Abstract
Introduction:
Mild traumatic brain injury (MTBI) can cause persistent functional deficits and healthcare burden. Intracerebral contusions cause permanent parenchymal injury, and understanding their association with outcomes may aid in treatment and prognosis.
Methods:
Adult MTBI patients with arrival GCS 13–15 and six-month outcomes [Glasgow Outcome Scale Extended (GOSE)], without polytrauma or operative neurosurgery from the prospective TRACK-TBI Pilot study were analyzed. Intracranial contusions detected by computed tomography (CT) were coded by location. Multivariable regression evaluated associations between intracranial injury type (temporal contusion (TC), frontal contusion, extraaxial [epidural/subdural/subarachnoid], other-intraaxial [intracerebral/intraventricular hemorrhage, axonal injury]) and GOSE. Odds ratios (OR) are reported.
Results:
Overall, 260 MTBI subjects were aged 44.4±18.1-years and 67.7%-male. Ninety-seven subjects were CT-positive, of which 46 had contusions (41.3%-frontal, 30.4%-temporal, 21.7%-frontal+temporal, 2.2%-parietal, 2.2%-occipital, 2.2%-brainstem); 95.7% had concurrent extraaxial hemorrhage. Mortality was 0% at discharge and 2.3% by six months.
Six-month GOSE was distributed with 2.3%-death, 1.5%-severe disability, 27.7%-moderate disability, 68.5%-good recovery. Nearly 46% of TC-positive subjects suffered moderate disability or worse (GOSE≤6) and 41.7% were unable to return to baseline work capacity (RTBWC), compared to 29.1%/20.4% for CT-negative and 26.1%/20.9% for CT-positive subjects without TC. On multivariable regression, TC associated with OR=3.33 (95% CI [1.16–9.60], p=0.026) for GOSE≤6, and OR=4.48 ([1.49–13.51], p=0.008) for inability to RTBWC.
Conclusions:
Parenchymal contusions in MTBI are often accompanied by extraaxial hemorrhage. TCs may be associated with six-month functional impairment. Their presence on imaging should alert the clinician to the need for heightened surveillance of sequelae complicating RTBWC, with low threshold for referral to services.
INTRODUCTION
Traumatic brain injury (TBI) creates a major public health burden. TBI annually affects 50–60 million worldwide, of whom 90% suffer mild (MTBI) [1]. MTBI is defined as trauma or whiplash injury to the head resulting in loss of consciousness (LOC) 0–30 minutes, retrograde or anterograde amnesia 0–24 hours, alteration in mental status, and/or focal neurologic deficits at time of injury, with a Glasgow Coma Scale (GCS) score of 13–15 in the emergency department (ED) [2]. The incidence of MTBI is likely underestimated as patients without LOC are less likely to seek attention [3–5]. Symptoms following MTBI can be broadly divided into physical (e.g. headache, nausea, vision changes), cognitive (e.g. memory, concentration, executive function), and behavioral (e.g. irritability, emotional lability). While symptoms typically resolve across days and weeks to months, it is estimated that 15–25% of patients with MTBI continue to experience symptoms more than a year after the initial insult [6–9].
Predictors of functional recovery following MTBI remain under investigation [10–12]. Multiple variables including type and severity of injury, comorbidities, socioeconomic status, quality of medical care, and postinjury rehabilitation and support may all contribute to recovery [1]. Imaging findings have been given special attention in analyses of MTBI outcome. Patients with MTBI and positive brain computed tomography (CT) findings on ED or hospital admission (e.g., extra-axial hemorrhage (epidural, subdural, or subarachnoid), cerebral contusion, axonal shear, intraparenchymal or intraventricular hemorrhage) are classified as “complicated MTBI”, which comprise 5%−39% of all MTBIs [13]. Some studies report that complicated MTBI patients suffer early cognitive deficits [14,15] and worse long-term functional outcomes [16], though these findings are debated [17,18]. While extra-axial hemorrhages can be evacuated or, if minor, resolve over time with medical management, contusional and shear injuries in the brain parenchyma usually cause permanent damage. Contusions in inferior, lateral, and anterior aspects of the frontal and temporal lobes, axonal shear in lobar white matter, and extra-axial hemorrhages have all been reported as common imaging findings in complicated MTBI [13,19]; however, precise associations of lesion type and location with functional outcome remains understudied.
Objective measures of functional recovery include disability and return to baseline work status. Functional limitations in these areas directly relate to reduced quality of life and financial impact to the patient and to society [1]. Delineating the relationships between lesion type/location and functional outcome may yield valuable information on management, post-acute rehabilitation and surveillance strategies, and resources and counseling for MTBI patients at risk for impaired recovery. In the current analysis, we utilize the prospective Transforming Research and Clinical Knowledge in Traumatic Brain Injury Pilot (TRACK-TBI Pilot) study to characterize the types of intracranial pathology in a cohort of isolated MTBI patients, with emphasis on evaluating contusion location as an independent risk factor for impaired functional recovery at six months.
