Abstract
The recently identified mechanically activated (MA) Piezo1 and Piezo2 channels play major roles in various aspects of mechanosensation in mammals, and their mutations are associated with human diseases. Recent reports show that activation of cell surface receptors coupled to heterotrimeric Gq proteins, as well as activation of the cAMP pathway potentiate Piezo2 channels. This phenomenon may play a role in mechanical allodynia or hyperalgesia during inflammation. Both Piezo1 and Piezo2 channels are inhibited upon depletion of plasma membrane phosphoinositides, in response to PLC activation by Ca2+ influx via the Transient Receptor Potential Vanilloid 1 (TRPV1) channels. This review will discuss current knowledge on regulation of Piezo channels by these intracellular signaling pathways.
Keywords: Piezo1, Piezo2, cAMP, EPAC, phosphoinositides, TRPV1
1. Introduction
Piezo1 and Piezo2 channels (Volkers et al., 2014) were identified in 2010 in an siRNA screen to knock down endogenous MA currents in the neuronal cell line Neuro2A (Coste et al., 2010). Unlike previous candidates for excitatory mammalian MA channels (Sachs, 2010), the expression of Piezo1 heterologous systems results in large MA currents (Coste et al., 2010; Gottlieb and Sachs, 2012; Bae et al., 2013b; Borbiro et al., 2015). Piezo2 is more difficult to express, probably due to its cytotoxicity, but there are reports from several laboratories reporting MA currents from heterologously expressed Piezo2 (Coste et al., 2010; Eijkelkamp et al., 2013; Borbiro et al., 2015). Piezos are Ca2+-permeable non-selective cation channels, with no similarity to any other protein and they contain no known protein domains. Piezo1 and Piezo2 share 47% identity with each other; they are large proteins, predicted to have 24–36 transmembrane domains, and the recently determined cryoEM structure of Piezo1 shows a trimeric structure (Ge et al., 2015). Piezo1 is expressed in several organs that have important mechanosensory functions, including the skin, the bladder and the lungs (Coste et al., 2010). Piezo2 is highly expressed in DRG neurons, where its siRNA mediated knockdown selectively eliminated rapidly adapting MA currents, while leaving intermediate and slowly adapting MA currents intact (Coste et al., 2010).
The global deletion of either Piezo1 (Ranade et al., 2014a) or Piezo2 (Dubin et al., 2012) in mice is embryonic lethal. Deletion of Piezo1 in vascular endothelium leads to severe defects in vascular development (Li et al., 2014). Skin specific deletion of Piezo2 leads to defects in Merkel cell mechanosensation (Maksimovic et al., 2014; Woo et al., 2014), whereas combined deletion of Piezo2 in Merkel cells and DRG neurons lead to marked defects in sensation of light touch (Ranade, 2014). Specific deletion of Piezo2 in proprioceptive sensory neurons lead to severely uncoordinated body movement, demonstrating the importance of Piezo2 in proprioception (Woo et al., 2015).
Mutations in Piezo channels have been shown to cause a variety of diseases in humans. Gain-of-function mutations in Piezo1 with reduced inactivation (Bae et al., 2013a) are associated with hereditary xerocytosis (Zarychanski et al., 2012; Andolfo et al., 2013), characterized by dehydration of red blood cells and hemolytic anemia. Gain of function mutations in Piezo2 cause Distal Arthrogryposis with restrictive pulmonary disease, and various musculoskeletal abnormalities (Coste et al., 2013). Loss of function mutations in Piezo1 in humans cause autosomal recessive congenital lymphatic dysplasia (Lukacs et al., 2015).
Intracellular second messengers, such as cyclic nucleotides and phospholipid-derived signaling molecules, regulate many ion channels. Compared to other channels, relatively little is known on the modulation of Piezo channels by intracellular signaling pathways. This chapter will summarize the current knowledge on this topic. We will discuss the effects of phosphoinositide-derived signals, and cAMP on Piezo channels, thus we first briefly introduce these two signaling pathways.
2. Phospholipase C (PLC) and cAMP Signaling
2.1. Phosphoinositide signaling
Phosphatidylinositol 4,5-bisphosphate [PI(4,5)P2] is generated by two phosphorylation steps from phosphatidylinositol (PI) (Balla, 2013). PLC enzymes catalyze the hydrolysis of PI(4,5)P2 and the formation of the two classical second messengers inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (DAG). IP3 induces Ca2+ release from the endoplasmic reticulum, by binding to its receptor, which is a Ca2+ permeable ion channel. DAG is an activator of Protein Kinase C (PKC). Conventional PKC isoforms (PKCα, β1, β2 and γ) are activated by DAG and Ca2+. Novel PKC isoforms (PKCδ, ε, θ, and η) are Ca2+ insensitive, but they are activated by DAG, and their affinity for this lipid is much higher than that of conventional isoforms. The regulation of atypical (ζ,ι) isoforms is less well understood (Newton, 2010).
PLCβ isoforms (PLCβ1–4) are activated by G-protein coupled receptors (GPCR) that couple to the Gq family of heterotrimeric G-proteins; PLCγ-s (PLCγ1 and 2) are activated by receptor tyrosine kinases, but PLCδ isoforms (PLCδ1, δ3 and δ4) do not have any obvious activators. While all PLC-s need some Ca2+ for activity, PLCδ-s are the most sensitive to Ca2+ among the three classical PLC groups, and they can be activated by Ca2+ influx alone (Allen et al., 1997; Lukacs et al., 2013). In addition to the classical PLC-s, newer isoforms have also been cloned more recently (PLCη1 and η2 and PLCε); their regulation is less well understood (Fukami et al., 2010).
