Summary
Studies over the last decade uncovered overlapping niches for hematopoietic stem cells (HSCs), multipotent progenitor cells, common lymphoid progenitors, and early B cell progenitors. HSC and lymphoid niches are predominantly composed by mesenchymal progenitor cells (MPCs), and by a small subset of endothelial cells. Niche cells create specialized microenvironments through the concomitant production of short-range acting cell-fate determining cytokines such as Interleukin (IL)-7 and Stem Cell Factor (SCF) and the potent chemoattractant C-X-C Motif Chemokine Ligand 12 (CXCL12). This type of cellular organization allows for the cross-talk between hematopoietic stem and progenitor cells with niche cells, such that niche cell activity can be regulated by the quality and quantity of hematopoietic progenitors being produced. For example, pre-leukemic B cell progenitors and preB acute lymphoblastic leukemias interact directly with MPCs, and downregulate IL-7 expression and the production of non-leukemic lymphoid cells. In this review, we discuss a novel model of B cell development that is centered on cellular circuits formed between B cell progenitors and lymphopoietic niches.
1–. Introduction
The immune system is composed of multiple cell types produced in primary lymphoid organs. With the exception of T cells, all other blood cell types develop from hematopoietic progenitor cells in the bone marrow. In humans, the bone marrow produces about 200 billion blood cells daily, of which 90% are red blood cells, and the remaining 10% being composed of granulocytes (neutrophils being the most abundant), monocytes, dendritic cell precursors, lymphocytes, and platelets. This remarkable capacity to produce large numbers of distinct cell types with specialized functions is, perhaps, unmatched by any other organ in adult mammals. Besides ensuring daily blood cell production, specialized microenvironments in the bone marrow also contribute to the long-term maintenance of two types of stem cells, hematopoietic stem cells (HSCs) and mesenchymal stem and progenitor cells (1, 2), and to other differentiated cells, such as memory T cell subsets and antibody producing plasma cells (3), that are critical for ensuring long-term immunity against previously encountered antigens.
Hematopoietic cells are commonly separated into two major lineages, lymphoid and myeloid, with each lineage composed by multiple cell subsets with different cellular functions, distribution throughout the body, and life spans. Lymphoid lineage cells, for example, develop from a common lymphoid progenitor (CLP) that has lost the capacity to differentiate into myeloid cells, but still retains the ability to differentiate into multiple types of lymphocytes. B lymphocytes are the most abundant lymphocyte subset produced in bone marrow (20%), followed by NK cells (5%), and some subsets of innate lymphoid cells (ILCs, < 1%). Although T cell development takes place in the thymus, a CLP subset with thymus-homing capacity periodically exits the bone marrow to seed the thymus and initiate T cell development (4). Similarly, the common myeloid progenitor (CMP) can differentiate into all types of myeloid cells, with erythroid cells and granulocyte subsets being the most abundant, followed by monocytes, dendritic cell precursors, and megakaryocytes.
The diversity of hematopoietic cells that are produced on a daily basis is dependent on a limited number of lineage instructive extracellular signals and/or growth factors. For example, T and B lymphoid lineages, and some ILC subsets, are almost strictly dependent on interleukin (IL)-7 for proper development in the adult bone marrow (5-7), while NK cell development requires IL-15 (8). Likewise, the cytokines Granulocyte-Colony Stimulating Factor (GCSF), Granulocyte-Monocyte Colony Stimulating Factor (GMCSF), and Macrophage Colony Stimulating Factor (MCSF) are critical for granulocyte, monocyte, and macrophage production, whereas thrombopoietin and erythropoietin stimulate megakaryocyte and erythroid cell development. Although lymphoid and myeloid cytokines are not exclusively produced by cells residing in the bone marrow, they act on hematopoietic progenitor cells that are predominantly localized in this organ. Furthermore, some of these cytokines are produced in limiting amounts, such as IL-7 and IL-15, and consumed by several types of cells (e.g. B cells, T cells, ILC subsets), making the bioavailability of lymphopoietic cytokines restricted to the niche that produces them. Lymphocyte progenitors require specific migratory cues, specifically CXCR4 and its ligand CXCL12, in order to localize near or in contact with cells that produce lymphopoietic cytokines (9). Although, a clear understanding of how myeloid cells are organized during development in bone marrow is still lacking, several studies suggest that specialized niches also exist for granulocyte monocyte precursors (GMPs), for osteoclast precursors, for megakaryocytes, and for erythrocyte production (10-14). In the case of osteoclast development, the finals steps are coordinated by oxysterol ligands of EBI2 (Gpr183) most likely produced by osteoblasts, which attract osteoclast progenitor cells to endosteal niches (12). These observations demonstrate that hematopoietic cell development is coordinated by chemotactic cues that guide distinct progenitor cells to specialized niches for differentiation.
One of the most studied bone marrow niches is the HSC niche. Initial studies pointed at osteoblasts forming the HSC niche (15, 16). However, recent studies characterized the HSC niche by focusing on the cells that produce essential cytokines (e.g. SCF) and chemokines (e.g. CXCL12) for HSC homeostasis, and demonstrated that the HSC niche is predominantly formed by a major population of perivascular mesenchymal stem and progenitor cells (MSPCs), by a small subset of sinusoidal endothelial cells, and to a smaller extent by a rare population of periarteriolar pericytes (2, 17-23). Furthermore, SCF or CXCL12-producing niche cells are broadly distributed in the bone marrow cavity with no obvious enrichment at the endosteum or osteoblast-rich trabecular areas (19, 20, 24). As some of the signals required for HSC maintenance act in short range (e.g. SCF), HSCs localize predominantly near or in direct contact with MSPCs and endothelial cells expressing SCF and CXCL12. HSC localization is critically controlled by CXCR4 in response to CXCL12, and SCF and CXCL12 producing cells overlap by ~ 99% (20). Deficiency in SCF or in CXCL12 causes major defects in HSC maintenance and hematopoietic cell development (19, 20).
Over the past several decades many studies attempted at defining the bone marrow niche for lymphoid progenitors, and particularly for B cell development. Using a variety of genetic approaches that target non-hematopoietic cells in the bone marrow, several studies converged on the conclusion that lymphocyte development requires important signals delivered by osteoblasts, including IL-7 (25-27). These findings were consistent with prior studies examining the distribution of early B cell precursors in the bone marrow of mice and rats by in situ microscopy, in which loosely defined B-lineage progenitors were mapped to sub-endosteal areas (28, 29). However, these findings were not easily compatible with other, and more recent, studies examining the distribution of B cell precursors in situ (30, 31), and the dynamic behavior of developing B cell subsets in vivo (32-34), in which the large majority of B-lineage cells localized in the central bone marrow cavity, often near or in contact with the sinusoidal endothelium. Furthermore, recent studies using several IL-7 reporter transgenic mice identified IL-7 producing cells as being predominantly Leptin receptor-expressing, CXCL12Hi, non-hematopoietic mesenhcymal cells with multilineage differentiation potential, including into osteoblasts and osteocytes (35). Combined, these studies challenged the idea that osteoblasts and endosteal niches control B-lymphopoiesis and demanded a new model for explaining where and how B lymphocytes are formed.
In this review, we propose that the major niche coordinating lymphoid progenitor development, and in particular B cell development, is predominantly composed by perivascular MPCs, and to a smaller extent by sinusoidal endothelial cells. Our findings discussed here in detail are compatible with a model in which HSC maintenance and hematopoietic progenitor differentiation into lymphoid lineages are predominantly controlled by soluble and membrane-bound factors produced by the same niche. Furthermore, our recent findings showed that IL-7 production is sensitive to the quality of B cell progenitors being produced. Pre-leukemic B cell progenitors and preB acute lymphoblastic leukemic cells reduce the amount of IL-7 produced by MPCs. This type of cross-talk between niche cells and hematopoietic progenitors reveals a highly organized and spatially controlled B cell development that is reminiscent of a cell circuit model of tissue homeostasis (36). This novel cell circuit-based model of B-lymphopoiesis predicts the existence of feed-forward and feedback loops to ensure the quality and possibly quantity of B cell progenitors being produced, and may have implications for understanding the impact of pre-leukemic and leukemic B-lineage cells in the local microenvironment.
2–. Niches for B lymphopoiesis in bone marrow.
In many biological systems, including the immune system, cellular localization is often a tightly regulated process characterized by feedforward and feedback mechanisms that are intimately associated with cellular decisions such as quiescence, activation and differentiation. The concept of microenvironmental niches, pioneered by Schofield in 1978 to explain the phenomenon of higher hematopoietic progenitor capacity for bone marrow resident cells when compared to splenic cells (37), illustrates this general principle quite well, and its formulation propelled numerous studies on the bone marrow niches for hematopoietic stem and progenitor cell populations.
Pioneer studies of B-lineage cell localization in the mouse bone marrow suggested that early B cell progenitors marked by B220 and lacking cytoplasmic Igμ expression were somewhat enriched in sub-endosteal niches, whereas preB cells defined by cytoplasmic but undetectable surface Igμ expression were more evenly distributed throughout sub-endosteal and central bone marrow cavities (38). Early lymphoid progenitors expressing Terminal deoxynucleotidyl transferase (TdT) were also slightly enriched in sub-endosteal areas, but TdT+ cells were abundantly detected in central marrow as well (38). Similar findings obtained in rat femur tissues gave further support to a sub-endosteal localization of TdT+ B cell progenitors (28). TdT expression is detectable at late CLP stages and increases to its highest at the proB cell stage, while preB cells express TdT at lower amounts than proB cells (39, 40). At later stages of B cell development, immature B lymphocytes localized mostly in central marrow with small caliber sinusoids often packed with immature B cells, suggesting that B cell development is finalized with lymphocyte loading of sinusoids (41, 42). Regardless of where B-lineage cell localized, it was frequently noted that reticular stromal cells were in direct association with small clusters of B cell progenitors (38, 41, 42), lending support to other studies showing that factors produced by stromal cells could support lymphoid and myeloid cell development in vitro (43).
Cell ablation strategies are commonly used for uncovering relationships between niches and hematopoietic cell fates. Using a 2.3Kb promoter element of the collagen type Ia gene Col1a (Col2.3) to promote the expression of an inducible suicide gene product (Herpes simplex thymidine kinase) in mature osteoblasts, two independent studies uncovered severe hematopoietic defects in transgenic mice treated with the thymidine kinase substrate analog, ganciclovir. B-lineage and erythrocyte lineage cells were among the most reduced (25, 26). Consistent with these findings, genetic deficiency of Gnas (encodes the small G alpha stimulatory (Gs) protein) in osteoblasts, using the Osterix (Sp7) promoter to drive constitutive Cre recombinase expression in osteolineage cells led to significant reductions in osteoblasts and in B-lineage cell subsets dependent on IL-7 (27). The fact that B cell deficiency was partially ameliorated by exogenous treatment with IL-7 in vivo suggested that B cell development depends on IL-7 produced by osteoblasts (27). Lending further support to this model, mouse osteoblasts engineered to overexpress human IL-7 could restore B cell development in an Il7−/− mouse (44), and Il7 deletion in osteolineage cells using tamoxifen-inducible Sp7-driven cre recombinase transgenic mice also led to significant reductions in B cell developmental subsets in 6-week old mice (45). Combined, these studies converged on a model where B cell development depends of signals provided by osteoblasts, particularly IL-7.
Using monoclonal antibodies to detect IL-7-producing cells in bone marrow Tokoyoda et al. proposed that IL-7+ cells are stromal cells that also produce Vascular Cell Adhesion Molecule-1 (VCAM-1), but these cells seemed to be distributed throughout the bone marrow parenchyma without evidence of being associated or proximal to endosteal niches (30, 46). However, comparative analyses of IL-7-deficient and sufficient bone marrow sections failed to reliably detect IL-7 in situ by immunohistology, a technical hurdle overcome by the generation of Il7 reporter mice (47). In these studies, intravital microscopy of calvaria bone marrow of Il7-eCFP Bacterial Artificial Chromosome transgenic mice identified IL-7-producing cells as static reticular stromal cells that seemed predominantly distributed around blood vessels. Likewise, femur sections of Il7GFP/+ knock-in mice identified IL-7+ reticular cells broadly distributed in the parenchyma, with some IL-7+ cells also localized in endosteal and sub-endosteal areas (48). However, none of these examined the lineage identity and/or phenotype of IL-7+ cells in bone marrow.
