Abstract
BACKGROUND
Minimally invasive surgery was adopted as an alternative to laparotomy (open surgery) for radical hysterectomy in patients with early-stage cervical cancer before high-quality evidence regarding its effect on survival was available. We sought to determine the effect of minimally invasive surgery on all-cause mortality among women undergoing radical hysterectomy for cervical cancer.
METHODS
We performed a cohort study involving women who underwent radical hysterectomy for stage IA2 or IB1 cervical cancer during the 2010–2013 period at Commission on Cancer–accredited hospitals in the United States. The study used inverse probability of treatment propensity-score weighting. We also conducted an interrupted time-series analysis involving women who underwent radical hysterectomy for cervical cancer during the 2000–2010 period, using the Surveillance, Epidemiology, and End Results program database.
RESULTS
In the primary analysis, 1225 of 2461 women (49.8%) underwent minimally invasive surgery. Women treated with minimally invasive surgery were more often white, privately insured, and from ZIP Codes with higher socioeconomic status, had smaller, lower-grade tumors, and were more likely to have received a diagnosis later in the study period than women who underwent open surgery. Over a median follow-up of 45 months, the 4-year mortality was 9.1% among women who underwent minimally invasive surgery and 5.3% among those who underwent open surgery (hazard ratio, 1.65; 95% confidence interval [CI], 1.22 to 2.22; P = 0.002 by the log-rank test). Before the adoption of minimally invasive radical hysterectomy (i.e., in the 2000–2006 period), the 4-year relative survival rate among women who underwent radical hysterectomy for cervical cancer remained stable (annual percentage change, 0.3%; 95% CI, −0.1 to 0.6). The adoption of minimally invasive surgery coincided with a decline in the 4-year relative survival rate of 0.8% (95% CI, 0.3 to 1.4) per year after 2006 (P = 0.01 for change of trend).
CONCLUSIONS
In an epidemiologic study, minimally invasive radical hysterectomy was associated with shorter overall survival than open surgery among women with stage IA2 or IB1 cervical carcinoma. (Funded by the National Cancer Institute and others.)
Women with early-stage cervical cancer can be treated with surgery or radiotherapy, but most undergo surgery.1-4 For women with stage IA2 or IB1 cervical cancer (tumors <4 cm in the greatest dimension that are confined to the cervix), radical hysterectomy is associated with cure rates in excess of 80%.5-8 Traditionally, radical hysterectomy has been performed as open surgery through a laparotomy incision; however, this approach is associated with considerable perioperative and long-term complications.7-9
Randomized clinical trials have shown that survival after minimally invasive surgery is similar to survival after open surgery among patients with early-stage uterine, colorectal, or gastric cancer.10-13 Furthermore, minimally invasive hysterectomy has led to a lower risk of infection and faster recovery than open surgery.14 The first laparoscopic radical hysterectomy for cervical cancer was reported in 1992.15 Since then, numerous single-institution series and observational cohort studies have shown that the procedure is feasible and is associated with less blood loss, shorter postoperative hospitalization, and fewer complications than open surgery.16-30 However, although some studies concluded that minimally invasive techniques did not negatively affect oncologic outcomes, these studies were limited by low power,19-30 uncertain generalizability,19-29 and probable residual confounding.27-30 Data on long-term survival assessed in randomized trials or large, well-designed observational studies have been limited.
Despite the paucity of high-quality evidence supporting the use of minimally invasive radical hysterectomy for cervical cancer, this approach has been broadly adopted in the United States and is considered to be a standard approach in national guidelines.1,31 In this study, we used data from two large cancer registries to compare all-cause mortality among patients with cervical cancer who underwent minimally invasive surgery and those who underwent open radical hysterectomy, and we evaluated whether the adoption of minimally invasive radical hysterectomy affected national trends in 4-year relative survival rates.
METHODS
DATA SOURCE
In the main patient-level analysis, we used the National Cancer Database, a cancer registry that is maintained by the American College of Surgeons and the American Cancer Society. This database includes data from patients who have been treated at Commission on Cancer–accredited centers and covers approximately 70% of newly diagnosed cancer cases in more than 1500 hospitals in the United States (see the Supplementary Appendix, available with the full text of this article at NEJM.org).32 Because the National Cancer Database public-use files lack data on patients who received a diagnosis before 2004, we also used the April 2017 release of the Surveillance, Epidemiology, and End Results (SEER) 18-registry database to perform an interrupted time-series analysis of how the adoption of minimally invasive radical hysterectomy affected survival trends in the United States. The SEER program is a population-based cancer registry that covers 28% of the U.S. population.
COHORT SELECTION
In patient-level analyses, we included women with stage IA2 or IB1 squamous-cell carcinoma, adenosquamous carcinoma, or adenocarcinoma of the cervix who had received a diagnosis during the 2010–2013 period and had undergone radical hysterectomy as primary treatment. We excluded women for whom the surgical approach was unknown, those who had a preexisting cancer diagnosis, those for whom there was a lack of pathological confirmation of cancer, those who had received neoadjuvant chemotherapy or radio-therapy, those who did not undergo complete pelvic lymphadenectomy, and those for whom the lymphadenectomy status was unknown. In the interrupted time-series analysis, we included all the patients in the SEER 18-registry database who had locoregionally confined cervical carcinoma and had undergone radical hysterectomy and lymphadenectomy during the 2000–2010 period (see the Supplementary Appendix).
MEASURES
Cancer registrars documented the primary surgical approach as open, laparoscopic, or robot-assisted. In the primary intention-to-treat analysis, all the patients whose surgical procedure was initiated by a laparoscopic or robot-assisted approach were categorized as having undergone minimally invasive surgery, even when conversion to open surgery occurred.
The primary outcome of interest was the time to death, as recorded by the cancer registrar and ascertained through the end of 2016. Additional outcomes included the 4-year survival rate, death within 90 days after surgery, number of lymph nodes evaluated, frequency of positive lymph nodes, parametrial involvement, and positive surgical margins.
