Abstract
Bilateral renal infarction is a rare phenomenon which can be difficult to diagnose because the symptoms may often mimic renal calculi, infection, muscle inflammation, genital diseases, myocardial infarction, or ischemia. We present the case of a 55-year-old male patient who presented with non-radiating, left-sided flank pain associated with nausea and vomiting. A computed tomography (CT) scan of the abdomen and pelvis with contrast demonstrated bilateral renal infarction. A thorough workup was initiated, and the thrombus formation due to left atrial enlargement from hypertrophic obstructive cardiomyopathy was considered as the cause of the bilateral renal infarction in this patient. The patient's renal function improved with treatment, and she was discharged on an anticoagulant, considering her left atrial enlargement and renal infarction.
Keywords: acute kidney injury, bilateral renal infarction, cardiogenic etiology, bilateral renal infarction, hypertrophic obstructive cardiomyopathy, renal blood flow
Introduction
Acute renal infarction is a rare entity with variable and possibly misleading manifestations. Misdiagnosis may lead to a delay in treatment resulting in permanent renal impairment or increased risk of embolism in other organs [1]. In previous studies, the incidence of renal infarction has been estimated to be 0.004% to 0.007% based on admissions to the emergency department, while the estimated prevalence in autopsy studies was 14 per 1,000 [1-3]. The infarction can result from the blockage of arterial or venous drainage, with compromised arterial supply being far more common than venous abnormalities. The incidence is higher in patients with atherosclerosis, kidney damage at baseline (nephritic syndrome, glomerulonephritis), fibromuscular disease, aneurysms, and dissections of the renal artery. Bilateral infarction was reported to be found in dissecting aneurysms of the aorta, septic emboli from endocarditis, lupus, vasculitis, sickle cell disease, or with fibromuscular dysplasia of the renal arteries [4-5]. Renal infarction in patients with a patent foramen ovale secondary to paradoxical emboli has also been reported in the literature. Herein, we report the case of a 55-year-old male diagnosed with a bilateral renal infarction caused by left atrial enlargement from hypertrophic obstructive cardiomyopathy (HOCM). To our knowledge, this is the first reported case of a bilateral renal infarction in a patient with hypertrophic obstructive cardiomyopathy.
Case presentation
A 55-year-old male with a history of hypertension and HOCM presented to the emergency department with the acute onset of sharp, non-radiating, left-sided flank pain associated with nausea and vomiting. On admission, his vital signs were unremarkable. Physical exam was significant for a Grade III/VI systolic murmur, loudest at the apex, with no radiation. Marked tenderness on superficial palpation of the left inferior costal margin was present. There was no rebound tenderness, no costovertebral angle tenderness, and no abdominal or flank erythema. Lab workup demonstrated leukocytosis at 13,000 and acute kidney injury (creatinine: 1.3 mg/dl from a baseline of 0.7 mg/dl). Urinalysis was positive for hematuria, whereas urine toxicology was negative for any illicit substances. Computed tomography (CT) scan of the abdomen and pelvis without contrast showed no evidence of nephrolithiasis. CT scan of the abdomen and pelvis with contrast demonstrated bilateral segmental hypoperfusion indicative of a bilateral renal infarction, the left greater than the right, with no evidence of hydronephrosis (Figure 1). An electrocardiogram (EKG) upon admission showed a normal sinus rhythm with no evidence of infarction, ischemia, or atrial fibrillation. The patient was started on a heparin drip soon after the infarction was noted. Further workup ruled out infection, a hypercoagulable state (anti-cardiolipin antibody, perinuclear antineutrophil cytoplasmic antibodies (P-ANCA), cytoplasmic antineutrophil cytoplasmic antibodies (C-ANCA), protein C, protein S, antithrombin antibody, and Factor V Leiden), autoimmune etiology, sickle cell disease, patent foramen ovale, and arrhythmias. A transthoracic echocardiogram (TTE) showed hyperdynamic left ventricle systolic function, a moderately dilated left atrium at 54 mm, and mild thickening of the anterior and posterior mitral valve leaflets. Later, transesophageal echocardiography (TEE) was performed which showed a peak subvalvular gradient around 20 mmHg with no obvious masses or vegetation. A small rupture in the subvalvular chord and a left ventricular outflow tract (LVOT) obstruction was also observed (Figure 2). Different blood cultures were obtained throughout the hospital stay and no microbial organism was isolated, including bacteria, fungus, or acid-fast bacilli. Serologic antibody titers for Bartonella, Rickettsia, and M. pneumoniae were also negative. No obvious source of embolic origin was identified on echocardiogram and imaging of the renal arteries. The patient was placed on telemetry throughout his hospital course, and there was no evidence of any underlying arrhythmia, such as atrial fibrillation. However, it was presumed that the left atrial enlargement might be a predisposing factor to thromboembolic renal infarction via the same mechanism by which it predisposes to stroke [6]. The patient's renal function improved with treatment, and he was discharged on an anticoagulant, considering his left atrial enlargement and renal infarction.