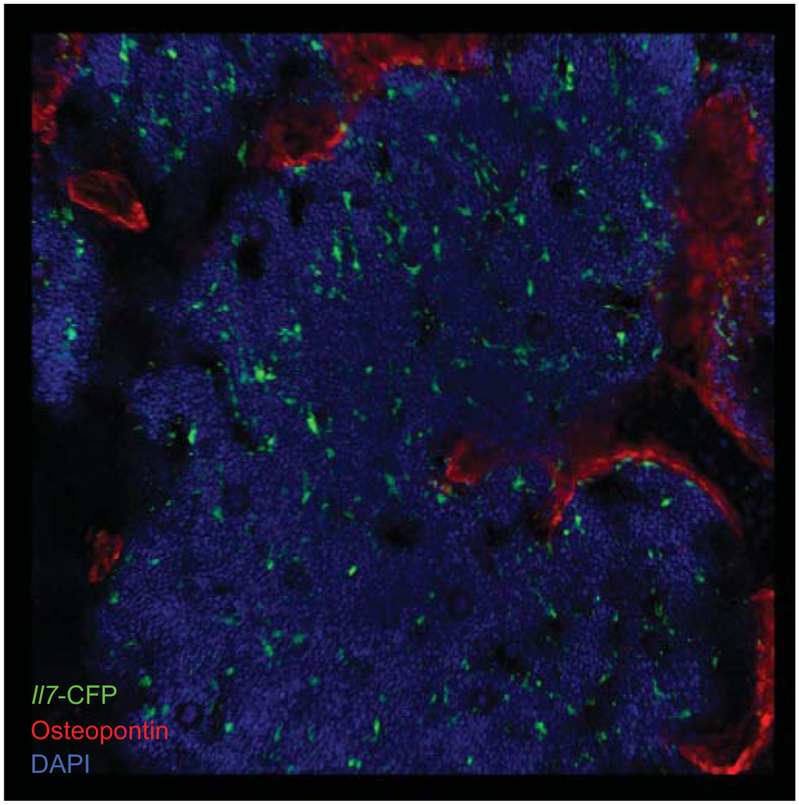
In recent studies, our laboratory characterized IL-7+ cells in bone marrow as an heterogeneous population composed by a majority of mesenchymal-lineage cells with multipotent differentiation capacity, and a minor population of sinusoidal endothelial cells. Using an Il7-cre fate mapping approach, we found that IL-7+ cells can differentiate into osteoblasts, osteocytes and adipocytes in vivo (35). However, careful examination of longitudinal femur sections and whole mounts from Il7GFP/+ reporter mice failed to detect GFP expression in osteocalcinHi osteoblasts (Figure 1). Furthermore, the majority of Il7-GFP+ reticular cells were mostly distributed throughout the bone marrow parenchyma, with some elongated reticular cells localizing near the endosteum, but without detectable osteopontin (35). Indeed, conditional deletion of Il7 in mature osteoblasts using constitutive Col2.3-cre transgenic mice showed no detectable differences in the number of CLPs and B cell developmental subsets in bone marrow (35). Combined, these studies ruled out mature osteoblasts as major sources of IL-7 in bone marrow. So, how can the discrepancies between our study and the previous studies be explained?
Figure 1: Osteoblasts do not express IL-7 under homeostasis.
Distribution of IL-7+ and osteopontin+ cells in bone marrow. Femur section of Il7-CFP BAC transgenic mouse stained to detect IL-7+ cells (green) and osteopontin-expressing cells (red).
Insight into this question came from the careful elucidation of the mesenchymal developmental stages targeted by the various Cre recombinase transgenic strains used. Several transgenic mouse lines driving cre recombinase expression from gene promoters previously thought to be osteolineage-specific (e.g. Bglap-cre, Dmp1-cre) were in fact already active at the mesenchymal-lineage multipotent progenitor stage (49). Similarly, genetic approaches that take advantage of Sp7- driven cre recombinase expression (constitutive or tamoxifen inducible) must also be interpreted with caution because this strategy also targets mesenchymal-lineage cells with multipotent progenitor potential (50-52), a stage in which these cells still express HSC maintenance genes (e.g. Kitl, Cxcl12) and lymphoid-lineage promoting genes (Il7). Therefore, in vivo studies using Cre-mediated transgenic approaches that target mesenchymal progenitor cells, or genetic deficiencies that alter MPC differentiation, often lead to the inaccurate conclusion that osteoblasts and/or osteolineage niches play major roles in HSC homeostasis and/or in lymphopoiesis (25-27, 45, 53-57).
An interesting feature of IL-7-producing mesenchymal progenitor cells (MPCs) is that these cells express the highest amounts of CXCL12 in the bone marrow (35). CXCR4, the chemokine receptor for migration towards CXCL12 gradients, is abundantly expressed in the vast majority of hematopoietic cells in the bone marrow, including HSCs. HSC homing from embryonic liver, spleen, and blood circulation to the developing bone marrow compartment requires CXCR4, and in the adult HSCs also require CXCR4 for homing and retention within bone marrow (58). The tight interplay between CXCR4 and hematopoietic cell migration is remarkably conserved throughout evolution, with evidence for its appearance before the branching of jawless (Agnathans) and jawed (Gnathostomes) vertebrates. CXCR4-mediated movement of hematopoietic cells and primordial germ cells has been reported in Zebrafish (59, 60), and in lamprey (a jawless fish) hematopoietic cells similar of mammalian B and T lymphocytes express a CXCR4 ortholog (61).
Of all the hematopoietic cell lineages, B cells are perhaps the most dependent on CXCR4 signaling for proper development in the bone marrow. Mice genetically deficient in CXCR4 are lethal at an embryonic/perinatal stage, but fetal-liver HSCs deficient in CXCR4 expression can still be recovered and transplanted into adult mice. Under these conditions, B-lineage cells were remarkably reduced, with defects also detected in myeloid and erythroid-lineage development (62-66). Importantly, inducible genetic deficiency in CXCR4 in HSCs, and thus in early progenitors and essentially all differentiated hematopoietic cells in adult mice, also caused marked reductions in B-lineage cell development, while myeloid and erythroid lineages were reduced to a lower extent (24, 67). These studies demonstrated that CXCR4 signaling is not only required for HSC maintenance but also for multilineage differentiation in adult bone marrow. However, when CXCR4 was conditionally deleted in early B cell subsets starting at the proB cell stage using a Cd19Cre/+ allele, there was only a small reduction in the number of immature B cell subsets in the bone marrow, with evidence of premature release of B cell precursors into the spleen (68), consistent with a known role for CXCR4-mediated retention of hematopoietic cells in the bone marrow (65). Using a Cd79aCre/+ allele to delete Cxcr4 in proB cells more efficiently, our laboratory also detected small but significant reductions in proB and preB cell subsets in the bone marrow (33). The fact that CXCR4 deficiency at the HSC stage led to a large defect in B-lineage development, whereas deletion at the proB cell stage led to a smaller defect in B cell development suggested that CXCR4 acts either at the HSC stage or at an early downstream hematopoietic progenitor stage prior to lymphoid-lineage commitment into B-lineage cells. To distinguish between these possibilities we analyzed mice in which Cxcr4 was deleted at the fms like tyrosine kinase 3 (FLT3)-expressing hematopoietic multipotent progenitor stage (MPP) using the Flk2-cre transgene (Flk2 encodes for FLT3), while leaving CXCR4 expression intact in HSCs. These studies revealed significant reductions in the MPP, CLP subsets and in particular the Ly6D+ B-lineage committed CLP stage (69), and downstream B-lineage cell subsets in the bone marrow, a defect that was further exacerbated when CXCR4-deficient MPPs competed with CXCR4-expressing hematopoietic precursors in the setting of mixed bone marrow chimeras (35).
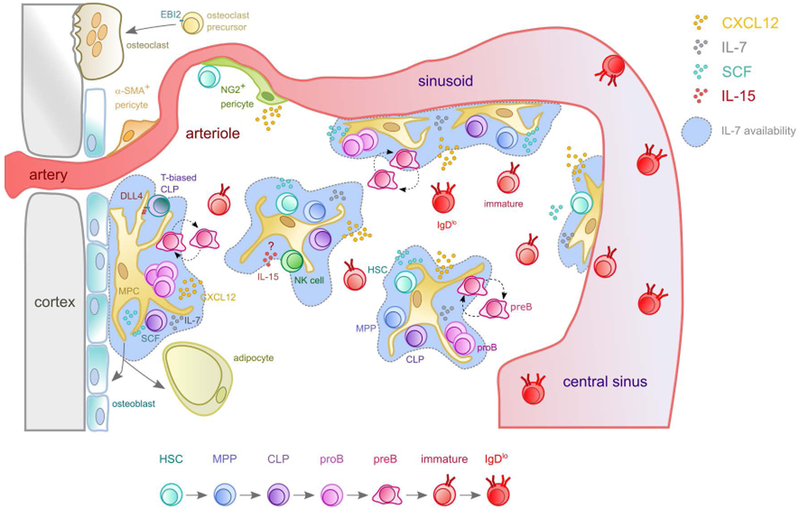
As mentioned previously, IL-7+ MPCs express the highest amount of CXCL12 in bone marrow. The fact that Ly6D+ CLPs were among the most reduced when CXCR4 was deleted at the MPP stage suggested that CXCR4 might be required for MPPs, CLPs, and downstream B-lineage cell subsets to localize in the vicinity of IL-7-expressing cells. Indeed, not only the vast majority of MPPs, Ly6D+ CLPs, and proB cells are in direct contact with IL-7+ cells, or within 10 μm away from the nearest IL-7+ cell in bone marrow, but also HSCs were frequently found in contact or the vicinity of IL-7+ cells. Defective CXCR4 signaling in MPPs resulted in significantly reduced STAT5 phosphorylation in Ly6D+ CLPs (35), a direct target of IL-7 receptor signaling (70). Reciprocally, Cxcl12 deletion from IL-7+ cells also led to measurable reductions in HSCs, MPPs, CLP subsets, and B-lineage cell subsets, while Kitl (encodes SCF) deletion from IL-7+ cells reduced HSC and MPP numbers by 2-3 fold (35). Combined, these studies demonstrated that IL-7+ MPCs play a key role in HSC and MPP maintenance through the production of SCF, but are also instrumental for lymphoid lineage development at least through the production of IL-7. These findings led us to propose a new model for the organization of HSCs, MPPs and lymphoid lineage development in bone marrow (Figure 2).
Figure 2: Overlapping niches for HSCs and lymphopoiesis in the bone marrow.
MPCs are the main source of IL-7 in bone marrow. Hematopoietic stem cells (HSC, cyan), multipotent progenitors (MPP, blue) and common lymphoid progenitors (CLP, violet) are distributed in proximity of CXCL12Hi IL-7+ SCF+ MPC. ProB (magenta) and motile preB cells (purple, amoeboid shaped cell with ruffled edges) predominantly localize near or in contact with IL-7+ MPCs, with proB cells spending longer periods of time in contact with IL-7+ cells than preB cells. MPCs can differentiate into osteolineage cells and to adipocytes. T-biased CLPs (blue-green) depend on Notch-ligand DLL4 possibly provided by MPCs, and natural killer (NK, green) cells on IL-15. Osteoclast precursor migration towards endosteal areas is mediated by the receptor EBI2 and oxysterol ligands. Larger arteries are surrounded by a-SMA+ pericytes, while NG2+ pericytes localize next to arterioles. Dashed arrows indicate movement, full arrows indicate differentiation. Circles represent chemokines and growth factors according to the color code shown in the upper right corner.
3. Cell circuits between proB, preB, and IL-7-producing cells.
After CLP commitment to the B lineage cell fate, B cell development proceeds through a series of stages characterized by surface receptor expression and the status of RAG-mediated D to J and V to DJ gene recombination events on the Igh chain locus. D-J rearrangements are initiated at the Ly6D+ CLP stage and continued over two to three B cell stages defined as Hardy’s fractions A, B and C. Productive VDJ recombination and expression of a functional Ig heavy chain that can pair with surrogate light chains VPreB1,2 and λ5 forms the preB cell receptor (preBCR). PreBCR signaling drives a rapid burst of proliferation (Hardy’s fraction C’) leading to a 6-8 fold expansion and progression into the small resting preB cell stage (Hardy’s fraction D). This stage is followed by recombination of V and J genes at the Ig light chain kappa and lamda loci, which if successful enables the assembly of a functional BCR and transition into the immature B cell stage (Hardy’s fraction E) (71). The following section is focused on the bone marrow niches required for early B cell progenitor development, and on the dynamic behavior of B cell progenitors in bone marrow before and after assembly of a functional preBCR. For simplicity, Hardy’s fractions A-C will be referred to as proB cells given the fact that at these stages B cell progenitors are highly dependent on IL-7 for proliferation and survival (72-75).
