Abstract
Previous studies have not adequately considered the influence of adiposity and activity-related energy expenditure (AEE) on gut microbe diversity. We determined associations of gut microbiota diversity with measures of cardiorespiratory fitness while accounting for the potential effects of %body fat and free-living AEE. Cancer treatment uniquely triggers multiple physiologic shifts detrimental to overall health. Though previous research indicates a link between gut microbiota and cardiorespiratory fitness, it is unclear whether these findings are due to potential underlying effects %body fat or free-living activity energy expenditure (AEE). Microbe composition of fecal specimens from 37 breast cancer survivors were determined using 16S microbiome analyses. Individual-sample microbiota diversity (α-diversity) and between-sample community differences (β-diversity) were examined. Peak oxygen uptake (V̇O2peak) was estimated from a graded exercise test (GXT) consistent with the modified-Naughton protocol, in which, exercise terminates at 85% age-predicted heart rate max (HRmax). AEE was measured over 10-days using doubly-labeled water wherein %body fat was calculated from total body water. Pearson correlations revealed α-diversity indices (Chao1, Observed Species, PD Whole Tree, Shannon) positively associated with V̇O2peak (r = 0.34 to 0.51; p < 0.05) whereas %HRmax during Stages 1–4 of the GXT (r = −0.34 to −0.50; p < 0.05) and %body fat (r = −0.32 to −0.41; p < 0.05) were negatively associated with the same α-diversity indices. Multiple linear regression models showed V̇O2peak accounted for 22% and 26% of the variance in taxonomic richness (Observed Species) and phylogenic diversity after adjustment for %body fat and menopausal status. Unweighted UniFrac (β-diversity) was significant for several outcomes involving cardiorespiratory fitness and significant taxa comparisons were found. Associations between gut microbiota and free-living AEE were not found. Results from the present work suggest cardiorespiratory fitness, not physical activity, is a superior correlate of gut microbiota diversity.
Keywords: cardiovascular, doubly-labeled water, energy expenditure, gut microbiome, maximal oxygen uptake
INTRODUCTION
Early detection and targeted therapies have improved 5-year relative survival rates (89%), such that, the number of breast cancer survivors (BCS) in United States is projected to soon exceed 4 million (Miller et al., 2016). Regrettably, among these women, the combined influence of cancer treatment and advancing age frequently coincides with several physiological shifts detrimental to cardio-metabolic health including increased adiposity (Vance et al., 2011), dysbiosis (Sheflin Whitney and Weir., 2014), and reduced cardiorespiratory fitness (Jones et al., 2012).
Compelling evidence indicates an active cross-talk exists between the gut and brain wherein endocrine cell-mediated interactions from gut microbiota and enterochromaffin cells perform neuro-immune response modulation and regulatory functions (Furness and Clerc, 2000; Rhee, Pothoulakis and Mayer, 2009). While approximately two-thirds of gut microbes are reflective of diet and lifestyle (Qin et al., 2010), species diversity is generally considered representative of a healthy commensal gut microbiota. Dysregulation of this relative balance (i.e., dysbiosis) may play an underlying role as previous research in murine models has shown that an increased ratio of Firmicutes (p) to Bacteriodetes (p) corresponds with obesity (Turnbaugh et al., 2006). In recent human studies, uneven clustering of gut microbes have been associated with cardiovascular disease (Kelly et al., 2016) and type II diabetes (Larsen et al., 2010). Thus, gut microbiota diversity has been proposed as an emerging biomarker of health status (Bian et al., 2017).
Since habitual exercise represents a cornerstone to the prevention of cardio-metabolic disease, several studies have explored the connection between cardiorespiratory fitness and gut microbiota diversity (Estaki et al., 2016; Yang et al., 2017). Of the few available human investigations, Estaki et al. (2016) reported peak oxygen uptake (V̇O2) explained ≈20% of the variance in gut microbiota diversity (i.e., species richness) among young, healthy men and women. Alternatively, others have reported that the association between cardiorespiratory fitness and gut microbiota diversity may be mediated by %body fat (Yang et al., 2017). Still, it is unknown if underlying differences in activity-related energy expenditure (AEE) are contributing to the relationship between cardiorespiratory fitness and gut microbiota, which is particularly important given the prevalence of obesity and insufficient physical activity among BCS. Given the myriad of direct/indirect consequences of cancer treatment, as well as, the propensity for obesity and insufficient physical activity, it remains unclear if cardiorespiratory fitness associates with gut microbiota diversity in BCS.
Gut microbes have long been theorized to modify estrogen metabolism (Adlercreutz et al., 1984). Consistent with this principle, Flores and colleagues (2012) reported taxonomic richness (e.g., Shannon index, Observed Species) positively associated with circulating, non-ovarian estrogens in men and postmenopausal women. While previous research has shown a positive association between body mass index (BMI) and estrogen (Boyapati et al., 2004), it is understandable that the risk of incident estrogen-related diseases, like breast cancer, are linked with obesity in postmenopausal women. Though habitual physical activity is thought to modulate this relationship (McTiernan et al., 2006), it is unclear whether the benefits of physical activity are driven by changes in adiposity or gut microbiota, as both associate with systemic estrogens. Recent comparative analyses of microbiota from urine and local breast tissue (Wang et al., 2017), as well as gut microbes (Goedert et al., 2015) have revealed compositionally distinct microbiomic profiles between women with and without breast cancer. Thus, a complex interaction exists between gut microbiota diversity, adiposity, menopausal status, and free-living physical activity.
Therefore, the current study sought to examine the associations between gut microbiota diversity with multiple measures of cardiorespiratory fitness while accounting for the potential influence of %body fat, menopausal status, and AEE among a cohort of post-primary treatment, non-metastatic BCS. Based on previous findings, gut microbiota diversity may be a correlate of health status, and thus, closely linked with substrate delivery and utilization as evidenced by peak V̇O2. Consequently, we hypothesized peak V̇O2 and percent of age-predicted maximal heart rate during exercise, would associate with species richness and evenness (α-diversity). Similar to previous work (Paulsen et al., 2017), it was hypothesized that measures of cardiorespiratory fitness (e.g., peak V̇O2) would associate with between-sample community differences (β-diversity) with the potential for significant taxa comparison differences.
METHODS
Ethical approval
All study procedures were conducted in accordance with the ethical guidelines set forth by the local institutional review board (IRB-121114008) at the University of Alabama at Birmingham and the Declaration of Helsinki (ClincalTrials.gov NCT00929617). Written informed consent was obtained from all participants prior to study involvement.
Participants and design
Thirty-seven (n = 37) post-primary treatment BCS volunteered for the present cross-sectional, proof-of-concept investigation. Several recruitment strategies were used including periodical advertisement, institutional cancer registry, and contact with local cancer support groups. Preliminary phone screenings were conducted with interested respondents. Inclusion criteria were: 1) English-speaking females between the ages of 18–70 with a previous diagnosis of ductal carcinoma in situ or stage I-IIIA breast cancer, 2) physician’s clearance for study participation, 3) ambulatory, 4) not currently receiving or planning to receive radiation/chemotherapy, and 5) participating in < 30 minutes of vigorous-intensity physical activity or < 60 minutes of moderate-intensity physical activity per week for at least 6 months. Exclusion criteria were: 1) contraindications to exercise, 2) recurrent or metastatic breast cancer, 3) antibiotic use within the previous week, 4) dementia/psychological disorders preventing study participation, 5) non-ambulatory, and 6) current participation in an exercise study. Eligible participants were scheduled for an orientation visit, during which, study expectations and testing procedures were explained. Testing procedures were performed over three visits within 10 days. With the exception of prescription medications, participants were asked to abstain from alcohol, caffeine, and smoking at least 12 hours before each visit. Vigorous-intensity physical activity was restricted at least 24 hours prior to testing. Following an overnight fast, participants reported to the Physical Activity Core Laboratory between 0730 and 0900.
