Abstract
Background
There are rising rates of multiple births worldwide with associated higher rates of complications and more hospital care, often due to prematurity. While there is strong evidence about the risks of not breastfeeding, rates of breastfeeding in women who have given birth to more than one infant are lower than with singleton births. Breastfeeding more than one infant can be more challenging because of difficulties associated with the birth or prematurity. The extra demands on the mother of frequent suckling, coordinating the needs of more than one infant or admission to the neonatal intensive care unit can lead to delayed initiation or early cessation. Additional options such as breast milk expression, the use of donor milk or different methods of supplementary feeding may be considered. Support and education about breastfeeding has been found to improve the duration of any breastfeeding for healthy term infants and their mothers, however evidence is lacking about interventions that are effective to support women with twins or higher order multiples.
Objectives
To assess effectiveness of breastfeeding education and support for women with twins or higher order multiples.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (30 June 2016), ClinicalTrials.gov (30 June 2016), the WHO International Clinical Trials Registry Platform (ICTRP) (1 July 2016), the excluded studies list from the equivalent Cochrane review of singletons, and reference lists of retrieved studies.
Selection criteria
Randomised or quasi‐randomised trials comparing extra education or support for women with twins or higher order multiples were included.
Data collection and analysis
Two review authors independently assessed trials for inclusion and risk of bias, extracted data and checked them for accuracy. We planned to assess the quality of evidence using the GRADE approach, but were unable to analyse any data.
Main results
We found 10 trials (23 reports) of education and support for breastfeeding that included women with twins or higher order multiples. The quality of evidence was mixed, and the risk of bias was mostly high or unclear. It is difficult to blind women or staff to group allocation for this intervention, so in all studies there was high risk of performance and high or unclear risk of detection bias. Trials recruited 5787 women (this included 512 women interviewed as part of a cluster randomised trial); of these, data were available from two studies for 42 women with twins or higher order multiples. None of the interventions were specifically designed for women with more than one infant, and the outcomes for multiples were not reported separately for each infant. Due to the scarcity of evidence and the format in which data were reported, a narrative description of the data is presented, no analyses are presented in this review, and we were unable to GRADE the evidence.
The two trials with data for women with multiple births compared home nurse visits versus usual care (15 women), and telephone peer counselling versus usual care (27 women). The number of women who initiated breastfeeding was reported (all 15 women in one study, 25 out of 27 women in one study). Stopping any breastfeeding before four to six weeks postpartum, stopping exclusive breastfeeding before four to six weeks postpartum, stopping any breastfeeding before six months postpartum andstopping exclusive breastfeeding before six months postpartum were not explicitly reported, and there were insufficient data to draw any meaningful conclusions from survival data.
Stopping breast milk expression before four to six weeks postpartum, andstopping breast milk expression before six months postpartum were not reported. Measures ofmaternal satisfaction were reported in one study of 15 women, but there were insufficient data to draw any conclusions; no other secondary outcomes were reported for women with multiple births in either study. No adverse events were reported.
Authors' conclusions
We found no evidence from randomised controlled trials about the effectiveness of breastfeeding education and support for women with twins or higher order multiples, or the most effective way to provide education and support . There was no evidence about the best way to deliver the intervention, the timing of care, or the best person to deliver the care. There is a need for well‐designed, adequately powered studies of interventions designed for women with twins or higher order multiples to find out what types of education and support are effective in helping these mothers to breastfeed their babies.
Plain language summary
Breastfeeding education and support for women with multiple pregnancies
What is the issue?
Breastfeeding has many benefits that include protecting the baby against inflammatory diseases of the gut, lungs or ears, and longer term health problems such as diabetes and obesity, improved cognitive outcomes, and protecting the mother against breast cancer. Rates of breastfeeding are lower in women who have given birth to more than one baby than for women who have a single baby. However, there are challenges to overcome in breastfeeding multiples (twins, triplets or more). Education and support have been found to increase the number of women who start breastfeeding and improve the duration of any breastfeeding for single healthy term babies. This education and support may come from lay workers or from health professionals. It could be given in preparation for birth or once the babies arrive.
Mothers who have more than one baby have many additional challenges to overcome to breastfeed their babies and they may need additional advice and support. They have extra demands of frequent suckling, coordinating the potentially differing needs of more than one baby, or the need to express milk and to feed different babies by different feeding methods. The mothers have a greater likelihood of giving birth preterm and their babies being admitted to the neonatal intensive care unit, which can lead to delayed starting or early stopping of breastfeeding.
Why is this important?
Breastfeeding helps babies’ health and development. Giving birth to more than one baby poses additional challenges for a mother planning to breastfeed. The mothers are also more likely to have to consider options such as breast milk expression, the use of donor milk or fortification of the milk and different methods of supplementary feeding. Some mothers may prefer feeding expressed breast milk because they can be certain about the volume of milk being fed and as a way of allowing others to assist with feeding. We wanted to find out if education and support helps mothers of multiples to breastfeed.
What evidence did we find?
We searched for randomised controlled trials on 30 June 2016 and 1 July 2016 and found 10 studies (23 reports) to include in our review. All the studies were of education and support for all mothers, not just those giving birth to more than one baby, which introduced methodological issues for looking specifically at multiple births. Trials recruited 5787 women (this included 512 women interviewed as part of a cluster randomised trial). The number of babies from multiple pregnancies was small and none of the studies had sufficient numbers to provide information about how interventions worked for mothers of multiples. There were several problems with how the studies had been done, including women knowing if they were in the group getting support.
The authors of two of the studies sent us their findings for women with multiple births (42 women in total). The trials compared home nurse visits versus usual care (15 women), and telephone peer counselling versus usual care (27 women). They looked at the number of women stopping any or exclusive breastfeeding before four weeks after giving birth and before six months, without any clear improvements provided by the intervention. All 15 women in one study and 25 out of 27 women in the other had started breastfeeding. There was no information on breast milk expression. Other outcome measures were reported, including a measure of maternal satisfaction in one study of 15 women, but there were not sufficient numbers to allow us to draw any conclusions. No adverse events were reported.
What does this mean?
We could not draw conclusions from the evidence available from randomised controlled trials about whether education and support helps mothers of multiples to breastfeed. None of the studies were designed to offer tailored support or education to women who give birth to more than one baby. More research is needed to find out what types of education and support could help mothers of multiples to breastfeed their babies. Data from these studies should be presented and analysed in an appropriate way for multiple babies.
Background
Description of the condition
Description of the condition (breastfeeding multiples)
The incidence of multiple births (birth of two or more infants) in high‐income countries has risen since the 1970s (Blondel 2002; Collins 2007). In the United States, rates increased from 19.3 to 30.7 per 1000 live births between 1980 and 1999 (Russell 2003), while in England and Wales the rate increased from 10 per 1000 in 1980, to 16 per 1000 in 2011 (NICE 2013). In 2013 in England and Wales, this figure was comprised of 10593 sets of twins, 187 triplets, and "three quads and above" (ONS 2014). European rates in 2000 varied from 12.2 per 1000 maternities in Italy to 19.4 per 1000 in the Netherlands (Blondel 2006). In low‐income countries, rates of between nine and 18 per 1000 live births have also been reported (Smits 2011). This rise and variation in rates is due to the use of reproductive techniques, and is partly accounted for by more births to older women and more multiple births in women from more affluent backgrounds (Smith 2014). More recently, rates of higher order births (three or more infants) have declined as changes in assisted reproductive techniques (ART) to reduce multiple pregnancies have been implemented (Smith 2014; Umstad 2013).
Multiple pregnancy carries greater risks for both mother and babies, with around a 50% risk of preterm birth in multiple pregnancy (Blondel 2006; NICE 2013). Additional fetal risks include feto‐fetal transfusion syndrome, intrauterine growth restriction and congenital abnormalities (NICE 2013). There is higher risk of operative delivery (Antsaklis 2013; Kyvernitakis 2013; Lee 2011). Infants conceived through assisted reproductive technology (ART) may be at even higher risk of complications (Murray 2014); consequently, admission to the neonatal unit is more likely. This is likely to cause stress and anxiety for parents, separation of the mother from her babies (Flacking 2006), and has a cost implication for the healthcare provider (Chambers 2014).
There is strong evidence that not breastfeeding or not being fed breast milk carries greater risks, both for the infant and for the mother, with small and sick babies having specific additional risks (Renfrew 2012a, Victora 2016). Breastfeeding protects the infant against gastrointestinal disease, respiratory disease, otitis media and necrotising enterocolitis (NEC), and protects the mother against breast cancer (Renfrew 2012a, Victora 2016). Breastfeeding is also likely to lead to improvements in IQ, reduce rates of Sudden Infant Death Syndrome (SIDS) and reduce obesity in young children, and there is growing evidence that it confers a number of other health and development benefits on the child and health benefits on the mother (Renfrew 2012a, Victora 2016). It has been estimated that even modest increases in the numbers of infants breastfed exclusively could have considerable cost savings for the health service (Renfrew 2012a, Rollins 2016). For preterm infants, the protection breast milk confers against NEC is particularly important.
All mothers have to make decisions about how to feed their baby, however mothers of multiples face more challenges feeding their infants than mothers of singletons and may need additional advice and support. Mothers of twins have been found to have lower intentions to breastfeed (Lustiv 2013), to be less likely to initiate breastfeeding (Yokoyama 2006), and to be less likely to offer any breastfeeding (Multiple Births Foundation 2011) or to breastfeed exclusively (McAndrew 2012; Multiple Births Foundation 2011). A European study found that an overall figure of 36.4% of twins were breastfed at discharge, compared to 39.3% of singleton infants, with the rate of breastfeeding at discharge varying widely between countries (Bonet 2011). In Japan at three to six months, 4.1% of twins or triplets were exclusively breastfed compared to 44.7% of singletons (Yokoyama 2006). A UK‐wide survey found 69% of twins were breastfed initially, compared to 81% of singletons (McAndrew 2012). This study also reported that twins were more likely to be given donor milk, and have formula introduced by one week old (McAndrew 2012). The study found that some mothers reported feeding each baby by differing methods; reasons included one baby being ill or in hospital and needing special formula or drip or tube feeding (8%), one baby starting solids earlier (8%), or babies taking differing amounts of milk (6%) (McAndrew 2012). Prematurity contributes to a higher percentage of twins starting partial breastfeeding and moving to full breastfeeding later (Flidel‐Rimon 2002). In the UK, older women and those living in more affluent circumstances are more likely to breastfeed (McAndrew 2012) and this difference also applies to women who have infants from multiple pregnancies (Ostlund 2010).
Some of the issues faced by mothers of multiples are related to the practicalities of feeding more than one infant at the breast, and this may mean that advice offered to mothers of singletons may not be appropriate (Bennington 2011). While some women may find that breastfeeding multiples is straightforward, mothers of multiples may have more difficulty offering early and continuous skin‐to‐skin contact with their infants, there may be delay in initiation of feeding at the breast, the infants may have a disorganised or immature sucking pattern as a result of prematurity and the demands of facilitating frequent feeding are more challenging (Bennington 2011; Cinar 2013). Women who have given birth by caesarean section are less likely to be mobile, less able to care for more than one infant at once, and may have more difficulty finding a comfortable position to feed (Bennington 2011; Flidel‐Rimon 2002). Decisions may have to be made about whether to feed the infants together or feed separately. There may be conflicting needs; accommodating the individual feeding preferences of each infant may be incompatible with the extra time needed to feed separately (Bennington 2011; Gromada 1998). Simultaneous feeding of two babies is likely to stimulate simultaneous let down, and can facilitate feeding if one infant has weak sucking, however the practicalities of this can be hard to manage without help in the early stages of breastfeeding, particularly if two hands are needed to encourage a satisfactory latch (Flidel‐Rimon 2002; Gromada 1998; Multiple Births Foundation 2011). Waking a sleeping baby for simultaneous feeding may not be easy and is unlikely to result in a satisfactory feed. Simultaneous feeding of more than two babies is not a practical possibility. It is important to note that the challenges of feeding more than one baby at a time also arise with feeding multiples with formula and, in addition, extra time is required to make up formula feeds (Bennington 2011).
Prematurity adds to the already significant challenges of initiating and maintaining lactation for twins and higher order multiples. Twin infants born prematurely are less likely than twins born at term to be breastfed (Ostlund 2010). Problems can be exacerbated by the degree of prematurity and the severity of any additional illness, and the consequences of an operative birth (Bennington 2011). Mothers of infants born early may be motivated to express breast milk as a way of providing a unique contribution to the care of their infants. There have been reports that, for premature infants, breast milk may be seen by staff as a commodity, leading to pressure from neonatal intensive care unit (NICU) staff on mothers to express milk (Flacking 2006), with the consequence that breast milk feeding may be favoured over breastfeeding (Niela‐Vilén 2014). There is some evidence that counselling women who intend to formula feed about the benefits of expressing milk for very low birthweight babies increases the incidence of lactation initiation and breast milk feeding without increasing maternal stress and anxiety (Sisk 2006). Expressing breast milk can lead to women who did not plan to breastfeed, eventually feeding their infants directly at the breast (Sisk 2006).
While feeding directly at the breast is optimal for stimulating a good supply of milk, the above practicalities and the additional challenges of prematurity may mean that feeding at the breast is not possible initially. The mother may express her milk and mother's own or donor expressed breast milk (EBM) can then be given by bottle, tube or cup (Damato 2005; Gromada 1998). The latter two options may increase the chances of a baby subsequently feeding successfully at the breast while in hospital, however cup feeding may increase length of stay and success is likely to be dependent on the experience of the staff (Collins 2008). Within the NICU, a lack of privacy can make expression of breast milk, initiation of skin contact, and breastfeeding difficult for many mothers (Alves 2013; Flacking 2006; Gromada 1998; Niela‐Vilén 2014). Facilities to pump and store EBM in the NICU are essential (Gromada 1998). However, the transition from bottle or alternative feeding methods to breastfeeding may be problematic and involve decisions about when and how to make the transition, particularly if the infants are discharged at different times (Bennington 2011; Gromada 1998). Some mothers report that feeding EBM by bottle is a preferable method because there is certainty about the volume of milk being fed (Niela‐Vilén 2014) and it is also a way of allowing others to assist with feeding, particularly with higher order multiples (Multiple Births Foundation 2011). Milk expression may be by hand or by pump (hand pump or electrical pump, single or double pumping); there is no strong evidence that one method is better than another (Becker 2015). An increase in milk supply can be achieved by early initiation of pumping, increased frequency of pumping, warming of breast, massage of breast, and relaxation and therapeutic touch (Becker 2015). If only one baby is able to latch, the mother can simultaneously pump on the other side (Gromada 1998). There are various options for changing sides or for supplementing and if mothers are advised and supported well, they are likely to find a pattern that suits their circumstances (Bennington 2011; Gromada 1998). Various positioning options such as the underarm hold or the use of a special V‐shaped pillow to support the babies may be helpful (Flidel‐Rimon 2002; Gromada 1998).
If a mother is unable to express sufficient milk, or does not wish to express milk, pasteurised donor breast milk can be used. This has been found to protect against NEC (ESPGHAN 2013). NICE guidelines make recommendations about the safe and effective operation of donor milk banks (NICE 2010), however not all areas operate this service.
When infants are premature or ill and admission to the NICU is required, the consequent likely (though not inevitable) separation of mother and babies, the possibility of long periods of hospitalisation, the mother being discharged home before the babies, her need for rest and recovery, the need to care for older siblings, long periods of pumping, staggered infant discharge and the involvement of many other caregivers can make establishing a good milk supply and initiating breastfeeding very challenging (Bennington 2011; Gromada 1998; Multiple Births Foundation 2011). Success in breastfeeding a premature infant has been reported to compensate for any perceived sense of ‘failure’ a mother may experience in regard to the premature birth, and can give her a sense of achievement (Flacking 2006; Flacking 2007; Niela‐Vilén 2014).
Kangaroo skin‐to‐skin mother care is an effective intervention to improve the duration of breastfeeding in all settings (Renfrew 2009). It is an alternative to conventional neonatal care for low birthweight (LBW) infants and has some benefits for breastfeeding outcomes (Conde‐Agudelo 2014). Early skin contact with the mother soon after birth improves breastfeeding at one to four months post birth and is associated with other benefits for the mother and baby including improvements in attachment and bonding (Moore 2012). However, achieving sufficient skin contact when there is more than one baby can be more challenging and the evidence for this intervention in multiple pregnancies is lacking.
Mothers of multiples can produce sufficient breast milk, especially if given appropriate help and support (Multiple Births Foundation 2011). Anxiety about adequacy of milk supply is a frequently reported concern for mothers of multiples who are breastfeeding, however (Cinar 2013; Flidel‐Rimon 2002; Multiple Births Foundation 2011). For mothers of preterm infants there may also be anxiety about the sufficiency of breast milk to meet the nutritional needs of their infants (Flacking 2007). This concern may be exacerbated by the often routine use of fortification of breast milk with artificial fortifier while the infant is in the NICU and being fed by nasogastric tube (Roze 2012). Supplementation with formula or donor EBM may be considered by staff if it is thought that there is insufficient supply of mother's own EBM, however inadequate pumping can lead to reduced stimulation of the breast, a reduced maternal milk supply and earlier cessation or less likelihood of exclusive breastfeeding (Gromada 1998). The use of supplementary artifical formula has been found to lead to a higher rate of short‐term growth, but also a higher rate of NEC (Quigley 2014).