METHODS
Study design
The TRACK-TBI Pilot study was conducted at three U.S. Level I trauma centers (Zuckerberg San Francisco General Hospital [San Francisco, CA], University of Pittsburgh Medical Center [Pittsburgh, PA], and University Medical Center Brackenridge [Austin, TX]) using the National Institute of Neurological Disorders and Stroke (NINDS) TBI Common Data Elements (CDEs) [20–24]. TRACK-TBI Pilot inclusion criteria were external force trauma to the head, presentation to one of the three trauma centers, and a clinically indicated head CT scan within 24 hours of injury; exclusion criteria were pregnancy, ongoing life-threatening disease (e.g., end-stage malignancy), police custody, involuntary psychiatric hold, and inability to speak English due to limitations in participation with outcome assessments. All head CT images were independently reviewed by one central board-certified neuroradiologist blinded to the clinical history of the subjects, and scored according to the NINDS TBI CDEs [20].
Eligible subjects were enrolled prospectively by convenience sampling from years 2010 to 2012. Institutional review board approval was obtained at the participating sites. Informed consent was obtained prior to study enrollment. For subjects unable to provide consent due to their injury, consent was obtained from their legally authorized representatives. Subjects were then re-consented, if cognitively able, during the course of their clinical care and/or follow-up visits for continued participation in the study.
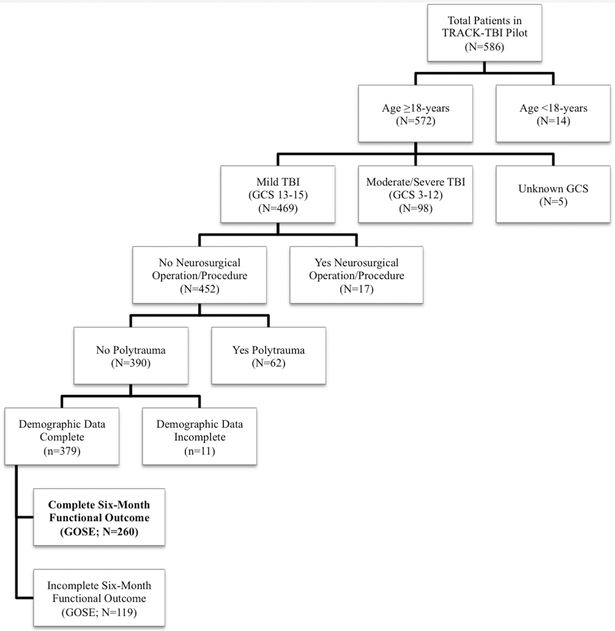
As the goal of the current analysis was to evaluate associations between contusion pathology and outcome following isolated MTBI, subjects were included if they were aged ≥18 years with an ED admission GCS score of 13–15, had no polytrauma (as defined by Abbreviated Injury Scale [AIS] score of >2 in any extracranial region [25,26]), underwent no inpatient neurosurgical operation, and completed six-month GOSE assessment. A flowchart of included subjects is shown in Figure 1.
Figure 1. Flowchart of included patients.
A total of 260 patients are included in the current analysis as shown. GCS = Glasgow Coma Scale; GOSE = Glasgow Outcome Scale-Extended; MTBI = mild traumatic brain injury; TRACK-TBI Pilot = Transforming Research and Clinical Knowledge in Traumatic Brain Injury Pilot study
Outcome measures
The GOSE provides an overall measure of functional disability based on cognition, independence, employability, and social/community participation. It is assessed via structured interview and has been widely used as a standard outcome measure for TBI [27,28]. Subjects are described by one of the eight categories: 1=dead, 2=vegetative state, 3=lower severe disability, 4=upper severe disability, 5=lower moderate disability, 6=upper moderate disability, 7=lower good recovery, and 8=upper good recovery. Good recovery is defined as GOSE 7–8, moderate disability as GOSE 5–6, and severe disability as GOSE 3–4. A GOSE of 8 reflects functional recovery to baseline status. For the current analysis, GOSE was dichotomized into “good recovery (GOSE 7–8)” vs. “moderate disability or worse (GOSE ≤6)”. Return to baseline work status was dichotomized as yes/no using GOSE question 5a: “Are they currently able to work (or look after others at home) to their previous capacity?” Answering “no” equates to a GOSE of 6 per scoring guidelines [27].
Statistical analysis
Descriptive statistics are presented as means and standard deviations (SD) for continuous variables and proportions for categorical variables. Group differences were assessed using Pearson’s chi-squared test for categorical variables. As variables of interest, traumatic intracranial pathology types were classified as follows: temporal contusion, frontal contusion, extra-axial lesion (epidural, subdural, or subarachnoid hemorrhage), and other intra-axial lesion (intracerebral hemorrhage, intraventricular hemorrhage, or axonal injury). Intracranial pathology types were assessed as independent variables using multivariable logistic regression for GOSE and return to work status, controlling for age, years of education, race (Caucasian, African-American/African, other races), preinjury employment status (employed, unemployed, retired/student/disabled), and preinjury psychiatric disorder (yes/no). Multivariable odds ratios (OR) and their associated 95% confidence intervals (CI) were reported for each predictor in the regression analyses. Significance was assessed at p<0.05. All analyses were performed using Statistical Package for the Social Sciences (SPSS) version 24 (IBM Corporation, Chicago, IL).
