Abstract
Background
Down's syndrome occurs when a person has three, rather than two copies of chromosome 21; or the specific area of chromosome 21 implicated in causing Down's syndrome. It is the commonest congenital cause of mental disability and also leads to numerous metabolic and structural problems. It can be life‐threatening, or lead to considerable ill health, although some individuals have only mild problems and can lead relatively normal lives. Having a baby with Down’s syndrome is likely to have a significant impact on family life.
Non‐invasive screening based on biochemical analysis of maternal serum or urine, or fetal ultrasound measurements, allows estimates of the risk of a pregnancy being affected and provides information to guide decisions about definitive testing.
Before agreeing to screening tests, parents need to be fully informed about the risks, benefits and possible consequences of such a test. This includes subsequent choices for further tests they may face, and the implications of both false positive and false negative screening tests (i.e. invasive diagnostic testing, and the possibility that a miscarried fetus may be chromosomally normal). The decisions that may be faced by expectant parents inevitably engender a high level of anxiety at all stages of the screening process, and the outcomes of screening can be associated with considerable physical and psychological morbidity. No screening test can predict the severity of problems a person with Down's syndrome will have.
Objectives
To estimate and compare the accuracy of first trimester ultrasound markers alone, and in combination with first trimester serum tests for the detection of Down’s syndrome.
Search methods
We carried out extensive literature searches including MEDLINE (1980 to 25 August 2011), Embase (1980 to 25 August 2011), BIOSIS via EDINA (1985 to 25 August 2011), CINAHL via OVID (1982 to 25 August 2011), and The Database of Abstracts of Reviews of Effects (the Cochrane Library 2011, Issue 7). We checked reference lists and published review articles for additional potentially relevant studies.
Selection criteria
Studies evaluating tests of first trimester ultrasound screening, alone or in combination with first trimester serum tests (up to 14 weeks' gestation) for Down's syndrome, compared with a reference standard, either chromosomal verification or macroscopic postnatal inspection.
Data collection and analysis
Data were extracted as test positive/test negative results for Down's and non‐Down's pregnancies allowing estimation of detection rates (sensitivity) and false positive rates (1‐specificity). We performed quality assessment according to QUADAS criteria. We used hierarchical summary ROC meta‐analytical methods to analyse test performance and compare test accuracy. Analysis of studies allowing direct comparison between tests was undertaken. We investigated the impact of maternal age on test performance in subgroup analyses.
Main results
We included 126 studies (152 publications) involving 1,604,040 fetuses (including 8454 Down's syndrome cases). Studies were generally good quality, although differential verification was common with invasive testing of only high‐risk pregnancies. Sixty test combinations were evaluated formed from combinations of 11 different ultrasound markers (nuchal translucency (NT), nasal bone, ductus venosus Doppler, maxillary bone length, fetal heart rate, aberrant right subclavian artery, frontomaxillary facial angle, presence of mitral gap, tricuspid regurgitation, tricuspid blood flow and iliac angle 90 degrees); 12 serum tests (inhibin A, alpha‐fetoprotein (AFP), free beta human chorionic gonadotrophin (ßhCG), total hCG, pregnancy‐associated plasma protein A (PAPP‐A), unconjugated oestriol (uE3), disintegrin and metalloprotease 12 (ADAM 12), placental growth factor (PlGF), placental growth hormone (PGH), invasive trophoblast antigen (ITA) (synonymous with hyperglycosylated hCG), growth hormone binding protein (GHBP) and placental protein 13 (PP13)); and maternal age. The most frequently evaluated serum markers in combination with ultrasound markers were PAPP‐A and free ßhCG.
Comparisons of the 10 most frequently evaluated test strategies showed that a combined NT, PAPP‐A, free ßhCG and maternal age test strategy significantly outperformed ultrasound markers alone (with or without maternal age) except nasal bone, detecting about nine out of every 10 Down's syndrome pregnancies at a 5% false positive rate (FPR). In both direct and indirect comparisons, the combined NT, PAPP‐A, free ßhCG and maternal age test strategy showed superior diagnostic accuracy to an NT and maternal age test strategy (P < 0.0001). Based on the indirect comparison of all available studies for the two tests, the sensitivity (95% confidence interval) estimated at a 5% FPR for the combined NT, PAPP‐A, free ßhCG and maternal age test strategy (69 studies; 1,173,853 fetuses including 6010 with Down's syndrome) was 87% (86 to 89) and for the NT and maternal age test strategy (50 studies; 530,874 fetuses including 2701 Down's syndrome pregnancies) was 71% (66 to 75). Combinations of NT with other ultrasound markers, PAPP‐A and free ßhCG were evaluated in one or two studies and showed sensitivities of more than 90% and specificities of more than 95%.
High‐risk populations (defined before screening was done, mainly due to advanced maternal age of 35 years or more, or previous pregnancies affected with Down's syndrome) showed lower detection rates compared to routine screening populations at a 5% FPR. Women who miscarried in the over 35 group were more likely to have been offered an invasive test to verify a negative screening results, whereas those under 35 were usually not offered invasive testing for a negative screening result. Pregnancy loss in women under 35 therefore leads to under‐ascertainment of screening results, potentially missing a proportion of affected pregnancies and affecting test sensitivity. Conversely, for the NT, PAPP‐A, free ßhCG and maternal age test strategy, detection rates and false positive rates increased with maternal age in the five studies that provided data separately for the subset of women aged 35 years or more.
Authors' conclusions
Test strategies that combine ultrasound markers with serum markers, especially PAPP‐A and free ßhCG, and maternal age were significantly better than those involving only ultrasound markers (with or without maternal age) except nasal bone. They detect about nine out of 10 Down’s affected pregnancies for a fixed 5% FPR. Although the absence of nasal bone appeared to have a high diagnostic accuracy, only five out of 10 affected Down's pregnancies were detected at a 1% FPR.
Plain language summary
Screening tests for Down’s syndrome in first 24 weeks of pregnancy
Background Down's syndrome (also known as Down's or Trisomy 21) is an incurable genetic disorder that causes significant physical and mental health problems, and disabilities. However, there is wide variation in how Down's affects people. Some individuals are severely affected whilst others have mild problems and are able to lead relatively normal lives. There is no way of predicting how badly a baby might be affected.
Expectant parents are given the choice to be tested for Down’s during pregnancy to assist them in making decisions. If a mother is carrying a baby with Down’s, then there is the decision about whether to terminate or continue with the pregnancy. The information offers parents the opportunity to plan for life with a Down’s child.
The most accurate tests for Down’s involve testing fluid from around the baby (amniocentesis) or tissue from the placenta (chorionic villus sampling (CVS)) for the abnormal chromosomes associated with Down’s. Both these tests involve inserting needles through the mother's abdomen and are known to increase the risk of miscarriage. Thus the tests are not suitable for offering to all pregnant women. Rather, tests that measure markers in the mother’s blood, urine or on ultrasound scans of the baby are used for screening. These screening tests are not perfect, they can miss cases of Down’s and also give a ‘high risk’ test results to a number of women whose babies are not affected by Down’s. Thus pregnancies identified as ‘high risk’ using these screening tests require further testing using amniocentesis (from 15 weeks' gestation) or CVS (from 10 + 0 to 13 + 6 weeks' gestation) to confirm a diagnosis of Down’s.
What we did The aim of this review was to find out which of the first trimester ultrasound screening tests, with or without first trimester serum tests done during the first 14 weeks of pregnancy are the most accurate at predicting the risk of a pregnancy being affected by Down's. We looked at 11 different ultrasound markers and 12 different serum markers that can be used alone, in ratios or in combination, taken before 14 weeks' gestation, thus creating 60 screening tests for Down’s. We found 126 studies, involving 1,604,040 fetuses (including 8454 fetuses affected by Down's syndrome).
What we found For the first 14 weeks of pregnancy, the evidence supports the use of first trimester ultrasound tests in combination with two serum (blood) markers ‐ especially pregnancy‐associated plasma protein A (PAPP‐A) and free beta human chorionic gonadotrophin (ßhCG) ‐ and maternal age, for Down's syndrome screening. In general, these tests are better than ultrasound markers on their own. They detect nine out of 10 pregnancies affected by Down's syndrome. Five per cent of women undertaking the test will have a high risk test result, however the majority of these pregnancies will not be affected by Down's syndrome. Other important information to consider The ultrasound tests themselves have no adverse effects for the woman, blood tests can cause discomfort, bruising and rarely infection. However some women who have a ‘high risk’ screening test result, and are given amniocentesis or CVS have a risk of miscarrying a baby unaffected by Down’s. Parents will need to weigh up this risk when deciding whether or not to have an amniocentesis or CVS following a ‘high risk’ screening test result.
Summary of findings
Background
This is one of a series of reviews on antenatal screening for Down's syndrome following a generic protocol (Alldred 2010) ‐ see Published notes for more details.
Target condition being diagnosed
Down’s syndrome
Down’s syndrome affects approximately one in 800 live‐born babies (Cuckle 1987). It results from a person having three, rather than two, copies of chromosome 21 — or the specific area of chromosome 21 implicated in causing Down's syndrome — as a result of trisomy or translocation. If not all cells are affected, the pattern is described as 'mosaic'. Down’s syndrome can cause a wide range of physical and mental problems. It is the commonest cause of mental disability, and is also associated with a number of congenital malformations, notably affecting the heart. There is also an increased risk of cancers such as leukaemia, and numerous metabolic problems including diabetes and thyroid disease. Some of these problems may be life‐threatening, or lead to considerable ill health, while some individuals with Down’s syndrome have only mild problems and can lead a relatively normal life.
There is no cure for Down’s syndrome, and antenatal diagnosis allows for preparation for the birth and subsequent care of a baby with Down’s syndrome, or for the offer of a termination of pregnancy. Having a baby with Down’s syndrome is likely to have a significant impact on family and social life, relationships and parents’ work. Special provisions may need to be made for education and care of the child, as well as accommodating the possibility of periods of hospitalisation.
Definitive invasive tests (amniocentesis and chorionic villus sampling (CVS)) exist that allow the diagnosis of Down's syndrome before birth but carry a risk of miscarriage. No test can predict the severity of problems a person with Down’s syndrome will have. Non‐invasive screening tests based on biochemical analysis of maternal serum or urine, or fetal ultrasound measurements, allow an estimate of the risk of a pregnancy being affected and provide parents with information to enable them to make choices about definitive testing. Such screening tests are used during the first and second trimester of pregnancy.
Screening tests for Down's syndrome
Initially, screening was determined solely by using maternal age to classify a pregnancy as high or low risk for trisomy 21, as it was known that older women had a higher chance of carrying a baby with Down’s syndrome (Penrose 1933).
Further advances in screening were made in the early 1980s, when Merkatz and colleagues investigated the possibility that low maternal serum alpha‐fetoprotein (AFP), obtained from maternal blood in the second trimester of pregnancy could be associated with chromosomal abnormalities in the fetus. Their retrospective case‐control study showed a statistically significant relationship between fetal trisomy, such as Down’s syndrome, and lowered maternal serum AFP (Merkatz 1984). This was further explored by Cuckle and colleagues in a larger retrospective trial using data collected as part of a neural tube defect (NTD) screening project (Cuckle 1984). This work was followed by calculation of risk estimates using maternal serum AFP values and maternal age, which ultimately led to the introduction of the two screening parameters in combination (Alfirevic 2004).
In 1987, in a small case‐control study of women carrying fetuses with known chromosomal abnormalities, Bogart and colleagues investigated maternal serum levels of human chorionic gonadotrophin (hCG) as a possible screening tool for chromosomal abnormalities in the second trimester (Bogart 1987). This followed the observations that low hCG levels were associated with miscarriages, which are commonly associated with fetal chromosomal abnormalities. They concluded that high hCG levels were associated with Down’s syndrome and because hCG levels plateau at 18 to 24 weeks, that this would be the most appropriate time for screening. Later work suggested that the ß subunit of hCG was a more effective marker than total hCG (Macri 1990; Macri 1993).
Second trimester unconjugated oestriol (uE3), produced by the fetal adrenals and the placenta, was also evaluated as a potential screening marker. In another retrospective case‐control study, uE3 was shown to be lower in Down’s syndrome pregnancies compared with unaffected pregnancies. When used in combination with AFP and maternal age, it appeared to identify more pregnancies affected by Down’s syndrome than AFP and age alone (Canick 1988). Further work suggested that all three serum markers (AFP, hCG and uE3) showed even higher detection rates when combined with maternal age (Wald 1988a; Wald 1988b) and appeared to be a cost‐effective screening strategy (Wald 1992a).
Two other serum markers, produced by the placenta, have been linked with Down’s syndrome, namely pregnancy‐associated plasma protein A or PAPP‐A, and Inhibin A. PAPP‐A has been shown to be reduced in the first trimester of Down’s syndrome pregnancies, with its most marked reduction in the early first trimester (Bersinger 1995). Inhibin A is high in the second trimester in pregnancies affected by Down’s syndrome (Cuckle 1995; Wallace 1995). There are some issues concerning the biological stability and hence reliability of this marker, and the effect this will have on individual risk.
In addition to serum and ultrasound markers for Down’s syndrome, work has been carried out looking at urinary markers. These markers include invasive trophoblast antigen, ß‐core fragment, free ßhCG and total hCG (Cole 1999). There is controversy about their value (Wald 2003a.
Screening and parental choice
Antenatal screening is used for several reasons (Alfirevic 2004), but the most important is to enable parental choice regarding pregnancy management and outcome. Before a woman and her partner opt to have a screening test, they need to be fully informed about the risks, benefits and possible consequences of such a test. This includes the choices they may have to face should the result show that the woman has a high risk of carrying a baby with Down’s syndrome and implications of both false positive and false negative screening tests. They need to be informed of the risk of a miscarriage due to invasive diagnostic testing, and the possibility that a miscarried fetus may be chromosomally normal. If, following invasive diagnostic testing, the fetus is shown to have Down’s syndrome, further decisions need to be made about continuation or termination of the pregnancy, the possibility of adoption and finally, preparation for parenthood. Equally, if a woman has a test that shows she is at a low risk of carrying a fetus with Down’s syndrome, it does not necessarily mean that the baby will be born with a normal chromosomal make up. This possibility can only be excluded by an invasive diagnostic test (Alfirevic 2003). The decisions that may be faced by expectant parents inevitably engender a high level of anxiety at all stages of the screening process, and the outcomes of screening can be associated with considerable physical and psychological morbidity. No screening test can predict the severity of problems a person with Down's syndrome will have.
Index test(s)
This review examined ultrasound and serum screening tests used in the first trimester of pregnancy (up to 14 weeks' gestation). The tests included the following individual ultrasound markers: nuchal translucency (NT), nasal bone, ductus venosus Doppler, maxillary bone length, fetal heart rate, aberrant right subclavian artery, frontomaxillary facial angle, presence of mitral gap, tricuspid regurgitation, tricuspid blood flow and iliac angle 90 degrees; and the following individual serum markers: inhibin A, AFP, free ßhCG, total hCG, pregnancy‐associated plasma protein A (PAPP‐A), uE3, a disintegrin and metalloprotease 12 (ADAM 12), placental growth factor (PlGF), placental growth hormone (PGH) invasive trophoblast antigen (ITA) (synonymous with hyperglycosylated hCG), growth hormone binding protein (GHBP) and placental protein 13 (PP13).
These markers can be used individually, in combination with age, and can also be used in combination with each other. The risks are calculated by comparing a woman's test result for each marker with values for an unaffected population, and multiplying this with her age‐related risk. Where several markers are combined, risks are computed using risk equations (often implemented in commercial software) that take into account the correlational relationships between the different markers and marker distributions in affected and unaffected populations.
Alternative test(s)
Down’s syndrome can be detected during pregnancy with invasive diagnostic tests such as amniocentesis or CVS, with or without prior screening. These tests are considered to be reference tests rather than index or screening tests. The ability to determine fetal chromosomal make up (also known as a karyotype) from amniotic fluid samples was demonstrated in 1966 by Steele and Breg (Steele 1966), and the first antenatal diagnosis of Down’s syndrome was made in 1968 (Valenti 1968). Amniocentesis is an invasive procedure which involves taking a small sample of the amniotic fluid (liquor) surrounding the baby, using a needle which goes through the abdominal wall into the uterus, and is usually performed after 15 weeks' gestation. Chorionic villus sampling involves taking a sample of the placental tissue using a needle which goes through the abdominal wall and uterus or a cannula through the cervix. It is usually performed between 10 and 13 weeks' gestation. Amniocentesis and CVS are both methods of obtaining fetal chromosome material, which are then used to diagnose Down’s syndrome. Both tests use ultrasound scans to guide placement of the needle. Amniocentesis carries a risk of miscarriage in the order of 1%; transabdominal CVS may carry a similar risk (Alfirevic 2003). A more recent systematic review suggests that the procedure‐related risk of pregnancy loss is lower than this (Akolekar 2015).
Recent developments in the use of cell‐free fetal DNA detection in maternal serum are paving the way for non‐invasive diagnosis of Down's syndrome and other trisomies, however these tests were not used as reference standards in any of the studies examined for this review, and were not included in the search strategy, which preceded their widespread introduction. A systematic review conducted by another group is currently in preparation, examining this newer screening technology ( Badeau 2015).
There are many different screening tests which are available and offered which are the subject of additional Cochrane reviews and there are other reviews looking at this area. Tests being assessed in the other Cochrane reviews include first trimester serum tests (Alldred 2015); urine tests (Alldred 2015a); second trimester serum markers (Alldred 2012); and tests that combine markers from the first trimester with markers from the second trimester (in press). Second trimester ultrasound markers have been assessed in a previous systematic review (Smith‐Bindman 2001).
Rationale
This is one of a suite of Cochrane reviews, the aim of which is to identify all screening tests for Down's syndrome used in clinical practice, or evaluated in the research setting, in order to try to identify the most accurate test(s) available, and to provide clinicians, policy‐makers and women with robust and balanced evidence on which to base decisions about interpreting test results and implementing screening policies to triage the use of invasive diagnostic testing. The full set of reviews is described in the generic protocol (Alldred 2010).
The topic has been split into several different reviews to allow for greater ease of reading and greater accessibility of data, and also to allow the reader to focus on separate groups of tests, for example, first trimester serum tests alone, first trimester ultrasound alone, first trimester serum and ultrasound, second trimester serum alone, first and second trimester serum, combinations of serum and ultrasound markers and urine markers alone. An overview review will compare the best tests, focusing on commonly used strategies, from each of these groups to provide comparative results between the best tests in the different categories. This review is written with the global perspective in mind, rather than to conform with any specific local or national policy, as not all tests will be available in all areas where screening for Down's syndrome is carried out.
A systematic review of second trimester ultrasound markers in the detection of Down’s syndrome fetuses was published in 2001 which concluded that nuchal fold thickening may be useful in detecting Down’s syndrome, but that it was not sensitive enough to use as a screening test. The review concluded that the other second trimester ultrasound markers did not usefully distinguish between Down’s syndrome and pregnancies without Down’s syndrome (Smith‐Bindman 2001). There has yet to be a systematic review and meta‐analysis of the observed data on serum, urine and first trimester ultrasound markers, in order to draw rigorous and robust conclusions about the diagnostic accuracy of available Down’s syndrome screening tests.
Objectives
The aim of this review was to estimate and compare the accuracy of first trimester ultrasound with and without serum markers for the detection of Down’s syndrome in the antenatal period, both as individual markers and as combinations of markers. Accuracy is described by the proportion of fetuses with Down’s syndrome detected by screening before birth (sensitivity or detection rate) and the proportion with a low‐risk screening test result (negative) from amongst babies born without Down's syndrome. We grouped our analyses to focus on investigating the value of adding increasing numbers of markers (comparing single, dual, triple, quadruple, quintuple and sextuple tests).
Investigation of sources of heterogeneity
We had planned to investigate whether a uniform screening test is suitable for all women, or whether different screening methods are more applicable to different groups, defined by advanced maternal age, ethnic groups and aspects of the pregnancy and medical history such as multiple (multifetal) pregnancy, diabetes and family history of Down's syndrome. We also planned to examine whether there was evidence of overestimation of test accuracy in studies evaluating risk equations in the derivation sample rather than in a separate validation sample.
Methods
Criteria for considering studies for this review
Types of studies
We included studies in which all women from a given population had one or more index test(s) compared to a reference standard. Both consecutive series and diagnostic case‐control study designs were included. Randomised trials where individuals were randomised to different screening strategies and all verified using a reference standard were also eligible for inclusion. Studies in which test strategies were compared head‐to‐head either in the same women, or between randomised groups were identified for inclusion in separate comparisons of test strategies. Studies were excluded if they included less than five Down's syndrome cases, or more than 20% of participants were not followed up.
Participants
Pregnant women at less than 14 weeks' gestation confirmed by ultrasound, who had not undergone previous testing for Down’s syndrome in their pregnancy were eligible. Studies were included if the pregnant women were unselected, or if they represented groups with increased risk of Down’s syndrome, or difficulty with conventional screening tests including maternal age greater than 35 years old, multifetal pregnancy, diabetes mellitus and a family history of Down’s syndrome.
Index tests
Improved diagnostic performance can be obtained by using several tests in combination, such as maternal age and serum marker combinations, or combinations of maternal age, serum markers and sonographic measurements. We examined individual first trimester ultrasound markers or combinations of these markers with one or more first trimester serum tests, with and without adjustment for maternal age.
The following ultrasound markers were examined: NT, nasal bone, ductus venosus Doppler, maxillary bone length, fetal heart rate, aberrant right subclavian artery, frontomaxillary facial angle, presence of mitral gap, tricuspid regurgitation, tricuspid blood flow and iliac angle 90 degrees.
The serum markers examined in different combinations with ultrasound markers were inhibin A, AFP, free ßhCG, total hCG, PAPP‐A, uE3, ADAM 12, PlGF, PGH, ITA (h‐hCG), GHBP and PP13.
We examined comparisons of ultrasound markers in isolation and in various combinations with or without serum markers. The combinations included one or two ultrasound markers with single (one marker), double (two markers), triple (three markers), quadruple (four markers), quintuple and sextuple (six markers) serum markers, with or without adjustment for maternal age.
Where tests were used in combinations, we examined the performance of test combinations according to predicted probabilities computed using risk equations and dichotomised into high risk and low risk at some standard high‐risk value. Risk equations are often coded into software to produce 'risk score' computations, which provide an individual's predicted probability of Down’s syndrome.
Target conditions
Down's syndrome in the fetus due to trisomy, translocation or mosaicism.
Reference standards
We considered several reference standards, involving chromosomal verification and postnatal macroscopic inspection.
Amniocentesis and chorionic villus sampling (CVS) are invasive chromosomal verification tests undertaken during pregnancy. They are highly accurate, but the process carries a 1% miscarriage rate, and therefore they are only used in pregnancies considered to be at high risk of Down's syndrome, or on the mother's request. All other types of testing (postnatal examination, postnatal karyotyping, birth registers and Down’s syndrome registers) are based on information available at the end of pregnancy. The greatest concern is not their accuracy, but the loss of the pregnancy to miscarriage between the urine test and the reference standard. Miscarriage with cytogenetic testing of the fetus is included in the reference standard where available. We anticipated that older studies, and studies undertaken in older women are more likely to have used invasive chromosomal verification tests in all women.
Studies undertaken in younger women and more recent studies were likely to use differential verification as they often only used prenatal karyotypic testing on fetuses considered screen positive/high risk according to the screening test; the reference standard for most unaffected infants being observing a phenotypically normal baby. Although the accuracy of this combined reference standard is considered high, it is methodologically a weaker approach as pregnancies that miscarry between the index test and birth are likely to be lost from the analysis, and miscarriage is more likely to occur in Down's than normal pregnancies. We investigated the impact of the likely missing false negative results in sensitivity analyses.
Search methods for identification of studies
Electronic searches
We applied a sensitive search strategy to search the following databases using the search strategies listed in Appendix 1. We used one generic search to identify studies for all reviews in this series.
We searched the following databases
MEDLINE via OVID (1980 to 25 August 2011)
Embase via Dialog Datastar (1980 to 25 August 2011)
BIOSIS via EDINA (1985 to 25 August 2011)
CINAHL via OVID (1982 to 25 August 2011)
The Database of Abstracts of Reviews of Effects (the Cochrane Library 2011, Issue 7)
MEDION (25 August 2011)
The Database of Systematic Reviews and Meta‐Analyses in Laboratory Medicine (www.ifcc.org/) (25 August 2011)
The National Research Register (archived 2007)
Health Services Research Projects in Progress database (HSRPROJ) (25 August 2011)
The search strategy combined three sets of search terms (seeAppendix 1). The first set was made up of named tests, general terms used for screening/diagnostic tests and statistical terms. Note that the statistical terms were used to increase sensitivity and were not used as a methodological filter to increase specificity. The second set was made up of terms that encompass Down's syndrome, and the third set made up of terms to limit the testing to pregnant women. All terms within each set were combined with the Boolean operator OR and then the three sets were combined using AND. The terms used were a combination of subject headings and free‐text terms. The search strategy was adapted to suit each database searched.
We attempted to identify cumulative papers that reported data from the same data set, and contacted authors to obtain clarification of the overlap between data presented in these papers, in order to prevent data from the same women being analysed more than once.
Searching other resources
In addition, we examined references cited in studies identified as being potentially relevant, and those cited by previous reviews. We contacted authors of studies where further information was required. We did not apply a diagnostic test filter, and we did not apply language restrictions to the search.
We carried out forward citation searching of relevant items, using the search strategy in ISI citation indices, Google Scholar and Pubmed ‘related articles’.
Data collection and analysis
Selection of studies
Two review authors screened the titles and abstracts (where available) of all studies identified by the search strategy. Full‐text versions of studies identified as being potentially relevant were obtained and independently assessed by two review authors for inclusion, using a study eligibility screening pro forma according to the pre‐specified inclusion criteria. Any disagreement between the two review authors was settled by consensus, or where necessary, by a third party.
Data extraction and management
A data extraction form was developed and piloted using a subset of 20 identified studies (from all identified studies in this suite of reviews). Two review authors independently extracted data, and where disagreement or uncertainty existed, a third review author validated the information extracted.
Data on each marker were extracted as binary test positive/test negative results for Down's and non‐Down's pregnancies, with a high‐risk result ‐ as defined by each individual study ‐ being regarded as test positive (suggestive or diagnostic of Down's syndrome), and a low‐risk result being regarded as test negative (suggestive of absence of Down's Syndrome). Where results were reported at several thresholds, we extracted data at each threshold.
We noted those in special groups that posed either increased risk of Down’s syndrome or difficulty with conventional screening tests including maternal age greater than 35 years old, multifetal pregnancy, diabetes mellitus and family history of Down’s syndrome.
Assessment of methodological quality
We used a modified version of the QUADAS tool (Whiting 2003), a quality assessment tool for use in systematic reviews of diagnostic accuracy studies, to assess the methodological quality of included studies. We anticipated that a key methodological issue would be the potential for bias arising from the differential use of invasive testing and follow‐up for the reference standard according to index test results, bias arising due to higher loss to miscarriage in false negatives than true negatives. We chose to code this issue as originating from differential verification in the QUADAS tool: we are aware that it could also be coded under delay in obtaining the reference standard, and reporting of withdrawals. We omitted the QUADAS item assessing quality according to length of time between index and reference tests, as Down's syndrome is either present or absent rather than a condition that evolves and resolves, and disregarding the differential reference standard issue, thus any length of delay is acceptable. Two review authors assessed each included study separately. Any disagreement between the two review authors was settled by consensus, or where necessary, by a third party. Each item in the QUADAS tool was marked as ‘yes’, ‘no’ or ‘unclear’, and scores were summarised graphically. We did not use a summary quality score.
QUADAS criteria included the following 10 questions.
Was the spectrum of women representative of the women who will receive the test in practice? (Criteria met if the sample was selected from a wide range of childbearing ages, or selected from a specified ‘high‐risk’ group such as over 35s, family history of Down’s syndrome, multifetal pregnancy or diabetes mellitus, provided all affected and unaffected fetuses included that could be tested at the time point when the screening test would be applied; criteria not met if the sample taken from a select or unrepresentative group of women (i.e. private practice), was an atypical screening population or recruited at a later time point when selection could be affected by selective fetal loss.)
Is the reference standard likely to correctly classify the target condition? (Amniocentesis, chorionic villus sampling, postnatal karyotyping, miscarriage with cytogenetic testing of the fetus, a phenotypically normal baby or birth registers are all regarded as meeting this criteria.)
Did the whole sample or a random selection of the sample receive verification using a reference standard of diagnosis?
Did women receive the same reference standard regardless of the index test result?
Was the reference standard independent of the index test result (i.e. the index test did not form part of the reference standard)?
Were the index test results interpreted without knowledge of the results of the reference standard?
Were the reference standard results interpreted without knowledge of the results of the index test?
Were the same clinical data (i.e. maternal age and weight, ethnic origin, gestational age) available when test results were interpreted as would be available when the test is used in practice?
Were uninterpretable/intermediate test results reported?
Were withdrawals from the study explained?
Statistical analysis and data synthesis
We initially examined each test or test strategy at each of the common risk thresholds used to define test positivity by plotting estimates of sensitivity and specificity from each study on forest plots and in receiver operating characteristic (ROC) space. Test strategies were selected for further investigation if they were evaluated in four or more studies or, if there were three or fewer studies, but the individual study results indicated performance likely to be superior to a sensitivity of 70% and specificity of 90%.
Estimation of average sensitivity and specificity
The analysis for each test strategy was undertaken first restricting to studies which reported a common threshold to estimate average sensitivity and specificity for each test at each threshold. Although data on all thresholds were extracted, we present only key common thresholds (historically reported in literature based on age‐related risk) close to risks of 1:384, 1:250 and the 5% false positive rate (FPR), unless other thresholds were more commonly reported. Where combinations of tests were used in a risk score, we extracted the result for the test combination using the risk score and not the individual components that made up the test.
Meta‐analyses were undertaken using hierarchical summary ROC (HSROC) models, which included estimation of random‐effects in accuracy and threshold parameters when there were four or more studies. When there was an insufficient number of studies to reliably estimate all the parameters in the HSROC model, univariate random‐effects logistic regression models were used to obtain pooled estimates of sensitivity and specificity. It is common in this field for studies to report sensitivity for a fixed specificity (usually a 5% FPR). This removes the requirement to account for the correlation between sensitivity and specificity across studies by using a bivariate model since all specificities are the same value. Thus, at a fixed specificity value, the summary estimate of sensitivity was obtained using a univariate random‐effects logistic regression model. This model was further simplified to a fixed‐effect model when there were only two or three studies and heterogeneity was not observed on the SROC plot. All analyses were undertaken using the NLMIXED procedure in SAS (version 9.2; SAS Institute, Cary, NC) and the xtmelogit command in Stata version 11.2 (Stata‐Corp, College Station, TX, USA).
Comparisons between tests
Comparisons between tests were first made utilising all available studies, selecting one threshold for each test from each study to estimate a SROC curve without restricting to a common threshold. The threshold for each test was chosen from each study according to the following order of preference: a) the risk threshold closest to one in 250; b) a multiples of the median (MoM) or presence/absence threshold; c) the performance closest to a 5% FPR or 95th percentile. The 5% FPR was chosen as a cut‐off point as this is the cut‐off most commonly reported in the literature. The analysis that used all available studies was performed by including the most evaluated or best performing test strategies in a single HSROC model. The model included two indicator terms for each test to allow for differences in accuracy and threshold. As there were very few studies for each test, a symmetric summary ROC curve was assumed. In addition, because the analysis failed to converge, we assumed fixed‐effect for the threshold and accuracy parameters. An estimate of the sensitivity of each test for a 5% FPR was derived from the SROC curve, and associated confidence intervals were obtained using the delta method.
Direct comparisons between tests were based on results of very few studies, and were analysed using a simplified HSROC model with fixed‐effect and symmetrical underlying SROC curves because the number of studies was insufficient to estimate between study heterogeneity in accuracy and threshold or asymmetry in the shape of the SROC curves. A separate model was used to make each pair‐wise comparison. Comparisons between tests were assessed by using likelihood ratio tests to test if the differences in accuracy were statistically significant or not. The differences were expressed as ratios of diagnostic odds ratios and were reported with 95% confidence intervals. As studies rarely report data cross‐classified by both tests for Down's and normal pregnancies, the analytical method did not take full account of the pairing of test results, but the restriction to direct head‐to‐head comparisons should have removed the potential confounding of test comparisons with other features of the studies. The strength of evidence for differences in performance of test strategies relied on evidence from both the direct and indirect comparisons.
Investigations of heterogeneity
If there were 10 or more studies available for a test, we had planned to investigate heterogeneity by adding covariate terms to the HSROC model (meta‐regression) to assess the effect of each factor stated in the Investigation of sources of heterogeneity section on accuracy and threshold.
Sensitivity analyses
Mothers with pregnancies identified as high risk for Down's syndrome by ultrasound and serum testing were often offered immediate definitive testing by amniocentesis, whereas those considered low risk were assessed for Down's syndrome by inspection at birth. Such delayed and differential verification will introduce bias most likely through there being greater loss to miscarriage in the Down's syndrome pregnancies that were not detected by the ultrasound and serum testing (the false negative diagnoses). Testing and detection of miscarriages is impractical in many situations, and no clear data are available on the magnitude of these miscarriage rates.
To account for potential bias introduced by such a mechanism, where possible, we performed sensitivity analyses by increasing the number of false negatives in studies where delayed verification in test negatives occurred (Mol 1999). We increased the number of false negatives in such studies by a multiplicative factor that we applied incrementally from 10% to 50%. The final value of 50% assumes the true number of false negatives is 1.5 times the observed number of false negatives, implying the observed number of false negatives.is 67% (i.e. 1/1.5) of the true number and the fetal loss rate is 33%. Since no increments were added to the number of true negatives, this represents a scenario where a third more pregnancies affected by Down’s syndrome is likely to miscarry compared to those unaffected by Down's syndrome. This is thought to be higher than the likely value.
We intended to conduct these sensitivity analyses on analyses investigating the effect of maternal age on test sensitivity. However, due to limited data, we performed the sensitivity analyses when comparing high‐risk populations with routine screening populations. This comparison was considered a proxy for the effect of maternal age because the main indication for referral for invasive testing was often increased risk due to advanced maternal age.
Results
Results of the search
After the results from each bibliographic database were combined and duplicates were removed, the search for the whole suite of reviews identified a total of 15,394 papers. After screening out obviously inappropriate papers based on their title and abstract, 1145 papers remained and we obtained full‐text copies for formal assessment of eligibility. From these, a total of 269 papers were deemed eligible and were included in the suite of reviews. A total of 126 studies (reported in 152 publications) were included in this review of first trimester ultrasound alone or in combination with first trimester serum screening. Since women with multifetal pregnancies were included in six of the 126 studies, where a study included multifetal pregnancies, we report fetuses rather than women or pregnancies. The review involved 1,604,040 fetuses including 8454 Down's syndrome cases.
A total of 60 different test strategies were evaluated in the 126 studies. These tests were formed from combinations of different ultrasound markers, serum tests and maternal age. The 11 individual ultrasound markers were nuchal translucency (NT), nasal bone, ductus venosus Doppler (ductus venosus a‐wave reversed, ductus venosus pulsivity index), maxillary bone length, fetal heart rate, aberrant right subclavian artery, frontomaxillary facial angle, presence of mitral gap, tricuspid regurgitation, tricuspid blood flow and iliac angle 90 degrees. The 12 individual serum markers were inhibin A, alpha‐fetoprotein (AFP), free beta human chorionic gonadotrophin (ßhCG), total hCG, pregnancy‐associated plasma protein A (PAPP‐A), unconjugated oestriol (uE3), disintegrin and metalloprotease 12 (ADAM 12), placental growth factor (PlGF), placental growth hormone (PGH), invasive trophoblast antigen (ITA) (h‐hCG), growth hormone binding protein (GHBP), and placental protein 13 (PP13). The strategies evaluated, with or without maternal age, included 13 single ultrasound markers; five combinations of two or more ultrasound markers; six ultrasound and single serum marker combinations; 22 ultrasound and double serum marker combinations; nine ultrasound and triple serum marker combinations; one ultrasound and quadruple serum marker combination; three ultrasound and quintuple serum marker combinations; and one ultrasound and sextuple serum marker combination. Seventy‐eight of the 126 studies only evaluated the performance of a single first trimester ultrasound or ultrasound and serum test or test strategy; 27 studies evaluated two tests, 10 evaluated three tests, four evaluated four tests, four evaluated five tests, one evaluated eight tests (Koster 2011), one evaluated 11 tests (Kagan 2010), and one evaluated 19 tests (Wald 2003).
The following test combinations were evaluated by four or more studies.
Ultrasound and triple serum markers
NT, PAPP‐A, free ßhCG, ADAM 12 and maternal age (four studies; 2571 women, including 256 Down's syndrome pregnancies)
Ultrasound and double serum markers
NT, PAPP‐A, free ßhCG and maternal age (69 studies; 1,173,853 fetuses, including 6010 Down's syndrome cases)
Ultrasound and single serum markers
NT, free ßhCG and maternal age (five studies; 10,795 women, including 421 Down's syndrome pregnancies)
NT, PAPP‐A and maternal age (five studies; 9,814 women including 372 Down's syndrome pregnancies)
Ultrasound markers alone
NT, nasal bone and maternal age (five studies, 29,699 women, including 221 Down's syndrome pregnancies)
NT and maternal age (50 studies; 530,874 fetuses including 2701 Down's syndrome cases)
Nasal bone and maternal age (four studies; 25,303 women, including 165 Down's syndrome pregnancies)
Ductus and maternal age (five studies; 5,331 women including 165 Down's syndrome pregnancies)
Nasal bone (11 studies; 48,279 fetuses including 290 Down's syndrome cases)
NT (13 studies; 90,978 fetuses, including 593 Down's syndrome cases)
Of the remaining test combinations, four were evaluated in three studies, six were evaluated in two studies and the remaining 40 in single studies only.
Methodological quality of included studies
The studies were judged to be of high methodological quality in most categories (Figure 1) and details are provided in the Characteristics of included studies. The spectrum of participants was judged to be representative in all study cohorts. The reference standard used was judged unclear in three studies (Hafner 1998; Krantz 2000; Orlandi 1997) and unacceptable in one study (Noble 1995). Due to the nature of testing for Down's syndrome screening and the potential side effects of invasive testing, differential verification is almost universal in the general screening population, as most women whose screening test result is defined as low risk (negative) will have their screening test verified at birth, rather than by invasive diagnosis in the antenatal period. Partial verification was avoided in 81 study cohorts (64%) and differential verification was avoided in 15 study cohorts (12%). Both differential and partial verification was avoided in 14 study cohorts (Biagiotti 1998; Borenstein 2008; Christiansen 2005; Cicero 2004a; De Graaf 1999; Hewitt 1996; Maiz 2007; Matias 1998; Matias 2001; Mavrides 2002; Molina 2010 high risk; Otaño 2002; Pajkrt 1998a; Prefumo 2005 ). Of the 14 study cohorts, the populations in 13 were high‐risk referral for invasive testing (prior to screening being undertaken), while one (Christiansen 2005) obtained maternal serum samples through screening programmes for syphilis and Down's syndrome. Reference standard results were unblinded in 124 study cohorts and unclear in three study cohorts. In contrast, index test results were blinded in 113 study cohorts and unclear in 14. It would be difficult to blind clinicians performing invasive diagnostic tests (reference standards) to the index test result, unless all women received the same reference standard, which would not be appropriate in most scenarios. Any biases secondary to a lack of clinician blinding are likely to be minimal.
1.
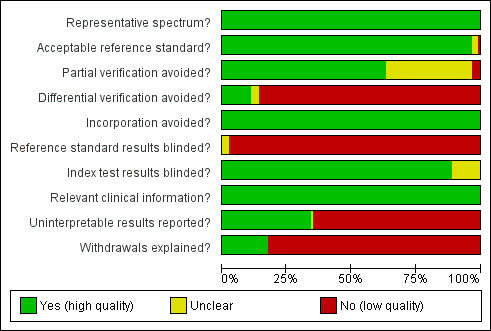
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
Most studies seemed to indicate 100% follow‐up, however there will inevitably be losses to follow‐up due to women moving out of area, for example. Studies sometimes accounted for these and it is unlikely that there were enough losses to follow‐up to have introduced significant bias. There was likely under‐ascertainment of miscarriage, and very few papers accounted for miscarriage or performed tissue karyotyping in pregnancies resulting in miscarriage. Some studies attempted to adjust for predicted miscarriage rate and the incidence of Down's syndrome in this specific population, but most did not. We have not attempted to adjust for expected miscarriage rate in this review. There is a higher natural miscarriage rate in the first trimester, however this will be uniform across studies and therefore unlikely to introduce significant bias.
Some studies which provided estimates of risk using multivariable equations used the same data set to evaluate performance of the risk equation as was used to derive the equation. This is often thought to lead to over‐estimation of test performance.
Findings
The results for the 10 most evaluated test strategies are presented in Table 1. Additional information and results at specific thresholds are provided below.
Summary of findings 1. Performance of the 10 most evaluated first trimester ultrasound markers alone or in combination with first trimester serum tests.
| Review question | What is the accuracy of ultrasound based markers alone and in combination with maternal age and/or first trimester serum markers for screening for Down's syndrome? | |||||
| Population | Pregnant women at less than 14 weeks' gestation confirmed by ultrasound, who had not undergone previous testing for Down’s syndrome. Some studies were undertaken in women identified to be at high risk based on maternal age. | |||||
| Settings | All settings. | |||||
| Numbers of studies, pregnancies and Down's syndrome cases | 126 studies (reported in 152 publications) involving 1,604,040 fetuses of which 8454 were Down's syndrome cases | |||||
| Index tests | Risk scores computed using maternal age and first trimester ultrasound and serum markers for ultrasound markers ‐ NT, nasal bone, ductus venosus Doppler, maxillary bone length, fetal heart rate, aberrant right subclavian artery, frontomaxillary facial angle, presence of mitral gap, tricuspid regurgitation, tricuspid blood flow and iliac angle 90 degrees ‐ and serum markers ‐ inhibin A, AFP, free ßhCG, total hCG, PAPP‐A, uE3, ADAM 12, PlGF, PGH, ITA (h‐hCG), GHBP and PP13. | |||||
| Reference standards | Chromosomal verification (amniocentesis and CVS undertaken during pregnancy, and postnatal karyotyping) and postnatal macroscopic inspection. | |||||
| Study limitations | 116 studies only used selective chromosomal verification during pregnancy, and were at risk of under‐ascertainment of Down's syndrome cases due to pregnancy loss between administering the serum test and the reference standard. | |||||
| Test strategy | Studies | Women (Down's cases) | Sensitivity (95% CI) |
Specificity (95% CI)* |
Consequences in a hypothetical cohort of 10,000 pregnant women assuming Down’s syndrome affects approximately one in 800 live‐born babies | |
| Missed cases | False positives | |||||
| Nasal bone | 11 | 48,279 (290) | 49 (34, 64) | 99 (99, 100) | 7 | 100 |
| NT | 13 | 90,978 (593) | 70 (61, 78) | 95 | 4 | 500 |
| NT and maternal age | 50 | 530,874 (2701) | 71 (66, 75) | 95 | 4 | 500 |
| Nasal bone and maternal age | 4 | 25,303 (165) | 68 (28, 92) | 95 | 4 | 500 |
| Ductus and maternal age | 5 | 5331 (165) | 68 (49, 83) | 95 | 4 | 500 |
| NT, nasal bone and maternal age | 5 | 29,699 (221) | 78 (55, 91) | 95 | 3 | 500 |
| NT, free ßhCG and maternal age | 5 | 10,795 (421) | 77 (72, 82) | 95 | 3 | 500 |
| NT, PAPP‐A and maternal age | 5 | 9814 (372) | 81 (75, 86) | 95 | 3 | 500 |
| NT, PAPP‐A, free ßhCG and maternal age | 69 | 1,173,853 (6010) | 87 (86, 89) | 95 | 2 | 500 |
| NT, PAPP‐A, free ßhCG, ADAM 12 and maternal age | 4 | 2571 (256) | 82 (75, 87) | 95 | 3 | 500 |
*We estimated sensitivity (with a 95% confidence interval) at a 5% false positive rate from the summary ROC curve obtained for each test except nasal bone. For nasal bone, the pooled specificity is reported because the cut‐point was absence or presence of nasal bone, and all studies reported false positive rates below 5% so estimation of sensitivity at a fixed 5% FPR was not appropriate.
1) NT, PAPP‐A, free ßhCG and maternal age (Figure 2)
2.
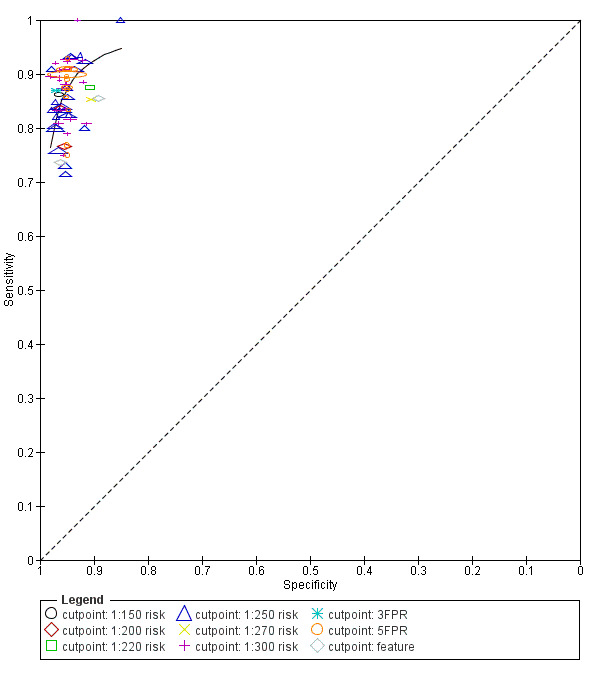
Study estimates of sensitivity and specificity with a summary ROC curve for the NT, PAPP‐A, free ßhCG and maternal age test combination at different cut‐points. Each symbol represents a pair of sensitivity and specificity at one cut‐point from each study.
This was the most evaluated test strategy and accounted for most (73%) of the fetuses in this systematic review. The test was evaluated by 69 studies and involved 1,173,853 fetuses (including 6010 Down's syndrome cases). Six studies (Cowans 2009; Ekelund 2008; Kagan 2010; Merz 2011; Nicolaides 2005; Wright 2010) contributed more than half the total number of fetuses affected by Down’s syndrome (3057); the largest study (Wright 2010) included 223,361 women in whom 886 pregnancies were affected by Down’s syndrome. Across the 69 studies, data were presented at 10 cut‐points (1% false positive rate (FPR), 3% FPR, 4.5% FPR, 5% FPR, 1:150 risk, 1:200 risk, 1:220 risk, 1:250 risk, 1:270 risk and 1:300 risk). At a cut‐point of 5% FPR (24 studies, 391,874 fetuses including 2521 fetuses affected by Down’s syndrome), the estimated sensitivity was 87% (95% CI 84 to 89); at a cut‐point of 1:250 risk (25 studies; 174,712 fetuses including 1032 fetuses affected by Down’s syndrome), the estimated sensitivity was 85% (95% CI 81 to 87) and the specificity was 95% (95% CI 95 to 96).
2) NT, PAPP‐A, free ßhCG, ADAM 12 and maternal age
This combination of NT, triple serum markers and maternal age was evaluated by four studies (Christiansen 2010; Koster 2011; Spencer 2008; Torring 2010) and included 2571 women (256 pregnancies were affected by Down’s syndrome). Studies presented data for cut‐points of 5% FPR (Christiansen 2010; Koster 2011; Spencer 2008; Torring 2010) and 1;250 risk (Christiansen 2010; Torring 2010). At a cut‐point of 5% FPR (four studies, 2571 women), the estimated sensitivity was 85% (95% confidence interval (CI) 75 to 91); at a cut‐point of 1:250 risk (two studies; 1222 women in whom 74 pregnancies were affected by Down’s syndrome), the estimated sensitivity was 86% (95% CI 77 to 93) and the specificity was 97% (95% CI 96 to 98).
3) NT, PAPP‐A and maternal age
This test strategy was evaluated by five studies (Biagiotti 1998; Habayeb 2010; Krantz 2000; Spencer 1999; Wald 2003) and involved 9814 women (including 372 Down's syndrome pregnancies). Data were presented at cut‐points of 5% FPR (Biagiotti 1998; Spencer 1999; Wald 2003), 1:100 risk (Habayeb 2010) and 1:185 risk (Krantz 2000). Habayeb 2010 estimated a sensitivity of 67% (95% CI 35 to 90) and specificity of 98% (95% CI 97 to 98) at a cut‐point of 1:100 risk based on 1507 women in whom 12 pregnancies were affected by Down’s syndrome. At a cut‐point of 1:185 risk, Krantz 2000 estimated a sensitivity of 82% (95% CI 65 to 93) and specificity of 95% (95% CI 94 to 96) based on 5809 women in whom 33 pregnancies were affected by Down’s syndrome. For the three studies (2498 women in whom 327 pregnancies were affected by Down’s syndrome) that reported a 5% FPR, the estimated sensitivity was 80% (95% CI 75 to 84).
4) NT, nasal bone and maternal age
This combination of two ultrasound markers and maternal age was evaluated by five studies (Has 2008; Kagan 2010; Prefumo 2005; Prefumo 2006; Sepulveda 2007) and involved 29,699 women (including 221 Down's syndrome pregnancies). Data were presented at cut‐points of 1:100 risk (Kagan 2010) and 1:300 risk (Has 2008; Prefumo 2005; Prefumo 2006; Sepulveda 2007). Kagan 2010 estimated a sensitivity of 83% (95% CI 75 to 89) and specificity of 97% (95% CI 97 to 97) based on 19,736 women in whom 122 pregnancies were affected by Down’s syndrome. At a cut‐point of 1:300 risk (four studies; 9963 women in whom 99 pregnancies were affected by Down’s syndrome), the estimated sensitivity was 61% (95% CI 22 to 89) and the specificity was 97% (95% CI 90 to 99).
5) NT, free ßhCG and maternal age
Results for this combination of NT, a single serum marker and maternal age were obtained from five studies (Biagiotti 1998; Krantz 2000; Noble 1995; Spencer 1999; Wald 2003) involving 10,975 women in whom 421 were affected by Down's syndrome pregnancies. Data were presented at cut‐points of 5% FPR (Biagiotti 1998; Noble 1995; Spencer 1999; Wald 2003) and 1:240 risk (Krantz 2000). At a cut‐point of 5% FPR (four studies; 4986 women in whom 388 pregnancies were affected by Down’s syndrome), the estimated sensitivity was 77% (95% CI 68 to 84). At a cut‐point of 1:240 risk, Krantz 2000 estimated a sensitivity of 79% (95% CI 61 to 91) and specificity of 95% (95% CI 94 to 96) based on 5799 women in whom 33 pregnancies were affected by Down’s syndrome.
6) NT and maternal age (Figure 3)
3.
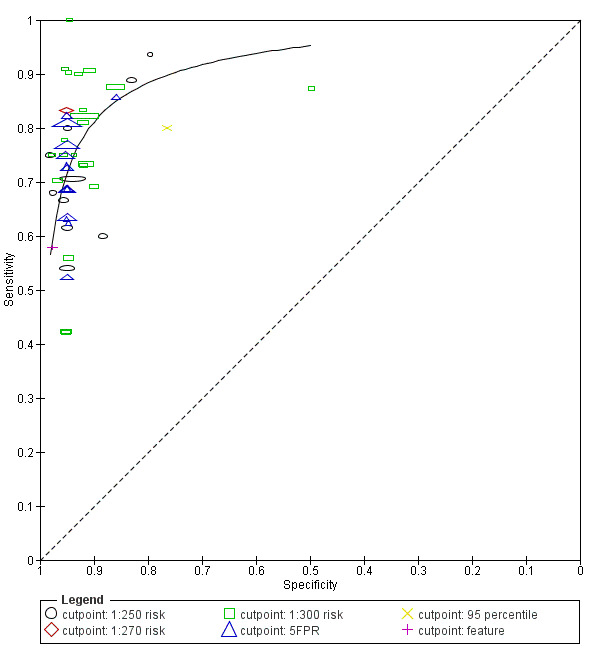
Study estimates of sensitivity and specificity with a summary ROC curve for NT and maternal age across different cut‐points. Each symbol represents a pair of sensitivity and specificity at one cut‐point from each study.
This ultrasound marker was evaluated in 50 studies that included 530,874 fetuses including 2701 fetuses affected by Down's syndrome. Seven studies (Bestwick 2010; Gasiorek‐Wiens 2001; Kagan 2010; O'Leary 2006; Snijders 1998; Wald 2003; Wright 2008) each included over 20,000 fetuses and contributed over half the data (296,481 fetuses including 1444 Down's syndrome cases); Snijders 1998 was the largest study (95,802 fetuses). The 50 studies reported diagnostic accuracy at five different cut‐points (1% FPR, 3% FPR, 5% FPR, 1:250 risk and 1:300 risk). At a cut‐point of 5% FPR (22 studies; 288,853 fetuses including 1784 Down's syndrome cases), the estimated sensitivity was 71% (95% CI 67 to 75); at a cut‐point of 1:250 risk, the estimated sensitivity was 72% (95% CI 62 to 80) and specificity was 94% (95% CI 90 to 96) based on 10 studies of 79,412 fetuses including 247 affected by Down’s syndrome.
7) NT (Figure 4)
4.
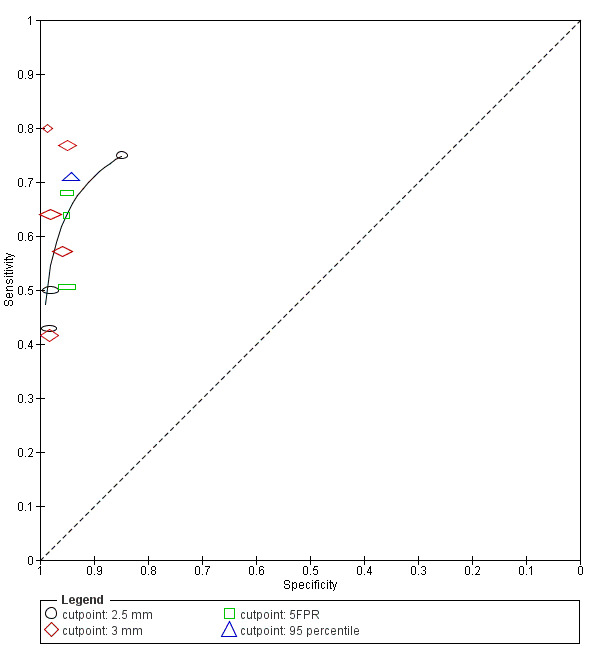
Study estimates of sensitivity and specificity with a summary ROC curve for NT. Each symbol represents a pair of sensitivity and specificity at one cut‐point from each study.
Thirteen studies (Acacio 2001; Babbur 2005; Bestwick 2010; Hafner 1998; Hewitt 1996; Kim 2006; Marsis 2004; Michailidis 2001; Nicolaides 1992; Pajkrt 1998a; Schuchter 2002; Spencer 1999; Wald 2003) evaluated NT in 90,978 fetuses including 593 affected by Down's syndrome. Of the 13 studies, two studies (Bestwick 2010; Wald 2003) had a sample size of more than 20,000 and contributed 69% (62,729 fetuses) of the data. Data were presented at cut‐points of 2.5 mm (Acacio 2001; Hafner 1998; Kim 2006; Schuchter 2002), 3 mm (Babbur 2005; Hewitt 1996; Kim 2006; Marsis 2004; Nicolaides 1992; Pajkrt 1998a), 5% FPR (Bestwick 2010; Spencer 1999; Wald 2003) and 99th centile (Michailidis 2001). At a 5% FPR, the estimated sensitivity from the three studies was 62% (95% CI 54 to 69), based on 63,885 fetuses including 401 affected by Down's syndrome. At the 2.5 mm cut‐point, the estimated sensitivity from the four studies was 61% (95% CI 42 to 77) and the specificity was 96% (95% CI 90 to 98) based on 64 affected cases and a total of 11,835 fetuses. For the 3 mm cut‐point, the estimated sensitivity from the six studies was 58% (95% CI 48 to 68) and the specificity was 97% (95% CI 96 to 98) based on 136 cases and a total of 10,381 fetuses.
8) Nasal bone and maternal age
Nasal bone adjusted for maternal age was evaluated in four studies (Monni 2005; Prefumo 2005; Prefumo 2006; Viora 2003) involving 25,303 women and included 165 Down's syndrome pregnancies.Monni 2005 accounted for 66% (16,641 women) of the data. The estimated summary sensitivity was 49% (95% CI 37 to 60) and the summary specificity was 98% (95% CI 95 to 99).
9) Ductus and maternal age
Five studies (Borrell 2005; Matias 2001; Mavrides 2002; Molina 2010 high risk; Prefumo 2005) evaluated this single ultrasound marker in 5,331 women including 165 Down's syndrome pregnancies. Borrell 2005 contributed 70% (3731 women) of the data. Data were presented at 5% FPR (Borrell 2005; Mavrides 2002), 1:250 risk (Borrell 2005), or fetuses were categorised as negative or positive for Down's syndrome based on normal or abnormal ductus venous flow (Matias 2001; Mavrides 2002; Prefumo 2005). At a 5% FPR, the estimated sensitivity from the two studies was 67% (95% CI 54 to 78) based on 3965 women in whom 55 were affected by Down's syndrome pregnancies.
10) Nasal bone
Results for this single marker were obtained from 11 studies (Cicero 2006; Has 2008; Leung 2009; Malone 2004; Molina 2010 high risk; Moon 2007; Orlandi 2003; Orlandi 2005; Otaño 2002; Ramos‐Corpas 2006; Sepulveda 2007) involving 48,279 fetuses including 290 affected by Down's syndrome. Cicero 2006 was the largest study (20,418 women including 140 affected cases), accounting for 42% of the data. The estimated summary sensitivity was 49% (95% CI 34 to 64) and the summary specificity was 99% (95% CI 99 to 100).
11) Other test strategies
The results for the remaining test strategies are presented in Table 2. Of the 50 test strategies evaluated in fewer than four studies, 33 test strategies showed estimated sensitivities of at least 70% and estimated specificities of 90%; none of the eight single tests without maternal age achieved this level of test performance. The following seven test strategies evaluated in one or two studies showed sensitivities of more than 90% and specificities of more than 95%.
Summary of findings 2. Performance of other first trimester ultrasound markers alone or in combination with first trimester serum tests.
| Test strategy | Studies | Women (Down's cases) | Sensitivity* (95% CI) | Specificity* (95% CI) | Threshold |
| Without maternal age | |||||
| Ultrasound markers alone | |||||
| Aberrant right subclavian artery | 1 | 425 (51) | 8 (2, 19) | 99 (98, 100) | Feature |
| Frontomaxillary facial angle | 1 | 242 (22) | 18 (5, 40) | 98 (95, 99) | > 95th percentile |
| Presence of mitral gap | 1 | 217 (20) | 20 (6, 44) | 87 (81, 91) | Feature |
| Maxillary bone length | 1 | 927 (88) | 24 (15, 34) | 95 (93, 96) | 5th centile |
| Tricuspid regurgitation | 1 | 312 (20) | 50 (27, 73) | 98 (96, 99) | Feature |
| Iliac angle 90 degrees | 1 | 2032 (52) | 60 (45, 73) | 98 (97, 98) | Feature |
| Ductus venosus a‐wave reversed | 1 | 378 (72) | 68 (56, 79) | 70 (64, 75) | Feature |
| Ductus venosus pulsivity index | 1 | 378 (72) | 81 (70, 89) | 58 (52, 63) | > 95th percentile |
| NT and nasal bone | 1 | 486 (38) | 89 (75, 97) | 93 (91, 95) | Absent nasal bone and NT ≥ 95th centile |
| Ultrasound and double serum markers | |||||
| NT, free ßhCG and PAPP‐A | 1 | 6508 (40) | 90 (76, 97) | 95 (95, 96) | First trimester incidence rate 63.3% |
| With maternal age | |||||
| Ultrasound markers alone | |||||
| NT‐adjusted risk > 1:300 and abnormal ductus venosus flow and absent nasal bones | 1 | 544 (47) | 21 (11, 36) | 100 (99, 100) | 1:300 risk |
| NT and ductus | 3 | 23,697 (177) | 76 to 93 | 73 to 99 | 5% FPR, 1:250 risk, feature |
| NT and tricuspid blood flow | 1 | 19,736 (122) | 85 (78, 91) | 97 (97, 98) | 1:100 risk |
| Ultrasound and single serum markers | |||||
| NT and inhibin A | 2 | 1150 (97) | 61 to 75 | 95 to 96 | 5% FPR, 1:250 risk |
| NT and AFP | 1 | 1110 (85) | 61 (50, 72) | 95 (94, 96) | 5% FPR |
| NT and total hCG | 1 | 1110 (85) | 61 (50, 72) | 95 (94, 96) | 5% FPR |
| NT and ITA | 1 | 278 (54) | 80 (66, 89) | 95 (91, 98) | 5% FPR |
| Ultrasound and double serum markers | |||||
| NT, AFP and free ßhCG | 2 | 2766 (90) | 66 to 100 | 93 to 95 | 5% FPR, 1:250 risk |
| NT, PAPP‐A and inhibin A | 2 | 1150 (97) | 80 to 83 | 95 to 96 | 5% FPR, 1:250 risk |
| NT, total hCG and inhibin A | 1 | 1110 (85) | 62 (51, 73) | 95 (94, 96) | 5% FPR |
| NT, free ßhCG and inhibin A | 1 | 1110 (85) | 66 (55, 76) | 95 (94, 96) | 5% FPR |
| NT, free ßhCG and ADAM 12 | 1 | 351 (31) | 68 (49, 83) | 95 (92, 97) | 5% FPR |
| NT, PAPP‐A and uE3 | 1 | 576 (24) | 79 (58, 93) | 95 (93, 97) | 5% FPR |
| NT, total hCG and PAPP‐A | 1 | 1110 (85) | 80 (70, 88) | 95 (94, 96) | 5% FPR |
| NT, AFP and PAPP‐A | 1 | 1110 (85) | 80 (70, 88) | 95 (94, 96) | 5% FPR |
| NT, PAPP‐A and ITA | 2 | 11,053 (77) | 83 (73, 90) | 95 | 5% FPR |
| NT, PAPP‐A and ADAM 12 | 2 | 1042 (77) | 83 (73, 90) | 95 | 5% FPR |
| Free ßhCG and PAPP‐A, if risk between 1:42 and 1:1000 (intermediate risk), NToffered, final composite risk !:250 | 1 | 10,189 (44) | 89 (75, 96) | 94 (94, 95) | 1:250 risk |
| NT, ductus, free ßhCG and PAPP‐A | 3 | 30,061 (212) | 83 to 96 | 97 to 99 | 1:100 risk, 1:250 risk |
| NT, nasal bone, free ßhCG and PAPP‐A | 3 | 41,842 (271) | 89 to 94 | 95 to 98 | 5% FPR, 1:100 risk, 1:300 risk |
| NT, PAPP‐A, free ßhCG and ductus venosus pulsivity index | 1 | 7,250 (66) | 89 (79, 96) | 95 (94, 95) | 5% FPR |
| NT, tricuspid blood flow, free ßhCG and PAPP‐A | 1 | 19,736 (122) | 91 (84, 95) | 97 (97, 98) | 1:100 risk |
| NT, fetal heart rate, free ßhCG and PAPP‐A | 2 | 76,385 (517) | 92 (89, 94) | 95 | 5% FPR |
| NT, fetal heart rate, nasal bone, free ßhCG and PAPP‐A | 1 | 19,736 (122) | 95 (90, 98) | 96 (95, 96) | 1:200 risk |
| NT, fetal heart rate, tricuspid blood flow, free ßhCG and PAPP‐A | 1 | 19,736 (122) | 96 (91, 99) | 95 (95, 95) | 5% FPR |
| NT, fetal heart rate, ductus, free ßhCG and PAPP‐A | 1 | 19,614 (122) | 97 (92, 99) | 95 (95, 95) | 5% FPR |
| Ultrasound and triple serum markers | |||||
| NT, AFP, free ßhCG and PAPP‐A | 3 | 6789 (135) | 73 to 84 | 95 | 5% FPR, 1:250 risk |
| NT, PAPP‐A, free ßhCG and PP13 | 1 | 998 (151) | 77 (69, 83) | 95 (93, 96) | 5% FPR |
| NT, PAPP‐A, free ßhCG and total hCG | 1 | 998 (151) | 77 (69, 83) | 95 (93, 96) | 5% FPR |
| NT, total hCG, inhibin A and PAPP‐A | 1 | 1110 (85) | 81 (71, 89) | 95 (94, 96) | 5% FPR |
| NT, free ßhCG, inhibin A and PAPP‐A | 1 | 1110 (85) | 84 (74, 91) | 95 (94, 96) | 5% FPR |
| NT, PAPP‐A, free ßhCG and PGH | 1 | 335 (74) | 86 (77, 93) | 95 (92, 97) | 5% FPR |
| NT, PAPP‐A, free ßhCG and PIGF | 2 | 1443 (221) | 88 (70, 95) | 95 | 5% FPR |
| NT, PAPP‐A, free ßhCG and GHBP | 1 | 335 (74) | 91 (81, 96) | 95 (92, 97) | 5% FPR |
| Ultrasound and quadruple serum markers | |||||
| NT, PAPP‐A, free ßhCG, ADAM 12 and PlGF | 1 | 998 (151) | 79 (72, 86) | 95 (93, 96) | 5% FPR |
| Ultrasound and quintuple serum markers | |||||
| NT, PAPP‐A, free ßhCG, ADAM 12, total hCG and PlGF | 1 | 998 (151) | 79 (72, 86) | 95 (93, 96) | 5% FPR |
| NT, total hCG, inhibin A, PAPP‐A, AFP and uE3 | 1 | 1110 (85) | 84 (74, 91) | 95 (94, 96) | 5% FPR |
| NT, free ßhCG, inhibin A, PAPP‐A, AFP and uE3 | 1 | 1110 (85) | 86 (77, 92) | 95 (94, 96) | 5% FPR |
| Ultrasound and sextuple serum markers | |||||
| NT, PAPP‐A, free ßhCG, ADAM 12, total hCG, PlGF and PP13 | 1 | 998 (151) | 80 (73, 86) | 95 (93, 96) | 5% FPR |
*Tests evaluated by at least one study are presented in the table. Where there were two studies at the same threshold, estimates of summary sensitivity and summary specificity were obtained by using univariate fixed‐effect logistic regression models to pool sensitivities and specificities separately. If the threshold used was a 5% FPR, then only the sensitivities were pooled. The range of sensitivities and specificities are presented where meta‐analysis was not performed because there were only two or three studies and no common threshold.
NT, free ßhCG and PAPP‐A evaluated in a single study (Hormansdorfer 2011) estimated a sensitivity of 90% (95% CI 76 to 97%) and specificity of 95% (95% CI 95 to 96) at a first trimester incidence rate of 63.3%.
NT, PAPP‐A, free ßhCG, GHBP and maternal age evaluated in a single study (Christiansen 2009) estimated a sensitivity of 91% (95% CI 81 to 96) at a cut‐point of 5% FPR.
NT, tricuspid blood flow, free ßhCG, PAPP‐A and maternal age evaluated in a single study (Kagan 2010) estimated a sensitivity of 91% (95% CI 84 to 95) and specificity of 97% (95% CI 97 to 98) at a cut‐point of 1:100 risk.
NT, fetal heart rate, free ßhCG, PAPP‐A and maternal age evaluated in two studies (Kagan 2010; Maiz 2009) estimated a sensitivity of 92% (95% CI 89 to 94) at a cut‐point of 5% FPR.
NT, fetal heart rate, nasal bone, free ßhCG, PAPP‐A and maternal age evaluated in a single study (Kagan 2010) estimated a sensitivity of 95% (95% CI 90 to 98) and specificity of 96% (95% CI 95 to 96) at a cut‐point of 1:200 risk.
NT, fetal heart rate, tricuspid blood flow, free ßhCG, PAPP‐A and maternal age evaluated in a single study (Kagan 2010) estimated a sensitivity of 96% (95% CI 91 to 99) at a cut‐point of 5% FPR.
NT, fetal heart rate, ductus, free ßhCG, PAPP‐A and maternal age evaluated in a single study (Maiz 2009) estimated a sensitivity of 97% (95% CI 92 to 99) at a cut‐point of 5% FPR.
Comparative analysis of the 10 selected test strategies
For each test we obtained the detection rate (sensitivity) for a fixed false positive rate (FPR) (1‐specificity), a metric which is commonly used in Down’s syndrome screening to describe test performance. We chose to estimate detection rates at a 5% FPR in common with much of the literature. However, because the 5% FPR was not within the range of the data for the nasal bone marker (the specificities were between 97% and 100%), we did not compute the detection rate at a 5% FPR for this test; the summary sensitivity was 49% (95% CI 34 to 64) and the summary specificity was 99% (95% CI 99 to 100). Figure 5 shows point estimates of the detection rate (and their 95% CIs) at a 5% FPR based on all available data for the remaining nine test strategies; the test strategies are ordered according to decreasing detection rates. The plot shows that for the combined NT, PAPP‐A, free ßhCG and maternal age test strategy, the estimated detection rate was 87% (95% CI 86 to 89) based on data from 69 studies with 6010 affected cases out of a total of 1,173,853 participants. The four single ultrasound markers (NT and maternal age; NT; nasal bone and maternal age; and ductus and maternal age) showed the worst performance, whereas, the three test strategies containing PAPP‐A showed the highest performance with detection rates above 80%. However, it should be noted that the confidence intervals around the estimates generally overlap though the confidence interval for the combined NT, PAPP‐A, free ßhCG and maternal age test strategy is very narrow and not overlapped by five of the other test strategies.
5.
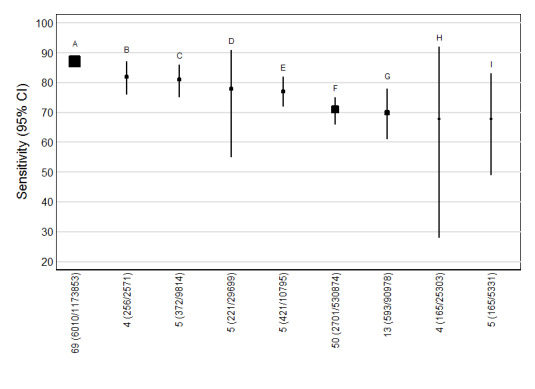
Detection rates (% sensitivity) at a 5% false positive rate for nine of the most evaluated first trimester ultrasound markers alone or in combination with first trimester serum tests. A = NT, PAPP‐A, free ßhCG and maternal age; B = NT, PAPP‐A, free ßhCG, ADAM 12 and maternal age; C = NT, PAPP‐A and maternal age; D = NT, nasal bone and maternal age; E= NT, free ßhCG and maternal age; F= NT and maternal age; G = NT; H = Nasal bone and maternal age; and I = Ductus and maternal age.
Each square represents the summary sensitivity for a test strategy at a 5% false positive rate. The size of each square is proportional to the number of Down's cases. The estimates are shown with 95% confidence intervals. The test strategies are ordered on the plot according to decreasing detection rate. For each test strategy, the number of included studies, Down's syndrome cases and pregnancies are shown on the horizontal axis.
The strength of evidence for differences in the diagnostic performance of the 10 test strategies relied on evidence from both direct and indirect comparisons. Table 3 shows pair‐wise direct comparisons (head‐to‐head), where studies were available. Such comparisons are regarded as providing the strongest evidence as differences between tests are unconfounded by study characteristics. The table shows the number of studies (K), the ratios of diagnostic odds ratios (DORs) with 95% CIs and P values for each test comparison. The diagnostic accuracy of NT (with or without maternal age) alone tended to be inferior unlike when combined with serum tests (PAPP‐A and free ßhCG). However, all comparisons in this table, except for the combined NT, PAPP‐A, free ßhCG and maternal age versus NT and maternal age test comparison (25 studies), were based on five or fewer studies and so are unlikely to be powered to detect differences in accuracy.
1. Direct (head‐to‐head) comparisons of the diagnostic accuracy of the 10 most evaluated first trimester ultrasound markers alone or in combination with first trimester serum tests.
|
Ratio of DORs (95% CI); P value (Studies) |
Nasal bone | NT | Nasal bone and age | Ductus and age | NT and age | NT, nasal bone and age | NT, free ßhCG and age | NT, PAPP‐A and age | NT, PAPP‐A, free ßhCG and age |
| NT | – | ||||||||
| Nasal bone and age | – | – | |||||||
| Ductus and age | 1.19 (0.12, 11.4); P = 0.84 (K = 1) |
– | 0.85 (0.21, 3.41); P = 0.76 (K = 1) |
||||||
| NT and age | 0.62 (0.13, 2.93); P = 0.50 (K = 2) |
1.25 (0.90, 1.74); P = 0.17 (K = 3) |
0.84 (0.48, 1.49); P = 0.52 (K = 3) |
1.07 (0.51, 2.23); P = 0.85 (K = 3) |
|||||
| NT, nasal bone and age | 0.61 (0.12, 3.10); P = 0.50 (K = 2) |
– | 4.01 (1.51, 10.6); P = 0.01 (K = 2) |
0.95 (0.23, 3.97); P = 0.93 (K = 1) |
1.05 (0.70, 1.56); P = 0.82 (K = 5) |
||||
| NT, free ßhCG and age | – | 2.15 (1.33, 3.50); P = 0.007 (K = 2) |
– | – | 1.47 (1.00, 2.15); P = 0.05 (K = 4) |
– | |||
| NT, PAPP‐A and age | – | 2.86 (1.73, 4.73); P = 0.001 (K = 2) |
– | – | 1.88 (1.27, 2.78); P = 0.004 (K = 4) |
– | 1.28 (0.84, 1.93); P = 0.23 (K = 4) |
||
| NT, PAPP‐A, free ßhCG and age | 3.83 (0.89, 16.4); P = 0.07 (K = 2) |
4.35 (2.00, 9.46); P = 0.015 (K = 4) |
– | 3.00 (0.42, 21.2); P = 0.19 (K = 1) |
3.19 (2.19, 4.66); P < 0.0001 (K = 25) |
1.23 (0.63, 2.40); P = 0.50 (K = 2) |
2.06 (1.31, 3.22); P = 0.004 (K = 4) |
1.61 (1.02, 2.55); P = 0.043 (K = 4) |
|
| NT, PAPP‐A, free ßhCG, ADAM 12 and age | – | – | – | – | – | – | – | – | 0.87 (0.49, 1.52); P = 0.60 (K = 4) |
– Indicates pairs of tests where there were no head‐to head comparisons of the two tests in a study. Direct comparisons were made using only data from studies that compared each pair of tests in the same population. Ratio of diagnostic odds ratios (DORs) were computed by division of the DOR for the test in the row by the DOR for the test in the column. If the ratio of DORs is greater than one, then the diagnostic accuracy of the test in the row is higher than that of the test in the column; if the ratio is less than one, the diagnostic accuracy of the test in the column is higher than that of the test in the row.