Instrumentation and Measurements
A self-reported questionnaire was administered to collect descriptive data concerning age, race, medical history (i.e., breast cancer status, months since diagnosis), menopausal status, antibiotic use history, and comorbidities. Dietary components (grams per day of carbohydrate, sugar, and fiber) were quantified using a 3-day food record that included two weekdays and one weekend. Weight (kg) and standing height (m) were measured to calculate BMI. Following exhalation, a tape measure with a 4 oz. tension indicator was used to determine waist circumference. Physical activity patterns over a 10-day period were measured by a hip-worn triaxial accelerometer (GT3X, Pensacola, FL). In agreement with accepted physical activity guidelines (Physical Activity Guidelines Advisory Committee, 2009), average minutes of vigorous-intensity (≥5725 counts∙min−1) were doubled then added to minutes of moderate-intensity (1952–5724 counts∙min−1) to quantitate moderate-to-vigorous physical activity (MVPA; min·week−1). Average MVPA was calculated from daily wear-time and used for analyses.
Resting Energy Expenditure
Resting energy expenditure (REE) was determined while participants laid quietly awake in a softly lit, well-ventilated room. Ambient temperature was maintained between 22–23 °C. Oxygen uptake (V̇O2) and carbon dioxide (V̇CO2) production were continuously measured for 30 minutes using indirect calorimetry (Vmax Encore, Loma Linda, CA) coupled with a ventilated canopy. Energy expenditure (kcals) and respiratory quotient (V̇CO2:V̇O2) were averaged in 60 second intervals by Vmax software using a modified-Weir equation. The concluding 20 minutes were used for analyses.
Standardized Treadmill Task
A two-stage, standardized treadmill task was used to evaluate physiological responses at submaximal intensities in a subset of participants (n = 18). Following the equilibration period, breath-by-breath V̇O2 and V̇CO2 were measured via indirect calorimetry (MAX II, Pittsburgh, PA). Participants performed two 4 minute stages of 0.89 m/s at 0% and 2.5% grade. Steady-state V̇O2, defined as the highest 30 second average during each stage was used for analyses. Oxygen-pulse, representing the V̇O2 used by peripheral tissues per heartbeat, was calculated from steady-state V̇O2 (mLO2∙kg−1∙min−1) divided by the corresponding heart rate (bpm). Units are expressed in mLO2∙kg−1∙beat−1 to account for weight-bearing nature of walking and variance in body mass. Higher values of oxygen-pulse indicate greater V̇O2 per heartbeat (index of stroke volume) (Oliveira, Myers, & de Araujo, 2011).
Graded Exercise Test
Within 5–6 days of completing the standardized treadmill task, a graded exercise test (GXT), consistent with the modified-Naughton protocol, was used to estimate peak V̇O2 from ACSM regression equations (American College of Sports Medicine, 2010). Treadmill workload was increased at two minute intervals until participants reached 85% of their age-predicted (220-age) maximum heart rate (HRmax). Heart rate was measured continuously (Polar Electro, Kempele, Finland) and used to calculate the percent of age-predicted HRmax (%HRmax) during each stage. Since Stage 4 (0.89 m/s at 7% grade) estimates a peak V̇O2 of 15.6 mLO2∙kg−1∙min−1, closely resembling the minimum peak V̇O2 needed for functional independence in older women (Shephard, 2009), Stages 1–4 were of interest and included for analyses. Heart rate recovery was measured at minutes one and two upon the termination of the GXT as indices of parasympathetic reactivation and sympathetic withdrawal, respectively.
Doubly-Labeled Water
Total energy expenditure (TEE) was calculated over a 10-day period under free-living conditions using doubly-labeled water (DLW) (Hunter et al., 2015). The DLW technique makes use of the inherent difference in elimination kinetics between deuterium (2H) and oxygen-18 (18O) in the body. Deuterium is lost solely through body water whereas 18O is lost from both water and carbon dioxide. Briefly, a baseline urine specimen was provided in the morning, after which, participants were administered an oral dose of 2H2O and H218O based on their measured body weight (54 g if ≤ 60 kg; 63 g if 60.1 to 75 kg; 74 g for 75.1 to 95 kg; and 89 g for > 95 kg). Post-dose urine samples were collected at +3 hours and +4 hours to permit isotopic equilibrium. Ten days later, two additional urine samples were collected in the morning. Carbon dioxide production rates were determined using a fixed constant for the dilution space ratio (1.0427) provided by Speakman et al. (Speakman, Nair and Goran., 1993) and converted to energy expenditure using the equation by de Weir (Weir, 1949): TEE (kcal∙d−1) = 3.9 (rCO2 / RQ) + 1.1, where rCO2 is the rate of carbon dioxide production in liters per day and RQ is the respiratory quotient (0.88) used for all participants. Urine samples were stored at −20 °C until measured in duplicate by isotope ratio mass spectrometry. Coefficient of variation (CV) for repeated-measures in our laboratory is 4.3%. Free-living AEE was estimated by initially reducing TEE by 10% to account for the thermogenic effects of digestion. AEE was then calculated by subtracting REE from the adjusted-TEE. Percent body fat was derived from total body water (Hunter et al., 2015).
Gut Microbiota Diversity
Gut microbiota were examined from fecal wipes processed according to the protocol described by Kumar et al. (2014) and Paulsen et al. (2017). In short, samples were collected and sent via overnight mail to our local Microbiome Resource Laboratory where they were stored at −80 °C until analyses were performed. Individual samples were dissolved in a buffer solution then processed with a DNA Miniprep Kit to acquire isolated fecal DNA. Polymerase chain reaction (PCR) was used to amplify the V4 region of the 16S rRNA gene then analyzed with Illumina MiSeq DNA sequencing. Two hundred fifty base paired-end sequences were obtained, and following filtering of low quality reads, the paired-end sequences were merged and analyzed. Quality control, operational taxonomic unit (OTU) picking, and taxonomic assignment were performed as previously described (Kumar et al., 2014). Within-sample microbiota diversity (α-diversity) was evaluated using Chao1, Shannon, and Simpson indices which measure gut microbiota richness and evenness. Additional α-diversity assessments were performed with Observed Species and PD Whole Tree which measure species richness and phylogeny, respectively. Between-sample microbiota community differences (β-diversity) were analyzed using weighted (quantitative) and unweighted (qualitative) UniFrac distance metrics (Lozupone and Knight., 2005). For β-diversity, the following continuous outcomes were divided into quartiles (i.e., the difference in the minimum and maximum values were divided by four): age, number of comorbidities, BMI, waist circumference, %body fat, months since cancer diagnosis, peak V̇O2, %HRmax Stages 1–4 of GXT, AEE, MVPA, sugar intake, carbohydrate intake, and fiber intake. The following outcomes were dichotomized: ethnicity (African-American vs. other), menopausal status (yes vs. no), history of chemotherapy (yes vs. no), and history of radiation (yes vs. no) with the original categories maintained for breast cancer stage (0 vs. 1 vs. 2 vs. 3 vs. 4) and hormonal therapy (none vs. ≤ 1 year vs. > 1 year).
