Abstract
Background
Breast milk provides optimal nutrition for term and preterm infants, and the ideal way for infants to receive breast milk is through suckling at the breast. Unfortunately, this may not always be possible for medical or physiological reasons such as being born sick or preterm and as a result requiring supplemental feeding. Currently, there are various ways in which infants can receive supplemental feeds. Traditionally in neonatal and maternity units, bottles and nasogastric tubes have been used; however, cup feeding is becoming increasingly popular as a means of offering supplemental feeds in an attempt to improve breastfeeding rates. There is no consistency to guide the choice of method for supplemental feeding.
Objectives
To determine the effects of cup feeding versus other forms of supplemental enteral feeding on weight gain and achievement of successful breastfeeding in term and preterm infants who are unable to fully breastfeed.
Search methods
We used the standard search strategy of the Cochrane Neonatal Review group to search the Cochrane Central Register of Controlled Trials (CENTRAL 2016, Issue 1), MEDLINE via PubMed (1966 to 31 January 2016), Embase (1980 to 31 January 2016), and CINAHL (1982 to 31 January 2016). We also searched clinical trials' databases, conference proceedings, and the reference lists of retrieved articles for randomised controlled trials and quasi‐randomised trials.
Selection criteria
Randomised or quasi‐randomised controlled trials comparing cup feeding to other forms of enteral feeding for the supplementation of term and preterm infants.
Data collection and analysis
Data collection and analysis was performed in accordance with the methods of Cochrane Neonatal. We used the GRADE approach to assess the quality of evidence.
The review authors independently conducted quality assessments and data extraction for included trials. Outcomes reported from these studies were: weight gain; proportion not breastfeeding at hospital discharge; proportion not feeding at three months of age; proportion not feeding at six months of age; proportion not fully feeding at hospital discharge; proportion not fully breastfeeding at three months of age; proportion not fully breastfeeding at six months of age; average time per feed (minutes); length of stay; and physiological events of instability such as bradycardia, apnoea, and low oxygen saturation. For continuous variables such as weight gain, mean differences and 95% confidence intervals (CIs) were reported. For categorical outcomes such as mortality, the relative risks (RR) and 95% CIs were reported.
Main results
The experimental intervention was cup feeding and the control intervention was bottle feeding in all five studies included in this review. One study reported weight gain as g/kg/day and there was no statistically significant difference between the two groups (MD −0.60, 95% CI −3.21 to 2.01); while a second study reported weight gain in the first seven days as grams/day and also showed no statistically significant difference between the two groups (MD −0.10, 95% CI −0.36 to 0.16). There was substantial variation in results for the majority of breastfeeding outcomes, except for not breastfeeding at three months (three studies) (typical RR 0.83, 95% CI 0.71 to 0.97) which favoured cup feeding. Where there was moderate heterogeneity meta‐analysis was performed: not breastfeeding at six months (two studies) (typical RR 0.83, 95% CI 0.72 to 0.95); not fully breastfeeding at hospital discharge (four studies) (typical RR 0.61, 95% CI 0.52 to 0.71).
Two studies reported average time to feed which showed no difference between the two groups. Two studies assessed length of hospital stay and there was considerable variation in results and in the direction of effect. Only one study has reported gestational age at discharge, which showed no difference between the two groups (MD −0.10, 95% CI −0.54 to 0.34).
Authors' conclusions
As the majority of infants in the included studies are preterm infants, no recommendations can be made for cup feeding term infants due to the lack of evidence in this population.
From the studies of preterm infants, cup feeding may have some benefits for late preterm infants and on breastfeeding rates up to six months of age. Self‐reported breastfeeding status and compliance to supplemental interventions may over‐report exclusivity and compliance, as societal expectations of breastfeeding and not wishing to disappoint healthcare professionals may influence responses at interview and on questionnaires.
The results for length of stay are mixed, with the study involving lower gestational age preterm infants finding that those fed by cup spent approximately 10 days longer in hospital, whereas the study involving preterm infants at a higher gestational age, who did not commence cup feeding until 35 weeks' gestation, did not have a longer length of stay, with both groups staying on average 26 days. This finding may have been influenced by gestational age at birth and gestational age at commencement of cup feeding, and their mothers' visits; (a large number of mothers of these late preterm infants lived regionally from the hospital and could visit at least twice per week).
Compliance to the intervention of cup feeding remains a challenge. The two largest studies have both reported non‐compliance, with one study analysing data by intention to treat and the other excluding those infants from the analysis. This may have influenced the findings of the trial. Non‐compliance issues need consideration before further large randomised controlled trials are undertaken as this influences power of the study and therefore the statistical results. In addition larger studies with better‐quality (especially blinded) outcome assessment with 100% follow‐up are needed.
Plain language summary
Cup feeding versus other forms of supplemental enteral feeding for newborn infants unable to fully breastfeed
Review question:
For both term and preterm infants we wanted to identify the best method for offering supplemental feeds and asked if cup feeding is a better way to supplementally feed rather than bottle feeding or feeding with a tube, when newborn infants are unable to fully breastfeed.
Background:
Most infants born at term or slightly preterm can fully breastfeed following birth. However for a number of reasons some term newborns and many preterm newborns may not be able to fully breastfeed and require supplemental feeding by alternative methods, such as a cup, syringe, bottle or feeding tube, until they are able to fully breastfeed.
Study characteristics:
Our search for eligible studies conducted on 31 January 2016 revealed five studies, all comparing cup and bottle feeding in newborn infants, which we were able to include in this review. These studies were conducted in neonatal and maternity units in hospitals in Australia, the United Kingdom, Brazil and Turkey. The mean gestational age of the infants in most of the studies were similar at the time of entry into the study. In four of the studies the intervention (cup or bottle) commenced from the time of enrolment into the study, when the infants first needed a supplemental feed and were as young as 30 weeks' gestation. In the study conducted in Turkey, supplemental feeding was not commenced on enrolment into the study and at the time of first supplemental feed but delayed until infants were at least 35 weeks of age.
Key results:
For some of the outcomes, the results of the different studies could not be combined. This included not breastfeeding at hospital discharge; not exclusively breastfeeding at three months and at six months; the average time taken for a feed; and the number of days spent in hospital. For each of these outcomes, the results from some studies favoured cup feeding, while the results from other studies favoured bottle feeding.
For some of the outcomes, the results of the different studies could be combined: there was no difference in weight gain or gestational age at discharge between those infants who recieved supplemental feeds by cup compared to bottle. However those infants who received supplemental feeds by cup were more likely to be exclusively breastfeeding at hospital discharge and were more likely to be receiving some breastfeeds at three and six months of age.
As the studies mostly included preterm infants and few term infants, no recommendations can be made for cup feeding term infants.
Quality of evidence:
The quality of evidence for weight gain, length of stay, not breastfeeding at hospital discharge and at six months of age and exclusively breastfeeding at hospital discharge and at six months of age is graded very low to low. In the studies included in this review, it is reported that many infants who were to receive supplemental feeds by cup received supplemental feeds by other means as either the parents or nurses did not like cup feeding.
Summary of findings
for the main comparison.
| Cup feeding compared with supplemental enteral feeding for newborn infants unable to fully breastfeed | ||||
|
Patient or population: Newborn infants unable to fully breastfeed Settings: Neonatal and Maternity Units Intervention: cup feeding Comparison: supplemental enteral feeding | ||||
| Outcomes | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments |
| Weight gain in first 7 days of study (g/day) | −0.10 (−0.36, 0.16) | 522 (one study) | ⊕⊕⊝⊝ low | The two earlier studies reported g/kg/day |
| Not breastfeeding at hospital discharge | 957 (four studies) | ⊕⊕⊝⊝ low | Studies have been limited by non‐compliance with the intervention. | |
| Not breastfeeding at six months | RR 0.83 (0.72, 0.95) | 803 (two studies) | ⊕⊝⊝⊝ very low | Future studies involving a wide range of gestational age preterm infants with 100% follow‐up are likely to impact the estimate of effect. |
| Not fully breastfeeding at hospital discharge | RR 0.61 (0.52, 0.71) | 893 (four studies) | ⊕⊕⊝⊝ low | Studies have been limited by non‐compliance with the intervention. |
| Not fully breastfeeding at six months | 803 (two studies) | ⊕⊝⊝⊝ very low | Future studies involving a wide range of gestational age preterm infants with 100% follow‐up are likely to impact the estimate of effect. | |
| Length of stay (days) | 823 (two studies) | ⊕⊕⊝⊝ low | Future studies involving a wide range of gestational age preterm infants with 100% follow‐up are likely to impact the estimate of effect. | |
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||
Background
Description of the condition
The optimal milk for term and preterm newborn infants is their mother's breast milk; and the ideal way for them to receive breast milk is by sucking on the breast. However, due to a variety of reasons including being born preterm or sick, newborns may not be able to fully breastfeed initially. For these newborns an alternative method for supplemental enteral feeding will be required which may include gastric tube feeding, bottle feeding or cup feeding.
Traditionally, bottles and gastric tubes have been used routinely in neonatal and maternity units to feed newborn infants who are unable to fully breastfeed, particularly at night and when mothers are unable to be present for all feeds (Lang 1994b). While this may not be desired by either staff or mothers, and gastric tube feeding does not satisfy the infant's psychological and social needs (Lang 1994b), there are limited options for when the mother is not available to breastfeed. Cup feeding has been suggested as an alternative.
Description of the intervention
Originally, cup feeding was used to feed term infants who were born with oral deformities such as a cleft lip or cleft palate (Fredeen 1948). Instruments to supplementally feed newborns have existed throughout history and include pap bowls (a bowl with a wide brimmed lip); feeding horns (a bowl with a funnel‐like horn); cups with lips; and bottles (Foote 1944; Lang 1994a). Cup feeding is successfully used in developing countries where the care and hygiene facilities for bottles and nipples are limited and gastric tubes are not readily available (Dowling 2002; Lang 1994b). Cup feeding is gaining increased use as an alternative feeding method in neonatal and maternity units for preterm and term infants who are unable to fully breastfeed (NANN 2004). It is argued that because cup feeding only requires the infant to 'lap' the milk and then coordinate swallowing and breathing, infants can be fed using a cup from as early as 30 weeks' gestation. In preterm infants, this is generally before the time that breast and bottle feeding can be introduced as this requires the coordination of sucking, swallowing and breathing, which are often uncoordinated until approximately 32 to 35 weeks of age (Lang 1994a; Lang 1994b; Palmer 1993).
How the intervention might work
The theoretical benefits of cup feeding include avoiding the confusion between breast and bottle (Dowling 2002; Gupta 1999; Thorley 1997); enhancing the infants ability to develop a suckling action for breastfeeding (Thorley 2004); and facilitating the infants ability to self‐regulate feeds and demand feed (Vallenas 1998). The Baby Friendly Hospital Initiative (BFHI) training literature and guideline recommend the use of cup feeding for infants intended for breastfeeding, so that no artificial nipples are introduced to infants (Lang 1994b; Vallenas 1998).
The literature suggests that there are many benefits to cup feeding (Cousins 1999; Fredeen 1948; Gupta 1999; Kuehl 1997; Lang 1994a; Lang 1994b): cup feeding is a simple procedure that can involve both parents; early positive body and eye contact is fostered; the infant receives positive tactile and olfactory stimulation; cardiorespiratory and oxygen saturation can be maintained (Dowling 2002; Lang 1994a; Lang 1994b); the infant controls the feed and can pace the intake and the total volume of milk taken; and there is minimal risk of aspiration and minimal energy expended (Lang 1994a; Lang 1994b; Thorley 2004). However, many of these benefits could also be claimed of supplemental enteral feeding including bottle and tube.
