicotine replacements, CBT and a minimal intervention. International Journal of Methods in Psychiatric Research 2011;20(1):28‐39. [CENTRAL: 799983; CRS: 9400123000009843; 6422; PUBMED: 21574208] [DOI] [PMC free article] [PubMed] [Google Scholar]
Woodruff 2002 {published data only}
- Woodruff SI, Talavera GA, Elder JP. Evaluation of a culturally appropriate smoking cessation intervention for Latinos. Tobacco Control 2002;11(4):361‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
Bonevski 2011 {published data only}
- Bonevski B, Paul C, D'Este C, Sanson‐Fisher R, West R, Girgis A, et al. RCT of a client‐centred, caseworker‐delivered smoking cessation intervention for a socially disadvantaged population. BMC Public Health 2011;11(1):70. [CENTRAL: 812695; CRS: 9400123000013400; 6412; PUBMED: 21281519] [DOI] [PMC free article] [PubMed] [Google Scholar]
Garvey 2012a {published data only}
- Garvey AJ, Kalman D, Hoskinson RA, Kinnunen T, Armour CD, Copp S, et al. Effects of extended‐duration counseling vs. shorter‐duration counseling after 1.5 years of follow‐up (POS4‐49). Society for Research on Nicotine and Tobacco 18th Annual Meeting March 13‐16, 2012, Houston, Texas. 2012:139. [CENTRAL: 814595; CRS: 9400103000000026]
Additional references
AHCPR 1996
- Fiore MC, Bailey WC, Cohen SJ, et al. Smoking Cessation. Clinical Practice Guideline No 18. AHCPR Publication No. 96‐0692. Rockville (MD): U.S. Department of Health and Human Services, Agency for Health Care Policy and Research, April 1996. [Google Scholar]
Cahill 2014
- Cahill K, Lancaster T. Workplace interventions for smoking cessation. Cochrane Database of Systematic Reviews 2014, Issue 2. [DOI: 10.1002/14651858.CD003440.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chamberlain 2013
- Chamberlain C, O'Mara‐Eves A, Oliver S, Caird JR, Perlen SM, Eades SJ, Thomas J. Psychosocial interventions for supporting women to stop smoking in pregnancy. Cochrane Database of Systematic Reviews 2013, Issue 10. [DOI: 10.1002/14651858.CD001055.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
de Bruin 2016
- Bruin M, Viechtbauer W, Eisma MC, Hartmann‐Boyce J, West R, Bull E, et al. Identifying effective behavioural components of Intervention and Comparison group support provided in SMOKing cEssation (IC‐SMOKE) interventions: a systematic review protocol. Systematic Reviews 2016;5:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fiore 2000
- Fiore MC, Bailey WC, Cohen SJ, et al. Treating Tobacco Use and Dependence. A Clinical Practice Guideline. AHRQ publication No. 00‐0032. Rockville, MD: US Dept of Health and Human Services, 2000. [Google Scholar]
Fiore 2008
- Fiore MC, Jaén CR, Baker TB, et al. Treating Tobacco Use and Dependence: 2008 Update. Clinical Practice Guideline; www.ncbi.nlm.nih.gov/books/NBK63952/. Rockville, MD: U.S. Department of Health and Human Services. Public Health Service, May 2008. [Google Scholar]
Greenland 1985
- Greenland S, Robbins J. Estimation of a common effect parameter from sparse follow‐up data. Biometrics 1985;41(1):55‐68. [PubMed] [Google Scholar]
Hartmann‐Boyce 2014
- Hartmann‐Boyce J, Lancaster T, Stead LF. Print‐based self‐help interventions for smoking cessation. Cochrane Database of Systematic Reviews 2014, Issue 6. [DOI: 10.1002/14651858.CD001118.pub3] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analysis. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lindson‐Hawley 2015
- Lindson‐Hawley N, Thompson TP, Begh R. Motivational interviewing for smoking cessation. Cochrane Database of Systematic Reviews 2015, Issue 3. [DOI: 10.1002/14651858.CD006936.pub3] [DOI] [PubMed] [Google Scholar]
Rice 2013
- Rice VH, Hartmann‐Boyce J, Stead LF. Nursing interventions for smoking cessation. Cochrane Database of Systematic Reviews 2013, Issue 8. [DOI: 10.1002/14651858.CD001188.pub4] [DOI] [PubMed] [Google Scholar]
Rigotti 2012
- Rigotti NA, Clair C, Munafò MR, Stead LF. Interventions for smoking cessation in hospitalised patients. Cochrane Database of Systematic Reviews 2012, Issue 5. [DOI: 10.1002/14651858.CD001837.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stead 2008
- Stead LF, Perera R, Bullen C, Mant D, Lancaster T. Nicotine replacement therapy for smoking cessation. Cochrane Database of Systematic Reviews 2008, Issue 1. [DOI: 10.1002/14651858.CD000146.pub3] [DOI] [PubMed] [Google Scholar]
Stead 2013a
- Stead LF, Buitrago D, Preciado N, Sanchez G, Hartmann‐Boyce J, Lancaster T. Physician advice for smoking cessation. Cochrane Database of Systematic Reviews 2013, Issue 5. [DOI: 10.1002/14651858.CD000165.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stead 2013b
- Stead LF, Hartmann‐Boyce J, Perera T, Lancaster T. Telephone counselling for smoking cessation. Cochrane Database of Systematic Reviews 2013, Issue 8. [DOI: 10.1002/14651858.CD002850.pub3] [DOI] [PubMed] [Google Scholar]
Stead 2015
- Stead LF, Koilpillai P, Lancaster T. Additional behavioural support as an adjunct to pharmacotherapy for smoking cessation. Cochrane Database of Systematic Reviews 2015, Issue 10. [DOI: 10.1002/14651858.CD009670.pub3] [DOI] [PubMed] [Google Scholar]
Stead 2016
- Stead LF, Koilpillai P, Fanshawe TR, Lancaster T. Combined pharmacotherapy and behavioural interventions for smoking cessation. Cochrane Database of Systematic Reviews 2016, Issue 3. [DOI: 10.1002/14651858.CD008286.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stead 2017
- Stead LF, Carroll AJ, Lancaster T. Group behaviour therapy programmes for smoking cessation. Cochrane Database of Systematic Reviews 2017, Issue 3. [DOI: 10.1002/14651858.CD001007.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Lancaster 2002
- Lancaster TR, Stead LF. Individual behavioural counselling for smoking cessation. Cochrane Database of Systematic Reviews 2002, Issue 3. [DOI: 10.1002/14651858.CD001292] [DOI] [PubMed] [Google Scholar]
Lancaster 2005b
- Lancaster T, Stead LF. Individual behavioural counselling for smoking cessation. Cochrane Database of Systematic Reviews 2005, Issue 2. [DOI: 10.1002/14651858.CD001292.pub2] [DOI] [PubMed] [Google Scholar]


