Abstract
Background
There is extensive evidence of important health risks for infants and mothers related to not breastfeeding. In 2003, the World Health Organization recommended that infants be breastfed exclusively until six months of age, with breastfeeding continuing as an important part of the infant’s diet until at least two years of age. However, current breastfeeding rates in many countries do not reflect this recommendation.
Objectives
To describe forms of breastfeeding support which have been evaluated in controlled studies, the timing of the interventions and the settings in which they have been used.
To examine the effectiveness of different modes of offering similar supportive interventions (for example, whether the support offered was proactive or reactive, face‐to‐face or over the telephone), and whether interventions containing both antenatal and postnatal elements were more effective than those taking place in the postnatal period alone.
To examine the effectiveness of different care providers and (where information was available) training.
To explore the interaction between background breastfeeding rates and effectiveness of support.
Search methods
We searched Cochrane Pregnancy and Childbirth's Trials Register (29 February 2016) and reference lists of retrieved studies.
Selection criteria
Randomised or quasi‐randomised controlled trials comparing extra support for healthy breastfeeding mothers of healthy term babies with usual maternity care.
Data collection and analysis
Two review authors independently assessed trials for inclusion and risk of bias, extracted data and checked them for accuracy. The quality of the evidence was assessed using the GRADE approach.
Main results
This updated review includes 100 trials involving more than 83,246 mother‐infant pairs of which 73 studies contribute data (58 individually‐randomised trials and 15 cluster‐randomised trials). We considered that the overall risk of bias of trials included in the review was mixed. Of the 31 new studies included in this update, 21 provided data for one or more of the primary outcomes. The total number of mother‐infant pairs in the 73 studies that contributed data to this review is 74,656 (this total was 56,451 in the previous version of this review). The 73 studies were conducted in 29 countries. Results of the analyses continue to confirm that all forms of extra support analyzed together showed a decrease in cessation of 'any breastfeeding', which includes partial and exclusive breastfeeding (average risk ratio (RR) for stopping any breastfeeding before six months 0.91, 95% confidence interval (CI) 0.88 to 0.95; moderate‐quality evidence, 51 studies) and for stopping breastfeeding before four to six weeks (average RR 0.87, 95% CI 0.80 to 0.95; moderate‐quality evidence, 33 studies). All forms of extra support together also showed a decrease in cessation of exclusive breastfeeding at six months (average RR 0.88, 95% CI 0.85 to 0.92; moderate‐quality evidence, 46 studies) and at four to six weeks (average RR 0.79, 95% CI 0.71 to 0.89; moderate quality, 32 studies). We downgraded evidence to moderate‐quality due to very high heterogeneity.
We investigated substantial heterogeneity for all four outcomes with subgroup analyses for the following covariates: who delivered care, type of support, timing of support, background breastfeeding rate and number of postnatal contacts. Covariates were not able to explain heterogeneity in general. Though the interaction tests were significant for some analyses, we advise caution in the interpretation of results for subgroups due to the heterogeneity. Extra support by both lay and professionals had a positive impact on breastfeeding outcomes. Several factors may have also improved results for women practising exclusive breastfeeding, such as interventions delivered with a face‐to‐face component, high background initiation rates of breastfeeding, lay support, and a specific schedule of four to eight contacts. However, because within‐group heterogeneity remained high for all of these analyses, we advise caution when making specific conclusions based on subgroup results. We noted no evidence for subgroup differences for the any breastfeeding outcomes.
Authors' conclusions
When breastfeeding support is offered to women, the duration and exclusivity of breastfeeding is increased. Characteristics of effective support include: that it is offered as standard by trained personnel during antenatal or postnatal care, that it includes ongoing scheduled visits so that women can predict when support will be available, and that it is tailored to the setting and the needs of the population group. Support is likely to be more effective in settings with high initiation rates. Support may be offered either by professional or lay/peer supporters, or a combination of both. Strategies that rely mainly on face‐to‐face support are more likely to succeed with women practising exclusive breastfeeding.
Plain language summary
Support for breastfeeding mothers
What is the issue?
The World Health Organization recommends that infants should be breastfed exclusively until six months of age with breastfeeding continuing as an important part of the infant’s diet until he or she is at least two years old. We know that breastfeeding is good for the short‐term and long‐term health of both infants and their mothers. Babies are less likely to develop infections in the digestive tract, lungs or airways, and ears. They are also less likely to become overweight and develop diabetes later in life. The mothers are less likely to develop diabetes and to experience breast or ovarian cancer. Many mothers may stop breastfeeding before they want to as a result of the problems they encounter. Good care and support may help women solve these problems so that they can continue to breastfeed.
Why is this important?
By knowing what kind of support can be provided to help mothers with breastfeeding, we can help them solve any problems and continue to breastfeed for as long as they want to, wherever they live. Stopping breastfeeding early may cause disappointment and distress for mothers and health problems for themselves and their infants. Support can be in the form of giving reassurance, praise, information, and the opportunity for women to discuss problems and ask questions as needed. This review looked at whether providing extra organised support for breastfeeding mothers would help mothers to continue to breastfeed when compared with standard maternity care. We were interested in support from health professionals including midwives, nurses and doctors, or from trained lay workers such as community health workers and volunteers.
What evidence did we find?
We searched for evidence on 29 February 2016 and identified a further 31 new trials for inclusion in the review. This updated review now includes 100 randomised controlled studies involving more than 83,246 women. The 73 trials that contributed to the analyses were from 29 countries and involved 74,656 women. Some 62% of the women were from high‐income countries, 34% from middle income countries and 4% from low‐income countries
All forms of extra organised support analyzed together showed an increase in the length of time women continued to breastfeed, either with or without introducing any other types of liquids or foods. This meant that fewer women stopped any breastfeeding or exclusively breastfeeding (moderate quality evidence) before four to six weeks and before six months. Both trained volunteers and doctors and nurses had a positive impact on breastfeeding.
Factors that may have contributed to the success for women who exclusively breastfed were face‐to‐face contact (rather than contact by telephone), volunteer support, a specific schedule of four to eight contacts and high numbers of women who began breastfeeding in the community or population (background rates).
The term 'high‐quality evidence' means that we are confident that further studies would provide similar findings. No outcome was assessed as being 'high‐quality'. The term 'moderate‐quality evidence' means that we found wide variations in the findings with some conflicting results in the studies in this review. New studies of different kinds of support for exclusive breastfeeding may change our understanding of how to help women to continue with exclusive breastfeeding.
The methodological quality of the studies was mixed and the components of the standard care interventions and extra support interventions varied a lot and were not always well described. Also, the settings for the studies and the women involved were diverse.
What does this mean?
Providing women with extra organised support helps them breastfeed their babies for longer. Breastfeeding support may be more helpful if it is predictable, scheduled, and includes ongoing visits with trained health professionals including midwives, nurses and doctors, or with trained volunteers. Different kinds of support may be needed in different geographical locations to meet the needs of the people within that location. We need additional randomised controlled studies to identify what kinds of support are the most helpful for women.
Summary of findings
Summary of findings for the main comparison. All forms of support versus usual care.
| All forms of support versus usual care | ||||||
| Patient or population: healthy breastfeeding mothers with healthy term babies Setting: outpatient settings in multiple countries (8% low‐ or lower‐middle income; 30% upper‐middle income; 60% high‐income countries) Intervention: all forms of support Comparison: usual care | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments1 | |
| Risk with usual care | Risk with all forms of support | |||||
| Stopping breastfeeding (any) before last study assessment up to 6 months | Study population | average RR 0.91 (0.88 to 0.95) | 21418 (51 RCTs) | ⊕⊕⊕⊝ MODERATE2 | We have not downgraded evidence for lack of blinding. However, no trial had adequate blinding of pregnant women or staff. | |
| 573 per 1000 | 510 per 1000 (487 to 532) | |||||
| Stopping exclusive breastfeeding before last study assessment up to 6 months | Study population | average RR 0.88 (0.85 to 0.92) | 18591 (46 RCTs) | ⊕⊕⊕⊝ MODERATE 3, 4 | ||
| 823 per 1000 | 732 per 1000 (707 to 765) | |||||
| Stopping breastfeeding (any) at up to 4‐6 weeks | Study population | average RR 0.87 (0.80 to 0.95) | 11264 (33 RCTs) | ⊕⊕⊕⊝ MODERATE5 | ||
| 353 per 1000 | 304 per 1000 (279 to 329) | |||||
| Stopping exclusive breastfeeding at up to 4‐6 weeks | Study population | RR 0.79 (0.71 to 0.89) | 10960 (32 RCTs) | ⊕⊕⊕⊝ MODERATE 4, 6 | ||
| 642 per 1000 | 507 per 1000 (443 to 571) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Sensitivity analyses restricted to trials of low risk of bias for allocation concealment showed similar effects for all four outcomes, with a reduction in effect size of (0 to 0.08) and minimal differences in confidence intervals.
2 Statistical heterogeneity, downgraded one level (I² = 55%).
3 Statistical heterogeneity, downgraded one level (I² = 96%).
4 There is some evidence of funnel plot asymmetry due to small studies with large effect sizes. Not downgraded.
5 Statistical heterogeneity, downgraded one level (I² = 54%).
6 Statistical heterogeneity, downgraded one level (I² = 97%).
Background
Description of the condition
Breastfeeding has a fundamental impact on the short‐, medium‐ and long‐term health of children and has an important impact on women’s health (Victora 2016). For children, good quality evidence demonstrates that in both low‐, middle‐ and high‐income settings not breastfeeding contributes to mortality due to infectious diseases (Sankar 2015), hospitalisation for preventable disease such as gastroenteritis, and respiratory disease (Horta 2013), otitis media (Bowatte 2015), increased rates of childhood diabetes and obesity (Horta 2015a), and increased dental disease (Peres 2015; Tham 2015). For women, there is good quality evidence that not breastfeeding is associated with increased risks of breast and ovarian cancer, and diabetes (Chowdhury 2015). Lactational amenorrhoea is associated with exclusive/predominant breastfeeding and increases birth spacing when other forms of contraception are not available (Chowdhury 2015). Not being breastfed has an adverse impact on intelligence quotient (IQ), and educational and behavioural outcomes for the child (Heikkilä 2014; Heikkilä 2011; Horta 2015b; Quigley 2012). For many outcomes a dose‐response relationship exists, with the greatest benefit resulting from breastfeeding exclusively, with no added food or fluids, for around six months, with breastfeeding continuing thereafter as an important component of the infant’s diet (Kramer 2012). The negative impact of not breastfeeding has been demonstrated in a range of settings and population groups, though the balance of risks and benefits varies from setting to setting; for example, gastroenteritis will result in much higher mortality in low‐income countries (Horta 2013).
Few health behaviours have such a broad‐spectrum and long‐lasting impact on population health, with the potential to improve life chances, health and well‐being. Victora 2016 estimated that each year, 823,000 deaths in children under five years and 20,000 deaths from breast cancer could be prevented by near universal breastfeeding. The cost burden of not breastfeeding was estimated by Rollins 2016 to represent 0.49% of world gross domestic product. The cost burden includes the cost of caring for children and women with chronic disease as well as short‐term illness (Bartick 2010; Smith 2010).
The established negative impact on a population of not breastfeeding has resulted in global and national support for encouraging the initiation and continuation of breastfeeding. The World Health Organization (WHO) recommends that, wherever possible, infants should be fed exclusively on breastmilk until six months of age (WHO 2003), with breastfeeding continuing as an important part of the infant’s diet until at least two years of age. Other agencies and countries have endorsed the recommendation to breastfeed exclusively to around six months of age (EFSA Panel 2009; National Center for Health Statistics 2012).
Due to the lack of standardised infant feeding indicators in high‐income countries, it is difficult to compare rates of breastfeeding across high‐income countries, or between high‐income, and low‐ and middle‐income countries. Therefore reported rates of breastfeeding need to be treated with caution. Victora 2016 suggest that, in general, there is an inverse relationship between breastfeeding rates and national wealth, though this relationship does not necessarily hold at the level of population subgroups. In high‐income countries, for example, the relationship is often seen to be the opposite, with rates higher among more affluent women (McAndrew 2012).
Although some high‐income countries such as, Norway and Finland have high rates of both initiation and continuation of breastfeeding (Cattaneo 2010), rates in many high‐income countries are low. Initiation rates have risen in some high‐income countries in recent years (NHS England 2014; U.S. Department of Health and Human Services 2011), but there remains a marked decline in breastfeeding within the first few weeks after initiation, and exclusive breastfeeding to six months is rare (Cattaneo 2010; McAndrew 2012).
In middle‐ and low‐income countries, while breastfeeding initiation and duration are generally higher than in high‐income countries, the average rate of exclusive breastfeeding for children younger than six months is only 37% (Victora 2016). However, rates of exclusive breastfeeding for children younger than six months vary widely; Peru and Rwanda report rates of 72% and 85% respectively (UNICEF 2012), while in Nigeria the rate is only 17%. In some low‐ and middle‐income countries, cultural practices such as prelacteal feeds, and giving water or teas alongside breastfeeding, account for the low rates of exclusive breastfeeding (Kimani‐Murage 2011). This is particularly important as when breastfeeding continues for long periods of time, infant and young child mortality are reduced in the second year of life in low‐ and middle‐income countries (Victora 2016).
Infant feeding is strongly related to inequalities in health, and, far from being an individual decision made by each woman, is influenced most strongly by structural determinants of health. The range of different rates of initiation and continuation of breastfeeding in different settings globally demonstrates that the key factors influencing infant feeding rates are likely to be sociocultural and related to societal and subgroup norms, public policy, and the availability of appropriate care and support, both professional and lay (EU Project on Promotion of Breastfeeding 2004; Rollins 2016). In high‐income countries, for example, young mothers and women in low‐income groups, or women who ceased full‐time education at an early age, are least likely either to start breastfeeding or to continue for a period of time sufficient to benefit from the greatest health gain (McAndrew 2012). Migrant women have been shown to adopt breastfeeding practices that are more similar to the country in which they live, than the country of their birth (McLachlan 2006).
The early discontinuation of breastfeeding is not a decision that is taken lightly by women; it is associated with a high prevalence of problems such as painful breasts and nipples, concern about adequacy of milk supply and about the baby’s behaviour, and, in some settings, embarrassment related to breastfeeding in public. Many mothers report distress related to the decision to discontinue breastfeeding (McAndrew 2012), even in cultures where breastfeeding rates are high (Almqvist‐Tangen 2012). A key factor is the widespread lack of appropriate education for health professionals in the prevention and treatment of breastfeeding problems, which means that in a wide range of settings women commonly do not receive the quality of care needed from the health services (Cattaneo 2010; Renfrew 2006). Enkin 2000 notes that industrial societies, on the whole, do not provide women with the opportunity to observe other breastfeeding women before they attempt breastfeeding themselves. In such societies, where breastfeeding is not normative behaviour and women may find it socially challenging to breastfeed, women are at particular risk of finding a serious lack of support to continue breastfeeding.
Description of the intervention
‘Support’ is complex and can include several elements such as emotional and esteem‐building support (including reassurance and praise), practical help, informational support (including the opportunity to discuss and respond to women’s questions) and social support (including signposting women to support groups and networks) (Dykes 2006; Schmied 2011). It can be offered in a range of ways, by health professionals or lay people, trained or untrained, in hospital and community settings. It can be offered to groups of women or one‐to‐one, it can involve mother‐to‐mother support, and it can include family members (typically fathers or grandmothers) and wider communities. Support can be offered proactively by contacting women directly, or reactively, by waiting for women to get in touch. It can be provided face‐to‐face, by telephone or through social media. It can involve only one contact or regular, ongoing contact over several months.
Support is a complex intervention that tackles the multifaceted challenge of enabling women to breastfeed, and it should not be surprising that it varies from setting to setting and from study to study. However, it is likely that different forms of support in different contexts will be differentially effective. The global Baby Friendly Hospital Initiative (Baby Friendly Initiative in some countries), which is a complex intervention incorporating 10 steps to successful breastfeeding, has been shown to be associated with increased breastfeeding rates (Labbok 2012; Pérez‐Escamilla 2016; Venancio 2011). Over 21,000 facilities in 198 countries have ever been accredited, representing 27.5% of maternities worldwide (Labbok 2012), but most babies are still not born in a Baby Friendly environment.
In many settings, the health professionals who provide standard maternity care lack in‐depth knowledge of the prevention and treatment of breastfeeding problems. Therefore training and education of health professionals and others who provide breastfeeding support is critical. To address this, WHO and UNICEF (the United Nations Children's Fund) have developed two breastfeeding training programmes: the 40‐hour Breastfeeding Counselling, and the five‐day Infant and Young Child Feeding Counselling, to train a cadre of health workers that can provide skilled support to breastfeeding mothers and help them overcome problems (WHO/UNICEF 1993; WHO/UNICEF 2006).
How the intervention might work
Support for breastfeeding women can work in different ways for different women. Timely, skilled support will help women to avoid or overcome breastfeeding problems that may lead to cessation of breastfeeding. In settings where breastfeeding is not the social norm, support can increase women’s belief in breastfeeding, and give them confidence to continue breastfeeding in the face of societal and family pressures that might undermine breastfeeding. In settings where exclusive breastfeeding is rare, support can dispel myths about the need for additional foods or fluids alongside breastfeeding to meet babies’ nutritional needs.
Why it is important to do this review
It is fundamentally important to examine the support that mothers receive when breastfeeding to determine what might be effective in helping women continue to breastfeed, whatever setting they live in. There is evidence that effective breastfeeding support interventions are cost‐effective and likely to realise a return on investment within a few years (Renfrew 2012a).
The purpose of this review is to examine interventions which provide extra support for mothers who are breastfeeding or considering breastfeeding; and to assess their impact on breastfeeding duration and exclusivity and, where recorded, on health outcomes and maternal satisfaction. This review is an update of the previously published version Renfrew 2012b. The focus of this review is support for mothers and babies who are part of the general healthy population of their countries; mothers of premature and sick babies and mothers with some medical conditions have additional issues with breastfeeding, and interventions to support these mothers need to be reviewed separately. A Cochrane Review of breastfeeding education and support for mothers with multiple pregnancies is in progress (Whitford 2015). Whilst many support interventions include breastfeeding education for mothers, our review excludes interventions described as solely educational in nature and interventions with no postnatal component. A Cochrane Review of antenatal breastfeeding education for increasing breastfeeding duration has been published (Lumbiganon 2012).
Specific objectives of this review are to describe forms of support which have been evaluated in controlled studies, and the settings in which they have been used. It was also of interest to examine the effectiveness of different modes of offering similar supportive interventions (for example, face‐to‐face or over the telephone), whether interventions containing both antenatal and postnatal elements were more effective than those taking place in the postnatal period alone, and whether the support was offered proactively to women, or whether they needed to seek it out. We also planned to examine the effectiveness of different care providers, and the possible impact of background breastfeeding rates in the countries or areas where the trials took place on the effectiveness of supportive interventions. It is important to note that the support interventions offered were in addition to standard care, which varied from setting to setting, though there are few settings in which standard care is consistently offered by people with training and skill in enabling women to breastfeed.
Objectives
To describe forms of breastfeeding support which have been evaluated in controlled studies, the timing of the interventions and the settings in which they have been used.
To examine the effectiveness of different modes of offering similar supportive interventions (for example, whether the support offered was proactive or reactive, face‐to‐face or over the telephone), and whether interventions containing both antenatal and postnatal elements were more effective than those taking place in the postnatal period alone.
To examine the effectiveness of different care providers and (where information was available) training.
To explore the interaction between background breastfeeding rates and effectiveness of support.
Methods
Criteria for considering studies for this review
Types of studies
All randomised or quasi‐randomised controlled trials, with or without blinding. Cluster‐randomised controlled trials were also eligible for inclusion.
Types of participants
Participants were healthy pregnant women considering or intending to breastfeed or healthy women who were breastfeeding healthy babies.Healthy women and babies were considered those who did not require additional medical care (e.g. women with diabetes, women with HIV/AIDs, overweight or obese women) or surgical care (e.g. women who required a Caesarean Section). Studies which focused specifically on women with additional care needs were excluded.
Types of interventions
Contact with an individual or individuals (either professional or volunteer) offering support which is supplementary to the standard care offered in that setting. ‘Support’ interventions eligible for this review could include elements such as reassurance, praise, information, and the opportunity to discuss and to respond to the mother’s questions, and could also include staff training to improve the supportive care given to women. It could be offered by health professionals or lay people, trained or untrained, in hospital and community settings. It could be offered to groups of women or one‐to‐one, including mother‐to‐mother support, and it could be offered proactively by contacting women directly, or reactively, by waiting for women to get in touch. It could be provided face‐to‐face or over the phone, and it could involve only one contact or regular, ongoing contact over several months. Studies were included if the intervention occurred in the postnatal period alone or also included an antenatal component. Interventions taking place in the antenatal period alone were excluded from this review, as were interventions described as solely educational in nature.
Types of outcome measures
The main outcome measure was the effect of the interventions on stopping breastfeeding by specified points in time. Primary outcomes were recorded for stopping any or exclusive breastfeeding before four to six weeks and before six months postpartum. Other outcomes of interest in previous versions of this review were stopping any or exclusive breastfeeding at other time points (two, three, four, nine and 12 months), measures of neonatal and infant morbidity (where available) and measures of maternal satisfaction with care or feeding method. Secondary outcomes were not considered in this update so that the review could be completed in time to inform the World Health Organisation’s review of the evidence and update of the WHO recommendations on breastfeeding in maternity facilities. A new set of core outcomes for Cochrane pregnancy and childbirth breastfeeding reviews is currently being developed and the outcomes from this core set may influence future outcomes chosen for this review.
Primary outcomes
Stopping breastfeeding before six months postpartum.
Stopping exclusive breastfeeding before six months postpartum.
Stopping any breastfeeding before four to six weeks postpartum.
Stopping exclusive breastfeeding before four to six weeks postpartum.
Secondary outcomes
We did not consider secondary outcomes in this 2016 update.
Search methods for identification of studies
The following methods section of this review is based on a standard template used by Cochrane Pregnancy and Childbirth.
Electronic searches
We searched the Cochrane Pregnancy and Childbirth’s Trials Register by contacting their Information Specialist (29 February 2016).
The Register is a database containing over 22,000 reports of controlled trials in the field of pregnancy and childbirth. For full search methods used to populate Pregnancy and Childbirth’s Trials Register including the detailed search strategies for CENTRAL, MEDLINE, Embase and CINAHL; the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service, please follow this link to the editorial information about Cochrane Pregnancy and Childbirth in the Cochrane Library and select the ‘Specialized Register ’ section from the options on the left side of the screen.
Briefly, Cochrane Pregnancy and Childbirth’s Trials Register is maintained by their Information Specialist and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE (Ovid);
weekly searches of Embase (Ovid);
monthly searches of CINAHL (EBSCO);
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals, plus monthly BioMed Central email alerts.
Search results are screened independently by two people and the full text of all relevant trial reports identified through the searching activities described above is reviewed. Based on the intervention described, each trial report is assigned a number that corresponds to a specific Pregnancy and Childbirth review topic (or topics), and is then added to the Register. The Information Specialist searches the Register for each review using this topic number rather than keywords. This results in a more specific search set, which has been fully accounted for in the relevant review sections (Included studies; Excluded studies; Studies awaiting classification; Ongoing studies).
(For details of search methods used in previous versions of this review, please see: Britton 2007; Renfrew 1995; Renfrew 2012b; Sikorski 1999; Sikorski 2002)
Searching other resources
We searched the reference lists of retrieved studies.
We did not apply any language or date restrictions.
Data collection and analysis
For methods used in the previous version of this review, seeRenfrew 2012b.
For this update, the following methods (based on a standard template used by Cochrane Pregnancy and Childbirth) were used for assessing the 162 reports that were identified as a result of the updated search.
Selection of studies
Two review authors independently assessed all the potential studies identified as a result of the search strategy for inclusion. We resolved any disagreement through discussion and consulted a third review author if required.
Data extraction and management
We designed and piloted a form to extract data. For eligible studies, two review authors extracted information using the agreed form. We resolved discrepancies through discussion. Data were entered into Review Manager 5 software (RevMan 2014), and checked for accuracy.
When information regarding study methods and results was unclear, we attempted to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (the Handbook) (Higgins 2011). Any disagreement was resolved by discussion or by involving a third assessor.
(1) Random sequence generation (checking for possible selection bias)
For each included study, we described the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
For each included study, we described the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
For each included study, we described the method used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
For each included study, we described the method used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
For each included study, and for each outcome or class of outcomes, we described the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we planned to re‐include missing data in the analyses that we undertook.
We assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
For each included study, we described how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it is clear that all of the study’s prespecified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s prespecified outcomes have been reported; one or more reported primary outcomes were not prespecified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included study any important concerns we had about other possible sources of bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Handbook (Higgins 2011).
Overall findings for our assessment of risk of bias in the included studies are set out in Figure 1 and Figure 2.
1.
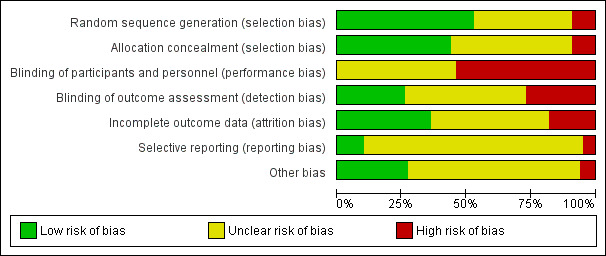
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
2.
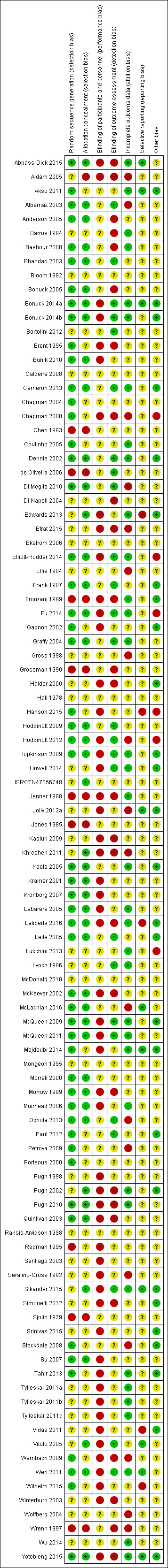
Risk of bias summary: review authors' judgements about each risk of bias item for each included study
Assessment of the quality of the evidence using the GRADE approach
For this update the quality of the evidence was assessed using the GRADE approach as outlined in the GRADE handbook in order to assess the quality of the body of evidence relating to the following primary outcomes for the comparison, All forms of support versus usual care.
Stopping breastfeeding before six months postpartum.
Stopping exclusive breastfeeding before six months postpartum.
Stopping any breastfeeding before four to six weeks postpartum.
Stopping exclusive breastfeeding before four to six weeks postpartum.
The GRADEpro Guideline Development Tool was used to import data from Review Manager 5.3 in order to create ’Summary of findings’ tables (RevMan 2014). A summary of the intervention effect and a measure of quality for each of the above outcomes was produced using the GRADE approach. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. The evidence can be downgraded from 'high quality' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratios with 95% confidence intervals.
Continuous data
There are no continuous data in this review.
Unit of analysis issues
Cluster‐randomised trials
There are 15 cluster‐randomised trials in the analyses. Their sample sizes have been adjusted using the methods described in the Handbook and by Donner 2000 incorporating an estimate of the intracluster correlation coefficient (ICC) derived from the trial (if possible). Where cluster adjusted confidence limits were presented but not the ICC, the design effect was estimated from comparison with limits based on the raw numbers. However, for Ochola 2013, outcome one of Elliott‐Rudder 2014, arm two of Yotebieng 2015, adjusting for clustering based on the summary statistic made the standard error larger and the width of the confidence interval increased which resulted in a design of <1. Therefore, the adjustment for clustering resulted in an increase of the error sum of squares for the raw numbers given. As this was nonsensical, no adjustment for clustering was made for these studies. We have synthesised the findings from individually‐ and cluster‐randomised trials provided that there was little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit was considered to be unlikely. We have carried out sensitivity analyses to investigate the effect of including cluster‐randomised trials where no adjustment was possible. For all trials where ICCs were not reported, study authors will be contacted in the next version of the review.
Trials with multiple groups
In order to avoid 'double counting' in studies involving one control group and two different interventions groups, we split the control group number of events and participants in half, so that we could include two independent comparisons, as per methods described the Handbook [section16.5.4].
Dealing with missing data
For all outcomes, analyses were carried out, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
For included studies, we have noted levels of attrition. We have not included outcomes in the analyses where more than 25% of the data were missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the Tau², I² and Chi² statistics. We regarded heterogeneity as substantial if I² was greater than 30% and either Tau² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity. If we identified substantial heterogeneity (above 30%), we planned to explore it by prespecified subgroup analysis.
Assessment of reporting biases
For all outcomes we have ordered studies in terms of weight, where a sufficient number of studies contributed data, we have generated funnel plots. We examined plots visually to see whether there was any evidence of asymmetry that might suggest different treatment effects in smaller studies, which may indicate publication bias (Harbord 2006). We note however, that there are many other reasons for asymmetry in Funnel Plots such as heterogeneity.
Data synthesis
We carried out statistical analysis using Review Manager 5 software (RevMan 2014). At the outset, we had anticipated that there would be some heterogeneity between studies in terms of the interventions and the populations studied, we therefore decided to use random‐effects meta‐analysis for combining data. Random‐effects meta‐analysis estimates the average treatment effect, and this may not always be clinically meaningful. Furthermore, where there is high heterogeneity the applicability of the overall effect estimate is likely to vary in different settings and we therefore advise caution in the interpretation of results. The random‐effects summary was treated as the average of the range of possible treatment effects and we discuss the clinical implications of treatment effects differing between trials. If the average treatment effect was not clinically meaningful, we planned not to combine trials. Since we used random‐effects analyses, the results were presented as the average treatment effect with 95% confidence intervals, and the estimates of Tau² and I².
Subgroup analysis and investigation of heterogeneity
If we identified substantial heterogeneity, we investigated it using subgroup analyses and sensitivity analyses. We considered whether an overall summary was meaningful, and if it was, we used random‐effects analysis to produce it.
We carried out the following subgroup analyses for the four primary outcomes.
By type of supporter (professional versus lay person, or both).
By type of support (face‐to‐face versus telephone support).
By timing of support (antenatal and postnatal versus postnatal alone).
By whether the support was proactive (scheduled contacts) or reactive (women needed to request support).
By background breastfeeding initiation rates (low, medium or high background rates).
By intensity of support (number of scheduled contacts).
Sensitivity analysis
We have carried out sensitivity analysis for primary outcomes by study quality; we did this by dividing the studies into subgroups according to whether they were at low risk of bias as opposed to unclear or high risk of bias. We have performed this for allocation concealment. Because we have excluded studies from any analyses if they had more than 25% attrition, we have not conducted sensitivity analyses for this item.
Results
Description of studies
Results of the search
In this updated version, we assessed 162 reports and have subsequently included a further 31 studies. We excluded 68 studies and have assigned the remainder as either an additional report of another study in the review, a study awaiting classification or an ongoing study (seeStudies awaiting classification and Characteristics of ongoing studies). This review now therefore includes 100 studies and has excluded 147 studies.
This updated review is only focused on two primary outcomes each at two different time points. Of the 31 new studies included in this update, 21 studies provided data for one or more of the primary outcomes (see Table 2). Ten new trials met the inclusion criteria for this review but were excluded from the analyses either because they did not present data in a useable form or because of attrition rates >25%. Eleven studies provided data for outcome 1.1; 13 studies for outcome 1.2; eight studies for outcome 1.3; and eight studies for outcome 1.4. The addition of these studies to the studies included in the previous version of the review meant that for this 2016 update a total of 51 studies contributed data for outcome 1.1; 46 studies for outcome 1.2; 33 studies for outcome 1.3; and 32 studies for outcome 1.4.
1. Summary of included studies from 2016 update.
| Study | RCT 2‐arm | RCT 3‐arm | RCT 4‐arm | Cluster | Background breastfeeding rates (low, medium, high) | Type of supporter (professional, lay person, both) | Type of support (face‐to‐face, telephone) | Timing of support (antenatal (ante) + postnatal (post), or post alone) | Whether support was: proactive (scheduled contacts) or reactive (women needed to request support) | Number of postnatal contacts (< 4, 4‐8, 9+) | Data included in outcome 1 | Data included in outcome 2 | Data included in outcome 3 | Data included in outcome 4 |
| Abbas‐Dick 2015 | x | High | Professional | Face‐to‐face and telephone | Post alone | Proactive | < 4 | N | N | Y | Y | |||
| Bonuck 2014 (BINGO trial) | x | Medium | Professional | Face‐to‐face and telephone | Post alone | Proactive | Unclear | Y | Y | Y | Y | |||
| Bonuck 2014a (PAIRINGS trial) | x | Medium | Professional | Face‐to‐face and telephone | Ante and post | Proactive | Unclear | Y | Y | Y | Y | |||
| Bortolini 2012 | x | High | Professional | Face‐to‐face | Post alone | Proactive | 9+ | Y | Y | N | Y | |||
| Cameron 2013 | x | High | Professional | Face‐to‐face | Ante and post | Proactive | < 4 | N | N | N | N | |||
| Chapman 2008 | x | Medium | Professional | Face‐to‐face and telephone | Ante and post | Proactive | 9+ | N | N | N | N | |||
| Edwards 2013 | x | High | Lay | Face‐to‐face | Ante and post | Unclear | 9+ | N | N | Y | N | |||
| Efrat 2015 | x | High | Professional | Telephone | Ante and post | Proactive | 9+ | N | N | N | N | |||
| Elliott‐Rudder 2014 | x | x | High | Professional | Face‐to‐face | Post alone | Proactive | < 4 | Y | Y | N | N | ||
| Fu 2014 | x | x | High | Professional | Telephone | Post alone | Proactive | 4 to 8 | N | N | Y | Y | ||
| Hanson 2015 | x | x | High | Lay | Face‐to‐face | Ante and post | Proactive | 4 to 8 | N | N | N | N | ||
| Hoddinott 2012 | x | Medium | Professional | Telephone | Post | Proactive | 9+ | N | N | N | N | |||
| Howell 2014 | x | High/low | Professional | Face‐to‐face and telephone | Post | Proactive | < 4 | N | Y | N | N | |||
| Jolly 2012 | x | x | Low | Lay | Face‐to‐face or telephone | Ante and post | Proactive | 4 to 8 | N | N | N | N | ||
| Laliberte 2016 | x | High | Professional | Face‐to‐face | Post alone | Proactive | Unclear | Y | Y | N | N | |||
| Lucchini 2013 | x | High | Professional | Face‐to‐face | Post | Proactive | < 4 | N | N | N | N | |||
| McLachlan 2016 | x | x | High | Professional | Face‐to‐face | Post alone | Proactive | Unclear | Y | N | N | N | ||
| McQueen 2009 | x | High | Professional | Face‐to‐face and telephone | Post alone | Proactive | < 4 | N | N | Y | Y | |||
| Mejdoubi 2014 | x | High | Professional | Face‐to‐face | Ante and post | Proactive | 9+ | Y | N | N | N | |||
| Ochola 2013 | x | x | High | Lay | Face‐to‐face | Ante and post | Proactive | 4 to 8 | N | Y | N | Y | ||
| Paul 2012 | x | Medium | Professional | Face‐to‐face | Post alone | Proactive | < 4 | Y | Y | N | N | |||
| Sikander 2015 | x | x | High | Professional | Face‐to‐face | Ante and post | Proactive | 4 to 8 | N | Y | N | N | ||
| Simonetti 2012 | x | High | Professional | Telephone | Post alone | Proactive | 4 to 8 | N | Y | N | N | |||
| Srinivas 2015 | x | Medium | Lay | Face‐to‐face and telephone | Ante and post | Proactive | > 9 | N | N | N | N | |||
| Stockdale 2008 | x | Medium | Professional | Face‐to‐face | Ante and post | Unclear | Unclear | N | N | N | N | |||
| Tahir 2013 | x | High | Professional | Face‐to‐face | Post alone | Proactive | 9+ | N | Y | Y | Y | |||
| Vidas 2011 | x | Unknown | Professional | Face‐to‐face | Post alone | Unclear | Unclear | N | Y | N | N | |||
| Wen 2011 | x | High | Professional | Face‐to‐face | Ante and post | Proactive | 4 to 8 | N | Y | N | N | |||
| Wilhelm 2015 | x | High | Professional | Face‐to‐face | Post alone | Proactive | 4 to 8 | Y | N | N | N | |||
| Wu 2014 | x | Unknown | Professional | Face‐to‐face and telephone | Post alone | Proactive | < 4 | N | N | Y | N | |||
| Yotebieng 2015 | x | x | High | Professional | Face‐to‐face | Post alone | Proactive | Unclear | N | Y | N | N | ||
| 25 | 5 | 2 | 8 | 11 | 13 | 8 | 8 |
Abbreviations
ante: antenatally N: no post: postnatally Y: yes
In the results section we will not discuss further those studies that did not contribute data to the review, but additional information about these trials is provided in the Characteristics of included studies table and further details about the eleven new trials from the update is also provided in Table 2.
Included studies
This updated review includes 100 trials involving more than 83,246 mother‐infant pairs of which 73 studies contribute data (58 individually‐randomised trials and 15 cluster‐randomised trials).
Description of included studies (n = 73)
Seventy‐three of the 100 included studies contribute data to this 2016 update of the review. It should be noted that two of the included trials were obtained via a single publication (Bonuck 2014a); one trial is called the BINGO trial and the other called the PAIRING trial. In order to differentiate between these two trials in this review the BINGO trial is identified via the reference (Bonuck 2014a) and the PAIRING trial via the reference (Bonuck 2014b).
The total number of mother‐infant pairs in these studies is 74,656 (this total was 56,451 in the previous version of this review (Renfrew 2012b). The 73 studies were published/conducted between 1979 and 2016 and show increases over time both in number of studies (five studies are dated before 1990, 10 between 1990 and 1999, 40 between 2000 and 2011, and 18 are dated between 2012 and 2016), and range of country settings (the seven studies with dates before 1994 were all undertaken in high‐income countries, and the eight studies from low‐/low‐middle income countries were published in 2000 or later). The data in this review come from participants living in 29 countries. Using the World Bank classification of countries by income (http://data.worldbank.org/about/country‐classifications/country‐and‐lending‐groups, accessed 30 June 2016):
four studies with 3260 participants (4.4% of the total number of participants) were conducted in low‐income countries (Bangladesh, Haider 2000; Burkina Faso and Uganda, Tylleskar 2011a and Tylleskar 2011b; and the Democratic Republic of the Congo, Yotebieng 2015);
four studies with 2534 participants (3.4%) were conducted in low‐middle income countries (India, Bhandari 2003; Kenya, Ochola 2013; Pakistan, Sikander 2015; and Syria, Bashour 2008);
15 studies with 22,477 participants (30.1%) were conducted in upper‐middle income countries (Belarus, Kramer 2001; Brazil, Albernaz 2003, Barros 1994, Bortolini 2012, Coutinho 2005, de Oliveira 2006, Leite 2005, Santiago 2003, and Vitolo 2005; China, Wu 2014; Iran, Froozani 1999; Malaysia, Tahir 2013; Mexico, Morrow 1999; Turkey, Aksu 2011; and South Africa, Tylleskar 2011c1);
52 studies with 46,390 participants (62.1%) were conducted in high‐income countries (Australia, Elliott‐Rudder 2014, McLachlan 2016, McDonald 2010, Quinlivan 2003, and Wen 2011; Canada, Abbass‐Dick 2015, Dennis 2002, Gagnon 2002, Laliberte 2016, Lynch 1986, Mongeon 1995, McQueen 2011, and Porteous 2000; Croatia, Vidas 2011; Denmark, Kronborg 2007; France, Labarere 2005, and McQueen 2009; Hong Kong, Wu 2014; Italy, Di Napoli 2004; Netherlands, Kools 2005, and Mejdoubi 2014; Singapore, Su 2007; Sweden, Ekstrom 2006, and Sjolin 1979; UK, Graffy 2004, Hoddinott 2009, Jones 1985, Jenner 1988, Morrell 2000, Muirhead 2006, ISRCTN47056748, and Winterburn 2003; USA, Bonuck 2014a, Bonuck 2014b, Bonuck 2005, Brent 1995, Bunik 2010, Chapman 2004, Di Meglio 2010, Edwards 2013, Frank 1987, Grossman 1990, Hopkinson 2009, Howell 2014, Paul 2012, Petrova 2009, Pugh 1998, Pugh 2002, Pugh 2010, Serafino‐Cross 1992, Wilhelm 2015, and Wrenn 1997).
1 Note: The Tylleskar study, Tylleskar 2011a, Tylleskar 2011b, and Tylleskar 2011c, was undertaken in three countries, two are in the low‐income and one in the upper‐middle income World Bank category. In this review, we have entered data into the analyses separately for each country.
Methods used in trials
The 73 studies include 58 individually‐randomised trials and 15 cluster‐randomised trials (Bhandari 2003; Ekstrom 2006; Elliott‐Rudder 2014; Fu 2014; Haider 2000; Hoddinott 2009; Kools 2005; Kramer 2001; Kronborg 2007; McLachlan 2016; Morrow 1999; Ochola 2013; Sikander 2015; Tylleskar 2011a; Tylleskar 2011b; Tylleskar 2011c; Yotebieng 2015).
Participants and setting
Socioeconomic and health status
Participants were women from the general healthy population of their countries. However, 28 of the 73 studies were undertaken with women from low‐income groups within their country. These 28 studies include 16 of the 20 USA studies, with four other studies from high‐income countries (Jones 1985; Mejdoubi 2014; Quinlivan 2003; Wen 2011), three of the studies from Brazil (Barros 1994; Coutinho 2005; Vitolo 2005), three of the studies from low‐middle income countries (Ochola 2013; Sikander 2015; Yotebieng 2015), and the two studies from low‐income countries. In one of these (Haider 2000, Bangladesh), participants were mainly of lower‐middle and low socioeconomic status. In the other (Tylleskar 2011a; Tylleskar 2011b; Tylleskar 2011c), participants came from three countries in sub‐Saharan Africa, with those in one country (Uganda) from low‐income groups within that country. With regard to health of the general population of countries, Tylleskar 2011a; Tylleskar 2011b; Tylleskar 2011c reported local HIV prevalence rates of 10% to 34% in the South Africa study sites; during recruitment, women who had not been HIV tested were encouraged to visit the antenatal clinic, and those who had HIV‐positive status were recruited into another study.
Background rates of breastfeeding initiation/'ever breastfed'
Among the 73 studies, World Bank country income group shows an inverse relationship with background rates of breastfeeding initiation ('ever breastfed'). All the studies with intermediate (60% to < 80%, n = 18) or low (< 60%, n = 11) background rates of breastfeeding initiation were undertaken in high‐income countries. Nine of the 11 studies with low background rates recruited women from low‐income groups in the USA (Brent 1995; Bonuck 2005; Bunik 2010; Chapman 2004; Di Meglio 2010; Frank 1987; Grossman 1990; Pugh 2002; Serafino‐Cross 1992); the remaining two (UK) studies were from areas of Scotland with lower breastfeeding initiation rates than the Scottish average (Hoddinott 2009; Muirhead 2006). All the country income groups are represented among the 24 studies with high (≥ 80%) rates, however the seven studies from low‐/low‐middle income countries all had high rates. Where background rates of 'ever breastfed' were not reported, we have used either rates published in the WHO Global Data Bank on Infant and Young Child Feeding (www.who.int/nutrition/databases/infantfeeding/countries/en/index; accessed July 2016), or those published in the supplementary material to Victora 2016, and for the two studies from Scotland (Hoddinott 2009; Muirhead 2006), we used www.isdscotlandarchive.scot.nhs.uk/isd/1914 (accessed November 2016). For one study that was conducted in China (Wu 2014), data were not presented in the paper or available in the WHO Global Data Bank on Infant and Young Child Feeding and so were therefore excluded from the sensitivity analysis.
Interventions
Level of the intervention
In 64/73 studies, women received the intervention. In eight studies the intervention was additional training in breastfeeding support for staff (five cluster‐randomised trials; Bhandari 2003; Ekstrom 2006; Elliott‐Rudder 2014; Kramer 2001; Yotebieng 2015; and three individually‐randomised trials; Labarere 2005; Santiago 2003; ISRCTN47056748). One cluster‐randomised trial evaluated a policy for providing breastfeeding groups (Hoddinott 2009).
Breastfeeding support: proactive/indirect
In 58 of the 64 studies where women received the intervention and seven of the eight studies of staff training, breastfeeding support was delivered directly to women. However, in two of these studies although the support was offered proactively initially, it was up to the women to request follow‐up support (Bonuck 2014a; Laliberte 2016). In five other studies (Graffy 2004; Hoddinott 2009; Labarere 2005; Morrell 2000; Winterburn 2003), breastfeeding support was not offered proactively; women were encouraged to access it, but breastfeeding support was not delivered directly to women as part of these interventions. One study evaluated a multi‐faceted intervention, of which breastfeeding support delivered directly to women was one component (Kools 2005). For two studies it was not clear if the support was offered proactively or not (Edwards 2013; Vidas 2011).
One‐to‐one/group support
In 57 of the 73 studies there was individual, one‐to‐one contact between the breastfeeding supporter and the breastfeeding mother. Two studies offered group support (Hoddinott 2009; Vidas 2011), one offered both individual and group support (Ekstrom 2006), one study offered support to couples (Abbass‐Dick 2015), and in two studies this aspect of support was unclear (Kools 2005; Kramer 2001).
Breastfeeding support from professional/lay supporters
In the previous version of this review, the people providing breastfeeding support were categorised as 'professional', 'lay and professional' or 'lay'. Using those categories, the 73 studies in this update comprise 49 studies of professional support, nine of lay and professional support and 15 of lay support. In view of the growing body of work evaluating breastfeeding peer support, we have distinguished between this and other kinds of lay support, following the definition by Dennis 2002: “Peer support is provided by lay individuals who are not part of the client’s own embedded network, who possess experiential knowledge of the targeted behaviour (i.e. successful breastfeeding skills) and similar qualities (i.e. age, socioeconomic status, ethnicity, residency etc.) in order to aid the client during a time of actual or potential stress (i.e. the initiation and continuation of breastfeeding)."
Professional
In 49 of the 73 studies a variety of medical, nursing and allied professionals (for example, nutritionists, lactation consultants and researchers) provided the breastfeeding support (Abbass‐Dick 2015; Albernaz 2003; Bashour 2008; Barros 1994Bonuck 2005; Bonuck 2014a; Bonuck 2014b; Bortolini 2012; Brent 1995; Bunik 2010; de Oliveira 2006; Di Napoli 2004; Ekstrom 2006; Elliott‐Rudder 2014; Frank 1987; Froozani 1999; Fu 2014; Gagnon 2002; Grossman 1990; Howell 2014; Jones 1985; Kramer 2001; Kronborg 2007; Laliberte 2016; Lynch 1986; McLachlan 2016; McDonald 2010; McQueen 2009; McQueen 2011; Mejdoubi 2014; Paul 2012; Petrova 2009; Porteous 2000; Pugh 1998; Quinlivan 2003; Santiago 2003; Serafino‐Cross 1992; Sikander 2015; ISRCTN47056748; Sjolin 1979; Su 2007; Tahir 2013; Vidas 2011; Vitolo 2005; Wen 2011; Wilhelm 2015; Wrenn 1997; Wu 2014; Yotebieng 2015).
Professional and lay
Professionals provided breastfeeding support with other people in a further nine studies; para‐professionals (Kools 2005; Morrell 2000), peer supporters (Bhandari 2003; Hopkinson 2009; Pugh 2002; Pugh 2010), and lay people (employees who had to be mothers in Barros 1994; someone chosen by the mother in Winterburn 2003; and a group of mothers in Hoddinott 2009).
Lay
Lay people provided breastfeeding support in 17 studies. In twelve of these, the lay people were peer supporters (Chapman 2004; Dennis 2002; Di Meglio 2010; Edwards 2013; Haider 2000; Leite 2005; Morrow 1999; Muirhead 2006; Ochola 2013; Tylleskar 2011a; Tylleskar 2011b; Tylleskar 2011c). The other five studies did not report that the lay supporters met the Dennis 2002 criteria for us to classify them as peer supporters (Aksu 2011; Coutinho 2005; Graffy 2004; Jenner 1988; Mongeon 1995).
Training in breastfeeding support
Overall, 50 of the 73 studies reported that the people providing breastfeeding support had additional training to provide breastfeeding support (33/49 professional, 3/9 professional and lay, and 14/15 peer/lay). In 10 studies the professionals were International Board Certified Lactation Consultants (IBCLC) (Bonuck 2014a; Bonuck 2014b; Bortolini 2012; Brent 1995; Fu 2014; Laliberte 2016; Petrova 2009; Pugh 1998; Tahir 2013; Yotebieng 2015).
In one of the studies of support from professionals and paraprofessionals, the professionals were lactation consultants (Kools 2005), and in the other they were midwives not stated to have had extra training in breastfeeding support (Morrell 2000); in both these studies the para‐professionals were trained to refer women with breastfeeding problems to the professionals. Two of the four studies of support from professionals and peers reported training; in Bhandari 2003 peer supporters received WHO‐based training, and in Hopkinson 2009 the professionals were IBCLCs and the peer supporters had three days' training in lactation management, 20 hours' training in peer counselling and at least one year’s work experience. One of the three studies study of professional and lay support stated that lay supporters received breastfeeding support training (Barros 1994).
All 10 studies of peer support (alone) reported that peer supporters were trained. The training was WHO 20 hours (Leite 2005), 40 hours (Haider 2000; Ochola 2013) or one week (Tylleskar 2011a; Tylleskar 2011b; Tylleskar 2011c); La Leche League (LLL) 30 hours (Chapman 2004), 20 hours (Di Meglio 2010); over 30 weeks (Edwards 2013), or not specified (Morrow 1999). Two studies reported the length but not the type of training; 2.5 hours and more than two days (Dennis 2002; Muirhead 2006, respectively). Three of the five studies of lay support (alone) reported breastfeeding training; WHO 18 hours plus five days (Coutinho 2005), WHO 18 hours (Aksu 2011), and National Childbirth Trust training (Graffy 2004).
Mode of support (face‐to‐face or by telephone, or both)
Forty‐seven of the 73 studies offered telephone support and all but two of these were undertaken in countries classified as high‐income countries by the World Bank (Albernaz 2003, Brazil; Wu 2014, China). Four studies offered breastfeeding support only by telephone (Bunik 2010; Dennis 2002; Di Meglio 2010; Fu 2014). Thirty offered both face‐to‐face and telephone support, with telephone support either predominating (e.g. Muirhead 2006; Petrova 2009), or as backup (e.g. Chapman 2004). In some studies (e.g. Kools 2005; Pugh 1998), telephone contact with the breastfeeding support specialist came after the women had been visited by someone else. Across the 27 studies examining telephone support, details about whether or not the telephone support was proactively offered by the peer or professional supporter were not reported consistently. Thirty‐eight studies offered only face‐to‐face support. In the one remaining study (Winterburn 2003), the support was not proactive and the mode of support was not specified.
Support with an antenatal component and intention to breastfeed
The outcomes of interventions intended to promote longer duration of breastfeeding could be expected to differ according to whether women were recruited before or after they started to breastfeed. Two‐thirds of the studies (49/73) included postnatal women at or after initiation of breastfeeding. In the one study of breastfeeding in groups (Hoddinott 2009), pregnant women and breastfeeding mothers could be invited to attend groups. The remaining 24 studies recruited women before the birth, not all of whom went on to initiate breastfeeding. Six of the 24 studies included only women who intended to breastfeed (Kramer 2001 in Belarus; Jenner 1988 and Winterburn 2003 in the UK; Serafino‐Cross 1992 in the USA; Mongeon 1995 in Canada; Tylleskar 2011a; Tylleskar 2011b; Tylleskar 2011c in Burkina Faso, Uganda and South Africa). In the Tylleskar 2011 study, this inclusion criterion was related to HIV/AIDS prevention and management in the country and study populations. The other studies that recruited before the birth did not specify that participants had to intend to breastfeed.
Intensity of the intervention
Sixty of the 73 studies reported the intensity of the intervention in terms of the number of postnatal contacts the mother could have for breastfeeding support. Twenty‐four studies specified three or fewer contacts, 21 specified four to eight contacts, and the remaining 17 studies specified nine or more contacts. We have performed a subgroup analysis and the results are described in the text.
Control group care
Seven of the 73 studies were undertaken in hospital settings with Baby Friendly accreditation (Aksu 2011; Chapman 2004; Coutinho 2005; de Oliveira 2006; ISRCTN47056748; Tahir 2013; Yotebieng 2015). However, in the study by Yotebieng 2015, the intervention was the Baby Friendly Hospital Initiative (BFHI) so the control group did not access this. For the other six studies undertaken in settings with Baby Friendly accreditation, study interventions were additional to care that met Baby Friendly standards and were received by everyone at the hospital including all the study participants in the intervention and control groups. In two community‐based cluster‐randomised trials (Hoddinott 2009; Kronborg 2007), most of the maternity hospitals in which the participants had given birth had Baby Friendly accreditation.
In 29 studies control group care was not specified (n = 9) or stated to be standard care but not described (n = 20). In the remaining studies there was some description of control group care (see Characteristics of included studies). Standard postnatal care varies both between and within countries. Care may have differed within the study period and may also have differed from that which is offered at the present time.
Outcomes
Level of data collection
In 66 of the 73 studies outcome data were collected from the women who had received the intervention. In the other seven studies, the relationship between the recipients of the intervention and the source of the outcome data varied. In the three individually‐randomised trials of staff training (ISRCTN47056748; Labarere 2005; Santiago 2003), outcome data came from all the women randomised to receive, or not to receive, a support intervention from trained staff. In one of the three cluster‐randomised trials of staff training (Ekstrom 2006), data came from mothers of singleton term healthy infants at centres where staff had been randomised, or not randomised, to receive training. In another (Bhandari 2003), trained staff visited all families in the intervention villages and outcome data were collected from all infants in the intervention and control villages, and in the third (Kramer 2001), staff in all intervention sites were trained and data were collected from mothers who intended to breastfeed in the intervention and control sites. In the cluster‐randomised trial that evaluated a policy for providing breastfeeding groups (Hoddinott 2009), the policy intervention was made at locality level. Pregnant or postnatal women could be invited to groups in intervention clusters; however, only 1310 pregnant or breastfeeding women out of more than 9000 births in the intervention localities attended any group.
Duration of any and/or exclusive breastfeeding
Breastfeeding duration was most commonly assessed at six months. A total of 51 studies measured any breastfeeding at six months and 46 studies measured exclusive breastfeeding at six months. For the other primary outcomes, 33 studies measured any breastfeeding at four to six weeks and 32 measured exclusive breastfeeding at four to six weeks. When data on both seven‐day and 24‐hour recall were provided, we selected the data for 24‐hour recall.
The breastfeeding outcomes reported reflect World Bank country income group of the countries in which the 73 studies were undertaken. Most studies (29/52) reported the effect of the intervention on rates of both any and exclusive breastfeeding. Some studies report details about data collection that make it clear that duration of exclusive breastfeeding at specific time points was not necessarily measured from birth (Tylleskar 2011a; Tylleskar 2011b; Tylleskar 2011c; Vitolo 2005); most studies did not report this level of detail.
Secondary outcomes
Details of secondary outcomes were not collected for this update but will be included in the next update in two years time.
In the previous version of this review (Renfrew 2012b), a few studies reported various infant morbidity and maternal satisfaction with feeding and care outcomes by intervention group: infant morbidity was reported in 11 studies (Bashour 2008; Bhandari 2003; Bunik 2010; Frank 1987; Froozani 1999; Kramer 2001; Morrow 1999; Petrova 2009; Pugh 2002; Quinlivan 2003; Tylleskar 2011a; Tylleskar 2011b; Tylleskar 2011c); maternal satisfaction with feeding in 11 studies (Bashour 2008; de Oliveira 2006; Dennis 2002; Hoddinott 2009; Hopkinson 2009; Kronborg 2007; Labarere 2005; McDonald 2010; McQueen 2011; Petrova 2009; Pugh 1998), and maternal satisfaction with care in six studies (Bashour 2008; Ekstrom 2006; Graffy 2004; Jones 1985; Kools 2005; Morrow 1999).
Excluded studies
The previous version of this review excluded 79 studies from the review and we have excluded a further 68 studies. Thus 147 studies have been excluded with reasons (see Characteristics of excluded studies). The main reason for exclusion was because studies were not randomised trials, or it was not clear that allocation to groups had been carried out randomly; we excluded 18 studies identified by the search for this reason (Caulfield 1998; Davies‐Adetugbo 1996; Ebbeling 2007; Garcia‐Montrone 1996; Hall 2007; Jang 2008; Kistin 1994; McInnes 2000; Moreno‐Manzanares 1997; Neyzi 1991; Nor 2009; Pascali‐Bonaro 2004; Perez‐Escamilla 1992; Segura‐Millan 1994; Sisk 2006; Susin 2008; Thussanasupap 2006; Valdes 2000). A further two papers were reviews rather than reports of a randomised controlled trials (Guise 2003; Lewin 2005).
We excluded 72 trials because the intervention was not relevant to this review. We excluded 42 trials on the grounds that studies examined educational interventions where the focus was on instruction rather than on support to women to encourage breastfeeding (Ahmed 2016; Beiler 2011; Benitez 1992; Bolam 1998; Cattaneo 2001; Christie 2011; Edwards 2013a; Ehrlich 2014; Finch 2015; Flax 2014; Forster 2004; Giglia 2015; Girish 2013; Hanafi 2014; Harari 2014; Hauck 1994; Henderson 2001; Isselmann 2006; Jakobsen 2008; Jones 2004; Labarere 2003; Labarere 2011; Lavender 2004; Louzada 2012; Mattar 2003; Perez‐Blasco 2013; Phillips 2011; Pollard 1998; Rea 1999; Rossiter 1994; Sakha 2008; Schy 1996; Svensson 2013; Szucs 2015; Tully 2012; Vianna 2011; Vitolo 2012; Vitolo 2014; Wallace 2006; Wan 2011; Westphal 1995; Williams 2014). We excluded a further 13 trials as the intervention was not designed to support continued breastfeeding; these studies examined more general interventions in the postnatal period (Ball 2011; Barlow 2006; Barnet 2002; Black 2001; Gagnon 1997; MacArthur 2002; Peterson 2002; Pollard 2011; Ratner 1999; Rush 1991; Serrano 2010; Thomson 2009; Wiggins 2005); a further trial by Baqui 2008 focused on breastfeeding initiation only, rather than on postnatal support to encourage continuation. Eleven of the studies examined interventions carried out in the antenatal period only, and had no postnatal support component (Forster 2006; Jahan 2014; Johnston 2001; Katepa‐Bwalya 2011; Kronborg 2012; MacArthur 2009; Olenick 2011; Otsuka 2012; Noel‐Weiss 2006; Reeve 2004; Wockel 2009).
We excluded 25 of the studies that we assessed for inclusion as they did not focus on healthy mothers with healthy, term infants. Five trials examined interventions for low birthweight babies (Agrasada 2005; Brown 2008; Junior 2007; Pinelli 2001; Thakur 2012), while the Ahmed 2008 study recruited only mothers of premature babies. The Davies‐Adetugbo 1997 and Haider 1996 studies recruited the mothers of babies with severe diarrhoea, the Merewood 2006, Phillips 2010 and Phillips 2012 studies recruited only mothers of babies admitted to neonatal intensive care units, and the Pound 2015 study only included babies with jaundice. The Ferrara 2008 and Stuebe 2016 trials focused on an intervention for mothers with diabetes, the Martin 2015 and Carlsen 2013 studies focused on overweight women, and the Gijsbers 2006 and Mesters 2013 trials on families with a history of asthma, while Moore 1985 looked at infants with a parent with eczema or asthma. Three other trials recruited only women in high‐risk groups (Chapman 2011; McLeod 2003; Rasmussen 2011). Two studies focused providing support for fathers (Byas 2011; Tohotoa 2012), and one study concerned training for student nurses (Davis 2014).
The remaining trials were excluded for other reasons (Bica 2014; Finch 2002; Haider 2014; Hives‐Wood 2013; Hoddinott 2012a; Lieu 2000; Mannan 2008; Nkonki 2014; Nor 2012; Ochola 2013a; Penfold 2014; Rasmussen 2011; Rowe 1990; Sciacca 1995; Steel O'Connor 2003; Thakur 2012; Wasser 2015). Further details of these, and other excluded studies, can be found in the Characteristics of excluded studies tables.
Risk of bias in included studies
Each trial was assessed for methodological quality as outlined in the Methods section (see Figure 1 and Figure 2).
Allocation
Random sequence generation: we considered that a little over half of the studies included in the review used methods that were at low risk of bias to generate the randomisation sequence: we deemed 54 studies to be at low risk; nine studies at high risk and 38 studies to be at unclear risk.
Allocation concealment: we considered that a little less than half of the studies included in the review used methods that were at low risk of bias to conceal allocation to experimental groups: we deemed 44 studies to be at low risk; nine studies at high risk and 48 studies to be at unclear risk.
Blinding
Blinding participants and personnel: with interventions of this type, it is very difficult to assess risk of bias associated with blinding. Both the mothers and the staff providing care would probably be aware that they were either receiving or delivering an intervention. In studies where there was randomisation at the clinic level, all women may have been exposed to the same intervention, and contamination between groups would thereby be reduced, but there may still have been a risk of response bias if outcomes were reported to staff providing care. Therefore, we assessed no studies as being at a low risk of bias for this domain.
Blinding of outcome assessment: we assessed approximately one‐quarter of studies as being at low risk of bias for blinding of outcome assessment: we deemed 27 studies to be at low risk, 27 studies to be at high risk, and 47 studies at unclear risk.
Incomplete outcome data
We had prespecified that we would not include data for any outcome where there were missing data for more than 25% of the randomised group. Loss to follow‐up was a particular problem in studies where women were recruited in the antenatal period and, as we have described above, we have not included any outcome data from studies that were otherwise eligible for inclusion in the review because of high levels of attrition for all outcomes. For some of those studies that contributed data there was still considerable loss to follow‐up, and loss was not always balanced across randomisation groups. When assessing incomplete outcome data, reasons for loss of data were not taken into consideration.
We judged approximately one‐third of studies to be at low risk of bias for incomplete outcome data: we deemed 36 studies to be at low risk; 18 studies at high risk and 47 studies to be at unclear risk.
Selective reporting
We assessed bias in most of the studies included in the review from published study reports. In most cases we did not have access to the trial registration or protocol. Under these circumstances assessing risk of bias due to selective reporting bias is very difficult. For this reason in the last version of this review all of the studies were deemed to be at unclear risk of bias for this domain. In this update we were able to access some protocols for newly included studies. Only 10 studies have been assessed as being at low risk of bias; five studies as high risk of bias and 86 studies as unclear.
Other potential sources of bias
We have noted any other concerns about bias (including any apparent baseline imbalance between randomised groups) in the Characteristics of included studies tables along with further information about the judgements we made about risk of bias for each included study. The quality of the studies was very mixed and most of the studies had some methodological weakness, or did not provide good information about methods. It is important that the mixed quality of the evidence is taken into account in the interpretation of results. We judged 28 studies to be at low risk of other potential sources of bias; six studies at high risk of bias and 67 studies at unclear risk.
Effects of interventions
See: Table 1
Interventions to support breastfeeding versus usual care: 73 studies
Primary outcomes
Outcome 1.1: Stopping any breastfeeding up to six months postpartum
The main summary outcome measure was cessation of any breastfeeding at the time of the last study assessment up to six months (Analysis 1.1).
1.1. Analysis.
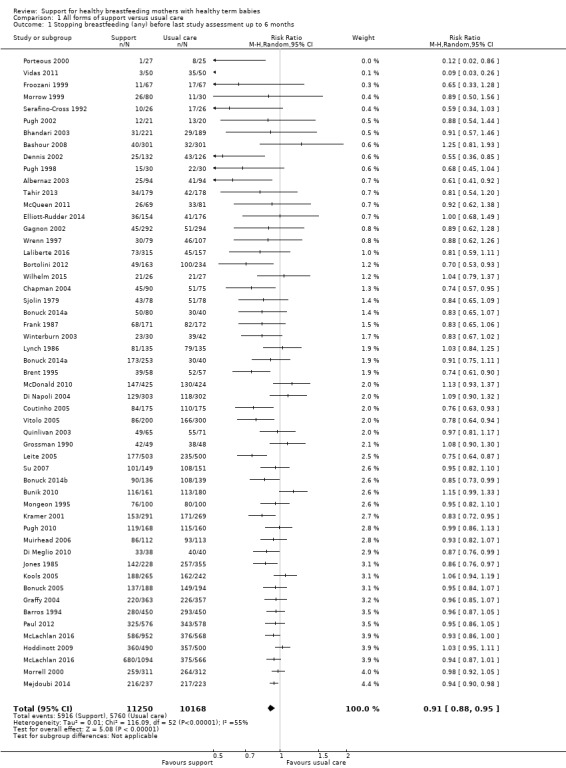
Comparison 1 All forms of support versus usual care, Outcome 1 Stopping breastfeeding (any) before last study assessment up to 6 months.
In the meta‐analysis for this outcome the previous version of this review we included 40 trials with 14,227 women (effective sample size). With the new studies we added in this update, there are now 51 studies with 21,418 women included. One new study (the BINGO trial, Bonuck 2014a), contributes two intervention arms to the analysis and the control group was split to avoid double counting. One of the cluster‐randomised trials (McLachlan 2016) also contributed two intervention arms and splitting over the control group was included in the adjustment for clustering.
Interventions to support breastfeeding appear to have a beneficial effect on the number of women who continue breastfeeding beyond six months, with fewer women in the groups that receive support stopping breastfeeding by this time (average risk ratio (RR) 0.91, 95% confidence interval (CI) 0.88 to 0.95;moderate‐quality evidence). Overall, 52.59% of those receiving support interventions had stopped any breastfeeding by six months compared with 56.64% of controls (unweighted percentages). However, there was high heterogeneity for this outcome and results should therefore be interpreted with caution (heterogeneity: Tau² = 0.01, I² = 55%, Chi² = 116.09, P < 0.00001).
Sensitivity analysis using only studies assessed as having a low risk of bias for allocation concealment demonstrated a similar positive treatment effect on breastfeeding at up to six months (Analysis 1.5). A sensitivity analysis omitting the cluster‐randomised study (Elliott‐Rudder 2014) for which a design effect could not be calculated demonstrated a similar positive treatment effect (average RR 0.91, 95% CI 0.88 to 0.94).