COVARIATES
Control variables included the demographic, socioeconomic, and clinical variables that are tabulated in Table S1 in the Supplementary Appendix. Disease was categorized according to the International Classification of Diseases for Oncology, third edition, as squamous-cell carcinoma, adenocarcinoma, or adenosquamous carcinoma. The stage of disease was categorized according to the International Federation of Gynecology and Obstetrics system for cervical cancer and defined according to the American Joint Committee on Cancer (AJCC), seventh edition, clinical-stage variable when available, Collaborative Stage Site-Specific Factor 1 when the AJCC clinical stage was unknown, and the AJCC pathologic stage when the former two variables were unknown.33
We categorized patients’ county of residence as metropolitan, metropolitan adjacent, or rural, using the U.S. Department of Agriculture 2003 Rural–Urban Continuum Codes classification.34 ZIP Code–level estimates of median income and the proportion of residents without a high-school diploma were categorized into quartiles and were used as proxy measures of patients’ income and educational level. Insurance status was categorized as private insurance, Medicare, Medicaid or other type of government insurance, or uninsured. The treating facility was categorized as academic or nonacademic, according to Commission on Cancer criteria.35 We identified patients who had one or more coexisting conditions using the Charlson Comorbidity Index value provided by the National Cancer Database.36
STATISTICAL ANALYSIS
In the main patient-level analysis, we used inverse probability of treatment weighting that was based on propensity score to construct a weighted cohort of patients who differed with respect to surgical approach but were similar with respect to other measured characteristics.37,38 To calculate the inverse probability of treatment weights, we estimated each patient’s propensity to under-go minimally invasive radical hysterectomy, using a logistic-regression model that included predictor variables that had been selected on the basis of their a priori possibility of confounding the relationship between surgical approach and survival (age, race or ethnic group, facility type, geographic region, rural or urban status, insurance status, ZIP Code–level income and educational levels, presence of coexisting conditions, histologic type, tumor grade, stage of disease, year of diagnosis, and tumor size). We assigned patients who underwent minimally invasive surgery a weight of 1 ÷ (propensity score) and those who underwent open surgery a weight of 1 ÷ (1 – propensity score).39 To reduce the variability in the inverse probability of treatment–weighted models, we used stabilized weights.40 We assessed balance among covariates using absolute standardized differences; a difference of 10% or less was considered to indicate a well-balanced result.39 Population-level (marginal) hazard-ratio effects (under the assumption of the absence of unmeasured confounding) that are estimated by propensity-score methods are more like the effects estimated in a randomized, controlled trial than those estimated by means of multivariable Cox regression.40,41
We compared the distributions of categorical variables using the chi-square test in the unweighted cohort and weighted logistic-regression models in the weighted cohort. In the propensity-score–weighted cohort, we compared survival, perioperative outcomes, and pathologic outcomes between the open-surgery group and the group that underwent minimally invasive surgery. We compared all-cause mortality using the inverse probability of treatment–weighted log-rank test and plotted weighted survival functions.42 We estimated the hazard ratio for death from any cause after minimally invasive radical hysterectomy, as compared with open surgery, with weighted Cox proportional-hazards models.
We performed several sensitivity analyses to assess the robustness of our findings. To ensure that treatment-related survival differences were not confounded by a differential use of adjuvant therapy, the survival model was refitted with postoperative treatment as a covariate (radiotherapy, chemoradiotherapy, chemotherapy, or no further treatment). We further evaluated whether the use of indicator variables for missing data introduced bias into our results by performing a multiple-imputation analysis. We also assessed the robustness of our main results by using alternative analytic strategies, such as model selection with the use of Hosmer and Lemeshow’s purposeful-selection approach43 and multivariable Cox regression, and propensity-score matching. To confirm that the observed associations were not the result of unobserved differences between hospitals that had adopted minimally invasive surgery and those that had not, we repeated the primary analysis after excluding patients who had been treated at centers that performed no minimally invasive radical hysterectomies during the study period. Details of the sensitivity analyses are provided in the Supplementary Appendix. To explore whether the observed association differed according to the minimally invasive method (traditional laparoscopy vs. robot-assisted laparoscopy), tumor size in the greatest dimension (≥2 cm vs. <2 cm), or histologic type, we estimated the hazard ratios that were associated with minimally invasive surgery after refitting separate propensity-score–weighted survival models for each subgroup.
To test whether the findings of the patient-level analysis might be due to a causal effect of minimally invasive radical hysterectomy, we conducted a quasi-experimental interrupted time-series analysis using the SEER 18-registry database.44 We hypothesized that if the association in the main analysis was due to a causal effect, the adoption of minimally invasive radical hysterectomy would influence survival trends among women undergoing radical hysterectomy for cervical cancer in the United States. The 4-year relative survival rate was used as the primary outcome in this analysis to adjust for the effect of noncancer-related mortality trends.45 According to published data,31 2006 was the year in which surgeons in the United States began to adopt minimally invasive radical hysterectomy for the treatment of cervical cancer; the use of the procedure increased from 1.8% of the cases in 2006 to 31.1% of the cases in 2010. We used data from the 2000–2006 period to estimate the trends before adoption, and we fitted a weighted least-squares model to test whether the trend in the 4-year relative survival rate changed in 2006 (see the Supplementary Appendix).
