Abstract
Background
Strict or partial bed rest in hospital or at home is commonly recommended for women with multiple pregnancy to improve pregnancy outcomes. In order to advise women to rest in bed for any length of time, a policy for clinical practice needs to be supported by reliable evidence and weighed against possible adverse effects resulting from prolonged activity restriction.
Objectives
The objective of this review is to assess the effectiveness of bed rest in hospital or at home to improve perinatal outcomes in women with a multiple pregnancy.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (30 May 2016), ClinicalTrials.gov, the WHO International Clinical Trials Registry Platform (ICTRP) (30 May 2016) and reference lists of retrieved studies.
Selection criteria
We selected all individual and cluster‐randomised controlled trials evaluating the effect of strict or partial bed rest at home or in hospital compared with no activity restriction during multiple pregnancy.
Data collection and analysis
Two review authors independently assessed trials for inclusion, extracted data and methodological quality. We evaluated the quality of the evidence using the GRADE approach and summarised it in 'Summary of findings' tables.
Main results
We included six trials, involving a total of 636 women with a twin or triplet pregnancy (total of 1298 babies). We assessed all of the included trials as having a low risk of bias for random sequence generation. Apart from one trial with an unclear risk of bias, we judged all remaining trials to be of low risk of bias for allocation concealment.
Five trials (495 women and 1016 babies) compared strict bed rest in hospital with no activity restriction at home. There was no difference in the risk of very preterm birth (risk ratio (RR) 1.02, 95% confidence interval (CI) 0.66 to 1.58, five trials, 495 women, assuming complete correlation between twins/triplets, low‐quality evidence), perinatal mortality (RR 0.65, 95% CI 0.35 to 1.21, five trials, 1016 neonates, assuming independence between twins/triplets, low‐quality evidence) and low birthweight (RR 0.95, 95% CI 0.75 to 1.21, three trials, 502 neonates, assuming independence between twins/triplets, low‐quality evidence). We observed no differences for the risk of small‐for‐gestational age (SGA) (RR 0.75, 95% CI 0.56 to 1.01, two trials, 293 women, assuming independence between twins/triplets, low‐quality evidence) and prelabour preterm rupture of the membrane (PPROM) (RR 1.30, 95% CI 0.71 to 2.38, three trials, 276 women, low‐quality evidence). However, strict bed rest in hospital was associated with increased spontaneous onset of labour (RR 1.05, 95% CI 1.02 to 1.09, P = 0.004, four trials, 488 women) and a higher mean birthweight (mean difference (MD) 136.99 g, 95% CI 39.92 to 234.06, P = 0.006, three trials, 314 women) compared with no activity restriction at home.
Only one trial (141 women and 282 babies) compared partial bed rest in hospital with no activity restriction at home. There was no evidence of a difference in the incidence of very preterm birth (RR 2.30, 95% CI 0.84 to 6.27, 141 women, assuming complete correlation between twins, low‐quality evidence) and perinatal mortality (RR 4.17, 95% CI 0.90 to 19.31, 282 neonates, assuming complete independence twins, low‐quality evidence) between the intervention and control group. Low birthweight was not reported in this trial. We found no differences in the risk of PPROM and SGA between women receiving partial bed rest and the control group (low‐quality evidence). Women on partial bed rest in hospital were less likely to develop gestational hypertension compared with women without activity restriction at home (RR 0.30, 95% CI 0.16 to 0.59, P = 0.0004, 141 women).
Strict or partial bed rest in hospital was found to have no impact on other secondary outcomes. None of the trials reported on costs of the intervention or adverse effects such as the development of venous thromboembolism or psychosocial effects.
Authors' conclusions
The evidence to date is insufficient to inform a policy of routine bed rest in hospital or at home for women with a multiple pregnancy. There is a need for large‐scale, multicenter randomised controlled trials to evaluate the benefits, adverse effects and costs of bed rest before definitive conclusions can be drawn.
Plain language summary
Bed rest with and without hospitalisation for women who are pregnant with twins or triplets for improving outcomes
What is the issue?
Twins, triplets or pregnancies with a greater number of babies have a higher risk of preterm births (birth before 37 weeks of gestation) and poor growth of the babies compared with single baby pregnancies. Women with a multiple pregnancy are often advised to rest in bed at home or in hospital to reduce the risk of preterm birth and other pregnancy complications.
Why is this important?
Although bed rest is widely used in multiple pregnancies currently there is insufficient evidence to support the routine use of bed rest to reduce the risk of preterm birth. Furthermore, many studies have reported on adverse effects of bed rest. It is important to evaluate bed rest and weigh up the potential benefits and risks for women with multiple pregnancies.
What evidence did we find?
We searched for evidence on 30 May 2016. We identified six randomised controlled trials involving a total of 636 women and 1298 babies. The women were at 17 to 33 weeks pregnant when they entered the trials. The overall risk of bias of the trials was low and the evidence in general was of low quality.
Advising women with a multiple pregnancy to either continuously rest in bed (five trials, 495 women and 1016 babies) or rest in bed for several hours during the day but with some physical activity allowed (one trial, 141 women and 282 babies) in hospital did not reduce the risk of very preterm birth (birth before 34 weeks of gestation), infant deaths before or up to one week after the birth or, low birthweight babies (strict bed rest only) compared with women who maintained daily activities at home. Women receiving strict bed rest in hospital were more likely to go into labour normally (four trials, 488 women) and had babies with a higher mean birthweight (three trials, 314 women) compared with women without activity restrictions at home. Partial bed rest in hospital reduced the number of pregnant women developing high blood pressure (one trial, 141 women, low‐quality evidence) but the same benefit was not observed with strict bed rest (five trials, 495 women).
Adverse effects such as the development of venous thromboembolism or mental, emotional, social and spiritual well‐being (psychosocial) effects, and women’s views and experiences of bed rest were not reported in the included trials. Neither were the costs of the intervention reported on.
What does this mean?
We did not find sufficient evidence to support or refute bed rest for women with a multiple pregnancy as a way of preventing preterm birth and other pregnancy complications.
Summary of findings
Summary of findings for the main comparison. Strict bed rest with and without hospitalisation in multiple pregnancy for improving outcomes.
| Population: women with multiple pregnancy Setting: Australia, Zimbabwe Intervention: strict bed rest in hospital Comparison: no activity restriction at home | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with no activity restriction at home | Risk with strict bed rest in hospital | |||||
| Very preterm birth (less than 34 weeks) ‐ assuming complete correlation between twins/triplets | Study population | RR 1.02 (0.66 to 1.58) | 495 (5 RCTs) | ⊕⊕⊝⊝ Low 1,2 | ||
| 120 per 1000 | 123 per 1000 (80 to 190) | |||||
| Perinatal mortality ‐ assuming independence between twins/triplets | Study population | RR 0.65 (0.35 to 1.21) | 1016 (5 RCTs) | ⊕⊕⊝⊝ Low 1,2 | ||
| 47 per 1000 | 31 per 1000 (16 to 57) | |||||
| Low birthweight (less than 2500 g) ‐ assuming independence between twins/triplets | Study population | RR 0.95 (0.75 to 1.21) | 502 (3 RCTs) | ⊕⊕⊝⊝ Low 2,3,4 | ||
| 502 per 1000 | 477 per 1000 (376 to 607) | |||||
| Prelabour preterm rupture of the membrane | Study population | RR 1.30 (0.71 to 2.38) | 276 (3 RCTs) | ⊕⊕⊝⊝ Low 1,2 | ||
| 116 per 1000 | 151 per 1000 (82 to 276) | |||||
| Small‐for‐gestational age ‐ assuming independence between twins/triplets | Study population | RR 0.75 (0.56 to 1.01) | 293 (2 RCTs) | ⊕⊕⊝⊝ Low 1,2 | ||
| 442 per 1000 | 332 per 1000 (248 to 447) | |||||
| Psychosocial effects of bed rest (depression, anxiety, stress) | See comments | No studies reported this outcome | ||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Wide 95% CI with crossing the line with no effect (‐2).
2 We did not downgrade for the lack of blinding because the outcomes were not likely to be influenced by lack of blinding.
3 Wide 95% CI with crossing the line with no effect (‐1). 4 High heterogeneity (I² > 60%) (‐1).
Summary of findings 2. Partial bed rest with or without hospitalisation in multiple pregnancy for improving outcomes.
| Population: women with multiple pregnancy Setting: Australia, Zimbabwe Intervention: partial bed rest in hospital Comparison: no activity restriction at home | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with no activity restriction at home | Risk with partial bed rest in hospital | |||||
| Very preterm birth (less than 34 weeks) ‐ assuming complete correlation between twins | Study population | RR 2.30 (0.84 to 6.27) | 141 (1 RCT) | ⊕⊕⊝⊝ Low 1,2 | ||
| 69 per 1000 | 160 per 1000 (58 to 435) | |||||
| Perinatal mortality ‐ assuming independence between twins | Study population | RR 4.17 (0.90 to 19.31) | 282 (1 RCT) | ⊕⊕⊝⊝ Low 1,2 | ||
| 14 per 1000 | 58 per 1000 (12 to 268) | |||||
| Low birthweight (less than 2500 g) ‐ assuming independence between twins/triplets | See comments | No studies reported this outcome | ||||
| Prelabour preterm rupture of the membrane | Study population | RR 1.51 (0.69 to 3.30) | 141 (1 RCT) | ⊕⊕⊝⊝ Low 1,2 | ||
| 125 per 1000 | 189 per 1000 (86 to 413) | |||||
| Small‐for‐gestational age ‐ assuming independence between twins | Study population | RR 0.93 (0.69 to 1.26) | 282 (1 RCT) | ⊕⊕⊝⊝ Low 1,2 | ||
| 389 per 1000 | 362 per 1000 (268 to 490) | |||||
| Psychosocial effects of bed rest (depression, anxiety, stress) | See comments | No studies reported this outcome | ||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Very wide 95% CI with very small number of events (‐2). 2 We did not downgrade for the lack of blinding due to the outcomes was not likely to be influenced by lack of blinding.
Background
Description of the condition
Since the mid‐1970s, the frequency and rate of multiple births has continued to rise due to the increase in maternal age and the use of fertility treatments (Pison 2006). In the USA, Australia and many European countries, the twin rate rose from under 10 per 1000 births in the 1970s, to 13 to 16 per 1000 births around the year 2000 (Imaizumi 2003; Pison 2006). The incidence of multiple pregnancies varies worldwide, ranging from 6 to 9 per 1000 births in South and South‐East Asia to 18 and more per 1000 births in most Central‐African countries (Smits 2011). The incidence of monozygotic twinning (arising from the fertilisation of one egg) occurs at a relatively constant rate of 3.5 to 4 per 1000 births across different populations, suggesting that it is a random and less genetically influenced event (Campbell 1998). On the other hand, dizygotic twinning (arising from the fertilisation of two eggs) and higher‐order multiple pregnancy vary among populations (Campbell 1998; Imaizumi 2003). Two main reasons associated with multiple births are advanced maternal age (35 years or older at the time of delivery) and assisted reproductive technologies, such as in vitro fertilisation, intrauterine insemination, and ovulation induction (Beemsterboer 2006; Black 2010).
Multiple births represent a small percentage of all newborns, but are at very high risk for pregnancy complications and neonatal morbidity compared with singletons. Multiple pregnancies account for 9% to 12% of all perinatal deaths, that is, death before, during and up to the first week after birth (Norwitz 2005). The higher the order of multiples, the greater the risk: the main risk factors of multiple mortality and morbidity are preterm birth (before 37 completed weeks of gestation), low birthweight (less than 2500 g), and fetal growth restriction. About 40% to 50% of twins and 90% of triplets are born preterm compared to 5% to 10% of singletons (Blondel 2002). Preterm labour, premature preterm rupture of the membranes or cervical effacement and dilatation can lead to preterm delivery. Cervical dilatation is a frequent complication, particularly in multiple pregnancy, and women with this condition are at higher risk for preterm birth (Brubaker 2012; Neilson 1988). Fetal growth is restricted from 30 weeks in twins, from 27 weeks in triplets and from 26 weeks in quadruplets; in singletons, fetal growth is linear between 30 and 36 weeks (McKeown 1952). Preterm birth and low birthweight substantially contribute to perinatal mortality and long‐term complications (e.g. learning disabilities and developmental delays), as well as an increase in the risk of non‐communicable diseases later in life (Barker 2004). Among women, multiple pregnancy also involves an increased risk of mortality (MacKay 2006). In addition, multiple births are associated with maternal complications such as pregnancy‐induced hypertension, gestational diabetes, caesarean delivery and postpartum haemorrhage (Senat 1998).
Description of the intervention
Bed rest at home or in hospital is a very common therapeutic intervention in obstetric practice to prevent preterm birth in women at risk. Strict bed rest refers to the confinement to rest in bed as much as possible with minimum physical activity. Women who are recommended to partially rest are not restricted in physical activity, but encouraged to stay in bed for a few hours during the day (Maloni 2010; Sciscione 2010).
How the intervention might work
Bed rest has been traditionally used for preventing preterm birth as well as in the treatment of other pregnancy complications such as threatened miscarriage, multiple gestations, hypertensive diseases, fetal growth restriction and oedema (Goldenberg 1994). The common prescription of the intervention is justified with the assumption that it effectively prevents preterm birth by reducing uterine activity and that bed rest is safe for mother and infants (Maloni 2010). In singleton pregnancies, current evidence does not support or refute bed rest for the prevention of preterm birth (Sosa 2015). Benefits from bed rest could include prolongation of the multiple pregnancy to achieve greater maturation of the fetuses with improvement in growth and weight at birth, and optimal management of early labour in the case of bed rest in the hospital setting. However, adverse effects in relation to bed rest have been observed. This includes an increase in the risk of thrombosis, loss of muscle mass and cardiovascular deconditioning for women (Kovacevich 2000; Maloni 1993; Maloni 2002). Besides physiological effects, there is evidence that bed rest has negative psychological (i.e. stress and depression) and financial effects for women and their families (Maloni 1993; May 2001). Additionally, the associated healthcare costs for a long stay in the hospital or at home are of significance for the public sector (Goldenberg 1994).