1.5. Analysis.
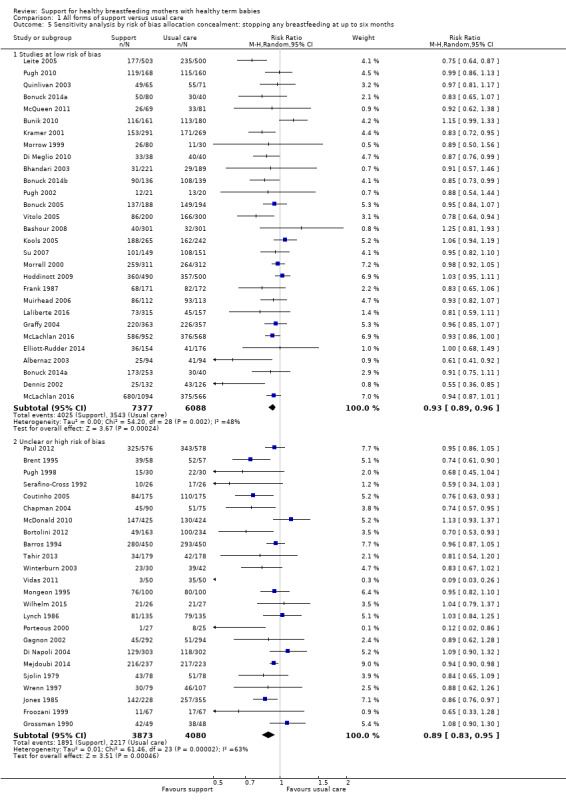
Comparison 1 All forms of support versus usual care, Outcome 5 Sensitivity analysis by risk of bias allocation concealment: stopping any breastfeeding at up to six months.
Visual inspection of the funnel plot generated for this outcome suggested that there was some asymmetry with results from smaller studies tending to show a greater positive treatment effect (Figure 3).
3.
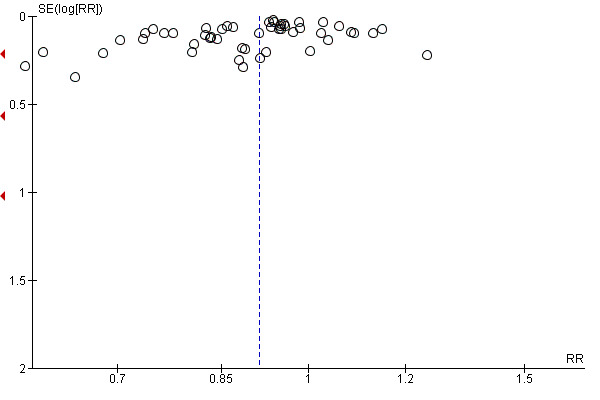
Funnel plot of comparison: 1 All forms of support versus usual care, outcome: 1.1 Stopping breastfeeding (any) before last study assessment up to 6 months
Outcome 1.2: Stopping exclusive breastfeeding up to six months postpartum
In the previous version of this review we included 33 studies with 11,961 women in the analysis of women who had stopped exclusive breastfeeding at up to six months. With the new studies we added in this update, there are now 46 studies with 18,591 women included. Two new studies (the BINGO trial, Bonuck 2014a;Yotebieng 2015), contribute two intervention arms to the analysis; we split the control group to avoid double‐counting.
Women in the intervention groups were less likely to have stopped exclusive breastfeeding before six months (average RR 0.88, 95% CI 0.85 to 0.92; moderate‐quality evidence) (Heterogeneity: Tau² = 0.01, I² = 96%, Chi² = 1076.75, P<0.00001; Analysis 1.2); although 74.9% of women in the intervention groups had stopped exclusive breastfeeding by this time, a greater proportion of women in the control groups had stopped (83.4%; unweighted percentages).
1.2. Analysis.
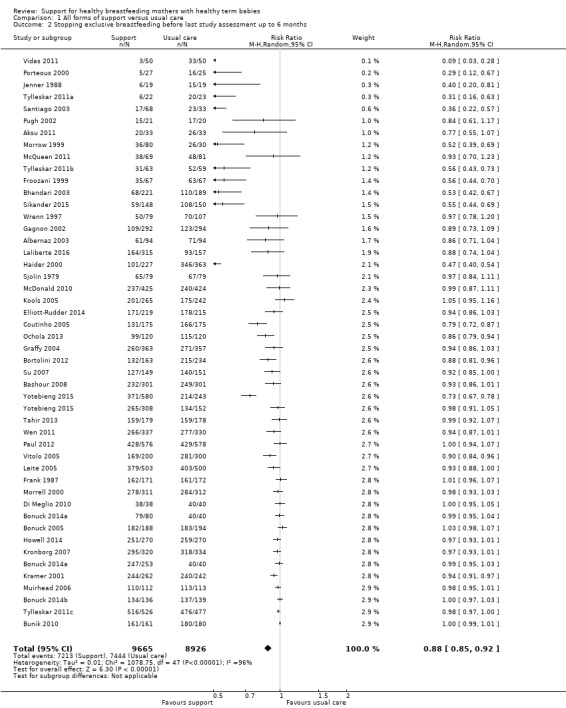
Comparison 1 All forms of support versus usual care, Outcome 2 Stopping exclusive breastfeeding before last study assessment up to 6 months.
Sensitivity analysis using only those studies assessed as having a low risk of bias for allocation concealment revealed that results still significantly favoured the intervention groups (Analysis 1.6), although the effect size was reduced in the studies at low risk of bias (average RR 0.93, 95% CI 0.89 to 0.96). A sensitivity analysis omitting the one arm of the cluster‐randomised study (Yotebieng 2015) for which a design effect could not be calculated demonstrated the same positive treatment effect (average RR 0.88, 95% CI 0.84 to 0.91).
1.6. Analysis.
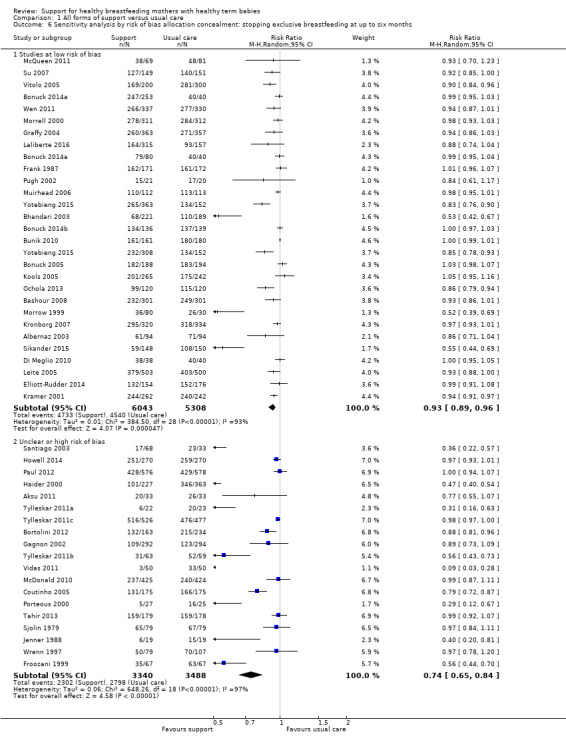
Comparison 1 All forms of support versus usual care, Outcome 6 Sensitivity analysis by risk of bias allocation concealment: stopping exclusive breastfeeding at up to six months.
Visual examination of a funnel plot for this outcome suggested that the treatment effect may have been more pronounced in smaller studies (Figure 4).
4.
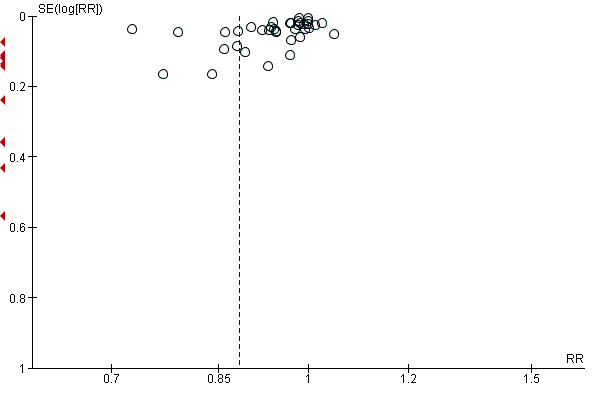
Funnel plot of comparison: 1 All forms of support versus usual care, outcome: 1.2 Stopping exclusive breastfeeding before last study assessment up to 6 months
Outcome 1.3: Stopping any breastfeeding before four to six weeks postpartum
In the previous version of this review we included 25 studies with 8513 women in the analysis of women stopping breastfeeding before four to six weeks. With the new studies we added in this update, there are now 33 studies with 10,776 women included. Two new studies (the BINGO trial, Bonuck 2014a; Fu 2014), contribute two intervention arms to the analysis, and we have split the control arm to avoid double‐counting.
Women receiving support interventions were less likely to stop breastfeeding before six weeks (average RR 0.87, 95% CI 0.80 to 0.95; moderate‐quality evidence): while 31.3% of women in the intervention groups had stopped exclusive breastfeeding by this time, 34.8% of women in control groups had also stopped (unweighted percentages). There was considerable variation in the results from individual studies (heterogeneity: Tau² = 0.02, I² = 54%, Chi² = 74.65, P = 0.0001; Analysis 1.3).
1.3. Analysis.
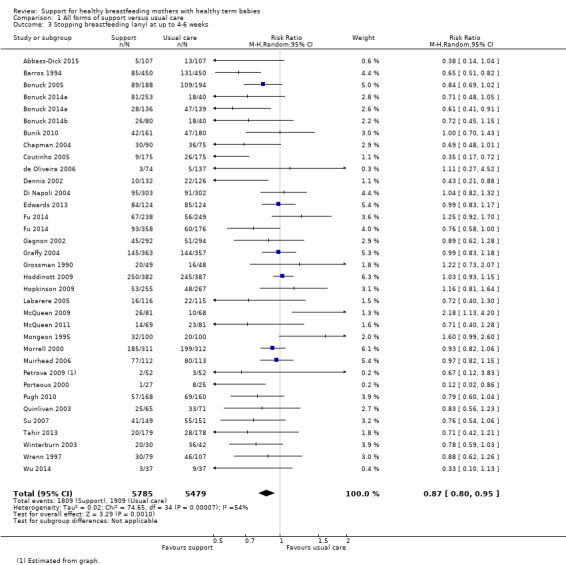
Comparison 1 All forms of support versus usual care, Outcome 3 Stopping breastfeeding (any) at up to 4‐6 weeks.
Sensitivity analysis using only those studies assessed as having a low risk of bias for allocation concealment revealed that results still significantly favoured the intervention groups (Analysis 1.7), although the effect size was reduced in the studies at low risk of bias (average RR 0.88, 95% CI 0.81 to 0.96).
1.7. Analysis.
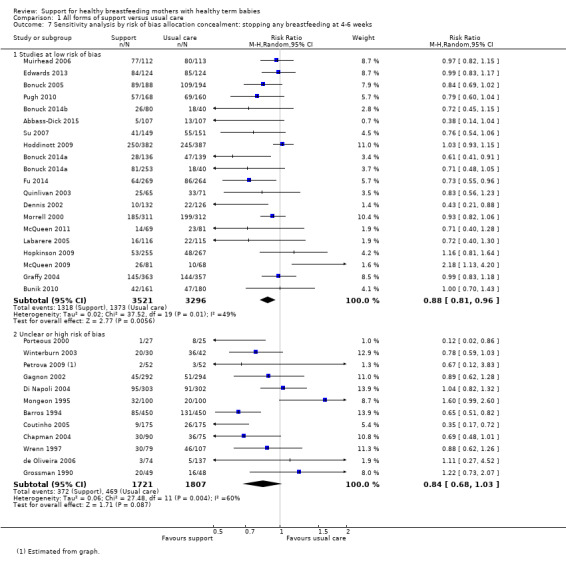
Comparison 1 All forms of support versus usual care, Outcome 7 Sensitivity analysis by risk of bias allocation concealment: stopping any breastfeeding at 4‐6 weeks.
Visual examination of a funnel plot for this outcome suggested that the treatment effect may have been more pronounced in smaller studies (Figure 5).
5.
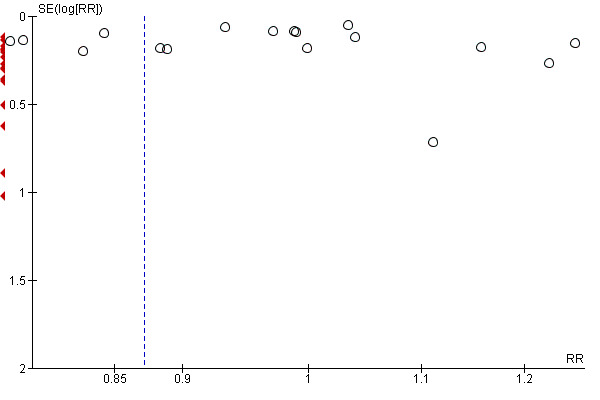
Funnel plot of comparison: 1 All forms of support versus usual care, outcome: 1.3 Stopping breastfeeding (any) at up to 4‐6 weeks
Outcome 1.4: Stopping exclusive breastfeeding before four to six weeks postpartum
In the previous version of this review, we included 24 studies with 7693 women in the analysis of women who had stopped exclusive breastfeeding before four to six weeks. With the new studies we added in this update, there are now 32 studies with 10,960 women included. Two new studies (the BINGO trial, Bonuck 2014a; Fu 2014), contribute two intervention arms to the analysis, and we have split the controls to avoid double‐counting.
Women in the intervention groups were less likely to stop exclusive breastfeeding by six weeks compared with women in the control groups (average RR 0.79, 95% CI 0.71 to 0.89; moderate‐quality evidence); and while 57.2% of women in the intervention groups had stopped exclusive breastfeeding by this time, 65.0% of women in control groups had also stopped (unweighted percentages); (heterogeneity: Tau² = 0.10, I² = 97%, Chi² = 1160.22, P < 0.00001; Analysis 1.4).
1.4. Analysis.
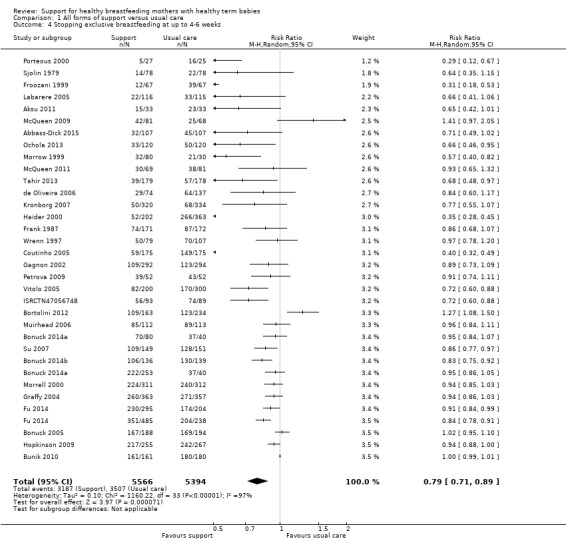
Comparison 1 All forms of support versus usual care, Outcome 4 Stopping exclusive breastfeeding at up to 4‐6 weeks.
Again, there was some evidence that the treatment effect may be partly due to bias; sensitivity analysis including only those studies assessed as being at low risk of bias for allocation concealment showed that results still favoured the intervention group although the treatment effect was less pronounced in the studies at lower risk of bias (Analysis 1.8). A sensitivity analysis omitting the cluster‐randomised study (Ochola 2013) for which a design effect could not be calculated demonstrated a similar positive treatment effect (average RR 0.80, 95% CI 0.71 to 0.89).
1.8. Analysis.
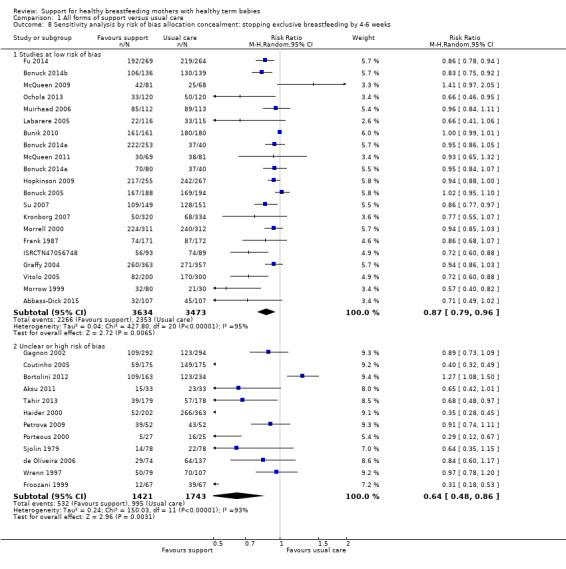
Comparison 1 All forms of support versus usual care, Outcome 8 Sensitivity analysis by risk of bias allocation concealment: stopping exclusive breastfeeding by 4‐6 weeks.
Visual examination of the funnel plot for this outcome suggested that the treatment effect may have been more pronounced in smaller studies and that there may be smaller studies missing which do not find an effect in favour of the intervention (Figure 6).
6.
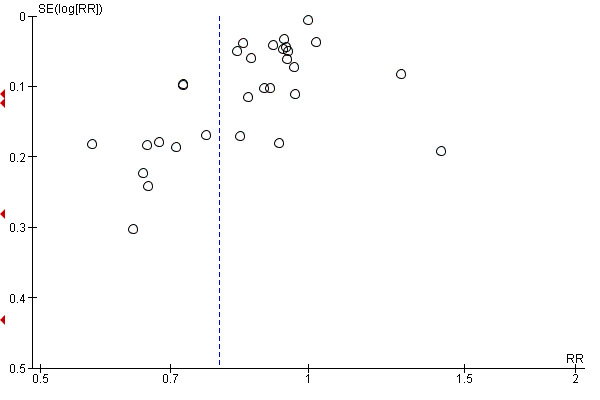
Funnel plot of comparison: 1 All forms of support versus usual care, outcome: 1.4 Stopping exclusive breastfeeding at up to 4‐6 weeks
Secondary outcomes
This update of the review does not include an analysis of secondary outcomes.
Secondary outcomes were not considered in this update so that the review could be completed in time to inform the World Health Organisation’s review of the evidence and update of the WHO recommendations on breastfeeding in maternity facilities. A new set of core outcomes for Cochrane pregnancy and childbirth breastfeeding reviews is currently being developed and the outcomes from this core set may influence future outcomes chosen for this review.
Subgroup analysis
There was considerable variation between the trials in terms of the interventions examined, the standard care offered to women, and the background breastfeeding initiation rates in the various study settings. We wanted to explore whether different types of support and settings were associated with different or more pronounced treatment effects. Therefore, for the review's four primary outcomes we carried out subgroup analysis to explore the impact of interventions involving different types of supporter (professional versus lay person, or both); types of support (face‐to‐face versus telephone support or both); timing of support (antenatal and postnatal versus postnatal alone); whether the support was proactive (scheduled contacts) or reactive (women needed to request support); and whether support interventions had similar effects in settings with different background breastfeeding initiation rates (low, medium or high background rates).
For all subgroup analyses, the covariate chosen does little to explain the high heterogeneity; most within‐group heterogeneity remains high.
Who delivered the support
For our four primary outcomes, we examined whether the treatment effect was similar where the support was delivered by professionals as opposed to non‐professionals (lay support) or both. It should be noted that most studies involved delivery of the intervention by professionals (37 of the 51 studies).
For cessation of any breastfeeding at up to six months it appeared that support from non‐professionals was associated with a broadly similar treatment effect to that for support from professionals (Analysis 2.1).
2.1. Analysis.
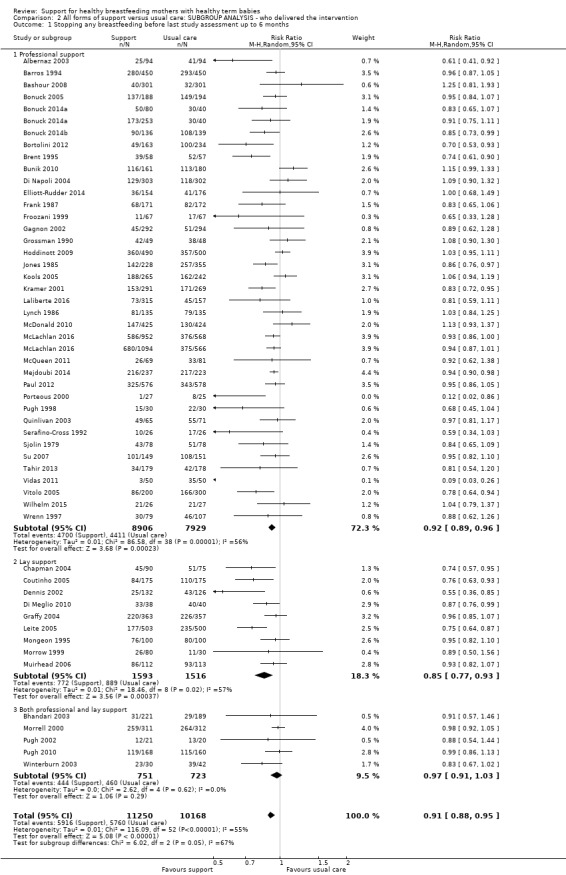
Comparison 2 All forms of support versus usual care: SUBGROUP ANALYSIS ‐ who delivered the intervention, Outcome 1 Stopping any breastfeeding before last study assessment up to 6 months.
The test for subgroup differences suggests a differential treatment effect according to who delivers the support, but we are not confident in this result due to very different subgroup sizes and high within‐group heterogeneity in two of three groups and zero heterogeneity in the other (test for subgroup differences: Chi² = 6.02, degrees of freedom (df) = 2 (P = 0.05), I² = 66.8%). When the smaller, mixed support group is removed from the analysis, there is no evidence of a difference between lay and professional support (test for subgroup differences: Chi² = 2.89, df = 1 (P = 0.09), I² = 65.4%).
For cessation of exclusive breastfeeding at up to six months the treatment effect appears to be greater when the intervention was delivered by non‐professionals (lay support) compared with professionals or mixed support (test for subgroup differences: Chi² = 7.74, df = 2 (P = 0.02), I² = 73.1%; Analysis 2.2). The confidence intervals for professional and lay support do not overlap, but due to the high heterogeneity remaining within the subgroups, we advise caution when interpreting this result.
2.2. Analysis.
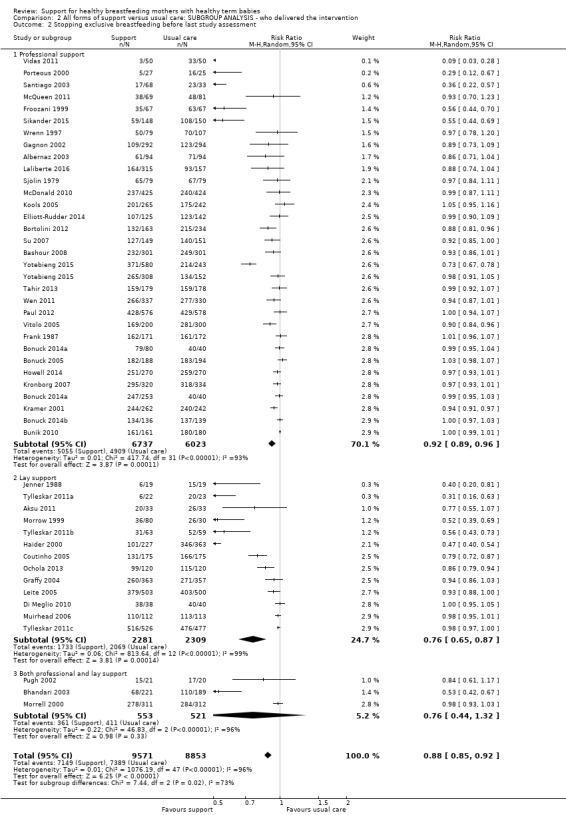
Comparison 2 All forms of support versus usual care: SUBGROUP ANALYSIS ‐ who delivered the intervention, Outcome 2 Stopping exclusive breastfeeding before last study assessment.
For cessation of any breastfeeding by four to six weeks there is no evidence for a differential effect when professionals, lay or both deliver support (test for subgroup differences: Chi² = 1.47, df = 2 (P = 0.48), I² = 0%; Analysis 2.3).
2.3. Analysis.
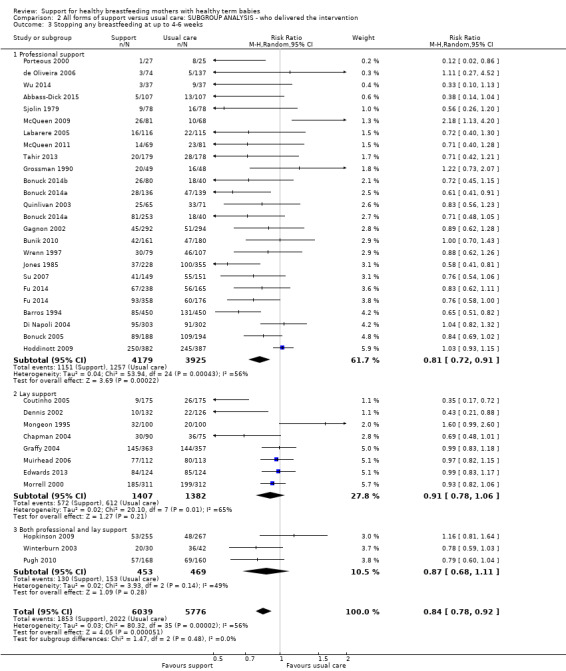
Comparison 2 All forms of support versus usual care: SUBGROUP ANALYSIS ‐ who delivered the intervention, Outcome 3 Stopping any breastfeeding at up to 4‐6 weeks.
For cessation of exclusive breastfeeding by four to six weeks the test for subgroup differences indicates a possible differential treatment effect (test for subgroup differences: Chi² = 7.12, df = 2 (P = 0.03), I² = 71.9%). However, we are not confident in this result, because the mixed subgroup is disproportionately small. When this third group is removed from the analysis there is no evidence for a differential effect between professional and lay support test for subgroup differences: Chi² = 2.31, df = 1 (P = 0.13), I² = 56.7%; Analysis 2.4).
2.4. Analysis.
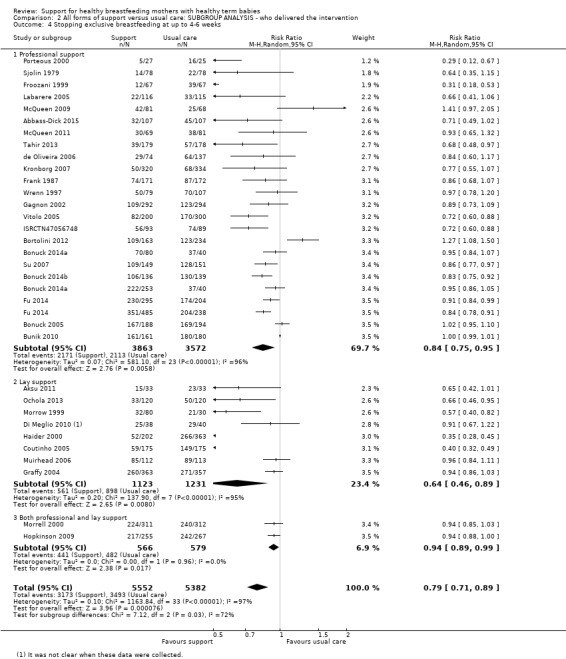
Comparison 2 All forms of support versus usual care: SUBGROUP ANALYSIS ‐ who delivered the intervention, Outcome 4 Stopping exclusive breastfeeding at up to 4‐6 weeks.
Type of support
We compared different types of intervention (support provided predominantly by face‐to‐face contact, predominantly by telephone, or by both face‐to‐face and telephone contact) for our primary outcomes.
For cessation of any breastfeeding at up to six months there was no evidence of a differential effect according to type of support (test for subgroup differences: Chi² = 0.40, df = 2 (P = 0.82), I² = 0%; Analysis 3.1).
3.1. Analysis.
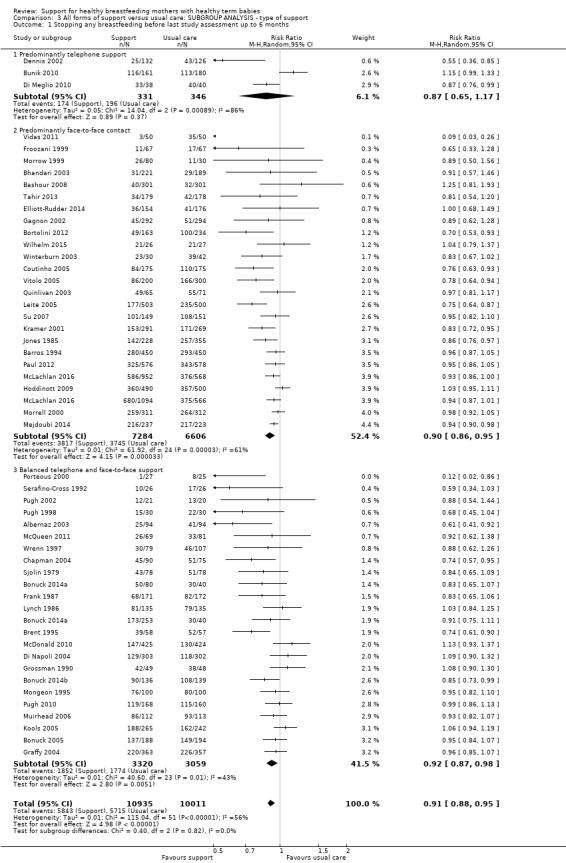
Comparison 3 All forms of support versus usual care: SUBGROUP ANALYSIS ‐ type of support, Outcome 1 Stopping any breastfeeding before last study assessment up to 6 months.
For cessation of exclusive breastfeeding at up to six months face‐to‐face interventions may be associated with greater effects than other types of support; however, very high within‐group heterogeneity remains in the analysis, and we advise caution when interpreting this result (test for subgroup differences: Chi² = 37.55, df = 2 (P<.00001, I² = 94.7%; Analysis 3.2).
3.2. Analysis.
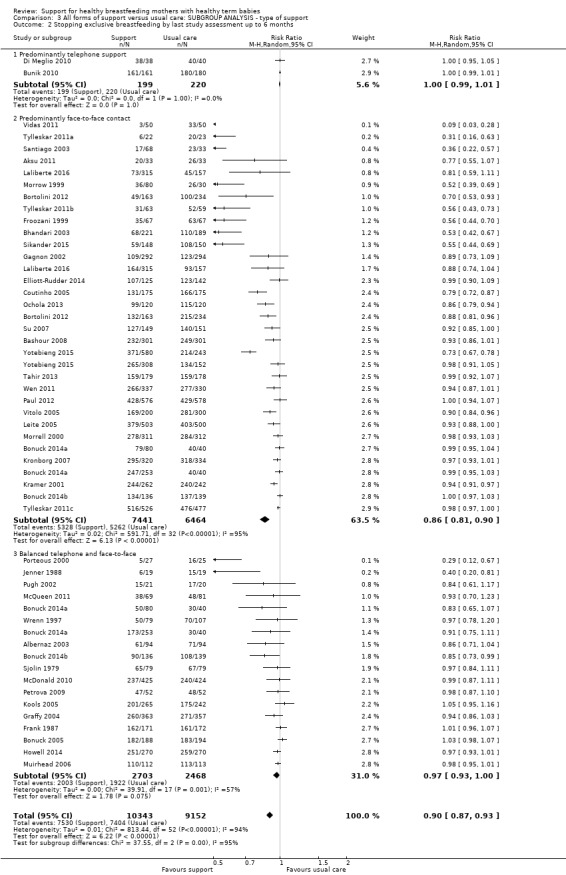
Comparison 3 All forms of support versus usual care: SUBGROUP ANALYSIS ‐ type of support, Outcome 2 Stopping exclusive breastfeeding by last study assessment up to 6 months.
For cessation of any breastfeeding by four to six weeks, there is no evidence of a differential effect according to type of support (test for subgroup differences: Chi² = 0.91, df = 2 (P = 0.64), I² = 0%; Analysis 3.3).
3.3. Analysis.
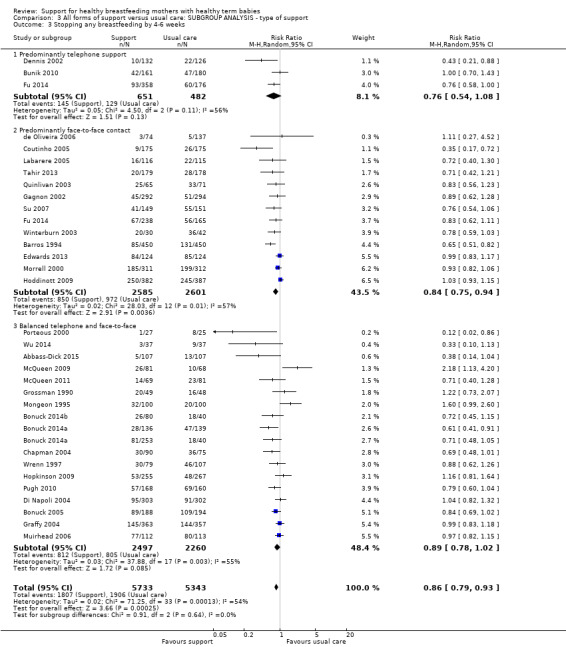
Comparison 3 All forms of support versus usual care: SUBGROUP ANALYSIS ‐ type of support, Outcome 3 Stopping any breastfeeding by 4‐6 weeks.
For cessation of exclusive breastfeeding at up to four to six weeks, face‐to‐face interventions may be associated with greater effects than other types of support; however very high within‐group heterogeneity remains in the analyses, and we advise caution when interpreting this result (test for subgroup differences: Chi² = 10.63, df = 2 (P = 0.005), I² = 81.2%; Analysis 3.4).
3.4. Analysis.
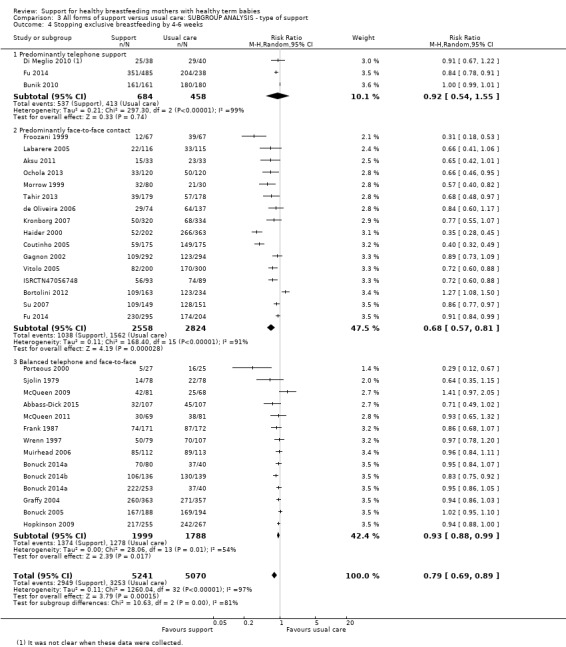
Comparison 3 All forms of support versus usual care: SUBGROUP ANALYSIS ‐ type of support, Outcome 4 Stopping exclusive breastfeeding by 4‐6 weeks.
When the support was offered
We examined whether offering support with an antenatal component rather than postnatal support alone was associated with any difference in treatment effect. The results were similar in both subgroups for all of our four primary outcomes and there were no significant subgroup differences according to the interaction tests (Analysis 4.1 test for subgroup differences: test for subgroup differences: Chi² = 0.15, df = 1 (P = 0.70), I² = 0%); (Analysis 4.2 test for subgroup differences: Chi² = 1.54, df = 1 (P = 0.21), I² = 35.1%); (Analysis 4.3 test for subgroup differences: Chi² = 1.05, df = 1 (P = 0.31), I² = 4.4%); (Analysis 4.4 test for subgroup differences: Chi² = 2.07, df = 1 (P = 0.15), I² = 51.6%).
4.1. Analysis.
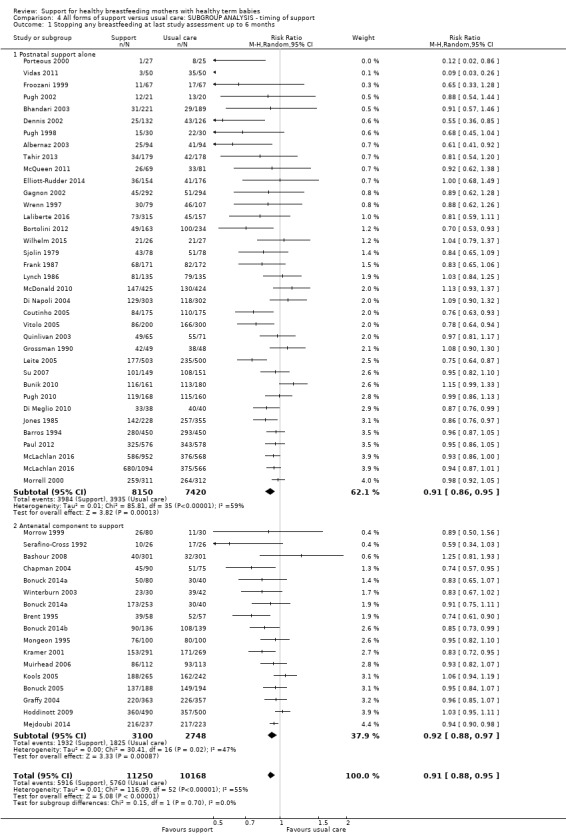
Comparison 4 All forms of support versus usual care: SUBGROUP ANALYSIS ‐ timing of support, Outcome 1 Stopping any breastfeeding at last study assessment up to 6 months.
4.2. Analysis.
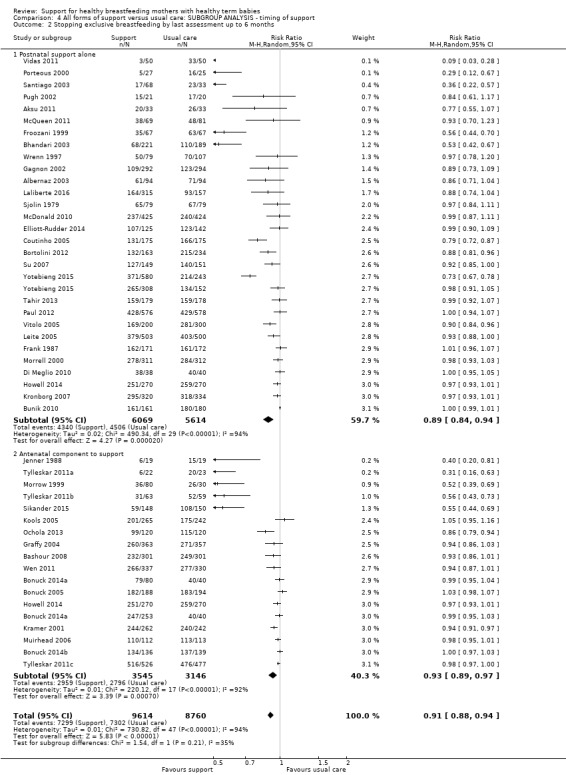
Comparison 4 All forms of support versus usual care: SUBGROUP ANALYSIS ‐ timing of support, Outcome 2 Stopping exclusive breastfeeding by last assessment up to 6 months.
4.3. Analysis.
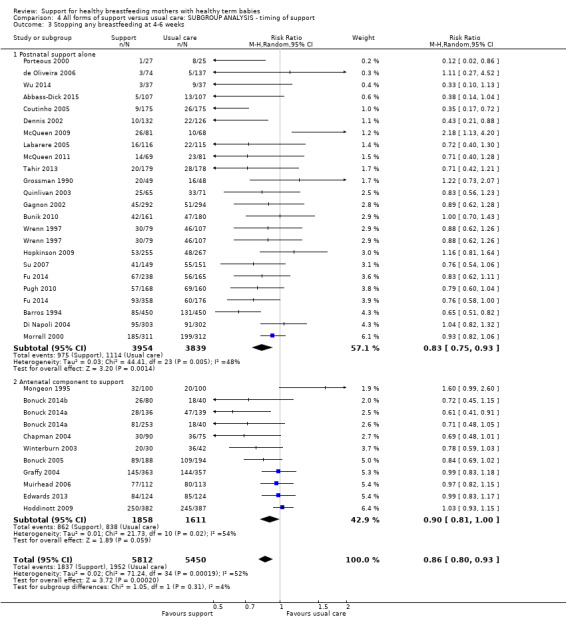
Comparison 4 All forms of support versus usual care: SUBGROUP ANALYSIS ‐ timing of support, Outcome 3 Stopping any breastfeeding at 4‐6 weeks.
4.4. Analysis.
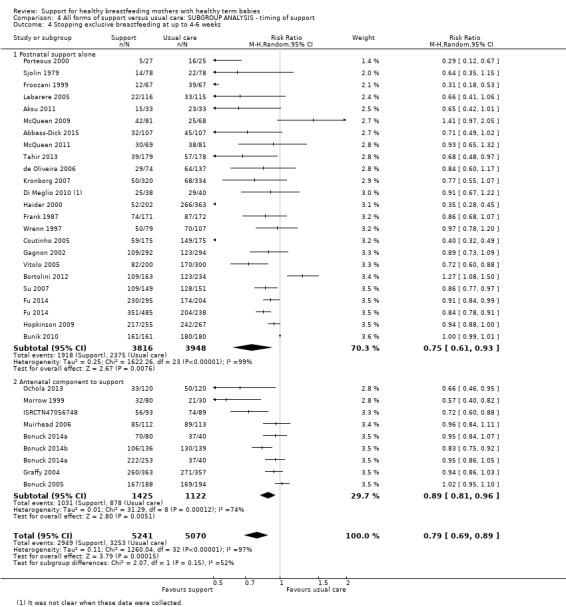
Comparison 4 All forms of support versus usual care: SUBGROUP ANALYSIS ‐ timing of support, Outcome 4 Stopping exclusive breastfeeding at up to 4‐6 weeks.
Proactive versus reactive support
We had planned to carry out formal subgroup analysis by whether support was proactive or reactive, but due to the fact that most interventions included at least one scheduled contact (proactive), we did not think that this way of categorising studies would shed light on types of interventions that were effective or ineffective.
Background breastfeeding initiation rates in study settings
We were interested in whether or not background rates of breastfeeding in different settings had any impact on the success of interventions. We divided the studies into three groups: those carried out in settings where 80% or more women initiated breastfeeding (high background initiation), where between 60% to 80% initiated breastfeeding (intermediate) or where breastfeeding initiation rates were less than 60% (low). These groups showed an inverse relationship with World Bank country income groups. The studies with high background rates of breastfeeding initiation were set in countries from all the World Bank country income groups, however, the four studies from low‐/low‐middle income countries had the highest rates (more than 95%). All the studies with intermediate or low background rates of breastfeeding initiation were undertaken in high‐income countries.
We found the interventions had a greater effect of preventing women stopping exclusive breastfeeding at both time points in countries where background rates were already high; there was no similar effect on any breastfeeding. Because within‐group heterogeneity remains high in all analyses, we advise caution when interpreting this result.
For cessation of any breastfeeding at up to six months, there is no evidence of a difference in the effectiveness of the intervention according to the background breastfeeding rate (test for subgroup differences: Chi² = 0.56, df = 2 (P = 0.76), I² = 0%; Analysis 5.1).
5.1. Analysis.
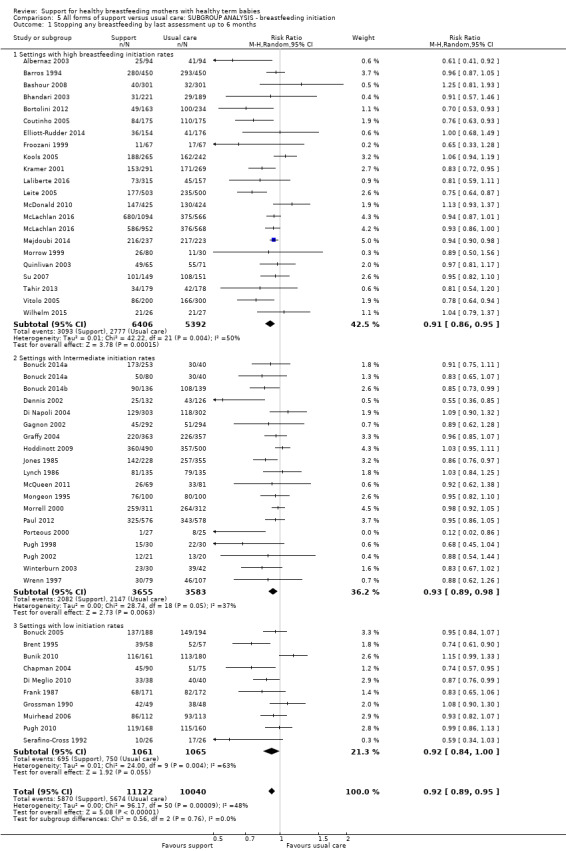
Comparison 5 All forms of support versus usual care: SUBGROUP ANALYSIS ‐ breastfeeding initiation, Outcome 1 Stopping any breastfeeding by last assessment up to 6 months.
For cessation of exclusive breastfeeding at up to six months, the intervention effect appears greater in studies where breastfeeding initiation rates were high (test for subgroup differences: Chi² = 30.73, df = 2 (P <.00001, I² = 93.5%; Analysis 5.2). However, within‐group heterogeneity remains very high, and we advise caution when interpreting this result.
5.2. Analysis.
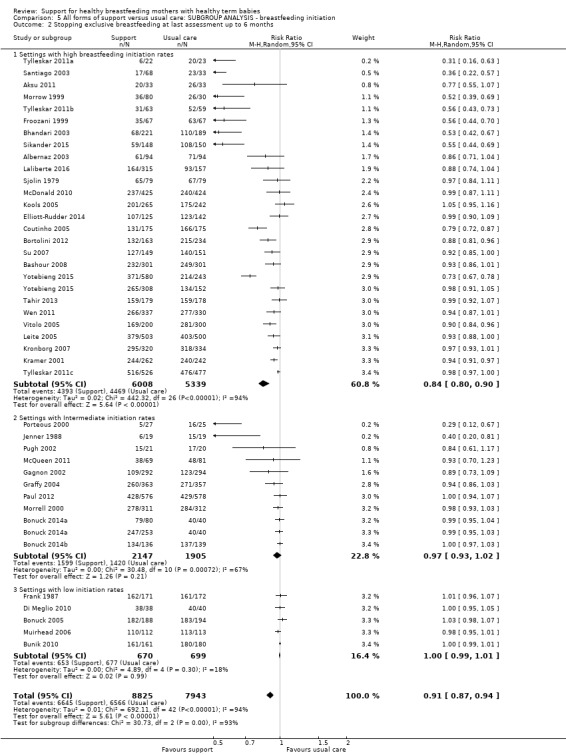
Comparison 5 All forms of support versus usual care: SUBGROUP ANALYSIS ‐ breastfeeding initiation, Outcome 2 Stopping exclusive breastfeeding at last assessment up to 6 months.
For cessation of any breastfeeding by four to six weeks, there is no evidence that the interventions worked differently in trials with different background rates of breastfeeding (test for subgroup differences: Chi² = 2.72, df = 2 (P = 0.26), I² = 26.6%; Analysis 5.3).
5.3. Analysis.
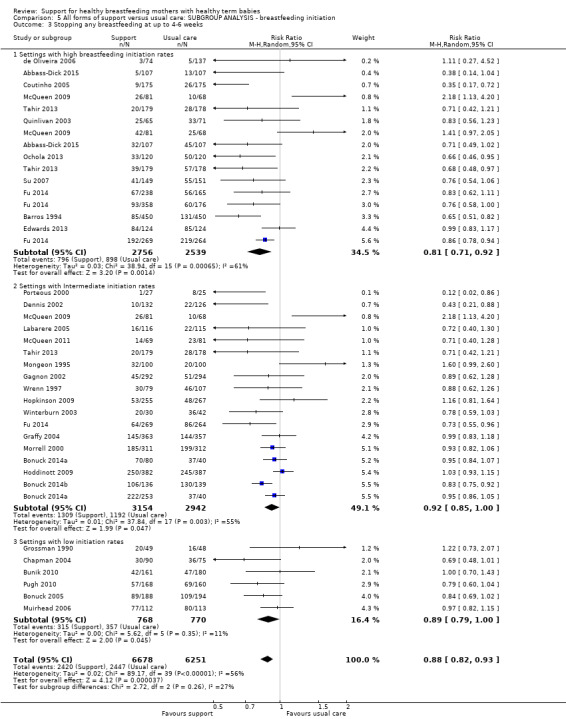
Comparison 5 All forms of support versus usual care: SUBGROUP ANALYSIS ‐ breastfeeding initiation, Outcome 3 Stopping any breastfeeding at up to 4‐6 weeks.
For cessation of exclusive breastfeeding at up to four to six weeks, the intervention effect appears stronger in studies where initiation rates were high (test for subgroup differences: Chi² = 9.24, df = 2 (P = 0.010), I² = 78.4% Analysis 5.4). However, within‐group heterogeneity remains very high in all subgroups, and we advise caution when interpreting this result.
5.4. Analysis.
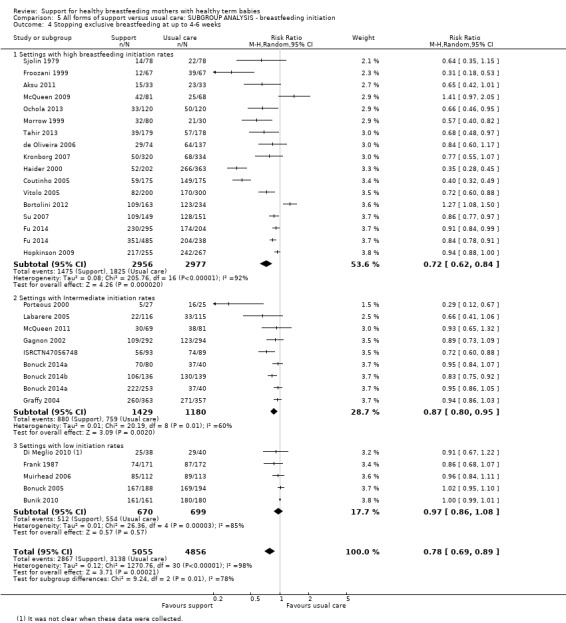
Comparison 5 All forms of support versus usual care: SUBGROUP ANALYSIS ‐ breastfeeding initiation, Outcome 4 Stopping exclusive breastfeeding at up to 4‐6 weeks.
Intensity of the intervention: the number of postnatal contacts
We examined whether different numbers of postnatal contacts were associated with any difference in treatment effect. We divided the studies into four subgroups: unspecified or no direct contacts (for example in studies that involved staff training rather than direct contacts with women); less than four postnatal contacts; between four and eight contacts; and more than eight contacts. Trials including antenatal contacts are included here, and we used the number of postnatal contacts to determine the appropriate subgroup.
For cessation of any breastfeeding at the final study assessment up to six months, there was no evidence of subgroup differences (test for subgroup differences: Chi² = 1.45, df = 3 (P = 0.69, I² = 0%; Analysis 6.1).
6.1. Analysis.
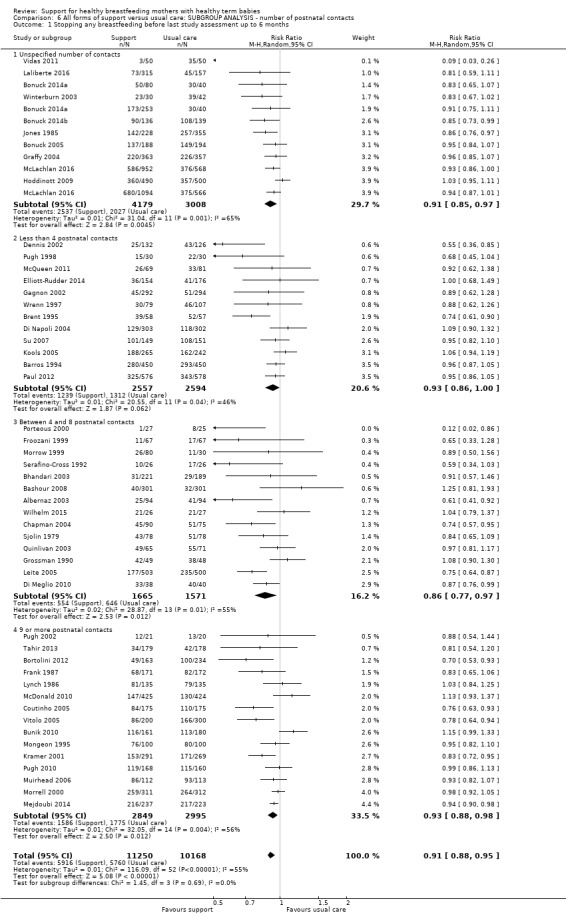
Comparison 6 All forms of support versus usual care: SUBGROUP ANALYSIS ‐ number of postnatal contacts, Outcome 1 Stopping any breastfeeding before last study assessment up to 6 months.
For cessation of exclusive breastfeeding at up to six months, there appears to be a differential effect of the number of postnatal contacts, with four to eight contacts performing best. The confidence intervals for this subgroup do not overlap with any other subgroup, but within‐group heterogeneity for all subgroups remains very high, and we advise caution when interpreting this result (Analysis 6.2; test for subgroup differences: Chi² = 13.78, df = 3 (P = 0.003), I² = 78.2%).
6.2. Analysis.
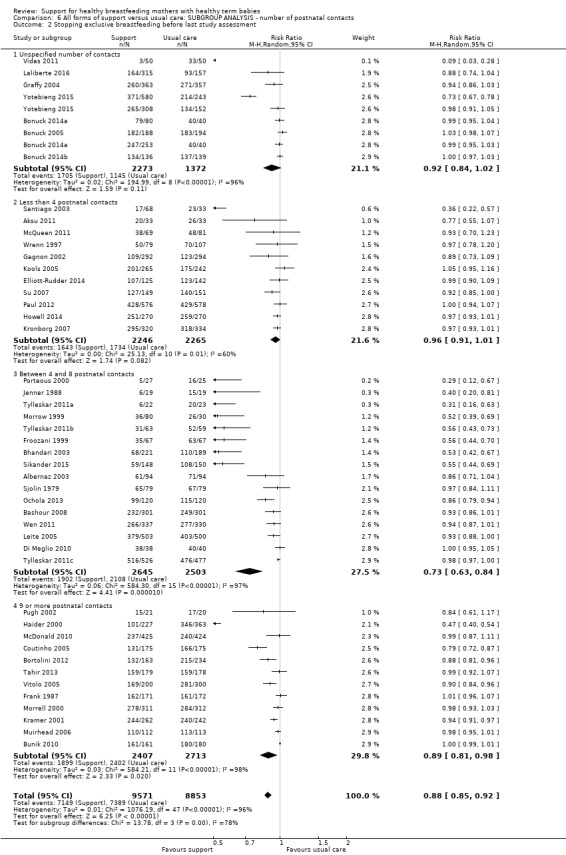
Comparison 6 All forms of support versus usual care: SUBGROUP ANALYSIS ‐ number of postnatal contacts, Outcome 2 Stopping exclusive breastfeeding before last study assessment.
For cessation of any breastfeeding by four to six weeks, there was no evidence of a differential treatment effect according to the number of postnatal contacts (test for subgroup differences: Chi² = 1.59, df = 3 (P = 0.66), I² = 0%; Analysis 6.3).
6.3. Analysis.
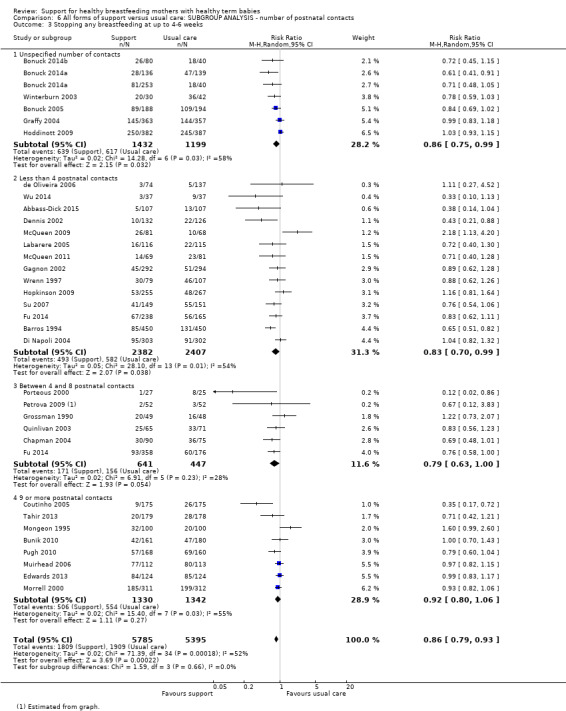
Comparison 6 All forms of support versus usual care: SUBGROUP ANALYSIS ‐ number of postnatal contacts, Outcome 3 Stopping any breastfeeding at up to 4‐6 weeks.
For cessation of exclusive breastfeeding at up to four to six weeks there appears to be differential treatment effect according to the number of support contacts, with four to eight contacts the most effective schedule. However, within‐group heterogeneity remains very high in all subgroups, and we advise caution when interpreting this result (test for subgroup differences: Chi² = 7.62, df = 3 (P = 0.05), I² = 60.6%; Analysis 6.4).
6.4. Analysis.
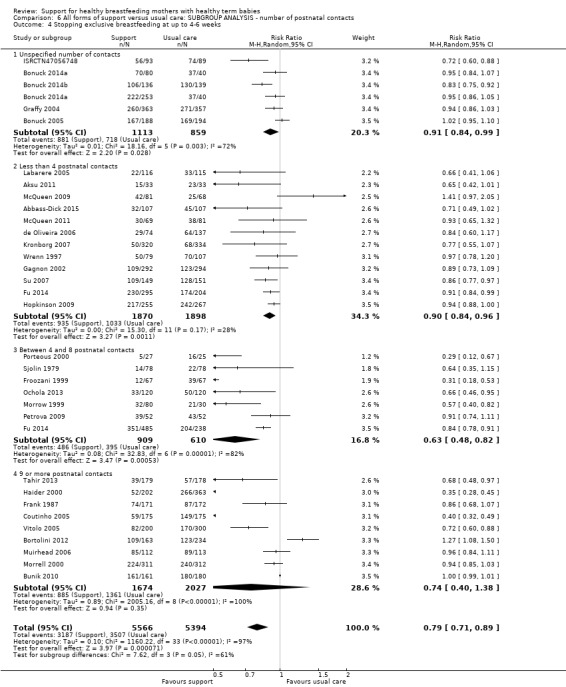
Comparison 6 All forms of support versus usual care: SUBGROUP ANALYSIS ‐ number of postnatal contacts, Outcome 4 Stopping exclusive breastfeeding at up to 4‐6 weeks.
Discussion
Summary of main results
This update of the review considered the evidence of the effect of breastfeeding support interventions on primary outcomes of stopping any or exclusive breastfeeding before four to six weeks and at up to six months postpartum. The review includes 100 trials published from 1979 to 2016, 73 of which contributed data to the analyses. The 73 trials that contributed data to the analyses were conducted in 29 countries; 52 studies (62.1% of participants) in high‐income countries, 15 (30.1% of participants) in upper‐middle income countries, four (3.4% of participants) in lower‐middle income countries, and four (4.4% of participants) in low‐income countries. The numbers of trials include one (Tylleskar 2011a; Tylleskar 2011b; Tylleskar 2011c) that was conducted in three countries; two low‐income and one upper‐middle income countries. This number and location of trials indicates that the challenge of supporting women to breastfeed is both longstanding and international; this is also reflected in the continuing low rates of duration and exclusivity of breastfeeding in many countries, despite increasing availability of good‐quality evidence of the scale of its public health impact.
This updated review provides evidence that interventions to support breastfeeding appear to reduce the risk of women stopping any breastfeeding at up to six months and exclusive breastfeeding at up to six months. Similarly the review provides evidence that women receiving breastfeeding support interventions were less likely to stop any breastfeeding before six weeks and exclusive breastfeeding at up to four to six weeks. The size of the treatment effects varied considerably in different trials, and average treatment effects may not be applicable in different settings. The subgroup analyses suggested that face‐to‐face support was associated with a greater treatment effect for exclusive breastfeeding than either telephone support alone or mixed telephone and face‐to‐face support. Similarly, support interventions were associated with greater effect on exclusive breastfeeding in settings with high background breastfeeding initiation rates compared to settings with low or intermediate breastfeeding initiation rates. Lay support and more contact in the form of scheduled visits (4 to 8 visits) were also associated with greater treatment effects. However, for all of these subgroup results, the within‐group heterogeneity remains high, and we advise caution when interpreting these results.
A striking aspect of this updated review is the heterogeneity of the support interventions, and the diversity of settings and of standard care. Interventions deemed by researchers to be ‘supportive’ included some where it was difficult to see how women might actually feel supported, especially when the support service provided was one they had to ask for, or travel a distance to get to (Graffy 2004; Hoddinott 2009), or where there was only one scheduled contact with the support person. However, this updated review, like the previous update (Renfrew 2012b), has shown that the effect of supportive interventions is robust across settings and population groups, and results from a wide range of interventions.
Overall completeness and applicability of evidence
This review adds 21 trials contributing data to its evidence base compared to its predecessors (Britton 2007; Renfrew 2012b; Sikorski 2002). The number of mother‐infant pairs in these studies has increased to 74,656 from 56,451 in Renfrew 2012b. The reporting of the included studies was, however, often not comprehensive ‐ lacking, for example, in terms of a description of the components of the support intervention, details of the training and qualifications of the supporters, the definitions used of the extent of breastfeeding and in the description of adherence to the support protocol. There was also a failure to present details of the interventions and of the standard care available to both intervention and comparison groups. Very few of the trials described a theoretical basis for the intervention tested, with the result that the findings are difficult to explain or to replicate. There has been slight improvement in study reporting or quality, with 44 out of the 73 trials that contributed data (60%) reporting an approach to allocation concealment that we considered to be at low risk of bias compared to 26 of the 52 trials (50%) in the previous review (Renfrew 2012b).
It is possible that not all existing trials have been included in this meta‐analysis. Funnel plot analyses conducted for the primary outcomes all show marked asymmetry, with each suggesting that smaller studies showing a less beneficial effect of the intervention may be missing. This may be the result of publication bias although it is also possible that few or no such trials exist. Nevertheless, caution should be taken when interpreting the evidence as funnel plot asymmetry can also be the result of heterogeneity.
Interventions offered across all included studies were very diverse, as was the provision of standard care. Interventions included, for example, one individual session in hospital, offering women the opportunity to attend a group session in community settings, telephone support, and multiple one‐to‐one visits at home over several months. Five studies offered the intervention in the context of Baby Friendly accreditation of the hospital, and are unlikely to be generalisable to settings where this standard of care is not available to all women.
Study endpoints also varied widely, with some substantial gaps of many months between the completion of the intervention and the last study outcome assessed. Many only offered support in the early days or weeks. These factors, together with the range of different settings and population subgroups studied, should urge caution in the interpretation of the analysis of pooled data.
Despite this caution, the overall benefit identified from all forms of support interventions has been explored with subgroup and sensitivity analyses and is moderately robust following exclusion of the methodologically weaker trials. In this review, the greatest effect of breastfeeding support interventions on reducing cessation of exclusive breastfeeding before six months occurred in communities with high (over 80%) levels of breastfeeding initiation. This suggests that work to promote breastfeeding at a population level should continue as one strategy to increase breastfeeding duration and exclusivity (Dyson 2009; Rollins 2016).
While the effect size of support interventions on reducing the cessation of any breastfeeding is modest, there is evidence of a greater effect on the prolongation of exclusive breastfeeding. There was a reduction in the cessation of exclusive breastfeeding within the first six months and at up to four to six weeks when lay support was used, although in view of considerable within‐subgroup heterogeneity, these findings should be interpreted with caution.
We have explored a range of possible reasons to explain the significant heterogeneity in the findings. As noted above, included studies were very varied in setting, population group studied, content, timing and intensity of the intervention, whether it was proactively offered to women or available only if they asked for it, the standard care available, staff training programmes, and the type and timing of the outcomes measured.
Strategies that depend mainly on face‐to‐face support appear more effective than those that rely primarily on telephone contact for women who practice exclusive breastfeeding. The duration of the intervention also seems to be an important factor for exclusive breastfeeding. Interventions that relied on one session in hospital are very different from interventions where women receive repeated home visits. We attempted to examine this by assessing the intensity of the intervention, and we found studies with four to eight visits to be associated with a more pronounced treatment effect on exclusive breastfeeding at final study assessment. However there was some evidence that more pronounced treatment effects were associated with studies at higher risk of bias; this could potentially confound any differences between subgroups. Caution is also needed in the interpretation of this finding as there is inconsistent reporting due to variations in the timing of outcome assessments, and the settings of studies and the population groups included in studies with more face‐to‐face visits. There was no evidence found for a difference between solely postnatal interventions and those interventions with both an antenatal and postnatal component.
It is likely that support will be most effective when it reflects the local needs of the population. It was notable that none of the five studies where women were expected to access support without any proactive element found a difference in outcomes between control and intervention groups. Four of these five were UK‐based studies, which may help to explain the lack of effect seen in recent UK trials (Hoddinott 2011).
The findings of the subgroup analysis that suggests that interventions with four to eight contacts are best compared to those with either fewer contacts or nine or more contacts seems counter‐intuitive. Given the heterogeneity of the somewhat complex interventions being tested, however, we might assume that it is other aspects of the interventions that may be responsible. One of the ways to test this would be to conduct meta‐regression of the effects of the number of contacts; thus ensuring that the exact number of contacts is used for each study rather than the categorization we have used. This is something we will explore in a future update of this review. We will also consider, in a future update, adopting an analysis strategy which would treat the outcomes as time‐to‐event data (i.e. 'time to breastfeeding cessation' and 'time to cessation of exclusive breastfeeding'). However, whilst some studies do provide such data, many currently do not.
Quality of the evidence
We considered that the overall risk of bias of trials included in the review was mixed. We graded fewer than half of the studies (44/100) as being at low risk of bias for allocation concealment. However, when we carried out sensitivity analysis which included only those studies at low risk of bias for allocation concealment, the results were not substantially different. A potentially important source of bias in these studies was the general lack of blinding. However, given the nature of the intervention it is unlikely that participants or personnel, or both, would be blinded, as for the support interventions, trialists would face considerable difficulties in blinding staff and women. We graded blinding of outcome assessment as being at low risk in about a quarter of studies. However, even where an attempt is made to blind outcome assessment, there is still a risk of response bias for outcomes relying on self‐report such as any or exclusive breastfeeding. A further possible source of bias was loss to follow‐up and missing outcome data. In the 17 studies with an attrition of more than 25%, the reasons for attrition were unclear, and these studies did not contribute data to the review. However, we are aware that even lower levels of attrition are problematic, particularly where loss was not balanced across different arms of trials. To avoid problems associated with attrition, we carried out intention‐to‐treat analysis for our primary outcomes; that is, we assumed that all women who were lost to follow‐up had stopped breastfeeding by given time points. This is likely to have diluted overall treatment effects but these estimates may be more appropriate given the possibility of response bias and the increased likelihood of women who stopped breastfeeding dropping out before those who continued.
We assessed the four primary outcomes with GRADE criteria. We did not downgrade any evidence for lack of blinding during this assessment; neither did we downgrade trials for other risk of bias domains (our sensitivity analyses were robust, as reported above). We judged all outcomes to be of moderate quality ‐ stopping 'any' breastfeeding at up to six months; 'any' breastfeeding between four to six weeks; stopping exclusive breastfeeding at up to six months; or stopping exclusive breastfeeding between four to six weeks; all analyses had substantial heterogeneity even with a random‐effects model. An assessment of moderate quality highlights our uncertainty in the summary estimate presented here. Included trials of breastfeeding support had mixed results for preventing women from stopping exclusive breastfeeding.