IL-7R signaling through the JAK1/3 and STAT5a/b pathway is critical for proB cell survival and proliferation. However, once the rearrangement of genes encoding the immunoglobulin (Ig) heavy chain succeeds and preB cells are formed, IL-7R signaling needs to be attenuated. PreB cells continue to depend on IL-7 for proliferation and expression of anti-apoptotic genes (e.g. Bcl2, Bcl2l1 and Mcl1). However, excessive IL-7R signaling arrests preB cell development into the immature B cell stage due to a near complete block in the rearrangements of Ig light chain genes. This is because IL-7R signaling represses Igκ germline transcription due to the binding of activated STAT5 to Eκi, which is followed by recruitment of Polycomb repressive complex 2 into the Igκ locus. These sequence of events result in histone H3 lysine 27 trimethylation thus rendering Jκ and Cκ regions inaccessible to the RAG protein complex (76). Furthermore, IL-7R driven cyclin D3 expression in preB cells prevents Vκ transcription, which also reduced the accessibility of RAG protein complexes to Vκ genes (77). Besides repressing the overall accessibility to the Igκ locus, IL-7R signaling in preB cells has also been proposed to repress Rag1 and Rag2 transcription (78) via the phosphatidylinositol-3-OH kinase (PI3K)-mediated Foxo1 phosphorylation, followed by its nuclear export, ubiquitination and degradation by the proteasome (79). The positive effects of IL-7R signaling in preB cell proliferation and survival, and repressive effects in the rearrangement of Ig light chain genes occur despite the fact that IL-7R expression is considerably reduced from the proB to the preB cell stage (80). So, how can preB cells achieve a balanced threshold of IL-7R signaling that allows developmental progression to occur?
Insight into this problem came from the finding that Interferon Responsive Factor (IRF)-4 induced by preBCR signaling led to a ~ 2-fold increase in CXCR4 expression and chemotaxis towards CXCL12 (78). This change in chemotactic behavior was proposed to allow preB cells to move away from IL-7-expressing stromal cells (78, 79, 81) because of previous studies suggesting, inaccurately, that the stromal cells producing IL-7 were distinct and spatially distributed away from CXCL12-producing cells (30). Instead, as IL-7+ cells express the highest amounts of CXCL12 in the bone marrow (35), the increased CXCR4 expression and function in preB cells would predict these cells localize in close proximity to IL-7+ cells. Indeed, using two independent strategies for in situ analysis of progenitor B cell localization in relationship to Il7-GFP+ Cxcl12-dsRed+ bone marrow cells, our studies revealed that 90% of proB cells and 85% of preB cells are in direct contact with IL-7+ CXCL12+ cells (82). Furthermore, the difference in localization between proB and preB cells, albeit small, was in fact statistically significant and raised another developmental conundrum. How are proB cells more frequently found in direct association with IL-7+ cells than preB cells that express higher amounts of CXCR4 and respond more vigorously to CXCL12? And could such difference in proximity to IL-7+ cells be sufficient to attenuate IL-7R signaling in preB cells? Answers to both questions came from in vivo visualization of proB cells and preB cells in the calvaria bone marrow of Cxcl12dsRed/+ by intravital two-photon microscopy. While proB cells were found predominantly non-motile and spent large periods of time in close association with CXCL12+ cells, preB cells were mostly motile in the bone marrow parenchyma and spent significantly reduced periods of time in direct contact with CXCL12+ cells (82). Mechanistically, proB cells express higher amounts of Focal Adhesion Kinase (FAK) and of VLA4 integrins, and are consequently more adherent to VCAM-1 (an adhesion molecule abundantly expressed in CXCL12-producing cells (30)) than preB cells, a combination of changes in adhesion and chemokine receptor expression that presumably renders the preB cell more freedom for traveling longer distances within the bone marrow parenchyma. Importantly, preBCR signaling controls the switch from high FAK/VLA4- to a lower FAK/VLA4 expression and adhesion to VCAM-1 while promoting higher CXCR4 expression and migration. In a simple analogy, preBCR signaling accelerates preB cell motility by releasing the break (FAK/VLA4) while stepping on the gas pedal (CXCR4). These findings are consistent with prior studies showing a relationship between BCR signaling and CXCR4 responsiveness (83), and with other studies implicating FAK, VLA4 and CXCR4 signaling in B cell progenitor localization and movement within the bone marrow (31-33, 84).
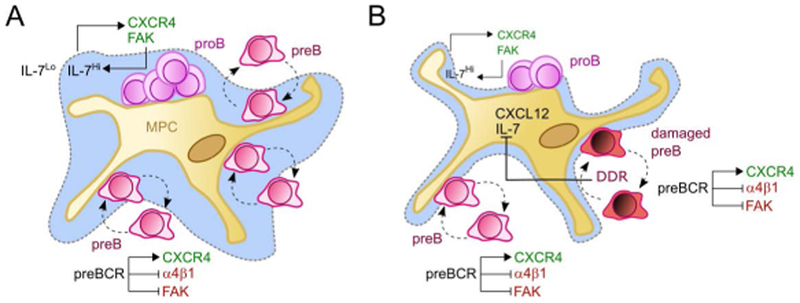
It has long been appreciated that proB and preB cells display remarkable differences in responsiveness to CXCL12 and in integrin-mediated adhesion to the extracellular matrix (85). ProB and preB cells also differ in their ability to respond to limiting amounts of IL-7, with proB cells being considerably more dependent on high IL-7 concentrations for efficient proliferation to occur (80). Importantly, IL-7R signaling in proB cells controls the expression of CXCR4 and FAK such that it regulates proB cell static behavior, high adhesion to IL-7-producing cells, and consequently control their bone marrow retention (82). This type of feed-forward control of proB cell proliferation whereby IL7R controls the expression of CXCR4, which in turn controls access to IL-7, is reminiscent of an unstable type of a two-cell circuit system (36), as it would predict proB cell proliferate in an uncontrolled manner. However, once proB cells acquire a productive rearrangement of IgH genes, preBCR signaling alters the chemotactic behavior of the newly formed preB cell such that it presumably changes IL7R signaling from a constitutively high into an intermittent low state. In agreement with this model, preB cell development into the immature and mature B cell stages was significantly blocked by overexpression of a constitutively active desensitization mutant CXCR4 (R334X), presumably due to reduced RAG protein expression (79, 82). Thus, we propose that preBCR signaling breaks the proB and IL-7+ cell circuit to form a new, and perhaps more stable, circuit formed by preB cells and IL-7+ cells that reduces the proliferative effects of IL-7R signaling (Figure 3). It is possible that other unidentified feedback mechanisms between proB cells and IL-7+ MPCs also contribute to prevent an uncontrolled expansion of B cell progenitors in the bone marrow.
Figure 3: Cell circuit models for early B cell development under homeostasis (A) and in preleukemic states (B).
(A) CXCL12 attracts proB cells (magenta) to the MPC niche, while IL-7 promotes CXCR4 and FAK expression in proB cells. This IL-7R/CXCR4 feed-forward loop keeps proB cells in a low motility state in IL-7Hi environments. preBCR signaling induces even higher CXCR4 expression while reducing FAK/ α4β1-mediated adhesion. Then, motile preB cells (purple) transit rapidly between IL-7lo and IL-7Hi microenvironments (indicated by dashed grey lines). (B) In preleukemic states, excessive DNA damage response (DDR) in preB cells with unrepaired DSBs (dark red) results in IL-7 and CXCL12 downregulation. Dashed arrows indicate movement or adhesion. Full pointed arrows indicate induction, blunt-end arrows inhibition.
Signaling from the preBCR is a critical checkpoint in B cell development that is commonly defined as positive selection. Signaling is initiated when a newly generated Igμ chain assembles a protein complex with surrogate light chains (VpreB1,2 and λ5) and with the transmembrane signaling components Igα and Igβ. This is followed by preBCR internalization and degradation (86, 87), which together with downregulation of surrogate light chain transcription contributes to termination of preBCR signaling (88). There is still uncertainty about whether pre‑BCR signaling is induced by self-ligands or whether signal initiation is due to autonomous pre‑BCR complex assembly (89, 90). Single preB cells cultured in the absence of stromal cells proliferate upon expression of a functional preBCR (89). However, a soluble form of the preBCR could bind to heparan sulfate expressed on cultured stromal cell lines via the tail of the surrogate light chain λ5 (90, 91). Schiff and colleagues identified Galectin 1 (an S-type lectin which binds β-galactoside glycoconjugates) as a candidate ligand for the preBCR (92). Structural studies identified hydrophobic residues in the unique region or tail of λ5 required for the interaction with Galectin-1 and for pre-BCR clustering (93). Galectin-1-expressing stromal cells were characterized as a mixture of osteoblasts and reticular stromal cells. To examine the localization of proB and preB cells relative to Galectin-1+ cells, Mourcin et al. used a short-term adoptive transfer approach of sorted and labeled proB and preB cells into actin-GFP transgenic mice treated with hydroxyurea to eliminate hematopoietic cycling cells. Large cycling preB cells were often found in contact with Galectin-1+ stromal cells, whereas proB cells were mostly found in association with Il7-cre fate mapped cells (94). The finding that ~85% of proB cells were in contact with Il7-cre fate mapped cells (94) agrees with our findings that proB cells are mostly found in contact with IL-7-expressing cells (82). In contrast, only ~ 12% of preB cells could be found in contact with Il7-cre fate mapped cells (94), whereas our studies of “static” confocal microscopy and of intravital 2-photon microscopy showed that the large majority of preB cells are in direct contact with IL-7-expressing cells (82). The latter findings are consistent with the fact that IL-7-expressing cells express the highest amounts of CXCL12 in bone marrow and that preB cells express the highest CXCR4 amounts during B cell development (82). It is possible that differences in methodology between these studies accounted for discrepancies in preB cell localization. For example, it is currently unknown if hydroxyurea treatment affects CXCL12 expression in bone marrow and/or alters CXCR4 expression and/or function in preB cells. Regardless of these discrepancies, the finding that Il7-cre fate mapping failed to mark Galectin-1+ cells, and the fact that Galectin-1+ cells express little or no Il7 and Cxcl12, suggests that Galectin expression marks a unique subset of bone marrow stromal cells (94). Future studies are needed to characterize the relationship between IL-7+, Galectin-1+, CXCL12+ and Nestin-GFP+ stromal cells in the bone marrow.
Given the motile behavior of preB cells in the bone marrow it is likely that preB cells establish multiple contacts with Galectin-1. This raises the possibility that a specialized niche allows sampling of preBCR ligands (95). This model is not without controversy though given the finding that Galectin-1 deficient mice showed no detectable defects in developing B cell subsets under homeostatic conditions (96). Furthermore, high-resolution structural studies of the preBCR complexed with λ5 or VpreB showed that the unique region of λ5 (the domain that interacts with Galectin 1) bends over the top of the complementarity-determining region 3 and obscures the antigen-binding region of the Ig heavy chain (97). Further biochemical studies confirmed that a pre-BCR formed by pairing of the surrogate light chain with an Ig heavy chain of defined antigen specificity could not bind cognate antigen at physiological concentrations (97). Finally, a model in which self-ligands modulate preBCR signaling and positively influence preB cell development is not easily compatible with the fact that BCR signaling induced by self-antigens at the immature B cell stage (Hardy’s fraction E) completely prevents further development (98, 99), unless the autoreactive Ig heavy and/or light chains are replaced.
Our recent studies support a model in which early stages of B cell development are controlled by cell circuits established between proB, preB and IL-7+ cells (82). An advantage of a model based on cell circuit control of B cell development is that it incorporates feed-forward loops and predicts the existence of feed-back mechanisms to ensure the quality and quantity of B cells being produced. B cells progenitors coordinate rapid cell cycling with RAG-mediated genetic rearrangements. When the RAG proteins cleave DNA it generates four broken ends that are repaired by the non-homologous end joining (NHEJ) pathway thus forming coding and signal joints. However, on rare occasions errors occur during the DNA repair steps that result in chromosomal lesions that have the potential to cause leukemias and lymphomas. This time sensitive sequence of steps is tightly regulated by the cell cycle status such that RAG-mediated double strand DNA (DSBs) are only formed during the G1 phase of the cell cycle and must be repaired before progression into the S phase (100). A major regulatory step in ensuring DSB formation in G1 is a mechanism involving RAG2 phosphorylation, nuclear export and degradation by the proteasome (101). Once formed, DSBs are repaired by NHEJ pathway via a sequence of steps involving the NHEJ core proteins XRCC4, DNA Ligase IV, Ku70, and Ku80, which catalyze the formation of signal joints and coding joints (100, 102). However, the coding ends are hairpin sealed, and must be opened in order to allow for coding joints to be ligated. Artemis catalyzes the opening of hairpin-sealed coding ends during V(D)J recombination, and in Artemis-deficient mice B and T cell development is severely defective due to a block in coding joint formation and an accumulation of unrepaired DSBs (103). In turn, unrepaired DSBs activate the ataxia-telangiectasia mutated (ATM) DNA damage response kinase, which phosphorylates numerous targets that lead to the activation of specific transcriptional pathways, such as p53, SpiC and NF-κB (104, 105). Among the multiple genes that are differentially expressed by the DNA damage response program are several involved in controlling cell localization in lymphoid tissues, such as SWAP-70, L-Selectin and CD69 (106). These observations led us to consider the possibility that preB cells deficient in Artemis (and thus activated with high levels of DNA damage response) might localize in different bone marrow niches than normal preB cells. While in situ analyses of Artemis-deficient and sufficient preB cells failed to detect significant differences in their localization in relationship to IL-7+ CXCL12+ cells in bone marrow, we noted that IL-7 expression was considerably reduced in Artemis-deficient mice (82). Therefore, these findings suggest that signals provided by preB cells with unrepaired DSBs are sensed by IL-7+ MPCs, which in turn reduce IL-7 production. Although the DNA damage response pathway efficiently blocks preB cell entry into the S phase (106), it is at present unclear if IL-7 downregulation also contributes to prevent preB cell division. It is possible that normal preB cells undergoing transient RAG-mediated DSB responses are also capable of relaying signals that modulate IL-7 production, and that Artemis deficiency simply allows DSBs to persist long enough for changes in IL-7 expression to be detected. If correct, this would predict that proB and preB cells could conceivably control the size of the progenitor B cell compartment through negative feed-back regulation of IL-7 production in vivo. Although no direct evidence for this model has been reported thus far, the fact that increased IL-7 production by transgenic approaches results in a markedly increased size of the progenitor B cell pool suggests that access to IL-7 is a major regulatory checkpoint controlling the size of the progenitor B cell compartment (44, 107-109).