Why it is important to do this review
While there may be many benefits of feeding preterm and term newborn infants with a cup, there are also potential risks that need to be considered when introducing this practice into neonatal and maternity units (NANN 2004). Some authors have reported that cup feeding is awkward and that the infant is at risk of aspiration pneumonia when the improper technique is used resulting in the milk being 'poured into' the infants mouth rather than allowing the infant to 'lap' or sip the milk (Lang 1994b; Thorley 2004). Other potential risks include physiological instability (bradycardia, apnoea, low oxygen saturation) (Freer 1999), and choking and poor weight gain (Kuehl 1997), which can result in extended hospitalizations and additional cost of care. Lastly, undesirable outcomes have been reported: nursing workload may be increased as a result of extra nursing time needed to cup feed, and term infants may refuse the breast, becoming addicted to the cup if use is prolonged and they are not given the opportunity to breastfeed (Lang 1994b; Thorley 1997; Thorley 2004). If infants require treatment as a result of an adverse event or if term infants reject the breast, this may result in increased stress and anxiety for the parents and family.
Before the introduction of cup feeding into neonatal and maternity units, this practice must be evaluated for efficacy and safety in terms of clinical outcomes, human resource use, cost and time.
Objectives
To determine the effects of cup feeding versus other forms of supplemental enteral feeding on weight gain and achievement of successful breastfeeding in term and preterm newborn infants that are unable to fully breastfeed.
Further subgroup analysis was planned to determine outcomes according to:
gestational age: born preterm (less than 37 weeks' gestation) versus term (greater than or equal to 37 weeks' gestation);
oral‐facial abnormalities: born with oral‐facial abnormalities (e.g. cleft lip or cleft palate or both) versus no oral‐facial abnormalities.
Methods
Criteria for considering studies for this review
Types of studies
Randomised and some types of non‐randomised (i.e. quasi‐randomised) controlled trials that evaluated the effects of cup feeding versus other forms of supplemental enteral feeding in term or preterm newborn infants who are unable to fully breastfeed. Cross‐over studies were excluded.
Types of participants
Term and preterm newborn infants, up to 44 weeks' postmenstrual age or 28 days' postnatal age, who were unable to fully breastfeed.
Types of interventions
Oral feeding of either expressed breast milk or a combination of expressed breast milk and artificial formula via a cup (or of a similar design so that the infant 'laps' the milk) versus other forms of supplemental enteral feeding (such as tube feeds and bottle feeds).
Types of outcome measures
Full breastfeeding is defined in this review as only having breast feeds and no other supplemental feeds.
Primary outcomes
Weight gain (g/kg/day)
Time to full breastfeeding with acceptable weight gain (15 to 30 grams/day)
Proportion not breastfeeding at hospital discharge and at three and six months of age
Proportion not fully breastfeeding at hospital discharge and at three and six months of age
Secondary outcomes
Average time per feed (minutes)
Number of reported choking events per infant or per cup feed over the duration of cup‐feeding period, depending on how described in individual studies
Number of reported aspiration events per infant or per cup feed over the duration of cup‐feeding period, depending on how described in individual studies
Number of reported infection events per infant or per cup feed over the duration of cup‐feeding period, depending on how described in individual studies
Number of reported physiological instability events i.e. bradycardia, apnoea, low oxygen saturations per infant or per cup feed over the duration of the cup‐feeding period, depending on how described in individual studies
Gestational age at discharge (weeks)
Length of hospital stay (days)
Cost
Parental satisfaction (however assessed in individual studies)
Parental anxiety (however assessed in individual studies)
Neurodevelopmental outcomes at 18 and 24 months of age (measured by, for example, the Bayley Scales of Infant and Toddler Development or the Griffiths Mental Development Scales)
Death prior to discharge
Death by 28 days of age
Death by 12 months of age
Search methods for identification of studies
We used the standard methods of the Cochrane Neonatal Review Group guidelines.
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL, the Cochrane Library, Issue 1, 2016), MEDLINE via PubMed (1948 to 31 January 2016), Embase (1980 to 31 January 2016), and CINAHL (1982 to 31 January 2016), using the search terms Infant, Newborn, Neonatal Intensive Care, Cup feeding, Neonatal, Maternity Units, Postnatal, Oral feeding and Bottle feeding. All languages were included. Full search strategies are detailed in Appendix 1, Appendix 2.
Searching other resources
Published abstracts: we electronically searched the abstracts of the Society for Pediatric Research from 2000 to 2015 through the PAS website (abstract archive). We also searched clinical trials' registries for ongoing or recently completed trials (ClinicalTrials.gov; Controlled‐Trials.com; and the World Health Organization (WHO) International Clinical Trials Registry Platform (who.int/ictrp/en/)). The results of the search of trials registries are detailed in Appendix 3, Appendix 4 and Appendix 5.
Data collection and analysis
We used the standard methods of the Cochrane Neonatal Review Group guidelines.
Selection of studies
Independently, two review authors (AF and KN) assessed titles and abstracts of retrieved studies for relevance. After this initial assessment, we retrieved full versions of all potentially eligible studies. Independently, the same two review authors checked the full papers for eligibility and resolved disagreements through discussion. When we could not reach consensus, we consulted the third independent review author (MD).
Data extraction and management
Two of the review authors (AF and KN) independently extracted data. Using an agreed form designed to extract data, we assessed study quality using four key criteria — blinding of allocation, blinding of intervention, completeness of follow up and blinding of outcome measurement — and assigned a rating of 'Yes', 'No' or 'Can't tell' for each. we resolved differences by discussion to reach consensus; and when required we consulted the third independent review author (MD). we contacted the trial authors of studies to clarify published results that were unclear and for missing data.
Assessment of risk of bias in included studies
Two of the three review authors (AF and KN) independently assessed studies for methodological quality and bias using the Cochrane's 'Risk of bias' assessment tool. This tool addresses the following six specific domains and other issues that have the potential to bias the study (Higgins 2011):
selection bias
performance bias
detection bias
attrition bias
reporting bias
or any other bias
We resolved any disagreements by discussion or by consulting a third assessor. See Appendix 6 for a more detailed description of risk of bias for each domain.
Measures of treatment effect
For dichotomous data, we presented results as summary risk ratio (RR) with 95% confidence intervals (CIs). For continuous data, we used the mean difference (MD) with 95% CIs if outcomes are measured in the same way between trials.
For individual trials and for continuous variables such as weight gain, we reported MDs and 95% CIs. For categorical outcomes such as mortality, we reported the RR and 95% CIs.
For pooled results, we reported continuous variables, MD and 95% CIs. For categorical outcomes, we reported the RR and 95% CIs. We tested each treatment effect for heterogeneity using the I² test. We used the fixed‐effect model for meta‐analysis and examined sources of statistical heterogeneity.
Dealing with missing data
We contacted study authors for missing data and to clarify data that was unclear in the published articles. For included studies, we noted levels of attrition.
Review authors extracted data independently and resolved differences by discussion.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the I² and Chi² statistics. We regarded heterogeneity to be substantial if either I² was greater than or equal to 60% or there was a low P value (less than 0.10) in the Chi² test for heterogeneity (Higgins 2002).
Assessment of reporting biases
As there were fewer than 10 studies included in analyses we were unable to investigate reporting biases (such as publication bias) using funnel plots.
Data synthesis
We carried out statistical analyses using Review Manager 5 (RevMan) software.
We used fixed‐effect meta‐analysis where it was reasonable to assume that studies are estimating the same underlying treatment effect: i.e. where studies are examining the same intervention, and the trials' populations and methods are judged to be sufficiently similar.
Quality of evidence
We used the GRADE approach, as outlined in the GRADE Handbook (Schünemann 2013), to assess the quality of evidence for the following (clinically relevant) outcomes: weight in the first seven days of the study (g/day); not breastfeeding at hospital discharge; not breastfeeding at six months; not fully breastfeeding at hospital discharge; not fully breastfeeding at six months; length of stay (days).
Two authors independently assessed the quality of the evidence for each of the outcomes above. We considered evidence from randomised controlled trials as high quality but downgraded the evidence one level for serious (or two levels for very serious) limitations based upon the following: design (risk of bias), consistency across studies, directness of the evidence, precision of estimates, and presence of publication bias. We used the GRADEpro 2008 Guideline Development Tool to create a ‘Summary of findings’ table to report the quality of the evidence.
The GRADE approach results in an assessment of the quality of a body of evidence in one of four grades:
High: We are very confident that the true effect lies close to that of the estimate of the effect.
Moderate: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect.
Very low: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
Subgroup analysis and investigation of heterogeneity
Subgroup analyses were planned a priori on the following:
Gestational age: born preterm (less than 37 weeks' gestation) versus term (greater than or equal to 37 weeks' gestation);
Oral‐facial abnormalities: born with oral‐facial abnormalities (e.g. cleft lip or cleft palate or both) versus no oral‐facial abnormalities
Sensitivity analysis
We planned to carry out sensitivity analyses to explore the effects of adequacy of allocation concealment (including quasi‐randomisation) and other risk of bias components, but there were insufficient data to do this.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies.
Results of the search
For the update of this review a further five studies were identified, of which four were excluded (Abouelfettoh 2008; Aloysius 2007; Al‐Sahab 2010; Huang 2009); and one was included (Yilmaz 2014) (Figure 1). Therefore the included studies total five and excluded studies total sixteen.
1.
Study flow diagram: review update
Included studies
The five included studies (Collins 2004; Gilks 2004; Mosley 2001; Rocha 2002; Yilmaz 2014), with a total of 971 participants are described in the Characteristics of included studies.
The studies are RCTs conducted in four countries: the United Kingdom (Gilks 2004; Mosley 2001); Australia (Collins 2004); Brazil (Rocha 2002); and Turkey (Yilmaz 2014, the most recent study).
Studies enrolled preterm infants with mean gestational ages at birth from 29 to 35 weeks, and all studies compared cup versus bottle feeds as a supplement to breastfeeding when transitioning from full nasogastric feeds to full breastfeeds.
Collins 2004 included 319 preterm infants (mean gestational ages 29.3 and 30.0, mean birth weights 1334 g and 1446 g for the respective study groups 'cup' and 'bottle') of mothers who had indicated that they intended to breastfeed. Infants born at less than 34 weeks' gestational age were eligible for inclusion in the study and were randomised to supplemental feeds either via a cup or via a bottle. This study also randomised infants to either dummy (pacifier) or no dummy within each of the two study groups. The initiation of the allocated supplemental feeds was determined by the attending nurse/midwife or neonatologist and occurred when the mother was unavailable to breastfeed or when additional oral feeds of milk were required after a breastfeed.
The authors report that compliance to the allocated intervention, in particular to cup feeding, was poor and therefore reduced the power to identify a real treatment effect. The tertiary hospital that had previously been using cup feeding was more compliant with the intervention than the other tertiary recruiting hospital (where cup feeding was introduced for the study) or the participating 54 peripheral hospitals (where the use of cup feeding was uncommon).
The main outcome measures were partial or fully breastfeeding or no breastfeeding on discharge home. Secondary outcomes included length of hospital stay and prevalence of breastfeeding at three and six months post discharge.