RESULTS
STUDY POPULATION
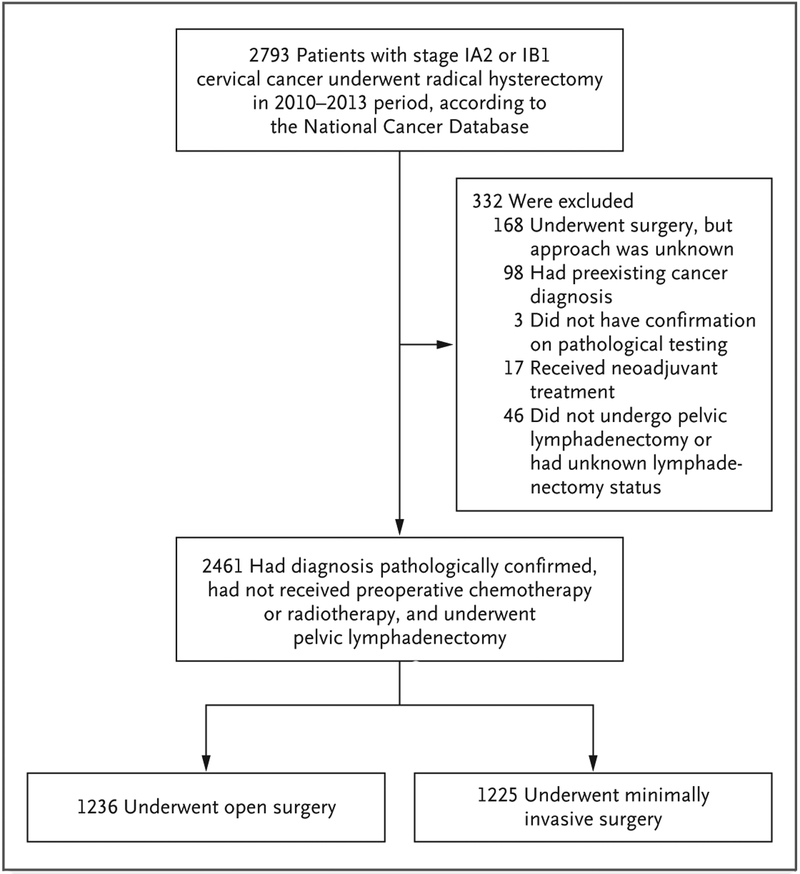
During the 2010–2013 period, 2461 women with data in the National Cancer Database underwent radical hysterectomy for stage IA2 or IB1 cervical carcinoma and met the inclusion criteria (Fig. 1); of these women, 1225 (49.8%) underwent minimally invasive surgery. Of the women who underwent minimally invasive surgery, 978 (79.8%) underwent robot-assisted laparoscopy. Conversion from minimally invasive surgery to open surgery was rare overall (2.9% of the cases) but was more frequent among cases that were initiated with traditional laparoscopy (8.9%; 95% confidence interval [CI], 5.4 to 12.5) than among those initiated with robot-assisted laparoscopy (1.3%; 95% CI, 0.6 to 2.1).
Figure 1. Study Population.
Table 1 summarizes selected demographic and clinical characteristics of the study population before and after propensity-score weighting. (Additional characteristics are shown in Table S1 in the Supplementary Appendix.) Women who underwent minimally invasive surgery were more likely to be white, were more often privately insured, were more likely to have received a diagnosis later in the study period, and were more likely to reside in ZIP Codes that were associated with higher income and educational levels than those who underwent open surgery. Women who underwent minimally invasive surgery were also more likely to have undergone treatment in the Northeast and South and at nonacademic facilities and more often had smaller, lower-grade tumors and adenocarcinomas. The covariates were well balanced in the propensity-weighted cohort, with all the standardized differences less than 10% (data not shown).
Table 1.
Selected Characteristics of Patients Who Underwent Radical Hysterectomy for Stage IA2 or IB1 Cervical Carcinoma, According to Surgical Approach, before and after Inverse Probability of Treatment Weighting.*
| Characteristic | Cohort before Inverse Probability of Treatment Weighting |
Cohort after Inverse Probability of Treatment Weighting |
||||
|---|---|---|---|---|---|---|
| Open Surgery (N=1236) |
Minimally Invasive Surgery (N = 1225) |
P Value† | Open Surgery (N = 1340) |
Minimally Invasive Surgery (N = 1334) |
P Value‡ | |
| number (percent) | number (percent) | |||||
| Year of diagnosis | <0.001 | 1.00 | ||||
| 2010 | 408 (33.0) | 211 (17.2) | 338 (25.2) | 336 (25.2) | ||
| 2011 | 310 (25.1) | 317 (25.9) | 336 (25.1) | 334 (25.1) | ||
| 2012 | 268 (21.7) | 356 (29.1) | 344 (25.7) | 342 (25.6) | ||
| 2013 | 250 (20.2) | 341 (27.8) | 323 (24.1) | 322 (24.1) | ||
| Race or ethnic group§ | <0.001 | 1.00 | ||||
| White | 789 (63.8) | 853 (69.6) | 899 (67.1) | 896 (67.2) | ||
| Black | 160 (12.9) | 95 (7.8) | 140 (10.4) | 140 (10.5) | ||
| Hispanic | 196 (15.9) | 169 (13.8) | 196 (14.6) | 191 (14.3) | ||
| Asian | 71 (5.7) | 82 (6.7) | 83 (6.2) | 84 (6.3) | ||
| Other or unknown | 20 (1.6) | 26 (2.1) | 23 (1.7) | 23 (1.7) | ||
| Facility type | <0.001 | 0.94 | ||||
| Nonacademic | 544 (44.0) | 654 (53.4) | 657 (49.0) | 656 (49.2) | ||
| Academic | 692 (56.0) | 571 (46.6) | 683 (51.0) | 678 (50.8) | ||
| Stage of disease | 0.04 | 0.94 | ||||
| IA2 | 127 (10.3) | 159 (13.0) | 157 (11.7) | 155 (11.6) | ||
| IB1 | 1109 (89.7) | 1066 (87.0) | 1183 (88.3) | 1179 (88.4) | ||
| Histologic type | 0.01 | 1.00 | ||||
| Squamous cell | 789 (63.8) | 709 (57.9) | 820 (61.2) | 815 (61.1) | ||
| Adenocarcinoma | 381 (30.8) | 452 (36.9) | 450 (33.6) | 450 (33.7) | ||
| Adenosquamous | 66 (5.3) | 64 (5.2) | 70 (5.2) | 69 (5.2) | ||
| Tumor size | 0.005 | 0.99 | ||||
| <2 cm | 459 (37.1) | 534 (43.6) | 543 (40.5) | 541 (40.6) | ||
| ≥2 cm | 615 (49.8) | 543 (44.3) | 626 (46.7) | 624 (46.8) | ||
| Unknown | 162 (13.1) | 148 (12.1) | 171 (12.8) | 169 (12.6) | ||
Counts in the weighted cohort may not sum to expected totals owing to rounding. Percentages may not total 100 because of rounding, and disagreements between numbers and percentages in the weighted cohort are the result of rounding of noninteger number values.
The P value was calculated by the chi-square test.
The P values were calculated by inverse probability of treatment–weighted logistic-regression models.
Race and ethnic group were ascertained from the medical records by cancer registrars.
