Abstract
Background
Hepatocellular carcinoma is the most common liver neoplasm, the sixth most common cancer worldwide, and the third most common cause of cancer mortality. Moreover, its incidence has increased dramatically in the past decade. While surgical resection and liver transplantation are the main curative treatments, only around 20% of people with early hepatocellular carcinoma may benefit from these therapies. Current treatment options for unresectable hepatocellular carcinoma include various ablative and transarterial therapies in addition to the drug sorafenib.
Objectives
To assess the benefits and harms of external beam radiotherapy in the management of localised unresectable hepatocellular carcinoma.
Search methods
We searched the Cochrane Hepato‐Biliary Group Controlled Trials Register, Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library, MEDLINE (OvidSP), Embase (OvidSP), Science Citation Index Expanded (Web of Science), and clinicaltrials.gov registry. We also checked reference lists of primary original studies and review articles manually for further related articles (cross‐references) up to October 6, 2016.
Selection criteria
Eligible studies included all randomised clinical trials comparing external beam radiotherapy either as a monotherapy or in combination with other systemic or locoregional therapies versus placebo, no treatment, or other systemic or locoregional therapies for people with unresectable hepatocellular carcinoma.
Data collection and analysis
We used standard methodological procedures expected by Cochrane. We used a random‐effects model as well as a fixed‐effect model meta‐analysis but in case of discrepancy between the two models (e.g. one giving a significant intervention effect, the other no significant intervention effect), we reported both results; otherwise, we reported only the results from the fixed‐effect model meta‐analysis. We assessed risk of bias of the included trials using predefined risk of bias domains; assessed risks of random errors with Trial Sequential Analysis; and presented the review results incorporating the methodological quality of the trials using GRADE.
Main results
Nine randomised clinical trials with 879 participants fulfilled our inclusion criteria. All trials were at high risk of bias, and we rated the evidence as low to very low quality. All of the included trials compared combined external beam radiotherapy plus chemoembolisation versus chemoembolisation alone in people with unresectable hepatocellular carcinoma; moreover, three of the trials compared external beam radiotherapy alone versus chemoembolisation alone. All trials were conducted in China. The median age in most of the included trials was around 52 years, and most trial participants were male. The median follow‐up duration ranged from one to three years. None of the trials reported data on cancer‐related mortality, quality of life, serious adverse events, or time to progression of the tumour. For the comparison of radiotherapy plus chemoembolisation versus chemoembolisation alone, the risk ratio for one‐year all‐cause mortality was 0.51 (95% confidence interval (CI) 0.41 to 0.62; P < 0.001; 9 trials; low‐quality evidence); for complete response rate was 2.14 (95% CI 1.47 to 3.13; P < 0.001; 7 trials; low‐quality evidence); and for overall response rate defined as complete response plus partial response was 1.58 (95% CI 1.40 to 1.78; P < 0.001; 7 trials; low‐quality evidence), all in favour of combined treatment with external beam radiotherapy plus transarterial chemoembolisation and seemingly supported by our Trial Sequential Analysis. Additionally, the combined treatment was associated with a higher risk of elevated total bilirubin and elevated alanine aminotransferase. The risk ratio for the risk of elevated alanine aminotransferase was 1.41 (95% CI 1.08 to 1.84; P = 0.01; very low‐quality evidence), while for elevated total bilirubin it was 2.69 (95% CI 1.34 to 5.40; P = 0.005; very low‐quality evidence). For the comparison of radiotherapy versus chemoembolisation, the risk ratio for one‐year all‐cause mortality was 1.21 (95% CI 0.97 to 1.50; 3 trials; I2 = 0%; very low‐quality evidence) which was not supported by our Trial Sequential Analysis.
In addition, we found seven ongoing randomised clinical trials evaluating different external beam radiotherapy techniques for people with unresectable hepatocellular carcinoma.
Authors' conclusions
We found very low‐ and low‐quality evidence suggesting that combined external beam radiotherapy and chemoembolisation may be associated with lower mortality and increased complete and overall response rates, despite an increased toxicity as expressed by a higher rise of bilirubin and alanine aminotransferase. A high risk of systematic errors (bias) as well as imprecision and inconsistency suggest that these findings should be considered cautiously and that high‐quality trials are needed to assess further the role of external beam radiotherapy for unresectable hepatocellular carcinoma.
Plain language summary
Radiotherapy administered externally for advanced hepatocellular carcinoma (primary liver cancer)
Review question
What are the benefits and harms of radiotherapy administered externally in people with advanced liver cancer compared with other available therapies or no therapy?
Background
Hepatocellular carcinoma (primary liver cancer) is the most common cancerous tumour of the liver and the sixth most common cancerous tumour worldwide. In the majority of people with hepatocellular carcinoma, the disease is diagnosed at the advanced stage. Treatment options for these people include ablation (which destroys the tumour), embolisation (the use of substances to block or decrease the flow of blood through the hepatic artery to the tumour), radiotherapy, or sorafenib, which is a targeted drug therapy (a treatment that uses a substance to identify and attack cancer cells while avoiding normal cells).
Study characteristics
We searched the medical literature for randomised clinical trials (where people are allocated at random to one of two or more treatment groups) in order to perform an analysis of the role of radiotherapy administered externally for advanced liver cancer. We found nine randomised clinical trials including a total of 879 people with advanced liver cancer. All of the included trials were conducted in China. The average age in most of the included studies was around 52 years, and most trial participants were male. The average follow‐up duration ranged from one to three years. All trials were at high risk of bias, and we rated the evidence as low to very low quality. Most of the included trials compared combined radiotherapy and chemoembolisation versus chemoembolisation alone. We also identified seven ongoing randomised clinical trials. The evidence is current to October 2016.
Key results
When compared with chemoembolisation alone, combined radiotherapy plus chemoembolisation may be associated with fewer deaths and more tumour size reduction, despite being associated with an increased risk for non‐life‐threatening adverse effects such as a higher rise of bilirubin and alanine aminotransferase.
Quality of the evidence and conclusions
Combined radiotherapy and chemoembolisation may be associated with fewer deaths and increased overall response, but also an increased risk of adverse effects, when compared with chemoembolisation alone.
The low quality of evidence suggests that these results should be considered cautiously and that high‐quality randomised trials should be performed to assess further the role of external beam radiotherapy for unresectable hepatocellular carcinoma.
Summary of findings
Summary of findings for the main comparison. External beam radiotherapy (EBRT) plus transarterial chemoembolisation (TACE) versus TACE alone for unresectable hepatocellular carcinoma.
| External beam radiotherapy (EBRT) plus transarterial chemoembolisation(TACE) versus TACE alone for unresectable hepatocellular carcinoma | ||||||
| Patient or population: people with unresectable hepatocellular carcinoma Setting: specialist hospitals Intervention: EBRT + TACE Comparison: TACE | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with TACE | Risk with EBRT + TACE | |||||
| All‐cause mortality (at maximum 1‐year follow‐up) | Study population | RR 0.51 (0.41 to 0.62) | 786 (9 RCTs) | ⊕⊕⊝⊝ LOW 1 | ||
| 456 per 1000 | 233 per 1000 (187 to 283) | |||||
| Health‐related quality of life | No data were available for this outcome. | |||||
| Serious adverse events | No data were available for this outcome. | |||||
|
Complete response plus partial response Length of follow‐up: 1 year |
Study population | RR 1.58 (1.40 to 1.78) | 620 (7 RCTs) | ⊕⊕⊝⊝ LOW 1 | ||
| 518 per 1000 | 819 per 1000 (726 to 923) | |||||
|
Elevated alanine aminotransferase Length of follow‐up: 1 year |
Study population | RR 1.41 (1.08 to 1.84) | 232 (3 RCTs) | ⊕⊝⊝⊝ VERY LOW 2 | ||
| 319 per 1000 | 449 per 1000 (344 to 586) | |||||
|
Elevated total bilirubin Length of follow‐up: 1 year |
Study population | RR 2.69 (1.34 to 5.40) | 172 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 3 | ||
| 108 per 1000 | 292 per 1000 (145 to 586) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; RCT: randomised clinical trial | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. | ||||||
1Downgraded two levels (‐2) due to: i) within‐study risk of bias: high risk of bias in all included trials; ii) publication bias: cannot be assessed. 2Downgraded three levels (‐3) due to: i) within‐study risk of bias: high risk of bias in all included trials; ii) publication bias: cannot be assessed; iii) heterogeneity: large heterogeneity index (93%); iv) imprecision: small number of trials. 3Downgraded three levels (‐3) due to: i) within‐study risk of bias: high risk of bias in all included trials; ii) publication bias: cannot be assessed; iii) imprecision: small number of trials.
Summary of findings 2. External beam radiotherapy (EBRT) versus transarterial chemoembolisation (TACE) for unresectable hepatocellular carcinoma.
| External beam radiotherapy (EBRT) versus transarterial chemoembolisation(TACE) for unresectable hepatocellular carcinoma | ||||||
| Patient or population: people with unresectable hepatocellular carcinoma Setting: specialist hospitals Intervention: EBRT Comparison: TACE | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with TACE | Risk with EBRT | |||||
| All‐cause mortality (at 1 year) | Study population | RR 1.21 (0.97 to 1.50) | 152 (3 RCTs) | ⊕⊝⊝⊝ VERY LOW1 | ||
| 570 per 1000 | 689 per 1000 (553 to 854) | |||||
| Serious adverse events | No data were available for this outcome. | |||||
|
Complete response plus partial response Length of follow‐up: 1 year |
10 out of 20 trial participants attained partial response in the TACE arm. | 3 out of 21 trial participants attained a response in the EBRT arm. | ‐ | 41 (1 RCT) | ⊕⊝⊝⊝ VERY LOW1 | |
| Elevated alanine aminotransferase | No data were available for this outcome. | |||||
| Elevated total bilirubin | No data were available for this outcome. | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; RCT: randomised clinical trial | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. | ||||||
1Downgraded three levels (‐3) due to i) within‐study risk of bias: high risk of bias in all included trials; ii) publication bias: cannot be assessed; iii) imprecision: small number of trials.
Background
Description of the condition
Liver cancer is the sixth most common cancer worldwide and the second most common cause of cancer mortality, with more than 500,000 deaths annually (Globocan 2012). Rarely detected early, hepatocellular carcinoma, which comprises most primary liver cancers, is usually fatal within a few months of diagnosis (Hassan 2011). According to guidelines from the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver (Bruix 2011; EASL/EORTC 2012), hepatocellular carcinoma diagnosis can be based on biopsy or imaging techniques fulfilling some well‐defined criteria. However, given recent modifications on diagnostic algorithm and different sensitivities of some investigation techniques such as magnetic resonance imaging (MRI), some heterogeneity in the classification of patients and in the prognostic assessment may have been introduced.
Hepatocellular carcinoma is preceded by cirrhosis of the liver in most patients, and, unsurprisingly, common causes of cirrhosis have been identified as key risk factors for hepatocellular carcinoma. Of particular importance is chronic infection with hepatitis B or C virus, as well as chronic alcohol consumption. Indeed, it has been estimated that hepatitis B virus is responsible for 50% to 80% of hepatocellular carcinoma cases worldwide, with 10% to 25% of cases thought to be the result of hepatitis C virus infection (Venook 2010; Maida 2014). Such aetiological issues raise additional safety concerns and considerations with the use of various locoregional (particularly radiation) and systemic therapies and, accordingly, this may limit the available therapeutic options for these patients.
Potentially effective treatments for hepatocellular carcinoma include liver resection, transplantation, radiofrequency ablation, transarterial chemoembolisation and radioembolisation, external beam radiotherapy, in addition to systemic treatment (Oliveri 2011; Taefi 2013; Weis 2013; Abdel‐Rahman 2016). Surgical resection and liver transplantation are the main curative treatments. Unfortunately, only around 20% of patients, mostly diagnosed by regular screening, may benefit from these therapies (Yau 2008; Abdel‐Rahman 2013).
Description of the intervention
Historically, radiotherapy has always played a limited role in the treatment of hepatocellular carcinoma due to the low radiation tolerance of the liver and the subsequent risk of radiation‐induced liver disease (Lee 2014). Technological advancement in radiation planning and treatment delivery, such as stereotactic body radiotherapy combined with image‐guided radiotherapy, has permitted a further increase radiation dose while maximally sparing the surrounding non‐involved liver tissue (Jihye 2012). This along with the growing knowledge of radiobiological models in liver disease have facilitated several mono‐institutional retrospective and prospective series, which are reporting encouraging results. Consequently, external beam radiotherapy might play a significant role for the treatment of unresectable hepatocellular carcinoma, alone or combined with another locoregional treatment such as transarterial chemoembolisation (Oliveri 2011; Ursino 2012). Available techniques currently employed for external beam radiotherapy in hepatocellular carcinoma include conformal radiotherapy, intensity‐modulated radiotherapy, and stereotactic body radiotherapy coupled with image‐guided technologies to account for tumour and normal tissue motion (Dawson 2011; Klein 2013; Tsai 2013).
How the intervention might work
The basic strategy of radiotherapy is based on tumour control and consideration of normal tissue toxicity. In radiotherapy of hepatocellular carcinoma, radiosensitivity of tumour and normal tissues has not been traditionally considered in favour of therapeutic success (Hoffe 2010). Until recently, radiosensitivity of hepatocellular carcinoma was considered low, a notion based on the poor clinical outcomes of early trials that used an insufficient radiation dose for fear of radiation‐induced liver toxicity (Wigg 2010). However, accumulated experience using image‐guided radiotherapy technology with substantially increased radiation doses has resulted in a reconsideration of hepatocellular as radiosensitive, and thus with potential of therapeutic success (Seong 2009).
Why it is important to do this review
Both three‐dimensional conformal radiotherapy and stereotactic body radiation therapy have emerged as promising non‐invasive therapeutic modalities for people with unresectable hepatocellular carcinoma and may potentially serve as a bridging therapy for patients awaiting transplantation (Cárdenes 2009; Ma 2010). External beam radiotherapy can thus be considered as a potentially competitive alternative to other available locoregional therapies such as radiofrequency ablation, transarterial chemoembolisation, and radioembolisation. Moreover, external beam radiotherapy can be used in combination with any of the above mentioned modalities as well as systemic therapies like sorafenib (Abdel‐Rahman 2013). A limited number of clinical trials examining the use of both three‐dimensional conformal radiotherapy and stereotactic body radiation therapy in people with hepatocellular carcinoma have reported very good local tumour control and acceptable toxicities (Merle 2009). Therefore, we considered the conduct of a well‐designed systematic review on the benefits and harms of three‐dimensional conformal radiotherapy or stereotactic body radiotherapy in the management of hepatocellular carcinoma to be of paramount significance. We have been unable to identify meta‐analyses or systematic reviews on the topic based on randomised clinical trials only.