Statistical Analyses
Participant characteristics are presented as means and standard deviations unless noted otherwise (Table 1). The Shapiro-Wilk test was used to confirm the normality of distributions. Two-tailed parametric (Pearson) correlations were performed on variables of interest. Based on consequent results, multiple linear regression was used to examine the associations for peak V̇O2 and %HRmax (Stage 3, GXT) on α-diversity while adjusting for %body fat and menopausal status. Of note, adjusting variables were included following significant bivariate association and/or plausible physiologic link to gut microbiota. Additionally, Stage 3 of the GXT was used as surrogate for cardiorespiratory fitness since all 37 participants completed the stage and workload matched a vigorous-intensity effort as evidenced by an average 70 ± 10% %HRmax. Collinearity of diagnostics for all variables were within acceptable limits as variable inflation factors for each model were less than 2.22. Separate univariate analyses were used to evaluate differences in α-diversity between participants in the highest quartile for peak V̇O2 (≥ 22.4 mLO2∙kg−1∙min−1) versus those below. all others. Substantive differences were determined with Hedges’ g as a measure of effect size: 0.2 as small; 0.5 as medium; and 0.8 as large. Principal coordinate analysis (PCoA) was used to visualize β-diversity differences among outcome categories or quartiles. Statistical significance of the β-diversity/outcomes relationships were determined using permutational multivariate analysis of variance (PERMANOVA). Kruskal-Wallis testing with false discovery rate (FDR) correction procedures compared relative taxa level frequencies using QIIME for variables with statistically significant β-diversity. The threshold of statistical significance for all tests was set a priori and defined as two-sided p-value ≤ 0.05 (or q-value ≤ 0.05 for analyses using FDR correction).
Table 1.
Participants characteristics (n = 37).
| Demographics | |
| Age (yrs.) | 55 ±9 |
| Race (no.(%)) | |
| African American | 16(43) |
| European American | 19(51) |
| Other | 2(6) |
| Comorbidities | 2.1 ± 1.9 |
| BMI (kg/m2) | 31.8 ± 7.8 |
| Waist circumference (cm) | 92 ± 16 |
| Body fat (%)† | 46 ± 7 |
| Cancer status | |
| Months since diagnosis | 53 ± 60 |
| Breast cancer stage (no.(%)) | |
| DCIS | 5 (14) |
| I | 11 (29) |
| II | 17(46) |
| III | 4(11) |
| Chemotherapy (yes) | 26(70) |
| Radiation (yes) | 20(54) |
| Fitness and heart rate responses | |
| Peak V02 (mL02 kg"1min1) | 20 ±5 |
| %HRmax stage 1 | 61 ± 7 |
| %HRmax stage 2 | 64 ± 8 |
| %HRmax stage 3 | 70 ± 10 |
| %HRmax stage 4 | 75 ± 11 |
| Physical activity measures | |
| TEE (kcal day_1)† | 2012 ±302 |
| AEE (kcal day_1)† | 650 ± 229 |
| MVPA (min week1) | 173 ± 90 |
| Diet‡ | |
| Sugar (g) | 85 ± 42 |
| Carbohydrates (g) | 206 ± 66 |
| Fiber (g) | 16 ± 8 |
| Alpha diversity (au) | |
| Chaol | 341 ± 44 |
| Observed species | 277± 50 |
| PD whole tree | 23± 4 |
| Shannon | 5± 1 |
| Simpson | 0.9± 0.08 |
n = 32;
n = 36;
BMI, body mass index; ductual incarcinoman in situ; Peak oxygen uptake (VO2) estimated from the graded exercise test; % HRmax stage 1–4, percent of age-predicted heart rate (HR) max derived(from 220-age)during stage 1–4 of modified-Naughton protocol; TEE, total energy expenditure; AEE, activity-related energy expenditure; MVPA,moderate-to-vigrorous physical activity. Diet represents daily averages. Values are shown as means and SD’s unless noted otherwise.
RESULTS
Overview
Descriptive data are shown in Table 1. Coinciding with established BMI stratifications, 21 of 37 (57%) of participants were obese (> 30 kg/m2) while 19 of 37 (51%) had an increased risk of cardio-metabolic disease as evidenced by a waist circumference > 88 cm (Janssen, Katzmarzyk & Ross., 2004). Accelerometry-based measurement showed 20 of 37 (54%) participants appeared to be meeting the minimum recommendation of accumulating at least 150 minutes of MVPA per week. Mean cohort data indicated peak V̇O2 was 20 ± 5 mLO2∙kg−1∙min−1 or approximately 33% greater than the minimum cardiorespiratory fitness needed for independent-living among older women (Shephard, 2009). Food record data showed that just 6 of 36 (17%) met the American Dietary Guidelines (22.4 g/day) for daily fiber intake in women aged 51 years and older (Department of Health and Human Services, 2015).
α-Diversity
Consistent with our hypotheses, zero-order correlation coefficients revealed several α-diversity indices significantly associated with markers of cardiorespiratory fitness including peak V̇O2 and %HRmax during the GXT (Table 2). Surprisingly, free-living AEE and TEE were not associated with α-diversity. Percent body fat, but not waist circumference, negatively associated (r = −0.32 to −0.41; p < 0.05) with Chao1, Observed Species, and PD Whole Tree. Menopausal status trended toward significance for Chao1 (r = −0.32; p = 0.057), PD Whole Tree (p = 0.10) and Observed Species (p = 0.11) suggesting premenopausal participants tended to have greater species richness. However, no other demographic or diet variable associated with α-diversity.
Table 2.
Bivariate correlation matrix (n = 37)
| Variables | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1) Peak VO2 | - | |||||||||||||||
| 2) %HRmax stage 1 | −0.77† | - | ||||||||||||||
| 3) %HRmax stage 2 | −0.83† | 0.97† | - | |||||||||||||
| 4) %HRmax stage 3 | −0.88† | 0.90† | 0.94† | - | ||||||||||||
| 5) %HRmax stage 4 | −0.53† | 0.56† | 0.62† | 0.56† | - | |||||||||||
| 6) Body fat (%) | −0.74† | 0.52† | 0.63† | 0.70† | 0.43* | - | ||||||||||
| 7) Waist circumference | −0.64† | 0.36* | 0.51† | 0.60† | 0.43* | 0.80† | - | |||||||||
| 8) TEE | −0.19 | −0.03 | 0.12 | 0.20 | 0.01 | 0.54† | 0.59† | - | ||||||||
| 9) AEE | −0.07 | −0.01 | 0.07 | 0.15 | −0.15 | 0.40† | 0.28 | 0.76† | - | |||||||
| 10) MVPA | −0.05 | 0.04 | 0.06 | 0.03 | 0.03 | 0.15 | 0.12 | 0.21 | 0.24 | - | ||||||
| 11)Chao1 | 0.45† | −0.38* | −0.41* | −0.44† | −0.35* | −0.41* | −0.23 | −0.24 | −0.03 | 0.02 | - | |||||
| 12) Observed species | 0.51† | −0.45† | −0.49† | −0.50† | −0.36* | −0.32* | −0.25 | −0.05 | 0.17 | 0.03 | 0.86† | - | ||||
| 13) PD whole tree | 0.51† | −0.40† | −0.45† | −0.47† | −0.28 | −0.38* | −0.23 | −0.10 | 0.04 | 0.01 | 0.89† | 0.94† | - | |||
| 14)Shannon | 0.34* | −0.30 | −0.34* | −0.35* | −0.37* | −0.33 | −0.18 | −0.01 | −0.04 | 0.01 | 0.54† | 0.68† | 0.66† | - | ||
| 15) Simpson | 0.17 | −0.19 | −0.24 | −0.21 | −0.34* | −0.27 | −0.15 | 0.02 | 0.01 | 0.13 | 0.30 | 0.42† | 0.40* | 0.89† | - | |
| 16) Menopausal status | −0.01 | 0.01 | 0.01 | −0.10 | 0.18 | −0.02 | 0.06 | 0.03 | −0.09 | −0.11 | −0.32 | −0.28 | −0.27 | −0.17 | −0.15 | - |
Peak oxygen uptake (V O2) estimated from the graded exercise test (mLO2kg−1min−1); %HRmax stage 1–4, percent of age-predicted heart rate (HR) max derived (from 220-age) during stage 1–4 of the modified-Naughton protocol; Body fat (%) (n = 32); TEE, total energy expenditure (n = 32); AEE, activity- related energy expenditure (n = 32); MVPA, moderate-to-vigorous physical activity measured with accelerometry; Menopausal status, participants dichotomized by 0 (no) and 1 (yes) indicating whether they experienced menopause.