Participants in Gilks 2004 were 54 preterm infants (median gestational ages 31 weeks and 32 weeks, median birth weights 1560 g and 1750 g for the respective study groups 'cup' and 'bottle') of mothers who had indicated an intention to breastfeed. However 14 infants (11 in cup feeding group and 3 in bottle group) withdrew prior to discharge and data is not presented for these infants. For infants to be eligible for the study, they needed to be born at less than 35 weeks' completed gestation and more than 30 weeks' gestation. Once enrolled into the study, the infant was randomised to receive supplemental feeds either by cup or bottle in addition to nasogastric tube feeds and breastfeeds. It is unclear who determined when supplemental feeds by cup or bottle were begun, the criteria for assessing which feed would be given by cup or bottle, and how often the infants received the allocated treatments as opposed to receiving feeds by nasogastric tube or the breast. Cup feeding had been introduced into the hospital six months prior to the initiation of the study, and staff had received information sheets and attended a teaching programme during the introduction phase.
The main outcome measure was partial or exclusively breastfeeding rates at discharge. Secondary outcomes included breastfeeding rates at term, at six weeks post term and postconceptional age at which the nasogastric tube was withdrawn. Mosley 2001 was a pilot study to establish the feasibility of conducting a randomised controlled trial of supplemental feeding methods. Recruitment of infants took place over a three‐month period, resulting in the recruitment of 16 preterm infants, only 14 infants of which had data presented. The study compared two methods of supplementary feeding (bottle versus cup) for preterm infants of mothers who indicated a desire to breastfeed. The initiation of oral feeding or supplemental feeding was at the discretion of the physician or advanced neonatal nurse practitioner, which was the normal practice in the study hospital.
The main outcome measure was breastfeeding rates at discharge. Other outcomes were examined retrospectively, following assessment of the data set. These included the use of a pacifier (dummy), influence of assisted delivery on breastfeeding, previous experience of breastfeeding, influence of prematurity on breastfeeding rates, influence of support to breastfeed and the impact of delayed breastfeeding initiation.
Rocha 2002 was a stratified randomised controlled trial; 83 infants were randomised to either cup or bottle, however data for only 78 infants is reported. Infants were between 32 and 36 weeks' gestation and weighing less than 1700 grams. Stratification encompassed three groups: 500 g to 999 g; 1000 g to 1499 g, and 1500 g to 1699 g. All infants were fed by nasogastric tube until they weighed 1600 grams, at which time breastfeeding was encouraged. If supplemental feeds were required, they were offered feeding by the assigned method. Prior to the study, there was education of all staff about cup feeding technique. After a week of oral feeds, monitoring was begun by an investigator who examined oxygen saturation before, during and after the feed. Weight gain and feed interval were also recorded. Follow‐up was conducted until the third month or when the infant weaned.
The main outcome measure was to examine the impact of cup and bottle feeding on subsequent breastfeeding of preterm infants. Secondary outcomes examined the difference between oxygen saturation levels in bottle, cup and breastfed infants.
Yilmaz 2014 conducted a randomised controlled trial in three hospitals in Turkey comparing the effect of cup versus bottle feeding on exclusive breastfeeding rates in infants between 32 and 35 weeks' gestation; however cup feeding was not initiated until infants were 35 weeks of age. Infants became eligible for the study once intermittent feeding by gastric tube had commenced, thus the infants were receiving some breastfeeds. A large number of mothers lived regionally from the recruitment hospitals and were able to visit their infants at least twice per week. Infants were randomised to either bottle or cup feeding and commencement of the allocated feeding method was determined by the attending physician; all infants were late preterm on commencement. A power calculation based on the exclusive breastfeeding rate at six months determined that 766 infants (383 per group) were required. The total number of infants randomised was 607, of which 40 (13%) in the bottle feeding group and 45 (15%) in the cup feeding group were excluded from the analysis due to non‐compliance or development of a disease preventing oral feeding. Follow‐up was at one week and then monthly until infants were six months of age, at which time breastfeeding status was recorded and compliance to assigned feeding methods was assessed using a questionnaire.
The main outcome measure was weight gain at day seven of the study and the proportion of exclusive or any breastfeeding on discharge. Secondary outcomes included length of stay and prevalence of exclusive or any breastfeeding at three and six months of age.
Excluded studies
Thirteen studies were excluded because they were neither randomised controlled trials nor non‐randomised controlled trials that used quasi‐randomised group allocation (Abouelfettoh 2008; Aloysius 2007; Al‐Sahab 2010; Brown 1999; Davis 1948; Dowling 2001; Dowling 2002; Fredeen 1948; Freer 1999; Gupta 1999; Huang 2009; Malhotra 1999; Marinelli 2001). Howard 1999 was excluded since the study randomised exclusively formula‐feeding infants to either bottle or cup (which did not meet our definition for study participants), and an exclusively breastfeeding group of infants were used as a comparison group. Howard 2003 was excluded since this study did not meet the inclusion criteria for this review since participants were not infants that were unable to fully breastfeed. One further study — Schubiger 1997 — was excluded as this study did not meet the inclusion criteria for this review as the intervention was supplements by either cup or spoon, not solely via a cup. Reasons for exclusion can be seen in the table 'Characteristics of excluded studies'.
Risk of bias in included studies
Three studies had a high risk of bias and two studies had a low risk of bias (Figure 2; Figure 3).
2.
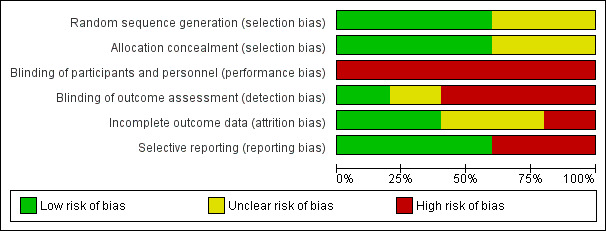
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
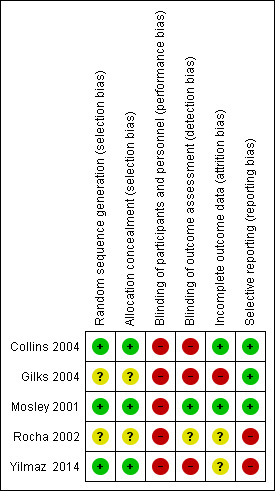
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Three of the five studies described their method of allocation concealment in detail and specified an adequate method of sequence generation (Collins 2004; Mosley 2001; Yilmaz 2014).
Blinding
The intervention of cup feeding versus other forms of supplemental enteral feeding was not feasible to blind; and although assessment of some outcomes could have been blinded, only Mosley 2001 reported doing so.
Incomplete outcome data
Two studies had complete outcome data (Collins 2004; Mosley 2001). Rocha 2002 enrolled 83 infants, five were excluded, thus data were presented for 78 infants. Of the five infants excluded, two were excluded from the cup‐feeding group due to a break in protocol, and three infants from the bottle‐feeding group due to reflux and bronchopulmonary dysplasia, although these were not specified as exclusion criteria for enrolment into the study. The outcome data for three‐month follow‐up does not report number weaned, therefore it is unclear if complete data is presented. Gilks 2004 enrolled 54 infants; however 14 infants were withdrawn post‐randomisation, 11 from the cup feeding group and three from the bottle feeding group. Of those who withdrew from the cup‐feeding group, eight were because they no longer wished to breastfeed. Yilmaz 2014 analysed data for 522 of the 607 infants randomised. Data analysis was not by intention to treat and the outcome data for 85 infants was not reported. These infants were excluded from the data analysis for either non‐compliance or developing a disease that prevented oral feeding for longer than two days.
Selective reporting
One study — Rocha 2002 — was at high risk of selective reporting. Rocha 2002 reported that outcomes would be evaluated monthly until the third month, however no data is reported for the first or second months.
Other potential sources of bias
A self‐reporting questionnaire for compliance to the assigned feeding method was used by Yilmaz 2014 during the six months of follow‐up which may have introduced response bias. While it is reported that all cup feedings were given by NICU staff nurses or parents the authors report that "the same researcher recorded feed duration for both bottle and cup feeding groups for each patient".
Effects of interventions
See: Table 1
Supplemental feed using cup versus bottle (Comparison 01)
Primary Outcome Measures
Weight gain (Outcome 1.1 and Outcome 1.2)
Two studies reported results for weight gain (Rocha 2002; Yilmaz 2014). However Rocha 2002 reports g/kg/day (Analysis 1.1) and Yilmaz 2014 reports g/day (Analysis 1.2). Both studies report no significant difference between groups: Rocha 2002 reports RR of −0.60 (95% CI −3.21 to 2.01); Yilmaz 2014 reports RR of −0.10 (95% CI −0.36 to 0.16).
1.1. Analysis.
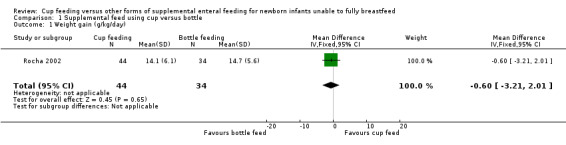
Comparison 1 Supplemental feed using cup versus bottle, Outcome 1 Weight gain (g/kg/day).
1.2. Analysis.
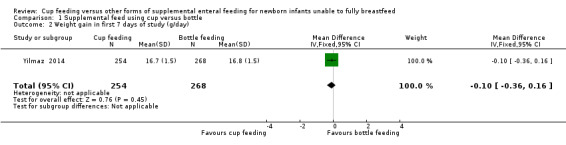
Comparison 1 Supplemental feed using cup versus bottle, Outcome 2 Weight gain in first 7 days of study (g/day).
Proportion not breastfeeding at hospital discharge (Outcome 1.3)
Four studies reported this outcome:
Yilmaz 2014 reported RR of 0.09 (95% CI 0.02 to 0.37); Collins 2004 reported RR of 0.80 (95% CI 0.56 to 1.14); Gilks 2004 reported RR of 0.87 (95% CI 0.52 to 1.45); and Rocha 2002 reported RR of 0.88 (95% CI 0.36 to 2.19).
The meta‐analysis (I² = 72%) of the four trials showed a typical RR of 0.64 (95% CI 0.49 to 0.85) demonstrating significant heterogeneity (Analysis 1.3). The results from Yilmaz 2014 included in this meta‐analysis indicated higher breastfeeding rates at hospital discharge, however this needs to be considered with caution given the variation in results.
1.3. Analysis.
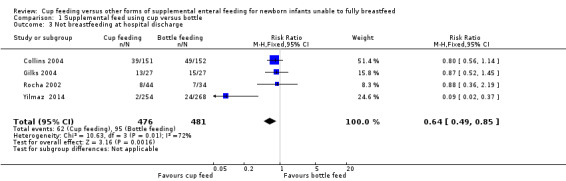
Comparison 1 Supplemental feed using cup versus bottle, Outcome 3 Not breastfeeding at hospital discharge.
Proportion not breastfeeding at three months of age (Outcome 1.4)
Three studies reported this outcome:
Yilmaz 2014 reported RR of 0.70 (95% CI 0.46 to 1.06); Collins 2004 reported RR of 0.90 (95% CI 0.75 to 1.09); Rocha 2002 reported RR of 0.83 (95% CI 0.65 to 1.05). The meta‐analysis (I² = 0%) of the three trials showed a typical RR of 0.83 (CI 0.71 to 0.97) (Analysis 1.4). The results from Collins 2004 included in this meta‐analysis is an evaluation of infants seen at follow‐up, not all infants who were randomised. The analysis demonstrates no significant reduction in the proportion of infants not breastfeeding at three months of age.