Besides being a precursor for second messengers, PI(4,5)P2 has several important direct biological roles, for example it is involved in cytoskeletal organization and membrane trafficking (Saarikangas et al., 2010). PI(4,5)P2 and other phosphoinositides are emerging as a regulators of many different mammalian ion channels (Hilgemann et al., 2001; Suh and Hille, 2008; Logothetis et al., 2015), including most sensory and other TRP channels (Rohacs, 2014).
To put the effects of various phosphoinositides in context, we need to consider their in situ concentrations. PI(4,5)P2 constitutes up to 1 % of the phospholipids in the plasma membrane; its precursor PI(4)P is found in comparable quantities (Fruman et al., 1998). Their precursor PI constitutes up to 10% of membrane lipids (Fruman et al., 1998), but it has no effect on most PI(4,5)P2 sensitive channels, and its concentration is not expected to change significantly upon PLC activation. PI(3,4,5)P2 and PI(3,4)P2, the products of PI3-Kinase enzymes may also activate some PI(4,5)P2 sensitive ion channels, but their concentrations in the plasma membrane do not exceed 0.1 % (Fruman et al., 1998), thus their effect is generally overridden by the much higher concentration of PI(4,5)P2. Therefore the two most likely phosphoinositides regulating ion channels are PI(4,5)P2 and PI(4)P; both these lipids are substrates for PLC, even though most PLC isoforms prefer PI(4)P. Most attention has been paid so far to PI(4,5)P2, but PI(4)P also regulates certain ion channels, and its role as an independent signaling entity is beginning to be appreciated (Hammond et al., 2012; Lukacs et al., 2013).
2.2. cAMP signaling
Cyclic adenosine monophosphate (cAMP) is formed by Adenylate cyclase enzymes, which are activated by receptors that couple to Gs heterotrimeric G-proteins. The three major targets of cAMP are Protein Kinase A (PKA) enzymes, cyclic nucleotide gated (CNG) ion channels, and EPAC (exchange protein directly activated by cAMP) (Borland et al., 2009; Gloerich and Bos, 2010). EPAC is the most recently described target; it was identified in a database screen to explain the PKA-independent activation of the small G-protein Rap by cAMP (Gloerich and Bos, 2010). EPAC1 and EPAC2 are present in most tissues, and they function as guanine nucleotide exchange factors (GEFs) for both Rap1 and Rap2, which belong to the Ras family of small G proteins. These G-proteins cycle between the inactive GDP-bound state and the active GTP-bound state. GEFs accelerate the exchange of GDP for GTP and thus activate the G protein, whereas GTPase-activating proteins (GAPs) enhance GTP hydrolysis, thus inactivate the G-protein. Several cAMP analogues, such as 8-pCPT are available that selectively interact with EPAC1 and EPAC2. The rationale for this selective agonism is that EPAC proteins lack the glutamate that interacts with the 2-OH group of the ribose of cAMP in PKA and cAMP-gated ion channels (Borland et al., 2009; Gloerich and Bos, 2010).
3. Sensitization of sensory ion channels by inflammatory pathways
Under inflammatory conditions neurons show enhanced sensitivity to painful stimuli (hyperalgesia) and abnormal pain sensitivity to non-painful stimuli (allodynia). There are multiple inflammatory signaling pathways known to sensitize sensory neurons to both thermal and mechanical stimuli (Linley et al., 2012), here we briefly discuss sensitization of the heat- and capsaicin-dependent Transient Receptor Potential Vanilloid 1 (TRPV1) channels.
Thermal hyperalgesia in mice is largely dependent on TRPV1 (Caterina et al., 2000; Davis et al., 2000). While the expression level of these channels may increase in chronic inflammation, there are also important acute signaling events that increase the activity of TRPV1 downstream of the activation of both Gq and Gs coupled receptors. Bradykinin, the classic, very well studied sensitizing agent is a pro-inflammatory peptide is generated after tissue injury and noxious stimulation (Petho and Reeh, 2012). Bradykinin receptors (B1 and B2) are GPCR-s; they stimulate PLCβ enzymes through the Gαq subunits of heterotrimeric G-proteins. Extracellular ATP acting on Gq coupled purinergic receptors also sensitizes TRPV1 (Tominaga et al., 2001). The downstream activation of PKC will lead to the phosphorylation of TRPV1 on S501 and S800 residues (Numazaki et al., 2002), and thus sensitize the channel to heat and chemical activation. This phosphorylation shifts the capsaicin concentration-response to the left, without substantial effect on maximal currents, leading to selective enhancement of TRPV1 activity at moderate stimulation levels. Sensitivity to heat and low extracellular pH also increases during sensitization (Tominaga et al., 2001).
Gq-coupled receptors were also proposed to sensitize TRPV1 channels by decreasing PI(4,5)P2 levels, and relieving TRPV1 from tonic inhibition by this lipid (Chuang et al., 2001). Later it was found by several laboratories that PI(4,5)P2 activates, rather than inhibits TRPV1 in excised inside out patches (Stein et al., 2006; Lukacs et al., 2007; Poblete et al., 2014). Nevertheless, in a cellular context, there may be an indirect inhibitory effect of PI(4,5)P2, relieve from which by PI(4,5)P2 hydrolysis potentiates the effect of PKC activation (Lukacs et al., 2013). Detailed discussion of this complex topic is beyond the scope of this chapter, and has been reviewed in detail recently (Rohacs, 2015).