Why it is important to do this review
Effective interventions for preterm birth would have a significant impact on the outcome of multiple pregnancies. Bed rest, with or without hospitalisation, may have the potential to reduce the risk of preterm birth, fetal and neonatal mortality and long‐term morbidity. Advising women with a multiple pregnancy to rest in bed for any length of time and restricting their lifestyle to reduce the risk of preterm delivery, needs to be supported by reliable evidence. In order to guide clinical decisions, this new review replaced a previous review on the topic first published in 2001, which was updated in 2010 (Crowther 2001; Crowther 2010). This review found that routine hospitalisation and bed rest for women with multiple pregnancy may improve fetal growth, but did not find sufficient evidence to reduce the risk of preterm birth and perinatal mortality (Crowther 2010). We systematically evaluated the latest evidence on the effectiveness of bed rest in hospital or at home in women with multiple pregnancy across pregnancy outcomes.
Objectives
The objective of this review is to assess the effectiveness of bed rest in hospital or at home to improve perinatal outcomes in women with a multiple pregnancy.
Methods
Criteria for considering studies for this review
Types of studies
We included all published, unpublished, and ongoing randomised controlled trials (RCTs), including individual‐RCTs and cluster‐RCTs, that compared pregnancy outcomes in women who were offered bed rest in hospital or at home during pregnancy with women who did not receive bed rest in hospital or at home during pregnancy. We excluded quasi‐RCTs and cross‐over trials.
Types of participants
All women with a multiple pregnancy.
Types of interventions
We considered any comparisons (in hospital or at home) for the following interventions.
Strict bed rest: women are encouraged to rest in bed as much as possible, with minimal physical activity such as ambulation for toileting needs or healthcare‐related visiting, etc.
Partial bed rest: women are advised to rest for a few hours during the day, but physical activity is not restricted.
No activity restriction.
Types of outcome measures
Primary outcomes
Very preterm birth (less than 34 weeks' gestation)
Perinatal mortality (defined by trialists)
Low birthweight (less than 2500 g)
Secondary outcomes
Maternal outcomes
Prelabour preterm rupture of the membranes (PPROM)
Spontaneous onset of labour
Caesarean delivery
Development of maternal hypertension (e.g. pregnancy‐induced hypertension, pre‐eclampsia)
Development of venous thromboembolism
Women's assessment and satisfaction of care
Quality of life (experience and feeling) during bed rest
Psychosocial effects of bed rest (depression, anxiety, stress)
Fetal or infant outcomes
Stillbirth (death after 20 weeks' gestation and before birth)
Early neonatal death (death within the first seven days after birth)
Preterm birth (less than 37 weeks' gestation)
Birthweight (g)
Very low birthweight (less than 1500 g)
Gestational age at delivery
Small‐for‐gestational age (SGA) (defined by trialists)
Apgar score less than seven (at five minutes)
Admission to neonatal intensive care unit (NICU)
Neonatal stay at NICU (stay of seven days or more)
Other outcomes
Costs to health service
Costs to women and their families
Search methods for identification of studies
The methods section of this review is based on a standard template used by Cochrane Pregnancy and Childbirth.
Electronic searches
We searched Cochrane Pregnancy and Childbirth’s Trials Register by contacting their Information Specialist (30 May 2016).
The Register is a database containing over 22,000 reports of controlled trials in the field of pregnancy and childbirth. For full search methods used to populate Pregnancy and Childbirth’s Trials Register including the detailed search strategies for CENTRAL, MEDLINE, Embase and CINAHL; the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service, please follow this link to the editorial information about Cochrane Pregnancy and Childbirth in the Cochrane Library and select the ‘Specialized Register’ section from the options on the left side of the screen.
Briefly, Cochrane Pregnancy and Childbirth’s Trials Register is maintained by their Information Specialist and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE (Ovid);
weekly searches of Embase (Ovid);
monthly searches of CINAHL (EBSCO);
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Search results are screened by two people and the full text of all relevant trial reports identified through the searching activities described above is reviewed. Based on the intervention described, each trial report is assigned a number that corresponds to a specific Pregnancy and Childbirth review topic (or topics), and is then added to the Register. The Information Specialist searches the Register for each review using this topic number rather than keywords. This results in a more specific search set which has been fully accounted for in the relevant review sections (Included studies; Excluded studies; Studies awaiting classification).
In addition, we searched ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP) for unpublished, planned and ongoing trial reports (30 May 2016) (see: Appendix 1 for terms used).
Searching other resources
We searched the reference lists of retrieved studies.
We did not apply any language or date restrictions.
Data collection and analysis
We used the following methods for assessing the reports identified as a result of the search. These methods are based on a standard template used by Cochrane Pregnancy and Childbirth.
Selection of studies
Review authors Katharina da Silva Lopes (KL), Yo Takemoto (YT) and Shinji Tanigaki (ST) independently assessed for inclusion all the potential studies we identified as a result of the search strategy for this update. We resolved any disagreement through discussion or, if required, we consulted with Erika Ota (EO). We planned to include studies published as abstracts and to contact authors if further information was needed. We marked studies as 'awaiting classification' if we could not assess study quality and extract information (after attempting to contact study authors).
We created a study flow diagram to map out the number of records identified, included and excluded
Data extraction and management
For eligible studies, KL, YT and ST independently extracted the data. We resolved discrepancies through discussion or, if required, we consulted with EO. We entered data into Review Manager 5 (RevMan 5) software (RevMan 2014) and checked for accuracy. When information regarding any of the above was unclear, we attempted to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
KL, YT and ST independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). We resolved any disagreement by discussion or by involving Rintaro Mori.
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding would be unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
low, high or unclear risk of bias for assessors.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we re‐included missing data in the analyses which we undertook.
We assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as‐treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it was clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review were reported);
high risk of bias (where not all the study’s pre‐specified outcomes were reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest were reported incompletely and so could not be used; study failed to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included study any important concerns we had about other possible sources of bias.
We assessed whether each study was free of other problems that could put it at risk of bias:
low risk of other bias;
high risk of other bias;
unclear whether there is risk of other bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). With reference to (1) to (6) above, we to assessed the likely magnitude and direction of the bias and whether we considered it was likely to impact on the findings. In future updates, we will explore the impact of the level of bias through undertaking sensitivity analyses ‐ see Sensitivity analysis.
Assessment of the quality of the evidence using the GRADE approach
We assessed the quality of the evidence using the GRADE approach as outlined in the GRADE handbook in order to assess the quality of the body of evidence relating to the following outcomes for the main comparisons.
Very preterm birth (less than 34 weeks' gestation)
Perinatal mortality (defined by trialists)
Low birthweight (less than 2500 g)
Prelabour preterm rupture of the membrane
Small‐for‐gestational age
Psychosocial effects of bed rest (depression, anxiety, stress)
We used the GRADEpro GDT (Guideline Development Tool) to import data from RevMan 5 (RevMan 2014) in order to create ’Summary of findings’ tables. We produced a summary of the intervention effect and a measure of quality for each of the above outcomes using the GRADE approach. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. The evidence can be downgraded from 'high quality' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratios (RR) with 95% confidence intervals (CI).
Continuous data
For continuous data, we used the mean difference (MD) if outcomes were measured in the same way between trials. We planned to use the standardised mean difference (SMD) to combine trials that measured the same outcome, but used different methods.
Unit of analysis issues
Cluster‐randomised trials
We did not identify any cluster‐randomised trials in this review. In future updates, if identified, we plan to include cluster‐randomised trials in the analyses along with individually‐randomised trials. We will adjust their standard errors using the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Section 16.3.4 or 16.3.6) and using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population (Higgins 2011b). If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster‐randomised trials and individually‐randomised trials, we plan to synthesise the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention, and the choice of randomisation unit is considered to be unlikely.
We will also acknowledge heterogeneity in the randomisation unit and perform a sensitivity analysis to investigate the effects of the randomisation unit.
Other unit of analysis issues
For maternal outcomes, we used the number of women as the denominator for the incidence of PPROM, spontaneous onset of labour, caesarean delivery and development of maternal hypertension. Treating babies from multiple pregnancies as if they were independent, when they were more likely to have similar outcomes than babies form different pregnancies, would overestimate the sample size and give confidence intervals that are too narrow. For fetal and infant outcomes, to avoid incorrect conclusions due to the non‐independence of babies from multiple pregnancies, we performed sensitivity analyses assuming complete correlation between multiples for most outcomes (i.e. assuming the outcomes for all multiples from the same pregnancy would be the same; this is a very conservative assumption). To make adjustments to take account of the assumed correlation we divided both numerator and denominator by one, two or three for singleton, twin and triplet pregnancies respectively. For some outcomes the numerators were small and not easily divisible; for example a single event when divided by two or three resulted in a fraction and only whole numbers can be entered into RevMan 5 (RevMan 2014). For these outcomes, for the adjusted analyses, we have entered whole numbers (rounded up) and acknowledge that this will lead to some inaccuracy. We only adjusted the outcomes by the number of babies where the individual babies from the twin or triplet pregnancy could potentially have different outcomes. Therefore, we carried out sensitivity analyses for the following fetal and infant outcomes: perinatal mortality, low birthweight, stillbirth, early neonatal death, very low birthweight, small‐for‐gestational age, Apgar score less than seven at five minutes and admission to neonatal intensive care unit. If we carried out adjustments, we considered the unadjusted outcomes, that is, assuming independence of twins/triplets, as the main outcomes, but have also reported adjusted risk ratios and noted if adjustments led to any substantial changes in the confidence intervals. For the continuous outcome, infant birthweight, we have reported unadjusted figures. For very preterm birth and preterm birth we assumed total correlation between multiples and have only reported adjusted figures.
Multiple‐arm studies
We did not identify any multi‐arm studies (more than two intervention arms). In future updates, if identified, we will avoid 'double counting' the participants by combining groups to create a single pair‐wise comparison if possible.
Where a trial has an intervention arm that is not relevant to our review question, we will comment on this in the table 'Characteristics of included studies', but only include intervention and control groups that meet the eligibility criteria in the analysis.
Dealing with missing data
For included studies, we noted levels of attrition. In future updates, if more eligible studies are included, we will explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes, we carried out analyses on an intention‐to‐treat basis, that is, we included all participants randomised to each group in the analyses, and all participants were analysed in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the Tau², I² (Higgins 2003) and Chi² statistics. We regarded heterogeneity as substantial if an I² was greater than 30% and either a Tau² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity. Where we identified substantial heterogeneity (above 30%), we explored it by pre‐specified subgroup analysis.
Assessment of reporting biases
In future updates, if there are 10 or more studies in the meta‐analysis, we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using the RevMan 5 software (RevMan 2014). We used fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect: that is, where trials were examining the same intervention, and we judged the trials’ populations and methods sufficiently similar. If there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or if substantial statistical heterogeneity was detected, we used random‐effects meta‐analysis to produce an overall summary, if we considered an average treatment effect across trials to be clinically meaningful. We treated the random‐effects summary as the average of the range of possible treatment effects and we discussed the clinical implications of treatment effects differing between trials. If the average treatment effect was not clinically meaningful, we did not combine trials.
Where we used random‐effects analyses, we presented the results as the average treatment effect with 95% CIs, and the estimates of Tau² and I².
Subgroup analysis and investigation of heterogeneity
If we identified substantial heterogeneity, we planned to investigate it using subgroup analyses and to consider whether an overall summary was meaningful, and if it was, use random‐effects analysis to produce it.
We planned to carry out the following subgroup analyses.
Women with twin pregnancy versus triplet or higher‐order multiple
Women with cervical dilation versus women without cervical dilation
Women with short cervix (25 mm or less) versus women with cervix greater than 25 mm
Women with monochorionic twin pregnancy versus dichorionic twin pregnancy
Women with monochorionic triplet pregnancy versus di‐ or trichorionic triplet pregnancy
We planned to include all of the following primary outcomes in subgroup analysis. However, we actually performed subgroup analysis only for twin pregnancy versus triplet or higher‐order multiple for the outcome low birthweight because there were not enough data to analyse the other subgroups.
Very preterm birth (less than 34 weeks' gestation)
Perinatal mortality (defined by trialists)
Low birthweight (less than 2500 g)
We assessed subgroup differences by interaction tests available within RevMan 5 (RevMan 2014). We reported the results of subgroup analyses quoting the Chi² statistic and P value, and the interaction test I² value.
Sensitivity analysis
We planned to carry out sensitivity analysis for aspects of the review that might affect the results, for example, where there was risk of bias associated with the quality of some of the included trials. We also planned to carry out sensitivity analysis to explore the effects of fixed‐effect or random‐effects analyses for outcomes with statistical heterogeneity. However, there were too few studies included in any meta‐analysis to carry out meaningful sensitivity analysis in this review.
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies and Characteristics of studies awaiting classification.
Results of the search
See: Figure 1.
1.