1.4. Analysis.
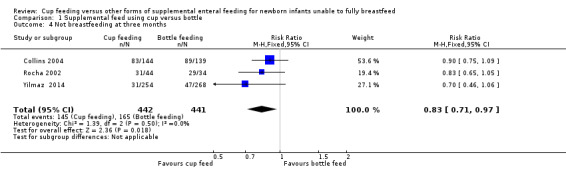
Comparison 1 Supplemental feed using cup versus bottle, Outcome 4 Not breastfeeding at three months.
Proportion not breastfeeding at six months of age (Outcome 1.5)
Only two studies reported this outcome:
Yilmaz 2014 reported RR of 0.75 (95% CI 0.59 to 0.95); Collins 2004 reported RR of 0.90 (95% CI 0.78 to 1.05).
The meta‐analysis (I² = 55%) of the two trials showed a typical RR of 0.83 (0.72 to 0.95) (Analysis 1.5). The results from Collins 2004 is an evaluation of infants seen at follow‐up, not all infants who were randomised.
1.5. Analysis.
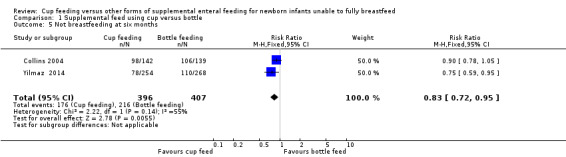
Comparison 1 Supplemental feed using cup versus bottle, Outcome 5 Not breastfeeding at six months.
Proportion not fully breastfeeding at hospital discharge (Outcome 1.6)
Four studies reported this outcome:
Yilmaz 2014 reported RR of 0.51 (95% CI 0.41 to 0.64); Collins 2004 reported RR of 0.74 (95% CI 0.58 to 0.95); Gilks 2004 reported RR of 0.74 (95% CI 0.53 to 1.03); Mosley 2001 reported RR of 1.33 (95% CI 0.26 to 6.94). The meta‐analysis (I² = 57%) of the four trials showed a typical RR of 0.61 (95% CI 0.52 to 0.71) with a number needed to treat for an additional beneficial outcome (NNTB) of 5 (95% CI 3.6 to 6.8) (Analysis 1.6). The analysis demonstrates that the group of infants who were cup fed had a reduction in the proportion of infants not fully breastfeeding at hospital discharge (i.e. an increase in the proportion of infants exclusively breastfeeding at discharge).
1.6. Analysis.
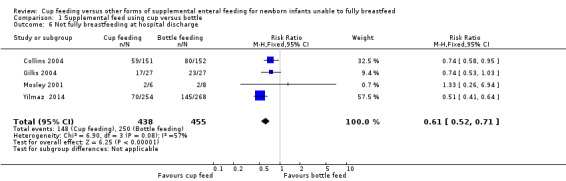
Comparison 1 Supplemental feed using cup versus bottle, Outcome 6 Not fully breastfeeding at hospital discharge.
Proportion not fully breastfeeding at three months of age (Outcome 1.7)
Only two studies reported this outcome:
Yilmaz 2014 reported RR of 0.43 (95% CI 0.33 to 0.55); Collins 2004 reported RR of 1.18 (95% CI 0.88 to 1.58).
The intention was to undertake a meta‐analysis; however there was considerable variation in results, with substantial heterogeneity (I² = 96%) therefore a meta‐analysis was not undertaken.
Proportion not fully breastfeeding at six months of age (Outcome 1.8)
Only two studies reported this outcome:
Yilmaz 2014 reported RR of 0.74 (95% CI 0.62 to 0.88) Collins 2004 reported RR of 1.31 (95% CI 0.89 to 1.92).
The intention was to undertake a meta‐analysis; however there was considerable variation in results, with substantial heterogeneity (I² = 86%) therefore a meta‐analysis was not undertaken.
Secondary Outcomes
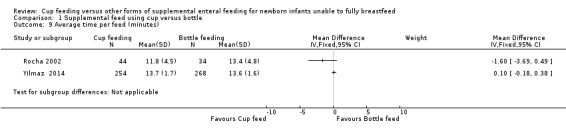
Average time per feed (minutes) (Outcome 1.9)
Only two studies reported this outcome: Yilmaz 2014 reported MD of 0.10 (95% CI −0.18 to 0.38); Rocha 2002 reported MD of −1.60 (95% CI −3.69 to 0.49).
The intention was to undertake a meta‐analysis; however there was considerable variation in results, with substantial heterogeneity (I² = 60%) therefore a meta‐analysis was not undertaken.
Number of reported physiological instability events
Rocha 2002 reported episodes of oxygen desaturation. The outcome 'lowest oxygen saturations (%) during feeding' was reported. Mean (SD) oxygen saturation in the cup‐feeding group was 90.8% (4.8%) and mean (SD) oxygen saturation in the bottle feeding group was 87.7% (7.6%). The difference between means was not statistically significant. They also reported desaturation episodes of less than 85% and 90%. However, it is not clear whether the data reported are the proportion of time spent less than the cut‐off oxygen saturation (85% or 90%) or the proportion of infants who had an oxygen saturation less than the cut‐off at some stage.
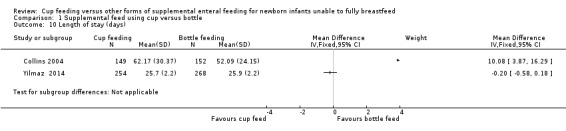
Length of hospital stay (days) (Outcome 1.10)
Yilmaz 2014 reported MD of −0.20 (95% CI −0.58 to 0.18); Collins 2004 reported MD of 10.08 (95% CI 3.87 to 16.29). The original report included only median days and interquartile range (IQR). In the cup‐feeding group, the median (IQR) length of stay was 59 (37 to 85) days and in the bottle‐feeding group it was 48 (33 to 65) days. On request, the authors provided these data as means and standard deviations.
The intention was to undertake a meta‐analysis; however there was considerable variation in results, with substantial heterogeneity (I² = 90%) therefore a meta‐analysis was not undertaken.
Gestational age at discharge (Outcome 1.11)
Yilmaz 2014 reported MD of −0.10 (−0.54 to 0.34) for gestational age at discharge (Analysis 1.11).
1.11. Analysis.
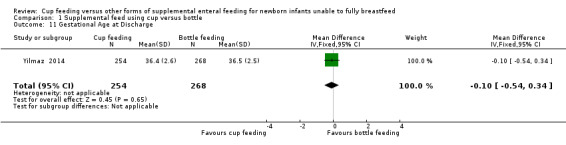
Comparison 1 Supplemental feed using cup versus bottle, Outcome 11 Gestational Age at Discharge.
Unreported outcomes
None of the following outcomes were reported in any of the included studies for primary outcome: time to full breastfeeding with acceptable weight gain. None of the following outcomes were reported in any of the included studies for secondary outcomes: number of reported choking events; number of reported aspiration events; number of reported infection events; cost; parental satisfaction; parental anxiety; neurodevelopmental outcomes at 18 and 24 months of age; death prior to discharge; death by 28 days of age; death by 12 months of age.
Other outcomes reported
Collins 2004 reported that non‐compliance to the experimental intervention was high, with 56% (85/151) of cup‐feeding infants having a bottle introduced. Of the 44% of mothers who decided to introduce a bottle, 39% reported that they did not like, or had problems with, cup feeding. These problems included the infant not managing cup feeds, spilling a lot, not being satisfied, or taking too long to feed. Twelve per cent of mothers reported that staff refused to cup feed their infant. Yilmaz 2014 report in the flow diagram that 26 infants in the cup‐feeding group and 21 in the bottle‐feeding group were excluded for non‐compliance and report that a limitation of the study was failure to analyse by intention to treat.
Collins 2004 reported no adverse events and Yilmaz 2014 and Rocha 2002 reported that no case of aspiration and apnoea were observed. Rocha 2002 reported that there was no difference in mean oxygen saturations between cup‐fed and bottle‐fed infants during feeds.
Discussion
Summary of main results
This review sought to determine the effect of cup feeding as a supplemental feeding method on weight gain and achievement of exclusive breastfeeding in term and preterm infants. We identified five studies that compared cup feeding to other methods of supplemental feeding (e.g. bottle feeding). All five studies enrolled only preterm infants.
Of the five studies, only two reported results for weight gain in the first week (Rocha 2002; Yilmaz 2014), which found no significant difference between the groups.
The results of this update demonstrate considerable variation in results for some breastfeeding outcomes while others showed statistically significant differences favouring cup feeding over bottle feeding. Four studies reported 'not breastfeeding at hospital discharge' (Collins 2004;Gilks 2004; Rocha 2002; Yilmaz 2014); three studies reported 'not breastfeeding at three months' (Collins 2004;Rocha 2002;Yilmaz 2014); two studies reported 'not breastfeeding at six months' (Collins 2004;Yilmaz 2014); four studies reported 'not fully breastfeeding at hospital discharge' (Collins 2004;Gilks 2004;Mosley 2001;Yilmaz 2014); two studies reported 'not fully breastfeeding at three months; or six months' (Collins 2004;Yilmaz 2014). For the important outcomes of exclusive breastfeeding at three and six months the meta‐analysis favours cup feeding; however these results should be treated with caution given the high degree of heterogeneity and the very low quality of evidence particularly for these outcomes (Table 1).
Further secondary outcomes were average time to feed which was reported in two studies and showed no difference between the two groups (Rocha 2002; Yilmaz 2014). Two studies assessed length of hospital stay and there was considerable variation in results and in the direction of effect (Collins 2004;Yilmaz 2014). Only one study has reported gestational age at discharge which showed no difference between the two groups (Yilmaz 2014).
Overall completeness and applicability of evidence
We found no randomised controlled trials that compared the intervention in the term newborn population. Therefore the results of this review are only applicable to preterm infants.
All of the included trials were conducted in upper‐middle income and high‐income countries, therefore these data are relevant to current clinical practice in the care of preterm infants in such countries. Evidence was provided on the effect on weight gain and breastfeeding outcomes for preterm infants receiving supplemental feeds by cup. In an environment where hospitals are attempting to reduce length of stay, a finding such as this will significantly affect financial and bed‐management resources. The cost implications related to length of stay need to be considered against a short‐term gain in exclusive breastfeeding at discharge.
It is unclear how applicable this evidence is to neonatal care practices in low‐income countries and in those countries culturally familiar with traditional implements, such as the paladai cup, compared to 'cups' used in high‐income countries. Additionally in those countries with limited or no clean water supplies, or limited sterilisation equipment, cup feeding may still be the practice of choice.
Quality of the evidence
The methodological quality of the included studies was low to very low, suggesting that the results of the meta‐analyses need to be treated with extreme caution (Table 1).
Generally it was not possible to mask caregivers to the nature of the intervention, thus increasing the likelihood of performance bias. Lack of blinding also increases the likelihood of contamination. All studies included in this review had high rates of contamination in the intervention group, with many infants randomised to the cup‐feeding group actually receiving bottle feeds. This may have diluted any effects of cup feeding — both positive and negative. However this may have been due to the impracticality of the intervention: for example in Collins 2004, 56% of those allocated to cup feeding had some bottle feeds (with over 90% of those mothers not liking, or having significant problems with, cup feeding).
There was also little blinding of any outcome assessments in four of the five studies (especially the single dominant study by Collins 2004). Such blinding could have been possible, and if done would have decreased the likelihood of detection bias.
Agreements and disagreements with other studies or reviews
Abouelfettoh 2008 studied cup versus bottle feeding in a preterm population in Egypt. This was a quasi‐experimental cohort design. The intention of the study was to report breastfeeding outcomes up to six weeks post hospital discharge. This paper reports a difference between the two groups at one week post discharge only and cites that outcome data up to six weeks was not feasible due to low maternal education. That is, maternal recall regarding breastfeeding practices could not be relied upon over the six weeks. Additionally the authors report that the cup‐feeding group displayed more mature breastfeeding behaviours over the six weeks; however this needs to be viewed with caution as only 13 or the 30 infants were assessed at six weeks.