p < 0.01;
p < 0.05.
Note all variables were analyzed using Pearson’s correlation.
In Table 3, multivariate regression modeling showed that peak V̇O2 was shown to account accounted for 22% and 26% of the variance in richness and phylogenic diversity as represented by Observed Species and PD Whole Tree, respectively. Participants were stratified into quartiles based on peak V̇O2 wherein analyses revealed participants (n = 13) in the highest quartile (≥ 22.4 mLO2∙kg−1∙min−1) exhibited significantly higher α-diversity scores compared to participants (n = 24) occupying the lower quartiles (Figure 1). Unadjusted scatterplots shown in Figure 2, illustrate the distribution of alpha diversity with respect to cardiorespiratory fitness. Given the resultant negative associations, it appears participants with greater cardiorespiratory fitness, as evidenced by a lower percentage of age-adjusted %HRmax, had higher gut microbiota diversity.
Table 3.
Modeling alpha diversity indices adjusted for peak V O2, percent body fat, and menopausal status (n = 32).
| R2 | partial r | p- value | |
|---|---|---|---|
| Model 1: Chaol | 0.38 | ||
| Peak VO2 | 0.34 | 0.07 | |
| %Body fat | −0.09 | 0.63 | |
| Menopausal status | −0.39* | 0.03 | |
| Model 2: Observed species | 0.37 | ||
| Peak VO2 | 0.47* | 0.01 | |
| %Body fat | 0.11 | 0.55 | |
| Menopausal status | −0.34 | 0.07 | |
| Model 3: PD Whole Tree | 0.43 | ||
| Peak VO2 | 0.51* | 0.004 | |
| %Body fat | 0.10 | 0.60 | |
| Menopausal status | −0.33 | 0.07 | |
| Model 4: Shannon | 0.21 | ||
| Peak VO2 | 0.24 | 0.19 | |
| Body fat | −0.06 | 0.74 | |
| Menopausal status | −0.24 | 0.21 | |
| Model 5: Simpson | 0.12 | ||
| Peak VO2 | 0.08 | 0.68 | |
| %Body fat | −0.14 | 0.47 | |
| Menopausal status | −0.21 | 0.27 | |
Peak oxygen uptake (V O2) estimated from the graded exercise test; percent (%) body fat calculated from isotopic total body water; menopausal status, participants dichotomized by 0 (no) and 1 (yes) indicating whether they experienced menopause.
p < 0.05.
Figure 1.
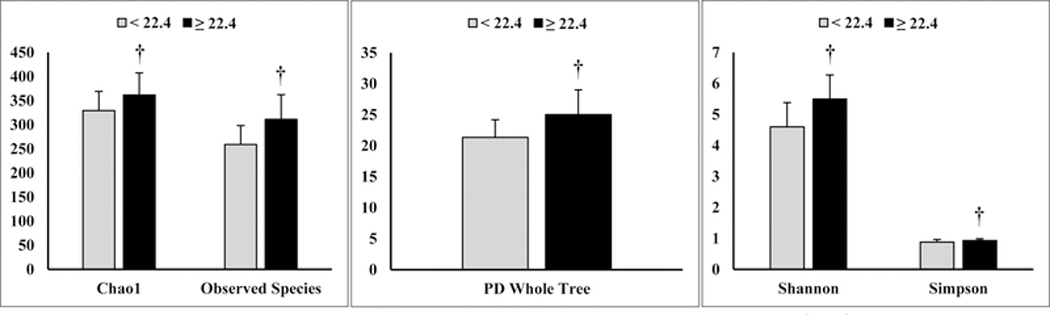
Unadjusted, between-groups comparisons of alpha diversity among participants above and below 22.4 mLO2·kg−1·min−1 VO2peak. Note participants (n = 13) above 22.4 mLO2·kg−1·min−1 had higher alpha diversity compared to those (n = 24) with a lower VO2peak. Substantive differences were determined by Hedges’ g as a measure of effect size: 0.77 (Chao1), 1.21 (Observed Species), 1.12 (PD Whole Tree), 1.15 (Shannon), 0.72 (Simpson). Data are represented by means and S.D. †denotes significance at p ≤ 0.05.
Figure 2:
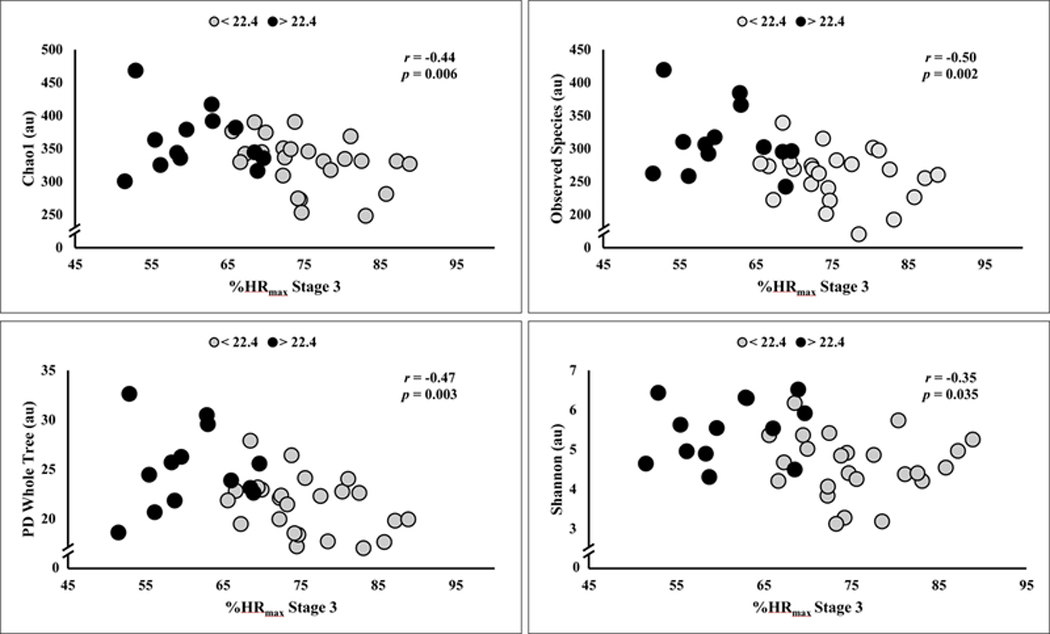
Unadjusted scatterplot of alpha diversity (e.g., Chao1, Observed Species, PD Whole Tree, and Shannon) and percent of age-predicted maximal heart rate (%HRmax) during Stage 3 of the graded exercise test. Mean intensity corresponded to 70 +/− 10% %HRmax. Note the tendency for greater aerobic fitness, evidenced by lower %HRmax associated with higher alpha diversity. Groups are dichotomized by VO2peak above and below 22.4 mLO2·kg−1·min-1. n = 37.