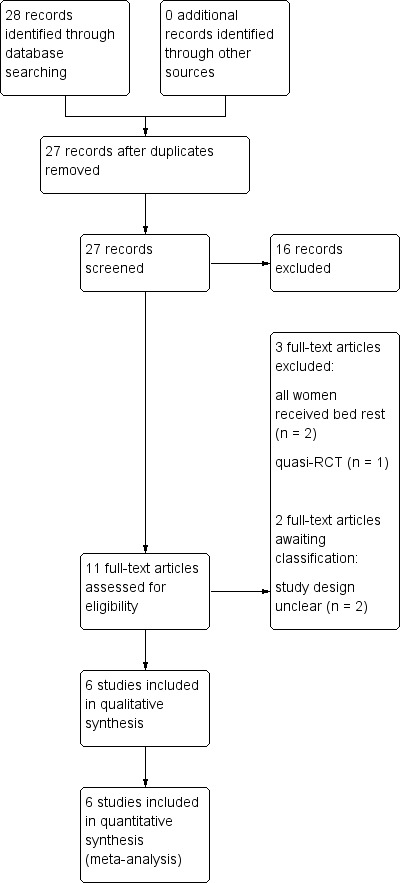
Study flow diagram
The search of Cochrane Pregnancy and Childbirth's Register retrieved 11 reports. Additional searching retrieved a further 17, all of which were screened out. We included six trials (Crowther 1989; Crowther 1990; Crowther 1991; Dodd 2005; MacLennan 1990; Saunders 1985), excluded three trials (Gummerus 1985; Gummerus 1987; Hartikainen‐Sorri 1984) and two studies are awaiting classification (Al‐Najashi 1996; Younis 1990).
Included studies
Six trials published between 1985 and 2005 met our inclusion criteria. For full details see Characteristics of included studies.
Participants
A total of 636 women and 1298 babies were included in the six trials. Four trials recruited women with a twin pregnancy (610 women and 1220 babies) (Crowther 1989; Crowther 1990; MacLennan 1990; Saunders 1985). The remaining two trials recruited women with a triplet pregnancy (26 women and 78 babies) (Crowther 1991; Dodd 2005). In five trials, only women with an uncomplicated twin or triplet pregnancy were included, while in Crowther 1989, women with a twin pregnancy and a cervical score of ‐2 or less which is calculated by length minus dilatation (cm) of the cervix were included into the trial. The mean age of participants in the intervention group ranged from 25.2 to 33.2 years and in the control group from 26.7 to 36.2 years. The gestational age at study entry ranged from 17.0 to 33.3 weeks in the intervention group and from 17.5 to 33.5 in the control group.
Interventions and comparisons
Five trials compared strict bed rest in hospital with normal activity at home (Crowther 1989; Crowther 1990; Crowther 1991; Dodd 2005; Saunders 1985). One trial compared partial bed rest in hospital with normal activity at home (MacLennan 1990).
Outcomes
All of the included studies focused on assessing the effect of routine hospital admission for bed rest in multiple pregnancy on pregnancy outcomes, that is, length of gestation. All included trials reported the incidence of very preterm birth and perinatal mortality, but only three trials reported the incidence of low birthweight (Crowther 1991; Dodd 2005; Saunders 1985). None of the included trials assessed maternal outcomes such as the development of thromboembolism, satisfaction of care, quality of life and psychosocial effects of bed rest. In MacLennan 1990, women were asked about their quality of life during bed rest. However, we did not include this outcome in our analysis as only the intervention group was questioned and not the control group. All the included trials reported most fetal/infant outcomes. None of the trials assessed the costs of the intervention to the health system or to women and their families.
Setting
Four studies were conducted in a single centre in Harare, Zimbabwe (Crowther 1989; Crowther 1990; Crowther 1991; Saunders 1985). The remaining two trials were undertaken in Australia. One was a single centre study (Dodd 2005) and the other a multicenter study involving 11 hospitals (MacLennan 1990).
Excluded studies
We excluded three trials. In Gummerus 1985, participants were allocated to receive bed rest in hospital or betamimetic treatment. In Gummerus 1987, all women with a multiple pregnancy were admitted to hospital to receive either bed rest or salbutamol treatment. In both trials, there was no group of women without bed rest or drug treatment for comparison. We excluded the other trial because participants were allocated to the intervention or control group according to their year of birth (Hartikainen‐Sorri 1984). See Characteristics of excluded studies.
Studies awaiting classification
Two studies (Al‐Najashi 1996; Younis 1990) are still awaiting classification because the type of study design was unclear. We contacted the study authors, but we did not receive a reply. See Characteristics of studies awaiting classification.
Risk of bias in included studies
Figure 2 and Figure 3 illustrate the overall high quality of the trials.
2.
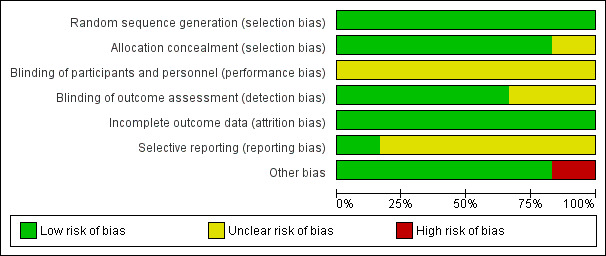
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
3.
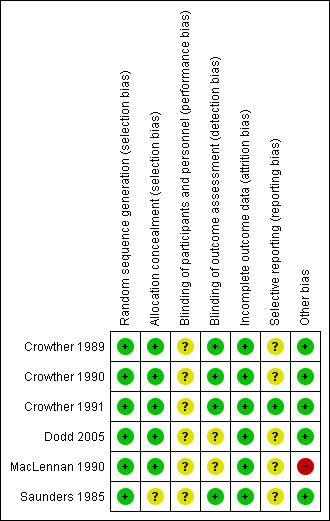
Risk of bias summary: review authors' judgements about each risk of bias item for each included study
Allocation
Sequence generation
All six trials adequately randomised women to the intervention and control group and were therefore judged to be at low risk of bias.
Allocation concealment
Five trials were at low risk of bias for allocation concealment (Crowther 1989; Crowther 1990; Crowther 1991; Dodd 2005; MacLennan 1990). Saunders 1985 was of unclear risk of bias because the method for allocation concealment was not described.
Blinding
Participants and personnel
We judged all six trials to be at unclear risk of bias due to the type of intervention (hospitalisation and bed rest compared with no activity restriction at home), where blinding of participants and personnel was not possible.
Outcome assessment
Four trials stated that they blinded outcome assessors (Crowther 1989; Crowther 1990; Crowther 1991; Saunders 1985) and blinding of outcome assessors was unclear in two (Dodd 2005; MacLennan 1990).
Incomplete outcome data
There was no loss to follow‐up in any of the included trials and analyses were done on an intention‐to‐treat basis. Therefore, we considered all included trials to have a low risk of attrition bias.
Selective reporting
We assessed Crowther 1991 as being at low risk of reporting bias. Risk of bias in the remaining five trials was unclear due to insufficient details being provided about pre‐specified outcomes or no study protocol being available (Crowther 1989; Crowther 1990; Dodd 2005; MacLennan 1990; Saunders 1985).
Other potential sources of bias
One trial was at high risk of bias as the enrolment of the study was stopped following an interim analysis after the first participants were recruited (MacLennan 1990). The other trials appeared to be free of other sources of bias and we judged them to be low risk.
Effects of interventions
Comparison one: strict bed rest in hospital versus no activity restriction at home
Five trials, including 495 women and 1016 babies, studied the effect of strict bed rest in hospital compared with normal activity at home (Crowther 1989; Crowther 1990; Crowther 1991; Dodd 2005; Saunders 1985).
Primary outcomes
Very preterm birth (less than 34 weeks' gestation)
Five trials reported this outcome (Crowther 1989; Crowther 1990; Crowther 1991; Dodd 2005; Saunders 1985). Strict bed rest in hospital showed no evidence of an effect in reducing the incidence of birth at less than 34 weeks' gestation compared with no activity restriction at home (risk ratio (RR) 1.02, 95% confidence interval (CI) 0.66 to 1.58, five trials, 495 women, moderate‐quality evidence; Analysis 1.1).
1.1. Analysis.
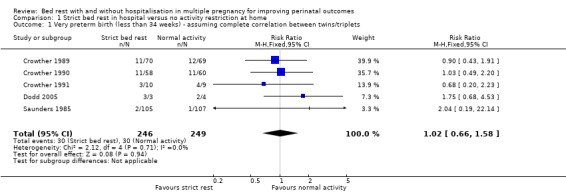
Comparison 1 Strict bed rest in hospital versus no activity restriction at home, Outcome 1 Very preterm birth (less than 34 weeks) ‐ assuming complete correlation between twins/triplets.
Perinatal mortality
This outcome was reported by five trials (Crowther 1989; Crowther 1990; Crowther 1991; Dodd 2005; Saunders 1985). There was no evidence of a difference in the risk of perinatal mortality between women assigned to strict bed rest in hospital compared with women not restricted in their activities at home (RR 0.65, 95% CI 0.35 to 1.21, five trials, 1016 neonates; I² = 20%, moderate‐quality evidence). Assuming complete correlation between twins/triplets, there was also no evidence of an effect (RR 0.70, 95% CI 0.30 to 1.63, five trials, 495 twins/triplets, I² = 0%; Analysis 1.2).
1.2. Analysis.
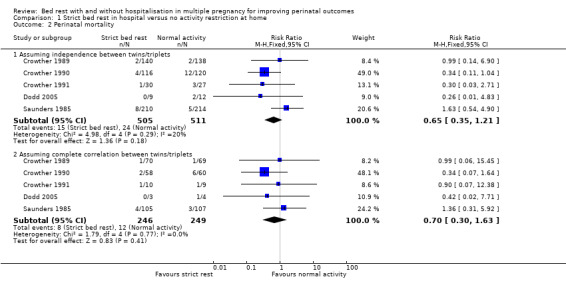
Comparison 1 Strict bed rest in hospital versus no activity restriction at home, Outcome 2 Perinatal mortality.
Low birthweight (less than 2500 g)
Three trials reported on low birthweight (Crowther 1991; Dodd 2005; Saunders 1985). There was no effect of strict bed rest in hospital in multiple pregnancy in reducing the risk of low birthweight (RR 0.95, 95% CI 0.75 to 1.21, three trials, 502 neonates, I² = 64%, moderate‐quality evidence; Analysis 1.3). Subgroup analysis of twin or triplet pregnancy alone indicated no differences in the rate of low birthweight (test for subgroup differences: Chi² = 0.73, df = 1 (P = 0.39), I² = 0%), although it should be noted that there were too few data for meaningful subgroup analysis (Analysis 1.4). The assumption of complete correlation between twins/triplets showed no differences in the rate of low birthweight between intervention and control group (RR 0.91, 95% CI 0.74 to 1.11, three trials, 238 twins/triplets, I² = 0%; Analysis 1.3).
1.3. Analysis.
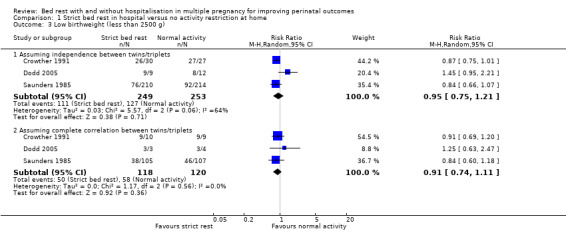
Comparison 1 Strict bed rest in hospital versus no activity restriction at home, Outcome 3 Low birthweight (less than 2500 g).
1.4. Analysis.
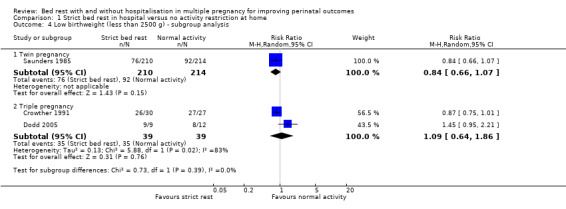
Comparison 1 Strict bed rest in hospital versus no activity restriction at home, Outcome 4 Low birthweight (less than 2500 g) ‐ subgroup analysis.
Maternal secondary outcomes
Prelabour preterm rupture of the membrane (PPROM)
PPROM was reported by three trials (Crowther 1989; Crowther 1990; Crowther 1991). The results showed no evidence of an effect of strict bed rest in hospital to reduce PPROM (RR 1.30, 95% CI 0.71 to 2.38, three trials, 276 women, moderate‐quality evidence; Analysis 1.5).
1.5. Analysis.
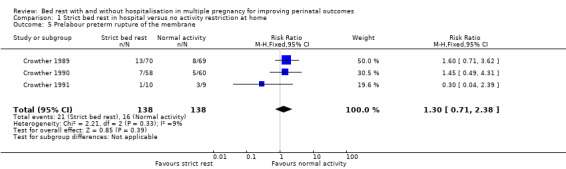
Comparison 1 Strict bed rest in hospital versus no activity restriction at home, Outcome 5 Prelabour preterm rupture of the membrane.
Spontanenous onset of labour
Four trials reported on this outcome (Crowther 1989; Crowther 1990; Crowther 1991; Saunders 1985), and the results showed an increase the spontaneous onset of labour in the strict bed‐rest group (RR 1.05, 95% CI 1.02 to 1.09, P = 0.004, four trials, 488 women; Analysis 1.6).
1.6. Analysis.
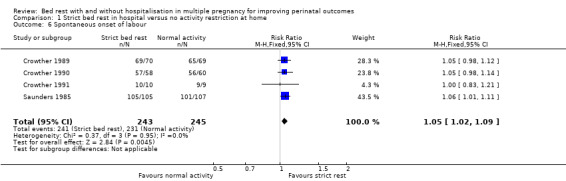
Comparison 1 Strict bed rest in hospital versus no activity restriction at home, Outcome 6 Spontaneous onset of labour.
