Abstract
Background
Allergy is common and may be associated with foods, including cow's milk formula (CMF). Formulas containing hydrolysed proteins have been used to treat infants with allergy. However, it is unclear whether hydrolysed formulas can be advocated for prevention of allergy in infants.
Objectives
To compare effects on allergy and food allergy when infants are fed a hydrolysed formula versus CMF or human breast milk. If hydrolysed formulas are effective, to determine what type of hydrolysed formula is most effective, including extensively or partially hydrolysed formula (EHF/PHF). To determine which infants at low or high risk of allergy and which infants receiving early, short‐term or prolonged formula feeding may benefit from hydrolysed formulas.
Search methods
We used the standard search strategy of the Cochrane Neonatal Review Group supplemented by cross referencing of previous reviews and publications (updated August 2016).
Selection criteria
We searched for randomised and quasi‐randomised trials that compared use of a hydrolysed formula versus human milk or CMF. Trials with ≥ 80% follow‐up of participants were eligible for inclusion.
Data collection and analysis
We independently assessed eligibility of studies for inclusion, methodological quality and data extraction. Primary outcomes included clinical allergy, specific allergy and food allergy. We conducted meta‐analysis using a fixed‐effect (FE) model.
Main results
Two studies assessed the effect of three to four days' infant supplementation with an EHF whilst in hospital after birth versus pasteurised human milk feed. Results showed no difference in infant allergy or childhood cow's milk allergy (CMA). No eligible trials compared prolonged hydrolysed formula versus human milk feeding.
Two studies assessed the effect of three to four days' infant supplementation with an EHF versus a CMF. One large quasi‐random study reported a reduction in infant CMA of borderline significance among low‐risk infants (risk ratio (RR) 0.62, 95% confidence interval (CI) 0.38 to 1.00).
Prolonged infant feeding with a hydrolysed formula compared with a CMF was associated with a reduction in infant allergy (eight studies, 2852 infants; FE RR 0.82, 95% CI 0.72 to 0.95; risk difference (RD) ‐0.04, 95% CI ‐0.08 to ‐0.01; number needed to treat for an additional beneficial outcome (NNTB) 25, 95% CI 12.5 to 100) and infant CMA (two studies, 405 infants; FE RR 0.38, 95% CI 0.16 to 0.86). We had substantial methodological concerns regarding studies and concerns regarding publication bias, as substantial numbers of studies including those in high‐risk infants have not comprehensively reported allergy outcomes (GRADE quality of evidence 'very low').
Prolonged infant feeding with a hydrolysed formula compared with a CMF was not associated with a difference in childhood allergy and led to no differences in specific allergy, including infant and childhood asthma, eczema and rhinitis and infant food allergy. Many of the analyses assessing specific allergy are underpowered.
Subroup analyses showed that infant allergy was reduced in studies that enrolled infants at high risk of allergy who used a hydrolysed formula compared with a CMF; used a PHF compared with a CMF; used prolonged and exclusive feeding of a hydrolysed formula compared with a CMF; and used a partially hydrolysed whey formula compared with a CMF. Studies that enrolled infants at high risk of allergy; used a PHF compared with a CMF; used prolonged and exclusive feeding of a hydrolysed formula compared with a CMF; and used a partially hydrolysed whey formula compared with a CMF found a reduction in infant CMA.
Authors' conclusions
We found no evidence to support short‐term or prolonged feeding with a hydrolysed formula compared with exclusive breast feeding for prevention of allergy. Very low‐quality evidence indicates that short‐term use of an EHF compared with a CMF may prevent infant CMA.
In infants at high risk of allergy not exclusively breast fed, very low‐quality evidence suggests that prolonged hydrolysed formula feeding compared with CMF feeding reduces infant allergy and infant CMA. Studies have found no difference in childhood allergy and no difference in specific allergy, including infant and childhood asthma, eczema and rhinitis and infant food allergy.
Very low‐quality evidence shows that prolonged use of a partially hydrolysed formula compared with a CMF for partial or exclusive feeding was associated with a reduction in infant allergy incidence and CMA incidence, and that prolonged use of an EHF versus a PHF reduces infant food allergy.
Keywords: Humans; Infant; Infant, Newborn; Dietary Proteins; Food Hypersensitivity; Food Hypersensitivity/prevention & control; Hydrolysis; Infant Formula; Infant Formula/chemistry; Milk Hypersensitivity; Milk Hypersensitivity/prevention & control; Milk, Human; Protein Hydrolysates; Protein Hydrolysates/administration & dosage; Randomized Controlled Trials as Topic; Synapsins
Formulas containing hydrolysed protein for prevention of allergy and food allergy in infants
Review question
Does feeding infants with a formula containing hydrolysed protein result in decreased risk of developing allergy such as asthma, dermatitis/eczema, hay fever and food allergy during infancy and childhood?
Background
Allergy is responsible for a substantial health burden among infants, children and adults. Early dietary intake may influence the development of allergic disease. When babies are not exclusively breast fed, use of hydrolysed formula instead of ordinary cow's milk formula may reduce allergy among babies and children, although additional studies are needed to confirm this. Infant formulas have been designed to lower the chance of infants developing allergy or food allergy. These include hydrolysed cow's milk and soy milk formulas. Hydrolysed formulas break down milk proteins into smaller, potentially less allergy‐producing proteins.
Results
This review of trials found no evidence to support feeding with a hydrolysed formula to prevent allergy in preference to exclusive breast feeding. For infants at high risk of allergy who are unable to be completely breast fed, limited evidence indicates that feeding with a hydrolysed formula compared with a cow's milk formula reduces allergy among babies and children, including cow's milk allergy (CMA). Concerns regarding quality of the evidence and consistency of the results indicate that continued study is needed.
Conclusions
Among infants at high risk of allergy who cannot be exclusively breast fed, very low‐quality evidence suggests that prolonged supplementation with a hydrolysed formula compared with a cow's milk formula reduces the risk of infant allergy and infant CMA. However, further research is needed to confirm these findings.
Summary of findings
Summary of findings for the main comparison.
Early short‐term feeding: hydrolysed formula versus human milk feeding ‐ low‐risk infants for prevention of allergic disease and food allergy
| Early short‐term feeding of hydrolysed formula versus human milk for prevention of allergic disease and food allergy | ||||||
| Patient or population: infants not selected for allergy risk Settings: hospitals Intervention: early short‐term feeding: hydrolysed formula versus human milk feeding for prevention of allergic disease and food allergy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Early short‐term feeding: hydrolysed formula vs human milk ‐ low‐risk infants | |||||
| Any allergy ‐ childhood (incidence) Follow‐up: mean 3 years | Study population | RR 1.43 (0.38 to 5.37) | 90 (1 study) | ⊕⊝⊝⊝ very lowa,b,c | ||
| 75 per 1000 | 108 per 1000 (29 to 405) | |||||
| Moderate | ||||||
| 76 per 1000 | 109 per 1000 (29 to 408) | |||||
| Asthma ‐ childhood (incidence) Follow‐up: mean 3 years | Study population | RR 0.48 (0.05 to 4.41) | 90 (1 study) | ⊕⊝⊝⊝ very lowa,b,c | ||
| 57 per 1000 | 27 per 1000 (3 to 250) | |||||
| Moderate | ||||||
| 57 per 1000 | 27 per 1000 (3 to 251) | |||||
| Eczema ‐ childhood (incidence) Follow‐up: mean 3 years | Study population | RR 0.48 (0.05 to 4.41) | 90 (1 study) | ⊕⊝⊝⊝ very lowa,b,c | ||
| 57 per 1000 | 27 per 1000 (3 to 250) | |||||
| Moderate | ||||||
| 57 per 1000 | 27 per 1000 (3 to 251) | |||||
| Food allergy ‐ childhood (incidence) Follow‐up: mean 3 years | Study population | RR 1.43 (0.38 to 5.37) | 90 (1 study) | ⊕⊝⊝⊝ very lowa,b,c | ||
| 75 per 1000 | 108 per 1000 (29 to 405) | |||||
| Moderate | ||||||
| 76 per 1000 | 109 per 1000 (29 to 408) | |||||
| Cow's milk allergy ‐ infancy (incidence) Follow‐up: mean 27 months | Study population | RR 0.87 (0.52 to 1.46) | 3559 (1 study) | ⊕⊝⊝⊝ very lowc,d,e | ||
| 17 per 1000 | 15 per 1000 (9 to 25) | |||||
| Moderate | ||||||
| 17 per 1000 | 15 per 1000 (9 to 25) | |||||
| Cow's milk allergy ‐ childhood (incidence) Follow‐up: mean 3 years | Study population | RR 7.11 (0.35 to 143.84) | 90 (1 study) | ⊕⊝⊝⊝ very lowa,b,c | ||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: We are very uncertain about the estimate | ||||||
aMethodological concerns including quasi‐random sequence allocation, incomplete outcome data and imbalances at baseline bImprecision of estimate ‐ single small study cReported by only a single study dMethodological concerns including quasi‐random sequence allocation and incomplete outcome data eImpression of estimate ‐ low incidence of outcome
Summary of findings 2.
Early short‐term feeding: hydrolysed formula versus cow's milk formula ‐ low‐risk infants for prevention of allergic disease and food allergy
| Early short‐term feeding of hydrolysed formula versus cow's milk formula for prevention of allergic disease and food allergy | ||||||
| Patient or population: infants not selected for allergy risk Settings: hospitals Intervention: early short‐term feeding of hydrolysed formula versus cow's milk formula for prevention of allergic disease and food allergy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Early short‐term feeding: hydrolysed formula vs cow's milk formula ‐ low‐risk infants | |||||
| Any allergy ‐ childhood (incidence) Follow‐up: mean 3 years | Study population | RR 1.37 (0.33 to 5.71) | 77 (1 study) | ⊕⊝⊝⊝ very lowa,b,c | ||
| 77 per 1000 | 105 per 1000 (25 to 439) | |||||
| Moderate | ||||||
| 77 per 1000 | 105 per 1000 (25 to 440) | |||||
| Asthma ‐ childhood (incidence) Follow‐up: mean 3 years | Study population | RR 3.08 (0.13 to 73.26) | 77 (1 study) | ⊕⊝⊝⊝ very lowa,b,c | ||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Eczema ‐ childhood (incidence) Follow‐up: mean 3 years | Study population | RR 0.34 (0.04 to 3.15) | 77 (1 study) | ⊕⊝⊝⊝ very lowa,b,c | ||
| 77 per 1000 | 26 per 1000 (3 to 242) | |||||
| Moderate | ||||||
| 77 per 1000 | 26 per 1000 (3 to 243) | |||||
| Food allergy ‐ childhood (incidence) Follow‐up: mean 3 years | Study population | RR 1.37 (0.33 to 5.71) | 77 (1 study) | ⊕⊝⊝⊝ very lowa,b,c | ||
| 77 per 1000 | 105 per 1000 (25 to 439) | |||||
| Moderate | ||||||
| 77 per 1000 | 105 per 1000 (25 to 440) | |||||
| Cow's milk allergy ‐ infancy (incidence) Follow‐up: mean 3 years | Study population | RR 0.62 (0.38 to 1) | 3473 (1 study) | ⊕⊝⊝⊝ very lowa,c,d | ||
| 24 per 1000 | 15 per 1000 (9 to 24) | |||||
| Moderate | ||||||
| 25 per 1000 | 15 per 1000 (9 to 25) | |||||
| Cow's milk allergy ‐ childhood (incidence) Follow‐up: mean 3 years | Study population | RR 5.13 (0.25 to 103.43) | 77 (1 study) | ⊕⊝⊝⊝ very lowa,b,c | ||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: We are very uncertain about the estimate | ||||||
aMethodological concerns including quasi‐random sequence allocation, incomplete outcome data and imbalances at baseline bImprecision of estimate ‐ single small study cReported only by a single study dImprecision of estimate ‐ low incidence of outcome
Summary of findings 3.
Prolonged feeding: hydrolysed formula versus cow's milk formula for prevention of allergic disease and food allergy
| Prolonged feeding of hydrolysed formula versus cow's milk formula for prevention of allergic disease and food allergy | ||||||
| Patient or population: infants Settings: infant feeding Intervention: prolonged feeding of hydrolysed formula versus cow's milk formula for prevention of allergic disease and food allergy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Prolonged feeding: hydrolysed formula vs cow's milk formula | |||||
| Any allergy ‐ infancy (incidence) Follow‐up: 2 years | Study population | RR 0.82 (0.72 to 0.95) | 2852 (8 studies) | ⊕⊝⊝⊝ very lowa,b | ||
| 294 per 1000 | 241 per 1000 (212 to 279) | |||||
| Moderate | ||||||
| 395 per 1000 | 324 per 1000 (284 to 375) | |||||
| Any allergy ‐ childhood (incidence) Follow‐up: 3 years | Study population | RR 0.85 (0.69 to 1.05) | 950 (2 studies) | ⊕⊝⊝⊝ very lowa,b,c,d | ||
| 353 per 1000 | 300 per 1000 (244 to 371) | |||||
| Moderate | ||||||
| 381 per 1000 | 324 per 1000 (263 to 400) | |||||
| Asthma ‐ infancy (incidence) Follow‐up: 2 years | Study population | RR 0.57 (0.31 to 1.04) | 318 (4 studies) | ⊕⊝⊝⊝ very lowa,b,d | ||
| 175 per 1000 | 100 per 1000 (54 to 182) | |||||
| Moderate | ||||||
| 149 per 1000 | 85 per 1000 (46 to 155) | |||||
| Eczema ‐ infancy (incidence) Follow‐up: 2 years | Study population | RR 0.86 (0.73 to 1.01) | 2896 (9 studies) | ⊕⊝⊝⊝ very lowa,b,d | ||
| 223 per 1000 | 191 per 1000 (162 to 225) | |||||
| Moderate | ||||||
| 200 per 1000 | 172 per 1000 (146 to 202) | |||||
| Rhinitis ‐ infancy (incidence) Follow‐up: 2 years | Study population | RR 0.52 (0.14 to 1.85) | 256 (3 studies) | ⊕⊝⊝⊝ very lowa,b,d | ||
| 58 per 1000 | 30 per 1000 (8 to 107) | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Food allergy ‐ infancy (incidence) Follow‐up: 2 years | Study population | RR 0.95 (0.58 to 1.54) | 479 (2 studies) | ⊕⊝⊝⊝ very lowa,b,d | ||
| 139 per 1000 | 132 per 1000 (81 to 214) | |||||
| Moderate | ||||||
| 119 per 1000 | 113 per 1000 (69 to 183) | |||||
| Cow's milk allergy ‐ infancy (incidence) Follow‐up: 2 years | Study population | RR 0.38 (0.16 to 0.86) | 405 (2 studies) | ⊕⊝⊝⊝ very lowa,b,d | ||
| 80 per 1000 | 30 per 1000 (13 to 68) | |||||
| Moderate | ||||||
| 222 per 1000 | 84 per 1000 (36 to 191) | |||||
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: We are very uncertain about the estimate | ||||||
aSubstantial methodological concerns. Sensitivity analysis found no study considered at low risk of bias bNot reported by all studies cSignificant heterogeneity between studies dImprecision of estimate
Background
Description of the condition
Food allergy and allergic disease are prevalent and represent a substantial health problem that may be increasing in developed countries (Burr 1989; Halken 2004; Prescott 2005; Schultz Larsen 1996). Although less than half of those who develop childhood allergic disease have a first‐degree relative with a history of allergy, the risk of allergy increases substantially with a positive family history (Bergmann 1994; Sears 1996; Tariq 1998). Approximately 10% of children without an allergic first‐degree relative develop allergic disease compared with 20% to 30% with an allergic first‐degree relative (parent or sibling) and 40% to 50% with two affected relatives (Arshad 2005; Bergmann 1997; Hansen 1993; Kjellman 1977). The predictive value of family history is increased with the addition of cord blood immunoglobulin (Ig) E antibody testing, although its accuracy may not be adequate for population screening (Bergmann 1997; Bergmann 1998; Tariq 1998).
Manifestations of allergic disease are age dependent. Infants commonly present with symptoms and signs of atopic eczema, gastrointestinal symptoms and recurrent wheezing. Asthma and rhinoconjunctivitis become prevalent in later childhood. Sensitisation to allergens tends to follow a characteristic pattern (Halken 2004), with sensitisation to food allergens in the first two to three years of life, followed by indoor allergens (e.g. house dust mite, pets) and subsequently outdoor allergens (e.g. rye, timothy grass). The cumulative prevalence of allergic disease during childhood is high, with up to 7% to 8% developing a food allergy, 15% to 20% atopic eczema and 31% to 34% asthma or recurrent wheezing (Halken 2004). Of these, 7% to 10% will continue to have asthma symptoms beyond five years of age (Halken 2004). Food hypersensitivities affect approximately 6% of infants younger than three years, and prevalence decreases over the first decade (Osterballe 2005; Sampson 2004).
Allergy may be diagnosed by questionnaire or clinician assessment, and the diagnosis may be confirmed by specific skin or serological testing, or by allergen challenge. Diagnostic criteria for different allergic diseases are not uniform, and the mode of ascertainment of allergic disease is variable. Although tests of bronchial hyperresponsiveness, challenge tests and classical tests of IgE‐mediated allergy have an imperfect correlation with allergy symptoms and clinical signs (Darsow 2000; Peat 2000), they are associated with an increased likelihood of allergy and disease (Ronmark 2001; Sears 1998; Sly 1999; Strachan 1996). In addition, some evidence suggests that questionnaires, although compromised by selection and recall bias (Peat 2001), are suitable for allergy screening (Kilpelainen 2001; Ravault 2001). This review includes trials that diagnosed allergy by questionnaire or by clinician assessment, with or without confirmation by laboratory testing. Criteria for diagnosis of allergic disease should include typical symptoms and/or signs, with evidence of precipitants, persistence or recurrence typical of allergic disease, or with test evidence confirming atopy or bronchial hyperreactivity.
The World Allergy Organization 2003 consensus (Johansson 2004) recommended that the term 'hypersensitivity' should be used to describe objectively reproducible symptoms or signs initiated by exposure to a defined stimulus at a dose tolerated by normal persons. 'Allergy' is a hypersensitivity reaction initiated by specific immunological mechanisms. The term 'food allergy' is used when immunological mechanisms have been demonstrated. Food‐specific IgG antibodies in serum are not of clinical importance but merely indicate previous exposure to a specific food. If IgE is involved in the reaction, the term 'IgE‐mediated food allergy' is appropriate. Food hypersensitivity is diagnosed by resolution of typical symptoms with elimination from the diet, with confirmation by blinded challenge. Around 2% to 3% of babies develop hypersensitivity to a particular food. Principal symptoms among infants with proven cow's milk protein hypersensitivity (CMPH) are gastrointestinal (˜ 50%), dermatological (˜ 31%) and respiratory (˜ 19%) in nature (Host 1994; Host 1995; Schrander 1993). Two of every three infants with CMPH have a family history of atopy (Schrander 1993). CMPH is strongly associated with feeding of cow's milk formula (CMF) to infants during the first month of life (Host 1991). Many infants with CMPH become tolerant over time, with approximately 30% at one year, 50% at two years and 70% at three years tolerant to cow's milk challenge. The risk of persisting hypersensitivity is increased with evidence of atopy (Host 1995).
Description of the intervention
Measures to prevent allergy and food allergy have included maternal allergen avoidance during pregnancy (Custovic 2000; Custovic 2001; Kramer 2001; Zeiger 1989) and/or lactation (Custovic 2000; Custovic 2001; Zeiger 1989), periods of exclusive breast feeding (Custovic 2000; Custovic 2001; Gruskay 1982; Oddy 1999; Saarinen 1995; Saarinen 2000) and avoidance of potential allergens including food and environmental antigens during the first year of life and beyond (Custovic 2000). Formulas prescribed for infants with the intention of preventing allergy and food allergy include hydrolysed cow's milk and elemental formula, as well as soy or hydrolysed soy formula. These formulas may be produced from cow's milk or soy milk, may be derived predominantly from whey or casein proteins and may be partially or extensively hydrolysed. Protein modification is performed through a variety of physiochemical processes including ultraheating and enzymatic cleavage, most often with trypsin and chymotrypsin.
The American Academy of Pediatrics (AAP) recommends that hypoallergenic formulas should be tested in trials in which investigators examine human infants for toxicity and suitability to maintain a positive nitrogen balance, while attempting to predict whether infants allergic to cow’s milk will react adversely to these formulas (AAP 2000). These formulas are studied in infants with cow’s milk or cow’s milk‐based formula allergic reactions verified by double‐blind placebo‐controlled challenge (DBPCC) (Bock 1988). At a minimum, these tests should ensure with 95% confidence that 90% of infants with documented cow’s milk allergy respond to treatment and do not react during challenge (Kleinman 1991). Protein particle size does not appear to be a prerequisite for defining a formula as hypoallergenic, although amino acid‐based formulas and those with more extensive hydrolysis are less likely to produce reactions among infants with cow's milk allergy (Hill 2007). Although universal agreement has not been reached on the definition (Chaffen 2010; Host 1999), for the purposes of this review an extensively hydrolysed formula (EHF) will be regarded as one meeting the AAP definition for hypoallergenic formula (AAP 2000), and those with less extensive hydrolysis will be regarded as partially hydrolysed.
How the intervention might work
Infants' immune systems become sensitised or tolerant to allergens in the order in which they are exposed. Early life exposure to allergens occurs frequently through ingested protein, particularly cow's milk protein in formula (Muraro 2004). Amino acid‐based and extensively hydrolysed protein formulas are produced so as to substantially reduce the antigenicity of the protein and prevent sensitisation of infants to commonly ingested antigens, including cow's milk protein. However, low concentrations of food allergens, especially cow's milk proteins, are present in human milk. It has been suggested that the low incidence of cow's milk protein allergy among exclusively breast fed infants ‐ at 0.5% in unselected infants and 1.3% in high‐risk infants ‐ in prospective birth cohort studies was due to low‐level exposure‐induced tolerance rather than to disease (Halken 2004). It has been proposed that prolonged exposure to allergenic proteins or to proteins with reduced but not absent allergenicity may induce tolerance over time (Allen 2009). Although most infants with cow’s milk hypersensitivity exhibit this in the first year of life, more than 80% subsequently develop clinical tolerance (Katz 2011; Sampson 2004). The concern is that early avoidance of cow's milk protein may reduce the likelihood that infants will subsequently develop tolerance to the allergen (Katz 2010).
Why it is important to do this review
The aim of this review is to gather evidence on the use of hydrolysed formulas for prevention of allergy and food allergy. This review does not include treatment of infants with clinically recognised allergy or food allergy.
Objectives
To compare effects on allergy and food allergy when infants are fed a hydrolysed formula versus CMF or human breast milk. If hydrolysed formulas are effective, to determine what type of hydrolysed formula is most effective, including extensively or partially hydrolysed formula (EHF/PHF). To determine which infants at low or high risk of allergy and which infants receiving early, short‐term or prolonged formula feeding may benefit from hydrolysed formulas.
Methods
Criteria for considering studies for this review
Types of studies
We searched for randomised and quasi‐randomised trials that compared the use of a hydrolysed formula versus human milk or cow's milk formula. Randomised and quasi‐randomised (e.g. using alternation) trials with ≥ 80% follow‐up of participants were eligible for inclusion.
Types of participants
Infants in the first six months of life without clinical evidence of allergy.
Types of interventions
Hydrolysed formulas included:
hydrolysed cow's milk and soy formulas; and
extensively and partially hydrolysed formulas.
Hydrolysed formulas may be used for:
early, short‐term supplementary or sole formula feeding of infants unable to be exclusively breast fed in the first days of life;
prolonged supplementation of breast fed infants or infants fed solely with formula in the first months of life; and
weaning from the breast with infant formula.
The control group may include infants who receive:
exclusive human milk (breast fed or expressed); and
cow's milk formula.
Study authors had to prespecify the following comparisons.
Early short‐term hydrolysed formula versus human milk.
Prolonged use of a hydrolysed formula versus human milk.
Early short‐term hydrolysed formula versus cow's milk formula.
Prolonged use of a hydrolysed formula versus cow's milk formula.
Prespecified subgroup analyses included the following (see Methods for definitions).
Infant risk of allergy or food allergy.
Low‐risk infants (no family history of allergy or food allergy among first‐degree relatives).
High‐risk infants (family history of allergy or food allergy among first‐degree relatives or high cord IgE level).
Extent of protein hydrolysis.
Extensively hydrolysed formula versus cow's milk formula.
Partially hydrolysed formula versus cow's milk formula.
Extensively hydrolysed formula versus partially hydrolysed formula.
Indication for use.
Prolonged sole formula feeding.
Supplemental feeding or weaning from the breast using infant formula.
Method of ascertainment of allergy.
Allergy/Food allergy confirmed by test.
Blinded measurement for allergy or food allergy.
Type of protein hydrolysate used.
Partially hydrolysed whey formula versus cow's milk formula.
Partially hydrolysed casein formula versus cow's milk formula.
Extensively hydrolysed whey formula versus cow's milk formula.
Extensively hydrolysed casein formula versus cow's milk formula.
Hydrolysed soy formula versus cow's milk formula.
We excluded studies that included other allergy prevention interventions (e.g. maternal dietary avoidance measures, environmental allergy reduction measures) in the treatment group and not in the control group. Studies that provided other allergy prevention interventions for both treatment and control groups were eligible.
Types of outcome measures
Primary outcomes
All allergies, including asthma, atopic dermatitis, allergic rhinitis and food allergy
Secondary outcomes
Asthma
Atopic dermatitis/Eczema
Allergic rhinitis
Cow's milk or soy protein allergy
Food allergy
Urticaria
Anaphylaxis
In the 2013 review update, we no longer reported previously reported food hypersensitivity and potential harms, including growth parameters, cost and infant feed refusal.
Researchers may have diagnosed a specific allergy on the basis of:
history of recurrent and persistent symptoms typical of the allergy;
clinician diagnosis of allergy; or
clinical allergy confirmed by testing including detection of allergen sensitisation by skin testing or by serological testing for specific IgE (e.g. radio‐allergosorbent (RAST), enzyme‐allergosorbent (EAST)), or asthma confirmed by respiratory function testing for the presence of bronchial hyperresponsiveness confirmed by elimination/challenge.
Investigators used the following definitions of age of allergy.
Infant allergy incidence: allergy occurring up to two years of age.
Childhood allergy incidence: allergy occurring up to 10 years of age (or up to age of latest report between two and 10 years).
Childhood allergy prevalence: reported allergy that was present between two and 10 years of age.
Adolescent allergy: allergy present from 10 to 18 years of age.
Adult allergy: allergy present after 18 years of age.
Search methods for identification of studies
Electronic searches
See the Collaborative Review Group search strategy.
2016 update: We performed an updated search of the Cochrane Central Register of Controlled Trials (CENTRAL; August 2016), MEDLINE (1948 to August 2016) and Embase (1974 to August 2016). We also searched citations of authors of included studies and citation lists of articles and reviews.
We documented the search strategies in Appendix 1,Appendix 2 and Appendix 3.
Searching other resources
We performed a search of previous reviews including cross references (all articles referenced), abstracts, conferences (Pediatric Academic Societies 2003 to 2016; Perinatal Society of Australia and New Zealand 2003 to 2016), recent review citations and expert informants.
We also updated in August 2016 our search of clinical trials registries for ongoing or recently completed trials (clinicaltrials.gov; controlled‐trials.com; who.int/ictrp).
Data collection and analysis
This review updates previous versions (Osborn 2003; Osborn 2006).
Each review author independently assessed eligibility of studies for inclusion. We included only studies with ≥ 80% reporting of randomised infants. We used the criteria and standard methods of the Cochrane Neonatal Review Group to assess the methodological quality of included trials regarding adequacy of randomisation and allocation concealment, blinding of parents or caregivers and assessors to intervention and completeness of assessment of all randomised individuals. We used a data collection form to aid extraction of relevant information and data from each included study. Each review author extracted the data separately, and review authors compared data and resolved differences by consensus. We used the standard methods of the Cochrane Neonatal Review Group to synthesise the data and expressed effects as risk ratio (RR), risk difference (RD) and 95% confidence intervals (CIs) for categorical data, and as mean difference (MD) and 95% CIs for continuous data. We used the Chi² test to examine data for heterogeneity, and we quantified heterogeneity using the I² statistic. We used the fixed‐effect model for meta‐analysis when enrolled infants and interventions were similar and no significant heterogeneity was found. We explored sources of heterogeneity by performing subgroup analysis.
The term 'hydrolysed formula' used without a reference to type refers to both extensively and partially hydrolysed formulas. We did not pool studies that used hydrolysed formula for early (first few days of life) supplemental or sole infant feeding with studies that used hydrolysed formula for prolonged feeding. We performed all comparisons by including only studies with no different co‐interventions prescribed for prevention of allergy in either study arm (e.g. in treatment group but not in control group). Allergy‐preventing co‐interventions included modifications to mother's diet when pregnant or breast feeding and environmental modifications such as avoidance of pet hair and host dust mite reduction measures. The protocol did not originally prespecify that we should restrict analyses to studies with no differential co‐interventions.
Selection of studies
We included all randomised and quasi‐randomised controlled trials fulfilling the selection criteria described in the previous section. Each review author reviewed the search results and separately selected studies for inclusion. Review authors resolved disagreements by discussion.
Data extraction and management
Each review author extracted the data separately. Review authors compared data and resolved differences by consensus.
We obtained additional method details and data from the authors of two studies (Hill 2000; von Berg 2003). For one study (Lam 1992), we extracted methods and data from a thesis.
For the 2013 update, we performed all analyses using Review Manager software (RevMan 2011).
Assessment of risk of bias in included studies
We used the criteria and standard methods of the Cochrane Neonatal Review Group to assess the methodological quality of included trials. We evaluated the quality of included trials in terms of adequacy of randomisation and allocation concealment, blinding of parents or caregivers and assessors to intervention and completeness of assessment in all randomised individuals.
For the 2016 update, we incorporated previous assessments into RevMan 5 'Risk of bias' tables. We assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) by examining the following.
Sequence generation (checking for possible selection bias)
Adequate (any truly random process, e.g. random number table; computer random number generator)
Inadequate (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number)
Unclear
Allocation concealment (checking for possible selection bias)
Adequate (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes)
Inadequate (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth)
Unclear
Blinding (checking for possible performance bias)
Adequate, inadequate or unclear for participants
Adequate, inadequate or unclear for personnel
Adequate, inadequate or unclear for outcome assessors
Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations)
Adequate (< 20% missing data)
Inadequate
Unclear
Selective reporting bias
Adequate (when it is clear that all of the study’s prespecified outcomes and all expected outcomes of interest to the review have been reported)
Inadequate (when not all of the study’s prespecified outcomes have been reported; one or more reported primary outcomes were not prespecified; outcomes of interest were reported incompletely and so cannot be used; study failed to include results of a key outcome that would have been expected to have been reported)
Unclear
Other sources of bias
We assessed the possibility of other sources of bias (e.g. early termination of trial due to data‐dependent process, extreme baseline imbalance) as follows.
Yes.
No.
Unclear.
Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). With reference to the criteria above, we assessed the likely magnitude and direction of bias, and whether it was likely to have an impact on study findings. We explored the impact of the level of bias by undertaking sensitivity analyses (see Sensitivity analysis section).
Measures of treatment effect
We used the standard methods of the Cochrane Neonatal Review Group to synthesise data and expressed effects as risk ratio (RR) and risk difference (RD) with 95% confidence intervals (CIs) for categorical data, and as mean difference (MD) with 95% CIs for continuous data.
Unit of analysis issues
The unit of analysis was the individual participant (infant).
Dealing with missing data
We recorded missing data in 'Risk of bias' tables and assessed the effect of missing data by performing sensitivity analysis.
We performed all analyses by 'intention to treat' when data were available. When intention‐to‐treat data were not available, we reported data as infants assessed by group of assignment as well as losses after randomisation.
Assessment of heterogeneity
We used the two formal statistics described here.
Chi2 test: to assess whether observed variability in effect sizes between studies is greater than would be expected by chance. This test has low power when the number of studies included in the meta‐analysis is small, so we planned to set the probability at the 10% level of significance.
I2 statistic: to ensure that pooling of data is valid. We planned to grade the degree of heterogeneity as follows: 0% to 30%: might not be important; 31% to 50%: moderate heterogeneity; 51% to 75%: substantial heterogeneity; 76% to 100%: considerable heterogeneity.
When we found evidence of apparent or statistical heterogeneity, we planned to assess the source of the heterogeneity by conducting sensitivity and subgroup analyses to look for evidence of bias or methodological differences between trials.
Assessment of reporting biases
We documented in the Characteristics of excluded studies table all studies that reported use of a prebiotic in a potentially eligible infant population but did not report allergy‐related outcomes. We assessed reporting and publication bias by examining the degree of asymmetry of a funnel plot.
Data synthesis
We used the fixed‐effect model and Mantel‐Haenszel methods for meta‐analysis.
Quality of evidence
We used the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach, as outlined in the GRADE Handbook (Schünemann 2013), to assess the quality of evidence for the following (clinically relevant) outcomes: all allergy; specific allergies including asthma, atopic dermatitis/eczema, allergic rhinitis, cow's milk or soy protein allergy, food allergy, urticarial allergy and anaphylaxis.
Two review authors independently assessed the quality of the evidence for each of the outcomes above. We considered evidence from randomised controlled trials as high quality but downgraded the evidence one level for serious (and two levels for very serious) limitations on the basis of the following: design (risk of bias), consistency across studies, directness of evidence, precision of estimates and presence of publication bias. We used the GRADEpro Guideline Development Tool to create a ‘Summary of findings’ table to report the quality of the evidence.
The GRADE approach yields an assessment of the quality of a body of evidence by one of four grades.
High: We are very confident that the true effect lies close to the estimate of effect.
Moderate: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of effect but may be substantially different.
Low: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of effect.
Very low: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect.
Subgroup analysis and investigation of heterogeneity
We performed subgroup analyses according to the following.
Infant risk of allergy or food allergy: low‐risk infants (no family history of allergy or food allergy in first‐degree relatives); high‐risk infants (family history of allergy or food allergy in first‐degree relatives or high cord blood IgE level).
Extent of protein hydrolysis: EHF versus CMF; PHF versus CMF; EHF versus PHF. An EHF should meet the definition provided by the AAP Committee on Nutrition (AAP 2000) ‐ extensively hydrolysed proteins derived from cow's milk in which most of the nitrogen is present in the form of free amino acids and peptides ≤ 1500 kDaltons ‐ and should, at a minimum, ensure with 95% confidence that 90% of infants with documented CMA will not react with defined symptoms to the formula under double‐blind, placebo‐controlled conditions.
Indication for use: prolonged sole formula feeding; supplemental formula feeding; weaning from the breast with infant formula.
Method of ascertainment of allergy or food allergy: clinical allergy confirmed by challenge testing or by testing for atopy (e.g. skin testing or serological testing for specific IgE, asthma confirmed by testing for presence of bronchial hyperresponsiveness, food allergy confirmed by elimination/challenge). Included in this definition is clinical allergy in a patient for whom atopy has been confirmed by testing (e.g. asthma when atopy has been confirmed by skin prick testing or RAST for specific IgE); blinded measurement for allergy or food allergy ‐ when measurement of outcome was blinded to treatment allocation (this analysis was not prespecified).
Type of protein hydrolysate used: partially hydrolysed whey formula versus CMF; partially hydrolysed casein formula versus CMF; extensively hydrolysed whey formula versus CMF; extensively hydrolysed casein formula versus CMF; hydrolysed soy formula versus CMF.
Sensitivity analysis
We prespecified a sensitivity analysis to determine whether review findings were affected by including only studies at low risk of bias, defined as those with adequate randomisation and allocation concealment and < 10% loss to follow‐up.
Results
Description of studies
Results of the search
We performed searches on 22 January 2016 (see Figure 1 for study flow diagram). We searched MEDLINE using OVID and retrieved 198 reports (Appendix 1); CENTRAL and retrieved 242 reports (Appendix 2); and Embase and retrieved 199 reports (Appendix 3). We identified eight ongoing or unpublished studies (Baldassarre 2013; Barber 2009; Del Moral 2010; Knip 2012; Mennella 2012; Sorensen 2010; Vivatvakin 2009; Yin 2015) (see Appendix 3 and Appendix 4). We updated the search on 30 August 2016 and found one additional excluded study (Boyle 2016) and an additional 15‐year follow‐up report from a previously included study (von Berg 2003).
Figure 1.
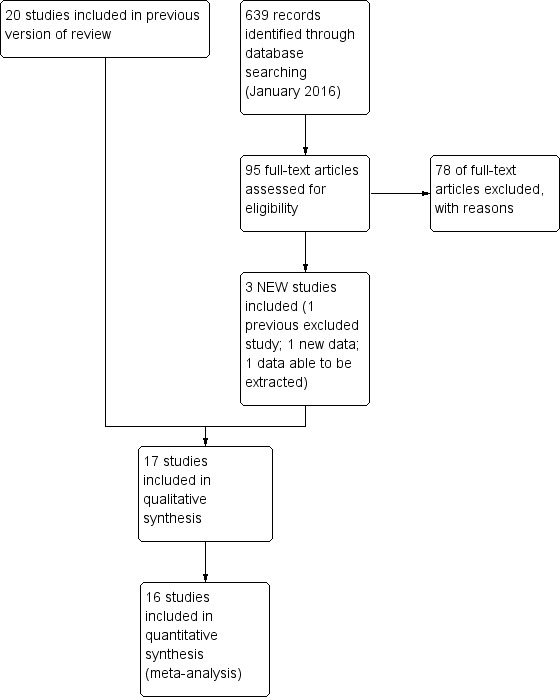
Study flow diagram: review update.