Shown in Table 4, %HRmax (Stage 3, GXT), adjusted for %body fat and menopausal status, accounted for 28% to 35% of the variance in three of the five models examining α-diversity. Oxygen-pulse during Stage 1 (r = 0.52 to 0.70) and 2 (r = 0.62 to 0.75) of the standardized treadmill task positively associated with several α-diversity indices. Increased aerobic capacity (represented by a greater V̇O2 per heartbeat) positively associated (r = 0.75; p = 0.001; n = 17) with PD Whole Tree. Interestingly, the correlation coefficients between oxygen-pulse and α-diversity were reliably stronger during the graded walking task. Additionally, heart rate recovery during minutes 1 and 2 were found to be negatively associated (r = −0.47 to −0.60; p < 0.05) with indices of α-diversity. As shown in Figure 3, a greater reduction in heart rate 1-minute post-exercise, indicative of greater parasympathetic reactivation, and thus, increased aerobic fitness associated with the Shannon index.
Table 4.
Modeling alpha diversity indices adjusted for heart rate max, percent body fat, and menopausal status (n = 32).
| R2 | partial r | p-value | |
|---|---|---|---|
| Model 1: Chao1 | 0.49 | ||
| %HRmax | −0.53* | 0.003 | |
| %Body fat | 0.01 | 0.96 | |
| Menopausal status | −0.49* | 0.006 | |
| Model 2: Observed species | 0.45 | ||
| %HRmax | −0.55* | 0.001 | |
| %Body fat | 0.15 | 0.43 | |
| Menopausal status | −0.43* | 0.02 | |
| Model 3: PD Whole Tree | 0.50 | ||
| %HRmax | −0.59* | 0.001 | |
| %Body fat | 0.12 | 0.53 | |
| Menopausal status | −0.44* | 0.02 | |
| Model 4: Shannon | 0.26 | ||
| %HRmax | −0.34 | 0.07 | |
| %Body fat | −0.03 | 0.89 | |
| Menopausal status | −0.29 | 0.12 | |
| Model 5: Simpson | 0.15 | ||
| %HRmax | −0.20 | 0.30 | |
| %Body fat | −0.07 | 0.70 | |
| Menopausal status | −0.23 | 0.21 | |
%HRmax, percent of age-predicted heart rate (HR) max derived (from 220-age) during stage 3 of the modified-Naughton protocol;
%body fat calculated from isotopic total body water; menopausal status, participants dichotomized by 0 (no) and 1 (yes) indicating whether they experienced menopause.
p ≤ 0.05.
Figure 3.
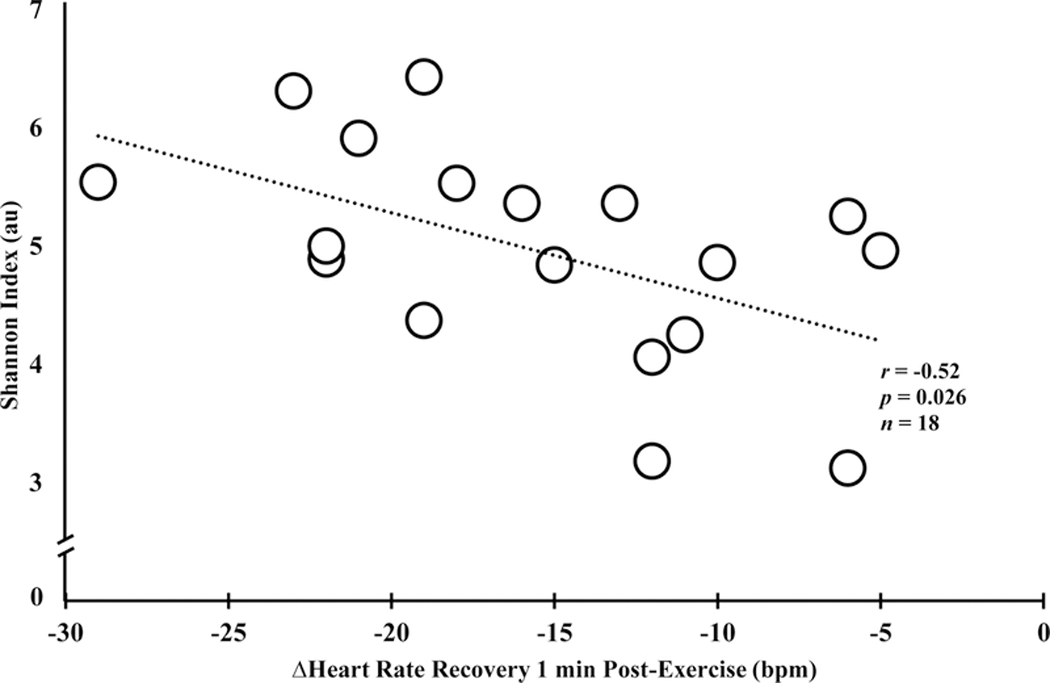
Unadjusted scatterplot of Shannon index and the change (Δ) in heart rate recovery 1-minute post-graded exercise test. Note the negative association depicting higher alpha diversity and greater parasympathetic reactivation, evidenced by more pronounced heart rate recovery. **significance at p < 0.05.
β-Diversity
With unweighted UniFrac clustering used to detect differences in specific taxa present, β-diversity differences were found for BMI (p = 0.043), waist circumference (p = 0.017), %HRmax during Stage 2 (p = 0.046) and Stage 3 (p = 0.016) of the GXT, along with peak V̇O2 (p = 0.04). Similar to our previous work (Paulsen, et al, 2017), quartiles for continuous variables were dichotomized (1 and 2 vs. 3 and 4) to test taxa differences responsible for the β-diversity findings. Quartiles 1 and 2 vs. 3 and 4 for %HRmax during Stage 2 and 3 of the GXT, exhibited statistically significant taxa differences following FDR correction for organisms within the phyla of Actinobacteria (p), Bacteroidetes (p), and Proteobacteria (p). Because visualization of the 3D PCoA plot showed distinct differences between quartile 1 and 4 of peak V̇O2, taxa comparisons were performed wherein significant taxa differences for Verrucomicrobia (p) and Verrucomicrobiae (c) were found after FDR correction (0.002737 vs. 0.047163 for quartile 1 vs. quartile 4; FDR corrected q-value = 0.024 and 0.002737 vs. 0.047163, q = 0.048, respectively).