More widely, just as for singletons, other issues can impact on attitudes and practices towards breastfeeding multiples. These include: cultural beliefs and pressures (e.g. anxiety about breastfeeding in public, beliefs about adequacy of milk supply); lack of availability of trained support; legislation to protect women who are breastfeeding; and commercial pressures from marketing and advertising of formula by manufacturers (Save the Children 2013).
In general, studies are lacking in details about the complexities of feeding multiples and do not specify details of the feeding method such as direct breastfeeding, use of tube, cup or bottle, the use of fortifiers, the use of supplementary milks, the use of donor breast milk or expressed maternal breast milk, and the differences in feeding method between different babies (Renfrew 2009).
Description of the intervention
Education and support for breastfeeding multiples
Critical to success with breastfeeding multiples is information and support from staff (Multiple Births Foundation 2011). Support might be given by a trained healthcare professional (such as a midwife, lactation consultant, or nurse), a peer counsellor or a lay advisor. Advice might include early anticipatory advice in pregnancy, and multidisciplinary support or advice consistent with the mother’s goals and pace (Gromada 1998; Szucs 2009). Specific advice might be needed to help mothers distinguish normal infant feeding behaviour from issues related to caring for multiples (Gromada 1998). Staff may be able to advise about patterns of feeding, avoidance of painful feeding, feeding from both breasts equally, expressing after feeds, and providing sufficient stimulation (both direct feeding and regular and frequent expression of milk) (Bennington 2011). Advice may be provided on a one‐to‐one basis or as part of a group and could take place in hospital or at home. Following discharge from hospital, contact could be face‐to‐face, over the phone or using teleconferencing facilities. Increasingly mothers are turning to websites or online sources of information and support (Newby 2015). For healthy term infants, extra support has been shown to improve the duration of 'any breastfeeding' and the duration of exclusive breastfeeding (McFadden 2017). However, much of this evidence relates to singleton infants; although some studies include multiples, results are not usually reported separately, therefore applicability to infants from multiple pregnancies is uncertain (Renfrew 2009). Many staff lack experience or confidence about feeding multiples, and incorrect or discouraging advice can be detrimental (Cinar 2013; Damato 2005). Staff in NICU may view formula feeding as a way of ensuring faster growth of the preterm baby and therefore quicker discharge home (Niela‐Vilén 2014).
At an organisational level, the UNICEF Baby Friendly Hospital Initiative (BFHI) accreditation of the hospital results in improvement in breastfeeding outcomes for infants including those in NICU (Dall'Oglio 2007; Entwistle 2013; Parker 2013; Renfrew 2009). Other organisational interventions in NICU such as Family Centred Care may have an impact on maternal confidence and success with breastfeeding (POPPY Steering Group; Wataker 2012).
While successfully breastfeeding twins and higher order multiples can be straightforward, it can also be time consuming for the mother, who is likely to need good support at home to ensure she gets sufficient rest and adequate nutrition (Multiple Births Foundation 2011). Feeding babies with formula does, of course, require additional preparation time and equipment when there are two or more babies and, even for mothers with adequate help at home (which not all women have), this can be a significant burden of both time and expense. As with breastfeeding singletons (McFadden 2017), adequate help and support during the weeks after birth is likely to be important for success in breastfeeding. Support can come from non‐professional sources such as peer (mother‐to‐mother) support, community support groups, support groups for multiples (Tamba (Twins & Multiple Births Association), MBF (Multiple Births Foundation), or similar organisations) and support from family. These aspects may be even more important for multiples. Support may take the form of practical support (help with housework, cooking, etc.) or emotional support. Enhancing the mother’s trust and confidence in her ability to sustain a sufficient milk supply is crucial (Gromada 1998). As the babies get older, workplace support and facilities may be especially important for mothers of multiples in long‐term maintenance of breastfeeding (Gromada 1998), while further advice and support may be needed during weaning to help prevent problems with milk stasis and mastitis (Gromada 1998). Women may require advice on specific interventions which may facilitate or inhibit successful breastfeeding of multiples. For example, the use of pacifiers for multiples who may have the additional breastfeeding problems identified above is unknown.
How the intervention might work
Breastfeeding is important for all babies, including multiples, and especially for those born too soon or too small (Renfrew 2012a, Victora 2016). Women with multiples are known to be less likely to intend to breastfeed, and to be less likely to initiate and sustain breastfeeding compared with those with singletons (Lustiv 2013; Yokoyama 2006). Tailored advice on initiating and sustaining breastfeeding or breast milk feeding may be needed for women with multiples, particularly in cultures where breastfeeding is not the norm. The practical challenges of caring for two or more infants may also mean that women require encouragement and emotional support in order to breastfeed their babies (Multiple Births Foundation 2011). It is possible that education and support for women with multiples may increase the number of women initiating breastfeeding and reduce the risk of early discontinuation.
Why it is important to do this review
This review is one in a series of Cochrane reviews examining education and support interventions to promote the initiation of breastfeeding and to increase the duration of breastfeeding and exclusive breastfeeding (Balogun 2016; Lumbiganon 2016; McFadden 2017). Findings of these related reviews suggest that support interventions can be effective in singleton pregnancies. There is some evidence that breastfeeding education and peer and professional support can increase the initiation of breastfeeding (Balogun 2016), and there is good evidence that support interventions by professionals or peers are effective in increasing the duration of any and exclusive breastfeeding for mothers of healthy term singletons (McFadden 2017). There is less conclusive evidence that antenatal education alone is effective to increase the duration of breastfeeding (Lumbiganon 2016). An earlier review examined the promotion and support of breastfeeding in the neonatal unit (Renfrew 2009). While the findings of these related reviews have some relevance for breastfeeding multiples, interventions were not necessarily designed to take account of the special needs of women attempting to feed twins or higher order multiples. A review to guide health professionals about breastfeeding multiples was carried out by the Multiple Births Foundation, but the evidence was low quality and is dated (Denton 2011).
Rates of breastfeeding of twins or higher order multiples are lower than rates of breastfeeding singletons suggesting that further evidence is needed about how to support this group of women. Women have reported that the help and support they received with infant feeding for multiples was insufficient; a UK study found that 34% of mothers of twins said further support with feeding would have helped (McAndrew 2012). Similarly, in Turkey, mothers of twins reported they would have liked more support and better advice during pregnancy (Cinar 2013).
Women who are breastfeeding more than one infant face particular challenges; there is a need for evidence‐based recommendations about what works to help women with multiples initiate and continue to breastfeed.
Objectives
To assess effectiveness of breastfeeding education and support for women with twins or higher order multiples. More detailed specific objectives are:
To describe the forms of breastfeeding education and support for women with twins and high order multiples examined in randomised controlled studies.
To examine the effectiveness of different modes of interventions (e.g. face‐to‐face or over the telephone, or by different sorts of healthcare or lay practitioners), and whether interventions containing both antenatal and postnatal elements are more effective than those taking place in the antenatal or postnatal period alone.
To examine the effectiveness of education and support from different care providers and (where information was available) training for care providers.
Methods
Criteria for considering studies for this review
Types of studies
Randomised or quasi‐randomised trials examining breastfeeding education and support interventions for women with twins and high order multiples. We included cluster‐randomised trials.
We included studies reported in brief abstracts, provided sufficient information was reported to allow us to assess risk of bias; those studies that did not provide sufficient information were considered in the review under the section, Studies awaiting classification, pending publication of the full study report.
Cross‐over studies are not an appropriate research design for this type of intervention and were not included.
Types of participants
Women with multiple pregnancies and births, during pregnancy or after birth and regardless of gestation at time of birth.
We included trials that recruited both women with multiple and singleton births provided that there were separate data available for women with multiples.
Types of interventions
The intervention was defined as breastfeeding education and support during pregnancy, the postnatal period (including immediately after delivery), or both for women with multiples. This included contact with an individual or individuals (either professional or volunteer) offering support which is supplementary to the standard care offered in that setting. ‘Support’ interventions eligible for this review included elements such as reassurance, praise, information, and the opportunity to discuss and to respond to the mother’s questions, and they also included staff training to improve the supportive care given to women. They could be offered by health professionals or lay people, trained or untrained, in hospital and community settings. They could be offered to groups of women or one‐to‐one, including mother‐to‐mother support, and the could be offered proactively by contacting women directly, or reactively, by waiting for women to get in touch. They could be provided face‐to‐face or over the phone, and they could involve only one contact or regular, ongoing contact over several months. Studies were included if the intervention occurred in the postnatal period alone or also included an antenatal component.
We included studies examining interventions which included education and support as part of a broader package of care provided that these elements were an important part of the package of care. We included education and training interventions aimed at staff providing care, provided that these interventions were designed to improve the education and support offered to women.
We included education or support for using any intervention designed to increase breastfeeding or breast milk feeding. This could include education or support interventions to encourage women to express breast milk either in the antenatal or postnatal period, or maternal education and support about other interventions which might increase or interfere with breastfeeding (such as pacifier use or kangaroo skin‐to‐skin mother care).
Comparisons: we compared different modes of interventions with each other or with standard care offered in that setting, and we identified the components of that standard care, wherever possible.
Types of outcome measures
The main outcome measure was the effect of the interventions on stopping breastfeeding or breast milk feeding by specified points in time. Primary outcomes were recorded for stopping any or exclusive breastfeeding before four to six weeks and at the last study assessment (up to six months). Other outcomes of interest were stopping any or exclusive breastfeeding at other time points (two, three, four, nine and 12 months), measures of neonatal and infant morbidity (where available), and measures of maternal satisfaction with care or feeding method.
Primary outcomes
Initiation of breastfeeding (baby put to the breast, even if on one occasion only McAndrew 2012) or breast milk feeding for each baby.
Initiation of breast milk expression by the mother.
Stopping any breastfeeding or any breast milk feeding before four to six weeks postpartum for each baby.
Stopping exclusive breastfeeding or exclusive breast milk feeding (baby has only ever been given breast milk and never given formula, solid foods or any other liquids McAndrew 2012) before four to six weeks postpartum for each baby.
Stopping breast milk expression before four to six weeks postpartum.
Stopping any breastfeeding or any breast milk feeding before six months postpartum for each baby.
Stopping exclusive breastfeeding or exclusive breast milk feeding before six months postpartum for each baby.
Stopping breast milk expression before six months postpartum.
Breast milk feeding could include expressed maternal breast milk, or expressed donor breast milk.
Secondary outcomes
Stopping any breastfeeding or any breast milk feeding before two, three, nine and 12 months postpartum.
Stopping exclusive breastfeeding or exclusive breast milk feeding before two, three, nine and 12 months postpartum.
Frequency of milk collection or number of participants expressing milk, or volume of expressed breast milk at any time point.
Maternal satisfaction with care.
Maternal satisfaction with feeding method.
All‐cause infant or neonatal morbidity (trialist defined).
Maternal morbidity (trialist defined).
Duration of NICU stay (days).
Psycho‐social outcomes including measures of attachment, self‐esteem, mental health, etc.
Search methods for identification of studies
The methods section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Review Group.
Electronic searches
We searched Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting the Information Specialist (30 June 2016).
The Register is a database containing over 22,000 reports of controlled trials in the field of pregnancy and childbirth. For full search methods used to populate Cochrane Pregnancy and Childbirth Group’s Trials Register including the detailed search strategies for CENTRAL, MEDLINE, EMBASE and CINAHL, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service, please follow this link to the editorial information about the Cochrane Pregnancy and Childbirth Group in the Cochrane Library and select the ‘Specialized Register ’ section from the options on the left side of the screen.
Briefly, Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Information Specialist and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE (Ovid);
weekly searches of EMBASE (Ovid);
monthly searches of CINAHL (EBSCO);
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Search results are screened by two people and the full text of all relevant trial reports identified through the searching activities described above is reviewed. Based on the intervention described, each trial report is assigned a number that corresponds to a specific Pregnancy and Childbirth Group review topic (or topics), and is then added to the Register. The Information Specialist searches the Register for each review using this topic number rather than keywords. This results in a more specific search set which has been fully accounted for in the relevant review sections (Included studies; Excluded studies; Ongoing studies).
We searched ClinicalTrials.gov (30 June 2016) and the WHO International Clinical Trials Registry Platform (ICTRP) (1 July 2016) for unpublished, planned and ongoing trial reports using the terms given in Appendix 1.
Searching other resources
In addition, one review author (T Dowswell) checked excluded studies from 'Support for healthy breastfeeding mothers with healthy term babies' (McFadden 2017) for any studies that included sick or preterm infants and which might have included multiples, and we checked reference lists of retrieved studies (H Whitford, T Dowswell and S Wallis).
We did not apply any language or date restrictions.
Data collection and analysis
The methods section of this review is based on a standard template used by Cochrane Pregnancy and Childbirth Group.
Selection of studies
Two review authors (H Whitford and T Dowswell) independently assessed for inclusion all the potential studies we identified as a result of the search strategy. We resolved any disagreement through discussion and, as required, we consulted a third review author.
We created a study flow diagram to map out the number of records identified, included and excluded,Figure 1
1.
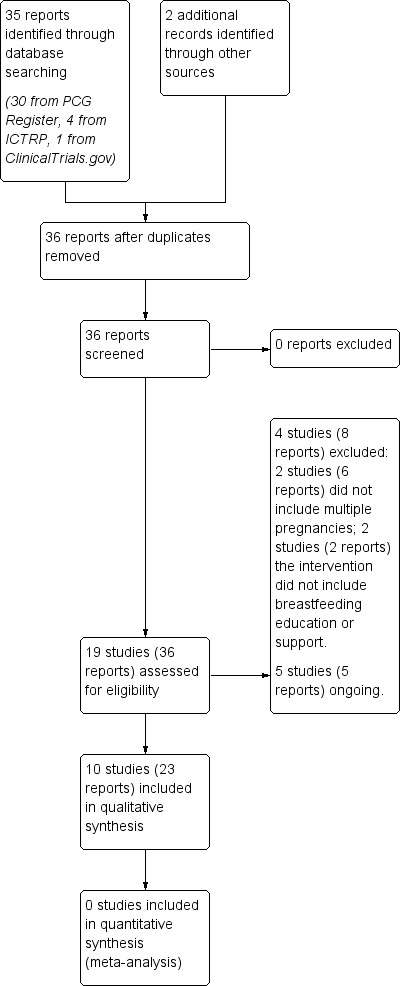
Study flow diagram.
Data extraction and management
We designed a form to extract data. For eligible studies, two review authors (H Whitford, H West, T Dowswell or S Wallis) independently extracted the data using the agreed form. We resolved any discrepancies through discussion and, as required, we consulted another member of the review team. We entered data into Review Manager software (RevMan 2014) and checked for accuracy. When information regarding any of the above was unclear, we attempted to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors (H Whitford, T Dowswell, H West, or S Wallis) independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion or by involving a third assessor.
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
Blinding of women and staff to support and education interventions is not straightforward and is usually not attempted. However, we described for each included study any methods used to blind or partially blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if we judged that any attempted blinding was likely to be effective or if we judged that lack of blinding would be unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We state whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or was supplied by the trial authors, we planned to re‐include missing data in the analyses which we undertook.
We assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias (even with fairly low levels of attrition there may be some bias because sample loss may be related to outcome).
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it is clear that all of the study’s prespecified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s prespecified outcomes have been reported; one or more reported primary outcomes were not prespecified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included study any important concerns we have about other possible sources of bias such as baseline imbalance between groups.
We assessed whether each study was free of other problems that could put it at risk of bias:
low risk of other bias;
high risk of other bias;
unclear whether there is risk of other bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at overall high risk of bias, according to the criteria given in the Handbook (Higgins 2011). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether it was likely to impact on the findings. We planned to explore the impact of the level of bias through undertaking sensitivity analyses, if appropriate ‐ seeSensitivity analysis.
Assessment of the quality of the evidence using the GRADE approach
We did not have data to assess the quality of the evidence. In future updates, for our main comparison (any education or support intervention versus standard care/no intervention), we plan to assess the quality of the evidence using the GRADE approach as outlined in the GRADE Handbook in order to assess the quality of the body of evidence relating to the following outcomes.
Initiation of breastfeeding or breast milk feeding for each baby.
Stopping any breastfeeding or any breast milk feeding before four to six weeks postpartum for each baby.
Stopping exclusive breastfeeding or exclusive breast milk feeding before four to six weeks postpartum for each baby.
Stopping any breastfeeding or any breast milk feeding before six months postpartum for each baby.
Stopping exclusive breastfeeding or exclusive breast milk feeding before six months postpartum for each baby.
Maternal satisfaction with care.
Maternal satisfaction with feeding method.
We plan to include women's views of feeding methods and care as outcomes to be graded as the focus of the review was on the quality of care for women as well as breastfeeding outcomes for the baby.
In future updates, we will use the GRADEpro Guideline Development Tool to import data from Review Manager (RevMan 2014) in order to create ’Summary of findings’ tables. We plan to summarise the intervention effect and a measure of quality for each of the above outcomes will be produced using the GRADE approach. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. The evidence can be downgraded from 'high quality' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Measures of treatment effect
Dichotomous data
In future updates, for dichotomous data, we will present results as summary risk ratios with 95% confidence intervals.