Table 4 shows the same comparisons made using all available data. Results are generally in agreement with the direct comparisons, and in addition, showed some statistically significance differences (P < 0.05) suggesting that nasal bone outperformed other ultrasound markers and had similar accuracy with strategies comprising NT and serum markers. Nasal bone was the best performing ultrasound marker (DOR (95% CI): 132 (71 to 245)), and the combined NT, PAPP‐A, free ßhCG and maternal age test strategy was the best performing ultrasound and serum test combination (DOR (95% CI): 133 (114 to 155)). Both tests had a much higher diagnostic accuracy than the other tests, and the difference in accuracy was statistically significant in several comparisons especially when compared with single ultrasound markers with or without maternal age. The difference in accuracy between the nasal bone marker and test strategies that included at least one serum test was statistically significant (P = 0.04) for only the comparison with the combined NT, free ßhCG and maternal age test strategy. There were no statistically significant differences in accuracy between combinations that included nasal bone and NT with or without maternal age, and test strategies that included both NT and one or more serum markers. However, these comparisons are potentially confounded by differences between the studies.
2. Indirect comparisons of the diagnostic accuracy of the 10 most evaluated first trimester ultrasound markers alone or in combination with first trimester serum tests.
|
Ratio of DORs (95% CI); P value |
Nasal bone | NT | Nasal bone and age | Ductus and age | NT and age | NT, nasal bone and age | NT, free ßhCG and age | NT, PAPP‐A and age | NT, PAPP‐A, free ßhCG and age | |
|
DOR (95% CI) Studies |
132 (71, 245) K = 11 | 45 (31, 67) K = 13 | 40 (7, 224) K = 4 | 41 (18, 92) K = 5 | 46 (37, 57) K = 50 | 66 (24, 180) K = 5 | 65 (51, 84) K = 5 |
80 (59, 109) K = 5 |
133 (114, 155) K = 69 |
|
| NT | 45 (31, 67) K = 13 | 0.34 (0.16, 0.71); P = 0.006 | ||||||||
| Nasal bone and age | 40 (7, 224) K = 4 | 0.31 (0.05, 1.90); P = 0.18 | 0.90 (0.16, 5.05); P = 0.89 | |||||||
| Ductus and age | 41 (18, 92) K = 5 | 0.31 (0.11, 0.87); P = 0.03 | 0.90 (0.37, 2.20); P = 0.80 | 1.00 (0.11, 9.34); P = 1.00 | ||||||
| NT and age | 46 (37, 57) K = 50 | 0.35 (0.19, 0.66); P = 0.002 | 1.02 (0.66, 1.58); P = 0.92 | 1.14 (0.23, 5.61); P = 0.87 | 1.14 (0.52, 2.49); P = 0.74 | |||||
| NT, nasal bone and age | 66 (24, 180) K = 5 | 0.50 (0.14, 1.81); P = 0.26 | 1.47 (0.47, 4.58); P = 0.48 | 1.64 (0.12, 21.5); P = 0.62 | 1.64 (0.33, 8.08); P = 0.46 | 1.43 (0.52, 3.98); P = 0.48 | ||||
| NT, free ßhCG and age | 65 (51, 84) K = 5 |
0.49 (0.25, 0.98); P = 0.04 | 1.44 (0.89, 2.34); P = 0.12 | 1.61 (0.26, 10.1); P = 0.56 | 1.61 (0.65, 3.99); P = 0.26 | 1.41 (1.02, 1.96); P = 0.04 | 0.98 (0.30, 3.19); P = 0.98 | |||
| NT, PAPP‐A and age | 80 (59, 109) K = 5 | 0.61 (0.29, 1.25); P = 0.16 | 1.77 (1.05, 3.00); P = 0.04 | 1.98 (0.30, 13.1); P = 0.42 | 1.98 (0.76, 5.15); P = 0.14 | 1.73 (1.19, 2.53); P = 0.005 | 1.21 (0.35, 4.13); P = 0.73 | 1.23 (0.74, 2.05); P = 0.35 | ||
| NT, PAPP‐A, free ßhCG and age | 133 (114, 155) K = 69 |
1.00 (0.55, 1.84); P = 1.00 | 2.93 (1.96, 4.40); P < 0.0001 | 3.27 (0.68, 15.8); P = 0.14 | 3.27 (1.53, 7.00); P = 0.003 | 2.87 (2.21, 3.72); P < 0.0001 | 2.00 (0.73, 5.45); P = 0.17 | 2.03 (1.52, 2.72) P < 0.0001 |
1.65 (1.17, 2.34) P = 0.005 |
|
| NT, PAPP‐A, free ßhCG, ADAM 12 and age | 85 (58, 124) K = 4 | 0.64 (0.30, 1.37); P = 0.23 | 1.88 (1.07, 3.32); P = 0.03 | 2.10 (0.31, 14.1); P = 0.39 | 2.10 (0.78, 5.63); P = 0.12 | 1.84 (1.19, 2.84); P = 0.007 | 1.28 (0.37, 4.47); P = 0.65 | 1.30 (0.81, 2.09) P = 0.26 |
1.06 (0.61, 1.86) P = 0.81 |
0.64 (0.43, 0.96) P = 0.03 |
Indirect comparisons were made using all available data for each pair of tests. Ratios of diagnostic odds ratios (DORs) were computed by division of the DOR for the test in the row by the DOR for the test in the column. If the ratio of DORs is greater than one, then the diagnostic accuracy of the test in the row is higher than that of the test in the column; if the ratio is less than one, the diagnostic accuracy of the test in the column is higher than that of the test in the row.
Investigation of heterogeneity and sensitivity analyses
We explored the effect of advanced maternal age (< 35 years versus ≥ 35 years) on test performance. However, we were unable to use meta‐regression to formally investigate the effect of advanced maternal age due to limited data. Of the 126 included studies, 13 did not report maternal age. The available data for all studies are summarised in Table 5 which also shows the four test combinations (NT, PAPP‐A, free ßhCG and maternal age; NT and maternal age; nasal bone alone; and NT alone) that included 10 or more studies. Two studies included only pregnant women with maternal age of 35 years or more; one study (Centini 2005) evaluated the NT, PAPP‐A, free ßhCG and maternal age test combination and the other study (Marsis 2004) evaluated NT. Across the four tests there were 12 studies of women considered high‐risk referrals; one of the studies (Centini 2005), included only pregnant women ≥ 35 years old. The main indication for referral for invasive testing was often increased risk due to advanced maternal age and so we compared high‐risk populations with routine screening populations. The analysis was not performed for nasal bone because only two of the 11 studies were conducted in high‐risk populations. The results of the investigation for the remaining three tests together with the sensitivity analyses inflating the false negatives from 10% to 50% in studies where delayed verification in test negatives occurred are shown in Table 6.
3. Summary of study characteristics.
| Study | NT, PAPP‐A, free ßhCG and age | Nasal bone | NT and age | NT | Maternal age (range) in years | Reference standard | Population | Study design | Study location |
| Acacio 2001 | X | Mean 35.8 (21‐45) | CVS biopsy, amniocentesis or blood or placenta used for fetal karyotyping | High‐risk referral for invasive testing | Retrospective study of patient notes | South America | |||
| Audibert 2001 | X | Mean 30.1, all < 38, 86% < 35, 14% ≥ 35 | Prenatal karyotype conducted (in 7.6% of patients) depending on presence of risk > 125, high maternal age, parental anxiety, history of chromosomal defects or parental translocation or abnormal second trimester scan age | Routine screening | Prospective consecutive series | France | |||
| Babbur 2005 | X | Median 37 (19‐46) | Invasive testing offered to women with NT > 3 mm or risk > 1:250 as defined by combined NT and serum results (CVS from 11 weeks, amniocentesis from 15 weeks). Rapid in situ hybridisation test in patients with risk > 1:30. No details given of any follow‐up to birth | Women requesting screening (self‐paying service) and women attending on account of previous pregnancy history of fetal abnormality | Prospective cohort | UK | |||
| Barrett 2008 | X | Mean 34.9 for screen positives, 30.5 for screen negatives | Karyotyping or follow‐up to birth | Routine screening | Cohort | Australia | |||
| Belics 2011 | Mean 36.4 (15‐46) for Down's cases, 29.8 (15‐49) for unaffected pregnancies | Amniocentesis or CVS (85% of women) or follow‐up to birth | High‐risk referral for invasive testing | Cohort | Budapest | ||||
| Benattar 1999 | X | Mean 32 (16‐46), 8.3% > 35 | Amniocentesis due to maternal age > 38 years (6.1% or women). Karyotyping encouraged for women with positive result on one or more index test. No details of reference standard for index test negative women | Routine screening | Prospective cohort | France | |||
| Bestwick 2010 | X | X | X | Median 39 for Down's cases, 34 for unaffected pregnancies | Karyotyping or follow‐up to birth | Routine screening | Retrospective cohort | UK | |
| Biagiotti 1998 | X | X | Unclear (maybe all ≥ 38) | Amniocentesis or CVS | High‐risk referral for invasive testing | Case control | Italy | ||
| Borenstein 2008 | Median 35 (17‐49) | CVS | High‐risk referral for invasive testing | Prospective cohort | UK | ||||
| Borrell 2005 | X | X | Not reported | CVS (high‐risk women) or follow‐up to birth | Routine screening | Retrospective cohort | Spain | ||
| Borrell 2009 | X | Mean 32 | Karyotyping or follow‐up to birth | Routine screening and high‐risk referral | Prospective cohort | Spain | |||
| Brameld 2008 | X | Median 31 (14‐47), 20% ≥ 35 | Karyotyping or follow‐up to birth | Routine screening | Retrospective cohort | Australia | |||
| Brizot 2001 | X | Median 28 (13‐46), 19.4% ≥ 35 | Antenatal karyotyping (5.9% of pregnancies: 62% of high‐risk, 29% of medium‐risk and 3% of the low‐risk women). Follow‐up to birth (85.3% of women) | Routine screening | Prospective cohort | Brazil | |||
| Centini 2005 | X | ≥ 35 (35‐44) | Amniocentesis in women high risk on screening (16.2%). Follow‐up at birth in women who were low risk on screening | High‐risk patients undergoing routine screening | Retrospective cohort | Italy | |||
| Chasen 2003 | X | Median 33 (IQR 31‐36), 36.2% ≥ 35 | Karyotyping or follow‐up to birth in 96.1% of patients | Routine screening | Prospective consecutive cohort | USA | |||
| Chen 2009 | Median 30 (20‐44) for Down's cases, 32 (19‐40) for controls | Karyotyping or follow‐up to birth | Routine screening | Case control | China | ||||
| Christiansen 2005 | X | Not reported | Karyotyping | Screening programmes for syphilis and Down's syndrome | Case control | Denmark | |||
| Christiansen 2009 | X | Median 37.5 for Down's cases, 36.4 for controls | Karyotyping or follow‐up to birth | Routine screening | Case control | Denmark | |||
| Christiansen 2010 | X | Median 36 (25‐44) for Down's cases, 29 (17‐45) for controls | Karyotyping or follow‐up to birth | Routine screening | Case control | Denmark | |||
| Cicero 2004a | Median 37 (16‐48) | CVS | High‐risk referral for invasive testing | Prospective cohort | USA | ||||
| Cicero 2006 | X | Median 35 (18‐50) | CVS or amniocentesis (in high risk women) or follow‐up to birth | Routine screening | Prospective cohort | UK | |||
| Cocciolone 2008 (first trimester screening cohort) | X | Median 31.3 | Karyotyping or follow‐up to birth | Routine screening | Cohort | Australia | |||
| Cowans 2009 | X | Mean 38 (16‐49) for Down's cases, 29 (13‐56) for unaffected pregnancies | Karyotyping or follow‐up to birth | Routine screening | Cohort | UK | |||
| Cowans 2010 | X | Mean 37.0 (IQR 32.9‐40.5) for Down's cases, 32.4 (IQR 29.0‐35.9) for controls | Karyotyping or follow‐up to birth | Routine screening | Case control | UK | |||
| Crossley 2002 | X | X | Median 29.9, 15.4% ≥ 35 | CVS (offered where women had high NT measurements), amniocentesis or follow‐up to birth | Routine screening | Prospective cohort | UK | ||
| De Graaf 1999 | X | X | Not reported | CVS and amniocentesis | High‐risk referral for invasive testing | Case control | Netherlands | ||
| Ekelund 2008 | X | Not reported | Karyotyping or follow‐up to birth | Routine screening | Cohort | Denmark | |||
| Gasiorek‐Wiens 2001 | X | Median 33 (15‐49), 36.1% > 35 | CVS, amniocentesis or follow‐up to birth | Routine screening | Prospective cohort | Germany, Switzerland and Austria | |||
| Gasiorek‐Wiens 2010 | X | Median 35.1 (13.2‐46.7) | Karyotyping or follow‐up to birth | Routine screening | Cohort | Germany | |||
| Go 2005 | X | 49% ≤ 35, 51% ≥ 36 | Invasive testing or follow‐up to birth | Routine screening | Retrospective cohort | Netherlands | |||
| Gyselaers 2005 | X | X | Not reported | CVS, amniocentesis or follow‐up to birth | Routine screening | Prospective cohort | Belgium | ||
| Habayeb 2010 | Median 35.4 (18‐49) | Karyotyping or follow‐up to birth | Routine screening | Cohort | UK | ||||
| Hadlow 2005* | X | Mean 30.7, 21.2% ≥ 35 | CVS, amniocentesis or follow‐up to birth | Routine screening | Prospective cohort | Australia | |||
| Hafner 1998* | X | Median 28 (15‐49) 6.9% ≥ 35 | Amniocentesis or CVS in patients with previous Down’s pregnancy, > 35 years or with a positive biochemical test result. Other women underwent scan at 22 weeks and, if NT >2.5 mm special examination directed to examination of fetal heart. Follow‐up to birth | Routine screening | Prospective cohort | Austria | |||
| Has 2008 | X | X | X | Median 28.3 (17‐45) | Karyotyping or follow‐up to birth | Routine screening | Cohort | Turkey | |
| Hewitt 1996 | X | Median 37 (21‐48) | CVS | High‐risk referral for invasive testing | Prospective cohort | Australia | |||
| Hormansdorfer 2011 | X | Mean 31.1 (16‐46), 22% ≥ 35 | Karyotyping or follow‐up to birth | Routine screening | Prospective cohort | Germany | |||
| Huang 2010 | X | Median 30 (15‐47), mean 29.8 (SD 3.3) | Karyotyping or follow‐up to birth | Routine screening | Cohort | Taiwan | |||
| Jaques 2007 | X | Mean 33 (16‐51), 18.5% ≥ 37 | Karyotyping or follow‐up to birth | Routine screening | Retrospective cohort | Australia | |||
| Jaques 2010 FTS (first trimester screening) | X | Mean 16.3% ≥ 37 | Karyotyping or follow‐up to birth | Routine screening | Retrospective cohort | Australia | |||
| Kagan 2010 | X | X | Mean 35.4 (14.1‐52.2) | Karyotyping or follow‐up to birth | Routine screening | Prospective cohort | UK | ||
| Kim 2006 | X | Mean 29.9 (SD 3.3) | Amniocentesis or CVS in patients considered high risk (NT > 2.5, aged > 35 years, positive biochemical test result, history or chromosomal abnormality, fetal structural abnormality at ultrasound or other reason). Follow‐up to birth | Routine screening | Retrospective cohort | South Korea | |||
| Koster 2011 | X | Median 37 (IQR 36‐39) | Karyotyping or follow‐up to birth | Routine screening | Case control | Netherlands | |||
| Kozlowski 2007 GC (Gynaecologists' practices) | X | X | Median 32 (15‐48), 26.4% ≥ 35 | Karyotyping or follow‐up to birth | Routine screening | Cohort | Germany | ||
| Kozlowski 2007 PC (Prenatal centre) | X | X | Median 34 (14‐46), 43.2% ≥ 35 | Karyotyping or follow‐up to birth | Routine screening | Cohort | Germany | ||
| Krantz 2000* | X | X | 34.7% ≥ 35 | Not reported | Routine screening | Prospective cohort | USA | ||
| Kublickas 2009 | X | 51% ≥ 35 | Karyotyping or follow‐up to birth | Routine screening | Prospective cohort | Sweden | |||
| Kuc 2010 | X | Not reported | Karyotyping or follow‐up to birth | Routine screening | Case control | Netherlands | |||
| Lam 2002 | X | Mean 30.5 (19% ≥ 35) for unaffected pregnancies | Women considered high risk offered CVS (0.7%) or amniocentesis (11.8%). Follow‐up to birth | Routine screening | Prospective cohort | Hong Kong | |||
| Leung 2009 | X | X | Median 32 (IQR 30‐35), 27.4% ≥ 35 | Amniocentesis or follow‐up to birth | Routine screening | Prospective cohort | China | ||
| MacRae 2008 | X | Not reported | Karyotyping or follow‐up to birth | Routine screening | Retrospective cohort | UK | |||
| Maiz 2007 | Median 35 (17‐49) | CVS | High‐risk referral for invasive testing | Prospective cohort | UK | ||||
| Maiz 2009 | Median 34.5 (14.1‐50.1) | Karyotyping or follow‐up to birth | Routine screening | Prospective cohort | UK | ||||
| Malone 2004 | X | Mean 30.1 (16‐47), 22.1% ≥ 35 | Amniocentesis (in women considered high risk, n = 510) or follow‐up to birth | Routine screening | Prospective cohort | USA | |||
| Malone 2005 | X | 21.6% ≥ 35 | Amniocentesis offered to women with positive results from any screening test. Follow‐up to birth | Routine screening | Prospective cohort | USA | |||
| Marchini 2010* | X | Median 31.3 (18‐45), 19.7% ≥ 35 | Karyotyping or follow‐up to birth | Routine screening | Retrospective cohort | Italy | |||
| Marsis 2004 | X | Mean 37.8 (35‐43) | Amniocentesis (unclear in which patients this was conducted) or follow‐up to birth | Screening of patients ≥ 35 years of age | Prospective cohort | Indonesia | |||
| Marsk 2006 | X | X | Mean 38.5 (SD 4.0) for Down's cases, 35.5 (SD 4.0) for controls | Not reported | Routine screening | Case control | Sweden | ||
| Matias 1998 | Median 35 (17‐46) | Fetal karyotyping. In cases where NT above 95th percentile or abnormal ductus venousus flow, follow‐up scan conducted at 14‐16 weeks | High‐risk referral for invasive testing | Prospective cohort | UK and Portugal | ||||
| Matias 2001 | Median 35 (17‐46) | Fetal karyotyping. In cases where NT above 95th percentile or abnormal ductus venousus flow, follow‐up scan conducted at 14‐16 weeks | High‐risk referral for invasive testing | Prospective cohort | Portugal | ||||
| Mavrides 2002 | X | Median 35 (15‐42) | CVS or follow‐up | High‐risk referral for invasive testing | Prospective cohort | UK | |||
| Maxwell 2011 FTS (first trimester screening cohort) | X | Median 31 (14‐48), 24.3% ≥ 35 | Karyotyping or follow‐up to birth | Routine screening | Prospective cohort | Australia | |||
| Maymon 2005 | Mean 33.7 (SD 4.9) for Down's cases, 30.3 (SD 4.5) for controls | Amniocentesis (recommended for women with higher risk on first or second trimester testing) or follow‐up to birth | Routine screening | Case control | Israel | ||||
| Maymon 2008 | X | X | Not reported | Karyotyping or follow‐up to birth | Routine screening | Case control | USA | ||
| Merz 2011 | X | Not reported | Karyotyping or follow‐up to birth | Routine screening | Retrospective cohort | Germany | |||
| Michailidis 2001 | X | Mean 30.1 (13‐50), 21.1% ≥ 35, 11.9% ≥ 37 | Karyotyping in women considered at risk due to index test results, age or family history or those with considerable anxiety (632 women, 8.5%) or follow‐up to birth | Routine screening | Prospective cohort | UK | |||
| Molina 2010 high risk (High‐risk cohort) | X | Mean 32.7 (16.7‐47.5) | CVS | High‐risk referral for invasive testing | Cohort | Spain | |||
| Molina 2010 screening (Screening cohort) | X | Not reported | Karyotyping or follow‐up to birth | Routine screening | Cohort | Spain | |||
| Monni 2005 | X | Median 32 (14‐49) | Karyotyping or follow‐up to birth | Routine screening | Prospective cohort | Italy | |||
| Montalvo 2005 | X | Mean 31.1 (14‐49), 25.9% ≥35 | Invasive testing offered to women considered high risk from screening results or follow‐up to birth | Routine screening | Prospective cohort | Spain | |||
| Moon 2007 | X | Mean 35.5 (SD 4.8) for Down's cases, 31.7 (SD 3.4) for unaffected pregnancies | Karyotyping or follow‐up to birth | Routine screening | Prospective cohort | Korea | |||
| Muller 2003 | X | X | Not reported | Invasive testing (offered to women with high NT measurement) or follow‐up to birth | Routine screening | Retrospective cohort | France | ||
| Nicolaides 1992 | X | Median 38 (22‐47) | Fetal karyotyping by amniocentesis (52%) or CVS (48%) | High‐risk referral for invasive testing | Prospective cohort | UK | |||
| Nicolaides 2005 | X | Median 31 (13‐49) | Amniocentesis or CVS (patients considered high risk based on screening). First trimester presence/absence of nasal bone, presence/absence of tricuspid regurgitation or normal/abnormal Doppler studies (patients of intermediate risk on first trimester screening and did not undergo CVS or amniocentesis. With the addition of information from these tests, if adjusted risk was high, CVS was performed). Follow‐up to birth | Routine screening | Prospective cohort | UK | |||
| Niemimaa 2001 | X | X | 17.5% ≥ 35 | Invasive testing (patients considered high risk based on NT screening) or follow‐up to birth. | Routine screening | Prospective cohort | Finland | ||
| Noble 1995 | Median 34 (15‐47), 47% ≥ 35 | Karyotyping performed (27% of women) due to increased NT (14%), advanced maternal age (10%), previous chromosomally abnormal child (0.5%) or parental anxiety (2%). Ultrasound examination at 20 weeks (65% of patients). Follow‐up to birth (9% of women) | Routine screening in a high risk population | Prospective cohort | UK | ||||
| O'Callaghan 2000 | X | Median 32 | CVS, amniocentesis or neonatal karyotyping or follow‐up to birth | Routine screening | Prospective cohort | Australia | |||
| O'Leary 2006 | X | X | Median 31 (14‐47), 20% ≥ 35 | CVS or amniocentesis (women assessed to be high risk on screening) or follow‐up to birth | Routine screening | Prospective cohort | Australia | ||
| Okun 2008 FTS (first trimester screening cohort) | X | Mean 34 | Karyotyping or follow‐up to birth | Routine screening | Prospective cohort | Canada | |||
| Orlandi 1997 | X | X | Range 15 to 46, 35% ≥ 35 | Not reported | Routine screening | Prospective cohort | Italy | ||
| Orlandi 2003 | X | Median 31.7 (SD 4.0) for Down's cases, 36.5 (SD 4.1) for unaffected pregnancies | CVS or amniocentesis (women considered high risk on screening on the basis of NT and biochemical results, but not on nasal bone screening, or if requested due to age or anxiety) or follow‐up to birth | Routine screening (2 centres) or in referred patients (1 centre) | Prospective cohort | Italy and Netherlands | |||
| Orlandi 2005 | X | Median 30.5 (SD 8.2) | Not reported | Routine screening | Retrospective cohort | Italy | |||
| Otaño 2002 | X | Median 36 (19‐44) | CVS | High‐risk referral for invasive testing | Prospective cohort | Argentina | |||
| Pajkrt 1998 | X | Mean 31.4 (SD 5.7), 24% ≥ 35 | Prenatal karyotyping offered to patients considered high risk or maternal anxiety (conducted in 24%) or follow‐up to birth | Routine screening | Prospective cohort | Netherlands | |||
| Pajkrt 1998a | X | Mean 37.6 (22‐46) | Prenatal karyotyping | High‐risk referral for invasive testing | Consecutive cohort | Netherlands | |||
| Palomaki 2007 FTS (first trimester screening cohort) | Mean 32.3 (SD 4.6) | Karyotyping or follow‐up to birth | Routine screening | Prospective cohort | Canada | ||||
| Perni 2006 | X | Median 33.0 (IQR 31.0‐36.0) | CVS or amniocentesis. Cytogenetic testing in cases of miscarriage. Follow‐up to birth. | Routine screening | Retrospective cohort | USA | |||
| Prefumo 2005 | X | Median 37 (19‐46) | CVS | High‐risk referral for invasive testing | Prospective cohort | UK | |||
| Prefumo 2006 | X | Mean 31.4 (14.5‐50.2) | Karyotyping or follow‐up to birth | Routine screening | Prospective cohort | UK | |||
| Ramos‐Corpas 2006 | X | Mean 30.1 (15‐46) (SD 5.37), 18% ≥ 35 | Invasive testing offered to patients considered high risk at screening (> 1:300) or follow‐up to birth | Routine screening | Prospective cohort | Spain | |||
| Rissanen 2007 | X | 29.5, 17.7% ≥35 | Karyotyping or follow‐up to birth | Routine screening | Prospective cohort | Finland | |||
| Rozenberg 2002 | X | Median 30.5 (18‐37) | Amniocentesis offered to patients with NT >3mm or serum marker risk was > 1:250, or follow‐up to birth | Routine screening | Prospective cohort | France | |||
| Rozenberg 2007 | X | Mean 30.9 (SD 4.5) | Karyotyping or follow‐up to birth | Routine screening | Prospective cohort | Canada | |||
| Sahota 2010 | X | X | Median 33.1, 30.1% ≥ 35 | Karyotyping or follow‐up to birth | Routine screening | Prospective cohort | China | ||
| Salomon 2010 | X | Median 30.7 (18.0‐46.3) | Karyotyping or follow‐up to birth | Routine screening | Prospective cohort | France | |||
| Santiago 2007 | X | X | Mean 30.6 (14‐46) | Karyotyping or follow‐up to birth | Routine screening | Retrospective cohort | Spain | ||
| Sau 2001 | X | Mean 28 (SD 5) | Invasive testing (women with high risk on screening) or follow‐up to birth | Routine screening | Prospective cohort | UK | |||
| Schaelike 2009 | X | X | 31.0% ≥35 | Karyotyping or follow‐up to birth | Routine screening | Prospective cohort | Germany | ||
| Schielen 2006* | X | Median 36.5 (18‐47) | Invasive testing or follow‐up to birth | Routine screening | Retrospective cohort | Netherlands | |||
| Schuchter 2001 | X | Mean 28 (15‐46), 10.7% ≥ 35 | CVS (offered to patients with first trimester NT > 3.5 mm), amniocentesis (offered to patients with first trimester NT 2.5‐3.4 mm, high risk on second trimester serum testing (> 1:250) and those > 35 years) or follow‐up to birth | Routine screening | Retrospective cohort | Austria | |||
| Schuchter 2002 | X | X | 13% > 35 | CVS and amniocentesis (offered to patients with increased risk (> 1:400) at first trimester screening. CVS recommended when NT > 3.5 or when women did not want to wait until the 15th week for amniocentesis), or follow‐up to birth | Routine screening | Prospective cohort | Austria | ||
| Schwarzler 1999 | X | Mean 29.4 (16‐47) | Invasive testing (women considered high risk on screening) or follow‐up to birth | Routine screening | Prospective consecutive cohort | UK | |||
| Scott 2004 | X | X | Median 32 (15‐44), 29% ≥ 35 | Invasive testing or follow‐up to birth | Routine screening | Prospective cohort | Australia | ||
| Sepulveda 2007 | X | X | Median 33 (14‐47), 35.4% ≥ 35 | CVS, amniocentesis, cordocentesis or follow‐up to birth | Routine screening | Prospective cohort | Chile | ||
| Snijders 1998 | X | Median 31 (14‐49) | CVS and amniocentesis (9.6% of women) or follow‐up to birth | Routine screening | Prospective cohort | UK | |||
| Sorensen 2011 | X | Median 34 (23‐44) for Down's cases; mean 30.4 (16‐45), 16.5% ≥ 35 for unaffected pregnancies | Karyotyping or follow‐up to birth | Routine screening | Retrospective cohort | Denmark | |||
| Spencer 1999 | X | X | X | Median 38 (19‐46) for Down's cases, 36 (15‐47) for controls | Invasive testing (high‐risk women) or follow‐up to birth | Routine screening | Case control | UK | |
| Spencer 2002 | Median 36 (20‐44) for Down's cases, 30 (16‐41) for controls | Not reported | Routine screening | Case control | UK | ||||
| Spencer 2008 | X | Median 35.8 for Down's cases, 29.3 for controls | Karyotyping or follow‐up to birth | Routine screening | Case control | Denmark | |||
| Stenhouse 2004 | X | Median 32 (14‐45), 27% ≥ 35 | Invasive testing offered to women with screening risk of > 1:250 or follow‐up to birth | Routine screening | Prospective cohort | UK | |||
| Strah 2008 | X | Median 28.6 (15‐42) | Karyotyping or follow‐up to birth | Routine screening | Cohort | Slovenia | |||
| Theodoropoulos 1998 | X | Median 29 (16‐48), 7.8% ≥ 37 | CVS or amniocentesis or follow‐up to birth. Unclear reference standard in cases of intrauterine death, miscarriages and terminations. | Routine screening | Prospective cohort | Greece | |||
| Thilaganathan 1999 | X | Mean 29 (15‐45) | CVS (offered to patients considered high risk on screening) or follow‐up to birth | Routine screening | Prospective cohort | UK | |||
| Timmerman 2010 | Mean 34.5 (19‐45) | Karyotyping or follow‐up to birth | Routine screening | Prospective cohort | Netherlands | ||||
| Torring 2010 | X | Mean 35 for Down's cases, 31 for controls | Karyotyping or follow‐up to birth | Routine screening | Case control | Denmark | |||
| Vadiveloo 2009 | X | Median 33.1, 36.9% ≥ 35 | Karyotyping or follow‐up to birth | Routine screening | Retrospective cohort | UK | |||
| Valinen 2007 | X | Mean 29.6, 18.6% ≥ 35 | Karyotyping or follow‐up to birth | Routine screening | Retrospective cohort | Finland | |||
| Viora 2003 | Median 32 (18‐47) | CVS or follow‐up to birth | Routine screening | Prospective cohort | Italy | ||||
| Wald 2003 | X | X | X | Not reported | Invasive testing (following second trimester screening) or follow‐up to birth | Routine screening | Case control | UK and Austria | |
| Wapner 2003* | X | X | Mean 35 (SD 4.6), 50% ≥ 35 | Invasive testing. Miscarriage with cytogenetic testing. Follow‐up to birth | Routine screening | Prospective cohort | USA | ||
| Wax 2009 | X | Mean 36.7 (SD 3.2) | Karyotyping or follow‐up to birth | Routine screening | Retrospective cohort | USA | |||
| Wojdemann 2005 | X | X | Mean 29, 10.8% ≥ 35 | Invasive testing (in cases of increased risk) or follow‐up to birth | Referrals for screening | Prospective cohort | Denmark | ||
| Wortelboer 2009 | X | Median 34.9 (15‐48) | Karyotyping or follow‐up to birth | Routine screening | Cohort | Netherlands | |||
| Wright 2008 | X | Median 35.2 (16‐52) | Karyotyping or follow‐up to birth | Routine screening | Cohort | UK | |||
| Wright 2010 | X | Median 31.9 (IQR 27.7‐35.8) | Karyotyping or follow‐up to birth | Routine screening | Cohort | UK, Denmark and Cyprus | |||
| Zoppi 2001 | X | Median 33 (14‐48) | Amniocentesis, CVS or follow‐up to birth | Routine screening | Prospective cohort | Italy |
*The study provided data for the subset of women with maternal age of 35 or more.
X indicates that the test was evaluated in the study.
CVS = chorionic villus sampling; IQR = interquartile range; SD = standard deviation.
4. Investigation of the effect of type of population.
| Correction made for missing false negatives in studies with delayed verification of test negatives | NT | NT and maternal age | NT, PAPP‐A, free ßhCG and maternal age | ||||||
|
Ratio of DORs (95% CI); P value |
Sensitivity at 5% FPR (95% CI) (studies) |
Ratio of DORs (95% CI); P value |
Sensitivity at 5% FPR (95% CI) (studies) |
Ratio of DORs (95% CI); P value |
Sensitivity at 5% FPR (95% CI) (studies) | ||||
| Screening (n = 9) | High risk (n = 4) | Screening (n = 46) | High risk (n = 4) | Screening (n = 66) | High risk (n =3) | ||||
| No FN correction | 0.68 (0.26, 1.77); P = 0.40 |
73 (62, 81) | 64 (45, 80) | 0.34 (0.17, 0.69); P = 0.003 |
72 (68, 76) | 47 (31, 63) | 0.41 (0.16, 1.00); P = 0.05 | 88 (86, 89) | 74 (54, 88) |
| FN increased +10% | 0.69 (0.27, 1.78); P = 0.40 |
70 (59, 79) | 62 (42, 78) | 0.40 (0.20, 0.82); P = 0.01 |
69 (64, 73) | 47 (31, 64) | 0.48 (0.19, 1.20); P = 0.11 | 86 (84, 87) | 74 (53, 88) |
| FN increased +20% | 0.74 (0.29, 1.92); P = 0.50 |
69 (57, 78) | 62 (42, 78) | 0.43 (0.21, 0.89); P = 0.02 |
67 (63, 71) | 47 (31, 64) | 0.51 (0.20, 1.28); P = 0.15 | 85 (83, 87) | 74 (54, 88) |
| FN increased +30% | 0.81 (0.31, 2.09); P = 0.63 |
67 (55, 76) | 62 (42, 78) | 0.46 (0.22, 0.97); P = 0.04 |
66 (61, 70) | 47 (30, 64) | 0.55 (0.22, 1.38); P = 0.20 | 84 (82, 86) | 74 (54, 88) |
| FN increased +40% | 0.76 (0.29, 2.02); P = 0.55 |
66 (53, 76) | 59 (39, 77) | 0.50 (0.24, 1.02); P = 0.06 |
64 (60, 68) | 47 (31, 64) | 0.59 (0.24, 1.48); P = 0.26 | 83 (81, 85) | 74 (54, 88) |
| FN increased +50% | 0.81 (0.30, 2.15): P = 0.65 | 64 (52, 75) | 59 (39, 77) | 0.52 (0.25, 1.08); P = 0.08 |
63 (58, 67) | 47 (30, 64) | 0.62 (0.25, 1.56); P = 0.31 | 82 (80, 84) | 74 (54, 88) |
DOR = diagnostic odds ratio
Delayed verification was not common in high‐risk referral studies as women tended to be offered invasive testing on the basis of the increased risk, and the corrections to the false negatives made very little or no difference to the estimates of sensitivity. However, in screening populations the correction reduced sensitivity, and consequently reduced the apparent relationship between type of population and test performance, observed through the ratio of DORs approaching one. Up to an increase of 40% in the false negatives, the difference in sensitivity between high risk and screening populations for the NT and maternal age test strategy remained statistically significant; the magnitude of the difference dropping from 25% to 17%. However, it should be noted that there were few high‐risk referral studies for each of the three tests and the results should be interpreted with caution.
In six studies (Hadlow 2005; Hafner 1998; Krantz 2000; Marchini 2010; Schielen 2006; Wapner 2003), we were able to extract data for the subset of women ≥ 35 years old (≥ 36 years for Schielen 2006). The five NT, PAPP‐A, free ßhCG and maternal age test combination studies all showed higher sensitivity and higher FPR for the ≥ 35 years subgroup compared to the < 35 years subgroup as shown on the forest plot (Figure 6) and summary ROC plot (Figure 7). We did not formally compare the two age groups in a meta‐analysis because the younger age group had very few cases, thresholds were mixed and there were few studies.
6.
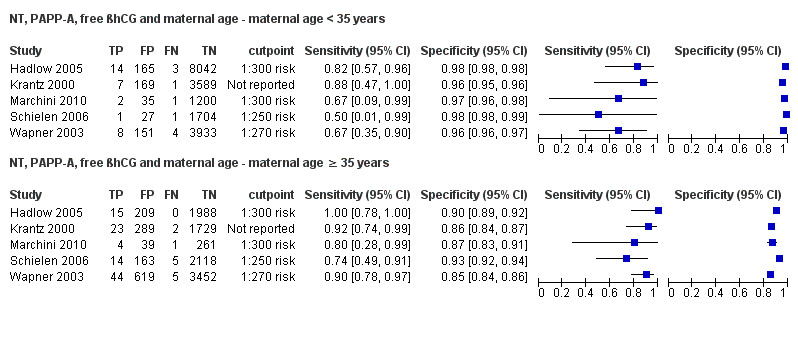
Forest plot of the NT, PAPP‐A, free ßhCG and maternal age test strategy by maternal age group (< 35 years versus ≥ 35 years).
7.
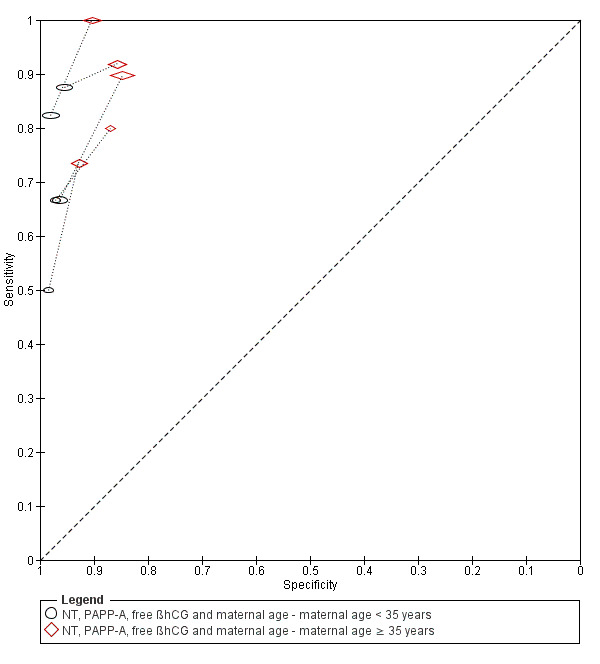
Summary ROC plot of the NT, PAPP‐A, free ßhCG and maternal age test strategy by maternal age group (< 35 years versus ≥ 35 years).
Women with multifetal pregnancies were included in six studies (Chasen 2003; Hewitt 1996; Leung 2009; Marchini 2010; Moon 2007; O'Callaghan 2000). Hewitt 1996 evaluated NT alone. Chasen 2003 and O'Callaghan 2000 evaluated the combination of NT and maternal age. Both Leung 2009 and Moon 2007 evaluated nasal bone. Leung 2009 and Marchini 2010 both evaluated the combination of NT, PAPP‐A, free βhCG and maternal age. We excluded both studies in a sensitivity analysis to determine the effect on our estimates of test accuracy, due to the potential effect of multifetal pregnancy on serum marker levels. Our findings were unchanged.
Discussion
Summary of main results
We found a large number of studies evaluating first trimester Down’s syndrome ultrasound markers with or without first trimester serum screening tests. Few studies compared two or more test strategies in the same population; the majority of studies only evaluated a single test strategy. However, the comparison between NT and the combined NT, PAPP‐A, free ßhCG test strategy, both with maternal age, was evaluated in 25 studies. Few studies were available to assess the performance of test strategies involving newer serum markers such as ADAM 12. A summary of results for the 10 most commonly evaluated test strategies is given in Table 1, and the remaining 50 test strategies are given in Table 2.
Four key findings were noted.
The combined test comprised of NT, PAPP‐A, free βhCG and maternal age appears to have significantly better test accuracy than the tests comprised of NT and maternal age with or without either PAPP‐A or free βhCG. This combined test detects around nine out of every 10 Down's affected pregnancies for a fixed 5% false positive rate (FPR). By comparison, the tests comprised of NT and maternal age and either PAPP‐A or free βhCG, and NT alone or with maternal age detects between seven and eight out of every 10 Down's affected pregnancies for a fixed 5% FPR.
While the test combinations that include nasal bone showed good detection rates when combined with PAPP‐A and free βhCG, the evidence was limited (three studies) and the variation in threshold precluded meta‐analysis.
The evidence for combining NT with higher numbers of serum markers showed similar detection rates to combinations of NT and double or triple serum markers that include PAPP‐A, but were based on data from only one or two studies. Therefore further evaluation of these tests is needed. Furthermore, there were combinations of NT and other ultrasound markers with serum markers that showed superior detection rates to combinations of NT with standard double markers commonly used in clinical practice, which may warrant further study.
Detection rates were lower in high‐risk pregnancies (mainly due to advanced maternal age) compared to routine screening populations. Evidence was available for three tests at a fixed 5% FPR and showed reductions in detection rates of between 5% and 25%. Part of this effect may be explained by studies in routine screening populations missing false negative cases lost through increased miscarriage in Down’s pregnancies, but this does not fully explain the effect. We were unable to draw any conclusions as to why this may be the case, especially since the analyses were based on few high‐risk referral studies. This finding also contradicts the observation we made in five studies where data were available to compare the performance of the NT, PAPP‐A, free βhCG and maternal age test strategy between women younger than 35 years and those 35 years or more within the same study. In these studies, the ≥ 35 years age group showed higher detection rates and FPRs compared to the group less than 35 years old. It should be noted that very few cases contributed to the analysis of the younger age group.
Strengths and weaknesses of the review
This review is the first comprehensive review of first trimester ultrasound and serum screening. We examined papers from around the world (32 countries), covering a wide cross‐section of women in varying populations. We contacted authors to verify data where necessary to give as complete a picture as possible while trying to avoid replication of data.
There were a number of factors that made meta‐analysis of the data difficult, which we tried to adapt for in order to allow for comparability of data presented in different studies.
There were many different cut‐points used to define pregnancies as high or low risk for Down's syndrome. This means that direct comparison is more difficult than if all studies used the same cut‐point to dichotomise their populations. This is less of an issue for first trimester serum screening, compared to second trimester serum screening, as the majority of authors chose a cut‐point of 5% FPR.
There were many different risk equations and software applications in use for combination of multiple markers, which were often not described in the papers. This means that risks may be calculated by different formulae and they may not be directly comparable for this reason. It is possible that this is responsible for unexplained heterogeneity in results.
Different laboratories and clinics run different assays and use different machines and methods. This may influence raw results and subsequent risk calculations. Many laboratories have a quality assessment or audit trail, however, this may not necessarily be standard across the board. For example, how many assays are run, how often medians are calculated and adjusted for a given population and how quickly samples are tested from initially being taken.
Few studies made direct comparisons between tests, making it difficult to detect if a real difference exists between tests (i.e. how different tests perform in the same population). There were differences in populations, with assay medians being affected, for example, by race. It is not certain whether it is appropriate to make comparisons between populations that are inherently different.
We were unable to perform all the investigations of heterogeneity that we had originally intended to because the data simply were not available. The vast majority of papers looking at pregnancies conceived by IVF, affected by diabetes, multiple gestation or a family history of Down's syndrome involved unaffected pregnancies only.
In addition, the search for this review was last updated in August 2011, and it is possible that new studies may have been published which have not been included. Since the search was completed we have kept a watching brief on outputs and are not aware of any studies with substantial sample sizes which could substantially affect the findings.
Applicability of findings to the review question
Potentially, when planning screening policy or a clinical screening programme, clinicians and policy makers need to make decisions about a finite number of tests or type of tests that can be offered. These policies are often driven by both the needs of a specific population and by financial resources. Economic analysis was considered to be outside of the scope of this review. Many of the tests examined as part of this review are already commercially available and in use in the clinical setting. The studies were carried out on populations of typical pregnant women and therefore, the results should be considered comparable with most pregnant populations encountered in every day clinical practice.
We were unable to extract information about harms of testing, information about miscarriage rates and uptake of definitive testing as the data were not available the majority of the time. While it is unlikely that major differences between the tests evaluated here exist in terms of direct harms of testing, as they are all based on ultrasound, with or without a blood sample, differences in accuracy may lead to differences in the use of definitive testing and its consequent adverse outcomes.
In some countries with a defined screening policy (i.e. the UK), first trimester serum screening plays a major role, usually in combination with first trimester ultrasound scanning. In others however, there may only be a limited range of tests or markers available—often second trimester markers, rather than first trimester markers. The results of this review should be interpreted and applied in the context of test availability and local restrictions, populations or policies.
Authors' conclusions
Implications for practice.
The evidence supports the use of the first trimester test comprised of nuchal translucency (NT), pregnancy‐associated plasma protein A (PAPP‐A), free beta human chorionic gonadotrophin (βhCG) and maternal age; there is little evidence to recommend the use of first trimester ultrasound markers alone, combinations with single serum tests or those that exclude PAPP‐A. However, the data available on the addition of more that more than two serum markers to ultrasound markers are limited, and based on generally small populations of women. We would not recommend that these tests be introduced into wider clinical practice without careful consideration of cost.
The review has shown that tests involving NT and two or three markers in combination with maternal age are significantly better than those involving ultrasound markers alone. We would therefore recommend that ultrasound markers alone, or combinations involving a single serum marker are not used for Down's syndrome screening. The choice of multiple serum markers will depend on the availability of certain assays in local laboratories. On the basis of this review we would recommend the combination of NT, PAPP‐A, free βhCG and maternal age, as it significantly outperforms NT and maternal age or NT and maternal age with either of the two serum markers, and is widely available. The data for other test combinations limits our ability to make any other recommendations about specific test combinations. Alternative screening methods should also be considered when making policy decisions, and are the subject of other reviews in this suite.
Implications for research.
Further evaluation of test combinations involving ultrasound markers with three or more serum markers are required to determine whether they offer superior test performance. Further study of the performance of test combinations in women over 35 is required, as this age group has the highest incidence of Down’s syndrome and has the greatest requirement for tests with high detection rates.
Future studies should ensure that adequate sample sizes are recruited, and take opportunities to make comparisons of test performance testing several alternative test combinations on the same population. Such direct comparison removes issues of confounding when making test comparisons, and allows a clear focus on testing the incremental benefit of increasingly complex and expensive testing strategies. The reporting of studies of test accuracy can be improved and more closely adhere to the standards for the reporting of diagnostic accuracy studies (STARD) guideline. Three key aspects of this are: 1) formally testing the statistical significance of differences in test performance in direct comparisons and estimating incremental changes in detection rates (together with confidence intervals); 2) clearly reporting the number of mothers studied and their results; and 3) reporting the numbers of women who are lost to follow‐up. Many authors reported results of extrapolating findings to age‐standardised national cohorts to demonstrate the performance of the test, and failed to report the actual numbers studied and evaluated.
For the purposes of meta‐analysis and to allow for comparisons to be made between different tests and combinations, we would recommend the publication of consensus standard algorithms for estimating risk, and reporting of test performance at a standard set of thresholds. This would be difficult to achieve and implement, but an attempt at consensus should be made.
Notes
This review belongs to a suite of reviews examining antenatal screening for Down's syndrome which includes:
First trimester serum tests for Down's syndrome screening (Alldred 2015);
Urine tests for Down's syndrome screening (Alldred 2015a)
Second trimester serum tests for Down's syndrome screening (Alldred 2012);
First trimester ultrasound tests alone or in combination with first trimester serum tests for Down's syndrome screening (this review)
First and second trimester serum tests with and without first trimester ultrasound tests for Down's syndrome screening (in press).
The plans for these reviews were described in a generic protocol (Alldred 2010) published in the Cochrane Library in 2010. The project as a whole has been much larger than initially anticipated, both in terms of size and statistical complexity. The initial search was completed in 2007 and an updated search in August 2011. After identifying studies appropriate for inclusion, a significant amount of time has been devoted to data management and analysis.
The authors are conscious of the time lag from the latest literature search to publication, and the potential for the introduction of new urine tests in this time frame. The authors are also conscious of the potential for publication of new data pertaining to tests included in this review. Whilst not fulfilling the usual Cochrane up‐to‐date criteria, this review is published because it provides historical context in what is a rapidly‐changing field, and because it is unlikely to ever be repeated.
Acknowledgements
We acknowledge the assistance of the Pregnancy and Childbirth Cochrane Review Group Editorial base with writing the searches and other aspects of this review.
As part of the pre‐publication editorial process, this review has been commented on by three peers (an editor and two referees who are external to the editorial team).
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Appendices
Appendix 1. Search strategy
Database: Ovid MEDLINE
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 exp Prenatal Diagnosis/
2 nuchal translucency.mp.
3 exp Pregnancy‐Associated Plasma Protein‐A/
4 pregnancy associated plasma protein a.mp.
5 papp‐a.mp.
6 exp Chorionic Gonadotropin, beta Subunit, Human/
7 (b‐hcg or bhcg).mp.
8 human chorionic gonadotropin.mp.
9 exp alpha‐Fetoproteins/
10 alphafetoprotein$.mp.
11 alpha‐fetoprotein$.mp.
12 afp.mp.
13 (unconjugated estriol or unconjugated oestriol).mp.
14 ue3.mp.
15 exp INHIBINS/
16 inhibin a.mp.
17 ultrasound.mp.
18 amniocentesis/
19 chorion$ vill$ sampling.mp.
20 Chorionic Villi‐Sampling/
21 nasal bone.mp.
22 tricuspid regurgitation.mp.
23 ductus venosus.mp
24 marker$.mp.
25 screen$.mp.
26 detect$.mp.
27 accura$.mp.
28 predict$.mp.
29 ROC.mp.
30 ROC curve/
31 AUC.mp.
32 Area under curve/
33 exp false negative reactions/ or exp false positive reactions/
34 (false positive$ or false negative$).mp.
35 likelihood ratio$.mp.
36 sensitiv$.mp.
37 specific$.mp.
38 diagnos$.ti,ab.
39 "reproducibility of results".mp.
40 reference value$.mp.
41 reference standard$.mp.
42 exp Down Syndrome/
43 downs syndrome.mp.
44 down syndrome.mp.
45 trisomy 21.mp.
46 Aneuploidy/
47 aneuploidy.mp.
48 Mosaicism/
49 mosaicism.mp.
50 or/1‐41
51 or/42‐49
52 50 and 51
53 (antenatal$ or prenatal$ or trimester$ or pregnan$ or fetus or foetus or fetal or foetal).mp.
54 52 and 53
55 animal/ not (humans/ and animal/)
56 54 not 55
*******************************************************
Embase via Dialog Datastar
1. PRENATAL‐DIAGNOSIS#.DE.
2. FETUS‐ECHOGRAPHY#.DE.
3. PREGNANCY‐ASSOCIATED‐PLASMA‐PROTEIN‐A#.DE.
4. CHORIONIC‐GONADOTROPIN‐BETA‐SUBUNIT#.DE.
5. HCG.AB.
6. PAPP.AB.
7. ALPHA‐FETOPROTEIN#.DE.
8. AFP.AB.
9. ALPHA ADJ FETOPROTEIN$
10. ALPHAFETOPROTEIN$
11. BETA ADJ HUMAN ADJ CHORIONIC ADJ GONADOTROPIN
12. PREGNANCY ADJ ASSOCIATED ADJ PLASMA ADJ PROTEIN
13. (UNCONJUGATED ADJ ESTRIOL OR UNCONJUGATED ADJ OESTRIOL).TI.
14. (UNCONJUGATED ADJ ESTRIOL OR UNCONJUGATED ADJ OESTRIOL).AB.
15. UE3
16. INHIBIN‐A#.DE.
17. INHIBIN ADJ A
18. ULTRASOUND
19. AMNIOCENTESIS
20. CHORION‐VILLUS‐SAMPLING.DE.
21. NASAL ADJ BONE
22. TRICUSPID ADJ REGURGITATION
23. DUCTUS ADJ VENOSUS
24. MARKER OR MARKERS
25. SCREEN OR SCREENING
26. DETECT OR DETECTING OR DETECTION
27. FALSE ADJ POSITIVE$
28. FALSE ADJ NEGATIVE$
29. SENSITIVITY OR SENSITIVE OR SENSITIVITIES
30. SPECIFICITY OR SPECIFICITIES
31. (DIAGNOSE OR DIAGNOSIS OR DIAGNOSTIC OR DIAGNOSTICS OR DIAGNOSES
OR DIAGNOSED).TI.
32. (DIAGNOSE OR DIAGNOSIS OR DIAGNOSTIC OR DIAGNOSTICS OR DIAGNOSES
OR DIAGNOSED).AB.
33. ROC.AB.
34. AUC.AB.
35. AREA‐UNDER‐THE‐CURVE.DE.
36. ROC‐CURVE.DE.
37. ACCURA$
38. PREDICT$
39. REPRODUCIBILITY.DE.
40. REFERENCE ADJ VALUE$
41. REFERENCE‐VALUE.DE.
42. REFERENCE ADJ STANDARD$
43. DOWN‐SYNDROME#.DE.
44. DOWN ADJ SYNDROME OR DOWNS ADJ SYNDROME
45. TRISOMY ADJ '21'
46. MOSAICISM
47. ANEUPLOIDY
48. ANTENATAL$ OR PRENATAL$ OR PREGNANCY OR PREGNANT OR TRIMESTER$ OR MATERNAL OR FETUS OR FOETUS OR FOETAL OR FETAL
49. 1 OR 2 OR 3 OR 4 OR 5 OR 6 OR 7 OR 8 OR 9 OR 10 OR 11 OR 12 OR 13 OR 14 OR 15 OR 16 OR 17 OR 18 OR 19 OR 20 OR 21 OR 22 OR 23 OR 24 OR 25 OR 26 OR 27 OR 28 OR 29 OR 30 OR 31 OR 32 OR 33 OR 34 OR 35 OR 36 OR 37 OR 38 OR 39 OR 40 OR 41 OR 42
50. 43 OR 44 OR 45 OR 46 OR 47
51. 48 AND 49 AND 50
52. HUMAN=YES
53. 51 AND 52
ADJ = adjacent AB = abstract
TI = title $ = truncation symbol DE = descriptor (similar to MeSH)
*******************************************************
CINAHL via OVID
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 exp Prenatal Diagnosis/
2 nuchal translucency.mp.
3 pregnancy associated plasma protein.mp.
4 papp$.ti,ab.
5 exp Gonadotropins, chorionic/
6 (b‐hcg or bhcg).mp.
7 human chorionic gonadotropin.mp.
8 exp alpha‐Fetoproteins/
9 alphafetoprotein$.mp.
10 alpha‐fetoprotein$.mp.
11 afp.mp.
12 (unconjugated estriol or unconjugated oestriol).mp.
13 ue3.mp.
14 inhibin$.mp.
15 ultrasound.mp.
16 amniocentesis/
17 chorion$ vill$ sampling.mp.
18 Chorionic Villi‐Sampling/
19 nasal bone.mp.
20 tricuspid regurgitation.mp.
21 ductus venosus.mp.
22 marker$.mp.
23 screen$.mp.
24 detect$.mp.
25 accura$.mp.
26 predict$.mp.
27 ROC.mp.
28 ROC curve/
29 AUC.mp.
30 "area under curve".mp.
31 exp false negative reactions/ or exp false positive reactions/
32 (false positive$ or false negative$).mp.
33 likelihood ratio$.mp.
34 sensitiv$.mp.
35 specific$.mp.
36 diagnos$.ti,ab.
37 "reproducibility of results".mp.
38 reference value$.mp.
39 reference standard$.mp.
40 exp Down Syndrome/
41 downs syndrome.mp.
42 down syndrome.mp.
43 trisomy 21.mp.
44 aneuploidy.mp.
45 mosaicism.mp.
46 (antenatal$ or prenatal$ or trimester$ or pregnan$ or fetus or foetus or fetal or foetal).mp.
47 or/1‐39
48 or/40‐45
49 47 and 48 and 46
*******************************************************
Search terms and instructions for Biosis
The following search terms were entered separately in standard search box (select ‘Titles/subject/abstract’ from the drop‐down box on the right of the search box).
“reference standard*”
“reference value*”
“reproducibility of results”
diagnos*
sensitiv*
specific*
“likelihood ratio*”
“false negative*"
“false positive*”
“area under curve”
ROC
AUC
predict*
detect*
marker*
screen*
accura*
“ductus venosus”
“nasal bone”
“tricuspid regurgitation”
“chorion* vill* sampling”
amniocentesis
ultrasound
inhibin*
“unconjugated oestriol”
“unconjugated estriol”
afp
“alpha fetoprotein*”
alphafetoprotein*
“ b hcg”
“human chorionic gonadotrophin”
“papp a”
“pregnancy associated plasma protein”
“nuchal translucency”
foetal
fetal
foetus
fetus
prenatal*
antenatal*
pregnan*
trimester*
“trisomy 21”
mosaicism
“down* syndrome”
The search then used the history function to combine terms:
1‐34 – combine using OR
35 – 42 – combine using OR
43 – 45 – combine using OR
The three sets were combined using AND
The combined search strategy had the form
(((((((al: "trisomy 21") or (al: (mosaicism))) or (al: "down* syndrome"))) and (((((((((((((((((((((((((((((((((((al: "reference standard*") or (al: "reference value*")) or (al: "reproducibility of results")) or (al: (diagnos*))) or (al: (specific*))) or (al: (sensitiv*))) or (al: "likelihood ratio*")) or (al: "false negative*")) or (al: "false positive*")) or (al: "area under curve")) or (al: (auc))) or (al: (roc))) or (al: (predict*))) or (al: (accura*))) or (al: (detect*))) or (al: (screen*))) or (al: (marker*))) or (al: "ductus venosus")) or (al: "tricuspid regurgitation")) or (al: "nasal bone")) or (al: "chorion* vill* sampling")) or (al: (amniocentesis))) or (al: (ultrasound))) or (al: (inhibin*))) or (al: "unconjugated oestriol")) or (al: "unconjugated estriol")) or (al: (afp))) or (al: "alpha feto protein*")) or (al: "alpha fetoprotein*")) or (al: "b hcg")) or (al: "human chorionic gonadotropin")) or (al: "papp a")) or (al: "pregnancy associated plasma protein")) or (al: "nuchal translucency")))) and (((((((((al: (foetal)) or (al: (fetal))) or (al: (foetus))) or (al: (fetus))) or (al: (pregnan*))) or (al: (trimester*))) or (al: (prenatal*))) or (al: (antenatal*))))))
Data
Presented below are all the data for all of the tests entered into the review.
Tests. Data tables by test.
1. Test.
Aberrant right subclavian artery.
2. Test.
Frontomaxillary facial angle >95 percentile.
3. Test.
Presence of mitral gap.
4. Test.
Maxillary bone length, 5% percentile.
5. Test.
Tricuspid regurgitation.
6. Test.
Iliac angle 90 degrees.
7. Test.
Ductus venosus a‐wave reversed.
8. Test.
Ductus venosus pulsivity index > 95 percentile.
9. Test.
Nasal bone, mixed cut‐points.
10. Test.
NT, 2.5 mm.
11. Test.
NT, 3 mm.
12. Test.
NT, 5FPR.
13. Test.
NT, mixed cut‐points.
14. Test.
NT and age, risk 1:100.
15. Test.
NT and age, risk 1:250.
16. Test.
NT and age, risk 1:300.
17. Test.
NT and age, 1FPR.
18. Test.
NT and age, 3FPR.
19. Test.
NT and age, 5FPR.
20. Test.
NT and age, mixed cut‐points.
21. Test.
NT and nasal bone, Absent NB + NT ≥ 95th centile.
22. Test.
Ductus and age, risk 1:250.
23. Test.
Ductus and age, 5FPR.
24. Test.
Ductus and age, mixed cut‐points.
25. Test.
Ductus, NT and age, risk 1:100.
26. Test.
Ductus, NT and age, risk 1:250.
27. Test.
Ductus, NT and age, 5FPR.
28. Test.
Ductus, NT and age, mixed cut‐points.
29. Test.
Age and nasal bone, mixed cut‐points.
30. Test.
Age, NT and tricuspid blood flow, risk 1:100.
31. Test.
Age, NT and nasal bone, risk 1:100.
32. Test.
Age, NT and nasal bone, risk 1:300.
33. Test.
Age, NT and nasal bone, mixed cut‐points.
34. Test.
Age, NT, nasal bone and ductus, risk NT>1:300 AND abnormal DV flow AND absent NB.
35. Test.
Age, NT, nasal bone, free ßhCG and PAPP‐A, 1st trimester, 5FPR.
36. Test.
Age, NT, nasal bone, free ßhCG and PAPP‐A, 1st trimester, mixed cut‐points.
37. Test.
Age, NT and free ßhCG, 1st trimester, 5FPR.
38. Test.
Age, NT and free ßhCG, 1st trimester, risk 1:240.
39. Test.
Age, NT and free ßhCG, 1st trimester, mixed cut‐points.
40. Test.
Age, NT and PAPP‐A, 1st trimester, risk 1:100.
41. Test.
Age, NT and PAPP‐A, 1st trimester, risk 1:185.
42. Test.
Age, NT and PAPP‐A, 1st trimester, 5FPR.
43. Test.
Age, NT and PAPP‐A, 1st trimester, mixed cut‐points.
44. Test.
Age, NT and total hCG, 1st trimester, 5FPR.
45. Test.
Age, NT and AFP, 1st trimester, 5FPR.
46. Test.
Age, NT and ITA, 1st trimester, 5FPR.
47. Test.
Age, NT and inhibin, 1st trimester, risk 1:100.
48. Test.
Age, NT and inhibin, 1st trimester, risk 1:250.
49. Test.
Age, NT and inhibin, 1st trimester, risk 1:400.
50. Test.
Age, NT and inhibin, 1st trimester, 5FPR.
51. Test.
Age, NT and inhibin, 1st trimester, mixed cut‐points.
52. Test.
Age, NT, PAPP‐A and free ßhCG, 1st trimester, risk 1:100.
53. Test.
Age, NT, PAPP‐A and free ßhCG, 1st trimester, risk 1:150.
54. Test.
Age, NT, PAPP‐A and free ßhCG, 1st trimester, risk 1:200.
55. Test.
Age, NT, PAPP‐A and free ßhCG, 1st trimester, risk 1:220.
56. Test.
Age, NT, PAPP‐A and free ßhCG, 1st trimester, risk 1:250.
57. Test.
Age, NT, PAPP‐A and free ßhCG, 1st trimester, risk 1:300.
58. Test.
Age, NT, PAPP‐A and free ßhCG, 1st trimester, 1FPR.
59. Test.
Age, NT, PAPP‐A and free ßhCG, 1st trimester, 3FPR.
60. Test.
Age, NT, PAPP‐A and free ßhCG, 1st trimester, 5FPR.
61. Test.
Age, NT, PAPP‐A and free ßhCG, 1st trimester, mixed cut‐points.
62. Test.
Age, NT, PAPP‐A and uE3, 1st trimester, 5FPR.
63. Test.
Age, NT, PAPP‐A and ITA, 1st trimester, 5FPR.
64. Test.
Age, NT, PAPP‐A and inhibin, 1st trimester, risk 1:100.
65. Test.
Age, NT, PAPP‐A and inhibin, 1st trimester, risk 1:250.
66. Test.
Age, NT, PAPP‐A and inhibin, 1st trimester, risk 1:400.
67. Test.
Age, NT, PAPP‐A and inhibin, 1st trimester, 5FPR.
68. Test.
Age, NT, PAPP‐A and inhibin, 1st trimester, mixed cut‐points.
69. Test.
Age, NT, PAPP‐A and ADAM12, 1st trimester, 5FPR.
70. Test.
Age, NT, PAPP‐A and ADAM12, 1st trimester, risk 1:250.
71. Test.
Age, NT, free ßhCG and ADAM12, 1st trimester, 5FPR.
72. Test.
Age, NT, AFP and free ßhCG, 1st trimester, risk 1:250.
73. Test.
Age, NT, AFP and free ßhCG, 1st trimester, 5FPR.
74. Test.
Age, NT, AFP and free ßhCG, 1st trimester, mixed cut‐points.
75. Test.
Age, NT, AFP and PAPP‐A, 1st trimester, 5FPR.
76. Test.
Age, NT, total hCG and PAPP‐A, 1st trimester, 5FPR.
77. Test.
Age, NT, total hCG and inhibin, 1st trimester, 5FPR.
78. Test.
Age, NT, free ßhCG and inhibin, 1st trimester, 5FPR.
79. Test.
Age, NT, PAPP‐A, free ßhCG, 1st trimester serum, ductus venosus pulsivity index, 5FPR.
80. Test.
Age, free ßhCG and PAPP‐A, if risk 1:42‐1:1000, NT, final 1:250 risk.
81. Test.
Age, NT, ductus, free ßhCG and PAPP‐A, 1st trimester, risk 1:100.
82. Test.
Age, NT, ductus, free ßhCG and PAPP‐A, 1st trimester, risk 1:250.
83. Test.
Age, NT, ductus, free ßhCG and PAPP‐A, 1st trimester, 5FPR.
84. Test.
Age, NT, ductus, free ßhCG and PAPP‐A, 1st trimester, mixed cut‐points.
85. Test.
Age, NT, nasal bone, free ßhCG and PAPP‐A, 1st trimester, risk 1:100.
86. Test.
Age, NT, nasal bone, free ßhCG and PAPP‐A, 1st trimester, risk 1:300.
87. Test.
Age, NT, tricuspid blood flow, free ßhCG and PAPP‐A, 1st trimester, risk 1:100.
88. Test.
Age, NT, fetal heart rate, free ßhCG and PAPP‐A, 1st trimester, 5FPR.
89. Test.
Age, NT, fetal heart rate, nasal bone, free ßhCG and PAPP‐A, 1st trimester, risk 1:200.
90. Test.
age, NT, fetal heart rate, ductus, free ßhCG and PAPP‐A, 1st trimester, 5FPR.
91. Test.
Age, NT, fetal heart rate, tricuspid blood flow, free ßhCG and PAPP‐A,1st trimester, 5FPR.
92. Test.
Age, NT, AFP, free ßhCG and PAPP‐A, 1st trimester, risk 1:250.
93. Test.
Age, NT, AFP, free ßhCG and PAPP‐A, 1st trimester, 5FPR.
94. Test.
Age, NT, AFP, free ßhCG and PAPP‐A, 1st trimester, mixed cut‐points.
95. Test.
Age, NT, total hCG, inhibin and PAPP‐A, 1st trimester, 5FPR.
96. Test.
Age, NT, PAPP‐A, free ßhCG and PGH, 1st trimester, 5FPR.
97. Test.
Age, NT, PAPP‐A, free ßhCG and GHBP, 1st trimester, 5FPR.
98. Test.
Age, NT, PAPP‐A, free ßhCG and PIGF, 1st trimester, 5FPR.
99. Test.
Age, NT, PAPP‐A, free ßhCG and total hCG, 1st trimester, 5FPR.
100. Test.
Age, NT, PAPP‐A, free ßhCG and PP13, 1st trimester, 5FPR.
101. Test.
Age, NT, PAPP‐A, free ßhCG and ADAM12, 1st trimester, 5FPR.
102. Test.
Age, NT, PAPP‐A, free ßhCG and ADAM12, 1st trimester, risk 1:250.
103. Test.
Age, NT, PAPP‐A, free ßhCG and ADAM12, 1st trimester, mixed cut‐points.
104. Test.
Age, NT, free ßhCG, PAPP‐A and inhibin, 1st trimester, risk 1:100.
105. Test.
Age, NT, free ßhCG, PAPP‐A and inhibin, 1st trimester, risk 1:250.
106. Test.
Age, NT, free ßhCG, PAPP‐A and inhibin, 1st trimester, risk 1:400.
107. Test.
Age, NT, free ßhCG, PAPP‐A and inhibin, 1st trimester, 5FPR.
108. Test.
Age, NT, PAPP‐A, free ßhCG, ADAM12 and PlGH, 1st trimester, 5FPR.
109. Test.
Age, NT, total hCG, inhibin, PAPP‐A, AFP and uE3, 1st trimester, 5FPR.
110. Test.
Age, NT, free ßhCG, inhibin, PAPP‐A, AFP and uE3,1st trimester, 5FPR.
111. Test.
Age, NT, PAPP‐A, free ßhCG, ADAM12, total hCG and PlGF, 1st trimester, 5FPR.
112. Test.
Age, NT, PAPP‐A, free ßhCG, ADAM12, total hCG, PlGF and PP13, 1st trimester, 5FPR.
113. Test.
NT, free ßhCG and PAPP‐A, 1st trimester incidence rate 63.3%.
114. Test.
NT, PAPP‐A, free ßhCG and maternal age ‐ maternal age < 35 years.
