Abstract
Background
Foot ulceration is a major problem in people with diabetes and is the leading cause of hospitalisation and limb amputations. Skin grafts and tissue replacements can be used to reconstruct skin defects for people with diabetic foot ulcers in addition to providing them with standard care. Skin substitutes can consist of bioengineered or artificial skin, autografts (taken from the patient), allografts (taken from another person) or xenografts (taken from animals).
Objectives
To determine the benefits and harms of skin grafting and tissue replacement for treating foot ulcers in people with diabetes.
Search methods
In April 2015 we searched: The Cochrane Wounds Specialised Register; the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library); Ovid MEDLINE; Ovid MEDLINE (In‐Process & Other Non‐Indexed Citations); Ovid EMBASE and EBSCO CINAHL. We also searched clinical trial registries to identify ongoing studies. We did not apply restrictions to language, date of publication or study setting.
Selection criteria
Randomised clinical trials (RCTs) of skin grafts or tissue replacements for treating foot ulcers in people with diabetes.
Data collection and analysis
Two review authors independently extracted data and assessed the quality of the included studies.
Main results
We included seventeen studies with a total of 1655 randomised participants in this review. Risk of bias was variable among studies. Blinding of participants, personnel and outcome assessment was not possible in most trials because of obvious differences between the treatments. The lack of a blinded outcome assessor may have caused detection bias when ulcer healing was assessed. However, possible detection bias is hard to prevent due to the nature of the skin replacement products we assessed, and the fact that they are easily recognisable. Strikingly, nearly all studies (15/17) reported industry involvement; at least one of the authors was connected to a commercial organisation or the study was funded by a commercial organisation. In addition, the funnel plot for assessing risk of bias appeared to be asymmetrical; suggesting that small studies with 'negative' results are less likely to be published.
Thirteen of the studies included in this review compared a skin graft or tissue replacement with standard care. Four studies compared two grafts or tissue replacements with each other. When we pooled the results of all the individual studies, the skin grafts and tissue replacement products that were used in the trials increased the healing rate of foot ulcers in patients with diabetes compared to standard care (risk ratio (RR) 1.55, 95% confidence interval (CI) 1.30 to 1.85, low quality of evidence). However, the strength of effect was variable depending on the specific product that was used (e.g. EpiFix® RR 11.08, 95% CI 1.69 to 72.82 and OrCel® RR 1.75, 95% CI 0.61 to 5.05). Based on the four included studies that directly compared two products, no specific type of skin graft or tissue replacement showed a superior effect on ulcer healing over another type of skin graft or tissue replacement.
Sixteen of the included studies reported on adverse events in various ways. No study reported a statistically significant difference in the occurrence of adverse events between the intervention and the control group.
Only two of the included studies reported on total incidence of lower limb amputations. We found fewer amputations in the experimental group compared with the standard care group when we pooled the results of these two studies, although the absolute risk reduction for amputation was small (RR 0.43, 95% CI 0.23 to 0.81; risk difference (RD) ‐0.06, 95% CI ‐0.10 to ‐0.01, very low quality of evidence).
Authors' conclusions
Based on the studies included in this review, the overall therapeutic effect of skin grafts and tissue replacements used in conjunction with standard care shows an increase in the healing rate of foot ulcers and slightly fewer amputations in people with diabetes compared with standard care alone. However, the data available to us was insufficient for us to draw conclusions on the effectiveness of different types of skin grafts or tissue replacement therapies. In addition, evidence of long term effectiveness is lacking and cost‐effectiveness is uncertain.
Keywords: Humans; Wound Healing; Amputation, Surgical; Amputation, Surgical/statistics & numerical data; Diabetic Foot; Diabetic Foot/surgery; Foot Ulcer; Foot Ulcer/surgery; Randomized Controlled Trials as Topic; Skin Transplantation; Skin Transplantation/adverse effects; Skin Transplantation/methods
Plain language summary
Skin grafting and tissue replacement for treating foot ulcers in people with diabetes
Background
Foot ulceration is a major problem in people with diabetes and is the leading cause of hospital admissions and limb amputations. Despite the current variety of strategies available for the treatment of foot ulcers in people with diabetes, not all ulcers heal completely. Additional treatments with skin grafts and tissue replacement products have been developed to help complete wound closure.
Review question
What are the benefits and harms of skin grafting and tissue replacement for treating foot ulcers in people with diabetes?
What we found
We included thirteen randomised studies that compared two types of skin grafts or tissue replacements with standard care and four randomised studies that compared two grafts or tissue replacements with each other. In total 1655 patients were randomised in these seventeen trials. Risk of bias was variable among studies. The biggest drawbacks were the lack of blinding (i.e. patients and investigators were aware who was receiving the experimental therapy and who was receiving the standard therapy), industry involvement and the possibility that small studies were less likely to be published if they reported 'negative' results. Adverse advent rates (harm due to the treatment) varied widely.
Conclusion
Based on the seventeen studies included in this review, skin grafts and tissue replacements, used in conjunction with standard care, increase the healing rate of foot ulcers and lead to slightly fewer amputations in people with diabetes compared with standard care alone. However, evidence of long term effectiveness is lacking and cost‐effectiveness is uncertain. There was not enough evidence for us to be able to recommend a specific type of skin graft or tissue replacement.
This plain language summary is up‐to‐date as of 9 April 2015.
Summary of findings
Summary of findings for the main comparison. Skin grafts and tissue replacements compared to placebo or standard care for treating foot ulcers in people with diabetes.
| Skin grafts and tissue replacements compared to placebo or standard care for treating foot ulcers in people with diabetes | ||||||
| Patient or population: People with diabetes who have foot ulcers Intervention: Skin grafts and tissue replacements Comparison: Standard care | ||||||
| Outcomes | Illustrative comparative risks (95% CI) | Relative effect (95% CI) | No. of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Standard care | Skin grafts and tissue replacement products | |||||
|
Incidence of complete closure of the ulcer (healing rate) Follow‐up: 6 to 16 weeks |
273 per 1000 | 423 per 1000 (354 to 504) | RR 1.55 (1.30 to 1.85) | 1472 (13 studies) | ⊕⊕⊝⊝ LOW | Downgraded to low quality of evidence due to lack of blinding, industry involvement and possible publication bias. Furthermore we found wide confidence intervals for a number of comparisons (imprecision). |
| Time to complete closure of the ulcer | N/A | N/A | N/A | 0 (0 studies) |
N/A | Time to compete healing of the ulcer was reported very heterogeneously. The majority of studies did not used survival analysis and reported hazard ratios, so meta‐analysis was not possible for this outcome and grading the quality of the evidence was not applicable |
| Total incidence of lower limb amputations Follow‐up: 12 weeks |
109 per 1000 | 47 per 1000 (25 to 89) | RR 0.43 (0.23 – 0.81) | 522 (2 studies) | ⊕⊝⊝⊝ VERY LOW | Downgraded by three levels because only two studies reported on this outcome (imprecision) and possible publication bias is present. Furthermore, a longer follow‐up period is necessary to estimate the effect more precisely. |
| The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio; N/A: not applicable | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
Background
Description of the condition
Foot ulceration is a major problem in people with diabetes, and is often caused by a combination of factors such as neuropathy (nerve damage), foot deformity, external trauma or peripheral arterial disease (Boulton 2008; Falanga 2005; Quattrini 2008; Szabo 2009). A diabetic foot ulcer has been defined as a wound of full‐thickness (into the subcutaneous tissue, the innermost layer of the skin) below the ankle, or as a lesion of the foot penetrating through the dermis (the inner layer of the skin), in people with type 1 or type 2 diabetes (Apelqvist 1999; Schaper 2004).
Worldwide, almost 350 million people have been diagnosed with diabetes mellitus, and this number is still increasing (Danaei 2011). The annual incidence of development of a foot ulcer in people with diabetes is 1% to 4%, and the lifetime risk is approximately 12% to 25% (Abbot 2005; Singh 2005). These ulcers are a leading cause of hospitalisation and major amputations (above the ankle joint) (Levin 1998; Pham 2000). About 85% of amputations are preceded by ulceration (Boulton 2008). After amputation there is a high risk of re‐amputation at a higher level on the same limb (Izumi 2009; Skoutas 2009). It is estimated that worldwide there is an amputation due to diabetes every 30 seconds (Game 2012).
Current treatment of foot ulcers in people with diabetes usually consists of pressure off‐loading (keeping weight off the area) (Lewis 2013), debridement (removal of dead tissue) (Edwards 2010), infection control, the use of wound dressings or topical agents (Bergin 2006; Dumville 2013a; Dumville 2013b; Dumville 2013c; Dumville 2013d; Jull 2013), intensive regulation of blood glucose (Fernando 2013), and, in the case of ischaemia, vascular reconstruction (Falanga 2005). Additional treatments such as hyperbaric oxygen therapy (Kranke 2015; Stoekenbroek 2014) and granulocyte‐colony stimulating factor (Cruciani 2013) may also be used. Despite these multidisciplinary treatments, complete healing is not accomplished in 24% to 60% of ulcers (Hinchliffe 2012; Margolis 1999).
Diabetic foot ulceration has a great impact on quality of life and poses a significant burden on the healthcare budget (Nabuurs‐Franssen 2005; Valensi 2005). The direct medical costs of each ulcer can frequently exceed USD 45,000 (Jeffcoate 2003; Jeffcoate 2004; Stockl 2004). The overall long‐term costs attributable to diabetic foot ulceration were analysed over a period of three years (Apelqvist 1995). This showed that the costs, including inpatient care, outpatient care, home care and social services, ranged from USD 16,100 in a person with a healed ulcer without critical ischaemia to USD 63,100 in people who underwent a major amputation.
Description of the intervention
Skin grafts and tissue replacement can be used to treat foot ulcers in people with diabetes by reconstructing the skin defect. Skin substitutes need to be placed on a prepared wound bed to ensure contact between the wound bed and the graft and they take on the functions of the missing skin layer. Before the skin substitute is applied ulcers are usually rinsed and debrided to remove hyperkeratinised (abnormally horny or thickened skin) or necrotic tissue. The method of clinical application of the graft/tissue replacement and the frequency of application depends on the specific product used. Some skin substitutes are designed for temporary wounds coverage and some as a permanent replacement.
Different types of skin grafts and tissue replacements are currently available. These are generally divided into the following categories: autografts (taken from the patient), allografts (taken from one person, given to another) and xenografts (taken from animals), and bioengineered tissue or artificial skin. They are used in a number of ways.
Autografts: skin taken from the patient and placed directly in the bed of the target ulcer (e.g. split‐skin or full‐thickness skin from pinch or mesh grafts).
Allografts and xenografts: skin taken from other humans or animals with a similar skin structure, placed directly in the bed of the target ulcer.
Bioengineered or artificial skin: skin replacement products created in a laboratory from cultures of skin components and cells (e.g. fibroblasts or keratinocytes), and then placed in the bed of the target ulcer.
Grafting and tissue replacement of allogeneic skin are associated with some risk of transmission of infections such as hepatitis or the human immunodeficiency virus (HIV). Even with screening for these diseases in donors, this risk is not eliminated entirely (Falanga 1998).
How the intervention might work
Despite the current variety of strategies available for the treatment of foot ulcers in people with diabetes, not all ulcers achieve complete healing. Additional treatments with skin grafts and tissue replacement products have been developed that aim to promote complete wound closure by reconstructing the skin defect. It is believed that tissue replacements promote complete closure of the ulcer through the addition of extracellular matrices that induce growth factors and cytokine expression, although the exact mechanism underlying the process remains unclear.
Why it is important to do this review
The treatment of foot ulcers in people with diabetes is complex and challenging. Foot ulceration continues to be the leading risk factor for major amputation and is a significant burden to the healthcare system. Delayed ulcer healing is an example of the impaired process of wound healing (inflammation, tissue formation and tissue remodelling) characteristic of people with diabetes. Skin grafts and tissue replacements could function as a temporary cover for ulcers and aid normal wound healing alongside usual care that includes, for example, mechanical pressure relief and, in the case of ischaemia, vascular reconstruction.
There are some reviews on the use of skin replacement therapies for treating foot ulcers in people with diabetes (Blozik 2008; Langer 2009). One review suggests that tissue‐engineered artifical skin products may be cost‐effective in selected patients with chronic wounds (Langer 2009), however no recent review has included a rigorous quality assessment.
This systematic review will examine current evidence for skin grafts and tissue replacement for treating foot ulcers in people with diabetes to inform current practice about effectiveness, costs and safety. The review will help clinicians to make informed decisions about the use of grafting and tissue replacement alongside usual care.
Objectives
To determine the benefits and harms of skin grafting and tissue replacement for treating foot ulcers in people with diabetes.
Methods
Criteria for considering studies for this review
Types of studies
Randomised clinical trials (RCTs), in any setting. We included cross‐over trials only if the outcomes at the point of cross‐over were given.
Types of participants
People (18 years of age or older) with diabetes mellitus types 1 or 2, who have been diagnosed with an open foot ulcer of ischaemic, neuropathic or neuroischaemic aetiology. We excluded trials that also include wounds of different aetiologies, such as burns, if the data for the diabetic foot ulcer subgroups were not reported separately.
Types of interventions
Skin grafts or tissue replacements applied to foot ulcers in people with diabetes. We included studies if they compared different types of skin grafts or tissue replacements, or compared skin grafts or tissue replacements with standard care or placebo.
Types of outcome measures
Primary outcomes
Incidence of complete closure of the foot ulcer (healing rate).
Time to complete closure of the foot ulcer.
