Abstract
Background
In general, in vitro fertilisation (IVF) or intracytoplasmic sperm injection (ICSI) implies a single fresh and one or more frozen‐thawed embryo transfers. Alternatively, the 'freeze‐all' strategy implies transfer of frozen‐thawed embryos only, with no fresh embryo transfers. In practice, both strategies can vary technically including differences in freezing techniques and timing of transfer of cryopreservation, that is vitrification versus slow freezing, freezing of two pro‐nucleate (2pn) versus cleavage‐stage embryos versus blastocysts, and transfer of cleavage‐stage embryos versus blastocysts.
In the freeze‐all strategy, embryo transfers are disengaged from ovarian stimulation in the initial treatment cycle. This could avoid a negative effect of ovarian hyperstimulation on the endometrium and thereby improve embryo implantation. It could also reduce the risk of ovarian hyperstimulation syndrome (OHSS) in the ovarian stimulation cycle by avoiding a pregnancy.
We compared the benefits and risks of the two treatment strategies.
Objectives
To evaluate the effectiveness and safety of the freeze‐all strategy compared to the conventional IVF/ICSI strategy in women undergoing assisted reproductive technology.
Search methods
We searched the Cochrane Gynaecology and Fertility Group Trials Register, the Cochrane Central Register of Studies (CRSO), MEDLINE, Embase, PsycINFO, CINAHL, and two registers of ongoing trials in November 2016 together with reference checking and contact with study authors and experts in the field to identify additional studies.
Selection criteria
We included randomised clinical trials comparing a freeze‐all strategy with a conventional IVF/ICSI strategy which includes fresh transfer of embryos in women undergoing IVF or ICSI treatment.
Data collection and analysis
We used standard methodological procedures recommended by Cochrane. The primary review outcomes were cumulative live birth and OHSS. Secondary outcomes included other adverse effects (miscarriage rate).
Main results
We included four randomised clinical trials analysing a total of 1892 women comparing a freeze‐all strategy with a conventional IVF/ICSI strategy. The evidence was of moderate to low quality due to serious risk of bias and (for some outcomes) serious imprecision. Risk of bias was associated with unclear blinding of investigators for preliminary outcomes of the study, unit of analysis error, and absence of adequate study termination rules.
There was no clear evidence of a difference in cumulative live birth rate between the freeze‐all strategy and the conventional IVF/ICSI strategy (odds ratio (OR) 1.09, 95% confidence interval (CI) 0.91 to 1.31; 4 trials; 1892 women; I2 = 0%; moderate‐quality evidence). This suggests that if the cumulative live birth rate is 58% following a conventional IVF/ICSI strategy, the rate following a freeze‐all strategy would be between 56% and 65%.
The prevalence of OHSS was lower after the freeze‐all strategy compared to the conventional IVF/ICSI strategy (OR 0.24, 95% CI 0.15 to 0.38; 2 trials; 1633 women; I2 = 0%; low‐quality evidence). This suggests that if the OHSS rate is 7% following a conventional IVF/ICSI strategy, the rate following a freeze‐all strategy would be between 1% and 3%.
The freeze‐all strategy was associated with fewer miscarriages (OR 0.67, 95% CI 0.52 to 0.86; 4 trials; 1892 women; I2 = 0%; low‐quality evidence) and a higher rate of pregnancy complications (OR 1.44, 95% CI 1.08 to 1.92; 2 trials; 1633 women; low‐quality evidence). There was no difference in multiple pregnancies per woman after the first transfer (OR 1.11, 95% CI 0.85 to 1.44; 2 trials; 1630 women; low‐quality evidence), and no data were reported for time to pregnancy.
Authors' conclusions
We found moderate‐quality evidence showing that one strategy is not superior to the other in terms of cumulative live birth rates. Time to pregnancy was not reported, but it can be assumed to be shorter using a conventional IVF/ICSI strategy in the case of similar cumulative live birth rates, as embryo transfer is delayed in a freeze‐all strategy. Low‐quality evidence suggests that not performing a fresh transfer lowers the OHSS risk for women at risk of OHSS.
Keywords: Female; Humans; Pregnancy; Cryopreservation; Embryo, Mammalian; Abortion, Spontaneous; Abortion, Spontaneous/epidemiology; Embryo Transfer; Embryo Transfer/methods; Live Birth; Live Birth/epidemiology; Ovarian Hyperstimulation Syndrome; Ovarian Hyperstimulation Syndrome/epidemiology; Ovarian Hyperstimulation Syndrome/prevention & control; Pregnancy Complications; Pregnancy Complications/epidemiology; Pregnancy Rate; Pregnancy, Multiple; Pregnancy, Multiple/statistics & numerical data; Randomized Controlled Trials as Topic
Fresh versus frozen embryo transfers for assisted reproduction
Review question
We reviewed the evidence about the effectiveness and safety of a 'freeze‐all' strategy for women undergoing in vitro fertilisation (IVF) or intracytoplasmic sperm injection (ICSI) compared to a conventional IVF/ICSI strategy, in terms of cumulative live birth rate and risk of ovarian hyperstimulation syndrome (OHSS).
Background
Embryo transfer in IVF/ICSI can be performed using either fresh or frozen‐thawed embryos. There are therefore two embryo transfer strategies in IVF: 1) the conventional IVF/ICSI strategy with a single transfer of fresh and one or more transfers of frozen‐thawed embryos, and 2) the 'freeze‐all' strategy with transfer of frozen‐thawed embryos only, and no fresh embryo transfer. Differences in freezing technique and timing of cryopreservation and transfer exist within both transfer strategies. In the freeze‐all strategy, embryo transfers are disengaged from ovarian stimulation in the ovarian stimulation cycle. This strategy may be beneficial, as the ovarian hyperstimulation is suggested to have a negative effect on the receptivity of the endometrium for embryo implantation. The freeze‐all strategy would lower the risk of OHSS since pregnancies do not occur in the cycle with ovarian stimulation.
Study characteristics
We included four studies comparing a freeze‐all strategy with a conventional IVF/ICSI strategy in a total of 1892 women undergoing assisted reproductive technology. The evidence is current to November 2016.
Key results
We found evidence showing seemingly no difference between the strategies in cumulative live birth rate per woman. Our findings suggest that if the cumulative live birth rate is 58% following a conventional IVF/ICSI strategy, the rate following a freeze‐all strategy would be between 56% and 65%. Time to pregnancy was not reported as an outcome in in the included studies, but it can be assumed to be shorter using a conventional IVF/ICSI strategy including fresh transfer in the case of similar cumulative live birth rates, as embryo transfer is delayed in a freeze‐all strategy. Not performing a fresh transfer (freeze‐all strategy) lowers the OHSS risk for women at risk of OHSS. Our findings suggest that if the OHSS rate is 7% following a conventional IVF/ICSI strategy, the rate following a freeze‐all strategy would be between 1% and 3%.
Quality of the evidence
The evidence was of moderate to low quality due to serious risk of bias and (for some outcomes) serious imprecision. Risk of bias was associated with unclear blinding of investigators for preliminary outcomes of the study, unit of analysis error, and absence of adequate study termination rules.
Summary of findings
Summary of findings for the main comparison.
Fresh versus frozen embryo transfers in assisted reproduction
| Fresh versus frozen embryo transfers in assisted reproduction | ||||||
| Patient or population: women undergoing assisted reproduction Setting: assisted reproduction clinic Intervention: frozen embryo transfers only Comparison: fresh and frozen embryo transfers | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with fresh and frozen embryo transfers | Risk with frozen embryo transfers only | |||||
| Live birth rate cumulatively for all embryo stages of transfer |
579 per 1000 | 600 per 1000 (556 to 643) | OR 1.09 (0.91 to 1.31) | 1892 (4 RCTs) | ⊕⊕⊕⊝ MODERATE 1 | |
| Ovarian hyperstimulation syndrome per cycle with ovarian hyperstimulation |
70 per 1000 | 18 per 1000 (11 to 28) | OR 0.24 (0.15 to 0.38) | 1633 (2 RCTs) | ⊕⊕⊝⊝ LOW 1,2 | |
| Multiple pregnancy per woman after first ET |
161 per 1000 | 176 per 1000 (141 to 217) |
OR 1.11 (0.85 to 1.44) |
1630 (2 RCTs) |
⊕⊕⊝⊝ LOW 1,2 | |
| Miscarriage per woman after first ET |
184 per 1000 | 131 per 1000 (105 to 162) |
OR 0.67 (0.52 to 0.86) |
1892 (4 RCTs) | ⊕⊕⊝⊝ LOW 1,2 | |
| Pregnancy complications per woman after first ET | 110 per 1000 | 151 per 1000 (118 to 191) |
OR 1.44 (1.08 to 1.92) |
1633 (2 RCTs) | ⊕⊕⊝⊝ LOW 1,2 | |
| Time to pregnancy | Not reported in any of the included studies | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the mean risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; ET: embryo transfer; OR: odds ratio; RCT: randomised clinical trial | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. | ||||||
1Downgraded one level due to serious risk of bias associated with lack of power calculation (unclear what determined end of study) and/or use of interim analysis that was calculated per transfer (unit of analysis error) with absence of adequate stopping rules (possible overestimation of treatment effect). 2Downgraded one level due to serious imprecision: event rate < 300.
Background
Description of the condition
Subfertility is defined as the failure to conceive after one year of unprotected intercourse (Van Voorhis 2007). One in six couples experience subfertility at least once during their reproductive lifetime, and approximately 10% of couples worldwide are subfertile (ESHRE 2010; CDC 2011). Common causes of subfertility include poor semen quality, obstruction of the fallopian tubes, absence of ovulation, and endometriosis (Hull 1985). Poor semen quality can manifest itself as low sperm concentration, low motility, or low numbers of sperm with normal morphology. Fallopian tubes can be blocked or damaged by infection, or there can be adhesions of the tubes or ovaries caused by surgery, chlamydia, or endometriosis. Couples who fail to conceive naturally are diagnosed as having unexplained infertility if no cause can be found after standard fertility tests.
Description of the intervention
Assisted reproductive technology has rapidly evolved as an intervention to improve pregnancy rates. It involves the handling of gametes and embryos outside the human body and consists of in vitro fertilisation (IVF) with or without intracytoplasmic sperm injection (ICSI). After fertilisation, fresh transfer of the morphologically best embryo(s) into the uterine cavity is performed. Embryos suitable for transfer, but not transferred fresh, are frozen for future use.
Even so, many women fail to achieve a pregnancy after transfer of one or more fresh embryos. Recent technical improvements in cryopreservation have led to increased chances of embryo survival after thawing and subsequently increased pregnancy rates per frozen‐thawed embryo transfer (CDC 2011; Wong 2014). In fact, pregnancy rates after frozen‐thawed embryo transfer are now almost equal to pregnancy rates after fresh transfer when calculated per transfer. This has fuelled the call for a new IVF/ICSI strategy where no fresh embryo transfer is conducted and all available embryos are cryopreserved, thawed, and transferred in a subsequent cycle. This would reduce any residual chance of ovarian hyperstimulation syndrome (OHSS) and possibly increase the cumulative live birth rates (Mastenbroek 2011; Maheshwari 2013).
How the intervention might work
In contrast to the conventional strategy, in a 'freeze‐all' strategy there are no fresh embryo transfers in the cycle with ovarian stimulation, but only frozen‐thawed embryo transfers in subsequent cycles without ovarian stimulation. This avoids possible adverse effects of ovarian stimulation. The underlying reason here is the claim that ovarian stimulation reduces endometrial receptivity for the implanting embryo (Kolibianakis 2002; Bourgain 2003). Studies on the molecular level comparing stimulated with unstimulated endometrium samples have shown distinct gene‐expression profiles between the two conditions (Haouzi 2009). Transfer of frozen‐thawed embryos only would thus circumvent a possible negative effect of gonadotropins on the endometrium in the cycle with ovarian stimulation, and consequently increase live birth rates, the main outcome of interest to subfertile couples.
Ovarian stimulation with exogenous gonadotropins in IVF also increases the risk of OHSS when a pregnancy occurs in such a cycle with ovarian stimulation. Avoiding a pregnancy in the cycle with ovarian stimulation by only transferring frozen‐thawed embryos in subsequent unstimulated cycles would eliminate the residual risks of OHSS, and OHSS would therefore be self limiting. Mild OHSS symptoms can still occur as a result of the human chorionic gonadoptropin trigger in the hyperstimulated cycle in the freeze‐all strategy, but OHSS in its severe form should be rare.
Why it is important to do this review
Nowadays, an increasing number of clinics apply the freeze‐all strategy as a standard treatment strategy in their practice. However, the relative effectiveness and safety of IVF treatment with the freeze‐all strategy compared to the conventional IVF/ICSI strategy is unclear. A previous non‐Cochrane systematic review reported that a freeze‐all strategy was associated with higher ongoing and clinical pregnancy rates, and lower miscarriage rates than the conventional IVF/ICSI strategy (Roque 2013). However, this review did not report live birth or safety outcomes. This review aimed to provide a systematic, up‐to‐date summary of reliable evidence of the benefits and risks of a freeze‐all strategy.
Objectives
To evaluate the effectiveness and safety of the freeze‐all strategy compared to the conventional IVF/ICSI strategy in women undergoing assisted reproductive technology.
Methods
Criteria for considering studies for this review
Types of studies
We included published randomised clinical trials and excluded quasi‐ and pseudo‐randomised clinical trials. We excluded trials published only as abstract. We planned to include cross‐over trials for completeness, but would only pool the data from the first phase in the meta‐analysis (Vail 2003).
Types of participants
All women undergoing IVF or ICSI.
Types of interventions
Trials comparing the freeze‐all strategy with transfer of frozen‐thawed embryos only versus the conventional IVF/ICSI strategy with transfer of fresh and subsequent frozen‐thawed embryos until a live birth occurred or until all embryos from the initial cycle were transferred.
