Abstract
Background
Kangaroo mother care (KMC), originally defined as skin‐to‐skin contact between a mother and her newborn, frequent and exclusive or nearly exclusive breastfeeding, and early discharge from hospital, has been proposed as an alternative to conventional neonatal care for low birthweight (LBW) infants.
Objectives
To determine whether evidence is available to support the use of KMC in LBW infants as an alternative to conventional neonatal care before or after the initial period of stabilization with conventional care, and to assess beneficial and adverse effects.
Search methods
We used the standard search strategy of the Cochrane Neonatal Review Group. This included searches in CENTRAL (Cochrane Central Register of Controlled Trials; 2016, Issue 6), MEDLINE, Embase, CINAHL (Cumulative Index to Nursing and Allied Health Literature), LILACS (Latin American and Caribbean Health Science Information database), and POPLINE (Population Information Online) databases (all from inception to June 30, 2016), as well as the WHO (World Health Organization) Trial Registration Data Set (up to June 30, 2016). In addition, we searched the web page of the Kangaroo Foundation, conference and symposia proceedings on KMC, and Google Scholar.
Selection criteria
Randomized controlled trials comparing KMC versus conventional neonatal care, or early‐onset KMC versus late‐onset KMC, in LBW infants.
Data collection and analysis
Data collection and analysis were performed according to the methods of the Cochrane Neonatal Review Group.
Main results
Twenty‐one studies, including 3042 infants, fulfilled inclusion criteria. Nineteen studies evaluated KMC in LBW infants after stabilization, one evaluated KMC in LBW infants before stabilization, and one compared early‐onset KMC with late‐onset KMC in relatively stable LBW infants. Sixteen studies evaluated intermittent KMC, and five evaluated continuous KMC.
KMC versus conventional neonatal care: At discharge or 40 to 41 weeks' postmenstrual age, KMC was associated with a statistically significant reduction in the risk of mortality (risk ratio [RR] 0.60, 95% confidence interval [CI] 0.39 to 0.92; eight trials, 1736 infants), nosocomial infection/sepsis (RR 0.35, 95% CI 0.22 to 0.54; five trials, 1239 infants), and hypothermia (RR 0.28, 95% CI 0.16 to 0.49; nine trials, 989 infants; moderate‐quality evidence). At latest follow‐up, KMC was associated with a significantly decreased risk of mortality (RR 0.67, 95% CI 0.48 to 0.95; 12 trials, 2293 infants; moderate‐quality evidence) and severe infection/sepsis (RR 0.50, 95% CI 0.36 to 0.69; eight trials, 1463 infants; moderate‐quality evidence). Moreover, KMC was found to increase weight gain (mean difference [MD] 4.1 g/d, 95% CI 2.3 to 5.9; 11 trials, 1198 infants; moderate‐quality evidence), length gain (MD 0.21 cm/week, 95% CI 0.03 to 0.38; three trials, 377 infants) and head circumference gain (MD 0.14 cm/week, 95% CI 0.06 to 0.22; four trials, 495 infants) at latest follow‐up, exclusive breastfeeding at discharge or 40 to 41 weeks' postmenstrual age (RR 1.16, 95% CI 1.07 to 1.25; six studies, 1453 mothers) and at one to three months' follow‐up (RR 1.20, 95% CI 1.01 to 1.43; five studies, 600 mothers), any (exclusive or partial) breastfeeding at discharge or at 40 to 41 weeks' postmenstrual age (RR 1.20, 95% CI 1.07 to 1.34; 10 studies, 1696 mothers; moderate‐quality evidence) and at one to three months' follow‐up (RR 1.17, 95% CI 1.05 to 1.31; nine studies, 1394 mothers; low‐quality evidence), and some measures of mother‐infant attachment and home environment. No statistically significant differences were found between KMC infants and controls in Griffith quotients for psychomotor development at 12 months’ corrected age (low‐quality evidence). Sensitivity analysis suggested that inclusion of studies with high risk of bias did not affect the general direction of findings nor the size of the treatment effect for main outcomes.
Early‐onset KMC versus late‐onset KMC in relatively stable infants: One trial compared early‐onset continuous KMC (within 24 hours post birth) versus late‐onset continuous KMC (after 24 hours post birth) in 73 relatively stable LBW infants. Investigators reported no significant differences between the two study groups in mortality, morbidity, severe infection, hypothermia, breastfeeding, and nutritional indicators. Early‐onset KMC was associated with a statistically significant reduction in length of hospital stay (MD 0.9 days, 95% CI 0.6 to 1.2).
Authors' conclusions
Evidence from this updated review supports the use of KMC in LBW infants as an alternative to conventional neonatal care, mainly in resource‐limited settings. Further information is required concerning the effectiveness and safety of early‐onset continuous KMC in unstabilized or relatively stabilized LBW infants, as well as long‐term neurodevelopmental outcomes and costs of care.
Plain language summary
Kangaroo mother care to reduce morbidity and mortality in low birthweight infants
Review question: Does kangaroo mother care (KMC) reduce morbidity and mortality in low birthweight (LBW) infants?
Background: Conventional neonatal care of LBW infants (< 2500 g) is expensive and requires both highly skilled personnel and permanent logistical support. KMC has been proposed as an alternative to conventional neonatal care of LBW infants. The major component of KMC is skin‐to‐skin contact between mother and newborn. The other two components of KMC are frequent and exclusive or nearly exclusive breastfeeding and attempted early discharge from hospital.
Study characteristics: We identified 21 randomized controlled trials (3042 infants) for inclusion in this review by searching medical databases in June 2016.
Key results: Compared with conventional neonatal care, KMC was found to reduce mortality at discharge or at 40 to 41 weeks' postmenstrual age and at latest follow‐up, severe infection/sepsis, nosocomial infection/sepsis, hypothermia, severe illness, and lower respiratory tract disease. Moreover, KMC increased weight, length, and head circumference gain, breastfeeding at discharge or at 40 to 41 weeks' postmenstrual age and at one to three months' follow‐up, mother satisfaction with method of infant care, some measures of maternal‐infant attachment, and home environment. Researchers noted no differences in neurodevelopmental and neurosensory outcomes at 12 months' corrected age.
Quality of evidence: Most critical and important outcomes had moderate‐quality evidence.
Conclusions: KMC is an effective and safe alternative to conventional neonatal care for LBW infants, mainly in resource‐limited countries.
Summary of findings
Summary of findings for the main comparison. Kangaroo mother care versus conventional neonatal care for reducing morbidity and mortality in low birthweight infants.
| Kangaroo mother care versus conventional neonatal care for reducing morbidity and mortality in low birthweight infants | ||||||
| Patient or population: infants with low birthweight Settings: neonatal intensive care unit/newborn nursery/home Intervention: kangaroo mother care Comparison: conventional neonatal care | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Conventional neonatal care | Kangaroo mother care | |||||
| Mortality at latest follow‐up | Study population | RR 0.67 (0.48 to 0.95) | 2293 (12 studies) | ⊕⊕⊕⊝ moderatea | ||
| 60 per 1000 | 40 per 1000 (29 to 57) | |||||
| Moderate | ||||||
| 30 per 1000 | 20 per 1000 (14 to 28) | |||||
| Severe infection/sepsis at latest follow‐up ‐ stabilized infants | Study population | RR 0.5 (0.36 to 0.69) | 1463 (8 studies) | ⊕⊕⊕⊝ moderatea | ||
| 131 per 1000 | 65 per 1000 (47 to 90) | |||||
| Moderate | ||||||
| 162 per 1000 | 81 per 1000 (58 to 112) | |||||
| Hypothermia at discharge or at 40 to 41 weeks’ postmenstrual age ‐ stabilized infants | Study population | RR 0.28 (0.16 to 0.49) | 989 (9 studies) | ⊕⊕⊕⊝ moderateb | ||
| 271 per 1000 | 76 per 1000 (43 to 133) | |||||
| Moderate | ||||||
| 333 per 1000 | 93 per 1000 (53 to 163) | |||||
| Weight gain at latest follow‐up (g/d) ‐ stabilized infants | Mean weight gain at latest follow‐up (g/d) ‐ stabilized infants in the intervention groups ‐ was 4.08 higher (2.3 to 5.86 higher) | 1198 (11 studies) | ⊕⊕⊕⊝ moderatec | |||
| Any breastfeeding at discharge or at 40 to 41 weeks' postmenstrual age ‐ stabilized infants | Study population | RR 1.2 (1.07 to 1.34) | 1696 (10 studies) | ⊕⊕⊕⊝ moderated | ||
| 762 per 1000 | 914 per 1000 (815 to 1000) | |||||
| Moderate | ||||||
| 743 per 1000 | 892 per 1000 (795 to 996) | |||||
| Any breastfeeding at 1 to 3 months' follow‐up ‐ stabilized infants | Study population | RR 1.17 (1.05 to 1.31) | 1394 (9 studies) | ⊕⊕⊝⊝ lowa,e | ||
| 711 per 1000 | 832 per 1000 (747 to 932) | |||||
| Moderate | ||||||
| 622 per 1000 | 728 per 1000 (653 to 815) | |||||
| Griffith quotient for psychomotor development (all subscales) at 12 months' corrected age (copy) | Mean Griffith quotient for psychomotor development (all subscales) at 12 months' corrected age (copy) in the intervention groups was 1.05 higher (0.75 lower to 2.85 higher) | 579 (1 study) | ⊕⊕⊝⊝ lowf,g | |||
| *The basis for the assumed risk (eg, median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI = confidence interval; RR = risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: We are very uncertain about the estimate | ||||||
aMost of the pooled effect provided by studies with moderate or high risk of bias bSubstantial heterogeneity (I2 = 52%) cSubstantial heterogeneity (I2 = 86%) dSubstantial heterogeneity (I2 = 80%) eSubstantial heterogeneity (I2 = 62%) fEffect provided by 1 study with moderate risk of bias gWide 95% CI
Background
Description of the condition
Low birthweight (LBW), defined as weight at birth of less than 2500 g, irrespective of gestational age, has an adverse effect on child survival and development, and may even be an important risk factor for adult disease (Barker 1995). Overall, it is estimated that 15% to 20% of all births worldwide are LBW, representing more than 20 million births a year, the great majority of them reported in low‐ and middle‐income countries (WHO 2014). LBW is a major contributor to both neonatal and child mortality (Lawn 2014; UNICEF 2015). A complex process of care named conventional or modern neonatal care includes interventions already proven to lower the burden of neonatal morbidity and mortality. Conventional neonatal care of LBW infants is expensive and requires both trained personnel and permanent logistical support. This complexity is critical, mainly during the stabilization period, until the infant has adapted to autonomous extrauterine life. In low‐ and middle‐income countries, financial and human resources for neonatal care are limited, and hospital wards for LBW infants are often overcrowded. Thus, interventions for LBW infants that reduce neonatal morbidity and mortality and costs would signify an important advance in care.
Description of the intervention
In 1978, Edgar Rey (Rey 1983) proposed and developed kangaroo mother care (KMC) at Instituto Materno Infantil in Santa Fe de Bogotá, Colombia, as an alternative to the conventional contemporary method of care for LBW infants. KMC was initially conceived to address the lack of incubators, the high rate of nosocomial infection, and the occurrence of infant abandonment at the local hospital. The term KMC is derived from similarities to marsupial caregiving. Mothers are used as "incubators" to maintain infants' body temperature, and as the main source of food and stimulation for LBW infants, while they mature enough to face extrauterine life in similar conditions as those born at term. Initially, the method was applied only after the LBW infant had stabilized, because LBW infants need a variable period of conventional care before they are eligible for KMC. Stabilization of respiratory, thermal, and feeding functions has been considered crucial for the success of this intervention. The definition of stabilization is not precise; stabilization has been defined as independent of gestational age and weight, which are the main variables associated with those vital functions. Some recent studies have evaluated the effectiveness of early‐onset KMC (as soon as possible after birth) for LBW infants born in hospitals with little neonatal intensive care capacity (Nagai 2010; Worku 2005). Currently, the definition of KMC is characterized by significant heterogeneity (Chan 2016). The major component of KMC is skin‐to‐skin contact (SSC), by which infants are placed vertically between the mother's breasts firmly attached to the chest and below her clothes. SSC is offered to infants as far as the mother‐infant dyad can tolerate it. Mothers can share the role of provider of SSC with others, especially the babies' fathers. The aim is to empower the mother (parents or caregivers) by gradually transferring the skills and responsibility for becoming the child's primary caregiver and meeting every physical and emotional need (Nyqvist 2010). The other two components of KMC are frequent and exclusive or nearly exclusive breastfeeding and attempts at early discharge from hospital, regardless of weight or gestational age, with strict follow‐up. However, the last two components are less frequently identified as part of KMC (Chan 2016).
Different modalities of KMC have been adopted around the world (Charpak 1996), according to the needs of various settings. This diversity includes exclusive and non‐exclusive breastfeeding, breast or gavage feedings, completely or partially naked, continuous (≥ 20 hours per day) or intermittent (for short periods once or a few times per day and for a variable number of days) SSC with variable duration of exposure, and early‐or‐not hospital discharge. KMC has been reported to be associated with similar neonatal mortality after stabilization, some reduction of neonatal morbidity, greater quality of mother‐to‐child bonding, and shorter hospital stay and lower costs compared with standard, conventional care of LBW infants. Some researchers have claimed that KMC is the best option if neonatal care units are unavailable; if they are available but are overwhelmed by demand, KMC would allow rationalization of resources by freeing up incubators for sicker infants (Ruiz‐Peláez 2004). This review covered all randomized controlled trials (RCTs) of KMC with all its components, irrespective of duration of intervention, breastfeeding patterns, and time to discharge from hospital. We performed subgroup analyses for the primary outcome of mortality at discharge or at 40 to 41 weeks' postmenstrual age and at latest follow‐up according to type of KMC (intermittent vs continuous), infant age at initiation of KMC (≤ 10 days vs >10 days), setting in which the trial was conducted (low/middle‐income countries vs high‐income countries), and infant stabilization (before vs after). For all outcomes in stabilized LBW infants, we performed subgroup analyses according to type of KMC (intermittent vs continuous). In addition, we included RCTs that compared early‐onset (starting within 24 hours after birth) versus late‐onset (starting after 24 hours after birth) KMC.
How the intervention might work
The intervention assumes that the mother maintains the infant's body temperature and is the main source of nutrition and stimulation, which are the main components of conventional neonatal care (Rey 1983). SSC would allow that an infant´s demands for care may trigger neuropsychobiological paths that increase maternal behavior and immediate response to infant needs, as well as increased lactogenesis (Diaz‐Rossello 2008). In addition, KMC would empower the mother (parents or caregivers) by gradually transferring the skills and responsibility for becoming the child’s primary caregiver and meeting every physical and emotional need (Nyqvist 2010).
Why it is important to do this review
We undertook this systematic review to determine whether KMC reduces morbidity and mortality in LBW infants. We believe that this review provides a valuable resource for clinicians and policy makers in summarizing current best evidence and highlighting gaps in research.
Objectives
To determine whether evidence is available to support the use of KMC in LBW infants as an alternative to conventional neonatal care before or after the initial period of stabilization with conventional care, and to assess beneficial and adverse effects.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials, including cluster‐randomized trials, in which KMC (continuous or intermittent) was compared with conventional neonatal care in stabilized or non‐stabilized LBW infants. Moreover, we included randomized trials that compared early‐onset KMC versus late‐onset KMC. We excluded trials if they were quasi‐randomized, or if they evaluated effects of KMC in healthy full‐term infants or in those with birthweight ≥ 2500 g, which is the topic of a separate review (Moore 2012), or if they used a cross‐over design, or if they reported only results for physiological parameters, or if they evaluated only the effect of KMC on procedural pain in infants, which is the topic of a separate review (Johnston 2014), or if they assessed the effect of KMC on infant colic or on neonatal transport. In addition, we did not include studies in which KMC was part of a package of interventions for newborn care.
When trials were reported in abstracts, we planned to include them, provided information on study methods was sufficient to allow us to assess eligibility and risk of bias. If insufficient information was reported, we attempted to contact trial authors to request further information before deciding to exclude any study.
Types of participants
LBW Infants (defined as birthweight < 2500 g), regardless of gestational age.
Types of interventions
Comparisons of KMC with conventional neonatal care in LBW infants, regardless of infant stabilization status, duration of intervention, and breastfeeding patterns, and irrespective of whether or not discharge from hospital was early.
Comparisons of early‐onset KMC with late‐onset KMC in LBW infants, irrespective of infant stabilization status.
Types of outcome measures
We chose primary outcomes to be most representative of the clinically important measures of effectiveness and safety for these infants. Secondary outcomes included other clinical measures of effectiveness, mother‐infant attachment or interaction, satisfaction with care, home environment and father involvement, and costs of care.
Primary outcomes
Mortality.
At discharge or at 40 to 41 weeks' postmenstrual age (from randomization until discharge or 40 to 41 weeks' postmenstrual age).
At six months of age or at six months' follow‐up (from randomization until six months of age or six months' follow‐up).
At 12 months' corrected age (from randomization until 12 months' corrected age).
At latest follow‐up (from randomization until last follow‐up).
Severe infection/sepsis (as defined in individual studies).
Severe illness (as defined in individual studies).
Infant growth.
Weight at discharge or at 40 to 41 weeks' postmenstrual age, and at six and 12 months' corrected age.
Weight gain at latest follow‐up.
Length at discharge or at 40 to 41 weeks' postmenstrual age, and at six and 12 months' corrected age.
Length gain at latest follow‐up.
Head circumference at discharge or at 40 to 41 weeks' postmenstrual age, and at six and 12 months' corrected age.
Head circumference gain at latest follow‐up.
Neurodevelopmental and neurosensory impairment.
Psychomotor development (measured by a validated tool/instrument).
Cerebral palsy.
Deafness.
Visual impairment.
Secondary outcomes
Nosocomial infection/sepsis (as defined in individual studies).
Mild/moderate infection or illness (as defined in individual studies).
Lower respiratory tract disease (as defined in individual studies).
Diarrhea (as defined in individual studies).
Hypothermia (as defined in individual studies).
Hyperthermia (as defined in individual studies).
Length of hospital stay.
Re‐admission to hospital.
Breastfeeding.
Exclusive breastfeeding at discharge or at 40 to 41 weeks' postmenstrual age, and at one to three and at six to 12 months' follow‐up.
Any breastfeeding at discharge or at 40 to 41 weeks' postmenstrual age, and at one to two, three, six, and 12 months' follow‐up.
Onset of breastfeeding.
Mother‐infant attachment (measured by a validated tool/instrument).
Mother‐infant interaction (measured by a validated tool/instrument).
Parental and familial satisfaction (measured by interviews).
Home environment and father involvement (measured by a validated tool/instrument).
Costs of care.
Search methods for identification of studies
Electronic searches
Appendix 1 shows the search strategy for the 2014 update. For the 2016 update, we used criteria and standard methods of The Cochrane Collaboration and the Cochrane Neonatal Review Group (see the Cochrane Neonatal Group search strategy for specialized register). This included searches of the CENTRAL (Cochrane Central Register of Controlled Trials; 2016, Issue 6) in The Cochrane Library; and MEDLINE via PubMed, Embase, LILACS (Latin American Caribbean Health Sciences Literature), POPLINE (Population Information Online), and CINAHL (Cumulative Index to Nursing and Allied Health Literature) databases (all from inception to June 30, 2016) using the following search terms: (Kangaroo OR skin‐to‐skin OR [skin to skin]), plus database‐specific limiters for RCTs and neonates (see Appendix 2 for the full search strategies for each database). We applied no language restrictions.
We searched clinical trials registries for ongoing or recently completed trials (clinicaltrials.gov), the World Health Organization International Trials Registry and Platform (www.whoint/ictrp/search/en/), and the ISRCTN Registry (International Standard Randomized Controlled Trial Number Registry).
Searching other resources
We also searched the Web page of the Kangaroo Foundation, the International Network of Kangaroo Care, conference and symposia proceedings on KMC, reference lists of identified studies, textbooks, review articles, and Google Scholar. In addition, we performed journal handsearching and contacted investigators involved in the field to locate unpublished studies.
Data collection and analysis
Selection of studies
We used standard methods of The Cochrane Collaboration and its Neonatal Review Group. Two review authors retrieved and reviewed independently all studies deemed suitable to determine inclusion. We resolved disagreements through consensus.
Data extraction and management
Two review authors independently extracted data in duplicate from all reports and recorded them on a piloted form. We performed no blinding of authorship. We extracted the following data for each trial: authors; year of publication; country; level of care; human resources used; inclusion and exclusion criteria; study characteristics; mean or median weight and gestational age at birth, and infant age at enrollment by group; description of interventions; co‐interventions; mean or median duration of KMC; criteria for infant discharge from the hospital; scheme for follow‐up of infants after discharge; numbers randomized and analyzed; numbers of and reasons for withdrawal; and outcomes. Review authors resolved differences in data extracted by discussion and reached consensus. We sought additional information from individual investigators when published information did not include the required details. One review author (AC‐A) entered data into Review Manager software (RevMan 2014), and the other review author (JLD‐R) checked data for accuracy. We processed included trial data as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Assessment of risk of bias in included studies
Two review authors who were not associated with any of the trials assessed risk of bias individually in each included trial. Review authors were not blind to author, institution, journal of publication, or results when conducting methodological assessments, as they were familiar with most of the studies. When differences in assessment of risk of bias arose, we reached a consensus. We assessed risk of bias by using the dimensions outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We assessed seven domains related to risk of bias in each included trial because evidence suggests that these are associated with biased estimates of treatment effect: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias. We made a judgment about risk of bias for each of the seven domains as "low risk," "high risk," or "unclear risk."
Random sequence generation
"Low risk" of bias: Investigators described a random component in the sequence generation process such as random number table, computer random number generator, shuffling of cards or envelopes, drawing of lots, or computerized minimization.
"High risk" of bias: Investigators described a non‐random component in the sequence generation process, such as odd or even date of birth, based on date or day of admission, based on hospital or clinical record number, or allocated by judgment of the clinician; preference of the participant; availability of the intervention; or results of laboratory tests.
"Unclear risk" of bias: information insufficient to permit judgment of "low risk" or "high risk."
Allocation concealment
"Low risk" of bias: Investigators used an adequate method to conceal allocation, such as central allocation (including telephone or web‐based randomization) or sequentially numbered, opaque, sealed envelopes.
"High risk" of bias: Investigators used a non‐adequate method to conceal allocation, such as open random allocation schedule (eg, a list of random numbers), assignment envelopes without appropriate safeguards, alternation or rotation, date of birth, or case record number.
"Unclear risk" of bias: information insufficient to permit judgment of "low risk" or "high risk."
Blinding of participants and personnel
"Low risk" of bias: As KMC cannot be implemented when masked, we considered adequate blinding of participants and personnel as either of the following: (1) no blinding or incomplete blinding, but review authors judged that the outcome was not likely to be influenced by lack of blinding; or (2) blinding of participants and key study personnel ensured, and unlikely that blinding could have been broken.
"High risk" of bias: either of the following: (1) no blinding or incomplete blinding, and the outcome was likely to be influenced by lack of blinding; or (2) blinding of key study participants and personnel attempted, but likely that blinding could have been broken, and the outcome was likely to be influenced by lack of blinding.
"Unclear risk" of bias: information insufficient to permit judgment of "low risk" or "high risk."
We assessed blinding of participants and personnel separately for each outcome or each class of outcomes (objective and subjective).
Blinding of outcome assessment
"Low risk" of bias: We considered blinding of outcome assessment to be adequate in either of the following: (1) no blinding of outcome assessment, but review authors judged that outcome measurement was not likely to be influenced by lack of blinding; or (2) blinding of outcome assessment ensured, and unlikely that blinding could have been broken.
"High risk" of bias: either of the following: (1) no blinding of outcome assessment, and outcome measurement was likely to be influenced by lack of blinding; or (2) blinding of outcome assessment, but likely that blinding could have been broken, and that outcome measurement was likely to be influenced by lack of blinding.
"Unclear risk" of bias: information insufficient to permit judgment of "low risk" or "high risk."
We assessed blinding of outcome assessment separately for each outcome or each class of outcomes (objective and subjective).
Incomplete outcome data
"Low risk" of bias: any one of the following: (1) no missing outcome data; (2) reasons for missing outcome data unlikely to be related to true outcome; (3) missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; (4) for dichotomous outcome data, proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate; (5) for continuous outcome data, plausible effect size among missing outcomes not enough to have a clinically relevant impact on observed effect size; or (6) missing data imputed by appropriate methods.
"High risk" of bias: any one of the following: (1) reasons for missing outcome data likely to be related to true outcome, with imbalance in numbers or reasons for missing data across intervention groups; (2) for dichotomous outcome data, proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; (3) for continuous outcome data, plausible effect size among missing outcomes enough to induce clinically relevant bias impact on observed effect size; (4) "as‐treated" analysis done with substantial departure of the intervention received from that assigned at randomization; or (5) potentially inappropriate application of simple imputation.
"Unclear risk" of bias: reporting of attrition/exclusions insufficient to permit judgment of "low risk" or "high risk."
Selective reporting
"Low risk" of bias: any one of the following: (1) Study protocol was available, and all of the study's prespecified outcomes that were of interest in the review were reported in the prespecified way; or (2) the study protocol was not available, but it was clear that published reports included all expected outcomes, including those that were prespecified.
"High risk" of bias: any one of the following: (1) Not all of the study's prespecified primary outcomes were reported; (2) one or more primary outcomes were reported using measurements, analysis methods, or subsets of data that were not prespecified; (3) one or more reported primary outcomes were not prespecified; (4) one or more outcomes of interest in the review were reported incompletely, so that they could not be entered into a meta‐analysis; or (5) the study report failed to include results for a key outcome that would be expected to have been reported for such a study.
"Unclear risk" of bias: information insufficient to permit judgment of "low risk" or "high risk."
Other bias
"Low risk" of bias: Study appeared to be free of other sources of bias.
"High risk" of bias: At least one important risk of bias was present. For example, the study (1) had a potential source of bias related to the specific study design used; or (2) has been claimed to have been fraudulent; or (3) had extreme baseline imbalance; or (4) used blocked randomization in unblinded trials; or (5) had differential diagnostic activity; or (6) had some other problem.
"Unclear risk" of bias: information insufficient to assess whether an important risk of bias existed, or rationale or evidence insufficient to suggest that an identified problem will introduce bias.
Review authors independently assessed risk of bias in included studies and resolved discrepancies through discussion. We made explicit judgments about whether studies were at high risk of bias, according to the criteria given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), and we explored the impact of the level of bias by undertaking sensitivity analyses (see Sensitivity analysis).
Measures of treatment effect
For dichotomous data, we have presented results as risk ratios (RRs) with 95% confidence intervals (CIs). For continuous data, we have used mean differences (MDs) with 95% CIs. We calculated the number needed to treat for an additional beneficial or harmful outcome (NNTB or NNTH) for outcomes for which investigators reported a statistically significant reduction or increase in risk difference based on control event rates in the included studies.
Unit of analysis issues
The unit of analysis was the participating infant in individually randomized trials. We had planned to include cluster‐randomized trials in the analyses, along with individually randomized trials, but no such trials met our inclusion criteria.
We considered that cross‐over trials would not be feasible for this intervention, and consequently, we did not include such trials.
Dealing with missing data
For included studies, we noted levels of attrition in the Characteristics of included studies tables. We analyzed outcomes on an intention‐to‐treat basis. If this was not clear from the original article, we carried out re‐analysis when possible. We contacted study authors for missing data.
Assessment of heterogeneity
We tested heterogeneity of results among studies by using the quantity I2, which describes the percentage of total variation across studies that is due to heterogeneity rather than to chance (Higgins 2003). A value of 0% indicates no observed heterogeneity, whereas I2 values of 50% or greater indicate a substantial level of heterogeneity. We planned to pool data across studies using the fixed‐effect model if substantial statistical heterogeneity was not present. If we noted substantial heterogeneity (I2 values ≥ 50%), we used a random‐effects model to pool data and made an attempt to identify potential sources of heterogeneity based on subgroup analysis by type of KMC, infant age at initiation of KMC, setting in which the trial was conducted, and risk of bias of trials.
Assessment of reporting biases
We assessed publication and related biases visually by examining the symmetry of funnel plots, and statistically by using Egger's test (Egger 1997). The larger the deviation of the intercept of the regression line from zero, the greater was the asymmetry, and the more likely it was that the meta‐analysis would yield biased estimates of effect. We considered a P value < 0.1 to indicate significant asymmetry, as suggested by Egger.
Data synthesis
We performed statistical analyses using Review Manager software (RevMan 2014) and analyzed outcomes on an intention‐to‐treat basis. If data for similar outcomes from two or more separate studies were available, we combined data in a meta‐analysis and calculated a pooled RR or MD with associated 95% CIs.
Quality of evidence
We used the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach, as outlined in the GRADE Handbook (Schünemann 2013), to assess the quality of evidence for the following (clinically relevant) outcomes: mortality at latest follow‐up, severe infection/sepsis at latest follow‐up, hypothermia at discharge or at 40 to 41 weeks’ postmenstrual age, weight gain at latest follow‐up, any breastfeeding at discharge or at 40 to 41 weeks' postmenstrual age and at one to three months' follow‐up, and psychomotor development at 12 months' corrected age (Griffith quotient for all subscales).
Two authors independently assessed the quality of the evidence for each of the outcomes above. We considered evidence from randomized controlled trials as high quality but downgraded the evidence one level for serious (or two levels for very serious) limitations based upon the following: design (risk of bias), consistency across studies, directness of the evidence, precision of estimates and presence of publication bias. We used the GRADEpro 2008 Guideline Development Tool to create a ‘Summary of findings’ table to report the quality of the evidence.
The GRADE approach results in an assessment of the quality of a body of evidence in one of four grades:
High: We are very confident that the true effect lies close to that of the estimate of the effect.
Moderate: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect.
Very low: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
Subgroup analysis and investigation of heterogeneity
We performed prespecified subgroup analyses for the primary outcome of mortality at discharge or at 40 to 41 weeks' postmenstrual age and at latest follow‐up according to type of KMC (intermittent vs continuous), daily duration of KMC (< 2 hours vs 6 to 15 hours vs ≥ 20 hours), infant age at initiation of KMC (≤ 10 days vs > 10 days), setting in which the trial was conducted (low/middle‐income countries vs high‐income countries), and infant stabilization status at trial entry (before vs after). For all outcomes in stabilized LBW infants, we performed subgroup analyses according to type of KMC (intermittent vs continuous). We also compared early‐onset KMC (starting within 24 hours post birth) against late‐onset KMC (starting after 24 hours post birth).
It was not possible to perform planned subgroup analyses according to birthweight, gestational age, and type of LBW owing to limited available information.
Sensitivity analysis
We carried out a planned sensitivity analysis to explore the impact of risk of bias on the general direction of findings or on the size of the treatment effect for main outcomes when more than one study contributed data. We did this by excluding trials with high risk of bias in their results as judged by the review authors. For the primary outcomes of "mortality at discharge or at 40 to 41 weeks' postmenstrual age," "mortality at latest follow‐up," ''severe infection/sepsis at latest follow‐up," and "infant growth," we performed sensitivity analyses by excluding trials with unclear allocation concealment and high levels of attrition (> 20%).
Results
Description of studies
See Characteristics of included studies and Characteristics of excluded studies.
Results of the search
In the previous version of this review (Conde‐Agudelo 2014), we included 18 trials (Ali 2009; Blaymore Bier 1996; Boo 2007; Cattaneo 1998; Charpak 1997; Eka Pratiwi 2009; Gathwala 2008; Ghavane 2012; Kadam 2005; Nagai 2010; Neu 2010; Ramanathan 2001; Roberts 2000; Rojas 2003; Sloan 1994; Suman 2008; Whitelaw 1988; Worku 2005) and excluded 38 trials (Ahn 2010; Anderson 2003; Arandia 1993; Bera 2014; Bergman 1994; Bergman 2004; Charpak 1994; Chiu 2009; Christensson 1998; Chwo 2002; Dala Sierra 1994; Darmstadt 2006; de Almeida 2010; de Macedo 2007; Feldman 2002; Gregson 2011; Hake Brooks 2008; Huang 2006; Ibe 2004; Kambarami 1998; Kumar 2008; Lai 2006; Lamy Filho 2008; Legault 1993; Legault 1995; Lincetto 2000; Lizarazo‐Medina 2012; Ludington‐Hoe 1991; Ludington‐Hoe 2000; Ludington‐Hoe 2004; Ludington‐Hoe 2006; Miles 2006; Miltersteiner 2005; Mitchell 2013; Ohgi 2002; Sloan 2008; Tallandini 2006; Udani 2008). For this update, the search strategy identified 16 additional studies for possible inclusion, of which we included three (Acharya 2014; Kumbhojkar 2016; Nimbalkar 2014), added one study to awaiting assessment (Holditch‐Davis 2014), and excluded 12 (Badiee 2014; Broughton 2013; Dehghani 2015; Karimi 2014; Kashaninia 2015; Kristoffersen 2016; Lamy Filho 2015; Lyngstad 2014; Mörelius 2015; Samra 2015; Silva 2016; Swarnkar 2016) (Figure 1). One paper by Neu et al (published in 2013) reported additional results of a previously included study (Neu 2010).