115. Test.
NT, PAPP‐A, free ßhCG and maternal age ‐ maternal age ≥ 35 years.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Acacio 2001.
| Clinical features and settings | High‐risk referral for invasive testing | |
| Participants | 230 participants Brazil ‐ private centres Dates not specified Pregnant women Mean age 35.8 years (21‐45 years) Singleton pregnancies Karyotyping performed at same time as NT 10‐14 weeks' gestation |
|
| Study design | Diagnostic validation study to determine the best ROC cut‐off for NT Retrospective study of patient notes |
|
| Target condition and reference standard(s) | Down's syndrome: 12 cases Reference standards: chorionic villus biopsy, amniocentesis or blood or placenta used for fetal karyotyping |
|
| Index and comparator tests | NT with cut‐off of 2.5 mm (found to be optimum cut‐off from ROC) (Sequoia, Aspen 128XP10‐Acuson and Toshiba SH140) | |
| Follow‐up | 100% karyotyping | |
| Aim of study | To define the best fixed cut‐off point for NT, and the accuracy of this cut‐off for all fetal aneuploidy screening and for trisomy of chromosome 21 | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Selective testing of high‐risk women as done in practice |
| Acceptable reference standard? All tests | Yes | Karyotyping |
| Partial verification avoided? All tests | Yes | All patients received a reference standard |
| Differential verification avoided? All tests | No | Women had different reference standard |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? All tests | No | No details of withdrawals given |
Audibert 2001.
| Clinical features and settings | Routine screening | |
| Participants | 4130 participants France ‐ single centre May 1994 to December 1997 Pregnant women Mean maternal age 30.1 years (all under 38 years), 86% < 35, 14% ≥ 35 Singleton pregnancies 10‐14 weeks' gestation Crown‐rump length between 38 mm and 84 mm |
|
| Study design | Prospective consecutive series study | |
| Target condition and reference standard(s) | Down's syndrome: 12 cases Reference standards: prenatal karyotyping conducted (in 7.6% of patients) depending on presence of risk > 125, high maternal age, parental anxiety, history of chromosomal defects or parental translocation or abnormal second trimester scan age Cytogenetic testing of newborns with suspected abnormalities Postmortem on terminations of pregnancy or miscarriages Follow‐up to neonatal examination in newborn |
|
| Index and comparator tests | Maternal age First trimester NT planned at 12‐13 weeks, 3 mm risk cut‐off Second trimester serum hCG between 14 and 17 weeks (Amerlite, Orthoclinical diagnostics machine), cut‐off 1:250 (Prenata software) Second trimester serum AFP between 14 and 17 weeks (Amerlite, Orthoclinical diagnostics machine), cut‐off 1:250 (Prenata software) Serum tests in 3790 women |
|
| Follow‐up | Delivery and postnatal paediatric examination 35 lost to follow‐up and excluded from analysis Pregnancy loss in 37 women due to spontaneous abortion (n = 21) or intrauterine death (n = 16) 340 women had first trimester NT but not second trimester serum testing |
|
| Aim of study | To compare first trimester NT and second trimester maternal serum measurements as alternative methods of antenatal screening in a low‐risk population and to evaluate the consequence of combining the results in the estimation of risk. | |
| Notes | Women lost to follow‐up are excluded in the final analysis. All antenatally detected cases were terminated. | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? All tests | Yes | All women received a reference standard |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | Yes | NT was not measured or not recorded in 219 women and these patients were excluded from the study |
| Withdrawals explained? All tests | Yes | 340 women who did not want second trimester serum screening withdrew from that part of the study |
Babbur 2005.
| Clinical features and settings | Women requesting screening (self‐paying service) and women attending on account of previous pregnancy history of fetal abnormality | |
| Participants | 3188 participants Cambridge, UK ‐ Maternity Hospital August 2001‐March 2004 Singleton pregnancies Pregnant women Median age 37 years (19‐46 years) 11‐14 weeks' gestation 45‐84 mm crown‐rump length Viable fetus |
|
| Study design | Prospective cohort study | |
| Target condition and reference standard(s) | Down's syndrome: 25 cases Reference standards: invasive testing offered to women with NT > 3 mm or risk > 1:250 as defined by combined NT and serum results (chorionic villus sampling from 11 weeks, amniocentesis from 15 weeks). Rapid in situ hybridisation test in patients with risk > 1:30. No details given of any follow‐up to birth |
|
| Index and comparator tests | First trimester NT in all women (FMF methods) Second trimester serum biochemistry (AutoDELFIA(TM) time‐resolved fluorimmunoassay (Perkin Elmer)) at 14 weeks. Offered to patients with negative first trimester NT (2725 accepted, 85%) |
|
| Follow‐up | Details of follow‐up to birth not given | |
| Aim of study | To determine the detection and false positive rates for trisomy 21 using 2‐stage combined nuchal translucency and triple testing whilst disclosing abnormal NT measurements at the scan | |
| Notes | Women with miscarriages excluded | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? All tests | Unclear | Unclear if all women received a reference standard |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? All tests | Yes | 463 patients having NT did not go on to have serum testing |
Barrett 2008.
| Clinical features and settings | Routine screening | |
| Participants | 10,273 participants with complete screening and outcome data Australia ‐ screening programme, independent ultrasound practices 24‐month period (dates not specified) Pregnant women Mean maternal age 34.9 years (screen positive) and 30.5 years (screen negative) Singleton pregnancies 11‐13 weeks' gestation |
|
| Study design | Cohort study | |
| Target condition and reference standard(s) | Down's syndrome: 32 cases Reference standard: karyotyping or follow‐up to birth |
|
| Index and comparator tests | NT (FMF protocol) First trimester PAPP‐A and free ßhCG (90.2% by time resolved amplified cryptate emission technology, Kryptor random access immunoassay analyzer, Brahms, 9.8% by manual Ortho Clinical Diagnostic Immunometric I125 immunoassay for PAPP‐A, and Ortho Clinical Diagnostics Vitros ECi automated analyzer for ßhCG) Risk cut‐off 1:300 |
|
| Follow‐up | Linkage to data collected by the Midwives Notification System and the Western Australia Birth Defects Registry and by searching laboratory records of all prenatal cytogenetics services in the state. 162 women lost to follow‐up were excluded Pregnancy loss in 54 women due to miscarriage (n = 35), stillbirth (n = 17) and neonatal death (n = 2) |
|
| Aim of study | To investigate associations between combined first‐trimester screen result, pregnancy associated plasma protein level and adverse fetal outcomes in women | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? All tests | Yes | All women received a reference standard |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? All tests | No | No details of withdrawals given |
Belics 2011.
| Clinical features and settings | High‐risk referral for invasive testing | |
| Participants | 2032 participants with adequate imaging on ultrasound screening Budapest ‐ single centre January 2003 ‐ February 2010 Pregnant women Mean age 36.4 years (15‐46 years) (Down's syndrome) and 29.8 years (15‐49 years) (no Down's syndrome) 11‐20 weeks' gestation |
|
| Study design | Cohort study | |
| Target condition and reference standard(s) | Down's syndrome: 52 cases Reference standards: amniocentesis or CVS (85% of women), or follow‐up to birth |
|
| Index and comparator tests | First and second trimester fetal iliac angle (GE Medical System Kretztechnik GmbH & Co OHG, AC2‐5 transabdominal and IC5‐9 transvaginal curved array transducer and Medison Co., LTD EC4‐9ES transvaginal and C3‐7IM transabdominal curved array transducer) Measurement taken from a transverse section of the fetal pelvis Cut‐off angles of 75‐100o |
|
| Follow‐up | Followed up to delivery (no cases were detected at birth) | |
| Aim of study | To present results of the sonographic measurement of the fetal iliac angle during the first and second trimesters of pregnancy | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Selective testing of high‐risk women as done in practice |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? All tests | Yes | All women received a reference standard |
| Differential verification avoided? All tests | No | Different reference standards used |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | Yes | 95.2% had adequate imaging |
| Withdrawals explained? All tests | No | No details of withdrawals given |
Benattar 1999.
| Clinical features and settings | Routine screening | |
| Participants | 1656 participants France ‐ single centre January to December 1995 Singleton pregnancies Pregnant women Mean age 32 years (16‐46 years), 8.3% > 35 years Enrolled before 13 weeks' gestation |
|
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 5 cases Reference standards: amniocentesis due to maternal age > 38 years (6.1% or women). Karyotyping encouraged for women with positive result on 1 or more index test. No details of reference standard for index test negative women |
|
| Index and comparator tests | Maternal age NT at 12‐14 weeks (Toshiba SSA 270), risk cut‐point 1:250 First trimester (12‐14 weeks) serum AFP and free ßhCG (Elsa AFP and Elsa free ßhCG; Cis‐Bio International) Second trimester (15‐18 weeks) serum AFP and total hCG (AFP‐2T and hCG‐60; Ortho‐Clinical Diagnostics) All women had NT and serum testing |
|
| Follow‐up | Details of follow‐up are not stated. Unclear whether women were followed up to birth. Of the 1656 women, 12 (0.7%) were lost to follow‐up, 2 had miscarriages, 2 had preterm premature ruptures of the membranes and 2 had intrauterine deaths. |
|
| Aim of study | To evaluate the sequential combination of ultrasound screening for fetal aneoploidy at 11‐14 weeks with maternal biochemistry at 12‐14 and 15‐18 weeks of gestation | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? All tests | Unclear | Unclear if all women received a reference standard |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? All tests | No | No details of withdrawals given |
Bestwick 2010.
| Clinical features and settings | Routine screening | |
| Participants | 22,746 participants London ‐ 2 antenatal clinics January 2003 ‐ December 2008 Pregnant women Median age 39 years (Down's syndrome) and 34 years (non‐Down's syndrome) 11‐13 and 14‐22 weeks' gestation |
|
| Study design | Retrospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 106 cases Reference standards: karyotyping or follow‐up to birth |
|
| Index and comparator tests | First trimester NT, PAPP‐A and free ßhCG (details not reported) Second trimester AFP, uE3, free ßhCG and inhibin A (details not reported) Results in multiple publications |
|
| Follow‐up | Data obtained from the Hospitals, the regional cytogenetic unit and the National Down Syndrome Cytogenetic Register | |
| Aim of study | To determine whether the standard deviation of NT measurements has decreased over time and, if so, to revise the estimate and assess the effect of revising the estimate of the standard deviation on the performance of antenatal screening for Down's syndrome | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? All tests | Unclear | Unclear if all women received a reference standard |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? All tests | No | No details of withdrawals given |
Biagiotti 1998.
| Clinical features and settings | High‐risk referral for invasive testing | |
| Participants | 232 participants (all had NT and serum testing) 32 cases of Down's and 200 randomly selected controls (selected from series of 3731 women) Italy ‐ single centre July 1993 ‐ December 1996 Pregnant women 10 to 13 weeks' gestation |
|
| Study design | Case‐control study | |
| Target condition and reference standard(s) | Down's syndrome: 32 cases Reference standards: CVS or amniocentesis |
|
| Index and comparator tests | Maternal age First trimester NT (in longitudinal section of the fetus with caliper measurements to the nearest 0.1 mm) First trimester PAPP‐A (Amerlex‐M PAPP‐A IRMA, Ortho‐Clinical Diagnostics) First trimester free ßhCG (Elsa9free ßhCG CIS) |
|
| Follow‐up | 100% karyotyping | |
| Aim of study | To evaluate the potential effectiveness of maternal serum PAPP‐A and free ßhCG in combination with NT measurement in the first trimester of pregnancy | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Selective testing of high‐risk women as done in practice |
| Acceptable reference standard? All tests | Yes | Karyotyping |
| Partial verification avoided? All tests | Yes | All women received a reference standard |
| Differential verification avoided? All tests | Yes | All women had the same reference standard |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? All tests | No | No details of withdrawals given |
Borenstein 2008.
| Clinical features and settings | High‐risk referral for invasive testing | |
| Participants | 516 participants London ‐ hospital birth centre Dates not reported Pregnant women Median maternal age 35 years (range 17‐49 years) 11‐13 weeks' gestation 16‐24 weeks' gestation in a sub‐sample of 183 women |
|
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 51 cases Reference standard: CVS |
|
| Index and comparator tests | First trimester fetal echocardiography (transabdominally with a 4‐8 MHz curvilinear transducer, Voluson 730 Expert, GE Medical Systems) in all women (425 successfully examined) and in the second trimester in 183 women | |
| Follow‐up | 100% karyotyping | |
| Aim of study | To establish the feasibility of examining the subclavian artery at 11 + 0 to 13 + 6 weeks of gestation and to determine the prevalence of aberrant right subclavian artery (ARSA) in chromosomally normal and abnormal fetuses | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? All tests | Yes | Karyotyping |
| Partial verification avoided? All tests | Yes | All women received a reference standard |
| Differential verification avoided? All tests | Yes | All women received the same reference standard |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | Yes | 425/516 (82.4%) of women were successfully examined |
| Withdrawals explained? All tests | No | No details of withdrawals given |
Borrell 2005.
| Clinical features and settings | Routine screening | |
| Participants | 3731 participants Spain October 1999 ‐ December 2002 Pregnant women 10 to 14 weeks' gestation |
|
| Study design | Retrospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 25 cases Reference standards: CVS (high‐risk women) or follow‐up to birth |
|
| Index and comparator tests | Maternal age First trimester (10‐14 weeks) Ductus venous Doppler studies First trimester (10‐14 weeks) NT (FMF method) First trimester (10 weeks) serum PAPP‐A and free ßhCG (time‐resolved fluorescent assays, Perkin‐Elmer Life Sciences) Risk cutoffs 1:200, 1:250 or 1:300 DV ‐ Saggital view of quiescent fetus. When optimal record of DV obtained, measured only once. When reversed end diastolic flow present, 3 separated samples obtained. Maximum velocity manually drawn in 3 waveforms and PIV automatically obtained by software linked to equipment |
|
| Follow‐up | Details given in Borrel 2004: follow‐up through phone enquiry, contact with attending obstetrician, births defects registry of Barcelona. Cases with missing follow‐up or unknown karyotype excluded from further analysis | |
| Aim of study | To estimate the improvement in screening efficiency when fetal ductus venosus Doppler studies are added to existing first trimester Down's syndrome screening protocols | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth (described in Borrel 2004) |
| Partial verification avoided? All tests | Yes | All patients received a reference standard |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | Yes | 4 unaffected pregnancies could not be assessed with NT |
| Withdrawals explained? All tests | No | No details of withdrawals given |
Borrell 2009.
| Clinical features and settings | Routine screening and high‐risk referral | |
| Participants | 7250 participants: 6940 women undergoing routine screening (October 1999 ‐ December 2006) 310 women referred for CVS (October 1999 ‐ December 2007) Barcelona ‐ hospital clinic Pregnant women Mean maternal age 32 years 10‐13 and 15‐20 weeks' gestation |
|
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 66 cases Reference standards: karyotyping or follow‐up to birth |
|
| Index and comparator tests | Maternal age First trimester NT and ductus venosus pulsivity index (DVPI) (transabdominal ultrasound, Eccocee SSA and Power‐Vision 400, Toshiba Medical Systems, Voluson PRO, General Electrics Healthcare) First trimester PAPP‐A and free ßhCG (details not reported) Second trimester AFP, uE3, free ßhCG and inhibin A (details not reported) |
|
| Follow‐up | From hospital clinic records, telephoning women or from the attending obstetrician. Obtained in 97.4% of pregnancies | |
| Aim of study | To assess the value of ductus venosus blood flow (expressed as pulsatility index, DVPI) in antenatal Down's syndrome screening when used with the combined and integrated tests | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population and selective testing of high‐risk women as done in practice |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? All tests | Unclear | Unclear if all women received a reference standard |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | Yes | Ductus venosus measurements were not obtained in 3.3% of pregnancies |
| Withdrawals explained? All tests | No | No details of withdrawals given |
Brameld 2008.
| Clinical features and settings | Routine screening | |
| Participants | 22,280 participants with complete screening results and outcome data August 2001 ‐ October 2003 Australia ‐ State‐wide screening programme evaluation Pregnant women Median maternal age 31 years (range 14‐47 years), 20% ≥ 35 Singleton pregnancies 10‐14 weeks' gestation |
|
| Study design | Retrospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 60 cases Reference standards: karyotyping or follow‐up to birth |
|
| Index and comparator tests | Maternal age First trimester PAPP‐A, free ßhCG and NT (details not reported) Risk cut‐point 1:300 |
|
| Follow‐up | Data on outcome from the Western Australia Midwives data collection, Birth Defects Registry and hospital morbidity and mortality data | |
| Aim of study | To identify first trimester indicators of adverse pregnancy outcomes | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐ up to birth |
| Partial verification avoided? All tests | Yes | All women received a reference standard |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? All tests | No | No details of withdrawals given |
Brizot 2001.
| Clinical features and settings | Routine screening | |
| Participants | 2996 participants Brazil ‐ University Hospital Estimated date of delivery pre December 1999 Pregnant women Median age 28 years (13‐46 years), 19.4% ≥ 35 years Singleton pregnancies 10‐14 weeks' gestation (mean 12 weeks) |
|
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 10 cases Reference standards: antenatal karyotyping (5.9% of pregnancies: 62% of high‐risk, 29% of medium‐risk and 3% of the low‐risk women) or follow‐up to birth (85.3% of women) |
|
| Index and comparator tests | Maternal age First trimester (10‐14 weeks) NT Risk cut‐off 1:300 |
|
| Follow‐up | 85.3% of women were followed up to birth. Of these, 65 were spontaneous miscarriages or intrauterine death with no karyotyping | |
| Aim of study | To assess the detection rate of chromosomal abnormalities using NT | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? All tests | Unclear | Unclear if all women received a reference standard |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? All tests | No | No details of withdrawals given |
Centini 2005.
| Clinical features and settings | High‐risk patients undergoing routine screening | |
| Participants | 408 participants Italy Dates not reported Pregnant women Singleton pregnancies Aged ≥ 35 years (range 35‐44 years) 10‐13 weeks' gestation |
|
| Study design | Retrospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 6 cases Reference standards: amniocentesis in women high risk on screening (16.2%) or follow‐up to birth in women who were low risk on screening |
|
| Index and comparator tests | Maternal age NT with cut‐point 3 mm Serum free ßhCG (Schering RIA) and PAPP‐A (Chematil ELISA) Risk score cut‐point 1:250 |
|
| Follow‐up | Follow‐up at birth in all by collaboration with mothers Women who miscarried were excluded from the study |
|
| Aim of study | To evaluate the combined test of NT, serum markers and age in pregnant women 35 years of age and over to detect Down's syndrome | |
| Notes | No live births were Down's syndrome. All detected cases were terminated. 7 women were excluded due to miscarriages | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? All tests | Yes | All women received a reference standard |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? All tests | No | No details of withdrawals given |
Chasen 2003.
| Clinical features and settings | Routine screening | |
| Participants | 2131 women with 2339 fetuses New York ‐ single centre April 2000 to November 2002 Pregnant women Singleton or multifetal pregnancies Median age 33 years (interquartile range 31‐36), 36.2% ≥ 35 years |
|
| Study design | Prospective consecutive cohort | |
| Target condition and reference standard(s) | Down's syndrome: 12 cases Reference standards: karyotyping or follow‐up to birth in 96.1% of patients |
|
| Index and comparator tests | Maternal age NT (FMF methods) Combined risk score cut‐point 1:300 Each fetus with a separate chorion was considered individually when calculating the performance of NT but for monochorionic twins, only the fetus with the higher risk calculation was included |
|
| Follow‐up | Attempted to obtain results for cytogenetic testing following miscarriage or termination or where Down's suspected at birth. Karyotype results or documented evidence of phenotypically normal baby was recorded in 96.1% of patients | |
| Aim of study | To examine the detection rate of chromosomal abnormalities using a combination of nuchal translucency and maternal age | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? All tests | No | Reference standard results were available for only 96% of patients |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | Yes | 19 patients could not be imaged |
| Withdrawals explained? All tests | No | No details of withdrawals given |
Chen 2009.
| Clinical features and settings | Routine screening | |
| Participants | 242 participants: 22 cases and 220 randomly selected controls China ‐ hospital screening programme August 2003 ‐ March 2007 Pregnant women Median maternal age, cases 30 years (20‐44 years) and controls 32 years (19‐40 years) 12‐14 weeks' gestation |
|
| Study design | Case‐control study | |
| Target condition and reference standard(s) | Down's syndrome: 22 cases Reference standards: karyotyping or follow‐up to birth |
|
| Index and comparator tests | First trimester frontomaxillary facial (FMF) angle (transabdominal ultrasound, ATL HDI 5000, Philips Medical Systems or Voluson 730 Pro, GE Medical systems, by clinicians accredited by the FMF) Measured with a protractor from printed and filed images Angle > 95th percentile taken as positive test result |
|
| Follow‐up | Pregnancy outcome obtained from obstetric and neonatal files | |
| Aim of study | To evaluate the measurement of FMF angle at 11‐13 weeks, 6 days in a Chinese population and its applicability in screening for fetal trisomy 21 | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? All tests | Yes | All women received a reference standard |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Unclear | Unclear if index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | Unclear | Only the most optimal images were included in the study and the proportion of images that were not included is not stated |
| Withdrawals explained? All tests | No | No details of withdrawals given |
Christiansen 2005.
| Clinical features and settings | Screening programmes for syphilis and Down's syndrome | |
| Participants | 108 participants (27 cases of Down's syndrome, 81 controls) Denmark ‐ Statens Serum Institute Dates not specified Pregnant women 5‐11 weeks' gestation |
|
| Study design | Case‐control study | |
| Target condition and reference standard(s) | Down's syndrome: 27 affected cases (18 diagnosed in 2nd trimester, 9 at birth) Reference standard: karyotyping |
|
| Index and comparator tests | Maternal age First trimester (week 11‐14) NT Frozen samples tested for: First trimester (week 5‐11) inhibin A (dimer assay kit MCA 950KZZ, Serotec) First trimester (week 5‐11) ßhCG (available for some samples) First trimester (week 5‐11) PAPP‐A (available for some samples) (combined PAPP‐A and ßhCG TrIFMA assay) Risk cutpoints of 1:100, 1:250 and 1:400 Performance assessed with SPlus algorithm |
|
| Follow‐up | All diagnosis were verified by karyotyping | |
| Aim of study | To investigate whether inhibin A can be used in the first trimester for Down's syndrome screening | |
| Notes | Identified through the Danish central cytogenetic registry as part of quality assurance programme | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? All tests | Yes | Karyotyping |
| Partial verification avoided? All tests | Yes | All women had a reference standard |
| Differential verification avoided? All tests | Yes | All women had the same reference standard |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Unclear | Unclear if all index tests interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? All tests | No | No details of withdrawals given |
Christiansen 2009.
| Clinical features and settings | Routine screening | |
| Participants | 335 participants: 74 cases and 261 controls matched for length of sample storage and maternal age Denmark ‐ screening programme Dates not reported Pregnant women Singleton pregnancies Median maternal age cases 37.5 years and controls 36.4 years 8‐13 weeks' gestation |
|
| Study design | Case‐control study | |
| Target condition and reference standard(s) | Down's syndrome: 74 cases Reference standards: karyotyping or follow‐up to birth |
|
| Index and comparator tests | Maternal age First trimester NT (details not reported) Fresh serum samples tested for: First trimester PAPP‐A and free ßhCG (AutoDelfia, PerkinElmer, Turku or Kryptor, Brahms) Frozen serum samples tested for: First trimester placental growth hormone (double monoclonal ELISA, DSL‐10‐19 200, Diagnostic Systems Laboratory Inc) Growth hormone binding protein (enzyme‐amplified ELISA, DSL‐10‐48 100, Diagnostic Systems Laboratory Inc) |
|
| Follow‐up | Cross‐referencing with the Danish Cytogenetic Central Registry | |
| Aim of study | To examine the potential of placental growth hormone and growth hormone binding protein as maternal serum screening markers for Down's syndrome | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? All tests | Yes | All women received a reference standard |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of some index test results |
| Index test results blinded? All tests | Unclear | Unclear if all index tests interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? All tests | No | No details of withdrawals given |
Christiansen 2010.
| Clinical features and settings | Routine screening | |
| Participants | 531 participants: 28 cases and 503 controls Denmark ‐ screening programme Dates not specified Pregnant women Singleton pregnancies Median age cases 36 years (range 25‐44 years) and controls 29 years (range 17‐45 years) 8‐14 weeks' gestation |
|
| Study design | Case‐control study | |
| Target condition and reference standard(s) | Down's syndrome: 28 cases Reference standards: karyotyping or follow‐up to birth |
|
| Index and comparator tests | Maternal age First trimester NT (details not reported) First trimester PAPP‐A and free ßhCG (details not reported) First trimester ADAM12s (AutoDELFIA/Delfia ADAM12 Research kit 4025‐0010, PerkinElmer Life and Analytical Sciences, on the 1235 AutoDELFIA automatic immunoassay system) |
|
| Follow‐up | Cross‐referencing with the Danish Cytogenetic Central Registry | |
| Aim of study | To examine the efficiency of a second generation assay for ADAM12 | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? All tests | Yes | All women received a reference standard |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of some index test results |
| Index test results blinded? All tests | Unclear | Unclear if all index tests interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? All tests | No | No details of withdrawals given |
Cicero 2004a.
| Clinical features and settings | High‐risk referral for invasive testing | |
| Participants | 970 fetuses (20 twin and 1 triplet pregnancy) UK Dates not specified Pregnant women Median age 37 years (16‐48 years) 11‐14 weeks' gestation (median 12 weeks) |
|
| Study design | Prospective cohort study | |
| Target condition and reference standard(s) | Down's syndrome: 88 cases Reference standard: CVS |
|
| Index and comparator tests | Maxillary bone length Mid‐saggital view of fetal profile obtained for nasal bone. Transducer angled laterally so that the maxillary bone and mandible including the ramus and condylar process can be seen. Maxillary length measured with callipers. Magnified to 0.1 mm increment |
|
| Follow‐up | 100% karyotyping | |
| Aim of study | To determine the value of measuring maxillary length at 11‐14 weeks' gestation in screening for trisomy 21 | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Selective testing of high‐risk women as done in practice |
| Acceptable reference standard? All tests | Yes | CVS |
| Partial verification avoided? All tests | Yes | All women had a reference standard |
| Differential verification avoided? All tests | Yes | All women had the same reference standard |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | Yes | Study reports that measurements were made successfully in all cases |
| Withdrawals explained? All tests | No | No details of withdrawals given |
Cicero 2006.
| Clinical features and settings | Routine screening | |
| Participants | 20, 418 participants UK ‐ Fetal Medicine Centre October 2001‐2004 Pregnant women Singleton pregnancies Median age 35 years (18‐50 years) 11‐13 weeks' gestation |
|
| Study design | Prospective cohort study | |
| Target condition and reference standard(s) | Down's syndrome: 140 cases Reference standards: CVS or amniocentesis in high‐risk women, or follow‐up to birth |
|
| Index and comparator tests | Maternal age Presence of nasal Bone (FMF methods) First trimester NT (FMF methods) First trimester serum free ßhCG (Kryptor analyser, Brahms AG) First trimester serum PAPP‐A (Kryptor analyser, Brahms AG) |
|
| Follow‐up | Data on pregnancy outcome from cytogenetics laboratory and by letters and telephone calls to patients, GPs and maternity units 656 patients excluded because karyotype was not known due to miscarriage (n = 185), termination of pregnancy (n = 85) or loss to follow‐up (n = 386) |
|
| Aim of study | To investigate the impact of incorporating assessment of the nasal bone into first trimester combined screening by fetal nuchal translucency thickness and maternal serum biochemistry | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? All tests | Yes | All women received a reference standard |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | Yes | Reported that fetal NT and serum markers were successfully measured in all cases |
| Withdrawals explained? All tests | Yes | Patients lost to follow‐up reported |
Cocciolone 2008 FTS.
| Clinical features and settings | Routine screening | |
| Participants | 18,901 participants Australia ‐ South Australian Maternal Serum Antenatal Screening Program Dates not reported Pregnant women Median age 31.3 years Maternal and gestational age not reported |
|
| Study design | Cohort study | |
| Target condition and reference standard(s) | Down's syndrome: 66 cases Reference standards: karyotyping or follow‐up to birth |
|
| Index and comparator tests | Maternal age First trimester NT PAPP‐A and free ßhCG (details not reported) |
|
| Follow‐up | Details not reported | |
| Aim of study | To compare different screening strategies for the detection of Down's syndrome and to consider the practical implications of using multiple screening protocols | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? All tests | Unclear | Unclear if all women received a reference standard |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? All tests | No | No details of withdrawals given |
Cowans 2009.
| Clinical features and settings | Routine screening | |
| Participants | 57,057 participants June 1998 ‐ July 2007 UK ‐ 6 Hospitals Pregnant women Singleton pregnancies Mean age: Down's syndrome 38 years (range 16‐49 years) and healthy 29 years (range 13‐56 years) 10‐14 weeks' gestation |
|
| Study design | Cohort study | |
| Target condition and reference standard(s) | Down's syndrome: 723 cases (307 from original cohort and 416 supplemented cases screened at the Fetal Medicine centre or Harris Birthright Research Centre for Fetal Medicine) Reference standards: karyotyping or follow‐up to birth |
|
| Index and comparator tests | Maternal age First trimester NT (FMF certified sonographers) First trimester PAPP‐A and free ßhCG (Kryptor analyser, Brahms) Rick cut‐point 1:300 |
|
| Follow‐up | Birth data collected at birth by the delivering hospital and stored in several databases which were merged. Only women with full records for screening and birth outcome included in the study | |
| Aim of study | To investigate if fetal sex has an impact on first trimester combined screening for aenuploidy | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? All tests | Yes | All women received a reference standard |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? All tests | No | No details of withdrawals given |
Cowans 2010.
| Clinical features and settings | Routine screening | |
| Participants | 445 participants: 70 cases and 375 controls matched for storage time and gestational age January 2007 ‐ October 2008 UK Pregnant women Singleton pregnancies Mean maternal age cases 37.0 years (IQR 32.9 to 40.5 years) and controls 32.4 years (IQR 29.0 to 35.9 years) 11‐13 weeks' gestation |
|
| Study design | Case‐control study | |
| Target condition and reference standard(s) | Down's syndrome: 70 cases Reference standards: karyotyping or follow‐up to birth |
|
| Index and comparator tests | Maternal age First trimester NT (FMF certified sonographers) Fresh serum samples tested for: First trimester PAPP‐A and free ßhCG (Kryptor analyser, Brahms) Frozen serum samples tested for: First trimester placental growth factor (Solid‐phase, 2‐site fluoroimmunometric research assay (4083‐0010) on 6000 DELFIA Xpress random access platform, PerkinElmer) |
|
| Follow‐up | Karyotype and results for pregnancy outcome received from cytogenetics laboratories and maternity units where deliveries took place | |
| Aim of study | To examine placental growth factor levels in first trimester maternal serum in trisomy 21 pregnancies and to investigate the potential value of PIGF in a first trimester screening test | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? All tests | Yes | All women received a reference standard |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of some index test results |
| Index test results blinded? All tests | Unclear | Unclear if all index tests interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? All tests | No | No details of withdrawals given |
Crossley 2002.
| Clinical features and settings | Routine screening | |
| Participants | 17,229 participants UK ‐ 15 centres Dates not specified Pregnant women Median age 29.9 years, 15.4% ≥ 35 years 10‐14 weeks' gestation |
|
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down’s syndrome: 45 cases Reference standards: CVS (offered where women had high NT measurements), amniocentesis or follow‐up to birth |
|
| Index and comparator tests | Maternal age NT (FMF method) in 73% of patients Clotted blood samples tested for: Free ßhCG and PAPP‐A (Kryptor analyser) in 98.4% of patients |
|
| Follow‐up | Reported that the outcome of all pregnancies was followed up | |
| Aim of study | To evaluate the use of NT measurement in combination with biochemical markers as a first trimester test for Down's syndrome in routine antenatal setting | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? All tests | Yes | All women received a reference standard |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | Yes | Report average success rate of NT (72.9%) |
| Withdrawals explained? All tests | Yes | Numbers of patients not undergoing NT and biochemical testing given |
De Graaf 1999.
| Clinical features and settings | High‐risk referral for invasive testing | |
| Participants | 292 participants (207 participants before 14 weeks' gestation) The Netherlands ‐ single centre 19 84‐1997 Pregnant women Cases: 37 with Down's syndrome Controls: 255 matched 5:1 with cases for maternal age (within 2 years), gestational age (within 2 weeks) and duration of sample storage (within 2 months) 9‐15 weeks' gestation (in a few cases, blood samples for serum testing taken at 15‐19 weeks) |
|
| Study design | Case‐control study | |
| Target condition and reference standard(s) | Down's syndrome: 37 cases (24 affected pregnancies in women with NT testing enrolled before 14 weeks' gestation) Reference standards: CVS and amniocentesis |
|
| Index and comparator tests | Maternal age NT (FMF methods) with cut‐off > 3 mm Frozen serum samples tested for: First trimester free ßhCG and AFP (DELFIA dual labelled time resolved fluorescent assay) First trimester serum PAPP‐A (DELFIA research assay (CR61‐105)) First trimester serum AFP |
|
| Follow‐up | 100% karyotyping | |
| Aim of study | To determine the expected detection rate and false positive rate for Down's syndrome achievable by early pregnancy screening with combined measurements of serum PAPP‐A, free ßhCG and fetal nuchal translucency, with the addition of AFP | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Selective testing of high‐risk women as done in practice |
| Acceptable reference standard? All tests | Yes | Karyotyping |
| Partial verification avoided? All tests | Yes | All women had a reference standard |
| Differential verification avoided? All tests | Yes | All women had karyotyping |
| Incorporation avoided? All tests | Yes | Index test did not form part of the reference standard |
| Reference standard results blinded? All tests | No | Reference standard interpreted without knowledge of index test results |
| Index test results blinded? All tests | Unclear | Unclear if index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | Yes | In 11 controls, failed to measure NT |
| Withdrawals explained? All tests | No | No details of withdrawals given |
Ekelund 2008.
| Clinical features and settings | Routine screening | |
| Participants | 95,645 participants (40,815 in 2005 and 54,830 in 2006) Denmark ‐ 19 obstetrics and gynaecology departments January 2005 ‐ December 2006 Pregnant women Maternal and gestational age not reported First trimester |
|
| Study design | Cohort | |
| Target condition and reference standard(s) | Down's syndrome: 225 cases (121 in 2005 and 104 in 2006) Reference standards: karyotyping or follow‐up to birth |
|
| Index and comparator tests | Maternal age First trimester NT (by nurses, midwives and doctors in accordance with FMF guidelines) First trimester PAPP‐A and free ßhCG (Brahms Kryptor, Brahms Immunodiagnostic Systems or Delfia Xpress, PerkinElmer) Risk cut‐point 1:300 |
|
| Follow‐up | Information obtained from the Danish central cytogenetic registry. No details of follow‐up for women without pre or post‐natal chromosome analysis | |
| Aim of study | To evaluate the impact of a screening strategy in the first trimester, introduced in Denmark during 2004 to 2006, on the number of infants born with Down's syndrome and the number of CVS and amniocentesis, and to determine detection and false positive rates in the screened population in 2005 and 2006 | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? All tests | Unclear | Unclear if all women received a reference standard |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? All tests | Yes | Information given on the proportion of women not undergoing screening |
Gasiorek‐Wiens 2001.
| Clinical features and settings | Routine screening | |
| Participants | 21,959 participants Germany, Switzerland and Austria ‐ multicentre study June 1995‐May 2000 Pregnant women Median age 33 years (15‐49 years), 36.1% > 35 years Singleton pregnancies 10‐14 weeks' gestation |
|
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 210 cases Reference standards: CVS, amniocentesis or follow‐up to birth |
|
| Index and comparator tests | Maternal age NT (FMF methods) Risk cut‐points of 1:100 and 1:300 |
|
| Follow‐up | Follow‐up in 92.2% of women. Loss to follow‐up was due to miscarriage (n = 258), termination of pregnancy (n = 125) or absence of antenatal karyotyping (n = 1463). Only those with follow‐up information included in the study | |
| Aim of study | To examine the effectiveness of screening for Down's syndrome using age and NT at 10‐14 weeks of gestation | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? All tests | Yes | All women received a reference standard |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | Yes | Reported that NT successfully measured in all cases |
| Withdrawals explained? All tests | No | No details of withdrawals given |
Gasiorek‐Wiens 2010.
| Clinical features and settings | Routine screening | |
| Participants | 4097 participants with complete data on pregnancy outcome Germany ‐ single examiner December 1997 ‐ November 2006 Pregnant women Singleton pregnancies Median age 35.1 years (range 13.2‐46.7 years) 11‐13 weeks' gestation |
|
| Study design | Cohort study | |
| Target condition and reference standard(s) | Down's syndrome: 34 cases Reference standards: Karyotyping or follow‐up to birth |
|
| Index and comparator tests | Maternal age First trimester NT (FMF methods) Mixture model, Delta NT and multiple of the median methods |
|
| Follow‐up | Patient history and ultrasound results were entered into a database and pregnancy outcome or chromosomal results added as they became available 74 (1.8%) of women were excluded from the study because of incomplete follow‐up information |
|
| Aim of study | To validate the mixture model in a single operator dataset and to compare the detection rates for fetal chromosomal defects obtained from the mixture model with those obtained from either the delta NT or log multiple of the median approach | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? All tests | Yes | All women received a reference standard |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? All tests | No | No details of withdrawals given |
Go 2005.
| Clinical features and settings | Routine screening | |
| Participants | 1759 participants The Netherlands ‐ private practice (VU medical centre) May 2001‐October 2003 Pregnant women 49% ≤ 35 years, 51% ≥ 36 years 9‐14 weeks' gestation |
|
| Study design | Retrospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 21 cases Reference standards: Invasive testing or follow‐up to birth |
|
| Index and comparator tests | Maternal age First trimester NT (FMF methods using own medians) First trimester PAPP‐A and free ßhCG (ELIPS Perkin Elmer, Finland) |
|
| Follow‐up | Follow‐up data from medical records and patient reports. Data from 242 patients (12%) were not available and these patients were excluded from the study. | |
| Aim of study | To determine the diagnostic value of the combination screening test for Down's syndrome in the first trimester of pregnancy | |
| Notes | Dutch language | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? All tests | Yes | All women received a reference standard |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Unclear | Unclear if all index tests interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | Yes | Incomplete investigation reported in 25 patients (1.2%) |
| Withdrawals explained? All tests | No | No details of withdrawals given |
Gyselaers 2005.
| Clinical features and settings | Routine screening | |
| Participants | 13,267 participants (13,207 participant received both NT test and serum testing) Belgium ‐ multicentre study (35 centres) Data from January 2004‐April 2004 added to previous database from before 2003 Pregnant women First and second trimester testing |
|
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 26 cases Reference standards: CVS, amniocentesis or follow‐up to birth |
|
| Index and comparator tests | Maternal age First trimester NT (FMF methods) First trimester PAPP‐A (ELISA 2397, DRG International Inc) and free ßhCG (IRMA K1P1001) Second trimester PAPP‐A and free ßhCG Risk cut‐points of 1:200 and 1:300 |
|
| Follow‐up | Follow‐up to birth reported by mail by obstetricians. Non‐responding obstetricians contacted personally to obtain missing data. Results of follow‐up reported by mail by obstetricians. Non‐responding obstetricians contacted personally to obtain missing data Cases of miscarriages (n = 49) and other fetal chromosomal abnormalities excluded from the study. Unclear if other patients lost to follow‐up |
|
| Aim of study | To evaluate the performance of a first trimester fetal aneuploidy screening programme | |
| Notes | Women with miscarriages or cases of other chromosomal defects were excluded from the study. 9 live births of babies with Down's syndrome | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? All tests | Yes | All women received a reference standard |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? All tests | Yes | Numbers of women excluded due to miscarriage or other chromosomal defects and numbers not undergoing NT and biochemical testing reported. |
Habayeb 2010.
| Clinical features and settings | Routine screening | |
| Participants | 1507 participants UK ‐ fetal medicine unit September 2007 ‐ December 2008 Pregnant women Median maternal age 35.4 years (range 18‐49 years) 9‐10, 11‐13 and > 14 weeks' gestation |
|
| Study design | Cohort study | |
| Target condition and reference standard(s) | Down's syndrome: 12 cases Reference standards: karyotyping or follow‐up to birth |
|
| Index and comparator tests | Maternal age Early first trimester PAPP‐A (9 weeks' gestation) (AutoDELFIA PAPP‐A kit, PerkinElmer LAS (UK) Ltd) First trimester NT (11‐13 weeks' gestation) (General Electric E8, Voluson 730 Pro, GE Healthcare) Second trimester AFP, free ßhCG and uE3 (at or after 14 weeks' gestation) (AutoDELFIA(TM) time‐resolved fluorimmunoassay, PerkinElmer Life Sciences) Second trimester tests given if first trimester risk low (< 1:100) or invasive testing declined Cut‐point for second‐stage risk 1:250 |
|
| Follow‐up | Data recorded on a fetal medicine database and combined with data held on separate databases for pregnancy outcome and the regional cytogenetic laboratory. Cytogenetic test results available for all women delivering in the region | |
| Aim of study | To audit a model combining early PAPP‐A with NT and early triple test | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? All tests | Unclear | Unclear if all women received a reference standard |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? All tests | No | No details of withdrawals given |
Hadlow 2005.
| Clinical features and settings | Routine screening | |
| Participants | 10,436 participants receiving both NT and serum testing and with complete follow‐up data Australia Data from 2‐year period (dates not specified) Pregnant women Mean age 30.7 years, 21.2% ≥ 35 years Singleton pregnancies 11‐14 weeks' gestation |
|
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 32 cases Reference standards: CVS, amniocentesis or follow‐up to birth |
|
| Index and comparator tests | Maternal age First trimester NT (FMF methods) Clotted blood samples tested for: First trimester PAPP‐A (Kryptor random access immunoassay analyser or manual Ortho Clinical Diagnostics Immunometric I125 immunoassay) First trimester free ßhCG (Kryptor random access immunoassay analyser or Ortho Clinical Diagnostics Vitros ECi automated analyser) Risk cut‐point 1:300 |
|
| Follow‐up | Data obtained from WA Midwives notification system and WA Birth defects registry. Missing information sought from referring doctor and ultrasound practice. Data linkage achieved in 10,436 (99.6%) of patients In index test negative patients, outcome for 160 women not known In index test positive patients, outcome in 2 women not known |
|
| Aim of study | To audit the initial 2 years of conduct of the combined first trimester screening | |
| Notes | Women with miscarriages or multiple pregnancies were excluded from the study | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? All tests | Yes | All women received a reference standard |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? All tests | No | No details of withdrawals given |
Hafner 1998.
| Clinical features and settings | Routine screening | |
| Participants | 4233 participants Austria ‐ single hospital June 1993 to July 1996 Pregnant women Median age 28 years (15‐49 years), 6.9% ≥ 35 years 10‐13 weeks' gestation |
|
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down’s syndrome: 7 cases Reference standards: amniocentesis or CVS in patients with previous Down’s pregnancy, > 35 years or with a positive biochemical test result. Other women underwent scan at 22 weeks and, if NT > 2.5 mm special examination directed to examination of fetal heart. Follow‐up to birth |
|
| Index and comparator tests | First trimester NT (cut‐off 2.5 mm) NT taken in saggital section. Distance between the end of the echogenic muscles of the c spine and the inner layer of echogenic skin with callipers on the line |
|
| Follow‐up | No details given of methods of follow‐up. 138 women lost to follow‐up | |
| Aim of study | To determine the value of NT measurement for the detection of aneuploidies and other malformations in a low‐risk population | |
| Notes | It appears that Down’s syndrome was only picked up in cases where CVS or amniocentesis had been conducted and it s not clear if patients were followed up to birth | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? All tests | Unclear | Amniocentesis or anomalies scan at 22 weeks. Unclear if women were also followed up to birth. |
| Partial verification avoided? All tests | Unclear | Unclear if all women received a reference standard |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | Yes | NT measurement was not possible in 2% of cases |
| Withdrawals explained? All tests | No | No details of withdrawals given |
Has 2008.
| Clinical features and settings | Routine screening | |
| Participants | 1807 participants with successful scans Turkey September 2003 ‐ December 2005 Pregnant women Singleton pregnancies Median maternal age 28.3 years (range 17‐45 years) 11‐14 weeks' gestation |
|
| Study design | Cohort study | |
| Target condition and reference standard(s) | Down's syndrome: 9 cases Reference standards: karyotyping or follow‐up to birth |
|
| Index and comparator tests | Maternal age First trimester NT (FMF methods) First trimester fetal nasal bone (experienced maternal fetal specialists) First trimester PAPP‐A and free ßhCG (details not reported) Combined cut‐point 1:300 |
|
| Follow‐up | Findings recorded in a computer database. Karyotype results obtained directly from the genetics department. Pregnancy outcomes obtained from hospital records or from parents via telephone interview. 110 women (5%) with terminations, miscarriages or malformations and unknown outcome were excluded from the study | |
| Aim of study | To evaluate the contribution of nasal bone assessment in first trimester Down's syndrome screening | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? All tests | Unclear | Unclear if all women received a reference standard |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | Yes | Evaluation of nasal bone was not possible in 9 (0.5%) cases |
| Withdrawals explained? All tests | No | No details of withdrawals given |
Hewitt 1996.
| Clinical features and settings | High‐risk referral for invasive testing | |
| Participants | 1306 women with 1317 fetuses (11 sets of twins) Australia ‐ 2 hospitals and 2 private practices September 1993 to September 1994 Pegnant women Singleton or multifetal pregnancies Median age 37 years (21‐48 years) 10 to 14 weeks' gestation |
|
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down’s syndrome: 21 cases Reference standard: CVS |
|
| Index and comparator tests | First trimester NT (ATL HDI ESP Diagnostic Ultrasound system), cut‐point 3 mm or more | |
| Follow‐up | 100% karyotyping | |
| Aim of study | To evaluate the accuracy of ultrasound measurement of nuchal thickness in first trimester fetuses for predicting fetal karyotype | |
| Notes | No measurement of NT was recorded in 126 cases (9.6%). All down’s syndrome fetuses terminated | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Selective testing of high‐risk women as done in practice |
| Acceptable reference standard? All tests | Yes | CVS |
| Partial verification avoided? All tests | Yes | All women received a reference standard |
| Differential verification avoided? All tests | Yes | All women had the same reference standard |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | Yes | No measurement of NT was recorded in 126 cases (9.6%) |
| Withdrawals explained? All tests | No | No details of withdrawals given |
Hormansdorfer 2011.
| Clinical features and settings | Routine screening | |
| Participants | 6508 participants with known fetal outcome Germany ‐ 3 prenatal health centres August 1999 ‐ May 2007 Pregnant women Mean maternal age 31.1 years (16‐46 years), 22% ≥ 35 years First trimester |
|
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 40 cases Reference standards: karyotyping or follow‐up to birth |
|
| Index and comparator tests | Maternal age First trimester NT (FMF standards) First trimester PAPP‐A and free ßhCG (no details given) Different software programmes used with and without modification to exclude the role of maternal age |
|
| Follow‐up | Methods of follow‐up not reported. Stated that only women with known fetal outcome were included in the study | |
| Aim of study | To analyse the impact in test performance of 3 widely used first trimester screening software programs if the maternal age was excluded from their calculation algorithm | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? All tests | Yes | All women received a reference standard |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? All tests | No | No details of withdrawals given |
Huang 2010.
| Clinical features and settings | Routine screening | |
| Participants | 7118 participants undergoing combined first trimester screening and a fetal abnormality scan Taiwan ‐ single hospital January 2004 ‐ December 2007 Pregnant women Median maternal age 30 years (range 15‐47 years) 8‐13 weeks' gestation |
|
| Study design | Cohort study | |
| Target condition and reference standard(s) | Down's syndrome: 25 cases Reference standards: karyotyping or follow‐up to birth |
|
| Index and comparator tests | Maternal age First trimester NT (11‐13 weeks' gestation) (FMF accredited obstetricians) First trimester free ßhCG and PAPP‐A (8‐12 weeks' gestation) (time resolved amplified cryptate emission, automated Kryptor Analyser, Brahms) Combined cut‐point 1:300 Second trimester fetal abnormality scan (18‐22 weeks' gestation) for intracardiac echogenic focus (ICEF) (In accordance with the American Institute of Ultrasound in Medicine Practice Guideline) |
|
| Follow‐up | All neonates examined postnatally and Hospital records reviewed | |
| Aim of study | To determine the relation between intracardiac echogenic focus and trisomy 21 in a population of fetuses previously evaluated by first trimester combined screening | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? All tests | Yes | All women received a reference standard |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? All tests | No | No details of withdrawals given |
Jaques 2007.
| Clinical features and settings | Routine screening | |
| Participants | 16,153 participants Australia ‐ State screening programme February 2000 ‐ June 2002 Pregnant women Mean maternal age 33 years (range 16‐51 years), 18.5% ≥ 37 years 10‐13 weeks' gestation |
|
| Study design | Retrospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 63 cases Reference standards: karyotyping or follow‐up to birth |
|
| Index and comparator tests | Maternal age First trimester NT (FMF accredited ultrasonologists) First trimester PAPP‐A and free ßhCG (details not reported) First trimester AFP, inhibin A and uE3 added to first trimester results for women who were screened at 13 weeks' gestation (augmented screening, number not reported) |
|
| Follow‐up | Probabilistic record linkage was used to link health records from the Genetic Health prenatal screening database, Perinatal Data Collection Unit and the Birth Defects Register. Written requests for pregnancy outcome were sent to referring health professionals. Pathology and cytogenetics reports were collected for confirmation of birth defects and/or karyotype 151 women were lost to follow‐up and these were excluded in the analysis Of the 16,003 women, pregnancy loss in 71 due to miscarriage (n = 68), stillbirth (n = 1) and neonatal death (n = 2) |
|
| Aim of study | To follow up and evaluate the state‐wide first trimester combined screening programme for Down's syndrome and trisomy 18 at Genetic Health Services Victoria, Australia | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? All tests | Yes | All women received a reference standard |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? All tests | No | No details of withdrawals given |
Jaques 2010 FTS.
| Clinical features and settings | Routine screening | |
| Participants | 38,584 participants Australia ‐ State screening programme 2003 ‐ 2004 Pregnant women Maternal age ≥ 37 years in 16.3% of women First and second trimester |
|
| Study design | Retrospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 110 cases Reference standards: karyotyping (CVS = 774, amniocentesis =1644) or follow‐up to birth |
|
| Index and comparator tests | Maternal age First trimester NT, PAPP‐A and free ßhCG (n = 38,584) (details not reported) |
|
| Follow‐up | Probabilistic record linkage was used to link health records from the Prenatal Screening Database, prenatal diagnostic data from cytogenetic laboratories, the Victoria Birth Register (Perinatal Data collection Unit) and the Victoria Birth Defects Register | |
| Aim of study | To map prenatal screening and diagnostic testing pathways in Victorian pregnant women during 2003‐2004; measure the impact of prenatal diagnostic testing uptake on the effectiveness of prenatal screening for Down's syndrome; and assess factors influencing uptake of diagnostic testing following screening | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? All tests | Unclear | Unclear if all women received a reference standard |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | Yes | Invalid results obtained for 7.4% of first and 0.1% of second trimester screenings |
| Withdrawals explained? All tests | Yes | 48% of pregnant women in the state did not undergo prenatal testing |
Kagan 2010.
| Clinical features and settings | Routine screening | |
| Participants | 56,954 participants with available outcome data UK ‐ multicentre July 1999 ‐ April 2007 Pregnant women Singleton pregnancies Mean maternal age 35.4 years (range 14.1 to 52.2 years) 11‐13 weeks' gestation |
|
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 395 cases Reference standards: karyotyping or follow‐up to birth |
|
| Index and comparator tests | Maternal age First trimester NT First trimester fetal heart rate (pulsed‐wave Doppler) First trimester nasal bone (FMF certified sonographers) First trimester ductus venous flow (FMF certified sonographers) First trimester flow across tricuspid valve (FMF certified sonographers) First trimester PAPP‐A and free ßhCG (Kryptor, Brahms AG or Delfia Express, Perkin Elmer) Multiple publications with different test evaluations |
|
| Follow‐up | Karyotype results and details of pregnancy outcome added to databases as they became available. Women without complete screening and outcome data (n = 3053, 5.1%) were excluded from the study | |
| Aim of study | To examine the performance of first‐trimester screening for trisomies 21, 18 and 13 by maternal age, fetal nuchal translucency thickness, fetal heart rate and maternal serum free ßhCG and PAPP‐A Other objectives in related publications |
|
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? All tests | Yes | All women received a reference standard |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? All tests | No | No details of withdrawals given |
Kim 2006.
| Clinical features and settings | Routine screening | |
| Participants | 2570 participants with available outcome data Korea ‐ hospital and womens healthcare centre January 2001 to December 2001 Pregnant women Mean age 29.9 years (SD 3.3 years) Singleton pregnancies 10‐14 weeks' gestation |
|
| Study design | Retrospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 31 cases Reference standard: amniocentesis or CVS in 419 patients considered high risk (NT > 2.5, aged > 35 years, positive biochemical test result, history of chromosomal abnormality, fetal structural abnormality at ultrasound or other reason). Follow‐up to birth |
|
| Index and comparator tests | First trimester NT (FMF methods) (HDI 3000, ATL, Bothell, WA, USA) 3 measurements taken, largest one used for risk calculation Cut‐off 2.5 mm, 3.0 mm or 95th percentile of each CRL |
|
| Follow‐up | Pregnancy outcomes ascertained from obstetric and neonatal medical records of live or stillborn babies Only patients with known pregnancy outcome included in the study 8 patients who terminated their pregnancies because of structural abnormalities on ultrasound with no karyotyping results were excluded. Karyotyping was performed in intrauterine fetal death (n = 4) cases |
|
| Aim of study | To determine the value of NT with different cut‐offs for the detection of chromosomal aberrations | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? All tests | Yes | All women received a reference standard |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? All tests | No | No details of withdrawals given |
Koster 2011.
| Clinical features and settings | Routine screening | |
| Participants | 998 participants: 151 cases and 847 controls matched for gestational age, maternal weight, maternal age and storage time The Netherlands ‐ National institute for Public Health and the Environment 2004 ‐ 2006 Pregnant women Singleton pregnancies Median maternal age 37 years (interquartile range 36‐39 years) 8‐13 weeks' gestation |
|
| Study design | Case‐control study | |
| Target condition and reference standard(s) | Down's syndrome: 151 cases Reference standards: karyotyping or follow‐up to birth |
|
| Index and comparator tests | Maternal age First trimester NT Fresh serum samples tested for: First trimester free ßhCG and PAPP‐A (AutoDELFIA, PerkinElmer) Frozen serum samples tested for: First trimester ADAM 12s, total hCG, placental protein 13 (PP13) and placental growth factor (PlGF) (AutoDELFIA or DelfiaXpress, PerkinElmer) |
|
| Follow‐up | Pregnancy outcome was recorded via questionnaires and self‐reporting by the participating women. Only samples for pregnancies with known outcome were selected as controls | |
| Aim of study | To evaluate the modelled predictive value of 3 current screening markers (PAPP‐A, free ßhCG and NT) and 4 potential screening markers (ADAM 12, total hCG, PP13 and PIGF) for Down's syndrome using different screening strategies | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? All tests | Yes | All women received a reference standard |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of some index test results |
| Index test results blinded? All tests | Unclear | Unclear if all index tests interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? All tests | No | No details of withdrawals given |
Kozlowski 2007 GC.
| Clinical features and settings | Routine referral | |
| Participants | 6906 participants with complete outcome data Germany ‐ gynaecologists practices January 2000 ‐ December 2003 Pregnant women Median maternal age 32 years (15‐48 years), 26.4% ≥ 35 years 11‐14 weeks' gestation |
|
| Study design | Cohort study | |
| Target condition and reference standard(s) | Down's syndrome: 19 cases in gynaecologists practices Reference standard: karyotyping or follow‐up to birth |
|
| Index and comparator tests | Maternal age First trimester NT (FMF certified gynaecologists) First trimester free ßhCG and PAPP‐A (Kryptor analyser, Brahms) Risk cut‐point 1:300 |
|
| Follow‐up | Data on pregnancy outcome were obtained by contacting the patient or their general gynaecologist. Women without complete outcome data (36%) were excluded from the study | |
| Aim of study | To evaluate and compare the screening performance for fetal trisomy 21 in the first trimester of pregnancy in general gynaecologists practices and specialised centres for prenatal care in Germany | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? All tests | Yes | All women received a reference standard |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? All tests | Yes | 146 women (including 11 with down's syndrome) excluded as results could not be assigned to gynaecologists' or prenatal centre group |
Kozlowski 2007 PC.
| Clinical features and settings | Routine referral | |
| Participants | 3862 participants with complete outcome data Germany ‐ tertiary level prenatal centres January 2000 ‐ December 2003 Pregnant women Median maternal age 34 years (range 14‐46 years), 43.2% ≥ 35 years 11‐14 weeks' gestation |
|
| Study design | Cohort study | |
| Target condition and reference standard(s) | Down's syndrome: 26 cases Reference standard: karyotyping or follow‐up to birth |
|
| Index and comparator tests | Maternal age First trimester NT (FMF certified sonographers) First trimester free ßhCG and PAPP‐A (Kryptor analyser, Brahms) Risk cut‐point 1:300 |
|
| Follow‐up | Data on pregnancy outcome were obtained by contacting the patient or their general gynaecologist. Women without complete outcome data (8%) were excluded from the study | |
| Aim of study | To evaluate and compare the screening performance for fetal trisomy 21 in the first trimester of pregnancy in general gynaecologists practices and specialised centres for prenatal care in Germany | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? All tests | Yes | All women received a reference standard |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? All tests | Yes | 146 women (including 11 with down's syndrome) excluded as results could not be assigned to gynaecologists' or prenatal centre group |
Krantz 2000.
| Clinical features and settings | Routine screening | |
| Participants | 10,251 participants USA September 1995 to June 1998 Pregnant women 34.7% ≥ 35 years Singleton pregnancies No diabetes 9‐13 weeks' gestation |
|
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 50 cases (33 had undergone biochemical testing) Reference standards: not reported |
|
| Index and comparator tests | Maternal age First trimester NT in 5,809 (2018 ≥ 35 years).patients (FMF methods) Dried blood samples tested for: First trimester free ßhCG and PAPP‐A in 10,251 patients (enzyme‐linked immunosorbent asay procedures) |
|
| Follow‐up | No details of follow‐up reported | |
| Aim of study | To assess the effectiveness of free ßhCG, PAPP‐A and NT for first trimester screening for Down's syndrome and trisomy 18 | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? All tests | Unclear | Unclear reference standard |
| Partial verification avoided? All tests | Unclear | Unclear if all patients had a reference standard |
| Differential verification avoided? All tests | Unclear | Unclear if choice of reference depended on index test results |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? All tests | No | No details of withdrawals given |
Kublickas 2009.
| Clinical features and settings | Routine screening | |
| Participants | 3907 participants Sweden 2005 ‐ 2006 Pregnant women 51% of women aged ≥ 35 years 9‐14 weeks' gestation |
|
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 29 cases Reference standards: karyotyping or follow‐up to birth |
|
| Index and comparator tests | Maternal age First trimester NT (FMF trained sonographers) First trimester free ßhCG and PAPP‐A (AutoDELFIA, PerkinElmer) |
|
| Follow‐up | The dataset used contained outcomes for all pregnancies | |
| Aim of study | To provide the necessary mathematical formulae to construct a risk calculation package for Down's syndrome using maternal serum free ßhCG, PAPP‐A and NT measurements in the first trimester for use in a web‐based system | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? All tests | Yes | All women received a reference standard |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? All tests | No | No details of withdrawals given |
Kuc 2010.
| Clinical features and settings | Routine screening | |
| Participants | 27,291 participants: 223 cases and 22,157 controls (not matched) The Netherlands ‐ The Dutch National Institute for Public Health and the Environment Dates not specified Pregnant women Maternal age not reported 8‐13 weeks' gestation |
|
| Study design | Case‐control study | |
| Target condition and reference standard(s) | Down's syndrome: 223 cases Reference standards: karyotyping or follow‐up to birth |
|
| Index and comparator tests | Maternal age First trimester NT (FMF trained sonographers) First trimester free ßhCG and PAPP‐A (automated dissociation‐enhanced lanthanide fluorescent immunoassay, AutoDELFIA, PerkinElmer) |
|
| Follow‐up | Known outcomes for cases and controls | |
| Aim of study | To estimate the effect of timing of serum collection on screening performance | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? All tests | Yes | All women received a reference standard |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? All tests | No | No details of withdrawals given |
Lam 2002.
| Clinical features and settings | Routine screening | |
| Participants | 16,237 participants Hong Kong ‐ multicentre study 1997 to 2000 Pregnant women Mean age 30.5 years (19% ≥ 35 years) (unaffected pregnancies) 10‐14 weeks and 15‐18 weeks' gestation |
|
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 35 cases Reference standards: women considered high risk offered CVS (0.7%) or amniocentesis (11.8%). Follow‐up to birth |
|
| Index and comparator tests | Maternal age First trimester NT (FMF methods) Second trimester free ßhCG and AFP (methods not stated) (All women underwent both NT and biochemical testing) |
|
| Follow‐up | By review of hospital and laboratory records and by directly telephoning women. Participants who defaulted the second trimester serum tests (n = 1015) and those who miscarried after NT but before serum testing (n = 91) were excluded from the study. Outcome obtained in only 15,253 patients (93.9%) |
|
| Aim of study | To report data on participants undergoing both first and second trimester methods of screening to assess the relative efficacy of different methods of screening | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? All tests | No | Not all women received a reference standard (6.1% had no ascertainment of pregnancy outcome) |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | Yes | NT successful in 99.8% of cases |
| Withdrawals explained? All tests | No | No details of withdrawals given |
Leung 2009.
| Clinical features and settings | Routine screening | |
| Participants | 10,185 participants (178 twin pregnancies; 10,363 fetuses) Hong Kong ‐ University Hospital June 2003 ‐ March 2007 Pregnant women Singleton or multifetal pregnancies Median maternal age 32 years (IQR 30‐35 years), 27.4% of women aged ≥ 35 years 11‐13 weeks' gestation |
|
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 34 cases Reference standards: amniocentesis or follow‐up to birth |
|
| Index and comparator tests | Maternal age First trimester NT (FMF accredited doctors, HDI 5000 or HDI 3000, Philips Medical System) First trimester nasal bone assessment (7925 women) (FMF accredited doctors, HDI 5000 or HDI 3000, Philips Medical System) First trimester PAPP‐A and free ßhCG (Kryptor analyser, Brahms Diagnostica GmbH) Risk cut‐point 1:300 For twin pregnancies, a risk was calculated for each fetus based on the individual NT and maternal serum biochemistry corrected for twin pregnancies |
|
| Follow‐up | Specific staff were allocated to contact all women for pregnancy and fetal outcome. Women were contacted by phone and mail. 5 screen positive and 50 screen negative cases had unknown outcome. | |
| Aim of study | To examine the effectiveness of first trimester fetal trisomy 21 screening using a combination of maternal age, NT and maternal serum free ßhCG and PAPP‐A levels in a predominantly Chinese population in Hong Kong | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? All tests | Yes | All women received a reference standard |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | Yes | Nasal bone status could not be determined in 176 women (2.2%) (2 with Down's syndrome) |
| Withdrawals explained? All tests | No | No details of withdrawals given |
MacRae 2008.
| Clinical features and settings | Routine screening | |
| Participants | 18,965 pregnancies UK ‐ University Hospital July 1998 ‐ January 2004 Maternal age not reported 10‐13 weeks' gestation |
|
| Study design | Retrospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 37 cases Reference standards: karyotyping or follow‐up to birth |
|
| Index and comparator tests | Maternal age First trimester NT (trained sonographers) Risk cut‐point 1:300 |
|
| Follow‐up | Information on birth outcome from Harris birthright Research Centre database, the North East Regional Cytogenetic Laboratory, the National Down's syndrome register and the Basildon and Thurrock University Hospital database and, in some cases, maternal and paediatric records. For each case, screening results were linked to cytogenetic results/pregnancy outcome | |
| Aim of study | To evaluate NT scans with a view to comparing findings with other research centres | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? All tests | Yes | All women received a reference standard |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results |
| Incorporation avoided? All tests | Yes | Index tests did not form part of the reference standard |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? All tests | No | No details of withdrawals given |
Maiz 2007.
| Clinical features and settings | High‐risk referral for invasive testing | |
| Participants | 227 participants UK ‐ single centre Pregnant women Singleton pregnancies Median maternal age 35 years (17‐49 years) 11‐13 weeks' gestation |
|
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 20 cases Reference standard: CVS |
|
| Index and comparator tests | First trimester presence of mitral gap (Doppler flow traces) | |
| Follow‐up | 100% karyotyping | |
| Aim of study | To investigate the possible association between a particular pulsed Doppler waveform pattern, mitral gap and trisomy 21 at 11 + 0 to 13 + 6 weeks | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? All tests | Yes | Karyotyping |
| Partial verification avoided? All tests | Yes | All women received a reference standard |
| Differential verification avoided? All tests | Yes | Choice of reference standard did not depend on index test results |
| Incorporation avoided? All tests | Yes | Index tests did not form part of the reference standard |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? All tests | No | No details of withdrawals given |
Maiz 2009.
| Clinical features and settings | Routine screening | |
| Participants | 19,614 participants with complete screening and outcome data UK ‐ multicentre January 2006 ‐ May 2007 Pregnant women Singleton pregnancies Median maternal age 34.5 years (14.1‐50.1 years) 11‐13 weeks' gestation |
|
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 122 cases Reference standards: karyotyping or follow‐up to birth |
|
| Index and comparator tests | Maternal age First trimester NT and fetal heart rate First trimester ductus venous blood flow velocity waveforms (FMF certified sonographers) First trimester PAPP‐A and free ßhCG (Delfia Xpress, PerkinElmer) |
|
| Follow‐up | Karyotype results and details on pregnancy outcome were added to the database as soon as they became available. Women without complete outcome data (5.3%) were excluded from the study | |
| Aim of study | To investigate the performance of first trimester screening for aneuploidies by including assessment of ductus venosus flow in the combined test of maternal age, fetal NT thickness, fetal heart rate and serum free ßhCG and PAPP‐A | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? All tests | Yes | All women received a reference standard |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? All tests | No | No details of withdrawals given |
Malone 2004.
| Clinical features and settings | Routine screening | |
| Participants | 6324 participants USA ‐ multicentre study (15 centres) May 2002 to December 2002 Pregnant women Mean age 30.1 years (16‐47 years), 22.1% ≥ 35 years Singleton pregnancies 10‐13 weeks' gestation |
|
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 11 cases Reference standards: karyotyping in 587 (amniocentesis n = 510; neonatal cord blood n = 41; products of conception and autopsy material n = 31), or follow‐up to birth |
|
| Index and comparator tests | Nasal bone imaging Fetal image in a perfect saggital plane with fetal spine down. Angle of insonation of ultrasound beam with fetal profile close to 45 degrees. Image magnified significantly until 2 echogenic lines are visible in region of fetal nose. Transducer tilted from side to side to distinguish fetal skin from nasal bone. Deeper echogenic line noted to become more echolucent at its distal end |
|
| Follow‐up | A tracking programme with up to 10 contact options for each patient used for follow‐up Follow‐up to birth in 6228 patients (98.5%) and adequate nasal bone imaging in 4801 (75.9%) |
|
| Aim of study | To evaluate first trimester nasal bone imaging as a screening tool for aneuploidy | |
| Notes | Only 17% of patients who had miscarriage or termination of pregnancy had karyotype information available | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? All tests | No | Not all women received a reference standard (1.5% had no ascertainment of pregnancy outcome) |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | Yes | Nasal bone screening successful in 4801 cases (75.9%) |
| Withdrawals explained? All tests | No | No details of withdrawals given |
Malone 2005.
| Clinical features and settings | Routine screening | |
| Participants | 38,033 participants USA ‐ multicentre study (15 centres) October 1999 to December 2002 Pregnant women Mean maternal age 30.1 years (SD 5.8 years.); 8199 (21.6%) aged ≥ 35 years Singleton pregnancies Live fetuses 10‐13 and 15‐18 weeks' gestation |
|
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down’s syndrome: 92 cases Reference standards: amniocentesis offered to women with positive results from any screening test or follow‐up to birth |
|
| Index and comparator tests | Maternal age First trimester NT in 36,306 patients (92.9%) First trimester PAPP‐A and free ßhCG in 37,843 patients (99.5%) Second trimester AFP, total hCG, uE3 and inhibin A in 35,236 patients (92.6%) All data in 33,546 patients (88.2%) |
|
| Follow‐up | Follow‐up with computerised tracking system. Medical records were reviewed in cases of 1) possible medical problem suspected 2) positive screening test results with no karyotype data, 3) 10% random sample of all enrolled patients Follow‐up to birth in 36,378 patients (97%) |
|
| Aim of study | To evaluate first trimester and/or second trimester screening tool for Down's syndrome | |
| Notes | Unclear which types of patients did not have follow‐up data. Appears that aborted/miscarried fetuses did not have follow‐up | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? All tests | No | Not all women received a reference standard (3% had no ascertainment of pregnancy outcome, patients not excluded from study) |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | Yes | NT failed or rejected at review in only 7.1% |
| Withdrawals explained? All tests | Yes | Details given for patients who did not undergo different index tests |
Marchini 2010.
| Clinical features and settings | Routine screening | |
| Participants | 1521 participants (18 twin and 2 triplet pregnancies; 1543 fetuses) Italy Pregnant women Singleton or multifetal pregnancies Median maternal age 31.3 years (range 18‐45 years), 19.7% ≥ 35 years 11‐14 weeks' gestation |
|
| Study design | Retrospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 8 cases Reference standards: karyotyping or follow‐up to birth |
|
| Index and comparator tests | Maternal age First trimester NT (FMF accredited sonographers) First trimester serum free ßhCG and PAPP‐A (Kryptor analyser, Brahms) Risk cut‐point 1:300 |
|
| Follow‐up | Follow‐up obtained by analysis of fetal karyotype, from patient notes and by telephoning patients | |
| Aim of study | To evaluate the performance of the combined test compared to the NT measurement alone, in fetal aneuploidy screening in the general population and in pregnant women aged 35 years and over | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? All tests | Unclear | Unclear if all women received a reference standard |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? All tests | No | No details of withdrawals given |
Marsis 2004.
| Clinical features and settings | Screening of patients ≥ 35 years of age | |
| Participants | 262 participants Indonesia ‐ 4 hospitals January 2001 to January 2003 Pregnant women Mean age 37.7 years (35‐43 years) Singleton pregnancies 11‐13 weeks' gestation |
|
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 8 cases Reference standards: amniocentesis (unclear in which patients this was conducted) or follow‐up to birth |
|
| Index and comparator tests | First trimester NT (all patients) with > 3.0 mm cut‐off (FMF methods, Apoge 800‐ATL, SSD 680‐Aloka, Logic alpha 200 GE, Veluson 730 Pro GE) First trimester nasal bone assessment (97 (55%) patients who also had NT) |
|
| Follow‐up | Follow‐up to birth in patients with no nasal bone and NT > 3 mm. Unclear if screen‐negative patients had follow‐up to birth | |
| Aim of study | Evaluation of a non‐invasive method to screen for Down's syndrome at a maternal age of 35 years or more | |
| Notes | No cases of Down’s detected that were not picked up in screening tests | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Selective testing of high‐risk women as done in practice |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? All tests | Unclear | Unclear if all women received a reference standard |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? All tests | No | No details of withdrawals given |
Marsk 2006.
| Clinical features and settings | Routine screening | |
| Participants | 139 participants: 31 cases and 108 controls (3:1 with cases, matched for time of study, geographic location and to be within 5‐year age interval) Sweden ‐ data from Swedish Nuchal Translucency Trial Dates not reported Pregnant women Mean age cases 38.5 years (SD 4.0 years) and controls 35.5 years (SD 4.0 years) Singleton pregnancies 8‐14 weeks' gestation |
|
| Study design | Case‐control study | |
| Target condition and reference standard(s) | Down's syndrome: 31 cases Reference standards: not reported |
|
| Index and comparator tests | Maternal age First trimester NT (12‐14 weeks) (method not specified) Frozen serum samples PAPP‐A and free ßhCG in sample taken at 8‐14 weeks (Auto Delfia Instrument) Risk cut‐points of 1:250 and 1:350 (Lifecycle software used to calculate risk) |
|
| Follow‐up | No details of methods used to follow women‐up | |
| Aim of study | To determine to what extent adding first trimester serum screening to NT would change the detection rate and test positive rate for Down's syndrome | |
| Notes | Part of NUPP trial | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? All tests | Yes | All women received a reference standard |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Unclear | Unclear if all index tests interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? All tests | Yes | Details given for women who did not agree to take part |
Matias 1998.
| Clinical features and settings | High‐risk referral for invasive testing | |
| Participants | 486 participants UK and Portugal Dates not reported Pregnant women Singleton pregnancies Median age 35 years (17‐46 years) 10‐14 weeks' gestation |
|
| Study design | Prospective cohort study | |
| Target condition and reference standard(s) | Down's syndrome: 38 cases Reference standard: fetal karyotyping. In cases where NT above 95th percentile or abnormal ductus venousus flow, follow‐up scan conducted at 14‐16 weeks |
|
| Index and comparator tests | Maternal age First trimester NT (SSD, Aloka) First trimester ductus venosus flow velocity: measured transabdominally (5‐MHz curvilinear probe, Ecocee, Toshiba) or transvaginally (SSD 2000, Aloka) |
|
| Follow‐up | 100% karyotyping | |
| Aim of study | To assess the possible role of Doppler ultrasound assessment of ductus venous blood flow in screening for chromosomal abnormalities at 11 to 14 weeks of gestation | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Selective testing of high‐risk women as done in practice |
| Acceptable reference standard? All tests | Yes | Karyotyping |
| Partial verification avoided? All tests | Yes | All women received a reference standard |
| Differential verification avoided? All tests | Yes | All women had the same reference standard |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | Yes | Reported that measurements made successfully in all cases |
| Withdrawals explained? All tests | No | No details of withdrawals given |
Matias 2001.
| Clinical features and settings | High‐risk referral for invasive testing | |
| Participants | 515 participants Portugal Dates not reported Pregnant women Median age 35 years (17‐46 years) Singleton pregnancies 11‐14 weeks' gestation |
|
| Study design | Prospective cohort study | |
| Target condition and reference standard(s) | Down's syndrome: 43 cases Reference standards: fetal karyotyping. In cases where NT above 95th percentile, follow‐up scan conducted at 14‐16 weeks |
|
| Index and comparator tests | Maternal age First trimester NT (SSD, Aloka) First trimester ductus venous Doppler evaluation ‐ ductus venosus flow velocity ‐ abnormal flow is defined as absent or reversed flow of blood in the ductus venosus, normal flow defined as presence. Measurement made by obtaining the right ventral midsaggital plane of the fetal trunk in fetal quiescence. Pulsed Doppler gate placed in distal portion of umbilical sinus. 5 consecutive high‐quality waveforms used to measure peak velocity during ventricular systole and diastole, the lowest forward velocity during atrial contraction in late diastole and the pulsatility index. Up to 10 minutes allowed for measurements |
|
| Follow‐up | All women received karyotyping. Unclear if patients followed up to birth | |
| Aim of study | To review the role of Doppler ultrasound in screening for chromosomal abnormalities at 11 to 14 weeks of gestation | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Selective testing of high‐risk women as done in practice |
| Acceptable reference standard? All tests | Yes | Karyotyping |
| Partial verification avoided? All tests | Yes | All women had a reference standard |
| Differential verification avoided? All tests | Yes | All women had the same reference standard |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | Yes | Reported that Doppler measurements made successfully in all cases |
| Withdrawals explained? All tests | No | No details of withdrawals given |
Mavrides 2002.
| Clinical features and settings | High‐risk referral for invasive testing | |
| Participants | 256 participants who were referred to unit for fetal karyotyping and had NT and Doppler studies UK ‐ tertiary referral fetal medicine unit Conducted over 18 months, dates not reported Pregnant women Median age 35 years (15‐42 years) 11‐14 weeks' gestation |
|
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 30 cases Reference standard: CVS or follow‐up |
|
| Index and comparator tests | Maternal age First trimester NT First trimester ductus venous Doppler studies (ATI HDL 5000 US machine with curvilinear TV probe) |
|
| Follow‐up | Follow‐up based on ultrasounds findings, examination at birth, postmortem examination in cases of intrauterine death or termination of pregnancy and by telephone interviews with parents | |
| Aim of study | To assess the role of first trimester Doppler assessment of the ductus venosus in screening for fetal aneuploidy in pregnancies at 11‐14 weeks of gestation | |
| Notes | 2 live births with Down’s syndrome. Appears to be a high‐risk invasive testing study but some people did not appear to get karyotyping but were followed up. Probably the majority got karyotyping | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Selective testing of high‐risk women as done in practice |
| Acceptable reference standard? All tests | Yes | Karyotyping |
| Partial verification avoided? All tests | Yes | All participants had a reference standard |
| Differential verification avoided? All tests | Yes | All participants had the same reference standard |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | Yes | Doppler studies failed in 4 cases (1.5%) |
| Withdrawals explained? All tests | No | No details of withdrawals given |
Maxwell 2011 FTS.
| Clinical features and settings | Routine screening | |
| Participants | 32,478 participants with available outcome data Australia ‐ screening programme 2005 ‐ 2006 Pregnant women Singleton pregnancies Median maternal age 31 years (14‐48 years), 24.3% of women aged ≥ 35 years 10‐13 weeks' gestation |
|
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 94 cases Reference standards: karyotyping or follow‐up to birth |
|
| Index and comparator tests | Maternal age First trimester NT PAPP‐A and free ßhCG (details not reported) Risk cut‐point 1:300 |
|
| Follow‐up | Diagnostic data collected from cytogenetic laboratories. Screening data linked to Western Australia diagnostic data, hospital morbidity and mortality data, midwives notification data and the Birth Defects Registry data through the Department of Health Western Australias Data Linkage Branch. Outcome data available for 92.3% of screened women | |
| Aim of study | To investigate socio‐demographic characteristics in the uptake of prenatal aneuploidy screening in Western Australia and to identify potential barriers to screening access | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? All tests | Yes | All women received a reference standard |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? All tests | No | No details of withdrawals given |
Maymon 2005.
| Clinical features and settings | Routine screening | |
| Participants | 595 participants Israel January 1999 ‐ January 2004 Pregnant women Mean age, healthy 30.3 years (SD 4.5), Down's syndrome 33.7 years (SD 4.9) Singleton pregnancies 11‐14 weeks' gestation and second trimester screening |
|
| Study design | Case‐control study | |
| Target condition and reference standard(s) | Down's syndrome: 24 cases Reference standards: amniocentesis (recommended for women with higher risk on first or second trimester testing) or follow‐up to birth |
|
| Index and comparator tests | Maternal age First trimester NT (11‐14 weeks) First trimester PAPP‐A and free ßhCG (methods detailed in Maymon 2001) (some analysed retrospectively from banked samples) Second trimester PAPP‐A and free ßhCG (methods detailed in Maymon 2001) |
|
| Follow‐up | Delivery outcome obtained by telephone interview or medical records. Information was available for all uneventful pregnancies and delivery outcomes. It is unclear whether information on terminations of pregnancy or miscarriages was available. | |
| Aim of study | To evaluate the cross‐trimester multiple marker correlation and the minimum marker combination needed for detecting various chromosomal aneuploides | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? All tests | Yes | All women received a reference standard |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? All tests | No | No details of withdrawals given |
Maymon 2008.