The Baby Friendly Health Initiative (BFHI) is “a global standard based on evidence” WHO 1998. One of the 10 steps of the BHFI initiative includes the need to offer supplemental feeds without the use of bottles and teats and therefore BFHI promotes cup feeding as the alternative when infants are not able to breastfeed (WHO 1998) and that “current evidence related to alternative methods of feeding undergoes extensive review by the World Health Organization” (personal communication, Rachel Ford, Australian College of Midwives). Whilst this is appreciated, there is limited evidence and no high‐quality randomised controlled trials involving only term infants in either high‐ or low‐resource settings. In addition there are reports in the literature that cup feeding can create problems in the term population. If accreditation processes such as BFHI state evidence as the basis of practice, the interpretation of current evidence about cup feeding is of a biased perspective. Evidence put forward by BFHI is not 'gold standard' and does not address the issue of increased length of stay and parental choice. Organisations and individuals should be supported in choosing alternative supplemental methods, in addition to cup feeding, until successful breastfeeding can be achieved.
Huang 2009, a longitudinal study undertaken in Northern Taiwan, used questionnaires and telephone calls to obtain information on full‐term infant feeding behaviour and mother's milk supply. Exclusively breast‐fed infant outcomes were compared to those infants who received supplemental feeds via cup or bottle. Infants were recruited at different times to avoid interaction of the groups. The cup‐feeding group was recruited over a one‐month period at the end of 2005. The bottle‐feeding group was recruited during February 2006. The reported result in the abstract was that "the bottle group was significantly more fretful during breastfeeding". The authors measured negative sucking behaviours, in which there were no difference in negative behaviour on initial feeding or at two and four weeks, only on day 3. Thus it is difficult to ascertain how and when 'fretful' was measured as fretful was not a measured outcome presented in the paper. Additionally the authors conclude that "cup‐feeding was better than bottle‐feeding", although there is a lack of data in this paper to draw such a conclusion. Furthermore the authors state that they found "no significant correlation between infant feeding method and infant sucking competence". Thus the commentary by Han 2010 reporting that the study by Huang 2009 showed cup‐feeding had beneficial effects over bottle feeding needs to be viewed with caution. Additionally the second study referred to in this commentary (Gupta 1999), conducted in India, may suggest that cup feeding is a viable option in under‐resourced countries; however the scientific data are inconclusive and further robust, well‐designed studies are needed.
Authors' conclusions
Implications for practice.
The current evidence from randomised controlled trials does not reliably demonstrate that cup feeding over bottle feeding confers a benefit in maintaining breastfeeding beyond hospital discharge for newborn infants. Supplemental feeding by cup may result in a longer stay in hospital.
Implications for research.
While the limitations of the studies included in the review might lead us to conclude that further large high‐quality randomised controlled trials should be undertaken in developed countries, the issue of high rates of non‐compliance with the intervention of cup feeding by both practitioners and parents, as reported in the majority of previous studies, may make this a futile undertaking. Should nipple confusion and supplementation with bottles, and impact on breastfeeding rates and duration remain a concern, then this should be the focus for future research rather than cup feeding. Traditional interventions aimed at maintaining breastfeeding longer term (e.g. early breastfeeds, early and regular skin‐to‐skin contact, rooming in, non‐separation of mother and baby as much as possible, non‐introduction of supplemental feeds unless medically indicated, and antenatal breastfeeding education as documented in WHO 1998) should also be given due consideration before further trials of cup feeding are undertaken.
Feedback
Rajiv Bahl Feedback October/07 and Review Author Response
Summary
Date of Submission: 19‐Oct‐2007
Name: Rajiv Bahl Email Address: bahlr@who.int Personal Description: Occupation Medical Officer WHO Feedback: Conclusions of the Cochrane review on cup feeding for newborns unable to fully breastfeed are not supported by the findings
This recently published systematic Cochrane review based on four relatively small studies makes a general conclusion "Cup feeding cannot be recommended over bottle feeding as a supplement to breastfeeding because it confers no significant benefit in maintaining breastfeeding beyond hospital discharge and carries the unacceptable consequence of longer stay in hospital" (1). We feel that the findings of the review do not support this conclusion. Justification for this feeling is provided below.
The risk of infection should be an important consideration in the choice of feeding method. One of the major potential advantages of cup feeding over bottle feeding is in reducing the risk of infection, particularly in developing countries. All studies (2‐4) included in the review except a small study from Brazil (5) are from developed countries and none has reported on the risk of infection. Although infection was included as an outcome in the protocol for this Cochrane review, the lack of data on this important outcome is not discussed.
On the outcomes on which data is presented, the number of participants in all studies is small. The total number of infants included in the meta‐analysis on any breastfeeding is about 400. For comparison, in another study in term infants, Howard et al estimated that at least 700 infants would be needed to detect a 10% difference in breastfeeding cessation with 90% power and 5% significance level (6). The lack of significant effect on many outcomes could therefore have been just because of lack of statistical power. We think that the "lack of evidence of effect" cannot be taken as the "evidence of lack of effect".
The authors base their conclusions on their findings related to the lack of significant benefit of cup feeding beyond hospital discharge. However, all the studies included in the review were hospital studies with the primary outcomes limited to the time of hospital discharge. Only two studies reported effect at 3 months and one study at 6 months after discharge, as a secondary outcome. It is clear that even in these studies, sample sizes were not calculated, follow up was not complete and the quality of data cannot therefore be considered to be same as that for the primary outcome. Considering only the primary outcomes, there was a 18% non‐significant benefit in any breastfeeding and 25% significant benefit in full breastfeeding rates associated with cup feeding. While these findings cannot be considered conclusive in favour of cup feeding, they are certainly indicative of a benefit. In any case, this is clearly not evidence of lack of benefit.
One of the possible reasons for only a modest benefit of cup feeding on subsequent breastfeeding could be the lack of compliance with the allocated intervention. Although the authors of the Cochrane review have recognized this, we think that they have not discussed it appropriately. For example, the researchers in the largest included study (2) considered the lack of compliance as the main limitation of their study which could have lead to an underestimate of the effect. Indeed, the researchers state in their paper that "Compliance analysis showed a significant increase in the prevalence of any breastfeeding with cup feeding (Odds ratio 21.09, 95% CI 2.62 to 169.75, P = 0.004) with no significant difference in length of hospital stay (hazard ratio 0.82, 95% CI 0.58 to 1.17, P = 0.27). Such compliance analysis needs to be interpreted with caution and highlight the need for further research." Further, they state that "Compliance differed between recruiting hospitals, the hospital with the better compliance has used cup feeding before, in the other it was introduced for the trial. Most peripheral hospitals had not used cup feeding before. Some staff had strong feelings against cup feeding?". The authors of the Cochrane review have not considered the lack of previous experience of staff with a new feeding method as one of the potential causes of lack of compliance with cup feeding.
The finding related to length of hospital stay (about a 15% increase in the duration of stay) needs to be interpreted with caution as it comes from a single study. Further, a possible reason for this could again be the lack of confidence of the treating physicians about the ability of the mother to feed the infant related to less experience in having used cup feeding relative to bottle feeding.
The conclusions and the plain language summary seem to indicate that the findings of this review are generalizable to all infants, in all settings. However, the studies reviewed included only those preterm infants who are not able to fully breastfeed. In this regard, it is important to consider the findings of an excluded study in term, healthy, breast fed infants (Howard et al), which show that for infants who received more than 2 supplemental feeds per day, cup feeding has distinct advantage over bottle feeding on breastfeeding duration. Also the findings may not be applicable to preterm infants in developing country settings.
Finally, we find the authors' conclusion that conducting further large, high quality RCTs on this issue may be a "futile undertaking" highly questionable. As stated above the problem of compliance in previous studies is an argument for doing better designed studies in health facilities that have experience in both cup and bottle feeding. Further, the importance of other factors like skin to skin contact, rooming in etc. should not be used an argument for not conducting research on appropriate feeding methods for infants who are not able to fully breastfeed. In our opinion, this meta‐analysis underscores the need for further well‐designed studies on this subject, in both developing and developed countries.
Rajiv Bahl, Constanza Vallenas, Jose Martines Department of Child and Adolescent Health and Development World Health Organization, Geneva Disclaimer: The authors of this feedback are staff members of the World Health Organization. The authors alone are responsible for the views expressed in this publication and they do not necessarily represent the decisions or the stated policy of the World Health Organization.
References
1. Flint A, New K, Davies MW. Cup feeding versus other forms of supplemental enteral feeding for newborn infants unable to fully breastfeed. Cochrane Database of Systematic Reviews 2007, Issue 2. Art. No.: CD005092. DOI: 10.1002/14651858.CD005092.pub2.
2. Collins CT, Ryan P, Crowther CA, McPhee AJ, Paterson S, Hiller JE. Effect of bottles, cups and dummies on breast feeding in preterm infants: a randomized controlled trial. BMJ Online First bmj.com 18 June 2004; BMJ, doi:10.1136/bmj.38131.675914.55.
3. Gilks J, Watkinson M. Improving breast feeding in preterm babies: Cup feeding versus bottle feeding. Journal of Neonatal Nursing 2004; 10:118‐20.
4. Mosley C, Whittle C, Hicks C. A pilot study to assess the viability of a randomized controlled trial of methods of supplementary feeding of breast‐fed pre‐term babies. Midwifery 2001;17:150‐7.
5. Rocha NM, Martinez FE, Jorge SM. Cup or bottle for preterm infants: effects on oxygen saturation, weight gain, and breastfeeding. Journal of Human Lactation 2002;18:132‐8.
6. Howard CR, Howard FM, Lanphear BP, Ederly S, de Blieck EA, Oakes D, Lawrence RA. Randomized clinical trial of pacifier use and bottle‐feeding or cupfeeding and their effect on breastfeeding. Pediatrics 2003;111:511‐18.
Reply
Thank you for your comment on our review "Cup feeding versus other forms of supplemental enteral feeding for newborn infants unable to fully breastfeed".
The authors disagree with the statement from Rajiv B et al that the findings from the review do not support the conclusion. The conclusions were drawn from the evidence presented in the four included randomised control trials.
The author’s acknowledge that infection is important and thus, it was included as an outcome. A thorough literature search was undertaken on all published and unpublished studies and no data from either randomised nor non‐randomised trials was found for this outcome. Several other papers from India (Gupta 1999, Malhotrata 1998) were found. Whilst they were not eligible to be included in the review, again neither of these two papers considered or discussed risks of infection, despite one discussing different utensils for delivering milk. Given that milk delivered by any utensil, irrespective of whether it is a bottle, teat, spoon or cup, each require cleaning. The authors do not feel that conducting a trial on comparing different delivery utensils and infection rates is of benefit. Without any supporting evidence or discussion in any papers regarding cup feeding in developing countries, the authors are reluctant to discuss and draw conclusions regarding this outcome We have acknowledged in the text of the review under unreported outcomes, "that none of the following outcomes were reported in any of the included studies".
Rajiv B et al comment on methodological influences which the authors have no impact on, such as sample size calculation. The statement made by the authors in the review is based on the evidence available; inferences cannot be made about design and sample size estimation by the authors. At this point in time there is a lack of evidence of effect.