Some inflammatory agents such as prostaglandin E2 act via Gs coupled receptors. While less studied than the PLC pathway, activation of the cAMP pathways also sensitizes TRPV1 via PKA mediated phosphorylation (Rathee et al., 2002). Interestingly, the A-kinase anchoring protein AKAP150 is required for TRPV1 sensitization by not only the PKA, but also the PKC pathway (Zhang et al., 2008).
Little is known about the molecular targets of inflammatory agents in mechanical hyperalgesia and allodynia, but there is evidence that Piezo2 channel activity is enhanced in sensory neurons by various inflammatory signaling pathways. Below we will give an overview of Piezo2 channel sensitizing pathways.
3.1. Piezo2 potentiation by Gq-coupled receptors
Shortly after the discovery of Piezo channels the Patapoutian group reported that activation of bradykinin receptors increased Piezo2-mediated rapidly adapting MA current amplitudes in sensory DRG neurons (Dubin et al., 2012). The B2 bradykinin receptor antagonist HOE-140 (icatibant) blocked the bradykinin-induced modulation, indicating that endogenous B2 receptors were responsible for the observed effects. The effect of Bradykinin was abolished by combined inhibition of PKC with bisindolylmaleimide (BIM) and PKA using H89. When B2 bradykinin receptors were heterologously expressed together with Piezo2 in HEK cells, the amplitude of piezo2- mediated MA currents increased and their inactivation slowed upon exposure to bradykinin. The Gβγ inhibitor gallein did not inhibit the potentiating effect of Bradykinin (Dubin et al., 2012), but including the non-hydrolyzable GTP analogue GTPγS in the patch pipette potentiated the currents, suggesting the role of Gα subunits (Dubin et al., 2012). Consistent with this, a subsequent study showed that including GTP in the patch pipette potentiated rapidly adapting MA currents in DRG neurons (Jia et al., 2013). The potentiating effect of Bradykinin was abolished by the combined inhibition of PKC and PKA, but not by applying one, or the other protein kinase inhibitor alone (Dubin et al., 2012). The PKA activator 8-BrcAMP and the PKC agonist PMA also potentiates Piezo2 currents, suggesting that both pathways are capable of similarly modulating Piezo2. The PLC inhibitor U73122, and depleting intracellular Ca2+ stores using thapsigargin had no effect on Bradykinin induced changes in Piezo2 currents. The PLC activator m-3M3FBS had no effect on Piezo2 current amplitudes.
The overall conclusion of the paper by (Dubin et al., 2012) is that bradykinin receptor activation potentiates Piezo2 currents by activating both PKC and PKA. There are several open questions, however. Both B1 and B2 receptors act via Gq and to some extent Gi heterotrimeric G-proteins (Leeb-Lundberg et al., 2005). Gαq activates PLC directly, and Gi signaling may also activate PLC via Gβγ subunits, but Gi inhibits the formation of cAMP. How PKA is activated upon bradykinin receptor stimulation, is unclear. It is also not clear, how neither PLC inhibitors nor a PLC activator had any effect on Piezo2 currents, when the downstream PKC was concluded to be involved in potentiation by bradykinin.
Extracellular ATP when released from damaged tissue can directly activate pain-sensing neurons through P2X ionotropic receptors, and the metabotropic P2Y receptor activation by ATP results in sensitization to noxious stimuli (Tominaga et al., 2001). Lechner & Lewin found that application of ATP and UTP potentiated rapidly adapting MA currents in a nociceptive subset of mouse DRG neurons (Lechner and Lewin, 2009). Rapidly adapting currents in large mechanoreceptors, as well as intermediate and slowly adapting MA currents were not potentiated by UTP. These results were obtained before the cloning of Piezo2, channels, but they are in line with the bradykinin-induced sensitization described previously. Extracellular nucleotides also potentiated mechanically evoked action potentials in 41% of c-fibers in the skin-nerve preparation. Responses of low-threshold mechanoreceptors and D-hair mechanoreceptors were not altered by the addition UTP. The potentiating effect of ATP on MA currents was not sensitive to inhibitors of P2X channels. UTP, which has low affinity for P2X receptors, was equally effective as ATP, thus the authors concluded that the potentiating effect was due to P2Y signaling (Lechner and Lewin, 2009). P2Y receptors couple to Gαq and are known to activate PKC, but the authors did not perform experiments to study the mechanism of potentiation of MA currents. They argued however that the potentiating effect of P2Y activation is independent of PKC based on an earlier report with pharmacological activation of PKC using the phorbol ester PMA (Di Castro et al., 2006). The effect of PMA developed in 1 hour, and was essentially irreversible (Di Castro et al., 2006), while UTP acted within minutes and the effect was quickly reversible (Lechner and Lewin, 2009).
Overall, there is independent evidence from two laboratories showing that activation of Gq-coupled receptors potentiates Piezo2 currents, but deciphering the mechanism of this phenomenon will require further work.
3.2. Piezo2 regulation by the cAMP-EPAC pathway
During inflammation prostaglandin (PG) levels increase and these short-lived tissue hormones add to the scope of sensory neuron sensitization when continuously formed and thus having a long lasting sustained effect. They are derived from arachidonic acid by cyclooxygenase (COX) enzymes locally. Some prostanoids, such as PGE2 act via Gs-coupled receptors, thus increase intracellular cAMP levels. Prostanoids induce prolonged mechanical hyperalgesia, the mechanism of which is not very well established (Petho and Reeh, 2012). Two studies discussed below found that cAMP acting via EPAC1 potentiates Piezo2 currents, and this phenomenon may be involved in mechanical hyperalgesia / allodynia.