Artemis-deficient B cell progenitors do not readily undergo malignant transformation. However, these cells are in a pre-leukemic state given the fact that acquisition of a second hit mutation in tumor suppressor genes, such as p53, is sufficient to develop lethal leukemia (110). The fact that Artemis-deficient cells could modulate Il7-expression in the bone marrow led us to ask if primary preB cell leukemias (e.g. BCR-ABL expressing preB acute lymphoblastic leukemias) could also cross-talk with IL-7+ cells. Intravital microscopy of BCR-ABL+ preB cells revealed that these cells are migratory and interact closely with CXCL12+ cells in the bone marrow, similarly to normal preB cells in vivo (82). Furthermore, Il7 expression was also significantly downregulated in mice transplanted with BCR-ABL+ preB cells, such that it reduced by ~ 3-fold the production of non-leukemic host B cell progenitors, whereas myeloid cells were only reduced by ~ 20% (82). The inhibitory effect of leukemic cells in host B cell development is not exclusive to B cell leukemias as other types of cancers include solid tumors had similar inhibitory effects in B and NK cell development (111, 112). It is tempting to speculate that leukemic cells and other tumors might explore homeostatic mechanisms of growth factor production to turn-off the development of highly proliferative hematopoietic progenitor cells as a strategy for reducing competition for, for example, anabolic nutrients. Consistent with this possibility, mice carrying a genetic deficiency in Dicer1 (DICER-1 is an RNase III endonuclease required for the generation of microRNAs and for RNA processing (113)) in bone marrow MPCs develop a secondary acute myeloid leukemia (114). Future studies are needed to delineate further the mechanisms involved in IL-7 downregulation in MPCs by pre-leukemic, leukemic, and possibly other types of tumor cells, and the extent to which these mechanisms safe-guard against tumor development.
Besides IL-7, other extracellular factors also contribute to the development of lymphocyte subsets, and in particular to B cell development, albeit with a significantly lower impact. ProB cells are commonly distinguished from preB cells due to their expression of cKit and FLT3. Deficiency in cKit signaling cause an age-dependent reduction in proB cell development that may be due to defects in MPP to CLP to proB cell transition (115, 116). As SCF-producing cells overlap by > 99% with CXCL12-expressing cells in the bone marrow (20), it is likely that B-lineage progenitors encounter SCF and IL-7 simultaneously. Signaling from the receptor tyrosine kinase FLT3 is also required for MPP and CLP development and/or maintenance (117), and combined FLT3 and IL7Ra deficiency resulted in a complete block in B cell development due to a near complete absence of CLPs (118). The IL7Ra chain can heterodimerize with the common γ chain to form the signaling receptor for IL-7, but it can also heterodimerize with the Thymic Stromal-derived Lymphopoietin (TSLP) receptor chain to form the TSLP receptor (119). Interestingly, TSLP seems to contribute to fetal B cell development, and adult preB cells can respond to TSLP under in vitro conditions (120). Whether TSLP makes measurable contributions to B cell development during fetal and adult stages still remains controversial (121). The cellular sources for FLT3L and for TSLP in bone marrow also remain to be identified.
A recent study uncovered a small but significant role for insulin growth factor 1 (IGF-1) in early stages of B cell development, particularly at the preB cell stage (55). How deficiency in IGF-1 reduces the preB cell compartment size remains unknown. Also unclear are the exact cellular sources of IGF-1 in bone marrow. The fact that Sp7-cre-mediated Igf1 deletion reduced the number of preB cells in bone marrow (55) suggests that the IL-7+ CXCL12+ MPC is an important cellular source of IGF-1. Some evidence suggests that mature osteoblasts can contribute to B and T lymphopoiesis. Mice carrying a Col2.3-cre-mediated conditional deletion of Cxcl12 in osteoblasts showed a ~ 2 fold reduction in lymphoid-primed MPPs (LMPPs) and in CLPs that did not cause any measurable reductions in early B cell progenitors (20). These findings contrast with data obtained in mice with Bglap-mediated Cxcl12 deletion in osteoblasts in which no measurable differences were found in CLPs, but is consistent with no measurable differences in subsequent B-lineage progenitor subsets (21). Paradoxically, only ~ half of osteoblasts express Cxcl12 transcripts, but at levels that are 1,000 fold lower than in CXCL12+ MPCs. This finding raises an interesting mechanistic problem: how can hematopoietic progenitor cells depend on minute amounts of CXCL12 produced by a subset of osteoblasts while seemingly ignoring 1,000 fold higher amounts of CXCL12 produced by CXCL12+ MPCs? In any case, in situ visualization of lineage-negative IL-7Ra-expressing cells (a heterogeneous mixture of lymphoid progenitor cells that include CLPs and LMPPs) indicated that Lin−IL7Ra+ cells preferentially localize near the endosteum (20). Whether CXCL12 produced by osteoblasts is required for lymphoid progenitor localization near the endosteum remains unknown.
4. Regulation of IL-7 production in bone marrow.
Multiple studies suggest that IL-7 production in bone marrow is regulated by a variety of homeostatic and inflammatory soluble mediators. As previously discussed, Lepr+ MPCs produce about 90% of the total IL-7 in bone marrow. Like Lepr, MPCs express several cellular receptors that can sense hormones produced in the local microenvironment (e.g. norepinephrin secreted by sympathetic nerve terminals), or distally produced by endocrine organs such as thyroid and adipose tissue (e.g. parathyroid hormone (PTH), Leptin). Hormones such as norepinephrin and PTH signal from Gs protein coupled receptors, and bone marrow MPCs express physiologically relevant levels of β2 and β3 adrenergic receptors and of PTH receptors. Conditional deletion of Gnas (encodes Gs) in MPCs using a constitutive Sp7-cre allele reduced B-lymphopoiesis, possibly because of reduced IL-7 levels in the bone marrow (27). Although the Gs-coupled receptors involved in controlling IL-7 production were not identified, several studies suggest that PTH can mediate such effects (26, 122). Whether these effects are direct or indirect remains unclear. Some studies suggest that retinoic acids (RA) regulate B-lymphopoiesis through modulation of IL-7 production. Retinoid acids signal through heterodimers of retinoid acid receptors (α, β and γ) with retinoid X receptors bound to DNA motifs called retinoic acid response elements in the cell nucleus. RARγ deficient mice showed reduced numbers of IL-7-responsive progenitor B cells in the bone marrow (123), which correlated with reduced Il7 transcripts in total bone marrow (124). However, this phenotype was not detected in mice with conditional Rarg deletion in bone marrow MPCs (125), suggesting that the defects seen in early B cell progenitors and IL-7 production might have been caused by indirect effects of global Rarg deletion. A likely candidate indirect mechanism for the effects seen in early B cell progenitors and IL-7 expression is the effect of TNFα, a cytokine overly produced in RARγ-deficient mice (123), in B lymphopoiesis (126).
As briefly mentioned above, inflammatory cytokines alone or produced in response to microbial products have relatively strong inhibitory effects on IL-7 (and CXCL12) production and B-lymphopoiesis (126, 127). Mice immunized with incomplete Freund’s adjuvant showed a two to three-fold reduction in IL-7, and a near 10-fold reduction in CXCL12 mRNA levels, 4 days after immunization, a combination that is likely to synergize towards a near complete inhibition of B cell development. The B-lymphopenia could be recapitulated by systemic treatment with TNFα and IL-1β, with TNFα alone having a stronger inhibitory effect on B cell development than single IL-1β treatment (127). Other inflammatory cytokines, such as type I and type II interferons (IFNs), or microbial products that induce IFN production can inhibit B cell development directly (128-130), but it remains unclear if these cytokines can act via modulation of IL-7 production in vivo. The myelopoietic cytokine G-CSF, abundantly produced during systemic infection and/or inflammation, can induce a strong downregulation of IL-7 and CXCL12 expression in CXCL12+ cells (131-133). Given that IL-7+ MPCs do not express G-CSF receptors these effects are also presumably indirect. An attractive possibility explaining IL-7-downregulation is that inflammatory mediators promote MPC differentiation into cellular states that no longer express IL-7. Indirect support for this possibility is the finding that sterile or microbial-induced systemic inflammation promotes a dramatic loss of osteoblasts with G-CSF playing an important role in this process (45, 134, 135). Although the kinetics of osteoblast recovery is presently unknown, it is likely that signals sensed by MPCs induce their differentiation into osteolineage cells that no longer express IL-7.
Systemic inflammation caused by microbial products or by inflammatory cytokines commonly produced during microbial infections trigger a rapid shift in hematopoiesis from a balanced lymphoid and myeloid cell production to a myeloid skewed output (126, 127, 136). While the cytokines (and chemokines) involved in lymphoid versus myeloid lineage development do not overlap, both hematopoietic cell lineages are characterized by highly proliferative intermediate developmental stages. Therefore, it is tempting to speculate that proliferative progenitor subsets compete for limiting factors, such as anabolic nutrients, in the bone marrow microenvironment. In this case, the mechanism(s) involved in prioritizing the development of one lineage over other lineages (e.g. infection and increased production of granulocytes; exposure to reduced oxygen levels such as at higher altitudes and the concomitant increase in red blood cell production) are likely intertwined with mechanisms that halt or reduce the production of resource-consuming hematopoietic progenitors. It is possible that some types of blood cell cancers (e.g. preB cell leukemias (35)) gain competitive advantages over non-malignant hematopoietic progenitor cells by engaging molecular switches that turn-off the expression of cytokines promoting cell proliferation, such as IL-7.
5. Mechanisms of bone marrow B cell egress.
In a series of studies based on light, electron and autoradiographic microscopy Osmond and colleagues analyzed the distribution of lymphocytes in femur bone marrow of guinea pig and rats and noted that lymphocytes were frequently found inside bone marrow sinusoids at frequencies greater than what was typically found in the blood circulation (137, 138). While the typical ratio of lymphocyte to red blood cell in peripheral blood is about 1:1000, in the marrow sinusoids lymphocytes often out number red blood cells, and in the case of some small sinusoids lymphocytes are the only cells found in the lumen. The accumulation of lymphocytes in the sinusoids was defined as “lymphocyte loading”. Bone marrow sinusoids were found peculiar due to the fact that the endothelial cells lacked basement membrane, were fenestrated, with some of the fenestrations resulting in large gaps the size of a lymphocyte diameter (137-139). Intravenous injection of various types of small particles shortly before animal sacrifice led to the perfusion of numerous particles into the bone marrow parenchyma. Although it was not possible to resolve the direction of cell migration, these studies provided compelling evidence for a simple model: that cells produced in the bone marrow parenchyma exited into peripheral blood circulation via the vast collecting network of bone marrow sinusoids.
Direct evidence supporting this model came from another series of elegant studies of autoradiographic microscopy of radiolabeled anti-IgM antibodies injected intravenously into 3-week old mice. This approach allowed labeling lymphocytes expressing IgM on the cell surface (sIgM) selectively, which led to the observation that a significant fraction of sIgM+ cells were localized inside sinusoids. In 3-week old mice, because most of sIgM+ cells are recently developed immature B cells, it was suggested that the “lymphocyte loading” of sinusoids is a hallmark of the final stages of B cell maturation in bone marrow (41, 42). Insight into the mechanisms controlling B cell egress from parenchyma into the sinusoids came from the identification of the chemoattractant lipid Sphingosine 1-phosphate (S1P) and its receptors (mostly S1PR1, but also S1PR3 and S1PR5) as an essential guidance cue for lymphocyte exit from lymphoid organs (140). In mice deficient in S1PR1 or in mice deficient in S1P synthesis enzymes (Sphingosine kinase-1 and 2) naïve T cells and naïve mature B cells are reduced by ~100-1000 fold in circulatory fluids (blood and lymph), whereas mature CD4+ and CD8+ thymocytes accumulate in the thymus (141-143). Importantly, disruption of S1P gradients via small molecule inhibitors or genetic deficiency of the S1P degrading enzyme S1P lyase also causes severe peripheral T cell lymphopenia with concomitant mature thymocyte accumulation, demonstrating that disruption of S1P gradients could block lymphocyte egress from lymphoid organs just as well as S1P deficiency (144). The fact that S1P enforces a complete S1PR1 desensitization from the cell surface (145) is a logical explanation for the importance of S1P gradient distribution in tissues for mediating lymphocyte egress. But, in contrast to its critical role in T cell egress from thymus and T and B cell egress from lymph nodes, the S1P/S1PR1 pathway played a significantly smaller role in mediating immature B cell egress from the bone marrow, given that their numbers only marginally accumulate in bone marrow and are reduced by only 2-3 fold in peripheral blood (146). A likely explanation for such minor role is the fact that S1P concentration in the bone marrow parenchyma is high (as evaluated by the very low amounts of S1PR1 expressed on the surface of the few naïve T cells that normally transit through the bone marrow (147)), which presumably prevents sharp S1P gradients being formed between bone marrow parenchyma and sinusoids. This model is consistent with rapid and efficient plasma perfusion of the bone marrow parenchyma through via fenestrated sinusoidal endothelium. It is also in agreement with the finding that red blood cells do not express S1P lyase (144), which may contribute to high S1P interstitial concentration in bone marrow due to inefficient degradation.