Caesarean delivery
Four trials evaluated the effect of strict bed rest in hospital versus no activity restriction at home on the rate of caesarean delivery (Crowther 1989; Crowther 1990; Crowther 1991; Dodd 2005). There was no evidence of a difference between intervention and control group (RR 0.69, 95% CI 0.41 to 1.17, four trials, 283 women; Analysis 1.7).
1.7. Analysis.
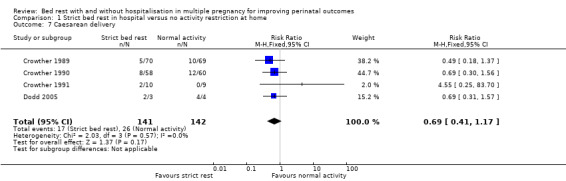
Comparison 1 Strict bed rest in hospital versus no activity restriction at home, Outcome 7 Caesarean delivery.
Development of maternal hypertension
The development of maternal hypertension was reported by five trials (Crowther 1989; Crowther 1990; Crowther 1991; Dodd 2005; Saunders 1985) and no evidence of an effect of strict bed rest in hospital was shown (RR 0.68, 95% CI 0.37 to 1.23, five trials, 495 women; Analysis 1.8).
1.8. Analysis.
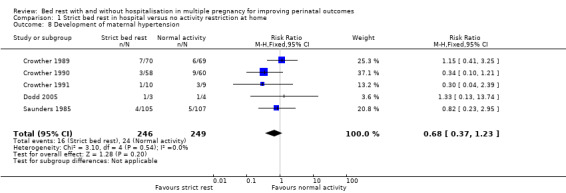
Comparison 1 Strict bed rest in hospital versus no activity restriction at home, Outcome 8 Development of maternal hypertension.
Other maternal secondary outcomes
No trials reported on development of venous thromboembolism, women's assessment and satisfaction of care, quality of life (experience and feeling) during bed rest or physiological effects such as depression, anxiety or stress.
Fetal or infant secondary outcomes
Stillbirth
Five trials reported stillbirth (death after 20 weeks' gestation and before birth) (Crowther 1989; Crowther 1990; Crowther 1991; Dodd 2005; Saunders 1985). There was no evidence of a difference between the bed‐rest group and control group (RR 0.78, 95% CI 0.20 to 2.98, five trials, 1016 neonates). Assuming complete correlation between twins/triplets also showed no evidence of a difference between groups (RR 0.81, 95% CI 0.25 to 2.69, five trials, 495 twins/triplets, I² = 9%; Analysis 1.9).
1.9. Analysis.
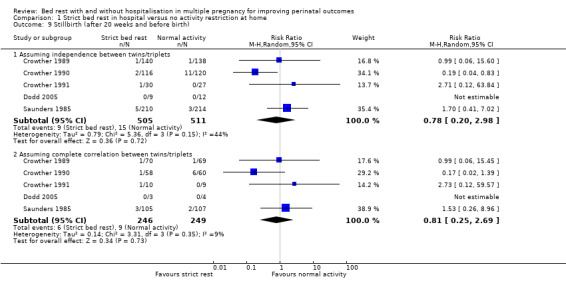
Comparison 1 Strict bed rest in hospital versus no activity restriction at home, Outcome 9 Stillbirth (after 20 weeks and before birth).
Early neonatal death
Five trials assessed early neonatal death (death within seven days after birth) (Crowther 1989; Crowther 1990; Crowther 1991; Dodd 2005; Saunders 1985) and the results showed no effect of strict bed rest in hospital on this outcome (RR 0.72, 95% CI 0.28 to 1.87, five trials, 1016 neonates). Even if we supposed complete correlation between twins/triplets, there was no evidence of a difference in early neonatal death between groups (RR 0.86, 95% CI 0.27 to 2.74, five trials, 495 twins/triplets, I² = 0%; Analysis 1.10).
1.10. Analysis.
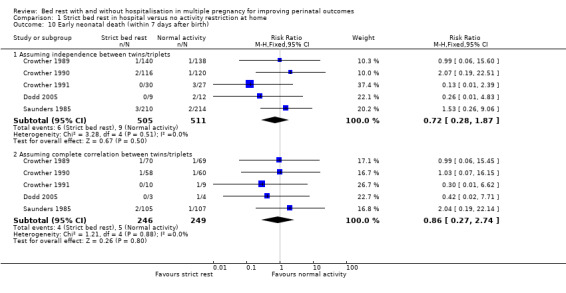
Comparison 1 Strict bed rest in hospital versus no activity restriction at home, Outcome 10 Early neonatal death (within 7 days after birth).
Preterm birth (less than 37 weeks' gestation)
Five trials reported this outcome (Crowther 1989; Crowther 1990; Crowther 1991; Dodd 2005; Saunders 1985). Strict bed rest in hospital did not reduce the incidence of preterm birth compared with normal activity at home (RR 0.97, 95% CI 0.80 to 1.18, five trials, 495 women; Analysis 1.11).
1.11. Analysis.
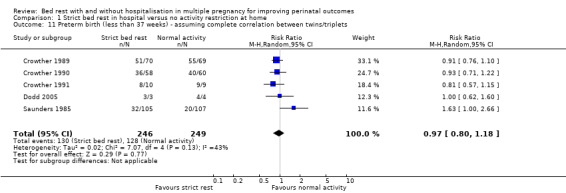
Comparison 1 Strict bed rest in hospital versus no activity restriction at home, Outcome 11 Preterm birth (less than 37 weeks) ‐ assuming complete correlation between twins/triplets.
Birthweight (g)
Three trials reported mean birthweight (Crowther 1990; Crowther 1991; Dodd 2005). Strict bed rest in hospital was associated with an increase in mean birthweight compared with no restriction in activity at home (fixed‐effect mean difference (MD) 136.99, 95% CI 39.92 to 234.06, P = 0.006, three trials, 314 women; Analysis 1.12).
1.12. Analysis.
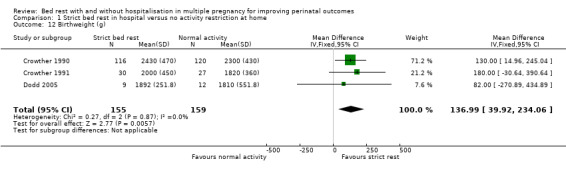
Comparison 1 Strict bed rest in hospital versus no activity restriction at home, Outcome 12 Birthweight (g).
Verly low birthweight (less than 1500 g)
Two trials reported very low birthweight (Dodd 2005; Saunders 1985). There was no evidence of a difference between the bed‐rest group and control group (RR 1.32, 95% CI 0.15 to 11.70, two trials, 445 neonates). Assuming complete correlation between twins/triplets, there was also no difference in the incidence of very low birthweight (RR 1.64, 95% CI 0.31 to 8.70, two trials, 219 twins/triplets, I² = 0%; Analysis 1.13).
1.13. Analysis.
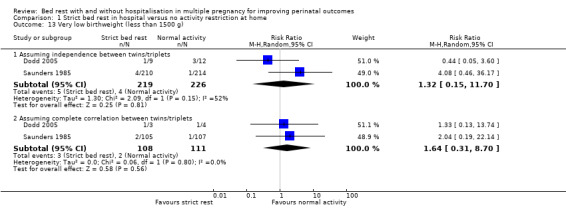
Comparison 1 Strict bed rest in hospital versus no activity restriction at home, Outcome 13 Very low birthweight (less than 1500 g).
Gestational age at delivery (weeks)
Five trials reported this outcome (Crowther 1989; Crowther 1990; Crowther 1991; Dodd 2005; Saunders 1985). Strict bed rest in hospital had no effect on mean gestational age at delivery compared with normal activity at home (fixed‐effect MD ‐0.14, 95% CI ‐0.51 to 0.24, five trials, 495 women; Analysis 1.14).
1.14. Analysis.
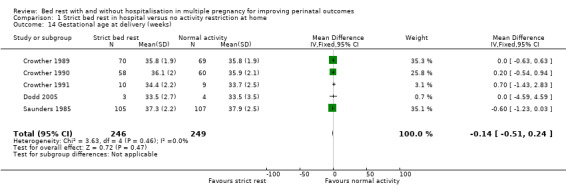
Comparison 1 Strict bed rest in hospital versus no activity restriction at home, Outcome 14 Gestational age at delivery (weeks).
Small‐for‐gestational age
Two trials reported this outcome (Crowther 1990; Crowther 1991). There was no evidence of a difference between the intervention and control groups (RR 0.75, 95% CI 0.56 to 1.01, P = 0.05, two trials, 293 neonates, moderate‐quality evidence). Assuming complete correlation between twins/triplets also showed no difference in the incidence of small‐for‐gestational age (RR 0.71, 95% CI 0.46 to 1.10, two trials, 137 twins/triplets, I² = 0%; Analysis 1.15).
1.15. Analysis.
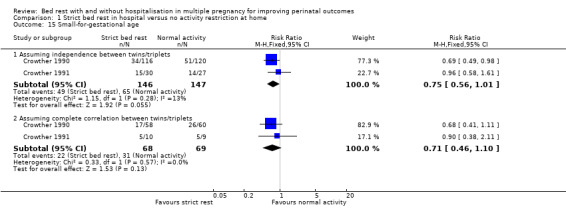
Comparison 1 Strict bed rest in hospital versus no activity restriction at home, Outcome 15 Small‐for‐gestational age.
Apgar score of less than seven at five minutes
Two trials reported Apgar score of less than seven at five minutes (Crowther 1989; Dodd 2005). The results showed no evidence of a difference between the intervention and control group when assuming independence between twins/triplets (RR 1.60, 95% 0.55 to 4.62, two trials, 299 neonates) and when assuming complete correlation between twins/triplets (RR 1.35, 95% CI 0.34 to 5.32, two trials, 146 twins/triplets, I² = 0%; Analysis 1.16).
1.16. Analysis.
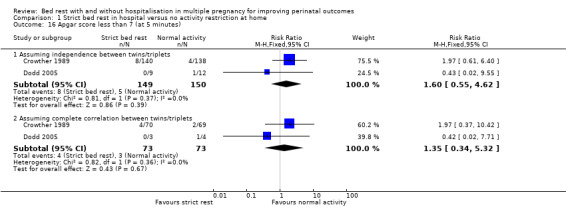
Comparison 1 Strict bed rest in hospital versus no activity restriction at home, Outcome 16 Apgar score less than 7 (at 5 minutes).
Admission to neonatal intensive care unit (NICU)
Three trials reported on the admission of infants to the NICU (Crowther 1989; Crowther 1990; Crowther 1991). Strict bed rest in hospital had no effect on this outcome (RR 0.89, 95% CI 0.74 to 1.06, three trials, 571 neonates). Assuming complete correlation between twins/triplets also showed no evidence of a difference between the intervention and control group (RR 0.88, 95% CI 0.68 to 1.14, three trials, 276 twins/triplets, I² = 0%; Analysis 1.17).
1.17. Analysis.
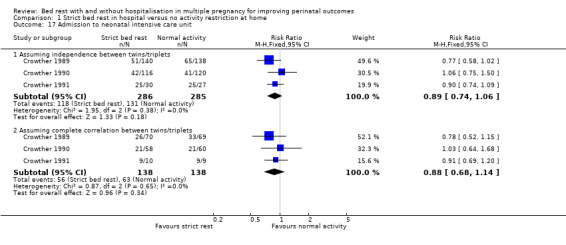
Comparison 1 Strict bed rest in hospital versus no activity restriction at home, Outcome 17 Admission to neonatal intensive care unit.
Neonatal stay at NICU (seven days or more)
None of the included trials reported this outcome.
Other secondary outcomes
No trials reported on costs of strict bed rest in hospital to health services or costs of the intervention to women and their families.
Comparison two: partial bed rest in hospital versus no activity restriction at home
Partial bed rest in hospital, where women were encouraged to rest for several hours during the day, was compared with no activity restriction at home in one trial including 141 women with an uncomplicated twin pregnancy (MacLennan 1990).
Primary outcomes
Very preterm birth (less than 34 weeks' gestation)
There was no evidence of an effect of partial bed rest in hospital on the incidence of very preterm birth (RR 2.30, 95% CI 0.84 to 6.27, one trial, 141 women, low‐quality evidence; Analysis 2.1).
2.1. Analysis.
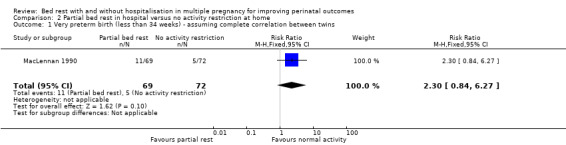
Comparison 2 Partial bed rest in hospital versus no activity restriction at home, Outcome 1 Very preterm birth (less than 34 weeks) ‐ assuming complete correlation between twins.
Perinatal mortality
No evidence of a difference between partial bed rest in hospital compared with normal activity at home was observed when assuming independence between twins (RR 4.17, 95% CI 0.90 to 19.31, one trial, 282 neonates, low‐quality evidence) and complete correlation between twins (RR 4.17, 95% CI 0.48 to 36.42, one trial, 141 twins; Analysis 2.2).
2.2. Analysis.
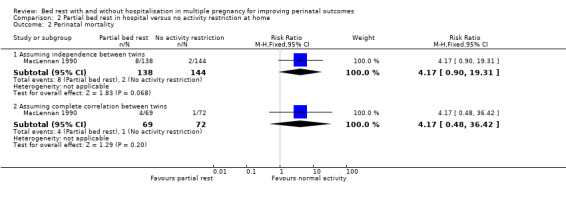
Comparison 2 Partial bed rest in hospital versus no activity restriction at home, Outcome 2 Perinatal mortality.
Low birthweight (less than 2500 g)
This outcome was not reported by MacLennan 1990.