| Clinical features and settings | Routine screening | |
| Participants | 243 participants: 19 cases and 224 consecutive controls USA ‐ antenatal sonographic unit October 2005 ‐ May 2007 Pregnant women Singleton pregnancies 11‐13 and 14‐28 weeks' gestation |
|
| Study design | Case‐control study | |
| Target condition and reference standard(s) | Down's syndrome: 19 cases Reference standards: karyotyping or follow‐up to birth |
|
| Index and comparator tests | Maternal age First trimester NT (according to FMF criteria) Second trimester nuchal skin‐fold (according to published criteria) First trimester free ßhCG and PAPP‐A (details not reported) |
|
| Follow‐up | Cases detected through karyotyping. Stated that controls had normal pregnancies | |
| Aim of study | To assess whether there is a correlation between nuchal translucency and nuchal skin‐fold measurements in Down's syndrome and in normal pregnancies | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? All tests | Yes | All women received a reference standard |
| Differential verification avoided? All tests | No | Different reference standards used |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of some index test results |
| Index test results blinded? All tests | Unclear | Unclear if all index tests interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? All tests | No | No details of withdrawals given |
Merz 2011.
| Clinical features and settings | Routine referral | |
| Participants | 124,205 participants Germany Dates not reported Pregnant women Maternal age not reported Singleton pregnancies First trimester |
|
| Study design | Retrospective cohort study | |
| Target condition and reference standard(s) | Down's syndrome: 500 cases Reference standard: karyotyping or follow‐up to birth |
|
| Index and comparator tests | Maternal age First trimester NT (only data obtained by level II or III certified sonographers included) First trimester free ßhCG and PAPP‐A (Brahms Kryptor system) FMF Germany risk calculation Risk cut‐point 1:150 |
|
| Follow‐up | Details not reported | |
| Aim of study | To demonstrate that the variability of the FPR can be reduced through adjusting the concentrations of free ßhCG and PAPP‐A measured in the maternal serum by meaning of a nonlinear regression function modelling the dependence of these variables on maternal weight | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? All tests | Unclear | Unclear if all women received a reference standard |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? All tests | No | No details of withdrawals given |
Michailidis 2001.
| Clinical features and settings | Routine screening | |
| Participants | 7447 participants UK ‐ hospital maternity unit January 1995 to January 2000 Pregnant women Mean age 30.1 years (13‐50 years), 21.1% ≥ 35 years, 11.9% ≥ 37 years 10‐14 weeks' gestation |
|
| Study design | Prospective cohort study | |
| Target condition and reference standard(s) | Down’s syndrome: 23 cases Reference standards: karyotyping in women considered at risk due to index test results, age or family history or those with considerable anxiety (632 women, 8.5%). Follow‐up to birth |
|
| Index and comparator tests | Maternal age First trimester NT in all patients (fetus in mid‐sagittal section. Maximum thickness of subcutaneous translucency between skin and soft tissue overlying the C‐spine with the fetus in the ventral position) Second trimester AFP, free ßhCG in 65% of patients with NT (radio‐immunoassay and immunoradiometric assays) |
|
| Follow‐up | Outcome at birth assess from hospital database, labour ward records or directly from patients. Follow‐up data in 7447 patients (87% of initial patient cohort). Patients without follow‐up excluded |
|
| Aim of study | To asses the effectiveness of antenatal screening for trisomy 21 by first trimester sonography followed by second trimester biochemical screening | |
| Notes | 2nd trimester data not analysed 4 live births: 1 diagnosed before birth and chose not to abort. 3 diagnosed after birth (no invasive testing was conducted) |
|
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? All tests | Yes | All women received a reference standard |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? All tests | No | No details of withdrawals given |
Molina 2010 high risk.
| Clinical features and settings | High‐risk referral for invasive testing | |
| Participants | 333 participants Spain ‐ fetal medicine unit February 2007 ‐ January 2009 Pregnant women Singleton pregnancies Mean maternal age 32.7 years (range 16.7‐47.5 years) 11‐14 weeks' gestation |
|
| Study design | Cohort | |
| Target condition and reference standard(s) | Down's syndrome: 20 cases Reference standard: CVS |
|
| Index and comparator tests | First trimester nasal bone (FMF certified sonographer) First trimester ductus venosus (FMF certified sonographer) First trimester tricuspid regurgitation (FMF certified sonographer) |
|
| Follow‐up | 100% karyotyping | |
| Aim of study | To evaluate detection and false positive rates of the ultrasound markers ‐ nasal bone, ductus venosus flow and tricuspid regurgitation, during the first trimester in a population at high‐genetic risk and to study the influence of a 2‐stage screening policy after previous combined screening on the rate of invasive procedures | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Selective testing of high‐risk women as done in practice |
| Acceptable reference standard? All tests | Yes | Karyotyping |
| Partial verification avoided? All tests | Yes | All women received a reference standard |
| Differential verification avoided? All tests | Yes | Choice of reference standard did not depend on index test results |
| Incorporation avoided? All tests | Yes | Index tests did not form part of the reference standard |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | Yes | 5 (1.5%) women did not have measurements obtained for nasal bone and tricuspid regurgitation and 10 (3%) did not have measurements obtained for ductus venosus |
| Withdrawals explained? All tests | No | No details of withdrawals given |
Molina 2010 screening.
| Clinical features and settings | Routine screening | |
| Participants | 6831 participants Spain ‐ fetal medicine unit February 2007 ‐ January 2009 Pregnant women Maternal age not reported 9‐11 weeks' gestation |
|
| Study design | Cohort | |
| Target condition and reference standard(s) | Down's syndrome: 23 cases Reference standard: karyotyping or follow‐up to birth |
|
| Index and comparator tests | Maternal age First trimester NT (FMF certified sonographer) First trimester PAPP‐A and free ßhCG (DELFIA Xpress random access platform, PerkinElmer) |
|
| Follow‐up | Details not reported | |
| Aim of study | To evaluate detection and false positive rates of the ultrasound markers ‐ nasal bone, ductus venosus flow and tricuspid regurgitation, during the first trimester in a population at high‐genetic risk and to study the influence of a 2‐stage screening policy after previous combined screening on the rate of invasive procedures | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? All tests | Unclear | Unclear if all women received a reference standard |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results |
| Incorporation avoided? All tests | Yes | Index tests did not form part of the reference standard |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? All tests | No | No details of withdrawals given |
Monni 2005.
| Clinical features and settings | Routine screening | |
| Participants | 16,654 participants Italy ‐ single centre 2001‐2004 Pregnant women Median age 32 years (14‐49 years) Singleton pregnancies 10‐14 weeks' gestation |
|
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 96 cases Reference standards: karyotyping or follow‐up to birth |
|
| Index and comparator tests | Maternal age First trimester NT (FMF methods, No information given regarding machines used) First trimester nasal bone examination (transabdominal ultrasound in mid‐sagittal view) Annual audit of screening performance (medians) |
|
| Follow‐up | Outcome at birth as recorded in hospital database (provided by outcome sheets or telephone interviews). Of 32,000 cases in the database, 16,654 (52%) patients had NT, nasal bone assessment and follow‐up data available. Patients without follow‐up data were excluded from the study | |
| Aim of study | To evaluate the feasibility and diagnostic accuracy of fetal NT and nasal bone assessment at 11‐14 weeks for screening of trisomy 21 | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? All tests | Yes | All women received a reference standard |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | Yes | In 13 cases (1.3%) not possible to ascertain if nasal bone was visible |
| Withdrawals explained? All tests | No | No details of withdrawals given |
Montalvo 2005.
| Clinical features and settings | Routine screening | |
| Participants | 4538 participants who had follow‐up data available Spain ‐ tertiary hospital July 1999 ‐ October 2004 Pregnant women Mean age 31.1 years (14‐49 years), 25.9% of patients ≥ 35 years Singleton pregnancies 10‐14 weeks' gestation |
|
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 19 cases Reference standards: invasive testing offered to women considered high risk from screening results or follow‐up to birth |
|
| Index and comparator tests | Maternal age First trimester NT (Methods described by Nicholaides) First trimester PAPP‐A and free ßhCG (Kryptor Trace system, CIS Bio International) Risk cut‐point 1:270 |
|
| Follow‐up | Only patients with postnatal results available are included in the study | |
| Aim of study | To report the experience of using of use of the combined first trimester screening test | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? All tests | Yes | All women received a reference standard |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? All tests | No | No details of withdrawals given |
Moon 2007.
| Clinical features and settings | Routine screening | |
| Participants | 6471 fetuses with available outcome data Korea July 2004 ‐ March 2006 Pregnant women Singleton or multifetal pregnancies Mean maternal age: Down's syndrome 35.5 years (SD 4.8 years), non‐Down's syndrome 31.7 years (SD 3.4 years) 11‐14 weeks' gestation |
|
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 15 cases Reference standard: karyotyping or follow‐up to birth |
|
| Index and comparator tests | First trimester fetal nasal bone assessment (Voluson 730, LOGIQ 400 or 5, GE Medical Systems or HDI 500, Philips Medical systems) (American Registry of Diagnostic Medical Sonographers certified Sonographers) | |
| Follow‐up | Obstetric and neonatal outcome obtained from medical records, karyotyping reports and, when needed, telephone conversations with parents or physicians. A total of 7834 fetuses were included in the study but 1047 fetuses (13.4%) without available outcome data were excluded. The remaining 6787 fetuses included 154 twin pregnancies. Assessment of fetal nasal bone was possible in 6490 (95.6%) of the 6787 fetuses. Comparison of nasal assessments between the control population and Down's cases was performed in 6471 fetuses | |
| Aim of study | To evaluate the role of nasal bone assessment in first‐trimester screening for Down's syndrome in the Korean population | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? All tests | Yes | All women received a reference standard |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results |
| Incorporation avoided? All tests | Yes | Index tests did not form part of the reference standard |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | Yes | Assessment of fetal nasal bone was not possible in 297 women (4.4%) |
| Withdrawals explained? All tests | No | No details of withdrawals given |
Muller 2003.
| Clinical features and settings | Routine screening | |
| Participants | 5694 participants who had first trimester NT and biochemical testing France ‐ 9 centres serving 12 maternity units January 1998 ‐ June 2001 Pregnant women Singleton pregnancies Maternal age not reported 11‐13 weeks' gestation |
|
| Study design | Retrospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 26 cases Reference standards: invasive testing (offered to women with high NT measurement) or follow‐up to birth |
|
| Index and comparator tests | Maternal age First trimester nuchal translucency in 98% of patients (methods not specified. 60 sonographers ‐ 2 trained by Fetal Medicine Foundation, who trained 30 in turn. 8 received specific training in France, and 20 were self‐taught. Machines not specified) Frozen serum tested for: First trimester PAPP‐A (99% of patients), free ßhCG 99% of patients and AFP (93% of patients) (time‐resolved fluorescent assay, Perkin‐Elmer Life sciences) Risk cut‐point 1:250 |
|
| Follow‐up | Data from the French national screening programme used for follow‐up at birth. 211 women (3.7%) who did not return after NT or were found to be > 14 weeks were excluded. It is unclear how many patients had follow‐up to birth | |
| Aim of study | Prospective study of NT and retrospective evaluation of serum (in same patient population) to evaluate whether or not to move the national French Down's screening programme to a first trimester programme | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? All tests | Unclear | Unclear if all women received a reference standard |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | Yes | Women with NT too small to measure assumed to have NT of < 0.5 mm. |
| Withdrawals explained? All tests | Yes | Women failing to return or who more than 14 weeks pregnant were excluded (214). |
Nicolaides 1992.
| Clinical features and settings | High‐risk referral for invasive testing | |
| Participants | 827 participants UK ‐ research centre for fetal medicine January 1990 ‐ October 1991 Pregnant women Median age 38 years (22‐47 years) 10‐14 weeks' gestation |
|
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 13 cases Reference standards: fetal karyotyping by amniocentesis (52%) or CVS (48%) |
|
| Index and comparator tests | Maternal age First trimester NT (curvilinear 5MHz transducer, Aloka 650 CO Limited) |
|
| Follow‐up | 100% karyotyping | |
| Aim of study | To examine the significance of fetal NT at 10‐14 weeks' gestation in the prediction of abnormal fetal karyotype | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Selective testing of high‐risk women as done in practice |
| Acceptable reference standard? All tests | Yes | Amniocentesis or CVS |
| Partial verification avoided? All tests | Yes | All women had a reference standard |
| Differential verification avoided? All tests | No | Women had different reference standards |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? All tests | No | No details of withdrawals given |
Nicolaides 2005.
| Clinical features and settings | Routine screening | |
| Participants | 75,821 participants with available information on outcome UK ‐ Various hospitals and a fetal medicine centre June 1998 ‐ December 2003 Pregnant women Median age 31 years (13‐49 years) Singleton pregnancies 11‐13 weeks' gestation |
|
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 325 cases Reference standards: amniocentesis or CVS (patients considered high risk based on screening). First trimester presence/absence of nasal bone, presence/absence of tricuspid regurgitation or normal/abnormal Doppler studies (patients of intermediate risk on first trimester screening and did not undergo CVS or amniocentesis. With the addition of information from these tests, if adjusted risk was high, CVS was performed). Follow‐up to birth |
|
| Index and comparator tests | Maternal age First trimester NT (FMF methods) First trimester free ßhCG and PAPP‐A (Kryptor analyser, Brahms AG) Risk cut‐point 1:300 |
|
| Follow‐up | Follow‐up data from cytogenetics laboratories, patients, GPs or maternity units where they delivered. Patients without follow‐up information due to miscarriage or termination (n = 490) or loss to follow‐up (n = 2117) were excluded from the study. | |
| Aim of study | To evaluate the performance of first trimester screening for trisomy 21 by a combination of maternal age, fetal NT and maternal serum free ßhCG and PAPP‐A. In addition, the impact of a new individual risk orientated 2‐stage approach to first trimester screening was examined | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? All tests | Yes | All women received a reference standard |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? All tests | Yes | Exclusions due to loss to follow‐up and missing information for women with miscarriages or terminations of pregnancy explained |
Niemimaa 2001.
| Clinical features and settings | Routine screening | |
| Participants | 2515 participants Finland ‐ primary care centres and maternity clinics of hospitals During 1999 Pregnant women 17.5% aged ≥ 35 years 10‐13 weeks' gestation |
|
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 8 cases Reference standards: invasive testing (patients considered high risk based on NT screening) or follow‐up to birth |
|
| Index and comparator tests | Maternal age First trimester NT (≥ 3 mm) (64% of women) (method not described) Fresh serum tested for: First trimester free ßhCG and PAPP‐A (Wallac analytes and 1st trimester risk calculation programme maternal weight correction) Risk cut‐point 1:250 |
|
| Follow‐up | Follow‐up data from maternity clinics and the National Research and Development Centre for Welfare and Health. Test negative patients followed up by contacting all maternity clinics and the National Research and Development Centre for Welfare and Health. Unclear if follow‐up information was obtained in all cases | |
| Aim of study | To evaluate efficacy of combining first trimester maternal serum and fetal NT measurement in screening for Down's syndrome in Finland | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? All tests | Unclear | Unclear if all women received a reference standard |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? All tests | No | No details of withdrawals given |
Noble 1995.
| Clinical features and settings | Routine screening in a high‐risk population | |
| Participants | 2529 participants UK October 1994 to April 1995 Pregnant women Singleton pregnancies Median age 34 years (15‐47 years), 47% ≥ 35 years 10‐14 weeks' gestation |
|
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 61 cases Reference standards: karyotyping performed (27% of women) due to increased NT (14%), advanced maternal age (10%), previous chromosomally abnormal child (0.5%) or parental anxiety (2%). Ultrasound examination at 20 weeks (65% of patients). Follow‐up to birth (9% of women) |
|
| Index and comparator tests | Maternal age First trimester NT (methods not stated) Fresh serum (or serum frozen over a weekend) tested for: First trimester free ßhCG (immunoradiometric assay, CIS) |
|
| Follow‐up | Pregnancy outcome obtained from maternity units or the patients themselves. Follow‐up information only appears to have been obtained in 9% of cases (second trimester ultrasound used as reference standard for other women) | |
| Aim of study | To measure the contribution of maternal serum free beta hCG in a screening programme for fetal trisomy 21 based on fetal NT in the first trimester of pregnancy | |
| Notes | No proper results data are presented for this study | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? All tests | No | Invasive testing, ultrasound at 20 weeks or follow‐up to birth |
| Partial verification avoided? All tests | Unclear | Unclear if all women received a reference standard |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? All tests | No | No details of withdrawals given |
O'Callaghan 2000.
| Clinical features and settings | Routine screening | |
| Participants | 1000 participants Australia ‐ public and private sector venues September 1997 to September 1999 Pregnant women Singleton or multifetal pregnancies (2000 fetuses including 25 sets of dichorionic twins, 7 sets of monochorionic twins and 4 sets of triplets but the numbers amongst the 1000 fetuses reported in the paper were not stated) Median age 32 years 11‐14 weeks' gestation |
|
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down’s syndrome: 8 cases Reference standards: CVS, amniocentesis, neonatal karyotyping or follow‐up to birth |
|
| Index and comparator tests | Maternal age NT (FMF methods) |
|
| Follow‐up | Follow‐up from cytogenetics laboratory records but the completeness of follow‐up is not reported | |
| Aim of study | To evaluate a risk assessment tool based on first trimester NT | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? All tests | Unclear | Unclear if all women had a reference standard |
| Differential verification avoided? All tests | Unclear | Unclear if the choice of reference standard depended on index test results |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? All tests | No | No details of withdrawals given |
O'Leary 2006.
| Clinical features and settings | Routine screening | |
| Participants | 22,340 participants Australia ‐ 13 ultrasound practices August 2001 to October 2003 Singleton pregnancies Pregnant women Median age 31 years (14‐47 years), 20% ≥ 35 years 11‐13 weeks' gestation |
|
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 60 cases Reference standards: CVS or amniocentesis (women assessed to be high risk on screening) or follow‐up to birth |
|
| Index and comparator tests | Maternal age First trimester NT (FMF methods) First trimester free ßhCG and PAPP‐A (machine not stated) All study participants underwent all tests Risk cut‐point 1:300 |
|
| Follow‐up | Follow‐up data obtained by review of the Midwives Notification System and the Birth Defects Registry. 415 patients (1.8%) excluded due to no follow‐up data. Patients with multiple pregnancies or incomplete screens (n = 3946) were also excluded from the study | |
| Aim of study | To assess fetal outcomes for pregnancies identified at increase risk for Down's syndrome by first trimester combined ultrasound examination and maternal serum biochemistry | |
| Notes | Appears likely that patients with miscarriages and terminations excluded | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? All tests | Yes | All women received a reference standard |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? All tests | Yes | Details given of patients excluded due to incomplete screening data or loss to follow‐up |
Okun 2008 FTS.
| Clinical features and settings | Routine screening | |
| Participants | 14,487 participants undergoing first trimester screening (a separate cohort of 30,792 pregnancies were evaluated for integrated screening) November 2002 ‐ December 2005 Canada ‐ 2 hospitals Pregnant women Singleton pregnancies Mean maternal age 34 years 11‐14 weeks' gestation |
|
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 62 cases Reference standards: karyotyping or follow‐up to birth |
|
| Index and comparator tests | Maternal age First trimester NT (most sonographers had FMF certification) First trimester free ßhCG and PAPP‐A (DSX Four Plate Automated ELISA Processing system, Dynex Technologies and DPC Immulite 2000 automated immunoassay analyser, Siemens Medical Solutions Diagnostics) Risk cut‐point 1:200 or NT ≥ 3.5 mm Results presented with and without adjustment for bias due to miscarriages (viability bias) |
|
| Follow‐up | From cytogenetics databases in both Hospitals, the Canadian Institute for Health Information, labour and delivery databases, written and phone follow‐up with care providers and phone follow‐up with women after birth | |
| Aim of study | To evaluate the performance of integrated prenatal screening and first trimester combined screening for trisomy 21 in a large Canadian urban centre | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? All tests | Unclear | Unclear if all women received a reference standard |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? All tests | No | No details of withdrawals given |
Orlandi 1997.
| Clinical features and settings | Routine screening of general and high‐risk women | |
| Participants | 2010 participants (744 in subgroup undergoing NT testing) Italy Dates not reported Recruited through private physician or genetic counselling program for women of advanced maternal age Pregnant women Aged 15‐46 years, 35% ≥ 35 years Singleton pregnancies 9‐13 weeks' gestation |
|
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 11 cases (7 in subgroup with NT testing) Reference standards: not reported |
|
| Index and comparator tests | Maternal age First trimester NT (37% of patients) (FMF methods, Toshiba SSA 250A or Acuson XP 10) First trimester free ßhCG and PAPP‐A (all patients) (dried blood samples, enzyme‐linked immunosorbent assays) Risk cut‐point 1:380 |
|
| Follow‐up | Not reported | |
| Aim of study | To evaluate first trimester combined screening for Down's syndrome | |
| Notes | Unclear as to what reference standard (if any) was used. All cases of Down's syndrome identified had been picked up by screening | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? All tests | Unclear | Reference standard not reported |
| Partial verification avoided? All tests | Unclear | Unclear if all women received a reference standard |
| Differential verification avoided? All tests | Unclear | Unclear if the choice of reference standard depended on screening results |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? All tests | Yes | Details given of women undergoing NT but not biochemical testing |
Orlandi 2003.
| Clinical features and settings | Routine screening (2 centres) or in referred patients (1 centre) | |
| Participants | 1089 participants undergoing fetal nasal bone assessment Italy/The Netherlands ‐ 3 centres February 2002 to April 2002 Pregnant women Singleton pregnancies Median age 31.7 years (SD 4.0) in unaffected cases and 36.5 years (SD 4.1) in affected cases 11‐14 weeks' gestation |
|
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 15 cases Reference standards: CVS or amniocentesis (women considered high risk on screening on the basis of NT and biochemical results, but not on nasal bone screening, or if requested due to age or anxiety), or follow‐up to birth |
|
| Index and comparator tests | Maternal age First trimester nasal bone assessment First trimester NT First trimester free ßhCG First trimester PAPP‐A |
|
| Follow‐up | Reported that karyotyping was performed postnatally. It is unclear in which cases this was conducted | |
| Aim of study | To assess the feasibility of measuring nasal bone length in first trimester pregnancy and to confirm if the absence of fetal nasal bone is a marker for Down's syndrome | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? All tests | Unclear | Unclear if all women received a reference standard |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | Yes | Nasal bone assessment was successfully conducted in 94.3% of women |
| Withdrawals explained? All tests | No | No details of withdrawals given |
Orlandi 2005.
| Clinical features and settings | Routine screening | |
| Participants | 2411 participants Italy Dates not reported Pregnant women Median age 30.5 years (SD 8.2) First trimester (gestational weeks not reported) |
|
| Study design | Retrospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 15 cases Reference standard: not reported |
|
| Index and comparator tests | Maternal age First trimester nasal bone assessment (FMF methods) First trimester NT, free ßhCG and PAPP‐A Data from other studies used to generate statistical parameters to estimate performance of first trimester screening with and without nasal bone evaluation) Risk cut‐point 1:250 |
|
| Follow‐up | No details reported for any follow‐up to birth | |
| Aim of study | To determine the benefit of including nasal bone assessment in addition to standard first trimester markers as a screening test for Down's syndrome | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? All tests | Unclear | Unclear if all women received a reference standard |
| Differential verification avoided? All tests | Unclear | Unclear if the choice of reference standard depended on screening results |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | Unclear | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? All tests | No | No details of withdrawals given |
Otaño 2002.
| Clinical features and settings | High‐risk referral for invasive testing | |
| Participants | 194 participants Argentina October 2001 ‐ January 2002 Pregnant women Median age 36 years (19‐44 years) Singleton pregnancies 11‐14 weeks' gestation |
|
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 5 cases Reference standard: CVS |
|
| Index and comparator tests | First trimester nasal bone assessment (frontal saggital section of the fetal face. Angle of insonation of fetal nose close to 90 degree angle) | |
| Follow‐up | 100% karyotyping | |
| Aim of study | To evaluate the association of nasal bone on ultrasound and Down's syndrome fetuses at 11‐14 weeks' gestation | |
| Notes | States in text that there were 6 cases of trisomy 21 | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Selective testing of high‐risk women as done in practice |
| Acceptable reference standard? All tests | Yes | CVS |
| Partial verification avoided? All tests | Yes | All women received a reference standard |
| Differential verification avoided? All tests | Yes | All women had the same reference standard |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | Unclear | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | Yes | Unsuccessful nasal bone assessment in 6% |
| Withdrawals explained? All tests | No | No details of withdrawals given |
Pajkrt 1998.
| Clinical features and settings | Routine screening | |
| Participants | 1473 participants The Netherlands tertiary maternity unit June 1994 to March 1997 Pregnant women Mean age 31.4 years (SD 5.7), 24% ≥ 35 years Singleton pregnancies 10‐14 weeks' gestation |
|
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 9 cases Reference standards: prenatal karyotyping offered to patients considered high risk or maternal anxiety (conducted in 24%) or follow‐up to birth |
|
| Index and comparator tests | Maternal age NT (FMF method, Hitachi machines, 6 sonographers instructed to take 'sufficient time') Risk cut‐point ≥ 3 mm |
|
| Follow‐up | Follow‐up to outcome assessment in the delivery room. 68 women (4.4%) were excluded from the study due to loss to follow‐up | |
| Aim of study | To evaluate the effectiveness of NT measurement in the detection of trisomy 21 in a low‐risk population | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? All tests | Yes | All women received a reference standard |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | Unclear | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | Yes | Unsuccessful NT measurement in 4.3% |
| Withdrawals explained? All tests | No | No details of withdrawals given |
Pajkrt 1998a.
| Clinical features and settings | High‐risk referral for invasive testing | |
| Participants | 2247 participants undergoing NT and fetal karyotyping The Netherlands ‐ prenatal diagnostic centre February 1994 to July 1997 Singleton pregnancies Pregnant women Mean age 37.6 years (22‐46 years) 10‐14 weeks' gestation |
|
| Study design | Consecutive cohort | |
| Target condition and reference standard(s) | Down's syndrome: 36 cases Reference standard: prenatal karyotyping |
|
| Index and comparator tests | Maternal age FT NT (maximal saggital thickness of NT, corrected for gestational age) |
|
| Follow‐up | 100% karyotyping | |
| Aim of study | To examine the discriminatory capacity of NT measurement in the detection of trisomy 21 and other chromosomal anomalies | |
| Notes | No follow‐up information on 12 miscarriages | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Selective testing of high‐risk women as done in practice |
| Acceptable reference standard? All tests | Yes | Karyotyping |
| Partial verification avoided? All tests | Yes | All women received a reference standard |
| Differential verification avoided? All tests | Yes | All women had the same reference standard |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | Yes | Unsuccessful NT measurement in 2.4% |
| Withdrawals explained? All tests | Yes | Patients excluded due to sonographically detected fetal abnormalities at NT measurement, no karyotyping or miscarriages |
Palomaki 2007 FTS.
| Clinical features and settings | Routine screening | |
| Participants | 10,775 participants Canada ‐ General Hospital October 2003 ‐ November 2004 Pregnant women Mean maternal age 32.3 years (SD 4.6 years) 10‐13 weeks' gestation |
|
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 23 cases Reference standards: karyotyping or follow‐up to birth |
|
| Index and comparator tests | Maternal age FT NT (encouraged to only accept measurements from sonographers with FMF certification) FT PAPP‐A (AutoDELFIA, PerkinElmer) FT hyperglycosylated‐hCG (Nichols Advantage Specialty system, Nochols Institute Diagnosics) |
|
| Follow‐up | From electronic record searches of local patient and cytogenetic records and case finding of local and regional birth records | |
| Aim of study | To validate Down's syndrome screening protocols that include hyperglycosylated‐hCG measurements | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? All tests | Unclear | Unclear if all women received a reference standard |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? All tests | No | No details of withdrawals given |
Perni 2006.
| Clinical features and settings | Routine screening | |
| Participants | 4615 participants USA ‐ single institution January 2003 to September 2004 Pregnant women Singleton pregnancies Mean age 33.0 years (IQR 31.0‐36.0) 10‐13 weeks' gestation |
|
| Study design | Retrospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 22 cases Reference standards: CVS or amniocentesis. Cytogenetic testing in cases of miscarriage. Follow‐up to birth. |
|
| Index and comparator tests | Maternal First trimester NT (FMF methods) First trimester PAPP‐A and free ßhCG (dried blood spots, methodology described elsewhere) |
|
| Follow‐up | Outcome information from computerised medical record review. Numbers of patients lost to follow‐up not reported | |
| Aim of study | To evaluate the performance of maternal age, fetal NT, PAPP‐A and free ßhCG for aneuploidy screening | |
| Notes | Appears that all cases of Down’s were diagnosed prenatally by karyotyping | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? All tests | Unclear | Unclear if all women received a reference standard |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? All tests | No | No details of withdrawals given |
Prefumo 2005.
| Clinical features and settings | High‐risk referral for invasive testing | |
| Participants | 544 participants UK ‐ tertiary referral fetal medicine unit December 2001 to November 2003 Pregnant women Median age 37 years (19‐46 years) Singleton pregnancies 11‐14 weeks' gestation |
|
| Study design | Prospective cohort study | |
| Target condition and reference standard(s) | Down’s syndrome: 47 cases Reference standard: CVS |
|
| Index and comparator tests | Maternal age First trimester NT (methods not reported), risk cut‐point 1:300 First trimester nasal bone examination (mid‐sagittal view with beam of the ultrasound transducer being parallel to the nasal bones, previously described) First trimester ductus venous flow (abnormal defined as absent or reversed flow. Angle of insonation < 30 degrees. 3 minutes allotted time. NB previously described) |
|
| Follow‐up | 100% karyotyping | |
| Aim of study | To assess the role of fetal ductus venous and nasal bone evaluation in first trimester screening for Down’s syndrome | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Selective testing of high‐risk women as done in practice |
| Acceptable reference standard? All tests | Yes | CVS |
| Partial verification avoided? All tests | Yes | All women received a reference standard |
| Differential verification avoided? All tests | Yes | All women had the same reference standard |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | Yes | Not possible to satisfactorily assess ductus venous flow in 4 cases (0.6%) and nasal bones in 52 cases (8.3%) |
| Withdrawals explained? All tests | Yes | 158 patients not included in the study due to time restrictions or due to the patient declining taking part |
Prefumo 2006.
| Clinical features and settings | Routine screening | |
| Participants | 7116 participants UK ‐ single institution December 2001 to November 2003 Pregnant women Singleton pregnancies Mean age 31.4 years (14.5‐50.2 years) 10‐14 weeks' gestation |
|
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 12 cases Reference standards: karyotyping or follow‐up to birth |
|
| Index and comparator tests | Maternal age First trimester NT (all patients) (mid‐sagittal view) First trimester nasal bone assessment |
|
| Follow‐up | Outcome information from computerised hospital records. Results cross‐matched with the registry of the Regional Genetics Service. No report of how many patients lost to follow‐up | |
| Aim of study | To assess the role of fetal nasal bone evaluation in first trimester screening for trisomy 21 in selected and unselected pregnancies | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? All tests | Unclear | Unclear if all women received a reference standard |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | Yes | Nasal bones could not be satisfactorily assessed in 9.9% of fetuses |
| Withdrawals explained? All tests | No | No details of withdrawals given |
Ramos‐Corpas 2006.
| Clinical features and settings | Routine screening | |
| Participants | 1800 participants Spain ‐ hospital fetal medicine department June 2003 to April 2004 Pregnant women Singleton pregnancies Mean age 30.1 years (15‐46 years) (SD 5.37), 18% ≥ 35 years First trimester (before week 14) |
|
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down’s syndrome: 7 cases Reference standards: invasive testing offered to patients considered high risk at screening (> 1:300) or follow‐up to birth |
|
| Index and comparator tests | Maternal age First trimester NT (FMF method (Accuson XP10, Mountain View, California) Maximum allotted time of 20 minutes) First trimester nasal bone assessment (in 93.4% of patients) Risk cut‐point 1:300 PAPP‐A and free ßhCG (Delfia Xpress 6000 immunoanalyzer, Perkin Elmer) ‐ not used in study Published population parameters used (Wald 2003) |
|
| Follow‐up | Follow‐up in all patients without invasive testing by 1) monitoring all births and miscarriages at the hospital, 2) continued contact with the genetics departments and 3) telephone follow‐up. States in abstract that only fetuses with complete follow‐up results included in the study | |
| Aim of study | To evaluate the utility of determining the presence or absence of nasal bone in a low‐risk fetal population | |
| Notes | 5 cases diagnosed by invasive testing, 2 by follow‐up | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? All tests | Yes | All women received a reference standard |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | Yes | Nasal bones could not be satisfactorily assessed in 6.6% of fetuses |
| Withdrawals explained? All tests | No | No details of withdrawals given |
Rissanen 2007.
| Clinical features and settings | Routine screening | |
| Participants | 4776 participants undergoing NT and/or biochemical screening Finland ‐ hospitals or health care centres 1999 ‐ 2000 Pregnant women Singleton pregnancies Mean maternal age 29.5 years, 17.7% ≥ 35 years 10‐13 weeks' gestation |
|
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 13 cases Reference standards: karyotyping or follow‐up to birth |
|
| Index and comparator tests | Maternal age First trimester NT (Trained personnel) First trimester PAPP‐A and free ßhCG (AutoDelfia kits, PerkinElmer) Risk cut‐point 1:250 |
|
| Follow‐up | Outcomes obtained from all maternity clinics, the Finnish Register of Congenital Malformations and the National Research and Development Centre for Welfare and Health. Follow‐up was complete in 99% of live‐born infants. Data on miscarriages (n = 68) received from the National Research and Development Centre for Welfare and Health | |
| Aim of study | To evaluate whether first trimester screening markers are altered in pregnancies affected both by other chromosomal defects than trisomy 21 and structural anomalies and whether it is possible to detect these pregnancies by combined ultrasound and biochemical screening | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? All tests | Yes | All women received a reference standard |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? All tests | No | No details of withdrawals given |
Rozenberg 2002.
| Clinical features and settings | Routine screening | |
| Participants | 9118 participants France ‐ 2 tertiary and 4 primary referral centres March 1994 to December 1997 Pregnant women Median age 30.5 years (18‐37 years) Singleton pregnancies 12‐14 and 14‐17 weeks' gestation |
|
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down’s syndrome: 21 cases Reference standards: amniocentesis offered to patients with NT > 3 mm or serum marker risk was > 1:250, or follow‐up to birth |
|
| Index and comparator tests | Maternal age First trimester NT in 98.6% of women (FMF methods) Second trimester free ßhCG (beta hCG ELISA immunoradiometric assay) and AFP (AFP ELISA immunoradiometric assay) in 91.1% of women Both NT and biochemical testing in 60.4% of women |
|
| Follow‐up | Details of the method of follow‐up not given. 3.4% of patients were lost to follow‐up and were excluded from the study. This included 113 women (1.2%) with miscarriages | |
| Aim of study | To assess the performance of combined first trimester sonographic screening and second trimester serum screening | |
| Notes | Includes cost‐effectiveness analysis | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? All tests | Yes | All women had a reference standard |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | Yes | NT was not able to be measured in 93 women (1.5%) |
| Withdrawals explained? All tests | No | No details of withdrawals given |
Rozenberg 2007.
| Clinical features and settings | Routine screening | |
| Participants | 14,934 participants Canada ‐ multicentre study Pregnant women Singleton pregnancies Mean maternal age 30.9 (SD 4.5) years 11‐13 weeks' gestation |
|
| Study design | Prospective cohort study | |
| Target condition and reference standard(s) | Down's syndrome: 51 cases Reference standards: karyotyping or follow‐up to birth |
|
| Index and comparator tests | Maternal age First trimester NT (trained assessors following protocol) First trimester PAPP‐A and free ßhCG (PerkinElmer Life Sciences) Second trimester ultrasound and/or serum markers (free ßhCG and AFP or total hCG, AFP and uE3) performed in some cases Risk cut‐point 1:250 |
|
| Follow‐up | Notebooks in maternity hospitals used to record information on patient characteristics, screening and outcome at birth. Data obtained from cytogenetic laboratories and DASDY database (contains results of birth examinations). Letters sent to women with missing outcome information and, after 3 months, if there was no response, they were contacted by telephone | |
| Aim of study | To evaluate the performance, acceptability and cost‐effectiveness ratio of a pragmatic approach to screening for Down's syndrome based on the combined first trimester test supplemented by routine ultrasound at 20‐22 weeks in the general population | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? All tests | Unclear | Unclear if all women received a reference standard |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? All tests | Yes | 554 women (3.7%) did not undergo screening |
Sahota 2010.
| Clinical features and settings | Routine screening | |
| Participants | 10,854 pregnancies with complete outcome data China ‐ University Hospital January 2005 ‐ May 2008 Pregnant women Singleton pregnancies Median maternal age 33.1 years, 30.1% of women aged ≥ 35 years 10‐13 weeks' gestation |
|
| Study design | Retrospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 32 cases Reference standards: karyotyping or follow‐up to birth |
|
| Index and comparator tests | Maternal age First trimester NT (FMF accredited sonographers, HDI 5000, Philips Medical System) First trimester PAPP‐A and free ßhCG (kryptor analyser, Brahms Diagnostica GmbH) Contingent screening strategies
Intermediate risk cut‐points 1:50 to 1:1000 |
|
| Follow‐up | Fetal karyotypes entered into database when available. Data on pregnancy outcomes obtained either from local maternity database for those who delivered in the unit or via telephone calls to patients | |
| Aim of study | To assess the relative performance of a multi‐stage first trimester screening protocol for fetal Down's syndrome | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? All tests | Yes | All women received a reference standard |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? All tests | No | No details of withdrawals given |
Salomon 2010.
| Clinical features and settings | Routine screening | |
| Participants | 21,492 participants France ‐ Single Health Authority district January 2001 ‐ December 2003 Pregnant women Median maternal age 30.7 years (18.0‐46.3 years) 11‐13 weeks' gestation |
|
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 80 cases Reference standards: karyotyping or follow‐up to birth |
|
| Index and comparator tests | Maternal age First trimester NT (sonographers trained to FMF standards) First trimester PAPP‐A and free ßhCG (time resolved fluorescent assay, PerkinElmer Life Sciences) Routine abnormality scan for structural malformations (20‐24 weeks) Femur length at routine abnormality scan |
|
| Follow‐up | Case report forms completed by attending obstetrician or midwife throughout pregnancy and delivery. Databases of certified laboratories cross‐checked with delivery and outcome data in all maternity units, the databases of all cytogenetic laboratories, the database of the health authority (DASDY), contact with women by mail 3 months after expected delivery and direct telephone with women. | |
| Aim of study | To evaluate the performance of the contingent use of femur length at routine mid‐trimester scan in screening for Down's syndrome in women having previously undergone first trimester screening with disclosure of risk estimates | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? All tests | Unclear | Unclear if all women received a reference standard |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? All tests | No | No details of withdrawals given |
Santiago 2007.
| Clinical features and settings | Routine screening | |
| Participants | 4248 participants Spain ‐ Screening database managed by the Fetaltest project To December 2005 Pregnant women Singleton pregnancies Mean maternal age 30.6 years (14‐46 years) 11‐13 weeks' gestation |
|
| Study design | Retrospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 13 cases Reference standards: karyotyping or follow‐up to birth |
|
| Index and comparator tests | Maternal age First trimester NT (trained sonographers) First trimester PAPP‐A and free ßhCG (details not reported) Cut‐point 1:300 and detection rate at 5% FPR |
|
| Follow‐up | Follow‐up by supervising the live births and miscarriages at the Hospital together with continuous contact with the genetics department. 24 pregnancies ended in miscarriage and were lost to follow‐up. In 269 women not giving birth at that Hospital, only those karyotyped were followed up. In total, 287 women (6.8%) were lost to follow‐up | |
| Aim of study | To determine whether delta‐NT could be extrapolated successfully from 1 centre‐specific NT reference curve to another and thus to empirically calculate the likelihood ratios of delta‐NT | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? All tests | Unclear | Unclear if all women received a reference standard |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? All tests | No | No details of withdrawals given |
Sau 2001.
| Clinical features and settings | Routine screening | |
| Participants | 3185 participants UK ‐ single hospital November 1996 to November 1998 Pregnant women Mean age 28 years (SD 5) 11‐14 and 6‐20 weeks' gestation |
|
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down’s syndrome: 8 cases Reference standards: invasive testing (women with high risk on screening) or follow‐up to birth |
|
| Index and comparator tests | Maternal age First trimester NT (FMF methods, transabdominal route) in 84% of women. NT risk cut‐point of 1:100 or if NT measurement > 95th centile for that particular CRL considered screen positive. Confirmatory NT test conducted in all women positive on first NT screening Second trimester AFP, ßhCG and uE3 in 49% of women. Serum risk cut‐point 1:250 |
|
| Follow‐up | Follow‐up from computerised maternity records, the neonatal database and the hospital termination of pregnancy and miscarriage record books | |
| Aim of study | To present data on the performance of biochemical screening in a population with a prior low‐risk screening result | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? All tests | Yes | All women received a reference standard |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | Yes | In 122 (4.3%) of women, a second NT scan was needed since the first 1 failed to obtain a measurement |
| Withdrawals explained? All tests | Yes | Of 3704 women booked for hospital delivery, 3185 had at least 1 screening test and were included in the study |
Schaelike 2009.
| Clinical features and settings | Routine screening | |
| Participants | 10,668 participants with complete outcome data Germany ‐ private centre November 2000 ‐ December 2006 Pregnant women Singleton pregnancies Maternal age ≥ 35 years in 31.0% of women 11‐13 weeks' gestation |
|
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 59 cases Reference standards: karyotyping or follow‐up to birth |
|
| Index and comparator tests | Maternal age First trimester NT (FMF certified physicians) First trimester PAPP‐A and free ßhCG (Kryptor analyser, Brahms GmbH) Cut‐point 1:300 |
|
| Follow‐up | Information provided by either obstetric departments or obstetricians. Results from CVS and amniocentesis, as well as karyotypes from aborted fetal tissue or from postnatal investigations were used. 3.9% of women were lost to follow‐up and were excluded from the study | |
| Aim of study | To assess the performance of a combined first trimester screening concept for trisomies 21, 18 and 13 applied to a low‐ and high‐risk patient sample in a specialised private centre for prenatal medicine | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? All tests | Yes | All women received a reference standard |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? All tests | No | No details of withdrawals given |
Schielen 2006.
| Clinical features and settings | Routine screening | |
| Participants | 4033 participants The Netherlands ‐ multicentre (44 centres) study July 2002 to May 2004 Singleton pregnancies Pregnant women aged 18‐47 years (median 36.5 years) 10‐14 weeks' gestation |
|
| Study design | Retrospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 21 cases Reference standards: invasive testing or follow‐up to birth |
|
| Index and comparator tests | Maternal age First trimester NT (FMF methods) First trimester free ßhCG and PAPP‐A (AutoDELFIA analyser) |
|
| Follow‐up | Women were asked to fill in a questionnaire about outcome of pregnancy. A second request was sent by mail if necessary 784 patients were lost to follow‐up (16.2%) and were excluded from the study |
|
| Aim of study | To report the results of a first trimester combined‐test screening programme in a multicentre routine clinical setting | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? All tests | Yes | All women received a reference standard |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? All tests | No | No details of withdrawals given |
Schuchter 2001.
| Clinical features and settings | Routine screening | |
| Participants | 9342 participants Austria ‐ single institution January 1994 to December 1998 Pregnant women Singleton pregnancies Mean age 28 years (15‐46 years), 10.7% ≥ 35 years 10‐13 weeks' gestation |
|
| Study design | Retrospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 19 cases Reference standards: CVS (offered to patients with first trimester NT > 3.5 mm), amniocentesis (offered to patients with first trimester NT 2.5‐3.4 mm, high risk on second trimester serum testing (> 1:250) and those > 35 years) or follow‐up to birth |
|
| Index and comparator tests | Maternal age First trimester NT (5‐MHz transducer, Acuson Corp) Second trimester AFP, uE3 and hGC (triple test) offered to patients not undergoing first trimester invasive testing (99.7% of women) (AMERLEX‐M 2nd Trimester kits, Ortho Clinical Diagnostics) |
|
| Follow‐up | Patients included in study if they were delivered in the same hospital where they were screened. All newborns were examined for malformations by a paediatrician after delivery. | |
| Aim of study | To evaluate screening for trisomy 21 in a low‐risk population utilising a combination of NT measurement in the first trimester and the triple test in the second trimester | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? All tests | Yes | All women received a reference standard |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? All tests | No | No details of withdrawals given |
Schuchter 2002.
| Clinical features and settings | Routine screening | |
| Participants | 4802 participants Austria ‐ single institution December 1997 to April 2000 Singleton pregnancies Pregnant women 13.0% > 35 years 10‐12 weeks' gestation |
|
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 14 cases Reference standards: CVS and amniocentesis (offered to patients with increased risk (> 1:400) at first trimester screening. CVS recommended when NT > 3.5 or when women did not want to wait until the 15th week for amniocentesis), or follow‐up to birth |
|
| Index and comparator tests | Maternal age First trimester NT (transabdominal transducer, 5‐MHz curvilinear Transducer, Acuson, Mountain View), cut‐point 2.5 mm First trimester PAPP‐A and free ßhCG (done radioimmunologically, kits by Ortho Clinical Diagnostics) Combined risk cut‐point 1:250 |
|
| Follow‐up | Patients without follow‐up information (n = 92, 2%) were excluded from the study. 27 women with spontaneous abortions were also excluded from the study | |
| Aim of study | To determine the detection rate of the combined test, NT alone and maternal age alone in a non‐selected population at a false positive rate of about 5% | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? All tests | Yes | All patients received a reference standard |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? All tests | Yes | Women not attending visits were excluded from the study |
Schwarzler 1999.
| Clinical features and settings | Routine screening | |
| Participants | 4523 participants UK ‐ single institution July 1996 to November 1997 Pregnant women Mean age 29.4 years (16‐47 years) 10‐14 weeks' gestation |
|
| Study design | Prospective consecutive cohort | |
| Target condition and reference standard(s) | Down's syndrome: 12 cases Reference standards: invasive testing (women considered high risk on screening) or follow‐up to birth |
|
| Index and comparator tests | Maternal age NT (Sagittal plane by transabdominal (92.7%) and transvaginal (7.3%) sonography) Adjusted risk cut‐point 1:270 |
|
| Follow‐up | Pregnancy outcome obtained via questionnaires, examination by neonatologist and outcome cross‐referenced with regional cytogenetics registry. 26 test‐negative patients lost to follow‐up and excluded from the study | |
| Aim of study | To evaluate first trimester pregnancy screening for fetal aneuploidy and congenital heart defects by maternal age and NT measurement in an unselected population | |
| Notes | 3 live births, 9 termination of pregnancy | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? All tests | Yes | All women received a reference standard |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? All tests | No | No details of withdrawals given |
Scott 2004.
| Clinical features and settings | Routine screening | |
| Participants | 2053 participants Australia ‐ private practice (Sydney Ultrasound for Women) July 2000 to May 2002 Pregnant women Median age 32 years (15‐44 years), 29% ≥ 35 years Singleton pregnancies 11‐14 weeks' gestation |
|
| Study design | Prospective cohort study | |
| Target condition and reference standard(s) | Down’s syndrome: 5 affected cases Reference standards: invasive testing or follow‐up to birth |
|
| Index and comparator tests | Maternal age First trimester NT (FMF methods, sagittal plane, ATL 5000; Philips) First trimester free ßhCG and PAPP‐A (kryptor analyser, Brahms Diagnostics) All participants had all tests Risk cut‐point 1:300 |
|
| Follow‐up | Data obtained from referring doctors or patients via letter, phone or completed feedback form given at the time of consultation. Only cases of known outcome included in the study. 68 (1.3%) lost to follow‐up, largely due to miscarriage (n = 20) and loss to follow‐up (n = 40). | |
| Aim of study | To report the sensitivity of combined first trimester biochemistry and ultrasound screening for Down's syndrome in an Australian private practice specialising in obstetric ultrasound | |
| Notes | Only women having biochemical testing before NT were included in the study. This was done to avoid bias from women declining biochemical testing following negative NT. | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? All tests | Yes | All women received a reference standard |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? All tests | No | No details of withdrawals given |
Sepulveda 2007.
| Clinical features and settings | Routine screening | |
| Participants | 1287 participants Chile ‐ fetal medicine centre January 2003 ‐ January 2006 Pregnant women Median maternal age 33 years (range 14‐47 years), 35.4% ≥ 35 years Singleton pregnancies 11‐14 weeks' gestation |
|
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 31 cases Reference standards: CVS, amniocentesis, cordocentesis or follow‐up to birth |
|
| Index and comparator tests | Maternal age First trimester NT and nasal bone assessment (Accuvix XQ, Medison or Voluson 730, GE Healthcare) (only included in study if scanned by 1 of 2 fetal medicine specialists following FMF guidelines) |
|
| Follow‐up | Cases of chromosomal abnormality were identified from the cytogenetics laboratory logbook, which recorded all the cytogenetic studies performed prenatally, after a spontaneous abortion or fetal death, or in neonates with physical abnormalities. Information from the remaining cases was obtained from the delivery records and neonatal discharge summaries, which recorded the condition of the neonate at birth and the physical examination performed by a neonatologist | |
| Aim of study | To report their experience with first trimester screening for trisomy 21 by using the combination of NT thickness and nasal bone assessment | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? All tests | Unclear | Unclear if all women received a reference standard |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? All tests | No | No details of withdrawals given |
Snijders 1998.
| Clinical features and settings | Routine screening | |
| Participants | 96,127 participants UK ‐ multicentre study (22 centres) Women due to deliver before June 1997 Pregnant women Median age 31 years (14‐49 years) Singleton pregnancies 10 to 14 weeks' gestation |
|
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 326 cases Reference standards: CVS and amniocentesis (9.6% of women) or follow‐up to birth |
|
| Index and comparator tests | Maternal age First trimester NT (sagittal section) Risk cut‐point 1:300 |
|
| Follow‐up | Each women given a request form to complete about the outcome of pregnancy. 4184 women (4.2%) were excluded due to loss to follow‐up or due to miscarriages that were not karyotyped | |
| Aim of study | To investigate the assessment of risk by a combination of maternal age and fetal NT thickness, measured by ultrasonography at 10‐14 weeks of gestation | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? All tests | Yes | All women received a reference standard |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? All tests | No | No details of withdrawals given |
Sorensen 2011.
| Clinical features and settings | Routine screening | |
| Participants | 19,694 participants Denmark ‐ 2 centres July 2005 ‐ June 2007 Pregnant women Singleton pregnancies Maternal age: healthy mean age 30.4 years (16‐45 years), 16.5% ≥ 35 years, Down's syndrome median age 34 years (23‐44 years) 8‐13 weeks' gestation |
|
| Study design | Retrospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 100 cases Reference standards: karyotyping or follow‐up to birth |
|
| Index and comparator tests | Maternal age First trimester NT (FMF certified sonographers) First trimester PAPP‐A and free ßhCG (TRACE technology, Kryptor instrument, Brahms AG) |
|
| Follow‐up | Details not reported. It was stated that, for non‐Down's syndrome pregnancies, only those with known healthy fetus were included | |
| Aim of study | To develop 2 alternative risk calculation programmes to assess whether the screening efficacies for T13, T18 and T21 could be improved by using our locally estimated medians | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? All tests | Unclear | Unclear if all women received a reference standard |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? All tests | No | No details of withdrawals given |
Spencer 1999.
| Clinical features and settings | Women referred for invasive testing or self‐referred for screening | |
| Participants | 1156 participants: 210 cases and 946 controls matched for gestational and maternal age UK ‐ fetal medicine research centre Dates not specified Pregnant women Median maternal age 38 years (19‐46 years) (cases) and 36 years (15‐47 years) (controls) 10‐14 weeks' gestation |
|
| Study design | Case‐control study | |
| Target condition and reference standard(s) | Down's syndrome: 210 cases Reference standards: invasive testing (high‐risk women) or follow‐up to birth |
|
| Index and comparator tests | Maternal age First trimester NT (methods not reported) Frozen serum samples tested for: First trimester free ßhCG and PAPP‐A (Kryptor analyser, time resolved amplified cryptate emission (TRACE)) |
|
| Follow‐up | Stated that pregnancy outcome was ascertained in all women | |
| Aim of study | To examine the potential impact of combining maternal age with fetal NT thickness and maternal serum free ßhCG and PAPP‐A in screening for trisomy 21 at 10‐14 weeks of gestation | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Selective testing of high‐risk women as done in practice |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? All tests | Yes | All women received a reference standard |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Unclear | Unclear if all index tests interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? All tests | No | No details of withdrawals given |
Spencer 2002.
| Clinical features and settings | Routine screening | |
| Participants | 278 participants: 54 cases and 224 controls (no details of how selected) UK ‐ OSCAR screening program Samples collected since 1998 Pregnant women Median maternal age 36 years (20‐44 years) (cases) and 30 years (16‐41 years) (controls) 11‐13 weeks' gestation |
|
| Study design | Case‐control study | |
| Target condition and reference standard(s) | Down's syndrome: 54 cases Reference standards: details not reported |
|
| Index and comparator tests | Maternal age First trimester NT (FMF methods) Frozen serum samples tested for: First trimester free ßhCG, PAPP‐A and ThCG (Kryptor Analyser (TRACE) and automated immunofluorescent assays) |
|
| Follow‐up | Methods for follow‐up to birth not reported | |
| Aim of study | To assess serum hyperglycosylated hCG for use in the first trimester of pregnancy as a marker of Down's syndrome | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth (Nicolaides 2005(OSCAR screening program)) |
| Partial verification avoided? All tests | Yes | All women received a reference standard |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Unclear | Unclear of all index tests interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? All tests | No | No details of withdrawals given |
Spencer 2008.
| Clinical features and settings | Routine screening | |
| Participants | 622 participants: 55 cases and 567 controls matched for gestational age Denmark ‐ screening programme Dates not reported Pregnant women Median maternal age cases 35.8 years, controls 29.3 years 8‐13 weeks' gestation (results modelled on only cases where testing conducted before 10 weeks' gestation) |
|
| Study design | Case‐control study | |
| Target condition and reference standard(s) | Down's syndrome: 55 cases (31 tested before 10 weeks' gestation) Reference standards: karyotyping or follow‐up to birth |
|
| Index and comparator tests | Maternal age First trimester NT (details not reported) Fresh serum samples tested for: First trimester PAPP‐A and free ßhCG (Kryptor analyser, Brahms) Frozen samples tested for: First trimester ADAM 12 (measured blind to clinical outcome) (manual DELFIA assay, PerkinElmer Life & Analytical Sciences) |
|
| Follow‐up | Details not reported | |
| Aim of study | To establish the effectiveness or otherwise of ADAM 12 as an early screening marker | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? All tests | Yes | All women received a reference standard |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? All tests | No | No details of withdrawals given |
Stenhouse 2004.
| Clinical features and settings | Routine screening | |
| Participants | 5000 participants UK ‐ maternity clinic Over a 3 year period ‐ dates not specified Pregnant women Singleton pregnancies Median age 32 years (14‐45 years), 27% ≥ 35 years 11 to 14 weeks' gestation |
|
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 15 cases Reference standards: invasive testing offered to women with screening risk of > 1:250 or follow‐up to birth |
|
| Index and comparator tests | Maternal age First trimester NT (FMF methods, ATL HDI 3500, ATL HDI 3000, Toshiba SSA‐340A and Kretz Voluson) First trimester free ßhCG and PAPP‐A (Clotted venous blood samples, AutoDELFIA immunoassy, Perkin Elmer) |
|
| Follow‐up | Details not reported | |
| Aim of study | To assess the effectiveness of combined ultrasound and biochemical screening for chromosomal abnormalities in singleton pregnancies in a routine antenatal clinic and laboratory setting | |
| Notes | Fetal loss rate for invasive testing was 1.4% (3/212) | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? All tests | Unclear | Unclear if all women received a reference standard |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | Yes | NT not successfully measured in 25 patients (0.5%) |
| Withdrawals explained? All tests | No | No details of withdrawals given |
Strah 2008.
| Clinical features and settings | Routine screening | |
| Participants | 7096 participants with information available on pregnancy outcome Slovenia ‐ 2 outpatient clinics November 1999 ‐ May 2006 Pregnant women Singleton pregnancies Median maternal age 28.6 years (range 15‐42 years), 2.5% ≥ 36 years 11‐14 weeks' gestation |
|
| Study design | Cohort | |
| Target condition and reference standard(s) | Down's syndrome: 12 cases Reference standards: karyotyping or follow‐up to birth |
|
| Index and comparator tests | Maternal age First trimester NT (2 FMF certified sonographers) (3.5‐5 MHz and 8‐4 MHz transducers Toshiba Corevision Pro and 2‐5 MHz and 9.3‐3.7 MHz transducers GE Healthcare Voluson 730 Pro) Cut‐off 1/300 |
|
| Follow‐up | Pregnancy outcomes were obtained from participating women, referring gynaecologists, paediatricians and maternity units and were missing in 3% (n = 225) of cases. Karyotype results were reported from the cytogenetics laboratory. Only women with known outcome were included in the study analysis | |
| Aim of study | To evaluate screening for trisomy 21 by maternal age and NT in a low‐risk population | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? All tests | Yes | All women received a reference standard |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? All tests | No | No details of withdrawals given |
Theodoropoulos 1998.
| Clinical features and settings | Routine screening | |
| Participants | 4611 women due to deliver before July 1996 Greece ‐ 4 medical centres Dates not specified Singleton pregnancies Median maternal age 29 years (16‐48 years), 7.8% ≥ 37 years 10 to 14 weeks' gestation |
|
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 10 cases Reference standards: CVS or amniocentesis or follow‐up to birth. Unclear reference standard in cases of intrauterine death, miscarriages and terminations |
|
| Index and comparator tests | Maternal age First trimester NT (FMF methods, transabdominally with 5 or 3.5 MHz curvilinear translucer or transvaginally with 5 MHz transducer) Pandya's risk criteria |
|
| Follow‐up | Results of fetal karyotyping and pregnancy outcome were entered into the database when they became available | |
| Aim of study | To evaluate first trimester screening for chromosomal defects by fetal NT thickness at 10‐14 weeks of gestation in 4 medical centres in Greece | |
| Notes | 1 set of parents continued with diagnosed Down’s pregnancy to birth, 9 terminated. 1 case of Down’s syndrome only detected at birth. | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? All tests | Unclear | Unclear if all women received a reference standard |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? All tests | No | No details of withdrawals given |
Thilaganathan 1999.
| Clinical features and settings | Routine screening | |
| Participants | 9802 participants UK ‐ district general hospital November 1994 to November 1998 Pregnant women Singleton pregnancies Mean age 29 years (15‐45 years) 10 to 14 weeks' gestation |
|
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 21 cases Reference standards: CVS (offered to patients considered high risk on screening) or follow‐up to birth |
|
| Index and comparator tests | Maternal age First trimester NT (transabdominally, Toshiba SSA‐250, Accuson 128XP/4 or Aloka 650CL with 3.5‐7.5 curvilinear transducers) |
|
| Follow‐up | Pregnancy outcomes from hospital records and general practitioners. Karyotype results or postnatal tests were provided by the local Regional Cytoenetics laboratory. The proportion of patients who were followed up is not reported (49 patients had not given birth at the time of analysis of outcomes) | |
| Aim of study | To evaluate the effectiveness of 10‐14 week NT measurement in routine ultrasounds screening for Down's syndrome | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? All tests | Unclear | Unclear if all women received a reference standard |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | Yes | Unsuccessful NT in 10.1% of patients |
| Withdrawals explained? All tests | Yes | Patients not included due to ineligibility described |
Timmerman 2010.
| Clinical features and settings | High‐risk referral | |
| Participants | 445 fetuses with increased risk based on NT or biochemical testing and information available on pregnancy outcome The Netherlands ‐ fetal medicine unit September 1996 ‐ March 2008 Mean maternal age 34.5 years (19‐45 years) First trimester |
|
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 72 cases Reference standards: karyotyping or follow‐up to birth |
|
| Index and comparator tests | First trimester ductus venosus pulsatility index and Ductus venosus a‐wave (methods reported elsewhere) | |
| Follow‐up | Pregnancy outcome was obtained from standard follow‐up forms filled in and returned by patients, maternity wards or midwife practices and by reviewing neonatal, pathology and clinical paediatric notes. When the baby was born without structural defects or dysmorphic features, the chromosomes were assumed to be normal. In all cases of enlarged NT or antenatal suspicion of abnormal development, the infant was investigated by a neonatologist, paediatric cardiologist or geneticist. | |
| Aim of study | To investigate if ductus venosus pulsatility index for veins and a‐wave measurements can increase the accuracy of first trimester Down's syndrome screening in a high‐risk population | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Selective testing of high‐risk women as done in practice |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? All tests | Yes | All women received a reference standard |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | Yes | Satisfactory waveform measurements made in 98% of cases |
| Withdrawals explained? All tests | No | No details of withdrawals given |
Torring 2010.
| Clinical features and settings | Routine screening | |
| Participants | 691 participants: 46 cases and 645 controls Denmark ‐ nationwide screening programme Dates not reported Pregnant women Singleton pregnancies Mean maternal age cases 35 years, controls 31 years 8‐11 weeks' gestation |
|
| Study design | Case‐control study | |
| Target condition and reference standard(s) | Down's syndrome: 46 cases Reference standards: karyotyping or follow‐up to birth |
|
| Index and comparator tests | Maternal age First trimester NT (11‐13 weeks' gestation) (FMF certified sonographers) First trimester PAPP‐A and free ßhCG (fresh serum, 8‐11 weeks' gestation) (Kryptor analyser, Brahms) First trimester ADAM 12 (frozen serum, 8‐11 weeks' gestation) (Kyptor analyser, assay by Cezanne SAS, TRACE technology) |
|
| Follow‐up | Not reported | |
| Aim of study | To determine whether ADAM 12 is a useful serum marker for fetal trisomy 21 using the mixture model | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? All tests | Yes | All women received a reference standard |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of some index test results |
| Index test results blinded? All tests | Unclear | Unclear if all index tests interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? All tests | No | No details of withdrawals given |
Vadiveloo 2009.
| Clinical features and settings | Routine screening | |
| Participants | 10,189 participants UK ‐ screening programme July 2000 ‐ October 2005 Pregnant women Median maternal age 33.1 years, 36.9% ≥ 35 years 9‐14 weeks' gestation |
|
| Study design | Retrospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 44 cases Reference standards: karyotyping or follow‐up to birth |
|
| Index and comparator tests | Maternal age First trimester NT (trained sonographers) First trimester PAPP‐A and free ßhCG (DELFIA fluoroimmunoassay, PerkinElmer LAS) Contingent: biochemistry high risk cut‐off 1:42, low risk cut‐off 1:1000. If biochemical/maternal age risk between 1:42 and 1:1000, NT results added and combined risk calculated. Final cut‐point 1:250 |
|
| Follow‐up | Not reported | |
| Aim of study | To assess the performance of a 2‐stage screening protocol for Down's syndrome based on initial serum marker analysis for all women and NT measurement only in women with intermediate risks | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? All tests | Unclear | Unclear if all women received a reference standard |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? All tests | No | No details of withdrawals given |
Valinen 2007.
| Clinical features and settings | Routine screening | |
| Participants | 7534 participants Finland ‐ screening programme 2002‐2004 Pregnant women Singleton pregnancies Mean maternal age 29.6 years, 18.6% ≥ 35 years 10‐12 weeks' gestation |
|
| Study design | Retrospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 30 cases (24 underwent NT as well as biochemical testing) Reference standards: karyotyping or follow‐up to birth |
|
| Index and comparator tests | Maternal age First trimester NT (trained nurses, midwives and doctors) (4765 women) First trimester PAPP‐A and free ßhCG (details not reported) (all women) Cut‐point 1:250 |
|
| Follow‐up | Contacted chromosome laboratory at the department of clinical genetics in the Oulu university clinic and the Finish Register of Congenital Malformation and the National Research and Development Centre for Welfare and Health | |
| Aim of study | To compare the efficacy of both separate and combined maternal serum testing and fetal NT measurement in the first trimester screening for Down's syndrome in northern Finland | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? All tests | Unclear | Unclear if all women received a reference standard |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? All tests | No | No details of withdrawals given |
Viora 2003.
| Clinical features and settings | Routine screening | |
| Participants | 1752 participants Italy ‐ ultrasound and prenatal diagnosis unit December 2001 to June 2002 Pregnant women Median age 32 years (18‐47 years) 11 to 14 weeks' gestation |
|
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 10 cases Reference standards: CVS or follow‐up to birth |
|
| Index and comparator tests | Maternal age Nasal bone assessment (ultrasound examinations with Aloka SSD‐1700 or ATL‐Philips 5000 HCD) |
|
| Follow‐up | Follow‐up to birth in all cases of abnormalities. Not reported if there was follow‐up in screen‐negative patients | |
| Aim of study | To evaluate the significance of nasal bone ossification as a marker fir trisomy 21 at 11 to 14 weeks' gestation in an unselected population | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? All tests | Yes | All women received a reference standard |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | Yes | In 154 cases (8.1%) fetal profile was not obtained |
| Withdrawals explained? All tests | No | No details of withdrawals given |
Wald 2003.
| Clinical features and settings | Routine screening | |
| Participants | 39,983 participants UK and Austria ‐ multicentre trial September 1996 to April 2000 Pregnant women 9‐13 and 14‐20 weeks' gestation |
|
| Study design | Case‐control study | |
| Target condition and reference standard(s) | Down's syndrome: 85 cases Reference standards: invasive testing (following second trimester screening) or follow‐up to birth |
|
| Index and comparator tests | First trimester NT (midsaggital section, optimal magnification of thickness of translucent space between inner skin surface and fascia covering cervical spine (white black interface (oute) ‐ black white interface (inner), 41 models of ultrasound machine, 20 minutes allotted scanning time) First and second trimester serum AFP, hCG, uE3, PAPP‐A, free ßhCG (time resolved fluoroimmunoassay, AutoDELFIA) First and second trimester inhibin A (Sandwich enzyme linked immunosorbent assay, Oxford bioinnovation) First and second trimester urinary beta core fragment, total hCG, ITA and free ßhCG (ITA and beta core fragment, Quest diagnostics USA) |
|
| Follow‐up | Follow‐up by: 1) staff at local hospitals completed a study outcome form at, or just after delivery, 2) study records of CVS, amniocentesis or karyotype at birth linked to information from cytogenic laboratories, 3) study records linked to records of cases of Down's syndrome from the National Down's Syndrome Cytogenetic Register, 4) information obtained from local obstetrical outcome records, 5) forms sent to all women with a request to return details of the outcome of their pregnancy, 6) individual searches in respect of women whose outcomes of pregnancy had not been obtained by any of the previous methods. 4% of total patient cohort did not have a documented outcome of pregnancy. Unclear if any of these were included in the nested case‐control study | |
| Aim of study | To identify the most effective, safe and cost‐effective strategy for antenatal screening for Down's syndrome using NT, maternal serum and urine markers in the first and second trimesters of pregnancy and maternal age in various combinations | |
| Notes | Performance of screening assessed at 17 weeks' gestation. Study tried to be non‐interventional in the first trimester ‐ second trimester testing was aimed to be used as the basis for any referral for invasive testing | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? All tests | Unclear | Unclear if all women received a reference standard |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Unclear | Unclear if all index tests interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | Yes | Rates of NT failure on average 9%. Pre‐10 weeks' gestation, > 33% failure rate, declined to 7% at 12 weeks |
| Withdrawals explained? All tests | No | No details of withdrawals given |
Wapner 2003.
| Clinical features and settings | Routine screening | |
| Participants | 8216 participants USA multicentre study (12 prenatal diagnostic centres) Dates not specified Singleton pregnancies Pregnant women Mean age 35 years (SD 4.6), 50% ≥ 35 years 11 to 14 weeks' gestation |
|
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 61 cases Reference standards: invasive testing. Miscarriage with cytogenetic testing. Follow‐up to birth |
|
| Index and comparator tests | Maternal age First trimester NT (FMF methods) Dried blood samples tested for: First trimester free ßhCG and PAPP‐A (dried blood samples, enzyme‐linked immunoadsorbent assay as previously described) Risk cut‐point 1:270 |
|
| Follow‐up | Follow‐up to birth by directly following up women and reviewing delivery records. An effort was also made to obtain information on terminated or miscarried pregnancies. 196 (2.3%) of patients without follow‐up information were excluded and women with a previous trisomy 18 or 21 pregnancy were also excluded | |
| Aim of study | To evaluate the use of combined first trimester markers for aneuploidy in clinical practice | |
| Notes | 16 live Down’s syndrome births | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? All tests | Yes | All women received a reference standard |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? All tests | No | No details of withdrawals given |
Wax 2009.
| Clinical features and settings | Routine screening | |
| Participants | 2231 participants USA January 2005 ‐ January 2008 Pregnant women Singleton pregnancies Mean maternal age 36.7 years (SD 3.2 years) First and second trimester |
|
| Study design | Retrospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 8 cases Reference standards: karyotyping or follow‐up to birth |
|
| Index and comparator tests | Maternal age First trimester NT (Sonographers credited by FMF or Nuchal Translucency Quality Review Program) First trimester PAPP‐A and free ßhCG (details not reported) Second trimester ultrasound (in 884 women) Cut‐point for combined test 1:220 |
|
| Follow‐up | Down's syndrome cases ascertained from pre‐natal genetic database, including prenatal and newborn testing or physical examination at birth | |
| Aim of study | To evaluate the trisomy 21 screening performance of the first trimester combined test followed by second trimester genetic sonography | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? All tests | Unclear | Unclear if all women received a reference standard |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? All tests | No | No details of withdrawals given |
Wojdemann 2005.
| Clinical features and settings | Referrals for screening | |
| Participants | 8622 participants Denmark ‐ 3 obstetrics departments March 1998 to June 2001 Pregnant women Mean age 29 years, 10.8% ≥ 35 years Singleton pregnancies 11 to 14 weeks' gestation |
|
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 12 cases Reference standards: invasive testing (in cases of increased risk) or follow‐up to birth |
|
| Index and comparator tests | Maternal age First trimester NT (FMF methods, Logic 700 MR machine) (all women) First trimester free ßhCCG (AFP/ßhCG Auto Delfia kit) and PAPP‐A (In‐house ELISA (Sandwich)) in 6,441 women (75%) Risk cut‐point 1:250 |
|
| Follow‐up | Cross‐checking with all the chromosome laboratories in Denmark. Follow‐up in 96.2% of pregnancies through patients records | |
| Aim of study | To determine the performance of screening for Down's syndrome and other major chromosomal abnormalities using NT, free ßhCG and PAPP‐A in a prospective study of a non‐selected population | |
| Notes | Uptake of screening was 73% (9,941 accepted out of 13,621 offered screening) Women with miscarriages excluded from the study 3 live Down’s syndrome births |
|
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? All tests | Unclear | Unclear if all women received a reference standard |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | Yes | NT could not be measured in 2.5% of cases |
| Withdrawals explained? All tests | No | No details of withdrawals given |
Wortelboer 2009.
| Clinical features and settings | Routine screening | |
| Participants | 20,293 participants with complete outcome data The Netherlands ‐ nationwide screening programme May 2004 ‐ July 2006 Pregnant women Singleton pregnancies Median maternal age 34.9 years (15‐48 years) 8‐14 weeks' gestation |
|
| Study design | Cohort | |
| Target condition and reference standard(s) | Down's syndrome: 87 cases Reference standards: karyotyping or follow‐up to birth |
|
| Index and comparator tests | Maternal age First trimester NT (FMF protocols) First trimester PAPP‐A and free ßhCG (AutoDELFIA analyser, PerkinElmer, Turku) Cut‐point for combined test 1:250 |
|
| Follow‐up | Pregnancy outcome was evaluated by questionnaire and collected through self‐reporting of the participating women. Due to strict privacy rules of the Dutch Personal Data Protection Act, the researchers were allowed to send a reminder letter to collect missing data only once. Women without complete information on outcome were excluded from the study | |
| Aim of study | To study the performance of the first‐trimester combined test between 2004 and 2006 compared to a previous period to investigate changes in time and identify reasons for sub‐optimal performance | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? All tests | Yes | All women received a reference standard |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? All tests | Yes | Only 65% of biochemistry screened women (n = 41,782) had NT results |
Wright 2008.
| Clinical features and settings | Routine screening | |
| Participants | 37,488 participants with complete outcome data UK ‐ single centre July 1999 ‐ July 2005 Pregnant women Singleton pregnancies Median maternal age 35.2 years (16‐52 years) 11‐13 weeks' gestation |
|
| Study design | Cohort | |
| Target condition and reference standard(s) | Down's syndrome: 264 cases Reference standards: karyotyping or follow‐up to birth |
|
| Index and comparator tests | Maternal age First trimester NT First trimester PAPP‐A and free ßhCG (Kryptor system, Brahms AG) |
|
| Follow‐up | Maternal characteristics and test results were recorded in a computer database and karyotype results and details on pregnancy outcomes added as they became available. Women without complete outcome data (n = 1231, 3.2%) were excluded from the study | |
| Aim of study | To examine the validity of methods used to derive patient‐specific risks form NT measurements | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? All tests | Yes | All women received a reference standard |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? All tests | No | No details of withdrawals given |
Wright 2010.
| Clinical features and settings | Routine screening | |
| Participants | 223,361 pregnant women UK, Denmark and Cyprus – multicentre Some data from UK and Denmark in previous publications Dates not reported Singleton pregnancies Median maternal age 31.9 years (IQR 27.7‐35.8 years) 7‐14 weeks' gestation |
|
| Study design | Cohort | |
| Target condition and reference standard(s) | Down’s syndrome: 886 cases Reference standards: karyotyping or follow‐up to birth |
|
| Index and comparator tests | Maternal age First trimester NT (details not reported) First trimester PAPP‐A and free βhCG (Kryptor system, Brahms AG or Delfia Express sustem, PerkinElmer, Waltham) |
|
| Follow‐up | Karyotype results and details on pregnancy outcomes were added to databases as soon as they became available | |
| Aim of study | To establish an algorithm for first trimester combined screening for trisomy 21 with biochemical testing from 7 to 14 weeks’ gestation and ultrasound testing at 11‐13 weeks | |
| Notes | Taken results modelled for PAPP‐A and free ßhcg at 12 weeks as that was most common time for testing (44% of women) | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? All tests | Unclear | Unclear if all women received a reference standard |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | No | |
| Withdrawals explained? All tests | No | |
Zoppi 2001.