Total incidence of lower limb amputations (major and minor amputations with a minimum of one toe removed, defined as amputation above or below the ankle joint, respectively).
Secondary outcomes
Recurrence rate of foot ulcers.
Change in ulcer area.
Incidence of infection.
Quality of life (as measured by a valid scale such as EQ‐5D or SF‐36).
Safety (treatment‐related adverse events).
Costs of treatment.
Search methods for identification of studies
Electronic searches
We searched the following electronic databases to identify reports of relevant clinical trials:
The Cochrane Wounds Specialised Register (searched 09 April 2015);
The Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2015, Issue 3);
Ovid MEDLINE (1946 to 09 April 2015);
Ovid MEDLINE (In‐Process & Other Non‐Indexed Citations) (searched 09 April 2015);
Ovid EMBASE (1974 to 09 April 2015);
EBSCO CINAHL (1982 to 09 April 2015).
The search strategies we used can be found in Appendix 1. We combined the Ovid MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximising version (2008 revision) (Lefebvre 2011). We combined the Ovid EMBASE search with the Ovid EMBASE filter developed by the UK Cochrane Centre (Lefebvre 2011). We combined the EBSCO CINAHL searches with the trial filters developed by the Scottish Intercollegiate Guidelines Network (SIGN 2014). We did not impose any restrictions with respect to language, date of publication or study setting.
We searched the following clinical trials registries in an effort to identify published, unpublished and ongoing trials:
ClinicalTrials.gov (clinicaltrials.gov);
ISRCTN registry (www.controlled‐trials.com);
Trials Central (www.trialscentral.org);
World Health Organization (WHO) International Clinical Trials Registry (ICTR) (http://apps.who.int/trialsearch/Default.aspx);
The European Union (EU) Clinical Trials Register (www.clinicaltrialsregister.eu).
Searching other resources
We handsearched the bibliographies of all relevant articles for further relevant studies.
Data collection and analysis
We summarised data using standard Cochrane Collaboration methodologies (Higgins 2011a).
Selection of studies
Independently, two review authors (TS and DU) assessed titles and abstracts of the studies identified by the search strategy. We retrieved the full‐text of all potentially relevant abstracts and citations. Independently, these two review authors checked all full‐text papers for eligibility. Disagreements were resolved by discussion between the review authors, with the third review author (PP) acting as an arbitrator, if necessary. The selection process was recorded in sufficient detail to complete a PRISMA flow diagram (Moher 2009), as shown in Figure 1.
1.

Study flow diagram of the number of records identified, included and excluded, and the reasons for exclusion
Data extraction and management
Details of eligible studies were extracted independently and summarised by two review authors using a standardised data extraction sheet. Discrepancies between review authors were resolved by discussion to achieve a final consensus, with the third author acting as an arbitrator, if necessary. Where data were missing from reports, we attempted to obtain the missing information by contacting the study author. One review author (TS) entered the data into Review Manager 5 (RevMan 2014), and two other authors (DU and PP) checked the entered data.
We extracted the following data.
Country of origin.
Patient inclusion and exclusion criteria.
Type of ulcer.
Study setting.
A priori sample size calculation.
Unit of allocation and number of participants randomised to each intervention.
Baseline participant data.
Description of intervention and comparison.
Details of any cointerventions.
Types of primary and secondary outcome measures (with definitions).
Primary and secondary outcome data.
Duration of follow‐up.
Number of withdrawals and reasons for withdrawal (by group).
Adverse events (including amputations).
Source of funding of the trial.
Assessment of risk of bias in included studies
Independently, two review authors assessed the risk of bias for each included study using the Cochrane tool for assessing risk of bias (Higgins 2011b). This tool addresses six specific domains, namely: sequence generation, allocation concealment, blinding, incomplete outcome data, selective reporting and other issues (e.g. extreme baseline imbalance or bias related to the specific study design). See Appendix 2 for the criteria on which we based our judgement.
The authors assessed blinding and completeness of outcome data separately for each outcome. We completed a 'Risk of bias' table for each eligible study. We discussed any disagreement amongst all review authors to achieve a consensus. We presented the assessment of risk of bias using a 'Risk of bias' summary figure, which presents all of the judgements in a cross‐tabulation of study by entry (Figure 2). This display of internal validity indicates the weight the reader may give the results of each study. Also see Figure 3 for a 'Risk of bias' graph.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Measures of treatment effect
We calculated risk ratios (RRs) with 95% confidence intervals (CIs) for dichotomous outcomes, such as incidence of complete closure of the ulcer and incidence of amputation. For continuous outcomes, such as quality of life and cost, we would have calculated the difference in means (mean difference; MD) with 95% CIs as recommended by Higgins 2011a. For time to complete wound healing we planned to extract hazard ratios (HRs) with 95% CIs.
Unit of analysis issues
We dealt with unit of analysis issues according to guidance provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). We reported whether studies measured outcomes per person or per ulcer, and whether multiple ulcers on the same person were studied. Since the healing of multiple ulcers on an individual person cannot be considered to be independent events, we reported outcomes per person whenever possible.
Dealing with missing data
We contacted the study authors when data were incomplete or missing. Where trials reported outcomes only for participants who completed the follow‐up period, we treated the participants who were not included in the analysis as if their wound did not heal (e.g. withdrawals, participants lost‐to follow up or otherwise excluded from analysis).
We evaluated whether an intention‐to‐treat (ITT) analysis was performed or could have been performed. An ITT analysis includes all participants randomised into a trial irrespective of what happened subsequently (Higgins 2011a).
Assessment of heterogeneity
We evaluated clinical and methodological heterogeneity by comparing population, methods, interventions, and outcomes of studies.
We assessed statistical heterogeneity by visual inspection of the forest plots (overlap of CIs) and by using the Chi² test and the I² statistic. If the P value of the Chi² test was greater than 0.1, no significant statistical heterogeneity was present. The I² statistic was interpreted as suggested by Higgins: 0% to 40% might not be important, 30% to 60% may represent moderate heterogeneity, 50% to 90% may represent substantial heterogeneity and 75% to 100% may represent considerable heterogeneity (Higgins 2003).
Assessment of reporting biases
We used a funnel plot of primary outcomes to illustrate variability between trials visually and to assess whether the review was subject to publication bias.
Data synthesis
Details of the studies that were eligible for inclusion in this review were presented narratively or, whenever appropriate, by using meta‐analysis. If trials were similar in terms of population, methods, interventions and outcomes, we considered pooling them using a fixed‐effects model to summarise their results. If the I² was less than 50%, we also presented the results of a random effects model and discussed which measure we believed to be the most appropriate. If I² was substantial (over 75%) we did not pool these studies. We presented risk ratios (RRs) with 95% confidence intervals (CIs) for dichotomous outcomes. If meta‐analysis had been possible for continuous outcomes, we would have calculated a pooled difference in means (mean difference; MD) with 95% CIs as recommended by Higgins 2011a. For time to complete wound healing we planned to plot hazard ratios (HRs) with 95% CIs using the generic inverse variance method in RevMan 5.3.
'Summary of findings' tables
We presented the main results of this review in 'Summary of findings' tables. These tables present key information concerning the quality of the evidence, the magnitude of the effects of the interventions examined, and the sum of the available data for the main outcomes (Schunemann 2011a). The 'Summary of findings' tables also include an overall grading of the evidence related to each of the main outcomes using the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) approach. The GRADE approach defines the quality of a body of evidence as the extent to which one can be confident that an estimate of effect or association is close to the true quantity of specific interest. The quality of a body of evidence involves consideration of within‐trial risk of bias (methodological quality), directness of evidence, heterogeneity, precision of effect estimates and risk of publication bias (Schunemann 2011b).
Subgroup analysis and investigation of heterogeneity
We conducted subgroup analyses to examine the treatment effects of the different types of skin grafts or skin replacements. When subgroup analyses were not possible due to a lack of data, we reported the results narratively, according to the types of skin graft or replacement.
Sensitivity analysis
We conducted a sensitivity analysis to assess the impact of excluding trials assessed as being at high, or unclear, risk of bias. We excluded trials from this sensitivity analysis if they were at high risk or unclear risk of bias in at least two of the following three domains, namely: generation of the allocation sequence, concealment of allocation and blinding of outcome assessor.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies.
Results of the search
The initial search resulted in 283 potentially relevant records. After the first screening we selected 22 full‐text articles for a more detailed assessment. Seventeen studies were eligible for inclusion in this review (Figure 1). We identified five ongoing studies (NCT01693133; NCT02070835; NCT02120755; NCT02331147; NCT02399826) and added these to Characteristics of ongoing studies for future assessment (checked ISRCTN register 14 August 2015).
Included studies
We included seventeen RCTs with a total of 1655 randomised participants. Study size ranged from 23 to 314 included patients. Thirteen studies compared a skin graft or tissue replacement with standard care (Brigido 2006; Caravaggi 2003; Edmonds 2009; Gentzkow 1996; Lipkin 2003; Marston 2003; Naughton 1997; Reyzelman 2009; Uccioli 2011; Veves 2001; You 2012; You 2014; Zelen 2013). Four studies investigated the effectiveness of two different types of grafts (DiDomenico 2011; Landsman 2008; Puttirutvong 2004; Sanders 2014).
Study setting
Four studies were single‐centred (Brigido 2006; DiDomenico 2011; Puttirutvong 2004; Zelen 2013) and thirteen were multi‐centred (Caravaggi 2003; Edmonds 2009; Gentzkow 1996; Landsman 2008; Lipkin 2003; Marston 2003; Naughton 1997; Reyzelman 2009; Sanders 2014; Uccioli 2011; Veves 2001; You 2012; You 2014). Most (11/17) studies were undertaken in the United States. One study was multinational, taking place in the European Union and Australia (Edmonds 2009), one was conducted in Thailand (Puttirutvong 2004), two in Korea (You 2012; You 2014) and two in Italy (Caravaggi 2003; Uccioli 2011). None of the studies provided a detailed description of the study setting, but it appears that in general, studies were conducted in an outpatient hospital setting.
Inclusion and exclusion criteria
Inclusion and exclusion criteria were clearly listed in most trials (15/17). Two publications lacked a complete description of the selected patients (Naughton 1997; Puttirutvong 2004).
The majority of studies (15/17) excluded patients with an infection of the target ulcer. One study allowed the inclusion of ulcers with limited infection, as only patients with cellulitis or osteomyelitis were excluded (Landsman 2008). In another study, only ulcers with the absence of deep abscesses, gangrene or necrotising fasciitis were included (Puttirutvong 2004).
Adequate arterial perfusion of the foot was required for inclusion in all fifteen trials that described their inclusion and exclusion criteria.
More than half of the studies (10/17) included chronic, or hard‐to‐heal ulcers that were present for at least four to six weeks (Brigido 2006; Caravaggi 2003; DiDomenico 2011; Landsman 2008; Lipkin 2003; Sanders 2014; Uccioli 2011; You 2012; You 2014; Zelen 2013). Four studies included ulcers that were present for at least two weeks (Edmonds 2009; Marston 2003; Naughton 1997; Veves 2001). Three studies did not specify the minimum period of ulcer duration in their inclusion criteria (Gentzkow 1996; Puttirutvong 2004; Reyzelman 2009).
Follow‐up period
The follow‐up period ranged from six weeks (Zelen 2013) to 14 months (Gentzkow 1996), but most trials (11/17) reported a follow‐up period of 12 weeks (Edmonds 2009; Gentzkow 1996; Landsman 2008, Lipkin 2003; Marston 2003; Naughton 1997; Reyzelman 2009; Uccioli 2011; Veves 2001; You 2012; You 2014).
Sample size
An a priori sample size calculation was described in only three studies (Caravaggi 2003, Marston 2003, You 2012). In only one of these trials (Caravaggi 2003) the chosen effect size was clearly described. In this trial they calculated a sample size of 78 participants to detect a difference in healing rate after 30 days with 70% of ulcers healing in the intervention group and 30% of ulcers healing in the control group.
Most trials included less than 100 participants, whereas four trials included more than 100 participants (Marston 2003, Naughton 1997, Uccioli 2011, Veves 2001). The largest included trial in this review had 314 participants (Marston 2003), while the smallest had only 23 participants (Sanders 2014).
Excluded studies
We excluded five studies from this review. We excluded three single‐centre studies since the results were incorporated in multicentre studies already included in this review (Hanft 2002; Pham 1999; Sams 2002). Also the use of a recombinant human platelet‐derived growth factor in the control group (Niezgoda 2005), and the lack of outcome data before treatment switch in a cross‐over study led to exclusion (Moustafa 2007).
Risk of bias in included studies
Further details of each study are documented in the Characteristics of included studies and the ‘Risk of bias’ summary figures (Figure 2 and Figure 3).
Allocation
Randomisation sequence generation
Ten studies reported a random component in the sequence generation process, such as a computer‐generated random number table or a randomisation schedule (Caravaggi 2003; Edmonds 2009; Gentzkow 1996; Lipkin 2003; Sanders 2014; Uccioli 2011; Veves 2001; You 2012; You 2014; Zelen 2013).
In one study, an error in the randomisation scheme led to an uneven patient distribution. We considered the risk of bias to be high for this study (DiDomenico 2011). The remaining six studies were described as being randomised, but provided insufficient information about the method of random sequence generation. We therefore classified these studies at an unclear risk of bias for this domain (Brigido 2006; Landsman 2008; Marston 2003; Naughton 1997; Puttirutvong 2004; Reyzelman 2009).
