Abstract
Background
Health services often manage agitated or violent people, and such behaviour is particularly prevalent in emergency psychiatric services (10%). The drugs used in such situations should ensure that the person becomes calm swiftly and safely.
Objectives
To examine whether haloperidol plus promethazine is an effective treatment for psychosis‐induced aggression.
Search methods
On 6 May 2015 we searched the Cochrane Schizophrenia Group's Register of Trials, which is compiled by systematic searches of major resources (including MEDLINE, EMBASE, AMED, BIOSIS, CINAHL, PsycINFO, PubMed, and registries of clinical trials) and their monthly updates, handsearches, grey literature, and conference proceedings.
Selection criteria
All randomised clinical trials with useable data focusing on haloperidol plus promethazine for psychosis‐induced aggression.
Data collection and analysis
We independently extracted data. For binary outcomes, we calculated risk ratio (RR) and its 95% confidence interval (CI), on an intention‐to‐treat basis. For continuous data, we estimated the mean difference (MD) between groups and its 95% CI. We employed a fixed‐effect model for analyses. We assessed risk of bias for included studies and created 'Summary of findings' tables using GRADE.
Main results
We found two new randomised controlled trials (RCTs) from the 2015 update searching. The review now includes six studies, randomising 1367 participants and presenting data relevant to six comparisons.
When haloperidol plus promethazine was compared with haloperidol alone for psychosis‐induced aggression for the outcome not tranquil or asleep at 30 minutes, the combination treatment was clearly more effective (n=316, 1 RCT, RR 0.65, 95% CI 0.49 to 0.87, high‐quality evidence). There were 10 occurrences of acute dystonia in the haloperidol alone arm and none in the combination group. The trial was stopped early as haloperidol alone was considered to be too toxic.
When haloperidol plus promethazine was compared with olanzapine, high‐quality data showed both approaches to be tranquillising. It was suggested that the combination of haloperidol plus promethazine was more effective, but the difference between the two approaches did not reach conventional levels of statistical significance (n=300, 1 RCT, RR 0.60, 95% CI 0.22 to 1.61, high‐quality evidence). Lower‐quality data suggested that the risk of unwanted excessive sedation was less with the combination approach (n=116, 2 RCTs, RR 0.67, 95% CI 0.12 to 3.84).
When haloperidol plus promethazine was compared with ziprasidone all data were of lesser quality. We identified no binary data for the outcome tranquil or asleep. The average sedation score (Ramsay Sedation Scale) was lower for the combination approach but not to conventional levels of statistical significance (n=60, 1 RCT, MD ‐0.1, 95% CI ‐ 0.58 to 0.38). These data were of low quality and it is unclear what they mean in clinical terms. The haloperidol plus promethazine combination appeared to cause less excessive sedation but again the difference did not reach conventional levels of statistical significance (n=111, 2 RCTs, RR 0.30, 95% CI 0.06 to 1.43).
We found few data for the comparison of haloperidol plus promethazine versus haloperidol plus midazolam. Average Ramsay Sedation Scale scores suggest the combination of haloperidol plus midazolam to be the most sedating (n=60, 1 RCT, MD ‐ 0.6, 95% CI ‐1.13 to ‐0.07, low‐quality evidence). The risk of excessive sedation was considerably less with haloperidol plus promethazine (n=117, 2 RCTs, RR 0.12, 95% CI 0.03 to 0.49, low‐quality evidence). Haloperidol plus promethazine seemed to decrease the risk of needing restraints by around 12 hours (n=60, 1 RCT, RR 0.24, 95% CI 0.10 to 0.55, low‐quality evidence). It may be that use of midazolam with haloperidol sedates swiftly, but this effect does not last long.
When haloperidol plus promethazine was compared with lorazepam, haloperidol plus promethazine seemed to more effectively cause sedation or tranquillisation by 30 minutes (n=200, 1 RCT, RR 0.26, 95% CI 0.10 to 0.68, high‐quality evidence). The secondary outcome of needing restraints or seclusion by 12 hours was not clearly different between groups, with about 10% in each group needing this intrusive intervention (moderate‐quality evidence). Sedation data were not reported, however, the combination group did have less 'any serious adverse event' in 24‐hour follow‐up, but there were not clear differences between the groups and we are unsure exactly what the adverse effect was. There were no deaths.
When haloperidol plus promethazine was compared with midazolam, there was clear evidence that midazolam is more swiftly tranquillising of an aggressive situation than haloperidol plus promethazine (n=301, 1 RCT, RR 2.90, 95% CI 1.75 to 4.8, high‐quality evidence). On its own, midazolam seems to be swift and effective in tranquillising people who are aggressive due to psychosis. There was no difference in risk of serious adverse event overall (n=301, 1 RCT, RR 1.01, 95% CI 0.06 to 15.95, high‐quality evidence). However, 1 in 150 participants allocated haloperidol plus promethazine had a swiftly reversed seizure, and 1 in 151 given midazolam had swiftly reversed respiratory arrest.
Authors' conclusions
Haloperidol plus promethazine is effective and safe, and its use is based on good evidence. Benzodiazepines work, with midazolam being particularly swift, but both midazolam and lorazepam cause respiratory depression. Olanzapine intramuscular and ziprasidone intramuscular do seem to be viable options and their action is swift, but resumption of aggression with subsequent need to re‐inject was more likely than with haloperidol plus promethazine. Haloperidol used on its own without something to offset its frequent and serious adverse effects does seem difficult to justify.
Plain language summary
Haloperidol plus promethazine for psychosis‐induced aggression
Review question
How effective is giving a combination of haloperidol and promethazine for calming people who are aggresssive due to psychosis?
Background
Emergency psychiatric services are often required to help calm people who are aggressive because they are experiencing distressing psychoses. In such situations quick‐acting medication is usually given. Haloperidol is an antipsychotic typically used to treat schizophrenia, and promethazine is a strong tranquilliser that can help to reduce nervous tension.
Searches
We searched the Cochrane Schizophrenia Group Trials Register on 6 May 2015 for randomised controlled trials that compared the use of haloperidol and promethazine with other drugs for the treatment of psychosis‐induced aggression.
Key results
We found six trials that randomised 1367 participants to receive haloperidol plus promethazine or either haloperidol, midazolam, lorazepam, olanzapine, ziprasidone, or a combination of haloperidol plus midazolam.
Haloperidol plus promethazine effectively manages aggressive behaviour swiftly and safely, and is more effective after 30 minutes than haloperidol on its own.
Midazolam has a sedative effect and reduces anxiety, and was shown to be more effective in offering swift sedation than haloperidol plus promethazine. However, the risk of serious side effects when taking midazolam (in particular breathing problems) should be noted.
Haloperidol plus midazolam had a greater sedative effect than haloperidol plus promethazine. Haloperidol and midazolam didn't make people feel excessively sleepy and reduced the need for restraints or seclusion.
Haloperidol plus promethazine had a greater sedative effect than lorazepam (which is typically used to treat anxiety). There was no difference in the number of people requiring restraints or seclusion.
Olanzapine offered effective sedation but had a higher risk of making people feel excessively sleepy.
The results comparing haloperidol plus promethazine with the antipsychotic ziprasidone were unclear and of low quality.
Quality of the evidence
Overall the quality of the evidence was high. The data provided demonstrate that a haloperidol plus promethazine is effective and safe for use in situations where people are aggressive due to psychoses.
Summary of findings
Background
Description of the condition
Most people live in low‐ or middle‐income countries, and rates of severe mental illnesses are consistent across the world (Jablensky 1992). As there is no evidence that the prevalence of psychiatric emergencies differs across the globe, it follows that most episodes of aggression in severely mentally ill people must take place in these lower‐income countries. Although new preparations of atypical antipsychotic drugs may be available for use in the acute emergency, these drugs are expensive and are unlikely to be commonplace for the majority of people in need of emergency tranquillisation in the near future.
Health services often manage agitated or violent people, and such behaviour is particularly prevalent for emergency psychiatric services (10%) (McAllister 2002). Most incidents in the psychiatric setting are secondary to severe illnesses such as schizophrenia or substance abuse (Kaplan 1994). Guidelines recommend that patients should be calmed by use of words and reassurance, a diagnostic history acquired, and physical and laboratory tests completed before starting any pharmacological treatment (Expert 1999; RCPsych 1998). The acute danger of the situation often makes this impossible, and emergency room staff work in circumstances where histories may be short and fragmented, diagnoses speculative, and physical examination impossible. Nevertheless, clinicians have a responsibility to ensure the safety of everyone involved, and so rapid pharmacological tranquillisation of aggressive/violent patients may be unavoidable.
The drugs used in this situation should ensure that the person becomes calm safely and swiftly. However, guidelines are usually statements of consensus and differ on which drugs to use (Expert 1999; NICE 2015; RCPsych 1998). Surveys also show variation in clinicians' preferred drug treatments (Binder 1999; Cunnane 1994), which is confirmed by audit (Moritz 1999; Pilowsky 1992), although the broad class of the older‐generation antipsychotics or benzodiazepines, or both are most frequently used (Huf 2002a; McAllister 2002). The combination haloperidol plus promethazine is used commonly and consistently in Brazil (Huf 2002a) (Table 7) and India (Alexander 2003). This medication combination is inexpensive, and each drug is on the World Health Organization's Model List of Essential Drugs (WHO 2002). The NICE 2015 updated guidance now recommends the combination as one possible approach.
1. Survey of rapid tranquillisation in Rio de Janeiro 2002.
| Drug of choice | Frequency of use | mean mg (range) |
| Haloperidol + promethazine | 61% | 5 (2.5 to 10) + 50 (25 to 100) |
| Haloperidol + promethazine + diazepam | 15% | 5 (2.5 to 10) + 50 (25 to 100) + 10 |
| Diazepam | 9% | 10 |
| Haloperidol + promethazine + chlorpromazine | 7% | 5 + 50 + 25 |
| Chlorpromazine + diazepam + promethazine | 1% | 25 + 10 + 50 |
| Chlorpromazine + promethazine | 1% | 25 + 50 |
| Chlorpromazine | 1% | 25 |
| Diazepam + promethazine | 1% | 10 + 50 |
| Haloperidol + diazepam | 1% | 5 + 10 |
| Promethazine | 1% | 50 |
Description of the intervention
As has already been stated in this review's sibling (Adams 2013), haloperidol was developed in the late 1950s for use in the field of anaesthesia and was initially used to prevent surgical shock (Figure 1). Research subsequently demonstrated beneficial effect on hallucinations, delusions, aggressiveness, impulsiveness, and states of excitement (Ayd 1972; Ayd 1978). These findings led to the introduction of haloperidol as an antipsychotic. However, haloperidol has many adverse effects, particularly problematic of which, when used in the acute situation, are the acute dystonias. These are intermittent spasmodic or sustained involuntary contractions of muscles all over the body including the face, neck, trunk, pelvis, extremities, and even the larynx. While not often life‐threatening, they are most distressing. Opisthotonus is one such dystonia resulting in an out‐of‐control arching of the head, neck, and spinal column that is particularly dramatic and unpleasant and frightening to the patient. The prevalence of this adverse effect is not clear, but we are aware that TREC‐Rio‐II was stopped early because haloperidol alone was thought to cause dystonias too often for continuation of the the trial to be ethical. The acute dystonias are successfully and swiftly treated with use of anticholinergic medication.
1.
Haloperidol structure.
Promethazine is a phenothiazine. It is thought to be antipsychotic in itself ‐ although weakly so compared with others ‐ but has strong antihistamine and moderately potent anticholinergic properties (Figure 2). Promethazine is known to be sedating, which is likely due to its antihistaminic properties.
2.
Promethazine structure.
How the intervention might work
Although widely used in situations where a person is acutely aggressive thought to be due to psychotic illness, haloperidol given parenterally does have important adverse effects. It is also not particularly sedating in itself (Adams 2013). However, it is a potent antipsychotic drug. The combination of both drugs is theoretically attractive. Promethazine could conceivably introduce often‐welcome sedation in the acute situation whilst offsetting any acute dystonic reaction through its anticholinergic effects.
We are unclear of the history of the combination of the two drugs. We have been told that it was a common practice in British psychiatry decades ago but have no reference to corroborate this. It is, however, used in Brazil, Huf 2002a, and India, Alexander 2003, and now, with support of NICE 2015, it may become more prevalent in the UK.
Why it is important to do this review
We have been made aware of new trials relevant to this review. Also, the previous version of this review was outdated, both in its text, appraisal of the trials, and how it synthesised the available data. We felt it timely to improve the review (Huf 2004). This is one of a family of reviews relevant to this difficult area of care (Table 8).
2. Other relevant Cochrane reviews.
| Focus of review | Reference |
| Completed and maintained reviews | |
| 'As required' medication regimens for seriously mentally ill people in hospital | Chakrabarti 2007 |
| Benzodiazepines for psychosis‐induced aggression or agitation | Gillies 2005 |
| Chlorpromazine for psychosis‐induced aggression or agitation | Ahmed 2010 |
| Clotiapine for acute psychotic illnesses | Berk 2004 |
| Containment strategies for people with serious mental illness | Muralidharan 2006 |
| Droperidol for acute psychosis | Rathbone 2004 |
| Haloperidol for psychosis‐induced aggression or agitation (rapid tranquillisation) | Powney 2012 |
| Olanzapine IM or velotab for acutely disturbed/agitated people with suspected serious mental illnesses | Belgamwar 2005 |
| Seclusion and restraint for serious mental illnesses | Sailas 2000 |
| Zuclopenthixol acetate for acute schizophrenia and similar serious mental illnesses | Gibson 2004 |
| Reviews in the process of being completed | |
| Risperidone for psychosis‐induced aggression or agitation | Ahmed 2011 |
| Haloperidol for long‐term aggression in psychosis | Khushu 2012 |
| Loxapine inhaler for psychosis‐induced aggression | Vangala 2012 |
| Clozapine for people with schizophrenia and recurrent physical aggression | Toal 2012 |
| Quetiapine for psychosis‐induced aggression or agitation | Wilkie 2012 |
| De‐escalation techniques for psychosis‐induced aggression | Rao 2012 |
Objectives
To examine whether haloperidol plus promethazine is an effective treatment for psychosis‐induced aggression.
Methods
Criteria for considering studies for this review
Types of studies
All relevant randomised control trials. We excluded quasi‐randomised trials, such as those where allocation was undertaken on surname. If a trial had been described as double blind, but it was implied it had been randomised, we would have included these trials in a sensitivity analysis (see Sensitivity analysis). Randomised cross‐over trials were eligible, but only data up to the point of first cross‐over because of the instability of the problem behaviours and the likely carry‐over effects of all treatments.
Types of participants
We included people currently within an aggressive episode thought to be due to psychotic illness. We included trials that also involved people with other diagnoses such as drug or alcohol intoxication, organic problems including dementia, non‐psychotic mental illnesses, or learning disabilities as long as the proportion of the other groups did not exceed that for people with psychosis.
Types of interventions
1. Haloperidol plus promethazine
Given intramuscularly: any dose, compared with:
a. Haloperidol alone
Given intramuscularly: any dose
b. Other antipsychotic
Given intramuscularly: any dose
c. Benzodiazepine alone
Given intramuscularly: any dose
d. Anticonvulsive alone
Given intramuscularly: any dose
e. Haloperidol plus benzodiazepine
Given intramuscularly: any dose
f. Placebo or no intervention
Types of outcome measures
We predefined the primary outcomes of interest as tranquil or asleep, global state, and specific serious adverse effects. We grouped all outcomes by time: by 30 minutes, up to two hours, up to four hours, up to 24 hours, and over 24 hours.
We knew that some of our own work was eligible for this review and that this potentially biases our choice of primary outcome. Countering this, however, is that the primary outcomes for our trials were not chosen by the trialists, but by the clinicians working in front‐line psychiatric emergency services of Rio de Janeiro, Brazil. This clinical grounding, we suggest, protects the review from a biased choice of outcomes.
Primary outcomes
1. Not tranquil or asleep by up to 30 minutes
2. Global state: needing restraints or seclusion by 24 hours
3. Specific and serious adverse effects by 24 hours
Secondary outcomes
We recorded and grouped these as follows:
1. Tranquillisation or asleep
1.1 Not tranquil or asleep 1.2 Not tranquil 1.3 Not asleep 1.4 Time to tranquillisation/sleep 1.5 Time to tranquillisation 1.6 Time to sleep
2. Global state
2.1 No overall improvement 2.2 Use of additional medication 2.3 Use of restraints/seclusion 2.4 Relapse ‐ as defined by each study 2.5 Recurrence of violent incidents 2.6 Needing extra visits from the doctor 2.7 Refusing oral medication 2.8 Not accepting treatment 2.9 Average endpoint acceptance score 2.10 Average change in acceptance score
3. Mental state
3.1 No clinically important change in general mental state 3.2 Not any change in general mental state 3.3 Average endpoint general mental state score 3.4 Average change in general mental state scores
4. Adverse effects
4.1 Death 4.2 Other clinically important general adverse effects 4.3 Any general adverse effects 4.4 Any serious, specific adverse effects 4.5 Average endpoint general adverse effect score 4.6 Average change in general adverse effect scores 4.7 No clinically important change in specific adverse effects 4.8 Not any change in specific adverse effects 4.9 Average endpoint specific adverse effects 4.10 Average change in specific adverse effects
5. Service outcomes
5.1 Duration of hospital stay 5.2 Re‐admission 5.3 No clinically important engagement with services 5.4 Not any engagement with services 5.5 Average endpoint engagement score 5.6 Average change in engagement scores
6. Specific behaviours
6.1 Self harm, including suicide 6.2 Injury to others 6.3 Aggression 6.3.1 Other episode of aggression 6.3.2 No clinically important change in aggression 6.3.3 Not any change in aggression 6.3.4 Average endpoint aggression score 6.3.5 Average change in aggression scores
7. Leaving the study early
7.1 For specific reasons 7.2 For general reasons
8. Satisfaction with treatment
8.1 Recipient of treatment not satisfied with treatment 8.2 Recipient of treatment average satisfaction score 8.3 Recipient of treatment average change in satisfaction scores 8.4 Informal treatment providers not satisfied with treatment 8.5 Informal treatment providers' average satisfaction score 8.6 Informal treatment providers' average change in satisfaction scores 8.7 Professional providers not satisfied with treatment 8.8 Professional providers' average satisfaction score 8.9 Professional providers' average change in satisfaction scores
9. Acceptance of treatment
9.1 Not accepting treatment 9.2 Average endpoint acceptance score 9.3 Average change in acceptance score
10. Quality of life
10.1 No clinically important change in quality of life 10.2 Not any change in quality of life 10.3 Average endpoint quality of life score 10.4 Average change in quality of life scores 10.5 No clinically important change in specific aspects of quality of life 10.6 Not any change in specific aspects of quality of life 10.7 Average endpoint specific aspects of quality of life 10.8 Average change in specific aspects of quality of life
11. Economic outcomes
11.1 Direct costs 11.2 Indirect costs
Summary of findings table
We used the GRADE approach to interpret findings, in Schünemann 2008, and GRADEpro to import data from Review Manager 5 to create 'Summary of findings' tables. These tables provide outcome‐specific information concerning the overall quality of evidence from each included study in the comparison, the magnitude of effect of the interventions examined, and the sum of available data on all outcomes we rate as important to patient care and decision making. We selected the following main outcomes for inclusion in the 'Summary of findings' table.
Tranquil or asleep: Not tranquil or asleep by up to 30 minutes
Global state: Needing restraints or seclusion by 24 hours
Adverse effect: Specific and serious adverse effects by 24 hours (not death)
Adverse effect: Specific and serious adverse effects (death)
Service outcome: Not discharged
Specific behaviours: Aggression
Economic outcomes: Direct costs
Search methods for identification of studies
Electronic searches
Cochrane Schizophrenia Group’s Trials Register
On 6 May 2015, the information specialist searched the Cochrane Schizophrenia Group’s Study‐Based Register of Trials using the following search strategy:
*Promethazine* in Intervention Field of STUDY
In such study‐based register, searching the major concept retrieves all the synonym keywords and relevant studies because all of the studies have already been organised based on their interventions and linked to the relevant topics.
The Cochrane Schizophrenia Group’s Register of Trials is compiled by systematic searches of major resources (including MEDLINE, EMBASE, AMED, BIOSIS, CINAHL, PsycINFO, PubMed, and registries of clinical trials) and their monthly updates, handsearches, grey literature, and conference proceedings (see Group’s Module). There are no language, date, document type, or publication status limitations for inclusion of records into the register.
For previous searches please see Appendix 1.
Searching other resources
1. Handsearching
We also searched reference lists of included and excluded studies for additional relevant trials. We planned to handsearch specific journals not previously hand searched that gave a high yield of studies. We did not identify any journal with a high yield of relevant articles.
2. Personal contacts
If necessary we contacted the author of each included study for information regarding unpublished data.
Data collection and analysis
We have presented the methods used in the 2015 update below; for previous methods please see Appendix 2.
Selection of studies
Review author PG inspected all abstracts of studies identified in the 2015 search and identified potentially relevant reports. CEA (Acknowledgements) helped and provided guidance. We resolved any disagreements by discussion, or where there was still doubt, we acquired the full article for further inspection. We acquired the full articles of relevant reports/abstracts meeting initial criteria for reassessment and carefully inspected for a final decision on inclusion (see Criteria for considering studies for this review). PG and CEA were not blinded to the names of the authors, institutions, or journal of publication. If difficulties or disputes had arisen, we would have asked author GH for help, and where it was impossible to decide or if adequate information was not available to make a decision, we would have added these studies to those awaiting assessment and contacted the authors of the paper for clarification.
Data extraction and management
1. Extraction
Review author PG independently extracted data from trials found in the update search. CEA (Acknowledgements) gave advice and help. If disagreements had arisen, we would have discussed and documented decisions. We extracted data presented only in graphs and figures whenever possible, but we only included such data if we independently had the same result. We attempted to contact authors through an open‐ended request in order to obtain missing information or for clarification whenever necessary. If studies were multicentre, where possible we extracted data relevant to each component centre separately.
2. Management
2.1 Forms
We extracted data onto standard, simple forms.
2.2 Scale‐derived data
We included continuous data from rating scales only if: a. the psychometric properties of the measuring instrument have been described in a peer‐reviewed journal (Marshall 2000); and b. the measuring instrument was not written or modified by one of the trialists for that particular trial. Ideally the measuring instrument should either be i. a self report or ii. completed by an independent rater or relative (not the therapist). We realise that this is not often reported clearly; we noted in Description of studies if this was the case or not.
2.3 Endpoint versus change data
Both endpoint and change data have advantages. Change data can remove a component of between‐person variability from the analysis. On the other hand, calculation of change needs two assessments (baseline and endpoint), which can be difficult in unstable and hard‐to‐measure conditions such as schizophrenia. We decided to primarily use endpoint data, and only use change data if the former were not available. We combined endpoint and change data in the analysis, as we preferred to use mean differences rather than standardised mean differences throughout (Higgins 2011).
2.4 Skewed data
Continuous data on clinical and social outcomes are often not normally distributed. To avoid the pitfall of applying parametric tests to non‐parametric data, we applied the following standards to relevant data before inclusion.
(Note that we entered data from studies of at least 200 participants in the analysis irrespective of the following rules, because skewed data pose less of a problem in large studies. We also entered all relevant change data, as when continuous data are presented on a scale that includes a possibility of negative values (such as change data), it is difficult to tell whether data are skewed or not.)
For endpoint data N < 200:
a. when a scale starts from the finite number zero, we subtracted the lowest possible value from the mean, and divided this by the standard deviation (SD). If this value was lower than 1, it strongly suggests a skew, and we excluded these data. If this ratio was higher than 1 but below 2, there is suggestion of skew. We entered these data and tested whether its inclusion or exclusion changed the results substantially. Finally, if the ratio was larger than 2, we included these data, because skew is less likely (Altman 1996; Higgins 2011);
b. if a scale starts from a positive value (such as the Positive and Negative Syndrome Scale (PANSS), which can have values from 30 to 210) (Kay 1986), we modified the calculation described above to take the scale starting point into account. In these cases skew is present if 2 SD > (S ‐ S min), where S is the mean score and 'S min' is the minimum score.
2.5 Common measure
Where relevant, to facilitate comparison between trials, we converted variables that can be reported in different metrics, such as days in hospital (mean days per year, per week, or per month) to a common metric.
2.6 Conversion of continuous to binary
Where possible, we converted continuous outcome measures to dichotomous data. This can be done by identifying cutoff points on rating scales and dividing participants accordingly into 'clinically improved' or 'not clinically improved'. It is generally assumed that if there is a 50% reduction in a scale‐derived score such as the Brief Psychiatric Rating Scale (BPRS), in Overall 1962, or the PANSS (Kay 1986), this can be considered to be a clinically significant response (Leucht 2005; Leucht 2005a). Where data based on these thresholds were not available, we used the primary cutoff presented by the original authors.
2.7 Direction of graphs
Where possible, we entered data in such a way that the area to the left of the line of no effect indicated a favourable outcome for haloperidol plus promethazine. Where keeping to this made it impossible to avoid outcome titles with clumsy double‐negatives (for example 'Not un‐improved'), we presented data where the left of the line indicated an unfavourable outcome and noted this in the relevant graphs.
Assessment of risk of bias in included studies
Review author PG independently assessed risk of bias within the included studies found in the update search by using criteria described in the Cochrane Handbook for Systematic Reviews of Interventions to assess trial quality (Higgins 2011). This set of criteria is based on evidence of associations between overestimate of effect and high risk of bias of the article such as sequence generation, allocation concealment, blinding, incomplete outcome data, and selective reporting. CEA provided help and advice.
Where the raters disagreed, we made the final rating by consensus. Where details of randomisation and other characteristics of trials were inadequate, we contacted authors of the studies to obtain additional information. If non‐concurrence occurred, we reported this.
We noted the level of risk of bias within included studies in the text of the review and in Figure 3, Figure 4, and Table 1.
3.
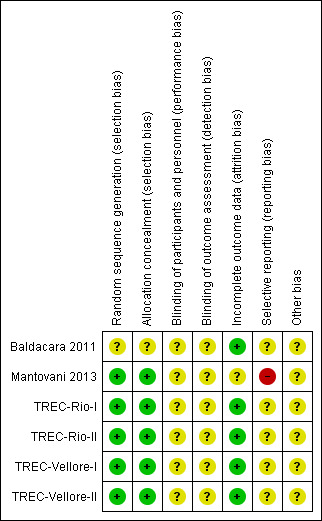
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
4.
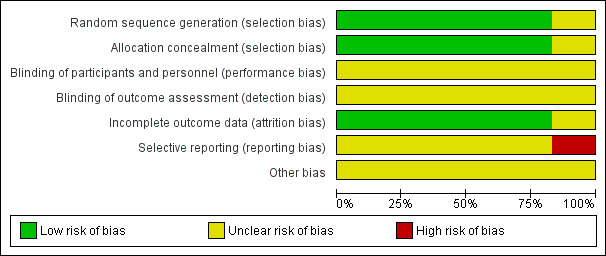
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Summary of findings for the main comparison. HALOPERIDOL + PROMETHAZINE compared to ANTIPSYCHOTIC ‐ HALOPERIDOL for psychosis‐induced aggression.
| HALOPERIDOL + PROMETHAZINE compared to ANTIPSYCHOTIC ‐ HALOPERIDOL for psychosis‐induced aggression | ||||||
| Patient or population: people with psychosis‐induced aggression Settings: Intervention: HALOPERIDOL + PROMETHAZINE Comparison: ANTIPSYCHOTIC ‐ HALOPERIDOL | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| ANTIPSYCHOTIC ‐ HALOPERIDOL | HALOPERIDOL + PROMETHAZINE | |||||
| Tranquil or asleep: Not tranquil or asleep ‐ by 30 minutes | Moderate1 | RR 0.65 (0.49 to 0.87) | 316 (1 study) | ⊕⊕⊕⊕ high | ||
| 500 per 1000 | 325 per 1000 (245 to 435) | |||||
| Global state: Needing restraints or seclusion by 12 hours | Moderate1 | RR 0.83 (0.28 to 2.44) | 60 (1 study) | ⊕⊕⊝⊝ low2,3 | ||
| 200 per 1000 | 166 per 1000 (56 to 488) | |||||
| Adverse effects: Specific and serious adverse effects by 24 hours (not death) Central nervous system ‐ seizure | Moderate1 | RR 0.95 (0.06 to 15.01) | 298 (1 study) | ⊕⊕⊝⊝ low4,5 | ||
| 10 per 1000 | 9 per 1000 (1 to 150) | |||||
| Adverse effect: Specific and serious ‐ Death | See comment | See comment | Not estimable | 0 (0) | See comment | No study reported this outcome |
| Service outcomes: Not discharged ‐ by 2 weeks | Moderate1 | RR 0.83 (0.64 to 1.07) | 310 (1 study) | ⊕⊕⊕⊕ high | ||
| 500 per 1000 | 415 per 1000 (320 to 535) | |||||
| Specific behaviours: Average aggression score ‐ by 12 hours Overt Aggression Scale | The mean specific behaviours: average aggression score in the intervention groups was 1.8 lower (1.93 to 1.67 lower) | 60 (1 study) | ⊕⊕⊝⊝ low3,6 | |||
| Economics: Costs of care | See comment | See comment | Not estimable | 0 (0) | See comment | No study reported this outcome |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Moderate control risk approximates to that of the included trial(s). 2Indirectness: rated 'serious' ‐ pre‐stated outcome was 'another episode of aggression' ‐ proxy outcome used. 3Imprecision: rated 'serious' ‐ sample size is small and confidence intervals wide. 4Indirectedness: rated 'serious' ‐ pre‐stated outcome was 'serious adverse event' ‐ proxy outcome used. 5Imprecision: rated 'serious' ‐ wide confidence intervals ‐ rare events. 6Indirectedness: rated 'serious' ‐ pre‐stated outcome was 'specific behaviours' ‐ proxy outcome used.
Measures of treatment effect
1. Binary data
For binary outcomes, we calculated a standard estimation of the risk ratio (RR) and its 95% confidence interval (CI). It has been shown that RR is more intuitive than odds ratios (Boissel 1999), and that clinicians tend to interpret odds ratios as RR (Deeks 2000).
2. Continuous data
For continuous outcomes, we estimated mean difference (MD) between groups. We preferred not to calculate effect size measures (standardised mean difference (SMD)). However, if scales of very considerable similarity were used, we presumed there was a small difference in measurement, calculated effect size, and transformed the effect back to the units of one or more of the specific instruments.
Unit of analysis issues
1. Cluster trials
Studies increasingly employ 'cluster randomisation' (such as randomisation by clinician or practice), but analysis and pooling of clustered data pose problems. Firstly, authors often fail to account for intraclass correlation in clustered studies, leading to a 'unit of analysis' error (Divine 1992), whereby P values are spuriously low, confidence intervals unduly narrow, and statistical significance overestimated. This causes type I errors (Bland 1997; Gulliford 1999).
Had we found cluster studies, where clustering had not been accounted for in primary studies, we would have presented data in a table, with a (*) symbol to indicate the presence of a probable unit of analysis error. If in subsequent versions of this review we find cluster studies, we will attempt to contact first authors of studies to obtain intraclass correlation coefficients for their clustered data and adjust for this by using accepted methods (Gulliford 1999). Where clustering has been incorporated into the analysis of primary studies, we will present these data as if from a non‐cluster randomised study, but adjust for the clustering effect.
We have sought statistical advice and have been advised that the binary data as presented in a report should be divided by a 'design effect'. This is calculated using the mean number of participants per cluster (m) and the intraclass correlation coefficient (ICC) [Design effect=1+(m‐1)*ICC] (Donner 2002). If the ICC is not reported, we will assume it to be 0.1 (Ukoumunne 1999).
If cluster studies have been appropriately analysed taking into account intraclass correlation coefficients and relevant data documented in the report, synthesis with other studies will be possible using the generic inverse variance technique.
2. Cross‐over trials
A major concern of cross‐over trials is the carry‐over effect, which occurs if an effect (for example pharmacological, physiological, or psychological) of the treatment in the first phase is carried over to the second phase. As a consequence, on entry to the second phase the participants can differ systematically from their initial state despite a wash‐out phase. For the same reason, cross‐over trials are not appropriate if the condition of interest is unstable (Elbourne 2002). As both effects are very likely in severe mental illness, we only used data from the first phase of any cross‐over studies.
3. Studies with multiple treatment groups
Where a study involved more than two treatment arms, if relevant, we presented the additional treatment arms in comparisons. If data were binary, we simply added and combined within the two‐by‐two table. If data were continuous, we combined data following the formulae in Section 7.7.3.8 (Combining groups) of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We did not use data where the additional treatment arms were not relevant.
Dealing with missing data
1. Overall loss of credibility
At some degree of loss of follow‐up data must lose credibility (Xia 2009). We chose that, for any particular outcome, should more than 50% of data be unaccounted for, we would not use them within analyses. However, if more than 50% of data in one arm of a study were lost, but the total loss was less than 50%, we addressed this within the 'Summary of findings' table/s by down‐rating quality. We also downgraded quality within the 'Summary of findings' table/s where the total loss was 25% to 50%.
2. Binary
In the case where attrition for a binary outcome was between 0 and 50% and where data were not clearly described, we presented such data on a 'once‐randomised‐always‐analyse' basis (an intention‐to‐treat (ITT) analysis). We assumed all those leaving the study early to have the same rates of negative outcome as those who completed, except for the outcomes of death and adverse effects, for which we used the rate of those who stayed in the study (in that particular arm of the trial) for those who did not. We undertook a sensitivity analysis testing how prone the primary outcomes were to change when data only from people who completed the study to that point were compared to the ITT analysis using the above assumptions.
3. Continuous
3.1 Attrition
We used data where attrition for a continuous outcome was between 0 and 50%, and data only from people who completed the study to that point were available.
3.2 Standard deviations
If SDs were not reported, we first tried to obtain the missing values from the authors. If these were not available, where there were missing measures of variance for continuous data, but an exact standard error and confidence intervals available for group means, and either P value or t value available for differences in mean, we calculated SDs according to the rules described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011): When only the standard error (SE) is reported, SDs are calculated by the formula SD=SE * square root (n). Sections 7.7.3 and 16.1.3 of the Cochrane Handbook for Systematic Reviews of Interventions present detailed formulae for estimating SDs from P values, t or F values, confidence intervals, ranges, or other statistics (Higgins 2011). If these formulae did not apply, we calculated the SDs according to a validated imputation method that is based on the SDs of the other included studies (Furukawa 2006). Although some of these imputation strategies can introduce error, the alternative would be to exclude a given study’s outcome and thus to lose information. We nevertheless examined the validity of the imputations in a sensitivity analysis excluding imputed values.
3.3 Assumptions about participants who left the trials early or who were lost to follow‐up
Various methods are available to account for participants who left the trials early or who were lost to follow‐up. Some trials just present the results of study completers, others use the method of last observation carried forward (LOCF), while more recently methods such as multiple‐imputation or mixed effects models for repeated measurements (MMRM) have become more of a standard. While the latter methods seem to be somewhat better than LOCF (Leon 2006), we feel that the high percentage of participants leaving the studies early and differences in the reasons for leaving the studies early between groups is often the core problem in randomised schizophrenia trials. We therefore did not exclude studies based on the statistical approach used. However, we preferred to use the more sophisticated approaches (for example MMRM or multiple‐imputation) and only presented completer analyses if no ITT data were available at all. Moreover, we addressed this issue in the item 'incomplete outcome data' of the 'Risk of bias' tool.
Assessment of heterogeneity
1. Clinical heterogeneity
We considered all included studies initially, without seeing comparison data, to judge clinical heterogeneity. We simply inspected all studies for clearly outlying people or situations that we had not predicted would arise and discussed these in the text.
2. Methodological heterogeneity
We considered all included studies initially, without seeing comparison data, to judge methodological heterogeneity. We simply inspected all studies for clearly outlying methods that we had not predicted would arise and discussed these in the text.
3. Statistical heterogeneity
3.1 Visual inspection
We visually inspected graphs to investigate the possibility of statistical heterogeneity.
3.2 Employing the I2 statistic
We investigated heterogeneity between studies by considering the I2 method alongside the Chi2 P value. The I2 provides an estimate of the percentage of inconsistency thought to be due to chance (Higgins 2003). The importance of the observed value of I2 depends on i. magnitude and direction of effects and ii. strength of evidence for heterogeneity (for example P value from Chi2 test, or a confidence interval for I2). We interpreted an I2 estimate greater than or equal to around 50% accompanied by a statistically significant Chi2 statistic as evidence of substantial levels of heterogeneity (Higgins 2011). We explored and discussed in the text potential reasons for substantial levels of heterogeneity (see Subgroup analysis and investigation of heterogeneity).
Assessment of reporting biases
Reporting biases arise when the dissemination of research findings is influenced by the nature and direction of results (Egger 1997). These are described in Chapter 10 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We are aware that funnel plots may be useful in investigating reporting biases but are of limited power to detect small‐study effects. We did not use funnel plots for outcomes where there were 10 or fewer studies, or where all studies were of similar size. In future versions of this review, if funnel plots are possible, we will seek statistical advice in their interpretation.
Data synthesis
We understand that there is no closed argument for preference for use of fixed‐effect or random‐effects models. The random‐effects method incorporates an assumption that the different studies are estimating different, yet related, intervention effects. This often seems to be true to us, and the random‐effects model takes into account differences between studies even if there is no statistically significant heterogeneity. However, there is a disadvantage to the random‐effects model: it puts added weight onto small studies, which often are the most biased ones. Depending on the direction of effect, these studies can either inflate or deflate the effect size. We chose the fixed‐effect model for all analyses.