SURVIVAL ANALYSIS
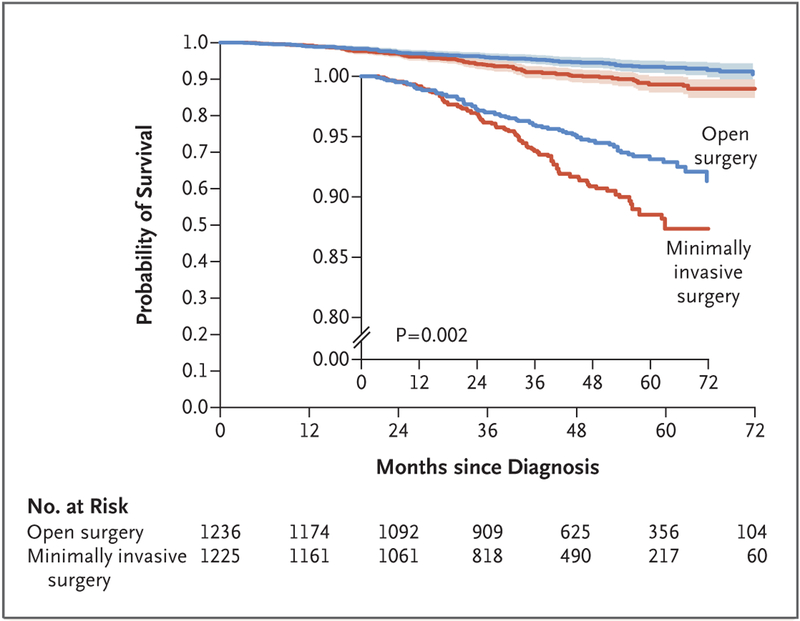
The median follow-up in the propensity-score–weighted cohort was 45 months. A total of 94 deaths occurred in the minimally invasive surgery group and 70 in the open-surgery group. Weighted survival functions for the minimally invasive surgery group and the open-surgery group are plotted in Figure 2. In propensity-score–weighted analyses, the risk of death within 4 years after diagnosis was 9.1% in the minimally invasive surgery group and 5.3% in the open-surgery group. Women who underwent minimally invasive surgery had shorter overall survival than those who underwent open surgery, which corresponds to a 65% higher risk of death from any cause (hazard ratio, 1.65; 95% CI, 1.22 to 2.22; P=0.002 by the log-rank test).
Figure 2. Inverse Probability of Treatment–Weighted Survival Curves among Women with Stage IA2 or IB1 Cervical Cancer, According to Type of Surgery.
Shaded bands represent the 95% confidence interval. Women who underwent minimally invasive surgery had shorter overall survival than those who underwent open surgery (P = 0.002 by the log-rank test). The at-risk table shows the actual number of patients at risk. The inset shows the same data on an enlarged y axis.
HISTOPATHOLOGICAL OUTCOMES AND ADJUVANT THERAPY
After propensity-score weighting, we found that women who underwent minimally invasive surgery were similar to those who underwent open surgery in terms of histopathological variables. In the minimally invasive surgery group and the open-surgery group, respectively, the mean numbers of lymph nodes evaluated were 20.2 (95% CI, 19.6 to 20.8) and 19.2 (95% CI, 18.6 to 19.8), the rates of parametrial invasion were 11.0% (95% CI, 9.1 to 13.2) and 9.5% (95% CI, 7.7 to 11.6), the rates of positive margins were 5.0% (95% CI, 3.7 to 6.6) and 4.4% (95% CI, 3.2 to 6.0), the rates of lymph-node involvement were 10.7% (95% CI, 8.9 to 12.9) and 8.9% (95% CI, 7.2 to 11.0), and the rates of lymphovascular space invasion were 31.9% (95% CI, 28.9 to 35.0) and 28.0% (95% CI, 25.1 to 31.0). Perioperative deaths were rare, with 1 death occurring in the minimally invasive surgery group and 3 deaths in the open-surgery group. We observed similar rates of administration of adjuvant radiotherapy and adjuvant chemotherapy in the minimally invasive surgery group and the open-surgery group (radiotherapy: 22.1% [95% CI, 19.5 to 24.9] and 20.9% [95% CI, 18.4 to 23.7], respectively; chemotherapy: 16.8% [95% CI, 14.5 to 19.4] and 13.6% [95% CI, 11.6 to 16.0], respectively).
SENSITIVITY ANALYSES
In sensitivity analyses, all-cause mortality remained higher in the minimally invasive surgery group than in the open-surgery group, after adjustment for adjuvant treatment (hazard ratio, 1.62; 95% CI, 1.20 to 2.19). The exclusion of patients who were treated in hospitals that did not perform minimally invasive radical hysterectomy did not alter our findings substantially (hazard ratio, 1.55; 95% CI, 1.22 to 1.96). Alternative analytic strategies yielded consistent results, including the multiple imputation of missing variables followed by inverse probability of treatment weighting (hazard ratio, 1.65; 95% CI, 1.23 to 2.23), multivariable Cox regression after model selection (hazard ratio, 1.76; 95% CI, 1.29 to 2.41), propensity-score matching (hazard ratio, 1.64; 95% CI, 1.14 to 2.35), and propensity-score matching with covariate adjustment (hazard ratio, 1.74; 95% CI, 1.21 to 2.49) (see the Supplementary Appendix).
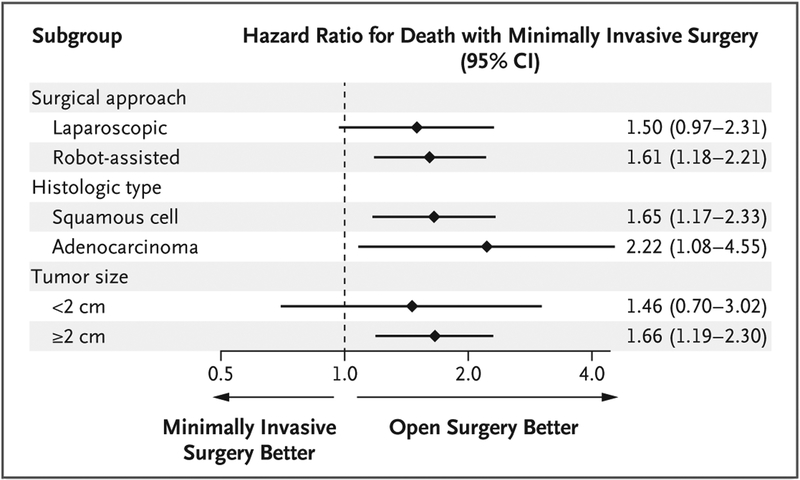
In exploratory subgroup analyses, robot-assisted radical hysterectomy was associated with a higher risk of death than open surgery (hazard ratio, 1.61; 95% CI, 1.18 to 2.21), as was traditional laparoscopic radical hysterectomy (hazard ratio, 1.50; 95% CI, 0.97 to 2.31) (Fig. 3). The greater relative hazard that was associated with minimally invasive surgery than with open surgery was evident across histologic types and tumor sizes.