RESULTS
Demographic and injury profiles
Overall, 260 MTBI subjects were aged 44.4±18.1 years, 67.7% were male, and 78.1% were Caucasian. Baseline education was 14.1±3.0 years, 21.2% were unemployed, and 34.2% had a history of psychiatric disorder. Mechanisms of injury included fall (50.8%), motor vehicle accident (MVA, 18.4%), pedestrian vs. auto (PVA, 10.8%), assault (15.8%), or other mechanisms (4.2%). Traumatic intracranial injury on brain CT was present in 97 subjects (37.3%), and the types of intracranial lesions were as follows: 14.6% extra-axial-only, 16.5% contusion+extra-axial, 0.8% contusion-only, 0.4% contusion+extra-axial+other intra-axial, 3.1% other intra-axial-only, 1.9% extra-axial+other intra-axial. Forty-six subjects had intracranial contusion, which constituted 17.7% of all MTBI subjects and 47.4% of CT-positive MTBI subjects. Of the subjects with contusion, 41.3% (n=19) were frontal-only, 30.4% (n=14) were temporal-only, 21.7% (n=10) were frontal+temporal, 2.2% (n=1) were parietal-only, 2.2% (n=1) were occipital-only, and 2.2% (n=1) were brainstem; of the 24 total patients with temporal contusions, 12 were left temporal-only and 12 were right temporal-only. Nearly 96% of subjects with presence of contusion had concurrent extra-axial hemorrhage. Detailed demographic and injury information are presented in Table 1.
Table 1.
Demographic data and injury characteristics of 260 isolated MTBI subjects
| Variable | Value* |
|---|---|
| Mean age in years (SD)† | 44.4 (18.1) |
| M/F ratio (%) | 176 (67.7)/84 (32.3) |
| Race | |
| Caucasian | 203 (78.1) |
| African-American/African | 22 (8.5) |
| Other races | 35 (13.4) |
| Education in years (SD)‡ | 14.1 (3.0) |
| Employment status | |
| Employed | 147 (56.5) |
| Retired, student, disabled | 58 (22.3) |
| Unemployed | 55 (21.2) |
| History of psychiatric disorder | 89 (34.2) |
| Mechanism of injury | |
| Fall | 132 (50.8) |
| Motor vehicle accident | 48 (18.5) |
| Pedestrian versus auto | 28 (10.8) |
| Assault | 41 (15.8) |
| Other | 11 (4.2) |
| CT-positive intracranial injury | 97 (37.3) |
| Extra-axial only | 38 (14.6) |
| Contusion only | 2 (0.8) |
| Contusion + extra-axial | 43 (16.5) |
| Other intra-axial only | 8 (3.1) |
| Contusion + extra-axial + other intra-axial | 1 (0.4) |
| Extra-axial + other intra-axial | 5 (1.9) |
| CT-positive contusiona | 46 (17.7) |
| Frontal only | 19 (41.3) |
| Temporal only | 14 (30.4) |
| Frontal + temporal | 10 (21.7) |
| Parietal only | 1 (2.2) |
| Occipital only | 1 (2.2) |
| Brainstem | 1 (2.2) |
Data represent number of patients and (%), unless indicated otherwise.
The age range of the patients was 18-93.
The range of education is 2-24 years.
Contusion locations are indicated as individual N’s with (%) of total subjects with contusions (N=46). CT = computed tomography; SD = standard deviation
Univariable analyses
Mortality rate was 0% at hospital discharge. On six-month GOSE, 2.3% died (GOSE 1), 1.5% had severe disability (GOSE 3–4), 27.7% had moderate disability (GOSE 5–6), 31.2% had lower good recovery (GOSE 7), and 37.3% had upper good recovery (GOSE 8).
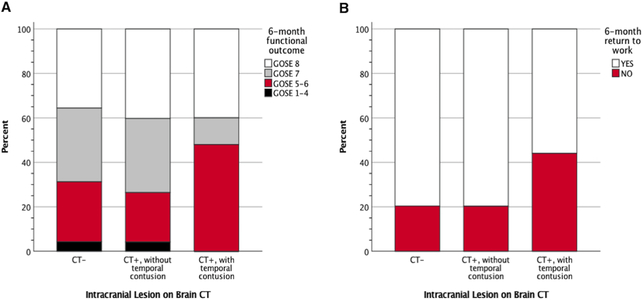
Of the subjects with temporal lobe contusions, 45.8% had moderate disability or worse at six months (GOSE ≤6), compared to 26.1% for CT-positive subjects without temporal lobe contusions, and to 29.1% of CT-negative subjects (Figure 2A). Nearly 42% of subjects with temporal lobe contusions were unable to return to baseline work capacity at six months, compared to 20.9% of CT-positive subjects without temporal lobe contusions, and 20.4% of CT-negative subjects (Figure 2B). In those with temporal lobe contusions, no statistically significant differences were observed for laterality and six-month disability or return to work status.
Figure 2. Six-month functional disability by intracranial lesion status.
Panel A: Functional ability at six-months postinjury are shown for 260 MTBI subjects who had either [1] no evidence of acute intracranial lesion on emergency department brain CT scan (CT-; N=163), [2] evidence of acute intracranial lesion(s) but without a temporal lobe contusion (CT+, without temporal contusion; N=73), or [3] evidence of acute intracranial lesion(s) including a temporal lobe contusion (CT+, with temporal lobe contusion; N=24). GOSE 8 = back to baseline functional status; GOSE 7 = lower good recovery; GOSE 5–6 = moderate disability; GOSE 1–4 = death or severe disability. Panel B: Return to work status at six-months postinjury, which describes the ability to return to baseline level of work (either outside or inside the home), is shown for subject groups who were CT−, CT+ without, and CT+ with evidence of temporal lobe contusion. CT = computed tomography; GOSE = Glasgow Outcome Scale-Extended; MTBI = mild traumatic brain injury
Multivariable analyses
On multivariable regression, presence of a temporal contusion conferred an OR of 3.17 (95% CI [1.11–9.07], p=0.031) for moderate disability or worse on GOSE, and an OR of 4.48 ([1.49–13.51], p=0.008) for inability to return to baseline work capacity. Frontal contusion, extra-axial injury, and other intra-axial injury did not show statistically significant associations with disability on GOSE or return to work status (Table 2).