Subgroup analysis and investigation of heterogeneity
1. Subgroup analyses
1.1 Primary outcomes
We did not anticipate any subgroup analyses.
1.2 Clinical state, stage, or problem
We proposed to undertake this review and provide an overview of the effects of haloperidol plus promethazine for people with psychosis‐induced aggression in general. In addition, however, we tried to report data on subgroups of people in the same clinical state, stage, and with similar problems.
2. Investigation of heterogeneity
We reported where inconsistency was high. First we investigated whether data were entered correctly. Second, if data were correct, we visually inspected the graph and successively removed studies outside of the company of the rest to see if homogenity was restored. For this update, we decided that should this occur with data contributing to the summary finding of no more than around 10% of the total weighting, we would present data. If not, we did not pool such data and discussed issues. We know of no supporting research for this 10% cutoff but are investigating use of prediction intervals as an alternative to this unsatisfactory state.
When unanticipated clinical or methodological heterogeneity was obvious, we simply discussed. We did not undertake sensitivity analyses relating to these.
Sensitivity analysis
1. Implication of randomisation
If trials were described in some way as to imply randomisation, we planned to undertake sensitivity analyses for the primary outcomes. We would include primary outcome data in the analyses, and if there was no substantive difference when we added data from the implied randomised studies to those with better description of randomisation, then we would use relevant data from these studies.
2. Assumptions for lost binary data
2.1 High attrition
Where assumptions had to be made regarding people lost to follow‐up (see Dealing with missing data), we compared the findings of the primary outcomes when we used our assumption compared with completer data only. If there was a substantial difference, we reported and discussed these results but continued to employ our assumption.
2.2 Missing SDs
Where assumptions had to be made regarding missing SDs data (see Dealing with missing data), we compared the findings on primary outcomes when we used our assumption compared with completer data only. We undertook a sensitivity analysis testing how prone results were to change when completer data only were compared to the imputed data using the above assumption. If there was a substantial difference, we reported and discussed these results but continued to employ our assumption.
3. Risk of bias
We analysed the effects of excluding trials that we judged to be at high risk of bias across one or more of the domains of randomisation (implied as randomised with no further details available, allocation concealment, blinding, and outcome reporting). If excluding trials at high risk of bias did not substantially alter the direction of effect or the precision of the effect estimates, we included data from these trials in the analysis.
4. Imputed values
Had we found cluster studies, we would have undertaken a sensitivity analysis to assess the effects of including data from trials where we used imputed values for ICC in calculating the design effect in cluster randomised trials.
Had we found substantial differences in the direction or precision of effect estimates in any of the sensitivity analyses listed above, we would not have pooled data from the excluded trials with the other trials contributing to the outcome, but would have presented them separately.
5. Fixed effect and random effects
We synthesised data using a fixed‐effect model, however we also synthesised data for the primary outcome using a random‐effects model to evaluate whether this altered the significance of the results.
Results
Description of studies
Results of the search
Previous versions of this review included four studies (TREC‐Rio‐I; TREC‐Rio‐II; TREC‐Vellore‐I; TREC‐Vellore‐II). The 2015 search identified two more studies (Baldacara 2011; Mantovani 2013) (Figure 5).
5.
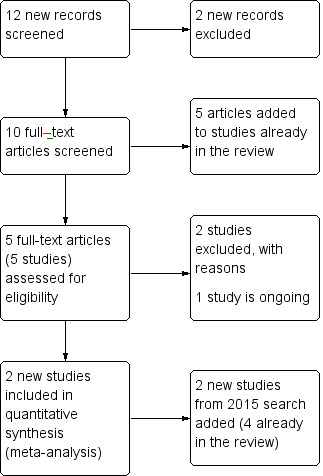
Study flow diagram 2015 update.
Included studies
This review now includes six randomised studies.
As four studies were undertaken by authors of this review, objectivity is difficult. The TREC studies (TREC=Tranquilização Rápida‐Ensaio Clínico, translation: Rapid Tranquillisation‐Clinical Trial) were undertaken in response to clinical need for good evidence, the first in Rio de Janeiro, Brazil, and the second in Vellore, Tamil Nadu, India. A preliminary survey in the psychiatric emergency rooms of Rio found haloperidol plus promethazine to be the drug combination of choice (Huf 2002a). Midazolam, a benzodiazepine, was another option. Trialists worked in conjunction with the clinicians of these emergency rooms to design a randomised trial which was then published and followed (Huf 2002b). Psychiatrists in Vellore, Southern India also use haloperidol plus promethazine, but use lorazepam and, more latterly, olanzapine as an alternative. They essentially used the same protocol for their work. Also in Rio the original protocol, Huf 2002b, was reused in a new trial comparing the benchmark haloperidol plus promethazine with haloperidol alone.
1. Length of trials
All four TREC studies followed people for up to two weeks, though the primary outcomes of interest were all within the first few hours (see below). The two new studies reported outcomes within 24 hours.
2. Participants
The TREC‐Rio‐I and TREC‐Rio‐II trials included any person for whom rapid tranquillisation was being considered in the psychiatric emergency rooms of a very large city. Over two‐thirds of participants suffered from psychosis. In TREC‐Vellore‐I and TREC‐Vellore‐II trials, only around 16% of participants had schizophrenia, but nearly half had mania; substance misuse was less prevalent. The TREC‐Vellore‐I and TREC‐Vellore‐II trials employed diagnostic criteria (International Classification of Diseases, Tenth Revision); TREC‐Rio‐I and TREC‐Rio‐II did not. In TREC‐Rio‐II, initial diagnoses at presentation were stable by two weeks or the time of discharge if that was less than two weeks (Huf 2002b). The two new studies were very similar (Baldacara 2011; Mantovani 2013), also focussing on people who were very disturbed and agitated whose condition was thought to be due to psychosis. The Baldacara 2011 study, similar to the four TREC studies, involved people who were determined to likely have psychosis (60%), with the other 40% likely to be agitated as a result of a manic episode as part of bipolar disorder. In Mantovani 2013, there were more participants with bipolar disorder and other less clear causes of the agitation. Overall the majority of participants were designated as severely agitated or worse and average age was around the early to mid‐30s.
3. Setting
TREC‐Rio‐I and TREC‐Rio‐II trials were set in specialist psychiatric emergency rooms that serve about half of the city of Rio de Janeiro (population 6 million). The TREC‐Vellore‐I and TREC‐Vellore‐II trials were set in the psychiatric emergency rooms of a large general hospital that serves both the city and its environs (population 1 million). Baldacara 2011 and Mantovani 2013 were both Brazilian and seem to have similar settings to the TREC‐Rio trials. All the hospital settings had very limited funding and deal with a rapid turnover of patients.
4. Study size
The table illustrates the study size, with studies ordered by both overall size as well as the size of the intervention groups.
| Study | Total N | Number of interventions | Approximate number of people per intervention group |
| TREC‐Rio‐II | 316 | 2 | 158 |
| TREC‐Rio‐I | 301 | 2 | 150 |
| TREC‐Vellore‐II | 300 | 2 | 150 |
| TREC‐Vellore‐I | 200 | 2 | 100 |
| Baldacara 2011 | 150 | 5 | 30 |
| Mantovani 2013 | 100 | 4 | 25 |
5. Interventions
5.1 Haloperidol plus promethazine
All studies had one arm in which haloperidol could be given by intramuscular (IM) injection (dose up to 10 mg) along with promethazine (dose up to 50 mg). In effect, TREC‐Rio‐I clinicians gave half the participants in the combination arm 5 mg haloperidol and the other half 10 mg. All but one person got the higher dose of 50 mg of promethazine. In TREC‐Vellore‐I, all 100 people allotted to the combination were given 10 mg of haloperidol combined with 50 mg (96 out of 100) or 25 mg (4 out of 100) promethazine. In TREC‐Vellore‐II, 148 people received 10 mg of haloperidol combined with 50 mg of promethazine, and two received a lower 5 mg dose of haloperidol combined with 25 mg of promethazine. In Baldacara 2011, the participants in the combination arm (n=30) were given 5 mg haloperidol and 50 mg of promethazine. Mantovani 2013 used smaller doses of 2.5 haloperidol IM and 25 mg promethazine IM (n=27). In total, 617 people have been allocated to this combination in the included trials.
5.2 Haloperidol plus midazolam
Baldacara 2011 included one arm where haloperidol (5 mg) was given in conjunction with midazolam (15 mg) (n=30). Mantovani 2013 had a similar group, but doses of each drug were less (2.5 mg haloperidol, 7.5 mg midazolam; n=25).
5.3 Haloperidol alone
TREC‐Rio‐II used an IM injection of haloperidol alone as its comparator drug (up to 10 mg as a single dose). Baldacara 2011 had one arm where people were allocated up to 5 mg of haloperidol alone (total N in trials' haloperidol‐alone arms=186).
5.4 Benzodiazepine
TREC‐Rio‐I included a midazolam arm. All doses were at the clinician's discretion and could have been administered by IM in a dose up to 15 mg. Of the 150 people allocated to midazolam, 124 were given 15 mg and 26 were given 7.5 mg. TREC‐Vellore‐I administered lorazepam IM in a dose up to 4 mg (n=100).
5.5 Olanzapine
TREC‐Vellore‐II compared haloperidol plus promethazine with olanzapine. Administration was by intramuscular injection. The majority of participants (148) received a 10 mg dose of IM olanzapine, while two received a 5 mg dose. In both Baldacara 2011 and Mantovani 2013, one intervention arm was olanzapine, again given at 10 mg (total N in the two trials' olanzapine arms=55).
5.6 Ziprasidone
Both trials new to this review had a ziprasidone IM arm: Baldacara 2011 employed 20 mg (n=30) and Mantovani 2013 10 mg (n=23).
6. Outcomes
The primary outcome of all studies was essentially 'tranquil or asleep'. TREC‐Rio‐I and TREC‐Rio‐II followed up at 20 minutes, 40 minutes, one hour, and two hours. TREC‐Vellore‐I specified the primary outcome to be at four hours, but measured tranquillisation or sleep every 30 minutes. TREC‐Vellore‐II specified the primary outcomes to be at 15 minutes and two hours. Having the advantage of a common protocol, all TREC studies recorded other episodes of aggression; use of additional medication; use of restraints or seclusion; needing extra visits from the doctor; refusing oral medication; hospital discharge; serious adverse effects; and leaving the study early. However, TREC‐Vellore‐I and TREC‐Vellore‐II also used the scales listed below for some outcomes.
6.1 Outcome scales
a. Agitation‐Calmness Evaluation Scale (ACES) (Breier 2002) The ACES is a single‐item rating scale developed by Eli Lilly and Company. On this scale, 1=marked agitation, 4=normal, 9=unable to be aroused.
b. Barnes Akathisia Scale (BAS) (Barnes 1989) Akathisia is a distressing subjective experience of restlessness associated with restless movements that may occur after commencing antipsychotic medication. The BAS includes an objective and a subjective component and a global impression rating for akathisia; these are rated on a scale of 0 to 3 for the objective and subjective items and 0 to 5 for the global clinical assessment.
c. Clinical Global Impression Scale (CGI Scale) (Guy 1976) One of the most widely used brief assessment tools in psychiatry, the CGI Scale assesses both severity of illness and clinical improvement by comparing the conditions of the person standardised against other people with the same diagnosis. The scale includes three items: the first two rate severity of illness and global improvement on a seven‐point scoring system, with low scores showing decreased severity or overall improvement, respectively, and the less frequently used third item assesses therapeutic response, which is rated as a combination of therapeutic effectiveness and adverse events. Each item is scored separately.
d. Overt Aggression Scale (OAS) (Yudofsky 1986) The OAS is a 16‐item rating scale used to measure the intensity of verbal and physical aggression. Clinicians comment on the duration of the aggressive incident as well as the intervention required to control it. High scores are indicative of higher levels of aggression.
e. Overt Agitation Severity Scale (OASS) (Yudofsky 1997) The OASS is designed to define and objectively rate the severity of agitated behaviour. Its rating is confined exclusively to observable behavioural manifestations of agitation, which comprise three categories: vocalisations and oral/facial movements; upper torso and upper extremity movements; and lower extremity movements. Under each of these categories four types of agitated behaviour are listed, which are rated on a 0 to 4 point scale, with 0=not present and 4=always present. High scores indicate worse agitated behaviour.
f. Positive and Negative Syndrome Scale ‐ Excited Component (PANSS‐EC) (Montoya 2011) The PANSS‐EC is a five‐item scale (excitement, tension, hostility, unco‐operativeness, and poor impulse control). The items are rated from 1 (not present) to 7 (extremely severe). Scores range from 5 to 35, with mean scores ≥ 20 indicating agitation. A high score indicates high levels of agitation.
g. Ramsay Sedation Scale (RSS) (Ramsay 1974) The RSS is a six‐item rating scale used to assess levels of sedation by selecting the most appropriate level of response. A rating of 1 indicates an agitated, anxious state; a rating of 6 indicates an unresponsive state.
h. Simpson‐Angus Scale ‐ Hillside/Long Island Jewish Hospital modification (SAS) (Simpson 1970) This SAS is a 10‐item scale used to evaluate the presence and severity of drug‐induced parkinsonian symptomatology. The 10 items focus on rigidity rather than bradykinesia, and do not assess subjective rigidity or slowness. Items are rated for severity on a scale of 0 to 4. A low score indicates low levels of parkinsonism.
7. Funding
All studies were undertaken by researchers and clinicians who were already receiving support from their home institutions. Industry funding was not involved.
Excluded studies
We have excluded 15 studies identified by the searches. Thirteen of these did not focus on a group of people who were specifically aggressive or agitated. However, Srinath 2010 focusses on the evaluation of haloperidol plus promethazine versus lorazepam (n=60). We regret having to exclude this study as it is clearly relevant. We have been unable to identify the full publication, no data were available in the report we identified, and we have had no response from our emails to the authors. Hou 2011 is relevant to the treatment of people whose aggression is thought to be due to psychosis, but this study compared risperidone plus lorazepam with haloperidol alone.
Ongoing
TREC‐Vellore‐III is ongoing and compares zuclopenthixol acetate with an IM injection of a combination of haloperidol plus promethazine in people with violence or agitation presenting to a psychiatric hospital as an emergency. We will include this trial in this review once data are accessible.
Awaiting assessment
No studies currently await assessment.
Risk of bias in included studies
The estimates of the risk of bias are graphically illustrated in Figure 3 and Figure 4.
Allocation
All but one trial had low risk of bias for allocation. Full details of the randomisation process and the concealment of allocation in TREC‐Rio‐I are published (Huf 2002b). The TREC‐Vellore‐I trial also involved randomisation codes being generated, away from the site, from a table of random numbers, and the allocation sequence being supplied to colleagues who designed the serially numbered intervention packs completely independently from those administering the treatments or recording the outcomes. TREC‐Rio‐II and TREC‐Vellore‐II generated the allocation sequences using a free online system (www.randomization.com/) but employed the same technique to conceal allocation. Concealment of allocation has convincingly been shown to be of key importance in excluding selection biases (Juni 2001). In Mantovani 2013 randomisation was well conducted and described, but for Baldacara 2011 this was unclear. With the exception of Baldacara 2011, we graded all trials as at low risk of selection bias ‐ (see Methods).
Blinding
All TREC studies were blind only until the point of treatment assignment to minimise selection bias. In TREC‐Rio‐I, all ratings were not blind, and in TREC‐Vellore‐I, ratings for the first two hours were not blind, as management teams needed to know the prescribed medications. In both Indian studies, however, the study co‐ordinators were blind and undertook ratings at 240 minutes. At this time, they also guessed the allocated intervention to assess their blinding. In any event, the TREC studies were designed to evaluate real‐world interventions that are not routinely given blind. In TREC‐Rio‐I, a medical student ("Dr Stopwatch"), blinded to group allocation, accurately recorded the time from injection to when they felt the person to be tranquil or asleep. This blinded rating concurred with that of the unblinded assessment, suggesting that, at least for these outcomes, blinding may not be necessary. The Baldacara 2011 and Mantovani 2013 studies are both described as blinded, but they do not say how successful this was. We rated all included trials as at unclear risk of blinding bias.
Incomplete outcome data
All studies have nearly complete data sets. Over 90% of people in all trials had their outcomes directly recorded. Follow‐up of this level of completeness is unusual, and the reasons for loss to follow‐up are also well‐reported. We gave only one trial, Mantovani 2013, an unclear rating, as for this trial, all attrition was before full enrolment and it was unclear how data were handled.
Selective reporting
Overall reporting bias is unclear across the majority of included trials. It should be noted that the review authors who extracted data from 2004 and 2008 searches (GH, JA, NR) were also trialists involved in the trials found in these searches. However, other observers have noted the TREC trials to be the most methodologically rigorous randomised trials of aggressive people with mental health problems (NICE 2004). The data are also avaiable in full for re‐analysis. The two new studies found in the 2015 search were extracted by a different review author (PG). Baldacara 2011 and Mantovani 2013 do mirror the TREC trials and, although using scale‐derived data more than the original studies, reporting seemed to be clear and largely complete. There was some difficulty with Mantovani 2013, where scales used to collect data were complicated and methods used for measures of standard deviation and standard error were unclear.
Other potential sources of bias
This is typically where review authors comment on funding sources within the trials and where they might have had an influence on the findings. Unusually none of the included trials were commercially funded.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6
Summary of findings 2. HALOPERIDOL + PROMETHAZINE compared to ANTIPSYCHOTIC ‐ OLANZAPINE for psychosis‐induced aggression.
| HALOPERIDOL + PROMETHAZINE compared to ANTIPSYCHOTIC ‐ OLANZAPINE for psychosis‐induced aggression | ||||||
| Patient or population: people with psychosis‐induced aggression Settings: Intervention: HALOPERIDOL + PROMETHAZINE Comparison: ANTIPSYCHOTIC ‐ OLANZAPINE | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| ANTIPSYCHOTIC ‐ OLANZAPINE | HALOPERIDOL + PROMETHAZINE | |||||
| Tranquil or asleep: Not tranquil or asleep ‐ by 30 mins | Moderate1 | RR 0.60 (0.22 to 1.61) | 300 (1 study) | ⊕⊕⊕⊕ high | ||
| 100 per 1000 | 60 per 1000 (22 to 161) | |||||
| Global state: Needing restraints or seclusion by 12 hours | Moderate | RR 5.00 (0.62 to 40.28) | 60 (1 study) | ⊕⊕⊝⊝ low2,3 | ||
| 50 per 10001 | 250 per 1000 (31 to 1000) | |||||
| Adverse effects: Specific and serious adverse effects by 24 hours Central nervous system ‐ excessive sedation. | Moderate | RR 0.67 (0.12 to 3.84) | 116 (2 studies) | ⊕⊕⊝⊝ low4,5 | ||
| 100 per 10001 | 64 per 1000 (11 to 364) | |||||
| Adverse effect: Specific ‐ Death | See comment | See comment | Not estimable | 0 (0) | See comment | No study reported this outcome |
| Service outcomes: Not discharged ‐ by 4 hours | Moderate | RR 0.94 (0.77 to 1.16) | 300 (1 study) | ⊕⊕⊕⊕ high | ||
| 600 per 10001 | 564 per 1000 (462 to 696) | |||||
| Specific behaviours: Average aggression score ‐ by 12 hours Overt Aggression Scale | The mean specific behaviours: average aggression score in the intervention groups was 2 lower (2.21 to 1.79 lower) | 60 (1 study) | ⊕⊕⊝⊝ low5,6 | |||
| Economics: Costs of care7 | See comment | See comment | Not estimable | 0 (0) | See comment | No study reported this outcome |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Moderate control risk approximates to that of the included trial(s). 2Indirectness: rated 'serious' ‐ pre‐stated outcome was 'another episode of aggression' ‐ proxy outcome used. 3Imprecision: rated 'serious' as sample size too small and confidence interval too wide. 4Indirectness: rated 'serious' ‐ pre‐stated outcome was 'serious adverse effect' ‐ proxy outcome used. 5Imprecision: rated 'serious' ‐ sample size too small and confidence interval wide. 6Indirectness: rated 'serious' ‐ pre‐stated outcome was 'specific behaviours' ‐ proxy outcome used.
Summary of findings 3. HALOPERIDOL + PROMETHAZINE compared to ANTIPSYCHOTIC ‐ ZIPRASIDONE for psychosis‐induced aggression.
| HALOPERIDOL + PROMETHAZINE compared to ANTIPSYCHOTIC ‐ ZIPRASIDONE for psychosis‐induced aggression | ||||||
| Patient or population: people with psychosis‐induced aggression Settings: Intervention: HALOPERIDOL + PROMETHAZINE Comparison: ANTIPSYCHOTIC ‐ ZIPRASIDONE | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| ANTIPSYCHOTIC ‐ ZIPRASIDONE | HALOPERIDOL + PROMETHAZINE | |||||
| Tranquil or asleep: Average sedation score ‐ by 30 minutes Ramsay Sedation Scale | The mean tranquil or asleep: average sedation score in the intervention groups was 0.1 lower (0.58 lower to 0.38 higher) | 60 (1 study) | ⊕⊕⊝⊝ low1,2 | |||
| Global state: Needing restraints or seclusion ‐ by 12 hours | Moderate | RR 0.5 (0.19 to 1.29) | 60 (1 study) | ⊕⊕⊕⊝ moderate4 | ||
| 400 per 10003 | 200 per 1000 (76 to 516) | |||||
| Adverse effects: Specific and serious adverse effect ‐ by 24 hours Central nervous system ‐ excessive sedation. | Moderate | RR 0.30 (0.06 to 1.43) | 111 (2 studies) | ⊕⊕⊝⊝ low2,5 | ||
| 150 per 10003 | 47 per 1000 (11 to 219) | |||||
| Adverse effect: Specific ‐ Death | See comment | See comment | Not estimable | 0 (0) | See comment | No study reported this outcome |
| Service outcomes: Not discharged ‐ by 2 weeks | See comment | See comment | Not estimable | 0 (0) | See comment | No study reported this outcome |
| Specific behaviours: Average aggression score ‐ by 12 hours Overt Aggression Scale | The mean specific behaviours: average aggression score in the intervention groups was 1.6 lower (1.75 to 1.45 lower) | 60 (1 study) | ⊕⊕⊕⊝ moderate2,4 | |||
| Economics: Costs of care | See comment | See comment | Not estimable | 0 (0) | See comment | No study reported this outcome |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Indirectness: rated 'serious' ‐ pre‐stated outcome 'Tranquil or asleep' ‐ proxy outcome used. 2Imprecision: rated 'serious' ‐ sample size small. 3Moderate control risk approximates to that of the included trial. 4Indirectness: rated 'serious' ‐ pre‐stated outcome 'another episode of aggression' ‐ proxy outcome used. 5Indirectness: rated 'serious' ‐ pre‐stated outcome 'serious adverse effect' ‐ proxy outcome used.
Summary of findings 4. HALOPERIDOL + PROMETHAZINE compared to ANTIPSYCHOTIC & BENZODIAZEPINE ‐ HALOPERIDOL + MIDAZOLAM for psychosis‐induced aggression.
| HALOPERIDOL + PROMETHAZINE compared to ANTIPSYCHOTIC & BENZODIAZEPINE ‐ HALOPERIDOL + MIDAZOLAM for psychosis‐induced aggression | ||||||
| Patient or population: people with psychosis‐induced aggression Settings: Intervention: HALOPERIDOL + PROMETHAZINE Comparison: ANTIPSYCHOTIC & BENZODIAZEPINE ‐ HALOPERIDOL + MIDAZOLAM | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| ANTIPSYCHOTIC & BENZODIAZEPINE ‐ HALOPERIDOL + MIDAZOLAM | HALOPERIDOL + PROMETHAZINE | |||||
| Tranquil or asleep: Average sedation score ‐ by 1 hour Ramsay Sedation Scale | The mean tranquil or asleep: average sedation score in the intervention groups was 0.6 lower (1.13 to 0.07 lower) | 60 (1 study) | ⊕⊕⊝⊝ low1 | |||
| Global state: Needing restraints or seclusion ‐ by 12 hours | Moderate | RR 0.24 (0.1 to 0.55) | 60 (1 study) | See comment | ||
| 700 per 10002 | 168 per 1000 (70 to 385) | |||||
| Adverse effects: Specific and serious adverse effect ‐ by 24 hours Central nervous system ‐ excessive sedation | Moderate | RR 0.12 (0.03 to 0.49) | 117 (2 studies) | ⊕⊕⊝⊝ low3,4 | ||
| 300 per 10002 | 33 per 1000 (9 to 141) | |||||
| Adverse effect: Specific ‐ Death | See comment | See comment | Not estimable | 0 (0) | See comment | No study reported this outcome |
| Service outcomes: Not discharged ‐ by 2 weeks | See comment | See comment | Not estimable | 0 (0) | See comment | No study reported this outcome |
| Specific behaviours: Average aggression score ‐ by 12 hours Overt Aggression Scale | The mean specific behaviours: average aggression score in the intervention groups was 3.7 lower (4.39 to 3.01 lower) | 60 (1 study) | See comment | |||
| Economics: Costs of care | See comment | See comment | Not estimable | 0 (0) | See comment | No study reported this outcome |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Indirectness: rated 'serious' ‐ pre‐stated outcome 'tranquil or asleep' ‐ proxy outcome used. 2Moderate control risk approximates to that of the included trial. 3Indirectness: rated 'serious' ‐ pre‐stated outcome was 'another episode of aggression' ‐ proxy outcome used. 4Imprecision: rated 'serious' ‐ sample size small.
Summary of findings 5. HALOPERIDOL + PROMETHAZINE compared to BENZODIAZEPINES ‐ LORAZEPAM for psychosis‐induced aggression.
| HALOPERIDOL + PROMETHAZINE compared to BENZODIAZEPINES ‐ LORAZEPAM for psychosis‐induced aggression | ||||||
| Patient or population: people with psychosis‐induced aggression Settings: Intervention: HALOPERIDOL + PROMETHAZINE Comparison: BENZODIAZEPINES ‐ LORAZEPAM | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| BENZODIAZEPINES ‐ LORAZEPAM | HALOPERIDOL + PROMETHAZINE | |||||
| Tranquil or asleep: Not tranquil or asleep ‐ by 30 mins Follow‐up: to 30 minutes | Moderate | RR 0.26 (0.1 to 0.68) | 200 (1 study) | ⊕⊕⊕⊕ high | ||
| 200 per 10001 | 52 per 1000 (20 to 136) | |||||
| Global state: Needing restraints or seclusion ‐ by 12 hours | Moderate | RR 0.82 (0.35 to 1.89) | 200 (1 study) | ⊕⊕⊕⊝ moderate2 | ||
| 150 per 10001 | 123 per 1000 (52 to 283) | |||||
| Adverse effects: Specific and serious adverse effect ‐ by 24 hours Central nervous system ‐ excessive sedation | See comment | Not estimable | 0 (0) | See comment | No study reported for this outcome | |
| Adverse effect: Specific ‐ Death | See comment | Not estimable | 0 (0) | See comment | No study reported for this outcome | |
| Service outcomes: Not discharged ‐ by 4 hours | Moderate | RR 1.13 (0.85 to 1.5) | 200 (1 study) | ⊕⊕⊕⊕ high | ||
| 500 per 10001 | 565 per 1000 (425 to 750) | |||||
| Specific behaviours: Average aggression score | See comment | See comment | Not estimable | 0 (0) | See comment | No study reported for this outcome |
| Economics: Costs of care | See comment | See comment | Not estimable | 0 (0) | See comment | No study reported for this outcome |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Moderate control risk approximates to that of the included trial. 2Indirectness: rated 'serious' ‐ pre‐stated outcome was 'another episode of aggression' ‐ proxy outcome used. 3Imprecision: rated 'serious' ‐ confidence interval is wide.
Summary of findings 6. HALOPERIDOL + PROMETHAZINE compared to BENZODIAZEPINES ‐ MIDAZOLAM for psychosis‐induced aggression.
| HALOPERIDOL + PROMETHAZINE compared to BENZODIAZEPINES ‐ MIDAZOLAM for psychosis‐induced aggression | ||||||
| Patient or population: people with psychosis‐induced aggression Settings: Intervention: HALOPERIDOL + PROMETHAZINE Comparison: BENZODIAZEPINES ‐ MIDAZOLAM | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| BENZODIAZEPINES ‐ MIDAZOLAM | HALOPERIDOL + PROMETHAZINE | |||||
| Tranquil or asleep: Not tranquil or asleep Follow‐up: to 30 minutes | Moderate | RR 2.9 (1.75 to 4.8) | 301 (1 study) | ⊕⊕⊕⊕ high | ||
| 200 per 10001 | 580 per 1000 (350 to 960) | |||||
| Global state: Needing restraints or seclusion‐ by 2 hours | Moderate | RR 1.22 (0.82 to 1.82) | 301 (1 study) | ⊕⊕⊕⊝ moderate2 | ||
| 250 per 10001 | 305 per 1000 (205 to 455) | |||||
| Adverse effect: Specific ‐ Death | See comment | See comment | Not estimable | 0 (0) | See comment | No study reported for this outcome |
| Service outcomes: Not discharged ‐ Follow‐up: to 2 weeks | Moderate | RR 1.05 (0.84 to 1.29) | 301 (1 study) | ⊕⊕⊕⊕ high | ||
| 550 per 10001 | 577 per 1000 (462 to 709) | |||||
| Specific behavioursAggression Follow‐up: to 12 hours | Moderate | RR 0.89 (0.62 to 1.29) | 301 (1 study) | ⊕⊕⊕⊝ moderate4 | ||
| Economics: Costs of care | See comment | See comment | Not estimable | 0 (0) | See comment | No study reported for this outcome |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Moderate control risk approximates to that of the included trial. 2Indirectness: rated 'serious' ‐ pre‐stated outcome was 'another episode of aggression' ‐ proxy outcome used. 3Imprecision: rated 'serious' ‐ confidence interval wide. 4Indirectness: rated 'serious' ‐ pre‐stated outcome was 'specific behaviours' ‐ proxy outcome used.
For this review we generated seven comparisons. We identified six randomised trials from which it was possible to extract numerical data.
1. COMPARISON 1: HALOPERIDOL + PROMETHAZINE vs ANTIPSYCHOTIC ‐ HALOPERIDOL
This comparison has 14 outcomes.
1.1 Tranquil or asleep: 1. Not tranquil or asleep
We identified one study relevant to this outcome (n=316).
1.1.1 by 30 minutes
For this outcome, we did find evidence that 'haloperidol + promethazine' was clearly different in its effects compared with 'antipsychotic ‐ haloperidol' (risk ratio (RR) 0.65, 95% confidence interval (CI) 0.49 to 0.87; Analysis 1.1).
1.1. Analysis.
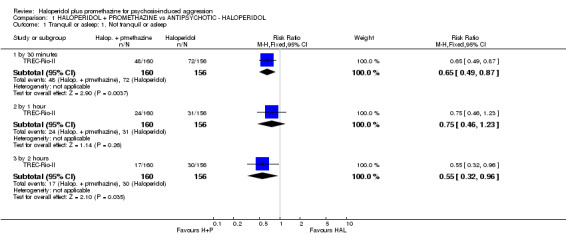
Comparison 1 HALOPERIDOL + PROMETHAZINE vs ANTIPSYCHOTIC ‐ HALOPERIDOL, Outcome 1 Tranquil or asleep: 1. Not tranquil or asleep.
1.1.2 by 1 hour
We found no clear difference between 'haloperidol + promethazine' and 'antipsychotic ‐ haloperidol' within this subgroup (RR 0.75, 95% CI 0.46 to 1.23; Analysis 1.1).
1.1.3 by 2 hours
We found evidence of a clear difference between 'haloperidol + promethazine' and 'antipsychotic ‐ haloperidol' (RR 0.55, 95% CI 0.32 to 0.96). This subgroup had important levels of heterogeneity (Chi2 =0.0; df=0.0; P=0.0; I2 =100%; Analysis 1.1).
1.2 Tranquil or asleep: 2. Not asleep
For this outcome, we found a single study (n=316).
1.2.1 by 30 minutes
For this outcome, within this subgroup, we did find evidence that 'haloperidol + promethazine' was clearly different in its effects compared with 'antipsychotic ‐ haloperidol' (RR 0.89, 95% CI 0.82 to 0.96; Analysis 1.2).
1.2. Analysis.
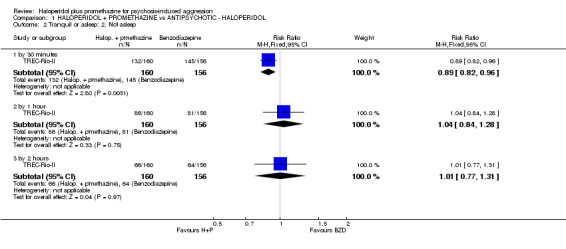
Comparison 1 HALOPERIDOL + PROMETHAZINE vs ANTIPSYCHOTIC ‐ HALOPERIDOL, Outcome 2 Tranquil or asleep: 2. Not asleep.
1.2.2 by 1 hour
We found no clear difference between 'haloperidol + promethazine' and 'antipsychotic ‐ haloperidol' within this subgroup (RR 1.04, 95% CI 0.84 to 1.28; Analysis 1.2).
1.2.3 by 2 hours
We found no clear difference between 'haloperidol + promethazine' and 'antipsychotic ‐ haloperidol' within this subgroup (RR 1.01, 95% CI 0.77 to 1.31; Analysis 1.2).
1.3 Tranquil or asleep: 3. Time until tranquil or asleep (RSS, high score=good)
We identified one study (n=60) relevant to this outcome.
1.3.1 by 1 hour
We found evidence of a clear difference between 'haloperidol + promethazine' and 'antipsychotic ‐ haloperidol' within this subgroup (mean difference (MD) ‐0.10, 95% CI ‐0.58 to 0.38; Analysis 1.3).
1.3. Analysis.
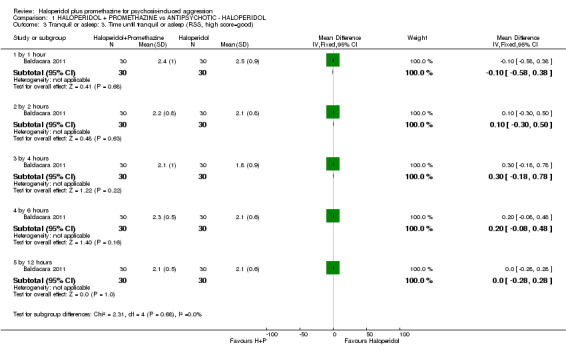
Comparison 1 HALOPERIDOL + PROMETHAZINE vs ANTIPSYCHOTIC ‐ HALOPERIDOL, Outcome 3 Tranquil or asleep: 3. Time until tranquil or asleep (RSS, high score=good).
1.3.2 by 2 hours
For this outcome, within this subgroup, we did find evidence that 'haloperidol + promethazine' was clearly different in its effects compared with 'antipsychotic ‐ haloperidol' (MD 0.10, 95% CI ‐0.30 to 0.50; Analysis 1.3).
1.3.3 by 4 hours
We found evidence of a clear difference between 'haloperidol + promethazine' and 'antipsychotic ‐ haloperidol' within this subgroup (MD 0.30, 95% CI ‐0.18 to 0.78; Analysis 1.3).
1.3.4 by 6 hours
We found evidence of a clear difference between 'haloperidol + promethazine' and 'antipsychotic ‐ haloperidol' within this subgroup (MD 0.20, 95% CI ‐0.08 to 0.48; Analysis 1.3).
1.3.5 by 12 hours
We found evidence of a clear difference between 'haloperidol + promethazine' and 'antipsychotic ‐ haloperidol' within this subgroup (MD 0.00, 95% CI ‐0.28 to 0.28; Analysis 1.3).
1.4 Global state: 1. Needing restraints or seclusion
We identified two studies relevant to this outcome.
1.4.1 by 2 hours
We found one trial to be relevant to this subgroup, with a total of 311 participants. For this subgroup, we did not find evidence of a clear difference between the two treatments (RR 0.80, 95% CI 0.54 to 1.18; Analysis 1.4).
1.4. Analysis.
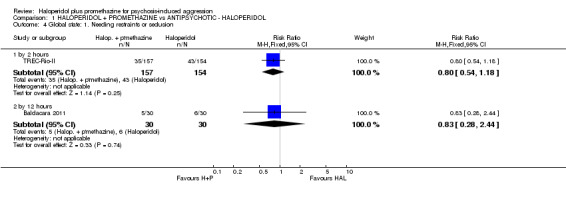
Comparison 1 HALOPERIDOL + PROMETHAZINE vs ANTIPSYCHOTIC ‐ HALOPERIDOL, Outcome 4 Global state: 1. Needing restraints or seclusion.
1.4.2 by 12 hours
There was a single trial in this subgroup, with a total of 60 participants. For this subgroup, we did not find evidence of a clear difference between the two treatments (RR 0.83, 95% CI 0.28 to 2.44; Analysis 1.4).
1.5 Global state: 2. Various measures
For this outcome we found a single study.
1.5.1 requiring additional drugs during initial phase ‐ by 2 hours
There was a single trial in this subgroup, with a total of 311 participants. We found no clear difference between 'haloperidol + promethazine' and 'antipsychotic ‐ haloperidol' within this subgroup (RR 0.45, 95% CI 0.16 to 1.25; Analysis 1.5).