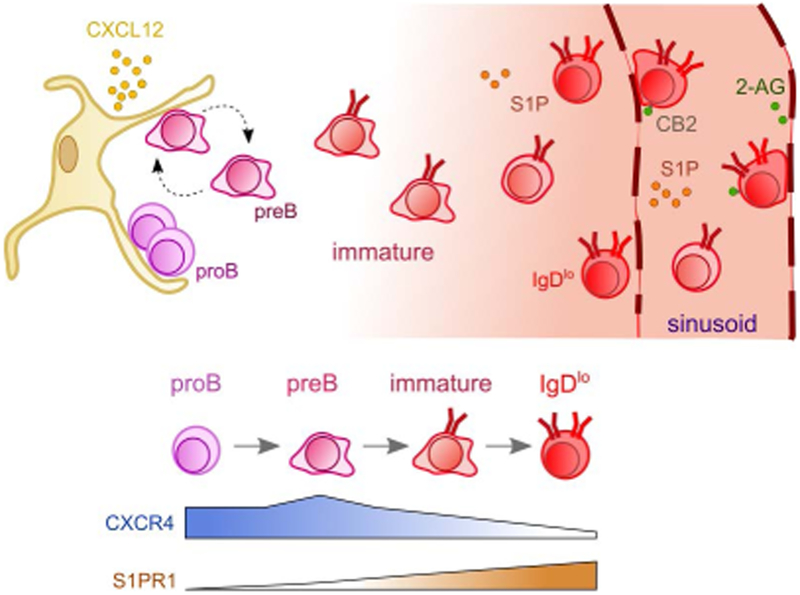
In lymph nodes, T cell egress is regulated by a balance between responsiveness to egress versus retention-promoting chemoattractants (148). In the bone marrow, some evidence suggests that S1PR1 signaling in immature B cells counteracts CXCR4-mediated retention (149). Such balanced responsiveness between CXCR4-mediated retention and egress-promoting cues seems to be a common mechanism controlling bone marrow residence of a variety of hematopoietic cells, including monocytes, granulocytes and lymphocytes (147, 150-156). However, bone marrow cell egress cannot be solely explained by balanced responsiveness to retention versus egress cues. Immature B cells (and other hematopoietic cells) can still exit the bone marrow when egress-promoting chemoattractants are not expressed, or when their gradients are disrupted in vivo. Thus, alternative mechanisms likely operate to enable cell export from bone marrow. In agreement with this possibility, B cells engineered to express the S1 subunit of Pertussis toxin (PTX) and thus cannot respond to most chemoattractants due to inactivation of Gαi proteins and impairment of Gαi protein-coupled receptor signaling could nevertheless exit the bone marrow even more efficiently than “normal” B cells (33). Furthermore, intravital 2-photon microscopy of PTX-expressing B cells in calvaria bone marrow showed that they were mostly non-motile and often with rounded cell morphology, suggesting that PTX-expressing B cells can egress the bone marrow in a passive manner i.e. independently of cell-intrinsic mechanisms (33). Similar observations were made with B-lineage cells conditionally deficient in CXCR4 (33). A possible cell-extrinsic mechanism that helps hematopoietic cells to exit the bone marrow is shear forces generated by interstitial fluid draining into the central collecting sinusoids that occupy the center cavity of long-bones. Of the many types of hematopoietic cells being produced, red blood cells are unique in their inability to migrate in response to tissue chemoattractants, yet millions of red blood cells are exported from the bone marrow on a daily basis. Thus, it is reasonable to hypothesize that interstitial fluid drainage into collecting and fenestrated sinusoids also carry non-motile and poorly adherent hematopoietic cells into peripheral circulation. Thus, in this model the major regulatory step in the control of immature B cell export from the bone marrow (and possibly other hematopoietic cells) is attenuation of CXCR4 signaling and of integrin-mediated adhesion to extracellular matrix (Figure 4).
Figure 4: Immature B cell egress is predominantly controlled by attenuation of CXCR4 signaling.
ProB cells (magenta) are predominantly non-motile and highly adherent to VCAM-1 expressed on CXCL12Hi MPCs. These cells express very little amounts of egress-promoting S1P receptor-1 but are highly dependent on CXCR4 signaling for bone marrow retention. PreB cells (purple) are motile (indicated by amoeboid shape with ruffled edges) due to high levels of CXCR4 expression, which also promotes their retention in the bone marrow. PreB also express very little amounts of egress-promoting S1P receptors. Immature B cells (red) gradually reduce CXCR4 expression while S1PR1 expression is markedly increased. Reduced CXCL12-mediated retention of immature B cells, particularly at the IgDlo B cell stage, causes the cells to exit into the sinusoids. S1P plays a small but detectable role in promoting immature B cell egress into sinusoids. Upon egress, immature B cells are temporarily retained inside sinusoids via a mechanism primarily dependent on CB2-mediated α4β1 transactivation and adhesion to VCAM-1 expressed on luminal sinusoidal endothelium. The CB2 ligand 2-Ag is presumably enriched in the perisinusoidal/intrasinusoidal space.
While IL-7R signaling controls CXCR4 expression in proB cells, preBCR signaling further increased CXCR4 in preB cells (82). Importantly, BCR signaling at the immature B cell stage also controls CXCR4 expression and increases B cell motility and retention in the bone marrow parenchyma, such that it reduces immature B egress from the bone marrow by nearly 10-fold (33). These observations are compatible with other findings showing that negative selection of autoreactive BCRs by RAG-mediated recombination of alternative Ig light chains (receptor editing) preferentially occurs in the bone marrow (157, 158). Furthermore, the fact that BCR signaling promotes CXCR4-mediated migration within bone marrow suggests that autoreactive immature B cells interact with IL-7+ CXCL12Hi MPCs while receptor editing is occurring, and lend support to the possibility that IL-7 contributes to this process (159).
In humans, compelling evidence in support of a model in which bone marrow egress is preferentially regulated via attenuation of CXCR4 signaling comes from the finding that mutations resulting in the loss of the cytoplasmic tail of CXCR4 (a region required for CXCR4 desensitization) cause peripheral B lymphopenia and neutropenia due to impaired egress from the bone marrow (160-162). Similar observations were also made in mice carrying an equivalent mutation in CXCR4 cytoplasmic tail to that found in most human patients (163).
As CXCR4 prevents bone marrow egress, signaling modules that alter CXCR4 responsiveness to CXCL12 may also play non-redundant roles in controlling cell residence in bone marrow. The circadian clock, for example, controls oscillation of CXCL12 expression in bone marrow MPCs such that it regulates trafficking of HSCs and other leukocytes in and out of the bone marrow (156, 164, 165). Although the mechanism underlying circadian control of bone marrow and peripheral leukocyte trafficking is through oscillations in CXCR4 signaling, it is possible that other mechanisms, such as adrenergic control of vascular permeability also contribute to regulating bone marrow cell trafficking (166). Other mechanisms acting at the level of CXCR4 signaling may also be involved in controlling cell egress from bone marrow. For example, several studies suggested that Suppressor Of Cytokine Signaling-3 (SOCS3) negatively regulates CXCR4, and FAK and integrin-mediated adhesion to the extracellular matrix (167, 168). Furthermore, conditional deletion of Socs3 in hematopoietic and some non-hematopoietic cells, led to an accumulation of immature B cells in bone marrow parenchyma, implicating SOCS3 signaling in the control of bone marrow B-lineage cell egress (168). However, conditional Socs3 deletion exclusively in B-lineage cells did not reveal an intrinsic requirements for SOCS3 in B-lineage cell motility and/or distribution between bone marrow parenchyma, sinusoids and periphery (34).
Before exiting into peripheral blood, immature B cells (and mature B cells) are temporarily retained inside bone marrow sinusoids via a mechanism regulated by Cannabinoid receptor (CB)-2, and by S1PR3 (32, 169). The endocannabinoid ligand of CB2, 2-arachidonoylglycerol (2-AG), is abundant in bone marrow interstitium and nearly absent in plasma (32). The cellular sources of 2-AG are unknown. However, indirect evidence suggests it is active near or inside bone marrow sinusoids, where it transactivates α4β1 integrins and promotes immature and mature B adhesion to VCAM-1 expressed on the luminal side of the sinusoidal endothelium (32). Even though CB2 also promotes immature and mature B cell migration towards 2-AG gradients in vitro, CB2 signaling does not seem to contribute to promote immature B cell egress into the sinusoids (neither it seems to promote mature B cell homing to the bone marrow). Instead, CB2 signaling is mostly required for the development and/or selection of Igλ-expressing immature and mature B cells, suggesting that residence in sinusoidal niches represents an important and perhaps final stage of B cell development in bone marrow. How CB2 promotes Igλ+ B cell homeostasis is unknown. CB2 signaling in preB acute lymphoblastic leukemia has been proposed to restrict glucose metabolism (170). It is possible that Igλ+ B cells are particularly dependent on CB2-regulated ATP production via glucose metabolism for survival.
6. Conclusions and Future Perspectives
It has been more than 50 years since the niche concept was proposed to explain fundamental differences between bone marrow and spleen microenvironments in supporting hematopoiesis (37). Since then, many studies provided a plethora of molecular details explaining how bone marrow niches work to control HSC homeostasis and also to enable differentiation of early progenitors into all hematopoietic cell lineages. Central to most cell-fate decisions are the cytokines (e.g. SCF and IL-7) and chemokines (mostly CXCL12) produced by the MPC network and by a small subset of sinusoidal endothelial cells. The concomitant production of CXCL12, SCF, and lymphopoietic cytokines helps HSCs, MPPs, CLPs, and B cell progenitors to access important growth factors that act predominantly as short-range signals. IL-7 bioavailability is presumably restricted to the vicinity of MPCs and ECs such that lymphoid progenitors and early B cell precursors are highly dependent on CXCR4-mediated migration for optimal IL-7R signaling and further differentiation. The CXCL12/IL-7 axis is not only key for ensuring B cell production but it may also be an important layer of regulation against the development of B cell leukemias. The findings that MPCs also provide essential signals for the development of T-lineage committed CLPs and NK cells (171, 172) is consistent with a model in which MPCs play a central role in early lymphoid progenitor development and/or differentiation. Besides HSC homeostasis and lymphopoiesis, mature lymphocyte subsets, such as regulatory T cells (173, 174), memory CD8+ (175-178), CD4+ T cells (179, 180) and long-lived plasma cells (181-184), migrate to the bone marrow and possibly share the MPC niche with hematopoietic stem and progenitor cells. It is likely that the cell circuit interactions described for proB, preB and IL-7+ MPCs also contribute to the homeostasis of other hematopoietic cells. It will be important in future studies to determine if (and how) cross-talk between HSCs, MPPs, CLPs, or downstream hematopoietic cells with MPCs can regulate the production of individual cytokines (e.g. SCF, FLT3L, IL-15) similarly to how pre-leukemic and leukemic B-lineage cells regulate IL-7 production. Acute T lymphoblastic leukemias are also dependent on bone marrow microenvironments controlled by CXCR4/CXCL12 CXCR4/CXCL12 for tumor growth (185). It is possible that acute T lymphoblastic leukemias regulate cytokine production by niche cells in a similar manner as pre-lekemic and leukemic B-lineage cells. Future studies are needed to unravel the mechanisms and magnitude of cross-talk between normal and pre-leukemic hematopoietic progenitors, and various types of leukemias, with the specialized microenvironments formed by MPCs and sinusoidal endothelial cells.
Acknowledgements
S. Zehentmeier was partly funded by the German Research Foundation (DFG ZE1060/1-1). The National Institutes of Health funded these studies (RO1AI113040 and R21AI133060-01A1 to J.P. Pereira). The authors declare no competing financial interests.