Table 2.
Multivariable logistic regression of six-month functional disability and inability to return to work
| Predictor | Moderate disability or worse at six-months (GOSE ≤6) |
Inability to return to baseline work status |
||
|---|---|---|---|---|
| Odds Ratio (95% CI) | Sig. (p) | Odds Ratio (95% CI) | Sig. (p) | |
| Age (per-unit-increase) | 1.001 (0.99-1.02) | 0.528 | 1.01 (1.00-1.04) | 0.148 |
| Sex (vs. female) | 1.22 (0.66-2.30) | 0.524 | 0.70 (0.34-1.44) | 0.331 |
| Race | 0.109 | 0.174 | ||
| Caucasian | Reference | --- | Reference | --- |
| African-American/African | 2.43 (1.04-5.66) | 0.039 | 2.34 (0.93-5.81) | 0.069 |
| Other races | 1.47 (0.56-3.91) | 0.435 | 0.93 (0.30-2.87) | 0.897 |
| Education (per-unit-increase) | 0.92 (0.83-1.01) | 0.081 | 0.91 (0.82-1.01) | 0.088 |
| History of psychiatric disorder (vs. none) | 3.12 (1.72-5.69) | < 0.001 | 2.15 (1.10-4.22) | 0.026 |
| Employment | 0.354 | 0.685 | ||
| Employed | Reference | --- | Reference | --- |
| Retired, student, or disabled | 1.46 (0.67-3.17) | 0.337 | 1.41 (0.62-3.24) | 0.423 |
| Unemployed | 1.63 (0.78-3.40) | 0.196 | 0.96 (0.41-2.23) | 0.917 |
| Frontal contusion (vs. CT-negative) | 0.97 (0.34-2.78) | 0.955 | 0.58 (0.18-1.88) | 0.366 |
| Temporal contusion (vs. CT-negative) | 3.17 (1.11-9.07) | 0.031 | 4.48 (1.49-13.51) | 0.008 |
| Extra-axial injury (vs. CT-negative) | 0.80 (0.35-1.81) | 0.588 | 0.93 (0.38-2.25) | 0.866 |
| Other Intra-axial injury (vs. CT-negative) | 1.17 (0.33-4.17) | 0.810 | 1.17 (0.285-4.76) | 0.831 |
Functional disability is assessed using the Glasgow Outcome Scale-Extended (GOSE) at six-months post-injury in 260 MTBI subjects. GOSE ≤6 is classified as “moderate disability or worse”. CI = confidence interval; CT = computed tomography
DISCUSSION
MTBI patients suffer heterogeneous injuries that differ in pathology and evolution. These differences may alter the trajectory of recovery and outcome. Guidelines and management strategies for nonoperative MTBI suffer from lack of consensus, and remedying this requires improved understanding of structure-function relationships between lesion type along with location and injury progression and outcome. In the current analysis, we characterized the demographics and injury profiles of a cohort of isolated MTBI patients without neurosurgical intervention based on the presence of contusive parenchymal injury, and we evaluated the risks associated with contusion location on six-month disability and return to work status while controlling for other intracranial lesion types, demographics and pre-injury characteristics. We found that contusions rarely exist without extra-axial injury (namely subdural and subarachnoid hemorrhage), and that temporal lobe contusions are independently associated with increased likelihood of moderate disability or worse on the GOSE, as well as the inability to return to baseline work status. To our knowledge, this is the first study to evaluate the relationship between contusion type and location following isolated MTBI. Investigating the possible reasons for these associations may lead to improved management, surveillance and counseling.
The short- and long-term sequelae of parenchymal contusive injury are mediated through separate mechanisms. The acute injury sustained in contusion results from direct mechanical force upon impact, causing immediate rupture of neurons, astrocytes and oligodendrocytes. This cell death is accompanied by simultaneous mechanical damage to small blood vessels [29]. It is possible for cell membranes to be stretched rather than ruptured. However, both forms of injury lead to long-term secondary effects [30]. The contusive mechanical force arises from contact between the parenchyma and bony protuberances on the inner surface of the skull. As such, immediate necrosis and small blood vessel damage are typically located in the inferior frontal or anterior temporal lobes at the sites of irregular bony protuberances inside the cranial vault [31]. Unsurprisingly, in our study the vast majority of the contusions (~93%) were frontal, temporal, or frontotemporal.
Because it is impossible to prevent primary mechanical damage of contusive MTBI after it has occurred, it is particularly important to examine the evolution of subsequent pathophysiologies of injury. The mechanisms of this secondary damage may be clinical targets for improved interventions in acute and chronic settings. Necrotic cells in the contused region release excitotoxic substances (such as glutamate), triggering deleterious biochemical cascades that result in further neuronal damage [32]. Extravasated blood from microvessel rupture causes free radical damage in addition to ischemia. Vasogenic edema, endothelial swelling, and vasospasm can also lead to ischemia. Cerebral blood flow (CBF) decreases after MTBI, especially in contused regions [5], as heightened vasoresponsivity to CO2, decreased oxygenation, and impaired autoregulation increase the vulnerability of the contused parenchyma to further rounds of secondary damage via inflammation, increased metabolic demand, mitochondrial dysfunction, and disrupted calcium homeostasis, potentially triggering apoptosis [33–36].
