Abstract
Background
A dacryocystorhinostomy (DCR) procedure aims to restore drainage of tears by bypassing a blockage in the nasolacrimal duct, through the creation of a bony ostium that allows communication between the lacrimal sac and the nasal cavity. It can be performed using endonasal or external approaches. The comparative success rates of these two approaches have not yet been established and this review aims to evaluate the relevant up‐to‐date research.
Objectives
The primary aim of this review is to compare the success rates of endonasal DCR with that of external DCR. The secondary aim is to compare the complication rates between the two procedures.
Search methods
We searched CENTRAL (which contains the Cochrane Eyes and Vision Group Trials Register) (2016, Issue 8), Ovid MEDLINE, Ovid MEDLINE In‐Process and Other Non‐Indexed Citations, Ovid MEDLINE Daily, Ovid OLDMEDLINE (January 1946 to 22 August 2016), Embase (January 1980 to 22 August 2016), Latin American and Caribbean Health Sciences Literature Database (LILACS) (January 1982 to 22 August 2016), Web of Science Conference Proceedings Citation Index‐ Science (CPCI‐S) (January 1990 to 22 August 2016), the ISRCTN registry (www.isrctn.com/editAdvancedSearch), ClinicalTrials.gov (www.clinicaltrials.gov) and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en). We did not use any date or language restrictions in the electronic searches for trials. We last searched the electronic databases on 22 August 2016. We requested or examined relevant conference proceedings for appropriate trials.
Selection criteria
We included all randomised controlled trials (RCTs) comparing endonasal and external DCRs.
Data collection and analysis
Two review authors independently assessed studies for eligibility and extracted data on reported outcomes. We attempted to contact investigators to clarify the methodological quality of the studies. We graded the certainty of the evidence using GRADE.
Main results
We included two trials in this review. One trial from Finland compared laser‐assisted endonasal DCR with external DCR, and one trial from India compared mechanical endonasal DCR (using punch forceps) with external DCR. The trials were poorly reported and it was difficult to judge the extent to which bias had been avoided.
Anatomic success was defined as the demonstration of a patent lacrimal passage on syringing, or endoscopic visualisation of fluorescein dye at the nasal opening of the anastomoses after a period of at least six months following surgery. Subjective success was defined as the resolution of symptoms of watering following surgery after a period of at least six months. Both included trials used anatomic patency demonstrated by irrigation as a measure of anatomic success. Different effects were seen in these two trials (I2 = 76%). People receiving laser‐assisted endonasal DCR were less likely to have a successful operation compared with external DCR (63% versus 91%; risk ratio (RR) 0.69, 95% confidence intervals (CI) 0.52 to 0.92; 64 participants). There was little or no difference in success comparing mechanical endonasal DCR and external DCR (90% in both groups; RR 1.00, CI 0.81 to 1.23; 40 participants). We judged this evidence on success to be very low‐certainty, downgrading for risk of bias, imprecision and inconsistency. The trial from Finland also assessed subjective improvement in symptoms following surgery. Resolution of symptoms of watering in outdoor conditions was reported by 84% of the participants in the external DCR group and 59% of those in the laser‐assisted endonasal DCR group (RR 0.70, CI 0.51 to 0.97; 64 participants, low‐certainty evidence).
There were no cases of intraoperative bleeding in any participant in the trial that compared laser‐assisted endonasal DCR to external DCR. This was in contrast to the trial comparing mechanical endonasal DCR to external DCR in which 45% of participants in both groups experienced intraoperative bleeding (RR 1.00, 95% CI 0.50 to 1.98; 40 participants). We judged this evidence on intraoperative bleeding to be very low‐certainty, downgrading for risk of bias, imprecision and inconsistency.
There were only two cases of postoperative bleeding, both in the external DCR group (RR 0.33, 95% CI 0.04 to 3.10; participants = 104; studies = 2). There were only two cases of wound infection/gaping, again both in the external DCR group (RR 0.20, CI 0.01 to 3.92; participants = 40; studies = 1). We judged this evidence on complications to be very low‐certainty, downgrading one level for risk of bias and two levels for imprecision due to the very low number of cases.
Authors' conclusions
There is uncertainty as to the relative effects of endonasal and external DCR. Differences in effect seen in the two trials included in this review may be due to variations in the endonasal technique, but may also be due to other differences between the trials. Future larger RCTs are required to further assess the success and complication rates of endonasal and external DCR. Different techniques of endonasal DCR should also be assessed, as the choice of endonasal technique can influence the outcome. Strict outcome criteria should be adopted to assess functional and anatomical outcomes with a minimal follow‐up of six months.
Plain language summary
Different surgical techniques for treating blockage of the tear duct
What is the aim of this review? The aim of this Cochrane Review was to compare two different surgical techniques for treating blockage of the tear (nasolacrimal) duct. Cochrane researchers collected and analysed all relevant studies to answer this question and found two studies.
Key messages It is unclear whether or not endonasal dacryocystorhinostomy (DCR) is a better way of treating tear duct obstruction than external DCR (very low‐certainty evidence), nor is it clear whether endonasal DCR reduces the chance of complications such as bleeding or wound infection (very low‐certainty evidence).
What was studied in the review? The tear duct, or nasolacrimal passage, allows excess tears to drain away from the eye. If the tear duct gets blocked then the eye can water too much. Doctors can use a surgical procedure known as dacryocystorhinostomy (DCR) to treat the blocked tear duct. This operation creates a way for the tears to drain from the eye that bypasses the blockage. There are two ways of doing this operation: either by making a cut on the outside of the nose (external DCR); or by operating inside the nose, using an endoscope (a flexible tube with a light at the end) to see inside the nose (endonasal DCR) and creating an alternate drainage pathway using instruments (such as forceps or drill) or laser.
What are the main results of the review? The review authors found two relevant studies. One study was from Finland and compared laser‐assisted endonasal DCR with external DCR. One study was from India and compared mechanical endonasal DCR (using punch forceps) with external DCR.
The Cochrane researchers are uncertain whether endonasal DCR increases the chance of success compared with external DCR, or whether endonasal DCR reduces the chance of complications such as bleeding or wound infection. They judged the certainty of the evidence to be very low.
How up‐to‐date is this review? The Cochrane researchers searched for studies that had been published up to 22 August 2016.
Summary of findings
for the main comparison.
| Endonasal dacryocystorhinostomy (DCR) compared with external DCR for nasolacrimal duct obstruction | ||||||
|
Patient or population: People with nasolacrimal duct obstruction Settings: Hospital Intervention: Endonasal DCR Comparison: External DCR | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of Participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk1 | Corresponding risk | |||||
| External DCR | Endonasal DCR | |||||
|
Anatomic success (i.e. patent lacrimal passage after a period of at least six months after operation) |
900 per 1000 | Laser‐assisted endonasal DCR | ⊕⊝⊝⊝ VERY LOW2,3,4 | |||
| 621 per 1000 (468 to 828) | RR 0.69 (0.52 to 0.92) | 64 (1) |
||||
| Mechanical endonasal DCR | ||||||
| 900 per 1000 (729 to 1000) | RR 1.00 (0.81 to 1.23) | 40 (1) | ||||
|
Subjective success (i.e. resolution of symptoms of watering following surgery) |
840 per 1000 | Laser‐assisted endonasal DCR5 | ⊕⊕⊝⊝ LOW2,3 | |||
| 588 per 1000 (428 to 815) | RR 0.70 (0.51 to 0.97) | 64 (1) |
||||
| Intraoperative bleeding | 170 per 1000 | Laser‐assisted endonasal DCR | ⊕⊝⊝⊝ VERY LOW2,3,6 | No cases of intraoperative bleeding reported in trial of laser‐assisted endonasal DCR | ||
| Not estimable | Not estimable | 64 (1) |
||||
| Mechanical endonasal DCR | ||||||
| 170 per 1000 (85 to 337) | RR 1.00 (0.50 to 1.98) | 40 (1) |
||||
| Postoperative bleeding | 40 per 1000 | 13 per 1000 (2 to 124) | RR 0.33 (0.04 to 3.10) | 104 (2) |
⊕⊝⊝⊝ VERY LOW2,7 | |
| Wound infection/gaping | 40 per 1000 | Laser‐assisted endonasal DCR | ⊕⊝⊝⊝ VERY LOW2,7 | No cases of wound infection/gaping reported in trial of laser‐assisted endonasal DCR | ||
| Not estimable | Not estimable | 64 (1) |
||||
| Mechanical endonasal DCR | ||||||
| 8 per 1000 (0 to 157) | RR 0.20 (0.01 to 3.92) | 40 (1) |
||||
| CI: confidence interval; DCR: dacryocystorhinostomy; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High‐certainty: Further research is very unlikely to change our confidence in the estimate of effect. Moderate‐certainty: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low‐certainty: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low‐certainty: We are very uncertain about the estimate. | ||||||
1The assumed control risk was estimated from the control group in the included studies. 2We downgraded one level for study limitations because the methods used for random sequence generation, allocation concealment and masking were not clearly described. 3We downgraded one level for imprecision because the number of participants enrolled in these trials was low and the estimate of effect was imprecise. 4We downgraded one level for inconsistency as there was clinical and statistical heterogeneity in the two trials (test for interaction P = 0.04). 5Subjective success was not reported in the trial of mechanical endonasal DCR (Moras 2011). 6We downgraded one level for inconsistency as there was clinical heterogeneity in the two trials. There were no cases of intraoperative haemorrhage in the trial of laser‐assisted endonasal DCR. 7We downgraded two levels for imprecision as there were only two events recorded, both in the external DCR group.