Figure 3. Subgroup Analyses.
Subgroup analyses show the associations between minimally invasive radical hysterectomy and all-cause mortality according to mode of minimally invasive surgery (laparoscopic approach vs. robot-assisted approach), histologic type (squamous-cell carcinoma vs. adenocarcinoma), and tumor size in the greatest dimension (<2 cm vs. ≥2 cm). Diamonds represent point estimates for the hazard ratio as compared with open surgery, and horizontal lines indicate the associated 95% confidence intervals. Separate propensity-score models were fitted to predict the probability of minimally invasive surgery for each subgroup, and hazard ratios were estimated with the use of inverse probability of treatment–weighted Cox proportional-hazards models.
INTERRUPTED TIME-SERIES ANALYSIS
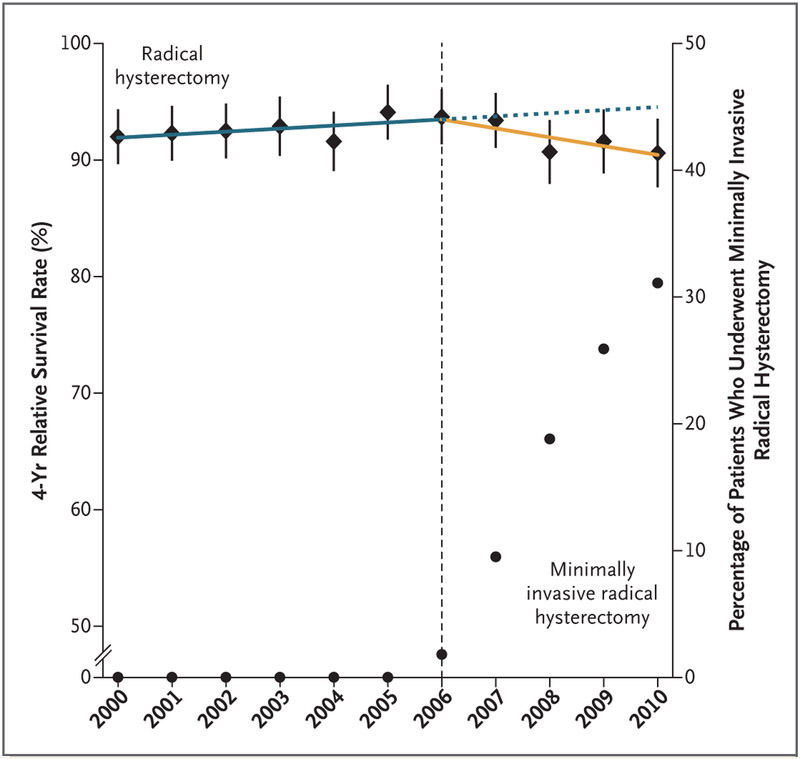
Results of the interrupted time-series analysis are shown in Figure 4. Before the adoption of minimally invasive radical hysterectomy in the United States (i.e., in the 2000–2006 period), a nonsignificant trend toward longer survival over time was noted among women who underwent radical hysterectomy for early-stage cervical cancer (annual percentage change, 0.3%; 95% CI, −0.1 to 0.6). The adoption of minimally invasive surgery was associated with a significant change of trend (P = 0.01) and coincided with the beginning of a decline in the 4-year relative survival rate of 0.8% (95% CI, 0.3 to 1.4) per year between 2006 and 2010 in this population.
Figure 4. Interrupted Time-Series Evaluation of the Effect of Adoption of Minimally Invasive Radical Hysterectomy on 4-Year Relative Survival Rate.
Shown are the 4-year relative survival rates among women who underwent radical hysterectomy for cervical cancer by any surgical approach (diamonds) with 95% confidence intervals (error bars) and the percentages of radical hysterectomies that were undertaken with the use of a minimally invasive approach (circles). The adoption of minimally invasive radical hysterectomy in 2006 was associated with a significant change of temporal trend (as indicated by the dotted blue line) (P=0.01) and a declining 4-year relative survival rate after 2006 (yellow line) (annual percentage change, 0.8%; 95% CI, 0.3 to 1.4).
DISCUSSION
Our findings suggest that minimally invasive surgery was associated with a higher risk of death than open surgery among women who underwent radical hysterectomy for early-stage cervical cancer. This association was apparent regardless of laparoscopic approach (robot-assisted or traditional), tumor size, or histologic type. This finding was consistent across several analytic approaches and robust to multiple sensitivity analyses. Furthermore, we observed that the adoption of minimally invasive surgery in the United States, starting in 2006, coincided with the beginning of a decline in 4-year relative survival rates among women undergoing radical hysterectomy for cervical cancer.
The association between minimally invasive surgery and shorter survival that was observed in the patient-level analysis could be due to the selection of patients or to unmeasured confounding. However, patients who underwent minimally invasive surgery would be predicted to have longer survival than those who underwent open surgery on the basis of their younger age, higher socioeconomic status, and lower tumor grade. Furthermore, unmeasured confounders such as human immunodeficiency virus (HIV) infection and tobacco use, which are risk factors for poor survival among patients with cervical cancer, are associated with low socioeconomic status46,47 and were therefore likely to be more common among women who underwent open surgery than among those who underwent minimally invasive surgery. In addition, previous National Cancer Database studies that compared minimally invasive surgery and open surgery in patients with early-stage ovarian or endometrial cancer did not show that minimally invasive surgery was associated with inferior survival, which suggests that the observed effect is unique to cervical cancer.48,49 Finally, the interrupted time-series analysis, which showed that the adoption of minimally invasive techniques coincided with a decline in cancer-related survival, is not subject to a patient-selection bias.