Prior studies of mild and moderate TBI report that patients with focal cortical contusions differ from patients without focal pathology in behavioral measurements one month after injury, resulting in difficulties with professional and psychosocial reintegration fort the affected individuals [19,37]. Therefore, clinical characterization of long-term deficits attributable to sprcifc types and locations of focal pathology is necessary for prognostication and postinjury mangement. In our patients presenting with parenchymal contusions, we observed that the force of the initial trauma also induces extra-axial hemorrhage in 96% (44 of 46) of subjects; however, in the multivariate analysis we find that the presence of a meningeal hemorrhage in MTBI patients does not associate with long-term functional impairment. While a majority of patients with focal contusions present with extra-axial injuries such as subdural hematoma or subarachnoid hemorrhage, it is important to highlight that the clinical severity of the meningeal hemorrhage, as is often the case with MTBI, is mild and nonoperative, with no significant implications for long-term outcomes.
Notably, we observe that, regardless of laterality, a temporal contusion (12 right-sided, 12 left-sided, no bilateral) is associated with significant disability as measured by the GOSE at six months and the inability to return to work. Clarification of whether the GOSE is sufficient to capture differences in functional disability due to temporal lobe laterality would require a larger cohort and the utilization of more sensitive imaging modalities such as MRI, diffusion tensor imaging (DTI), and/or single-photon emission computed tomography (SPECT) to further localize cortical networks within the temporal lobe that might be susceptible to injury [38].
Given the risk that contusions in both frontal and temporal lobes might have effects on executive function, memory, neuropsychiatric status, and emotional processing, our finding of an association between temporal lobe contusion after MTBI and six-month functional deficit is intriguing. Animal studies have shown that the temporal lobe -- the hippocampus in particular -- is especially susceptible to injury from mild or moderate TBI [39,40]. The ventromedial temporal lobe is susceptible to TBI due to its proximity to the floor of the middle fossa, and differential risks for ischemia have been described for both CA1 and CA3 neurons in settings of global cerebral ischemia vs. limbic seizures [41]. The mechanisms of this are not fully understood but may be related to dysfunctional apoptosis and autophagy [42]. In contrast, while direct impact and contra-coup injuries commonly jeopardize the frontal lobe, the relative thickness of the frontal bone and insulation from the sinuses to direct impact may mitigate the mechanical force of trauma to reduce the susceptibility of lobar injury in ways that the thinner temporal bone cannot reproduce. The delicate networks of memory-related structures in the medial temporal lobe are likely especially prone to irreversible disruption in ways that non-memory structures are not [43,44]
Analyses of contusive parenchymal injury remain sparse in MTBI and mostly consist of retrospective studies without delineation of effects of contusion location. Results are mixed when for outcomes such as rehospitalization, neurosurgical intervention, metabolic disturbances, and a range of functional, neuropsychological and neurocognitive assessments. In contrast, studies of moderate to severe contusive TBI discuss temporal lobe vulnerability in the context of hydrocephalus and seizure risk rather than functional outcome on the GOSE [45,46]. Studies investigating contusive parenchymal injury rarely engage in thorough discussions of rehabilitation or post-acute services, and the data remain mixed on benefits associated with different types of rehabilitative, cognitive or neuropsychiatric services. The reasons for this are manifold, including difficulty in obtaining consistent follow-up as MTBI patients become more removed from time of injury, lack of guidelines and/or consensus on residual MTBI symptomatology and its treatment options, and presence of socioeconomic resources and health insurance that enable pursuit of such services. Inconsistency of conclusions may be related to small sample sizes, inadequate follow-up times, and the clinically vague distinction between different categories of TBI in terms of severity and injury pattern [18,47–49]. Hence in our study we focused on functional disability at the level of moderate disability or worse, which for the MTBI patient is a rather dismal recovery in light of the relative severity of the initial injury in conjunction with patient expectations for recovery. Additionally, the increased risk of inability to return to baseline work capacity and/or employment status for subjects with temporal lobe contusions may lead to further decline and reinjury, loss of quality of life, economic debilitation, and costs to the healthcare system. An increased focus on contusion location may ameliorate a subset of these issues and inform clinical practices based on MTBI pathophysiology, e.g., the susceptibility of the temporal lobe to contusive injury and its sequelae compared to the frontal lobe.
Lastly, there is the need for evidence-based targeted rehabilitation programs after discharge from hospital in order to optimize long-term functional recovery [50–52]. In addition, the substantial heterogeneity of contusive MTBI, as seen in our current study, complicates the issue of proper rehabilitation referral. Given the findings in this study, it is possible that tailored rehabilitation services may be necessary for contusive MTBI patients, with lower threshold for surveillance, earlier intervention for susceptible and/or symptomatic individuals, and the need to anticipate longer recovery trajectories for patients with temporal lobe injuries.
Limitations
Although we have begun to bridge the knowledge gap on the heterogeneous pathophysiology of MTBI, there are inherent limitations to retrospective data analyses based on prospective multicenter observational studies. First, our sample size remains relatively small, in part due to inclusion only of MTBI subjects without neurosurgical intervention or polytrauma. A consequence of this is wider confidence intervals which can decrease the precision of risk factor estimates in predicting outcomes, In consideration for not overfitting our regression models, we were limited to controlling for known predictors of MTBI outcomes rather than the full extent of possible predictors available in our dataset. Patient recruitment is limited to Level I trauma centers, which captures more of an urbanized population. Our findings may not extrapolate to all MTBI patients. We did not observe differences in outcome by the GOSE between right versus left-sided temporal contusion, which as previously stated could be due to the sample size or to the sensitivity of the GOSE.