Objectives
To assess the benefits and harms of external beam radiotherapy in the management of localised unresectable hepatocellular carcinoma.
Methods
Criteria for considering studies for this review
Types of studies
Randomised clinical trials comparing external beam radiotherapy used as a monotherapy or in combination with other treatment modalities (if they are also used in the control group) versus placebo, no treatment, or other treatment modalities, and irrespective of publication status (unpublished or published as an article, abstract, or letter), language (both English and non‐English trials), or blinding.
If our searches for randomised clinical trials retrieved quasi‐randomised studies or other observational studies, we considered these for the report on harms.
Types of participants
All trial participants with histologically or radiologically diagnosed advanced (unresectable) hepatocellular carcinoma who were 18 years of age or older.
Types of interventions
External beam radiotherapy (whether used as monotherapy or in combination with other systemic or locoregional therapies and whether used as fractionated three‐dimensional conformal radiotherapy or stereotactic body radiotherapy) versus placebo, no treatment, other systemic therapies, or locoregional therapies.
We included trials using co‐interventions if they were administered equally to all trial intervention groups.
Types of outcome measures
Primary outcomes
All‐cause mortality.
Health‐related quality of life (as reported by the participants and assessed by a standard grading systems).
Serious adverse events as defined according to the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) Guidelines for Good Clinical Practice as any untoward medical occurrence that at any dose resulted in death, was life‐threatening, required inpatient hospitalisation or prolongation of existing hospitalisation, or resulted in persistent or significant disability or incapacity, or was a congenital anomaly/birth defect, or any medical event that might have jeopardised the patient, or required intervention to prevent it (ICH‐GCP 1997).
Secondary outcomes
Cancer‐related mortality.
Time to progression of the tumour (reported as median time to progression).
-
Tumour response assessments (as recommended by the Response Evaluation Criteria In Solid Tumours (RECIST)) (Eisenhauer 2009).
Complete response: disappearance of all target lesions. Any pathological lymph nodes (whether target or non‐target) must have reduction in short axis to less than 10 mm.
Partial response: at least a 30% decrease in the sum of diameters of target lesions, taking as reference the baseline sum diameters.
Progressive disease: at least a 20% increase in the sum of diameters of target lesions, taking as reference the smallest sum on study (this includes the baseline sum if that is the smallest on study). In addition to the relative increase of 20%, the sum must also demonstrate an absolute increase of at least 5 mm. (Note: the appearance of one or more new lesions is also considered progression.)
Stable disease: neither sufficient shrinkage to qualify for partial response nor sufficient increase to qualify for progressive disease, taking as reference the smallest sum diameters while on study.
We also planned to consider the European Association for the Study of the Liver disease response evaluation criteria and the positron emission tomography RECIST whenever appropriate (Riaz 2011; Maffione 2013). Note that we planned to use these criteria as the main tool, and will report if the authors have used other methods.
Non‐serious adverse events: all adverse events not fulfilling the above definition of serious adverse events were considered as non‐serious adverse events.
Liver‐related adverse events: these involved clinical (e.g. encephalopathy or ascites) or laboratory (e.g. elevated total bilirubin, elevated alanine aminotransferase) evidence of liver‐related morbidity.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Hepato‐Biliary Group Controlled Trials Register (Gluud 2017), the Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library (2016, Issue 9), MEDLINE (OvidSP), Embase (Ovid SP), Science Citation Index Expanded (Web of Science), and clinicaltrials.gov registry (Royle 2003), from their dates of inception to October 6, 2016. Search strategies with the time spans of the searches can be found in Appendix 1.
Searching other resources
We checked reference lists of primary original studies and review articles manually to identify further related articles (cross‐references).
Data collection and analysis
We performed the review according to the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions and the Cochrane Hepato‐Biliary Group Module (Higgins 2011; Gluud 2017). We performed data analysis using the Cochrane statistical software, Review Manager 5, RevMan 2014, and Trial Sequential Analysis software (TSA 2011). Any disagreements were resolved by consensus.
Selection of studies
The two review authors independently identified the trials for inclusion. We listed the excluded studies with their reasons for exclusion. We excluded duplicate records based on review of titles. We reviewed the abstracts of the remaining, excluding studies using duplicate participant data sets. We reviewed the full text of the remaining articles for relevancy to the review.
Data extraction and management
The two review authors independently extracted the required data. We extracted details of study population, interventions, and outcomes using a standardised data extraction form, which included at least the following items.
Publication year
Country
Year of conduct of the trial
Inclusion and exclusion criteria
Whether sample size calculation was performed
Population characteristics such as age and sex ratio
Baseline characteristics including proportion of cirrhosis expressed as the Child‐Pugh class, Eastern Cooperative Oncology Group (ECOG) performance status, Barcelona Clinic Liver Cancer stage, proportion of participants positive for hepatitis B or C virus, or both
Outcomes (Types of outcome measures)
Risk of bias (Assessment of risk of bias in included studies)
Whether intention‐to‐treat analysis was performed
Radiotherapy modality, target tumour dose, and criteria used for toxicity grading
Assessment of risk of bias in included studies
The two review authors independently assessed the risk of bias of each included trial according to the recommendations given in the Cochrane Handbook for Systematic Reviews of Interventions and the Cochrane Hepato‐Biliary Group Module (Higgins 2011; Gluud 2017). We used the following definitions in the assessment of risk of bias (Schulz 1995; Moher 1998; Kjaergard 2001; Wood 2008; Lundh 2017; Savović 2012; Savović 2012a).
Allocation sequence generation
Low risk of bias: sequence generation was achieved using computer random number generation or a random number table. Drawing lots, tossing a coin, shuffling cards, and throwing dice are adequate if performed by an independent person not otherwise involved in the trial.
Unclear risk of bias: the method of sequence generation was not specified.
High risk of bias: the sequence generation method was not random. Such studies were included only for assessments of harms.
Allocation concealment
Low risk of bias: the participant allocations could not have been foreseen in advance of, or during, enrolment. Allocation was controlled by a central and independent randomisation unit. The allocation sequence was unknown to the investigators (e.g. if the allocation sequence was hidden in sequentially numbered, opaque, and sealed envelopes).
Unclear risk of bias: the method used to conceal the allocation was not described, so that intervention allocations may have been foreseen in advance of, or during, enrolment.
High risk of bias: the allocation sequence was likely to be known to the investigators who assigned the participants. Such studies were included only for assessments of harms.
Blinding of participants and personnel
Low risk of bias: it was mentioned that both participants and personnel providing the interventions were blinded, and the method of blinding was described, so that knowledge of allocation was prevented during the trial.
Unclear risk of bias: it was not mentioned if the trial was blinded, or the trial was described as blinded, but the method or extent of blinding was not described, so that knowledge of allocation was possible during the trial.
High risk of bias: the trial was not blinded, so that the allocation was known during the trial.
Blinded outcome assessment
Low risk of bias: outcome assessment was carried out blinded for all relevant outcomes, and the method of blinding was described, so that knowledge of allocation was prevented.
Unclear risk of bias: blinding of outcome assessment was not described, or the outcome assessment was described as blinded, but the method of blinding was not described, so that knowledge of allocation was possible.
High risk of bias: outcome assessment was not blinded, so that the allocation was known to outcome assessors.
Incomplete outcome data
Low risk of bias: missing data were unlikely to make treatment effects depart from plausible values. Sufficient methods, such as multiple imputation, were employed to handle missing data.
Unclear risk of bias: there was insufficient information to assess whether missing data in combination with the method used to handle missing data were likely to induce bias on the results.
High risk of bias: the results were likely to be biased due to missing data.
Selective outcome reporting
Low risk: the trial reported the following predefined outcomes: all‐cause mortality, serious adverse events, and cancer‐related mortality. If the original trial protocol was available, the outcomes should be those called for in that protocol. If the trial protocol was obtained from a trial registry (e.g. ClinicalTrials.gov), the outcomes sought should have been those enumerated in the original protocol if the trial protocol was registered before or at the time that the trial was begun. If the trial protocol was registered after the trial was begun, those outcomes were not considered reliable.
Unclear risk: not all predefined outcomes were reported fully, or it was unclear whether data on these outcomes were recorded or not.
High risk: one or more predefined outcomes was not reported.
For‐profit bias
Low risk of bias: the trial appeared to be free of industry sponsorship or other type of for‐profit support that may manipulate the design, conductance, or results of the trial.
Unclear risk of bias: the trial may or may not be free of for‐profit bias, as no information on clinical trial support or sponsorship was provided.
High risk of bias: the trial was sponsored by industry or received other type of for‐profit support.
Other bias
Low risk of bias: the trial appeared to be free of other factors that could put it at risk of bias.
Unclear risk of bias: the trial may or may not have been free of other factors that could put it at risk of bias.
High risk of bias: there were other factors in the trial that could put it at risk of bias.
We considered trials assessed as having 'low risk of bias' in all of the specified individual domains as 'trials at low risk of bias'. We considered trials assessed as having 'uncertain risk of bias' or 'high risk of bias' in one or more of the specified individual domains as trials at 'high risk of bias'.
Measures of treatment effect
For dichotomous variables, we planned to calculate risk ratios (RR) with 95% confidence intervals (CI). For continuous variables, we planned to calculate mean differences (MD) or standardised mean differences (SMD) with 95% CI. We planned to base time‐to‐event data analyses on hazard ratios (HR) (Parmar 1998). As no time to event data were reported in the included trials, we could not calculate HRs in this systematic review.
Unit of analysis issues
The unit of analysis were the participants with unresectable hepatocellular carcinoma according to the intervention groups they were randomised to. In the case of cross‐over trials, we planned to use the data from the first trial period only. In case of trials with more than two intervention groups, we produced two different analyses, comparing the intervention group with the common control group.
Dealing with missing data
When we could not extract data from the text, or a statistic was missing, we contacted the authors of the original article to provide the necessary information.
Intention‐to‐treat analyses
Regarding the primary outcomes, we planned to include participants with incomplete or missing data in sensitivity analyses by imputing them according to the following scenarios (Hollis 1999).
Poor outcome analysis: assuming that dropouts/participants lost from both the experimental and control arms experienced the outcome, including all randomised participants in the denominator.
Good outcome analysis: assuming that none of the dropouts/participants lost from the experimental and control arms experienced the outcome, including all randomised participants in the denominator.
Extreme case analysis favouring the experimental intervention ('best‐worse' case scenario): none of the dropouts/participants lost from the experimental arm, but all of the dropouts/participants lost from the control arm experienced the outcome, including all randomised participants in the denominator.
Extreme case analysis favouring the control ('worst‐best' case scenario): all dropouts/participants lost from the experimental arm, but none from the control arm experienced the outcome, including all randomised participants in the denominator.
Assessment of heterogeneity
We used the Chi2 test to assess between‐trial heterogeneity. In addition, we quantified the degree of heterogeneity observed in the results using the I2 statistic, which can be interpreted as the percentage of variation observed between the trials attributable to between‐trial differences rather than sampling error (chance). We planned to perform a subgroup analysis in order to compare the intervention effect in trials with low risk of bias to that of trials with unclear or high risk of bias (i.e. trials that lack one or more adequate domain). We based analysis on intention‐to‐treat data, to the degree permitted by the published data.
Assessment of reporting biases
We carried out a comprehensive search in order to minimise publication bias. We planned to use a funnel plot to explore bias in the case of at least 10 included trials (Egger 1997; Macaskill 2001). However, as the number of included trials was less than 10, we did not perform funnel plot analysis.
Data synthesis
Meta‐analysis
For meta‐analyses, we used a random‐effects model as well as a fixed‐effect model meta‐analysis (Mantel 1959; DerSimonian 1986; DeMets 1987). In case of discrepancy between the two models (e.g. one giving a significant intervention effect, the other no significant intervention effect), we reported both results; otherwise, we reported only the results from the fixed‐effect model meta‐analysis.
Trial Sequential Analysis
We examined apparently significant beneficial and harmful intervention effects and neutral effects with Trial Sequential Analysis in order to evaluate if these apparent effects could be caused by random error ('play of chance') (Brok 2008; Wetterslev 2008; Brok 2009; Thorlund 2009; Wetterslev 2009; Thorlund 2010; Thorlund 2011; TSA 2011).
We applied Trial Sequential Analysis (as cumulative meta‐analyses are at risk of producing random errors due to sparse data and repetitive testing of the accumulating data) (Wetterslev 2008; Wetterslev 2009). To control random errors, we calculated the required information size (i.e. the number of participants needed in a meta‐analysis to detect or reject a certain intervention effect). The required information size calculation should also account for the diversity present in the meta‐analysis (Wetterslev 2009). In our meta‐analysis, the diversity‐adjusted required information size was based on the event proportion in the control group; assumption of a plausible risk reduction of 20%; a risk of type I error of 5%; a risk of type II error of 10%; and the assumed diversity of the meta‐analysis. The underlying assumption of Trial Sequential Analysis is that testing for significance may be performed each time a new trial is added to the meta‐analysis. We added the trials according to the year of publication, and if more than one trial had been published in a year, trials were added alphabetically according to the last name of the first author. On the basis of the diversity‐adjusted required information size, we constructed the trial sequential monitoring boundaries (Thorlund 2011). These boundaries determine the statistical inference one may draw regarding the cumulative meta‐analysis that has not reached the required information size. If the cumulative Z‐curve crosses the trial sequential monitoring boundary for benefit or harm before the diversity‐adjusted required information size was reached, firm evidence may perhaps be established and further trials may turn out to be superfluous. On the other hand, if the boundary is not surpassed, it is most likely necessary to continue performing trials in order to detect or reject a certain intervention effect, which could be determined by assessing if the cumulative Z‐curve crossed the trial sequential monitoring boundaries for futility.
Subgroup analysis and investigation of heterogeneity
We planned to perform subgroup analysis to explore treatment effect differences by comparing:
trials at low risk of bias compared to trials at high risk of bias;
participants with Child‐Pugh class A unresectable hepatocellular carcinoma compared to participants with Child‐Pugh class B unresectable hepatocellular carcinoma;
ECOG performance score 0 compared to score 1 or 2;
trials using stereotactic body radiotherapy compared to trials using three‐dimensional conformal radiotherapy;
trials using radiotherapy as a monotherapy compared to trials using a combination of radiotherapy and other modalities.
Sensitivity analysis
We planned to perform sensitivity analyses according to identified clinical and methodological variations, such as tumour response assessments, adequacy of allocation concealment, and incomplete reporting of the first primary outcome. However, we were unable to perform these sensitivity analyses due to missing data.