Application of the EPAC-selective cAMP analogue 8-pCPT increased peak amplitudes of rapidly adapting MA currents in large mouse DRG neurons (Eijkelkamp et al., 2013). In HEK cells, coexpressing human Piezo2 and EPAC1, 8-pCPT potentiated rapidly adapting MA currents. Interestingly, 8-pCPT had only a minimal effect on MA currents in HEK cells co-expressing hPiezo2 and EPAC2 (Eijkelkamp et al., 2013).
The same study found that EPAC1 levels increased in a nerve injury model, and mechanical allodynia induced by nerve damage was attenuated in EPAC−/− mice. Intraplantar injection of 8-pCPT induced long lasting mechanical allodynia, which persisted when Nav1.8 expressing nociceptors were depleted using diphtheria-toxin, but was attenuated by intrathecal injection of Piezo2 antisense oligonucleotides. Interestingly, mechanical allodynia induced by a PKA-selective cAMP analogue (6-Bnz-cAMP) was attenuated in mice in which Nav1.8+ neurons were depleted, pointing to different mechanisms for the PKA and EPAC pathways in inducing hypersensitivity (Eijkelkamp et al., 2013).
Eijkelkamp et al also found that the PKC activator PMA did not potentiate human Piezo2 currents, a finding contradicting the results of (Dubin et al., 2012), who as mentioned earlier found that PMA potentiated mouse Piezo2 currents. The PKA specific cAMP analogue 6-Bnz-cAMP also did not increase Piezo2 current amplitudes (Eijkelkamp et al., 2013).
Another recent study confirmed the potentiation of Piezo2 channels by 8-pCPT in HEK cells expressing EPAC1, and also found that coexpressing G-protein kinase 2 (GRK2) attenuated this effect (Singhmar et al., 2016). Additionally EPAC1−/− mice were protected against inflammatory hyperalgesia induced by complete Freund’s adjuvant (CFA). Moreover intrathecal administration of Piezo2 antisense oligonucleotides inhibited mechanical, but not thermal hyperalgesia. The inhibitory effect of Piezo2 antisense injection started on day 3 after injection of CFA. One day after CFA injection the Piezo2 antisense had no effect (Singhmar et al., 2016), which is consistent with an earlier finding that combined genetic deletion of Piezo2 in DRG and in Merkel cells in mice, had no effect on mechanical allodynia after 24 hours of CFA injection (Ranade et al., 2014b).
The studies discussed here (Eijkelkamp et al., 2013; Singhmar et al., 2016) agree that cAMP signaling via EPAC1 stimulate Piezo2 activity, which plays a role in mechanical allodynia. While the agreement from two independent reports makes a strong case, open questions remain. For example, none of the studies identified the effector downstream of EPAC1, and the mechanism by which Piezo2 is potentiated. Also, they did not demonstrate that receptor mediated increase in cAMP is sufficient to potentiate Piezo2 currents via this pathway.
4. Calcium-induced inhibition of Piezo channels – the role of phosphoinositides
The heat- and capsaicin-sensitive TRPV1 channels, and Piezo2 are coexpressed in a subset of small sensory neurons (Coste et al., 2010). We found that in these cells application of capsaicin abrogated the rapidly adapting MA currents (Borbiro et al., 2015). This phenomenon was observed only in neurons with the characteristic capsaicin-induced TRPV1 currents. When Piezo2 and TRPV1 were coexpressed in HEK cells, capsaicin robustly inhibited MA Piezo2 currents, and this effect was dependent on the presence of extracellular Ca2+, showing the importance of Ca2+ influx through TRPV1 channels. Similarly, in a heterologous system coexpressing TRPV1 and Piezo1, capsaicin inhibited MA currents, and this effect disappeared in a Ca2+ free extracellular medium.
We have shown earlier that both in an expression system (Lukacs et al., 2007) and in native DRG neurons (Lukacs et al., 2013), Ca2+ influx via TRPV1 leads to the activation of a PLCδ isoform, with a concomitant robust decrease in PI(4,5)P2 and PI(4)P levels in the plasma membrane. TRPV1 requires phosphoinositides for activity, and this phenomenon plays an important role in the desensitization of this channel (Rohacs, 2015). As PI(4,5)P2 is required for the activity of many different ion channels, we tested if phosphoinositide depletion was responsible for Piezo channel inhibition (Borbiro et al., 2015).
We found that intracellular application of excess PI(4,5)P2 or PI(4)P in whole cell patch clamp attenuated the inhibitory effect of capsaicin on mechanically evoked Piezo1 currents. Activation of chemically inducible phosphoinositide phosphatases, which deplete phosphoinositides (Varnai et al., 2006; Hammond et al., 2012) without the formation of IP3 or DAG, also inhibited Piezo1 currents (Borbiro et al., 2015). Pseudojanin, the inducible phosphatase that dephosphorylates both PI(4,5)P2 and PI(4)P was more effective than a 5’ phosphatase, arguing for the importance of both PI(4,5)P2 and PI(4)P. Note that the inhibition by the inducible phosphatases took several minutes to develop, in contrast to the almost immediate inhibition upon the activation of TRPV1. Finally, Piezo1 current activity ran down in excised patches, a phenomenon characteristic of PI(4,5)P2 dependent ion channels (Rohacs et al., 2002). Phosphoinositides, PI(4,5)P2 and PI(4)P applied together inhibited rundown of MA Piezo1 currents in inside-out patches (Borbiro et al., 2015). Overall, these data show that Piezo channels require phosphoinositides for activity. Attempts to reactivate Piezo1 currents after rundown, however, resulted in variable results (Borbiro et al., 2015), in contrast to the very reliable reactivation of most PI(4,5)P2 dependent ion channels in the excised patch configuration (Rohacs, 2014). This shows that the relationship between phosphoinositides and Piezo channels, may be more complex than the lipid being a cofactor directly activating the channel, and points to the potential importance of other cellular components.