7 References
- 1.Frenette PS, Pinho S, Lucas D, Scheiermann C. Mesenchymal stem cell: keystone of the hematopoietic stem cell niche and a stepping-stone for regenerative medicine. Annual review of immunology.2013;31:285–316. [DOI] [PubMed] [Google Scholar]
- 2.Morrison SJ, Scadden DT. The bone marrow niche for haematopoietic stem cells. Nature.2014;505:327–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang HD, Tokoyoda K, Radbruch A. Immunological memories of the bone marrow. Immunol Rev.2018;283:86–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boehm T, Bleul CC. Thymus-homing precursors and the thymic microenvironment. Trends Immunol.2006;27:477–484. [DOI] [PubMed] [Google Scholar]
- 5.Mebius RE. Organogenesis of lymphoid tissues. Nat Rev Immunol.2003;3:292–303. [DOI] [PubMed] [Google Scholar]
- 6.Milne CD, Paige CJ. IL-7: a key regulator of B lymphopoiesis. Semin Immunol.2006;18:20–30. [DOI] [PubMed] [Google Scholar]
- 7.Rochman Y, Spolski R, Leonard WJ. New insights into the regulation of T cells by gamma(c) family cytokines. Nat Rev Immunol.2009;9:480–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kennedy MK, Glaccum M, Brown SN, et al. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. The Journal of experimental medicine.2000;191:771–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lim VY, Zehentmeier S, Fistonich C, Pereira JP. A Chemoattractant-Guided Walk Through Lymphopoiesis: From Hematopoietic Stem Cells to Mature B Lymphocytes. Adv Immunol.2017;134:47–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Avecilla ST, Hattori K, Heissig B, et al. Chemokine-mediated interaction of hematopoietic progenitors with the bone marrow vascular niche is required for thrombopoiesis. Nat Med.2004;10:64–71. [DOI] [PubMed] [Google Scholar]
- 11.Chasis JA, Mohandas N. Erythroblastic islands: niches for erythropoiesis. Blood.2008;112:470–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nevius E, Pinho F, Dhodapkar M, et al. Oxysterols and EBI2 promote osteoclast precursor migration to bone surfaces and regulate bone mass homeostasis. The Journal of experimental medicine.2015;212:1931–1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nevius E, Gomes AC, Pereira JP. Inflammatory Cell Migration in Rheumatoid Arthritis: A Comprehensive Review. Clin Rev Allergy Immunol.2016;51:59–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herault A, Binnewies M, Leong S, et al. Myeloid progenitor cluster formation drives emergency and leukaemic myelopoiesis. Nature.2017;544:53–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Calvi LM, Adams GB, Weibrecht KW, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature.2003;425:841–846. [DOI] [PubMed] [Google Scholar]
- 16.Zhang J, Niu C, Ye L, et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature.2003;425:836–841. [DOI] [PubMed] [Google Scholar]
- 17.Mendez-Ferrer S, Michurina TV, Ferraro F, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature.2010;466:829–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Omatsu Y, Sugiyama T, Kohara H, et al. The Essential Functions of Adipo-osteogenic Progenitors as the Hematopoietic Stem and Progenitor Cell Niche. Immunity.2010;33:387–399. [DOI] [PubMed] [Google Scholar]
- 19.Ding L, Saunders TL, Enikolopov G, Morrison SJ. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature.2012;481:457–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ding L, Morrison SJ. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature.2013;495:231–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greenbaum A, Hsu YM, Day RB, et al. CXCL12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance. Nature.2013;495:227–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kunisaki Y, Bruns I, Scheiermann C, et al. Arteriolar niches maintain haematopoietic stem cell quiescence. Nature.2013;502:637–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Birbrair A, Frenette PS. Niche heterogeneity in the bone marrow. Annals of the New York Academy of Sciences.2016;1370:82–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sugiyama T, Kohara H, Noda M, Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity.2006;25:977–988. [DOI] [PubMed] [Google Scholar]
- 25.Visnjic D, Kalajzic Z, Rowe DW, et al. Hematopoiesis is severely altered in mice with an induced osteoblast deficiency. Blood.2004;103:3258–3264. [DOI] [PubMed] [Google Scholar]
- 26.Zhu J, Garrett R, Jung Y, et al. Osteoblasts support B-lymphocyte commitment and differentiation from hematopoietic stem cells. Blood.2007;109:3706–3712. [DOI] [PubMed] [Google Scholar]
- 27.Wu JY, Purton LE, Rodda SJ, et al. Osteoblastic regulation of B lymphopoiesis is mediated by Gs{alpha}-dependent signaling pathways. Proc Natl Acad Sci U S A.2008;105:16976–16981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hermans MH, Hartsuiker H, Opstelten D. An in situ study of B-lymphocytopoiesis in rat bone marrow. Topographical arrangement of terminal deoxynucleotidyl transferase-positive cells and pre-B cells. J Immunol.1989;142:67–73. [PubMed] [Google Scholar]
- 29.Jacobsen K, Osmond DG. Microenvironmental organization and stromal cell associations of B lymphocyte precursor cells in mouse bone marrow. Eur J Immunol.1990;20:2395–2404. [DOI] [PubMed] [Google Scholar]
- 30.Tokoyoda K, Egawa T, Sugiyama T, Choi BI, Nagasawa T. Cellular niches controlling B lymphocyte behavior within bone marrow during development. Immunity.2004;20:707–718. [DOI] [PubMed] [Google Scholar]
- 31.Park SY, Wolfram P, Canty K, et al. Focal adhesion kinase regulates the localization and retention of pro-B cells in bone marrow microenvironments. J Immunol.2013;190:1094–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pereira JP, An J, Xu Y, Huang Y, Cyster JG. Cannabinoid receptor 2 mediates the retention of immature B cells in bone marrow sinusoids. Nature immunology.2009;10:403–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beck TC, Gomes AC, Cyster JG, Pereira JP. CXCR4 and a cell-extrinsic mechanism control immature B lymphocyte egress from bone marrow. The Journal of experimental medicine.2014;211:2567–2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nadrah K, Beck TC, Pereira JP. Immature B Cell Egress from Bone Marrow Is SOCS3 Independent. PLoS One.2015;10:e0136061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cordeiro Gomes A, Hara T, Lim VY, et al. Hematopoietic Stem Cell Niches Produce Lineage-Instructive Signals to Control Multipotent Progenitor Differentiation. Immunity.2016;45:1219–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou X, Franklin RA, Adler M, et al. Circuit Design Features of a Stable Two-Cell System. Cell.2018;172:744–757 e717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schofield R The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells.1978;4:7–25. [PubMed] [Google Scholar]
- 38.Osmond DG, Jacobsen K, Park YH, Lamontagne L. In vivo localization of B lymphocyte progenitor cells in mouse bone marrow. Adv Exp Med Biol.1988;237:45–51. [DOI] [PubMed] [Google Scholar]
- 39.Wei C, Zeff R, Goldschneider I. Murine pro-B cells require IL-7 and its receptor complex to up-regulate IL-7R alpha, terminal deoxynucleotidyltransferase, and c mu expression. J Immunol.2000;164:1961–1970. [DOI] [PubMed] [Google Scholar]
- 40.Bendall SC, Davis KL, Amir el AD, et al. Single-cell trajectory detection uncovers progression and regulatory coordination in human B cell development. Cell.2014;157:714–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Batten SJ, Osmond DG. The localization of B lymphocytes in mouse bone marrow: radioautographic studies after in vivo perfusion of radiolabelled anti-IgM antibody. J Immunol Methods.1984;72:381–399. [DOI] [PubMed] [Google Scholar]
- 42.Osmond DG, Batten SJ. Genesis of B lymphocytes in the bone marrow: extravascular and intravascular localization of surface IgM-bearing cells in mouse bone marrow detected by electron-microscope radioautography after in vivo perfusion of 125I anti-IgM antibody. Am J Anat.1984;170:349–365. [DOI] [PubMed] [Google Scholar]
- 43.Dorshkind K Regulation of hemopoiesis by bone marrow stromal cells and their products. Annual review of immunology.1990;8:111–137. [DOI] [PubMed] [Google Scholar]
- 44.Aguila HL, Mun SH, Kalinowski J, et al. Osteoblast-specific overexpression of human interleukin-7 rescues the bone mass phenotype of interleukin-7-deficient female mice. J Bone Miner Res.2012;27:1030–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Terashima A, Okamoto K, Nakashima T, et al. Sepsis-Induced Osteoblast Ablation Causes Immunodeficiency. Immunity.2016;44:1434–1443. [DOI] [PubMed] [Google Scholar]
- 46.Tokoyoda K, Zehentmeier S, Hegazy AN, et al. Professional memory CD4+ T lymphocytes preferentially reside and rest in the bone marrow. Immunity.2009;30:721–730. [DOI] [PubMed] [Google Scholar]
- 47.Mazzucchelli RI, Warming S, Lawrence SM, et al. Visualization and identification of IL-7 producing cells in reporter mice. PLoS One.2009;4:e7637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hara T, Shitara S, Imai K, et al. Identification of IL-7-producing cells in primary and secondary lymphoid organs using IL-7-GFP knock-in mice. J Immunol.2012;189:1577–1584. [DOI] [PubMed] [Google Scholar]
- 49.Zhang J, Link DC. Targeting of Mesenchymal Stromal Cells by Cre-Recombinase Transgenes Commonly Used to Target Osteoblast Lineage Cells. J Bone Miner Res.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu Y, Strecker S, Wang L, et al. Osterix-cre labeled progenitor cells contribute to the formation and maintenance of the bone marrow stroma. PLoS One.2013;8:e71318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mizoguchi T, Pinho S, Ahmed J, et al. Osterix marks distinct waves of primitive and definitive stromal progenitors during bone marrow development. Dev Cell.2014;29:340–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ono N, Ono W, Mizoguchi T, et al. Vasculature-associated cells expressing nestin in developing bones encompass early cells in the osteoblast and endothelial lineage. Dev Cell.2014;29:330–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cain CJ, Rueda R, McLelland B, et al. Absence of sclerostin adversely affects B-cell survival. J Bone Miner Res.2012;27:1451–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cao J, Zhang L, Wan Y, et al. Ablation of Wntless in endosteal niches impairs lymphopoiesis rather than HSCs maintenance. Eur J Immunol.2015;45:2650–2660. [DOI] [PubMed] [Google Scholar]
- 55.Yu VW, Lymperi S, Oki T, et al. Distinctive Mesenchymal-Parenchymal Cell Pairings Govern B Cell Differentiation in the Bone Marrow. Stem cell reports.2016;7:220–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Martin SK, Fitter S, El Khawanky N, et al. mTORC1 plays an important role in osteoblastic regulation of B-lymphopoiesis. Sci Rep.2018;8:14501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang Y, Xiao M, Tao C, et al. Inactivation of mTORC1 Signaling in Osterix-Expressing Cells Impairs B-cell Differentiation. J Bone Miner Res.2018;33:732–742. [DOI] [PubMed] [Google Scholar]
- 58.Nagasawa T CXCL12/SDF-1 and CXCR4. Frontiers in immunology.2015;6:301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Walters KB, Green JM, Surfus JC, Yoo SK, Huttenlocher A. Live imaging of neutrophil motility in a zebrafish model of WHIM syndrome. Blood.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Boldajipour B, Doitsidou M, Tarbashevich K, et al. Cxcl12 evolution - subfunctionalization of a ligand through altered interaction with the chemokine receptor. Development.2011;138:2909–2914. [DOI] [PubMed] [Google Scholar]
- 61.Mayer WE, Uinuk-Ool T, Tichy H, et al. Isolation and characterization of lymphocyte-like cells from a lamprey. Proc Natl Acad Sci U S A.2002;99:14350–14355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nagasawa T, Hirota S, Tachibana K, et al. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature.1996;382:635–638. [DOI] [PubMed] [Google Scholar]
- 63.Ma Q, Jones D, Borghesani PR, et al. Impaired B-lymphopoiesis, myelopoiesis, and derailed cerebellar neuron migration in CXCR4- and SDF-1-deficient mice. Proc Natl Acad Sci U S A.1998;95:9448–9453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman DR. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature.1998;393:595–599. [DOI] [PubMed] [Google Scholar]
- 65.Ma Q, Jones D, Springer TA. The chemokine receptor CXCR4 is required for the retention of B lineage and granulocytic precursors within the bone marrow microenvironment. Immunity.1999;10:463–471. [DOI] [PubMed] [Google Scholar]
- 66.Egawa T, Kawabata K, Kawamoto H, et al. The earliest stages of B cell development require a chemokine stromal cell-derived factor/pre-B cell growth-stimulating factor. Immunity.2001;15:323–334. [DOI] [PubMed] [Google Scholar]