Summary of findings tables
We presented the evidence in 'Summary of findings' tables using GRADEprofiler software (GRADEpro 2008). For the GRADE outcomes, we used the definitions given in Table 3. We evaluated the quality of the evidence for outcomes reported in the review considering the within‐study risk of bias (methodological quality), indirectness of evidence, heterogeneity, imprecision of effect estimate, and risk of publication bias (Balshem 2011; Guyatt 2011; Guyatt 2011a; Guyatt 2011b; Guyatt 2011c; Guyatt 2011d; Guyatt 2011e; Guyatt 2011f; Guyatt 2011g; Guyatt 2013; Guyatt 2013a; Guyatt 2013b; Guyatt 2013c; Mustafa 2013). We presented the following outcomes in the 'Summary of findings' tables: all‐cause mortality at one year, health‐related quality of life, serious adverse events, complete response plus partial response, elevated alanine aminotransferase, and elevated total bilirubin (Table 1; Table 2).
1. Explanations for the 'Summary of findings' table.
| Examples from table | Explanation |
| Outcomes | The tables provide the findings for the most important outcomes for someone making a decision. These include potential benefits and harms, whether the included studies provide data for these outcomes or not. Additional findings may be reported elsewhere in the review. |
| Assumed control group risk | Assumed control group risks can be based either on the control group risks reported in the included studies or on epidemiological data from elsewhere. When only one control group risk is provided, it is normally the median control group risk across the studies that provided data for that outcome. Risk is the probability of an outcome occurring. The control group risk is the risk of an outcome occurring in the comparison group (without the intervention). |
| Corresponding intervention group risk | Risk is the probability of an outcome occurring. The intervention group risk is the risk of an outcome occurring in the group receiving the intervention. |
| Relative effect |
Relative effect or RR (risk ratio) Relative effects are ratios. Here the relative effect is expressed as a risk ratio. Risk is the probability of an outcome occurring. A RR is the ratio between the risk in the intervention group and the risk in the control group. If the risk in the control group is 10% (100 per 1000) and the risk in the intervention group is 1% (10 per 1000), the RR is 10/100 or 0.10. If the RR is exactly 1.0, this means that there is no difference between the occurrence of the outcome in the intervention and the control group. It is unusual for the RR to be exactly 1.0, and understanding what it means if it is above or below this value depends on whether the outcome being counted is judged to be good or bad. If the RR is greater than 1.0, the intervention increases the risk of the outcome. If it is a good outcome (e.g. the birth of a healthy baby), a RR > 1.0 indicates a desirable effect for the intervention, whereas if the outcome is bad (e.g. death), a RR > 1.0 indicates an undesirable effect. If the RR is less than 1.0, the intervention decreases the risk of the outcome. This indicates a desirable effect if it is a bad outcome (e.g. death) and an undesirable effect if it is a good outcome (e.g. birth of a healthy baby). |
|
What is the difference between absolute and relative effects? The effect of an intervention can be described by comparing the risk of the intervention group with the risk of the control group. Such a comparison can be made in different ways. One way to compare two risks is to calculate the difference between the risks. This is the absolute effect. Consider the risk for blindness in a person with diabetes over a five‐year period. If the risk for blindness is found to be 20 in 1000 (2%) in a group of people treated conventionally and 10 in 1000 (1%) in people treated with a new drug, the absolute effect is derived by subtracting the intervention group risk from the control group risk: 2% ‐ 1% = 1%. Expressed in this way, it can be said that the new drug reduces the five‐year risk for blindness by 1% (absolute effect is 10 fewer per 1000). Another way to compare risks is to calculate the ratio of the two risks. Given the data above, the relative effect is derived by dividing the two risks, with the intervention risk being divided by the control risk: 1% ÷ 2% = ½ (0.50). Expressed in this way, as the 'relative effect', the five‐year risk for blindness with the new drug is 1/2 the risk with the conventional drug. Here the table presents risks as x per 1000 (or 100, etc.) instead of %, as this tends to be easier to understand. Whenever possible, the table presents the relative effect as the RR. The absolute effect is usually different for groups that are at high and low risk, whereas the relative effect is often the same, therefore, when it is relevant, we have reported indicative risks for groups at different levels of risk. Two or three indicative control group risks and the corresponding intervention group risks are presented when there are important differences across different populations. | |
| Mean difference | The mean difference (MD) is the average difference between the intervention group and the control group across studies. Here a weighted MD is used, which means the results of some of the studies make a greater contribution to the average than the results of others. Studies with more precise estimates for their results (narrower confidence intervals) are given more weight. This way of measuring effect is used when combining or comparing data for continuous outcomes such as weight, blood pressure, or pain measured on a scale. When different scales are used to measure the same outcome, e.g. different pain scales, a standardised mean difference (SMD) may be provided. This is a weighted mean difference standardised across studies giving the average difference in standard deviations for the measures of that outcome. |
| Confidence interval | A confidence interval (CI) is a range around an estimate that conveys how precise the estimate is; in this example the result is the estimate of the intervention group risk. The CI is a guide to how sure we can be about the quantity we are interested in (here the true absolute effect). The narrower the range between the two numbers, the more confident we can be about what the true value is; the wider the range, the less sure we can be. The width of the CI reflects the extent to which chance may be responsible for the observed estimate (with a wider interval reflecting more chance). |
| 95% confidence interval | As explained above, the CI indicates the extent to which chance may be responsible for the observed numbers. In the simplest terms, a 95% CI means that we can be 95% confident that the true size of effect is between the lower and upper confidence limit (e.g. 0 and 3 in the blindness drugs example mentioned above). Conversely, there is a 5% chance that the true effect is outside of this range. |
| Not statistically significant | Statistically significant means that a result is unlikely to have occurred by chance. The usual threshold for this judgement is that the results, or more extreme results, would occur by chance with a probability of less than 0.05 if the null hypothesis (no effect) was true. When results are not statistically significant, as in this example, this is stated to alert users to the possibility that the results may have occurred by chance. |
| No. of participants (studies) | The table provides the total number of participants across studies and the number of studies that provided data for that outcome. This indicates how much evidence there is for the outcome. |
| Quality of the evidence | The quality of the evidence is a judgement about the extent to which we can be confident that the estimates of effect are correct. These judgements are made using the GRADE system, and are provided for each outcome. The judgements are based on the type of study design (randomised trials versus observational studies), the risk of bias, the consistency of the results across studies, and the precision of the overall estimate across studies. For each outcome, the quality of the evidence is rated as high, moderate, low, or very low using the following definitions:
|
| ‐ | A "‐" indicates that the information is not relevant. |
Results
Description of studies
We identified 6415 citations from our database searches. See: Characteristics of included studies; Characteristics of excluded studies.
Results of the search
Among the identified 6415 citations, we removed 3304 references that were duplicates. We scanned a final number of 3111 references. We excluded 3097 references based on titles and abstracts and retrieved 14 full‐text papers for review (Figure 1). Based on the full papers, we found nine randomised clinical trials eligible for inclusion in our systematic review (Xue 1995; Leng 2000; Peng 2000; Wang 2000; Zhao 2006; Shang 2007; Xiao 2008; Liao 2010; Zhang 2012). All trials were conducted in China. The median age in most of the included trials was around 52 years, and most of the trial participants were male. The median follow‐up duration varied among the trials, ranging from one to three years.
1.
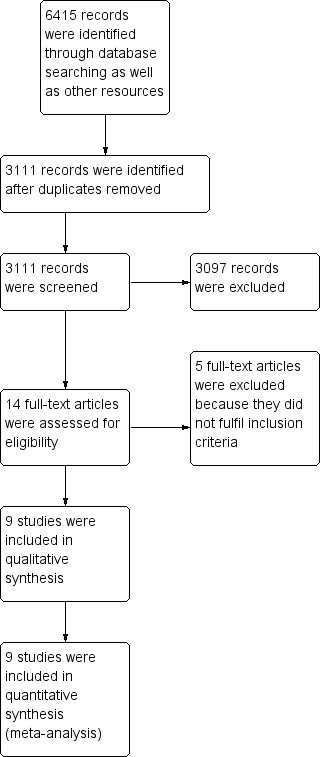
Study flow diagram.
We excluded the five remaining studies (Li 2003; Lan 2005; Wang 2006; Kang 2014; Bush 2016). We identified no additional trials from other sources. Seven trials are still ongoing (see Characteristics of ongoing studies)
Included studies
We included and analysed nine trials in this systematic review: six trials randomised participants to two treatment groups, two trials randomised participants to three treatment groups, and one trial randomised participants to four treatment groups. The trials included a total of 879 randomised participants. All of the included trials compared combined external beam radiotherapy plus chemoembolisation versus chemoembolisation alone in people with unresectable hepatocellular carcinoma. Additionally, three of the nine trials compared external beam radiotherapy alone versus chemoembolisation alone (Xue 1995; Leng 2000; Wang 2000). Five trials with 371 participants compared three‐dimensional conformal radiotherapy plus transarterial chemoembolisation versus transarterial chemoembolisation (Peng 2000; Zhao 2006; Shang 2007; Xiao 2008; Liao 2010). Two trials with 167 participants compared three‐dimensional conformal radiotherapy plus transarterial chemoembolisation versus radiotherapy alone or versus transarterial chemoembolisation alone (Leng 2000; Wang 2000). Another trial compared gamma knife irradiation plus transarterial chemoembolisation versus transarterial chemoembolisation alone (Zhang 2012). The Xue 1995 trial included 82 participants, allocated to four groups evaluating technically different combinations of external beam radiotherapy with transarterial chemoembolisation. We contacted the study authors for whom we found contact emails, but we have not yet received a reply (see Notes of Characteristics of included studies). We found no eligible trials that compared external beam radiotherapy versus best supportive care, systemic chemotherapy, radioembolisation, cryotherapy, laser‐induced thermotherapy, radiofrequency ablation, or high‐frequency ultrasound.
Excluded studies
We excluded five studies as they did not fulfil our inclusion criteria. Three studies were not randomised clinical trials (Li 2003; Lan 2005; Wang 2006), while the fourth study included radiotherapy treatment in the three randomised arms, which prevents meaningful assessment of radiotherapy benefits and harms (Kang 2014). All participants included in the fifth study were eligible for transplantation (participants were required to have disease within the Milan or San Francisco criteria, without vascular invasion) (Bush 2016).
Risk of bias in included studies
Allocation
Allocation sequence generation
Generation of the allocation sequence was unclearly reported in six of the included trials (Xue 1995; Leng 2000; Zhao 2006; Shang 2007; Liao 2010; Zhang 2012). Three trials were at low risk of bias for allocation sequence generation (Peng 2000; Wang 2000; Xiao 2008).
Allocation concealment
Allocation concealment was unclear in the nine included trials.
Blinding
Due to the differences in the nature of the procedures, reasonable blinding to an investigator or an informed patient could not be achieved. We thus judged there to be a high risk of performance bias for all of the included trials. We found detection bias to be low in four of the included trials (Shang 2007; Xiao 2008; Liao 2010; Zhang 2012); high in four trials (Xue 1995; Leng 2000; Peng 2000; Zhao 2006); and unclear in one trial (Wang 2006).
Incomplete outcome data
There was insufficient information to assess whether there were missing data in all of the included trials except for the Liao 2010 trial, where there were no missing data.
Selective reporting
There was a high risk of reporting bias in all of the included trials.
Other potential sources of bias
For‐profit bias
Profit bias was unclear in all included trials except for the Liao 2010 trial, which was assessed as at low risk of bias.
Other bias
We judged the domain other sources of bias as unclear in all of the included trials.
Overall risk of bias
The overall risk of bias was high in all of the included trials (Figure 2; Figure 3).
2.
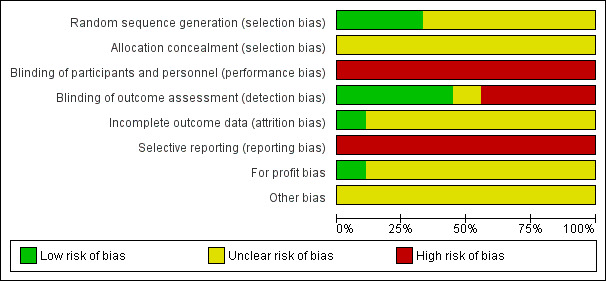
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
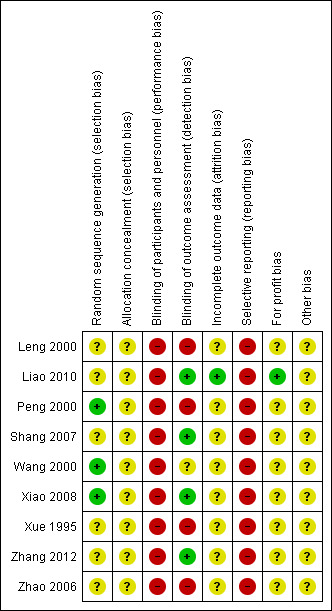
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Effects of interventions
All‐cause mortality
All nine included trials reported total number of deaths at one year (and thus, all‐cause mortality at one year). We conducted a meta‐analysis of one‐year all‐cause mortality among all trials that compared external beam radiotherapy plus transarterial chemoembolisation versus transarterial chemoembolisation alone. The meta‐analysed risk ratio for one‐year all‐cause mortality was 0.51 (95% CI 0.41 to 0.62; 786 participants; 9 trials; I2 = 0%; P < 0.001; low‐quality evidence) There was no statistically significant subgroup difference between treatment with three‐dimensional conformal radiotherapy compared to stereotactic body radiotherapy (see Analysis 1.1).
1.1. Analysis.
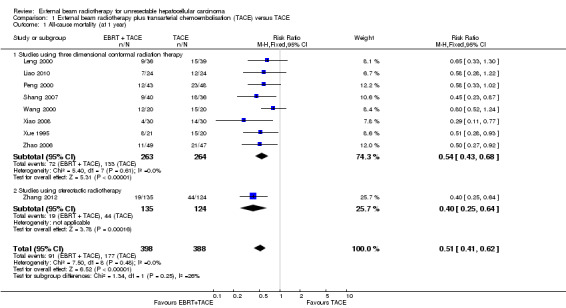
Comparison 1 External beam radiotherapy plus transarterial chemoembolisation (TACE) versus TACE, Outcome 1 All‐cause mortality (at 1 year).