Second, intracranial imaging in our study was limited to only the initial CT after acute injury. Therefore, the evolution of intracranial trauma or the presence of occult injury using serial CTs or advanced neuroimaging (e.g., MRI, DTI, or SPECT) remains a topic for future studies. Additionally, the significance of other intracranial injuries localized to the temporal region, such as temporal subarachnoid hemorrhage, also remains a question for future research.
Thirdly, the focus of the current analysis was on assessment of the relationship between intracranial pathology and functional outcome using the GOSE, and we did not evaluate sensory processing (e.g. visual memory, emotional connection, response to pain, and others). Evaluation of intracranial lesion types and measures of processing sensory input constitutes an important future direction.
Lastly, there remains the need for data on the influence on long-term recovery of rehabilitation programs, or lack thereof, within the first six months following hospital discharge, which in our current study was only assessed at a single follow-up time point. Also, evaluation of outcomes beyond six-months will be useful in assessing long-term recovery and trajectories of recovery. Our findings are exploratory and associative, and they provide a starting point for recognizing different subpopulations among those with MTBI and cerebral contusions.
Conclusions
Parenchymal contusions occur predominantly in the frontal and/or temporal lobes after MTBI and are often accompanied by extra-axial hemorrhage. Temporal lobe contusions may be associated with clinically substantive functional impairment at six months. Their presence on acute imaging should alert the clinician to the potential need for heightened surveillance for sequelae that can complicate return to baseline work capacity, as well as to the importance of a low threshold for follow-up with primary care and/or referral to specialty services. Future studies are warranted.
Acknowledgements:
Amy J. Markowitz, JD provided editorial support. The authors would like to thank the following contributors to the development of the TRACK-TBI database and repositories by organization and in alphabetical order by last name: One Mind for Research: General Peter Chiarelli, U.S. Army (Ret.), Garen Staglin, MBA; QuesGen Systems, Inc.: Vibeke Brinck, MS, Michael Jarrett, MBA; Thomson Reuters: Sirimon O’Charoen, PhD
Funding Details: This work was supported by the following grants: NINDS 1RC2NS069409–01, 3RC2NS069409–02S1, 5RC2NS069409–02, 1U01NS086090–01, 3U01NS086090–02S1, 3U01NS086090–02S2, 3U01NS086090–03S1, 5U01NS086090–02, 5U01NS086090–03; US DOD W81XWH-13–1-0441, US DOD W81XWH-14–2-0176 (to G. T. M.)
Footnotes
Disclosure Statement: No potential conflict of interest was reported by the authors.
The TRACK-TBI Investigators include: Shelly R. Cooper, MS (Department of Psychology, Washington University in St. Louis, St. Louis, MO, USA), Kristen Dams-O’Connor, PhD (Department of Rehabilitation Medicine, Mount Sinai School of Medicine, New York, NY, USA), Wayne A. Gordon, PhD (Department of Rehabilitation Medicine, Mount Sinai School of Medicine, New York, NY, USA), Allison J. Borrasso, MS (Department of Neurosurgery, University of Pittsburgh Medical Center, Pittsburgh, PA, USA), Andrew I. R. Maas, MD, PhD (Department of Neurosurgery, Antwerp University Hospital, Edegem, Belgium), David K. Menon, MD, PhD (Division of Anaesthesia, University of Cambridge, Addenbrooke’s Hospital, Cambridge, United Kingdom)
REFERENCES
- [1].Maas AIR, Menon DK, Adelson PD, et al. Traumatic brain injury: integrated approaches to improve prevention, clinical care, and research. Lancet Neurol. 2017;16:987–1048. [DOI] [PubMed] [Google Scholar]
- [2].Ruff RM, Iverson GL, Barth JT, et al. Recommendations for diagnosing a mild traumatic brain injury: a National Academy of Neuropsychology education paper. Arch. Clin. Neuropsychol. 2009;24:3–10. [DOI] [PubMed] [Google Scholar]
- [3].Langlois JA, Rutland-Brown W, Wald MM. The epidemiology and impact of traumatic brain injury: a brief overview. J. Head Trauma Rehabil. 2006;21:375–378. [DOI] [PubMed] [Google Scholar]
- [4].Daneshvar DH, Nowinski CJ, McKee AC, et al. The epidemiology of sport-related concussion. Clin. Sports Med. 2011;30:1–17, vii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Centers for Disease Control and Prevention (CDC). Nonfatal traumatic brain injuries from sports and recreation activities--United States, 2001–2005. MMWR Morb. Mortal. Wkly. Rep. 2007;56:733–737. [PubMed] [Google Scholar]
- [6].Hall RCW, Hall RCW, Chapman MJ. Definition, diagnosis, and forensic implications of postconcussional syndrome. Psychosomatics. 2005;46:195–202. [DOI] [PubMed] [Google Scholar]