1.
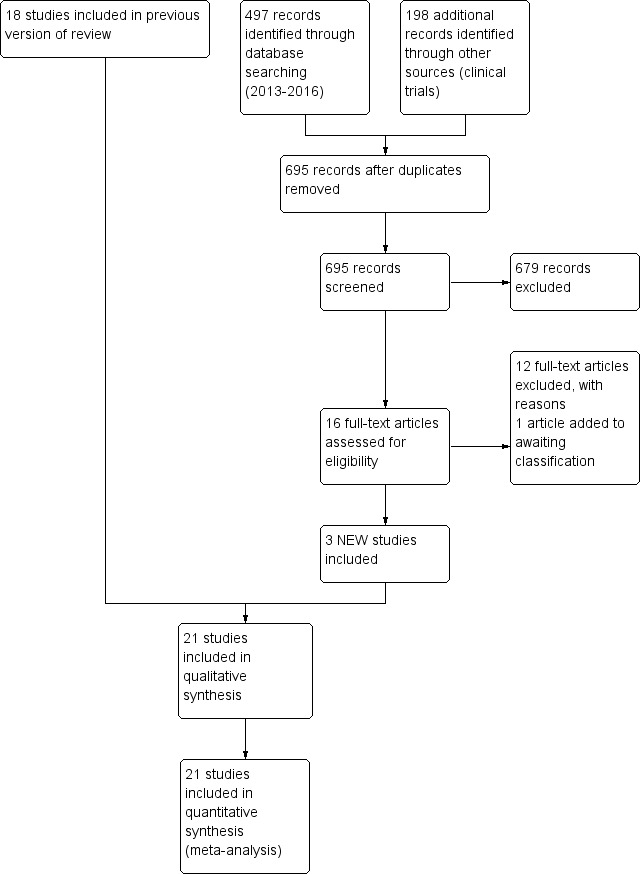
Study flow diagram: review update
Included studies
Twenty‐one studies, including 3042 infants, fulfilled inclusion criteria, of which 19 evaluated KMC in LBW infants after stabilization (Acharya 2014; Ali 2009; Blaymore Bier 1996; Boo 2007; Cattaneo 1998; Charpak 1997; Eka Pratiwi 2009; Gathwala 2008; Ghavane 2012; Kadam 2005; Kumbhojkar 2016; Neu 2010; Nimbalkar 2014; Ramanathan 2001; Roberts 2000; Rojas 2003; Sloan 1994; Suman 2008; Whitelaw 1988), one evaluated KMC in LBW infants before stabilization (Worku 2005), and one compared early‐onset KMC with late‐onset KMC (Nagai 2010) in relatively stable LBW infants. Sixteen studies were conducted in low‐ or middle‐income countries (India (Ali 2009; Gathwala 2008; Ghavane 2012; Kadam 2005; Kumbhojkar 2016; Nimbalkar 2014; Ramanathan 2001; Suman 2008); Ethiopia (Cattaneo 1998; Worku 2005); Malaysia (Boo 2007); Madagascar (Nagai 2010); Indonesia (Cattaneo 1998; Eka Pratiwi 2009); Nepal (Acharya 2014); Ecuador (Sloan 1994); Colombia (Charpak 1997); and Mexico (Cattaneo 1998)), and five in high‐income countries (United States (Blaymore Bier 1996; Neu 2010; Rojas 2003); United Kingdom (Whitelaw 1988); and Australia (Roberts 2000)). The sample size ranged from 28 (Ramanathan 2001) to 777 (Charpak 1997) (median, 110). Five studies included infants from multiple pregnancies (Ali 2009; Blaymore Bier 1996; Boo 2007; Charpak 1997; Whitelaw 1988), and six included only infants with birthweight ≤ 1500 g (Blaymore Bier 1996; Boo 2007; Ghavane 2012; Ramanathan 2001; Rojas 2003; Whitelaw 1988). Infants with major congenital malformations or severe perinatal complications and parental refusal to participate in the study were reported as meeting exclusion criteria in the great majority of included studies.
Eight studies did not provide data on the percentage of LBW infants meeting eligibility criteria. Among studies conducted in low‐ or middle‐income countries, 37% (Eka Pratiwi 2009) to 87% (Acharya 2014) of LBW infants met eligibility criteria, whereas for studies conducted in high‐income countries, percentages ranged from 19% (Rojas 2003) to 50% (Whitelaw 1988). The mean or median age of LBW infants at enrollment varied from < one hour (Nimbalkar 2014) to 32 days (Roberts 2000) (median, seven days). Median or mean infant age at enrollment was < one day in three studies (Eka Pratiwi 2009; Nimbalkar 2014; Worku 2005), one to 10 days in eight studies (Ali 2009; Cattaneo 1998; Charpak 1997; Gathwala 2008; Kadam 2005; Kumbhojkar 2016; Nagai 2010; Suman 2008), 11 to 20 days in six studies (Ghavane 2012; Neu 2010; Ramanathan 2001; Rojas 2003; Sloan 1994; Whitelaw 1988), and 21 to 32 days in three studies (Blaymore Bier 1996; Boo 2007; Roberts 2000). One study did not report data on infant age at enrollment (Acharya 2014). In the study that compared early‐onset KMC with late‐onset KMC (Nagai 2010), mean age at initiation of KMC was 19.8 hours in the early‐onset KMC group, and 33.0 hours in the late‐onset KMC. Mean or median weight of infants at recruitment ranged from 968 g (Blaymore Bier 1996) to 2076 g (Nagai 2010) (median, 1611 g).
Trials were conducted under a variety of hospital conditions, regulations, and routines. However, descriptions of the KMC intervention shows remarkable consistency across trials. In all instances, the intervention included SSC and encouraged breastfeeding. Early neonatal discharge from hospital was considered only in the Colombian study (Charpak 1997). Among studies evaluating KMC in stabilized LBW infants, 16 used intermittent KMC (Acharya 2014; Ali 2009; Blaymore Bier 1996; Boo 2007; Eka Pratiwi 2009; Gathwala 2008; Ghavane 2012; Kadam 2005; Kumbhojkar 2016; Neu 2010; Nimbalkar 2014; Ramanathan 2001; Roberts 2000; Rojas 2003; Suman 2008; Whitelaw 1988), and three used continuous KMC (Cattaneo 1998; Charpak 1997; Sloan 1994). Only one study provided a detailed definition of stabilization (Nagai 2010). The mean or median duration of KMC per day was < two hours in six studies (Blaymore Bier 1996; Boo 2007; Neu 2010; Roberts 2000; Rojas 2003; Whitelaw 1988), four to seven hours in three studies (Acharya 2014; Ali 2009; Ramanathan 2001), eight to 17 hours in seven studies (Eka Pratiwi 2009; Gathwala 2008; Ghavane 2012; Kadam 2005; Kumbhojkar 2016; Nimbalkar 2014; Suman 2008), and ≥ 20 hours in three studies (Cattaneo 1998; Charpak 1997; Sloan 1994). Studies that evaluated KMC in LBW infants before stabilization (Worku 2005) and compared early‐onset KMC with late‐onset KMC (Nagai 2010) used continuous KMC. In studies evaluating intermittent KMC, the intervention was a combination of SSC and radiant warmer/incubator. Standard neonatal care included infant stay in incubator only (Blaymore Bier 1996; Boo 2007; Charpak 1997; Neu 2010; Roberts 2000; Rojas 2003; Whitelaw 1988) or in radiant warmer only (Acharya 2014; Ali 2009; Kadam 2005; Kumbhojkar 2016; Nimbalkar 2014; Suman 2008; Worku 2005) or in incubator or radiant warmer (Cattaneo 1998; Eka Pratiwi 2009; Gathwala 2008; Ghavane 2012; Ramanathan 2001; Sloan 1994). Information provided to mothers in the conventional neonatal care group on promotion of breastfeeding and on facilitation and promotion of maternal involvement in the care of the neonate, which are critical for the outcomes measured, was not reported in eight trials (Acharya 2014; Blaymore Bier 1996; Charpak 1997; Eka Pratiwi 2009; Kumbhojkar 2016; Nagai 2010; Suman 2008; Worku 2005).
Eleven studies were performed in neonatal intensive care units of tertiary care, public, maternity, or university hospitals (Ali 2009; Boo 2007; Eka Pratiwi 2009; Kadam 2005; Kumbhojkar 2016; Ramanathan 2001; Roberts 2000; Rojas 2003; Sloan 1994; Suman 2008; Whitelaw 1988), four in neonatal units of university hospitals (Cattaneo 1998; Gathwala 2008; Nagai 2010; Worku 2005), two in "kangaroo wards" (KMC infants) and neonatal intensive/intermediate care units of tertiary care hospitals (controls) (Charpak 1997; Ghavane 2012), two in newborn nurseries (Acharya 2014; Blaymore Bier 1996), one in a maternity ward (Nimbalkar 2014), and one in both hospital and home (Neu 2010). Infants were cared for by both doctors and nurses in all but two studies (Ghavane 2012; Neu 2010). In the Ghavane 2012 study, infants in the KMC group were cared for solely by their mothers, who was supervised by a trained nurse. In the Neu 2010 study, the supportive intervention that promoted kangaroo holding of preterm infants by their mothers was performed by an experienced nurse. Nine studies reported clearly on criteria for discharging infants from the hospital (Ali 2009; Boo 2007; Cattaneo 1998; Charpak 1997; Ghavane 2012; Kadam 2005; Kumbhojkar 2016; Ramanathan 2001; Suman 2008). The most commonly reported criteria were (1) good general health of the infant without overt illness; (2) feeding well on exclusive or predominant breastfeeding; (3) weight gain of 10 to 15 g/kg/d for ≥ three consecutive days; (4) stable temperature for ≥ three consecutive days; and (5) mother confident of taking care of the infant at home. In addition, three studies included an infant weight of ≥ 1300 to 1500 g as a discharge criterion. Thirteen studies reported on schemes for follow‐up of infants after discharge from the hospital (Ali 2009; Blaymore Bier 1996; Cattaneo 1998; Charpak 1997; Gathwala 2008; Ghavane 2012; Kumbhojkar 2016; Nagai 2010; Neu 2010; Roberts 2000; Sloan 1994; Suman 2008; Whitelaw 1988). In summary, the most common scheme for follow‐up was the following: weekly until 40 weeks' postmenstrual age, and monthly thereafter until three to six months of age or corrected age. In five studies, the last follow‐up was provided at six months of age or corrected age (Ali 2009; Blaymore Bier 1996; Neu 2010; Roberts 2000; Sloan 1994). Infants were followed up to 12 months of age or corrected age in only two studies (Charpak 1997; Whitelaw 1988).
The main characteristics of the included studies are shown in the table Characteristics of included studies.
Excluded studies
We excluded 50 studies: 21 because they were non‐randomized trials (Ahn 2010; Arandia 1993; Bera 2014; Bergman 1994; Broughton 2013; Charpak 1994; Dala Sierra 1994; de Almeida 2010; de Macedo 2007; Feldman 2002; Gregson 2011; Ibe 2004; Kashaninia 2015; Kristoffersen 2016; Lamy Filho 2008; Legault 1995; Lincetto 2000; Lizarazo‐Medina 2012; Ohgi 2002; Silva 2016; Tallandini 2006), 10 because they included infants with birthweight ≥ 2500 g and did not report results separately for the subgroup of infants with birthweight < 2500 g (Anderson 2003; Chiu 2009; Chwo 2002; Hake Brooks 2008; Huang 2006; Karimi 2014; Lai 2006; Mörelius 2015; Samra 2015; Sloan 2008), seven because they reported only physiological outcomes (Bergman 2004; Dehghani 2015; Ludington‐Hoe 1991; Ludington‐Hoe 2000; Ludington‐Hoe 2004; Ludington‐Hoe 2006; Mitchell 2013), three because the method of generation of allocation to treatment was quasi‐randomized (Kambarami 1998; Miltersteiner 2005; Swarnkar 2016), three because allocation was performed by a cross‐over design (Legault 1993; Lyngstad 2014; Miles 2006), two because KMC was part of a preventive package of interventions for essential newborn care (Darmstadt 2006; Kumar 2008), one because it evaluated only KMC for rewarming hypothermic infants (Christensson 1998), one because it assessed only the effect of KMC on the mental health of mothers (Badiee 2014), one because it evaluated only the effect of KMC on colonization status of newborns’ nostrils (Lamy Filho 2015), and one because it was published as an abstract only, and our attempts to locate full publications or to contact study authors were unsuccessful (Udani 2008).
We have presented the main characteristics of the excluded studies in the table Characteristics of excluded studies.
Risk of bias in included studies
We have depicted the risk of bias in included studies in Figure 2. We judged that no study adequately addressed all seven domains. We judged only two studies to adequately address six domains. The methodological quality of the included trials was mixed, and we carried out a sensitivity analysis to examine the impact of excluding trials at high risk of bias. See Sensitivity analysis. The main threats to validity were performance bias (by lack of blinding of participants, personnel, and outcomes assessors) and selection bias (by lack of information on methods used for concealment of treatment allocation).
2.
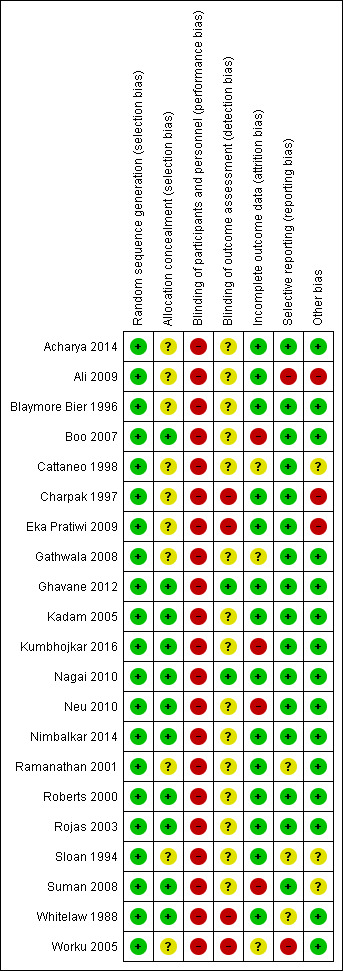
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Most of the included studies used adequate methods to generate allocation sequence. Ten studies used random number tables (Acharya 2014; Cattaneo 1998; Charpak 1997; Eka Pratiwi 2009; Gathwala 2008; Nimbalkar 2014; Ramanathan 2001; Rojas 2003; Sloan 1994; Worku 2005), and four studies used shuffling of envelopes (Blaymore Bier 1996; Boo 2007; Roberts 2000; Whitelaw 1988). Other methods of sequence generation used included web‐based random number generator (Ghavane 2012), computer random number generator (Neu 2010), minimization computerized technique (Nagai 2010), block randomization technique (Ali 2009), the sealed envelope method (Kadam 2005), and simple randomization (Kumbhojkar 2016; Suman 2008).
Ten studies used sealed envelopes for concealment of treatment allocation (Boo 2007; Ghavane 2012; Kadam 2005; Kumbhojkar 2016; Neu 2010; Nimbalkar 2014; Roberts 2000; Rojas 2003; Suman 2008; Whitelaw 1988), although only five studies (Ghavane 2012; Neu 2010; Nimbalkar 2014; Rojas 2003; Whitelaw 1988) explicitly stated that the envelopes were opaque, sealed, and numbered. Investigators concealed allocation by using a software that provided automatically random allocation (minimization method) in only one study (Nagai 2010). Ten studies did not report the method of allocation concealment (Acharya 2014; Ali 2009; Blaymore Bier 1996; Cattaneo 1998; Charpak 1997; Eka Pratiwi 2009; Gathwala 2008; Ramanathan 2001; Sloan 1994; Worku 2005).
Blinding
As KMC cannot be implemented when masked, all included studies reported lack of blinding of participants and clinical staff. Only two studies (Ghavane 2012; Nagai 2010) reported that outcome assessors were masked to the intervention group of infants. Neu 2010 reported that four researchers assessed outcome measures, two of whom were blinded to the hypotheses of the study but not to group assignment of mother‐infant dyads. The other two researchers were blinded to group assignment and hypotheses. The remaining trials did not state whether any attempt was made to "blind" outcome assessment.
We consider that performance and observer bias cannot be excluded owing to lack of blinding of participants and clinicians. However, although this could affect assessment of subjective outcomes such as parental and familial satisfaction, mother‐infant attachment, and social and home environment, or objective outcomes such as breastfeeding, length of hospital length, and re‐admission to hospital after discharge, it is much less likely to have affected the primary outcomes (infant mortality, severe infection/sepsis, severe illness, infant growth, and neurodevelopmental disability) and some secondary outcomes of this review (nosocomial infection, mild/moderate infection or illness, hypothermia, and hyperthermia).
Incomplete outcome data
Eight trials had no losses to follow‐up and no exclusions post randomization (Acharya 2014; Kadam 2005; Nagai 2010; Nimbalkar 2014; Ramanathan 2001; Roberts 2000; Rojas 2003; Whitelaw 1988). In seven studies, 1% to 10% of recruited infants were lost to follow‐up (Ali 2009; Blaymore Bier 1996; Charpak 1997; Eka Pratiwi 2009; Gathwala 2008; Ghavane 2012; Sloan 1994). Boo 2007 excluded 12.3% of infants in the KMC group because SSC sessions were carried out on less than 50% of hospital stay days after recruitment. Two trials (Cattaneo 1998; Worku 2005) did not report the number of infants lost to follow‐up or excluded after randomization. Kumbhojkar 2016 did not report the number of infants lost to follow‐up or exclusions, but investigators stated in the Discussion section of the article that "poor follow‐up" was provided in the control group. Suman 2008 had high risk of attrition bias because 22.3% of infants were lost to follow‐up. Moreover, imbalance across intervention groups was evident in numbers for losses to follow‐up (KMC 10.2%; control 33.9%). In addition, 6.4% of infants were omitted from reports of analyses because they did not receive assigned care. Neu 2010 had high risk of attrition bias because 9.2% of infants were lost to follow‐up and 16.1% were excluded post randomization.
Selective reporting
No study protocols were available. We compared outcomes listed in the Methods section of articles against those reported in the Results section. Sixteen studies (Acharya 2014; Blaymore Bier 1996; Boo 2007; Cattaneo 1998; Charpak 1997; Eka Pratiwi 2009; Gathwala 2008; Ghavane 2012; Kadam 2005; Kumbhojkar 2016; Nagai 2010; Neu 2010; Nimbalkar 2014; Roberts 2000; Rojas 2003; Suman 2008) reported all outcomes listed in the Methods section, and we assume that these reports probably included all prespecified variables. Two studies (Ali 2009; Worku 2005) had high risk of bias owing to selective outcome reporting. Worku 2005 did not report the great majority of outcomes listed in the Methods section, such as mild/moderate and severe illness, sepsis, diarrhea, pneumonia, aspiration, weight gain, and mother's feelings. In Ali 2009, non‐significant results such as infant mortality (primary outcome) and weight, length, and head circumference at discharge and follow‐up (secondary outcomes) were mentioned but were not reported adequately. In the remaining three studies, some secondary outcomes listed in the Methods section were not reported (Ramanathan 2001), or they were mentioned but were not reported adequately (Sloan 1994; Whitelaw 1988).
Other potential sources of bias
We did not identify other potential sources of bias in 15 studies (Acharya 2014; Blaymore Bier 1996; Boo 2007; Gathwala 2008; Ghavane 2012; Kadam 2005; Kumbhojkar 2016; Nagai 2010; Neu 2010; Nimbalkar 2014; Ramanathan 2001; Roberts 2000; Rojas 2003; Whitelaw 1988; Worku 2005). Three studies (Ali 2009; Charpak 1997; Eka Pratiwi 2009) used blocked randomization for sequence generation. When blocked randomization is used in an unblinded trial, and when assignments are revealed after individuals are recruited into the trial, it is sometimes possible to predict future assignments. This is particularly the case when blocks are of a fixed size. Cattaneo 1998 carried out randomization in blocks of six with stratification by weight at one of the three participating centers. The trial performed by Sloan 1994 was stopped early because investigators found a highly significant difference in severe morbidity at two months and at six months. Randomized controlled trials that are stopped early are more likely to be associated with greater effect sizes than RCTs not stopped early (Bassler 2010). This difference is independent of the presence of statistical stopping rules and is greatest in smaller studies. In the study by Suman 2008, groups were significantly different at baseline in weight and age at enrollment.
Effects of interventions
See: Table 1
Comparison 1. Kangaroo mother care versus conventional neonatal care
The comparison between KMC and conventional neonatal care included 20 studies (2969 infants) and 49 outcomes, of which 24 were reported in more than one study.
Mortality (outcomes 1.1 to 1.4)
Kangaroo mother care was associated with a statistically significant reduction in risk of mortality at discharge or at 40 to 41 weeks’ postmenstrual age (3.2% vs 5.3%; RR 0.60, 95% CI 0.39 to 0.92; I2 = 0%; NNTB = 47, 95% CI 31 to 236; eight trials, 1736 infants) (Analysis 1.1), and at latest follow‐up (4.0% vs 6.0%; RR 0.67, 95% CI 0.48 to 0.95; I2 = 0%; NNTB = 50, 95% CI 32 to 331; 12 trials, 2293 infants; moderate‐quality evidence) (Analysis 1.4) (Figure 3). The significantly decreased risk of death at discharge or at 40 to 41 weeks’ postmenstrual age, and at latest follow‐up, was also demonstrated in the subgroup of studies that used continuous (≥ 20 hours/d) KMC (mortality at discharge or at 40 to 41 weeks’ postmenstrual age: RR 0.60, 95% CI 0.38 to 0.96; I2 = 0%; three trials, 1117 infants; mortality at latest follow‐up: RR 0.67, 95% CI 0.46 to 0.98; I2 = 0%; four trials, 1384 infants), the subgroup of studies in which KMC was initiated within 10 days post birth (mortality at discharge or at 40 to 41 weeks’ postmenstrual age: RR 0.56, 95% CI 0.36 to 0.88; I2 = 0%; five trials, 1412 infants; mortality at latest follow‐up: RR 0.56, 95% CI 0.37 to 0.85; I2 = 0%; six trials, 1489 infants), the subgroup of studies conducted in low/middle‐income countries (mortality at discharge or at 40 to 41 weeks’ postmenstrual age: RR 0.57, 95% CI 0.37 to 0.89; I2 = 0%; seven studies, 1676 infants; mortality at latest follow‐up: RR 0.65, 95% CI 0.45 to 0.93; I2 = 0%; 10 trials, 2162 infants), and the trial in which KMC was used in unstabilized infants (RR 0.57, 95% CI 0.33 to 1.00). The statistically significant beneficial effect of KMC on mortality at discharge or at 40 to 41 weeks’ postmenstrual age and on mortality at latest follow‐up was not demonstrated in the subgroup of trials that used intermittent KMC (< 2 hours/d and between 6 and 15 hours/d), or that initiated KMC after 10 days post birth, or that were conducted in high‐income countries, or that used KMC in stabilized infants.
1.1. Analysis.
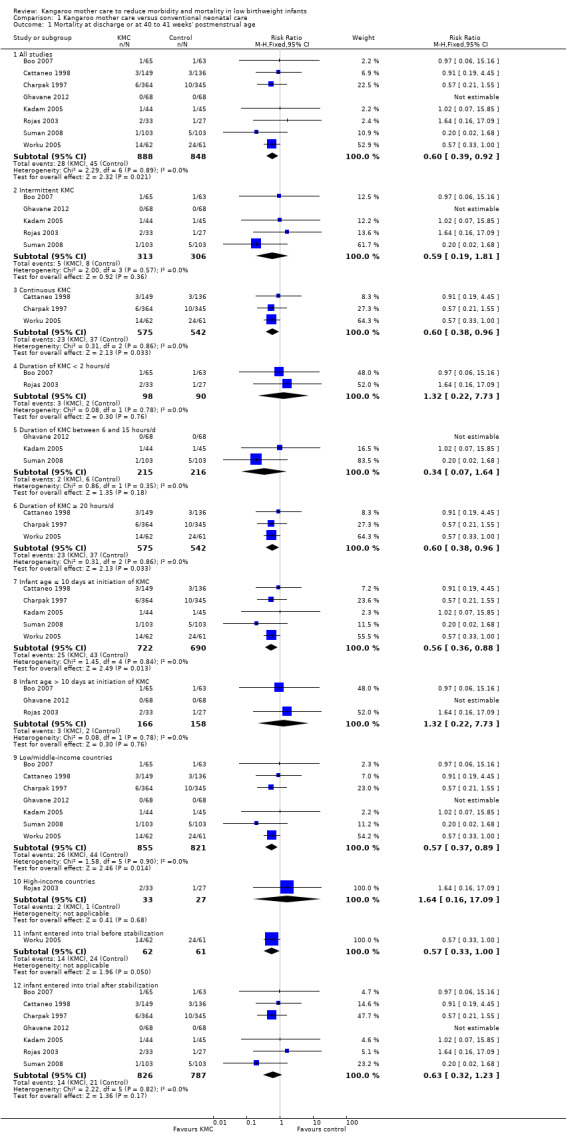
Comparison 1 Kangaroo mother care versus conventional neonatal care, Outcome 1 Mortality at discharge or at 40 to 41 weeks' postmenstrual age.
1.4. Analysis.
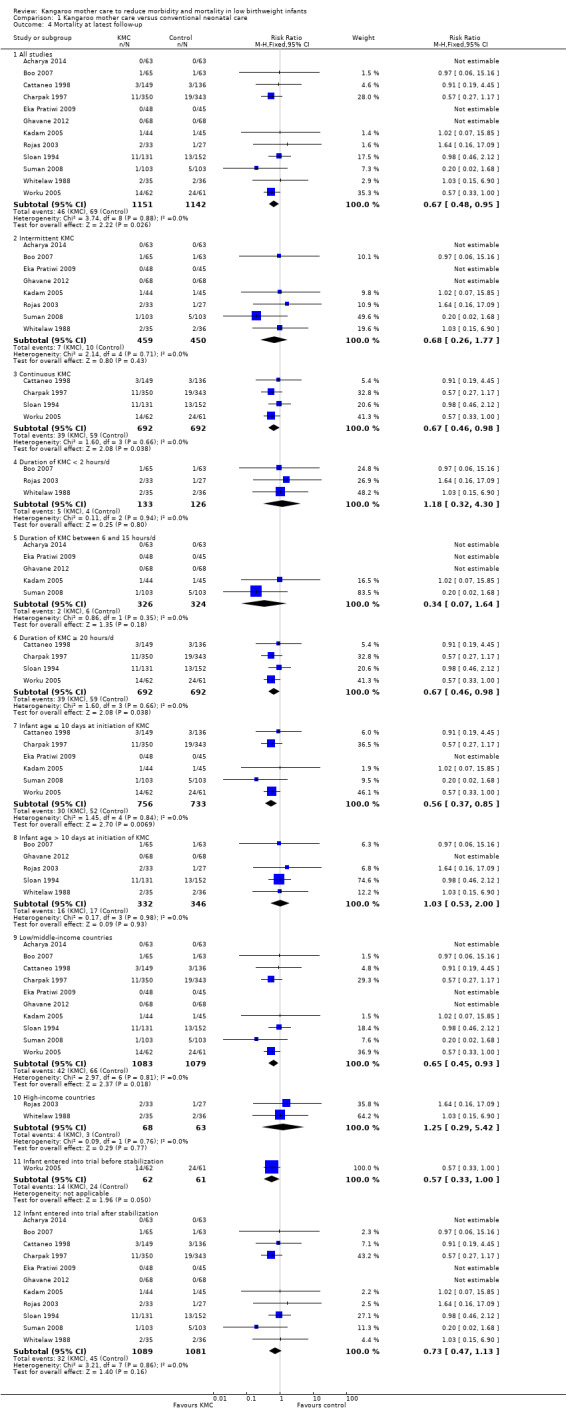
Comparison 1 Kangaroo mother care versus conventional neonatal care, Outcome 4 Mortality at latest follow‐up.
3.
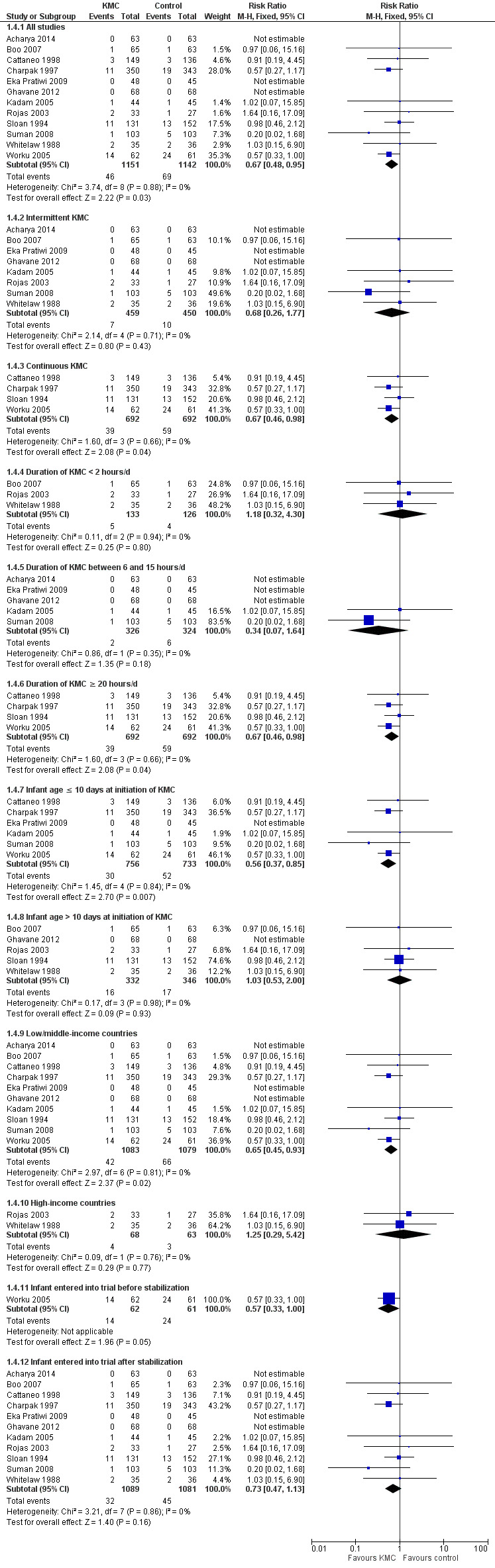
Forest plot of comparison: 1 Kangaroo mother care versus conventional neonatal care, outcome: 1.1 Mortality at latest follow‐up.
The sensitivity analysis limited to studies with adequate concealment of allocation revealed a similar reduction in mortality at discharge or at 40 to 41 weeks’ postmenstrual age, and at latest follow‐up, although this was not statistically significant (mortality at discharge or at 40 to 41 weeks’ postmenstrual age: RR 0.59, 95% CI 0.19 to 1.81; I2 = 0%; five trials; mortality at latest follow‐up: RR 0.68, 95% CI 0.26 to 1.77; I2 = 0%; six trials). Similar results were obtained when we excluded studies with high risk of attrition bias (mortality at discharge or at 40 to 41 weeks’ postmenstrual age: RR 0.64, 95% CI 0.41 to 1.00; I2 = 0%; six studies; mortality at latest follow‐up: RR 0.71, 95% CI 0.49 to 1.01; I2 = 0%; 10 studies).
We found no overall difference in risk of mortality at six months of age or at six months' follow‐up (RR 0.99, 95% CI 0.48 to 2.02; two trials, 354 infants) (Analysis 1.2), and at 12 months’ corrected age (RR 0.57, 95% CI 0.27 to 1.17; one trial, 693 infants) (Analysis 1.3) between KMC infants and controls.
1.2. Analysis.
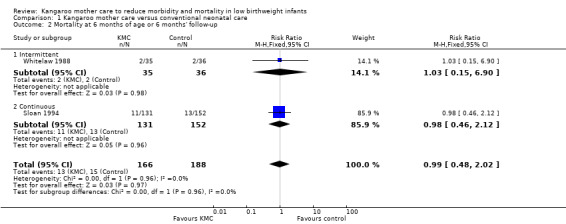
Comparison 1 Kangaroo mother care versus conventional neonatal care, Outcome 2 Mortality at 6 months of age or 6 months' follow‐up.