Unweighted UniFrac clustering revealed significant differences for heart rate recovery at minute one (p = 0.047) and minute two (p = 0.018) post-exercise. However, following FDR correction significant taxa differences in heart rate recovery remained for minute one (parasympathetic reactivation) but not minute two (sympathetic withdrawal). Given that 30 different taxa were different, we report those OTUs at the genus level with differences noted for organisms within the phyla of Actinobacteria (p), Firmicutes (p), and Proteobacteria (p). Among the four aerobic fitness measures with taxa differences, Since several taxa were detected at a very low mean relative abundance suggesting these taxa make up a very minor population of microbes in the sample; hence, To aid reader interpretation, we provided the top 10 genera for peak V̇O2 (Figure 4). As indicated greater aerobic capacity is associated with higher relative abundance of Bacteroides (g) and Prevotella (g), lower relative abundance of Escherichia (g), and variable differences in multiple organisms within Firmicutes (p).
Figure 4.
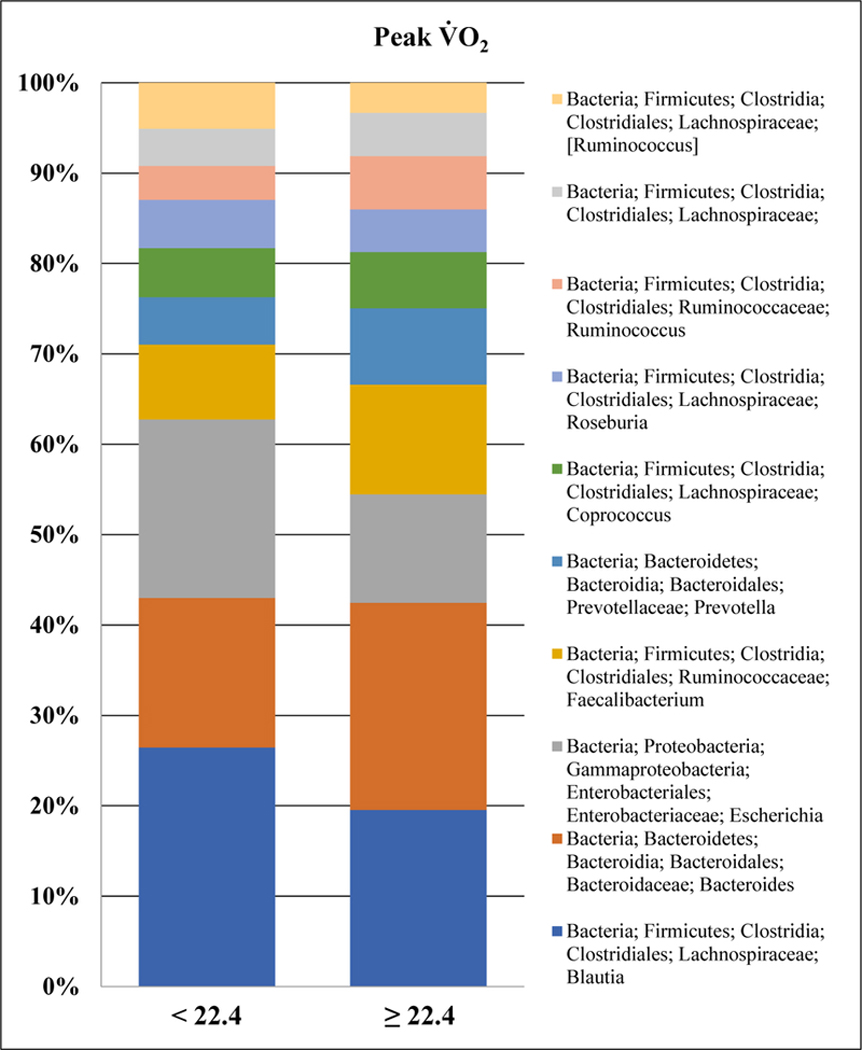
Top 10 genera truncated for peak V̇O2. Note that groups are dichotomized by VO2peak above and below 22.4 mLO2·kg−1·min-1.
DISCUSSION
Though previous work (Estaki et al., 2016; Yang et al., 2017) has shown a link between cardiorespiratory fitness and gut microbiota diversity, the underlying effects of %body fat and free-living AEE have not been previously examined. To this end, the hypotheses of the present investigation were based on the widely-accepted premise that %body and AEE both associate with cardiorespiratory fitness. Hence, we sought to explore the inter-associations between gut microbiota diversity and measures representative of cardiorespiratory fitness (e.g., peak V̇O2, %HRmax during exercise) while accounting for the potential influence of %body fat, menopausal status, and AEE. Consistent with our hypotheses, our findings revealed that peak V̇O2 accounted for 22% and 26% of the variance in taxonomic richness and phylogenic diversity, respectively, after adjustment for %body fat and menopausal status. Age-adjusted %HRmax during Stage 3 of the GXT accounted for 28% to 35% of the variance in 3 of 5 α-diversity regression models. Remarkably similar findings, linking higher aerobic fitness and greater gut microbiota diversity, were also found for oxygen-pulse during exercise and heart rate recovery. After FDR correction, significant taxa differences were observed for four aerobic fitness measures (peak V̇O2, %HRmax during Stages 2 and 3 of the GXT, and heart rate recovery at 1-minute post-exercise). Collectively, the present findings suggest aerobic fitness associates with gut microbiota diversity among post-primary treatment, non-metastatic BCS.
Whereas a high cardiorespiratory fitness is known to attenuate cardiovascular disease risk/progression, the contributing features are generally thought to involve favorable arterial compliance, autonomic tone, and endothelial function. However, our data adds further support to the probability that a high peak V̇O2 may also offset the risk of various chronic disease states through interactions with the gut microbiota. Similar to previous work (Estaki et al., 2016, Yang et al., 2017), our results demonstrate that peak V̇O2 associates with microbiota species richness and phylogenic diversity after adjustment for %body fat and menopausal status, while participants with peak V̇O2 ≥ 22.4 mLO2∙kg−1∙min−1 had greater α-diversity. Although our cross-sectional design restricts causal inferences we can make concerning peak V̇O2 and gut microbiota diversity, Allen and colleagues (2018) recently showed six weeks of aerobic training (in the absence of dietary modifications), altered gut microbiota and microbial-derived short chain fatty acids in a group of lean and obese participants. In their study, participants exhibited compositionally distinct microbiomic profiles at baseline (lean vs. obese) that disappeared following exercise training. Interestingly, among the obese participants, the changes in gut microbiota diversity corresponded with an improved maximal V̇O2. Certainly, these data highlight the responsive nature of gut microbiota to exercise training and strong link to cardiorespiratory fitness.