1.5. Analysis.
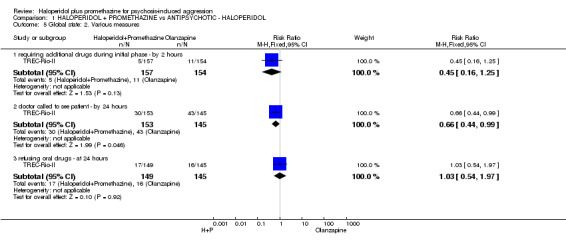
Comparison 1 HALOPERIDOL + PROMETHAZINE vs ANTIPSYCHOTIC ‐ HALOPERIDOL, Outcome 5 Global state: 2. Various measures.
1.5.2 doctor called to see patient ‐ by 24 hours
We found one trial to be relevant to this subgroup, with a total of 298 participants. For this outcome, within this subgroup, we did find evidence that 'haloperidol + promethazine' was clearly different in its effects compared with 'antipsychotic ‐ haloperidol' (RR 0.66, 95% CI 0.44 to 0.99; Analysis 1.5).
1.5.3 refusing oral drugs ‐ at 24 hours
There was a single trial in this subgroup, with a total of 294 participants. For this subgroup, we did not find evidence of a clear difference between the two treatments (RR 1.03, 95% CI 0.54 to 1.97; Analysis 1.5).
1.6 Global state: 3. Average value of additional medication ‐ after initial dose (skewed data)
These continuous data, from a single trial, were too skewed to report in a graphic. We have therefore presented them in an 'Other data' table (Analysis 1.6).
1.6. Analysis.
Comparison 1 HALOPERIDOL + PROMETHAZINE vs ANTIPSYCHOTIC ‐ HALOPERIDOL, Outcome 6 Global state: 3. Average value of additional medication ‐ after initial dose (skewed data).
| Global state: 3. Average value of additional medication ‐ after initial dose (skewed data) | ||||
|---|---|---|---|---|
| Study | Intervention | Mean | SD | Total |
| Baldacara 2011 | Haloperidol + Promethazine | 1.10 | 1.03 | 30 |
| Baldacara 2011 | Haloperidol | 1.53 | 1.19 | 30 |
1.7 Adverse effects: 1. General ‐ Any serious adverse effect
We identified one study (n=298) relevant to this outcome.
1.7.1 by 24 hours
We found evidence of a clear difference between 'haloperidol + promethazine' and 'antipsychotic ‐ haloperidol' within this subgroup (RR 0.09, 95% CI 0.01 to 0.66; Analysis 1.7).
1.7. Analysis.
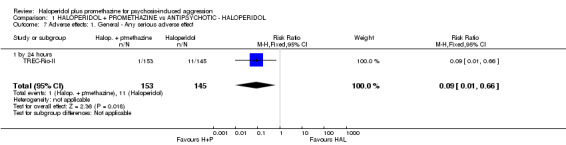
Comparison 1 HALOPERIDOL + PROMETHAZINE vs ANTIPSYCHOTIC ‐ HALOPERIDOL, Outcome 7 Adverse effects: 1. General ‐ Any serious adverse effect.
1.8 Adverse effects: 2. Specific ‐ a. Cardiovascular ‐ hypotension
For this outcome we found a single study, with a total of 60 participants. There were no subgroups in this outcome. We found no clear difference between 'haloperidol + promethazine' and 'antipsychotic ‐ haloperidol' (RR 7.00, 95% CI 0.38 to 129.93; Analysis 1.8).
1.8. Analysis.
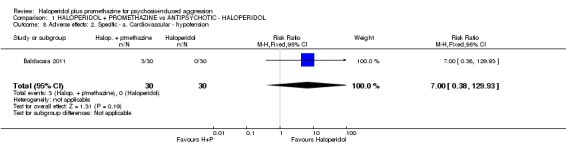
Comparison 1 HALOPERIDOL + PROMETHAZINE vs ANTIPSYCHOTIC ‐ HALOPERIDOL, Outcome 8 Adverse effects: 2. Specific ‐ a. Cardiovascular ‐ hypotension.
1.9 Adverse effects: 2. Specific ‐ b. Central nervous system
We identified two studies relevant to this outcome and divided the data into two subgroups.
1.9.1 seizure ‐ by 24 hours
We found one trial to be relevant to this subgroup, with a total of 298 participants. We found no clear difference between 'haloperidol + promethazine' and 'antipsychotic ‐ haloperidol' within this subgroup (RR 0.95, 95% CI 0.06 to 15.01; Analysis 1.9).
1.9. Analysis.
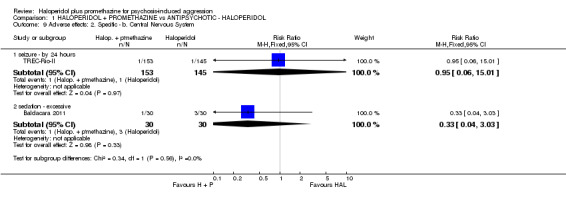
Comparison 1 HALOPERIDOL + PROMETHAZINE vs ANTIPSYCHOTIC ‐ HALOPERIDOL, Outcome 9 Adverse effects: 2. Specific ‐ b. Central Nervous System.
1.9.2 sedation ‐ excessive
We found one trial to be relevant to this subgroup, with a total of 60 participants. We found no clear difference between 'haloperidol + promethazine' and 'antipsychotic ‐ haloperidol' within this subgroup (RR 0.33, 95% CI 0.04 to 3.03; Analysis 1.9).
1.10 Adverse effects: 2. Specific ‐ c. Extrapyramidal problems
We identified two studies relevant to this outcome and divided the data into two subgroups in accordance with our protocol (n=358). For this outcome, we did find evidence that 'haloperidol + promethazine' was clearly different in its effects compared with 'antipsychotic ‐ haloperidol' (RR 0.35, 95% CI 0.14 to 0.88). This outcome had important levels of heterogeneity (Chi2 =5.35; df=1.0; P=0.02; I2 =81%).
1.10.1 acute dystonia ‐ by 24 hours
We found one trial to be relevant to this subgroup, with a total of 298 participants. For this outcome, within this subgroup, we did find evidence that 'haloperidol + promethazine' was clearly different in its effects compared with 'antipsychotic ‐ haloperidol' (RR 0.05, 95% CI 0.00 to 0.76; Analysis 1.10).
1.10. Analysis.
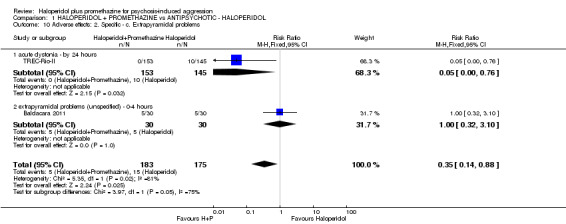
Comparison 1 HALOPERIDOL + PROMETHAZINE vs ANTIPSYCHOTIC ‐ HALOPERIDOL, Outcome 10 Adverse effects: 2. Specific ‐ c. Extrapyramidal problems.
1.10.2 extrapyramidal problems (unspecified) ‐ 0 to 4 hours
There was a single trial in this subgroup, with a total of 60 participants. For this subgroup, we found no evidence of a clear difference between the two treatments (RR 1.00, 95% CI 0.32 to 3.1; Analysis 1.10).
1.11 Service outcomes: Not discharged ‐ by 2 weeks
We identified one study relevant to this outcome with a total of 310 participants. This outcome had no subgroups. We found no evidence of a clear difference between the two treatments (RR 0.83, 95% CI 0.64 to 1.07).
1.12 Specific behaviours: 1. Aggression ‐ a. Other episode of aggression
We found one trial to be relevant to this subgroup, with a total of 298 participants. For this subgroup, we found no evidence of a clear difference between the two treatments (RR 1.17, 95% CI 0.68 to 2.01; Analysis 1.12).
1.12. Analysis.
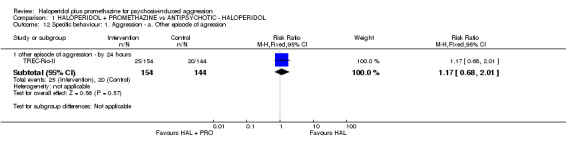
Comparison 1 HALOPERIDOL + PROMETHAZINE vs ANTIPSYCHOTIC ‐ HALOPERIDOL, Outcome 12 Specific behaviour: 1. Aggression ‐ a. Other episode of agression.
1.13 Specific behaviours: 1. Aggression ‐ b. Average aggression score (OAS, high score=bad)
For this outcome we found a single study.
1.13.1 by 1 hour
There was a single trial in this subgroup, with a total of 60 participants. We found evidence of a clear difference between 'haloperidol + promethazine' and 'antipsychotic ‐ haloperidol' within this subgroup (MD 4.50, 95% CI 2.72 to 6.28; Analysis 1.13).
1.13. Analysis.
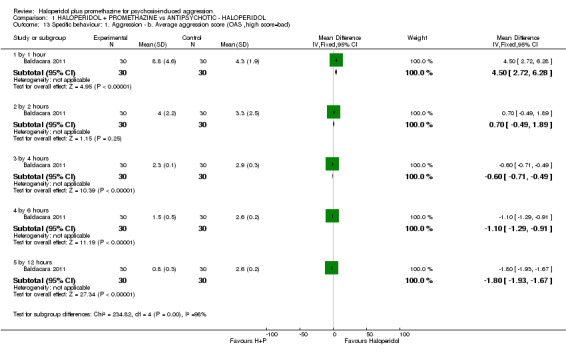
Comparison 1 HALOPERIDOL + PROMETHAZINE vs ANTIPSYCHOTIC ‐ HALOPERIDOL, Outcome 13 Specific behaviour: 1. Aggression ‐ b. Average aggression score (OAS ,high score=bad).
1.13.2 by 2 hours
There was a single trial in this subgroup, with a total of 60 participants. We found no clear difference between 'haloperidol + promethazine' and 'antipsychotic ‐ haloperidol' within this subgroup (MD 0.70, 95% CI ‐0.49 to 1.89; Analysis 1.13).
1.13.3 by 4 hours
We found one trial to be relevant to this subgroup, with a total of 60 participants. We found evidence of a clear difference between 'haloperidol + promethazine' and 'antipsychotic ‐ haloperidol' within this subgroup (MD ‐0.60, 95% CI ‐0.71 to ‐0.49; Analysis 1.13).
1.13.4 by 6 hours
There was a single trial in this subgroup, with a total of 60 participants. We found evidence of a clear difference between 'haloperidol + promethazine' and 'antipsychotic ‐ haloperidol' within this subgroup (MD ‐1.10, 95% CI ‐1.29 to ‐0.91; Analysis 1.13).
1.13.5 by 12 hours
We found one trial to be relevant to this subgroup, with a total of 60 participants. For this outcome, within this subgroup, we did find evidence that 'haloperidol + promethazine' was clearly different in its effects compared with 'antipsychotic ‐ haloperidol' (MD ‐1.80, 95% CI ‐1.93 to ‐1.67; Analysis 1.13).
1.14 Specific behaviours: 1. Aggression ‐ c. Average agitation score (OASS, high score=bad)
For this outcome we found a single study and divided the data into five subgroups.
1.14.1 by 1 hour
There was a single trial in this subgroup, with a total of 60 participants. We found evidence of a clear difference between 'haloperidol + promethazine' and 'antipsychotic ‐ haloperidol' within this subgroup (MD 24.50, 95% CI 21.68 to 27.32; Analysis 1.14).
1.14. Analysis.
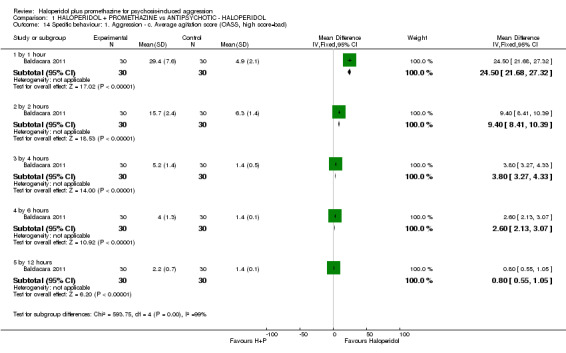
Comparison 1 HALOPERIDOL + PROMETHAZINE vs ANTIPSYCHOTIC ‐ HALOPERIDOL, Outcome 14 Specific behaviour: 1. Aggression ‐ c. Average agitation score (OASS, high score=bad).
1.14.2 by 2 hours
We found one trial to be relevant to this subgroup, with a total of 60 participants. We found evidence of a clear difference between 'haloperidol + promethazine' and 'antipsychotic ‐ haloperidol' within this subgroup (MD 9.40, 95% CI 8.41 to 10.39; Analysis 1.14).
1.14.3 by 4 hours
There was a single trial in this subgroup, with a total of 60 participants. We found evidence of a clear difference between 'haloperidol + promethazine' and 'antipsychotic ‐ haloperidol' within this subgroup (MD 3.80, 95% CI 3.27 to 4.33; Analysis 1.14).
1.14.4 by 6 hours
We found one trial to be relevant to this subgroup, with a total of 60 participants. We found evidence of a clear difference between 'haloperidol + promethazine' and 'antipsychotic ‐ haloperidol' within this subgroup (MD 2.60, 95% CI 2.13 to 3.07; Analysis 1.14).
1.14.5 by 12 hours
We found one trial to be relevant to this subgroup, with a total of 60 participants. We found no clear difference between 'haloperidol + promethazine' and 'antipsychotic ‐ haloperidol' within this subgroup (MD 0.80, 95% CI 0.55 to 1.05; Analysis 1.14).
1.15 Leaving the study early
We identified two studies relevant to this outcome.
1.15.1 before treatment
We found one trial to be relevant to this subgroup, with a total of 316 participants. We found no clear difference between 'haloperidol + promethazine' and 'antipsychotic ‐ haloperidol' within this subgroup (RR 1.46, 95% CI 0.25 to 8.63; Analysis 1.15).
1.15. Analysis.
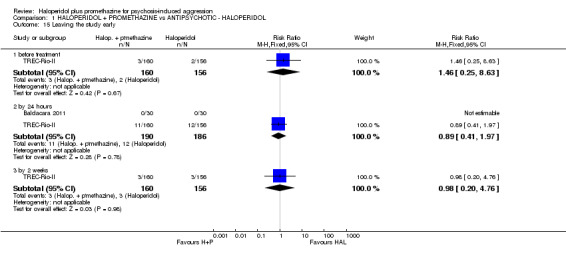
Comparison 1 HALOPERIDOL + PROMETHAZINE vs ANTIPSYCHOTIC ‐ HALOPERIDOL, Outcome 15 Leaving the study early.
1.15.2 by 24 hours
We found two trials to be relevant to this subgroup, with a total of 376 participants. We found no clear difference between 'haloperidol + promethazine' and 'antipsychotic ‐ haloperidol' within this subgroup (RR 0.89, 95% CI 0.41 to 1.97; Analysis 1.15).
1.15.3 by 2 weeks
We found one trial to be relevant to this subgroup, with a total of 316 participants. We found no clear difference between 'haloperidol + promethazine' and 'antipsychotic ‐ haloperidol' within this subgroup (RR 0.97, 95% CI 0.20 to 4.76; Analysis 1.15).
2. COMPARISON 2: HALOPERIDOL + PROMETHAZINE vs ANTIPSYCHOTIC ‐ OLANZAPINE
This comparison has 21 outcomes.
2.1 Tranquil or asleep: 1. Not tranquil or asleep
For this outcome we found a single study and divided the data into four subgroups.
2.1.1 by 30 minutes
There was a single trial in this subgroup, with a total of 300 participants. For this subgroup, we found no evidence of a clear difference between the two treatments (RR 0.60, 95% CI 0.22 to 1.61; Analysis 2.1).
2.1. Analysis.
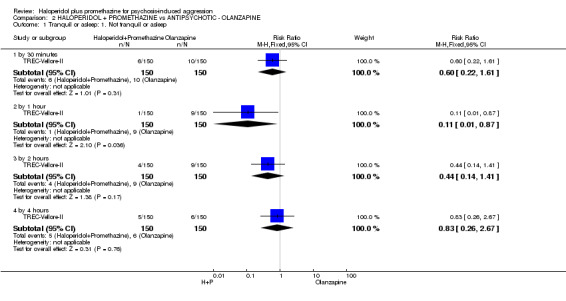
Comparison 2 HALOPERIDOL + PROMETHAZINE vs ANTIPSYCHOTIC ‐ OLANZAPINE, Outcome 1 Tranquil or asleep: 1. Not tranquil or asleep.
2.1.2 by 1 hour
We found one trial to be relevant to this subgroup, with a total of 300 participants. For this outcome, within this subgroup, we did find evidence that 'haloperidol + promethazine' was clearly different in its effects compared with 'antipsychotic ‐ olanzapine' (RR 0.11, 95% CI 0.01 to 0.87; Analysis 2.1).
2.1.3 by 2 hours
We found one trial to be relevant to this subgroup, with a total of 300 participants. For this subgroup, we found no evidence of a clear difference between the two treatments (RR 0.44, 95% CI 0.14 to 1.41; Analysis 2.1).
2.1.4 by 4 hours
We found one trial to be relevant to this subgroup, with a total of 300 participants. For this subgroup, we found no evidence of a clear difference between the two treatments (RR 0.83, 95% CI 0.26 to 2.67; Analysis 2.1).
2.2 Tranquil or asleep: 2. Not asleep
For this outcome we found a single study.
2.2.1 by 30 minutes
We found one trial to be relevant to this subgroup, with a total of 300 participants. For this outcome, within this subgroup, we did find evidence that 'haloperidol + promethazine' was clearly different in its effects compared with 'antipsychotic ‐ olanzapine' (RR 0.65, 95% CI 0.46 to 0.93; Analysis 2.2).
2.2. Analysis.
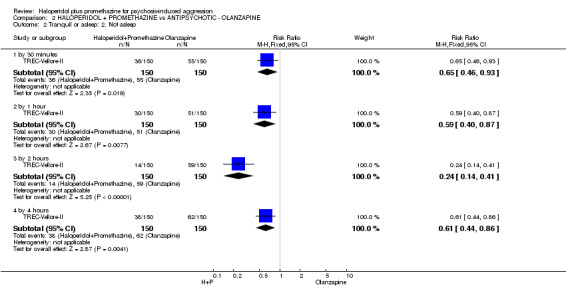
Comparison 2 HALOPERIDOL + PROMETHAZINE vs ANTIPSYCHOTIC ‐ OLANZAPINE, Outcome 2 Tranquil or asleep: 2. Not asleep.
2.2.2 by 1 hour
We found one trial to be relevant to this subgroup, with a total of 300 participants. For this outcome, within this subgroup, we did find evidence that 'haloperidol + promethazine' was clearly different in its effects compared with 'antipsychotic ‐ olanzapine' (RR 0.59, 95% CI 0.40 to 0.87; Analysis 2.2).
2.2.3 by 2 hours
We found one trial to be relevant to this subgroup, with a total of 300 participants. We found evidence of a clear difference between 'haloperidol + promethazine' and 'antipsychotic ‐ olanzapine' within this subgroup (RR 0.24, 95% CI 0.14 to 0.41; Analysis 2.2).
2.2.4 by 4 hours
There was a single trial in this subgroup, with a total of 300 participants. We found evidence of a clear difference between 'haloperidol + promethazine' and 'antipsychotic ‐ olanzapine' within this subgroup (RR 0.61, 95% CI 0.44 to 0.86; Analysis 2.2).
2.3 Tranquil or asleep: 3. Never tranquil or asleep during first 4 hours
We identified one study relevant to this outcome involving 300 participants. There were no subgroups in this outcome. We found no clear difference between 'haloperidol + promethazine' and 'antipsychotic ‐ olanzapine' (RR 0.25, 95% CI 0.03 to 2.21; Analysis 2.3).
2.3. Analysis.
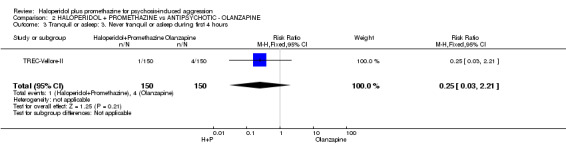
Comparison 2 HALOPERIDOL + PROMETHAZINE vs ANTIPSYCHOTIC ‐ OLANZAPINE, Outcome 3 Tranquil or asleep: 3. Never tranquil or asleep during first 4 hours.
2.4 Tranquil or asleep: 4. Average sedation score (RSS, high score=good)
We identified one study relevant to this outcome and divided the data into five subgroups.
2.4.1 by 1 hour
We found one trial to be relevant to this subgroup, with a total of 60 participants. We found evidence of a clear difference between 'haloperidol + promethazine' and 'antipsychotic ‐ olanzapine' within this subgroup (MD 0.20, 95% CI ‐0.26 to 0.66; Analysis 2.4).
2.4. Analysis.
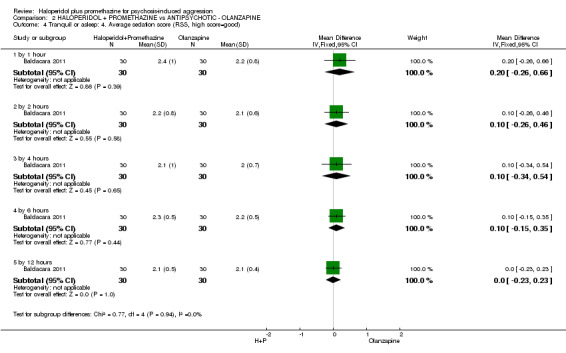
Comparison 2 HALOPERIDOL + PROMETHAZINE vs ANTIPSYCHOTIC ‐ OLANZAPINE, Outcome 4 Tranquil or asleep: 4. Average sedation score (RSS, high score=good).
2.4.2 by 2 hours
There was a single trial in this subgroup, with a total of 60 participants. We found evidence of a clear difference between 'haloperidol + promethazine' and 'antipsychotic ‐ olanzapine' within this subgroup (MD 0.10, 95% CI ‐0.26 to 0.46; Analysis 2.4).
2.4.3 by 4 hours
We found one trial to be relevant to this subgroup, with a total of 60 participants. For this outcome, within this subgroup, we did find evidence that 'haloperidol + promethazine' was clearly different in its effects compared with 'antipsychotic ‐ olanzapine' (MD 0.10, 95% CI ‐0.34 to 0.54; Analysis 2.4).
2.4.4 by 6 hours
We found one trial to be relevant to this subgroup, with a total of 60 participants. For this outcome, within this subgroup, we did find evidence that 'haloperidol + promethazine' was clearly different in its effects compared with 'antipsychotic ‐ olanzapine' (MD 0.10, 95% CI ‐0.15 to 0.35; Analysis 2.4).
2.4.5 by 12 hours
We found one trial to be relevant to this subgroup, with a total of 60 participants. We found evidence of a clear difference between 'haloperidol + promethazine' and 'antipsychotic ‐ olanzapine' within this subgroup (MD 0.00, 95% CI ‐0.23 to 0.23; Analysis 2.4).
2.5 Tranquil or asleep: 5. Time (skewed data)
These continuous data (one RCT) had such large standard deviations as to suggest that analysis within Review Manager would be inadvisable. We have therefore reported these data in an 'Other data' table (Analysis 2.5).
2.5. Analysis.
Comparison 2 HALOPERIDOL + PROMETHAZINE vs ANTIPSYCHOTIC ‐ OLANZAPINE, Outcome 5 Tranquil or asleep: 5. Time (skewed data).
| Tranquil or asleep: 5. Time (skewed data) | ||||||
|---|---|---|---|---|---|---|
| Study | Intervention | Mean (mins) | SD | N | Statistical test | p |
| time until tranquil or asleep | ||||||
| TREC‐Vellore‐II | Haloperidol + Promethazine | 12.8 | 16.7 | 150 | Mann‐Whitney U | 0.4 |
| TREC‐Vellore‐II | Olanzapine | 20.5 | 34.5 | 150 | ||
| time until asleep | ||||||
| TREC‐Vellore‐II | Haloperidol + Promethazine | 26.2 | 32.2 | 150 | Mann‐Whitney U | 0.2 |
| TREC‐Vellore‐II | Olanzapine | 34.9 | 42.2 | 150 | ||
2.6 Tranquil or asleep: 6. Effect of tranquillisation (PANSS‐EC, high=bad) (skewed data)
These continuous data, from a single trial, had such large standard deviations as to suggest that analysis within Review Manager would be inadvisable. We have therefore reported these data in an 'Other data' table (Analysis 2.6).
2.6. Analysis.
Comparison 2 HALOPERIDOL + PROMETHAZINE vs ANTIPSYCHOTIC ‐ OLANZAPINE, Outcome 6 Tranquil or asleep: 6. Effect of tranquillisation (PANSS‐EC, high=bad) (skewed data).
| Tranquil or asleep: 6. Effect of tranquillisation (PANSS‐EC, high=bad) (skewed data) | ||||
|---|---|---|---|---|
| Study | Intervention | Mean | SD | N |
| at 30 minutes | ||||
| Mantovani 2013 | Haloperidol + Promethazine | 10.9 | 6.7 | 27 |
| Mantovani 2013 | Olanzapine | 10.1 | 6.4 | 25 |
| at 60 minutes | ||||
| Mantovani 2013 | Haloperidol + Promethazine | 11.1 | 7.6 | 27 |
| Mantovani 2013 | Olanzapine | 8 | 3.8 | 25 |
| at 90 minutes | ||||
| Mantovani 2013 | Haloperidol + Promethazine | 10.7 | 6.7 | 27 |
| Mantovani 2013 | Olanzapine | 9.2 | 5.3 | 25 |
2.7 Tranquil or asleep: 7. Level of tranquillisation/agitation (ACES) (skewed data)
These continuous data (one RCT) were too skewed to report in a graphic. We have therefore presented them in an 'Other data' table (Analysis 2.7).
2.7. Analysis.
Comparison 2 HALOPERIDOL + PROMETHAZINE vs ANTIPSYCHOTIC ‐ OLANZAPINE, Outcome 7 Tranquil or asleep: 7. Level of tranquillisation / agitation (ACES) (skewed data).
| Tranquil or asleep: 7. Level of tranquillisation / agitation (ACES) (skewed data) | ||||
|---|---|---|---|---|
| Study | Intervention | Mean | SD | Nl |
| at 30 minutes | ||||
| Mantovani 2013 | Haloperidol + Promethazine | 5.2 | 8.1 | 27 |
| Mantovani 2013 | Olanzapine | 5.5 | 7.5 | 25 |
| at 90 minutes | ||||
| Mantovani 2013 | Haloperidol + Promethazine | 5.0 | 10.8 | 27 |
| Mantovani 2013 | Olanzapine | 5.8 | 10 | 25 |
2.8 Global state: 1. No overall improvement
For this outcome we found a single study and divided the data into four subgroups.
2.8.1 by 30 minutes
There was a single trial in this subgroup, with a total of 300 participants. For this outcome, within this subgroup, we did find evidence that 'haloperidol + promethazine' was clearly different in its effects compared with 'antipsychotic ‐ olanzapine' (RR 0.57, 95% CI 0.36 to 0.91; Analysis 2.8).
2.8. Analysis.
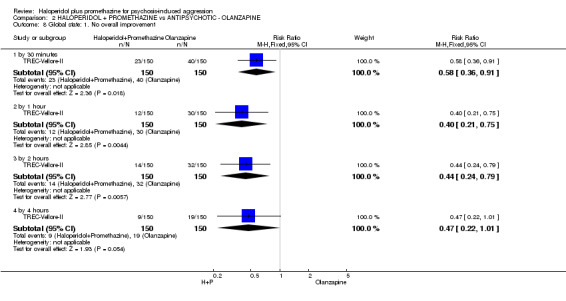
Comparison 2 HALOPERIDOL + PROMETHAZINE vs ANTIPSYCHOTIC ‐ OLANZAPINE, Outcome 8 Global state: 1. No overall improvement.
2.8.2 by 1 hour
We found one trial to be relevant to this subgroup, with a total of 300 participants. For this outcome, within this subgroup, we did find evidence that 'haloperidol + promethazine' was clearly different in its effects compared with 'antipsychotic ‐ olanzapine' (RR 0.40, 95% CI 0.21 to 0.75; Analysis 2.8).
2.8.3 by 2 hours
There was a single trial in this subgroup, with a total of 300 participants. For this outcome, within this subgroup, we did find evidence that 'haloperidol + promethazine' was clearly different in its effects compared with 'antipsychotic ‐ olanzapine' (RR 0.44, 95% CI 0.24 to 0.79; Analysis 2.8).
2.8.4 by 4 hours
We found one trial to be relevant to this subgroup, with a total of 300 participants. For this subgroup, we found no evidence of a clear difference between the two treatments (RR 0.47, 95% CI 0.22 to 1.01; Analysis 2.8).
2.9 Global state: 2. Needing restraints or seclusion
For this outcome we found three relevant studies and divided the data into five subgroups.
2.9.1 by 30 minutes
We found one trial to be relevant to this subgroup, with a total of 300 participants. We found no clear difference between 'haloperidol + promethazine' and 'antipsychotic ‐ olanzapine' within this subgroup (RR 1.02, 95% CI 0.71 to 1.47; Analysis 2.9).
2.9. Analysis.
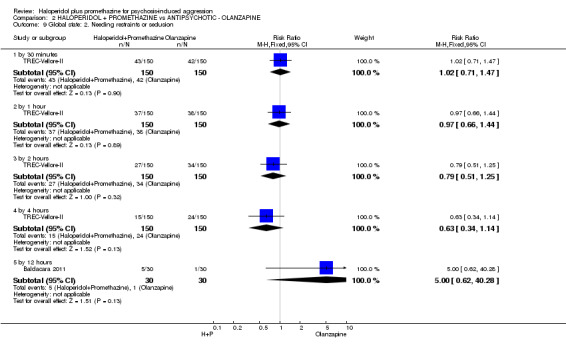
Comparison 2 HALOPERIDOL + PROMETHAZINE vs ANTIPSYCHOTIC ‐ OLANZAPINE, Outcome 9 Global state: 2. Needing restraints or seclusion.
2.9.2 by 1 hour
We found one trial to be relevant to this subgroup, with a total of 300 participants. For this subgroup, we found no evidence of a clear difference between the two treatments (RR 0.97, 95% CI 0.66 to 1.44; Analysis 2.9).
2.9.3 by 2 hours
We found one trial to be relevant to this subgroup, with a total of 300 participants. We found no clear difference between 'haloperidol + promethazine' and 'antipsychotic ‐ olanzapine' within this subgroup (RR 0.79, 95% CI 0.51 to 1.25; Analysis 2.9).
2.9.4 by 4 hours
We found one trial to be relevant to this subgroup, with a total of 300 participants. We found no clear difference between 'haloperidol + promethazine' and 'antipsychotic ‐ olanzapine' within this subgroup (RR 0.63, 95% CI 0.34 to 1.14; Analysis 2.9).
2.9.5 by 12 hours
We found one trial to be relevant to this subgroup, with a total of 60 participants. For this subgroup, we found no evidence of a clear difference between the two treatments (RR 5.00, 95% CI 0.62 to 40.28; Analysis 2.9).
2.10 Global state: 3. Various measures
For this outcome we found two relevant studies and divided the data into four subgroups.
2.10.1 requiring additional drugs during initial phase ‐ by 4 hours
There were two relevant trials in this subgroup, with a total of 356 participants. We found evidence of a clear difference between 'haloperidol + promethazine' and 'antipsychotic ‐ olanzapine' within this subgroup (RR 0.52, 95% CI 0.37 to 0.74). For this outcome heterogeneity was high (Chi2 =2.25; df=1.0; P=0.13; I2 =55%; Analysis 2.10).
2.10. Analysis.
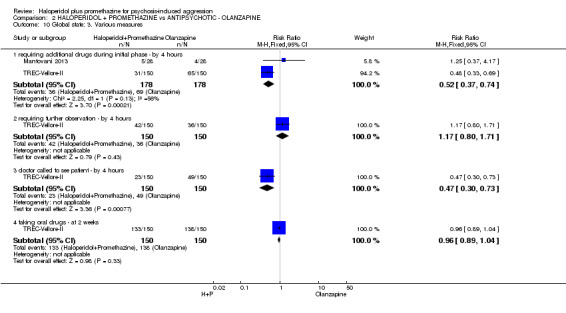
Comparison 2 HALOPERIDOL + PROMETHAZINE vs ANTIPSYCHOTIC ‐ OLANZAPINE, Outcome 10 Global state: 3. Various measures.
2.10.2 requiring further observation ‐ by 4 hours
There was a single trial in this subgroup, with a total of 300 participants. We found no clear difference between 'haloperidol + promethazine' and 'antipsychotic ‐ olanzapine' within this subgroup (RR 1.17, 95% CI 0.80 to 1.71; Analysis 2.10).
2.10.3 doctor called to see patient ‐ by 4 hours
There was a single trial in this subgroup, with a total of 300 participants. For this outcome, within this subgroup, we did find evidence that 'haloperidol + promethazine' was clearly different in its effects compared with 'antipsychotic ‐ olanzapine' (RR 0.47, 95% CI 0.30 to 0.73; Analysis 2.10).
2.10.4 taking oral drugs ‐ at 2 weeks
There was a single trial in this subgroup, with a total of 300 participants. We found no clear difference between 'haloperidol + promethazine' and 'antipsychotic ‐ olanzapine' within this subgroup (RR 0.96, 95% CI 0.89 to 1.04; Analysis 2.10).
2.11 Global state: 4. Average improvement (CGI, high score=bad)
We identified one study relevant to this outcome and divided the data into four subgroups.
2.11.1 by 30 minutes
We found one trial to be relevant to this subgroup, with a total of 300 participants. For this outcome, within this subgroup, we did find evidence that 'haloperidol + promethazine' was clearly different in its effects compared with 'antipsychotic ‐ olanzapine' (MD ‐0.35, 95% CI ‐0.58 to ‐0.12; Analysis 2.11).
2.11. Analysis.
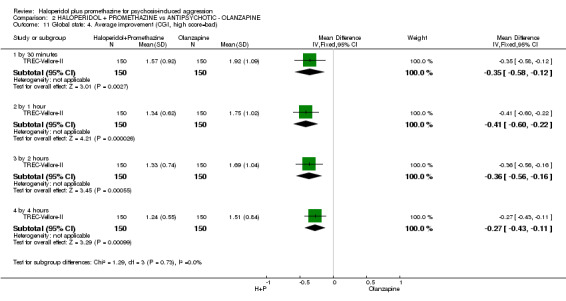
Comparison 2 HALOPERIDOL + PROMETHAZINE vs ANTIPSYCHOTIC ‐ OLANZAPINE, Outcome 11 Global state: 4. Average improvement (CGI, high score=bad).
2.11.2 by 1 hour
There was a single trial in this subgroup, with a total of 300 participants. For this outcome, within this subgroup, we did find evidence that 'haloperidol + promethazine' was clearly different in its effects compared with 'antipsychotic ‐ olanzapine' (MD ‐0.41, 95% CI ‐0.6 to ‐0.22; Analysis 2.11).
2.11.3 by 2 hours
There was a single trial in this subgroup, with a total of 300 participants. For this outcome, within this subgroup, we did find evidence that 'haloperidol + promethazine' was clearly different in its effects compared with 'antipsychotic ‐ olanzapine' (MD ‐0.36, 95% CI ‐0.56 to ‐0.16). For this outcome heterogeneity was high (Chi2 =0.0; df=0.0; P=0.0; I2 =100%; Analysis 2.11).
2.11.4 by 4 hours
There was a single trial in this subgroup, with a total of 300 participants. For this outcome, within this subgroup, we did find evidence that 'haloperidol + promethazine' was clearly different in its effects compared with 'antipsychotic ‐ olanzapine' (MD ‐0.27, 95% CI ‐0.43 to ‐0.11; Analysis 2.11).
2.12 Global state: 5. Average value of additional medication ‐ after initial dose (skewed data)
These continuous data (one RCT) were too skewed to report in a graphic. We have therefore presented them in an 'Other data' table (Analysis 2.12).
2.12. Analysis.
Comparison 2 HALOPERIDOL + PROMETHAZINE vs ANTIPSYCHOTIC ‐ OLANZAPINE, Outcome 12 Global state: 5. Average value of additional medication ‐ after initial dose (skewed data).
| Global state: 5. Average value of additional medication ‐ after initial dose (skewed data) | ||||
|---|---|---|---|---|
| Study | Intervention | Mean | SD | N |
| Baldacara 2011 | Haloperidol + Promethazine | 1.10 | 1.03 | 30 |
| Baldacara 2011 | Olanzapine | 0.37 | 0.77 | 30 |
2.13 Adverse effects: 1. General ‐ serious adverse effect
For this outcome we found a single study.
2.13.1 by 4 hours
We found one trial to be relevant to this subgroup, with a total of 300 participants. We found no clear difference between 'haloperidol + promethazine' and 'antipsychotic ‐ olanzapine' within this subgroup (RR 0.33, 95% CI 0.04 to 3.17; Analysis 2.13).
2.13. Analysis.
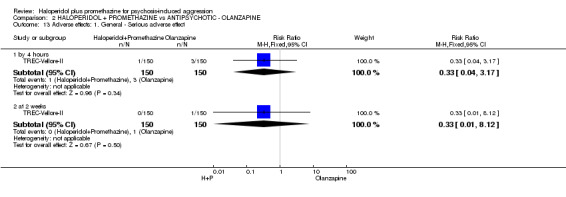
Comparison 2 HALOPERIDOL + PROMETHAZINE vs ANTIPSYCHOTIC ‐ OLANZAPINE, Outcome 13 Adverse effects: 1. General ‐ Serious adverse effect.