Our findings differ from those of earlier retrospective studies and conflict with a consensus within the field of gynecologic oncology that supports the use of minimally invasive surgery in early-stage cervical cancer.2,3,16-29,50 However, previous studies were considerably smaller than the present study, and most had shorter follow-up as well.19-30 For example, in one of the largest published investigations reporting the results of long-term follow-up, Nam and colleagues23 studied data from 526 patients with stage IA2 or IIA cervical cancer who underwent laparoscopic surgery or open surgery and found that, after a median follow-up of 91 months, women who underwent minimally invasive surgery did not have a significantly higher risk of death than those who underwent open surgery (hazard ratio, 1.46; 95% CI, 0.6 to 3.4). However, the hazard ratio that they observed was similar to ours, and given that there were only 23 deaths, the study was underpowered to detect a clinically meaningful difference between groups.
An important limitation of the present study is our inability to explain why minimally invasive surgery was associated with shorter survival. There may be limits to the extent of resection that can be achieved that are inherent to minimally invasive radical hysterectomy. For example, open surgery may allow for greater anterior traction on the uterus, thereby facilitating wider resection at the uterosacral ligaments and parametria. Although we observed no meaningful difference in the frequency of positive margins, close surgical margins may have been more common in the minimally invasive surgery group than in the open-surgery group, which could explain the observed difference in survival.51 It is also possible that uterine manipulators, which are frequently used for retraction and visualization during minimally invasive hysterectomy, may disseminate tumor cells. Alternatively, it may be that minimally invasive surgery is not inherently inferior to open surgery but that the patients in this study were treated by surgeons who were more experienced with open radical hysterectomy than with minimally invasive surgery. Cancer control after radical prostatectomy appears to be sensitive to surgeon experience,52 and a similar effect may exist in minimally invasive radical hysterectomy. If the observed findings are the result of a learning curve, we would expect that survival differences among patients undergoing open surgery and those undergoing minimally invasive surgery would diminish as surgeons gain experience with minimally invasive surgery. Further studies are needed to better understand the mechanisms leading to shorter survival.
Another limitation of this study is the absence of information about recurrence or cause of death in the National Cancer Database. In addition, although the National Cancer Database includes 70% of new cancer diagnoses, our findings may not generalize to patients who were treated in other settings.32 On the other hand, SEER registries, although they are population-based, are located in regions that have greater proportions of nonwhite and economically disadvantaged residents than are in the general U.S. population.53 Although the populations that were included in the main analysis of the National Cancer Database and the interrupted time-series may overlap, they were distinct with respect to geographic region, study period, treating facilities, and definition of disease stage. We were unable to confirm the accuracy of clinical, pathologic, exposure, or outcome data in either database directly. In addition, we were unable to estimate precisely the associations between minimally invasive surgery and all-cause mortality among subgroups in which few deaths occurred, such as the subgroup of women who had tumors smaller than 2 cm in the greatest dimension. Finally, although we are not aware of another change in cervical-cancer treatment that coincided with the adoption of minimally invasive surgery, if such a change had occurred, it could be responsible for the trends that were observed in the interrupted time-series analysis.
In conclusion, among women with stage IA2 or IB1 cervical cancer who underwent radical hysterectomy, minimally invasive surgery was associated with shorter survival than open surgery. The results of a prospective, randomized assessment of minimally invasive radical hysterectomy for cervical cancer that are reported in this issue of the Journal are consistent with our findings.54 The reasons for this differential effect on survival are not clear from our work.
Supplementary Material
Acknowledgments
Supported by grants (P30CA016672, 4P30CA060553-22, and R25CA092203) from the National Cancer Institute, by a grant (K12HD050121-12) from the National Institute of Child Health and Human Development, and by the American Association of Obstetricians and Gynecologists Foundation, the Foundation for Women’s Cancer, the Jean Donovan Estate, and the Phebe Novakovic Fund.
Dr. Wright reports receiving consulting fees from Clovis Oncology and Tesaro; and Dr. Kocherginsky, holding patents (U.S. patent numbers, 8710035, 9149485, and 9623032), licensed to Corcept Therapeutics, on methods and compositions related to glucocorticoid-receptor antagonists and breast cancer, for which she receives royalties, and a pending patent (U.S. patent number, 15448827), licensed to Corcept Therapeutics, on methods and compositions related to glucocorticoid-receptor antagonists and breast cancer, for which she receives royalties. No other potential conflict of interest relevant to this article was reported.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
Contributor Information
Alexander Melamed, Division of Gynecologic Oncology, Vincent Department of Obstetrics and Gynecology, Massachusetts General Hospital, Harvard Medical School, Boston
Daniel J. Margul, Division of Gynecologic Oncology, Department of Obstetrics and Gynecology, Prentice Women’s Hospital, Northwestern University, Feinberg School of Medicine, Chicago
Ling Chen, Department of Obstetrics and Gynecology and Herbert Irving Comprehensive Cancer Center, Columbia University, and New York Presbyterian Hospital, New York
Nancy L. Keating, Department of Health Care Policy, Harvard Medical School, and the Division of General Internal Medicine, Brigham and Women’s Hospital, Boston
Marcela G. del Carmen, Division of Gynecologic Oncology, Vincent Department of Obstetrics and Gynecology, Massachusetts General Hospital, Harvard Medical School, Boston