Types of outcome measures
Primary outcomes
Effectiveness: cumulative live birth rate per randomised woman, i.e. the rate of live birth following the transfer of all (fresh or frozen‐thawed) embryos available from the stimulated cycle.
Safety: OHSS per randomised woman.
Secondary outcomes
Cumulative ongoing pregnancy rate, defined as the number of ongoing pregnancies per woman randomised (demonstrated by the presence of a gestational sac with fetal heartbeat on ultrasound at ≥ 12 weeks of gestation).
Clinical pregnancy, defined as the cumulative number of clinical pregnancies per woman randomised (demonstrated by a pregnancy confirmed by ultrasonographic visualisation of one or more gestational sacs.
Time to pregnancy, defined as the time between the first day of the last menstrual period and clinical pregnancy.
Multiple‐pregnancy rate, defined as the number of multiple pregnancies per woman.
Miscarriage rate, defined as the number of miscarriages per woman.
Pregnancy complications (including ectopic pregnancy, foetal growth disorders, preterm birth < 37 weeks, pregnancy‐induced hypertension, (pre‐) eclampsia, women with haemolysis, elevated liver enzymes, and low platelets in the blood (HELLP syndrome) per woman.
Birth weight of babies born per baby.
Congenital disorders, defined as the number of congenital abnormalities at birth per live‐born children plus number of foetuses therapeutically terminated.
We also calculated multiple pregnancy, miscarriage, pregnancy complications, and birth weight per clinical pregnancy in a secondary analysis.
Search methods for identification of studies
We searched for all published randomised clinical trials on the freeze‐all strategy, without language restriction and in consultation with the Cochrane Gynaecology and Fertility Group (CGF) Information Specialist.
Electronic searches
We searched the following electronic databases, trial registers, and websites from inception to 14 November 2016 without language restriction and in consultation with the CGF Information Specialist: Cochrane Gynaecology and Fertility Group Specialised Register, Cochrane Central Register of Studies (CENTRAL CRSO), MEDLINE, Embase, PsycINFO, and CINAHL. These search strategies are presented in Appendix 1; Appendix 2; Appendix 3; Appendix 4; Appendix 5; Appendix 6.
Other electronic sources of trials included:
trial registers for ongoing and registered trials: ClinicalTrials.gov, a service of the US National Institutes of Health (clinicaltrials.gov/ct2/home) and the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (www.who.int/trialsearch/Default.aspx); see Appendix 7; Appendix 8.
DARE (Database of Abstracts of Reviews of Effects) in the Cochrane Library for reference lists from relevant non‐Cochrane reviews (onlinelibrary.wiley.com/o/cochrane/cochrane_cldare_articles_fs.html);
conference abstracts in the Web of Knowledge (wokinfo.com/);
OpenGrey (System for Information on Grey Literature in Europe) (www.opengrey.eu/);
PubMed (www.ncbi.nlm.nih.gov/pubmed/).
Searching other resources
We examined the reference lists of eligible articles and contacted study authors where necessary to obtain additional relevant data. We also handsearched relevant journals and conference abstracts that were not covered in the CGF Register.
Data collection and analysis
Selection of studies
Two review authors (KMW and SM) screened the titles and abstracts retrieved by the search and retrieved the full texts of all potentially eligible studies. We independently examined these full‐text articles for compliance with the inclusion criteria and selected studies eligible for inclusion in the review. We corresponded with study investigators as required to clarify study eligibility. Disagreements as to study eligibility were resolved by discussion or by consulting a third review author (SR). We documented the selection process with a PRISMA flow chart (Figure 1).
Figure 1.
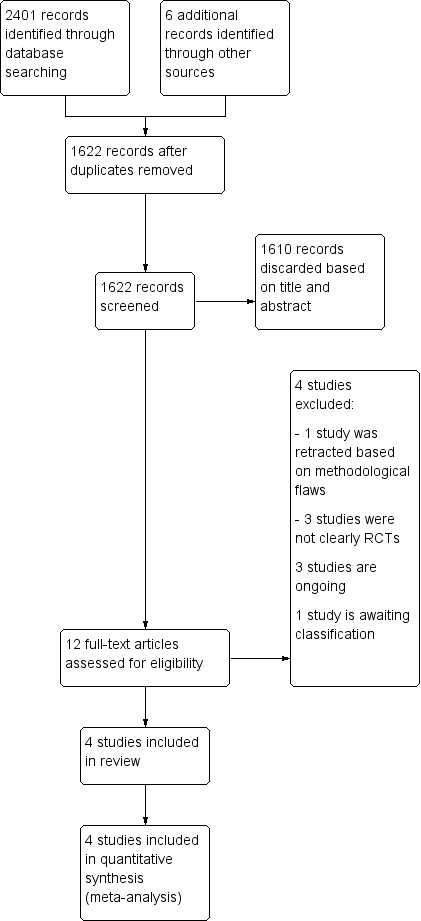
Study flow diagram.
Data extraction and management
Two review authors (KMW and SM) independently extracted data from the eligible studies using a data extraction form designed and pilot‐tested by the authors. Any discrepancies were resolved by discussion. The data extraction forms included methodological quality and allocation information. We included this information in the review and presented it in the Characteristics of included studies and Characteristics of excluded studies tables.
We corresponded with study investigators to request further data on methods or results, or both, as required.
Assessment of risk of bias in included studies
Two review authors (KMW and SM) independently assessed the included studies for risk of bias using the Cochrane 'Risk of bias' assessment tool for the following domains (Higgins 2011).
Sequence generation
We allocated a low risk of bias if the investigators described a random component in the sequence generation process, such as:
using a computerised random number generator;
using a random numbers table.
Allocation concealment
We allocated a low risk of bias if the participants and investigators enrolling participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal allocation:
central computer randomisation;
serially numbered, sealed, opaque envelopes.
Blinding
We allocated a low risk of bias if blinding of participants, scientists, and clinicians or nurses had been ensured. However, in this study design it was ethically not possible to blind participants and clinicians. Lack of blinding may not increase the risk of bias if follow‐up is complete and outcomes are unequivocal (live birth).
Completeness of outcome data
We allocated a low risk of bias if there were no missing data, which meant live birth rate and length of follow‐up were stated, loss to follow‐up was accounted for, and an intention‐to‐treat analysis had been carried out.
Selective outcome reporting
We allocated a low risk of bias if all of the study's primary, secondary, and additional outcomes that were of interest in the review had been reported in a prespecified way.
Other sources of bias
We allocated a low risk of bias if the study:
was free of commercial funding;
reported multiple‐pregnancy rate in the case of an embryo transfer policy of multiple embryos per treatment cycle;
had no other source of bias identified (e.g. imbalance in prognostic factors at baseline).
Two review authors (KMW and SM) assessed these domains and resolved any disagreements by consensus or by consulting a third review author (SR). We described the judgements and presented the conclusions in the 'Risk of bias' figures. We took into account all judgements in the interpretation of review findings.
Measures of treatment effect
For dichotomous data (e.g. live birth rates), we used the numbers of events in the freeze‐all strategy and in the conventional IVF/ICSI strategy group of each study to calculate Mantel‐Haenszel odds ratios (ORs) with 95% confidence intervals (CI). We used Peto ORs where the event was very rare (less than 1%) or in the case of zero cell counts. For continuous data (e.g. birth weight), we calculated mean difference (MD) between treatment groups provided that the same measure was used. We reversed the direction of effect of individual studies if required to ensure consistency across trials. We treated ordinal data as continuous data. Where data to calculate ORs or MDs were not available, we utilised the most detailed numerical data available that would facilitate similar analyses of included studies (e.g. test statistics, P values). We compared the magnitude and direction of effect reported by studies with how they were presented in the review, taking into account legitimate differences.
We planned to analyse the outcome 'time to pregnancy' using hazard ratios (HRs).
Unit of analysis issues
We performed the analyses with data per woman randomised, apart from birth weight, which we analysed per baby. If data of the primary analysis were reported per embryo, per oocyte, per cycle, or per transfer, we contacted the authors of the studies for per‐woman data for completeness.
We counted reported multiple live births as one live birth event.
We planned to include only first‐phase data from cross‐over trials.
We also performed secondary analyses for multiple pregnancy, miscarriage, pregnancy complications, and birth weight per clinical pregnancy since these conditions only occur in pregnant women.
Dealing with missing data
We analysed the data on an intention‐to‐treat basis, and contacted the authors of three included studies, Shapiro 2011a, Shapiro 2011b, and Ferraretti 1999, and one excluded study, Absalan 2013, for missing data. We queried the study authors about these missing data and about bias (e.g. randomisation and blinding). One author did not reply to our request for information (Absalan 2013). The remaining authors very kindly responded to our request for additional information, and we were able to include these data in our analysis.
We assumed that live births had not occurred in women without a reported outcome. If studies reported sufficient detail to calculate MDs, but provided no information on associated standard deviations (SD), we assumed that the outcome had a SD equal to the highest SD from other studies within the same analysis.
Assessment of heterogeneity
We considered heterogeneity when the clinical and methodological characteristics of the included studies were sufficiently similar for a meta‐analysis to provide a clinically meaningful summary. We performed statistical analyses in accordance with the guidelines developed by Cochrane (Higgins 2003; Higgins 2011). We assessed heterogeneity between the results of different studies by the I2 statistic, considering an I2 value greater than 50% to indicate substantial heterogeneity (Higgins 2003; Higgins 2011).
Assessment of reporting biases
We aimed to minimise the potential impact of publication and reporting biases by performing a comprehensive search for eligible studies and looking for duplication of data. We planned to perform a funnel plot to investigate the possibility of small‐study effects if 10 or more studies were included in an analysis.
If included studies reported neither the primary outcome measure of live birth nor interim outcomes such as clinical pregnancy, we undertook informal assessment as to whether studies reporting the primary outcome measures reflected typical findings for the interim outcomes. We considered within‐study reporting bias by looking at the protocols.
We addressed the assessment of reporting biases in the Risk of bias in included studies section of the Results.
Data synthesis
We used Review Manager 5 software to perform the meta‐analyses with a fixed‐effect model to calculate pooled ORs and 95% CIs (RevMan 2014). To aid interpretation, we translated findings for primary outcomes to absolute risks, expressed as percentages based on the 95% CIs. We combined results for continuous outcomes using MDs.
Prospectively, we planned to present the analyses as:
cumulative live birth rates for IVF/ICSI cycles with frozen‐thawed embryo transfers until live birth was achieved or when all frozen embryos originating from the cycle with ovarian stimulation were transferred in the freeze‐all strategy versus IVF/ICSI cycles with fresh and subsequent frozen‐thawed embryo transfers until live birth was achieved or when all frozen embryos originating from the cycle with ovarian stimulation were transferred in the conventional IVF/ICSI strategy;
pregnancy and live birth rates for one IVF/ICSI cycle with the first frozen‐thawed embryo transfer in the freeze‐all strategy versus one IVF/ICSI cycle with the first fresh embryo transfer in the conventional IVF/ICSI strategy (as an additional table).
Subgroup analysis and investigation of heterogeneity
We had planned to perform subanalyses on timing of cryopreservation (e.g. day of embryo development) and method of cryopreservation (e.g. slow freezing or vitrification). However, data were insufficient to conduct all planned subgroup analyses. Should more data become available in the future, we will conduct additional subgroup analyses in later updates of this review.
Sensitivity analysis
We conducted sensitivity analyses for the primary outcome. These analyses included consideration of whether the review conclusions would have differed if:
eligibility was restricted to studies without high risk of bias;
a random‐effects model had been adopted;
the summary effect measure was risk ratio rather than OR.
Overall quality of the body of evidence: 'Summary of findings' table
We prepared a 'Summary of findings' table using GRADEpro software and Cochrane methods (GRADEpro GDT 2014; Higgins 2011). This table evaluates the overall quality of the body of evidence for the main review outcomes. Two review authors independently evaluated the overall quality of the evidence for the outcomes (live birth, OHSS, multiple pregnancy, miscarriage, pregnancy complications and time to pregnancy) using GRADE criteria (study limitations such as risk of bias, consistency of effect, imprecision, indirectness, and publication bias). We justified, documented, and took into account judgements about evidence quality (high, moderate, low, or very low) in the results for each outcome.
Results
Description of studies
Results of the search
Our searches on 14 November 2016 revealed 2401 reports, of which 785 were duplicates, leaving 1622 reports. After screening the title and abstract, we found 12 reports to be potentially eligible, and retrieved these reports in full text.
We excluded four studies: one randomised women to a different intervention that was not clear from the abstract (Boostanfar 2016); two were considered not properly randomised (Absalan 2013; Yang 2015); and one has been retracted (Aflatoonian 2010).
Three studies were ongoing trials and awaiting data (ACTRN12612000422820; NCT02148393; NTR3187).
One study did not clearly report the methods used; it has been classified as awaiting classification and will be reassessed in the next iteration of this review (Chandel 2016).
We included four studies in the review.
See the study flow diagram (Figure 1) and study tables (Characteristics of included studies; Characteristics of excluded studies; Characteristics of ongoing studies).
Included studies
Study design and setting
We included four parallel‐design randomised clinical trials (RCTs) in the review. Three were single‐centre studies, conducted in reproductive medical centres in Italy and the United States (Ferraretti 1999; Shapiro 2011a; Shapiro 2011b), and one was a multicentre trial conducted in 14 reproductive medical centres throughout China (Chen 2016).
Participants
The four studies enrolled a total of 1892 women, with 934 women undergoing the freeze‐all strategy and 958 women undergoing the conventional IVF/ICSI strategy. The inclusion criteria of the two studies of Shapiro and colleagues were based on the number of antral follicles observed at baseline ultrasound examination: Shapiro 2011a included normal responders (8 to 15 antral follicles), and Shapiro 2011b included high responders (> 15 antral follicles). Ferraretti 1999 included women at risk of developing OHSS, based on level of oestradiol (E2) and number of retrieved eggs (≥ 15 oocytes). In all trials, the baseline characteristics were similar between the two strategies. Chen 2016 included women with polycystic ovary syndrome. The ages of the women included by Shapiro ranged from 18 to 41 years. The mean age for the women included in Ferraretti 1999 ranged from 31.6 to 31.4 years. Women in Chen 2016 were between the ages of 20 and 34 years. For details, see Characteristics of included studies.