Maternal secondary outcomes
PPROM
There was no evidence of a difference in PPROM between the intervention and control group (RR 1.51, 95% CI 0.69 to 3.30, one trial, 141 women, low‐quality evidence; Analysis 2.3).
2.3. Analysis.
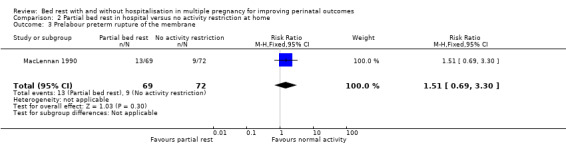
Comparison 2 Partial bed rest in hospital versus no activity restriction at home, Outcome 3 Prelabour preterm rupture of the membrane.
Spontanenous onset of labour
There was no difference in the spontaneous onset of labour between groups (RR 1.03, 95% CI 0.89 to 1.18, one trial, 141 women; Analysis 2.4).
2.4. Analysis.
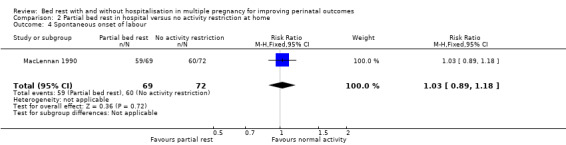
Comparison 2 Partial bed rest in hospital versus no activity restriction at home, Outcome 4 Spontaneous onset of labour.
Caesarean delivery
Partial bed rest in hospital compared with no activity restriction at home showed no effect on the rate of caesarean delivery (RR 1.10, 95% CI 0.81 to 1.49, one trial, 141 women; Analysis 2.5).
2.5. Analysis.
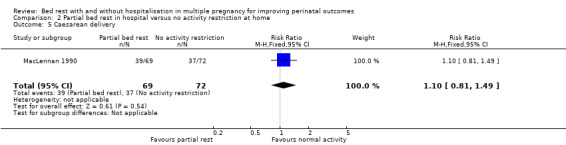
Comparison 2 Partial bed rest in hospital versus no activity restriction at home, Outcome 5 Caesarean delivery.
Development of maternal hypertension
Partial bed rest in hospital reduced the development of maternal hypertension compared with women who were encouraged to perform normal activity at home (RR 0.30, 95% CI 0.16 to 0.59, P = 0.0004, one trial, 141 women; Analysis 2.6).
2.6. Analysis.
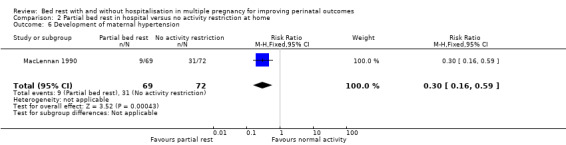
Comparison 2 Partial bed rest in hospital versus no activity restriction at home, Outcome 6 Development of maternal hypertension.
Other maternal secondary outcomes
The trial did not report on development of venous thromboembolism, women's assessment and satisfaction of care, quality of life (experience and feeling) during bed rest or physiological effects. Only women who had been hospitalised for partial bed rest were questioned about their inpatient experience: four women appreciated admission and nine women found it psychologically distressing and left the hospital early.
Fetal or infant secondary outcomes
Stillbirth
There was no effect of partial bed rest in hospital on the incidence of stillbirth (RR 5.22, 95% CI 0.62 to 44.09, one trial, 282 neonates). Assuming complete correlation between twins, the results also showed no evidence of an effect of the intervention on stillbirth (RR 3.13, 95% CI 0.33 to 29.37, one trial, 141 twins; Analysis 2.7).
2.7. Analysis.
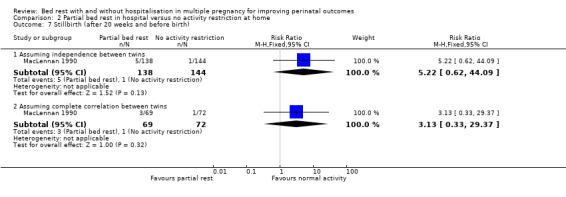
Comparison 2 Partial bed rest in hospital versus no activity restriction at home, Outcome 7 Stillbirth (after 20 weeks and before birth).
Early neonatal death
Compared with control, partial bed rest in hospital showed no evidence of an effect on the incidence of early neonatal death when assuming independence of twins (RR 3.13, 95% CI 0.33 to 29.73, one trial, 282 neonates) or complete correlation (RR 2.09, 95% CI 0.19 to 22.50, one trial, 141 twins; Analysis 2.8).
2.8. Analysis.
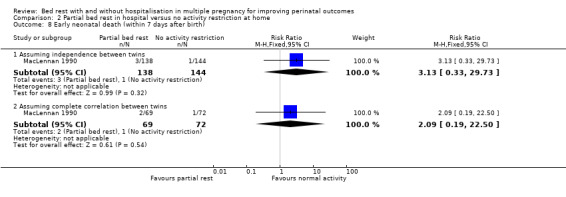
Comparison 2 Partial bed rest in hospital versus no activity restriction at home, Outcome 8 Early neonatal death (within 7 days after birth).
Preterm birth (less than 37 weeks' gestation)
There was no evidence of a difference between intervention and control group in the incidence of birth before 37 weeks' gestation (RR 1.07, 95% CI 0.79 to 1.46, one trial, 141 women; Analysis 2.9).
2.9. Analysis.
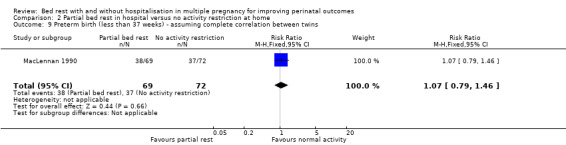
Comparison 2 Partial bed rest in hospital versus no activity restriction at home, Outcome 9 Preterm birth (less than 37 weeks) ‐ assuming complete correlation between twins.
Birthweight (g)
Partial bed rest in hospital versus normal activity at home did not result in changes in the mean birthweight for twin I (fixed‐effect MD ‐90.00, 95% CI ‐299.40 to 119.40, one trial, 141 neonates; Analysis 2.10) and twin II (fixed‐effect MD ‐80.00, 95% CI ‐294.84 to 134.84, one trial, 141 neonates; Analysis 2.11).
2.10. Analysis.
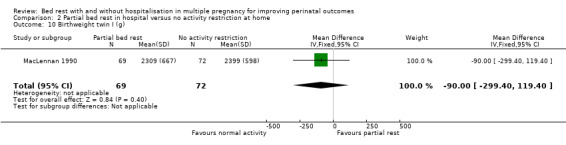
Comparison 2 Partial bed rest in hospital versus no activity restriction at home, Outcome 10 Birthweight twin I (g).
2.11. Analysis.
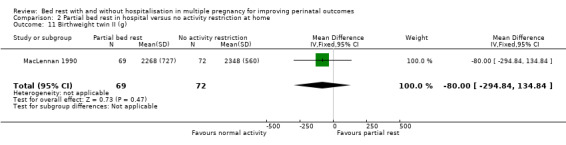
Comparison 2 Partial bed rest in hospital versus no activity restriction at home, Outcome 11 Birthweight twin II (g).
Very low birthweight (less than 1500 g)
Assuming independence between twins as well as complete correlation, there was no evidence of a difference between the partial bed‐rest group and control group (RR 1.74, 95% CI 0.88 to 3.42, one trial 282 neonates, low‐quality evidence, and RR 1.74, 95% CI 0.67 to 4.53, one trial, 141 twins, respectively) (Analysis 2.12).
2.12. Analysis.
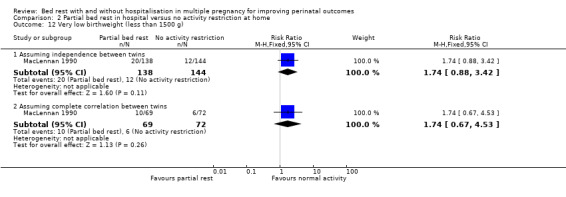
Comparison 2 Partial bed rest in hospital versus no activity restriction at home, Outcome 12 Very low birthweight (less than 1500 g).
Gestational age at delivery (weeks)
No differences were observed in mean gestational age at delivery between intervention and control group (MD ‐0.60, 95% CI ‐1.56 to 0.36, one trial, 141 women; Analysis 2.13).
2.13. Analysis.
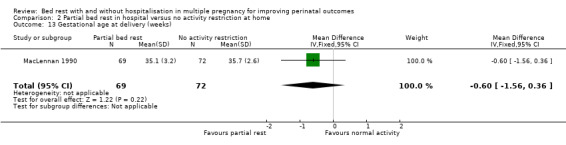
Comparison 2 Partial bed rest in hospital versus no activity restriction at home, Outcome 13 Gestational age at delivery (weeks).
Small‐for‐gestational age
There was no evidence of a difference in small‐for‐gestational age between the intervention and control group (RR 0.93, 95% CI 0.69 to 1.26, one trial, 282 neonates, low‐quality evidence). Assuming complete correlation between twins showed a similar result (RR 0.93, 95% CI 0.61 to 1.43, one trial, 141 twins; Analysis 2.14).
2.14. Analysis.
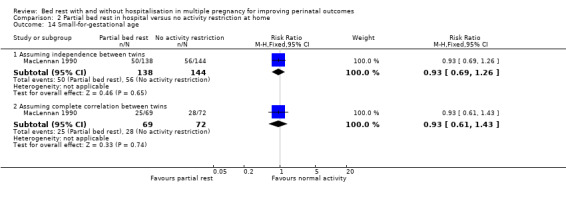
Comparison 2 Partial bed rest in hospital versus no activity restriction at home, Outcome 14 Small‐for‐gestational age.
Apgar score of less than seven at five minutes
The results for comparing partial bed rest in hospital with no activity restriction at home showed no evidence of a difference in Apgar score of less than seven at five minutes for twin I (RR 2.09, 95% CI 0.19 to 22.50, one trial, 141 neonates; Analysis 2.15) or twin II (RR 2.09, 95% CI 0.39 to 11.03, one trial, 141 neonates; Analysis 2.16).
2.15. Analysis.
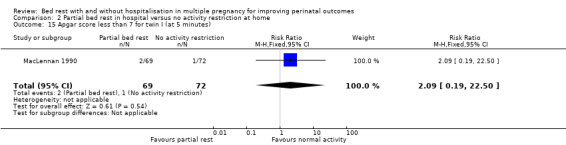
Comparison 2 Partial bed rest in hospital versus no activity restriction at home, Outcome 15 Apgar score less than 7 for twin I (at 5 minutes).
2.16. Analysis.
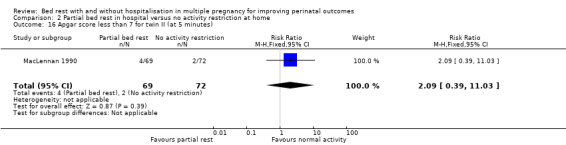
Comparison 2 Partial bed rest in hospital versus no activity restriction at home, Outcome 16 Apgar score less than 7 for twin II (at 5 minutes).
Admission to NICU and neonatal stay at NICU (seven days or more)
This trial did not report admission to NICU and neonatal stay at NICU of more than seven days.
Other secondary outcomes
The trials did not report on costs of strict bed rest in hospital to health services or costs of the intervention to women and their families.
Discussion
Summary of main results
We aimed to assess the effectiveness of bed rest in hospital or at home to improve pregnancy outcomes in women with a multiple pregnancy. This review replaces an earlier Cochrane review (Crowther 2010). We included six trials, involving 636 women and 1298 babies. Four trials evaluated the potential effect of the intervention for women with a twin pregnancy (610 women and 1220 babies) and two trials for women with a triplet pregnancy (26 women and 78 babies). Five trials compared strict bed rest in hospital with normal activity at home. We adjusted fetal and infant outcomes for non‐independence where outcomes for individual babies from one pregnancy could potentially be different and there were no differences between unadjusted (assuming independence of twins/triplets) and adjusted (assuming complete correlation between twins/triplets) results. There were no differences in the incidence of very preterm birth, perinatal mortality or low birthweight between women assigned to strict bed rest in hospital compared with no activity restriction at home. However, the rate of spontaneous onset of labour was increased in the strict bed‐rest group. Furthermore, women assigned to strict bed rest in hospital had twins/triplets with a higher mean birthweight compared with women encouraged to continue with their normal activity at home. Only one trial assessed the effect of partial bed rest in hospital compared with no activity restriction at home. There was no evidence of a difference in the incidence of very preterm birth or perinatal mortality. Low birthweight was not reported in this trial. Although there was evidence of decreased risk for development of maternal hypertension for women admitted to hospital for partial bed rest, this result needs to be interpreted with caution as it was derived from only one study with a small number of women. Currently, the available evidence from the studies is insufficient to support or refute routine bed rest in hospital or at home for women with a multiple pregnancy to improve outcomes.
Overall completeness and applicability of evidence
All included trials focused primarily on the prevention of preterm birth in multiple pregnancy and the prolongation of gestation through routine admission to hospital for bed rest. All trials reported on preterm and very preterm birth, perinatal mortality (stillbirth and early neonatal death), mean gestational age at delivery and development of pregnancy‐induced hypertension.
Women may be advised to rest in bed for variable durations which can lead to the development of adverse effects due to the prolonged immobilisation. None of the trials reported on adverse outcomes such as the development of thromboembolism, loss of muscle mass or cardiovascular deconditioning. Likewise, the included studies did not assess long‐term effects of the intervention for women and infants. Women's assessment and satisfaction of care, women's views about quality of life during bed rest and psychosocial effects were not evaluated in any of the included trials. However, the limited information provided from one trial (MacLennan 1990) suggested that women admitted to hospital for bed rest more often experienced psychosocial distress. Hospitalisation for bed rest also had a financial impact for women and their families, as well as for their public health system, but there were no trials exploring the costs of the intervention.