1.3. Analysis.
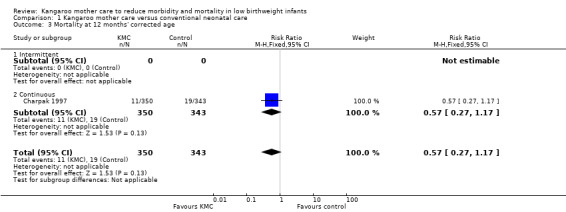
Comparison 1 Kangaroo mother care versus conventional neonatal care, Outcome 3 Mortality at 12 months' corrected age.
Infection/illness (outcomes 1.5 to 1.14)
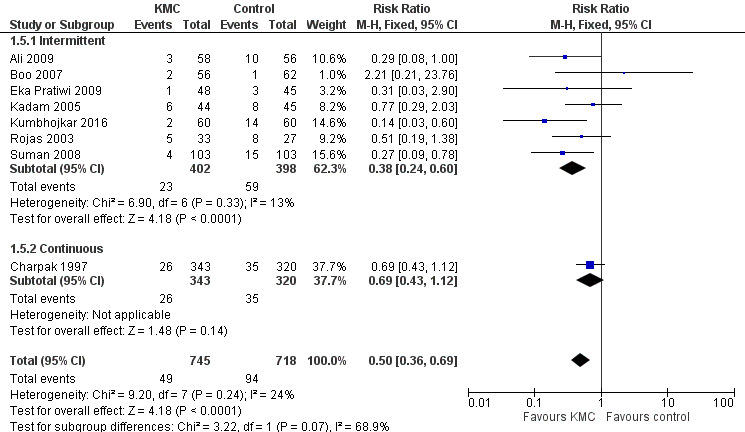
In stabilized LBW infants, KMC was associated with a statistically significant reduction in severe infection/sepsis at latest follow‐up (6.6% vs 13.1%; RR 0.50, 95% CI 0.36 to 0.69; I2 = 24%; NNTB = 15, 95% CI 12 to 25; eight trials, 1463 infants; moderate‐quality evidence) (Analysis 1.5) (Figure 4), severe illness at six months' follow‐up (5.3% vs 17.8%; RR 0.30, 95% CI 0.14 to 0.67; NNTB = 8, 95% CI 7 to 17; one trial, 283 infants) (Analysis 1.6), nosocomial infection/sepsis at discharge or at 40 to 41 weeks’ postmenstrual age (4.0% vs 11.4%; RR 0.35, 95% CI 0.22 to 0.54; I2 = 0%; NNTB = 14, 95% CI 11 to 19; five trials, 1239 infants) (Analysis 1.7), lower respiratory tract disease at six months' follow‐up (4.6% vs 12.5%; RR 0.37, 95% CI 0.15 to 0.89; NNTB = 13, 95% CI 9 to 73; one trial, 283 infants) (Analysis 1.9), and hypothermia at discharge or at 40 to 41 weeks' postmenstrual age (7.6% vs 27.1%; RR 0.28, 95% CI 0.16 to 0.49; I2 = 52%; NNTB = 5, 95% CI 4 to 7; nine trials, 989 infants; moderate‐quality evidence) (Analysis 1.11).
1.5. Analysis.
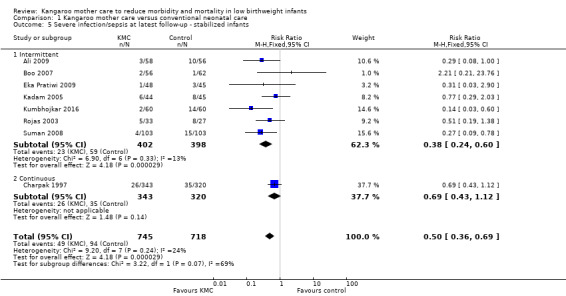
Comparison 1 Kangaroo mother care versus conventional neonatal care, Outcome 5 Severe infection/sepsis at latest follow‐up ‐ stabilized infants.
4.
Forest plot of comparison: 1 Kangaroo mother care versus conventional neonatal care, outcome: 1.2 Severe infection/sepsis at latest follow‐up ‐ stabilized infants.
1.6. Analysis.
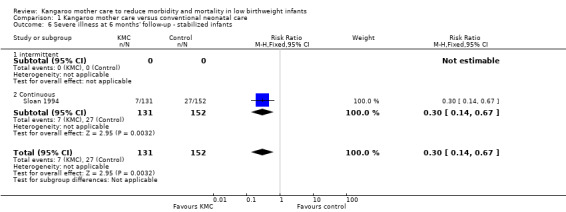
Comparison 1 Kangaroo mother care versus conventional neonatal care, Outcome 6 Severe illness at 6 months' follow‐up ‐ stabilized infants.
1.7. Analysis.
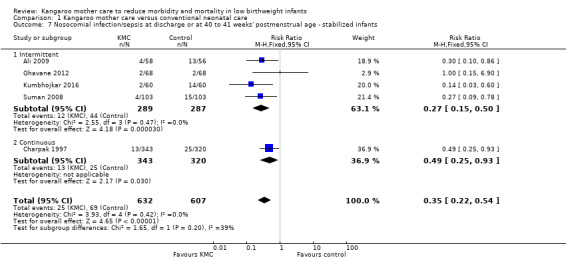
Comparison 1 Kangaroo mother care versus conventional neonatal care, Outcome 7 Nosocomial infection/sepsis at discharge or at 40 to 41 weeks' postmenstrual age ‐ stabilized infants.
1.9. Analysis.
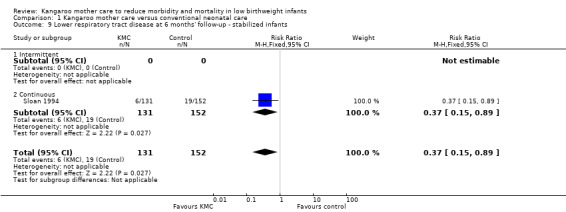
Comparison 1 Kangaroo mother care versus conventional neonatal care, Outcome 9 Lower respiratory tract disease at 6 months' follow‐up ‐ stabilized infants.
1.11. Analysis.
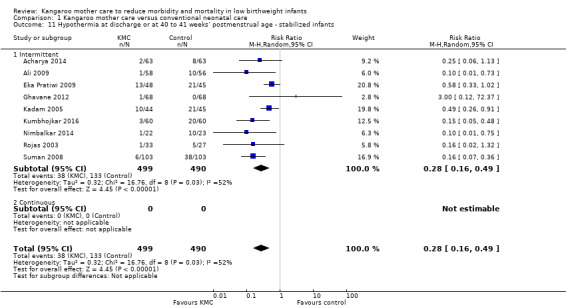
Comparison 1 Kangaroo mother care versus conventional neonatal care, Outcome 11 Hypothermia at discharge or at 40 to 41 weeks’ postmenstrual age ‐ stabilized infants.
Only the subgroup of trials that used intermittent KMC demonstrated significantly reduced risk of severe infection/sepsis at latest follow‐up and hypothermia at discharge or at 40 to 41 weeks' postmenstrual age. Subgroups of trials that used intermittent or continuous KMC showed a statistically significantly reduced risk of nosocomial infection/sepsis at discharge or at 40 to 41 weeks’ postmenstrual age.
We found no overall difference between KMC infants and controls in risk of mild/moderate infection or illness at latest follow‐up (RR 1.28, 95% CI 0.87 to 1.88) (Analysis 1.8), diarrhea at six months' follow‐up (RR 0.65, 95% CI 0.35 to 1.20) (Analysis 1.10), hyperthermia at discharge or at 40 to 41 weeks' postmenstrual age (RR 0.79, 95% CI 0.59 to 1.05) (Analysis 1.12), and re‐admission to hospital at latest follow‐up (RR 0.60, 95% CI 0.34 to 1.06) (Analysis 1.14).
1.8. Analysis.
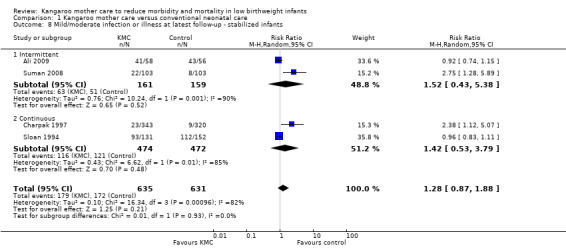
Comparison 1 Kangaroo mother care versus conventional neonatal care, Outcome 8 Mild/moderate infection or illness at latest follow‐up ‐ stabilized infants.
1.10. Analysis.
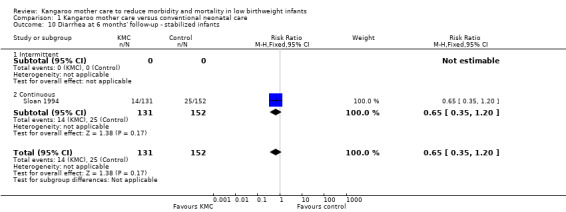
Comparison 1 Kangaroo mother care versus conventional neonatal care, Outcome 10 Diarrhea at 6 months' follow‐up ‐ stabilized infants.
1.12. Analysis.
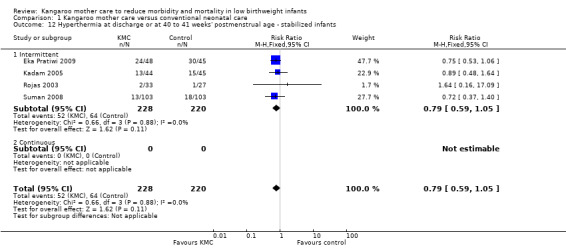
Comparison 1 Kangaroo mother care versus conventional neonatal care, Outcome 12 Hyperthermia at discharge or at 40 to 41 weeks' postmenstrual age ‐ stabilized infants.
1.14. Analysis.
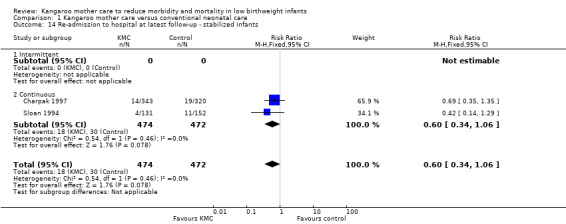
Comparison 1 Kangaroo mother care versus conventional neonatal care, Outcome 14 Re‐admission to hospital at latest follow‐up ‐ stabilized infants.
Intermittent KMC decreased length of hospital stay by 1.6 days, although this difference was not statistically significant (95% CI ‐0.2 to 3.4; P value = 0.08; 11 studies, 1057 infants) (Analysis 1.13). Mean hospital stay from randomization to 41 weeks' postmenstrual age was 4.5 days for KMC infants and 5.6 days for control infants in Charpak 1997. Investigators provided no standard deviations. Cattaneo 1998 reported only median hospital stay, which was 11 days in the KMC group versus 13 days in the control group. Length of hospital stay was two days greater in KMC infants than in control infants in Sloan 1994.
1.13. Analysis.
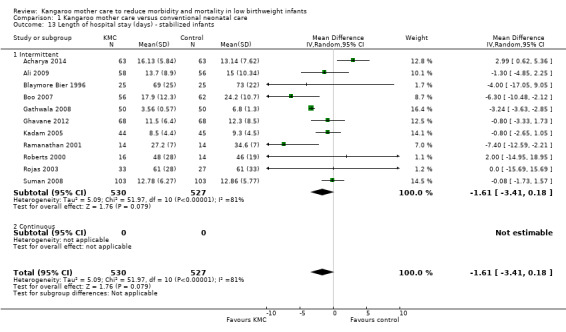
Comparison 1 Kangaroo mother care versus conventional neonatal care, Outcome 13 Length of hospital stay (days) ‐ stabilized infants.
Sensitivity analyses using only studies with adequate allocation concealment demonstrated a similar result for severe infection/sepsis at latest follow‐up (RR 0.40, 95% CI 0.24 to 0.66) and for hypothermia at discharge or at 40 to 41 weeks' postmenstrual age (RR 0.24, 95% CI 0.16 to 0.36). Additional sensitivity analyses did not indicate that removing the study with high risk of attrition bias (Suman 2008) had any important impact on overall effects of KMC on severe infection/sepsis at latest follow‐up (RR 0.54, 95% CI 0.38 to 0.76) and on hypothermia at discharge or at 40 to 41 weeks' postmenstrual age (RR 0.33, 95% CI 0.23 to 0.48).
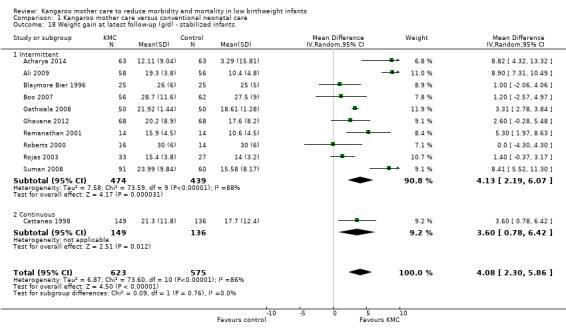
Infant growth (outcomes 1.15 to 1.26)
Infants given kangaroo mother care gained more weight per day (MD 4.1 g, 95% CI 2.3 to 5.9; 11 trials, 1198 infants; moderate‐quality evidence) (Analysis 1.18) (Figure 5) and had greater increases in length (MD 0.21 cm, 95% CI 0.03 to 0.38; three trials, 377 infants) (Analysis 1.22) and head circumference (MD 0.14 cm, 95% CI 0.06 to 0.22; four trials, 495 infants) (Analysis 1.26) per week than controls. Nevertheless, considerable heterogeneity was evident (I2 > 70%) among trials reporting gain in weight, length, and head circumference. One trial (Charpak 1997) reported that KMC infants had a larger head circumference at 6 months' corrected age than controls (MD 0.34 cm, 95% CI 0.11 to 0.57; 592 infants) (Analysis 1.24). Investigators observed no differences in weight, length, or head circumference at discharge or at 40 to 41 weeks' postmenstrual age (Analysis 1.15; Analysis 1.19; Analysis 1.23) or at 12 months' corrected age (Analysis 1.17; Analysis 1.21; Analysis 1.25), or in weight or length at 6 months’ corrected age (Analysis 1.16; Analysis 1.20). Sloan 1994 reported, "there were no significant differences between the groups in growth indices during the six‐month follow up."
1.18. Analysis.
Comparison 1 Kangaroo mother care versus conventional neonatal care, Outcome 18 Weight gain at latest follow‐up (g/d) ‐ stabilized infants.
5.
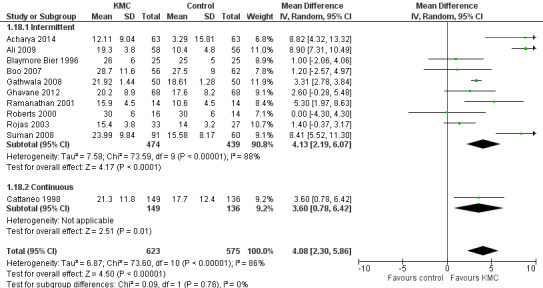
Forest plot of comparison: 1 Kangaroo mother care versus conventional neonatal care, outcome: 1.10 Weight gain at latest follow‐up (g/d) ‐ stabilized infants.
1.22. Analysis.
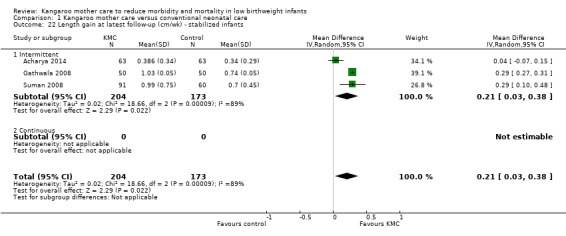
Comparison 1 Kangaroo mother care versus conventional neonatal care, Outcome 22 Length gain at latest follow‐up (cm/wk) ‐ stabilized infants.
1.26. Analysis.
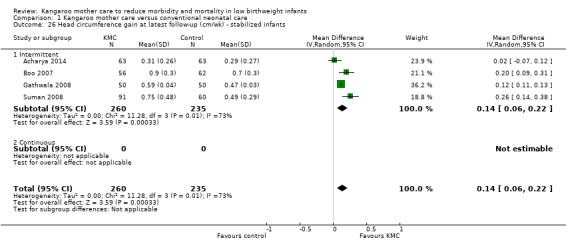
Comparison 1 Kangaroo mother care versus conventional neonatal care, Outcome 26 Head circumference gain at latest follow‐up (cm/wk) ‐ stabilized infants.
1.24. Analysis.
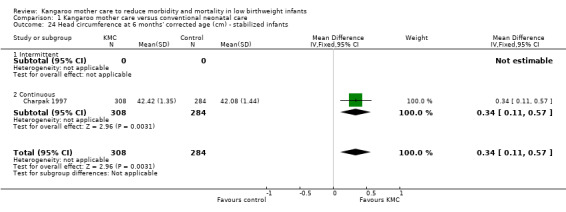
Comparison 1 Kangaroo mother care versus conventional neonatal care, Outcome 24 Head circumference at 6 months' corrected age (cm) ‐ stabilized infants.
1.15. Analysis.
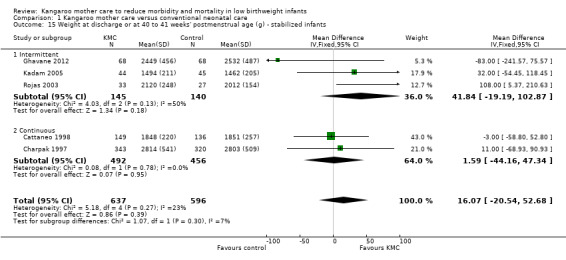
Comparison 1 Kangaroo mother care versus conventional neonatal care, Outcome 15 Weight at discharge or at 40 to 41 weeks' postmenstrual age (g) ‐ stabilized infants.
1.19. Analysis.
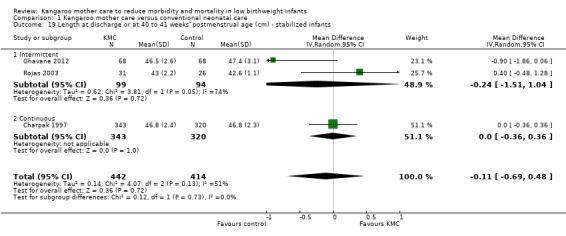
Comparison 1 Kangaroo mother care versus conventional neonatal care, Outcome 19 Length at discharge or at 40 to 41 weeks' postmenstrual age (cm) ‐ stabilized infants.
1.23. Analysis.
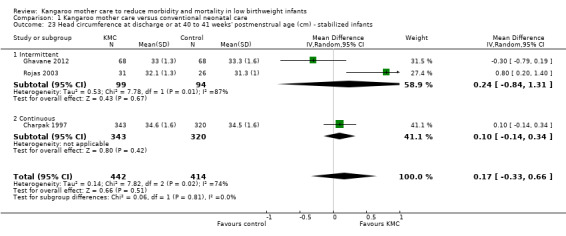
Comparison 1 Kangaroo mother care versus conventional neonatal care, Outcome 23 Head circumference at discharge or at 40 to 41 weeks' postmenstrual age (cm) ‐ stabilized infants.
1.17. Analysis.
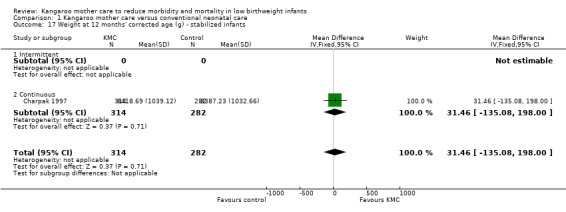
Comparison 1 Kangaroo mother care versus conventional neonatal care, Outcome 17 Weight at 12 months' corrected age (g) ‐ stabilized infants.
1.21. Analysis.
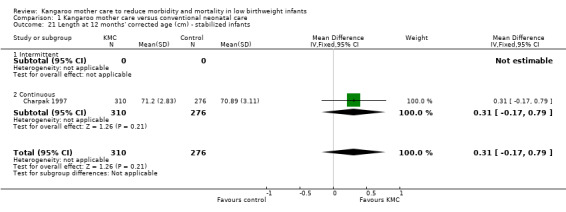
Comparison 1 Kangaroo mother care versus conventional neonatal care, Outcome 21 Length at 12 months' corrected age (cm) ‐ stabilized infants.
1.25. Analysis.
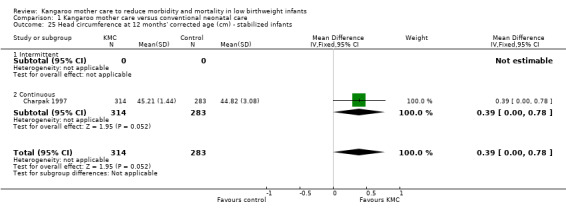
Comparison 1 Kangaroo mother care versus conventional neonatal care, Outcome 25 Head circumference at 12 months' corrected age (cm) ‐ stabilized infants.
1.16. Analysis.
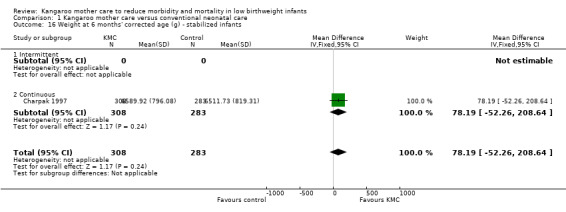
Comparison 1 Kangaroo mother care versus conventional neonatal care, Outcome 16 Weight at 6 months' corrected age (g) ‐ stabilized infants.
1.20. Analysis.
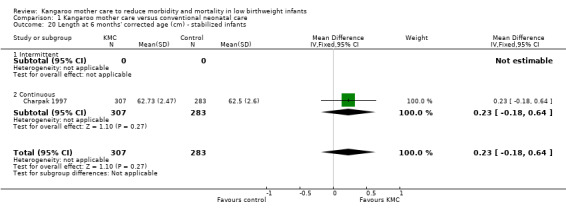
Comparison 1 Kangaroo mother care versus conventional neonatal care, Outcome 20 Length at 6 months' corrected age (cm) ‐ stabilized infants.
We undertook sensitivity analysis by excluding studies with unclear allocation concealment and high risk of attrition bias to examine the impact on increases in both weight and head circumference. We found no differences in the overall direction of findings.
Neurodevelopmental and neurosensory impairment (outcomes 1.27 to 1.30)
Only one study (Charpak 1997) reported results for neurodevelopmental and neurosensory impairment at one year of corrected age. Researchers found no statistically significant differences between KMC infants and controls in Griffith quotients for psychomotor development (low‐quality evidence) (Analysis 1.27), cerebral palsy (Analysis 1.28), deafness (Analysis 1.29), and visual impairment (Analysis 1.30). A secondary publication of the Charpak 1997 trial reported that the subgroup of KMC infants with birthweight ≤ 1800 g had a higher general developmental quotient than controls at one year of corrected age (P value < 0.01).
1.27. Analysis.
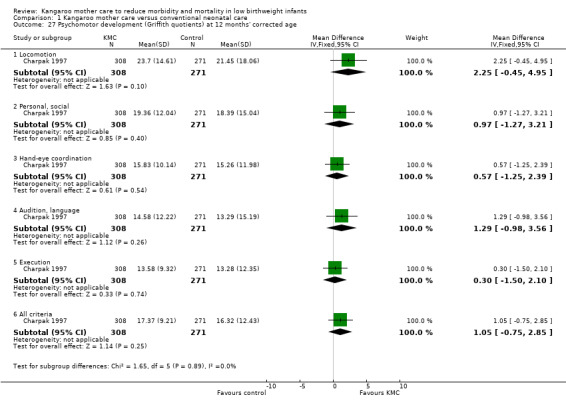
Comparison 1 Kangaroo mother care versus conventional neonatal care, Outcome 27 Psychomotor development (Griffith quotients) at 12 months' corrected age.
1.28. Analysis.
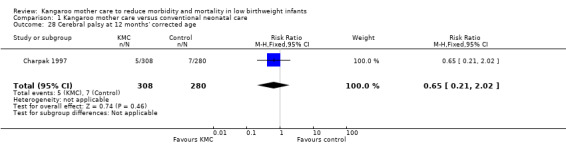
Comparison 1 Kangaroo mother care versus conventional neonatal care, Outcome 28 Cerebral palsy at 12 months' corrected age.
1.29. Analysis.
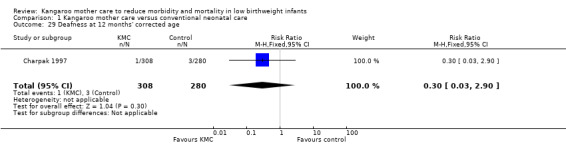
Comparison 1 Kangaroo mother care versus conventional neonatal care, Outcome 29 Deafness at 12 months' corrected age.
1.30. Analysis.
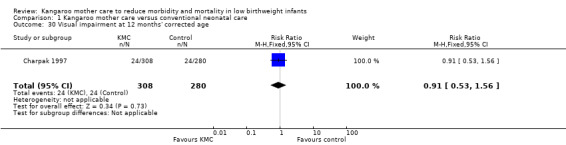
Comparison 1 Kangaroo mother care versus conventional neonatal care, Outcome 30 Visual impairment at 12 months' corrected age.
Breastfeeding (outcomes 1.31 to 1.40)
Mothers of KMC infants were more likely to be breastfeeding at discharge or at 40 to 41 weeks' postmenstrual age and at one to three months' follow‐up than mothers in the control group. Compared with conventional care, KMC was associated with an increase in the likelihood of exclusive breastfeeding at discharge or at 40 to 41 weeks' postmenstrual age (66.3% vs 56.3%; RR 1.16, 95% CI 1.07 to 1.25; I2 = 39%; NNTB = 11, 95% CI 7 to 25; six studies, 1453 mothers) (Analysis 1.31), and at one to three months' follow‐up (86.9% vs 76.5%; RR 1.20, 95% CI 1.01 to 1.43; I2 = 76%; NNTB = 7, 95% CI 3 to 131; five studies, 600 mothers) (Analysis 1.32), or any (exclusive or partial) breastfeeding at discharge or at 40 to 41 weeks' postmenstrual age (88.9% vs 76.2%; RR 1.20, 95% CI 1.07 to 1.34; I2 = 80%; NNTB = 7, 95% CI 4 to 19; 10 studies, 1696 mothers; moderate‐quality evidence) (Analysis 1.34) (Figure 6), at one to two months' follow‐up (77.9% vs 67.9%; RR 1.33, 95% CI 1.00 to 1.78; I2 = 78%; six studies, 538 mothers) (Analysis 1.35), at 3 months' follow‐up (79.7% vs 69.8%; RR 1.14, 95% CI 1.06 to 1.23; I2 = 41%; five studies, 924 mothers) (Analysis 1.36), and at one to three months' follow‐up (80.4% vs 71.1%; RR 1.17, 95% CI 1.05 to 1.31; I2 = 62%; NNTB = 8, 95% CI 5 to 28; nine studies, 1394 mothers; low‐quality evidence) (Analysis 1.37). It should be noted that heterogeneity was substantial (I2 > 50%) among trials reporting breastfeeding. Overall, investigators found no statistically significant differences between KMC and control for exclusive or any breastfeeding at six to 12 months' follow‐up (Analysis 1.33; Analysis 1.38; Analysis 1.39) and at onset of breastfeeding (Analysis 1.40). However, subgroup analyses showed that intermittent KMC was associated with a significant increase in exclusive breastfeeding at six to 12 months' follow‐up (84.6% vs 55.6%; RR 1.52, 95% CI 1.10 to 2.10; one study, 75 women) and in any breastfeeding at six months' follow‐up (54.7% vs 36.8%; RR 1.50, 95% CI 1.08‐2.08; three studies, 143 women).
1.31. Analysis.
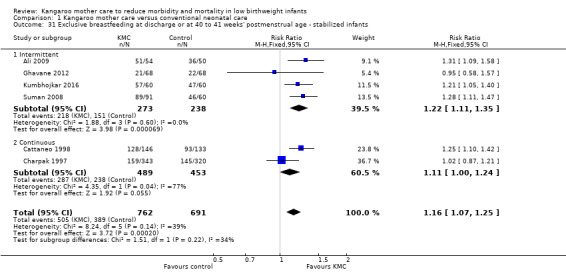
Comparison 1 Kangaroo mother care versus conventional neonatal care, Outcome 31 Exclusive breastfeeding at discharge or at 40 to 41 weeks' postmenstrual age ‐ stabilized infants.
1.32. Analysis.
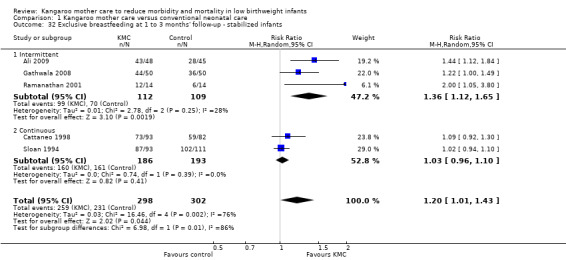
Comparison 1 Kangaroo mother care versus conventional neonatal care, Outcome 32 Exclusive breastfeeding at 1 to 3 months' follow‐up ‐ stabilized infants.
1.34. Analysis.
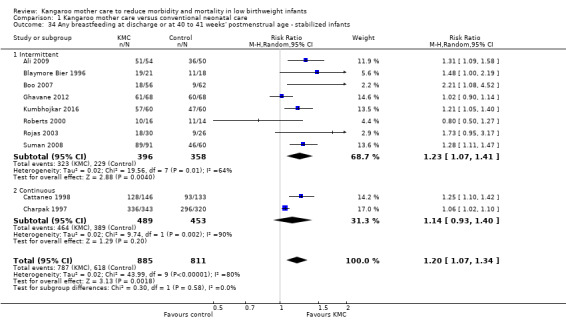
Comparison 1 Kangaroo mother care versus conventional neonatal care, Outcome 34 Any breastfeeding at discharge or at 40 to 41 weeks' postmenstrual age ‐ stabilized infants.
6.
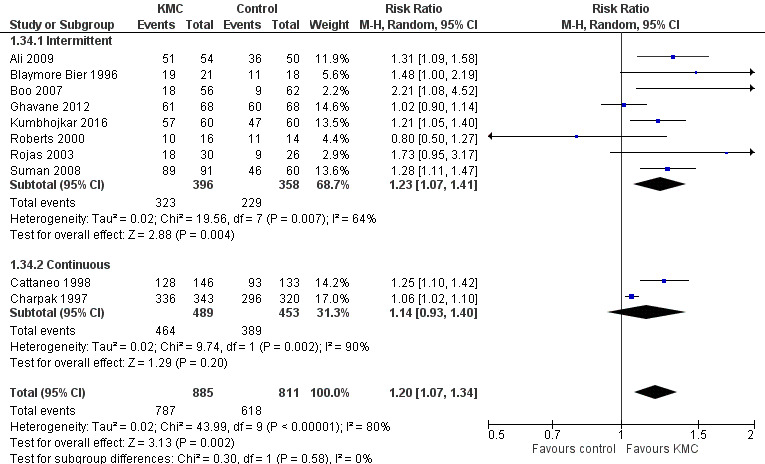
Forest plot of comparison: 1 Kangaroo mother care versus conventional neonatal care, outcome: 1.34 Any breastfeeding at discharge or at 40 to 41 weeks' postmenstrual age ‐ stabilized infants.
1.35. Analysis.
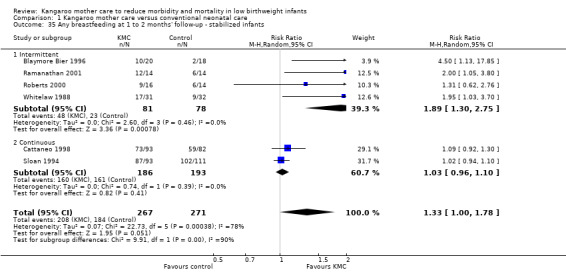
Comparison 1 Kangaroo mother care versus conventional neonatal care, Outcome 35 Any breastfeeding at 1 to 2 months' follow‐up ‐ stabilized infants.
1.36. Analysis.
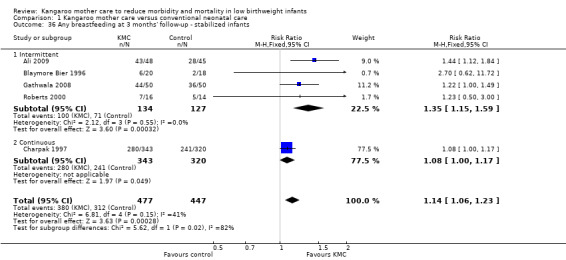
Comparison 1 Kangaroo mother care versus conventional neonatal care, Outcome 36 Any breastfeeding at 3 months' follow‐up ‐ stabilized infants.