2.13.2 at 2 weeks
There was a single trial in this subgroup, with a total of 300 participants. For this subgroup, we found no evidence of a clear difference between the two treatments (RR 0.33, 95% CI 0.01 to 8.12; Analysis 2.13).
2.14 Adverse effects: 2. Specific ‐ a. Cardiovascular ‐ hypotension
For this outcome we found two relevant studies involving 116 participants. This outcome had no subgroups. We found no evidence of a clear difference between the two treatments (RR 3.00, 95% CI 0.49 to 18.31; Analysis 2.14).
2.14. Analysis.
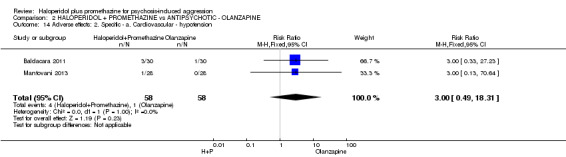
Comparison 2 HALOPERIDOL + PROMETHAZINE vs ANTIPSYCHOTIC ‐ OLANZAPINE, Outcome 14 Adverse effects: 2. Specific ‐ a. Cardiovascular ‐ hypotension.
2.15 Adverse effects: 2. Specific ‐ b. Central nervous system ‐ sedation ‐ excessive
We identified two studies relevant to this outcome involving 116 participants. This outcome had no subgroups. We found no clear difference between 'haloperidol + promethazine' and 'antipsychotic ‐ olanzapine' (RR 0.67, 95% CI 0.12 to 3.84; Analysis 2.15).
2.15. Analysis.
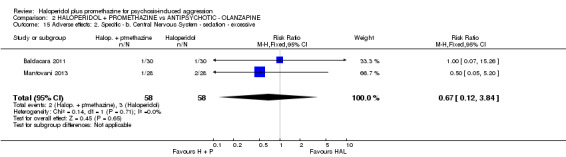
Comparison 2 HALOPERIDOL + PROMETHAZINE vs ANTIPSYCHOTIC ‐ OLANZAPINE, Outcome 15 Adverse effects: 2. Specific ‐ b. Central Nervous System ‐ sedation ‐ excessive.
2.16 Adverse effects: 2. Specific ‐ c. Extrapyramidal problems ‐ 0 to 4 hours
For this outcome we found three relevant studies and categorised the data into one subgroup.
2.16.1 any change in scale‐rated extrapyramidal problems (Simpson‐Angus Scale)
There were three relevant trials in this subgroup, with a total of 416 participants. We found evidence of a clear difference between 'haloperidol + promethazine' and 'antipsychotic ‐ olanzapine' within this subgroup (RR 1.76, 95% CI 1.12 to 2.77). This subgroup had important levels of heterogeneity (Chi2 =2.45; df=1.0; P=0.12; I2 =59%; Analysis 2.16).
2.16. Analysis.
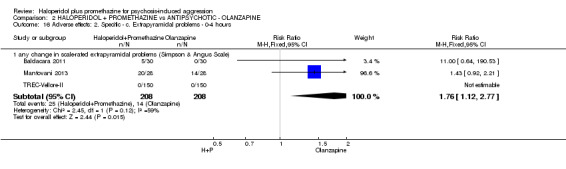
Comparison 2 HALOPERIDOL + PROMETHAZINE vs ANTIPSYCHOTIC ‐ OLANZAPINE, Outcome 16 Adverse effects: 2. Specific ‐ c. Extrapyramidal problems ‐ 0‐4 hours.
2.17 Specific behaviours: 1. Severe agitation
For this outcome we found a single study involving 56 participants. There were no subgroups in this outcome. We found no evidence of a clear difference between the two treatments (RR 7.00, 95% CI 0.38 to 129.55; Analysis 2.17).
2.17. Analysis.
Comparison 2 HALOPERIDOL + PROMETHAZINE vs ANTIPSYCHOTIC ‐ OLANZAPINE, Outcome 17 Specific behaviour: 1. Severe agitation.
2.18 Specific behaviours: 2. Average aggression score (OAS, high score=bad)
We identified one study relevant to this outcome.
2.18.1 by 1 hour
There was a single trial in this subgroup, with a total of 60 participants. We found evidence of a clear difference between 'haloperidol + promethazine' and 'antipsychotic ‐ olanzapine' within this subgroup (MD 5.40, 95% CI 3.72 to 7.08; Analysis 2.18).
2.18. Analysis.
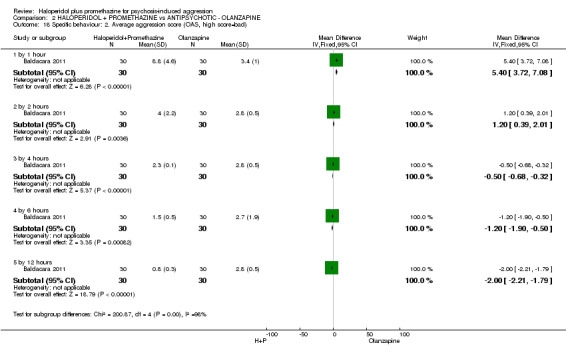
Comparison 2 HALOPERIDOL + PROMETHAZINE vs ANTIPSYCHOTIC ‐ OLANZAPINE, Outcome 18 Specific behaviour: 2. Average aggression score (OAS, high score=bad).
2.18.2 by 2 hours
We found one trial to be relevant to this subgroup, with a total of 60 participants. We found no clear difference between 'haloperidol + promethazine' and 'antipsychotic ‐ olanzapine' within this subgroup (MD 1.20, 95% CI 0.39 to 2.01; Analysis 2.18).
2.18.3 by 4 hours
There was a single trial in this subgroup, with a total of 60 participants. For this outcome, within this subgroup, we did find evidence that 'haloperidol + promethazine' was clearly different in its effects compared with 'antipsychotic ‐ olanzapine' (MD ‐0.50, 95% CI ‐0.68 to ‐0.32; Analysis 2.18).
2.18.4 by 6 hours
We found one trial to be relevant to this subgroup, with a total of 60 participants. We found evidence of a clear difference between 'haloperidol + promethazine' and 'antipsychotic ‐ olanzapine' within this subgroup (MD ‐1.20, 95% CI ‐1.90 to ‐0.50; Analysis 2.18).
2.18.5 by 12 hours
There was a single trial in this subgroup, with a total of 60 participants. We found evidence of a clear difference between 'haloperidol + promethazine' and 'antipsychotic ‐ olanzapine' within this subgroup (MD ‐2.00, 95% CI ‐2.21 to ‐1.79; Analysis 2.18).
2.19 Specific behaviours: 3. Average agitation score (OASS, high score=bad)
We identified one study relevant to this outcome and divided the data into five subgroups.
2.19.1 by 1 hour
We found one trial to be relevant to this subgroup, with a total of 60 participants. We found evidence of a clear difference between 'haloperidol + promethazine' and 'antipsychotic ‐ olanzapine' within this subgroup (MD 26.50, 95% CI 23.76 to 29.24; Analysis 2.19).
2.19. Analysis.
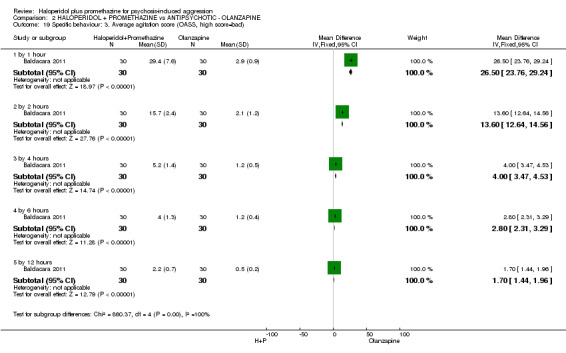
Comparison 2 HALOPERIDOL + PROMETHAZINE vs ANTIPSYCHOTIC ‐ OLANZAPINE, Outcome 19 Specific behaviour: 3. Average agitation score (OASS, high score=bad).
2.19.2 by 2 hours
We found one trial to be relevant to this subgroup, with a total of 60 participants. For this outcome, within this subgroup, we did find evidence that 'haloperidol + promethazine' was clearly different in its effects compared with 'antipsychotic ‐ olanzapine' (MD 13.60, 95% CI 12.64 to 14.56; Analysis 2.19).
2.19.3 by 4 hours
We found one trial to be relevant to this subgroup, with a total of 60 participants. We found evidence of a clear difference between 'haloperidol + promethazine' and 'antipsychotic ‐ olanzapine' within this subgroup (MD 4.00, 95% CI 3.47 to 4.53; Analysis 2.19).
2.19.4 by 6 hours
There was a single trial in this subgroup, with a total of 60 participants. For this outcome, within this subgroup, we did find evidence that 'haloperidol + promethazine' was clearly different in its effects compared with 'antipsychotic ‐ olanzapine' (MD 2.80, 95% CI 2.31 to 3.29; Analysis 2.19).
2.19.5 by 12 hours
There was a single trial in this subgroup, with a total of 60 participants. We found evidence of a clear difference between 'haloperidol + promethazine' and 'antipsychotic ‐ olanzapine' within this subgroup (MD 1.7, 95% CI 1.44 to 1.96; Analysis 2.19).
2.20 Service outcomes
We identified one study relevant to this outcome.
2.20.1 admitted ‐ by 4 hours
We found one trial to be relevant to this subgroup, with a total of 300 participants. We found no clear difference between 'haloperidol + promethazine' and 'antipsychotic ‐ olanzapine' within this subgroup (RR 0.81, 95% CI 0.56 to 1.16; Analysis 2.20).
2.20. Analysis.
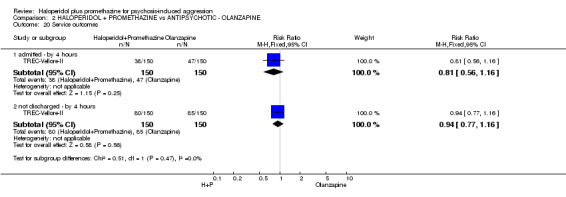
Comparison 2 HALOPERIDOL + PROMETHAZINE vs ANTIPSYCHOTIC ‐ OLANZAPINE, Outcome 20 Service outcomes.
2.20.2 not discharged ‐ by 4 hours
There was a single trial in this subgroup, with a total of 300 participants. For this subgroup, we found no evidence of a clear difference between the two treatments (RR 0.94, 95% CI 0.77 to 1.16; Analysis 2.20).
2.21 Leaving the study early
For this outcome we found three relevant studies and divided the data into five subgroups.
2.21.1 by 30 minutes
We found one trial to be relevant to this subgroup, with a total of 300 participants. For this subgroup, we found no evidence of a clear difference between the two treatments (RR 0.33, 95% CI 0.01 to 8.12; Analysis 2.21).
2.21. Analysis.
Comparison 2 HALOPERIDOL + PROMETHAZINE vs ANTIPSYCHOTIC ‐ OLANZAPINE, Outcome 21 Leaving the study early.
2.21.2 by 2 hours
There was a single trial in this subgroup, with a total of 300 participants. For this subgroup, we found no evidence of a clear difference between the two treatments (RR 0.14, 95% CI 0.01 to 2.74; Analysis 2.21).
2.21.3 by 4 hours
There was a single trial in this subgroup, with a total of 300 participants. We found no clear difference between 'haloperidol + promethazine' and 'antipsychotic ‐ olanzapine' within this subgroup (RR 0.09, 95% CI 0.01 to 1.63; Analysis 2.21).
2.21.4 by 24 hours
We found two trials to be relevant to this subgroup, with a total of 116 participants. We found no clear difference between 'haloperidol + promethazine' and 'antipsychotic ‐ olanzapine' within this subgroup (RR 0.33, 95% CI 0.04 to 3.01; Analysis 2.21).
2.21.5 by 2 weeks
There was a single trial in this subgroup, with a total of 300 participants. We found no clear difference between 'haloperidol + promethazine' and 'antipsychotic ‐ olanzapine' within this subgroup (RR 0.71, 95% CI 0.33 to 1.56; Analysis 2.21).
3. COMPARISON 3: HALOPERIDOL + PROMETHAZINE vs ANTIPSYCHOTIC ‐ ZIPRASIDONE
This comparison has 13 outcomes.
3.1 Tranquil or asleep: 1. Average sedation score (RSS, high score=good)
For this outcome we found a single study and divided the data into five subgroups.
3.1.1 by 1 hour
There was a single trial in this subgroup, with a total of 60 participants. We did not find evidence of a clear difference between 'haloperidol + promethazine' and 'antipsychotic ‐ ziprasidone' within this subgroup (MD ‐0.10, 95% CI ‐0.58 to 0.38; Analysis 3.1).
3.1. Analysis.
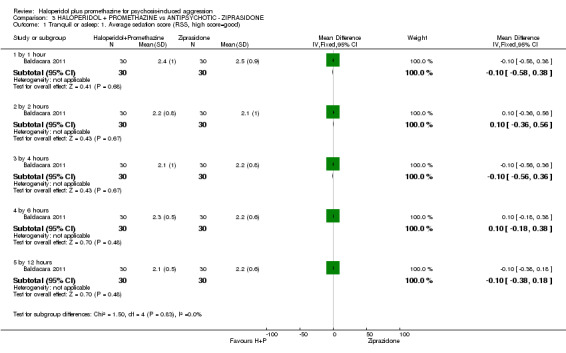
Comparison 3 HALOPERIDOL + PROMETHAZINE vs ANTIPSYCHOTIC ‐ ZIPRASIDONE, Outcome 1 Tranquil or asleep: 1. Average sedation score (RSS, high score=good).
3.1.2 by 2 hours
There was a single trial in this subgroup, with a total of 60 participants. We found evidence of a clear difference between 'haloperidol + promethazine' and 'antipsychotic ‐ ziprasidone' within this subgroup (MD 0.10, 95% CI ‐0.36 to 0.56; Analysis 3.1).
3.1.3 by 4 hours
We found one trial to be relevant to this subgroup, with a total of 60 participants. For this outcome, within this subgroup, we did find evidence that 'haloperidol + promethazine' was clearly different in its effects compared with 'antipsychotic ‐ ziprasidone' (MD ‐0.10, 95% CI ‐0.56 to 0.36; Analysis 3.1).
3.1.4 by 6 hours
We found one trial to be relevant to this subgroup, with a total of 60 participants. For this outcome, within this subgroup, we did find evidence that 'haloperidol + promethazine' was clearly different in its effects compared with 'antipsychotic ‐ ziprasidone' (MD 0.10, 95% CI ‐0.18 to 0.38; Analysis 3.1).
3.1.5 by 12 hours
There was a single trial in this subgroup, with a total of 60 participants. We found evidence of a clear difference between 'haloperidol + promethazine' and 'antipsychotic ‐ ziprasidone' within this subgroup (MD ‐0.10, 95% CI ‐0.38 to 0.18; Analysis 3.1).
3.2 Tranquil or asleep: 2. Effect of tranquillisation (PANSS‐EC, high=bad) (skewed data)
These continuous data, from a single trial, were too skewed to report in a graphic. We have therefore presented them in an 'Other data' table (Analysis 3.2).
3.2. Analysis.
Comparison 3 HALOPERIDOL + PROMETHAZINE vs ANTIPSYCHOTIC ‐ ZIPRASIDONE, Outcome 2 Tranquil or asleep: 2. Effect of tranquilisation (PANSS‐EC, high=bad) (skewed data).
| Tranquil or asleep: 2. Effect of tranquilisation (PANSS‐EC, high=bad) (skewed data) | ||||
|---|---|---|---|---|
| Study | Intervention | Mean | SD | N |
| at 30 minutes | ||||
| Mantovani 2013 | Haloperidol + Promethazine | 10.9 | 6.7 | 27 |
| Mantovani 2013 | Ziprasidone | 12.6 | 9.1 | 23 |
| at 60 minutes | ||||
| Mantovani 2013 | Haloperidol + Promethazine | 11.1 | 7.6 | 27 |
| Mantovani 2013 | Ziprasidone | 10.5 | 8 | 23 |
| at 90 minutes | ||||
| Mantovani 2013 | Haloperidol + Promethazine | 10.7 | 9.2 | 27 |
| Mantovani 2013 | Ziprasidone | 11.2 | 8.3 | 23 |
3.3 Tranquil or asleep: 3. Level of tranquillisation/agitation (ACES) (skewed data)
These continuous data (one RCT) had such large standard deviations as to suggest that analysis within Review Manager would be inadvisable. We have therefore presented them in an 'Other data' table (Analysis 3.3).
3.3. Analysis.
Comparison 3 HALOPERIDOL + PROMETHAZINE vs ANTIPSYCHOTIC ‐ ZIPRASIDONE, Outcome 3 Tranquil or asleep: 3. Level of tranquilisation / agitation (ACES) (skewed data).
| Tranquil or asleep: 3. Level of tranquilisation / agitation (ACES) (skewed data) | ||||
|---|---|---|---|---|
| Study | Intervention | Mean | SD | N |
| at 30 minutes | ||||
| Mantovani 2013 | Haloperidol + Promethazine | 5.2 | 8.1 | 27 |
| Mantovani 2013 | Ziprasidone | 4.8 | 4.6 | 23 |
| at 90 minutes. | ||||
| Mantovani 2013 | Haloperidol + Promethazine | 5.0 | 10.8 | 27 |
| Mantovani 2013 | Ziprasidone | 5.1 | 6.9 | 23 |
3.4 Global state: 1. Needing restraints or seclusion
For this outcome we found a single study, with a total of 60 participants. There were no subgroups in this outcome. We found no clear difference between 'haloperidol + promethazine' and 'antipsychotic ‐ ziprasidone' (RR 0.50, 95% CI 0.19 to 1.29; Analysis 3.4).
3.4. Analysis.
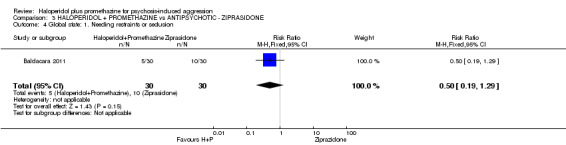
Comparison 3 HALOPERIDOL + PROMETHAZINE vs ANTIPSYCHOTIC ‐ ZIPRASIDONE, Outcome 4 Global state: 1. Needing restraints or seclusion.
3.5 Global state: 2. Additional tranquillising drugs
We identified one study relevant to this outcome, with a total of 51 participants. This outcome had no subgroups. We found no evidence of a clear difference between the two treatments (RR 0.51, 95% CI 0.19 to 1.36; Analysis 3.5).
3.5. Analysis.
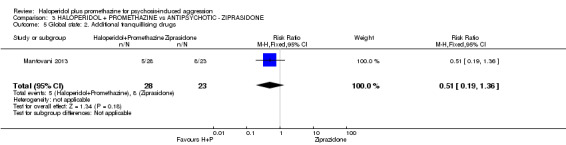
Comparison 3 HALOPERIDOL + PROMETHAZINE vs ANTIPSYCHOTIC ‐ ZIPRASIDONE, Outcome 5 Global state: 2. Additional tranquillising drugs.
3.6 Global state: 3. Average value of additional medication ‐ after initial dose (skewed data)
These continuous data (one RCT) had such large standard deviations as to suggest that analysis within Review Manager would be inadvisable. We have therefore reported these data in an 'Other data' table (Analysis 3.6).
3.6. Analysis.
Comparison 3 HALOPERIDOL + PROMETHAZINE vs ANTIPSYCHOTIC ‐ ZIPRASIDONE, Outcome 6 Global state: 3. Average value of additional medication ‐ after initial dose (skewed data).
| Global state: 3. Average value of additional medication ‐ after initial dose (skewed data) | ||||
|---|---|---|---|---|
| Study | Intervention | Mean | SD | N |
| Baldacara 2011 | Haloperidol + Promethazine | 1.10 | 1.03 | 30 |
| Baldacara 2011 | Ziprasidone | 0.77 | 0.98 | 30 |
3.7 Adverse effects: 1. Specific ‐ a. Cardiovascular ‐ hypotension
For this outcome we found two relevant studies, with a total of 111 participants. There were no subgroups in this outcome. We found no evidence of a clear difference between the two treatments (RR 0.55, 95% CI 0.17 to 1.75; Analysis 3.7).
3.7. Analysis.
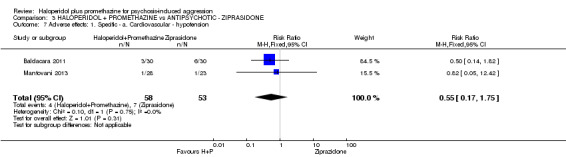
Comparison 3 HALOPERIDOL + PROMETHAZINE vs ANTIPSYCHOTIC ‐ ZIPRASIDONE, Outcome 7 Adverse effects: 1. Specific ‐ a. Cardiovascular ‐ hypotension.
3.8 Adverse effects: 1. Specific ‐ b. Central nervous system ‐ excessive sedation
For this outcome we found two relevant studies involving 111 participants. This outcome had no subgroups. We found no clear difference between 'haloperidol + promethazine' and 'antipsychotic ‐ ziprasidone' (RR 0.30, 95% CI 0.06 to 1.43; Analysis 3.8).
3.8. Analysis.
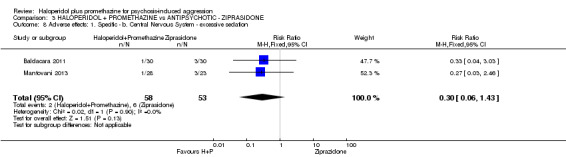
Comparison 3 HALOPERIDOL + PROMETHAZINE vs ANTIPSYCHOTIC ‐ ZIPRASIDONE, Outcome 8 Adverse effects: 1. Specific ‐ b. Central Nervous System ‐ excessive sedation.
3.9 Adverse effects: 1. Specific ‐ c. Extrapyramidal problems ‐ 0 to 4 hours
We identified two studies relevant to this outcome, with a total of 111 participants. This outcome had no subgroups. For this outcome, we did find evidence that 'haloperidol + promethazine' was clearly different in its effects compared with 'antipsychotic ‐ ziprasidone' (RR 1.72, 95% CI 1.07 to 2.76). For this outcome heterogeneity was high (Chi2 =2.59; df=1.0; P=0.11; I2 =61%; Analysis 3.9).
3.9. Analysis.
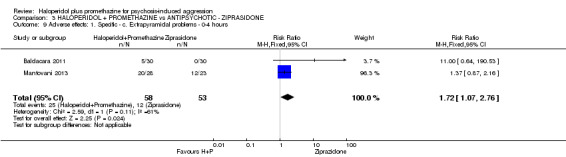
Comparison 3 HALOPERIDOL + PROMETHAZINE vs ANTIPSYCHOTIC ‐ ZIPRASIDONE, Outcome 9 Adverse effects: 1. Specific ‐ c. Extrapyramidal problems ‐ 0‐4 hours.
3.10 Specific behaviours: 1. Severe agitation
For this outcome we found a single study, with a total of 51 participants. There were no subgroups in this outcome. We found no clear difference between 'haloperidol + promethazine' and 'antipsychotic ‐ ziprasidone' (RR 0.82, 95% CI 0.18 to 3.69; Analysis 3.10).
3.10. Analysis.
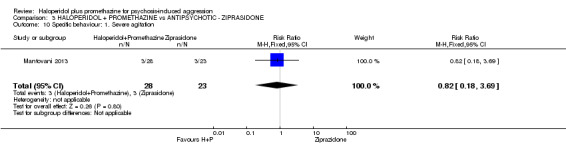
Comparison 3 HALOPERIDOL + PROMETHAZINE vs ANTIPSYCHOTIC ‐ ZIPRASIDONE, Outcome 10 Specific behaviour: 1. Severe agitation.
3.11 Specific behaviours: 2. Average aggression score (OAS, high score=bad)
For this outcome we found a single study.
3.11.1 by 1 hour
We found one trial to be relevant to this subgroup, with a total of 60 participants. For this outcome, within this subgroup, we did find evidence that 'haloperidol + promethazine' was clearly different in its effects compared with 'antipsychotic ‐ ziprasidone' (MD 4.50, 95% CI 2.82 to 6.18; Analysis 3.11).
3.11. Analysis.
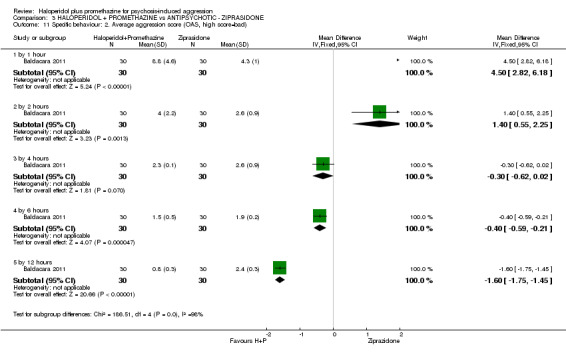
Comparison 3 HALOPERIDOL + PROMETHAZINE vs ANTIPSYCHOTIC ‐ ZIPRASIDONE, Outcome 11 Specific behaviour: 2. Average aggression score (OAS, high score=bad).
3.11.2 by 2 hours
We found one trial to be relevant to this subgroup, with a total of 60 participants. We found no clear difference between 'haloperidol + promethazine' and 'antipsychotic ‐ ziprasidone' within this subgroup (MD 1.40, 95% CI 0.55 to 2.25; Analysis 3.11).
3.11.3 by 4 hours
There was a single trial in this subgroup, with a total of 60 participants. For this outcome, within this subgroup, we did find evidence that 'haloperidol + promethazine' was clearly different in its effects compared with 'antipsychotic ‐ ziprasidone' (MD ‐0.30, 95% CI ‐0.62 to 0.02; Analysis 3.11).
3.11.4 by 6 hours
We found one trial to be relevant to this subgroup, with a total of 60 participants. We found evidence of a clear difference between 'haloperidol + promethazine' and 'antipsychotic ‐ ziprasidone' within this subgroup (MD ‐0.40, 95% CI ‐0.59 to ‐0.21; Analysis 3.11).
3.11.5 by 12 hours
We found one trial to be relevant to this subgroup, with a total of 60 participants. We found evidence of a clear difference between 'haloperidol + promethazine' and 'antipsychotic ‐ ziprasidone' within this subgroup (MD ‐1.60, 95% CI ‐1.75 to ‐1.45; Analysis 3.11).
3.12 Specific behaviours: 3. Average agitation score (OASS, high score=bad)
We identified one study relevant to this outcome and divided the data into five subgroups.
3.12.1 by 1 hour
We found one trial to be relevant to this subgroup, with a total of 60 participants. For this outcome, within this subgroup, we did find evidence that 'haloperidol + promethazine' was clearly different in its effects compared with 'antipsychotic ‐ ziprasidone' (MD 16.8, 95% CI 13.68 to 19.92; Analysis 3.12).
3.12. Analysis.
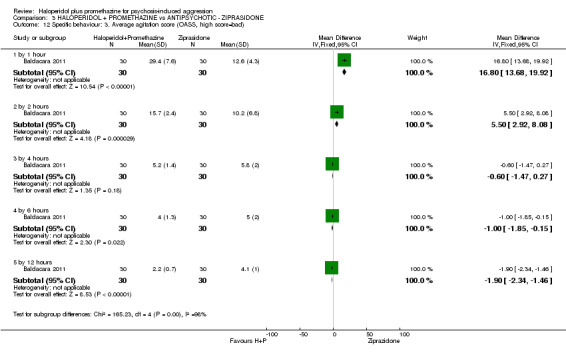
Comparison 3 HALOPERIDOL + PROMETHAZINE vs ANTIPSYCHOTIC ‐ ZIPRASIDONE, Outcome 12 Specific behaviour: 3. Average agitation score (OASS, high score=bad).
3.12.2 by 2 hours
There was a single trial in this subgroup, with a total of 60 participants. For this outcome, within this subgroup, we did find evidence that 'haloperidol + promethazine' was clearly different in its effects compared with 'antipsychotic ‐ ziprasidone' (MD 5.50, 95% CI 2.92 to 8.08; Analysis 3.12).
3.12.3 by 4 hours
We found one trial to be relevant to this subgroup, with a total of 60 participants. For this outcome, within this subgroup, we did find evidence that 'haloperidol + promethazine' was clearly different in its effects compared with 'antipsychotic ‐ ziprasidone' (MD ‐0.60, 95% CI ‐1.47 to 0.27; Analysis 3.12).
3.12.4 by 6 hours
We found one trial to be relevant to this subgroup, with a total of 60 participants. We found evidence of a clear difference between 'haloperidol + promethazine' and 'antipsychotic ‐ ziprasidone' within this subgroup (MD ‐1.00, 95% CI ‐1.85 to ‐0.15; Analysis 3.12).
3.12.5 by 12 hours
There was a single trial in this subgroup, with a total of 60 participants. For this outcome, within this subgroup, we did find evidence that 'haloperidol + promethazine' was clearly different in its effects compared with 'antipsychotic ‐ ziprasidone' (MD ‐1.90, 95% CI ‐2.34 to ‐1.46; Analysis 3.12).
3.13 Leaving the study early
For this outcome we found two relevant studies, with a total of 111 participants. We found no clear difference between 'haloperidol + promethazine' and 'antipsychotic ‐ ziprasidone' (RR 2.48, 95% CI 0.11 to 58.2).
3.13.1 by 24 hours
We found two trials to be relevant to this subgroup, with a total of 111 participants. We found no clear difference between 'haloperidol + promethazine' and 'antipsychotic ‐ ziprasidone' within this subgroup (RR 2.48, 95% CI 0.11 to 58.2; Analysis 3.13).
3.13. Analysis.
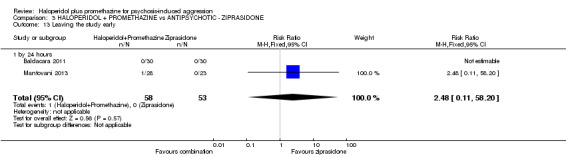
Comparison 3 HALOPERIDOL + PROMETHAZINE vs ANTIPSYCHOTIC ‐ ZIPRASIDONE, Outcome 13 Leaving the study early.
4. COMPARISON 4: HALOPERIDOL + PROMETHAZINE vs ANTIPSYCHOTIC & BENZODIAZEPINE ‐ HALOPERIDOL + MIDAZOLAM
This comparison has 12 outcomes.
4.1 Tranquil or asleep: 1. Average sedation score (RSS, high score=good)
For this outcome we found a single study.
4.1.1 by 1 hour
We found one trial to be relevant to this subgroup, with a total of 60 participants. For this outcome, within this subgroup, we did find evidence that 'haloperidol + promethazine' was clearly different in its effects compared with 'antipsychotic & benzodiazepine ‐ haloperidol + midazolam' (MD ‐0.60, 95% CI ‐1.13 to ‐0.07; Analysis 4.1).
4.1. Analysis.
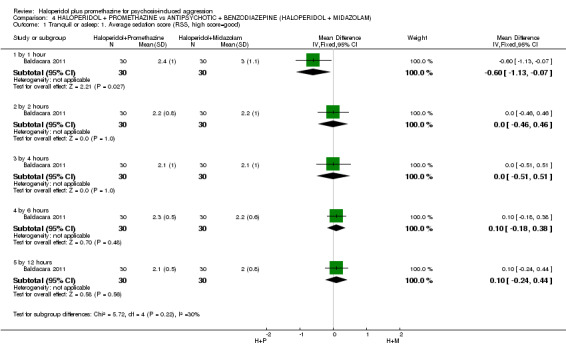
Comparison 4 HALOPERIDOL + PROMETHAZINE vs ANTIPSYCHOTIC + BENZODIAZEPINE (HALOPERIDOL + MIDAZOLAM), Outcome 1 Tranquil or asleep: 1. Average sedation score (RSS, high score=good).
4.1.2 by 2 hours
There was a single trial in this subgroup, with a total of 60 participants. We found evidence of a clear difference between 'haloperidol + promethazine' and 'antipsychotic & benzodiazepine ‐ haloperidol + midazolam' within this subgroup (MD 0.00, 95% CI ‐0.46 to 0.46; Analysis 4.1).
4.1.3 by 4 hours
We found one trial to be relevant to this subgroup, with a total of 60 participants. For this outcome, within this subgroup, we did find evidence that 'haloperidol + promethazine' was clearly different in its effects compared with 'antipsychotic & benzodiazepine ‐ haloperidol + midazolam' (MD 0.00, 95% CI ‐0.51 to 0.51; Analysis 4.1).
4.1.4 by 6 hours
We found one trial to be relevant to this subgroup, with a total of 60 participants. For this outcome, within this subgroup, we did find evidence that 'haloperidol + promethazine' was clearly different in its effects compared with 'antipsychotic & benzodiazepine ‐ haloperidol + midazolam' (MD 0.10, 95% CI ‐0.18 to 0.38; Analysis 4.1).
4.1.5 by 12 hours
There was a single trial in this subgroup, with a total of 60 participants. We found evidence of a clear difference between 'haloperidol + promethazine' and 'antipsychotic & benzodiazepine ‐ haloperidol + midazolam' within this subgroup (MD 0.10, 95% CI ‐0.24 to 0.44; Analysis 4.1).
4.2 Tranquil or asleep: 2. Effect of tranquillisation (PANSS‐EC, high score=bad) (skewed data)
These continuous data, from a single trial, had such large standard deviations as to suggest that analysis within Review Manager would be inadvisable. We have therefore reported these data in an 'Other data' table (Analysis 4.2).
4.2. Analysis.
Comparison 4 HALOPERIDOL + PROMETHAZINE vs ANTIPSYCHOTIC + BENZODIAZEPINE (HALOPERIDOL + MIDAZOLAM), Outcome 2 Tranquil or asleep: 2. Effect of tranquilisation (PANSS‐EC, high score=bad) (skewed data).
| Tranquil or asleep: 2. Effect of tranquilisation (PANSS‐EC, high score=bad) (skewed data) | ||||
|---|---|---|---|---|
| Study | Intervention | Mean | SD | N |
| at 30 minutes | ||||
| Mantovani 2013 | Haloperidol + Promethazine | 10.9 | 6.7 | 27 |
| Mantovani 2013 | Haloperidol + Midazolam | 8.7 | 4.1 | 25 |
| at 60 minutes | ||||
| Mantovani 2013 | Haloperidol + Promethazine | 11.1 | 7.6 | 27 |
| Mantovani 2013 | Haloperidol + Midazolam | 8.8 | 6.1 | 25 |
| at 90 minutes | ||||
| Mantovani 2013 | Haloperidol + Promethazine | 10.7 | 9.2 | 27 |
| Mantovani 2013 | Haloperidol + Midazolam | 9.4 | 9.4 | 25 |
4.3 Tranquil or asleep: 3. Level of tranquillisation/agitation (ACES) (skewed data)
These continuous data, from a single trial, had such large standard deviations as to suggest that analysis within Review Manager would be inadvisable. We have therefore presented them in an 'Other data' table (Analysis 4.3).
4.3. Analysis.
Comparison 4 HALOPERIDOL + PROMETHAZINE vs ANTIPSYCHOTIC + BENZODIAZEPINE (HALOPERIDOL + MIDAZOLAM), Outcome 3 Tranquil or asleep: 3. Level of tranquilisation / agitation (ACES) (skewed data).
| Tranquil or asleep: 3. Level of tranquilisation / agitation (ACES) (skewed data) | ||||
|---|---|---|---|---|
| Study | Intervention | Mean | SD | N |
| at 30 minutes | ||||
| Mantovani 2013 | Haloperidol + Promethazine | 5.2 | 8.1 | 27 |
| Mantovani 2013 | Haloperidol + Midazolam | 6 | 7.5 | 25 |
| at 90 minutes | ||||
| Mantovani 2013 | Haloperidol + Promethazine | 5.0 | 10.8 | 27 |
| Mantovani 2013 | Haloperidol + Midazolam | 5.8 | 12.5 | 25 |
4.4 Global state: 1. Needing restraints or seclusion
There was a single trial in this subgroup, with a total of 60 participants. For this outcome, within this subgroup, we did find evidence that 'haloperidol + promethazine' was clearly different in its effects compared with 'antipsychotic & benzodiazepine ‐ haloperidol + midazolam' (RR 0.24, 95% CI 0.10 to 0.55; Analysis 4.4).
4.4. Analysis.
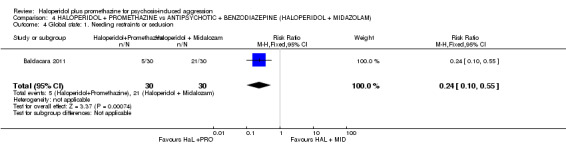
Comparison 4 HALOPERIDOL + PROMETHAZINE vs ANTIPSYCHOTIC + BENZODIAZEPINE (HALOPERIDOL + MIDAZOLAM), Outcome 4 Global state: 1. Needing restraints or seclusion.
4.5 Global state: 2. Additional tranquillising drugs
We found one trial to be relevant to this subgroup, with a total of 57 participants. We found no clear difference between 'haloperidol + promethazine' and 'antipsychotic & benzodiazepine ‐ haloperidol + midazolam' within this subgroup (RR 1.04, 95% CI 0.34 to 3.19; Analysis 4.5).
4.5. Analysis.
Comparison 4 HALOPERIDOL + PROMETHAZINE vs ANTIPSYCHOTIC + BENZODIAZEPINE (HALOPERIDOL + MIDAZOLAM), Outcome 5 Global state: 2. Additional tranquilising drugs.