Background
Description of the condition
Tears from the conjunctival sac pass through the lacrimal puncta in the upper and lower lids to the upper and lower lacrimal canaliculi, then to the common canaliculi to empty into the lacrimal sac, located in the lacrimal fossa. From the lacrimal sac, tears pass to the nasolacrimal duct along the lateral wall of the nose to open at the inferior meatus. Obstruction anywhere along this course can result in excessive watering from the eyes as well as recurrent infections. In a retrospective study of 150 patients who underwent external DCR for nasolacrimal duct obstruction (Tarbet 1995), it was found that the most common presenting symptoms included excessive watering (86%), and either acute or chronic infections (about 30%). The prevalence of nasolacrimal duct obstruction was also found to increase with age and to have a female preponderance.
Description of the intervention
Nasolacrimal duct obstruction is treated by dacryocystorhinostomy (DCR). This is a surgical technique that involves the creation of an alternative route for drainage of tears, between the lacrimal sac and nasal cavity, bypassing the nasolacrimal duct. This can be done either by an external approach (external DCR) or through the nasal cavity using an endoscope or a microscope (endonasal DCR). Functional nasolacrimal duct obstruction is due to poor functioning of the lacrimal pump mechanism. Treatment with DCR in this situation can give variable results.
External DCR
The ophthalmologist generally performs this procedure. The technique was originally described in 1904 (Toti 1904), and was later modified by the addition of suturing of the mucosal flaps (Dupuy‐Dutemps 1921), thus forming an epithelium‐lined fistula. Several case series have estimated the success rate of external DCR to be between 70% and 95% (Ben Simon 2005; Cokkeser 2000; Dolman 2003; Tarbet 1995; Tsirbas 2004; Yigit 2007).
Endonasal DCR
The endonasal approach was introduced in 1893 by Caldwell (Caldwell 1893), and modified by West (West 1910), and later, Halle (Halle 1914). The approach failed to gain popularity due to poor access to the nasal cavity. With the advent of the nasal endoscope (Stammberger 1986), and functional endoscopic sinus surgery in the early 1990s (Kennedy 1985), there was renewed interest in endonasal DCR. McDonogh 1989 introduced endonasal DCR in its present form. The reported success rate for endonasal DCR ranges from 63% to 96% (Ben Simon 2005; Hartikainen 1998; Sham 2000; Yuce 2013). Endonasal DCR can also be performed by using a microscope or by direct visualisation using a surgical loupe that magnifies the surgical field. Various techniques, such as bone drills and lasers to vaporise bone have been used to create the functioning passage from the lacrimal sac into the nasal cavity in the endonasal procedure. Argon blue green; potassium titanyl phosphate; carbon dioxide; holmium: yttrium‐aluminium‐garnet (YAG); neodymium: YAG; and combined carbon dioxide neodymium (CO²‐Nd): YAG are some examples of the lasers used (Boush 1994; Kong 1994; Metson 1994; Muellner 2000; Pearlman 1997; Reifler 1993; Seppa 1994; Woog 1993). The outcome of endonasal DCR can vary depending on the method employed to create the ostium (Huang 2014;Maini 2007).
Endonasal versus external DCR
The advantages of endonasal DCR over external DCR are: limited invasiveness, less intraoperative bleeding, shorter operative time and preservation of pump function of the orbicularis oculi muscle. Absence of an external scar, minimal morbidity and low complication rate have made endonasal DCR popular (a newer technique of external DCR has recently been described, whereby a periciliary incision is made, avoiding the external cutaneous scar of conventional external DCR (Ng 2015)). The disadvantages of endonasal DCR include: a relatively smaller opening between the lacrimal sac and nasal cavity, higher recurrence rate, high equipment cost, and the fact that it is a more difficult procedure to learn. The relatively smaller opening, steeper learning curve and technique used to create the opening may affect the success rates.
Intraoperative factors
A few intraoperative factors are thought to influence the outcome of the surgery. For example, augmentation with antimitotic agents (5‐Fluorouracil (5‐FU) or mitomycin C (MMC)) has been reported in a few cohort studies. Henson 2007 reported a success rate of 87.5% in their non‐comparative study investigating the use of endocanalicular DCR employing diode laser and MMC. Watts 2001 found external DCR gave better surgical outcomes (95%) compared with 5‐FU augmented Holmium‐YAG laser‐assisted endonasal DCR (64%). Roozitalab 2004 found no benefit to using intraoperative MMC in external DCR in their comparative study; success in the group randomised to MMC was 90.5% compared to 92.4% in the group randomised to no MMC. A controlled study by Qin 2010a yielded a significantly higher rate of success with the use of MMC in endoscopic endonasal DCR using nasolacrimal duct stent placement (95.2% in treatment group versus 85.8% in control group).
The use of silicone intubation of the lacrimal passages is another option available to ophthalmologists performing DCR. However, there appears to be no evidence in support of this practice. Studies that compared the effectiveness of DCR with and without silicone intubation did not find any advantage to intubation (Saiju 2009; Smirnov 2006; Unlu 2009). The added cost and follow‐up for the patient can be avoided by omitting this step. Chen 2009 described a new technique of recanalisation of nasolacrimal duct obstruction using a lacrimal canaliser. The authors claim this technique to be simple, non‐invasive and with comparable success rates and adverse events to external DCR.
Other adjunctive measures to improve the success of DCR include concomitant endonasal procedures such as middle turbinectomy/endonasal mechanical enlargement of the neo‐ostium, as well as the suturing of mucosal flaps.
How the intervention might work
DCR creates an alternate passage between the lacrimal sac and nasal cavity, thus bypassing the obstruction along the nasolacrimal duct. This helps the tears to drain away from the eye.
Why it is important to do this review
There is no clear consensus on the choice of surgery type for treatment of nasolacrimal duct obstruction.
Objectives
The primary aim of this review is to compare the success rates of endonasal DCR with that of external DCR. The secondary aim is to compare the complication rates between the two procedures.
Methods
Criteria for considering studies for this review
Types of studies
We included all randomised controlled trials (RCTs) which compared the success rates of endonasal DCR with that of external DCR.
Types of participants
We included participants of all age groups, diagnosed with primary post‐canalicular obstruction of the lacrimal passages. We excluded studies that included participants who had previous surgical procedures to the lacrimal apparatus.
Types of interventions
We compared endonasal dacryocystorhinostomy (DCR) using a drill, curette or laser to create a communication between the lacrimal sac and nasal cavity, with external DCR using the standard technique. We included studies with a follow‐up period of six months to two years.
Types of outcome measures
Primary outcomes
Anatomic success (defined as patent lacrimal passage on syringing, or endoscopic visualisation of fluorescein dye at the nasal opening of the anastomoses, after a period of at least six months following surgery).
Subjective success (resolution of symptoms of watering following surgery, after a period of at least six months).
We considered recurrence of dacryocystitis as failure.
Secondary outcomes
We collected and collated data on the following adverse events for the two types of interventions.
Intraoperative bleeding requiring intervention.
Postoperatve bleeding requiring intervention (within seven days of surgery).
Wound infection/gaping.
Search methods for identification of studies
Electronic searches
We searched CENTRAL (which contains the Cochrane Eyes and Vision Group Trials Register) (2016, Issue 8), Ovid MEDLINE, Ovid MEDLINE In‐Process and Other Non‐Indexed Citations, Ovid MEDLINE Daily, Ovid OLDMEDLINE (January 1946 to 22 August 2016), Embase (January 1980 to 22 August 2016), Latin American and Caribbean Health Sciences Literature Database (LILACS) (January 1982 to 22 August 2016), Web of Science Conference Proceedings Citation Index‐ Science (CPCI‐S) (January 1990 to 22 August 2016), the ISRCTN registry (www.isrctn.com/editAdvancedSearch), ClinicalTrials.gov (www.clinicaltrials.gov) and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en). We did not use any date or language restrictions in the electronic searches for trials. We last searched the electronic databases on 22 August 2016.