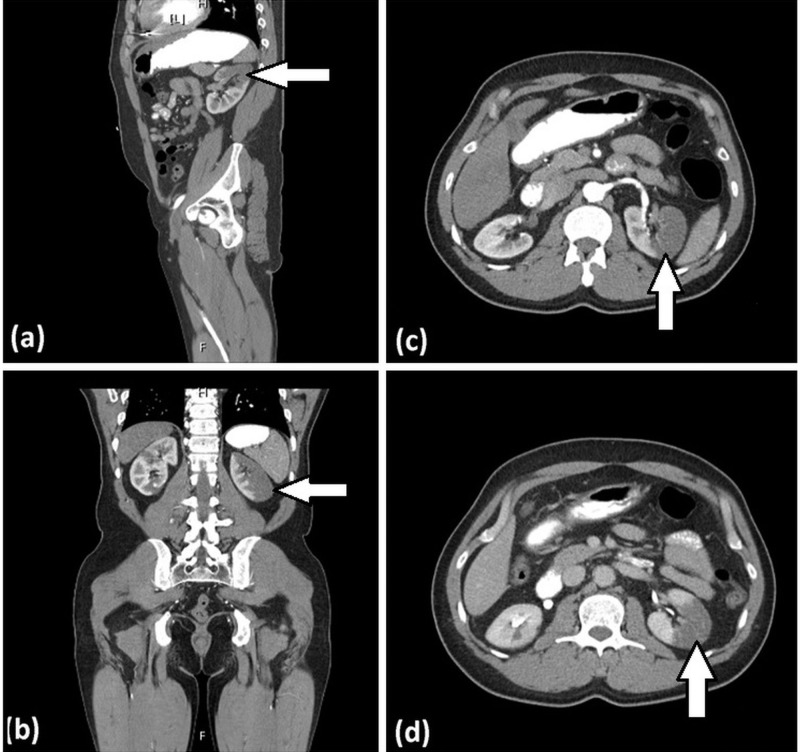
Figure 1. Computed tomography (CT) of the abdomen and pelvis with contrast.
Figure 1 shows a bilateral renal infarction. The arrows depict filling defects in the (a) sagittal, (b) coronal, and (c, d) axial images of the CT scan of the abdomen and pelvis with contrast.
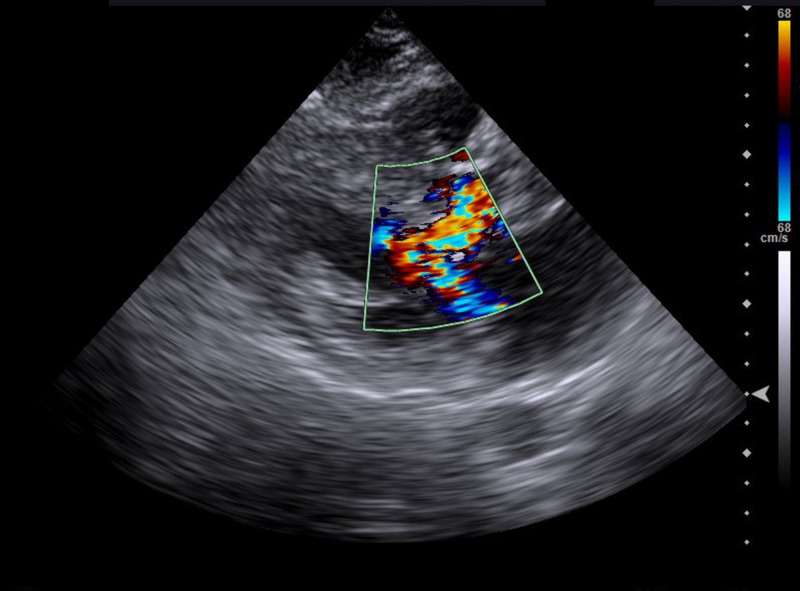
Figure 2. Color Doppler echocardiography.
Color Doppler echocardiography (systolic frame) shows marked turbulence in the left ventricular outflow tract (LVOT) and a posteriorly directed jet of mitral regurgitation. LVOT during systole is due to systolic anterior motion (SAM) of the mitral valve, resulting in a Venturi effect.
Discussion
Herein, we have presented a case of bilateral renal infarction and HOCM. The diagnosis of renal infarction may be difficult or delayed because of the rarity of the disorder and the non-specific signs and symptoms with which it presents. Symptoms can be misleading and mimic other pathology, including renal colic, mesenteric ischemia, spinal disorders, genitourinary disorders, and muscular strain. Based on the etiology, four main types of renal infarction were proposed: cardiac origin, renal artery injury, hypercoagulable state, and idiopathic [1, 7]. Renal infarction commonly results from thromboembolic events related to dysrhythmias or cardiac structural disease. The most common cause is cardiac in origin, such as secondary to atrial fibrillation. Vegetations in infective endocarditis and cardiac tumors are other possible sources of emboli.