1.37. Analysis.
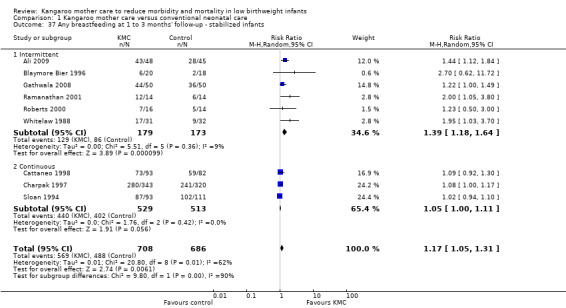
Comparison 1 Kangaroo mother care versus conventional neonatal care, Outcome 37 Any breastfeeding at 1 to 3 months' follow‐up ‐ stabilized infants.
1.33. Analysis.
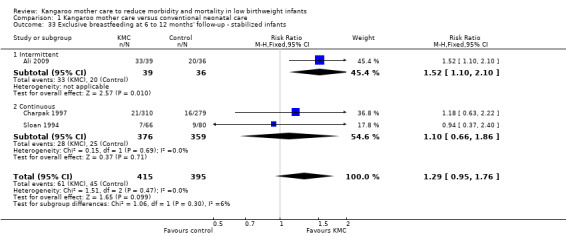
Comparison 1 Kangaroo mother care versus conventional neonatal care, Outcome 33 Exclusive breastfeeding at 6 to 12 months' follow‐up ‐ stabilized infants.
1.38. Analysis.
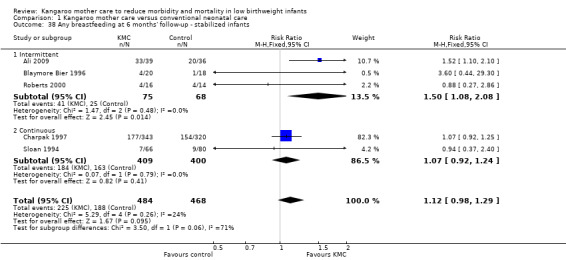
Comparison 1 Kangaroo mother care versus conventional neonatal care, Outcome 38 Any breastfeeding at 6 months' follow‐up ‐ stabilized infants.
1.39. Analysis.
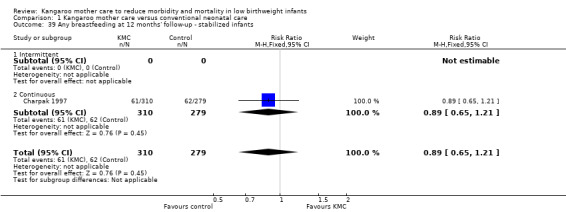
Comparison 1 Kangaroo mother care versus conventional neonatal care, Outcome 39 Any breastfeeding at 12 months' follow‐up ‐ stabilized infants.
1.40. Analysis.
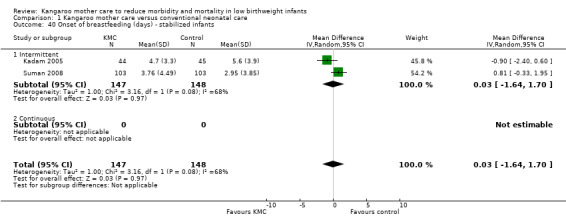
Comparison 1 Kangaroo mother care versus conventional neonatal care, Outcome 40 Onset of breastfeeding (days) ‐ stabilized infants.
Statistically significant positive effects of KMC on breastfeeding at discharge or at 40 to 41 weeks' postmenstrual age and at one to three and six months' follow‐up were demonstrated in the subgroup of trials that used intermittent KMC but not in the subgroup of trials that used continuous KMC. In addition, an increase in the likelihood of any breastfeeding at one to two months' follow‐up was demonstrated in the subgroup of three trials (131 infants) conducted in high‐income countries (RR 2.02, 95% CI 1.28 to 3.21; I2 = 23%).
Parental and familial satisfaction (outcome 1.41)
Only one study (Cattaneo 1998) evaluated parental and familial satisfaction with method of infant care. Mothers in the KMC group were more satisfied with the method of care than were mothers in the control group (91% vs 78%; RR 1.17, 95% CI 1.05 to1.30; 269 mothers) (Analysis 1.41). Investigators found no significant differences in satisfaction with method of care between fathers and families of KMC and control groups.
1.41. Analysis.
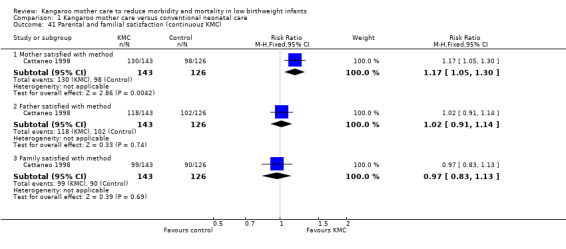
Comparison 1 Kangaroo mother care versus conventional neonatal care, Outcome 41 Parental and familial satisfaction (continuous KMC).
Mother‐infant attachment or interaction (outcomes 1.42 to 1.49)
Three studies (Charpak 1997; Gathwala 2008; Roberts 2000) reported results on mother‐infant attachment, and one (Neu 2010) on mother‐infant interaction.
A secondary publication of the Charpak 1997 trial reported two series of outcomes that were assessed as manifestations of mother‐infant attachment. The first was the mother's feelings and perceptions of her premature birth experience, measured through a "mother's perception of premature birth questionnaire" using a Likert scale (1 to 5), 24 hours after birth and when the infant reached 41 weeks' postmenstrual age. The second outcome was derived from observations of the mother's and child's responsiveness to each other during breastfeeding, using a "nursing child assessment feeding scale." Researchers compared a total of nine items between KMC and control groups according to the interval between birth and start of the intervention (one to two days, three to 14 days, and longer than 14 days), as well as admission of the infant to the neonatal intensive care unit (NICU) (yes or not), for a total of 45 comparisons. Overall, scores on six comparisons (mother's sense of competence [interval between birth and start of intervention of one to two days], mother's sense of competence [infant admitted to NICU], mother's sense of competence [infant not admitted to NICU], mother’s feelings of worry and stress [interval between birth and start of intervention of one to two days], mother’s sensitivity [interval between birth and start of intervention > 14 days], and infant responsiveness [interval between birth and start of intervention > 14 days]) were significantly higher in the KMC group than in the control group. Scores on two comparisons (mother’s perceptions of social support [interval between birth and start of intervention > 14 days, and infant not admitted to NICU]) were significantly lower in the KMC group than in the control group. Results showed no significant differences in scores for the remaining 37 comparisons (Analysis 1.42; Analysis 1.43; Analysis 1.44).
1.42. Analysis.
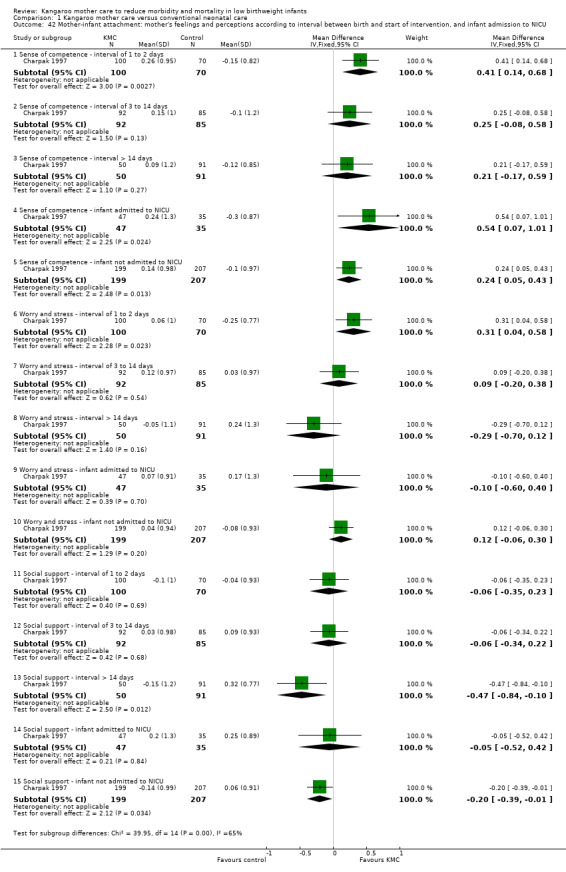
Comparison 1 Kangaroo mother care versus conventional neonatal care, Outcome 42 Mother‐infant attachment: mother's feelings and perceptions according to interval between birth and start of intervention, and infant admission to NICU.
1.43. Analysis.
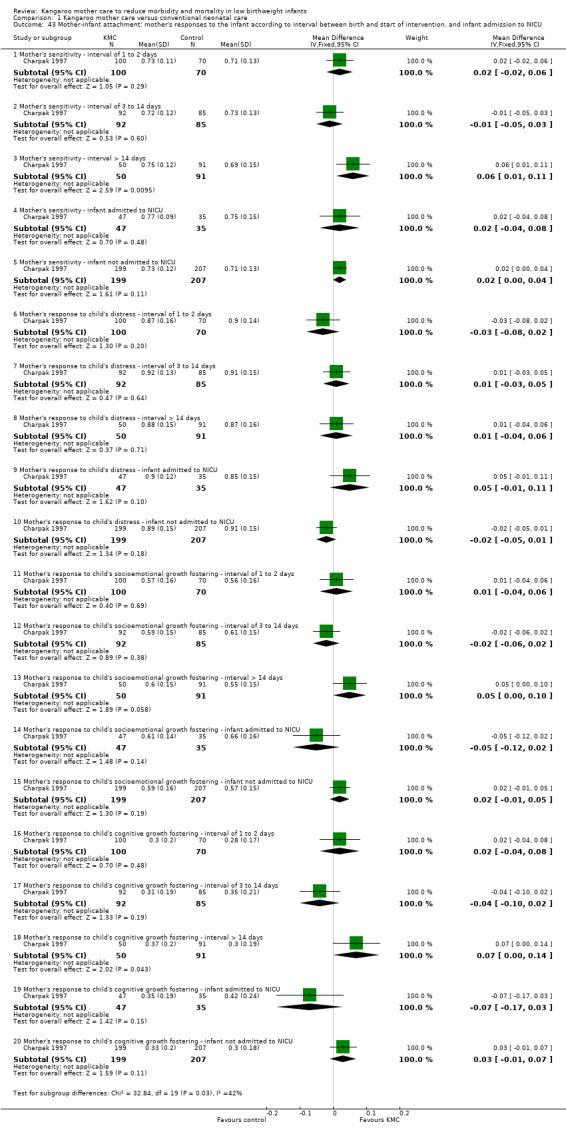
Comparison 1 Kangaroo mother care versus conventional neonatal care, Outcome 43 Mother‐infant attachment: mother's responses to the infant according to interval between birth and start of intervention, and infant admission to NICU.
1.44. Analysis.
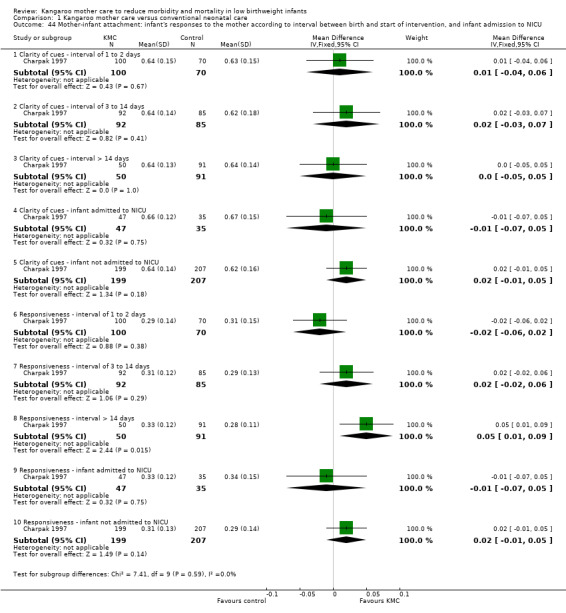
Comparison 1 Kangaroo mother care versus conventional neonatal care, Outcome 44 Mother‐infant attachment: infant's responses to the mother according to interval between birth and start of intervention, and infant admission to NICU.
Gathwala 2008 evaluated mother‐infant attachment at three months' follow‐up through a structured maternal interview that used attachment questions scored in such a manner that a higher score indicated greater attachment. The total attachment score for the KMC group (24.46 ± 1.64) was significantly higher than that obtained for the control group (18.22 ± 1.79) (Analysis 1.45).
1.45. Analysis.
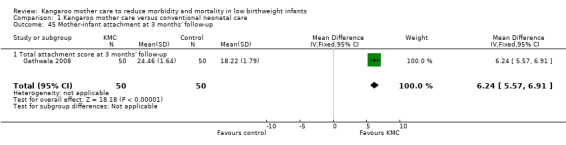
Comparison 1 Kangaroo mother care versus conventional neonatal care, Outcome 45 Mother‐infant attachment at 3 months' follow‐up.
Roberts 2000 measured maternal stress levels in the NICU and mothers' perceptions of their maternal competence. Only the score on the scale for "relationship with the infant" was significantly higher in the KMC group (4.4 ± 0.46) than in the control group (3.4 ± 1.16). Researchers found no significant differences between KMC and control group scores on nursery environment, infant appearance, staff behavior and communication, and parental confidence in their parenting abilities (Analysis 1.46; Analysis 1.47).
1.46. Analysis.
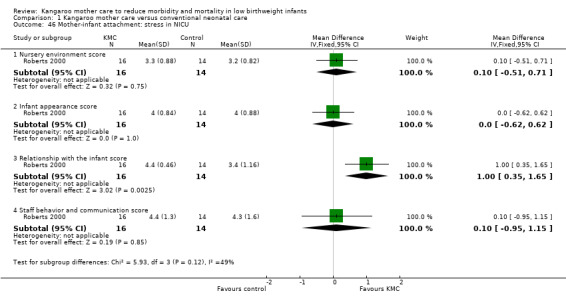
Comparison 1 Kangaroo mother care versus conventional neonatal care, Outcome 46 Mother‐infant attachment: stress in NICU.
1.47. Analysis.
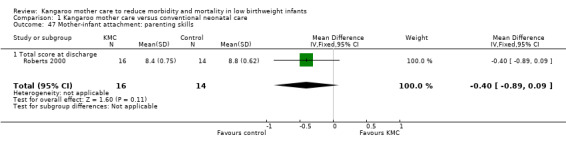
Comparison 1 Kangaroo mother care versus conventional neonatal care, Outcome 47 Mother‐infant attachment: parenting skills.
Neu 2010 evaluated the mother‐infant interaction at six months of age by using the Stiil‐Face Paradigm tool. Mother‐infant dyads in the KMC group showed more symmetrical, and less asymmetrical, co‐regulation than mother‐infant dyads in the control group (Analysis 1.48). Multivariate analysis showed no differences between groups in infant vitality during the neutral face portion of the Stiil‐Face procedure. A secondary publication of the Neu 2010 study reported that KMC infants had similar scores for behavioral regulation and development to those of infants who experienced nurse‐supported blanket holding at 40 to 44 weeks' postmenstrual age (Analysis 1.49).
1.48. Analysis.
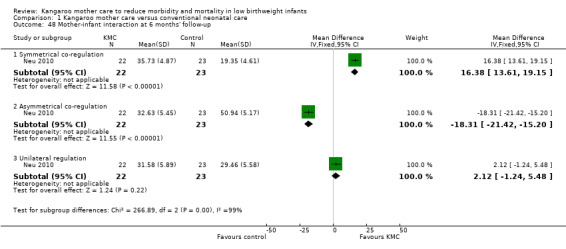
Comparison 1 Kangaroo mother care versus conventional neonatal care, Outcome 48 Mother‐infant interaction at 6 months' follow‐up.
1.49. Analysis.
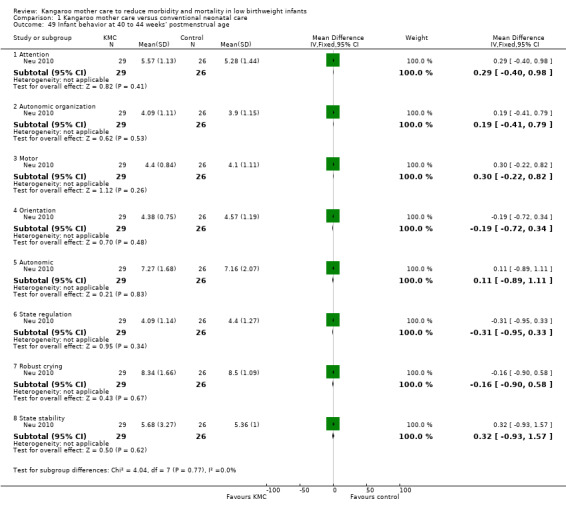
Comparison 1 Kangaroo mother care versus conventional neonatal care, Outcome 49 Infant behavior at 40 to 44 weeks’ postmenstrual age.
Home environment and father involvement (outcome 1.50)
One trial (Charpak 1997) evaluated home environment and father involvement at 12 months' corrected age through a structured interview administered to parents during a home visit. The total Home Observation for Measurement of the Environment (HOME) score was significantly higher among kangaroo families (0.28 ± 0.24) than in conventional care families (‐0.51 ± 0.26) (Analysis 1.50). Scores on father involvement were not reported, but study authors claimed that KMC increased father involvement (the father's sense of responsibility and competence).
1.50. Analysis.
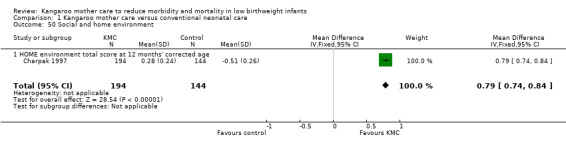
Comparison 1 Kangaroo mother care versus conventional neonatal care, Outcome 50 Social and home environment.
Costs of care
No study reported data on mean (SD) total medical and non‐medical costs for KMC and control groups. The overall cost was "about 50% less for KMC" in the Cattaneo 1998 study. Specifically, the cost was US $19,289 for KMC and US $39,764 for conventional care. In the Sloan 1994 study, "costs of neonatal care were greater in the control than in the KMC group." Overall, the cost of hospital stay and postneonatal care at five months was US $741 greater for the control than the KMC group. However, data were available for only 49 infants (24 KMC, 25 control) at six months' follow‐up.
All funnel plots showed no asymmetry, either visually or in terms of statistical significance (P value > 0.10 for all, by Egger's test).
Comparison 2. Early‐onset kangaroo mother care versus late‐onset kangaroo mother care in relatively stable infants
Only one trial (Nagai 2010), which was considered at low risk of bias, compared early‐onset KMC versus late‐onset KMC in relatively stable LBW infants. Early continuous KMC was begun as soon as possible, within 24 hours post birth, and late continuous KMC was begun after complete stabilization, generally after 24 hours post birth. This study included a total of 73 LBW infants (early 37, late 36). Investigators reported no statistically significant differences between early‐onset KMC and late‐onset KMC for mortality at four weeks of age (RR 1.95, 95% CI 0.18 to 20.53) (Analysis 2.1) and at six months of age (RR 1.00, 95% CI 0.15 to 6.72) (Analysis 2.10), morbidity (RR 0.49, 95% CI 0.18 to 1.28) (Analysis 2.2) and severe infection (RR 0.42, 95% CI 0.12 to 1.49) (Analysis 2.3) at four weeks of age, re‐admission to hospital at four weeks of age (RR 1.95, 95% CI 0.18 to 20.53) (Analysis 2.4) and at six to 12 months of age (RR 1.00, 95% CI 0.32 to 3.16) (Analysis 2.11), hypothermia (RR 0.58, 95% CI 0.15 to 2.27) (Analysis 2.5), hyperthermia (RR 1.05, 95% CI 0.56 to 1.99) (Analysis 2.6), weight gain at four weeks of age (MD 58.9 g, 95% CI ‐116.9 to 234.6) (Analysis 2.7), exclusive breastfeeding at four weeks of age (RR 0.94, 95% CI 0.85 to 1.04) (Analysis 2.8), and stunting (RR 0.83, 95% CI 0.46 to 1.48) (Analysis 2.12), severe stunting (RR 0.67, 95% CI 0.17 to 2.73) (Analysis 2.13), wasting (RR 0.10, 95% CI 0.01 to 1.77) (Analysis 2.14), severe wasting (RR 0.00, 95% CI 0.00 to 0.00) (Analysis 2.15), underweight (RR 0.49, 95% CI 0.21 to 1.14) (Analysis 2.16), and severe underweight (RR 0.22, 95% CI 0.03 to 1.88) (Analysis 2.17) at six to 12 months of age. However, compared with late‐onset KMC, early‐onset KMC was associated with a statistically significant reduction in body weight loss from birth to 48 hours post birth (MD 43.3 g, 95% CI 5.5 to 81.1) (Analysis 2.7) and in length of hospital stay (MD 0.9 days, 95% CI 0.6 to 1.2) (Analysis 2.9). In addition, early‐onset KMC was associated with a non‐significant increase in the likelihood of exclusive breastfeeding at six months of age (41.4% vs 15.4%; RR 2.69, 95% CI 0.99 to 7.31) (Analysis 2.8).
2.1. Analysis.
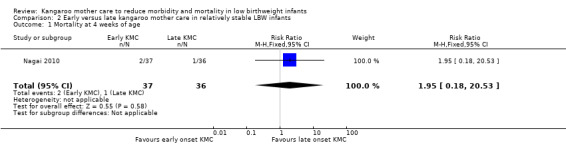
Comparison 2 Early versus late kangaroo mother care in relatively stable LBW infants, Outcome 1 Mortality at 4 weeks of age.
2.10. Analysis.
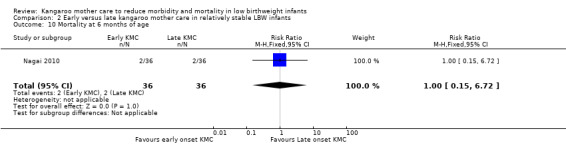
Comparison 2 Early versus late kangaroo mother care in relatively stable LBW infants, Outcome 10 Mortality at 6 months of age.
2.2. Analysis.
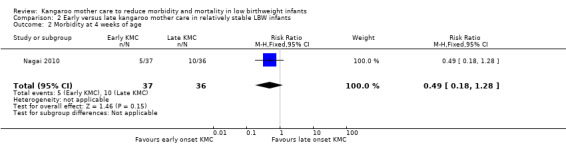
Comparison 2 Early versus late kangaroo mother care in relatively stable LBW infants, Outcome 2 Morbidity at 4 weeks of age.
2.3. Analysis.
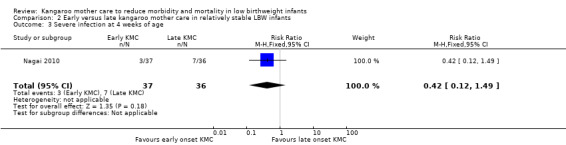
Comparison 2 Early versus late kangaroo mother care in relatively stable LBW infants, Outcome 3 Severe infection at 4 weeks of age.
2.4. Analysis.
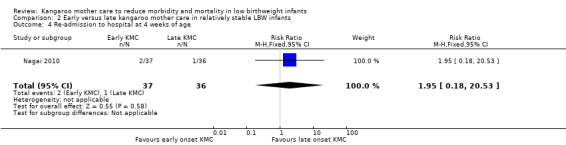
Comparison 2 Early versus late kangaroo mother care in relatively stable LBW infants, Outcome 4 Re‐admission to hospital at 4 weeks of age.
2.11. Analysis.
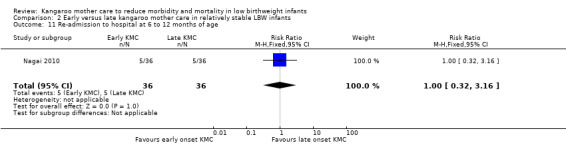
Comparison 2 Early versus late kangaroo mother care in relatively stable LBW infants, Outcome 11 Re‐admission to hospital at 6 to 12 months of age.
2.5. Analysis.
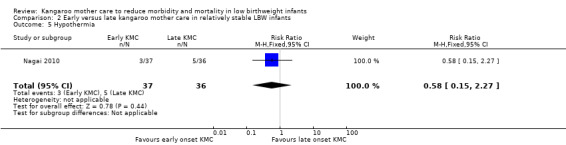
Comparison 2 Early versus late kangaroo mother care in relatively stable LBW infants, Outcome 5 Hypothermia.
2.6. Analysis.
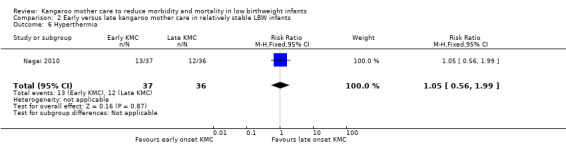
Comparison 2 Early versus late kangaroo mother care in relatively stable LBW infants, Outcome 6 Hyperthermia.
2.7. Analysis.
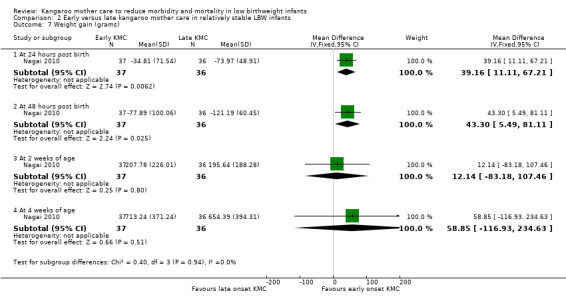
Comparison 2 Early versus late kangaroo mother care in relatively stable LBW infants, Outcome 7 Weight gain (grams).
2.8. Analysis.
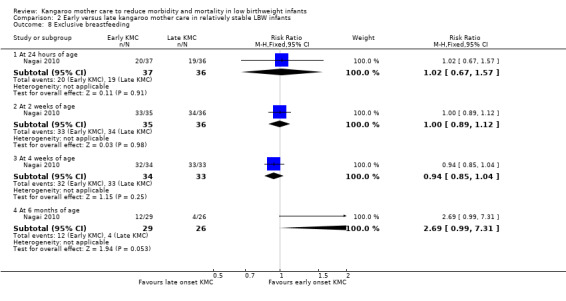
Comparison 2 Early versus late kangaroo mother care in relatively stable LBW infants, Outcome 8 Exclusive breastfeeding.
2.12. Analysis.
Comparison 2 Early versus late kangaroo mother care in relatively stable LBW infants, Outcome 12 Stunting at 6 to 12 months of age.
2.13. Analysis.
Comparison 2 Early versus late kangaroo mother care in relatively stable LBW infants, Outcome 13 Severe stunting at 6 to 12 months of age.
2.14. Analysis.
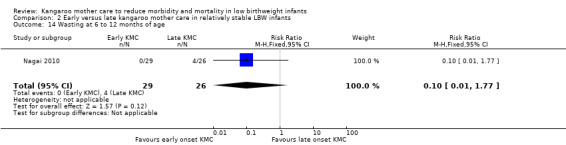
Comparison 2 Early versus late kangaroo mother care in relatively stable LBW infants, Outcome 14 Wasting at 6 to 12 months of age.
2.15. Analysis.
Comparison 2 Early versus late kangaroo mother care in relatively stable LBW infants, Outcome 15 Severe wasting at 6 to 12 months of age.
2.16. Analysis.
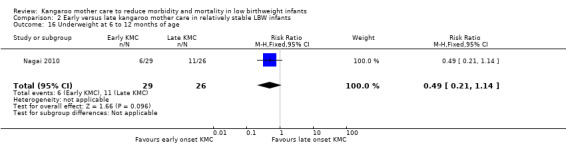
Comparison 2 Early versus late kangaroo mother care in relatively stable LBW infants, Outcome 16 Underweight at 6 to 12 months of age.
2.17. Analysis.
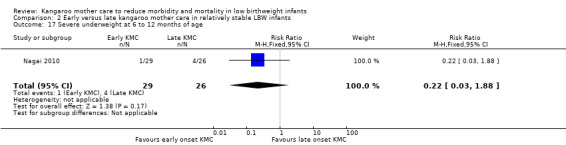
Comparison 2 Early versus late kangaroo mother care in relatively stable LBW infants, Outcome 17 Severe underweight at 6 to 12 months of age.
2.9. Analysis.
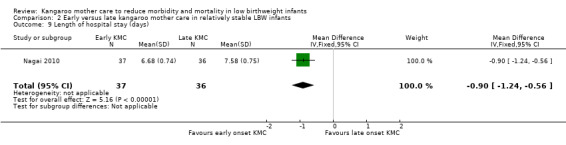
Comparison 2 Early versus late kangaroo mother care in relatively stable LBW infants, Outcome 9 Length of hospital stay (days).
Discussion
Summary of main results
This updated systematic review of 20 randomized controlled trials (RCTs) comparing kangaroo mother care (KMC) and conventional neonatal care found compelling evidence that KMC is associated with a reduction in mortality at discharge or at 40 to 41 weeks' postmenstrual age and at latest follow‐up, severe infection/sepsis, and hypothermia, and an increase in weight gain and in exclusive or any breastfeeding at discharge or at 40 to 41 weeks' postmenstrual age and at one to three months' follow‐up. Moreover, growing evidence indicates that KMC reduces the risk of nosocomial infection/sepsis at discharge or at 40 to 41 weeks’ postmenstrual age, and increases the gain in length and head circumference, maternal satisfaction with the method, maternal‐infant attachment, and home environment. One trial (Charpak 1997) reported no significant differences between KMC infants and controls in a variety of neurodevelopmental and neurosensory outcomes at one year of corrected age.
Overall, continuous KMC led to a reduction in mortality at discharge or at 40 to 41 weeks' postmenstrual age and at latest follow‐up, and in nosocomial infection/sepsis, severe illness, and lower respiratory tract disease, and an increase in weight gain, maternal satisfaction with the method, and some measures of mother‐infant attachment and home environment. On the other hand, intermittent KMC was associated with a decrease in the risk of severe infection/sepsis, nosocomial infection/sepsis, and hypothermia, and an increase in weight, length, and head circumference gain, exclusive or any breastfeeding at discharge or 40 to 41 weeks' postmenstrual age and at one to three months' follow‐up, and mother‐infant attachment at three months' follow‐up.
Subgroup analyses showed that decreased risk of death at discharge or at 40 to 41 weeks' postmenstrual age and at latest follow‐up was demonstrated in the subgroup of trials that used continuous KMC (≥ 20 hours/d), the subgroup of trials in which KMC was initiated within 10 days post birth, the subgroup of trials conducted in low/middle‐income countries, and the trial in which KMC was used in unstabilized infants. Sensitivity analysis suggested that inclusion of studies with high risk of bias did not affect the general direction of findings nor the size of the treatment effect, although the beneficial effect of KMC on mortality turned non‐significant or marginally significant.
One small high‐quality trial (Nagai 2010) suggested that early‐onset KMC, compared with late‐onset KMC, is associated with a significant reduction in body weight loss from birth to 48 hours post birth and in length of hospital stay, and a marginally significant increase in the likelihood of exclusive breastfeeding at six months of age, with no significant difference in mortality, morbidity, severe infection, re‐admission to hospital, hypothermia, hyperthermia, exclusive breastfeeding at four weeks of age, or infant nutritional indicators at six to 12 months of age.
Overall completeness and applicability of evidence
Participants in the included trials reflect the population for which this intervention is currently considered, that is, low birthweight (LBW)/preterm infants. Sixteen trials, including all five trials that evaluated continuous KMC, were conducted in hospitals in low/middle‐income countries. Mortality at discharge was the only outcome reported in the sole trial (Worku 2005) that compared KMC with conventional neonatal care in LBW infants before stabilization. The remaining 48 outcomes were reported in 19 trials that evaluated KMC in stabilized LBW infants. We were unable to draw conclusions about the effectiveness of KMC in unstabilized LBW infants. Given these factors, the great majority of results of our meta‐analysis can be applied only to stabilized LBW infants in low/middle‐income countries. However, the beneficial effect of KMC on any breastfeeding at one to two months' follow‐up was also found among stabilized LBW infants in high‐income countries.
As only a small trial compared early‐onset KMC with late‐onset KMC, review authors could draw no firm conclusions regarding apparent differences between these two types of management.
One randomized controlled cluster trial (Sloan 2008) assessed the effect of community‐based KMC on overall neonatal mortality, infant mortality, and LBW neonatal mortality; investigators assigned 4165 infants in rural Bangladesh to community‐based KMC or control without KMC. Unfortunately, we did not include this study in the review because 40% overall and 65% of newborns who died were not weighed at birth, and missing birthweight was differential for study group. Results show no difference in overall neonatal mortality rate or infant mortality rate. However, for infants whose modeled birthweight was ≤ 2000 g, the neonatal mortality rate was 9.5% in the community‐based KMC group and 22.5% in the control group (adjusted odds ratio 0.37, 95% confidence interval [CI] 0.16 to 0.86).