Continuous data
In future updates, for continuous data, we will use the mean difference if outcomes are measured in the same way between trials. We will use the standardised mean difference to combine trials that measure the same outcome, but use different measurement methods.
Unit of analysis issues
Cluster‐randomised trials
If we had found any cluster‐randomised trials, we would have included them in the analyses along with individually‐randomised trials, if such trials were identified and were otherwise eligible. We would have adjusted their sample sizes using the methods described in the Handbook using an estimate of the intracluster correlation coefficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population. If we had used ICCs from other sources, we would have reported this and conducted sensitivity analyses to investigate the effect of variation in the ICC. If we had identified both cluster‐randomised trials and individually‐randomised trials, we planned to synthesise the relevant information. We would have considered it reasonable to combine the results from both if there was little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit was considered to be unlikely.
We also would have acknowledged heterogeneity in the randomisation unit and would have performed a subgroup analysis to investigate the effects of the randomisation unit.
Cross‐over trials
We did not include cross‐over trials; such trials are not appropriate for this type of intervention.
Trials with more than two arms
If we had identified trials with more than two arms, we would have pooled results using the methods set out in the Handbook (Higgins 2011) to avoid double‐counting.
Other unit of analysis issues
This review focuses on twins and higher order multiples. Outcomes for these babies are not independent. For some outcomes (e.g. preterm birth), outcomes for babies from the same pregnancy are likely to be the same, or very highly correlated. For other outcomes, there will be a lower correlation (e.g. fetal death or infant anomaly). For breastfeeding outcomes, outcomes for twins or higher multiples are likely to be highly correlated although women may use different feeding methods for their babies, depending on infant birthweight, behaviour or other considerations. We were not able to include any data for analysis and so did not make any adjustments. In future updates, to take account of the non‐independence of outcomes for babies from multiple pregnancies, we will treat each multiple pregnancy as a cluster, and analyse data using methods described above for cluster‐randomised trials. We will seek ICCs for outcomes for twins and higher multiples from trials (if available) from similar trials or from observational studies. Where published ICCs are not available, we will consult with experts in the field to estimate ICCs, and conduct sensitivity analysis using a range of ICC values.
Dealing with missing data
For included studies, we noted levels of attrition. In future updates we will explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes, in future updates, we plan to carry out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we would attempt to include all participants randomised to each group in the analyses, and all participants would be analysed in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial would be the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
In future updates, we will assess statistical heterogeneity in each meta‐analysis using the Tau², I² and Chi² statistics. We will regard heterogeneity as moderate if the I² is greater than 30% and either the Tau² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity.
Assessment of reporting biases
If there were 10 or more studies in a future meta‐analysis, we would investigate reporting biases (such as publication bias) using funnel plots. We would assess funnel plot asymmetry visually. If asymmetry was suggested by a visual assessment, we would perform exploratory analyses to investigate it.
Data synthesis
We planned to carry out statistical analysis using the Review Manager software (RevMan 2014). In future updates, we will use fixed‐effect meta‐analysis for combining data where it is reasonable to assume that studies are estimating the same underlying treatment effect: i.e. where trials are examining the same intervention, and the trials’ populations and methods are judged sufficiently similar. If there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or if substantial statistical heterogeneity was detected, we would use random‐effects meta‐analysis to produce an overall summary, if an average treatment effect across trials is considered clinically meaningful. The random‐effects summary would be treated as the average of the range of possible treatment effects and we would discuss the clinical implications of treatment effects differing between trials. If the average treatment effect was not clinically meaningful, we would not combine trials.
If we used random‐effects analyses, the results would be presented as the average treatment effect with 95% confidence intervals, and the estimates of Tau² and I².
Subgroup analysis and investigation of heterogeneity
We did not combine data in meta‐analysis, due to insufficient data. In future updates, if we identify substantial heterogeneity, we will investigate it using subgroup analyses and sensitivity analyses. We will consider whether an overall summary is meaningful, and if it is, we will use random‐effects analysis to produce it.
As data permit, we will plan to carry out the following subgroup analysis:
by type of supporter (professional versus lay person, or both);
by type of support (face‐to‐face versus telephone support);
by timing of support (antenatal alone, postnatal alone, or both);
by whether the support was proactive (scheduled contacts) or reactive (women needed to request support);
by background breastfeeding initiation rates (low, medium, or high background rates);
by intensity of support (number of scheduled contacts);
by whether babies were premature or sick or healthy babies delivered at term (> 37 weeks’ gestation);
by whether babies were twins or higher order multiples.
We will use only primary outcomes in subgroup analysis.
We plan to assess subgroup differences by interaction tests available within RevMan (RevMan 2014). We will report the results of subgroup analyses quoting the Chi² statistic and P value, and the interaction test I² value.
Sensitivity analysis
There were insufficient data to carry out any sensitivity analyses to examine any possible effect of risk of bias on results. In future updates of this review, if appropriate, for our primary outcomes, we will temporarily remove studies at high or unclear risk of bias (using the allocation concealment domain) to examine whether this has an impact on results. We will also carry out sensitivity analysis to examine the effects of varying the ICC when adjusting data for a cluster design effect.
Results
Description of studies
Results of the search
The search identified 19 studies (36 reports), see Figure 1. Searching the Cochrane PCG Register retrieved 30 reports, searching ClinicalTrials.gov retrieved one report, searching the WHO International Clinical Trials Registry Platform (ICTRP) retrieved four, and the PCG search identified two additional reports for included studies (Graffy 2004; Paul 2012). We did not identify any studies from those excluded from the publication 'Support for healthy breastfeeding mothers with healthy term babies' (McFadden 2017), or the reference lists of retrieved studies.
We included ten studies (23 reports) (Collins 2004; Graffy 2004; Hoddinott 2012; Junior 2007; Paul 2012; Penfold 2014; Reeder 2014; Sellen 2013; Serwint 1996; Winterburn 2003). See Characteristics of included studies.
Five studies (five reports) are ongoing and only the protocol was available. These studies (Brizot 2009; Kikuchi 2015; Kimani‐Murage 2013; Martin‐Iglesias 2011; Yang 2016) may be eligible for future updates. See Characteristics of ongoing studies.
We excluded four studies (eight reports) (Bonuck 2005; Robling 2012; Simmer 2015; Tomlinson 2014). See Characteristics of excluded studies.
Included studies
See Characteristics of included studies.
Design
Seven studies were two‐arm randomised controlled trials. One compared volunteer counsellor support in antenatal and postnatal period to usual care (Graffy 2004), one compared proactive with reactive telephone support for mothers living in disadvantaged circumstances (Hoddinott 2012), one compared breastfeeding support and encouragement for mothers of preterm infants to usual care (Junior 2007), one compared home nurse visits to usual care for ‘well’ breastfeeding newborns and mothers (Paul 2012), one compared prenatal paediatric home visits to usual care (Serwint 1996), and one compared support from a close female confidante to usual care (Winterburn 2003). One two‐arm cluster‐randomised controlled trial compared home‐based counselling visits to usual care (Penfold 2014). There were two, three‐arm randomised controlled trials. One compared telephone peer counselling (low and high frequency) to usual care (Reeder 2014) and the other compared continuous cell phone support to monthly peer support and usual care (Sellen 2013). One four‐arm randomised controlled trial compared the use of cup +/‐ dummy and bottle +/‐ dummy for infants delivered before 34 weeks, and whose mothers wanted to breastfeed (Collins 2004). This study was included because the intervention was part of a package of care and was designed to find out the effect of cups/dummies on breastfeeding. It included education and support for the hospitals and might have had an effect on the mother, but this detail was not specified.
Sample sizes
Trials recruited 5787 women (this included 512 women interviewed as part of a cluster randomised trial). Studies had sample sizes ranging from 69 (Hoddinott 2012) to 1948 (Reeder 2014). However, women with multiples were a small subgroup within these studies, and the only data provided that reported on women with twins and higher order births were from two studies, one with 27 women (Paul 2012) and the other with 15 women (Reeder 2014).
Setting
Seven studies were carried out in high‐income countries: Australia (Collins 2004), Scotland (Hoddinott 2012), England (Graffy 2004; Winterburn 2003), and the USA (Paul 2012; Reeder 2014; Serwint 1996). One took place in an upper‐middle income country: Brazil (Junior 2007), and two in low‐income countries: Tanzania (Penfold 2014) and Kenya (Sellen 2013).
Participants
We included studies in the review if they did not exclude twins or multiples. However in some studies this was not explicitly stated in the methods and further information was sought from authors. Some authors (Paul 2012; Reeder 2014) provided this information.
Participant inclusion specified infant criteria in four studies. Collins 2004 included babies born before 34 weeks' gestation whose mothers wanted to breastfeed, Junior 2007 included babies weighing less than 1500g at birth, Graffy 2004 excluded women if the baby was born before 36 weeks' gestation and Paul 2012 specified ‘well’ newborns born after 34 weeks' gestation who were breastfeeding at discharge and their mothers intended to continue after discharge.
Serwint 1996 recruited women in pregnancy who were between eight to 28 weeks' gestation, Graffy 2004 recruited women between 28 to 36 weeks' gestation and Sellen 2013 recruited women between 32 to 36 weeks' gestation.
The other studies stated criteria for the mother at recruitment. Hoddinott 2012 included women who had started breastfeeding and Graffy 2004 included women who were considering breastfeeding and who did not plan to consult a counsellor anyway. Women were defined by sociodemographic criteria in three studies: living in the three most disadvantaged postcode areas and over the age of 16 (Hoddinott 2012), over 18, nulliparous, low income families, who had not yet selected a paediatrician (Serwint 1996), and low‐income urban women (Sellen 2013). Graffy 2004 excluded women if they intended to move away within four months of the birth or if the home was considered unsafe for home visits. Communication criteria were stated in two studies: an ability to communicate in English on the phone (Hoddinott 2012), and being able to speak English (Paul 2012).
Women or their infants were excluded from participating if the mother was under 16 years (Hoddinott 2012), had a medical contraindication (Hoddinott 2012; Junior 2007; Paul 2012), the physical condition of baby prevented breastfeeding (Junior 2007), if the hospital stay was longer than usual, there were any postnatal complications in the mother or newborn, no phone number, living outside the area, or child protection concerns (Paul 2012), and any prenatal drug use, psychiatric illness, or HIV positive status (Serwint 1996).
Three studies included all women and did not specify any exclusion criteria; all women living in the intervention ward (Penfold 2014), all antenatal women (Winterburn 2003), and no exclusion criteria stated (Sellen 2013).
Interventions and comparisons
Timing of intervention
In two studies, the intervention took place in the antenatal period only (Serwint 1996; Winterburn 2003); five included both the antenatal and postnatal periods (Graffy 2004; Junior 2007; Penfold 2014; Reeder 2014; Sellen 2013), and three took place in the postnatal period only (Collins 2004; Hoddinott 2012; Junior 2007).
Where was the intervention carried out
In eight studies, the intervention was offered to the women at home (Graffy 2004, Hoddinott 2012; Paul 2012; Penfold 2014; Reeder 2014; Sellen 2013; Serwint 1996; Winterburn 2003), one study carried out interventions in hospital only (Collins 2004), and one study carried out interventions, both in hospital and the outpatient clinic (Junior 2007).
Method of intervention
Phone support only was provided in two studies; proactive phone contact was compared to reactive contact in one study (Hoddinott 2012) (from a specialised feeding team for all women), and one study compared proactive phone contact (high frequency or low frequency) to usual care (Reeder 2014). Sellen 2013 compared cell phone support, to monthly peer‐led support groups or usual care. Two studies compared a combination of face‐to‐face and phone contact to usual care (Graffy 2004; Junior 2007). Four studies offered face‐to‐face contact only (Paul 2012; Penfold 2014; Serwint 1996; Winterburn 2003). One study provided a combination of education and support to staff as part of a package of care (Collins 2004)
Breastfeeding support: training and experience
Breastfeeding support was provided by healthcare staff in four studies. In one study, support was provided by a feeding team of staff who had breastfeeding induction and a recognised two‐day training course (Hoddinott 2012), in one study, by a nurse who had extra breastfeeding training (Paul 2012), in one study, by a paediatrician with training in breastfeeding techniques and support to encourage breastfeeding (Serwint 1996), and in one study, by a community midwife who had no extra training (Winterburn 2003).
Support was provided by lay personnel in four studies. In one study, volunteers with five days training provided the support (Penfold 2014), in one study, peer supporters had training in counselling which included breastfeeding support (Reeder 2014), in one study, support was provided by National Childbirth Trust volunteer counsellors (women who had breastfed and had training in non‐directive counselling and strengthening mothers' confidence in their own abilities) (Graffy 2004) and Sellen 2013 used trained peer leaders to provide support.
The training of the supporter was not specified in one study (Junior 2007), and in Collins 2004, details of the personnel who provided training to the staff were not given.
Frequency of contact
In three studies, the extra support was provided during one visit (Paul 2012; Serwint 1996; Winterburn 2003), in two studies it was offered as often as needed (Graffy 2004; Hoddinott 2012), and in one study, it was ongoing while the infant was in hospital (Collins 2004). Sellen 2013 provided cell phone support on a continuous basis, while the comparison support group was held monthly. One study did not specify the frequency of contact; it was delivered as often as the researcher was available while the infant was in hospital, then monthly after discharge (Junior 2007). Two studies had a prescribed regimen of visits: three visits in pregnancy and two in the early postnatal period with extra visits for small babies (Penfold 2014), four visits (two in pregnancy and two up to two weeks postnatal) compared to calls (four extra up to four months) (Reeder 2014).
Outcomes
Although in all studies multiple pregnancies or births (women or their infants) were eligible for inclusion, no outcome data were reported separately for multiples in the published reports. Email enquiries elicited data for multiples from Paul 2012 (see Appendix 2) and Reeder 2014 (see Appendix 3). These authors provided data on initiation of breastfeeding, survival data on duration of any breastfeeding, and duration of exclusive breastfeeding. Paul 2012 also reported infant morbidity and measures of maternal satisfaction for mothers of multiples. However, the outcomes for infants from the same pregnancy were not reported separately, therefore the data could not be reported in the review. If the studies had reported data for multiples individually, correction would need to be applied for correlation between babies with the same mother. The degree of correlation would be likely to vary depending on the outcome, so intracluster correlation coefficients would need to be established.
In most studies, outcomes were reported for all women (including mothers of multiples but not for these women or infants separately). Seven studies collected data about exclusive breastfeeding. The timing of these data varied: at discharge to home (Collins 2004), at three days (Penfold 2014), at six weeks (Graffy 2004), at six to eight weeks (Hoddinott 2012), at discharge to home, then monthly until six months (Junior 2007), at one, three, and six months (Reeder 2014) and at three months of age (Sellen 2013).
Six studies collected data about any breastfeeding: at discharge to home, three and six months (Collins 2004), at six weeks and four months (Graffy 2004), at six to eight weeks (Hoddinott 2012), at discharge to home, then monthly until six months (Junior 2007), at two weeks, two months and six months (Paul 2012), and at one, three, and six months (Reeder 2014).
Two studies reported ‘breastfeeding’ rates, but did not specify whether this was any or exclusive; Serwint 1996 reported rates at 30 and 60 days, while Winterburn 2003 reported initiation at birth, and duration at 10 days, one month, six weeks, three months and six months.
The definition of exclusive breastfeeding was given in six studies: no other types of milk or solids, except vitamins and minerals (Collins 2004), no other liquids of solid foods (Graffy 2004), no other liquids except medicines in past 24 hours (Hoddinott 2012), only mother’s milk (Junior 2007), only breast milk (Penfold 2014), and non‐exclusive breastfeeding derived by date of introduction of formula (Reeder 2014). In four studies the definition of exclusive breastfeeding was not specified (Paul 2012; Sellen 2013; Serwint 1996; Winterburn 2003).
The method of breastfeeding was specified in one study: direct at breast or other feeding device (Collins 2004), but not in any other study (Graffy 2004; Hoddinott 2012; Junior 2007; Paul 2012; Penfold 2014; Reeder 2014; Sellen 2013; Serwint 1996; Winterburn 2003).
Excluded studies
See Characteristics of excluded studies.
Four studies (eight reports) were excluded (Bonuck 2005; Robling 2012; Simmer 2015; Tomlinson 2014). Two studies (six reports) were excluded because women with multiples were excluded from the analysis (Bonuck 2005; Tomlinson 2014). One study (one report) was excluded because the intervention did not include breastfeeding education or support (Robling 2012). One study (Simmer 2015) included insufficient information in the paper about the element of education and support for mothers. As it was potentially eligible, the authors were contacted and data were provided for twins, but no further information was supplied about the intervention and it was still not clear if the intervention included education or support, so it was subsequently excluded.
Risk of bias in included studies
2.