| Clinical features and settings | Routine screening | |
| Participants | 10,001 participants Italy ‐ genetic diagnosis centre May 1996 to unspecified date Pregnant women Median age 33 years (14‐48 years) Singleton pregnancies 10 to 14 weeks' gestation |
|
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 64 cases Reference standards: amniocentesis, CVS or follow‐up to birth |
|
| Index and comparator tests | Maternal age First trimester NT (FMF methods) Risk cut‐points of 1:100, 1:200 and 1:300 |
|
| Follow‐up | Outcome obtained from women themselves. 1422 patients (11%) with no data on follow‐up outcome and 202 patients with miscarriages were excluded from the study | |
| Aim of study | To examine the distribution of fetal NT thickness in normal and abnormal fetuses in Sardinia and to determine its effectiveness as a screening tool | |
| Notes | Study design unclear (maybe a case‐control study) | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? All tests | Unclear | Unclear if all women received a reference standard |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? All tests | Yes | NT could not be measured in 25 (0.2%) of cases |
| Withdrawals explained? All tests | No | No details of withdrawals given |
AFP:alpha‐fetoprotein ßhCG: beta human chorionic gonadotrophin CVS: chorionic villus sampling DELFIA: dual labelled time resolved fluorescent assay DVPI: ductus venosus pulsivity index FMF: frontomaxillary facial hCG: human chorionic gonadotrophin NT: nuchal translucency PAPP‐A: pregnancy‐associated plasma protein A PIGF: placental growth factor uE3: unconjugated oestriol
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abbas 1995 | Unable to extract useful data |
| Abdul‐Hamid 2004 | No Down's syndrome pregnancies |
| Abraha 1999 | Unable to extract useful data |
| Abu‐Rustum 2010 | Not Down’s syndrome specific |
| Achiron 2010 | Study only includes cases of Down’s syndrome |
| Adekunle 1999 | Unable to extract useful information |
| Agaard‐Tillery 2010 | Results presented in another study |
| Aitken 1993 | Unable to extract useful data |
| Aitken 1996 | Fewer than 80% of pregnancies had gestational age confirmed by USS |
| Aitken 1996a | Fewer than 80% of pregnancies had gestational age confirmed by USS |
| Ajayi 2011 | No diagnostic data |
| Akbas 2001 | Less than 5 Down's syndrome pregnancies |
| Alexioy 2009 | Study only includes test‐positives |
| Allingham‐Hawkins 2011 | Quantitative fluorescent polymerase chain reaction study |
| American College 2009 | Discussion article |
| Antona 1998 | Likely fewer than 80% of pregnancies dated by USS |
| Antsaklis 1999 | Women screened at greater than 24 weeks' gestation |
| Anuwutnavin 2009 | Second trimester ultrasound |
| Ashwood 1987 | Unable to extract useful data |
| Asrani 2005 | Review article |
| Audibert 2001b | Unable to ascertain whether part of screening population in Rozenberg et al. No response from authors, therefore excluded to reduce risk of data replication. |
| Axt‐Fleidner 2006 | Unable to extract useful data |
| Azuma 2002 | Unable to extract useful data |
| Baghagho 2004 | Unable to obtain paper |
| Bahado‐Singh 1995 | USS markers greater than 14 weeks' gestation |
| Bahado‐Singh 1996 | USS markers greater than 14 weeks' gestation |
| Bahado‐Singh 1999b | USS markers greater than 14 weeks' gestation |
| Bahado‐Singh 2002 | USS markers greater than 14 weeks' gestation |
| Bahado‐Singh 2003 | Review article |
| Ball 2007 | Data from the FASTER trial |
| Bar‐Hava 2001 | No Down's pregnancies in study population |
| Barkai 1996 | No Down's pregnancies in study population |
| Barnabei 1995 | No Down's pregnancies in study population |
| Bartels 1988 | Unable to extract useful data |
| Bartels 1993 | No Down's pregnancies in study population |
| Barth 1991 | Second trimester ultrasound study |
| Bas‐Budecka 2007 | No diagnostic data |
| Baviera 2004 | Unclear method of confirmation of gestational age |
| Bazzett 1998 | Male versus female fetuses |
| Beke 2008 | Results are not specific to Down’s syndrome |
| Bellver 2005 | No Down's syndrome pregnancies in study |
| Benn 1995 | Less than 80% follow‐up |
| Benn 1996 | Less than 80% follow‐up |
| Benn 1997 | No Down's pregnancies in study population |
| Benn 1998 | Less than 80% follow‐up |
| Benn 2001 | Statistical modelling (computer simulation) |
| Benn 2002 | Modelled data |
| Benn 2003 | Less than 80% of pregnancies dated by USS |
| Benn 2003a | Editorial |
| Benn 2005 | No Down's pregnancies included |
| Benn 2005a | Mathematical model |
| Benn 2007 | No follow‐up information |
| Berry 1995 | Less than 80% of pregnancies USS dated |
| Berry 1997 | Less than 80% of pregnancies USS dated |
| Bersinger 1994 | Gestational age not USS estimated |
| Bersinger 2000 | Unable to extract useful data |
| Bersinger 2001 | No Down's syndrome pregnancies in study population |
| Bersinger 2003 | Unable to extract useful data |
| Bersinger 2004 | No Down's syndrome pregnancies in study population |
| Bersinger 2005 | No Down's syndrome pregnancies in study population |
| Bestwick 2008 | All healthy pregnancies |
| Biggio 2004 | Cost‐effectiveness analysis |
| Bilardo 2011 | Not a proper sample ‐ most had elevated NT |
| Bindra 2002 | Review article |
| Blundell 1999 | Unable to extract useful data |
| Boormans 2010 | Study of testing on amniocentesis samples |
| Boots 1989 | Population risk factor calculations |
| Bornstein 2009 | No diagnostic data |
| Bornstein 2009a | No diagnostic data |
| Bornstein 2010 | No diagnostic data |
| Borowski 2007 | No diagnostic data |
| Borrell 2007 | No follow‐up data |
| Borrell 2009a | Based on SURUSS data ‐ second trimester serum parameters not actually measured |
| Borruto 2002 | Unable to extract useful data |
| Bottalico 2009 | Second trimester ultrasound |
| Boue 1990 | Review article |
| Bradley 1994 | Screen‐negative population gestations not confirmed by ultrasound |
| Braithwaite 1996 | Review article |
| Brambati 1995 | USS screening inclusive of women greater than 14 weeks' gestation |
| Brambati 1996 | Review article |
| Brizot 1995 | Unable to extract useful data |
| Brizot 1995a | Unable to extract useful data |
| Brizzi 1989a | Second trimester ultrasound |
| Brock 1990 | Unable to extract useful data |
| Calda 2010 | No data for false positive rates |
| Campogrande 2001 | Unable to extract useful data |
| Canick 1988 | Unable to extract useful data |
| Canick 1995b | Unable to extract useful data |
| Canini 2002 | No Down's syndrome pregnancies in study population |
| Cans 1998 | Second trimester ultrasound |
| Carreras 1991 | Second trimester ultrasound |
| Caughey 2007 | No diagnostic data |
| Cebesoy 2008 | No diagnostic data |
| Chelli 2008 | No follow‐up for false negatives |
| Chen 1999 | Review article |
| Chen 2002 | No Down's syndrome pregnancies in study population |
| Chen 2004 | Less than 5 Down's cases in study population |
| Chen 2005 | Unable to extract useful data |
| Chen 2008 | No diagnostic data |
| Cheng 1993 | Likely that fewer than 80% of gestational age confirmed by USS |
| Cheng 1999 | Case series No Down's syndrome pregnancies in study population |
| Cheng 2004 | No Down's syndrome pregnancies in study population |
| Cheng 2004a | No Down's syndrome pregnancies in study population |
| Chitayat 2002 | Less than 5 Down's cases in study population |
| Chiu 2011 | Study of maternal DNA testing |
| Cho 2009 | Study of testing amniotic fluid |
| Chou 2009 | Not possible to calculate specificity |
| Christiansen 2002 | Unable to extract useful data |
| Christiansen 2007a | Unable to extract useful data |
| Christiansen 2008 | No diagnostic data |
| Chung 2000 | Less than 5 Down's syndrome pregnancies in study population |
| CNGOF 1996 | Unable to obtain translation |
| Cocciolone 2008 | Unable to extract useful data ‐ attempted to contact author |
| Cole 1996 | Review article |
| Comas 2001 | USS at greater than 14 weeks |
| Comas 2002 | USS at greater than 14 weeks |
| Comas 2002a | USS at greater than 14 weeks |
| Comstock 2006 | Unable to extract useful data |
| Conde‐Agudelo 1998 | Review article |
| Cowans 2011 | No diagnostic data |
| Crossley 1991 | Less than 80% of pregnancies had gestational age confirmation by ultrasound |
| Crossley 1993 | Less than 80% of pregnancies had gestational age confirmation by ultrasound |
| Crossley 1996 | No Down's syndrome pregnancies in study population |
| Crossley 2002a | Adjustment factors for smokers |
| Cuckle 1984 | Gestational age not confirmed by USS |
| Cuckle 1987 | Gestational age not confirmed by USS |
| Cuckle 1987a | No gestational age limits given |
| Cuckle 1990 | Paper presenting adjustment factors |
| Cuckle 1996 | Data modelled on 4 meta‐analysed studies |
| Cuckle 1999b | Unable to extract useful data |
| Cuckle 1999c | Review article |
| Cullen 1990 | Abnormal scans only in study population |
| Cusick 2004 | Less than 5 Down's syndrome pregnancies in study population |
| Cusick 2007 | ST ultrasound |
| Dancoine 2001 | No Down's syndrome pregnancies in study population |
| Dane 2008 | Not specific to Down’s syndrome |
| De Biasio 2000 | Unable to extract useful information |
| De Biasio, 1999 | Unable to ascertain whether overlapping populations between several papers ‐ attempted to contact author with no response |
| De Biasio, 2001 | Unable to ascertain whether overlapping populations between several papers ‐ attempted to contact author with no response |
| De Graaf 1991 | Unable to extract useful data |
| Del Carmen Saucedo 2009 | No follow‐up information |
| DeVore 2001 | Second trimester ultrasound |
| Dhaifalah 2007 | Unable to obtain translation |
| Dhaifalah 2007a | Unable to obtain translation |
| Dhallan 2007 | DNA testing of blood samples from parents |
| Dickerson 1994 | Comment |
| Dimaio 1987 | Gestational age by USS only in screen‐positive population |
| Doran 1986 | Ultrasound confirmation of gestational age performed in screen‐positive women only |
| Dreux 2008 | No information for specificity |
| Drugan 1996 | Second trimester ultrasound |
| Drugan 1996a | Unable to extract useful data |
| Drysdale 2002 | Fewer than 5 Down's syndrome pregnancies in population |
| Dugoff 2008 | Not specific to Down’s syndrome |
| Ebell 1999 | Review article |
| Economides 1998 | Unable to extract useful data |
| Erickson 2004 | No Down's syndrome pregnancies in population |
| Evans 1996 | No Down's syndrome pregnancies in population |
| Evans 2007 | Data previously presented in another study |
| Falcon 2005 | Unable to extract useful data |
| Falcon 2006 | Unable to extract useful data |
| Ford 1998 | Audit |
| Frishman 1997 | No Down's syndrome pregnancies in population |
| Fukada 2000 | Unable to extract useful data |
| Gaudry 2009 | Study of karyotyping |
| Gebb 2009 | Study only examines screen‐positives |
| Geerts 2008 | Study only examines abnormal fetuses |
| Geipel 2010 | ST ultrasound |
| Gekas 2009 | Diagnostic data from other studies |
| Gekas 2011 | Diagnostic data from other studies |
| Gekas 2011a | Diagnostic parameters from other studies |
| Gerovassili 2007 | No diagnostic data |
| Ghidini 1998 | Comparison of male versus female fetuses |
| Goetzinger 2010 | Second trimester ultrasound |
| Goldie 1995 | Fewer than 80% of study population had gestational age confirmed by USS |
| Gollo 2008 | Only 1 case of Down’s syndrome |
| Gonçalves 2004 | Greater than 14 weeks USS screening |
| Goodburn 1994 | Likely that fewer than 80% of pregnancies had gestational age estimated by USS |
| Gorduza 2007 | Study of FISH technique |
| Grace 2010 | ST ultrasound |
| Grati 2010 | No diagnostic data |
| Gray 2009 | ST ultrasound |
| Gregor 2007 | Unable to obtain translation |
| Gregor 2009 | Unable to obtain translation |
| Grether 2009 | Systematic review and guidelines |
| Grozdea 2002 | Unable to extract useful data |
| Guo 2010 | Study of fetal samples |
| Gyselaers 2004 | Less than 80% follow‐up |
| Gyselaers 2004a | Less than 80% follow‐up |
| Gyselaers 2006 | Unaffected pregnancies only |
| Gyselaers 2006a | Unable to extract useful data |
| Hackshaw 1995 | No Down's syndrome pregnancies in population |
| Hackshaw 2001 | No Down's syndrome pregnancies in population |
| Haddow 1992 | Less than 80% of pregnancies had gestational age confirmed by ultrasound scan |
| Hadzsiev 2007 | Study of FISH technique |
| Hafner 1995 | Less than 5 Down's pregnancies in study population |
| Hallahan 1998 | Gestational age greater than 24 weeks |
| Han 2008 | Study of findings on amniocentesis |
| Harper 2010 | Second trimester ultrasound |
| Harrison 2006 | Less than 80% of pregnancies had gestational age confirmed by ultrasound scan |
| Harry 2006 | Editorial |
| Hayashi 1995 | Unable to extract useful data |
| Hayashi 1996 | Less than 5 Down's pregnancies in study population |
| Heikkila 1997 | Fewer than 80% of pregnancies had gestational age confirmed by USS |
| Heinig 2007 | No Down’s syndrome data |
| Heinonen 1996 | No Down's syndrome pregnancies in population |
| Herman 2000 | No Down's syndrome pregnancies in study population |
| Herman 2003 | Correlation between markers, not evaluation of screening tests |
| Herrou 1992 | Unable to extract useful data |
| Hershey 1985 | Gestation unclear |
| Hershey 1986 | Gestation based on LMP |
| Hewitt 1993 | Unable to extract useful data |
| Hills 2010 | Study of testing on CVS and amniocentesis samples |
| Ho 2010 | Study of FISH diagnosis |
| Hogdall 1992 | Unclear method of determination of gestational age Unable to extract useful data |
| Hong Kong Practitioner | CME |
| Hoogendoorn 2008 | Diagnostic data from other studies used |
| Howe 2000 | Second trimester ultrasound scans |
| Hsiao 1991 | Unable to obtain translation |
| Hsieh 1999 | No Down's syndrome pregnancies in study population |
| Hsu 1997a | Adjustment factors |
| Hsu 1998a | No Down's syndrome pregnancies in study population |
| Hsu 1999b | No Down's pregnancies |
| Hu 2007 | Same data as Liu 2010 |
| Huang 2003 | No Down's syndrome pregnancies in study population |
| Huang 2007 | Not possible to obtain detection rate |
| Huang 2007a | No diagnostic data |
| Huggon 2004 | Study of cardiac function in pregnancies with normal and abnormal NT results |
| Hui 2003 | No Down's syndrome pregnancies in population |
| Hui 2005 | No Down's syndrome pregnancies in population |
| Hultén 2004 | Editorial/commentary |
| Hung 2003 | Modelling |
| Hung 2008 | Second trimester ultrasound |
| Hurley 1993 | Unable to extract useful data |
| Huttly 2004 | No Down's syndrome pregnancies in population |
| Hwa 2004 | Less than 5 Down's pregnancies in population |
| Iles 1996 | Review |
| Ind 1994 | Unable to extract useful data |
| Ivorra‐Deleuze 2010 | No diagnostic data |
| Jakobsen 2011 | Not Down’s syndrome specific |
| Jean‐Pierre 2005 | Review article |
| Johnson 1991 | Gestational age estimated by USS in fewer than 80% of cases |
| Johnson 1993 | Normal pregnancies only |
| Jorgensen 1999 | Gestation greater than 14 weeks for USS |
| Jorgez 2007 | Study of DNA testing on maternal blood |
| Josefsson 1998 | No Down's syndrome pregnancies in study population |
| Jou 2001 | Less than 5 Down's syndrome pregnancies in study population |
| Jun‐Tao 2003 | Unable to obtain translation |
| Jung 2007 | ST ultrasound |
| Kagan 2006 | Screen‐positive pregnancies only |
| Kagan 2007 | No diagnostic data |
| Kagan 2008 | Not Down’s syndrome detection |
| Kalelioglu 2007 | ST ultrasound |
| Kautzmann 1995 | Fewer than 80% pregnancies had gestational age estimated by USS |
| Kazerouni 2009 | Not possible to obtain complete diagnostic data |
| Keith 1992 | Summary article |
| Kelekci 2004 | Less than 5 Down's syndrome pregnancies in population |
| Kellner 1995 | Less than 5 Down's syndrome pregnancies in population |
| Kellner 1995a | Less than 80% follow‐up Unable to ascertain proportion of population with gestational age confirmed by USS |
| Kellner 1997 | Assumption of normal karyotype without reference standard in significant proportion of control pregnancies |
| Kirkegaard 2008 | FPR only calculated for subset of the cohort |
| Kjaergaard 2008 | Unable to obtain translation |
| Knight 1990 | Review article |
| Knight 2001 | Validation of a specific assay |
| Knight 2005 | Less than 80% of pregnancies had gestational age confirmed by ultrasound scan |
| Koos 2006 | Review article |
| Kornman 1996 | Less than 5 Down's syndrome pregnancies in population |
| Kornman 1997 | Unable to extract useful information |
| Kotaska 2007 | No new data |
| Kramer 1998 | No Down's syndrome pregnancies in study population |
| Krantz 1996 | Modelled data |
| Krantz 2005 | Adjustment factor |
| Krantz 2007 | Uses data from other published studies |
| Kulch 1993 | No Down's cases in population |
| Lai 1998 | Modelled population |
| Lai 2003 | No Down's syndrome pregnancies in study population |
| Laigaard 2006 | Unable to extract useful data |
| Laigaard 2006b | Simulation |
| Lam 1997 | Unable to extract useful data |
| Lam 1998 | Fewer than 80% pregnancies had gestational age estimated by USS |
| Lam 1999 | No Down's syndrome pregnancies in population |
| Lam 1999a | Unable to extract useful data |
| Lam 2000 | Study of women's decisions about screening |
| Lam 2001 | Male versus female fetuses |
| Lambert‐Messerlian 1996 | Fewer than 80% of pregnancies USS dated |
| Lambert‐Messerlian 1998 | Unable to extract useful data |
| Lauria 2007 | No diagnostic data |
| Lehavi 2005 | Down's syndrome pregnancies only |
| Leung 2006 | Unable to separate twins from singletons therefore unable to extract useful data |
| Leymarie 1993 | Appears to be a review article (French) |
| Li 1998 | Unable to obtain translation |
| Li 1999 | Unable to obtain translation |
| Li 2010 | No diagnostic data |
| Liao 1997 | Unable to obtain translation |
| Liao 2001 | Unable to extract useful data |
| Lim 2002 | Second trimester ultrasound |
| Lippman 1987 | Editorial |
| Liu 2010 | Not possible to separate out data for cases of Down’s syndrome |
| Lo 2010 | Pooled test results |
| Lustig 1988 | Gestational age by LMP only |
| Luthgens 2008 | FPR and DR obtained from different cohorts |
| MacDonald 1991 | Fewer than 80% of gestational ages estimated by USS |
| Macintosh 1994 | Unable to extract useful data |
| Macintosh 1997 | Unable to extract useful data |
| MacRae 2010 | Pooled test results |
| Macri 1994 | Likely fewer than 80% evaluated for gestational age by ultrasound examination |
| Macri 1996 | Likely fewer than 80% evaluated for gestational age by ultrasound examination |
| Malone 1998 | Review article |
| Malone 2003 | Review article |
| Mandryka‐Stankewycz 2009 | No diagnostic data |
| Mangione 2001 | Abnormal screening results only |
| Markov 2008 | Unable to obtain paper |
| Maymon 2001 | No Down's syndrome pregnancies in study population |
| Maymon 2001a | No normal test results included therefore unable to extract meaningful data |
| Maymon 2002 | No Down's syndrome pregnancies in study population |
| Maymon 2004a | No Down's syndrome pregnancies in study population |
| Maymon 2005a | Modelled data |
| McDuffie 1996 | USS dating on screen positive women only |
| Meier 2002 | Observed versus expected cases of Down's syndrome in a population |
| Merkatz 1984 | Gestational age not confirmed by ultrasound scan |
| Merz 2005 | Editorial |
| Merz 2008 | Part of Merz 2011 cohort |
| Metzenbauer 2001 | Normal pregnancies only |
| Metzenbauer 2002 | Unable to extract useful data |
| Mikic 1999 | No Down's syndrome pregnancies in study population |
| Miller 1991 | Unable to extract useful data |
| Milunsky 1989 | Fewer than 80% gestational age estimated by USS |
| Milunsky 1996 | Fewer than 80% gestational age estimated by USS |
| Minobe 2002 | Gestational age greater than specified limits |
| Miron 2008 | No diagnostic data |
| Miron 2009 | No diagnostic data |
| Miron 2010 | No diagnostic data |
| Miyamura 1999 | Unable to extract useful data |
| Moghadam 1998 | Unable to extract useful data |
| Monni 2000 | Less than 5 Down's syndrome pregnancies |
| Monni 2002 | Review article |
| Mooney 1994 | Greater than 24 weeks' gestation |
| Muhcu 2008 | No diagnostic data |
| Muller 1994 | No Down's syndrome pregnancies in study population |
| Muller 1996a | Unable to extract useful data |
| Muller 1999 | Unable to extract useful data |
| Muller 2002 | Gestational age greater than 24 weeks |
| Muller 2002a | Unable to extract meaningful data ‐ unable to separate double and triple test data |
| Muller 2003b | No Down's syndrome pregnancies in study population |
| Murta 2002 | Unable to extract useful data |
| Musone 2000 | Unable to extract useful data |
| Musto 1986 | Fewer than 80% USS dated |
| Myrick 1990 | Unable to extract useful data |
| Naidoo 2008 | Not specific Down’s syndrome results |
| Nau 2009 | No diagnostic data |
| Nau 2009a | No diagnostic data |
| Neveux 1996 | No Down's syndrome pregnancies in population |
| Neveux 1996a | Unable to extract useful data |
| Ng 2004 | Unable to extract useful data |
| Nicolaides 1992a | Study of outcomes of abnormal NT results |
| Nicolaides 2000 | Review article |
| Nicolaides 2004 | Review article |
| Nicolaides 2005a | Unable to obtain translation ‐ appears to be a review article |
| Nicolaides 2005b | Unable to obtain translation ‐ appears to be a review article |
| Nicolaides 2005c | Unable to obtain translation ‐ appears to be a review article |
| Nicolaides 2005d | Unable to obtain translation ‐ appears to be a review article |
| Nicolaides 2005e | Unable to obtain translation ‐ appears to be a review article |
| Nicolaides 2005f | Review article |
| Niemimaa 2001b | No Down's pregnancies in study population |
| Niemimaa 2002 | No Down's syndrome pregnancies in population |
| Niemimaa 2003 | No Down's syndrome pregnancies in population |
| Noble 1997b | Unable to extract useful data |
| Norgaard 1990 | Less than 80% of gestational ages confirmed by USS |
| Norton 1992 | Unable to extract useful data |
| Novakov‐Mikic 2007 | Out of FT screening time frame |
| O'Brien 1997a | No Down's syndrome pregnancies in population |
| O'Brien 1997b | No Down's syndrome pregnancies in population |
| Odibo 2004 | Gestational age of greater than 14 weeks in USS population |
| Odibo 2007 | ST ultrasound |
| Odibo 2008 | ST ultrasound |
| Odibo 2009 | No results presented |
| Offerdal 2008 | ST ultrasound |
| Ognibene 1999 | Unable to extract useful data |
| Oh 2007 | No diagnostic data |
| Olajide 1989 | Unable to extract useful data |
| Onda 1996 | Unable to extract useful data |
| Onda 1998 | Unable to extract useful data |
| Onda 2000 | Less than 80% follow‐up |
| Orlandi 2002 | No Down's syndrome pregnancies in study population |
| Ottavio 1997 | Second trimester USS |
| Ozkaya 2010 | Only healthy pregnancies |
| Paladini 2007 | No diagnostic data |
| Palka 1998 | Twin data used in calculation of the median |
| Palomaki 1989 | Fewer than 80% USS dated |
| Palomaki 1993 | No Down's syndrome pregnancies in population |
| Palomaki 1994 | No Down's syndrome pregnancies in population |
| Palomaki 1996 | Meta‐analysis |
| Palomaki 2005 | Unable to extract meaningful data |
| Panburana 2001 | Less than 5 Down's syndrome pregnancies in population |
| Pandya 1994 | Study of outcomes of abnormal NT results |
| Pandya 1995b | Review article |
| Papadopoulou 2008 | No diagnostic data |
| Parra‐Cordero 2007 | ST ultrasound |
| Paterlini‐Brechot 2007 | Editorial, no new data |
| Paul 2001 | Unable to extract useful data |
| Peralta 2005 | Unable to extract useful data |
| Perenc 1998 | No Down's syndrome pregnancies in study population |
| Perheentupa 2002 | No Down's syndrome pregnancies in population |
| Perona 1998 | Smokers versus non smokers |
| Persico 2008 | ST ultrasound |
| Petervari 2000 | Unable to extract useful data |
| Petrocik 1989 | Likely fewer than 80% USS dated |
| Phillips 1992 | Gestational age confirmed by USS in less than 80% of population |
| Phillips 1993 | Gestational age confirmed by USS in less than 80% of population |
| Pihl 2008 | Only 2 cases of Down's syndrome |
| Pinette 2003 | Women screened prior to recruitment |
| Platt 2004 | Unable to extract useful data |
| Podobnik 1995 | Abnormal results only |
| Poon 2009 | No diagnostic data |
| Prefumo 2002 | Comparison of prevalence and predicition |
| Prefumo 2004 | Comparison of a marker in women of different ethnic origins |
| Price 1998 | Unable to extract useful data |
| Páez 2004 | Unable to obtain translation |
| Raty 2000 | No Down's syndrome pregnancies in population |
| Rembouskos 2004 | Unable to extract useful data |
| Ren 1992 | Review article |
| Renier 1998 | Method of ascertainment of gestational age unclear Twin gestations included in general population |
| Resta 1990 | Second trimester USS |
| Reynders 1997 | Fewer than 5 Down's cases |
| Reynolds 1989 | Explanation of mathematical techniques |
| Reynolds 1999 | Unable to extract useful data |
| Reynolds 2008 | Not full diagnostic data |
| Ribbert 1996 | No Down's syndrome pregnancies in study population |
| Rice 2005 | Down's syndrome pregnancies excluded from study |
| Rich 1991 | Unable to extract useful data |
| Roberts 1995 | No Down's syndrome pregnancies in study population |
| Robertson 1991 | Editorial |
| Rode 2003 | No Down's pregnancies |
| Ronge 2006 | Editorial ‐ summary of FASTER results |
| Rose 1995 | Review article |
| Ross 1997 | Review article |
| Rotmensch 1996 | Unable to extract useful data |
| Rotmensch 1999 | No Down's syndrome pregnancies in study population |
| Rozenberg 2006 | USS greater than 14 weeks' gestation |
| Rudnicka 2002 | No Down's syndrome pregnancies in population |
| Ryall 1992 | Unable to determine method of confirmation of gestational age |
| Ryall 2001 | High‐risk results only included (i.e. no screen‐negative group for comparison) |
| Räty 2002 | No Down's pregnancies in population |
| Sabriá 2002 | Unable to ascertain how numbers calculated and from which populations |
| Sacchini 2003 | Unable to extract useful data |
| Sahota 2009 | No diagnostic data |
| Sahota 2010a | Included in Sahota 2010 |
| Salazar 2007 | Unable to obtain paper |
| Salazar 2008 | Only 1 case of Down’s syndrome |
| Saller 1997 | Down's syndrome secondary to Robertsonian translocation only. No controls |
| Salomon 2001 | No Down's syndrome pregnancies in population |
| Salonen 1997 | Fewer than 80% had gestational age estimated by USS |
| Saltvedt 2005 | Gestation greater than 14 weeks for nuchal scanning |
| Saridogan 1996 | Down's syndrome and Edward's syndrome affected pregnancies only |
| Savoldelli 1993 | Unable to extract useful data |
| Schielen 2009 | Full study information not given |
| Schiott 2006 | Unable to extract useful data |
| Schmidt 2007a | Not specific to Down’s syndrome |
| Schmidt 2007b | No separate Down’s syndrome data |
| Schmidt 2007c | No diagnostic data |
| Schmidt 2008a | Not specific to Down’s syndrome |
| Schmidt 2008b | Not specific to Down’s syndrome |
| Schmidt 2008c | Not specific to Down’s syndrome |
| Schmidt 2010 | No follow‐up data for test negatives |
| Schuchter 1998 | No Down's pregnancies in study population |
| Scott 1995 | Less than 5 Down's syndrome pregnancies in study population |
| Seeds 1990 | Review article |
| Seki 1995 | No Down's syndrome pregnancies in study population |
| Shenhav 2003 | No Down's syndrome pregnancies |
| Shintaku 1989 | Unable to extract useful data |
| Shulman 2003 | No Down's syndrome pregnancies in population |
| Sieroszewski 2008 | No Down’s syndrome specific information for specificity |
| Simon‐Bouy 1999 | Review article |
| Simpson 1986 | Gestational age confirmed by USS in less than 80% of population |
| Smith 1990 | Analysis of screen‐positive results |
| Smith 1996 | Review/meta‐analysis |
| Smith 1999 | Unable to extract useful data |
| Smith‐Bindman 2001 | Meta‐analysis of second trimester ultrasound markers |
| Smith‐Bindman 2003 | Population study, not examining DTA |
| Snijders 1995 | Study of prevalence, not screening |
| Snijders 1999 | Study of prevalence, not screening |
| Soergel 2006 | Less than 80% follow‐up |
| Sokol 1998 | Observation of Down's prevalence stratified by age |
| Sonek 2003 | Editorial |
| Sonek 2007 | ST ultrasound |
| Sood 2010 | No diagnostic data |
| Sooklim 2010 | ST ultrasound |
| Spencer 1985 | Fewer than 80% USS dated |
| Spencer 1991a | Likely fewer than 80% USS dated |
| Spencer 1991b | Unable to extract useful data |
| Spencer 1992 | Unable to extract useful data |
| Spencer 1993a | Fewer than 80% USS dated |
| Spencer 1993b | No Down's pregnancies in study population |
| Spencer 1993c | Unable to extract useful data |
| Spencer 1993d | Fewer than 80% of pregnancies had gestational age confirmed by USS |
| Spencer 1993e | Unable to extract useful data |
| Spencer 1995a | No Down's pregnancies in population |
| Spencer 1996b | Fewer than 80% of pregnancies had gestational age confirmed by USS |
| Spencer 1997 | Statistical modelling, aneuploid pregnancies only in study population |
| Spencer 1998a | No Down's pregnancies in population |
| Spencer 1998b | Unable to extract useful data |
| Spencer 1999a | Review |
| Spencer 1999b | Statistical methods paper |
| Spencer 2000a | Examination of median shifts rather than an evaluation of screening |
| Spencer 2000b | No Down's syndrome pregnancies in population |
| Spencer 2000c | No Down's syndrome pregnancies in population |
| Spencer 2000d | No Down's cases |
| Spencer 2000e | Male versus female fetuses |
| Spencer 2000f | No Down's cases in population |
| Spencer 2000g | No Down's pregnancies in population |
| Spencer 2000h | No Down's pregnancies in population |
| Spencer 2000i | Comparsison of fetal sex |
| Spencer 2001a | No Down's syndrome pregnancies in population |
| Spencer 2001b | Unable to extract useful data |
| Spencer 2001c | Unable to extract useful data |
| Spencer 2001d | Unable to extract useful data |
| Spencer 2001e | No Down's syndrome pregnancies in population |
| Spencer 2002a | No Down's pregnancies |
| Spencer 2002b | Risk validation study |
| Spencer 2002c | No Down's syndrome pregnancies in population |
| Spencer 2002d | Demonstration of median changes with time, rather than evaluation of screening |
| Spencer 2003a | No Down's pregnancies in population |
| Spencer 2003b | No Down's pregnancies in population |
| Spencer 2003c | Calculation of weight correction factor |
| Spencer 2003d | Fewer than 5 Down's syndrome pregnancies |
| Spencer 2004 | Calculation of smoking correction factor |
| Spencer 2005a | No Down's pregnancies |
| Spencer 2005b | No Down's pregnancies |
| Spencer 2005c | Comparison of 2 different assays ‐ not actual screening evaluation |
| Spencer 2008a | Unable to extract appropriate data for unaffected pregnancies |
| Spong 1999 | Comparison of male and female fetuses |
| Staboulidou 2009 | No diagnostic data |
| Stevens 1998 | Literature review |
| Stoll 1992 | Review article |
| Stressig 2011 | ST ultrasound |
| Su 2002a | Unable to extract useful data |
| Suchet 1995 | Review article |
| Suchy 1990 | Unable to ascertain method of confirmation of gestational age |
| Summers 2003a | Only 55% gestational ages estimated by USS |
| Summers 2003b | No Down's syndrome pregnancies in study population |
| Suntharasaj 2005 | Examination of inter‐observer variation in NT scanning |
| Susman 2010 | No diagnostic data |
| Sutton 2004 | Unable to extract useful data |
| Suzuki 1998 | Unable to extract useful data |
| Tabor 1987 | Gestational age not confirmed by USS |
| Tanski 1999 | Information on screen‐positive pregnancies only |
| Thilaganathan 1998 | No Down's syndrome pregnancies in study population |
| Thilaganathan 1999b | Editorial |
| Tislaric 2002 | No Down's syndrome pregnancies in population |
| Torok 1997 | Unable to extract useful data |
| Torring 2009 | Not possible to obtain full diagnostic data |
| Trninic‐Pjevic 2007 | Unable to obtain translation |
| Tsai 2001 | Less than 5 Down's syndrome pregnancies in study population |
| Valerio 1996 | Fewer than 80% pregnancies had gestational age estimated by USS |
| Van Blerk 1992 | Unable to extract useful data |
| Van Dyke 2007 | Not possible to obtain full diagnostic data |
| Van Heesch, 2006 | No Down's syndrome pregnancies in study population Software comparison study |
| Van Lith 1991 | Unable to extract useful data |
| Van Lith, 1993 | Unable to extract useful data |
| Van Lith, 1994 | Unable to extract useful data |
| Veress 1986 | Unable to extract useful data |
| Veress 1988 | Unable to extract useful data |
| Vergani 2008 | ST ultrasound |
| Vintzileos 2003 | Second trimester USS |
| Wald 1988a | Less than 80% had gestational age confirmed by ultrasound |
| Wald 1988b | Gestational age not confirmed by USS |
| Wald 1991 | No Down's pregnancies in study |
| Wald 1992a | Less than 80% had gestational age confirmed by ultrasound |
| Wald 1992b | No Down's pregnancies in study |
| Wald 1992c | No Down's pregnancies in study |
| Wald 1993 | No USS dating |
| Wald 1994a | No Down's syndrome pregnancies in population |
| Wald 1994b | Review article |
| Wald 1996a | No Down's pregnancies |
| Wald 1996b | Dated by LMP |
| Wald 1996c | No Down's syndrome pregnancies in population |
| Wald 1996d | Gestational age greater than 24 weeks |
| Wald 1997 | Data modelled on 3 separate populations of women |
| Wald 1998 | Unable to extract useful data |
| Wald 1999a | Unable to extract useful data |
| Wald 1999b | Gestational age not confirmed by USS |
| Wald 1999c | No Down's syndrome pregnancies |
| Wald 1999d | Modelled on several studies, some of which have no USS dating |
| Wald 2003b | No cases |
| Wald 2003c | Less than 80% had gestational age confirmed by USS |
| Wald 2006 | Modelled on SURRUS data |
| Wallace 1994 | Unable to extract useful data |
| Wallace 1997 | No Down's syndrome pregnancies in study population |
| Wang 2010 | ST ultrasound |
| Ward 2005 | Review article |
| Watt 1996a | No Down's syndrome pregnancies in study population |
| Watt 1996b | No Down's syndrome pregnancies in study population |
| Wax 2007 | No diagnostic data |
| Weinans 2001 | Unable to extract useful data |
| Weinans 2004 | Study of women's views on screening |
| Weisz 2007 | Cohort split into people having different tests and non‐representative samples of women assessed for each test |
| Welborn 1994 | Abnormal results only (cystic hygroma) |
| Wenstrom 1993 | Less than 80% of pregnancies had gestational age confirmed by USS |
| Wenstrom 1995a | Adjustment factors |
| Wenstrom 1995b | Less than 80% of pregnancies had gestational age confirmed by USS |
| Wetta 2011 | No diagnostic data |
| Whitlow 1998a | Unable to extract useful data |
| Whitlow 1998b | Unable to extract useful data |
| Whitlow 1999 | Unable to extract useful data |
| Williamson 1994 | Likely fewer than 80% USS dated |
| Wilson 2000 | Review |
| Wojdemann 2001 | No Down's syndrome pregnancies in study population |
| Wong 2003 | Less than 5 Down's syndrome pregnancies in population |
| Wright 2006 | Mathematical model |
| Wright 2007 | Simulation study, no new data |
| Xie 2010 | Only cases of false negatives and true negatives included |
| Yagel 1998 | Second trimester USS |
| Yamamoto 2001a | Unable to extract useful data |
| Yamamoto 2001b | Method of determination of gestational age unclear |
| Yamamoto 2001c | Unable to extract useful data |
| Yaron 2001 | Male versus female fetuses |
| Ye 1995 | Unable to obtain translation |
| Yoshida 2000 | Fewer than 80% pregnancies had gestational age estimated by USS |
| Zalel 2008 | No diagnostic data |
| Zeitune 1991 | Only aneuploid pregnancies included in study |
| Zelop 2005 | No Down's cases in population |
| Zhang 2011 | No diagnostic data |
| Zhao 1998 | Unable to obtain translation |
| Zhong 2011 | Second trimester ultrasound |
| Zoppi 2003a | Inappropriate study design |
CVS: chorionic villus sampling DR: detection rate FPR: false positive rate LMP: last menstrual period NT: nuchal translucency USS: ultrasound scan
Differences between protocol and review
The protocol intended to investigate several additional outcomes downstream from test accuracy, should they be reported in the test accuracy studies. When we attempted to extract this information however, it was found to be available in very few studies, and where such information was found, it was difficult to extract meaningful data to allow for comparison between studies, as data were not reported in a universal manner. In several studies such outcomes were estimated rather than measured. Often they were not reported at all. The outcomes stated in the protocol which have not been included are: harms of testing; need for further testing; side effects of test; interventions and side effects; other abnormalities detected by testing; spontaneous miscarriage; miscarriage subsequent to invasive procedure, with or without normal karyotype; fetal karyotype; termination of pregnancy (prior to definitive testing or in a karyotypically normal pregnancy and following confirmation of Down’s syndrome or following detection of other chromosomal abnormalities); stillbirth; livebirth of affected and unaffected fetus; uptake of definitive testing by women.
The following refinements to the eligibility criteria were imposed to ensure that the quality of the included literature remained high. We excluded studies that identified fewer than five Down's syndrome pregnancies in their study population. We excluded studies that had less than 80% follow‐up of participants.
In addition, the analytical strategy was informed by the volume of tests and studies included, and developed so that we focused on key tests and test combinations by a) only meta‐analysed tests that were included in four or more studies or b) showed more than 70% sensitivity for more than 90% specificity. In addition, a requirement that a minimum of 10 studies for a single test was required before subgroup analysis was undertaken. Consequently, several potential sources of heterogeneity were not investigated due to lack of data. To investigate the impact of multifetal pregnancies, we excluded studies in a sensitivity analysis to determine the effect on our estimates of test accuracy. This was done because data were limited for meta‐regression analyses.
We intended to conduct sensitivity analyses on the analysis investigating the effect of maternal age on test sensitivity. This was not possible due to limited data. Instead we performed the sensitivity analyses when comparing high‐risk populations with routine screening populations. This comparison was considered a proxy for the effect of maternal age because the main indication for referral for invasive testing was often increased risk due to advanced maternal age. Due to lack of information, we were unable to consider the impact of age standardisation and improvements in technology on the estimates of test performance.
Contributions of authors
KA undertook the searches, applied eligibility criteria, extracted and entered data and wrote the first and second draft of the review.
ZA applied eligibility criteria, provided senior clinical input, oversaw the review process, and approved the final draft of the review.
JD supervised and planned the review, checked data extraction, supervised statistical analyses and wrote the second draft of the review.
JP applied eligibility criteria, provided senior clinical input, oversaw the review process, and approved the final draft of the review.
BG checked data extraction and undertook statistical analyses.
MP applied eligibility criteria, extracted and entered data for the updated literature search, and entered characteristics of studies information.
YT checked data extraction, undertook statistical analyses and wrote parts of the first draft of the review.
Sources of support
Internal sources
-
University of Birmingham, UK.
Funding of Research time for BG, MP, SW, YT and JD
External sources
-
NIHR Health Technology Assessment Programme, UK.
Project grant
-
NIHR Health Technology Assessment Programme, UK.
- Funding for the Cochrane Reviews of Diagnostic Test Accuracy Support Unit, based at the University of Birmingham (JD).
Declarations of interest
S Kate Alldred was supported by a project grant from the NIHR Health Technology Assessment Programme
Boliang Guo: none known.
Jonathan J Deeks : none known.
Zarko Alfirevic (ZA) is Director of Harris Wellbeing Preterm Birth Centre which is grant funded by the charity Wellbeing of Women. This grant is administered by University of Liverpool and Zarko Alfirevic is not paid directly. He is the principal investigator or co‐investigator on several grants from public funders including National Institute of Health Research, British Medical Association, European Commission and WHO. He has received research support in the past from Perkin Elmer and Alere for research related to pre‐eclampsia and preterm birth prevention. These grants were administered by his employers and ZA did not benefit directly. ZA is also a Co‐coordinating Editor of Cochrane Pregnancy and Childbirth.
James P Neilson received an award from the UK NIHR to facilitate a panel of Cochrane systematic reviews on Down's syndrome.
Mary Pennant: none known.
Yemisi Takwoingi was supported by an award from the United Kingdom National Institute for Health Research [DRF‐2011‐04‐135].
New
References
References to studies included in this review
Acacio 2001 {published data only}
- Acacio GL, Barini R, Pinto Júnior W, Ximenes RL, Pettersen H, Faria M. Nuchal translucency: an ultrasound marker for fetal chromosomal abnormalities. Sao Paulo Medical Journal = Revista Paulista de Medicina. 2001;119(1):19‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Audibert 2001 {published data only}
- Audibert F, Dommergues M, Benattar C, Taieb J, Thalabard JC, Frydman R. Screening for Down syndrome using first‐trimester ultrasound and second‐trimester maternal serum markers in a low‐risk population: a prospective longitudinal study. Ultrasound in Obstetrics & Gynecology. 2001;18(1):26‐31. [DOI] [PubMed] [Google Scholar]
Babbur 2005 {published data only}
- Babbur V, Lees CC, Goodburn SF, Morris N, Breeze AC, Hackett GA. Prospective audit of a one‐centre combined nuchal translucency and triple test programme for the detection of trisomy 21. Prenatal Diagnosis 2005;25(6):465‐9. [DOI] [PubMed] [Google Scholar]
Barrett 2008 {published data only}
- Barrett SL, Bower C, Hadlow NC. Use of the combined first‐trimester screen result and low PAPP‐A to predict risk of adverse fetal outcomes. Prenatal Diagnosis 2008;28(1):28‐35. [DOI] [PubMed] [Google Scholar]
Belics 2011 {published data only}
- Belics Z, Fekete T, Beke A, Szabo I. Prenatal ultrasonographic measurement of the fetal iliac angle during the first and second trimester of pregnancy. Prenatal Diagnosis 2011;31(4):351‐5. [DOI] [PubMed] [Google Scholar]
Benattar 1999 {published data only}
- Benattar C, Audibert F, Taieb J, Ville Y, Roberto A, Lindenbaum A, et al. Efficiency of ultrasound and biochemical markers for Down's syndrome risk screening. A prospective study. Fetal Diagnosis and Therapy 1999;14(2):112‐7. [DOI] [PubMed] [Google Scholar]
Bestwick 2010 {published data only}
- Bestwick JP, Huttly WJ, Wald NJ. Distribution of nuchal translucency in antenatal screening for Down's syndrome.[Erratum appears in J Med Screen. 2010 Jun;17(2):106]. Journal of Medical Screening 2010;17(1):8‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bestwick JP, Huttly WJ, Wald NJ. Evaluation of a proposed mixture model to specify the distributions of nuchal translucency measurements in antenatal screening for Down's syndrome. Journal of Medical Screening 2010;17(1):13‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Biagiotti 1998 {published data only}
- Biagiotti R, Brizzi L, Periti E, d'Agata A, Vanzi E, Cariati E. First trimester screening for Down's syndrome using maternal serum PAPP‐A and free beta‐hCG in combination with fetal nuchal translucency thickness. British Journal of Obstetrics and Gynaecology 1998;105(8):917‐20. [DOI] [PubMed] [Google Scholar]
Borenstein 2008 {published data only}
- Borenstein M, Cavoretto P, Allan L, Huggon I, Nicolaides KH. Aberrant right subclavian artery at 11 + 0 to 13 + 6 weeks of gestation in chromosomally normal and abnormal fetuses. Ultrasound in Obstetrics & Gynecology 2008;31(1):20‐4. [DOI] [PubMed] [Google Scholar]
Borrell 2005 {published data only}
- Borrell A, Casals E, Fortuny A, Farre MT, Gonce A, Sanchez A, et al. First‐trimester screening for trisomy 21 combining biochemistry and ultrasound at individually optimal gestational ages. An interventional study. Prenatal Diagnosis 2004;24(7):541‐5. [DOI] [PubMed] [Google Scholar]
- Borrell A, Gonce A, Martinez JM, Borobio V, Fortuny A, Coll O, et al. First‐trimester screening for Down syndrome with ductus venosus Doppler studies in addition to nuchal translucency and serum markers. Prenatal Diagnosis 2005;25(10):901‐5. [DOI] [PubMed] [Google Scholar]
Borrell 2009 {published data only}
- Borrell A, Borobio V, Bestwick JP, Wald NJ. Ductus venosus pulsatility index as an antenatal screening marker for Down's syndrome: use with the Combined and Integrated tests. Journal of Medical Screening 2009;16(3):112‐8. [DOI] [PubMed] [Google Scholar]
Brameld 2008 {published data only}
- Brameld KJ, Dickinson JE, O'Leary P, Bower C, Goldblatt J, Hewitt B, et al. First trimester predictors of adverse pregnancy outcomes. Australian & New Zealand Journal of Obstetrics & Gynaecology 2008;48(6):529‐35. [DOI] [PubMed] [Google Scholar]
Brizot 2001 {published data only}
- Brizot ML, Carvalho MH, Liao AW, Reis NS, Armbruster‐Moraes E, Zugaib M. First‐trimester screening for chromosomal abnormalities by fetal nuchal translucency in a Brazilian population. Ultrasound in Obstetrics & Gynecology 2001;18(6):652‐5. [DOI] [PubMed] [Google Scholar]
Centini 2005 {published data only}
- Centini G, Rosignoli L, Scarinci R, Faldini E, Morra C, Centini G, et al. Re‐evaluation of risk for Down syndrome by means of the combined test in pregnant women of 35 years or more. Prenatal Diagnosis 2005;25(2):133‐6. [DOI] [PubMed] [Google Scholar]
Chasen 2003 {published data only}
- Chasen ST, Sharma G, Kalish RB, Chervenak FA. First‐trimester screening for aneuploidy with fetal nuchal translucency in a United States population. Ultrasound in Obstetrics & Gynecology 2003;22(2):149‐51. [DOI] [PubMed] [Google Scholar]
Chen 2009 {published data only}
- Chen M, Yang X, Leung TY, Sahota DS, Fung TY, Chan LW, et al. Study on the applicability of frontomaxillary facial angle in the first‐trimester trisomy 21 fetuses in Chinese population. Prenatal Diagnosis 2009;29(12):1141‐4. [DOI] [PubMed] [Google Scholar]
Christiansen 2005 {published data only}
- Christiansen M, Norgaard‐Pedersen B. Inhibin A is a maternal serum marker for Down's syndrome early in the first trimester. Clinical Genetics 2005;68(1):35‐9. [DOI] [PubMed] [Google Scholar]
Christiansen 2009 {published data only}
- Christiansen M. Placental growth hormone and growth hormone binding protein are first trimester maternal serum markers of Down syndrome. Prenatal Diagnosis 2009;29(13):1249‐55. [DOI] [PubMed] [Google Scholar]
Christiansen 2010 {published data only}
- Christiansen M, Pihl K, Hedley PL, Gjerris AC, Lind PO, Larsen SO, et al. ADAM 12 may be used to reduce the false positive rate of first trimester combined screening for Down syndrome. Prenatal Diagnosis 2010;30(2):110‐4. [DOI] [PubMed] [Google Scholar]
Cicero 2004a {published data only}
- Cicero S, Curcio P, Rembouskos G, Sonek J, Nicolaides KH. Maxillary length at 11‐14 weeks of gestation in fetuses with trisomy 21. Ultrasound in Obstetrics & Gynecology 2004;24(1):19‐22. [DOI] [PubMed] [Google Scholar]
Cicero 2006 {published data only}
- Cicero S, Avgidou K, Rembouskos G, Kagan KO, Nicolaides KH. Nasal bone in first‐trimester screening for trisomy 21.[see comment]. American Journal of Obstetrics and Gynecology 2006;195(1):109‐14. [DOI] [PubMed] [Google Scholar]
- Cicero S, Bindra R, Rembouskos G, Spencer K, Nicolaides KH. Integrated ultrasound and biochemical screening for trisomy 21 using fetal nuchal translucency, absent fetal nasal bone, free beta‐hCG and PAPP‐A at 11 to 14 weeks. Prenatal Diagnosis 2003;23(4):306‐10. [DOI] [PubMed] [Google Scholar]
- Cicero S, Bindra R, Rembouskos G, Tripsanas C, Nicolaides KH. Fetal nasal bone length in chromosomally normal and abnormal fetuses at 11‐14 weeks of gestation. Journal of Maternal‐Fetal & Neonatal Medicine 2002;11(6):400‐2. [DOI] [PubMed] [Google Scholar]
- Cicero S, Curcio P, Papageorghiou A, Sonek J, Nicolaides K. Absence of nasal bone in fetuses with trisomy 21 at 11‐14 weeks of gestation: an observational study. Lancet 2001;358(9294):1665‐7. [DOI] [PubMed] [Google Scholar]
- Cicero S, Longo D, Rembouskos G, Sacchini C, Nicolaides KH. Absent nasal bone at 11‐14 weeks of gestation and chromosomal defects. Ultrasound in Obstetrics & Gynecology 2003;22(1):31‐5. [DOI] [PubMed] [Google Scholar]
- Cicero S, Rembouskos G, Vandecruys H, Hogg M, Nicolaides KH. Likelihood ratio for trisomy 21 in fetuses with absent nasal bone at the 11‐14‐week scan. Ultrasound in Obstetrics & Gynecology 2004;23(3):218‐23. [DOI] [PubMed] [Google Scholar]
- Cicero S, Spencer K, Avgidou K, Faiola S, Nicolaides KH. Maternal serum biochemistry at 11‐13(+6) weeks in relation to the presence or absence of the fetal nasal bone on ultrasonography in chromosomally abnormal fetuses: An updated analysis of integrated ultrasound and biochemical screening. Prenatal Diagnosis 2005;25(11):977‐83. [DOI] [PubMed] [Google Scholar]
Cocciolone 2008 FTS {published data only}
- Cocciolone R, Brameld K, O'Leary P, Haan E, Muller P, Shand K. Combining first and second trimester markers for Down syndrome screening: think twice. Australian & New ZealandJournal of Obstetrics & Gynaecology 2008;48(5):492‐500. [DOI] [PubMed] [Google Scholar]
Cowans 2009 {published data only}
- Cowans NJ, Stamatopoulou A, Maiz N, Spencer K, Nicolaides KH. The impact of fetal gender on first trimester nuchal translucency and maternal serum free beta‐hCG and PAPP‐A MoM in normal and trisomy 21 pregnancies. Prenatal Diagnosis 2009;29(6):578‐81. [DOI] [PubMed] [Google Scholar]
Cowans 2010 {published data only}
- Cowans NJ, Stamatopoulou A, Spencer K. First trimester maternal serum placental growth factor in trisomy 21 pregnancies. Prenatal Diagnosis 2010;30(5):449‐53. [DOI] [PubMed] [Google Scholar]
Crossley 2002 {published data only}
- Crossley JA, Aitken DA, Cameron AD, McBride E, Connor JM. Combined ultrasound and biochemical screening for Down's syndrome in the first trimester: a Scottish multicentre study. BJOG: international journal of obstetrics and gynaecology 2002;109(6):667‐76. [DOI] [PubMed] [Google Scholar]
De Graaf 1999 {published data only}
- Graaf I, Pajkrt E, Bilardo CM, Leschot NJ, Cuckle HS, Lith JM. Early pregnancy screening for fetal aneuploidy with serum markers and nuchal translucency. Prenatal Diagnosis 1999;19(5):458‐62. [DOI] [PubMed] [Google Scholar]
Ekelund 2008 {published data only}
- Ekelund CK, Jorgensen FS, Petersen OB, Sundberg K, Tabor A, Danish Fetal Medicine Research Group. Impact of a new national screening policy for Down's syndrome in Denmark: population based cohort study. BMJ 2008;337:a2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gasiorek‐Wiens 2001 {published data only}
- Gasiorek‐Wiens A, Tercanli S, Kozlowski P, Kossakiewicz A, Minderer S, Meyberg H, et al. Screening for trisomy 21 by fetal nuchal translucency and maternal age: a multicenter project in Germany, Austria and Switzerland. Ultrasound in Obstetrics & Gynecology. 2001;18(6):645‐8. [DOI] [PubMed] [Google Scholar]
Gasiorek‐Wiens 2010 {published data only}
- Gasiorek‐Wiens A, Kotsis S, Staboulidou I, Stumm M, Wegner RD, Soergel P, et al. A mixture model of nuchal translucency thickness in screening for chromosomal defects: validation of a single operator dataset. Prenatal Diagnosis 2010;30(11):1100‐6. [DOI] [PubMed] [Google Scholar]
Go 2005 {published data only}
- Go AT, Hupkes HW, Lomecky M, Twisk J, Blankenstein JM, Vugt JM. [Evaluation of a programme for the prenatal screening for Down's syndrome by ultrasonographic nuchal translucency measurement and serum determinations in the first trimester of pregnancy].[see comment]. [Dutch]. Nederlands Tijdschrift voor Geneeskunde 2005;149(50):2795‐9. [PubMed] [Google Scholar]
Gyselaers 2005 {published data only}
- Gyselaers WJ, Vereecken AJ, Herck EJ, Straetmans DP, Jonge ET, Ombelet WU, et al. Population screening for fetal trisomy 21: easy access to screening should be balanced against a uniform ultrasound protocol. Prenatal Diagnosis 2005;25(11):984‐90. [DOI] [PubMed] [Google Scholar]
Habayeb 2010 {published data only}
- Habayeb O, Goodburn S, Chudleigh T, Brockelsby J, Missfelder‐Lobos H, Hackett G, et al. The NTplus method of screening for Down syndrome: achieving the 2010 targets?. Prenatal Diagnosis 2010;30(5):434‐7. [DOI] [PubMed] [Google Scholar]
Hadlow 2005 {published data only}
- Hadlow NC, Hewitt BG, Dickinson JE, Jacoby P, Bower C. Community‐based screening for Down's Syndrome in the first trimester using ultrasound and maternal serum biochemistry. BJOG: international journal of obstetrics and gynaecology 2005;112(11):1561‐4. [DOI] [PubMed] [Google Scholar]
Hafner 1998 {published data only}
- Hafner E, Schuchter K, Liebhart E, Philipp K. Results of routine fetal nuchal translucency measurement at weeks 10‐13 in 4233 unselected pregnant women. Prenatal Diagnosis 1998;18(1):29‐34. [PubMed] [Google Scholar]
Has 2008 {published data only}
- Has R, Kalelioglu I, Yuksel A, Ibrahimoglu L, Ermis H, Yildirim A. Fetal nasal bone assessment in first trimester down syndrome screening. Fetal Diagnosis and Therapy 2008;24(1):61‐6. [DOI] [PubMed] [Google Scholar]
Hewitt 1996 {published data only}
- Hewitt BG, Crespigny L, Sampson AJ, Ngu AC, Shekleton P, Robinson HP. Correlation between nuchal thickness and abnormal karyotype in first trimester fetuses. Medical Journal of Australia 1996;165(7):365‐8. [DOI] [PubMed] [Google Scholar]
Hormansdorfer 2011 {published data only}
- Hormansdorfer C, Corral A, Scharf A, Vaske B, Hillemanns P, Schmidt P. [Comparison of current methods of prenatal screening for down syndrome]. [Spanish]. Revista Espanola de Salud Publica 2010;84(1):43‐51. [DOI] [PubMed] [Google Scholar]
- Hormansdorfer C, Golatta M, Scharf A, Hillemanns P, Schmidt P. Age‐independent first trimester screening for Down syndrome: analysis of three modified software programs with 6,508 pregnancies. Archives of Gynecology and Obstetrics 2011;283(4):749‐54. [DOI] [PubMed] [Google Scholar]
- Hormansdorfer C, Scharf A, Golatta M, Vaske B, Corral A, Hillemanns P, et al. Comparison of Prenatal Risk Calculation (PRC) with PIA Fetal Database software in first‐trimester screening for fetal aneuploidy. Ultrasound in Obstetrics & Gynecology 2009;33(2):147‐51. [DOI] [PubMed] [Google Scholar]
- Hormansdorfer C, Scharf A, Golatta M, Vaske B, Hillemanns P, Schmidt P. Preliminary analysis of the new 'Prenatal Risk Calculation (PRC)' software. Archives of Gynecology and Obstetrics 2009;279(4):511‐5. [DOI] [PubMed] [Google Scholar]
Huang 2010 {published data only}
- Huang SY, Shaw SW, Cheuh HY, Cheng PJ. Intracardiac echogenic focus and trisomy 21 in a population previously evaluated by first‐trimester combined screening. Acta Obstetricia et Gynecologica Scandinavica 2010;89(8):1017‐23. [DOI] [PubMed] [Google Scholar]
Jaques 2007 {published data only}
- Jaques AM, Halliday JL, Francis I, Bonacquisto L, Forbes R, Cronin A, et al. Follow up and evaluation of the Victorian first‐trimester combined screening programme for Down syndrome and trisomy 18. BJOG: international journal of obstetrics and gynaecology 2007;114(7):812‐8. [DOI] [PubMed] [Google Scholar]
Jaques 2010 FTS {published data only}
- Jaques AM, Collins VR, Muggli EE, Amor DJ, Francis I, Sheffield LJ, et al. Uptake of prenatal diagnostic testing and the effectiveness of prenatal screening for Down syndrome. Prenatal Diagnosis 2010;30(6):522‐30. [DOI] [PubMed] [Google Scholar]
Kagan 2010 {published data only}
- Kagan KO, Cicero S, Staboulidou I, Wright D, Nicolaides KH. Fetal nasal bone in screening for trisomies 21, 18 and 13 and Turner syndrome at 11‐13 weeks of gestation. Ultrasound in Obstetrics & Gynecology 2009;33(3):259‐64. [DOI] [PubMed] [Google Scholar]
- Kagan KO, Staboulidou I, Cruz J, Wright D, Nicolaides KH. Two‐stage first‐trimester screening for trisomy 21 by ultrasound assessment and biochemical testing. Ultrasound in Obstetrics & Gynecology 2010;36(5):542‐7. [DOI] [PubMed] [Google Scholar]
- Kagan KO, Valencia C, Livanos P, Wright D, Nicolaides KH. Tricuspid regurgitation in screening for trisomies 21, 18 and 13 and Turner syndrome at 11+0 to 13+6 weeks of gestation. Ultrasound in Obstetrics & Gynecology 2009;33(1):18‐22. [DOI] [PubMed] [Google Scholar]
- Kagan KO, Wright D, Valencia C, Maiz N, Nicolaides KH. Screening for trisomies 21, 18 and 13 by maternal age, fetal nuchal translucency, fetal heart rate, free beta‐hCG and pregnancy‐associated plasma protein‐A. Human Reproduction 2008;23(9):1968‐75. [DOI] [PubMed] [Google Scholar]
Kim 2006 {published data only}
- Kim MH, Park SH, Cho HJ, Choi JS, Kim JO, Ahn HK, et al. Threshold of nuchal translucency for the detection of chromosomal aberration: comparison of different cut‐offs. Journal of Korean Medical Science 2006;21(1):11‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Koster 2011 {published data only}
- Koster MP, Wortelboer EJ, Stoutenbeek P, Visser GH, Schielen PC. Modeling Down syndrome screening performance using first‐trimester serum markers. Ultrasound in Obstetrics & Gynecology 2011;38(2):134‐9. [DOI] [PubMed] [Google Scholar]
Kozlowski 2007 GC {published data only}
- Kozlowski P, Knippel AJ, Stressig R. Comparing first trimester screening performance: routine care gynaecologists' practices vs. prenatal centre. Ultraschall in der Medizin 2007;28(3):291‐5. [DOI] [PubMed] [Google Scholar]
Kozlowski 2007 PC {published data only}
- Kozlowski P, Knippel AJ, Stressig R. Comparing first trimester screening performance: routine care gynaecologists' practices vs. prenatal centre. Ultraschall in der Medizin 2007;28(3):291‐5. [DOI] [PubMed] [Google Scholar]
Krantz 2000 {published data only}
- Krantz DA, Hallahan TW, Orlandi F, Buchanan P, Larsen JW Jr, Macri JN. First‐trimester Down syndrome screening using dried blood biochemistry and nuchal translucency. Obstetrics & Gynecology 2000;96(2):207‐13. [DOI] [PubMed] [Google Scholar]
Kublickas 2009 {published data only}
- Kublickas M, Crossley J, Aitken D. Screening for Down's syndrome in the first trimester: combined risk calculation, methodology, and validation of a web‐based system. Acta Obstetricia et Gynecologica Scandinavica 2009;88(6):635‐8. [DOI] [PubMed] [Google Scholar]
Kuc 2010 {published data only}
- Kuc S, Koster MP, Visser GH, Schielen PC. Performance of first‐trimester serum screening for trisomy 21 before and from 11 + 0 weeks of gestational age in The Netherlands. Prenatal Diagnosis 2010;30(9):906‐8. [DOI] [PubMed] [Google Scholar]
Lam 2002 {published data only}
- Lam YH, Lee CP, Sin SY, Tang R, Wong HS, Wong SF, et al. Comparison and integration of first trimester fetal nuchal translucency and second trimester maternal serum screening for fetal Down syndrome. Prenatal Diagnosis 2002;22(8):730‐5. [DOI] [PubMed] [Google Scholar]
Leung 2009 {published data only}
- Leung TY, Chan LW, Law LW, Sahota DS, Fung TY, Leung TN, et al. First trimester combined screening for Trisomy 21 in Hong Kong: outcome of the first 10,000 cases. Journal of Maternal‐Fetal & Neonatal Medicine 2009;22(4):300‐4. [DOI] [PubMed] [Google Scholar]
MacRae 2008 {published data only}
- MacRae R, Ojutiku D, Duke‐MacRae J, Usifo F, Ekong M. Evaluating nuchal translucency scans performed for trisomy screening in a district general hospital between July 1998 and January 2004. Journal of Obstetrics and Gynaecology 2008;28(7):683‐7. [DOI] [PubMed] [Google Scholar]
Maiz 2007 {published data only}
- Maiz N, Dagklis T, Huggon I, Allan L, Nicolaides KH. The mitral gap at 11 + 0 to 13 + 6 weeks: marker of trisomy 21 or artifact?. Ultrasound in Obstetrics & Gynecology 2007;30(6):813‐8. [DOI] [PubMed] [Google Scholar]
Maiz 2009 {published data only}
- Maiz N, Valencia C, Kagan KO, Wright D, Nicolaides KH. Ductus venosus Doppler in screening for trisomies 21, 18 and 13 and Turner syndrome at 11‐13 weeks of gestation. Ultrasound in Obstetrics & Gynecology 2009;33(5):512‐7. [DOI] [PubMed] [Google Scholar]
Malone 2004 {published data only}
- Malone FD, Ball RH, Nyberg DA, Comstock CH, Saade G, Berkowitz RL, et al. First‐trimester nasal bone evaluation for aneuploidy in the general population. Obstetrics and Gynecology 2004 Dec;104(6):1222‐8. [DOI] [PubMed] [Google Scholar]
Malone 2005 {published data only}
- Malone FD, Canick JA, Ball RH, Nyberg DA, Comstock CH, Bukowski R, et al. First‐trimester or second‐trimester screening, or both, for Down's syndrome.[see comment]. New England Journal of Medicine 2005;353(19):2001‐11. [DOI] [PubMed] [Google Scholar]
Marchini 2010 {published data only}
- Marchini G, Rosati A, Ribiani E, Romanelli M, Porcaro G, Clerici G. [Nuchal translucency and combined test: what are the implications in clinical practice?]. [Italian]. Minerva Ginecologica 2010;62(3):187‐93. [PubMed] [Google Scholar]
Marsis 2004 {published data only}
- Marsis IO. Screening for down syndrome using nuchal translucency thickness and nasal bone examination at advanced maternal age in Jakarta: A preliminary report. Journal of Medical Ultrasound 2004;12(1):1‐6. [Google Scholar]
Marsk 2006 {published data only}
- Marsk A, Grunewald C, Saltvedt S, Valentin L, Almstrom H. If nuchal translucency screening is combined with first‐trimester serum screening the need for fetal karyotyping decreases. Acta Obstetricia et Gynecologica Scandinavica 2006;85(5):534‐8. [DOI] [PubMed] [Google Scholar]
Matias 1998 {published data only}
- Matias A, Gomes C, Flack N, Montenegro N, Nicolaides KH. Screening for chromosomal abnormalities at 10‐14 weeks: the role of ductus venosus blood flow. Ultrasound in Obstetrics & Gynecology 1998;12(6):380‐4. [DOI] [PubMed] [Google Scholar]
Matias 2001 {published data only}
- Matias A, Montenegro N. Ductus venosus blood flow in chromosomally abnormal fetuses at 11 to 14 weeks of gestation. Seminars in Perinatology 2001;25(1):32‐7. [DOI] [PubMed] [Google Scholar]
Mavrides 2002 {published data only}
- Mavrides E, Sairam S, Hollis B, Thilaganathan B. Screening for aneuploidy in the first trimester by assessment of blood flow in the ductus venosus. BJOG: an international journal of obstetrics and gynaecology 2002;109(9):1015‐9. [DOI] [PubMed] [Google Scholar]
Maxwell 2011 FTS {published data only}
- Maxwell S, Brameld K, Bower C, Dickinson JE, Goldblatt J, Hadlow N, et al. Socio‐demographic disparities in the uptake of prenatal screening and diagnosis in Western Australia. Australian & New Zealand Journal of Obstetrics & Gynaecology 2011;51(1):9‐16. [DOI] [PubMed] [Google Scholar]
Maymon 2005 {published data only}
- Maymon R, Sharony R, Grinshpun‐Cohen J, Itzhaky D, Herman A, Reish O. The best marker combination using the integrated screening test approach for detecting various chromosomal aneuploidies. Journal of Perinatal Medicine 2005;33(5):392‐8. [DOI] [PubMed] [Google Scholar]
Maymon 2008 {published data only}
- Maymon R, Zimerman AL, Weinraub Z, Herman A, Cuckle H. Correlation between nuchal translucency and nuchal skin‐fold measurements in Down syndrome and unaffected fetuses. Ultrasound in Obstetrics & Gynecology 2008;32(4):501‐5. [DOI] [PubMed] [Google Scholar]
Merz 2011 {published data only}
- Merz E, Thode C, Eiben B, Faber R, Hackeloer BJ, Huesgen G, et al. Individualized correction for maternal weight in calculating the risk of chromosomal abnormalities with first‐trimester screening data. Ultraschall in der Medizin 2011;32(1):33‐9. [DOI] [PubMed] [Google Scholar]
Michailidis 2001 {published data only}
- Michailidis GD, Spencer K, Economides DL. The use of nuchal translucency measurement and second trimester biochemical markers in screening for Down's syndrome. BJOG: an international journal of obstetrics and gynaecology 2001;108(10):1047‐52. [DOI] [PubMed] [Google Scholar]
Molina 2010 high risk {published data only}
- Molina Garcia FS, Carrillo Badillo MP, Zaragoza Garcia EA, Fernandez de Santos AG, Montoya Ventoso F. Analysis of secondary ultrasound markers in the first trimester before chorionic villus sampling. Prenatal Diagnosis 2010;30(12):1117‐20. [DOI] [PubMed] [Google Scholar]
Molina 2010 screening {published data only}
- Molina Garcia FS, Carrillo Badillo MP, Zaragoza Garcia EA, Fernandez de Santos AG, Montoya Ventoso F. Analysis of secondary ultrasound markers in the first trimester before chorionic villus sampling. Prenatal Diagnosis 2010;30(12):1117‐20. [DOI] [PubMed] [Google Scholar]
Monni 2005 {published data only}
- Monni G, Zoppi MA, Ibba RM, Floris M, Manca F, Axiana C. Nuchal translucency and nasal bone for trisomy 21 screening: single center experience. Croatian Medical Journal 2005;46(5):786‐91. [PubMed] [Google Scholar]