Interventions
All four studies compared the freeze‐all strategy versus the conventional IVF/ICSI strategy.
Women in the Ferraretti 1999 study received a down‐regulation protocol with gonadotropin‐releasing hormone (GnRH) analogue (0.3 mg subcutaneous buserelin acetate (Suprefact) two times a day) and ovarian stimulation with urinary gonadotropin (4 ampoules of follicle‐stimulating hormone (FSH) on the first and second days of treatment, and 2 ampoules of FSH plus 2 ampoules of human menopausal gonadotropins (hMG) on the third and fourth treatment days, followed by an adjusted dosage of gonadotropins according to the individual response measured by plasma concentration of E2 and follicular growth assessed by ultrasound) (Ferraretti 1996). All women received 7500 IU of human chorionic gonadotropin (hCG) 34 to 36 hours before follicle aspiration followed by 20 g of intravenous albumin. Embryos were frozen at the pronuclear stage. All embryos were transferred at the early cleavage stage (day 3) in artificial cycles. The scheme included oral administration of oestradiol valerate, 2 mg daily for the first 5 days of the cycle; 4 mg/day from day 6 to day 10; 6 mg/day from day 11 to day 13; then 4 mg/day from day 14 onward. On day 15 of the cycle, 50 mg of progesterone in oil was administered daily, and on day 17 the dose was increased to 100 mg/day.
In Shapiro 2011a and Shapiro 2011b, women received down‐regulation with a GnRH antagonist and a combination of recombinant FSH and highly purified urinary FSH. Human chorionic gonadotropin (5 to 15 IU per pound body weight (11 to 33 IU/kg)) was administered 34 to 36 hours prior to follicle aspiration. In those women with greater ovarian response, 4 mg leuprolide acetate was added concomitant to the hCG. Embryos were vitrified at the pronuclear stage. All embryos were transferred as blastocysts in artificial cycles. Women with fresh embryo transfers received 6.0 mg daily E2 and daily progesterone injections (typically 100 mg), with progesterone supplementation beginning one to two days after follicle aspiration and E2 initiated as needed. Women with frozen‐thawed embryo transfers were down‐regulated with leuprolide acetate in a subsequent cycle and received oral 6.0 mg daily E2 and E2 patches as needed starting 10 to 14 days before thawing to achieve a target endometrial thickness of at least 8 mm. Daily progesterone injections (typically 100 mg) were started the day before thawing. In both groups, E2 and progesterone supplements were adjusted as needed to sustain serum levels of at least 200 pg/mL and 15 ng/mL, respectively, until increasing serum levels indicated placental production, typically at 9 to 10 weeks’ gestation.
In Chen 2016, women received recombinant FSH at a daily dose of 112.5 IU for those weighing less than 60 kg and 150 IU for those weighing over 60 kg starting on day 2 or 3 of the menstrual cycle. This was adjusted following ovarian response. Human menopausal gonadotropin could be added when considered to appropriate. On the day of oocyte retrieval, women had to have more than 3 and fewer than 30 oocytes with a low risk of OHSS to be randomised. Intramuscular progesterone at a daily dose of 80 mg was administered for luteal‐phase support in the fresh‐transfer group. Embryos were cryopreserved at day 3 of development. Oral oestradiol valerate was used for endometrial preparation on day 2 or 3 of the second menstrual cycle after oocyte retrieval. Intramuscular progesterone (80 mg/day) was added when endometrial thickness reached 8 mm or more or at the physician’s discretion. On day 4 of progesterone administration, two day 3 frozen embryos were thawed and transferred. Luteal‐phase support with oestradiol valerate and intramuscular progesterone for endometrium preparation continued until 10 weeks after conception.
Outcomes
Data were extracted from study reports or provided by authors for the following outcomes.
Primary outcomes
Effectiveness: Cumulative live birth per woman. Two studies did not report on live birth in their published article (Shapiro 2011a; Shapiro 2011b), but we were able to obtain these data by personal communication with the authors. One study did not report on live birth rate after the first embryo transfer (Ferraretti 1999), but we were able to obtain these data by personal communication with the authors. Chen 2016 reported these data.
Safety: OHSS. One study reported OHSS per woman if hospitalisation was required (Ferraretti 1999). Two studies did not report on OHSS (Shapiro 2011a; Shapiro 2011b), but we were able to obtain these data by personal communication with the authors. However, we did not include the data from these two studies in the analysis, as women with high risk of OHSS were excluded and standardly received the freeze‐all strategy. Chen 2016 reported these data.
Secondary outcomes
Two studies reported ongoing pregnancy rate determined at 10 weeks of gestational age (Shapiro 2011a; Shapiro 2011b).
Three studies reported clinical pregnancy rate (Ferraretti 1999; Shapiro 2011a; Shapiro 2011b), but only one study reported this outcome cumulative per woman (Ferraretti 1999).
None of the studies reported time to pregnancy or the results for each menstrual cycle following randomisation.
Two studies reported multiple‐pregnancy rate (Shapiro 2011b; Chen 2016).
All four studies reported the number of miscarriages (Ferraretti 1999; Shapiro 2011a; Shapiro 2011b; Chen 2016).
One study reported on congenital disorders (Chen 2016).
Two studies reported pregnancy complications (Ferraretti 1999; Chen 2016).
One study reported birth weight of the newborn (Chen 2016).
Excluded studies
We excluded four potentially eligible studies from the review, for the following reasons.
Aflatoonian 2010, as this study was retracted.
Absalan 2013, as it was unclear whether it was truly a RCT. This study compared the clinical and delivery rates between the freeze‐all strategy and the conventional strategy in women at risk for OHSS. In their abstract it was stated that women with OHSS were randomly divided into two groups, with fresh embryo transfer and with frozen transfer. However, nothing is mentioned in the methods section about the method of randomisation (sequence generation or allocation concealment) or which method was used to divide women into the two groups. Nothing was reported on the occurrence of OHSS in these women. The authors did not respond to our request for additional information.
Yang 2015, as one‐third of all randomised women chose to be in group 3 (fresh transfer of a day 3 embryo followed by frozen‐thawed embryos) after randomisation. We did not consider the study to be a properly randomised RCT.
Boostanfar 2016 randomised women to a different intervention that was not clear from the abstract.
Awaiting classification
Chandel 2016 is awaiting classification (see Characteristics of studies awaiting classification); we await more information from the study authors.
Ongoing studies
We identified 12 ongoing studies from trial registers that may have results for inclusion in future versions of this review (ACTRN12612000422820; ACTRN12616000643471; ISRCTN61225414; NCT02000349; NCT02133950; NCT02148393; NCT02471573; NCT02570386; NCT02681367; NCT02712840; NCT02746562; NTR3187). Note that studies that were registered in the trial registers but that were not started or that were withdrawn or stopped were not included in this review.
Risk of bias in included studies
See the 'Risk of bias’ summary (Figure 2) and graph (Figure 3) for the four included trials. See also Characteristics of included studies.
Figure 2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Figure 3.
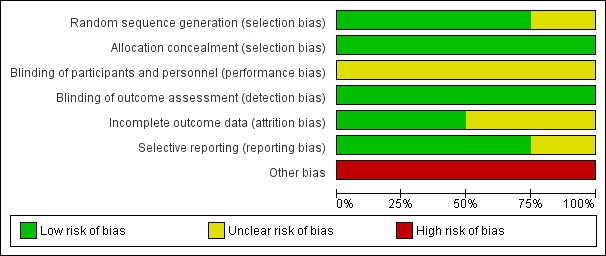
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Sequence generation
The randomisation procedure was well described in Shapiro 2011a. In the second study of Shapiro 2011b, the authors referred to the randomisation procedure in Shapiro 2011a. These two studies used randomly chosen envelopes, and we judged these two studies to be at low risk of selection bias related to sequence generation. Ferraretti 1999 did not describe the method of randomisation in the published article, but replied in a personal communication that the method of randomisation was performed with random sealed envelopes; we judged this study to be at unclear risk of this bias, as random sequence was used but it was unclear whether envelopes were opaque and sequentially numbered. Randomisation in Chen 2016 was well described; an online central randomisation system was used. We considered risk of selection bias related to sequence generation to be low.
Allocation concealment
Shapiro 2011a and Shapiro 2011b performed allocation concealment by using identical, opaque, unmarked, sealed envelopes, and we therefore judged both studies to be at low risk of selection bias related to allocation concealment. The first author of the Ferraretti 1999 study provided additional information on allocation concealment. This study performed participant allocation by sealed envelopes, and we therefore judged it to be at low risk of bias for this domain. There was low risk of selection bias related to allocation concealment in Chen 2016 due to the use of an online central randomisation system.
Blinding
As described in the Methods section, blinding of the participant or the clinician is technically not possible due to the nature of the intervention in this study design. We felt that lack of blinding was not likely to influence findings for the primary outcomes live birth or OHSS. However, blinding for the primary outcome was not reported for the investigators of the four studies, which could have influenced the decision to terminate a trial. The risk of performance bias was unclear in all four studies.
Incomplete outcome data
Three studies did not report intention‐to‐treat analysis in the methodological or analysis sections (Ferraretti 1999; Shapiro 2011a; Shapiro 2011b), while one study did report intention‐to‐treat analysis (Chen 2016).
We judged the studies by Shapiro 2011a and Shapiro 2011b to be at high risk of attrition bias. These studies did not take into account withdrawals or exclusions of randomised women in the reported analyses. Both studies also analysed the outcomes per embryo transferred instead of per woman. However, sufficient data were available for analysis per woman in meta‐analysis. We prespecified ongoing pregnancy as a viable pregnancy at 12 weeks' gestation. These two studies defined ongoing pregnancy at 10 weeks' gestation, which could slightly overestimate the results for this outcome. Taken these issues into account, we considered the risk of bias to be unclear in these two studies.
Ferraretti 1999 and Chen 2016 did analyse all randomised women. Risk of attrition bias was low.
Selective reporting
One study was registered in a prospective trial register under number NCT01841528, including an automatically indexed link on the published report on the study, and the study protocol was published beforehand (Chen 2016). Prespecified outcomes were generally reported, although some prespecified outcomes (e.g. time to pregnancy) were missing from the report. Considering this, we judged this study to be at low risk of reporting bias. Two studies were registered in a prospective trial register with the respective trial numbers NCT00963625 and NCT00963079 (Shapiro 2011a; Shapiro 2011b). Data on the follow‐up of the studies were available in the trial register. The prespecified outcomes of interest were reported in the two studies, and we judged these studies to be at low risk of this bias. We could not assess reporting bias for Ferraretti 1999, as trial registers did not exist at that time, therefore the risk of reporting bias for this study was unclear.
Other potential sources of bias
Three of the studies did not clearly report their prespecified criteria for early termination of their trial. Ferraretti 1999 did not prespecify rules as to when to terminate the study. In the two studies by Shapiro 2011a and Shapiro 2011b, an interim analysis was planned after 100 completed blastocyst transfers. While women were randomised, the interim analyses were based on completed blastocyst transfers (unit of analysis error). They did not report whether the interim analysis was performed by an independent committee that was blinded for the primary outcome. In addition, Shapiro 2011b pre‐terminated the study after an interim analysis based on differences in embryo quality between the two strategies. This reason was not mentioned as one of the criteria to terminate the study. All three studies cryopreserved embryos at the two pro‐nucleate (2pn) stage with slow freezing, which is not currently a common freezing protocol in IVF centres.
After freezing and thawing, the four studies transferred embryos at a different developmental stage: Ferraretti 1999 and Chen 2016 transferred cleavage embryos, and Shapiro 2011a and Shapiro 2011b transferred blastocysts. None of the four studies reported time to pregnancy or (separate or incremental) data per subsequent menstrual or cryo‐transfer cycle (relevant for time‐to‐pregnancy comparison). The difference in technical protocols (some of which are not common practice) between studies in day of cryopreservation and embryo developmental stage of transfer, together with the differences in study population, complicates the comparison between freeze‐all and conventional IVF/ICSI strategies and could introduce heterogeneity between studies. We therefore judged all studies to be at high risk of this bias.
Effects of interventions
See: Table 1
We included four studies involving 1892 women in this review. See Table 1.
1. Comparison of the freeze‐all strategy versus the conventional IVF/ICSI strategy
Primary outcomes
1.1 Effectiveness: Cumulative live birth per woman
All studies collected data on cumulative live birth rates (Ferraretti 1999; Shapiro 2011a; Shapiro 2011b; Chen 2016). There was no clear evidence of a difference between the freeze‐all strategy and the conventional IVF/ICSI strategy in cumulative live birth rates (OR 1.09, 95% CI 0.91 to 1.31; 4 trials; 1892 women; I2 = 0%; moderate‐quality evidence).
It was unclear whether there was any difference between the two strategies in cumulative live birth rate when the studies were analysed per cleavage stage (OR 1.11, 95% CI 0.91 to 1.35; 2 trials; 1633 women; low‐quality evidence) or blastocyst transfer stage (OR 0.99, 95% CI 0.60 to 1.62; 2 trials; 259 women; low‐quality evidence) (Analysis 1.1; Figure 4).
Analysis 1.1.
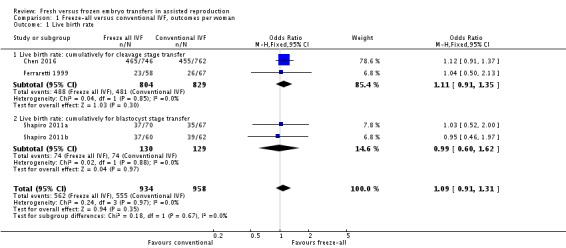
Comparison 1 Freeze‐all versus conventional IVF, outcomes per woman, Outcome 1 Live birth rate.