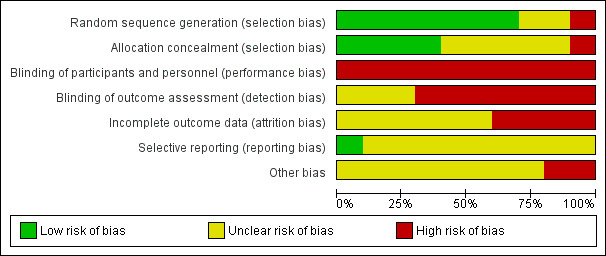
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
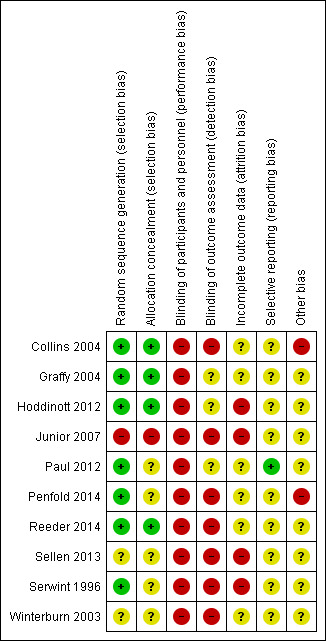
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
We considered that only four of the studies used methods that were at low risk of bias to generate the random sequence and conceal allocation to the experimental groups (Collins 2004; Graffy 2004; Hoddinott 2012; Reeder 2014). Three studies used adequate methods for random sequence generation, but their methods for allocation concealment were not clear (Paul 2012; Penfold 2014; Serwint 1996). In two studies, with respect to sequence generation and allocation concealment, these domains were either not described or were unclear (Sellen 2013; Winterburn 2003). One study was at high risk of bias for both domains (Junior 2007).
Blinding
All included studies were at high risk of bias for blinding of participants or staff. In all studies, both women and staff were aware of group allocation and were therefore potentially subject to performance bias (Collins 2004; Graffy 2004; Hoddinott 2012; Junior 2007; Paul 2012; Penfold 2014; Reeder 2014; Sellen 2013; Serwint 1996; Winterburn 2003). With this kind of intervention, it is very difficult for women or staff to be blind to group allocation. Three studies were judged to be at unclear risk of detection bias (Graffy 2004; Hoddinott 2012; Paul 2012). In these studies, investigators made an attempt to blind outcome assessors, however, because women knew their group allocation, and all data were self‐reported, the group allocation could have been revealed during data collection. All the other studies were judged to be at high risk of detection bias because the blinding of outcome assessors was not described (Collins 2004; Junior 2007; Penfold 2014; Reeder 2014; Sellen 2013; Serwint 1996; Winterburn 2003).
Incomplete outcome data
In this review, we considered even low levels of attrition as potentially problematic in terms of risk of bias because sample loss may be related to the true outcome. All studies were assessed at high or unclear risk of attrition bias. Four studies were assessed at high risk of attrition bias: one because there was high loss to follow up and imbalance between arms of the study (Hoddinott 2012), and three because of high loss to follow up (Junior 2007; Sellen 2013; Serwint 1996). The others were at unclear risk of attrition bias: two because of incomplete reporting (Penfold 2014; Winterburn 2003) and four because although there was low attrition, there was no information on loss of twins and any missing data could relate to the true outcome (Collins 2004; Graffy 2004; Paul 2012; Reeder 2014).
Selective reporting
Paul 2012 was assessed at low risk of reporting bias. In all other studies, the protocol was not available or there was insufficient information provided to be able to assess risk of selective reporting (Collins 2004; Graffy 2004; Hoddinott 2012; Junior 2007; Penfold 2014; Reeder 2014; Sellen 2013; Serwint 1996; Winterburn 2003).
Other potential sources of bias
All studies raised concerns about other potential sources of bias. Two were assessed at high risk of bias: Collins 2004 because of baseline imbalance between the groups and high noncompliance with the intervention, and Penfold 2014 because leakage from the intervention to control areas was possible and there was lack of clarity about whether adjustment was made for clusters. All other studies were judged at unclear risk of other sources of bias. Baseline differences were noted between groups in two studies (Graffy 2004; Hoddinott 2012), in one study, the intervention was not delivered to all participants in the intervention group (Reeder 2014), and in one study, the study group differed significantly from the background population (Winterburn 2003). Sellen 2013 was assessed from a series of abstracts, with no full report of the study available.
None of the studies included in this review looked specifically at women with multiples, but instead considered breastfeeding education and support for all women, whether they had one or more babies. Randomisation was not stratified by singleton/multiple birth, and small numbers of women with multiples were recruited. This may result in chance baseline imbalance between the randomised groups. However, as baseline characteristics were not reported separately for multiples, it is not possible to gauge whether the groups were comparable. Sample size power calculations were based on all women, so all the studies were underpowered to show differences in the subgroup of women with multiples.
Where additional data on multiples were provided by trialists (Paul 2012; Reeder 2014), the data were not presented separately for each individual baby. To interpret breastfeeding outcomes, this introduces an assumption that individual multiples were fed in the same manner. This may not be the case, for example, if one baby was admitted to NICU or had difficulty feeding. Paul 2012 reported infant morbidity, however this was presented for only one baby out of each set of multiples. This may have inflated the number of adverse events and introduces a high risk of bias. Where data were given for just one baby of the multiples, we need to know if it was decided beforehand which baby would contribute data (e.g. the first born), or if any adverse event was reported for whichever baby it happened to.
Effects of interventions
The following is a narrative description of the data on the effects of interventions for women with multiples in Paul 2012 and Reeder 2014. Data tables are presented in Appendix 2 and Appendix 3. The methodological and reporting limitations of these studies prevented us from analysing the data in RevMan (RevMan 2014).
Home nurse visits versus usual care
Primary outcomes
One study (Paul 2012) randomised 576 mothers, including 15 women with multiples. Data were not reported separately for mothers of multiples in the paper but were obtained from the authors. A single response from each mother was used to assess outcomes in all babies.
All women included in the study had initiated breastfeeding and intended to breastfeed post discharge (seven out of seven women in the home nurse visit group, eight out of eight women in the routine care group). The duration of breastfeeding was reported using survival data, so stopping any breastfeeding before four to six weeks postpartum was not explicitly reported, however it appears that two out of six women in the home nurse visit group and one out of seven women in the usual care group stopped any breastfeeding before four weeks. Likewise, stopping exclusive breastfeeding before four to six weeks postpartum was not explicitly reported, however it appears that five out of five women in the home nurse visit group and five out of seven women in the usual care group stopped exclusive breastfeeding before four weeks. Similarly, stopping any breastfeeding before six months postpartum andstopping exclusive breastfeeding before six months postpartum were not explicitly reported, however it appears that five out of six women in the home nurse visit group and four out of six women in the usual care group stopped exclusive breastfeeding before six months, and all women in the home nurse visit group and six out of seven women in the usual care group stopped exclusive breastfeeding before six months.
Women in the home nurse group breastfed their babies for an average duration of 67.00 days (standard deviation (SD) 62.00, n = 6), compared with 132.86 days (SD 79.57, n = 7) in the usual care group. The duration of exclusive breastfeeding was 7.58 days (SD 8.32, n = 6) compared with 33.14 days (SD 66.51, n = 7) in the control group. However, the small number of women and wide variation in duration means that these data are very unreliable.
Initiation of breast milk expression, stopping breast milk expression before four to six weeks postpartum, andstopping breast milk expression before six months postpartum were not reported.
Secondary outcomes
The study authors provided data from multiples for infant morbidity (jaundice, infant feeding difficulty, weight loss, dehydration, illness not related to jaundice/feeding, ER visit, and hospitalisation) at two weeks after discharge, and two months after discharge, and measures ofmaternal satisfaction (amount of information on feeding your baby, clarity of information on feeding your baby, amount of help with feeding your baby, and total satisfaction with care), assessed in hospital, two weeks after discharge, and two months after discharge. These data are presented in Appendix 2.
Other secondary outcomes were not reported for women with multiples.
Telephone peer counselling (low and high frequency) versus usual care
Primary outcomes
One study (Reeder 2014) randomised 1948 mothers, including 27 women with a multiples, to three study arms: low frequency telephone contact (two prenatal and two early postpartum calls from a peer counsellor, eight women), high frequency telephone contact (two prenatal and up to six postpartum calls from a peer counsellor, nine women), or usual care (10 women). High and low frequency support were reported together in the main publication, as there was no difference in the number of contacts women received in these groups. Data were not reported separately for multiples in the paper but were obtained from the authors. A single response from each mother was used to assess outcomes in all babies.
The number of women who initiated breastfeeding was 16 out of 17 in the telephone contact groups, and nine out of 10 in the usual care group. The duration of breastfeeding was reported using survival data, so stopping any breastfeeding before four to six weeks postpartum was not explicitly reported, however, it appears that four out of 13 women in the telephone support group and six out of nine women in the usual care group stopped any breastfeeding before four weeks. Likewise, stopping exclusive breastfeeding before four to six weeks postpartum was not explicitly reported, however, it appears that 12 out of 16 women in the telephone support group and six out of six women in the usual care group stopped exclusive breastfeeding before four weeks. Similarly, stopping any breastfeeding before six months postpartum andstopping exclusive breastfeeding before six months postpartum were not explicitly reported, however, it appears that 11 out of 13 women in the telephone support group and eight out of nine women in the usual care group stopped exclusive breastfeeding before six months, and 14 out of 16 women in the telephone support group and six out of six women in the usual care group stopped exclusive breastfeeding before six months.
Initiation of breast milk expression, stopping breast milk expression before four to six weeks postpartum, andstopping breast milk expression before six months postpartum were not reported.
Secondary outcomes
None of the secondary outcomes were reported for women with a multiples.
Discussion
Summary of main results
We identified 10 studies of education and support for breastfeeding that included multiples. All the studies were interventions aimed at all women (or babies), and were studies which did not exclude multiple births. Therefore, none of the forms of breastfeeding education or support included specific advice or expertise relating to the challenges of breastfeeding multiples. Numbers of infants from multiple pregnancies were small and none of the studies were powered to answer questions about multiples. Out of the 10 included studies (a total of 5787 women, including 512 women interviewed as part of a cluster randomised trial), only two studies gathered data for multiples separately and were able to provide us with the data (Paul 2012; Reeder 2014, a total of 42 women with multiples). Due to the scarcity of evidence and the format in which data were reported, we were unable to present any analyses in this review, and we were unable to GRADE the evidence.
The two trials with data for women with multiple births compared home nurse visits versus usual care (15 women), and telephone peer counselling versus usual care (27 women). The number of women who initiated breastfeeding was reported (all 15 women in one study, 25 out of 27 women in one study). Stopping any breastfeeding before four to six weeks postpartum, stopping exclusive breastfeeding before four to six weeks postpartum, stopping any breastfeeding before six months postpartum and stopping exclusive breastfeeding before six months postpartum were not explicitly reported, and there were insufficient data to draw any meaningful conclusions from survival data.
Stopping breast milk expression before four to six weeks postpartum, and stopping breast milk expression before six months postpartum were not reported. Measures of maternal satisfaction were reported in one study of 15 women, but there were insufficient data to draw any conclusions; no other secondary outcomes were reported for women with a multiple pregnancy in either study. No adverse events were reported.
Overall completeness and applicability of evidence
In no study did the training of breastfeeding supporters explicitly detail training specific to breastfeeding multiples. None of the studies were designed to offer tailored support or education about breastfeeding for women who had given birth to more than one infant. We were unable to answer questions about the effectiveness of education and support for breastfeeding multiples from different care providers and the training of care providers, nor of the timing, intensity or form of support, as none of the studies examined these issues. We identified two ongoing studies (Brizot 2009; Yang 2016) which appeared to offer interventions specifically designed for twins, but results were not available.
The interventions in the studies that we identified may not be applicable or appropriate for women with multiples. Support and education for women with a singleton may not be relevant to address the particular challenges of breastfeeding more than one infant. Support provided in a single visit or only in the antenatal period may not meet the needs of women breastfeeding more than one infant. Women who have delivered more than one baby may spend more time in hospital or the babies may be premature; interventions provided only at home may not be appropriate. The particular challenges of prematurity and breastfeeding more than one infant were not addressed.
There are other factors related to breastfeeding multiples which were not included in this review, and where evidence is lacking. Evidence is needed about positioning for the babies or the mother while breastfeeding more than one infant at the same time, particularly when the infants are premature or when the mother has undergone operative delivery. Questions about simultaneous or separate feeding remain. For feeding premature multiples (as for premature singleton infants), there is uncertainty about methods of breast milk expression, the use of donor breast milk, the use of supplementary milks or fortifiers and the best way to transition to direct feeding at the breast once the infants are ready. Our review found that evidence is lacking about the optimum timing, frequency, method or person to provide support and education to mothers to promote and encourage breastfeeding of multiples.
Many studies of breastfeeding support or education specifically exclude women or infants who have experienced complications. Women expecting multiple births are more likely to experience complicated births, such as caesarean section, and their infants are more likely to be delivered early or be admitted to special care. These women and infants would therefore be more likely to be excluded from studies of predominantly normal or healthy dyads, and the effectiveness of interventions to address their particular needs not assessed.
The 10 studies were heterogeneous in terms of the setting of the studies, the method of delivery of the intervention, the location of the intervention, the frequency of the intervention, the timing of the intervention, the training of the person or people delivering the intervention, the timing of the data collection, and the definitions used for breastfeeding. These differences limit conclusions that can be drawn about what works for breastfeeding support of multiples. Differences in cultural attitudes to infant feeding and background levels of breastfeeding initiation can have a strong influence on outcomes.
Quality of the evidence
The overall quality of the evidence was mixed, and the risk of bias was mostly high or unclear. In some studies we were unable to judge risk of bias because of a lack of information, for example, when only abstracts were available. Blinding of participants and assessors in this kind of intervention is difficult and response bias is likely. Due to the scarcity of evidence and the format in which data were reported, a narrative description of the data is presented, no analyses are presented in this review, and we were unable to GRADE the evidence.
None of the studies included in this review looked specifically at women with multiples, but instead considered breastfeeding education and support for all women, whether they had one or more babies. Randomisation was not stratified by singleton/multiples, and small numbers of women with multiples were recruited. This may result in chance baseline imbalance between the randomised groups. However, as baseline characteristics were not reported separately for multiples, it is not possible to gauge whether the groups were comparable. Sample size power calculations were based on all women, so the studies are underpowered to show differences in the subgroup of women with multiples.
Potential biases in the review process
Two authors independently assessed eligibility for inclusion, carried out data extraction and assessed risk of bias. Disagreements were resolved by discussion, and by involving a third assessor. The lack of available information in many studies meant the risk of bias was unclear. Our reliance on authors to send unpublished data on multiples may have been open to bias, as authors might be more likely to respond when interventions had shown a positive effect.
Agreements and disagreements with other studies or reviews
A systematic review of feeding twins, triplets and higher order multiples was carried out by Denton 2011 to inform a booklet of information and advice for parents (Multiple Births Foundation 2011). The authors found good quality evidence of breastfeeding rates in multiples. However, most of the evidence for specific practices was of low quality, from small descriptive studies and case reports. Guidance for parents was therefore based on a summary of the options available and recommended good practice points, where available. This review informed the development of our protocol.
Related Cochrane reviews have examined education and support interventions to promote breastfeeding in singletons, and findings suggest that education and support interventions by professionals and lay supporters or peers may increase the initiation of breastfeeding and increase the duration of exclusive or any breastfeeding (Balogun 2016; McFadden 2017). The current review needs to be interpreted in the light of these findings, whilst recognising that women with multiple pregnancies may face additional challenges to initiate and sustain breastfeeding.
Authors' conclusions
Implications for practice.
We found no evidence from randomised controlled trials about forms of breastfeeding support and education for women with multiples, or the most effective way to provide support or education. There was no evidence about the best way to deliver support and education, the timing of the intervention, or the best person to deliver the intervention. Therefore, conclusions for practice cannot be made on the basis of the findings of this review.
Implications for research.
Well designed, adequately powered research is needed to answer questions about the education and support that can help mothers of multiples to breastfeed their babies. Trials are needed that examine interventions designed specifically for women with multiples and delivered by people with training about how to overcome the particular challenges of breastfeeding more than one infant. Outcome data needs to be gathered for the mothers of twins and higher order multiples separately so that conclusions can be drawn about what works for all mothers of multiples.
Acknowledgements
Thanks to Mars Lord, mother of twins, for providing helpful feedback on the review protocol.
This project was supported by the National Institute for Health research, via Cochrane Infrastructure and Cochrane Programme Grant funding to the Cochrane Pregnancy and Childbirth Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Therese Dowswell and Helen West's work is supported by an NIHR Cochrane Programme Grant Project: 13/89/05 – Pregnancy and childbirth systematic reviews to support clinical guidelines.
As part of the pre‐publication editorial process, this review has been commented on by two peers (an editor and referee who is external to the editorial team), a member of the Pregnancy and Childbirth Group's international panel of consumers and the Group's Statistical Adviser.