Allocation concealment
In eight studies the treatment allocation was adequately concealed. Five of these studies reported the use of sealed envelopes (Edmonds 2009; Gentzkow 1996; Sanders 2014; Uccioli 2011; Veves 2001). One study reported a central independent allocation method (Landsman 2008), and another study concealed the allocation by using a computer‐generated randomisation code (Lipkin 2003). Caravaggi 2003 reported that the "randomisation schedule was held by the sponsor." The remaining nine trials did not describe the method of allocation concealment. We classified these studies at an unclear risk of bias for this domain.
Blinding
Blinding of participants and personnel
None of the included studies described blinding of personnel. Participants were blinded to the treatment allocation in three of the included studies (Marston 2003; Naughton 1997; You 2012) and we therefore classified them at a low risk of bias for this domain. We considered nine studies to be at a high risk of bias for this domain because they were described as open‐label or single‐blinded studies (Caravaggi 2003; Edmonds 2009; Gentzkow 1996; Landsman 2008; Lipkin 2003; Sanders 2014; Uccioli 2011; Veves 2001; Zelen 2013). The remaining five studies provided no information regarding blinding of participants and personnel (Brigido 2006; DiDomenico 2011; Puttirutvong 2004; Reyzelman 2009; You 2014) and we classified them as having an unclear risk of bias for this domain.
Blinding of outcome assessment
Blinded assessment of the outcome was described in only one study Lipkin 2003). Nine studies specifically stated the lack of outcome assessment and we classified them at a high risk of bias for this domain (Landsman 2008; Marston 2003; Naughton 1997; Reyzelman 2009; Sanders 2014; Uccioli 2011; Veves 2001; You 2012; Zelen 2013). The remaining seven studies provided no information regarding blinding of outcome assessment (Brigido 2006; Caravaggi 2003; DiDomenico 2011; Edmonds 2009; Gentzkow 1996; Puttirutvong 2004; You 2014) and we classified them as having an unclear risk of bias for this domain.
Incomplete outcome data
We classified 9 studies at low risk of bias for incomplete outcome data because the numbers and reasons for withdrawals were adequately described (Brigido 2006; Caravaggi 2003; Edmonds 2009; Gentzkow 1996; Lipkin 2003; Marston 2003; Reyzelman 2009; Veves 2001; Zelen 2013). Four trials reported dropouts but failed to include all patients in the ITT analysis (Naughton 1997; Uccioli 2011; You 2012; You 2014). We judged these four studies to be at high risk of bias for this domain. The remaining four trials did not provide a statement regarding missing data and we classified them as having an unclear risk of bias for this domain (DiDomenico 2011; Landsman 2008; Puttirutvong 2004; Sanders 2014).
Selective reporting
The majority of studies (14/17) reported all clinically relevant outcomes without suggestion of selective reporting and we classified them at a low risk of bias for this domain (Brigido 2006; Caravaggi 2003; DiDomenico 2011; Edmonds 2009; Gentzkow 1996; Lipkin 2003; Marston 2003; Puttirutvong 2004; Reyzelman 2009; Sanders 2014; Uccioli 2011; Veves 2001; You 2014; Zelen 2013).
Primary outcomes were related to ulcer healing. Only Veves 2001 reported on incidence of amputation as a separate outcome parameter. However, given the short follow‐up time, no statistically significant differences could be expected for this outcome.
We classified three studies as having an unclear risk of bias for selective reporting. Landsman 2008 described that the wounds were measured at each time point. But data on the outcome ‘wound closure’ was only specified as incidence of ‘healed wounds’ and not as a measured change in wound size. In a second study, the primary outcome parameter was changed to the number of 'active implants', which resulted in less patients receiving the 'best treatment' (Naughton 1997). In the third study, You 2012 left out the exact numbers of patients in the ITT analysis.
Some studies (7/17) failed to report outcomes based on ITT analyses or failed to include all randomised patients in the ITT analysis (Edmonds 2009; Marston 2003; Naughton 1997; Reyzelman 2009; Uccioli 2011; You 2012; You 2014). Because total number of participants was stated in each study, we were able to easily calculate the effects based on the ITT. We treated the participants who were not included in the analysis as if their wound did not heal (worst case scenario).
Other potential sources of bias
We classified all studies as having an unclear risk of bias for this domain as no trial was completely free of components that could put it at risk of bias. For example, in three studies the baseline characteristics were not provided (Brigido 2006; Naughton 1997; Uccioli 2011), while one study showed baseline differences (Gentzkow 1996).
Furthermore, nearly all studies (15/17) reported industry involvement; at least one of the authors was connected to a commercial organisation or the study was funded by a commercial organisation (Brigido 2006; Caravaggi 2003; DiDomenico 2011; Edmonds 2009; Gentzkow 1996; Lipkin 2003; Marston 2003; Naughton 1997; Reyzelman 2009; Sanders 2014; Uccioli 2011; Veves 2001; You 2012; You 2014; Zelen 2013).
Effects of interventions
See: Table 1
See: Table 1.
Primary outcomes
Incidence of complete closure of the ulcer (healing rate)
Thirteen studies compared a skin graft or tissue replacement with standard wound care and reported on incidence of complete closure of the ulcer. Compared products were Apligraf® or Graftskin® (Edmonds 2009; Veves 2001), Dermagraft® (Marston 2003; Naughton 1997; Gentzkow 1996), EpiFix® (Zelen 2013), Graftjacket® (Brigido 2006; Reyzelman 2009), Hyalograft 3D® (Caravaggi 2003; Uccioli 2011; You 2014), Kaloderm® (You 2012) and OrCel® (Lipkin 2003). Pooling of the results was possible because all trials reported on incidence of complete closure at similar time points. Eleven trials reported on incidence of complete closure after 12 weeks (Edmonds 2009; Gentzkow 1996; Lipkin 2003; Marston 2003; Naughton 1997; Reyzelman 2009; Sanders 2014; Uccioli 2011; Veves 2001; You 2012; You 2014), one after 11 weeks (Caravaggi 2003), one after 16 weeks (Brigido 2006) and one after six weeks (Zelen 2013).
Pooling of these results by using a random‐effects model yielded a significant effect in favour of the intervention group (risk ratio (RR) 1.55, 95% CI 1.30 to 1.85, low quality of evidence) (Analysis 1.1). Because there was only limited statistical heterogeneity for this outcome (I2 = 30%) we also pooled the effects by using a fixed‐effect model (RR 1.55, 95% CI 1.35 to 1.79). However, we believe the use of a random‐effects model is more appropriate because of the small clinical differences between the products that were used in the intervention groups.
1.1. Analysis.

Comparison 1 Skin grafts or tissue replacements compared with standard care, Outcome 1 Incidence of complete closure of the foot ulcer.
In the sensitivity analysis we excluded the trials with high, or unclear risk of bias for two of the following three domains, namely: generation of the allocation sequence, concealment of allocation and blinding of outcome assessor. Pooling the results from the six trials (Caravaggi 2003; Edmonds 2009; Gentzkow 1996; Lipkin 2003; Uccioli 2011; Veves 2001) that were assessed at low risk of bias showed similar results in favour of the intervention group (RR 1.49, 95% CI 1.21 to 1.85;) (Analysis 1.2).
1.2. Analysis.
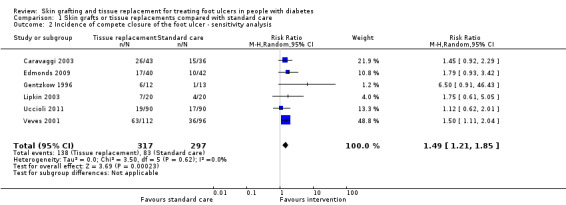
Comparison 1 Skin grafts or tissue replacements compared with standard care, Outcome 2 Incidence of compete closure of the foot ulcer ‐ sensitivity analysis.
Four studies compared two grafts or tissue replacements and reported on incidence of complete closure (DiDomenico 2011; Landsman 2008; Puttirutvong 2004; Sanders 2014). One of these four studies compared a meshed skin graft with a split‐skin graft in 80 participants (Puttirutvong 2004). Incidence of complete ulcer closure was not specifically stated as an outcome parameter, but trialists reported that all wounds were healed after six months (Analysis 2.1).
2.1. Analysis.

Comparison 2 Meshed skin graft compared with split‐skin graft, Outcome 1 Incidence of complete closure of the foot ulcer.
Landsman 2008 compared a living skin equivalent (Dermagraft®) with an extracellular collagen wound dressing (OASIS®). In this trial no significant differences were found as to the incidence of complete ulcer closure (RR 1.10, 95% CI 0.75 to 1.60) (Analysis 3.1).
3.1. Analysis.

Comparison 3 Dermagraft® compared with OASIS®, Outcome 1 Incidence of complete closure of the foot ulcer.
One study (DiDomenico 2011) reported a higher incidence of ulcer healing after 20 weeks in the TheraSkin® group (66.7%) compared with the Apligraf® group (46.1%), although this difference was not statistically significant (RR 0.71, 95% CI 0.37 to 1.34) (Analysis 4.1).
4.1. Analysis.
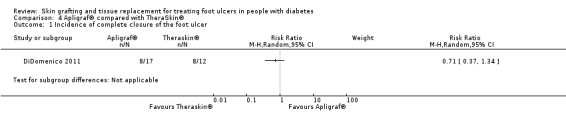
Comparison 4 Apligraf® compared with TheraSkin®, Outcome 1 Incidence of complete closure of the foot ulcer.
Sanders 2014 reported a higher incidence of ulcer healing after 12 weeks in the TheraSkin® group (63.6%) compared with the Dermagraft® group (33.3%), but this difference was not statistically significant (RR 1.91, 95% CI 0.76 to 4.77) (Analysis 5.1).
5.1. Analysis.

Comparison 5 Dermagraft® compared with TheraSkin®, Outcome 1 Incidence of complete closure of the foot ulcer.
Time to complete closure of the foot ulcer
Sixteen studies reported on time to complete healing. Lipkin 2003 was the only study that did not report on time to complete closure. However, the majority of studies had not been using survival analysis and reported hazard ratios, so meta‐analysis was not possible for this outcome.
Uccioli 2011 was the only study that reported the estimated hazard ratio (HR) for time to complete healing after two weeks. Trialists reported a HR of 2.17 of achieving complete closure per week in favour of the intervention group.
We reported all other statements on this outcome in the Characteristics of included studies tables.
Total incidence of lower limb amputations
Only two studies reported on the total incidence of amputations. Veves 2001 reported a lower amputation rate after 12 weeks in the Graftskin® group (6.3%) compared with the control group (15.6%). Frykberg 2015 (the same study as Marston 2003) reported a lower total number of amputations and bone resections in the Dermagraft group (5.5%) compared with the control group (12.6%). However, when we excluded the bone resections and define 'lower limb amputation' as a below knee amputation, foot amputation or toe amputation, there was no statistical significant difference (3.7% and 7.9%, respectively). By pooling the results of these two studies, we still found that there were fewer lower limb amputations after 12 weeks in the experimental groups; this is a small but statistically significant difference (RR 0.43, 95% CI 0.23 to 0.81, very low quality of evidence) (Analysis 1.3).
1.3. Analysis.

Comparison 1 Skin grafts or tissue replacements compared with standard care, Outcome 3 Incidence of lower limb amputations.
Reyzelman 2009 and Edmonds 2009 reported on three amputations as being an adverse event, but it is not clear whether all amputations were reported so these studies were not included in the meta‐analysis. Reyzelman 2009 stated that two hallux amputations occurred during the study period, but it was not clear to which group these patients were allocated. Edmonds reported one transmetatarsal amputation in the group that was not treated with a tissue replacement.
Secondary outcomes
Recurrence rate of foot ulcers
Six studies reported on recurrence rates of foot ulcers (Edmonds 2009; Gentzkow 1996; Naughton 1997; Puttirutvong 2004; Veves 2001; You 2012). Four of these studies found no differences in ulcer recurrence rates between the study groups (Edmonds 2009; Gentzkow 1996; Veves 2001; You 2012). Edmonds 2009 reported one recurrent ulcer in the Apligraf® group (1/15, 7.0%) and one recurrent ulcer in the control group (1/10, 10%). Veves 2001 reported a recurrence percentage of 5.9% in the Graftskin® group (3/112) and 12.9% in the control group (4/96) during the first six months. You 2012 showed an ulcer recurrence rate of 6.3% in the intervention group (1/16) and 6.7% in the control group (1/15) among patients who were monitored for 6 months. Gentzkow 1996 reported that none of the healed ulcers (n = 11 intervention group, n = 1 control group) had recurred during the follow‐up period (mean 14 months, range two to 22 months). Pooling of the results of these four studies showed no statistically significant difference in recurrence rates between intervention and control groups (RR 0.69, 95% CI 0.22 to 2.22) (Analysis 1.4).
1.4. Analysis.

Comparison 1 Skin grafts or tissue replacements compared with standard care, Outcome 4 Ulcer recurrence.
Naughton 1997 did not report exact numbers of recurred ulcers, but stated “whereas ulcers recurred in a comparable minority of both groups, it is noteworthy that Dermagraft® tended to delay recurrence (median time to recurrence: 12 weeks for Dermagraft® versus 7 weeks for control)”.
One study that compared two grafts reported on ulcer recurrence. Puttirutvong 2004 reported one recurrent ulcer in the split‐skin group and no recurrence in the meshed skin group after six months.
Change in ulcer area
Nine studies reported on change or reduction in ulcer area in various ways, which precluded meta‐analysis (Brigido 2006;Caravaggi 2003; Gentzkow 1996; Marston 2003; Reyzelman 2009; Uccioli 2011; You 2012; You 2014; Zelen 2013).
Without presenting the exact numbers, Brigido 2006 did show a figure of the average percentage of wound area that healed each week.