4.6 Global state: 3. Average value of additional medication ‐ after initial dose (skewed data)
These continuous data, from a single trial, had such large standard deviations as to suggest that analysis within Review Manager would be inadvisable. We have therefore presented them in an 'Other data' table (Analysis 4.6).
4.6. Analysis.
Comparison 4 HALOPERIDOL + PROMETHAZINE vs ANTIPSYCHOTIC + BENZODIAZEPINE (HALOPERIDOL + MIDAZOLAM), Outcome 6 Global state: 3. Average value of additional medication ‐ after initial dose (skewed data).
| Global state: 3. Average value of additional medication ‐ after initial dose (skewed data) | ||||
|---|---|---|---|---|
| Study | Intervention | Mean | SD | N |
| Baldacara 2011 | Haloperidol + Promethazine | 1.10 | 1.03 | 30 |
| Baldacara 2011 | Haloperidol + Midazolam | 1.73 | 0.87 | 30 |
4.7 Adverse effects: 1. Specific ‐ a. Cardiovascular ‐ hypotension
We identified two studies relevant to this outcome involving 117 participants. This outcome had no subgroups. We found no evidence of a clear difference between the two treatments (RR 0.51, 95% CI 0.16 to 1.58; Analysis 4.7).
4.7. Analysis.
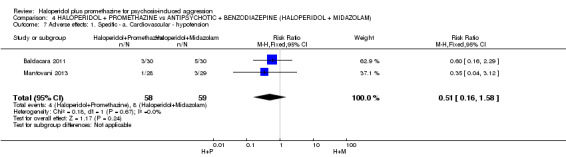
Comparison 4 HALOPERIDOL + PROMETHAZINE vs ANTIPSYCHOTIC + BENZODIAZEPINE (HALOPERIDOL + MIDAZOLAM), Outcome 7 Adverse effects: 1. Specific ‐ a. Cardiovascular ‐ hypotension.
4.8 Adverse effects: 1. Specific ‐ b. Central nervous system ‐ excessive sedation
We identified two studies relevant to this outcome involving 117 participants. There were no subgroups in this outcome. We found evidence of a clear difference between 'haloperidol + promethazine' and 'antipsychotic & benzodiazepine ‐ haloperidol + midazolam' (RR 0.12, 95% CI 0.03 to 0.49; Analysis 4.8).
4.8. Analysis.
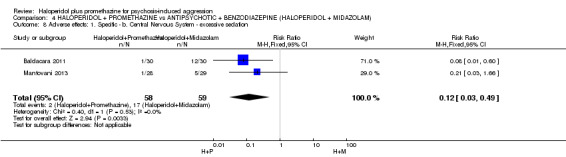
Comparison 4 HALOPERIDOL + PROMETHAZINE vs ANTIPSYCHOTIC + BENZODIAZEPINE (HALOPERIDOL + MIDAZOLAM), Outcome 8 Adverse effects: 1. Specific ‐ b. Central Nervous System ‐ excessive sedation.
4.9 Adverse effects: 1. Specific ‐ c. Extrapyramidal problems ‐ 0 to 4 hours
We identified two studies relevant to this outcome, with a total of 117 participants. This outcome had no subgroups. For this outcome, we did find evidence that 'haloperidol + promethazine' was clearly different in its effects compared with 'antipsychotic & benzodiazepine ‐ haloperidol + midazolam' (RR 1.84, 95% CI 1.12 to 3.02; Analysis 4.9).
4.9. Analysis.
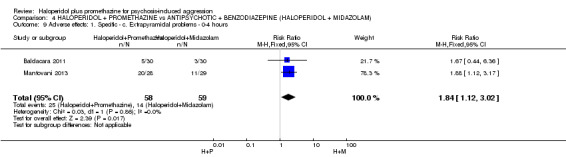
Comparison 4 HALOPERIDOL + PROMETHAZINE vs ANTIPSYCHOTIC + BENZODIAZEPINE (HALOPERIDOL + MIDAZOLAM), Outcome 9 Adverse effects: 1. Specific ‐ c. Extrapyramidal problems ‐ 0‐4 hours.
4.10 Specific behaviours: 1. Average aggression score (OAS, high score=bad)
We identified one study relevant to this outcome.
4.10.1 by 1 hour
We found one trial to be relevant to this subgroup, with a total of 60 participants. We found evidence of a clear difference between 'haloperidol + promethazine' and 'antipsychotic & benzodiazepine ‐ haloperidol + midazolam' within this subgroup (MD 3.30, 95% CI 1.35 to 5.25; Analysis 4.10).
4.10. Analysis.
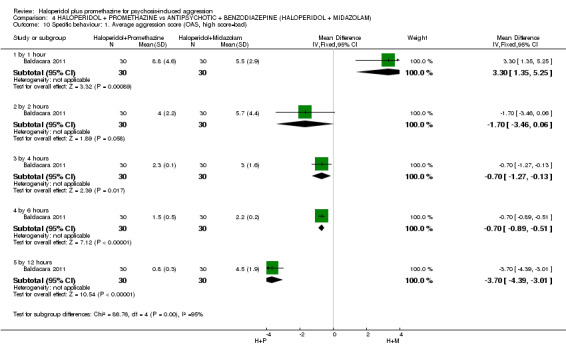
Comparison 4 HALOPERIDOL + PROMETHAZINE vs ANTIPSYCHOTIC + BENZODIAZEPINE (HALOPERIDOL + MIDAZOLAM), Outcome 10 Specific behaviour: 1. Average aggression score (OAS, high score=bad).
4.10.2 by 2 hours
We found one trial to be relevant to this subgroup, with a total of 60 participants. We found evidence of a clear difference between 'haloperidol + promethazine' and 'antipsychotic & benzodiazepine ‐ haloperidol + midazolam' within this subgroup (MD ‐1.70, 95% CI ‐3.46 to 0.06; Analysis 4.10).
4.10.3 by 4 hours
There was a single trial in this subgroup, with a total of 60 participants. For this outcome, within this subgroup, we did find evidence that 'haloperidol + promethazine' was clearly different in its effects compared with 'antipsychotic & benzodiazepine ‐ haloperidol + midazolam' (MD ‐0.70, 95% CI ‐1.27 to ‐0.13). This subgroup had important levels of heterogeneity (Chi2 =0.0; df=0.0; P=0.0; I2 =100%; Analysis 4.10).
4.10.4 by 6 hours
We found one trial to be relevant to this subgroup, with a total of 60 participants. We found evidence of a clear difference between 'haloperidol + promethazine' and 'antipsychotic & benzodiazepine ‐ haloperidol + midazolam' within this subgroup (MD ‐0.70, 95% CI ‐0.89 to ‐0.51; Analysis 4.10).
4.10.5 by 12 hours
There was a single trial in this subgroup, with a total of 60 participants. For this outcome, within this subgroup, we did find evidence that 'haloperidol + promethazine' was clearly different in its effects compared with 'antipsychotic & benzodiazepine ‐ haloperidol + midazolam' (MD ‐3.70, 95% CI ‐4.39 to ‐3.01; Analysis 4.10).
4.11 Specific behaviours: 2. Average agitation score (OASS, high score=bad)
For this outcome we found a single study.
4.11.1 by 1 hour
There was a single trial in this subgroup, with a total of 60 participants. We found evidence of a clear difference between 'haloperidol + promethazine' and 'antipsychotic & benzodiazepine ‐ haloperidol + midazolam' within this subgroup (MD 16.00, 95% CI 13.02 to 18.98; Analysis 4.11).
4.11. Analysis.
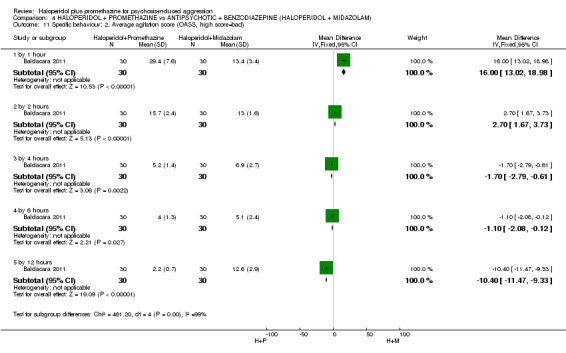
Comparison 4 HALOPERIDOL + PROMETHAZINE vs ANTIPSYCHOTIC + BENZODIAZEPINE (HALOPERIDOL + MIDAZOLAM), Outcome 11 Specific behaviour: 2. Average agitation score (OASS, high score=bad).
4.11.2 by 2 hours
There was a single trial in this subgroup, with a total of 60 participants. For this outcome, within this subgroup, we did find evidence that 'haloperidol + promethazine' was clearly different in its effects compared with 'antipsychotic & benzodiazepine ‐ haloperidol + midazolam' (MD 2.70, 95% CI 1.67 to 3.73). This subgroup had important levels of heterogeneity (Chi2 =0.0; df=0.0; P=0.0; I2 =100%; Analysis 4.11).
4.11.3 by 4 hours
We found one trial to be relevant to this subgroup, with a total of 60 participants. We found evidence of a clear difference between 'haloperidol + promethazine' and 'antipsychotic & benzodiazepine ‐ haloperidol + midazolam' within this subgroup (MD ‐1.70, 95% CI ‐2.79 to ‐0.61). This subgroup had important levels of heterogeneity (Chi2 =0.0; df=0.0; P=0.0; I2 =100%; Analysis 4.11).
4.11.4 by 6 hours
There was a single trial in this subgroup, with a total of 60 participants. For this outcome, within this subgroup, we did find evidence that 'haloperidol + promethazine' was clearly different in its effects compared with 'antipsychotic & benzodiazepine ‐ haloperidol + midazolam' (MD ‐1.10, 95% CI ‐2.08 to ‐0.12; Analysis 4.11).
4.11.5 by 12 hours
We found one trial to be relevant to this subgroup, with a total of 60 participants. For this outcome, within this subgroup, we did find evidence that 'haloperidol + promethazine' was clearly different in its effects compared with 'antipsychotic & benzodiazepine ‐ haloperidol + midazolam' (MD ‐10.4, 95% CI ‐11.47 to ‐9.33; Analysis 4.11).
4.12 Specific behaviours: 3. Severe agitation
For this outcome we found a single study involving 57 participants. This outcome had no subgroups. We found no evidence of a clear difference between the two treatments (RR 1.55, 95% CI 0.28 to 8.61; Analysis 4.12).
4.12. Analysis.
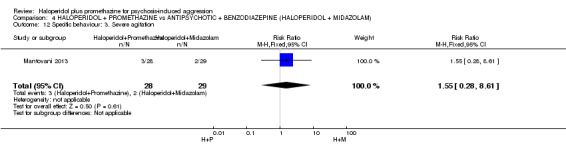
Comparison 4 HALOPERIDOL + PROMETHAZINE vs ANTIPSYCHOTIC + BENZODIAZEPINE (HALOPERIDOL + MIDAZOLAM), Outcome 12 Specific behaviour: 3. Severe agitation.
4.13 Leaving the study early
4.13.1 by 24 hours
We found two trials to be relevant to this subgroup, with a total of 117 participants. We found no clear difference between 'haloperidol + promethazine' and 'antipsychotic & benzodiazepine ‐ haloperidol + midazolam' within this subgroup (RR 0.26, 95% CI 0.03 to 2.18; Analysis 4.13).
4.13. Analysis.
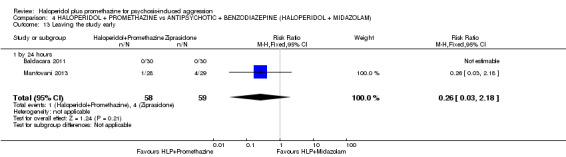
Comparison 4 HALOPERIDOL + PROMETHAZINE vs ANTIPSYCHOTIC + BENZODIAZEPINE (HALOPERIDOL + MIDAZOLAM), Outcome 13 Leaving the study early.
5. COMPARISON 5: HALOPERIDOL + PROMETHAZINE vs BENZODIAZEPINES ‐ LORAZEPAM
This comparison has 12 outcomes.
5.1 Tranquil or asleep: 1. Not tranquil or asleep
We identified one study relevant to this outcome.
5.1.1 by 30 minutes
We found one trial to be relevant to this subgroup, with a total of 200 participants. For this outcome, within this subgroup, we did find evidence that 'haloperidol + promethazine' was clearly different in its effects compared with 'benzodiazepines ‐ lorazepam' (RR 0.26, 95% CI 0.10 to 0.68; Analysis 5.1).
5.1. Analysis.
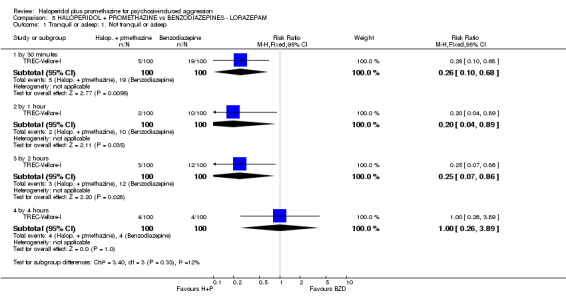
Comparison 5 HALOPERIDOL + PROMETHAZINE vs BENZODIAZEPINES ‐ LORAZEPAM, Outcome 1 Tranquil or asleep: 1. Not tranquil or asleep.
5.1.2 by 1 hour
We found one trial to be relevant to this subgroup, with a total of 200 participants. We found evidence of a clear difference between 'haloperidol + promethazine' and 'benzodiazepines ‐ lorazepam' within this subgroup (RR 0.20, 95% CI 0.04 to 0.89; Analysis 5.1).
5.1.3 by 2 hours
There was a single trial in this subgroup, with a total of 200 participants. For this outcome, within this subgroup, we did find evidence that 'haloperidol + promethazine' was clearly different in its effects compared with 'benzodiazepines ‐ lorazepam' (RR 0.25, 95% CI 0.07 to 0.86; Analysis 5.1).
5.1.4 by 4 hours
There was a single trial in this subgroup, with a total of 200 participants. For this subgroup, we found no evidence of a clear difference between the two treatments (RR 1.00, 95% CI 0.26 to 3.89; Analysis 5.1).
5.2 Tranquil or asleep: 2. Not asleep
We identified one study relevant to this outcome.
5.2.1 by 30 minutes
There was a single trial in this subgroup, with a total of 200 participants. For this outcome, within this subgroup, we did find evidence that 'haloperidol + promethazine' was clearly different in its effects compared with 'benzodiazepines ‐ lorazepam' (RR 0.40, 95% CI 0.29 to 0.54; Analysis 5.2).
5.2. Analysis.
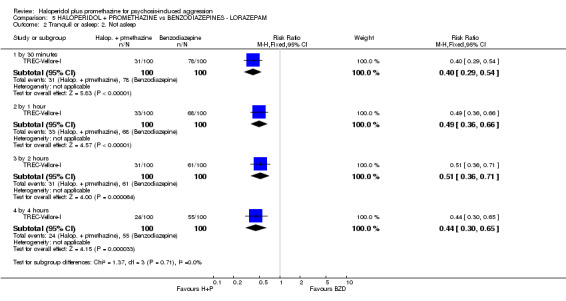
Comparison 5 HALOPERIDOL + PROMETHAZINE vs BENZODIAZEPINES ‐ LORAZEPAM, Outcome 2 Tranquil or asleep: 2. Not asleep.
5.2.2 by 1 hour
There was a single trial in this subgroup, with a total of 200 participants. We found evidence of a clear difference between 'haloperidol + promethazine' and 'benzodiazepines ‐ lorazepam' within this subgroup (RR 0.49, 95% CI 0.36 to 0.66; Analysis 5.2).
5.2.3 by 2 hours
We found one trial to be relevant to this subgroup, with a total of 200 participants. For this outcome, within this subgroup, we did find evidence that 'haloperidol + promethazine' was clearly different in its effects compared with 'benzodiazepines ‐ lorazepam' (RR 0.51, 95% CI 0.36 to 0.71; Analysis 5.2).
5.2.4 by 4 hours
There was a single trial in this subgroup, with a total of 200 participants. We found evidence of a clear difference between 'haloperidol + promethazine' and 'benzodiazepines ‐ lorazepam' within this subgroup (RR 0.44, 95% CI 0.3 to 0.65; Analysis 5.2).
5.3 Tranquil or asleep: 3. Time (skewed data)
These continuous data, from a single trial, were too skewed to report in a graphic. We have therefore presented them in an 'Other data' table (Analysis 5.3).
5.3. Analysis.
Comparison 5 HALOPERIDOL + PROMETHAZINE vs BENZODIAZEPINES ‐ LORAZEPAM, Outcome 3 Tranquil or asleep: 3. Time (skewed data).
| Tranquil or asleep: 3. Time (skewed data) | ||||||
|---|---|---|---|---|---|---|
| Study | Intervention | Mean (mins) | SD | N | Statistical test | p |
| time until tranquil or asleep | ||||||
| TREC‐Vellore‐I | Haloperidol + Promethazine | 29.7 | 35.6 | 100 | Mann‐Whitney U 327.0 | <0.001 |
| TREC‐Vellore‐I | Lorazepam | 47.8 | 46.7 | 100 | ||
| time until asleep | ||||||
| TREC‐Vellore‐I | Haloperidol + Promethazine | 37.4 | 42.9 | 100 | Mann‐Whitney U 1893.5 | <0.001 |
| TREC‐Vellore‐I | Lorazepam | 80.6 | 64.3 | 100 | ||
5.4 Global state: 1. No overall improvement
We identified one study relevant to this outcome.
5.4.1 by 30 minutes
We found one trial to be relevant to this subgroup, with a total of 200 participants. For this outcome, within this subgroup, we did find evidence that 'haloperidol + promethazine' was clearly different in its effects compared with 'benzodiazepines ‐ lorazepam' (RR 0.4, 95% CI 0.25 to 0.66; Analysis 5.4).
5.4. Analysis.
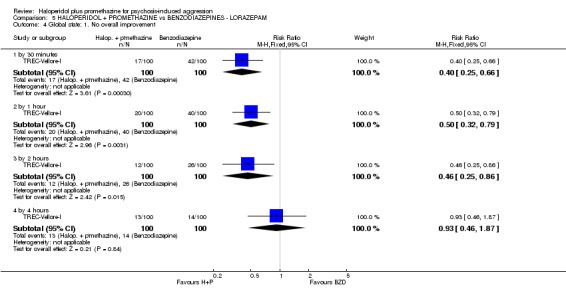
Comparison 5 HALOPERIDOL + PROMETHAZINE vs BENZODIAZEPINES ‐ LORAZEPAM, Outcome 4 Global state: 1. No overall improvement.
5.4.2 by 1 hour
There was a single trial in this subgroup, with a total of 200 participants. For this outcome, within this subgroup, we did find evidence that 'haloperidol + promethazine' was clearly different in its effects compared with 'benzodiazepines ‐ lorazepam' (RR 0.5, 95% CI 0.32 to 0.79; Analysis 5.4).
5.4.3 by 2 hours
We found one trial to be relevant to this subgroup, with a total of 200 participants. For this outcome, within this subgroup, we did find evidence that 'haloperidol + promethazine' was clearly different in its effects compared with 'benzodiazepines ‐ lorazepam' (RR 0.46, 95% CI 0.25 to 0.86; Analysis 5.4).
5.4.4 by 4 hours
There was a single trial in this subgroup, with a total of 200 participants. We found no clear difference between 'haloperidol + promethazine' and 'benzodiazepines ‐ lorazepam' within this subgroup (RR 0.93, 95% CI 0.46 to 1.87; Analysis 5.4).
5.5 Global state: 2. Needing restraints or seclusion
For this outcome we found a single study and divided the data into four subgroups.
5.5.1 by 30 minutes
We found one trial to be relevant to this subgroup, with a total of 200 participants. For this subgroup, we found no evidence of a clear difference between the two treatments (RR 0.55, 95% CI 0.28 to 1.09; Analysis 5.5).
5.5. Analysis.
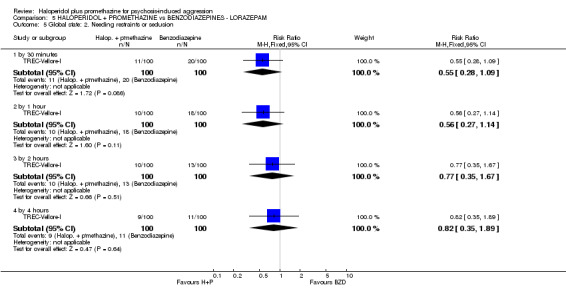
Comparison 5 HALOPERIDOL + PROMETHAZINE vs BENZODIAZEPINES ‐ LORAZEPAM, Outcome 5 Global state: 2. Needing restraints or seclusion.
5.5.2 by 1 hour
There was a single trial in this subgroup, with a total of 200 participants. We found no clear difference between 'haloperidol + promethazine' and 'benzodiazepines ‐ lorazepam' within this subgroup (RR 0.56, 95% CI 0.27 to 1.14; Analysis 5.5).
5.5.3 by 2 hours
We found one trial to be relevant to this subgroup, with a total of 200 participants. For this subgroup, we found no evidence of a clear difference between the two treatments (RR 0.77, 95% CI 0.35 to 1.67; Analysis 5.5).
5.5.4 by 4 hours
We found one trial to be relevant to this subgroup, with a total of 200 participants. For this subgroup, we found no evidence of a clear difference between the two treatments (RR 0.82, 95% CI 0.35 to 1.89; Analysis 5.5).
5.6 Global state: 3. Additional tranquillising drugs
For this outcome we found a single study.
5.6.1 by 30 minutes
We found one trial to be relevant to this subgroup, with a total of 200 participants. For this subgroup, we found no evidence of a clear difference between the two treatments (RR 0.33, 95% CI 0.01 to 8.09; Analysis 5.6).
5.6. Analysis.
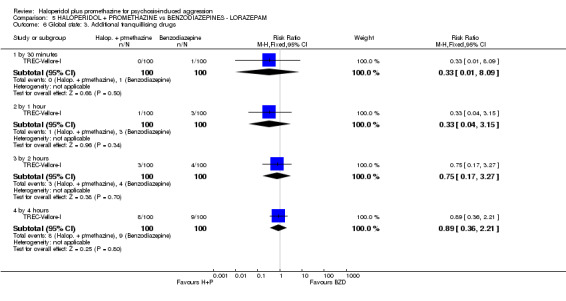
Comparison 5 HALOPERIDOL + PROMETHAZINE vs BENZODIAZEPINES ‐ LORAZEPAM, Outcome 6 Global state: 3. Additional tranquillising drugs.
5.6.2 by 1 hour
There was a single trial in this subgroup, with a total of 200 participants. For this subgroup, we found no evidence of a clear difference between the two treatments (RR 0.33, 95% CI 0.04 to 3.15; Analysis 5.6).
5.6.3 by 2 hours
There was a single trial in this subgroup, with a total of 200 participants. For this subgroup, we found no evidence of a clear difference between the two treatments (RR 0.75, 95% CI 0.17 to 3.27; Analysis 5.6).
5.6.4 by 4 hours
There was a single trial in this subgroup, with a total of 200 participants. We found no clear difference between 'haloperidol + promethazine' and 'benzodiazepines ‐ lorazepam' within this subgroup (RR 0.89, 95% CI 0.36 to 2.21; Analysis 5.6).
5.7 Global state: 4. Various measures
For this outcome we found a single study and divided the data into two subgroups.
5.7.1 doctor called to see patient ‐ by 4 hours
There was a single trial in this subgroup, with a total of 200 participants. For this subgroup, we found no evidence of a clear difference between the two treatments (RR 0.72, 95% CI 0.37 to 1.39; Analysis 5.7).
5.7. Analysis.

Comparison 5 HALOPERIDOL + PROMETHAZINE vs BENZODIAZEPINES ‐ LORAZEPAM, Outcome 7 Global state: 4. Various measures.
5.7.2 refusing oral medication ‐ by 2 weeks
We found one trial to be relevant to this subgroup, with a total of 200 participants. We found no clear difference between 'haloperidol + promethazine' and 'benzodiazepines ‐ lorazepam' within this subgroup (RR 1.63, 95% CI 0.7 to 3.75; Analysis 5.7).
5.8 Global state: 5. Average improvement (CGI, high score=bad) )
For this outcome we found a single study and divided the data into four subgroups.
5.8.1 by 30 minutes
There was a single trial in this subgroup, with a total of 200 participants. We found evidence of a clear difference between 'haloperidol + promethazine' and 'benzodiazepines ‐ lorazepam' within this subgroup (MD ‐0.60, 95% CI ‐0.86 to ‐0.34; Analysis 5.8).
5.8. Analysis.
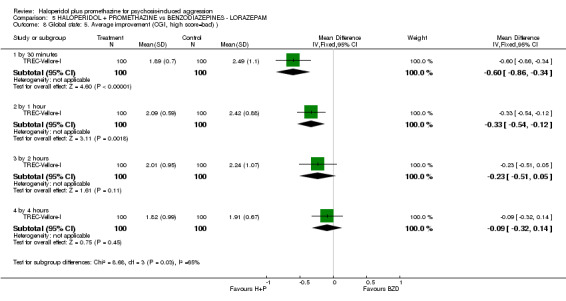
Comparison 5 HALOPERIDOL + PROMETHAZINE vs BENZODIAZEPINES ‐ LORAZEPAM, Outcome 8 Global state: 5. Average improvement (CGI, high score=bad) ).
5.8.2 by 1 hour
There was a single trial in this subgroup, with a total of 200 participants. For this outcome, within this subgroup, we did find evidence that 'haloperidol + promethazine' was clearly different in its effects compared with 'benzodiazepines ‐ lorazepam' (MD ‐0.33, 95% CI ‐0.54 to ‐0.12; Analysis 5.8).
5.8.3 by 2 hours
There was a single trial in this subgroup, with a total of 200 participants. We found evidence of a clear difference between 'haloperidol + promethazine' and 'benzodiazepines ‐ lorazepam' within this subgroup (MD ‐0.23, 95% CI ‐0.51 to 0.05; Analysis 5.8).
5.8.4 by 4 hours
We found one trial to be relevant to this subgroup, with a total of 200 participants. We found evidence of a clear difference between 'haloperidol + promethazine' and 'benzodiazepines ‐ lorazepam' within this subgroup (MD ‐0.09, 95% CI ‐0.32 to 0.14; Analysis 5.8).
5.9 Adverse effects: 1. General ‐ serious adverse effect
For this outcome we found a single study.
5.9.1 by 30 minutes
We found one trial to be relevant to this subgroup, with a total of 200 participants. For this subgroup, we found no evidence of a clear difference between the two treatments (RR 0.33, 95% CI 0.01 to 8.09; Analysis 5.9).
5.9. Analysis.
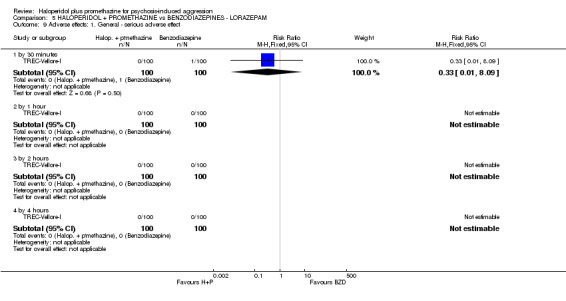
Comparison 5 HALOPERIDOL + PROMETHAZINE vs BENZODIAZEPINES ‐ LORAZEPAM, Outcome 9 Adverse effects: 1. General ‐ serious adverse effect.
5.9.2 by 1 to 4 hours
There was a single trial in each time subgroup, with a total of 200 participants. No further serious adverse effects were reported (Analysis 5.9).
5.10 Adverse effects: 2. Specific ‐ extrapyramidal problems ‐ 0 to 4 hours
We identified one study relevant to this outcome.
5.10.1 akathisia (Barnes Akathisia Scale)
There was a single trial in this subgroup, with a total of 200 participants. No akathisia was reported (Analysis 5.10).
5.10. Analysis.
Comparison 5 HALOPERIDOL + PROMETHAZINE vs BENZODIAZEPINES ‐ LORAZEPAM, Outcome 10 Adverse effects: 2. Specific ‐ Extrapyramidal problems ‐ 0‐4 hours.
5.10.2 any change in scale‐rated extrapyramidal problems (Simpson‐Angus Scale)
There was a single trial in this subgroup, with a total of 200 participants. No extrapyramidal problems were reported (Analysis 5.10).
5.11 Service outcomes: Not discharged
For this outcome we found a single study, involving 200 participants.
5.11.1 by 4 hours
We found one trial to be relevant to this subgroup, with a total of 200 participants. For this subgroup, we found no evidence of a clear difference between the two treatments (RR 1.13, 95% CI 0.85 to 1.50; Analysis 5.11).
5.11. Analysis.
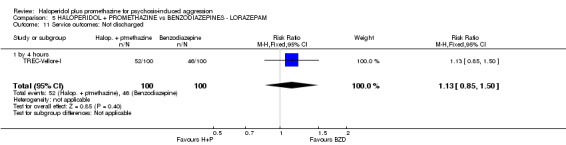
Comparison 5 HALOPERIDOL + PROMETHAZINE vs BENZODIAZEPINES ‐ LORAZEPAM, Outcome 11 Service outcomes: Not discharged.
5.12 Leaving the study early
For this outcome we found a single study and divided the data into two subgroups.
5.12.1 by 4 hours
There was a single trial in this subgroup, with a total of 200 participants. For this subgroup, we found no evidence of a clear difference between the two treatments (RR 3.00, 95% CI 0.12 to 72.77; Analysis 5.12).
5.12. Analysis.

Comparison 5 HALOPERIDOL + PROMETHAZINE vs BENZODIAZEPINES ‐ LORAZEPAM, Outcome 12 Leaving the study early.
5.12.2 by 2 weeks
There was a single trial in this subgroup, with a total of 200 participants. For this subgroup, we found no evidence of a clear difference between the two treatments (RR 1.25, 95% CI 0.51 to 3.04; Analysis 5.12).
6. COMPARISON 6: HALOPERIDOL + PROMETHAZINE vs BENZODIAZEPINES ‐ MIDAZOLAM
This comparison has six outcomes.
6.1 Tranquil or asleep: 1. Not tranquil or asleep
We identified one study relevant to this outcome and divided the data into three subgroups.
6.1.1 by 30 minutes
There was a single trial in this subgroup, with a total of 301 participants. We found evidence of a clear difference between 'haloperidol + promethazine' and 'benzodiazepines ‐ midazolam' within this subgroup (RR 2.90, 95% CI 1.75 to 4.8; Analysis 6.1).
6.1. Analysis.
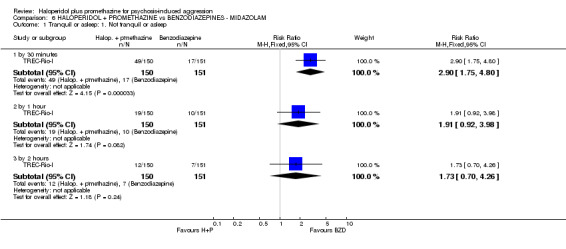
Comparison 6 HALOPERIDOL + PROMETHAZINE vs BENZODIAZEPINES ‐ MIDAZOLAM, Outcome 1 Tranquil or asleep: 1. Not tranquil or asleep.
6.1.2 by 1 hour
There was a single trial in this subgroup, with a total of 301 participants. For this subgroup, we found no evidence of a clear difference between the two treatments (RR 1.91, 95% CI 0.92 to 3.98). This subgroup had important levels of heterogeneity (Chi2 =0.0; df=0.0; P=0.0; I2 =100%; Analysis 6.1).
6.1.3 by 2 hours
There was a single trial in this subgroup, with a total of 301 participants. For this subgroup, we found no evidence of a clear difference between the two treatments (RR 1.73, 95% CI 0.70 to 4.26; Analysis 6.1).
6.2 Tranquil or asleep: 2. Not asleep
We identified one study relevant to this outcome and divided the data into three subgroups.
6.2.1 by 30 minutes
There was a single trial in this subgroup, with a total of 301 participants. For this outcome, within this subgroup, we did find evidence that 'haloperidol + promethazine' was clearly different in its effects compared with 'benzodiazepines ‐ midazolam' (RR 1.86, 95% CI 1.48 to 2.33; Analysis 6.2).
6.2. Analysis.
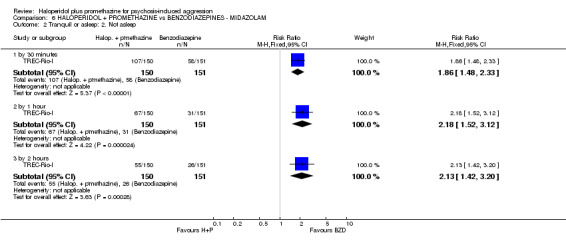
Comparison 6 HALOPERIDOL + PROMETHAZINE vs BENZODIAZEPINES ‐ MIDAZOLAM, Outcome 2 Tranquil or asleep: 2. Not asleep.
6.2.2 by 1 hour
There was a single trial in this subgroup, with a total of 301 participants. For this outcome, within this subgroup, we did find evidence that 'haloperidol + promethazine' was clearly different in its effects compared with 'benzodiazepines ‐ midazolam' (RR 2.18, 95% CI 1.52 to 3.12). For this outcome heterogeneity was high (Chi2 =0.0; df=0.0; P=0.0; I2 =100%; Analysis 6.2).
6.2.3 by 2 hours
We found one trial to be relevant to this subgroup, with a total of 301 participants. We found evidence of a clear difference between 'haloperidol + promethazine' and 'benzodiazepines ‐ midazolam' within this subgroup (RR 2.13, 95% CI 1.42 to 3.2; Analysis 6.2).
6.3. Global state: 1. Needing restraints or seclusion ‐ by 2 hours
We found one trial to be relevant to this subgroup, with a total of 301 participants. We found no clear difference between 'haloperidol + promethazine' and 'benzodiazepines ‐ midazolam' within this subgroup (RR 1.22, 95% CI 0.82 to 1.82; Analysis 6.3).
6.3. Analysis.
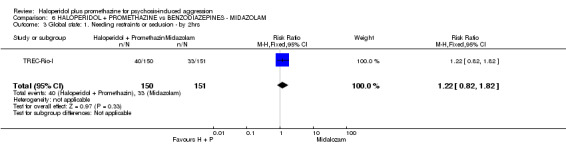
Comparison 6 HALOPERIDOL + PROMETHAZINE vs BENZODIAZEPINES ‐ MIDAZOLAM, Outcome 3 Global state: 1. Needing restraints or seclusion ‐ by 2hrs.
6.4 Global state: 2. Requiring additional drugs during initial phase ‐ by 2 hours
There was a single trial in this subgroup, with a total of 301 participants. For this subgroup, we found no evidence of a clear difference between the two treatments (RR 3.52, 95% CI 0.74 to 16.69; Analysis 6.4).
6.4. Analysis.
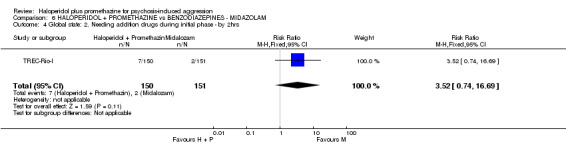
Comparison 6 HALOPERIDOL + PROMETHAZINE vs BENZODIAZEPINES ‐ MIDAZOLAM, Outcome 4 Global state: 2. Needing addition drugs during initial phase ‐ by 2hrs.
6.5 Global state: 3. Various measures
We identified one study relevant to this outcome.
6.5.1 doctor called to see patient ‐ by 24 hours
There was a single trial in this subgroup, with a total of 301 participants. We found no clear difference between 'haloperidol + promethazine' and 'benzodiazepines ‐ midazolam' within this subgroup (RR 0.85, 95% CI 0.61 to 1.19; Analysis 6.5).
6.5. Analysis.
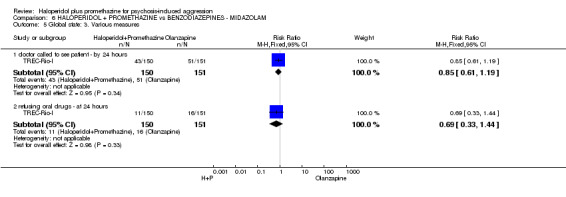
Comparison 6 HALOPERIDOL + PROMETHAZINE vs BENZODIAZEPINES ‐ MIDAZOLAM, Outcome 5 Global state: 3. Various measures.
6.5.2 refusing oral drugs ‐ at 24 hours
We found one trial to be relevant to this subgroup, with a total of 301 participants. For this subgroup, we found no evidence of a clear difference between the two treatments (RR 0.69, 95% CI 0.33 to 1.44; Analysis 6.5).
6.6 Adverse effects: Serious adverse effect
For this outcome we found a single study.
6.6.1 by 30 minutes
We found one trial to be relevant to this subgroup, with a total of 301 participants. For this subgroup, we found no evidence of a clear difference between the two treatments (RR 1.01, 95% CI 0.06 to 15.95; Analysis 6.6).
6.6. Analysis.
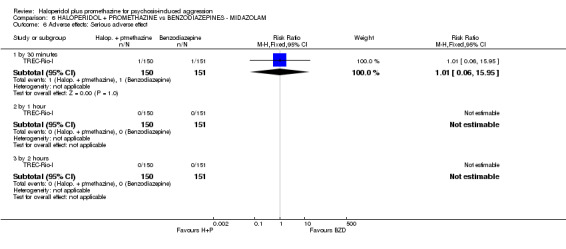
Comparison 6 HALOPERIDOL + PROMETHAZINE vs BENZODIAZEPINES ‐ MIDAZOLAM, Outcome 6 Adverse effects: Serious adverse effect.