Appendices
Appendix 1. Search terms for ClinicalTrials.gov and ICTRP
ClinicalTrials.gov and ICTRP (search was run as separate lines in ICTRP)
(breastfeeding OR breast‐feeding OR breastfeed OR breast‐feed OR lactation) AND (twin OR twins or "multiple pregnancy" OR multiple pregnancies" OR "higher order pregnancy" OR "higher order pregnancies")
Appendix 2. Data: Paul 2012
| group allocation (individual participant data) |
duration of any breastfeeding (days) | stopped any breastfeeding (at 6 months follow up) (1 = stopped; 0 = still breastfeeding at follow up) |
duration of exclusive breastfeeding (days) | stopped exclusive breastfeeding (at 6 months follow up) (1 = stopped; 0 = still breastfeeding at follow up) |
| usual care | 150 | 1 | 1 | 1 |
| usual care | 7 | 1 | 3 | 1 |
| usual care | 30 | 1 | 1 | 1 |
| usual care | 182 | 0 | 182 | 0 |
| usual care | 181 | 0 | 14 | 1 |
| usual care | 192 | 0 | 30 | 1 |
| usual care (missing data) | ||||
| usual care | 188 | 0 | 1 | 1 |
| Mean | 132.8571 | 33.14286 | ||
| SD | 79.56638 | 66.51172 | ||
| home nurse visit | 182 | 0 | 3 | 1 |
| home nurse visit | 13 | 1 | 8.5 | 1 |
| home nurse visit | 60 | 1 | 0 | 1 |
| home nurse visit | 42 | 1 | 0 | 1 |
| home nurse visit (missing data) | ||||
| home nurse visit | 84 | 1 | 21 | 1 |
| home nurse visit | 21 | 1 | 13 | 0 |
| Mean | 67 | 7.583333 | ||
| SD | 62 | 8.321158 | ||
| Infant visit for jaundice at 2 weeks | Infant visit for feeding difficulty at 2 weeks | Infant visit for weight loss at 2 weeks | Infant visit for dehydration at 2 weeks | Infant visit for illness not related to jaundice/feeding at 2 weeks | ER visit at 2 weeks | hospitalisation at 2 weeks | |
| Usual care | 3 | 0 | 0 | 0 | 0 | 1 | 0 |
| Home nurse visit | 1 | 0 | 1 | 0 | 0 | 0 | 0 |
| Infant visit for jaundice at 2 months | Infant visit for feeding difficulty at 2 months | Infant visit for weight loss at 2 months | Infant visit for dehydration at 2 months | Infant visit for illness not related to jaundice/feeding at 2 months | ER visit at 2 months | hospitalisation at 2 months | |
| Usual care | 0 | 0 | 0 | 0 | 3 | 2 | 2 |
| Home nurse visit | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| home nurse visit | usual care | |||||
| Maternal satisfaction with: | total | mean | SD | total | mean | SD |
| Amount of information on feeding your baby (hosp) | 7 | 4.285 | 1.25 | 8 | 4.125 | 0.83 |
| Clarity of information on feeding your baby (hosp) | 7 | 4.142 | 1.21 | 8 | 4.0 | 0.75 |
| Amount of help with feeding your baby (time hosp) | 7 | 4.142 | 1.07 | 8 | 4.25 | 1.03 |
| Total satisfaction with care (time 2 wk) | 7 | 49.57 | 7.66 | 8 | 50.625 | 6.93 |
| Amount of information on feeding your baby (time 2 wk) | 6 | 4.5 | 0.84 | 7 | 4 | 1.15 |
| Clarity of information on feeding your baby (time 2 wk) | 6 | 4.33 | 0.82 | 7 | 4 | 0.82 |
| Amount of help with feeding your baby (2 wk) | 6 | 4.5 | 0.84 | 7 | 3.57 | 0.79 |
| Total satisfaction with care (time 2 wk) | 6 | 44.83 | 8.38 | 7 | 44.85 | 7.82 |
| Amount of information on feeding your baby (time 2 month) | 6 | 4 | 0.89 | 7 | 3.86 | 0.69 |
| Clarity of information on feeding your baby (time 2 month) | 6 | 4 | 0.89 | 7 | 3.86 | 0.69 |
| Amount of help with feeding your baby (time 2 month) | 6 | 3.66 | 1.50 | 7 | 3.71 | 0.75 |
| Total satisfaction with care (time 2 month) | 6 | 46.66 | 5.68 | 7 | 45.28 | 6.15 |
Appendix 3. Data: Reeder 2014
| Control (no peer counsellor) N = 10 women |
Peer counsellor (low frequency) N = 8 women |
Peer counsellor (high frequency) N = 9 women |
|
| Initiated breastfeeding | 9 | 7 | 9 |
| Any breastfeeding duration (weeks) | |||
| 0.5 | 4 | 0 | 0 |
| 2 | 1 | 0 | 3 |
| 3 | 1 | 1 | 0 |
| 5 | 1 | 0 | 1 |
| 9 | 0 | 0 | 2 |
| 22 | 0 | 3 | 1 |
| 26 | 1 | 1 | 0 |
| 39 | 1 | 0 | 0 |
| 52 | 0 | 1 | 0 |
| Unknown | 0 | 1 | 2 |
| none | 1 | 1 | 0 |
| Exclusive breastfeeding duration (weeks) | |||
| 0.5 | 5 | 3 | 5 |
| 1 | 0 | 1 | 0 |
| 2 | 1 | 1 | 1 |
| 3 | 0 | 0 | 0 |
| 5 | 0 | 0 | 1 |
| 13 | 0 | 1 | 0 |
| 22 | 0 | 1 | 0 |
| 26 | 0 | 0 | 0 |
| 39 | 0 | 0 | 1 |
| 52 | 0 | 0 | 1 |
| Unknown | 3 | 0 | 0 |
| none | 1 | 1 | 0 |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Collins 2004.
| Methods | 4‐arm randomised controlled trial to determine the effect of artificial teats on breastfeeding. Postnatal interventions while preterm infants were in hospital. Breastfeeding education and support was provided to staff. | |
| Participants | Setting: Australia, 2 tertiary referral hospitals. Recruitment: April 1996‐November 1999. Inclusion criteria: women with singleton or twin infants < 34 weeks’ gestation who wanted to breastfeed. Exclusion criteria: infants with congenital abnormalities precluding enteral feeding. |
|
| Interventions | Cup/no dummy n = 82. Cup/dummy n = 69. Bottle/no dummy n = 70. Bottle/dummy n = 82. “Cup or bottle feeding commenced at the discretion of the attending nurse/midwife or neonatologist and occurred when the mother was unavailable to breastfeed or when additional milk, given orally, was required after a breastfeed. Small plastic medicine cups were used as described by Lang. Infants randomised to the dummy groups had dummies available on trial entry; their use was encouraged during tube feeds and when the infant was restless. For infants who did not receive a dummy, alternate soothing methods were promoted (for example, facilitation of hand to mouth action promoting self quieting behaviour). Recruiting hospital received education, written instructions, literature, and 1‐to‐1 support. Written instructions, literature, and telephone contact were provided to participating peripheral hospitals.” |
|
| Outcomes | The primary outcomes were the proportion of infants fully breastfeeding (compared with partially and not) and the proportion receiving any breastfeeding (compared with none) on discharge home. Breastfeeding was defined as mother’s milk given by direct breastfeeding or other feeding device. Full breastfeeding meant that no other types of milk or solids were given except vitamins and minerals. Secondary outcomes included the length of hospital stay and prevalence of breastfeeding at 3 and 6 months after discharge. | |
| Notes | This study was included because the intervention was part of a package of care and was designed to find out the effect of cups/dummies on breastfeeding. It included education and support for the hospitals and might have had an effect on the mother, but this detail was not specified. The authors were contacted about data for multiples in March 2016; mail was returned and other contact addresses unavailable (T Dowswell). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “An independent researcher developed a separate randomisation schedule for each recruiting hospital by using a random number table to select balanced blocks of varying size with stratification for gestation (<28 weeks, 28‐<34 weeks).” |
| Allocation concealment (selection bias) | Low risk | “Researchers determined allocation by telephoning an independent ward, available 24 hours a day, within the recruiting hospitals.” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Participants, care providers, and researchers were not blinded to treatment allocation.” Lack of blinding may have affected outcomes and treatment decisions. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | “Data entry and analysis were undertaken unblinded.” Lack of blinding may have affected interpretation of the data. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | All women appear to be accounted for, both in a study flow diagram and in the text. Attrition of women with multiple pregnancies is not described separately. |
| Selective reporting (reporting bias) | Unclear risk | This study was assessed from a published report without access to the protocol, therefore we cannot be certain whether all prespecified outcomes were reported. |
| Other bias | High risk | The report states that noncompliance was high: 56% of infants randomised to cup feeding had a bottle introduced, and 31% of the infants randomised to no dummy had a dummy introduced. These figures for noncompliance were not presented for multiple pregnancies. The results appear to be presented and analysed by intention‐to‐treat. “We accounted for the dependence due to inclusion of twins by using robust variance estimates clustering on the mother.” Results for multiple pregnancies will be underpowered, as the sample size was calculated to show differences in all babies (singletons and multiples). “Most maternal and neonatal characteristics were balanced between groups. There was, however, a ≥10% difference between dummy and no dummy for primiparity and number who had breastfed before and between cup and bottle for primiparity”. This possible baseline imbalance may have affected the results. Elsewhere, the study reported that primiparous women were more likely to have complied with the study protocol, and that previous breastfeeding may have influenced successful breastfeeding with this baby. |
Graffy 2004.
| Methods | Randomised controlled trial of volunteer support from counsellors in breastfeeding. | |
| Participants | Setting: 32 General Practitioner (GP) practices in London and south Essex, England, with mixed or deprived population. Recruitment between April 1995 and August 1998. |
|
| Interventions | Inclusion criteria: considering breastfeeding. Exclusion criteria: having breastfed a previous child for 6 weeks, planning to move out of area within 4 months of the birth, planning to consult a counsellor anyway, considered unsafe for home visits, or delivery before 36 weeks. Recruitment 28‐36 weeks' gestation. Multiples were not excluded from the study, however the number of women with multiple pregnancies who participated in the study were not reported. Intervention group: n = 363. Counselling provided by 28 National Childbirth Trust (NCT) accredited counsellors, by women who had breastfed and had training in counselling (non‐directive approach and strengthening mothers' confidence in own abilities). Visited once before birth and offered postnatal support by phone or home visits. Control group: n = 357, details of care not described. |
|
| Outcomes | The main outcome was the prevalence of any breastfeeding at 6 weeks. Secondary outcomes included the proportion of women giving any breast feeds, or bottle feeds at 4 months, the duration of any breastfeeding, time to introduction of bottle feeds, and satisfaction with breastfeeding at 6 weeks. | |
| Notes | Multiples were not excluded from the study, however data were not reported separately. Authors were contacted for data about multiples in July 2016; response is still awaited (H Whitford). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was achieved using numbered, sealed envelopes prepared by the statistical adviser from random permuted blocks and held in the study office. The sample was stratified by practice and birth order using separate sets of envelopes for mothers of first and subsequent babies. |
| Allocation concealment (selection bias) | Low risk | Central allocation was used using numbered sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Women and counsellors were not blind to group allocation. Unclear if other staff were aware of group allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The identity of participants was held separately from the data records prepared when questionnaires were returned. Responses were coded blind to treatment allocation. Counsellors played no part in assessing feeding outcomes, but women were aware of group allocation and outcome data were self reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Loss to follow‐up: Intervention group: 13/363 at birth, 14/350 at 6 weeks, 26/336 at 4 months. Control group: 17/357 at birth, 14/350 at 6 weeks, 26/336 at 4 months. All women accounted for, but missing data could be related to true outcome. |
| Selective reporting (reporting bias) | Unclear risk | This study was assessed from a published report without access to the protocol, therefore we cannot be certain whether all prespecified outcomes were reported. |
| Other bias | Unclear risk | There were differences in the baseline characteristics of the participants. More women in the intervention group were undecided about breastfeeding. Results for multiple pregnancies will be underpowered, as the sample size was calculated to show differences in all babies (singletons and multiples). |
Hoddinott 2012.
| Methods | 2‐arm randomised controlled trial. The FEeding Support Team (FEST) trial, comparing proactive and reactive telephone support with reactive‐only telephone support for breastfeeding women living in disadvantaged areas. | |
| Participants | Setting: Scotland. Hospital maternity unit, mix of urban and rural population. Inclusion criteria: women admitted to the ward between 26 July and 18 October 2010 who lived in the 3 most disadvantaged postcode area quintiles for the Scottish Index of Multiple Deprivation in 2009 and who were breastfeeding. Exclusion criteria: women aged < 16 years, with serious medical or psychiatric problems, or with insufficient spoken English to communicate by telephone. |
|
| Interventions | Experimental group: proactive and reactive telephone support (n = 35). Daily proactive phone calls for 1 week with further calls if needed up to 14 days. Women could call the team at any time for 2 weeks. Control group: reactive calls only (n = 34). Women had support from the feeding team in hospital, and the option of reactive calls after discharge. “All postnatal ward staff (including the FEST team) had a breastfeeding induction and completed a 2‐day Unicef accredited training programme.” |
|
| Outcomes | Any breastfeeding at 6‐8 weeks, exclusive breastfeeding at 6‐8 weeks (no other liquids except medicines within the previous 24 hours), satisfaction with breastfeeding help in hospital and at home (using a rating scale where 0 was least satisfied and 10 was most satisfied), number of days readmitted to hospital (mother or baby) and contact with health professionals following hospital discharge. | |
| Notes | Authors were contacted for data about multiples in March 2016 and they reported that no separate data were available for multiples (H Whitford). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Women were randomised “immediately after discharge using a website randomisation sequence service set up by an independent statistician. Randomisation was stratified to ensure balance of primiparous and multiparous women across both trial arms”. |
| Allocation concealment (selection bias) | Low risk | A website randomisation sequence service set up by an independent statistician was used. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | “Although not informed of the randomisation outcome, women knew if they had been randomised to the proactive group as they received a phone call from the feeding team within 24h of hospital discharge”. Participants and caregivers were not blinded, and this may have influenced outcomes. It is not clear if womens' ‘usual’ caregivers knew of allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | “Outcomes were collected by telephone by a researcher who was blind to randomisation and who had no other contact with study women”. However, women knew their group allocation and may have revealed this during the data collection interview. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Lost to follow‐up at 6‐8 weeks: 3/35 in the proactive group, 8/34 in the reactive group: overall 16%. Missing data could be related to the true outcome and loss to follow up was not balanced across groups. |
| Selective reporting (reporting bias) | Unclear risk | This study was assessed from a published report without access to the protocol, therefore we cannot be certain whether all prespecified outcomes were reported. However, the report states that "no changes to outcome measurement were made during the study". |
| Other bias | Unclear risk | There were differences in the baseline characteristics of the groups that may have affected the outcomes. Women in the proactive call group were older on average, and more lived in the most disadvantaged postcode areas. Results for multiple pregnancies will be underpowered, as the sample size was calculated to show differences in all babies (singletons and multiples). |
Junior 2007.
| Methods | 2‐arm non‐blind randomised controlled trial. Comparing breastfeeding support and encouragement to mothers of preterm infants. | |
| Participants | Setting: Urban hospital in Brazil. Inclusion criteria: preterm newborn, weighing less than 1500 g at birth. Exclusion criteria: all situations that prevented breastfeeding due to the infant’s or the mother’s conditions: severe neurologic problems or facial malformations that made breast sucking difficult, digestive tract malformations, hospitalisation for longer than 4 months, HIV+ mother, death. Twins were included, but multiple births were excluded. |
|
| Interventions | “If the infant belonged to the intervention group, the researcher was called and stayed with the mother in the labor room” “(T)he researcher would provide information about the infant’s conditions to the mother” “At the mother’s hospital discharge, support was offered again and instructions about the continuation of milk production and treatment were repeated” “When an infant of the intervention group was discharged, the researcher gave the parents a cell phone number for contact. The first meeting was scheduled one week after discharge”. |
|
| Outcomes | "Exclusive breastfeeding was defined as feeding the infant with only the mother’s milk, and breastfeeding was any situation in which the infant received the mother’s milk, regardless of whether exclusively or not." Assessed at discharge. Follow‐up after discharge was monthly until 6 months |
|
| Notes | Authors were contacted about data for multiples in March 2016: no response was received and other contact addresses were unavailable (H Whitford). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | “(A)llocation followed the consecutive and alternate order of birth of the infants. By drawing lots, it was determined that the first infant would be assigned to the routine group”. This method appears to be alternate randomisation. |
| Allocation concealment (selection bias) | High risk | Allocation was not concealed if alternate. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel were not blind to group allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | “Data about the two groups were recorded and reviewed by the researcher; therefore, the study was not blinded”. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 15/51 in the routine group, 13/49 in the intervention group were excluded. The main causes were prolonged hospitalisation (32%) and death (43%); causes were similar in the 2 groups. There was no loss to follow‐up at 6 months after discharge or up to weaning in the remaining infants. |
| Selective reporting (reporting bias) | Unclear risk | Not clear what data were recorded. |
| Other bias | Unclear risk | “Mothers and infants in the two groups had similar characteristics”. Results for multiple pregnancies will be underpowered, as the sample size was calculated to show differences in all babies (singletons and multiples). |
Paul 2012.