Rajiv B et al again comment on design and methodology. The authors disagree with the comments and are adamant conclusions can only be based on the evidence found to be included in the review.
It is not the role of authors of Cochrane reviews to hypothesise on potential causes for lack of compliance for any intervention.
Again, the authors have presented the data available from included studies.
In reference to the excluded paper of healthy term newborn infants; this study was excluded because the population studied were healthy, term infants that were able to fully breastfeed; with supplemental feeds being offered as maternal choice, not because these were infants who were unable to fully breastfeed. This review is addressing the issue of supplemental feeds in the population of infants (irrespective of gestational age) who are unable to fully breastfeed. The authors have the view that term infants who can be fully breast fed should not be offered supplemental feeds as per the Baby Friendly Hospital Initiative (WHO1998). In addition, there are reports in the literature that caution needs to be exercised when cup feeding term infants due to the different tongue action required from that of breastfeeding, and term infants may reject the breast.
In the majority of the articles reviewed, the authors of these papers made a comment about the difficulty of ensuring compliance with this intervention. This is often the reality of conducting clinical trials in clinical settings with clinical staff. In this case, the authors do not feel that it was a question of poorly designed clinical trials.
Based on the current literature and the evidence that was reviewed, the authors disagree with the closing comment and maintain their conclusion.
The authors look forward to updating this review should further randomised controlled trials be conducted.
In conclusion, if WHO have any unpublished data regarding term infants that are unable to fully breastfeed, we would be happy to include it in future updates of the review.
References
Gupta, A., Khanna, K., & Chattree, S. (1999). Cup Feeding: An Alternative to Bottle Feeding in a Neonatal Intensive Care Unit. Journal of Tropical Paediatrics, 45, P108‐110.
Malhotra, N., Vishwambaran, L., Sunaram, K.R., & Narayanan, I. (1999). A controlled trial of methods of feeding in neonates. Early Human Dvelopment, 54, p. 29‐38.
World Health Organisation, (1998). Evidence for the Ten Steps to successful breastfeeding. Division of Child Health and Development, World Health Organisation. 1998.
Contributors
A. Flint, K. New, M. Davies
What's new
| Date | Event | Description |
|---|---|---|
| 6 February 2017 | Amended | Add external source of support |
History
Protocol first published: Issue 1, 2005 Review first published: Issue 2, 2007
| Date | Event | Description |
|---|---|---|
| 22 April 2016 | New citation required but conclusions have not changed | This updates the review 'Cup feeding versus other forms of supplemental enteral feeding for newborn infants unable to fully breastfeed' (Flint 2007). Protocol indicated would report secondary outcome of postnatal age at discharge; changed to report gestational age at discharge, |
| 31 January 2016 | New search has been performed | Updated literature searches were performed in January 2016. Protocol indicated would report secondary outcome of postnatal age at discharge; changed to report gestational age at discharge. |
| 8 May 2013 | Amended | Change of wording. The term nurseries did not adequately convey that all infants in neonatal units and maternity units in which trials have been conducted would be included in this review. Therefore the word nurseries/nursery has been changed to neonatal unit and maternity units. Change of wording. Due to feedback regarding that this review did not include term infants, there appears to be some confusion over the use of the word newborn especially referring to preterm infants only. Therefore the term 'newborn' has been replaced with term and preterm infants. |
| 14 February 2008 | Amended | Converted to new review format. |
| 14 February 2008 | Feedback has been incorporated | Feedback comments and response to comments included in review. |
Acknowledgements
Our thanks to Carmel Collins for providing additional data for the initial review.
Appendices
Appendix 1. Search strategy for CINAHL
Intensive Care Units, Neonatal.mp. or newborn intensive care/ (1912)
1 neonatal intensive care.mp (4894)
2 infant, newborn.mp (53119)
3 cup feeding.mp (27)
4 1 and 3 (7)
5 2 and 3 (20)
6 cup feeding AND newborns (4)
7 cup feeding AND Neonatal (17)
8 cup feeding AND maternity units (3)
9 cup feeding AND postnatal (3)
10 cup feeding AND oral feeding (13)
11 cup feeding AND bottle feeding (40)
Appendix 2. Search strategy for MEDLINE
Intensive Care Units, Neonatal.mp. or newborn intensive care/ (3771)
1 neonatal intensive care.mp (10736)
2 infant, newborn.mp (462199)
3 cup feeding.mp (33)
4 1 and 3 (6)
5 2 and 3 (21)
6 cup feeding AND newborns (2)
7 cup feeding AND Neonatal (10)
8 cup feeding AND maternity units (0)
9 cup feeding AND postnatal (2)
10 cup feeding AND oral feeding (22)
11 cup feeding AND bottle feeding (45)
Appendix 3. Search strategy for http://clinicaltrials.gov
# cup feeding AND infants ‐ result 7
# cup feeding AND newborns ‐ result 1
Appendix 4. Search strategy for http://current controlled‐trials.com
# cup feeding AND infants ‐ result 0
# cup feeding AND newborns ‐ result 0
# cup feeding ‐ result 1
Appendix 5. Search strategy for International Clincial Trial Registry Platform (http://who.int/ictrp/en)
# cup feeding AND newborns ‐ result 0
# cup feeding AND infants ‐ result 1
# cup feeding ‐ result 2
Appendix 6. Risk of bias tool
1. Sequence generation: was the allocation sequence adequately generated? For each included study we described the method used to generate the allocation sequence. We assessed the methods as:
low risk (any truly random process, e.g. random number table; computer random number generator);
high risk (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk.
2. Allocation concealment: Was allocation adequately concealed? For each included study, we described the method used to conceal the allocation sequence and determined whether intervention allocation could have been foreseen in advance of, or during recruitment, of changed after assignment. We assessed the methods as:
low risk (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk.
3. Blinding of participants, personnel and outcome assessors: was knowledge of the allocated intervention adequately prevented during the study? Of the four included studies blinding of the intervention was not possible for participants or personnel. However blinded assessment of some outcome measures were possible.
4. Incomplete outcome data: were incomplete outcome data adequately addressed? For each included study and for each outcome, we described the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. We assessed the methods as:
low risk;
high risk;
unclear risk.
5. Selective outcome reporting: are reports of the study free of suggestion of selective outcome reporting? For each included study we described how we examined the possibility of selective outcome reporting bias and what we found. We assessed the methods as:
low risk (where it is clear that all of the study's pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk (where not all the study's pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk.
6. Other sources of bias: Was the study apparently free of other problems that could put it at a high risk of bias? For each included study, we described any important concerns regarding other possible sources of bias. We assessed whether each study was free of other problems that could put it at risk of bias:
low risk;
high risk;
unclear risk.
Data and analyses
Comparison 1. Supplemental feed using cup versus bottle.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Weight gain (g/kg/day) | 1 | 78 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐3.21, 2.01] |
| 2 Weight gain in first 7 days of study (g/day) | 1 | 522 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.36, 0.16] |
| 3 Not breastfeeding at hospital discharge | 4 | 957 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.49, 0.85] |
| 4 Not breastfeeding at three months | 3 | 883 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.71, 0.97] |
| 5 Not breastfeeding at six months | 2 | 803 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.72, 0.95] |
| 6 Not fully breastfeeding at hospital discharge | 4 | 893 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.52, 0.71] |
| 7 Not fully breastfeeding at three months | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8 Not fully breastfeeding at six months | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9 Average time per feed (minutes) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 10 Length of stay (days) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 11 Gestational Age at Discharge | 1 | 522 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.54, 0.34] |
1.7. Analysis.
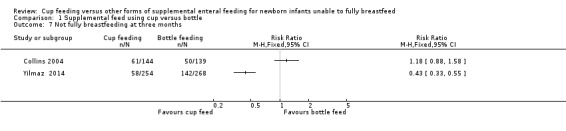
Comparison 1 Supplemental feed using cup versus bottle, Outcome 7 Not fully breastfeeding at three months.
1.8. Analysis.
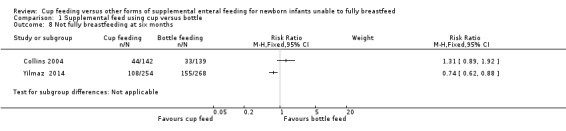
Comparison 1 Supplemental feed using cup versus bottle, Outcome 8 Not fully breastfeeding at six months.
1.9. Analysis.
Comparison 1 Supplemental feed using cup versus bottle, Outcome 9 Average time per feed (minutes).
1.10. Analysis.
Comparison 1 Supplemental feed using cup versus bottle, Outcome 10 Length of stay (days).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Collins 2004.
| Methods | Randomised Controlled Trial. Allocation concealment (blinding of randomisation): yes Blinding of intervention: no Completeness of follow up: yes Blinding of outcome measurement: no | |
| Participants | 319 preterm infants randomised (cup‐feeding group n = 161; bottle‐feeding group n = 158) (mean gestational ages 29.3 and 30.0 weeks); conducted in Australia; involved 2 tertiary hospitals and 54 peripheral hospitals (this number of peripheral hospitals were involved as they were the receiving hospitals for the babies from the tertiary hospitals). Eligibility criteria: preterm infants less than 34 weeks' gestational age whose mothers wanted to breastfeed. | |
| Interventions | Randomised to supplemental feeds via cup or bottle | |
| Outcomes | Not breastfeeding at hospital discharge: number assessed ‐ cup‐feeding group N = 151; bottle‐feeding group N = 152. Not breastfeeding at 3 months: number assessed ‐ cup‐feeding group N = 144; bottle‐feeding group N = 139 Not breastfeeding at 6 months: number assessed ‐ cup‐feeding group N = 142; bottle‐feeding group N = 139 Not fully breastfeeding at hospital discharge: number assessed ‐ cup‐feeding group N = 151; bottle‐feeding group N = 152 Not fully breastfeeding at 3 months: number assessed ‐ cup‐feeding group N = 144; bottle‐feeding group N = 139 Not fully breastfeeding at 6 months: number assessed ‐ cup‐feeding group N = 142; bottle‐feeding group N = 139 Length of hospital stay: number assessed ‐ cup‐feeding group N = 149; bottle‐feeding group N = 152 | |
| Notes | Results are an evaluation of infants followed at 3 and 6 months and not all infants randomised | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "an independent researcher developed a separate randomisation schedule for each recruiting hospital by using a random number table to select balanced blocks of varying size with stratification for gestation" |
| Allocation concealment (selection bias) | Low risk | "assignments were sealed in sequentially numbered opaque envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to the nature of the intervention, participants and personnel cannot be blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "data entry and analysis were undertaken unblinded" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
Gilks 2004.
| Methods | Randomised Controlled Trial Allocation concealment (blinding of randomisation): yes (assignments concealed in sequentially numbered opaque envelopes held in an independent ward to the nursery within each hospital). Blinding of intervention: no Completeness of follow up: yes at hospital discharge; no thereafter Blinding of outcome measurement: no | |
| Participants | 54 preterm infants randomised (cup‐feeding group n = 27; bottle‐feeding group n = 27) (mean gestational ages 31.0 and 32.0 weeks); conducted in the UK; single centre trial Eligibility criteria: preterm infants who were less than 35 weeks' completed gestation and more than 30 weeks' gestation whose mothers intended to breastfeed. | |
| Interventions | Randomised to supplemental feeds via cup or bottle | |
| Outcomes | Not breastfeeding at hospital discharge Not fully breastfeeding at hospital discharge Breast feeding rates: at term; at six weeks post‐term and post‐conceptual age. | |
| Notes | 11 mothers in the cup‐feeding group withdrew prior to discharge and 3 in the bottle‐feeding group yet data have been presented as number assessed at discharge as 27 in each group. Total number assessed for each outcome is unclear. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomised, non‐blinded, stratified controlled trial" |
| Allocation concealment (selection bias) | Unclear risk | "concealed cards in envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to the nature of the intervention, participants and personnel cannot be blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | This was an "unblinded randomised trial" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | More than 25% withdrew from the study |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
Mosley 2001.