Figure 4.
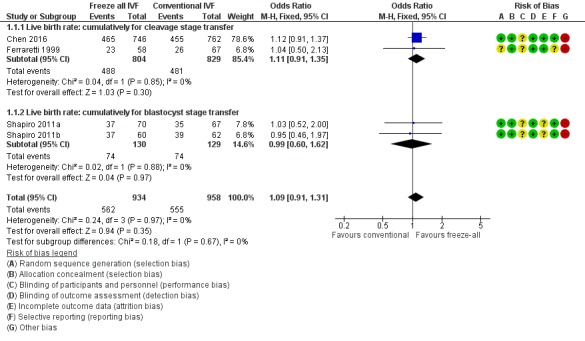
Forest plot of comparison: 1 Freeze‐all vs conventional IVF, outcomes per woman, outcome: 1.1 Live birth rate.
1.2 Safety: Ovarian hyperstimulation syndrome per woman
One study reported OHSS per woman if hospitalisation was required (Ferraretti 1999). Two studies did not report on OHSS, but we were able to obtain these data by personal communication with the authors (Shapiro 2011a; Shapiro 2011b). However, we did not include the data from these two studies in the analysis as women with high risk of OHSS were excluded and standardly received the freeze‐all strategy. Chen 2016 reported these data. The prevalence of OHSS was lower after the freeze‐all strategy compared to the conventional IVF/ICSI strategy (OR 0.24, 95% CI 0.15 to 0.38; 2 trials; 1633 women; I2 = 0%; low‐quality evidence) (Analysis 1.2; Figure 5).
Analysis 1.2.
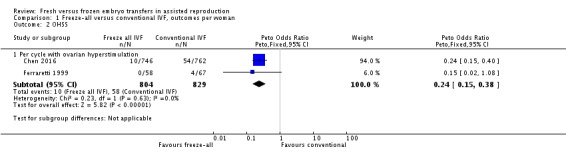
Comparison 1 Freeze‐all versus conventional IVF, outcomes per woman, Outcome 2 OHSS.
Figure 5.
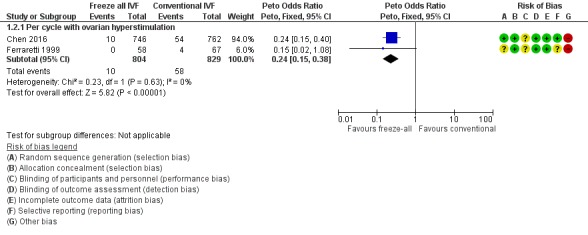
Forest plot of comparison: 1 Freeze‐all vs conventional IVF, outcomes per woman, outcome: 1.2 OHSS.
Secondary outcomes
1.3 Ongoing pregnancy rate per woman
Two studies reported on the cumulative ongoing pregnancy rates (Shapiro 2011a; Shapiro 2011b). There was no evidence of a difference between the two strategies in the cumulative ongoing pregnancy rate (OR 1.05, 95% CI 0.64 to 1.73; 2 trials; 259 women; I2 = 0%; low‐quality evidence) (Analysis 1.3; Figure 6).
Analysis 1.3.
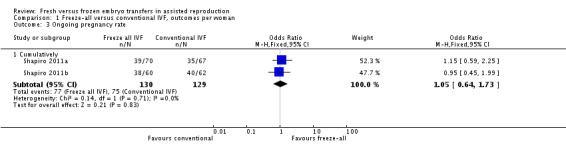
Comparison 1 Freeze‐all versus conventional IVF, outcomes per woman, Outcome 3 Ongoing pregnancy rate.
Figure 6.
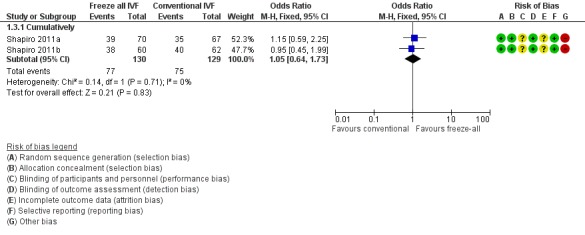
Forest plot of comparison: 1 Freeze‐all vs conventional IVF, outcomes per woman, outcome: 1.3 Ongoing pregnancy rate.
1.4 Clinical pregnancy rate per woman
One study reported the cumulative clinical pregnancy rates (Ferraretti 1999), therefore pooling was not possible. There was no evidence of a difference between the two strategies in clinical pregnancy rate (OR 1.08, 95% CI 0.54 to 2.19; 1 trial; 125 women; low‐quality evidence) (Analysis 1.4; Figure 7).
Analysis 1.4.
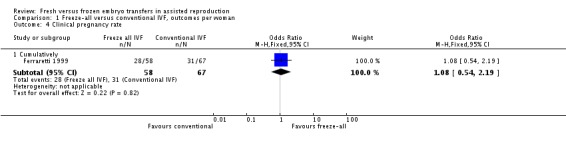
Comparison 1 Freeze‐all versus conventional IVF, outcomes per woman, Outcome 4 Clinical pregnancy rate.
Figure 7.
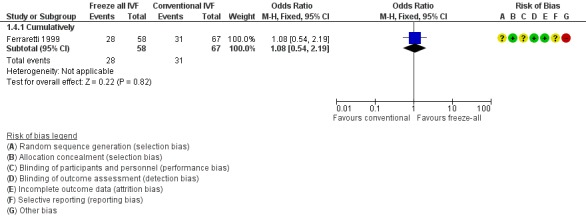
Forest plot of comparison: 1 Freeze‐all vs conventional IVF, outcomes per woman, outcome: 1.4 Clinical pregnancy rate.
1.5 Time to pregnancy
No study reported the time to pregnancy or (separate or incremental) data per subsequent menstrual or cryo‐transfer cycle (relevant for time‐to‐pregnancy comparison).
1.6 Multiple‐pregnancy rate
Two studies reported on the multiple‐pregnancy rate (Shapiro 2011b; Chen 2016). There was no evidence of a difference between the two strategies in multiple‐pregnancy rate (OR 1.11, 95% CI 0.85 to 1.44; 2 trials; 1630 women; low‐quality evidence) (Analysis 1.5; Figure 8).
Analysis 1.5.
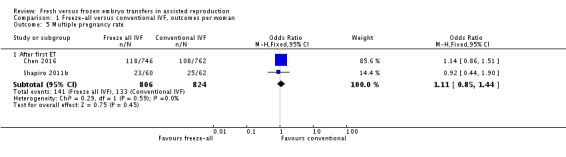
Comparison 1 Freeze‐all versus conventional IVF, outcomes per woman, Outcome 5 Multiple pregnancy rate.
Figure 8.
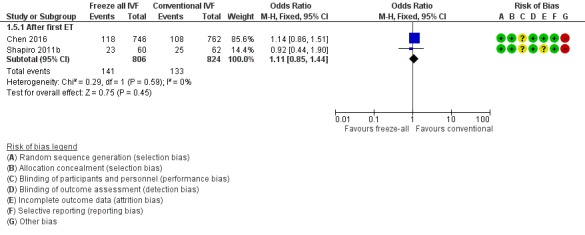
Forest plot of comparison: 1 Freeze‐all vs conventional IVF, outcomes per woman, outcome: 1.5 Multiple pregnancy rate.
1.7 Miscarriage rate
All studies reported the miscarriage rate (Ferraretti 1999; Shapiro 2011a; Shapiro 2011b; Chen 2016). Miscarriage rate was lower in the freeze‐all group (OR 0.67, 95% CI 0.52 to 0.86; 4 trials; 1892 women; I2 = 0%, low‐quality evidence) (Analysis 1.6; Figure 9).
Analysis 1.6.
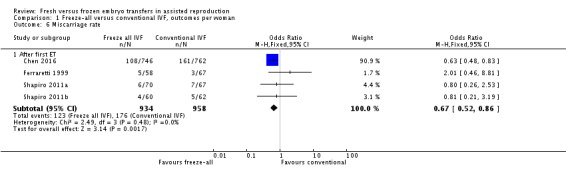
Comparison 1 Freeze‐all versus conventional IVF, outcomes per woman, Outcome 6 Miscarriage rate.
Figure 9.
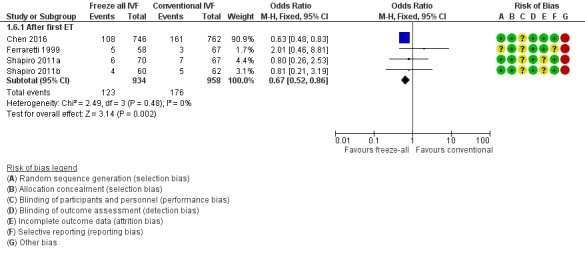
Forest plot of comparison: 1 Freeze‐all vs conventional IVF, outcomes per woman, outcome: 1.6 Miscarriage rate.
1.8 Pregnancy complications
Two studies reported on pregnancy complications (Ferraretti 1999; Chen 2016). There were more pregnancy complications in the freeze‐all group (OR 1.44, 95% CI 1.08 to 1.92; 2 trials; 1633 women; low‐quality evidence) (Analysis 1.7; Figure 10).
Analysis 1.7.
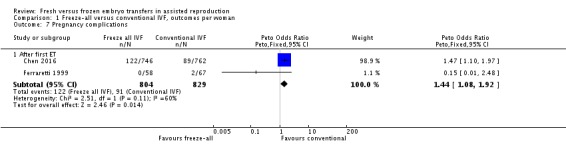
Comparison 1 Freeze‐all versus conventional IVF, outcomes per woman, Outcome 7 Pregnancy complications.
Figure 10.
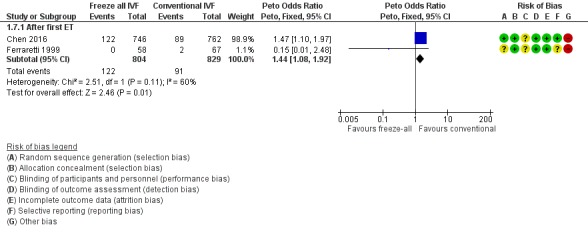
Forest plot of comparison: 1 Freeze‐all vs conventional IVF, outcomes per woman, outcome: 1.7 Pregnancy complications.
1.9 Birth weight
One study reported on birth weight (Chen 2016). A higher birth weight of singleton babies born was reported for the freeze‐all strategy (MD 161.8 g, 95% CI 57.1 to 266.5; 1 trial; 462 singletons; low‐quality evidence). Birth weight of multiples was similar between strategies (MD ‐2.00 g, 95% CI ‐94.08 to 90.08; 1 trial; 453 multiples; low‐quality evidence) (Analysis 1.8; Figure 11).
Analysis 1.8.
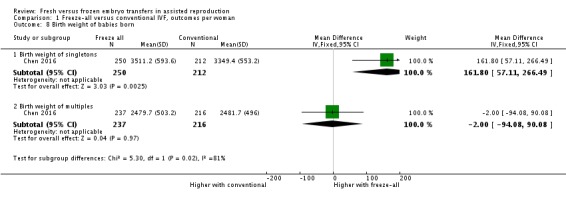
Comparison 1 Freeze‐all versus conventional IVF, outcomes per woman, Outcome 8 Birth weight of babies born.
Figure 11.
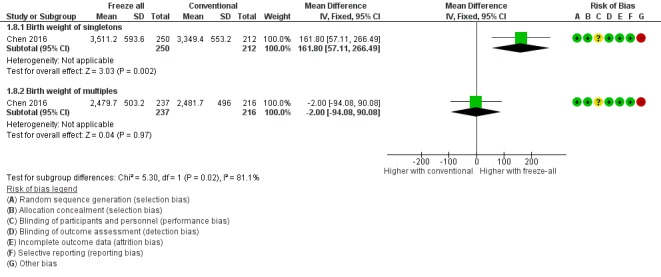
Forest plot of comparison: 1 Freeze‐all vs conventional IVF, outcomes per woman, outcome: 1.8 Birth weight of babies born.
1.10 Congenital abnormalities
One study reported on congenital abnormalities (Chen 2016). There was no evidence of a difference between the two strategies for congenital abnormalities per live‐born children plus number of fetuses therapeutically terminated (OR 1.25, 95% CI 0.66 to 2.37; 1 trial; 923 live‐born children plus number of fetuses therapeutically terminated; low‐quality evidence) (Analysis 3.1; Figure 12).
Analysis 3.1.
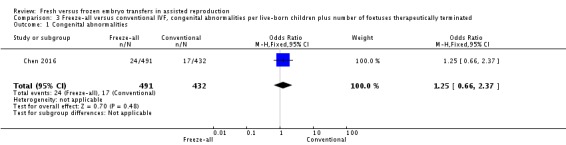
Comparison 3 Freeze‐all versus conventional IVF, congenital abnormalities per live‐born children plus number of foetuses therapeutically terminated, Outcome 1 Congenital abnormalities.
Figure 12.
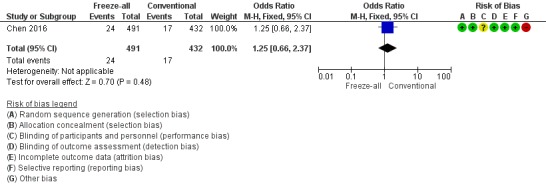
Forest plot of comparison: 3 Freeze‐all vs conventional IVF, congenital abnormalities per live‐born children plus number of foetuses therapeutically terminated, outcome: 3.1 Congenital abnormalities.