In meta‐analyses with a large number of trials, as is the case in this review, there is a strong possibility that a large number of small and possibly poor quality trials will have a substantial influence on the result. In such circumstances it is preferable, therefore, to conduct a sensitivity analysis confined to the trials of the best methodological quality, which would be assumed to be the most reliable and unbiased. Apart from allocation concealment, we have not performed such an analysis, since none of the studies were assessed as being low risk of bias across all of the domains of bias we assessed.
Potential biases in the review process
There is a potential for bias to be introduced at any stage of the review process. In order to minimise the bias in the review process, two review authors independently screened studies for inclusion and any disagreements were resolved by a third review author. Data extraction and risk of bias assessment was performed by one reviewer and then checked by a second review author. Again any discrepancies were resolved by a third review author. It must be stressed that 'Risk of bias' assessment is subjective in nature and therefore another team of review authors may have graded studies differently. It is also worth noting that we did not formerly assess risk of bias in the 15 included cluster‐randomized trials. Particular biases are unique to cluster designs, as described in the Handbook [section16.3.2] and formal assessments will be made of these in future updates. To minimise language bias any study not reported in English was translated into English and included in the review provided it met the inclusion criteria. This update was limited to primary outcomes so that it could be completed in time to inform important international guidance on infant feeding. All primary and secondary outcomes will be considered in the next update of this review. The development of a core outcome set for breastfeeding reviews is currently underway and this may influence the choice of outcomes in subsequent updates. This update, consistent with the previous versions of the review, combined different levels of interventions, so that trials in which breastfeeding mothers received the intervention were combined with trials where the intervention was directed at the staff providing the support. We will reconsider this approach in the next update. It is also of concern that there was missing data for 28 studies. Whilst we attempted to identify all the evidence on interventions to support breastfeeding (including published abstracts from conference proceedings) and followed‐up ongoing studies, it is feasible that relevant research that is unpublished or not registered in a clinical trials register could have been missed.
Agreements and disagreements with other studies or reviews
The overall findings of this review, that breastfeeding support interventions have been shown to be effective in reducing the risk of cessation of any breastfeeding and of exclusive breastfeeding, are similar to the findings of other reviews (Rollins 2016; Sinha 2015). We concur with others, e.g. Hoddinott 2011 and Renfrew 2007, that it is critically important to identify the characteristics of support that may make this important but heterogenous intervention more or less effective in different circumstances and settings. For example, Jolly 2012b found that peer support had a greater effect on reducing the risk of non‐exclusive breastfeeding in low‐ and middle‐income countries compared to high‐income countries, especially in the UK. Other recent reviews have found that interventions to increase breastfeeding duration and exclusivity are more effective when delivered as multi‐component structured programmes such as the Baby Friendly Hosptial Initiative/Baby Friendly Initiative (BFHI/BFI), in a combination of settings (Beake 2012; Pérez‐Escamilla 2016; Rollins 2016; Sinha 2015).
Authors' conclusions
Implications for practice.
When breastfeeding support is offered to women, the duration and exclusivity of breastfeeding is increased. Characteristics of effective support include: that it is offered as standard by trained personnel during antenatal or postnatal care, that it includes ongoing scheduled visits so that women can predict when support will be available, and that it is tailored to the setting and the needs of the population group. Support is likely to be more effective in settings with high initiation rates. Support may be offered either by professional or lay/peer supporters, or a combination of both. Strategies that rely mainly on face‐to‐face support are more likely to succeed with women practising exclusive breastfeeding.
Implications for research.
There is a very large number of trials in this field and this number continues to grow. This has resulted in a great deal of research time, energy and funding. While there are still questions to address about how best to provide support, the key messages are clear – we have ample evidence to know that women need support to be available and to be provided using the characteristics we have identified to increase the duration and exclusivity of breastfeeding. The key research question for the future is to identify how such support can best be provided consistently, for all women, in all countries. This becomes a scaling‐up issue, which needs implementation and quality improvement approaches rather than effectiveness studies.
Any future studies should describe in detail the attributes of the intervention (who delivered it, setting, intensity, proactive or reactive); standard care (Baby Friendly accreditation or not, staff trained in breastfeeding or not); the population group studied (low‐ versus high‐income, any selection criteria); and the background breastfeeding rates in the population studies. Studies should also examine the potential for synergy between support and other interventions that aim to increase breastfeeding rates, as it may be that a package of interventions is more effective than single interventions in tackling the multifaceted challenge of increasing breastfeeding rates. Packages to be tested could include peer and professional support along with, for example, antenatal education, staff training, and mother‐to‐mother support. Studies should also assess the effectiveness of lay, professional and combined support in different settings ‐ in particular in those communities with low rates of breastfeeding initiation. Implementation of the Baby Friendly Initiative should be accompanied by the continued monitoring of breastfeeding rates to explore whether its effect is similar in countries with differing rates of initiation and prevalence of breastfeeding.
Further study is also required to:
test the effectiveness of different training programmes (which should be well‐defined and reproducible) and should attempt to address impact on both exclusive and any breastfeeding where possible;
analyse and develop the theoretical basis for their approach, and analyse the elements of their approach that appear to have an impact, including training, timing, and intensity of the intervention, and differential impact on different population subgroups;
establish the cost‐effectiveness of different interventions;
investigate appropriate strategies for supporting women who wish to breastfeed for longer than six months;
What's new
| Date | Event | Description |
|---|---|---|
| 29 February 2016 | New search has been performed | Search updated and 31 new studies included. The review now includes a total of 100 studies, with 73 studies providing data. A 'Summary of findings' table has been incorporated in this update. In order to expedite this review rapidly to be ready to inform the World health Organisation recommendations on breastfeeding in maternity facilities, we have restricted the outcomes analysed in this update to the primary outcomes only. Secondary outcomes analysed in the previously published version of this review will be added in the next update of this review in two years time.. |
| 29 February 2016 | New citation required but conclusions have not changed | Conclusions broadly similar. |
History
Protocol first published: Issue 3, 1998 Review first published: Issue 1, 1999
| Date | Event | Description |
|---|---|---|
| 12 December 2011 | New search has been performed | In the previous version of this review (Britton 2007) we included 34 trials in 14 countries. In this updated version, we assessed 218 reports; corresponding to 150 separate studies. We have included 67 studies and excluded 79. Four studies are still ongoing or awaiting further assessment. In this updated version we have added further subgroup analysis and discuss the impact of different types of support interventions. |
| 12 December 2011 | New citation required but conclusions have not changed | The update was prepared by a new author team. Studies were carried out in 21 countries. Overall conclusions have not changed, but we include more evidence on the effect of interventions in different settings and for different types of interventions; proactive interventions that rely mainly on face‐to‐face support are more likely to succeed. |
| 27 July 2009 | Amended | Search updated, 68 reports added to Studies awaiting classification. |
| 6 November 2008 | Amended | Converted to new review format. |
| 30 January 2006 | New citation required and conclusions have changed | New review team prepared this update. Previous versions of this review categorised support as 'professional' or 'lay'. This edition introduces a new category: combined lay and professional support. Studies in this category demonstrated a significant effect on duration of any breastfeeding, especially in the first two months. |
| 30 January 2006 | New search has been performed | Searches updated. We have included fourteen new studies and excluded an additional 30 studies. |
Acknowledgements
The review authors wish to thank those study authors who were very helpful in responding to queries.
As part of the prepublication editorial process, this review has been commented on by two peers (an editor and referee who is external to the editorial team), a member of the Pregnancy and Childbirth Group's international panel of consumers and the Group's Statistical Adviser.
Work on this review was supported in part by a grant from the National Institute for Health Research Health Technology Assessment programme, grant number 10/106/01.
This research was supported by a grant from the Evidence and Programme Guidance Unit, Department of Nutrition for Health and Development, World Health Organization. The findings, interpretations and conclusions expressed in this paper are entirely those of the authors and should not be attributed in any manner whatsoever to WHO.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Thanks to Hannah Soley for translating Lucchini 2013.
Data and analyses
Comparison 1. All forms of support versus usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Stopping breastfeeding (any) before last study assessment up to 6 months | 51 | 21418 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.88, 0.95] |
| 2 Stopping exclusive breastfeeding before last study assessment up to 6 months | 46 | 18591 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.85, 0.92] |
| 3 Stopping breastfeeding (any) at up to 4‐6 weeks | 33 | 11264 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.80, 0.95] |
| 4 Stopping exclusive breastfeeding at up to 4‐6 weeks | 32 | 10960 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.71, 0.89] |
| 5 Sensitivity analysis by risk of bias allocation concealment: stopping any breastfeeding at up to six months | 51 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Studies at low risk of bias | 27 | 13465 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.89, 0.96] |
| 5.2 Unclear or high risk of bias | 24 | 7953 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.83, 0.95] |
| 6 Sensitivity analysis by risk of bias allocation concealment: stopping exclusive breastfeeding at up to six months | 46 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Studies at low risk of bias | 27 | 11351 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.89, 0.96] |
| 6.2 Unclear or high risk of bias | 19 | 6828 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.65, 0.84] |
| 7 Sensitivity analysis by risk of bias allocation concealment: stopping any breastfeeding at 4‐6 weeks | 31 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Studies at low risk of bias | 19 | 6817 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.81, 0.96] |
| 7.2 Unclear or high risk of bias | 12 | 3528 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.68, 1.03] |
| 8 Sensitivity analysis by risk of bias allocation concealment: stopping exclusive breastfeeding by 4‐6 weeks | 32 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 Studies at low risk of bias | 20 | 7107 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.79, 0.96] |
| 8.2 Unclear or high risk of bias | 12 | 3164 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.48, 0.86] |
Comparison 2. All forms of support versus usual care: SUBGROUP ANALYSIS ‐ who delivered the intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Stopping any breastfeeding before last study assessment up to 6 months | 51 | 21418 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.88, 0.95] |
| 1.1 Professional support | 37 | 16835 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.89, 0.96] |
| 1.2 Lay support | 9 | 3109 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.77, 0.93] |
| 1.3 Both professional and lay support | 5 | 1474 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.91, 1.03] |
| 2 Stopping exclusive breastfeeding before last study assessment | 46 | 18424 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.85, 0.92] |
| 2.1 Professional support | 30 | 12760 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.89, 0.96] |
| 2.2 Lay support | 13 | 4590 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.65, 0.87] |
| 2.3 Both professional and lay support | 3 | 1074 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.44, 1.32] |
| 3 Stopping any breastfeeding at up to 4‐6 weeks | 34 | 11815 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.78, 0.92] |
| 3.1 Professional support | 23 | 8104 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.72, 0.91] |
| 3.2 Lay support | 8 | 2789 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.78, 1.06] |
| 3.3 Both professional and lay support | 3 | 922 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.68, 1.11] |
| 4 Stopping exclusive breastfeeding at up to 4‐6 weeks | 32 | 10934 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.71, 0.89] |
| 4.1 Professional support | 22 | 7435 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.75, 0.95] |
| 4.2 Lay support | 8 | 2354 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.46, 0.89] |
| 4.3 Both professional and lay support | 2 | 1145 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.89, 0.99] |
Comparison 3. All forms of support versus usual care: SUBGROUP ANALYSIS ‐ type of support.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Stopping any breastfeeding before last study assessment up to 6 months | 50 | 20946 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.88, 0.95] |
| 1.1 Predominantly telephone support | 3 | 677 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.65, 1.17] |
| 1.2 Predominantly face‐to‐face contact | 24 | 13890 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.86, 0.95] |
| 1.3 Balanced telephone and face‐to‐face support | 23 | 6379 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.87, 0.98] |
| 2 Stopping exclusive breastfeeding by last study assessment up to 6 months | 46 | 19495 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.87, 0.93] |
| 2.1 Predominantly telephone support | 2 | 419 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.99, 1.01] |
| 2.2 Predominantly face‐to‐face contact | 29 | 13905 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.81, 0.90] |
| 2.3 Balanced telephone and face‐to‐face | 17 | 5171 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.93, 1.00] |
| 3 Stopping any breastfeeding by 4‐6 weeks | 32 | 11076 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.79, 0.93] |
| 3.1 Predominantly telephone support | 3 | 1133 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.54, 1.08] |
| 3.2 Predominantly face‐to‐face contact | 13 | 5186 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.75, 0.94] |
| 3.3 Balanced telephone and face‐to‐face | 17 | 4757 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.78, 1.02] |
| 4 Stopping exclusive breastfeeding by 4‐6 weeks | 31 | 10311 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.69, 0.89] |
| 4.1 Predominantly telephone support | 3 | 1142 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.54, 1.55] |
| 4.2 Predominantly face‐to‐face contact | 16 | 5382 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.57, 0.81] |
| 4.3 Balanced telephone and face‐to‐face | 13 | 3787 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.88, 0.99] |
Comparison 4. All forms of support versus usual care: SUBGROUP ANALYSIS ‐ timing of support.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Stopping any breastfeeding at last study assessment up to 6 months | 51 | 21418 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.88, 0.95] |
| 1.1 Postnatal support alone | 35 | 15570 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.86, 0.95] |
| 1.2 Antenatal component to support | 16 | 5848 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.88, 0.97] |
| 2 Stopping exclusive breastfeeding by last assessment up to 6 months | 45 | 18374 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.88, 0.94] |
| 2.1 Postnatal support alone | 29 | 11683 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.84, 0.94] |
| 2.2 Antenatal component to support | 17 | 6691 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.89, 0.97] |
| 3 Stopping any breastfeeding at 4‐6 weeks | 32 | 11262 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.80, 0.93] |
| 3.1 Postnatal support alone | 22 | 7793 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.75, 0.93] |
| 3.2 Antenatal component to support | 10 | 3469 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.81, 1.00] |
| 4 Stopping exclusive breastfeeding at up to 4‐6 weeks | 31 | 10311 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.69, 0.89] |
| 4.1 Postnatal support alone | 23 | 7764 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.61, 0.93] |
| 4.2 Antenatal component to support | 8 | 2547 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.81, 0.96] |
Comparison 5. All forms of support versus usual care: SUBGROUP ANALYSIS ‐ breastfeeding initiation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Stopping any breastfeeding by last assessment up to 6 months | 49 | 21162 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.89, 0.95] |
| 1.1 Settings with high breastfeeding initiation rates | 21 | 11798 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.86, 0.95] |
| 1.2 Settings with Intermediate initiation rates | 18 | 7238 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.89, 0.98] |
| 1.3 Settings with low initiation rates | 10 | 2126 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.84, 1.00] |
| 2 Stopping exclusive breastfeeding at last assessment up to 6 months | 41 | 16768 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.87, 0.94] |
| 2.1 Settings with high breastfeeding initiation rates | 26 | 11347 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.80, 0.90] |
| 2.2 Settings with Intermediate initiation rates | 10 | 4052 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.93, 1.02] |
| 2.3 Settings with low initiation rates | 5 | 1369 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.99, 1.01] |
| 3 Stopping any breastfeeding at up to 4‐6 weeks | 31 | 12929 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.82, 0.93] |
| 3.1 Settings with high breastfeeding initiation rates | 11 | 5295 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.71, 0.92] |
| 3.2 Settings with Intermediate initiation rates | 17 | 6096 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.85, 1.00] |
| 3.3 Settings with low initiation rates | 6 | 1538 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.79, 1.00] |
| 4 Stopping exclusive breastfeeding at up to 4‐6 weeks | 29 | 9911 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.69, 0.89] |
| 4.1 Settings with high breastfeeding initiation rates | 16 | 5933 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.62, 0.84] |
| 4.2 Settings with Intermediate initiation rates | 8 | 2609 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.80, 0.95] |
| 4.3 Settings with low initiation rates | 5 | 1369 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.86, 1.08] |
Comparison 6. All forms of support versus usual care: SUBGROUP ANALYSIS ‐ number of postnatal contacts.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Stopping any breastfeeding before last study assessment up to 6 months | 51 | 21418 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.88, 0.95] |
| 1.1 Unspecified number of contacts | 10 | 7187 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.85, 0.97] |
| 1.2 Less than 4 postnatal contacts | 12 | 5151 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.86, 1.00] |
| 1.3 Between 4 and 8 postnatal contacts | 14 | 3236 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.77, 0.97] |
| 1.4 9 or more postnatal contacts | 15 | 5844 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.88, 0.98] |
| 2 Stopping exclusive breastfeeding before last study assessment | 46 | 18424 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.85, 0.92] |
| 2.1 Unspecified number of contacts | 7 | 3645 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.84, 1.02] |
| 2.2 Less than 4 postnatal contacts | 11 | 4511 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.91, 1.01] |
| 2.3 Between 4 and 8 postnatal contacts | 16 | 5148 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.63, 0.84] |
| 2.4 9 or more postnatal contacts | 12 | 5120 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.81, 0.98] |
| 3 Stopping any breastfeeding at up to 4‐6 weeks | 33 | 11180 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.79, 0.93] |
| 3.1 Unspecified number of contacts | 6 | 2631 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.75, 0.99] |
| 3.2 Less than 4 postnatal contacts | 14 | 4789 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.70, 0.99] |
| 3.3 Between 4 and 8 postnatal contacts | 6 | 1088 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.63, 1.00] |
| 3.4 9 or more postnatal contacts | 8 | 2672 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.80, 1.06] |
| 4 Stopping exclusive breastfeeding at up to 4‐6 weeks | 32 | 10960 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.71, 0.89] |
| 4.1 Unspecified number of contacts | 5 | 1972 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.84, 0.99] |
| 4.2 Less than 4 postnatal contacts | 12 | 3768 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.84, 0.96] |
| 4.3 Between 4 and 8 postnatal contacts | 7 | 1519 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.48, 0.82] |
| 4.4 9 or more postnatal contacts | 9 | 3701 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.40, 1.38] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abbass‐Dick 2015.
| Methods | 2‐arm RCT, single‐site, n = 214 | |
| Participants | Large urban teaching hospital in Toronto, Canada Background rates of breastfeeding initiation: 89% Inclusion criteria: primiparous mothers in the first 2 days postpartum, singleton birth, ≥ 18 years old, ≥ 37 weeks’ gestation at delivery, able to speak and read English, and living with a male partner Exclusion criteria: women sharing a hospital room with a current study participant, a medical problem that could interfere with breastfeeding, infant not discharged from hospital with them, no access to the Internet or a telephone, planning to breastfeed for < 12 weeks, and had a partner who would not be available to participate in the study |
|
| Interventions | Intervention: the trial intervention was a multifaceted coparenting breastfeeding support intervention, provided face‐to‐face on the postpartum unit, at which time the couples were provided with breastfeeding information, the information package was reviewed, and couples were given the option of watching a video. The session took ∼15 min in the majority of cases. Couples had a take‐home breastfeeding booklet, developed by Best Start: Ontarios Maternal, Newborn and Early Child Development Resource Centre, access to a secure study web site that consisted of extensive information on breastfeeding and coparenting and contained links to related information and resources on the Internet including a copy of the video to watch at home. The couples were followed up at home with emails at 1 and 3 weeks postpartum and a telephone call at 2 weeks postpartum to answer any questions or concerns about the information provided. Control: couples received usual care, which included standard in‐hospital breastfeeding support and any breastfeeding assistance that was proactively sought in the community. |
|
| Outcomes | Primary: Exclusive breastfeeding at 6 weeks and 12 weeks postpartum Secondary: Breastfeeding duration at 6 and 12 weeks postpartum Maternal perceptions of breastfeeding support Maternal perception of the coparenting relationship at 12 weeks postpartum |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Intervention group by sequentially numbered randomly generated numbers |
| Allocation concealment (selection bias) | Low risk | Sealed, opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Envelopes were constructed by a research assistant who was not involved in any other trial procedure. Participants not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Participants in intervention group were known to assessors because they were interviewed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data were < 25%. Complete follow‐up data were collected from 87.9% (n = 188) of fathers at 6 weeks and 88.3% (n = 189) of mothers at 6 weeks and 91.6% (n = 196) at 12 weeks. |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcomes detailed in the study protocol were reported. |
| Other bias | Unclear risk | There were no significant differences in baseline characteristics between the groups except prenatal education. However, there was a non‐significant difference between the 2 groups in attendance at a prenatal breastfeeding class. |
Aidam 2005.
| Methods | 3‐arm RCT, with individual randomisation n=231 | |
| Participants | The study was carried out in the Tema area of Ghana (sub‐Saharan Africa). Women were recruited in prenatal clinics in 2 hospitals (1 government and 1 private) that served urban areas (an industrial city and a commercial town). High baseline prevalence of breastfeeding in Ghana, the median duration of breastfeeding was reported as being 22 months and 53.4% of women with babies < 6 months breastfeed exclusively. It was reported that "almost all" mothers initiated breastfeeding. 231 women randomized (136 eligible at the beginning of the intervention period). Inclusion criteria: pregnant women in the last trimester planning delivery in the study hospitals and to stay in study area for 6 months after delivery. After delivery: singleton babies with normal birthweight (> 2500 g) and Apgar scores ≥ 6 at 1 min and 5 min Exclusion criteria: multiple birth, low Apgar score or planning to move out of area Participant characteristics: 38% of the women had only primary level or no formal education; 90% were married or living with a partner; 46% were primiparous; 73% had vaginal birth; 24% lived in households with access to a car; 74% were described as trader/artisan |
|
| Interventions | All 3 groups (intervention 1, intervention 2 and control) were allocated to 2 educational group sessions during pregnancy by trained nurses and 9 proactive home visits by trained nurse counsellors at 1, 2, 4, 6, 8, 12, 16, 20 and 24 weeks postpartum. These were in addition to standard care. The content of the sessions differed between the 3 groups. 63% of intervention 1, 73% of intervention 2 and 65% of the control group women received all 9 scheduled home follow‐up visits. Intervention 1 (n = 74): 43 followed up. Content of sessions was breastfeeding and exclusive breastfeeding. Trained local nurses with experience of breastfeeding gave 2 educational sessions, of approximately 20 min each, to groups of 2‐4 women during their third trimester. At postpartum home visits women received individual counselling and nurses were advised to respond to concerns. Materials were developed from WHO/UNICEF breastfeeding counselling training manual. Intervention 2 (n = 72): 44 followed up. Content of the pregnancy sessions was general health and childcare as for control group. Content of the postpartum home visits was breastfeeding and exclusive breastfeeding as for intervention 1. Control (n = 85): 49 followed up. Content of sessions was general health and childcare topics such as immunisation, HIV/AIDS, nutrition and family planning. |
|
| Outcomes | Breastfeeding status at 1, 2, 3, 4, 5, and 6 months, exclusive breastfeeding up to 6 months, infant morbidity and growth | |
| Notes | We have not included data from this study in the review due to high levels of attrition (> 25% loss to follow‐up). Most data were reported in graphs and difficult to interpret. Several measures of exclusive breastfeeding were reported; at 1 and 6 months women were asked about breastfeeding since birth, during previous month and on previous day. In this review we have reported figures for exclusive breastfeeding since birth for both time points. Figures in the paper were expressed as percentage of women still exclusively breastfeeding; in order to use the data we used subtraction to calculate a figure for women who had stopped breastfeeding. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Randomization was achieved by writing numbers 1 to 3 on folded pieces of paper." |
| Allocation concealment (selection bias) | High risk | Quote: "The numbers were not viewed by either study staff or mothers and the pieces of paper looked the same on the outside. Before offering papers to mothers, they were shuffled in the interviewer’s palm.” Quote: “The randomisation scheme used was not a formal one. It was one that could be conducted easily in the field. Despite this, it functionally produced balanced groups with no evidence of bias.” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | It was stated that women were informed only that they would receive “health education” that would be beneficial to their infants and themselves, but were not aware of their group allocation or of differences in the content of the health education. However, it is not possible to blind. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "It was impossible to keep counsellors unaware of study design.... research assistants [collecting outcome data] were aware of mothers group allocation." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 231 women randomized during the third trimester. At delivery 95 women were excluded as they were no longer eligible (41% lost before the intervention). A further 13 women were lost to follow‐up during the intervention period. 123 completed the final follow‐up at 6 months (i.e. 53% of the original randomized sample but 90% of those still eligible at delivery). Results were reported in graphs and percentages and it was not clear how many women commenced breastfeeding, so group denominators are not clear. Loss to follow‐up appeared balanced across groups. |
| Selective reporting (reporting bias) | Unclear risk | Failure to provide denominators for results means that they are very difficult to interpret. |
| Other bias | Unclear risk | Women in the 3 arms of the trial appeared similar at baseline. Analysis was according to group allocation. |
Aksu 2011.
| Methods | RCT, single site, a Baby‐Friendly hospital, March‐July 2008, n = 66 | |
| Participants | Urban state maternity hospital in Turkey Background rates of breastfeeding initiation: high Inclusion criteria: primaparous, live vaginal birth, healthy term singleton infant, living in study area, able to speak Turkish, no history of chronic diseases, non‐smoker, intending to breastfeed Exclusion criteria: infant birthweight < 2500 g, Apgar score ≤ 7, congenital anomalies, serious disease or needing intensive care Baseline prevalence of "ever breastfed" in Turkey: 96.7% (WHO Global data bank 2010, accessed 6 October 2011). |
|
| Interventions | Intervention: women received standard breastfeeding support plus support from trained lay supporters who had undergone WHO/UNICEF 18‐h training. The intervention was a single home visit on day 3 after the birth (in hospital), by 2 lay breastfeeding supporters, that lasted about 30 min and covered the same topics as routine support. Control: at this Baby‐Friendly hospital, a standard breastfeeding education session lasting 20‐30 min was provided to all mothers before standard discharge home at 24 h after the birth. The session included the topics covered by the 18‐h WHO/Unicef training. |
|
| Outcomes | Exclusive breastfeeding at 2 and 6 weeks and 6 months postpartum; breastfeeding duration (any/exclusive) to 18 months; breastfeeding knowledge scores at 2 and 6 weeks postpartum | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information provided to enable a judgement to be made |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Women were contacted either through home visits or via the phone and data on breastfeeding was collected, however, not reported whether the assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 82% follow‐up at 18 months. Reasons for loss were explained and were balanced across groups. |
| Selective reporting (reporting bias) | Low risk | Not apparent |
| Other bias | Low risk | Groups appeared similar at baseline. |
Albernaz 2003.
| Methods | Primary care facilities, recruitment over 5 months, n = 169 | |
| Participants | 3 hospitals in the city of Pelotas, in southern Brazil Background rates of breastfeeding initiation: 88% Ethnic composition not described. Inclusion criteria: term healthy baby, family income ≥ USD 500 per month (no economic constraints to baby's growth), mother intended to breastfeed and did not smoke. Exclusion criteria: multiple birth, gestational age not 37‐42 weeks, significant perinatal morbidity, maternal smoking and family income USD 500 per month. |
|
| Interventions | Intervention: hospital visit, home visits at 5, 15, 30, 45, 90 and 120 days, and 24‐h telephone hotline for help or to arrange visits. 2 members of the lactation support team had received the 40‐h WHO lactation support training course. Control: attended paediatric clinics where general advice on advantages of breastfeeding may have been offered, but specific lactation counselling was not provided. |
|
| Outcomes | Breastfeeding pattern and duration up to age of 4 months. Breastmilk intake for a subgroup of 68 infants at 4 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated code |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information provided to enable a judgement to be made |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The interviewers were not informed about the intervention or control status of each mother, and did not know the study's objectives. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 188 women were randomized. 21 were excluded after 2 weeks as they had introduced formula milk. A further 26 withdrew (some data were available for some of these women). 141 women completed the trial (75%). |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol. |
| Other bias | Unclear risk | Excluding women who introduced formula within 2 weeks of randomisation is likely to have introduced bias although similar numbers were excluded from both groups (9 women lost from the intervention group for this reason and 11 women from the control group and an additional control was withdrawn for smoking). |
Anderson 2005.
| Methods | 2‐arm RCT, with individual randomisation N=135 | |
| Participants | Hartford area of Connecticut, USA in a hospital providing care for predominantly Latina low‐income women Inclusion criteria: age ≥18 years; gestational age < 32 weeks at first approach; healthy, considering breastfeeding, planning delivery at study hospital and resident in area for 3 months after the birth, 185% of the federal poverty level, available for telephone contact and willing to participate Exclusion criteria for mothers: medical conditions such as diabetes or hypertension; drug use that could impair breastfeeding Exlusion criteria for infants: preterm, low birthweight (< 2500 g), any complications requiring admission to special care, Apgar score < 7 at 1 min and 5 min Participant characteristics: at baseline: intervention n = 63; control n = 72 Participant characteristics: Married/cohabiting: intervention 40%; control 26% Hispanic race: intervention 81%; control 64% Education less than high school: intervention 31%; control 38% Received welfare: intervention 31%; control 38% Primiparous: intervention 92%; control 89% Planned breastfeeding duration < 6 months: intervention 20%; control 46% Planned breastfeeding duration 6‐12 months or longer: intervention 80%; control 54% |
|
| Interventions | Intervention: in addition to standard care, women received 3 prenatal home visits, daily in‐hospital visits and 9 postpartum home visits from peer counsellors: 3 in first week, 2 in second week and 1 in each week for weeks 3‐6. Women could also phone peer counsellors. Peer counsellors were mothers from the area with experience of successful breastfeeding and training from a lactation consultant (LC). Control: women received what would have been standard care for private patients (these women may not have normally qualified to receive this care as many were participating in welfare programmes). This consisted of: breastfeeding support line open to mothers after delivery staffed by a lactation specialist. Usual in‐patient care and support for breastfeeding was provided by hospital staff. |
|
| Outcomes | Infant feeding practices (weekly for first month) breastfeeding and exclusive breastfeeding. Infant morbidity (diarrhoea and ear infection). Breastfeeding outcomes measured in 3 different ways – over the past 24 h, over the past week and since the birth (ever given). | |
| Notes | We have not included data from this study in the review due to high levels of attrition (> 25% loss to follow‐up). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | SPSS software was used to randomly assign subjects to study groups. |
| Allocation concealment (selection bias) | Low risk | Quote: "Recruited subjects were entered into the database at the end of every week” and then random allocation by computer software. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated if women or peer counsellors were blinded, but unlikely. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not a double‐blind study and the interviewer knew the study hypotheses. Steps were taken to prevent interviewer bias by asking questions regarding peer counsellor contact at the very end of each follow‐up interview session. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 182 women were recruited and randomized. 162 were still eligible at delivery and 135 completed the trial (84% of those still eligible at delivery and 74% of the total randomized). |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol. |
| Other bias | Unclear risk | Groups appeared similar at baseline although women in the control group were more likely (46%) to plan to breastfeed for < 6 months than women in the intervention group (20.4%). This difference in breastfeeding intentions means that the results are more difficult to interpret. |
Barros 1994.
| Methods | 2‐arm RCT, single‐site, n = 900 | |
| Participants | Urban setting in Brazil: in‐patient maternity unit Background rates of breastfeeding initiation: high Ethnic composition not described Inclusion criteria: family income < twice the minimum Brazilian wage; hospital stay < 5 days; wanting to breastfeed: living within the city of Pelotas Baseline prevalence in Pelotas (1993) for any breastfeeding: 85% at 1 month, 66% at 3 months and 38% at 6 months. |
|
| Interventions | Intervention: 3 home visits at 5, 10 and 20 days postpartum by a social assistant or nutritionist. The visitor was required to have a personal history of successfully breastfeeding a child and received training in breastfeeding physiology and common breastfeeding problems and their solutions. Control: usual care, a social assistant would not normally make routine home visits but would visit only when requested to do so by the hospital team. |
|
| Outcomes | Breastfeeding at monthly intervals to 6 months and median duration of breastfeeding Time to introduction of artificial feeds Difficulties encountered during breastfeeding and reasons for weaning also recorded. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Record in Portugese and no information in the translation regarding blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The nurse collecting outcome data was not aware of previous contacts, but the authors stated that s/he may have been made aware of group assignment as women were likely to talk about the intervention. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 900 randomized, approximately 8% lost to follow‐up in the intervention and control groups. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol. |
| Other bias | Unclear risk | No baseline imbalance apparent. Assessment of risk of bias was made from translation notes. The original paper is in Portuguese. |
Bashour 2008.
| Methods | 3‐arm RCT, with individual randomisation N=903 | |
| Participants | Recruited from Maternity Teaching Hospital in Damascus, Syria Background rates of breastfeeding initiation: high Inclusion criteria: consenting women who delivered a healthy newborn by vaginal delivery or caesarean section, who lived within 30 km from hospital, and were available for follow‐up for the next 6 months Exclusion criteria for infants: premature, low birthweight (< 2500 g), with apparent congenital anomalies Participant characteristics: Age not clear. Approximately 37% primiparous 90% had normal labour > 99% of the women were married Home conditions were described as bad (number of rooms, poor sanitation or water, etc.) in 28.5% of control group and approximately 20% of the intervention groups Few of the women (approximately 5%) worked outside the home. |
|
| Interventions | Intervention 1 (n = 301): 4 structured home visits from trained midwives at 1, 3 and 7 days and 4 weeks after the birth. Midwives examined mothers and infants and provided and advice and support on a range of healthcare issues including breastfeeding support and education. Intervention 2 (n = 301): a single postnatal visit from a trained midwife at 3 days which included advice and education on breastfeeding. Control (n=301): received standard care in Syria (no postnatal visits). |
|
| Outcomes | Primary: Maternal postpartum morbidities, postnatal care uptake, contraceptive uptake and type, infant morbidities, infant immunisation according to the national schedule at 3 months and Infant feeding (specifically exclusive breastfeeding during the first 4 months of life) Secondary: Women’s perceptions of their health, impressions about the home visit and perceptions of its quality |
|
| Notes | Some baseline imbalance, women in the control groups were more likely to have poor home conditions and were less likely to have received antenatal care. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomized into blocks to either of the intervention groups (4 home visits or 1 home visit) or to the control group (no home visits). Randomisation was in blocks of 7 where a caseload of 21 eligible deliveries per day was assumed, based on the average daily number of deliveries in the hospital (ranging from 30 to 35) after excluding non‐eligible cases. |
| Allocation concealment (selection bias) | Low risk | Quote: "Numbered, opaque and sealed envelopes..” Group allocation was carried out by a senior midwife not involved in the rest of the study. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The midwives carrying out the intervention were not blinded. It was not stated whether the participants were blinded, but this is unlikely. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The interviewers carrying out outcome assessment were not informed of groups, but would be aware of which group women were in from the interviews. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 903 women met the inclusion criteria. After randomisation (301 in each arm), 27 women were excluded (18 due to lack of address detail and 9 refusals). A total of 876 women were followed up in the 3 study groups: Intervention 1 (285 women), Intervention 2 (294 women) and Control (297 women). Incomplete data were addressed. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol. |
| Other bias | Unclear risk | Some baseline imbalance, women in the control groups were more likely to have poor home conditions and were less likely to have received antenatal care. Outcome data were collected at 4 months, but it is likely that there may have been recall bias for some outcomes, e.g. breast engorgement – women in the intervention groups would have discussed this and maybe it was recorded at the time it occurred, women in the control group would not have been asked until 4 months postpartum. Outcome data were collected for a large number of variables, so any differences may have occurred by chance. |
Bhandari 2003.
| Methods | Cluster‐randomised study with 8 sites, n = 1115 | |
| Participants | 8 village communities located 3 km‐5 km from the main highway in Haryana, India Background rates of breastfeeding initiation: high Inclusion criteria: born in a study village within 9 months of start of intervention Exclusion criteria: not reported Baseline breastfeeding prevalence stated to be high |
|
| Interventions | Intervention: health and nutrition workers in the intervention communities received training based on Integrated Management of Childhood Illnesses Training Manual on Breastfeeding Counseling (WHO 1997). Messages ‐ feed only breast milk for first 6 months of life; breastfeed the infant day and night, at least 8 times in 24 h; possible adverse effects of other foods and fluids given to breastfeeding infants ‐ given to mothers at birth, plus monthly home visits, immunisation clinics and neighbourhood meetings. Control: at the control sites, the research team provided routine services, in which, according to national policy, workers are required to advise exclusive breastfeeding for 4‐6 months. |
|
| Outcomes | Feeding at 3 months Anthropometry and diarrhoea prevalence at 3 and 6 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Communities were paired on the basis of similar scores for socioeconomic, mortality and morbidity indicators. 1 of each pair was allocated to the intervention using a random number table. 8 areas were randomized (4 to each condition). |
| Allocation concealment (selection bias) | Low risk | Statistician independent of project carried out randomisation. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated whether participants and peer counsellors were blinded but unlikely. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Authors state "we attempted to keep to a minimum reporting bias by use of a separate team for assessment of outcomes; this team did not take part in the intervention and was unaware of the hypothesis being tested". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Reasons for drop‐out recorded. 1151 births within the study period (not clear how many in each area). 588 families received the intervention and 527 no intervention. 895 completed 3 months follow‐up (80%) and 880 6 months (79%). |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol. |
| Other bias | Unclear risk | Areas were paired, but it was not clear whether this achieved similar baseline characteristics between groups. Results were reported to have been adjusted for clustering. |
Bloom 1982.
| Methods | 2‐arm RCT, with individual randomisation (although the study also included a non‐randomised comparison group) | |
| Participants | 100 breastfeeding mothers randomized; recruited 3 days after the birth Inclusion criteria: married, primiparous with healthy infants born at a maternity hospital in Nova Scotia, Canada Exclusion criteria: infants with birthweight < 2500 g, with Apgar scores < 5, twins, women having operative deliveries, women who did not speak English |
|
| Interventions | Women in both groups received a pamphlet on breastfeeding. Intervention: weekly telephone calls beginning 10 days after the birth made by a nurse interviewer, offering support and advice and referral if necessary. Calls lasted 5‐10 min and were described as friendly. Women received up to 3 calls up to 6 weeks postpartum. Calls ceased when women discontinued breastfeeding. Control: women received usual care (not specified) |
|
| Outcomes | Interviews at 6 weeks postpartum. Women were asked about infant behaviour and infant feeding and breastfeeding duration. | |
| Notes | We have not included data from this study, because results in this paper were not reported in a form in which we could use them in the review. Most of the results were not reported according to randomisation group (rather authors described factors and associations with, e.g. breastfeeding). Breastfeeding in the randomized groups at 6 weeks was not reported and it was not possible to contact the authors to obtain this information. It was stated that average breastfeeding duration was 28.6 days in the intervention group vs 21.0 days for controls, but no SDs were reported. It was not clear when or how breastfeeding duration data were collected; if at the 6‐week postpartum interviews this suggests that figures for average breastfeeding duration only apply to those women who had discontinued breastfeeding and denominators are therefore not clear. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Described as “randomly assigned”. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The interviewer who recruited women also carried out the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The interviewer carrying out outcome assessment was reported not to be aware of the initial feeding choice (but may have been made aware of the intervention allocation by women). |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Rates of follow‐up at 6 weeks were high (97%). However, denominators for breastfeeding duration results were not reported. |
| Selective reporting (reporting bias) | Unclear risk | Most results were not reported by randomisation group and are difficult to interpret. |
| Other bias | Unclear risk | Unclear ‐ no baseline characteristics table for randomized groups. |
Bonuck 2005.
| Methods | 2‐arm RCT, n = 382 | |
| Participants | From 2 prenatal care centres in the Bronx, New York (reported to be the county in the USA with the highest poverty rate) Background rates of breastfeeding initiation: low Inclusion criteria: able to speak English or Spanish, singleton or twin pregnancy < 24 weeks (twins subsequently excluded), intending to keep infant and attend for prenatal and postnatal care at centre and affiliated hospital, telephone contact numbers available Exclusion criteria: HIV‐positive status, chronic disease with medication not compatible with breastfeeding, diabetes, serious illness, or breast reduction surgery Participant characteristics: 57% Hispanic, 36% African‐American, 62% multiparous (70% of these had previous breastfeeding experience), mean age 25 years (SD 6.23), 51.5% married or living with a partner, 57% receiving Medicaid |
|
| Interventions | Intervention (n = 188): delivered by a trained LC. Women were recruited when < 24 weeks pregnant, and had 2 prenatal LC visits scheduled. During late pregnancy there was telephone contact, and hospital and home visits and telephone support (up to 12 months postpartum) were planned for the postnatal period. In the postnatal period 25% of the intervention group received at least 1 hospital contact; approximately 50% had telephone and/or home visits; but 36% received no home or hospital visits and no telephone support. Control (n = 194): women had no contact with the LC. Standard care varied between the sites and neither site followed an established protocol for breastfeeding. Women enrolled in women and child nutrition programmes (WIC) had the opportunity to visit a breastfeeding co‐ordinator. |
|
| Outcomes | Infant health outcomes: Duration of breastfeeding and exclusive breastfeeding was presented mostly in graphical form and was difficult to interpret. Breastfeeding was categorised on a 7‐point scale from 7 = exclusive breastfeeding (which was defined as no other milk or food, but infants may have received water and other liquids) through to exclusive formula, between these extremes of the scale there were various 'intensities' of breastfeeding (e.g. > 50% breast milk). This meant that results were complicated and not easy to interpret. Women were followed up for up to 12 months and detailed (graphical) weekly data were reported for weeks 1‐26 postpartum. |
|
| Notes | Results estimated from graphs. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “The project’s biostatistical office generated and maintained a list of random codes for subjects... undisclosed blocking factor and stratification according to center.” |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes, numbered and opened sequentially. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | It was not stated whether the women were blinded. The LC providing the intervention was not blinded with respect to treatment group. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The research assistant collecting breastfeeding outcome data was not blinded with respect to treatment group. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Women were recruited in the antenatal period. 382 women were randomized. Loss to follow‐up included 10 women who miscarried or terminated the pregnancy. 304 women were followed up into the postnatal period (80% of those randomized). There were further missing data for longer term follow‐up. Loss to follow‐up was balanced across groups. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol. |
| Other bias | Unclear risk | The intervention did not appear to be standardised and many women in the intervention group (36%) did not receive any postnatal visits. |
Bonuck 2014a.
| Methods | Parallel 2‐arm participant‐level RCT, n = 666 | |
| Participants | Women who attended an urban medical centre providing prenatal care to a low income population in the Bronx, New York City. Background rates of breastfeeding initiation:79% Inclusion criteria: English‐ or Spanish‐speaking women aged ≥18 years, in the first or second trimester of a singleton pregnancy Exclusion criteria: risk factors for premature birth maternal or infant conditions that would preclude or complicate breastfeeding (e.g. mother HIV‐positive, infant congenital anomaly) |
|
| Interventions | 666 women were randomized in a 1:3:3:1 ratio to: usual care, electronic prompt (EP) alone, Lactation Consultant (LC) + EP, or LC alone. Only the LC and EP+LC arms are included in this review as the EP arm (n=253) was antental only and therefore does meet the review inclusion criteria for a breastfeeding support intervention. LC intervention (n= 80): Two LCs were allocated to this intervention.The LC protocol included 2 prenatal sessions, a hospital visit, and regular phone calls postpartum for 3 months or until breastfeeding ceased. The prenatal sessions occurred in the examination room, during the 30‐plus min of 'downtime' while waiting for the prenatal care provider. Attempts were made to complete interrupted sessions after the examination. The first session focused on rapport building and education, and the second was on the practical aspects of breastfeeding. The study provided nursing bras and breast pumps to LC group participants as needed. LCs met mothers and their infants at the 1‐week routine paediatric visit, modelling practice on a recent review. Postpartum home visits were optional, based upon participant and LC preference and comfort. LC + EP intervention (n=253): Included the LC protocol detailed above and electronic prompts for healthcare providers to ask three brief open‐ended questions which portrayed breastfeeding as the norm. This was done during pre‐natal care appointments. Control (n=80): usual care |
|
| Outcomes | For BINGO, the prespecified primary outcome measure was 3‐month breastfeeding intensity. Quote: "We categorized breastfeeding intensity as < 20% (low), 20% to 80% (medium), and greater than 80% (high) of all feeds from breast milk consistent with previous studies and Infant Feeding Practices Survey II analyses. Other analysis was planned. Power calculations were affected by the finding ‐ “we found that breastfeeding intensity was not normally distributed, and most women stopped breastfeeding altogether during follow‐up". Other outcomes: Quote: “Study staff assessed infant feeding at 1, 3, and 6 months postpartum during phone interviews using items adapted from the Infant Feeding Practices Survey II”; exclusive breastfeeding, breastfeeding intensity (“defined as the percentage of all feedings in the past 7 days that were breast milk”), breastfeeding initiation, and total duration data collected. |
|
| Notes | The paper reported 2 trials that appear in this review (PAIRINGS and BINGO). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Women were randomized using sequentially numbered opaque sealed envelopes, generated by the study’s biostatistician". Quote: "Randomization incorporated an undisclosed blocking factor and nativity status (US‐born vs foreign‐born)." |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered opaque sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "It was infeasible to blind participants and clinical staff to treatment group." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "we sought to minimize bias by restricting access to allocation assignment, stripping group assignment from study databases to which research staff had access, and omitting group identifiers from participant interview form." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The BINGO analytic sample included 94% of those randomized (628 of 666 participants). |
| Selective reporting (reporting bias) | Low risk | We checked the Clinicaltrials.gov record and the key breastfeeding outcome data seemed to be reported in this paper. |
| Other bias | Low risk | |
Bonuck 2014b.
| Methods | Parallel 2‐arm, participant‐level RCT, (n=275) | |
| Participants | Women who attended an urban medical centre providing prenatal care for a economically diverse population in the Bronx, New York City. Background rates of breastfeeding initiation:79% Inclusion criteria: English‐ or Spanish‐speaking women aged ≥18 years, in the first or second trimester of a singleton pregnancy Exclusion criteria: risk factors for premature birth maternal or infant conditions that would preclude or complicate breastfeeding (e.g. mother HIV‐positive, infant congenital anomaly) |
|
| Interventions | Intervention (n=136): lactation counselling and electronic pumps. One LC was allocated to this intervention.The LC protocol included 2 prenatal sessions, a hospital visit, and regular phone calls postpartum for 3 months or until breastfeeding ceased. The prenatal sessions occurred in the examination room, during the 30‐plus min of 'downtime' while waiting for the prenatal care provider. Attempts were made to complete interrupted sessions after the examination. The first session focused on rapport building and education, and the second was on the practical aspects of breastfeeding. The study provided nursing bras and breast pumps to LC group participants as needed. LCs met mothers and their infants at the 1‐week routine paediatric visit, modelling practice on a recent review. Postpartum home visits were optional, based upon participant and LC preference and comfort. electronic prompts for healthcare providers to ask three brief open‐ended questions which portrayed breastfeeding as the norm. This was done during pre‐natal care appointments. Control (n=139): usual practice. |
|
| Outcomes | For PAIRINGS, the prespecified primary outcome was exclusive breastfeeding at 3 months. Other outcomes: Quote: “Study staff assessed infant feeding at 1, 3, and 6 months postpartum during phone interviews using items adapted from the Infant Feeding Practices Survey II”; exclusive breastfeeding, breastfeeding intensity (“defined as the percentage of all feedings in the past 7 days that were breast milk”), breastfeeding initiation, and total duration data collected. |
|
| Notes | The paper reported 2 trials that appear in this review (PAIRINGS and BINGO). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomized using sequentially numbered opaque sealed envelopes, generated by the study’s biostatistician. |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered opaque sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "It was infeasible to blind participants and clinical staff to treatment group." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "we sought to minimize bias by restricting access to allocation assignment, stripping group assignment from study databases to which research staff had access, and omitting group identifiers from participant interview form." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Analytic sample included 95% of those randomized (262 of 275 participants. |
| Selective reporting (reporting bias) | Unclear risk | Breastfeeding outcomes reported in Clinicaltrials.gov were reported in this paper. Other outcomes on weight and length were not reported in this paper, but they are not included as outcomes in this systematic review. |
| Other bias | Low risk | |
Bortolini 2012.
| Methods | 2‐arm RCT, 1 site setting, n = 397 (unclear if this was the total number in the study overall) | |
| Participants | Children recruited at birth at the Hospital Centenário (the only hospital in the city of São Leopoldo, state of Rio Grande do Sul, Brazil), only children in Brazilian National Health Service (Sistema Único de Saúde, SUS) wards were enrolled, between October 2001‐June 2002. Background rates of breastfeeding initiation: 95.8% Inclusion criteria: newborn infants with birthweight ≥ 2500 g and gestational age ≥ 37 weeks Exclusion criteria: none noted |
|
| Interventions | Intervention: mothers provided with dietary counselling based on the guidance provided in the Ten Steps for Healthy Feeding of Children Younger Than Two Years. Counseling took place through 10 home visits: one in the first 10 days after birth, monthly up to 6 months and then at 8, 10 and 12 months. The dietary recommendations that the mothers were given prioritised exclusive breastfeeding up to 6 months and introduction of complementary foods at the age of 6 months. Mothers were advised not to give their children bottles or pacifiers. 12 undergraduate students of nutrition conducted the home visits in pairs. The entire team was trained in the dietary guidelines and in techniques for counselling mothers about the Ten Steps for Healthy Feeding of Children Younger Than Two Years. Each dietary counselling session lasted 30 min‐40 min. Control: women were visited at 6 and 12 months for collection of anthropometric, dietary and sociodemographic data and to collect data on the infants’ health status. Interviewers, who were not involved in the intervention process and who were blinded to the group to which the children belonged, conducted home visits at 6 and 12 months in order to collect data on the study variables. Interviewers informed mothers about the anthropometric results and instructed them to attend the nearest health service if nutritional problems were detected. |
|
| Outcomes | Following outcomes at 6 months of age for both groups: proportion of children exclusively breastfed for < 1 month; proportion of children exclusively breastfed for 4 months or more; proportion of children exclusively breastfed at 6 months; proportion of children recieving breastmilk at 6‐12 months and age at introduction of cows milk. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported in another paper (Vitolo 2005) which is in Spanish and needs to be translated. |
| Allocation concealment (selection bias) | Unclear risk | Reported in another paperVitolo 2005 which is in Spanish and needs to be translated. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | It is not stated whether the women and undergraduate students providing the intervention were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Interviewers who were not involved in the intervention process and were blind to the group to which the children belonged conducted home visits at 6 and 12 months in order to collect data on the study variables. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unable to assess with the information given. |
| Selective reporting (reporting bias) | Unclear risk | Outcomes not prespecified in clinicaltrials.gov record. |
| Other bias | Unclear risk | None noted. |
Brent 1995.
| Methods | 2‐arm RCT with individual randomisation, single‐site, duration not stated, n = 115 | |
| Participants | Urban USA ‐ ambulatory care centre and in‐patient maternity unit Background rates of breastfeeding initiation: low Baseline prevalence of breastfeeding at birth in national WIC sample = 33% (1991) Inclusion criteria: English‐speaking; nulliparous Exclusion criteria: separated from child at birth; preterm delivery; child in NICU > 72 h Ethnic composition: described as 71% white 90% of participants were eligible for WIC programmes for those on low income. Study population not limited to those intending to breastfeed. |
|
| Interventions | Intervention: package of: 2‐4 prenatal sessions with LC (10 min‐15 min each); telephone call 48 h after discharge; visit to lactation clinic at 1 week postpartum (staffed by paediatrician or LC); contact with LC at each health supervision visit until weaning or 1 year; professional education of nursing and medical staff. Control: women were offered optional prenatal breastfeeding classes, postpartum breastfeeding instruction by nurses and physicians and outpatient follow‐up by nurses and physicians in the paediatric ambulatory department. |
|
| Outcomes | Rates of breastfeeding at 2 months and median duration of breastfeeding | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sample stratified by age with block randomisation in blocks of 8. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Women and LC were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome data were collected by questionnaire administered by the LC who was not blinded to group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Follow‐up 94%. It appeared that 115 women were randomized. It was stated that 7 in the intervention group were excluded as they did not receive the intervention. 8 women in the control group were subsequently excluded from the analysis for at least some outcomes as the treatment they received deviated from protocol. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol. |
| Other bias | Unclear risk | Potential confounders: women were excluded from intervention group following randomisation if they had received fewer than 2 prenatal lactation consultations; ITT analysis not performed (8 women in control group who met LC excluded); intervention included input by staff caring for both intervention and control groups. |
Bunik 2010.
| Methods | 2‐arm RCT, with individual randomisation, with add‐on qualitative study, n = 339 | |
| Participants | Denver, USA; a clinic providing care for a predominantly Hispanic, medically underserved population Background rates of breastfeeding initiation: low Inclusion criteria: women ≥ 18 years, primiparous with healthy, term, singleton baby who were willing to consider breastfeeding Exclusion criteria: primary language not English or Spanish, medical complication that interfered with breastfeeding, hospital stay > 72 h following vaginal births or > 96 h following caesarean section, baby with medical problems, admitted to NICU or had a hospital stay > 72 h Participant characteristics: Mean age 22 years; 88% Hispanic or Latino; 77% vaginal delivery Planned to breastfeed only: intervention group 50%, control group 55% (other women planned to combine breastfeeding with formula) > 60% were participating in WIC programmes at 1 month and 74% of these women were provided with formula at WIC clinics. |
|
| Interventions | Intervention: daily telephone support, from the day following hospital discharge until 2 weeks postpartum, from trained nurses following a specific protocol covering advantages and disadvantages of breastfeeding, cultural issues, technique, problems and with referral for any lactation or medical problems. Control: usual hospital care (pamphlets on breastfeeding, a breast pump, lanolin cream and a water bottle); usual discharge care (commercial discharge packs) and scheduled healthcare visits at 3‐5 days and at 2 weeks at the local community health centre. |
|
| Outcomes | Any breastfeeding or predominantly breastfeeding Maternal satisfaction Healthcare utilisation Reasons for stopping breastfeeding |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated block random allocation |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered opaque sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding for participants or caregivers. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding not described for outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 341 women were randomized. At 1 month there was approximately 8% loss to follow‐up, By 6 months 27% loss. 73% were described as included in the analyses; women in the intervention group that did not receive the intervention as planned were not included. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol. |
| Other bias | Unclear risk | Groups appeared similar at baseline. |
Caldeira 2008.
| Methods | Study methods were not clear. This appeared to be a cluster‐randomised trial in 35 clinics. The intervention was carried out with healthcare workers. Results were for women attending intervention and control clinics before and after the intervention period. | |
| Participants | Setting: family healthcare teams from Montes Claros city in South East Brazil Baseline prevalence of breastfeeding initiation in country/setting: not clear 1423 women recruited (unclear). Follow‐up for 12 months Inclusion criteria: mothers with children between 0 and 2 years old registered with the family health teams Participant characteristics: Approximately 20% under 20 years, 38% primiparous, 27% vaginal deliveries, 90% with > 4 years' education |
|
| Interventions | Intervention: 20 healthcare teams received staff training to promote breastfeeding, based on the Baby Friendly Hospital Initiative. Duration of the intervention was unclear; there was an initial interview before the study and a second interview 12 months after the start of training. Control: healthcare teams (n = 15 ‐ unclear) in control clinics did not receive the training. |
|
| Outcomes | Number of exclusive breastfeeding days; survival curves | |
| Notes | We have not included data from this study. Data were not reported in a way in which we could incorporate results into the review. Authors reported the number of days, not the number of participants, for exclusive breastfeeding. It is reported that the median duration of exclusive breastfeeding was 106 days before and 107 days after the intervention period for the control group. For the intervention group the median duration of exclusive breastfeeding was reported to be104 days before and 125 days after the intervention period; the difference was reported to be statistically significant. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described; it was reported that half of the women were assigned to the Intervention group and the other half to the control group. |
| Allocation concealment (selection bias) | Unclear risk | Not described; 20 intervention clinics and 15 control (not clear). |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described in translation form. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described in translation form. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not clear. Authors reported that dropouts were negligible because all children registered were contacted with the help of community health agent. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol. |
| Other bias | Unclear risk | Data extraction from translation (original paper in Portuguese). Cluster trial with no apparent adjustment for design effect. |
Cameron 2013.
| Methods | 4‐arm RCT, 1 study site, n = 802 | |
| Participants | Maternity hospital in Dunedin, New Zealand Background rates of breastfeeding initiation: not specified. Inclusion criteria: all mothers who had booked into the single maternity hospital (> 97% of all births) serving the city of Dunedin, New Zealand, between May 2009‐November 2010, as well as mothers who planned to give birth at home and were invited to participate by their midwife. Mothers were invited to participate at 28‐30 weeks gestation and an 'opt out' recruitment strategy (eligible participants were contacted and excluded only when they said they were unwilling to participate) was used. Exclusion criteria before birth: home address outside the greater Dunedin area, planning to move away from Dunedin in the next 2 years, booked into the maternity centre after 34‐week gestation, or unable to communicate in English or Te Reo Maori [language of the indigenous (Maori) ethnic group of New Zealand]. Exclusion criteria after birth: identification of a congenital abnormality that was likely to affect feeding or growth, or the infant being born before 36.5 weeks gestation. When a mother delivered twins, the oldest child was recruited into the study. There were no triplets born during the study recruitment period. |
|
| Interventions | Interventions: various types of support: 1) infant sleep education only intervention (sleep); 2) Food, physical activity and breastfeeding (FAB) intervention: LC providing food, activity and breastfeeding help intervention; 3) combination of both 1 and 2 (Combo), participants received both the sleep and FAB interventions. Total number randomized: n = 802 (sleep 192, FAB 205, Combo 196) Control: usual care n = 209 |
|
| Outcomes | Outcomes: delayed introduction of complementary foods at 5 months and preferably until 6 months Complementary foods were defined as foods other than breast milk or infant formula (p 1483) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used a computerised random‐number generator, which assigned blocks of participants to the 4 arms. |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed and performed after application of the prebirth exclusion criteria, stratified by socioeconomic status with use of the New Zealand Deprivation Index 2006. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Group allocation was revealed to the participant after consent to participate had been obtained. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Most of the breastfeeding and other data were collected by a researcher who was not aware of the participants' group, and no data were collected by the LC who delivered the intervention. The statistician remained blinded to group allocation codes until primary analyses were conducted. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | < 25% attrition |
| Selective reporting (reporting bias) | Unclear risk | This study is a sub‐sample of results from a larger study and the outcomes reported in the trial registration document are not reported in this study, However, the full study results have not yet been published to be able to judge this outcome. |
| Other bias | Low risk | Appears to be free of demographic variables, looks comparable across the groups. |
Chapman 2004.
| Methods | 2‐arm RCT, with individual randomisation; recruitment July 2000‐August 2002 at an urban USA hospital with BFI accreditation, n = 219 | |
| Participants | Urban USA hospital prenatal clinic serving a low‐income, predominantly Latina population Background rates of breastfeeding initiation: low Antenatal inclusion criteria: low‐income women ≥ 18 years old, at ≤ 26 weeks' gestation, considering breastfeeding, not yet enrolled in peer counselling programme, resident in hospital area, available for telephone follow‐up Postnatal inclusion criteria: healthy, full term singleton infants, no congenital abnormalities, no maternal history of HIV and no admission to NICU Exclusion criteria: none specified After birth, n = 165 women remained in the study, 90 in the intervention group and 75 controls. Participant characteristics: ethnic composition 80% Hispanic (61% Puerto Rican origin), 9% African American, 3% white, 8% other |
|
| Interventions | Intervention: 1 prenatal home visit, daily visits during postpartum hospitalisation, home visit within 24 h and at least 2 more home visits as requested, and telephone/pager contact. Intervention from peer counsellors with 30 h classroom training that covered La Leche League International Peer Counseling Program and Hispanic Health Council's curricula. Peer counsellors had to score 85% in a written exam and work for 3‐6 months with experienced peer counsellors to demonstrate competence before working independently with clients. Peer counsellors had 1 h per month continuing education and were paid for their work. Control: routine breastfeeding education offered by the study hospital, and the same breastfeeding services as women paying privately. A small amount of exposure to peer counsellors among the control group was reported. |
|
| Outcomes | Breastfeeding rates at birth and 1, 3 and 6 months postpartum Subgroups most responsive to breastfeeding peer counselling | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | By computer programme |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No detail provided about whether participants and personnel were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It was not stated whether outcome assessors were blinded, but to minimise bias, data related to peer counsellor contact were collected at the end of each interview. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Loss to follow‐up appeared reasonably balanced although there was more loss from the control group. Reasons for loss to follow‐up stated. 219 were randomized, 72% followed up at 1 month, 70% at 3 months and 66% at 6 months. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol. |
| Other bias | Unclear risk | Groups appeared similar at baseline. It was reported that many women in the intervention group received less than half of the planned visits. |
Chapman 2008.
| Methods | 2‐arm RCT , 1 site, n = 206 | |
| Participants | Hartford Hospital prenatal clinic. Hospital with BFI status, the prenatal clinic serves a low‐income, predominantly Latina population. Study population (82% Latina, with Puerto Ricans comprising 50% of Latinas) Background rates of breastfeeding initiation: not specified Inclusion criteria at prenatal recruitment: be considering breastfeeding and have a prepregnancy BMI ≥ 27, ≥ 18 years of age, ≤ 36 weeks' gestation, singleton pregnancy, absence of medical conditions that would interfere with breastfeeding, planning to remain in the area for 6 months postpartum, income < 185% of the federal poverty level, and have telephone access Inclusion criteria at delivery: ≥ 36 weeks' gestation, birthweight ≥ 2.5 kg and ≤ 3.9 kg, 1 min and 5 min Apgar scores of ≥ 6, and no NICU admission Exclusion criteria: none stated |
|
| Interventions | Intervention: specialised breastfeeding peer counsellor (SBFPC) made 3 prenatal visits, plus daily in‐hospital support, and up to 11 postpartum home visits promoting exclusive breastfeeding and addressing potential obesity‐related breastfeeding barriers. Prenatal visits involved assessment of previous breastfeeding experiences/knowledge, personalised education about breastfeeding logistics, the risks of formula feeding, and anticipatory guidance. During hospitalisation, women received 1 SBFPC visit per day, which were similar in content to those provided by 'Breastfeeding: Heritage and Pride' (BHP) peer counsellors. The SBFPC ensured that intervention participants received a manual breast pump before discharge. Control: standard breastfeeding support and staff peer counsellors (see below), which involved routine access to breastfeeding support from hospital personnel, including staff peer counsellors, plus prenatal breastfeeding education that included brief breastfeeding discussions during routine clinic appointments and receipt of written educational materials. Staff nurses provided routine perinatal breastfeeding assistance, with LCs available as needed. After discharge, participants could call the hospital telephone hotline with breastfeeding questions. Standard care also included optional breastfeeding support from Breastfeeding: Heritage and Pride (BHP) peer counsellors (PC), who provided the following: up to 3 prenatal visits (covering breastfeeding benefits, breastfeeding myths, positioning, and common breastfeeding problems and to educate with up to 7 personalised home visits during the first year postpartum; and telephone support. If available, electric breast pumps were loaned as needed. To receive prenatal PC visits, controls could self‐refer or be referred to the BHP program. |
|
| Outcomes | Primary: breastfeeding initiation and the rates of exclusive and any breastfeeding at 2 weeks, 1 month, 3 months, and 6 months postpartum Secondary: infant morbidity (diarrhoea, otitis media, emergency department visits, hospitalisation), maternal amenorrhoea, and breastfeeding intensity |
|
| Notes | High attrition due to randomisation prior to birth resulting in high exclusion (Intervention n = 76, 26.2%; Control n = 78, 24.3%) with further study attrition at 6 months (intervention n = 55; control n = 53 (> 25% attrition for various stated reasons)). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | All participants provided written informed consent. Each week, the study co‐ordinator used SPSS software (SPSS Inc, Chicago, IL) to randomly assign 50% of newly recruited participants to the intervention group, thus preserving allocation concealment. |
| Allocation concealment (selection bias) | Unclear risk | Each week, the study co‐ordinator used SPSS software (SPSSInc, Chicago, IL) to randomly assign 50% of newly recruited participants to the intervention group, thus preserving allocation concealment (not sure how they did this using SPSS). |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind participants. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The interviewer was not informed of participants' group assignment, but was not completely blinded because she collected participant contact data. To minimise potential bias, participant contact questions were asked at the end of each interview. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | All attrition > 25% |
| Selective reporting (reporting bias) | Unclear risk | The paper contains additional outcomes that were not included in the clinicaltrials.gov record. |
| Other bias | High risk | The intervention group was significantly younger and differed in delivery mode, compared with the control group. |
Chen 1993.
| Methods | 3‐arm quasi‐RCT, with sequential allocation, n = 180? | |
| Participants | 180 women (not clear) attending a hospital in Southern Taiwan Inclusion criteria: breastfeeding at hospital discharge, term, healthy infant, able to read Chinese (hospital discharge at approximately 5 days) |
|
| Interventions | Intervention 1 ‐ telephone support: weekly phone calls for 2 weeks after hospital discharge then at 4 and 8 weeks postpartum by maternity nurse. The calls were to increase women’s self confidence. Intervention 2 ‐ home visits intervention: same schedule as phone support group with visits at home by the maternity nurse Control: usual care |
|
| Outcomes | Breastfeeding duration and analysis of factors affecting duration of breastfeeding | |
| Notes | We have not included data from this study in the review as data were not reported in a way that allowed us to enter them into RevMan 2014 for meta‐analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Sequentially to 1 of 3 groups |
| Allocation concealment (selection bias) | High risk | In sequence (could be anticipated and changed by the person carrying out randomisation) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient detail provided to judge this. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient detail provided to judge this. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not clear: 180 women were followed up. It was not clear whether this number was randomized. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol. |
| Other bias | Unclear risk | Baseline characteristics of 3 groups were similar. |
Coutinho 2005.
| Methods | 2‐arm RCT, with individual randomisation N=350 | |
| Participants | The study was carried out in 2 hospitals serving urban areas and neighbouring small towns in the interior of the State of Pernambuco, north‐eastern Brazil. Background rates of breastfeeding initiation: high Inclusion criteria: singleton infants Exclusion criteria: infants with congenital anomalies or serious illness necessitating intensive care and those whose mothers had serious disease or mental illness or were planning to leave the area within 6 months Approximately 60% had an income lower than the minimum wage; 33% did not have access to a flush toilet, approximately 35% of the mothers were < 20 years, 39% primiparous, approximately 28% had a caesarean delivery |
|
| Interventions | 90% of maternity staff in both hospitals received the 18‐h UNICEF/WHO Baby Friendly Hospital Initiative training course. All participants in the intervention and control groups received their hospital postnatal care from these Baby‐Friendly trained staff. Intervention: women (N=175) received 10 postnatal home visits (mean duration 30 min); 4 during the first month, 2 during the second month and 1 per month during the third to sixth months. Each mother was given an illustrated booklet. At each visit the home visitors observed the positioning of the infant at the breast, flow of milk and the baby’s satisfaction; encouraged exclusive breastfeeding for 6 months and continued breastfeeding for at least 2 years, and used the booklet as a basis for discussions of key topics relevant to the infant’s age. If there were any difficulties home visitors could not resolve he/she referred the mother for more specialist help at the hospital. If other family members were present, their attitude towards exclusive breastfeeding was assessed and their support was sought, including help with household chores. Control: (N=175) usual care with no postnatal home visits |
|
| Outcomes | Primary outcome: exclusive breastfeeding. Data collected prospectively at 1, 10, 30, 60, 90, 120,150 and 180 days after birth. Any breastfeeding at same time points. The type of other fluids introduced were also recorded at each time point. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised in blocks of 10 per town by use of a random numbers table. The random numbers were generated by the project manager, and enrolment and group assignment were made by 2 maternity‐based research assistants. |
| Allocation concealment (selection bias) | Unclear risk | Concealment was achieved by drawing numbers from envelopes at the time of assignment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "Mothers in the trial were not close neighbours, so discussion with other mothers is unlikely, but we did not formally assess whether masking was maintained". It was not stated whether the personnel delivering the intervention were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Data were collected in the trial by 4 researchers who were not aware of group allocation and were unconnected with the delivery of the interventions, however, authors did not formally assess whether masking was maintained. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 350 women were randomized, 175 in each group. 20 women (6%) were lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol. |
| Other bias | Unclear risk | The random numbers were generated by the project manager and so this may lead to bias. Results were presented in graphs and aggregated results were not simple to interpret. |
de Oliveira 2006.