In order to detect or reject a risk reduction of 20%, the diversity‐adjusted required information size was n = 1029 trial participants, calculated based upon a proportion of death of 45% of participants in the transarterial chemoembolisation group, an alpha (type I error) of 5%, a beta (type II error) of 10%, and a diversity of 0%. Using the random‐effects model Trial Sequential Analysis, the resulting cumulative test statistic (Z‐score) crossed the adjusted threshold for statistical significance, thus confirming the significant difference between three‐dimensional conformal external beam radiotherapy plus transarterial chemoembolisation compared with transarterial chemoembolisation monotherapy regarding all‐cause mortality at one year (Figure 4).
4.
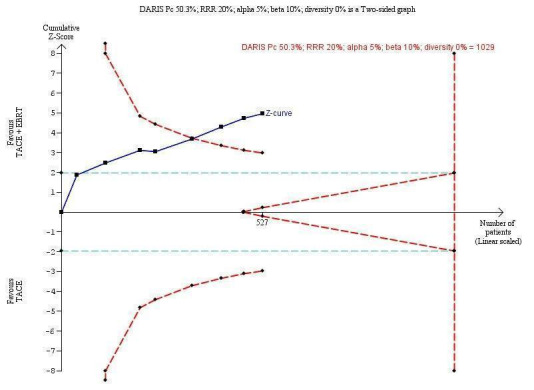
Trial Sequential Analysis comparing external beam radiotherapy (EBRT) plus transarterial chemoembolisation (TACE) versus TACE alone on the outcome 'all‐cause mortality at one year'. A subgroup of studies used three‐dimensional conformal radiotherapy. The diversity‐adjusted required information size (DARIS) of n = 1029 patients was calculated based upon a proportion of mortality of 50.3% of patients in the TACE group, a relative risk reduction of 20% in the EBRT + TACE group, an alpha (type I error) of 5%, a beta (type II error) of 10%, and a diversity of 0%. The blue curve presents the cumulative meta‐analysis Z‐score, and the inward‐sloping dotted red curves present the adjusted threshold for statistical significance according to the two‐sided trial sequential monitoring boundaries.
Moreover, in three of the nine included trials an additional group received external beam radiotherapy as a monotherapy (Xue 1995; Leng 2000; Wang 2000). It was thus feasible to compare one‐year all‐cause mortality for external beam radiotherapy as a monotherapy versus transarterial chemoembolisation alone. The risk ratio for one‐year all‐cause mortality using a fixed‐effect model was 1.21 (95% CI 0.97 to 1.50; 152 participants; 3 trials; P = 0.09; I2 = 0%) and using a random‐effects model was 1.22 (95% CI 1.01 to 1.48; 152 participants; 3 trials; P = 0.04; I2 = 0%) (see Analysis 2.1). The quality of evidence was very low.
2.1. Analysis.
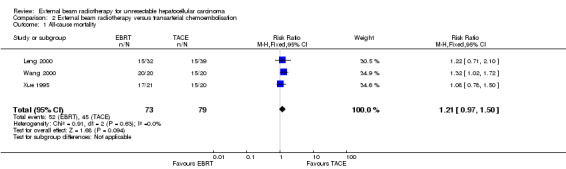
Comparison 2 External beam radiotherapy versus transarterial chemoembolisation, Outcome 1 All‐cause mortality.
In order to detect or reject a risk reduction of 20%, the diversity‐adjusted required information size was n = 808 trial participants, calculated based upon a proportion of death of 57% of participants in the transarterial chemoembolisation group, an alpha (type I error) of 5%, a beta (type II error) of 10%, and a diversity of 0%. Using the random‐effects model Trial Sequential Analysis, the resulting cumulative test statistic (Z‐score) did not cross the adjusted threshold for statistical significance (Figure 5).
5.
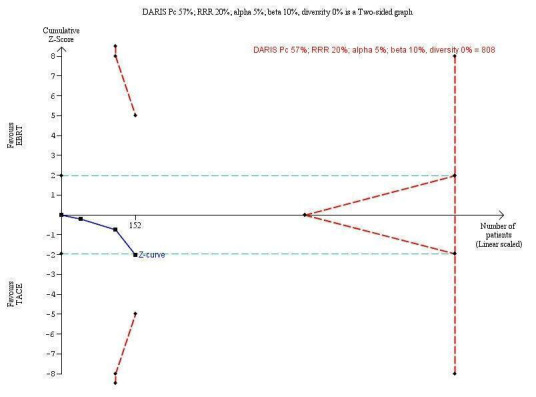
Trial Sequential Analysis comparing external beam radiotherapy (EBRT) versus transarterial chemoembolisation (TACE) on the outcome 'all‐cause mortality at one year'. The diversity‐adjusted required information size (DARIS) of n = 808 patients was calculated based upon a proportion of mortality of 57% of patients in the TACE group, a relative risk reduction of 20% in the EBRT group, an alpha (type I error) of 5%, a beta (type II error) of 10%, and a diversity of 0%. The blue curve presents the cumulative meta‐analysis Z‐score, and the inward‐sloping dotted red curves present the adjusted threshold for statistical significance according to the two‐sided trial sequential monitoring boundaries.
Health‐related quality of life
None of the included trials reported health‐related quality of life.
Serious adverse events
None of the included trials reported serious adverse events.
Cancer‐related mortality
None of the included trials reported cancer‐related mortality.
Time to progression of the tumour
None of the included trials reported time to progression of the tumour.
Tumour response assessments
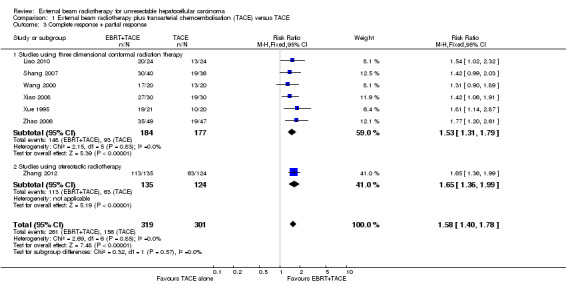
Seven of the included trials reported on tumour response (Xue 1995; Wang 2000; Zhao 2006; Shang 2007; Xiao 2008; Liao 2010; Zhang 2012). We conducted meta‐analyses of complete response rate and overall response rate (defined as complete response plus partial response) among these seven trials, all of which compared external beam radiotherapy plus transarterial chemoembolisation versus transarterial chemoembolisation alone. The risk ratio for complete response rate was 2.14 (95% CI 1.47 to 3.13; 620 participants; 7 trials; P < 0.001; I2 = 0%; low‐quality evidence). There was no statistically significant subgroup difference between treatment with three‐dimensional conformal radiotherapy and stereotactic body radiotherapy (see Analysis 1.2). The risk ratio for overall response rate was 1.58 (95% CI 1.40 to 1.78; 620 participants; 7 trials; P < 0.001; I2 = 0%; low‐quality evidence). There was no statistically significant subgroup difference between treatment with three‐dimensional conformal radiotherapy and stereotactic body radiotherapy (see Analysis 1.3).
1.2. Analysis.
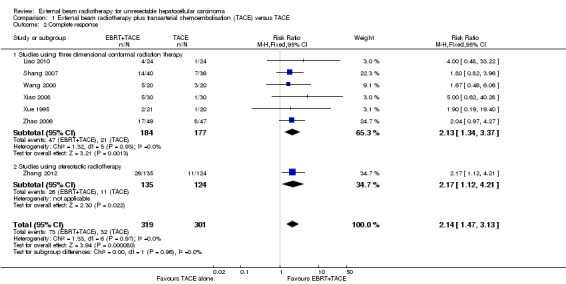
Comparison 1 External beam radiotherapy plus transarterial chemoembolisation (TACE) versus TACE, Outcome 2 Complete response.
1.3. Analysis.
Comparison 1 External beam radiotherapy plus transarterial chemoembolisation (TACE) versus TACE, Outcome 3 Complete response + partial response.
In order to detect or reject a risk reduction of 20% for overall response rate (complete response plus partial response), the diversity‐adjusted required information size was n = 951 participants based upon a proportion of overall response of 51.8% of participants in the transarterial chemoembolisation group, an alpha (type I error) of 5%, a beta (type II error) of 10%, and a diversity of 0%. Using the random‐effects model Trial Sequential Analysis, the resulting cumulative test statistic (Z‐score) crossed the adjusted threshold for statistical significance, thus confirming the significant difference between three‐dimensional conformal external beam radiotherapy plus transarterial chemoembolisation versus transarterial chemoembolisation alone regarding overall response rate (Figure 6).
6.
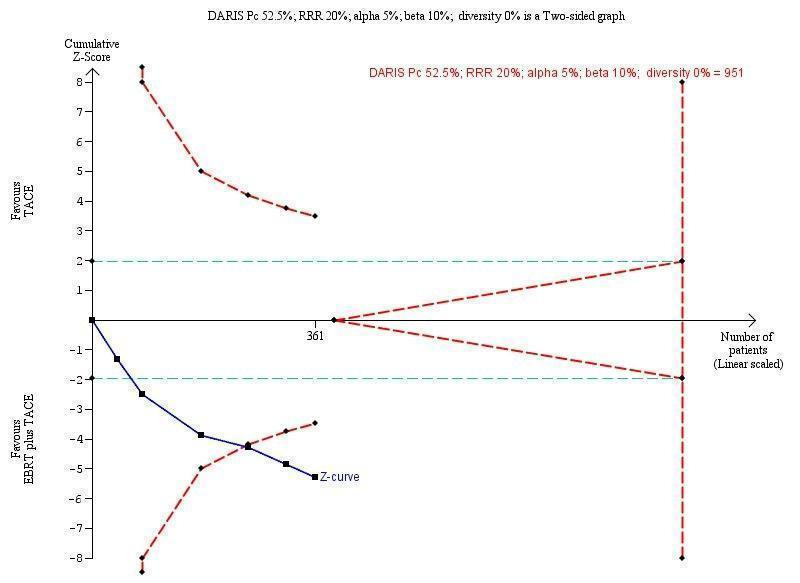
Trial Sequential Analysis comparing external beam radiotherapy (EBRT) plus transarterial chemoembolisation (TACE) versus TACE alone on the outcome 'complete response plus partial response ‐ subgroup of studies using three‐dimensional conformal radiotherapy'. The diversity‐adjusted required information size (DARIS) of n = 951 patients was calculated based upon a proportion of response of 52.5% of patients in the TACE group, a relative risk reduction of 20% in the TACE + EBRT group, an alpha (type I error) of 5%, a beta (type II error) of 10%, and a diversity of 0%. The blue curve presents the cumulative meta‐analysis Z‐score, and the inward‐sloping red curves present the adjusted threshold for statistical significance according to the two‐sided trial sequential monitoring boundaries.
Among the three studies comparing external beam radiotherapy as a monotherapy versus transarterial chemoembolisation alone (Xue 1995; Leng 2000; Wang 2000), only Xue 1995 reported on tumour response. It was therefore not feasible to establish a meta‐analysed risk ratio for this comparison.
European Association for the Study of the Liver disease response evaluation criteria and the positron emission tomography response criteria in solid tumours
None of the included trials reported on this outcome.
Non‐serious adverse events
Collectively the reporting of non‐serious adverse events in the included trials was poor and inconsistent, thus formal evaluation of this outcome was not possible.
Liver‐related adverse events
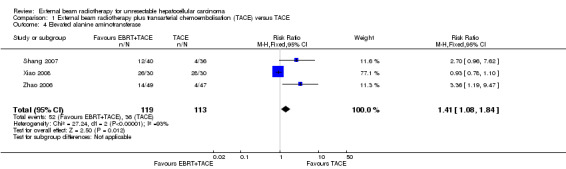
Three trials reported elevated alanine aminotransferase (Zhao 2006; Shang 2007; Xiao 2008), and the meta‐analysed risk ratio for elevated alanine aminotransferase was 1.41 (95% CI 1.08 to 1.84; P = 0.01; very low‐quality evidence) (see Analysis 1.4). It should be noted that the I2 statistic was 93%, due to the Xiao 2008 trial reporting more elevated alanine aminotransferase in the transarterial chemoembolisation group compared with the combined external beam radiotherapy plus transarterial chemoembolisation.
1.4. Analysis.
Comparison 1 External beam radiotherapy plus transarterial chemoembolisation (TACE) versus TACE, Outcome 4 Elevated alanine aminotransferase.
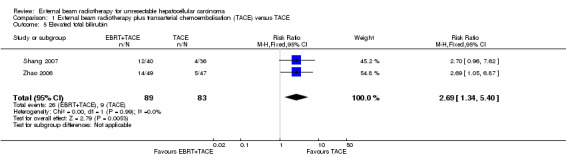
Two trials reported elevated total bilirubin (Zhao 2006; Shang 2007), and the risk ratio for elevated total bilirubin was 2.69 (95% CI 1.34 to 5.40; P = 0.005; very low‐quality evidence) (see Analysis 1.5).
1.5. Analysis.
Comparison 1 External beam radiotherapy plus transarterial chemoembolisation (TACE) versus TACE, Outcome 5 Elevated total bilirubin.
Clinical liver‐related adverse events (e.g. encephalopathy or ascites) were not consistently reported in the included trials, preventing their formal evaluation.
None of the three trials comparing external beam radiotherapy as a monotherapy versus transarterial chemoembolisation alone reported on liver‐related adverse events (Xue 1995; Leng 2000; Wang 2000). It was therefore not feasible to establish meta‐analysed risk ratio for this comparison.
Subgroup analyses
We could not perform subgroup analysis for risk of bias because all of the included trials were judged to be high risk of bias.
We could not perform subgroup analyses for the Child‐Pugh class or ECOG performance score due to insufficient data.
We added a separate comparison for all‐cause mortality in the three studies reporting on external beam radiotherapy as a monotherapy (Analysis 2.1)
We performed subgroup analysis for participants treated with stereotactic radiotherapy versus those treated with three‐dimensional conformal radiotherapy (Analysis 1.1; Analysis 1.2; Analysis 1.3).
Harmful effects reported from non‐randomised studies
As per our protocol, we additionally evaluated the harmful effects from the following non‐randomised studies.
Hong 2016 is a phase II study of high‐dose hypofractionated proton beam therapy in people with localised, unresectable hepatocellular carcinoma and intrahepatic cholangiocarcinoma. Of the 83 evaluable participants (44 participants with hepatocellular carcinoma, 37 participants with intrahepatic cholangiocarcinoma, and two participants with mixed histology), 71 participants (85.5%) experienced at least one radiation‐related toxicity event while in the study, most commonly fatigue (54/83), rash (51/83), nausea (25/83), or anorexia (21/83). Four participants experienced at least one grade 3 radiation‐related toxicity. One participant with hepatocellular carcinoma developed grade 3 thrombocytopenia. There were no grade 4 or 5 radiation‐related toxicities.