Other analyses
We also analysed the adverse events multiple pregnancy, miscarriage and pregnancy complications per clinical pregnancy (Analysis 2.1 , Analysis 2.2 , Analysis 2.3). There was no evidence of a difference between the two strategies for multiple pregnancy per clinical pregnancy after the first transfer (OR 1.02, 95% CI 0.77 to 1.37; 2 trials; 939 clinical pregnancies) (Analysis 2.1). Miscarriage rate was lower in the freeze‐all group per clinical pregnancy after the first transfer (OR 0.56, 95% CI 0.41 to 0.77; 4 trials; 1058 clinical pregnancies) (Analysis 2.2).There were more pregnancy complications in the freeze‐all group per clinical pregnancy after the first transfer (OR 1.43, 95% CI 1.05 to 1.95; 2 trials; 914 clinical pregnancies) (Analysis 2.3).
Analysis 2.1.
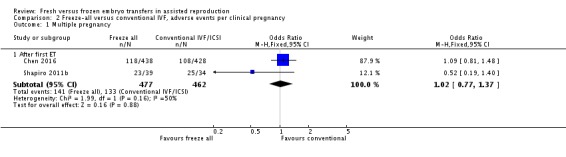
Comparison 2 Freeze‐all versus conventional IVF, adverse events per clinical pregnancy, Outcome 1 Multiple pregnancy.
Analysis 2.2.
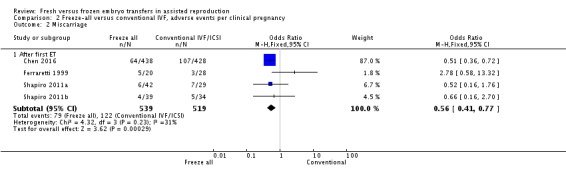
Comparison 2 Freeze‐all versus conventional IVF, adverse events per clinical pregnancy, Outcome 2 Miscarriage.
Analysis 2.3.
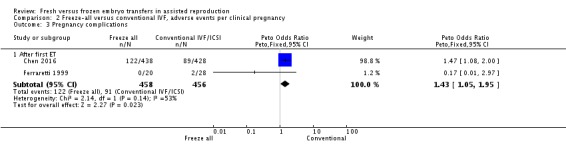
Comparison 2 Freeze‐all versus conventional IVF, adverse events per clinical pregnancy, Outcome 3 Pregnancy complications.
Sensitivity analysis
We did not undertake sensitivity analysis by risk of bias because all studies in the analyses were at high risk of bias in at least one domain. We undertook sensitivity analyses of the primary outcome using 1) adoption of a random‐effects model and 2) using the summary effect measure of risk ratio (RR) rather than OR. Neither of the sensitivity analyses made any material difference to the findings (Table 5).
Table 1.
Sensitivity analysis for cumulative live birth rate
| Studies, number of participants | OR, 95% CI, fixed effect | OR, 95% CI, random effect | RR, 95% CI, fixed effect | RR, 95% CI, random effect |
|
Ferraretti 1999 (n = 125) Shapiro 2011a (n = 103) Shapiro 2011b (n = 122) Chen 2016 (n = 1508) |
1.09 (0.91, 1.31) | 1.09 (0.91, 1.31) | 1.04 (0.96, 1.12) | 1.04 (0.96, 1.12) |
CI: confidence interval OR: odds ratio RR: risk ratio
Discussion
Summary of main results
There was no clear evidence of a difference between the freeze‐all strategy and the conventional IVF/ICSI strategy in cumulative live birth rates per woman, but the prevalence of OHSS appears to be lower after the freeze‐all strategy. The freeze‐all strategy appears to be associated with fewer miscarriages and a higher birth weight of singleton babies (MD 161.80 g, 95% CI 57.11 to 266.49), but also with an increased rate of pregnancy complications.
Overall completeness and applicability of evidence
All trials provided data on the primary outcome live birth rate, but for OHSS we could use data from only two studies.
Three out of four included studies involved a small number of women. All studies had specific and differing technical protocols, and studies had differing inclusion criteria leading to the inclusion of select groups of women ('normal responders', 'high responders', women with polycystic ovary syndrome, women with high OHSS risk). No study included women with low ovarian response.
Quality of the evidence
We rated the quality of evidence using GRADE methods and judged it to be moderate to low, due to serious risk of bias and (for some outcomes) serious imprecision. Risk of bias was associated with unclear blinding of investigators for preliminary outcomes of the study, unit of analysis error, and absence of adequate study termination rules.
The four included studies involved a total of 934 women undergoing the freeze‐all strategy and 958 women undergoing the conventional IVF/ICSI strategy. Varying protocols between studies (some not common in routine practice), varying study population (select groups of women undergoing IVF), one study without power calculation reported (unclear what determined the end of study), and two studies with interim analysis that was calculated per transfer (unit of analysis error) with absence of adequate stopping rules (possible overestimation of treatment effect) resulted in an overall judgement of the evidence as low quality.
Our searches identified 12 ongoing studies. We anticipate that the evidence from these will provide a more definitive answer on the relative effectiveness and safety of a freeze‐all strategy.
Potential biases in the review process
We tried to reduce potential bias in the review process to a minimum by identifying all eligible trials for inclusion in this meta‐analysis. We were able to retrieve additional information on three included trials where required, which helped us in providing accurate study outcomes.
Agreements and disagreements with other studies or reviews
Three out of four included studies reported higher pregnancy or live birth rates for the freeze‐all strategy than for conventional IVF/ICSI treatment including fresh transfer in the published manuscripts (Shapiro 2011b; Shapiro 2011a; Chen 2016), while our review concluded that there was no difference in live birth rates between the strategies. This discrepancy in conclusion is attributed to the fact that these publications focussed on outcomes that were reported per first transfer, whereas in our review we focused on the cumulative live birth rate per woman randomised. The live birth rate calculated per first transfer possibly shows differences in outcome for a stimulated and an unstimulated uterus, although this does not take into account the number of embryos that were thawed for transfer. But for women undergoing treatment, the live birth rate per first transfer is less relevant since at the same time of first transfer in a freeze‐all strategy, they would already have received the second transfer (in case of sufficient number of embryos) in a conventional strategy including fresh transfer. We therefore used the cumulative live birth rate as a primary outcome. In case cumulative live birth rates are comparable, as found in our review, then the difference between strategies could be time to pregnancy. Unfortunately, this outcome was not reported in any of the included studies, but by design time to pregnancy is shorter in the conventional strategy than in the freeze‐all strategy when the cumulative live birth rate is comparable. For illustrative purposes we also calculated and presented the live birth rate per first transfer (Table 6).
Table 2.
Live birth rate after first transfer
| Outcome | Number of studies | Number of participants | Analysis method | OR |
| Live birth rate after first embryo transfer for all embryo stages of transfer | 4 | 1892 | Odds ratio (Mantel‐Haenszel, fixed, 95% confidence interval) | 1.34 (1.12, 1.61) |
| Live birth rate after first transfer with cleavage‐stage embryos | 2 | 1633 | Odds ratio (Mantel‐Haenszel, fixed, 95% confidence interval) | 1.31 (1.08, 1.59) |
| Live birth rate after first transfer with blastocyst‐stage embryo | 2 | 259 | Odds ratio (Mantel‐Haenszel, fixed, 95% confidence interval) | 1.54 (0.94, 2.52) |
Live birth rate calculated per first transfer is added for illustrative purposes as this comparison is often reported in the literature. It possibly shows differences in outcome for a stimulated and an unstimulated uterus, although this does not take into account the number of embryos that were thawed for transfer. This outcome is less relevant for women undergoing treatment since at the same time of first transfer in a freeze‐all strategy, they would already have received the second transfer (in case of sufficient number of embryos) in a conventional strategy that includes fresh transfer.
The same difference in primary outcome (cumulative live birth rate versus live birth rate per first transfer) explains the difference from previous reviews that found improved IVF/ICSI outcomes with the freeze‐all strategy, such as Roque 2013, although the included studies also differed. Roque and colleagues did not include the study of Ferraretti 1999 in their analysis, for reasons that are unclear. The authors did include the retracted study of Aflatoonian 2010 in their analysis. The Chen 2016 study was not yet published when this review was written.
Although we reported pregnancy and live birth rates only cumulatively for the above reasons, for other outcomes, such as the number of multiples and the number of miscarriages, we did report the numbers per first transfer, as cumulative rates for these outcomes were not available from any of the studies.
The lower rate of OHSS found in our review is in agreement with previous studies, and is to be expected, as avoiding a pregnancy in the initial cycle with ovarian stimulation by only transferring frozen‐thawed embryos in subsequent unstimulated cycles would eliminate the residual risks of OHSS, and OHSS would therefore be self limiting. Mild OHSS symptoms can still occur as a result of the hCG trigger in the hyperstimulated cycle in the freeze‐all strategy, but OHSS in its severe form should be rare.
Authors' conclusions
Moderate‐quality evidence does not show one strategy to be superior to the other in terms of cumulative live birth rates. Time to pregnancy was not reported, but can be assumed to be shorter using a conventional IVF/ICSI strategy in case of similar cumulative live birth rates, as embryo transfer is delayed in a freeze‐all strategy. Low‐quality evidence suggests that not performing a fresh transfer lowers the OHSS risk for women at risk of OHSS.
Well‐designed RCTs reporting on cumulative live birth rate and OHSS per hyperstimulated cycle are required. Participant characteristics (e.g. women with good prognosis versus poor prognosis), treatment characteristics (e.g. number of available embryos, number of embryos transferred, results for first and every subsequent transfer, time to pregnancy), and protocols used (e.g. timing and method of cryopreservation) should be properly reported, as these are relevant for future meta‐analyses. Subanalyses of RCTs with data on freezing and transferring frozen‐thawed embryos at the same developmental phase as in the conventional IVF/ICSI strategy would be a better way to compare the two strategies. These RCTs should be performed with well‐described randomisation and allocation concealment methods, and should include intention‐to‐treat analyses. Outcome measures should be expressed as cumulative live birth rate per woman rather than per first transfer.
Acknowledgements
We would like to acknowledge the team at the Cochrane Gynaecology and Fertility Group for their assistance, and especially Information Specialist Marian Showell for the literature search.