In contrast to the robust and complete inhibition of Piezo1 currents by TRPV1 activation, activation of the Gq coupled M1 muscarinic receptors by carbachol lead only to a slow and marginal inhibition of Piezo1 currents (Borbiro et al., 2015). When we measured PI(4,5)P2 and PI(4)P levels with fluorescence based sensors, we found that TRPV1 activation lead to somewhat larger decrease in PI(4,5)P2 and a much larger decrease in PI(4)P levels than carbachol (Borbiro et al., 2015). This is consistent with our earlier finding that in DRG neurons, TRPV1 activation by capsaicin induced a robust decrease of both PI(4,5)P2 and PI(4)P, but activation of bradykinin receptors only induced a small decrease in PI(4,5)P2 levels, and no detectable change in PI(4)P (Lukacs et al., 2013). Overall, it is likely that Piezo1 channels have high apparent affinity for phosphoinositides, and only robust and combined depletion of PI(4,5)P2 and PI(4)P inhibits them. Consistent with this idea, when we used ATP free intracellular pipette solutions, a maneuver shown to make phosphoinositide depletion irreversible (Suh and Hille, 2002), carbachol induced a much larger, inhibition of Piezo1 currents, compared to experiments with ATP in the patch pipette (Borbiro et al., 2015).
Capsaicin has long been used as a local analgesic, after an initial burning sensation, it relieves pain (Szallasi and Blumberg, 1999). While the effects of capsaicin are complex, they involve desensitization of TRPV1 itself, inhibition of other ion channels, and even local nerve degeneration, inhibition of Piezo2 channels may also contribute to its local analgesic effect.
Inhibition of Piezo1 currents was not specific to capsaicin-induced activation of TRPV1, both activation of TRPA1 with mustard oil, and activation of TRPV1 by low extracellular pH was capable of inhibiting the channel. The level of inhibition of Piezo1 by these latter maneuvers was smaller than by capsaicin, correlating with the smaller TRPA1 or TRPV1 currents (Borbiro et al., 2015). An earlier study showed that increase of cytoplasmic Ca2+ from 50 nM to 1 μM induced a small increase in Piezo2 current activity (Eijkelkamp et al., 2013). Inactivation kinetics of Piezo1 was not significantly affected by the absence or presence of extracellular Ca2+ (Coste et al., 2010; Gottlieb et al., 2012). Overall, the effects of Ca2+ on Piezo channel activity may be complex, and probably only very large increases in cytoplasmic Ca2+ will inhibit their activity via phosphoinositide depletion.
It will require further studies whether Ca2+ influx via other pathways, such as voltage gated Ca2+ channels will modulate Piezo channel activity. It also has to be noted that the presence of excess PI(4,5)P2 or PI(4)P in the patch pipette, delayed, rather than eliminated Piezo1 current inhibition by TRPV1 activation, pointing to the potential involvement of other signaling pathways (Borbiro et al., 2015).
5. Conclusions
The recent identification of Piezo1 and Piezo2 channels induced a flurry of research activity to understand the physiological importance, structure, gating mechanism and regulation of these channels. On the topic of Piezo channel regulation by signaling pathways, an emerging picture shows that Piezo2 channels are potentiated by inflammatory signals, but the exact mechanism how channel activity increases is not yet clear. Data also show that the Piezo channels require phosphoinositides for activity, but whether the lipids act directly on the channel, or via an indirect mechanism, is not yet clear. Integrating regulation of Piezos by Ca2+, phosphoinositides, and by GPCR activation will also require further studies. Overall, in less than 6 years, at the time of writing of this chapter, the research community has accumulated an amazing amount of knowledge about Piezo channels. Nevertheless, this is a still a new field, full of open questions, and the following years are expected to bring new and exciting results.
Figure 1.
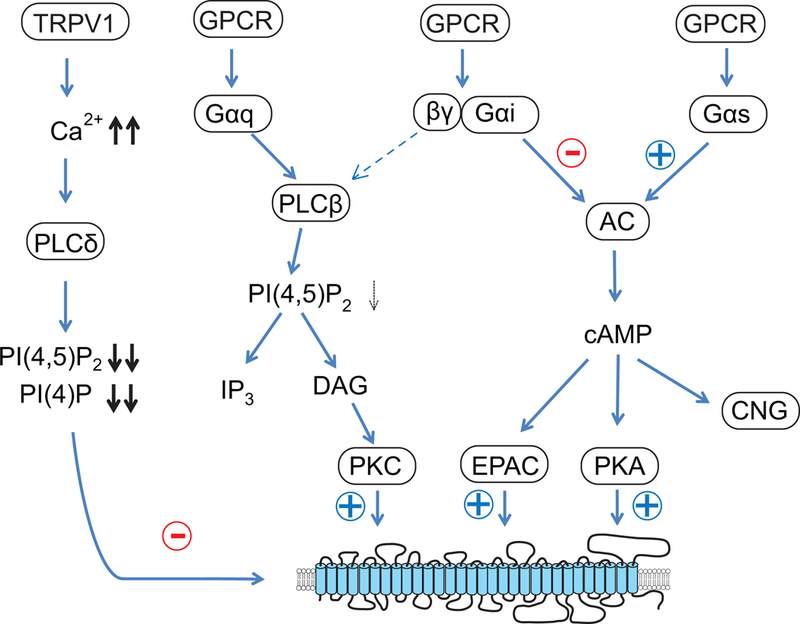
Summary of signaling pathways regulating Piezo channels. Activation of TRPV1 channels by capsaicin leads to a massive Ca2+ influx, which activated PLCδ isoforms. The concomitant robust decrease in PI(4,5)P2 and PI(4)P levels inhibits Piezo channel activity. Activation of Gq-coupled GPCR-s lead to activation of PLCβ isoforms, with only a modest decrease in PI(4,5)P2 levels, which is not enough to inhibit channel activity, and the downstream activation of PKC will stimulate Piezo2 activity. Activation Gs-coupled receptors potentiate Piezo2 channel activity, via the EPAC pathway. Activation of PKA may also contribute to potentiation of channel activity.