| Methods | 2‐arm RCT, with individual randomisation, methods unclear N=211 | |
| Participants | From maternity ward of the Hospital de Clinicas de Porto Alegre in Brazil, a university general hospital with Baby Friendly accreditation Background rates of breastfeeding initiation high, however median duration of exclusive breastfeeding 29 days Inclusion criteria: mothers living in the city of Porto Alegre, healthy non twin newborns with a birthweight ≥ 2500 g Exclusion criteria: mother‐infant pairs that were unable to stay together due to a health concern for either the mother or infant Participant characteristics: ≥ 20 years old 76%, vaginal delivery 72%, white mothers 70%, > 8 years' education 64%, living with partner 83% |
|
| Interventions | Intervention (n=74) in hospital, 2 nurses reinforced the orientation about breastfeeding technique routinely given to mothers, following the WHO breastfeeding counselling principles, in a 30‐min session with no more than 2 mother‐infant pairs. Topics included comfortable and proper mother and infant positioning, correct attachment of the child to the breast and manual milk expression. Pictures, dolls and a model breast were used for demonstrating proper breastfeeding technique. Women also received 2 home visits from the same nurse, when the child reached 7 and 30 days of age. Infant feeding patterns, positioning, attachment, milk expression and breastfeeding problems were discussed, and breast examination performed. Control (n=137): standard hospital care met Baby‐Friendly standards. The control group appear not to have received home visits. |
|
| Outcomes | Primary outcome: number of mothers who breastfed and exclusively breastfed on maternity ward and at 30 days Secondary outcome: frequency of breastfeeding‐related problems |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Following interviews and feeding assessments, mother‐infant pairs were randomly assigned by pulling coloured balls from a bag indicating either the control or experimental group. After the number of mothers for the experimental group were selected, all women eligible for the study were added to the control group until the sample was complete. |
| Allocation concealment (selection bias) | High risk | By drawing coloured balls from bags – this could be changed and it was not clear that all women in the control group were randomly allocated. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No detail provided regarding whether participants or personnel were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The researchers responsible for the breastfeeding evaluations did not participate in the intervention and were blinded to the group to which the mother infant pairs had been assigned.” |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 233 women were eligible, 211 followed up. (It was not clear how many were randomized.) |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol. |
| Other bias | Unclear risk | Groups were described as similar at baseline, although it appeared that more women in the control group that had had previous breastfeeding experience were more likely to feed for 6 months (65%) compared to women in the intervention group (47.5%). Unequal numbers in the intervention and control group. The groups were not balanced (74 in the intervention group and 137 controls). It was not clear that all the women in the control group were randomly allocated. |
Dennis 2002.
| Methods | 2‐arm RCT, with individual randomisation, single‐site study, recruiting over 10 months, n = 258 | |
| Participants | Women at home in Toronto, Canada Background rates of breastfeeding initiation: intermediate Inclusion criteria: English‐speaking, primiparous, ≥ 16 years, single full‐term baby, intending to breastfeed, Exclusion criteria: none specified. Breastfeeding initiation 79% |
|
| Interventions | Intervention: telephone support by briefly‐trained volunteers (2.5 h session) who had personal breastfeeding experience for at least 6 months. First contact within 48 h of hospital discharge and then as required. Mean number of contacts in those completing log‐books = 5.4. Mean duration of telephone contact = 16.2 min. 97% of contacts by telephone, 3% at home. Control: not described |
|
| Outcomes | Breastfeeding (any or exclusive) at 1, 2 and 3 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly generated numbers were provided by a statistician who was not involved in the recruitment process. |
| Allocation concealment (selection bias) | Low risk | Consecutively numbered, sealed opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not explicitly stated if peer counsellors were blinded but as they were recruited for the study, it is unlikely. No detail provided on blinding participants. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “A research assistant blinded to group allocation telephoned the participants to collect data regarding current infant feeding status, breast problems encountered and health services used.” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Very little loss to follow‐up. 258 women randomized and 2 women lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol. |
| Other bias | Low risk | No apparent differences between groups at baseline. |
Di Meglio 2010.
| Methods | 2‐arm RCT, with individual randomisation, n = 78 | |
| Participants | Setting: 2 hospitals in Rochester, NY, USA Background rates of breastfeeding initiation: low Inclusion criteria: maternal age < 20 years, uncomplicated postpartum, breastfeeding singleton infant born at gestational age > 36 weeks and weighing > 2000 g, mothers and infants discharged home together Exclusion criteria: maternal contraindications to breastfeeding (HIV, active substance abuse), postpartum transfusion or intensive care; infants in intensive or special care unit > 6 h, infants with anomalies that interfered with breastfeeding (e.g. cleft lip or palate) Participant characteristics: mean age 18.3 years, approximately half were African Americans, approximately one‐third had private or health maintenance organisation insurance, the rest were on Medicaid or with Medicaid health maintenance organisations, > 80% were first time mothers and gave birth vaginally. |
|
| Interventions | Intervention: telephone support from trained peer supporters (teen mothers who had breastfed for > 4 weeks). Peer supporters telephoned the new teen mothers at 2, 4, 7 days and 2, 3, 4 and 5 weeks postdischarge. Peers introduced themselves and talked about the breastfeeding experience. No specific discussion topics were assigned. Peers offered their telephone numbers so that new mothers could call for support. They were advised to refer anyone with a problem to telephone resources for breastfeeding information or to their physician. Peers and women received gift voucher incentives to complete assessments and training. Control: usual care included access to paediatric care providers and hospital LCs. The control group did not receive telephone peer support. |
|
| Outcomes | Primary outcome: ‘any breastfeeding’ duration, as measured by the age in days at complete breastfeeding cessation Secondary outcome: exclusive breastfeeding duration, as measured by the time to the first introduction of any other supplement (water, juice, vitamins or formula) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers |
| Allocation concealment (selection bias) | Low risk | Quote: “The assignment was recorded in a sealed and numbered envelope. Envelopes were sequentially opened.” |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: “In order to blind subjects to the study hypothesis, recruiters explained that this study was about: how young mothers who breastfeed in the hospitals feed their babies at home; how young mothers make feeding decisions and who helps them make those decisions.” Not clear if this attempt was successful. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “a single research assistant conducted all the telephone interviews, using standardised, closed ended questionnaires. The interviewer had no knowledge of the study hypothesis or design.” |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 78 randomized (38 intervention, 40 control) In intervention group: 6 dropouts before first interview; 3 dropouts before 8‐week interview; 7 dropouts between 8 and 37 weeks In control group: 5 dropouts before first interview; 2 dropouts before 8‐week interview; 9 dropouts between 8 and 21 weeks Overall, 11 women dropped out immediately after recruitment (14%). By 8 weeks 21% lost to follow‐up. 46/78 (61%) were successfully followed up to complete breastfeeding cessation (22 intervention and 24 control). |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol. |
| Other bias | Unclear risk | Of the 5 adolescents who completed peer support training, there was only 1 that remained involved for the entire duration of the study. There was very poor compliance with possibly only half of the intervention group receiving the planned intervention. The analysis is presented in diagrams that are not simple to interpret. Study results published in 2010, data collected 1996‐1997 |
Di Napoli 2004.
| Methods | 2‐arm RCT, single‐site study, mothers recruited March 2000‐December 2001, n = 605 | |
| Participants | Urban Italy Background rates of breastfeeding initiation: intermediate Inclusion criteria: mothers in public maternity ward in Rome, intending to breastfeed Exclusion criteria: mothers who did not speak Italian, had no phone, breastfeeding medically contraindicated, baby in SCBU Ethnic composition not defined. Baseline national breastfeeding initiation rate 70%. |
|
| Interventions | Intervention (home visit and telephone contact): home visit, from 1 of the 6 midwives from the maternity ward of the study hospital, took place within 7 days of hospital discharge. Telephone breastfeeding counselling session provided by the same midwife. These midwives had attended the UNICEF 18‐h intensive training course on breastfeeding techniques and management. Control: standard care (not described) |
|
| Outcomes | Any breastfeeding up to 60 days | |
| Notes | Extra information about reported numbers requested and received from author. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sample was stratified "for age and parity, and finally randomly assigned to either the intervention or control group". |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details were provided about blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | A trained interviewer conducted the interviews, but was not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 605 women were randomized. Full data were available for 278 women (46%) and partial data available for a further 264 (44%). Follow‐up rates for breastfeeding outcomes collected up to 180 days, but after 60 days follow‐up rates were < 75% so only outcomes up to 60 days are included in this review. Reasons for drop‐out were reported by group. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol. |
| Other bias | Unclear risk | Baseline characteristics similar and no apparent differences between those who refused intervention and those who received it, see Table 1. |
Edwards 2013.
| Methods | 2‐arm RCT, single site, n = 248 | |
| Participants | A major USA urban university hospital community doula intervention Participant: low‐income, African‐American mothers < 22 years old Breastfeeding rates: young African‐American mothers continue to breastfeed at low rates, and commonly introduce complementary foods earlier than recommended. In the 2006 National Health and Nutrition Study, for example, only 30% of black adolescent mothers had ever attempted to breastfeed their infants. Background rates of breastfeeding initiation:79% Inclusion criteria: women who were < 34 weeks pregnant, < 21 years of age, and planning to deliver at the affiliated hospital were eligible to participate in the study. Exclusion criteria: mothers who were aware at the time of recruitment that they would require a surgical delivery, who planned to move from the area, or who planned to give up custody of the infant |
|
| Interventions | Intervention (n=124): Women received additional care from doulas who were women from the same communities as the women attending the clinic. During pregnancy women received weekly home visits (average = 10) where the doula focused on building a relationship with the mother and discussed pregnancy health, childbirth preparation, and bonding with the unborn infant.Doulas were present during labour to provide support and help initiate breastfeeding after birth.Doulas continued to provide face‐to‐face breastfeeding support in the post‐natal period (average 12 home visits). Doulas undertook a 20‐week doula training course provided by the Chicago Health Connection (Health Connect One) and a 10‐week breastfeeding peer counsellor training programme from the same organisation. Control (n=124): mothers received usual prenatal care; no doula input |
|
| Outcomes | Primary outcomes: data on breastfeeding attempts were collected by mother report at the hospital the second morning after the birth and from review of the nursing notes in the mothers medical chart after the mothers discharge. mothers were considered to have attempted breastfeeding if breastfeeding was indicated by either self report or nursing notes. At 4 months postpartum, the mothers participated in an interview on feeding practices. Mothers reported on whether they were currently breastfeeding and, if not, when they had stopped breastfeeding. Secondary: mothers were also asked about whether they had started feeding their infants cereal, either in the bottle or by spoon, or other solid foods, and reported the infant age. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomization took place in blocks of 4, 6, or 8, with equal numbers assigned to the intervention and control groups." |
| Allocation concealment (selection bias) | Low risk | Quote: "A biostatistician prepared a set of opaque envelopes, each labelled with a subject ID number and containing a group assignment. Envelopes were opened by the interviewer in the presence of the mother at the completion of the baseline interview." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind participants. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Data were collected by research staff through interviews with mothers and by chart review. Not stated whether research staff were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A total of 221 mothers,113 in the control group and 108 in the doula participated in the 4‐month interview. Attrition < 25% for both groups. |
| Selective reporting (reporting bias) | High risk | Breastfeeding at 12 months was reported as a secondary outcome in the Clinicaltrials.gov record, but this was not reported in the paper. |
| Other bias | Low risk | Mothers in the 2 groups were compared on a variety of demographic, psychological, and health variables measured before randomisation and no significant differences were found. |
Efrat 2015.
| Methods | Parallel 2‐arm RCT, single‐site study, n = 298 | |
| Participants | Community health centres in Los Angeles County, USA Participants: low‐income, Hispanic women Breastfeeding rates: local breastfeeding rates not reported but authors state that within the Hispanic population the exclusive breastfeeding rate in hospital is 27.9% and at 1 week postpartum 33% of breastfeeding Hispanic women also give their babies formula. Inclusion criteria: women 26–34 weeks pregnant, Medicaid recipient, self‐identified Hispanic, available via telephone, not assigned to a WIC peer counsellor, gave birth to a healthy full‐term singleton, absence of congenital abnormality, the infant was not admitted to a NICU Exclusion criteria: participants whose babies had medical conditions that could significantly interfere with breastfeeding. The researchers also avoided recruiting participants from health clinics located near WIC sites that offered peer support. |
|
| Interventions | Intervention (n = 146): WIC Supplemental Nutrition Programmes. The standard WIC programmes provide monthly food vouchers, nutrition education and breastfeeding support to women, infants and children aged ≤ 5 years. Breastfeeding support includes breastfeeding classes, access to a free breastfeeding helpline, breast pumps and LC service. Some programmes also offered breastfeeding and support and education using peer counsellors. The intervention group received additional support from LCs who were undergraduate students who had completed a semester‐long lactation education course and 10 h of postcourse training. The lactation education course included content knowledge on the normal breastfeeding process and cultural sensitivity training. The intervention entailed 4 prenatal and 17 postpartum phone calls (first call initiated when mothers were in the third trimester of pregnancy and the last call when mother was 6 months postpartum. The intervention participants were also provided with the lactation educator’s phone number so they could contact her more frequently if need be. On occasion, text messages were used to implement phone contacts with participants. Control (n = 143): standard WIC programme |
|
| Outcomes | Primary and secondary outcomes not distinguished. Exclusive breastfeeding at 72 h Any breastfeeding at 72 h Exclusive breastfeeding at 1 month Any breastfeeding at 1 month Exclusive breastfeeding at 3 months Any breastfeeding at 3 months Exclusive breastfeeding at 6 months Any breastfeeding at 6 months |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided to enable judgement of this. |
| Allocation concealment (selection bias) | Unclear risk | No details provided to enable judgement of this. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome data collected by the research assistants who also acted as lactation educators and were not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Follow‐up varied between 61% and 38%. |
| Selective reporting (reporting bias) | Unclear risk | Outcomes not specified in trial registration document. |
| Other bias | Unclear risk | None noted. |
Ekstrom 2006.
| Methods | Longitudinal study, 2‐arm cluster‐randomised trial, 10 Swedish municipalities randomized n=540 | |
| Participants | Setting: Antenatal Centres and Child Health Centres in 10 municipalities in southwest Sweden Background rates of breastfeeding initiation: high Inclusion criteria: Swedish‐speaking mothers who gave birth to singleton, healthy, term infants spontaneously, by vacuum extraction or by caesarean section Participant characteristics: mean age approximately 27 years, married 61%‐69%, vaginal delivery 70%‐75%, university educated 36% |
|
| Interventions | Intervention: the intervention included continuity of care at the antenatal and child centres, and a process‐oriented training program of 7 sessions for health professionals. The staff training included reflection on personal experience of breastfeeding and breastfeeding counselling, management and promotion. Staff were encouraged to develop a common breastfeeding policy between the antenatal and child health centres. The family classes were also kept together before and after childbirth. Control: offered standard family classes, usually discontinued at birth |
|
| Outcomes | Maternal perceptions of the relationship with the infant, maternal feelings for the infant and duration of exclusive/any breastfeeding | |
| Notes | 10 centres randomized. A total of 540 women took part in the study (intervention group 206 women; 2 control groups 162 + 172 = 334 women). Data collection took place at different times for the 2 control groups. We have included data from 378 women; the intervention group (206 women) and 1 control group (172 women), from whom data were collected at the same time as from the intervention group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The 10 largest municipalities were classified in pairs that were similar in size and had similar figures of breastfeeding duration. The municipalities were randomized pair‐wise to either an intervention or control group. |
| Allocation concealment (selection bias) | Unclear risk | Trial report did not report. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No detail regarding blinding of participants or personnel, but appears unlikely. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Maternity staff distributed the first questionnaire. Follow‐up questionnaires were sent to women. It was not stated whether there were any blinding procedures. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The sample included women cared for in intervention clinics and then 2 control groups. However, data collection in 1 of the control groups was carried out before the intervention period, so in the analyses we have included only the control group data that were collected simultaneously with the intervention group (total 540 women, 378 included in analysis). Response rates at 3 days 84% and 93% in the intervention and control groups, by 9 months postpartum 64% and 73%. There was no adjustment for cluster design. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol. |
| Other bias | Unclear risk | No baseline imbalance was apparent, although duration of exclusive breastfeeding was presented as a baseline characteristic. |
Elliott‐Rudder 2014.
| Methods | 2‐arm cluster‐RCT, n = 330 in 15 clusters | |
| Participants | NSW, Australia, primary care setting of general practice in rural agricultural settings. Maternity hospitals were not Baby Friendly accredited, although at each hospital an International Board Certified LC and registered midwives encouraged mothers to breastfeed. 35.3% of infants were currently fed solids at 4 months, while 52.9% had received solids, infant formula or other nonhuman milk, at least once, by 4 months. Background rates of breastfeeding initiation:92% Inclusion criteria: all pregnant women who had registered to give birth at 1 of the 3 local hospitals (n = 3127) over 14 months, had reached 24–36 weeks of pregnancy, who planned to have their postnatal care at a participating general practice and who were still breastfeeding at8 weeks were randomised. Exclusion criteria: not reported |
|
| Interventions | Intervention (n = 154): a structured conversation to support continuation of breastfeeding following a Conversation Tool flowchart that used a motivational interviewing approach. The Conversation Tool was used with each breastfeeding mother who attended a general practice intervention site for her infant to be immunised at 2, 4 or 6 months. Mothers were informed of the recommendation for breastfeeding exclusively to 6 months and maintenance to 1‐2 years and asked ‘How would that work for you?’ According to the mother’s response, the practice nurse provided a targeted proactive conversational action. Intervention practice nurses attended 2 x 5‐h training workshops that were delivered by a team of a midwife/LC/trainer and a family doctor/breastfeeding counsellor and based on WHO‐based resource that presented breastfeeding maintenance as appropriate and physiological. In addition, training addressed motivational interviewing and reflective practice. Information about local government and community breastfeeding support services, and handout literature for mothers, were provided. Control (n = 176): mothers received usual care from nurses who had not received WHO breastfeeding support training, and who commonly asked whether the mother had any problems. |
|
| Outcomes | Outcomes not clearly stated Exclusive and full/predominant (substitution of breastmilk with water‐based substances allowed) breastfeeding at 4 and 6 months |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Clusters were coded, computer randomized and assigned to the intervention or control group. |
| Allocation concealment (selection bias) | Low risk | Clusters randomized at same time, so concealment was not an issue. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was not possible for the practice nurses. Participants were unaware of the group allocation process, but not clear if this was effective. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Research assistants, who were not otherwise associated with the study, collected blinded outcome data by telephone interview. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2% attrition in intervention group and 3% in control group. |
| Selective reporting (reporting bias) | Unclear risk | The protocol did not state the predefined outcomes clearly. |
| Other bias | High risk | Difference in prenatal intentions to rejoin employment within 12 months between the 2 groups (70% intervention, 56% control). |
Ellis 1984.
| Methods | 2‐arm RCT, with individual randomisation n=120 | |
| Participants | Setting not clear: women expecting to give birth in an urban maternity unit, Canada. 120 women recruited in late pregnancy. | |
| Interventions | Intervention: in addition to usual care, prenatal breastfeeding class and postnatal drop‐in breastfeeding session. Telephone follow‐up by nurse at 2, 6 and 12 weeks postpartum. Control: usual care in hospital with assistance from nurses who had received breastfeeding education. |
|
| Outcomes | Exclusive breastfeeding at 1 and 3 months and any breastfeeding at 3 and 6 months | |
| Notes | We have not included data from this study in the review due to high levels of attrition (> 25% loss to follow‐up). Recruitment to the study took place during pregnancy and by 1 month postpartum there was high loss to follow‐up and loss was not balanced across groups. At 1 month 42% of controls and 22% of the intervention group were not available for follow‐up. The high level and unbalanced attrition means that results from this study were difficult to interpret. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Described as “randomly assigned”. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details provided about blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Did not state who collected the data. No details provided about blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Recruitment to the study took place during pregnancy and by 1 month postpartum there was high loss to follow‐up and this was not balanced across groups. At 1 month 42% of controls and 22% of the intervention group were not available for follow‐up. The high level and unbalanced attrition means that results from this study are difficult to interpret. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol. |
| Other bias | Unclear risk | There was very little information about study methods and most of the results in the paper were not reported by randomisation group. |
Frank 1987.
| Methods | 4‐arm (factorial design) RCT, single site, recruiting over 17 months, n = 343 | |
| Participants | Urban USA: inpatient maternity unit Background rates of breastfeeding initiation: low Inclusion criteria: breastfed once in hospital; able to speak Spanish or English; baby needed < 48 h on NICU; able to be contacted by telephone after discharge Participant characteristics: 57% primiparous Ethnic composition: black 65%, Hispanic 19%, white 13%, other 4% Socioeconomic status defined by: < 100% poverty level ‐ 69%; 100%‐200% poverty level ‐ 21%; > 200% poverty level ‐ 10% Mean age of participants 25.7 years |
|
| Interventions | Intervention: women received postpartum breastfeeding counselling in hospital by trained counsellor (20‐40 min) and by telephone at 5, 7, 14, 21, 28, days and 6, 8 and 12 weeks, also 24‐h advice by pager. Given research discharge pack in English and Spanish. Routine care consisted of postpartum staff nursing contacts (including discharge teaching session on infant care), infrequent breastfeeding classes, written information on breastfeeding management and the opportunity to access a midwife‐run telephone advice line. |
|
| Outcomes | Exclusive breastfeeding at 1, 2, 3 and 4 months Any breastfeeding at 4 months Median duration of breastfeeding Time to introduction of formula or solids Rehospitalisation of infants |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised block design (block size 8) with computer‐generated list of random numbers |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered opaque sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants were aware of the overall goal of the interventions but not aware of the study hypotheses. It is not detailed whether personnel were blinded but appears unlikely. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | For follow‐up at 4 months it was stated that the investigator was not aware of group assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Participants received a fee to minimise sample attrition. 343 women were recruited. There were a small number of protocol deviations (7 women received the wrong type of discharge pack and were analyzed according to treatment received rather than by randomisation group). 19 women were lost to follow‐up. Attrition and reasons for attrition were described as similar across groups. Follow‐up 94%. Appropriate randomisation procedures. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol. |
| Other bias | Low risk | No baseline imbalance apparent. |
Froozani 1999.
| Methods | Single‐site study recruiting over 7 months, n = 134 | |
| Participants | Urban Iran Background rates of breastfeeding initiation: high Inclusion criteria: women without breastfeeding experience or chronic disease giving birth normally at term to a healthy baby ≥ 2.5 kg |
|
| Interventions | Intervention: nutritionist trained using WHO Breastfeeding Counseling training course (40 h). Contact in hospital immediately after birth, between 10 and 15 days, after 30 days and monthly to the fourth month at home or in a lactation clinic Control: standard care (not described) |
|
| Outcomes | Exclusive breastfeeding at 1, 2, 3 and 4 months Mean number of days illness with diarrhoea |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Allocation by day of the week of birth. |
| Allocation concealment (selection bias) | High risk | Allocation could be anticipated in advance and different days of the week may have had different characteristics (e.g. staff on duty). |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Women were not told directly which group they had been assigned to but would be aware of whether or not they had received the intervention. The nutritionist carrying out the intervention would have been aware of allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The nutritionist carrying out the intervention also carried out all the measurements and noted breastfeeding pattern at each visit. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 134 randomized and 120 followed up. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol. |
| Other bias | Low risk | No baseline imbalance apparent. |
Fu 2014.
| Methods | Multicentre, 3‐arm cluster‐RCT, n = 722 (clusters (hospitals) n = 3) | |
| Participants | Mother–infant pairs were recruited from the postnatal units of 3 geographically distributed public hospitals providing obstetrical services in Hong Kong. Participants: 722 primiparous breastfeeding mothers with uncomplicated, full‐term pregnancies Background rates of breastfeeding initiation: 80% In Hong Kong current breastfeeding patterns are similar to those of other developed countries, with > 80% of women initiating breastfeeding, but with only 20% continuing to breastfeed exclusively for 3 months. Inclusion criteria (mother): Hong Kong Chinese primiparas, ≥ 18 years old, intending to breastfeed, and without any major obstetric complications (i.e. severe postpartum haemorrhage) or serious medical problems (i.e. psychiatric illness) Inclusion criteria (baby): gestational age ≥ 37 weeks; birthweight ≥ 2500 g, 5‐min Apgar score ≥ 8, and no physical anomalies that would contraindicate or complicate breastfeeding Exclusion criteria: mothers who were planning to live in mainland China after delivery |
|
| Interventions | Intervention 1: standard care plus 3 in‐hospital professional breastfeeding support sessions, of 30–45 min in duration Intervention 2: standard care plus weekly postdischarge breastfeeding telephone support, of 20–30 min duration, for 4 weeks. Both interventions were delivered by 4 trained research nurses, who were either highly experienced registered midwives or certified LCs. Control: standard postnatal maternity care that consisted of routine perinatal care according to the type of delivery, group postnatal lactation education provided by a midwife or LC, 1‐on‐1 assistance with breastfeeding if problems arose and time permitted, and postdischarge follow‐up, either at the outpatient clinic of the delivery hospital or at the nearest Maternal and Child Health Centre. Information on available peer‐support groups was also provided upon hospital discharge. |
|
| Outcomes | Primary: prevalence of any and exclusive breastfeeding at 1, 2, and 3 months postpartum. Classified infant feeding status into 3 categories: exclusive breastfeeding; any breastfeeding; and exclusive formula feeding. Secondary: overall duration of any and exclusive breastfeeding. Measured the duration of any and exclusive breastfeeding as the age of the infant in weeks when the participant completely stopped breastfeeding and first introduced infant formula, respectively. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The allocation sequence was generated using an online program (www.randomization.com). All participants at each study site were allocated to the intervention to which the hospital was randomly assigned for that week. Cluster‐randomisation was used, with hospitals being the unit of randomisation. Each week, a study hospital was randomly assigned each study hospital to 1 of the 3 treatment groups. |
| Allocation concealment (selection bias) | Low risk | Conducted by a person not involved in the subject recruitment or data collection. Assignments placed in sequentially numbered opaque sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The blinding of either participants or those delivering the intervention was not possible for this type of study design. For the control and telephone support group, a research nurse not involved with delivering the intervention, recruited the participants. However, authors state that for the inpatients, the same nurse who recruited the participants also delivered the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | A study research assistant, who was blinded to the participants’ treatment allocation, conducted the telephone follow‐up. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 97% of participants had complete follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | No evidence of predefined outcome measures so unable to make a judgement. |
| Other bias | High risk | Not all intervention groups received the full intervention.Of the 191 participants allocated to the in‐hospital support group, 137 (71.7%) received all 3 sessions, 52 (27.2%) received 2 sessions, and 2 (1.0%) received only 1 session before hospital discharge. Of the 268 participants in the telephone support group, 199 (74.3%) received all support sessions for which they were eligible; 27 (10.1%), 24 (9.0%), 13 (4.9%), and 5 (1.9%). Baseline characteristics and maternal and birth data were similar across the 3 groups although there were some minor variations in maternal education, family income, intention to exclusively breastfeed, and antenatal breastfeeding class attendance. |
Gagnon 2002.
| Methods | 2‐arm RCT, with individual randomisation, n = 586, 292 assigned to intervention and 294 to control | |
| Participants | Study conducted at a university teaching hospital and affiliated community health centres in urban Quebec, Canada. Recruitment January 1997‐September 1998 Background rates of breastfeeding initiation: intermediate Inclusion criteria: mothers participating in hospital short‐stay programme Ethnic and socioeconomic composition of sample not reported Baseline prevalence of breastfeeding initiation in Canada (excluding territories) 1994‐5 = 73% |
|
| Interventions | Intervention: home visit from community nurse 3‐4 days postpartum. Nurses were Baccalaureate prepared, had a minimum of 3 years' clinical experience in maternal‐child health, and had attended training to ensure assessment skills of maternal‐newborn and breastfeeding support. Contact with the nurse continued if required. Control: usual care was a 48‐h postpartum contact and 1 postpartum hospital clinic visit (day 3) following a standard plan of care and lasting up to 45 min. Referral for continued care was available. |
|
| Outcomes | Breastfeeding frequency and infant weight gain assessed at 2 weeks postpartum | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation (block size 8) stratified by parity, by computer‐generated random numbers. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "masking of the women and health professionals was not possible." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It was reported that outcome data were collected by blind investigators. It was not clear whether planned blinding was effective, although investigators apparently asked women "not to divulge their group status". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 586 randomized. 21 protocol deviations, but analysis performed according to randomisation. 499 completed trial and provided information on primary outcome (15% attrition). Some further missing data for some outcomes. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol, so were unable to evaluate. |
| Other bias | Low risk | Groups described as similar at baseline. |
Graffy 2004.
| Methods | 2‐arm RCT with individual randomisation, conducted in 32 general practices in the UK; recruitment April 1995‐August 1998, n = 720; 363 assigned to intervention and 357 to control | |
| Participants | Urban south‐east England Background rates of breastfeeding initiation: intermediate Inclusion criteria: mothers considering breastfeeding who had not breastfed a previous child for 6 weeks after birth Exclusion criteria: planning to contact a breastfeeding counsellor, address considered unsafe to visit, baby born before 36 weeks' gestation Ethnic composition of sample: 59% white (UK) participants, 11% white (other) participants, 16% African or Caribbean, 8% Indian subcontinent, 6% other Socioeconomic status on RG classification: 10% I, 26% II, 19% IIINM, 26% IIIM, 12% IV, 3% V, 5% other First baby: 74% National baseline prevalence 66% breastfeeding at birth |
|
| Interventions | Intervention: women received 1 antenatal visit from a National Childbirth Trust trained breastfeeding counsellor, who offered postnatal support by telephone or further visits if the mother requested this after the birth Control: standard care (UK standard care includes postnatal home visits from midwives and health visitors) |
|
| Outcomes | Prevalence of any breastfeeding to 6 weeks; duration of any breastfeeding to 4 months; time to introduction of formula feeds; maternal satisfaction and common feeding problems; mothers' perspectives on support from counsellors; association between counselling uptake and feeding behaviour | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Permuted block design stratified by GP practice and parity, randomisation schedule prepared by statistician. |
| Allocation concealment (selection bias) | Low risk | Numbered sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Did not state whether participants or personnel were blinded, however, it seems unlikely due to the nature of the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Reported that responses to follow‐up questionnaires were coded by blinded assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 720 women recruited and randomized. 97% available for follow‐up at birth, 93% at 6 weeks and 86% at 4 months. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol, so could not evaluate this. |
| Other bias | Unclear risk | Groups were similar at baseline although more women in the intervention group (16) than the control group (6) were undecided about breastfeeding intention at the antenatal assessment. It was reported that a sensitivity analysis was carried out to adjust for this possible confounder. |
Gross 1998.
| Methods | Cluster‐randomised trial. 4 clinics were 'randomly assigned' to 4 different interventions n=548 | |
| Participants | Setting: 4 WIC clinics in Baltimore USA Women were predominantly African American (> 90%) 548 women attending study clinics enrolled at between 6 and 24 weeks’ gestation. Women were WIC eligible with singleton pregnancies, planning to keep the baby and to stay in study areas |
|
| Interventions | The study was carried out in 4 clinics. Each clinic offered a different intervention. Clinic 1: standard care (usual breastfeeding promotion by clinic staff) Clinic 2: standard care plus a motivational video (encouraging breastfeeding) that was played repeatedly in the clinic waiting area Clinic 3: peer support by a mother who had breastfed and undertaken training. Peer supporters contacted pregnant women and discussed breastfeeding. Women were offered a 1‐h group breastfeeding support session in the WIC clinic before the birth. After the birth, peer counsellors contacted women and remained in contact with breastfeeding women (phone or visits) until 16 weeks after the birth. Clinic 4: standard care plus video plus peer support |
|
| Outcomes | Infant feeding method at 8 weeks and 16 weeks postpartum and maternal work status | |
| Notes | We were not able to include data from this study in the review due to very high levels of attrition. The study was at a high risk of bias. This was a cluster trial with 4 clinics each allocated to a different intervention and with no adjustment for study design effect. Women were recruited in the antenatal period. 548 women enrolled but information was only available for 273 women at 7‐10 days postpartum (50%); of the 275 women lost to follow‐up 31% (74) were excluded due to pregnancy complications, the remaining 73% (201 women) refused or could not be contacted – these women represented 37% of the original randomized sample. It was not clear whether loss was similar in the 4 clinics. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Cluster trial. 4 clinics; method of randomisation not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Did not state whether participants or personnel were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Did not state who the interviewers were or if any attempt at blinding outcome assessment was made. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 548 women were enrolled on the study, but information was only available for 273 women at 7 to 10 days postpartum (50%); of the 275 women lost to follow‐up 31% (74) were excluded due to pregnancy complications, the remaining 73% (201 women) refused or could not be contacted – these women represented 37% of the original randomized sample. It was not clear whether loss was similar in the 4 clinics. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol, so could not evaluate this. |
| Other bias | Unclear risk | Baseline characteristics: imbalance for educational status, employment and parity ‐ although these were adjusted for in the analysis. |
Grossman 1990.
| Methods | 2‐arm quasi‐RCT, with individual randomisation, single‐site study recruiting over 10 months, n = 97, follow‐up 90%. Quasi‐randomisation via coin toss, with women sharing same room allocated by 1 toss | |
| Participants | Urban USA ‐ inpatient maternity unit Background rates of breastfeeding initiation: low WIC breastfeeding prevalence at birth 1991 = 33% Inclusion criteria: women eligible for WIC programme services for those on low incomes; women intending to breastfeed Participant characteristics: approximately one‐third were primiparous. Ethnic composition described as 54% black. Mean age was 25.4 years. |
|
| Interventions | Intervention: package included 30‐45 min face‐to‐face meeting in hospital with LC (a registered nurse) after birth ‐ educational booklet given; telephone contacts on days 2, 4, 7, 10 and 21; a telephone help‐line staffed by a nurse or paediatrician; and back‐up support for those with problems from a lactation clinic Control: routine postnatal teaching on infant care and feeding by obstetric nursing staff |
|
| Outcomes | Rates of breastfeeding at 6 weeks and 3 and 6 months Median duration of breastfeeding |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Coin toss at the point of randomisation. |
| Allocation concealment (selection bias) | High risk | Coin toss at the point of randomisation, so allocation could be altered. If 2 women occupied the same room they were allocated to the same group. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Did not state whether women or personnel were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Some data were derived from medical records, but telephone outcome assessment was not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 97 women randomized, by 6 weeks 4 control group women could not be contacted (> 90% follow‐up but loss not balanced across groups). |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol, so could not evaluate this. |
| Other bias | Unclear risk | Groups appeared similar at baseline. |
Haider 2000.
| Methods | Community‐based cluster‐randomised study (40 adjacent areas randomized), recruitment over 10 months, n = 726 | |
| Participants | Setting: Dakka, Bangladesh Background rates of breastfeeding initiation: high Socioeconomic status: mainly lower‐middle and low Inclusion criteria: women aged 16‐35 years with ≤ 3 children (or ≤ 6 pregnancies) and no serious illness Exclusion criteria: multiple births, children with congenital abnormalities, and those weighing < 1800 g National baseline prevalence reported in paper was similar to the control group rates; UNICEF quoted higher rates ‐ 53% exclusive breastfeeding at 0‐3 months |
|
| Interventions | Intervention: peer counselling by women with personal breastfeeding experience trained over 40 h with the WHO/UNICEF Breastfeeding Counseling course. Paid honorarium. Supervised caseload of 12‐25 mothers. 15 home visits: 2 in last trimester/4 in month 1/2‐weekly in months 2‐5. Duration of visits 20‐40 min. Control:not specified |
|
| Outcomes | Exclusive breastfeeding at birth, 4 days, 4 weeks, 2, 3, 4 and 5 months. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random number tables |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Women and counsellors aware of group assignment. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Interviewers collecting outcome data would also be aware of assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 40 areas randomized (20 intervention, 20 control) 726 women randomized. 653 available to follow‐up at delivery (90%). 573 available at 5 months (79%). Loss appeared balanced across groups. No ITT analysis. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol, so could not evaluate this. |
| Other bias | Low risk | No differences in baseline characteristics apparent. Staed that results were based on individual level analysis, but with adjustment for cluster level of randomisation. |
Hall 1978.
| Methods | 3‐arm RCT n=49 | |
| Participants | 49 women giving birth in a small community hospital in the USA planning to breastfeed for at least 6 weeks and breastfeeding for the first time. All women had healthy babies. Women were described as married and middle class aged 17–31 years | |
| Interventions | 3 groups: Intervention 1: 15 randomized, 13 followed up (not clear): usual care plus an educational session Intervention 2: 16 randomized, 15 followed up (not clear): usual care plus education plus daily visits by nurse while in hospital and telephone support 2 days after discharge and 1 week later and further support if necessary (up to 5 weeks postpartum). Control: 18 randomized, 12 followed up (not clear) |
|
| Outcomes | Outcomes were unclear, but included breastfeeding at 6 weeks and breastfeeding problems. | |
| Notes | We have not included data from this study in the review due to methodological weakness and high and unbalanced levels of attrition. More than 30% of the control group were lost to follow‐up and results were therefore difficult to interpret. Most results were not reported according to randomisation group and the only result for breastfeeding duration was approximate, stating: “Approximately 50% of the control group and 50% of the group which received the teaching unit were still nursing at 6 weeks. Of the group who received the teaching plus support 80% were still nursing at 6 weeks.” | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Described as 'randomly assigned'. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessment was not by the same nurse as the 1 delivering the intervention, but unclear if they were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | There was high attrition in this small study, > 30% of the control group were lost to follow‐up and results were therefore difficult to interpret. |
| Selective reporting (reporting bias) | Unclear risk | Most results were not reported by randomisation group. |
| Other bias | Unclear risk | No baseline characteristics reported. |
Hanson 2015.
| Methods | 2‐arm cluster‐RCT, whole population in 132 wards (clusters) | |
| Participants | Setting: rural Tanzania. Most residents were subsistence farmers living in small settlements (subvillages) and 90% lived within 5 km of primary facilities. Background rates of breastfeeding initiation: not stated Inclusion criteria: households in intervention and comparison wards with live births. If the village had fewer than 130 households, all households in the village were included. Exclusion criteria: If the village had greater than 130 households, segmentation was used to limit the sample to a maximum of 131 households. |
|
| Interventions | Intervention: home‐based counselling ‐ this strategy, branded Mtunze Mtoto Mchanga, which means "protect your newborn baby" in Swahili, was developed in 2008–2009. The strategy was designed in consultation with the Ministry of Health and members of the WHO, UNICEF, and professional organisations. Key counselling messages were selected on the basis of the frequency of the behaviour in 2007 the feasibility of change, and the likely impact on survival on the basis of evidence published at the time. They included hygiene during childbirth, early and exclusive breastfeeding, and extra care for low‐birthweight babies, including skin‐to‐skin care. Control: usual practice |
|
| Outcomes | Primary: all‐cause neonatal mortality rate, per 1000 live births, defined as the proportion of all live births who died in the first 28 days of life Secondary: Breastfeeding within an hour of delivery; Soap or use of gloves for those attending home deliveries; Exclusive breastfeeding for 3 days after birth; Skilled attendance at birth; Birth preparedness; Immediate drying and covering of the baby; Clean cord care; Delayed bathing; Identification and extra care for small babies, including skin‐to‐skin care for small babies and referral to hospital for very small babies |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation was performed using STATA" (p.8). |
| Allocation concealment (selection bias) | Unclear risk | No information provided to enable us to judge this. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were not blinded to the intervention. Volunteers who provided the intervention were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "The survey team was unaware of cluster allocation. The data analyst was masked to the cluster allocation until data cleaning was complete and a copy of the data lodged with the data and safety monitoring board". Unclear if these procedures were effective. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Probably high as the data were obtained retrospectively in the 2013 survey and results from the previous year were used in the data analysis. Six of the sampled sub‐villages refused to participate. In 6% of households no one was present, 1% of households refused to participate. |
| Selective reporting (reporting bias) | High risk | Breastfeeding was not a prespecified outcome in the clinicaltrials.gov record. |
| Other bias | High risk | Risk of contamination, quote: "Methodological limitations include our inability to rule out some degree of leakage of the intervention into the comparison areas and response bias for newborn care behaviours". |
Hoddinott 2009.
| Methods | 2‐arm cluster‐RCT with prospective mixed method embedded case studies to evaluate implementation processes. 14 localities randomized; recruitment 2002‐2004 n=18858 | |
| Participants | Setting: women registered at GP practices in 14 localities (of 66) in Scotland Background rates of breastfeeding initiation: low In Scotland, in 2005, only 44% of babies had received any breast milk at 6 weeks. 14 clusters randomized, birth records supplied data for n = 9747 in intervention group and n = 9111 in control group. Inclusion criteria: pregnant women and breastfeeding mothers Exclusion criteria: not stated In intervention localities 25.2% of the populations were in the most deprived social groups, compared with 32.1% in the control localities. Mean age of mothers at the first child health record was 28‐29 years. In 7 areas (3 intervention, 4 control) women gave birth at Baby‐Friendly hospitals. |
|
| Interventions | Intervention: a policy intervention aimed at locality areas rather than at individual women. The policy aimed to double the number of local breastfeeding support groups and to make weekly support groups open to all pregnant women and breastfeeding mothers. These local breastfeeding support groups were facilitated by health professionals taking a woman‐centred approach and aiming to provide breastfeeding support and social interaction for women. Control: control localities received no additional intervention; however, breastfeeding support groups existed in some control areas. |
|
| Outcomes | Primary: number of babies receiving any breast milk at 6‐8 weeks, as reported in routinely collected data for the 2 pretrial years and 2 trial years Secondary: any breastfeeding at birth, 5‐7 days and 8‐9 months, and maternal satisfaction Results were not presented in a way which allowed us to enter them into data and analysis tables, but we have summarised findings in the text. |
|
| Notes | When we updated our search in October 2011, Hoddinott 2009 was the only evaluation we found: a) of a policy‐level intervention; b) of breastfeeding in groups; and c) that used routinely collected locality‐level outcome data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Cluster‐RCT; 14 localities randomized. Localities varied in size, baseline breastfeeding rates and numbers of pre‐existing groups and how pregnancy and postnatal care were organised. Localities were matched on breastfeeding rates and existing support groups: quote: “An independent statistician used random number tables to randomise locality pairs to either intervention or control”. |
| Allocation concealment (selection bias) | Low risk | Researchers analysing primary and secondary outcomes were blinded to allocation, ensured by coding of localities. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Cluster‐randomised trial, so women may not have been aware of the study although they would be aware of the intervention. Not stated whether personnel were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Researchers analysing primary and secondary outcomes were reported to be blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | According to flow chart no clusters discontinued the intervention or were lost to follow‐up and there was follow‐up of national data in all localities included in the trial. The amount of data missing varied for different outcomes (e.g. birth and 6 week postpartum records were available for most of the eligible population but child health records at 8‐9 months were only available for approximately a quarter of the children). |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol, so could not assess this. |
| Other bias | Unclear risk | Analysis took into account aspects of design effect. It appeared that there were some differences in the localities at baseline. Control localities may have had higher levels of social deprivation. |
Hoddinott 2012.
| Methods | 2‐arn RCT, single‐site study, n = 69 | |
| Participants | Setting: a maternity unit serving a mixed urban and rural population in Scotland Background rates of breastfeeding initiation: at hospital discharge, 54% of babies were exclusively breastfed and 6% were receiving breast and formula milk. In the most disadvantaged areas, 39% exclusively breastfed compared with 63% in the most advantaged areas. Inclusion criteria: women admitted to the ward between 26 July‐18 October 2010 who lived in the 3 most disadvantaged postcode area quintiles for the Scottish Index of Multiple Deprivation (SIMD 1‐3) in 2009 and who were breastfeeding Exclusion criteria: women aged < 16 years with serious medical or psychiatric problems or with insufficient spoken English to communicate by telephone |
|
| Interventions | Intervention (n = 35): proactive telephone calls (intervention) daily for 1 week following hospital discharge. Calls were terminated at the woman’s request or if breastfeeding ceased. At 1 week following discharge, women could choose to continue receiving daily calls for a further week, change the frequency of calls, or have no further calls. Women could telephone the feeding team at any point over the 2 weeks following discharge. Text and answer phone messaging was available. All proactive calls stopped 14 days after hospital discharge. Control (n = 34): reactive telephone calls; women could telephone the feeding team at any point over the 2 weeks following discharge. Text and answer‐phone messaging was available. |
|
| Outcomes | Any breastfeeding at 6‐8 weeks | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Using a website randomisation sequence service set up by an independent statistician. Randomisation was stratified to ensure balance of primiparous and multiparous women across both trial arms." |
| Allocation concealment (selection bias) | Low risk | Performed by an independent statistician. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Although not informed of the randomisation outcome, women knew if they had been randomized to the proactive group as they received a phone call from the feeding team within 24 h of hospital discharge". Healthcare professionals providing intervention would have been aware of allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes were collected by telephone by a researcher who was blind to randomisation and who had no other contact with study women. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 3/35 in the intervention group and 8/34 of those in the control group did not have data at follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | No evidence of outcomes being prespecified anywhere, so difficult to judge this. |
| Other bias | High risk | Women in the proactive call group were a year older on average, with more living in the most disadvantaged postcode areas (SIMD 1). Hospital stays were half a day longer on average in the proactive call group; however, data were imbalanced by a small number of women with unusually long hospital stays. Otherwise the randomized groups were similar for parity, method of delivery, gestation and admission to the neonatal special care unit. |
Hopkinson 2009.
| Methods | 2‐arm RCT, n = 522 | |
| Participants | A large metropolitan hospital in Houston, Texas, USA, serving predominantly immigrant Hispanic women (85% monolingual Hispanic) Background rates of breastfeeding initiation were high in this study population. Inclusion criteria: mothers of low‐risk infants, mixed feeding in hospitals, had telephones and access to transportation Exclusion criteria: infants with elevated risk for hyperbilirubinaemia (preterm, discharged at < 48 h old, jaundice within 24 h of birth, Rhesus‐incompatibility, cephalohematoma, positive Coombs test, family history of disorders of red blood cell enzyme defects, or defects of red blood cell shape and size) Participant characteristics: Mean maternal age: intervention group 26.8 years, control group 27.1 years Mean parity: intervention group 1.5, control group 1.5 Mothers born in the USA: intervention group 2.8%, control group 1.1% (most of the women were born in Mexico or Central America) |
|
| Interventions | Intervention: mothers were given an appointment to visit the hospital‐based breastfeeding clinic at 3‐7 days postpartum. At the breastfeeding clinic, peer counselling sessions included a breastfeeding history, breast examination, infant oral‐motor assessment, measurement of infant weight, evaluation of latch and milk transfer, and discussion of the mother's concerns and support system. Additional visits and telephone consultations were provided if deemed necessary by the mother and the clinic staff. Women who missed appointments received a telephone call. Control: received usual care, which included bedside breastfeeding assistance before hospital discharge and the phone number of the hospital breastfeeding clinic with instructions to call if needed. |
|
| Outcomes | Primary: exclusive breastfeeding at 1 month Secondary: volume of formula given by mothers who were mixed feeding, and incidence of breastfeeding problems |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Generated by random number table. |
| Allocation concealment (selection bias) | Low risk | After participants had given informed consent, the group was determined using opaque, sealed envelopes containing assignments generated by random table number. The envelope was opened by the mother. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were not blinded as the envelope was opened by the mother. Caregivers were also not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | For outcome assessors, outcomes were determined by telephone survey at 4 weeks postpartum by interviewers blinded to group assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 522 randomized (255 in intervention group and 267 in control group. 55 women were lost to follow‐up at 4 weeks (10.5%). Loss was balanced in the 2 groups. Issues around incomplete data were addressed. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol so could not judge this. |
| Other bias | Low risk | There were slight baseline differences between the control group and the experimental group. Women in the intervention group were more likely to have an emergency caesarean, and were taller. Due to low compliance with the intervention, secondary analysis was carried out, but we have reported data from the primary analysis (unadjusted). |
Howell 2014.
| Methods | 2‐arm RCT, single site, n = 540 | |
| Participants | Black and Latina women who had delivered at a large tertiary hospital located in New York City, USA Background rates of breastfeeding initiation: not stated Inclusion criteria: participants were black/African American or Latina/Hispanic, spoke English or Spanish, had a working telephone, ≥ 18 years old, and had infants with birthweights > 2500 g and 5‐min Apgar scores > 7 Exclusion criteria: not specified |
|
| Interventions | Intervention (n = 270): The intervention used a behavioural educational approach and aimed to prepare and educate mothers about postpartum symptoms and experiences provide social support, and develop self management skills. The intervention was delivered in two parts. The first part was delivered whilst the women was in hospital by a social worker who reviewed an education pamphlet and partner summary sheet with each mother. Education materials provided information about post‐partum care and included information on breastfeeding and breast/nipple pain.. Additional information was provided on social support. The second part was a 2‐week postdelivery call, where the social worker assessed patients’ symptoms, skills in symptom management, and other needs. Patients and the social worker created action plans to address current needs that included assessment of community resources. Control: enhanced usual care; participants received a list of community resources and received a 2‐week control call |
|
| Outcomes | Duration and exclusivity of breastfeeding at 6 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Data were collected in person at baseline and by telephone during follow‐up interviews by bilingual clinical research co‐ordinators who were blinded to intervention status. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 79.3% of intervention group and 77.4% of control group had data available at 6 month follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Not clear |
| Other bias | Low risk | Baseline sociodemographic, clinical, psychosocial, and breastfeeding characteristics were similar among intervention vs control subjects. There were no clinically important differences between intervention and control groups in baseline sociodemographic, clinical, and psychosocial characteristics, except that comorbid conditions were more prevalent among the control subjects than intervention subjects (27% vs 20%; P < .05). |
ISRCTN47056748.
| Methods | 2‐arm RCT, single centre, single blind, recruitment 2005‐2006 n=182 | |
| Participants | Setting: maternity unit in Northern Ireland with Baby‐Friendly accreditation Background rates of breastfeeding initiation: intermediate, one‐fifth of women were reported to have stopped breastfeeding before hospital discharge. Participants randomized n = 182 Inclusion criteria: primigravid women who attended for antenatal care when 20 weeks pregnant, intended to give birth at the study hospital and consented to participate Exclusion criteria: women < 20 years old who had commenced the ‘young mums’ parentcraft programme prior to the 20‐week visit. Vulnerable women, e.g. women who neither spoke nor understood English. Mothers separated from their babies, for example when a baby was admitted to the neonatal unit, who did not receive routine instruction (postrandomisation exclusion). Sample characteristics for n = 144 who completed the research (not reported by randomized group): Age 21‐30 years: 79/144 (55%) Age 31‐40 years: 53/144 (37%) Socioeconomic status: 38 (26%) professionals; 20 (14%) not working |
|
| Interventions | All study participants received standard care at the study hospital, this met Baby‐Friendly standards and complied with National Institute for Clinical Excellence (NICE) guidelines. Intervention: staff training; women (n = 93) received a 'motivationally enhanced' version of control group care from staff who had been trained in a programme called ‘Designer Breastfeeding’. Control (n = 89): women received a 2‐h antenatal infant feeding class, a breastfeeding book and midwife support for the first 3 postnatal weeks. |
|
| Outcomes | Primary: women’s motivational profile was measured, using 7‐point Likert scales, in relation to 3 motivational factors: total value placed on breastfeeding, perceived midwife support, and expectancy for successful breastfeeding. Secondary: breastfeeding behaviour on discharge from hospital and at 3 weeks postnatally. Breastfeeding initiation was defined according to the Department of Health as giving 1 breastfeed or 1 episode of expressed breast milk. Duration of breastfeeding was categorised in accordance with the Index of Breastfeeding Status, which classifies breastfeeding on a scale in accordance with the amount of breast milk the infant receives |
|
| Notes | Only 53/89 women randomized to the control group were known to have initiated breastfeeding. In the intervention group 57/93 women randomized initiated breastfeeding. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Prior to recruitment a randomized table was created." |
| Allocation concealment (selection bias) | Low risk | The authors state: "Neither the researcher, nor the research participants could predict their allocated treatment". |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Described as single blind. Women were said to be not aware of groups, but there were stickers on the notes so care providers would be aware of group assignment. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details available about who collected outcome data to enable us to judge this. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 234 assessed for eligibility, 182 consented and 144 completed. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol, so could not assess this. |
| Other bias | Unclear risk | There were some baseline differences between groups. Women in the control group were more likely to be discharged from hospital early and were less likely to attend antenatal infant feeding classes. It is not clear what impact these differences had on the results. |
Jenner 1988.
| Methods | Quasi‐RCT, recruitment location/duration not stated, n = 38 | |
| Participants | UK white, working‐class women 19‐32 years old, living with partner and intending to breastfeed Background rates of breastfeeding initiation: intermediate: prevalence of breastfeeding in 1985 = 64% at birth and 26% at 4 months |
|
| Interventions | Intervention: 3 antenatal home visits/1 hospital visit/1 'immediate' home visit and 1 or 2 further home visits 'in the early weeks'; plus face‐to‐face and telephone support by a single lay supporter (mother/previous breastfeeding experience, but no indication of training) Control: 1 antenatal home visit and 1 postnatal hospital visit |
|
| Outcomes | Breastfeeding at 3 months. Partial breastfeeding grouped with formula feeding as 'breastfeeding failure' | |
| Notes | Moderate‐to‐high risk of bias | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Alternation |
| Allocation concealment (selection bias) | High risk | Alternate allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was not attempted. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding was not attempted. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 38 women included. It appeared that all were followed up. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol to judge this. |
| Other bias | Unclear risk | Very little information available about study methods. |
Jolly 2012a.
| Methods | 2‐arm cluster‐controlled trial, n = 1267 | |
| Participants | Primary Care Trust health district (PCT) in Birmingham, UK Background rates of breastfeeding initiation: 58%, with continuation rates poorly collected, but considered to be lower than national average Inclusion criteria: all pregnant women registered with a GP within the PCT, with an estimated delivery date between 1 February 2007‐31 July 2007 Exclusion criteria: none stated |
|
| Interventions | Intervention (n = 1267): antenatal peer support offered to all women in intervention clusters to encourage breastfeeding initiation, and postnatal peer support for women who initiated breastfeeding to increase continuation. Community peer support workers were employed and trained by the breastfeeding personnel in the PCT in line with WHO/UNICEF Baby Friendly breastfeeding management course. Antenatal support was aimed to be 2 support sessions (at least 1 at home, although almost all actually took place in the clinic/Children’s Centre setting). The support workers were informed when the women were discharged from hospital so that they could contact and visit them within 24 h–48 h. Further contact would be needs‐based, but with a minimum of 1 more contact in the first week. Additional needs‐based contacts could be by telephone or home visits. Number randomized = 1267 (416 consented to follow‐up at 6 months, and 271 of these responded at 6 months) Control (n = 1370): routine maternity care. Number randomized = 1370 (432 consented to follow‐up at 6 months, 301 responded at 6 months) |
|
| Outcomes | Any breastfeeding and exclusive breastfeeding at 10–14 days, 6 weeks and 6 months and 6 months | |
| Notes | The numbers randomized in this paper do match the MacArthur paper which states: Intervention: n = 1140 deliveries Women with data on initiation of breastfeeding n = 1083 Women who initiated breastfeeding n = 747 Comparison: n = 1371 deliveries Women with data on initiation of breastfeeding n = 1315 Women who initiated breastfeeding n = 896 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Practices were "randomized by the trial statistician with stratification by midwifery team and numbers of deliveries per clinic". Unclear how the sequence was generated. |
| Allocation concealment (selection bias) | Unclear risk | No details provided to allow us to assess this. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Women were aware of allocation at recruitment. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Women’s options for 6 month follow‐up were by postal questionnaire in English, or by telephone in their language of choice by a researcher blinded to the trial allocation. Unclear whether the questionnaire would have contained identification related to allocation. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | When based upon the number of women who consented to be followed up at 6 months, the follow‐up was 69.7% in the intervention group and 65.1% in control group. When based on the number actually in the clusters this number is 21.4% in intervention group and 20.5% in the control group. |
| Selective reporting (reporting bias) | Low risk | Outcomes in paper match those prespecified in ISRCTN registry. |
| Other bias | Low risk | None identified |
Jones 1985.
| Methods | Quasi‐RCT, individual randomisation, single‐site study; recruitment period 18 months, n = 678 | |
| Participants | Maternity department of UK district general hospital Background rates of breastfeeding initiation: intermediate Inclusion criteria: all women who attempted at least 1 breastfeed Exclusion criteria: birth of child overlapped intervention and control periods 55% of the sample were primiparous. Ethnic composition not stated. Socioeconomic status defined by UK census categories (I and II 22%, III 46%, IV and V 13%) |
|
| Interventions | Intervention (n = 228): individual support and problem solving by lactation nurse in hospital and at home. Duration of the intervention not specified. Control (n = 355): not specified |
|
| Outcomes | Breastfeeding rates at 4 weeks, and 3, 6 and 12 months Satisfaction with care and intention to breastfeed after next pregnancy |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Alternating 2 week periods (i.e. 2 week intervention recruitment period, 2 week control recruitment period) |
| Allocation concealment (selection bias) | High risk | The randomisation method did not achieve balanced group size; 228 in the intervention group vs 355 controls. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of participants and personnel not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Stated that 12 month follow‐up was conducted by an independent interviewer. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 678 women randomized and 649 available to follow‐up (96%). Potential confounder: late exclusion of 66 women because of overlap of recruitment periods, and group sizes were uneven. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol, so could not assess this. |
| Other bias | Unclear risk | The method of recruiting intervention and control women appeared different; possibly face‐to‐face for intervention group but records not clear for the control group. |
Kaojuri 2009.
| Methods | Not clear; described as 'case control randomized trial', 2‐armed n=120 | |
| Participants | Setting: Tehran, Iran; mothers and babies recruited in a Baby Friendly accredited hospital. 120 women (baseline characteristics not described) Inclusion criteria: women giving birth to singletons by caesarean section only Exclusion criteria: infants with congenital abnormalities or serious illness necessitating intensive care, and mothers who had a serious illness or were planning to leave the area within 6 months, infants weighing < 2500 g at birth |
|
| Interventions | Intervention: 4 postnatal home visits (not clear) Control: standard care (not clear) |
|
| Outcomes | Follow‐up interviews by telephone on days 90, 120, 150 and 180 Results were not reported in a way in which we can include them in the review. Authors reported that "the patterns of exclusive breastfeeding in the 2 groups for days 3 to 180 differed significantly (P < 0.0001) with a mean aggregated of 67.72% among the group assigned home visits compared with 31.78% for the group assigned none". |
|
| Notes | We have not included data from this study in the review. Results were difficult to interpret and data were not reported in a way that allowed us to include them in meta‐analyses. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants/professionals reported. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of participants/professionals reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not clear, 120 women were recruited but it was not clear how many were followed up. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol, so could not assess this. |
| Other bias | Unclear risk | Did not state what was included in the telephone interview. Results reported on exclusive breastfeeding (not reported in a form we can use in the review). |
Khresheh 2011.
| Methods | 2‐arm RCT, recruited August 2008‐April 2009, n = 140 | |
| Participants | Recruitment from postnatal wards of 2 hospitals in South Jordan Prevalence of 'ever breastfed' in country: 93% (WHO Global Data Bank on Breastfeeding, accessed 12 Oct 2011). Paper stated that traditionally most women initiate breastfeeding and breastfeed for up to 2 years, with 32% fully breastfeeding for > 6 months. Inclusion criteria: primiparous women following vaginal delivery with term infants Exclusion criteria: women who lived outside the study area or who could not be contacted by phone |
|
| Interventions | Intervention: women received a 1‐h education session approximately 2 h after the birth. The session included demonstrations of breastfeeding. Mothers were encouraged to ask questions and were given a pamphlet on breastfeeding. At 2 and 4 months postpartum women were contacted by phone by the same researcher/nurse. The purpose of calls was to offer support, monitor breastfeeding practices and identify any problems. Control: usual care; women were given an appointment for 6 weeks after discharge to attend the maternal and child health services for support and follow‐up. Paper states most women did not return for these appointments and were not receiving any postnatal care. Control group women did not receive postnatal home visits from a midwife or a child health nurse. |
|
| Outcomes | Primary: exclusive breastfeeding at 6 months and breastfeeding knowledge Secondary: infant hospital admissions for diarrhoea and vomiting or respiratory tract infections |
|
| Notes | Due to high levels of attrition (36%) we have not included outcome data from this study in the review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | Paper stated randomisation occurred by women selecting 1 envelope from a group of sealed opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The researchers carried out the intervention and would have been aware of allocation. No details provided regarding whether mothers were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding described and it appears that the same researchers who collected data also carried out recruitment and delivered the intervention. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Serious loss to follow‐up. At 6 months, follow‐up was 62.5% in the intervention group and 66.2% in the control group. |
| Selective reporting (reporting bias) | Unclear risk | Not apparent |
| Other bias | Unclear risk | Data collection procedures varied at 6 months. Some women were visited at home while others were telephoned. It was not clear how many women in the control and intervention groups were telephoned vs visited. The different data collection procedures may have affected responses. |
Kools 2005.
| Methods | 2‐arm cluster‐randomised study with 10 sites, divided into 2 groups, which had similar numbers of births and breastfeeding rates. Recruitment December 2000‐December 2002, n = 781, 408 women in sites assigned to the intervention and 373 in sites assigned to the control | |
| Participants | Child healthcare centres in Limbourg province, the Netherlands Background rates of breastfeeding initiation: high Inclusion criteria: mothers applying for maternity care at any of the 10 centres Exclusion criteria: birthweight < 2000 g Ethnic composition not defined. Baseline prevalence of breastfeeding initiation was 80% in the Netherlands in 2002. |
|
| Interventions | Intervention: programme with 3 elements: structured health counselling by maternity and child healthcare nurses and physicians; booklet to transfer information between caregivers and between mother and caregivers and used at each consultation; lactation consultancy available via caregiver faxing consultant with details of problem (LC would then contact the caregiver or mother within 24 h of receiving the fax). Control: not specified |
|
| Outcomes | Exclusive and complementary breastfeeding rates at 3 months; determinants of breastfeeding at 3 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | By coin flip |
| Allocation concealment (selection bias) | Low risk | Clusters were randomized after sites were paired for similarity of breastfeeding rates and the number of births in each centre. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details provided about whether women and personnel were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details provided about whether the caregivers (who collected some of the data) or those responsible for conducting the questionnaires were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 20 centres and 781 women were randomized. Data available for 701 for the first follow‐up (90%) and 683 (87%) at 6 months postpartum. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol, so could not assess this. |
| Other bias | Low risk | Analyses adjusted for cluster effect by multi‐level analysis. No baseline imbalance apparent. |
Kramer 2001.
| Methods | Multi‐site cluster‐randomised study, recruitment period 19 months, n = 17,046 | |
| Participants | Urban and rural sites within Belarus Background rates of breastfeeding initiation: high Inclusion criteria: intention to breastfeed, healthy mother, child ≥ 2500 g at term, Apgar ≥ 5 at 5 min Baseline breastfeeding prevalence 50% at 3 months |
|
| Interventions | Intervention: WHO/UNICEF BFI training for all staff dealing with mothers and babies in hospitals and community polyclinics. Infants seen monthly for polyclinic well‐child visits and whenever ill. Control: staff did not receive the training |
|
| Outcomes | Any breastfeeding at 3, 6, 9 and 12 months Incidence of respiratory, gastrointestinal infection, and atopic eczema in first year |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Cluster‐randomised trial with double randomisation procedure. Random number tables were used to ascribe numbers to sites and higher/lower numbers were used to allocate sites to A or B interventions. Then later, in public, a coin flip was used to determine whether A or B would be intervention or control sites. |
| Allocation concealment (selection bias) | Low risk | 2‐stage randomisation procedure |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Personnel working in the hospital were not blinded. It was not stated whether the women were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The paediatricians carrying out the interventions were aware of the status of the study infants. An audit was carried to assess data validity, but it was not clear what this identified. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 34 sites randomized, 2 of which refused to carry out allocated intervention and 1 clinic falsified outcome data and was excluded. 31 sites contributed data. In addition, follow‐up data were missing for 3.3% of women. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol, so could not assess this |
| Other bias | Unclear risk | A steering group ensured that "control sites did not institute any changes that would render their maternity hospitals or polyclinics more baby friendly". Analysis took account of cluster design. Groups appeared similar at baseline. |
Kronborg 2007.
| Methods | Cluster‐randomised, 2‐community‐based trial; 22 municipalities randomized to intervention and control clusters n= 109 Health visitors and 1588 women | |
| Participants | Western Denmark, urban and agricultural areas Background rates of breastfeeding initiation: high. The 5 hospitals serving the area had adopted UNICEF/ WHO Baby Friendly Hospital Initiative standards, and 3 of the 5 were accredited at the time of the study. Inclusion criteria: Danish mothers who lived in the 22 municipalities and gave birth to a single child with gestational age of ≥ 37 completed weeks Participant characteristics: 36% primiparous, 7.5% multiparous with previous short breastfeeding experience |
|
| Interventions | Usual care included hospital care at hospitals working to Baby‐Friendly standards, and an existing Health Visitor service in all municipalities. Intervention cluster: 1‐3 structured home visits within the first 5 postnatal weeks from Health Visitors with additional training. Main topics for the first visit were effective breastfeeding technique and learning to know the baby; for the second visit, self‐regulated feeding and interpretation of the baby's cues; for the third visit, sufficient milk and interaction with the baby. Mothers were also given a booklet about how to breastfeed and how to read the baby's cues. Control cluster: Health Visitors' usual practice consisting of 1 or more non‐standardised visits |
|
| Outcomes | Duration of exclusive breastfeeding and mother’s satisfaction with breastfeeding | |
| Notes | The authors did not adjust for cluster design effect. In our data and analysis tables we have adjusted the sample size and event rates to take account of the design effect. We calculated an effective sample size by dividing figures by the design effect – calculated using the ICC for breastfeeding cessation given in the paper: ICC = 0.02. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The 22 clusters were stratified according to their number of births the year before the trial, and within 3 strata, 11 municipalities were randomized to the intervention group and 11 to the comparison group. The randomisation was computerised and done independently of the investigators. |
| Allocation concealment (selection bias) | Low risk | As for the sequence generation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and caregivers were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The health visitors provided the mothers with questionnaires which appear to have been self‐completed as the mothers were asked to return the questionnaire in a stamped addressed envelope. The identity of the health visitors was blinded to the investigators and it is not clear whether this partial blinding was effective. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 22 municipalities were randomized. 2186 women had babies during the study time period. 1760 women were followed up. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol, so could not assess this. |
| Other bias | Unclear risk | Reported that there were no significant differences between groups at baseline. This was a cluster‐randomised trial and authors stated they did not make allowance for clustering in the sample size calculation as the cluster effect was expected to be small. Elsewhere in the paper an ICC value was provided, which the authors said indicated that cluster effect was small. It was not clear that the cluster design effect was taken into account in any of the analyses. |
Labarere 2005.