| Methods | 2‐armed randomised controlled trial "to compare office‐based care (OBC) with a care model using a home nursing visit (HNV) as the initial postdischarge encounter for “well” breastfeeding newborns and mothers". | |
| Participants | Setting: deliveries in 1 medical centre in Pennsylvania, USA, Sept 2006‐Aug 2009. Inclusion criteria: “Eligible newborns were singletons and twins born after at least 34 weeks’ gestation to English‐ speaking mothers attempting to breastfeed during the maternity stay and with intent to continue breastfeeding after discharge". Exclusion criteria: "Dyads were excluded for atypical stays characterized by (1) a 2‐night or longer stay after a vaginal delivery; (2) a 4‐night stay or longer after a cesarean section; (3) a hospital course with atypical complications (e.g., ambiguous genitalia, endometritis); or (4) newborn hyperbilirubinaemia requiring phototherapy during the nursery stay. Mothers were also excluded for major morbidities and/or preexisting conditions that would affect postpartum care, lack of a telephone number, previous study participation, residence outside the coverage region of the Visiting Nurse Association of Central Pennsylvania (VNA), or if an HNV was specifically requested by a hospital social worker or child protective services owing to social concerns". |
|
| Interventions | Randomised n = 1154. Home nurse visits n = 576 (n = 7 twins). Control group n = 578 (n = 8 twins). Nurses had received additional training on supporting breastfeeding. Women also had a clinic visit 1 week after the nurse visit to check recovery after the birth and newborn weight (5‐14 days after the birth). The nurses provided information on a range of issues including safety, infant care and infant feeding. |
|
| Outcomes | Primary outcomes: maternal satisfaction with care, maternal mental health, infant morbidity, emergency room visits, and hospital admission. Secondary outcomes: data provided by the research team on maternal satisfaction, breastfeeding outcomes (duration and exclusivity) and infant health outcomes at 2 weeks and 2 months following birth. |
|
| Notes | Authors were contacted about data for multiples in March 2016: data were received (T Dowswell). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “The computer‐generated randomization sequence included stratification for delivery type (vaginal delivery, forceps‐ or vacuum‐assisted vaginal delivery, or cesarean section)." |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Women and staff providing care could be aware of randomisation group. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It was stated that follow‐up was by telephone interviews carried out by staff unaware of the randomisation group – it is possible women may have revealed group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | There were some missing data for questionnaires. |
| Selective reporting (reporting bias) | Low risk | Outcome reporting bias is unlikely. Protocol was available and all relevant outcomes appear to be reported. |
| Other bias | Unclear risk | Groups appeared balanced at baseline. (However, there were limited data for twin deliveries, so baseline comparability not clear for this group. Data were not adjusted for twins, rather a single outcome for each set of twin was reported.) Results for multiple pregnancies will be underpowered, as the sample size was calculated to show differences in all babies (singletons and multiples). |
Penfold 2014.
| Methods | 2‐arm cluster‐randomised trial, to compare home based counselling visits to usual care. | |
| Participants | Setting: community areas of Southern Tanzania, in 2009‐2011. Inclusion criteria: ward in 1 of the 6 districts: Newala, Randahimba, Lindi Rural, Ruangwa and Nachingwea, Mtwara Rural. Exclusion criteria: “There were no exclusion criteria for clusters, households, or women, after randomisation”. |
|
| Interventions | Wards randomised n = 132, in 6 district study areas. Home‐based counselling intervention to improve newborn care, in addition to routine care. Volunteers were recruited from the villages to implement the counselling intervention. They initiated the contact and provided face‐to‐face contacts in 3 visits during pregnancy, 2 in the early neonatal period, with additional visits for small babies. Volunteers were trained for 5 days by their district health teams and followed up in their villages after starting conducting home visits. |
|
| Outcomes | "Breastfed within 1 hour of birth, fed only breast milk in first 3 days." | |
| Notes | Authors were contacted about data for multiples in March 2016: no response was received (T Dowswell). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “(I)mplicit stratification to maximise balance in intervention and comparison groups. We listed the 114 wards in order of district, division, tertile of baseline neonatal mortality, and population, splitting them into 57 pairs. We allocated the wards in each ‘pair’ to intervention or control using random numbers generated by Microsoft Excel. This is equivalent to 57 tosses of the coin”. |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | “The nature of the intervention prevented blinding researchers, community members or health staff to the allocation”. This may have introduced bias in treatment decisions, and assessment of outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The nature of the intervention prevented blinding researchers, community members or health staff to the allocation”. This may have introduced bias in the assessment of outcomes during household interviews. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | “If no household members were present at the time the interviewer visited, the household was visited again later the same day. Households were not replaced in cases of refusal or absence. Logical checks and skip patterns took place at data entry”. Difficult to tell if there were incomplete data. |
| Selective reporting (reporting bias) | Unclear risk | Newborn morbidity is prespecified in the protocol, but does not appear to have been reported (mortality is reported, however). Supervisors undertook quality control activities: accompanying interviewers to 3 households each day, and revisiting households where interviewers had reported either that there were no residents or they refused to participate. 2 houses were revisited daily and a small number of interview questions were repeated, to compare with the responses collected. 'Random' method of selection of houses to collect outcome measures using throw of a pen. |
| Other bias | High risk | Not sure if adjustment was made for clusters. Some leakage from intervention to control areas was possible. Results for multiple pregnancies will be underpowered, as the sample size was calculated to show differences in all babies (singletons and multiples). |
Reeder 2014.
| Methods | 3‐arm randomised trial of telephone peer counselling (low frequency contact, high frequency contact compared to usual care). | |
| Participants | Setting: Oregon, USA. Recruitment July 2005‐July 2007 (stopped after December 2005 in 1 Local WIC Agency (LWA) because of no peer counsellor). Inclusion criteria: English or Spanish‐speaking women attending a new pregnancy appointment for WIC (Supplemental Nutrition Program for Women, Infants, and Children) who indicated that they intended to breastfeed or who were undecided about breastfeeding. Women were not excluded on the basis of age, multiple gestations, or previous birth history. Exclusion criteria: none. |
|
| Interventions | 4 telephone contacts (n = 646) or 8 telephone contacts (n = 645) (subsequently combined because there was no difference in the distribution of peer contacts) compared to usual care (n = 657). Contact from peers who had “breastfed at least 1 infant for a minimum of 6 months, were currently or had been a WIC client within the past 5 years, were able to devote at least 10 hours per week to peer counselling, were able to access transportation to bring them to the clinic several times per week, and were fluent in Spanish if service Spanish‐speaking clients”. Proactive contact, by telephone during pregnancy and postnatal period. Counsellors received training which was “grounded in the Loving Support curriculum, covered technical breastfeeding topics, methods of providing peer support, scope of practice, and the benefits of breastfeeding”. “Women assigned to the low‐frequency peer counselling group were scheduled to receive 4 planned, peer‐initiated contacts: the first after initial prenatal assignment, the second 2 weeks before the expected due date, and the third and 4th at 1 and 2 weeks postpartum. Women in the higher‐frequency treatment group were to receive 8 scheduled calls. The first 4 calls were the same as those in the low‐frequency treatment group and the last 4 calls were scheduled at months 1, 2, 3 and 4.” | |
| Outcomes | Initiation, duration, and exclusivity of breastfeeding at 1, 3 and 6 months. Recorded weekly for first month, then monthly to 1 year. "Duration of exclusive and nonexclusive breastfeeding was derived from the first time that the mother reported to WIC that she had stopped breastfeeding or introduced formula and the timing of each." | |
| Notes | Authors were contacted about data for multiples in March 2016: data were received (H Whitford). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “(C)onsent forms were sent to the state’s WIC office. The forms were sorted between Spanish‐ and English‐speaking clients, after which they were randomly allocated to 1 of 3 study arms by using a computer‐generated random number function”. |
| Allocation concealment (selection bias) | Low risk | Randomisation happened at a central office. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants: “participants knew how many contacts they were eligible to receive”. Caregiver: “Peer counsellors were not blinded to the treatment status of their clients”. This lack of blinding may have affected decisions about feeding. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome assessor not described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 17 women were lost to follow‐up, 46 women lost their infant or pregnancy: 3.2% attrition (1885/1948 were retained). Breastfeeding duration data were missing for women who reported breastfeeding and then left WIC. The percentages of unknown breastfeeding duration and exclusivity differed significantly between the 3 study arms. |
| Selective reporting (reporting bias) | Unclear risk | “There was no difference in the number of contacts between the high‐ and low‐frequency treatment groups for women with non‐missing data on breastfeeding outcomes. Nor did we find any difference in breastfeeding outcomes between those in the high‐ and low‐frequency treatment groups. As a result we combined the 2 peer counselling treatment arms into a single category”. This change to the prespecified method is well justified, but may have introduced bias. |
| Other bias | Unclear risk | Figures presented for when peer counsellors contacted women suggest that 52% were reached within 1 week after delivery, and 72% by 2 weeks. 78% of all women in the treatment group had multiple telephone contacts with a peer counsellor, with at least 1 prenatal and 1 postpartum call. This means that 28% of women had not been reached at 2 weeks postpartum, and 22% had only 1 contact (or all contact was prenatal or postnatal). This is a sizeable proportion of women who did not receive the intervention. Results appeared to be intention‐to treat. The groups of women appeared to be comparable at baseline:
“(T)here was no evidence of pretreatment covariate imbalance among women assigned to a treatment arm”. Results for multiple pregnancies will be underpowered, as the sample size was calculated to show differences in all babies (singletons and multiples). |
Sellen 2013.
| Methods | 3‐arm randomised trial of telephone peer counselling (low frequency contact, high frequency contact compared to usual care). Randomised controlled trial of women in attending antenatal care at a large hospital to receive 1 of 3 support strategies from late pregnancy (32‐26 weeks) to 3 months postpartum: cell phone based peer support (CPS) or monthly peer‐led support groups (PSG) or Control |
|
| Participants | Setting: a large hospital in Kenya, Africa. 753 randomised. Inclusion criteria: low‐income urban women (32‐36 weeks pregnant). Exclusion criteria: none known. |
|
| Interventions | Women were randomised to community‐based continuous cell phone‐based peer support (CPS) or monthly peer‐led support groups (PSG) or Control (usual care). Trained peer leaders supported both pregnant and postpartum women randomised to CPS and PSG from late pregnancy (32‐26 weeks) to 3 months postpartum and were retrained mid intervention using FAQ of participants. (Data available for 504 women.) | |
| Outcomes | Exclusive breastfeeding (EBF) estimated by maternal 7 day recall of use of infant foods at 3 months age by intervention group. Process evaluation results were also collected. | |
| Notes | Authors were contacted about data for multiples in March and April 2016: no response was received (H Whitford).Only conference abstracts were available. Unable to find any published papers with full report of details. Attempted to contact authors on 3 occasions with no reply. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described, other than ‘randomised’. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants: not possible due to the interventions. Caregiver: not possible due to the interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome assessor not described. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 753 women randomised; 504 (66.6%) infants completed, including twins (Sellen 2014). “There was no difference in dropout by baseline cell‐phone access or intervention allocation” (Mbugua 2013). |
| Selective reporting (reporting bias) | Unclear risk | Assessed from several abstracts, with no protocol available. Few outcomes were reported. |
| Other bias | Unclear risk | Full study report unavailable and assessment made on basis of abstracts only. Supported by Bill & Melinda Gates Foundatin to FHI360, through the Alive & Thrive Small Grants Program managed by UC Davis; Global Alliance for Improved Nutrition; Canada Research Chair program award. |
Serwint 1996.
| Methods | 2‐armed randomised control trial of a prenatal paediatric home visit for urban low‐income families. | |
| Participants | Setting: USA, urban, low‐income families. Recruitment from February 1992 to July 1993. Inclusion criteria: nulliparous pregnant women, 18 years or older, 8‐28 weeks gestational age, who "had not yet selected a paediatrician or wanted their infant to receive paediatric care at the hospital‐based paediatric clinic were recruited from the hospital‐based obstetrics clinic". Exclusion criteria: "Women were excluded if they admitted to prenatal drug use or had a recognized psychiatric illness or human immunodeficiency virus infection". |
|
| Interventions | Intervention: women received an appointment by mail for a prenatal paediatric visit to occur between 32 to 36 weeks' gestation and a telephone reminder 24‐48 hours before the appointment, n = 81. Comparison: women were not offered a prenatal paediatric visit, but received standard care, n = 75. Both groups received a letter informing them of the paediatrician's name, and clinic brochures. Paediatricians received training in components of the prenatal paediatric visit, counselling parents of a newborn infant, and breastfeeding techniques and strategies to encourage breastfeeding initiation. |
|
| Outcomes | Breastfeeding intent, initiation and duration (at 30 and 60 days); car safety seat use; circumcision; health service use; relationship between mother and paediatrician. | |
| Notes | "In the one instance where a mother delivered twins, only twin A infant was included in the study." Authors were contacted about data for multiples in March 2016: no response was received (S Wallis). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Random number table with blocks of 10”; “block randomization was chosen to control for any variation that might occur over time in recruitment strategies or resident education”. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants or caregivers was described. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of outcome assessors was described. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | All women were accounted for, but high attrition at 2 months may have introduced bias. “A total of 156 women enrolled in the study; 81 were randomised to the prenatal visit intervention group and 75 to the control group.” “By the time of delivery, 74 mother‐infant dyads remained in the intervention group and 70 in the control group.” (miscarriage or transferred obstetric care elsewhere) “When the infants were 2 months, 54 intervention mothers and 51 control mothers were interviewed” (mostly because of transferred care). 4.4% lost at delivery, 33% lost at 2 months. |
| Selective reporting (reporting bias) | Unclear risk | This study was assessed from a published report, without access to the protocol. It is therefore not possible to be certain if all prespecified outcomes were reported. |
| Other bias | Unclear risk | The 2 groups of women appeared to have similar baseline characteristics: “Dyads in the intervention and control group did not differ with regard to maternal age, education, type of medical coverage, week at which prenatal care was initiated, infant gestational age at birth, race, or rate of vaginal delivery”. “The data were analysed using the “intention to treat” model. Mothers remained in the intervention group once they were randomized whether or not they made a prenatal pediatric visit”. Results for multiple pregnancies will be underpowered, as the sample size was calculated to show differences in all babies (singletons and multiples). |
Winterburn 2003.
| Methods | 2‐armed randomised controlled trial. | |
| Participants | Setting: primary care in North Trent, UK. Inclusion criteria: "All women attending hospital and general practice antenatal clinics in one locality in North Trent were invited to participate". Exclusion criteria: none described. |
|
| Interventions | Intervention: pregnant women "were asked in the antenatal period to identify a close female confidante who could support them to breastfeed following the birth of their babies". "The midwife visited both mother and confidante together during the antenatal period to discuss breastfeeding." Most women chose their mothers as their breastfeeding supporter, n = 30. Control: "Routine antenatal care which also included a home visit to discuss breastfeeding, although this was without a female confidante", n = 42. |
|
| Outcomes | Breastfeeding initiation and duration at birth, 10 days, 1 month, 6 weeks, 3 months and 6 months. Not specified if any or exclusive breastfeeding recorded. | |
| Notes | Authors were contacted about data for multiples in March 2016: no response was received (S Wallis). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Randomised”, but no further information given. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and caregivers were not blinded to the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The results were not recorded as raw data, so numerators and denominators were not clear. 4/30 women did not receive a home visit to discuss breastfeeding. It is unclear whether these women were subsequently included in analyses (intention‐to‐treat) or not. |
| Selective reporting (reporting bias) | Unclear risk | The study was assessed from a brief published report without access to the protocol. It is unclear whether all prespecified outcomes were reported. |
| Other bias | Unclear risk | “The breastfeeding initiation rate for women participating in the study was significantly higher than the background Trust rate”. This suggests that women taking part in the study were not representative of the population being studied. Results for multiple pregnancies will be underpowered, as the sample size was calculated to show differences in all babies (singletons and multiples). |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bonuck 2005 | Women with multiple pregnancies were excluded from the analyses. |
| Robling 2012 | The intervention did not include breastfeeding education or support. |
| Simmer 2015 | The intervention did not include breastfeeding education or support. |
| Tomlinson 2014 | Women with multiple pregnancies were excluded from the study. |
Characteristics of ongoing studies [ordered by study ID]
Brizot 2009.
| Trial name or title | Effect of prenatal counselling on breastfeeding rates in twins. |
| Methods | RCT |
| Participants | Setting: Sao Paulo? Inclusion criteria: twin pregnancies with both fetuses alive between 18 and 34 weeks of gestational age and followed at our twin clinic; absence of absolute contraindications for breastfeeding, such as positive serology for HIV, HTLV; absence of use of lactation inhibitors to treat prolactinoma, such as cabergoline and bromocriptine; and absence of major malformation of 1 or both twins that could preclude breastfeeding, including gastrointestinal defects, cleft lip and cleft palate, or malformations that would require neonatal surgery with prolonged hospital stay. Exclusion criteria: fetal or postnatal death of 1 or both twins; delivery before 32 weeks; major malformation diagnosed postnatally; and lost to follow‐up or no breastfeeding information. |
| Interventions | Behavioral: prenatal counselling group. |
| Outcomes | Primary outcomes: mother's total and exclusive breastfeeding rates (time frame: 5 years). Secondary outcomes: twin infant's breastfeeding rates (time frame: 5 years). |
| Starting date | Sept 2009. |
| Contact information | MARIA DE LOURDES BRIZOT, University of Sao Paulo. |
| Notes | Authors were contacted July 2016 for reports or data (H Whitford). |
Kikuchi 2015.