Due the systemic, cardio-metabolic benefits of physical activity, it is logical that insufficient quantities contribute to poorer health outcomes including dysbiosis (Nehra et al., 2016). However, in humans, the underlying mechanisms whereby physical activity and gut microbiota interact to effect host physiology remains largely unexplored. Data from animal models offers intriguing evidence supporting voluntary physical activity (i.e., wheel running) as an effective modulator of gut microbes (Allen et al., 2015; Matsumoto et al., 2008). While compelling, the translational implications to humans are less straight-forward, as it is difficult to disentangle the true physical activity/exercise-mediated effects on gut microbiota (or vice versa) under free-living conditions. Since variation in non-exercise activity thermogenesis is a key feature to overall health and weight maintenance, our group was surprised to discover weekly minutes of MVPA and AEE (kcals∙day−1) were unrelated to indices of α-diversity and β-diversity. Nevertheless, our data appears suggestive that cardiorespiratory fitness, not physical activity, is a better correlate of gut microbiota diversity. It is worth noting, our relatively small sample size may have restricted our ability to identify possible relationships between AEE and gut microbiota diversity, as the precision to measure free-living AEE via DLW is less than that to measure heart rate and V̇O2 (e.g., CV < 3% in our laboratory). It is intriguing to consider how genetic endowment for high cardiorespiratory fitness may interact with gut microbiota in defense against cardio-metabolic disease through the lifecycle.
While Estaki et al. (2016) did not report a significant association between cardiorespiratory fitness and β-diversity, it is possible youthful age (mean < 30 yrs.) and higher cardiorespiratory fitness may have contributed to these findings. In the present work, we identified a greater relative abundance of several organisms within the phyla of Bacteriodetes (p), Proteobacteria (p), Actinobacteria (p), and Firmicutes (p). These phyla include organisms involved in formation of short-chain fatty acids, possibly linking gut microbiota to health (Chakraborti, 2015), yet the organisms with significant taxa comparisons reported here are not among the more prevalent and known butyrate-producing bacteria (Louis and Flint, 2009). It is noteworthy that increased Bacteroidetes (p) and Verrucomicrobia (p), as found here with greater aerobic fitness, have been reported in mice after Roux-en Y (RYGB) gastric bypass surgery along with alterations in short chain fatty acid production (↑propionate and ↓acetate) (Liou et al., 2013). This same study reported an increase in Escherichia (g) in mice after RYGB, which is in contrast, to our finding that cardiorespiratory fitness may be associated with a decrease in the Escherichia (g) supporting the need for further research (Liou et al., 2013). Although no prior human study has reported an association between greater relative abundance of Verrucomicrobia (p) and cardiorespiratory fitness, this organism has been associated with obesity in humans (Zhang et al., 2009). Furthermore, several potential taxa differences (e.g., Proteobacteria (p)) warrant further study as potential links between exercise and improvements in psychosocial outcomes like depression (Jiang et al., 2015).
Despite the rapid increase in studies linking gut microbiota to health status, gut microbes have been known to influence estrogen metabolism for many decades (Adlercreutz et al., 1984). More recently, Flores et al. (2012) reported gut microbiota diversity positively associated with systemic, non-ovarian estrogens in men and postmenopausal women but not premenopausal women. Researchers from that study indicated negligible effects when adjustments were made for age and BMI. However, they did not have a measure for aerobic fitness. Similarly, age was not associated with gut microbiota α-diversity in the present work. Interestingly, self-reported menstrual status was a significant, independent correlate in 3 of 5 α-diversity regression models. In other words, participants who specified they had not experienced menopause exhibited greater α-diversity. While our results differ from previous work (Flores et al., 2012), it is interesting that menopausal status remained significant after adjusting for %HRmax and %body fat. It is important to note that men tend to have higher absolute aerobic capacities than women, and as such, the sample of men (n = 25) and postmenopausal women (n = 7) reported by Flores et al. (2012) may have simply had a greater aerobic fitness compared to the premenopausal women (n = 19). Still, it is difficult to completely reconcile our divergent findings since we did not have a direct measure of estrogen metabolites or control for menstrual phase, as this would require a specifically designed study.
Limitations
We recognize several limitations in the present investigation. Inherent with our cross-sectional design, we lack the ability to establish cause-and-effect or direction for the reported associations. Certainly the present outcomes are correlative in nature and should therefore be considered hypothesis-generating. Our participant sample was restricted to women with a history of breast cancer which tended to exhibit low cardiorespiratory fitness and multiple comorbidities. As such, generalizability of our results to other demographics should be performed with caution. Additionally, we do not include any functional outcomes (e.g., fecal concentrations of short chain fatty acids) of gut microbiota which are likely important for gut health. Nevertheless, strengths include the use of DLW to objectively measure free-living EE, as well as, evaluating taxa comparisons with several measures representative of aerobic capacity.
Conclusions
To our knowledge, this is the first study to examine the link between cardiorespiratory fitness and gut microbiota diversity while accounting for the underlying effects of %body fat and free-living AEE. Our primary findings showed that peak V̇O2 associated with taxonomic richness and phylogenic diversity while %HRmax during exercise accounted for 28% to 35% of the variance in α-diversity adjusted for %body fat and menopausal status. Consequently, it seems cardiorespiratory fitness, not physical activity, is a better correlate of gut microbiota diversity. Future work should include controlled-feeding (i.e., diet standardization) and exercise training to properly examine the longitudinal changes in gut microbiota. In doing so, researchers may be able to identify strategies and optimize therapeutic approaches to enhance health outcomes among clinical populations.
New Findings: What is the central question of this study? Does the link between cardiorespiratory fitness and gut microbiota diversity persist after adjusting for the potential effects of %body fat and activity-related energy expenditure (AEE)? What is the main finding and its importance? This is the first study to examine the link between cardiorespiratory fitness and gut microbiota diversity while accounting for the underlying effects of %body fat and free-living AEE. Results from the present work suggest cardiorespiratory fitness, not physical activity, is a superior correlate of gut microbiota diversity among post-primary treatment, non-metastatic breast cancer survivors.
Acknowledgements and Funding Information
We acknowledge the following funding sources from the National Institute of Health: R01CA136859, U01CA136859, R25CA76023 (CaRES), R25CA047888 (Cancer Prevention and Control Training Grant), and P30DK056336 (University of Alabama at Birmingham Nutrition Obesity Research Center). Additional support was provided by the Microbiome Resource at the University of Alabama at Birmingham: School of Medicine, Comprehensive Cancer Center (P30AR050948), Center for Clinical Translational Science (UL1TR000165), Multidisciplinary Clinical Research Center (P60AR064172), Microbiome Center and Heflin Center.
Footnotes
Competing Interests
None declared.
REFERENCES
- [1].Physical Activity Guidelines Advisory Committee report, 2008. To the Secretary of Health and Human Services. Part A: executive summary (2009). Nutr.Rev. 67, 114–120. [DOI] [PubMed] [Google Scholar]
- [2].U.S. Department of Health and Human Services and U.S. Department of Agriculture. 2015–2020 Dietary Guidelines for Americans. 8th Edition. December 2015. Available at http://health.gov/dietaryguidelines/2015/guidelines/.