Four out of the six studies were conducted in Harare, Zimbabwe. The remaining two trials were set in Australia. Due to the small number of trials and limited geographical distribution of the study population, the results are not directly generalisable and applicable to other settings.
Quality of the evidence
For the six included studies, the overall risk of bias was low. Random sequence generation and allocation concealment was adequate in almost all trials. Most of the studies were judged to be low risk of bias for detection and attrition bias as well as other sources of bias. All studies were unclear risk of bias for performance bias due to the type of intervention.
The quality of the available evidence was evaluated using the GRADE methodology and is presented in the 'Summary of findings' tables (Table 1; Table 2) for the primary outcomes, very preterm birth, perinatal mortality, low birthweight, and the secondary outcomes, small‐for‐gestational age and PPROM. We planned to use GRADE for psychosocial effects of bed rest; however, none of the trials reported this outcome.
The quality of the evidence as assessed using GRADE was low for very preterm birth, perinatal mortality, low birthweight, small‐for‐gestational age, and PPROM in the comparison of strict bed rest in hospital versus no activity restriction at home. These outcomes were downgraded due to wide 95% confidence intervals crossing the line of no effect (‐2), with the exception of low birthweight which was downgraded for wide 95% CIs crossing the line of no effect (‐1) and also due to high heterogeneity (I²=64%) (‐1). For the comparison of partial bed rest in hospital versus no activity restriction at home, the quality of evidence was low for all listed outcomes due to very wide 95% confidence intervals, a very small number of events, and the results coming from only a single study. We did not downgrade for the lack of blinding (unclear for participants and personnel for all studies) because the outcomes were not likely to be influenced by lack of blinding.
Potential biases in the review process
We attempted to minimise potential biases in the review process by following the Cochrane Pregnancy and Childbirth search strategies and recommended review processes. We did not apply language or date restrictions to the search. Two review authors assessed identified studies for inclusion. Additionally, two review authors independently performed data extraction and risk of bias assessment.
Agreements and disagreements with other studies or reviews
Several Cochrane reviews have evaluated the use of bed rest for various pregnancy complications to improve maternal and infant outcomes. In Sosa 2015, the effect of bed rest for women with a singleton pregnancy and high risk of preterm birth was compared with no intervention. The analysis resulted from one study and found no evidence of a difference in the risk of preterm birth. The review concluded that there was no evidence to support or refute the policy of bed rest at home or in hospital and further evaluation was needed.
In another review, comparing bed rest in hospital to ambulatory management for 107 women with suspected impaired fetal growth, no differences for fetal growth parameters and neonatal outcomes were observed (Say 1996). In contrast, Bell 1994 reported that moderate to high levels of sustained maternal exercise was associated with reduced birthweight due to changes in uteroplacental flow and reduced levels of maternal glucose. Bed rest with or without hospitalisation was also recommended for women with hypertension during pregnancy.
Meher 2005 assessed the effect of different levels of bed rest in a hospital setting or at home for pregnancy‐induced hypertension. Two included trials compared strict bed rest with some bed rest in hospital and showed no evidence of a difference between groups for outcomes such as severe hypertension, stillbirth, perinatal and neonatal death, and preterm birth. Another two trials included in this review compared some bed rest in hospital with routine activity at home. Results from one trial involving 218 women showed a reduced risk of severe hypertension and borderline reduction in the risk of preterm birth. This is in agreement with our results, where women on partial bed rest in hospital developed less maternal hypertension compared with women with no activity restriction at home. However, due to the limited number of trials with small numbers of participants and few reported outcomes, the authors concluded that there was insufficient evidence to routinely recommend bed rest for women with hypertension during pregnancy (Meher 2005).
Two small trials including 106 women evaluated the effect of rest during pregnancy for preventing pre‐eclampsia in women with normal blood pressure (Meher 2006). Resting for four to six hours a day, as described in one trial with 32 women, was associated with a risk reduction in pre‐eclampsia, but not pregnancy‐induced hypertension. In the second trial involving 76 women, a 30‐minute rest each day in combination with nutritional supplementation showed a reduction in both outcomes. Other outcomes such as preterm birth, perinatal mortality, women's views or costs were not reported in either trial. Rest during pregnancy may reduce the risk of pre‐eclampsia for women with normal blood pressure, but the small number of participants made it difficult to reach a reliable conclusion. Therefore, the trial authors concluded that there was insufficient evidence to support a policy for bed rest to prevent pre‐eclampsia and taking a rest remains a matter of choice for each woman.
In women at high risk of miscarriage, bed rest is commonly advocated to prevent fetal loss. In a review including two trials with 84 women, a positive effect of bed rest on reducing the risk of miscarriage could not be confirmed and therefore, a policy of bed rest could not be encouraged (Aleman 2005).
In agreement with our study, all these reviews concluded that there was no or insufficient evidence to support the recommendation of bed rest in pregnancy. Results were inconclusive and significant benefits for guiding clinical practice could not be shown. It remains unclear if bed rest causes adverse effects in women with multiple pregnancy or pregnancy complications as these outcomes were poorly reported.
Authors' conclusions
Implications for practice.
There was insufficient evidence to support or refute the routine use of bed rest in hospital to improve pregnancy outcomes for women with a multiple pregnancy. There was no effect of strict or partial bed rest in hospital on very preterm birth, perinatal mortality or low birthweight.
Advising strict bed rest in hospital for women with multiple pregnancy had some positive effects on some of the maternal and infant outcomes. Women with strict bed rest experienced spontaneous onset of labour more often and had babies with a higher mean birthweight compared to women with no activity restriction. However, findings for other maternal and infant outcomes showed no benefit for strict bed rest in hospital for women with a twin or triplet pregnancy.
For women with a twin pregnancy on partial bed rest in hospital, the intervention had no effect on infant outcomes and most maternal outcomes. The development of maternal hypertension was reduced for women advised to partially rest in bed compared with no activity restriction. However, the result was retrieved from a single trial with a limited number of women and needs to be investigated further in order to draw firm conclusions.
Implications for research.
Bed rest with or without hospitalisation is commonly recommended for women with a multiple pregnancy to improve outcomes even though sufficient evidence to support this is not available.
Any further studies should be of high quality, well‐designed, with a large sample size and well‐controlled to investigate the risks and benefits of bed rest. Trials are needed that evaluate different degrees of bed rest with a clear and consistent definition of strict and partial bed rest. Geographically, the studies included in this review were limited to Zimbabwe and Australia. Additional trials should be expanded to other areas and include several centres to increase the number of women with a multiple pregnancy so that the findings can be applied to various populations and settings. Further studies especially need to evaluate adverse effects of the intervention as well as consider long‐term follow‐up of mothers and infants. The focus should also include women's views and experience of care, their quality of life during bed rest, and psychosocial effects. Evaluating the costs for healthcare systems and for women and their families may be an important issue to consider in future studies.
What's new
| Date | Event | Description |
|---|---|---|
| 21 April 2017 | Amended | A citation for the protocol has been added. |
Acknowledgements
We would like to thank Ms Emma Barber for her editorial support.
We acknowledge Cochrane Pregnancy and Childbirth's Information Specialist for her contribution to the template for the search strategy for identification of studies.
As part of the pre‐publication editorial process, this review was commented on by four peers (an editor and three referees who are external to the editorial team), a member of Cochrane Pregnancy and Childbirth's international panel of consumers and the Group's statistical adviser.
This project was supported by the National Institute for Health Research, via Cochrane programme Grant funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Rintaro Mori's institution receives government funding from the Clinical Research Program for Child Health and Development, AMED, Japan to provide support for the PCG Satellite in Japan.
Appendices
Appendix 1. Search terms for ICTRP and ClinicalTrials.gov
bedrest AND pregnancy
bedrest AND twin(s)
Data and analyses
Comparison 1. Strict bed rest in hospital versus no activity restriction at home.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Very preterm birth (less than 34 weeks) ‐ assuming complete correlation between twins/triplets | 5 | 495 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.66, 1.58] |
| 2 Perinatal mortality | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Assuming independence between twins/triplets | 5 | 1016 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.35, 1.21] |
| 2.2 Assuming complete correlation between twins/triplets | 5 | 495 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.30, 1.63] |
| 3 Low birthweight (less than 2500 g) | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Assuming independence between twins/triplets | 3 | 502 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.75, 1.21] |
| 3.2 Assuming complete correlation between twins/triplets | 3 | 238 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.74, 1.11] |
| 4 Low birthweight (less than 2500 g) ‐ subgroup analysis | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Twin pregnancy | 1 | 424 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.66, 1.07] |
| 4.2 Triple pregnancy | 2 | 78 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.64, 1.86] |
| 5 Prelabour preterm rupture of the membrane | 3 | 276 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.71, 2.38] |
| 6 Spontaneous onset of labour | 4 | 488 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [1.02, 1.09] |
| 7 Caesarean delivery | 4 | 283 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.41, 1.17] |
| 8 Development of maternal hypertension | 5 | 495 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.37, 1.23] |
| 9 Stillbirth (after 20 weeks and before birth) | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 9.1 Assuming independence between twins/triplets | 5 | 1016 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.20, 2.98] |
| 9.2 Assuming complete correlation between twins/triplets | 5 | 495 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.25, 2.69] |
| 10 Early neonatal death (within 7 days after birth) | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 Assuming independence between twins/triplets | 5 | 1016 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.28, 1.87] |
| 10.2 Assuming complete correlation between twins/triplets | 5 | 495 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.27, 2.74] |
| 11 Preterm birth (less than 37 weeks) ‐ assuming complete correlation between twins/triplets | 5 | 495 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.80, 1.18] |
| 12 Birthweight (g) | 3 | 314 | Mean Difference (IV, Fixed, 95% CI) | 136.99 [39.92, 234.06] |
| 13 Very low birthweight (less than 1500 g) | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 13.1 Assuming independence between twins/triplets | 2 | 445 | Risk Ratio (M‐H, Random, 95% CI) | 1.32 [0.15, 11.70] |
| 13.2 Assuming complete correlation between twins/triplets | 2 | 219 | Risk Ratio (M‐H, Random, 95% CI) | 1.64 [0.31, 8.70] |
| 14 Gestational age at delivery (weeks) | 5 | 495 | Mean Difference (IV, Fixed, 95% CI) | ‐0.14 [‐0.51, 0.24] |
| 15 Small‐for‐gestational age | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 15.1 Assuming independence between twins/triplets | 2 | 293 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.56, 1.01] |
| 15.2 Assuming complete correlation between twins/triplets | 2 | 137 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.46, 1.10] |
| 16 Apgar score less than 7 (at 5 minutes) | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 16.1 Assuming independence between twins/triplets | 2 | 299 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.60 [0.55, 4.62] |
| 16.2 Assuming complete correlation between twins/triplets | 2 | 146 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.34, 5.32] |
| 17 Admission to neonatal intensive care unit | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 17.1 Assuming independence between twins/triplets | 3 | 571 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.74, 1.06] |
| 17.2 Assuming complete correlation between twins/triplets | 3 | 276 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.68, 1.14] |
Comparison 2. Partial bed rest in hospital versus no activity restriction at home.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Very preterm birth (less than 34 weeks) ‐ assuming complete correlation between twins | 1 | 141 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.30 [0.84, 6.27] |
| 2 Perinatal mortality | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Assuming independence between twins | 1 | 282 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.17 [0.90, 19.31] |
| 2.2 Assuming complete correlation between twins | 1 | 141 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.17 [0.48, 36.42] |
| 3 Prelabour preterm rupture of the membrane | 1 | 141 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.51 [0.69, 3.30] |
| 4 Spontaneous onset of labour | 1 | 141 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.89, 1.18] |
| 5 Caesarean delivery | 1 | 141 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.81, 1.49] |
| 6 Development of maternal hypertension | 1 | 141 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.16, 0.59] |
| 7 Stillbirth (after 20 weeks and before birth) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Assuming independence between twins | 1 | 282 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.22 [0.62, 44.09] |
| 7.2 Assuming complete correlation between twins | 1 | 141 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.13 [0.33, 29.37] |
| 8 Early neonatal death (within 7 days after birth) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 Assuming independence between twins | 1 | 282 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.13 [0.33, 29.73] |
| 8.2 Assuming complete correlation between twins | 1 | 141 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.09 [0.19, 22.50] |
| 9 Preterm birth (less than 37 weeks) ‐ assuming complete correlation between twins | 1 | 141 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.79, 1.46] |
| 10 Birthweight twin I (g) | 1 | 141 | Mean Difference (IV, Fixed, 95% CI) | ‐90.0 [‐299.40, 119.40] |
| 11 Birthweight twin II (g) | 1 | 141 | Mean Difference (IV, Fixed, 95% CI) | ‐80.0 [‐294.84, 134.84] |
| 12 Very low birthweight (less than 1500 g) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 12.1 Assuming independence between twins | 1 | 282 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.74 [0.88, 3.42] |
| 12.2 Assuming complete correlation between twins | 1 | 141 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.74 [0.67, 4.53] |
| 13 Gestational age at delivery (weeks) | 1 | 141 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐1.56, 0.36] |
| 14 Small‐for‐gestational age | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 14.1 Assuming independence between twins | 1 | 282 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.69, 1.26] |
| 14.2 Assuming complete correlation between twins | 1 | 141 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.61, 1.43] |
| 15 Apgar score less than 7 for twin I (at 5 minutes) | 1 | 141 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.09 [0.19, 22.50] |
| 16 Apgar score less than 7 for twin II (at 5 minutes) | 1 | 141 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.09 [0.39, 11.03] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Crowther 1989.