Kim 2015 is a phase I dose‐escalation study of proton beam therapy for inoperable hepatocellular carcinoma. Of the 27 included participants, 22 participants showed no change in Child‐Pugh score; four participants showed a one‐point decrease, and one participant showed a one‐point increase. A grade 1 late skin and pulmonary reaction was observed in five and four participants. No participants experienced a grade ≥ 2 late toxicity associated with treatment (e.g. mucosal toxicities of the gastrointestinal tract or radiation‐induced liver disease).
Bujold 2013 represents sequential phase I and II trials of stereotactic body radiotherapy for locally advanced hepatocellular carcinoma. No significant (≥ grade 3) liver enzyme elevation was observed during treatment. No classic radiation‐induced liver disease was observed. Of participants without progressive disease, a decline in Child class was seen in 29% at 3 months and in 6% at 12 months.
Bush 2011 is a phase II study of high‐dose proton beam radiotherapy for hepatocellular carcinoma. Acute toxicity during proton therapy was minimal and included mild fatigue and skin reactions consisting of erythema (grade 1). There were no acute toxicities requiring interruption or discontinuation of the three‐week treatment course.
Kawashima 2005 is a phase II study of radiotherapy employing proton beam for hepatocellular carcinoma. Development of hepatic insufficiency within six months after completion of therapy was observed in eight participants. Moreover, a total of 10 participants experienced transient grade 3 leukopenia or thrombocytopenia, or both without infection or bleeding necessitating treatment. Andolino 2011 is a retrospective study evaluating stereotactic body radiotherapy for primary hepatocellular carcinoma. There were no ≥ grade 3 non‐haematologic toxicities. Thirteen per cent of participants experienced an increase in haematologic/hepatic dysfunction greater than grade 1, and 20% experienced progression in Child class within three months of treatment.
Ben‐Josef 2005 is a phase II trial of high‐dose conformal radiation therapy with concurrent hepatic artery floxuridine for unresectable intrahepatic malignancies. Overall toxicity was acceptable, with 27 participants (21%) and 11 participants (9%) developing grade 3 and 4 toxicity, and one treatment‐related death.
Li 2003 is a study of local three‐dimensional conformal radiotherapy combined with transcatheter arterial chemoembolisation for people with stage III hepatocellular carcinoma. Nine participants developed radiation‐induced liver disease. Three participants had treatment‐related gastrointestinal bleeding. There were two treatment‐related deaths, one from radiation‐induced liver disease and one from gastrointestinal bleeding.
Discussion
Summary of main results
We found evidence that the combined treatment of external beam radiotherapy plus transarterial chemoembolisation versus transarterial chemoembolisation alone appears to be associated with less probability of one‐year all‐cause mortality and a higher complete response rate. The combined treatment seems also to be associated with a higher risk of elevated total bilirubin and alanine aminotransferase. By using Trial Sequential Analysis, we saw that for the analyses of all‐cause mortality and overall response, the cumulative test statistic crossed the adjusted threshold for statistical significance, thus confirming the significant difference between external beam radiotherapy plus chemoembolisation versus chemoembolisation. However, the data for all comparisons were of very low quality, thus we could not reach any definitive conclusions.
We await the results of seven ongoing randomised trials to provide additional data regarding the role of external beam radiotherapy for unresectable hepatocellular carcinoma (Characteristics of ongoing studies).
Overall completeness and applicability of evidence
None of the included trials provided data on all the primary outcomes of interest specified in our review protocol. Moreover, the global applicability of the evidence is limited, considering that most relevant comparators are surgery and other non‐surgical ablation methods, and that all of the evidence came from Chinese populations.
Quality of the evidence
We assessed the overall evidence as low to very low quality using the GRADE approach (GRADEpro 2008); GRADE factors that support the judgement of low to very low quality include risk of bias, substantial heterogeneity (inconsistency) between trial results, and a small number of trials, which causes imprecision of our effect estimates. Among the 'Risk of bias' domains, generation of the allocation sequence was unclearly reported in five included trials, while allocation concealment was unclearly reported in all of the included trials. Reporting and attrition biases were unclearly reported in all of the included trials. We found a high risk of performance bias in seven of the included trials.
Potential biases in the review process
This review could be at risk for publication bias, however we did not perform funnel plot analysis to examine publication bias because our meta‐analyses included fewer than 10 trials.
We did not conduct searches in the US Food and Drug Administration and European Medicines Agency databases for trials. However, we will include search results from these databases in future updates of this review.
Agreements and disagreements with other studies or reviews
We found three meta‐analyses on the combination of radiotherapy plus transarterial chemoembolisation versus transarterial chemoembolisation alone for the treatment of hepatocellular carcinoma (Meng 2009; Zou 2014; Huo 2015). In the meta‐analysis by Huo 2015, the authors stated that they had included 25 studies, of which 11 were randomised trials. However, when we reassessed the included trials in their meta‐analysis, we found that three of the 11 studies were not randomised trials and thus could not be included in our systematic review (Li 2003; Lan 2005; Wang 2006). We have included the other eight trials in our systematic review. In the meta‐analysis by Meng 2009, the authors stated that they had included 17 studies, of which five were randomised trials. All of these five randomised studies are included in our systematic review. In the meta‐analysis by Zou 2014, the authors stated that they had included three randomised studies. All of these three randomised studies are included in our systematic review. All three meta‐analyses reach similar conclusions to ours, that is suggesting a beneficial impact of combined external beam radiotherapy with chemoembolisation. Two of the three meta‐analyses also questioned the overall quality of the evidence, and hence recommended further randomised clinical trials to better assess this intervention (Meng 2009; Zou 2014).
Authors' conclusions
Implications for practice.
This review provides very low‐ to low‐quality evidence that combined external beam radiotherapy and chemoembolisation may lower mortality and increase complete and overall response rates, despite an increased toxicity as expressed by a higher rise of bilirubin and alanine aminotransferase, in comparison with chemoembolisation alone. The high risk of systematic error (bias) in all of the included trials, as well as the imprecision and inconsistency of the results, suggest that these findings should be considered cautiously and that high‐quality trials are needed to further assess the role of external beam radiotherapy in the treatment of unresectable hepatocellular carcinoma.
Implications for research.
There is a need for large, high‐quality, randomised clinical trials of external beam radiotherapy for people with unresectable hepatocellular carcinoma. Currently, the most relevant comparators are surgery and other non‐surgical ablation methods, especially radiofrequency ablation and percutaneous ethanol injection (Taefi 2013; Weis 2013). It is also important that the randomisation process and the interventions are clearly described. The participant flow and data handling should be well specified. The trials must be designed following the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) statement (spirit‐statement.org) and reported following the CONSORT statement (consort‐statement.org).
Seven ongoing randomised clinical trials are currently evaluating different techniques of external beam radiotherapy for people with unresectable hepatocellular carcinoma. We expect to include their results in future updates of the present review.
Acknowledgements
To the Cochrane Hepato‐Biliary Group and its supporting editorial team. Special thanks go to Dr. Rouhan Wu (China) for translating the Chinese articles.
Peer Reviewers: Francesco Dionisi, Italy; Zehaui Wen, China. Contact Editors: Rosa Simonetti, Italy; Christian Gluud, Denmark. Sign‐off Editor: Christian Gluud, Denmark.
Cochrane Review Group funding acknowledgement: The Danish State is the largest single funder of the Cochrane Hepato‐Biliary Group through its investment in The Copenhagen Trial Unit, Centre for Clinical Intervention Research, Rigshospitalet, Copenhagen University Hospital, Denmark. Disclaimer: The views and opinions expressed in this review are those of the authors and do not necessarily reflect those of the Danish State or The Copenhagen Trial Unit.
Appendices
Appendix 1. Search strategies
| Database | Time span | Search strategy |
| The Cochrane Hepato‐Biliary Group Controlled Trials Register | October 2016 | (radiotherapy) AND (hepato* or liver*) and (carcinom* or cancer* or neoplasm* or malign* or tumo*)) |
| Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library | 2016, Issue 9 | #1 radiotherapy #4 MeSH descriptor: [liver neoplasms] explode all trees #5 MeSH descriptor: [Hepatocellular carcinoma] explode all trees #6 MeSH descriptor: [carcinoma,liver] explode all trees #7 (liver* or hepato*) and (carcinom* or cancer* or neoplasm* or malign* or tumo*) #8 #2 or #3 or #4 or #5 or #6 or #7 #9 #1 and #8 |
| MEDLINE (OvidSP) | 1946 to October 2016 | 1. radiotherapy.mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier] 4. exp liver Neoplasms/ 5. hepatocellular carcinoma/ 6. carcinoma, liver/ 7. ((liver* or hepato*) and (carcinom* or cancer* or neoplasm* or malign* or tumo*)).mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier] 8. 2 or 3 or 4 or 5 or 6 or 7 9. 1 and 8 10. (random* or blind* or placebo* or meta‐analys*).mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier] 11. 9 and 10 |
| Embase (OvidSP) | 1974 to October 2016 | 1. exp radiotherapy/ 2. radiotherapy.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] 3. 1 or 2 6. exp liver tumor/ 7. exp liver carcinoma/ 8. ((liver* or hepato*) and (carcinom* or cancer* or neoplasm* or malign* or tumo*)).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] 9. 4 or 5 or 6 or 7 or 8 10. 3 and 9 11. (random* or blind* or placebo* or meta‐analys*).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] 12. 10 and 11 |
| Science Citation Index Expanded (Web of Science) | 1900 to October 2016 | #7 #6 AND #5 #6 TS=(random* or blind* or placebo* or meta‐analys*) #5 #4 AND #1 #4 #3 OR #2 #3 TS=((liver* or hepato*) and (carcinom* or cancer* or neoplasm* or malign* or tumo*)) #1 TS=(radiotherapy) |
Data and analyses
Comparison 1. External beam radiotherapy plus transarterial chemoembolisation (TACE) versus TACE.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality (at 1 year) | 9 | 786 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.41, 0.62] |
| 1.1 Studies using three dimensional conformal radiation therapy | 8 | 527 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.43, 0.68] |
| 1.2 Studies using stereotactic radiotherapy | 1 | 259 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.25, 0.64] |
| 2 Complete response | 7 | 620 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.14 [1.47, 3.13] |
| 2.1 Studies using three dimensional conformal radiation therapy | 6 | 361 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.13 [1.34, 3.37] |
| 2.2 Studies using stereotactic radiotherapy | 1 | 259 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.17 [1.12, 4.21] |
| 3 Complete response + partial response | 7 | 620 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.58 [1.40, 1.78] |
| 3.1 Studies using three dimensional conformal radiation therapy | 6 | 361 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.53 [1.31, 1.79] |
| 3.2 Studies using stereotactic radiotherapy | 1 | 259 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.65 [1.36, 1.99] |
| 4 Elevated alanine aminotransferase | 3 | 232 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [1.08, 1.84] |
| 5 Elevated total bilirubin | 2 | 172 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.69 [1.34, 5.40] |
Comparison 2. External beam radiotherapy versus transarterial chemoembolisation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality | 3 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.97, 1.50] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Leng 2000.
| Methods | Randomised clinical trial with three arms: transarterial chemoembolisation (TACE) (arm A), or radiotherapy (arm B), or radiotherapy +TACE (arm C). Parrallel group design | |
| Participants | 107 people with primary liver cancer Median age: 46 years Male/female: 80/27 TACE = 39; RT = 32; TACE + RT = 36 Recruitment: September 1990 to June 1995 Inclusion criteria:
|
|
| Interventions | TACE: hepatic artery infusion. Two drugs from (cisplatin 60 mg to 120 mg, doxorubicin , THP 50 mg to 100 mg, mitomycin 16 mg to 20 mg, 5‐fluorouracil 1.0 g to 2.0 g, and cyclophosphamide 1.2 g) were chosen. 40% iodinated oil 10 mL to 30 mL, gel foam particles 1 mm to 2 mm every 4 to 8 weeks; average 3.2 times. Radiotherapy: cobalt‐60 exposure, 57.5 ˜ 70.0 cGy twice a day, 5 days a week; average total dose: 50 Gy. TACE + radiotherapy: first, TACE 1 to 4 times; after 4 to 8 weeks take radiotherapy. | |
| Outcomes |
|
|
| Notes | Country of the study: China We were unable to locate a contact email for the corresponding author to request missing data. Funding: unclear |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The authors did not mention the method of randomisation. |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation was not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The trial was not blinded, so that the allocation was known during the trial. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome assessment was not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | There was insufficient data to assess attrition bias. |
| Selective reporting (reporting bias) | High risk | The study did not report cancer‐related mortality, quality of life, or serious adverse events. |
| For profit bias | Unclear risk | Unclear funding source |
| Other bias | Unclear risk | Unclear risk of other biases |
Liao 2010.
| Methods | Randomised clinical trial with two arms: transarterial chemoembolisation (TACE) versus three dimensional conformal radiotherapy (DCRT) and TACE). Parrallel group design. | |
| Participants | 48 people with unresectable liver cancer TACE = 24; 3‐DCRT and TACE = 24 Recruitment: November 2005 to November 2007 |
|
| Interventions | TACE: femoral artery by Seldinger technique. 5‐fluorouracil 1000 mg to 1250 mg, DDP 70 mg to 90 mg, Epi‐ADM 50 mg to 60 mg, iodinated oil 5 mL to 20 mL. Radiotherapy: three‐dimensional conformal radiotherapy using 6MV X‐ray; total dose: 40 to 66 Gy over 20 to 33 fractions. | |
| Outcomes |
|
|
| Notes | Country of the study: China We were unable to locate a contact email for the corresponding author to request missing data. Funding: Quzhou Science and Technology Bureau (NO. 20051120) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The article mentioned that the study was "randomised", but they did not provide the randomisation method. |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation was unclearly reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes were assessed by apparatus. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing participants |
| Selective reporting (reporting bias) | High risk | The study did not report cancer‐related mortality, quality of life, or serious adverse events. |
| For profit bias | Low risk | The study was funded by Quzhou Science and Technology Bureau (grant NO. 20051120). |
| Other bias | Unclear risk | Unclear risk of other biases |
Peng 2000.