- Zoppi MA, Ibba RM, Axiana C, Floris M, Manca F, Monni G. Absence of fetal nasal bone and aneuploidies at first‐trimester nuchal translucency screening in unselected pregnancies. Prenatal Diagnosis 2003;23(6):496‐500. [DOI] [PubMed] [Google Scholar]
Montalvo 2005 {published data only}
- Montalvo J, Gómez ML, Ortega MD, Soler P, Herraiz I, Herraiz MA. First trimester combined screening for chromosomal defects: Our results in a population with a high percent of women aged 35 or older. Ultrasound Review of Obstetrics and Gynecology 2005;5(3):178‐85. [Google Scholar]
Moon 2007 {published data only}
- Moon MH, Cho JY, Lee YM, Jung SI, Yang JH, Kim MY, et al. First‐trimester screening for Down syndrome; the role of nasal bone assessment in the Korean population. Prenatal Diagnosis 2007;27(9):830‐4. [DOI] [PubMed] [Google Scholar]
Muller 2003 {published data only}
- Muller F, Benattar C, Audibert F, Roussel N, Dreux S, Cuckle H. First‐trimester screening for Down syndrome in France combining fetal nuchal translucency measurement and biochemical markers. Prenatal Diagnosis 2003;23(10):833‐6. [DOI] [PubMed] [Google Scholar]
Nicolaides 1992 {published data only}
- Nicolaides K, Azar G, Byrne D, Mansur C, Marks K. Fetal nuchal translucency: ultrasound screening for chromosomal defects in the first trimester of pregnancy. BMJ 1992;304:867‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nicolaides 2005 {published data only}
- Avgidou K, Papageorghiou A, Bindra R, Spencer K, Nicolaides KH. Prospective first‐trimester screening for trisomy 21 in 30,564 pregnancies. American Journal of Obstetrics and Gynecology 2005;192(6):1761‐7. [DOI] [PubMed] [Google Scholar]
- Bindra R, Heath V, Liao A, Spencer K, Nicolaides KH. One‐stop clinic for assessment of risk for trisomy 21 at 11‐14 weeks: a prospective study of 15 030 pregnancies. Ultrasound in Obstetrics & Gynecology. 2002;20(3):219‐25. [DOI] [PubMed] [Google Scholar]
- Nicolaides KH, Spencer K, Avgidou K, Faiola S, Falcon O. Multicenter study of first‐trimester screening for trisomy 21 in 75 821 pregnancies: results and estimation of the potential impact of individual risk‐orientated two‐stage first‐trimester screening. Ultrasound in Obstetrics & Gynecology 2005;25(3):221‐6. [DOI] [PubMed] [Google Scholar]
- Spencer K, Spencer CE, Power M, Dawson C, Nicolaides K. Screening for chromosomal abnormalities in the first trimester using ultrasound and maternal serum biochemistry in a one‐stop clinic: a review of three years prosepctive experience. BJOG: an international journal of obstetrics and gynaecology 2003;110:281‐6. [PubMed] [Google Scholar]
- Spencer K, Spencer CE, Power M, Moakes A, Nicolaides KH. One stop clinic for assessment of risk for fetal anomalies: a report of the first year of prospective screening for chromosomal anomalies in the first trimester. BJOG: an international journal of obstetrics and gynaecology 2000;107(10):1271‐5. [DOI] [PubMed] [Google Scholar]
Niemimaa 2001 {published data only}
- Niemimaa M, Suonpaa M, Perheentupa A, Seppala M, Heinonen S, Laitinen P, et al. Evaluation of first trimester maternal serum and ultrasound screening for Down's syndrome in Eastern and Northern Finland. European Journal of Human Genetics 2001;9(6):404‐8. [DOI] [PubMed] [Google Scholar]
Noble 1995 {published data only}
- Brizot ML, Snijders RJM, Butler J, Bersinger NA, Nicolaides KH. Maternal serum hCG and fetal nuchal translucency thickness for the prediction of fetal trisomies in the first trimester of pregnancy. British Journal of Obstetrics and Gynaecology 1995;102:127‐32. [DOI] [PubMed] [Google Scholar]
- Noble PL, Abraha HD, Snijders RJ, Sherwood R, Nicolaides KH. Screening for fetal trisomy 21 in the first trimester of pregnancy: maternal serum free beta‐hCG and fetal nuchal translucency thickness. Ultrasound in Obstetrics & Gynecology 1995;6(6):390‐5. [DOI] [PubMed] [Google Scholar]
O'Callaghan 2000 {published data only}
- O'Callaghan SP, Giles WB, Raymond SP, McDougall V, Morris K, Boyd J. First trimester ultrasound with nuchal translucency measurement for Down syndrome risk estimation using software developed by the Fetal Medicine Foundation, United Kingdom‐‐the first 2000 examinations in Newcastle, New South Wales, Australia. Australian & New Zealand Journal of Obstetrics & Gynaecology 2000;40(3):292‐5. [DOI] [PubMed] [Google Scholar]
O'Leary 2006 {published data only}
- O'Leary P, Breheny N, Dickinson JE, Bower C, Goldblatt J, Hewitt B, et al. First‐trimester combined screening for Down syndrome and other fetal anomalies. Obstetrics & Gynecology 2006;107(4):869‐76. [DOI] [PubMed] [Google Scholar]
Okun 2008 FTS {published data only}
- Okun N, Summers AM, Hoffman B, Huang T, Winsor E, Chitayat D, et al. Prospective experience with integrated prenatal screening and first trimester combined screening for trisomy 21 in a large Canadian urban center. Prenatal Diagnosis 2008;28(11):987‐92. [DOI] [PubMed] [Google Scholar]
Orlandi 1997 {published data only}
- Orlandi F, Damiani G, Hallahan TW, Krantz DA, Macri JN. First‐trimester screening for fetal aneuploidy: biochemistry and nuchal translucency. Ultrasound in Obstetrics & Gynecology 1997;10(6):381‐6. [DOI] [PubMed] [Google Scholar]
Orlandi 2003 {published data only}
- Orlandi F, Bilardo CM, Campogrande M, Krantz D, Hallahan T, Rossi C, et al. Measurement of nasal bone length at 11‐14 weeks of pregnancy and its potential role in Down syndrome risk assessment. Ultrasound in Obstetrics & Gynecology 2003;22(1):36‐9. [DOI] [PubMed] [Google Scholar]
Orlandi 2005 {published data only}
- Orlandi F, Rossi C, Orlandi E, Jakil MC, Hallahan TW, Macri VJ, et al. First‐trimester screening for trisomy‐21 using a simplified method to assess the presence or absence of the fetal nasal bone. American Journal of Obstetrics and Gynecology 2005;192(4):1107‐11. [DOI] [PubMed] [Google Scholar]
Otaño 2002 {published data only}
- Otaño L, Aiello H, Igarzábal L, Matayoshi T, Gadow EC. Association between first trimester absence of fetal nasal bone on ultrasound and Down syndrome. Prenatal Diagnosis 2002;22(10):930‐2. [DOI] [PubMed] [Google Scholar]
Pajkrt 1998 {published data only}
- Pajkrt E, Lith JM, Mol BW, Bleker OP, Bilardo CM. Screening for Down's syndrome by fetal nuchal translucency measurement in a general obstetric population. Ultrasound in Obstetrics & Gynecology 1998;12(3):163‐9. [DOI] [PubMed] [Google Scholar]
Pajkrt 1998a {published data only}
- Pajkrt E, Bilardo CM, Lith JM, Mol BW, Hansson K, Prooijen Knegt AC, et al. Ultrasound screening for fetal trisomies at 10‐14 weeks' gestation. Early Human Development 1996;47 Suppl:S35‐6. [DOI] [PubMed] [Google Scholar]
- Pajkrt E, Mol BW, Lith JM, Bleker OP, Bilardo CM. Screening for Down's syndrome by fetal nuchal translucency measurement in a high‐risk population. Ultrasound in Obstetrics & Gynecology 1998;12(3):156‐62. [DOI] [PubMed] [Google Scholar]
Palomaki 2007 FTS {published data only}
- Palomaki GE, Neveux LM, Haddow JE, Wyatt P. Hyperglycosylated‐hCG (h‐hCG) and Down syndrome screening in the first and second trimesters of pregnancy. Prenatal Diagnosis 2007;27(9):808‐13. [DOI] [PubMed] [Google Scholar]
Perni 2006 {published data only}
- Perni SC, Predanic M, Kalish RB, Chervenak FA, Chasen ST. Clinical use of first‐trimester aneuploidy screening in a United States population can replicate data from clinical trials. American Journal of Obstetrics and Gynecology 2006;194(1):127‐30. [DOI] [PubMed] [Google Scholar]
Prefumo 2005 {published data only}
- Prefumo F, Sethna F, Sairam S, Bhide A, Thilaganathan B. First‐trimester ductus venosus, nasal bones, and Down syndrome in a high‐risk population. Obstetrics & Gynecology 2005;105(6):1348‐54. [DOI] [PubMed] [Google Scholar]
Prefumo 2006 {published data only}
- Prefumo F, Sairam S, Bhide A, Thilaganathan B. First‐trimester nuchal translucency, nasal bones, and trisomy 21 in selected and unselected populations. American Journal of Obstetrics and Gynecology 2006;3:828‐33. [DOI] [PubMed] [Google Scholar]
Ramos‐Corpas 2006 {published data only}
- Ramos Corpas D, Santiago JC, Montoya F. Ultrasonographic evaluation of fetal nasal bone in a low‐risk population at 11‐13 + 6 gestational weeks. Prenatal Diagnosis 2006;26(2):112‐7. [DOI] [PubMed] [Google Scholar]
Rissanen 2007 {published data only}
- Rissanen A, Niemimaa M, Suonpaa M, Ryynanen M, Heinonen S. First trimester Down's syndrome screening shows high detection rate for trisomy 21, but poor performance in structural abnormalities‐‐regional outcome results. Fetal Diagnosis and Therapy 2007;22(1):45‐50. [DOI] [PubMed] [Google Scholar]
Rozenberg 2002 {published data only}
- Rozenberg P, Malagrida L, Cuckle H, Durand‐Zaleski I, Nisand I, Audibert F, et al. Down's syndrome screening with nuchal translucency at 12(+0)‐14(+0) weeks and maternal serum markers at 14(+1)‐17(+0) weeks: a prospective study. Human Reproduction 2002;17(4):1093‐8. [DOI] [PubMed] [Google Scholar]
Rozenberg 2007 {published data only}
- Rozenberg P, Bussieres L, Chevret S, Bernard JP, Malagrida L, Cuckle H, et al. [Screening for Down syndrome using first‐trimester combined screening followed by second trimester ultrasound examination in an unselected population]. [French]. Gynecologie, Obstetrique & Fertilite 2007;35(4):303‐11. [DOI] [PubMed] [Google Scholar]
Sahota 2010 {published data only}
- Sahota DS, Leung TY, Chan LW, Law LW, Fung TY, Chen M, et al. Comparison of first‐trimester contingent screening strategies for Down syndrome. Ultrasound in Obstetrics & Gynecology 2010;35(3):286‐91. [DOI] [PubMed] [Google Scholar]
Salomon 2010 {published data only}
- Salomon LJ, Chevret S, Bussieres L, Ville Y, Rozenberg P. Down syndrome screening using first‐trimester combined tests and contingent use of femur length at routine anomaly scan. Prenatal Diagnosis 2010;30(8):783‐9. [DOI] [PubMed] [Google Scholar]
Santiago 2007 {published data only}
- Santiago JC, Ramos‐Corpas D. Delta‐NT and center‐specific ultrasound nuchal translucency medians. Ultrasound in Obstetrics & Gynecology 2007;30(7):934‐40. [DOI] [PubMed] [Google Scholar]
Sau 2001 {published data only}
- Sau A, Langford K, Auld B, Maxwell D. Screening for trisomy 21: The significance of a positive second trimester serum screen in women screen negative after a nuchal translucency scan. Journal of Obstetrics and Gynaecology 2001;21(2):145‐8. [DOI] [PubMed] [Google Scholar]
Schaelike 2009 {published data only}
- Schaelike M, Kossakiewicz M, Kossakiewicz A, Schild RL. Examination of a first‐trimester Down syndrome screening concept on a mix of 11,107 high‐ and low‐risk patients at a private center for prenatal medicine in Germany. European Journal of Obstetrics, Gynecology, & Reproductive Biology 2009;144(2):140‐5. [DOI] [PubMed] [Google Scholar]
Schielen 2006 {published data only}
- Schielen PC, Leeuwen‐Spruijt M, Belmouden I, Elvers LH, Jonker M, Loeber JG. Multi‐centre first‐trimester screening for Down syndrome in the Netherlands in routine clinical practice. Prenatal Diagnosis 2006;26(8):711‐8. [DOI] [PubMed] [Google Scholar]
Schuchter 2001 {published data only}
- Schuchter K, Hafner E, Stangl G, Ogris E, Philipp K. Sequential screening for trisomy 21 by nuchal translucency measurement in the first trimester and maternal serum biochemistry in the second trimester in a low‐risk population. Ultrasound in Obstetrics & Gynecology 2001;18(1):23‐5. [DOI] [PubMed] [Google Scholar]
Schuchter 2002 {published data only}
- Schuchter K, Hafner E, Stangl G, Metzenbauer M, Hofinger D, Philipp K. The first trimester 'combined test' for the detection of Down syndrome pregnancies in 4939 unselected pregnancies. Prenatal Diagnosis 2002;22(3):211‐5. [DOI] [PubMed] [Google Scholar]
Schwarzler 1999 {published data only}
- Schwarzler P, Carvalho JS, Senat MV, Masroor T, Campbell S, Ville Y. Screening for fetal aneuploidies and fetal cardiac abnormalities by nuchal translucency thickness measurement at 10‐14 weeks of gestation as part of routine antenatal care in an unselected population. British Journal of Obstetrics and Gynaecology 1999;106(10):1029‐34. [DOI] [PubMed] [Google Scholar]
Scott 2004 {published data only}
- Scott F, Peters H, Bonifacio M, McLennan A, Boogert A, Kesby G, et al. Prospective evaluation of a first trimester screening program for Down syndrome and other chromosomal abnormalities using maternal age, nuchal translucency and biochemistry in an Australian population. Australian & New Zealand Journal of Obstetrics & Gynaecology 2004;44(3):205‐9. [DOI] [PubMed] [Google Scholar]
Sepulveda 2007 {published data only}
- Sepulveda W, Wong AE, Dezerega V. First‐trimester ultrasonographic screening for trisomy 21 using fetal nuchal translucency and nasal bone. Obstetrics & Gynecology 2007;109(5):1040‐5. [DOI] [PubMed] [Google Scholar]
Snijders 1998 {published data only}
- Nicolaides KH, Brizot ML, Snijders RJM. Fetal nuchal translucency: Ultrasound screening for fetal trisomy in the first trimester of pregnancy. British Journal of Obstetrics and Gynaecology 1994;101(9):782‐6. [DOI] [PubMed] [Google Scholar]
- Pandya PP, Snijders RJ, Johnson SP, Lourdes Brizot M, Nicolaides KH. Screening for fetal trisomies by maternal age and fetal nuchal translucency thickness at 10 to 14 weeks of gestation.[see comment]. British Journal of Obstetrics and Gynaecology 1995;102(12):957‐62. [DOI] [PubMed] [Google Scholar]
- Snijders RJ, Noble P, Sebire N, Souka A, Nicolaides KH. UK multicentre project on assessment of risk of trisomy 21 by maternal age and fetal nuchal‐translucency thickness at 10‐14 weeks of gestation. Fetal Medicine Foundation First Trimester Screening Group.[see comment][comment]. Lancet 1998;352(9125):343‐6. [DOI] [PubMed] [Google Scholar]
Sorensen 2011 {published data only}
- Sorensen S, Momsen G, Sundberg K, Friis‐Hansen L, Jorgensen FS. First‐trimester risk calculation for trisomy 13, 18, and 21: comparison of the screening efficiency between 2 locally developed programs and commercial software. Clinical Chemistry 2011;57(7):1023‐31. [DOI] [PubMed] [Google Scholar]
Spencer 1999 {published data only}
- Spencer K, Souter V, Tul N, Snijders R, Nicolaides KH. A screening program for trisomy 21 at 10‐14 weeks using fetal nuchal translucency, maternal serum free beta‐human chorionic gonadotropin and pregnancy‐associated plasma protein‐A.[see comment]. Ultrasound in Obstetrics & Gynecology 1999;13(4):231‐7. [DOI] [PubMed] [Google Scholar]
Spencer 2002 {published data only}
- Spencer K, Talbot JA, Abushoufa RA. Maternal serum hyperglycosylated human chorionic gonadotrophin (HhCG) in the first trimester of pregnancies affected by Down syndrome, using a sialic acid‐specific lectin immunoassay.[see comment]. Prenatal Diagnosis 2002;22(8):656‐62. [DOI] [PubMed] [Google Scholar]
Spencer 2008 {published data only}
- Spencer K, Cowans NJ, Uldbjerg N, Torring N. First‐trimester ADAM12s as early markers of trisomy 21: a promise still unfulfilled?. Prenatal Diagnosis 2008;28(4):338‐42. [DOI] [PubMed] [Google Scholar]
Stenhouse 2004 {published data only}
- Stenhouse EJ, Crossley JA, Aitken DA, Brogan K, Cameron AD, Connor JM. First‐trimester combined ultrasound and biochemical screening for Down syndrome in routine clinical practice. Prenatal Diagnosis 2004;24(10):774‐80. [DOI] [PubMed] [Google Scholar]
Strah 2008 {published data only}
- Strah DM, Pohar M, Gersak K. Risk assessment of trisomy 21 by maternal age and fetal nuchal translucency thickness in 7,096 unselected pregnancies in Slovenia. Journal of Perinatal Medicine 2008;36(2):145‐50. [DOI] [PubMed] [Google Scholar]
Theodoropoulos 1998 {published data only}
- Theodoropoulos P, Lolis D, Papageorgiou C, Papaioannou S, Plachouras N, Makrydimas G. Evaluation of first‐trimester screening by fetal nuchal translucency and maternal age. Prenatal Diagnosis 1998;18(2):133‐7. [PubMed] [Google Scholar]
Thilaganathan 1999 {published data only}
- Thilaganathan B, Sairam S, Michailidis G, Wathen NC. First trimester nuchal translucency: effective routine screening for Down's syndrome. British Journal of Radiology 1999;72(862):946‐8. [DOI] [PubMed] [Google Scholar]
Timmerman 2010 {published data only}
- Timmerman E, Rengerink KO, Pajkrt E, Opmeer BC, Post JA, Bilardo CM. Ductus venosus pulsatility index measurement reduces the false‐positive rate in first‐trimester screening. Ultrasound in Obstetrics & Gynecology 2010;36(6):661‐7. [DOI] [PubMed] [Google Scholar]
Torring 2010 {published data only}
- Torring N, Ball S, Wright D, Sarkissian G, Guitton M, Darbouret B. First trimester screening for trisomy 21 in gestational week 8‐10 by ADAM12‐S as a maternal serum marker. Reproductive Biology & Endocrinology 2010;8:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vadiveloo 2009 {published data only}
- Vadiveloo T, Crossley JA, Aitken DA. First‐trimester contingent screening for Down syndrome can reduce the number of nuchal translucency measurements required. Prenatal Diagnosis 2009;29(1):79‐82. [DOI] [PubMed] [Google Scholar]
Valinen 2007 {published data only}
- Valinen Y, Rapakko K, Kokkonen H, Laitinen P, Tekay A, Ahola T, et al. Clinical first‐trimester routine screening for Down syndrome in singleton pregnancies in northern Finland. American Journal of Obstetrics and Gynecology 2007;196(3):278‐5. [DOI] [PubMed] [Google Scholar]
Viora 2003 {published data only}
- Viora E, Masturzo B, Errante G, Sciarrone A, Bastonero S, Campogrande M. Ultrasound evaluation of fetal nasal bone at 11 to 14 weeks in a consecutive series of 1906 fetuses. Prenatal Diagnosis 2003;23(10):784‐7. [DOI] [PubMed] [Google Scholar]
Wald 2003 {published data only}
- Wald NJ, Rodeck C, Hackshaw AK, Rudnicka A. SURUSS in perspective. Seminars in Perinatology 2005;29(4):225‐35. [DOI] [PubMed] [Google Scholar]
- Wald NJ, Rodeck C, Hackshaw AK, Rudnicka A. SURUSS in perspective.[see comment]. BJOG: an international journal of obstetrics and gynaecology 2004;111(6):521‐31. [DOI] [PubMed] [Google Scholar]
- Wald NJ, Rodeck C, Hackshaw AK, Walters J, Chitty L, Mackinson AM. First and second trimester antenatal screening for Down's syndrome: the results of the Serum, Urine and Ultrasound Screening Study (SURUSS). Journal of Medical Screening 2003;10(2):56‐104. [DOI] [PubMed] [Google Scholar]
- Wald NJ, Rodeck C, Hackshaw AK, Walters J, Chitty L, Mackinson AM, et al. First and second trimester antenatal screening for Down's syndrome: the results of the Serum, Urine and Ultrasound Screening Study (SURUSS). Health Technology Assessment (Winchester, England) 2003;7(11):1‐77. [DOI] [PubMed] [Google Scholar]
Wapner 2003 {published data only}
- Wapner R, Thom E, Simpson JL, Pergament E, Silver R, Filkins K, et al. First‐trimester screening for trisomies 21 and 18.[see comment]. New England Journal of Medicine 2003;349(15):1405‐13. [DOI] [PubMed] [Google Scholar]
- Wapner RJ. First trimester screening: the BUN study. Seminars in Perinatology 2005;29(4):236‐9. [DOI] [PubMed] [Google Scholar]
Wax 2009 {published data only}
- Wax JR, Pinette MG, Cartin A, Blackstone J. Second‐trimester genetic sonography after first‐trimester combined screening for trisomy 21. Journal of Ultrasound in Medicine 2009;28(3):321‐5. [DOI] [PubMed] [Google Scholar]
Wojdemann 2005 {published data only}
- Wojdemann KR, Shalmi AC, Christiansen M, Larsen SO, Sundberg K, Brocks V, et al. Improved first‐trimester Down syndrome screening performance by lowering the false‐positive rate: a prospective study of 9941 low‐risk women. Ultrasound in Obstetrics & Gynecology 2005;25(3):227‐33. [DOI] [PubMed] [Google Scholar]
Wortelboer 2009 {published data only}
- Wortelboer EJ, Koster MP, Stoutenbeek P, Elvers LH, Loeber JG, Visser GH, et al. First‐trimester Down syndrome screening performance in the Dutch population; how to achieve further improvement?. Prenatal Diagnosis 2009;29(6):588‐92. [DOI] [PubMed] [Google Scholar]
Wright 2008 {published data only}
- Wright D, Kagan KO, Molina FS, Gazzoni A, Nicolaides KH. A mixture model of nuchal translucency thickness in screening for chromosomal defects. Ultrasound in Obstetrics & Gynecology 2008;31(4):376‐83. [DOI] [PubMed] [Google Scholar]
Wright 2010 {published data only}
- Wright D, Spencer K, Kagan KK, Torring N, Petersen OB, Christou A, et al. First‐trimester combined screening for trisomy 21 at 7‐14 weeks' gestation. Ultrasound in Obstetrics & Gynecology 2010;36(4):404‐11. [DOI] [PubMed] [Google Scholar]
Zoppi 2001 {published data only}
- Zoppi MA, Ibba RM, Floris M, Monni G. Fetal nuchal translucency screening in 12495 pregnancies in Sardinia. Ultrasound in Obstetrics & Gynecology 2001;18(6):649‐51. [DOI] [PubMed] [Google Scholar]
- Zoppi MA, Ibba RM, Putzolu M, Floris M, Monni G. Assessment of risk for chromosomal abnormalities at 10‐14 weeks of gestation by nuchal translucency and maternal age in 5210 fetuses at a single centre. Fetal Diagnosis and Therapy 2000;15(3):170‐3. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abbas 1995 {published data only}
- Abbas A, Chard T, Nicolaides K. Fetal and maternal hCG concentration in aneuploid pregnancies. British Journal of Obstetrics and Gynaecology 1995;102(7):561‐3. [DOI] [PubMed] [Google Scholar]
Abdul‐Hamid 2004 {published data only}
- Abdul‐Hamid S, Fox R, Martin I. Maternal serum screening for trisomy 21 in women with a false positive result in last pregnancy. Journal of Obstetrics & Gynaecology 2004;24(4):374‐6. [DOI] [PubMed] [Google Scholar]
Abraha 1999 {published data only}
- Abraha HD, Noble PL, Nicolaides KH, Sherwood RA. Maternal serum S100 protein in normal and Down syndrome pregnancies. Prenatal Diagnosis 1999;19(4):334‐6. [PubMed] [Google Scholar]
Abu‐Rustum 2010 {published data only}
- Abu‐Rustum RS, Daou L, Abu‐Rustum SE. Role of first‐trimester sonography in the diagnosis of aneuploidy and structural fetal anomalies. Journal of Ultrasound in Medicine 2010;29(10):1445‐52. [DOI] [PubMed] [Google Scholar]
Achiron 2010 {published data only}
- Achiron R, Gindes L, Gilboa Y, Weissmann‐Brenner A, Berkenstadt M. Umbilical vein anomaly in fetuses with Down syndrome. Ultrasound in Obstetrics & Gynecology 2010;35(3):297‐301. [DOI] [PubMed] [Google Scholar]
Adekunle 1999 {published data only}
- Adekunle O, Gopee A, el‐Sayed M, Thilaganathan B. Increased first trimester nuchal translucency: pregnancy and infant outcomes after routine screening for Down's syndrome in an unselected antenatal population. British Journal of Radiology 1999;72(857):457‐60. [DOI] [PubMed] [Google Scholar]
Agaard‐Tillery 2010 {published data only}
- Agaard‐Tillery KM, Flint Porter T, Malone FD, Nyberg DA, Collins J, Comstock CH, et al. Influence of maternal BMI on genetic sonography in the FaSTER trial. Prenatal Diagnosis 2010;30(1):14‐22. [DOI] [PubMed] [Google Scholar]
Aitken 1993 {published data only}
- Aitken DA, McCaw G, Crossley JA, Berry E, Connor JM, Spencer K, et al. First‐trimester biochemical screening for fetal chromosome abnormalities and neural tube defects. Prenatal Diagnosis 1993;13(8):681‐9. [DOI] [PubMed] [Google Scholar]
Aitken 1996 {published data only}
- Aitken DA, Syvertsen BS, Crossley JA, Berry E, Connor JM. Heat‐stable and immunoreactive placental alkaline phosphatase in maternal serum from Down's syndrome and trisomy 18 pregnancies.[see comment]. Prenatal Diagnosis 1996;16(11):1051‐4. [DOI] [PubMed] [Google Scholar]
Aitken 1996a {published data only}
- Aitken DA, Wallace EM, Crossley JA, Swanston IA, Pareren Y, Maarle M, et al. Dimeric Inhibin A as a marker for Down's syndrome in early pregnancy. New England Journal of Medicine 1996;334(19):1231‐6. [DOI] [PubMed] [Google Scholar]
Ajayi 2011 {published data only}
- Ajayi GO. Is there any effect of fetal gender on the markers of first trimester Down's syndrome screening?. Clinical & Experimental Obstetrics & Gynecology 2011;38(2):162‐4. [PubMed] [Google Scholar]
Akbas 2001 {published data only}
- Akbas SH, Ozben T, Alper O, Ugur A, Yucel G, Luleci G. Maternal serum screening for Down's syndrome, open neural tube defects and trisomy 18. Clinical Chemistry & Laboratory Medicine 2001;39(6):487‐90. [DOI] [PubMed] [Google Scholar]
Alexioy 2009 {published data only}
- Alexioy E, Alexioy E, Trakakis E, Kassanos D, Farmakidis G, Kondylios A, et al. Predictive value of increased nuchal translucency as a screening test for the detection of fetal chromosomal abnormalities. Journal of Maternal‐Fetal & Neonatal Medicine 2009;22(10):857‐62. [DOI] [PubMed] [Google Scholar]
Allingham‐Hawkins 2011 {published data only}
- Allingham‐Hawkins DJ, Chitayat D, Cirigliano V, Summers A, Tokunaga J, Winsor E, et al. Prospective validation of quantitative fluorescent polymerase chain reaction for rapid detection of common aneuploidies. Genetics in Medicine 2011;13(2):140‐7. [DOI] [PubMed] [Google Scholar]
American College 2009 {published data only}
- American College of Nurse‐Midwives. [Share with women. Prenatal tests for Down syndrome]. [Spanish]. Journal of Midwifery & Women's Health 2009;54(6):527‐8. [PubMed] [Google Scholar]
Antona 1998 {published data only}
- Antona D, Wallace EM, Shearing C, Ashby JP, Groome NP. Inhibin A and pro‐alphaC Inhibin Ain Down syndrome and normal pregnancies. Prenatal Diagnosis 1998;18(11):1122‐6. [DOI] [PubMed] [Google Scholar]
Antsaklis 1999 {published data only}
- Antsaklis A, Papantoniou N, Mesogitis S, Michalas S, Aravantinos D. Pregnant women of 35 years of age or more: Maternal serum markers or amniocentesis?. Journal of Obstetrics and Gynaecology 1999;19(3):253‐6. [DOI] [PubMed] [Google Scholar]
Anuwutnavin 2009 {published data only}
- Anuwutnavin S, Wanitpongpan P, Chanprapaph P. Specificity of fetal tricuspid regurgitation in prediction of Down syndrome in Thai fetuses at 17‐23 weeks of gestation. Journal of the Medical Association of Thailand 2009;92(9):1123‐30. [PubMed] [Google Scholar]
Ashwood 1987 {published data only}
- Ashwood ER, Cheng E, Luthy DA. Maternal serum alpha‐fetoprotein and fetal trisomy‐21 in women 35 years and older: implications for alpha‐fetoprotein screening programs. American Journal of Medical Genetics 1987;26(3):531‐9. [DOI] [PubMed] [Google Scholar]
Asrani 2005 {published data only}
- Asrani CH. Triple marker. National Journal of Homoeopathy 2005;7(3):174. [Google Scholar]
Audibert 2001b {published data only}
- Audibert F, Dommergues M, Benattar C, Taieb J, Thalabard JC, Frydman R. Screening for Down syndrome using first‐trimester ultrasound and second‐trimester maternal serum markers in a low‐risk population: a prospective longitudinal study. Ultrasound in Obstetrics & Gynecology 2001;18(1):26‐31. [DOI] [PubMed] [Google Scholar]
Axt‐Fleidner 2006 {published data only}
- Axt Fliedner, Schwarze A, Kreiselmaier P, Krapp M, Smrcek J, Diedrich K. Umbilical cord diameter at 11‐14 weeks of gestation: Relationship to nuchal translucency, ductus venous blood flow and chromosomal defects. Fetal Diagnosis and Therapy 2006;21(4):390‐5. [DOI] [PubMed] [Google Scholar]
Azuma 2002 {published data only}
- Azuma M, Yamamoto R, Wakui Y, Minobe S, Satomura S, Fujimoto S. A novel method for the detection of Down syndrome with the use of four serum markers. American Journal of Obstetrics and Gynecology 2002;187(1):197‐201. [DOI] [PubMed] [Google Scholar]
Baghagho 2004 {published data only}
- Baghagho EE, Kharboush IF, El‐Kaffash DM, KarKour TA, Ismail SR, Mortada MM. Maternal serum alpha fetoprotein among pregnant females in Alexandria. Journal of the Egyptian Public Health Association 2004;79(1‐2):59‐81. [PubMed] [Google Scholar]
Bahado‐Singh 1995 {published data only}
- Bahado Singh R, Goldstein I, Uerpairojkit B, Copel JA, Mahoney MJ, Baumgarten A. Normal nuchal thickness in the midtrimester indicates reduced risk of Down syndrome in pregnancies with abnormal triple‐screen results. American Journal of Obstetrics and Gynecology 1995;173(4):1106‐10. [DOI] [PubMed] [Google Scholar]
Bahado‐Singh 1996 {published data only}
- Bahado‐Singh R, Tan A, Deren O, Hunter D, Copel J, Mahoney MJ. Risk of Down syndrome and any clinically significant chromosome defect in pregnancies with abnormal triple‐screen and normal targeted ultrasonographic results. American Journal of Obstetrics and Gynecology 1996;175(4 I):824‐9. [DOI] [PubMed] [Google Scholar]
Bahado‐Singh 1999b {published data only}
- Bahado‐Singh R, Oz AU, Flores D, Cermik D, Acuna E, Mahoney MJ, et al. Nuchal thickness, urine ß‐core fragment level, and maternal age for down syndrome screening. American Journal of Obstetrics and Gynecology 1999;180(2 I):491‐5. [DOI] [PubMed] [Google Scholar]
Bahado‐Singh 2002 {published data only}
- Bahado‐Singh R, Shahabi S, Karaca M, Mahoney MJ, Cole L, Oz UA. The comprehensive midtrimester test: High‐sensitivity Down syndrome test. American Journal of Obstetrics and Gynecology 2002;186(4):803‐8. [DOI] [PubMed] [Google Scholar]
Bahado‐Singh 2003 {published data only}
- Bahado‐Singh R, Cheng CC, Matta P, Small M, Mahoney MJ. Combined serum and ultrasound screening for detection of fetal aneuploidy. Seminars in Perinatology 2003;27(2):145‐51. [DOI] [PubMed] [Google Scholar]
Ball 2007 {published data only}
- Ball RH, Caughey AB, Malone FD, Nyberg DA, Comstock CH, Saade GR, et al. First‐ and second‐trimester evaluation of risk for Down syndrome. Obstetrics & Gynecology 2007;110(1):10‐7. [DOI] [PubMed] [Google Scholar]
Bar‐Hava 2001 {published data only}
- Bar‐Hava I, Yitzhak M, Krissi H, Shohat M, Shalev J, Czitron B, et al. Triple‐test screening in in vitro fertilization pregnancies. Journal of Assisted Reproduction and Genetics 2001;18(4):226‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Barkai 1996 {published data only}
- Barkai G, Goldman B, Ries L, Chaki R, Dor J, Cuckle H. Down's syndrome screening marker levels following assisted reproduction. Prenatal Diagnosis 1996;16(12):1111‐4. [DOI] [PubMed] [Google Scholar]
Barnabei 1995 {published data only}
- Barnabei VM, Krantz DA, Macri JN, Larsen JW Jr. Enhanced twin pregnancy detection within an open neural tube defect and Down syndrome screening protocol using free‐ß hCG and AFP. Prenatal Diagnosis 1995;15(12):1131‐4. [DOI] [PubMed] [Google Scholar]
Bartels 1988 {published data only}
- Bartels I, Lindemann A. Maternal levels of pregnancy‐specific ß 1‐glycoprotein (SP‐1) are elevated in pregnancies affected by Down's syndrome. Human Genetics 1988;80(1):46‐8. [DOI] [PubMed] [Google Scholar]
Bartels 1993 {published data only}
- Bartels I, Hoppe‐Sievert B, Bockel B, Herold S, Caesar J. Adjustment formulae for maternal serum alpha‐fetoprotein, human chorionic gonadotropin, and unconjugated oestriol to maternal weight and smoking. Prenatal Diagnosis 1993;13(2):123‐30. [DOI] [PubMed] [Google Scholar]
Barth 1991 {published data only}
- Barth WH Jr, Frigoletto FD Jr, Krauss CM, MacMillin MD, Stryker JM, Benacerraf BR. Ultrasound detection of fetal aneuploidy in women with elevated maternal serum alpha‐fetoprotein. Obstetrics & Gynecology 1991;77(6):897‐900. [PubMed] [Google Scholar]
Bas‐Budecka 2007 {published data only}
- Bas‐Budecka E, Perenc M, Sieroszewski P. [Abnormal second trimester screening for fetal chromosomal abnormalities as a predictor of adverse pregnancy outcome]. [Polish]. Ginekologia Polska 2007;78(11):877‐80. [PubMed] [Google Scholar]
Baviera 2004 {published data only}
- Baviera G, Carbone C, Corrado F, Mastrantonio P. Placental growth hormone in Down's syndrome screening. Journal of Maternal‐Fetal & Neonatal Medicine 2004;16(4):241‐3. [DOI] [PubMed] [Google Scholar]
Bazzett 1998 {published data only}
- Bazzett LB, Yaron Y, O'Brien JE, Critchfield G, Kramer RL, Ayoub M, et al. Fetal gender impact on multiple‐marker screening results. American Journal of Medical Genetics 1998;76(5):369‐71. [PubMed] [Google Scholar]
Beke 2008 {published data only}
- Beke A, Barakonyi E, Belics Z, Joo JG, Csaba A, Papp C, et al. Risk of chromosome abnormalities in the presence of bilateral or unilateral choroid plexus cysts. Fetal Diagnosis and Therapy 2008;23(3):185‐91. [DOI] [PubMed] [Google Scholar]
Bellver 2005 {published data only}
- Bellver J, Lara C, Soares SR, Ramirez A, Pellicer A, Remohi J, et al. First trimester biochemical screening for Down's syndrome in singleton pregnancies conceived by assisted reproduction. Human Reproduction 2005;20(9):2623‐7. [DOI] [PubMed] [Google Scholar]
Benn 1995 {published data only}
- Benn PA, Horne D, Briganti S, Greenstein RM. Prenatal diagnosis of diverse chromosome abnormalities in a population of women identified by triple‐marker testing as screen positive for Down syndrome. American Journal of Obstetrics and Gynecology 1995;173(2):496‐501. [DOI] [PubMed] [Google Scholar]
Benn 1996 {published data only}
- Benn PA, Horne D, Craffey A, Collins R, Ramsdell L, Greenstein R. Maternal serum screening for birth defects: results of a Connecticut regional program. Connecticut Medicine 1996;60(6):323‐7. [PubMed] [Google Scholar]
Benn 1997 {published data only}
- Benn PA, Clive JM, Collins R. Medians for second‐trimester maternal serum alpha‐fetoprotein, human chorionic gonadotropin, and unconjugated estriol; differences between races or ethnic groups. Clinical Chemistry 1997;43(2):333‐7. [PubMed] [Google Scholar]
Benn 1998 {published data only}
- Benn PA. Preliminary evidence for associations between second‐trimester human chorionic gonadotropin and unconjugated oestriol levels with pregnancy outcome in Down syndrome pregnancies. Prenatal Diagnosis 1998;18(4):319‐24. [PubMed] [Google Scholar]
Benn 2001 {published data only}
- Benn PA, Ying J, Beazoglou T, Egan JF. Estimates for the sensitivity and false‐positive rates for second trimester serum screening for Down syndrome and trisomy 18 with adjustment for cross‐identification and double‐positive results. Prenatal Diagnosis 2001;21(1):46‐51. [PubMed] [Google Scholar]
Benn 2002 {published data only}
- Benn PA, Kaminsky LM, Ying J, Borgida AF, Egan JF. Combined second‐trimester biochemical and ultrasound screening for Down syndrome. Obstetrics & Gynecology 2002;100(6):1168‐76. [DOI] [PubMed] [Google Scholar]
Benn 2003 {published data only}
- Benn PA, Fang M, Egan JFX, Horne D, Collins R. Incorporation of inhibin‐A in second‐trimester screening for Down syndrome. Obstetrics and Gynecology 2003;101(3):451‐4. [DOI] [PubMed] [Google Scholar]
Benn 2003a {published data only}
- Benn P. Improved antenatal screening for Down's syndrome. Lancet 2003;361(9360):794‐5. [DOI] [PubMed] [Google Scholar]
Benn 2005 {published data only}
- Benn P, Wright D, Cuckle H. Practical strategies in contingent sequential screening for Down syndrome. Prenatal Diagnosis 2005;25(8):645‐52. [DOI] [PubMed] [Google Scholar]
Benn 2005a {published data only}
- Benn P, Donnenfeld AE. Sequential Down syndrome screening: the importance of first and second trimester test correlations when calculating risk. Journal of Genetic Counseling 2005;14(6):409‐13. [DOI] [PubMed] [Google Scholar]
Benn 2007 {published data only}
- Benn PA, Campbell WA, Zelop CM, Ingardia C, Egan JF. Stepwise sequential screening for fetal aneuploidy. American Journal of Obstetrics and Gynecology 2007;197(3):312‐5. [DOI] [PubMed] [Google Scholar]
Berry 1995 {published data only}
- Berry E, Aitken DA, Crossley JA, Macri JN, Connor JM. Analysis of maternal serum alpha‐fetoprotein and free ß human chorionic gonadotrophin in the first trimester: implications for Down's syndrome screening. Prenatal Diagnosis 1995;15(6):555‐65. [DOI] [PubMed] [Google Scholar]
Berry 1997 {published data only}
- Berry E, Aitken DA, Crossley JA, Macri JN, Connor JM. Screening for Down's syndrome: changes in marker levels and detection rates between first and second trimesters. British Journal of Obstetrics and Gynaecology 1997;104(7):811‐7. [DOI] [PubMed] [Google Scholar]
Bersinger 1994 {published data only}
- Bersinger NA, Brizot ML, Johnson A, Snijders RJ, Abbott J, Schneider H, et al. First trimester maternal serum pregnancy‐associated plasma protein A and pregnancy‐specific ß 1‐glycoprotein in fetal trisomies. British Journal of Obstetrics and Gynaecology 1994;101(11):970‐4. [DOI] [PubMed] [Google Scholar]
Bersinger 2000 {published data only}
- Bersinger NA, Xin WZ. Glycosylation of pregnancy‐associated plasma protein a (PAPP‐A) and pregnancy‐specific (ß)(1)‐glycoprotein (SP1): Relevance for fetal down syndrome screening and for placental function studies. Immuno‐Analyse et Biologie Specialisee 2000;15(6):402‐8. [Google Scholar]
Bersinger 2001 {published data only}
- Bersinger NA, Chanson A, Crazzolara S, Hänggi W, Pescia G, Scheier M, et al. Serum levels of placenta protein markers: The relevance of differences between spontaneous and after in vitro fertilization pregnancies for fetal trisomy screening. Journal fur Fertilitat und Reproduktion 2001;11(3):7‐13. [Google Scholar]
Bersinger 2003 {published data only}
- Bersinger NA, Noble P, Nicolaides KH. First‐trimester maternal serum PAPP‐A, SP1 and M‐CSF levels in normal and trisomic twin pregnancies. Prenatal Diagnosis 2003;23(2):157‐62. [DOI] [PubMed] [Google Scholar]
Bersinger 2004 {published data only}
- Bersinger NA, Wunder D, Vanderlick F, Chanson A, Pescia G, Janecek P, et al. Maternal serum levels of placental proteins after in vitro fertilisation and their implications for prenatal screening. Prenatal Diagnosis 2004;24(6):471‐7. [DOI] [PubMed] [Google Scholar]
Bersinger 2005 {published data only}
- Bersinger NA, Vanderlick F, Birkhäuser MH, Janecek P, Wunder D. First trimester serum concentrations of placental proteins in singleton and multiple IVF pregnancies: Implications for Down syndrome screening. Immuno‐Analyse et Biologie Specialisee 2005;20(1):21‐7. [Google Scholar]
Bestwick 2008 {published data only}
- Bestwick JP, Huttly WJ, Wald NJ. First trimester Down's syndrome screening marker values and cigarette smoking: new data and a meta‐analysis on free beta human chorionic gonadotophin, pregnancy‐associated plasma protein‐A and nuchal translucency. Journal of Medical Screening 2008;15(4):204‐6. [DOI] [PubMed] [Google Scholar]
Biggio 2004 {published data only}
- Biggio Jr, Morris TC, Owen J, Stringer JSA. An outcomes analysis of five prenatal screening strategies for trisomy 21 in women younger than 35 years. American Journal of Obstetrics and Gynecology 2004;190(3):721‐9. [DOI] [PubMed] [Google Scholar]
Bilardo 2011 {published data only}
- Bilardo CM, Timmerman E, Medina PG, Clur SA. Low‐resistance hepatic artery flow in first‐trimester fetuses: an ominous sign. Ultrasound in Obstetrics & Gynecology 2011;37(4):438‐43. [DOI] [PubMed] [Google Scholar]
Bindra 2002 {published data only}
- Bindra R, Heath V, Nicolaides KH. Screening for chromosomal defects by fetal nuchal translucency at 11 to 14 weeks. Clinical Obstetrics and Gynecology 2002;45(3):661‐70. [DOI] [PubMed] [Google Scholar]
Blundell 1999 {published data only}
- Blundell G, Ashby JP, Martin C, Shearing CH, Langdale‐Brown B, Keeling J, et al. Clinical follow‐up of high mid‐trimester maternal serum intact human chorionic gonadotrophin concentrations in singleton pregnancies. Prenatal Diagnosis 1999;19(3):219‐23. [DOI] [PubMed] [Google Scholar]
Boormans 2010 {published data only}
- Boormans EM, Birnie E, Oepkes D, Galjaard RJ, Schuring‐Blom GH, Lith JM, et al. Comparison of multiplex ligation‐dependent probe amplification and karyotyping in prenatal diagnosis. Obstetrics & Gynecology 2010;115(2 Pt 1):297‐303. [DOI] [PubMed] [Google Scholar]
Boots 1989 {published data only}
- Boots LR, Davis RO, Foster JM, Goldenberg RL. Maternal serum alpha‐fetoprotein prenatal screening for Down syndrome. Alabama Medicine 1989;59(1):25‐7. [PubMed] [Google Scholar]
Bornstein 2009 {published data only}
- Bornstein E, Lenchner E, Donnenfeld A, Barnhard Y, Seubert D, Divon MY. Advanced maternal age as a sole indication for genetic amniocentesis; risk‐benefit analysis based on a large database reflecting the current common practice. Journal of Perinatal Medicine 2009;37(2):99‐102. [DOI] [PubMed] [Google Scholar]
Bornstein 2009a {published data only}
- Bornstein E, Lenchner E, Donnenfeld A, Kapp S, Keeler SM, Divon MY. Comparison of modes of ascertainment for mosaic vs complete trisomy 21. American Journal of Obstetrics and Gynecology 2009;200(4):440‐5. [DOI] [PubMed] [Google Scholar]
Bornstein 2010 {published data only}
- Bornstein E, Lenchner E, Donnenfeld A, Jodicke C, Keeler SM, Kapp S, et al. Complete trisomy 21 vs translocation Down syndrome: a comparison of modes of ascertainment. American Journal of Obstetrics and Gynecology 2010;203(4):391‐5. [DOI] [PubMed] [Google Scholar]
Borowski 2007 {published data only}
- Borowski D, Czuba B, Cnota W, Hincz P, Czekierdowski A, Gajewska J, et al. [Evaluation of pregnancy‐associated plasma protein A (PAPP‐A) and free beta subunit of human chorionic gonadotropin (beta hCG) levels and sonographic assesement of fetal nuchal translucency (NT) in singleton pregnancies between 11 and 14 weeks of gestation‐‐Polish multi‐centre research]. [Polish]. Ginekologia Polska 2007;78(5):384‐7. [PubMed] [Google Scholar]
Borrell 2007 {published data only}
- Borrell A, Mercade I, Casals E, Borobio V, Seres A, Soler A, et al. Combining fetal nuchal fold thickness with second‐trimester biochemistry to screen for trisomy 21. Ultrasound in Obstetrics & Gynecology 2007;30(7):941‐5. [DOI] [PubMed] [Google Scholar]
Borrell 2009a {published data only}
- Borrell A, Borobio V, Bestwick JP, Wald NJ. Ductus venosus pulsatility index as an antenatal screening marker for Down's syndrome: use with the Combined and Integrated tests. Journal of Medical Screening 2009;16(3):112‐8. [DOI] [PubMed] [Google Scholar]
Borruto 2002 {published data only}
- Borruto F, Comparetto C, Acanfora L, Bertini G, Rubaltelli FF. Role of ultrasound evaluation of nuchal translucency in prenatal diagnosis. Clinical & Experimental Obstetrics & Gynecology 2002;29(4):235‐41. [PubMed] [Google Scholar]
Bottalico 2009 {published data only}
- Bottalico JN, Chen X, Tartaglia M, Rosario B, Yarabothu D, Nelson L. Second‐trimester genetic sonogram for detection of fetal chromosomal abnormalities in a community‐based antenatal testing unit. Ultrasound in Obstetrics & Gynecology 2009;33(2):161‐8. [DOI] [PubMed] [Google Scholar]
Boue 1990 {published data only}
- Boue A, Muller F. Screening for Down's syndrome with maternal serum human chorionic gonadotropin at midtrimester. Current Opinion in Pediatrics 1990;2(6):1157‐60. [Google Scholar]
Bradley 1994 {published data only}
- Bradley LA, Horwitz JA, Dowman AC, Ponting NR, Peterson LM. Triple marker screening for fetal Down syndrome. International Pediatrics 1994;9(3):168‐74. [Google Scholar]
Braithwaite 1996 {published data only}
- Braithwaite JM, Economides DL. Nuchal translucency and screening for Down's syndrome. Contemporary Reviews in Obstetrics and Gynaecology 1996;8(2):75‐81. [Google Scholar]
Brambati 1995 {published data only}
- Brambati B, Cislaghi C, Tului L, Alberti E, Amidani M, Colombo U, et al. First‐trimester Down's syndrome screening using nuchal translucency: a prospective study in women undergoing chorionic villus sampling. Ultrasound in Obstetrics & Gynecology 1995;5(1):9‐14. [DOI] [PubMed] [Google Scholar]
Brambati 1996 {published data only}
- Brambati B, Tului L, Alberti E. Sonography in the first trimester screening of trisomy 21 and other fetal aneuploidies. Early Pregnancy 1996;2(3):155‐67. [PubMed] [Google Scholar]
Brizot 1995 {published data only}
- Brizot ML, Bersinger NA, Xydias G, Snijders RJ, Nicolaides KH. Maternal serum Schwangerschafts protein‐1 (SP1) and fetal chromosomal abnormalities at 10‐13 weeks' gestation. Early Human Development 1995;43(1):31‐6. [DOI] [PubMed] [Google Scholar]
Brizot 1995a {published data only}
- Brizot ML, Kuhn P, Bersinger NA, Snijders RJ, Nicolaides KH. First trimester maternal serum alpha‐fetoprotein in fetal trisomies. British Journal of Obstetrics and Gynaecology 1995;102(1):31‐4. [DOI] [PubMed] [Google Scholar]
Brizzi 1989a {published data only}
- Brizzi L, Cariati E, Periti E, Nannini R, Torricelli F, Cappelli G, et al. Evaluation of maternal serum alpha‐fetoprotein and ultrasound examination to screen fetal chromosomal abnormalities. Journal of Nuclear Medicine & Allied Sciences 1989;33(3 Suppl):85‐8. [PubMed] [Google Scholar]
Brock 1990 {published data only}
- Brock DJ, Barron L, Holloway S, Liston WA, Hillier SG, Seppala M. First‐trimester maternal serum biochemical indicators in Down syndrome. Prenatal Diagnosis 1990;10(4):245‐51. [DOI] [PubMed] [Google Scholar]
Calda 2010 {published data only}
- Calda P, Sipek A, Gregor V. Gradual implementation of first trimester screening in a population with a prior screening strategy: population based cohort study. Acta Obstetricia et Gynecologica Scandinavica 2010;89(8):1029‐33. [DOI] [PubMed] [Google Scholar]
Campogrande 2001 {published data only}
- Campogrande M, Viora E, Errante G, Bastonero S, Sciarrone A, Grassi Pirrone P, et al. Correlations between first and second trimester markers for Down's syndrome screening. Journal of Medical Screening 2001;8(3):163‐4. [DOI] [PubMed] [Google Scholar]
Canick 1988 {published data only}
- Canick JA, Knight GJ, Palomaki GE, Haddow JE, Cuckle HS, Wald NJ. Low second trimester maternal serum unconjugated oestriol in pregnancies with Down's syndrome. British Journal of Obstetrics and Gynaecology 1988;95(4):330‐3. [DOI] [PubMed] [Google Scholar]
Canick 1995b {published data only}
- Canick JA, Kellner LH, Saller Jr, Palomaki GE, Walker RP, Osathanondh R. Second‐trimester levels of maternal urinary gonadotropin peptide in down syndrome pregnancy. Prenatal Diagnosis 1995;15(8):739‐44. [DOI] [PubMed] [Google Scholar]
Canini 2002 {published data only}
- Canini S, Prefumo F, Famularo L, Venturini PL, Palazzese V, Biasio P. Comparison of first trimester, second trimester and integrated Down's syndrome screening results in unaffected pregnancies. Clinical Chemistry & Laboratory Medicine 2002;40(6):600‐3. [DOI] [PubMed] [Google Scholar]
Cans 1998 {published data only}
- Cans C, Amblard F, Devillard F, Pison H, Jalbert P, Jouk PS. Population screening for aneuploidy using maternal age and ultrasound. Prenatal Diagnosis 1998;18(7):683‐92. [PubMed] [Google Scholar]
Carreras 1991 {published data only}
- Carreras de Paz JJ, Silva Mendoza JM, Violante Diaz M, Cerrillo Hinojosa M, Ahued Ahued JR. [Proposed normal values for alpha fetoprotein in maternal serum for the detection of neural tube closure defects and Down syndrome. Preliminary study]. [Spanish]. Ginecologia y Obstetricia de Mexico 1991;59:261‐4. [PubMed] [Google Scholar]
Caughey 2007 {published data only}
- Caughey AB, Musci TJ, Belluomini J, Main D, Otto C, Goldberg J. Nuchal translucency screening: how do women actually utilize the results?. Prenatal Diagnosis 2007;27(2):119‐23. [DOI] [PubMed] [Google Scholar]
Cebesoy 2008 {published data only}
- Cebesoy FB. Combining 'nasal bone length assessment as MoM' with other markers for trisomy 21 screening: could it be more effective?. American Journal of Obstetrics and Gynecology 2008;198(6):726‐7. [DOI] [PubMed] [Google Scholar]
Chelli 2008 {published data only}
- Chelli D, Dimassi K, Chaabouni M, Ben Saad M, Mssaed H, Bchir F, et al. [Prenatal diagnosis of trisomy 21: the Tunisian experience]. [French]. Sante 2008;18(4):199‐203. [PubMed] [Google Scholar]
Chen 1999 {published data only}
- Chen FM. Integrated screening for Down's syndrome. Journal of Family Practice 1999;48(11):846‐7. [PubMed] [Google Scholar]
Chen 2002 {published data only}
- Chen M, Lam YH, Tang MH, Lee CP, Sin SY, Tang R, et al. The effect of ethnic origin on nuchal translucency at 10‐14 weeks of gestation. Prenatal Diagnosis 2002;22(7):576‐8. [DOI] [PubMed] [Google Scholar]
Chen 2004 {published data only}
- Chen M, Lam YH, Lee CP, Tang MHY. Ultrasound screening of fetal structural abnormalities at 12 to 14 weeks in Hong Kong. Prenatal Diagnosis 2004;24(2):92‐7. [DOI] [PubMed] [Google Scholar]
Chen 2005 {published data only}
- Chen CP, Lin CJ, Wang W. Impact of second‐trimester maternal serum screening on prenatal diagnosis of Down syndrome and the use of amniocentesis in the Taiwanese population. Taiwanese Journal of Obstetrics and Gynecology 2005;44(1):31‐5. [Google Scholar]
Chen 2008 {published data only}
- Chen M, Lee CP, Lam YH, Tang RY, Chan BC, Wong SF, et al. Comparison of nuchal and detailed morphology ultrasound examinations in early pregnancy for fetal structural abnormality screening: a randomized controlled trial. Ultrasound in Obstetrics & Gynecology 2008;31(2):136‐46. [DOI] [PubMed] [Google Scholar]
Cheng 1993 {published data only}
- Cheng EY, Luthy DA, Zebelman AM, Williams MA, Lieppman RE, Hickok DE. A prospective evaluation of a second‐trimester screening test for fetal Down syndrome using maternal serum alpha‐fetoprotein, hCG, and unconjugated estriol. Obstetrics & Gynecology 1993;81(1):72‐7. [PubMed] [Google Scholar]
Cheng 1999 {published data only}
- Cheng PJ, Liu CM, Chang SD, Lin YT, Soong YK. Elevated second‐trimester maternal serum hCG in women undergoing haemodialysis. Prenatal Diagnosis 1999;19(10):955‐8. [DOI] [PubMed] [Google Scholar]
Cheng 2004 {published data only}
- Cheng CC, Bahado‐Singh RO, Chen SC, Tsai MS. Pregnancy outcomes with increased nuchal translucency after routine Down syndrome screening. International Journal of Gynaecology & Obstetrics 2004;84(1):5‐9. [DOI] [PubMed] [Google Scholar]
Cheng 2004a {published data only}
- Cheng PJ, Chu DC, Chueh HY, See LC, Chang HC, Weng DR. Elevated maternal midtrimester serum free ß‐human chorionic gonadotropin levels in vegetarian pregnancies that cause increased false‐positive Down syndrome screening results. American Journal of Obstetrics and Gynecology. 2004;190(2):442‐7. [DOI] [PubMed] [Google Scholar]
Chitayat 2002 {published data only}
- Chitayat D, Farrell SA, Huang T, Meier C, Wyatt PR, Summers AM. Double‐positive maternal serum screening results for down syndrome and open neural tube defects: An indicator for fetal structural or chromosomal abnormalities and adverse obstetric outcomes. American Journal of Obstetrics and Gynecology 2002;187(3):758‐63. [DOI] [PubMed] [Google Scholar]
Chiu 2011 {published data only}
- Chiu RW, Akolekar R, Zheng YW, Leung TY, Sun H, Chan KC, et al. Non‐invasive prenatal assessment of trisomy 21 by multiplexed maternal plasma DNA sequencing: large scale validity study. BMJ 2011;342:c7401. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cho 2009 {published data only}
- Cho EH, Park BY, Kang YS, Lee EH. Validation of QF‐PCR in a Korean population. Prenatal Diagnosis 2009;29(3):213‐6. [DOI] [PubMed] [Google Scholar]
Chou 2009 {published data only}
- Chou CY, Hsieh FJ, Cheong ML, Lee FK, She BQ, Tsai MS. First‐trimester Down syndrome screening in women younger than 35 years old and cost‐effectiveness analysis in Taiwan population. Journal of Evaluation in Clinical Practice 2009;15(5):789‐96. [DOI] [PubMed] [Google Scholar]
Christiansen 2002 {published data only}
- Christiansen M, Hogdall EV, Larsen SO, Hogdall C. The variation of risk estimates through pregnancy in second trimester maternal serum screening for Down syndrome. Prenatal Diagnosis 2002;22(5):385‐7. [DOI] [PubMed] [Google Scholar]
Christiansen 2007a {published data only}
- Christiansen M, Spencer K, Laigaard J, Cowans NJ, Larsen SO, Wewer UM. ADAM 12 as a second‐trimester maternal serum marker in screening for Down syndrome. Prenatal Diagnosis 2007;27(7):611‐5. [DOI] [PubMed] [Google Scholar]
Christiansen 2008 {published data only}
- Christiansen M, Sorensen TL, Larsen SO, Norgaard‐Pedersen B. First‐trimester maternal serum progesterone in aneuploid pregnancies. Prenatal Diagnosis 2008;28(4):319‐22. [DOI] [PubMed] [Google Scholar]
Chung 2000 {published data only}
- Chung BL, Kim YP, Nam MH. The application of three‐dimensional ultrasound to nuchal translucency thickness measurement at 10‐14 weeks of gestation. Prenatal and Neonatal Medicine 2000;5(1):17‐21. [Google Scholar]
CNGOF 1996 {published data only}
- Anon. Blood screening of Down's syndrome (Trisomy 21) and reimbursement of karyotype for women under 38. Revue Francaise de Gynecologie et d'Obstetrique 1996;91(11):575‐7. [Google Scholar]
Cocciolone 2008 {published data only}
- Cocciolone R, Brameld K, O'Leary P, Haan E, Muller P, Shand K. Combining first and second trimester markers for Down syndrome screening: think twice. Australian & New Zealand Journal of Obstetrics & Gynaecology 2008;48(5):492‐500. [DOI] [PubMed] [Google Scholar]
Cole 1996 {published data only}
- Cole L, Isozaki T, Palomaki G, Canick J, Iles R, Kellner L, et al. Detection of ß‐core fragment in second trimester Down's syndrome pregnancies. [Review]. Early Human Development 1996;47(Suppl):S47‐8. [DOI] [PubMed] [Google Scholar]
Comas 2001 {published data only}
- Comas C, Antolín E, Torrents M, Muñoz A, Figueras F, Echevarría M, et al. Early screening for chromosomal abnormalities: New strategies combining biochemical, sonographic and doppler parameters. Prenatal and Neonatal Medicine 2001;6(2):95‐102. [Google Scholar]
Comas 2002 {published data only}
- Comas C, Torrents M, Munoz A, Antolin E, Figueras F, Echevarria M. Measurement of nuchal translucency as a single strategy in trisomy 21 screening: should we use any other marker?. Obstetrics & Gynecology 2002;100(4):648‐54. [DOI] [PubMed] [Google Scholar]
Comas 2002a {published data only}
- Comas C, Carrera JM. Early sonographic screening for chromosomal abnormalities. Ultrasound Review of Obstetrics and Gynecology 2002;2(2):88‐91. [Google Scholar]
Comstock 2006 {published data only}
- Comstock CH, Malone FD, Ball RH, Nyberg DA, Saade GR, Berkowitz RL, et al. Is there a nuchal translucency millimeter measurement above which there is no added benefit from first trimester serum screening?. American Journal of Obstetrics and Gynecology. 2006;195(3):843‐7. [DOI] [PubMed] [Google Scholar]
Conde‐Agudelo 1998 {published data only}
- Conde‐Agudelo A, Kafury‐Goeta AC. Triple‐marker test as screening for down syndrome: A meta‐analysis. Obstetrical and Gynecological Survey 1998;53(6):369‐76. [DOI] [PubMed] [Google Scholar]
Cowans 2011 {published data only}
- Cowans NJ, Stamatopoulou A, Torring N, Spencer K. Early first‐trimester maternal serum placental growth factor in trisomy 21 pregnancies. Ultrasound in Obstetrics & Gynecology 2011;37(5):515‐9. [DOI] [PubMed] [Google Scholar]
Crossley 1991 {published data only}
- Crossley JA, Aitken DA, Connor JM. Prenatal screening for chromosome abnormalities using maternal serum chorionic gonadotrophin, alpha‐fetoprotein, and age. Prenatal Diagnosis 1991;11(2):83‐101. [DOI] [PubMed] [Google Scholar]
Crossley 1993 {published data only}
- Crossley JA, Aitken DA, Connor JM. Second‐trimester unconjugated oestriol levels in maternal serum from chromosomally abnormal pregnancies using an optimized assay.[see comment]. Prenatal Diagnosis 1993;13(4):271‐80. [DOI] [PubMed] [Google Scholar]
Crossley 1996 {published data only}
- Crossley JA, Berry E, Aitken DA, Connor JM. Insulin‐dependent diabetes mellitus and prenatal screening results: current experience from a regional screening programme. Prenatal Diagnosis 1996;16(11):1039‐42. [DOI] [PubMed] [Google Scholar]
Crossley 2002a {published data only}
- Crossley JA, Aitken DA, Waugh SM, Kelly T, Connor JM. Maternal smoking: age distribution, levels of alpha‐fetoprotein and human chorionic gonadotrophin, and effect on detection of Down syndrome pregnancies in second‐trimester screening. Prenatal Diagnosis 2002;22(3):247‐55. [DOI] [PubMed] [Google Scholar]
Cuckle 1984 {published data only}
- Cuckle HS, Wald NJ, Lindenbaum RH. Maternal serum alpha‐fetoprotein measurement: a screening test for Down syndrome. Lancet 1984;i(8383):926‐9. [DOI] [PubMed] [Google Scholar]
Cuckle 1987 {published data only}
- Cuckle HS, Wald NJ, Thompson SG. Estimating a woman's risk of having a pregnancy associated with Down's syndrome using her age and serum alpha‐fetoprotein level. British Journal of Obstetrics and Gynaecology 1987;94(5):387‐402. [DOI] [PubMed] [Google Scholar]
Cuckle 1987a {published data only}
- Cuckle HS, Nanchahal K, Wald NJ. Maternal serum alpha‐fetoprotein and ethnic origin. British Journal of Obstetrics and Gynaecology 1987;94(11):1111‐2. [DOI] [PubMed] [Google Scholar]
Cuckle 1990 {published data only}
- Cuckle HS, Wald NJ, Densem JW, Royston P, Knight GJ, Haddow JE, et al. The effect of smoking in pregnancy on maternal serum alpha‐fetoprotein, unconjugated oestriol, human chorionic gonadotrophin, progesterone and dehydroepiandrosterone sulphate levels. British Journal of Obstetrics and Gynaecology 1990;97(3):272‐4. [DOI] [PubMed] [Google Scholar]
Cuckle 1996 {published data only}
- Cuckle HS, Holding S, Jones R, Groome NP, Wallace EM. Combining Inhibin A with existing second‐trimester markers in maternal serum screening for Down's syndrome. Prenatal Diagnosis 1996;16(12):1095‐100. [DOI] [PubMed] [Google Scholar]
Cuckle 1999b {published data only}
- Cuckle HS, Sehmi I, Jones R, Evans LW. Maternal serum activin A and follistatin levels in pregnancies with Down syndrome. Prenatal Diagnosis 1999;19(6):513‐6. [PubMed] [Google Scholar]
Cuckle 1999c {published data only}
- Cuckle HS, Lith JM. Appropriate biochemical parameters in first‐trimester screening for Down syndrome.[see comment]. Prenatal Diagnosis 1999;19(6):505‐12. [DOI] [PubMed] [Google Scholar]
Cullen 1990 {published data only}
- Cullen MT, Gabrielli S, Green JJ, Rizzo N, Mahoney MJ, Salafia C, et al. Diagnosis and significance of cystic hygroma in the first trimester. Prenatal Diagnosis 1990;10(10):643‐51. [DOI] [PubMed] [Google Scholar]
Cusick 2004 {published data only}
- Cusick W, Provenzano J, Sullivan CA, Gallousis FM, Rodis JF. Fetal nasal bone length in euploid and aneuploid fetuses between 11 and 20 weeks' gestation: a prospective study. Journal of Ultrasound in Medicine 2004;23(10):1327‐33. [DOI] [PubMed] [Google Scholar]
Cusick 2007 {published data only}
- Cusick W, Shevell T, Duchan LS, Lupinacci CA, Terranova J, Crombleholme WR. Likelihood ratios for fetal trisomy 21 based on nasal bone length in the second trimester: how best to define hypoplasia?. Ultrasound in Obstetrics & Gynecology 2007;30(3):271‐4. [DOI] [PubMed] [Google Scholar]
Dancoine 2001 {published data only}
- Dancoine F, Couplet G, Mainardi A, Sukno F, Jaumain P, Nowak E, et al. Antenatal screening for Dawn's syndrome with serum markers: Influence of maternal weight, smoking habits and diabetes. Immuno‐Analyse et Biologie Specialisee 2001;16(6):381‐9. [Google Scholar]
Dane 2008 {published data only}
- Dane B, Dane C, Cetin A, Kiray M, Sivri D, Yayla M. Pregnancy outcome in fetuses with increased nuchal translucency. Journal of Perinatology 2008;28(6):400‐4. [DOI] [PubMed] [Google Scholar]
De Biasio, 1999 {published data only}
- Biasio P, Siccardi M, Volpe G, Famularo L, Santi F, Canini S. First‐trimester screening for down syndrome using nuchal translucency measurement with free ß‐hCG and PAPP‐A between 10 and 13 weeks of pregnancy ‐ The combined test. Prenatal Diagnosis 1999;19(4):360‐3. [DOI] [PubMed] [Google Scholar]
De Biasio, 2001 {published data only}
- De BiasioP, Ferrero S, Prefumo F, Canini S, Marchini P, Bruzzone I, et al. Down's syndrome: First trimester approach. Italian Journal of Gynaecology and Obstetrics 2001;13(1):22‐6. [Google Scholar]
De Biasio 2000 {published data only}
- Biasio P, Canini S, Prefumo F, Famularo L, Venturini PL. Extent of correlation between first and second trimester markers for Down's syndrome screening. Journal of Medical Screening 2000;7(3):163. [DOI] [PubMed] [Google Scholar]
De Graaf 1991 {published data only}
- Graaf I, Cuckle HS, Pajkrt E, Leschot NJ, Bleker OP, Lith JM. Co‐variables in first trimester maternal serum screening. Prenatal Diagnosis 1991;20(3):186‐9. [PubMed] [Google Scholar]
Del Carmen Saucedo 2009 {published data only}
- Carmen Saucedo M, DeVigan C, Vodovar V, Lelong N, Goffinet F, Khoshnood B. Measurement of nuchal translucency and the prenatal diagnosis of Down syndrome. Obstetrics & Gynecology 2009;114(4):829‐38. [DOI] [PubMed] [Google Scholar]
DeVore 2001 {published data only}
- DeVore GR, Romero R. Combined use of genetic sonography and maternal serum triple‐marker screening: an effective method for increasing the detection of trisomy 21 in women younger than 35 years.[see comment]. Journal of Ultrasound in Medicine 2001;20(6):645‐54. [DOI] [PubMed] [Google Scholar]
Dhaifalah 2007 {published data only}
- Dhaifalah I, Mickova I, Vrbicka D, Santavy J, Curtisova V. [Advanced maternal age as an indication for invasive prenatal diagnostics?]. [Czech]. Ceska Gynekologie 2007;72(3):181‐4. [PubMed] [Google Scholar]
Dhaifalah 2007a {published data only}
- Dhaifalah I, Mickova I, Santavy J, Vrbicka D, Zapletalova D, Curtisova V. [Efficiency of measuring nasal bone as an ultrasound marker of Down syndrome in 11th to 13th+6 week of pregnancy]. [Czech]. Ceska Gynekologie 2007;72(1):19‐23. [PubMed] [Google Scholar]
Dhallan 2007 {published data only}
- Dhallan R, Guo X, Emche S, Damewood M, Bayliss P, Cronin M, et al. A non‐invasive test for prenatal diagnosis based on fetal DNA present in maternal blood: a preliminary study. Lancet 2007;369(9560):474‐81. [DOI] [PubMed] [Google Scholar]
Dickerson 1994 {published data only}
- Dickerson VM. Multiple marker screening. Western Journal of Medicine 1994;161(2):161. [PMC free article] [PubMed] [Google Scholar]
Dimaio 1987 {published data only}
- Dimaio MS, Baumgarten A, Greenstein RM, Saal HM, Mahoney MJ. Screening for fetal Down's syndrome in pregnancy by measuring maternal serum alpha‐fetoprotein levels. New England Journal of Medicine 1987;317(6):342‐6. [DOI] [PubMed] [Google Scholar]
Doran 1986 {published data only}
- Doran TA, Cadesky K, Wong PY, Mastrogiacomo C, Capello T. Maternal serum alpha‐fetoprotein and fetal autosomal trisomies. American Journal of Obstetrics and Gynecology 1986;154(2):277‐81. [DOI] [PubMed] [Google Scholar]
Dreux 2008 {published data only}
- Dreux S, Olivier C, Dupont JM, Leporrier N, Study Group, Oury JF, et al. Maternal serum screening in cases of mosaic and translocation Down syndrome. Prenatal Diagnosis 2008;28(8):699‐703. [DOI] [PubMed] [Google Scholar]
Drugan 1996 {published data only}
- Drugan A, Reichler A, Bronstein M, Johnson MP, Sokol RJ, Evans MI. Abnormal biochemical serum screening versus 2nd‐trimester ultrasound‐detected minor anomalies as predictors of aneuploidy in low‐risk women. Fetal Diagnosis and Therapy 1996;11(5):301‐5. [DOI] [PubMed] [Google Scholar]
Drugan 1996a {published data only}
- Drugan A, O'Brien JE, Dvorin E, Krivchenia EL, Johnson MP, Sokol RJ, et al. Multiple marker screening in multifetal gestations: failure to predict adverse pregnancy outcomes. Fetal Diagnosis and Therapy 1996;11(1):16‐9. [DOI] [PubMed] [Google Scholar]
Drysdale 2002 {published data only}
- Drysdale K, Ridley D, Walker K, Higgins B, Dean T. First‐trimester pregnancy scanning as a screening tool for high‐risk and abnormal pregnancies in a district general hospital setting. Journal of Obstetrics and Gynaecology 2002;22(2):159‐65. [DOI] [PubMed] [Google Scholar]
Dugoff 2008 {published data only}
- Dugoff L, Cuckle HS, Hobbins JC, Malone FD, Belfort MA, Nyberg DA, et al. Prediction of patient‐specific risk for fetal loss using maternal characteristics and first‐ and second‐trimester maternal serum Down syndrome markers. American Journal of Obstetrics and Gynecology 2008;199(3):290‐6. [DOI] [PubMed] [Google Scholar]
Ebell 1999 {published data only}
- Ebell M. Is the integrated test better for screening for Down's syndrome than the traditional triple test?. Evidence‐Based Practice 1999;2(11):4‐5. [Google Scholar]
Economides 1998 {published data only}
- Economides DL, Whitlow BJ, Kadir R, Lazanakis M, Verdin SM. First trimester sonographic detection of chromosomal abnormalities in an unselected population. British Journal of Obstetrics and Gynaecology 1998;105(1):58‐62. [DOI] [PubMed] [Google Scholar]
Erickson 2004 {published data only}
- Erickson JA, Ashwood ER, Gin CA. Evaluation of a dimeric inhibin‐A assay for assessing fetal Down syndrome: establishment, comparison, and monitoring of median concentrations for normal pregnancies. Archives of Pathology & Laboratory Medicine 2004;128(4):415‐20. [DOI] [PubMed] [Google Scholar]
Evans 1996 {published data only}
- Evans MI, O'Brien JE, Dvorin E, Krivchenia EL, Drugan A, Hume RF Jr, et al. Similarity of insulin‐dependent diabetics' and non‐insulin‐dependent diabetics' levels of ß‐hCG and unconjugated estriol with controls: no need to adjust as with alpha‐fetoprotein. Journal of the Society for Gynecologic Investigation 1996;3(1):20‐2. [PubMed] [Google Scholar]
Evans 2007 {published data only}
- Evans MI, Galen RS. Comparison of serum markers in first‐trimester down syndrome screening. Obstetrics & Gynecology 2007;109(3):782. [DOI] [PubMed] [Google Scholar]
Falcon 2005 {published data only}
- Falcon O, Cavoretto P, Peralta CF, Csapo B, Nicolaides KH. Fetal head‐to‐trunk volume ratio in chromosomally abnormal fetuses at 11 + 0 to 13 + 6 weeks of gestation. Ultrasound in Obstetrics & Gynecology 2005;26(7):755‐60. [DOI] [PubMed] [Google Scholar]
Falcon 2006 {published data only}