| Methods | 2‐arm RCT, with individual randomisation, recruitment October 2001‐May 2002, n = 231 | |
| Participants | Setting: the maternity section at the Chambery Teaching hospital in Chambery, France Background rates of breastfeeding initiation: intermediate. Breastfeeding prevalence rates were 70.8% in hospital and 58.1% at 1 month of infant age. Inclusion criteria: mothers of healthy singleton infants (gestational age: > 37 completed weeks), breastfeeding on the day of discharge and consenting to participate in the study. Exclusion criteria: infants admitted to neonatal unit, mothers transferred to ICU, mothers < 18 years old, living outside the area, unable to speak French, or unable to complete follow‐up monitoring because of psychosocial problems such as homelessness Participant characteristics: Age: mean (SD): intervention: 29.3 years (4.1); control: 29.7 years (4.8) Education beyond high school graduate level: intervention: 87 (75.0); control 84 (73.0) White collar worker: intervention: 92 (79.3); control 87 (75.6) Primiparous: intervention: 58 (50.0); control: 63 (54.8) |
|
| Interventions | Intervention (n = 116): in addition to standard care, mothers were invited to an outpatient visit in a primary care physician’s office within 2 weeks of the birth to see a primary care doctor who had received special breastfeeding education. Topics covered included general health assessment, lactation physiology, feeding position and latch on assessment, management of common lactation problems (nipple pain, nipple cracks, sore nipples, mastitis, and maternal concern regarding low milk supply), management of infant problems (insufficient weight gain, breastfeeding jaundice, diarrhoea and dehydration), maternal medication use while breastfeeding and sources of support. The physicians' training programme was delivered through lectures, panel discussions, role playing exercises and printed educational materials. Control (n = 115): standard care; mothers received verbal encouragement from maternity ward staff to maintain breastfeeding. On discharge, the infant was examined by the paediatrician working in the department, for a general health assessment and an evaluation for evidence of successful breastfeeding behaviour. The mothers were also provided with the telephone number of a peer support group that they could call to ask questions and request help. The postdischarge follow‐up monitoring consisted of routine, preventive, outpatient visits in a primary care physician’s office at 1, 2, 3, 4, 5 and 6 months of infant age. |
|
| Outcomes | Primary: exclusive breastfeeding at 4 weeks (exclusive breastfeeding defined as giving maternal milk as the only food source, with no other liquids or foods) Secondary: any breastfeeding at 4 weeks, median duration of breastfeeding, breastfeeding difficulties and maternal satisfaction with the infant feeding experience |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The allocation sequence was generated by the statistical adviser of the study with random permuted blocks with a block size of 8. |
| Allocation concealment (selection bias) | Low risk | The randomisation assignments were unknown to any of the investigators and were concealed in consecutively numbered, sealed, opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants or personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcomes were assessed using self‐completed questionnaires, however, it was not stated whether the investigators analysing the data were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | (1080 women assessed for eligibility, 849 deemed not eligible) 231 women randomized, outcome data were available for all but 5 of the woman randomized, and a sensitivity analysis was carried out where the most conservative values were assumed for those women lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol, so could not judge this |
| Other bias | Unclear risk | The majority of women assessed were not eligible for inclusion in this trial and so the results may not be generalisable. |
Laliberte 2016.
| Methods | 2‐arm, RCT, single site, n = 472 | |
| Participants | Ottawa, Canada Background breastfeeding rates: not stated Inclusion criteria: women ≥ 18 years, with no diagnosed medical problems, with a healthy singleton infant at a gestational age of over 36 weeks and 6 days who were breastfeeding their baby and intended to continue upon discharge, and could be contacted by phone or email after hospital discharge Exclusion criteria: women who did not speak English or French, were unable to present to the clinic (transport not available), with multiple, preterm or adopted babies, with no plan or desire to breastfeed, women who had had breast surgery or a psychological risk that might impede their ability to attend the first appointment at the clinic. Out‐of‐province women were also excluded given the geographic distance and difference in social services. |
|
| Interventions | Intervention (n = 315): within 48 h of discharge, women attended the postpartum clinic. Clinic staff followed up with participants if they failed to keep the mandatory follow‐up appointment. The first appointment included maternal assessment and care (e.g. wound care, prescriptions), neonatal care (e.g. weight gain assessment, jaundice screening), blood work including total serum bilirubin (TSB), and breastfeeding assessment and support. Family physicians were available for on‐site consultations in the mornings, and LCs and registered nurses were at the clinic throughout the day. Additional follow‐up visits were offered to participants as clinically indicated and as many times as they desired up to a maximum of 6 weeks following the birth of their baby. Control (n= 157): after hospital discharge, participants were entitled to receive follow‐up care and seek breastfeeding support currently available in the community (e.g. through their family doctor, Public Health Unit or private services), but could not attend the postpartum clinic. |
|
| Outcomes | Primary: exclusive breastfeeding at 12 weeks postbirth (additional breastfeeding information regarding partial breastfeeding, expressed breast milk and formula feeding was also collected) Secondary: Mother Satisfaction Survey Breastfeeding self‐efficacy Postpartum depression Use of healthcare resources |
|
| Notes | All phases of this study were supported by the Ontario Ministry of Health and Long‐Term Care. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Group designation was given from a randomisation list, generated using a permuted randomized block design, with permutation block sizes of 3, 6, and 9 units, prior to study initiation by an external statistician. |
| Allocation concealment (selection bias) | Low risk | Study researchers, recruiters, and participants were blinded to the randomisation allocations prior to patient randomisation and enrolment into the trial. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Women were informed of their randomisation group. Clinicans were also aware of group allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Study staff were not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition to 6 months was 14% in the intervention group and 12% in control group. |
| Selective reporting (reporting bias) | High risk | Breastfeeding at 24 weeks is not identified as an outcome in the paper or the protocol, but was reported in table 4. |
| Other bias | Low risk | |
Leite 2005.
| Methods | 2‐arm RCT, with individual randomisation, n = 1003. Participants recruited from 8 public health maternity units, duration of recruitment 6 months | |
| Participants | Urban Brazil Background rates of breastfeeding initiation: high Inclusion criteria: healthy babies, weighing < 3000 g Exclusion criteria: twins, important health problems in mother or child |
|
| Interventions | Peer counsellor home visits lasting 30‐40 min at 5, 15, 30, 60, 90 and 120 days. Counsellors were from same social group as women they supported, had personal experience of breastfeeding and had been associated with maternity unit milk bank for a minimum of 5 years. Trained with adapted WHO breastfeeding counselling course (20 h). Paid BRL4 per visit. Each counsellor supported 25 mothers. | |
| Outcomes | Rates of exclusive, predominant, partial and artificial feeding at 4 months | |
| Notes | This is the only study in this review that targeted babies with birthweight below 3000 g. We considered excluding it from this review as the paper did not state the babies had to be born at term and did not specify a lower limit for birthweight. However as the babies had to be 'free of important health problems' we considered them to be healthy and therefore included this study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number table |
| Allocation concealment (selection bias) | Low risk | Study secretary opened a sealed envelope that contained the study code. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Did not describe whether mothers, lay workers or health professionals were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Authors state that the "interviewers had not had any prior contact with the mothers and were also unaware as to the objectives of the research (blinding)". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 1003 women randomized. 14% lost to follow‐up by the end of 4 months. Reasons for loss to follow‐up were not described but the loss appeared balanced across the 2 groups. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol, so could not assess this. |
| Other bias | Low risk | No baseline imbalance apparent. |
Lucchini 2013.
| Methods | Parallel 2‐arm RCT, single site, n = 770 | |
| Participants | Maternity ward at the Sotero del Rio Hospital, Santiago, Chile. Programme delivered by South East Metropolitan Health Service in conjunction with the Catholic University of Chile. Background breastfeeding initiation rates: on this ward 79.4% of the live births were fed with exclusive breastfeeding (EBF) up to 1 month of age and 67.3% were fed in this manner until 3 months of age. This was similar to national figures. Inclusion criteria: pregnancy without illness or risk factors which required more intensive maternal and/or perinatal monitoring during the process of labour and delivery, spontaneous initiation of labour , gestational age between 37 + 0 and 41 + 0 weeks, single pregnancy, live fetus and cephalic presentation Exclusion criteria: women with illnesses or risk factors that required more intensive maternal and/or perinatal monitoring |
|
| Interventions | Intervention (n = 384): 'comprehensive care' consisting of family member who accompanied the woman from admission to discharge, 24 h/day, and who participated actively throughout the period. Labour took place in a comprehensive room with constant care, with early skin‐to‐skin contact of at least 1 h and encouragement of early initiation of breastfeeding (positive covariates for EBF). During the immediate postpartum period personalised educational support was delivered by the healthcare team. Early discharge with comprehensive intervention (after 24 h) was complemented by a home visit (after 48 h) where the mother's and baby’s care was reinforced, as well as the breastfeeding technique in a family setting. Control (n = 386): 'traditional care', i.e. standard care from the public health system. This involved labour management interventions (negative covariates for EBF) with intermittent and passive family participation. Early skin‐to‐skin contact was performed without any standardised guidelines and mother and child room‐sharing began once the newborn had received immediate care. During the postpartum period, professional and technical support was given for the start of breastfeeding in the postnatal unit. |
|
| Outcomes | Prevalence of exclusive breastfeeding at 8, 16 and 24 weeks | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The pregnant women were assigned randomly to both forms of intervention, using a randomized block design of 6‐8 women, so that in each block an equal number of women were assigned to each group but not clear how sequence was generated. |
| Allocation concealment (selection bias) | Unclear risk | No details provided to enable us to judge this. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Did not state whether women or staff providing intervention were blinded, but unlikely. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It was not stated whether the data collectors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Follow‐up was 85.9% in the intervention group and 82.6% in the control group. There were no significant differences between the women lost to follow‐up and those who remained in the study. The number of cases lost to follow‐up (15.7%) was mainly due to a change of telephone number and address (Figure 1) For this reason it was expected that those lost to follow‐up were 'missing at random'. |
| Selective reporting (reporting bias) | Unclear risk | No protocol or document with predefined outcomes available. This paper focused on data collected at 8 weeks. Data collected at 16 and 24 weeks were not reported and no explanation was given. |
| Other bias | High risk | Intervention contained other components which may influence breastfeeding, including 24‐h family participation during hospital stay and these different birth experiences are also important. |
Lynch 1986.
| Methods | 2‐arm RCT, with individual randomisation, single‐site study, duration of recruitment not stated, n = 270 | |
| Participants | Urban Canada ‐ maternity unit of regional general hospital Background rates of breastfeeding initiation: intermediate. Baseline prevalence (1984) = 69% breastfeeding initiation (75% stopping by 6 months) Inclusion criteria: intending to breastfeed; English‐speaking Exclusion criteria: multiple births; birthweight < 2500 g; birth before 37 weeks Participant characteristics: 41% were primiparous; ethnic composition not described; socioeconomic status defined by Blishen scale for husband's occupation (62% groups 2‐3) |
|
| Interventions | Intervention: combination of home visit by breastfeeding consultant within 5 days of hospital discharge (duration 2 h) and weekly telephone calls by the consultant for 1 month, then monthly from 2‐6 months Control: postpartum home visit by public health nurse who gave breastfeeding advice determined largely by the questions and concerns of the mother |
|
| Outcomes | Duration of breastfeeding | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described "we randomly allocated 270 new mothers". |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | It was not stated whether the women, public health nurses or LCs were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The interviewer conducting the questionnaire was not aware of the study group status. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There appeared to be little loss to follow‐up; 270 women were randomized and questionnaire data were acquired from 256 (5% attrition); 3 women were lost from the intervention group vs 11 from the control group. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol, so could not assess this. |
| Other bias | Unclear risk | Little information about the methods. Possible confounders included: significant differences in baseline characteristics for parity (P = 0.02) and intention to return to work (P = 0.05). |
McDonald 2010.
| Methods | 2‐arm RCT, recruitment March 2000‐October 2001 n=849 | |
| Participants | Large university teaching hospital in Victoria, Australia Background rates of breastfeeding initiation: high. Baseline prevalence of breastfeeding in Australia = 83% at hospital discharge Participants were women intending to breastfeed their term infants, and were stratified by tertiary education and parity |
|
| Interventions | Intervention (n = 425): Extended Midwifery Support (EMS); women received an in‐hospital postnatal education session. Postdischarge, they were offered home support visits with a research midwife once a week and telephone contact at least twice a week for 6 weeks Control (n = 424): Standard Midwifery Support (SMS); women received routine midwifery support and information according to the hospital protocol. The study hospital was working towards Baby‐Friendly accreditation during data collection (achieved in 2004) |
|
| Outcomes | Any breastfeeding and exclusive breastfeeding at 6 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sample stratified by educational level and parity. Methods not clear. |
| Allocation concealment (selection bias) | Unclear risk | Paper stated "Women were asked to select an envelope from a group of at least 6 sealed, opaque envelopes, replenished in blocks of 12. The envelopes contained the allocation to either the intervention or control group". |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No detail provided on blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No detail provided on data collection so judgement not possible. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 849 women randomized. Loss to follow‐up was reported by group at 2 months (intervention 83/425, 19.5% vs control 124/424, 29.2%) and at 6 months (intervention 8/425, 1.9% vs. control 4/424, 0.9%). |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol, so could not assess this. |
| Other bias | Unclear risk | Abstract did not include details of allocation concealment, outcome assessment or loss to follow‐up. Outcomes included in the abstract were reported by ITT. |
McKeever 2002.
| Methods | 2‐arm RCT, individual randomisation, n = 101 | |
| Participants | Setting: study carried out in Canada Inclusion criteria: live, singleton, term or near term infant delivered in 12 h before recruitment; women ≥ 21 years residing in defined study area, intending to breastfeed and with satisfactory home circumstances (assessed by postpartum nurses) Exclusions: non‐English‐speaking women, caesarean delivery, postpartum complications, infants with congenital abnormalities or morbidity |
|
| Interventions | Intervention: planned early discharge from hospital (24 h‐36 h postpartum) and up to 3 home visits by community nurse LCs. Content of support unclear. The study aimed to compare of breastfeeding support in home and hospital settings. Control: planned hospital discharge 48 h‐60 h postpartum (usual care) with hospital based support for breastfeeding |
|
| Outcomes | Exclusive breastfeeding at 5‐10 days postpartum and satisfaction with care | |
| Notes | We have not included data from this study in the review. Outcomes were not assessed at the same time in the intervention and control groups (mean day of follow‐up was 8.4 days in the intervention group vs 7.8 days for controls) and there was high attrition (26% overall, with 33% loss to follow‐up in the control group). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central randomisation |
| Allocation concealment (selection bias) | Low risk | Central randomisation by staff not concerned with the study. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Blinding was not possible for the mothers or nurses as the experimental treatment (i.e. discharge to the home‐based lactation support) was known." |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Interviewers were originally blinded to group status. However, in the course of answering questions about postpartum care and satisfaction, mothers inadvertently revealed their group status." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Outcomes were not assessed at the same time in both groups and there was high attrition (26% overall, with 33% loss to follow‐up in the control group). |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol, so could not assess this. |
| Other bias | Unclear risk | Quote: "At baseline, no differences in maternal age, parity or gestational age were found in the two groups." |
McLachlan 2016.
| Methods | 3‐arm cluster‐controlled trial, single site, n = 9675 | |
| Participants | Local government authorities (LGA) in Victoria, Australia ‐ community‐based maternal and child health centres Background rates of breastfeeding initiation: only rates at 6 months detailed. Ranged from 32% to 68% in different LGAs in Victoria. Inclusion criteria: LGAs in Victoria with a lower rate of any breastfeeding at discharge from hospital than the Victorian state average; and > 450 births per year. For the postal survey women were recruited on the basis of giving birth during the intervention time‐frame in all participating LGAs. Exclusion criteria: LGAs with breastfeeding initiatives in place similar to the proposed interventions. Women living in participating LGAs were not sent an invitation to take part in th postal survey if it was known that either they or the infant died, they had moved to another LGA since the birth or they were not enrolled in the Maternal and Child Health Service. |
|
| Interventions | Intervention 1 (n = 3335): home visiting only (HV) ‐ early home‐based visiting by a maternal and child health nurse (MCHN) to women identified at risk of breastfeeding cessation. Aimed to provide proactive breastfeeding assistance as early as possible after birth. The focus of the visits were was the normalisation of breastfeeding, building women’s confidence to breastfeed, reassurance, development of an infant feeding plan (where needed), and provision of a list of useful web sites and telephone numbers. The topics covered at individual visits were driven by the specific needs of the woman. Intervention 2 (n = 2891): HV + access to a drop‐in centre ‐ women received home visit service as above and could attend local community breastfeeding drop‐in centre staffed by a MCHN, and where possible with a trained peer supporter or community educator or counsellor. Also provided mothers with the opportunity to meet and learn from other mothers. The centre was widely advertised. Control (n=3449): Usual care. Midwife visit 1‐2 days after discharge and then MCHN visit 10‐12 days after discharge (breastfeeding assessment, support and advice a core component of care). Then MCH centre based care thereafter. |
|
| Outcomes | Primary: any breastfeeding at 4 months Secondary: any breastfeeding at 3 and 6 months |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Envelopes shuffled |
| Allocation concealment (selection bias) | Low risk | Allocation to trial arms took place using opaque envelopes at a state‐wide MCH forum. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding "was not possible at the LGA (randomisation) or cluster levels; however, individual women in the LGAs were not aware of the intervention allocation—the intention was that any trial arm allocation was ‘standard’ care within the LGA during the intervention period". However it is not clear if this was successful and whether staff were blinded or not. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessment by participant‐completed questionnaire sent by mail, but not stated if those analysing the data were blinded to allocation. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High loss to follow‐up at 4 months in control group (69% of women followed‐up) and home visiting group (68% of women followed up), Follow‐up was higher in home visiting plus group, with 81% of women followed up. |
| Selective reporting (reporting bias) | Low risk | Not all secondary outcomes listed in protocol were reported, but these were not outcomes of interest in this review so we marked this trial as being at low risk of bias. |
| Other bias | Unclear risk | Significant differences in proportion of Australian‐born women in across the groups (69% in comparison LGAs; 58% in home‐visiting LGAs; 73% in home‐visiting plus drop‐in centre LGAs). Unclear whether this could have an impact. |
McQueen 2009.
| Methods | 2‐arm RCT, single site, n = 150 | |
| Participants | Conducted at a tertiary care centre located in Northwestern Ontario, Canada. Background breastfeeding initiation rates: 87.3% Inclusion criteria: English‐speaking, primiparous mothers who gave birth to a single, healthy, term infant whom they were planning on breastfeeding Exclusion criteria: any condition that could significantly interfere with breastfeeding, such as a serious illness, an infant with a congenital anomaly, or requiring special care that would not be discharged home with the mother |
|
| Interventions | Intervention (n = 69): participants received 3 individualized, self‐efficacy enhancing sessions with the researcher: 2 in‐hospital and 1 by telephone in the early postpartum period. The first session occurred after randomisation and within 24 h of delivery. The second session also took place in‐hospital, ideally within 24 h of the first session. In addition, observation of breastfeeding at 1 of the 2 in‐hospital sessions was planned to try to maximize performance accomplishment (successful breastfeeding). The third session occurred via telephone within 1 week of hospital discharge. Control (n = 81): standard in‐hospital and community care |
|
| Outcomes | Primary: feasibility, compliance, and the acceptability of the breastfeeding self‐efficacy intervention Secondary: breastfeeding self‐efficacy, duration, and exclusivity |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was achieved using consecutively numbered, sealed opaque envelopes containing group allocations generated by an experienced researcher not involved in the trial. |
| Allocation concealment (selection bias) | Low risk | Randomisation was achieved using consecutively numbered, sealed opaque envelopes containing group allocations generated by an experienced researcher not involved in the trial. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was not possible. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Mothers were telephoned by a research assistant who was blinded to group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition in intervention group was 11.5% and 13.6% in the control group. |
| Selective reporting (reporting bias) | Unclear risk | No trial registration document with prespecified outcomes. |
| Other bias | Low risk | None identified |
McQueen 2011.
| Methods | Pilot RCT (n = 150), March‐July 2008 | |
| Participants | Recruitment from 1 hospital in Northwestern Ontario, Canada, the sole provider of maternity care for the city and regional referral centre Background rates of breastfeeding initiation for Canada: intermediate, however, baseline prevalence of 'ever breastfed' in Ontario 90.6% (WHO Global Data Bank on Infant and Young Child Feeding accessed 12 Oct 2011) Inclusion criteria: English‐speaking, primiparous, planning on breastfeeding, with single, healthy, term infants Exclusion criteria: conditions that could significantly interfere with breastfeeding such as serious illness, infant with congenital anomaly or admitted to special care |
|
| Interventions | Intervention: standard in‐hospital and community postpartum care plus a 1‐to‐1 self‐efficacy intervention from the researcher (a Registered Nurse with practice, education, and research experience working with breastfeeding mothers). The intervention included assessment of the mother’s breastfeeding goals and breastfeeding self‐efficacy and her general physiologic and affective state; strategies to increase breastfeeding self‐efficacy; evaluation, and planning the next session. There were 3 contacts, 2 face‐to‐face in hospital on days 1 and 2 after the birth, and 1 phone call up to 7 days after hospital discharge. Control: standard in‐hospital and community postpartum care, which included a visit by a public health nurse after hospital discharge |
|
| Outcomes | Feasibility, compliance, and acceptability of the intervention, breastfeeding confidence (self‐efficacy scores), any and exclusive breastfeeding at 4 and 8 weeks | |
| Notes | Paper stated "Observation of breastfeeding at 1 of the 2 in‐hospital sessions was planned, to try to maximise performance accomplishment (successful breastfeeding)”. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Group allocations generated by an experienced researcher not involved in the trial. |
| Allocation concealment (selection bias) | Low risk | Consecutively numbered, sealed opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and the caregivers were not blinded due to the nature of the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes at 4 and 8 weeks postpartum were collected during phone interview by research assistant reported to be blind to group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes measured at 8 weeks in 134/150 (89%). Loss to follow‐up was balanced across groups. For breastfeeding outcomes we have carried out an ITT analysis. |
| Selective reporting (reporting bias) | Unclear risk | No baseline differences between groups apparent. |
| Other bias | Unclear risk | Baseline characteristics were similar. |
Mejdoubi 2014.
| Methods | 2‐arm parallel RCT, single site study, n = 460 | |
| Participants | 20 municipalities in the Netherlands, demographics not described Background rates of breastfeeding initiation: not described Inclusion criteria: ≤ 25 years, low educational level (primary school or prevocational secondary school), maximum 28 weeks of gestation, no previous live birth, understood Dutch, and at least 1 of the following additional risk factors: no social support, previously or currently experiencing domestic violence, psychosocial symptoms, unwanted and/or unplanned pregnancy, financial problems, housing difficulties, no education and/or employment and alcohol and/or drug use Exclusion criteria: previous live births, no additional risk factor as detailed above |
|
| Interventions | Intervention (n = 237): the VoorZorg programme ‐ a home visitation programme translated and culturally adapted from the Nurse Family Partnership (NFP) programme. In addition to usual care, women received approximately 10 home visits during pregnancy, 20 during the first life year of the child and 20 during the second by trained, specialised VoorZorg nurses. 6 domains were discussed during the home visits: 1) the health status of the mother, 2) the child's health and safety, 3) the personal development of the mother, 4) the role of the mother, 5) the mother's relation with her partner, family and friends, and 6) the use of (health) care organizations. During pregnancy, women receiving the VoorZorg intervention were encouraged to initiate and continue breastfeeding after childbirth. The VoorZorg nurse also discussed the problems women encountered when breastfeeding their child and worked together with the mother to seek solutions to continue breastfeeding. The VoorZorg nurses also aimed to reduce smoking with the V‐MIS smoking cessation programme. Control (n = 223): usual care provided by the Dutch Youth Health Care Organizations. Every pregnant women received care by a midwife including health education, physical examination and monitoring fetal development. The baby was automatically registered at an ambulatory well baby clinic for monitoring after birth and the parents were supported in parenthood. |
|
| Outcomes | Primary: Prevalence of cigarette smoking (percentage of smokers and average number of cigarettes smoked a day) Average number of cigarettes smoked a day near the baby Birthweight Weeks of gestation Adverse pregnancy outcome (LBW, prematurity) Breastfeeding initiation Breastfeeding at 6 months Secondary: Any breastfeeding at 3 and 6 months |
|
| Notes | The Netherlands Organisation for Health Research and Development (ZonMw), Academic Collaborative Centre, Child Health Care‐North HollandVU University Medical Center, participating Youth Health Care organizations and ZonMw Geestkracht, and participating city councils provided funding for the implementation of this study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated list of random numbers |
| Allocation concealment (selection bias) | Unclear risk | Independent randomisation procedure performed by a researcher at VU university |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Women and staff were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The interviewers were blinded regarding allocation but this may have been disclosed during interview |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up in control group was 18% and 8.4% in intervention group; authors stated baseline characteristics of women who were lost to follow‐up in each measurement were similar to women who remained in the study. |
| Selective reporting (reporting bias) | Low risk | Protocol included domestic violence, child development and child abuse as primary outcomes, but these were not reported in the results section. Breastfeeding was not reported as an outcome in the protocol, but was included as a primary outcome in the results paper. Authors stated that prevalence of babies with low birthweight, being premature or being small for gestational age, was similar in both groups. |
| Other bias | Low risk | None identified |
Mongeon 1995.
| Methods | Quasi‐RCT (drawing numbered tickets), single site, duration of recruitment not stated, n = 200, follow‐up 97% | |
| Participants | Urban Canada Background rates of breastfeeding initiation: intermediate Inclusion criteria: women who wished to breastfeed and who had not previously breastfed Participant characteristics: 97% primiparous, ethnic composition not stated, 57% had received education to college or university level, no specific socioeconomic classification used |
|
| Interventions | Intervention: home visit by volunteer during last month of pregnancy followed by telephone contacts weekly for 6 weeks and then 2 weekly to 5 months or until weaning. Volunteers were women who had breastfed themselves and had received 3 training sessions of 3 h duration followed by on‐going monthly supervision sessions. Average caseload was 1‐3 cases at any 1 time. Control: received home visit from public health nurse during the first month after birth followed by other contacts (face‐to‐face or by telephone) as determined by the mother |
|
| Outcomes | Breastfeeding rates at 1, 2, 3, 4 and 6 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation by "drawing numbered papers" |
| Allocation concealment (selection bias) | Unclear risk | Not clear |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Article in French, unable to judge blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | There was an attempt to blind outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Reasons for drop‐out recorded; 200 randomized, 3 babies died and 3 other women lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol, so could not assess this |
| Other bias | Unclear risk | Not clear over what time period women were recruited or whether controls and intervention women were recruited at the same time. Quote: "Subjects were recruited during various periods of time, depending on the availability of volunteers" |
Morrell 2000.
| Methods | 2‐arm RCT, individual randomisation, single‐site study recruiting over 14 months, n = 632 | |
| Participants | Urban UK Background rates of breastfeeding initiation: intermediate. National baseline prevalence 66% breastfeeding at birth and 42% at 4 months. Exclusive breastfeeding 21% at 4 months. Inclusion criteria: English‐speaking women, ≥ 17 years, who gave birth at the study hospital Exclusion criteria: baby spent > 48 h on the SCBU |
|
| Interventions | Intervention: community postnatal support worker with 8 weeks' training provided home‐based support of up to 10 visits in the first 28 days (maximum of 3 h/visit) Control: standard UK care (includes postnatal home visits from midwives and health visitors) |
|
| Outcomes | Exclusive or any breastfeeding at 6 weeks and 6 months | |
| Notes | Study population not limited to those intending to breastfeed Women consenting to participation more likely to be white and have had a CS |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number tables |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered sealed opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details provided about blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details provided about blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 623 women randomized; stated that analysis was by ITT; 30 women who declined visits were included in the analysis; 78% follow‐up |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol, so could not assess this. |
| Other bias | Unclear risk | There was some baseline imbalance between groups. Women in the intervention group were more likely to have twins (9 vs 1), to have another adult resident in their household and to have used TENS in labour. |
Morrow 1999.
| Methods | Community‐based cluster‐randomised study; recruitment over 18 months, n = 130 | |
| Participants | Peri‐urban Mexican community Background rates of breastfeeding initiation: high All pregnant or postnatal women were in 39 geographical clusters. Perinatal death only clinical exclusion criterion. Baseline breastfeeding prevalence: 92% initiation; 4% exclusivity at 2 weeks and 3 months; 50% cessation by 6 months |
|
| Interventions | Home visits were conducted by peer‐counsellors trained by La Leche League (7 days theoretical teaching/2 months in lactation clinics and with mother‐to‐mother support groups), personal breastfeeding experience was not essential. Intervention 1: 6 visits (mid and late pregnancy and at 1, 2, 4 and 8 weeks) Intervention 2: 3 visits (late pregnancy and 1 and 2 weeks) Control: not specified |
|
| Outcomes | Breastfeeding at 3 and 6 months Incidence of diarrhoea in infants 0‐3 months |
|
| Notes | Subgroup analysis: antenatal and postpartum support; proactive intervention with scheduled contacts at home; initial face‐to‐face contact; intervention delivered by trained counsellors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Cluster‐randomisation, clusters stratified by area, randomisation schedule generated by computer. |
| Allocation concealment (selection bias) | Low risk | Clusters randomized by computer. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Clusters randomized to avoid contamination, but women and counsellors would have been aware of intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome measurement was by staff who were aware of group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 130 women from 31 cluster areas randomized; 125 followed up at 3 months and 104 at 6 months (20% attrition at 6 months). |
| Selective reporting (reporting bias) | Unclear risk | How cluster design was taken into account was not clear. It was stated that ICC values were 'negligible' and the authors stated "these results show that the cluster‐randomisation design achieved the equivalent of individual randomisation". |
| Other bias | Unclear risk | No baseline imbalance apparent, although group size was uneven (this may have been due to chance). |
Muirhead 2006.
| Methods | 2‐arm RCT, with individual randomisation, n = 225 | |
| Participants | Setting: general practice in Ayrshire, Scotland Background rates of breastfeeding initiation: low Inclusion criteria: women at 28 weeks' gestation attending for antenatal care at a GP practice Exclusion criteria: not described Participant characteristics: Mean age of intervention group: 28.5 years; SD 5.2; range 17‐43. Mean age of control group: 27.8 years; SD 5.5; range 16‐40 Parity: 53% primiparous |
|
| Interventions | Intervention (n = 112): women were assigned 2 peer supporters (women with previous breastfeeding experience) who contacted them at least once in the antenatal period and provided further antenatal support on request. In the postnatal period after hospital discharge peer supporters contacted women who were still breastfeeding at least every 2 days by phone or by home visit up until 28 days, and further support was available up to 16 weeks postpartum. Control (n = 113): standard care that included visits from community midwife for the first 10 days, health visitor after 10 days; breastfeeding support groups and breastfeeding workshops were available. |
|
| Outcomes | Initiation of breastfeeding, any and exclusive breastfeeding at 6 weeks and 6 months, median breastfeeding duration and reasons for giving up breastfeeding | |
| Notes | The researchers noted that "health professionals varied in their commitment to breastfeeding and also in their acceptance of lay assistance, such as peer support". | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocation sequences for each stratum (primagravidae, previous formula feeding, previously breastfed < 6 weeks, previously breastfed > 6 weeks) were generated at the start of the trial by computer in blocks of 10 (that is, 5 random allocations to each of the peer support and control groups in each different block of 10) to give approximate numerical balance between groups. |
| Allocation concealment (selection bias) | Low risk | Allocation to control or peer support group was by post‐recruitment concealed allocation, with a telephone call for the next allocation on the list. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "There was no post‐allocation concealment as once a woman was allocated to the peer support or control group this was known to the peer supporters and others associated with the trial.” |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The questionnaire were completed in the presence of a GP or practice nurse. It is also stated that trial team were not involved in the questionnaire completion. Unclear if the GP or practice nurse would have been aware of group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low loss to follow‐up. Peer support group (intervention group) (n = 112): at 16 week follow‐up, n = 110; control group (n = 113): at 16 week follow‐up, n = 110 |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol, so could not assess this. |
| Other bias | Unclear risk | Planned recruitment was for 320 women but ended after 225 women recruited, therefore the study had reduced power to detect differences between groups. Few demographic data were reported so it was not clear whether or not there was baseline imbalance, although recruitment was balanced for parity by stratification. |
Ochola 2013.
| Methods | 3‐arm cluster‐controlled trial, single‐site study, n = 360 (note only 2 arms included in analysis) | |
| Participants | Kiberia slum, Nairobi, Kenya ‐ a densely populated area that was not well served with basic services such as health facilities, adequate safe water and sanitation services. Background rates of breastfeeding initiation: no data on initiation, but for Kenya the exclusive breastfeeding rate for infants under 6 months was 32.0%. Inclusion criteria: in the third trimester of pregnancy (34–36 weeks’ gestation), HIV‐negative, intention to stay in Kibera for at least 6 months after delivery, willing to be visited at home, willing to be included in the study Exclusion criteria: documented chronic diseases such as diabetes mellitus, renal disease, heart disease or any other chronic disease, and eclampsia in a previous pregnancy |
|
| Interventions | Intervention 1 (n = 120): home‐based intensive counselling group (HBICG); mothers received 7 counselling sessions: prenatally, the first week after delivery and then monthly up to 5 months postpartum. The content was similar to the facility‐based semi‐intensive counselling group (FBSICG; see Intervention 2) but was more tailored to the mother’s needs and more detailed. Women also had more practical exposure with regard to supporting breastfeeding (e.g. positioning, attachment, expression of milk). Counsellor training was the same as for FBSICG. Intervention 2 (n = 120): FBSICG; note this intervention was an antenatal one only, so not included in this review). Consisted of 1 session of 1‐to‐1 counselling at the health centre conducted by the investigator and breastfeeding counsellors. The breastfeeding counsellors were 3 local women trained in accordance with the WHO/UNICEF counselling course (40 h). The counselling content was structured around the benefits of exclusive breastfeeding; preparation for breastfeeding initiation and sustainability of breastfeeding; positioning and attachment of baby to the breast during feeding; and prevention and management of breastfeeding challenges. The single session took place after enrolment into the study.. Control (n = 120): usual standard health and nutrition education offered at the health centre. This was a group‐based education programme which covered breastfeeding and a range of other topics. |
|
| Outcomes | Primary: exclusive breastfeeding at 1, 3 and 6 months Secondary: cumulative (since birth exclusive breastfeeding at 6 months). |
|
| Notes | Funded by Nestle. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated using Excel for randomisation of the clusters. |
| Allocation concealment (selection bias) | Low risk | Method of concealment not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Paper stated that only the investigator and peer counsellors were aware of the treatment given and knew the hypothesis. The nurse in charge was blinded to the intervention allocation. It was not clear if the women were aware of the allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The enumerators conducting the interviews to determine breastfeeding practices were blinded to the study hypotheses to avoid any likelihood of bias in the way they asked questions, even though they were trained to ask questions in a standard way. There was no contact between the enumerators and the breastfeeding counsellors. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Follow‐up in both intervention and control groups was 74.2%. The analysis was as‐treated and not ITT. Younger women were significantly more likely to be lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | No evidence of a study protocol to judge whether all predefined outcomes were assessed. |
| Other bias | Unclear risk | Funded by Nestle. |
Paul 2012.
| Methods | 2‐arm RCT, n = 1154 | |
| Participants | Pennsylvania, USA Background rates of breastfeeding initiation: no details provided Inclusion criteria: singletons and twins born after at least 34 weeks’ gestation to English‐speaking mothers attempting to breastfeed during the maternity stay and with intent to continue breastfeeding after discharge Exclusion criteria: atypical stays characterised by: 1) a 2‐night or longer stay after a vaginal delivery; 2) a 4‐night stay or longer after a cesarean section; 3) a hospital course with atypical complications (e.g. ambiguous genitalia, endometritis); or 4) newborn hyperbilirubinemia requiring phototherapy during the nursery stay. Mothers were also excluded for major morbidities and/or pre‐existing conditions that would affect postpartum care, lack of a telephone number, previous study participation, residence outside the coverage region of the Visiting Nurse Association of Central Pennsylvania (VNA), or if a home nursing visit was specifically requested by a hospital social worker or child protective services owing to social concerns. |
|
| Interventions | Intervention (n = 576): 1 home nursing visit within 48 h of hospital discharge (typically 3‐5 days post birth). All nurses received continuing education related to breastfeeding support and cultural competency prior to study initiation. All newborns in intervention group were scheduled for an office‐based visit 1 week after the visit to assess weight and recovery. Control (n = 578): office‐base care; postdischarge visit timing for newborns was determined by the newborn nursery physician |
|
| Outcomes | Primary: maternal and infant use of unplanned health care services in the 14 days after delivery Secondary: Breastfeeding duration and exclusivity Maternal postpartum depression State of anxiety Percieved social support Parenting self‐efficacy |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation sequence |
| Allocation concealment (selection bias) | Unclear risk | No information provided about allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not detailed whether mothers and/or home visiting nurses were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Telephone interviews with mothers conducted by study co‐ordinators blinded to study group. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 8% attrition by 2‐week telephone interview; 13% attrition at 2‐month telephone interview. However, at 6 months attrition was 31% in the home nursing visit group and 38% in the office‐based care group. |
| Selective reporting (reporting bias) | Unclear risk | Outcomes not stated in Clinicaltrials.gov record. Unclear whether ‘any breastfeeding’ or ‘exclusive breastfeeding’ were reported, but both should have been, however, additional information received from author included both. |
| Other bias | Low risk | |
Petrova 2009.
| Methods | 2‐arm RCT, with individual randomisation, n = 104 | |
| Participants | Setting: maternal and paediatric clinic for low‐income inner‐city population (New Jersey, USA) Background rates of breastfeeding initiation in this population: high Inclusion criteria: WIC program‐qualified pregnant women in the third trimester of a singleton pregnancy without HIV, cancer, or illegal drug use Participant characteristics: 87.5% of the women were of Hispanic origin, 89% spoke Spanish at home, 30% were single, approximately 70% were educated to less than high school level. 37% of the intervention group, compared with 42% of controls, were expecting their first child. |
|
| Interventions | Intervention (n = 52): in addition to routine care, allocated to 2 individual educational/support sessions with a LC in the third trimester of pregnancy lasting 15‐20 min. After birth the LC provided support at the hospital or by phone soon after discharge, with further phone support after the first or second week then after 1 and 2 months. The participants were asked to contact the LC if they experienced any breastfeeding problems. Control (n = 52): routine breastfeeding education and support during the pregnancy and postpartum. LC services were available for all postpartum women if any breastfeeding problems arose during the hospital stay. |
|
| Outcomes | Exclusive and any breastfeeding at 7 days and 1, 2 and 3 months postpartum | |
| Notes | Among multiparous participants, 27/29 (93%) in the intervention group had previously breastfed, compared with 17/25 (68%) in the control group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “We used computer generated random numbers to assign women to the control and intervention groups. Each random number was related to an ordinal number that was assigned to the woman once she assigned the informed consent." |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details provided to enable a judgement. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details provided to enable a judgement. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 104 women randomized. 82% available to follow‐up at 1 month (data included in the review) 70% of women followed up for 3 months (35/52 in intervention group completed the 3‐month follow‐up (loss of 17); 38/52 in the control group completed the 3‐month follow‐up (loss of 14)). High attrition, but reasons for loss were given and balanced across groups (e.g. phone disconnected; women did not answer phone; some women did not notify the research team about their delivery). |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol, so could not assess this. |
| Other bias | Unclear risk | There was some baseline imbalance between groups that meant that differences between groups were difficult to interpret. Of the multiparous women 93% in the intervention group had previous breastfeeding experience compared with 68% in the control group. More women in the control group had a CS (40% vs 14%). Both of these differences possibly relate to breastfeeding outcomes. |
Porteous 2000.
| Methods | 2‐arm RCT with individual randomisation, single‐site study, recruiting over 3 months, n = 52 | |
| Participants | Urban Canada Background rates of breastfeeding initiation: intermediate Baseline breastfeeding prevalence at 4 months: approximately 33% Inclusion criteria: singleton pregnancy, healthy mother and child, vaginal delivery, self‐identified on breastfeeding questionnaire as unsupported |
|
| Interventions | Intervention: breastfeeding support from the researcher, a community midwife, consisting of daily visits in hospital, telephone call within 72 h of discharge and weekly through the fourth week postpartum, and at least 1 home visit (in the first week), with further home visits as required. Home visits lasted 60‐90 min. Control: hospital care from any member of the mother‐child nursing team |
|
| Outcomes | Exclusive and partial breastfeeding at 4 weeks | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised block randomisation procedure (stratified by planned length of breastfeeding, parity and education). |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 52 randomized, 51 appeared to complete the study, follow‐up was 98%. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol, so could not assess this. |
| Other bias | Unclear risk | Recruitment limited by availability of investigator. No baseline imbalance apparent. |
Pugh 1998.
| Methods | 2‐arm RCT, with individual randomisation n=60 | |
| Participants | Women were recruited at a community hospital in the USA and had diverse socioeconomic status. Background rates of breastfeeding initiation: intermediate Inclusion criteria: women who experienced vaginal deliveries after full‐term pregnancies Exclusion criteria: not stated Participant characteristics: mean age: 24.4 years; married n = 47 (78%); white n = 55 (93%); completed high school n = 58 (97%); income of USD ≤ 20,000 n = 13 (22%) |
|
| Interventions | Standard care included routine breastfeeding support in hospital following delivery. Intervention: 2 home visits by a professional community health nurse and phone call from a qualified LC. The nurse provided a structured teaching and support protocol. The focus of the first visit was to enhance breastfeeding. For the second visit, of up to 2 h duration, mothers could choose the content from options including help with dishes or laundry. Most chose education or infant assessment; 2 asked for child care help so they could rest and/or spend time with a partner. Control: home visit on day 3 or 4 by a hospital nurse (not specifically about breastfeeding). |
|
| Outcomes | Primary: duration of breastfeeding Secondary: fatigue, symptoms of anxiety and depression |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly assigned to the treatment (n = 30) or control group (n = 30). |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and caregivers were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome data were collected by a research assistant (by telephone). It was not clear whether blinding was achieved. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not clear. Loss to follow‐up was not mentioned and denominators were not provided for the results. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol, so could not assess this |
| Other bias | Unclear risk | Little information was provided on study methods. No information provided about how many women were followed up, blinding, or how randomisation occurred. |
Pugh 2002.
| Methods | 2‐arm RCT, single‐site study, recruitment April 1999‐February 2000, n = 41; 21 assigned to intervention and 20 to control group | |
| Participants | Community intervention in urban USA Background rates of breastfeeding initiation: low Inclusion criteria: low‐income women receiving financial medical assistance Exclusion criteria: not stated Ethnic composition: 95.2% African American |
|
| Interventions | Intervention: breastfeeding support visits by community health nurse/peer counsellor team. Support offered daily when in hospital, and at home during weeks 1, 2 and 4 and at the team's discretion. Telephone support from peer counsellor twice weekly through to week 8 and monthly through to month 6. Control: usual breastfeeding support consisted of support from hospital nurses, assistance by means of a telephone 'warm line' and if mothers gave birth on a weekday, 1 hospital visit from a LC. |
|
| Outcomes | Duration of breastfeeding to 6 months; healthcare services use by infants; costs | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as "assigned randomly". |
| Allocation concealment (selection bias) | Low risk | Described as "a sealed envelope technique". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Peer supporters would have been aware of intervention. Not clear if women were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome assessment by person or by phone; not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 41 women randomized, all appeared to have been followed up. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol. |
| Other bias | Low risk | Groups similar at baseline. |
Pugh 2010.
| Methods | 2‐arm RCT, with individual randomisation, n = 328 | |
| Participants | Setting: 2 hospitals (1 university and 1 community hospital) serving urban areas in Baltimore, Maryland, USA Background rates of breastfeeding initiation: intermediate Inclusion criteria: mother English‐speaking, with phone access and living within 25 miles of the hospital, intending to breastfeed, family eligible for WIC program, singleton term infant (> 37 weeks’ gestation) Exclusion criteria: infants or mothers with positive drug screen, infants with craniofacial abnormalities, infants admitted to NICU Participant characteristics: all enrolled in WIC program; mean age 23.1 years; 87% African Americans; 26.5% with less than high school education; 79.6% single; 17.4% not employed or in school; 26.6% caesarean births; 50.6% first time mothers; 32.3% with previous breastfeeding experience |
|
| Interventions | Intervention (n = 168): in addition to usual care, a structured programme of education and support comprising postnatal visits by a breastfeeding team (community nurse and peer counsellor) daily in hospital, 2 home visits in the first week after discharge, a third visit at approximately 4 weeks, then scheduled phone calls by the peer counsellor at least fortnightly until 24 weeks and phone access to the community nurse (24 h) for 24 weeks. Home visits lasted approximately 45‐60 min and the average length of phone calls was approximately 20 min. Control (n = 160): usual care included access to a LC in hospital and phone access after discharge home |
|
| Outcomes | Any breastfeeding (breastfed at least once during the previous 24 h) at 6, 12, and 24 weeks postpartum | |
| Notes | Baseline variables were measured using established valid instruments and were used as covariates to adjust for differences between randomisation groups in some of the analyses in the paper. In our analyses we have reported unadjusted figures. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation sequence. Block randomisation (block size 10). |
| Allocation concealment (selection bias) | Low risk | “sealed envelope technique” ... not entirely clear, not described in detail but probably adequate. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Baseline data were collected before randomisation therefore this was collected in a blind fashion, however following randomisation women and staff would be aware of group assignment. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | There was a serious risk of bias associated with the lack of blinding of outcome assessors. In the intervention group outcome data were collected by the staff carrying out the intervention whereas in the control group outcome data were collected by a research interviewer who the women will not have met. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 70% of those approached randomized. 328 randomized and followed up, 29% lost to follow‐up by 24 weeks but all women included in the analyses. Women who withdrew from the study early in the project were assumed not to be breastfeeding and those who were lost subsequently were assumed not to be breastfeeding since their last contact. Both I and C groups were treated in the same way and loss was similar in the 2 groups. The numbers recorded as still breastfeeding therefore represent a conservative estimate. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol. |
| Other bias | Unclear risk | There was no apparent baseline imbalance although baseline characteristics were used in regression analysis to determine adjusted treatment effect. In our results we have reported the unadjusted data. |
Quinlivan 2003.
| Methods | 2‐arm RCT, single‐site study, recruitment July 1998‐December 2000, n = 136 | |
| Participants | Urban Australia Background rates of breastfeeding initiation: high. Baseline prevalence of breastfeeding in Australia = 83% at hospital discharge Participants were recruited at a teenage pregnancy clinic serving mostly disadvantaged young women. The intervention was offered regardless of feeding intention or practice. Inclusion criteria: teenagers aged < 18 years; attending first antenatal appointment at public‐care teenage pregnancy clinic for first‐time mothers; English‐speaking; intending to continue with the pregnancy and not relinquish the infant Exclusion criteria: residence > 150 km from the study hospital; known fetal abnormality Participant characteristics: Ethnic composition of sample: 24% indigenous Australian Socioeconomic status: 86.5% of sample scored low or destitute on score derived from educational level of participant and her parents, and family income |
|
| Interventions | Intervention: structured home visits in weeks 1 and 2 by certified nurse‐midwives to teach feeding and maternal‐infant bonding skills. Further visits at months 1, 2, 3 and 4 to provide advice and support. Control: routine postnatal support, counselling and information services provided by the hospital including access to routine hospital domiciliary home‐visiting services |
|
| Outcomes | Adverse neonatal outcomes (infant death, severe non‐accidental injury and non‐voluntary foster care); knowledge and practice of contraception, vaccination schedules and breastfeeding | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | By computer‐generated randomized allocation schedule |
| Allocation concealment (selection bias) | Low risk | Concealed in numbered, sealed opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Women and staff aware of intervention group. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome assessors aware of intervention group. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 65 assigned to the intervention and 71 to the control group. Reasons for drop out recorded, 124 completed trial (91%). |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol, so could not assess this. |
| Other bias | Unclear risk | It was not clear how the intervention related to some of the outcomes (e.g. early infant death). No baseline imbalance apparent with similar numbers of women in the 2 groups initiating breastfeeding. |
Ransjo‐Arvidson 1998.
| Methods | 2‐arm quasi‐RCT, recruitment 1989‐1992 n=408 | |
| Participants | Setting: study in a hospital in Zambia 408 women recruited 1 h following delivery at the study hospital Inclusion criteria: normal birth, term, singleton, Apgar score > 7 at 1 min, no visible malformation and mother and baby assessed as healthy |
|
| Interventions | Intervention (n = 208): home visits by a midwife at 3, 7, 28 and 42 days. Home visits lasted about 1 h. Midwives examined women and infants and asked about their health; any health problems and related actions; breastfeeding patterns; social support (if any). If indicated, midwives referred women for medical help. Control (n = 200): home visit by a midwife at 42 days only |
|
| Outcomes | Maternal and infant health problems | |
| Notes | We have not included data from this study in the review as they were not reported in a way that allowed us to enter them into RevMan 2014 for meta‐analysis. Numbers of breastfeeding women were not reported by randomisation group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 2‐stage randomisation process with recruitment on certain days, when women were randomly selected to be randomized to treatment groups. |
| Allocation concealment (selection bias) | Unclear risk | It was not clear whether the person carrying out the randomisation had any control over the sample selection and the randomisation process. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Hospital staff were unaware of group allocation. Unclear if midwife delivering intervention and women were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Data were collected by research midwives but unclear if they were blinded to group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Participants seen at follow‐up for the intervention group‐ 98.5% at day 3, 97.5% at day 7, 87% at day 28 and 89% at day 42. Participants seen at follow‐up for the control group ‐ 87% at day 42. Loss to follow‐up < 20% at each follow‐up visit. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol, so could not assess this. |
| Other bias | Unclear risk | Baseline characteristics were similar. |
Redman 1995.
| Methods | 2‐arm RCT n=235 | |
| Participants | Setting: 235 eligible and consenting women booked for delivery at an Australian hospital in 1989 Inclusion criteria: primiparous women who expressed a wish to breastfeed, who booked for delivery before 20 weeks’ gestation, aged between 18‐35 years and lived within 20 km of the hospital Exclusion criteria: women who received additional care from independent midwives |
|
| Interventions | Intervention: programme of care based on health belief model and cognitive‐behavioural principles, including a 3‐h group teaching session in the antenatal period and a visit by a LC shortly after hospital birth, phone support 2‐3 weeks later and at 3 months, with a home visit if needed. The LC was available to provide telephone support at other times. Control: usual breastfeeding care and advice along with routine antenatal classes |
|
| Outcomes | Breastfeeding at 6 weeks and 4 months postdelivery, reasons for stopping breastfeeding, satisfaction with the intervention | |
| Notes | We have not included data from this study in the review due to very high attrition rates which meant results were difficult to interpret. In this study women were recruited in the antenatal period. 235 women were randomized; 30% were lost to follow‐up by 6 weeks postpartum (and full interview data were available for only 56% of the sample). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Alternate, by odd or even numbered consent forms. It was stated that forms were given out sequentially. |
| Allocation concealment (selection bias) | Unclear risk | Odd or evenly numbered consent forms. It was stated that those carrying out recruitment and the women were not aware of the code for allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Women and personnel were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Data were collected using a self‐completed questionnaire. it is not detailed if the questionnaire contained any information that could identify allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | High loss to follow‐up with interview data at 6 weeks for only 56% of the sample randomized. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol, so could not assess this. |
| Other bias | Unclear risk | Baseline characteristics similar ‐ no significant differences between control and intervention groups on any of these variables. |
Santiago 2003.
| Methods | 3‐arm RCT, with individual randomisation, single‐site study, recruitment August 2000‐July 2002, n = 101 | |
| Participants | Urban setting in Minas Gerais, Brazil Background rates of breastfeeding initiation: high. Baseline prevalence of breastfeeding in Brazil in the first 30 days = 88% Inclusion criteria: mother breastfeeding her well, term baby when appointment for paediatric clinic made; first clinic consultation took place at ≤ 30 days Exclusion criteria: mothers who expressed a preference to see a particular paediatrician; babies no longer breastfed at the first appointment Ethnic composition: 62% of babies white |
|
| Interventions | Intervention 1: babies were monitored by a paediatrician working with a multidisciplinary breastfeeding team. The paediatrician and team had all received training to promote exclusive breastfeeding (PNIAM: Programa de Incentivo ao Aleitamento Materno, Brazil). Intervention 2: babies were monitored by the same paediatrician, in individual consultations. Control: babies were monitored by a paediatrician who did not have formal training to promote exclusive breastfeeding. |
|
| Outcomes | Exclusive breastfeeding to 4 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | (Risk of bias assessment from translation notes.) Random assignment by drawing lots. |
| Allocation concealment (selection bias) | Unclear risk | Random assignment by drawing lots. Described as simple randomisation in translation notes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | It was stated that staff were aware of group assignment. It is unclear if women were aware of allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It is stated that "information was collected by the author of each child's medical record. it is unclear if it was 1 of the paediatricians that completed or if it was someone who was blinded to allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | It was not clear at what time randomisation took place or the number randomized to each group "the exclusion percentages were similar in the three groups". 190 were eligible and 101 completed the study. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol, so could not assess this. |
| Other bias | Unclear risk | No baseline imbalance apparent. It was not clear how many women were randomized. |
Serafino‐Cross 1992.
| Methods | 2‐arm RCT, with individual randomisation, recruitment 1986‐1987, n = 52 | |
| Participants | Volunteers attending prenatal clinics in Massachusetts USA who intended to breastfeed their babies for 2 months or longer Background rates of breastfeeding initiation: low Inclusion criteria: breastfeeding for the first time, or unsuccessful previous attempts; English‐speaking |
|
| Interventions | All women received prenatal breastfeeding information. Intervention (n = 26): home visits and telephone contacts up to 2 months postpartum from an experienced breastfeeding counsellor (who also recruited women to the study). Women received 5‐8 visits lasting 30‐60 min. Control (n = 26): usual care; women were given contact details for the clinic nutritionist to use if problems arose. |
|
| Outcomes | Breastfeeding at 2 months postpartum and 6 months postpartum | |
| Notes | We were only able to include 1 reported result in the review: numbers breastfeeding (any) at 8 weeks postpartum. The remaining data were in a graph and were not easy to interpret or data were not reported by randomisation group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Method not described as “randomized the clients”. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The researcher provided the intervention and would have been aware of group allocation. Women would have been aware if they were to receive the intervention or not. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Women were contacted by phone or mail to complete questionnaires. Not stated who did the data collection and whether they were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 52 women were recruited. It appeared that all women were followed up at 8 weeks postpartum, but that approximately half of the comparison group were lost to follow‐up by 6 months. We have not included any data in the review relating to the outcomes measured at 6 months. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol, so could not assess this. |
| Other bias | Unclear risk | Very low recruitment rate “it took 14 months to enrol just 52 participants from 4 clinics serving in total approximately 1000 pregnant women per year”. Results may not be generalisable. |
Sikander 2015.
| Methods | 2‐arm cluster‐RCT, n = 452 | |
| Participants | Union Councils in a rural, resource‐poor district in the northwest province of Pakistan with high infant mortality Background rates of breastfeeding initiation: Pakistan has 8% exclusive breastfeeding before 6 months. inclusion criteria: women aged 17‐40 years, married, in their third trimester of pregnancy, and intending to reside in the study area for the duration of the study Exclusion criteria: women with diagnosed serious medical/psychiatric condition requiring treatment, pregnancy‐related illness (except for common conditions, such as anaemia), and substantial physical/learning disability |
|
| Interventions | Intervention (n = 224): 7 psycho‐educational sessions integrated into the routine work of lady health workers (LHWs) and delivered to all women in their Union Council catchment areas. First session delivered before birth, second session immediately after birth, and the remaining 5 sessions monthly thereafter. The intervention had 6 components: developing an empathic relationship: a trusting, safe, alliance with the mother and other family members; collaborating with the family in an equal partnership; using guided discovery: a style of engagement to gently probe for the individual and family’s health beliefs, and also to stimulate alternative ideas; putting knowledge into practice and behavioural activation; and problem solving. LHWs underwent 2‐day (12 h) training in simplified cognitive behavioural therapy principles using participatory approaches. Control (n = 228): women received an equal number of visits in exactly the same way as those in the intervention arm, but by routinely trained LHWs. |
|
| Outcomes | Primary: rate and duration of exclusive breastfeedingin the first 6 months Secondary: impact on traditional practices impeding exclusive breastfeeding |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Simple unmatched randomization" |
| Allocation concealment (selection bias) | Low risk | Randomisation by an independent researcher |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and LHWs were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The assessors were blind to the allocation status of the mother |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 19% attrition in intervention group and 22% attrition in control group in 6 months after birth. |
| Selective reporting (reporting bias) | Low risk | All outcomes in protocol were reported on. |
| Other bias | Low risk | None identified |
Simonetti 2012.
| Methods | 2‐arm RCT, n = 114 | |
| Participants | Public maternity hospital in Italy Background rates of breastfeeding initiation: not stated Inclusion criteria: healthy primiparous women without breastfeeding problems, with a healthy baby born at full term (37–41 weeks, birthweight > 2500 g) and who agreed to be enrolled Exclusion criteria: multiparous women, premature baby (born before the 37th week), low birth weight baby (< 2500 g), admission to neonatal intensive care unit or transfer to another hospital, medical condition which could permanently or temporarily counter‐indicate breastfeeding (e.g. acute tuberculosis, psychosis, acute phase hepatitis A or B, hepatitis C, HIV), women who did not speak Italian, and women who could not be contacted by telephone). |
|
| Interventions | Intervention (n = 55): structured telephonic counselling (STC); each mother received telephone calls during the first 6 weeks after delivery. The phone call timing was planned by both the mother and the licensed midwife (LM) with at least one a week; in addition, mothers were invited to call the LM when necessary to solve any breastfeeding problem. During every phone call, the LM gave support and all information on fully breastfeeding. No weekly calls were missed. Control (n = 59): conventional counselling ‐ consisting of programmed periodical visits with the physician at 1, 3 and 5 months after delivery. Participants were also invited to call the LM in case of breastfeeding problems. |
|
| Outcomes | Primary: breastfeeding at 1,3 and 5 months after delivery Secondary: influence of mother’s educational level and employment status on exclusive breastfeeding |
|
| Notes | None identified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Women who consented to participate were randomly assigned to 1 of the 2 groups, 55 women were enrolled in the experimental group (receiving STC) and 59 were enrolled in the control group (receiving conventional counselling)". Sequence generation not described. |
| Allocation concealment (selection bias) | Unclear risk | No detail provided to enable judgement of this. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not possible due to nature of intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The data were collected by a specialist nurse who monitored all subjects. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not clear if all women who were randomized completed study as numbers not provided |
| Selective reporting (reporting bias) | Unclear risk | No evidence that outcomes were prespecified |
| Other bias | Low risk | |
Sjolin 1979.
| Methods | Quasi‐RCT, single site, duration 12 months, n = 146 | |
| Participants | Urban Sweden ‐ maternity ward of University Hospital Background rates of breastfeeding initiation: high. Baseline prevalence (1972): 4% breastfeeding at 24 weeks Inclusion criteria: resident in Uppsala; normal birth; healthy babies weighing > 3 kg Exclusion criteria: none specified Ethnic composition not stated. 28% of mothers had completed college or university education |
|
| Interventions | Intervention: 'Interview' with paediatrician in hospital on days 1 and 4, and at home at 2 and 6 weeks and 3 months; telephone contact weekly while breastfeeding followed by home visit if problem noted. Control. Usual care, |
|
| Outcomes | Partial and exclusive breastfeeding at 2, 4, 8, 12, 16, 20 and 24 weeks | |
| Notes | Primarily designed as a study of the reasons for breastfeeding difficulties and the cessation of breastfeeding. Recruitment halted during holidays. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quasi‐randomisation depending on time of day of birth |
| Allocation concealment (selection bias) | High risk | Quasi‐randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Women in the control group were not told about the study until the 6 months follow‐up interview. Not stated if women in the intervention group were aware of study. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | All interviews were carried out by the same investigator who was aware of group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Interviews took place while women continued to breastfeed and it was not clear how many women remained to follow‐up at different points although no drop‐out was reported for the final data collection interview at 6 months. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol, so could not assess this. |
| Other bias | Unclear risk | Women in the intervention group reported outcomes at scheduled interviews, whereas the control group were interviewed at 6 months postpartum only. Recall and response bias may have been different in the 2 groups. |
Srinivas 2015.
| Methods | 2‐arm RCT, n = 120 | |
| Participants | Westown Physician Center (WPC), a hospital‐affiliated urban clinic, USA, where most patients received public insurance or charity care Background rates of breastfeeding initiation: data from an inner‐city Cleveland clinic with a similar population reported lower rates with any and exclusive breastfeeding at 5 days at 40.8% and 22.0%, respectively. Inclusion criteria: aged18 years or older, with no contraindications to breastfeeding, ≥ 28 weeks’ gestation at recruitment stage Exclusion criteria: women < 18 years old, non‐English speakers, and those with a diagnosis that was an absolute contraindication to breastfeeding (HIV/AIDS, herpes simplex on the breast, tuberculous lesions of the breast) |
|
| Interventions | Intervention (n = 50): peer counselling WIC support programme; WIC definition of a breastfeeding peer counsellor: a woman who breastfed her own infant(s) to 1 year with exclusive breastfeeding for 6 months or was currently breastfeeding an infant following recommended practice, who received 20 h training. Counsellor was resident in the local area and received care herself from WPC. Antenatal: peer counsellor initiated contact once during third trimester of pregnancy with additional contacts at mother's request (mostly by telephone). Postnatal: peer counsellor contact within 3‐5 days of birth weekly to 1 month, every 2 weeks up to 3 months, and once at 4 months, in person during clinic visits or via telephone. No home visits for safety reasons. Control (n = 53): standard care (available to both intervention and control group), included access in hospital to International Board Certified LCs and outpatient lactation support from the clinic paediatricians and the WIC nutritionist. The in‐office WIC site had a peer helper available less than once a month. |
|
| Outcomes | Breastfeeding initiation Any breastfeeding at 1 month and 6 months Exclusive breastfeeding at hospital discharge, 1 month, 6 months Breastfeeding attitude and self‐efficacy Perception of breastfeeding support |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "participants were ran‐domized within these strata in blocks of 4 participants in a 1:1 ratio to intervention (PC) or control (usual care) group". Method of sequence generation not described. |
| Allocation concealment (selection bias) | Unclear risk | No detail provided to enable judgement of this. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The peer counsellor was blinded to self‐efficacy and attitude towards breastfeeding, but due to the nature of the intervention would have been aware of the allocation of the women. It was not stated whether the women were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Study co‐ordinator administered exit interview and it was not stated whether the co‐ordinator was blinded to group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number initially randomized not provided, so unable to calculate attrition rate. |
| Selective reporting (reporting bias) | Unclear risk | No evidence of a record of predefined outcomes to judge this. |
| Other bias | Low risk | None identified |
Stockdale 2008.
| Methods | 2‐arm RCT, n = 182 | |
| Participants | Suburban hospital and community health and social services trust that served both urban and rural areas in Northern Ireland Background rates of breastfeeding initiation: not detailed Inclusion criteria: primigravid women who intended to have their baby within the trust and who attended the routine 20‐week antenatal appointment during the recruitment phase Exclusion criteria: women who did not speak English (or had interpretation services available), women who experienced infant‐maternal separation and incidences of newborn abnormalities that required additional infant feeding support, or teenagers who had already attended a breastfeeding workshop |
|
| Interventions | Intervention (n = 93): motivationally‐enhanced version of midwife instruction as a means of increasing women's expectancy for successful breastfeeding, compared to best practice. The intervention had 4 components: antenatal feeding class (32‐36 weeks' gestation), a breastfeeding information book (provided in the antenatal phase), a breastfeeding CD‐ROM, postnatal instructional support provided by midwives (up to 3 weeks postnatal) and additional lactation consultancy on request. The postnatal midwives who supported the intervention attended an additional 1‐day training session that focused on the role of human motivation and the use of effective strategies to increase participants' expectancy for success. Control (n = 89): local best practice |
|
| Outcomes | Primary: women's motivation towards breastfeeding Secondary: breastfeeding on discharge from hospital and at 3 weeks |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were assigned using computer‐generated random numbers to the intervention or control groups. |
| Allocation concealment (selection bias) | Unclear risk | No details provided to enable judgement of this. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants were blinded to group membership but unclear if this was successful. Midwives were informed of the allocation through a colour‐coded sticker on the women‐held records. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated whether researcher or parent education co‐ordinator who collected the data were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Attrition in intervention group was 26% and in control group was 16%. The withdrawal rate was higher in the intervention group (n = 13) compared to the control group (n = 2). |
| Selective reporting (reporting bias) | Unclear risk | No evidence of prespecified outcomes to judge this. |
| Other bias | Low risk | None identified |
Su 2007.
| Methods | 3‐arm RCT, with individual randomisation, n = 450 | |
| Participants | National University Hospital, Singapore Background rates of breastfeeding initiation: high Inclusion criteria: healthy pregnant women attending antenatal clinics at the study hospital, with no illness that would contraindicate breastfeeding or severely compromise its success; intending to breastfeed; birth at 34 weeks' gestation or later Exclusion criteria: women with high risk and multiple pregnancies Participant characteristics: 40% primiparous, mixed ethnicity (Chinese 31%‐44%, Malay 46%‐54%, Indian and other), approximately a third educated beyond secondary school, approximately half employed outside the home, 56% had previously breastfed |
|
| Interventions | Intervention 1: antenatal education: in addition to routine care, women received 1 session of antenatal breastfeeding education and printed guides on breastfeeding. Intervention 2: postnatal lactation support: in addition to routine care, women received 2 postnatal sessions with a LC, 1 in hospital within the first 3 postnatal days (when they received the same printed guides on breastfeeding as the antenatal education group) and 1 during the first routine postnatal visit 1 to 2 weeks after the birth. Each session lasted about 30 min and covered latching on, proper positioning and other techniques to avoid common breastfeeding complications. Control: women received routine antenatal, intrapartum and postnatal care, including optional antenatal classes and postnatal visits by a LC should any problems with breastfeeding arise. |
|
| Outcomes | Exclusive and any breastfeeding at hospital discharge and 2 weeks, 6 weeks, 3 and 6 months after the birth. Exclusive breastfeeding was defined as giving breast milk as the only food source, with no other foods or liquids, other than vitamins and minerals being given. | |
| Notes | Intervention group 1, who received the antenatal intervention, are not included in the analysis in this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation sequence by external clinical trials unit |
| Allocation concealment (selection bias) | Low risk | Telephone allocation by external trials unit (with envelope back up used only on 4 occasions) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | For participants, blinding was not mentioned, but women would be aware of allocation. The caregivers who delivered the intervention would be aware of allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Data collection was on standard forms and was entered by remote unit, therefore outcome assessment may have been partially blinded. Not clear who conducted the actual interviews. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Low attrition in all arms. In total 450 randomized 347 completed follow‐up at 6 months (82%). In the data and analyses, 2 arms included 299 randomized, 245 followed up at 6 months (82%). |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol, so could not assess this. |
| Other bias | Unclear risk | There was an imbalance in the groups due to 4 women being randomized by using back up envelopes because of dysfunction in web randomisation, but groups appeared similar at baseline. Some of the data were based on assumptions. Sensitivity analyses were based on the assumption that none of the women lost to follow‐up were exclusively breastfeeding at any time point. |
Tahir 2013.