Appendices
Appendix 1. Cochrane Gynaecology and Fertility Group specialised register search strategy
From inception to 27 July 2016
PROCITE platform
Keywords CONTAINS "cryopreservation"or "frozen embryo transfer" or "frozen embryos" or "frozen‐thawed cycle" or "frozen‐thawed embryo transfer" or "frozen‐thawed embryos" or "FET" or "cryopreserved embryos" or "cryopreserved‐thawed embryos" or "vitrified"or "vitrification"or"fresh v cryopreserved" or "freeze all" or Title CONTAINS "cryopreservation"or "frozen embryo transfer" or "frozen embryos" or "frozen‐thawed cycle" or "frozen‐thawed embryo transfer" or "frozen‐thawed embryos" or "FET" or "cryopreserved embryos" or "cryopreserved‐thawed embryos" or "vitrified"or "vitrification"or "fresh v cryopreserved"or "freeze all"
AND
Keywords CONTAINS "fresh"or "fresh blastocyst transfer"or "fresh cycle"or "fresh embryos"or "fresh v cryopreserved"or "fresh versus frozen" or Title CONTAINS "fresh"or "fresh blastocyst transfer"or "fresh cycle"or "fresh embryos"or "fresh v cryopreserved"or "fresh versus frozen" (89 hits)
Appendix 2. CENTRAL CRSO search strategy
From inception until 14th November 2016
CRSO Web platform
#1 MESH DESCRIPTOR Embryo Transfer EXPLODE ALL TREES 917
#2 MESH DESCRIPTOR Fertilization in Vitro EXPLODE ALL TREES 1782
#3 MESH DESCRIPTOR Sperm Injections, Intracytoplasmic EXPLODE ALL TREES 445
#4 embryo*: TI,AB,KY 3959
#5 (vitro fertili?ation):TI,AB,KY 1873
#6 ivf:TI,AB,KY 2946
#7 icsi:TI,AB,KY 1307
#8 (intracytoplasmic sperm injection*):TI,AB,KY 1000
#9 blastocyst*:TI,AB,KY 518
#10 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 6032
#11 MESH DESCRIPTOR Cryopreservation EXPLODE ALL TREES 447
#12 MESH DESCRIPTOR Vitrification EXPLODE ALL TREES 23
#13 ((cryopreservat* or cryofixation or cryonic suspension)):TI,AB,KY 558
#14 (freez* or frozen):TI,AB,KY 2815
#15 Vitrif*:TI,AB,KY 193
#16 Thaw*:TI,AB,KY 494
#17 #11 OR #12 OR #13 OR #14 OR #15 OR #16 3332
#18 #10 AND #17 625
23 10 and 22 (451)
Appendix 3. MEDLINE search strategy
From 1946 until 14th November 2016
Ovid platform
1 exp Cryopreservation/ (33440) 2 exp Freezing/ (23063) 3 (cryopreservat$ or cryofixation or cryonic suspension).tw. (13490) 4 freez$.tw. (60485) 5 thaw$.tw. (22148) 6 exp Vitrification/ (970) 7 Vitrif$.tw. (4029) 8 froze$.tw. (71374) 9 disengage$.tw. (4166) 10 or/1‐9 (158372) 11 exp embryo transfer/ or exp fertilization in vitro/ or exp sperm injections, intracytoplasmic/ or exp ovulation induction/ (44353) 12 embryo$.tw. (344559) 13 blastocyst$.tw. (20065) 14 vitro fertili?ation.tw. (20204) 15 ivf.tw. (20159) 16 icsi.tw. (6895) 17 intracytoplasmic sperm injection$.tw. (6056) 18 ovulation induc$.tw. (3841) 19 (ovar$ adj3 hyperstim$).tw. (4654) 20 (ovar$ adj3 stimulat$).tw. (6873) 21 exp Superovulation/ or Superovulat$.tw. (3796) 22 or/11‐21 (382656) 23 10 and 22 (12133) 24 randomized controlled trial.pt. (469524) 25 controlled clinical trial.pt. (95062) 26 randomized.ab. (403302) 27 placebo.tw. (196678) 28 clinical trials as topic.sh. (189460) 29 randomly.ab. (284766) 30 trial.ti. (178345) 31 (crossover or cross‐over or cross over).tw. (75863) 32 or/24‐31 (1176650) 33 exp animals/ not humans.sh. (4668056) 34 32 not 33 (1083487) 35 23 and 34 (550)
Appendix 4. Embase search strategy
From 1980 until 14th November 2016
Ovid platform
1 exp Cryopreservation/ (33582) 2 exp Freezing/ (33013) 3 (cryopreservat$ or cryofixation or cryonic suspension).tw. (17116) 4 freez$.tw. (64880) 5 thaw$.tw. (26477) 6 exp Vitrification/ (4378) 7 Vitrif$.tw. (5937) 8 froze$.tw. (87650) 9 disengage$.tw. (4337) 10 or/1‐9 (182265) 11 exp embryo transfer/ or exp fertilization in vitro/ or exp intracytoplasmic sperm injection/ (63530) 12 in vitro fertili?ation.tw. (24581) 13 icsi.tw. (12341) 14 intracytoplasmic sperm injection$.tw. (7704) 15 (blastocyst adj2 transfer$).tw. (1593) 16 ivf.tw. (31218) 17 exp superovulation/ (2550) 18 superovulat$.tw. (3423) 19 exp ovulation induction/ (12957) 20 blastocyst$.tw. (23859) 21 embryo$.tw. (351069) 22 vitro fertili?ation.tw. (24607) 23 ovulation induc$.tw. (5059) 24 (ovar$ adj3 stimulat$).tw. (9775) 25 (ovar$ adj3 hyperstim$).tw. (6460) 26 or/11‐25 (404447) 27 10 and 26 (18175) 28 Clinical Trial/ (990930) 29 Randomized Controlled Trial/ (460474) 30 exp randomization/ (83533) 31 Single Blind Procedure/ (27015) 32 Double Blind Procedure/ (136735) 33 Crossover Procedure/ (53739) 34 Placebo/ (321486) 35 Randomi?ed controlled trial$.tw. (149041) 36 Rct.tw. (22285) 37 random allocation.tw. (1626) 38 randomly allocated.tw. (26549) 39 allocated randomly.tw. (2206) 40 (allocated adj2 random).tw. (843) 41 Single blind$.tw. (18625) 42 Double blind$.tw. (172648) 43 ((treble or triple) adj blind$).tw. (645) 44 placebo$.tw. (247067) 45 prospective study/ (385364) 46 or/28‐45 (1770419) 47 case study/ (92640) 48 case report.tw. (322778) 49 abstract report/ or letter/ (985370) 50 or/47‐49 (1391670) 51 46 not 50 (1720179) 52 27 and 51 (1488)
Appendix 5. PsycINFO search strategy
From 1806 to 14th November 2016
Ovid platform
1 (cryopreservat$ or cryofixation or cryonic suspension).tw. (66) 2 freez$.tw. (3756) 3 thaw$.tw. (124) 4 Vitrif$.tw. (11) 5 froze$.tw. (1321) 6 disengage$.tw. (5591) 7 or/1‐6 (10628) 8 exp reproductive technology/ (1573) 9 icsi.tw. (61) 10 intracytoplasmic sperm injection$.tw. (47) 11 (blastocyst adj2 transfer$).tw. (4) 12 assisted reproduct$.tw. (730) 13 ovulation induc$.tw. (26) 14 (ovari$ adj2 stimulat$).tw. (55) 15 COH.tw. (86) 16 superovulat$.tw. (6) 17 infertil$.tw. (2923) 18 subfertil$.tw. (77) 19 (ovari$ adj2 induction).tw. (6) 20 ivf.tw. (466) 21 vitro fertili?ation.tw. (630) 22 (ovar$ adj3 hyperstimulat$).tw. (11) 23 or/8‐22 (4438) 24 7 and 23 (98) 25 random.tw. (47258) 26 control.tw. (365925) 27 double‐blind.tw. (19960) 28 clinical trials/ (9713) 29 placebo/ (4602) 30 exp Treatment/ (656349) 31 or/25‐30 (1011580) 32 24 and 31 (24)
Appendix 6. CINAHL search strategy
From inception to 14th November 2016
Ebsco platform
| # | Query | Results |
| S32 | S19 AND S31 | 98 |
| S31 | S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 | 954,451 |
| S30 | TX allocat* random* | 4,243 |
| S29 | (MH "Quantitative Studies") | 13,282 |
| S28 | (MH "Placebos") | 9,173 |
| S27 | TX placebo* | 33,620 |
| S26 | TX random* allocat* | 4,243 |
| S25 | (MH "Random Assignment") | 38,985 |
| S24 | TX randomi* control* trial* | 85,907 |
| S23 | TX ( (singl* n1 blind*) or (singl* n1 mask*) ) or TX ( (doubl* n1 blind*) or (doubl* n1 mask*) ) or TX ( (tripl* n1 blind*) or (tripl* n1 mask*) ) or TX ( (trebl* n1 blind*) or (trebl* n1 mask*) ) | 763,614 |
| S22 | TX clinic* n1 trial* | 170,899 |
| S21 | PT Clinical trial | 77,668 |
| S20 | (MH "Clinical Trials+") | 186,062 |
| S19 | S17 AND S18 | 436 |
| S18 | S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 | 13,480 |
| S17 | S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 | 7,170 |
| S16 | TX ovulation induc* | 574 |
| S15 | TX icsi | 251 |
| S14 | TX ivf | 1,142 |
| S13 | TX vitro fertili?ation | 2,914 |
| S12 | TX blastocyst* | 603 |
| S11 | TX embryo* | 10,541 |
| S10 | TX intracytoplasmic sperm injection* | 234 |
| S9 | (MM "Fertilization in Vitro") | 1,445 |
| S8 | (MM "Embryo Transfer") | 261 |
| S7 | TX disengage* | 917 |
| S6 | TX frozen | 3,308 |
| S5 | TX Vitrif* | 72 |
| S4 | TX thaw* | 576 |
| S3 | TX freez* | 2,163 |
| S2 | TX (cryopreservat* or cryofixation or cryonic suspension) | 1,111 |
| S1 | (MH "Cryopreservation+") | 1,143 |
Appendix 7. ClinicalTrials.gov search string
search terms https://clinicaltrials.gov/
(IVF OR ICSI OR embryo transfer) AND (freeze‐all OR frozen thawed embryo transfer OR cryopreservation OR disengage)
Appendix 8. WHO ICTRP search string
search terms who.int/trialsearch
(IVF OR ICSI OR embryo transfer) AND (freeze‐all OR frozen thawed embryo transfer OR cryopreservation OR disengage)
Data and analyses
Comparison 1.
Freeze‐all versus conventional IVF, outcomes per woman
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Live birth rate | 4 | 1892 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.91, 1.31] |
| 1.1 Live birth rate: cumulatively for cleavage stage transfer | 2 | 1633 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.91, 1.35] |
| 1.2 Live birth rate: cumulatively for blastocyst stage transfer | 2 | 259 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.60, 1.62] |
| 2 OHSS | 2 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 2.1 Per cycle with ovarian hyperstimulation | 2 | 1633 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.24 [0.15, 0.38] |
| 3 Ongoing pregnancy rate | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Cumulatively | 2 | 259 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.64, 1.73] |
| 4 Clinical pregnancy rate | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Cumulatively | 1 | 125 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.54, 2.19] |
| 5 Multiple pregnancy rate | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 After first ET | 2 | 1630 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.85, 1.44] |
| 6 Miscarriage rate | 4 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 After first ET | 4 | 1892 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.52, 0.86] |
| 7 Pregnancy complications | 2 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 7.1 After first ET | 2 | 1633 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.44 [1.08, 1.92] |
| 8 Birth weight of babies born | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.1 Birth weight of singletons | 1 | 462 | Mean Difference (IV, Fixed, 95% CI) | 161.80 [57.11, 266.49] |
| 8.2 Birth weight of multiples | 1 | 453 | Mean Difference (IV, Fixed, 95% CI) | ‐2.0 [‐94.08, 90.08] |
Comparison 2.
Freeze‐all versus conventional IVF, adverse events per clinical pregnancy
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Multiple pregnancy | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 After first ET | 2 | 939 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.77, 1.37] |
| 2 Miscarriage | 4 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 After first ET | 4 | 1058 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.41, 0.77] |
| 3 Pregnancy complications | 2 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 3.1 After first ET | 2 | 914 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.43 [1.05, 1.95] |
Comparison 3.
Freeze‐all versus conventional IVF, congenital abnormalities per live‐born children plus number of foetuses therapeutically terminated
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Congenital abnormalities | 1 | 923 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.66, 2.37] |
Differences between protocol and review
We added a method of analysing time to pregnancy (by hazard ratios), as this was not reported in the protocol; in the event, no data were available for this outcome.
We performed a subgroup analysis by timing of embryo transfer for the primary outcome of cumulative live birth.
We changed the unit of analysis for birth weight (from per woman to per baby).
We added some details to the section specifying our plans for the summary of findings table.
Congenital disorders, defined as the number of congenital abnormalities at birth, were reported per live‐born children plus number of foetuses therapeutically terminated in stead of per all clinical pregnancies.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Multicentre randomised controlled trial 14 reproductive medical centres throughout China Enrolment: June 2013 to May 2014 Power calculation: stated Randomisation: an online central randomisation system (www.medresman.org) was used. Nature of intervention: day 3 embryo cryopreservation by means of vitrification. Local investigators had the option to transfer day 2 embryos if there were fewer than 3 embryos on day 2. Follow‐up: cumulative live birth (including all frozen‐embryo transfers performed within 12 months after the initial transfer) |
|
| Participants | 1508 women (746 freeze‐all, 762 control) Inclusion criteria:
Exclusion criteria: history of unilateral oophorectomy, recurrent spontaneous abortion (defined as 3 or more previous spontaneous pregnancy losses), congenital or acquired uterine malformations, abnormal results on parental karyotyping, or medical conditions that contraindicated assisted reproductive technology or pregnancy |
|
| Interventions | For women who were assigned to the fresh embryo group, on day 3, 2 high‐quality embryos were picked out for fresh transfer and supernumerary embryos were transferred by means of vitrification. For women who were assigned to the frozen embryo group, there was no fresh transfer as all day 3 embryos were cryopreserved for later transfer. Local investigators had the option to transfer day 2 embryos if there were fewer than 3 embryos on day 2. In cycles following the menstrual cycle with ovum pickup, on day 4 of the progesterone regimen, 2 day 3 frozen embryos were thawed and transferred. |
|
| Outcomes | Primary outcome was a live birth, defined as delivery of any viable infant at 28 weeks or more of gestation during the first embryo transfer. Prespecified secondary outcomes included biochemical pregnancy, clinical pregnancy, ongoing pregnancy, singleton live birth, cumulative live birth (including subsequent frozen embryo transfer), pregnancy loss, moderate or severe OHSS, ectopic pregnancy, pregnancy and neonatal complications, and congenital anomalies. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | An online central randomisation system (www.medresman.org) was used to automatically generate the assignment sequence |
| Allocation concealment (selection bias) | Low risk | Assignment sequence was unknown to the clinical investigators. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of doctors and participants was not possible due to the nature of the intervention. Blinding of doctors to interim analyses of outcomes of the study was not reported. Blinding of investigators was not reported (which is relevant for determining end of study), therefore judged to be unclear risk. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessor blinding was not reported, however primary outcome is not likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were analysed for all randomised women. |
| Selective reporting (reporting bias) | Low risk | All registered outcomes reported. |
| Other bias | High risk | (Cumulative) data per subsequent menstrual or cryo‐transfer cycle not reported (relevant for time‐to‐pregnancy comparison and the related comparison of results after first transfer in frozen group vs results after first 2 transfers in fresh group). |
| Methods | Single‐centre randomised controlled trial Conducted: in Italy, from January 1996 until July 1997 Power calculation: not reported Randomisation: allocation was performed with sealed envelopes, timing of randomisation was not reported. Nature of intervention: slow freezing Follow‐up: until no cryopreserved embryos were left or delivery of child |
|
| Participants | 125 women (58 freeze‐all, 67 control) Inclusion criteria: all women with a high level of oestradiol the day of hCG administration (oestradiol ≥ 1500 pg/mL or ≥ 5.500 mmol/mL (conversion factor to SI unit 53.671)) and a high number of retrieved eggs (≥ 15 oocytes) |
|