| Trial name or title | EMBRACE. |
| Methods | Cluster‐randomised controlled trial using an effectiveness‐implementation hybrid design. Adequate randomisation, unblinded. Unit of randomisation: sub‐district, Unit of analysis: individual mother/newborn. |
| Participants | Setting: Ghana (Dodowa, Kintampo, and Navrongo Health and Demographic Surveillance System (HDSS) sites). Mostly rural, although 1 area is 40km from Accra so pregnant women often prefer to deliver at health facilities there. Women who have given birth between 1 October 2014 and 30 September 2015. Inclusion criteria: women of reproductive age between the ages of 15 and 49 years who live in the areas covered by the Dodowa, Kintampo, and Navrongo HDSS sites. Women who have given birth between 1 October 2014 and 30 September 2015. Exclusion criteria: women who refuse to participate in the intervention. |
| Interventions | Intervention: package including use of a new continuum of care card, continuum of care orientation for health workers, 24‐hour health facility retention of mothers and newborns after delivery, and postnatal care by home visits. |
| Outcomes | Maternal, newborn, and child health outcomes. Continuum of care completion rate, rate of postnatal care within 48 hours, complication rate requiring mothers' and newborns' hospitalisations, and perinatal and neonatal mortality. |
| Starting date | Women giving birth between 1 October 2014 and 30 September 2015. |
| Contact information | Masamine Jimba: mjimba@m.u‐tokyo.ac.jp |
| Notes |
Kimani‐Murage 2013.
| Trial name or title | |
| Methods | Cluster‐randomised controlled trial of personalised, home‐based nutritional counselling. Adequate randomisation, unblinded. |
| Participants | Setting: Nairobi, Kenya. Inclusion criteria: "all pregnant women aged between 12 to 49 years old, who are residents of community units in Korogocho and Viwandani slums that fall within the Nairobi Urban Health and Demographic Surveillance System (NUHDSS) area, and their respective children (when born)". Exclusion criteria: "women of reproductive age who will have given birth before the proposed intervention starts; (b) women with a disability that makes delivery of the intervention difficult (for example, a hearing or sight problem, or mental handicap)". |
| Interventions | Personalised home based, face‐to‐face nutrition counselling during pregnancy and up to 1 year re breastfeeding – positioning, attachment, exclusive breastfeeding, expressing, complementary feeding. During pregnancy ‐ monthly to 34 weeks, then weekly. After delivery, weekly for first month, then monthly. Existing community health workers trained in counselling using UNICEF developed package of training. |
| Outcomes | The primary outcome measure was exclusive breastfeeding for 6 months. Data on breastfeeding practices will be collected longitudinally from birth every 2 months through an interviewer‐administered questionnaire to the mother. |
| Starting date | Recruitment Sept 2012‐Dec 2013. |
| Contact information | Elizabeth W Kimani‐Murage: ekimani@aphrc.org |
| Notes |
Martin‐Iglesias 2011.
| Trial name or title | |
| Methods | Community parallel 2‐arm cluster‐randomised controlled trial. Adequate randomisation, unblinded. Unit of randomisation: PHCC (Primary Healthcare Centre). |
| Participants | Setting: primary care in Leganés, Madrid, Spain. Inclusion criteria: all mothers of infants born during the study period (6 months) who come to the health centre on the first visit of the childcare programme. Able to participate in the trial, localisable for the next year, able to understand the questionnaires. Exclusion criteria: mothers and infants with contraindications for breastfeeding. Mothers' contraindications: HIV positive, substance abuse, chemotherapy, radioactive isotope treatments until the elimination of the isotope from the mother's body, active tuberculosis, active chickenpox, active Herpes lesions, Chagas disease. Infants' contraindications: galactosaemia. |
| Interventions | Intervention: implementation strategy for a breastfeeding guideline in primary care, including training sessions, information distribution, and opinion leader, (planned n = 120). Comparison: usual diffusion of a breastfeeding guideline in primary care, (planned n = 120). |
| Outcomes | Exclusive or predominant breastfeeding at 6 months. |
| Starting date | Planned to recruit between 1st June 2010 and 1st December 2010. |
| Contact information | Susana Martín‐Iglesias: smartin.gapm09@salud.madrid.org |
| Notes | Plan to use intention‐to‐treat analysis. Funded by the Spanish Ministry of Science and Innovation via the Instituto de Salud Carlos III (PI08/90680). |
Yang 2016.
| Trial name or title | The intervention for breastfeeding of twins: a randomised controlled trial. |
| Methods | RCT. |
| Participants | Setting: The First People's Hospital of Yichang, China. Inclusion criteria: participants were recruited from the prenatal clinics of The First People's Hospital of Yichang, they had completed the first malformation screening before 24 weeks' gestation, congenital fetal malformation had been excluded by ultrasound, delivered between 37‐42 weeks' gestation and the weigh of infant >= 2500 g. Exclusion criteria: women were experiencing psychosis and serious disease; dependent on substances such as tobacco, alcohol and drugs; not married; age < 18; existing congenital fetus malformation; the infant had important helminthic disease; mother of her infant could not be discharged at the same time because of health; women could not access internet or smartphone every day. |
| Interventions | Twins Intervention (TI group): take part in the Wechat [counselling intervention] of breastfeeding; Twins Control(TC group): follow up through telephone call |
| Outcomes | Primary: the rates of exclusive breastfeeding during 6 months postpartum for each baby.;breastfeeding self‐efficacy of mothers; breastfeeding knowledge of mothers. |
| Starting date | Registered with ChiCTR 21.3.216. |
| Contact information | Yang Huaijie, 2 Jiefang Road, Xilin District, Yichang, Hubei, China, yanghuaijie2011@163.com |
| Notes | ChiCTR‐IOR‐16008124. |
Differences between protocol and review
The title was changed from 'Breastfeeding education and support for women with multiple pregnancies' to 'Breastfeeding education and support for women with twins or higher order multiples'.
Minor changes to the background were made in response to author feedback.
The subgroup analysis "by whether babies were twins or higher order multiples" was added to the planned analyses.
Reports excluded from the review 'Support for healthy breastfeeding mothers with healthy term babies' (McFadden 2017) were checked for any studies that included sick or preterm infants and which might have included multiples.
Helen West was added as an author.
Contributions of authors
Heather Whitford produced the first draft of the protocol, revised the protocol, assessed studies, extracted data for the review, wrote and revised the text.
Selina Wallis commented on drafts of the protocol, assessed studies, extracted data for the review, and contributed to the text of the review.
Therese Dowswell contributed to the methods section of the protocol, revised drafts of the protocol, and assessed studies.
Mary Renfrew contributed to outcome selection and commented on drafts of the protocol and text of the review.
Helen West assessed studies, extracted data for the review, wrote and revised the text.
Sources of support
Internal sources
(T Dowswell & H West) Cochrane Pregnancy and Childbirth Group, Department of Women's and Children's Health, The University of Liverpool, Liverpool, UK.
External sources
-
National Institute for Health Research (NIHR), UK.
T Dowswell & H West are supported by the NIHR Cochrane Programme Grant Project: 13/89/05 – Pregnancy and childbirth systematic reviews to support clinical guidelines.
Declarations of interest
Heather Whitford: none known.
Selina Wallis: none known.
Therese Dowswell: is employed through a grant from NIHR to her institution (The University of Liverpool) for her work on this protocol, and in the last 36 months has received funding from WHO to work on other Cochrane reviews.
Mary J Renfrew: has received grants for quality improvement work related to feeding infants in neonatal units; small amounts of money, in regard to co‐authoring a book; and been an invited speaker at a range of professional and academic organisations, for which no fee was received. None of these activities are in conflict with the subject of this review.
H West: is employed through a grant from NIHR to her institution (The University of Liverpool) for her work on this review. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
New
References
References to studies included in this review
Collins 2004 {published data only (unpublished sought but not used)}
- Collins CT, Ryan P, Crowther CA, McPhee AJ, Paterson S, Hiller JE. Effect of bottles, cups, and dummies on breast feeding in preterm infants: a randomised controlled trial. BMJ 2004;329(7459):193‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Graffy 2004 {published data only (unpublished sought but not used)}
- Graffy J, Taylor J, Williams A, Eldridge S. Randomised controlled trial of support from volunteer counsellors for mothers considering breast feeding. BMJ 2004;328(7430):26. [doi: http://dx.doi.org/10.1136/bmj.328.7430.26] [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISRCTN37327292. A randomised controlled trial of the effectiveness of support from breastfeeding counsellors for women who want to breastfeed. isrctn.com/ISRCTN37327292 (date first received 23 January 2014).
Hoddinott 2012 {published data only (unpublished sought but not used)}
- Hoddinott P, Craig L, MacLennan G, Boyers D, Vale L. Process evaluation for the FEeding Support Team (FEST) randomised controlled feasibility trial of proactive and reactive telephone support for breastfeeding women living in disadvantaged areas. BMJ Open 2012;2(2):e001039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoddinott P, Craig L, MacLennan G, Boyers D, Vale L. The FEeding Support Team (FEST) randomised controlled feasibility trial of proactive and reactive telephone support for breastfeeding women living in disadvantaged areas. BMJ Open 2012;2(2):e000652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISRCTN27207603. Proactive telephone support for breastfeeding women in disadvantaged areas provided by a postnatal ward feeding support team. isrctn.com/ISRCTN27207603 (date first received June 2010). [DOI 10.1186/ISRCTN27207603]
Junior 2007 {published data only (unpublished sought but not used)}
- Junior WS, Martinez FE. Effect of intervention on the rates of breastfeeding of very low birth weight newborns. Jornal de Pediatria 2007;83(6):541‐6. [DOI] [PubMed] [Google Scholar]
Paul 2012 {published and unpublished data}
- Beiler JS, Schaefer EW, Alleman N, Paul IM. Newborn anticipatory guidance delivered at office‐based vs. home nurse visits. Pediatric Academic Societies and Asian Society for Pediatric Research Joint Meeting; 2011 April 30‐May 3; Denver, Colorado, USA. 2011.
- Paul IM, Beiler JS, Schaefer EW, Hollenbeak CS, Alleman N, Sturgis SA. A randomized trial of nurse home visits vs. office‐based care after nursery/maternity discharge. Pediatric Academic Societies and Asian Society for Pediatric Research Joint Meeting; 2011 April 30‐May 3; Denver, Colorado, USA. 2011:2300.6.
- Paul IM, Beiler JS, Schaefer EW, Hollenbeak CS, Alleman N, Sturgis SA, et al. A randomized trial of single home nursing visits vs office‐based care after nursery/maternity discharge: the Nurses for Infants Through Teaching and Assessment after the NurserY (NITTANY) study. Archives of Pediatrics & Adolescent Medicine 2012;166(3):263‐70. [DOI] [PubMed] [Google Scholar]
Penfold 2014 {published data only}
- Hanson C, Manzi F, Mkumbo E, Shirima K, Penfold S, Hill Z, et al. Effectiveness of a home‐based counselling strategy on neonatal care and survival: a cluster‐randomised trial in six districts of rural Southern Tanzania. Plos Medicine 2015;12(9):e1001881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01022788. Improving newborn survival in rural southern Tanzania: a study to evaluate the impact and cost of a scaleable package of interventions at community level with health system strengthening. clinicaltrials.gov/show/record/NCT01022788 (date first received 29 November 2009).
- Penfold S, Manzi F, Mkumbo E, Temu S, Jaribu J, Shamba DD, et al. Effect of home‐based counselling on newborn care practices in southern Tanzania one year after implementation: a cluster‐randomised controlled trial. BMC Pediatrics 2014;14(1):187. [DOI] [PMC free article] [PubMed] [Google Scholar]
Reeder 2014 {published and unpublished data}
- Reeder JA, Joyce T, Sibley K, Arnold D, Altindag O. Telephone peer counseling of breastfeeding among WIC participants: a randomized controlled trial. Pediatrics 2014;134(3):e700‐e709. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sellen 2013 {published data only (unpublished sought but not used)}
- Kamau‐Mbuthia E, Mbugua S, Webb Girard A, Kalungu S, Sarange C, Lou W, et al. Cell phone based peer counseling to support exclusive breastfeeding is associated with more frequent help and decreased breastfeeding problems. Annals of Nutrition & Metabolism 2013;63(Suppl 1):196‐7, Abstract no: O079. [Google Scholar]
- Mbugua S, Kamau‐Mbuthia E, Webb A, Kalungu S, Sarange C, Lou W, et al. Process indicators for a randomized trial of cell phone based peer counseling to support exclusive breastfeeding in Kenya. Annals of Nutrition & Metabolism 2013;63(Suppl 1):693, Abstract no: PO905. [Google Scholar]
- Mbugua S, Kamau‐Mbuthia E, Webb Girard A, Kalungu S, Sarange C, Lou W, et al. Process indicators for a randomized trial of cell phone based peer counseling to support exclusive breastfeeding in Kenya. Annals of Nutrition & Metabolism 2013;63(Suppl 1):751, Abstract no: PO1033. [Google Scholar]
- Sellen D, Mbugua S, Webb Girard A, Kalungu S, Sarange C, Lou W, et al. A randomized controlled trial indicates benefits of cell phone based peer counseling to support exclusive breastfeeding in Kenya. Annals of Nutrition & Metabolism 2013;63(Suppl 1):751, Abstract no: PO1032. [Google Scholar]
- Sellen D, Mbugua S, Webb‐Girard A, Lou W, Duan W, Kamau‐Mbuthia E. Cell phone based peer counselling can support exclusive breastfeeding: A randomized controlled trial in Kenya. FASEB Journal 2014;28(1 Suppl 1):[Abstract no. 119.5]. [Google Scholar]
- Sellen DW, Kamau‐Mbuthia E, Mbugua S, Webb Girard AL, Lou W, Dennis CL, et al. Lessons learned in providing peer support through cell phones and group meetings to increase exclusive breastfeeding in Kenya. Breastfeeding and the use of human milk. Science and Practice. Proceedings of the 16th ISRHML Conference; 2012 September 27th‐October 1st; Trieste, Italy. 2012:Abstract no. A18.
- Webb Girard A, Kamau‐Mbuthia E, Mbugua S, Kalungu S, Sarange C, Lou W, et al. Infant medication, illness and growth in a randomized controlled trial of exclusive breastfeeding support in Kenya. Annals of Nutrition & Metabolism 2013;63(Suppl 1):752, Abstract no: PO1034. [Google Scholar]
Serwint 1996 {published data only (unpublished sought but not used)}
- Serwint JR, Wilson MEH, Vogelhut JW, Repke JT, Seidel HM. A randomized controlled trial of prenatal pediatric visits for urban low income families. Pediatrics 1996;98(6):1069‐75. [PubMed] [Google Scholar]
Winterburn 2003 {published data only (unpublished sought but not used)}
- Winterburn S, Moyez J, Thompson J. Maternal grandmothers and support for breastfeeding. Journal of Community Nursing 2003;17(12):4‐9. [Google Scholar]
References to studies excluded from this review
Bonuck 2005 {published data only}
- Bonuck KA, Freeman K, Trombley M. Randomized controlled trial of a prenatal and postnatal lactation consultant intervention on infant health care use. Archives of Pediatrics & Adolescent Medicine 2006;160(9):953‐60. [DOI] [PubMed] [Google Scholar]
- Bonuck KA, Trombley M, Freeman K, McKee D. Randomized, controlled trial of a prenatal and postnatal lactation consultant intervention on duration and intensity of breastfeeding up to 12 months. Pediatrics 2005;116(6):1413‐26. [DOI] [PubMed] [Google Scholar]
- Memmott MM, Bonuck KA. Mother's reactions to a skills‐based breastfeeding promotion intervention. Maternal & Child Nutrition 2006;2(1):40‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Robling 2012 {published data only}
- ISRCTN23019866. Evaluating the family nurse partnership programme in England: a randomised controlled trial. http://www.controlled‐trials.com/ISRCTN23019866 (date first received 20 April 2009).
Simmer 2015 {published data only}
- Simmer K, Kok C, Nancarrow K, Hepworth AR, Geddes DT. Novel feeding system to promote establishment of breastfeeds after preterm birth: a randomized controlled trial. Journal of Perinatology 2016; Vol. 36:210‐5. [DOI] [PMC free article] [PubMed]
Tomlinson 2014 {published data only}
- ISRCTN41046462. An effectiveness study of an integrated, community based package for maternal, newborn, child and HIV care in a disadvantaged community in South Africa. isrctn.com/ISRCTN41046462 (date first received 3 November 2009).
- Ijumba P, Doherty T, Jackson D, Tomlinson M, Sanders D, Swanevelder S, et al. Effect of an integrated community‐based package for maternal and newborn care on feeding patterns during the first 12 weeks of life: a cluster‐randomized trial in a South African township. Public Health Nutrition 2015;18(14):2660‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson M, Doherty T, Ijumba P, Jackson D, Lawn J, Persson LA, et al. Goodstart: a cluster randomised effectiveness trial of an integrated, community‐based package for maternal and newborn care, with prevention of mother‐to‐child transmission of HIV in a South African township. Tropical Medicine & International Health 2014;19(3):256‐66. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Brizot 2009 {published data only (unpublished sought but not used)}
- NCT02738957. Effect of prenatal counseling on breastfeeding rates in twins. clinicaltrials.gov/show/NCT02738957 (date first received 11 April 2016).