Included studies
We assessed 17 studies as eligible for inclusion ‐ see Characteristics of included studies table. The 2016 search revealed two additional studies that we assessed as included studies (Kwinta 2009; Sorensen 2007). We found an additional publication (Hill 2000) of a study that we had previously assessed as an excluded study as it had reported a per‐protocol analysis with excess losses. We combined a new report of an 'intention‐to‐treat' analysis with trial data obtained from study authors and have now assessed this trial as an included study. Sorensen 2007 (n = 242) has not reported extractable data to date that can be included in a meta‐analysis. This study compared a CMF versus a PHF versus an EHF in infants at high risk of allergy and is not included in the risk of bias assessment and analyses. Data from sequential publications of the GINI study (von Berg 2003) beyond three years remain ineligible for inclusion in this review owing to excess losses to follow‐up. As no new data have been made available, we have moved to the excluded studies list four previously included studies (Maggio 2005; Picaud 2001; Szajewska 2001; Vandenplas 1993) that provided no outcome data for the previous version (Osborn 2006).
Types of infants enrolled
High risk of allergy: A total of 14 studies (Chirico 1997; de Seta 1994; Halken 2000; Hill 2000; Lam 1992; Mallet 1992; Marini 1996; Nentwich 2001; Oldaeus 1997; Sorensen 2007; Tsai 1991; Vandenplas 1992; von Berg 2003; Willems 1993) enrolled infants at high risk of allergy on the basis of a history of allergy in a first‐degree relative and/or a high cord IgE level, although Lam 1992 did not report 'high risk' criteria.
Risk of allergy not specified: Three studies did not enrol infants on the basis of risk of allergy: Juvonen 1996 enrolled healthy term infants, although 62% had a family history of allergy; Kwinta 2009 enrolled very low birth weight infants (≤ 1500 g); and Saarinen 1999 enrolled healthy term infants requiring supplemental feeding in hospital.
Low risk of allergy: No study reporting allergy outcomes enrolled infants at low risk of allergy.
Types of interventions
See Characteristics of included studies for types of formula used in each study.
Early short‐term feeding: hydrolysed formula versus human milk feeding ‐ low‐risk infants
Two studies (Juvonen 1996; Saarinen 1999) compared a hydrolysed formula versus pasteurised donor human milk used for early short‐term infant feeding. Juvonen 1996 gave sole bottle feeds for three days, then all infants were exclusively breast fed. Saarinen 1999 gave supplemental feeds when required while infants were in hospital (average four days). Mothers were then encouraged to breast feed.
Early short‐term feeding: hydrolysed formula versus cow's milk formula ‐ low‐risk infants
Two studies (Juvonen 1996; Saarinen 1999) compared a hydrolysed formula versus CMF for early short‐term infant feeding. Juvonen 1996 gave sole bottle feeds for three days, then all infants were exclusively breast fed. Saarinen 1999 gave supplemental feeds when required while infants were in hospital (average four days). Mothers were then encouraged to breast feed.
Prolonged feeding: hydrolysed formula versus human milk feeding
We found no studies for this comparison.
Prolonged feeding: hydrolysed formula versus cow's milk formula
Thirteen studies compared prolonged supplemental or sole feeding with a hydrolysed formula versus CMF without differential co‐interventions (Chirico 1997; de Seta 1994; Hill 2000; Kwinta 2009; Lam 1992; Mallet 1992; Marini 1996; Oldaeus 1997; Sorensen 2007; Tsai 1991; Vandenplas 1992; von Berg 2003; Willems 1993). Three studies (Chirico 1997; Marini 1996; Oldaeus 1997) reported additional allergy avoidance measures in both hydrolysed formula and CMF groups.
Prolonged feeding: hydrolysed formula versus cow's milk formula ‐ low‐risk infants
Kwinta 2009 compared prolonged feeding with a hydrolysed formula versus CMF in low‐risk infants.
Prolonged feeding: hydrolysed formula versus cow's milk formula ‐ high‐risk infants
Twelve studies (Chirico 1997; de Seta 1994; Hill 2000; Lam 1992; Mallet 1992; Marini 1996; Oldaeus 1997; Sorensen 2007; Tsai 1991; Vandenplas 1992; von Berg 2003; Willems 1993) compared prolonged feeding with a hydrolysed formula versus CMF in high‐risk infants.
Prolonged feeding: partially hydrolysed formula versus cow's milk formula
Twelve studies (Chirico 1997; de Seta 1994; Kwinta 2009; Hill 2000; Lam 1992; Marini 1996; Oldaeus 1997; Sorensen 2007; Tsai 1991; Vandenplas 1992; von Berg 2003; Willems 1993) compared prolonged feeding with a PHF versus CMF.
Prolonged feeding: extensively hydrolysed formula versus cow's milk formula
Five studies (Kwinta 2009; Mallet 1992; Oldaeus 1997; Sorensen 2007; von Berg 2003) compared prolonged feeding with an EHF versus CMF.
Prolonged feeding: extensively hydrolysed formula versus partially hydrolysed formula
Five studies (Halken 2000; Nentwich 2001; Oldaeus 1997; Sorensen 2007; von Berg 2003) compared prolonged feeding with an EHF versus a PHF.
Prolonged exclusive feeding: hydrolysed formula versus cow's milk formula
Seven studies reported prolonged exclusive hydrolysed formula versus CMF (Chirico 1997; de Seta 1994; Kwinta 2009; Lam 1992; Marini 1996; Vandenplas 1992; Willems 1993). It is unclear whether the period of formula feeding was exclusive for one study (Sorensen 2007).
Prolonged feeding: hydrolysed formula versus cow's milk formula ‐ studies with blinded measurement
Four studies assessed allergy without knowledge of participant allocation (Kwinta 2009; Oldaeus 1997; Vandenplas 1992; von Berg 2003).
Prolonged feeding: hydrolysed formula versus cow's milk formula ‐ studies at low risk of bias
We assessed no studies as eligible for inclusion in the sensitivity analysis of adequate study methods (adequate randomisation, allocation concealment and < 10% losses to follow‐up).
Prolonged feeding: partially hydrolysed whey formula versus cow's milk formula
Ten studies (Chirico 1997; de Seta 1994; Hill 2000; Lam 1992; Marini 1996; Sorensen 2007; Tsai 1991; Vandenplas 1992; von Berg 2003; Willems 1993) compared a partially hydrolysed whey formula versus CMF.
Prolonged feeding: partially hydrolysed casein‐containing formula versus cow's milk formula
Two studies (Kwinta 2009; Oldaeus 1997) compared a PHF containing casein versus CMF.
Prolonged feeding: extensively hydrolysed whey formula versus cow's milk formula
Two studies (Sorensen 2007; von Berg 2003) compared an extensively hydrolysed whey formula versus CMF.
Prolonged feeding: extensively hydrolysed casein formula versus extensively hydrolysed whey formula
Two studies (Halken 2000; von Berg 2003) compared an extensively hydrolysed casein formula versus CMF.
No study compared a hydrolysed soy formula versus CMF. No study compared a hydrolysed soy formula versus hydrolysed CMF.
Types of outcomes
Definitions for allergy varied between studies but usually required persistent or recurring symptoms and signs in the absence of another obvious clinical explanation. For definitions of 'any allergy' and type of allergy for each study, see Characteristics of included studies.
Sorensen 2007 has not reported extractable data to date that can be included in a meta‐analysis. This study enrolled infants at high risk of allergy.
Studies (with timing) reporting clinician‐diagnosed allergy included Chirico 1997 (six months); de Seta 1994 (six and 24 months); Halken 2000 (six, 12 and 18 months); Hill 2000 (two and six to seven years); Juvonen 1996 (three years); Kwinta 2009 (five to seven years); Lam 1992 (six months); Mallet 1992 (one, two and four years); Marini 1996 (six months, one and three years); Nentwich 2001 (six and 12 months); Oldaeus 1997 (nurse examination at three, six, nine, 12 and 18 months and doctor visit at 18 months); Saarinen 1999 (mean age at follow‐up of 27 months, range 18 to 34 months); Sorensen 2007 (12 months); Tsai 1991 (one, two, four, six and 12 months); Vandenplas 1992 (12 months); and von Berg 2003 (12 months and three, six and 10 years ‐ note excess losses from three years of age).
Three studies reported using questionnaire‐diagnosed allergy: Hill 2000 (six to seven years); Kwinta 2009 (five to seven years); and Willems 1993 (three months and one year).
Seven studies reported specific food allergy (Halken 2000; Hill 2000; Juvonen 1996; Oldaeus 1997; Saarinen 1999; Vandenplas 1992; von Berg 2003).
Excluded studies
We excluded 79 studies and documented reasons for exclusion in the Characteristics of excluded studies table. Here, we document controlled trials of hydrolysed formula according to types of participants and reasons for exclusion.
Preterm or low birth weight infants
Agosti 2003 did not report allergy; Florendo 2009 did not report allergy; Maggio 2005 did not report allergy; Mihatsch 1999 (cross‐over trial) did not report allergy; Mihatsch 2002 (excess losses) did not report allergy; Pauls 1996 did not report allergy (abstract format only); Picaud 2001 did not report allergy; Raupp 1995 did not report allergy; Riezzo 2001 did not report allergy; Szajewska 2001 did not report allergy; and Yu 2014 (likely non‐random, cross‐over) did not report allergy.
Term healthy infants
Akimoto 1993 reported allergy (non‐random); Berseth 2009 (multiple formula differences) did not report allergy; Borschel 2013 did not report allergy; Borschel 2014 (multiple formula differences) did not report allergy; Borschel 2014a (multiple formula differences) did not report allergy; Burks 2008 (excess losses) did not report allergy; de Jong 1998 used protein‐free formula; Decsi 1992 did not report allergy; Decsi 1998 did not report allergy; Exl 1998 reported allergy (non‐random); Fukushima 1997 reported allergy (excess losses); Hartman 1994 reported intolerance not allergy and reported unclear losses (abstract format only); Hernell 2003 (unclear allocation) did not report allergy; Keller 1996 (unclear allocation) reported allergy; Lasekan 2006 did not report allergy; Medjad‐Guillou 1992 (cross‐over trial) did not report allergy; Mennella 2011a did not report allergy; Moran 1992 reported excess losses; Paronen 2000 enrolled infants at risk of diabetes and did not report allergy; Rigo 1994a (unclear allocation) did not report allergy; Rigo 1994b (unclear allocation) did not report allergy; Scalabrin 2009 (excess losses) reported adverse events including allergy; Schmelzle 2003 (excess losses) did not report allergy; Schmitz 1992 did not report allergy (excess losses); Schrander 1993 was observational; Silva Rey 1996 (unclear allocation; excess losses) reported in a thesis only; Staelens 2008 did not report allergy; Tariq 1998 was observational; Vaarala 1995 did not report allergy; Vaarala 2012 (enrolled infants at risk of diabetes) did not report allergy; and Vandenplas 1993 did not report allergy.
Term infants at high risk of allergy
Arshad 1992a reported multiple differential allergy‐reducing co‐interventions; Barberi 1993 reported unclear allocation, excess losses and allergy (abstract format only); Bergmann 1996a reported allergy and non‐random allocation; Boyle 2016 reported multiple differential allergy‐reducing co‐interventions ‐ prebiotic and hydrolysed formulas ‐ and reported allergy; Chan 2002 reported allergy and excess losses; Chan‐Yeung 2000 reported multiple differential allergy‐reducing co‐interventions; Chandra 1989a reported data that could not be verified after allegations of fraud; Chandra 1989b reported data that could not be verified after allegations of fraud; D'Agata 1996 reported unclear allocation, imbalances between groups and allergy; Giovannini 1994 reported excess losses and did not report allergy; Halken 1992 reported allergy and excess losses; Kuo 2011 was observational; Iikura 1995 reported unclear allocation and imbalances between groups (abstract format only); Martinez‐Valverde reported unclear allocation in a thesis only; Moran 1992 reported excess losses and allergy; Nentwich 2003 was observational; Odelram 1996 reported excess losses and allergy; Porch 1998 reported excess losses; Shao 2006 reported multiple differential allergy‐reducing co‐interventions; Szajewska 2004 reported excess losses and allergy; TRIGR study 2011 did not report allergy; Vandenplas 1988 reported unclear allocation and losses; Wopereis 2014 did not report allergy to date (abstract format only); and Zeiger 1989 reported multiple co‐interventions and excess losses.
Infants with infantile colic, gastro‐oesophageal reflux symptoms or feed intolerance
Arikan 2008 did not report allergy; Campbell 1989 did not report allergy (non‐random); Corvaglia 2013 did not report allergy; Corvaglia 2013a did not report allergy; Hill 1995b did not report allergy; Lucassen 2000 did not report allergy; Nocerino 2012 did not report allergy (abstract format only); Savino 2003 was observational; Savino 2006 reported multiple formula differences; Taubman 1988 did not report allergy; and Zeiger 1989 reported allergy and excess losses.
Risk of bias in included studies
We have summarised risk of bias in included studies in Figure 2; and Figure 3. Overall, we considered no studies to be at 'low risk' of bias.
Figure 2.
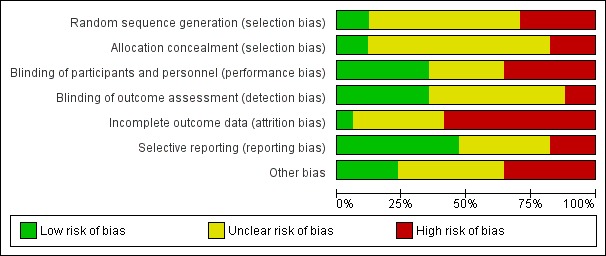
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Figure 3.
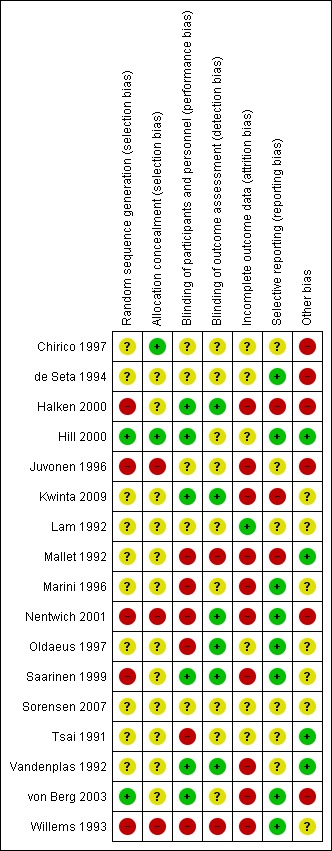
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Randomisation
Two studies reported an adequate method of randomisation (Hill 2000; von Berg 2003). Ten studies reported random allocation of infants but not the method of randomisation used (Chirico 1997; de Seta 1994; Lam 1992; Mallet 1992; Marini 1996; Oldaeus 1997; Tsai 1991; Vandenplas 1992), so we assessed their risk as 'unclear'. Five studies reported quasi‐random methods of participant allocation, including Halken 2000 (by date of birth), Juvonen 1996 (by day of month), Nentwich 2001 (odd and even numbers), Saarinen 1999 and Willems 1993 (month of birth), so we assessed them as having 'high risk'.
Allocation concealment
We assessed three studies as having 'low risk' of allocation concealment (Chirico 1997; Hill 2000; Tsai 1991) and 10 studies as having 'unclear' risk, including de Seta 1994 (did not report method of allocation), Halken 2000 (used quasi‐random allocation method but blinded intervention), Kwinta 2009 (used unclear allocation method), Lam 1992 (did not report method), Mallet 1992 (did not report method), Marini 1996 (did not report method), Oldaeus 1997 (did not report method), Saarinen 1999 (used quasi‐random allocation method but blinded intervention), von Berg 2003 (did not report method) and Vandenplas 1992 (used unclear allocation method). We assessed three studies as having 'high risk' for allocation concealment ‐ Juvonen 1996 (quasi‐random allocation, unblinded study), Nentwich 2001 (quasi‐random allocation, unblinded prescribing) and Willems 1993 (quasi‐random allocation, unblinded study).
Blinding
Six studies reported blinding of participants and personnel (Halken 2000; Hill 2000; Kwinta 2009; Saarinen 1999; Vandenplas 1992; von Berg 2003); we assessed four studies as having 'unclear' risk, as they did not report details (Chirico 1997; de Seta 1994; Juvonen 1996; Lam 1992) and six studies as unblinded (Mallet 1992; Marini 1996; Nentwich 2001; Oldaeus 1997; Tsai 1991; Willems 1993).
Six studies reported blinding of measurement (Halken 2000; Kwinta 2009; Nentwich 2001; Oldaeus 1997; Saarinen 1999; Vandenplas 1992). Eight studies did not report blinding of measurement of clinical allergy (Chirico 1997; de Seta 1994; Hill 2000; Juvonen 1996; Lam 1992; Marini 1996; Tsai 1991; von Berg 2003). Mallet 1992 and Willems 1993 performed unblinded measurements.
Incomplete outcome data
Studies reported losses to follow‐up. We included in this review only studies with < 20% loss to follow‐up. Studies reported the following losses to follow‐up: Chirico 1997 ‐ unclear; de Seta 1994 ‐ none reported; Halken 2000 ‐ 20% at 18 months; Hill 2000 ‐ 7.3% at two years and 20% at six to seven years; Juvonen 1996 ‐ 10% at three years; Kwinta 2009 ‐ 16% at five to seven years; Lam 1992 ‐ 8% at six months; Mallet 1992 ‐ 5% to 8% at four months but > 20% at one to four years; Marini 1996 ‐ 13% at two years and 19% at three years; Nentwich 2001 ‐ 19% at 12 months; Oldaeus 1997 ‐ 9% at 18 months; Saarinen 1999 ‐ unclear, although all infants were reported to be seen routinely in well baby clinics; Tsai 1991 ‐ 9% at 12 months; Vandenplas 1992 ‐ 11% at 12 months and > 20% at three and five years; von Berg 2003 ‐ for infants born in Wesel: 14.5% at one year and 19% at three years (all other analyses and time points > 20%); and Willems 1993 ‐ 13% at one year.
Studies with < 10% loss to follow‐up included de Seta 1994 ‐ none reported, Hill 2000 ‐ 7.3% at two years, Lam 1992 ‐ 8% at six months, Mallet 1992 ‐ 5% to 8% at four months, Oldaeus 1997 ‐ 9% at 18 months and Tsai 1991 ‐ 9% at 12 months.
Selective reporting
Eight studies were at 'low risk' of reporting bias with prespecified primary outcomes reported (de Seta 1994; Hill 2000; Marini 1996; Nentwich 2001; Oldaeus 1997; Saarinen 1999; von Berg 2003; Willems 1993). We assessed five studies as having 'unclear' risk of reporting bias (Chirico 1997; Juvonen 1996; Lam 1992; Tsai 1991; Vandenplas 1992) and three as having 'high risk' (Halken 2000; Kwinta 2009; Mallet 1992).
Other potential sources of bias
Six studies had imbalances between groups after randomisation, so we considered them to be at 'high risk' of bias (Chirico 1997; de Seta 1994; Halken 2000; Juvonen 1996; Nentwich 2001; von Berg 2003). Six studies did not report sufficient details of baseline characteristics or described differences of 'unclear' importance (Kwinta 2009; Lam 1992; Marini 1996; Oldaeus 1997; Saarinen 1999; Willems 1993). We considered four studies to have well‐balanced groups after randomisation with no other identified source of bias (Hill 2000; Mallet 1992; Tsai 1991; Vandenplas 1992).
We assessed no studies as having 'low risk' of bias overall ('low risk' of selection bias, performance and measurement bias and attrition bias with < 10% loss to follow‐up).
Effects of interventions
See: Table 1; Table 2; Table 3
Analyses
Comparison 1. Early short‐term feeding: hydrolysed formula versus human milk feeding ‐ low‐risk infants
We included two studies (Juvonen 1996; Saarinen 1999) that compared a short duration (three to four days whilst in hospital) of early supplemental or sole hydrolysed formula versus donor human milk feeds in infants who were subsequently encouraged to breast feed.
Juvonen 1996 (90 infants) reported no difference in childhood incidence of allergy (risk ratio (RR) 1.43, 95% confidence interval (CI) 0.38 to 5.37) and asthma (RR 0.48, 95% CI 0.05 to 4.41) and no difference in eczema (RR 0.48, 95% CI 0.05 to 4.41), no difference in food allergy (RR 1.43, 95% CI 0.38 to 5.37) and no difference in CMA (RR 7.11, 95% CI 0.35 to 143.84) at three years. Saarinen 1999 reported no difference in childhood incidence of CMA (3559 infants; RR 0.87, 95% CI 0.52 to 1.46) up to a mean age of 27 months.
We considered the following subgroup analyses, but as no significant benefits were reported, we did not wish to duplicate the results.
Both studies enrolled infants irrespective of family history of allergy or food allergy in first‐degree relatives.
Extent of protein hydrolysis: Juvonen 1996 and Saarinen 1999 compared an EHF versus pasteurised donor human milk.
Indication for use: Both studies used formula for early short‐term infant formula feeding.
Method of ascertainment of allergy: Saarinen 1999 reported outcomes of an unblinded elimination/challenge for CMA. Juvonen 1996 did not report criteria for diagnosis of allergy.
Type of protein hydrolysate used: Juvonen 1996 compared an extensively hydrolysed casein formula versus pasteurised donor human milk. Saarinen 1999 compared an extensively hydrolysed whey formula versus pasteurised donor human milk.
Sensitivity analysis
We considered neither study to be at 'low risk' of bias.
Comparison 2. Early short‐term feeding: hydrolysed formula versus cow's milk formula ‐ low‐risk infants
Two studies (Juvonen 1996; Saarinen 1999) compared a short duration (three to four days whilst in hospital) of early supplemental or sole feeding with a hydrolysed formula versus CMF. Both trials subsequently encouraged all mothers to breast feed.
Juvonen 1996 (77 infants) reported no difference in childhood allergy incidence (RR 1.37, 95% CI 0.33 to 5.71), no difference in childhood asthma incidence (RR 3.08, 95% CI 0.13 to 73.26), no difference in childhood eczema incidence (RR 0.34, 95% CI 0.04 to 3.15), no difference in childhood food allergy (RR 1.37, 95% CI 0.33 to 5.71) and no difference in childhood CMA (RR 5.13, 95% CI 0.25 to 103.43). Saarinen 1999 reported a reduction in infant CMA incidence of borderline significance (3478 infants; RR 0.62, 95% CI 0.38 to 1.00; risk difference (RD) ‐0.01, 95% CI ‐0.02 to 0.00; P = 0.05).
We considered the following subgroup analyses, but as no significant benefits were reported, we did not wish to duplicate the results.
Both studies enrolled infants irrespective of family history allergy or food allergy in first‐degree relatives.
Extent of protein hydrolysis: Juvonen 1996 and Saarinen 1999 compared an EHF versus CMF.
Indication for use: Both studies used formula for early short‐term infant formula feeding.
Method of ascertainment of allergy: Saarinen 1999 reported outcomes of an unblinded elimination/challenge for CMA. Juvonen 1996 did not report criteria for diagnosis of allergy.
Type of protein hydrolysate used: Juvonen 1996 compared an extensively hydrolysed casein formula versus CMF. Saarinen 1999 compared an extensively hydrolysed whey formula versus CMF.
Sensitivity analysis
We considered neither study to use adequate methods.
Comparison 3. Prolonged feeding: hydrolysed formula versus human milk feeding
We found no study that compared prolonged feeding with hydrolysed formula versus human milk feeding.
Comparison 4. Prolonged feeding: hydrolysed formula versus cow's milk formula
Twelve studies (Chirico 1997; de Seta 1994; Hill 2000; Kwinta 2009; Lam 1992; Mallet 1992; Marini 1996; Oldaeus 1997; Tsai 1991; Vandenplas 1992; von Berg 2003; Willems 1993) reported outcomes upon comparing prolonged hydrolysed formula versus CMF feeding.
Meta‐analysis showed a reduction in infant allergy (eight studies, 2852 infants; fixed‐effect (FE) RR 0.82, 95% CI 0.72 to 0.95; RD ‐0.04, 95% CI ‐0.08 to ‐0.01; number needed to treat for an additional beneficial outcome (NNTB) 25, 95% CI 12.5 to 100; I² = 18%). Meta‐analysis revealed no difference in childhood allergy incidence (two studies, 950 infants; RR 0.85, 95% CI 0.69 to 1.05; heterogeneity P = 0.05; I² = 73%). One study (Kwinta 2009) reported no difference in childhood allergy prevalence (62 infants; RR 1.76, 95% CI 0.76 to 4.09). (See Figure 4 for funnel plot.)
Figure 4.
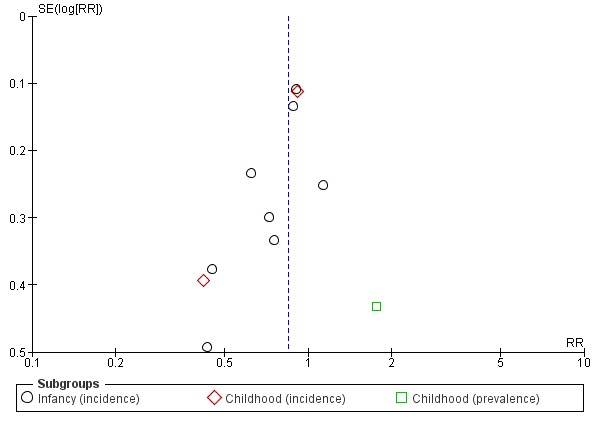
Funnel plot of comparison: 4 Prolonged feeding: hydrolysed formula versus cow's milk formula, outcome: 4.1 Any allergy.
Meta‐analysis showed no difference in infant asthma (four studies, 318 infants; FE RR 0.57, 95% CI 0.31 to 1.04; I² = 0%). Marini 1996 reported no difference in childhood asthma incidence (78 infants; RR 0.38, 95% CI 0.08 to 1.84). Meta‐analysis revealed no difference in childhood asthma prevalence (three studies, 1229 infants; FE RR 1.15, 95% CI 0.89 to 1.50; I² = 0%).
Meta‐analysis showed no difference in infant eczema (nine studies, 2896 infants; FE RR 0.86, 95% CI 0.73 to 1.01; heterogeneity P = 0.05; I² = 0%). Meta‐analysis showed no difference in childhood eczema incidence (two studies, 950 infants; FE RR 0.83, 95% CI 0.63 to 1.10; heterogeneity P = 0.20; I² = 40%). Meta‐analysis revealed no difference in childhood eczema prevalence (three studies, 1228 infants; FE RR 0.78, 95% CI 0.60 to 1.02; I² = 8%). (See Figure 5 for funnel plot.)
Figure 5.
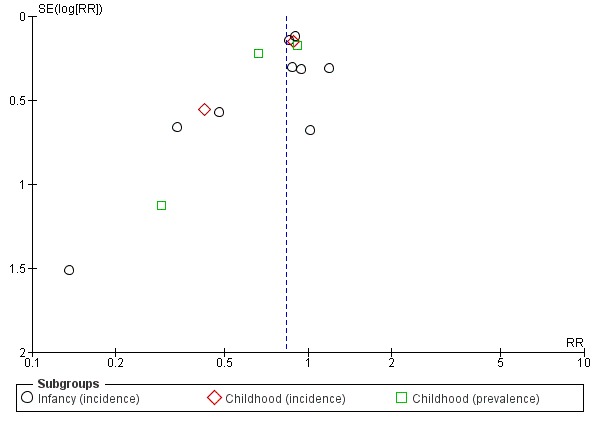
Funnel plot of comparison: 4 Prolonged feeding: hydrolysed formula versus cow's milk formula, outcome: 4.3 Eczema.
Meta‐analysis showed no difference in infant rhinitis (three studies, 256 infants; FE RR 0.52, 95% CI 0.14 to 1.85; I² = 0%). Marini 1996 reported no difference in childhood rhinitis incidence (78 infants; RR 0.48, 95% CI 0.04 to 5.03). Meta‐analysis revealed no difference in childhood rhinitis prevalence (two studies, 357 infants; FE RR 1.14, 95% CI 0.78 to 1.67; I² = 0%).
Meta‐analysis showed no difference in infant food allergy (two studies, 479 infants; FE RR 1.95, 95% CI 0.58 to 1.54; heterogeneity P = 0.15; I² = 52%). Meta‐analysis revealed a reduction in infant CMA (two studies, 405 infants; FE RR 0.38, 95% CI 0.16 to 0.86; I² = 0%). (See Figure 6 for funnel plot.)
Figure 6.
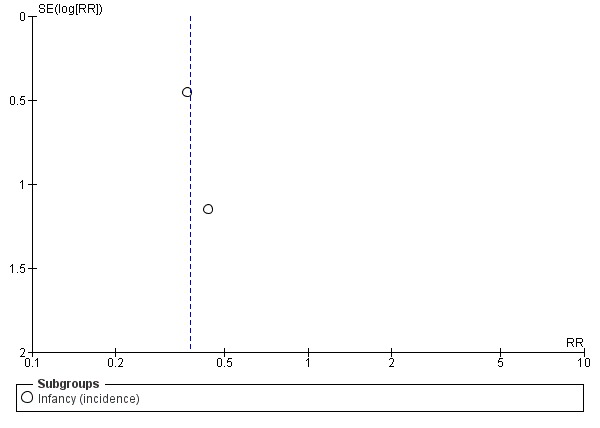
Funnel plot of comparison: 4 Prolonged feeding: hydrolysed formula versus cow's milk formula, outcome: 4.6 Cow's milk allergy.
Subgroup analyses (Comparisons 5 to 10)
Comparison 5. Prolonged feeding: hydrolysed formula versus cow's milk formula ‐ low‐risk infants
Kwinta 2009 compared prolonged feeding with a hydrolysed formula versus CMF in low‐risk infants. Kwinta 2009 reported no difference in childhood prevalence of allergy (62 infants; RR 1.76, 95% CI 0.76 to 4.09), asthma (62 infants; RR 2.20, 95% CI 0.77 to 6.26), eczema (62 infants; RR 0.29, 95% CI 0.03 to 2.66) and rhinitis (62 infants; RR 1.32, 95% CI 0.53 to 3.26).
Comparison 6. Prolonged feeding: hydrolysed formula versus cow's milk formula ‐ high‐risk infants
Eight studies (de Seta 1994; Hill 2000; Lam 1992; Marini 1996; Oldaeus 1997; Vandenplas 1992; von Berg 2003; Willems 1993) compared prolonged hydrolysed formula feeding versus CMF feeding in high‐risk infants.
Meta‐analysis showed a reduction in infant allergy incidence (eight studies, 2852 infants; FE RR 0.82, 95% CI 0.72 to 0.95; I² = 18%). Meta‐analysis revealed no difference in childhood allergy incidence (two studies, 950 infants; FE RR 0.85, 95% CI 0.69 to 1.05; heterogeneity P = 0.05; I² = 73%).
Meta‐analysis showed no difference in infant asthma incidence (four studies, 318 infants; FE RR 0.57, 95% CI 0.31 to 1.04; I² = 0%). Marini 1996 reported no difference in childhood asthma incidence (78 infants; RR 0.38, 95% CI 0.08 to 1.84). Meta‐analysis revealed no difference in childhood asthma prevalence (two studies, 1167 infants; RR 1.09, 95% CI 0.83 to 1.44; I² = 0%).
Meta‐analysis showed no difference in infant eczema incidence (nine studies, 2896 infants; FE RR 0.86, 95% CI 0.73 to 1.01; I² = 0%), no difference in childhood eczema incidence (two studies, 950 infants; FE RR 0.83, 95% CI 0.63 to 1.10) and no difference in childhood eczema prevalence (two studies, 1166 infants; FE RR 0.80, 95% CI 0.61 to 1.04; I² = 26%).
Meta‐analysis showed no significant difference in infant rhinitis incidence (three studies, 256 infants; FE RR 0.52, 95% CI 0.14 to 1.85; I² = 0%). Marini 1996 reported no difference in childhood rhinitis incidence (78 infants; RR 0.47, 95% CI 0.04 to 5.03). Hill 2000 found no difference in childhood rhinitis prevalence (295 infants; RR 1.11, 95% CI 0.73 to 1.68).
Meta‐analysis showed no difference in infant food allergy incidence (two studies, 479 infants; FE RR 0.95, 95% CI 0.58 to 1.54; heterogeneity P = 0.15; I² = 54%).
Meta‐analysis showed no difference in infant cow's milk allergy incidence (two studies, 405 infants; FE RR 0.38, 95% CI 0.16 to 0.86; I² = 0%).
Comparison 7. Prolonged feeding: partially hydrolysed formula versus cow's milk formula
Eleven studies (Chirico 1997; de Seta 1994; Kwinta 2009; Hill 2000; Lam 1992; Marini 1996; Oldaeus 1997; Tsai 1991; Vandenplas 1992; von Berg 2003; Willems 1993) reported outcomes that compared prolonged feeding with a PHF versus a CMF.
Meta‐analysis showed a reduction in infant allergy incidence (eight studies, 1820 infants; FE RR 0.83, 95% CI 0.72 to 0.96; RD ‐0.05, 95% CI ‐0.09 to ‐0.01; NNTB 20, 95% CI 11, 100; heterogeneity P = 0.16; I² = 33%) and no difference in childhood allergy incidence (two studies, 510 infants; FE RR 0.86, 95% CI 0.67 to 1.10; heterogeneity P = 0.04; I² = 75%). Marini 1996 reported a reduction in childhood allergy incidence (78 infants; RR 0.42, 95% CI 0.19 to 0.90), whereas von Berg 2003 found no difference (432 infants; RR 0.95, 95% CI 0.73 to 1.25).
Meta‐analysis showed no difference in infant asthma (four studies, 268 infants; FE RR 0.54, 95% CI 0.28 to 1.04; I² = 0%). Marini 1996 reported no difference in childhood asthma incidence (78 infants; RR 0.38, 95% CI 0.08 to 1.84). Meta‐analysis revealed no difference in childhood asthma prevalence (three studies, 789 infants; FE RR 1.20, 95% CI 0.91 to 1.58).
Meta‐analysis showed no difference in infant eczema (eight studies, 1699 infants; FE RR 0.89, 95% CI 0.75 to 1.06; I² = 0%), no difference in childhood eczema incidence (two studies, 510 infants; FE RR 0.85, 95% CI 0.61 to 1.19; heterogeneity P = 0.17; I² = 46%) and no difference in childhood eczema prevalence (three studies, 788 infants; FE RR 0.82, 95% CI 0.61 to 1.09; I² = 0%).
Meta‐analysis showed no difference in infant rhinitis (three studies, 206 infants; FE RR 0.40, 95% CI 0.09 to 1.70). Marini 1996 reported no difference in childhood rhinitis incidence (78 infants; RR 0.48, 95% CI 0.04 to 5.03). Meta‐analysis revealed no difference in childhood rhinitis prevalence (two studies, 257 infants; FE RR 1.14, 95% CI 0.78 to 1.67; I² = 0%).
Meta‐analysis showed no difference in infant food allergy (two studies, 429 infants; FE RR 1.00, 95% CI 0.62 to 1.63; heterogeneity P = 0.05; I² = 74%). Neither study reported a difference.
Meta‐analysis showed a reduction in infant CMA incidence (two studies, 405 infants; FE RR 0.38, 95% CI 0.16 to 0.86; I² = 0%).
Comparison 8. Prolonged feeding: extensively hydrolysed formula versus cow's milk formula
Five studies (Kwinta 2009; Mallet 1992; Oldaeus 1997; Sorensen 2007; von Berg 2003) compared prolonged feeding with an EHF versus CMF.
Meta‐analysis showed no difference in infant allergy (two studies, 1561 infants; FE RR 0.87, 95% CI 0.68 to 1.13; I² = 0%). von Berg 2003 reported no difference in childhood allergy incidence (651 infants; RR 0.89, 95% CI 0.71 to 1.13). Kwinta 2009 found no difference in childhood allergy prevalence (62 infants; RR 1.76, 95% CI 0.76 to 4.09).
Oldaeus 1997 reported no difference in infant asthma (96 infants; RR 0.61, 95% CI 0.18 to 2.04). Meta‐analysis revealed no difference in childhood asthma prevalence (two studies, 713 infants; FE RR 1.15, 95% CI 0.76 to 1.72; heterogeneity P = 0.18, I² = 43%).
Meta‐analysis showed no difference in infant eczema (three studies, 1726 infants; FE RR 0.83, 95% CI 0.63 to 1.08). von Berg 2003 reported no difference in childhood eczema incidence (651 infants; RR 0.86, 95% CI 0.63 to 1.17). Meta‐analysis revealed a reduction in childhood eczema prevalence (two studies, 713 infants; FE RR 0.61, 95% CI 0.39 to 0.97; I² = 0%).
Oldaeus 1997 reported no difference in infant rhinitis incidence (96 infants; RR 2.76, 95% CI 0.12 to 66.22). Kwinta 2009 found no difference in childhood rhinitis prevalence (62 infants; RR 1.32, 95% CI 0.53 to 3.26).
Oldaeus 1997 reported no difference in food allergy (96 infants; RR 1.15, 95% CI 0.33 to 4.02).