Caravaggi 2003 reported the mean percentage in wound size reduction for the ulcers that did not heal during the study period. The mean percentage wound reduction in the intervention group was 61.1% (SD 26.0) for plantar ulcers and 68.0% (SD 37.3) for dorsal ulcers. In the control group these percentages were 64.7% (SD 34.7) and 32.9% (SD 35.1) respectively.
Gentzkow 1996 reported on percentage of wounds achieving 50% closure after 12 weeks. In the group that received one piece of Dermagraft® weekly for a total of eight pieces and eight applications, 75% of patients achieved 50% wound closure. In the control group 23.1% achieved 50% closure by week 12.
Marston 2003 reported on the median percentage of wound closure by week 12. They found a median percent wound closure of 91% in the Dermagraft® group and 78% in the control group.
Reyzelman 2009 reported on the percentage of wounds that decreased in ulcer size but did not heal within 12 weeks. They reported that 85.7% of the patients in the intervention group and 71.4% in the control group experienced a decrease in ulcer size.
One study reported on a weekly ulcer size reduction percentage after two weeks (Uccioli 2011). They reported a weekly reduction percentage of 29% in the intervention group and 14% in the control group.
You 2012 reported the mean percentage in wound area reduction for all wounds, including the healed ones. They reported a mean wound size reduction of 87% (SD 49%) in the intervention group and 74% (SD 67%) in the control group in their intention‐to‐treat analysis.
You 2014 reported on the mean overall wound size reduction compared to the baseline size. In the intervention group they reported 3.0 cm2 (SD 2.6) wound size reduction and 2.1 cm2 (SD 1.5) in the control group.
Zelen 2013 reported on the average ulcer surface area reduction. After 6 weeks they reported an average reduction of ‐1.8% (SD 70.3) in the control group and 98.4% (SD 5.8) in the intervention group.
Incidence of infection
In general, the incidence of infection was poorly reported as a separate outcome. Nine of the studies that compared a skin graft or tissue replacement mentioned ulcer‐related infections (e.g. incidence of wound infection, osteomyelitis or cellulitis) (Brigido 2006; Gentzkow 1996; Lipkin 2003; Marston 2003; Naughton 1997; Uccioli 2011; Veves 2001; You 2012; Zelen 2013). We included these studies and meta‐analysis showed less infections in the intervention group (RR 0.82, 95% CI 0.53 to 0.98) (Analysis 1.5).
1.5. Analysis.

Comparison 1 Skin grafts or tissue replacements compared with standard care, Outcome 5 Incidence of infection.
DiDomenico 2011 reported the total number of adverse events without exact numbers of patients with infection or an increase in wound size. The exact number of infections was therefore unknown.
Quality of life
None of the included studies reported on this outcome.
Safety/adverse events
Landsman 2008 was the only study not to report on adverse events. The sixteen other trials mentioned adverse events in various ways. Some studies stated that there were no adverse events (Gentzkow 1996; Lipkin 2003), while Marston 2003, for example, reported up to 73% adverse events in the control group. One trial reported a fatal adverse event (myocardial infarction) in the intervention which was unrelated to the study treatment (Edmonds 2009). No study reported a statistical difference in the occurrence of adverse events between the intervention and the control group.
Costs of treatment
Only one study included a comparison of costs (Landsman 2008). A separate cost‐effectiveness analysis of this clinical trial is published by Gilligan 2015. Trialists estimated the total costs for the treatment by multiplying the average number of dressings necessary for complete healing and the costs per dressings. On average, treatment with Dermagraft® (total USD 3505; USD 1380 per dressing) was four times more expensive than treatment with OASIS® (total USD 807; USD 125 per dressing). In the cost‐effectiveness analysis, the predicted 12‐week cost per diabetic foot ulcer was USD 2522 for OASIS® and USD 3889 for treatment with Dermagraft® (Gilligan 2015).
Discussion
Summary of main results
This Cochrane systematic review for skin grafting and tissue replacements for treating foot ulcers in people with diabetes included seventeen RCTs that randomised 1655 participants. Most studies were multicentred (13/17) and were undertaken in the United States (11/17). Risk of bias was variable among studies. For the outcome 'incidence of complete closure of the ulcer' quality of evidence was low and for 'total incidence of lower limb amputations' quality of evidence was very low. Most trials lacked blinding of participants, personnel and outcome assessment because of obvious differences between the treatment strategies. Furthermore, nearly all studies (15/17) reported industry involvement, while at least one of the authors was connected to a commercial organisation or the study was funded by a commercial organisation. Possible publication bias was present because the funnel plot for assessing risk of bias appeared to be asymmetrical; suggesting that small studies with 'negative' results are less likely to be published.
Of the included trials in this review, thirteen studies compared a skin graft or tissue replacement with standard care. Four studies compared two grafts or tissue replacements with each other. When pooling the results of all individual studies the skin grafts and tissue replacement products that were used in the trials increased the healing rate of foot ulcers in patients with diabetes compared to standard care (RR 1.55, 95% CI 1.30 to 1.85). Based on the four included studies that directly compared two products, no specific type of skin graft or tissue replacement showed a superior effect on ulcer healing over another type of skin graft or tissue replacement. No statistically significant differences were found for ‘ulcer recurrence’ and ‘incidence of infection’.
Sixteen of the included studies reported on adverse events in various ways. Adverse event rates varied widely. No study reported a statistically significant difference in the occurrence of adverse events between the intervention and the control group.
Only two of the included studies reported on total incidence of lower limb amputations. Pooling the results of these two studies, we found fewer amputations in the experimental group compared with the standard care group, although the absolute risk reduction for amputation is small (RR 0.43, 95% CI 0.23 to 0.81; RD ‐0.06, 95% CI ‐0.10 to ‐0.01).
Overall completeness and applicability of evidence
Most included trials focused on wound healing speed or reduction in wound size. These seem relevant endpoints, but these data should be interpreted with caution. When wound size reduction is presented in percentages, e.g. 10% after 12 weeks, the clinical relevance for the patient is at best unclear.
Not all studies provided sufficient information about baseline characteristics. Baseline information is not only useful to check for baseline imbalances, but it also allows for comparison with other studies.
The ultimate goal of treating foot ulcers in people with diabetes is the prevention of (lower) limb amputations. Long‐term follow‐up is therefore not only necessary to assess the potential beneficial effect of these products on healing rates and ulcer recurrence, but also to evaluate amputation rates. Furthermore, no cost information on skin grafts or tissue replacement products was available in the selected trials; it is likely that these products are more expensive than current treatment alternatives and this difference should be taken into account when deciding the treatment strategy.
Currently, conventional (or standard) therapy remains the mainstay in the treatment of foot ulcers in people with diabetes. This multidisciplinary approach consists of pressure off‐loading (keeping weight off the area; Lewis 2013), debridement (removal of dead tissue; Edwards 2010), infection control, the use of wound dressings or topical agents (Bergin 2006; Dumville 2013a; Dumville 2013b; Dumville 2013c; Dumville 2013d; Jull 2013), intensive regulation of blood glucose (Fernando 2013), and ‐ in the case of ischaemia ‐ vascular reconstruction (Falanga 2005). However, due to the variety in treatment modalities, treatment intensity and patient adherence, it is likely that there are differences in the effectiveness of standard care as well. This hampers comparing the effectiveness of skin grafts and tissue replacements with standard care. Furthermore, the majority of the included studies only included non‐infected ulcers with an adequate arterial perfusion of the foot, which affects how generalisable the results are.
Quality of the evidence
GRADE assessments were conducted for the three key outcomes. Quality of the evidence was low for the outcome 'incidence of complete closure of the ulcer' and very low for the outcome 'total incidence of lower limb amputations'. For the outcome 'time to complete closure of the ulcer', quality assessment was not possible. Overall, quality of the evidence was downgraded because of risk of bias (lack of blinding, industry involvement and possible publication bias) and imprecision. See Table 1 for a complete assessment and rationale for ratings.
We judged some studies to be at ‘unclear risk of bias’ due to the lack of information concerning the methodology stated in the paper. None of the trials used reporting guidelines, e.g. the CONSORT statement (Schulz 2010).The lack of a blinded outcome assessor may have caused detection bias when assessing ulcer healing. However, possible detection bias is hard to prevent due to the nature of the skin replacement products we assessed and the fact that they are easily recognisable. Study sizes were generally small, but pooling yielded a positive effect on ulcer healing although some confidence intervals were wide for a number of comparisons (imprecision).
Potential biases in the review process
We believe that this review has included all RCTs currently available in the field of skin grafts or tissue replacements for treating foot ulcers in people with diabetes. In order to minimise bias in the review process, two of the review authors selected studies, extracted the study data and assessed the risk of bias and quality of the evidence independently.
In the assessment of publication bias, the funnel plot appeared to be asymmetrical. Small but statistically significant results, suggesting a beneficial effect for an experimental therapy, are more likely to be published. We may therefore have missed unpublished studies that found 'negative' results (Figure 4).
4.
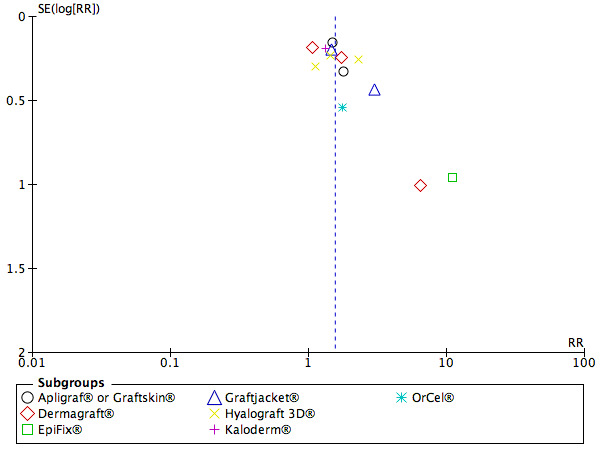
Funnel plot of comparison: 1 Skin grafts or tissue replacements compared with standard care, outcome: 1.1 Incidence of complete closure of the foot ulcer.
Agreements and disagreements with other studies or reviews
Although some published reviews and meta‐analyses mentioned the possible beneficial effect of skin grafts or tissue replacements, no rigorous quality assessment was provided in their analysis (Blozik 2008; Teng 2010). In this review, we also included newer and more relevant trials. Furthermore, single‐centre results of multicentre studies were sometimes included as if they were separate trials with unrelated results. Despite this pooling, and similar to our pooled results, these reviews reported favourable effects of skin substitutes on ulcer healing. Blozik 2008 found a pooled odds ratio of 1.46, 95% CI 1.21 to 1.76, showing a statistically significant difference in favour of the skin replacement products compared with standard care. Teng 2010 reported a pooled odds ratio of 1.88, 95% CI 1.41 to 2.51, also in favour of the skin substitutes.
Authors' conclusions
Implications for practice.
Overall, the therapeutic effect of skin grafts and tissue replacements, in conjunction with standard care, shows an increase in the healing rate of foot ulcers and slightly fewer amputations in people with diabetes compared with standard care alone. However, the data available to us was insufficient for us to draw conclusions on the effectiveness of different types of skin grafts or tissue replacement therapies, and evidence of long‐term effectiveness is lacking. Furthermore, the potential benefits of skin grafts and tissue replacements should be weighed against the high costs of these products. Finally, it is important to note that skin grafts and tissue replacements cannot be seen as a treatment on their own, but should always be part of the multidisciplinary approach to this complex, chronic disease.
Implications for research.
Most of the evidence which is currently available is derived from small studies with short follow‐up periods. Further research is needed to assess long‐term effectiveness of skin grafts and tissue replacement products. Recurrence rates, adverse events and amputation rates are essential outcomes and should be implemented in future studies. We also recommend quality of life and cost‐effectiveness as important outcomes. Cost‐effectiveness could also be assessed in observational studies when evidence for the effectiveness is more solid.
Feedback
Query re exclusion of potentially relevant study from Georgina Michael, Osiris Therapeutics Inc. 3 March 2017
Summary
Upon review of Skin grafting and tissue replacement for treating foot ulcers in people with diabetes by Santema et al we have noted that a randomized clinical trial (RCT) with a full‐length publication which appears to meet all specified criteria for inclusion was omitted from the reported results. The efficacy and safety of Grafix® for the treatment of chronic diabetic foot ulcers: results of a multi‐centre, controlled, randomised, blinded, clinical trial by Lavery et al. (Int Wound J, 11: 554–560.doi:10.1111/iwj.12329) was first published online by John Wiley & Sons, Ltd. on 21 July 2014.
Reply
This study was not found by the initial search sent to review authors. We agree with Ms Michael that the paper is potentially suitable for inclusion in our review. We are currently reviewing updated search results which include this trial and intend to update our review accordingly.
Contributors
Katrien Santema, Lead author of this review.
Georgina Michael, Senior Medical Science Liaison, Department of Medical Affairs, Osiris Therapeutics, Inc. Columbia, USA.
Declaration of interest: Osiris Therapeutics manufacture the intervention which is the subject of the trial we have identified as missing from this systematic review.
What's new
| Date | Event | Description |
|---|---|---|
| 3 March 2017 | Feedback has been incorporated | Feedback submitted and review author response added to the review. |
Acknowledgements
The authors would like to thank editors Andrea Nelson and Andrew Jull and peer reviewers Debra Fayter, Joyce Black, Devi Prasad Mohapatra, Sharon Van Wicklin, Duncan Chambers, Malcolm Brewster and Gill Worthy. Thanks also to copy editors Elizabeth Royle and Clare Dooley.