Acknowledgements:
The discussions and comments of the members of the Rohacs lab are highly appreciated. Work in TR’s laboratory is supported by NIH grants: NS055159 and GM093290.
ABBREVIATIONS
- cAMP
Cyclic adenosine monophosphate
- CNG
cyclic nucleotide gated ion channels
- DAG
Diacylglycerol
- DRG
Dorsal Root Ganglion
- EPAC
exchange protein directly activated by cAMP
- GPCR
G-protein coupled receptor
- IP3
Inositol 1,4,5 trisphosphate
- MA
Mechanically activated
- PI(4,5)P2
Phosphatidylinositol 4,5-bisphosphate
- PKA
Protein Kinase A
- PKC
Protein Kinase C
- PLC
Phospholipase C
- TRPV1
Transient Receptor Potential Vanilloid 1
REFERENCES
- Allen V, Swigart P, Cheung R, Cockcroft S, and Katan M (1997). Regulation of inositol lipid-specific phospholipase C-delta by changes in Ca2+ ion concentrations. Biochem J 327 ( Pt 2), 545–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andolfo I, Alper SL, De Franceschi L, Auriemma C, Russo R, De Falco L, Vallefuoco F, Esposito MR, Vandorpe DH, Shmukler BE, Narayan R, Montanaro D, D’Armiento M, Vetro A, Limongelli I, Zuffardi O, Glader BE, Schrier SL, Brugnara C, Stewart GW, Delaunay J, and Iolascon A (2013). Multiple clinical forms of dehydrated hereditary stomatocytosis arise from mutations in PIEZO1. Blood 121, 3925–3935. [DOI] [PubMed] [Google Scholar]
- Bae C, Gnanasambandam R, Nicolai C, Sachs F, and Gottlieb PA (2013a). Xerocytosis is caused by mutations that alter the kinetics of the mechanosensitive channel PIEZO1. Proc Natl Acad Sci U S A 110, E1162–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae C, Gottlieb PA, and Sachs F (2013b). Human PIEZO1: removing inactivation. Biophys J 105, 880–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balla T (2013). Phosphoinositides: tiny lipids with giant impact on cell regulation. Physiol Rev 93, 1019–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borbiro I, Badheka D, and Rohacs T (2015). Activation of TRPV1 channels inhibit mechanosensitive Piezo channel activity by depleting membrane phosphoinositides. Sci Signal 8, ra15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borland G, Smith BO, and Yarwood SJ (2009). EPAC proteins transduce diverse cellular actions of cAMP. Br J Pharmacol 158, 70–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, and Julius D (2000). Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 288, 306–313. [DOI] [PubMed] [Google Scholar]
- Chuang HH, Prescott ED, Kong H, Shields S, Jordt SE, Basbaum AI, Chao MV, and Julius D (2001). Bradykinin and nerve growth factor release the capsaicin receptor from PtdIns(4,5)P2-mediated inhibition. Nature 411, 957–962. [DOI] [PubMed] [Google Scholar]
- Coste B, Houge G, Murray MF, Stitziel N, Bandell M, Giovanni MA, Philippakis A, Hoischen A, Riemer G, Steen U, Steen VM, Mathur J, Cox J, Lebo M, Rehm H, Weiss ST, Wood JN, Maas RL, Sunyaev SR, and Patapoutian A (2013). Gain-of-function mutations in the mechanically activated ion channel PIEZO2 cause a subtype of Distal Arthrogryposis. Proc Natl Acad Sci U S A 110, 4667–4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coste B, Mathur J, Schmidt M, Earley TJ, Ranade S, Petrus MJ, Dubin AE, and Patapoutian A (2010). Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science 330, 55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JB, Gray J, Gunthorpe MJ, Hatcher JP, Davey PT, Overend P, Harries MH, Latcham J, Clapham C, Atkinson K, Hughes SA, Rance K, Grau E, Harper AJ, Pugh PL, Rogers DC, Bingham S, Randall A, and Sheardown SA (2000). Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature 405, 183–187. [DOI] [PubMed] [Google Scholar]
- Di Castro A, Drew LJ, Wood JN, and Cesare P (2006). Modulation of sensory neuron mechanotransduction by PKC- and nerve growth factor-dependent pathways. Proc Natl Acad Sci U S A 103, 4699–4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubin AE, Schmidt M, Mathur J, Petrus MJ, Xiao B, Coste B, and Patapoutian A (2012). Inflammatory signals enhance piezo2-mediated mechanosensitive currents. Cell Rep 2, 511–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eijkelkamp N, Linley JE, Torres JM, Bee L, Dickenson AH, Gringhuis M, Minett MS, Hong GS, Lee E, Oh U, Ishikawa Y, Zwartkuis FJ, Cox JJ, and Wood JN (2013). A role for Piezo2 in EPAC1-dependent mechanical allodynia. Nat Commun 4, 1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fruman DA, Meyers RE, and Cantley LC (1998). Phosphoinositide kinases. Annu Rev Biochem 67, 481–507. [DOI] [PubMed] [Google Scholar]
- Fukami K, Inanobe S, Kanemaru K, and Nakamura Y (2010). Phospholipase C is a key enzyme regulating intracellular calcium and modulating the phosphoinositide balance. Prog Lipid Res 49, 429–437. [DOI] [PubMed] [Google Scholar]