- [3].Adlercreutz H, Pulkkinen MO, Hamalainen EK & Korpela JT, (1984). Studies on the role of intestinal bacteria in metabolism of synthetic and natural steroid hormones. J.Steroid Biochem. 20, 217–229. [DOI] [PubMed] [Google Scholar]
- [4].Allen JM, Berg Miller ME, Pence BD, et al. , (2015). Voluntary and forced exercise differentially alters the gut microbiome in C57BL/6J mice. J.Appl.Physiol.( 1985) 118, 1059–1066. [DOI] [PubMed] [Google Scholar]
- [5].Allen JM, Mailing LJ, Niemiro GM, et al. , (2018). Exercise Alters Gut Microbiota Composition and Function in Lean and Obese Humans. Med.Sci.Sports Exerc. 50, 747–757. [DOI] [PubMed] [Google Scholar]
- [6].American College of Sports Medicine, (2010). ACSM’s Guidelines for Exercise Testing and Prescription. Lippincott, Williams, and Wilkins, Baltimore. [Google Scholar]
- [7].Bian G, Gloor GB, Gong A, et al. , (2017). The Gut Microbiota of Healthy Aged Chinese Is Similar to That of the Healthy Young. mSphere 2, 10.1128/mSphere.00327-17. eCollection 2017 Sep-Oct. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Boyapati SM, Shu XO, Gao YT, et al. , (2004). Correlation of blood sex steroid hormones with body size, body fat distribution, and other known risk factors for breast cancer in post-menopausal Chinese women. Cancer Causes Control 15, 305–311. [DOI] [PubMed] [Google Scholar]
- [9].Chakraborti CK, (2015). New-found link between microbiota and obesity. World J.Gastrointest.Pathophysiol. 6, 110–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Estaki M, Pither J, Baumeister P, et al. , (2016). Cardiorespiratory fitness as a predictor of intestinal microbial diversity and distinct metagenomic functions. Microbiome 4, 42–016-0189–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Flores R, Shi J, Fuhrman B, et al. , (2012). Fecal microbial determinants of fecal and systemic estrogens and estrogen metabolites: a cross-sectional study. J.Transl.Med 10, 253–5876-10–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Furness JB & Clerc N, (2000). Responses of afferent neurons to the contents of the digestive tract, and their relation to endocrine and immune responses. Prog.Brain Res. 122, 159–172. [DOI] [PubMed] [Google Scholar]
- [13].Goedert JJ, Jones G, Hua X, et al. , (2015). Investigation of the association between the fecal microbiota and breast cancer in postmenopausal women: a population-based case-control pilot study. J.Natl.Cancer Inst. 107, 10.1093/jnci/djv147 Print 2015 Aug [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Heiss CN & Olofsson LE, (2017). Gut Microbiota-Dependent Modulation of Energy Metabolism. J.Innate Immun. November 8. doi: 20.2259/000481519 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hunter GR, Fisher G, Neumeier WH, Carter SJ & Plaisance EP, (2015). Exercise Training and Energy Expenditure following Weight Loss. Med.Sci.Sports Exerc. 47, 1950–1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Janssen I, Katzmarzyk PT & Ross R, (2004). Waist circumference and not body mass index explains obesity-related health risk. Am.J.Clin.Nutr. 79, 379–384. [DOI] [PubMed] [Google Scholar]
- [17].Jiang H, Ling Z, Zhang Y, et al. , (2015). Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav.Immun. 48, 186–194. [DOI] [PubMed] [Google Scholar]
- [18].Jones LW, Courneya KS, Mackey JR, et al. , (2012). Cardiopulmonary function and age-related decline across the breast cancer survivorship continuum. J.Clin.Oncol. 30, 2530–2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kelly TN, Bazzano LA, Ajami NJ, et al. , (2016). Gut Microbiome Associates With Lifetime Cardiovascular Disease Risk Profile Among Bogalusa Heart Study Participants. Circ.Res. 119, 956–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kumar R, Eipers P, Little RB, et al. , (2014). Getting started with microbiome analysis: sample acquisition to bioinformatics. Curr.Protoc.Hum.Genet. 82, 18.8.1–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Larsen N, Vogensen FK, van den Berg FW, et al. , (2010). Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS One 5, e9085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Liou AP, Paziuk M, Luevano JM,Machineni S Jr, Turnbaugh PJ & Kaplan LM, (2013). Conserved shifts in the gut microbiota due to gastric bypass reduce host weight and adiposity. Sci.Transl.Med. 5, 178ra41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Louis P & Flint HJ, (2009). Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol.Lett. 294, 1–8. [DOI] [PubMed] [Google Scholar]
- [24].Lozupone C & Knight R, (2005). UniFrac: a new phylogenetic method for comparing microbial communities. Appl.Environ.Microbiol. 71, 8228–8235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Matsumoto M, Inoue R, Tsukahara T, et al. , (2008). Voluntary running exercise alters microbiota composition and increases n-butyrate concentration in the rat cecum. Biosci.Biotechnol.Biochem. 72, 572–576. [DOI] [PubMed] [Google Scholar]
- [26].McTiernan A, Wu L, Chen C, et al. , (2006). Relation of BMI and physical activity to sex hormones in postmenopausal women. Obesity (Silver Spring) 14, 1662–1677. [DOI] [PubMed] [Google Scholar]
- [27].Miller KD, Siegel RL, Lin CC, et al. , (2016). Cancer treatment and survivorship statistics, 2016. CA Cancer.J.Clin. 66, 271–289. [DOI] [PubMed] [Google Scholar]
- [28].Nehra V, Allen JM, Mailing LJ, Kashyap PC & Woods JA, (2016). Gut Microbiota: Modulation of Host Physiology in Obesity. Physiology (Bethesda) 31, 327–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Oliveira RB, Myers J, & de Araujo CGS, (2011). Long-term stability of the oxygen pulse curve during maximal exercise. Clinics (Sao Paulo) 66, 203–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Paulsen JA, Ptacek TS, Carter SJ, et al. , (2017). Gut microbiota composition associated with alterations in cardiorespiratory fitness and psychosocial outcomes among breast cancer survivors. Support.Care Cancer 25, 1563–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Qin J, Li R, Raes J, et al. , (2010). A human gut microbial gene catalogue established by metagenomic sequencing. Nature 464, 59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Rhee SH, Pothoulakis C & Mayer EA, (2009). Principles and clinical implications of the brain-gut-enteric microbiota axis. Nat.Rev.Gastroenterol.Hepatol. 6, 306–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Sheflin AM, Whitney AK & Weir TL, (2014). Cancer-promoting effects of microbial dysbiosis. Curr.Oncol.Rep. 16, 406–014-0406–0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Shephard RJ, (2009). Maximal oxygen intake and independence in old age. Br.J.Sports Med. 43, 342–346. [DOI] [PubMed] [Google Scholar]
- [35].Speakman JR, Nair KS & Goran MI, (1993). Revised equations for calculating CO2 production from doubly labeled water in humans. Am.J.Physiol. 264, E912–7. [DOI] [PubMed] [Google Scholar]
- [36].Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER & Gordon JI, (2006). An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444, 1027–1031. [DOI] [PubMed] [Google Scholar]
- [37].Vance V, Mourtzakis M, McCargar L & Hanning R, (2011). Weight gain in breast cancer survivors: prevalence, pattern and health consequences. Obes.Rev. 12, 282–294. [DOI] [PubMed] [Google Scholar]
- [38].Wang H, Altemus J, Niazi F, et al. , (2017). Breast tissue, oral and urinary microbiomes in breast cancer. Oncotarget 8, 88122–88138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].WEIR JB, (1949). New methods for calculating metabolic rate with special reference to protein metabolism. J.Physiol. 109, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Yang Y, Shi Y, Wiklund P, et al. , (2017). The Association between Cardiorespiratory Fitness and Gut Microbiota Composition in Premenopausal Women. Nutrients 9, 10.3390/nu9080792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Zhang H, DiBaise JK, Zuccolo A, et al. , (2009). Human gut microbiota in obesity and after gastric bypass. Proc.Natl.Acad.Sci.U.S.A. 106, 2365–2370. [DOI] [PMC free article] [PubMed] [Google Scholar]