Appendices
Appendix 1. Search strategies
The Cochrane Wounds Specialised Register
#1 ((diabet* NEAR5 (foot or feet or ulcer* or wound* or amputat*))) AND (INREGISTER) #2 (((skin and graft*) or (pinch and graft*) or (split and thickness) or (full and thickness) or (allograft* or dermagraft* or apligraf* or autograft* or xenograft*) or (tissue NEAR5 (engineer* or bio‐engineer* or bioengineer* or scaffold* or replac*)) or (cultured and keratinocyte*) or (artificial and skin) or (bio‐engineer* and skin) or (bioengineer* and skin) or (replac* and skin) or ( substitut* and skin))) AND (INREGISTER) #3 #1 AND #2
The Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library)
#1 MeSH descriptor: [Diabetic Foot] explode all trees #2 MeSH descriptor: [Foot Ulcer] explode all trees #3 (diabet* near/3 ulcer*):ti,ab,kw #4 (diabet* near/3 (foot or feet)):ti,ab,kw #5 (diabet* near/3 wound*):ti,ab,kw #6 (diabet* near/3 defect*):ti,ab,kw #7 (#1 or #2 or #3 or #4 or #5 or #6) #8 MeSH descriptor: [Skin Transplantation] explode all trees #9 (skin next graft* or pinch next graft*):ti,ab,kw #10 (split next thickness or full next thickness):ti,ab,kw #11 (allograft* or dermagraft* or apligraf* or autograft* or xenograft*):ti,ab,kw #12 MeSH descriptor: [Tissue Engineering] explode all trees #13 MeSH descriptor: [Biocompatible Materials] explode all trees #14 MeSH descriptor: [Tissue Scaffolds] explode all trees #15 (tissue near/5 (engineer* or bio‐engineer* or bioengineer* or scaffold* or replac*)):ti,ab,kw #16 MeSH descriptor: [Keratinocytes] explode all trees #17 MeSH descriptor: [Cells, Cultured] explode all trees #18 (cultured near/2 keratinocyte*):ti,ab,kw #19 MeSH descriptor: [Skin, Artificial] this term only #20 (artificial near/2 skin):ti,ab,kw #21 ((bio‐engineer* or bioengineer*) near/2 skin):ti,ab,kw #22 (skin near/2 (replac* or substitut*)):ti,ab,kw #23 #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 #24 #7 and #23 in Trials
Ovid MEDLINE
1 exp Diabetic Foot/ 2 exp Foot Ulcer/ 3 (diabet* adj3 ulcer*).tw. 4 (diabet* adj3 (foot or feet)).tw. 5 (diabet* adj3 wound*).tw. 6 (diabet* adj3 defect*).tw. 7 or/1‐6 8 exp Skin Transplantation/ 9 (skin graft* or pinch graft*).tw. 10 (split thickness or full thickness).tw. 11 (allograft* or dermagraft* or apligraf* or autograft* or xenograft*).tw. 12 exp Tissue Engineering/ 13 exp Biocompatible Materials/ 14 exp Tissue Scaffolds/ 15 (tissue adj5 (engineer* or bio‐engineer* or bioengineer* or scaffold* or replac*)).tw. 16 exp Keratinocytes/ 17 exp Cells, Cultured/ 18 (cultured adj2 keratinocyte*).tw. 19 Skin, Artificial/ 20 (artificial adj2 skin).tw. 21 ((bio‐engineer* or bioengineer*) adj2 skin).tw. 22 (skin adj2 (replac* or substitut*)).tw. 23 or/8‐22 24 7 and 23 25 randomized controlled trial.pt. 26 controlled clinical trial.pt. 27 randomi?ed.ab. 28 placebo.ab. 29 clinical trials as topic.sh. 30 randomly.ab. 31 trial.ti. 32 or/25‐31 33 exp animals/ not humans.sh. 34 32 not 33 35 24 and 34
Ovid EMBASE
1 diabetic foot/ 2 foot ulcer/ 3 (diabet* adj3 ulcer*).tw. 4 (diabet* adj3 (foot or feet)).tw. 5 (diabet* adj3 wound*).tw. 6 (diabet* adj3 defect*).tw. 7 or/1‐6 (16213) 8 exp skin transplantation/ 9 (skin graft* or pinch graft*).tw. 10 (split thickness or full thickness).tw. 11 (allograft* or dermagraft* or apligraf* or autograft* or xenograft*).tw. 12 tissue engineering/ 13 biomaterial/ 14 tissue scaffold/ 15 (tissue adj5 (engineer* or bio‐engineer* or bioengineer* or scaffold* or replac*)).tw. 16 keratinocyte/ 17 exp cell culture/ 18 (cultured adj2 keratinocyte*).tw. 19 artificial skin/ 20 (artificial adj2 skin).tw. 21 ((bio‐engineer* or bioengineer*) adj2 skin).tw. 22 (skin adj2 (replac* or substitut*)).tw. 23 or/8‐22 24 7 and 23 25 Randomized controlled trials/ 26 Single‐Blind Method/ 27 Double‐Blind Method/ 28 Crossover Procedure/ 29 (random$ or factorial$ or crossover$ or cross over$ or cross‐over$ or placebo$ or assign$ or allocat$ or volunteer$).ti,ab. 30 (doubl$ adj blind$).ti,ab. 31 (singl$ adj blind$).ti,ab. 32 or/25‐31 33 exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/ 34 human/ or human cell/ 35 and/33‐34 36 33 not 35 37 32 not 36 38 24 and 37
EBSCO CINAHL
S36 S23 AND S35 S35 S24 or S25 or S26 or S27 or S28 or S29 or S30 or S31 or S32 or S33 or S34 S34 MH "Quantitative Studies" S33 TI placebo* or AB placebo* S32 MH "Placebos" S31 TI random* allocat* or AB random* allocat* S30 MH "Random Assignment" S29 TI randomi?ed control* trial* or AB randomi?ed control* trial* S28 AB ( singl* or doubl* or trebl* or tripl* ) and AB ( blind* or mask* ) S27 TI ( singl* or doubl* or trebl* or tripl* ) and TI ( blind* or mask* ) S26 TI clinic* N1 trial* or AB clinic* N1 trial* S25 PT Clinical trial S24 MH "Clinical Trials+" S23 S7 AND S22 S22 S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 S21 TI ( skin N2 (replac* or substitut*) ) OR AB ( skin N2 (replac* or substitut*) ) S20 TI ( (bio‐engineer* or bioengineer*) N2 skin ) OR AB ( (bio‐engineer* or bioengineer*) N2 skin ) S19 TI artificial N2 skin OR AB artificial N2 skin S18 (MH "Skin, Artificial") S17 TI cultured N2 keratinocyte* OR AB cultured N2 keratinocyte* S16 (MH "Cells, Cultured+") S15 (MH "Keratinocytes") S14 TI ( tissue N5 (engineer* or bio‐engineer* or bioengineer* or scaffold* or replac*) ) OR AB ( tissue N5 (engineer* or bio‐engineer* or bioengineer* or scaffold* or replac*) ) S13 (MH "Biocompatible Materials") S12 (MH "Tissue Engineering") S11 TI ( allograft* or dermagraft* or apligraf* or autograft* or xenograft* ) OR AB ( allograft* or dermagraft* or apligraf* or autograft* or xenograft* ) S10 TI ( split N1 thickness OR full N1 thickness ) OR AB ( split N1 thickness OR full N1 thickness ) S9 TI ( skin N1 graft* OR pinch N1 graft* ) OR AB ( skin N1 graft* OR pinch N1 graft* ) S8 (MH "Skin Transplantation") S7 S1 OR S2 OR S3 OR S4 OR S5 OR S6 S6 TI diabet* N3 defect* or AB diabet* N3 defect* S5 TI diabet* N3 wound* or AB diabet* N3 wound* S4 TI ( diabet* N3 foot OR diabet* N3 feet ) or AB ( diabet* N3 foot OR diabet* N3 feet ) S3 TI diabet* N3 ulcer* or AB diabet* N3 ulcer* S2 MH "Foot Ulcer+" S1 MH "Diabetic Foot"
Appendix 2. Risk of bias
1. Random sequence generation (selection bias)
Low risk of bias
The investigators describe a random component in the sequence generation process such as: referring to a random number table; using a computer random‐number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots.
High risk of bias
The investigators describe a non‐random component in the sequence generation process. Usually, the description would involve some systematic, non‐random approach, for example: sequence generated by odd or even date of birth; sequence generated by some rule based on date (or day) of admission; sequence generated by some rule based on hospital or clinic record number.
Unclear
Insufficient information about the sequence generation process provided to permit a judgement of low or high risk of bias.
2. Allocation concealment (selection bias)
Low risk of bias
Participants and investigators enrolling participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal allocation: central allocation (including telephone, web‐based and pharmacy‐controlled randomisation); sequentially‐numbered drug containers of identical appearance; sequentially‐numbered, opaque, sealed envelopes.
High risk of bias
Participants or investigators enrolling participants could possibly foresee assignments and thus introduce selection bias, such as allocation based on: using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or non‐opaque or not sequentially‐numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure.
Unclear
Insufficient information provided to permit a judgement of low or high risk of bias. This is usually the case if the method of concealment is not described or not described in sufficient detail to allow a definite judgement, for example if the use of assignment envelopes is described, but it remains unclear whether envelopes were sequentially‐numbered, opaque and sealed.
3. Blinding or masking of participants, personnel and outcome assessment (performance and detection bias)
Low risk of bias
Any one of the following.
No blinding, but the review authors judge that the outcome and the outcome measurement are not likely to be influenced by lack of blinding.
Blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken.
Either participants or some key study personnel were not blinded, but outcome assessment was blinded and the non‐blinding of others was unlikely to introduce bias.
High risk of bias
Any one of the following.
No blinding or incomplete blinding, and the outcome or outcome measurement is likely to be influenced by lack of blinding.
Blinding of key study participants and personnel attempted, but likely that the blinding could have been broken.
Either participants or some key study personnel were not blinded, and the non‐blinding of others was likely to introduce bias.
Unclear
Either of the following.
Insufficient information provided to permit a judgement of low or high risk of bias.
The study did not address this outcome.
4. Incomplete outcome data
Low risk of bias
Any one of the following.
No missing outcome data.
Reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias).
Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups.
For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk was not enough to have a clinically relevant impact on the intervention effect estimate.
For continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes was not enough to have a clinically relevant impact on observed effect size.
Missing data have been imputed using appropriate methods.
High risk of bias
Any one of the following.
Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups.
For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk was enough to induce clinically relevant bias in intervention effect estimate.
For continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes was enough to induce clinically relevant bias in observed effect size.
‘As‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation.
Potentially inappropriate application of simple imputation.
Unclear
Either of the following.
Insufficient reporting of attrition/exclusions to permit a judgement of low or high risk of bias (e.g. number randomised not stated, no reasons for missing data provided).
The study did not address this outcome.
5. Selective outcome reporting
Low risk of bias
Either of the following.
The study protocol is available and all of the study’s prespecified (primary and secondary) outcomes that are of interest in the review have been reported in the prespecified way.
The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were prespecified (convincing text of this nature may be uncommon).
High risk of bias
Any one of the following.
Not all of the study’s prespecified primary outcomes have been reported.
One or more primary outcomes are reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not prespecified.
One or more reported primary outcomes were not prespecified (unless clear justification for their reporting is provided, such as an unexpected adverse effect).
One or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis.
The study report fails to include results for a key outcome that would be expected to have been reported for such a study.
Unclear
Insufficient information provided to permit judgement of low or high risk of bias. It is likely that the majority of studies will fall into this category.
6. Other sources of potential bias
Low risk of bias
The study appears to be free of other sources of bias.
High risk of bias
There is at least one important risk of bias. For example, the study:
Had a potential source of bias related to the specific study design used; or
Has been claimed to have been fraudulent; or
Had some other problem.
Unclear
There may be a risk of bias, but there is either:
Insufficient information to assess whether an important risk of bias exists; or
Insufficient rationale or evidence that an identified problem will introduce bias.