| Interventions | Intervention: zygotes were cryopreserved, 3 or 4 zygotes were thawed and cultured for 36 to 40 h before embryo transfer. If 2 or more zygotes did not cleave 24 h after being cultured, 1 or 2 additional zygotes were thawed. Control: zygotes were cultured for a subsequent 48 h, 3 or 4 fresh embryos were transferred, surplus embryos were cryopreserved. |
|
| Outcomes | Clinical pregnancies: gestational sac and foetal heartbeat by ultrasound | |
| Notes | Funding was not reported. Additional information was obtained from the authors by email. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence was used, but it is unclear whether envelopes were opaque and sequentially numbered. |
| Allocation concealment (selection bias) | Low risk | Allocation concealment was performed with sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of doctors and participants was not possible due to the nature of the intervention. Blinding of doctors to interim analyses of outcomes of the study was not reported. Blinding of investigators was not reported (which is relevant for determining end of study), therefore judged to be unclear risk. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessor blinding was not reported, however primary outcome is not likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were analysed for all randomised women. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. |
| Other bias | High risk | No power calculation reported. Unclear what determined the end of study. (Cumulative) data per subsequent menstrual or cryo‐transfer cycle not reported (relevant for time‐to‐pregnancy comparison and the related comparison of results after first transfer in frozen group vs results after first 2 transfers in fresh group). |
| Methods | Single‐centre randomised controlled trial Conducted: in the United States, from October 2007 until October 2010 Power calculation: stated. However, study was prematurely terminated after interim analysis. Randomisation: performed after retrieval by random drawing among identical, opaque, unmarked sealed envelopes Nature of intervention: slow freezing Follow‐up: clinical pregnancy after first embryo transfer |
|
| Participants | 137 women (70 freeze‐all, 67 fresh transfer) Inclusion criteria:
Exclusion criteria: genetic testing of embryos was excluded. |
|
| Interventions | Intervention: 2pn oocytes were frozen, and entire cohorts of frozen 2pn oocytes were thawed and subsequently cultured to the blastocyst stage. The morphologically best 1 or 2 blastocysts were transferred on the first day on which at least 1 good expanded blastocyst appeared. Supernumerary expanded blastocysts of high quality were cryopreserved. Control: fresh blastocysts transfer |
|
| Outcomes |
|
|
| Notes | Funding: research grant from the Investigator‐Initatiated trial research grant from Ferring Pharmaceuticals, Parsippany, NJ. Medications for this study were also provided by Ferring Pharmaceuticals. Time period was obtained from trial register. Additional information was obtained from authors by email. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Drawing randomly among identical, opaque, unmarked, sealed envelopes. |
| Allocation concealment (selection bias) | Low risk | Drawing randomly among identical, opaque, unmarked, sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of doctors and participants was not possible due to the nature of the intervention. Blinding of doctors to interim analyses of outcomes of the study was not reported. Blinding of investigators was not reported (which is relevant for determining end of study), therefore judged to be unclear risk. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessor blinding was not reported, however primary outcome is not likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Data were not reported for all women randomised, but per transfer. Dropouts and loss to follow‐up were not accounted for in the analysis. No ITT analysis was performed. Sufficient data available for analysis per woman in meta‐analysis. Ongoing pregnancy was determined at 10 weeks' gestation instead of 12 weeks' gestation. |
| Selective reporting (reporting bias) | Low risk | All registered outcomes reported. |
| Other bias | High risk | Trial was pre‐terminated after interim analysis. Interim analysis was preplanned, but calculated per transfer (unit of analysis error) with a P value of 0.03, overestimating possible effects. (Cumulative) data per subsequent menstrual or cryo‐transfer cycle not reported (relevant for time‐to‐pregnancy comparison and the related comparison of results after first transfer in frozen group vs results after first 2 transfers in fresh group). |
| Methods | Single‐centre randomised controlled trial Conducted: in the United States, from July 2007 until July 2010 Power calculation: Power calculation: stated (referred to Shapiro 2011a). However, study was terminated because of differing embryo quality between the 2 groups. Randomisation: performed after retrieval by random drawing among identical, opaque, unmarked, sealed envelopes Nature of intervention: slow freezing Follow‐up: clinical pregnancy after 1 embryo transfer |
|
| Participants | 122 women (60 freeze‐all, 62 control) Inclusion criteria:
Exclusion criteria: genetic testing of embryos was excluded. |
|
| Interventions | Intervention: 2pn oocytes were frozen, and entire cohorts of frozen 2pn oocytes were thawed and subsequently cultured to the blastocyst stage. The morphologically best 1 or 2 blastocysts were transferred on the first day on which at least 1 good expanded blastocyst appeared. Supernumerary expanded blastocysts of high quality were cryopreserved. Control: fresh blastocysts transfer |
|
| Outcomes |
|
|
| Notes | Funding: research grant from the Investigator‐Initatiated Studies Program of Merck Sharp & Dohme Time period was obtained from trial register. Additional information was obtained from authors by email. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Drawing randomly among identical, opaque, unmarked, sealed envelopes. |
| Allocation concealment (selection bias) | Low risk | Drawing randomly among identical, opaque, unmarked, sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of doctors and participants was not possible due to the nature of the intervention. Blinding of doctors to interim analyses of outcomes of the study was not reported. Blinding of investigators was not reported (which is relevant for determining end of study), therefore judged to be unclear risk. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessor blinding was not reported, however primary outcome is not likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Data were not reported for all women randomised, but per transfer. Dropouts and loss to follow‐up were not accounted for in the analysis. No ITT analysis was performed. Sufficient data available for analysis per woman in meta‐analysis. Ongoing pregnancy was determined at 10 weeks' gestation instead of 12 weeks' gestation. |
| Selective reporting (reporting bias) | Low risk | All registered outcomes reported. |
| Other bias | High risk | Trial was pre‐terminated after interim analysis. Interim analysis was preplanned, but calculated per transfer (unit of analysis error) with a P value of 0.03, overestimating possible effects. Stopping rules for interim analysis (embryo quality) were unclear. (Cumulative) data per subsequent menstrual or cryo‐transfer cycle not reported (relevant for time‐to‐pregnancy comparison and the related comparison of results after first transfer in frozen group vs results after first 2 transfers in fresh group). |
2pn: 2 pro‐nucleate FSH: follicle‐stimulating hormone hCG: human chorionic gonadotropin ITT: intention‐to‐treat IVF: in vitro fertilisation OHSS: ovarian hyperstimulation syndrome
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Absalan 2013 | Unclear from report whether trial was a RCT, and the authors did not respond to our request for further information. |
| Aflatoonian 2010 | This article has been retracted from the literature at the request of the Editor and the ASRM Publications Committee. |
| Boostanfar 2016 | Randomised a different intervention |
| Yang 2015 | One‐third of participants chose to be in group 3 after randomisation. Not considered a properly randomised RCT. |
ASRM: American Society for Reproductive Medicine RCT: randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Single‐centre randomised controlled trial Conducted: in India from September 2013 until September 2014 Power calculation: not reported Randomisation: computer‐based sequence generation, remote allocation Nature of intervention: day 3 frozen embryo transfer (FET), using rapid freezing Follow‐up: unclear (see notes) |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Controlled ovarian stimulation was achieved mainly using the gonadotropin‐releasing hormone (GnRH) antagonist for pituitary suppression and recombinant follicle‐stimulating hormone. Women underwent pituitary desensitisation with the use of GnRH antagonist. Immediately after the ovum pick‐up, intracytoplasmic sperm injection was performed for all the oocytes. The day 3 embryos were either transferred in the same cycle or were frozen using vitrification technique and transferred in the next cycle. |
| Outcomes | Conception/pregnancy: definition unclear |
| Notes | Attempts to contact authors unsuccessful to date (January 2017). Unclear whether follow‐up continued until a live birth occurred or until all embryos from the initial cycle were transferred. Poor reporting of results: both groups labelled as FET in study tables, no clear definition of pregnancy. |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | A randomized study of IVF patients to assess whether freezing all of the embryos and transferring them in a later natural, unstimulated cycle results in a higher pregnancy rate than transferring an embryo 5 days after egg collection |
| Methods | RCT Target enrolment: 200 |
| Participants | Included:
Excluded:
|
| Interventions | Both study groups will undertake a stimulated IVF cycle. The first (intervention) group will have all embryos cryostored electively for transfer in a later natural menstrual cycle. The second group will have the best‐quality embryo transferred to the endometrial cavity fresh and all remaining embryos cryostored. The protocol for the second group is standard practice today. Both groups will undertake the same drug regimen, therefore there is no difference in drug intervention. |
| Outcomes |
|
| Starting date | 1 May.2012 |
| Contact information | Mark Livingstone: ecosse@ihug.com.au |
| Notes | www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=362361 |
| Trial name or title | Comparison of the probability of live birth after elective freezing of all embryos versus standard fresh embryo transfer in patients undergoing in‐vitro fertilisation (IVF) |
| Methods | RCT Target enrolment: 400 |
| Participants | Women aged 18 to 39 with indication for COS and IVF or ICSI with autologous gametes Key inclusion criteria:
Key exclusion criteria:
|
| Interventions | |
| Outcomes |
|
| Starting date | May 2016 |
| Contact information | Christos Venetis: c.venetis@unsw.edu.au |
| Notes | www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12616000643471 |
| Trial name or title | Freezing of embryos in assisted conception: a randomized controlled trial evaluating the clinical and cost‐effectiveness of a policy of freezing embryos followed by thawed frozen embryo transfer, compared with a policy of fresh embryo transfer in women undergoing in‐vitro fertilization |
| Methods | RCT Estimated enrolment: 1086 |
| Participants | Inclusion criteria:
Exclusion criteria: Couples in whom:
|
| Interventions | Intervention arm: All good‐quality embryos will be frozen and couples will undergo frozen‐thawed embryo transfer within 3 months of the egg collection process. Couples will attend a clinic visit and additional monitoring visits before frozen embryo transfer is performed. Standard‐care arm: Women will undergo fresh embryo transfer on day 3 or 5 (after egg collection). |
| Outcomes |
|
| Starting date | 1 March 2015 |
| Contact information | Christina Cole: christina.cole@npeu.oxa.c.uk |
| Notes | www.isrctn.com/ISRCTN61225414 |
| Trial name or title | Comparison of frozen‐thawed embryo transfers and fresh embryo transfers with whole chromosome analysis using next generation sequencing |
| Methods | RCT Estimated enrolment: 186 |
| Participants | Women aged 18 to 42 Inclusion criteria (pre‐stimulation):
Exclusion criteria (pre‐stimulation):
|
| Interventions | Frozen embryo transfer with PGD: All embryos will be hatched on day 3. Women will have hatching blastocysts* biopsied on day 5 or day 6, embryos will then be vitrified, analysed by NGS, and women will have 1 or 2 euploid embryo(s) thawed and transferred on a FET cycle, before noon. *If more than 2 euploid blastocysts are available, the one(s) to be transferred will be selected based on morphology. Fresh embryo transfer with PGD: All embryos will be hatched on day 3. Women will have hatching blastocysts* biopsied on day 5, analysed by NGS, and will have 1 or 2 euploid embryo(s) transferred on day 6, in the a.m. Any morulas developing to hatching blastocyst on day 6 will be also analysed but vitrified for use in a future cycle. *If more than 2 euploid blastocysts are available, the one(s) to be transferred will be selected based on morphology. |
| Outcomes |
|
| Starting date | September 2013 |
| Contact information | Study director: Santiago Munne, Reprogenetics |
| Notes | clinicaltrials.gov/ct2/show/NCT02000349 |
| Trial name or title | Efficacy study of segmentation of PGD treatment |
| Methods | RCT Estimated enrolment: 240 |
| Participants | Women aged 20 to 40 Inclusion criteria:
Exclusion criteria:
|
| Interventions | Elective cryopreservation of available embryos after PGD PGD and elective fresh embryo transfer plus cryopreservation of supernumerary available embryos after PGD |
| Outcomes | Cumulative live birth rate of a single PGD treatment |
| Starting date | May 2014 |
| Contact information | Willem Verpoest, Centre for Reproductive Medicine UZ Brussel |
| Notes |
| Trial name or title | Implantation enhancement by elective cryopreservation of all viable embryos (ICE) |
| Methods | RCT Estimated enrolment: 212 |
| Participants | Women aged 18 to 40 Inclusion criteria:
Exclusion criteria:
|
| Interventions | Intervention: Elective vitrification with subsequent‐cycle embryo thawing/transfer (Cryo Bio System) will be performed. Hence, no luteal‐phase support will be provided immediately after oocyte retrieval. Instead, women will wait for a subsequent cycle before starting exogenous hormone therapy for endometrial preparation. On the day of embryo transfer, blastocyst(s) will be warmed one by one until 1 or 2 blastocysts are suitable for transfer. Embryo transfer to the uterine cavity will be performed under ultrasound guidance whenever possible. Control: Following oocyte retrieval, intensified luteal‐phase support for fresh embryo transfer (with hCG (Pregnyl), progesterone (Utrogestan), and oestradiol valerate (Progynova)) will be performed. Fresh embryo transfer in the uterine cavity will be performed on the 5th day of embryo development at blastocyst stage under ultrasound guidance whenever possible. |
| Outcomes |
|
| Starting date | May 2014 |