6.6.2 by 1 to 2 hours
There was a single trial in this subgroup, with a total of 301 participants. No further serious adverse effects were reported (Analysis 6.6).
6.7 Service outcomes: Not discharged
We identified one study relevant to this outcome.
6.7.1 by 15 days
There was a single trial in this subgroup, with a total of 301 participants. For this subgroup, we found no evidence of a clear difference between the two treatments (RR 1.05, 95% CI 0.84 to 1.29; Analysis 6.7).
6.7. Analysis.
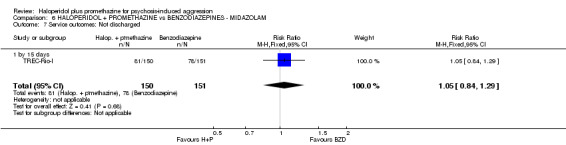
Comparison 6 HALOPERIDOL + PROMETHAZINE vs BENZODIAZEPINES ‐ MIDAZOLAM, Outcome 7 Service outcomes: Not discharged.
6.8. Specific behaviours: 1. Aggression ‐ a. Other episode of aggression ‐ within 24 hours
There was a single trial in this subgroup, with a total of 301 participants. We found no clear difference between 'haloperidol + promethazine' and 'benzodiazepines ‐ midazolam' within this subgroup (RR 0.89, 95% CI 0.62 to 1.29). This subgroup had important levels of heterogeneity (Chi2 =0.0; df=0.0; P=0.0; I2 =100%; Analysis 6.8).
6.8. Analysis.
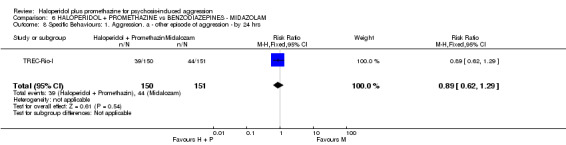
Comparison 6 HALOPERIDOL + PROMETHAZINE vs BENZODIAZEPINES ‐ MIDAZOLAM, Outcome 8 Specific Behaviours: 1. Aggression. a ‐ other episode of aggression ‐ by 24 hrs.
6.9 Leaving the study early
For this outcome we found a single study.
6.9.1 by 2 hours
We found one trial to be relevant to this subgroup, with a total of 301 participants. We found no clear difference between 'haloperidol + promethazine' and 'benzodiazepines ‐ midazolam' within this subgroup (RR 2.01, 95% CI 0.18 to 21.97; Analysis 6.9).
6.9. Analysis.
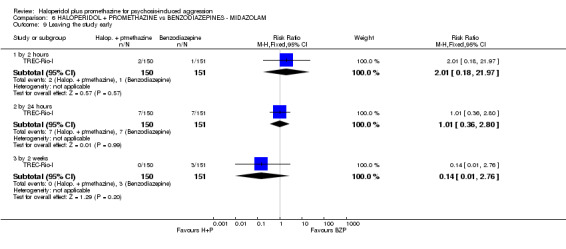
Comparison 6 HALOPERIDOL + PROMETHAZINE vs BENZODIAZEPINES ‐ MIDAZOLAM, Outcome 9 Leaving the study early.
6.9.2 by 24 hours
There was a single trial in this subgroup, with a total of 301 participants. We found no clear difference between 'haloperidol + promethazine' and 'benzodiazepines ‐ midazolam' within this subgroup (RR 1.01, 95% CI 0.36 to 2.80; Analysis 6.9).
6.9.3 by 2 weeks
We found one trial to be relevant to this subgroup, with a total of 301 participants. For this subgroup, we found no evidence of a clear difference between the two treatments (RR 0.14, 95% CI 0.01 to 2.76; Analysis 6.9).
7. COMPARISON 7: HALOPERIDOL + PROMETHAZINE vs ANTIPSYCHOTIC ‐ HALOPERIDOL ‐ additional 40 minutes data
We added this comparison post hoc, and it has two outcomes. There were data for 40 minutes that we did not stipulate to be included in other versions of this review, but have included them here for completeness. They do not add any major new information to the review.
7.1 Tranquil or asleep: 1. Not tranquil or asleep
For this outcome we found a single study.
7.1.1 by 40 minutes
We found one trial to be relevant to this subgroup, with a total of 316 participants. We found no clear difference between 'haloperidol + promethazine' and 'antipsychotic ‐ haloperidol ‐ additional 40 minutes data' within this subgroup (RR 0.83, 95% CI 0.56 to 1.24; Analysis 7.1).
7.1. Analysis.
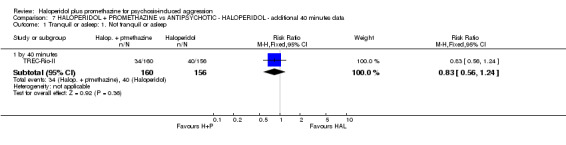
Comparison 7 HALOPERIDOL + PROMETHAZINE vs ANTIPSYCHOTIC ‐ HALOPERIDOL ‐ additional 40 minutes data, Outcome 1 Tranquil or asleep: 1. Not tranquil or asleep.
7.2 Tranquil or asleep: 2. Not asleep
For this outcome we found a single study and categorised the data into one subgroup.
7.2.1 by 40 minutes
There was a single trial in this subgroup, with a total of 316 participants. For this subgroup, we found no evidence of a clear difference between the two treatments (RR 0.99, 95% CI 0.85 to 1.16; Analysis 7.2).
7.2. Analysis.
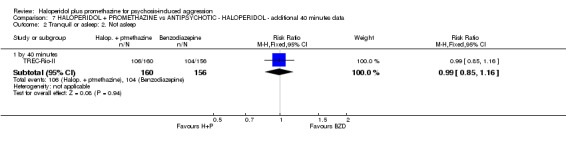
Comparison 7 HALOPERIDOL + PROMETHAZINE vs ANTIPSYCHOTIC ‐ HALOPERIDOL ‐ additional 40 minutes data, Outcome 2 Tranquil or asleep: 2. Not asleep.
Discussion
To summarise the main findings we used the list of outcomes chosen at review protocol stage for presentation in the 'Summary of findings' tables.
Summary of main results
1. HALOPERIDOL + PROMETHAZINE compared to ANTIPSYCHOTIC ‐ HALOPERIDOL for psychosis‐induced aggression
Please see Table 1.
1.1 Tranquil or asleep: Not tranquil or asleep ‐ by 30 minutes
The primary outcome of this review and that of the single trial contributing to this result was 'tranquil/asleep'. We found high‐quality evidence that the combination of haloperidol and promethazine is more effective than the use of haloperidol alone (RR 0.65, 95% CI 0.49 to 0.87).
1.2 Adverse effects
For adverse effects there was no difference in risk of seizure and no deaths. However, in about 150 per group of allocation there were 10 occurrences of acute dystonia in the haloperidol‐alone arm and none in the group given haloperidol plus promethazine (RR 0.05, 95% CI 0.00 to 0.76). Acute dystonia is most unpleasant, and it was for this reason that the study was stopped early, as the Steering Group of this trial felt that it was unethical to continue with the haloperidol alone (TREC‐Rio‐II). Haloperidol alone has been used for decades in the acute clinical situation. In the context of a randomised trial where adverse effects are scrutinised and more people are being monitored centrally than usual, the properties of a treatment can be properly estimated. We therefore agree with the Steering Group of TREC‐Rio‐II that there are better treatments available than the unprotected use of this toxic drug. The combination of haloperidol and promethazine seems to be one such better treatment.
1.3 Other outcomes
All other outcomes were less convincing, but overall favour the combination of haloperidol and promethazine when compared with haloperidol alone. The risk of another episode of aggression (by 24 hours) was somewhat reduced (RR 1.17 95% CI 0.68 to 2.01), but data were few and of low quality. The risk of 'not being discharged by 2 weeks' was also reduced, but not to conventional levels of statistical significance (RR 0.83, 95% CI 0.64 to 1.07, high‐quality evidence). Although difficult to interpret from a clinical perspective, the average OAS aggression scores favoured the combination of haloperidol and promethazine (MD ‐1.8 95% CI ‐1.93 to ‐1.67), but the data were of low quality. We have no economic data to analyse, but both haloperidol and promethazine are low‐cost treatments that may reduce the time spent in hospital, and can therefore be suggested to provide in combination the most cost‐effective treatment.
2. HALOPERIDOL + PROMETHAZINE compared to ANTIPSYCHOTIC ‐ OLANZAPINE for psychosis‐induced aggression
Please see Table 2.
2.1 Tranquil or asleep: Not tranquil or asleep ‐ by 30 minutes
Although the evidence suggested that the combination of haloperidol and promethazine was more effective, the difference between the two approaches did not reach statistical significance (RR 0.60, 95% CI 0.22 to 1.61). We found high‐quality data that both approaches are tranquillising.
2.2 Global state: Needing restraints or seclusion
By 12 hours fewer participants allocated to olanzapine had another episode of aggression necessitating restraints or seclusion but this difference did not reach conventional levels of statistical significance (n=60, 1 RCT, RR 5.00, 95% CI 0.62 to 40.28). These low‐quality data do fit with other outcomes such as 'requiring additional drugs during initial phase' (RR 0.52, 95% CI 0.37 to 0.74), 'being restrained by 4 hours' (RR 0.63, 95% CI 0.34 to 1.14), and 'no global improvement by 4 hours' (RR 0.47, 95% CI 0.22 to 1.01) ‐ data for these outcomes do tend to favour the haloperidol plus promethazine combination but some do not reach conventional levels of statistical significance and all results are from small trials. This work does merit reinvestigation in new trials.
2.3 Adverse effects
Lower ‐quality data suggested that the risk of unwanted excessive sedation was less with the combination approach (RR 0.67, 95% CI 0.12 to 3.84)
2.4 Other outcomes
There seemed to be no clear difference in the numbers of people discharged from hospital at two‐week follow‐up (high‐quality data). There were no deaths. Aggression scores were lower for the he combination approach, but the clinical meaning of these scores is not clear. There were no economic analyses, but olanzapine intramuscular is a much more expensive treatment than the combination approach and seems to be less effective.
3. HALOPERIDOL + PROMETHAZINE compared to ANTIPSYCHOTIC ‐ ZIPRASIDONE for psychosis‐induced aggression
Please see Table 3.
3.1 Tranquil or asleep: Average sedation score (RSS) ‐ by 30 minutes
We identified no binary data. The average sedation score (RSS) was lower for the combination approach, although not to conventional levels of statistical significance (MD ‐0.10, 95% CI ‐0.58 to 0.38). These data were of low quality, and the meaning of these findings in clinical terms is unclear.
3.2 Global state: Needing restraints or seclusion ‐ by up to 12 hours
Fewer participants receiving the combination treatment needed restraints or seclusion in the hours following the aggressive index incident, but there was not a clear difference between the groups (n=60, 1 RCT, RR 0.50, 95% CI 0.19 to 1.29, moderate‐quality evidence). The average aggression score (OAS) initially favoured ziprasidone, but after 12 hours the results favoured the combination treatment (MD ‐1.6, 95% CI ‐1.75 to ‐1.45, moderate‐quality evidence). This may fit with the increased risk of excessive sedation with ziprasidone ‐ an early effect that then disappears.
3.3 Adverse effects
Both new trials contributed to the outcome 'excessive sedation' (RR 0.30, 95% CI 0.06 to 1.43). It appears that ziprasidone may be effective but less desirable than the combination treatment of haloperidol plus promethazine, although the total numbers were small (n=110), and data were of low quality.
3.3 Other outcomes
No one died in these short, small trials. We have no data on service outcomes such as 'discharged'. There was no economic data or consideration of outcomes. Ziprasidone intramuscular is an expensive treatment compared with haloperidol plus promethazine.
4. HALOPERIDOL + PROMETHAZINE compared to ANTIPSYCHOTIC & BENZODIAZEPINE ‐ HALOPERIDOL + MIDAZOLAM for psychosis‐induced aggression
Please see Table 4.
4.1 Tranquil or asleep: Average sedation score (RSS) ‐ by 30 minutes
The primary outcomes for this review were binary, but we have only the proxy measure of average score for sedation (RSS). Low‐quality data from one small trial (n=60) suggest haloperidol plus midazolam to be more sedating by one hour (MD ‐0.60, 95% CI ‐1.13 to ‐0.07). The risk of excessive sedation was considerably less with haloperidol plus promethazine (n=112, 2 RCTs, RR 0.11, 95% CI 0.03 to 0.47, low‐quality evidence). These findings would fit with the better‐quality data of the comparison haloperidol plus promethazine versus midazolam alone (Comparison 6). Midazolam seems to be a highly sedating, swift‐acting intervention. The OAS average aggression scores (including changes during the 12‐hour follow‐up) are also in keeping with this impression. Combining midazolam with haloperidol does not seem to add ‐ or subtract ‐ much.
4.2 Global state: Needing restraints or seclusion ‐ by 12 hours
Low‐quality data from one small trial suggests that the combination of haloperidol plus promethazine reduces the need for restraints or seclusion (n=60, RR 0.24, 95% CI 0.1 to 0.55). It may be that use of midazolam ‐ in this case with the addition of haloperidol ‐ swiftly sedates, but the effects do not last over a longer period.
4.3 Other outcomes
There were no deaths in the short, small trial providing data for this outcome. Follow‐up was for a matter of hours, so service outcomes such as 'not discharged' were not relevant. We do not know the effects of haloperidol plus midazolam in the longer term. There were no economic data. Both combinations are inexpensive, but the differences in outcomes complex. Costing of short, deep sedation with the possibility of resumption of the aggressive incident (haloperidol plus midazolam) compared to treatments providing slower sedative effects that last longer (haloperidol plus promethazine) has not been undertaken.
5. HALOPERIDOL + PROMETHAZINE compared to BENZODIAZEPINES ‐ LORAZEPAM for psychosis‐induced aggression
Please see Table 5.
5.1 Tranquil or asleep: Not tranquil or asleep ‐ by 30 minutes
The combination of haloperidol plus promethazine seems to cause sedation or tranquillisation more effectively than lorazepam (n=200, 1 RCT, RR 0.26, 95% CI 0.1 to 0.68, high‐quality evidence). The secondary outcome of needing restraints or seclusion by 12 hours was not clearly different between groups, with about 10% needing this intrusive intervention in each group (moderate‐quality evidence). Unlike for other comparisons, we have no aggression scores, but in any event, such data are often problematic and almost impossible to interpret from a clinical perspective.
5.2 Adverse effects
The group administered haloperidol plus promethazine had fewer 'any serious adverse event' during the 24‐hour follow‐up, but there are no clear differences between the groups, and we were unable to determine exactly what the adverse effect was. There were no deaths.
5.3 Other outcomes
We found good data for two‐week follow‐up for service outcomes. There was no clear difference between groups; around half were discharged from both groups. There were no economic data. Haloperidol plus promethazine is a highly accessible, inexpensive combination. Lorazepam is more expensive and not heat stable, so it must be stored in cool conditions. This may be crudest of economic considerations, but in the absence of anything better, does suggest that lorazepam is likely to be the more expensive of the two approaches.
6. HALOPERIDOL + PROMETHAZINE compared to BENZODIAZEPINES ‐ MIDAZOLAM for psychosis‐induced aggression
Please see Table 6.
6.1 Tranquil or asleep: Not tranquil or asleep ‐ by 30 minutes
We found clear evidence that midazolam is more swiftly tranquillising of an aggressive situation than haloperidol plus promethazine (RR 2.90, 95% CI 1.75 to 4.8, high‐quality evidence). There was no clear difference in the risk of needing restraints or seclusion by around 12 hours, nor in the resumption of aggressive behaviour during the same period. On its own, midazolam seems to be swift and effective in terms of tranquillising difficult aggressive situations thought to be due to psychotic illness.
6.2 Adverse effects
As there was one serious adverse event in each group of around 150 people, there was no difference in risk overall (n=301, RR 1.01, 95% CI 0.06 to 15.95). However, the devil is in the details ‐ and this is where being authors of this review as well as being involved in some of the original and included trials could ‐ while potentially biasing our report ‐ also help. Our careful reading of the reports found detail lost in the reviewing process. One person in the group allocated to the combination of haloperidol plus promethazine had a major epileptic seizure. This was quickly brought under control. One person allocated to midazolam had profound respiratory depression ‐ again quickly reversed by use of flumazenil. We believe that mental health services would tend to be more comfortable and safe managing seizures than respiratory depression. There were no deaths.
6.3 Other outcomes
There was no difference in recurrence of aggression at 12 hours. By two weeks, there was no clear difference between the groups for service outcomes (not discharged). There were no economic data.
Overall completeness and applicability of evidence
1. Completeness
Before 2003 there were no trials of this widely used combination treatment for aggression thought to be caused by psychotic illness. There are now trials involving 1367 people with excellent follow‐up. This makes haloperidol plus promethazine the most evaluated treatment for people in this extreme situation.
1.1 Comparisons
That there are many comparisons is understandable, as there are many choices open to clinicians and few supported by robust evidence. We suggest that the question regarding haloperidol plus promethazine versus haloperidol alone is largely closed. The latter is too toxic, and this toxicity was well‐illustrated by the trial.
Data for the comparison with midazolam is also convincing, and further trials would be difficult to justify in light of the existing evidence. The comparison with haloperidol plus midazolam seems entirely to reflect that of that of the comparison with midazolam alone; adding haloperidol seems to offer little benefit. The comparison with lorazepam could perhaps be re‐undertaken to supplement the data presented in this review. The use of lorazepam is certainly grossly under‐researched (Gillies 2005; Powney 2012), considering the prevalence of its use. More information could be gained by further comparisons with olanzapine and ziprasidone, but a pattern does seem to be emerging that the newer compounds are swiftly effective, result in fast sedation, but also resumption of the aggression, whereas haloperidol plus promethazine calms the situation more gently and over a longer period of time.
The ongoing study (TREC‐Vellore‐III), comparing haloperidol plus promethazine with zuclopenthixol acetate, will add important information to this review.
1.2 Outcomes
We found no clear data on satisfaction of the patient or carers with the outcomes of treatment after the episode was over. There were no proper economic outcomes. Many of the outcomes for which there were data remain limited in their value. Certainly the rating scales have added little to this area in which clear binary outcomes are available, easy to record, and clinically meaningful.
2. Applicability
These studies were undertaken in largely poor, very busy hospitals of Brazil (middle‐income country) and India (low‐income country). Resource in these settings is very different to healthcare services of high‐income countries. However, we suggest that the patient groups experiencing aggression and the service provided at the acute point of care do not differ to richer countries. In India the body mass may be, on average, lower than in many other countries, and drug doses used may not be fully applicable to situations where people are larger, on average. Both countries use restraints as a possible addition to use of the medications, although Brazil is testing whether less restrictive options are a viable replacement for restraints (Huf 2011; Huf 2012). It is possible that the lower doses used, especially in the Mantovani 2013 trial, may not be as applicable in situations where restraints are less prevalent.
Quality of the evidence
1. Trials
For more information on quality of trials please see Figure 3 and Figure 4. We recognise that as authors of the TREC trials we could be biased in our [positive] appraisal. However, this concurs with NICE 2004, which described the available TREC studies of the time as "Unlike most of the other studies in this review, both [TREC‐Rio‐I; TREC‐Vellore‐I] were of high methodological quality" [p77]. The more recent TREC studies, TREC‐Rio‐II and TREC‐Vellore‐II, differ only to the original trials in their interventions. We did think that the newer studies, Baldacara 2011 and Mantovani 2013, did not match the methodological quality of the other studies. With inclusion of rating scales there was the loss of data, lack of binary important outcomes, and some selective reporting.
2. Outcomes
For more information please see the 'Summary of findings' tables. Our overall impression was that, unusually, this review contains much data of high quality ‐ but also some of the more familiar lower level of excellence. However, for the most partdata were collected in an understandable way, reported reasonably clearly, and about which we can draw some clinically meaningful conclusions.
Potential biases in the review process
We are aware that we invested considerable time and effort in the conduct of some of the trials relevant to this review. We do think that our knowledge helped the review (for example Summary of main results Section 6.2), but it could also have biased our appraisal of the literature. We have tried to work only with published reports and make all data extraction and analyses open to scrutiny. We tried repeatedly to contact the authors of Srinath 2010, as this study seemed to meet all inclusion criteria apart from reporting any useable data.
Agreements and disagreements with other studies or reviews
This review substantially updates and improves past work (Huf 2004; Huf 2009). It splits each comparison, where in previous versions all were lumped together. We felt uncomfortable with this initial combination of all data, and the heterogeneity of the findings at the time reinforced this discomfort. Lorazepam and midazolam are both benzodiazepines but are very different in their pharmacology. We are happier with this version where data are presented and commented upon separately. This work is largely concordant with recent NICE 2015 guidance.
Authors' conclusions
Implications for practice.
1. For people with schizophrenia and their families
People with schizophrenia and their families should be confident that if the person with mental illness becomes aggressive and out of control, they will swiftly receive effective and safe treatment.
Haloperidol plus promethazine seems an excellent combination to help manage acute aggression with few adverse effects in the very short term. Anyone given haloperidol in these difficult circumstances is at risk of seizure (about 1 in 100 to 150), but adding promethazine greatly offsets the risk of other distressing effects. It also helps swiftly calm the dangerous situation. The combination has a fast onset of action with the majority of episodes of aggression over safely within 30 minutes of it being administered. Re‐escalation of the aggression seems less common than with other options.
Compared with haloperidol plus promethazine, midazolam has an even faster onset of action, and, with close supervision, should be safe. The drug's swift action decreases everyone's exposure to the danger of unbridled aggression. However, close and skilled observation with midazolam is required as the respiratory depression is dangerous. Lorazepam used on its own does not compare favourably to the combination treatment. Use of haloperidol alone brings with it additional risk of avoidable periods of aggression and serious adverse effects. Olanzapine given as an intramuscular injection compares well with the less expensive, older combination of haloperidol plus promethazine in terms of tranquillisation, but the effects of the combination treatment are of longer duration. With olanzapine, re‐escalation of the aggression and re‐injection are more frequent compared with haloperidol plus promethazine. Like olanzapine, ziprasidone compares well for pace of tranquillisation but also seems to wear off quickly with the danger of re‐escalation of the aggression that the combination seems to avoid. Haloperidol plus midazolam seems to provide little difference to the use of midazolam alone. This particular combination probably carries the disadvantage of the use of haloperidol without anything to offset the acute dystonia that haloperidol causes.
2. For clinicians
All drug interventions included in the review are effective in calming agitation or aggression thought to be due to mental illness. Haloperidol plus promethazine is effective and safe. Benzodiazepines work, with midazolam being particularly swift, but both midazolam and lorazepam cause respiratory depression (probably midazolam more so than lorazepam), and we would question the use of this group of drugs outside of those services fully confident of observing for and managing the consequences of respiratory distress. Olanzapine intramuscular and ziprasidone intramuscular do seem to be viable options and are swift acting, but neither seems to last very long, and resumption of the aggression with subsequent need to re‐inject seems more likely than with haloperidol plus promethazine. Haloperidol used on its own without something to offset its frequent and serious adverse effects does seem difficult to justify.
3. For policymakers
There is compelling evidence that the combination treatment of haloperidol plus promethazine is effective and safe, at least within the context of a system that employs the use of restraints. The combination has been the focus of six trials (total n=1367) and compares favourably with all other drugs, including newer, more expensive treatments. All options are welcome for clinicians in this most difficult of situations, but the combination treatment of haloperidol plus promethazine is now becoming a gold standard against which others must be compared.
Implications for research.
1. General
The TREC studies' simplicity of design, real‐world practicality, and attention to outcomes of interest to both healthcare providers and recipients of care should be a model for other urgently needed studies. We think it important to ensure that all reporting meets highest CONSORT standards and that all data are available for future researchers (AllTrials).
2. Specific
2.1 Reviews
The two new included studies and excluded studies show that this area is beginning to be active and that there are more reviews to undertake in this area. Also, several of the excluded studies, although clearly not relevant for this review, could find value in other existing Cochrane reviews or suggest new titles (Table 9).
3. Included or excluded studies and Cochrane reviews.
| Study tag | Participants ‐ people with schizophrenia | Relevant Cochrane reviews | ||||
| + additional problems | ‐ not specifically aggressive/agitated | ‐ aggressive/agitated | ||||
| Comparison | Comparison | |||||
| Intervention #1 | Intervention #2 | Intervention #1 | Intervention #2 | |||
| Baldacara 2011 | + none specified | Not applicable | Haloperidol | Haloperidol + midazolam | Gillies 2013; Powney 2012 | |
| Olanzapine | Powney 2012 | |||||
| Ziprasidone | Powney 2012 | |||||
| Hou 2011 | Risperidone + lorazepam | Gillies 2013; Powney 2012 | ||||
| Baldacara 2011, Mantovani 2013 | Haloperidol + midazolam | Olanzapine | Gillies 2013; Powney 2012 | |||
| Ziprasidone | Gillies 2013; Powney 2012 | |||||
| Srinath 2010 | Haloperidol + promethazine | Lorazepam | Gillies 2013; Powney 2012 | |||
| Baldacara 2011, Mantovani 2013 | Olanzapine | Ziprasidone | Belgamwar 2005 | |||
| Levin 1959 | Chlorpromazine | Phenobarbital | Not applicable | ‐ | ||
| Placebo | Adams 2014 | |||||
| Promethazine | ‐ | |||||
| Bender 2003 | Perazine | Trimipramine | ‐ | |||
| Levin 1959 | Phenobarbital | Placebo | ‐ | |||
| Promethazine | Phenobarbital | ‐ | ||||
| Placebo | ‐ | |||||
| Merlo 2002 | Risperidone (2 mg) | Risperidone (4 mg) | Li 2009 | |||
| Brannen 1969 | Trifluoperazine | Placebo | Koch 2014 | |||
| St. Jean 1967 | + “mental deficiency" [? learning disability] | Chlorpromazine | Periciazine | ‐ | ||
| Yagi 1973 | + drug‐induced parkinsonism/EPS | Mazaticol hydrochloride | Promethazine | ‐ | ||
| Trihexyphenidyl | ‐ | |||||
| Otsuka 1978 | Methixene | Promethazine | ‐ | |||
| Trihexyphenidyl | ‐ | |||||
| Itoh 1972 | Piroheptine | Promethazine | ‐ | |||
| Trihexyphenidyl | ‐ | |||||
| Perenyi 1989 | Procyclidine | Promethazine | Essali 2013 | |||
| St. Jean 1964 | Promethazine | Placebo | ‐ | |||
| Otsuka 1978, Itoh 1972, Yagi 1973 | + drug‐induced parkinsonism/EPS | Promethazine | Trihexyphenidyl | ‐ | ||
| Claveria 1975 | + tardive dyskinesia | Pimozide | Placebo | Mothi 2013 | ||
| Yang 1999 | Promethazine | ‐ | ||||
EPS: extrapyramidal symptoms
2.2 Trials
The 2012 update stated "Many more studies are needed in this area. Replication of the work of the TREC trialists in different settings should be undertaken." We are pleased to see that new studies have been undertaken that can be added to this review and others. There is still much work to do and many more evaluations in this area to undertake. This is a delicate and difficult area, and all care should be based firmly on good trial evidence. Local practice will differ, and it can be problematic to accept and apply evidence from different care cultures. We provide no specific recommendation for which interventions should be evaluated, as there are many that justify randomisation within studies that have a focus on real‐world outcomes. However, some may feel that use of haloperidol unaccompanied by medication to specifically offset the acute dystonia is difficult to justify. We also feel that the new studies, Baldacara 2011 and Mantovani 2013, underpowered themselves by including many comparisons. It is important to keep the design in this area pragmatic (Thorpe 2009; Tosh 2011). We have suggested an outline design in Table 10 but recommend no scales or measures as these are largely not used in routine care and are problematic to collect, analyse, and interpret.
4. Design of a future study.
| Methods | Allocation: randomised (clearly described). Blinding: single blind (outcomes assessor). Duration: up to 2 weeks. Design: parallel. Setting: emergency settings |
| Participants | Diagnosis: anyone whose aggressive behaviour is thought to be due to psychotic illness. N=300. Age: > 18 years. Sex: N/A. Inclusion criteria: other measures failed. Exclusion criteria: specific contra‐indication to evaluated treatments |
| Interventions | 1. Drug intervention of choice. N=150. 2. Drug intervention of choice. N=150. Both drugs should be known to be effective, but the comparative effectiveness is unclear |
| Outcomes | Tranquil/asleep: binary outcomes, time. Behaviour: need for additional medication, additional aggressive episode. Adverse events. Acceptability of treatment. Costs: cost of services, cost of care. Service outcomes: days in hospital, discharged, transfer to secure unit |
What's new
| Date | Event | Description |
|---|---|---|
| 28 June 2016 | New citation required but conclusions have not changed | Update completed, conclusions unchanged. |
| 24 September 2015 | New search has been performed | Results from update search added to review, new trials identified and, where possible, incorporated. Structure substantially changed. |
History
Protocol first published: Issue 4, 2004 Review first published: Issue 4, 2004
| Date | Event | Description |
|---|---|---|
| 5 October 2011 | Amended | Contact details updated. |
| 11 May 2009 | New citation required and conclusions have changed | Highlighting addition of two new included studies and changed conclusions in the 2008 update. |
| 18 December 2008 | Amended | Plain language summary added. |
| 24 April 2008 | New search has been performed | Converted to new review format. |
| 4 July 2004 | New citation required and conclusions have changed | Substantive amendment. |
Acknowledgements
2009 version: The review authors would like to thank Edwardo da Silveira Martins (Pharmacy of Pinel hospital, Rio de Janeiro, Brazil) for his detailed provision of pharmacokinetic data on lorazepam and midazolam. We would also like to thank Clive E Adams, Tessa Grant, and Gill Rizzello of the Cochrane Schizophrenia Group for their editorial help.
Without the help of Clive E Adams for the 2015 update, we would not have been able to re‐extract all data, find the new studies, and complete the update in such a timely manner. We would also like to thank Nirmal Raveendran who helped update a previous version of this review of 2008.
Parts of this review were generated using RevMan HAL v 4.2. You can find more information about RevMan HAL here.
We would also like to thank and acknowledge Joanna Hubert, Sarah Barber, and Suravi Patra for peer reviewing this version of the review.
Appendices
Appendix 1. Previous searches
2.1 Search in 2004
We searched the Cochrane Schizophrenia Group's Register (July 2004) using the phrase'*Promethaz* in title, abstract or indexing terms of REFERENCE or *Promethaz* in interventions of STUDY
2.2 Search in 2008
We searched the Cochrane Schizophrenia Group's Register (January 2008) using the phrase
'*Promethaz* in title, abstract or indexing terms of REFERENCE or *Promethaz* in interventions of STUDY
Appendix 2. Methods ‐ as used in the 2009 version
1. Study selection We (GH and JA) independently inspected the citations identified from the search. We identified potentially relevant abstracts and ordered full papers for reassessment for inclusion and methodological quality. We discussed and reported any disagreements and contacted study authors for further clarification where necessary.
2. Assessment of quality Again working independently, we allocated trials to three quality categories, as described in the Cochrane Collaboration Handbook (Higgins 2005). Concealment of allocation remains the key aspect of methodology that predicts how susceptible results are to the inclusion of biases. Although adherence to blinding at outcome is also important, it is the ability of everyone involved to predict who will receive the next intervention that most substantially influences results. Category A studies in this review, where good concealment of allocation was made explicit, employed techniques that ensured that researchers and recipients could not have known the next intervention to be used. We considered these studies at low risk of bias. Any plausible biases were felt to be unlikely to seriously affect results. Category B studies assured the reader that studies were randomised but they did not make concealment of allocation explicit. We would have included these studies but these would have been at moderate risk of bias with some doubts about the results. We did not include Category C studies, where the process of allocation is neither described nor implied. These studies are at high risk of bias and the plausible bias seriously weakens confidence in the results.
If disputes had arisen as to which category a trial was to be allocated, again, resolution was to have been attempted by discussion. We included only trials in Category A or B in the review. Other dimensions of quality were not reasons for exclusion.
3. Data management 3.1 Data extraction We (GH, JA) independently extracted data from included studies. Again, any disagreement was discussed, decisions documented and, if necessary, we contacted the authors of the studies for clarification. We documented justifications for excluding references from the review.
3.2 Intention to treat assumptions For studies that did not specify the reasons for people leaving the study early, we assumed that these people had no change in clinical outcome variables. We excluded data from outcomes where attrition was greater than 50%.
5. Data analysis 5.1 Binary data For binary outcomes we calculated a standard estimation of the fixed‐effect risk ratio (RR) and its 95% confidence interval (CI). Where binary results were statistically significant we calculated the number needed to treat/harm statistic (NNT/H), and its 95% confidence interval (CI) using Visual Rx (http://www.nntonline.net/) which takes account of the event rate in the control group.
5.2 Continuous data 5.2.1 Skewed data: continuous data on clinical and social outcomes are often not normally distributed. To avoid the pitfall of applying parametric tests to non‐parametric data, we applied the following standards to all data before inclusion: (a) standard deviations and means were reported in the paper or were obtainable from the authors; (b) when a scale started from the finite number zero, the standard deviation, when multiplied by two, was less than the mean (as otherwise the mean is unlikely to be an appropriate measure of the centre of the distribution, (Altman 1996); (c) if a scale started from a positive value (such as PANSS which can have values from 30 to 210) the calculation described above was modified to take the scale starting point into account. In these cases skew is present if 2SD>(S‐Smin), where S is the mean score and Smin is the minimum score. Endpoint scores on scales often have a finite start and end point and these rules can be applied. When continuous data are presented on a scale which includes a possibility of negative values (such as change data), it is difficult to tell whether data are skewed or not. Skewed data from studies of less than 200 participants would have been entered in additional tables rather than into an analysis. Skewed data pose less of a problem when looking at means if the sample size is large and would have been entered into a synthesis.
5.2.2 Summary statistic: for continuous outcomes we estimated a fixed‐effect weighted mean difference (WMD) between groups.
5.2.3 Valid scales: we included continuous data from rating scales only if the measuring instrument had been described in a peer‐reviewed journal (Marshall 2000) and the instrument was either a self‐report or completed by an independent rater or relative (not the therapist).
5.2.4 Endpoint versus change data: we find it preferable to use scale endpoint data, which typically cannot have negative values and is easier to interpret from a clinical point of view. Change data are often not ordinal and are very problematic to interpret. If endpoint data had not been available, we would have used change data.
5.2.5 Cluster trials: studies increasingly employ 'cluster randomisation' (such as randomisation by clinician or practice) but analysis and pooling of clustered data poses problems. Firstly, authors often fail to account for intraclass correlation in clustered studies, leading to a 'unit of analysis' error (Divine 1992) whereby p values are spuriously low, confidence intervals unduly narrow and statistical significance overestimated. This causes type I errors (Bland 1997, Gulliford 1999).
Where clustering was not accounted for in primary studies, we presented the data in a table, with a (*) symbol to indicate the presence of a probable unit of analysis error. In subsequent versions of this review we will seek to contact first authors of studies to obtain intraclass correlation coefficients of their clustered data and to adjust for this by using accepted methods (Gulliford 1999). Where clustering has been incorporated into the analysis of primary studies, we will also present these data as if from a non‐cluster randomised study, but adjusted for the clustering effect.
We have sought statistical advice and have been advised that the binary data as presented in a report should be divided by a 'design effect'. This is calculated using the mean number of participants per cluster (m) and the intraclass correlation coefficient (ICC) Design effect=1+(m‐1)*ICC (Donner 2002). If the ICC was not reported it was assumed to be 0.1 (Ukoumunne 1999).
If cluster studies had been appropriately analysed taking into account intraclass correlation coefficients and relevant data documented in the report, synthesis with other studies would have been possible using the generic inverse variance technique.
6. Test for heterogeneity Firstly, we considered all the included studies within any comparison to judge clinical heterogeneity. Then we visually inspected graphs to investigate the possibility of statistical heterogeneity. This was supplemented, primarily, by employing the I‐squared statistic. This provides an estimate of the percentage of inconsistency thought to be due to chance. Where the I‐squared estimate was greater than or equal to 75%, this was interpreted as evidence of high levels of heterogeneity (Higgins 2003). Data were then re‐analysed using a random‐effects model to see if this made a substantial difference. If it did, and results became more consistent, i.e. falling below 75% in the estimate, the studies were added to the main body of trials. If using the random‐effects model did not make a difference and inconsistency remained high, data were not summated, but were presented separately and reasons for heterogeneity investigated.
7. Addressing small study bias We were to have entered data from all included studies into a funnel graph (trial effect against trial size) in an attempt to investigate the likelihood of overt publication bias (Egger 1997).
8. Sensitivity analyses If necessary, we analysed the effect of including studies with high attrition rates in a sensitivity analysis. We also included trials in a sensitivity analysis if they were described as 'double blind' but only implied randomisation. If we found no substantive differences within primary outcomes when these high attrition and 'implied randomisation' studies were added to the overall results, we included them in the final analysis. However, if there was a substantive difference we only used clearly randomised trials, and those with attrition lower than 50%.
9. General Where possible, we entered data in such a way that the area to the left of the line of no effect indicated a favourable outcome for haloperidol plus promethazine.