Data and analyses
Comparison 1. Skin grafts or tissue replacements compared with standard care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence of complete closure of the foot ulcer | 13 | 1472 | Risk Ratio (M‐H, Random, 95% CI) | 1.55 [1.30, 1.85] |
| 1.1 Apligraf® or Graftskin® | 2 | 290 | Risk Ratio (M‐H, Random, 95% CI) | 1.55 [1.17, 2.04] |
| 1.2 Dermagraft® | 3 | 620 | Risk Ratio (M‐H, Random, 95% CI) | 1.50 [0.85, 2.65] |
| 1.3 EpiFix® | 1 | 25 | Risk Ratio (M‐H, Random, 95% CI) | 11.08 [1.69, 72.82] |
| 1.4 Graftjacket® | 2 | 114 | Risk Ratio (M‐H, Random, 95% CI) | 1.90 [0.97, 3.71] |
| 1.5 Hyalograft 3D® | 3 | 324 | Risk Ratio (M‐H, Random, 95% CI) | 1.57 [1.06, 2.33] |
| 1.6 Kaloderm® | 1 | 59 | Risk Ratio (M‐H, Random, 95% CI) | 1.32 [0.90, 1.92] |
| 1.7 OrCel® | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 1.75 [0.61, 5.05] |
| 2 Incidence of compete closure of the foot ulcer ‐ sensitivity analysis | 6 | 614 | Risk Ratio (M‐H, Random, 95% CI) | 1.49 [1.21, 1.85] |
| 3 Incidence of lower limb amputations | 2 | 522 | Risk Difference (M‐H, Random, 95% CI) | ‐0.06 [‐0.10, ‐0.01] |
| 3.1 Graftskin® | 1 | 208 | Risk Difference (M‐H, Random, 95% CI) | ‐0.09 [‐0.18, ‐0.01] |
| 3.2 Dermagraft® | 1 | 314 | Risk Difference (M‐H, Random, 95% CI) | ‐0.04 [‐0.09, 0.01] |
| 4 Ulcer recurrence | 4 | 276 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.22, 2.22] |
| 4.1 Apligraf® or Graftskin® | 2 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.18, 2.35] |
| 4.2 Dermagraft® | 1 | 12 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 Kaloderm® | 1 | 31 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.06, 13.68] |
| 5 Incidence of infection | 9 | 845 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.53, 0.98] |
| 5.1 Apligraf® or Graftskin® | 2 | 280 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.43, 1.76] |
| 5.2 Dermagraft® | 2 | 270 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.40, 0.93] |
| 5.3 EpiFix® | 1 | 25 | Risk Ratio (M‐H, Random, 95% CI) | 0.19 [0.01, 3.52] |
| 5.4 Graftjacket® | 1 | 28 | Risk Ratio (M‐H, Random, 95% CI) | 0.6 [0.18, 2.04] |
| 5.5 Hyalograf 3D® | 1 | 171 | Risk Ratio (M‐H, Random, 95% CI) | 1.35 [0.62, 2.90] |
| 5.6 Kaloderm® | 1 | 31 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.12, 3.24] |
| 5.7 OrCel® | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.5 [0.10, 2.43] |
Comparison 2. Meshed skin graft compared with split‐skin graft.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence of complete closure of the foot ulcer | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only |
Comparison 3. Dermagraft® compared with OASIS®.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence of complete closure of the foot ulcer | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only |
Comparison 4. Apligraf® compared with TheraSkin®.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence of complete closure of the foot ulcer | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only |
Comparison 5. Dermagraft® compared with TheraSkin®.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence of complete closure of the foot ulcer | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Brigido 2006.
| Methods | Single‐centre RCT (one foot and ankle clinic in the USA) with 16 weeks' follow‐up | |
| Participants | Twenty‐eight diabetic patients with Wagner grade 2, chronic non‐healing lower extremity wounds and present for at least 6 weeks Mean age: (SD) 61.43 (7.18) in intervention group, 66.21 (4.37) in control group Mean ulcer size: not stated Mean ulcer duration: not stated |
|
| Interventions | Group 1 (n = 14): a human acellular regenerative tissue matrix onlay (Graft jacket) combined with sharp debridement Group 2 (n = 14): gauze dressings and sharp debridement |
|
| Outcomes | 1. Incidence of complete wound closure after 16 weeks: Group 1: 12/14 (85.7%) Group 2: 4/14 (28.6%) 2. Mean time to complete wound closure: Group 1: 11.92 (2.87) weeks Group 2: 13.50 (3.42) weeks 3. Total incidence of lower limb amputations: not reported |
|
| Notes | Definition of complete closure: ''full epithelization of the wound with the absence of drainage'' | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: ''Patients were then randomized to one of two treatment groups'' Comment: method of generating the random schedule was not reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: not stated, but blinding not likely |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: not stated, but blinding not likely |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: “All patients completed the 16‐week study” Comment: there were no dropouts or withdrawals |
| Selective reporting (reporting bias) | Low risk | Comment: all clinically relevant and reasonably expected outcomes were reported |
| Other bias | Unclear risk | Quote: "Stephen A. Brigido, DPM is a consultant for Wright Medical Technology" Comment: not clear if variables (e.g. ulcer size, ulcer duration) were balanced at baseline At least one of the authors is connected to a commercial organisation |
Caravaggi 2003.
| Methods | Multicentred RCT (6 centres in Italy) with 11 weeks' follow‐up | |
| Participants | Seventy‐nine patients with non‐infected diabetic plantar and dorsal foot ulcers > 2 cm², Wagner grade 1 or 2 and without signs of healing for at least one month Mean age: not stated Mean ulcer size (SD): 5.3 cm² (6.76) in intervention group, 6.2 cm² (7.58) in control group Mean ulcer duration (SD): 4.0 months (10.0) in intervention group, 4.0 months (6.0) in control group |
|
| Interventions | Group 1 (n = 43): autologous tissue‐engineered grafts (fibroblasts on Hyalograft 3D® and keratinocytes grown on Laserskin) Group 2 (n = 36): non‐adherent paraffin gauze with traditional absorbent secondary dressing |
|
| Outcomes | 1. Incidence of complete wound closure after 11 weeks: Group 1: 60.4% (total 26/43; plantar 12/22 and dorsal 14/21) Group 2: 41.7% (total 15/36; plantar 10/20 and dorsal 15/36) 2. Median time to complete wound closure: Group 1: 57 days Group 2: 77 days 3. Total incidence of lower limb amputations: not reported |
|
| Notes | Definition of complete closure: ''complete re‐epithelialization without residual exudate or crusting'' | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: ''Randomization was done by telephone, and the randomization list was generated and held by the sponsor'' |
| Allocation concealment (selection bias) | Low risk | Quote: ''Randomization was done by telephone, and the randomization list was generated and held by the sponsor'' Comment: allocation concealed using an independent central randomisation service |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: ''This was an open, stratified, randomized and controlled trial'' Comment: open‐label RCT, so no blinding of participants or personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: ''The primary efficacy parameters […] were evaluated by the investigators at every weekly visit'' Comment: blinding of investigators not likely in this open‐label RCT |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote:''Details of discontinued patients are presented in Table 1'' Comment: the numbers and reasons for dropouts and withdrawals were balanced and described |
| Selective reporting (reporting bias) | Low risk | Comment: all clinically relevant and reasonably expected outcomes were reported |
| Other bias | Unclear risk | Quote: "This study was supported by a research grant from Fidia Advanced Biopolymers" Comment: funded by commercial organisation |
DiDomenico 2011.
| Methods | Single‐centre RCT (podiatric practice in the USA) with 20 weeks' follow‐up | |
| Participants | Twenty‐nine wounds from 28 diabetic patients with Wagner 1 or Texas 1 diabetic foot ulcers with a size between 0.5 and 4 cm² and present for at least four weeks Mean age: not stated Mean ulcer size: 1.89 cm² in Apligraf®/BSS group, 1.82 cm² in TheraSkin®/SSA group Mean ulcer duration: not stated |
|
| Interventions | Group 1 (n = 17): a bioengineered skin substitute (BSS; Apligraf®) Group 2 (n = 12): a split‐skin allograft (SSA; TheraSkin®) |
|
| Outcomes | 1. Incidence of complete wound closure after 20 weeks: Group 1: 47.1% (exact numbers not stated, most likely 8/17) Group 2: 66.7% (exact numbers not stated, most likely 8/12) 2. Mean time to complete wound closure: Group 1: 6.86 (4.12) weeks Group 2: 5.00 (3.43) weeks 3. Total incidence of lower limb amputations: not reported |
|
| Notes | Definition of complete closure: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: ''Due to an unintentional error in the randomization scheme, more patients were enrolled in the BSS group than in the SSA group.'' Comment: randomised, but with errors |
| Allocation concealment (selection bias) | Unclear risk | Comment: apparent block randomisation, but not specifically stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: authors gave the impression that there had been no dropouts or withdrawals, but this was not specifically stated |
| Selective reporting (reporting bias) | Low risk | Comment: all clinically relevant and reasonably expected outcomes were reported |
| Other bias | Unclear risk | Quote: "This study was funded by Soluble Systems, LLC" Comment: funded by commercial organisation |
Edmonds 2009.
| Methods | International multicentre RCT (24 hospitals in the European Union and Australia) with 12 weeks' follow‐up for efficacy | |
| Participants | Eighty‐two patients with neuropathic diabetic foot ulcers with a size between 1 and 16 cm² and present for at least two weeks Mean age (SD) 56.4 (11.6) in intervention group, 60.6 (9.8) in control group Median ulcer size (range): 2.5 cm² ( 0.8 ‐ 9.3) in intervention group, 2.25 cm² (0.5 ‐ 10.0) in control group Median ulcer duration (range): 1.1 years (0.1 ‐ 8.0) in intervention group, 1.2 (2 weeks ‐ 7.0 years) in control group |
|
| Interventions | Group 1 (n = 40; 33 in per‐protocol analysis): Apligraf®, living keratinocytes and fibroblasts Group 2 (n = 42; 39 in per‐protocol analysis): Standard therapy; polyamide and saline‐moistened gauze dressings |
|
| Outcomes | 1. Incidence of complete wound closure after 12 weeks: Group 1: 17/40 (42.5%) Group 2: 10/42 (23.8%) 2. Median time to complete wound closure: Group 1: 84 days Group 2: median could not be estimated 3. Total incidence of lower limb amputations: Not reported as separate outcome, but at least 1 transmetatarsal amputation occurred in group 2 |
|
| Notes | Definition of complete closure: ''full epithelialization with no drainage'' | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: ''Eligible patients were randomized in a 1:1 ratio to either the Apligraf® group or the control group by means of sealed allocation cards'' |
| Allocation concealment (selection bias) | Low risk | Quote: ''Eligible patients were randomized in a 1:1 ratio to either the Apligraf® group or the control group by means of sealed allocation cards'' Comment: allocation concealed using sealed allocation cards |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: ''This was a prospective, multicenter, randomized, controlled, open‐label study'' Comment: No blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: Not stated, but Apligraf® was applied in addition to standard treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: ''One of the standard therapy subjects had a fractured femur following the baseline treatment visit, did not have a follow‐up efficacy visit, subsequently dropped out of the study'' Comment: all withdrawals and protocol violations are described |
| Selective reporting (reporting bias) | Low risk | Comment: all clinically relevant and reasonably expected outcomes were reported. |
| Other bias | Unclear risk | Quote: ''Because of difficulties encountered during the registration process in the European Union, the study sponsor elected to prematurely halt enrolment in the study. This was done for external reasons, and was not due to any safety concerns'' Comment: study was stopped prematurely and at least one of the authors is connected to a commercial organisation |
Gentzkow 1996.
| Methods | Multicentred RCT (5 centres in the USA) with a follow‐up period of 12 weeks for wound healing and a mean follow‐up period of 14 months for ulcer recurrence assessment | |
| Participants | Fifty patients with full‐thickness, diabetic foot ulcers of the plantar surface or heel > 1 cm² Mean age: group 1 62.7, group 2 66.2 and 62.7 years in group 3. In the control group the mean age was 53.8 years Median ulcer size was respectively 2.2, 2.3 and 3.3 cm² in the intervention groups and 1.9 cm² in the control group Mean ulcer duration in weeks was respectively 50.4, 40.7 and 43.2 weeks in the intervention groups and 87.0 weeks in the control group |
|
| Interventions | Group 1 (n = 12): one piece of Dermagraft® applied weekly for a total of eight pieces and eight applications Group 2 (n = 14): two pieces of Dermagraft® applied every 2 weeks for a total of eight pieces and four applications Group 3 (n = 11): one piece of Dermagraft® applied every 2 weeks for a total of four pieces and four applications Group 4 (n = 13): conventional therapy |
|
| Outcomes | 1. Incidence of complete wound closure after 12 weeks: Group 1: 6/12 (50.0%) Group 2: 3/14 (21.4%) Group 3: 2/11 (18.2%) Group 4: 1/13 (7.7%) 2. Median time to complete wound closure: Group 1: 12 weeks; Group 2, 3 and 4: > 12 weeks 3. Total incidence of lower limb amputations: not reported |
|
| Notes | Definition of complete closure: ''full epithelialization with no drainage'' | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: ''When patients and their wounds met study criteria, they were given a study number, and sealed randomization envelopes were used to assign them to one of four study treatments'' |
| Allocation concealment (selection bias) | Low risk | Quote: ''When patients and their wounds met study criteria, they were given a study number, and sealed randomization envelopes were used to assign them to one of four study treatments" Comment: allocation concealed using sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: “This was a controlled prospective multicenter randomized single‐blinded pilot study'' |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: “This was a controlled prospective multicenter randomized single‐blinded pilot study'' Comment: single‐blinded but not specifically explained the blinding process |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Three Dermagraft patients died after 2, 6, and 11 months of follow‐up, respectively, at which time their ulcers had not recurred. […] the one healed control‐treated ulcer had not recurred after 2 months, after which the patient was lost to follow up" Comment: all patients completed the 12 week follow‐up, patients died or lost‐to‐follow‐up were described |
| Selective reporting (reporting bias) | Low risk | Comment: all clinically relevant and reasonably expected outcomes were reported |
| Other bias | Unclear risk | Quote: "Advances Tissue Sciences, Inc., La Jolla, California, provided financial support for this study" Comment: ulcer duration and age were not balanced at baseline and the study was funded by a commercial organisation. Furthermore, there is no information about how these patients were randomised over the five institutions, which is relevant in this small patient sample |
Landsman 2008.