Comparison 9. Prolonged feeding: extensively hydrolysed formula versus partially hydrolysed formula
Five studies (Halken 2000; Nentwich 2001; Oldaeus 1997; Sorensen 2007; von Berg 2003) compared prolonged feeding with an EHF versus a PHF.
Meta‐analysis showed no difference in infant allergy (three studies, 1806 infants; FE RR 0.93, 95% CI 0.75 to 1.16; I² = 0%). von Berg 2003 reported no difference in childhood allergy incidence (661 infants; RR 0.89, 95% CI 0.58 to 1.35).
Meta‐analysis showed no difference in infant asthma incidence (two studies, 341 infants; FE RR 1.72, 95% CI 0.74 to 3.96; I² = 0%). von Berg 2003 reported no difference in childhood asthma prevalence (661 infants; RR 0.89, 95% CI 0.58 to 1.35).
Meta‐analysis showed no difference in infant eczema (four studies, 1865 infants; FE RR 0.89, 95% CI 0.73 to 1.10; I² = 0%). von Berg 2003 reported no difference in childhood eczema incidence (661 infants; RR 0.92, 95% CI 0.69 to 1.26) and no difference in childhood eczema prevalence (661 infants; RR 0.90, 95% CI 0.54 to 1.52).
Meta‐analysis revealed no difference in infant rhinitis (two studies, 341 infants; FE RR 1.25, 95% CI 0.36 to 4.29; I² = 0%).
Meta‐analysis showed a reduction in infant food allergy (two studies, 341 infants; FE RR 0.43, 95% CI 0.19, 0.99; I² = 0%).
Halken 2000 reported no difference in infant CMA (246 infants; RR 0.13, 95% CI 0.01 to 1.16) and no difference in infant urticaria (246 infants; RR 1.32, 95% CI 0.26 to 6.66).
Comparison 10. Prolonged exclusive feeding: hydrolysed formula versus cow's milk formula
Seven studies reported prolonged exclusive hydrolysed formula versus CMF (Chirico 1997; de Seta 1994; Kwinta 2009; Lam 1992; Marini 1996; Vandenplas 1992; Willems 1993).
Meta‐analysis showed no difference in infant allergy (five studies, 425 infants; FE RR 0.61, 95% CI 0.46 to 0.80; I² = 0%). Marini 1996 reported a reduction in childhood allergy incidence (78 infants; RR 0.42, 95% CI 0.19 to 0.90). Kwinta 2009 found no difference in childhood allergy prevalence (62 infants; RR 1.76, 95% CI 0.76 to 4.09).
Meta‐analysis revealed no difference in infant asthma (two studies, 144 infants; FE RR 0.57, 95% CI 0.25 to 1.31; I² = 0%). Marini 1996 reported no difference in childhood asthma incidence (78 infants; RR 0.38, 95% CI 0.08 to 1.84). Kwinta 2009 found no difference in childhood asthma prevalence (62 infants; RR 2.20, 95% CI 0.77 to 6.26).
Meta‐analysis showed no difference in infant eczema (four studies, 271 infants; FE RR 0.74, 95% CI 0.45 to 1.21; I² = 0%). Marini 1996 reported no difference in childhood eczema incidence (78 infants; RR 0.42, 95% CI 0.14 to 1,26). Kwinta 2009 found no difference in childhood eczema prevalence (62 infants; RR 0.29, 95% CI 0.03 to 2.66).
Marini 1996 reported that no infants had rhinitis and found no difference in childhood rhinitis incidence (82 infants; RR 0.48, 95% CI 0.04 to 5.03). Kwinta 2009 reported no difference in childhood rhinitis prevalence (62 infants; RR 1.32, 95% CI 0.53 to 3.26).
Vandenplas 1992 reported a reduction in infant CMA (67 infants; RR 0.36, 95% CI 0.15 to 0.89).
Sensitivity analyses (Comparisons 11 and 12)
Comparison 11. Prolonged feeding: hydrolysed formula versus cow's milk formula ‐ studies with blinded measurement
Four studies reported assessment for allergy without knowledge of participant allocation (Kwinta 2009; Oldaeus 1997; Vandenplas 1992; von Berg 2003).
Meta‐analysis showed no difference in infant allergy (three studies, 2156 infants; FE RR 0.87, 95% CI 0.69 to 1.08; heterogeneity P = 0.13; I² = 52%). von Berg 2003 reported no difference in childhood allergy incidence (872 infants; RR 0.91, 95% CI 0.73 to 1.14). Kwinta 2009 found no difference in childhood allergy prevalence (62 infants; RR 0.91, 95% CI 0.73 to 1.14) .
Oldaeus 1997 reported no difference in infant asthma (141 infants; RR 0.48, 95% CI 0.17 to 1.42). Meta‐analysis revealed no difference in childhood asthma prevalence (two studies; 934 infants; FE RR 1.17, 95% CI 0.80 to 1.73; heterogeneity P = 0.21; I² = 38%).
Meta‐analysis showed no difference in infant eczema (two studies, 2089 infants; FE RR 0.90, 95% CI 0.70 to 1.16; I² = 0%). von Berg 2003 reported no difference in childhood eczema incidence (872 infants; RR 0.88, 95% CI 0.66 to 1.18). Meta‐analysis revealed a reduction in childhood eczema prevalence (two studies, 934 infants; FE RR 0.64, 95% CI 0.42 to 0.97; I² = 0%).
Oldaeus 1997 reported no difference in infant rhinitis (141 infants; RR 1.47, 95% CI 0.06 to 35.37). Kwinta 2009 (62 infants) found no difference in childhood rhinitis prevalence (62 infants; RR 1.32, 95% CI 0.53 to 3.26).
Oldaeus 1997 reported no difference in infant food allergy (141 infants; RR 1.82, 95% CI 0.64 to 5.16). Vandenplas 1992 found a reduction in infant CMA (67 infants; RR 0.36, 95% CI 0.15 to 0.89).
Comparison 12. Prolonged feeding: hydrolysed formula versus cow's milk formula ‐ studies at low risk of bias
We assessed no studies as eligible for inclusion in the sensitivity analysis of studies of adequate methods (adequate randomisation, allocation concealment and < 10% loss to follow‐up).
Additional analyses (Comparisons 13 to 17)
Comparison 13. Prolonged feeding: partially hydrolysed whey formula versus cow's milk formula
Nine studies compared a partially hydrolysed whey formula versus CMF (Chirico 1997; de Seta 1994; Hill 2000; Lam 1992; Marini 1996; Tsai 1991; Vandenplas 1992; von Berg 2003; Willems 1993).
Meta‐analysis showed a reduction in infant allergy (seven studies, 1729 infants; FE RR 0.78, 95% CI 0.66 to 0.94; I² = 15%) and no difference in childhood allergy incidence (two studies, 510 infants; FE RR 0.68, 95% CI 0.31 to 1.52) but showed significant (P = 0.04) and substantial (I² = 75%) heterogeneity.
Meta‐analysis showed no difference in infant asthma (three studies, 177 infants; FE RR 0.61, 95% CI 0.29 to 1.28; I² = 0%). Marini 1996 reported no difference in childhood asthma incidence (78 infants; RR 0.38, 95% CI 0.08 to 1.84). Meta‐analysis revealed no difference in childhood asthma prevalence (two studies, 727 infants; FE RR 1.13, 95% CI 0.85 to 1.51; I² = 0%).
Meta‐analysis showed no difference in infant eczema (seven studies, 1608 infants; FE RR 0.86, 95% CI 0.72 to 1.03; I² = 0%), no difference in childhood eczema incidence (two studies, 510 infants; FE RR 0.85, 95% CI 0.61 to 1.19; heterogeneity P = 0.17; I² = 46%) and no difference in childhood eczema prevalence (two studies, 726 infants; FE RR 0.84, 95% CI 0.63 to 1.12; I² = 0%).
Meta‐analysis showed no difference in infant rhinitis (two studies, 115 infants; FE RR 0.4, 95% CI 0.09 to 1.70). Marini 1996 reported no difference in childhood rhinitis incidence (78 infants; RR 0.47, 95% CI 0.04 to 5.03). Hill 2000 found no difference in childhood rhinitis prevalence (295 infants; RR 1.11, 95% CI 0.73 to 1.68).
Hill 2000 reported no difference in infant food allergy (338 infants; RR 0.76, 95% CI 0.44 to 1.33).
Meta‐analysis revealed a reduction in infant CMA (two studies, 405 infants; RR 0.38, 95% CI 0.16 to 0.86; I² = 0%).
Comparison 14. Prolonged feeding: partially hydrolysed casein‐containing formula versus cow's milk formula
Two studies compared a PHF containing casein versus CMF (Kwinta 2009; Oldaeus 1997).
Oldaeus 1997 reported no difference in infant allergy incidence (91 infants; RR 1.36, 95% CI 0.80 to 2.31). Kwinta 2009 found no difference in childhood allergy prevalence (62 infants; RR 1.76, 95% CI 0.76 to 4.09).
Oldaeus 1997 reported no difference in infant asthma (91 infants; RR 0.34, 95% CI 0.07 to 1.60). Kwinta 2009 found no difference in childhood asthma prevalence (62 infants; RR 2.20, 95% CI 0.77 to 6.26).
Oldaeus 1997 reported no difference in infant eczema (91 infants; RR 1.30, 95% CI 0.66 to 2.55). Kwinta 2009 found no difference in childhood eczema prevalence (62 infants; RR 0.29, 95% CI 0.03 to 2.66).
Oldaeus 1997 reported that no infant had rhinitis. Kwinta 2009 reported no difference in childhood rhinitis prevalence (62 infants; RR 1.32, 95% CI 0.53 to 3.26).
Oldaeus 1997 reported no difference in infant food allergy (91 infants; RR 2.56, 95% CI 0.86 to 7.56).
Comparison 15. Prolonged feeding: extensively hydrolysed whey formula versus cow's milk formula
von Berg 2003 compared an extensively hydrolysed whey formula versus CMF.
von Berg 2003 reported no difference in infant allergy (972 infants; RR 0.97, 95% CI 0.71 to 1.34) and no difference in childhood allergy incidence (431 infants; RR 1.07, 95% CI 0.82 to 1.38).
von Berg 2003 reported no difference in childhood asthma prevalence (431 infants; RR 1.19, 95% CI 0.73 to 1.94).
von Berg 2003 reported no difference in infant eczema (972 infants; RR 1.00, 95% CI 0.72 to 1.40), no difference in childhood eczema incidence (431 infants; RR 1.06, 95% CI 0.75 to 1.49) and no difference in childhood eczema prevalence (431 infants; RR 0.78, 95% CI 0.46 to 1.33).
Comparison 16. Prolonged feeding: extensively hydrolysed casein formula versus cow's milk formula
von Berg 2003 compared an extensively hydrolysed casein formula versus CMF.
von Berg 2003 reported no difference in infant allergy (976 infants; RR 0.76, 95% CI 0.54 to 1.07) and a reduction in childhood allergy incidence (431 infants; RR 0.72, 95% CI 0.53 to 0.97).
von Berg 2003 reported no difference in childhood asthma prevalence (431 infants; RR 0.84, 95% CI 0.49 to 1.45).
von Berg 2003 reported no difference in infant eczema (976 infants; RR 0.69, 95% CI 0.47 to 1.00), a reduction in childhood eczema incidence (431 infants; RR 0.66, 95% CI 0.44 to 0.98) and a reduction in childhood eczema prevalence (431 infants; RR 0.50, 95% CI 0.27 to 0.92).
Comparison 17. Prolonged feeding: extensively hydrolysed casein formula versus extensively hydrolysed whey formula
Two studies compared an extensively hydrolysed casein formula versus an extensively hydrolysed whey formula (Halken 2000; von Berg 2003).
Meta‐analysis showed no difference in infant allergy (two studies, 1143 infants; FE RR 0.96, 95% CI 0.73 to 1.26). Results revealed significant (P = 0.02) and substantial (I² = 82%) heterogeneity between studies, although neither study reported a difference. von Berg 2003 reported a reduction in childhood allergy incidence (440 infants; RR 0.68, 95% CI 0.50 to 0.90).
Halken 2000 reported no difference in infant asthma (161 infants; RR 2.28, 95% CI 0.83 to 6.28). von Berg 2003 reported no difference in childhood asthma prevalence (440 infants; RR 0.71, 95% CI 0.42 to 1.19).
Meta‐analysis showed no difference in infant eczema (two studies, 1143 infants; FE RR 0.81, 95% CI 0.59 to 1.10; heterogeneity P = 0.07; I² = 70%). von Berg 2003 found a reduction in childhood eczema incidence (440 infants; RR 0.62, 95% CI 0.42 to 0.92) and no difference in childhood eczema prevalence (440 infants; RR 0.64, 95% CI 0.33 to 1.21).
Halken 2000 reported no difference in infant rhinitis (161 infants; RR 2.08, 95% CI 0.39 to 11.02), no difference in infant food allergy (161 infants; RR 1.45, 95% CI 0.48 to 4.39), no difference in infant CMA (161 infants; RR 5.19, 95% CI 0.25 to 106.38) and no difference in urticaria (161 infants; RR 4.15, 95% CI 0.47 to 36.34).
Discussion
Summary of main results
Early short‐term infant feeding
Two studies (Juvonen 1996; Saarinen 1999) assessed the effect of three to four days' supplementation with a hydrolysed formula versus cow's milk formula (CMF) versus pasteurised human milk feeds whilst in hospital after birth.
Juvonen 1996 reported no difference in rates of allergy, asthma, eczema, food allergy and cow's milk allergy (CMA) up to three years of age upon comparing feeding a hydrolysed formula versus pasteurised human milk (GRADE quality of evidence very low ‐ see Figure 4). Saarinen 1999 found no difference in the rate of CMA at a mean age of 27 months when comparing feeding a hydrolysed formula versus pasteurised human milk (GRADE quality of evidence very low ‐ see Figure 4).
Juvonen 1996 reported no difference in rates of allergy, asthma, eczema, food allergy and CMA up to three years of age upon comparing feeding a hydrolysed formula versus CMF (GRADE quality of evidence very low ‐ see Figure 5). Saarinen 1999 found a reduction in CMA at a mean age of 27 months when comparing feeding a hydrolysed formula versus CMF (GRADE quality of evidence very low ‐ see Figure 5). Substantial methodological concerns regarding these studies and imprecision in estimates of effect suggest the potential for confounding to influence the statistical significance of effects.
Prolonged infant feeding
Prolonged infant feeding with a hydrolysed formula compared with CMF was associated with a reduction in infant allergy (eight studies, 2852 infants; fixed‐effect risk ratio (FE RR) 0.82, 95% confidence interval (CI) 0.72 to 0.95; risk difference (RD) ‐0.04, 95% CI ‐0.08 to ‐0.01; number needed to treat for an additional beneficial outcome (NNTB) 25, 95% CI 12.5 to 100) (GRADE quality of evidence very low ‐ see Figure 6). However, methodological concerns regarding these studies (see Figure 2; Figure 3) and concerns regarding the potential for publication bias were substantial ‐ substantial numbers of studies including those examining high‐risk infants have not comprehensively reported allergy outcomes. We considered no studies to be at 'low risk' of bias.
Prolonged infant feeding with a hydrolysed formula compared with CMF was associated with a reduction in infant CMA (two studies, 405 infants; FE RR 0.38, 95% CI 0.16 to 0.86) (GRADE quality of evidence very low ‐ see Figure 6). However, methodological concerns regarding studies were substantial (see Figure 2; Figure 3). Concerns regarding publication bias include that substantial numbers of studies including those in high‐risk infants have not comprehensively reported allergy outcomes; other concerns involve imprecision, with only two studies reporting outcomes for 405 infants included in the meta‐analysis.
Prolonged infant feeding with a hydrolysed formula compared with CMF was not associated with a difference in childhood allergy incidence or prevalence. Investigators found no difference in specific allergy including infant and childhood asthma, infant and childhood eczema, infant and childhood rhinitis and infant food allergy. Many of the analyses assessing specific allergy are underpowered and do not preclude the possibility of benefit from prolonged use of a hydrolysed formula compared with CMF, especially given the finding of reduced infant allergy and reduced infant CMA.
Subgroup analyses
These analyses identified groups that may benefit from use of hydrolysed formulas and potential benefits associated with specific types of hydrolysed formulas.
Infant allergy was reduced in analyses of studies that enrolled infants at high risk of allergy and compared a hydrolysed formula with CMF; compared a partially hydrolysed formula (PHF) with CMF; compared prolonged and exclusive feeding (i.e. no breast feeding) of a hydrolysed formula with CMF; and compared a partially hydrolysed whey formula with CMF.
A single study (von Berg 2003) that compared an extensively hydrolysed casein formula versus CMF; and that compared an extensively hydrolysed casein formula versus an extensively hydrolysed whey formula reported a reduction in childhood allergy incidence.
A single study (von Berg 2003) that compared an extensively hydrolysed casein formula with an extensively hydrolysed whey formula reported a reduction in childhood eczema incidence. Studies that compared an extensively hydrolysed formula (EHF) with CMF; and that compared an extensively hydrolysed casein formula versus CMF found a reduction in childhood eczema prevalence.
Studies that compared an EHF with a PHF found a reduction in infant food allergy.
Studies that enrolled infants at high risk of allergy and compared a PHF with CMF; compared prolonged and exclusive feeding (i.e. no breast feeding) of a hydrolysed formula with CMF; and compared a partially hydrolysed whey formula with CMF found a reduction in infant CMA.
Summary
Prolonged infant feeding with a hydrolysed formula compared with CMF was associated with a reduction in infant allergy and infant CMA, but the GRADE quality of evidence was very low owing to methodological concerns regarding studies and the potential for publication bias.
Subgroup analyses identified potential reductions in infant allergy associated with using a PHF, feeding a hydrolysed formula to infants at high risk of allergy (first‐degree relative with allergy) and providing exclusive formula feeding (i.e. when infants are unable to be breast fed). A single study (von Berg 2003) also reported a reduction in infant and childhood incidence of eczema and in childhood prevalence of eczema resulting from use of an extensively hydrolysed casein formula compared with CMF. This study also reported a reduction in infant and childhood incidence of eczema from use of an extensively hydrolysed casein formula compared with an extensively hydrolysed whey formula. Additional high‐quality studies are needed to reproduce these findings.
Summary of subgroup analyses
Analyses showed a reduction in infant allergy in studies that:
enrolled infants at high risk of allergy and compared a hydrolysed formula versus CMF (eight studies, 2852 infants; FE RR 0.82, 95% CI 0.72 to 0.95; I² = 18%);
compared a PHF with CMF (eight studies, 1820 infants; FE RR 0.83, 95% CI 0.72 to 0.96; RD ‐0.05, 95% CI ‐0.09 to ‐0.01; NNTB 20, 95% CI 11 to 100; heterogeneity P = 0.16; I² = 33%);
compared prolonged and exclusive feeding (i.e. no breast feeding) of a hydrolysed formula versus CMF (five studies, 425 infants; FE RR 0.61, 95% CI 0.46 to 0.80; I² = 0%); and
compared a partially hydrolysed whey formula with CMF (seven studies, 1729 infants; FE RR 0.78, 95% CI 0.66 to 0.94; I² = 15%).
Analyses showed a reduction in childhood allergy incidence in a single study (von Berg 2003) that compared:
an extensively hydrolysed casein formula versus CMF (431 infants; RR 0.72, 95% CI 0.53 to 0.97); and
an extensively hydrolysed casein formula versus an extensively hydrolysed whey formula (440 infants; RR 0.68, 95% CI 0.50 to 0.90).
Analyses showed a reduction in childhood eczema prevalence in studies that:
compared an EHF with CMF (two studies, 713 infants; FE RR 0.61, 95% CI 0.39 to 0.97; I² = 0%); and
compared an extensively hydrolysed casein formula versus CMF (431 infants; RR 0.50, 95% CI 0.27 to 0.92).
Analyses showed a reduction in infant food allergy in studies that:
compared an EHF versus a PHF (two studies, 341 infants; FE RR 0.43, 95% CI 0.19 to 0.99; I² = 0%).
Analyses revealed a reduction in infant CMA in studies that:
enrolled infants at high risk of allergy (two studies, 405 infants; FE RR 0.38, 95% CI 0.16 to 0.86; I² = 0%);
compared a PHF versus CMF (two studies, 405 infants; FE RR 0.38, 95% CI 0.16 to 0.86; I² = 0%);
used prolonged and exclusive feeding (i.e. no breast feeding) of a hydrolysed formula versus CMF (one study, 67 infants; RR 0.36, 95% CI 0.15 to 0.89); or
compared a partially hydrolysed whey formula versus CMF (two studies, 405 infants; RR 0.38, 95% CI 0.16, 0.86; I² = 0%).
Overall completeness and applicability of evidence
For short‐term feeding (e.g. for three to four days whilst in hospital), a single small study (Juvonen 1996) is underpowered to determine an effect of hydrolysed formula compared with human milk or CMF on childhood allergy, asthma, eczema or rhinitis. A much larger study enrolling 5713 infants reported no difference in CMA upon comparing infants receiving short‐term feeding with an extensively hydrolysed whey formula versus human milk, and a reduction in CMA of borderline statistical significance when comparing infants fed an extensively hydrolysed whey formula versus CMF. Additional studies are needed to determine if infants benefit from short‐term feeding with a hydrolysed formula in hospital when human milk is unavailable for prevention of allergy and CMA. No studies have reported allergy outcomes resulting from short‐term use of a PHF for infants in hospital. Additional studies are needed to determine if infants derive benefit from using a PHF for short‐term feeding in hospital when human milk is unavailable for prevention of allergy and CMA.
For women unable to exclusively breast feed, no studies have compared prolonged use of a hydrolysed formula versus human milk (e.g. from a donor milk bank) for prevention of allergy. This review has not compared the effect of human milk versus CMF feeding so cannot provide a conclusion about potential benefit derived from use of a human milk bank for prevention of allergy.
Evidence suggests that prolonged use of a hydrolysed formula reduces allergy overall and CMA in infants unable to be exclusively breast fed. Evidence of benefit is limited to infants at high risk of allergy (first‐degree relative with allergy). Evidence is insufficient to show whether use of a hydrolysed formula or any specific hydrolysed formula reduces the prevalence of childhood allergy or any specific allergy. Few studies have reported the prevalence of allergy in childhood. Benefits in preventing childhood allergy and eczema following use of an extensively hydrolysed casein formula are largely restricted to a single study (von Berg 2003).
Quality of the evidence
Early short‐term infant feeding: Two studies reported the effect of short‐term infant feeding of a hydrolysed formula versus human milk and cow's milk. Both studies (Juvonen 1996; Saarinen 1999) had substantial methodological concerns (Figure 3). Analyses of the larger study (Saarinen 1999), which enrolled 5713 infants, were restricted to reporting of CMA. The other study (Juvonen 1996) was underpowered to detect important differences in other allergy outcomes. We assessed evidence for an effect on allergy and CMA as being at high risk of bias and underpowered ‐ we graded the quality of evidence as 'very low'.
Prolonged infant feeding with a hydrolysed formula compared with CMF: We assessed the GRADE quality of evidence as 'very low' (see Figure 6) for all outcomes. We had substantial methodological concerns regarding these studies (see Figure 2; Figure 3) and considered none of them to be at 'low risk' of bias. We had substantial concerns regarding the potential for publication bias in that substantial numbers of studies, including those in high‐risk infants, have not comprehensively reported allergy outcomes (see the description of excluded studies section). We also had concerns regarding imprecision of several analyses, including childhood allergy incidence and prevalence, as well as analyses of infant and childhood asthma, eczema, rhinitis, food allergy and CMA. We found substantial heterogeneity in analyses of childhood incidence of allergy (P = 0.05; I² = 73%) and infant food allergy (P = 0.15; I² =52%).
This review includes studies that used random and quasi‐random methods of participant allocation. Several studies reported methods of sequence generation that were at high risk of bias (Halken 2000; Juvonen 1996; Nentwich 2001; Saarinen 1999; Willems 1993). Several other studies failed to report the method of sequence generation used (Chirico 1997; de Seta 1994; Kwinta 2009; Lam 1992; Mallet 1992; Marini 1996; Oldaeus 1997; Sorensen 2007; Tsai 1991; Vandenplas 1992), leaving only two studies (Hill 2000; von Berg 2003) assessed as being at low risk of selection bias. In addition, for von Berg 2003, we had concerns regarding selection bias (in the extensively hydrolysed casein formula group, disproportionately more children had to be excluded as a result of non‐compliance with the study formula) and losses to follow‐up. As a result of methodological concerns regarding included studies, we downgraded the GRADE quality of evidence for all outcomes.
Potential biases in the review process
This review had strict prespecified inclusion criteria that included reporting data only when outcomes for ≥ 80% of infants were noted. A substantial number of studies had excess losses to follow‐up, so a substantial quantity of data is not included in this review. Losses to follow‐up do not necessarily bias a study. However, when losses do bias a study, it is not possible to predict the direction of effect. The review authors have consistently applied this inclusion criterion through all updates of this review.
This review includes unpublished data to reduce concern regarding publication bias. Not all studies have been reported in peer‐reviewed journals. Some studies were provided as university theses or by formula companies. Only one company to date has provided additional studies (Nestle). We excluded several studies after allegations of fraud were made and the authors of these published articles did not respond to requests for data (Chandra 1989a; Chandra 1989b). Despite this, a substantial number of studies have failed to report or comprehensively report outcomes. We consider the analyses reported in this review to be at risk of publication bias.
This review includes studies that use random and quasi‐random methods of participant allocation. See Quality of the evidence.
We obtained data from study authors for several analyses to overcome reporting problems with publications and losses. One study (Hill 2000) reported data for infants who were not contemporaneously randomised, and study authors kindly provided data for contemporaneously randomised infants, which the review authors believe overcame our concern regarding selection bias. For a second study (von Berg 2003) that published outcomes to 10 years, to overcome problems related to excess losses, study authors kindly provided additional data from one centre (Wessel) that reported an intention‐to‐treat analysis up to three years; we have included these data in this review. Losses to follow‐up after three years were excessive (> 20%), so we have excluded from this review follow‐up data from this study beyond three years.
Agreements and disagreements with other studies or reviews
A Cochrane protocol 'Hypoallergenic formula milk versus cow's milk for prevention of wheeze and asthma in children with a family history of atopy' is awaiting completion.
The European Academy of Allergy and Clinical Immunology (EAACI) has published systematic reviews and guidelines for primary prevention of food allergy and anaphylaxis (de Seta 2014; EAACI 2014; Muraro 2014). For prevention of food allergy, two systematic reviews and four randomised trials found benefit from extensively hydrolysed whey or casein formula, although one study found no benefit. Two systematic reviews, two randomised trials and two non‐randomised comparisons noted benefit when partially hydrolysed formula was compared with CMF. One randomised trial and one non‐randomised study found no effect (de Seta 2014).
Recommendations that have been provided for primary prevention of food allergy include the following.
"Recommendations for all infants:
no special diet during pregnancy or for the lactating mother;
exclusive breastfeeding for four to six months.
Further recommendations for high‐risk infants:
if supplement is needed during the first four months, a documented hypoallergenic formula is recommended.
Introduction of complementary foods after the age of four months according to normal standard weaning practices and nutrition recommendations, for all children irrespective of atopic heredity" (Muraro 2014).
Three published systematic reviews have examined hydrolysed formula for prevention of allergy (Alexander 2010; Boyle 2016a; de Silva 2014; Iskedjian 2010; Szajewska 2010); all had potential conflicts of interest. A systematic review (Alexander 2010) studied 100% whey protein partially hydrolysed formula (PHF‐W) compared with intact protein CMF in healthy infants. A meta‐analysis revealed a reduction in atopic dermatitis (11 studies; RR 0.56, 95% CI 0.40 to 0.76). A published funnel plot appears asymmetrical, suggesting lack of small negative studies. Meta‐analysis limited to 'top‐tier studies' showed a similar reduction in atopic dermatitis (four studies; RR 0.45, 95% CI 0.30 to 0.70). Review authors concluded, .."exclusive breast‐feeding should be encouraged as the standard for infant nutrition in the first months of life. For infants who are not exclusively breast‐fed, feeding with PHF‐W instead of CMF reduces the risk of AD in infants, particularly in infants with a family history of allergy." This work was partially funded by Nestle.
A recent systematic review (Boyle 2016a) found 37 eligible trials of hydrolysed formula including more than 19,000 participants. Review authors found evidence of conflict of interest and high or unclear risk of bias in most studies of allergic outcomes and evidence of publication bias in studies of eczema and wheeze. Overall, no consistent evidence indicated that PHFs or EHFs reduce the risk of allergic or autoimmune outcomes among infants at high pre‐existing risk for these outcomes. Odds ratios for eczema from birth to four years of age were 0.84 (95% CI 0.67 to 1.07) for PHF; 0.55 (95% CI 0.28 to 1.09) for extensively hydrolysed casein‐based formula; and 1.12 (95% CI 0.88 to 1.42) for extensively hydrolysed whey‐based formula compared with CMF. Review authors concluded that findings do not support current guidelines recommending the use of hydrolysed formula to prevent allergic disease in high‐risk infants. Methodologically, the Boyle 2016a review differs from this review in that it included studies with > 20% loss to follow‐up and reported infant allergy assessed up to four years of age. This review found very low‐quality evidence that prolonged supplementation of infants at high risk of allergy with hydrolysed formula compared with CMF reduces risk of infant allergy and infant CMA; very low‐quality evidence that prolonged use of a partially hydrolysed formula compared with CMF for partial or exclusive feeding was associated with a reduction in infant allergy incidence and infant CMA incidence; and very low‐quality evidence that prolonged use of an EHF versus a PHF reduces infant food allergy. Studies reported infant allergy outcomes at two years of age. This review found no evidence of benefit at later time points, which is consistent with the findings of Boyle 2016a.
Another systematic review (de Silva 2014) reported mixed findings regarding the preventive benefits of breast feeding for infants at high or normal risk, but evidence suggested that recommendations should include avoiding cow's milk and substituting extensively or partially hydrolysed whey or casein formulas for infants at high risk for the first four months.
Another systematic review (Szajewska 2010) assessed the effect of using a partially hydrolysed 100% whey formula (pHF) in reducing risk of allergy among healthy infants at high risk for allergy. The summary of findings reports that "meta‐analysis showed that pHF compared to SF (standard formula) reduced the risk of all allergic diseases, particularly atopic dermatitis/eczema, at some time points among children at high risk for allergy. Limited data suggest that the use of pHF compared with SF reduced the risk of gastrointestinal symptoms and food allergy. The pooled results did not provide evidence of a difference in the effect of pHF versus SF on the incidence of either wheezing/asthma or rhinitis. Few significant differences in outcomes were found between children who received pHF versus an extensively hydrolyzed whey formula. No significant differences in outcomes were found between children who received pHF versus an extensively hydrolyzed casein formula. These results should be interpreted with caution due to a lack of methodological rigor in many trials." A grant from Nestle Nutrition Institute supported this review.
Another review (Iskedjian 2010) compared a partially hydrolysed 100% whey‐based infant formula (PHF‐W) versus extensively hydrolysed whey‐ (EHF‐Whey) or casein‐based (EHF‐Casein) infant formula for prevention of atopic dermatitis (AD) among infants who cannot be breast fed exclusively. Meta‐analysis revealed no difference in AD when PHF‐W was compared with EHF‐Whey (RR 0.75, 95% CI 0.54 to 1.05 at 0 to 12 months; and RR 0.80, 95% CI 0.63 to 1.02 at 0 to 36 months); and no difference when PHF‐W was compared with EHF‐Casein (RR 1.06, 95% CI 0.74 to 1.53 at 0 to 12 months; and RR 1.13, 95% CI 0.87 to 1.47 at 0 to 36 months). Review authors concluded that the efficacy of PHF‐W falls within the range of efficacy of both EHF formulas (whey and casein). The Nestle Nutrition Institute supported this review.
In a report (Chung 2012) on the FDA Health Claim Review on whey‐protein partially hydrolysed infant formula and atopic dermatitis, "the FDA evaluated human intervention studies that evaluated the role of W‐PHF in reducing the risk of AD. The FDA concluded there is little to very little evidence, respectively, to support a qualified health claim concerning the relationship between intake of W‐PHF and a reduced risk of AD in partially breast fed and exclusively formula‐fed infants throughout the first year after birth and up to 3 years of age. In addition, the FDA required a warning statement be displayed along with the health claim to indicate to consumers that partially hydrolyzed infant formulas are not hypoallergenic and should not be fed to infants who are allergic to milk or to infants with existing milk allergy symptoms."
Evidence from this review for PHF versus CMF
This systematic review found evidence that use of a partially hydrolysed formula compared with CMF for partial or exclusive feeding was associated with a reduction in infant allergy incidence (eight studies, 1820 infants; FE RR 0.83, 95% CI 0.72 to 0.96; RD ‐0.05, 95% CI ‐0.09 to ‐0.01; NNTB 20, 95% CI 11 to 100; heterogeneity P = 0.16; I² = 33%); no difference in childhood allergy incidence (two studies, 510 infants; FE RR 0.86, 95% CI 0.67 to 1.10; heterogeneity P = 0.04; I² = 75%); no difference in infant asthma; no difference in childhood asthma incidence or prevalence; no difference in infant eczema (eight studies, 1699 infants; FE RR 0.89, 95% CI 0.75 to 1.06; I² = 0%); no difference in childhood eczema incidence or prevalence; no difference in infant rhinitis; no difference in infant food allergy; and a reduction in infant CMA incidence (two studies, 405 infants; FE RR 0.38, 95% CI 0.16 to 0.86; I² = 0%). We advise caution regarding these findings in view of the methodological concerns of included studies and the potential for publication bias. We also have concerns regarding imprecision of the estimate in most analyses, suggesting the potential for confounders to influence the statistical significance of findings in relation to infant allergy and CMA.
Evidence from this review for EHF versus PHF
This systematic review found no difference in infant allergy (three studies, 1806 infants; FE RR 0.93, 95% CI 0.75 to 1.16; I² = 0%); no difference in childhood allergy incidence (one study, 661 infants; RR 0.89, 95% CI 0.58 to 1.35); no difference in infant asthma incidence; no difference in childhood asthma prevalence; no difference in infant eczema (four studies, 1865 infants; FE RR 0.89, 95% CI 0.73 to 1.10; I² = 0%); no difference in childhood eczema incidence or prevalence; no difference in infant rhinitis; a reduction in infant food allergy (two studies, 341 infants; FE RR 0.43, 95% CI 0.19 to 0.99; I² = 0%); no difference in infant CMA (one study, 246 infants; RR 0.13, 95% CI 0.01 to 1.16); and no difference in infant urticaria. We advise caution regarding these findings in view of the methodological concerns of included studies and the potential for publication bias. We also have concerns regarding imprecision of the estimate in most analyses, suggesting the potential for confounders to influence the statistical significance of findings.
Authors' conclusions
We found no evidence to support feeding with a hydrolysed formula compared with exclusive breast feeding for prevention of allergy or food allergy. Until high‐quality trials compare prolonged hydrolysed formula feeding versus breast or expressed human milk feeding, hydrolysed formula should not be routinely offered to infants for prevention of allergy or food allergy in preference to breast milk.
We found no evidence of benefit from use of a hydrolysed formula in preference to human milk for early, short‐term feeding of low‐risk infants. When an infant requires a formula for short‐term feeding (e.g. three to four days in hospital), very low‐quality evidence indicates that use of an EHF may prevent CMA. No included studies assessed the effect of short‐term feeding with a PHF.
For infants at high risk of allergy who cannot be exclusively breast fed, very low‐quality evidence suggests that prolonged supplementation of infants at high risk of allergy (first‐degree relative with allergy) with hydrolysed formula compared with CMF reduces the risk of infant allergy and infant CMA. Results show no difference in childhood allergy incidence or prevalence and no difference in specific allergy, including infant and childhood asthma, infant and childhood eczema, infant and childhood rhinitis and infant food allergy.
Very low‐quality evidence indicates that prolonged use of a partially hydrolysed formula compared with CMF for partial or exclusive feeding was associated with a reduction in infant allergy incidence and infant CMA incidence.
Very low‐quality evidence also suggests that prolonged use of an EHF versus a PHF reduces infant food allergy.
Given that not all infants are able to be exclusively breast fed during the postpartum period, an adequately powered trial of supplemental hydrolysed formula compared with CMF feeding in infants at high risk of allergy requiring early supplemental feeding is warranted.
Given that this review found substantial methodological concerns among studies assessing effects of prolonged hydrolysed formula feeding and the potential for publication bias, additional large independent trials are required to determine the efficacy of specific hydrolysed formulas compared with CMF in infants not exclusively breast fed.
Acknowledgements
We acknowledge the authors of studies who kindly responded to requests for additional methodological information and unpublished data that have been included in this review (Hill 2000; von Berg 2003). We acknowledge Nestle, which provided copies of unpublished studies, theses and publications.
Appendices
Appendix 1. MEDLINE search strategy
Ovid MEDLINE 1946 to January 30/08/2016
1 hydrolyzed.mp.
2 hydrolysed.mp.
3 protein hydrolysate.mp. or exp protein hydrolysate/
4 formula.mp. or exp artificial milk/
5 1 or 2 or 3
6 4 and 5
7 limit 6 to (humans and clinical trial, all)
Appendix 2. CENTRAL search strategy
Cochrane Central Register of Controlled Trials 30/08/2016
1 hydrolyzed.mp.