| Methods | 2‐arm RCT, n = 357 | |
| Participants | Public maternity hospital in Kuala Lumpur Background rates of breastfeeding initiation: 92.2% of mothers were exclusively breastfeeding at the study site before discharge. Inclusion criteria: 18 years of age or older; of Malaysian nationality; delivered a single infant at ≥ 37 weeks' gestation; an intention to breastfeed and the ability to understand and communicate in spoken Malay or English; had received a prenatal breastfeeding education programme at least once; had telephone access; and gave informed consent Exclusion criteria: women with multiple pregnancies or medical problems that might hinder breastfeeding; women that delivered via caesarean section; or women whose baby subsequently required prolonged care in a Special Care Nursery |
|
| Interventions | Intervention (n = 179): lactation counselling given by certified LCs via telephone twice monthly to each lactating mother, in addition to the current conventional care (as descried below). Each mother was expected to receive 12 lactation counselling sessions by the end of the study. Contact was discontinued any time that a mother decided to stop breastfeeding completely. Contact was also discontinued if the mother had given the baby up for foster care and/or had no physical contact to enable her to breastfeed. LCs in this study were registered nurses from the Maternity Hospital Kuala Lumpur who had post‐basic training in midwifery and were certified as LCs. All 12 LCs had undergone a 40‐h lactation management and counselling course based on the WHO module. Control (n = 178): mothers received current conventional care for postnatal breastfeeding promotion or support from thier own public healthcare provider. This conventional care included breastfeeding talks during immunisation follow ups, a mothers’ communication with the LCs through information or pamphlets received during antenatal or postnatal follow‐ups, and advice regarding breastfeeding received at any time from any healthcare workers, the media, peer counsellors, family members or friends. |
|
| Outcomes | Exclusive breastfeeding at 1, 4 and 6 months Stopped any breastfeeding at 1, 4 and 6 months |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Generation of the group assignments was conducted using a blocked randomisation method with a block size of four by a random allocation software program" |
| Allocation concealment (selection bias) | Unclear risk | No details provided to enable judgement of this. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The women and LCs were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Only the Research Enumerator who collected the breastfeeding outcome data was blinded with respect to the treatment group" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition in intervention and control group was 89.4% and 88.8% respectively. |
| Selective reporting (reporting bias) | Unclear risk | No evidence of predefined outcomes to judge this. |
| Other bias | Low risk | None identified |
Tylleskar 2011a.
| Methods | One of the 3 country sites that completed this cluster‐randomised trial ‐ Burkino Faso in French‐speaking West Africa (24 clusters: 12 intervention, 12 control). Mother‐infant pairs enrolled: 392 intervention and 402 control (794 total). Followed up at 24 weeks: 359/392 (92%) intervention and 372/402 (93%) control | |
| Participants | Rural area where main source of income was farming. 60 primary care facilities and a regional hospital Baseline prevalence of breastfeeding initiation: high (98.4%). Exclusive breastfeeding for babies under 6 months estimated at 16% Inclusion criteria: women living in trial area at least 7 months pregnant and intending to breastfeed, singleton live birth, no serious congenital malformations Exclusion criteria: mothers or infants who died were not included in the analysis Participant characteristics: Mean age of women 25 years. None of the women had any formal education and more than half had had a previous child death. 99% of women had no toilet or an open toilet and < 1% had piped water in yard or home. Monthly income was approximately EUR 3. |
|
| Interventions | Intervention: peer counselling by supporters who received a modified version of WHO/UNICEF training (1 week training). Women were given information about breastfeeding and peers provided support and addressed problems or referred women for specialist help. The intervention involved a minimum of 5 home visits, 1 in the third trimester and at least 4 in the postnatal period up to 6 months postpartum. The supporters were local residents, literate, able to travel to visit women in their homes and had a good reputation in the community. Peer counsellors visited the same women each time to achieve continuity of care. The intervention varied in the 3 study areas and was adapted to local circumstances. Control: mothers and infants in control clusters in Burkina Faso were given standard healthcare only. |
|
| Outcomes | Prevalence of exclusive breastfeeding and prevalence of diarrhoea, reported by mothers for infants aged 12 weeks and 24 weeks | |
| Notes | The paper reported that current breastfeeding was assessed at all scheduled postpartum visits using past 24‐h and 7‐day recalls. Babies who were reported to have received no other food or liquids than breast milk (they may have been administered drugs) were classified as exclusively breastfed. This may have been during the last 24 h or 7 days rather than since birth. Prevalence of diarrhoea was based on the mothers’ reports of the past 2 weeks. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear (different procedures in different areas and the procedure in 1 of the 4 areas was not clear) . |
| Allocation concealment (selection bias) | Unclear risk | There was no allocation concealment within clusters and participants would be aware of assignment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | There was no participant or staff blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | There was an attempt to mask/blind outcome assessors to randomisation group, although it is possible women would have revealed whether or not they received support. The success of attempted blinding was not formally evaluated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was flooding in 1 of the 4 original study area and no results were reported for this area. For the remaining 82 clusters in 3 countries for primary outcomes the authors carried out an ITT analysis (i.e. those that were missing were recorded as non‐events, i.e. NOT exclusive breastfeeding and no diarrhoea). 2579 women enrolled. Missing data and missed visits at various data collection points. |
| Selective reporting (reporting bias) | Unclear risk | Not apparent |
| Other bias | Unclear risk | Recruitment procedures and intervention delivery was slightly different in each of the study countries which meant that results were difficult to interpret. It was reported that the ICC in each country for primary outcomes varied considerably and therefore results were reported separately for each country. Authors stated, “The community‐based approach could possibly have resulted in socially desirable answers, and the results were based on self‐reports. A bias towards desirable answers and thereby an increased effect size cannot be ruled out. We also noted some questionnaire fatigue in the Ugandan site—i.e. reluctance to fully engage in answering similar questions after a few interviews". |
Tylleskar 2011b.
| Methods | Second of 3 country sites that completed the cluster‐randomised trial ‐ Mbale district in Eastern Uganda (24 clusters: 12 intervention, 12 control). Mother‐infant pairs enrolled: 396 intervention and 369 control (765 total). Followed up at 24 weeks: 368/396 (93%) intervention and 329/369 (89%) control | |
| Participants | Urban and rural areas: urban area included “large slum migrant settlements”. Background rates of breastfeeding initiation: high (> 95%) Inclusion criteria: women living in trial area at least 7 months pregnant and intending to breastfeed, singleton live birth, no serious congenital malformations Exclusion criteria: mothers or infants who died were not included in the analysis Participant characteristics: HIV prevalence for fertile women was 6.2%. 26% of women had no toilet or an open toilet and 5% had piped water in yard or home. Mean age 25 years. Women had approximately 6 years of formal education and approximately a third had had a previous child death. Monthly income was approximately EUR 12. |
|
| Interventions | Intervention: peer counselling as in Burkina Faso (Tylleskar 2011a). Paper stated the intervention varied in the 3 study areas and was adapted to local circumstances. Control: mothers and infants in control clusters in Uganda were given standard healthcare only. |
|
| Outcomes | Prevalence of exclusive breastfeeding and prevalence of diarrhoea, reported by mothers for infants aged 12 weeks and 24 weeks | |
| Notes | The paper stated that current breastfeeding was assessed at all scheduled postpartum visits using past 24‐h and 7‐day recalls. Babies reported to have received no other food or liquids than breast milk (they may have been administered drugs) were classified as exclusively breastfed. This may have been during the last 24 h or 7 days rather than since birth. Prevalence of diarrhoea was based on the mothers’ reports of the past 2 weeks. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear (different procedures in different areas and procedure in 1 of the 4 areas was not clear) |
| Allocation concealment (selection bias) | Unclear risk | There was no allocation concealment within clusters and participants would be aware of assignment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | There was no participant or staff blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | There was an attempt to mask/blind outcome assessors to randomisation group, although it was possible women would have revealed whether or not they received support. The success of attempted blinding was not formally evaluated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was flooding in 1 of the 4 original study area and no results were reported for this area. For the remaining 82 clusters in 3 countries for primary outcomes the authors carried out an ITT analysis (i.e. those that were missing were recorded as non‐events, i.e. NOT exclusive breastfeeding and no diarrhoea). 2579 women enrolled. Missing data and missed visits at various data collection points. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol, so could not assess this. |
| Other bias | Unclear risk | Recruitment procedures and intervention delivery was slightly different in each of the study countries which meant that results were difficult to interpret. It was reported that the ICC in each country for primary outcomes varied considerably and therefore results were reported separately for each country. Authors stated, “The community‐based approach could possibly have resulted in socially desirable answers, and the results were based on self‐reports. A bias towards desirable answers and thereby an increased effect size cannot be ruled out. We also noted some questionnaire fatigue in the Ugandan site—i.e. reluctance to fully engage in answering similar questions after a few interviews". |
Tylleskar 2011c.
| Methods | Third of 3 country sites that completed this cluster‐randomised trial ‐ 3 geographically separate sites in South Africa (Paarl, a town at the centre of a farming district near Cape Town; Umlazi, a large periurban township near Durban; and Rietvlei, 1 of the country's poorest rural districts: 34 clusters: 17 intervention, 17 control). Mother‐infant pairs enrolled: 535 intervention and 485 control (1020 total). Followed up at 24 weeks: 461/535 (86%) intervention and 410/485 (85%) control | |
| Participants | South Africa (3 areas including 1 of the poorest rural area in South Africa) Under 5 mortality rate in South Africa was 67/1000 and infant mortality rate was 48/1000. Background rates of breastfeeding initiation: high (> 95%). Exclusive breastfeeding at 6 months was estimated at 8% in 2005‐2009. Inclusion criteria: women living in trial area at least 7 months pregnant and intending to breastfeed, singleton live birth, no serious congenital malformations Exclusion criteria: mothers or infants who died were not included in the analysis Participant characteristics: 16% of women had no toilet or open toilets and 66% had piped water in yard or home. Mean age 23 years. Women had approximately 10 years of formal education and approximately 7% had had a previous child death. Monthly income was approximately EUR 103. |
|
| Interventions | Intervention: peer counselling as in Burkina Faso and Uganda (Tylleskar 2011a; Tylleskar 2011b). Paper stated the intervention varied in the 3 study areas and was adapted to local circumstances. Control: control clusters were visited by peer counsellors, with the same schedule as the intervention clusters, but they assisted families in obtaining birth certificates and social welfare grants. The peer counsellors for the intervention and control clusters in South Africa were kept separate during the study. |
|
| Outcomes | Prevalence of exclusive breastfeeding and prevalence of diarrhoea, reported by mothers for infants aged 12 weeks and 24 weeks | |
| Notes | The paper stated that current breastfeeding was assessed at all scheduled postpartum visits using past 24‐h and 7‐day recalls. Babies reported to have received no other food or liquids than breast milk (they may have been administered drugs) were classified as exclusively breastfed. This may have been during the last 24 h or 7 days rather than since birth. Prevalence of diarrhoea was based on the mothers’ reports of the past 2 weeks. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear (different procedures in different areas and procedure in 1 of the 4 areas was not clear) |
| Allocation concealment (selection bias) | Unclear risk | There was no allocation concealment within clusters and participants would be aware of assignment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | There was no participant or staff blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | There was an attempt to mask/blind outcome assessors to randomisation group although it is possible women would have revealed whether or not they received support. The success of attempted blinding was not formally evaluated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was flooding in 1 of the 4 original study area and no results were reported for this area. For the remaining 82 clusters in 3 countries for primary outcomes the authors carried out an ITT analysis (i.e. those that were missing were recorded as non‐events, i.e. NOT exclusive breastfeeding and no diarrhoea). 2579 women enrolled. Missing data and missed visits at various data collection points. |
| Selective reporting (reporting bias) | Unclear risk | Not apparent |
| Other bias | Unclear risk | Recruitment procedures and intervention delivery was slightly different in each of the study countries which meant that results were difficult to interpret. It was reported that the ICC in each country for primary outcomes varied considerably and therefore results were reported separately for each country. Authors stated, “The community‐based approach could possibly have resulted in socially desirable answers, and the results were based on self‐reports. A bias towards desirable answers and thereby an increased effect size cannot be ruled out. We also noted some questionnaire fatigue in the Ugandan site—i.e. reluctance to fully engage in answering similar questions after a few interviews". |
Vidas 2011.
| Methods | 2‐arm RCT, n = 100 | |
| Participants | Setting not clear. "Our research was conducted by the Association for a healthy and happy childhood‐Counseling center for mother and child in Bjelovar, Croatia”– not obvious what type of setting this is, but we infer it is a community‐based setting of some sort. Background rates of breastfeeding initiation: 50% of women give‐up breastfeeding after 6 months in Croatia Inclusion criteria: "the criterion for inclusion in the study was that the mother was nursing her child and the child had up to two months" Exclusion criteria: none stated |
|
| Interventions | Intervention (n = 50): “six basic exercises of autogenic training". Not clear what this is but article states "every two weeks mothers were practicing a new exercise. The 6 basic exercises of autogenic training were taught for 12 weeks in small groups to 10 members". "After mothers have learned all the exercises of autogenic training, they have continued to practice until their child reached six months of life". The exercises seem to be delivered in a group setting and promoted breastfeeding. Control (n = 50): unclear; Quote "mothers of both groups were advised to successful breastfeeding up to 6 months of age" |
|
| Outcomes | Attitude, decision and duration of breastfeeding Mother’s level of confidence Motivation for successful breastfeeding Motivation for autogenic training Possible factors influencing breastfeeding Risk factors for postpartum mental disorders, anxiety and postpartal depression Degree of satisfaction with practising autogenic training and its possible role in promoting successful breastfeeding in the examined group |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information given on sequence generation. Only information about randomisation process was “Mothers were randomly divided into two groups‐examined and control". |
| Allocation concealment (selection bias) | Unclear risk | No information given on allocation concealment. Only information about randomisation process was “Mothers were randomly divided into two groups‐examined and control". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Nature of trial meant that participants and personnel would have been aware of group assignment. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information given about blinding of outcome assessment for any outcome. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information given in paper on the numbers assessed for outcomes, other than for breastfeeding at 6 months where the authors reported numbers for each arm and the data. Fig 4 implied they had complete outcome data for this – for all other outcomes it was unclear what number of participants in each arm were assessed for each outcome. |
| Selective reporting (reporting bias) | High risk | No statement of the original prespecified primary and secondary outcomes for the trial. No link to trial registry or protocol information which would enable discernment of any selective outcome reporting (or the lack of it). |
| Other bias | Low risk | None identified |
Vitolo 2005.
| Methods | 2‐arm RCT, with individual randomisation, n = 500 | |
| Participants | Setting: urban, a low‐income area of the city of São Leopoldo, Rio Grande do Sul, Brazil. Recruitment from maternity wards of the city's only publicly funded hospital, which mainly serves the low‐income population. Background rates of breastfeeding initiation: high Inclusion criteria: low‐income mothers with healthy, singleton, full‐term (> 37 week) babies with birthweight > 2500 g Exclusion criteria: impediments to breastfeeding, HIV/AIDS, or congenital malformation Demographics: 57% male children; 60% of intervention and 52% of control mothers had less than 8 years schooling; 73% of intervention and 67% of controls had low annual incomes (< USD 3000); 34% of mothers were not in paid work; 70% of children were living with mother and father; almost half of the mothers were overweight |
|
| Interventions | Both groups received routine assistance from paediatricians in the health service. Intervention (n = 200): dietary advice about breastfeeding and the adequate introduction of complementary foods, given monthly for 6 months in home visits starting within 10 days of the child’s birth then at 8, 10, and 12 months by 12 trained field‐workers (undergraduate students in groups of 2) who counselled mothers on the Ten Steps for Healthy Feeding Children from Birth to Two Years of Age (Brazilian Ministry of Health). Control (n = 300): standard care (not described) |
|
| Outcomes | Exclusive breastfeeding at 4 and 6 months; any breastfeeding at 12 months; also diarrhoea, respiratory problems, dental caries, anaemia, hospitalisation and nutritional status at 12‐16 months | |
| Notes | Although the paper called this intervention 'dietary counselling', we have included it as a breastfeeding support intervention because its main purpose was to promote exclusive breastfeeding for 6 months followed by healthy complementary foods, and it involved regular visits during the first year of life. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Block randomisation in groups of 5. |
| Allocation concealment (selection bias) | Low risk | Randomisation was conducted by an investigator not involved in the eligibility and entry of participants into the study. Fieldworkers were informed of this allocation and then proceeded with the study. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Women and care staff would be aware of group assignment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Interviewers were blinded to the group status to the mother‐infant pair. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 500 women were randomized (200 to the intervention and 300 to the control group). By 12 months 163 intervention group (81%) and 234 control group (78%) remained available to follow‐up. Reasons for loss to follow‐up were give by group with reasons. However there were some discrepancies between publications and information provided by the author in the numbers followed up. |
| Selective reporting (reporting bias) | Low risk | Not apparent |
| Other bias | Unclear risk | Method of randomisation led to imbalanced groups. Mothers and children appeared similar at baseline. There were some discrepancies between publications and information provided by the author in the numbers followed up and in the results. We have used information provided by the author. |
Wambach 2009.
| Methods | 3‐arm RCT 3‐arm, with individual randomisation, n = 390 | |
| Participants | The study was carried out in 7 prenatal clinics in the American Midwest. Clinics provided services to low‐income adolescent mothers Baseline prevalence of breastfeeding initiation in country/setting: low Inclusion criteria: age 15‐18 years, in second trimester of pregnancy, expecting first birth, planning to keep baby, able to read and speak English, with access to phone; at birth, only mothers of singleton, term healthy babies were included Exclusion criteria: women who had birth complications that prohibited or delayed breastfeeding beyond 48 h Sample characteristics: mean age 17 years (SD 0.9); 61% African American; 75% low‐income; 74% single and living with their families, and 71% were in school. |
|
| Interventions | Intervention (n = 128): 2 antenatal classes (1.5–2 h) on benefits of breastfeeding and practical issues run by the LC and the peer counsellor, followed up by phone calls. After the birth, phone calls made to those who had initiated breastfeeding, at 4, 7, 11, 18 days and 4 weeks to provide support. Control 1 (n = 128): the same contact schedule of classes and phone calls as the intervention group, with content concentrating on more general pregnancy and health issues Control 2 (n = 134): usual care with no special intervention |
|
| Outcomes | Data on breastfeeding were available for women who initiated breastfeeding – this meant results were difficult to interpret. | |
| Notes | We have not included outcome data from this study in the review due to very high levels of attrition. This was a study where women were recruited in the second trimester and interventions took place both prenatally and postnatally. For postnatal outcomes only those women who initiated breastfeeding were followed up. There was considerable loss to follow‐up. 390 were randomly assigned. Women who did not attend at least 1 of the study classes were dropped from the study. Follow‐up data on duration of breastfeeding were available for 201 women who initiated breastfeeding (51%). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “list of random codes generated by the study bio‐statistician." |
| Allocation concealment (selection bias) | Unclear risk | It was not clear how allocation was concealed at the point of randomisation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study described as being unblinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Study described as being unblinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 390 women were randomized and those who did not attend at least 1 of the study classes were excluded. Follow‐up data on duration of breastfeeding was available for 201 who initiated breastfeeding (51%). |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol, so could not assess this. |
| Other bias | Unclear risk | Unclear ‐ data limited ‐ only reported in the form of an abstract. |
Wen 2011.
| Methods | 2‐arm RCT, n = 667 | |
| Participants | The trial was conducted in socially and economically disadvantaged areas of Sydney, Australia, during 2007‐2010. Background rates of breastfeeding initiation: no information provided Inclusion criteria: ≥16 years old, expecting first child, between weeks 24‐34 of pregnancy, able to communicate in English, and lived in the local area Exclusion criteria: women were excluded from the study if they had severe medical conditions as evaluated by their physicians |
|
| Interventions | Intervention (n = 337): 5 or 6 home visits from a specifically trained research nurse delivering a staged home–based intervention in the antenatal period and at 1, 3, 5, 9 and 12 months. At each visit the research nurse spent 1 h‐2 h with the mother and infant. (The nurse addressed 4 key areas: infant feeding practices, infant nutrition and active play, family physical activity and nutrition, as well as social support). The was delivered by trained research nurses in accordance with a protocol (www.healthybeginnings.net.au/). Each visit involved standard information with key discussion points. and appropriate resources to reinforce the information. Control (n = 330): received the usual childhood nursing service, comprising 1 home visit within a month of birth if needed. Additional visits at baseline and 12 months were conducted by a research assistant for the purpose of data collection only. |
|
| Outcomes | Exclusive breastfeeding at 6 months Prevalence of any breastfeeding at 6 months and 12 months Median breastfeeding duration Time at introduction of solids |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "group allocation, which was determined by a computer‐generated random number" |
| Allocation concealment (selection bias) | Low risk | Random allocation was concealed by sequentially numbered, sealed, opaque envelopes containing the group allocation. A research assistant who had no direct contact with participating mothers was responsible for generating the random numbers and preparing the envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and those delivering the intervention were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The outcome data were collected by telephone at 6 months and by face‐to‐face interview in the home at 12 months. The data collectors and the research staff who dealt with data entry and analysis were masked to treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Follow‐up in intervention group was 82.5% at 6 months and 85.8% in the control group. "Those lost to follow‐up were significantly younger and less educated and were more likely to be unemployed or have low income (Table 1). The main reasons for loss to follow‐up were as follows: could not be contacted (67.8%), moved out of the area (14.2%), no longer interested (8.9%), too busy (4.0%), and illness or death (5.0%). This was similar across both groups" |
| Selective reporting (reporting bias) | Low risk | The breastfeeding outcomes were prespecified in the trial registry record. |
| Other bias | Unclear risk | Authors noted that they were unable to complete the baseline assessment and randomisation before birth, as planned, for 190 women (93 in the intervention group and 97 in the control group). There was no significant difference between these 190 and the 337 who were assessed and randomized before birth (175 in the intervention group and 162 in the control group) for any of the characteristics. Of the 268 participating mothers remaining in the intervention group at 12 months, 34.7% received 5 home visits after giving birth and 35.3% received 6 home visits, including an antenatal visit |
Wilhelm 2015.
| Methods | 2‐arm RCT, n = 53 | |
| Participants | Mexican–American women (American women of Mexican ethnicity/ancestry) residing in rural western Nebraska in the central USA Background rates of breastfeeding initiation: initiation 80% in Hispanic/Latino women. Duration and exclusivity of breastfeeding at 6 months was 45.2% and 14% respectively. Inclusion criteria: self‐identified Mexican‐American mothers between the ages of 15‐50 years who were breastfeeding at the time of recruitment/consent Exclusion criteria: admission of the mother to the ICU, multiple births, congenital abnormalities in the infant, or infant admitted to NICU |
|
| Interventions | Intervention (n = 26): motivational interviewing (MI); MI was operationalised by asking the participant to rank the importance of breastfeeding for 6 months (1–10 scale) and her confidence in her ability to continue breastfeeding (1–10 scale). The researcher focused on the lower score and asked the woman why she did not choose a higher number and what she thought it would take to increase the number. Initial intervention delivered at day 3 visit, MI booster sessions delivered at week 2 and week 6 visits to promote behavioural change. Control (n = 27): attention control (AC); mothers in the AC group were given educational information about infant safety including information on fall prevention, poisoning, fires, and burns during the first visit, about choking/aspiration, suffocation, drowning, and smoking during the second visit, and about car seat safety during the final visit. The principle investigator conducted all AC sessions. |
|
| Outcomes | Intention to breastfeed for 6 months Breastfeeding self‐efficacy Duration of breastfeeding |
|
| Notes | Feasibility study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The process was unclear (e.g. was a random number table or computerised randomisation used? However, because the authors stated that a randomisation schedule was prepared by the statistician, we can probably be confident there was true randomisation here) |
| Allocation concealment (selection bias) | Unclear risk | No information provided about what happened with the sequence, e.g. sealed numbered envelopes or not – unclear if randomisation could have been subverted. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants aware of group assignment given nature of the interventions. The principal investigator conducted the intervention and was aware of the group assignment. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear – report did not state who collected outcomes data and whether they were masked to intervention/control. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Authors stated, "High levels of attrition (69%, n = 18, in the MI group and 63%, n = 17, in the AC group) by week 6 impaired our ability to evaluate the potential of our MI intervention. 7 (27%) of the MI mothers and 13 (48%) of the AC mothers were no longer participating because they discontinued breastfeeding prior to week 6. We were unable to reach the remaining mothers (11 in the MI group [42%] and 4 in the AC group [15%]) in person or by phone to conduct the remaining assessments and interventions/control sessions. Subsequently, we reestablished contact with all but 3 mothers (all in the MI group) and determined their duration of breastfeeding through 6 months". |
| Selective reporting (reporting bias) | High risk | No trial registry information given or protocol available; paper did not state if the outcomes were prespecified, so not possible to compare outcomes reported in this paper vs those preplanned, and thus evaluate the possibility of selective outcome reporting. Authors did say however, “Our primary goal was to evaluate the effectiveness of MI by comparing intent to breastfeed, breastfeeding self efficacy, and duration of breastfeeding between mothers receiving the MI intervention and those receiving attention alone" |
| Other bias | Unclear risk | None noted |
Winterburn 2003.
| Methods | Single‐site study, duration of recruitment not reported, n = 72; 30 allocated to the intervention and 42 to the control group | |
| Participants | Community study in North Trent, England, UK Background rates of breastfeeding initiation: intermediate. National baseline prevalence 66% breastfeeding at birth Inclusion criteria: mothers attending for antenatal care on 1 area. Other details not reported. |
|
| Interventions | Intervention: the midwife asked mothers during their pregnancy to identify a close female confidante who could support them to breastfeed, and visited the mother and confidante together during the third trimester to discuss breastfeeding. | |
| Outcomes | Duration of breastfeeding to 3 months; women's satisfaction with the intervention; midwives' assessments of the intervention | |
| Notes | Numerical outcome data were provided by the researcher. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly allocated" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Women and the health visitors and community midwives would have been aware of the group allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Data (including details of the intervention) were collected by the health visitor. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 72 randomized; it was not clear whether full data were available for all women at 3 months. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol, so could not assess this. |
| Other bias | Unclear risk | No baseline characteristics reported. |
Wolfberg 2004.
| Methods | 2‐arm RCT, with individual randomisation; few details of study methods reported | |
| Participants | Partners of women attending for antenatal care at Baltimore Hospital USA 2001‐2002 (567 pregnant women were approached) | |
| Interventions | Intervention: 1 group session for fathers, lasting 2 h, to encourage them to support their partners to breastfeed Control: usual care; fathers received classes on child safety and baby care |
|
| Outcomes | Breastfeeding at 4, 6 and 8 weeks and breastfeeding duration | |
| Notes | We have not included data from this study in the review due to very high levels of attrition. 567 pregnant women were approached, of the 431 that agreed to participate only 59 fathers completed the study (14%). It was not clear at what point randomisation occurred. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not clear if women or fathers were aware of allocation/study hypotheses. The person delivering the class would have been aware of the intervention, but unclear if health professionals providing care would be aware of allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Data were collected through phone calls or questionnaires. It was not stated who collected data and whether or not they were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 431 women agreed to participate, but only 14% were followed up. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol, so could not assess this. |
| Other bias | Unclear risk | Quote: "The expectant mothers and fathers who were assigned randomly to the 2 study groups were demographically similar." Baseline characteristics tables were presented. |
Wrenn 1997.
| Methods | 2‐arm quasi‐RCT (even numbers to intervention and odd numbers to control group), single‐site, recruitment April 1999‐February 2000, n = 186, with 79 assigned to the intervention and 107 to the control group | |
| Participants | Urban USA ‐ military hospital in Texas Background rates of breastfeeding initiation: intermediate. Baseline breastfeeding rate in Texas at hospital discharge = 67% in 1999. Inclusion criteria: mothers on postpartum ward of study hospital; aged > 18 years, primiparous, uncomplicated delivery and postpartum, healthy baby, mother planned to breastfeed for at least 6 weeks Exclusion criteria: hospitalisation of mother or baby for > 4 days; mothers who did not speak English Ethnic composition of sample: 63% white, 11% black, 20% Hispanic, 2% Asian, 3% other All participants were members of the armed forces or their dependents. |
|
| Interventions | Intervention: breastfeeding support in hospital visit lasting approximately 30 min, home visit 2‐4 days after discharge lasting 45‐60 min, and phone call 10‐14 days after the home visit Control: standard care (not described) |
|
| Outcomes | Breastfeeding attrition to 6 weeks | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Allocation by odd and even numbers in groups of 10 |
| Allocation concealment (selection bias) | High risk | Could be anticipated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The person delivering the intervention would have been aware of allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The person delivering the intervention seems to have collected outcome data. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Information on drop‐outs incomplete and loss to follow‐up not balanced across groups. 79 in intervention group, 5 were lost to follow‐up, data at 6 weeks from 68. Outcome data were not obtained from 32 women in the control group at 6 weeks so more women were enrolled (107 enrolled to this group). Some breastfeeding duration data were obtained from drop‐outs by phone. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol, so could not assess this. |
| Other bias | Unclear risk | Replacing women lost to follow‐up in the control group means that this study is at high risk of bias. |
Wu 2014.
| Methods | 2‐arm quasi‐RCT, n = 74 | |
| Participants | Participants were recruited from the maternity department of a tertiary hospital in a major city of central China, Wuhan Background rates of breastfeeding initiation: not reported for Wuhan but 95.6% in Shanghai. In Whuhan 67% of mothers have stopped breastfeeding by 4‐6 months. Inclusion criteria: ≥ 18 years of age, able to read and understand Mandarin, new mother with a single, healthy term infant, and intending to breastfeed Exclusion criteria: any condition that would interfere with breastfeeding, such as a serious illness, mental illness, or an infant requiring special care that could not be discharged with the mother |
|
| Interventions | Intervention (n = 37): self‐efficacy intervention.Women received three sessions post‐partum: one within 1 day of delivery, 1 the next day and third 1 week after discharge. The sessions involved assessment of breastfeeding goals and self‐efficacy, self‐efficacy‐enhancing strategies, and evaluation. Assessment enabled individualization of the intervention to meet the woman's needs. The self‐efficacy strategies were informed by the WHO breastfeeding counselling course. At the end of each session women completed an evaluation form which was used to identify any changes needed and plan the following session. Control (n = 37): standard care that included in‐hospital care and follow‐up by a community nurse after discharge |
|
| Outcomes | Breastfeeding self‐efficacy Breastfeeding duration and exclusivity at 4 and 8 weeks postpartum |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient detail provided "a quasi‐random, point‐of‐reference sample of participants". |
| Allocation concealment (selection bias) | Unclear risk | No details provided to enable judgement of this. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details provided to enable judgement of this. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details provided to enable judgement of this. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Follow‐up in intervention group was 89% and 92% in the control group. |
| Selective reporting (reporting bias) | Unclear risk | No evidence of documentation of prespecified outcomes available to enable judgement of this. |
| Other bias | Unclear risk | Authors noted a potential risk of social desirability bias, as the intervention was delivered by the first author. |
Yotebieng 2015.
| Methods | 3‐arm cluster‐controlled trial, n = 975 | |
| Participants | Health‐care clinics in Kinshasa, DR Congo Background rates of breastfeeding initiation: near‐universal initiation of breastfeeding 90% breastfeeding at age 1 year; 69% of babies aged 0–1 month and 35% of those aged 2–3 months (about 10–14 weeks) were exclusively breastfed. Inclusion criteria: all mothers who gave birth to 1 healthy child in 1 of the participating facilities between 24 May‐25 August 2012 and who intended to attend well‐baby clinic visits in the same facility Exclusion criteria: intended to attend well‐baby clinic visits in a different health facility, or to travel before the child was aged at least 6 months |
|
| Interventions | Intervention 1 (n = 363): Baby Friendly Hospital Intiative (BFHI) steps 1‐9; healthcare staff from antenatal and maternity care (i.e. delivery rooms and postpartum wards) in the intervention facilities were trained using the WHO/UNICEF course. Session 14 of the training on 'Ongoing support for mothers' was limited only to 'Describe how to prepare a mother for discharge'. Session 15 on 'Making your hospital baby friendly' was not covered. Additional material in French developed as part of a different project was distributed to staff in clinics. Implementation of steps 1–9 was assessed at the end of the study using the hospital self‐appraisal questionnaire and each of the clinics randomized to intervention groups met at least 80% of the global criteria for each step. Intervention 2 (n = 308): BFHI steps 1‐10; staff training as for Intervention 1 and staff from well‐child clinics also received the same training. Flyers distributed to mothers before discharge from the postpartum ward and during well‐child clinic visits. These were developed locally and contained culturally appropriate messages addressing behaviours that had been identified as the main contributors to suboptimum breastfeeding practices (such as giving the baby water in the first 6 months of life) in a pretrial survey. These were published in 2 languages (French and local language). Additional material in French that had been developed as part of a different project was distributed to staff in clinics Implementation of steps 1–10 was assessed at the end of the study using the hospital self‐appraisal questionnaire and each of the clinics randomized to intervention groups met at least 80% of the global criteria for each step. Control (n = 304): standard care |
|
| Outcomes | Primary: Breastfeeding initiation within 1 h Exclusive breastfeeding at 14 and 24 weeks Secondary: Prevalence of infants with reported diarrhoea between 10‐14 weeks postpartum and 18‐24 weeks postpartum Prevalence of infants with respiratory illness between 10‐14 weeks postpartum and 18‐24 weeks postpartum |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The 3 pairs of facilities were ranked alphabetically and a computer was used to generate 3 random numbers |
| Allocation concealment (selection bias) | Low risk | The randomisation was done by the study statisticians who had no involvement in enrolment or follow‐up of participants |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Staff in participating clinics could not be masked to the interventions to group assignments because of the nature of the interventions". Mothers were masked to group assignment and this worked "quite well". |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Attempts were made to blind interviewers but this "did not work so well". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 12% of total participants randomized lost to follow‐up by 24 weeks postpartum. |
| Selective reporting (reporting bias) | Low risk | Breasteeding outcomes were prespecified in study protocol. |
| Other bias | Low risk | None identified |
Abbreviations
AIDS: acquired immunodeficency syndrome BFI: Baby Friendly Initiative (UNICEF) BFHI:Baby Friendly Hospital Initiative BINGO:Best Infant Nutrition for Good OutcomesBMI: body mass index CS: caesarean section d: day(s) EP: electronic prompt FAB: Food, physical activity and breastfeeding FBSICG: Facility‐based semi‐intensive counselling group GP: general practitioner h: hour(s) HBICG: Home‐based semi‐intensive counselling group HIV: human immunodeficiency virus HV: Health Visitor ICC: intra‐cluster correlation coefficient ICU: intensive care unit ITT: intention‐to‐treat analysis LBW: low birth weigh LC: lactation consultant LHW: lady health worker MB training: maternal breastfeeding training MCH: Maternal and Child Health MCHN: Maternal and Child Health Nurse min: minute(s) NICU: neonatal intensive care unit PAIRINGS: Provider Approaches to Improved Rates of Infant Nutrition & Growth StudyPC: Primary Care PCT: primary care trust RCT: randomized controlled trial RG: Registrar General SCBU: special care baby unit SD: standard deviation SPSS: Statistical Package for the Social Sciences TENS: transcutaneous electrical nerve stimulation vs: versus WHO: World Health Organization WIC: Special Supplemental Nutrition Programme for Women, Infants and Children (US Department of Agriculture, Food and Nutrition Service) UNICEF: the United Nations Children's Fund
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| ACTRN12614000605695 | Intervention targeted at fathers only. |
| ACTRN12615000063516 | Study of breastfeeding promotion not breastfeeding support. |
| Agrasada 2005 | Low birthweight infants. Under consideration for review of support for mothers breastfeeding infants who are not term and healthy. |
| Ahmed 2008 | Premature infants. Under consideration for review of support for mothers breastfeeding infants who are not term and healthy. |
| Ahmed 2016 | Web‐based intervention |
| Ball 2011 | The intervention examined in this trial was not a breastfeeding support intervention. The trial examined the use of a baby cot that was clamped onto the side of the mother's bed so that the baby was within easy reach of the mother at all times. |
| Baqui 2008 | Intervention was given antenatally and postnatally by community health workers giving home visits to promote newborn health and. Comparison was group sessions with same aim. This study was assessing general health and clinical outcomes, not breastfeeding support. |
| Barlow 2006 | Educational intervention not intended to facilitate continued breastfeeding. |
| Barnet 2002 | Intervention did not have the purpose of facilitating continued breastfeeding. |
| Beiler 2011 | Not a breastfeeding support intervention. |
| Benitez 1992 | Intervention was educational, not support. |
| Bica 2014 | Study comparing mothers who live with their mothers and mothers who do not. |
| Black 2001 | Intervention did not have the purpose of facilitating continued breastfeeding. |
| Blixt 2014 | Not a trial. |
| Bolam 1998 | Evaluated an educational intervention. |
| Brown 2008 | Low birthweight infants. Under consideration for review of support for mothers breastfeeding infants who are not term and healthy. |
| Byas 2011 | Intervention targeted at fathers. |
| Carlsen 2013 | Specifically focused on overweight or obese women so does not meet healthy women inclusion criteria. |
| Cattaneo 2001 | Intervention was staff training, and participants were hospitals. |
| Caulfield 1998 | Not a randomized controlled trial (see Dyson et al). |
| Chapman 2011 | This study specifically focused on women with obesity. The study will be considered for inclusion in a proposed review on breastfeeding support for women at high risk of health problems that affect breastfeeding. |
| Christie 2011 | Not a breastfeeding support intervention. |
| Davies‐Adetugbo 1996 | Controlled study of breastfeeding counselling intervention without randomisation. |
| Davies‐Adetugbo 1997 | Infants with diarrhoea. Under consideration for review of support for mothers breastfeeding infants who are not term and healthy. |
| Davis 2014 | Participants were students nurses. Outcomes were their knowledge and attitudes. |
| Ebbeling 2007 | Not a randomized controlled trial. |
| Edwards 2013a | Delivered by a computer agent (not health professional or lay worker) and appeared to give educational advice, not support. |
| Ehrlich 2014 | Not a breastfeeding support intervention. |
| Eneroth 2007 | Not a study of a breastfeeding support intervention. |
| Ferrara 2008 | Participants were women with gestational diabetes. Under consideration for review of support for mothers with conditions affecting/affected by breastfeeding. |
| Finch 2002 | Evaluated an antenatal educational and marketing intervention. Both groups received postnatal breastfeeding support. Under consideration for the review Interventions for promoting the initiation of breastfeeding (Dyson et al). |
| Finch 2015 | Not focused on breastfeeding support. |
| Flax 2014 | Educational intervention. |
| Forster 2004 | Evaluated an educational intervention. |
| Forster 2006 | Antenatal intervention with no postnatal component. |
| Gagnon 1997 | Intervention not relevant for this review. The intervention was an alternative to standard care. The intervention was not aimed at facilitating breastfeeding, rather the trial compared women who were randomized to early hospital discharge with telephone follow‐up (with home visits by nurses only for those women who left hospital within 36 h of the birth “to encourage them to leave the hospital early”) versus usual care with later discharge from hospital. It was not clear that the intervention included any breastfeeding support. Although outcomes included breastfeeding the main focus was on “maternal competence” and infant outcomes. 44% post‐randomisation exclusions. |
| Garcia‐Montrone 1996 | Educational intervention. Controls were matched, but not randomized. |
| Giglia 2015 | Web‐based programme. Not over the phone or face‐to‐face. |
| Gijsbers 2006 | This study focused on families with a history of asthma. |
| Girish 2013 | Not breastfeeding support. |
| Guise 2003 | A review |
| Haider 1996 | Infants with diarrhoea. Under consideration for review of support for mothers breastfeeding infants who are not term and healthy. |
| Haider 2014 | Non‐randomised selected study participants. |
| Hall 2007 | Not an RCT (groups not concurrent). Substudy asking open‐ended questions. |
| Hanafi 2014 | Intervention was education rather than support, and only measured breastfeeding initiation and attitudes towards and knowledge of breastfeeding. No measures of sustained breastfeeding. |
| Harari 2014 | Texting intervention. Not face‐to‐face or over the phone. |
| Hauck 1994 | Intervention was a booklet and did not involve contact with an individual. |
| Henderson 2001 | Evaluated an educational intervention. |
| Hives‐Wood 2013 | News article |
| Hoddinott 2012a | This was a secondary report on feasibility, acceptability and fidelity of the intervention within the RCT. No breastfeeding data reported. |
| Ijumba 2015 | Study participants included HIV‐positive women. |
| Israel‐Ballard 2014 | Study participants were breastfeeding women. |
| Isselmann 2006 | Educational intervention which did not have the purpose of facilitating continued breastfeeding. |
| Jahan 2014 | Antenatal educational intervention only with no postnatal component. |
| Jakobsen 2008 | Educational intervention |
| Jang 2008 | Not an RCT (groups not concurrent). |
| Johnston 2001 | This study examined an intervention carried out in the antenatal period. |
| Jones 2004 | Evaluated an education intervention. In this study women were offered specialist lactation advice by the researcher regarding returning to work and milk expression. This was a 1‐h evidence‐based session and was reinforced with a written leaflet. Results were reported for those women still breastfeeding on their return to work. |
| Junior 2007 | Very low birthweight infants. Under consideration for review of support for mothers breastfeeding infants who are not term and healthy. |
| Katepa‐Bwalya 2011 | Trial about using materials (counselling cards) as part of an antenatal counselling session. HIV‐positive women. |
| Kistin 1994 | Non‐randomised observational study. |
| Kronborg 2012 | Antenatal education programme with no postnatal component. |
| Labarere 2003 | Evaluated an educational intervention. |
| Labarere 2011 | Educational intervention delivered via CD‐ROM. |
| Lavender 2004 | Evaluated an educational intervention. |
| Lewin 2005 | A review |
| Lieu 2000 | Support was not supplementary to standard care. |
| Louzada 2012 | Not a breastfeeding support intervention. |
| MacArthur 2002 | Intervention was not breastfeeding support. No breastfeeding outcomes reported. |
| MacArthur 2009 | Antenatal intervention with no postnatal component. |
| Mannan 2008 | All the women in this study received the breastfeeding intervention and were analysed on the basis of intervention intensity and not on the basis of comparator versus intervention. |
| Martin 2015 | Study with overweight and obese mothers (thus not healthy). |
| Martin‐Iglesias 2011 | Study was of a healthcare professional education intervention. |
| Mattar 2003 | Evaluated an educational intervention. |
| Maycock 2013 | Intervention aimed at fathers. |
| Maycock 2015 | Ongoing trial of an educational intervention. |
| McInnes 2000 | Geographical controls |
| McLeod 2003 | This study specifically focused on smoking and the aim of the support intervention was to encourage women to quit or reduce smoking in pregnancy, although breastfeeding outcomes were reported. The study will be considered for inclusion in a proposed review on breastfeeding support for women at high risk of health problems that affect breastfeeding. |
| Merewood 2006 | Infants in neonatal intensive care. Under consideration for review of support for mothers breastfeeding infants who are not term and healthy. |
| Mesters 2013 | Participants had a family history of asthma so may not meet inclusion criteria as 'healthy'. |
| Moore 1985 | Participants in this study may not have been healthy mothers. We excluded this study as it focused on parents with eczema or asthma and was examining the impact of a breastfeeding intervention on the occurrence of these diseases in babies. |
| Moreno‐Manzanares 1997 | Correspondence with author established study was controlled, but not randomized. |
| Nasehi 2012 | Early breastfeeding initiation was the intervention rather than an outcome. This study aimed to assess the effect of early breastfeeding initiation on exclusive breastfeeding duration. |
| Nekavand 2014 | Trial of an educational intervention. |
| Neyzi 1991 | It was not clear whether this was a randomized trial. Only 66% follow‐up in intervention group. |
| Nguyen 2014 | Did not report a trial |
| Nkonki 2014 | Economic evaluation ‐ part of PROMISE‐EBF trial. |
| Noel‐Weiss 2006 | Antenatal intervention with no postnatal component. |
| Nor 2009 | Not an RCT. Qualitative study looking at women’s views of peer counselling. |
| Nor 2012 | Qualitative study (mothers' experiences) embedded within cluster‐RCT (Nkonki 2014). |
| Ochola 2013a | Abstract only ‐ qualitative aspects of cluster‐RCT above in (Nkonki 2014). |
| Olenick 2011 | The intervention took place before the birth; there was no postnatal component. |
| Otsuka 2012 | The intervention took place before the birth; there was no postnatal component. |
| Otsuka 2014 | Intervention was primarily educational. |
| Pascali‐Bonaro 2004 | Paper was not about a trial. |
| Paul 2011 | Not breastfeeding support. |
| Penfold 2014 | The follow‐up was only 3 days, so the outcomes did not meet the inclusion criteria. |
| Perez‐Blasco 2013 | Not breastfeeding support. |
| Perez‐Escamilla 1992 | Study controlled, but not randomized. |
| Peterson 2002 | Both groups received WIC breastfeeding education. The intervention group received social support for maternal diet, activity and weight loss outcomes. |
| Phillips 2010 | This study only recruited women whose babies were admitted to neonatal intensive care. Breastfeeding support for mothers of poorly babies will be considered in a separate review. |
| Phillips 2011 | Intervention about smoking cessation and both groups got breastfeeding support. |
| Phillips 2012 | Babies in neonatal intensive care unit. |
| Pinelli 2001 | Very low birthweight infants. Under consideration for review of support for mothers breastfeeding infants who are not term and healthy. |
| Pollard 1998 | Self monitoring, not support. |
| Pollard 2011 | This study did not examine a breastfeeding support intervention by professionals or peers. The intervention group completed daily feeding logs recording breastfeeding practices. |
| Pound 2015 | Hospitalised jaundiced infants. |
| Rasmussen 2010 | Did not report a study. |
| Rasmussen 2011 | This study specifically focused on women with obesity. The study will be considered for inclusion in a proposed review on breastfeeding support for women at high risk of health problems that affect breastfeeding. |
| Ratner 1999 | Intervention did not have the purpose of facilitating continued breastfeeding. |
| Rea 1999 | Training intervention with no data on breastfeeding women. |
| Reeve 2004 | Evaluated an antenatal education intervention. |
| Rojjanasrirat 1987 | Study changed methodology part way through. |
| Rossiter 1994 | Educational intervention |
| Rowe 1990 | Abstract only available. No information on intervention used. |
| Rush 1991 | Trial of hospital telephone help‐line. The intervention was an invitation to call a general telephone support line in the postnatal period; the help‐line was available to women in the control group, but this new service development was not promoted with this group. The aim of the study was to examine the uptake of this service (i.e. reasons for and number of calls to the help‐line and to other hospital departments from control and intervention women). The intervention was general and was not specifically to encourage breastfeeding; breastfeeding and breastfeeding duration were not measured. |
| Sakha 2008 | All the participants had given birth by caesarean section. Both groups received an educational intervention. 1 group received a drug to promote lactation. |
| Sakkaki 2013 | Study participants were all women who had received caesarean section. |
| Schlomer 1999 | Assessing effectiveness of breastfeeding assessment tools (LATCH or Infant Breastfeeding Assessment Tool (IBFAT)), and their correlation with scales of breastfeeding problems (Maternal Breastfeeding Evaluation Scale (MBFES) and Potential Early Breastfeeding Problem Tool (PEBPT)) not supporting breastfeeding. |
| Schy 1996 | Evaluated a purely educational intervention. |
| Sciacca 1995 | Support intervention available to all women in the trial. |
| Segura‐Millan 1994 | Study controlled, but not randomized. |
| Serrano 2010 | The intervention examined in this study was baby massage and not breastfeeding support. Breastfeeding was reported as a secondary outcome. |
| Sisk 2006 | Not an RCT. |
| Steel O'Connor 2003 | Support was not supplementary to standard care. |
| Stuebe 2016 | Women had gestational diabetes. |
| Susin 2008 | Participants were not randomized. |
| Svensson 2013 | The intervention was focused on skin‐to‐skin positioning for babies with latch problems. Breastfeeding counselling was given to both randomized groups. |
| Szucs 2015 | Monitoring system, breastfeeding outcomes |
| Talukder 2012 | After 6 months of intervention, an endline survey was conducted on a different sample of mothers from those assessed at baseline. |
| Talukder 2016 | Trial of an educational intervention. |
| Thakur 2012 | Based on low birthweight babies ‐ recruited postnatally. |
| Thomson 2009 | Both groups received support as part of standard care. The intervention was not breastfeeding support. |
| Thussanasupap 2006 | Educational intervention. Not an RCT (assignment was 30 then another 30). |
| Tohotoa 2012 | The intervention targeted fathers' anxieties. Though breastfeeding was an aim of the trial, the recruitment and eligibility was based solely on fathers' characteristics, so there is no way of knowing whether women and babies were healthy. |
| Tully 2012 | Intervention was a side cot attached to bed, not directly a breastfeeding intervention. |
| Valdes 2000 | Study was controlled, but not randomized. |
| Vianna 2011 | The intervention consisted of music therapy rather than breastfeeding support; women and their premature babies (< 1750 g) were included in the trial. |
| Vitolo 2012 | A study of dietary counselling in reducing the intake of energy‐dense foods by infants. |
| Vitolo 2014 | The intervention involved training health professionals about healthy feeding practices, including breastfeeding. Women were approached directly for outcome measurement only ‐ what support if any they received from health staff was unclear, but we only have the abstract. |
| Wallace 2006 | This study was excluded as it examined a brief educational intervention by midwives advising mothers on the correct positioning of the baby for breastfeeding. |
| Wan 2011 | This study was excluded as it compared two models of nursing care (continuous versus task orientated) and was not a study of breastfeeding support interventions.. |
| Wasser 2015 | Proposal for a trial. |
| Westphal 1995 | Intervention was training, and participants were hospitals. |
| Wiggins 2005 | Evaluated a social support intervention. |
| Williams 2014 | Not breastfeeding support intervention. |
| Wockel 2009 | This study examined an intervention aimed at fathers which was offered as part of antenatal childbirth preparation classes. There was no postnatal component to the intervention. |
Abbreviations
h: hour(s) RCT: randomised controlled trial WIC: Special Supplemental Nutrition Programme for Women, Infants and Children (US Department of Agriculture, Food and Nutrition Service)
Characteristics of studies awaiting assessment [ordered by study ID]
Babakazo 2015.
| Methods | 2‐arm cluster‐RCT, n = 422 |
| Participants | Kinshasa, Democratic Repbulic of the Congo Background rates of breastfeeding initiation: 52.4% No details about inclusion and exclusion criteria available in English abstract. |
| Interventions | Intervention: training of healthcare providers through the Baby Friendly Hospital Initative using the "20 hour course for Maternity Staff". Total number randomised: details not provided in English abstract Control: details not provided in English abstract |
| Outcomes | Exclusive breastfeeding at 6 months Median duration of breastfeeding |
| Notes | Needs to be translated from French |
Bahri 2013.
| Methods | 3‐arm, parallel RCT, n = 90 |
| Participants | Health centres in Gonbad, Iran Background rates of breastfeeding imitation: > 90% Inclusion criteria: pregnant women. No further details provided in English abstract. Exclusion criteria: no details provided in English abstract. |
| Interventions | Intervention 1 (n = 30): 3‐h workshop on breastfeeding training Intervention 2 (n = 30): booklet about breastfeeding provided. Control (n = 30): no special training on breastfeeding |
| Outcomes | Knowledge about breastfeeding Health beliefs about postpartum breastfeeding Breastfeeding behaviour in first 24 h after delivery |
| Notes | Needs to be translated from Arabic |
Cabezas 2014.
| Methods | 2‐arm, parallel RCT, n = 220 |
| Participants | Women receiving care from the Sexual and Reproductive Health Centre in Barcelona, Spain Background rates of breastfeeding imitation: 77% Inclusion criteria: low‐risk pregnancy and being cared for in the Sexual and Reproductive Health Centre Exclusion criteria: not specified |
| Interventions | Intervention: usual care plus telephone support from a community midwife. Control: usual care Total number randomised: not specified for either group. |
| Outcomes | Frequency of difficulties that women experienced breastfeeding Satisfaction with telephone support |
| Notes | Conference abstract. Unable to locate authors. |
Kamau‐Mbuthia 2013.
| Methods | 3‐arm, parallel RCT, n = 505 |
| Participants | Low income women attending for antenatal care at a large hospital in Kenya Background rates of breastfeeding imitation: 56.1% No details about inclusion and exclusion criteria provided. |
| Interventions | Intervention 1: continuous cell phone‐based peer support (CPS); support was provided by trained peer support leaders from late pregnancy (32‐36 weeks) until 3 months postpartum Intervention 2: monthly peer support group (PSG). Support was provided by trained peer support leaders from late pregnancy (32‐36 weeks) until 3 months postpartum. Control: standard care by existing facility‐based support No details provided about the total number randomised in each group. |
| Outcomes | Exclusive breastfeeding at 3 months |
| Notes | Conference abstract only. SM contacted authors for more information 20 July 2016. |
Li 2014.
| Methods | 2‐arm, cluster randomised trial, n = 308 |
| Participants | Women attending community health clinics in Shanghai at 11‐22 weeks gestation Background rates of breastfeeding imitation: 41.0% No details provided in abstract about inclusion/exclusion criteria. |
| Interventions | Intervention: weekly SMS messages from 28 weeks gestation until the children were 1 year old. ‘Message bank’ development was based on literature review and in‐depth interviews/focus group discussion with pregnant women, new mothers and healthcare providers. Control: no details provided in abstract No details provided about the total number randomised in each group. |
| Outcomes | Exclusive breastfeeding at 4 months |
| Notes | Conference abstract only. SM contacted authors for more information 21 July 2016. |
Mortazavi 2014.
| Methods | 2‐arm RCT, n = 186 |
| Participants | Women attending health centres in Sabzevar, Iran Background rates of exclusive breastfeeding at 6 months: 53.1% Inclusion criteria: wanted pregnancy and primigravidity Exclusion criteria: no details provided in English abstract |
| Interventions | Intervention: husbands attended prenatal care Control: women attended prenatal care alone No details provided about total number randomised in each group |
| Outcomes | Satisfaction of husband involvement Husband taking care of baby in absence of mother Husband's support of breastfeeding |
| Notes | Needs to be translated from Arabic. |
Raisi 2012.
| Methods | 2‐arm., parallel RCT, n = 140 |
| Participants | Primiparous women attending the selected health centres of Tehran University of Medical Sciences, Iran Background rates of breastfeeding imitation: > 90% Details of inclusion/exclusion criteria not provided in English abstract. |
| Interventions | Intervention (n = 70): telephone counselling on breastfeeding provided by one of the researchers Control (n = 70): routine care |
| Outcomes | Exclusive breastfeeding at 1 and 3 months Duration of continuity and exclusitivity of breastfeeding |
| Notes | Needs to be translated from Arabic |
Reeder 2014.
| Methods | 3‐arm, parallel RCT, n = 1948 |
| Participants | English‐ or Spanish‐speaking recipients of the Supplemental Nutrition Program for Women, Infants, and Children (WIC) in Oregon, USA Background breastfeeding imitation rates: 90% Inclusion criteria: English‐ and Spanish‐speaking women attending a new pregnancy appointment for the Supplemental Nutrition Program for Women, Infants, and Children (WIC) programme between July 2005 and July 2007; intending to breastfeed or undecided about breastfeeding Exclusion criteria: no exclusions on the basis of age, multiple gestations, known risk factors or previous birth history |
| Interventions | Intervention 1 (n = 646): low frequency peer counselling; women received 4 planned, peer‐initiated contacts: the first after initial prenatal assignment, the second 2 weeks before the expected due date, and the third and fourth at 1 and 2 weeks postpartum Intervention 2: (n = 645): high frequency peer counselling; women received 8 scheduled calls. The first 4 calls were the same as those in the low‐frequency treatment group and the last 4 calls were scheduled at months 1, 2, 3, and 4. |
| Outcomes | Breastfeeding imitation Exclusive breastfeeding at 1, 3 and 6 months Any breastfeeding at 1, 3 and 6 months |
| Notes | SM contacted authors for data 21 July 2016. |
Taylor 2014.
| Methods | 4‐arm RCT, n = 802 |
| Participants | Multiparous and primiparous women recruited during pregnancy. No other details provided about participants or study setting. |
| Interventions | Intervention 1: 'Sleep'; education sessions antenatally and at 3 weeks targeting the prevention of sleep problems, followed by an intervention from 6 months postpartum targeting the treatment of sleep problems Intervention 2: 'FAB'; provision of a LC to promote breastfeeding to 6 months, and education sessions at 3, 5, 7, 9, 12 and 18 months targeting healthy eating, sedentary time and active play for families Intervention 3: 'Combo'; Sleep and FAB interventions Control: standard Well Child Care (note all groups received this) No details provided in abstract about numbers randomised to each group. |
| Outcomes | BMI (at 2 years) Levels of physical activity Infant feeding Sleep Dietry intake |
| Notes | Conference abstract only. SM contacted authors for more information 21 July 2016. |
Whalen 2011.
| Methods | 2‐arm RCT, n = 206 |
| Participants | Mother‐baby dyads attending a 2‐week well‐baby visit. Study mothers were mainly white, non‐Hispanic, highly educated, married, of higher socioeconomic status, planned to return to work or school after their baby’s birth, and reported good to excellent baseline confidence in breastfeeding. No details provided about inclusion/exclusion criteria. |
| Interventions | Intervention (n = 100): online breastfeeding tutorial and maternal needs assessment administered at 2‐week, 2‐, 4‐, and 6‐month well‐baby visits with provider counselling targeted to the mother’s needs Control (n = 106): usual care |
| Outcomes | Exclusive breastfeeding at 2 months Exclusive breastfeeding at 2 months Any breastfeeding at 2 months |
| Notes | Conference abstract only. SM contacted authors for further details 21 July 2016. |
Abbreviations
BMI: body mass index FAB: Food, physical activity and breastfeeding LC: lactation consultant RCT: randomised controlled trial WIC: Special Supplemental Nutrition Programme for Women, Infants and Children (US Department of Agriculture, Food and Nutrition Service)
Characteristics of ongoing studies [ordered by study ID]
Forster 2014.
| Trial name or title | Ringing Up about Breastfeeding: a randomised controlled trial exploring earlY telephone peer support for breastfeeding (RUBY) – trial protocol |
| Methods | 2‐arm RCT |
| Participants | All eligible women having a baby at the Women’s, Monash Medical Centre and Sunshine Hospital during the recruitment period will be offered participation. Women attending these hospitals, although from a wide range of backgrounds, tend to be relatively disadvantaged, with low income and of culturally diverse backgrounds (even among those women who do speak English). Inclusion criteria: women admitted to the postnatal wards as public patients who have had a first live birth; are proficient in English; and breastfeeding or intending to breastfeed Exclusion criteria: serious illness (e.g. severe pre‐eclampsia/eclampsia, significant postpartum haemorrhage, severe psychiatric disturbance, pulmonary embolus); infant remaining in hospital after the mother’s postnatal discharge; multiple birth; mother has chosen to formula feed; or antenatal membership of the Australian Breastfeeding Association (ABA), as this may be associated with a higher breastfeeding intention |
| Interventions | Intervention: proactive peer support will be provided by telephone Control: usual care; all women recruited to the trial will receive usual hospital postnatal care and infant feeding support. The usual length of hospital stay postpartum is 2 nights following a vaginal birth and 3 for caesarean births. All women are eligible for 1 or more home visits by a hospital midwife in the early postnatal period as well as ongoing support from their local Maternal and Child Health (MCH) nurse. |
| Outcomes | Primary: the proportion of infants who are breastfed for at least 6 months |
| Starting date | Unclear |
| Contact information | Australian and New Zealand Clinical Trials Registry ACTRN12612001024831 |
| Notes |
Karanja 2012.
| Trial name or title | A community‐based intervention to prevent obesity beginning at birth among American Indian children: study design and rationale for the PTOTS study |
| Methods | A cluster‐RCT |
| Participants | A birth cohort of 577 children (infants and toddlers aged 0‐2 years) from 5 American Indian tribes randomised by tribe to either the intervention (3 tribes) or the comparison condition (control; 2 tribes) |
| Interventions | Intervention: includes nutrition and physical activity goals, and consists of a community‐wide component coupled with an individualised family‐counselling
component to improve nutrition and physical activity in infants and toddlers. The nutrition goals are presented in 4 modules: 1) breastfeeding, 2) curtailment of sugar sweetened beverage consumption, 3) introduction of healthy solid foods, and 4) parental management of feeding behaviours. Control: parents and guardians in the control tribes consent to provide study data for their children. Nondiagnostic dental screenings are offered to children aged 1–5 years as a service to these comparison communities. |
| Outcomes | Breastfeeding initiation and duration rates |
| Starting date | Unclear |
| Contact information | N Karanja Center for Health Research, Kaiser Permanente– Northwest/Hawaii/Southeast, 3800 N. Interstate Avenue, Portland, OR 97227, USA e‐mail: Njeri.Karanja@kpchr.org |
| Notes |
Kikuchi 2015.
| Trial name or title | Ghana’s Ensure Mothers and Babies Regular Access to Care (EMBRACE) programme: study protocol for a cluster‐randomised controlled trial |
| Methods | Cluster‐RCT using an effectiveness‐implementation hybrid design in Dodowa, Kintampo, and Navrongo, Ghana |
| Participants | The study population is women of reproductive age between the ages of 15 and 49 years. |
| Interventions | The provision of an intervention package to women living in randomly allocated intervention clusters. The package includes: 1) use of a new continuum of care card, 2) continuum of care orientation for health workers, 3) 24‐h health facility retention of mothers and newborns after delivery, and 4) postnatal care by home visits. |
| Outcomes | Maternal, newborn, and child health outcomes for both intervention and implementation impacts. Intervention outcomes: continuum of care completion rate, rate of postnatal care within 48 h, complication rate requiring mothers' and newborns' hospitalisations, and perinatal and neonatal mortality Implementation outcomes: intervention coverage of the target population, intervention adoption and fidelity, implementation cost, and sustainability |
| Starting date | Unclear |
| Contact information | Current Controlled Trials ISRCTN90618993. Registered on 3 September 2014. |
| Notes |
Kimani‐Murage 2013.
| Trial name or title | Effectiveness of personalised, home‐based nutritional counselling on infant feeding practices, morbidity and nutritional outcomes among infants in Nairobi slums: study protocol for a cluster‐randomised controlled trial |
| Methods | A cluster‐randomised study design, will be conducted in 2 slums in Nairobi, Korogocho and Viwandani, where 14 community units (defined by the Government’s healthcare system) will form the unit of randomisation. |
| Participants | A total of 780 pregnant women and their respective child will be recruited into the study. The mother‐child pair will be followed up until the child is 1 year old. Study participants will include all pregnant women aged 12‐49 years old, who are residents of CUs in Korogocho and Viwandani slums that fall within the Nairobi Urban Health and Demographic Surveillance System area, and their respective children (when born). These will be recruited during pregnancy on a rolling basis until the desired sample size is achieved. |
| Interventions | The mothers will receive regular, personalised, home‐based counselling by trained Community Health Workers on maternal, infant and young child nutrition. Regular assessment of knowledge, attitudes and practices will be done, coupled with assessments of nutritional status of the mother‐child pairs and diarrhoea morbidity for the children. |
| Outcomes | Primary: exclusive breastfeeding for 6 months |
| Starting date | September 2012 |
| Contact information | *Correspondence: ekimani@aphrc.org: African Population and Health Research Center (APHRC), PO 10787, 00100 Nairobi, Kenya |
| Notes |
Kimani‐Murage 2015.
| Trial name or title | Feasibility and effectiveness of the baby friendly community initiative in rural Kenya: study protocol for a randomised controlled trial |
| Methods | A formative study using participatory action research design will first be conducted, followed by a cluster‐randomised trial utilising both qualitative and quantitative data collection methods. 12 CUs will constitute the clusters to be included in the study. CUs are geographically defined units, mostly equal to a village and usually have a population size of approximately 5000 people. |
| Participants | This trial will include women of reproductive age (15–49 years) who are pregnant at the time of recruitment, and their respective children from the pregnancies aged less than 6 months in Koibatek sub‐county in Baringo county. |
| Interventions | The intervention will involve implementation of the BFCI in the intervention clusters. The proposed BFCI in Kenya is a multifaceted program for promotion of optimal breastfeeding and infant and young child nutrition, and other practices including maternal nutrition in the community. The BFCI is based on the principles of the BFHI, but extends them to the community in order to provide women with a comprehensive support system to improve breastfeeding practices and other maternal, infant and young child nutrition practices at the community level. The BFCI package (unpublished) adapted for implementation in Kenya involves an 8‐step plan. |
| Outcomes | Primary: proportion of children being exclusively breastfed for the first 6 months |
| Starting date | January 2015 |
| Contact information | ISRCTN03467700; Date of registration: 24 September 2014 |
| Notes |
Nabulsi 2014.
| Trial name or title | A complex breastfeeding promotion and support intervention in a developing country: study protocol for a randomised clinical trial |
| Methods | A randomised controlled single‐blind parallel‐arm clinical trial to investigate whether a complex intervention targeting new mothers’ breastfeeding knowledge, skills and social support within a Social Network and Social Support theory framework will increase exclusive breastfeeding duration among women in Lebanon. |
| Participants | Healthy pregnant women who are in their first or second trimester and who intend to breastfeed after delivery will be eligible to participate in this study. |
| Interventions | Intervention: women will receive, in addition to standard clinical care, a complex intervention starting in early pregnancy until 6 months postdelivery. The intervention is composed of the following elements: 1) prenatal breastfeeding education to raise knowledge and awareness, 2) postpartum professional lactation support to improve maternal skills and self‐efficacy, 3) postpartum peer (lay) support to build social support, and enhance social capital within women’s social networks. These include skill building activities for the provision of effective breastfeeding support. Control: women will receive standard prenatal and postnatal care that is usually offered to mothers at both study sites. |
| Outcomes | Primary: percentage difference in 6‐month breastfeeding exclusivity rates between the intervention and control groups |
| Starting date | Unclear |
| Contact information | Current Controlled Trials ISRCTN17875591 |
| Notes |
Nair 2015.
| Trial name or title | Participatory women’s groups and counselling through home visits to improve child growth in rural eastern India: protocol for a cluster‐randomised controlled trial |
| Methods | A cluster‐RCT in 2 rural districts of Jharkhand and Odisha (Eastern India) |
| Participants | The unit of randomisation is a purposively selected cluster of approximately 1000 population. A total of 120 geographical clusters covering an estimated population of 121,531 were randomised to 2 trial arms: 60 clusters in the intervention arm and 60 clusters in the control arm. The study participants are pregnant women identified in the third trimester of pregnancy and their children (n = 2520). |
| Interventions | Intervention: a community‐based worker carrying out 2 activities: 1) 1 home visit to all pregnant women in the third trimester, followed by subsequent monthly home visits to all infants aged 0–24 months to support appropriate feeding, infection control, and care‐giving; 2) a monthly women’s group meeting using participatory learning and action to catalyse individual and community action for maternal and child health and nutrition. Also receive an intervention to strengthen Village Health Sanitation and Nutrition Committees. Control: receive an intervention to strengthen Village Health Sanitation and Nutrition Committees only |
| Outcomes | Mothers and their children are followed up at 7 time points: during pregnancy, within 72 h of delivery, and at 3, 6, 9, 12 and 18 months after birth. Primary: children’s mean length‐for‐age Z scores at 18 months Secondary: wasting and underweight at all time points, birthweight, growth velocity, feeding, infection control, and care‐giving practices. Additional qualitative and quantitative data are collected for process and economic evaluations. |
| Starting date | July 2013 |
| Contact information | ISRCTN register 51505201; Clinical Trials Registry of India number 2014/06/004664 |
| Notes |
NCT01383070.
| Trial name or title | Evaluation of the effectiveness of cell phone technology as community based intervention to improve exclusive breastfeeding and reduce infant morbidity rates |
| Methods | Cluster‐randomised trial |
| Participants | Staff training to promote breastfeeding |
| Interventions | All the women in the trial will receive hospital maternity care at hospitals using BFHI (WHO/UNICEF Baby Friendly Hospital Initiative) training for staff. Intervention clusters: in addition to counselling in the hospitals during the scheduled antenatal visits, women will receive personalised lactation consultation and support via cell phone (handsets provided). Cell phone counselling will continue until 24 weeks after the birth. Control clusters: existing staff at the hospitals will be encouraged to set up their own systems to continue counselling of women during the antenatal period, at delivery and during immunisation visits. |
| Outcomes | Primary: exclusive breastfeeding at 24 weeks Secondary: Timely initiation of breastfeeding Timely initiation of complimentary feeding Duration of any breastfeeding Infant growth Hospital admissions/mortality for infants and mothers Maternal satisfaction Cost effectiveness |
| Starting date | August 2010 |
| Contact information | ceuiggmc@yahoo.co.in |
| Notes | Clinical Trials.gov accessed 14 December 2011 showed "This study is currently recruiting participants" with the verification date June 2011. |
Abbreviations
BFCI: Baby Friendly Community Initiative BFHI: Baby Friendly Hospital Initiative CU: Community Unit h: hour(s) MIYCN: Maternal and Young Child Nutrition RCT: randomised controlled trial UNICEF: the United Nations Children's Fund WHO: World Health Organization
Differences between protocol and review
In the original protocol, (1998), Odds ratios (ORs) with 95% confidence intervals (CIs) were specified as being the measure of treatment effect. However, in the first published version of the review, Sikorski 1999, and in all subsequent versions of the review, Risk Ratios (RRs) with 95% CIs have been presented. In the original protocol, the subgroups specified for investigation of heterogeneity were reported as being income group and both ante‐ and post‐natal time periods. Income group was removed from subsequent versions and with the addition of many more studies, the following subgroups have been incorporated:
By type of supporter (professional versus lay person, or both).