Patients with renal infarction generally complain of flank or back pain which may be accompanied by nausea/vomiting or hematuria, along with fever. Fevers have been reported in about half of the reported cases of renal infarction [8]. Our patient’s hospital course was complicated by an acute kidney injury, fevers up to 39 degrees C, and worsening leukocytosis, all of which were attributed to renal infarction and inflammatory state. These laboratory and objective parameters improved throughout his hospital stay, and their improvement corresponded with improvement in the patient’s pain.
Vascular and hypercoagulable causes for the bilateral renal infarction were ruled out after an extensive non-revealing workup. In our patient, no obvious source of embolic origin was identified on echocardiogram nor renal arterial imaging. He had no history of atrial dysrhythmia or any events on cardiac telemetry. A TEE did reveal a ruptured subvalvular chord, which might have caused an injured endothelial surface serving as a nidus for a thrombus to form and embolize bilaterally. Another plausible cause could be attributed to the severely dilated left atrium to 54 mm from the HOCM. It is known that patients with enlargement of the left atrium are prone to develop various complications, including thrombus formation and thromboembolic events. This is because an enlarged left atrium is associated with the stasis of blood, promoting thrombus formation [1]. The risk of thromboembolism increases with increasing left atrial size regardless of the degree of anticoagulation. A review of patients from the Framingham Heart Study revealed that left atrial enlargement was a significant predictor of stroke in men [6]; thus, it is possible that left atrial enlargement is a predisposing factor to thromboembolic renal infarction via the same mechanisms by which it predisposes to stroke.
To our knowledge, there has not been a case of bilateral renal infarction in a patient with underlying HOCM reported in the literature. It appears that left atrial enlargement in a patient with HOCM increases the risk of peripheral embolism. The risk is heightened in the presence of a known bilateral renal infarction. Thus, we believed it was prudent to anticoagulate our patient. He was discharged on Rivaroxaban to reduce the likelihood of future embolic sequelae and was prescribed to take it indefinitely, given the high likelihood of a primary thromboembolic event being the cause of his renal infarction.
Conclusions
Renal infarction is a rare entity and has various presenting symptoms which can make the diagnosis difficult. It is important to obtain a detailed history, perform a thorough physical exam, and obtain diagnostic imaging to establish the diagnosis early as any delay in treatment can result in irreversible renal impairment. To our knowledge, no cases of bilateral renal infarction secondary to thromboembolic disorders in the absence of cardiac dysrhythmia have been reported in the literature. Given the findings of the left atrial enlargement, HOCM, and bilateral renal infarction, we believe it was essential for our patient to be discharged on a lifelong anticoagulant to decrease the likelihood of future embolic sequelae.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained by all participants in this study
References
- 1.Acute renal infarction: a case series. Bourgault M, Grimbert P, Verret C, et al. Clin J Am Soc Nephrol. 2013;8:392–398. doi: 10.2215/CJN.05570612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.ED presentations of acute renal infarction. Huang CC, Lo HC, Huang HH, et al. Am J Emerg Med. 2007;25:164–169. doi: 10.1016/j.ajem.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 3.Bilateral renal artery thrombosis in inherited thrombophilia: a rare cause of acute kidney injury. [Jan;2019 ];Wiles KS, Hastings L, Muthuppalaniappan VM, Hanif M, Abeygunasekara S. Int J Nephrol Renovasc Dis. 2014 7:35–38. doi: 10.2147/IJNRD.S50948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Renal infarction in the ED: 10-year experience and review of the literature. Antopolsky M, Simanovsky N, Stalnikowicz R, Salameh S, Hiller N. Am J Emerg Med. 2012;30:1055–1060. doi: 10.1016/j.ajem.2011.06.041. [DOI] [PubMed] [Google Scholar]
- 5.The clinical spectrum of acute renal infarction. Korzets Z, Plotkin E, Bernheim J, Zissin R. https://pdfs.semanticscholar.org/8630/b6eece600aaa1aa94ed6fa0e179906c8655d.pdf. Isr Med Assoc J. 2002;4:781–784. [PubMed] [Google Scholar]
- 6.Left atrial size and the risk of stroke and death. The Framingham Heart Study. Benjamin EJ, D'Agostino RB, Belanger AJ, Wolf PA, Levy D. Circulation. 1995;92:835–841. doi: 10.1161/01.cir.92.4.835. [DOI] [PubMed] [Google Scholar]
- 7.Renal infarction due to combination of fibromuscular dysplasia and factor V Leiden mutation. Kirchgatterer A, Lugmayr H, Aspöck G, Wallner M, Knoflach P. Nephrol Dial Transplant. 2004;19:512–513. doi: 10.1093/ndt/gfg536. [DOI] [PubMed] [Google Scholar]
- 8.Bilateral renal infarction following atrial fibrillation and thromboembolism and presenting as acute abdominal pain: a case report. Bouassida K, Hmida W, Zairi A, et al. J Med Case Rep. 2012;6:153. doi: 10.1186/1752-1947-6-153. [DOI] [PMC free article] [PubMed] [Google Scholar]