- [7].Carroll L, David Cassidy J, Peloso P, et al. Prognosis for mild traumatic brain injury: results of the who collaborating centre task force on mild traumatic brain injury. J. Rehabil. Med. 2004;36:84–105. [DOI] [PubMed] [Google Scholar]
- [8].Rees PM. Contemporary issues in mild traumatic brain injury. Arch. Phys. Med. Rehabil. 2003;84:1885–1894. [DOI] [PubMed] [Google Scholar]
- [9].McMahon P, Hricik A, Yue JK, et al. Symptomatology and functional outcome in mild traumatic brain injury: results from the prospective TRACK-TBI study. J. Neurotrauma. 2014;31:26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Jacobs B, Beems T, Stulemeijer M, et al. Outcome prediction in mild traumatic brain injury: age and clinical variables are stronger predictors than CT abnormalities. J. Neurotrauma. 2010;27:655–668. [DOI] [PubMed] [Google Scholar]
- [11].Lingsma HF, Yue JK, Maas AIR, et al. Outcome prediction after mild and complicated mild traumatic brain injury: external validation of existing models and identification of new predictors using the TRACK-TBI pilot study. J. Neurotrauma. 2015;32:83–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].van der Naalt J, Timmerman ME, de Koning ME, et al. Early predictors of outcome after mild traumatic brain injury (UPFRONT): an observational cohort study. Lancet Neurol. 2017;16:532–540. [DOI] [PubMed] [Google Scholar]
- [13].Isokuortti H, Iverson GL, Silverberg ND, et al. Characterizing the type and location of intracranial abnormalities in mild traumatic brain injury. J. Neurosurg. 2018;1–10. [DOI] [PubMed] [Google Scholar]
- [14].Iverson GL. Complicated vs uncomplicated mild traumatic brain injury: acute neuropsychological outcome. Brain Inj. 2006;20:1335–1344. [DOI] [PubMed] [Google Scholar]
- [15].Kurca E, Sivák S, Kucera P. Impaired cognitive functions in mild traumatic brain injury patients with normal and pathologic magnetic resonance imaging. Neuroradiology. 2006;48:661–669. [DOI] [PubMed] [Google Scholar]
- [16].Temkin NR, Machamer JE, Dikmen SS. Correlates of functional status 3–5 years after traumatic brain injury with CT abnormalities. J. Neurotrauma. 2003;20:229–241. [DOI] [PubMed] [Google Scholar]
- [17].Iverson GL, Lange RT, Wäljas M, et al. Outcome from Complicated versus Uncomplicated Mild Traumatic Brain Injury. Rehabil. Res. Pract. 2012;2012:415740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lee H, Wintermark M, Gean AD, et al. Focal lesions in acute mild traumatic brain injury and neurocognitive outcome: CT versus 3T MRI. J. Neurotrauma. 2008;25:1049–1056. [DOI] [PubMed] [Google Scholar]
- [19].Gentry LR, Godersky JC, Thompson B. MR imaging of head trauma: review of the distribution and radiopathologic features of traumatic lesions. AJR Am. J. Roentgenol. 1988;150:663–672. [DOI] [PubMed] [Google Scholar]
- [20].Duhaime A-C, Gean AD, Haacke EM, et al. Common data elements in radiologic imaging of traumatic brain injury. Arch. Phys. Med. Rehabil. 2010;91:1661–1666. [DOI] [PubMed] [Google Scholar]
- [21].Maas AI, Harrison-Felix CL, Menon D, et al. Common data elements for traumatic brain injury: recommendations from the interagency working group on demographics and clinical assessment. Arch. Phys. Med. Rehabil. 2010;91:1641–1649. [DOI] [PubMed] [Google Scholar]
- [22].Manley GT, Diaz-Arrastia R, Brophy M, et al. Common data elements for traumatic brain injury: recommendations from the biospecimens and biomarkers working group. Arch. Phys. Med. Rehabil. 2010;91:1667–1672. [DOI] [PubMed] [Google Scholar]
- [23].Wilde EA, Whiteneck GG, Bogner J, et al. Recommendations for the use of common outcome measures in traumatic brain injury research. Arch. Phys. Med. Rehabil. 2010;91:1650–1660.e17. [DOI] [PubMed] [Google Scholar]
- [24].Yue JK, Vassar MJ, Lingsma HF, et al. Transforming research and clinical knowledge in traumatic brain injury pilot: multicenter implementation of the common data elements for traumatic brain injury. J. Neurotrauma. 2013;30:1831–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Butcher N, Balogh ZJ. AIS>2 in at least two body regions: a potential new anatomical definition of polytrauma. Injury. 2012;43:196–199. [DOI] [PubMed] [Google Scholar]
- [26].Butcher NE, Balogh ZJ. Update on the definition of polytrauma. Eur. J. Trauma Emerg. Surg. 2014;40:107–111. [DOI] [PubMed] [Google Scholar]
- [27].Wilson JT, Pettigrew LE, Teasdale GM. Structured interviews for the Glasgow Outcome Scale and the extended Glasgow Outcome Scale: guidelines for their use. J. Neurotrauma. 1998;15:573–585. [DOI] [PubMed] [Google Scholar]
- [28].Teasdale GM, Pettigrew LE, Wilson JT, et al. Analyzing outcome of treatment of severe head injury: a review and update on advancing the use of the Glasgow Outcome Scale. J. Neurotrauma. 1998;15:587–597. [DOI] [PubMed] [Google Scholar]