| Methods | Multicentred RCT (4 foot clinics in the USA) with a follow‐up duration of 20 weeks | |
| Participants | Twenty‐six patients with neuropathic, full‐thickness diabetic foot ulcers with a size between 1 and 16 cm² and present for at least four weeks Mean age (SD) 63.4 (9.84) in Dermagraft group, 62.17 (12.17) in OASIS group. Mean ulcer size (SD): 1.88 cm² (1.39) in Dermagraft group, 1.85 cm² (1.83) in OASIS group. Mean ulcer duration: not stated |
|
| Interventions | Group 1 (n = 13): Dermagraft, a living skin equivalent Group 2 (n = 13): OASIS, an acellular, porcine‐derived, bioactive collagen matrix material |
|
| Outcomes | 1. Incidence of complete wound closure after 12 weeks: Group 1: 11/13 (84.6%) Group 2: 10/13 (76.9%) 2. Mean time to complete wound closure: Group 1: 40.90 (32.32) days Group 2: 35.67 (41.47) days 3. Total incidence of lower limb amputations: not reported |
|
| Notes | Definition of complete closure: ''full epithelialization without any evidence of drainage or bleeding'' A cost‐effectiveness analysis of this clinical trial is published by Gilligan 2015 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Randomization was accomplished when investigative site identified a qualified candidate and contacted an independent site that randomly assessed patients to one of the two study arms” Comment: the method of generating the random sequence was not reported |
| Allocation concealment (selection bias) | Low risk | Quote: “Randomization was accomplished when investigative site identified a qualified candidate and contacted an independent site that randomly assessed patients to one of the two study arms” Comment: allocation concealed using an central independent unit |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: “In a randomized, non‐blinded study” Comment: no blinding of participants or personnel |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: “In a randomized, non‐blinded study” Comment: no blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: authors gave the impression that there had been no dropouts or withdrawals, but this was not specifically stated |
| Selective reporting (reporting bias) | Unclear risk | Quote: “At subsequent weekly evaluations, the wound was cleaned, evaluated, photographed, and measured at each time point" Comment: change in ulcer area was not stated, but measurements are described in the method section |
| Other bias | Unclear risk | Comment: there is a different maximum number of treatments allowed in each group. This influences costs and, possibly, outcome; furthermore, at least one of the authors is connected to a commercial organisation |
Lipkin 2003.
| Methods | Multicentred RCT (8 centres in the USA) with 12 weeks’ follow‐up | |
| Participants | Forty patients with neuropathic diabetic foot ulcers, Texas grade 1A, with a size between 1 and 12 cm² and present for at least 30 days Mean age (SD) 59.0 (12.7) in intervention group, 57.4 (10.6) in control group Mean ulcer size (SD): 6.0 cm² (7.6) in intervention group, 5.5 cm² (4.3) in control group Mean ulcer duration (SD): 11.9 months (11.8) in intervention group, 12.2 months (10.8) in control group |
|
| Interventions | Group 1 (n = 20): BCM Group 2 (n = 20): Standard care |
|
| Outcomes | 1. Incidence of complete wound closure after 12 weeks (all wounds): Group 1: 7/20 (35.0%) Group 2: 4/20 (20.0%) 1a. Incidence of complete wound closure after 12 weeks for ulcers with baseline size ≤ 6 cm² Group 1: 7/15 (46.6%) Group 2: 3/13 (23.1%) 1b. Incidence of complete wound closure after 12 weeks for ulcers with baseline size ≥ 6cm² Group 1: 0/5 (0.0%) Group 2: 1/7 (14.3%) 2. Average time to complete wound closure: not reported 3. Total incidence of lower limb amputations: not reported |
|
| Notes | Definition of complete closure: ''100% epithelialization with no drainage or need for absorbent dressing'' | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Those patients who continued to meet eligibility criteria were individually assigned to the BCM treatment group or to the control group according to a computer‐generated randomization code” |
| Allocation concealment (selection bias) | Low risk | Quote: “Those patients who continued to meet eligibility criteria were individually assigned to the BCM treatment group or to the control group according to a computer‐generated randomization code” Comment: allocation concealed using a computer‐generated randomization code |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: “This study was an open label, multicenter, controlled, randomized, parallel‐group pilot study'' Comment: no blinding of participants or personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “Film was processed at a central facility and read in randomized order by two separate wound care experts who were blinded to specific protocol, patient, and visit date.” Comment: the primary outcomes were assessed blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: the numbers and reasons for dropouts and withdrawals were balanced and described |
| Selective reporting (reporting bias) | Low risk | Comment: all clinically relevant and reasonably expected outcomes were reported |
| Other bias | Unclear risk | Quote: "This work was supported by a grant from Ortec International" Comment: funded by commercial organisation |
Marston 2003.
| Methods | Multicentred RCT (35 centres in the USA) with 12‐week follow‐up | |
| Participants | 314 patients with chronic diabetic foot ulcers sized ≥ 1 cm² and present for at least two weeks Mean age (range): 55.8 (27‐83) in intervention group, 55.5 (31‐79) in control group Mean ulcer size (range): 2.31 cm² (0.75 ‐ 16.7) in intervention group, 2.53 cm² (0.5 ‐ 18.0) in control group Mean ulcer duration: 41 weeks in intervention group, 67 weeks in control group |
|
| Interventions | Group 1 (n = 163; 130 per‐protocol): Dermagraft®, a cryopreserved human fibroblast derived dermal substitute Group 2 (n = 151: 115 per‐protocol): Saline‐moistened gauze, dry gauze and fixation sheets (Hypafix) |
|
| Outcomes | 1. Incidence of complete wound closure after 12 weeks: Group 1: 39/163 (23.9%) Group 2: 21/151 (13.9%) 2. Average time to complete wound closure: exact numbers not stated Quote: ''The Dermagraft treated group had a significantly faster time to complete wound closure than the control group (P = 0.04)'' 3. Total incidence of lower limb amputations: Group 1: 9/163 amputations or bone resections (5.5%) Group 2: 19/151 amputations or bone resections (12.6%) |
|
| Notes | Definition of complete closure: ''full epithelialization of the wound with the absence of drainage" Results of this study are also published by Frykberg 2015 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomized into either the Dermagraft or the control group. Patients were not informed as to which treatment they received" Comment: multicentre study design suggests central randomisation procedure, but this was not stated |
| Allocation concealment (selection bias) | Unclear risk | Comment: concealment method not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “The study was a prospective, single‐blind, randomized, controlled investigation'' Comment: patient was blinded, blinding of personnel was not possible |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: “The study was a prospective, single‐blind study'' Comment: the outcome assessment was not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: “The reasons for discontinuation were comparable between the two treatment groups. The majority of the patients who discontinued had an adverse event requiring' treatment that warranted withdrawal from the study'' Comment: numbers and reasons of discontinuation are stated |
| Selective reporting (reporting bias) | Low risk | Comment: all clinically relevant and reasonably expected outcomes were reported |
| Other bias | Unclear risk | Quote: "This study was supported by a research grant from Advanced Tissue Sciences, Inc., and Smith & Nephew, Inc" Comment: funded by commercial organisation |
Naughton 1997.
| Methods | Multicentred RCT (20 centres in the USA) with 32 weeks of follow‐up | |
| Participants | 281 patients with neuropathic, full‐thickness diabetic foot ulcers with a size ≥ 1 cm² and present for at least two weeks Baseline comparability for age, ulcer size and ulcer duration was not reported |
|
| Interventions | Group 1 (n = 139 randomised, 109 per‐protocol): Dermagraft®, a three‐dimensionally cultivated human diploid fibroblast cells on a polymer scaffold Group 2 (n = 142 randomised; 126 per‐protocol): standard therapy only |
|
| Outcomes | 1. Incidence of complete wound closure after 12 weeks: Group 1: 42/109 (38.5%; 30.2% of all 139 patients randomised) Group 2: 40/126 (31.7%; 28.2% of all 142 patients randomised) 2. Median time to complete wound closure: Group 1: 13 weeks Group 2: 28 weeks 3. Total incidence of lower limb amputations: not reported |
|
| Notes | Definition of complete closure: ''full epithelialization of the wound with absence of drainage'' | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: ''A prospective, randomised controlled single‐blind design was used'' Comment: insufficient information on method of randomisation |
| Allocation concealment (selection bias) | Unclear risk | Comment: concealment method not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “Single‐blind study'' Comment: patient was blinded, blinding of personnel was not possible |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: “The study was a prospective, single‐blind study'' Comment: the outcome assessment was not blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: ''A total of 281 patients were enrolled in this study (139 Dermagraft®, 142 control); of these, 235 patients (83.6%) could be evaluated for the primary effectiveness endpoint (109 Dermagraft®, 126 control)'' Comments: reasons for drop‐outs were not adequately described |
| Selective reporting (reporting bias) | Unclear risk | Comment: primary outcome changed to the number of 'active implants' resulting in less patients receiving the best treatment |
| Other bias | Unclear risk | Quote: ''The study enrolled diabetic patients with neuropathic full‐thickness plantar surface foot ulcers of the forefoot or heel, 31.0 cm2 in size, and eliminated ulcers that showed initial rapid healing in response to standard care during a screening period'' Comment: patients were included in the study with ulcers that initially did not respond to standard treatment. This might result in ulcers being in the control group that already had not responded to standard treatment. Furthermore, basic demographic information is not shown and the study was funded by a commercial organisation as authors are supported by Advanced Tissue Sciences, Inc |
Puttirutvong 2004.
| Methods | Single‐centre RCT (one foot centre in Thailand) with 6 months of follow‐up | |
| Participants | Eighty diabetic patients with infected lower extremity wounds Mean age (SD): 56.84 (8.96) in meshed skin graft group, 55.02 (10.12) in split‐skin graft group Mean ulcer size (SD): 104.24 cm² (152.0) in meshed skin graft group, 82.0 cm² (73.07) in split‐skin graft group Mean ulcer duration: not stated |
|
| Interventions | Group 1 (n = 36): Meshed skin graft Group 2 (n = 44): Split‐skin graft |
|
| Outcomes | 1. Incidence of complete wound closure after 6 months: not specifically stated, but this seems to be 100% in both groups 2. Mean time to complete wound closure: Group 1: 19.84 (7.37) days Group 2: 20.36 (7.21) days 3. Total incidence of lower limb amputations: not reported |
|
| Notes | Definition of complete closure: Excellent (95% < 14 days with smooth scar); good (< 21 days), fair (> 21 days), or poor (> 28 days with poor scar or recurrence) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “This prospective randomized control study'' Comment: insufficient information about the method of randomisation |
| Allocation concealment (selection bias) | Unclear risk | Quote: “This prospective randomized control study'' Comment: insufficient information about the randomisation procedure |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: not stated, but blinding not likely |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: not stated, but blinding not likely |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: insufficient information about dropouts |
| Selective reporting (reporting bias) | Low risk | Comment: complete wound healing was not assessed as primary outcome parameter |
| Other bias | Unclear risk | Comment: statistical analysis is dubious (t‐test) |
Reyzelman 2009.
| Methods | Multicentred RCT (11 centres in the USA) with 12 weeks’ follow‐up | |
| Participants | Eighty‐six patients with diabetic foot ulcers, University of Texas grade 1 or 2, with a size between 1 and 25 cm² Mean age (SD): 55.4 (9.6) in intervention group, 58.9 (11.6) in control group Mean ulcer size (SD): 3.6 cm² (4.3) in intervention group, 5.1 cm² (4.8) in control group Mean ulcer duration (SD): 23.3 weeks (22.4) in intervention group, 22.9 weeks (29.8) in control group |
|
| Interventions | Group 1 (n = 47; 46 per‐protocol): Graftjacket®, a human acellular dermal tissue matrix Group 2 (n = 39): Standard care |
|
| Outcomes | 1. Incidence of complete wound closure after 12 weeks: Group 1: 32/47 (68.1%) Group 2: 18/39 (46.2%) 2. Mean time to complete wound closure: Group 1: 5.7 weeks (SD 3.5) Group 2: 6.8 weeks (SD 3.3) 3. Total incidence of lower limb amputations: not reported |
|
| Notes | Definition of complete closure: ''100% re‐epithelialisation without drainage'' | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote:''A prospective, randomised, multicenter study'' Comment: insufficient information about the method of randomisation |
| Allocation concealment (selection bias) | Unclear risk | Quote:''A prospective, randomised, multicenter study'' Comment: insufficient information about the method of randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: not stated, but blinding not likely |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Patients were evaluated by the investigators at least once every 7 days to obtain ulcer measurements and to perform dressing changes" Comment: investigators actively participated in treatment and assessments |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: numbers and reasons of discontinuation are shown in figure 1 |
| Selective reporting (reporting bias) | Low risk | Comment: all clinically relevant and reasonably expected outcomes were reported |
| Other bias | Unclear risk | Quote: "This clinical trial was supported by Wright Medical Technology, Inc." Comment: funded by commercial organisation |
Sanders 2014.
| Methods | Multicentred RCT (2 hospital‐based wound care centres in the USA) with 20 weeks of follow‐up | |
| Participants | Twenty‐three patients with full‐thickness diabetic foot ulcers with a size between 1 and 10 cm² and present for at least 30 days Mean age (SD): 56.58 (14.96) in HFDS group, 60.0 (15.74) in HSA group Mean ulcer size (SD): 4.78 cm² (3.95) in HFDS group, 5.45 cm² (5.58) in HSA group Mean ulcer duration (SD): 11.71 weeks (8.02) HFDS group, 43.58 weeks (78.08) in HSA |
|
| Interventions | Group 1 (n = 12): HFDS, an invitro‐engineered, human fibroblast‐derived dermal skin substitute (Dermagraft®) Group 2 (n = 11): HSA, a biologically active cryopreserved human skin allograft (TheraSkin®) |
|
| Outcomes | 1. Incidence of complete wound closure after 12 weeks: Group 1: 4/12 (33.3%) Group 2: 7/11 (63.6%) 2. Mean time to complete wound closure: Group 1: 12.5 weeks (range 7‐20 weeks) Group 2: 8.9 (range 5‐20) 3. Total incidence of lower limb amputations: not reported |
|
| Notes | Definition of complete closure: ''only fully epithelialized wounds were considered healed'' | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed using a series of sealed envelopes that designated the biologically active treatment to be applied" Comment: allocation concealed using sealed envelopes |
| Allocation concealment (selection bias) | Low risk | Quote: "Envelopes were randomized in blocks of six; however, the investigators were unaware of the block size or randomization scheme" Comment: allocation concealed using sealed envelopes and the investigators were unaware of the randomisation scheme |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Because the grafts have a different physical appearance, it was not possible to disguise the type of graft used at the time of evaluation" Comment: blinding of participants and personnel was not possible |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Because the grafts have a different physical appearance, it was not possible to disguise the type of graft used at the time of evaluation" Comment: blinding of outcome assessment was not possible |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: authors gave the impression that there had been no dropouts or withdrawals, but this was not specifically stated |
| Selective reporting (reporting bias) | Low risk | Comment: all clinically relevant and reasonably expected outcomes were reported |
| Other bias | Unclear risk | Quote: "Dr. Sanders and Dr. A. Landsman are paid consultants" Comment: at least one of the authors is connected to a commercial organisation |
Uccioli 2011.