Junhua Yang, Division of Gynecologic Oncology, Department of Obstetrics and Gynecology, Prentice Women’s Hospital, Northwestern University, Feinberg School of Medicine, Chicago
Brandon-Luke L. Seagle, Division of Gynecologic Oncology, Department of Obstetrics and Gynecology, Prentice Women’s Hospital, Northwestern University, Feinberg School of Medicine, Chicago
Amy Alexander, Division of Gynecologic Oncology, Department of Obstetrics and Gynecology, Prentice Women’s Hospital, Northwestern University, Feinberg School of Medicine, Chicago
Emma L. Barber, Division of Gynecologic Oncology, Department of Obstetrics and Gynecology, Prentice Women’s Hospital, Northwestern University, Feinberg School of Medicine, Chicago
Laurel W. Rice, Division of Gynecologic Oncology, Department of Obstetrics and Gynecology, University of Wisconsin School of Medicine and Public Health, Madison
Jason D. Wright, Department of Obstetrics and Gynecology and Herbert Irving Comprehensive Cancer Center, Columbia University, and New York Presbyterian Hospital, New York
Masha Kocherginsky, Division of Gynecologic Oncology, Department of Obstetrics and Gynecology, Prentice Women’s Hospital, Northwestern University, Feinberg School of Medicine, Chicago; Division of Biostatistics, Department of Preventive Medicine, Northwestern University, Feinberg School of Medicine, Chicago
Shohreh Shahabi, Division of Gynecologic Oncology, Department of Obstetrics and Gynecology, Prentice Women’s Hospital, Northwestern University, Feinberg School of Medicine, Chicago
J. Alejandro Rauh-Hain, Departments of Gynecologic Oncology and Reproductive Medicine and Health Services Research, University of Texas M.D. Anderson Cancer Center, Houston
REFERENCES
- 1.National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: cervical cancer, version 1.2018. (http://oncolife.com.ua/doc/nccn/Cervical_Cancer.pdf). [DOI] [PubMed]
- 2.Colombo N, Carinelli S, Colombo A, Marini C, Rollo D, Sessa C. Cervical cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2012;23:Suppl 7:vii27–vii32. [DOI] [PubMed] [Google Scholar]
- 3.Petignat P, Roy M. Diagnosis and management of cervical cancer. BMJ 2007;335:765–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bansal N, Herzog TJ, Shaw RE, Burke WM, Deutsch I, Wright JD. Primary therapy for early-stage cervical cancer: radical hysterectomy vs radiation. Am J Obstet Gynecol 2009;201(5):485.e1–9. [DOI] [PubMed] [Google Scholar]
- 5.Peters WA III, Liu PY, Barrett RJ II, et al. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J Clin Oncol 2000;18:1606–13. [DOI] [PubMed] [Google Scholar]
- 6.Sedlis A, Bundy BN, Rotman MZ, Lentz SS, Muderspach LI, Zaino RJ. A randomized trial of pelvic radiation therapy versus no further therapy in selected patients with stage IB carcinoma of the cervix after radical hysterectomy and pelvic lymphadenectomy: a Gynecologic Oncology Group study. Gynecol Oncol 1999;73:177–83. [DOI] [PubMed] [Google Scholar]
- 7.Averette HE, Nguyen HN, Donato DM, et al. Radical hysterectomy for invasive cervical cancer: a 25-year prospective experience with the Miami technique. Cancer 1993;71:Suppl:1422–37. [DOI] [PubMed] [Google Scholar]
- 8.Landoni F, Maneo A, Colombo A, et al. Randomised study of radical surgery versus radiotherapy for stage Ib-IIa cervical cancer. Lancet 1997;350:535–40. [DOI] [PubMed] [Google Scholar]
- 9.Brooks RA, Wright JD, Powell MA, et al. Long-term assessment of bladder and bowel dysfunction after radical hysterectomy. Gynecol Oncol 2009;114:75–9. [DOI] [PubMed] [Google Scholar]
- 10.Walker JL, Piedmonte MR, Spirtos NM, et al. Recurrence and survival after random assignment to laparoscopy versus laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group LAP2 study. J Clin Oncol 2012;30:695–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fleshman J, Sargent DJ, Green E, et al. Laparoscopic colectomy for cancer is not inferior to open surgery based on 5-year data from the COST Study Group trial. Ann Surg 2007;246:655–62. [DOI] [PubMed] [Google Scholar]
- 12.Janda M, Gebski V, Davies LC, et al. Effect of total laparoscopic hysterectomy vs total abdominal hysterectomy on disease-free survival among women with stage I endometrial cancer: a randomized clinical trial. JAMA 2017;317:1224–33. [DOI] [PubMed] [Google Scholar]
- 13.Bonjer HJ, Deijen CL, Abis GA, et al. A randomized trial of laparoscopic versus open surgery for rectal cancer. N Engl J Med 2015;372:1324–32. [DOI] [PubMed] [Google Scholar]
- 14.Aarts JW, Nieboer TE, Johnson N, et al. Surgical approach to hysterectomy for benign gynaecological disease. Cochrane Database Syst Rev 2015;8:CD003677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nezhat CR, Burrell MO, Nezhat FR, Benigno BB, Welander CE. Laparoscopic radical hysterectomy with paraaortic and pelvic node dissection. Am J Obstet Gynecol 1992;166:864–5. [DOI] [PubMed] [Google Scholar]
- 16.Magrina JF, Kho RM, Weaver AL, Montero RP, Magtibay PM. Robotic radical hysterectomy: comparison with laparoscopy and laparotomy. Gynecol Oncol 2008;109:86–91. [DOI] [PubMed] [Google Scholar]
- 17.Malzoni M, Tinelli R, Cosentino F, Fusco A, Malzoni C. Total laparoscopic radical hysterectomy versus abdominal radical hysterectomy with lymphadenec-tomy in patients with early cervical cancer: our experience. Ann Surg Oncol 2009;16:1316–23. [DOI] [PubMed] [Google Scholar]
- 18.Geisler JP, Orr CJ, Khurshid N, Phibbs G, Manahan KJ. Robotically assisted laparoscopic radical hysterectomy compared with open radical hysterectomy. Int J Gynecol Cancer 2010;20:438–42. [DOI] [PubMed] [Google Scholar]
- 19.Hoogendam JP, Verheijen RH, Wegner I, Zweemer RP. Oncological outcome and long-term complications in robot-assisted radical surgery for early stage cervical cancer: an observational cohort study. BJOG 2014;121:1538–45. [DOI] [PubMed] [Google Scholar]
- 20.Chiantera V, Vizzielli G, Lucidi A, et al. Laparoscopic radical hysterectomy in cervical cancer as total mesometrial resection (L-TMMR): a multicentric experience. Gynecol Oncol 2015;139:47–51. [DOI] [PubMed] [Google Scholar]
- 21.Roque DR, Wysham WZ, Soper JT. The surgical management of cervical cancer: an overview and literature review. Obstet Gynecol Surv 2014;69:426–41. [DOI] [PubMed] [Google Scholar]
- 22.Geetha P, Nair MK. Laparoscopic, robotic and open method of radical hysterectomy for cervical cancer: a systematic review. J Minim Access Surg 2012;8:67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nam JH, Park JY, Kim DY, Kim JH, Kim YM, Kim YT. Laparoscopic versus open radical hysterectomy in early-stage cervical cancer: long-term survival out-comes in a matched cohort study. Ann Oncol 2012;23:903–11. [DOI] [PubMed] [Google Scholar]