2 hydrolysed.mp.
3 protein hydrolysate.mp. or exp protein hydrolysate/
4 formula.mp. or exp artificial milk/
5 1 or 2 or 3
6 4 and 5
Appendix 3. Clinicaltrials.gov search strategy
Searched 30/08/2016 :
(hydrolysed OR hydrolyzed) AND formula limited to recruiting studies and child.
Appendix 4. EU Clinical Trials Register search strategy
Searched 30/08/2016
Separate searches for 'hydrolysed'; hydrolyzed.
Data and analyses
Comparison 1.
Early short‐term feeding: hydrolysed formula versus human milk feeding ‐ low‐risk infants
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Any allergy | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Childhood (incidence) | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.38, 5.37] |
| 2 Asthma | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Childhood (incidence) | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.05, 4.41] |
| 3 Eczema | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Childhood (incidence) | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.05, 4.41] |
| 4 Food allergy | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Childhood (incidence) | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.38, 5.37] |
| 5 Cow's milk allergy | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Infancy (incidence) | 1 | 3559 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.52, 1.46] |
| 5.2 Childhood (incidence) | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.11 [0.35, 143.84] |
Analysis 1.1.
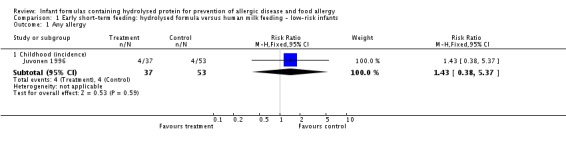
Comparison 1 Early short‐term feeding: hydrolysed formula versus human milk feeding ‐ low‐risk infants, Outcome 1 Any allergy.
Analysis 1.2.
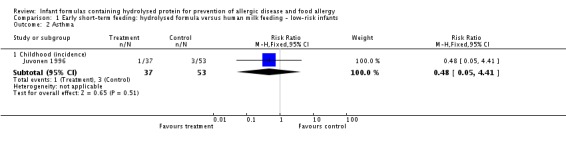
Comparison 1 Early short‐term feeding: hydrolysed formula versus human milk feeding ‐ low‐risk infants, Outcome 2 Asthma.
Analysis 1.3.
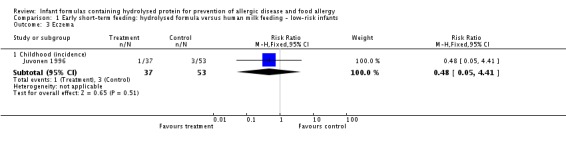
Comparison 1 Early short‐term feeding: hydrolysed formula versus human milk feeding ‐ low‐risk infants, Outcome 3 Eczema.
Analysis 1.4.
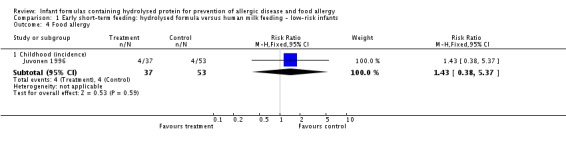
Comparison 1 Early short‐term feeding: hydrolysed formula versus human milk feeding ‐ low‐risk infants, Outcome 4 Food allergy.
Analysis 1.5.
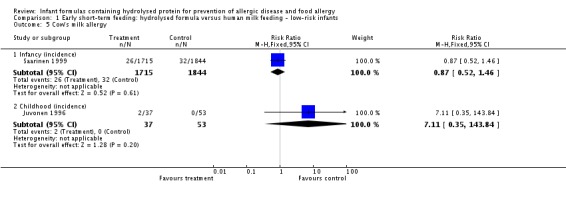
Comparison 1 Early short‐term feeding: hydrolysed formula versus human milk feeding ‐ low‐risk infants, Outcome 5 Cow's milk allergy.
Comparison 2.
Early short‐term feeding: hydrolysed formula versus cow's milk formula ‐ low‐risk infants
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Any allergy | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Childhood (incidence) | 1 | 77 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [0.33, 5.71] |
| 2 Asthma | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Childhood (incidence) | 1 | 77 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.08 [0.13, 73.26] |
| 3 Eczema | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Childhood (incidence) | 1 | 77 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.04, 3.15] |
| 4 Food allergy | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Childhood (incidence) | 1 | 77 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [0.33, 5.71] |
| 5 Cow's milk allergy | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Infancy (incidence) | 1 | 3473 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.38, 1.00] |
| 5.2 Childhood (incidence) | 1 | 77 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.13 [0.25, 103.43] |
Analysis 2.1.
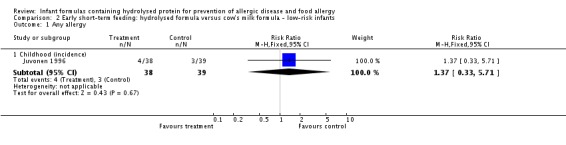
Comparison 2 Early short‐term feeding: hydrolysed formula versus cow's milk formula ‐ low‐risk infants, Outcome 1 Any allergy.
Analysis 2.2.
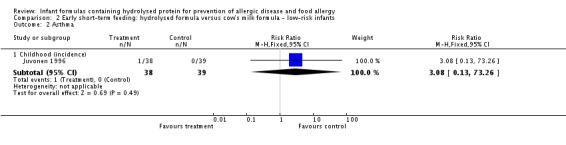
Comparison 2 Early short‐term feeding: hydrolysed formula versus cow's milk formula ‐ low‐risk infants, Outcome 2 Asthma.
Analysis 2.3.
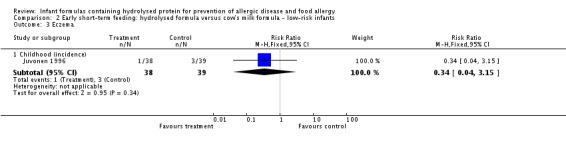
Comparison 2 Early short‐term feeding: hydrolysed formula versus cow's milk formula ‐ low‐risk infants, Outcome 3 Eczema.
Analysis 2.4.
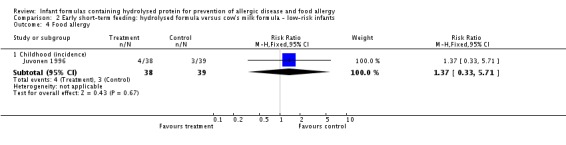
Comparison 2 Early short‐term feeding: hydrolysed formula versus cow's milk formula ‐ low‐risk infants, Outcome 4 Food allergy.
Analysis 2.5.
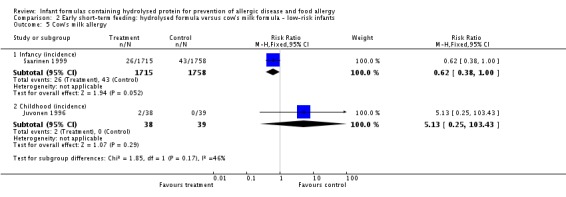
Comparison 2 Early short‐term feeding: hydrolysed formula versus cow's milk formula ‐ low‐risk infants, Outcome 5 Cow's milk allergy.
Comparison 4.
Prolonged feeding: hydrolysed formula versus cow's milk formula
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Any allergy | 9 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Infancy (incidence) | 8 | 2852 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.72, 0.95] |
| 1.2 Childhood (incidence) | 2 | 950 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.69, 1.05] |
| 1.3 Childhood (prevalence) | 1 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.76 [0.76, 4.09] |
| 2 Asthma | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Infancy (incidence) | 4 | 318 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.31, 1.04] |
| 2.2 Childhood (incidence) | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.08, 1.84] |
| 2.3 Childhood (prevalence) | 3 | 1229 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.89, 1.50] |
| 3 Eczema | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Infancy (incidence) | 9 | 2896 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.73, 1.01] |
| 3.2 Childhood (incidence) | 2 | 950 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.63, 1.10] |
| 3.3 Childhood (prevalence) | 3 | 1228 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.60, 1.02] |
| 4 Rhinitis | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Infancy (incidence) | 3 | 256 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.14, 1.85] |
| 4.2 Childhood (incidence) | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.04, 5.03] |
| 4.3 Childhood (prevalence) | 2 | 357 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.78, 1.67] |
| 5 Food allergy | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Infancy (incidence) | 2 | 479 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.58, 1.54] |
| 6 Cow's milk allergy | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Infancy (incidence) | 2 | 405 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.16, 0.86] |
Analysis 4.1.
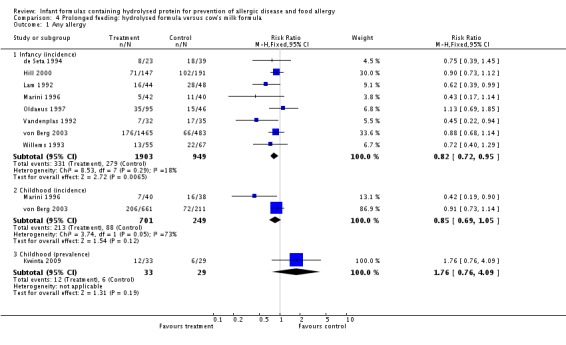
Comparison 4 Prolonged feeding: hydrolysed formula versus cow's milk formula, Outcome 1 Any allergy.
Analysis 4.2.
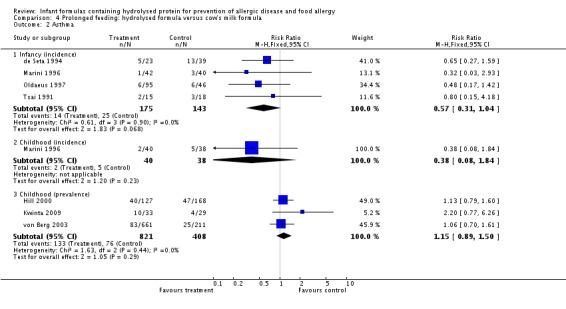
Comparison 4 Prolonged feeding: hydrolysed formula versus cow's milk formula, Outcome 2 Asthma.
Analysis 4.3.
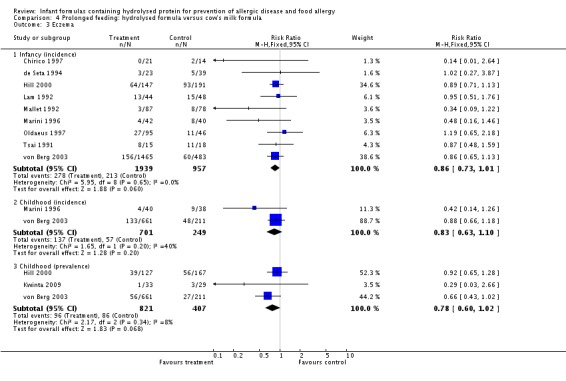
Comparison 4 Prolonged feeding: hydrolysed formula versus cow's milk formula, Outcome 3 Eczema.
Analysis 4.4.
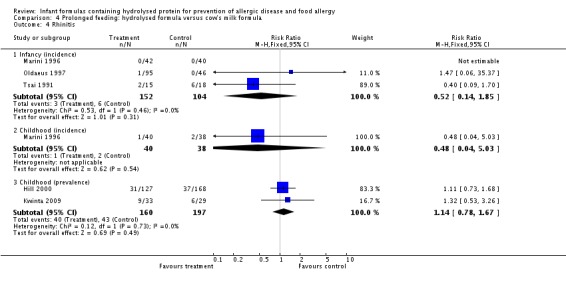
Comparison 4 Prolonged feeding: hydrolysed formula versus cow's milk formula, Outcome 4 Rhinitis.
Analysis 4.5.
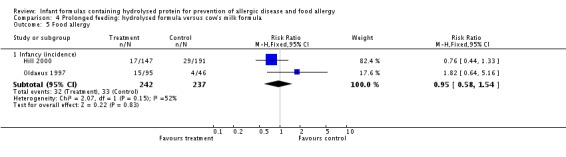
Comparison 4 Prolonged feeding: hydrolysed formula versus cow's milk formula, Outcome 5 Food allergy.
Analysis 4.6.
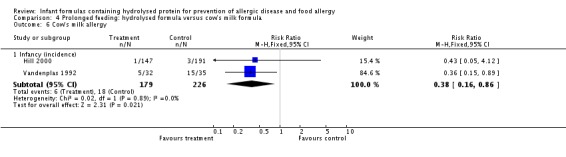
Comparison 4 Prolonged feeding: hydrolysed formula versus cow's milk formula, Outcome 6 Cow's milk allergy.
Comparison 5.
Prolonged feeding: hydrolysed formula versus cow's milk formula ‐ low‐risk infants
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Any allergy | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Childhood (prevalence) | 1 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.76 [0.76, 4.09] |
| 2 Asthma | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Childhood (prevalence) | 1 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.20 [0.77, 6.26] |
| 3 Eczema | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Childhood (prevalence) | 1 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.03, 2.66] |
| 4 Rhinitis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Childhood (prevalence) | 1 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.53, 3.26] |
Analysis 5.1.
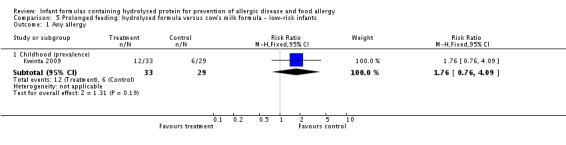
Comparison 5 Prolonged feeding: hydrolysed formula versus cow's milk formula ‐ low‐risk infants, Outcome 1 Any allergy.
Analysis 5.2.
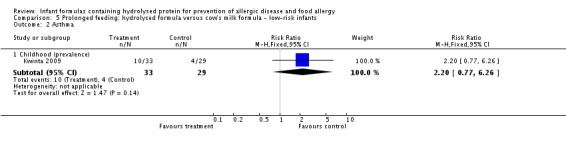
Comparison 5 Prolonged feeding: hydrolysed formula versus cow's milk formula ‐ low‐risk infants, Outcome 2 Asthma.
Analysis 5.3.
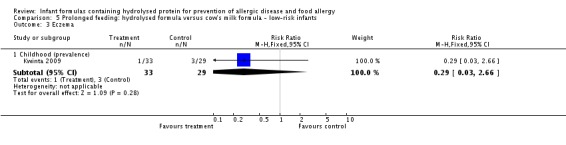
Comparison 5 Prolonged feeding: hydrolysed formula versus cow's milk formula ‐ low‐risk infants, Outcome 3 Eczema.
Analysis 5.4.
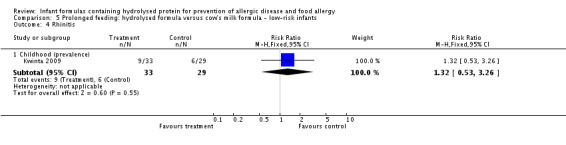
Comparison 5 Prolonged feeding: hydrolysed formula versus cow's milk formula ‐ low‐risk infants, Outcome 4 Rhinitis.
Comparison 6.
Prolonged feeding: hydrolysed formula versus cow's milk formula ‐ high‐risk infants
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Any allergy | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Infancy (incidence) | 8 | 2852 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.72, 0.95] |
| 1.2 Childhood (incidence) | 2 | 950 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.69, 1.05] |
| 2 Asthma | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Infancy (incidence) | 4 | 318 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.31, 1.04] |
| 2.2 Childhood (incidence) | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.08, 1.84] |
| 2.3 Childhood (prevalence) | 2 | 1167 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.83, 1.44] |
| 3 Eczema | 9 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Infancy (incidence) | 9 | 2896 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.73, 1.01] |
| 3.2 Childhood (incidence) | 2 | 950 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.63, 1.10] |
| 3.3 Childhood (prevalence) | 2 | 1166 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.61, 1.04] |
| 4 Rhinitis | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Infancy (incidence) | 3 | 256 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.14, 1.85] |
| 4.2 Childhood (incidence) | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.04, 5.03] |
| 4.3 Childhood (prevalence) | 1 | 295 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.73, 1.68] |
| 5 Food allergy | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Infancy (incidence) | 2 | 479 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.58, 1.54] |
| 5.2 Childhood (incidence) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.3 Childhood (prevalence) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Cow's milk allergy | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Infancy (incidence) | 2 | 405 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.16, 0.86] |
| 6.2 Childhood (prevalence) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Analysis 6.1.
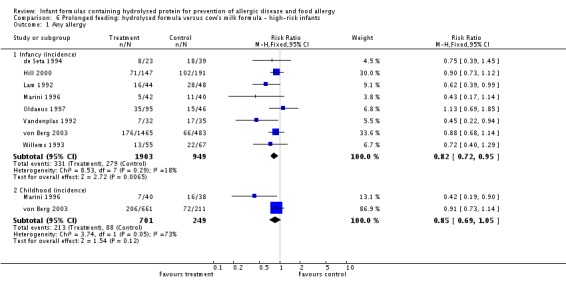
Comparison 6 Prolonged feeding: hydrolysed formula versus cow's milk formula ‐ high‐risk infants, Outcome 1 Any allergy.
Analysis 6.2.
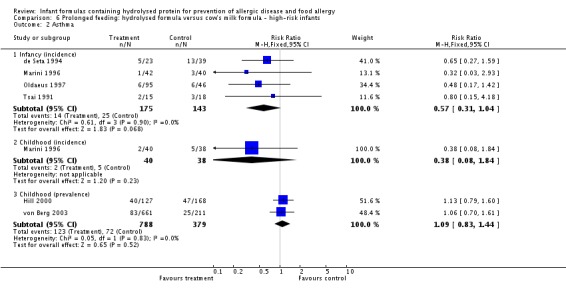
Comparison 6 Prolonged feeding: hydrolysed formula versus cow's milk formula ‐ high‐risk infants, Outcome 2 Asthma.
Analysis 6.3.
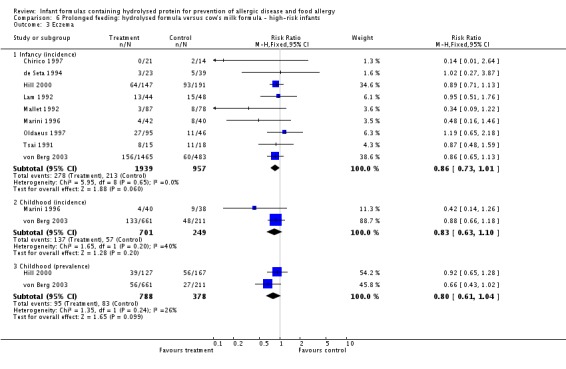
Comparison 6 Prolonged feeding: hydrolysed formula versus cow's milk formula ‐ high‐risk infants, Outcome 3 Eczema.
Analysis 6.4.
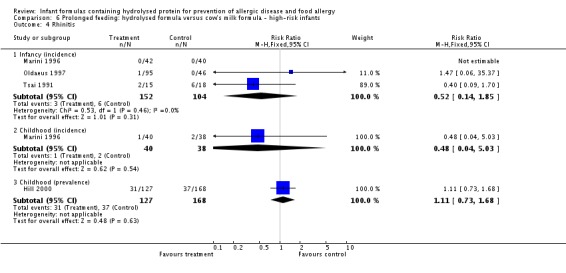
Comparison 6 Prolonged feeding: hydrolysed formula versus cow's milk formula ‐ high‐risk infants, Outcome 4 Rhinitis.
Analysis 6.5.
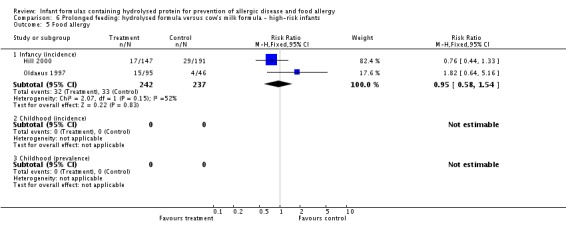
Comparison 6 Prolonged feeding: hydrolysed formula versus cow's milk formula ‐ high‐risk infants, Outcome 5 Food allergy.
Analysis 6.6.
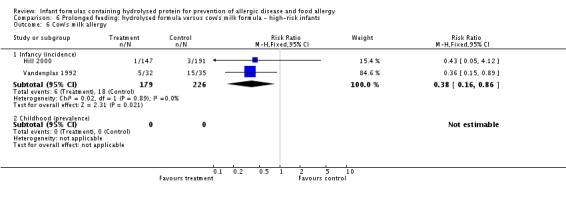
Comparison 6 Prolonged feeding: hydrolysed formula versus cow's milk formula ‐ high‐risk infants, Outcome 6 Cow's milk allergy.
Comparison 7.
Prolonged feeding: partially hydrolysed formula versus cow's milk formula
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Any allergy | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Infancy (incidence) | 8 | 1820 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.72, 0.96] |
| 1.2 Childhood (incidence) | 2 | 510 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.67, 1.10] |
| 2 Asthma | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Infancy (incidence) | 4 | 268 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.28, 1.04] |
| 2.2 Childhood (incidence) | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.08, 1.84] |
| 2.3 Childhood (prevalence) | 3 | 789 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.91, 1.58] |
| 3 Eczema | 9 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Infancy (incidence) | 8 | 1699 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.75, 1.06] |
| 3.2 Childhood (incidence) | 2 | 510 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.61, 1.19] |
| 3.3 Childhood (prevalence) | 3 | 788 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.61, 1.09] |
| 4 Rhinitis | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Infancy (incidence) | 3 | 206 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.4 [0.09, 1.70] |
| 4.2 Childhood (incidence) | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.04, 5.03] |
| 4.3 Childhood (prevalence) | 2 | 357 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.78, 1.67] |
| 5 Food allergy | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Infancy (incidence) | 2 | 429 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.62, 1.63] |
| 6 Cow's milk allergy | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Infancy (incidence) | 2 | 405 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.16, 0.86] |
Analysis 7.1.
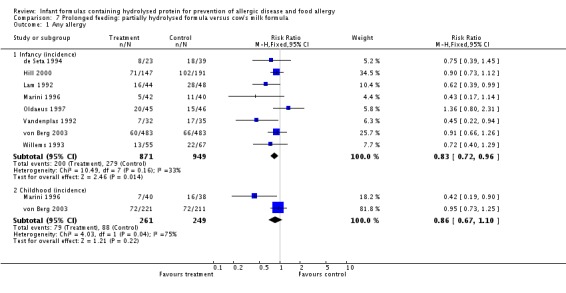
Comparison 7 Prolonged feeding: partially hydrolysed formula versus cow's milk formula, Outcome 1 Any allergy.
Analysis 7.2.
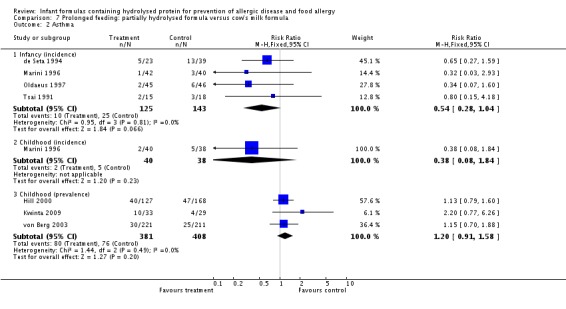
Comparison 7 Prolonged feeding: partially hydrolysed formula versus cow's milk formula, Outcome 2 Asthma.
Analysis 7.3.
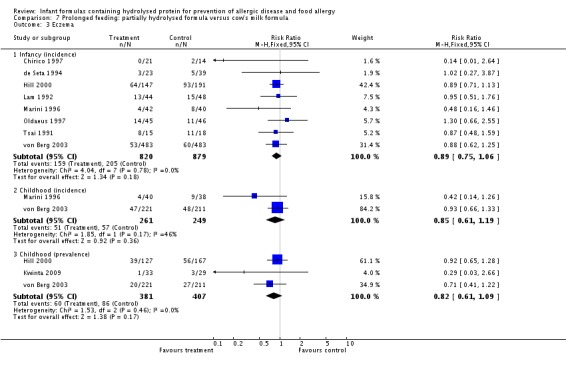
Comparison 7 Prolonged feeding: partially hydrolysed formula versus cow's milk formula, Outcome 3 Eczema.
Analysis 7.4.
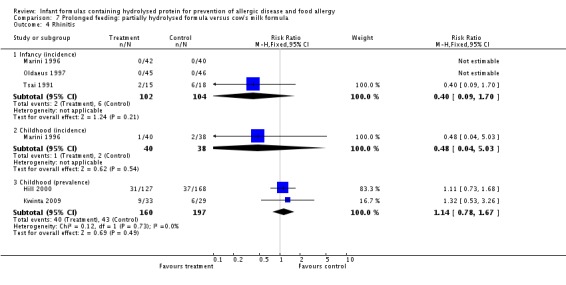
Comparison 7 Prolonged feeding: partially hydrolysed formula versus cow's milk formula, Outcome 4 Rhinitis.
Analysis 7.5.
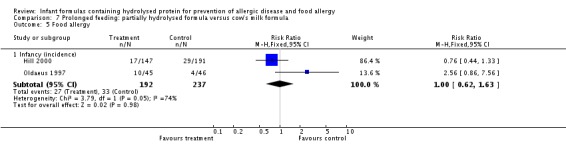
Comparison 7 Prolonged feeding: partially hydrolysed formula versus cow's milk formula, Outcome 5 Food allergy.
Analysis 7.6.
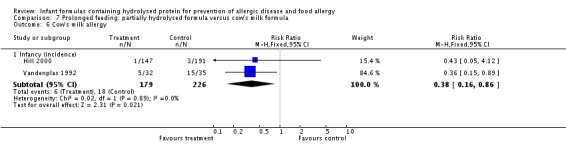
Comparison 7 Prolonged feeding: partially hydrolysed formula versus cow's milk formula, Outcome 6 Cow's milk allergy.
Comparison 8.
Prolonged feeding: extensively hydrolysed formula versus cow's milk formula
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Any allergy | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Infancy (incidence) | 2 | 1561 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.68, 1.13] |
| 1.2 Childhood (incidence) | 1 | 651 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.71, 1.13] |
| 1.3 Childhood (prevalence) | 1 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.76 [0.76, 4.09] |
| 2 Asthma | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Infancy (incidence) | 1 | 96 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.18, 2.04] |
| 2.2 Childhood (prevalence) | 2 | 713 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.76, 1.72] |
| 3 Eczema | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Infancy (incidence) | 3 | 1726 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.63, 1.08] |
| 3.2 Childhood (incidence) | 1 | 651 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.63, 1.17] |
| 3.3 Childhood (prevalence) | 2 | 713 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.39, 0.97] |
| 4 Rhinitis | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Infancy (incidence) | 1 | 96 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.76 [0.12, 66.22] |
| 4.2 Childhood (prevalence) | 1 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.53, 3.26] |
| 5 Food allergy | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Infancy (incidence) | 1 | 96 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.33, 4.02] |
Analysis 8.1.
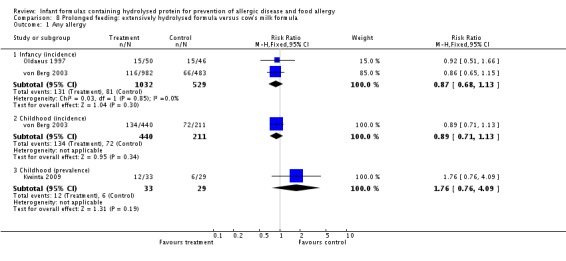
Comparison 8 Prolonged feeding: extensively hydrolysed formula versus cow's milk formula, Outcome 1 Any allergy.
Analysis 8.2.
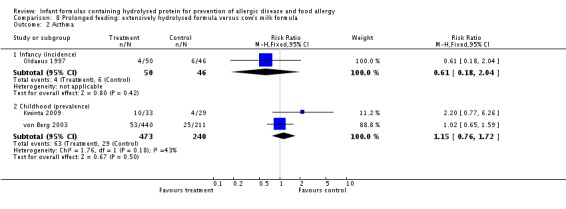
Comparison 8 Prolonged feeding: extensively hydrolysed formula versus cow's milk formula, Outcome 2 Asthma.
Analysis 8.3.
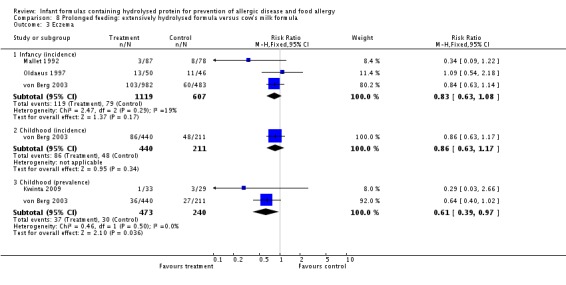
Comparison 8 Prolonged feeding: extensively hydrolysed formula versus cow's milk formula, Outcome 3 Eczema.
Analysis 8.4.
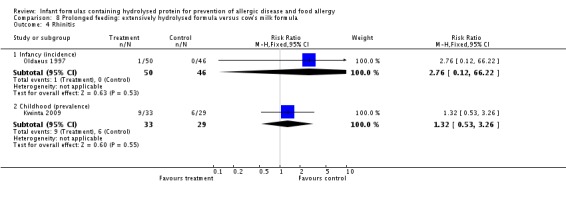
Comparison 8 Prolonged feeding: extensively hydrolysed formula versus cow's milk formula, Outcome 4 Rhinitis.
Analysis 8.5.
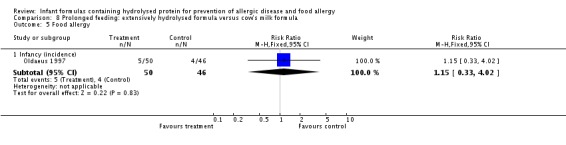
Comparison 8 Prolonged feeding: extensively hydrolysed formula versus cow's milk formula, Outcome 5 Food allergy.
Comparison 9.
Prolonged feeding: extensively hydrolysed formula versus partially hydrolysed formula
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Any allergy | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Infancy (incidence) | 3 | 1806 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.75, 1.16] |
| 1.2 Childhood (incidence) | 1 | 661 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.74, 1.18] |
| 2 Asthma | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Infancy (incidence) | 2 | 341 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.72 [0.74, 3.96] |
| 2.2 Childhood (prevalence) | 1 | 661 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.58, 1.35] |
| 3 Eczema | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Infancy (incidence) | 4 | 1865 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.73, 1.10] |
| 3.2 Childhood (incidence) | 1 | 661 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.67, 1.26] |
| 3.3 Childhood (prevalence) | 1 | 661 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.54, 1.52] |
| 4 Rhinitis | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Infancy (incidence) | 2 | 341 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.36, 4.29] |
| 5 Food allergy | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Infancy (incidence) | 2 | 341 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.19, 0.99] |
| 6 Cow's milk allergy | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Infancy (incidence) | 1 | 246 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.01, 1.16] |
| 7 Urticaria | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Infancy (incidence) | 1 | 246 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.26, 6.66] |
Analysis 9.1.
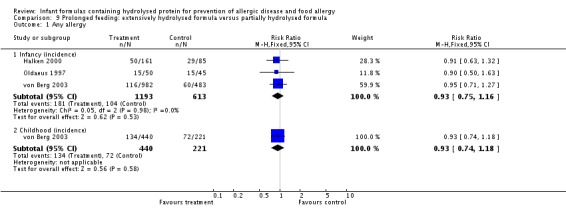
Comparison 9 Prolonged feeding: extensively hydrolysed formula versus partially hydrolysed formula, Outcome 1 Any allergy.
Analysis 9.2.
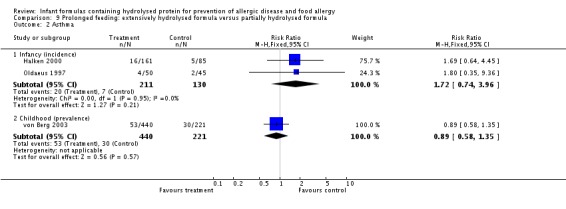
Comparison 9 Prolonged feeding: extensively hydrolysed formula versus partially hydrolysed formula, Outcome 2 Asthma.
Analysis 9.3.
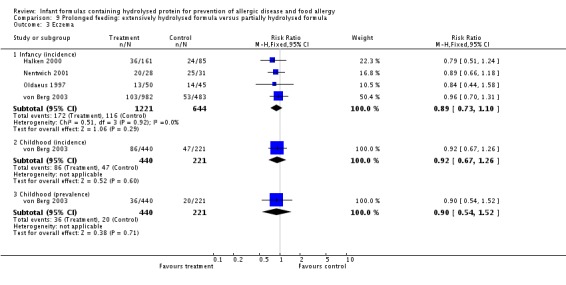
Comparison 9 Prolonged feeding: extensively hydrolysed formula versus partially hydrolysed formula, Outcome 3 Eczema.
Analysis 9.4.
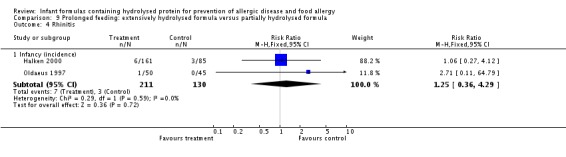
Comparison 9 Prolonged feeding: extensively hydrolysed formula versus partially hydrolysed formula, Outcome 4 Rhinitis.
Analysis 9.5.
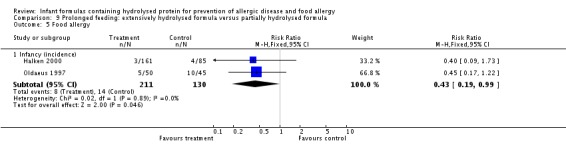
Comparison 9 Prolonged feeding: extensively hydrolysed formula versus partially hydrolysed formula, Outcome 5 Food allergy.
Analysis 9.6.
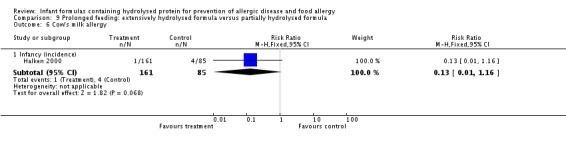
Comparison 9 Prolonged feeding: extensively hydrolysed formula versus partially hydrolysed formula, Outcome 6 Cow's milk allergy.
Analysis 9.7.
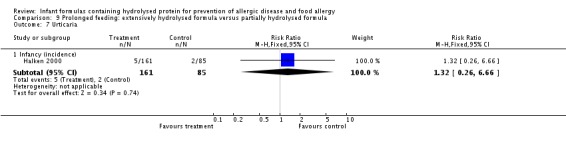
Comparison 9 Prolonged feeding: extensively hydrolysed formula versus partially hydrolysed formula, Outcome 7 Urticaria.
Comparison 10.
Prolonged exclusive feeding: hydrolysed formula versus cow's milk formula
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Any allergy | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Infancy (incidence) | 5 | 425 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.46, 0.80] |
| 1.2 Childhood (incidence) | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.19, 0.90] |
| 1.3 Childhood (prevalence) | 1 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.76 [0.76, 4.09] |
| 2 Asthma | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Infancy (incidence) | 2 | 144 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.25, 1.31] |
| 2.2 Childhood (incidence) | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.08, 1.84] |
| 2.3 Childhood (prevalence) | 1 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.20 [0.77, 6.26] |
| 3 Eczema | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Infancy (incidence) | 4 | 271 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.45, 1.21] |
| 3.2 Childhood (incidence) | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.14, 1.26] |
| 3.3 Childhood (prevalence) | 1 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.03, 2.66] |
| 4 Rhinitis | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Infancy (incidence) | 1 | 82 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Childhood (incidence) | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.04, 5.03] |
| 4.3 Childhood (prevalence) | 1 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.53, 3.26] |
| 5 Cow's milk allergy | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Infancy (incidence) | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.15, 0.89] |
Analysis 10.1.
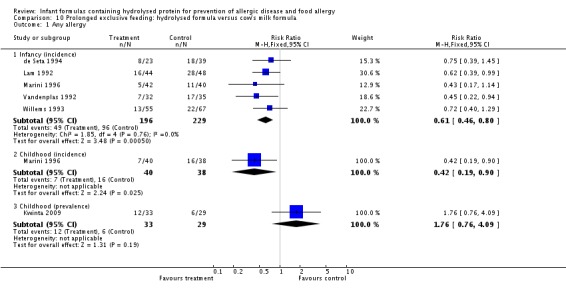
Comparison 10 Prolonged exclusive feeding: hydrolysed formula versus cow's milk formula, Outcome 1 Any allergy.
Analysis 10.2.
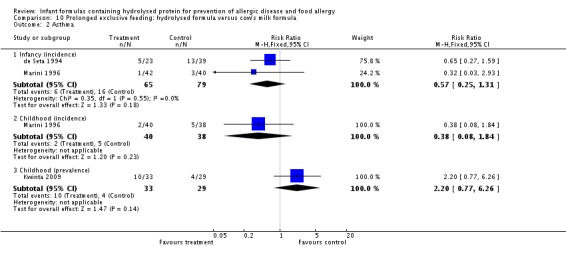
Comparison 10 Prolonged exclusive feeding: hydrolysed formula versus cow's milk formula, Outcome 2 Asthma.
Analysis 10.3.
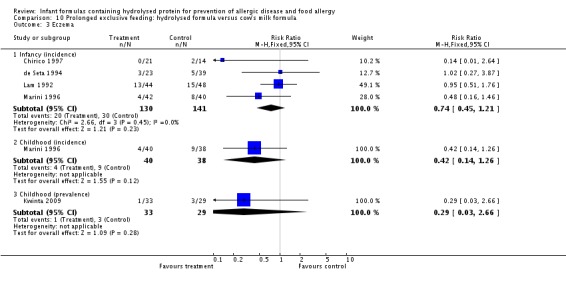
Comparison 10 Prolonged exclusive feeding: hydrolysed formula versus cow's milk formula, Outcome 3 Eczema.