| Methods | Randomised Controlled Trial Allocation concealment (blinding of randomisation): yes (concealed cards in envelopes) Blinding of intervention: no Completeness of follow up: yes at hospital discharge; no thereafter Blinding of outcome measurement: yes | |
| Participants | 16 preterm infants (cup‐feeding group n = 8; bottle‐feeding group n = 8) (mean gestational age of 35.2 to 35.5 weeks); conducted in the UK; single centre trial Eligibility criteria: preterm infants who were between 30 and 37 weeks' gestation, admitted to the special care nursery, whose mothers intended to breastfeed. | |
| Interventions | Randomised to supplemental feeds via cup or bottle | |
| Outcomes | Not fully breastfeeding at hospital discharge: number assessed ‐ cup‐feeding group N = 6; bottle‐feeding group N = 8. | |
| Notes | 2 infants (randomised to cup‐feeding group) were excluded as they had been given a supplementary feed prior to starting cup feeds. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "control and intervention details (10 of each) placed in envelopes and then shuffled thoroughly and then the envelopes were numbered sequentially" |
| Allocation concealment (selection bias) | Low risk | "sealed, sequentially numbered, opaque envelope" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to the nature of the intervention, participants and personnel cannot be blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Blinded assessment of outcome was undertaken by a midwife who was unaware of the study's purpose" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
Rocha 2002.
| Methods | Randomised Controlled Trial Allocation concealment (blinding of randomisation): yes (sealed numbered opaque envelope) Blinding of intervention: no Completeness of follow up: yes at hospital discharge; no thereafter Blinding of outcome measurement: no | |
| Participants | 83 preterm infants (cup‐feeding group n = 46; bottle feeding‐group n = 37) (mean gestational age of 32.5 to 32.7 weeks); conducted in Brazil; single centre trial Eligibility criteria: preterm infants who were born between 32 and 36 weeks' gestation, and weighing less than 1700 g, admitted to the intensive care nursery, whose mothers intended to breastfeed. | |
| Interventions | Randomised to supplemental feeds via cup or bottle | |
| Outcomes | Not breastfeeding at hospital discharge: number assessed ‐ cup‐feeding group N = 44; bottle‐feeding group N = 34. Not breastfeeding at 3 months: number assessed ‐ cup‐feeding group N = 44; bottle‐feeding group N = 34. Weight gain: number assessed ‐ cup‐feeding group N = 44; bottle‐feeding group N = 34. Average time per feed: number assessed ‐ cup‐feeding group N = 44; bottle‐feeding group N = 34. Other outcomes assessed: differences between oxygen saturation levels in bottle, cup and breastfed infants. | |
| Notes | 3 infants excluded from the bottle‐feeding group for medical and social reasons and 2 from the cup‐feeding group due to break of protocol | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "controlled experimental study with stratified randomisation" "within each stratum, the infants were randomly assigned to 1 of 2 feeding groups by drawing lots" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to the nature of the intervention, participants and personnel cannot be blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not clearly stated, report on only those that attended the outpatient clinic. |
| Selective reporting (reporting bias) | High risk | Do not report outcomes for each of the monthly visits |
Yilmaz 2014.
| Methods | Randomised Controlled Trial
Allocation concealment (blinding of randomisation): yes (sequentially numbered, opaque envelopes
Blinding of intervention: no
Completeness of follow up: yes at hospital discharge, data collected at 3 and 6 months Eligibility criteria: preterm infants were singletons, between 32 to 35 weeks' gestation, maternal intention to breastfeed, no supplemental oxygen and being fed intermittently by a nasogastric tube. The calculated sample size was not achieved. |
|
| Participants | Preterm infants of 32 to 35 weeks' gestation fed only by intermittent gastric tube at the time of recruitment; mean gestational ages 32.8 and 32.8 weeks; 607 infants were randomly assigned to 2 groups: the cup‐fed group (n = 299) and bottle‐fed group (n = 308). Conducted in Turkey in 3 neonatal intensive care units. | |
| Interventions | Randomised to cup or bottle supplemental feeding. | |
| Outcomes | Weight gain (gram per day) at day 7 of the study: number assessed ‐ cup feeding N = 254, bottle feeding N = 268 Exclusive Breasfeeding at discharge: number assessed ‐ cup feeding N = 254, bottle feeding N = 268 Exclusive breastfeeding at 3 months: number assessed ‐ cup feeding N = 254, bottle feeding N = 268 Exclusive breastfeeding at 6 months: number assessed ‐ cup feeding N = 254, bottle feeding N = 268 Length of stay: number assessed ‐ cup feeding N = 254, bottle feeding N = 268 Feeding time, min/feeding in the first week of life: number assessed ‐ cup feeding N = 254, bottle feeding N = 268 Gestational age at discharge: number assessed ‐ cup feeding N = 254, bottle feeding N = 268 |
|
| Notes | The primary author was contacted to confirm that 100% of infants were followed up at 3 and 6 months for any breastfeeding and exclusive breastfeeding. The primary author confirmed via e‐mail that 100% of infants were followed up at 3 and 6 months for any breastfeeding and exclusive breastfeeding. Initiation of cup feeding commenced when infants were 35 weeks. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | An Independent researcher developed a separate randomisation schedule for each recruiting hospital by using a random number table to select balanced blocks and stratification for gestation. |
| Allocation concealment (selection bias) | Low risk | Sealed sequentially numbered opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to the nature of the intervention, participants and personnel cannot be blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Researcher recorded the duration of feeds whilst in hospital and collected follow‐up questionnaires and interview data for all infants. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | It is not clearly described that 100% follow‐up was achieved for mothers and infants at 3 and 6 months. Also data on number of infants by gestational age group stratification is not reported. |
| Selective reporting (reporting bias) | High risk | Weight and breastfeeding status were to be recorded at follow‐up but weight data was not reported post 7 days. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abouelfettoh 2008 | Was neither a randomised controlled trial nor a non‐randomised controlled trial using quasi‐randomised group allocation. A quasi‐experimental cohort design. |
| Al‐Sahab 2010 | Was neither a randomised controlled trial nor a non‐randomised controlled trial using quasi‐randomised group allocation. A cross‐sectional study. |
| Aloysius 2007 | Was neither a randomised controlled trial nor a non‐randomised controlled trial using quasi‐randomised group allocation. An observational study. |
| Brown 1999 | Was neither a randomised controlled trial nor a non‐randomised controlled trial using quasi‐randomised group allocation. A retrospective chart review. |
| Davis 1948 | Was neither a randomised controlled trial nor a non‐randomised controlled trial using quasi‐randomised group allocation. |
| Dowling 2001 | Was neither a randomised controlled trial nor a non‐randomised controlled trial using quasi‐randomised group allocation. Descriptive literature on nipple confusion and alternative feeding methods. |
| Dowling 2002 | Was neither a randomised controlled trial nor a non‐randomised controlled trial using quasi‐randomised group allocation. A non‐experimental convenience sample. |
| Fredeen 1948 | Was neither a randomised controlled trial nor a non‐randomised controlled trial using quasi‐randomised group allocation. A descriptive report on experience with cup feeding of newborn infants. |
| Freer 1999 | Was neither a randomised controlled trial nor a non‐randomised controlled trial using quasi‐randomised group allocation. A convenience sample of newborn infants exposed to breast and cup feeding. |
| Gupta 1999 | Was neither a randomised controlled trial nor a non‐randomised controlled trial using quasi‐randomised group allocation. A retrospective chart review. |
| Howard 1999 | A randomised controlled trial of formula feeding infants only. Infants randomised to either receive feeds via cup or bottle. A group of exclusively breastfeeding infants were used as a comparison group. |
| Howard 2003 | This study did not meet the inclusion criteria for this review as participants were not infants that were unable to fully breastfeed. Participants were infant‐mother dyads. Unborn infants were randomised on maternal admission to either early or late pacifier use or cup or bottle supplemental feeding if required. A large proportion of the babies randomised were part of the study because of maternal choice to offer supplemental feeds not because the infants were unable to fully breastfeed. |
| Huang 2009 | Was neither a randomised controlled trial nor a non‐randomised controlled trial using quasi‐randomised group allocation. A prospective longitudinal study. |
| Ize‐Iyamu 2011 | This study did not meet the inclusion criteria for this review as participants received supplements by either a syringe (intervention group) or cup and spoon (control group). The intervention did not include supplementation solely via a cup. |
| Malhotra 1999 | Was neither a randomised controlled trial nor a non‐randomised controlled trial using quasi‐randomised group allocation. Cross‐over design was employed. |
| Marinelli 2001 | Was neither a randomised controlled trial nor a non‐randomised controlled trial using quasi‐randomised group allocation. Cross‐over design was employed. |
| Schubiger 1997 | This study did not meet the inclusion criteria for this review as participants received supplements by either cup or spoon (intervention group) or by bottle after breastfeeding (control group). Data were not presented for those supplemented by cup alone and therefore we were unable to elicit data for those infants who received supplementation solely via a cup. |
Characteristics of studies awaiting assessment [ordered by study ID]
NCT00703950.
| Methods | Randomised block design |
| Participants | Infants: very low birthweight, < 1500 grams and between 26 and 32 weeks' gestation. Enrolled 56 infants |
| Interventions | Cup feeding and bottle feeding |
| Outcomes | Primary outcome: Sucking patterns after use of bottle or cup Secondary outcomes: Weight gain, days of life to begin full oral feeding, length of hospital stay and breast feeding rates |
| Notes | Study details listed at URL: ClinicalTrials.gov/show/NCT00703950. Study completion date: December 2013. Author contacted 28 February 2016 |
Puapornpong 2015.
| Methods | Simple randomisation method |
| Participants | Newborns weighing > 2500 grams with no complications |
| Interventions | Feeding tube (intervention group), cup feedings (control group) |
| Outcomes | Latch scores were significantly higher in the tube‐feeding group |
| Notes | Author contacted on 28 February 2016, awaiting further clarification on randomisation and data |
Differences between protocol and review
We added the methodology and plan for 'Summary of findings' tables and GRADE recommendations, which were not included in the original protocol nor in the last version of the review.
Contributions of authors
Conceiving the review ‐ AF, KN Data collection for the review ‐ AF, KN Designing search strategies ‐ AF, KN, MWD Undertaking searches ‐ AF, KN, MWD Screening search results ‐ AF, KN Organising retrieval of papers ‐ AF, KN Screening retrieved papers against inclusion criteria ‐ AF, KN Appraising quality of papers ‐ AF, KN Extracting data from papers ‐ AF, KN Writing to authors of papers for additional information ‐ AF, KN Entering data into RevMan ‐ AF, KN Analysis of data ‐ AF, KN Interpretation of data ‐ AF, KN, MWD Writing the review ‐ AF, KN Revising review ‐ MWD Providing general advice on the review ‐ MWD
Sources of support
Internal sources
Grantley Stable Neonatal Unit, Royal Brisbane & Women's Hospital, Brisbane, Australia.
Dept of Paediatrics and Child Health, University of Queensland, Brisbane, Australia.
External sources
-
Eunice Kennedy Shriver National Institute of Child Health and Human Development National Institutes of Health, Department of Health and Human Services, USA.