- 67.Nie Y, Han YC, Zou YR. CXCR4 is required for the quiescence of primitive hematopoietic cells. The Journal of experimental medicine.2008;205:777–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nie Y, Waite J, Brewer F, et al. The role of CXCR4 in maintaining peripheral B cell compartments and humoral immunity. The Journal of experimental medicine.2004;200:1145–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Inlay MA, Bhattacharya D, Sahoo D, et al. Ly6d marks the earliest stage of B-cell specification and identifies the branchpoint between B-cell and T-cell development. Genes Dev.2009;23:2376–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lin JX, Migone TS, Tsang M, et al. The role of shared receptor motifs and common Stat proteins in the generation of cytokine pleiotropy and redundancy by IL-2, IL-4, IL-7, IL-13, and IL-15. Immunity.1995;2:331–339. [DOI] [PubMed] [Google Scholar]
- 71.Hardy RR, Hayakawa K. B cell development pathways. Annual review of immunology.2001;19:595–621. [DOI] [PubMed] [Google Scholar]
- 72.Hardy RR, Carmack CE, Shinton SA, Kemp JD, Hayakawa K. Resolution and characterization of pro-B and pre-pro-B cell stages in normal mouse bone marrow. The Journal of experimental medicine.1991;173:1213–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Carvalho TL, Mota-Santos T, Cumano A, Demengeot J, Vieira P. Arrested B lymphopoiesis and persistence of activated B cells in adult interleukin 7(-/)- mice. The Journal of experimental medicine.2001;194:1141–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dias S, Silva H Jr., Cumano A, Vieira P Interleukin-7 is necessary to maintain the B cell potential in common lymphoid progenitors. The Journal of experimental medicine.2005;201:971–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kikuchi K, Lai AY, Hsu CL, Kondo M. IL-7 receptor signaling is necessary for stage transition in adult B cell development through up-regulation of EBF. The Journal of experimental medicine.2005;201:1197–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mandal M, Powers SE, Maienschein-Cline M, et al. Epigenetic repression of the Igk locus by STAT5-mediated recruitment of the histone methyltransferase Ezh2. Nature immunology.2011;12:1212–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Powers SE, Mandal M, Matsuda S, et al. Subnuclear cyclin D3 compartments and the coordinated regulation of proliferation and immunoglobulin variable gene repression. The Journal of experimental medicine.2012;209:2199–2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Johnson K, Hashimshony T, Sawai CM, et al. Regulation of immunoglobulin light-chain recombination by the transcription factor IRF-4 and the attenuation of interleukin-7 signaling. Immunity.2008;28:335–345. [DOI] [PubMed] [Google Scholar]
- 79.Ochiai K, Maienschein-Cline M, Mandal M, et al. A self-reinforcing regulatory network triggered by limiting IL-7 activates pre-BCR signaling and differentiation. Nature immunology.2012;13:300–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Marshall AJ, Fleming HE, Wu GE, Paige CJ. Modulation of the IL-7 dose-response threshold during pro-B cell differentiation is dependent on pre-B cell receptor expression. J Immunol.1998;161:6038–6045. [PubMed] [Google Scholar]
- 81.Clark MR, Mandal M, Ochiai K, Singh H. Orchestrating B cell lymphopoiesis through interplay of IL-7 receptor and pre-B cell receptor signalling. Nat Rev Immunol.2014;14:69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fistonich C, Zehentmeier S, Bednarski JJ, et al. Cell circuits between B cell progenitors and IL-7(+) mesenchymal progenitor cells control B cell development. The Journal of experimental medicine.2018;215:2586–2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.de Gorter DJJ, Beuling EA, Kersseboom R, et al. Bruton’s Tyrosine Kinase and Phospholipase Cγ2 Mediate Chemokine-Controlled B Cell Migration and Homing. Immunity.2007;26:93–104. [DOI] [PubMed] [Google Scholar]
- 84.Glodek AM, Honczarenko M, Le Y, Campbell JJ, Silberstein LE. Sustained activation of cell adhesion is a differentially regulated process in B lymphopoiesis. The Journal of experimental medicine.2003;197:461–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Glodek AM, Honczarenko M, Le Y, Campbell JJ, Silberstein LE. Sustained activation of cell adhesion is a differentially regulated process in B lymphopoiesis. The Journal of experimental medicine.2003;197:461–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Brouns GS, de Vries E, Borst J. Assembly and intracellular transport of the human B cell antigen receptor complex. Int Immunol.1995;7:359–368. [DOI] [PubMed] [Google Scholar]
- 87.Winkler TH, Rolink A, Melchers F, Karasuyama H. Precursor B cells of mouse bone marrow express two different complexes with the surrogate light chain on the surface. Eur J Immunol.1995;25:446–450. [DOI] [PubMed] [Google Scholar]
- 88.Parker MJ, Licence S, Erlandsson L, et al. The pre-B-cell receptor induces silencing of VpreB and lambda5 transcription. EMBO J.2005;24:3895–3905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rolink AG, Winkler T, Melchers F, Andersson J. Precursor B cell receptor-dependent B cell proliferation and differentiation does not require the bone marrow or fetal liver environment. The Journal of experimental medicine.2000;191:23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bradl H, Jack HM. Surrogate light chain-mediated interaction of a soluble pre-B cell receptor with adherent cell lines. J Immunol.2001;167:6403–6411. [DOI] [PubMed] [Google Scholar]
- 91.Bradl H, Wittmann J, Milius D, Vettermann C, Jack HM. Interaction of murine precursor B cell receptor with stroma cells is controlled by the unique tail of lambda 5 and stroma cell-associated heparan sulfate. J Immunol.2003;171:2338–2348. [DOI] [PubMed] [Google Scholar]
- 92.Gauthier L, Rossi B, Roux F, Termine E, Schiff C. Galectin-1 is a stromal cell ligand of the pre-B cell receptor (BCR) implicated in synapse formation between pre-B and stromal cells and in pre-BCR triggering. Proc Natl Acad Sci U S A.2002;99:13014–13019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Elantak L, Espeli M, Boned A, et al. Structural basis for galectin-1-dependent pre-B cell receptor (pre-BCR) activation. The Journal of biological chemistry.2012;287:44703–44713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mourcin F, Breton C, Tellier J, et al. Galectin-1-expressing stromal cells constitute a specific niche for pre-BII cell development in mouse bone marrow. Blood.2011;117:6552–6561. [DOI] [PubMed] [Google Scholar]
- 95.Aurrand-Lions M, Mancini SJC. Murine Bone Marrow Niches from Hematopoietic Stem Cells to B Cells. Int J Mol Sci.2018;19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Espeli M, Mancini SJ, Breton C, Poirier F, Schiff C. Impaired B-cell development at the pre-BII-cell stage in galectin-1-deficient mice due to inefficient pre-BII/stromal cell interactions. Blood.2009;113:5878–5886. [DOI] [PubMed] [Google Scholar]
- 97.Bankovich AJ, Raunser S, Juo ZS, et al. Structural insight into pre-B cell receptor function Science (New York, NY: 2007;316:291–294. [DOI] [PubMed] [Google Scholar]
- 98.Healy JI, Goodnow CC. Positive versus negative signaling by lymphocyte antigen receptors. Annual review of immunology.1998;16:645–670. [DOI] [PubMed] [Google Scholar]
- 99.Nemazee D Mechanisms of central tolerance for B cells. Nat Rev Immunol.2017;17:281–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Helmink BA, Sleckman BP. The response to and repair of RAG-mediated DNA double-strand breaks. Annual review of immunology.2012;30:175–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Desiderio S Temporal and spatial regulatory functions of the V(D)J recombinase. Semin Immunol.2010;22:362–369. [DOI] [PubMed] [Google Scholar]
- 102.Lieber MR. The mechanism of double-strand DNA break repair by the nonhomologous DNA end-joining pathway. Annu Rev Biochem.2010;79:181–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rooney S, Sekiguchi J, Zhu C, et al. Leaky Scid phenotype associated with defective V(D)J coding end processing in Artemis-deficient mice. Mol Cell.2002;10:1379–1390. [DOI] [PubMed] [Google Scholar]
- 104.Bredemeyer AL, Helmink BA, Innes CL, et al. DNA double-strand breaks activate a multi-functional genetic program in developing lymphocytes. Nature.2008;456:819–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bednarski JJ, Pandey R, Schulte E, et al. RAG-mediated DNA double-strand breaks activate a cell type-specific checkpoint to inhibit pre-B cell receptor signals. The Journal of experimental medicine.2016;213:209–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bednarski JJ, Sleckman BP. Lymphocyte development: integration of DNA damage response signaling. Adv Immunol.2012;116:175–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fisher AG, Burdet C, LeMeur M, et al. Lymphoproliferative disorders in an IL-7 transgenic mouse line. Leukemia.1993;7 Suppl 2:S66–68. [PubMed] [Google Scholar]
- 108.Rich BE, Campos-Torres J, Tepper RI, Moreadith RW, Leder P. Cutaneous lymphoproliferation and lymphomas in interleukin 7 transgenic mice. The Journal of experimental medicine.1993;177:305–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fisher AG, Burdet C, Bunce C, Merkenschlager M, Ceredig R. Lymphoproliferative disorders in IL-7 transgenic mice: expansion of immature B cells which retain macrophage potential. Int Immunol.1995;7:415–423. [DOI] [PubMed] [Google Scholar]
- 110.Rooney S, Sekiguchi J, Whitlow S, et al. Artemis and p53 cooperate to suppress oncogenic N-myc amplification in progenitor B cells. Proc Natl Acad Sci U S A.2004;101:2410–2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Richards J, McNally B, Fang X, et al. Tumor growth decreases NK and B cells as well as common lymphoid progenitor. PLoS One.2008;3:e3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Moreau JM, Mielnik M, Berger A, Furlonger C, Paige CJ. Tumor-secreted products repress B-cell lymphopoiesis in a murine model of breast cancer. Eur J Immunol.2016;46:2835–2841. [DOI] [PubMed] [Google Scholar]
- 113.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell.2004;116:281–297. [DOI] [PubMed] [Google Scholar]
- 114.Raaijmakers MH, Mukherjee S, Guo S, et al. Bone progenitor dysfunction induces myelodysplasia and secondary leukaemia. Nature.2010;464:852–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Waskow C, Paul S, Haller C, Gassmann M, Rodewald HR. Viable c-Kit(W/W) mutants reveal pivotal role for c-kit in the maintenance of lymphopoiesis. Immunity.2002;17:277–288. [DOI] [PubMed] [Google Scholar]
- 116.Agosti V, Corbacioglu S, Ehlers I, et al. Critical role for Kit-mediated Src kinase but not PI 3-kinase signaling in pro T and pro B cell development. The Journal of experimental medicine.2004;199:867–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sitnicka E, Bryder D, Theilgaard-Monch K, et al. Key role of flt3 ligand in regulation of the common lymphoid progenitor but not in maintenance of the hematopoietic stem cell pool. Immunity.2002;17:463–472. [DOI] [PubMed] [Google Scholar]
- 118.Sitnicka E, Brakebusch C, Martensson IL, et al. Complementary signaling through flt3 and interleukin-7 receptor alpha is indispensable for fetal and adult B cell genesis. The Journal of experimental medicine.2003;198:1495–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Park LS, Martin U, Garka K, et al. Cloning of the murine thymic stromal lymphopoietin (TSLP) receptor: Formation of a functional heteromeric complex requires interleukin 7 receptor. The Journal of experimental medicine.2000;192:659–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Vosshenrich CA, Cumano A, Muller W, Di Santo JP, Vieira P. Thymic stromal-derived lymphopoietin distinguishes fetal from adult B cell development. Nature immunology.2003;4:773–779. [DOI] [PubMed] [Google Scholar]
- 121.Jensen CT, Kharazi S, Boiers C, et al. FLT3 ligand and not TSLP is the key regulator of IL-7-independent B-1 and B-2 B lymphopoiesis. Blood.2008;112:2297–2304. [DOI] [PubMed] [Google Scholar]
- 122.Panaroni C, Fulzele K, Saini V, et al. PTH Signaling in Osteoprogenitors Is Essential for B-Lymphocyte Differentiation and Mobilization. J Bone Miner Res.2015;30:2273–2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Walkley CR, Olsen GH, Dworkin S, et al. A microenvironment-induced myeloproliferative syndrome caused by retinoic acid receptor gamma deficiency. Cell.2007;129:1097–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Green AC, Poulton IJ, Vrahnas C, et al. RARgamma is a negative regulator of osteoclastogenesis. The Journal of steroid biochemistry and molecular biology.2015;150:46–53. [DOI] [PubMed] [Google Scholar]