- Ge J, Li W, Zhao Q, Li N, Chen M, Zhi P, Li R, Gao N, Xiao B, and Yang M (2015). Architecture of the mammalian mechanosensitive Piezo1 channel. Nature 527, 64–69. [DOI] [PubMed] [Google Scholar]
- Gloerich M, and Bos JL (2010). Epac: defining a new mechanism for cAMP action. Annu Rev Pharmacol Toxicol 50, 355–375. [DOI] [PubMed] [Google Scholar]
- Gottlieb PA, Bae C, and Sachs F (2012). Gating the mechanical channel Piezo1: a comparison between whole-cell and patch recording. Channels (Austin) 6, 282–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb PA, and Sachs F (2012). Piezo1: properties of a cation selective mechanical channel. Channels (Austin) 6, 214–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond GR, Fischer MJ, Anderson KE, Holdich J, Koteci A, Balla T, and Irvine RF (2012). PI4P and PI(4,5)P2 Are Essential But Independent Lipid Determinants of Membrane Identity. Science 337, 727–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgemann DW, Feng S, and Nasuhoglu C (2001). The complex and intriguing lives of PIP2 with ion channels and transporters. Sci STKE 2001, re19. [DOI] [PubMed] [Google Scholar]
- Jia Z, Ikeda R, Ling J, and Gu JG (2013). GTP-dependent run-up of Piezo2-type mechanically activated currents in rat dorsal root ganglion neurons. Mol Brain 6, 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechner SG, and Lewin GR (2009). Peripheral sensitisation of nociceptors via G-protein-dependent potentiation of mechanotransduction currents. J Physiol 587, 3493–3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeb-Lundberg LM, Marceau F, Muller-Esterl W, Pettibone DJ, and Zuraw BL (2005). International union of pharmacology. XLV. Classification of the kinin receptor family: from molecular mechanisms to pathophysiological consequences. Pharmacol Rev 57, 27–77. [DOI] [PubMed] [Google Scholar]
- Li J, Hou B, Tumova S, Muraki K, Bruns A, Ludlow MJ, Sedo A, Hyman AJ, McKeown L, Young RS, Yuldasheva NY, Majeed Y, Wilson LA, Rode B, Bailey MA, Kim HR, Fu Z, Carter DA, Bilton J, Imrie H, Ajuh P, Dear TN, Cubbon RM, Kearney MT, Prasad RK, Evans PC, Ainscough JF, and Beech DJ (2014). Piezo1 integration of vascular architecture with physiological force. Nature. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linley JE, Ooi L, Pettinger L, Kirton H, Boyle JP, Peers C, and Gamper N (2012). Reactive oxygen species are second messengers of neurokinin signaling in peripheral sensory neurons. Proc Natl Acad Sci U S A 109, E1578–1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis DE, Petrou VI, Zhang M, Mahajan R, Meng XY, Adney SK, Cui M, and Baki L (2015). Phosphoinositide Control of Membrane Protein Function: A Frontier Led by Studies on Ion Channels. Annu Rev Physiol 77, 81–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukacs V, Mathur J, Mao R, Bayrak-Toydemir P, Procter M, Cahalan SM, Kim HJ, Bandell M, Longo N, Day RW, Stevenson DA, Patapoutian A, and Krock BL (2015). Impaired PIEZO1 function in patients with a novel autosomal recessive congenital lymphatic dysplasia. Nat Commun 6, 8329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukacs V, Thyagarajan B, Varnai P, Balla A, Balla T, and Rohacs T (2007). Dual regulation of TRPV1 by phosphoinositides. J Neurosci 27, 7070–7080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukacs V, Yudin Y, Hammond GR, Sharma E, Fukami K, and Rohacs T (2013). Distinctive changes in plasma membrane phosphoinositides underlie differential regulation of TRPV1 in nociceptive neurons. Journal of Neuroscience 33, 11451–11463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maksimovic S, Nakatani M, Baba Y, Nelson AM, Marshall KL, Wellnitz SA, Firozi P, Woo SH, Ranade S, Patapoutian A, and Lumpkin EA (2014). Epidermal Merkel cells are mechanosensory cells that tune mammalian touch receptors. Nature 509, 617–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton AC (2010). Protein kinase C: poised to signal. Am J Physiol Endocrinol Metab 298, E395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numazaki M, Tominaga T, Toyooka H, and Tominaga M (2002). Direct phosphorylation of capsaicin receptor VR1 by protein kinase Cepsilon and identification of two target serine residues. J Biol Chem 277, 13375–13378. [DOI] [PubMed] [Google Scholar]
- Petho G, and Reeh PW (2012). Sensory and signaling mechanisms of bradykinin, eicosanoids, platelet-activating factor, and nitric oxide in peripheral nociceptors. Physiol Rev 92, 1699–1775. [DOI] [PubMed] [Google Scholar]