| Methods | Randomised clinical trial with 2 arms: hepatic arterial embolisation versus hyperfractionated radiotherapy and hepatic arterial embolisation. Parallel‐group design | |
| Participants | 91 people with unresectable liver cancer Male/female: 82/9 Average age: 52 years Hepatic arterial embolisation = 48; hyperfractionated radiotherapy and hepatic arterial embolisation = 43 Recruitment: September 1988 to December 1997 |
|
| Interventions | The total dose is 4000 to 5000 cGy, 34 to 42 fractions, over 3 to 4 weeks. | |
| Outcomes |
|
|
| Notes | Country of the study: China We were unable to locate a contact email for the corresponding author to request missing data. Funding: unclear |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sampling |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation was not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The trial was not blinded, so that the allocation was known during the trial. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome assessment was not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | There were insufficient data to assess attrition bias. |
| Selective reporting (reporting bias) | High risk | The study did not report cancer‐related mortality, quality of life, or serious adverse events. |
| For profit bias | Unclear risk | Unclear funding source |
| Other bias | Unclear risk | Unclear risk of other biases |
Shang 2007.
| Methods | Randomised clinical trial with 2 arms: three dimensional conformal radiotherapy (DCRT) + transarterial chemoembolisation (TACE) versus TACE. Parrallel group design | |
| Participants | 76 people with primary liver cancer 3‐DCRT + TACE = 40; TACE = 36 Median age: 52 years Male/female: 48/28 Recruitment: May 2003 to March 2007 Inclusion criteria: Chinese Medical Association guideline:
3‐DCRT + TACE:
TACE:
|
|
| Interventions | TACE: femoral artery by Seldinger technique. 3‐DCRT: 2 Gy once daily, 5 days a week, for 4 to 6 weeks; average ≤ 50 Gy. | |
| Outcomes |
|
|
| Notes | Country of the study: China We were unable to make contact with the corresponding author to provide missing data. Funding: unclear |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The article was described as a "prospective randomised clinical study", but randomisation method was not mentioned. |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation was not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The trial was not blinded, so that the allocation was known during the trial. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes were assessed by apparatus. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | There were insufficient data to assess attrition bias. |
| Selective reporting (reporting bias) | High risk | The study did not report cancer‐related mortality, quality of life, or serious adverse events. |
| For profit bias | Unclear risk | Unclear funding source |
| Other bias | Unclear risk | Unclear risk of other bias |
Wang 2000.
| Methods | Randomised clinical trial with 3 arms: radiotherapy versus transarterial chemoembolisation (TACE) versus radiotherapy + TACE. Parallel‐group design | |
| Participants | 60 people with histologically proven inoperable/unresectable advanced primary hepatocellular carcinoma. Radiotherapy = 20 participants; TACE = 20 participants; radiotherapy + TACE = 20 participants. Median age: 52 years Male/female: 45/16 Recruitment: January 1990 to January 1998 Inclusion criteria: Histologically or cytologically proven hepatocellular carcinoma and measurable bipolar disease. Patients with solitary lesion were also included if they were not candidates for surgery. Exclusion criteria: Patients were excluded if they were older than 75 years, had bilirubin level more than 3, TLC less than 3000, platelet less than 60,000, creatinine more than 2. |
|
| Interventions | Radiotherapy: given as whole‐liver irradiation with the moving‐strip technique 150 to 180 cGy until tumour dose at the centre reached 20 to 25 Gy, then the residual foci as localised by ultrasound were treated with a boost until 50 Gy with cobalt‐60 machine. TACE: Seldinger technique was used. Chemotherapy consisted of cisplatin, adriamycin, and mitomycin or floxuridine. The embolisation agent was 40% iodised oil. Treatment was repeated at 4, 6, and 8 weeks. Participants received TACE 4 to 5 times. Radiotherapy + TACE: radiotherapy started 2 weeks after TACE until 20 to 25 Gy, then 1 week rest followed by the 2nd TACE. Another 2‐week gap, then radiotherapy boost until total dose up to 50 Gy. A further 2‐week gap, then 3rd TACE was given. 4th TACE was given after 6 to 8 weeks. TACE was administered in this group until 5 times. |
|
| Outcomes |
|
|
| Notes | Country of the study: China We were unable to locate a contact email for the corresponding author to request missing data. Funding: unclear |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Envelope method |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation was not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The trial was not blinded, so that the allocation was known during the trial. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Detection bias was unclear. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | There were insufficient data to assess attrition bias. |
| Selective reporting (reporting bias) | High risk | The study did not report cancer‐related mortality, quality of life, or serious adverse events. |
| For profit bias | Unclear risk | Unclear funding source |
| Other bias | Unclear risk | Unclear risk of other bias |
Xiao 2008.
| Methods | Randomised clinical trial with 2 arms: three dimensional conformal radiotherapy (3‐DCRT) plus transarterial chemoembolisation (TACE) versus TACE. Parallel‐group design | |
| Participants | 60 people with unresectable primary hepatic carcinoma. TACE + 3‐DCRT = 30; TACE = 30 Median age: not reported Male/female: 45/15 Recruitment: January 2002 to June 2006 Inclusion criteria: Chinese Medical Association guideline:
|
|
| Interventions | TACE: femoral artery by Seldinger technique 3‐DCRT: total average dose = 55 Gy |
|
| Outcomes |
|
|
| Notes | Country of the study: China We were unable to make a contact with the corresponding author to request missing data. Funding: unclear |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocation sequence was generated by random table number. |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation was not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The trial was not blinded, so that the allocation was known during the trial. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome was assessed by apparatus. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | There were insufficient data to assess attrition bias. |
| Selective reporting (reporting bias) | High risk | The study did not report cancer‐related mortality, quality of life, or serious adverse events. |
| For profit bias | Unclear risk | Unclear funding source. |
| Other bias | Unclear risk | Unclear risk of other bias. |
Xue 1995.
| Methods | Randomised clinical trial including 4 arms: transarterial chemoembolisation (TACE) with immediate administration of doxorubicin versus TACE with delayed administration of doxorubicin versus TACE plus external beam radiotherapy (EBRT) versus EBRT alone. Parallel‐group design | |
| Participants | 82 people with hepatocellular carcinoma Average age: 48.3 years Male/female: 69/13 Recruitment: March 1991 to April 1993 |
|
| Interventions | TACE with immediate administration of doxorubicin (20 participants) versus TACE with delayed administration of doxorubicin (20 participants) versus EBRT + TACE (21 participants) versus EBRT alone (21 participants) | |
| Outcomes |
|
|
| Notes | Child‐Pugh A: 36
Child‐Pugh B: 46 Country of the study: China We were unable to locate a contact email for the corresponding author to request missing data. Funding: unclear |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study was described as a randomised clinical trial, but the method of randomisation was not mentioned. |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation was not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The trial was not blinded, so that the allocation was known during the trial. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome assessment was not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | There were insufficient data to assess attrition bias. |
| Selective reporting (reporting bias) | High risk | The trial did not report cancer‐related mortality, quality of life, or serious adverse events. |
| For profit bias | Unclear risk | Unclear funding source |
| Other bias | Unclear risk | Unclear risk of other bias |
Zhang 2012.
| Methods | Randomised clinical trial with 2 arms: transarterial chemoembolisation (TACE) + Gamma Knife versus TACE. Parallel‐group design | |
| Participants | 259 people with primary hepatocellular carcinoma. Gamma Knife + TACE = 135; TACE = 124 Median age: 52 years Male/female: 240/55 Recruitment: not mentioned Inclusion criteria Chinese Medical Association guideline:
TACE + Gamma Knife: elevated alpha‐fetoprotein: 101; single lesion: 92 TACE: elevated alpha‐fetoprotein: 95; single lesion: 86. |
|
| Interventions | TACE: femoral artery by Seldinger technique. 5‐fluorouracil 500 mg to 1000 mg, DDP 200 mg to 300 mg, Epi‐ADM 30 mg to 50 mg, iodinated oil 5 mL to 20 mL. Gamma Knife: cobalt‐60, 3 to 5 Gy once every other day, 8 to 12 times; total average rate 36 to 50 Gy. | |
| Outcomes |
|
|
| Notes | Country of the study: China We were unable to make contact with the corresponding author to request missing data. Funding: unclear |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study is described as a randomised clinical trial, but the method of randomisation is not mentioned. |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation was not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The trial was not blinded, so that the allocation was known during the trial. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes were assessed by apparatus. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | There were insufficient data to assess attrition bias. |
| Selective reporting (reporting bias) | High risk | The study did not report cancer‐related mortality, quality of life, or serious adverse events. |
| For profit bias | Unclear risk | Unclear funding source |
| Other bias | Unclear risk | Unclear risk of other bias |
Zhao 2006.
| Methods | Randomised clinical trial with 2 arms: three dimensional conformal radiotherapy (3‐DCRT) plus transarterial chemoembolisation (TACE) versus TACE | |
| Participants | 96 people with inoperable primary liver cancer. TACE + 3‐DCRT = 49; TACE = 47 Median age: 52 years Male/female: 59/37 Recruitment: January 1998 to April 2000 Diagnostic criteria:
79 participants had pathologic diagnosis; others were diagnosed by clinical symptoms, imaging, and alpha‐fetoprotein. |
|
| Interventions | Planning scan by spiral computed tomography, design CTV, and PTV. The machine is 2100C linear accelerator. 3‐DCRT: 45 to 55 Gy; treatment is administered every other day | |
| Outcomes |
|
|
| Notes | Country of the study: China We contacted the corresponding author on 19 March 2016 to provide missing data, but have not yet received any feedback. Funding: unclear |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study is described as a randomised clinical trial, but the method of randomisation is not mentioned. |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation was not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The trial was not blinded, so that the allocation was known during the trial. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome assessment was not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | There were insufficient data to assess attrition bias. |
| Selective reporting (reporting bias) | High risk | The study did not report cancer‐related mortality, quality of life, or serious adverse events. |
| For profit bias | Unclear risk | Unclear funding source |
| Other bias | Unclear risk | Unclear risk of other bias |
Epi‐ADM: doxorubicin and epirubicin; cGy: centigray; CTV: clinical target volume; DDP: cisplatin; Gy: gray; KPS: Karnofsky performance score; PTV: planning target volume; THP: tetrahydropalmatine; TLC: total leukocytic count; TNM: tumour, node and metastasis.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bush 2016 | All included participants were eligible for transplantation (participants were required to have disease within the Milan or San Francisco criteria, without vascular invasion). |
| Kang 2014 | Radiotherapy was part of the therapeutic strategy in the 3 randomised arms, thus it was not possible to formally assess the added benefit or harm of radiotherapy in these trial participants. |
| Lan 2005 | Not randomised |
| Li 2003 | Not randomised |
| Wang 2006 | Not randomised |
Characteristics of ongoing studies [ordered by study ID]
NCT01730937.
| Trial name or title | Sorafenib tosylate with or without stereotactic body radiation therapy in treating patients with liver cancer |
| Methods | Phase III trial |
| Participants | People with hepatocellular carcinoma unsuitable for resection, radiofrequency ablation, or transarterial chemoembolisation (TACE) |
| Interventions | Experimental: Arm 1 (sorafenib tosylate): Sorafenib tosylate given orally twice a day on days 1 to 28. Treatment repeats every 28 days for up to 5 years in the absence of disease progression or unacceptable toxicity. Experimental: Arm 2 (stereotactic body radiotherapy and sorafenib tosylate): stereotactic body radiotherapy administered every 24 to 72 hours for a total of 5 fractions over 5 to 15 days. Within 1 to 5 days post‐stereotactic body radiotherapy, treatment with sorafenib tosylate commences, given orally twice a day on days 1 to 28. Treatment repeats every 28 days for up to 5 years in the absence of disease progression or unacceptable toxicity. |
| Outcomes | Primary outcome measures:
Secondary outcome measures:
Health‐related quality of life assessments measured by FACT‐Hep |
| Starting date | April 2013 |
| Contact information | Christopher M Iannuzzi; twhite@stvincents.org |
| Notes | Inclusion criteria:
Exclusion criteria:
|
NCT01901692.
| Trial name or title | Transarterial chemoembolisation plus radiotherapy or sorafenib in hepatocellular carcinoma with major vascular invasion (START) |
| Methods | Randomised phase II trial |
| Participants | People with hepatocellular carcinoma invading major intrahepatic vessels Country: Korea |
| Interventions | transarterial chemoembolisation (TACE) + external beam radiotherapy versus sorafenib |
| Outcomes | Primary endpoint
Secondary endpoints
|
| Starting date | July 2013 |
| Contact information | Young‐Suk Lim, Associate Professor, Asan Medical Center |
| Notes | Estimated enrolment: 90 Inclusion criteria:
|
NCT01963429.
| Trial name or title | Comparison between radiofrequency ablation and hypofractionated proton beam radiation for recurrent/residual hepatocellular carcinoma |
| Methods | Phase III trial |
| Participants | People with recurrent/residual hepatocellular carcinoma |
| Interventions | Experimental: Arm A (radiofrequency ablation) Experimental: Arm B (proton beam radiotherapy) |
| Outcomes | Primary outcome measures:
Secondary outcome measures:
Other outcome measures:
|
| Starting date | October 2013 |
| Contact information | Joong Won Park, PhD; jwpark1@ncc.re.kr |
| Notes | Estimated enrolment: 144 Inclusion criteria:
Exclusion criteria:
|
NCT02323360.
| Trial name or title | A trial on stereotactic body radiotherapy after incomplete transarterial chemoembolisation (TACE) versus exclusive TACE for treatment of inoperable hepatocellular carcinoma |
| Methods | Phase III trial |
| Participants | People with inoperable hepatocellular carcinoma |
| Interventions | Experimental: Stereotactic body radiation therapy Active comparator: transarterial chemoembolisation (TACE) |
| Outcomes | Primary outcome measures:
Secondary outcome measures:
|
| Starting date | November 2014 |
| Contact information | Marta Scorsetti, MD, PhD; marta.scorsetti@humanitas.it |
| Notes | Estimated enrolment: 80 Inclusion criteria:
Exclusion criteria:
|
NCT02640924.
| Trial name or title | Proton radiotherapy versus radiofrequency ablation for patients with medium or large hepatocellular carcinoma |
| Methods | Phase III trial |
| Participants | People with medium (> 3, ≤ 5 cm) or large (> 5, ≤ 7 cm) treatment‐naive hepatocellular carcinoma |
| Interventions | Proton beam radiotherapy versus radiofrequency ablation |
| Outcomes | Primary outcome measures:
Secondary outcome measures:
|
| Starting date | January 2016 |
| Contact information | Bing‐Shen Huang, MD; beanson.tw@gmail.com |
| Notes | Inclusion criteria:
Exclusion criteria:
*Baseline laboratories results must be within the protocol range prior to signing informed consent. Repeat lab tests are permitted to evaluate eligibility during the screening period. |
NCT02762266.
| Trial name or title | Transarterial chemoembolisation compared with stereotactic body radiation therapy or stereotactic ablative radiation therapy in treating patients with residual or recurrent liver cancer undergone initial transarterial chemoembolisation |
| Methods | Phase III trial |
| Participants | People with residual or recurrent liver cancer who have undergone initial transarterial chemoembolisation |
| Interventions | Active comparator: Arm I ‐ transarterial chemoembolisation (TACE) Experimental: Arm II (stereotactic body radiotherapy) |
| Outcomes | Primary objectives:
Secondary objectives:
|
| Starting date | May 2016 |
| Contact information | Rachel Freiberg; rachelf@stanford.edu |
| Notes | Inclusion criteria:
Exclusion criteria:
|
NCT02794337.
| Trial name or title | Transarterial chemoembolisation (TACE) versus TACE + stereotactic body radiotherapy for unresectable hepatocellular cancer (TACE ‐ stereotactic body radiotherapy) |
| Methods | Phase III trial |
| Participants | People with unresectable hepatocellular carcinoma |
| Interventions | Active comparator: drug‐eluting beads ‐ TACE arm Experimental: drug‐eluting beads ‐ TACE + stereotactic body radiotherapy arm |
| Outcomes | Primary outcome measures:
Secondary outcome measures:
|
| Starting date | December 2014 |
| Contact information | Supriya Chopra; schopra@actrec.gov.in |
| Notes | Inclusion criteria:
Exclusion criteria:
|
CTCAE: Common Terminology Criteria for Adverse Events; EASL: European Association for the Study of the Liver; ECOG: Eastern Cooperative Oncology Group; FACT‐Hep: Functional Assessment of Cancer Therapy ‐ Hepatobiliary; MRI: magnetic resonance imaging; RECIST: Response Evaluation Criteria in Solid Tumours.