By type of support (face‐to‐face versus telephone support).
By timing of support (antenatal and postnatal versus postnatal alone) ‐ in original protocol.
By whether the support was proactive (scheduled contacts) or reactive (women needed to request support).
By background breastfeeding initiation rates (low, medium or high background rates).
By intensity of support (number of scheduled contacts.
No sensitivity analyses were specified in the original protocol, 1998. In subsequent versions of the review and in the current update, sensitivity analyses looking at the effect of allocation concealment by comparing results from studies at low risk of bias as opposed to unclear or high risk of bias were incorporated.
The methods section has been updated to the current standard methods for Cochrane Pregnancy and Childbirth reviews and the review now focuses on healthy mothers with healthy term infants. A 'Summary of findings' table has been incorporated for this 2016 update.
In this update, 2016, we have not included data for the following secondary outcomes. Primary outcomes were recorded for stopping any or exclusive breastfeeding before four to six weeks and before six months postpartum. Other outcomes of interest in previous versions of this review were stopping any or exclusive breastfeeding at other time points (two, three, four, nine and 12 months), measures of neonatal and infant morbidity (where available) and measures of maternal satisfaction with care or feeding method. Secondary outcomes were not considered in this update so that the review could be completed in time to inform the World Health Organisation’s review of the evidence and update of the WHO recommendations on breastfeeding in maternity facilities. A new set of core outcomes for Cochrane pregnancy and childbirth breastfeeding reviews is currently being developed and the outcomes from this core set may influence future outcomes chosen for this review.
Secondary outcomes included in last update, 2012:
Stopping breastfeeding before two, three, nine and 12 months postpartum.
Stopping exclusive breastfeeding before two, three, nine and 12 months postpartum.
Maternal satisfaction with care.
Maternal satisfaction with feeding method.
All‐cause infant or neonatal morbidity.
In this update, 2016, "healthy" in terms of types of participants has been more clearly defined: "Participants were healthy pregnant women considering or intending to breastfeed or healthy women who were breastfeeding healthy babies.Healthy women and babies were considered those who did not require additional medical care (e.g. women with diabetes, women with HIV/AIDs, overweight or obese women) or surgical care (e.g. women who required a Caesarean Section). Studies which focused specifically on women with additional care needs were excluded."
Contributions of authors
This update is based on the previous Cochrane Review, Renfrew 2012b, and has involved new authors.
Alison McFadden contributed to planning its restructure, assessment of study eligibility, data extraction and analysis and drafting text for the Background, Discussion and Conclusions, and commented on review drafts.
Anna Gavine contributed to planning its restructure, assessment of study eligibility, data extraction and analysis, drafting text for the Description of included studies, and commented on review drafts.
Mary Renfrew was co‐author of earlier versions of this review and lead author of the previous version. In this update of the review she contributed to planning its restructure, and drafting text for the Background, Discussion and Conclusions, and commented on review drafts.
Angela Wade provided statistical advice for this and all the earlier versions of this review. She advised about including cluster‐randomised trials in the analyses and commented on review drafts.
Phyll Buchanan contributed to assessment of study eligibility, data extraction, and commented on drafts.
Jane Taylor contributed to assessment of study eligibility, data extraction, and commented on drafts.
Emma Veitch contributed to assessment of study eligibility, data extraction, and commented on drafts.
Anne‐Marie Rennie contributed to assessment of study eligibility, data extraction, and commented on drafts.
Susan Crowther contributed to assessment of study eligibility, data extraction, and commented on drafts.
Sara Neiman contributed to assessment of study eligibility, data extraction, and commented on drafts.
Steve MacGillivray co‐ordinated this update and contributed to planning its restructure, assessment of study eligibility and data extraction. He set up conducted and reported the analyses, drafted text for the Methods and Results sections and commented on review drafts.
Sources of support
Internal sources
University of York, UK.
External sources
Cochrane Pregnancy and Childbirth received a grant from the Evidence and Programme Guidance Unit, Department of Nutrition for Health and Development, World Health Organization, Switzerland.
National Institute for Health Research Health Technology Assessment programme, grant number 10/106/01, UK.
Declarations of interest
Alison McFadden: nothing to declare.
Anna Gavine: my institution (University of Dundee) has received two small grants from WHO to support my contribution to this review.
Mary Renfrew: my institution (University of Dundee) received two small grants from WHO to help with completion of this review. This paid for some research support for data extraction and analysis.
Angela Wade: nothing to declare.
Phyll Buchanan: I am a trustee of the Breastfeeding Network and therefore interested in the effectiveness of support for breastfeeding mothers, especially peer support.
Jane Taylor: I am a volunteer in the Breastfeeding Network and therefore interested in the effectiveness of support for breastfeeding mothers.
Emma Veitch: I am a volunteer in the Breastfeeding Network and therefore interested in the effectiveness of support for breastfeeding mothers.
Sara Neiman: I am a volunteer in the Breastfeeding Network and therefore interested in the effectiveness of support for breastfeeding mothers. I work for the NHS as a Registered Midwife and therefore the evidence base is very important to me in my work.
Anne‐Marie Rennie: as a midwife I have an interest in support for breastfeeding mothers, and my role as Infant Feeding Co‐ordinator is also interested in support for breastfeeding mothers.
Susan Crowther: nothing to declare.
Steve MacGillivray: my institution (University of Dundee) has received two small grants from WHO to support my contribution to this review.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Abbass‐Dick 2015 {published data only}
- Abbass‐Dick J, Stern SB, Nelson LE, Watson W, Dennis CL. Coparenting breastfeeding support and exclusive breastfeeding: a randomized controlled trial. Pediatrics 2015;135(1):102‐10. [DOI] [PubMed] [Google Scholar]
Aidam 2005 {published data only}
- Aidam BA, Perez‐Escamilla R, Lartey A. Lactation counseling increases exclusive breastfeeding rates in Ghana. Journal of Nutrition 2005;135(7):1691‐5. [DOI] [PubMed] [Google Scholar]
Aksu 2011 {published data only}
- Aksu H, Kucuk M, Duzgun G. The effect of postnatal breastfeeding education/support offered at home 3 days after delivery on breastfeeding duration and knowledge: a randomized trial. Journal of Maternal‐Fetal and Neonatal Medicine 2011;24(2):354‐61. [DOI] [PubMed] [Google Scholar]
Albernaz 2003 {published data only}
- Albernaz E, Victora C. Impact of face‐to‐face counselling on duration of exclusive breastfeeding: a review. Pan American Journal of Public Health 2003;14(1):17‐24. [DOI] [PubMed] [Google Scholar]
- Albernaz E, Victora CG, Haisma H, Wright A, Coward WA. Lactation counseling increases breast‐feeding duration but not breast milk intake as measured by isotopic methods. Journal of Nutrition 2003;133(1):205‐10. [DOI] [PubMed] [Google Scholar]
Anderson 2005 {published data only}
- Anderson AK, Damio G, Chapman DJ, Perez‐Escamilla R. Differential response to an exclusive breastfeeding peer counseling intervention: the role of ethnicity. Journal of Human Lactation 2007;23(1):16‐23. [DOI] [PubMed] [Google Scholar]
- Anderson AK, Damio G, Young S, Chapman DJ, Perez‐Escamilla R. A randomised trial assessing the efficacy of peer counseling on exclusive breastfeeding in a predominantly Latina low‐income community. Archives of Pediatric and Adolescent Medicine 2005;159(9):836‐41. [DOI] [PubMed] [Google Scholar]
Barros 1994 {published data only}
- Barros FC, Halpern R, Victora CG, Teixera AM, Beria J. A randomised intervention study to increase breastfeeding prevalence in southern Brazil. Revista de Saude Publica 1994;28(4):277‐83. [DOI] [PubMed] [Google Scholar]
Bashour 2008 {published data only}
- Bashour HN, Kharouf MH, Abdulsalam AA, Asmar K, Tabbaa MA, Cheikha SA. Effect of postnatal home visits on maternal/infant outcomes in Syria: a randomized controlled trial. Public Health Nursing 2008;25(2):115‐25. [DOI] [PubMed] [Google Scholar]
Bhandari 2003 {published data only}
- Bhandari N. Promotion and consequences of exclusive breastfeeding in India [abstract]. Journal of Human Lactation 2007;23(1):75. [Google Scholar]
- Bhandari N, Bahl R, Mazumdar S, Martines J, Black RE, Bhan MK, et al. Effect of community‐based promotion of exclusive breastfeeding on diarrhoeal illness and growth: a cluster randomised controlled trial. Lancet 2003;361:1418‐23. [DOI] [PubMed] [Google Scholar]
- Bhandari N, Mazumder S, Bahl R, Martines J, Black RE, Bhan MK, et al. An educational intervention to promote appropriate complementary feeding practices and physical growth in infants and young children in rural Haryana, India. Journal of Nutrition 2004;134(9):2342‐8. [DOI] [PubMed] [Google Scholar]
- Bhandari N, Mazumder S, Bahl R, Martines J, Black RE, Bhan MK, et al. Use of multiple opportunities for improving feeding practices in under‐twos within child health programmes. Health Policy and Planning 2005;20(5):328‐36. [DOI] [PubMed] [Google Scholar]
Bloom 1982 {published data only}
- Bloom K, Goldbloom RB, Robinson SC, Stevens FE. II. Factors affecting the continuance of breast feeding. Acta Paediatrica Scandinavica 1982;71(Suppl 300):9‐14. [PubMed] [Google Scholar]
Bonuck 2005 {published data only}
- Bonuck KA, Freeman K, Trombley M. Randomized controlled trial of a prenatal and postnatal lactation consultant intervention on infant health care use. Archives of Pediatrics & Adolescent Medicine 2006;160(9):953‐60. [DOI] [PubMed] [Google Scholar]
- Bonuck KA, Trombley M, Freeman K, McKee D. Randomized, controlled trial of a prenatal and postnatal lactation consultant intervention on duration and intensity of breastfeeding up to 12 months. Pediatrics 2005;116(6):1413‐26. [DOI] [PubMed] [Google Scholar]
- Memmott MM, Bonuck KA. Mother's reactions to a skills‐based breastfeeding promotion intervention. Maternal & Child Nutrition 2006;2(1):40‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bonuck 2014a {published data only}
- Bonuck K, Stuebe A, Barnett J, Fletcher J, Bernstein P. Routine, primary‐care based interventions to increase breastfeeding: results of two randomized controlled trials. Breastfeeding Medicine 2013;8(Suppl 1):S‐19. [Google Scholar]
- Bonuck K, Stuebe A, Barnett J, Labbok MH, Fletcher J, Bernstein PS. Effect of primary care intervention on breastfeeding duration and intensity. American Journal of Public Health 2014;104 Suppl 1:S119‐S127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00619632. Boosting breastfeeding in low‐income, multi‐ethnic women: a primary care based RCT (BINGO). clinicaltrials.gov/show/NCT00619632 Date first received: 1 February 2008.
Bonuck 2014b {published data only}
- Bonuck K, Stuebe A, Barnett J, Fletcher J, Bernstein P. Routine, primary‐care based interventions to increase breastfeeding: results of two randomized controlled trials. Breastfeeding Medicine 2013;8(Suppl 1):S‐19. [Google Scholar]
- Bonuck K, Stuebe A, Barnett J, Labbok MH, Fletcher J, Bernstein PS. Effect of primary care intervention on breastfeeding duration and intensity. American Journal of Public Health 2014;104 Suppl 1:S119‐S127. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bortolini 2012 {published data only}
- Bortolini GA, Vitolo MR. The impact of systematic dietary counseling during the first year of life on prevalence rates of anemia and iron deficiency at 12‐16 months. Jornal de Pediatria 2012;88(1):33‐9. [DOI] [PubMed] [Google Scholar]
Brent 1995 {published data only}
- Brent NB, Redd B, Dworetz A, D'Amico FD, Greenberg J. Breastfeeding in a low‐income population. Archives of Pediatric and Adolescent Medicine 1995;149(7):798‐803. [DOI] [PubMed] [Google Scholar]
Bunik 2010 {published data only}
- Bunik M, Beaty B, Dickinson M, Shobe P, Kempe A, O'Connor ME. Early formula supplementation in breastfeeding mothers: how much is too much for BF duration success?. Breastfeeding Medicine 2007;2(3):184. [Google Scholar]
- Bunik M, Shobe P, Crane L, Kempe A. Low‐income Latina mothers' perspectives on breastfeeding issues and participation in a telephone based support intervention. Breastfeeding Medicine 2007;2(3):184. [Google Scholar]
- Bunik M, Shobe P, O'Connor ME, Beaty B, Langendoerfer S, Crane L, et al. Are 2 weeks of daily breastfeeding support insufficient to overcome the influences of formula?. Academic Pediatrics 2010;10(1):21‐8. [DOI] [PubMed] [Google Scholar]
- Bunik M, Shobe P, O'Connor ME, Beaty B, Langendoerfer S, Crane L, et al. Randomized controlled trial to evaluate a telephone support intervention for breastfeeding in low‐income Latina mothers. Breastfeeding Medicine 2007;2(3):183. [Google Scholar]
- Bunik M, Shobe P, O'Connor ME, Beaty B, Langendoerfer S, Crane L, et al. Telephone support intervention for breastfeeding in low‐income Latina mothers. Pediatric Academic Societies Annual Meeting; 2007 May 5‐8; Toronto, Canada. 2007.
Caldeira 2008 {published data only}
- Caldeira AP, Fagundes GC, Aguiar GN. Educational intervention on breastfeeding promotion to the Family Health Program team [Intervencao educacional em equipes de Programa de Saude de Familia para promocao da amamentacao]. Revista de Saude Publica 2008;42(6):1027‐33. [DOI] [PubMed] [Google Scholar]
Cameron 2013 {published data only}
- Cameron SL, Heath AM, Gray AR, Churcher B, Davies RS, Newlands A, et al. Lactation consultant support from late pregnancy with an educational intervention at 4 months of age delays the introduction of complementary foods in a randomized controlled trial. Journal of Nutrition 2015;145:1481‐90. [DOI] [PubMed] [Google Scholar]
- Cameron SL, Taylor RW, Gray AR, Taylor BJ, Heath AL. Exclusive breastfeeding to six months: Results from a randomised controlled trial including lactation consultant support. FASEB Journal 2013;27(Suppl):[Abstract no: lb345]. [Google Scholar]
- Fangupo LJ, Heath AM, Williams SM, Somerville MR, Lawrence JA, Gray AR, et al. Impact of an early‐life intervention on the nutrition behaviors of 2‐y‐old children: A randomized controlled trial. American Journal of Clinical Nutrition 2015;102(3):704‐12. [DOI] [PubMed] [Google Scholar]
Chapman 2004 {published data only}
- Chapman D, Damio G, Young S, Perez‐Escamilla R. Association of degree and timing of exposure to breastfeeding peer counseling services with breastfeeding duration. Advances in Experimental Medicine and Biology 2004;554:303‐6. [DOI] [PubMed] [Google Scholar]
- Chapman DJ, Damio G, Perez‐Escamilla R. Differential response to breastfeeding peer counseling within a low‐Income, predominantly Latina population. Journal of Human Lactation 2004;20(4):389‐96. [DOI] [PubMed] [Google Scholar]
- Chapman DJ, Damio GD, Young S, Perez‐Escamilla R. Effectiveness of breastfeeding peer counseling in a low‐income, predominantly Latina population. Archives of Pediatric and Adolescent Medicine 2004;158(9):897‐902. [DOI] [PubMed] [Google Scholar]
- Chapman DJ, Perez‐Escamilla R. Acculturative type is associated with breastfeeding duration among low‐income Latinas. Maternal and Child Nutrition 2013;9(2):188‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chapman 2008 {published data only}
- Chapman DJ, Bermudez‐Millan A, Wetzel K, Damio G, Kyer N, Young S, et al. Breastfeeding education and support trial for obese women. FASEB 2008;22:1080.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman DJ, Morel K, Bermudez‐Millan A, Young S, Damio G, Perez‐Escamilla R. Breastfeeding education and support trial for overweight and obese women: a randomized trial. Pediatrics 2013;131(1):e162‐e170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel K, Chapman DJ, Kyer N, Bermudez‐Millan A, Young S, Perez‐Escamilla R. Peer counselors improve breastfeeding technique among low‐income, obese women. FASEB Journal 2010;24(Suppl):[Abstract no. 91.7]. [Google Scholar]
Chen 1993 {published data only}
- Chen CH. Effects of home visits and telephone contacts on breastfeeding compliance in Taiwan. Maternal‐Child Nursing Journal 1993;21(3):82‐90. [PubMed] [Google Scholar]
Coutinho 2005 {published data only}
- Bechara Coutinho S, Cabral de Lira P, Carvalho Lima M, Ashworth A. Comparison of the effects of two systems for the promotion of exclusive breastfeeding. Lancet 2005;366:1094‐100. [DOI] [PubMed] [Google Scholar]
Dennis 2002 {published and unpublished data}
- Dennis CL. A Randomized Controlled Trial Evaluating the Effect of Peer (Mother‐to‐Mother) Support on Breastfeeding Duration Among Primiparous Women [PhD dissertation]. Toronto, Ontario, Canada: University of Toronto, 1999. [Google Scholar]
- Dennis CL. Breastfeeding peer support: maternal and volunteer perceptions from a randomised controlled trial. Birth 2002;29:169‐76. [DOI] [PubMed] [Google Scholar]
- Dennis CL, Hodnett E, Gallop R, Chalmers B. The effect of peer support on breastfeeding duration among primiparous women: a randomized controlled trial. Canadian Medical Association Journal 2002;166(1):21‐8. [PMC free article] [PubMed] [Google Scholar]
de Oliveira 2006 {published data only}
- Oliveira LD, Giugliani ER, do Espirito Santo LC, Franca MC, Weigert EM, Kohler CV, et al. Effect of intervention to improve breastfeeding technique on the frequency of exclusive breastfeeding and lactation‐related problems. Journal of Human Lactation 2006;22(3):315‐21. [DOI] [PubMed] [Google Scholar]
Di Meglio 2010 {published data only}
- Meglio GD, McDermott MP, Klein JD. A randomized controlled trial of telephone peer support's influence on breastfeeding duration in adolescent mothers. Breastfeeding Medicine 2010;5:41‐7. [DOI] [PubMed] [Google Scholar]
Di Napoli 2004 {published and unpublished data}
- Napoli A, Lallo D, Fortes C, Franceschelli C, Armeni E, Guasticchi G. Home breastfeeding support by health professionals: findings of a randomised controlled trial in a population of Italian women. Acta Paediatrica 2004;93:1108‐14. [PubMed] [Google Scholar]
Edwards 2013 {published data only}
- Edwards C, Thullen J, Korfmacher J, Lantos D, Henson G, Hans L. Breastfeeding and complementary food: randomized trial of community doula home visiting. Pediatrics 2013;132:S160‐6. [DOI] [PubMed] [Google Scholar]
Efrat 2015 {published data only}
- Efrat MW, Esparza S, Mendelson SG, Lane CJ. The effect of lactation educators implementing a telephone‐based intervention among low‐income Hispanics: a randomised trial. Health Education Journal 2015;74(4):424‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ekstrom 2006 {published data only}
- Ekstrom A, Nissen E. A mother's feelings for her infant are strengthened by excellent breastfeeding counseling and continuity of care. Pediatrics 2006;118(2):e309‐14. [DOI] [PubMed] [Google Scholar]
- Ekstrom A, Widstrom AM, Nissen E. Does continuity of care by well‐trained breastfeeding counselors improve a mother's perception of support?. Birth 2006;33(2):123‐30. [DOI] [PubMed] [Google Scholar]
Elliott‐Rudder 2014 {published data only}
- Elliott‐Rudder M, Pilotto L, McIntyre E, Ramanathan S. Motivational interviewing improves exclusive breastfeeding in an Australian randomised controlled trial. Acta Paediatrica 2014;103(1):e11‐6. [DOI] [PubMed] [Google Scholar]
Ellis 1984 {published data only}
- Ellis DJ, Hewat RJ. Factors related to breastfeeding duration. Canadian Family Physician 1984;30:1479‐84. [PMC free article] [PubMed] [Google Scholar]
Frank 1987 {published data only}
- Frank DA, Wirtz SJ, Sorensen JR, Heeren T. Commercial hospital discharge packs and breastfeeding counseling: effects on infant feeding practices in a randomized trial. Pediatrics 1987;80(6):845‐54. [PubMed] [Google Scholar]
Froozani 1999 {published data only}
- Froozani MD, Permehzadeh K, Motlagh AR, Golestan B. Effect of breastfeeding education on the feeding pattern and health of infants in their first 4 months in the Islamic Republic of Iran. Bulletin of the World Health Organization 1999;77(5):381‐5. [PMC free article] [PubMed] [Google Scholar]
Fu 2014 {published data only}
- Fu IC, Fong DY, Heys M, Lee IL, Sham A, Tarrant M. Professional breastfeeding support for first‐time mothers: a multicentre cluster randomised controlled trial. BJOG: an International Journal of Obstetrics and Gynaecology 2014;121:1673‐84. [DOI] [PubMed] [Google Scholar]
- Tarrant M, Fong DY, Heys M, Lee IL, Sham A, Hui Choi EW. Professional breastfeeding support to increase the exclusivity and duration of breastfeeding: a randomised controlled trial. Hong Kong Medical Journal = Xianggang Yi Xue Za Zhi / Hong Kong Academy of Medicine 2014;20(6 Suppl 7):34‐5. [PubMed] [Google Scholar]
Gagnon 2002 {published data only}
- Gagnon AJ, Dougherty G, Jimenez V, Leduc N. Randomized trial of postpartum care after hospital discharge. Pediatrics 2002;109(6):1074‐80. [DOI] [PubMed] [Google Scholar]
Graffy 2004 {published data only}
- Graffy J, Taylor J. What information, advice and support do women want with breastfeeding?. Birth 2005;32(3):179‐86. [DOI] [PubMed] [Google Scholar]
- Graffy J, Taylor J, Williams A, Eldridge S. Randomised controlled trial of support from volunteer counsellors for mothers considering breast feeding. BMJ 2004;328(7430):26‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gross 1998 {published data only}
- Gross SM, Caulfield LE, Bentley ME, Bronner Y, Kessler L, Jensen J, et al. Counseling and motivational videotapes increase duration of breast‐feeding in African‐American WIC participants who initiate breast‐feeding. Journal of the American Dietetic Association 1998;98:143‐8. [DOI] [PubMed] [Google Scholar]
Grossman 1990 {published data only}
- Grossman LK, Harter C, Kay A. Postpartum lactation counseling for low‐income women. American Journal of Diseases of Children 1987;141:375. [DOI] [PubMed] [Google Scholar]
- Grossman LK, Harter C, Kay A. The effect of postpartum lactation counseling on the duration of breastfeeding in low‐income women. American Journal of Diseases in Childhood 1990;144(4):471‐4. [DOI] [PubMed] [Google Scholar]
Haider 2000 {published data only}
- Haider R, Ashworth A, Kabir I, Huttly S. Effects of community‐based peer counsellors on exclusive breastfeeding practices in Dhaka, Bangladesh: a randomised controlled trial. Lancet 2000;356:1643‐7. [DOI] [PubMed] [Google Scholar]
- Haider R, Kabir I, Huttley SRA, Ashworth A. Training peer counselors to promote and support exclusive breastfeeding in Bangladesh. Journal of Human Lactation 2002;18(1):7‐12. [DOI] [PubMed] [Google Scholar]
Hall 1978 {published data only}
- Hall JM. Influencing breastfeeding success. Journal of Obstetric, Gynecologic and Neonatal Nursing 1978;7:28‐32. [DOI] [PubMed] [Google Scholar]
Hanson 2015 {published data only}
- Hanson C, Manzi F, Mkumbo E, Shirima K, Penfold S, Hill Z, et al. Effectiveness of a home‐based counselling strategy on neonatal care and survival: a cluster‐randomised trial in six districts of rural Southern Tanzania. PLOS Medicine 2015;12(9):e1001881. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hoddinott 2009 {published data only}
- Hoddinott P. A randomised controlled trial to evaluate the clinical and cost effectiveness of breastfeeding peer support groups in improving breastfeeding initiation, duration and satisfaction. National Research Register (www.nrr.nhs.uk) (accessed 6 July 2006) 2006.
- Hoddinott P, Britten J, Pill R. Why do interventions work in some places and not others: a breastfeeding support group trial. Social Science & Medicine 2010;70(5):769‐78. [DOI] [PubMed] [Google Scholar]
- Hoddinott P, Britten J, Prescott GJ, Tappin D, Ludbrook A, Godden DJ. Effectiveness of policy to provide breastfeeding groups (BIG) for pregnant and breastfeeding mothers in primary care: cluster randomised controlled trial. BMJ 2009;338:a3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hoddinott 2012 {published data only}
- Hoddinott P, Craig L, MacLennan G, Boyers D, Vale L. The FEeding Support Team (FEST) randomised, controlled feasibility trial of proactive and reactive telephone support for breastfeeding women living in disadvantaged areas. BMJ Open 2012;2(2):e000652. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hopkinson 2009 {published data only}
- Hopkinson J, Konefal Gallagher M. Assignment to a hospital‐based breastfeeding clinic and exclusive breastfeeding among immigrant Hispanic mothers: a randomized, controlled trial. Journal of Human Lactation 2009;25(3):287‐96. [DOI] [PubMed] [Google Scholar]
Howell 2014 {published data only}
- Howell EA, Bodnar‐Deren S, Balbierz A, Parides M, Bickell N. An intervention to extend breastfeeding among black and Latina mothers after delivery. American Journal of Obstetrics & Gynecology 2014;210:239.e1‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
ISRCTN47056748 {published data only}
- ISRCTN47056748. Successful breastfeeding promotion: a motivational instructional model applied and tested. isrctn.com/ISRCTN47056748 Date first received: 16 July 2007.
Jenner 1988 {published data only}
- Jenner S. The influence of additional information, advice and support on the success of breast feeding in working class primiparas. Child Care, Health and Development 1988;14(5):319‐28. [DOI] [PubMed] [Google Scholar]
Jolly 2012a {published data only}
- Jolly K, IngramL, Freemantle N, Khan K, Chambers J, Hamburger R, et al. Effect of a peer support service on breast‐feeding continuation in the UK: a randomised controlled trial. Midwifery 2012;28(6):740‐5. [DOI] [PubMed] [Google Scholar]
Jones 1985 {published data only}
- Jones D, West R. Effect of a lactation nurse on the success of breast‐feeding: a randomised controlled trial. Journal of Epidemiology & Community Health 1986;40(1):45‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DA, West RR. Lactation nurse increases duration of breastfeeding. Archives of Disease in Childhood 1985;60(8):772‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kaojuri 2009 {published data only}
- Kaojuri DM, Sakakky M, Hosseini F, Kherkhah M. Comparison of the effect of two methods of home visit for the promotion of exclusive breastfeeding in caesarean section mothers in Iran university of medical sciences 2008. International Journal of Gynecology & Obstetrics 2009;107(Suppl 2):S150. [Google Scholar]
Khresheh 2011 {published data only}
- Khresheh R, Suhaimat A, Jalamdeh F, Barclay L. The effect of a postnatal education and support program on breastfeeding among primiparous women: a randomized controlled trial. International Journal of Nursing Studies 2011;48(9):1058‐66. [DOI] [PubMed] [Google Scholar]
Kools 2005 {published data only}
- Kools EJ, Thijs C, Kester ADM, Brandt PA, Vries H. A breast‐feeding promotion and support program a randomized trial in the Netherlands. Preventive Medicine 2005;40:60‐70. [DOI] [PubMed] [Google Scholar]
Kramer 2001 {published and unpublished data}
- Kramer M, Matush L, Vanilovich I, Platt R, Mazer B. Does breastfeeding help prevent asthma and allergy? Evidence from a randomized trial in Belarus. American Journal of Epidemiology 2006;163(Suppl 11):S85. [Google Scholar]
- Kramer MS. "Breast is best": the evidence. Early Human Development 2010;86(11):729‐32. [DOI] [PubMed] [Google Scholar]
- Kramer MS, Aboud F, Mironova E, Vanilovich I, Platt RW, Matush L, et al. Breastfeeding and child cognitive development: new evidence from a large randomized trial. Archives of General Psychiatry 2008;65(5):578‐84. [DOI] [PubMed] [Google Scholar]
- Kramer MS, Chalmers B, Hodnett E, Sevkovskaya Z, Dzikovich I, Shapiro S, et al. Promotion of breastfeeding intervention trial (PROBIT): a randomized trial in the Republic of Belarus. JAMA 2001;285(4):413‐20. [DOI] [PubMed] [Google Scholar]
- Kramer MS, Fombonne E, Igumnov S, Vanilovich I, Matush L, Mironova E, et al. Effects of prolonged and exclusive breastfeeding on child behavior and maternal adjustment: evidence from a large, randomized trial. Pediatrics 2008;121(3):e435‐40. [DOI] [PubMed] [Google Scholar]
- Kramer MS, Matush L, Bogdanovich N, Aboud F, Mazer B, Fombonne E, et al. Health and development outcomes in 6.5‐y‐old children breastfed exclusively for 3 or 6 mo. American Journal of Clinical Nutrition 2009;90(4):1070‐4. [DOI] [PubMed] [Google Scholar]
- Kramer MS, Matush L, Vanilovich I, Platt R, Bogdanovich N, Sevkovskaya Z, et al. Effect of prolonged and exclusive breast feeding on risk of allergy and asthma: cluster randomised trial. BMJ 2007;335(7624):815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer MS, Matush L, Vanilovich I, Platt RW, Bogdanovich N, Sevkovskaya Z, et al. A randomized breast‐feeding promotion intervention did not reduce child obesity in Belarus. Journal of Nutrition 2009;139(2):417S‐21S. [DOI] [PubMed] [Google Scholar]
- Kramer MS, Vanilovich I, Matush L, Bogdanovich N, Zhang X, Shishko G, et al. The effect of prolonged and exclusive breast‐feeding on dental caries in early school‐age children. New evidence from a large randomized trial. Caries Research 2007;41(6):484‐8. [DOI] [PubMed] [Google Scholar]
- Lawrence RA. Promotion of Breastfeeding Intervention Trial (PROBIT) a randomized trial in the Republic of Belarus. Journal of Pediatrics 2001;139(1):164‐5. [PubMed] [Google Scholar]
- Martin RM, Patel R, Kramer MS, Guthrie L, Vilchuck K, Bogdanovich N, et al. Effects of promoting longer‐term and exclusive breastfeeding on adiposity and insulin‐like growth factor‐I at age 11.5 years: a randomized trial. JAMA 2013;309(10):1005‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin RM, Patel R, Kramer MS, Vilchuck K, Bogdanovich N, Sergeichick N, et al. Effects of promoting longer‐term and exclusive breastfeeding on cardiometabolic risk factors at age 11.5 years: a cluster‐randomized, controlled trial. Circulation 2014;129(3):321‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oken E, Patel R, Guthrie LB, Vilchuck K, Bogdanovich N, Sergeichick N, et al. Effects of an intervention to promote breastfeeding on maternal adiposity and blood pressure at 11.5 y postpartum: results from the Promotion of Breastfeeding Intervention Trial, a cluster‐randomized controlled trial. American Journal of Clinical Nutrition 2013;98(4):1048‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skugarevsky O, Wade KH, Richmond RC, Martin RM, Tilling K, Patel R, et al. Effects of promoting longer‐term and exclusive breastfeeding on childhood eating attitudes: a cluster‐randomized trial. International Journal of Epidemiology 2014;43(4):1263‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Platt RW, Dahhou M, Kramer MS. Do population‐based interventions widen or narrow socioeconomic inequalities? The case of breastfeeding promotion. International Journal of Epidemiology 2014;43(4):1284‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kronborg 2007 {published data only}
- Kronborg H, Vaeth M. How are effective breastfeeding technique and pacifier use related to breastfeeding problems and breastfeeding duration?. Birth 2009;36(1):34‐42. [DOI] [PubMed] [Google Scholar]
- Kronborg H, Vaeth M, Olsen J, Harder I. Health visitors and breastfeeding support: influence of knowledge and self‐efficacy. European Journal of Public Health 2008;18(3):283‐8. [DOI] [PubMed] [Google Scholar]
- Kronborg H, Vaeth M, Olsen J, Iversen L, Harder I. Effect of early postnatal breastfeeding support: a cluster‐randomized community based trial. Acta Paediatrica 2007;96(7):1064‐70. [DOI] [PubMed] [Google Scholar]
Labarere 2005 {published data only}
- Labarere J, Gelbert‐Baudino N, Ayral AS, Duc C, Berchotteau M, Bouchon N, et al. Efficacy of breastfeeding support provided by trained clinicians during an early, routine, preventive visit: a prospective, randomized, open trial of 226 mother‐infant pairs. Pediatrics 2005;115(2):e139‐46. [DOI] [PubMed] [Google Scholar]
Laliberte 2016 {published data only}
- Laliberte C, Dunn S, Pound C, Sourial N, Yasseen AS, Millar D, et al. A randomized controlled trial of innovative postpartum care model for mother‐baby dyads. PLOS One 2016;11(2):e0148520. [DOI] [PMC free article] [PubMed] [Google Scholar]
Leite 2005 {published data only}
- Leite AJ, Puccini RF, Atallah AN, Alves da Cunha AL, Machado MT. Effectiveness of home‐based peer counselling to promote breastfeeding in the northeast of Brazil: a randomised clinical trial. Acta Paediatrica 2005;94:741‐6. [DOI] [PubMed] [Google Scholar]
- Leite AJM, Puccini R, Atallah A, Cunha A, Machado M, Capiberibe A, et al. Impact on breastfeeding practices promoted by lay counselors: a randomized and controlled clinical trial. Journal of Clinical Epidemiology 1998;51(Suppl 1):S10. [Google Scholar]
Lucchini 2013 {published data only}
- Lucchini C, Uribe TC, Villarroel PL, Rojas RA. Randomized controlled clinical trial evaluating determinants of successful breastfeeding: follow‐up two months after comprehensive intervention versus standard care delivery [Determinantes para una lactancia materna exitosa: Intervencion integral vs cuidado estandar. Ensayo clinico aleatorio controlado]. Revista Chilena de Pediatria 2013;84(2):138‐44. [Google Scholar]
Lynch 1986 {published data only}
- Lynch SA, Koch AM, Hislop TG, Coldman AJ. Evaluating the effect of a breastfeeding consultant on the duration of breastfeeding. Canadian Journal of Public Health 1986;77(3):190‐5. [PubMed] [Google Scholar]
McDonald 2010 {published data only}
- McDonald SJ, Henderson JJ, Evans SF, Faulkner S, Hagan R. Effect of an extended midwifery support program on the duration of breastfeeding: a randomised controlled trial. [abstract]. Perinatal Society of Australia and New Zealand 7th Annual Congress; 2003 March 9‐12; Tasmania, Australia. 2003:A68.
- McDonald SJ, Henderson JJ, Faulkner S, Evans SF, Hagan R. Effect of an extended midwifery postnatal support programme on the duration of breast feeding: a randomised controlled trial. Midwifery 2010;26(1):88‐100. [DOI] [PubMed] [Google Scholar]
McKeever 2002 {published data only}
- McKeever P, Stevens B, Miller KL, MacDonell K, Gibbins S, Guerriere D, et al. Home versus hospital breastfeeding support for newborns: a randomized controlled trial. Birth 2002;29(4):258‐65. [DOI] [PubMed] [Google Scholar]
- Stevens B, Guerriere D, McKeever P, Croxford R, Miller KL, Watson‐MacDonell J, et al. Economics of home vs. hospital breastfeeding support for newborns. Journal of Advanced Nursing 2006;53(2):233‐43. [DOI] [PubMed] [Google Scholar]
- Stevens B, McKeever P, Coyte P, Daub S, Dunn M, Gibbins S, et al. The impact of home versus hospital support of breastfeeding on neonatal outcomes. Pediatric Research 2001;49 Suppl(4):261A. [Google Scholar]
McLachlan 2016 {published data only}
- Forster D, Mclachlan H. Supporting breastfeeding in local communities (SILC): a cluster randomised controlled trial in Victoria, Australia. International Confederation of Midwives 30th Triennial Congress. Midwives: Improving Women’s Health; 2014 June 1‐4; Prague, Czech Republic. 2014:C138.
- McLachlan H, Forster D, Amir L, Small R, Cullinane M, Watson L, et al. Supporting breastfeeding in local communities (silc): results of a cluster randomised trial. Journal of Paediatrics and Child Health 2015;51:48. [Google Scholar]
- McLachlan HL, Forster DA, Amir LH, Cullinane M, Shafiei T, Watson LF, et al. Supporting breastfeeding in local communities (silc) in Victoria, Australia: a cluster randomised controlled trial. BMJ Open 2016;6(2):e008292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan HL, Forster DA, Amir LH, Small R, Cullinane M, Watson LF, et al. Supporting breastfeeding In Local Communities (SILC): protocol for a cluster randomised controlled trial. BMC Pregnancy and Childbirth 2014;14(1):346. [DOI] [PMC free article] [PubMed] [Google Scholar]
McQueen 2009 {published data only}
- McQueen KA. Improving Breastfeeding Outcomes: a Pilot Randomized Controlled Trial of a Self‐Efficacy Intervention with Primiparous Mothers [thesis]. Toronto: University of Toronto, 2009. [Google Scholar]
McQueen 2011 {published data only}
- McQueen KA, Dennis CL, Stremler R, Norman CD. A pilot randomized controlled trial of a breastfeeding self‐efficacy intervention with primiparous mothers. JOGNN: Journal of Obstetric, Gynecologic and Neonatal Nursing 2011;40:35‐46. [DOI] [PubMed] [Google Scholar]
Mejdoubi 2014 {published data only}
- Mejdoubi J, Heijkant SC, Leerdam FJ, Crone M, Crijnen A, HiraSing RA. Effects of nurse home visitation on cigarette smoking pregnancy outcomes: a randomized controlled trial. Midwifery 2014;30:688‐95. [DOI] [PubMed] [Google Scholar]
Mongeon 1995 {published data only}
- Mongeon M, Allard R. A controlled study with regular telephonic support given by volunteers on the progress and outcome of breast‐feeding [Essai controle d'un soutien telephonique regulier donne par une benevole sur le deroulment et l'issus de l'allaitment]. Revue Canadienne de Sante Publique 1995;86(2):124‐7. [PubMed] [Google Scholar]
Morrell 2000 {published data only}
- Morrell CJ, Spiby H, Stewart P, Walters S, Morgan A. Costs and effectiveness of community postnatal support workers: randomised controlled trial. BMJ 2000;321(7261):593‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Morrow 1999 {published data only}
- Morrow AL, Lourdes Guerrero M. From bio‐active substances to research on breastfeeding promotion. In: Newburg editor(s). Bioactive Components of Human Milk. New York: Kluwer Academic/Plenum Publishers, 2001:447‐55. [Google Scholar]
- Morrow AL, Lourdes Guerrero M, Shults J, Calva JJ, Lutter C, Ruiz‐Palacios GM, et al. Efficacy of home‐based peer counselling to promote exclusive breastfeeding: a randomised controlled trial. Lancet 1999;353(9160):1226‐31. [DOI] [PubMed] [Google Scholar]
Muirhead 2006 {published data only}
- Muirhead PE, Butcher G, Rankin J, Munley A. The effect of a programme of organised and supervised peer support on the initiation and duration of breastfeeding: a randomised trial. British Journal of General Practice 2006;56(524):191‐7. [PMC free article] [PubMed] [Google Scholar]
Ochola 2013 {published data only}
- Ochola A, Labadarios D, Nduati W. Impact of counselling on exclusive breast‐feeding practices in a poor urban setting in Kenya: a randomized controlled trial. Public Health Nutrition 2013;16(10):1732‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Paul 2012 {published data only}
- Paul IM, Beiler JS, Schaefer EW, Hollenbeak CS, Alleman N, Sturgis SA. A randomized trial of nurse home visits vs. office‐based care after nursery/maternity discharge. Pediatric Academic Societies and Asian Society for Pediatric Research Joint Meeting; 2011 April 30‐May 3; Denver, Colorado, USA. 2011:2300.6.
- Paul IM, Beiler JS, Schaefer EW, Hollenbeak CS, Alleman N, Sturgis SA, et al. A randomized trial of single home nursing visits vs office‐based care after nursery/maternity discharge: the Nurses for Infants Through Teaching and Assessment After the Nursery (NITTANY) Study. Archives of Pediatrics & Adolescent Medicine 2012;166(3):263‐70. [DOI] [PubMed] [Google Scholar]
Petrova 2009 {published data only}
- Petrova A, Ayers C, Stechna S, Gerling JA, Mehta R. Effectiveness of exclusive breastfeeding promotion in low‐income mothers: a randomized controlled study. Breastfeeding Medicine 2009;4(2):63‐9. [DOI] [PubMed] [Google Scholar]
Porteous 2000 {published data only}
- Porteous R, Kaufman K, Rush J. The effect of individualized professional support on duration of breastfeeding: a randomized controlled trial. Journal of Human Lactation 2000;16(4):303‐8. [DOI] [PubMed] [Google Scholar]
Pugh 1998 {published data only}
- Pugh LC, Milligan RA. Nursing intervention to increase the duration of breastfeeding. Applied Nursing Research 1998;11(4):190‐4. [DOI] [PubMed] [Google Scholar]
Pugh 2002 {published data only}
- Pugh L, Milligan R, Frick K, Spatz D, Bronner Y. Breastfeeding duration, costs, and benefits of a support program for low‐income breastfeeding women. Birth 2002;29(2):95‐100. [DOI] [PubMed] [Google Scholar]
Pugh 2010 {published data only}
- Frick D, Pugh C, Milligan A. Costs related to promoting breastfeeding among urban low‐income women. JOGNN: Journal of Obstetric, Gynecologic & Neonatal Nursing 2012;41(1):144‐51. [DOI] [PubMed] [Google Scholar]
- Pugh LC, Nanda JP, Frick KD, Sharps PW, Spatz DL, Serwint JR, et al. A randomized controlled community‐based trial to improve breastfeeding among urban low‐income mothers. Pediatric Academic Societies Annual Meeting; 2007 May 5‐8; Toronto, Canada 2007. [DOI] [PMC free article] [PubMed]
- Pugh LC, Serwint JR, Frick KD, Nanda JP, Sharps PW, Spatz DL, et al. A randomized controlled community‐based trial to improve breastfeeding rates among urban low‐income mothers. Academic Pediatrics 2010;10(1):14‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Quinlivan 2003 {published data only}
- Quinlivan JA, Box H, Evans SF. Postnatal home visits in teenage mothers: a randomised controlled trial. Lancet 2003;361(9361):893‐900. [DOI] [PubMed] [Google Scholar]
Ransjo‐Arvidson 1998 {published data only}
- Ransjo‐Arvidson AB, Chintu K, Ng'andu N, Eriksson B, Susu B, Christensson K, et al. Maternal and infant health problems after normal childbirth: a randomised controlled study in Zambia. Journal of Epidemiology & Community Health 1998;52:385‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Redman 1995 {published data only}
- Redman S, Watkins J, Evans L, Lloyd D. Evaluation of an Australian intervention to encourage breast feeding in primiparous women. Health Promotion International 1995;10(2):101‐13. [Google Scholar]
Santiago 2003 {published data only}
- Santiago LB, Bettiol H, Barbieri MA, Guttierrez MR, Ciampo LA. Promotion of breastfeeding: the importance of pediatricians with specific training [Incentivo ao aleitamento materno: a importancia do pediatra com treinamento especifico]. Jornal de Pediatria 2003;79(6):504‐12. [PubMed] [Google Scholar]
Serafino‐Cross 1992 {published data only}
- Serafino‐Cross P, Donovan P. Effectiveness of professional breastfeeding home‐support. Society for Nutrition Education 1992;24(3):117‐22. [Google Scholar]
Sikander 2015 {published data only}
- Sikander S, Maselko J, Zafar S, Haq Z, Ahmad I, Ahmad M, et al. Cognitive‐behavioral counseling for exclusive breastfeeding in rural pediatrics: a cluster RCT. Pediatrics 2015;135(2):e424‐e431. [DOI] [PubMed] [Google Scholar]
Simonetti 2012 {published data only}
- Simonetti V, Palma E, Giglio A, Mohn A, Cicolini G. A structured telephonic counselling to promote the exclusive breastfeeding of healthy babies aged zero to six months: a pilot study. International Journal of Nursing Practice 2012;18(3):289‐94. [DOI] [PubMed] [Google Scholar]
Sjolin 1979 {published data only}
- Sjolin S, Hofvander Y, Hillervik C. A prospective study of individual courses of breastfeeding. Acta Paediatrica Scandinavica 1979;68(4):521‐9. [DOI] [PubMed] [Google Scholar]
Srinivas 2015 {published data only}
- Srinivas GL, Benson M, Worley S, Schulte E. A clinic‐based breastfeeding peer counselor intervention in an urban, low‐income population: interaction with breastfeeding attitude. Journal of Human Lactation 2015;31(1):120‐8. [DOI] [PubMed] [Google Scholar]
- Srinivas GL, Worley S. Effect of office‐based peer counselor on breastfeeding rates in an urban low‐income clinic. Pediatric Academic Societies Annual Meeting; 2013 May 4‐7; Washington DC, USA. 2013.
Stockdale 2008 {published data only}
- Stockdale J, Sinclair M, Kernohan G, Keller JM, Dunwoody L, Cunningham JB, et al. Feasibility study to test Designer Breastfeeding: a randomised controlled trial. Evidence Based Midwifery 2008;6(3):76‐82. [Google Scholar]
Su 2007 {published data only}
- Su LL, Chong YS, Chan YH, Chan YS, Fok D, Tun KT, et al. Antenatal education and postnatal support strategies for improving rates of exclusive breast feeding: randomised controlled trial. BMJ 2007;335(7620):596. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tahir 2013 {published data only}
- Tahir NM, Al‐Sadat N. Does telephone lactation counselling improve breastfeeding practices? A randomised controlled trial. International Journal of Nursing Studies 2013;50(1):16‐25. [DOI] [PubMed] [Google Scholar]
Tylleskar 2011a {published data only}
- Birungi N, Fadnes LT, Okullo I, Kasangaki A, Nankabirwa V, Ndeezi G, et al. Effect of breastfeeding promotion on early childhood caries and breastfeeding duration among 5 year old children in Eastern Uganda: a cluster randomized trial. PLOS One 2015;10(5):e0125352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chola L, Fadnes LT, Engebretsen IM, Nkonki L, Nankabirwa V, Sommerfelt H, et al. Cost‐effectiveness of peer counselling for the promotion of exclusive breastfeeding in Uganda. PLOS One 2015;10(11):e0142718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chola L, Fadnes LT, Engebretsen IM, Tumwine JK, Tylleskar T, Robberstad B, et al. Infant feeding survival and Markov transition probabilities among children under age 6 months in Uganda. American Journal of Epidemiology 2013;177(5):453‐62. [DOI] [PubMed] [Google Scholar]
- Doherty T, Sanders D, Jackson D, Swanevelder S, Lombard C, Zembe W, et al. Early cessation of breastfeeding amongst women in South Africa: an area needing urgent attention to improve child health. BMC Pediatrics 2012;12:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engebretsen I, Nankunda J, Nankabirwa V, Diallo A, Fadnes L, Doherty T, et al. Early infant feeding practices in the Promise‐EBF trial: promotion of exclusive breastfeeding by peer counsellors in three countries in Africa. Annals of Nutrition & Metabolism 2013;63(Suppl 1):709, Abstract no: PO940. [Google Scholar]
- Engebretsen IM, Jackson D, Fadnes LT, Nankabirwa V, Diallo AH, Doherty T, et al. Growth effects of exclusive breastfeeding promotion by peer counsellors in sub‐Saharan Africa: the cluster‐randomised PROMISE EBF trial. BMC Public Health 2014;14(1):633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engebretsen IM, Jackson D, Fadnes LT, Nankabirwa V, Diallo AH, Doherty T, et al. Is promotion of exclusive breastfeeding safe in sub‐Sharan Africa with respect to child growth? Results from the cluster‐randomised PROMISE EBF‐trial. Proceedings of the 16th ISRHML Conference "Breastfeeding and the Use of Human Milk. Science and Practice"; 2012 September 27‐October 1; Trieste, Italy. 2012.
- Engebretsen IMS, Nankabirwa V, Doherty T, Diallo AH, Nankunda J, Fadnes LT, et al. Early infant feeding practices in three African countries: the PROMISE‐EBF trial promoting exclusive breastfeeding by peer counsellors. International Breastfeeding Journal 2014;9:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00397150. PROMISE EBF: safety and efficacy of exclusive breastfeeding promotion in the era of HIV in sub‐Saharan Africa. clinicaltrials.gov/show/NCT00397150 Date first received: 7 November 2006.
- Nankabirwa V, Tylleskar T, Nankunda J, Engebretsen IM, Sommerfelt H, Tumwine JK, et al. Malaria parasitaemia among infants and its association with breastfeeding peer counselling and vitamin A supplementation: a secondary analysis of a cluster randomized trial.. PLOS ONE 2011;6(7):e21862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nankunda J, Turnwine JK, Nakabirwa V, Tylleskar T, PROMISE‐EBF SG. "She would sit with me": mothers' experiences of individual peer support for exclusive breastfeeding in Uganda. International Breastfeeding Journal 2010;5:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tylleskar T, Jackson D, Meda N, Engebretsen IM, Chopra M, Diallo AH, et al. Exclusive breastfeeding promotion by peer counsellors in sub‐Saharan Africa (PROMISE‐EBF): a cluster‐randomised trial. Lancet 2011;378(9789):420‐7. [DOI] [PubMed] [Google Scholar]
Tylleskar 2011b {published data only}
- NCT00397150. PROMISE EBF: safety and efficacy of exclusive breastfeeding promotion in the era of HIV in sub‐Saharan Africa. clinicaltrials.gov/show/NCT00397150 Date first received: 7 November 2006.
- Nankunda J, Turnwine JK, Nakabirwa V, Tylleskar T, PROMISE‐EBF SG. "She would sit with me": mothers' experiences of individual peer support for exclusive breastfeeding in Uganda. International Breastfeeding Journal 2010;5:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tylleskar T, Jackson D, Meda N, Engebretsen IM, Chopra M, Diallo AH, et al. Exclusive breastfeeding promotion by peer counsellors in sub‐Saharan Africa (PROMISE‐EBF): a cluster‐randomised trial. Lancet 2011;378(9789):420‐7. [DOI] [PubMed] [Google Scholar]
Tylleskar 2011c {published data only}
- Nankunda J, Turnwine JK, Nakabirwa V, Tylleskar T, PROMISE‐EBF SG. "She would sit with me": mothers' experiences of individual peer support for exclusive breastfeeding in Uganda. International Breastfeeding Journal 2010;5:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tylleskar T. PROMISE EBF: safety and efficacy of exclusive breastfeeding promotion in the era of HIV in sub‐Saharan Africa. ClinicalTrials.gov (clinicaltrials.gov/) (accessed 20 February 2008).
- Tylleskar T, Jackson D, Meda N, Engebretsen IM, Chopra M, Diallo AH, et al. Exclusive breastfeeding promotion by peer counsellors in sub‐Saharan Africa (PROMISE‐EBF): a cluster‐randomised trial. Lancet 2011;378(9789):420‐7. [DOI] [PubMed] [Google Scholar]
Vidas 2011 {published data only}
- Vidas M, Folnegovic‐Smalc V, Catipovic M, Kisic M. The application of autogenic training in counseling center for mother and child in order to promote breastfeeding. Collegium Antropologicum 2011;35(3):723‐31. [PubMed] [Google Scholar]
Vitolo 2005 {published and unpublished data}
- Vitolo MR, Bortolini GA, Dal Bo Campagnolo P, Feldens CA. Effectiveness of a nutrition program in reducing symptoms of respiratory morbidity in children: a randomized field trial. Preventive Medicine 2008;47(4):384‐8. [DOI] [PubMed] [Google Scholar]
- Vitolo MR, Bortolini GA, Feldens CA, Drachler Mde L. Impacts of the 10 Steps to Healthy Feeding in Infants: a randomized field trial [Impactos da implementacao dos dez passos da alimentacao saudavel para criancas: ensaio de campo randomizado]. Cadernos de Saude Publica 2005;21(5):1448‐57. [DOI] [PubMed] [Google Scholar]
- Vitolo MR, Rauber F, Campagnolo PD, Feldens CA, Hoffman DJ. Maternal dietary counseling in the first year of life is associated with a higher healthy eating index in childhood. Journal of Nutrition 2010;140(11):2002‐7. [DOI] [PubMed] [Google Scholar]
Wambach 2009 {published data only}
- NCT00222118. Kansas University Teen Mothers Project. clinicaltrials.gov/show/NCT00222118 Date first received: 13 September 2005.
- Wambach K, Rojjanasrirat W, Williams Domian E, Aaronson L, Breedlove G, Yeh HW. Effects of a peer counselor and lactation consultant on breastfeeding initiation and duration. Journal of Human Lactation 2009;25(1):101‐2. [Google Scholar]
- Wambach KA, Aaronson L, Breedlove G, Domian EW, Rojjanasrirat W, Yeh HW. A randomized controlled trial of breastfeeding support and education for adolescent mothers. Western Journal of Nursing Research 2011;33(4):486‐505. [DOI] [PubMed] [Google Scholar]
Wen 2011 {published data only}
- Wen LM, Baur LA, Rissel C, Simpson JM. A randomized controlled trial of an early intervention on childhood obesity: results from the first 12 months. Obesity (Silver Spring, Md.) 2011;19(Suppl 1):S67. [Google Scholar]
- Wen LM, Baur LA, Simpson JM, Rissel C, Flood VM. Effectiveness of an early intervention on infant feeding practices and "tummy time": a randomized controlled trial. Archives of Pediatrics and Adolescent Medicine 2011;165(8):701‐7. [DOI] [PubMed] [Google Scholar]
- Wen LM, Baur LA, Simpson JM, Rissel C, Wardle K, Flood VM. Effectiveness of home based early intervention on children's BMI at age 2: randomised controlled trial. BMJ (Online) 2012;345(7865):e3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wilhelm 2015 {published data only}
- Wilhelm L, Aguirre M, Koehler E, Rodehorst TK. Evaluating motivational interviewing to promote breastfeeding by rural Mexican‐American mothers: the challenge of attrition. Issues in Comprehensive Pediatric Nursing 2015;38(1):7‐22. [DOI] [PubMed] [Google Scholar]
Winterburn 2003 {published and unpublished data}
- Winterburn S, Moyez J, Thompson J. Maternal grandmothers and support for breastfeeding. Journal of Community Nursing 2003;17(12):4‐9. [Google Scholar]
Wolfberg 2004 {published data only}
- Wolfberg AJ, Michels KB, Shields W, O'Campo P, Bronner Y, Bienstock J. Dads as breastfeeding advocates: results from a randomized controlled trial of an educational intervention. American Journal of Obstetrics and Gynecology 2004;191:708‐12. [DOI] [PubMed] [Google Scholar]
Wrenn 1997 {published data only}
- Wrenn SE. Effects of a model‐based intervention on breastfeeding attrition [dissertation]. San Antonio: University of Texas, 1997. [Google Scholar]
Wu 2014 {published data only}
- Wu DS, Hu J, McCoy TP, Efird JT. The effects of a breastfeeding self‐efficacy intervention on short‐term breastfeeding outcomes among primiparous mothers in Wuhan, China. Journal of Advanced Nursing 2014;70(8):1867‐79. [DOI] [PubMed] [Google Scholar]
Yotebieng 2015 {published data only}
- Yotebieng M, Labbok M, Soeters HM, Chalachala JL, Lapika B, Vitta BS, et al. Ten steps to successful breastfeeding programme to promote early initiation and exclusive breastfeeding in dr congo: a cluster‐randomised controlled trial. Lancet Global Health 2015;3(9):e546‐55. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
ACTRN12614000605695 {published data only}
- ACTRN12614000605695. Parent Infant Feeding Initiative: a randomised controlled trial involving fathers and mothers to enhance breastfeeding duration with two medium level intervention groups, one high level intervention group and a control group receiving usual care. anzctr.org.au/Trial/Registration/TrialReview.aspx?id=366435. ACTRN12614000605695 Date first received: 6 June 2014.
ACTRN12615000063516 {published data only}
- ACTRN12615000063516. Improving infant feeding practices of women in Yangon region: a randomized controlled trial of a mobile phone communications intervention and evaluation of its effectiveness in Myanmar. anzctr.org.au/Trial/Registration/TrialReview.aspx?id=367704 Date first received: 23 January 2015.
Agrasada 2005 {published data only}
- Agrasada GV, Ewald U, Kylberg E, Gustafsson J. Exclusive breastfeeding of low birth weight infants for the first six months: infant morbidity and maternal and infant anthropometry. Asia Pacific Journal of Clinical Nutrition 2011;20(1):62‐8. [PubMed] [Google Scholar]
- Agrasada GV, Gustafsson J, Kylberg E, Ewald U. Postnatal peer counselling on exclusive breastfeeding or low‐birthweight infants: a randomised, controlled trial. Acta Paediatrica 2005;94:1109‐15. [DOI] [PubMed] [Google Scholar]
- Agrasada GV, Kylberg E. When and why Filipino mothers of term low birth weight infants interrupted breastfeeding exclusively. Breastfeeding Review 2009;17(3):5‐10. [PubMed] [Google Scholar]
Ahmed 2008 {published data only}
- Ahmed AH. Breastfeeding preterm infants: an educational program to support mothers of preterm infants in Cairo, Egypt. Pediatric Nursing 2008;34(2):125‐30. [PubMed] [Google Scholar]
- Ahmed AH. Effect of breastfeeding educational program based on Bandura's social cognitive theory on breastfeeding outcomes among mothers of preterm infants to support breastfeeding mothers. Journal of Human Lactation 2010;26(1):60. [Google Scholar]
Ahmed 2016 {published data only}
- Ahmed AH, Roumani AM, Szucs K, Zhang L, King D. The effect of interactive web‐based monitoring on breastfeeding exclusivity, intensity, and duration in healthy, term infants after hospital discharge. Journal of Obstetric, Gynecologic, & Neonatal Nursing 2016;45(2):143‐154. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ball 2011 {published data only}
- Ball HL, Ward‐Platt MP, Howel D, Russell C. Randomised trial of sidecar crib use on breastfeeding duration (NECOT). Archives of Disease in Childhood 2011;96(7):630‐4. [DOI] [PubMed] [Google Scholar]
Baqui 2008 {published data only}
- Baqui AH, Arifeen SE, Rosen HE, Mannan I, Rahman SM, Al‐Mahmud AB, et al. Community‐based validation of assessment of newborn illnesses by trained community health workers in Sylhet district of Bangladesh. Tropical Medicine and International Health 2009;14(12):1448‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baqui AH, El‐Arifeen S, Darmstadt GL, Ahmed S, Williams EK, Seraji HR, et al. Effect of community‐based newborn‐care intervention package implemented through two service‐delivery strategies in Sylhet district, Bangladesh: a cluster‐randomised controlled trial. Lancet 2008;371(9628):1936‐44. [DOI] [PubMed] [Google Scholar]
Barlow 2006 {published data only}
- Barlow A, Varipatis‐Baker E, Speakman K, Ginsburg G, Friberg I, Goklish N, et al. Home‐visiting intervention to improve child care among American Indian adolescent mothers: a randomized trial. Archives of Pediatrics and Adolescent Medicine 2006;160(11):1101‐7. [DOI] [PubMed] [Google Scholar]
Barnet 2002 {published data only}
- Barnet B, Duggan AK, Devoe M, Burrell L. The effect of volunteer home visitation for adolescent mothers on parenting and mental health outcomes: a randomized trial. Archives of Pediatric and Adolescent Medicine 2002;156:1216‐22. [DOI] [PubMed] [Google Scholar]
Beiler 2011 {published data only}
- Beiler JS, Schaefer EW, Alleman N, Paul IM. Newborn anticipatory guidance delivered at office‐based vs. home nurse visits. Pediatric Academic Societies and Asian Society for Pediatric Research Joint Meeting; 2011 April 30‐May 3; Denver, Colorado, USA. 2011.
Benitez 1992 {published data only}
- Benitez I, Cruz J, Suplido A, Oblepias V, Kennedy K, Visness C. Extending lactational amenorrhoea in Manila: a successful breast‐feeding education programme. Journal of Biosocial Science 1992;24(2):211‐31. [DOI] [PubMed] [Google Scholar]
Bica 2014 {published data only}
- Bica OC, Giugliani ER. Influence of counseling sessions on the prevalence of breastfeeding in the first year of life: a randomized clinical trial with adolescent mothers and grandmothers. Birth (Berkeley, Calif.) 2014;41(1):39‐45. [DOI] [PubMed] [Google Scholar]
- Nunes LM, Giugliani ER, Santo LC, Oliveira LD. Reduction of unnecessary intake of water and herbal teas on breast‐fed infants: a randomized clinical trial with adolescent mothers and grandmothers. Journal of Adolescent Health 2011;49(3):258‐65. [DOI] [PubMed] [Google Scholar]
- Schwartz R, Vigo A, Oliveira LD, Giugliani ERJ. The effect of a pro‐breastfeeding and healthy complementary feeding intervention targeting adolescent mothers and grandmothers on growth and prevalence of overweight of preschool children. PLOS One 2015;10(7):e0131884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soldateli B, Vigo A, Giugliani ER. Effect of pattern and duration of breastfeeding on the consumption of fruits and vegetables among preschool children. PLOS One 2016;11(2):e0148357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira LD, Giugliani ER, Santo LC, Nunes LM. Impact of a strategy to prevent the introduction of non‐breast milk and complementary foods during the first 6months of life: a randomized clinical trial with adolescent mothers and grandmothers. Early Human Development 2012;88(6):357‐61. [DOI] [PubMed] [Google Scholar]
- Oliveira LD, Giugliani ER, do Espirito Santo LC, Nunes LM. Counselling sessions increased duration of exclusive breastfeeding: a randomized clinical trial with adolescent mothers and grandmothers. Nutrition Journal 2014;13(1):73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Black 2001 {published data only}
- Black MM, Siegel EH, Abel Y, Bentley ME. Home and videotape intervention delays early complementary feeding among adolescent mothers. Pediatrics 2001;107:E67. [DOI] [PubMed] [Google Scholar]
Blixt 2014 {published data only}
- Blixt I, Martensson LB, Ekstrom AC. Process‐oriented training in breastfeeding for health professionals decreases women's experiences of breastfeeding challenges. International Breastfeeding Journal 2014;9:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrom A, Kylberg E, Nissen E, Ekstrom A, Kylberg E, Nissen E. A process‐oriented breastfeeding training program for healthcare professionals to promote breastfeeding: an intervention study. Breastfeeding Medicine 2012;7(2):85‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrom AC, Thorstensson S. Nurses and midwives professional support increases with improved attitudes ‐ design and effects of a longitudinal randomized controlled process‐oriented intervention. BMC Pregnancy and Childbirth 2015;15(1):275. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bolam 1998 {published data only}
- Bolam A, Manandhar DS, Shrestha P, Ellis M, Costello AM. The effects of postnatal health education for mothers on infant care and family planning practices in Nepal: a randomised controlled trial. BMJ 1998;316(7134):805‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brown 2008 {published data only}
- Brown LP. Breastfeeding services for LBW infants ‐ outcomes and cost. CRISP (http://crisp.cit.nih.gov) (accessed 17 June 2008) 2008.