See: Appendices for details of search strategies for CENTRAL (Appendix 1), MEDLINE (Appendix 2), Embase (Appendix 3), LILACS (Appendix 4), CPCI‐S (Appendix 5), ISRCTN (Appendix 6), ClinicalTrials.gov (Appendix 7) and the ICTRP (Appendix 8).
Searching other resources
We searched the proceedings of the Association for Research in Otolaryngology (ARO), available from 1992 to 2008; there were no relevant abstracts. Abstracts from other conferences, including the British Oculoplastic Surgery Society (BOPSS), the European Society of Ophthalmic Plastic and Reconstructive Surgery (ESOPRS) and the American Society of Ophthalmic Plastic and Reconstructive Surgery (ASOPRS) were not available for searching.
Data collection and analysis
We followed the review protocol, published in 2008 (Anijeet 2008), to select trials for this update.
Selection of studies
For the 2017 update, two review authors (LJ and DA) independently screened the titles and abstracts retrieved by the searches to establish whether they met the criteria as defined in the section, 'Criteria for considering studies for this review'. The two review authors resolved any disagreement by discussion and obtained the full‐text copies of definitely or potentially relevant studies.
Data extraction and management
For this update, two review authors (LJ and DA) independently assessed studies for eligibility and extracted data into Review Manager 5 on reported outcomes (RevMan 2014). Disagreements were resolved by discussion among review authors. We attempted to contact trial authors of the included studies if any clarification of study details were required.
We extracted the following data from the included studies.
Trial characteristics: design, randomisation, allocation concealment, masking (blinding).
Interventions: standard procedures for endonasal DCR and external DCR.
Outcomes: success rates as described in the primary outcomes, intraoperative and postoperative complications (intraoperative and postoperative bleeding; wound infection or wound dehiscence).
Assessment of risk of bias in included studies
Two review authors (LJ and DA) independently assessed study quality according to the guidelines in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). The quality parameters we assessed were allocation concealment, method of allocation, completeness of follow‐up, masking of participants and outcome assessors, and documentation of complications. The review authors were not masked to the study authors or results of the study. We graded these parameters as 'low' risk of bias, 'high' risk of bias or 'unclear'. The review authors made attempts to contact study authors to clarify data.
Measures of treatment effect
We summarised data from studies collecting similar outcome measures and with similar follow‐up times (minimum of six months). We summarised the outcome data using risk ratios (RRs).
Unit of analysis issues
We attempted to contact the authors of the included studies to clarify possible unit of analysis issues (method of randomisation/allocation sequence concealment/limiting performance or detection bias), but were unsuccessful.
Dealing with missing data
We attempted to contact the trial authors to try and retrieve relevant data. If we were unable to retrieve the data, we included the study, but designated it as a study with missing data when discussing the results.
Assessment of heterogeneity
We formally assessed heterogeneity using Chi2 test. We also calculated the I2 statistic, which describes the percentage of the variability in effect estimates that is due to heterogeneity rather than chance, and interpreted it as follows: 0% to 40% ‐ might not be important; 30% to 60% ‐ may represent moderate heterogeneity; 50% to 90% ‐ may represent substantial heterogeneity; 75% to 100% ‐ considerable heterogeneity.
Assessment of reporting biases
As only two trials were included, we did not construct a funnel plot.
Data synthesis
We pooled data from the individual studies using a fixed‐effect model. We used the fixed‐effect model (Mantel‐Haenszel) to determine RRs of dichotomous data from the included study. We chose the Mantel‐Haenszel model because of the low event rates and small trial sizes.
Subgroup analysis and investigation of heterogeneity
We conducted one subgroup analysis which was specified in our protocol (Anijeet 2008). We compared external DCR and endonasal DCR using either the laser or mechanical technique.The I2 statistic for subgroup differences was 76% (considerable heterogeneity).
Sensitivity analysis
In our protocol we planned to conduct a sensitivity analysis to assess the consequence of including missing data, or data with ambiguous results, or data analysed using different statistical methods. However, we did not conduct any sensitivity analyses because of the low number of included trials.
'Summary of findings' tables
We prepared a 'Summary of findings' table, presenting relative and absolute risks. The following outcomes were included in the table: anatomic success, subjective success, intraoperative bleeding, postoperative bleeding, and wound infection/gaping. Using GRADEpro software (GRADEpro GDT 2014), two review authors independently graded the overall certainty of the evidence for each outcome by applying the GRADE classification (Atkins 2004). GRADE includes consideration of study limitations, imprecision, inconsistency, indirectness and publication bias. We used the following four grades.
High‐certainty: Further research is very unlikely to change our confidence in the estimate of effect. Moderate‐certainty: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low‐certainty: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low‐certainty: We are very uncertain about the estimate.
Results
Description of studies
Results of the search
The electronic searches from December 2010 identified 881 references. The Cochrane Information Specialist scanned the search results and removed any references which were not relevant to the scope of the review. Two review authors independently reviewed the remaining references and obtained the full‐text copies of four studies. We included one randomised controlled trial (RCT) that met the inclusion criteria of the review and excluded three studies.
In an updated search, run in August 2016, we identified 898 new records (Figure 1). The Cochrane Information Specialist removed 186 duplicate records, screened the remaining 712 records and removed 582 references that were not relevant to the review. We screened the remaining 130 references and discarded 123 reports, as they were not relevant. We obtained seven full‐text reports for further assessment. We included one new study, Moras 2011, and excluded the following four studies: Balikoglu‐Yilmaz 2015; Derya 2013; Tang 2015; Taskin 2011 as they did not meet the inclusion criteria, see 'Characteristics of excluded studies' table for details. In the previous version of the review (Anijeet 2011) , a Chinese study by Qin 2010 was awaiting assessment. Having reviewed the abstract, it was agreed that this study was found to pertain to the effects of using mitomycin C in endonasal DCR, and was therefore removed from the shortlisted articles. In this update, we identified two studies (Cui 2013; Zhou 2015) for which we required further clarification regarding the methods. We have therefore placed these studies in the Characteristics of studies awaiting classification section. For Cui 2013, we are unsure of the methods of randomisation and for Zhou 2015, we would like details of how the participants were randomised as this information is missing from the full‐text report. We attempted to contact the authors, but to date have not received responses for these two trials.
1.
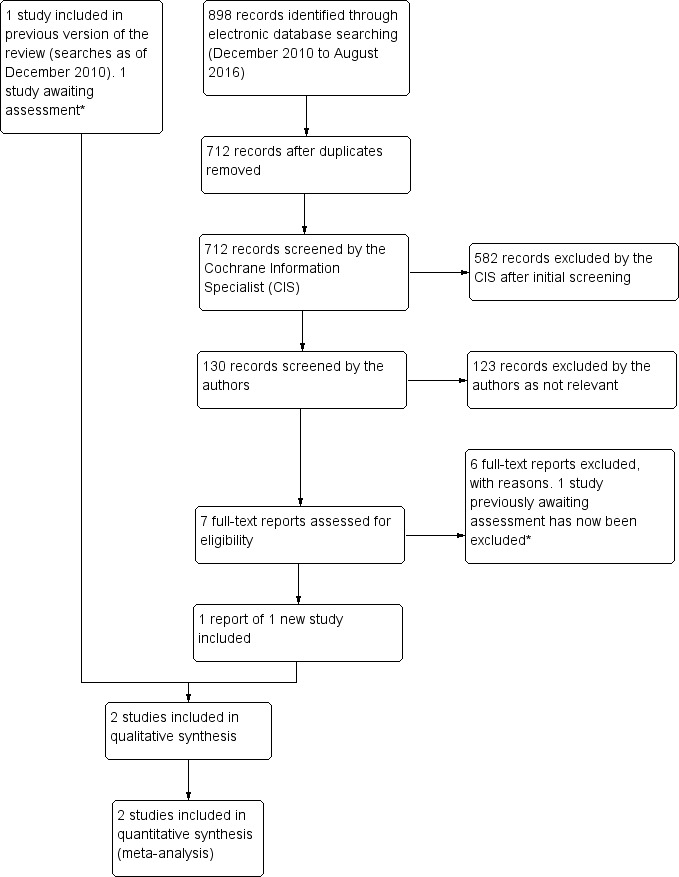
Study flow diagram.
Included studies
See: 'Characteristics of included studies' tables.
We included two studies that met our inclusion criteria (Hartikainen 1998; Moras 2011).
Design
Both studies were RCTs.
Sample sizes
Hartikainen 1998 randomised participants to laser‐assisted endonasal dacryocystorhinostomy (DCR) (32 procedures) or external DCR (32 procedures). Moras 2011 randomised 40 participants to be treated either by mechanical endonasal DCR) (20 procedures) or EXT DCR (20 procedures).