Quality of the evidence
Overall, we assessed the quality of the evidence as moderate for most critical and important outcomes (Table 1). We evaluated the risk of bias in included studies by addressing seven specific domains (random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias) discussed in section Risk of bias in included studies. Review authors judged that 12 studies adequately addressed at least four domains (Acharya 2014; Blaymore Bier 1996; Boo 2007; Ghavane 2012; Kadam 2005; Kumbhojkar 2016; Nagai 2010; Neu 2010; Nimbalkar 2014; Roberts 2000; Rojas 2003; Whitelaw 1988). Five studies adequately addressed three domains (Charpak 1997; Eka Pratiwi 2009; Gathwala 2008; Ramanathan 2001; Suman 2008), and four adequately addressed two or fewer domains (Ali 2009; Cattaneo 1998; Sloan 1994; Worku 2005).
Overall, the quality of the studies was mixed, although sensitivity analysis suggests that inclusion of studies with high risk of bias did not affect the general direction of findings nor the size of the treatment effect. Nevertheless, lack of blinding of outcome assessors in most studies and the unclear method of allocation concealment might present problems in terms of the overall quality of evidence. Investigators must make every effort to improve research quality.
For some of the results described in the review (hypothermia, weight gain, breastfeeding, and length of hospital stay), evidence shows high levels of statistical heterogeneity. Some of this heterogeneity may have occurred as a result of clinical heterogeneity, for example, different definitions of hypothermia were used, or women may not have been asked about breastfeeding in the same way in different trials. Results of meta‐analysis with substantial heterogeneity should be interpreted cautiously.
Potential biases in the review process
We attempted to reduce bias in the review process wherever possible. Two review authors independently assessed the risk of bias and findings of included studies. We tried to contact authors of studies with missing data but obtained limited response. Despite differences in the timing of outcome measurements among studies, we proceeded with meta‐analyses for several outcomes, as intervention effects were consistent among studies, although to varying degrees. Only one study reported about 50% of outcomes evaluated in the review, precluding convincing conclusions on the effect of KMC on such outcomes.
The beneficial effects of KMC on mortality at discharge or at 40 to 41 weeks' postmenstrual age and at latest follow‐up, severe infection/sepsis, and nosocomial infection found in our meta‐analyses are enhanced by the impressive statistical homogeneity observed among trials (I2 = 0% to 7%).
To date, only one study (Charpak 1997) reported neurodevelopmental results at one year of corrected age. Longer‐term assessments of neurodevelopmental outcomes have not been published yet, and some caution should perhaps be exercised in applying these findings at 12 months' corrected age, because it has been suggested that assessments done at a relatively young age may be insufficiently predictive of longer‐term neurodevelopmental outcomes, particularly with regard to cognitive functioning (Roberts 2010).
Agreements and disagreements with other studies or reviews
Previous versions of this review
Our assessment of the evidence was similar to that provided in the previous version of this review (Conde‐Agudelo 2014), which concluded that "the evidence from this updated review supports the use of KMC in LBW infants as an alternative to conventional neonatal care mainly in resource‐limited settings." In the current version of this review, we included three additional trials and results of infant neurobehavior at 40 to 44 weeks' postmenstrual age of a previously included study. Notwithstanding, the conclusions of this updated review have not changed in relation to those of the previous version of the review. It should be noted that the first two versions of this review (Conde‐Agudelo 2000; Conde‐Agudelo 2003), including only three trials (n = 1362 infants), had concluded that "although KMC appears to reduce severe infant morbidity without any serious deleterious effect reported, there is still insufficient evidence to recommend its routine use in LBW infants." In these earlier versions, biases related to blinding of participants, personnel and outcome assessors were assessed within a single domain. In the current version , we assessed blinding of participants and personnel in a domain that was separate from bias related to blinding of outcome assessment, as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
The findings of the current updated version of this review allow us to reassert that evidence is sufficient for review authors to recommend the use of KMC in stabilized LBW infants.
Other systematic reviews on KMC
Lawn 2010 performed a systematic review and meta‐analysis to estimate the effect of KMC on neonatal mortality due to direct complications of preterm birth. This review included observational studies and excluded RCTs that initiated KMC after the first week of life. In the meta‐analysis of RCTs, which included three studies (Charpak 1997; Suman 2008; Worku 2005) that provided data on neonatal specific mortality, KMC was associated with a reduction in neonatal death among infants < 2000 g (risk ratio [RR] 0.49, 95% CI 0.29 to 0.82; I2 = 0%; 988 infants). In the meta‐analysis of three observational studies, KMC was associated with decreased risk of neonatal death in infants < 2000 g (RR 0.68, 95% CI 0.58 to 0.79; I2 = 54%; 8151 infants). Another meta‐analysis, which included five RCTs, showed that KMC reduced significantly the risk of severe morbidity (RR 0.34, 95% CI 0.17 to 0.65; I2 = 70%; 1520 infants).
Boundy 2016 conducted a systematic review and meta‐analysis of RCTs and observational studies to assess the effect of KMC on neonatal outcomes among infants of any birthweight or gestational age. Studies with fewer than 10 participants, lack of a comparison group without KMC, and not reporting a quantitative association were excluded. Among LBW infants < 2000 g, KMC was associated with a significant decrease in the risk of mortality at latest follow‐up (RR 0.64, 95% CI 0.46 to 0.89; I2 = 72%; 15 studies [9 RCTs and 6 observational studies]).
The results of our review suggest that KMC reduces the risk of mortality at discharge or at 40 to 41 weeks' postmenstrual age and at latest follow‐up. Our estimated effects were similar to those of Boundy 2016 and were smaller than those of Lawn 2010. Differences between the findings of this updated review and those of Lawn 2010 and Boundy 2016 are explained by inclusion of only RCTs and of a greater number of studies in our review.
Authors' conclusions
Implications for practice.
Results of this updated review indicate that, currently, evidence is sufficient to support the use of kangaroo mother care (KMC) in stabilized low birthweight (LBW) infants as an alternative to conventional neonatal care in resource‐limited settings. Although current evidence is mainly limited to the use of KMC in low/middle‐income countries, emerging evidence suggests that use of KMC could improve breastfeeding rates in high‐income countries. Subgroup analyses suggest that both continuous KMC and intermittent KMC are beneficial for stabilized LBW infants. Given that the control group in studies evaluating continuous KMC was kept in incubators or radiant warmers, the potential beneficial effects of KMC on morbidity and mortality of LBW infants would be expected to be greatest in settings in which conventional neonatal care is unavailable.
To date, early‐onset continuous KMC in unstabilized or relatively stabilized LBW infants cannot be recommended on the basis of evidence provided by two small trials.
Implications for research.
Several areas require further study in light of the results of this review.
Methodologically rigorous trials are needed to further explore the effectiveness of early‐onset continuous KMC in unstabilized or relatively stabilized LBW infants in low‐income settings. Studies should provide detailed information on inclusion and exclusion criteria, methods used to generate and conceal the allocation sequence, measures used to blind outcome assessors to allocation of participants, completeness of outcome data for each main outcome (attritions and exclusions), definition of infant stabilization, infant age at initiation of KMC, and frequency, daily duration, and total duration of the intervention, and investigators should report adequately all prespecified outcomes in the study protocol. We are aware that a planned RCT (OMWaNA study) will assess the effect of early KMC among unstable infants weighing ≤ 2000g in eastern Uganda.
Only five RCTs, including a total of 256 infants, which were conducted in developed countries and reported clinical outcome measures, met minimal inclusion criteria (Blaymore Bier 1996; Neu 2010; Roberts 2000; Rojas 2003; Whitelaw 1988). Therefore, randomized trials with an adequate sample size are clearly needed to evaluate the use of continuous or intermittent KMC in high‐income settings and to report results mainly on infant morbidity.
Although some data on long‐term neurodevelopmental and neurosensory outcomes are available, continuing follow‐up and additional data for randomized children are justified, as more subtle differences may become apparent in later childhood (Roberts 2010).
Additional studies are needed to investigate effects of early‐onset KMC on breastfeeding.
Well‐designed economic evaluations are needed to assess the cost‐effectiveness of KMC in low‐, middle‐, and high‐income settings.
Exploration of mother‐infant attachment should be pursued in future trials, as this element has been inconsistently evaluated across studies.
Additional trials in different settings ensuring baseline comparability of mortality, adequate KMC implementation, and birthweight assessment are required to clarify the effect of community‐based KMC on LBW neonatal mortality before community‐based KMC programs are implemented and before community‐based KMC is included in essential newborn care.
What's new
| Date | Event | Description |
|---|---|---|
| 6 February 2017 | Amended | Amended to add source of support. |
History
Protocol first published: Issue 3, 1999 Review first published: Issue 4, 2000
| Date | Event | Description |
|---|---|---|
| 4 August 2016 | New citation required but conclusions have not changed | We updated the search in June 2016 and found three new studies for inclusion (Acharya 2014; Kumbhojkar 2016; Nimbalkar 2014). The conclusions of the review are unchanged. |
| 15 July 2016 | New search has been performed | This updates the review, "Kangaroo mother care to reduce morbidity and mortality in low birthweight infants," published in the Cochrane Database of Systematic Reviews (Conde‐Agudelo 2014) |
| 31 March 2014 | New search has been performed | This updates the review, "Kangaroo mother care to reduce morbidity and mortality in low birthweight infants," published in the Cochrane Database of Systematic Reviews (Conde‐Agudelo 2011) |
| 31 March 2014 | New citation required but conclusions have not changed | A new search has been performed. In addition to the 16 studies included in the previous version of the review, we have included 2 new studies (Eka Pratiwi 2009; Ghavane 2012) and a report on additional outcomes of a previously included study (Nagai 2010). This updated review includes a new secondary outcome measure (hyperthermia at discharge or at 40 to 41 weeks' postmenstrual age) and additional data regarding the external validity of each included study, such as level of care, human resources used, criteria for infant discharge from the hospital, and scheme for follow‐up of infants after discharge |
| 26 September 2008 | Amended | Converted to new review format |
Acknowledgements
We thank the following trial authors, who provided additional information on request: Drs Charpak, Sloan, Ludington‐Hoe, Neu, Suman, Miltersteiner, and Murki.
Appendices
Appendix 1. Search strategy for the 2014 update
Electronic searches The standard search strategy for the Cochrane Neonatal review Group was used.This included searches of MEDLINE, EMBASE, LILACS, POPLINE, and CINAHL databases (all from inception to March 31, 2014), and the Cochrane Central Register of Controlled Trials (The Cochrane Library, Issue 3, 2014) using a combination of keywords and text words related to KMC or SSC and LBW or preterm infants. To ensure maximum sensitivity we placed no limits or filters on the searches.
INDEX TERMS Text words Kangaroo mother care; kangaroo mother method; kangaroo care; skin‐to‐skin contact, skin‐to‐skin care Medical subject headings (MeSH) *Infant, Low Birth Weight; *Infant Mortality; *Breast Feeding; *Mother‐Child Relations; Infant, Newborn; Infant care [*Methods]; Length of Stay; Physical Stimulation; [*Methods]; Randomized Controlled Trials as Topic; Weight Gain
MeSH check words Humans; Infant We searched for ongoing trials most recently in September 2013 in the following databases using the terms “kangaroo care” and “skin‐to‐skin contact” : • The metaRegister of Controlled Trials www.controlledtrials.com. • The US National Institutes of Health ongoing trials register www.clinicaltrials.gov. • The National Research Register (NRR) Archive http://www.nihr.ac.uk, • The Australian and New Zealand Clinical Trials Registry www.anzctr.org.au. • UMIN Clinical Trials Registry www.umin.ac.jp/ctr. • The World Health Organization International Clinical Trials Registry platform www.who.int/trialsearch.
Searching other resources Web page of the Kangaroo Foundation, International Network of Kangaroo Care, conference and symposia proceedings on KMC, reference lists of identified studies, textbooks, review articles, and Google scholar were also searched. In addition,we performed journal hand searching and contacted investigators involved in the field to locate unpublished studies. No language restrictions were applied. For studies with multiple publications, the data from the most complete report were used and supplemented if additional information appeared in other publications.
Appendix 2. Standard search methodology
PubMed: ((infant, newborn[MeSH] OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or infan* or neonat*) AND (randomized controlled trial [pt] OR controlled clinical trial [pt] OR Clinical Trial [ptyp] OR randomized [tiab] OR placebo [tiab] OR clinical trials as topic [mesh: noexp] OR randomly [tiab] OR trial [ti]) NOT (animals [mh] NOT humans [mh]))
Embase: (infant, newborn or newborn or neonate or neonatal or premature or very low birth weight or low birth weight or VLBW or LBW or Newborn or infan* or neonat*) AND (human not animal) AND (randomized controlled trial or controlled clinical trial or randomized or placebo or clinical trials as topic or randomly or trial or clinical trial)
CINAHL: (infant, newborn OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or Newborn or infan* or neonat*) AND (randomized controlled trial OR controlled clinical trial OR randomized OR placebo OR clinical trials as topic OR randomly OR trial OR PT clinical trial)
Cochrane Library: (infant or newborn or neonate or neonatal or premature or very low birth weight or low birth weight or VLBW or LBW)
Data and analyses
Comparison 1. Kangaroo mother care versus conventional neonatal care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality at discharge or at 40 to 41 weeks' postmenstrual age | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 All studies | 8 | 1736 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.39, 0.92] |
| 1.2 Intermittent KMC | 5 | 619 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.19, 1.81] |
| 1.3 Continuous KMC | 3 | 1117 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.38, 0.96] |
| 1.4 Duration of KMC < 2 hours/d | 2 | 188 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.22, 7.73] |
| 1.5 Duration of KMC between 6 and 15 hours/d | 3 | 431 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.07, 1.64] |
| 1.6 Duration of KMC ≥ 20 hours/d | 3 | 1117 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.38, 0.96] |
| 1.7 Infant age ≤ 10 days at initiation of KMC | 5 | 1412 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.36, 0.88] |
| 1.8 Infant age > 10 days at initiation of KMC | 3 | 324 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.22, 7.73] |
| 1.9 Low/middle‐income countries | 7 | 1676 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.37, 0.89] |
| 1.10 High‐income countries | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [0.16, 17.09] |
| 1.11 infant entered into trial before stabilization | 1 | 123 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.33, 1.00] |
| 1.12 infant entered into trial after stabilization | 7 | 1613 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.32, 1.23] |
| 2 Mortality at 6 months of age or 6 months' follow‐up | 2 | 354 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.48, 2.02] |
| 2.1 Intermittent | 1 | 71 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.15, 6.90] |
| 2.2 Continuous | 1 | 283 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.46, 2.12] |
| 3 Mortality at 12 months' corrected age | 1 | 693 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.27, 1.17] |
| 3.1 Intermittent | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Continuous | 1 | 693 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.27, 1.17] |
| 4 Mortality at latest follow‐up | 12 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 All studies | 12 | 2293 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.48, 0.95] |
| 4.2 Intermittent KMC | 8 | 909 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.26, 1.77] |
| 4.3 Continuous KMC | 4 | 1384 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.46, 0.98] |
| 4.4 Duration of KMC < 2 hours/d | 3 | 259 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.32, 4.30] |
| 4.5 Duration of KMC between 6 and 15 hours/d | 5 | 650 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.07, 1.64] |
| 4.6 Duration of KMC ≥ 20 hours/d | 4 | 1384 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.46, 0.98] |
| 4.7 Infant age ≤ 10 days at initiation of KMC | 6 | 1489 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.37, 0.85] |
| 4.8 Infant age > 10 days at initiation of KMC | 5 | 678 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.53, 2.00] |
| 4.9 Low/middle‐income countries | 10 | 2162 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.45, 0.93] |
| 4.10 High‐income countries | 2 | 131 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.29, 5.42] |
| 4.11 Infant entered into trial before stabilization | 1 | 123 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.33, 1.00] |
| 4.12 Infant entered into trial after stabilization | 11 | 2170 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.47, 1.13] |
| 5 Severe infection/sepsis at latest follow‐up ‐ stabilized infants | 8 | 1463 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.36, 0.69] |
| 5.1 Intermittent | 7 | 800 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.24, 0.60] |
| 5.2 Continuous | 1 | 663 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.43, 1.12] |
| 6 Severe illness at 6 months' follow‐up ‐ stabilized infants | 1 | 283 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.14, 0.67] |
| 6.1 intermittent | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Continuous | 1 | 283 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.14, 0.67] |
| 7 Nosocomial infection/sepsis at discharge or at 40 to 41 weeks' postmenstrual age ‐ stabilized infants | 5 | 1239 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.22, 0.54] |
| 7.1 Intermittent | 4 | 576 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.15, 0.50] |
| 7.2 Continuous | 1 | 663 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.25, 0.93] |
| 8 Mild/moderate infection or illness at latest follow‐up ‐ stabilized infants | 4 | 1266 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [0.87, 1.88] |
| 8.1 Intermittent | 2 | 320 | Risk Ratio (M‐H, Random, 95% CI) | 1.52 [0.43, 5.38] |
| 8.2 Continuous | 2 | 946 | Risk Ratio (M‐H, Random, 95% CI) | 1.42 [0.53, 3.79] |
| 9 Lower respiratory tract disease at 6 months' follow‐up ‐ stabilized infants | 1 | 283 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.15, 0.89] |
| 9.1 Intermittent | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 Continuous | 1 | 283 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.15, 0.89] |
| 10 Diarrhea at 6 months' follow‐up ‐ stabilized infants | 1 | 283 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.35, 1.20] |
| 10.1 Intermittent | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 Continuous | 1 | 283 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.35, 1.20] |
| 11 Hypothermia at discharge or at 40 to 41 weeks’ postmenstrual age ‐ stabilized infants | 9 | 989 | Risk Ratio (M‐H, Random, 95% CI) | 0.28 [0.16, 0.49] |
| 11.1 Intermittent | 9 | 989 | Risk Ratio (M‐H, Random, 95% CI) | 0.28 [0.16, 0.49] |
| 11.2 Continuous | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Hyperthermia at discharge or at 40 to 41 weeks' postmenstrual age ‐ stabilized infants | 4 | 448 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.59, 1.05] |
| 12.1 Intermittent | 4 | 448 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.59, 1.05] |
| 12.2 Continuous | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Length of hospital stay (days) ‐ stabilized infants | 11 | 1057 | Mean Difference (IV, Random, 95% CI) | ‐1.61 [‐3.41, 0.18] |
| 13.1 Intermittent | 11 | 1057 | Mean Difference (IV, Random, 95% CI) | ‐1.61 [‐3.41, 0.18] |
| 13.2 Continuous | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Re‐admission to hospital at latest follow‐up ‐ stabilized infants | 2 | 946 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.34, 1.06] |
| 14.1 Intermittent | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14.2 Continuous | 2 | 946 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.34, 1.06] |
| 15 Weight at discharge or at 40 to 41 weeks' postmenstrual age (g) ‐ stabilized infants | 5 | 1233 | Mean Difference (IV, Fixed, 95% CI) | 16.07 [‐20.54, 52.68] |
| 15.1 Intermittent | 3 | 285 | Mean Difference (IV, Fixed, 95% CI) | 41.84 [‐19.19, 102.87] |
| 15.2 Continuous | 2 | 948 | Mean Difference (IV, Fixed, 95% CI) | 1.59 [‐44.16, 47.34] |
| 16 Weight at 6 months' corrected age (g) ‐ stabilized infants | 1 | 591 | Mean Difference (IV, Fixed, 95% CI) | 78.19 [‐52.26, 208.64] |
| 16.1 Intermittent | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16.2 Continuous | 1 | 591 | Mean Difference (IV, Fixed, 95% CI) | 78.19 [‐52.26, 208.64] |
| 17 Weight at 12 months' corrected age (g) ‐ stabilized infants | 1 | 596 | Mean Difference (IV, Fixed, 95% CI) | 31.46 [‐135.08, 198.00] |
| 17.1 Intermittent | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17.2 Continuous | 1 | 596 | Mean Difference (IV, Fixed, 95% CI) | 31.46 [‐135.08, 198.00] |
| 18 Weight gain at latest follow‐up (g/d) ‐ stabilized infants | 11 | 1198 | Mean Difference (IV, Random, 95% CI) | 4.08 [2.30, 5.86] |
| 18.1 Intermittent | 10 | 913 | Mean Difference (IV, Random, 95% CI) | 4.13 [2.19, 6.07] |
| 18.2 Continuous | 1 | 285 | Mean Difference (IV, Random, 95% CI) | 3.60 [0.78, 6.42] |
| 19 Length at discharge or at 40 to 41 weeks' postmenstrual age (cm) ‐ stabilized infants | 3 | 856 | Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.69, 0.48] |
| 19.1 Intermittent | 2 | 193 | Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐1.51, 1.04] |
| 19.2 Continuous | 1 | 663 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.36, 0.36] |
| 20 Length at 6 months' corrected age (cm) ‐ stabilized infants | 1 | 590 | Mean Difference (IV, Fixed, 95% CI) | 0.23 [‐0.18, 0.64] |
| 20.1 Intermittent | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20.2 Continuous | 1 | 590 | Mean Difference (IV, Fixed, 95% CI) | 0.23 [‐0.18, 0.64] |
| 21 Length at 12 months' corrected age (cm) ‐ stabilized infants | 1 | 586 | Mean Difference (IV, Fixed, 95% CI) | 0.31 [‐0.17, 0.79] |
| 21.1 Intermittent | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 21.2 Continuous | 1 | 586 | Mean Difference (IV, Fixed, 95% CI) | 0.31 [‐0.17, 0.79] |
| 22 Length gain at latest follow‐up (cm/wk) ‐ stabilized infants | 3 | 377 | Mean Difference (IV, Random, 95% CI) | 0.21 [0.03, 0.38] |
| 22.1 Intermittent | 3 | 377 | Mean Difference (IV, Random, 95% CI) | 0.21 [0.03, 0.38] |
| 22.2 Continuous | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 23 Head circumference at discharge or at 40 to 41 weeks' postmenstrual age (cm) ‐ stabilized infants | 3 | 856 | Mean Difference (IV, Random, 95% CI) | 0.17 [‐0.33, 0.66] |
| 23.1 Intermittent | 2 | 193 | Mean Difference (IV, Random, 95% CI) | 0.24 [‐0.84, 1.31] |
| 23.2 Continuous | 1 | 663 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.14, 0.34] |
| 24 Head circumference at 6 months' corrected age (cm) ‐ stabilized infants | 1 | 592 | Mean Difference (IV, Fixed, 95% CI) | 0.34 [0.11, 0.57] |
| 24.1 Intermittent | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 24.2 Continuous | 1 | 592 | Mean Difference (IV, Fixed, 95% CI) | 0.34 [0.11, 0.57] |
| 25 Head circumference at 12 months' corrected age (cm) ‐ stabilized infants | 1 | 597 | Mean Difference (IV, Fixed, 95% CI) | 0.39 [‐0.00, 0.78] |
| 25.1 Intermittent | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 25.2 Continuous | 1 | 597 | Mean Difference (IV, Fixed, 95% CI) | 0.39 [‐0.00, 0.78] |
| 26 Head circumference gain at latest follow‐up (cm/wk) ‐ stabilized infants | 4 | 495 | Mean Difference (IV, Random, 95% CI) | 0.14 [0.06, 0.22] |
| 26.1 Intermittent | 4 | 495 | Mean Difference (IV, Random, 95% CI) | 0.14 [0.06, 0.22] |
| 26.2 Continuous | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 27 Psychomotor development (Griffith quotients) at 12 months' corrected age | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 27.1 Locomotion | 1 | 579 | Mean Difference (IV, Fixed, 95% CI) | 2.25 [‐0.45, 4.95] |
| 27.2 Personal, social | 1 | 579 | Mean Difference (IV, Fixed, 95% CI) | 0.97 [‐1.27, 3.21] |
| 27.3 Hand‐eye coordination | 1 | 579 | Mean Difference (IV, Fixed, 95% CI) | 0.57 [‐1.25, 2.39] |
| 27.4 Audition, language | 1 | 579 | Mean Difference (IV, Fixed, 95% CI) | 1.29 [‐0.98, 3.56] |
| 27.5 Execution | 1 | 579 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐1.50, 2.10] |
| 27.6 All criteria | 1 | 579 | Mean Difference (IV, Fixed, 95% CI) | 1.05 [‐0.75, 2.85] |
| 28 Cerebral palsy at 12 months' corrected age | 1 | 588 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.21, 2.02] |
| 29 Deafness at 12 months' corrected age | 1 | 588 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.03, 2.90] |
| 30 Visual impairment at 12 months' corrected age | 1 | 588 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.53, 1.56] |
| 31 Exclusive breastfeeding at discharge or at 40 to 41 weeks' postmenstrual age ‐ stabilized infants | 6 | 1453 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [1.07, 1.25] |
| 31.1 Intermittent | 4 | 511 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [1.11, 1.35] |
| 31.2 Continuous | 2 | 942 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [1.00, 1.24] |
| 32 Exclusive breastfeeding at 1 to 3 months' follow‐up ‐ stabilized infants | 5 | 600 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [1.01, 1.43] |
| 32.1 Intermittent | 3 | 221 | Risk Ratio (M‐H, Random, 95% CI) | 1.36 [1.12, 1.65] |
| 32.2 Continuous | 2 | 379 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.96, 1.10] |
| 33 Exclusive breastfeeding at 6 to 12 months' follow‐up ‐ stabilized infants | 3 | 810 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.95, 1.76] |
| 33.1 Intermittent | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.52 [1.10, 2.10] |
| 33.2 Continuous | 2 | 735 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.66, 1.86] |
| 34 Any breastfeeding at discharge or at 40 to 41 weeks' postmenstrual age ‐ stabilized infants | 10 | 1696 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [1.07, 1.34] |
| 34.1 Intermittent | 8 | 754 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [1.07, 1.41] |
| 34.2 Continuous | 2 | 942 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.93, 1.40] |
| 35 Any breastfeeding at 1 to 2 months' follow‐up ‐ stabilized infants | 6 | 538 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [1.00, 1.78] |
| 35.1 Intermittent | 4 | 159 | Risk Ratio (M‐H, Random, 95% CI) | 1.89 [1.30, 2.75] |
| 35.2 Continuous | 2 | 379 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.96, 1.10] |
| 36 Any breastfeeding at 3 months' follow‐up ‐ stabilized infants | 5 | 924 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [1.06, 1.23] |
| 36.1 Intermittent | 4 | 261 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [1.15, 1.59] |
| 36.2 Continuous | 1 | 663 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [1.00, 1.17] |
| 37 Any breastfeeding at 1 to 3 months' follow‐up ‐ stabilized infants | 9 | 1394 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [1.05, 1.31] |
| 37.1 Intermittent | 6 | 352 | Risk Ratio (M‐H, Random, 95% CI) | 1.39 [1.18, 1.64] |
| 37.2 Continuous | 3 | 1042 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [1.00, 1.11] |
| 38 Any breastfeeding at 6 months' follow‐up ‐ stabilized infants | 5 | 952 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.98, 1.29] |
| 38.1 Intermittent | 3 | 143 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.50 [1.08, 2.08] |
| 38.2 Continuous | 2 | 809 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.92, 1.24] |
| 39 Any breastfeeding at 12 months' follow‐up ‐ stabilized infants | 1 | 589 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.65, 1.21] |
| 39.1 Intermittent | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 39.2 Continuous | 1 | 589 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.65, 1.21] |
| 40 Onset of breastfeeding (days) ‐ stabilized infants | 2 | 295 | Mean Difference (IV, Random, 95% CI) | 0.03 [‐1.64, 1.70] |
| 40.1 Intermittent | 2 | 295 | Mean Difference (IV, Random, 95% CI) | 0.03 [‐1.64, 1.70] |
| 40.2 Continuous | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 41 Parental and familial satisfaction (continuous KMC) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 41.1 Mother satisfied with method | 1 | 269 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [1.05, 1.30] |
| 41.2 Father satisfied with method | 1 | 269 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.91, 1.14] |
| 41.3 Family satisfied with method | 1 | 269 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.83, 1.13] |
| 42 Mother‐infant attachment: mother's feelings and perceptions according to interval between birth and start of intervention, and infant admission to NICU | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 42.1 Sense of competence ‐ interval of 1 to 2 days | 1 | 170 | Mean Difference (IV, Fixed, 95% CI) | 0.41 [0.14, 0.68] |
| 42.2 Sense of competence ‐ interval of 3 to 14 days | 1 | 177 | Mean Difference (IV, Fixed, 95% CI) | 0.25 [‐0.08, 0.58] |
| 42.3 Sense of competence ‐ interval > 14 days | 1 | 141 | Mean Difference (IV, Fixed, 95% CI) | 0.21 [‐0.17, 0.59] |
| 42.4 Sense of competence ‐ infant admitted to NICU | 1 | 82 | Mean Difference (IV, Fixed, 95% CI) | 0.54 [0.07, 1.01] |
| 42.5 Sense of competence ‐ infant not admitted to NICU | 1 | 406 | Mean Difference (IV, Fixed, 95% CI) | 0.24 [0.05, 0.43] |
| 42.6 Worry and stress ‐ interval of 1 to 2 days | 1 | 170 | Mean Difference (IV, Fixed, 95% CI) | 0.31 [0.04, 0.58] |
| 42.7 Worry and stress ‐ interval of 3 to 14 days | 1 | 177 | Mean Difference (IV, Fixed, 95% CI) | 0.09 [‐0.20, 0.38] |
| 42.8 Worry and stress ‐ interval > 14 days | 1 | 141 | Mean Difference (IV, Fixed, 95% CI) | ‐0.29 [‐0.70, 0.12] |
| 42.9 Worry and stress ‐ infant admitted to NICU | 1 | 82 | Mean Difference (IV, Fixed, 95% CI) | ‐0.1 [‐0.60, 0.40] |
| 42.10 Worry and stress ‐ infant not admitted to NICU | 1 | 406 | Mean Difference (IV, Fixed, 95% CI) | 0.12 [‐0.06, 0.30] |
| 42.11 Social support ‐ interval of 1 to 2 days | 1 | 170 | Mean Difference (IV, Fixed, 95% CI) | ‐0.06 [‐0.35, 0.23] |
| 42.12 Social support ‐ interval of 3 to 14 days | 1 | 177 | Mean Difference (IV, Fixed, 95% CI) | ‐0.06 [‐0.34, 0.22] |
| 42.13 Social support ‐ interval > 14 days | 1 | 141 | Mean Difference (IV, Fixed, 95% CI) | ‐0.47 [‐0.84, ‐0.10] |
| 42.14 Social support ‐ infant admitted to NICU | 1 | 82 | Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐0.52, 0.42] |
| 42.15 Social support ‐ infant not admitted to NICU | 1 | 406 | Mean Difference (IV, Fixed, 95% CI) | ‐0.2 [‐0.39, ‐0.01] |
| 43 Mother‐infant attachment: mother's responses to the infant according to interval between birth and start of intervention, and infant admission to NICU | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 43.1 Mother's sensitivity ‐ interval of 1 to 2 days | 1 | 170 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.02, 0.06] |
| 43.2 Mother's sensitivity ‐ interval of 3 to 14 days | 1 | 177 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.05, 0.03] |
| 43.3 Mother's sensitivity ‐ interval > 14 days | 1 | 141 | Mean Difference (IV, Fixed, 95% CI) | 0.06 [0.01, 0.11] |
| 43.4 Mother's sensitivity ‐ infant admitted to NICU | 1 | 82 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.04, 0.08] |
| 43.5 Mother's sensitivity ‐ infant not admitted to NICU | 1 | 406 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.00, 0.04] |
| 43.6 Mother's response to child's distress ‐ interval of 1 to 2 days | 1 | 170 | Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.08, 0.02] |
| 43.7 Mother's response to child's distress ‐ interval of 3 to 14 days | 1 | 177 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.03, 0.05] |
| 43.8 Mother's response to child's distress ‐ interval > 14 days | 1 | 141 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.04, 0.06] |
| 43.9 Mother's response to child's distress ‐ infant admitted to NICU | 1 | 82 | Mean Difference (IV, Fixed, 95% CI) | 0.05 [‐0.01, 0.11] |
| 43.10 Mother's response to child's distress ‐ infant not admitted to NICU | 1 | 406 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.05, 0.01] |
| 43.11 Mother's response to child's socioemotional growth fostering ‐ interval of 1 to 2 days | 1 | 170 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.04, 0.06] |
| 43.12 Mother's response to child's socioemotional growth fostering ‐ interval of 3 to 14 days | 1 | 177 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.06, 0.02] |
| 43.13 Mother's response to child's socioemotional growth fostering ‐ interval > 14 days | 1 | 141 | Mean Difference (IV, Fixed, 95% CI) | 0.05 [‐0.00, 0.10] |
| 43.14 Mother's response to child's socioemotional growth fostering ‐ infant admitted to NICU | 1 | 82 | Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐0.12, 0.02] |
| 43.15 Mother's response to child's socioemotional growth fostering ‐ infant not admitted to NICU | 1 | 406 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.01, 0.05] |
| 43.16 Mother's response to child's cognitive growth fostering ‐ interval of 1 to 2 days | 1 | 170 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.04, 0.08] |
| 43.17 Mother's response to child's cognitive growth fostering ‐ interval of 3 to 14 days | 1 | 177 | Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.10, 0.02] |
| 43.18 Mother's response to child's cognitive growth fostering ‐ interval > 14 days | 1 | 141 | Mean Difference (IV, Fixed, 95% CI) | 0.07 [0.00, 0.14] |
| 43.19 Mother's response to child's cognitive growth fostering ‐ infant admitted to NICU | 1 | 82 | Mean Difference (IV, Fixed, 95% CI) | ‐0.07 [‐0.17, 0.03] |
| 43.20 Mother's response to child's cognitive growth fostering ‐ infant not admitted to NICU | 1 | 406 | Mean Difference (IV, Fixed, 95% CI) | 0.03 [‐0.01, 0.07] |
| 44 Mother‐infant attachment: infant's responses to the mother according to interval between birth and start of intervention, and infant admission to NICU | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 44.1 Clarity of cues ‐ interval of 1 to 2 days | 1 | 170 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.04, 0.06] |
| 44.2 Clarity of cues ‐ interval of 3 to 14 days | 1 | 177 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.03, 0.07] |
| 44.3 Clarity of cues ‐ interval > 14 days | 1 | 141 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.05, 0.05] |
| 44.4 Clarity of cues ‐ infant admitted to NICU | 1 | 82 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.07, 0.05] |
| 44.5 Clarity of cues ‐ infant not admitted to NICU | 1 | 406 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.01, 0.05] |
| 44.6 Responsiveness ‐ interval of 1 to 2 days | 1 | 170 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.06, 0.02] |
| 44.7 Responsiveness ‐ interval of 3 to 14 days | 1 | 177 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.02, 0.06] |
| 44.8 Responsiveness ‐ interval > 14 days | 1 | 141 | Mean Difference (IV, Fixed, 95% CI) | 0.05 [0.01, 0.09] |
| 44.9 Responsiveness ‐ infant admitted to NICU | 1 | 82 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.07, 0.05] |
| 44.10 Responsiveness ‐ infant not admitted to NICU | 1 | 406 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.01, 0.05] |
| 45 Mother‐infant attachment at 3 months' follow‐up | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | 6.24 [5.57, 6.91] |
| 45.1 Total attachment score at 3 months' follow‐up | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | 6.24 [5.57, 6.91] |
| 46 Mother‐infant attachment: stress in NICU | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 46.1 Nursery environment score | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.51, 0.71] |
| 46.2 Infant appearance score | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.62, 0.62] |
| 46.3 Relationship with the infant score | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | 1.00 [0.35, 1.65] |
| 46.4 Staff behavior and communication score | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.95, 1.15] |
| 47 Mother‐infant attachment: parenting skills | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐0.89, 0.09] |
| 47.1 Total score at discharge | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐0.89, 0.09] |
| 48 Mother‐infant interaction at 6 months' follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 48.1 Symmetrical co‐regulation | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | 16.38 [13.61, 19.15] |
| 48.2 Asymmetrical co‐regulation | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | ‐18.31 [‐21.42, ‐15.20] |
| 48.3 Unilateral regulation | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | 2.12 [‐1.24, 5.48] |
| 49 Infant behavior at 40 to 44 weeks’ postmenstrual age | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 49.1 Attention | 1 | 55 | Mean Difference (IV, Fixed, 95% CI) | 0.29 [‐0.40, 0.98] |
| 49.2 Autonomic organization | 1 | 55 | Mean Difference (IV, Fixed, 95% CI) | 0.19 [‐0.41, 0.79] |
| 49.3 Motor | 1 | 55 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐0.22, 0.82] |
| 49.4 Orientation | 1 | 55 | Mean Difference (IV, Fixed, 95% CI) | ‐0.19 [‐0.72, 0.34] |
| 49.5 Autonomic | 1 | 55 | Mean Difference (IV, Fixed, 95% CI) | 0.11 [‐0.89, 1.11] |
| 49.6 State regulation | 1 | 55 | Mean Difference (IV, Fixed, 95% CI) | ‐0.31 [‐0.95, 0.33] |
| 49.7 Robust crying | 1 | 55 | Mean Difference (IV, Fixed, 95% CI) | ‐0.16 [‐0.90, 0.58] |
| 49.8 State stability | 1 | 55 | Mean Difference (IV, Fixed, 95% CI) | 0.32 [‐0.93, 1.57] |
| 50 Social and home environment | 1 | 338 | Mean Difference (IV, Fixed, 95% CI) | 0.79 [0.74, 0.84] |
| 50.1 HOME environment total score at 12 months' corrected age | 1 | 338 | Mean Difference (IV, Fixed, 95% CI) | 0.79 [0.74, 0.84] |
Comparison 2. Early versus late kangaroo mother care in relatively stable LBW infants.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality at 4 weeks of age | 1 | 73 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.95 [0.18, 20.53] |
| 2 Morbidity at 4 weeks of age | 1 | 73 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.18, 1.28] |
| 3 Severe infection at 4 weeks of age | 1 | 73 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.12, 1.49] |
| 4 Re‐admission to hospital at 4 weeks of age | 1 | 73 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.95 [0.18, 20.53] |
| 5 Hypothermia | 1 | 73 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.15, 2.27] |
| 6 Hyperthermia | 1 | 73 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.56, 1.99] |
| 7 Weight gain (grams) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 At 24 hours post birth | 1 | 73 | Mean Difference (IV, Fixed, 95% CI) | 39.16 [11.11, 67.21] |
| 7.2 At 48 hours post birth | 1 | 73 | Mean Difference (IV, Fixed, 95% CI) | 43.3 [5.49, 81.11] |
| 7.3 At 2 weeks of age | 1 | 73 | Mean Difference (IV, Fixed, 95% CI) | 12.14 [‐83.18, 107.46] |
| 7.4 At 4 weeks of age | 1 | 73 | Mean Difference (IV, Fixed, 95% CI) | 58.85 [‐116.93, 234.63] |
| 8 Exclusive breastfeeding | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 At 24 hours of age | 1 | 73 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.67, 1.57] |
| 8.2 At 2 weeks of age | 1 | 71 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.89, 1.12] |
| 8.3 At 4 weeks of age | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.85, 1.04] |
| 8.4 At 6 months of age | 1 | 55 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.69 [0.99, 7.31] |
| 9 Length of hospital stay (days) | 1 | 73 | Mean Difference (IV, Fixed, 95% CI) | ‐0.90 [‐1.24, ‐0.56] |
| 10 Mortality at 6 months of age | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.15, 6.72] |
| 11 Re‐admission to hospital at 6 to 12 months of age | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.32, 3.16] |
| 12 Stunting at 6 to 12 months of age | 1 | 55 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.46, 1.48] |
| 13 Severe stunting at 6 to 12 months of age | 1 | 55 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.17, 2.73] |
| 14 Wasting at 6 to 12 months of age | 1 | 55 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.1 [0.01, 1.77] |
| 15 Severe wasting at 6 to 12 months of age | 1 | 55 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Underweight at 6 to 12 months of age | 1 | 55 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.21, 1.14] |
| 17 Severe underweight at 6 to 12 months of age | 1 | 55 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.03, 1.88] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Acharya 2014.