- 24.Bogani G, Cromi A, Uccella S, et al. Laparoscopic versus open abdominal management of cervical cancer: long-term results from a propensity-matched analysis. J Minim Invasive Gynecol 2014;21:857–62. [DOI] [PubMed] [Google Scholar]
- 25.Ditto A, Martinelli F, Bogani G, et al. Implementation of laparoscopic approach for type B radical hysterectomy: a comparison with open surgical operations. Eur J Surg Oncol 2015;41:34–9. [DOI] [PubMed] [Google Scholar]
- 26.Diver E, Hinchcliff E, Gockley A, et al. Minimally invasive radical hysterectomy for cervical cancer is associated with reduced morbidity and similar survival outcomes compared with laparotomy. J Minim Invasive Gynecol 2017;24:402–6. [DOI] [PubMed] [Google Scholar]
- 27.Lee EJ, Kang H, Kim DH. A comparative study of laparoscopic radical hysterectomy with radical abdominal hysterectomy for early-stage cervical cancer: a long-term follow-up study. Eur J Obstet Gynecol Reprod Biol 2011;156:83–6. [DOI] [PubMed] [Google Scholar]
- 28.Gil-Moreno A, Carbonell-Socias M, Salicrú S, et al. Radical hysterectomy: efficacy and safety in the dawn of minimally invasive techniques. J Minim Invasive Gynecol 2018. June 13 (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 29.Mendivil AA, Rettenmaier MA, Abaid LN, et al. Survival rate comparisons amongst cervical cancer patients treated with an open, robotic-assisted or laparoscopic radical hysterectomy: a five year experience. Surg Oncol 2016;25:66–71. [DOI] [PubMed] [Google Scholar]
- 30.Sert BM, Boggess JF, Ahmad S, et al. Robot-assisted versus open radical hysterectomy: a multi-institutional experience for early-stage cervical cancer. Eur J Surg Oncol 2016;42:513–22. [DOI] [PubMed] [Google Scholar]
- 31.Wright JD, Herzog TJ, Neugut AI, et al. Comparative effectiveness of minimally invasive and abdominal radical hysterectomy for cervical cancer. Gynecol Oncol 2012;127:11–7. [DOI] [PubMed] [Google Scholar]
- 32.Boffa DJ, Rosen JE, Mallin K, et al. Using the National Cancer Database for Outcomes Research: a review. JAMA Oncol 2017;3:1722–8. [DOI] [PubMed] [Google Scholar]
- 33.Pecorelli S, Zigliani L, Odicino F. Revised FIGO staging for carcinoma of the cervix. Int J Gynaecol Obstet 2009;105:107–8. [DOI] [PubMed] [Google Scholar]
- 34.United States Department of Agriculture Economic Research Service. Rural-urban continuum codes (http://www.ers.usda.gov/data-products/rural-urban-continuum-codes.aspx).
- 35.Commission on Cancer. About cancer program categories (https://www.facs.org/quality-programs/cancer/accredited/about/categories).
- 36.Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol 2000;53:1258–67. [DOI] [PubMed] [Google Scholar]
- 37.Rosenbaum PR. Model-based direct adjustment. J Am Stat Assoc 1987;82:387–94. [Google Scholar]
- 38.Lunceford JK, Davidian M. Stratification and weighting via the propensity score in estimation of causal treatment effects: a comparative study. Stat Med 2004;23:2937–60. [DOI] [PubMed] [Google Scholar]
- 39.Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med 2015;34:3661–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Austin PC. The use of propensity score methods with survival or time-to-event outcomes: reporting measures of effect similar to those used in randomized experiments. Stat Med 2014;33:1242–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Robins JM, Hernán MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology 2000;11:550–60. [DOI] [PubMed] [Google Scholar]
- 42.Xie J, Liu C. Adjusted Kaplan–Meier estimator and log-rank test with inverse probability of treatment weighting for survival data. Stat Med 2005;24:3089–110. [DOI] [PubMed] [Google Scholar]
- 43.Hosmer DW, Lemeshow S, Sturdivant RX. Applied logistic regression. 3rd ed. Hoboken, NJ: Wiley, 2013. [Google Scholar]
- 44.Kontopantelis E, Doran T, Springate DA, Buchan I, Reeves D. Regression based quasi-experimental approach when randomisation is not an option: interrupted time series analysis. BMJ 2015;350:h2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cho H, Howlander N, Mariotto AB, Cronin KA. Estimating relative survival for cancer patients from the SEER Program using expected rates based on Ederer I versus Ederer II method. NCI Surveillance Research Program technical report #2011-01 (https://surveillance.cancer.gov/reports/tech2011.01.pdf). [Google Scholar]
- 46.Dryden-Peterson S, Bvochora-Nsingo M, Suneja G, et al. HIV infection and survival among women with cervical cancer. J Clin Oncol 2016;34:3749–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Coker AL, DeSimone CP, Eggleston KS, Hopenhayn C, Nee J, Tucker T. Smoking and survival among Kentucky women diagnosed with invasive cervical cancer: 1995-2005. Gynecol Oncol 2009;112:365–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Melamed A, Keating NL, Clemmer JT, et al. Laparoscopic staging for apparent stage I epithelial ovarian cancer. Am J Obstet Gynecol 2017;216(1):50.e1–50.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bregar AJ, Melamed A, Diver E, et al. Minimally invasive staging surgery in women with early-stage endometrial cancer: analysis of the National Cancer Database. Ann Surg Oncol 2017;24:1677–87. [DOI] [PubMed] [Google Scholar]
- 50.Conrad LB, Ramirez PT, Burke W, et al. Role of minimally invasive surgery in gynecologic oncology: an updated survey of members of the Society of Gynecologic Oncology. Int J Gynecol Cancer 2015;25:1121–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McCann GA, Taege SK, Boutsicaris CE, et al. The impact of close surgical margins after radical hysterectomy for early-stage cervical cancer. Gynecol Oncol 2013;128:44–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vickers AJ, Bianco FJ, Serio AM, et al. The surgical learning curve for prostate cancer control after radical prostatectomy. J Natl Cancer Inst 2007;99:1171–7. [DOI] [PubMed] [Google Scholar]
- 53.Kuo TM, Mobley LR. How generalizable are the SEER registries to the cancer populations of the USA? Cancer Causes Control 2016;27:1117–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ramirez PT, Frumovitz M, Pareja R, et al. Minimally invasive versus abdominal radical hysterectomy for cervical cancer. N Engl J Med 2018;379:1895–904. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.