- Falcon O, Faiola S, Huggon I, Allan L, Nicolaides KH. Fetal tricuspid regurgitation at the 11 + 0 to 13 + 6‐week scan: association with chromosomal defects and reproducibility of the method. Ultrasound in Obstetrics & Gynecology 2006;27(6):609‐12. [DOI] [PubMed] [Google Scholar]
Ford 1998 {published data only}
- Ford C, Moore AJ, Jordan PA, Bartlett WA, Wyldes MP, Jones AF, et al. The value of screening for Down's syndrome in a socioeconomically deprived area with a high ethnic population.[see comment]. British Journal of Obstetrics and Gynaecology 1998;105(8):855‐9. [DOI] [PubMed] [Google Scholar]
Frishman 1997 {published data only}
- Frishman GN, Canick JA, Hogan JW, Hackett RJ, Kellner LH, Saller DN Jr. Serum triple‐marker screening in in vitro fertilization and naturally conceived pregnancies. Obstetrics & Gynecology 1997;90(1):98‐101. [DOI] [PubMed] [Google Scholar]
Fukada 2000 {published data only}
- Fukada Y, Takizawa M, Amemiya A, Yoda H, Kohno K, Hoshi K. Detection of aneuploidy with fetal nuchal translucency and maternal serum markers in Japanese women. Acta Obstetricia et Gynecologica Scandinavica 2000;79(12):1124‐5. [PubMed] [Google Scholar]
Gaudry 2009 {published data only}
- Gaudry P, Lebbar A, Choiset A, Girard S, Lewin F, Tsatsaris V, et al. Is rapid aneuploidy screening used alone acceptable in prenatal diagnosis? An evaluation of the possible role of ultrasound examination. Fetal Diagnosis and Therapy 2009;25(2):285‐90. [DOI] [PubMed] [Google Scholar]
Gebb 2009 {published data only}
- Gebb J, Dar P. Should the first‐trimester aneuploidy screen be maternal age adjusted? Screening by absolute risk versus risk adjusted to maternal age. Prenatal Diagnosis 2009;29(3):245‐7. [DOI] [PubMed] [Google Scholar]
Geerts 2008 {published data only}
- Geerts L. Prenatal diagnosis of chromosomal abnormalities in a resource‐poor setting. International Journal of Gynaecology & Obstetrics 2008;103(1):16‐21. [DOI] [PubMed] [Google Scholar]
Geipel 2010 {published data only}
- Geipel A, Willruth A, Vieten J, Gembruch U, Berg C. Nuchal fold thickness, nasal bone absence or hypoplasia, ductus venosus reversed flow and tricuspid valve regurgitation in screening for trisomies 21, 18 and 13 in the early second trimester. Ultrasound in Obstetrics & Gynecology 2010;35(5):535‐9. [DOI] [PubMed] [Google Scholar]
Gekas 2009 {published data only}
- Gekas J, Gagne G, Bujold E, Douillard D, Forest JC, Reinharz D, et al. Comparison of different strategies in prenatal screening for Down's syndrome: cost effectiveness analysis of computer simulation. BMJ 2009;338:b138. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gekas 2011 {published data only}
- Gekas J, Berg DG, Durand A, Vallee M, Wildschut HI, Bujold E, et al. Rapid testing versus karyotyping in Down's syndrome screening: cost‐effectiveness and detection of clinically significant chromosome abnormalities. European Journal of Human Genetics 2011;19(1):3‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gekas 2011a {published data only}
- Gekas J, Durand A, Bujold E, Vallee M, Forest JC, Rousseau F, et al. Cost‐effectiveness and accuracy of prenatal Down syndrome screening strategies: should the combined test continue to be widely used?. American Journal of Obstetrics and Gynecology 2011;204(2):175‐8. [DOI] [PubMed] [Google Scholar]
Gerovassili 2007 {published data only}
- Gerovassili A, Garner C, Nicolaides KH, Thein SL, Rees DC. Free fetal DNA in maternal circulation: a potential prognostic marker for chromosomal abnormalities?. Prenatal Diagnosis 2007;27(2):104‐10. [DOI] [PubMed] [Google Scholar]
Ghidini 1998 {published data only}
- Ghidini A, Spong CY, Grier RE, Walker CN, Pezzullo JC. Is maternal serum triple screening a better predictor of Down syndrome in female than in male fetuses?. Prenatal Diagnosis 1998;18(2):123‐6. [DOI] [PubMed] [Google Scholar]
Goetzinger 2010 {published data only}
- Goetzinger KR, Dicke JM, Gray DL, Stamilio DM, Macones GA, Odibo AO. The effect of fetal gender in predicting Down syndrome using long bone ultrasonographic measurements. Prenatal Diagnosis 2010;30(10):950‐5. [DOI] [PubMed] [Google Scholar]
Goldie 1995 {published data only}
- Goldie DJ, Astley JP, Beaman JM, Bickley DA, Gunneberg A, Jones SR. Screening for Down's syndrome: the first two years experience in Bristol. Journal of Medical Screening 1995;2(4):207‐10. [DOI] [PubMed] [Google Scholar]
Gollo 2008 {published data only}
- Gollo CA, Murta CG, Bussamra LC, Santana RM, Moron AF. [Predictive value for fetal outcome of Doppler velocimetry of the ductus venosus between the 11th and the 14th gestation week]. [Portuguese]. Revista Brasileira de Ginecologia e Obstetricia 2008;30(1):5‐11. [DOI] [PubMed] [Google Scholar]
Gonçalves 2004 {published data only}
- Gonçalves LF, Espinoza J, Lee W, Schoen ML, Devers P, Mazor M, et al. Phenotypic characteristics of absent and hypoplastic nasal bones in fetuses with down syndrome: Description by 3‐dimensional ultrasonography and clinical significance. Journal of Ultrasound in Medicine 2004;23(12):1619‐27. [DOI] [PubMed] [Google Scholar]
Goodburn 1994 {published data only}
- Goodburn SF, Yates JR, Raggatt PR, Carr C, Ferguson‐Smith ME, Kershaw AJ, et al. Second‐trimester maternal serum screening using alpha‐fetoprotein, human chorionic gonadotrophin, and unconjugated oestriol: experience of a regional programme. Prenatal Diagnosis 1994;14(5):391‐402. [DOI] [PubMed] [Google Scholar]
Gorduza 2007 {published data only}
- Gorduza EV, Onofriescu M, Martiniuc V, Grigore M, Mihalceanu E, Iliev G. [FISH technique in aneuplodies prenatal diagnosis]. [Romanian]. Revista Medico‐Chirurgicala a Societatii de Medici Si Naturalisti Din Iasi 2007;111(4):990‐5. [PubMed] [Google Scholar]
Grace 2010 {published data only}
- Grace D, Eggers P, Glantz JC, Ozcan T. Mitral valve‐tricuspid valve distance as a sonographic marker of trisomy 21. Ultrasound in Obstetrics & Gynecology 2010;35(2):172‐7. [DOI] [PubMed] [Google Scholar]
Grati 2010 {published data only}
- Grati FR, Barlocco A, Grimi B, Milani S, Frascoli G, Meco AM, et al. Chromosome abnormalities investigated by non‐invasive prenatal testing account for approximately 50% of fetal unbalances associated with relevant clinical phenotypes. American Journal of Medical Genetics 2010;Part A. 152A(6):1434‐42. [DOI] [PubMed] [Google Scholar]
Gray 2009 {published data only}
- Gray DL, Dicke JM, Dickerson R, McCourt C, Odibo AO. Reevaluating humeral length for the detection of fetal trisomy 21. Journal of Ultrasound in Medicine 2009;28(10):1325‐30. [DOI] [PubMed] [Google Scholar]
Gregor 2007 {published data only}
- Gregor V, Sipek A, Horacek J. [Birth defects in the Czech Republic‐‐the prenatal diagnostic]. [Czech]. Ceska Gynekologie 2007;72(4):262‐8. [PubMed] [Google Scholar]
Gregor 2009 {published data only}
- Gregor V, Sipek A, Sipek AJ, Horacek J, Langhammer P, Petrzilkova L, et al. [Prenatal diagnostics of chromosomal aberrations Czech Republic: 1994‐2007]. [Czech]. Ceska Gynekologie 2009;74(1):44‐54. [PubMed] [Google Scholar]
Grether 2009 {published data only}
- Grether Gonzalez P, Aguinaga Rios M, Colegio Mexicano de Especialistas en Ginecologia. [Prenatal genetic screening: biochemical markers of the first and second quarter]. [Spanish]. Ginecologia y Obstetricia de Mexico 2009;77(2):S27‐46. [PubMed] [Google Scholar]
Grozdea 2002 {published data only}
- Grozdea J, Farge F, Bourrouillou G, Calot M, Cambus JP, Valdiguie P. Maternal serum urea resistant alkaline phosphatase in Down syndrome pregnancy. Early Human Development 2002;67(1‐2):55‐9. [DOI] [PubMed] [Google Scholar]
Guo 2010 {published data only}
- Guo Q, Zhou Y, Wang X, Li Q. Simultaneous detection of trisomies 13, 18, and 21 with multiplex ligation‐dependent probe amplification‐based real‐time PCR. Clinical Chemistry 2010;56(9):1451‐9. [DOI] [PubMed] [Google Scholar]
Gyselaers 2004 {published data only}
- Gyselaers WJ, Vereecken AJ, Herck EJ, Straetmans DP, Martens GE, Jonge ET, et al. Screening for trisomy 21 in Flanders: a 10 years review of 40.490 pregnancies screened by maternal serum. European Journal of Obstetrics, Gynecology, & Reproductive Biology 2004;115(2):185‐9. [DOI] [PubMed] [Google Scholar]
Gyselaers 2004a {published data only}
- Gyselaers WJA, Vereecken AJ, Herck E, Straetmans DPL, Jonge ET, Ombelet WUA, et al. Single‐step maternal serum screening for trisomy 21 in the era of combined or integrated screening. Gynecologic and Obstetric Investigation 2004;58(4):221‐4. [DOI] [PubMed] [Google Scholar]
Gyselaers 2006 {published data only}
- Gyselaers WJ, Vereecken AJ, Herck EJ, Straetmans DP, Ombelet WU, Nijhuis JG. Nuchal translucency thickness measurements for fetal aneuploidy screening: Log NT‐MoM or Delta‐NT, performer‐specific medians and ultrasound training. Journal of Medical Screening 2006;13(1):4‐7. [DOI] [PubMed] [Google Scholar]
Gyselaers 2006a {published data only}
- Gyselaers WJ, Roets ER, Holsbeke CD, Vereecken AJ, Herck EJ, Straetmans DP, et al. Sequential triage in the first trimester may enhance advanced ultrasound scanning in population screening for trisomy 21. Ultrasound in Obstetrics & Gynecology 2006;27(6):622‐7. [DOI] [PubMed] [Google Scholar]
Hackshaw 1995 {published data only}
- Hackshaw AK, Densem J, Wald NJ. Repeat maternal serum testing for Down's syndrome screening using multiple markers with special reference to free alpha and free ß‐hCG. Prenatal Diagnosis 1995;15(12):1125‐30. [DOI] [PubMed] [Google Scholar]
Hackshaw 2001 {published data only}
- Hackshaw AK, Wald NJ. Repeat testing in antenatal screening for Down syndrome using dimeric inhibin‐A in combination with other maternal serum markers. Prenatal Diagnosis 2001;21(1):58‐61. [PubMed] [Google Scholar]
Haddow 1992 {published data only}
- Haddow JE, Palomaki GE, Knight GJ, Williams J, Pulkkinen A, Canick J, et al. Prenatal screening for Down's syndrome with use of maternal serum markers. New England Journal of Medicine 1992;327(9):588‐93. [DOI] [PubMed] [Google Scholar]
Hadzsiev 2007 {published data only}
- Hadzsiev K, Czako M, Veszpremi B, Kosztolanyi G. [Rapid diagnosis of fetal chromosomal abnormalities by fluorescence in situ hybridization]. [Hungarian]. Orvosi Hetilap 2007;148(30):1401‐4. [DOI] [PubMed] [Google Scholar]
Hafner 1995 {published data only}
- Hafner E, Schuchter K, Philipp K. Screening for chromosomal abnormalities in an unselected population by fetal nuchal translucency. Ultrasound in Obstetrics & Gynecology 1995;6(5):330‐3. [DOI] [PubMed] [Google Scholar]
Hallahan 1998 {published data only}
- Hallahan TW, Krantz DA, Tului L, Alberti E, Buchanan PD, Orlandi F, et al. Comparison of urinary free ß (hCG) and ß‐core (hCG) in prenatal screening for chromosomal abnormalities. Prenatal Diagnosis 1998;18(9):893‐900. [DOI] [PubMed] [Google Scholar]
Han 2008 {published data only}
- Han SH, An JW, Jeong GY, Yoon HR, Lee A, Yang YH, et al. Clinical and cytogenetic findings on 31,615 mid‐trimester amniocenteses. Korean Journal of Laboratory Medicine 2008;28(5):378‐85. [DOI] [PubMed] [Google Scholar]
Harper 2010 {published data only}
- Harper LM, Gray D, Dicke J, Stamilio DM, Macones GA, Odibo AO. Do race‐specific definitions of short long bones improve the detection of down syndrome on second‐trimester genetic sonograms?. Journal of Ultrasound in Medicine 2010;29(2):231‐5. [DOI] [PubMed] [Google Scholar]
Harrison 2006 {published data only}
- Harrison G, Goldie D. Second‐trimester Down's syndrome serum screening: double, triple or quadruple marker testing?. Annals of Clinical Biochemistry 2006;43(1):67‐72. [DOI] [PubMed] [Google Scholar]
Harry 2006 {published data only}
- Harry WG, Reed KL. Nuchal translucency and first‐trimester screening. Journal of the Society for Gynecologic Investigation 2006;13(3):153‐4. [DOI] [PubMed] [Google Scholar]
Hayashi 1995 {published data only}
- Hayashi M, Kozu H. Maternal urinary ß‐core fragment of hCG/creatinine ratios and fetal chromosomal abnormalities in the second trimester of pregnancy. Prenatal Diagnosis 1995;15(1):11‐6. [DOI] [PubMed] [Google Scholar]
Hayashi 1996 {published data only}
- Hayashi M, Kozu H, Takei H. Maternal urinary free ß‐subunit of human chorionic gonadotrophin: Creatinine ratios and fetal chromosomal abnormalities in the second trimester of pregnancy. British Journal of Obstetrics and Gynaecology 1996;103(6):577‐80. [DOI] [PubMed] [Google Scholar]
Heikkila 1997 {published data only}
- Heikkila A, Ryynanen M, Kirkinen P, Saarikoski S. Results and views of women in population‐wide pregnancy screening for trisomy 21 in east Finland. Fetal Diagnosis and Therapy 1997;12(2):93‐6. [DOI] [PubMed] [Google Scholar]
Heinig 2007 {published data only}
- Heinig J, Steinhard J, Schmitz R, Nofer JR, Kiesel L, Klockenbusch W. Maternal serum free beta‐hCG and PAPP‐A in patients with habitual abortion‐influence on first‐trimester screening for chromosomal abnormalities. Prenatal Diagnosis 2007;27(9):814‐6. [DOI] [PubMed] [Google Scholar]
Heinonen 1996 {published data only}
- Heinonen S, Ryynanen M, Kirkinen P, Hippelainen M, Saarikoski S. Effect of in vitro fertilization on human chorionic gonadotropin serum concentrations and Down's syndrome screening. Fertility and Sterility 1996;66(3):398‐403. [DOI] [PubMed] [Google Scholar]
Herman 2000 {published data only}
- Herman A, Weinraub Z, Dreazen E, Arieli S, Rozansky S, Bukovsky I, et al. Combined first trimester nuchal translucency and second trimester biochemical screening tests among normal pregnancies. Prenatal Diagnosis 2000;20(10):781‐4. [DOI] [PubMed] [Google Scholar]
Herman 2003 {published data only}
- Herman Ae, Dreazen E, Tovbin Y, Reish O, Bukovsky I, Maymon R. Correlation and overlapping between nuchal translucency and triple test among Down syndrome‐affected pregnancies. Fetal Diagnosis and Therapy 2003;18(3):196‐200. [DOI] [PubMed] [Google Scholar]
Herrou 1992 {published data only}
- Herrou M, Leporrier N, Leymarie P. Screening for fetal Down syndrome with maternal serum hCG and oestriol: a prospective study. Prenatal Diagnosis 1992;12(11):887‐92. [DOI] [PubMed] [Google Scholar]
Hershey 1985 {published data only}
- Hershey DW, Crandall BF, Schroth PS. Maternal serum alpha‐fetoprotein screening of fetal trisomies. American Journal of Obstetrics and Gynecology 1985;153(2):224‐5. [DOI] [PubMed] [Google Scholar]
Hershey 1986 {published data only}
- Hershey DW, Crandall BF, Perdue S. Combining maternal age and serum alpha‐fetoprotein to predict the risk of Down syndrome. Obstetrics & Gynecology 1986;68(2):177‐80. [PubMed] [Google Scholar]
Hewitt 1993 {published data only}
- Hewitt B. Nuchal translucency in the first trimester. Australian & New Zealand Journal of Obstetrics & Gynaecology 1993;33(4):389‐91. [DOI] [PubMed] [Google Scholar]
Hills 2010 {published data only}
- Hills A, Donaghue C, Waters J, Waters K, Sullivan C, Kulkarni A, et al. QF‐PCR as a stand‐alone test for prenatal samples: the first 2 years' experience in the London region. Prenatal Diagnosis 2010;30(6):509‐17. [DOI] [PubMed] [Google Scholar]
Ho 2010 {published data only}
- Ho SS, Choolani MA. FlashFISH: "same day" prenatal diagnosis of common chromosomal aneuploidies. Methods in Molecular Biology 2010;659:261‐8. [DOI] [PubMed] [Google Scholar]
Hogdall 1992 {published data only}
- Hogdall CK, Hogdall EV, Arends J, Norgaard‐Pedersen B, Smidt‐Jensen S, Larsen SO. CA‐125 as a maternal serum marker for Down's syndrome in the first and second trimesters. Prenatal Diagnosis 1992;12(3):223‐7. [DOI] [PubMed] [Google Scholar]
Hong Kong Practitioner {published data only}
- Anon. Screening tests in pregnancy. Hong Kong Practitioner 2001;23(10):461‐5. [Google Scholar]
Hoogendoorn 2008 {published data only}
- Hoogendoorn M, Evers SM, Schielen PC, Genugten ML, Wit GA, Ament AJ. Costs and effects of prenatal screening methods for Down syndrome and neural tube defects. Community Genetics 2008;11(6):359‐67. [DOI] [PubMed] [Google Scholar]
Howe 2000 {published data only}
- Howe DT, Gornall R, Wellesley D, Boyle T, Barber J. Six year survey of screening for Down's syndrome by maternal age and mid‐trimester ultrasound scans.[see comment]. BMJ 2000;320(7235):606‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hsiao 1991 {published data only}
- Hsiao KJ, Lee SY, Chuang HC. [Antenatal screening of maternal alpha‐fetoprotein with dried‐blood spot samples on filter paper]. [Chinese]. Journal of the Formosan Medical Association 1991;90(6):598‐604. [PubMed] [Google Scholar]
Hsieh 1999 {published data only}
- Hsieh TT, Hsu JJ, Lo LM, Liou JD, Soong YK. Maternal urine alpha‐fetoprotein concentrations between 14 and 21 weeks of gestation. Changgeng Yi Xue Za Zhi 1999;22(2):234‐9. [PubMed] [Google Scholar]
Hsu 1997a {published data only}
- Hsu JJ, Hsieh TT, Soong YK. Influence of maternal age and weight on second‐trimester serum alpha‐fetoprotein, total and free ß human chorionic gonadotropin levels. Changgeng Yi Xue Za Zhi. 1997;20(3):181‐6. [PubMed] [Google Scholar]
Hsu 1998a {published data only}
- Hsu JJ, Hsieh TT, Hung TH, Chiang CH. Midtrimester maternal serum free ß‐human chorionic gonadotropin levels: normal reference values for Taiwanese women. Changgeng Yi Xue Za Zhi 1998;21(3):277‐82. [PubMed] [Google Scholar]
Hsu 1999b {published data only}
- Hsu JJ, Hsieh TT, Hung TH, Chen KC, Soong YK. Urine free ß‐human chorionic gonadotropin levels between 14 and 21 weeks of gestation in Taiwanese pregnancies. Changgeng Yi Xue Za Zhi 1999;22(1):11‐6. [PubMed] [Google Scholar]
Hu 2007 {published data only}
- Hu YL, Birth Defect Intervention Group of Jiangsu Province. [Serum screening of fetal chromosome abnormality during second pregnancy trimester: results of 26,803 pregnant women in Jiangsu Province]. [Chinese]. Chung‐Hua i Hsueh Tsa Chih [Chinese Medical Journal] 2007;87(35):2476‐80. [PubMed] [Google Scholar]
Huang 2003 {published data only}
- Huang T, Summers AM, Wyatt PR, Meier C, Cote GB. Maternal serum marker medians in Aboriginal Canadian women. Prenatal Diagnosis 2003;23(2):98‐100. [DOI] [PubMed] [Google Scholar]
Huang 2007 {published data only}
- Huang T, Boucher K, Summers AM. Second trimester prenatal screening for Down syndrome: the associations between the levels of serum markers in successive pregnancies. Prenatal Diagnosis 2007;27(12):1138‐42. [DOI] [PubMed] [Google Scholar]
Huang 2007a {published data only}
- Huang T, Wang FL, Boucher K, O'Donnell A, Rashid S, Summers AM. Racial differences in first trimester nuchal translucency. Prenatal Diagnosis 2007;27(12):1174‐6. [DOI] [PubMed] [Google Scholar]
Huggon 2004 {published data only}
- Huggon IC, Turan O, Allan LD. Doppler assessment of cardiac function at 11‐14 weeks' gestation in fetuses with normal and increased nuchal translucency. Ultrasound in Obstetrics & Gynecology 2004;24(4):390‐8. [DOI] [PubMed] [Google Scholar]
Hui 2003 {published data only}
- Hui PW, Tang MH, Lam YH, NG EH, Yeung WS, Ho PC. Maternal serum hCG and alpha‐fetoprotein levels in pregnancies conceived after IVF or ICSI with fresh and frozen‐thawed embryos. Human Reproduction 2003;18(3):572‐5. [DOI] [PubMed] [Google Scholar]
Hui 2005 {published data only}
- Hui PW, Tang MH, Lam YH, Yeung WS, NG EH, Ho PC. Nuchal translucency in pregnancies conceived after assisted reproduction technology. Ultrasound in Obstetrics & Gynecology 2005;25(3):234‐8. [DOI] [PubMed] [Google Scholar]
Hultén 2004 {published data only}
- Hultén M. Combined serum and nuchal translucency screening in the first trimester achieves 85% to 90% detection rate for Down and Edward syndromes. Evidence‐Based Healthcare 2004;8(2):82‐4. [Google Scholar]
Hung 2003 {published data only}
- Hung JH, Fu CY, Yuan CC, Chen CL, Yang ML, Shu LP, et al. Nuchal translucence incorporated into a one‐stage multifactorial screening model for Down syndrome prediction at second‐trimester pregnancy. Ultrasound in Medicine & Biology 2003;29(12):1667‐74. [DOI] [PubMed] [Google Scholar]
Hung 2008 {published data only}
- Hung JH, Fu CY, Chen CY, Chao KC, Hung J. Fetal nasal bone length and Down syndrome during the second trimester in a Chinese population. Journal of Obstetrics & Gynaecology Research 2008;34(4):518‐23. [DOI] [PubMed] [Google Scholar]
Hurley 1993 {published data only}
- Hurley PA, Ward RH, Teisner B, Iles RK, Lucas M, Grudzinskas JG. Serum PAPP‐A measurements in first‐trimester screening for Down syndrome. Prenatal Diagnosis 1993;13(10):903‐8. [DOI] [PubMed] [Google Scholar]
Huttly 2004 {published data only}
- Huttly W, Rudnicka A, Wald NJ. Second‐trimester prenatal screening markers for Down syndrome in women with insulin‐dependent diabetes mellitus. Prenatal Diagnosis 2004;24(10):804‐7. [DOI] [PubMed] [Google Scholar]
Hwa 2004 {published data only}
- Hwa HL, Yen MF, Hsieh FJ, Ko TM, Chen TH. Evaluation of second trimester maternal serum screening for Down's Syndrome using the Spiegelhalter‐Knill‐Jones (S‐KJ) approach. Journal of Perinatal Medicine 2004;32(5):407‐12. [DOI] [PubMed] [Google Scholar]
Iles 1996 {published data only}
- Iles RK. Urinary analysis for Down's syndrome: Is the measurement of urinary ß‐core the future of biochemical screening for Down's syndrome. Early Human Development 1996;47(Suppl):S41‐S45. [DOI] [PubMed] [Google Scholar]
Ind 1994 {published data only}
- Ind TEJ, Iles RK, Cuckle HS, Chard T. Second trimester maternal serum placental alkaline phosphatase concentrations in Down's syndrome. Journal of Obstetrics and Gynaecology 1994;14(5):305‐8. [Google Scholar]
Ivorra‐Deleuze 2010 {published data only}
- Ivorra‐Deleuze D, Bretelle F, Heinemann M, Levy A, Toga C, Philip N, et al. [Combined screening for Down syndrome in Marseille multidisciplinary prenatal centers]. [French]. Gynecologie, Obstetrique & Fertilite 2010;38(12):786‐8. [DOI] [PubMed] [Google Scholar]
Jakobsen 2011 {published data only}
- Jakobsen TR, Sogaard K, Tabor A. Implications of a first trimester Down syndrome screening program on timing of malformation detection. Acta Obstetricia et Gynecologica Scandinavica 2011;90(7):728‐36. [DOI] [PubMed] [Google Scholar]
Jean‐Pierre 2005 {published data only}
- Jean Pierre. Fetal nasal bone: Review of first trimester findings. Ultrasound Review of Obstetrics and Gynecology 2005;5(2):102‐4. [Google Scholar]
Johnson 1991 {published data only}
- Johnson A, Cowchock FS, Darby M, Wapner R, Jackson LG. First‐trimester maternal serum alpha‐fetoprotein and chorionic gonadotropin in aneuploid pregnancies. Prenatal Diagnosis 1991;11(7):443‐50. [DOI] [PubMed] [Google Scholar]
Johnson 1993 {published data only}
- Johnson MP, Johnson A, Holzgreve W, Isada NB, Wapner RJ, Treadwell MC, et al. First‐trimester simple hygroma: cause and outcome. American Journal of Obstetrics and Gynecology 1993;168(1):156‐61. [DOI] [PubMed] [Google Scholar]
Jorgensen 1999 {published data only}
- Jorgensen FS, Valentin L, Salvesen KA, Jorgensen C, Jensen FR, Bang J, et al. MULTISCAN‐‐a Scandinavian multicenter second trimester obstetric ultrasound and serum screening study. Acta Obstetricia et Gynecologica Scandinavica 1999;78(6):501‐10. [PubMed] [Google Scholar]
Jorgez 2007 {published data only}
- Jorgez CJ, Dang DD, Wapner R, Farina A, Simpson JL, Bischoff FZ. Elevated levels of total (maternal and fetal) beta‐globin DNA in maternal blood from first trimester pregnancies with trisomy 21. Human Reproduction 2007;22(8):2267‐72. [DOI] [PubMed] [Google Scholar]
Josefsson 1998 {published data only}
- Josefsson A, Molander E, Selbing A. Nuchal translucency as a screening test for chromosomal abnormalities in a routine first trimester ultrasound examination. Acta Obstetricia et Gynecologica Scandinavica 1998;77(5):497‐9. [PubMed] [Google Scholar]
Jou 2001 {published data only}
- Jou HJ, Shih JC, Wu SC, Li TC, Tzeng CY, Hsieh FJ. First‐trimester Down's syndrome screening by fetal nuchal translucency measurement in Taiwan. Journal of the Formosan Medical Association 2001;100(4):257‐61. [PubMed] [Google Scholar]
Jung 2007 {published data only}
- Jung E, Won HS, Lee PR, Kim A. Ultrasonographic measurement of fetal nasal bone length in the second trimester in Korean population. Prenatal Diagnosis 2007;27(2):154‐7. [DOI] [PubMed] [Google Scholar]
Jun‐Tao 2003 {published data only}
- Liu JT, Hao N, Sun NH, Wang FY, Xu YH, Gai MY, et al. [Screening by maternal serum markers for Down's syndrome]. [Chinese]. Chung‐Kuo i Hsueh Ko Hsueh Yuan Hsueh Pao Acta Academiae Medicinae Sinicae 2003;25(2):156‐9. [PubMed] [Google Scholar]
Kagan 2006 {published data only}
- Kagan KO, Avgidou K, Molina FS, Gajewska K, Nicolaides KH. Relation between increased fetal nuchal translucency thickness and chromosomal defects.[see comment]. Obstetrics & Gynecology 2006;107(1):6‐10. [DOI] [PubMed] [Google Scholar]
Kagan 2007 {published data only}
- Kagan KO, Frisova V, Nicolaides KH, Spencer K. Dose dependency between cigarette consumption and reduced maternal serum PAPP‐A levels at 11‐13+6 weeks of gestation. Prenatal Diagnosis 2007;27(9):849‐53. [DOI] [PubMed] [Google Scholar]
Kagan 2008 {published data only}
- Kagan KO, Anderson JM, Anwandter G, Neksasova K, Nicolaides KH. Screening for triploidy by the risk algorithms for trisomies 21, 18 and 13 at 11 weeks to 13 weeks and 6 days of gestation. Prenatal Diagnosis 2008;28(13):1209‐13. [DOI] [PubMed] [Google Scholar]
Kalelioglu 2007 {published data only}
- Kalelioglu IH. Humerus length measurement in Down syndrome screening. Clinical & Experimental Obstetrics & Gynecology 2007;34(2):93‐5. [PubMed] [Google Scholar]
Kautzmann 1995 {published data only}
- Kautzmann M, Solis RL, Luberta A, Fernandez JL, Navarro J, Rodriguez L, et al. Study of the efficiency of screening for trisomy 21 based on maternal serum levels of AFP and hCG combined with maternal age. Journal of Clinical Ligand Assay 1995;18(3):181‐5. [Google Scholar]
Kazerouni 2009 {published data only}
- Kazerouni NN, Currier B, Malm L, Riggle S, Hodgkinson C, Smith S, et al. Triple‐marker prenatal screening program for chromosomal defects. Obstetrics & Gynecology 2009;114(1):50‐8. [DOI] [PubMed] [Google Scholar]
Keith 1992 {published data only}
- Keith D. Maternal serum screening for neural tube defects and Down syndrome. Clinical Laboratory Science 1992;5(5):274‐6. [PubMed] [Google Scholar]
Kelekci 2004 {published data only}
- Kelekci S, Yazicioglu HF, Oguz S, Inan I, Yilmaz B, Sonmez S. Nasal bone measurement during the 1st trimester: is it useful?. Gynecologic & Obstetric Investigation 2004;58(2):91‐5. [DOI] [PubMed] [Google Scholar]
Kellner 1995 {published data only}
- Kellner LH, Weiner Z, Weiss RR, Neuer M, Martin GM, Mueenuddin M, et al. Triple marker (alpha‐fetoprotein, unconjugated estriol, human chorionic gonadotropin) versus alpha‐fetoprotein plus free‐ß subunit in second‐trimester maternal serum screening for fetal Down syndrome: a prospective comparison study.[see comment]. American Journal of Obstetrics and Gynecology 1995;173(4):1306‐9. [DOI] [PubMed] [Google Scholar]
Kellner 1995a {published data only}
- Kellner LH, Weiss RR, Weiner Z, Neuer M, Martin GM, Schulman H, et al. The advantages of using triple‐marker screening for chromosomal abnormalities. American Journal of Obstetrics and Gynecology 1995;172(3):831‐6. [DOI] [PubMed] [Google Scholar]
Kellner 1997 {published data only}
- Kellner LH, Canick JA, Palomaki GE, Neveux LM, Saller DN Jr, Walker RP, et al. Levels of urinary ß‐core fragment, total oestriol, and the ratio of the two in second‐trimester screening for Down syndrome. Prenatal Diagnosis 1997;17(12):1135‐41. [DOI] [PubMed] [Google Scholar]
Kirkegaard 2008 {published data only}
- Kirkegaard I, Petersen OB, Uldbjerg N, Torring N. Improved performance of first‐trimester combined screening for trisomy 21 with the double test taken before a gestational age of 10 weeks. Prenatal Diagnosis 2008;28(9):839‐44. [DOI] [PubMed] [Google Scholar]
Kjaergaard 2008 {published data only}
- Kjaergaard S, Hahnemann JM, Skibsted L, Jensen LN, Sperling L, Zingenberg H, et al. [Prenatal diagnosis of chromosome aberrations after implementation of screening for Down's syndrome]. [Danish]. Ugeskrift for Laeger 2008;170(14):1152‐6. [PubMed] [Google Scholar]
Knight 1990 {published data only}
- Knight GJ, Palomaki GE. Maternal serum alpha fetoprotein screening for fetal down syndrome. Journal of Clinical Immunoassay 1990;13(1):23‐9. [Google Scholar]
Knight 2001 {published data only}
- Knight GJ, Palomaki GE, Neveux LM, Haddow JE, Lambert‐Messerlian GM. Clinical validation of a new dimeric inhibin‐A assay suitable for second trimester Down's syndrome screening. Journal of Medical Screening 2001;8(1):2‐7. [DOI] [PubMed] [Google Scholar]
Knight 2005 {published data only}
- Knight GJ, Palomaki GE, Neveux LM, Smith DE, Kloza EM, Pulkkinen A, et al. Integrated serum screening for Down syndrome in primary obstetric practice. Prenatal Diagnosis 2005;25(12):1162‐7. [DOI] [PubMed] [Google Scholar]
Koos 2006 {published data only}
- Koos BJ. First‐trimester screening: Lessons from clinical trials and implementation. Current Opinion in Obstetrics and Gynecology 2006;18(2):152‐5. [DOI] [PubMed] [Google Scholar]
Kornman 1996 {published data only}
- Kornman LH, Morssink LP, Beekhuis JR, Wolf BT, Heringa MP, Mantingh A. Nuchal translucency cannot be used as a screening test for chromosomal abnormalities in the first trimester of pregnancy in a routine ultrasound practice.[see comment]. Prenatal Diagnosis 1996;16(9):797‐805. [DOI] [PubMed] [Google Scholar]
Kornman 1997 {published data only}
- Kornman LH, Morssink LP, Wortelboer MJ, Beekhuis JR, Wolf BT, Pratt JJ, et al. Maternal urinary ß‐core hCG in chromosomally abnormal pregnancies in the first trimester. Prenatal Diagnosis 1997;17(2):135‐9. [DOI] [PubMed] [Google Scholar]
Kotaska 2007 {published data only}
- Kotaska A. Prenatal screening for fetal aneuploidy. Journal of Obstetrics & Gynaecology Canada: JOGC 2007;29(6):499‐500. [DOI] [PubMed] [Google Scholar]
Kramer 1998 {published data only}
- Kramer RL, Yaron Y, O'Brien JE, Critchfield G, Ayoub M, Johnson MP, et al. Effect of adjustment of maternal serum alpha‐fetoprotein levels in insulin‐dependent diabetes mellitus. American Journal of Medical Genetics 1998;75(2):176‐8. [PubMed] [Google Scholar]
Krantz 1996 {published data only}
- Krantz DA, Larsen JW, Buchanan PD, Macri JN. First‐trimester Down syndrome screening: free ß‐human chorionic gonadotropin and pregnancy‐associated plasma protein A. American Journal of Obstetrics and Gynecology 1996;174(2):612‐6. [DOI] [PubMed] [Google Scholar]
Krantz 2005 {published data only}
- Krantz DA, Hallahan TW, Macri VJ, Macri JN. Maternal weight and ethnic adjustment within a first‐trimester Down syndrome and trisomy 18 screening program. Prenatal Diagnosis 2005;25(8):635‐40. [DOI] [PubMed] [Google Scholar]
Krantz 2007 {published data only}
- Krantz DA, Hallahan TW, Macri VJ, Macri JN. Genetic sonography after first‐trimester Down syndrome screening. Ultrasound in Obstetrics & Gynecology 2007;29(6):666‐70. [DOI] [PubMed] [Google Scholar]
Kulch 1993 {published data only}
- Kulch P, Keener S, Matsumoto M, Crandall BF. Racial differences in maternal serum human chorionic gonadotropin and unconjugated oestriol levels. Prenatal Diagnosis 1993;13(3):191‐5. [DOI] [PubMed] [Google Scholar]
Lai 1998 {published data only}
- Lai FM, Yeo GS. Down syndrome screening in Singapore‐‐the effectiveness of a second trimester serum screening policy modelled on 29,360 pregnancies in KK Women's and Children's Hospital. Singapore Medical Journal 1998;39(2):69‐75. [PubMed] [Google Scholar]
Lai 2003 {published data only}
- Lai TH, Chen SC, Tsai MS, Lee FK, Wei CF. First‐trimester screening for down syndrome in singleton pregnancies achieved by intrauterine insemination. Journal of Assisted Reproduction and Genetics 2003;20(8):327‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Laigaard 2006 {published data only}
- Laigaard J, Cuckle H, Wewer UM, Christiansen M. Maternal serum ADAM12 levels in Down and Edwards' syndrome pregnancies at 9‐12 weeks' gestation. Prenatal Diagnosis 2006;26(8):689‐91. [DOI] [PubMed] [Google Scholar]
Laigaard 2006b {published data only}
- Laigaard J, Spencer K, Christiansen M, Cowans NJ, Larsen SO, Pedersen BN, et al. ADAM 12 as a first‐trimester maternal serum marker in screening for Down syndrome. Prenatal Diagnosis 2006;26(10):973‐9. [DOI] [PubMed] [Google Scholar]
Lam 1997 {published data only}
- Lam YH, Tang MH, Tang LC, Lee CP, Ho PK. Second‐trimester maternal urinary gonadotrophin peptide screening for fetal Down syndrome in Asian women. Prenatal Diagnosis 1997;17(12):1101‐6. [PubMed] [Google Scholar]
Lam 1998 {published data only}
- Lam YH, Ghosh A, Tang MH, Tang LC, Lee CP, Sin SY, et al. Second‐trimester maternal serum alpha‐fetoprotein and human chorionic gonadotrophin screening for Down's syndrome in Hong Kong. Prenatal Diagnosis 1998;18(6):585‐9. [PubMed] [Google Scholar]
Lam 1999 {published data only}
- Lam YH, Yeung WS, Tang MH, NG EH, So WW, Ho PC. Maternal serum alpha‐fetoprotein and human chorionic gonadotrophin in pregnancies conceived after intracytoplasmic sperm injection and conventional in‐vitro fertilization. Human Reproduction 1999;14(8):2120‐3. [DOI] [PubMed] [Google Scholar]
Lam 1999a {published data only}
- Lam YH, Tang MH. Second‐trimester maternal serum inhibin‐A screening for fetal Down syndrome in Asian women. Prenatal Diagnosis 1999;19(5):463‐7. [DOI] [PubMed] [Google Scholar]
Lam 2000 {published data only}
- Lam YH, Tang MH, Lee CP, Sin SY, Tang R, Wong HS, et al. Acceptability of serum screening as an alternative to cytogenetic diagnosis of down syndrome among women 35 years or older in Hong Kong. Prenatal Diagnosis 2000;20(6):487‐90. [DOI] [PubMed] [Google Scholar]
Lam 2001 {published data only}
- Lam YH, Tang MH. The effect of fetal gender on second‐trimester maternal serum inhibin‐A concentration. Prenatal Diagnosis 2001;21(8):662‐4. [PubMed] [Google Scholar]
Lambert‐Messerlian 1996 {published data only}
- Lambert‐Messerlian GM, Canick JA, Palomaki GE, Schneyer AL. Second trimester levels of maternal serum Inhibin A, total inhibin, alpha Inhibin Aprecursor, and activin in Down's syndrome pregnancy. Journal of Medical Screening 1996;3(2):58‐62. [DOI] [PubMed] [Google Scholar]
Lambert‐Messerlian 1998 {published data only}
- Lambert Messerlian GM, Luisi S, Florio P, Mazza V, Canick JA, Petraglia F. Second trimester levels of maternal serum total activin A and placental inhibin/activin alpha and ßA subunit messenger ribonucleic acids in Down syndrome pregnancy. European Journal of Endocrinology 1998;138(4):425‐9. [DOI] [PubMed] [Google Scholar]
Lauria 2007 {published data only}
- Lauria MR, Branch MD, LaCroix VH, Harris RD, Baker ER. Clinical impact of systematic genetic sonogram screening in a low‐risk population. Journal of Reproductive Medicine 2007;52(5):359‐64. [PubMed] [Google Scholar]
Lehavi 2005 {published data only}
- Lehavi O, Aizenstein O, Evans MI, Yaron Y. 2nd‐trimester maternal serum human chorionic gonadotropin and alpha‐fetoprotein levels in male and female fetuses with Down syndrome. Fetal Diagnosis and Therapy 2005;20(3):235‐8. [DOI] [PubMed] [Google Scholar]
Leung 2006 {published data only}
- Leung TY, Spencer K, Leung TN, Fung TY, Lau TK. Higher median levels of free ß‐hCG and PAPP‐A in the first trimester of pregnancy in a Chinese ethnic group. Implication for first trimester combined screening for Down's syndrome in the Chinese population. Fetal Diagnosis and Therapy 2006;21(1):140‐3. [DOI] [PubMed] [Google Scholar]
Leymarie 1993 {published data only}
- Leymarie P, Leporrier N. Maternal serum markers and prenatal screening for Down syndrome. Archives Francaises de Pediatrie 1993;50(5):455‐7. [PubMed] [Google Scholar]
Li 1998 {published data only}
- Li G, Huang X. [Clinical uses of maternal serum markers in the prenatal diagnosis] [Chinese]. Chung‐Hua Fu Chan Ko Tsa Chih 1998;33(4):252‐4. [PubMed] [Google Scholar]
Li 1999 {published data only}
- Li W, Zhou Y. [Measurement of pregnancy‐associated plasma protein A in maternal peripheral blood and Down syndrome] [Chinese]. Chung‐Hua Fu Chan Ko Tsa Chih 1999;34(10):631‐3. [PubMed] [Google Scholar]
Li 2010 {published data only}
- Li HW, Hui PW, Tang MH, Lau ET, Yeung WS, Ho PC, et al. Maternal serum anti‐Mullerian hormone level is not superior to chronological age in predicting Down syndrome pregnancies. Prenatal Diagnosis 2010;30(4):320‐4. [DOI] [PubMed] [Google Scholar]
Liao 1997 {published data only}
- Liao S, Wang Y, Ye G. [AFP, uE3, ß‐hCG levels applied for prenatal diagnosis of Down's syndrome]. [Chinese]. Chung‐Hua Fu Chan Ko Tsa Chih 1997;32(11):655‐8. [PubMed] [Google Scholar]
Liao 2001 {published data only}
- Liao AW, Heath V, Kametas N, Spencer K, Nicolaides KH. First‐trimester screening for trisomy 21 in singleton pregnancies achieved by assisted reproduction. Human Reproduction 2001;16(7):1501‐4. [DOI] [PubMed] [Google Scholar]
Lim 2002 {published data only}
- Lim KI, Pugash D, Dansereau J, Wilson RD. Nuchal index: a gestational age independent ultrasound marker for the detection of Down syndrome. Prenatal Diagnosis 2002;22(13):1233‐7. [DOI] [PubMed] [Google Scholar]
Lippman 1987 {published data only}
- Lippman A, Evans JA. Screening for maternal serum alpha‐fetoprotein: what about the low side?. CMAJ: Canadian Medical Association Journal 1987;136(8):801‐4. [PMC free article] [PubMed] [Google Scholar]
Liu 2010 {published data only}
- Liu YH, Li LF, Wu YM. [Analysis of Down syndrome screening by maternal serum detection in mid‐pregnancy]. [Chinese]. Nan Fang Yi Ke Da Xue Xue Bao = Journal of Southern Medical University 2010;30(3):532‐4. [PubMed] [Google Scholar]
Lo 2010 {published data only}
- Lo TK, Lai FK, Leung WC, Lau WL, Tang LC, Chin RK. A new policy for prenatal screening and diagnosis of Down syndrome for pregnant women with advanced maternal age in a public hospital. Journal of Maternal‐Fetal & Neonatal Medicine 2010;23(8):914‐9. [DOI] [PubMed] [Google Scholar]
Lustig 1988 {published data only}
- Lustig L, Clarke S, Cunningham G, Schonberg R, Tompkinson G. California's experience with low MS‐AFP results. American Journal of Medical Genetics 1988;31(1):211‐22. [DOI] [PubMed] [Google Scholar]
Luthgens 2008 {published data only}
- Luthgens K. Comparison of the new PRC software with the established algorithm of the FMF UK for the detection of trisomy 21 and 18/13. Fetal Diagnosis and Therapy 2008;24(4):376‐84. [DOI] [PubMed] [Google Scholar]
MacDonald 1991 {published data only}
- MacDonald ML, Wagner RM, Slotnick RN. Sensitivity and specificity of screening for Down syndrome with alpha‐fetoprotein, hCG, unconjugated estriol, and maternal age.[see comment]. Obstetrics & Gynecology 1991;77(1):63‐8. [PubMed] [Google Scholar]
Macintosh 1994 {published data only}
- Macintosh MCM, Iles R, Teisner B, Sharma K, Chard T, Grudzinskas J, et al. Maternal serum human chorionic gonadotrophin and pregnancy‐associated plasma protein A, markers for fetal Down syndrome at 8‐14 weeks. Prenatal Diagnosis 1994;14(3):203‐8. [DOI] [PubMed] [Google Scholar]
Macintosh 1997 {published data only}
- Macintosh MCM, Nicolaides KH, Noble P, Chard T, Gunn L, Iles R. Urinary ß‐core hCG: Screening for aneuploidies in early pregnancy (11‐14 weeks' gestation). Prenatal Diagnosis 1997;17(5):401‐5. [PubMed] [Google Scholar]
MacRae 2010 {published data only}
- MacRae AR, Chodirker BN, Davies GA, Palomaki GE, Knight GJ, Minett J, et al. Second and first trimester estimation of risk for Down syndrome: implementation and performance in the SAFER study. Prenatal Diagnosis 2010;30(5):459‐66. [DOI] [PubMed] [Google Scholar]
Macri 1994 {published data only}
- Macri JN, Kasturi RV, Krantz DA, Cook EJ, Moore ND, Young JA, et al. Maternal serum Down syndrome screening: free ß‐protein is a more effective marker than human chorionic gonadotropin.[see comment]. American Journal of Obstetrics and Gynecology 1990;163(4):1248‐53. [DOI] [PubMed] [Google Scholar]
- Macri JN, Spencer K, Garver K, Buchanan PD, Say B, Carpenter NJ, et al. Maternal serum free ß hCG screening: results of studies including 480 cases of Down syndrome.[see comment]. Prenatal Diagnosis 1994;14(2):97‐103. [DOI] [PubMed] [Google Scholar]
- Spencer K, Macri JN. Early detection of Down's syndrome using free ß human choriogonadotropin. Annals of Clinical Biochemistry 1992;19(3):349‐50. [DOI] [PubMed] [Google Scholar]
Macri 1996 {published data only}
- Macri JN, Anderson RW, Krantz DA, Larsen JW, Buchanan PD. Prenatal maternal dried blood screening with alpha‐fetoprotein and free ß‐human chorionic gonadotropin for open neural tube defect and Down syndrome. American Journal of Obstetrics and Gynecology 1996;174(2):566‐72. [DOI] [PubMed] [Google Scholar]
Malone 1998 {published data only}
- Malone FD, D'Alton ME. Ultrasound clinics. Fetal nuchal fold translucency screening. Contemporary OB/GYN 1998;43(3):117‐8. [Google Scholar]
Malone 2003 {published data only}
- Malone FD, D'Alton ME. First‐trimester sonographic screening for Down syndrome. Obstetrics and Gynecology 2003;102(5):1066‐79. [DOI] [PubMed] [Google Scholar]
Mandryka‐Stankewycz 2009 {published data only}
- Mandryka‐Stankewycz S, Perenc M, Dec G, Sieroszewski P. [Noninvasive prenatal test in the first trimester of pregnancy (NT and estimation of beta‐hCG and PAPP‐A) in the diagnosis of fetal abnormalities in Polish population‐‐comparison of the biochemistry own normal ranges and literature reported data]. [Polish]. Ginekologia Polska 2009;80(11):851‐5. [PubMed] [Google Scholar]
Mangione 2001 {published data only}
- Mangione R, Guyon F, Taine L, Wen ZQ, Roux D, Vergnaud A, et al. Pregnancy outcome and prognosis in fetuses with increased first‐trimester nuchal translucency. Fetal Diagnosis and Therapy 2001;16(6):360‐3. [DOI] [PubMed] [Google Scholar]
Markov 2008 {published data only}
- Markov D, Dimitrova V. [Ultrasound screening for chromosomal anomalies by assessment of the fetal nasal bone during 11‐14 weeks of gestation‐‐a pilot study]. [Bulgarian]. Akusherstvo i Ginekologiia 2008;47(1):3‐9. [PubMed] [Google Scholar]
Maymon 2001 {published data only}
- Maymon R, Shulman A. Comparison of triple serum screening and pregnancy outcome in oocyte donation versus IVF pregnancies. Human Reproduction 2001;16(4):691‐5. [DOI] [PubMed] [Google Scholar]
Maymon 2001a {published data only}
- Maymon R, Dreazen E, Buckovsky I, Weinraub Z, Herman A. Does a 'notched' nuchal translucency indicate Down syndrome fetuses or other adverse pregnancy outcome?. Prenatal Diagnosis 2001;21(5):403‐8. [DOI] [PubMed] [Google Scholar]
Maymon 2002 {published data only}
- Maymon R, Shulman A. Serial first‐ and second‐trimester Down's syndrome screening tests among IVF‐versus naturally‐conceived singletons. Human Reproduction 2002;17(4):1081‐5. [DOI] [PubMed] [Google Scholar]
Maymon 2004a {published data only}
- Maymon R, Shulman A. Integrated first‐ and second‐trimester Down syndrome screening test among unaffected IVF pregnancies. Prenatal Diagnosis 2004;24(2):125‐9. [DOI] [PubMed] [Google Scholar]
Maymon 2005a {published data only}
- Maymon R, Cuckle H, Jones R, Reish O, Sharony R, Herman A. Predicting the result of additional second‐trimester markers from a woman's first‐trimester marker profile: A new concept in Down syndrome screening. Prenatal Diagnosis 2005;25(12):1102‐6. [DOI] [PubMed] [Google Scholar]
McDuffie 1996 {published data only}
- McDuffie Jr, Haverkamp AD, Stark CF, Haverkamp C, Barth CK. Prenatal screening using maternal serum alpha‐fetoprotein, human chorionic gonadotropin, and unconjugated estriol: Two‐year experience in a health maintenance organization. Journal of Maternal‐Fetal Medicine 1996;5(2):70‐3. [DOI] [PubMed] [Google Scholar]
Meier 2002 {published data only}
- Meier C, Huang T, Wyatt PR, Summers AM. Accuracy of expected risk of Down syndrome using the second‐trimester triple test. Clinical Chemistry 2002;48(4):653‐5. [PubMed] [Google Scholar]
Merkatz 1984 {published data only}
- Merkatz IR, Nitowsky HM, Macri JN, Johnson WE. An association between low maternal serum alpha‐fetoprotein and fetal chromosomal abnormalities. American Journal of Obstetrics and Gynecology 1984;148(7):886‐94. [DOI] [PubMed] [Google Scholar]
Merz 2005 {published data only}
- Merz E. The fetal nasal bone in the first trimester ‐ Precise assessment using 3D sonography. Ultraschall in der Medizin 2005;26(5):365‐6. [DOI] [PubMed] [Google Scholar]
Merz 2008 {published data only}
- Merz E, Thode C, Alkier A, Eiben B, Hackeloer BJ, Hansmann M, et al. A new approach to calculating the risk of chromosomal abnormalities with first‐trimester screening data. Ultraschall in der Medizin 2008;29(6):639‐45. [DOI] [PubMed] [Google Scholar]
Metzenbauer 2001 {published data only}
- Metzenbauer M, Hafner E, Hoefinger D, Schuchter K, Stangl G, Ogris E, et al. Three‐dimensional ultrasound measurement of the placental volume in early pregnancy: method and correlation with biochemical placenta parameters. Placenta 2001;22(6):602‐5. [DOI] [PubMed] [Google Scholar]
Metzenbauer 2002 {published data only}
- Metzenbauer M, Hafner E, Schuchter K, Philipp K. First‐trimester placental volume as a marker for chromosomal anomalies: preliminary results from an unselected population. Ultrasound in Obstetrics & Gynecology 2002;19(3):240‐2. [DOI] [PubMed] [Google Scholar]
Mikic 1999 {published data only}
- Mikic TS, Johnson P. Second trimester maternal serum ß human chorionic gonadotrophin and pregnancy outcome. British Journal of Obstetrics and Gynaecology 1999;106(6):598‐600. [DOI] [PubMed] [Google Scholar]
Miller 1991 {published data only}
- Miller CH, O'Brien TJ, Chatelain S, Butler BB, Quirk JG. Alteration in age‐specific risks for chromosomal trisomy by maternal serum alpha‐fetoprotein and human chorionic gonadotropin screening. Prenatal Diagnosis 1991;11(3):153‐8. [DOI] [PubMed] [Google Scholar]
Milunsky 1989 {published data only}
- Milunsky A, Jick SS, Bruell CL, Maclaughlin DS, Tsung Y‐K, Jick H, et al. Predictive values relative risks and overall benefits of high and low maternal serum alpha fetoprotein screening in singleton pregnancies ‐ new epidemiological data. American Journal of Obstetrics and Gynecology 1989;161(2):291‐7. [DOI] [PubMed] [Google Scholar]
Milunsky 1996 {published data only}
- Milunsky A, Nebiolo L. Maternal serum triple analyte screening and adverse pregnancy outcome. Fetal Diagnosis and Therapy 1996;11(4):249‐53. [DOI] [PubMed] [Google Scholar]
Minobe 2002 {published data only}
- Minobe S. [A study on the screening of prenatal trisomy 21 using the fucosylated alpha‐fetoprotein ratio measured by a liquid‐phase binding assay]. [Japanese]. Hokkaido Igaku Zasshi ‐ Hokkaido Journal of Medical Science 2002;77(6):527‐32. [PubMed] [Google Scholar]
Miron 2008 {published data only}
- Miron P, Cote YP, Lambert J. Effect of maternal smoking on prenatal screening for Down syndrome and trisomy 18 in the first trimester of pregnancy. Prenatal Diagnosis 2008;28(3):180‐5. [DOI] [PubMed] [Google Scholar]
Miron 2009 {published data only}
- Miron P, Cote YP, Lambert J. Nuchal translucency thresholds in prenatal screening for Down syndrome and trisomy 18. Journal of Obstetrics & Gynaecology Canada: JOGC 2009;31(3):227‐35. [DOI] [PubMed] [Google Scholar]
Miron 2010 {published data only}
- Miron P, Lambert J, Marcil A, Cowans NJ, Stamatopoulou A, Spencer K. Maternal plasma levels of follistatin‐related gene protein in the first trimester of pregnancies with Down syndrome. Prenatal Diagnosis 2010;30(3):224‐8. [DOI] [PubMed] [Google Scholar]
Miyamura 1999 {published data only}
- Miyamura T, Saito N, Touno A, Nagata S, Hidaki T, Ishimaru T, et al. Multicenter study for maternal serum triple markers to establish Japanese standards: Maternal serum marker study group, Japan Association of Prenatal Diagnostics. Acta Obstetrica et Gynaecologica Japonica 1999;51(11):1042‐8. [Google Scholar]
Moghadam 1998 {published data only}
- Moghadam S, Engel W, Bougoussa M, Hennen G, Igout A, Sancken U. Maternal serum placental growth hormone and insulinlike growth factor binding proteins 1 and 3 in pregnancies affected by fetal aneuploidy and other abnormalities: implications for prenatal diagnosis of trisomy 21. Fetal Diagnosis and Therapy 1998;13(5):291‐7. [DOI] [PubMed] [Google Scholar]
Monni 2000 {published data only}
- Monni G, Zoppi MA, Ibba RM, Putzolu M, Floris M. Nuchal translucency in multiple pregnancies. Croatian Medical Journal 2000;41(3):266‐9. [PubMed] [Google Scholar]
Monni 2002 {published data only}
- Monni G, Zoppi MA. New ultrasonographic markers of aneuploidies: Nasal bones. Ultrasound Review of Obstetrics and Gynecology 2002;2(4):229‐34. [Google Scholar]
Mooney 1994 {published data only}
- Mooney RA, Peterson CJ, French CA, Saller DN Jr, Arvan DA. Effectiveness of combining maternal serum alpha‐fetoprotein and hCG in a second‐trimester screening program for Down syndrome. Obstetrics and Gynecology 1994;84(2):298‐303. [PubMed] [Google Scholar]
Muhcu 2008 {published data only}
- Muhcu M, Mungen E, Atay V, Ipcioglu OM, Dundar O, Ergur R, et al. First trimester screening for Down syndrome in rhesus negative women. Prenatal Diagnosis 2008;28(5):404‐7. [DOI] [PubMed] [Google Scholar]
Muller 1994 {published data only}
- Muller F, Bussieres L, Pelissier MC, Oury JF, Boue C, Uzan S, et al. Do racial differences exist in second‐trimester maternal hCG levels? A study of 23,369 women. Prenatal Diagnosis 1994;14(7):633‐6. [DOI] [PubMed] [Google Scholar]
Muller 1996a {published data only}
- Muller F, Dommergues M, Bussieres L, Aegerter P, Fiblec B, Uzan S, et al. Prenatal screening for Down syndrome: should first trimester ultrasound replace maternal serum screening?. Early Human Development 1996;47(Suppl):S37‐9. [DOI] [PubMed] [Google Scholar]
Muller 1999 {published data only}
- Muller F, Ngo S, Rebiffe M, Oury JF, Uzan S, Satge D. Maternal serum s100b protein is ineffective for Down syndrome screening. Prenatal Diagnosis 1999;19(11):1086. [DOI] [PubMed] [Google Scholar]
Muller 2002 {published data only}
- Muller F, Dreux S, Oury JF, Luton D, Uzan S, Uzan M, et al. Down syndrome maternal serum marker screening after 18 weeks' gestation. Prenatal Diagnosis 2002;22(11):1001‐4. [DOI] [PubMed] [Google Scholar]
Muller 2002a {published data only}
- Muller F, Forestier F, Dingeon B, ABA Study Group. Second trimester trisomy 21 maternal serum marker screening. Results of a countrywide study of 854,902 women.[see comment]. Prenatal Diagnosis 2002;22(10):925‐9. [DOI] [PubMed] [Google Scholar]
Muller 2003b {published data only}
- Muller F, Dreux S, Lemeur A, Sault C, Desgres J, Bernard MA, et al. Medically assisted reproduction and second‐trimester maternal serum marker screening for Down syndrome. Prenatal Diagnosis 2003;23(13):1073‐6. [DOI] [PubMed] [Google Scholar]
Murta 2002 {published data only}
- Murta CG, Moron AF, Avila MA, Weiner CP. Application of ductus venosus Doppler velocimetry for the detection of fetal aneuploidy in the first trimester of pregnancy. Fetal Diagnosis and Therapy 2002;17(5):308‐14. [DOI] [PubMed] [Google Scholar]
Musone 2000 {published data only}
- Musone R, Bonafiglia R, Menditto A, Paccone M, Cassese E, Russo G, et al. Fetuses with cystic hygroma. A retrospective study. Panminerva Medica 2000;42(1):39‐43. [PubMed] [Google Scholar]
Musto 1986 {published data only}
- Musto JD, Pizzolante JM, Chesarone VP, Sassi AM, Sane R. Alpha‐fetoprotein: an enhanced‐sensitivity assay for neural tube defect and Down syndrome evaluation. Clinical Chemistry 1986;32(7):1412. [PubMed] [Google Scholar]
Myrick 1990 {published data only}
- Myrick JE, Caudill SP, Hubert IL, Robinson MK, Adams MJ Jr, Pueschel SM. Identification of haptoglobin alpha‐2FF variants in mid‐trimester maternal serum as potential markers for Down syndrome. Applied & Theoretical Electrophoresis 1990;1(5):233‐41. [PubMed] [Google Scholar]
Naidoo 2008 {published data only}
- Naidoo P, Erasmus I, Jeebodh J, Nicolaou E, Gelderen CJ. Nuchal translucency as a method of first‐trimester screening for aneuploidy. South African Medical Journal 2008;Suid‐Afrikaanse Tydskrif Vir Geneeskunde. 98(4):295‐9. [PubMed] [Google Scholar]
Nau 2009 {published data only}
- Nau JY. [Screening for trisomy 21 in France]. [French]. Revue Medicale Suisse 2009;5(211):1531. [PubMed] [Google Scholar]
Nau 2009a {published data only}
- Nau JY. [Trisomy 21, after a half century]. [French]. Revue Medicale Suisse 2009;5(190):380. [PubMed] [Google Scholar]
Neveux 1996 {published data only}
- Neveux LM, Palomaki GE, Larrivee DA, Knight GJ, Haddow JE. Refinements in managing maternal weight adjustment for interpreting prenatal screening results. Prenatal Diagnosis 1996;16(12):1115‐9. [DOI] [PubMed] [Google Scholar]
Neveux 1996a {published data only}
- Neveux LM, Palomaki GE, Knight GJ, Haddow JE. Multiple marker screening for Down syndrome in twin pregnancies. Prenatal Diagnosis 1996;16(1):29‐34. [DOI] [PubMed] [Google Scholar]
Ng 2004 {published data only}
- Ng EK, El‐Sheikhah A, Chiu RW, Chan KC, Hogg M, Bindra R, et al. Evaluation of human chorionic gonadotropin ß‐subunit mRNA concentrations in maternal serum in aneuploid pregnancies: a feasibility study. Clinical Chemistry 2004;50(6):1055‐7. [DOI] [PubMed] [Google Scholar]
Nicolaides 1992a {published data only}
- Nicolaides KH, Azar G, Snijders RJM, Gosden CM. Fetal nuchal oedema associated malformations and chromosomal defects. Fetal Diagnosis and Therapy 1992;7(2):123‐31. [DOI] [PubMed] [Google Scholar]
Nicolaides 2000 {published data only}
- Nicolaides KH, Cicero S, Liao AW. One‐stop clinic for assessment of risk of chromosomal defects at 12 weeks of gestation. Prenatal and Neonatal Medicine 2000;5(3):145‐54. [DOI] [PubMed] [Google Scholar]
Nicolaides 2004 {published data only}
- Nicolaides KH. Nuchal translucency and other first‐trimester sonographic markers of chromosomal abnormalities. American Journal of Obstetrics and Gynecology 2004;191(1):45‐67. [DOI] [PubMed] [Google Scholar]
Nicolaides 2005a {published data only}
- Nicolaides KH, Wegrzyn P. [First trimester diagnosis of chromosomal defects][Polish]. Ginekologia Polska 2005;76(1):1‐8. [PubMed] [Google Scholar]
Nicolaides 2005b {published data only}
- Nicolaides KH, Wegrzyn P. [Sonographic features of chromosomal defects at 11(+0) to 13(+6) weeks of gestation] [Polish]. Ginekologia Polska 2005;76(6):423‐30. [PubMed] [Google Scholar]
Nicolaides 2005c {published data only}
- Nicolaides KH, Wegrzyn P. [Increased nuchal translucency with normal karyotype]. [Polish]. Ginekologia Polska 2005;76(8):593‐601. [PubMed] [Google Scholar]
Nicolaides 2005d {published data only}
- Nicolaides KH, Wegrzyn P. [Fetal nuchal translucency]. [Polish]. Ginekologia Polska 2005;76(3):179‐86. [PubMed] [Google Scholar]
Nicolaides 2005e {published data only}
- Nicolaides KH, Wegrzyn P. [Fetal nuchal translucency thickness and risk for chromosomal defects]. [Polish]. Ginekologia Polska 2005;76(4):257‐63. [PubMed] [Google Scholar]
Nicolaides 2005f {published data only}
- Nicolaides Kypros H. First‐trimester screening for chromosomal abnormalities. Seminars in Perinatology (Philadelphia) 2005;29(4):190‐4. [DOI] [PubMed] [Google Scholar]
Niemimaa 2001b {published data only}
- Niemimaa M, Heinonen S, Seppala M, Hippelainen M, Martikainen H, Ryynanen M. First‐trimester screening for Down's syndrome in in vitro fertilization pregnancies. Fertility & Sterility 2001;76(6):1282‐3. [DOI] [PubMed] [Google Scholar]
Niemimaa 2002 {published data only}
- Niemimaa M, Suonpaa M, Heinonen S, Seppala M, Bloigu R, Ryynanen M. Maternal serum human chorionic gonadotrophin and pregnancy‐associated plasma protein A in twin pregnancies in the first trimester. Prenatal Diagnosis 2002;22(3):183‐5. [PubMed] [Google Scholar]
Niemimaa 2003 {published data only}
- Niemimaa M, Heinonen S, Seppala M, Ryynanen M. The influence of smoking on the pregnancy‐associated plasma protein A, free ß human chorionic gonadotrophin and nuchal translucency. BJOG: an international journal of obstetrics and gynaecology 2003;110(7):664‐7. [PubMed] [Google Scholar]
Noble 1997b {published data only}
- Noble PL, Snijders RJ, Abraha HD, Sherwood RA, Nicolaides KH. Maternal serum free ß‐hCG at 10 to 14 weeks of gestation in trisomic twin pregnancies. British Journal of Obstetrics and Gynaecology 1997;104(6):741‐3. [DOI] [PubMed] [Google Scholar]
Norgaard 1990 {published data only}
- Norgaard Pedersen B, Larsen SO, Arends J, Svenstrup B, Tabor A. Maternal serum markers in screening for Down syndrome. Clinical Genetics 1990;37(1):35‐43. [DOI] [PubMed] [Google Scholar]
Norton 1992 {published data only}
- Norton ME, Golbus MS. Maternal serum CA 125 for aneuploidy detection in early pregnancy. Prenatal Diagnosis 1992;12(9):779‐81. [DOI] [PubMed] [Google Scholar]
Novakov‐Mikic 2007 {published data only}
- Novakov‐Mikic A, Potic Z, Pjevic A. [Ultrasound screening program for chromosomal abnormalities‐‐the first 2000 women]. [Serbian]. Medicinski Pregled 2007;60(1‐2):66‐70. [DOI] [PubMed] [Google Scholar]
O'Brien 1997a {published data only}
- O'Brien JE, Dvorin E, Yaron Y, Ayoub M, Johnson MP, Hume RF Jr, et al. Differential increases in AFP, hCG, and uE3 in twin pregnancies: Impact on attempts to quantify Down syndrome screening calculations. American Journal of Medical Genetics 1997;73(2):109‐12. [DOI] [PubMed] [Google Scholar]
O'Brien 1997b {published data only}
- O'Brien JE, Dvorin E, Drugan A, Johnson MP, Yaron Y, Evans MI. Race‐ethnicity‐specific variation in multiple‐marker biochemical screening: Alpha‐fetoprotein, hCG, and estriol. Obstetrics and Gynecology 1997;89(3):355‐8. [DOI] [PubMed] [Google Scholar]
Odibo 2004 {published data only}
- Odibo AO, Sehdev HM, Dunn L, McDonald R, Macones GA. The association between fetal nasal bone hypoplasia and aneuploidy. Obstetrics & Gynecology 2004;104(6):1229‐33. [DOI] [PubMed] [Google Scholar]
Odibo 2007 {published data only}
- Odibo AO, Sehdev HM, Stamilio DM, Cahill A, Dunn L, Macones GA. Defining nasal bone hypoplasia in second‐trimester Down syndrome screening: does the use of multiples of the median improve screening efficacy?. American Journal of Obstetrics and Gynecology 2007;197(4):361‐4. [DOI] [PubMed] [Google Scholar]
Odibo 2008 {published data only}
- Odibo AO, Sehdev HM, Gerkowicz S, Stamilio DM, Macones GA. Comparison of the efficiency of second‐trimester nasal bone hypoplasia and increased nuchal fold in Down syndrome screening. American Journal of Obstetrics and Gynecology 2008;199(3):281‐5. [DOI] [PubMed] [Google Scholar]
Odibo 2009 {published data only}
- Odibo AO, Schoenborn JA, Haas K, Macones GA. Does the combination of fronto‐maxillary facial angle and nasal bone evaluation improve the detection of Down syndrome in the second trimester?. Prenatal Diagnosis 2009;29(10):947‐51. [DOI] [PubMed] [Google Scholar]
Offerdal 2008 {published data only}
- Offerdal K, Blaas HG, Eik‐Nes SH. Prenatal detection of trisomy 21 by second‐trimester ultrasound examination and maternal age in a non‐selected population of 49 314 births in Norway. Ultrasound in Obstetrics & Gynecology 2008;32(4):493‐500. [DOI] [PubMed] [Google Scholar]
Ognibene 1999 {published data only}
- Ognibene A, Ciuti R, Tozzi P, Messeri G. Maternal serum superoxide dismutase (SOD): a possible marker for screening Down syndrome affected pregnancies.[see comment]. Prenatal Diagnosis 1999;19(11):1058‐60. [PubMed] [Google Scholar]
Oh 2007 {published data only}
- Oh C, Harman C, Baschat AA. Abnormal first‐trimester ductus venosus blood flow: a risk factor for adverse outcome in fetuses with normal nuchal translucency. Ultrasound in Obstetrics & Gynecology 2007;30(2):192‐6. [DOI] [PubMed] [Google Scholar]
Olajide 1989 {published data only}
- Olajide F, Kitau MJ, Chard T. Maternal serum AFP levels in the first trimester of pregnancy. European Journal of Obstetrics, Gynecology, & Reproductive Biology 1989;30(2):123‐8. [DOI] [PubMed] [Google Scholar]
Onda 1996 {published data only}
- Onda T, Kitagawa M, Takeda O, Sago H, Kubonoya K, Iinuma K, et al. Triple marker screening in native Japanese women. Prenatal Diagnosis 1996;16(8):713‐7. [DOI] [PubMed] [Google Scholar]
Onda 1998 {published data only}
- Onda T, Tanaka T, Takeda O, Kitagawa M, Kuwabara Y, Yamamoto H, et al. Agreement between predicted risk and prevalence of Down syndrome in second‐trimester triple‐marker screening in Japan. Prenatal Diagnosis 1998;18(9):956‐8. [PubMed] [Google Scholar]
Onda 2000 {published data only}
- Onda T, Tanaka T, Yoshida K, Nakamura Y, Kudo R, Yamamoto H, et al. Triple marker screening for trisomy 21, trisomy 18 and open neural tube defects in singleton pregnancies of native Japanese pregnant women. Journal of Obstetrics & Gynaecology Research 2000;26(6):441‐7. [DOI] [PubMed] [Google Scholar]
Orlandi 2002 {published data only}
- Orlandi F, Rossi C, Allegra A, Krantz D, Hallahan T, Orlandi E, et al. First trimester screening with free ß‐hCG, PAPP‐A and nuchal translucency in pregnancies conceived with assisted reproduction. Prenatal Diagnosis 2002;22(8):718‐21. [DOI] [PubMed] [Google Scholar]
Ottavio 1997 {published data only}
- Ottavio G, Meir YJ, Rustico MA, Pecile V, Fischer Tamaro L, Conoscenti G, et al. Screening for fetal anomalies by ultrasound at 14 and 21 weeks. Ultrasound in Obstetrics and Gynecology 1997;10(6):375‐80. [DOI] [PubMed] [Google Scholar]
Ozkaya 2010 {published data only}
- Ozkaya O, Sezik M, Ozbasar D, Kaya H. Abnormal ductus venosus flow and tricuspid regurgitation at 11‐14 weeks' gestation have high positive predictive values for increased risk in first‐trimester combined screening test: results of a pilot study. Taiwanese Journal of Obstetrics & Gynecology 2010;49(2):145‐50. [DOI] [PubMed] [Google Scholar]
Páez 2004 {published data only}
- Páez L, Peña E, González F, Bello F, Bellorín J, Espinoza F, et al. Plasma protein "A" and chorionic gonadotropin at first trimester pregnancy. Informe Medico 2004;6(2):99‐109. [Google Scholar]
Paladini 2007 {published data only}
- Paladini D, Sglavo G, Penner I, Pastore G, Nappi C. Fetuses with Down syndrome have an enlarged anterior fontanelle in the second trimester of pregnancy. Ultrasound in Obstetrics & Gynecology 2007;30(6):824‐9. [DOI] [PubMed] [Google Scholar]
Palka 1998 {published data only}
- Palka G, Guanciali Franchi P, Papponetti M, Marcuccitti J, Morizio E, Calabrese G, et al. Prenatal diagnosis using the triple test. Minerva Ginecologica 1998;50(10):411‐5. [PubMed] [Google Scholar]
Palomaki 1989 {published data only}
- Palomaki GE, Williams J, Haddow JE. Combining maternal serum alpha‐fetoprotein measurements and age to screen for Down syndrome in pregnant women under age 35. American Journal of Obstetrics and Gynecology 1989;160(3):575‐81. [DOI] [PubMed] [Google Scholar]
Palomaki 1993 {published data only}
- Palomaki GE, Knight GJ, Haddow JE, Canick JA, Wald NJ, Kennard A. Cigarette smoking and levels of maternal serum alpha‐fetoprotein, unconjugated estriol, and hCG: Impact on Down syndrome screening. Obstetrics and Gynecology 1993;81(5):675‐8. [PubMed] [Google Scholar]
Palomaki 1994 {published data only}
- Palomaki GE, Knight GJ, Haddow JE. Human chorionic gonadotropin and unconjugated oestriol measurements in insulin‐dependent diabetic pregnant women being screened for fetal Down syndrome. Prenatal Diagnosis 1994;14(1):65‐8. [DOI] [PubMed] [Google Scholar]
Palomaki 1996 {published data only}
- Palomaki GE, Neveux LM, Haddow JE. Can reliable Down's syndrome detection rates be determined from prenatal screening intervention trials?. Journal of Medical Screening 1996;3(1):12‐7. [DOI] [PubMed] [Google Scholar]
Palomaki 2005 {published data only}
- Palomaki GE, Knight GJ, Neveux LM, Pandian R, Haddow JE. Maternal serum invasive trophoblast antigen and first‐trimester Down syndrome screening. Clinical Chemistry 2005;51(8):1499‐504. [DOI] [PubMed] [Google Scholar]
Panburana 2001 {published data only}
- Panburana P, Ajjimakorn S, Tungkajiwangoon P. First trimester Down Syndrome screening by nuchal translucency in a Thai population. International Journal of Gynaecology & Obstetrics 2001;75(3):311‐2. [DOI] [PubMed] [Google Scholar]
Pandya 1994 {published data only}
- Pandya PP, Brizot ML, Kuhn P, Snijders RJ, Nicolaides KH. First‐trimester fetal nuchal translucency thickness and risk for trisomies. Obstetrics & Gynecology 1994;84(3):420‐3. [PubMed] [Google Scholar]
Pandya 1995b {published data only}
- Pandya PP, Santiago C, Snijders RJM, Nicolaides KH. First trimester fetal nuchal translucency. Current Opinion in Obstetrics and Gynecology 1995;7(2):95‐102. [DOI] [PubMed] [Google Scholar]
Papadopoulou 2008 {published data only}
- Papadopoulou E, Sifakis S, Giahnakis E, Fragouli Y, Karkavitsas N, Koumantakis E, et al. Human placental growth hormone is increased in maternal serum in pregnancies affected by Down syndrome. Fetal Diagnosis and Therapy 2008;23(3):211‐6. [DOI] [PubMed] [Google Scholar]
Parra‐Cordero 2007 {published data only}
- Parra‐Cordero M, Quiroz L, Rencoret G, Pedraza D, Munoz H, Soto‐Chacon E, et al. Screening for trisomy 21 during the routine second‐trimester ultrasound examination in an unselected Chilean population. Ultrasound in Obstetrics & Gynecology 2007;30(7):946‐51. [DOI] [PubMed] [Google Scholar]
Paterlini‐Brechot 2007 {published data only}
- Paterlini‐Brechot P. [Non invasive prenatal diagnosis of trisomy 21: dream or reality?]. [French]. M S‐Medecine Sciences 2007;23(6‐7):592‐4. [DOI] [PubMed] [Google Scholar]