Data and analyses
Comparison 1. HALOPERIDOL + PROMETHAZINE vs ANTIPSYCHOTIC ‐ HALOPERIDOL.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Tranquil or asleep: 1. Not tranquil or asleep | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 by 30 minutes | 1 | 316 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.49, 0.87] |
| 1.2 by 1 hour | 1 | 316 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.46, 1.23] |
| 1.3 by 2 hours | 1 | 316 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.32, 0.96] |
| 2 Tranquil or asleep: 2. Not asleep | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 by 30 minutes | 1 | 316 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.82, 0.96] |
| 2.2 by 1 hour | 1 | 316 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.84, 1.28] |
| 2.3 by 2 hours | 1 | 316 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.77, 1.31] |
| 3 Tranquil or asleep: 3. Time until tranquil or asleep (RSS, high score=good) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 by 1 hour | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.58, 0.38] |
| 3.2 by 2 hours | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.30, 0.50] |
| 3.3 by 4 hours | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐0.18, 0.78] |
| 3.4 by 6 hours | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.08, 0.48] |
| 3.5 by 12 hours | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.28, 0.28] |
| 4 Global state: 1. Needing restraints or seclusion | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 by 2 hours | 1 | 311 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.54, 1.18] |
| 4.2 by 12 hours | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.28, 2.44] |
| 5 Global state: 2. Various measures | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 requiring additional drugs during initial phase ‐ by 2 hours | 1 | 311 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.16, 1.25] |
| 5.2 doctor called to see patient ‐ by 24 hours | 1 | 298 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.44, 0.99] |
| 5.3 refusing oral drugs ‐ at 24 hours | 1 | 294 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.54, 1.97] |
| 6 Global state: 3. Average value of additional medication ‐ after initial dose (skewed data) | Other data | No numeric data | ||
| 7 Adverse effects: 1. General ‐ Any serious adverse effect | 1 | 298 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.01, 0.66] |
| 7.1 by 24 hours | 1 | 298 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.01, 0.66] |
| 8 Adverse effects: 2. Specific ‐ a. Cardiovascular ‐ hypotension | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.0 [0.38, 129.93] |
| 9 Adverse effects: 2. Specific ‐ b. Central Nervous System | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 seizure ‐ by 24 hours | 1 | 298 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.06, 15.01] |
| 9.2 sedation ‐ excessive | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.04, 3.03] |
| 10 Adverse effects: 2. Specific ‐ c. Extrapyramidal problems | 2 | 358 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.14, 0.88] |
| 10.1 acute dystonia ‐ by 24 hours | 1 | 298 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.05 [0.00, 0.76] |
| 10.2 extrapyramidal problems (unspecified) ‐ 0‐4 hours | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.32, 3.10] |
| 11 Service outcomes: Not discharged ‐ by 2 weeks | 1 | 310 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.64, 1.07] |
| 12 Specific behaviour: 1. Aggression ‐ a. Other episode of agression | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 12.1 other episode of aggression ‐ by 24 hours | 1 | 298 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.68, 2.01] |
| 13 Specific behaviour: 1. Aggression ‐ b. Average aggression score (OAS ,high score=bad) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 13.1 by 1 hour | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 4.50 [2.72, 6.28] |
| 13.2 by 2 hours | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.70 [‐0.49, 1.89] |
| 13.3 by 4 hours | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐0.71, ‐0.49] |
| 13.4 by 6 hours | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐1.1 [‐1.29, ‐0.91] |
| 13.5 by 12 hours | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐1.8 [‐1.93, ‐1.67] |
| 14 Specific behaviour: 1. Aggression ‐ c. Average agitation score (OASS, high score=bad) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 14.1 by 1 hour | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 24.5 [21.68, 27.32] |
| 14.2 by 2 hours | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 9.40 [8.41, 10.39] |
| 14.3 by 4 hours | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 3.80 [3.27, 4.33] |
| 14.4 by 6 hours | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 2.6 [2.13, 3.07] |
| 14.5 by 12 hours | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.80 [0.55, 1.05] |
| 15 Leaving the study early | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 15.1 before treatment | 1 | 316 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [0.25, 8.63] |
| 15.2 by 24 hours | 2 | 376 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.41, 1.97] |
| 15.3 by 2 weeks | 1 | 316 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.20, 4.76] |
1.11. Analysis.
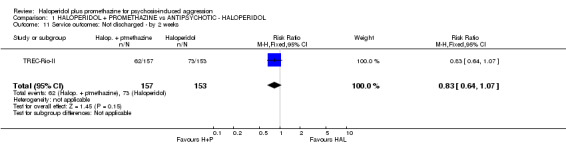
Comparison 1 HALOPERIDOL + PROMETHAZINE vs ANTIPSYCHOTIC ‐ HALOPERIDOL, Outcome 11 Service outcomes: Not discharged ‐ by 2 weeks.
Comparison 2. HALOPERIDOL + PROMETHAZINE vs ANTIPSYCHOTIC ‐ OLANZAPINE.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Tranquil or asleep: 1. Not tranquil or asleep | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 by 30 minutes | 1 | 300 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.6 [0.22, 1.61] |
| 1.2 by 1 hour | 1 | 300 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 0.87] |
| 1.3 by 2 hours | 1 | 300 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.14, 1.41] |
| 1.4 by 4 hours | 1 | 300 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.26, 2.67] |
| 2 Tranquil or asleep: 2. Not asleep | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 by 30 minutes | 1 | 300 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.46, 0.93] |
| 2.2 by 1 hour | 1 | 300 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.40, 0.87] |
| 2.3 by 2 hours | 1 | 300 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.14, 0.41] |
| 2.4 by 4 hours | 1 | 300 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.44, 0.86] |
| 3 Tranquil or asleep: 3. Never tranquil or asleep during first 4 hours | 1 | 300 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.03, 2.21] |
| 4 Tranquil or asleep: 4. Average sedation score (RSS, high score=good) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 by 1 hour | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.26, 0.66] |
| 4.2 by 2 hours | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.26, 0.46] |
| 4.3 by 4 hours | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.34, 0.54] |
| 4.4 by 6 hours | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.15, 0.35] |
| 4.5 by 12 hours | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.23, 0.23] |
| 5 Tranquil or asleep: 5. Time (skewed data) | Other data | No numeric data | ||
| 5.1 time until tranquil or asleep | Other data | No numeric data | ||
| 5.2 time until asleep | Other data | No numeric data | ||
| 6 Tranquil or asleep: 6. Effect of tranquillisation (PANSS‐EC, high=bad) (skewed data) | Other data | No numeric data | ||
| 6.1 at 30 minutes | Other data | No numeric data | ||
| 6.2 at 60 minutes | Other data | No numeric data | ||
| 6.3 at 90 minutes | Other data | No numeric data | ||
| 7 Tranquil or asleep: 7. Level of tranquillisation / agitation (ACES) (skewed data) | Other data | No numeric data | ||
| 7.1 at 30 minutes | Other data | No numeric data | ||
| 7.3 at 90 minutes | Other data | No numeric data | ||
| 8 Global state: 1. No overall improvement | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 by 30 minutes | 1 | 300 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.36, 0.91] |
| 8.2 by 1 hour | 1 | 300 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.4 [0.21, 0.75] |
| 8.3 by 2 hours | 1 | 300 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.24, 0.79] |
| 8.4 by 4 hours | 1 | 300 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.22, 1.01] |
| 9 Global state: 2. Needing restraints or seclusion | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 by 30 minutes | 1 | 300 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.71, 1.47] |
| 9.2 by 1 hour | 1 | 300 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.66, 1.44] |
| 9.3 by 2 hours | 1 | 300 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.51, 1.25] |
| 9.4 by 4 hours | 1 | 300 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.34, 1.14] |
| 9.5 by 12 hours | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.62, 40.28] |
| 10 Global state: 3. Various measures | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 requiring additional drugs during initial phase ‐ by 4 hours | 2 | 356 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.37, 0.74] |
| 10.2 requiring further observation ‐ by 4 hours | 1 | 300 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.80, 1.71] |
| 10.3 doctor called to see patient ‐ by 4 hours | 1 | 300 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.30, 0.73] |
| 10.4 taking oral drugs ‐ at 2 weeks | 1 | 300 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.89, 1.04] |
| 11 Global state: 4. Average improvement (CGI, high score=bad) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 11.1 by 30 minutes | 1 | 300 | Mean Difference (IV, Fixed, 95% CI) | ‐0.35 [‐0.58, ‐0.12] |
| 11.2 by 1 hour | 1 | 300 | Mean Difference (IV, Fixed, 95% CI) | ‐0.41 [‐0.60, ‐0.22] |
| 11.3 by 2 hours | 1 | 300 | Mean Difference (IV, Fixed, 95% CI) | ‐0.36 [‐0.56, ‐0.16] |
| 11.4 by 4 hours | 1 | 300 | Mean Difference (IV, Fixed, 95% CI) | ‐0.27 [‐0.43, ‐0.11] |
| 12 Global state: 5. Average value of additional medication ‐ after initial dose (skewed data) | Other data | No numeric data | ||
| 13 Adverse effects: 1. General ‐ Serious adverse effect | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 13.1 by 4 hours | 1 | 300 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.04, 3.17] |
| 13.2 at 2 weeks | 1 | 300 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.12] |
| 14 Adverse effects: 2. Specific ‐ a. Cardiovascular ‐ hypotension | 2 | 116 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.49, 18.31] |
| 15 Adverse effects: 2. Specific ‐ b. Central Nervous System ‐ sedation ‐ excessive | 2 | 116 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.12, 3.84] |
| 16 Adverse effects: 2. Specific ‐ c. Extrapyramidal problems ‐ 0‐4 hours | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 16.1 any change in scale‐rated extrapyramidal problems (Simpson & Angus Scale) | 3 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.76 [1.12, 2.77] |
| 17 Specific behaviour: 1. Severe agitation | 1 | 56 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.0 [0.38, 129.55] |
| 18 Specific behaviour: 2. Average aggression score (OAS, high score=bad) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 18.1 by 1 hour | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 5.4 [3.72, 7.08] |
| 18.2 by 2 hours | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 1.20 [0.39, 2.01] |
| 18.3 by 4 hours | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.5 [‐0.68, ‐0.32] |
| 18.4 by 6 hours | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐1.20 [‐1.90, ‐0.50] |
| 18.5 by 12 hours | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐2.21, ‐1.79] |
| 19 Specific behaviour: 3. Average agitation score (OASS, high score=bad) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 19.1 by 1 hour | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 26.5 [23.76, 29.24] |
| 19.2 by 2 hours | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 13.6 [12.64, 14.56] |
| 19.3 by 4 hours | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 4.0 [3.47, 4.53] |
| 19.4 by 6 hours | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 2.8 [2.31, 3.29] |
| 19.5 by 12 hours | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 1.70 [1.44, 1.96] |
| 20 Service outcomes | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 20.1 admitted ‐ by 4 hours | 1 | 300 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.56, 1.16] |
| 20.2 not discharged ‐ by 4 hours | 1 | 300 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.77, 1.16] |
| 21 Leaving the study early | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 21.1 by 30 minutes | 1 | 300 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.12] |
| 21.2 by 2 hours | 1 | 300 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.74] |
| 21.3 by 4 hours | 1 | 300 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.01, 1.63] |
| 21.4 by 24 hours | 2 | 116 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.04, 3.01] |
| 21.5 by 2 weeks | 1 | 300 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.33, 1.56] |
Comparison 3. HALOPERIDOL + PROMETHAZINE vs ANTIPSYCHOTIC ‐ ZIPRASIDONE.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Tranquil or asleep: 1. Average sedation score (RSS, high score=good) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 by 1 hour | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.58, 0.38] |
| 1.2 by 2 hours | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.36, 0.56] |
| 1.3 by 4 hours | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.56, 0.36] |
| 1.4 by 6 hours | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.18, 0.38] |
| 1.5 by 12 hours | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.38, 0.18] |
| 2 Tranquil or asleep: 2. Effect of tranquilisation (PANSS‐EC, high=bad) (skewed data) | Other data | No numeric data | ||
| 2.1 at 30 minutes | Other data | No numeric data | ||
| 2.2 at 60 minutes | Other data | No numeric data | ||
| 2.3 at 90 minutes | Other data | No numeric data | ||
| 3 Tranquil or asleep: 3. Level of tranquilisation / agitation (ACES) (skewed data) | Other data | No numeric data | ||
| 3.1 at 30 minutes | Other data | No numeric data | ||
| 3.2 at 90 minutes. | Other data | No numeric data | ||
| 4 Global state: 1. Needing restraints or seclusion | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.19, 1.29] |
| 5 Global state: 2. Additional tranquillising drugs | 1 | 51 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.19, 1.36] |
| 6 Global state: 3. Average value of additional medication ‐ after initial dose (skewed data) | Other data | No numeric data | ||
| 7 Adverse effects: 1. Specific ‐ a. Cardiovascular ‐ hypotension | 2 | 111 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.17, 1.75] |
| 8 Adverse effects: 1. Specific ‐ b. Central Nervous System ‐ excessive sedation | 2 | 111 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.06, 1.43] |
| 9 Adverse effects: 1. Specific ‐ c. Extrapyramidal problems ‐ 0‐4 hours | 2 | 111 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.72 [1.07, 2.76] |
| 10 Specific behaviour: 1. Severe agitation | 1 | 51 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.18, 3.69] |
| 11 Specific behaviour: 2. Average aggression score (OAS, high score=bad) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 11.1 by 1 hour | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 4.50 [2.82, 6.18] |
| 11.2 by 2 hours | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 1.4 [0.55, 2.25] |
| 11.3 by 4 hours | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐0.62, 0.02] |
| 11.4 by 6 hours | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐0.59, ‐0.21] |
| 11.5 by 12 hours | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐1.60 [‐1.75, ‐1.45] |
| 12 Specific behaviour: 3. Average agitation score (OASS, high score=bad) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 12.1 by 1 hour | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 16.80 [13.68, 19.92] |
| 12.2 by 2 hours | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 5.5 [2.92, 8.08] |
| 12.3 by 4 hours | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐1.47, 0.27] |
| 12.4 by 6 hours | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐1.85, ‐0.15] |
| 12.5 by 12 hours | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐1.90 [‐2.34, ‐1.46] |
| 13 Leaving the study early | 2 | 111 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.48 [0.11, 58.20] |
| 13.1 by 24 hours | 2 | 111 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.48 [0.11, 58.20] |
Comparison 4. HALOPERIDOL + PROMETHAZINE vs ANTIPSYCHOTIC + BENZODIAZEPINE (HALOPERIDOL + MIDAZOLAM).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Tranquil or asleep: 1. Average sedation score (RSS, high score=good) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 by 1 hour | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐1.13, ‐0.07] |
| 1.2 by 2 hours | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.46, 0.46] |
| 1.3 by 4 hours | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.51, 0.51] |
| 1.4 by 6 hours | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.18, 0.38] |
| 1.5 by 12 hours | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.24, 0.44] |
| 2 Tranquil or asleep: 2. Effect of tranquilisation (PANSS‐EC, high score=bad) (skewed data) | Other data | No numeric data | ||
| 2.1 at 30 minutes | Other data | No numeric data | ||
| 2.2 at 60 minutes | Other data | No numeric data | ||
| 2.3 at 90 minutes | Other data | No numeric data | ||
| 3 Tranquil or asleep: 3. Level of tranquilisation / agitation (ACES) (skewed data) | Other data | No numeric data | ||
| 3.1 at 30 minutes | Other data | No numeric data | ||
| 3.2 at 90 minutes | Other data | No numeric data | ||
| 4 Global state: 1. Needing restraints or seclusion | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.10, 0.55] |
| 5 Global state: 2. Additional tranquilising drugs | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.34, 3.19] |
| 6 Global state: 3. Average value of additional medication ‐ after initial dose (skewed data) | Other data | No numeric data | ||
| 7 Adverse effects: 1. Specific ‐ a. Cardiovascular ‐ hypotension | 2 | 117 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.16, 1.58] |
| 8 Adverse effects: 1. Specific ‐ b. Central Nervous System ‐ excessive sedation | 2 | 117 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.12 [0.03, 0.49] |
| 9 Adverse effects: 1. Specific ‐ c. Extrapyramidal problems ‐ 0‐4 hours | 2 | 117 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.84 [1.12, 3.02] |
| 10 Specific behaviour: 1. Average aggression score (OAS, high score=bad) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 10.1 by 1 hour | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 3.30 [1.35, 5.25] |
| 10.2 by 2 hours | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐1.70 [‐3.46, 0.06] |
| 10.3 by 4 hours | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.70 [‐1.27, ‐0.13] |
| 10.4 by 6 hours | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.70 [‐0.89, ‐0.51] |
| 10.5 by 12 hours | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐3.7 [‐4.39, ‐3.01] |
| 11 Specific behaviour: 2. Average agitation score (OASS, high score=bad) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 11.1 by 1 hour | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 16.00 [13.02, 18.98] |
| 11.2 by 2 hours | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 2.70 [1.67, 3.73] |
| 11.3 by 4 hours | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐1.70 [‐2.79, ‐0.61] |
| 11.4 by 6 hours | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐1.10 [‐2.08, ‐0.12] |
| 11.5 by 12 hours | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐10.40 [‐11.47, ‐9.33] |
| 12 Specific behaviour: 3. Severe agitation | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.55 [0.28, 8.61] |
| 13 Leaving the study early | 2 | 117 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.03, 2.18] |
| 13.1 by 24 hours | 2 | 117 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.03, 2.18] |
Comparison 5. HALOPERIDOL + PROMETHAZINE vs BENZODIAZEPINES ‐ LORAZEPAM.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Tranquil or asleep: 1. Not tranquil or asleep | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 by 30 minutes | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.10, 0.68] |
| 1.2 by 1 hour | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.04, 0.89] |
| 1.3 by 2 hours | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.07, 0.86] |
| 1.4 by 4 hours | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.26, 3.89] |
| 2 Tranquil or asleep: 2. Not asleep | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 by 30 minutes | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.29, 0.54] |
| 2.2 by 1 hour | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.36, 0.66] |
| 2.3 by 2 hours | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.36, 0.71] |
| 2.4 by 4 hours | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.30, 0.65] |
| 3 Tranquil or asleep: 3. Time (skewed data) | Other data | No numeric data | ||
| 3.1 time until tranquil or asleep | Other data | No numeric data | ||
| 3.2 time until asleep | Other data | No numeric data | ||
| 4 Global state: 1. No overall improvement | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 by 30 minutes | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.25, 0.66] |
| 4.2 by 1 hour | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.32, 0.79] |
| 4.3 by 2 hours | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.25, 0.86] |
| 4.4 by 4 hours | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.46, 1.87] |
| 5 Global state: 2. Needing restraints or seclusion | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 by 30 minutes | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.28, 1.09] |
| 5.2 by 1 hour | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.27, 1.14] |
| 5.3 by 2 hours | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.35, 1.67] |
| 5.4 by 4 hours | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.35, 1.89] |
| 6 Global state: 3. Additional tranquillising drugs | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 by 30 minutes | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.09] |
| 6.2 by 1 hour | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.04, 3.15] |
| 6.3 by 2 hours | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.17, 3.27] |
| 6.4 by 4 hours | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.36, 2.21] |
| 7 Global state: 4. Various measures | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 doctor called to see patient ‐ by 4 hours | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.37, 1.39] |
| 7.2 refusing oral medication ‐ by 2 weeks | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.63 [0.70, 3.75] |
| 8 Global state: 5. Average improvement (CGI, high score=bad) ) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.1 by 30 minutes | 1 | 200 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐0.86, ‐0.34] |
| 8.2 by 1 hour | 1 | 200 | Mean Difference (IV, Fixed, 95% CI) | ‐0.33 [‐0.54, ‐0.12] |
| 8.3 by 2 hours | 1 | 200 | Mean Difference (IV, Fixed, 95% CI) | ‐0.23 [‐0.51, 0.05] |
| 8.4 by 4 hours | 1 | 200 | Mean Difference (IV, Fixed, 95% CI) | ‐0.09 [‐0.32, 0.14] |
| 9 Adverse effects: 1. General ‐ serious adverse effect | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 by 30 minutes | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.09] |
| 9.2 by 1 hour | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.3 by 2 hours | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.4 by 4 hours | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Adverse effects: 2. Specific ‐ Extrapyramidal problems ‐ 0‐4 hours | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 akathisia (Barnes Akathisia Scale) | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 any change in scale‐rated extrapyramidal problems (Simpson & Angus Scale) | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Service outcomes: Not discharged | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.85, 1.50] |
| 11.1 by 4 hours | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.85, 1.50] |
| 12 Leaving the study early | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 12.1 by 4 hours | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.12, 72.77] |
| 12.2 by 2 weeks | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.51, 3.04] |
Comparison 6. HALOPERIDOL + PROMETHAZINE vs BENZODIAZEPINES ‐ MIDAZOLAM.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Tranquil or asleep: 1. Not tranquil or asleep | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 by 30 minutes | 1 | 301 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.90 [1.75, 4.80] |
| 1.2 by 1 hour | 1 | 301 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.91 [0.92, 3.98] |
| 1.3 by 2 hours | 1 | 301 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.73 [0.70, 4.26] |
| 2 Tranquil or asleep: 2. Not asleep | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 by 30 minutes | 1 | 301 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.86 [1.48, 2.33] |
| 2.2 by 1 hour | 1 | 301 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.18 [1.52, 3.12] |
| 2.3 by 2 hours | 1 | 301 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.13 [1.42, 3.20] |
| 3 Global state: 1. Needing restraints or seclusion ‐ by 2hrs | 1 | 301 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.82, 1.82] |
| 4 Global state: 2. Needing addition drugs during initial phase ‐ by 2hrs | 1 | 301 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.52 [0.74, 16.69] |
| 5 Global state: 3. Various measures | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 doctor called to see patient ‐ by 24 hours | 1 | 301 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.61, 1.19] |
| 5.2 refusing oral drugs ‐ at 24 hours | 1 | 301 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.33, 1.44] |
| 6 Adverse effects: Serious adverse effect | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 by 30 minutes | 1 | 301 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.06, 15.95] |
| 6.2 by 1 hour | 1 | 301 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.3 by 2 hours | 1 | 301 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Service outcomes: Not discharged | 1 | 301 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.84, 1.29] |
| 7.1 by 15 days | 1 | 301 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.84, 1.29] |
| 8 Specific Behaviours: 1. Aggression. a ‐ other episode of aggression ‐ by 24 hrs | 1 | 301 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.62, 1.29] |
| 9 Leaving the study early | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 by 2 hours | 1 | 301 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.01 [0.18, 21.97] |
| 9.2 by 24 hours | 1 | 301 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.36, 2.80] |
| 9.3 by 2 weeks | 1 | 301 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.76] |
Comparison 7. HALOPERIDOL + PROMETHAZINE vs ANTIPSYCHOTIC ‐ HALOPERIDOL ‐ additional 40 minutes data.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Tranquil or asleep: 1. Not tranquil or asleep | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 by 40 minutes | 1 | 316 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.56, 1.24] |
| 2 Tranquil or asleep: 2. Not asleep | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 by 40 minutes | 1 | 316 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.85, 1.16] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Baldacara 2011.
| Methods | Allocation: randomised. Blindness: double blind. Duration: 12 hours. Setting: psychiatric emergency rooms of Santa Casa de Sao Paula in Brazil |
|
| Participants | Diagnosis: psychotic disorder (60%), bipolar (40%). N=150. Age: average ˜ 32 years (SD ˜ 8). Sex: 60 women, 90 men. History: agitated people in emergency psychiatric rooms |
|
| Interventions | 1. Haloperidol plus promethazine: dose haloperidol 5 mg + promethazine 50 mg. N=30. 2. Olanzapine: dose 10 mg. N=30. 3. Ziprasidone: dose 20 mg. N=30. 4. Haloperidol plus midazolam: dose haloperidol 5 mg + midazolam 15 mg. N=30. 5. Haloperidol: dose 5 mg. N=30 |
|
| Outcomes | Tranquil or asleep: average sedation score (RSS). Specific behaviours: aggression, agitation (OAS, OASS). Global state: additional medication, mechanical restraint. Adverse effects: central nervous system, extrapyramidal side effects, hypotension. Leaving the study early. Unable to use ‐ Global state: value of additional medication (skewed data). Economic outcomes: cost of drug (no numerical data) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Randomly assigned under double blinded conditions. Method of randomization employed was allocation by permuted blocks.” Review author judgement: unclear exactly how people were randomised |
| Allocation concealment (selection bias) | Unclear risk | No information regarding concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: “Patients were assessed by two psychiatrists. Psychiatrists were all masked with regard to patient’s treatment assignment, double blinded and study medications were packaged in identical colour‐coded boxes." Review author judgement: Unclear information about participant blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All 150 were allocated and randomised. Review author judgement: People leaving early left before the enrolment and were well‐explained |
| Selective reporting (reporting bias) | Unclear risk | Review author judgement: No evidence of selective reporting |
| Other bias | Unclear risk | No clear evidence of other bias |
Mantovani 2013.
| Methods | Allocation: randomised. Blindness: blinded. Duration: 24 hours. Setting: psychiatric emergency unit of clinical hospital of Ribeirao Preto in Brazil |
|
| Participants | Diagnosis: psychotic disorder (21), bipolar/manic disorder (36), substance misuse (15), other (28). N=100 (120 selected). Age: 18 to 56 years (mean ˜ 31, SD ˜ 9). Sex: 53 women and 47 men. History: acute agitation requiring rapid tranquillisation |
|
| Interventions | 1. IM haloperidol + IM promethazine: dose 2.5 mg haloperidol + dose 25 mg promethazine. N=28. 2. IM haloperidol + IM midazolam: dose 2.5 mg haloperidol + dose 7.5 mg midazolam. N=29. 3. IM ziprasidone: dose 10 mg. N=23. 4. IM olanzapine: dose 10 mg. N=28 |
|
| Outcomes | Specific behaviour: severe agitation. Global state: needing additional medication. Adverse effects: UKU scale, extrapyramidal side effects, central nervous system (excessive sedation), reduction in heart rate. Unable to use ‐ Tranquil or asleep: ACES*, PANSS‐EC (data skewed). Physiological measures: no numerical data |
|
| Notes | *Data extracted solely from graph | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was generated by computer and cards with treatment option were prepared by researcher (M.E.S.B.S.), who was not directly participating in assessment.” |
| Allocation concealment (selection bias) | Low risk | Quote: "Allocation by cards numbered from 1 to 120 were kept in sealed envelopes identical in appearance and closed in specific box.” Review author judgement: Adequate blinding applied |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "Cards with treatment option were sealed in identical envelopes and picked by chance. Medications had same presentation and staff oriented not to tell patient about treatment. Neither patient nor relatives were aware of intervention. The rating psychiatrist, blinded to the treatment option applied the rating instruments." Review author judgement: Unclear information about participant blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No evidence |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "120 were allocated and 100 analysed". Review author judgement: Leaving the study early was before full enrolment, but we are unclear how data were handled |
| Selective reporting (reporting bias) | High risk | Quote: "The scaled used are complicated and measures standard deviation and standard error so they are picked and chosen". Review author judgement: Clear evidence of selective reporting |
| Other bias | Unclear risk | No evidence |
TREC‐Rio‐I.
| Methods | Allocation: randomised. Blindness: none. Duration: 14 days. Setting: 3 inner‐city emergency rooms of middle‐income country | |
| Participants | Diagnosis: psychosis (219), substance abuse (51), others (30). N=301. Sex: women 155, men 146. Age: mean ˜ 38 years (SD ˜ 11). History: agitation on presentation to emergency room, first psychiatric attendance (26), markedly severely agitated or worse (192) | |
| Interventions | 1. Haloperidol IM: dose up to 10 mg stat + promethazine IM: dose up to 50 mg stat. N=150. 2. Midazolam IM: dose up to 15 mg stat. N=151 | |
| Outcomes | Tranquil or asleep*. Specific behaviours: other episodes of aggression. Global state: use of additional medication, use of restraints/seclusion, needing extra visits from the doctor, refusing oral medication. Adverse effects: serious adverse effects. Service outcomes: no hospital discharge. Leaving the study early | |
| Notes | *Primary outcome chosen by emergency room staff (tranquil or asleep by 20 minutes) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization by excel generated numbers, block sizes were applied to a table of random numbers, sealed packs". Review author judgement: Adequate random sequence generation |
| Allocation concealment (selection bias) | Low risk | Quote: "Table of allocation sequence independent of block size produced to ensure correct drug was consecutively numbered and then sealed". Review author judgement: Adequate concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No participant blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No outcome blinding, outcomes selected to be robust to detection bias, and additional blinded student concurrently accurately timed their opinion of when tranquillisation occurred; this blind and accurate rating concurred with that of the unblinded staff |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Participants randomised were 301, and the follow‐up was explained well |
| Selective reporting (reporting bias) | Unclear risk | No evidence or information |
| Other bias | Unclear risk | None known |
TREC‐Rio‐II.
| Methods | Allocation: randomised. Blinding at outcome: none for primary outcomes (some non‐primary data extracted by rater blind to treatment). Duration: 14 days. Setting: emergency room of psychiatric hospital in Rio de Janeiro | |
| Participants | Diagnosis: psychosis (244), substance abuse (58), other (14). N=316*. Sex: women 146, men 170. Age: mean ˜ 39.8 years. History: agitation or aggression on presentation to emergency room, first psychiatric attendance (59), intensely or extremely agitated (204) | |
| Interventions | 1. Haloperidol IM: dose up to 10 mg stat + IM promethazine: dose up to 50 mg stat. N=160. 2. Haloperidol IM alone: dose up to 10 mg stat. N=156 | |
| Outcomes | **Tranqil or asleep.
Specific behaviours: other episodes of aggression.
Global state: use of additional medication, use of restraints/seclusion, needing extra visits from the doctor, refusing oral medication.
Adverse effects: serious adverse effects, central nervous system, extrapyramidal. Service outcomes: no hospital discharge. Leaving the study early |
|
| Notes | *5 people (2 from the haloperidol group and 3 from haloperidol + promethazine group) were randomised but left before treatment, we assumed poor outcome for the primary outcome tranquil or asleep but unable to analyse for adverse effects. **Primary outcome chosen by emergency room staff | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization by excel generated numbers, block sizes were applied to a table of random numbers, sealed packs". Review author judgement: Adequate random sequence generation |
| Allocation concealment (selection bias) | Low risk | Quote: "Table of allocation sequence independent of block size produced to ensure correct drug was consecutively numbered and then sealed". Review author judgement: Adequate concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No evidence of participant blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No outcome blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Participants randomised were 316, and the follow‐up was explained well |
| Selective reporting (reporting bias) | Unclear risk | No evidence or information |
| Other bias | Unclear risk | None evident |
TREC‐Vellore‐I.
| Methods | Allocation: randomised. Blinding at outcome: none. Duration: 14 days. Setting: inner‐city emergency rooms of middle‐income country | |
| Participants | Diagnosis: schizophrenia (37), acute psychosis (22), mania (97), depression (19), substance misuse (10), other (15) (ICD‐10). N=200. Sex: women 81, men 119. Age: mean ˜ 31 years (SD ˜ 9). History: agitation on presentation to emergency room, markedly severely agitated or worse (171), CGI mean ˜ 5.1 (SD ˜ 0.7) | |
| Interventions | 1. Haloperidol IM: dose up to 10 mg stat + promethazine IM: dose up to 50 mg stat. N=100. 2. Lorazepam IM: dose up to 4 mg stat. N=100 | |
| Outcomes | Tranquil or asleep*.
Specific behaviours: other episodes of aggression.
Global state: use of additional medication, use of restraints/seclusion, needing extra visits from the doctor, refusing oral medication, overall improvement, average improvement (CGI‐I).
Adverse effects: serious adverse effects, extrapyramidal (BAS, Simpson‐Angus). Service outcomes: no hospital discharge. Leaving the study early |
|
| Notes | *Primary outcome chosen by emergency room staff (tranquil or asleep by 4 hours) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization computer generated numbers and block sizes" Review author judgement: Adequate random sequence generation |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization by computer generated block sizes, prepared consequently numbered cardboard boxes identical in weight and appearance". Review author judgement: Adequate concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No evidence of participant blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "After assignment, rating was not blind as the management had to know the prescription medication". Review author judgement: No outcome blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 221 participants selected and 200 randomised, but excluded were explained and follow‐up was 100% after 4 hours. Review author judgement: Good follow‐up rate |
| Selective reporting (reporting bias) | Unclear risk | No evidence or information |
| Other bias | Unclear risk | None known |
TREC‐Vellore‐II.
| Methods | Allocation: randomised. Blinding at outcome: none. Duration: 14 days. Data entry: double. Setting: psychiatric unit of medical college, emergency room, open ward | |
| Participants | Diagnosis: schizophrenia (25), mania (188), acute psychosis (30), depression (31), substance misuse (20), other (3) (ICD‐10). N=300. Sex: women 112, men 188. Age: mean ˜ 30.5 years. History: agitation on presentation to emergency room, markedly severely agitated or worse (155), CGI mean ˜ 4.6 (SD ˜ 0.7) | |
| Interventions | 1. Haloperidol IM: dose up to 10 mg stat + promethazine IM: dose up to 50 mg stat. N=150. 2. Olanzapine IM: dose up to 10 mg stat. N=150 | |
| Outcomes | Tranquil or asleep*.
Specific behaviours: other episodes of aggression.
Global state: overall improvement, use of additional medication, use of restraints/seclusion, needing extra visits from the doctor, refusing oral medication, average improvement CGI‐I.
Adverse effects: serious adverse effects, extrapyramidal (BAS, Simpson‐Angus). Service outcomes: no hospital discharge. Leaving the study early. Unable to use ‐ Tranquil or asleep: time to sedation (skewed data) |
|
| Notes | *Primary outcome chosen by emergency room staff | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomization computer generated numbers and block sizes". Review author judgement: Not clear randomisation |
| Allocation concealment (selection bias) | Low risk | Quote: "randomization by computer generated block sizes, prepared consequently numbered cardboard boxes identical in weight and appearance". Review author judgement: Adequate concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No evidence of participant blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "after assignment, rating was not blind as the management had to know the prescription medication". Review author judgement: No outcome blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 364 participants selected and 300 randomised, but excluded were explained and follow‐up was 100% at primary outcome and 92% at 2 weeks. Review author judgement: Good follow‐up rate |
| Selective reporting (reporting bias) | Unclear risk | No evidence or information |
| Other bias | Unclear risk | None known |
ACES: Agitation‐Calmness Evaluation Scale BAS: Barnes Akathisia Scale CGI‐I: Clinical Global Impression Improvement ICD‐10: International Classification of Diseases, Tenth Revision IM: intramuscular OAS: Overt Aggression Scale OASS: Overt Agitation Severity Scale PANSS‐EC: Positive and Negative Syndrome Scale ‐ Excited Component RSS: Ramsay Sedation ScaleSD: standard deviation stat: immediately
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bender 2003 | Allocation: randomised. Participants: people with schizophrenia, not specifically agitated or aggressive. Interventions: trimipramine vs perazine, not haloperidol + promethazine |
| Brannen 1969 | Allocation: randomised. Participants: people with schizophrenia, chronically ill, acutely unwell but not specifically agitated or aggressive. Interventions: trifluoperazine vs placebo, not haloperidol + promethazine |
| Claveria 1975 | Allocation: randomised. Participants: people with tardive dyskinesia, not specifically agitated or aggressive. Interventions: pimozide vs placebo, not haloperidol + promethazine |
| Graupner 1972 | Allocation: randomised. Participants: healthy people, not acutely disturbed people |
| Hou 2011 | Allocation: randomised. Participants: people with schizophrenia + agitation. Interventions: risperidone + lorazepam vs haloperidol, not involving promethazine |
| Itoh 1972 | Allocation: randomised.
Participants: people with problematic extrapyramidal symptoms, not acutely disturbed people. Interventions: piroheptine, trihexyphenidyl, and promethazine, not haloperidol + promethazine |
| Levin 1959 | Allocation: randomised. Participants: chronically ill people, not acutely disturbed people. Interventions: phenobarbital vs promethazine vs chlorpromazine vs placebo, haloperidol not involved |
| Merlo 2002 | Allocation: randomised. Participants: acutely ill people with schizophrenia, not specifically aggressive. Interventions: risperidone 2 mg vs risperidone 4 mg, not haloperidol + promethazine |
| Otsuka 1978 | Allocation: randomised. Participants: people with schizophrenia + drug‐induced parkinsonism, not acutely ill. Interventions: methixene vs trihexyphenidyl vs promethazine, not involving haloperidol |
| Perenyi 1989 | Allocation: randomised. Participants: people with schizophrenia + drug‐induced parkinsonism, not acutely ill. Interventions: procyclidine vs promethazine, not involving haloperidol |
| Srinath 2010 | Allocation: randomised.
Participants: people with acute psychotic agitation (n=60).