- Poblete H, Oyarzun I, Olivero P, Comer J, Zuniga M, Sepulveda RV, Baez-Nieto D, Gonzalez Leon C, Gonzalez-Nilo F, and Latorre R (2014). Molecular Determinants of Phosphatidylinositol 4,5 Bisphosphate (PI(4,5)P2) Binding to Transient Receptor Potential V1 (TRPV1) Channels. J Biol Chem 290, 2086–2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranade SS, Qiu Z, Woo SH, Hur SS, Murthy SE, Cahalan SM, Xu J, Mathur J, Bandell M, Coste B, Li YS, Chien S, and Patapoutian A (2014a). Piezo1, a mechanically activated ion channel, is required for vascular development in mice. Proc Natl Acad Sci U S A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranade SS, Woo SH, Dubin AE, Moshourab RA, Wetzel C, Petrus M, Mathur J, Begay V, Coste B, Mainquist J, Wilson AJ, Francisco AG, Reddy K, Qiu Z, Wood JN, Lewin GR, and Patapoutian A (2014b). Piezo2 is the major transducer of mechanical forces for touch sensation in mice. Nature 516, 121–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranade SSWSH, Dubin AE;Moshourab RA; Wetzel C; Petrus M; Mathur J; Begay V; Coste B; Mainquist J; Wilson AJ; Francisco AG; Reddy K; Qui Z; Wood JN; Lewin GR; Patapoutian A (2014). Piezo2 Is Required for Touch Sensation in Mice. Journal of General Physiology 144, 14a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathee PK, Distler C, Obreja O, Neuhuber W, Wang GK, Wang SY, Nau C, and Kress M (2002). PKA/AKAP/VR-1 module: A common link of Gs-mediated signaling to thermal hyperalgesia. J Neurosci 22, 4740–4745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohacs T (2014). Phosphoinositide regulation of TRP channels. Handb Exp Pharmacol 233, 1143–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohacs T (2015). Phosphoinositide regulation of TRPV1 revisited. Pflugers Arch 467, 1851–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohacs T, Lopes C, Mirshahi T, Jin T, Zhang H, and Logothetis DE (2002). Assaying phosphatidylinositol bisphosphate regulation of potassium channels. Methods Enzymol 345, 71–92. [DOI] [PubMed] [Google Scholar]
- Saarikangas J, Zhao H, and Lappalainen P (2010). Regulation of the actin cytoskeleton-plasma membrane interplay by phosphoinositides. Physiol Rev 90, 259–289. [DOI] [PubMed] [Google Scholar]
- Sachs F (2010). Stretch-activated ion channels: what are they? Physiology (Bethesda) 25, 50–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhmar P, Huo X, Eijkelkamp N, Berciano SR, Baameur F, Mei FC, Zhu Y, Cheng X, Hawke D, Mayor F Jr., Murga C, Heijnen CJ, and Kavelaars A (2016). Critical role for Epac1 in inflammatory pain controlled by GRK2-mediated phosphorylation of Epac1. Proc Natl Acad Sci U S A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein AT, Ufret-Vincenty CA, Hua L, Santana LF, and Gordon SE (2006). Phosphoinositide 3-kinase binds to TRPV1 and mediates NGF-stimulated TRPV1 trafficking to the plasma membrane. J Gen Physiol 128, 509–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh BC, and Hille B (2002). Recovery from muscarinic modulation of M current channels requires phosphatidylinositol 4,5-bisphosphate synthesis. Neuron 35, 507–520. [DOI] [PubMed] [Google Scholar]
- Suh BC, and Hille B (2008). PIP2 is a necessary cofactor for ion channel function: how and why? Annu Rev Biophys 37, 175–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szallasi A, and Blumberg PM (1999). Vanilloid (Capsaicin) receptors and mechanisms. Pharmacol Rev 51, 159–212. [PubMed] [Google Scholar]
- Tominaga M, Wada M, and Masu M (2001). Potentiation of capsaicin receptor activity by metabotropic ATP receptors as a possible mechanism for ATP-evoked pain and hyperalgesia. Proc Natl Acad Sci U S A 98, 6951–6956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varnai P, Thyagarajan B, Rohacs T, and Balla T (2006). Rapidly inducible changes in phosphatidylinositol 4,5-bisphosphate levels influence multiple regulatory functions of the lipid in intact living cells. J Cell Biol 175, 377–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkers L, Mechioukhi Y, and Coste B (2014). Piezo channels: from structure to function. Pflugers Arch. [DOI] [PubMed] [Google Scholar]
- Woo SH, Lukacs V, de Nooij JC, Zaytseva D, Criddle CR, Francisco A, Jessell TM, Wilkinson KA, and Patapoutian A (2015). Piezo2 is the principal mechanotransduction channel for proprioception. Nat Neurosci 18, 1756–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo SH, Ranade S, Weyer AD, Dubin AE, Baba Y, Qiu Z, Petrus M, Miyamoto T, Reddy K, Lumpkin EA, Stucky CL, and Patapoutian A (2014). Piezo2 is required for Merkel-cell mechanotransduction. Nature 509, 622–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarychanski R, Schulz VP, Houston BL, Maksimova Y, Houston DS, Smith B, Rinehart J, and Gallagher PG (2012). Mutations in the mechanotransduction protein PIEZO1 are associated with hereditary xerocytosis. Blood 120, 1908–1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Li L, and McNaughton PA (2008). Proinflammatory mediators modulate the heat-activated ion channel TRPV1 via the scaffolding protein AKAP79/150. Neuron 59, 450–461. [DOI] [PubMed] [Google Scholar]