Editorial support of the Cochrane Neonatal Review Group has been funded with Federal funds from the Eunice Kennedy Shriver National Institute of Child Health and Human Development National Institutes of Health, Department of Health and Human Services, USA, under Contract No. HHSN275201100016C
-
National Institute for Health Research, UK.
Editorial support for Cochrane Neonatal has been funded with funds from a UK National Institute of Health Research Grant (NIHR) Cochrane Programme Grant (13/89/12). The views expressed in this publication are those of the authors and not necessarily those of the NHS, the NIHR, or the UK Department of Health.
Declarations of interest
None
Edited (no change to conclusions)
References
References to studies included in this review
Collins 2004 {published data only}
- Collins CT, Ryan P, Crowther CA, McPhee AJ, Paterson S, Hiller JE. Effect of bottles, cups and dummies on breast feeding in preterm infants: a randomised controlled trial. BMJ 2004 June 18 [Epub ahead of print]. [PUBMED: 15208209] [DOI] [PMC free article] [PubMed]
Gilks 2004 {published data only}
- Gilks J, Watkinson M. Improving breast feeding in preterm babies: Cup feeding versus bottle feeding. Journal of Neonatal Nursing 2004;10:118‐20. [Google Scholar]
Mosley 2001 {published data only}
- Mosley C, Whittle C, Hicks C. A pilot study to assess the viability of a randomised controlled trial of methods of supplementary feeding of breast‐fed pre‐term babies. Midwifery 2001;17(2):150‐7. [PUBMED: 11399136] [DOI] [PubMed] [Google Scholar]
Rocha 2002 {published data only}
- Rocha NM, Martinez FE, Jorge SM. Cup or bottle for preterm infants: effects on oxygen saturation, weight gain, and breastfeeding. Journal of Human Lactation 2002;18(2):132‐8. [PUBMED: 12033074] [DOI] [PubMed] [Google Scholar]
Yilmaz 2014 {published data only}
- Yilmaz G, Caylan N, Karacan CD, Bodur I, Gokcay G. Effect of cup feeding and bottle feeding on breastfeeding in late preterm infants: a randomized controlled study. Journal of Human Lactation 2014;30(1):174‐179. [PUBMED: 24442532] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abouelfettoh 2008 {published data only}
- Abouelfettoh AM, Dowling DA, Dabash AS, Elguindy SR, Seoud IA. Cup verus bottle feeding for hospitalized late preterm infants in Egypt: A quasi‐experimental study. International Breastfeeding Journal 2008;3:27. [PUBMED: 19025602] [DOI] [PMC free article] [PubMed] [Google Scholar]
Aloysius 2007 {published data only}
- Aloysius A, Hickson M. Evaluation of paladai cup feeding in breast‐fed preterm infants compared with bottle feeding. Early Human Development 2007;83(9):619‐21. [PUBMED: 17289306] [DOI] [PubMed] [Google Scholar]
Al‐Sahab 2010 {published data only}
- Al‐Sahab B, Feldman M, Macpherson A, Ohlsson A, Tamim H. Which method of breastfeeding supplementation is best? The beliefs and practices of paediatricians and nurses. Paediatrics & Child Health 2010;15(7):427‐31. [PUBMED: 21886446] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brown 1999 {published data only}
- Brown SJ, Alexander J, Thomas P. Feeding outcome in breast‐fed term babies supplemented by cup or bottle. Midwifery 1999;15(2):92‐6. [PUBMED: 10703411] [DOI] [PubMed] [Google Scholar]
Davis 1948 {published data only}
- Davis HV, Sears RR, Miller HC, Brodbeck AJ. Effects of cup, bottle and breast feeding on oral activities of newborn infants. Pediatrics 1948;2(5):549‐58. [PUBMED: 18893012] [PubMed] [Google Scholar]
Dowling 2001 {published data only}
- Dowling DA, Thanattherakul W. Nipple confusion, alternative methods, and breast‐feeding supplementation: State of the science. Newborn and Infant Nursing Reviews 2001;1:217‐23. [Google Scholar]
Dowling 2002 {published data only}
- Dowling DA, Meier PP, DiFiore JM, Blatz M, Martin RJ. Cup‐feeding for preterm infants: Mechanics and safety. Journal of Human Lactation 2002;18(1):13‐20. [PUBMED: 11845732] [DOI] [PubMed] [Google Scholar]
Fredeen 1948 {published data only}
- Fredeen RC. Cup feeding of newborn infants. Pediatrics 1948;2(5):544‐8. [PUBMED: 18893011] [PubMed] [Google Scholar]
Freer 1999 {published data only}
- Freer Y. A comparison of breast and cup feeding in preterm infants: Effects on physiological parameters. Journal of Neonatal Nursing 1999;5:16‐21. [Google Scholar]
Gupta 1999 {published data only}
- Gupta A, Khanna K, Chattree S. Cup feeding: An alternative to bottle feeding in a neonatal intensive care unit. Journal of Tropical Pediatrics 1999;45(2):108‐10. [PUBMED: 10341507] [DOI] [PubMed] [Google Scholar]
Howard 1999 {published data only}
- Howard CR, Blieck EA, Hoopen CB, Howard FM, Lanphear BP, Lawrence RA. Pyhsiologic stability of newborns during cup and bottle feeding. Pediatrics 1999;104(5 Pt 2):1204‐7. [PUBMED: 10545574] [PubMed] [Google Scholar]
Howard 2003 {published data only}
- Howard CR, Howard FM, Lanphear BP, Ederly S, Blieck EA, Oakes D, et al. Randomized clinical trial of pacifier use and bottle‐feeding or cupfeeding and their effect on breastfeeding. Pediatrics 2003;111(3):511‐18. [PUBMED: 12612229] [DOI] [PubMed] [Google Scholar]
Huang 2009 {published data only}
- Huang YY, Gau ML, Huang CM, Lee JT. Supplementation with cup feeding as a substitute for bottle feeding to promote breastfeeding. Chang Gung Medical Journal 2009;32(4):423‐31. [PUBMED: 19664349] [PubMed] [Google Scholar]
Ize‐Iyamu 2011 {published data only}
- Ize‐Iyamu IN, Saheeb BD. Feeding intervention in cleft lip and palate babies: a practical approach to feeding efficency and weight gain. International Journal Oral Maxillofacial Surgery 2011;40(9):916‐9. [DOI] [PubMed] [Google Scholar]
Malhotra 1999 {published data only}
- Malhotra N, Vishwambaran L, Sundaram KR, Narayanan I. A controlled trial of alternative methods of oral feeding in neonates. Early Human Development 1999;54(1):29‐38. [PUBMED: 10195713] [DOI] [PubMed] [Google Scholar]
Marinelli 2001 {published data only}
- Marinelli KA, Burke GS, Dodd VL. A comparison of the safety of cupfeedings and bottlefeedings in premature infants whose mothers intend to breastfeed. Journal of Perinatology 2001;21(6):350‐5. [PUBMED: 11593367] [DOI] [PubMed] [Google Scholar]
Schubiger 1997 {published data only}
- Schubiger G, Schwarz U, Tonz O. UNICEF/WHO baby‐friendly hospital initiative: does the use of bottles and pacifiers in the neonatal nursery prevent successful breastfeeding?. European Journal of Pedicatrics 1997;156(11):874‐7. [PUBMED: 9392404] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
NCT00703950 {unpublished data only}
- Lopas Moreira, M. Sucking Pattern of Preterm Infants Using Cup or Bottle Before Breastfeeding. https://ClinicalTrials.gov/show/NCT00703950 10 August 2015. [ClinicalTrials.gov Identifier:NCT00703950]
Puapornpong 2015 {published data only}
- Puapornpong P, Raungrongmorakot K, Hemachandra A, Ketsuwan S, Wongin S. Comparisons of Latching on between Newborns Fed with Feeding Tubes and CupFeedings. Journal of the Medical Association of Thailand 2015;98(Suppl 9):s61‐5. [PUBMED: 26817211] [PubMed] [Google Scholar]
Additional references
Cousins 1999
- Cousins R. Breast feeding the preterm infant in the special care baby unit: The first feed. Journal of Neonatal Nursing 1999;5:10‐14. [Google Scholar]
Foote 1944
- Foote HS. Silver in the service of medicine. Bulletin of the Medical Library Association 1944;32(3):369‐75. [PMC free article] [PubMed] [Google Scholar]
GRADEpro 2008 [Computer program]
- Brozek J, Oxman A, Schünemann H. GRADEpro [Version 3.2 for Windows]. The GRADE Working Group, 2008.
Han 2010
- Han AM. Cup‐feeding versus other forms of supplemental enteral feeding for newborn infants unable to fully breastfeed: RHL commentary. The WHO Reproductive Health Library. http://apps.who.int/rhl/newborn/cd005092_hanam_com/en/ (last revised: 1 August 2010).
Higgins 2002
- Higgins JP, Thompson SG. Quantifying heterogeneity in meta‐analysis. Statistics Medicine 2002;21(11):1539‐58. [PUBMED: 12111919] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Altman DG, Stern JA. Chapter 8: Assessing risk of bias in included studies. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. Available from www.cochrane‐handbook.org. The Cochrane Collaboration, 2011. [Google Scholar]
Kuehl 1997
- Kuehl J. Cup feeding the newborn: what you should know. The Journal of Perinatal & Neonatal Nursing 1997;11(2):56‐60. [PUBMED: 9391366] [DOI] [PubMed] [Google Scholar]
Lang 1994a
- Lang S. Cup‐feeding: An alternative method. Midwives Chronicle 1994;107(1276):171‐6. [PUBMED: 8007850] [PubMed] [Google Scholar]
Lang 1994b
- Lang S, Lawrence CJ, Orme RL. Cup feeding: An alternative method of infant feeding. Archives of Disease in Childhood 1994;71(4):365‐9. [PUBMED: 7979537] [DOI] [PMC free article] [PubMed] [Google Scholar]
NANN 2004
- National Association of Neonatal Nurses (NANN). Position Statement #3017: Cup and finger feeding of breast milk. www.nann.org/files/public/ (accessed 12 August 2004).
Palmer 1993
- Palmer MM. Identification and management of the transitional suck pattern in premature infants. The Journal of Perinatal & Neonatal Nursing 1993;7(1):66‐75. [PUBMED: 8336292] [DOI] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editors. GRADE Working Group. GRADE handbook for grading quality of evidence and strength of recommendations. Available from www.guidelinedevelopment.org/handbook Updated October 2013.
Thorley 1997
- Thorley V. Cup feeding: Problems created by incorrect use. Journal of Human Lactation 1997;13(1):54‐5. [PUBMED: 9233187] [DOI] [PubMed] [Google Scholar]
Thorley 2004
- Thorley V. Cup‐feeding. www.breastfeeding.asn.au/bfinfo/cupfeeding (accessed 12 August 2004).
Vallenas 1998
- Vallenas C, Savage F. Evidence for the ten steps to successful breastfeeding. Division of Child Health and Development, World Health Organization 1998.
WHO 1998
- Anonymous. Evidence for the Ten Steps to successful breastfeeding. Geneva: World Health Organisation, 1998. [Google Scholar]
References to other published versions of this review
Flint 2007
- Flint A, New K, Davies MW. Cup feeding versus other forms of supplemental enteral feeding for newborn infants unable to fully breastfeed. Cochrane Database of Systematic Reviews 2007, Issue 2. [DOI: 10.1002/14651858.CD005092.pub2] [DOI] [PubMed] [Google Scholar]