| Methods | Randomisation and allocation concealment: the study used block randomisation without stratification to provide balanced numbers in the study groups. Use of consecutively‐numbered, opaque, sealed envelopes. Researchers involved in allocating the women to the treatment groups were not involved in preparing the randomisation schedule. Blinding of outcome assessment: paediatrician examining newborn infants was unaware to which group the mother had been allocated. Documentation of exclusion: no losses to follow‐up. |
|
| Participants | 139 women with a twin pregnancy and a cervical score of ‐2 or less at or before 34 weeks' gestation attending a special antenatal clinic for multiple pregnancy. Cervical score is length minus dilatation (cm) of the cervix. |
|
| Interventions | Women allocated to the intervention group received bed rest in the hospital. Women allocated to the control group continued conventional outpatient management and were only admitted to the hospital if pregnancy complications occurred. |
|
| Outcomes | Primary outcomes
Seconcary outcomes
|
|
| Notes | Chorionicity: not reported Sample‐size calculation: reported, 44 women for a 40% (from 80%‐40%) reduction of preterm delivery rate at the 5% level Intention‐to‐treat analyses: performed Compliance: 2 women (3%) in the hospitalisation group did not attend the antenatal ward and 17 women (25%) in the control group were admitted to hospital because of pregnancy complications Location: single centre in Harare, Zimbabwe Timeframe: 1984 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation without stratification to provide balanced numbers in the study groups. Use of a series of consecutively‐numbered, opaque, sealed envelopes. p 851 |
| Allocation concealment (selection bias) | Low risk | "The researchers involved in allocating the women to the treatment groups were not involved in preparing the randomisation schedule." p 851 |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of participants and personnel was not possible due to the type of intervention (hospitalisation and bed rest compared with no activity restriction at home) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "All newborn infants were examined by a paediatrician who was unaware to which group the mother had been allocated." p 851 |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up and analysis on an intention‐to‐treat basis |
| Selective reporting (reporting bias) | Unclear risk | No information provided about trial protocol or predefined outcomes |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Crowther 1990.
| Methods | Randomisation and allocation concealment: block randomisation with no stratification and use of numbered, opaque, sealed envelopes. Researchers involved in treatment allocation were not involved in making the randomisation schedule. Blinding of outcome assessment: outcomes were assessed by staff who were unaware of participants' allocation to intervention and control group Documentation of exclusion: no loss to follow‐up |
|
| Participants | 118 women with an uncomplicated twin pregnancy between 28 and 30 weeks' gestation attending a special antenatal clinic for multiple pregnancy were recruited into the study. Women were not eligible for inclusion if they had a cervical suture, hypertension, a caesarean section scar, an antepartum haemorrhage or uncertain gestational age. |
|
| Interventions | Women allocated to the intervention group received bed rest in hospital and were encouraged to rest in bed as much as possible, although voluntary ambulation was allowed. Women in the control group were encouraged to continue their normal activities at home. They were admitted to the hospital only if complications arose such as hypertension, preterm labour or prelabour rupture of the membranes. All women received weekly antenatal assessment. |
|
| Outcomes | Primary outcomes
Secondary outcomes
|
|
| Notes | Chorionicity: not reported Sample‐size calculation: reported, 100 women for a reduction of preterm delivery from 50% to 28% and 200 women for a reduction in the incidence of small‐for‐gestational age from 35% to 17.5%, with 80% power Intention‐to‐treat analyses: performed Compliance: 4 women (7%) in the hospitalisation group did not attend for hospitalisation and 11 women (19%) required leave of absence from the hospital. In the control group, 22 women (37%) subsequently required hospitalisation because of pregnancy complications. Location: single centre in Harare, Zimbabwe Timeframe: 1984‐1986 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation with no stratification and use of numbered, opaque, sealed envelopes. p 873 |
| Allocation concealment (selection bias) | Low risk | "..the researchers involved in treatment allocation were not involved in making the randomisation schedule." p 873 |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of participants and personnel was not possible due to the type of intervention (hospitalisation and bed rest compared with no activity restriction at home) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "The gestational age assessments were made by a neonatologist who was unaware of the allocation treatment group." "The other primary measures of outcomes were measurements or decisions taken by staff who were unaware of the group to which the mother had been allocated." p 873 |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up and analysis on an intention‐to‐treat basis |
| Selective reporting (reporting bias) | Unclear risk | No information provided about trial protocol or predefined outcomes |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Crowther 1991.
| Methods | Randomisation and allocation concealment: block randomisation and use of consecutively‐numbered, opaque, sealed envelopes. Researchers involved in treatment allocation were not involved in preparing the randomisation schedule. Blinding of outcome assessment: the paediatricians examining the newborn infants were unaware of the participants' treatment allocation. Documentation of exclusion: no loss to follow‐up |
|
| Participants | 19 women with a confirmed triplet pregnancy from 24 weeks' gestation onwards attending a special antenatal clinic for multiple pregnancy were recruited into the study. Women with an uncertain gestational age, cervical suture, hypertension, caesarean section scar or antepartum haemorrhage were excluded. |
|
| Interventions | Women allocated to the intervention group received bed rest in hospital from 24 weeks' gestation onwards until delivery. Women were encouraged to rest in bed as much as possible although ambulation was allowed. Women allocated to the control group were encouraged to continue their normal activities at home. They were admitted to the hospital only if complications arose such as preterm labour, hypertension, or preterm rupture of membranes. All women received weekly antenatal assessment |
|
| Outcomes | Primary outcomes
Secondary outcomes
|
|
| Notes | Chorionicity: not reported Sample‐size calculation: not performed Intention‐to‐treat analyses: performed Compliance: no women in the hospitalisation group required leave of absence from the hospital. 6 women (67%) in the control group subsequently required admission to the hospital because of pregnancy complications Location: single centre in Harare, Zimbabwe Timeframe: 1984‐1986 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation and use of consecutively numbered, opaque, sealed envelopes. p 64 |
| Allocation concealment (selection bias) | Low risk | "...researchers involved in treatment allocation were not involved in preparing the randomisation schedule." p 64 |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of participants and personnel was not possible due to the type of intervention (hospitalisation and bed rest compared with no activity restriction at home) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "All newborn infants were examined by a paediatrician who was unaware to which group the mother had been allocated." p 64 |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up and analysis on an intention‐to‐treat basis |
| Selective reporting (reporting bias) | Low risk | "The main outcomes were prespecified..." p 64 |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Dodd 2005.
| Methods | Randomisation and allocation concealment: randomisation schedule used variable blocks with stratification by parity. An independent researcher responsible for the treatment allocation was contacted by telephone, and the next in a series of consecutively numbered, opaque, sealed envelopes opened. Blinding of outcome assessment: unclear, no information given about blinding of outcome assessors Documentation of exclusion: no loss to follow‐up |
|
| Participants | 7 women with a triplet pregnancy with ultrasound‐confirmed gestational age less than 24 weeks' gestation were recruited into the study. Women with a triplet pregnancy and any other condition requiring hospitalisation (e.g. placenta praevia) were excluded. |
|
| Interventions | Women allocated to the hospitalisation group were admitted from 24 weeks' gestation until 30 weeks' gestation, weekend leaves were allowed. Afterwards, women were encouraged to rest as much as possible at home. Women allocated to the control group were encouraged to continue with their normal activities at home. Routine antenatal care was provided. Women were admitted to the hospital if they developed any complications such as preterm labour, PPROM or pregnancy‐induced hypertension. |
|
| Outcomes | Primary outcomes
Secondary outcomes
|
|
| Notes | Chorionicity: not reported Sample‐size calculation: reported, 400 women for a reduction in the occurrence of very preterm birth from 44% to 30% and 52 women for a reduction of preterm birth from 100% to 80%, with 80% power Intention‐to‐treat analyses: performed Compliance: 3 of 4 women in the control group were admitted to the hospital during their antenatal course Location: single centre in Adelaide, Australia Timeframe: 1996‐2003 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomisation schedule used variable blocks with stratification by parity..." p 2 |
| Allocation concealment (selection bias) | Low risk | "...an independent researcher responsible for the treatment allocation was contacted by telephone, and the next in a series of consecutively numbered, opaque, sealed envelopes opened." p 2 |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of participants and personnel was not possible due to the type of intervention (hospitalisation and bed rest compared with no activity restriction at home) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It is unclear if outcome assessors were unaware of the participants' allocation to the intervention and control group |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up and analysis on an intention‐to‐treat basis |
| Selective reporting (reporting bias) | Unclear risk | No study protocol available |
| Other bias | Low risk | The study appears to be free of other sources of bias |
MacLennan 1990.
| Methods | Randomisation and allocation concealment: computer‐generated list of random numbers. The person designating the management was unaware of the management associated with the number until after enrolment. Blinding of outcome assessment: unclear, no information given about blinding of outcome assessors Documentation of exclusion: no loss to follow‐up |
|
| Participants | 141 women with an uncomplicated twin pregnancy (about 16‐19 weeks' gestation confirmed by ultrasound) were recruited into the study. Women were not enrolled when they would not accept possible hospital admission or had pre‐existing hypertension (> 90 mmHg diastolic), polyhydramnios, antepartum haemorrhage, preterm labour or membrane rupture in the current pregnancy |
|
| Interventions | Women allocated to the intervention group were admitted to the hospital at 26 weeks' gestation for 4 weeks. Weekend leave was allowed and strict bed rest in hospital was not advocated. Women allocated to the control group were advised to continue normal home duties and visit the clinic every 2 weeks. |
|
| Outcomes |
|
|
| Notes | Chorionicity: not reported Sample‐size calculation: reported, 188 women for each group to detect differences in percentage under 1500 g, 69 for percentage born before 32 weeks' gestation, and 40 for a reduction in the mean nursery stay. Intention‐to‐treat analyses: performed Compliance: 6 women (9%) in the intervention group were not able to follow hospitalisation and bed rest because 2 delivered before admission and for 4 women social circumstances changed. Of the 63 women admitted, 56 remained in the hospital for 3 or more weeks. In the control group, 4 women (6%) were admitted briefly between 26 and 30 weeks' gestation for less than a week Location: multicenter study (11 hospitals), Australia Timeframe: no information |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generated list of random numbers" p 268 |
| Allocation concealment (selection bias) | Low risk | "...the person designating the management was unaware of the management associated with the number until after enrolment." g 268 |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of participants and personnel was not possible due to the type of intervention (hospitalisation and bed rest compared with no activity restriction at home) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome assessors were unaware of participants' allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up and analysis on an intention‐to‐treat basis |
| Selective reporting (reporting bias) | Unclear risk | No information about predefined outcomes or protocol |
| Other bias | High risk | Enrolment of the study was stopped following an interim analysis after the first 128 pregnancies. p 268 |
Saunders 1985.
| Methods | Randomisation and allocation concealment: random allocation was achieved by reference to a consecutively‐numbered series of sealed envelopes. Blinding of outcome assessment: assessment of duration of gestation at delivery was made by labour‐ward staff who were unaware of the group to which individual women had been assigned. Documentation of exclusion: no loss to follow‐up |
|
| Participants | 212 women with a twin pregnancy around 30 weeks' gestation were recruited into the study | |
| Interventions | Women allocated to the intervention group were admitted to the hospital for bed rest from 32 weeks' gestation until the onset of labour Women allocated to the control group were admitted to the hospital only if pregnancy complications occurred |
|
| Outcomes |
|
|
| Notes | Chorionicity: not reported Sample‐size calculation: reported, 100 women in each arm would have a 40% chance of detecting a reduction in the risk of preterm birth from 30% to 20% Intention‐to‐treat analyses: performed Compliance: 11 women (10%) in the intervention group declined hospital admission and 2 women delivered before 32 weeks' gestation. In the control group, 58 women (54%) were admitted to the hospital before labour and 1 women delivered before 32 weeks' gestation Location: single centre in Harare, Zimbabwe Timeframe: no information |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Random allocation was achieved by reference to a consecutively numbered series of sealed envelopes." p 794 |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of participants and personnel was not possible due to the type of intervention (hospitalisation and bed rest compared with no activity restriction at home) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Assessment of duration of gestation at delivery was made by labour‐ward staff who were unaware of the group to which individual women had been assigned." p 794 |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up and analysis on an intention‐to‐treat basis |
| Selective reporting (reporting bias) | Unclear risk | No study protocol available |
| Other bias | Low risk | The study appears to be free of other sources of bias |
NICU: neonatal intensive care unit PPROM: prelabour preterm rupture of the membrane
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Gummerus 1985 | Women were allocated to receive bed rest in hospital or long‐term betamimetic treatment. There was no control group without bed rest or betamimetic treatment for comparison. |
| Gummerus 1987 | In this RCT, all women with a multiple pregnancy were admitted to the hospital for bed rest. Women were allocated to receive salbutamol (orally, 5 times/d) or to receive no drug. |
| Hartikainen‐Sorri 1984 | This is a quasi‐RCT where women were allocated to the intervention and control group according to their year of birth. Quasi‐RCTs were not eligible for inclusion. |
RCT: randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
Al‐Najashi 1996.
| Methods | Randomisation and allocation concealment: no information provided Blinding of outcome assessment: unclear Documentation of exclusion: no loss to follow‐up |
| Participants | 189 women with an uncomplicated twin pregnancy between 22‐26 weeks' gestation were recruited into the study. Women with hypertension, diabetes, cardiac disease or antepartum haemorrhage were excluded. |
| Interventions | Women were allocated into 2 intervention groups.