Analysis 10.4.
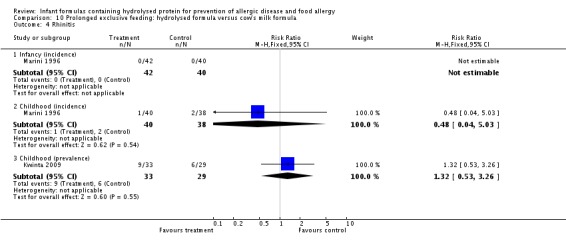
Comparison 10 Prolonged exclusive feeding: hydrolysed formula versus cow's milk formula, Outcome 4 Rhinitis.
Analysis 10.5.
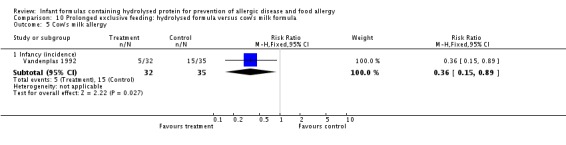
Comparison 10 Prolonged exclusive feeding: hydrolysed formula versus cow's milk formula, Outcome 5 Cow's milk allergy.
Comparison 11.
Prolonged feeding: hydrolysed formula versus cow's milk formula ‐ studies with blinded measurement
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Any allergy | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Infancy (incidence) | 3 | 2156 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.69, 1.08] |
| 1.2 Childhood (incidence) | 1 | 872 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.73, 1.14] |
| 1.3 Childhood (prevalence) | 1 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.76 [0.76, 4.09] |
| 2 Asthma | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Infancy (incidence) | 1 | 141 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.17, 1.42] |
| 2.2 Childhood (prevalence) | 2 | 934 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.80, 1.73] |
| 3 Eczema | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Infancy (incidence) | 2 | 2089 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.70, 1.16] |
| 3.2 Childhood (incidence) | 1 | 872 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.66, 1.18] |
| 3.3 Childhood (prevalence) | 2 | 934 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.42, 0.97] |
| 4 Rhinitis | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Infancy (incidence) | 1 | 141 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [0.06, 35.37] |
| 4.2 Childhood (prevalence) | 1 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.53, 3.26] |
| 5 Food allergy | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Infancy (incidence) | 1 | 141 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.82 [0.64, 5.16] |
| 6 Cow's milk allergy | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Infancy (incidence) | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.15, 0.89] |
Analysis 11.1.
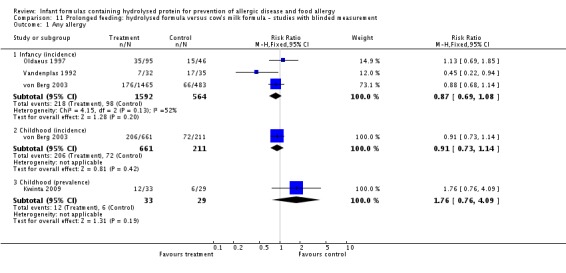
Comparison 11 Prolonged feeding: hydrolysed formula versus cow's milk formula ‐ studies with blinded measurement, Outcome 1 Any allergy.
Analysis 11.2.
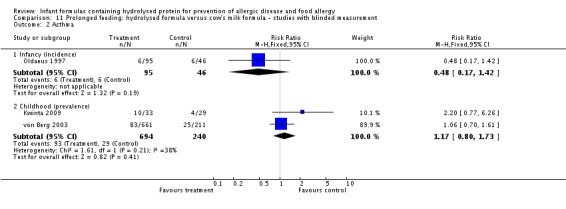
Comparison 11 Prolonged feeding: hydrolysed formula versus cow's milk formula ‐ studies with blinded measurement, Outcome 2 Asthma.
Analysis 11.3.
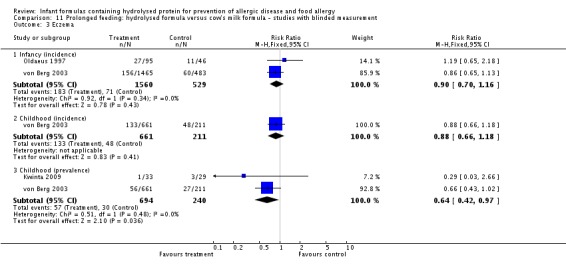
Comparison 11 Prolonged feeding: hydrolysed formula versus cow's milk formula ‐ studies with blinded measurement, Outcome 3 Eczema.
Analysis 11.4.
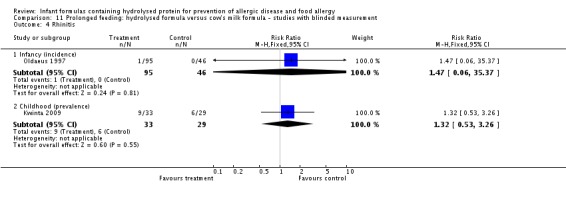
Comparison 11 Prolonged feeding: hydrolysed formula versus cow's milk formula ‐ studies with blinded measurement, Outcome 4 Rhinitis.
Analysis 11.5.
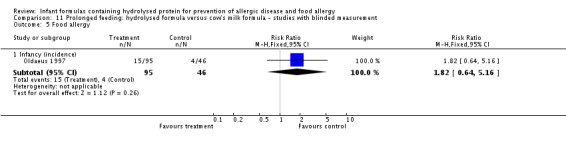
Comparison 11 Prolonged feeding: hydrolysed formula versus cow's milk formula ‐ studies with blinded measurement, Outcome 5 Food allergy.
Analysis 11.6.
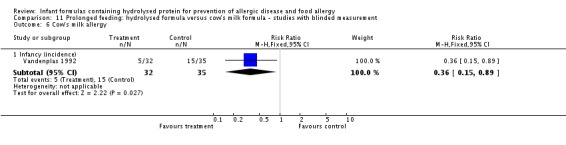
Comparison 11 Prolonged feeding: hydrolysed formula versus cow's milk formula ‐ studies with blinded measurement, Outcome 6 Cow's milk allergy.
Comparison 13.
Prolonged feeding: partially hydrolysed whey formula versus cow's milk formula
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Any allergy | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Infancy (incidence) | 7 | 1729 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.66, 0.94] |
| 1.2 Childhood (incidence) | 2 | 510 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.31, 1.52] |
| 2 Asthma | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Infancy (incidence) | 3 | 177 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.29, 1.28] |
| 2.2 Childhood (incidence) | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.08, 1.84] |
| 2.3 Childhood (prevalence) | 2 | 727 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.85, 1.51] |
| 3 Eczema | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Infancy (incidence) | 7 | 1608 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.72, 1.03] |
| 3.2 Childhood (incidence) | 2 | 510 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.61, 1.19] |
| 3.3 Childhood (prevalence) | 2 | 726 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.63, 1.12] |
| 4 Rhinitis | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Infancy (incidence) | 2 | 115 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.4 [0.09, 1.70] |
| 4.2 Childhood (incidence) | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.04, 5.03] |
| 4.3 Childhood (prevalence) | 1 | 295 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.73, 1.68] |
| 5 Food allergy | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Infancy (incidence) | 1 | 338 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.44, 1.33] |
| 6 Cow's milk allergy | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Infancy (incidence) | 2 | 405 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.16, 0.86] |
Analysis 13.1.
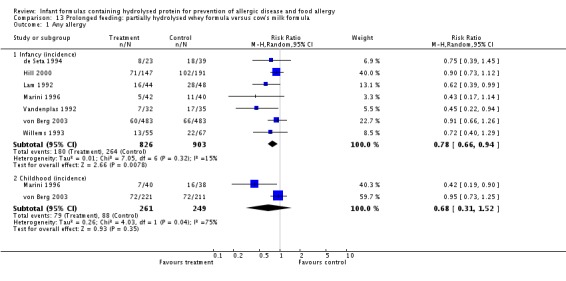
Comparison 13 Prolonged feeding: partially hydrolysed whey formula versus cow's milk formula, Outcome 1 Any allergy.
Analysis 13.2.
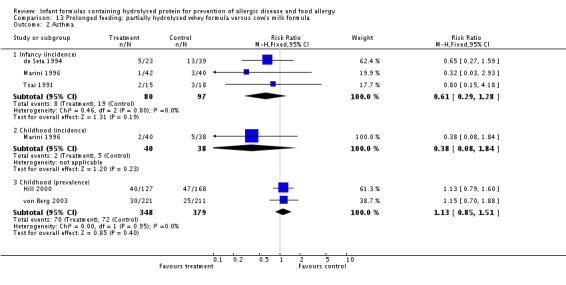
Comparison 13 Prolonged feeding: partially hydrolysed whey formula versus cow's milk formula, Outcome 2 Asthma.
Analysis 13.3.
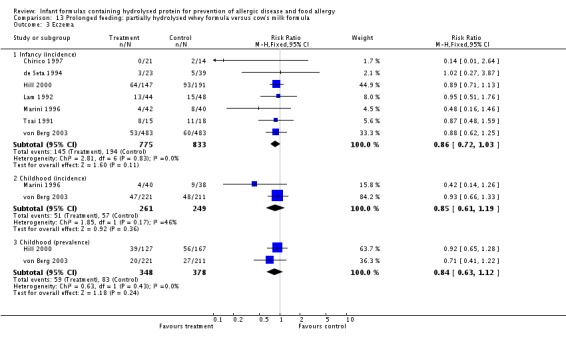
Comparison 13 Prolonged feeding: partially hydrolysed whey formula versus cow's milk formula, Outcome 3 Eczema.
Analysis 13.4.
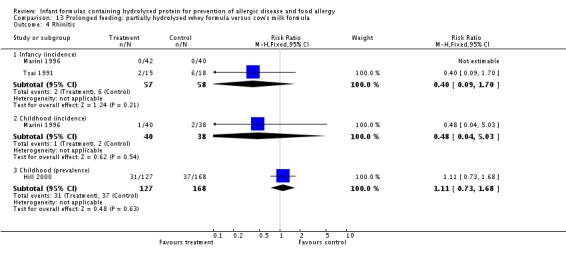
Comparison 13 Prolonged feeding: partially hydrolysed whey formula versus cow's milk formula, Outcome 4 Rhinitis.
Analysis 13.5.
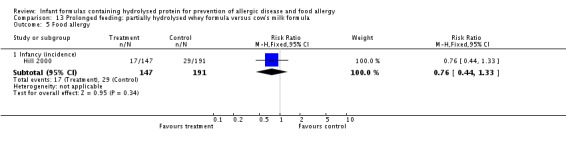
Comparison 13 Prolonged feeding: partially hydrolysed whey formula versus cow's milk formula, Outcome 5 Food allergy.
Analysis 13.6.
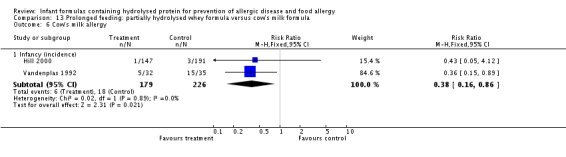
Comparison 13 Prolonged feeding: partially hydrolysed whey formula versus cow's milk formula, Outcome 6 Cow's milk allergy.
Comparison 14.
Prolonged feeding: partially hydrolysed casein‐containing formula versus cow's milk formula
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Any allergy | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Infancy (incidence) | 1 | 91 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.80, 2.31] |
| 1.2 Childhood (prevalence) | 1 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.76 [0.76, 4.09] |
| 2 Asthma | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Infancy (incidence) | 1 | 91 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.07, 1.60] |
| 2.2 Childhood (prevalence) | 1 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.20 [0.77, 6.26] |
| 3 Eczema | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Infancy (incidence) | 1 | 91 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.66, 2.55] |
| 3.2 Childhood (prevalence) | 1 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.03, 2.66] |
| 4 Rhinitis | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Infancy (incidence) | 1 | 91 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Childhood (prevalence) | 1 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.53, 3.26] |
| 5 Food allergy | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Infancy (incidence) | 1 | 91 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.56 [0.86, 7.56] |
Analysis 14.1.
Comparison 14 Prolonged feeding: partially hydrolysed casein‐containing formula versus cow's milk formula, Outcome 1 Any allergy.
Analysis 14.2.
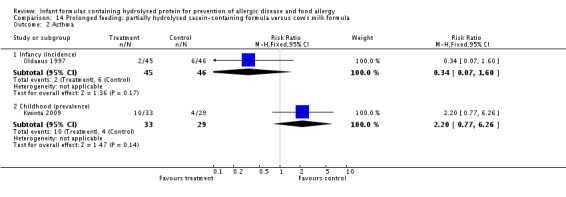
Comparison 14 Prolonged feeding: partially hydrolysed casein‐containing formula versus cow's milk formula, Outcome 2 Asthma.
Analysis 14.3.
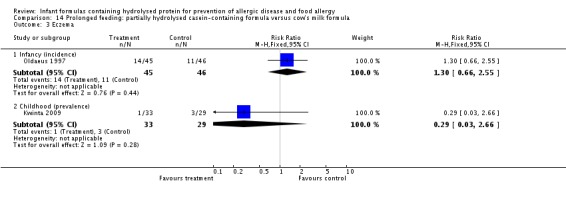
Comparison 14 Prolonged feeding: partially hydrolysed casein‐containing formula versus cow's milk formula, Outcome 3 Eczema.
Analysis 14.4.
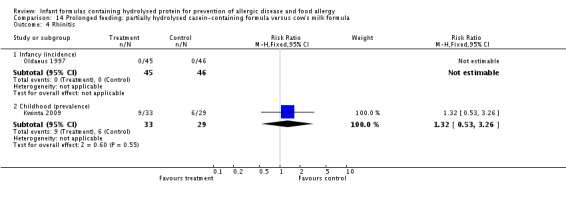
Comparison 14 Prolonged feeding: partially hydrolysed casein‐containing formula versus cow's milk formula, Outcome 4 Rhinitis.
Analysis 14.5.
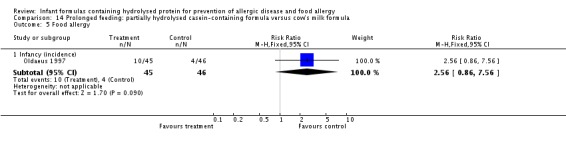
Comparison 14 Prolonged feeding: partially hydrolysed casein‐containing formula versus cow's milk formula, Outcome 5 Food allergy.
Comparison 15.
Prolonged feeding: extensively hydrolysed whey formula versus cow's milk formula
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Any allergy | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Infancy (incidence) | 1 | 972 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.71, 1.34] |
| 1.2 Childhood (incidence) | 1 | 431 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.82, 1.38] |
| 2 Asthma | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Childhood (prevalence) | 1 | 431 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.73, 1.94] |
| 3 Eczema | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Infancy (incidence) | 1 | 972 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.72, 1.40] |
| 3.2 Childhood (incidence) | 1 | 431 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.75, 1.49] |
| 3.3 Childhood (prevalence) | 1 | 431 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.46, 1.33] |
Analysis 15.1.

Comparison 15 Prolonged feeding: extensively hydrolysed whey formula versus cow's milk formula, Outcome 1 Any allergy.
Analysis 15.2.
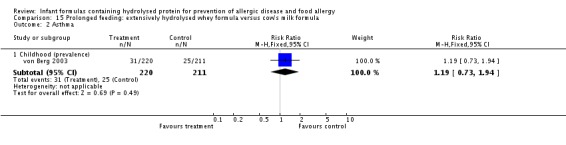
Comparison 15 Prolonged feeding: extensively hydrolysed whey formula versus cow's milk formula, Outcome 2 Asthma.
Analysis 15.3.
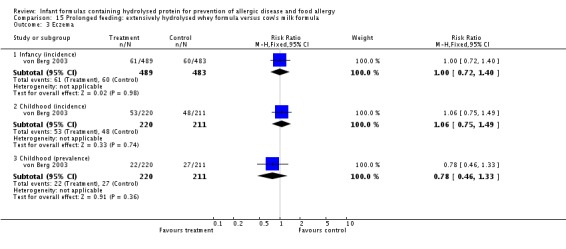
Comparison 15 Prolonged feeding: extensively hydrolysed whey formula versus cow's milk formula, Outcome 3 Eczema.
Comparison 16.
Prolonged feeding: extensively hydrolysed casein formula versus cow' milk formula
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Any allergy | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Infancy (incidence) | 1 | 976 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.54, 1.07] |
| 1.2 Childhood (incidence) | 1 | 431 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.53, 0.97] |
| 2 Asthma | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Childhood (prevalence) | 1 | 431 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.49, 1.45] |
| 3 Eczema | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Infancy (incidence) | 1 | 976 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.47, 1.00] |
| 3.2 Childhood (incidence) | 1 | 431 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.44, 0.98] |
| 3.3 Childhood (prevalence) | 1 | 431 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.27, 0.92] |
Analysis 16.1.
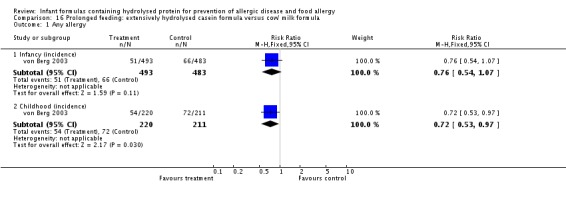
Comparison 16 Prolonged feeding: extensively hydrolysed casein formula versus cow' milk formula, Outcome 1 Any allergy.
Analysis 16.2.
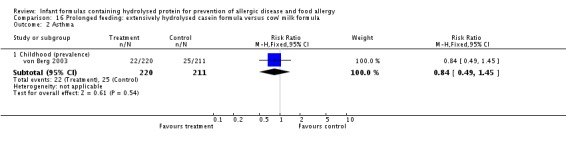
Comparison 16 Prolonged feeding: extensively hydrolysed casein formula versus cow' milk formula, Outcome 2 Asthma.
Analysis 16.3.
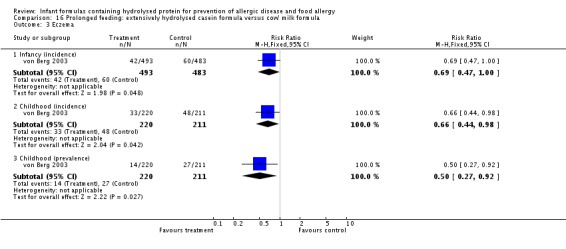
Comparison 16 Prolonged feeding: extensively hydrolysed casein formula versus cow' milk formula, Outcome 3 Eczema.
Comparison 17.
Prolonged feeding: extensively hydrolysed casein formula versus extensively hydrolysed whey formula
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Any allergy | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Infancy (incidence) | 2 | 1143 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.73, 1.26] |
| 1.2 Childhood (incidence) | 1 | 440 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.50, 0.90] |
| 2 Asthma | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Infancy (incidence) | 1 | 161 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.28 [0.83, 6.28] |
| 2.2 Childhood (prevalence) | 1 | 440 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.42, 1.19] |
| 3 Eczema | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Infancy (incidence) | 2 | 1143 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.59, 1.10] |
| 3.2 Childhood (incidence) | 1 | 440 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.42, 0.92] |
| 3.3 Childhood (prevalence) | 1 | 440 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.33, 1.21] |
| 4 Rhinitis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Infancy (incidence) | 1 | 161 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.08 [0.39, 11.02] |
| 5 Food allergy | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Infancy (incidence) | 1 | 161 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.45 [0.48, 4.39] |
| 6 Cow's milk allergy | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Infancy (incidence) | 1 | 161 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.19 [0.25, 106.38] |
| 7 Urticaria | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Infancy (incidence) | 1 | 161 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.15 [0.47, 36.34] |
Analysis 17.1.
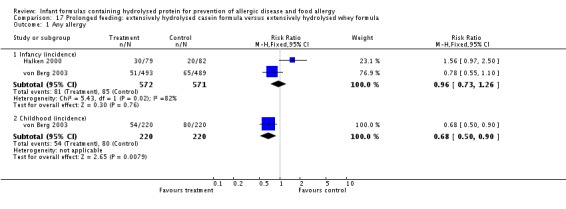
Comparison 17 Prolonged feeding: extensively hydrolysed casein formula versus extensively hydrolysed whey formula, Outcome 1 Any allergy.
Analysis 17.2.
Comparison 17 Prolonged feeding: extensively hydrolysed casein formula versus extensively hydrolysed whey formula, Outcome 2 Asthma.
Analysis 17.3.
Comparison 17 Prolonged feeding: extensively hydrolysed casein formula versus extensively hydrolysed whey formula, Outcome 3 Eczema.
Analysis 17.4.
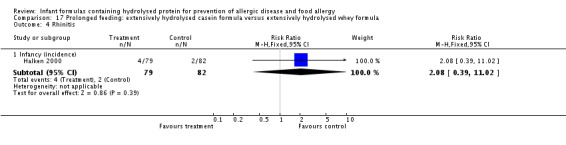
Comparison 17 Prolonged feeding: extensively hydrolysed casein formula versus extensively hydrolysed whey formula, Outcome 4 Rhinitis.
Analysis 17.5.
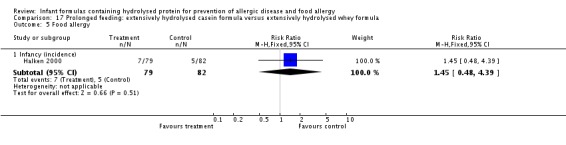
Comparison 17 Prolonged feeding: extensively hydrolysed casein formula versus extensively hydrolysed whey formula, Outcome 5 Food allergy.
Analysis 17.6.
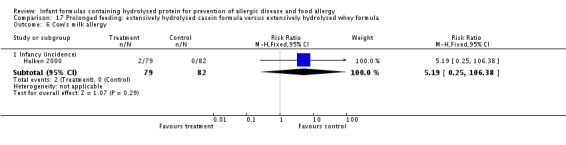
Comparison 17 Prolonged feeding: extensively hydrolysed casein formula versus extensively hydrolysed whey formula, Outcome 6 Cow's milk allergy.
Analysis 17.7.
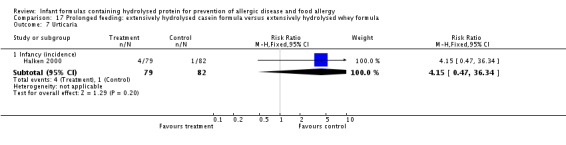
Comparison 17 Prolonged feeding: extensively hydrolysed casein formula versus extensively hydrolysed whey formula, Outcome 7 Urticaria.
What's new
Last assessed as up‐to‐date: 30 August 2016.
| Date | Event | Description |
|---|---|---|
| 31 October 2016 | New citation required and conclusions have changed | We identified 8 new ongoing or unpublished studies; we found 1 additional excluded study and an additional 15‐year follow‐up report from a previously included study; for 1 previously excluded study, trial authors provided additional data now included in the review. We added the GRADE method and SoF tables. We downgraded review conclusions. |
| 31 October 2016 | New search has been performed | See above |
History
Protocol first published: Issue 2, 2002 Review first published: Issue 4, 2003
| Date | Event | Description |
|---|---|---|
| 18 September 2008 | Amended | We converted this review to new review format |
| 27 July 2006 | New search has been performed | We reviewed the eligibility of all trials. We included several new studies and updated reports. We partially redid comparisons to better meet the objectives and methods specified in the protocol. Additionally, we performed previously specified subgroup analyses according to whether studies had blinded measurement for allergy and whether enrolled infants who were solely formula fed were fed 100% whey formula or casein‐containing formula (according to degree of hydrolysis) Exclusion of 2 previously included trials and inclusion of a new large trial resulted in substantial changes to the review and to the review conclusions |
| 27 July 2006 | New citation required and conclusions have changed | We made substantive amendments |
Differences between protocol and review
Review authors have deleted outcomes related to sensitisation in infants with clinical allergy that were incorporated in previous versions of the review to ensure that this review was focussed appropriately on clinical allergy ‐ not on surrogate testing.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Single‐centre randomised controlled trial in Italy | |
| Participants | Inclusion criteria: infants of mothers with atopy (rhinitis, asthma, eczema or food intolerance) who "could not breast feed" | |
| Interventions | Treatment (n = 21): partially hydrolysed cow's milk whey formula (Vivena HA‐Primigiorni HA) Control (n = 14): cow's milk formula (brand not reported) Co‐interventions (in all 'at‐risk infants'): avoidance of passive smoking, exposure to pets and mites, avoidance of nurseries, delayed weaning to 6 months of age | |
| Outcomes | Primary outcome(s): immunogenicity and antigenicity of partially hydrolysed whey formula in at‐risk infants, including RAST for milk and egg proteins, total and specific IgE and specific IgG and IgG4 subclass antibodies Other outcomes: eczema: defined as a pruritic, chronic or chronically relapsing dermatitis with typical features and distribution Follow‐up to 6 months (infant eczema incidence) | |
| Notes | Trial of sole prolonged partially hydrolysed whey cow's milk formula and environmental allergen avoidance vs cow's milk formula Conflict of interest: none reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not reported |
| Allocation concealment (selection bias) | Low risk | After informed consent was obtained from parents, infants considered at risk of atopy and who could not be breast fed were randomised (by the sealed‐envelope method) to treatment on the first day of life. Only infants who were exclusively breast fed, special formula fed or traditional formula fed and who were not exposed to passive smoking during the first 6 months of life were included in the study |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported for clinical outcomes. Reported for measurement of sensitisation (specific IgE) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Numbers of enrolled infants not reported. Only infants who received 6 months of allocated formula analysed |
| Selective reporting (reporting bias) | Unclear risk | Specific clinical outcomes not reported to be prespecified |
| Other bias | High risk | Unequal numbers in groups reported |
| Methods | Single‐centre randomised controlled trial in Italy | |
| Participants | Inclusion criteria: infants with at least 1 first‐degree relative with allergy. When history in doubt, skin prick tests or RAST performed | |
| Interventions | Treatment (n = 23): partially hydrolysed whey formula (Nidina‐HA, Nestle) Control (n = 39): cow's milk formula (Nidina, Nestle) Co‐interventions: none reported Formula only to 6 months, then 'normal' diet | |
| Outcomes | Primary outcome(s): allergic disease at 6 and 24 months (infant allergy) Other outcomes: physician clinical examination and/or telephone contact to determine incidence of allergic disease. CMPI, eczema and asthma diagnosed clinically according to standard criteria | |
| Notes | Trial of sole prolonged partially hydrolysed whey formula vs cow's milk formula Conflict of interest: none reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not reported |
| Allocation concealment (selection bias) | Unclear risk | Method not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | None reported |
| Selective reporting (reporting bias) | Low risk | Prespecified allergy and timing of reporting |
| Other bias | High risk | Group characteristics not reported. Group sizes unequal |
| Methods | Multi‐centre quasi‐randomised (alternation) controlled trial in Denmark | |
| Participants | Inclusion criteria: infants with bi‐parental atopy or uniparental atopy and cord IgE ≥ 0.3 kU/L | |
| Interventions | Supplemental or sole formula feeding with: Treatment 1 (n = 79): extensively hydrolysed casein formula (Nutramigen) Treatment 2 (n = 82): extensively hydrolysed whey formula (Profylac) Control (n = 85): partially hydrolysed whey formula (NAN‐HA) Recommended duration of feeding: 4 months Co‐interventions (all infants): delay solids and cow's milk to 4 months; avoid smoke, pets, damp housing | |
| Outcomes | Primary outcome(s): allergy Other outcomes: physician examination at 6, 12 and 18 months (infant allergy) Definitions: Any atopy: symptoms of asthma, atopic dermatitis, allergic rhino conjunctivitis or at least 2 episodes of allergic urticaria Asthma: clinician diagnosed, ≥ 3 episodes of recurrent wheezing needing bronchodilators Atopic dermatitis: physical examination, ≥ 3 months' duration Allergic rhinoconjunctivitis: ≥ 1 month or recurrent symptoms Food allergy: confirmed by unblinded elimination/challenge CMA/CMPI: confirmed by unblinded elimination/challenge and exclusion of lactose intolerance and infection | |
| Notes | Trial of supplemental or sole partially hydrolysed whey formula vs extensively hydrolysed casein formula vs extensively hydrolysed whey formula in high‐risk infants Control group of non‐randomly allocated breast fed infants not included in analysis Conflict of interest: funded by the Danish Dairy Foundation. Companies provided formula and funding | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quasi‐random. Postnatal allocation by date of birth |
| Allocation concealment (selection bias) | Unclear risk | Allocation predictable but intervention blinded |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Used formula tins labelled 'A, B or C' |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | ‘The blinded coding of the products was not revealed until all the data registration was complete’ |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Unclear as to exact numbers in each group not completing the study. Of initial population of 595 infants, 92% were included in study and 80% completed follow‐up. Reasons for losses included parental refusal (19), received other formula in first days (23), 'dropped out' (36), not seen at 18 months, did not fulfil inclusion criteria (4) and non‐compliance (32) |
| Selective reporting (reporting bias) | High risk | Not intention‐to‐treat analysis |
| Other bias | High risk | Some baseline differences between study groups (bi‐parental atopy: Nutramigen 38%, Profylac 22%, Nan HA 39%) |
| Methods | Multi‐centre randomised controlled trial in Australia | |
| Participants | Inclusion criteria: Mother‐baby pairs were enrolled if the unborn child had a first‐degree relative with a history of eczema, asthma, allergic rhinitis or food allergy | |
| Interventions |
Treatment 1 (n = 206): pHWF (NAN HA; Nestle, Biessenhoffen, Germany) Treatment 2 (n = 208): soy‐based formula (ProSobee; Mead Johnson Nutrition/Bristol Myers, Melbourne, Australia) Control CMF (n = 206): cow's milk formula (NAN; Nestle, Tongala, Australia) Mothers were encouraged to initiate and maintain breast feeding for at least 6 months Study formulas introduced at cessation or partial cessation of breast feeding or as a breast milk substitute if breast feeding was not intended Approximately 50% of infants received some allocated formula by 4 months of age; 16.5% never received allocated formula because of continuing breast feeding (13.6%; n = 78/575) or use of a non‐allocated formula (2.9%; n = 17/575) |
|
| Outcomes | Childhood outcomes based on parent report during telephone interviews up to age 2 (every 4 weeks until 64 weeks, then at 78 and 104 weeks), and at 6 or 7 years. Skin prick tests (SPTs) were performed at 6, 12 and 24 months according to a standard technique by 1 of 3 allergy‐trained research nurses Primary aim: to determine incidence of allergic manifestations (eczema and food reactions) up to 2 years of age in high‐risk infants Definitions: Eczema: doctor‐diagnosed eczema or any rash that was treated with topical steroid preparation (excluding rash that affected only the scalp or nappy region) Food reaction: Within 2 hours of ingesting that food, child developed an acute skin rash (urticaria, angioedema, erythematous or morbilliform), a flare of pre‐existing eczema, signs of anaphylaxis or vomiting Any allergic manifestation: presence of eczema or food reaction within first 2 years of life Positive SPT: a wheal of at least 3 mm (mean) diameter with a positive (histamine) control Childhood outcomes based on parent report during telephone interviews conducted when children were 6 or 7 years of age were defined as follows: ‐ Current childhood eczema: eczema diagnosed by family physician in previous 12 months ‐ Current childhood asthma: asthma diagnosed by family physician in previous 12 months ‐ Persistent childhood asthma: asthma diagnosed by family physician in previous 12 months on at least 2 occasions at follow‐up at 5, 6 or 7 years ‐ Current childhood allergic rhinitis: one or more episodes of nasal discharge and/or congestion in the absence of an upper respiratory tract infection in the previous 12 months that family physician or parent attributed to allergic rhinitis (hay fever) and that was treated with an antihistamine and/or nasal steroid |
|
| Notes |
Sponsor: Nestec Ltd, a subsidiary of Nestle Australia, provided the study formula and staff funding for the first 6 years of the study A.J. Lowe has received research support from Dairy Australia. K.J. Allen has received research support from Wyeth and Nutricia. M.J. Abramson has received research support from the National Health and Medical Research Council. D.J. Hill has received research support from Nestle Australia, SHS International and Nutricia Initial study reports were not intention‐to‐treat. Latest report is an intention‐to‐treat analysis so is now considered eligible for inclusion Data obtained from study authors excluded enrolments in the first 9 to 10 months, when infants were not randomised to a hydrolysed formula Executives from Nestlé Australia wrote to the editors of JACI on 12 May 2010 to raise scientific concerns about the study, including alleged discrepancies between the interim report and the publication accepted by JACI Before publication, and with full information on concerns raised by Nestlé, the following organisations reviewed these issues: ‐ Editorial Board of JACI ‐ American Academy of Allergy Asthma and Immunology (AAAAI) Board of Directors ‐ Committee on Publication Ethics (COPE – see http://publicationethics.org/) ‐ Mercy Hospital for Women Human Research Ethics Committee (HREC) ‐ Approving ethics committee for the MACS Project ‐ Mercy Hospital for Women Board of Directors Study authors document that internal audits of study data included re‐entry of infant feeding data for all infants and a random audit of outcome data for 100/620 infants |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | An independent statistician created each of the computer‐generated allocation schedules |
| Allocation concealment (selection bias) | Low risk | Mother‐baby pairs were allocated to the next sequential number as they were enrolled in the study and were assigned to the formula code allocated to that number |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Staff were blind to allocation codes and to group of allocation at the time of outcome assessment. Cans of formula were labelled at an independent location. Parents of participants were informed of the identity of the assigned formula only after the child’s second birthday |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Staff were blind to allocation codes and to group of allocation at the time of outcome assessment. However, random allocation list was available to research staff |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 2 years: 45/620 (7.3%) excluded; 6 to 7 years: 125/620 (20%) excluded. Excluded as lost contact or parent refused further follow‐up. Low risk 2 years, high risk 6 to 7 years owing to excess losses |
| Selective reporting (reporting bias) | Low risk | Prospective protocol registration (ACTRN12609000734268). Primary endpoints stated in the objectives reported in the results |
| Other bias | Low risk | Infants allocated to CMF and pHWF groups similar on baseline risk factors. No differences between groups in terms of duration of exclusive breast feeding or age of introduction of solids "The first 97 infants were randomized to either the CMF or soy study groups. When the pHWFbecame available, a new random allocation series was generated with a higher proportion allocated to the pHWF to obtain equal numbers in each formula group." This review incorporates only infants allocated to the 3 groups contemporaneously |
| Methods | Population derived (city of Malmo) quasi‐randomised (alternation) controlled trial in Sweden | |
| Participants | Inclusion criteria: 144 healthy term infants of pregnant mother volunteers. 62% had family history of atopy | |
| Interventions | Early sole feeding for 3 days. Subsequently, all infants exclusively breast fed Treatment 1 (n = 53): pasteurised human milk feeds from milk bank Treatment 2 (n = 38): casein extensively hydrolysed formula (Nutramigen) Control (n = 39): cow's milk formula (Baby Semp) | |
| Outcomes | Primary outcome(s): macromolecular absorption, antibody production and allergic symptoms Other outcomes: serum IgE at 4 days, 8 months, 1 and 2 years; skin prick test at 1 and 2 years; clinical allergy to 3 years (child allergy incidence) Criteria for allergy diagnosis not reported | |
| Notes | Trial of early (first 3 days) sole human milk vs cow's milk formula vs extensively hydrolysed casein formula Use of volunteers meant possible selection of high‐risk infants Conflict of interest: unclear; work supported by several foundations; affiliations not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quasi‐random, infants allocated according to day of the month |
| Allocation concealment (selection bias) | High risk | Predictable allocation; method of concealment not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Group numbers unequal. 15/144 (10%) lost to 3 years |
| Selective reporting (reporting bias) | Unclear risk | Aim to measure 'allergic symptoms' |
| Other bias | High risk | Potentially clinically important imbalances between groups (maternal smoking, bi‐parental atopy) |
| Methods | Single‐centre randomised controlled trial in Poland | |
| Participants |
Inclusion criteria: birth weight ≤ 1500 grams, age on admission < 72 hours, negative blood culture on admission Exclusion criteria: GI tract anomalies, early‐onset gram‐negative sepsis, necrotising enterocolitis before the beginning of enteral feeding, Apgar score < 3 at 5 minutes, intraventricular haemorrhage grade IV |
|
| Interventions | Infants fed for 1 month Treatment (n = 40): extensively hydrolysed protein‐based formula (casein hydrolysate formula; Pregestimil, Mead and Johnson) Control (n = 40): cow's milk‐based formula (Bebilon Neonatal, Nutricia) |
|
| Outcomes |
Primary outcome: at 5 to 7 years of age, presence of atopic disease according to the following categories: obvious atopic disease, possible atopic disease, no atopic disease Secondary outcomes: sensitisation status (atopic status) determined by IgE, by SPT or by CD4 + CCR4 + CD4 + CXCR3 + lymphocyte ratio Definitions: Parents asked to complete ISAAC (International Study of Asthma and Allergies in Childhood) questionnaire assessing past and current health status of the child All questions verified by physicians during face‐to‐face discussion All children examined by an investigator for the presence of atopic eczema, (rhino)conjunctivitis, wheezing and other clinical signs of allergy |
|
| Notes |
Sponsor: supported by unrestricted grant from Nutricia Research Foundation This is a follow‐on trial of a randomised controlled trial of feeding during first month of life with a formula containing lactose (Bebilon Nenatal) vs a lactose‐free formula (Pregestimil) (Kwinta 2002) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not reported |
| Allocation concealment (selection bias) | Unclear risk | 89 infants enrolled, 80 included ‐ unclear as to timing of allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Study formulas were prepared by the hospital pharmacy in the blind manner" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "All children were examined by an investigator in the blinded fashion for the presence of atopic eczema, (rhino)conjunctivitis, wheezing, and other clinical signs of allergy" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 9/89 excluded after enrolment. 6 infants died before discharge. Hydrolysed group 8/37 and CMF group 4/37 lost before follow‐up. Overall, 62/74 (84%) survivors reported |
| Selective reporting (reporting bias) | High risk | Study originally set up to evaluate the influence of different enteral feeding protocols on early morbidity of VLBW infants |
| Other bias | Unclear risk | Some clinically but not statistically significant differences in baseline family history of atopy: CMF 5/29 (17%); HF 3/33 (9%) |
| Methods | Single‐centre randomised controlled trial in Hong Kong | |
| Participants | Inclusion criteria: infants not breast fed or who stopped breast feeding in first 2 weeks. 'High‐risk infants' but criteria not reported | |
| Interventions | Allocated to: Treatment (n = 50): partially hydrolysed whey formula (Nan HA, Nestle) Control (n = 50): cow's milk formula (Nan, Nestle) Co‐interventions: none reported. Solids withheld for 6 months | |
| Outcomes | Primary outcome(s): allergic manifestations in first 6 months Other outcomes: growth parameters in first 6 months Definitions: Atopic symptoms included colic, respiratory atopy (wheeze and rhinitis) and skin atopy (eczema and urticaria). Eczema not defined | |
| Notes | Trial of prolonged feeding in infants at high risk of allergy with partially hydrolysed whey formula vs cow's milk formula Numbers of infants with atopic manifestations at 6 months converted from percentages Conflict of interest: internal report of Nestle. Data not published |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported 'double‐blind randomisation'; method not reported |
| Allocation concealment (selection bias) | Unclear risk | Method not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 8/100 (8%) ‐ 6 in hydrolysed formula group and 2 in cow's milk formula group |
| Selective reporting (reporting bias) | Unclear risk | Allergy definitions not reported |
| Other bias | Unclear risk | Group characteristics not reported |
| Methods | Single‐centre randomised controlled trial in France | |
| Participants | Inclusion criteria: 177 infants with immediate family history of allergy. Allergy score used | |
| Interventions | Sole or supplementary formula feeding for at least 4 months with: Treatment (n = 92): extensively hydrolysed casein formula (Pregestemil, Mead Johnson) Control (n = 85): cow's milk formula (Galliazyme, Gallia, France) No co‐interventions | |
| Outcomes | Primary outcome(s): allergy Other outcomes: clinician assessment for allergy Eczema and IgE assessed at 4 months; eczema, asthma and CMA assessed at 1, 2 and 4 years Definitions: Atopic eczema: graded as mild (< 4 patches), moderate or severe Asthmatic bronchitis: grade 1 (2 to 4 occurrences per year) and grade 2 (> 4 per year) CMA: confirmed by type 1 reagin allergy (specific IgE RAST) or malabsorption | |
| Notes | Excess losses at all time periods except 4 months (infant allergy incidence). Trial of supplemental or sole extensively hydrolysed casein formula vs cow's milk formula Results for only 4 months included (infant allergy incidence) Conflict of interest: Mead Johnson and Gallia supplied formula | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Infants randomised postnatally; method not reported |
| Allocation concealment (selection bias) | Unclear risk | Details not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The study design was not blinded.... and formulas were easily distinguishable by taste and smell |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Both parents and paediatricians knew which formula was fed to the infant |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Hydrolysed formula group (n = 92): 5 (5%) at 4 months, 21 (23%) at 1 year, 14 (15%) at 2 years, 22 (24%) at 4 years Cow's milk formula group (n = 85): 7 (8%) at 4 months, 32 (38%) at 1 year, 24 (28%) at 2 years, 31 (36%) at 4 years Three children failed to follow diet prescriptions and were excluded. No withdrawal seemed to be motivated by any abnormality linked to cow's milk intolerance |
| Selective reporting (reporting bias) | High risk | "Aim of assessing the allergy prevention effects". Reported multiple allergy outcomes, grades of severity and time points |
| Other bias | Low risk | Well‐balanced groups after allocation |
| Methods | Three‐centre randomised controlled trial in Italy | |
| Participants | Inclusion criteria: maternal questionnaire used to identify infants with well‐defined family history of allergy in either parent | |
| Interventions | Infants randomised were those whose mothers did not wish to breast feed or had insufficient milk Treatment (n = 48): 'moderately' hydrolysed formula (Nidina HA, Nestle) Control (n = 47): cow's milk formula (Nan, Nestle) Formula feeding advised to 5 months Co‐interventions (both groups): maternal cow's milk and food avoidance measures for breast feeding mothers. For infants, cow's milk and allergenic foods avoided to 1 year. Advice given to modify environmental exposure (smoking, pets, carpets, avoiding infant community care to 2 years) | |
| Outcomes | Primary outcome(s): allergic manifestations and nutritional adequacy of formula Other outcomes: weight, length and head circumference at 6 months, 1 and 3 years. Physician‐diagnosed allergy Definitions: Atopic dermatitis: typical rash in at least 2 areas Recurrent wheezing: ≥ 3 episodes and physician diagnosed Recurrent urticaria: ≥ 2 episodes after exposure to particular antigen GI symptoms: vomiting and/or diarrhoea after exclusion of infection and lactose intolerance, not confirmed by blinded elimination/challenge Allergic rhinitis: ≥ 3 weeks rhinorrhoea RAST and skin prick tests also performed in affected individuals Follow‐up performed at 1, 2 (infant allergy) and 3 years (child allergy) | |
| Notes | Trial of prolonged supplemental or sole 'moderately' hydrolysed whey formula vs cow's milk formula feeding in high‐risk infants Co‐interventions in both groups Conflict of interest: none reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Postnatal 'random' allocation of infants; method not reported |
| Allocation concealment (selection bias) | Unclear risk | Method not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Where artificial feeding was required... babies... randomly allocated to formula (a) or formula (b) ... (but the mothers were not blinded to the allocation)" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Physicians "unaware of the dietary regimen", but insufficient information reported on blinding of personnel to allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Hydrolysed group losses 5 (10%) at 1 year, 6 (13%) at 2 years, 8 (17%) at 3 years Cow's milk group losses 6 (13%) at 1 year, 7 (15%) at 2 years, 9 (19%) at 3 years |
| Selective reporting (reporting bias) | Low risk | Prespecified allergy outcomes. Standardised definitions |
| Other bias | Unclear risk | Insufficient data reported at baseline for allergy risk factors between study groups |
| Methods | Single‐centre quasi‐randomised (alternation) controlled trial in Czech Republic | |
| Participants | Inclusion criteria: pregnant women who themselves, husbands or children attended an allergy or dermatology outpatient clinic (i.e. family history of atopy in first‐degree relative) | |
| Interventions | Mothers encouraged to breast feed for at least 6 months to avoid cow's milk and highly allergenic foods. Allocated sole or supplemental formula if unable to solely breast feed according to prenatal treatment allocation Treatment 1 (n = 37): partially hydrolysed whey cow's milk formula (Beba HA, Nestle, Denmark) Treatment 2 (n = 35): extensively hydrolysed whey cow's milk formula (Hipp HA, Hipp GnbH, Gmunden, Austria) Co‐interventions: all mothers encouraged to breast feed for 6 months, avoid cow's milk for first 6 months, introduce solids after 6 months and delay allergenic foods to after 12 months. At 6 months: 24/37 fed partially hydrolysed formula and 21/35 fed extensively hydrolysed formula At 12 months: 31/37 fed partially hydrolysed formula and 28/35 extensively hydrolysed formula | |
| Outcomes | Primary outcome(s): antigen‐specific reactivity of mononuclear cells to cow's milk protein; cow's milk specific IgE and IgG; atopic skin symptoms Other outcomes: symptom diaries kept. Blinded paediatrician assessment for atopic dermatitis Reported weights up to 12 months (data not given) Definitions: Atopic dermatitis: typical rash in at least 2 locations relapsing for at least 3 months' duration. Standardised score used (SCORAD) Allergy reported at 6 and 12 months (infant allergy) | |
| Notes | Trial of sole or supplemental feeding partially hydrolysed whey formula vs extensively hydrolysed whey formula in high‐risk infants unable to be completely breast fed in first 6 months Conflict of interest: supported by research grants. The "study done independently of infant food companies" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quasi‐random ‐ prenatal randomisation by odd and even numbers |