Paul 2001 {published data only}
- Paul C, Krampl E, Skentou C, Jurkovic D, Nicolaides KH. Measurement of fetal nuchal translucency thickness by three‐dimensional ultrasound. Ultrasound in Obstetrics & Gynecology 2001;18(5):481‐4. [DOI] [PubMed] [Google Scholar]
Peralta 2005 {published data only}
- Peralta CF, Falcon O, Wegrzyn P, Faro C, Nicolaides KH. Assessment of the gap between the fetal nasal bones at 11 to 13 + 6 weeks of gestation by three‐dimensional ultrasound. Ultrasound in Obstetrics & Gynecology 2005;25(5):464‐7. [DOI] [PubMed] [Google Scholar]
Perenc 1998 {published data only}
- Perenc M, Dudarewicz L, Kaluzewski B. Analysis of triple test results in 27 cases of twin pregnancies. Acta Geneticae Medicae et Gemellologiae 1998;47(3‐4):249‐54. [DOI] [PubMed] [Google Scholar]
Perheentupa 2002 {published data only}
- Perheentupa A, Ruokonen A, Tuomivaara L, Ryynänen M, Martikainen H. Maternal serum (ß)‐HCG and (alpha)‐fetoprotein concentrations in singleton pregnancies following assisted reproduction. Human Reproduction 2002;17(3):794‐7. [DOI] [PubMed] [Google Scholar]
Perona 1998 {published data only}
- Perona M, Mancini G, Dall'Amico D, Guaraldo V, Carbonara A. Influence of smoking habits on Down's syndrome risk evaluation at mid‐trimester through biochemical screening. International Journal of Clinical & Laboratory Research 1998;28(3):179‐82. [DOI] [PubMed] [Google Scholar]
Persico 2008 {published data only}
- Persico N, Borenstein M, Molina F, Azumendi G, Nicolaides KH. Prenasal thickness in trisomy‐21 fetuses at 16‐24 weeks of gestation. Ultrasound in Obstetrics & Gynecology 2008;32(6):751‐4. [DOI] [PubMed] [Google Scholar]
Petervari 2000 {published data only}
- Petervari L, Varga A, Tanko A, Szabo L, Godo G. [Significance of nuchal edema in fetuses of pregnant women under 35 years of age]. [Hungarian]. Orvosi Hetilap 2000;141(8):399‐402. [PubMed] [Google Scholar]
Petrocik 1989 {published data only}
- Petrocik E, Wassman ER, Kelly JC. Prenatal screening for Down syndrome with maternal serum human chorionic gonadotropin levels.[see comment]. American Journal of Obstetrics and Gynecology 1989;161(5):1168‐73. [DOI] [PubMed] [Google Scholar]
Phillips 1992 {published data only}
- Phillips OP, Elias S, Shulman LP, Andersen RN, Morgan CD, Simpson JL. Maternal serum screening for fetal Down syndrome in women less than 35 years of age using alpha‐fetoprotein, hCG, and unconjugated estriol: a prospective 2‐year study. Obstetrics & Gynecology 1992;80(3):353‐8. [PubMed] [Google Scholar]
Phillips 1993 {published data only}
- Phillips OP, Shulman LP, Elias S, Simpson JL. Maternal serum screening for fetal Down syndrome using alpha‐ fetoprotein, human chorionic gonadotrophin, and unconjugated estriol in adolescents. Adolescent and Pediatric Gynecology 1993;6(2):91‐4. [Google Scholar]
Pihl 2008 {published data only}
- Pihl K, Larsen T, Jonsson L, Hougaard D, Krebs L, Norgaard‐Pedersen B, et al. [Quality control of prenatal screening]. [Danish]. Ugeskrift for Laeger 2008;170(35):2691‐5. [PubMed] [Google Scholar]
Pinette 2003 {published data only}
- Pinette MG, Egan JF, Wax JR, Blackstone J, Cartin A, Benn PA. Combined sonographic and biochemical markers for Down syndrome screening. Journal of Ultrasound in Medicine 2003;22(11):1185‐90. [DOI] [PubMed] [Google Scholar]
Platt 2004 {published data only}
- Platt LD, Greene N, Johnson A, Zachary J, Thom E, Krantz D, et al. Sequential pathways of testing after first‐trimester screening for trisomy 21. Obstetrics and Gynecology 2004;104(4):661‐6. [DOI] [PubMed] [Google Scholar]
Podobnik 1995 {published data only}
- Podobnik M, Singer Z, Podobnik Sarkanji S, Bulic M. First trimester diagnosis of cystic hygromata using transvaginal ultrasound and cytogenetic evaluation. Journal of Perinatal Medicine 1995;23(4):283‐91. [DOI] [PubMed] [Google Scholar]
Poon 2009 {published data only}
- Poon LC, Chelemen T, Minekawa R, Frisova V, Nicolaides KH. Maternal serum ADAM12 (A disintegrin and metalloprotease) in chromosomally abnormal pregnancy at 11‐13 weeks. American Journal of Obstetrics and Gynecology 2009;200(5):508‐6. [DOI] [PubMed] [Google Scholar]
Prefumo 2002 {published data only}
- Prefumo F, Thilaganathan B. Agreement between predicted risk and prevalence of Down syndrome in first trimester nuchal translucency screening. Prenatal Diagnosis 2002;22(10):917‐8. [DOI] [PubMed] [Google Scholar]
Prefumo 2004 {published data only}
- Prefumo F, Sairam S, Bhide A, Penna L, Hollis B, Thilaganathan B. Maternal ethnic origin and fetal nasal bones at 11‐14 weeks of gestation. BJOG: an international journal of obstetrics and gynaecology 2004;111(2):109‐12. [DOI] [PubMed] [Google Scholar]
Price 1998 {published data only}
- Price KM, Lith JM, Silman R, Mantingh A, Grudzinskas JG. First trimester maternal serum concentrations of fetal antigen 2 in normal pregnancies and those affected by trisomy 21. Human Reproduction 1998;13(6):1706‐8. [DOI] [PubMed] [Google Scholar]
Raty 2000 {published data only}
- Raty R, Virtanen A, Koskinen P, Laitinen P, Forsstrom J, Salonen R, et al. Maternal midtrimester serum AFP and free ß‐hCG levels in in vitro fertilization twin pregnancies. Prenatal Diagnosis 2000;20(3):221‐3. [PubMed] [Google Scholar]
Räty 2002 {published data only}
- Räty R, Virtanen A, Koskinen P, Anttila L, Forsström J, Laitinen P, et al. Serum free (ß)‐HCG and alpha‐fetoprotein levels in IVF, ICSI and frozen embryo transfer pregnancies in maternal mid‐trimester serum screening for Down's syndrome. Human Reproduction 2002;17(2):481‐4. [DOI] [PubMed] [Google Scholar]
Rembouskos 2004 {published data only}
- Rembouskos G, Cicero S, Longo D, Vandecruys H, Nicolaides KH. Assessment of the fetal nasal bone at 11‐14 weeks of gestation by three‐dimensional ultrasound. Ultrasound in Obstetrics & Gynecology 2004;23(3):232‐6. [DOI] [PubMed] [Google Scholar]
Ren 1992 {published data only}
- Ren S‐G, Braunstein GD. Human chorionic gonadotropin. Seminars in Reproductive Endocrinology 1992;10(2):95‐105. [Google Scholar]
Renier 1998 {published data only}
- Renier MA, Vereecken A, Herck E, Straetmans D, Ramaekers P, Buytaert P. Second trimester maternal dimeric inhibin‐A in the multiple‐marker screening test for Down's syndrome. Human Reproduction 1998;13(3):744‐8. [DOI] [PubMed] [Google Scholar]
Resta 1990 {published data only}
- Resta RG, Nyberg D. The role of ultrasound in screening for Down syndrome. Birth Defects: Original Article Series 1990;26(3):104. [PubMed] [Google Scholar]
Reynders 1997 {published data only}
- Reynders CS, Pauker SP, Benacerraf BR. First trimester isolated fetal nuchal lucency: significance and outcome. Journal of Ultrasound in Medicine 1997;16(2):101‐5. [DOI] [PubMed] [Google Scholar]
Reynolds 1989 {published data only}
- Reynolds TM, Penney MD. The mathematical basis of multivariate risk screening: with special reference to screening for Down's syndrome associated pregnancy. Annals of Clinical Biochemistry 1989;27(5):452‐8. [DOI] [PubMed] [Google Scholar]
Reynolds 1999 {published data only}
- Reynolds TM, Schaeffer HJ, Schlensker S. Estimation of Down's syndrome risks in the first trimester of pregnancy: Experience of testing with PAPP‐A, total hCG and free ß‐ hCG levels in maternal blood samples in a German population. Clinical Laboratory 1999;45(1‐2):49‐53. [Google Scholar]
Reynolds 2008 {published data only}
- Reynolds TM, Aldis J. Median parameters for Down's syndrome screening should be calculated using a moving time‐window method. Annals of Clinical Biochemistry 2008;45(Pt 6):567‐70. [DOI] [PubMed] [Google Scholar]
Ribbert 1996 {published data only}
- Ribbert LS, Kornman LH, Wolf BT, Simons AH, Jansen CA, Beekhuis JR, et al. Maternal serum screening for fetal Down syndrome in IVF pregnancies. Prenatal Diagnosis 1996;16(1):35‐8. [DOI] [PubMed] [Google Scholar]
Rice 2005 {published data only}
- Rice JD, McIntosh SF, Halstead AC. Second‐trimester maternal serum screening for Down syndrome in in vitro fertilization pregnancies. Prenatal Diagnosis 2005;25(3):234‐8. [DOI] [PubMed] [Google Scholar]
Rich 1991 {published data only}
- Rich N, Boots L, Davis R, Finley S. Efficiency of maternal serum hCG AFP and free estriol in the identification of trisomy 21 and other complications of pregnancy. Journal of the Alabama Academy of Science 1991;62(2‐3):135. [Google Scholar]
Roberts 1995 {published data only}
- Roberts LJ, Bewley S, Mackinson AM, Rodeck CH. First trimester fetal nuchal translucency: problems with screening the general population. 1. British Journal of Obstetrics and Gynaecology 1995;102(5):381‐5. [DOI] [PubMed] [Google Scholar]
Robertson 1991 {published data only}
- Robertson EF. Maternal serum screening for neural tube defects and Down's syndrome.[see comment]. Medical Journal of Australia 1991;155(2):67‐8. [PubMed] [Google Scholar]
Rode 2003 {published data only}
- Rode L, Wojdemann KR, Shalmi AC, Larsen SO, Sundberg K, Norgaard‐Pedersen B, et al. Combined first‐ and second‐trimester screening for Down syndrome: an evaluation of proMBP as a marker. Prenatal Diagnosis 2003;23(7):593‐8. [DOI] [PubMed] [Google Scholar]
Ronge 2006 {published data only}
- Ronge R. Combined first trimester screening for Down's syndrome is superior to quadruple test. Geburtshilfe und Frauenheilkunde 2006;66(4):332. [Google Scholar]
Rose 1995 {published data only}
- Rose NC, Mennuti MT. Multiple marker screening for women 35 and older. Contemporary OB/GYN 1995;40(9):55‐6. [Google Scholar]
Ross 1997 {published data only}
- Ross HL, Elias S. Maternal serum screening for fetal genetic disorders. Obstetrics & Gynecology Clinics of North America 1997;24(1):33‐47. [DOI] [PubMed] [Google Scholar]
Rotmensch 1996 {published data only}
- Rotmensch S, Liberati M, Kardana A, Copel JA, Ben‐Rafael Z, Cole LA. Nicked free ß‐subunit of human chorionic gonadotropin: A potential new marker for Down syndrome screening. American Journal of Obstetrics and Gynecology 1996;174(2):609‐11. [DOI] [PubMed] [Google Scholar]
Rotmensch 1999 {published data only}
- Rotmensch S, Celentano C, Shalev J, Vishne TH, Lipitz S, Ben‐Rafael Z, et al. Midtrimester maternal serum screening after multifetal pregnancy reduction in pregnancies conceived by in vitro fertilization. Journal of Assisted Reproduction and Genetics 1999;16(1):8‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rozenberg 2006 {published data only}
- Rozenberg P, Bussieres L, Chevret S, Bernard JP, Malagrida L, Cuckle H, et al. Screening for Down syndrome using first‐trimester combined screening followed by second‐trimester ultrasound examination in an unselected population. American Journal of Obstetrics and Gynecology 2006;195(5):1379‐87. [DOI] [PubMed] [Google Scholar]
Rudnicka 2002 {published data only}
- Rudnicka AR, Wald NJ, Huttly W, Hackshaw AK. Influence of maternal smoking on the birth prevalence of Down syndrome and on second trimester screening performance. Prenatal Diagnosis 2002;22(10):893‐7. [DOI] [PubMed] [Google Scholar]
Ryall 1992 {published data only}
- Ryall RG, Staples AJ, Robertson EF, Pollard AC. Improved performance in a prenatal screening programme for Down's syndrome incorporating serum‐free hCG subunit analyses. Prenatal Diagnosis 1992;12(4):251‐61. [DOI] [PubMed] [Google Scholar]
Ryall 2001 {published data only}
- Ryall RG, Callen D, Cocciolone R, Duvnjak A, Esca R, Frantzis N, et al. Karyotypes found in the population declared at increased risk of Down syndrome following maternal serum screening. Prenatal Diagnosis 2001;21(7):553‐7. [DOI] [PubMed] [Google Scholar]
Sabriá 2002 {published data only}
- Sabriá J, Cabrero D, Bach C. Aneuploidy screening: Ultrasound versus biochemistry. Ultrasound Review of Obstetrics and Gynecology 2002;2(4):221‐8. [Google Scholar]
Sacchini 2003 {published data only}
- Sacchini C, El‐Sheikhah A, Cicero S, Rembouskos G, Nicolaides KH. Ear length in trisomy 21 fetuses at 11‐14 weeks of gestation. Ultrasound in Obstetrics & Gynecology 2003;22(5):460‐3. [DOI] [PubMed] [Google Scholar]
Sahota 2009 {published data only}
- Sahota DS, Leung TY, Chan LW, Law LW, Fung TY, Chan OK, et al. First‐trimester fetal nasal bone length in an ethnic Chinese population. Ultrasound in Obstetrics & Gynecology 2009;34(1):33‐7. [DOI] [PubMed] [Google Scholar]
Sahota 2010a {published data only}
- Sahota DS, Leung TY, Chen M, Chan LW, Fung TY, Lau TK. Comparison of likelihood ratios of first‐trimester nuchal translucency measurements: multiples of median, delta or mixture. Ultrasound in Obstetrics & Gynecology 2010;36(1):15‐9. [DOI] [PubMed] [Google Scholar]
Salazar 2007 {published data only}
- Salazar Lopez R, Ibarra Gallardo AL, Iduma Melendrez M, Leyva Bojorquez R. [Specificity of biochemical markers of pregnancy second trimester]. [Spanish]. Ginecologia y Obstetricia de Mexico 2007;75(10):608‐14. [PubMed] [Google Scholar]
Salazar 2008 {published data only}
- Salazar Lopez R, Ibarra Gallardo AL, Iduma Melendrez M, Leyva R. [Evaluation of plasmatic A protein as only marker during first trimester of pregnancy]. [Spanish]. Ginecologia y Obstetricia de Mexico 2008;76(10):576‐81. [PubMed] [Google Scholar]
Saller 1997 {published data only}
- Saller DN Jr, Canick JA, Kellner LH, Rose NC, Garza J, French CA, et al. Maternal serum analyte levels in pregnancies with fetal Down syndrome resulting from translocations. American Journal of Obstetrics and Gynecology 1997;177(4):879‐81. [DOI] [PubMed] [Google Scholar]
Salomon 2001 {published data only}
- Salomon LJ, Bernard JP, Taupin P, Benard C, Ville Y. Relationship between nuchal translucency at 11‐14 weeks and nuchal fold at 20‐24 weeks of gestation. Ultrasound in Obstetrics & Gynecology 2001;18(6):636‐7. [DOI] [PubMed] [Google Scholar]
Salonen 1997 {published data only}
- Salonen R, Turpeinen U, Kurki L, Lappalainen M, Ammala P, Hiilesmaa V, et al. Maternal serum screening for Down's syndrome on population basis. Acta Obstetricia et Gynecologica Scandinavica 1997;76(9):817‐21. [DOI] [PubMed] [Google Scholar]
Saltvedt 2005 {published data only}
- Saltvedt S, Almstrom H, Kublickas M, Valentin L, Bottinga R, Bui TH, et al. Screening for Down syndrome based on maternal age or fetal nuchal translucency: a randomized controlled trial in 39,572 pregnancies. Ultrasound in Obstetrics & Gynecology 2005;25(6):537‐45. [DOI] [PubMed] [Google Scholar]
Saridogan 1996 {published data only}
- Saridogan E, Djahanbakhch O, Naftalin AA. Screening for Down's syndrome: experience in an inner city health district. British Journal of Obstetrics and Gynaecology 1996;103(12):1205‐11. [DOI] [PubMed] [Google Scholar]
Savoldelli 1993 {published data only}
- Savoldelli G, Binkert F, Achermann J, Schmid W. Ultrasound screening for chromosomal anomalies in the first trimester of pregnancy. Prenatal Diagnosis 1993;13(6):513‐8. [DOI] [PubMed] [Google Scholar]
Schielen 2009 {published data only}
- Schielen PC, Wildschut HI, Loeber JG. Down syndrome screening: determining the cutoff level of risk for invasive testing. Prenatal Diagnosis 2009;29(2):190‐2. [DOI] [PubMed] [Google Scholar]
Schiott 2006 {published data only}
- Schiott KM, Christiansen M, Petersen OB, Sorensen TL, Uldbjerg N. The "Consecutive Combined Test"‐‐using double test from week 8 + 0 and nuchal translucency scan, for first trimester screening for Down syndrome. Prenatal Diagnosis 2006;26(12):1105‐9. [DOI] [PubMed] [Google Scholar]
Schmidt 2007a {published data only}
- Schmidt P, Rom J, Maul H, Vaske B, Hillemanns P, Scharf A. Advanced first trimester screening (AFS): an improved test strategy for the individual risk assessment of fetal aneuploidies and malformations. Archives of Gynecology & Obstetrics 2007;276(2):159‐66. [DOI] [PubMed] [Google Scholar]
Schmidt 2007b {published data only}
- Schmidt P, Staboulidou I, Soergel P, Wustemann M, Hillemanns P, Scharf A. Comparison of Nicolaides' risk evaluation for Down's syndrome with a novel software: an analysis of 1,463 cases. Archives of Gynecology & Obstetrics 2007;275(6):469‐74. [DOI] [PubMed] [Google Scholar]
Schmidt 2007c {published data only}
- Schmidt P, Pruggmayer M, Steinborn A, Schippert C, Staboulidou I, Hillemanns P, et al. Are nuchal translucency, pregnancy associated plasma protein‐A or free‐beta‐human chorionic gonadotropin depending on maternal age? A multicenter study of 8,116 pregnancies. Archives of Gynecology & Obstetrics 2007;276(3):259‐62. [DOI] [PubMed] [Google Scholar]
Schmidt 2008a {published data only}
- Schmidt P, Hormansdorfer C, Pruggmayer M, Schutte C, Neumann A, Gerritzen A, et al. Improved prenatal aneuploidy screening using the novel advanced first‐trimester screening algorithm: a multicenter study of 10,017 pregnancies. Journal of Clinical Ultrasound 2008;36(7):397‐402. [DOI] [PubMed] [Google Scholar]
Schmidt 2008b {published data only}
- Schmidt P, Staboulidou I, Elsasser M, Vaske B, Hillemanns P, Scharf A. How imprecise may the measurement of fetal nuchal translucency be without worsening first‐trimester screening?. Fetal Diagnosis and Therapy 2008;24(3):291‐5. [DOI] [PubMed] [Google Scholar]
Schmidt 2008c {published data only}
- Schmidt P, Hormansdorfer C, Oehler K, Hartel H, Hillemanns P, Scharf A. [Three‐dimensional scatter plot analysis to estimate the risk of foetal aneuloidy]. [German]. Zeitschrift fur Geburtshilfe und Neonatologie 2008;212(4):127‐35. [DOI] [PubMed] [Google Scholar]
Schmidt 2010 {published data only}
- Schmidt P, Hormansdorfer C, Golatta M, Scharf A. Analysis of the distribution shift of detected aneuploidies by age independent first trimester screening. Archives of Gynecology & Obstetrics 2010;281(3):393‐9. [DOI] [PubMed] [Google Scholar]
Schuchter 1998 {published data only}
- Schuchter K, Wald N, Hackshaw AK, Hafner E, Liebhart E. The distribution of nuchal translucency at 10‐13 weeks of pregnancy. Prenatal Diagnosis 1998;18(3):281‐6. [PubMed] [Google Scholar]
Scott 1995 {published data only}
- Scott F, Boogert A, Smart S, Anderson J. Maternal serum screening and routine 18‐week ultrasound in the detection of all chromosomal abnormalities. Australian & New Zealand Journal of Obstetrics & Gynaecology 1995;35(2):165‐8. [DOI] [PubMed] [Google Scholar]
Seeds 1990 {published data only}
- Seeds JW, Watson WJ. Ultrasound and maternal serum alpha‐fetoprotein screening: A complementary relationship. Ultrasound Quarterly 1990;8(2):145‐66. [Google Scholar]
Seki 1995 {published data only}
- Seki K, Mitsui C, Nagata I. Measurement of urinary free ß‐human chorionic gonadotropin by immunoradiometric assay. Gynecologic and Obstetric Investigation 1995;40(3):162‐7. [DOI] [PubMed] [Google Scholar]
Shenhav 2003 {published data only}
- Shenhav S, Gemer O, Sherman DJ, Peled R, Segal S. Midtrimester triple‐test levels in women with chronic hypertension and altered renal function. Prenatal Diagnosis 2003;23(2):166‐7. [DOI] [PubMed] [Google Scholar]
Shintaku 1989 {published data only}
- Shintaku Y, Takabayashi T, Sasaki H, Ozawa N, Shinkawa O, Hamazaki Y, et al. [Screening for chromosomal anomalies with maternal serum alpha‐fetoprotein]. [Japanese]. Nippon Sanka Fujinka Gakkai Zasshi ‐ Acta Obstetrica et Gynaecologica Japonica 1989;41(2):185‐90. [PubMed] [Google Scholar]
Shulman 2003 {published data only}
- Shulman A, Maymon R. Mid‐gestation Down syndrome screening test and pregnancy outcome among unstimulated assisted‐conception pregnancies. Prenatal Diagnosis 2003;23(8):625‐8. [DOI] [PubMed] [Google Scholar]
Sieroszewski 2008 {published data only}
- Sieroszewski P, Perenc M, Budecka EB, Sobala W, Deutinger J. Sonographical integrated test for detection of chromosomal aberrations. Ultraschall in der Medizin 2008;29(2):190‐6. [DOI] [PubMed] [Google Scholar]
Simon‐Bouy 1999 {published data only}
- Simon‐Bouy B. [Markers for trisomy 21][French]. Fertilite Contraception Sexualite 1999;27(9):289‐91. [PubMed] [Google Scholar]
Simpson 1986 {published data only}
- Simpson JL, Baum LD, Marder R, Elias S, Ober C, Martin AO. Maternal serum alpha‐fetoprotein screening: low and high values for detection of genetic abnormalities. American Journal of Obstetrics and Gynecology 1986;155(3):593‐7. [DOI] [PubMed] [Google Scholar]
Smith 1990 {published data only}
- Smith C, Grube GL, Wilson S. Maternal serum alpha‐fetoprotein screening and the role of ultrasound. Journal of Diagnostic Medical Sonography 1990;6(6):312‐6. [Google Scholar]
Smith 1996 {published data only}
- Smith ER, Petersen J, Okorodudu AO, Bissell MG. Does the addition of unconjugated estriol in maternal serum screening improve the detection of trisomy 21? A meta‐analysis. Clinical Laboratory Management Review 1996;10(2):176‐81. [PubMed] [Google Scholar]
Smith 1999 {published data only}
- Smith NC, Hau C. A six year study of the antenatal detection of fetal abnormality in six Scottish health boards. British Journal of Obstetrics and Gynaecology 1999;106(3):206‐12. [DOI] [PubMed] [Google Scholar]
Smith‐Bindman 2001 {published data only}
- Smith‐Bindman R, Hosmer W, Feldstein VA, Deeks JJ, Goldberg JD. Second‐trimester ultrasound to detect fetuses with Down syndrome: a meta‐analysis.[see comment]. JAMA 2001;285(8):1044‐55. [DOI] [PubMed] [Google Scholar]
Smith‐Bindman 2003 {published data only}
- Smith‐Bindman R, Chu P, Bacchetti P, Waters JJ, Mutton D, Alberman E. Prenatal screening for Down syndrome in England and Wales and population‐based birth outcomes. American Journal of Obstetrics and Gynecology 2003;187(4):980‐5. [DOI] [PubMed] [Google Scholar]
Snijders 1995 {published data only}
- Snijders RJM, Sebire NJ, Nicolaides KH. Maternal age and gestational age‐specific risk for chromosomal defects. Fetal Diagnosis and Therapy 1995;10(6):356‐67. [DOI] [PubMed] [Google Scholar]
Snijders 1999 {published data only}
- Snijders RJM, Sundberg K, Holzgreve W, Henry G, Nicolaides KH. Maternal age‐ and gestation‐specific risk for trisomy 21. Ultrasound in Obstetrics and Gynecology 1999;13(3):167‐70. [DOI] [PubMed] [Google Scholar]
Soergel 2006 {published data only}
- Soergel P, Pruggmayer M, Schwerdtfeger R, Muhlhaus K, Scharf A. Screening for trisomy 21 with maternal age, fetal nuchal translucency and maternal serum biochemistry at 11‐14 weeks: a regional experience from Germany. Fetal Diagnosis and Therapy 2006;21(3):264‐8. [DOI] [PubMed] [Google Scholar]
Sokol 1998 {published data only}
- Sokol AI, Kramer RL, Yaron Y, O'Brien JE, Muller F, Johnson MP, et al. Age‐specific variation in aneuploidy incidence among biochemical screening programs. American Journal of Obstetrics and Gynecology 1998;179(4):971‐3. [DOI] [PubMed] [Google Scholar]
Sonek 2003 {published data only}
- Sonek JD. Nasal bone evaluation with ultrasonography: A marker for fetal aneuploidy. Ultrasound in Obstetrics and Gynecology 2003;22(1):11‐5. [DOI] [PubMed] [Google Scholar]
Sonek 2007 {published data only}
- Sonek J, Borenstein M, Downing C, McKenna D, Neiger R, Croom C, et al. Frontomaxillary facial angles in screening for trisomy 21 at 14‐23 weeks' gestation. American Journal of Obstetrics and Gynecology 2007;197(2):160‐5. [DOI] [PubMed] [Google Scholar]
Sood 2010 {published data only}
- Sood M, Rochelson B, Krantz D, Ravens R, Tam Tam H, Vohra N, et al. Are second‐trimester minor sonographic markers for Down syndrome useful in patients who have undergone first‐trimester combined screening?. American Journal of Obstetrics and Gynecology 2010;203(4):408‐4. [DOI] [PubMed] [Google Scholar]
Sooklim 2010 {published data only}
- Sooklim R, Manotaya S. Fetal facial sonographic markers for second trimester Down syndrome screening in a Thai population. International Journal of Gynaecology & Obstetrics 2010;111(2):144‐7. [DOI] [PubMed] [Google Scholar]
Spencer 1985 {published data only}
- Spencer K, Carpenter P. Screening for Down's syndrome using serum alpha fetoprotein: a retrospective study indicating caution. British Medical Journal Clinical Research Education 1985;290(6486):1940‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Spencer 1991a {published data only}
- Spencer K. Evaluation of an assay of the free ß‐subunit of choriogonadotropin and its potential value in screening for Down's syndrome. Clinical Chemistry 1991;37(6):809‐14. [PubMed] [Google Scholar]
Spencer 1991b {published data only}
- Spencer K. Maternal serum CA125 is not a second trimester marker for Down's syndrome. Annals of Clinical Biochemistry 1991;28(3):299‐300. [DOI] [PubMed] [Google Scholar]
Spencer 1992 {published data only}
- Spencer K, Coombes EJ, Mallard AS, Ward AM. Free ß human choriogonadotropin in Down's syndrome screening: a multicentre study of its role compared with other biochemical markers.[see comment]. Annals of Clinical Biochemistry 1992;29(5):506‐18. [DOI] [PubMed] [Google Scholar]
Spencer 1993a {published data only}
- Spencer K, Carpenter P. Prospective study of prenatal screening for Down's syndrome with free ß human chorionic gonadotrophin.[see comment]. BMJ 1993;307(6907):764‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Spencer 1993b {published data only}
- Spencer K, Macri JN, Carpenter P, Anderson R, Krantz DA. Stability of intact chorionic gonadotropin (hCG) in serum, liquid whole blood, and dried whole‐blood filter‐paper spots: impact on screening for Down syndrome by measurement of free ß‐hCG subunit. Clinical Chemistry 1993;39(6):1064‐8. [PubMed] [Google Scholar]
Spencer 1993c {published data only}
- Spencer K, Wood PJ, Anthony FW. Elevated levels of maternal serum Inhibin Aimmunoreactivity in second trimester pregnancies affected by Down's syndrome. Annals of Clinical Biochemistry 1993;30(2):219‐20. [DOI] [PubMed] [Google Scholar]
Spencer 1993d {published data only}
- Spencer K, Macri JN, Anderson RW, Aitken DA, Berry E, Crossley JA, et al. Dual analyte immunoassay in neural tube defect and Down's syndrome screening: results of a multicentre clinical trial. Annals of Clinical Biochemistry 1993;30(4):394‐401. [DOI] [PubMed] [Google Scholar]
Spencer 1993e {published data only}
- Spencer K. Free alpha‐subunit of human chorionic gonadotropin in Down syndrome. American Journal of Obstetrics and Gynecology 1993;168(1):132‐5. [DOI] [PubMed] [Google Scholar]
Spencer 1995a {published data only}
- Spencer K. The influence of gravidity on Down's syndrome screening with free ß hCG. Prenatal Diagnosis 1995;15(1):87‐9. [DOI] [PubMed] [Google Scholar]
Spencer 1996b {published data only}
- Spencer K, Wallace EM, Ritoe S. Second‐trimester dimeric inhibin‐A in Down's syndrome screening. Prenatal Diagnosis 1996;16(12):1101‐10. [DOI] [PubMed] [Google Scholar]
Spencer 1997 {published data only}
- Spencer K, Noble P, Snijders RJ, Nicolaides KH. First‐trimester urine free ß hCG, ß core, and total oestriol in pregnancies affected by Down's syndrome: implications for first‐trimester screening with nuchal translucency and serum free ß hCG. Prenatal Diagnosis 1997;17(6):525‐38. [DOI] [PubMed] [Google Scholar]
Spencer 1998a {published data only}
- Spencer K. The influence of smoking on maternal serum AFP and free ß hCG levels and the impact on screening for Down syndrome. Prenatal Diagnosis 1998;18(3):225‐34. [DOI] [PubMed] [Google Scholar]
Spencer 1998b {published data only}
- Spencer K, Carpenter P. Is prostate‐specific antigen a marker for pregnancies affected by Down syndrome?. Clinical Chemistry 1998;44(11):2362‐5. [PubMed] [Google Scholar]
Spencer 1999a {published data only}
- Spencer K. Second trimester prenatal screening for Down's syndrome using alpha‐fetoprotein and free ß hCG: a seven year review. British Journal of Obstetrics and Gynaecology 1999;106(12):1287‐93. [DOI] [PubMed] [Google Scholar]
Spencer 1999b {published data only}
- Spencer K. Accuracy of Down's syndrome risks produced in a prenatal screening program. Annals of Clinical Biochemistry 1999;36(1):101‐3. [DOI] [PubMed] [Google Scholar]
Spencer 2000a {published data only}
- Spencer K, Berry E, Crossley JA, Aitken DA, Nicolaides KH. Is maternal serum total hCG a marker of trisomy 21 in the first trimester of pregnancy?. Prenatal Diagnosis 2000;20(4):311‐7. [DOI] [PubMed] [Google Scholar]
Spencer 2000b {published data only}
- Spencer K. Screening for trisomy 21 in twin pregnancies in the first trimester using free ß‐hCG and PAPP‐A, combined with fetal nuchal translucency thickness. Prenatal Diagnosis 2000;20(2):91‐5. [DOI] [PubMed] [Google Scholar]
Spencer 2000c {published data only}
- Spencer K. The influence of smoking on maternal serum PAPP‐A and free ß hCG levels in the first trimester of pregnancy. Prenatal Diagnosis 1999;19(11):1065‐6. [DOI] [PubMed] [Google Scholar]
Spencer 2000d {published data only}
- Spencer K, Ong CY, Liao AW, Nicolaides KH. The influence of parity and gravidity on first trimester markers of chromosomal abnormality. Prenatal Diagnosis 2000;20(10):792‐4. [PubMed] [Google Scholar]
Spencer 2000e {published data only}
- Spencer K. The influence of fetal sex in screening for Down syndrome in the second trimester using AFP and free ß‐hCG. Prenatal Diagnosis 2000;20(8):648‐51. [DOI] [PubMed] [Google Scholar]
Spencer 2000f {published data only}
- Spencer K, Ong CY, Liao AW, Nicolaides KH. The influence of ethnic origin on first trimester biochemical markers of chromosomal abnormalities. Prenatal Diagnosis 2000;20(6):491‐4. [PubMed] [Google Scholar]
Spencer 2000g {published data only}
- Spencer K, Tul N, Nicolaides KH. Maternal serum free ß‐hCG and PAPP‐A in fetal sex chromosome defects in the first trimester. Prenatal Diagnosis 2000;20(5):390‐4. [DOI] [PubMed] [Google Scholar]
Spencer 2000h {published data only}
- Spencer K. Second‐trimester prenatal screening for Down syndrome and the relationship of maternal serum biochemical markers to pregnancy complications with adverse outcome. Prenatal Diagnosis 2000;20(8):652‐6. [PubMed] [Google Scholar]
Spencer 2000i {published data only}
- Spencer K, Ong CY, Liao AW, Papademetriou D, Nicolaides KH. The influence of fetal sex in screening for trisomy 21 by fetal nuchal translucency, maternal serum free ß‐hCG and PAPP‐A at 10‐14 weeks of gestation. Prenatal Diagnosis 2000;20(8):673‐5. [PubMed] [Google Scholar]
Spencer 2001a {published data only}
- Spencer K. Age related detection and false positive rates when screening for Down's syndrome in the first trimester using fetal nuchal translucency and maternal serum free ßhCG and PAPP‐A. BJOG: an international journal of obstetrics and gynaecology 2001;108(10):1043‐6. [DOI] [PubMed] [Google Scholar]
Spencer 2001b {published data only}
- Spencer K, Liao AW, Ong CY, Geerts L, Nicolaides KH. First trimester maternal serum placenta growth factor (PIGF)concentrations in pregnancies with fetal trisomy 21 or trisomy 18. Prenatal Diagnosis 2001;21(9):718‐22. [DOI] [PubMed] [Google Scholar]
Spencer 2001c {published data only}
- Spencer K, Liao AW, Ong CY, Geerts L, Nicolaides KH. Maternal serum levels of dimeric Inhibin A in pregnancies affected by trisomy 21 in the first trimester. Prenatal Diagnosis 2001;21(6):441‐4. [DOI] [PubMed] [Google Scholar]
Spencer 2001d {published data only}
- Spencer K, Liao AW, Skentou H, Ong CY, Nicolaides KH. Maternal serum levels of total activin‐A in first‐trimester trisomy 21 pregnancies. Prenatal Diagnosis 2001;21(4):270‐3. [DOI] [PubMed] [Google Scholar]
Spencer 2001e {published data only}
- Spencer K. Screening for trisomy 21 in twin pregnancies in the first trimester: does chorionicity impact on maternal serum free ß‐hCG or PAPP‐A levels?. Prenatal Diagnosis 2001;21(9):715‐7. [DOI] [PubMed] [Google Scholar]
Spencer 2002a {published data only}
- Spencer K, Nicolaides KH. A first trimester trisomy 13/trisomy 18 risk algorithm combining fetal nuchal translucency thickness, maternal serum free ß‐hCG and PAPP‐A. Prenatal Diagnosis 2002;22(10):877‐9. [DOI] [PubMed] [Google Scholar]
Spencer 2002b {published data only}
- Spencer K. Accuracy of Down syndrome risks produced in a first‐trimester screening programme incorporating fetal nuchal translucency thickness and maternal serum biochemistry. Prenatal Diagnosis 2002;22(3):244‐6. [DOI] [PubMed] [Google Scholar]
Spencer 2002c {published data only}
- Spencer K, Cuckle HS. Screening for chromosomal anomalies in the first trimester: does repeat maternal serum screening improve detection rates?. Prenatal Diagnosis 2002;22(10):903‐6. [DOI] [PubMed] [Google Scholar]
Spencer 2002d {published data only}
- Spencer K, Crossley JA, Aitken DA, Nix AB, Dunstan FD, Williams K. Temporal changes in maternal serum biochemical markers of trisomy 21 across the first and second trimester of pregnancy. Annals of Clinical Biochemistry 2002;39(6):567‐76. [DOI] [PubMed] [Google Scholar]
Spencer 2003a {published data only}
- Spencer K, Crossley JA, Aitken DA, Nix AB, Dunstan FD, Williams K. The effect of temporal variation in biochemical markers of trisomy 21 across the first and second trimesters of pregnancy on the estimation of individual patient‐specific risks and detection rates for Down's syndrome. Annals of Clinical Biochemistry 2003;40(3):219‐31. [DOI] [PubMed] [Google Scholar]
Spencer 2003b {published data only}
- Spencer K. The influence of different sample collection types on the levels of markers used for Down's syndrome screening as measured by the Kryptor Immunosassay system. Annals of Clinical Biochemistry 2003;40(2):166‐8. [DOI] [PubMed] [Google Scholar]
Spencer 2003c {published data only}
- Spencer K, Bindra R, Nicolaides KH. Maternal weight correction of maternal serum PAPP‐A and free ß‐hCG MoM when screening for trisomy 21 in the first trimester of pregnancy. Prenatal Diagnosis 2003;23(10):851‐5. [DOI] [PubMed] [Google Scholar]
Spencer 2003d {published data only}
- Spencer K, Nicolaides KH. Screening for trisomy 21 in twins using first trimester ultrasound and maternal serum biochemistry in a one‐stop clinic: a review of three years experience. BJOG: an international journal of obstetrics and gynaecology 2003;110(3):279‐80. [PubMed] [Google Scholar]
Spencer 2004 {published data only}
- Spencer K, Bindra R, Cacho AM, Nicolaides KH. The impact of correcting for smoking status when screening for chromosomal anomalies using maternal serum biochemistry and fetal nuchal translucency thickness in the first trimester of pregnancy. Prenatal Diagnosis 2004;24(3):169‐73. [DOI] [PubMed] [Google Scholar]
Spencer 2005a {published data only}
- Spencer K, Cicero S, Atzei A, Otigbah C, Nicolaides KH. The influence of maternal insulin‐dependent diabetes on fetal nuchal translucency thickness and first‐trimester maternal serum biochemical markers of aneuploidy. Prenatal Diagnosis 2005;25(10):927‐9. [DOI] [PubMed] [Google Scholar]
Spencer 2005b {published data only}
- Spencer K, Heath V, El‐Sheikhah A, Ong CY, Nicolaides KH. Ethnicity and the need for correction of biochemical and ultrasound markers of chromosomal anomalies in the first trimester: a study of Oriental, Asian and Afro‐Caribbean populations. Prenatal Diagnosis 2005;25(5):365‐9. [DOI] [PubMed] [Google Scholar]
Spencer 2005c {published data only}
- Spencer K. First trimester maternal serum screening for Down's syndrome: an evaluation of the DPC Immulite 2000 free ß‐hCG and pregnancy‐associated plasma protein‐A assays.[see comment]. Annals of Clinical Biochemistry 2005;42(1):30‐40. [DOI] [PubMed] [Google Scholar]
Spencer 2008a {published data only}
- Spencer K, Cowans NJ, Uldbjerg N, Vereecken A, Torring N. First trimester intact hCG as an early marker of trisomy 21: a promise unrecognised?. Prenatal Diagnosis 2008;28(12):1156‐9. [DOI] [PubMed] [Google Scholar]
Spong 1999 {published data only}
- Spong CY, Ghidini A, Stanley‐Christian H, Meck JM, Seydel FD, Pezzullo JC. Risk of abnormal triple screen for Down syndrome is significantly higher in women with female fetuses. Prenatal Diagnosis. 1999;19(4):337‐9. [DOI] [PubMed] [Google Scholar]
Staboulidou 2009 {published data only}
- Staboulidou I, Galindo A, Maiz N, Karagiannis G, Nicolaides KH. First‐trimester uterine artery Doppler and serum pregnancy‐associated plasma protein‐a in preeclampsia and chromosomal defects. Fetal Diagnosis and Therapy 2009;25(3):336‐9. [DOI] [PubMed] [Google Scholar]
Stevens 1998 {published data only}
- Stevens SL. The use of nuchal lucency as a screening tool in first trimester sonography. Journal of Diagnostic Medical Sonography 1998;14(6):251‐4. [Google Scholar]
Stoll 1992 {published data only}
- Stoll C. A new approach of prenatal prevention of constiutional disabilities ‐ the study of markers of maternal serum. Journal de Medecine de Strasbourg 1992;23(1):25‐7. [Google Scholar]
Stressig 2011 {published data only}
- Stressig R, Kozlowski P, Froehlich S, Siegmann HJ, Hammer R, Blumenstock G, et al. Assessment of the ductus venosus, tricuspid blood flow and the nasal bone in second‐trimester screening for trisomy 21. Ultrasound in Obstetrics & Gynecology 2011;37(4):444‐9. [DOI] [PubMed] [Google Scholar]
Su 2002a {published data only}
- Su YN, Hsu JJ, Lee CN, Cheng WF, Kung CC, Hsieh FJ. Raised maternal serum placenta growth factor concentration during the second trimester is associated with Down syndrome. Prenatal Diagnosis 2002;22(1):8‐12. [DOI] [PubMed] [Google Scholar]
Suchet 1995 {published data only}
- Suchet IB. Ultrasonography of the fetal neck in the first and second trimesters. Part 2. Anomalies of the posterior nuchal region. Canadian Association of Radiologists Journal 1995;46(5):344‐52. [PubMed] [Google Scholar]
Suchy 1990 {published data only}
- Suchy SF, Yeager MT. Down syndrome screening in women under 35 with maternal serum hCG. Obstetrics & Gynecology 1990;76(1):20‐4. [PubMed] [Google Scholar]
Summers 2003a {published data only}
- Summers AM, Farrell SA, Huang T, Meier C, Wyatt PR. Maternal serum screening in Ontario using the triple marker test. Journal of Medical Screening 2003;10(3):107‐11. [DOI] [PubMed] [Google Scholar]
Summers 2003b {published data only}
- Summers AM, Huang T, Meier C, Wyatt PR. The implications of a false positive second‐trimester serum screen for Down syndrome. Obstetrics & Gynecology 2003;101(6):1301‐6. [DOI] [PubMed] [Google Scholar]
Suntharasaj 2005 {published data only}
- Suntharasaj T, Ratanasiri T, Chanprapaph P, Kengpol C, Kor‐anantakul O, Leetanaporn R, et al. Variability of nuchal translucency measurement: a multicenter study in Thailand. Gynecologic & Obstetric Investigation 2005;60(4):201‐5. [DOI] [PubMed] [Google Scholar]
Susman 2010 {published data only}
- Susman MR, Amor DJ, Muggli E, Jaques AM, Halliday J. Using population‐based data to predict the impact of introducing noninvasive prenatal diagnosis for Down syndrome. Genetics in Medicine 2010;12(5):298‐303. [DOI] [PubMed] [Google Scholar]
Sutton 2004 {published data only}
- Sutton JM, Cole LA. Sialic acid‐deficient invasive trophoblast antigen (sd‐ITA): a new urinary variant for gestational Down syndrome screening. Prenatal Diagnosis 2004;24(3):194‐7. [DOI] [PubMed] [Google Scholar]
Suzuki 1998 {published data only}
- Suzuki Y, Takada J, Iwaki T, Isaka K, Takayama M. Screening for trisomy 21 in the first trimester by measurement of serum PAPP‐A and free ß‐hCG. Acta Obstetrica et Gynaecologica Japonica 1998;50(1):37‐40. [Google Scholar]
Tabor 1987 {published data only}
- Tabor A, Larsen SO, Nielsen J, Nielsen J, Philip J, Pilgaard B, et al. Screening for Down's syndrome using an iso‐risk curve based on maternal age and serum alpha‐fetoprotein level. British Journal of Obstetrics and Gynaecology 1987;94(7):636‐42. [DOI] [PubMed] [Google Scholar]
Tanski 1999 {published data only}
- Tanski S, Rosengren SS, Benn PA. Predictive value of the triple screening test for the phenotype of Down syndrome. American Journal of Medical Genetics 1999;85(2):123‐6. [DOI] [PubMed] [Google Scholar]
Thilaganathan 1998 {published data only}
- Thilaganathan B, Khare M, Williams B, Wathen NC. Influence of ethnic origin on nuchal translucency screening for Down's syndrome. Ultrasound in Obstetrics & Gynecology 1998;12(2):112‐4. [DOI] [PubMed] [Google Scholar]
Thilaganathan 1999b {published data only}
- Thilaganathan B. First‐trimester nuchal translucency and maternal serum biochemical screening for Down's syndrome: A happy union?. Ultrasound in Obstetrics and Gynecology 1999;13(4):229‐30. [DOI] [PubMed] [Google Scholar]
Tislaric 2002 {published data only}
- Tislaric D, Brajenovic‐Milic B, Ristic S, Latin V, Zuvic‐Butorac M, Bacic J, et al. The influence of smoking and parity on serum markers for Down's syndrome screening. Fetal Diagnosis and Therapy 2002;17(1):17‐21. [DOI] [PubMed] [Google Scholar]
Torok 1997 {published data only}
- Torok O, Veress L, Szabo M, Zsupan I, Buczko Z, Bolodar A, et al. [Biochemical and ultrasonic screening of chromosomal aneuploidies in the second trimester of pregnancy]. [Hungarian]. Orvosi Hetilap 1997;138(3):123‐7. [PubMed] [Google Scholar]
Torring 2009 {published data only}
- Torring N. Performance of first‐trimester screening between gestational weeks 7 and 13. Clinical Chemistry 2009;55(8):1564‐7. [DOI] [PubMed] [Google Scholar]
Trninic‐Pjevic 2007 {published data only}
- Trninic‐Pjevic A, Novakov‐Mikic A. [First trimester ultrasound screening of chromosomal abnormalities]. [Serbian]. Srpski Arhiv Za Celokupno Lekarstvo 2007;135(3‐4):153‐6. [DOI] [PubMed] [Google Scholar]
Tsai 2001 {published data only}
- Tsai MS, Huang YY, Hwa KY, Cheng CC, Lee FK. Combined measurement of fetal nuchal translucency, maternal serum free ß‐hCG, and pregnancy‐associated plasma protein A for first‐trimester Down's syndrome screening. Journal of the Formosan Medical Association 2001;100(5):319‐25. [PubMed] [Google Scholar]
Valerio 1996 {published data only}
- Valerio D, Aiello R, Altieri V, Fagnoni P. Maternal serum screening of fetal chromosomal abnormalities by AFP, UE3, hCG and free‐ß hCG. Prospective and retrospective results. Minerva Ginecologica 1996;48(5):169‐73. [PubMed] [Google Scholar]
Van Blerk 1992 {published data only}
- Blerk M, Smitz J, Catte L, Kumps C, Elst J, Steirteghem AC. Second‐trimester cancer antigen 125 and Down's syndrome.[see comment]. Prenatal Diagnosis 1992;12(12):1062‐6. [DOI] [PubMed] [Google Scholar]
Van Dyke 2007 {published data only}
- Dyke DL, Ebrahim SA, Al Saadi AA, Powell SA, Zenger‐Hain JL, Micale MA, et al. The impact of maternal serum screening programs for Down syndrome in southeast Michigan, 1988‐2003. Prenatal Diagnosis 2007;27(6):583‐4. [DOI] [PubMed] [Google Scholar]
Van Heesch, 2006 {published data only}
- Van Heesch, Schielen PCJ I, Wildhagen MF, Den Hollander, Steegers EAP, Wildschut HIJ. Combined first trimester screening for trisomy 21: Lack of agreement between risk calculation methods. Journal of Perinatal Medicine 2006;34(2):162‐5. [DOI] [PubMed] [Google Scholar]
Van Lith, 1993 {published data only}
- Van Lith, Mantingh A, De Bruijn. Maternal serum CA 125 levles in pregnancies with chromosomally‐normal and ‐abnormal fetuses. Prenatal Diagnosis 1993;13(12):1123‐31. [DOI] [PubMed] [Google Scholar]
Van Lith, 1994 {published data only}
- Van Lith, Mantingh A, Pratt JJ. First‐Trimester maternal serum immunoreactive Inhibin Ain chromosomally normal and abnormal pregnancies. Obstetrics and Gynecology 1994;83(5 I):661‐4. [PubMed] [Google Scholar]
Van Lith 1991 {published data only}
- Lith JM, Mantingh A, Beekhuis JR, Bruijn HW, Breed AS. First trimester CA 125 and Down's syndrome.[see comment]. British Journal of Obstetrics and Gynaecology 1991;98(5):493‐4. [DOI] [PubMed] [Google Scholar]
Veress 1986 {published data only}
- Veress L, Szabo M, Horvath K, Polgar K, Papp Z. [Low maternal serum alpha‐fetoprotein concentration and Down syndrome]. [Hungarian]. Orvosi Hetilap 1986;127(20):1232‐3. [PubMed] [Google Scholar]
Veress 1988 {published data only}
- Veress L, Szabo M, Polgar K, Takacs L, Papp Z. [Prenatal screening for Down's syndrome by measuring the AFP concentration in the maternal serum]. [Hungarian]. Orvosi Hetilap 1988;129(31):1677. [PubMed] [Google Scholar]
Vergani 2008 {published data only}
- Vergani P, Ghidini A, Weiner S, Locatelli A, Pozzi E, Biffi A. Risk assessment for Down syndrome with genetic sonogram in women at risk. Prenatal Diagnosis 2008;28(12):1144‐8. [DOI] [PubMed] [Google Scholar]
Vintzileos 2003 {published data only}
- Vintzileos A, Walters C, Yeo L. Absent nasal bone in the prenatal detection of fetuses with trisomy 21 in a high‐risk population. Obstetrics & Gynecology 2003;101(5):905‐8. [DOI] [PubMed] [Google Scholar]
Wald 1988a {published data only}
- Wald NJ, Cuckle HS, Densem JW, Nanchahal K, Royston P, Chard T, et al. Maternal serum screening for Down's syndrome in early pregnancy. BMJ 1988;297(6653):883‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wald 1988b {published data only}
- Wald NJ, Cuckle HS, Densem JW, Nanchahal K, Canick JA, Haddow JE, et al. Maternal serum unconjugated oestriol as an antenatal screening test for Down's syndrome. British Journal of Obstetrics and Gynaecology 1988;95(4):334‐41. [DOI] [PubMed] [Google Scholar]
Wald 1991 {published data only}
- Wald N, Cuckle H, Wu TS, George L. Maternal serum unconjugated oestriol and human chorionic gonadotrophin levels in twin pregnancies: implications for screening for Down's syndrome. British Journal of Obstetrics and Gynaecology 1991;98(9):905‐8. [DOI] [PubMed] [Google Scholar]
Wald 1992a {published data only}
- Wald NJ, Kennard A, Densem JW, Cuckle HS, Chard T, Butler L. Antenatal maternal serum screening for Down's syndrome: results of a demonstration project.[see comment]. BMJ 1992;305(6850):391‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wald 1992b {published data only}
- Wald NJ, Cuckle HS, Densem JW, Stone RB. Maternal serum unconjugated oestriol and human chorionic gonadotrophin levels in pregnancies with insulin‐dependent diabetes: implications for screening for Down's syndrome. British Journal of Obstetrics and Gynaecology 1992;99(1):51‐3. [DOI] [PubMed] [Google Scholar]
Wald 1992c {published data only}
- Wald NJ, Cuckle HS, Densem JW, Kennard A, Smith D. Maternal serum screening for Down's syndrome: the effect of routine ultrasound scan determination of gestational age and adjustment for maternal weight.[see comment]. British Journal of Obstetrics and Gynaecology 1992;99(2):144‐9. [DOI] [PubMed] [Google Scholar]
Wald 1993 {published data only}
- Wald N, Densem J, Stone R, Cheng R. The use of free ß‐hCG in antenatal screening for Down's syndrome.[see comment]. British Journal of Obstetrics and Gynaecology 1993;100(6):550‐7. [DOI] [PubMed] [Google Scholar]
Wald 1994a {published data only}
- Wald NJ, Densem JW. Maternal serum free alpha‐human chorionic gonadotrophin levels in twin pregnancies: implications for screening for Down's syndrome. Prenatal Diagnosis 1994;14(8):717‐9. [DOI] [PubMed] [Google Scholar]
Wald 1994b {published data only}
- Wald NJ, Watt HC. Choice of serum markers in antenatal screening for Down's syndrome. Journal of Medical Screening 1994;1(2):117‐20. [DOI] [PubMed] [Google Scholar]
Wald 1996a {published data only}
- Wald NJ, Watt HC. Serum markers for Down's syndrome in relation to number of previous births and maternal age. Prenatal Diagnosis 1996;16(8):699‐703. [DOI] [PubMed] [Google Scholar]
Wald 1996b {published data only}
- Wald NJ, George L, Smith D, Densem JW, Petterson K. Serum screening for Down's syndrome between 8 and 14 weeks of pregnancy. International Prenatal Screening Research Group.[see comment]. British Journal of Obstetrics and Gynaecology 1996;103(5):407‐12. [DOI] [PubMed] [Google Scholar]
Wald 1996c {published data only}
- Wald NJ, Watt HC, George L. Maternal serum inhibin‐A in pregnancies with insulin‐dependent diabetes mellitus: implications for screening for Down's syndrome. Prenatal Diagnosis 1996;16(10):923‐6. [DOI] [PubMed] [Google Scholar]
Wald 1996d {published data only}
- Wald NJ, Densem JW, George L, Muttukrishna S, Knight PG. Prenatal screening for Down's syndrome using inhibin‐A as a serum marker. Prenatal Diagnosis 1996;16(2):143‐53. [DOI] [PubMed] [Google Scholar]
Wald 1997 {published data only}
- Wald NJ, Hackshaw AK. Combining ultrasound and biochemistry in first‐trimester screening for Down's syndrome.[see comment]. Prenatal Diagnosis 1997;17(9):821‐9. [PubMed] [Google Scholar]
Wald 1998 {published data only}
- Wald NJ, Watt HC, Haddow JE, Knight GJ. Screening for Down syndrome at 14 weeks of pregnancy. Prenatal Diagnosis 1998;18(3):291‐3. [DOI] [PubMed] [Google Scholar]
Wald 1999a {published data only}
- Wald NJ, Hackshaw AK, Diamandis EP, Melegos DN. Maternal serum prostate‐specific antigen and Down syndrome in the first and second trimesters of pregnancy. Prenatal Diagnosis 1999;19(7):674‐6. [PubMed] [Google Scholar]
Wald 1999b {published data only}
- Wald NJ, Watt HC, Norgaard Pederson B, Christiansen M. SP1 in pregnancies with Down syndrome in the first trimester of pregnancy. Prenatal Diagnosis 1999;19(6):517‐20. [PubMed] [Google Scholar]
Wald 1999c {published data only}
- Wald NJ, White N, Morris JK, Huttly WJ, Canick JA. Serum markers for Down's syndrome in women who have had in vitro fertilisation: implications for antenatal screening. British Journal of Obstetrics and Gynaecology 1999;106(12):1304‐6. [DOI] [PubMed] [Google Scholar]
Wald 1999d {published data only}
- Wald NJ, Watt HC, Hackshaw AK. Integrated screening for Down's syndrome on the basis of tests performed during the first and second trimesters.[see comment]. New England Journal of Medicine 1999;341(7):461‐7. [DOI] [PubMed] [Google Scholar]
Wald 2003b {published data only}
- Wald NJ, Rish S, Hackshaw AK. Combining nuchal translucency and serum markers in prenatal screening for Down syndrome in twin pregnancies. Prenatal Diagnosis 2003;23(7):588‐92. [DOI] [PubMed] [Google Scholar]
Wald 2003c {published data only}
- Wald NJ, Huttly WJ, Hackshaw AK. Antenatal screening for Down's syndrome with the quadruple test.[see comment]. Lancet 2003;361(9360):835‐6. [DOI] [PubMed] [Google Scholar]
Wald 2006 {published data only}
- Wald NJ, Rudnicka AR, Bestwick JP. Sequential and contingent prenatal screening for Down syndrome. Prenatal Diagnosis 2006;26(9):769‐77. [DOI] [PubMed] [Google Scholar]
Wallace 1994 {published data only}
- Wallace EM, Harkness LM, Burns S, Liston WA. Evaluation of maternal serum immunoreactive Inhibin Aas a first trimester marker of Down's syndrome. Clinical Endocrinology 1994;41(4):483‐6. [DOI] [PubMed] [Google Scholar]
Wallace 1997 {published data only}
- Wallace EM, Crossley JA, Ritoe SC, Groome NP, Aitken DA. Maternal serum inhibin‐A in pregnancies complicated by insulin dependent diabetes mellitus. British Journal of Obstetrics and Gynaecology 1997;104(8):946‐8. [DOI] [PubMed] [Google Scholar]
Wang 2010 {published data only}
- Wang E, Chen C, Glimco E, Grobman W. The performance of second trimester long bone ratios for Down syndrome screening is influenced by gestational age. Journal of Maternal‐Fetal & Neonatal Medicine 2010;23(7):642‐5. [DOI] [PubMed] [Google Scholar]
Ward 2005 {published data only}
- Ward A. Nuchal translucency measurement. Synergy (http://www.highbeam.com/doc/1P3‐866108421.html) (accessed 2007) 2005.
Watt 1996a {published data only}
- Watt HC, Wald NJ, Smith D, Kennard A, Densem J. Effect of allowing for ethnic group in prenatal screening for Down's syndrome. Prenatal Diagnosis 1996;16(8):691‐8. [DOI] [PubMed] [Google Scholar]
Watt 1996b {published data only}
- Watt HC, Wald NJ, George L. Maternal serum inhibin‐A levels in twin pregnancies: implications for screening for Down's syndrome. Prenatal Diagnosis 1996;16(10):927‐9. [DOI] [PubMed] [Google Scholar]
Wax 2007 {published data only}
- Wax JR, Pinette MG, Cartin A, Blackstone J. Optimal crown‐rump length for measuring the nuchal translucency. Journal of Clinical Ultrasound 2007;35(6):302‐4. [DOI] [PubMed] [Google Scholar]
Weinans 2001 {published data only}
- Weinans MJN, Pratt JJ, De Wolf, Mantingh A. First‐trimester maternal serum human thyroid‐stimulating hormone in chromosomally normal and Down syndrome pregnancies. Prenatal Diagnosis 2001;21(9):723‐5. [PubMed] [Google Scholar]
Weinans 2004 {published data only}
- Weinans MJN, Kooij L, Müller MA, Bilardo KM, Van Lith, Tymstra T. A comparison of the impact of screen‐positive results obtained from ultrasound and biochemical screening for Down syndrome in the first trimester: A pilot study. Prenatal Diagnosis 2004;24(5):347‐51. [DOI] [PubMed] [Google Scholar]
Weisz 2007 {published data only}
- Weisz B, Pandya P, Chitty L, Jones P, Huttly W, Rodeck C. Practical issues drawn from the implementation of the integrated test for Down syndrome screening into routine clinical practice. BJOG: an international journal of obstetrics and gynaecology 2007;114(4):493‐7. [DOI] [PubMed] [Google Scholar]
Welborn 1994 {published data only}
- Welborn JL, Timm NS. Trisomy 21 and cystic hygromas in early gestational age fetuses. American Journal of Perinatology 1994;11(1):19‐20. [DOI] [PubMed] [Google Scholar]
Wenstrom 1993 {published data only}
- Wenstrom KD, Williamson RA, Grant SS, Hudson JD, Getchell JP. Evaluation of multiple‐marker screening for Down syndrome in a statewide population. American Journal of Obstetrics and Gynecology 1993;169(4):793‐7. [DOI] [PubMed] [Google Scholar]
Wenstrom 1995a {published data only}
- Wenstrom KD, Owen J, Boots L, Ethier M. The influence of maternal weight on human chorionic gonadotropin in the multiple‐marker screening test for fetal Down syndrome. American Journal of Obstetrics and Gynecology 1995;173(4):1297‐300. [DOI] [PubMed] [Google Scholar]
Wenstrom 1995b {published data only}
- Wenstrom KD, Desai R, Owen J, Dubard MB, Boots L. Comparison of multiple‐marker screening with amniocentesis for the detection of fetal aneuploidy in women greater than or equal35 years old. American Journal of Obstetrics and Gynecology 1995;173(4):1287‐92. [DOI] [PubMed] [Google Scholar]
Wetta 2011 {published data only}
- Wetta L, Biggio J Jr, Owen J. Use of ethnic‐specific medians for Hispanic patients reduces ethnic disparities in multiple marker screening. Prenatal Diagnosis 2011;31(4):331‐3. [DOI] [PubMed] [Google Scholar]
Whitlow 1998a {published data only}
- Whitlow BJ, Lazanakis ML, Kadir RA, Chatzipapas I, Economides DL. The significance of choroid plexus cysts, echogenic heart foci and renal pyelectasis in the first trimester. Ultrasound in Obstetrics & Gynecology 1998;12(6):385‐90. [DOI] [PubMed] [Google Scholar]
Whitlow 1998b {published data only}
- Whitlow BJ, Economides DL. First trimester detection of fetal abnormalities in an unselected population. Contemporary Reviews in Obstetrics and Gynaecology 1998;10(4):245‐53. [DOI] [PubMed] [Google Scholar]
Whitlow 1999 {published data only}
- Whitlow BJ, Chatzipapas IK, Lazanakis ML, Kadir RA, Economides DL. The value of sonography in early pregnancy for the detection of fetal abnormalities in an unselected population. British Journal of Obstetrics and Gynaecology 1999;106(9):929‐36. [DOI] [PubMed] [Google Scholar]
Williamson 1994 {published data only}
- Williamson R. Expanded maternal serum alpha fetoprotein screening. Iowa Medicine 1994;84(9):397‐400. [PubMed] [Google Scholar]
Wilson 2000 {published data only}
- Wilson K. New first‐trimester prenatal screening for down syndrome. Laboratory Medicine 2000;31(11):591. [Google Scholar]
Wojdemann 2001 {published data only}
- Wojdemann KR, Larsen SO, Shalmi A, Sundberg K, Christiansen M, Tabor A. First trimester screening for Down syndrome and assisted reproduction: no basis for concern. Prenatal Diagnosis 2001;21(7):563‐5. [DOI] [PubMed] [Google Scholar]
Wong 2003 {published data only}
- Wong SF, Choi H, Ho LC. Nasal bone hypoplasia: is it a common finding amongst chromosomally normal fetuses of southern Chinese women?. Gynecologic & Obstetric Investigation 2003;56(2):99‐101. [DOI] [PubMed] [Google Scholar]
Wright 2006 {published data only}
- Wright D, Bradbury I, Cuckle H, Gardosi J, Tonks A, Standing S, et al. Three‐stage contingent screening for Down syndrome. Prenatal Diagnosis 2006;26(6):528‐34. [DOI] [PubMed] [Google Scholar]
Wright 2007 {published data only}
- Wright D, Spencer K, Nix B. First trimester screening for Down syndrome using free beta hCG, total hCG and PAPP‐A: an exploratory study. Prenatal Diagnosis 2007;27(12):1118‐22. [DOI] [PubMed] [Google Scholar]
Xie 2010 {published data only}
- Xie Z, Lu S, Li H. Contingent triple‐screening for Down syndrome in the second trimester: a feasibility study in Mainland Chinese population. Prenatal Diagnosis 2010;30(1):74‐6. [DOI] [PubMed] [Google Scholar]
Yagel 1998 {published data only}
- Yagel S, Anteby EY, Hochner‐Celnikier D, Ariel I, Chaap T, Ben Neriah Z. The role of midtrimester targeted fetal organ screening combined with the "triple test" and maternal age in the diagnosis of trisomy 21: a retrospective study. American Journal of Obstetrics and Gynecology 1998;178(1):40‐4. [DOI] [PubMed] [Google Scholar]
Yamamoto 2001a {published data only}
- Yamamoto R, Azuma M, Kishida T, Yamada H, Satomura S, Fujimoto S. Total alpha‐fetoprotein and Lens culinaris agglutinin‐reactive alpha‐fetoprotein in fetal chromosomal abnormalities. BJOG: an international journal of obstetrics and gynaecology 2001;108(11):1154‐8. [DOI] [PubMed] [Google Scholar]
Yamamoto 2001b {published data only}
- Yamamoto R, Azuma M, Hoshi N, Kishida T, Satomura S, Fujimoto S. Lens culinaris agglutinin‐reactive alpha‐fetoprotein, an alternative variant to alpha‐fetoprotein in prenatal screening for Down's syndrome. Human Reproduction 2001;16(11):2438‐44. [DOI] [PubMed] [Google Scholar]
Yamamoto 2001c {published data only}
- Yamamoto R, Azuma M, Wakui Y, Kishida T, Yamada H, Okuyama K, et al. Alpha‐fetoprotein microheterogeneity: a potential biochemical marker for Down's syndrome. Clinica Chimica Acta 2001;304(1‐2):137‐41. [DOI] [PubMed] [Google Scholar]
Yaron 2001 {published data only}
- Yaron Y, Wolman I, Kupferminc MJ, Ochshorn Y, Many A, Orr‐Urtreger A. Effect of fetal gender on first trimester markers and on Down syndrome screening. Prenatal Diagnosis 2001;21(12):1027‐30. [DOI] [PubMed] [Google Scholar]
Ye 1995 {published data only}
- Ye Guoling Liao Shixiu, Zhao Xiaolan. The possibility of prenatal screening for fetal abnormalities in second‐trimester pregnancies by measuring AFP, ß‐HCG and uE‐3 levels. Xi'an Yike Daxue Xuebao 1995;16(4):408‐11. [Google Scholar]
Yoshida 2000 {published data only}
- Yoshida K, Kuwabara Y, Tanaka T, Onda T, Kudo R, Yamamoto H, et al. Dimeric Inhibin A as a fourth marker for Down's syndrome maternal serum screening in native Japanese women. Journal of Obstetrics and Gynaecology Research 2000;26(3):171‐4. [DOI] [PubMed] [Google Scholar]
Zalel 2008 {published data only}
- Zalel Y, Achiron R, Yagel S, Kivilevitch Z. Fetal aberrant right subclavian artery in normal and Down syndrome fetuses. Ultrasound in Obstetrics & Gynecology 2008;31(1):25‐9. [DOI] [PubMed] [Google Scholar]
Zeitune 1991 {published data only}
- Zeitune M, Aitken DA, Crossley JA, Yates JRW, Cooke A, Ferguson‐Smith A. Estimating the risk of a fetal autosomal trisomy at mid‐trimester using maternal serum alpha‐fetoprotein and age: A retrospective study of 142 pregnancies. Prenatal Diagnosis 1991;11(11):847‐57. [DOI] [PubMed] [Google Scholar]
Zelop 2005 {published data only}
- Zelop CM, Milewski E, Brault K, Benn P, Borgida AF, Egan JFX. Variation of fetal nasal bone length in second‐trimester fetuses according to race and ethnicity. Journal of Ultrasound in Medicine 2005;24(11):1487‐9. [DOI] [PubMed] [Google Scholar]
Zhang 2011 {published data only}
- Zhang J, Lambert‐Messerlian G, Palomaki GE, Canick JA. Impact of smoking on maternal serum markers and prenatal screening in the first and second trimesters. Prenatal Diagnosis 2011;31(6):583‐8. [DOI] [PubMed] [Google Scholar]
Zhao 1998 {published data only}
- Zhao Xiaolan, Ye Guoling, Liu Qi. Using maternal serum PAPP‐A and other pregnancy‐associated proteins in screening for fetal abnormalities. Xi'an Yike Daxue Xuebao 1998;19(1):94‐6, 110. [Google Scholar]
Zhong 2011 {published data only}
- Zhong Y, Longman R, Bradshaw R, Odibo AO. The genetic sonogram: comparing the use of likelihood ratios versus logistic regression coefficients for Down syndrome screening. Journal of Ultrasound in Medicine 2011;30(4):463‐9. [DOI] [PubMed] [Google Scholar]
Zoppi 2003a {published data only}
- Zoppi MA, Ibba RM, Floris M, Manca F, Axiana C, Monni G. Changes in nuchal translucency thickness in normal and abnormal karyotype fetuses. BJOG: an international journal of obstetrics and gynaecology 2003;110(6):584‐8. [PubMed] [Google Scholar]
Additional references
Akolekar 2015
- Akolekar R, Beta J, Picciarelli G, Ogilvie C, D'Antonio F. Procedure‐related risk of miscarriage following amniocentesis and chorionic villus sampling: a systematic review and meta‐analysis. Ultrasound in Obstetrics & Gynecology 2015;45(1):16‐26. [DOI] [PubMed] [Google Scholar]
Alfirevic 2003
- Alfirevic Z, Sundberg K, Brigham S. Amniocentesis and chorionic villus sampling for prenatal diagnosis. Cochrane Database of Systematic Reviews 2003, Issue 3. [DOI: 10.1002/14651858.CD003252] [DOI] [PMC free article] [PubMed] [Google Scholar]
Alfirevic 2004
- Alfirevic Z, Neilson JP. Antenatal screening for Down's syndrome. BMJ 2004;9(329(7470)):811‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Alldred 2010
- Alldred SK, Deeks JJ, Neilson JP, Alfirevic Z. Antenatal screening for Down's syndrome: generic protocol. Cochrane Database of Systematic Reviews 2010, Issue 4. [DOI: 10.1002/14651858.CD007384.pub2] [DOI] [Google Scholar]
Alldred 2012
- Alldred SK, Deeks JJ, Guo B, Neilson JP, Alfirevic Z. Second trimester serum tests for Down's Syndrome screening. Cochrane Database of Systematic Reviews 2012, Issue 6. [DOI: 10.1002/14651858.CD009925] [DOI] [PMC free article] [PubMed] [Google Scholar]
Alldred 2015
- Alldred SK, Takwoingi Y, Guo B, Pennant M, Deeks JJ, Neilson JP, et al. First trimester serum tests for Down's syndrome screening. Cochrane Database of Systematic Reviews 2015, Issue 11. [DOI: 10.1002/14651858.CD011975] [DOI] [PMC free article] [PubMed] [Google Scholar]
Alldred 2015a
- Alldred SK, Guo B, Takwoingi Y, Pennant M, Wisniewski S, Deeks JJ, Neilson JP, et al. Urine tests for Down's syndrome screening. Cochrane Database of Systematic Reviews 2015, Issue 12. [DOI: 10.1002/14651858.CD011984] [DOI] [PMC free article] [PubMed] [Google Scholar]
Badeau 2015
- Badeau M, Lindsay C, Blais J, Takwoingi Y, Langlois S, Légaré F, et al. Genomics‐based non‐invasive prenatal testing for detection of fetal chromosomal aneuploidy in pregnant women. Cochrane Database of Systematic Reviews. John Wiley & Sons, Ltd, 2015, issue 7. [DOI: 10.1002/14651858.CD011767] [DOI] [PMC free article] [PubMed]
Bersinger 1995
- Bersinger NA, Zakher A, Huber U, Pescia G, Schneider H. A sensitive enzyme immunoassay for pregnancy‐associated plasma protein A (PAPP‐A): a possible first trimester method of screening for Down syndrome and other trisomies. Archives of Gynecology and Obstetrics 1995;256(4):185‐92. [DOI] [PubMed] [Google Scholar]
Bogart 1987
- Bogart MH, Pandian MR, Jones OW. Abnormal maternal serum chorionic gonadotropin levels in pregnancies with fetal chromosome abnormalities. Prenatal Diagnosis 1987;7(9):623‐30. [DOI] [PubMed] [Google Scholar]
Cole 1999
- Cole LA, Rinne KM, Mahajan SM, Oz UA, Shahabi S, Mahoney MJ, et al. Urinary screening tests for fetal Down syndrome: I. Fresh beta‐core fragment.[see comment]. Prenatal Diagnosis 1999;19(4):340‐50. [DOI] [PubMed] [Google Scholar]
Cuckle 1995
Macri 1990
- Macri JN, Kasturi RV, Krantz DA, Cook EJ, Moore ND, Young JA, et al. Maternal serum Down syndrome screening: free beta‐protein is a more effective marker than human chorionic gonadotropin. American Journal of Obstetrics and Gynecology 1990;163(4 Pt 1):1248‐53. [DOI] [PubMed] [Google Scholar]
Macri 1993
- Macri JN, Spencer K, Aitken D, Garver K, Buchanan PD, Muller F, et al. First‐trimester free beta (hCG) screening for Down syndrome. Prenatal Diagnosis 1993;13(7):557‐62. [DOI] [PubMed] [Google Scholar]
Mol 1999
- Mol BW, Lijmer JG, Meulen J, Pajkrt E, Bilardo CM, Bossuyt PM. Effect of study design on the association between nuchal translucency measurement and Down syndrome. Obstetrics and Gynecology 1999;94(5 Pt 2):864‐9. [DOI] [PubMed] [Google Scholar]
Penrose 1933
- Penrose LS. The relative effects of parental and maternal age in mongolism. Journal of Genetics 1933;27:219‐24. [DOI] [PubMed] [Google Scholar]
Steele 1966
- Steele MW, Breg WR. Chromosome analysis of human amniotic‐fluid cells . Lancet 1966;i:383‐5. [DOI] [PubMed] [Google Scholar]
Valenti 1968
- Valenti C, Schutta EJ, Kehaty T. Prenatal diagnosis of Down's syndrome. Lancet 1968;ii:220. [DOI] [PubMed] [Google Scholar]
Wald 2003a
- Wald NJ, Rodeck C, Hackshaw AK, Walters J, Chitty L, Mackinson AM. First and second trimester antenatal screening for Down's syndrome: the results of the Serum, Urine and Ultrasound Screening Study (SURUSS). Health Technology Assessment 2003;7(11):1‐77. [DOI] [PubMed] [Google Scholar]
Wallace 1995
- Wallace EM, Grant VE, Swanston IA, Groome NP. Evaluation of maternal serum dimeric inhibin A as a first‐trimester marker of Down's syndrome. Prenatal Diagnosis 1995;15(4):359‐62. [DOI] [PubMed] [Google Scholar]
Whiting 2003
- Whiting P, Rutjes AW, Reitsma JB, Bossuyt PM, Kleijnen J. The development of QUADAS: A tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Medical Research Methodology 2003;3:25. [DOI] [PMC free article] [PubMed] [Google Scholar]