Differences between protocol and review
We added 'liver‐related adverse events' as a secondary outcome because we considered it to be an important additional point to clarify in any analysis of local or systemic therapies for hepatocellular carcinoma.
Contributions of authors
Omar Abdel‐Rahman: Collecting the data, performing the analyses, and writing the review. Zeinab Elsayed: Collecting the data and revising the review.
Both authors agreed to the final version of the review submitted for publication.
Sources of support
Internal sources
None, Other.
External sources
None, Other.
Declarations of interest
Omar Abdel‐Rahman: none known. Zeinab Elsayed: none known.
New
References
References to studies included in this review
Leng 2000 {published data only}
- Leng ZQ, Liang ZY, Shi S, Hu ZX. Comparison of treatment results of interventional therapy alone, radiotherapy alone, and combined interventional therapy plus radiotherapy for primary hepatic cancer. Chinese Journal of Radiation Oncology 2000;9:99‐101. [Google Scholar]
Liao 2010 {published data only}
- Liao X, He H, Zhou Z, Hu W, Zhu X. Three‐dimensional conformal radiotherapy combined with interventional therapy in treatment of primary hepatocellular carcinoma. Journal of Practical Oncology 2010;25:681‐3. [Google Scholar]
Peng 2000 {published data only}
- Peng KG, Han FS, Liu H, Song MZ. Clinical study of unresectable liver cancer treated by intraoperative hepatic arterial embolization and post‐operative hyperfractionation radiotherapy. Chinese Journal of Radiation Oncology 2000;9:11‐3. [Google Scholar]
Shang 2007 {published data only}
- Shang Y, You XX, Xu HY, Chen MC. Prospective randomized clinical study of transcatheter arterial chemoembolization, combined with three‐dimensional conformal radiotherapy for primary liver cancer: an analysis of 40 cases. Shijie Xiaohua Zazhi 2007;15:3140‐2. [Google Scholar]
Wang 2000 {published data only}
- Wang G, Shen W, Song M, Xu H. Results of combined treatment with transcatheter hepatic arterial chemoembolization and whole‐liver irradiation with the moving strip technique in unresectable hepatocellular carcinoma. International Journal of Clinical Oncology 2000;5:380‐5. [Google Scholar]
Xiao 2008 {published data only}
- Xiao Z, Ouyang T, Yu R, Jiang X, Reng H, Wu Z. Transcatheter arterial chemoembolization combined with 3‐dimensional conformal radiotherapy for patients with unresectable primary hepatic carcinoma. Chinese Journal of Clinical Oncology 2008;35(1):18‐21. [Google Scholar]
Xue 1995 {published data only}
- Xue HZ, Meng GD, Wang YW, Jiang QF. Transcatheter arterial chemoembolization plus radiotherapy in the treatment of hepatocellular carcinoma. Chinese Journal of Radiation Oncology 1995;4:84‐5. [Google Scholar]
Zhang 2012 {published data only}
- Zhang Z, Yang X, Wena M, Wan J. Evaluation of TACE combined with gamma‐knife radiotherapy for primary hepatocellular carcinoma. Journal of Interventional Radiology (China) 2012;7(21):596‐9. [Google Scholar]
Zhao 2006 {published data only}
- Zhao MH, Lang FP, Jiang QA, Ma JJ, Song YX. Three‐dimensional conformal radiotherapy combined with transcatheter arterial chemoembolization for inoperable primary liver cancer. Chinese Journal of Radiation Oncology 2006;15:39‐41. [Google Scholar]
References to studies excluded from this review
Bush 2016 {published data only}
- Bush DA, Smith JC, Slater JD, Volk ML, Reeves ME, Cheng J, et al. Randomized clinical trial comparing proton beam radiation therapy with transarterial chemoembolization for hepatocellular carcinoma: results of an interim analysis. International Journal of Radiation Oncology, Biology, Physics 2016;95(1):477‐82. [DOI] [PubMed] [Google Scholar]
Kang 2014 {published data only}
- Kang J, Nie Q, Du R, Zhang L, Zhang J, Li Q, et al. Stereotactic body radiotherapy combined with transarterial chemoembolization for hepatocellular carcinoma with portal vein tumor thrombosis. Molecular and Clinical Oncology 2014;2:43‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lan 2005 {published data only}
- Lan DQ, Gong XH, Wei XL. The efficacy analysis of transcatheter hepatic arterial chemoembolization combined with radiotherapy for primary liver cancer. Chinese Journal of Radiation Oncology 2005;14:152‐3. [Google Scholar]
Li 2003 {published data only}
- Li B, Yu J, Wang L, Li C, Zhou T, Zhai L, et al. Study of local three‐dimensional conformal radiotherapy combined with transcatheter arterial chemoembolization for patients with stage III hepatocellular carcinoma. American Journal of Clinical Oncology 2003;26(4):e92‐9. [DOI] [PubMed] [Google Scholar]
Wang 2006 {published data only}
- Wang XH, Li JX, Gao K. Radiotherapy combined with hepatic chemoembolization in the treatment of 54 primary liver cancer cases. Shaxi Yixue Zazhi 2006;35(4):461‐2. [Google Scholar]
References to ongoing studies
NCT01730937 {published data only}
- NCT01730937. Sorafenib tosylate with or without stereotactic body radiation therapy in treating patients with liver cancer [Randomized phase III study of sorafenib versus stereotactic body radiation therapy followed by sorafenib in hepatocellular carcinoma]. clinicaltrials.gov/ct2/show/NCT01730937 (first received November 15, 2012 ).
NCT01901692 {published data only}
- NCT01901692. Transarterial chemoembolization plus radiotherapy or sorafenib in hepatocellular carcinoma with major vascular invasion (START) [Randomized phase II trial comparing transarterial chemoembolization plus external beam radiotherapy versus sorafenib in patients with hepatocellular carcinoma with major vascular invasion]. clinicaltrials.gov/ct2/show/NCT01901692 (first received July 14, 2013 ).
NCT01963429 {published data only}
- NCT01963429. Comparison between radiofrequency ablation and hypofractionated proton beam radiation for recurrent/residual HCC [A randomized phase III study of the comparison between radiofrequency ablation and hypofractionated proton beam radiation in patients with recurrent/residual small hepatocellular carcinoma (APROH Study)]. clinicaltrials.gov/ct2/show/NCT01963429 (first received October 13, 2013 ).
NCT02323360 {published data only}
- NCT02323360. A trial on SBRT after incomplete TAE or TACE versus exclusive TAE or TACE for treatment of inoperable HCC [A randomised phase III trial on stereotactic body radiotherapy (SBRT) after incomplete transcatheter arterial embolization (TAE) or chemoembolization (TACE) versus exclusive TAE or TACE for inoperable hepatocellular carcinoma (HCC)]. clinicaltrials.gov/ct2/show/NCT02323360 (first received December 11, 2014 ).
NCT02640924 {published data only}
- NCT02640924. Proton radiotherapy versus radiofrequency ablation for patients with medium or large hepatocellular carcinoma [Proton beam radiotherapy versus switching control radiofrequency ablation for patients with medium (>3, ≦5 cm) or large (>5, ≦7cm) treatment‐naive hepatocellular carcinoma]. clinicaltrials.gov/ct2/show/NCT02640924 (first received December 7, 2015 ).
NCT02762266 {published data only}
- NCT02762266. Transarterial chemoembolization compared with stereotactic body radiation therapy or stereotactic ablative radiation therapy in treating patients with residual or recurrent liver cancer undergone initial transarterial chemoembolization [International randomized study of transarterial chemoembolization (TACE) versus stereotactic body radiotherapy (SBRT) / stereotactic ablative radiotherapy (SABR) for residual or recurrent hepatocellular carcinoma after initial TACE]. clinicaltrials.gov/ct2/show/NCT02762266 (first received April 5, 2016 ).
NCT02794337 {published data only}
- NCT02794337. TACE vs TACE+SBRT for unresectable hepatocellular cancer (TACE‐SBRT) [Integrated phase II/III randomized control trial of transarterial chemoembolisation alone or in combination with stereotactic body radiation in patients with unresectable hepatocellular cancer]. clinicaltrials.gov/ct2/show/NCT02794337 (first received March 8, 2016 ).
Additional references
Abdel‐Rahman 2013
- Abdel‐Rahman O, Elsayed Z. Combination trans arterial chemoembolization (TACE) plus sorafenib for the management of unresectable hepatocellular carcinoma: a systematic review of the literature. Digestive Diseases and Sciences 2013; Vol. 58, issue 12:3389‐96. [DOI: 10.1007/s10620-0132872-x] [DOI] [PubMed]
Abdel‐Rahman 2016
- Abdel‐Rahman OM, Elsayed Z. Yttrium‐90 microsphere radioembolisation for unresectable hepatocellular carcinoma. Cochrane Database of Systematic Reviews 2016, Issue 2. [DOI: 10.1002/14651858.CD011313.pub2] [DOI] [PubMed] [Google Scholar]
Andolino 2011
- Andolino DL, Johnson CS, Maluccio M, Kwo P, Tector AJ, Zook J, et al. Stereotactic body radiotherapy for primary hepatocellular carcinoma. International Journal of Radiation Oncology * Biology * Physics 2011;81(4):e447‐53. [DOI] [PubMed] [Google Scholar]
Balshem 2011
- Balshem H, Helfand M, Schunemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines 3: rating the quality of evidence ‐ introduction. Journal of Clinical Epidemiology 2011;64:401‐6. [DOI] [PubMed] [Google Scholar]
Ben‐Josef 2005
- Ben‐Josef E, Normolle D, Ensminger WD, Walker S, Tatro D, Haken RK, et al. Phase II trial of high‐dose conformal radiation therapy with concurrent hepatic artery floxuridine for unresectable intrahepatic malignancies. Journal of Clinical Oncology 2005;23(34):8739‐47. [DOI] [PubMed] [Google Scholar]
Brok 2008
- Brok J, Thorlund K, Gluud C, Wetterslev J. Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta‐analyses. Journal of Clinical Epidemiology 2008;61:763‐9. [DOI] [PubMed] [Google Scholar]
Brok 2009
- Brok J, Thorlund K, Wetterslev J, Gluud C. Apparently conclusive meta‐analyses may be inconclusive ‐ Trial sequential analysis adjustment of random error risk due to repetitive testing of accumulating data in apparently conclusive neonatal meta‐analyses. International Journal of Epidemiology 2009;38(1):287‐98. [DOI] [PubMed] [Google Scholar]
Bruix 2011
- Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology (Baltimore, Md.) 2011;53(3):1020‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bujold 2013
- Bujold A, Massey CA, Kim JJ, Brierley J, Cho C, Wong RKS, et al. Sequential phase I and II trials of stereotactic body radiotherapy for locally advanced hepatocellular carcinoma. Journal of Clinical Oncology 2013;31(13):1631‐9. [DOI] [PubMed] [Google Scholar]
Bush 2011
- Bush DA, Kayali Z, Grove R, Slater JD. The safety and efficacy of high‐dose proton beam radiotherapy for hepatocellular carcinoma: a phase 2 prospective trial. Cancer 2011;117(13):3053‐9. [DOI] [PubMed] [Google Scholar]
Cárdenes 2009
- Cárdenes HR. Role of stereotactic body radiotherapy in the management of primary hepatocellular carcinoma. Rationale, technique and results. Clinical Translational Oncology 2009;11(5):276‐83. [DOI] [PubMed] [Google Scholar]
Dawson 2011
- Dawson L. Overview: Where does radiation therapy fit in the spectrum of liver cancer local‐regional therapies?. Seminars in Radiation Oncology 2011;21(4):241‐6. [DOI] [PubMed] [Google Scholar]
DeMets 1987
- DeMets DL. Methods of combining randomised clinical trials: strengths and limitations. Statistics in Medicine 1987;6(3):341‐50. [DOI] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. [DOI] [PubMed] [Google Scholar]
EASL/EORTC 2012
- European Association for the Study of the Liver, European Organisation for Research and Treatment of Cancer. EASL‐EORTC clinical practice guidelines: management of hepatocellular carcinoma. Journal of Hepatology 2012;56(4):908‐43. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ (Clinical Research Ed.) 1997;315:629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Eisenhauer 2009
- Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). European Journal of Cancer 2009;45(2):228‐47. [DOI] [PubMed] [Google Scholar]
Globocan 2012
- International Agency for Research on Cancer. Globocan 2012: estimated cancer incidence, mortality and prevalence worldwide in 2012. globocan.iarc.fr/Default.aspx (accessed 20 June 2016).
Gluud 2017
- Gluud C, Nikolova D, Klingenberg SL. Cochrane Hepato‐Biliary Group. About Cochrane (Cochrane Review Groups (CRGs)) 2017, Issue 2. Art. No.: LIVER.
GRADEpro 2008 [Computer program]
- McMaster University (developed by Evidence Prime, Inc.). GRADEpro GDT: GRADEpro Guideline Development Tool. Hamilton (ON): McMaster University (developed by Evidence Prime, Inc.), 2015.