| Allocation concealment (selection bias) | High risk | Prenatal randomisation by odd and even numbers. Postnatal allocation to formula if unable to fully breast feed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Paediatrician prescribing treatment aware of allocation. Formula not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Second paediatrician unaware of allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 1/73 (1%) post randomisation loss. 13/72 (18%) not fed hydrolysed formula and reported in separate group |
| Selective reporting (reporting bias) | Low risk | Prespecified atopic skin symptoms in first 12 months of life. Standardised definitions |
| Other bias | High risk | Groups appeared well balanced after allocation. However, not intention‐to‐treat analysis |
| Methods | Population derived (well baby clinics in 3 towns) randomised controlled trial in Sweden | |
| Participants | Inclusion criteria: term newborn infants with 2 allergic family members or 1 allergic family member and cord IgE ≥ 0.5 U/L Mean age of weaning between 3 and 4 months | |
| Interventions | In infants weaning from the breast: Treatment 1 (n = 55): extensively hydrolysed casein formula (Nutramigen, Mead Johnson) Treatment 2 (n = 51): partially hydrolysed formula whey:casein ratio 60:40 (Mead Johnson) Control (n = 49): cow's milk formula (Enfamil, Mead Johnson) Co‐interventions: both groups advised to not smoke and avoid pets. Solids introduced after 4 months. Avoidance of cow's milk, eggs, fish and citrus until after 9 months | |
| Outcomes | Primary outcome(s): atopic and allergic disease at 18 months (infant allergy incidence) Other outcomes: nurse examination at 3, 6, 9, 12, 18 months and doctor visit at 18 months. Skin prick tests at each visit and specific IgE RAST at 9, 12, 18 months Definitions: Atopic dermatitis: standard scoring system used Food reactions: double‐blind placebo‐controlled challenges for formula milk reactions Asthma: recurrent wheeze with doctor confirmation Allergic rhinitis: doctor verified and allergen sensitisation proved Gastrointestinal allergy: positive unblinded oral challenge to food to which infant was sensitised | |
| Notes | Trial of extensively hydrolysed vs partially hydrolysed vs cow's milk formula for weaning high‐risk infants Conflict of interest: co‐investigator from formula company. Formula supplied by Mead Johnson. Study supported by Bristol‐Myers Inc | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stratified by age at weaning. Infants randomised when weaning commenced. Method of sequence generation not reported |
| Allocation concealment (selection bias) | Unclear risk | Method not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Identical coded tins used for extensively and partially hydrolysed formulas; no blinding of cow's milk formula tin |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Nurses and investigators blinded throughout the study" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 14/155 post‐randomisation losses (9%) at 18 months. Feeding problems resulted in 11 losses (EHF 3, PHF 6, CMF 2) |
| Selective reporting (reporting bias) | Low risk | Primary endpoints reported in results |
| Other bias | Unclear risk | Incomplete reporting of potential confounders in groups. Potential imbalance (furry animals at home: EHF 22%, PHF 6%, CMF 16%) between groups after allocation, although reported to be not statistically significant |
| Methods | Three‐hospital quasi‐random (allocation by month) study in Finland | |
| Participants | Inclusion criteria: healthy full‐term infants requiring supplemental feeding in hospital | |
| Interventions | Early supplementary feeding in hospital with: Control 1 (n = 1758): cow's milk formula (Tutteli, Vali, Finland) Control 2 (n = 1844): pasteurised donor human milk Treatment (n = 1715): extensively hydrolysed whey formula (Pepti‐Junior, Nutricia, Netherlands) Average duration hospital stay 4 days Mothers encouraged to breast feed. Supplemental cow's milk formula used after discharge when required. Solids introduced at 4 to 6 months No co‐interventions | |
| Outcomes |
Primary outcome(s): CMA Other outcomes: CMA ‐ mothers contacted researchers if symptoms suggestive of CMA appeared. Well baby clinics also informed of study (all infants seen average 8 times in first 12 months) Definitions: CMA: unblinded elimination/challenge performed Mean age follow‐up 27 months (range 18 to 34 months) (infant allergy) |
|
| Notes | Trial of early supplemental human milk vs extensively hydrolysed whey formula vs cow's milk formula Potential ascertainment bias as compliance with reporting not assessed Conflict of interest: supported by Nutricia | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quasi‐random allocation by month of birth and hospital born |
| Allocation concealment (selection bias) | Unclear risk | Possible if blinding maintained |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Colour‐coded bottles used |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding maintained until last follow‐up assessment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 6209/6267 (99%) eligible mothers returned baseline questionnaire. Mothers were asked to call study author if symptoms of CMA appeared. Compliance not assessed Diary of infant feeding regimen returned by 118/118 mothers of infants subsequently found to be hypersensitive to CM and 76% CM‐tolerant infants Well baby clinics of the area, in which every infant is seen an average of 8 times during the first 12 months of life, were informed of the study |
| Selective reporting (reporting bias) | Low risk | Primary endpoint was an adverse reaction to challenge with CM |
| Other bias | Unclear risk | Baseline characteristics not reported |
| Methods | Randomised controlled trial | |
| Participants | Inclusion criteria: Infants (n = 242) with family history of allergy were enrolled at birth | |
| Interventions | All infants were breast fed for 3 months, then study formula was given for 1 month. Then randomisation to groups Control (n = not reported): cow's milk formula Treatment 1 (n = not reported): extensively hydrolysed formula Treatment 2 (n = not reported): partially hydrolysed formula After 4 months of age, all formula groups received the same cow's milk formula given to 1 of the groups during the intervention period |
|
| Outcomes |
Primary outcomes: development of anti‐cow's milk IgE antibodies No data for clinical allergy outcomes reported to date |
|
| Notes |
Sponsor: Nestle Nutrition, Nestlec Ltd, Vevey Switzerland Reported in conference abstract only |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported randomised but method not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Unclear risk | No clinical allergy outcomes reported in abstract |
| Other bias | Unclear risk | Group characteristics not reported |
| Methods | Single‐centre randomised controlled trial in Taiwan | |
| Participants | Inclusion criteria: healthy term infants. Family history of allergy score used. Infants with score > 3 enrolled | |
| Interventions | Treatment (n = 15): infants breast fed for 1 to 2 months, then fed partially hydrolysed formula for subsequent 4 months (Nan HA, Nestle). All except 2 infants received formula Control (n = 18): regular formula from birth No co‐interventions reported | |
| Outcomes | Primary outcome(s): allergic diseases Other outcomes: seen at 1, 2, 4, 6, 12 months (infant allergy incidence) in well baby clinic. Total and specific IgE at 2, 6, 12 months. Skin prick tests in cases of suspected allergy. Growth in weight and height up to 12 months Definitions: Atopic dermatitis: grading score used (mild: faint lesions on forehead or cheek without treatment; moderate and severe: lesions required treatment) Allergic rhinitis: typical symptoms in early morning Wheezing: any | |
| Notes | Trial of prolonged supplementary or sole partially hydrolysed whey formula vs cow's milk formula Data not reported in group of allocation for clinical allergy confirmed by skin prick testing, and possibly for growth Conflict of interest: financial support and formula provided by ANPING Ltd | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The newborns were randomly allocated to two groups". Method not reported |
| Allocation concealment (selection bias) | Unclear risk | Method not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unlikely. Infants fed cow's milk formula from birth. Those fed partially hydrolysed formula breast fed for 1 to 2 months |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Cross‐over of 3 infants from hydrolysed to cow's milk formula group (unclear which reported outcomes this affected) |
| Selective reporting (reporting bias) | Unclear risk | Did not report prespecified definition of specific allergy or time of reporting |
| Other bias | Low risk | No reported differences between study groups for cord blood IgE, family history allergy score or duration of follow‐up |
| Methods | Single‐centre randomised controlled trial in Belgium | |
| Participants | Inclusion criteria: infants with at least 2 first‐degree relatives with allergy, whose mothers intended not to breast feed | |
| Interventions | Exclusive formula feeding for 6 months with: Treatment (n = 32): partially hydrolysed whey formula (Nan HA, Nestle) Control (n = 35): cow's milk formula (Nan, Nestle) Co‐interventions (both groups): grated apple from 4 months. 'Normal' diet after 6 months | |
| Outcomes | Primary outcome(s): atopic disease Other outcomes: blinded physician assessment for allergy monthly for first year of life. Total IgE, specific RAST, IgG4 antibodies, skin prick tests Definitions: Atopic dermatitis: at least 3 of 4 criteria including typical rash, recurrence or chronicity and specific IgE Urticaria: no definition given Allergic wheezing: cough without infection ≥ 24 hours Chronic rhinitis: clear nasal discharge CMPI: confirmed by unblinded elimination/challenge Allergic diarrhoea: infection excluded. Infants with diarrhoea had jejunal biopsy performed Follow‐up to 12 months (infant allergy incidence) | |
| Notes | Trial of prolonged sole partially hydrolysed whey formula vs cow's milk formula in high‐risk infants 3‐ and 5‐year results excluded owing to excess losses Data for cumulative specific allergy manifestations up to 12 months not extractable separately Conflict of interest: Nestle provided formula and performed statistical analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Infants postnatally allocated; method not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation process not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Infants were given 1 of 2 coded formulas... or an adapted formula with native cow's milk proteins, delivered in an unlabelled package |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Follow‐up was blinded as much as possible: neither the parents nor the physician(s) involved in the follow‐up were informed about the nature of the formula" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 12 months: 8/75 (11%) post‐randomisation losses. At 3 and 5 years: 17/75 (23%) lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Reported prespecified definitions of specific allergy but not time of reporting |
| Other bias | Low risk | Groups similar at baseline for risk factors for allergy |
| Methods | Multi‐centre randomised controlled trial in Germany | |
| Participants | Inclusion criteria: high risk of allergy in healthy infants with at least one first‐degree family member with allergy Exclusion criteria: severe acquired or congenital diseases, gestation < 37 weeks, birth weight < 2500 grams, > 14 days, intake cow's milk‐based formula before inclusion, incapability of parent to comply with study protocol | |
| Interventions | Mothers encouraged to breast feed for at least 4 months. Study formula provided for when sole breast feeding no longer continued and provided until infant 6 months of age. Infants (all centres: N = 2252; Wessel: n = 1087) randomised to: Treatment 1 (all centres: n = 557; Wessel: n = 273): partially hydrolysed 100% whey formula (Beba HA, Nestle, Vevey, Switzerland) Treatment 2 (all centres: n = 559; Wessel: n = 265): extensively hydrolysed 100% whey formula (Hipp HA, Hipp, Pfaffenhofen, Germany) Treatment 3 (all centres: n = 580; Wessel: n = 281): lactose‐free, extensively hydrolysed 100% casein formula (Nutramigen, Mead Johnson, Diezenbach, Germany) Control (all centres: n = 556; Wessel: n = 268): cow's milk formula with casein:whey ratio 40:60 (Nutrilon Premium, Nutricia/Numico, Zoetermeer, Netherlands) Co‐interventions: All groups received advice about breast feeding for at least 4 months, preferably 6; no dietary restrictions during lactation; not to feed solids during study period, and thereafter to add 1 food a week and avoid common allergenic foods in first year 58.4% of infants received study formula | |
| Outcomes |
Primary outcome(s): allergy Other outcomes: allergy (atopic manifestations), asthma and eczema Definitions: Allergy (atopic manifestations) diagnosed at 12 months (infant allergy) as atopic dermatitis, allergic urticaria or gastrointestinal food allergy Atopic dermatitis: typical morphology and distribution of skin lesions; pruritus; chronicity (duration ≥ 14 days, chronically relapsing or both); confirmed on skin examination by a second specially trained allergist; severity rated using the SCORAD method Allergic urticaria: at least 2 episodes of itching eruptions or swelling with typical appearance, observed by parents or physician, caused by the same allergen. In case of a single episode, immunological evidence (specific skin prick test or allergen‐specific IgE level ≥ 0.35 KU/L or positive provocation response) Gastrointestinal food allergy: suspected if gastrointestinal symptoms not explained by any other condition, and if unblinded elimination challenge reproduced symptoms. Gastrointestinal allergy definite if a positive standardised elimination/challenge procedure. Double‐blind, placebo‐controlled food challenge performed in cases of uncertain reactions At 3 years, childhood allergy included atopic dermatitis, urticaria, food allergy with manifestation in the gastrointestinal tract and asthma Allergic asthma: diagnosed from parental report of relevant symptoms (wheeze and/or cough without infection) or regular use of asthma medication in the child's third year of life. Asthma symptoms included wheezing or cough for at least 2 weeks (acute laryngotracheitis excluded); exercise‐induced wheeze or cough at any time (with crying, laughing or activity); and episodes of wheezing or dry night‐time cough At 6 years, reported physician‐diagnosed allergic diseases (atopic dermatitis, food allergy, allergic urticaria, asthma and hay fever/allergic rhinitis) At 10 years, reported physician‐diagnosed asthma, allergic or atopic eczema/dermatitis, hay fever/allergic rhinitis, urticaria and food allergy. Also, if present, at 10 years, parents reported a physician’s diagnosis during past 4 years, treatment in past 12 months or both At 15 years, parent‐reported physician‐diagnosed asthma, allergic rhinitis (AR) and eczema, spirometric indices and sensitisation |
|
| Notes | Data for intention‐to‐treat analyses of all infants (including breast fed infants) according to study centre provided by study authors Analyses meeting inclusion criteria for the review are intention‐to‐treat analyses including breast fed infants for all study centres at 1 year and infants enrolled in Wesel for 3 year outcomes Excess losses beyond 3 years, so data not included in analyses Trial of prolonged breast feeding with supplemental or sole formula feeding when required comparing use of cow's milk formula, partially hydrolysed whey formula, extensively hydrolysed whey formula and lactose‐free extensively hydrolysed casein formula Sponsor: study supported by Federal Ministry for Education, Science, Research and Technology and the Child Health Research Foundation. Formulas provided by Nestle, Hipp, Milupa, Numico and Mead Johnson |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated list stratified for single or double parental atopy and study region |
| Allocation concealment (selection bias) | Unclear risk | Method not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Blinding of parents and the study team was guaranteed by using identically labelled tins for the study formula coded with 4 different letters for each of the 4 formulas" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | None reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | In intention‐to‐treat analyses for study centres (Munich and Wesel) that included breast fed infants: 1 year: 304/2252 (13.5% lost to follow‐up) 3 years: 692/2254 (31% lost to follow‐up) 6 years: 572/2252 (25% lost to follow‐up) 10 years: 1115 to 1145/2522 (44% to 45% lost to follow‐up) 15 years: Response rates were 61.1% (1377/2252) ‐ lost to follow‐up 875/2252 (39%) Breast fed infants randomised to interventions who did not receive interventions were followed up only in Wesel In intention‐to‐treat analyses for Wesel only: 1 year: 158/1087 (14.5% lost to follow‐up) 3 years: 206/1087 (19% lost to follow‐up) |
| Selective reporting (reporting bias) | Low risk | Primary endpoints and specific timings stated in the methods were reported in the results in the original study report (von Berg 2003) |
| Other bias | High risk | ".. significant bias in the eHF‐C group because disproportionally more children had to be excluded as a result of noncompliance with the study formula (18% [47/257] in the eHF‐C group vs 10% to 12% in the other study formula groups, P = 0.02)" |
| Methods | Single‐centre quasi‐randomised (allocated by month) controlled trial in Belgium | |
| Participants | Inclusion criteria: infants not breast fed with a family history of allergy and cord IgE ≥ 0.5 IU/L | |
| Interventions | Prolonged sole formula feeding with: Control: (n = 55): cow's milk formula Treatment (n = 67): partially hydrolysed whey formula (Nan HA) Formula used for first 3 months, then unrestricted diet No co‐interventions | |
| Outcomes | Primary outcome(s): allergy Other outcomes: paediatrician‐administered questionnaire at 3 months and 1 year for allergy (infant allergy incidence) Definitions: Allergy included eczema, asthma, recurrent episodes of bronchitis, persistent rhinitis, persistent gastrointestinal symptoms (excluding infection) and sleeping difficulties No specific definitions given | |
| Notes | High rate (45%) of non‐compliance with formula Trial of prolonged sole partially hydrolysed whey formula vs cow's milk formula in high‐risk infants Conflict of interest: unclear; co‐investigator from FNRS, Brussels | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quasi‐random ‐ infants postnatally allocated by even or odd month of birth |
| Allocation concealment (selection bias) | High risk | Quasi‐random and unblinded |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Formulas not masked |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | None |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 17/135 (13%) high‐risk infants did not complete the study |
| Selective reporting (reporting bias) | Low risk | Prespecified allergy |
| Other bias | Unclear risk | Did not report baseline characteristics of groups |
CM: cow’s milk CMA: cow’s milk allergy CMF: cow’s milk formula CMPI: cow’s milk protein intolerance EHF: extensively hydrolysed formula GI: gastrointestinal HF: hydrolysed formula Ig: immunoglobulin ISAAC: International Study of Asthma and Allergies in Childhood PHF: partially hydrolysed formula pHWF: partially hydrolysed whey formula RAST: radio‐allergosorbent SPT: skin prick test VLBW: very low birth weight
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Agosti 2003 | AGA preterm infants allocated to low hydrolysed protein formula vs intact protein formula. Method of allocation not reported. Did not measure allergy. n = 20 |
| Akimoto 1993 | Cohort study |
| Arikan 2008 | Randomised families with colicky infants to 4 different intervention groups ‐ massage, sucrose solution, herbal tea and hydrolysed formula and control group. Did not report allergy. n = 35 per group |
| Arshad 1992a | Had multiple allergies preventing co‐interventions in treatment group and not in control group |
| Barberi 1993 | Infants at high risk of atopy randomised to HF vs cow's milk formula. 440 received allocated formula. Method of allocation not reported. Excess post‐randomisation losses ‐ 278/815 (34%). No intention‐to‐treat analysis. n = 815 |
| Bergmann 1996a | Infants at risk of atopy were assigned to 1 of 3 nutritional groups and were followed‐up through the first 6 months of life: 20 infants were breast fed, 28 received a predominantly whey protein‐based infant formula, 53 received an ultrafiltrated partially hydrolysed whey protein formula. Non‐random allocation. n = 101 |
| Berseth 2009 | Double‐blind randomised controlled trial in healthy formula fed term infants with no family history of allergy of 70% lactose, partially hydrolyzed cow milk protein formula supplemented with docosahexaenoic acid and arachidonic acid vs full lactose intact cow milk protein formula supplemented with docosahexaenoic acid and arachidonic acid. Did not report allergy. Measured adverse effects and tolerance. n = 335 |
| Borschel 2013 | Healthy term infants randomised to an amino acid–based formula or an extensively hydrolysed casein‐based formula. Did not report allergy. n = 213 |
| Borschel 2014 | RCT in healthy term infants fed whey‐based, palm olein oil–free partially hydrolysed formula vs whey‐based, palm olein oil‐containing partially hydrolysed formula (not eligible comparison). Did not report allergy. n = 209 |
| Borschel 2014a | RCT in healthy term infants fed ready‐to‐feed or powdered form of an extensively hydrolysed casein‐based formula (not eligible comparison). Did not report allergy. n = 195 |
| Boyle 2016 | RCT in infants at high risk of allergy fed partially hydrolysed formula supplemented with prebiotic vs standard cow's milk formula without prebiotic (not eligible comparison). Reported allergy. n = 863 |
| Burks 2008 | Randomised healthy term infants to amino acid‐based formula vs extensively hydrolysed formula. Excess post‐randomisation losses (33%). Reported adverse events. Did not report allergy. n = 165 |
| Campbell 1989 | Enrolled infants with colic. Not randomised |
| Chan 2002 | Trial of sole prolonged partially hydrolysed cow's milk formula vs cow's milk formula in infants at high risk of atopy. Excess losses (28%). n = 110 |
| Chan‐Yeung 2000 | Infants at high risk of allergy. Had multiple allergy‐preventing co‐interventions in treatment group and not in control group. n = 545 |
| Chandra 1989a | Original data unable to be verified |
| Chandra 1989b | Original data unable to be verified |
| Corvaglia 2013 | Randomised preterm infants with symptomatic gastroesophageal reflux to extensively hydrolysed preterm formula vs standard preterm formula. Did not report allergy. n = 40 |
| Corvaglia 2013a | Randomised cross‐over trial in preterm infants with symptoms of feeding intolerance allocated eHF vs SPF. Did not report allergy |
| D'Agata 1996 | Infants at high risk of allergy. 30 exclusively breast fed, 50 hypoallergenic milk fed, 30 soy milk fed and 15 fed with conventional milk formula. Method of allocation unspecified. Substantial imbalances in numbers. Reported allergy |
| de Jong 1998 | Trial of early supplementation of cow's milk formula vs a protein‐free placebo formula (not hydrolysed protein) in breast fed infants |
| Decsi 1992 | Healthy term infants who were breast fed or received conventional formula (Mildibe, EGIS; Pre‐Aptamil, Milupa), or a formula containing hydrolysed proteins (Aptamil H.A., Milupa). Did not report allergy. n = 10 per group |
| Decsi 1998 | Term infants fed conventional formula or PHF. Excess losses (27% hydrolysed formula group and 21% cow's milk formula group). Did not report allergy. n = 11 per group |
| Exl 1998 | Allocated to intervention (breast feeding, hydrolysed formula and delayed weaning) according to geographical area |
| Florendo 2009 | Preterm infants randomised to feeds with a standard non‐hydrolysed whey–casein vs partially hydrolysed whey preterm infant formula. Reported feed intolerance. Did not report allergy. n = 80 |
| Fukushima 1997 | Trial of maternal allergen avoidance and infant supplemental or sole hydrolysed or cow's milk formula feeding when required. Differential losses with excess losses in maternal and infant hydrolysed formula group (27%) and in maternal hydrolysed formula and infant cow's milk formula group (23%) |
| Giovannini 1994 | Study in infants at high risk of atopy given human milk vs soy formula vs whey‐based low‐degree hydrolysate vs casein‐based high‐degree hydrolysate vs soy plus collagen‐based high‐degree hydrolysate formula. Did not report allergy. Excess post allocation losses (56/138) not analysed in group of assignment (solely breast fed infants reported separately). n = 138 |
| Halken 1992 | Trial of prolonged supplementary or sole extensively vs partially hydrolysed ultrafiltrated formula in high‐risk infants. Only infants who received hydrolysed formula included in analysis. Excess losses after allocation (24%). n = 158 |
| Hartman 1994 | Randomised trial in healthy infants allocated to soy formula, cow's milk formula or partially hydrolysed formula. Abstract only. Losses unclear. Reported intolerance and allergy but data not extractable from abstract. n = 510 |
| Hattevig 1989 | Trial of maternal allergen avoidance. The diet for infants was the same in both groups. During first 6 months, only breast milk and or formula based on hydrolysed casein (Nutramigen) given |
| Hernell 2003 | Breast fed infants, infants fed hydrolysate formulas, infants fed milk formula. Method of allocation not reported. Allergy not reported. n = 55 |
| Hill 1995b | Enrolled infants with 'colic'. Randomised to casein hydroIysate or cow's milk formula. Did not report allergy. n = 151 |
| Host 1991 | Cohort study. Casein hydrolysate (Nutramigen) used in elimination/challenge |
| Iikura 1995 | Abstract form only. Method of allocation unclear. Substantial differences in group sizes |
| Keller 1996 | Allocation to various feeding regimens including hydrolysed formula performed by nurses 'at random'. "Maternal decision respected". Unlikely to be random allocation. Allergy reported |
| Kuo 2011 | Prospective, observational, uncontrolled cohort study in newborns who had at least 1 first‐degree family member with a history of atopy and could not breast feed. Fed with HF or CM for at least 6 months and monitored prospectively at 6, 18 and 36 months of age |
| Lasekan 2006 | Assessed growth efficacy, gastrointestinal tolerance and plasma biochemical measurements of healthy infants receiving a partially hydrolysed rice protein‐based formula vs cow's milk formula for the first 16 weeks after birth. Did not report allergy. n = 65 |
| Lucassen 2000 | Enrolled infants with excessive crying. Randomised healthy, thriving, formula‐fed infants, < 6 months old, crying > 3 hours per day on at least 3 days per week to whey hydrolysate formula or standard formula. Did not report allergy. n = 43 |
| Maggio 2005 | Randomised controlled trial of preterm formula with hydrolysed cow’s milk proteins reporting short‐term growth and urinary and plasma amino acid levels. Did not report allergy. n = 21 |
| Martinez‐Valverde | No definition of allergic symptoms reported in first 4 months. In Spanish version of thesis, method of treatment allocation not extractable independently |
| Medjad‐Guillou 1992 | Cross‐over trial of CMF vs hydrolysed formula in healthy term infants. Did not report allergy |
| Mennella 2011a | Healthy infants randomly assigned to CMF or PHF between 0.5 and 7.5 months of age. Did not report allergy. n = 79 |
| Mihatsch 1999 | Cross‐over trial examining effects of hydrolysed formula on plasma amino acids and gastrointestinal transit time in preterm infants. Did not report allergy |
| Mihatsch 2002 | Randomised trial of partially hydrolysed preterm infant formula vs cow's milk formula in VLBW infants at low risk of atopy establishing enteral feeds. Excess post‐randomisation exclusions 48/135 (36%). Did not report allergy. n = 135 |
| Mitchell 1977 | Trial of lactose hydrolysed milk, not protein hydrolysed. Enrolled slightly undernourished Aboriginal children < 3 years of age |
| Moran 1992 | Trial of supplementary or sole hydrolysed formula vs cow's milk formula in low‐risk infants. Excessive losses > 20% in both groups. n = 205 |
| Nentwich 2003 | Observational study of infants fed a whey hydrolysate formula compared with exclusively breast fed controls |
| Nocerino 2012 | Randomised trial of standard formula vs partially hydrolysed whey formula in infants with diagnosed infant colic. Did not report allergy. Published as conference abstract only. n = unclear (52 reported) |
| Odelram 1996 | Trial of extensively hydrolysed vs cow's milk formula for weaning of high‐risk infants. Excluded trial, as 13 losses in addition to 9 post‐randomisation exclusions (total 27%). n = 91 |
| Paronen 2000 | Enrolled infants at high genetic risk for diabetes. Did not report allergy. n = 119 |
| Pauls 1996 | Trial of formulas with hydrolysed vs non‐hydrolysed protein for starting enteral feedings in preterm infants < 1500 g. Did not report allergy. Only outcomes to day 6 reported. Reported in abstract format only |
| Picaud 2001 | Preterm infants randomly assigned to partially hydrolysed formula or conventional formula. Did not report allergy. n = 16 |
| Porch 1998 | Infants at high risk of allergy randomly assigned extensively hydrolysed casein formula (Nutramigen); partially hydrolysed whey formula (Good Start); and soy formula. Excess losses 51/181 (28%). n = 181 |
| Raupp 1995 | Trial of sole hydrolysed formula vs cow's milk formula in low birth weight infants. Excess post‐randomisation losses. Allergy not reported. n = 39 |
| Riezzo 2001 | Randomised trial of standard and hydrolysate formulas in preterm infants. Allergy not reported. n = 36 |
| Rigo 1994a | Method of treatment allocation unclear. Allocated successive infants to formulas. Trial of 5 different types of hydrolysed formula in healthy term infants. Extent of hydrolysis not reported. Allergy not reported. n = 74 |
| Rigo 1994b | Trial of hydrolysate formula in preterm infants. Method of allocation not stated. Allergy not reported. n = 19 |
| Savino 2003 | Observational study of whey hydrolysate formula enrolling infants with "minor feeding problems" |
| Savino 2006 | Randomised to receive formula containing partially hydrolysed whey proteins, prebiotic oligosaccharides, with a high beta‐palmitic acid content, or standard formula and simethicone (multiple formula differences) |
| Scalabrin 2009 | RCT of healthy term infants who received extensively hydrolysed casein formula, the same formula supplemented with Lactobacillus rhamnosus GG or partially hydrolysed whey:casein (60:40) formula supplemented with LGG (PHFLGG). Excess losses at all time points. Reported adverse events including allergy. n = 289 |
| Schmelzle 2003 | Randomised healthy formula fed term infant trial of partially hydrolysed whey infant formula vs standard infant cow's milk formula. Excess losses ‐ 52 (34%). Allergy not reported. n = 154 |
| Schmidt 1995 | Observational study (infants allocated formula at parents' discretion) |
| Schmitz 1992 | Trial in normal breast fed infants of early supplementary hydrolysed formula vs cow's milk formula. Did not report allergy. Excess losses at 1 year. n = 256 |
| Schrander 1993 | Cohort study of newborn infants to determine incidence of CMPI and response to Pregomin (Milupa) protein hydrolysate formula |
| Shao 2006 | Multiple dietary interventions including hydrolysed formula in treatment group for infants at high risk of atopy who were not able to be breast fed |
| Silva Rey 1996 | Trial of partially hydrolysed milk formula. Excess losses ‐ 124/276 (45%) ‐ 42 losses by 6 months and further 82 excluded post allocation. Method of allocation not reported. n = 276 |
| Staelens 2008 | Double‐blind randomised cross‐over study in healthy newborns fed cow's milk formula vs partially hydrolysed formula vs extensively hydrolysed formula (EHF). Allergy not reported. n = 17 |
| Szajewska 2001 | Low birth weight infants were assigned randomly to receive extensive protein hydrolysate preterm formula, partial protein hydrolysate preterm formula and standard preterm formula. Allergy not reported. n = 61 |
| Szajewska 2004 | Randomised trial of extensively hydrolysed preterm formula vs partially hydrolysed preterm formula vs cow's milk formula in high risk for atopy preterm infants. Excess post‐randomisation losses at all times ‐ 22/90 (24%) at 4 to 5 months. n = 90 |
| Tariq 1998 | Cohort study |
| Taubman 1988 | Enrolled infants with excessive crying ('colic'). Randomised to hydrolysed casein formula or counselling. Allergy not reported. n = 20 |
| TRIGR study 2011 | Double‐blind randomised clinical trial. 5156 randomised. 2159 infants with HLA‐conferred disease susceptibility and first‐degree relative with type 1 diabetes allocated to be weaned to extensively hydrolysed casein formula vs a conventional cows’ milk–based formula. Reported asthma and other allergy outcomes. Excluded as excess post‐randomisation exclusions (58%). n = 5156 |
| Vaarala 1995 | Double‐blind trial; 10 infants received cow's milk‐based formula and 10 received a casein hydrolysate formula until the age of 9 months. Allergy not reported. n = 20 |
| Vaarala 2012 | Infants with HLA‐conferred susceptibility to type 1 diabetes randomly assigned to receive CMF (n = 389), whey‐based hydrolysed formula (WHF) (n = 350) or whey‐based FINDIA formula essentially free of bovine insulin (n = 365). Allergy not reported. n = 1104 |
| Vandenplas 1988 | Retrospective study. Embedded intervention study. Trial of formula, breast milk or a hypoallergenic formula. Method of allocation reported to be "chronological". Losses unclear. n = 75 |
| Vandenplas 1993 | Randomised healthy term infants to whey intermediate hydrolysed formula vs cow's milk formula. Allergy not reported. n = 45 |
| Wopereis 2014 | Infants at high risk of allergy randomised to partially hydrolysed formula containing a specific mixture of oligosaccharides or to standard cow's milk formula for the first 6 months of life. Allergy not reported to date. Published abstract only. n = 108 |
| Yu 2014 | Likely non‐random allocation. Cross‐over design. Enrolled preterm infants. Did not report allergy |
| Zeiger 1989 | Trial in mothers and infants at high risk of atopy of maternal dietary avoidance in pregnancy and lactation, and infant allergen avoidance through encouragement of breast feeding with supplemental or weaning formula use and subsequent dietary restriction vs usual maternal diet and infant feeding with use of supplementary or weaning cow's milk formula. Excluded as multiple co‐interventions and excess losses |
AGA: appropriate for gestational age CM: cow’s milk CMF: cow’s milk formula CMPI: cow’s milk protein intolerance eHF: extensively hydrolysed formula HF: hydrolysed formula PHF: partially hydrolysed formula PHFLGG: partially hydrolysed formula supplemented with LGG RCT: randomised controlled trial SPF: specific pathogen free VLBW: very low birth weight WHF: whey‐based hydrolysed formula
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Tolerance of an Extensively Hydrolyzed Protein Infant Formula Versus a Premature Infant Formula |
| Methods | Randomised controlled trial |
| Participants | n = 60 Inclusion criteria:
Exclusion criteria:
|
| Interventions | Cow's milk‐based premature infant formula Extensively hydrolysed casein infant formula |
| Outcomes | Primary outcome measures: enteral intake Secondary outcome measures: body weight, feeding tolerance, respiratory status, gut Inflammation, confirmed or suspected sepsis or necrotising enterocolitis, date of hospital discharge, growth |
| Starting date | February 2014 |
| Contact information | |
| Notes | ClinicalTrials.gov Identifier: NCT01987154 |
| Trial name or title | Effects on Growth of an Extensively Hydrolyzed Formula Fed to Term |
| Methods | Randomised single‐group controlled trial (unclear) |
| Participants | n = 32 At birth: healthy term (37 to 42 weeks) infant/weight for length between 10th and 90th percentiles according to National Center for Health Statistics (NCHS) growth charts At time of enrolment: ≤ 21 days' postnatal age/weight for length between 10th and 90th percentiles according to National Center for Health Statistics (NCHS) growth charts. Exclusively formula fed. Written informed consent of parent/guardian Exclusion criteria:
|
| Interventions | Extensively hydrolysed infant formula |
| Outcomes | Growth |
| Starting date | July 2007; completion date: December 2008. Not published to date |
| Contact information | |
| Notes | ClinicalTrials.gov Identifier: NCT00936637 |
| Trial name or title | Hydrolized Protein Formula for Premature Infants |
| Methods | Randomised controlled trial |
| Participants | n = 118 Inclusion criteria:
Exclusion criteria:
|
| Interventions | Protein hydrolysed formula Standard premature formula |
| Outcomes | Primary outcome measure: time to achieve full feeds Secondary outcome measures: postnatal days to achieve full feeds |
| Starting date | July 2010; study completion date: September 2015 |
| Contact information | |
| Notes | ClinicalTrials.gov Identifier: NCT01156493 |
| Trial name or title | Early Dietary Intervention and Later Signs of Beta‐Cell Autoimmunity (EDIA) |
| Methods | Randomised controlled trial |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Experimental: extensively hydrolysed casein formula Experimental: cow's milk‐based infant formula |
| Outcomes | Primary outcome measure: intestinal permeability determined at the age of 3, 6, 9 and 12 months with the lactulose/mannitol test Secondary outcome measure: serum metabolic profile Serum metabolic profile will be analysed with metabolomics at the age of 3, 6, 9 and 12 months Other outcome measures: intestinal microbiome |
| Starting date | January 2013 |
| Contact information | Mikael Knip, MD; mikael.knip@helsinki.fi |
| Notes | ClinicalTrials.gov Identifier: NCT01735123 |
| Trial name or title | "Watch Your Baby Grow" Study (GRO) |
| Methods | Randomised controlled trial |
| Participants | n = 144 Inclusion criteria:
Exclusion criteria:
|
| Interventions | Extensively hydrolysed infant formula Standard cow's milk formula during first year of life |
| Outcomes | Primary outcome measures: growth and energy balance Secondary outcome measures: Intake and feeding behaviours, genotype |
| Starting date | October 2012. Estimated completion date: December 2017 |
| Contact information | Julie A. Mennella |
| Notes | ClinicalTrials.gov Identifier: NCT01700205 |
| Trial name or title | Growth of Infants Fed an Extensively Hydrolyzed Infant Formula |
| Methods | Randomised controlled trial |
| Participants | n = 282 Inclusion criteria:
Exclusion criteria:
|
| Interventions | Extensively hydrolysed whey infant formula vs extensively hydrolysed casein infant formula |
| Outcomes | Weight gain to 4 months Mean weight gain (grams/d) from enrolment to 4 months of age |
| Starting date | November 2010; study completion date: July 2013 |
| Contact information | Ricardo Sorensen, MD, Louisiana State University Health Sciences Center in New Orleans |
| Notes | ClinicalTrials.gov Identifier: NCT01210391 |
| Trial name or title | Digestive Tolerance of Slightly Hydrolyzed Starter Infant Formula With Probiotics |
| Methods | Randomised controlled trial |
| Participants | n = 480 Inclusion criteria:
Exclusion criteria:
|
| Interventions | Hydrolysed formula with probiotics Acidified hydrolysed formula Hydrolysed formula without probiotics Standard infant formula |
| Outcomes | Incidence of crying/fussing from 1 to 3 months Growth and night sleep |
| Starting date | December 2009; study completion date: April 2012 |
| Contact information | A/Prof. Boosba Vivatvakin |
| Notes | ClinicalTrials.gov Identifier:NCT01036243 |
| Trial name or title | Application Effect of Extensively Hydrolyzed Milk Protein Formula and Follow‐up in Preterm Children With a Gestational Age of Less Than 34 Weeks |
| Methods | Randomised single‐blind and controlled clinical trial |
| Participants | Preterm children of gestational age less than 34 weeks who cannot be breast fed |
| Interventions | Extensively hydrolysed milk protein (100% whey protein) formula vs preterm children’s formula until discharge from neonatal intensive care unit |
| Outcomes | First endpoint: food intolerance in preterm children. Second endpoint variables include (1) time to achieve full enteral nutrition; (2) time of parenteral nutrition; (3) time of NICU stay; (4) meconium drainage time; (5) daily spontaneous faecal discharge conditions; (6) growth; (7) total protein, albumin, calcium, phosphorus and alkaline phosphatase; and (8) neonatal necrotising enterocolitis, cholestasis, parenteral nutrition associated with cholestasis and congenital hypothyroidism |
| Starting date | 2014 |
| Contact information | Li‐Xing Qia; qiao_lixing@163.com |
| Notes | Chinese Clinical Trial Registry (http://www.chictr.org.cn/); ChiCTR‐IOR‐14005696 |
FiO2: fraction of inspired oxygen GA: gestational age GI: gastrointestinal
Contributions of authors
DO and JS independently performed the literature search, extracted data and checked the accuracy of the review. Both review authors independently performed the literature search, extracted data and checked the accuracy of the updated reviews. LJ reviewed risk of bias and GRADE summary of findings assessments.