Setting
Hartikainen 1998 was conducted in a single unit in Finland. Moras 2011 was conducted in India.
Participants
In Hartikainen 1998, the mean age of the study group was 65 years (range 23 to 89). The male to female ratio was 1:5.
Moras 2011 identified 40 participants with primary acquired nasolacrimal duct obstruction or chronic dacryocystitis who presented to the ophthalmology and Ear, Nose and Throat (ENT) outpatient departments of Fr. Muller Medical College, Mangalore, India. Eighty per cent of the participants were female, and the age of the participants ranged from 16 to 68 years, with the majority (62.5%) being in the 30 to 50 age group.
Interventions
Hartikainen 1998 Endonasal laser‐assisted DCR was compared to external DCR. The external DCR was performed by an ophthalmologist and the endonasal DCR was performed by an otolaryngologist and ophthalmologist. The endonasal DCR used a continuous wave CO2‐Nd: YAG combined laser to fashion the bony ostium and nasal mucosal opening.
Moras 2011 Endonasal mechanical DCR was compared to external DCR. The endonasal DCR was performed using punch forceps to create the ostium in the lacrimal bone.
Outcomes
The outcomes in Hartikainen 1998 were patent lacrimal passage on irrigation at one year (anatomic success), and patient‐reported symptomatic improvement.
The outcome in Moras 2011 was patent lacrimal drainage system on sac syringing at the end of six months.
Excluded studies
We excluded eight studies in total, and details (including reason for exclusion) can be found in the 'Characteristics of excluded studies' tables.
Risk of bias in included studies
2.
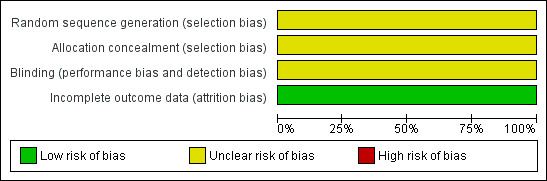
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Overall, the risk of bias for the two included studies was unclear. We could not contact the corresponding authors for the studies, and therefore we could not resolve unit of analysis issues, and errors may have been introduced.
Allocation
We assessed allocation concealment and the method of randomisation as unclear; no further information was available.
Blinding
Masking (blinding) with respect to the type of operation was not possible with the surgeons and there was no description of masking of either the participants or outcome assessors in either trial. As no further information was available, we assessed the risk of bias as unclear.
Incomplete outcome data
Outcome data for all three parameters ‐ success, intraoperative and postoperative bleeding appeared to be complete in the trials.
Effects of interventions
See: Table 1
Endonasal dacryocystorhinostomy (DCR) versus external DCR
Primary outcomes
Anatomic success
In the two included trials, 104 procedures were performed; 52 each of endonasal dacryocystorhinostomy (DCR) and external DCR.
In Hartikainen 1998, the anatomic success rates in the external DCR group were 100% (32/32) at six months and 91% (29/32) at 12 months postoperatively. In the laser‐assisted endonasal DCR group, they were 78% (25/32) at six months and 63% (20/32) at 12 months postoperatively. The risk ratio (RR) for the 12‐month anatomic success rates for external DCR versus laser‐assisted endonasal DCR was 0.69, 95% confidence interval (CI) 0.52 to 0.92, 64 participants, 1 study.
In Moras 2011, the success rate six months after the operation was 90% (18 out of 20 procedures) for both types of DCR: RR 1.00, 95% CI 0.81 to 1.23, 40 participants, 1 study (data for 12‐month follow‐up were not available, as in Hartikainen 1998).
We judged this evidence on success to be very low‐certainty, downgrading for risk of bias, imprecision and inconsistency (Table 1; Analysis 1.1).
1.1. Analysis.
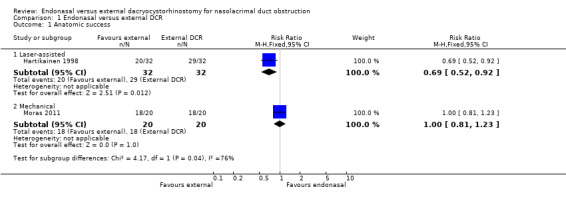
Comparison 1 Endonasal versus external DCR, Outcome 1 Anatomic success.
The six‐month success rate for endonasal laser‐assisted DCR in Hartikainen 1998 (32 participants) was lower than for endonasal mechanical DCR in Moras 2011 (20 participants) ‐ 78% versus 90%.
Subjective success
Hartikainen 1998 reported the rate of subjective success (resolution of watering in outdoor conditions) at the final postoperative visit. This was 84% (27/32) in the external DCR group and 59% (19/32) in the laser‐assisted endonasal DCR group: RR 0.70, 95% CI 0.51 to 0.97, 64 participants, 1 study. We judged this evidence to be low‐certainty, downgrading for risk of bias and imprecision (Table 1; Analysis 1.2).
1.2. Analysis.
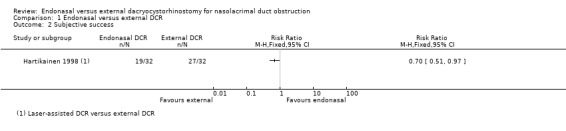
Comparison 1 Endonasal versus external DCR, Outcome 2 Subjective success.
The difference in effect between the two trials is discussed further in the 'Discussion' section below.
Secondary outcomes
Intraoperative bleeding
There were no cases of significant intraoperative bleeding in either the laser‐assisted endonasal (32 participants) or the external DCR group (32 participants) in Hartikainen 1998 (RR not estimable), but in Moras 2011, equal numbers of participants in the mechanical endonasal and external DCR group (9/20; 45%) experienced intraoperative bleeding (RR 1.00, 95% CI 0.50 to 1.98, 40 participants, 1 study). We judged this evidence to be very low‐certainty, downgrading for risk of bias, imprecision and uncertainty (Table 1; Analysis 1.3).
1.3. Analysis.
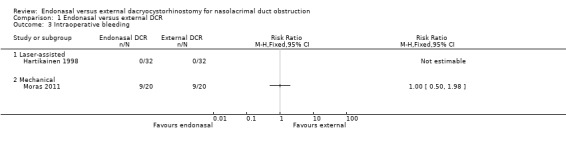
Comparison 1 Endonasal versus external DCR, Outcome 3 Intraoperative bleeding.
Postoperative bleeding
Overall, none of the 52 participants in the endonasal group experienced postoperative bleeding, whereas in the external DCR group, 1 participant in each study experienced bleeding. Of these 2 participants, one required hospitalisation and endonasal tamponade. The risk ratios for postoperative bleeding in endonasal versus external DCR was 0.33 (95% CI 0.01 to 7.89, 64 participants) for Hartikainen 1998, and 0.33 (95% CI 0.01 to 7.72, 40 participants) in Moras 2011.
We judged this evidence to be very low‐certainty, downgrading for risk of bias and imprecision (Table 1; Analysis 1.4).
1.4. Analysis.
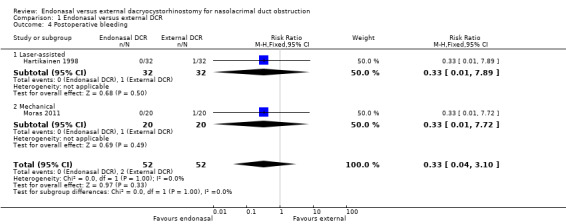
Comparison 1 Endonasal versus external DCR, Outcome 4 Postoperative bleeding.
Wound infection/gaping
In the external DCR group, 2/52 participants experienced wound infection/gaping (1 participant in each study). None of the 52 participants in the endonasal DCR group experienced these wound complications The risk ratios for wound infection/ gaping in endonasal versus external DCR was not estimable for Hartikainen 1998, and 0.20 (95% CI 0.01 to 3.92, 40 participants) in Moras 2011.We judged this evidence to be very low‐certainty, downgrading for risk of bias and imprecision (Table 1; Analysis 1.5).
1.5. Analysis.
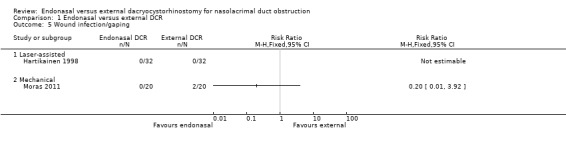
Comparison 1 Endonasal versus external DCR, Outcome 5 Wound infection/gaping.
Discussion
We included two randomised controlled trials (RCTs) comparing the successes and complications of endonasal versus external dacryocystorhinostomy (DCR) for nasolacrimal duct obstruction in this updated review.