Guyatt 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction ‐ GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [PUBMED: 21195583] [DOI] [PubMed] [Google Scholar]
Guyatt 2011a
- Guyatt GH, Oxman AD, Kunz R, Atkins D, Brozek J, Vist G, et al. GRADE guidelines: 2. Framing the question and deciding on important outcomes. Journal of Clinical Epidemiology 2011;64(4):395‐400. [PUBMED: 21194891] [DOI] [PubMed] [Google Scholar]
Guyatt 2011b
- Guyatt GH, Oxman AD, Vist G, Kunz R, Brozek J, Alonso‐Coello P, et al. GRADE guidelines: 4. Rating the quality of evidence ‐‐ study limitations (risk of bias). Journal of Clinical Epidemiology 2011;64(4):407‐15. [PUBMED: 21247734] [DOI] [PubMed] [Google Scholar]
Guyatt 2011c
- Guyatt GH, Oxman AD, Montori V, Vist G, Kunz R, Brozek J, et al. GRADE guidelines: 5. Rating the quality of evidence ‐‐ publication bias. Journal of Clinical Epidemiology 2011;64(12):1277‐82. [PUBMED: 21802904] [DOI] [PubMed] [Google Scholar]
Guyatt 2011d
- Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso‐Coello P, Rind D, et al. GRADE guidelines 6. Rating the quality of evidence ‐‐ imprecision. Journal of Clinical Epidemiology 2011;64(12):1283‐93. [PUBMED: 21839614] [DOI] [PubMed] [Google Scholar]
Guyatt 2011e
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 7. Rating the quality of evidence ‐‐ inconsistency. Journal of Clinical Epidemiology 2011;64(12):1294‐302. [PUBMED: 21803546] [DOI] [PubMed] [Google Scholar]
Guyatt 2011f
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 8. Rating the quality of evidence ‐‐ indirectness. Journal of Clinical Epidemiology 2011;64(12):1303‐10. [PUBMED: 21802903] [DOI] [PubMed] [Google Scholar]
Guyatt 2011g
- Guyatt GH, Oxman AD, Sultan S, Glasziou P, Akl EA, Alonso‐Coello P, et al. GRADE guidelines: 9. Rating up the quality of evidence. Journal of Clinical Epidemiology 2011;64(12):1311‐6. [PUBMED: 21802902] [DOI] [PubMed] [Google Scholar]
Guyatt 2013
- Guyatt G, Oxman AD, Sultan S, Brozek J, Glasziou P, Alonso‐Coello P, et al. GRADE guidelines: 11. Making an overall rating of confidence in effect estimates for a single outcome and for all outcomes. Journal of Clinical Epidemiology 2013;66(2):151‐7. [PUBMED: 22542023] [DOI] [PubMed] [Google Scholar]
Guyatt 2013a
- Guyatt GH, Oxman AD, Santesso N, Helfand M, Vist G, Kunz R, et al. GRADE guidelines: 12. Preparing summary of findings tables ‐‐ binary outcomes. Journal of Clinical Epidemiology 2013;66(2):158‐72. [PUBMED: 22609141] [DOI] [PubMed] [Google Scholar]
Guyatt 2013b
- Guyatt GH, Thorlund K, Oxman AD, Walter SD, Patrick D, Furukawa TA, et al. GRADE guidelines: 13. Preparing summary of findings tables and evidence profiles ‐‐ continuous outcomes. Journal of Clinical Epidemiology 2013;66(2):173‐83. [PUBMED: 23116689] [DOI] [PubMed] [Google Scholar]
Guyatt 2013c
- Guyatt G, Andrews J, Oxman AD, Alderson P, Dahm P, Falck‐Ytter Y, et al. GRADE guidelines: 15. Going from evidence to recommendations: the significance and presentation of recommendations. Journal of Clinical Epidemiology 2013;66(7):719‐25. [DOI] [PubMed] [Google Scholar]
Hassan 2011
- Hassan M, Kaseb A. Epidemiology and pathogenesis of hepatocellular carcinoma. Hepatocellular Carcinoma: Targeted Therapy and Multidisciplinary Care. Springer, 2011:15‐25. [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hoffe 2010
- Hoffe SE, Finkelstein SE, Russell MS, Shridhar R. Nonsurgical options for hepatocellular carcinoma: evolving role of external beam radiotherapy. Cancer Control 2010;17(2):100‐10. [DOI] [PubMed] [Google Scholar]
Hollis 1999
- Hollis S, Campbell F. What is meant by intention to treat analysis? Survey of published randomised controlled trials. BMJ (Clinical Research Ed.) 1999;319:670‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hong 2016
- Hong TS, Wo JY, Yeap BY, Ben‐Josef E, McDonnell EI, Blaszkowsky LS, et al. Multi‐institutional phase II study of high‐dose hypofractionated proton beam therapy in patients with localized, unresectable hepatocellular carcinoma and intrahepatic cholangiocarcinoma. Journal of Clinical Oncology 2016;34(5):460‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Huo 2015
- Huo Y, Eslick GD. Transcatheter arterial chemoembolization plus radiotherapy compared with chemoembolization alone for hepatocellular carcinoma: a systematic review and meta‐analysis. JAMA Oncology 2015;1(6):756‐65. [DOI] [PubMed] [Google Scholar]
ICH‐GCP 1997
- International Conference on Harmonisation Expert Working Group. International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. ICH Harmonised Tripartite Guideline. Guideline for Good Clinical Practice CFR & ICH Guidelines. Vol. 1, Philadelphia (PA): Barnett International/PAREXEL, 1997. [Google Scholar]
Jihye 2012
- Jihye C, Jinsil S. Application of radiotherapeutic strategies in the BCLC‐defined stages of hepatocellular carcinoma. Liver Cancer 2012;1(3‐4):216‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kawashima 2005
- Kawashima M, Furuse J, Nishio T, Konishi M, Ishii H, Kinoshita T, et al. Phase II study of radiotherapy employing proton beam for hepatocellular carcinoma. Journal of Clinical Oncology 2005;23(9):1839‐46. [DOI] [PubMed] [Google Scholar]
Kim 2015
- Kim TH, Park J‐W, Kim Y‐J, Kim BH, Woo SM, Moon SH, et al. Phase I dose‐escalation study of proton beam therapy for inoperable hepatocellular carcinoma. Cancer Research and Treatment 2015;47(1):34‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kjaergard 2001
- Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta‐analyses. Annals of Internal Medicine 2001;135(11):982‐9. [DOI] [PubMed] [Google Scholar]
Klein 2013
- Klein J, Dawson LA. Hepatocellular carcinoma radiation therapy: review of evidence and future opportunities. International Journal of Radiation Oncology * Biology * Physics 2013;87(1):22‐32. [DOI] [PubMed] [Google Scholar]
Lee 2014
- Lee D, Seong J. Radiotherapeutic options for hepatocellular carcinoma with portal vein tumour thrombosis. Liver Cancer 2014;3(1):18‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lundh 2017
- Lundh A, Lexchin J, Mintzes B, Schroll JB, Bero L. Industry sponsorship and research outcome. Cochrane Database of Systematic Reviews 2017, Issue 2. [DOI: 10.1002/14651858.MR000033.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ma 2010
- Ma S, Jiao B, Liu X, Yi H, Kong D, Gao L, et al. Approach to radiation therapy in hepatocellular carcinoma. Cancer Treatment Reviews 2010;36(2):157‐63. [DOI] [PubMed] [Google Scholar]
Macaskill 2001
- Macaskill P, Walter SD, Irwig L. A comparison of methods to detect publication bias in meta‐analysis. Statistics in Medicine 2001;20:641‐54. [DOI] [PubMed] [Google Scholar]
Maffione 2013
- Maffione AM, Ferretti A, Vinjamuri S, Rubello D. The PERCIST criteria: an insightful appraisal. Nuclear Medicine Communications 2013;34(7):619‐20. [DOI] [PubMed] [Google Scholar]
Maida 2014
- Maida M, Orlando E, Cammà C, Cabibbo G. Staging systems of hepatocellular carcinoma: a review of literature. World Journal of Gastroenterology 2014;20(15):4141‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mantel 1959
- Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. Journal of the National Cancer Institute 1959;2(4):719‐48. [PubMed] [Google Scholar]
Meng 2009
- Meng M‐B, Cui Y‐L, Lu Y, She B, Chen Y, Guan Y‐S, et al. Transcatheter arterial chemoembolization in combination with radiotherapy for unresectable hepatocellular carcinoma: a systematic review and meta‐analysis. Radiotherapy and Oncology 2009;92(2):184‐94. [DOI] [PubMed] [Google Scholar]
Merle 2009
- Merle P, Mornex F, Trepo C. Innovative therapy for hepatocellular carcinoma: three‐dimensional high‐dose photon radiotherapy. Cancer Letters 2009;286(1):129‐33. [DOI] [PubMed] [Google Scholar]
Moher 1998
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. Lancet 1998;352(9128):609‐13. [DOI] [PubMed] [Google Scholar]
Mustafa 2013
- Mustafa RA, Santesso N, Brozek J, Akl EA, Walter SD, Norman G, et al. The GRADE approach is reproducible in assessing the quality of evidence of quantitative evidence syntheses. Journal of Clinical Epidemiology 2013;66(7):736‐42; quiz 742.e1‐5. [PUBMED: 23623694] [DOI] [PubMed] [Google Scholar]
Oliveri 2011
- Oliveri RS, Wetterslev J, Gluud C. Transarterial (chemo)embolisation for unresectable hepatocellular carcinoma. Cochrane Database of Systematic Reviews 2011, Issue 3. [DOI: 10.1002/14651858.CD004787.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Parmar 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815‐34. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Riaz 2011
- Riaz A, Memon K, Miller FH, Nikolaidis P, Kulik LM, Lewandowski RJ, et al. Role of the EASL, RECIST, and WHO response guidelines alone or in combination for hepatocellular carcinoma: radiologic‐pathologic correlation. Journal of Hepatology 2011;54(4):695‐704. [DOI] [PMC free article] [PubMed] [Google Scholar]
Royle 2003
- Royle P, Milne R. Literature searching for randomised controlled trials used in Cochrane reviews: rapid versus exhaustive searches. International Journal of Technology Assessment in Health Care 2003;19(4):591‐603. [DOI] [PubMed] [Google Scholar]
Savović 2012
- Savović J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomised, controlled trials. Health Technology Assessment 2012;16(35):1‐82. [DOI] [PubMed] [Google Scholar]
Savović 2012a
- Savović J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Annals of Internal Medicine 2012;157(6):429‐38. [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. [DOI] [PubMed] [Google Scholar]
Seong 2009
- Seong J. Challenge and hope in radiotherapy of hepatocellular carcinoma. Yonsei Medical Journal 2009;50(5):601‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Taefi 2013
- Taefi A, Abrishami A, Nasseri‐Moghaddam S, Eghtesad B, Sherman M. Surgical resection versus liver transplant for patients with hepatocellular carcinoma. Cochrane Database of Systematic Reviews 2013, Issue 6. [DOI: 10.1002/14651858.CD006935.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Thorlund 2009
- Thorlund K, Devereaux PJ, Wetterslev J, Guyatt G, Ioannidis JP, Thabane L, et al. Can trial sequential monitoring boundaries reduce spurious inferences from meta‐analyses. International Journal of Epidemiology 2009;38(1):276‐86. [DOI] [PubMed] [Google Scholar]
Thorlund 2010
- Thorlund K, Anema A, Mills E. Interpreting meta‐analysis according to the adequacy of sample size. An example using isoniazid chemoprophylaxis for tuberculosis in purified protein derivative negative HIV‐infected individuals. Clinical Epidemiology 2010;2:57‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thorlund 2011
- Thorlund K, Engstrøm J, Wetterslev J, Brok J, Imberger G, Gluud C. User manual for Trial Sequential Analysis (TSA). ctu.dk/tsa/files/tsa_manual.pdf 2011 (accessed 20 June 2016).
TSA 2011 [Computer program]
- Copenhagen Trial Unit. TSA ‐ Trial Sequential Analysis. Version 0.9 Beta. Copenhagen: Copenhagen Trial Unit, 2011.
Tsai 2013
- Tsai CL, Koong AC, Hsu FM, Graber M, Chen IS, Cheng JC. Biomarker studies on radiotherapy to hepatocellular carcinoma. Oncology 2013;84(Suppl 1):64‐8. [DOI] [PubMed] [Google Scholar]
Ursino 2012
- Ursino S, Greco C, Cartei F, Colosimo C, Stefanelli A, Cacopardo B, et al. Radiotherapy and hepatocellular carcinoma: update and review of the literature. European Review of Medical and Pharmacological Sciences 2012;16(11):1599‐604. [PubMed] [Google Scholar]
Venook 2010
- Venook AP, Papandreou C, Furuse J, Guevara LL. The incidence and epidemiology of hepatocellular carcinoma: a global and regional perspective. Oncologist 2010;15(Suppl 4):5‐13. [DOI] [PubMed] [Google Scholar]
Weis 2013
- Weis S, Franke A, Mössner J, Jakobsen JC, Schoppmeyer K. Radiofrequency (thermal) ablation versus no intervention or other interventions for hepatocellular carcinoma. Cochrane Database of Systematic Reviews 2013, Issue 12. [DOI: 10.1002/14651858.CD003046.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wetterslev 2008
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta‐analysis. Journal of Clinical Epidemiology 2008;61(1):64‐75. [DOI] [PubMed] [Google Scholar]
Wetterslev 2009
- Wetterslev J, Thorlund K, Brok J, Gluud C. Estimating required information size by quantifying diversity in a random‐effects meta‐analysis. BMC Medical Research Methodology 2009;9:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wigg 2010
- Wigg AJ, Palumbo K, Wigg DR. Radiotherapy for hepatocellular carcinoma: systematic review of radiobiology and modelling projections indicate reconsideration of its use. Journal of Gastroenterology and Hepatology 2010;25(4):664‐71. [DOI] [PubMed] [Google Scholar]
Wood 2008
- Wood L, Egger M, Gluud LL, Schulz KF, Jüni P, Altman GD, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta‐epidemiological study. BMJ (Clinical Research Ed.) 2008;336:601‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yau 2008
- Yau T, Chan P, Epstein R, Poon RT. Evolution of systemic therapy of advanced hepatocellular carcinoma. World Journal of Gastroenterology 2008;14(42):6437‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zou 2014
- Zou L‐Q, Zhang B‐L, Chang Q, Zhu F‐P, Li Y‐Y, Wei Y‐Q, et al. 3D conformal radiotherapy combined with transcatheter arterial chemoembolization for hepatocellular carcinoma. World Journal of Gastroenterology 2014;20(45):17227‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]