| Contact information | Samuel Santos‐Ribeiro: samuel.ribeiro@uzbrussel.be |
| Notes | clinicaltrials.gov/ct2/show/NCT02148393 |
| Trial name or title | Freeze all protocol versus fresh embryo transfer in women undergoing in‐vitro fertilization (IVF) |
| Methods | RCT Estimated enrolment: 780 |
| Participants | Women aged 18 to 42 Inclusion criteria:
Exclusion criteria:
|
| Interventions | Freeze‐all protocol: Embryos are selected for cryopreservation using vitrification technique. 2 vitrified embryos will be warmed and transferred in subsequent cycle. Fresh transfer protocol: 2 embryos are selected and transferred fresh in the same cycle. |
| Outcomes |
|
| Starting date | June 2015 |
| Contact information | Lan TN Vuong: drlan@yahoo.com.vn Vinh Q Dang: bsvinh.dq@myduchospital.vn |
| Notes | clinicaltrials.gov/ct2/show/record/NCT02471573 |
| Trial name or title | Clinical effectiveness of frozen thawed embryo transfer compared to fresh embryo transfer |
| Methods | RCT Estimated enrolment: 800 |
| Participants | Women aged 18 to 42 Inclusion criteria:
Exclusion criteria:
|
| Interventions | Intervention: Fresh embryo transfer will not be undertaken in this group. Embryos will be frozen by vitrification or slow freezing at cleavage or blastocyst stage according to standard agreed local protocols. Women will be contacted after 4 weeks and arrangements made for frozen embryo transfer. Control: Women allocated to the control arm will either undergo fresh embryo transfer at cleavage stage or extended culture and transfer at blastocyst stage according to local policy. A maximum of 2 embryos or blastocysts will be replaced according to the standard protocol under transabdominal ultrasound guidance. Luteal‐phase support is given according to local protocols. |
| Outcomes |
|
| Starting date | October 2015 |
| Contact information | Ernest HY Ng: nghye@hku.hk |
| Notes | clinicaltrials.gov/ct2/show/record/NCT02570386 |
| Trial name or title | Management of recurrent implantation failure (RIF) |
| Methods | RCT Estimated enrolment: 200 |
| Participants | Women aged 20 to 39 Inclusion criteria:
Exclusion criteria: Couples with testicular or epididymal sperm were excluded. |
| Interventions | Fresh embryo transfer: ICSI cycle followed by day 5 fresh embryo transfer. Freeze‐all: ICSI cycle, all embryos were cryopreserved at day 5 and transferred in a consecutive natural cycle. |
| Outcomes |
|
| Starting date | February 2012 |
| Contact information | Yasmin Magdi, Research and Development Department Director, TopLab Company for ART Laboratories Consultation and Training |
| Notes | clinicaltrials.gov/ct2/show/record/NCT02681367 |
| Trial name or title | Comparing pregnancy outcomes in good prognosis patients between fresh and 'freeze‐all' single blastocyst transfers |
| Methods | RCT Estimated enrolment: 118 |
| Participants | Women aged 18 to 35 Inclusion criteria:
Exclusion criteria:
|
| Interventions | Freeze‐all protocol: All good morphologic quality blastocysts are vitrified on day 5 or 6. The best‐quality vitrified blastocyst frozen on day 5 will be warmed and transferred in a subsequent cycle. Fresh protocol: Women receive fresh embryo transfer of best morphologic quality blastocyst on day 5 and vitrification of all good‐quality supernumerary blastocysts. |
| Outcomes |
|
| Starting date | September 2015 |
| Contact information | Stephanie Jewell: sjewell@mtsinai.on.ca Jason E Elliott: jelliott@mtsinai.on.ca |
| Notes | Contact: Jason E Elliott, MD, MSc |
| Trial name or title | Study comparing outcomes between conventional IVF and a "freeze‐all"‐strategy in assisted reproductive technology |
| Methods | RCT Estimated enrolment: 424 |
| Participants | Women aged 18 to 39 Inclusion criteria:
Exclusion criteria:
|
| Interventions | Freeze‐all: transfer of a frozen‐thawed blastocyst in a subsequent natural menstrual cycle Fresh embryo transfer: standard procedure |
| Outcomes |
|
| Starting date | May 2016 |
| Contact information | Sacha Stormlund: sacha.stormlund.01@regionh.dk Anja Pinborg: anja.bisgaard.pinborg@regionh.dk |
| Notes | clinicaltrials.gov/ct2/show/record/NCT02746562 |
| Trial name or title | A single‐center non‐blinded randomised controlled trial on the effect of ovarian hyperstimulation on endometrial receptivity |
| Methods | RCT Target enrolment: 193 |
| Participants | Women aged < 43 years Inclusion criteria:
Exclusion criteria:
|
| Interventions | Experimental arm: All embryos will be cryopreserved for subsequent transfer in artificial cycles. Ovarian hyperstimulation, oocyte retrieval, and oocyte fertilisation will be performed using standard procedures. Control arm: 1 or 2 fresh embryo(s) will be transferred in the same cycle with cryopreservation of all supernumerary embryos and subsequent transfer of frozen‐thawed embryos in artificial cycles if pregnancy is not achieved after fresh transfer. |
| Outcomes |
|
| Starting date | January 2013 |
| Contact information | Sebastian Mastenbroek: S.Mastenbroek@amc.uva.nl |
| Notes | www.trialregister.nl/trialreg/admin/rctview.asp?TC=3187 |
AFC: antral follicle count AMH: anti‐Müllerian hormone BMI: body mass index COS: controlled ovarian stimulation FET: frozen embryo transfer FSH: follicle‐stimulating hormone GnRH: gonadotropin‐releasing hormone hCG: human chorionic gonadotropin ICSI: intracytoplasmic sperm injection IDDM: Insuline‐Dependent Diabetes Mellitus IVF: in vitro fertilisation LH: luteinising hormone MESA: microsurgical epididymal sperm aspiration NGS: next‐generation sequencing NIDDM: Non‐Insuline‐Dependent Diabetes Mellitus OHSS: ovarian hyperstimulation syndrome PGD: pre‐implantation genetic diagnosis PGS: pre‐implantation genetic screening PRL: prolactine RCT: randomised clinical trial SHBG: sex hormone‐binding globulin TESA: testicular sperm aspiration TSH: thyroid‐stimulating hormone
Contributions of authors
Kai Mee Wong and Sebastiaan Mastenbroek wrote the review. Kai Mee Wong, Sjoerd Repping, and Sebastiaan Mastenbroek developed the concept of the study. Madelon van Wely, Femke Mol, and Sjoerd Repping provided feedback on the review.
Sources of support
Internal sources
None, Other.
External sources
None, Other.
Declarations of interest
Kai Mee Wong: none known Madelon van Wely: none known Femke Mol: none known Sjoerd Repping: none known Sebastiaan Mastenbroek is principal investigator of one of the ongoing studies.
New
References
References to studies included in this review
- Chen ZJ, Shi Y, Sun Y, Zhang B, Liang X, Cao Y, et al. Fresh versus frozen embryos for infertility in the polycystic ovary syndrome. New England Journal of Medicine 2016;375(6):523‐33. [DOI] [PubMed] [Google Scholar]
- Ferraretti AP, Gianaroli L, Magli C, Fortini D, Selman HA, Feliciani E. Elective cryopreservation of all pronucleate embryos in women at risk of ovarian hyperstimulation syndrome: efficiency and safety. Human Reproduction 1999;14:1457‐60. [DOI: 10.1093/humrep/14.6.1457; PMID: 10357958 ] [DOI] [PubMed] [Google Scholar]
- Shapiro BS, Daneshmand ST, Garner FC, Aguirre M, Hudson C, Thomas S. Evidence of impaired endometrial receptivity after ovarian stimulation for in vitro fertilization: A prospective randomized trial comparing fresh and frozen‐thawed embryo transfer in normal responders. Fertility and Sterility 2011;96:344‐8. [DOI: 10.1016/j.fertnstert.2011.05.050; PMID: 21737072 ] [DOI] [PubMed] [Google Scholar]
- Shapiro BS, Daneshmand ST, Garner FC, Aguirre M, Hudson C, Thomas S. Evidence of impaired endometrial receptivity after ovarian stimulation for in vitro fertilization: A prospective randomized trial comparing fresh and frozen‐thawed embryo transfers in high responders. Fertility and Sterility 2011;96:516‐8. [DOI: 10.1016/j.fertnstert.2011.02.059; PMID: 21737071 ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
- Absalan F, Ghannadi A, Kazerooni M. Reproductive outcome following thawed embryo transfer in management of ovarian hyperstimulation syndrome. Journal of Reproduction and Infertility 2013;14(3):133‐7. [PMID: 24163797 ] [PMC free article] [PubMed] [Google Scholar]
- Aflatoonian A, Oskouian H, Ahmadi S, Oskouian L. Can fresh embryo transfers be replaced by cryopreserved‐thawed embryo transfers in assisted reproductive cycles? A randomized controlled trial. Journal of Assisted Reproduction and Genetics 2010;27:357‐63. [DOI: 10.1007/s10815-010-9412-9; PMID: 20373015 ] [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]; Editor and the ASRM Publications Committee. Retraction note to: Can fresh embryo transfers be replaced by cryopreserved‐thawed embryo transfers in assisted reproductive cycles? A randomized controlled trial. Journal of Assisted Reproduction and Genetics 2013;30(9):1245. [DOI: 10.1007; PMID: 23975193 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boostanfar R, Gates D, Guan Y, Gordon K, McCrary Sisk C, Stegmann B. Efficacy and safety of frozen‐thawed embryo transfer in women aged 35 to 42 years from the PURSUE randomized clinical trial. Fertility and Sterility 2016;106(2):300‐5. [DOI] [PubMed] [Google Scholar]
- Yang S, Pang T, Li R, Yang R, Zhen X, Chen X, et al. The individualized choice of embryo transfer timing for patients with elevated serum progesterone level on the HCG day in IVF/ICSI cycles: a prospective randomized clinical study. Gynecological Endocrinology: the official journal of the International Society of Gynecological Endocrinology 2015;31(5):355‐8. [PUBMED: 25558791] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
- Chandel N, Bhat V, Bhat B, Chandel S. Outcome analysis of day‐3 frozen embryo transfer v/s fresh embryo transfer in infertility: a prospective therapeutic study in Indian scenario. Journal of Obstetrics and Gynecology of India 2016;66(5):345‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
- ACTRN12612000422820. A randomised controlled trial to determine the effect of elective embryo cryopreservation and subsequent transfer in a natural menstrual cycle on clinical pregnancy rates in infertile females. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=362361 (first received 11 April 2012).
- ACTRN12616000643471. Fresh vs. elective frozen embryo transfer after IVF: a randomised controlled trial. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=370373 (first received 24 March 2016).
- ISRCTN61225414. Freezing of embryos in assisted conception: a randomised controlled trial evaluating the clinical and cost‐effectiveness of a policy of freezing embryos followed by thawed frozen embryo transfer, compared with a policy of fresh embryo transfer in women undergoing in‐vitro fertilization. www.isrctn.com/ISRCTN61225414 (first received 24 December 2015). [DOI] [PMC free article] [PubMed]
- NCT02000349. Comparison of frozen‐thawed embryo transfers and fresh embryo transfers with whole chromosome analysis using next generation sequencing. clinicaltrials.gov/ct2/show/NCT02000349 (first received 19 September 2013).
- NCT02133950. Efficacy study of segmentation of PGD treatment. clinicaltrials.gov/ct2/show/NCT02133950 (first received 6 May 2014).
- NCT02148393. Implantation enhancement by elective cryopreservation of all viable embryos (ICE). clinicaltrials.gov/ct2/show/NCT02148393 (first received 16 May 2014).
- NCT02471573. Freeze all protocol versus fresh embryo transfer in women undergoing in‐vitro fertilization (IVF). clinicaltrials.gov/ct2/show/NCT02471573 (first received 11 June 2015).
- NCT02570386. Clinical effectiveness of frozen thawed embryo transfer compared to fresh embryo transfer. clinicaltrials.gov/ct2/show/record/NCT02570386 (first received 29 September 2015).
- NCT02681367. Management of recurrent implantation failure (RIF). clinicaltrials.gov/ct2/show/NCT02681367 (first received 8 February 2016).
- NCT02712840. Comparing pregnancy outcomes in good prognosis patients between fresh and 'freeze‐all' single blastocyst transfers. clinicaltrials.gov/ct2/show/NCT02712840 (first received 9 September 2015).
- NCT02746562. Study comparing outcomes between conventional IVF and a "freeze‐all"‐strategy in assisted reproductive technology. clinicaltrials.gov/ct2/show/NCT02746562 (first received 18 April 2016).
- NTR3187. A single‐center non‐blinded randomized controlled trial on the effect of ovarian hyperstimulation on endometrial receptivity. www.trialregister.nl/trialreg/admin/rctview.asp?TC=3187 (first registered 9 December 2011).
Additional references
- Bourgain C, Devroey P. The endometrium in stimulated cycles for IVF. Human Reproduction Update 2003;9:515‐22. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Assisted Reproductive Technology Report; 2011. www.cdc.gov/art/ART2011/section5.htm (accessed 25 April 2014).
- European Society of Human Reproduction and Embryology (ESHRE). ART Fact Sheet; 2010. www.eshre.eu/ESHRE/English/Guidelines‐Legal/ART‐fact‐sheet/page.aspx/1061 (accessed 25 April 2014).
- Ferraretti AP, Magli C, Feliciani E, Montanaro N, Gianaroli L. Relationship of timing of agonist administration in the cycle phase to the ovarian response to gonadotropins in the long down‐regulation protocols for assisted reproductive technologies. Fertility and Sterility 1996;65(1):114‐21. [DOI] [PubMed] [Google Scholar]
- McMaster University. GRADEpro GDT. Version accessed XXX. Hamilton (ON): GRADE Working Group: McMaster University, 2014.
- Haouzi D, Assou S, Mahmoud K, Tondeur S, Reme T, Hedon B, et al. Gene expression profile of human endometrial receptivity: comparison between natural and stimulated cycles for the same patients. Human Reproduction 2009;24:1436‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
- Hull MG, Glazener CM, Kelly NJ, Conway DI, Foster PA, Hinton RA, et al. Population study of causes, treatment, and outcome of infertility. British Medical Journal (Clinical Research Ed.) 1985;291:1693‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolibianakis E, Bourgain C, Albano C, Osmanagaoglu K, Smitz J, Steirteghem A, et al. Effect of ovarian stimulation with recombinant follicle‐stimulating hormone, gonadotropin releasing hormone antagonists, and human chorionic gonadotropin on endometrial maturation on the day of oocyte pick‐up. Fertility and Sterility 2002;78:1025‐9. [DOI] [PubMed] [Google Scholar]
- Maheshwari A, Bhattacharya S. Elective frozen replacement cycles for all: ready for prime time?. Human Reproduction 2013;28:6‐9. [DOI] [PubMed] [Google Scholar]
- Mastenbroek S, Veen F, Aflatoonian A, Shapiro B, Bossuyt P, Repping S. Embryo selection in IVF. Human Reproduction 2011;26:964‐6. [DOI] [PubMed] [Google Scholar]
- The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre: The Cochrane Collaboration, 2014.
- Roque M, Lattes K, Serra S, Sola I, Geber S, Carreras R, et al. Fresh embryo transfer versus frozen embryo transfer in in vitro fertilization cycles: a systematic review and meta‐analysis. Fertility and Sterility 2013;99:156‐62. [DOI] [PubMed] [Google Scholar]
- Vail A, Gardener E. Common statistical errors in the design and analysis of subfertility trials. Human Reproduction 2003;18:1000‐4. [DOI] [PubMed] [Google Scholar]
- Voorhis BJ. Clinical practice. In vitro fertilization. New England Journal of Medicine 2007;356:379‐86. [DOI] [PubMed] [Google Scholar]
- Wong KM, Mastenbroek S, Repping S. Cryopreservation of human embryos and its contribution to IVF success rates. Fertility and Sterility 2014;102(1):19‐26. [DOI] [PubMed] [Google Scholar]