A few different ways exist of assessing postoperative success for nasolacrimal duct obstruction (Fayers 2009; Moore 2002). Objective methods include the demonstration of patency of lacrimal system by syringing, and demonstration of functional success by endoscopic visualisation of fluorescein dye at the nasal opening of the anastomoses. Alternatively, subjective success can be assessed by asking patients about the improvement in epiphora following the operation. Few studies exist regarding the long‐term success of DCR.
The impact on quality of life of the patients undergoing the procedures was not assessed in either included trial. Other studies have compared quality of life outcomes between external DCR and endonasal DCR. Mathew 2004 did a retrospective telephone interview with 20 patients who had undergone endonasal mechanical DCR and 42 who had undergone external DCR – they found that patient satisfaction rates (70% and 86% respectively) were comparable between the 2 procedures as there was no significant difference.
The avoidance of a facial scar is an important advantage of endonasal DCR. Two studies (Bakri 1999; Hii 2012) were found which assessed postoperative quality of life following endonasal or external DCR using the Glasgow Benefit Inventory. This validated questionnaire is used widely for otorhinolaryngological procedures. Although it does not directly assess patients’ satisfaction with cosmesis following the procedures, it does assess the patients’ perception of psychological, social and physical wellbeing by including outcomes such as feelings of self‐confidence, feelings of embarrassment in a group of people, confidence regarding job opportunities and participation in social activities.
Bakri 1999 reported mean scores of +16.8 for patients in the endonasal laser DCR and +23.2 for patients in the external DCR group ‐ this difference was not significant. Hii 2012 also found comparable outcomes between endonasal mechanical and external DCR groups (mean score of +24.1 and +16.1 respectively; no significant difference). This would suggest that the presence of the scar following external DCR does not appear to have a huge impact on patients’ levels of post‐operative satisfaction.
Summary of main results
Our review shows different anatomic success rates in the two included trials (I2 = 76%). For the study comparing endonasal laser‐assisted DCR with external DCR (Hartikainen 1998), the anatomic success rates 12 months postoperatively were 91% and 63%, respectively (risk ratio (RR) 0.69, 95% confidence interval (CI) 0.52 to 0.92, 64 participants), and for the study comparing endonasal mechanical DCR with external DCR (Moras 2011), the anatomic success rates six months postoperatively were 90% for both studies (RR 1.00, CI 0.81 to 1.23, 40 participants).
Hartikainen 1998 also assessed subjective outcomes. Resolution of watering in outdoor conditions was reported by 84% of the participants in the external DCR group and 59% of those in the laser‐assisted endonasal DCR group (RR 0.70, CI 0.51 to 0.97, 64 participants). The subjective success rates were slightly lower than the anatomic success rates. Of the three participants who were considered to be anatomical failures in the external DCR group, two were asymptomatic. This demonstrates that anatomical success does not always correlate with functional success ‐ it has been argued (Fayers 2009) that the patient experience is a more meaningful way of evaluating success of DCR than demonstration of patency of the lacrimal system.
There were no cases of intraoperative bleeding in Hartikainen 1998. In the Moras 2011 study (laser‐assisted endonasal DCR versus external DCR), 45% of participants in both groups experienced intraoperative bleeding (RR 1.00, 95% CI 0.50 to 1.98, 40 participants, 1 study). We judged this evidence on intraoperative bleeding to be very‐low certainty, downgrading for risk of bias, imprecision and inconsistency.
There were no postoperative complications in the endonasal group, while two cases of postoperative bleeding (1 requiring readmission) and two cases of wound infection/gaping occurred in the external DCR group.
Overall completeness and applicability of evidence
The periods of follow‐up in the two trials were different (12 months for the first trial and 6 months for the second trial), which presents a difficulty in comparing the data from both trials. Moreover, a six‐month follow‐up period (while being within the limit specified in the protocol) might not uncover some later‐onset failures.
In addition to different follow up periods in the two trials, different techniques were also used for endonasal DCR in the two trials. There was also some variability in study design and some difficulty in clarifying the randomisation process in both studies which further limits analysis of data.
Quality of the evidence
We judged the evidence on anatomic success to be very low‐certainty, downgrading for risk of bias, imprecision and inconsistency.
The evidence on complications was also judged to be very‐low certainty, downgrading one level for risk of bias and two levels for imprecision, due to the very low number of cases.
The moderate‐certainty of the included studies precludes us from drawing any firm conclusions. Endonasal DCR is evolving since its introduction more than a decade ago; as more efficient techniques develop, the success rates for this technique are likely to improve.
Potential biases in the review process
Several conference proceedings were unavailable for searching and therefore unpublished relevant studies could have been missed.
Future larger RCTs are required to further assess the success and complication rates of endonasal and external DCR. Different techniques of endonasal DCR should also be assessed, as the choice of endonasal technique can influence the outcome. Strict outcome criteria should be adopted to assess functional and anatomical outcomes with a minimal follow‐up of six months.
Agreements and disagreements with other studies or reviews
Huang 2014 reviewed four RCTs and 15 comparative cohort studies. They found that the relative success rates for endonasal mechanical DCR and external DCR were comparable, while success rates for endonasal laser‐assisted DCR were lower.
Ben Simon 2005 and Verma 2006 are two non‐randomised studies that found a significantly higher rate of success with endonasal mechanical DCR than external DCR (endonasal mechanical DCR was assisted by carbon dioxide laser in Verma 2006).
Cokkeser 2000 and Dolman 2003 are comparative non‐randomised studies that obtained comparable success rates between external and endonasal mechanical DCR.
Ibrahim 2001, a retrospective comparative cohort study, found a higher success rate with external DCR than endonasal laser‐assisted DCR. (82% versus 58%).
Authors' conclusions
Implications for practice.
The success rate for laser‐assisted endonasal dacryocystorhinostomy (DCR) was lower than for external DCR, while the success rates for mechanical endonasal and external DCR were similar. We judged the evidence for success to be very low. The differences in effect seen may be due to variations in the endonasal technique, but may also be due to other differences between the trials. Therefore, the relative effects of endonasal and external DCR remain uncertain.
There is clearly a need for robust randomised controlled trials (RCTs) comparing external and endonasal DCR that provide high‐certainty evidence which can influence practice.
Implications for research.
More RCTs comparing endonasal and external DCR techniques are required to determine if endonasal DCR provides better results. Double‐masking may be difficult due to the external DCR scar, but participants can be masked to the type of procedure, and postoperative evaluation should be by an independent assessor. There is also a need for trials comparing different techniques of endonasal DCR, as the choice of endonasal technique can influence the outcome. Strict outcome criteria should be adopted (Fayers 2009; Moore 2002). Subjective outcome criteria should be assessed based on resolution of patient reported symptoms. Objective outcomes should be based on anatomical success demonstrating lacrimal system patency (syringing of the lacrimal system) or on functional success demonstrated by functional endoscopic dye test or endoscopic inspection of the ostium, and fluorescein dye retention test. Trials that include patient reported outcomes, such as, impact on quality of life or appearance would offer valuable information, especially given the invasive nature of the techniques involved.
What's new
| Date | Event | Description |
|---|---|---|
| 22 August 2016 | New citation required but conclusions have not changed | Issue 2 2017: We identified one new trial that fulfilled the inclusion criteria (Moras 2011). |
| 22 August 2016 | New search has been performed | Issue 2 2017: We updated the electronic searches. |
History
Protocol first published: Issue 2, 2008 Review first published: Issue 1, 2011
| Date | Event | Description |
|---|---|---|
| 19 March 2008 | Amended | Converted to new review format. |
Acknowledgements
Cochrane Eyes and Vision created and executed the search strategies. We thank Catey Bunce and Daniel Morris for their comments, and Iris Gordon for her assistance with searches and obtaining articles. We also thank Anupa Shah and Jennifer Evans for author support, and Gianni Virgili for his help with statistical analysis.
We are also grateful to Hsin‐wen Wu for translating Chinese reports of trials.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor: [Dacryocystorhinostomy] explode all trees #2 dacryocystorhinostom* #3 ((endonasal or external or endoscopic or microscopic) near/5 DCR*) #4 MeSH descriptor: [Lacrimal Apparatus] explode all trees #5 MeSH descriptor: [Lacrimal Apparatus Diseases] this term only #6 MeSH descriptor: [Lacrimal Duct Obstruction] this term only #7 MeSH descriptor: [Nasolacrimal Duct] this term only #8 MeSH descriptor: [Dacryocystitis] this term only #9 dacryocystitis #10 lacrimal near/4 (obstruct* or block*) #11 nasolacrimal near/4 (obstruct* or block*) #12 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11
Appendix 2. MEDLINE Ovid search strategy
1. randomized controlled trial.pt. 2. (randomized or randomised).ab,ti. 3. placebo.ab,ti. 4. dt.fs. 5. randomly.ab,ti. 6. trial.ab,ti. 7. groups.ab,ti. 8. or/1‐7 9. exp animals/ 10. exp humans/ 11. 9 not (9 and 10) 12. 8 not 11 13. exp dacryocystorhinostomy/ 14. dacryocystorhinostom$.tw. 15. ((endonasal or external or endoscopic or microscopic) adj5 DCR$).tw. 16. exp Lacrimal Apparatus/ 17. Lacrimal Apparatus Diseases/ 18. Lacrimal duct obstruction/ 19. Nasolacrimal Duct/ 20. Dacryocystitis/ 21. dacryocystitis.tw. 22. (lacrimal adj4 (obstruct$ or block$)).tw. 23. (nasolacrimal adj4 (obstruct$ or block$)).tw. 24. or/13‐23 25. 12 and 24
The search filter for trials at the beginning of the MEDLINE strategy is from the published paper by Glanville 2006.