Sources of support
Internal sources
No sources of support supplied
External sources
Australian Satellite of the Cochrane Neonatal Review Group, Australia.
-
Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services, USA.
Editorial support of the Cochrane Neonatal Review Group has been funded with Federal funds from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services, USA, under Contract No. HHSN275201600005C
-
National Institute for Health Research, UK.
Editorial support for the Cochrane Neonatal Review Group has been funded with funds from a UK National Institute of Health Research Grant (NIHR) Cochrane Programme Grant (13/89/12). The views expressed in this publication are those of the review authors and are not necessarily those of the NHS, the NIHR or the UK Department of Health
Declarations of interest
DO and JS have been invited speakers at industry‐organised scientific meetings. Neither has accepted an honorarium. LJ has no conflicts of interest to declare.
Notes
This review update (2016) is a substantive update with delayed publication to obtain and clarify data received from study authors.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
- Chirico G, Gasparoni A, Ciardelli L, Amici M, Colombo A, Rondini G. Immunogenicity and antigenicity of a partially hydrolyzed cow's milk infant formula. Allergy 1997;52:82‐8. [DOI] [PubMed] [Google Scholar]
- Seta L, Siani P, Cirillo G, Gruttola M, Cimaduomo L, Coletta S. The prevention of allergic diseases with a hypoallergenic formula: a follow‐up at 24 months. The preliminary results. [Italian]. La Pediatria Medica e Chirurgica: Medical and Surgical Pediatrics 1994;16:251‐4. [PubMed] [Google Scholar]
- Halken S, Hansen KS, Jacobsen HP, Estmann A, Faelling AE, Hansen LG, et al. Comparison of a partially hydrolyzed infant formula with two extensively hydrolyzed formulas for allergy prevention: a prospective, randomized study. Pediatric Allergy and Immunology 2000;11:149‐61. [DOI] [PubMed] [Google Scholar]
- Hill D. A report on the analysis of the Melbourn Atopy Cohort Study. A study designed to test the effectiveness of different formula types on the development of atopic symptoms and signs on a cohort of atopy‐at‐risk infants. Report at year 2. Nestec Internal Report. ; Hill DJ, Sporik R, Thorburn J, Hosking CS. The association of atopic dermatitis in infancy with immunoglobulin E food sensitization. Journal of Pediatrics 2000;137:475‐9. [DOI] [PubMed] [Google Scholar]; Koplin J, Dharmage SC, Gurrin L, Osborne N, Tang ML, Lowe AJ, et al. Soy consumption is not a risk factor for peanut sensitization. Journal of Allergy and Clinical Immunology 2008;121:1455‐9. [DOI] [PubMed] [Google Scholar]; Lowe A, Hosking C, Bennett C, Allen K, Carlin J, Dharmage S, et al. The Melbourne Atopy Cohort Study: a RCT of a partially hydrolysed whey formula for the prevention of allergic disease. Internal Medicine Journal. 2008; Vol. 38:A158. [Google Scholar]; Lowe AJ, Abramson MJ, Hosking CS, Carlin JB, Bennett CM, Dharmage SC, et al. The temporal sequence of allergic sensitization and onset of infantile eczema. Clinical & Experimental Allergy 2007;37:536‐42. [DOI] [PubMed] [Google Scholar]; Lowe AJ, Carlin JB, Bennett CM, Hosking CS, Allen KJ, Robertson CF, et al. Paracetamol use in early life and asthma: prospective birth cohort study. BMJ 2010;341:c4616. [DOI] [PMC free article] [PubMed] [Google Scholar]; Lowe AJ, Hosking CS, Bennett CM, Allen KJ, Axelrad C, Carlin JB, et al. Effect of a partially hydrolyzed whey infant formula at weaning on risk of allergic disease in high‐risk children: a randomized controlled trial. Journal of Allergy and Clinical Immunology 2011;128:360‐5, e4. [DOI] [PubMed] [Google Scholar]; Stoney RM, Woods RK, Hosking CS, Hill DJ, Abramson MJ, Thien FC. Maternal breast milk long‐chain n‐3 fatty acids are associated with increased risk of atopy in breastfed infants. Clinical and Experimental Allergy 2004;34:194‐200. [DOI] [PubMed] [Google Scholar]; Thomson JA, Widjaja C, Darmaputra AA, Lowe A, Matheson MC, Bennett CM, et al. Early childhood infections and immunisation and the development of allergic disease in particular asthma in a high‐risk cohort: a prospective study of allergy‐prone children from birth to six years. Pediatric Allergy and Immunology 2010;21:1076‐85. [DOI] [PubMed] [Google Scholar]
- Juvonen P, Mansson M, Andersson C, Jakobsson I. Allergy development and macromolecular absorption in infants with different feeding regimens during the first three days of life. A three‐year prospective follow‐up. Acta Paediatrica 1996;85:1047‐52. [DOI] [PubMed] [Google Scholar]; Juvonen P, Mansson M, Jakobsson I. Does early diet have an effect on subsequent macromolecular absorption and serum IgE?. Journal of Pediatric Gastroenterology and Nutrition 1994;18:344‐9. [DOI] [PubMed] [Google Scholar]; Juvonen P, Mansson M, Kjellman NI, Bjorksten B, Jakobsson I. Development of immunoglobulin G and immunoglobulin E antibodies to cow's milk proteins and ovalbumin after a temporary neonatal exposure to hydrolyzed and whole cow's milk proteins. Pediatric Allergy and Immunology 1999;10:191‐8. [DOI] [PubMed] [Google Scholar]
- Kwinta P, Sawiec P, Klimek M, Lis G, Cichocka‐Jarosz E, Pietrzyk JJ. Correlation between early neonatal diet and atopic symptoms up to 5‐7 years of age in very low birth weight infants: follow‐up of randomized, double‐blind study. Pediatric Allergy and Immunology 2009;20:458‐66. [DOI] [PubMed] [Google Scholar]
- Lam BCC, Yeung CY. The effect of breast milk, infant formula and hypoallergenic formula on incidence of atopic manifestation in high risk infants. Nestle Internal Report1992.
- Mallet E, Henocq A. Long‐term prevention of allergic diseases by using protein hydrolysate formula in at‐risk infants. Journal of Pediatrics 1992;121:S95‐100. [DOI] [PubMed] [Google Scholar]
- Marini A, Agosti M, Motta G, Mosca F. Effects of a dietary and environmental prevention programme on the incidence of allergic symptoms in high atopic risk infants: three years' follow‐up. Acta Paediatrica (Suppl) 1996;414:1‐21. [DOI] [PubMed] [Google Scholar]
- Nentwich I, Michkova E, Nevoral J, Urbanek R, Szepfalusi Z. Cow's milk‐specific cellular and humoral immune responses and atopy skin symptoms in infants from atopic families fed a partially (pHF) or extensively (eHF) hydrolyzed infant formula. Allergy 2001;56:1144‐56. [DOI] [PubMed] [Google Scholar]
- Oldaeus G, Anjou K, Bjorksten B, Moran JR, Kjellman NI. Extensively and partially hydrolysed infant formulas for allergy prophylaxis. Archives of Disease in Childhood 1997;77:4‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]; Oldaeus G, Bjorksten B, Jenmalm MC, Kjellman NI. Cow's milk IgE and IgG antibody responses to cow's milk formulas. Allergy 1999;54:352‐7. [DOI] [PubMed] [Google Scholar]
- Saarinen KM, Juntunen‐Backman K, Jarvenpaa AL, Klemetti P, Kuitunen P, Lope L, et al. Breast‐feeding and the development of cows' milk protein allergy. Advances in Experimental Medicine and Biology 2000;478:121‐30. [DOI] [PubMed] [Google Scholar]; Saarinen KM, Juntunen‐Backman K, Jarvenpaa AL, Kuitunen P, Lope L, Renlund M, et al. Supplementary feeding in maternity hospitals and the risk of cow's milk allergy: a prospective study of 6209 infants. Journal of Allergy and Clinical Immunology 1999;104:457‐61. [DOI] [PubMed] [Google Scholar]; Saarinen KM, Savilahti E. Infant feeding patterns affect the subsequent immunological features in cow's milk allergy. Clinical and Experimental Allergy 2000;30:400‐6. [DOI] [PubMed] [Google Scholar]; Saarinen KM, Suomalainen H, Savilahti E. Diagnostic value of skin‐prick and patch tests and serum eosinophil cationic protein and cow's milk‐specific IgE in infants with cow's milk allergy. Clinical and Experimental Allergy 2001;31:423‐9. [DOI] [PubMed] [Google Scholar]; Saarinen KM, Vaarala O, Klemetti P, Savilahti E. Transforming growth factor‐beta1 in mothers' colostrum and immune responses to cows' milk proteins in infants with cows' milk allergy. Journal of Allergy and Clinical Immunology 1999;104:1093‐8. [DOI] [PubMed] [Google Scholar]; Savilahti E, Saarinen KM. Early infant feeding and type 1 diabetes. European Journal of Nutrition 2009;48:243‐9. [DOI] [PubMed] [Google Scholar]; Savilahti EM, Saarinen KM, Savilahti E. Specific antibodies to cow's milk proteins in infants: effect of early feeding and diagnosis of cow's milk allergy. European Journal of Nutrition 2010;49:501‐4. [DOI] [PubMed] [Google Scholar]; Siltanen M, Kajosaari M, Poussa T, Saarinen KM, Savilahti E. A dual long‐term effect of breastfeeding on atopy in relation to heredity in children at 4 years of age. Allergy 2003;58:524‐30. [DOI] [PubMed] [Google Scholar]
- Sorensen RU, Inostroza J, Hebal E, Vinet AM, Fierro J. Weaning to a partially hydrolyzed whey formula at three months of age protects against the later development of anti‐cow milk lgE antibodies in comparison to whole cows milk or extensively hydrolyzed whey formulas. Journal of Allergy and Clinical Immunology. 2007; Vol. 119:S120. [Google Scholar]
- Tsai YT, Chou CC, Hsieh KH. The effect of hypoallergenic formula on the occurrence of allergic diseases in high risk infants. Zhonghua Min Guo Xiao Er Ke Yi Xue Hui Za Zhi 1991;32:137‐44. [PubMed] [Google Scholar]
- Vandenplas Y. Atopy at 3 years in high‐risk infants fed whey hydrolysate or conventional formula. Lancet 1992;339:1118. [DOI] [PubMed] [Google Scholar]; Vandenplas Y, Hauser B, Borre C, Clybouw C, Mahler T, Hachimi‐Idrissi S, et al. The long‐term effect of a partial whey hydrolysate formula on the prophylaxis of atopic disease. European Journal of Pediatrics 1995;154:488‐94. [DOI] [PubMed] [Google Scholar]; Vandenplas Y, Hauser B, Borre C, Sacre L, Dab I. Effect of a whey hydrolysate prophylaxis of atopic disease. Annals of Allergy 1992;68:419‐24. [PubMed] [Google Scholar]
- Berg A, Kramer U, Link E, Bollrath C, Heinrich J, Brockow I, et al. Impact of early feeding on childhood eczema: development after nutritional intervention compared with the natural course ‐ the GINIplus study up to the age of 6 years. Clinical & Experimental Allergy 2010;40:627‐36. [DOI] [PubMed] [Google Scholar]; Brockow I, Zutavern A, Hoffmann U, Grubl A, Berg A, Koletzko S, et al. Early allergic sensitizations and their relevance to atopic diseases in children aged 6 years: results of the GINI study. Journal of Investigative Allergology and Clinical Immunology 2009;19:180‐7. [PubMed] [Google Scholar]; Laubereau B, Brockow I, Zirngibl A, Koletzko S, Gruebl A, Berg A, et al. Effect of breast‐feeding on the development of atopic dermatitis during the first 3 years of life ‐ results from the GINI‐birth cohort study. Journal of Pediatrics 2004;144:602‐7. [DOI] [PubMed] [Google Scholar]; Laubereau B, Filipiak‐Pittroff B, Berg A, Grubl A, Reinhardt D, Wichmann HE, et al. Caesarean section and gastrointestinal symptoms, atopic dermatitis, and sensitisation during the first year of life. Archives of Disease in Childhood 2004;89:993‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]; Long‐term effect of cow milk protein hydrolysates in high‐risk children. Results from the 10‐years follow‐up of the GINI study. Allergy: European Journal of Allergy and Clinical Immunology 2013;68:295‐6. [DOI] [PubMed] [Google Scholar]; Rzehak P, Sausenthaler S, Koletzko S, Bauer CP, Schaaf B, Berg A, et al. Period‐specific growth, overweight and modification by breastfeeding in the GINI and LISA birth cohorts up to age 6 years. European Journal of Epidemiology 2009;24:449‐67. [DOI] [PubMed] [Google Scholar]; Rzehak P, Sausenthaler S, Koletzko S, Reinhardt D, Berg A, Kramer U, et al. Long‐term effects of hydrolyzed protein infant formulas on growth ‐ extended follow‐up to 10 y of age: results from the German Infant Nutritional Intervention (GINI) study. American Journal of Clinical Nutrition 2011;94:1803S‐7S. [DOI] [PubMed] [Google Scholar]; Rzehak P, Sausenthaler S, Koletzko S, Reinhardt D, Berg A, Kramer U, et al. Short‐ and long‐term effects of feeding hydrolyzed protein infant formulas on growth. American Journal of Clinical Nutrition 2009;89:1846‐56. [DOI] [PubMed] [Google Scholar]; Sausenthaler S, Koletzko S, Koletzko B, Reinhardt D, Kramer U, Berg A, et al. Effect of hydrolysed formula feeding on taste preferences at 10 years. Data from the German Infant Nutritional Intervention Program Plus Study. Clinical Nutrition 2010;29:304‐6. [DOI] [PubMed] [Google Scholar]; Schoetzau A, Filipiak‐Pittroff B, Franke K, Koletzko S, Berg A, Gruebl A, et al. Effect of exclusive breast‐feeding and early solid food avoidance on the incidence of atopic dermatitis in high‐risk infants at 1 year of age. Pediatric Allergy and Immunology 2002;13:234‐42. [DOI] [PubMed] [Google Scholar]; Schoetzau A, Gehring U, Franke K, Grubl A, Koletzko S, Berg A, et al. Maternal compliance with nutritional recommendations in an allergy preventive programme. Archives of Disease in Childhood 2002;86:180‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]; Berg A, Filipiak‐Pittroff B, Krämer U, Link E, Heinrich J, Koletzko S, et al. The German Infant Nutritional Intervention Study (GINI) for the preventive effect of hydrolysed infant formulas in infants at high risk for allergic diseases. Design and selected results. Allergologie 2012;35:32‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]; Berg A, Filipiak‐Pittroff B, Schulz H, Hoffmann U, Link E, Sussmann M, et al. Allergic manifestation 15 years after early intervention with hydrolyzed formulas ‐ the GINI study. Allergy: European Journal of Allergy and Clinical Immunology 2016;71:210‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]; Berg A, Filipiak‐Pittroff B, Kramer U, Hoffmann B, Link E, Beckmann C, et al. Allergies in high‐risk schoolchildren after early intervention with cow's milk protein hydrolysates: 10‐year results from the German Infant Nutritional Intervention (GINI) study. Journal of Allergy and Clinical Immunology 2013;131:1565‐73. [DOI] [PubMed] [Google Scholar]; Berg A, Filipiak‐Pittroff B, Kramer U, Link E, Bollrath C, Brockow I, et al. Preventive effect of hydrolyzed infant formulas persists until age 6 years: long‐term results from the German Infant Nutritional Intervention Study (GINI). Journal of Allergy and Clinical Immunology 2008;121:1442‐7. [DOI] [PubMed] [Google Scholar]; Berg A, Koletzko S, Filipiak‐Pittroff B, Laubereau B, Grubl A, Wichmann HE, et al. Certain hydrolyzed formulas reduce the incidence of atopic dermatitis but not that of asthma: three‐year results of the German Infant Nutritional Intervention Study. Journal of Allergy and Clinical Immunology 2007;119:718‐25. [DOI] [PubMed] [Google Scholar]; Berg A, Koletzko S, Grubl A, Filipiak‐Pittroff B, Wichmann HE, Bauer CP, et al. The effect of hydrolyzed cow's milk formula for allergy prevention in the first year of life: the German Infant Nutritional Intervention Study, a randomized double‐blind trial. Journal of Allergy and Clinical Immunology 2003;111:533‐40. [DOI] [PubMed] [Google Scholar]
- Willems R, Duchateau J, Magrez P, Denis R, Casimir G. Influence of hypoallergenic milk formula on the incidence of early allergic manifestations in infants predisposed to atopic diseases. Annals of Allergy 1993;71:147‐50. [PubMed] [Google Scholar]
References to studies excluded from this review
- Agosti M, Pugni L, Ramenghi LA, Mosca F, Marini A. Hydrolysed proteins in preterm formula: influence on plasma aminoacids, blood fatty acids and insulinaemia. Acta Paediatrica Supplement 2003;91:34‐8. [DOI] [PubMed] [Google Scholar]
- Akimoto K, Saito H, Akasawa A, Iikura Y. [Preventative effect of a whey hydrolyzed formula (Nestle, NAN H.A.) on the development of allergic symptoms in infants]. [Japanese]. Arerugi ‐ Japanese Journal of Allergology 1997;46:1044‐51. [PubMed] [Google Scholar]
- Arikan D, Alp H, Gozum S, Orbak Z, Cifci EK. Effectiveness of massage, sucrose solution, herbal tea or hydrolysed formula in the treatment of infantile colic. Journal of Clinical Nursing 2008;17:1754‐61. [DOI] [PubMed] [Google Scholar]
- Arshad SH, Bateman B, Matthews SM. Primary prevention of asthma and atopy during childhood by allergen avoidance in infancy: a randomised controlled study. Thorax 2003;58:489‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]; Arshad SH, Matthews S, Gant C, Hide DW. Effect of allergen avoidance on development of allergic disorders in infancy. Lancet 1992;339:1493‐7. [DOI] [PubMed] [Google Scholar]; Hide DW, Matthews S, Matthews L, Stevens M, Ridout S, Twiselton R, et al. Effect of allergen avoidance in infancy on allergic manifestations at age two years. Journal of Allergy and Clinical Immunology 1994;93:842‐6. [DOI] [PubMed] [Google Scholar]; Hide DW, Matthews S, Tariq S, Arshad SH. Allergen avoidance in infancy and allergy at 4 years of age. Allergy 1996;51:89‐93. [PubMed] [Google Scholar]; Scott M, Kurukulaaratchy R, Roberts G, Clayton B, Matthews S, Arshad S. Primary prevention of asthma, outcomes at 17 year follow up. Allergy 2010;65; S92:315. [Google Scholar]; Scott M, Roberts G, Kurukulaaratchy R, Clayton B, Matthews S, Arshad S. Primary prevention of eczema, outcomes at 17 year follow up. Allergy 2010;65; S92:314. [Google Scholar]; Scott M, Roberts G, Kurukulaaratchy RJ, Matthews S, Nove A, Arshad SH. Multifaceted allergen avoidance during infancy reduces asthma during childhood with the effect persisting until age 18 years. Thorax 2012;67:1046‐51. [DOI] [PubMed] [Google Scholar]
- Barberi I, Salpietro DC, Catalioto G, Fulia F. Clinical trial of a new hypoallergenic formula for risk infants. 2nd World Congress of Perinatal Medicine. 1993. [Google Scholar]
- Bergmann RL, Dannemann A, Edenharter G, Bergmann KE, Monch E, Dudenhausen JW, et al. Influence of ultrafiltrated, partially hydrolysed formula on growth and health of young infants. [German]. Monatsschrift fur Kinderheilkunde 1996;144:152‐8. [Google Scholar]
- Berseth CL, Mitmesser SH, Ziegler EE, Marunycz JD, Vanderhoof J. Tolerance of a standard intact protein formula versus a partially hydrolyzed formula in healthy, term infants. Nutrition Journal 2009;8:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borschel MW, Ziegler EE, Wedig RT, Oliver JS. Growth of healthy term infants fed an extensively hydrolyzed casein‐based or free amino acid‐based infant formula: a randomized, double‐blind, controlled trial. Clinical Pediatrics (Phila) 2013;52:910‐7. [DOI] [PubMed] [Google Scholar]
- Borschel MW, Choe YS, Kajzer JA. Growth of healthy term infants fed partially hydrolyzed whey‐based infant formula: a randomized, blinded, controlled trial. Clinical Pediatrics (Phila) 2014;53:1375‐82. [DOI] [PubMed] [Google Scholar]
- Borschel MW, Baggs GE, Barrett‐Reis B. Growth of healthy term infants fed ready‐to‐feed and powdered forms of an extensively hydrolyzed casein‐based infant formula: a randomized, blinded, controlled trial. Clinical Pediatrics (Phila) 2014;53:585‐92. [DOI] [PubMed] [Google Scholar]
- Boyle RJ, Tang MLK, Chiang WC, Chua MC, Ismail I, Nauta A, et al. Prebiotic‐supplemented partially hydrolysed cow's milk formula for the prevention of eczema in high‐risk infants: a randomized controlled trial. Allergy: European Journal of Allergy and Clinical Immunology 2016;71:701‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burks W, Jones SM, Berseth CL, Harris C, Sampson HA, Scalabrin DM. Hypoallergenicity and effects on growth and tolerance of a new amino acid‐based formula with docosahexaenoic acid and arachidonic acid. Journal of Pediatrics 2008;153:266‐71. [DOI] [PubMed] [Google Scholar]
- Campbell JP. Dietary therapy of infant colic: a double‐blind study. [Czech]. Ceskoslovenska Pediatrie 1993;48:199‐202. [PubMed] [Google Scholar]; Campbell JP. Dietary treatment of infant colic: a double‐blind study. Journal of the Royal College of General Practitioners 1989;39:11‐4. [PMC free article] [PubMed] [Google Scholar]
- Chan YH, Shek LP, Aw M, Quak SH, Lee BW. Use of hypoallergenic formula in the prevention of atopic disease among Asian children. Journal of Paediatrics and Child Health 2002;38:84‐8. [DOI] [PubMed] [Google Scholar]
- Chandra RK, Puri S, Hamed A. Influence of maternal diet during lactation and use of formula feeds on development of atopic eczema in high risk infants. BMJ 1989;299:228‐30. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Chandra RK. Five‐year follow‐up of high‐risk infants with family history of allergy who were exclusively breast‐fed or fed partial whey hydrolysate, soy, and conventional cow's milk formulas. Journal of Pediatric Gastroenterology and Nutrition 1997;24:380‐8. [DOI] [PubMed] [Google Scholar]; Chandra RK, Hamed A. Cumulative incidence of atopic disorders in high risk infants fed whey hydrolysate, soy, and conventional cow milk formulas. Annals of Allergy 1991;67:129‐32. [PubMed] [Google Scholar]; Chandra RK, Singh G, Shridhara B. Effect of feeding whey hydrolysate, soy and conventional cow milk formulas on incidence of atopic disease in high risk infants. Annals of Allergy 1989;63:102‐6. [PubMed] [Google Scholar]
- Becker A, Watson W, Ferguson A, Dimich‐Ward H, Chan‐Yeung M. The Canadian asthma primary prevention study: outcomes at 2 years of age. Journal of Allergy and Clinical Immunology 2004;113:650‐6. [DOI] [PubMed] [Google Scholar]; Chan‐Yeung M, Manfreda J, Dimich‐Ward H, Ferguson A, Watson W, Becker A. A randomized controlled study on the effectiveness of a multifaceted intervention program in the primary prevention of asthma in high‐risk infants. Archives of Pediatrics and Adolescent Medicine 2000;154:657‐63. [DOI] [PubMed] [Google Scholar]
- Corvaglia L, Mariani E, Aceti A, Galletti S, Faldella G. Extensively hydrolyzed protein formula reduces acid gastro‐esophageal reflux in symptomatic preterm infants. Early Human Development 2013;89:453‐5. [DOI] [PubMed] [Google Scholar]
- Corvaglia L, Mariani E, Aceti A, Galletti S, Faldella G. Extensively hydrolyzed protein formula reduces acid gastro‐esophageal reflux in symptomatic preterm infants. Early Human Development 2013;89:453‐5. [DOI] [PubMed] [Google Scholar]
- D'Agata A, Betta P, Sciacca P, Morano C, Pratico G, Curreri R, et al. Role of dietary prevention in newborns at risk for atopy. Results of a follow‐up study. Pediatria Medica e Chirurgica 1996;18:469‐72. [PubMed] [Google Scholar]
- Decsi T, Volker V, Szasz M, Ezer E, Mehes K. [Comparison of human milk with 3 different types of infant food in the nutrition of full‐term neonates]. Orvosi Hetilap 1992;133:2087‐91. [PubMed] [Google Scholar]
- Decsi T, Veitl V, Burus I. Plasma amino acid concentrations, indexes of protein metabolism and growth in healthy, full‐term infants fed partially hydrolyzed infant formula. Journal of Pediatric Gastroenterology and Nutrition 1998;27:12‐6. [DOI] [PubMed] [Google Scholar]
- Jong MH, Scharp‐Van Der Linden VT, Aalberse R, Heymans HS, Brunekreef B. The effect of brief neonatal exposure to cows' milk on atopic symptoms up to age 5. Archives of Disease in Childhood. 2002;86:365‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]; Jong MH, Scharp‐van der Linden VT, Aalberse RC, Oosting J, Tijssen JG, Groot CJ. Randomised controlled trial of brief neonatal exposure to cows' milk on the development of atopy. Archives of Disease in Childhood. 1998;79:126‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exl BM, Deland U, Secretin MC, Preysch U, Wall M, Shmerling DH. Improved general health status in an unselected infant population following an allergen reduced dietary intervention programme. The ZUFF‐study‐programme. Part I: study design and 6‐month nutritional behaviour. European Journal of Nutrition 2000;39:89‐102. [DOI] [PubMed] [Google Scholar]; Exl BM, Deland U, Secretin MC, Preysch U, Wall M, Shmerling DH. Improved general health status in an unselected infant population following an allergen‐reduced dietary intervention programme: the ZUFF‐STUDY‐PROGRAMME. Part II: infant growth and health status to age 6 months. European Journal of Nutrition 2000;39:145‐56. [DOI] [PubMed] [Google Scholar]; Exl BM, Deland U, Wall M, Preysch U, Secretin MC, Shmerling DH. Zug‐Frauenfeld Nutritional Survey ('Zuff Study'): allergen‐reduced nutrition in a normal infant population and its health‐related effects. Results at the age of six months. Nutrition Research 1998;18:1443‐62. [Google Scholar]
- Florendo KN, Bellflower B, Zwol A, Cooke RJ. Growth in preterm infants fed either a partially hydrolyzed whey or an intact casein/whey preterm infant formula. Journal of Perinatology 2009;29:106‐11. [DOI] [PubMed] [Google Scholar]
- Fukushima Y, Iwamoto K, Takeuchi‐Nakashima A, Akamatsu N, Fujino‐Numata N, Yoshikoshi M, et al. Preventive effect of whey hydrolysate formulas for mothers and infants against allergy development in infants for the first 2 years. Journal of Nutritional Science and Vitaminology 1997;43:397‐411. [DOI] [PubMed] [Google Scholar]
- Giovannini M, Agostoni C, Fiocchi A, Bellu R, Trojan S, Riva E. Antigen‐reduced infant formulas versus human milk: growth and metabolic parameters in the first 6 months of life. Journal of the American College of Nutrition 1994;13:357‐63. [DOI] [PubMed] [Google Scholar]
- Halken S, Host A, Hansen LG, Osterballe O. Effect of an allergy prevention programme on incidence of atopic symptoms in infancy. A prospective study of 159 "high‐risk" infants. Allergy 1992;47:545‐53. [DOI] [PubMed] [Google Scholar]; Halken S, Host A, Hansen LG, Osterballe O. Prevention of allergy in infants. A prospective study of 159 high‐risk children [German]. Ugeskrift for Laeger 1994;156:308‐12. [PubMed] [Google Scholar]; Halken S, Host A, Hansen LG, Osterballe O. Preventive effect of feeding high‐risk infants a casein hydrolysate formula or an ultrafiltrated whey hydrolysate formula. A prospective, randomized, comparative clinical study. Pediatric Allergy and Immunology 1993;4:173‐81. [DOI] [PubMed] [Google Scholar]
- Hartman CT, Fredericks GL, Katz ES, Brown CA. Prevalence of symptomatic formula intolerance and allergy in a general infant population. Journal of Allergy and Clinical Immunology. 1994; Vol. 93:210 (A286). [Google Scholar]
- Hattevig G, Kjellman B, Sigurs N, Bjorksten B, Kjellman NI. Effect of maternal avoidance of eggs, cow's milk and fish during lactation upon allergic manifestations in infants. Clinical and Experimental Allergy 1989;19:27‐32. [DOI] [PubMed] [Google Scholar]; Hattevig G, Kjellman B, Sigurs N, Grodzinsky E, Hed J, Bjorksten B. The effect of maternal avoidance of eggs, cow's milk, and fish during lactation on the development of IgE, IgG, and IgA antibodies in infants. Journal of Allergy and Clinical Immunology 1990;85:108‐15. [DOI] [PubMed] [Google Scholar]; Hattevig G, Sigurs N, Kjellman B. Effects of maternal dietary avoidance during lactation on allergy in children at 10 years of age. Acta Paediatrica 1999;88:7‐12. [PubMed] [Google Scholar]; Paronen J, Bjorksten B, Hattevig G, Akerblom HK, Vaarala O. Effect of maternal diet during lactation on development of bovine insulin‐binding antibodies in children at risk for allergy. Journal of Allergy and Clinical Immunology 2000;106:302‐6. [DOI] [PubMed] [Google Scholar]; Sigurs N, Hattevig G, Kjellman B. Maternal avoidance of eggs, cow's milk, and fish during lactation: effect on allergic manifestations, skin‐prick tests, and specific IgE antibodies in children at age 4 years. Pediatrics 1992;89:735‐9. [PubMed] [Google Scholar]
- Hernell O, Lonnerdal B. Nutritional evaluation of protein hydrolysate formulas in healthy term infants: plasma amino acids, hematology, and trace elements. American Journal of Clinical Nutrition 2003;78:296‐301. [DOI] [PubMed] [Google Scholar]
- Hill DJ, Hudson IL, Sheffield LJ, Shelton MJ, Menahem S, Hosking CS. A low allergen diet is a significant intervention in infantile colic: results of a community‐based study. Journal of Allergy and Clinical Immunology 1995;96:886‐92. [DOI] [PubMed] [Google Scholar]
- Host A. Importance of the first meal on the development of cow's milk allergy and intolerance. Allergy Proceedings 1991;12:227‐32. [DOI] [PubMed] [Google Scholar]
- Iikura Y, Akimoto K, Ebisawa M, Onda T, Akazawa A, Saito H, et al. Effect of hydrolyzed whey protein formula for babies on development of allergic symptoms during infancy. Nestle Nutrition Workshop Intestinal Immunology and Food Allergy. Vol. 34, Baltimore, Maryland: Raven Press, 1995:269‐75. [Google Scholar]
- Keller KM, Burgin‐Wolff A, Lippold R, Wirth S, Lentze MJ. The diagnostic significance of IgG cow's milk protein antibodies re‐evaluated. European Journal of Pediatrics 1996;155:331‐7. [DOI] [PubMed] [Google Scholar]
- Kuo HC, Liu CA, Ou CY, Hsu TY, Wang CL, Huang HC, et al. Partial protein‐hydrolyzed infant formula decreased food sensitization but not allergic diseases in a prospective birth cohort study. International Archives of Allergy and Immunology 2011;154:310‐7. [DOI] [PubMed] [Google Scholar]
- Lasekan JB, Koo WW, Walters J, Neylan M, Luebbers S. Growth, tolerance and biochemical measures in healthy infants fed a partially hydrolyzed rice protein‐based formula: a randomized, blinded, prospective trial. Journal of the American College of Nutrition 2006;25:12‐9. [DOI] [PubMed] [Google Scholar]
- Lucassen PL, Assendelft WJ, Gubbels JW, Eijk JT, Douwes AC. Infantile colic: crying time reduction with a whey hydrolysate. A double‐blind, randomized, placebo‐controlled trial. Pediatrics 2000;106:1349‐54. [DOI] [PubMed] [Google Scholar]
- Maggio L, Zuppa AA, Sawatzki G, Valsasina R, Schubert W, Tortorolo G. Higher urinary excretion of essential amino acids in preterm infants fed protein hydrolysates. Acta Paediatrica 2005;94:75‐84. [DOI] [PubMed] [Google Scholar]; Zuppa AA, Girlando P, Scapillati ME, Maggio L, Romagnoli C, Tortorolo G. [Effects on growth, tolerability and biochemical parameters of two different human milk fortifiers in very low birth‐weight newborns]. Pediatria Medica e Chirurgica 2004;26:45‐9. [PubMed] [Google Scholar]
- Martinenez‐Valverde A, Aljma‐Garcia JM. Study and evaluation of serum IgE and specific total IgE against alpha‐lactalbumin, beta‐lactoglobulin and casein with hypoallergenic formulas (HA) and other types of feeding (Spanish). Thesis, Universidad de Malaga, Spain1993.