- [29].Kurland D, Hong C, Aarabi B, et al. Hemorrhagic progression of a contusion after traumatic brain injury: a review. J. Neurotrauma. 2012;29:19–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Pearn ML, Niesman IR, Egawa J, et al. Pathophysiology Associated with Traumatic Brain Injury: Current Treatments and Potential Novel Therapeutics. Cell. Mol. Neurobiol. 2017;37:571–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Mckee AC, Daneshvar DH. The neuropathology of traumatic brain injury. Handb. Clin. Neurol. 2015;127:45–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Yi J-H, Hazell AS. Excitotoxic mechanisms and the role of astrocytic glutamate transporters in traumatic brain injury. Neurochem. Int. 2006;48:394–403. [DOI] [PubMed] [Google Scholar]
- [33].Len TK, Neary JP. Cerebrovascular pathophysiology following mild traumatic brain injury. Clin. Physiol. Funct. Imaging. 2011;31:85–93. [DOI] [PubMed] [Google Scholar]
- [34].Forbes ML, Clark RS, Dixon CE, et al. Augmented neuronal death in CA3 hippocampus following hyperventilation early after controlled cortical impact. J. Neurosurg. 1998;88:549–556. [DOI] [PubMed] [Google Scholar]
- [35].Clark RS, Kochanek PM, Dixon CE, et al. Early neuropathologic effects of mild or moderate hypoxemia after controlled cortical impact injury in rats. J. Neurotrauma. 1997;14:179–189. [DOI] [PubMed] [Google Scholar]
- [36].Barkhoudarian G, Hovda DA, Giza CC. The Molecular Pathophysiology of Concussive Brain Injury - an Update. Phys. Med. Rehabil. Clin. N. Am. 2016;27:373–393. [DOI] [PubMed] [Google Scholar]
- [37].Wallesch C-W, Curio N, Kutz S, et al. Outcome after mild-to-moderate blunt head injury: effects of focal lesions and diffuse axonal injury. Brain Inj. 2001;15:401–412. [DOI] [PubMed] [Google Scholar]
- [38].Yuh EL, Mukherjee P, Lingsma HF, et al. Magnetic resonance imaging improves 3-month outcome prediction in mild traumatic brain injury. Ann. Neurol. 2013;73:224–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Hicks RR, Smith DH, Lowenstein DH, et al. Mild experimental brain injury in the rat induces cognitive deficits associated with regional neuronal loss in the hippocampus. J. Neurotrauma. 1993;10:405–414. [DOI] [PubMed] [Google Scholar]
- [40].Kotapka MJ, Gennarelli TA, Graham DI, et al. Selective vulnerability of hippocampal neurons in acceleration-induced experimental head injury. J. Neurotrauma. 1991;8:247–258. [DOI] [PubMed] [Google Scholar]
- [41].Van Hoesen GW, Augustinack JC, Redman SJ. Ventromedial temporal lobe pathology in dementia, brain trauma, and schizophrenia. Ann. N. Y. Acad. Sci. 1999;877:575–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Luo C-L, Li B-X, Li Q-Q, et al. Autophagy is involved in traumatic brain injury-induced cell death and contributes to functional outcome deficits in mice. Neuroscience. 2011;184:54–63. [DOI] [PubMed] [Google Scholar]
- [43].Umile EM, Sandel ME, Alavi A, et al. Dynamic imaging in mild traumatic brain injury: support for the theory of medial temporal vulnerability. Arch. Phys. Med. Rehabil. 2002;83:1506–1513. [DOI] [PubMed] [Google Scholar]
- [44].Urgolites ZJ, Hopkins RO, Squire LR. Medial temporal lobe and topographical memory. Proc. Natl. Acad. Sci. U. S. A. 2017;114:8626–8630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Yuan Q, Wu X, Yu J, et al. Subdural hygroma following decompressive craniectomy or non-decompressive craniectomy in patients with traumatic brain injury: Clinical features and risk factors. Brain Inj. 2015;29:971–980. [DOI] [PubMed] [Google Scholar]
- [46].Wang H, Xin T, Sun X, et al. Post-traumatic seizures--a prospective, multicenter, large case study after head injury in China. Epilepsy Res. 2013;107:272–278. [DOI] [PubMed] [Google Scholar]
- [47].Zare MA, Ahmadi K, Zadegan SA, et al. Effects of brain contusion on mild traumatic brain-injured patients. Int. J. Neurosci. 2013;123:65–69. [DOI] [PubMed] [Google Scholar]
- [48].Kim H, Jin ST, Kim YW, et al. Risk Factors for Early Hemorrhagic Progression after Traumatic Brain Injury: A Focus on Lipid Profile. J. Neurotrauma. 2015;32:950–955. [DOI] [PubMed] [Google Scholar]
- [49].Wallesch CW, Curio N, Galazky I, et al. The neuropsychology of blunt head injury in the early postacute stage: effects of focal lesions and diffuse axonal injury. J. Neurotrauma. 2001;18:11–20. [DOI] [PubMed] [Google Scholar]
- [50].Das-Gupta R, Turner-Stokes L. Traumatic brain injury. Disabil. Rehabil. 2002;24:654–665. [DOI] [PubMed] [Google Scholar]
- [51].Dara PK, Parakh M, Choudhary S, et al. Clinico-radiologic Profile of Pediatric Traumatic Brain Injury in Western Rajasthan. J. Neurosci. Rural Pract. 2018;9:226–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Salazar AM, Warden DL, Schwab K, et al. Cognitive rehabilitation for traumatic brain injury: A randomized trial. Defense and Veterans Head Injury Program (DVHIP) Study Group. JAMA. 2000;283:3075–3081. [DOI] [PubMed] [Google Scholar]