Interventions: injection haloperidol + promethazine vs injection lorazepam. Outcomes: tranquil or asleep, needing restraints, additional medication, absconding, adverse effects ‐ no data available. Tried to contact author via email but no reply |
| St. Jean 1964 | Allocation: randomised. Participants: people with schizophrenia + drug‐induced parkinsonism, not acutely ill. Interventions: promethazine vs placebo, not involving haloperidol |
| St. Jean 1967 | Allocation: randomised. Participants: people with "mental deficiency" not schizophrenia, not acutely ill. Interventions: periciazine vs chlorpromazine, not involving haloperidol |
| Yagi 1973 | Allocation: randomised. Participants: people with schizophrenia + drug‐induced parkinsonism, not acutely ill. Interventions: mazaticol hydrochloride vs trihexyphenidyl vs promethazine, not involving haloperidol |
| Yang 1999 | Allocation: randomised. Participants: people with schizophrenia + drug‐induced tardive dyskinesia, not acutely ill. Interventions: promethazine vs placebo, not involving haloperidol |
Characteristics of ongoing studies [ordered by study ID]
TREC‐Vellore‐III.
| Trial name or title | Rapid tranquillization of violent or agitated patients in a psychiatric setting: pragmatic, randomised, allocation concealed, participant and assessor blinded trial of intramuscular zuclopenthixol acetate versus intramuscular haloperidol plus promethazine |
| Methods | Allocation: randomised, permuted block randomisations. Blinding: participant and outcome assessor blinding. Duration: 14 days. Setting: emergency services of psychiatric department in South India |
| Participants | Diagnosis: ICD‐10 diagnosis of F10, F20‐29, F30‐39, and F60‐62 presenting agitation and aggression.
N=350. Age: adults between 18 and 65 years. History: agitation and violent behaviours requiring tranquillisation presenting in emergency |
| Interventions | Haloperidol plus promethazine IM: dose 10 mg haloperidol + dose 50 mg promethazine. N=100. Zuclopenthixol acetate IM: dose 100 mg. N=100. |
| Outcomes | Tranquil or asleep: by 30 mins, 120 mins, 24 hours, 48 hours, and 2 weeks.
Specific behaviours: other episodes of aggression over 2 weeks.
Global effects: use of additional medication, use of restraints/seclusion, needing extra visits from the doctor, refusing oral medication, CGI‐I, and absconding.
Service outcomes: hospital discharge.
Adverse effects: serious adverse effects over 48 hours, distonic and extrapyramidal symptoms.
Leaving the study early. Economic: costs of interventions |
| Starting date | 01/07/2014 |
| Contact information | Name: Dr Prathap Tharyan. Designation: Professor. Affliation: Christian Medical College. Address: Department of Psychiatry. CMC Vellore. Bagayam, Vellore 632002. Vellore. Tamil Nadu. India. Phone: 04162284520. Email: prathap@cmcvellore.ac.in |
| Notes |
CGI‐I: Clinical Global Impression Improvement ICD‐10: International Classification of Diseases, Tenth Revision
Differences between protocol and review
We consulted Clive E Adams (CEA) regarding the multiple time periods for which data are now available. We wanted to balance maximising the value of the efforts of trialists but not undermine the protocol by presenting data in such a way that promotes multiple and needless analysis. CEA, blind to data, suggested keeping to protocol for the 30‐minute outcomes and adding 1 hour as a time period, as the first 60 minutes are so important clinically. He also suggested presenting data for the longer‐term outcomes 'by > 2 to ≤ 6 hours' as one group. We recognise that this is driven by the trials and was not been pre‐stated in the original protocol. However, we think in this clinical situation the broad category has some meaning, and we did not come to this decision after assimilating the data.
For the 2015 update, we have added some data into a seventh comparison. This was just to ensure that this is the full repository of data from the relevant trial. This comparison contains some extra data at the 40‐minute stage that does not materially change any part of the review.
We have updated some methods to reflect current methodology of the Cochrane Schizophrenia Group. We moved the outcome 'specific behaviour' down the secondary outcomes list.
Contributions of authors
Gisele Huf helped write the protocol, formulate searches, select studies, extract data, format the review, and write the report.
Jacob Alexander helped write the protocol, formulate searches, select studies, extract data, format the review, and write the report.
Michael H Allen helped edit the protocol and final report.
Pinky Ghandi completed trial selection and data extraction for the 2015 update.
Sources of support
Internal sources
Universidade Federal de Rio de Janeiro, Brazil.
Christian Medical Centre, Vellore, India.
University of Colorado School of Medicine, USA.
National Institute of Quality Control in Health, Oswald Cruz Foundation, Brazil.
External sources
No sources of support supplied
Declarations of interest
GH: is an author of the included studies (TREC‐Rio‐I; TREC‐Rio‐II) see Potential biases in the review process for more information. No other conflict of interest.
JA: is an author of included studies (TREC‐Vellore‐I; TREC‐Vellore‐II) see Potential biases in the review process for more information. No other conflict of interest.
PG: no conflict of interest.
MHA: Michael was involved in the development of inhaled loxapine with Alexza and continued to work on the 'Phase 4 programme' with Ferrer in Europe. He was also involved in some advisory and educational programme development with Teva around inhaled loxapine. This might be considered an alternative to haloperidol and promethazine in the developed world.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Baldacara 2011 {published data only}
- Baldacara L, Sanches M, Cordeiro DC, Jackoswski AP. Rapid tranquilization for agitated patients in emergency psychiatric rooms: A randomized trial of olanzapine, ziprasidone, haloperidol plus promethazine, haloperidol plus midazolam and haloperidol alone. Revista Brasileira de Psiquiatria 2011;33(1):30‐9. [BIOSIS: PREV201100319442] [DOI] [PubMed] [Google Scholar]
Mantovani 2013 {published data only}
- Mantovani C, Labate CM, Sponholz AJ, Azevedo Marques JM, Guapo VG, Simone Brito dos Santos ME, et al. Are low doses of antipsychotics effective in the management of psychomotor agitation? A randomized, rated‐blind trial of 4 intramuscular interventions. Journal of Clinical Psychopharmacology 2013;33:306‐12. [DOI] [PubMed] [Google Scholar]
TREC‐Rio‐I {published data only}
- Coutinho ES, Huf G, Allen MH, Adams CE. Physical restraints for agitated patients in psychiatric emergency hospitals in Rio de Janeiro, Brazil: a predictive model. Schizophrenia Bulletin 2005;31:220. [Google Scholar]
- Huf G. Rapid safe tranquillisation for acutely disturbed people attending public psychiatric emergency clinics in Rio de Janeiro. http://www.isrctn.com/ISRCTN44153243 [accessed 7 January 2016].
- Huf G, Coutinho ESF, Adams CE. TREC I. Background. Schizophrenia Research 2002;53(3 Suppl 1):187. [Google Scholar]
- Huf G, Coutinho ESF, Adams CE. TREC III. The protocol and progress of TREC. Schizophrenia Research 2002;53(3 Suppl 1):187. [MEDLINE: ; PUBMED: 440538]440538 [Google Scholar]
- Huf G, Coutinho ESF, Adams CE. TREC‐Rio trial: a randomised controlled trial for rapid tranquillisation for agitated patients in emergency psychiatric rooms. BMC Psychiatry 2002;2:11. [EMBASE: 2002383527] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huf G, Coutinho ESF, Adams CE. The pharmacological management of agitated patients in emergency psychiatric hospitals in Rio de Janeiro, Brazil: the results of two pragmatic randomized clinical trials. 5th European Congress on Violence in Clinical Psychiatry; 25‐27 October 2007; Amsterdam, the Netherlands. 2007.
- Huf G, Coutinho ESF, Fagundes Jr HM, Carvalho AL, Ramos FA, Keusen AL, et al. TREC II. Current practices in managing acutely disturbed patients at three hospitals in Rio de Janeiro, Brazil. Schizophrenia Research 2002;53(3):236‐7. [MEDLINE: ; PUBMED: 4405388] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migon MN, Coutinho ES, Huf G, Adams CE, Cunha GM, Allen MH. Factors associated with the use of physical restraints for agitated patients in psychiatric emergency rooms. General Hospital Psychiatry 2008;30(3):263‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- TREC Collaborative Group. Rapid tranquillisation for agitated patients in emergency psychiatric rooms: a randomised trial of midazolam versus haloperidol plus promethazine. BMJ 2003;327(7417):708‐13. [EMBASE: 2003210869] [DOI] [PMC free article] [PubMed] [Google Scholar]
TREC‐Rio‐II {published data only}
- Barbui C. Intramuscular haloperidol plus promethazine is more effective and safer than haloperidol alone for rapid tranquillisation of agitated mentally ill patients. Evidence‐Based Mental Health 2008;11(3):86‐7. [DOI] [PubMed] [Google Scholar]
- Huf G. Haloperidol plus promethazine versus haloperidol for psychosis induced aggression. Unpublished protocol 2004.
- Huf G, Coutinho ESF, Adams CE. The pharmacological management of agitated patients in emergency psychiatric hospitals in Rio de Janeiro, Brazil: the results of two pragmatic randomized clinical trials. 5th European Congress on Violence in Clinical Psychiatry; 25‐27 October 2007; Amsterdam, the Netherlands. 2007.
- Huf G, Coutinho ESF, Adams CE, TREC Collaborative Group. Rapid tranquillisation in psychiatric emergency settings in Brazil: pragmatic randomised controlled trial of intramuscular haliperidol versus intramuscular haloperidol plus promethazine. BMJ 2007;335(7625):869‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISRCTN83261243. TREC2 ‐ Rapid tranquillisation for agitated patients in emergency psychiatric rooms in Rio de Janeiro. A randomised trial of intramuscular Haloperidol versus intramuscular Haloperidol + Promethazine. http://www.isrctn.com/ISRCTN83261243 [accessed 7 January 2016].
TREC‐Vellore‐I {published data only}
- Alexander J. Lorazepam Versus a Combination of Haloperidol and Promethazine in the Acute Management of Agitation and Aggression ‐ a Randomized Controlled Trial [MD Thesis]. Vellore, India: Christian Medical College, 2003. [Google Scholar]
- Alexander J, John T, Tharyan P, Adams CE. TREC‐India. A second arm of TREC. Schizophrenia Research 2002;53(3 Suppl 1):236. [MEDLINE: ; PUBMED: 4405388]4405388 [Google Scholar]
- Alexander J, Tharyan P, Adams CE, John T, Mol C, Philip J. Rapid tranquilisation of violent or agitated patients in a psychiatric emergancy setting: a pragmatic randomised trial of intramuscular lorazepam versus haloperidol plus promethazine. British Journal of Psychiatry 2004;185:63‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Tharyan P. A randomised controlled trial of intra‐muscular lorazepam versus intra‐muscular haloperidol+promethazine in the management of psychotic agitations and aggression. http://www.isrctn.com/ISRCTN29863938 [accessed 7 January 2016].
TREC‐Vellore‐II {published data only}
- Barbui C. Intramuscular haloperidol plus promethazine is more effective and safer than haloperidol alone for rapid tranquillisation of agitated mentally ill patients. Evidence‐Based Mental Health 2008;11(3):86‐7. [DOI] [PubMed] [Google Scholar]
- NCT00455234. Rapid tranquilization of violent or agitated people in psychiatric emergency settings ‐ a pragmatic randomized controlled trial of intramuscular olanzapine vs intramuscular haloperidol + promethazine. https://www.clinicaltrials.gov/ct/show/NCT00455234 [accessed 7 January 2016].
- Nirmal SR. Rapid tranquillization of acutely agitated patients: intramuscular olanzapine vs haloperidol + promethazine ‐ pragmatic randomized control trial. 5th European Congress on Violence in Clinical Psychiatry; 25‐27 October 2007; Amsterdam, the Netherlands. 2007.
- Raveendran NS, Tharyan P, Alexander J, Adams CE, Trec‐India II Collaborative Group. Rapid tranquillisation in psychiatirc emergency settings in India: a pragmatic randomised controlled trial of intramuscular olanzapine versus intramuscular haloperidol plus promethazine. BMJ 2007;335(7625):865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tharyan P, Alexander J. Haloperidol plus promethazine versus IM olanzapine. Data on file 2004.
- Tharyan P, Alexander J. Haloperidol plus promethazine versus IM olanzapine. Unpublished protocol 2004.
References to studies excluded from this review
Bender 2003 {published data only}
- Bender S, Olbrich HM, Fischer W, Hornstein C, Schoene W, Falkai P, et al. Antipsychotic efficacy of the antidepressant trimipramine: A randomized, double‐blind comparison with the phenothiazine perazine. Pharmacopsychiatry 2003;36(2):61‐9. [EMBASE: 2003210869] [DOI] [PubMed] [Google Scholar]
Brannen 1969 {published data only}
- Brannen JO, Jewett RE. Effects of selected phenothiazines on REM sleep in schizophrenics. Archives of General Psychiatry 1969;21(3):284‐90. [MEDLINE: ; PUBMED: 4308796] [DOI] [PubMed] [Google Scholar]
Claveria 1975 {published data only}
- Claveria LE, Teychenne PF, Calne DB, Haskayne L, Petrie A, Pallis CA, et al. Tardive dyskinesia treated with pimozide. Journal of the Neurological Sciences 1975;24(4):393‐401. [MEDLINE: ; PUBMED: 235013] [DOI] [PubMed] [Google Scholar]
Graupner 1972 {published data only}
- Graupner OK, Kalman EV. Effects of various drugs on flicker fusion frequency. II. Phenothiazine derivatives (with reference to the dependence of the results on the chronological course of experiments [Die Flimmer‐Verschmelzung‐Frequenz unter dem Einfluss verschiedener Pharmaka. II. Phenothiazinderivate (mit einem Hinweis auf die Abhangigkeit der Ergebnisse vom zeitlichen Verlauf der Versuche)]. Psychopharmacologia 1972;27(4):343‐7. [MEDLINE: ; PUBMED: 4405388] [DOI] [PubMed] [Google Scholar]
Hou 2011 {published data only}
- 侯春兰, 侯凌峰, 张东升, 董继雪. Risperidone oral solution with lorazepam treatment of schizophrenia excited state [利培酮口服液合并劳拉西泮治疗精神分裂症兴奋状态]. China Journal of Health Psychology [中国健康心理学杂志] 2011;19(07):787‐9. [Google Scholar]
Itoh 1972 {published data only}
- Itoh H, Miura S, Takesada M, Tanaka N, Tsuji E, Yagi G. Comparison of three kinds of antiparkinsonian agents (piroheptine=fk‐1190, trihexyphenidyl and promethazine) in drug‐induced extrapyramidal symptoms using double‐blind technique. Rinsho Hyoka (Clinical Evaluation) 1972;1(1):113‐36. [CENTRAL: CN‐00266949] [Google Scholar]
Levin 1959 {published data only}
- Levin ML. A comparison of the effects of phenobarbital, promethazine, chlorpromazine, and placebo upon mental hospital patients. Journal of Consulting Psychology 1959;23:167‐70. [MEDLINE: ; PUBMED: 235013] [DOI] [PubMed] [Google Scholar]
Merlo 2002 {published data only}
- Merlo MCG, Hofer H, Gekle W, Berger G, Ventura J, Panhuber I, et al. Risperidone, 2 mg/day vs. 4 mg/day, in first‐episode, acutely psychotic patients: Treatment efficacy and effects on fine motor functioning. Journal of Clinical Psychiatry 2002;63(10):885‐91. [EMBASE: 2002383527] [DOI] [PubMed] [Google Scholar]
Otsuka 1978 {published data only}
- Otsuka N, Yagi G, Kobayashi T, Sakurai S, Takeda A, Tashiro I, et al. Comparison of clinical efficacy of methixene, trihexyphenidyl and promethazine in drug‐induced parkinsonism using double blind cross‐over technique. Clinical Evaluation 1978;6:223‐72. [CENTRAL: CN‐00266949] [Google Scholar]
Perenyi 1989 {published data only}
- Perenyi A, Goswami U, Frecska E, Majlath E, Barcs G, Kassay Farkas A. A pilot study of the role of prophylactic antiparkinson treatment during neuroleptic therapy. Pharmacopsychiatry 1989;22(3):108‐10. [MEDLINE: ; PUBMED: 2568643] [DOI] [PubMed] [Google Scholar]
Srinath 2010 {published data only}
- Srinath G, Shailaja B, Sai PG, Reddy KA. A comparative study of injection haloperidol and promethazine vs. injection lorazepam in controlling acute psychotic agitation. Indian Journal of Psychiatry 2010;52:S21. [Google Scholar]
St. Jean 1964 {published data only}
- St. Jean A, Donald M, Ban TA. Interchangeability of antiparkinsonian medication. American Journal of Psychiatry 1964;120:1189‐90. [MEDLINE: ; PUBMED: 235013] [DOI] [PubMed] [Google Scholar]
St. Jean 1967 {published data only}
- St. Jean A, Sterlin C, Noe W, Ban TA. Clinical studies with propericiazine (RP. 8909). Diseases of the Nervous System 1967;28(8):526‐31. [MEDLINE: ; PUBMED: 4383004] [PubMed] [Google Scholar]
Yagi 1973 {published data only}
- Yagi G, Yanai N, Tokisawa T. Comparison of clinical efficacy of mazaticol hydrochloride, trihexyphenidyl and promethazine for drug‐induced parkinsonism: comparison by the double‐blind, cross‐over method. Seishin Igaku 1973;2(11):1311‐38. [CENTRAL: CN‐00266949] [Google Scholar]
Yang 1999 {published data only}
- Yang X, Meng F, Cui Y, Yang P, Liu Ro, Ma L, et al. Promethazine treatment of tardive dyskinesia: a double blind placebo controlled study. Chinese Mental Health Journal 1999;13(6):365‐7. [PsycINFO: 1999‐15232‐015] [Google Scholar]
References to ongoing studies
TREC‐Vellore‐III {unpublished data only}
- Mythri SV, Tharyan P, Sunder S, Kattula D, Kirubakaran R, Adams CE. CTRI‐2014‐07‐004712: Rapid tranquillization of violent or agitated patients in a psychiatric emergency setting: Pragmatic, randomized, allocation concealed, participant and assessor blinded trial of intramuscular zuclopenthixol acetate versus intramuscular haloperidol plus promethazine. http://apps.who.int/trialsearch/Trial.aspx?TrialID=CTRI/2014/07/004712 [accessed 7 January 2016].
- Mythri SV, Tharyan P, Sunder S, Kattula D, Kirubakaran R, Adams CE. Rapid tranquillization of violent or agitated patients in a psychiatric emergency setting: Pragmatic, randomized, allocation concealed, participant and assessor blinded trial of intramuscular zuclopenthixol acetate versus intramuscular haloperidol plus promethazine ‐ a comparative study of the effects of an intramuscular injection of zuclopenthixol acetate versus an intramuscular injection of a combination of haloperidol plus promethazine in people with violence or agitation presenting to a psychiatric hospital as an emergency. Study Protocol 2013.
Additional references
Adams 2013
- Adams CE, Bergman H, Irving CB, Lawrie S. Haloperidol versus placebo for schizophrenia. Cochrane Database of Systematic Reviews 2013, Issue 11. [DOI: 10.1002/14651858.CD003082.pub3; PUBMED: 24242360] [DOI] [PubMed] [Google Scholar]
Adams 2014
- Adams CE, Awad GA, Rathbone J, Thornley B, Soares‐Weiser K. Chlorpromazine versus placebo for schizophrenia. Cochrane Database of Systematic Reviews 2014, Issue 1. [DOI: 10.1002/14651858.CD000284.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ahmed 2010
- Ahmed U, Jones H, Adams CE. Chlorpromazine for psychosis‐induced aggression or agitation. Cochrane Database of Systematic Reviews 2010, Issue 4. [DOI: 10.1002/14651858.CD007445.pub2; PUBMED: 20393959] [DOI] [PubMed] [Google Scholar]
Ahmed 2011
- Ahmed U, Rehman F, Jones H, Adams CE. Risperidone for psychosis induced aggression or agitation. Cochrane Database of Systematic Reviews 2011, Issue 11. [DOI: 10.1002/14651858.CD009412] [DOI] [PMC free article] [PubMed] [Google Scholar]
Alexander 2003
- Alexander J. Lorazepam Versus a Combination of Haloperidol and Promethazine in the Acute Management of Agitation and Aggression ‐ a Randomized Controlled Trial [Thesis submitted for the Degree of MD Psychological Medicine]. Guindy, Chennai, Tamilnadu, India: MGR Medical University, 2003. [Google Scholar]
Altman 1996
- Altman DG, Bland JM. Detecting skewness from summary information. BMJ 1996;313(7066):1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ayd 1972
- Ayd FJ. Haloperidol: fifteen years of clinical experience. Diseases of the Nervous System 1972;33:459‐69. [PubMed] [Google Scholar]
Ayd 1978
- Ayd FJ. Haloperidol: twenty years' clinical experience. Journal of Clinical Psychiatry 1978;39:807‐14. [PubMed] [Google Scholar]
Barnes 1989
- Barnes TRE. A rating scale for drug‐induced akathisia. British Journal of Psychiatry 1989;154:672‐6. [DOI] [PubMed] [Google Scholar]
Belgamwar 2005
- Belgamwar RB, Fenton M. Olanzapine IM or velotab for acutely disturbed/agitated people with suspected serious mental illnesses. Cochrane Database of Systematic Reviews 2005, Issue 2. [DOI: 10.1002/14651858.CD003729.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Berk 2004
- Berk M, Rathbone J, Mandriota‐Carpenter SL. Clotiapine for acute psychotic illnesses. Cochrane Database of Systematic Reviews 2004, Issue 4. [DOI: 10.1002/14651858.CD002304.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Binder 1999
- Binder RL, McNiel DE. Emergency psychiatry: contemporary practices in managing acutely violent patients in 20 psychiatric emergency rooms. Psychiatric Services 1999;50:1553‐4. [DOI] [PubMed] [Google Scholar]
Bland 1997
- Bland JM. Statistics notes. Trials randomised in clusters. BMJ 1997;315:600. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boissel 1999
- Boissel JP, Cucherat M, Li W, Chatellier G, Gueyffier F, Buyse M, et al. The problem of therapeutic efficacy indices. 3. Comparison of the indices and their use [Apercu sur la problematique des indices d'efficacite therapeutique, 3: comparaison des indices et utilisation. Groupe d'Etude des Indices D'efficacite]. Therapie 1999;54(4):405‐11. [PUBMED: 10667106] [PubMed] [Google Scholar]
Breier 2002
- Breier A, Meehan K, Birkett M, David S, Ferchland I, Sutton V, et al. A double‐blind, placebo‐controlled dose‐response comparison of intramuscular olanzapine and haloperidol in the treatment of acute agitation in schizophrenia. Archives of General Psychiatry 2002;59(5):441‐8. [DOI] [PubMed] [Google Scholar]
Chakrabarti 2007
- Chakrabarti A, Whicher EV, Morrison M, Douglas‐Hall P. 'As required' medication regimens for seriously mentally ill people in hospital. Cochrane Database of Systematic Reviews 2007, Issue 3. [DOI: 10.1002/14651858.CD003441.pub2] [DOI] [PubMed] [Google Scholar]
Cunnane 1994
- Cunnane JG. Drug management of disturbed behaviour by psychiatrists. Psychiatric Bulletin 1994;18:138‐9. [Google Scholar]
Deeks 2000
- Deeks J. Issues in the selection for meta‐analyses of binary data. Proceedings of the 8th International Cochrane Colloquium. 25‐28 Oct 2000 Cape Town, South Africa. Cape Town: The Cochrane Collaboration.
Divine 1992
- Divine GW, Brown JT, Frazer LM. The unit of analysis error in studies about physicians' patient care behavior. Journal of General Internal Medicine 1992;7(6):623‐9. [DOI] [PubMed] [Google Scholar]
Donner 2002
- Donner A, Klar N. Issues in the meta‐analysis of cluster randomized trials. Statistics in Medicine 2002;21:2971‐80. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey‐Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315:629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne D, Altman DG, Higgins JPT, Curtina F, Worthingtond HV, Vaile A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. [DOI] [PubMed] [Google Scholar]
Essali 2013
- Essali A, Rihawi A, Altujjar M, Alhafez B, Tarboush A, Alhaj HN. Anticholinergic medication for non‐clozapine neuroleptic‐induced hypersalivation in people with schizophrenia. Cochrane Database of Systematic Reviews 2013, Issue 12. [DOI: 10.1002/14651858.CD009546.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Expert 1999
- Expert Consensus Guideline Group. Treatment of schizophrenia 1999. The expert consensus guideline series. Journal of Clinical Psychiatry 1999;60(Suppl 11):3‐80. [PubMed] [Google Scholar]
Furukawa 2006
- Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N. Imputing missing standard deviations in meta‐analyses can provide accurate results. Journal of Clinical Epidemiology 2006;59(7):7‐10. [DOI] [PubMed] [Google Scholar]
Gibson 2004
- Gibson RC, Fenton M, Coutinho ES, Campbell C. Zuclopenthixol acetate for acute schizophrenia and similar serious mental illnesses. Cochrane Database of Systematic Reviews 2004, Issue 3. [DOI: 10.1002/14651858.CD000525.pub2] [DOI] [PubMed] [Google Scholar]
Gillies 2005
- Gillies D, Beck A, McCloud A, Rathbone J. Benzodiazepines for psychosis‐induced aggression or agitation. Cochrane Database of Systematic Reviews 2005, Issue 4. [DOI: 10.1002/14651858.CD003079.pub2] [DOI] [PubMed] [Google Scholar]
Gillies 2013
- Gillies D, Sampson S, Beck A, Rathbone J. Benzodiazepines for psychosis‐induced aggression or agitation. Cochrane Database of Systematic Reviews 2013, Issue 4. [DOI: 10.1002/14651858.CD003079.pub3] [DOI] [PubMed] [Google Scholar]
Gulliford 1999
- Gulliford MC, Ukoumunne OC, Chinn S. Components of variance and intraclass correlations for the design of community‐based surveys and intervention studies: data from the Health Survey for England 1994. American Journal of Epidemiology 1999;149:876‐83. [DOI] [PubMed] [Google Scholar]
Guy 1976
- Guy W. Clinical Global Impressions. ECDEU Assessment Manual for Psychopharmacology. Rockville, MD: Department of Health, Education and Welfare, 1976:76‐338. [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2005
- Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Library. Chichester, UK: John Wiley & Sons, Ltd, 2005. [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Huf 2002a
- Huf G, Silva Freire Coutinho E, Fagundes Jr HM, Oliveira ES, Lopez JRRA, Gewandszajder M, et al. Current practices in managing acutely disturbed patients at three hospitals in Rio de Janeiro‐Brazil: a prevalence study. BMC Psychiatry 2002;2:4. [http://www.biomedcentral.com/1471‐244X/2/4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Huf 2002b
- Huf G, Coutinho ESF, Adams CE. TREC‐Rio trial: a randomised controlled trial for rapid tranquillisation for agitated patients in emergency psychiatric rooms. BMC Psychiatry 2002;2:11. [EMBASE: 2002383527] [DOI] [PMC free article] [PubMed] [Google Scholar]
Huf 2011
- Huf G, Coutinho ES, Ferreira MA, Ferreira S, Mello F, Adams CE. TREC‐SAVE: a randomised trial comparing mechanical restraints with use of seclusion for aggressive or violent seriously mentally ill people: study protocol for a randomised controlled trial. Trials 2011;12:180. [PUBMED: 21774823] [DOI] [PMC free article] [PubMed] [Google Scholar]
Huf 2012
- Huf G, Coutinho ES, Adams CE. Physical restraints versus seclusion room for management of people with acute aggression or agitation due to psychotic illness (TREC‐SAVE): a randomized trial. Psychological Medicine 2012;42(11):2265‐73. [PUBMED: 22405443] [DOI] [PubMed] [Google Scholar]
Jablensky 1992
- Jablensky A, Sartorius N, Ernberg G, Anker M, Korten A, Cooper J, et al. Schizophrenia: manifestations, incidence and course in different cultures. A World Health Organization ten‐country study. Psychological Medicine 1992;Monograph Supplement:1‐97. [DOI] [PubMed] [Google Scholar]
Juni 2001
- Juni P, Altman DG, Egger M. Assessing the quality of controlled clinical trials. BMJ 2001;323:42‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kaplan 1994
- Kaplan HI, Sadock BJ, Grebb JA. Kaplan and Sadock's Synopsis of Psychiatry. Baltimore, USA: Williams & Wilkins, 1994. [Google Scholar]
Kay 1986
- Kay SR, Opler LA, Fiszbein A. Positive and Negative Syndrome Scale (PANSS) Manual. North Tonawanda, NY: Multi‐Health Systems, 1986. [Google Scholar]
Khushu 2012
- Khushu A, Powney MJ, Adams CE. Haloperidol for long term aggression in psychosis. Cochrane Database of Systematic Reviews 2012, Issue 5. [DOI: 10.1002/14651858.CD009830] [DOI] [PMC free article] [PubMed] [Google Scholar]
Koch 2014
- Koch K, Mansi K, Haynes E, Adams CE, Sampson S, Furtado VA. Trifluoperazine versus placebo for schizophrenia. Cochrane Database of Systematic Reviews 2014, Issue 1. [DOI: 10.1002/14651858.CD010226.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Leon 2006
- Leon AC, Mallinckrodt CH, Chuang‐Stein C, Archibald DG, Archer GE, Chartier K. Attrition in randomized controlled clinical trials: methodological issues in psychopharmacology. Biological Psychiatry 2006;59(11):1001‐5. [PUBMED: 16905632] [DOI] [PubMed] [Google Scholar]
Leucht 2005
- Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel RR. What does the PANSS mean?. Schizophrenia Research 2005;79(2‐3):231‐8. [PUBMED: 15982856] [DOI] [PubMed] [Google Scholar]
Leucht 2005a
- Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel R. Clinical implications of brief psychiatric rating scale scores. British Journal of Psychiatry 2005;187:366‐71. [PUBMED: 16199797] [DOI] [PubMed] [Google Scholar]
Li 2009
- Li C, Xia J, Wang J. Risperidone dose for schizophrenia. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD007474.pub2] [DOI] [PubMed] [Google Scholar]
Marshall 2000
- Marshall M, Lockwood A, Bradley C, Adams CE, Joy C, Fenton M. Unpublished rating scales: a major source of bias in randomised controlled trials of treatments for schizophrenia. British Journal of Psychiatry 2000;176:249‐52. [DOI] [PubMed] [Google Scholar]
McAllister 2002
- McAllister‐Williams RH, Ferrier IN. Rapid tranquillisation: time for a reappraisal of options for parenteral therapy. British Journal of Psychiatry 2002;180:485‐9. [DOI] [PubMed] [Google Scholar]
Montoya 2011
- Montoya A, Valladares A, Lizán L, San L, Escobar R, Paz S. Validation of the Excited Component of the Positive and Negative Syndrome Scale (PANSS‐EC) in a naturalistic sample of 278 patients with acute psychosis and agitation in a psychiatric emergency room. Health and Quality of Life Outcomes 2011;9:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moritz 1999
- Moritz F, Bauer F, Boyer A, Lemarchand P, Kerleau JM, Moirot E, et al. Patients in a state of agitation at the admission service of a Rouen hospital emergency department. Presse Medicale 1999;28:1630‐4. [PubMed] [Google Scholar]
Mothi 2013
- Mothi M, Sampson S. Pimozide for schizophrenia or related psychoses. Cochrane Database of Systematic Reviews 2013, Issue 11. [DOI: 10.1002/14651858.CD001949.pub3] [DOI] [PubMed] [Google Scholar]
Muralidharan 2006
- Muralidharan S, Fenton M. Containment strategies for people with serious mental illness. Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858.CD002084.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
NICE 2004
- National Institute for Clinical Evidence (NICE). Disturbed (violent) behaviour: the short‐term management of disturbed (violent) behaviour in in‐patient psychiatric settings and emergency departments. Clinical Guideline 25. www.nice.org.uk/pdf/cg025niceguideline.pdf [accessed 2005].
NICE 2015
- National Collaborating Centre for Mental Health (UK). Violence and aggression: short‐term management in mental health, health and community settings: updated edition. National Institute for Health and Clinical Excellence: Guidance 2015. [PUBMED: 26180871] [PubMed]
Overall 1962
- Overall JE, Gorham DR. The brief psychiatric rating scale. Psychological Reports 1962;10:799‐812. [Google Scholar]
Pilowsky 1992
- Pilowsky LS, Ring H, Shine PJ, Battersby M, Lader M. Rapid tranquillisation. A survey of emergency prescribing in a general psychiatric hospital. British Journal of Psychiatry 1992;160:831‐5. [DOI] [PubMed] [Google Scholar]
Powney 2012
- Powney MJ, Adams CE, Jones H. Haloperidol for psychosis‐induced aggression or agitation (rapid tranquillisation). Cochrane Database of Systematic Reviews 2012, Issue 11. [DOI: 10.1002/14651858.CD009377.pub2; PUBMED: 23152276] [DOI] [PubMed] [Google Scholar]
Ramsay 1974
- Ramsay MA, Savege TM, Simpson BR, Goodwin R. Controlled sedation with alphaxalone‐alphadolone. British Medical Journal 1974;2:656‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rao 2012
- Rao H, Yeung W‐L, Jayaram MB. De‐escalation techniques for psychosis‐induced aggression or agitation. Cochrane Database of Systematic Reviews 2012, Issue 7. [DOI: 10.1002/14651858.CD009922] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rathbone 2004
- Rathbone J, Mandriota‐Carpenter SL, Cure SJ. Droperidol for acute psychosis. Cochrane Database of Systematic Reviews 2004, Issue 1. [DOI: 10.1002/14651858.CD002830.pub2] [DOI] [PubMed] [Google Scholar]
RCPsych 1998
- The Royal College of Psychiatrists. Management of Imminent Violence: Clinical Practice Guidelines to Support Mental Health services. London: Gaskill, 1998. [Google Scholar]
Sailas 2000
- Sailas E, Fenton M. Seclusion and restraint for people with serious mental illnesses. Cochrane Database of Systematic Reviews 2000, Issue 2. [DOI: 10.1002/14651858.CD001163] [DOI] [PMC free article] [PubMed] [Google Scholar]
Simpson 1970
- Simpson GM, Angus JW. A rating scale for extrapyramidal side effects. Acta Psychiatrica Scandinavica 1970;212 (Suppl 44):11‐9. [DOI] [PubMed] [Google Scholar]
Thorpe 2009
- Thorpe KE, Zwarenstein M, Oxman AD, Treweek S, Furberg CD, Altman DG, et al. A pragmatic‐explanatory continuum indicator summary (PRECIS): a tool to help trial designers. Journal of Clinical Epidemiology 2009;62(5):464‐75. [PUBMED: 19348971] [DOI] [PubMed] [Google Scholar]
Toal 2012
- Toal F, Roberts K. Clozapine for people with schizophrenia and recurrent physical aggression. Cochrane Database of Systematic Reviews 2012.
Tosh 2011
- Tosh G, Soares‐Weiser K, Adams CE. Pragmatic vs explanatory trials: the pragmascope tool to help measure differences in protocols of mental health randomized controlled trials. Dialogues in Clinical Neuroscience 2011;13(2):209‐15. [PUBMED: 21842618] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ukoumunne 1999
- Ukoumunne OC, Gulliford MC, Chinn S, Sterne JAC, Burney PGJ. Methods for evaluating area‐wide and organisation‐based interventions in health and health care: a systematic review. Health Technology Assessment 1999;3(5):iii‐92. [MEDLINE: ] [PubMed] [Google Scholar]
Vangala 2012
- Vangala R, Ahmed U, Ahmed R. Loxapine inhaler for psychosis‐induced aggression. Cochrane Database of Systematic Reviews 2012, Issue 11. [DOI: 10.1002/14651858.CD010190] [DOI] [Google Scholar]
WHO 2002
- WHO Expert Committee. World Health Organization/EDM/PAR/Procedures for Updating the WHO Model List of Essential Drugs. www.who.int/medicines/organization/par/edl/infedlmain.shtml [accessed 3 April 2002].
Wilkie 2012
- Wilkie F, Fenton M. Quetiapine for psychosis‐induced aggression or agitation. Cochrane Database of Systematic Reviews 2012, Issue 4. [DOI: 10.1002/14651858.CD009801] [DOI] [Google Scholar]
Xia 2009
- Xia J, Adams CE, Bhagat N, Bhagat V, Bhoopathi P, El‐Sayeh H, et al. Loss to outcomes stakeholder survey: the LOSS study. Psychiatric Bulletin 2009;33(7):254‐7. [Google Scholar]
Yudofsky 1986
- Yudofsky SC, Silver JM, Jackson W, Endicott J, Williams D. The Overt Aggression Scale for the objective rating of verbal and physical aggression. American Journal of Psychiatry 1986;143:35‐9. [DOI] [PubMed] [Google Scholar]
Yudofsky 1997
- Yudofsky SC, Kopecky HJ, Kunik M, Silver JM, Endicott J. The Overt Agitation Severity Scale for the objective rating of agitation. Journal of Neuropsychiatry 1997;9(4):541‐8. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Huf 2004
- Huf G, Alexander J, Allen MH. Haloperidol plus promethazine for psychosis induced aggression. Cochrane Database of Systematic Reviews 2004, Issue 4. [DOI: 10.1002/14651858.CD005146] [DOI] [PubMed] [Google Scholar]
Huf 2005
- Huf G, Alexander J, Allen MH. Haloperidol plus promethazine for psychosis induced aggression. Cochrane Database of Systematic Reviews 2005, Issue 1. [DOI: 10.1002/14651858.CD005146; PUBMED: 15654706] [DOI] [PubMed] [Google Scholar]
Huf 2009
- Huf G, Alexander J, Allen MH, Raveendran NS. Haloperidol plus promethazine for psychosis‐induced aggression. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD005146.pub2; PUBMED: 19588366] [DOI] [PubMed] [Google Scholar]
Huf 2009a
- Huf G, Coutinho ES, Adams CE. Haloperidol plus promethazine for agitated patients ‐ a systematic review [Haloperidol mais prometazina para pacientes agitados ‐ uma revisao sistematica]. Revista Brasileira de Psiquiatria 2009;31(3):265‐70. [PUBMED: 19784494] [DOI] [PubMed] [Google Scholar]