Women allocated to the control group received no medication or hospitalisation for bed rest and were seen regularly in the outpatient clinic until delivery. No restriction in any of their activities All women received abdominal ultrasound for confirmation of diagnosis of booking, and 1 or 2 subsequent ultrasounds to monitor fetal growth. |
| Outcomes |
|
| Notes | Study design is unclear Chorionicity: not reported Sample‐size calculation: not performed Intention‐to‐treat analyses: performed Compliance: 5 women (8%) in the ritodrine group did not take their tablets at the beginning due to side effects. 12 women (21%) in the hospitalisation group did not complete their hospital stay Location: single centre in Al‐Khobar, Saudi Arabia Timeframe: July 1986‐August 1994 |
Younis 1990.
| Methods | Randomisation and allocation concealment: no information provided Blinding of outcome assessment: unclear Documentation of exclusion: no loss to follow‐up |
| Participants | 132 women with a twin pregnancy between 30‐32 weeks' gestation were recruited into the study. Women suffering from pregnancy complications affecting time of delivery (e.g. hypertensive disorders, antepartum bleeding, premature uterine contractions) were excluded. Women who delivered before 32 weeks' gestation were also excluded. |
| Interventions | Women in the intervention group were electively admitted to the hospital from 30‐32 weeks' gestation and if not delivered, until the end of 36 weeks' gestation. Women were allocated into 2 control groups.
|
| Outcomes |
|
| Notes | Study design is unclear Chorionicity: not reported Sample‐size calculation: not performed Intention‐to‐treat analyses: performed Compliance: no information provided Location: single centre in Jerusalem, Israel Timeframe: November 1979‐October 1986 |
Differences between protocol and review
There are no differences between the protocol da Silva Lopes 2016 and the review.
Contributions of authors
Katharina da Silva Lopes: screened studies for inclusion/exclusion, data extraction, 'Risk of bias' assessment, data analysis, manuscript preparation.
Erika Ota: statistical support, 'Summary of findings' tables, manuscript revision, overall supervision.
Shinji Tanigaki: screened studies for inclusion/exclusion, data extraction, 'Risk of bias' assessment, manuscript revision.
Yo Takemoto: screened studies for inclusion/exclusion, data extraction, 'Risk of bias' assessment, manuscript revision.
Rintaro Mori: statistical support, overall supervision and guarantor of the review.
Sources of support
Internal sources
The Grant of the National Center for Child Health and Development 27B‐10, 26A‐5, Japan.
External sources
Clinical Research Program for Child Health and Development, Japan Agency for Medical Research and Development, AMED No.27300101, Japan.
NIHR Cochrane Programme Grant Project: 13/89/05 – Pregnancy and childbirth systematic reviews to support clinical guidelines, UK.
Grant‐in‐Aid for Young Scientists B Grant Number 26860428, Japan.
Declarations of interest
Katharina da Silva Lopes: none known.
Erika Ota: none known.
Shinji Tanigaki: none known.
Yo Takemoto: none known.
Rintaro Mori: none known.
Edited (no change to conclusions)
References
References to studies included in this review
Crowther 1989 {published data only}
- Crowther CA, Neilson JP, Verkuyl DAA, Bannerman C, Ashurst HM. Preterm labour in twin pregnancies: can it be prevented by hospital admission?. British Journal of Obstetrics and Gynaecology 1989;96:850‐3. [DOI] [PubMed] [Google Scholar]
Crowther 1990 {published data only}
- Crowther CA, Verkuyl DAA, Neilson JP, Bannerman C, Ashurst HM. The effects of hospitalization for rest on fetal growth, neonatal morbidity and length of gestation in twin pregnancy. British Journal of Obstetrics and Gynaecology 1990;97:872‐7. [DOI] [PubMed] [Google Scholar]
Crowther 1991 {published data only}
- Crowther CA, Verkuyl D, Ashworth M, Bannerman C, Ashurst H. The effects of hospitalisation for bed rest on duration of gestation, fetal growth and neonatal morbidity in triplet pregnancy. Acta Geneticae Medicae et Gemellologiae 1991;40:63‐8. [DOI] [PubMed] [Google Scholar]
Dodd 2005 {published data only}
- Dodd JM, Crowther CA. Hospitalisation for bed rest for women with a triplet pregnancy: an abandoned randomised controlled trial with meta‐analysis. BMC Pregnancy and Childbirth 2005;5:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
MacLennan 1990 {published data only}
- MacLennan AH, Green RC, O'Shea R, Brookes C, Morris D. Routine hospital admission in twin pregnancy between 26 and 30 weeks' gestation. Lancet 1990;335:267‐9. [DOI] [PubMed] [Google Scholar]
Saunders 1985 {published data only}
- Saunders MC, Dick JS, Brown IM, McPherson K, Chalmers I. The effects of hospital admission for bed rest on the duration of twin pregnancy: a randomised trial. Lancet 1985;2:793‐5. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Gummerus 1985 {published data only}
- Gummerus M, Halonen O. The value of bed rest and beta‐sympathomimetic treatment in multiple pregnancies. Duodecim 1985;101:1966‐71. [PubMed] [Google Scholar]
Gummerus 1987 {published data only}
- Gummerus M, Halonen O. Prophylactic long‐term oral tocolysis of multiple pregnancies. British Journal of Obstetrics and Gynaecology 1987;94:249‐51. [DOI] [PubMed] [Google Scholar]
Hartikainen‐Sorri 1984 {published data only}
- Hartikainen‐Sorri AL, Jouppila P. Is routine hospitalization needed in antenatal care of twin pregnancy?. Journal of Perinatal Medicine 1984;12:31‐4. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Al‐Najashi 1996 {published data only}
- Al‐Najashi SS, Al‐Mulhim AA. Prolongation of pregnancy in multiple pregnancy. International Journal of Gynecology & Obstetrics 1996;54:131‐5. [DOI] [PubMed] [Google Scholar]
Younis 1990 {published data only}
- Younis JS, Sadovsky E, Eldar‐Geva T, Mildwidsky A, Zeevi D, Zajicek G. Twin gestations and prophylactic hospitalization. International Journal of Gynecology & Obstetrics 1990;32:325‐30. [DOI] [PubMed] [Google Scholar]
Additional references
Aleman 2005
- Aleman A, Althabe F, Belizán J M, Bergel E. Bed rest during pregnancy for preventing miscarriage. Cochrane Database of Systematic Reviews 2005, Issue 2. [DOI: 10.1002/14651858.CD003576.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Barker 2004
- Barker DJ. The developmental origins of adult disease. Journal of the American College of Nutrition 2004;23(6 Suppl):588S‐95S. [PUBMED: 15640511] [DOI] [PubMed] [Google Scholar]
Beemsterboer 2006
- Beemsterboer SN, Homburg R, Gorter NA, Schats R, Hompes PG, Lambalk CB. The paradox of declining fertility but increasing twinning rates with advancing maternal age. Human Reproduction (Oxford, England) 2006;21(6):1531‐2. [PUBMED: 16497698] [DOI] [PubMed] [Google Scholar]
Bell 1994
- Bell R, O'Neill M. Exercise and pregnancy: a review. Birth 1994;21(2):85‐95. [DOI] [PubMed] [Google Scholar]
Black 2010
- Black M, Bhattacharya S. Epidemiology of multiple pregnancy and the effect of assisted conception. Seminars in Fetal & Neonatal Medicine 2010;15(6):306‐12. [PUBMED: 20630816] [DOI] [PubMed] [Google Scholar]
Blondel 2002
- Blondel B, Kaminski M. Trends in the occurrence, determinants, and consequences of multiple births. Seminars in Perinatology 2002;26(4):239‐49. [PUBMED: 12211614] [DOI] [PubMed] [Google Scholar]
Brubaker 2012
- Brubaker SG, Gyamfi C. Prediction and prevention of spontaneous preterm birth in twin gestations. Seminars in Perinatology 2012;36(3):190‐4. [PUBMED: 22713500] [DOI] [PubMed] [Google Scholar]
Campbell 1998
- Campbell DM. Epidemiology of twinning. Current Obstetrics and Gynecology 1998;8:126‐34. [Google Scholar]
Goldenberg 1994
- Goldenberg RL, Cliver SP, Bronstein J, Cutter GR, Andrews WW, Mennemeyer ST. Bed rest in pregnancy. Obstetrics and Gynecology 1994;84(1):131‐6. [PUBMED: 8008308] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
- Higgins JPT, Deeks JJ, Altman DG (editors). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Imaizumi 2003
- Imaizumi Y. A comparative study of zygotic twinning and triplet rates in eight countries, 1972‐1999. Journal of Biosocial Science 2003;35(2):287‐302. [PUBMED: 12664963] [DOI] [PubMed] [Google Scholar]
Kovacevich 2000
- Kovacevich GJ, Gaich SA, Lavin JP, Hopkins MP, Crane SS, Stewart J, et al. The prevalence of thromboembolic events among women with extended bed rest prescribed as part of the treatment for premature labor or preterm premature rupture of membranes. American Journal of Obstetrics and Gynecology 2000;182(5):1089‐92. [PUBMED: 10819836] [DOI] [PubMed] [Google Scholar]
MacKay 2006
- MacKay AP, Berg CJ, King JC, Duran C, Chang J. Pregnancy‐related mortality among women with multifetal pregnancies. Obstetrics and Gynecology 2006;107(3):563‐8. [PUBMED: 16507925] [DOI] [PubMed] [Google Scholar]
Maloni 1993
- Maloni JA, Chance B, Zhang C, Cohen AW, Betts D, Gange SJ. Physical and psychosocial side effects of antepartum hospital bed rest. Nursing Research 1993;42(4):197‐203. [PUBMED: 8337156] [PubMed] [Google Scholar]
Maloni 2002
- Maloni JA, Schneider BS. Inactivity: symptoms associated with gastrocnemius muscle disuse during pregnancy. AACN Clinical Issues 2002;13(2):248‐62. [PUBMED: 12011597] [DOI] [PubMed] [Google Scholar]
Maloni 2010
- Maloni JA. Antepartum bed rest for pregnancy complications: efficacy and safety for preventing preterm birth. Biological Research for Nursing 2010;12(2):106‐24. [PUBMED: 20798159] [DOI] [PubMed] [Google Scholar]
May 2001
- May KA. Impact of prescribed activity restriction during pregnancy on women and families. Health Care for Women International 2001;22(1‐2):29‐47. [PUBMED: 11813795] [DOI] [PubMed] [Google Scholar]
McKeown 1952
- McKeown T, Record RG. Observations on foetal growth in multiple pregnancy in man. Journal of Endocrinology 1952;8(4):386‐401. [PUBMED: 12990740] [DOI] [PubMed] [Google Scholar]
Meher 2005
- Meher S, Abalos E, Carroli G. Bed rest with or without hospitalisation for hypertension during pregnancy. Cochrane Database of Systematic Reviews 2005, Issue 4. [DOI: 10.1002/14651858.CD003514.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Meher 2006
- Meher S, Duley L. Rest during pregnancy for preventing pre‐eclampsia and its complications in women with normal blood pressure. Cochrane Database of Systematic Reviews 2006, Issue 2. [DOI: 10.1002/14651858.CD005939] [DOI] [PMC free article] [PubMed] [Google Scholar]
Neilson 1988
- Neilson JP, Verkuyl DA, Crowther CA, Bannerman C. Preterm labor in twin pregnancies: prediction by cervical assessment. Obstetrics and Gynecology 1988;72(5):719‐23. [PUBMED: 3173923] [PubMed] [Google Scholar]
Norwitz 2005
- Norwitz ER, Edusa V, Park JS. Maternal physiology and complications of multiple pregnancy. Seminars in Perinatology 2005;29(5):338‐48. [PUBMED: 16360493] [DOI] [PubMed] [Google Scholar]
Pison 2006
- Pison G, D'Addato AV. Frequency of twin births in developed countries. Twin Research and Human Genetics 2006;9(2):250‐9. [PUBMED: 16611495] [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Say 1996
- Say L, Gülmezoglu AM, Hofmeyr GJ. Bed rest in hospital for suspected impaired fetal growth. Cochrane Database of Systematic Reviews 1996, Issue 1. [DOI: 10.1002/14651858.CD000034] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sciscione 2010
- Sciscione AC. Maternal activity restriction and the prevention of preterm birth. American Journal of Obstetrics and Gynecology 2010;202(3):232.e1‐5. [PUBMED: 19766979] [DOI] [PubMed] [Google Scholar]
Senat 1998
- Senat MV, Ancel PY, Bouvier‐Colle MH, Breart G. How does multiple pregnancy affect maternal mortality and morbidity?. Clinical Obstetrics and Gynecology 1998;41(1):78‐83. [PUBMED: 9504226] [DOI] [PubMed] [Google Scholar]
Smits 2011
- Smits J, Monden C. Twinning across the Developing World. PLoS One 2011;6(9):e25239. [PUBMED: 21980404] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sosa 2015
- Sosa CG, Althabe F, Belizan JM, Bergel E. Bed rest in singleton pregnancies for preventing preterm birth. Cochrane Database of Systematic Reviews 2015, Issue 3. [DOI: 10.1002/14651858.CD003581.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Crowther 2001
- Crowther CA. Hospitalisation and bed rest for multiple pregnancy. Cochrane Database of Systematic Reviews 2001, Issue 1. [DOI: 10.1002/14651858.CD000110] [DOI] [PubMed] [Google Scholar]
Crowther 2010
- Crowther CA, Han S. Hospitalisation and bed rest for multiple pregnancy. Cochrane Database of Systematic Reviews 2010, Issue 7. [DOI: 10.1002/14651858.CD000110.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
da Silva Lopes 2016
- Silva Lopes K, Ota E, Tanigaki S, Mori R. Bed rest with and without hospitalisation in multiple pregnancy for improving outcomes. Cochrane Database of Systematic Reviews 2016, Issue 1. [DOI: 10.1002/14651858.CD012031] [DOI] [PMC free article] [PubMed] [Google Scholar]