Appendix 3. Embase Ovid search strategy
1. exp randomized controlled trial/ 2. exp randomization/ 3. exp double blind procedure/ 4. exp single blind procedure/ 5. random$.tw. 6. or/1‐5 7. (animal or animal experiment).sh. 8. human.sh. 9. 7 and 8 10. 7 not 9 11. 6 not 10 12. exp clinical trial/ 13. (clin$ adj3 trial$).tw. 14. ((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$)).tw. 15. exp placebo/ 16. placebo$.tw. 17. random$.tw. 18. exp experimental design/ 19. exp crossover procedure/ 20. exp control group/ 21. exp latin square design/ 22. or/12‐21 23. 22 not 10 24. 23 not 11 25. exp comparative study/ 26. exp evaluation/ 27. exp prospective study/ 28. (control$ or prospectiv$ or volunteer$).tw. 29. or/25‐28 30. 29 not 10 31. 30 not (11 or 23) 32. 11 or 24 or 31 33. dacryocystorhinostomy/ 34. dacryocystorhinostom$.tw. 35. ((endonasal or external or endoscopic or microscopic) adj5 DCR$).tw. 36. lacrimal duct/ 37. lacrimal duct occlusion/ 38. dacryocystitis/ 39. dacryocystitis.tw. 40. (lacrimal adj4 (obstruct$ or block$)).tw. 41. (nasolacrimal adj4 (obstruct$ or block$)).tw. 42. or/33‐41 43. 32 and 42
Appendix 4. LILACS search strategy
dacryocystorhinostom$ or dacryocystitis or DCR or lacrimal and obstruc$ or block$
Appendix 5. Web of Science CPCI‐S search strategy
#4 #1 OR #2 OR #3 #3 TS= lacrimal obstruc* #2 TS=dacryocystitis #1 TS=dacryocystorhinostom*
Appendix 6. ISRCTN search strategy
dacryocystorhinostomy OR dacryocystitis
Appendix 7. ClinicalTrials.gov search strategy
Dacryocystorhinostomy OR Dacryocystitis
Appendix 8. WHO ICTRP search strategy
Dacryocystorhinostomy OR Dacryocystitis
Data and analyses
Comparison 1. Endonasal versus external DCR.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Anatomic success | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Laser‐assisted | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.52, 0.92] |
| 1.2 Mechanical | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.81, 1.23] |
| 2 Subjective success | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Intraoperative bleeding | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Laser‐assisted | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Mechanical | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Postoperative bleeding | 2 | 104 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.04, 3.10] |
| 4.1 Laser‐assisted | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.89] |
| 4.2 Mechanical | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.72] |
| 5 Wound infection/gaping | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 Laser‐assisted | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Mechanical | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Hartikainen 1998.
| Methods | Randomised controlled trial; simple parallel group design with participants individually randomised to one of two intervention groups | |
| Participants | Country: Finland 64 eyes (63 participants) Age: range 23 to 89 years Mean age: 65 years |
|
| Interventions | Intervention 1: endonasal laser‐assisted dacryocystorhinostomy Intervention 2: external dacryocystorhinostomy | |
| Outcomes | Patent lacrimal passage on irrigation at one year | |
| Notes | Operations performed between January and December 1994, manuscript received May 1997. Study supported in part by a grant from the Turku University Foundation, Turku, Finland. The authors did not have any proprietary interest in any of the equipment mentioned in the article. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description of sequence generation is provided in the trial report. |
| Allocation concealment (selection bias) | Unclear risk | No description of allocation concealment is provided in the trial report. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No description of masking either the participants or outcome assessors is provided in the trial report. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | At 12 months follow‐up all participants in both groups were assessed for success. Complication rates were also determined for the whole study group. Therefore there was no incomplete data. |
Moras 2011.
| Methods | Randomised controlled trial; simple parallel group design with participants individually randomised to one of two intervention groups | |
| Participants | Country: India 40 eyes (40 participants) Age: 16‐68 Mean age: not specified |
|
| Interventions | Intervention 1: endonasal mechanical dacryocystorhinostomy using punch forceps Intervention 2: external dacryocystorhinostomy | |
| Outcomes | Patent lacrimal drainage system on sac syringing at the end of 6 months | |
| Notes | Date of submission: December 2010 (date of study not available from article). No competing interests declared by authors. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'They were randomised into two groups' with no further description. |
| Allocation concealment (selection bias) | Unclear risk | No description of allocation concealment is provided in the trial report. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Masking of surgeons not possible. No description of masking either the participants or outcome assessors is provided in the trial report. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | At 6 months there were no participants lost to follow‐up. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ajalloueyan 2007 | Even though the authors describe their surgery as endonasal, the technique described in the full‐text of the trial is that of transcanalicular rather than endonasal DCR. |
| Balikoglu‐Yilmaz 2015 | Prospective study comparing outcomes of external DCR, mechanical endonasal DCR and transcanalicular DCR using multidiode laser. Study excluded as it was non‐randomised, the procedure being chosen according to participant preference. |
| Derya 2013 | The endoscopic procedure involved a transcanalicular approach with the diode laser probe instead of an endonasal one. |
| Hartikainen 1998b | The same group conducted the two studies: Hartikainen 1998 and Hartikainen 1998b. The external DCR group in these two studies appear to be the same. We were unsuccessful in our attempts to contact the authors to clarify this matter. |
| Javate 2010 | This trial uses a lacrimal microendoscope with a trephine to remove fibrous obstruction along the lacrimal sac and nasolacrimal sac. It does not create an alternative drainage pathway using an endonasal technique which is what our review evaluates. |
| Tang 2015 | This study used a transcanalicular approach using a fifth generation lacrimal endoscope with a microdrill, instead of an endonasal technique. |
| Taskin 2011 | Alternate allocation used, therefore this study does not qualify as a randomised controlled trial. |
| Yigit 2007 | The full‐text article revealed that the study was not a randomised trial. |
DCR: dacryocystorhinostomy
Characteristics of studies awaiting assessment [ordered by study ID]
Cui 2013.
| Methods | Participants randomly divided into 3 treatment groups; details of randomisation process not available |
| Participants | 182 cases (202 eyes) |
| Interventions | External dacryocystorhinostomy Endonasal endoscopic dacryocystorhinostomy Nd:YAG laser dacryoplasty |
| Outcomes | Criteria for success not defined in abstract |
| Notes | Only Chinese version of article available. Chinese translator asked by CEV Information Specialist to contact study authors on June 4th 2015 for additional information about study (methods of randomisation etc). No response from study authors. |
Zhou 2015.
| Methods | Participants randomly divided into 2 treatment groups. Translation of methods section in full‐text Chinese article is as follows 'The sample is a collection of cases of people with chronic dacryocystitis from November 2010 to January 2011 within the author’s hospital ‐ Songjiang District Central Hospital. There are 2 males and 35 females, aged from 25 to 63 years of age (average 44.03±7.13)'. Unable to ascertain method of randomisation from this description. |
| Participants | 37 cases (37 eyes) with chronic dacryocystitis |
| Interventions | External dacryocystorhinostomy Endonasal endoscopic dacryocystorhinostomy combined with intubation of lacrimal ducts |
| Outcomes | Criteria of 'success' not defined in abstract |
| Notes | Study authors emailed by Chinese translator on 12 Oct 2015 ‐ no response from study authors to date |
Differences between protocol and review
We prepared a 'Summary of findings' table and a GRADE assessment which was an amendment to the protocol (Anijeet 2008).