| Methods | Randomized controlled trial conducted in Dharan, Nepal | |
| Participants |
Number of infants: 126 Inclusion criteria: stable infants with birthweight < 2000 g admitted to the newborn nursery Exclusion criteria: neonates critically ill requiring ventilatory or ionotropic support or radiant warmer, neonates with chromosomal and life‐threatening congenital anomalies, neonates whose mothers were critically ill, and neonates whose mothers did not provide consent for enrollment into the study Infant stabilization status at trial entry: stabilized Infant age and weight at trial entry: Mean weight at recruitment was 1362 ± 240 g and 1452 ± 175 g for KMC and control infants, respectively. No data on infant age at recruitment |
|
| Interventions |
KMC group: SSC between the mother's breasts in an upright position. Infants were dressed with diaper and a cap, and the mother's blouse covered the infant’s trunk and extremities but not the head. The duration of KMC was ≥ 6 hours per day in not more than 4 sittings, with each sitting lasting ≥ 1 hour. No data on total number of days that KMC was given after enrollment in the study (n = 63) Control group: Infants were adequately clothed, covered, and kept with their mother. If infants did not maintain temperature, they were kept under a radiant warmer (n = 63) Level of care: nursery of a tertiary care hospital Human resources: doctors and nurses Criteria for infant discharge from the hospital: unreported. However, it was mentioned that LBW infants were discharged when weight was > 1.600 g Scheme for follow‐up of infants after discharge: unreported |
|
| Outcomes | Gain in weight, length, and head circumference; hypothermia; apnea; hospital stay | |
| Notes | 87% of LBW infants met eligibility criteria | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants: no/unfeasible; blinding of clinical staff: no/unfeasible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No infants apparently lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in Methods section adequately reported or explained in Results |
| Other bias | Low risk | Other biases not identified |
Ali 2009.
| Methods | Randomized controlled trial carried out in Aligarh, India | |
| Participants |
Number of infants: 114 Inclusion criteria: hemodynamically stable infants delivered by vaginal route with birthweight between 1200 and 1800 g Exclusion criteria: neonates delivered by cesarean section, major life‐threatening congenital malformations, severe perinatal complications, parental refusal of KMC intervention Infant stabilization status at trial entry: stabilized Infant age and weight at trial entry: Mean age at recruitment was 4.7 ± 2.9 and 4.8 ± 2.4 days, and mean weight was 1607 ± 211 and 1615 ± 179 g, for KMC and control infants, respectively |
|
| Interventions |
KMC group: SSC between the mother's breasts in an upright position. Infants were dressed with a cap, socks, and a diaper and were supported at the bottom with a sling/binder. The duration of KMC during hospital stay was 6.3 ± 1.5 hours (range, 4 to 12) per day, and KMC was given for a period of 25.7 ± 6.9 (range, 15 to 43) days after enrollment in the study (n = 58) Control group: Infants were kept in radiant warmers or open cots in warm rooms (n = 56) In both groups, mothers were allowed to handle their babies at any hour of the day and to breastfeed them by nasogastric tube, by paladai, or directly. Babies in both groups were provided with vitamins and mineral supplementation Level of care: NICU of a tertiary care hospital Human resources: doctors and nurses Criteria for infant discharge from the hospital: weight gain for ≥ 3 consecutive days, no overt illness, no intravenous medications, exclusive breastfeeding Scheme for follow‐up of infants after discharge: weekly until 40 weeks' postmenstrual age, fortnightly until 3 months' corrected age, and monthly thereafter until 6 months' corrected age |
|
| Outcomes | Duration of hospital stay, weight gain, head circumference, length, exclusive breastfeeding, nosocomial sepsis, hypothermia, mild/moderate infection, severe infection, mortality | |
| Notes | 81% of LBW infants met eligibility criteria | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomization technique |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants: no/unfeasible; blinding of clinical staff: no/unfeasible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 10 infants (8.8%) lost at 40 weeks' corrected gestational age follow‐up (KMC 4, control 6), 21 (18.4%) lost at 3 months' corrected age (KMC 10, control 11), and 39 (34.2%) lost at 6 months' corrected age (KMC 19, control 20) |
| Selective reporting (reporting bias) | High risk | Non‐significant results such as infant mortality and weight, length, and head circumference at discharge and follow‐up (secondary outcomes listed in Methods) mentioned but not reported adequately |
| Other bias | High risk | Use of blocked randomization, which could make possible prediction of future assignments in an unblinded trial when assignments are revealed, subsequently to the person recruiting into the trial |
Blaymore Bier 1996.
| Methods | Randomized controlled trial conducted in Providence, Rhode Island, United States | |
| Participants |
Number of infants: 50 Inclusion criteria: medically stable infants from singleton or multiple pregnancy with birth weight < 1500 g, whose mothers planned to breastfeed. Infants were no longer ventilator dependent and were without chest tubes, and they no longer required continuous positive airway pressure, when the study was begun Exclusion criteria: mother's positive history of illicit drug use, mental illness, human immunodeficiency virus (HIV) infection, receiving any medications contraindicative to breastfeeding. In addition, any infants who had a positive toxicologic screen for cocaine or other illicit drugs or were showing drug withdrawal symptoms at birth were excluded Infant stabilization status at trial entry: stabilized Infant age and weight at trial entry: Mean age at recruitment was 29 and 30 days, and mean weight was 993 ± 275 and 942 ± 322 g, for KMC and control infants, respectively |
|
| Interventions |
KMC group: SSC involved included the infant clothed in only a diaper and hat, held upright between the mother's breasts, with the mother and infant covered with a blanket (n = 25) Control group: Standard contact involved a fully clothed infant wrapped in a blanket and held cradled in his or her mother's arms (n = 25) During the study, the mother‐infant dyad was observed participating in SSC or standard contact once each weekday until bottle feedings and breastfeedings were initiated, or for a maximum of 10 days. The duration of the SSC and of standard contact sessions was 10 minutes per day Level of care: special care nursery of a hospital Human resources: doctors and nurses Criteria for infant discharge from the hospital: unreported Scheme for follow‐up of infants after discharge: at 1, 3, and 6 months after hospital discharge |
|
| Outcomes | Breastfeeding and physiological data | |
| Notes | No data on percentage of LBW infants who met eligibility criteria | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Shuffling of envelopes |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants: no/unfeasible; blinding of clinical staff: no/unfeasible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 21 mothers of 25 infants allocated to KMC group, and 20 mothers of 25 infants to standard contact group. One mother in the KMC group lost to follow‐up after discharge. Two mothers in the control group excluded because they wanted to participate in the KMC group |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in Methods section adequately reported or explained in Results |
| Other bias | Low risk | Other biases not identified |
Boo 2007.
| Methods | Randomized controlled trial carried out in Kebangsaan, Malaysia | |
| Participants |
Number of infants: 128 Inclusion criteria: very low‐birthweight infants (< 1501 g) in stable condition, nursed in a closed incubator, not requiring ventilatory support other than nasal continuous positive airway pressure, able to tolerate enteral feeds of ≥ 50% of required fluid volume, having ≥ 1 parent or guardian who was willing to participate in the study Exclusion criteria: lethal or major malformations, severe perinatal asphyxia, with evidence of hypoxic ischemic encephalopathy, transfer to another hospital, abandoned by parents, parental refusal to participate Infant stabilization status at trial entry: stabilized Infant age and weight at trial entry: Median age at recruitment was 24.5 and 20.5 days, and median weight was 1514 and 1492 g, for KMC and control infants, respectively |
|
| Interventions |
KMC group: Parent held the infant prone on naked chest, in a semi‐upright position, and between his/her breasts. Infants wore only a nappy and a bonnet. Both parent and infant were covered with a thermal blanket. Median duration of SSC was 1 hour per day with a mean total duration of 12.7 ± 5.0 days (n = 65) Control group: Infants were not exposed to SSC while in the NICU All mothers were encouraged to breastfeed their infants (n = 63) Level of care: NICU of a tertiary care hospital Human resources: doctors and nurses Criteria for infant discharge from the hospital: clinically well, able to tolerate oral feeds totally, weight gain ≥ 10 g/d, no apnea, bradycardia, and/or desaturation for ≥ 5 consecutive days Scheme for follow‐up of infants after discharge: unreported |
|
| Outcomes | Duration of hospital stay, weight gain, weekly increase in head circumference, breastfeeding rate at discharge, sepsis, mortality at discharge | |
| Notes | 43% of LBW infants met eligibility criteria | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Shuffling of envelopes |
| Allocation concealment (selection bias) | Low risk | Numbered sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants: no/unfeasible; blinding of clinical staff: no/unfeasible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unreported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 8 infants in the KMC group (12.3%) excluded because SSC sessions were carried out on < 50% of hospital stay days after recruitment |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in Methods section adequately reported or explained in Results |
| Other bias | Low risk | Other biases not identified |
Cattaneo 1998.
| Methods | Multicenter randomized controlled trial conducted in Addis Ababa (Ethiopia), Yogyakarta (Indonesia), and Merida (Mexico) | |
| Participants |
Number of infants: 285 Inclusion criteria: infants with birthweight between 1000 and 1999 g without gestational age limits, no dependency on oxygen and/or i.v. fluids, ability (at least partial) to feed, no visible major malformation, mother present and willing to collaborate Exclusion criteria: unreported Infant stabilization status at trial entry: stabilized Infant age and weight at trial entry: Median age (range) at recruitment was 10 (1 to 74) and 8 (1 to 40) days, and and mean weight (SD) was 1584 (223) and 1574 (251) g, for KMC and control infants, respectively |
|
| Interventions |
KMC group: Infants were kept in close and continuous SSC, between the mother's breasts, naked except for a diaper and a hat covered across their backs with their mother's clothes, day and night, for an average of about 20 hours/d, including when the mother was asleep. The mother was replaced occasionally, for a few hours, by another person, usually the father or a member of the family. For short absences of the mother (< 1 hour), the baby was left on the mother's bed, covered by a blanket (n = 149) Control group: Infants were kept in a warm room in Addis Ababa, with open cribs and the possibility of rewarming in a bulb‐heated cot, and in incubators in the other 2 hospitals. SSC with their mothers was not allowed (n = 136) Level of care: neonatal units of teaching hospitals Human resources: doctors and nurses Criteria for infant discharge from the hospital: weight ≥ 1500 g, clear upward growth trend (≥ 15 g/kg/d) and stable temperature for ≥ 3 days, satisfactory ability to suck, good general conditions, mother considered capable of good home care Scheme for follow‐up of infants after discharge: ≥ 4 times, at 3, 10, 20, and 30 days, and as usually scheduled at each hospital afterward |
|
| Outcomes | Severe illness, hypothermia, hyperthermia, breastfeeding, weight gain, neonatal death, acceptability to health workers, acceptability to mothers, costs | |
| Notes | 44% of LBW infants met eligibility criteria | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants: no/unfeasible; blinding of clinical staff: no/unfeasible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unreported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number of infants lost to follow‐up or excluded after randomization not reported |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in Methods section adequately reported or explained in Results |
| Other bias | Unclear risk | In Indonesia, randomization was carried out in blocks of 6 with stratification by weight, which could make prediction of future assignments possible in an unblinded trial when assignments are revealed subsequent to recruitment into the trial |
Charpak 1997.
| Methods | Randomized controlled trial carried out in Bogotá, Colombia | |
| Participants |
Number of infants: 777 Inclusion criteria: infants from singleton or multiple pregnancies with birthweights ≤ 2000 g, with a mother or a relative able to understand and willing to follow general program instructions. Infants were eligible when they had overcome major problems of adaptation to extrauterine life, had received proper treatment for infection or a concomitant condition, sucked and swallowed properly, and had achieved a positive weight gain Exclusion criteria: referred to another institution, plans to leave Bogotá in the near future, life‐threatening or major malformations, early detected major conditions arising from perinatal problems, parental or family refusal to comply with the follow‐up program; for those assigned to the KMC group, refusal to comply with specifics of the intervention Infant stabilization status at trial entry: stabilized Infant age and weight at trial entry: At recruitment, median age (range) was 4 (1 to 60) and 3 (1 to 55) days, and mean weight (SD) was 1678 (226) and 1715 (228) g, for KMC and control infants, respectively |
|
| Interventions |
KMC group: Infants were kept 24 hours a day in a strict upright position, in SSC, while firmly attached to the mother's chest. Infants were breastfed regularly, although premature formula supplements were administered if necessary (n = 396) Control group: Infants were kept in an incubator until they were able to regulate temperature and were thriving. Parents' access to their babies was severely restricted (n = 381) Level of care: pediatric hospital (KMC infants) and NICU of a tertiary care hospital (controls) Human resources: doctors and nurses Criteria for infant discharge from the hospital: (1) For infants in the KMC group: temperature regulated in the kangaroo position, adequate weight gain, completion of treatment, if any; ability to be fed by direct suction from the breast or expressed milk, adequate sucking‐swallowing‐breathing coordination, and ability of mother to care for her baby using the kangaroo method at home. Infants were discharged from the hospital regardless of their weight or gestational age. (2) For infants in the control group: weight ≥ 1700 g Scheme for follow‐up of infants after discharge: at least once a week until 40 weeks' postmenstrual age; then, monthly up to 3 months' corrected age, every 6 weeks until at least 6 months' corrected age, and every third month until 12 months' corrected age |
|
| Outcomes | At 40 to 41 weeks' postmenstrual: mortality, infant growth, length of hospital stay, infection, breastfeeding, and mother‐infant attachment At 12 months' corrected age: neurodevelopmental disability, and social and home environment |
|
| Notes | 72% of LBW infants met eligibility criteria. Informed consent was not asked of parents of infants allocated to the control group. Additional data provided by Dr Nathalie Charpak | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Unclear risk | Person managing allocation aware of weight at birth and whether the infant was a twin or a triplet |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants: no/unfeasible; blinding of clinical staff: no/unfeasible |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Infants allocated to KMC group were managed in a pediatric hospital, whereas infants allocated to control group remained in an NICU of a tertiary care hospital |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 31 infants (4%) (KMC 14, control 17) excluded after randomization owing to pre‐existing neurological impairment, or fetal intrauterine infection not detected at time of randomization. Follow‐up at 40 to 41 weeks' corrected gestational age incomplete for 67 (8.6%) survivor infants (KMC 33, control 34), but mortality data available for 30 of these, yielding mortality data for 364 vs 345 |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in Methods section adequately reported or explained in Results |
| Other bias | High risk | Use of blocked randomization (block size of 4), which could make possible prediction of future assignments in an unblinded trial when assignments are revealed subsequent to recruitment of the person into the trial |
Eka Pratiwi 2009.
| Methods | Randomized controlled trial conducted in Bali, Indonesia | |
| Participants |
Number of infants: 93 Inclusion criteria: infants with birthweight between 1500 and 2250 g, with Apgar score > 6 at 5 minutes, and mother willing to follow study instructions Exclusion criteria: infants with major congenital malformations, cardiopulmonary problems, critical illness (sepsis, necrotizing enterocolitis, intracranial bleeding); twin gestation or complicated pregnancy and/or labor; mothers with history of drug abuse, psychiatric disorders, or cesarean section, or unable to take care of themselves or their babies Infant stabilization status at trial entry: stabilized Infant age and weight at trial entry: Mean birthweight at recruitment was 2034 ± 159 and 1988 ± 176 g for KMC and control infants, respectively. No data on infant age at recruitment. However, researchers mentioned that KMC was started "in the first day or in several hours after birth" |
|
| Interventions |
KMC group: Infants were kept in close SSC with the mother whilst in vertical position. Specially tailored kangaroo suits were used by mother‐infant pairs to enable SSC. Mean duration of KMC was 10.0 ± 1.8 hours per day (range, 5.3 to 13.5 hours) (n = 48) Control group: Infants were kept in incubators or open cribs in warm rooms (n = 45) Level of care: NICU of a public hospital Human resources: doctors and nurses Criteria for infant discharge from the hospital: unreported Scheme for follow‐up of infants after discharge: unreported |
|
| Outcomes | Hypothermia, birthweight regain, sepsis, mortality | |
| Notes | 37% of LBW infants met eligibility criteria | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants: no/unfeasible; blinding of clinical staff: no/unfeasible |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unblinded assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One infant (1%) lost to follow‐up; 4 (4.1%) excluded after randomization owing to sepsis |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in Methods section adequately reported or explained in Results |
| Other bias | High risk | Use of block randomization (block size of 6), which could make possible prediction of future assignments in an unblinded trial when assignments are revealed subsequent to recruitment of the person into the trial |
Gathwala 2008.
| Methods | Randomized controlled trial carried out in Rohtak, India | |
| Participants |
Number of infants: 110 Inclusion criteria: infants with birthweight ≤ 1800 g, stable cardiopulmonary status, Apgar score ≥ 7 at 1 and 5 minutes, tolerating enteral feeds, and maintaining temperature Exclusion criteria: infants sick, unstable, or with major congenital malformations, or whose mothers were unwell and unable to come or refused consent Infant stabilization status at trial entry: stabilized Infant age and weight at trial entry: Mean age at recruitment was 1.7 ± 0.5 days, and mean birthweight was 1690 ± 110 and 1690 ± 120 g, for KMC and control infants, respectively. No data on infant weight at recruitment |
|
| Interventions |
KMC group: Infants were kept in SSC, between the mother's breasts, naked except for a cap and nappy, for ≥ 6 hours per day. Duration of KMC in the first month was 10.2 ± 1.5 hours per day, in the second month 10.0 ± 1.6, and in the third month 9.0 ± 1.4. The gown covered the baby's trunk and extremities, but not the head. KMC was given for a minimum of 1 hour at a stretch and was continued for as long as it was comfortable for baby and mother. When not receiving KMC, infants received standard care under a warmer or incubator. Infants continued to receive KMC after they were shifted to the mother in the ward (n = 50) Control group: Infants were kept in a warmer or incubator. Mothers were allowed to visit their babies and touch and handle them. Infants were shifted to the mother in her bed but did not receive KMC (n = 50) Level of care: neonatal unit of a public hospital Human resources: doctors and nurses Criteria for infant discharge from the hospital: unreported Scheme for follow‐up of infants after discharge: weekly until 3 months of age |
|
| Outcomes | Attachment between mother and infant at 3 months' follow‐up; duration of hospital stay; breastfeeding; weight, length and circumference head gain | |
| Notes | No data on percentage of LBW infants who met eligibility criteria | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants: no/unfeasible; blinding of clinical staff: no/unfeasible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unreported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 10 infants (9.1%) lost to follow‐up. Number of infants lost to follow‐up in each intervention group not reported. Of the remaining 100, 50 received KMC and 50 standard care |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in Methods section adequately reported or explained in Results |
| Other bias | Low risk | Other biases not identified |
Ghavane 2012.
| Methods | Randomized controlled trial conducted in Hyderabad, India | |
| Participants |
Number of infants: 140 Inclusion criteria: infants with birthweight < 1500 g, tolerating spoon feeds of 150 mL/kg/d, and hemodynamically stable (not receiving oxygen or respiratory support, no apnea for 72 hours, not receiving intravenous fluids) Exclusion criteria: major malformations, refused consent Infant stabilization status at trial entry: stabilized Infant age and weight at trial entry: Mean age at recruitment was 14.1 ± 10.3 and 13.7 ± 10.2 days, and mean weight was 1191 ± 131 and 1223 ± 125 g, for KMC and control infants, respectively |
|
| Interventions |
KMC group: Infants were kept in SSC, between the mother's breasts, in an upright position, dressed with a cap, socks, and diaper, and supported at the bottom with a cloth sling/binder, for ≥ 8 hours per day. When not receiving KMC, infants were placed in open cribs (n = 71) Control group: Infants were kept in a warmer or incubator. Mothers were allowed to visit their babies and were encouraged to perform infant care activities such as diaper change, oil massage, and paladai feeding (n = 69) Level of care: "kangaroo ward" (KMC infants) and neonatal intermediate care unit (controls) at a level III tertiary care hospital Human resources: Infants in KMC group were cared for solely by their mothers, assisted by a trained nurse. Infants in control group were cared for by doctors and nurses Criteria for infant discharge from the hospital: (1) For infants in KMC group: weight ≥ 1300 g or weight gain ≥ 10 g/d on 3 consecutive days if weight at randomization was > 1300 g. (2) For infants in control group: weight ≥ 1300 g, weight gain ≥ 10 g/d on 3 consecutive days, and skin temperature of 36°C to 37°C in the servo mode of the incubator with heater output < 25% Scheme for follow‐up of infants after discharge: weekly until 40 weeks' postmenstrual age |
|
| Outcomes | At 40 weeks' postmenstrual age: infant growth At discharge: breastfeeding, sepsis, hypothermia, apnea, hypoglycemia, length of hospital stay, mortality |
|
| Notes | No data on percentage of LBW infants who met eligibility criteria. Additional data provided by Dr Srinivas Murki | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Web‐based random number generator |
| Allocation concealment (selection bias) | Low risk | Numbered sealed opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants: no/unfeasible; blinding of clinical staff: no/unfeasible |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Physician who assessed growth outcomes was blinded to infants' intervention group |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4 infants (2.9%) lost to follow‐up (KMC 3, control 1); no exclusions |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in Methods section adequately reported or explained in Results |
| Other bias | Low risk | Other biases not identified |
Kadam 2005.
| Methods | Randomized controlled trial carried out in Mumbai, India | |
| Participants |
Number of infants: 89 Inclusion criteria: infants with birthweight ≤ 1800 g, stable cardiopulmonary status, Apgar score ≥ 7 at 5 minutes, and on feeds (breastfeeds or spoon wati feeds with expressed breast milk) Exclusion criteria: infants sick and unstable, or with major congenital malformations, or whose parents refused consent Infant stabilization status at trial entry: stabilized Infant age and weight at trial entry: Mean age (range) at enrollment was 3.2 (1 to 8) days for both groups. Mean birthweight was 1467 ± 228 and 1461 ± 217 g for KMC and control infants, respectively. No data on infant weight at recruitment |
|
| Interventions |
KMC group: Infants were placed on mother's chest in between the breasts in a vertical position, supported by a cloth dupatta, with mothers seated in a semi reclining position, for a mean of 9.8 ± 3.7 hours per day. In case of any problem, the baby was transferred to conventional care, and after stabilization was transferred back to KMC, which was continued till discharge (n = 44) Control group: Infants were kept in radiant warmers (n = 45) More than 95% of infants in both groups received exclusive breastfeeding; the remaining were supplemented by banked human milk. Mothers in both groups were allowed to enter and handle the babies at any hour of the day, change diapers, and breastfeed the babies Level of care: NICU of a tertiary care hospital Human resources: doctors and nurses Criteria for infant discharge from the hospital: weight gain for ≥ 3 consecutive days, maintenance of temperature without the need for a warmer, feeding well on breastfeeds or wati spoon‐feeds, and mother confident of taking care of the infant at home Scheme for follow‐up of infants after discharge: unreported |
|
| Outcomes | Mortality, morbidity (hypothermia, hyperthermia, sepsis, apnea), onset of breastfeeding, duration of hospital stay, weight at discharge | |
| Notes | No data on percentage of LBW infants who met eligibility criteria | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sealed envelope method |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants: no/unfeasible; blinding of clinical staff: no/unfeasible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unreported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No infants lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in Methods section adequately reported or explained in Results |
| Other bias | Low risk | Other biases not identified |
Kumbhojkar 2016.
| Methods | Randomized controlled trial conducted in Kolhapur, India | |
| Participants |
Number of infants: 120 Inclusion criteria: stable infants with birthweight < 2000 g Exclusion criteria: infants critically ill requiring ventilator support or inotropic support, or with chromosomal and life‐threatening congenital anomalies, or whose mother was critically ill or unable to comply with the follow‐up schedule Infant stabilization status at trial entry: stabilized Infant age and weight at trial entry: Mean age at recruitment was 3 and 4 days, and mean weight was 1610 ± 200 and 1627 ± 204 g, for KMC and control infants, respectively |
|
| Interventions |
KMC group: Infants were kept in SSC using a specially tailored "kangaroo bag" made of soft flannel cloth on the reclining cot in the semi upright position with the help of pillows. Mothers were encouraged to keep the baby in KMC as long as possible during the day and night for a minimum period of 1 to 2 hours at a time. When the baby was receiving intravenous fluids, the mother provided kangaroo care while seated in a comfortable chair placed close to the baby's cradle. Mean duration of KMC was 11.5 hours per day. No data on total number of days that KMC was given after enrollment (n = 60) Control group: Infants were managed under a servo‐controlled radiant warmer or in a cradle under hot lamp in NICU, adequately clothed and covered (n = 60) All babies were exclusively breastfed and also received calcium, phosphorus, and multivitamin supplements. Infants who developed a life‐threatening event or required phototherapy were temporarily withdrawn from the KMC group Level of care: NICU of a tertiary care hospital Human resources: doctors and nurses Criteria for infant discharge from the hospital: weight gain of 10 to 15 g/kg/d for ≥ 3 consecutive days, maintenance of temperature without assistance, feeding well, and mother confident of taking care of the infant at home Scheme for follow‐up of infants after discharge: weekly until 40 weeks' postmenstrual age in preterm infants, or until a weight of 2500 g was reached in term SGA infants. Home visits were not possible |
|
| Outcomes | Gain in weight, length, and head circumference; hospital stay; hypothermia; sepsis; apnea; acceptability of KMC; breastfeeding | |
| Notes | No data on percentage of LBW infants who met eligibility criteria | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Simple randomization |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants: no/unfeasible; blinding of clinical staff: no/unfeasible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unreported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No information on infants lost to follow‐up nor on exclusions. However, it was stated in the Discussion section that poor follow‐up in the control group was a limitation of this study |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in Methods section reported or explained in Results |
| Other bias | Low risk | Other biases not identified |
Nagai 2010.