- Medjad‐Guillou N, Henocq A, Arnaud‐Battandier F. Does the hydrolysis of proteins change the acceptability and the digestive tolerance of milk for infants? The results of a comparative and randomized prospective study. [French]. Annales de Pediatrie 1992;39:202‐6. [PubMed] [Google Scholar]
- Mennella JA, Castor SM. Sensitive period in flavor learning: effects of duration of exposure to formula flavors on food likes during infancy. Clinical Nutrition 2012;31:1022‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]; Mennella JA, Forestell CA, Morgan LK, Beauchamp GK. Early milk feeding influences taste acceptance and liking during infancy. American Journal of Clinical Nutrition 2009;90:780S‐8S. [DOI] [PMC free article] [PubMed] [Google Scholar]; Mennella JA, Lukasewycz LD, Castor SM, Beauchamp GK. The timing and duration of a sensitive period in human flavor learning: a randomized trial. American Journal of Clinical Nutrition 2011;93:1019‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]; Mennella JA, Trabulsi JC, Papas MA. Effects of cow milk versus extensive protein hydrolysate formulas on infant cognitive development. Amino Acids 2016;48:697‐705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihatsch WA, Hogel J, Pohlandt F. Hydrolysed protein accelerates the gastrointestinal transport of formula in preterm infants. Acta Paediatrica 2001;90:196‐8. [DOI] [PubMed] [Google Scholar]; Mihatsch WA, Pohlandt F. Protein hydrolysate formula maintains homeostasis of plasma amino acids in preterm infants. Journal of Pediatric Gastroenterology and Nutrition 1999;29:406‐10. [DOI] [PubMed] [Google Scholar]
- Mihatsch WA, Franz AR, Hogel J, Pohlandt F. Hydrolyzed protein accelerates feeding advancement in very low birth weight infants. Pediatrics 2002;110:1199‐203. [DOI] [PubMed] [Google Scholar]
- Mitchell JD, Brand J, Halbisch J. Weight‐gain inhibition by lactose in Australian Aboriginal children. A controlled trial of normal and lactose hydrolysed milk. Lancet 1977;1:500‐2. [DOI] [PubMed] [Google Scholar]
- Moran JR. Effects of prolonged exposure to partially hydrolyzed milk protein. Journal of Pediatrics 1992;121:S90‐4. [DOI] [PubMed] [Google Scholar]
- Nentwich I, Pazdirkova A, Koberska I, Pokojska E, Szepfalusi Z, Lokaj J. Cow milk‐specific humoral and cellular immune response in infants with high risk of atopy under feeding a whey hydrolysate infant formula. Klinische Padiatrie 2003;215:275‐9. [DOI] [PubMed] [Google Scholar]
- Nocerino R, Marco G, Cosenza L, Pezzella V, Passariello A, Terrin G, et al. Efficacy of a new partially hydrolyzed whey formula on infant colic: a randomized controlled trial. Digestive and Liver Disease 2012;44:S272. [Google Scholar]
- Odelram H, Vanto T, Jacobsen L, Kjellman NI. Whey hydrolysate compared with cow's milk‐based formula for weaning at about 6 months of age in high allergy‐risk infants: effects on atopic disease and sensitization. Allergy 1996;51:192‐5. [PubMed] [Google Scholar]
- Paronen J, Knip M, Savilahti E, Virtanen SM, Ilonen J, Akerblom HK, et al. Effect of cow's milk exposure and maternal type 1 diabetes on cellular and humoral immunization to dietary insulin in infants at genetic risk for type 1 diabetes. Finnish Trial to Reduce IDDM in the Genetically at Risk Study Group. Diabetes 2000;49:1657‐65. [DOI] [PubMed] [Google Scholar]
- Pauls J, Bauer K, Versmold H. Randomized controlled trial of formulas with hydrolyzed versus non‐hydrolyzed protein for starting enteral feedings in preterm infants <1500g body weight. Journal of Pediatric Gastroenterology and Nutrition 1996;22:450 (abstract 164). [Google Scholar]
- Picaud JC, Lapillonne A, Rigo J, Normand S, Reygrobellet B, Claris O, et al. Nitrogen utilization and bone mineralization in very low birth weight infants fed partially hydrolyzed preterm formula. Seminars in Perinatology 2002;26:439‐46. [DOI] [PubMed] [Google Scholar]; Picaud JC, Rigo J, Normand S, Lapillonne A, Reygrobellet B, Claris O, et al. Nutritional efficacy of preterm formula with a partially hydrolyzed protein source: a randomized pilot study. Journal of Pediatric Gastroenterology and Nutrition 2001;32:555‐61. [DOI] [PubMed] [Google Scholar]
- Porch MC, Shahane AD, Leiva LE, Elston RC, Sorensen RU. Influence of breast milk, soy or two hydrolyzed formulas on the development of allergic manifestations in infants at risk. Nutrition Research 1998;18:1413‐24. [Google Scholar]
- Raupp P, Kries R, Gobel C, Fox A, Lemburg P, Schmidt E, et al. Bone density and biochemical parameters of bone mineral metabolism and acid load in preterm infants. Cow's milk formula versus hydrolysed protein preparation. Monatsschr Kinderheilkd 1995;143(S2):S140‐6. [Google Scholar]
- Riezzo G, Indrio F, Montagna O, Tripaldi C, Laforgia N, Chiloiro M, et al. Gastric electrical activity and gastric emptying in preterm newborns fed standard and hydrolysate formulas. Journal of Pediatric Gastroenterology and Nutrition 2001;33:290‐5. [DOI] [PubMed] [Google Scholar]
- Rigo J, Salle BL, Picaud JC, Putet G, Senterre J. Nutritional evaluation of protein hydrolysate formulas. European Journal of Clinical Nutrition 1995;49(Suppl 1):S26‐38. [PubMed] [Google Scholar]; Rigo J, Salle BL, Putet G, Senterre J. Nutritional evaluation of various protein hydrolysate formulae in term infants during the first month of life. Acta Paediatrica Supplement 1994;402:100‐4. [DOI] [PubMed] [Google Scholar]
- Rigo J, Senterre J. Metabolic balance studies and plasma amino acid concentrations in preterm infants fed experimental protein hydrolysate preterm formulas. Acta Paediatrica Supplement 1994;405:98‐104. [DOI] [PubMed] [Google Scholar]
- Savino F, Cresi F, Maccario S, Cavallo F, Dalmasso P, Fanaro S, et al. "Minor" feeding problems during the first months of life: effect of a partially hydrolysed milk formula containing fructo‐ and galacto‐oligosaccharides. Acta Paediatrica Supplement 2003;91:86‐90. [DOI] [PubMed] [Google Scholar]
- Savino F, Palumeri E, Castagno E, Cresi F, Dalmasso P, Cavallo F, et al. Reduction of crying episodes owing to infantile colic: a randomized controlled study on the efficacy of a new infant formula. European Journal of Clinical Nutrition 2006;60:1304‐10. [DOI] [PubMed] [Google Scholar]
- Scalabrin D, Berseth C, Marunycz J, Mitmesser S. Long term safety, growth, and health effects of lactobacillus GG (LGG) added to partially and extensively hydrolyzed infant formulas. Allergy: European Journal of Allergy and Clinical Immunology 2009;64:447. [Google Scholar]; Scalabrin DM, Johnston WH, Hoffman DR, P'Pool VL, Harris CL, Mitmesser SH. Growth and tolerance of healthy term infants receiving hydrolyzed infant formulas supplemented with Lactobacillus rhamnosus GG: randomized, double‐blind, controlled trial. Clinical Pediatrics 2009;48:734‐44. [DOI] [PubMed] [Google Scholar]; Scalabrin DMF, Harris C, Strong PV, Liu B, Berseth CL. Infant supplementation with Lactobacillus rhamnosus GG (LGG) and long‐term growth and health: a 5‐year follow‐up. Allergy: European Journal of Allergy and Clinical Immunology 2014;69:196‐7. [Google Scholar]
- Schmelzle H, Wirth S, Skopnik H, Radke M, Knol J, Bockler HM, et al. Randomized double‐blind study of the nutritional efficacy and bifidogenicity of a new infant formula containing partially hydrolyzed protein, a high beta‐palmitic acid level, and nondigestible oligosaccharides. Journal of Pediatric Gastroenterology and Nutrition 2003;36:343‐51. [DOI] [PubMed] [Google Scholar]
- Schmidt E, Eden‐Kohler J, Tonkaboni F, Tolle J. Alimentary allergy prevention in infants with increased allergic risk: the effect of different feeding regimens in the first 6 months on atopic manifestations during the first year of life. A large scale feeding trial. Nestle Nutritional Workshop: Intestinal Immunology and Food Allergy. Vol. 34, Baltimore, Maryland: Raven Press, 1995:231‐48. [Google Scholar]
- Schmitz J, Digeon B, Chastang C, Dupouy D, Leroux B, Robillard P, et al. Effects of brief early exposure to partially hydrolyzed and whole cow milk proteins. Journal of Pediatrics 1992;121:S85‐9. [DOI] [PubMed] [Google Scholar]
- Schrander JJ, Bogart JP, Forget PP, Schrander‐Stumpel CT, Kuijten RH, Kester AD. Cow's milk protein intolerance in infants under 1 year of age: a prospective epidemiological study. European Journal of Pediatrics 1993;152:640‐4. [DOI] [PubMed] [Google Scholar]
- Shao J, Sheng J, Dong W, Li YZ, Yu SC. Effects of feeding intervention on development of eczema in atopy high‐risk infants: an 18‐month follow‐up study. Zhonghua Er Ke Za Zhi 2006;44:684‐7. [PubMed] [Google Scholar]
- Silva Rey AL, Garcia G, Nogales A. Preventatibve effect of a partially hydrolyzed milk formula. Thesis, Madrid, Spain.1996.
- Staelens S, Driessche M, Barclay D, Carrie‐Faessler AL, Haschke F, Verbeke K, et al. Gastric emptying in healthy newborns fed an intact protein formula, a partially and an extensively hydrolysed formula. Clinical Nutrition 2008;27:264‐8. [DOI] [PubMed] [Google Scholar]
- Szajewska H, Albrecht P, Stoinska B, Prochowska A, Gawecka A, Laskowska‐Klita T. Extensive and partial protein hydrolysate preterm formulas: the effect on growth rate, protein metabolism indices, and plasma amino acid concentrations. Journal of Pediatric Gastroenterology and Nutrition 2001;32:303‐9. [DOI] [PubMed] [Google Scholar]
- Szajewska H, Mrukowicz JZ, Stoinska B, Prochowska A. Extensively and partially hydrolysed preterm formulas in the prevention of allergic diseases in preterm infants: a randomized, double‐blind trial. Acta Paediatrica 2004;93:1159‐65. [PubMed] [Google Scholar]
- Tariq SM, Matthews SM, Hakim EA, Stevens M, Arshad SH, Hide DW. The prevalence of and risk factors for atopy in early childhood: a whole population birth cohort study. Journal of Allergy and Clinical Immunology 1998;101:587‐93. [DOI] [PubMed] [Google Scholar]
- Taubman B. Parental counseling compared with elimination of cow's milk or soy milk protein for the treatment of infant colic syndrome: a randomized trial. Pediatrics 1988;81:756‐61. [PubMed] [Google Scholar]
- Akerblom HK, Krischer J, Virtanen SM, Berseth C, Becker D, Dupre J, et al. The Trial to Reduce IDDM in the Genetically at Risk (TRIGR) study: recruitment, intervention and follow‐up. Diabetologia 2011;54:627‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]; Knip M, Akerblom HK, Becker D, Dosch HM, Dupre J, Fraser W, et al. Hydrolyzed infant formula and early beta‐cell autoimmunity: a randomized clinical trial. JAMA 2014;311:2279‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]; Knip M, Virtanen SM, Becker D, Dupre J, Krischer JP, Akerblom HK. Early feeding and risk of type 1 diabetes: experiences from the Trial to Reduce Insulin‐dependent diabetes mellitus in the Genetically at Risk (TRIGR). American Journal of Clinical Nutrition 2011;94:1814S‐20S. [DOI] [PMC free article] [PubMed] [Google Scholar]; Knip M, Virtanen SM, Seppa K, Ilonen J, Savilahti E, Vaarala O, et al. Dietary intervention in infancy and later signs of beta‐cell autoimmunity. New England Journal of Medicine 2010;363.:1900‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]; Krischer JP. Hydrolyzed infant formula and early beta‐cell autoimmunity. Diabetes 2014;63:A7. [DOI] [PMC free article] [PubMed] [Google Scholar]; TRIGR Study Group. TRIGR ‐ Primary Prevention Study for Type 1 Diabetes in Children at Risk. ClinicalTrials.gov Identifier: NCT001797772005. ; Virtanen SM, Barlund S, Salonen M, Savilahti E, Reunanen A, Paronen J, et al. Feasibility and compliance in a nutritional primary prevention trial in infants at increased risk for type 1 diabetes. Acta Paediatrica 2011;100:557‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paronen J, Vaarala O, Savilahti E, Saukkonen T, Akerblom HK. Soluble adhesion molecules and oral antigen feeding in infants. Pediatric Research 1996;40:276‐9. [DOI] [PubMed] [Google Scholar]; Vaarala O, Saukkonen T, Savilahti E, Klemola T, Akerblom HK. Development of immune response to cow's milk proteins in infants receiving cow's milk or hydrolyzed formula. Journal of Allergy and Clinical Immunology 1995;96:917‐23. [DOI] [PubMed] [Google Scholar]
- Vaarala O, Ilonen J, Ruohtula T, Pesola J, Virtanen SM, Harkonen T, et al. Removal of bovine insulin from cow's milk formula and early initiation of beta‐cell autoimmunity in the FINDIA pilot study. Archives of Pediatrics and Adolescent Medicine 2012;166:608‐14. [DOI] [PubMed] [Google Scholar]
- Vandenplas Y, Deneyer M, Sacre L, Loeb H. Preliminary data on a field study with a new hypo‐allergenic formula. European Journal of Pediatrics 1988;148:274‐7. [DOI] [PubMed] [Google Scholar]; Vandenplas Y, Malfroot A, Dab I. Short‐term prevention of cow's milk protein allergy in infants. Immunology and Allergy Practice 1989;11:430‐7. [Google Scholar]
- Hauser B, Blecker U, Keymolen K, Suys B, Gerlo E, Vandenplas Y. Plasma amino acid concentrations in term‐born infants fed a whey predominant or a whey hydrolysate formula. Journal of Parenteral and Enteral Nutrition 1997;21:27‐30. [DOI] [PubMed] [Google Scholar]; Hauser B, Keymolen K, Blecker U, Suys B, Bougatef A, Loeb H, et al. A comparative evaluation of whey hydrolysate and whey‐predominant formulas. How well do infants accept and tolerate them?. Clinical Pediatrics 1993;62:433‐7. [DOI] [PubMed] [Google Scholar]; Vandenplas Y, Hauser B, Blecker U, Suys B, Peeters S, Keymolen K, Loeb H. The nutritional value of a whey hydrolysate formula compared with a whey‐predominant formula in healthy infants. Journal of Pediatric Gastroenterology and Nutrition 1993;17:92‐6. [DOI] [PubMed] [Google Scholar]
- Wopereis H, Sim K, Shaw A, Martin R, Oozeer R, Warner JO, et al. The developmental gut microbiota is modulated towards a pattern closer to breastfed infants by a partially hydrolyzed cow's milk formula supplemented with specific prebiotic oligosaccharides. Allergy: European Journal of Allergy and Clinical Immunology 2014;69:315. 24266710 [Google Scholar]
- Yu MX, Zhuang SQ, Wang DH, Zhou XY, Liu XH, Shi LP, et al. Effects of extensively hydrolyzed protein formula on feeding and growth in preterm infants: a multicenter controlled clinical study. Chinese Journal of Contemporary Pediatrics 2014;16:684‐90. [PubMed] [Google Scholar]
- Zeiger RS, Heller S. Development of nasal basophilic cells and nasal eosinophils from age 4 months through 4 years in children of atopic parents. Journal of Allergy and Clinical Immunology 1993;91:723‐34. [DOI] [PubMed] [Google Scholar]; Zeiger RS, Heller S. The development and prediction of atopy in high‐risk children: follow‐up at age seven years in a prospective randomized study of combined maternal and infant food allergen avoidance. Journal of Allergy and Clinical Immunology 1995;95:1179‐90. [DOI] [PubMed] [Google Scholar]; Zeiger RS, Heller S, Mellon MH, Forsythe AB, O'Connor RD, Hamburger RN, et al. Effect of combined maternal and infant food‐allergen avoidance on development of atopy in early infancy: a randomized study. Journal of Allergy and Clinical Immunology 1989;84:72‐89. [DOI] [PubMed] [Google Scholar]; Zeiger RS, Heller S, Mellon MH, Halsey JF, Hamburger RN, Sampson HA. Genetic and environmental factors affecting the development of atopy through age 4 in children of atopic parents: a prospective randomized study of food allergen avoidance. Pediatric Allergy and Immunology 1992;3:110‐27. [Google Scholar]
References to ongoing studies
- Baldassarre M. Tolerance of an Extensively Hydrolyzed Protein Infant Formula Versus a Premature Infant Formula. ClinicalTrials.gov Identifier: NCT019871542013.
- Barber CM. Effects on Growth of an Extensively Hydrolyzed Formula Fed to Term. ClinicalTrials.gov Identifier: NCT009366372009.
- Moral T. Hydrolized Protein Formula for Premature Infants. ClinicalTrials.gov Identifier: NCT011564932010.
- Knip M. Early Dietary Intervention and Later Signs of Beta‐Cell Autoimmunity (EDIA). ClinicalTrials.gov Identifier: NCT01735123 2012. [DOI] [PMC free article] [PubMed]
- Mennella JA. "Watch Your Baby Grow" Study (GRO). ClinicalTrials.gov Identifier: NCT017002052012.
- Sorensen R. Growth of Infants Fed an Extensively Hydrolyzed Infant Formula. ClinicalTrials.gov Identifier: NCT012103912010.
- Vivatvakin B. Digestive Tolerance of Slightly Hydrolyzed Starter Infant Formula With Probiotics. ClinicalTrials.gov Identifier: NCT010362432009.
- Yin LP, Qian LJ, Zhu H, Chen Y, Li H, Han JN, et al. Application effect of extensively hydrolyzed milk protein formula and follow‐up in preterm children with a gestational age of less than 34 weeks: study protocol for a randomized controlled trial. Trials 2015;16:498. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
- Americal Academy of Pediatrics: Committee on Nutrition. Hypoallergenic infant formulas. Pediatrics 2000;106:346‐9. [PubMed] [Google Scholar]
- Alexander DD, Cabana MD. Partially hydrolyzed 100% whey protein infant formula and reduced risk of atopic dermatitis: a meta‐analysis. Journal of Pediatric Gastroenterology and Nutrition 2010;50:422‐30. [DOI] [PubMed] [Google Scholar]
- Allen CW, Campbell DE, Kemp AS. Food allergy: is strict avoidance the only answer?. Pediatric Allergy and Immunology 2009;20:415‐22. [DOI] [PubMed] [Google Scholar]
- Arshad SH, Kurukulaaratchy RJ, Fenn M, Matthews S. Early life risk factors for current wheeze, asthma, and bronchial hyperresponsiveness at 10 years of age. Chest 2005;127:502‐8. [DOI] [PubMed] [Google Scholar]
- Bergmann RL, Bergmann KE, Lau‐Schadensdorf S, Luck W, Dannemann A, Bauer CP, et al. Atopic diseases in infancy. The German multicenter atopy study (MAS‐90). Pediatric Allergy and Immunology 1994;5(Suppl 6):19‐25. [DOI] [PubMed] [Google Scholar]
- Bergmann RL, Edenharter G, Bergmann KE, Guggenmoos‐Holzmann I, Forster J, Bauer CP, et al. Predictability of early atopy by cord blood‐IgE and parental history. Clinical and Experimental Allergy 1997;27:752‐60. [PubMed] [Google Scholar]
- Bergmann R, Woodcock A. Whole population or high‐risk group? Childhood asthma. European Respiratory Journal Suppl 1998;27:9S‐12S. [PubMed] [Google Scholar]
- Bock SA, Sampson HA, Atkins FM, Zeiger RS, Lehrer S, Sachs M, et al. Double‐blind, placebo‐controlled food challenge (DBPCFC) as an office procedure: a manual. Journal of Allergy and Clinical Immunology 1988;82:986‐97. [DOI] [PubMed] [Google Scholar]
- Boyle RJ, Ierodiakonou D, Khan T, Chivinge J, Robinson Z, Geoghegan N, et al. Hydrolysed formula and risk of allergic or autoimmune disease: systematic review and meta‐analysis. BMJ 2016;352:i974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burr ML, Butland BK, King S, Vaughan‐Williams E. Changes in asthma prevalence: two surveys 15 years apart. Archives of Disease in Childhood 1989;64:1452‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chafen JJ, Newberry SJ, Riedl MA, Bravata DM, Maglione M, Suttorp MJ, et al. Diagnosing and managing common food allergies: a systematic review. JAMA 2010;303:1848‐56. [DOI] [PubMed] [Google Scholar]
- Chung CS, Yamini S, Trumbo PR. FDA's health claim review: whey‐protein partially hydrolyzed infant formula and atopic dermatitis. Pediatrics 2012;130:e408‐14. [DOI] [PubMed] [Google Scholar]
- Custovic A, Simpson BM, Simpson A, Hallam C, Craven M, Brutsche M, et al. Manchester Asthma and Allergy Study: low‐allergen environment can be achieved and maintained during pregnancy and in early life. Journal of Allergy and Clinical Immunology 2000;105:252‐8. [DOI] [PubMed] [Google Scholar]
- Custovic A, Simpson BM, Simpson A, Kissen P, Woodcock A. NAC Manchester Asthma and Allergy Study Group. Effect of environmental manipulation in pregnancy and early life on respiratory symptoms and atopy during first year of life: a randomised trial. Lancet 2001;358:188‐93. [DOI] [PubMed] [Google Scholar]
- Darsow U, Ring J. Airborne and dietary allergens in atopic eczema: a comprehensive review of diagnostic tests. Clinical and Experimental Dermatology 2000;25:544‐51. [DOI] [PubMed] [Google Scholar]
- Silva D, Geromi M, Halken S, Host A, Panesar SS, Muraro A, et al. EAACI Food Allergy Anaphylaxis Guidelines Group. Primary prevention of food allergy in children and adults: systematic review. Allergy 2014;69:581‐9. [DOI] [PubMed] [Google Scholar]
- Silva D, Geromi M, Halken S, Host A, Panesar SS, Muraro A, Anaphylaxis Guidelines G. Primary prevention of food allergy in children and adults: systematic review. Allergy 2014;69:581‐9. [DOI] [PubMed] [Google Scholar]
- EAACI food allergy, anaphylaxis guidelines. Primary prevention of food allergy. Allergy 2014;69:590‐601. [DOI] [PubMed] [Google Scholar]
- McMaster University. GRADEpro [www.gradepro.org]. Ontario: McMaster University, 2014.
- Gruskay FL. Comparison of breast, cow, and soy feedings in the prevention of onset of allergic disease: a 15‐year prospective study. Clinical Pediatrics 1982;21:486‐91. [DOI] [PubMed] [Google Scholar]
- Halken S. Prevention of allergic disease in childhood: clinical and epidemiological aspects of primary and secondary allergy prevention. Pediatric Allergy and Immunology 2004;15(Suppl 16):4‐5. [DOI] [PubMed] [Google Scholar]
- Hansen LG, Halken S, Host A, Moller K, Osterballe O. Prediction of allergy from family history and cord blood IgE levels. A follow‐up at the age of 5 years. Cord blood IgE. IV. Pediatric Allergy and Immunology 1993;4:34‐40. [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
- Hill DJ, Murch SH, Rafferty K, Wallis P, Green CJ. The efficacy of amino acid‐based formulas in relieving the symptoms of cow's milk allergy: a systematic review. Clinical & Experimental Allergy 2007;37:808‐22. [DOI] [PubMed] [Google Scholar]
- Host A. Cow's milk protein allergy and intolerance in infancy. Some clinical, epidemiological and immunological aspects. Pediatric Allergy and Immunology 1994;5(Suppl 5):1‐36. [PubMed] [Google Scholar]
- Host A, Jacobsen HP, Halken S, Holmenlund D. The natural history of cow's milk protein allergy/intolerance. European Journal of Clinical Nutrition 1995;49(Suppl 1):S13‐8. [PubMed] [Google Scholar]
- Host A, Koletzko B, Dreborg S, Muraro A, Wahn U, Aggett P, et al. Dietary products used in infants for treatment and prevention of food allergy. Joint Statement of the European Society for Paediatric Allergology and Clinical Immunology (ESPACI) Committee on Hypoallergenic Formulas and the European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) Committee on Nutrition. Archives of Disease in Childhood 1999;81:80‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iskedjian M, Szajewska H, Spieldenner J, Farah B, Berbari J. Meta‐analysis of a partially hydrolysed 100%‐whey infant formula vs. extensively hydrolysed infant formulas in the prevention of atopic dermatitis. Current Medical Research and Opinion 2010;26:2599‐606. [DOI] [PubMed] [Google Scholar]
- Johansson SG, Bieber T, Dahl R, Friedmann PS, Lanier BQ, Lockey RF, et al. Revised nomenclature for allergy for global use. Report of the Nomenclature Review Committee of the World Allergy Organization, October 2003. Journal of Allergy and Clinical Immunology 2004;113:832‐6. [DOI] [PubMed] [Google Scholar]
- Katz Y, Rajuan N, Goldberg MR, Eisenberg E, Heyman E, Cohen A, et al. Early exposure to cow's milk protein is protective against IgE‐mediated cow's milk protein allergy. Journal of Allergy and Clinical Immunology 2010;126:77‐82 e1. [DOI] [PubMed] [Google Scholar]
- Katz Y, Goldberg MR, Rajuan N, Cohen A, Leshno M. The prevalence and natural course of food protein‐induced enterocolitis syndrome to cow's milk: a large‐scale, prospective population‐based study. Journal of Allergy and Clinical Immunology 2011;127:647‐53 e1‐3. [DOI] [PubMed] [Google Scholar]
- Kilpelainen M, Terho EO, Helenius H, Koskenvuo M. Validation of a new questionnaire on asthma, allergic rhinitis, and conjunctivitis in young adults. Allergy 2001;56:377‐84. [DOI] [PubMed] [Google Scholar]
- Kjellman NI. Atopic disease in seven‐year‐old children. Incidence in relation to family history. Acta Paediatrica Scandinavica 1997;66:465‐71. [DOI] [PubMed] [Google Scholar]
- Kleinman RE, Bahna S, Powell GF, Sampson HA. Use of infant formulas in infants with cow milk allergy. A review and recommendations. Pediatric Allergy and Immunology 1991;4:146‐55. [Google Scholar]
- Kramer MS. Maternal antigen avoidance during pregnancy for preventing atopic disease in infants of women at high risk. Cochrane Database of Systematic Reviews 2001, Issue 3. [DOI: 10.1002/14651858.CD000132] [DOI] [PubMed] [Google Scholar]
- Kwinta P, Mitkowska Z, Kruczek P, Tomasik T, Pietrzyk JJ. Influence of the lactose free and lactose containing diet on prevalence of gram‐negative sepsis and feeding intolerance in very low birth weight infants: double‐blind randomized trial. Przegl Lek 2002;59:63–6. [PubMed] [Google Scholar]
- Muraro A, Dreborg S, Halken S, Host A, Niggemann B, Aalberse R, et al. Dietary prevention of allergic diseases in infants and small children. Part II. Evaluation of methods in allergy prevention studies and sensitization markers. Definitions and diagnostic criteria of allergic diseases. Pediatric Allergy and Immunology 2004;15:196‐205. [DOI] [PubMed] [Google Scholar]
- Muraro A, Halken S, Arshad SH, Beyer K, Dubois AE, Du Toit G, EAACI Food Allergy Anaphylaxis Guidelines Group. EAACI food allergy and anaphylaxis guidelines. Primary prevention of food allergy. Allergy 2014;69:590‐601. [DOI] [PubMed] [Google Scholar]
- Oddy WH, Holt PG, Sly PD, Read AW, Landau LI, Stanley FJ, et al. Association between breast feeding and asthma in 6 year old children: findings of a prospective birth cohort study. BMJ 1999;319:815‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterballe M, Hansen TK, Mortz CG, Host A, Bindslev‐Jensen C. The prevalence of food hypersensitivity in an unselected population of children and adults. Pediatric Allergy and Immunology 2005;16:567‐73. [DOI] [PubMed] [Google Scholar]
- Peat JK, Toelle BG, Mellis CM. Problems and possibilities in understanding the natural history of asthma. Journal of Allergy & Clinical Immunology 2000;106(Suppl 3):S144‐52. [DOI] [PubMed] [Google Scholar]
- Peat JK, Toelle BG, Marks GB, Mellis CM. Continuing the debate about measuring asthma in population studies. Thorax 2001;56:406‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott SL, Tang ML, Australasian Society of Clinical Immunology and Allergy. The Australasian Society of Clinical Immunology and Allergy position statement: Summary of allergy prevention in children. Medical Journal of Australia 2005;182:464‐7. [DOI] [PubMed] [Google Scholar]
- Ravault C, Kauffmann F. Validity of the IUATLD (1986) questionnaire in the EGEA study. International Union Against Tuberculosis and Lung Disease. Epidemiological study on the genetics and environment of asthma, bronchial hyperresponsiveness and atopy. Journal of Tuberculosis and Lung Disease 2001;5:191‐6. [PubMed] [Google Scholar]
- The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen, The Nordic Cochrane Centre: The Cochrane Collaboration, 2011.
- Ronmark E, Jonsson E, Platts‐Mills T, Lundback B. Incidence and remission of asthma in schoolchildren: report from the obstructive lung disease in northern Sweden studies. Pediatrics 2001;107:E37 (http://www.pediatrics.org/cgi/content/full/107/3/e37). [DOI] [PubMed] [Google Scholar]
- Saarinen UM, Kajosaari M. Breastfeeding as prophylaxis against atopic disease: prospective follow‐up study until 17 years old. Lancet 1995;346:1065‐9. [DOI] [PubMed] [Google Scholar]
- Saarinen KM, Savilahti E. Infant feeding patterns affect the subsequent immunological features in cow's milk allergy. Clinical & Experimental Allergy 2000;30:400‐6. [DOI] [PubMed] [Google Scholar]
- Sampson HA. Update on food allergy. Journal of Allergy and Clinical Immunology 2004;113:805‐19. [DOI] [PubMed] [Google Scholar]
- Schultz Larsen F. Atopic dermatitis: an increasing problem. Pediatric Allergy and Immunology 1996;7:51‐3. [DOI] [PubMed] [Google Scholar]
- Schünemann H, Brożek J, Guyatt G, Oxman A, editors. GRADE Working Group. GRADE Handbook. Handbook for grading quality of evidence and strength of recommendations. www.guidelinedevelopment.org/handbook [Updated October 2013].
- Sears MR, Holdaway MD, Flannery EM, Herbison GP, Silva PA. Parental and neonatal risk factors for atopy, airway hyper‐responsiveness, and asthma. Archives of Disease in Childhood 1996;75:392‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears MR. Evolution of asthma through childhood. Clinical & Experimental Allergy 1998;28(Suppl 5):82‐91. [DOI] [PubMed] [Google Scholar]
- Sly RM. Changing prevalence of allergic rhinitis and asthma. Annals of Allergy, Asthma and Immunology 1999;82:233‐48. [DOI] [PubMed] [Google Scholar]
- Strachan DP, Butland BK, Anderson HR. Incidence and prognosis of asthma and wheezing illness from early childhood to age 33 in a national British cohort. BMJ 1996;312:1195‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szajewska H, Horvath A. Meta‐analysis of the evidence for a partially hydrolyzed 100% whey formula for the prevention of allergic diseases. Current Medical Research and Opinion 2010;26:423‐37. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
- Osborn DA, Sinn J. Formulas containing hydrolysed protein for prevention of allergy and food intolerance in infants. Cochrane Database of Systematic Reviews 2003, Issue 4. [DOI: 10.1002/14651858.CD003664.pub3] [DOI] [PubMed] [Google Scholar]
- Osborn DA, Sinn J. Formulas containing hydrolysed protein for prevention of allergy and food intolerance in infants. Cochrane Database of Systematic Reviews 2006, Issue CD003664. [DOI: 10.1002/14651858.CD003664.pub3] [DOI] [PubMed] [Google Scholar]