Contributions of authors
Conceiving the review: Cochrane Eyes and Vision
2011 version
Designing the review: DA
Co‐ordinating the review: DA
-
Data collection for the review
Designing electronic search strategies: Cochrane Eyes and Vision Group Trials Search Co‐ordinator
Undertaking manual searches: DA
Screening search results: DA, LD
Organising retrieval of papers: DA, LD
Screening retrieved papers against inclusion criteria: DA, LD
Appraising quality of papers: DA, LD
Extracting data from papers: DA, LD
Writing to authors of papers for additional information: DA
Providing additional data about papers: DA
Obtaining and screening data on unpublished studies: DA
-
Data management for the review:
Entering data into RevMan: DA, LD
Analysis of data: DA, LD
-
Interpretation of data
Providing a methodological perspective: DA, CJM
Providing a clinical perspective: DA, CJM
Providing a policy perspective: DA, CJM
Providing a consumer perspective: DA, CJM
Writing the review: DA, LD, CJM
Providing general advice on the review: CJM
2017 update
LJ and DA screened the search results, extracted data, assessed studies for bias and wrote to trial investigators for additional information. CJM provided general advice on the review. LJ wrote the text for the update of the review.
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Institute for Health Research (NIHR), UK.
- Richard Wormald, Co‐ordinating Editor for Cochrane Eyes and Vision (CEV) acknowledges financial support for his CEV research sessions from the Department of Health through the award made by the National Institute for Health Research to Moorfields Eye Hospital National Health Service (NHS) Foundation Trust and UCL Institute of Ophthalmology for a Specialist Biomedical Research Centre for Ophthalmology.
- The NIHR also funds the CEV Editorial Base in London.
The views expressed in this publication are those of the authors and not necessarily those of the NIHR, NHS, or the Department of Health.
Declarations of interest
Lona Jawaheer: none known Caroline MacEwen: none known Deepa Anijeet: none known
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Hartikainen 1998 {published data only (unpublished sought but not used)}
- Hartikainen J, Grenman R, Puukka P, Seppä H. Prospective randomized comparison of external dacryocystorhinostomy and endonasal laser dacryocystorhinostomy. Ophthalmology 1998;105(6):1106‐13. [DOI] [PubMed] [Google Scholar]
Moras 2011 {published data only}
- Moras K, Bhat M, Shreyas CS, Mendonca N, Pinto G. External dacryocystorhinostomy versus endoscopic dacryocystorhinostomy: A comparison. Journal of Clinical and Diagnostic Research 2011;5(2):182‐6. [Google Scholar]
References to studies excluded from this review
Ajalloueyan 2007 {published data only}
- Ajalloueyan M, Fartookzadeh M, Parhizgar H. Use of laser for dacrocystorhinostomy. Archives of Otolaryngology ‐ Head & Neck Surgery 2007;133(4):340‐3. [DOI] [PubMed] [Google Scholar]
Balikoglu‐Yilmaz 2015 {published data only}
- Balikoglu‐Yilmaz M, Yilmaz T, Taskin U, Taskapili M, Akcay M, Oktay MF, et al. Prospective comparison of 3 dacryocystorhinostomy surgeries: external versus endoscopic versus transcanalicular multidiode laser. Ophthalmic Plastic and Reconstructive Surgery 2015;31(1):13‐8. [DOI] [PubMed] [Google Scholar]
Derya 2013 {published data only}
- Derya K, Demirel S, Orman G, Cumurcu T, Gunduz A. Endoscopic transcanalicular diode laser dacryocystorhinostomy: is it an alternative method to conventional external dacryocystorhinostomy?. Ophthalmic Plastic and Reconstructive Surgery 2013;29(1):15‐7. [DOI] [PubMed] [Google Scholar]
Hartikainen 1998b {published data only}
- Hartikainen J, Antila J, Varpula M, Puukka P, Seppä H, Grénman R. Prospective randomized comparison of endonasal endoscopic dacryocystorhinostomy and external dacryocystorhinostomy. Laryngoscope 1998;108(12):1861‐6. [DOI] [PubMed] [Google Scholar]
Javate 2010 {published data only}
- Javate RM, Pamintuan FG, Cruz RT. Efficacy of endoscopic lacrimal duct recanalization using microendoscope. Ophthalmic Plastic and Reconstructive Surgery 2010;26(5):330‐3. [DOI] [PubMed] [Google Scholar]
Tang 2015 {published data only}
- Tang YZ, Lu HL, Yan SG, Kong XB, Liu XY, Liang KF, et al. Clinical research of the micro‐invasive treatments for chronic dacryocystitis with the fifth generation lacrimal endoscope. International Eye Science 2015;15(6):1046‐9. [Google Scholar]
Taskin 2011 {published data only}
- Taskin U, Yigit O, Sisman A, Eltutar K, Eryigit T. Comparison of outcomes between endoscopic and external dacryocystorhinostomy with a Griffiths nasal catheter. Journal of Otolaryngology ‐ Head & Neck Surgery 2011;40(3):216‐20. [PubMed] [Google Scholar]
Yigit 2007 {published data only}
- Yigit O, Samancioglu M, Taskin U, Ceylan S, Eltutar K, Yener M. External and endoscopic dacryocystorhinostomy in chronic dacryocystitis: Comparison of results. European Archives of Oto‐Rhino‐Laryngology 2007;264(8):879‐85. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Cui 2013 {published data only}
- Cui W, Jiang L, Jiang YH, Xi J. Effects analysis of three kinds of operation methods in treatment of dacryocystitis. International Eye Science 2013;13(7):1510‐1. [Google Scholar]
Zhou 2015 {published data only}
- Zhou J, Kong QJ, Li B. Effects comparison of two operation methods in treatment of dacryocystitis. International Eye Science 2015;15(3):565‐66. [Google Scholar]
Additional references
Atkins 2004
- Atkins D, Best D, Briss PA, Eccles M, Falck‐Ytter Y, Flottorp S, et al. GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bakri 1999
- Bakri SJ, Carney AS, Robinson K, Jones NS, Downes RN. Quality of life outcomes following dacryocystorhinostomy: External and endonasal laser techniques compared. Orbit 1999;18(2):83‐8. [DOI] [PubMed] [Google Scholar]
Ben Simon 2005
- Ben Simon GJ, Joseph J, Lee S, Schwarcz RM, McCann JD, Goldberg RA. External versus endoscopic dacryocystorhinostomy for acquired nasolacrimal duct obstruction in a tertiary referral centre. Ophthalmology 2005;112(8):1464‐8. [DOI] [PubMed] [Google Scholar]
Boush 1994
- Boush GA, Lemke BN, Dortzbach RK. Results of endonasal laser assisted dacryocystorhinostomy. Ophthalmology 1994;101(5):955‐9. [DOI] [PubMed] [Google Scholar]
Caldwell 1893
- Caldwell GW. Two new operations for obstruction of the nasal duct. New York Medical Journal 1893;57:581‐2. [Google Scholar]
Chen 2009
- Chen D, Ge J, Wang L, Gao Q, Ma P, Li N, et al. A simple and evolutional approach proven to recanalise the nasolacrimal duct obstruction. British Journal of Ophthalmology 2009;93(11):1438‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cokkeser 2000
- Cokkeser Y, Evereklioglu C, Er H. Comparative external versus endoscopic dacryocystorhinostomy: Results in 115 patients (130 eyes). Otolaryngology ‐ Head & Neck Surgery 2000;123(4):488‐91. [DOI] [PubMed] [Google Scholar]
Dolman 2003
- Dolman PJ. Comparison of external dacryocystorhinostomy with nonlaser endonasal dacryocystorhinostomy. Ophthalmology 2003;110(1):78‐84. [DOI] [PubMed] [Google Scholar]
Dupuy‐Dutemps 1921
- Dupuy‐Dutemps B. Procede plastique de dacryocystorhinostomie et ses resultants. Annales d'Ocullstique 1921;158:241‐61. [Google Scholar]
Fayers 2009
- Fayers T, Laverde T, Tay E, Olver JM. Lacrimal surgery success after external dacryocystorhinostomy: functional and anatomical results using strict outcome criteria. Ophthalmic Plastic and Reconstructive Surgery 2009;25(6):472‐5. [DOI] [PubMed] [Google Scholar]
Glanville 2006
- Glanville JM, Lefebvre C, Miles JN, Camosso‐Stefinovic J. How to identify randomized controlled trials in MEDLINE: ten years on. Journal of the Medical Library Association 2006;94(2):130‐6. [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT 2014 [Computer program]
- GRADE Working Group, McMaster University. GRADEpro GDT. Version accessed 14 September 2016. Hamilton (ON): GRADE Working Group, McMaster University, 2014.