| Methods | Randomized controlled trial conducted in Mahajanga, Madagascar | |
| Participants |
Number of infants: 73 Inclusion criteria: infants with birthweight < 2500 g, < 24 hours post birth, no serious malformation, relatively stable clinical condition (oxygen saturation ≥ 95%; heart rate > 100 beats/min; respiratory rate < 60 times/min; capillary refilling time < 3 seconds), and healthy mother and/or other family members willing to practice KMC Exclusion criteria: prolonged apnea (> 20 seconds) and intravenous infusion Infant stabilization status at trial entry: relatively stabilized Infant age and weight at trial entry: Mean age at recruitment was 19.8 ± 14.3 and 33.0 ± 13.2 hours, and mean weight was 2075 ± 272 and 2078 ± 292 g, for early‐onset KMC and late‐onset KMC infants, respectively |
|
| Interventions |
Early KMC group: Infants were kept in direct and continuous SSC (without underwear, except for a diaper, a warm hat, and socks for the baby) for as long as possible. SSC was begun as soon as possible, within 24 hours post birth (n = 37) Late KMC group: Initially, infants were kept in an incubator or radiant warmer. Later, infants were covered with cotton cloth and were laid beside their mothers. KMC was begun after complete stabilization (generally after 24 hours post birth) of infant (n = 36) After KMC was initiated, all participants were encouraged to continue KMC for as long as possible during hospitalization and after discharge. Other family members assisted the mother occasionally in performing continuous KMC Level of care: neonatal unit of a referral university hospital Human resources: doctors and nurses Criteria for infant discharge from the hospital: unreported Scheme for follow‐up of infants after discharge: at 14 and 28 days of age |
|
| Outcomes |
Primary outcome: mortality at 4 weeks of age Secondary outcomes: morbidity; severe infection; re‐admission to hospital; adverse events (hypothermia, hyperthermia, bradycardia and/or tachycardia, and prolonged apnea) at 4 weeks of age; body weight changes from birth to 24 hours, 48 hours, 14 days, and 28 days post birth; length of hospital stay; discharge within 7 days post birth; exclusive breastfeeding at 24 and 48 hours, 2 and 4 weeks, and 6 months post birth; mortality; re‐admission to hospital; nutritional indicators at 6 to 12 months of age |
|
| Notes | 52% of LBW infants met eligibility criteria | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Minimization method by software "minim" |
| Allocation concealment (selection bias) | Low risk | Software automatically provided random allocation for each participant |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants: no/unfeasible; blinding of clinical staff: no/unfeasible |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | A neonatologist who was masked to allocation of participants and had no contact with participants determined the classification of morbidities using interview records and medical charts |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No infants lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in Methods section adequately reported or explained in Results |
| Other bias | Low risk | Other biases not identified |
Neu 2010.
| Methods | Randomized controlled trial carried out in Aurora, Colorado, United States | |
| Participants |
Number of infants: 60 Inclusion criteria: healthy infants with gestational age between 32 and 34 weeks, oxygen requirement < ½ liter O2 per nasal cannula, infant without umbilical lines, intraventricular hemorrhage, physical anomalies or anticipated major surgery, mother fluent in English or Spanish without recorded or stated illicit drug use, or diagnosis of serious chronic illness Exclusion criteria: unreported Infant stabilization status at trial entry: stabilized Infant age and weight at trial entry: Mean age at recruitment was 15.0 ± 6.7 and 15.0 ± 4.9 days, and mean birthweight was 1990 ± 450 and 1880 ± 340 g, for KMC and control infants, respectively |
|
| Interventions |
KMC group: infant in SSC on mother's chest for 60 consecutive minutes at least once daily over 8 weeks (n = 31) Control group: infant wrapped in blanket and held in mother's arms for 60 consecutive minutes at least once daily over 8 weeks (n = 29) In both conditions, weekly home visits by an experienced registered nurse included encouragement to hold the infant, emotional support, and information about infant behavior and development. Other control group received brief social visits with no holding constraints and participated in all assessments. In the meta‐analysis, we excluded results from this last control group Level of care: initially at the hospital, then at home Human resources: nurses Criteria for infant discharge from the hospital: not applicable Scheme for follow‐up of infants after discharge: twice a week for 2 weeks, followed by weekly visits for 6 months |
|
| Outcomes | Mother‐infant interaction at 6 months' follow‐up and infant vitality during the neutral‐face period of the Still‐Face Procedure | |
| Notes | No data on percentage of LBW infants who met eligibility criteria. Approximately 60% of mothers who were approached declined to be included in the study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer random number generator |
| Allocation concealment (selection bias) | Low risk | Opaque sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants: no/unfeasible; blinding of clinical staff: no/unfeasible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Four researchers assessed outcome measures. Two outcome assessors were blinded to the hypotheses of the study but not to group assignment of mother‐infant dyads. The other 2 researchers were blinded to group assignment and hypotheses |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 87 infants were randomized: 31 to KMC, 29 to traditional holding, and 36 to control. At 6 months of age, 8 infants (9.2%) were lost to follow‐up and 14 (16.1%) were excluded (8 withdrawn for maternal reasons and 6 because of technical problems during videotaping) |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in Methods section adequately reported or explained in Results |
| Other bias | Low risk | Other biases not identified |
Nimbalkar 2014.
| Methods | Randomized controlled trial conducted in Karamsad, India | |
| Participants |
Number of infants: 100, of whom 45 were LBW Inclusion criteria: stable infants delivered vaginally with birthweight ≥ 1800 g Exclusion criteria: infants delivered by cesarean section or needing any resuscitation measures or with any congenital malformation at birth Infant stabilization status at trial entry: stabilized Infant age and weight at trial entry: Mean age was 43 ± 13 minutes in the KMC group and 30 to 60 minutes in the control group. Mean birthweight (and weight at recruitment) was 2622 ± 399 g and 2589 ± 443 g for KMC and control infants, respectively |
|
| Interventions |
KMC group: Mothers started SSC 30 minutes to 1 hour after delivery and continued for as long as possible in the first 24 hours, with each session lasting a minimum of 60 minutes. SSC was discontinued after 24 hours and conventional care was provided for next 24 hours of life. Mean duration of KMC was 17.0 ± 0.3 hours during first 24 hours (n = 22) Control group: Infants were kept clothed (including head cap) and covered with a blanket with their mother (bedding in) for first 48 hours (n = 23) In both groups, infants were taken under radiant warmers immediately after delivery and were exclusively breastfed Level of care: maternity ward of a tertiary care hospital Human resources: doctors and nurses Criteria for infant discharge from the hospital: unreported Scheme for follow‐up of infants after discharge: unreported |
|
| Outcomes | Hypothermia within first 48 hours of life | |
| Notes | 43% of infants met eligibility criteria. Results for the 45 LBW infants reported separately | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Web‐based software (WINPEPI) |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants: no/unfeasible; blinding of clinical staff: no/unfeasible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unreported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No infants lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in Methods section adequately reported or explained in Results |
| Other bias | Low risk | Other biases not identified |
Ramanathan 2001.
| Methods | Randomized controlled trial conducted in New Delhi, India | |
| Participants |
Number of infants: 28 Inclusion criteria: infants with birthweight < 1500 g, stable cardiopulmonary status, tolerating enteral feeds, and maintaining temperature in the thermoneutral environment Exclusion criteria: infants whose mothers were unable to come to the nursery because of illness or disability Infant stabilization status at trial entry: stabilized Infant age and weight at trial entry: Median age at initiation of KMC was 11.8 days. Mean birthweight was 1219 ± 186 and 1271 ± 170 g for KMC and control infants, respectively. No data on infant weight at recruitment |
|
| Interventions |
KMC group: Infants were kept between the mother's breasts for ≥ 4 hours per day in not more than 3 sittings. The gown covered the baby's trunk and extremities but not the head. When not receiving KMC, infants received standard care under a warmer or incubator (n = 14) Control group: Infants were kept in a warmer or incubator. Mothers were allowed to visit their babies and touch and handle them (n = 14) Breastfeeding guidelines were followed for both groups and lactational counseling was emphasized to ensure breast milk feeding Level of care: NICU of a tertiary care hospital Human resources: doctors and nurses Criteria for infant discharge from the hospital: weight > 1400 g, “adequate” weight gain, gestation over 34 weeks, only on enteral feeds, no intravenous medications, no overt illness, exclusive breastfeeding, and mother confident of taking care of the infant at home Scheme for follow‐up of infants after discharge: unreported |
|
| Outcomes | Weight gain, breastfeeding, duration of hospitalization | |
| Notes | No data on percentage of LBW infants who met eligibility criteria. Infants in KMC group required positive‐pressure ventilation, continuous positive airway pressure, and oxygen therapy over greater duration than infants in control group, indicating that these infants were sicker before enrollment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants: no/unfeasible; blinding of clinical staff: no/unfeasible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unreported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No infants lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Neonatal complications prospectively recorded but not reported |
| Other bias | Low risk | Other biases not identified |
Roberts 2000.
| Methods | Randomized controlled trial carried out in Darwin, Australia | |
| Participants |
Number of infants: 30 Inclusion criteria: premature or small for gestational age infants born at 30 or more weeks' gestation or corrected age, with 5‐minute Apgar of ≥ 5, medically stable, without congenital abnormalities or central nervous system impairment. Infants could have received nasal continuous positive airway pressure in place or a nasal cannula Exclusion criteria: phototherapy within previous 24 hours, resuscitated infants, mothers with a history of drug use Infant stabilization status at trial entry: stabilized Infant age and weight at trial entry: Mean age at recruitment was 31.5 ± 2.7 days and mean weight was 1690 ± 333 g, respectively |
|
| Interventions |
KMC group: Infants were dressed in only a diaper, with a bonnet added for smaller infants. They were placed on the mother's skin and covered with a light blanket. Mean duration of KMC was 1.6 ± 0.9 hours per day, 5 days a week (n = 16) Control group: Infants were swaddled in infant clothing and a light blanket. They had contact with the mother only through normal clothing (n = 14) Breastfeeding was permitted as desired in both groups Level of care: neonatal intensive care nurseries of 2 hospitals Human resources: doctors and nurses Criteria for infant discharge from the hospital: unreported Scheme for follow‐up of infants after discharge: at 6 weeks after discharge or at 3 months of age, whichever was later, and at 6 months of age |
|
| Outcomes | Weight gain, length of stay in hospital, temperature, breastfeeding | |
| Notes | No data on percentage of LBW infants who met eligibility criteria | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Shuffling envelopes |
| Allocation concealment (selection bias) | Low risk | Numbered envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants: no/unfeasible; blinding of clinical staff: no/unfeasible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unreported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No infants lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in Methods section adequately reported or explained in Results |
| Other bias | Low risk | Other biases not identified |
Rojas 2003.
| Methods | Randomized controlled trial conducted in Connecticut, United States | |
| Participants |
Number of infants: 60 Inclusion criteria: very low birthweight infants (< 1501 g) with gestational age ≤ 32 weeks, with minimal ventilatory support or extubated on nasal continuous positive airway pressure or nasal canula, with hemodynamic stability Exclusion criteria: mother's age < 18 years, history of illicit drug use during pregnancy, clinical evidence of perinatal asphyxia, potential transfer within the first month after birth, presence of a major congenital anomaly, planned adoption, grade III or IV intraventricular hemorrhage, fetal growth restriction, suspected sepsis Infant stabilization status at trial entry: stabilized Infant age and weight at trial entry: Mean age at trial entry was 19 days, and mean weight was 1021 ± 268 g and 1002 ± 219 g for KMC and control infants, respectively |
|
| Interventions |
KMC group: Infants were held in a prone semi upright position at approximately a 45° angle, in direct SSC with the parent's chest. Infants wore only a diaper, and their backs were covered with a blanket. Mean duration of KMC was 1.3 ± 0.7 hours per day for an average of 15 ± 16 days (n = 33) Control group: Parents removed their infants from the incubator and held them in their arms in supine position with eye‐to‐eye contact. Infants wore diapers and T‐shirts and were wrapped in a blanket (n = 27) Level of care: NICU of a hospital Human resources: doctors and nurses Criteria for infant discharge from the hospital: unreported Scheme for follow‐up of infants after discharge: not performed |
|
| Outcomes | Mortality at discharge; sepsis; necrotizing enterocolitis; intraventricular hemorrhage; weight, head circumference, and length at discharge; rate of weight gain and head circumference growth; total weight gain and head circumference growth; breastfeeding at discharge; hospital stay | |
| Notes | 19% of LBW infants met eligibility criteria | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Low risk | Numbered sealed opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants: no/unfeasible; blinding of clinical staff: no/unfeasible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unreported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No infants lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in Methods section adequately reported or explained in Results |
| Other bias | Low risk | Other biases not identified |
Sloan 1994.
| Methods | Randomized controlled trial carried out in Quito, Ecuador | |
| Participants |
Number of infants: 300 Inclusion criteria: singleton infants weighing < 2000 g, with no serious congenital abnormalities or respiratory, metabolic, or infectious disease. Infants had to be stabilized for 24 hours before enrollment (temperature between 36.5°C and 37.0°C); acceptable tolerance of food; stable weight Exclusion criteria: unreported Infant stabilization status at trial entry: stabilized Infant age and weight at trial entry: Mean age at recruitment was 13.0 ± 10.5 days, and mean weight was 1618 ± 317 g, respectively |
|
| Interventions |
KMC group: Infants were kept in an upright position, in SSC contact (diapers allowed) against the mother's breasts, and had frequent breastfeeding. SSC was reported by 68% of mothers at follow‐up of 1 month, 47% at 1.5 months, 20% at 2 months, and 7% at 3 months (n = 140) Control group: Infants stayed in an incubator or thermal crib and were breastfed at scheduled times (n = 160) Level of care: NICU of a maternity hospital Human resources: doctors and nurses Criteria for infant discharge from the hospital: unreported Scheme for follow‐up of infants after discharge: at 1, 1.5, 2, 3, 4, 5, and 6 months of age |
|
| Outcomes | Severe illness (lower respiratory tract disorder, apnea, aspiration, pneumonia, septicemia, general infection), moderate illness (urinary infection), mild illness (upper respiratory tract disorder, dermatitis, jaundice, hip displacement), diarrhea, infant growth (weight, length, upper arm and head circumference), duration of hospital stay, re‐admission, costs of care | |
| Notes | 53% of LBW infants met eligibility criteria. Additional data provided by Dr Nancy L. Sloan | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants: no/unfeasible; blinding of clinical staff: no/unfeasible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unreported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data reported for 131 KMC infants and 152 controls. 17 infants (5.7%) lost to follow‐up (KMC 9, control 8); no exclusions |
| Selective reporting (reporting bias) | Unclear risk | Secondary outcomes such as infant growth indices at follow‐up and costs of care were mentioned but were not reported adequately |
| Other bias | Unclear risk | Trial was stopped early because a highly significant difference (P value < 0.02 at 2 months, P value < 0.005 at 6 months) in severe morbidity arose. No information about whether this was a planned interim analysis |
Suman 2008.
| Methods | Randomized controlled trial conducted in Mumbai, India | |
| Participants |
Number of infants: 220 Inclusion criteria: singleton infants with birthweight < 2000 g Exclusion criteria: infants critically ill requiring ventilatory or inotropic support, or with chromosomal and life‐threatening congenital anomalies, or requiring transfer, or whose mothers were critically ill or unable to comply with the follow‐up schedule Infant stabilization status at trial entry: stabilized Infant age and weight at trial entry: Mean age at recruitment was 3.7 ± 2.8 and 2.3 ± 1.9 days, and mean weight was 1608 ± 278 and 1691 ± 273 g, for KMC and control infants, respectively |
|
| Interventions |
KMC group: Infants were kept in SSC using a specially tailored "kangaroo bag" made of soft flannel cloth on the reclining cot in the semi upright position with the help of pillows. Mothers were encouraged to keep the baby in KMC as long as possible during the day and night, for a minimum period of 1 to 2 hours at a time. When not in KMC, the baby was placed under a servo‐controlled radiant warmer or in a cradle under a hot lamp adequately clothed and covered. Mean duration of KMC was 13.5 hours per day, with a mean total duration of 33.8 ± 15.1 days (n = 108) Control group: Infants were managed under a servo‐controlled radiant warmer or in a cradle under a hot lamp in the NICU adequately clothed and covered (n = 112) All babies were exclusively breastfed. Infants who developed a life‐threatening event or required phototherapy were temporarily withdrawn from the KMC group Level of care: NICU of a tertiary care hospital Human resources: doctors and nurses Criteria for infant discharge from the hospital: weight gain of 10 to 15 g/kg/d for ≥ 3 consecutive days, maintenance of temperature without assistance, feeding well, and mother confident of taking care of the infant at home Scheme for follow‐up of infants after discharge: weekly until 40 weeks' postmenstrual age in preterm infants, or until a weight of 2500 g was reached in term SGA infants |
|
| Outcomes | Infant growth (weight, length, head, chest, mid‐arm circumference, and foot length), mortality, morbidity (hypothermia, hyperthermia, hypoglycemia, sepsis, apnea in < 1500 g, other minor illness), duration of hospital stay | |
| Notes | 63% of LBW infants met eligibility criteria | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Simple randomization |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants: no/unfeasible; blinding of clinical staff: no/unfeasible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unreported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 49 infants (22.3%) lost to follow‐up (KMC 11 [10.2%], control 38 [33.9%]); 14 babies (6.4%) were excluded (KMC 5, control 9) because they did not receive assigned care |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in Methods section adequately reported or explained in Results |
| Other bias | Unclear risk | Groups were different at baseline in 2 important variables: (1) weight at enrollment (1608 ± 278 g and 1691 ± 273 g for KMC and control infants, respectively; P value = 0.03), and (2) age at enrollment (3.7 ± 2.8 days and 2.3 ± 1.9 days for KMC and control infants, respectively; P value < 0.01) |
Whitelaw 1988.
| Methods | Randomized controlled trial carried out in London, United Kingdom | |
| Participants |
Number of infants: 71 Inclusion criteria: infants from singleton or twin pregnancy with weight < 1500 g, stable breathing with no oxygen requirement, and ≥ 1 parent speaking fluent English. Stable infants were not excluded if they had congenital malformations such as hydronephrosis or scoliosis, nor if they had intracranial lesions such as periventricular leukomalacia or ventricular dilatation Exclusion criteria: unreported Infant stabilization status at trial entry: stabilized Infant age and weight at trial entry: Mean (range) age at enrollment was 16 (1 to 66) days. Mean birthweight was 1152 ± 220 g and 1135 ± 263 g for KMC and control infants, respectively. No data on infant weight at recruitment |
|
| Interventions |
KMC group: Infants were kept in an upright position, in SSC between the mother's breasts, with a cardiac or respiration monitor attached. Mean (range) duration of KMC was 0.6 (0 to 1.5) hours per day (n = 35) Control group: Mother was encouraged to visit as much as she liked and helped to take her baby out of the incubator for a cuddle. However, baby and mother remained clothed Care was taken that the normal contact group would receive no less attention from the nursing staff (n = 36) Level of care: NICU of a hospital Human resources: doctors and nurses Criteria for infant discharge from the hospital: unreported Scheme for follow up of infants after discharge: at 6, 9, and 12 months of age |
|
| Outcomes | Breastfeeding and infant's behavior at 6 months of age, mother's feelings about the infant at discharge and at 6 months of age | |
| Notes | 50% of LBW infants met eligibility criteria | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Shuffling of envelopes |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered sealed opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants: no/unfeasible; blinding of clinical staff: no/unfeasible |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No infants lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Non‐significant results for some outcome measures (eg, mother's feelings about the infant at discharge and at 6 months' follow‐up) were mentioned but were not reported adequately |
| Other bias | Low risk | Other biases not identified |
Worku 2005.
| Methods | Randomized controlled trial carried out in Addis Ababa, Ethiopia | |
| Participants |
Number of infants: 123 Inclusion criteria: infants with birthweight < 2000 g, singletons unless 1 of the twins died, no major congenital malformations, and mother healthy and willing to participate Exclusion criteria: unreported Infant stabilization status at trial entry: non‐stabilized Infant age and weight at trial entry: Mean age at enrollment was 10.0 and 9.8 hours, and mean birthweight was 1515 g and 1472 g for KMC and control infants, respectively |
|
| Interventions |
Early KMC group: Infants were kept in continuous SSC with their mother beginning immediately after birth or within the first 24 hours of life (before stabilization). The mother kept her newborn infant between the breasts, in close contact with her body and covered with her clothes day and night. Breastfeeding was the standard feeding method. However, the mother could also feed her baby with formula milk using tube or cup when needed. KMC could be combined with a heated room during low environmental temperatures (n = 62) Control group: Infants were kept in a heated room with overhead lamp warmers and received oxygen therapy and breast, tube, cup, or mixed feeding (n = 61) The 2 methods of care were applied and continued until the baby was considered stabilized (stable temperature, stabilized cardiovascular status, satisfactory ability to suck, and good general condition); then both group of babies were transferred to the ward for routine kangaroo care service. KMC was continued at home after discharge in both groups Level of care: neonatal unit of a teaching hospital Human resources: doctors and nurses Criteria for infant discharge from the hospital: (1) for discharge from the study to the ward routine kangaroo care service: stable temperature, stabilized cardiovascular status, satisfactory ability to suck, and good general condition; (2) for discharge from the hospital: "according to the hospital's protocol" Scheme for follow‐up of infants after discharge: unreported |
|
| Outcomes | Death, serious illness (sepsis, diarrhea, pneumonia, aspiration, pneumonia), mothers' feeling about the method of care | |
| Notes | 48% of LBW infants met eligibility criteria | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants: no/unfeasible; blinding of clinical staff: no/unfeasible |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information on infants lost to follow‐up; no exclusions |
| Selective reporting (reporting bias) | High risk | Great majority of outcomes listed in Methods section of the article, such as weight gain, mild/moderate and severe illness, sepsis, diarrhea, pneumonia, aspiration, and mother's feelings, collected but not reported |
| Other bias | Low risk | Other biases not identified |
KMC = kangaroo mother care LBW = low birthweight SSC = skin‐to‐skin contact SGA = small for gestational age
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ahn 2010 | Not a randomized controlled trial |
| Anderson 2003 | Study compared SSC (N = 48) and standard care (N = 43) in preterm infants born at 32 to 36 weeks' gestation with birthweight between 1300 and 3000 g. No data on daily duration of KMC. Study did not report results for clinical outcomes |
| Arandia 1993 | Not a randomized controlled trial |
| Badiee 2014 | Study assessed effect of KMC (N = 25) vs standard care (N = 25) on mental health of mothers of LBW infants in the postpartum period. No data on neonatal morbidity and mortality |
| Bera 2014 | Not a randomized controlled trial |
| Bergman 1994 | Not a randomized controlled trial |
| Bergman 2004 | Study compared SSC (N = 21) from birth and standard care (N = 14) in LBW infants. Study period was 6 hours. Study reported results only for physiological parameters. Newborns receiving SSC from birth were significantly advantaged in some measures of cardiorespiratory stability |
| Broughton 2013 | Not a randomized controlled trial |
| Charpak 1994 | Not a randomized controlled trial |
| Chiu 2009 | Study compared early KMC (N = 52) and standard care (N = 48) in late preterm infants (32 to < 37 weeks' gestation). Study included infants with birthweight ≥ 2500 g. No data for subgroup of infants < 2500 g at birth. KMC infants had lower infant teaching scores at 6 months than controls ‐ a difference that disappeared thereafter. Feeding scores at 6 and 12 months' follow‐up were similar for KMC infants and controls |
| Christensson 1998 | Study compared SSC and incubator care for rewarming in 80 low‐risk hypothermic infants (clinically stable with admission weight ≥ 1500 g) |
| Chwo 2002 | Study compared SSC (N = 17) and standard contact (N = 17) in infants born at 34 to 36 weeks' gestation. 20 of 34 included infants (59%) had birthweight > 2500 g. No data for the remaining 14 LBW infants |
| Dala Sierra 1994 | Not a randomized controlled trial |
| Darmstadt 2006 | Study evaluated acceptance of KMC within a trial of impact of a package of essential newborn care |
| de Almeida 2010 | Not a randomized controlled trial |
| de Macedo 2007 | Not a randomized controlled trial |
| Dehghani 2015 | Study compared SSC (N = 27) and standard care (N = 26) in infants hospitalized in the NICU, and reported results only for physiological parameters. Newborns receiving SSC had a significant increase in average temperature and arterial oxygen saturation rate |
| Feldman 2002 | Not a randomized controlled trial |
| Gregson 2011 | Not a randomized controlled trial |
| Hake Brooks 2008 | Study compared KMC (N = 36) and standard care (N = 30) in preterm infants. Study included infants with birthweight of 1300 to 3000 g. 39% of included infants had a gestational age of 36 weeks. No data for subgroup of infants < 2500 g at birth. KMC was associated with a significantly longer breastfeeding duration and a higher frequency of exclusive breastfeeding at discharge and at 1.5, 3, and 6 months |
| Huang 2006 | Study compared early KMC (N = 39) and use of radiant warmers (N = 39) in term infants with hypothermia problems. Mean (SD) birthweight was 3072 (393) and 2808 (428) g for KMC and control infants, respectively. After 4 hours, more infants in the KMC group had reached normal body temperature |
| Ibe 2004 | Not a randomized controlled trial |
| Kambarami 1998 | Quasi‐random allocation to treatment (alternation). 74 (37 per group) infants were subjected to KMC or incubator care. Infants in the KMC group had higher mean daily weight gain, shorter stay in hospital, and better survival rates |
| Karimi 2014 | 72 infants born between 32 and 42 weeks' gestation were randomly assigned to KMC or routine care. Study included infants with birthweight > 2500 g and reported results only for breastfeeding self efficacy score at 3 months post partum. No data for subgroup of infants ≤ 2500 g at birth |
| Kashaninia 2015 | Not a randomized controlled trial |
| Kristoffersen 2016 | Not a randomized controlled trial |
| Kumar 2008 | Cluster‐randomized controlled trial in which SSC was part of a preventive package of interventions for essential newborn care |
| Lai 2006 | Study compared music during KMC (N = 15) and standard care (N = 15) in preterm infants. Study included infants with birthweight of 1505 to 3285 g. No data for subgroup of infants < 2500 g at birth. In addition, the study did not report results for clinical outcomes |
| Lamy Filho 2008 | Not a randomized controlled trial |
| Lamy Filho 2015 | Study compared SSC (N = 53) and no intervention (N = 49) in LBW infants hospitalized in NICU whose nostrils were colonized with methicillin‐oxacillin‐resistant Staphylococcus aureus or methicillin‐oxacillin‐resistant coagulase‐negative Staphylococcus aureus. Study reported results only for colonization status of newborns’ nostrils after 7 days of intervention |
| Legault 1993 | Participant allocation was by a cross‐over recruitment design. Study did not report results for clinical outcomes |
| Legault 1995 | Not a randomized controlled trial |
| Lincetto 2000 | Not a randomized controlled trial |
| Lizarazo‐Medina 2012 | Not a randomized controlled trial |
| Ludington‐Hoe 1991 | Randomized controlled trial that compared KMC and standard care in cardiorespiratory, thermal, and state effects in preterm infants. No data on neonatal morbidity and mortality |
| Ludington‐Hoe 2000 | Randomized controlled trial that compared KMC (N = 16) and standard care (N = 13) in maintenance of body warmth in preterm infants. No data on neonatal morbidity and mortality |
| Ludington‐Hoe 2004 | Randomized controlled trial that compared KMC (N = 11) and standard care (N = 13) for assessment of cardiorespiratory and thermal responses in preterm infants. No data on neonatal morbidity and mortality |
| Ludington‐Hoe 2006 | Randomized controlled trial that compared KMC (N = 14) and standard care (N = 14) for assessment of neonatal sleep organization in preterm infants. No data on neonatal morbidity and mortality |
| Lyngstad 2014 | Randomized controlled trial with a cross‐over design (N = 19), which assessed SSC for reducing stress of preterm infants during diaper change |
| Miles 2006 | Study was a pragmatic, controlled trial in which participant allocation was by a cross‐over, cluster recruitment design between 2 tertiary referral NICUs. Each hospital remained in KMC or control group for 4 months, then crossed over following a washout phase, during which no recruitment was undertaken. No significant difference was found in any infant or maternal measure at any time point |
| Miltersteiner 2005 | Quasi‐random allocation to treatment (even or odd number). Length of hospital stay was 8 ± 1 days for the KMC group and 10 ± 1.9 days for the control group (P value = 0.004) |
| Mitchell 2013 | 38 infants (27 to 30 weeks' gestational age) were randomly assigned to 2 hours of KMC daily between days of life 5 and 10, or to standard incubator care. Study reported results only for physiological parameters. Infants allocated to KMC had significantly fewer events of bradycardia and oxygen desaturation than infants allocated to standard care |
| Mörelius 2015 | Study compared SSC (N = 18) and standard care (N = 19) in preterm infants (32 to 36 weeks' gestation). Study included infants with birthweight ≥ 2500 g. No data for subgroup of infants < 2500 g at birth. Overall, SSC decreased infants' cortisol reactivity in response to handling, improved concordance between mothers' and infants' salivary cortisol levels, and decreased fathers' experiences of spouse relationship problems |
| Ohgi 2002 | Not a randomized controlled trial |
| Samra 2015 | Randomized controlled trial that assessed effects of skin‐to‐skin holding (N = 20) versus blanket holding (N = 20) on stress of mothers of late preterm infants (34 to 36 weeks' gestation). Study included infants with birthweight ≥ 2500 g. No data for subgroup of infants < 2500 g at birth. Overall, no significant differences in stress scores between study groups |
| Silva 2016 | Not a randomized controlled trial |
| Sloan 2008 | Randomized controlled cluster trial in which 4165 infants were assigned to community‐based KMC or control. 40% overall and 65% of newborns who died were not weighed at birth, and missing birthweight was differential for study group. 68.6% of weighed infants had a birthweight ≥ 2500 g. No difference in overall neonatal mortality rate nor infant mortality rate |
| Swarnkar 2016 | Quasi‐random allocation (alternation) to KMC (N = 30) or conventional care (N = 30). Infants in KMC group had greater weight, length, and head circumference gain, and decreased risk of hypothermia compared with infants in the control group |
| Tallandini 2006 | Not a randomized controlled trial |
| Udani 2008 | Published as abstract only. Insufficient information to include this study in the systematic review, and unsuccessful attempts to locate full publication or to contact study author |
KMC = kangaroo mother care LBW = low birthweight SSC = skin‐to‐skin contact SGA = small for gestational age
Characteristics of studies awaiting assessment [ordered by study ID]
Holditch‐Davis 2014.
| Methods | Randomized controlled trial carried out in North Carolina and Illinois, United States |
| Participants |
Number of infants: 162 Inclusion criteria: non‐critically ill preterm infants with birthweight < 1750 g Exclusion criteria: infants with congenital neurological problems (eg, congenital hydrocephalus, Down syndrome), mothers who had symptoms of substance exposure or who did not have custody of the infant or who had a risk factor that could affect their ability to administer the intervention (eg, age < 15 years; history of psychosis or bipolar disease; current diagnosis of major depression; non‐English speaking); follow‐up for 12 months unlikely Infant stabilization status at trial entry: stabilized |
| Interventions |
KMC group: Infants were kept in SSC in an upright position between the mother's breasts, dressed with a diaper and a hat. Mothers were instructed to perform the intervention at least once a day, 3 times a week, and for ≥ 15 minutes during infant hospitalization, and to continue at home until the infant was 2 months' corrected age (n = 81) Control group: Mothers spent a similar amount of time each week as KMC mothers with the study nurse, discussing how to select and locate safe equipment needed to care for preterm infants at home, for example, clothes, diapers, formula, and toys. Holding was not part of the control group intervention (n = 81) Level of care: initially at the NICU, then at home Human resources: nurses |
| Outcomes | Mother‐infant relationship, maternal psychological distress, social and home environment, mother's satisfaction |
| Notes | This study examined effects of KMC vs massage with auditory, tactile, visual, and vestibular (ATVV) stimulation vs an attention control group. If included in the review, we would exclude results of the ATVV intervention group |
KMC = kangaroo mother care LBW = low birthweight SSC = skin‐to‐skin contact SGA = small for gestational age
Differences between protocol and review
We have updated the Background and Methods sections. After the protocol was published, a new version of the Cochrane Handbook for Systematic Reviews of Interventions recommended a new approach to assess risk of bias. We changed our method of assessment to be consistent with these recommendations. We decided to group studies into continuous KMC and intermittent KMC after looking at variation in the interventions. We changed the labels for most primary and secondary outcomes and performed several new subgroup and sensitivity analyses. In the latest version of this review, we have included studies that evaluated KMC before stabilization, intermittent KMC, and early‐onset KMC.