- 125.Joseph C, Nota C, Fletcher JL, et al. Retinoic Acid Receptor gamma Regulates B and T Lymphopoiesis via Nestin-Expressing Cells in the Bone Marrow and Thymic Microenvironments. J Immunol.2016;196:2132–2144. [DOI] [PubMed] [Google Scholar]
- 126.Ueda Y, Yang K, Foster SJ, Kondo M, Kelsoe G. Inflammation controls B lymphopoiesis by regulating chemokine CXCL12 expression. The Journal of experimental medicine.2004;199:47–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ueda Y, Kondo M, Kelsoe G. Inflammation and the reciprocal production of granulocytes and lymphocytes in bone marrow. The Journal of experimental medicine.2005;201:1771–1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Grawunder U, Melchers F, Rolink A. Interferon-gamma arrests proliferation and causes apoptosis in stromal cell/interleukin-7-dependent normal murine pre-B cell lines and clones in vitro, but does not induce differentiation to surface immunoglobulin-positive B cells. Eur J Immunol.1993;23:544–551. [DOI] [PubMed] [Google Scholar]
- 129.Wang J, Lin Q, Langston H, Cooper MD. Resident bone marrow macrophages produce type 1 interferons that can selectively inhibit interleukin-7-driven growth of B lineage cells. Immunity.1995;3:475–484. [DOI] [PubMed] [Google Scholar]
- 130.Lalanne AI, Moraga I, Hao Y, et al. CpG inhibits pro-B cell expansion through a cathepsin B-dependent mechanism. J Immunol.2010;184:5678–5685. [DOI] [PubMed] [Google Scholar]
- 131.Lee MY, Fevold KL, Dorshkind K, et al. In vivo and in vitro suppression of primary B lymphocytopoiesis by tumor-derived and recombinant granulocyte colony-stimulating factor. Blood.1993;82:2062–2068. [PubMed] [Google Scholar]
- 132.Semerad CL, Christopher MJ, Liu F, et al. G-CSF potently inhibits osteoblast activity and CXCL12 mRNA expression in the bone marrow. Blood.2005;106:3020–3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Day RB, Bhattacharya D, Nagasawa T, Link DC. Granulocyte colony-stimulating factor reprograms bone marrow stromal cells to actively suppress B lymphopoiesis in mice. Blood.2015;125:3114–3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Shono Y, Ueha S, Wang Y, et al. Bone marrow graft-versus-host disease: early destruction of hematopoietic niche after MHC-mismatched hematopoietic stem cell transplantation. Blood.2010;115:5401–5411. [DOI] [PubMed] [Google Scholar]
- 135.Maltby S, Lochrin AJ, Bartlett B, et al. Osteoblasts Are Rapidly Ablated by Virus-Induced Systemic Inflammation following Lymphocytic Choriomeningitis Virus or Pneumonia Virus of Mice Infection in Mice. J Immunol.2018;200:632–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Manz MG, Boettcher S. Emergency granulopoiesis. Nat Rev Immunol.2014;14:302–314. [DOI] [PubMed] [Google Scholar]
- 137.Yoffey JM, Hudson G, Osmond DG. The lymphocyte in guinea-pig bone marrow. J Anat.1965;99:841–860. [PMC free article] [PubMed] [Google Scholar]
- 138.Hudson G, Yoffey JM. The passage of lymphocytes through the sinusoidal endothelium of guinea-pig bone arrow. Proc R Soc Lond B Biol Sci.1966;165:486–496. [DOI] [PubMed] [Google Scholar]
- 139.Hudson G, Yoffey JM. Ultrastructure of reticuloendothelial elements in guinea-pig bone marrow. J Anat.1968;103:515–525. [PMC free article] [PubMed] [Google Scholar]
- 140.Cyster JG, Schwab SR. Sphingosine-1-Phosphate and Lymphocyte Egress from Lymphoid Organs. Annual review of immunology.2011;30:69–94. [DOI] [PubMed] [Google Scholar]
- 141.Matloubian M, Lo CG, Cinamon G, et al. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature.2004;427:355–360. [DOI] [PubMed] [Google Scholar]
- 142.Pappu R, Schwab SR, Cornelissen I, et al. Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate Science (New York, NY: 2007;316:295–298. [DOI] [PubMed] [Google Scholar]
- 143.Schwab SR, Cyster JG. Finding a way out: lymphocyte egress from lymphoid organs. Nature immunology.2007;8:1295–1301. [DOI] [PubMed] [Google Scholar]
- 144.Schwab SR, Pereira JP, Matloubian M, et al. Lymphocyte sequestration through S1P lyase inhibition and disruption of S1P gradients. Science (New York, NY: 2005;309:1735–1739. [DOI] [PubMed] [Google Scholar]
- 145.Lo CG, Xu Y, Proia RL, Cyster JG. Cyclical modulation of sphingosine-1-phosphate receptor 1 surface expression during lymphocyte recirculation and relationship to lymphoid organ transit. The Journal of experimental medicine.2005;201:291–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Pereira JP, Cyster JG, Xu Y. A role for S1P and S1P1 in immature-B cell egress from mouse bone marrow. PLoS One.2010;5:e9277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Jenne CN, Enders A, Rivera R, et al. T-bet-dependent S1P5 expression in NK cells promotes egress from lymph nodes and bone marrow. The Journal of experimental medicine.2009;206:2469–2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Pham TH, Okada T, Matloubian M, Lo CG, Cyster JG. S1P1 receptor signaling overrides retention mediated by G alpha i-coupled receptors to promote T cell egress. Immunity.2008;28:122–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Allende ML, Tuymetova G, Lee BG, et al. S1P1 receptor directs the release of immature B cells from bone marrow into blood. The Journal of experimental medicine.2010;207:1113–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Serbina NV, Pamer EG. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nature immunology.2006;7:311–317. [DOI] [PubMed] [Google Scholar]
- 151.Bernardini G, Sciume G, Bosisio D, et al. CCL3 and CXCL12 regulate trafficking of mouse bone marrow NK cell subsets. Blood.2008;111:3626–3634. [DOI] [PubMed] [Google Scholar]
- 152.Eash KJ, Greenbaum AM, Gopalan PK, Link DC. CXCR2 and CXCR4 antagonistically regulate neutrophil trafficking from murine bone marrow. J Clin Invest.2010;120:2423–2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Sugita K, Kabashima K, Sakabe J, et al. FTY720 regulates bone marrow egress of eosinophils and modulates late-phase skin reaction in mice. Am J Pathol.2010;177:1881–1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Shi C, Jia T, Mendez-Ferrer S, et al. Bone Marrow Mesenchymal Stem and Progenitor Cells Induce Monocyte Emigration in Response to Circulating Toll-like Receptor Ligands. Immunity.2011;34:590–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Jung H, Mithal DS, Park JE, Miller RJ. Localized CCR2 Activation in the Bone Marrow Niche Mobilizes Monocytes by Desensitizing CXCR4. PLoS One.2015;10:e0128387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Chong SZ, Evrard M, Devi S, et al. CXCR4 identifies transitional bone marrow premonocytes that replenish the mature monocyte pool for peripheral responses. The Journal of experimental medicine.2016;213:2293–2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sandel PC, Monroe JG. Negative selection of immature B cells by receptor editing or deletion is determined by site of antigen encounter. Immunity.1999;10:289–299. [DOI] [PubMed] [Google Scholar]
- 158.Ota T, Ota M, Duong BH, Gavin AL, Nemazee D. Liver-expressed Ig{kappa} superantigen induces tolerance of polyclonal B cells by clonal deletion not {kappa} to {lambda} receptor editing. The Journal of experimental medicine.2011;208:617–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Rowland SL, Tuttle K, Torres RM, Pelanda R. Antigen and cytokine receptor signals guide the development of the naive mature B cell repertoire. Immunol Res.2013;55:231–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Gorlin RJ, Gelb B, Diaz GA, et al. WHIM syndrome, an autosomal dominant disorder: clinical, hematological, and molecular studies. Am J Med Genet.2000;91:368–376. [PubMed] [Google Scholar]
- 161.Hernandez PA, Gorlin RJ, Lukens JN, et al. Mutations in the chemokine receptor gene CXCR4 are associated with WHIM syndrome, a combined immunodeficiency disease. Nat Genet.2003;34:70–74. [DOI] [PubMed] [Google Scholar]
- 162.Balabanian K, Lagane B, Pablos JL, et al. WHIM syndromes with different genetic anomalies are accounted for by impaired CXCR4 desensitization to CXCL12. Blood.2005;105:2449–2457. [DOI] [PubMed] [Google Scholar]
- 163.Balabanian K, Brotin E, Biajoux V, et al. Proper desensitization of CXCR4 is required for lymphocyte development and peripheral compartmentalization in mice. Blood.2012;119:5722–5730. [DOI] [PubMed] [Google Scholar]
- 164.Mendez-Ferrer S, Lucas D, Battista M, Frenette PS. Haematopoietic stem cell release is regulated by circadian oscillations. Nature.2008;452:442–447. [DOI] [PubMed] [Google Scholar]
- 165.Scheiermann C, Kunisaki Y, Lucas D, et al. Adrenergic Nerves Govern Circadian Leukocyte Recruitment to Tissues. Immunity.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Golan K, Kumari A, Kollet O, et al. Daily Onset of Light and Darkness Differentially Controls Hematopoietic Stem Cell Differentiation and Maintenance. Cell Stem Cell.2018;23:572–585 e577. [DOI] [PubMed] [Google Scholar]
- 167.Soriano SF, Hernanz-Falcon P, Rodriguez-Frade JM, et al. Functional inactivation of CXC chemokine receptor 4-mediated responses through SOCS3 up-regulation. The Journal of experimental medicine.2002;196:311–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Le Y, Zhu BM, Harley B, et al. SOCS3 protein developmentally regulates the chemokine receptor CXCR4-FAK signaling pathway during B lymphopoiesis. Immunity.2007;27:811–823. [DOI] [PubMed] [Google Scholar]
- 169.Donovan EE, Pelanda R, Torres RM. S1P3 confers differential S1P-induced migration by autoreactive and non-autoreactive immature B cells and is required for normal B-cell development. Eur J Immunol.2010;40:688–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Chan LN, Chen Z, Braas D, et al. Metabolic gatekeeper function of B-lymphoid transcription factors. Nature.2017;542:479–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Noda M, Omatsu Y, Sugiyama T, et al. CXCL12-CXCR4 chemokine signaling is essential for NK-cell development in adult mice. Blood.2011;117:451–458. [DOI] [PubMed] [Google Scholar]
- 172.Yu VW, Saez B, Cook C, et al. Specific bone cells produce DLL4 to generate thymus-seeding progenitors from bone marrow. The Journal of experimental medicine.2015;212:759–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Fujisaki J, Wu J, Carlson AL, et al. In vivo imaging of T reg cells providing immune privilege to the haematopoietic stem-cell niche. Nature.2011;474:216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Zaretsky AG, Konradt C, Dépis F, et al. T regulatory cells support plasma cell populations in the bone marrow. Cell reports.2017;18:1906–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Sercan Alp Ö, Durlanik S, Schulz D, et al. Memory CD8+ T cells colocalize with IL‐7+ stromal cells in bone marrow and rest in terms of proliferation and transcription. European journal of immunology.2015;45:975–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Mazo IB, Honczarenko M, Leung H, et al. Bone marrow is a major reservoir and site of recruitment for central memory CD8+ T cells. Immunity.2005;22:259–270. [DOI] [PubMed] [Google Scholar]
- 177.Becker TC, Coley SM, Wherry EJ, Ahmed R. Bone marrow is a preferred site for homeostatic proliferation of memory CD8 T cells. The Journal of Immunology.2005;174:1269–1273. [DOI] [PubMed] [Google Scholar]
- 178.Herndler-Brandstetter D, Landgraf K, Jenewein B, et al. Human bone marrow hosts polyfunctional memory CD4+ and CD8+ T cells with close contact to IL-15–producing cells. The Journal of Immunology.2011:1100243. [DOI] [PubMed] [Google Scholar]
- 179.Tokoyoda K, Zehentmeier S, Hegazy AN, et al. Professional memory CD4+ T lymphocytes preferentially reside and rest in the bone marrow. Immunity.2009;30:721–730. [DOI] [PubMed] [Google Scholar]
- 180.Okhrimenko A, Grün JR, Westendorf K, et al. Human memory T cells from the bone marrow are resting and maintain long-lasting systemic memory. Proceedings of the National Academy of Sciences.2014:201318731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Manz RA, Thiel A, Radbruch A. Lifetime of plasma cells in the bone marrow. Nature.1997;388:133–134. [DOI] [PubMed] [Google Scholar]
- 182.Slifka MK, Antia R, Whitmire JK, Ahmed R. Humoral immunity due to long-lived plasma cells. Immunity.1998;8:363–372. [DOI] [PubMed] [Google Scholar]
- 183.Hargreaves DC, Hyman PL, Lu TT, et al. A coordinated change in chemokine responsiveness guides plasma cell movements. The Journal of experimental medicine.2001;194:45–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Zehentmeier S, Roth K, Cseresnyes Z, et al. Static and dynamic components synergize to form a stable survival niche for bone marrow plasma cells. Eur J Immunol.2014;44:2306–2317. [DOI] [PubMed] [Google Scholar]
- 185.Pitt LA, Tikhonova AN, Hu H, et al. CXCL12-Producing Vascular Endothelial Niches Control Acute T Cell Leukemia Maintenance. Cancer cell.2015;27:755–768. [DOI] [PMC free article] [PubMed] [Google Scholar]