Kikuchi 2015 {published data only}
- Kikuchi K, Ansah E, Okawa S, Shibanuma A, Gyapong M, Owusu‐Agyei S, et al. Ghana's Ensure Mothers and Babies Regular Access to Care (EMBRACE) program: study protocol for a cluster randomized controlled trial. Trials 2015;16:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kimani‐Murage 2013 {published data only}
- Kimani‐Murage EW, Kyobutungi C, Ezeh AC, Wekesah F, Wanjohi M, Muriuki P, et al. Effectiveness of personalised, home‐based nutritional counselling on infant feeding practices, morbidity and nutritional outcomes among infants in Nairobi slums: study protocol for a cluster randomised controlled trial. Trials (Electronic Resource) 2013;14:445. [DOI] [PMC free article] [PubMed] [Google Scholar]
Martin‐Iglesias 2011 {published data only}
- Martin‐Iglesias S, del‐Cura‐Gonzalez I, Sanz‐Cuesta T, Arana‐Canedo Arguelles C, Rumayor‐Zarzuelo M, Alvarez‐de la Riva M, et al. Effectiveness of an implementation strategy for a breastfeeding guideline in primary care: cluster randomised trial. BMC Family Practice 2011;12:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yang 2016 {published data only}
- Yang H. The intervention for breast feeding of twins: A randomized controlled trial. chictr.org.cn/showproj.aspx?proj=13743 (date first received 21 March 2016).
Additional references
Alves 2013
- Alves E, Rodrigues C, Fraga S, Barros H, Silva S. Parents’ views on factors that help or hinder breastmilk supply in neonatal care units: systematic review. Archives of Disease in Childhood. Fetal and Neonatal Edition 2013;98:F511–F517. [DOI: 10.1136/archdischild-2013-304029] [DOI] [PubMed] [Google Scholar]
Antsaklis 2013
- Antsaklis A, Malamas FM, Sindos M. Trends in twin pregnancies and mode of delivery during the last 30 years: inconsistency between guidelines and clinical practice. Journal of Perinatal Medicine 2013;41(4):355‐64. [DOI] [PubMed] [Google Scholar]
Balogun 2016
- Balogun OO, O'Sullivan EJ, McFadden A, Ota E, Gavine A, Garner CD, et al. Interventions for promoting the initiation of breastfeeding. Cochrane Database of Systematic Reviews 2016, Issue 11. [DOI: 10.1002/14651858.CD001688.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Becker 2015
- Becker G, Smith H, Cooney F. Methods of milk expression for lactating women. Cochrane Database of Systematic Reviews 2015, Issue 2. [DOI: 10.1002/14651858.CD006170.pub4] [DOI] [PubMed] [Google Scholar]
Bennington 2011
- Bennington, L. Breastfeeding multiples: it can be done. Newborn and Infant Nursing Reviews 2011;11(4):194‐7. [DOI: 10.1053/j.nainr.2011.09.012] [DOI] [Google Scholar]
Blondel 2002
- Blondel B, Kaminski M. Trends in the occurrence, determinants, and consequences of multiple births. Seminars in Perinatology 2002;26(4):239‐49. [DOI] [PubMed] [Google Scholar]
Blondel 2006
- Blondel B, Macfarlane A, Gissler M, Breart G, Zeitlin J, PERISTAT Study Group. Preterm birth and multiple pregnancy in European countries participating in the PERISTAT project. BJOG: an international journal of obstetrics and gynaecology 2006;113:528–35. [DOI: 10.1111/j.1471-0528.2006.00923.x] [DOI] [PubMed] [Google Scholar]
Bonet 2011
- Bonet M, Blondel B, Agostino R, Combier E, Maier R, Cuttini M, et al. Variations in breastfeeding rates for very preterm infants between regions and neonatal units in Europe: results from the MOSAIC cohort. Archives of Disease in Childhood. Fetal and Neonatal Edition 2011;96:F450–F452. [F450 10.1136/adc.2009.179564] [DOI] [PubMed] [Google Scholar]
Chambers 2014
- Chambers G, Ledger W. The economic implications of multiple pregnancy following ART. Seminars in Fetal & Neonatal Medicine 2014;9:254‐61. [DOI] [PubMed] [Google Scholar]
Cinar 2013
- Cinar N, Kose A, Nemut T. Breastfeeding twins: a qualitative study. Journal of Healthy Population Nutrition 2013;31(4):504‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Collins 2007
- Collins J. Global epidemiology of multiple birth. Reproductive BioMedicine Online 2007;15:45‐52. [DOI] [PubMed] [Google Scholar]
Collins 2008
- Collins CT, Makrides M, Gillis J, McPhee AJ. Avoidance of bottles during the establishment of breast feeds in preterm infants. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD005252] [DOI] [PubMed] [Google Scholar]
Conde‐Agudelo 2014
- Conde‐Agudelo A, Díaz‐Rossello JL. Kangaroo mother care to reduce morbidity and mortality in low birthweight infants. Cochrane Database of Systematic Reviews 2014, Issue 4. [DOI: 10.1002/14651858.CD002771.pub3] [DOI] [PubMed] [Google Scholar]
Dall'Oglio 2007
- Dall’Oglio I, Salvatori G, Bonci E, Nantini B, D’Agostino G, Dotta A. Breastfeeding promotion in neonatal intensive care unit: impact of a new program toward a BFHI for high‐risk infants. Acta Pædiatrica 2007;96:1626–31. [DOI: 10.1111/j.1651-2227.2007.00495.x] [DOI] [PubMed] [Google Scholar]
Damato 2005
- Damato E, Dowling D, Madigan E, Thanattherakul C. Duration of breastfeeding for mothers of twins. Journal of Obstetric, Gynecologic, and Neonatal Nursing 2005;34:201‐9. [DOI: 10.1177/088421750427367] [DOI] [PubMed] [Google Scholar]
Denton 2011
- Denton J, Quigley M, Ridley B. Appendices to the guidance for health professionals on feeding twins, triplets and higher order multiples. The Multiple Births Foundation (http://www.multiplebirths.org.uk/Appendicesfinal.pdf 2011 (accessed 11 July 2016).
Entwistle 2013
- Entwistle FM. The evidence and rationale for the UNICEF UK Baby Friendly Initiative standards. UNICEF UK, 2013. [Google Scholar]
ESPGHAN 2013
- Arslanoglu S, Corpeleijn W, Moro G, Braegger C, Campoy C, Colomb V, et al. ESPGHAN Committee on Nutrition. Donor human milk for preterm infants: current evidence and research directions. Journal of Pediatric Gastroenterology and Nutrition 2013;57(4):535‐42. [DOI: 10.1097/MPG.0b013e3182a3af0a] [DOI] [PubMed] [Google Scholar]
Flacking 2006
- Flacking R, Ewalda U, Hedberg Nyqvista KH, Starrin B. Trustful bonds: A key to ‘‘becoming a mother’’ and to reciprocal breastfeeding. Stories of mothers of very preterm infants at a neonatal unit. Social Science & Medicine 2006;62(1):70‐80. [DOI: 10.1016/j.socscimed.2005.05.026] [DOI] [PubMed] [Google Scholar]
Flacking 2007
- Flacking R, Ewalda U, Starrin B. ‘‘I wanted to do a good job’’: Experiences of ‘becoming a mother’ and breastfeeding in mothers of very preterm infants after discharge from a neonatal unit. Social Science & Medicine 2007;64:2405–16. [DOI: 10.1016/j.socscimed.2007.03.008] [DOI] [PubMed] [Google Scholar]
Flidel‐Rimon 2002
- Flidel‐Rimon O, Shinwell E. Breast‐feeding multiples. Seminars in Neonatology 2002;7:231–9. [DOI: 10.1053/siny.2002.0110] [DOI] [PubMed] [Google Scholar]
Gromada 1998
- Gromada K, Spangler A. Breastfeeding twins and higher‐order multiples. Journal of Obstetric, Gynecologic, and Neonatal Nursing 1998;27:441‐9. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Kyvernitakis 2013
- Kyvernitakis A, Kyvernitakis I, Karageorgiadis AS, Misselwitz B, Papaspyrou G, Kalder M, et al. Rising cesarean rates of twin deliveries in Germany from 1990 to 2012. Zeitschrift fur Geburtshilfe und Neonatologie 2013;217(5):177‐82. [DOI] [PubMed] [Google Scholar]
Lee 2011
- Lee HC, Gould JB, Boscardin WJ, El‐Sayed YY, Blumenfeld YJ. Trends in cesarean delivery for twin births in the United States: 1995‐2008. Obstetrics & Gynecology 2011;118(5):1095‐101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lumbiganon 2016
- Lumbiganon P, Martis R, Laopaiboon M, Festin MR, Ho JJ, Hakimi M. Antenatal breastfeeding education for increasing breastfeeding duration. Cochrane Database of Systematic Reviews 2016, Issue 12. [DOI: 10.1002/14651858.CD006425.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lustiv 2013
- Lustiv O, Pullenayegum E, Foster G, Vera C, Giglia L, Chapman B, et al. Women’s intentions to breastfeed: a population‐based cohort study. BJOG: an international journal of obstetrics and gynaecology 2013;120:1490‐9. [DOI: 10.1111/1471-0528.12376] [DOI] [PubMed] [Google Scholar]
McAndrew 2012
- McAndrew F, Thompson J, Fellows L. Infant Feeding Survey 2010, NHS Information Centre, IFF Research. http://www.hscic.gov.uk/catalogue/PUB08694 2012 (accessed 2015).
McFadden 2017
- McFadden A, Gavine A, Renfrew MJ, Wade A, Buchanan P, Taylor JL, et al. Support for healthy breastfeeding mothers with healthy term babies. Cochrane Database of Systematic Reviews 2017, Issue 2. [DOI: 10.1002/14651858.CD001141.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moore 2012
- Moore ER, Anderson GC, Bergman N, Dowswell T. Early skin‐to‐skin contact for mothers and their healthy newborn infants. Cochrane Database of Systematic Reviews 2012, Issue 5. [DOI: 10.1002/14651858.CD003519.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Multiple Births Foundation 2011
- Multiple Births Foundation. Guidance for Health Professionals on Feeding Twins, Triplets and Higher Order Multiples. http://www.multiplebirths.org.uk/MBFParentsFeedingGuideFINALVERSION.pdf. Multiple Births Foundation, 2011. [ISBN: 978‐1‐902068‐17‐6]
Murray 2014
- Murray S, Norman J. Multiple pregnancies following assisted reproductive technologies. A happy consequence or double trouble?. Seminars in Fetal & Neonatal Medicine 2014;19:222‐7. [DOI: 10.1016/j.siny.2014.03.001] [DOI] [PubMed] [Google Scholar]
Newby 2015
- Newby R, Brodribb W, Ware R, Davies P. Internet use by first‐time mothers for infant feeding support. Journal of Human Lactation 2015;31(3):416‐24. [DOI: 10.1177/0890334415584319] [DOI] [PubMed] [Google Scholar]
NICE 2010
- NICE. Donor Milk Banks: the Operation of Donor Milk Bank Services. NICE Guideline CG93. NICE, 2010. [PubMed] [Google Scholar]
NICE 2013
- NICE. Multiple pregnancy: twin and triplet pregnancies. NICE quality standard [QS46]. http://www.nice.org.uk/guidance/qs46/resources/guidance‐multiple‐pregnancy‐pdf 2013 (accessed 2016).
Niela‐Vilén 2014
- Niela‐Vilén H, Axelin A, Melender H, Salanterä S. Aiming to be a breastfeeding mother in a neonatal intensive care unit and at home: a thematic analysis of peer‐support group discussion in social media. Maternal & Child Nutrition 2015;11(4):712‐26. [DOI: 10.1111/mcn.12108] [DOI] [PMC free article] [PubMed] [Google Scholar]
ONS 2014
- Office for National Statistics. Births in England and Wales by Characteristics of Birth 2, 2013. http://www.ons.gov.uk/ons/rel/vsob1/characteristics‐of‐birth‐2‐‐england‐and‐wales/2013/sb‐characteristics‐of‐birth‐2.html 2014 (accessed 18 November 2015).
Ostlund 2010
- Östlund Å, Nordström M, Dykes F, Flacking R. Breastfeeding in preterm and term twins—maternal factors associated with early cessation: a population‐based study. Journal of Human Lactation 2010;26(3):235–41. [DOI: 10.1177/0890334409359627] [DOI] [PubMed] [Google Scholar]
Parker 2013
- Parker M, Burnham L, Cook J, Sanchez BA, Philipp BL, Merewood A. 10 Years after baby‐friendly designation: breastfeeding rates continue to increase in a US neonatal intensive care unit. Journal of Human Lactation 2013;29(3):354‐8. [DOI: 10.1177/0890334413489374] [DOI] [PubMed] [Google Scholar]
POPPY Steering Group
- POPPY Steering Group 2009. Family‐centred care in neonatal units. A summary of research results and recommendations from the POPPY project. http://www.poppy‐project.org.uk/resources/Poppy+report+for+PRINT.pdf 2009 (accessed 2015).
Quigley 2014
- Quigley M, McGuire W. Formula versus donor breast milk for feeding preterm or low birth weight infants. Cochrane Database of Systematic Reviews 2014, Issue 4. [DOI: 10.1002/14651858.CD002971.pub3] [DOI] [PubMed] [Google Scholar]
Renfrew 2009
- Renfrew M, Craig D, Dyson L, McCormick F, Rice S, King S, et al. Breastfeeding promotion for infants in neonatal units: a systematic review and economic analysis. Health Technology Assessment 2009;40:1‐146. [DOI: 10.3310/hta13400] [DOI] [PubMed] [Google Scholar]
Renfrew 2012a
- Renfrew M, Subhash P, Quigley M, McCormick F, Fox‐Rushby J, Dodds R, et al. Preventing disease and saving resources: the potential contribution of Increasing breastfeeding rates in the UK. UNICEF UK, 2012. [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rollins 2016
- Rollins N, Bhandari N, Hajeebhoy N, Horton S, Lutter C, Martines J, et al. The Lancet Breastfeeding Series Group. Why invest, and what it will take to improve breastfeeding practices?. Lancet 2016;387:491‐504. [DOI: 10.1016/S0140-6736(15)01044-2] [DOI] [PubMed] [Google Scholar]
Roze 2012
- Roze J, Darmaun D, Boquien C, Flamant C, Picaud J, Savagner C, et al. The apparent breastfeeding paradox in very preterm infants: relationship between breast feeding, early weight gain and neurodevelopment based on results from two cohorts, EPIPAGE and LIFT. BMJ Open 2012;2:e000834. [DOI: 10.1136/bmjopen-2012-000834] [DOI] [PMC free article] [PubMed] [Google Scholar]
Russell 2003
- Russell R, Petrini J, Damus K, Mattison D, Schwarz R. The changing epidemiology of multiple births in the United States. Obstetrics and Gynecology 2003;101(1):129‐35. [DOI] [PubMed] [Google Scholar]
Save the Children 2013
- Save the Children. Superfood for Babies: How overcoming barriers to breastfeeding will save children’s lives. http://www.savethechildren.org/atf/cf/%7B9def2ebe‐10ae‐432c‐9bd0‐df91d2eba74a%7D/SUPERFOOD%20FOR%20BABIES%20ASIA%20LOW%20RES%282%29.PDF 2013 (accessed 18 November 2015).
Sisk 2006
- Sisk PM, Lovelady CA, Dillard RG, Gruber KJ. Lactation counselling for mothers of very low birth weight infants: Effect on maternal anxiety and infant intake of human milk. Paediatrics 2006;117:e67‐75. [DOI] [PubMed] [Google Scholar]
Smith 2014
- Smith L, Manktelow B, Draper E, Boyle E, Johnson S, Field D. Trends in the incidence and mortality of multiple births by socioeconomic deprivation and maternal age in England: population‐based cohort study. BMJ Open 2014;4:e004514. [DOI: 10.1136/bmjopen-2013-004514] [DOI] [PMC free article] [PubMed] [Google Scholar]
Smits 2011
- Smits J, Mondon C. Twinning across the developing world. PLoS ONE 2011;6(9):e25239. [DOI: 10.1371/journal.pone.0025239] [DOI] [PMC free article] [PubMed] [Google Scholar]
Szucs 2009
- Szucs K, Axline S, Rosenman M. Quintuplets and a mother’s determination to provide human milk: it takes a village to raise a baby ‐ how about five?. Journal of Human Lactation 2009;25(1):79‐84. [DOI: 10.1177/0890334408328385] [DOI] [PubMed] [Google Scholar]
Umstad 2013
- Umstad M, Lyndon H, Wang Y, Sullivan E. Multiple deliveries: The reduced impact of in vitro fertilisation in Australia. Australian and New Zealand Journal of Obstetrics and Gynaecology 2013;53:158‐64. [DOI] [PubMed] [Google Scholar]
Victora 2016
- Victora C, Bahl R, Barros A, França G, Horton S, Krasevec J, et al. The Lancet Breastfeeding Series Group. Breastfeeding in the 21st century: epidemiology, mechanisms, and lifelong effect. Lancet 2016;387:475‐90. [DOI: 10.1016/S0140-6736(15)01024-7] [DOI] [PubMed] [Google Scholar]
Wataker 2012
- Wataker H, Meberg A, Nestaas E. Neonatal family care for 24 hours per day: effects on maternal confidence and breast‐feeding. Journal of Perinatal & Neonatal Nursing 2012;26(4):336‐42. [DOI: 10.1097/JPN.0b013e31826d928b] [DOI] [PubMed] [Google Scholar]
Yokoyama 2006
- Yokoyama Y, Wada S, Sugimoto M, Katayama M, Miyuki Saito M, Sono J. Breastfeeding rates among singletons, twins and triplets in Japan: a population‐based study. Twin Research and Human Genetics 2006;9(2):298–302. [DOI] [PubMed] [Google Scholar]