In this updated review, we have added the method and plan for 'Summary of findings' tables and GRADE (Grades of Recommendation, Assessment, Development and Evaluation Working Group) recommendations; these were not included in the original protocol.
Contributions of authors
The original review was carried out by Agustin Conde‐Agudelo, Jose L. Diaz‐Rossello, and Jose M. Belizán (Conde‐Agudelo 2000). The same review authors updated the review in 2003 (Conde‐Agudelo 2003) and 2011 (Conde‐Agudelo 2011). Agustin Conde‐Agudelo and Jose L. Diaz‐Rossello updated the review in 2014 (Conde‐Agudelo 2014). For this update, Dr Agustin Conde‐Agudelo conducted all statistical analyses, wrote the first draft of the review, and revised subsequent drafts in response to feedback. Dr Jose L. Diaz‐Rossello commented on the first draft of the updated review and contributed to the writing of the final draft.
Sources of support
Internal sources
(AC‐A) Perinatology Research Branch, Eunice Kennedy Shriver National Institute of Child Health and Human Development/National Institutes of Health/Department of Health and Human Services, Bethesda, MD, and Detroit, MI, and Department of Obstetrics and Gynecology, Wayne State University, Detroit, MI, USA.
(JLD‐R) Departamento de Neonatología del Hospital de Clínicas, Universidad de la República, Montevideo, Uruguay.
External sources
-
Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services, USA.
Editorial support of the Cochrane Neonatal Review Group has been funded with Federal funds from the Eunice Kennedy Shriver National Institute of Child Health and Human Development National Institutes of Health, Department of Health and Human Services, USA, under Contract No. HHSN275201100016C
-
National Institute for Health Research, UK.
Editorial support for Cochrane Neonatal has been funded with funds from a UK National Institute of Health Research Grant (NIHR) Cochrane Programme Grant (13/89/12). The views expressed in this publication are those of the authors and not necessarily those of the NHS, the NIHR, or the UK Department of Health.
Declarations of interest
None.
Edited (no change to conclusions)
References
References to studies included in this review
Acharya 2014 {published data only}
- Acharya N, Singh RR, Bhatta NK, Poudel P. Randomized control trial of Kangaroo Mother Care in low birth weight babies at a tertiary level hospital. Journal of Nepal Paediatric Society 2014;34(1):18‐23. [Google Scholar]
Ali 2009 {published data only}
- Ali SM, Sharma J, Sharma R, Alam S. Kangaroo mother care as compared to conventional care for low birth weight babies. Dicle Medical Journal 2009;36(3):155‐60. [Google Scholar]
Blaymore Bier 1996 {published data only}
- Blaymore Bier JA, Ferguson AE, Morales Y, Liebling JA, Archer D, Oh W, et al. Comparison of skin‐to‐skin contact with standard contact in low‐birth‐weight infants who are breast‐fed. Archives of Pediatrics & Adolescent Medicine 1996;150(12):1265‐9. [DOI] [PubMed] [Google Scholar]
Boo 2007 {published data only}
- Boo NY, Jamli FM. Short duration of skin‐to‐skin contact: effects on growth and breastfeeding. Journal of Paediatrics and Child Health 2007;43(12):831‐6. [DOI] [PubMed] [Google Scholar]
Cattaneo 1998 {published data only}
- Cattaneo A, Davanzo R, Worku B, Surjono A, Echeverria M, Bedri A, et al. Kangaroo mother care for low birthweight infants: a randomized controlled trial in different settings. Acta Paediatrica 1998;87(9):976‐85. [DOI] [PubMed] [Google Scholar]
Charpak 1997 {published data only}
- Charpak N, Ruiz‐Pelaez JG, Figueroa de CZ, Charpak Y. A randomized, controlled trial of kangaroo mother care: results of follow‐up at 1 year of corrected age. Pediatrics 2001;108(5):1072‐9. [DOI] [PubMed] [Google Scholar]
- Charpak N, Ruiz‐Peláez JG, Figueroa de CZ, Charpak Y. Kangaroo mother versus traditional care for newborn infants ≤2000 grams: a randomized, controlled trial. Pediatrics 1997;100(4):682‐8. [DOI] [PubMed] [Google Scholar]
- Tessier R, Charpak N, Giron M, Cristo M, Calume ZF, Ruiz‐Peláez JG. Kangaroo Mother Care, home environment and father involvement in the first year of life: a randomized controlled study. Acta Paediatrica 2009;98(9):1444‐50. [DOI] [PubMed] [Google Scholar]
- Tessier R, Cristo M, Velez S, Giron M, Calume ZF, Ruiz‐Palaez JG, et al. Kangaroo mother care and the bonding hypothesis. Pediatrics 1998;102(2):e17. [DOI] [PubMed] [Google Scholar]
- Tessier R, Cristo MB, Velez S, Giron M, Nadeau L, Figueroa Z, et al. Kangaroo mother care: a method for protecting high‐risk low birth weight and premature infants against developmental delay. Infant Behavior and Development 2003;26:384‐97. [Google Scholar]
Eka Pratiwi 2009 {published data only}
- Eka Pratiwi IGAP, Soetjiningsih, Made Kardane I. Effect of kangaroo method on the risk of hypothermia and duration of birth weight regain in low birth weight infants: a randomized controlled trial. Paediatrica Indonesiana 2009;49(5):253‐8. [Google Scholar]
Gathwala 2008 {published data only}
- Gathwala G, Singh B, Balhara B. KMC facilitates mother baby attachment in low birth weight infants. Indian Journal of Pediatrics 2008;75(1):43‐7. [DOI] [PubMed] [Google Scholar]
- Gathwala G, Singh B, Singh J. Effect of Kangaroo Mother Care on physical growth, breastfeeding and its acceptability. Tropical Doctor 2010;40(4):199‐202. [DOI] [PubMed] [Google Scholar]
Ghavane 2012 {published data only}
- Ghavane S, Murki S, Subramanian S, Gaddam P, Kandraju H, Thumalla S. Kangaroo Mother Care in Kangaroo ward for improving the growth and breastfeeding outcomes when reaching term gestational age in very low birth weight infants. Acta Paediatrica 2012;101(12):e545‐9. [DOI] [PubMed] [Google Scholar]
Kadam 2005 {published data only}
- Kadam S, Binoy S, Kanbur W, Mondkar JA, Fernandez A. Feasibility of kangaroo mother care in Mumbai. Indian Journal of Pediatrics 2005;72(1):35‐8. [DOI] [PubMed] [Google Scholar]
Kumbhojkar 2016 {published data only}
- Kumbhojkar S, Mokase Y, Sarawade S. Kangaroo mother care (KMC): an alternative to conventional method of care for low birth weight babies. International Journal of Health Sciences and Research 2016;6(3):36‐42. [Google Scholar]
Nagai 2010 {published data only}
- Nagai S, Andrianarimanana D, Rabesandratana N, Yonemoto N, Nakayama T, Mori R. Earlier versus later continuous Kangaroo Mother Care (KMC) for stable low‐birth‐weight infants: a randomized controlled trial. Acta Paediatrica 2010;99(6):827‐35. [DOI] [PubMed] [Google Scholar]
- Nagai S, Yonemoto N, Rabesandratana N, Andrianarimanana D, Nakayama T, Mori R. Long‐term effects of earlier initiated continuous Kangaroo Mother Care (KMC) for low‐birth‐weight (LBW) infants in Madagascar. Acta Paediatrica 2011;100(12):e241‐7. [DOI] [PubMed] [Google Scholar]
Neu 2010 {published data only}
- Neu M, Robinson J. Maternal holding of preterm infants during the early weeks after birth and dyad interaction at six months. Journal of Obstetric, Gynecologic and Neonatal Nursing 2010;39(4):401‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu M, Robinson J, Schmiege SJ. Influence of holding practice on preterm infant development. MCN. American Journal of Maternal Child Nursing 2013;83(3):136‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nimbalkar 2014 {published data only}
- Nimbalkar SM, Patel VK, Patel DV, Nimbalkar AS, Sethi A, Phatak A. Effect of early skin‐to‐skin contact following normal delivery on incidence of hypothermia in neonates more than 1800 g: randomized control trial. Journal of Perinatology 2014;34(5):364‐8. [DOI] [PubMed] [Google Scholar]
Ramanathan 2001 {published data only}
- Ramanathan K, Paul VK, Deorari AK, Taneja U, George G. Kangaroo Mother Care in very low birth weight infants. Indian Journal of Pediatrics 2001;68(11):1019‐23. [DOI] [PubMed] [Google Scholar]
Roberts 2000 {published data only}
- Roberts KL, Paynter C, McEwan B. A comparison of kangaroo mother care and conventional cuddling care. Neonatal Network 2000;19(4):31‐5. [DOI] [PubMed] [Google Scholar]
Rojas 2003 {published data only}
- Rojas MA, Kaplan M, Quevedo M, Sherwonit E, Foster LB, Ehrenkranz RA, et al. Somatic growth of preterm infants during skin‐to‐skin care versus traditional holding: a randomized, controlled trial. Journal of Developmental and Behavioral Pediatrics 2003;24(3):163‐8. [DOI] [PubMed] [Google Scholar]
Sloan 1994 {published data only}
- Sloan NL, Camacho LW, Rojas EP, Stern C. Kangaroo mother method: randomised controlled trial of an alternative method of care for stabilised low‐birthweight infants. Lancet 1994;344(8925):782‐5. [DOI] [PubMed] [Google Scholar]
Suman 2008 {published data only}
- Suman RP, Udani R, Nanavati R. Kangaroo mother care for low birth weight infants: a randomized controlled trial. Indian Pediatrics 2008;45(1):17‐23. [PubMed] [Google Scholar]
Whitelaw 1988 {published data only}
- Whitelaw A, Heisterkamp G, Sleath K, Acolet D, Richards M. Skin to skin contact for very low birthweight infants and their mothers. Archives of Disease in Childhood 1988;63(11):1377‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Worku 2005 {published data only}
- Worku B, Kassie A. Kangaroo mother care: a randomized controlled trial on effectiveness of early kangaroo mother care for the low birthweight infants in Addis Ababa, Ethiopia. Journal of Tropical Pediatrics 2005;51(2):93‐7. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Ahn 2010 {published data only}
- Ahn HY, Lee J, Shin HJ. Kangaroo care on premature infant growth and maternal attachment and post‐partum depression in South Korea. Journal of Tropical Pediatrics 2010;56(5):342‐4. [DOI] [PubMed] [Google Scholar]
Anderson 2003 {published data only}
- Anderson GC, Chiu SH, Dombrowski MA, Swinth JY, Albert JM, Wada N. Mother‐newborn contact in a randomized trial of kangaroo (skin‐to‐skin) care. Journal of Obstetric, Gynecologic and Neonatal Nursing 2003;32(5):604‐11. [DOI] [PubMed] [Google Scholar]
Arandia 1993 {published data only}
- Arandia R, Morales L. Program kangaroo mother [Programa Madre‐Canguro]. Gaceta Medica Boliviana 1993;17:51‐5. [Google Scholar]
Badiee 2014 {published data only}
- Badiee Z, Faramarzi S, MiriZadeh T. The effect of kangaroo mother care on mental health of mothers with low birth weight infants. Advanced Biomedical Research 2014;3:214. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bera 2014 {published data only}
- Bera A, Ghosh J, Singh AK, Hazra A, Mukherjee S, Mukherjee R. Effect of kangaroo mother care on growth and development of low birthweight babies up to 12 months of age: a controlled clinical trial. Acta Paediatrica 2014;103(6):643‐50. [DOI] [PubMed] [Google Scholar]
Bergman 1994 {published data only}
- Bergman NJ, Jürisoo LA. The 'kangaroo‐method' for treating low birth weight babies in a developing country. Tropical Doctor 1994;24(2):57‐60. [DOI] [PubMed] [Google Scholar]
Bergman 2004 {published data only}
- Bergman NJ, Linley LL, Fawcus SR. Randomized controlled trial of skin‐to‐skin contact from birth versus conventional incubator for physiological stabilization in 1200‐ to 2199‐gram newborns. Acta Paediatrica 2004;93(6):779‐85. [DOI] [PubMed] [Google Scholar]
Broughton 2013 {published data only}
- Broughton EI, Gomez I, Sanchez N, Vindell C. The cost‐savings of implementing kangaroo mother care in Nicaragua. Revista Panamericana de Salud Publica 2013;34(3):176‐82. [PubMed] [Google Scholar]
Charpak 1994 {published data only}
- Charpak N, Ruiz‐Peláez JG, Charpak Y. Rey‐Martinez. Kangaroo Mother Program: an alternative way of caring for low birth weight infants? One year mortality in a two cohort study. Pediatrics 1994;94(6):804‐10. [PubMed] [Google Scholar]
Chiu 2009 {published data only}
- Chiu SH, Anderson GC. Effect of early skin‐to‐skin contact on mother‐preterm infant interaction through 18 months: randomized controlled trial. International Journal of Nursing Studies 2009;46(9):1168‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Christensson 1998 {published data only}
- Christensson K, Bhat GJ, Amadi BC, Eriksson B, Höjer B. Randomised study of skin‐to‐skin versus incubator care for rewarming low‐risk hypothermic neonates. Lancet 1998;352(9134):1115. [DOI] [PubMed] [Google Scholar]
Chwo 2002 {published data only}
- Chwo MJ, Anderson GC, Good M, Dowling DA, Shiau SH, Chu DM. A randomized controlled trial of early kangaroo care for preterm infants: effects on temperature, weight, behavior, and acuity. Journal of Nursing Research 2002;10(2):129‐42. [DOI] [PubMed] [Google Scholar]
Dala Sierra 1994 {published data only}
- Dala Sierra E, Pineda Barahona E, Banegas RM. Kangaroo mother experience [Experiencia madre canguro]. Revista Medica Hondureña 1994;62:43‐6. [Google Scholar]
Darmstadt 2006 {published data only}
- Darmstadt GL, Kumar V, Yadav R, Singh V, Singh P, Mohanty S, et al. Introduction of community‐based skin‐to‐skin care in rural Uttar Pradesh, India. Journal of Perinatology 2006;26(10):597‐604. [DOI] [PubMed] [Google Scholar]
de Almeida 2010 {published data only}
- Almeida H, Venancio SI, Sanches MT, Onuki D. The impact of kangaroo care on exclusive breastfeeding in low birth weight newborns. Jornal de Pediatria (Rio J) 2010;86(3):250‐3. [DOI] [PubMed] [Google Scholar]
Dehghani 2015 {published data only}
- Dehghani K, Movahed ZP, Dehghani H, Nasiriani K. A randomized controlled trial of kangaroo mother care versus conventional method on vital signs and arterial oxygen saturation rate in newborns who were hospitalized in neonatal intensive care unit. Journal of Clinical Neonatology 2015;4(1):26‐31. [Google Scholar]
de Macedo 2007 {published data only}
- Macedo EC, Cruvinel F, Lukasova K, D'Antino ME. The mood variation in mothers of preterm infants in Kangaroo mother care and conventional incubator care. Journal of Tropical Pediatrics 2007;53(5):344‐6. [DOI] [PubMed] [Google Scholar]
Feldman 2002 {published data only}
- Feldman R, Eidelman AI, Sirota L, Weller A. Comparison of skin‐to‐skin (kangaroo) and traditional care: parenting outcomes and preterm infant development. Pediatrics 2002;110(1):16‐26.. [DOI] [PubMed] [Google Scholar]
Gregson 2011 {published data only}
- Gregson S, Blacker J. Kangaroo care in pre‐term or low birth weight babies in a postnatal ward. British Journal of Midwifery 2011;19(9):566‐75. [Google Scholar]
Hake Brooks 2008 {published data only}
- Hake‐Brooks SJ, Anderson GC. Kangaroo care and breastfeeding of mother‐preterm infant dyads 0‐18 months: a randomized, controlled trial. Neonatal Network 2008;27(3):151‐9. [DOI] [PubMed] [Google Scholar]
Huang 2006 {published data only}
- Huang YY, Huang CY, Lin SM, Wu SC. Effect of very early kangaroo care on extrauterine temperature adaptation in newborn infants with hypothermia problems [In Chinese]. Hu Li Za Zhi Journal of Nursing 2006;53(4):41‐8. [PubMed] [Google Scholar]
Ibe 2004 {published data only}
- Ibe OE, Austin T, Sullivan K, Fabanwo O, Disu E, Costello AM. A comparison of kangaroo mother care and conventional incubator care for thermal regulation of infants < 2000 g in Nigeria using continuous ambulatory temperature monitoring. Annals of Tropical Paediatrics 2004;24(3):245‐51. [DOI] [PubMed] [Google Scholar]
Kambarami 1998 {published data only}
- Kambarami RA, Chidede O, Kowo DT. Kangaroo care versus incubator care in the management of well preterm infants ‐ a pilot study. Annals of Tropical Paediatrics 1998;18(2):81‐6. [DOI] [PubMed] [Google Scholar]
Karimi 2014 {published data only}
- Karimi FZ, Bagheri S, Tara F, Khadivzadeh T, Mercer SMM. Effect of kangaroo mother care on breastfeeding self‐efficacy in primiparous women, 3 month after child birth [in Persian]. Iranian Journal of Obstetrics, Gynecology and Infertility 2014;17(120):1‐8. [Google Scholar]
Kashaninia 2015 {published data only}
- Kashaninia Z, Dehghan M. The effect of kangaroo care on weight gain of premature neonates hospitalized in neonatal intensive care units. Biosciences Biotechnology Research Asia 2015;12(2):1405‐10. [Google Scholar]
Kristoffersen 2016 {published data only}
- Kristoffersen L, Stoen R, Hansen LF, Wilhelmsen J, Bergseng H. Skin‐to‐skin care after birth for moderately preterm infants. Journal of Obstetric, Gynecologic and Neonatal Nursing 2016;45(3):339‐45. [DOI] [PubMed] [Google Scholar]
Kumar 2008 {published data only}
- Kumar V, Mohanty S, Kumar A, Misra RP, Santosham M, Awasthi S, et al. Saksham Study Group. Effect of community‐based behaviour change management on neonatal mortality in Shivgarh, Uttar Pradesh, India: a cluster‐randomised controlled trial. Lancet 2008;372(9644):1151‐62. [DOI] [PubMed] [Google Scholar]
Lai 2006 {published data only}
- Lai HL, Chen CJ, Peng TC, Chang FM, Hsieh ML, Huang HY, et al. Randomized controlled trial of music during kangaroo care on maternal state anxiety and preterm infants' responses. International Journal of Nursing Studies 2006;43(2):139‐46. [DOI] [PubMed] [Google Scholar]
Lamy Filho 2008 {published data only}
- Lamy Filho F, Silva AA, Lamy ZC, Gomes MA, Moreira ME. Evaluation of the neonatal outcomes of the kangaroo mother method in Brazil. Jornal de Pediatria (Rio J) 2008;84(5):428‐35. [DOI] [PubMed] [Google Scholar]
Lamy Filho 2015 {published data only}
- Lamy Filho F, Sousa SH, Freitas IJ, Lamy ZC, Simões VM, Silva AA, et al. Effect of maternal skin‐to‐skin contact on decolonization of methicillin‐oxacillin‐resistant Staphylococcus in neonatal intensive care units: a randomized controlled trial. BMC Pregnancy and Childbirth 2015;15:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Legault 1993 {published data only}
- Legault M, Goulet C. Comparative study of two methods of holding premature infants: the kangaroo method versus the traditional method [Etude comparative de deux méthodes de sortie du prématuré: méthode kangourou versus méthode traditionnelle]. Canadian Journal of Nursing Research 1993;25(4):67‐80. [PubMed] [Google Scholar]
Legault 1995 {published data only}
- Legault M, Goulet C. Comparison of kangaroo and traditional methods of removing preterm infants from incubators. Journal of Obstetric, Gynecologic and Neonatal Nursing 1995;24(6):501‐6. [DOI] [PubMed] [Google Scholar]
Lincetto 2000 {published data only}
- Lincetto O, Nazir AL, Cattaneo A. Kangaroo mother care with limited resources. Journal of Tropical Pediatrics 2000;46(5):293‐5. [DOI] [PubMed] [Google Scholar]
Lizarazo‐Medina 2012 {published data only}
- Lizarazo‐Medina JP, Ospina‐Diaz JM, Ariza‐Riaño NE. The kangaroo mothers' programme: a simple and cost‐effective alternative for protecting the premature newborn or low‐birth‐weight babies [Programa madre canguro: una alternativa sencilla y costo eficaz para la protección de los recién nacidos prematuros o con bajo peso al nacer]. Revista de Salud Publica 2012;14(Suppl 2):32‐45. [DOI] [PubMed] [Google Scholar]
Ludington‐Hoe 1991 {published data only}
- Ludington SM, Hadeed AJ, Anderson G. Cardiorespiratory, thermal and state effects of kangaroo care for preterm infants: randomized controlled trial. Pediatric Research 1991;29(4):223A. [Google Scholar]
Ludington‐Hoe 2000 {published data only}
- Ludington‐Hoe SM, Nguyen N, Swinth JY, Satyshur RD. Kangaroo care compared to incubators in maintaining body warmth in preterm infants. Biological Research for Nursing 2000;2(1):60‐73. [DOI] [PubMed] [Google Scholar]
Ludington‐Hoe 2004 {published data only}
- Ludington‐Hoe SM, Anderson GC, Swinth JY, Thompson C, Hadeed AJ. Randomized controlled trial of kangaroo care: cardiorespiratory and thermal effects on healthy preterm infants. Neonatal Network 2004;23(3):39‐48. [DOI] [PubMed] [Google Scholar]
Ludington‐Hoe 2006 {published data only}
- Ludington‐Hoe SM, Johnson MW, Morgan K, Lewis T, Gutman J, Wilson PD, et al. Neurophysiologic assessment of neonatal sleep organization: preliminary results of a randomized, controlled trial of skin contact with preterm infants. Pediatrics 2006;117(5):e909‐23. [DOI] [PubMed] [Google Scholar]
Lyngstad 2014 {published data only}
- Lyngstad LT, Tandberg BS, Storm H, Ekeberg BL, Moen A. Does skin‐to‐skin contact reduce stress during diaper change in preterm infants?. Early Human Development 2014;90(4):169‐72. [DOI] [PubMed] [Google Scholar]
Miles 2006 {published data only}
- Miles R, Cowan F, Glover V, Stevenson J, Modi N. A controlled trial of skin‐to‐skin contact in extremely preterm infants. Early Human Development 2006;82(7):447‐55. [DOI] [PubMed] [Google Scholar]
Miltersteiner 2005 {published data only}
- Miltersteiner AR, Dalle Molle L, Marchetto Claus S, Rotta NT. Length of hospital stay of preterm infants observed in the Kangaroo position and prone position in incubator [Tempo de internação hospitalar de bebês pré‐termos observados na Posição Mãe‐Canguru e na Posição Prona na incubadora]. Revista AMRIGS 2005;49(1):20‐6. [Google Scholar]
Mitchell 2013 {published data only}
- Mitchell AJ, Yates C, Williams K, Hall RW. Effects of daily kangaroo care on cardiorespiratory parameters in preterm infants. Journal of Neonatal‐Perinatal Medicine 2013;6(3):243‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mörelius 2015 {published data only}
- Mörelius E, Örtenstrand A, Theodorsson E, Frostell A. A randomised trial of continuous skin‐to‐skin contact after preterm birth and the effects on salivary cortisol, parental stress, depression, and breastfeeding. Early Human Development 2015;91(1):63‐70. [DOI] [PubMed] [Google Scholar]
Ohgi 2002 {published data only}
- Ohgi S, Fukuda M, Moriuchi H, Kusumoto T, Akiyama T, Nugent JK, et al. Comparison of kangaroo care and standard care: behavioral organization, development, and temperament in healthy, low‐birth‐weight infants through 1 year. Journal of Perinatology 2002;22(5):374‐9. [DOI] [PubMed] [Google Scholar]
Samra 2015 {published data only}
- Samra HA, Dutcher J, McGrath JM, Foster M, Klein L, Djira G, et al. Effect of skin‐to‐skin holding on stress in mothers of late‐preterm infants: a randomized controlled trial. Advances in Neonatal Care 2015;15(5):354‐64. [DOI] [PubMed] [Google Scholar]
Silva 2016 {published data only}
- Silva MG, Barros MC, Pessoa ÚM, Guinsburg R. Kangaroo‐mother care method and neurobehavior of preterm infants. Early Human Development 2016;95:55‐9. [DOI] [PubMed] [Google Scholar]
Sloan 2008 {published data only}
- Sloan NL, Ahmed S, Mitra SN, Choudhury N, Chowdhury M, Rob U, et al. Community‐based kangaroo mother care to prevent neonatal and infant mortality: a randomized, controlled cluster trial. Pediatrics 2008;121(5):e1047‐59. [DOI] [PubMed] [Google Scholar]
Swarnkar 2016 {published data only}
- Swarnkar K, Vagha J. Effect of kangaroo mother care on growth and morbidity pattern in low birth weight infants. Journal of Krishna Institute of Medical Sciences University 2016;5(1):91‐9. [Google Scholar]
Tallandini 2006 {published data only}
- Tallandini MA, Scalembra C. Kangaroo mother care and mother‐premature infant dyadic interaction. Infant Mental Health Journal 2006;27(3):251‐75. [DOI] [PubMed] [Google Scholar]
Udani 2008 {published data only}
- Udani R, Nanavati R, Mauskar AV, Suman R. Innovation: KEM Kangaroo bag for Kangaroo care. http://kangaroo.javeriana.edu.co/encuentros/7encuentro/workshop/Abstract‐Posters/Rekha%20Udani%205.pdf 2008 (accessed June 30, 2010).
References to studies awaiting assessment
Holditch‐Davis 2014 {published data only}
- Holditch‐Davis D, White‐Traut R, Levy J, Williams KL, Ryan D, Vonderheid S. Maternal satisfaction with administering infant interventions in the neonatal intensive care unit. Journal of Obstetric, Gynecologic and Neonatal Nursing 2013;42(6):641‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holditch‐Davis D, White‐Traut RC, Levy JA, O'Shea TM, Geraldo V, David RJ. Maternally administered interventions for preterm infants in the NICU: effects on maternal psychological distress and mother‐infant relationship. Infant Behavior and Development 2014;37(4):695‐710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tully KP, Holditch‐Davis D, White‐Traut RC, David R, O'Shea TM, Geraldo V. A test of kangaroo care on preterm infant breastfeeding. Journal of Obstetric, Gynecologic and Neonatal Nursing 2016;45(1):45‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Barker 1995
- Barker DJ. The fetal and infant origins of disease. European Journal of Clinical Investigation 1995;25(7):457‐63. [DOI] [PubMed] [Google Scholar]
Bassler 2010
- Bassler D, Briel M, Montori VM, Lane M, Glasziou P, Zhou Q, et al. Stopping randomized trials early for benefit and estimation of treatment effects: systematic review and meta‐regression analysis. Journal of the American Medical Association 2010;303(12):1180‐7. [DOI] [PubMed] [Google Scholar]
Boundy 2016
- Boundy EO, Dastjerdi R, Spiegelman D, Fawzi WW, Missmer SA, Lieberman E, et al. Kangaroo mother care and neonatal outcomes: a meta‐analysis. Pediatrics 2016;137(1):e20152238. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chan 2016
- Chan GJ, Valsangkar B, Kajeepeta S, Boundy EO, Wall S. What is kangaroo mother care? Systematic review of the literature. Journal of Global Health 2016;6(1):010701. [DOI] [PMC free article] [PubMed] [Google Scholar]
Charpak 1996
- Charpak N, Ruiz‐Peláez JG, Figueroa de Calume Z. Current knowledge of kangaroo mother Intervention. Current Opinion in Pediatrics 1996;8(2):108‐12. [DOI] [PubMed] [Google Scholar]
Diaz‐Rossello 2008
- Díaz‐Rossello JL, Ferreira‐Castro A. Maternology: when a baby is born, a mother is born. NeoReviews 2008;9(8):e326‐e331. [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analyses detected by a simple graphical test. British Medical Journal 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro 2008 [Computer program]
- Brozek J, Oxman A, Schünemann H. GRADEpro [Version3.2 for Windows]. McMaster University, 2008.
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. British Medical Journal 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration 2011; Vol. www.cochrane‐handbook.org.
Johnston 2014
- Johnston C, Campbell‐Yeo M, Fernandes A, Inglis D, Streiner D, Zee R. Skin‐to‐skin care for procedural pain in neonates. Cochrane Database of Systematic Reviews 2014, Issue 1. [DOI: 10.1002/14651858.CD008435.pub2] [DOI] [PubMed] [Google Scholar]
Lawn 2010
- Lawn JE, Mwansa‐Kambafwile J, Horta BL, Barros FC, Cousens S. 'Kangaroo mother care' to prevent neonatal deaths due to preterm birth complications. International Journal of Epidemiology 2010;39(Suppl 1):i144‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lawn 2014
- Lawn JE, Blencowe H, Oza S, You D, Lee AC, Waiswa P, et al. Lancet Every Newborn Study Group. Every Newborn: progress, priorities, and potential beyond survival. Lancet 2014;384(9938):189‐205. [DOI] [PubMed] [Google Scholar]
Moore 2012
- Moore ER, Anderson GC, Bergman N, Dowswell T. Early skin‐to‐skin contact for mothers and their healthy newborn infants. Cochrane Database of Systematic Reviews 2012, Issue 5. [DOI: 10.1002/14651858.CD003519.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nyqvist 2010
- Nyqvist KH, Anderson GC, Bergman N, Cattaneo A, Charpak N, Davanzo R, et al. Towards universal Kangaroo Mother Care: recommendations and report from the First European conference and Seventh International Workshop on Kangaroo Mother Care. Acta Paediatrica 2010;99(6):820‐6. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rey 1983
- Rey E, Martinez H. Rational management of the premature infant [Manejo racional del niño prematuro]. I Curso de Medicina Fetal y Neonatal. Bogota, Colombia: Universidad Nacional, 1983:137‐51. [Google Scholar]
Roberts 2010
- Roberts G, Anderson PJ, Doyle LW, the Victorian Infant Collaborative Study Group. The stability of the diagnosis of developmental disability between ages 2 and 8 in a geographic cohort of very preterm children born in 1997. Archives of Disease in Childhood 2010;95(10):786‐90. [DOI] [PubMed] [Google Scholar]
Ruiz‐Peláez 2004
- Ruiz‐Peláez JG, Charpak N, Cuervo LG. Kangaroo Mother Care, an example to follow from developing countries. British Medical Journal 2004;329(7475):1179‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Broek J, Guyatt G, Oxman A (editors). GRADE handbook for grading quality of evidence and strength of recommendations. http://gdt.guidelinedevelopment.org/central˙prod/˙design/client/handbook/handbook.html Updated October 2013.
UNICEF 2015
- United Nations Inter‐agency Group for Child Mortality Estimation (UN IGME). Levels & Trends in Child Mortality. Report 2015. New York, NY: UNICEF, 2015. [Google Scholar]
WHO 2014
- World Health Organization. Global Nutrition Targets 2025: Low Birth Weight Policy Brief (WHO/NMH/NHD/14.5). Geneva: World Health Organization,. New York, 2014.
References to other published versions of this review
Conde‐Agudelo 2000
- Conde‐Agudelo A, Diaz‐Rossello JL, Belizan JM. Kangaroo mother care to reduce morbidity and mortality in low birthweight infants. Cochrane Database of Systematic Reviews 2000, Issue 4. [DOI: 10.1002/14651858.CD002771] [DOI] [PubMed] [Google Scholar]
Conde‐Agudelo 2003
- Conde‐Agudelo A, Diaz‐Rossello JL, Belizan JM. Kangaroo mother care to reduce morbidity and mortality in low birthweight infants. Cochrane Database of Systematic Reviews 2003, Issue 2. [DOI: 10.1002/14651858.CD002771] [DOI] [PubMed] [Google Scholar]
Conde‐Agudelo 2011
- Conde‐Agudelo A, Belizán JM, Diaz‐Rossello J. Kangaroo mother care to reduce morbidity and mortality in low birthweight infants. Cochrane Database of Systematic Reviews 2011, Issue 3. [DOI: 10.1002/14651858.CD002771.pub2] [DOI] [PubMed] [Google Scholar]
Conde‐Agudelo 2014
- Conde‐Agudelo A, Diaz‐Rossello JL. Kangaroo mother care to reduce morbidity and mortality in low birthweight infants. Cochrane Database of Systematic Reviews 2014, Issue 4. [DOI: 10.1002/14651858.CD002771.pub3] [DOI] [PubMed] [Google Scholar]