- Zukowsky K. Breast‐fed low‐birth‐weight premature neonates: developmental assessment and nutritional intake in the first 6 months of life. Journal of Perinatal & Neonatal Nursing 2007;21(3):242‐9. [DOI] [PubMed] [Google Scholar]
Byas 2011 {published data only}
- Byas P, Du H. Dads championing breastfeeding. JOGNN: Journal of Obstetric, Gynecologic & Neonatal Nursing 2011;40(Suppl 1):S99‐S100. [Google Scholar]
Carlsen 2013 {published data only}
- Carlsen EM, Kyhnaeb A, Renault KM, Cortes D, Michaelsen KF, Pryds O. Telephone‐based support prolongs breastfeeding duration in obese women: a randomized trial. American Journal of Clinical Nutrition 2013;98(5):1226‐32. [DOI] [PubMed] [Google Scholar]
Cattaneo 2001 {published data only}
- Cattaneo A, Buzzetti R. Effect on rates of breast feeding of training for the Baby Friendly Hospital Initiative. BMJ 2001;323(7325):1358‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Caulfield 1998 {published data only}
- Caulfield LE, Gross SM, Bentley ME, Bronner Y, Kessler L, Jensen J, et al. WIC‐based interventions to promote breastfeeding among African‐American women in Baltimore: effects on breastfeeding initiation and continuation. Journal of Human Lactation 1998;14(1):15‐22. [DOI] [PubMed] [Google Scholar]
Chapman 2011 {published data only}
- Chapman DJ, Morel K, Bermudez‐Millan A, Young S, Damio G, Kyer N, et al. Breastfeeding education and support trial for obese women: effects of a specialized peer counseling intervention on breastfeeding and health outcomes. Journal of Human Lactation 2011;27(1):75‐6. [Google Scholar]
Christie 2011 {published data only}
- Christie J, Bunting B. The effect of health visitors' postpartum home visit frequency on first‐time mothers: cluster randomised trial. International Journal of Nursing Studies 2011;48(6):689‐702. [DOI] [PubMed] [Google Scholar]
Davies‐Adetugbo 1996 {published data only}
- Davies‐Adetugbo AA. Promotion of breastfeeding in the community: impact of health education programme in rural communities in Nigeria. Journal of Diarrhoeal Disease Research 1996;14(1):5‐11. [PubMed] [Google Scholar]
Davies‐Adetugbo 1997 {published data only}
- Davies‐Adetugbo AA, Adetugbo K, Orewole Y, Fabiyi AK. Breast‐feeding promotion in a diarrhoea programme in rural communities. Journal of Diarrhoeal Diseases Research 1997;15(3):161‐6. [PubMed] [Google Scholar]
Davis 2014 {published data only}
- Davis A. Effects of an Educational Intervention on Baccalaureate Nursing Students' Knowledge and Attitude in Providing Breastfeeding Support to Mothers [thesis]. University of Alabama, 2014. [Google Scholar]
Ebbeling 2007 {published data only}
- Ebbeling CB, Pearson MN, Sorensen G, Levine RA, Hebert JR, Salkeld JA, et al. Conceptualization and development of a theory‐based healthful eating and physical activity intervention for postpartum women who are low income. Health Promotion Practice 2007;8(1):50‐9. [DOI] [PubMed] [Google Scholar]
Edwards 2013a {published data only}
- Edwards RA, Bickmore T, Jenkins L, Foley M, Manjourides J. Use of an interactive computer agent to support breastfeeding. Maternal & Child Health Journal 2013;17(10):1961‐8. [DOI] [PubMed] [Google Scholar]
Ehrlich 2014 {published data only}
- Ehrlich SF, Hedderson MM, Feng J, Crites Y, Quesenberry CP, Ferrara A. Lifestyle intervention improves postpartum fasting glucose levels in women with gestational diabetes. Diabetes 2014;63(Suppl 1):A95, Abstract no: 363‐OR. [Google Scholar]
Eneroth 2007 {published data only}
- Eneroth H, Arifeen S, Kabir I, Persson LA, Lonnerdal B, Hossain MB, et al. Exclusive breastfeeding and infant iron and zinc status, the MINIMat study Bangladesh [abstract]. Journal of Human Lactation 2007;23(1):79‐80. [Google Scholar]
- Eneroth H, Arifeen S, Persson LA, Kabir I, Lonnerdal B, Hossain MB, et al. Duration of exclusive breast‐feeding and infant iron and zinc status in rural Bangladesh. Journal of Nutrition 2009;139(8):1562‐7. [DOI] [PubMed] [Google Scholar]
- Kabir I, Khan AI, Arifeen S, Alam DS, Persson LA. Effects of prenatal food and micronutrient supplementation and breastfeeding counseling on postnatal growth of rural Bangladeshi children. Pediatric Academic Societies Annual Meeting; 2009 May 2‐5; Baltimore, USA. 2009.
- Moore SE, Prentice AM, Coward WA, Wright A, Frongillo EA, Fulford AJ, et al. Use of stable‐isotope techniques to validate infant feeding practices reported by Bangladeshi women receiving breastfeeding counseling. American Journal of Clinical Nutrition 2007;85(4):1075‐82. [DOI] [PubMed] [Google Scholar]
Ferrara 2008 {published data only}
- Ferrara A, Hedderson MM, Albright CL, Ehrlich SF, Quesenberry CP Jr, Peng T, et al. A pregnancy and postpartum lifestyle intervention in women with gestational diabetes mellitus reduces diabetes risk factors: a feasibility randomized control trial. Diabetes Care 2011;34(7):1519‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00460018. Diet, exercise and breastfeeding intervention program for women with gestational diabetes (DEBI Trial). clinicaltrials.gov/show/NCT00460018 Date first received: 11 April 2007.
Finch 2002 {published data only}
- Finch C, Daniel EL. Breastfeeding education program with incentives increases exclusive breastfeeding among urban WIC participants. Journal of the American Dietetic Association 2002;102(7):981‐4. [DOI] [PubMed] [Google Scholar]
Finch 2015 {published data only}
- Finch M, Yoong SL, Thomson RJ, Seward K, Cooney M, Jones J, et al. A pragmatic randomised controlled trial of an implementation intervention to increase healthy eating and physical activity‐promoting policies, and practices in centre‐based childcare services: study protocol. BMJ Open 2015;5(5):e006706. [DOI] [PMC free article] [PubMed] [Google Scholar]
Flax 2014 {published data only}
- Flax V, Negerie M, Usman A, Leatherman S, Daza E, Bentley M. Nigerian women participating in an integrated microcredit and mhealth breastfeeding promotion intervention were more likely to adopt international breastfeeding recommendations. Annals of Nutrition & Metabolism 2013;63(Suppl 1):885, Abstract no: PO1294. [Google Scholar]
- Flax VL, Negerie M, Ibrahim AU, Leatherman S, Daza EJ, Bentley ME. Integrating group counseling, cell phone messaging, and participant‐generated songs and dramas into a microcredit program increases Nigerian women's adherence to international breastfeeding recommendations. Journal of Nutrition 2014;144(7):1120‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Forster 2004 {published data only}
- Forster D, McLachlan H, Lumley J, Beanland C, Waldenstrom U, Amir L. Two mid‐pregnancy interventions to increase the initiation and duration of breastfeeding: a randomized controlled trial. Birth 2004;31(3):176‐82. [DOI] [PubMed] [Google Scholar]
- Forster D, McLachlan H, Lumley J, Beanland C, Waldenstrom U, Harris H, et al. ABFAB. Attachment to the breast and family attitudes to breastfeeding. The effect of breastfeeding education in the middle of pregnancy on the initiation and duration of breastfeeding: a randomised controlled trial. BMC Pregnancy and Childbirth 2003;3(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster DA, McLachlan HL, Lumley J. Factors associated with breastfeeding at six months postpartum in a group of Australian women. International Breastfeeding Journal 2006;1:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster DA, McLachlan HL, Lumley J, Beanland CJ, Waldenstrom U, Short RV, et al. ABFAB: attachment to the breast and family attitudes towards breastfeeding. The effect of breastfeeding education in the middle of pregnancy on the duration of breastfeeding: a randomised controlled trial [abstract]. Perinatal Society of Australia and New Zealand 7th Annual Congress; 2003 March 9‐12; Tasmania, Australia. 2003:A70. [DOI] [PMC free article] [PubMed]
Forster 2006 {published data only}
- Forster DA, McLachlan HL, Lumley J. Risk factors for early cessation of breastfeeding: results from a randomised controlled trial. Perinatal Society of Australia and New Zealand 10th Annual Congress; 2006 April 3‐6; Perth, Australia. 2006:149.
Gagnon 1997 {published data only}
- Gagnon AJ, Edgar L, Kramer MS, Papageorgiou A, Waghorn K, Klein MC. A randomized trial of a program of early postpartum discharge with nurse visitation. American Journal of Obstetrics and Gynecology 1997;176:205‐11. [DOI] [PubMed] [Google Scholar]
Garcia‐Montrone 1996 {published data only}
- Garcia‐Montrone V, Rose JC. An education experience for promoting breast‐feeding and infant stimulation by low‐income women: a preliminary study. Cadernos de Saude Publica 1996;12(1):61‐8. [DOI] [PubMed] [Google Scholar]
Giglia 2015 {published data only}
- Giglia R, Cox K, Zhao Y, Binns CW. Exclusive breastfeeding increased by an internet intervention. Breastfeeding Medicine 2015;10(1):20‐5. [DOI] [PubMed] [Google Scholar]
Gijsbers 2006 {published data only}
- Gijsbers B, Mesters I, Knottnerus JA, Kester AD, Schayck CP. The success of an educational program to promote exclusive breastfeeding for 6 months in families with a history of asthma: a randomized controlled trial. Pediatric Asthma 2006;19(4):214‐22. [Google Scholar]
- Gijsbers B, Mesters I, Knottnerus JA, Schayck CP. Factors associated with the initiation of breastfeeding in asthmatic families: the attitude‐social influence‐self‐efficacy model. Breastfeeding Medicine 2006;1(4):236‐46. [DOI] [PubMed] [Google Scholar]
- Gijsbers B, Mesters I, Knottnerus JA, Schayck CP. Factors associated with the duration of exclusive breast‐feeding in asthmatic families. Health Education Research 2008;23(1):158‐69. [DOI] [PubMed] [Google Scholar]
Girish 2013 {published data only}
- Girish M, Mujawar N, Gotmare P, Paul N, Punia S, Pandey P. Impact and feasibility of breast crawl in a tertiary care hospital. Journal of Perinatology 2013;33(4):288‐91. [DOI] [PubMed] [Google Scholar]
Guise 2003 {published data only}
- Guise JM, Palda V, Westhoff C, Chan BK, Helfand M, Lieu TA, et al. The effectiveness of primary care‐based interventions to promote breastfeeding: systematic evidence review and meta‐analysis for the US Preventive Services Task Force. Annals of Family Medicine 2003;1(2):70‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Haider 1996 {published data only}
- Haider R, Islam A, Hamadani J, Amin NJ, Kabir I, Malek MA, et al. Breast‐feeding counseling in a diarrhoeal disease hospital. Revista Panamericana De Salud Publica/Pan American Journal of Public Health 1997;1:355‐61. [Google Scholar]
- Haider R, Islam A, Hamadani J, Amin NJ, Kabir I, Malek MA, et al. Breastfeeding counselling in a diarrhoeal disease hospital. Bulletin of the World Health Organization 1996;74(2):173‐9. [PMC free article] [PubMed] [Google Scholar]
Haider 2014 {published data only}
- Haider SJ, Chang LV, Bolton TA, Gold JG, Olson BH. An evaluation of the effects of a breastfeeding support program on health outcomes. Health Services Research 2014;49(6):2017‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hall 2007 {published data only}
- Hall WA, Hauck Y. Getting it right: Australian primiparas' views about breastfeeding: a quasi‐experimental study. International Journal of Nursing Studies 2007;44(5):786‐95. [DOI] [PubMed] [Google Scholar]
Hanafi 2014 {published data only}
- Hanafi MI, Shalaby SA, Falatah N, El‐Ammari H. Impact of health education on knowledge of, attitude to and practice of breastfeeding among women attending primary health care centres in Almadinah Almunawwarah, Kingdom of Saudi Arabia: controlled pre‐post study. Journal of Taibah University Medical Sciences 2014;9(3):187‐93. [Google Scholar]
Harari 2014 {published data only}
- Harari N, Rosenthal MS, Griswold M, Goeschel L, Bozzi V, Fenick AM, et al. Impact of a text message intervention used as an adjunct tool by WIC breastfeeding counselors: the LATCH project. Pediatric Academic Societies and Asian Society for Pediatric Research Joint Meeting; 2014 May 3‐6; Vancouver, Canada. 2014:Abstract no: 2195.6.
Hauck 1994 {published data only}
- Hauck YL, Dimmock JE. Evaluation of an information booklet on breastfeeding duration: a clinical trial. Journal of Advanced Nursing 1994;20(5):836‐43. [DOI] [PubMed] [Google Scholar]
Henderson 2001 {published data only}
- Henderson A, Stamp G, Pincombe J. Postpartum positioning and attachment education for increasing breastfeeding: a randomized trial. Birth 2001;28(4):236‐42. [DOI] [PubMed] [Google Scholar]
Hives‐Wood 2013 {published data only}
- Hives‐Wood S. Trial will test whether shopping vouchers encourage breast feeding. BMJ (Clinical research ed.) 2013;347:F6807. [DOI] [PubMed] [Google Scholar]
Hoddinott 2012a {published data only}
- Hoddinott P, Craig L, MacLennan G, Boyers D, Vale L. Process evaluation for the FEeding Support Team (FEST) randomised controlled feasibility trial of proactive and reactive telephone support for breastfeeding women living in disadvantaged areas. BMJ Open 2012;2(2):e001039. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ijumba 2015 {published data only}
- Ijumba P, Doherty T, Jackson D, Tomlinson M, Sanders D, Swanevelder S, et al. Effect of an integrated community‐based package for maternal and newborn care on feeding patterns during the first 12 weeks of life: a cluster‐randomized trial in a South African township. Public Health Nutrition 2015;18(14):2660‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Israel‐Ballard 2014 {published data only}
- NCT02162498. Effect of feeding buddies on adherence to WHO PMTCT guidelines in South Africa. clinicaltrials.gov/show/NCT02162498 Date first received: 10 June 2014.
Isselmann 2006 {published data only}
- Isselmann KF, Collins B, McCoy A. A prospective efficacy trial of a brief breastfeeding promotion intervention to prevent postpartum smoking relapse. American Public Health Association 134th Annual Meeting & Exposition 2006 Nov 4‐8; Boston, MA. 2006.
Jahan 2014 {published data only}
- Jahan K, Roy SK, Mihrshahi S, Sultana N, Khatoon S, Roy H, et al. Short‐term nutrition education reduces low birthweight and improves pregnancy outcomes among urban poor women in Bangladesh. Food and Nutrition Bulletin 2014;35(4):414‐21. [DOI] [PubMed] [Google Scholar]
Jakobsen 2008 {published data only}
- Jakobsen MS, Sodemann M, Biai S, Nielsen J, Aaby P. Promotion of exclusive breastfeeding is not likely to be cost effective in West Africa. A randomized intervention study from Guinea‐Bissau. Acta Paediatrica 2008;97:68‐75. [DOI] [PubMed] [Google Scholar]
Jang 2008 {published data only}
- Jang GJ, Kim SH. Effects of breast‐feeding education and support services on breast‐feeding rates and infant's growth. Journal of Korean Academy of Nursing 2010;40(2):277‐86. [DOI] [PubMed] [Google Scholar]
- Jang GJ, Kim SH, Jeong KS. Effect of postpartum breast‐feeding support by nurse on the breast‐feeding prevalence. Taehan Kanho Hakhoe Chi 2008;38(1):172‐9. [DOI] [PubMed] [Google Scholar]
Johnston 2001 {published data only}
- Johnston BD, Huebner CE, Anderson ML, Tyll LT, Thompson RS. Healthy steps in an integrated delivery system: child and parent outcomes at 30 months. Archives of Pediatrics & Adolescent Medicine 2006;160(8):793‐800. [DOI] [PubMed] [Google Scholar]
- Johnston BD, Thompson RS, Huebner CE, Barlow WE, Tyll L. Expanded well‐child care with a pre‐natal component: early results from the group health evaluation of "healthy steps" [abstract]. Pediatric Research 2001;49(4):132A. [Google Scholar]
Jones 2004 {published data only}
- Jones E, Jones P, Spencer A. Breastfeeding and returning to work. Practising Midwife 2004;7(11):17‐8, 20, 22. [PubMed] [Google Scholar]
Junior 2007 {published data only}
- Junior WS, Martinez FE. Effect of intervention on the rates of breastfeeding of very low birth weight newborns. Jornal de Pediatria 2007;83(6):541‐6. [DOI] [PubMed] [Google Scholar]
Katepa‐Bwalya 2011 {published data only}
- Katepa‐Bwalya M, Kankasa C, Babaniyi O, Siziya S. Effect of using HIV and infant feeding counselling cards on the quality of counselling provided to HIV positive mothers: a cluster randomized controlled trial. International Breastfeeding Journal 2011;6:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kistin 1994 {published data only}
- Kistin N, Abramson R, Dublin P. Effect of peer‐counsellors on breastfeeding initiation, exclusivity and duration among low‐income women. Journal of Human Lactation 1994;10(1):11‐5. [DOI] [PubMed] [Google Scholar]
Kronborg 2012 {published data only}
- Kronborg H, Maimburg RD, Vaeth M. Antenatal training to improve breast feeding: a randomised trial. Midwifery 2012;28(6):784‐90. [DOI] [PubMed] [Google Scholar]
Labarere 2003 {published data only}
- Labarere J, Bellin V, Fourny M, Gagnaire JC, Francois P, Pons JC. Assessment of a structured in‐hospital educational intervention addressing breastfeeding: a prospective randomised open trial. BJOG: an international journal of obstetrics and gynaecology 2003;110:847‐52. [PubMed] [Google Scholar]
Labarere 2011 {published data only}
- Labarere J, Gelbert‐Baudino N, Laborde L, Arragain D, Schelstraete C, Francois P. CD‐ROM‐based program for breastfeeding mothers. Maternal & Child Nutrition 2011;7(3):263‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lavender 2004 {published data only}
- Lavender T. Breastfeeding: expectations versus reality. 10th International Conference of Maternity Care Researchers; 2004 June 13‐16; Lund, Sweden. 2004:12.
- Lavender T, Baker L, Smyth R, Collins S, Spofforth A, Dey P. Breastfeeding expectations versus reality: a cluster randomised controlled trial. BJOG: an international journal of obstetrics and gynaecology 2005;112:1047‐53. [DOI] [PubMed] [Google Scholar]
Lewin 2005 {published data only}
- Lewin SA, Dick J, Pond P, Zwarenstein M, Aja G, Wyk B, et al. Lay health workers in primary and community health care. Cochrane Database of Systematic Reviews 2005, Issue 1. [DOI: 10.1002/14651858.CD004015.pub2] [DOI] [PubMed] [Google Scholar]
Lieu 2000 {published data only}
- Lieu TA, Braveman PA, Escobar GJ, Fischer AF, Jensvold NG, Capra AM. A randomized comparison of home and clinic follow‐up visits after early postpartum hospital discharge. Pediatrics 2000;105:1058‐65. [DOI] [PubMed] [Google Scholar]
Louzada 2012 {published data only}
- Louzada ML, Campagnolo PD, Rauber F, Vitolo MR. Long‐term effectiveness of maternal dietary counseling in a low‐income population: a randomized field trial. Pediatrics 2012;129(6):e1477‐e1484. [DOI] [PubMed] [Google Scholar]
MacArthur 2002 {published data only}
- MacArthur C, Winter HR, Bick DE, Knowles H, Lilford R, Henderson C, et al. Effects of redesigned community postnatal care on women's health 4 months after birth: a cluster randomised trial. Lancet 2002;359:378‐85. [DOI] [PubMed] [Google Scholar]
MacArthur 2009 {published data only}
- MacArthur C, Jolly K, Ingram L, Freemantle N, Dennis CL, Hamburger R, et al. Antenatal peer support workers and initiation of breast feeding: cluster randomised controlled trial. BMJ 2009;338:b131. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mannan 2008 {published data only}
- Mannan I, Rahman SM, Sania A, Seraji HR, Arifeen SE, Winch PJ, et al. Can early postpartum home visits by trained community health workers improve breastfeeding of newborns?. Journal of Perinatology 2008;28(9):632‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Martin 2015 {published data only}
- Martin J, MacDonald‐Wicks L, Hure A, Smith R, Collins CE. Reducing postpartum weight retention and improving breastfeeding outcomes in overweight women: a pilot randomised controlled trial. Nutrients 2015;7(3):1464‐79. [DOI] [PMC free article] [PubMed] [Google Scholar]
Martin‐Iglesias 2011 {published data only}
- Martin‐Iglesias S, del‐Cura‐Gonzalez I, Sanz‐Cuesta T, Arana‐Canedo Arguelles C, Rumayor‐Zarzuelo M, Alvarez‐de la Riva M, et al. Effectiveness of an implementation strategy for a breastfeeding guideline in primary care: cluster randomised trial. BMC Family Practice 2011;12:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mattar 2003 {published data only}
- Mattar CN, Chan YS, Chong YS. Breastfeeding: it's an important gift. Obstetrics and Gynecology 2003;102(6):1414. [DOI] [PubMed] [Google Scholar]
Maycock 2013 {published data only}
- Maycock B, Binns CW, Dhaliwal S, Tohotoa J, Hauck Y, Burns S, et al. Education and support for fathers improves breastfeeding rates: a randomized controlled trial. Journal of Human Lactation 2013;29(4):484‐90. [DOI] [PubMed] [Google Scholar]
Maycock 2015 {published data only}
- Maycock BR, Scott JA, Hauck YL, Burns SK, Robinson S, Giglia R, et al. A study to prolong breastfeeding duration: design and rationale of the Parent Infant Feeding Initiative (PIFI) randomised controlled trial. BMC Pregnancy and Childbirth 2015;15:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
McInnes 2000 {published data only}
- McInnes RJ, Love JG, Stone DH. Evaluation of a community‐based intervention to increase breastfeeding prevalence. Journal of Public Health Medicine 2000;22(2):138‐45. [DOI] [PubMed] [Google Scholar]
McLeod 2003 {published data only}
- McLeod D, Benn C, Pullon S, Viccars A, White S, Cookson T, et al. The midwife's role in facilitating smoking behaviour change during pregnancy. Midwifery 2003;19(4):285‐97. [DOI] [PubMed] [Google Scholar]
- McLeod D, Pullon S, Benn C, Cookson T, Dowell A, Viccars A, et al. Can support and education for smoking cessation and reduction be provided by midwives within primary care?. Midwifery 2004;20:37‐50. [DOI] [PubMed] [Google Scholar]
Merewood 2006 {published data only}
- Merewood A, Chamberlain LB, Cook JT, Philipp BL, Malone K, Bauchner H. The effect of peer counselors on breastfeeding rates in the neonatal intensive care unit: results of a randomized controlled trial. Archives of Pediatrics & Adolescent Medicine 2006;160(7):681‐5. [DOI] [PubMed] [Google Scholar]
- Merewood A, Philipp BL, Chamberlain LB, Malone KL, Cook JT, Bauchner H. Using peer support to improve breastfeeding rates among premature infants: an RCT [abstract]. Pediatric Academic Societies Annual Meeting; 2005 May 14‐17; Washington DC, USA. 2005:Abstract no: 2342.
- Philipp BL, Merewood A, Malone KL, Chamberlain LB, Cook JT, Bauchner H. Effect of NICU‐based peer counselors on breastfeeding duration among premature infants [abstract]. Pediatric Research 2004;55 Suppl:73. [Google Scholar]
Mesters 2013 {published data only}
- Mesters I, Gijsbers B, Bartholomew K, Knottnerus JA, Schayck OC. Social cognitive changes resulting from an effective breastfeeding education program. Breastfeeding Medicine 2013;8(1):23‐30. [DOI] [PubMed] [Google Scholar]
Moore 1985 {published data only}
- Moore WJ, Midwinter, Morris AF, Colley JR, Soothill JF. Infant feeding and subsequent risk of atopic eczema. Archives of Disease in Childhood 1985;60(8):722‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moreno‐Manzanares 1997 {published data only}
- Moreno‐Manzanares L, Cabrera‐Sanz MT, Garcia‐Lopez L. Breast feeding [Lactancia materna]. Revista Rol de Enfermeria 1997;20(227‐8):79‐84. [PubMed] [Google Scholar]
Nasehi 2012 {published data only}
- Nasehi MM, Farhadi R, Ghaffari V, Ghaffari‐Charati M. The effect of early breastfeeding after cesarean section on the success of exclusive breastfeeding. HealthMED 2012;6(11):3597‐601. [Google Scholar]
Nekavand 2014 {published data only}
- Nekavand M, Hoorsan R, Kerami A, Zohoor A. Effect of exclusive breast feeding education on breast‐feeding self‐efficacy and maternal stress. Research Journal of Obstetrics and Gynecology 2014;7(1):1‐5. [Google Scholar]
Neyzi 1991 {published data only}
- Neyzi O, Gulecyuz M, Dincer Z, Olgun P, Kutluay T, Uzel N, et al. An educational intervention on promotion of breast feeding complemented by continuing support. Paediatric and Perinatal Epidemiology 1991;5:299‐303. [DOI] [PubMed] [Google Scholar]
Nguyen 2014 {published data only}
- Nguyen PH, Menon P, Keithly SC, Kim SS, Hajeebhoy N, Tran LM, et al. Program impact pathway analysis of a social franchise model shows potential to improve infant and young child feeding practices in Vietnam. Journal of Nutrition 2014;144(10):1627‐36. [DOI] [PubMed] [Google Scholar]
Nkonki 2014 {published data only}
- Nkonki LL, Daviaud E, Jackson D, Chola L, Doherty T, Chopra M, et al. Costs of promoting exclusive breastfeeding at community level in three sites in South Africa. PLOS ONE 2014;9(1):e79784. [DOI] [PMC free article] [PubMed] [Google Scholar]
Noel‐Weiss 2006 {published data only}
- Noel‐Weiss J, Rupp A, Cragg B, Bassett V, Woodend AK. Randomized controlled trial to determine effects of prenatal breastfeeding workshop on maternal breastfeeding self‐efficacy and breastfeeding duration. Journal of Obstetric, Gynecologic and Neonatal Nursing 2006;35(5):616‐24. [DOI] [PubMed] [Google Scholar]
Nor 2009 {published data only}
- Nor B, Zembe Y, Daniels K, Doherty T, Jackson D, Ahlberg BM, et al. "Peer but not peer": considering the context of infant feeding peer counseling in a high HIV prevalence area. Journal of Human Lactation 2009;25(4):427‐34. [DOI] [PubMed] [Google Scholar]
Nor 2012 {published data only}
- Nor B, Ahlberg BM, Doherty T, Zembe Y, Jackson D, Ekstrom EC, et al. Mother's perceptions and experiences of infant feeding within a community‐based peer counselling intervention in South Africa. Maternal & Child Nutrition 2012;8(4):448‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ochola 2013a {published data only}
- Ochola S, Demetre L, Nduati R. Potentials and barriers to exclusive breastfeeding among women in an urban low‐resource setting in Nairobi, Kenya: a qualitative study. Annals of Nutrition & Metabolism 2013;63(Suppl 1):808, Abstract no: PO3318. [Google Scholar]
Olenick 2011 {published data only}
- Olenick PL. The effect of structured group prenatal education on breastfeeding confidence, duration, and exclusivity to 12 weeks postpartum. Journal of Human Lactation 2011;27(1):71‐2. [Google Scholar]
Otsuka 2012 {published data only}
- Otsuka K, Kitamura T, Jimba M. Can breastfeeding enhance maternal‐infant bonding?. Breastfeeding Medicine 2012;7(Suppl 1):S‐7. [Google Scholar]
Otsuka 2014 {published data only}
- Otsuka K, Taguri M, Dennis CL, Wakutani K, Awano M, Yamaguchi T, et al. Effectiveness of a breastfeeding self‐efficacy intervention: do hospital practices make a difference?. Maternal & Child Health Journal 2014;18(1):296‐306. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pascali‐Bonaro 2004 {published data only}
- Pascali‐Bonaro D, Kroeger M. Continuous female companionship during childbirth: a crucial resource in times of stress or calm. Journal of Midwifery & Women's Health 2004;49(4 Suppl 1):19‐27. [DOI] [PubMed] [Google Scholar]
Paul 2011 {published data only}
- Paul IM, Savage JS, Anzman SL, Beiler JS, Marini ME, Stokes JL, et al. Preventing obesity during infancy: a pilot study. Obesity (Silver Spring, Md.) 2011;19(2):353‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Penfold 2014 {published data only}
- Penfold S, Manzi F, Mkumbo E, Temu S, Jaribu J, Shamba DD, et al. Effect of home‐based counselling on newborn care practices in southern Tanzania one year after implementation: a cluster‐randomised controlled trial. BMC Pediatrics 2014;14(1):187. [DOI] [PMC free article] [PubMed] [Google Scholar]
Perez‐Blasco 2013 {published data only}
- Perez‐Blasco J, Viguer P, Rodrigo MF. Effects of a mindfulness‐based intervention on psychological distress, well‐being, and maternal self‐efficacy in breast‐feeding mothers: results of a pilot study. Archives of Women's Mental Health 2013;16(3):227‐36. [DOI] [PubMed] [Google Scholar]
Perez‐Escamilla 1992 {published data only}
- Perez‐Escamilla R, Segura‐Millan S, Pollitt E, Dewey K. Effect of the maternity ward system on the lactation success of low‐income urban Mexican women. Early Human Development 1992;31(1):25‐40. [DOI] [PubMed] [Google Scholar]
Peterson 2002 {published data only}
- Peterson KE, Sorensen G, Pearson M, Hebert JR, Gottlieb BR, McCormick MC. Design of an intervention addressing multiple levels of influence on dietary and activity patterns of low‐income, postpartum women. Health Education Research 2002;17(5):531‐40. [DOI] [PubMed] [Google Scholar]
Phillips 2010 {published data only}
- Phillips RM, Merritt TA, Goldstein MR, Deming DD, Job JS, Rudatsikira EM. Increasing the duration of breastfeeding by preventing postpartum smoking relapse in mothers of infants in the neonatal intensive care unit. American Academy of Pediatrics Annual Meeting; 2010 October 2‐5; San Francisco, California, USA. 2010.
Phillips 2011 {published data only}
- Phillips RM, Merritt TA, Goldstein MR, Deming DD, Slater LE, Angeles DM. Supporting mother‐infant bonding increases the duration of breastfeeding in mothers with newborns in the neonatal intensive care unit. Breastfeeding Medicine 2011;6 Suppl 1:S‐3‐S‐4. [Google Scholar]
Phillips 2012 {published data only}
- Phillips RM, Merritt TA, Goldstein MR, Deming DD, Slater LE, Angeles DM. Prevention of postpartum smoking relapse in mothers of infants in the neonatal intensive care unit. Journal of Perinatology 2012;32(5):374‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pinelli 2001 {published data only}
- Pinelli J, Atkinson SA, Saigal S. Randomized trial of breastfeeding support in very low‐birth‐weight infants. Archives of Pediatric and Adolescent Medicine 2001;155(5):548‐53. [DOI] [PubMed] [Google Scholar]
Pollard 1998 {published data only}
- Pollard DL. The Effect of Self‐Regulation on Breastfeeding Duration in Primiparous Mothers [thesis]. University of Pittsberg, 1998. [Google Scholar]
Pollard 2011 {published data only}
- Pollard DL. Impact of a feeding log on breastfeeding duration and exclusivity. Maternal and Child Health Journal 2011;15(3):395‐400. [DOI] [PubMed] [Google Scholar]
Pound 2015 {published data only}
- Pound C, Moreau K, Rohde K, Farion K, Barrowman N, Aglipay M, et al. The impact of a breastfeeding support intervention on breastfeeding duration in jaundiced infants admitted to a tertiary care centre hospital: a randomized controlled trial. Paediatrics and Child Health (Canada) 2014;19(6):e95, Abstract no: 172. [Google Scholar]
- Pound CM, Moreau K, Rohde K, Barrowman N, Aglipay M, Farion KJ, et al. Lactation support and breastfeeding duration in jaundiced infants: a randomized controlled trial. PLOS One 2015;10(3):e0119624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pound CM, Moreau K, Rohde K, Farion K, Barrowman N, Aglipay M, et al. The impact of a breastfeeding support intervention on breastfeeding duration in jaundiced infants admitted to a tertiary care centre hospital: a randomized controlled trial. Pediatric Academic Societies and Asian Society for Pediatric Research Joint Meeting; 2014 May 3‐6; Vancouver, Canada. 2014:Abstract no: 198.
Rasmussen 2010 {published data only}
- Rasmussen KM, Dieterich CM, Zelek ST, Altabet JD, Kjolhede CL. Interventions to increase the duration of breastfeeding in obese mothers. Journal of Human Lactation 2010;26(4):430. [DOI] [PubMed] [Google Scholar]
Rasmussen 2011 {published data only}
- Rasmussen KM, Dieterich CM, Zelek ST, Altabet JD, Kjolhede CL. Interventions to increase the duration of breastfeeding in obese mothers: the Bassett Improving Breastfeeding Study. Breastfeeding Medicine 2011;6:69‐75. [DOI] [PubMed] [Google Scholar]
Ratner 1999 {published data only}
- Ratner P, Johnson J, Bottorff J. Smoking relapse and early weaning among postpartum women: is there an association?. Birth 1999;26(1):76‐82. [DOI] [PubMed] [Google Scholar]
Rea 1999 {published data only}
- Rea MF, Venancio SI, Martines JC, Savage F. Counselling on breastfeeding: assessing knowledge and skills. Bulletin of the World Health Organization 1999;77(6):492‐8. [PMC free article] [PubMed] [Google Scholar]
Reeve 2004 {published data only}
- Reeve JR, Gull SE, Johnson MH, Hunter S, Streather M. A preliminary study on the use of experiential learning to support women's choices about infant feeding. European Journal of Obstetrics & Gynecology and Reproductive Biology 2004;113:199‐203. [DOI] [PubMed] [Google Scholar]
Rojjanasrirat 1987 {published data only}
- Rojjanasrirat W. The Effects of a Nursing Intervention on Breastfeeding Duration Among Primiparous Mothers Planning to Return to Work [thesis]. University of Kansas, 1987. [Google Scholar]
Rossiter 1994 {published data only}
- Rossiter JC. The effect of a culture‐specific education program to promote breastfeeding among Vietnamese women in Sydney. International Journal of Nursing Studies 1994;31(4):369‐79. [DOI] [PubMed] [Google Scholar]
Rowe 1990 {published data only}
- Rowe L, Hartmann PE. Comparison of two methods of breast feeding management. Proceedings of 6th Congress of the Federation of the Asia‐Oceania Perinatal Societies; 1990; Perth, Western Australia. 1990:236.
Rush 1991 {published data only}
- Rush JP, Kitch TL. A randomized, controlled trial to measure the frequency of use of a hospital telephone line for new parents. Birth 1991;18:193‐7. [DOI] [PubMed] [Google Scholar]
Sakha 2008 {published data only}
- Sakha K, Behbahan AG. Training for perfect breastfeeding or metoclopramide: which one can promote lactation in nursing mothers?. Breastfeeding Medicine 2008;3(2):120‐3. [DOI] [PubMed] [Google Scholar]
Sakkaki 2013 {published data only}
- Sakkaki M, Khairkhah M. Promotion of exclusive breastfeeding: teaching good positioning and support from fathers and families. Journal of Urmia Nursing & Midwifery Faculty 2013;10(6):824‐32. [Google Scholar]
Schlomer 1999 {published data only}
- Schlomer JA, Kemmerer J, Twiss JJ. Evaluating the association of two breastfeeding assessment tools with breastfeeding problems and breastfeeding satisfaction. Journal of Human Lactation 1999;15(1):35‐9. [DOI] [PubMed] [Google Scholar]
Schy 1996 {published data only}
- Schy DS, Maglaya CF, Mendelson SG, Race KE, Ludwig‐Beymer P. The effects of in‐hospital lactation education on breastfeeding practice. Journal of Human Lactation 1996;12(2):117‐22. [DOI] [PubMed] [Google Scholar]
Sciacca 1995 {published data only}
- Sciacca JP, Dube DA, Phipps BL, Ratliff MI. A breast feeding education and promotion program: effects on knowledge, attitudes, and support for breast feeding. Journal of Community Health 1995;20(6):473‐89. [DOI] [PubMed] [Google Scholar]
- Sciacca JP, Phipps B, Dube D, Ratliff MI. Influences on breast‐feeding by lower‐income women: an incentive, partner‐supported educational program. Journal of the American Dietetic Association 1995;95(3):323‐8. [DOI] [PubMed] [Google Scholar]
Segura‐Millan 1994 {published data only}
- Segura‐Millan S, Dewey KG, Perez‐Escamilla R. Factors associated with perceived insufficient milk in a low‐income urban population in Mexico. Journal of Nutrition 1994;124(2):202‐12. [DOI] [PubMed] [Google Scholar]
Serrano 2010 {published data only}
- Serrano MS, Doren FM, Wilson L. Teaching Chilean mothers to massage their full‐term infants: effects on maternal breast‐feeding and infant weight gain at age 2 and 4 months. Journal of Perinatal & Neonatal Nursing 2010;24(2):172‐81. [DOI] [PubMed] [Google Scholar]
Sisk 2006 {published data only}
- Sisk PM, Lovelady CA, Dillard RG, Gruber KJ. Lactation counseling for mothers of very low birthweight infants: effect on maternal anxiety and infant intake of human milk. Pediatrics 2006;117(1):E67‐E75. [DOI] [PubMed] [Google Scholar]
Steel O'Connor 2003 {published data only}
- Steel O'Connor KO, Mowat DL, Scott HM, Carr PA, Dorland JL, Young Tai KF. A randomized trial of two public health nurse follow‐up programs after early obstetrical discharge: an examination of breastfeeding rates, maternal confidence and utilization and costs of health services. Canadian Journal of Public Health 2003;94(2):98‐103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stuebe 2016 {published data only}
- Stuebe AM, Bonuck K, Adatorwovor R, Schwartz TA, Berry D. A tailored breastfeeding support intervention for women with gestational diabetes. American Journal of Obstetrics and Gynecology 2016;214(1 Suppl):S68, Abstract no: 97. [Google Scholar]
Susin 2008 {published data only}
- Susin LR, Giugliani ER. Inclusion of fathers in an intervention to promote breastfeeding: impact on breastfeeding rates. Journal of Human Lactation 2008;24(4):386‐92. [DOI] [PubMed] [Google Scholar]
Svensson 2013 {published data only}
- Svensson KE, Velandia MI, Matthiesen AS, Welles‐Nystrom BL, Widstrom AM. Effects of mother‐infant skin‐to‐skin contact on severe latch‐on problems in older infants: a randomized trial. International Breastfeeding Journal 2013;8(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Szucs 2015 {published data only}
- Szucs KA, Ahmed AH. The effect of interactive web‐based breastfeeding monitoring on maternal breastfeeding self‐efficacy and satisfaction: a randomized control trial. Pediatric Academic Socieities Annual Meeting; 2015 April 25‐28; San Diego, California, USA. 2015.
Talukder 2012 {published data only}
- Talukder SH, Greiner T, Dewey K, Haider R, Farhana D, Chowdhury SS. Cost and effectiveness of training and supervision of frontline workers on early breastfeeding practices in Bangladesh. Proceedings of the 16th ISRHML Conference "Breastfeeding and the Use of Human Milk. Science and Practice"; 2012 September 27‐ October 1; Trieste, Italy. 2012:Abstract A106.
Talukder 2016 {published data only}
- Talukder S, Farhana D, Vitta B, Greiner T. In a rural area of Bangladesh, traditional birth attendant training improved early infant feeding practices: a pragmatic cluster randomized trial. Maternal & Child Nutrition 2016 [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
Thakur 2012 {published data only}
- Thakur SK, Roy SK, Paul K, Khanam M, Khatun W, Sarker D. Effect of nutrition education on exclusive breastfeeding for nutritional outcome of low birth weight babies. European Journal of Clinical Nutrition 2012;66(3):376‐81. [DOI] [PubMed] [Google Scholar]
Thomson 2009 {published data only}
- Thomson T, Hall W, Balneaves L, Wong S. Waiting to be weighed: a pilot study of the effect of delayed newborn weighing on breastfeeding outcomes. Canadian Nurse 2009;105(6):24‐8. [PubMed] [Google Scholar]
Thussanasupap 2006 {published data only}
- Thussanasupap B. The effects of systematic instructional program on breastfeeding self‐efficacy, nipple pain, nipple skin changes and incision pain of cesarean mothers [abstract]. Care, Concern and Cure in Perinatal Health. 14th Congress of the Federation of Asia‐Oceania Perinatal Societies; 2006 Oct 1‐5; Bangkok, Thailand. 2006:138.
Tohotoa 2012 {published data only}
- Tohotoa J, Maycock B, Hauck YL, Dhaliwal S, Howat P, Burns S. Can father inclusive practice reduce paternal postnatal anxiety? A repeated measures cohort study using the hospital anxiety and depression scale. BMC Pregnancy and Childbirth 2012;12:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tully 2012 {published data only}
- Tully KP, Ball HL. Postnatal unit bassinet types when rooming‐in after cesarean birth: implications for breastfeeding and infant safety. Journal of Human Lactation 2012;28(4):495‐505. [DOI] [PMC free article] [PubMed] [Google Scholar]
Valdes 2000 {published data only}
- Valdes V, Pugin E, Schooley J, Catalan S, Aravena R. Clinical support can make the difference in exclusive breastfeeding success among working women. Journal of Tropical Pediatrics 2000;46(3):149‐54. [DOI] [PubMed] [Google Scholar]
Vianna 2011 {published data only}
- Vianna MN, Barbosa AP, Carvalhaes AS, Cunha AJ. Music therapy may increase breastfeeding rates among mothers of premature newborns: a randomized controlled trial [A musicoterapia pode aumentar os indices de aleitamento materno entre maes de recem‐nascidos prematuros: Um ensaio clinico randomizado controlado]. Jornal de Pediatria 2011;87(3):206‐12. [DOI] [PubMed] [Google Scholar]
Vitolo 2012 {published data only}
- Vitolo MR, Bortolini GA, Campagnolo PD, Hoffman DJ. Maternal dietary counseling reduces consumption of energy‐dense foods among infants: a randomized controlled trial. Journal of Nutrition Education and Behavior 2012;44(2):140‐7. [DOI] [PubMed] [Google Scholar]
Vitolo 2014 {published data only}
- Vitolo MR, Louzada ML, Rauber F. Positive impact of child feeding training program for primary care health professionals: a cluster randomized field trial [Atualizacao sobre alimentacao da crianca para profissionais de saude: estudo de campo randomizado por conglomerados]. Revista Brasileira De Epidemiologia 2014;17(4):873‐86. [DOI] [PubMed] [Google Scholar]
- Vitolo MR, Louzada ML, Rauber F, Grechi P, Gama CM. The impact of health workers's training on breastfeeding and complementary feeding practices. Cadernos De Saude Publica 2014;30(8):1695‐707. [DOI] [PubMed] [Google Scholar]
Wallace 2006 {published data only}
- Inch S, Law S, Wallace L. Hands off! The breastfeeding best start project (2). Practising Midwife 2003;6(11):24‐5. [PubMed] [Google Scholar]
- Wallace LM, Dunn OM, Alder EM, Inch S, Hills RK, Law SM. A randomised‐controlled trial in England of a postnatal midwifery intervention on breast‐feeding duration. Midwifery 2006;22:262‐73. [DOI] [PubMed] [Google Scholar]
Wan 2011 {published data only}
- Wan H, Hu S, Thobaben M, Hou Y, Yin T. Continuous primary nursing care increases satisfaction with nursing care and reduces postpartum problems for hospitalized pregnant women. Contemporary Nurse 2011;37(2):149‐59. [DOI] [PubMed] [Google Scholar]
Wasser 2015 {published data only}
- Wasser H, Bentley M. Mothers and others: designing a randomized trial to prevent obesity among infants and toddlers. FASEB Journal 2015;29(1 Suppl):[584.16]. [Google Scholar]
Westphal 1995 {published data only}
- Taddei JA, Westphal MF, Venancio S, Bogus C, Souza S. Breastfeeding training for health professionals and resultant changes in breastfeeding duration. Sao Paulo Medical Journal 2000;118:185‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westphal MF, Taddei JA, Venancio SI, Bogus CM. Breast‐feeding training for health professionals and resultant institutional changes. Bulletin of the World Health Organization 1995;73(4):461‐8. [PMC free article] [PubMed] [Google Scholar]
Wiggins 2005 {published data only}
- Wiggins M, Oakley A, Roberts I, Turner H, Rajan L, Austerberry H, et al. Postnatal support for mothers living in disadvantaged inner city areas: a randomised controlled trial. Journal of Epidemiology & Community Health 2005;59(4):288‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Williams 2014 {published data only}
- Williams A, Chantry C, Dentz H, Kiprotich M, Null C, Stewart C. Effectiveness of behavior change communication on maternal nutrition and breastfeeding practices within a cluster randomized trial in rural Western Kenya. Journal of Human Lactation 2015;31(3):534‐5. [Google Scholar]
- Williams AM, Chantry C, Dentz H, Kiprotich M, Null C, Stewart CP. Effectiveness of behavior change communication on maternal nutrition and breastfeeding practices within a cluster randomized trial in rural Western Kenya. 17th Conference of the International Society for Research in Human Milk and Lactation (ISRHML); 2014 Oct 23‐27; Kiawah Island, South Carolina, USA. 2014:140.
Wockel 2009 {published data only}
- Wockel A, Abou‐Dakn M. Influence of the partner on breastfeeding duration and breast diseases during lactation. Results of an intervention study [Einfluss des partners auf stilldauer und stillprobleme: Ergebnisse einer interventionsstudie]. Gynakologische Praxis 2009;33(4):643‐9. [Google Scholar]
- Wockel A, Abou‐Dakn M. Influence of the partner on breastfeeding duration and breast diseases during lactation. Results of an intervention study [Einfluss des partners auf stilldauer und stillprobleme. Ergebnisse einer interventionsstudie]. Padiatrische Praxis 2011;77(1):125‐31. [Google Scholar]
References to studies awaiting assessment
Babakazo 2015 {published data only}
- Babakazo P, Donnen P, Mapatano MA, Lulebo A, Okitolonda E. [Effect of the baby friendly hospital initiative on the duration of exclusive breastfeeding in Kinshasa: a cluster randomized trial]. Revue D'epidemiologie Et De Sante Publique 2015;63:285‐92. [DOI] [PubMed] [Google Scholar]
Bahri 2013 {published data only}
- Bahri N, Bagheri S, Erfani M, Rahmani R, Tolidehi H. The comparison of workshop‐training and booklet‐offering on knowledge, health beliefs and behavior of breastfeeding after delivery. Iranian Journal of Obstetrics, Gynecology and Infertility 2013;15(32):14‐22. [Google Scholar]
Cabezas 2014 {published data only}
- Cabezas PMA, Gomez RG, Ferrer AP. Midwives' mobile phone support for breastfeeding. International Confederation of Midwives 30th Triennial Congress. Midwives: Improving Women’s Health; 2014 June 1‐4; Prague, Czech Republic. 2014:P139.
Kamau‐Mbuthia 2013 {published data only}
- Kamau‐Mbuthia E, Mbugua S, Webb Girard A, Kalungu S, Sarange C, Lou W, et al. Cell phone based peer counseling to support exclusive breastfeeding is associated with more frequent help and decreased breastfeeding problems. Annals of Nutrition & Metabolism 2013;63(Suppl 1):196‐7, Abstract no: O079. [Google Scholar]
- Mbugua S, Kamau‐Mbuthia E, Webb A, Kalungu S, Sarange C, Lou W, et al. Process indicators for a randomized trial of cell phone based peer counseling to support exclusive breastfeeding in Kenya. Annals of Nutrition & Metabolism 2013;63(Suppl 1):693, Abstract no: PO905. [Google Scholar]
- Mbugua S, Kamau‐Mbuthia E, Webb Girard A, Kalungu S, Sarange C, Lou W, et al. Process indicators for a randomized trial of cell phone based peer counseling to support exclusive breastfeeding in Kenya. Annals of Nutrition & Metabolism 2013;63(Suppl 1):751, Abstract no: PO1033. [Google Scholar]
- Sellen D, Mbugua S, Webb Girard A, Kalungu S, Sarange C, Lou W, et al. A randomized controlled trial indicates benefits of cell phone based peer counseling to support exclusive breastfeeding in Kenya. Annals of Nutrition & Metabolism 2013;63(Suppl 1):751, Abstract no: PO1032. [Google Scholar]
- Sellen D, Mbugua S, Webb‐Girard A, Lou W, Duan W, Kamau‐Mbuthia E. Cell phone based peer counselling can support exclusive breastfeeding: a randomized controlled trial in Kenya. FASEB Journal 2014;28(1 Suppl 1):[Abstract no. 119.5]. [Google Scholar]
- Sellen DW, Kamau‐Mbuthia E, Mbugua S, Webb Girard AL, Lou W, Dennis CL, et al. Lessons learned in providing peer support through cell phones and group meetings to increase exclusive breastfeeding in Kenya. Proceedings of the 16th ISRHML Conference 'Breastfeeding and the Use of Human Milk. Science and Practice'; 2012 September 27‐October 1; Trieste, Italy. 2012:Abstract no. A18.
- Webb Girard A, Kamau‐Mbuthia E, Mbugua S, Kalungu S, Sarange C, Lou W, et al. Infant medication, illness and growth in a randomized controlled trial of exclusive breastfeeding support in Kenya. Annals of Nutrition & Metabolism 2013;63(Suppl 1):752, Abstract no: PO1034. [Google Scholar]
Li 2014 {published data only}
- Li M, Jiang H, Hu QZ, He GS, Wen LM, Dibley MJ, et al. Text message to promote breastfeeding and obesity‐protective eating behaviours in young children: 12 and 24 months BMI results. Obesity Research and Clinical Practice 2014;8(1):58. [Google Scholar]
Mortazavi 2014 {published data only}
- Mortazavi F, Delara M, Akaberi A. Male involvement in prenatal care: impacts on pregnancy and birth outcomes. Journal of Urmia Nursing & Midwifery Faculty 2014;12(1):63‐72. [Google Scholar]
Raisi 2012 {published data only}
- Raisi DZ, Raei M, Ghassab SM, Ahmad RS, Mirmohammadali M. Effect of telephone counseling on continuity and duration of breastfeeding among primiparus women. HAYAT 2012;18(2):9‐10. [Google Scholar]
Reeder 2014 {published data only}
- Reeder JA, Joyce T, Sibley K, Arnold D, Altindag O. Telephone peer counseling of breastfeeding among WIC participants: a randomized controlled trial. Pediatrics 2014;134(3):e700‐e709. [DOI] [PMC free article] [PubMed] [Google Scholar]
Taylor 2014 {published data only}
- Taylor R. Providing additional guidance and support to parents about sleep, diet and physical activity from birth to 2 years of age: The Prevention of Overweight in Infancy study. Obesity Research & Clinical Practice 2014;8:102‐3. [Google Scholar]
Whalen 2011 {published data only}
- Whalen B, Murray D, MacKenzie T, Bernstein H. Efficacy of an online breastfeeding tutorial and maternal needs assessment in increasing breastmilk‐only feeding in a pediatric practice: results of a randomized controlled trial. Pediatric Academic Societies and Asian Society for Pediatric Research Joint Meeting; 2011 April 30‐May 3; Denver, Colorado, USA. 2011:2902.36.
References to ongoing studies
Forster 2014 {published data only}
- Forster DA, McLachlan HL, Davey MA, Amir LH, Gold L, Small R, et al. Ringing Up about Breastfeeding: a randomised controlled trial exploring earlY telephone peer support for breastfeeding (RUBY) ‐ trial protocol. BMC Pregnancy and Childbirth 2014;14(1):177. [DOI] [PMC free article] [PubMed] [Google Scholar]
Karanja 2012 {published data only}
- Karanja N, Aickin M, Lutz T, Mist S, Jobe JB, Maupomé G, et al. A community‐based intervention to prevent obesity beginning at birth among American Indian children: study design and rationale for the PTOTS study. Journal of Primary Prevention 2012;33(4):161‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kikuchi 2015 {published data only}
- Kikuchi K, Ansah E, Okawa S, Shibanuma A, Gyapong M, Owusu‐Agyei S, et al. Ghana's Ensure Mothers and Babies Regular Access to Care (EMBRACE) program: study protocol for a cluster randomized controlled trial. Trials 2015;16:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kimani‐Murage 2013 {published data only}
- Kimani‐Murage EW, Kyobutungi C, Ezeh AC, Wekesah F, Wanjohi M, Muriuki P, et al. Effectiveness of personalised, home‐based nutritional counselling on infant feeding practices, morbidity and nutritional outcomes among infants in Nairobi slums: study protocol for a cluster randomised controlled trial. Trials (electronic resource) 2013;14:445. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kimani‐Murage 2015 {published data only}
- Kimani‐Murage EW, Kimiywe J, Kabue M, Wekesah F, Matiri E, Muhia N, et al. Feasibility and effectiveness of the baby friendly community initiative in rural Kenya: study protocol for a randomized controlled trial. Trials 2015;16(1):431. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nabulsi 2014 {published data only}
- Nabulsi M, Hamadeh H, Tamim H, Kabakian T, Charafeddine L, Yehya N, et al. A complex breastfeeding promotion and support intervention in a developing country: study protocol for a randomized clinical trial. BMC Public Health 2014;14:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nair 2015 {published data only}
- Nair N, Tripathy P, Sachdev HS, Bhattacharyya S, Gope R, Gagrai S, et al. Participatory women's groups and counselling through home visits to improve child growth in rural eastern India: protocol for a cluster randomised controlled trial. BMC Public Health 2015;15(1):384. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT01383070 {published data only}
- CTRI/2011/06/001822. Effectiveness of cell phone counseling to improve breast feeding indicators. ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=3060 Date first received: 21 June 2011.
- NCT01383070. Effectiveness of cell phone counseling to improve breast feeding indicators. clinicaltrials.gov/show/NCT01383070 first received 24 June 2011.
Additional references
Almqvist‐Tangen 2012
- Almqvist‐Tangen G, Bergman S, Dahlgren J, Roswall J, Alm B. Factors associated with discontinuation of breast feeding before one month of age. Acta Paediatrica 2012;101(1):55‐60. [DOI] [PubMed] [Google Scholar]
Bartick 2010
- Bartick M, Reinhold A. The burden of suboptimal breastfeeding in the United States: a pediatric cost analysis. Pediatrics 2010;125(5):e1048. [DOI] [PubMed] [Google Scholar]
Beake 2012
- Beake S, Pellowe C, Dykes F, Schmied V, Bick D. A systematic review of structured compared with non‐structured breastfeeding programmes to support the initiation and duration of exclusive and any breastfeeding in acute and primary health care settings. Maternal & Child Nutrition 2012;8:141‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bowatte 2015
- Bowatte G, Tham R, Allen K, Tan D, Lau M, Dai X, et al. Breastfeeding and childhood acute otitis media: a systematic review and meta‐analysis. Acta Paediatrica 2015;104:85‐95. [DOI] [PubMed] [Google Scholar]
Cattaneo 2010
- Cattaneo A, Burmaz T, Arendt M, Nilsson I, Mikiel‐Kostyra K, Kondrate I, et al. Protection, promotion and support of breast‐feeding in Europe: progress from 2002 to 2007. Public Health Nutrition 2010;13:751‐9. [DOI] [PubMed] [Google Scholar]
Chowdhury 2015
- Chowdhury R, Sinha B, Sankar MJ, Taneja S, Bhandari N, Rollins N, et al. Breastfeeding and maternal health outcomes: a systematic review and meta‐analysis. Acta Paediatrica 2015;104:96‐113. [DOI] [PMC free article] [PubMed] [Google Scholar]
Donner 2000
- Donner A, Klar N. Design and Analysis of Cluster Randomised Trials in Health Research. Milton Keynes: Open University Press, 2000. [Google Scholar]
Dykes 2006
- Dykes F. The education of health practitioners supporting breastfeeding women: time for critical reflection. Maternal & Child Nutrition 2006;2:204‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dyson 2009
- Dyson L, Renfrew MJ, McFadden A, McCormick F, Herbert G, Thomas J. Policy and public health recommendations to promote the initiation and duration of breast‐feeding in developed country settings. Public Health Nutrition 2009;13(1):137‐44. [DOI] [PubMed] [Google Scholar]
EFSA Panel 2009
- EFSA Panel on Dietetic Products, Nutrition, Allergies (NDA). Scientific Opinion on the appropriate age for introduction of complementary feeding of infants. EFSA Journal 2009;7(12):1423. [Google Scholar]
Enkin 2000
- Enkin M, Keirse MJ, Neilson JP, Crowther C, Duley L, Hodnett E, et al. A Guide to Effective Care in Pregnancy and Childbirth. Third Edition. Oxford: Oxford University Press, 2000. [Google Scholar]
EU Project on Promotion of Breastfeeding 2004
- EU Project on Promotion of Breastfeeding in Europe. Protection, promotion and support of breastfeeding in Europe: a blueprint for action. Luxembourg: European Commission, Directorate Public Health and Risk Assessment, 2004. [Google Scholar]
Heikkilä 2011
- Heikkilä K, Sacker A, Kelly Y, Renfrew MJ, Quigley MA. Breast feeding and child behaviour in the Millennium Cohort Study. Archives of Disease in Childhood 2011;96(7):635. [DOI] [PubMed] [Google Scholar]
Heikkilä 2014
- Heikkilä K, Kelly Y, Renfrew MJ, Sacker A, Quigley MA. Breastfeeding and educational achievement at age 5. Maternal & Child Nutrition 2014;10:92‐101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hoddinott 2011
- Hoddinott P, Seyara R, Marais D. Global evidence synthesis and UK idiosyncrasy; why have recent UK trials had no significant effects on breastfeeding rates?. Maternal and Child Nutrition 2011;7:221‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Horta 2013
- Horta B, Victora C. Short‐term Effects of Breastfeeding: a Systematic Review on the Benefits of Breastfeeding on Diarrhoea and Pneumonia Mortality. Geneva: World Health Organisation, 2013. [Google Scholar]
Horta 2015a
- Horta BL, Loret de Mola C, Victora CG. Long‐term consequences of breastfeeding on cholesterol, obesity, systolic blood pressure and type 2 diabetes: a systematic review and meta‐analysis. Acta Paediatrica 2015;104:30‐7. [DOI] [PubMed] [Google Scholar]
Horta 2015b
- Horta BL, Loret de Mola C, Victora CG. Breastfeeding and intelligence: a systematic review and meta‐analysis. Acta Paediatrica 2015;104:14‐9. [DOI] [PubMed] [Google Scholar]
Jolly 2012b
- Jolly K, Ingram L, Khan KS, Deeks JJ, Freemantle N, MacArthur C. Systematic review of peer support for breastfeeding continuation: metaregression analysis of the effect of setting, intensity, and timing. BMJ 2012;344:d8287. [DOI] [PubMed] [Google Scholar]
Kimani‐Murage 2011
- Kimani‐Murage EW, Madise NJ, Fotso J‐C, Kyobutungi C, Mutua MK, Gitau TM, et al. Patterns and determinants of breastfeeding and complementary feeding practices in urban informal settlements, Nairobi Kenya. BMC Public Health 2011;11:396. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kramer 2012
- Kramer MS, Kakuma R. Optimal duration of exclusive breastfeeding. Cochrane Database of Systematic Reviews 2012, Issue 8. [DOI: 10.1002/14651858.CD003517] [DOI] [PMC free article] [PubMed] [Google Scholar]
Labbok 2012
- Labbok MH. Global Baby‐friendly Hospital Initiative monitoring data: update and discussion. Breastfeeding Medicine 2012;7:210‐22. [DOI] [PubMed] [Google Scholar]
Lumbiganon 2012
- Lumbiganon P, Martis R, Laopaiboon M, Festin MR, Ho JJ, Hakimi M. Antenatal breastfeeding education for increasing breastfeeding duration. Cochrane Database of Systematic Reviews 2012, Issue 9. [DOI: 10.1002/14651858.CD006425] [DOI] [PubMed] [Google Scholar]
McAndrew 2012
- McAndrew F, Thompson J, Fellows L, Large A, Speed M, Renfrew MJ. Infant Feeding Survey 2010. Leeds, UK: Health and Social Care Information Centre, 2012. [Google Scholar]
McLachlan 2006
- McLachlan HL, Forster DA. Initial breastfeeding attitudes and practices of women born in Turkey, Vietnam and Australia after giving birth in Australia. International Breastfeeding Journal 2006;1:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
National Center for Health Statistics 2012
- National Center for Health Statistics. Healthy People 2010 Final Review. Hyattsville, MD: National Center for Health Statistics, 2012. [Google Scholar]
NHS England 2014
- NHS England. Statistical Release Breastfeeding Initiation & Breastfeeding prevalence 6‐8 weeks. London: NHS England, 2014. [Google Scholar]
Peres 2015
- Peres KG, Cascaes AM, Nascimento GG, Victora CG. Effect of breastfeeding on malocclusions: a systematic review and meta‐analysis. Acta Paediatrica 2015;104:54‐61. [DOI] [PubMed] [Google Scholar]
Pérez‐Escamilla 2016
- Pérez‐Escamilla R, Martinez JL, Segura‐Pérez S. Impact of the Baby‐friendly Hospital Initiative on breastfeeding and child health outcomes: a systematic review. Maternal & Child Nutrition 2016;12:402‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Quigley 2012
- Quigley MA, Hockley C, Carson C, Kelly Y, Renfrew MJ, Sacker A. Breastfeeding is associated with improved child cognitive development: a population‐based cohort study. Journal of Pediatrics 2012;160(1):25‐32. [DOI] [PubMed] [Google Scholar]
Renfrew 2006
- Renfrew MJ, McFadden A, Dykes F, Wallace LM, Abbott S, Burt S, et al. Addressing the learning deficit in breastfeeding: strategies for change. Maternal and Child Nutrition 2006;2(4):239‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Renfrew 2007
- Renfrew MJ, Spiby H, D’Souza L, Wallace LM, Dyson L, McCormick F. Rethinking research in breastfeeding: a critique of the evidence base identified in a systematic review of interventions to promote and support breastfeeding. Public Health Nutrition 2007;10(7):726–32. [DOI] [PubMed] [Google Scholar]
Renfrew 2012a
- Renfrew MJ, Pokhrel S, Quigley M, McCormick F, Fox‐Rushby J, Dodds R, et al. Preventing Disease and Saving Resources: the Potential Contribution of Increasing Breastfeeding Rates in the UK. London: UNICEF, 2012. [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rollins 2016
- Rollins N, Bhandari N, Hajeebhoy N, Horton S, Lutter C, Martines J, et al. Why invest, and what it will take to improve breastfeeding practices?. Lancet 2016;387:491‐504. [DOI] [PubMed] [Google Scholar]
Sankar 2015
- Sankar MJ, Sinha B, Chowdhury R, Bhandari N, Taneja S, Martines J, et al. Optimal breastfeeding practices and infant and child mortality: a systematic review and meta‐analysis. Acta Pædiatrica 2015;104:3‐13. [DOI] [PubMed] [Google Scholar]
Schmied 2011
- Schmied V, Beake S, Sheehan A, McCourt C, Dykes F. Women’s perceptions and experiences of breastfeeding support: a metasynthesis. Birth 2011;38:49‐60. [DOI] [PubMed] [Google Scholar]
Sinha 2015
- Sinha B, Chowdhury R, Sankar MJ, Martines J, Taneja S, Mazumder S, et al. Interventions to improve breastfeeding outcomes: a systematic review and meta‐analysis. Acta Paediatrica 2015;104:114‐34. [DOI] [PubMed] [Google Scholar]
Smith 2010
- Smith JP, Harvey PJ. Chronic disease and infant nutrition: is it significant to public health?. Public Health Nutrition 2011;14:279‐89. [DOI] [PubMed] [Google Scholar]
Tham 2015
- Tham R, Bowatte G, Dharmage S, Tan D, Lau M, Dai X, et al. Breastfeeding and the risk of dental caries: a systematic review and meta‐analysis. Acta Paediatrica 2015;104:62‐84. [DOI] [PubMed] [Google Scholar]
U.S. Department of Health and Human Services 2011
- U.S. Department of Health and Human Services. The Surgeon General’s Call to Action to Support Breastfeeding. Washington, DC: U.S. Department of Health and Human Services, Office of the Surgeon General, 2011. [Google Scholar]
UNICEF 2012
- UNICEF. State of the World’s Children 2015 Executive Summary. www.unicef.org/publications/files/SOWC_2015_Summary_and_Tables.pdf (accessed 10 June 2016). New York: UNICEF, 2012.
Venancio 2011
- Venancio SI, Saldiva SR, Escuder MM, Giugliani ER. The Baby‐Friendly Hospital Initiative shows positive effects on breastfeeding indicators in Brazil. Journal of Epidemiology and Community Health 2012;66:914–8. [DOI] [PubMed] [Google Scholar]
Victora 2016
- Victora C, Barros A, França G, Bahl R, Horton S, Krasevec J, et al. Breastfeeding in the 21st century: epidemiology, mechanisms, and lifelong effect. Lancet 2016;387:475‐90. [DOI] [PubMed] [Google Scholar]
Whitford 2015
- Whitford HM, Wallis SK, Dowswell T, Renfrew MJ. Breastfeeding education and support for women with multiple pregnancies. Cochrane Database of Systematic Reviews 2015, Issue 12. [DOI: 10.1002/14651858.CD012003] [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 1997
- World Health Organization, Division of Child Health and Development. Integrated Management of Childhood Illness: Management of the Sick Young Infant Age 1 Week up to 2 Months; 1997. Report No.: WHO/CHD/97.3F. Geneva: WHO, 1997. [Google Scholar]
WHO 2003
- World Health Organization. Global Strategy for Infant and Young Child Feeding. Geneva, Switzerland: World Health Organization, 2003. [Google Scholar]
WHO/UNICEF 1993
- WHO/UNICEF. Breastfeeding counselling: a training course. www.who.int/maternal_child_adolescent/documents/who_cdr_93_3/en/ (accessed 30 June 2016) 1993.
WHO/UNICEF 2006
- WHO/UNICEF. Infant and Young Child Feeding Counselling: An Integrated Course. http://www.who.int/maternal_child_adolescent/documents/9789241594745/en/ (accessed 30 June 2016) 2006.
References to other published versions of this review
Britton 2007
- Britton C, McCormick FM, Renfrew MJ, Wade A, King SE. Support for breastfeeding mothers. Cochrane Database of Systematic Reviews 2007, Issue 1. [DOI: 10.1002/14651858.CD001141.pub3] [DOI] [PubMed] [Google Scholar]
Renfrew 1995
- Renfrew MJ. Postnatal support for breastfeeding mothers (revised May 1994). In: Enkin MW, Keirse MJNC, Renfrew MJ, Neilson JP, Crowther C, editor(s) Pregnancy and Childbirth Module. In: The Cochrane Pregnancy and Childbirth Database (database on disk and CDROM). The Cochrane Collaboration; Issue 2, Oxford: Update Software; 1995.
Renfrew 2012b
- Renfrew MJ, McCormick FM, Wade A, Quinn B, Dowswell T. Support for healthy breastfeeding mothers with healthy term babies. Cochrane Database of Systematic Reviews 2012, Issue 5. [DOI: 10.1002/14651858.CD001141.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sikorski 1999
- Sikorski J, Renfrew MJ. Support for breastfeeding mothers. Cochrane Database of Systematic Reviews 1999, Issue 1. [DOI] [PubMed] [Google Scholar]
Sikorski 2002
- Sikorski J, Renfrew MJ, Pindoria P, Wade A. Support for breastfeeding mothers. Cochrane Database of Systematic Reviews 2002, Issue 1. [DOI: 10.1002/14651858.CD001141.pub2] [DOI] [PubMed] [Google Scholar]