Halle 1914
- Halle M. [Zur intranasalen operation am tranensack]. Archives of Oto‐Rhino‐Laryngology 1914;28:256‐66. [Google Scholar]
Henson 2007
- Henson RD, Henson RG Jr, Cruz HL Jr, Camara JG. Use of the diode laser with intraoperative mitomycin C in endocanalicular laser dacryocystorhinostomy. Ophthalmic Plastic and Reconstructive Surgery 2007;23(2):134‐7. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Altman DG, Sterne JAC editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hii 2012
- Hii BW, McNab AA, Friebel JD. A Comparison of External and Endonasal Dacryocystorhinostomy in Regard to Patient Satisfaction and Cost. Orbit 2012;31(2):67‐76. [DOI] [PubMed] [Google Scholar]
Huang 2014
- Huang J, Malek J, Chin D, Snidvongs K, Wilcsek G, Tumuluri K, et al. Systematic review and meta‐analysis on outcomes for endoscopic versus external dacryocystorhinostomy. Orbit 2014;33(2):81‐90. [DOI] [PubMed] [Google Scholar]
Ibrahim 2001
- Ibrahim HA, Batterbury M, Banhegyi G, McGalliard J. Endonasal laser dacryocystorhinostomy and external dacryocystorhinostomy outcome profile in a general ophthalmic service unit: a comparative retrospective study. Ophthalmic Surgery and Lasers 2001;32(3):220‐7. [PubMed] [Google Scholar]
Kennedy 1985
- Kennedy DW. Functional endoscopic sinus surgery technique. Archives of Otolaryngology ‐ Head & Neck Surgery 1985;111(10):643‐9. [DOI] [PubMed] [Google Scholar]
Kong 1994
- Kong YT, Kim TI, Kong BW. A report of 131 cases of endoscopic laser lacrimal surgery. Ophthalmology 1994;101(11):1793‐800. [DOI] [PubMed] [Google Scholar]
Maini 2007
- Maini S, Raghava N, Youngs R, Evans K, Trivedi S, Foy C, et al. Endoscopic endonasal laser versus endonasal surgical dacryocystorhinostomy for epiphora due to nasolacrimal duct obstruction: prospective, randomised, controlled trial. Journal of Laryngology and Otology 2007;121(2):1170‐6. [DOI] [PubMed] [Google Scholar]
Mathew 2004
- Mathew MR, McGuiness R, Webb LA, Murray SB, Esakowitz L. Patient satisfaction in our initial experience with endonasal endoscopic non‐laser dacryocystorhinostomy. Orbit 2004;23(2):77‐85. [DOI] [PubMed] [Google Scholar]
McDonogh 1989
- McDonogh M, Meiring JH. Endoscopic transnasal dacryocystorhinostomy. Journal of Laryngology and Otology 1989;103(6):585‐7. [DOI] [PubMed] [Google Scholar]
Metson 1994
- Metson R, Woog JJ, Puliafito CA. Endoscopic laser dacryocystorhinostomy. Laryngoscope 1994;104(3 Pt 1):269‐74. [DOI] [PubMed] [Google Scholar]
Moore 2002
- Moore WM, Bentley CR, Olver JM. Functional and anatomic results after two types of endoscopic endonasal dacryocystorhinostomy: surgical and holmium laser. Ophthalmology 2002;109(8):1575‐82. [DOI] [PubMed] [Google Scholar]
Muellner 2000
- Muellner K, Bodner E, Mannor GE, Wolf G, Hofmann T, Luxenberger W. Endolacrimal laser assisted lacrimal surgery. British Journal of Ophthalmology 2000;84(1):16‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ng 2015
- Ng DS, Chan E, Yu DK, Ko ST. Aesthetic assessment in periciliary "v‐incision" versus conventional external dacryocystorhinostomy in Asians. Graefe's Archive for Clinical and Experimental Ophthalmology 2015;253(10):1783‐90. [DOI] [PubMed] [Google Scholar]
Pearlman 1997
- Pearlman SJ, Michalos P, Leib ML, Moazed KT. Translacrimal transnasal laser‐assisted dacryocystorhinostomy. Laryngoscope 1997;107(10):1362‐5. [DOI] [PubMed] [Google Scholar]
Qin 2010
- Qin ZY, Lu ZM, Liang ZJ. Application of mitomycin C in nasal endoscopic dacryocystorhinostomy. International Journal of Ophthalmology 2010;10(8):1569‐71. [Google Scholar]
Reifler 1993
- Reifler DM. Results of endoscopic KTP laser assisted dacryocystorhinostomy. Ophthalmic Plastic and Reconstructive Surgery 1993;9(4):231‐6. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Roozitalab 2004
- Roozitalab MH, Amirahmadi M, Namazi MR. Results of the application of intraoperative mitomycin C in dacryocystorhinostomy. European Journal of Ophthalmology 2004;14(6):461‐3. [DOI] [PubMed] [Google Scholar]
Saiju 2009
- Saiju R, Morse LJ, Weinberg D, Shrestha MK, Ruit S. Prospective randomized comparison of external dacryocystorhinostomy with and without silicone intubation. British Journal of Ophthalmology 2009;93(9):1220‐2. [DOI] [PubMed] [Google Scholar]
Seppa 1994
- Seppa H, Grenman R, Hartikainen J. Endonasal CO2‐Nd: YAG laser dacryocystorhinostomy. Acta Ophthalmologica 1994;72(6):703‐6. [DOI] [PubMed] [Google Scholar]
Sham 2000
- Sham CL, Hasselt CA. Endoscopic terminal dacryocystorhinostomy. Laryngoscope 2000;110(6):1045‐9. [DOI] [PubMed] [Google Scholar]
Smirnov 2006
- Smirnov G, Tuomilehto H, Terasvirta M, Nuutinen J, Seppa J. Silicone tubing after endoscopic dacryocystorhinostomy: is it necessary?. American Journal of Rhinology 2006;20(6):600‐2. [DOI] [PubMed] [Google Scholar]
Stammberger 1986
- Stammberger H. Endoscopic endonasal surgery: concepts in treatment of recurring rhinosinusitis. Part II. Surgical technique. Otolaryngology ‐ Head and Neck Surgery 1986;94(2):147‐56. [DOI] [PubMed] [Google Scholar]
Tarbet 1995
- Tarbet KJ, Custer PL. External dacryocystorhinostomy: surgical success, patient satisfaction and economic cost. Ophthalmology 1995;102(7):1065‐70. [DOI] [PubMed] [Google Scholar]
Toti 1904
- Toti A. [Nuovo metodo conservatore di cura radicle delle suppurazoni croniche del sacco lacrimale (Dacriocistorinostomia)]. Clinica Moderna Firenze 1904;10:385‐7. [Google Scholar]
Tsirbas 2004
- Tsirbas A, Davis G, Wormald PJ. Mechanical endonasal dacryocystorhinostomy versus external dacryocystorhinostomy. Ophthalmic Plastic and Reconstructive Surgery 2004;20(1):50‐6. [DOI] [PubMed] [Google Scholar]
Unlu 2009
- Unlu HH, Gunhan K, Baser EF, Songu M. Long term results in endoscopic dacryocystorhinostomy: is intubation really required?. Otolaryngology ‐ Head and Neck Surgery 2009;140(7):589‐95. [DOI] [PubMed] [Google Scholar]
Verma 2006
- Verma A, Khabori M, Zutshi R. Endonasal carbon‐dioxide laser assisted dacryocystorhinostomy verses external dacryocystorhinostomy. Indian Journal of Otolaryngology and Head and Neck Surgery 2006;58(1):9‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Watts 2001
- Watts P, Ram AR, Nair R, Williams H. Comparison of external dacryocystorhinostomy and 5‐fluorouracil augmented endonasal laser dacryocystorhinostomy. A retrospective review. Indian Journal of Ophthalmology 2001;49(3):169‐72. [PubMed] [Google Scholar]
West 1910
- West JM. A window resection of the nasal duct in cases of stenosis. Transactions of the American Ophthalmological Society 1910;12(Pt 2):654‐8. [PMC free article] [PubMed] [Google Scholar]
Woog 1993
- Woog JJ, Metson R, Puliafito CA. Holmium: YAG endonasal laser dacryocystorhinostomy. American Journal of Ophthalmology 1993;116(1):1‐10. [DOI] [PubMed] [Google Scholar]
Yuce 2013
- Yuce S, Ali A, Dogan M, Uysal IO, Muderris S. Results of Endoscopic Endonasal Dacryocystorhinostomy. Journal of Craniofacial Surgery 2013;24(1):e11‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Anijeet 2011
- Anijeet D, Dolan L, MacEwen CJ. Endonasal versus external dacryocystorhinostomy for nasolacrimal duct obstruction. Cochrane Database of Systematic Reviews 2011, Issue 1. [DOI: 10.1002/14651858.CD007097.pub2] [DOI] [PubMed] [Google Scholar]
Anijeet 2008
- Anijeet D, Dolan L, MacEwen CJ. Endonasal versus external dacryocystorhinostomy for nasolacrimal duct obstruction. Cochrane Database of Systematic Reviews 2008, Issue 2. [DOI: 10.1002/14651858.CD007097] [DOI] [PMC free article] [PubMed] [Google Scholar]