| Methods | Multicentred RCT (7 specialised diabetic foot centres in Italy) with 20 weeks of follow‐up for efficacy and 18 months for safety | |
| Participants | One hundred and eighty patients with diabetic foot ulcers, Wagner grades 1 or 2, with a size ≥ 2 cm² and present for at least 1 month Mean age (SD): 61 (10) in intervention group, 62 (11) control group Mean ulcer size (SD): 8.8 cm² (9.4) in intervention group, 6.7 cm² (7.7) in control group Mean ulcer duration (SD): 6.82 months (5.09) in intervention group, 5.43 months (4.83) in control group |
|
| Interventions | Group 1 (n = 90, 80 in analyses): Hyalograft 3D® and Laserskin® autograft Group 2 (n = 90, 80 in analyses): Non‐adherent paraffin gauze, control group |
|
| Outcomes | 1. Incidence of complete wound closure after 12 weeks: Group 1: 19/90 (21.1%) Group 2: 17/90 (18.9%) 2. Mean time to complete wound closure: Group 1: 50 days Group 2: 58 days 3. Total incidence of lower limb amputations: not reported |
|
| Notes | Definition of complete closure: ''complete reepithelialization without exudates ad eschar'' | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: ''At the first visit, eligible patients were randomized in a 1:1 ratio, using a computer‐generated method, in a block size of 4 and stratified by center'' |
| Allocation concealment (selection bias) | Low risk | Quote: ''For randomization, each site used sealed envelopes opened in numerical order'' |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: ''This was an open, randomized, controlled study'' |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: investigators actively participated in treatment and assessments |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: ''A total of 180 patients were screened and randomized (n = 90 per group). Of these, 7 patients had an ulcer area < 1 cm2 after the run‐in period and were excluded, and 13 patients did not return to the investigational site after the baseline visit. Thus, 160 patients were included in the intention‐to treat analysis (n = 80 per group)'' Comment: all randomised patients should have been included in the intention‐to treat analysis |
| Selective reporting (reporting bias) | Low risk | Comment: all clinically relevant and reasonably expected outcomes were reported |
| Other bias | Unclear risk | Quote 1: ''It was ended prematurely because of the low enrolment with fewer number of randomized patients than initially planned, with larger ulcers at baseline in the treated group, which may have underpowered the trial and included hard‐to‐heal ulcers in the treated group'' Quote 2: "This study was supported by a research grant from Anika Therapeutics srl" Comment 1: the study was prematurely ended and funded by commercial organisation Comment 2: basic demographic information is not shown Comment 3: only patients not healed after 2 weeks of control treatment were enrolled |
Veves 2001.
| Methods | Multicentred (24 centres in the USA) RCT with 12 weeks of follow‐up | |
| Participants | 208 patients with non‐infected, non‐ischaemic neuropathic diabetic foot ulcers with a size between 1 and 16 cm² and present for at least two weeks Mean age (SD): 58 (10) in intervention group, 56 (10) control group Mean ulcer size (SD): 2.97 cm² (3.10) in intervention group, 2.83 cm² (2.45) in control group Mean ulcer duration (SD): 11.5 months (13.3) in intervention group, 11.1 months (12.5) in control group |
|
| Interventions | Group 1 (n = 112): Graftskin® Group 2 (n = 96): Saline‐moistened gauze, control group |
|
| Outcomes | 1. Incidence of complete wound closure after 12 weeks: Group 1: 63/112 (56.3%) Group 2: 36/96 (37.5%) 2. Median time to complete wound closure: Group 1: 65 days Group 2: 90 days 3. Total incidence of lower limb amputations (on study limb) Group 1: 7/112 (6.3%) Group 2: 15/96 (15.6%) |
|
| Notes | Definition of complete closure: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: ''Patients were randomized at the end of the screening visit according to a computer generated randomization schedule provided by the sponsor'' |
| Allocation concealment (selection bias) | Low risk | Quote: ''For randomization, each site used sealed envelopes opened in numerical order'' |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote:''Patients were informed about the results of randomization during their next visit'' Comment: participants and clinicians were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: ''Complete dressing changes were performed by the investigator at visits scheduled for study weeks 1, 2, 3, and 4'' Comment: investigators actively participated in treatment and assessments |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: numbers and reasons of discontinuation are described |
| Selective reporting (reporting bias) | Low risk | Comment: all clinically relevant and reasonably expected outcomes were reported |
| Other bias | Unclear risk | Comment: patients were pretreated with moist saline gauze and the non‐responders were then randomised; control patients again received moist saline, who had already shown not to respond Furthermore, the study was funded by a commercial organisation |
You 2012.
| Methods | Multicentred RCT (three university hospitals in Korea) with a follow‐up duration of 12 weeks for efficacy and 6 months for safety and recurrence | |
| Participants | Fifty‐nine patients with diabetic foot ulcers, Texas grade 1 or 2, with a size of ≥ 1 cm² and without signs of healing for at least six weeks Mean age (SD) in per‐protocol set: 63.5 (9.0) in intervention group, 62.4 (9.4) control group Mean ulcer size (SD) in per‐protocol set: 4.0 cm² (3.5) in intervention group, 5.2 cm² (6.4) in control group Mean ulcer duration (SD) in per‐protocol set: 0.33 years (0.24) in intervention group, 0.40 years (0.68) in control group |
|
| Interventions | Group 1 (n = 27, 20 in per‐protocol set): Allogenic keratinocyte treatment Group 2 (n = 32, 26 in per‐protocol set): Vaseline gauze, control group |
|
| Outcomes | 1. Incidence of complete wound closure after 12 weeks: Group 1: 20/27(100% in per‐protocol group; 74.1% in intention‐to treat analysis, authors report 85%) Group 2: 18/32 (69.2% in per‐protocol group; 56.3% in intention‐to treat analysis, authors report 59%) 2. Mean time to complete wound closure Group 1 (SD): 41.6 (26.1) days in intention‐to treat analysis Group 2 (SD): 43.6 (19.4) days in intention‐to treat analysis 3. Total incidence of lower limb amputations: not reported |
|
| Notes | Definition of complete closure: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: ''Randomization schedules were stratified according to clinical center with the use of a permuted‐block method with a block size of four to six using the Statistical Analysis System and treatment allocation ratio of 1:1 and stratification at the three sites'' |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information concerning method of concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: ''Wound evaluation was performed in a single‐blinded fashion. The patients did not know whether or not their wounds had been treated with the keratinocytes, but the wound evaluators were aware of the method of treatment'' Comment: patient was blinded, blinding of personnel was not possible |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: ''Wound evaluation was performed in a single‐blinded fashion. The patients did not know whether or not their wounds had been treated with the keratinocytes, but the wound evaluators were aware of the method of treatment'' Comment: the outcome assessment was not blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: numbers and reasons of discontinuation are shown in figure 1. However, not all exact numbers in intention‐to treat analysis were reported. |
| Selective reporting (reporting bias) | Unclear risk | Comment: not all exact numbers in intention‐to treat analysis were reported |
| Other bias | Unclear risk | Quote: "This study was supported by grants from Tego Science" Comment: funded by commercial organisation |
You 2014.
| Methods | Multicentre RCT (two university hospitals in Korea) with 12 weeks of follow‐up | |
| Participants | Sixty‐five patients with diabetic foot ulcers, Wagner grade 1 or 2, with a size ≥ 1 cm² and without signs of healing for at least six weeks Mean age (SD): 61.2 (11.4) in intervention group, 63.8 (10.7) in control group Mean ulcer size (SD): 3.5 cm² (3.7) in intervention group, 2.9 cm² (2.7) in control group Mean ulcer duration (SD): 6.1 months (16.4) in intervention group, 6.2 months (19.7) in control group |
|
| Interventions | Group 1 (n = 33; 31 per‐protocol): autologous fibroblast‐hyaluronic acid complex Group 2 (n = 32): polyurethane foam dressing |
|
| Outcomes | 1. Incidence of complete wound closure after 12 weeks: Group 1: 26/33 (78.79%) Group 2: 11/32 (34.38%) 2. Mean time to complete wound closure for patients that healed: Group 1: 36.4 days (SD 17.6) Group 2: 48.4 days (SD 13.1) 3. Total incidence of lower limb amputations: not reported |
|
| Notes | Definition of complete closure: ''a completely epithelialised state in the absence of any discharge and which allowed the patient to shower'' | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: Randomiation schedules were stratified with the use of a permuted block method with a block size of four to six using the statistical analysis system and a treatment allocation ratio of 1:1" |
| Allocation concealment (selection bias) | Unclear risk | Quote: Randomiation schedules were stratified with the use of a permuted block method with a block size of four to six using the statistical analysis system and a treatment allocation ratio of 1:1" Comment: insufficient information about the concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: not stated, but blinding not likely |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: not stated, but blinding not likely |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: ''Two patients in the treatment group were excluded before application of the autologous fibroblast‐hyaluronic acid complex owing to contamination during cell culture Comment: numbers and reasons of discontinuation are shown in figure 2A. However, all randomised patients should have been included in the intention‐to treat analysis |
| Selective reporting (reporting bias) | Low risk | Comment: all clinically relevant and reasonably expected outcomes were reported |
| Other bias | Unclear risk | Quote: "This study was supported by grants from ChaBio & Diostec" Comment: funded by commercial organisation |
Zelen 2013.
| Methods | Single‐centre RCT (one hospital in the USA) with 6 weeks of follow‐up | |
| Participants | Twenty‐five patients with diabetic foot ulcers between 1 and 25 cm² and present for at least four weeks Mean age (SD): 56.4 (14.7) in intervention group, 61.7 (10.3) in control group Mean ulcer size (SD): 2.6 cm² (1.9) in intervention group, 3.4 cm² (2.9) in control group Mean ulcer duration (SD): 14.1 weeks (13.0) in intervention group, 16.4 weeks (15.5) in control group |
|
| Interventions | Group 1 (n = 13): Dehydrated human amniotic membrane (EpiFix®) Group 2 (n = 12): Moist wound therapy, standard care |
|
| Outcomes | 1. Incidence of complete wound closure after 6 weeks: Group 1: 12/13 (92.3%) Group 2: 1/12 (8.3%) 2. Mean time to complete wound closure for patients that healed: Group 1: 2.5 weeks (SD 1.9, n = 12) Group 2: 5 weeks (n = 1) 3. Total incidence of lower limb amputations: no amputations reported |
|
| Notes | Definition of complete closure: ''complete epithelialisation of the open area of the wound'' | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A prospective, stratified, randomised, comparative, parallel group, non blinded clinical trial [...] The randomisation schedule was balanced and permuted in blocks of 10'' |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information concerning the concealment of the allocation schedule |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: ''A prospective, stratified, randomised, comparative, parallel group, non blinded clinical trial'' Comment: participants and personnel were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: ''A prospective, stratified, randomised, comparative, parallel group, non blinded clinical trial'' Comment: the outcome assessment was not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: ''Patients were exited from the study and allowed to seek alternative treatment if the index ulcer did not achieve 50% area reduction at 6 weeks'' 'Comment: no dropouts were reported at the 6‐week time point, one dropout was reported at the final endpoint after 12 weeks. After 65 weeks there were still 12 patients in the EpiFix® and 13 in the standard care group |
| Selective reporting (reporting bias) | Low risk | Comment: all clinically relevant and reasonably expected outcomes were reported |
| Other bias | Unclear risk | Comment: surgical debridement of all necrotic tissue was performed only in EpiFix® group. Furthermore, at least one of the authors is connected to a commercial organisation |
BCM: bilayered cellular matrix BSS: bioengineered skin substitute HFDS: human fibroblast‐derived dermal skin HSA: human skin allograft RCT: randomised controlled trial SD: standard deviation SSA: split‐thickness skin substitute
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Hanft 2002 | Single‐centre results from multicentre study (Marston 2003) |
| Moustafa 2007 | No outcome data before cross‐over point |
| Niezgoda 2005 | The use of a recombinant human platelet‐derived growth factor in the control group |
| Pham 1999 | Single‐centre results from multicentre study (Veves 2001) |
| Sams 2002 | Single‐centre results from multicentre study (Veves 2001) |
Characteristics of ongoing studies [ordered by study ID]
NCT01693133.
| Trial name or title | Trial of dehydrated human amnion/chorion membrane (dHACM) In the management of diabetic foot ulcers |
| Methods | Randomised controlled trial |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | EpiFix® and standard of care |
| Outcomes | Percentage of subjects with complete closure of the study ulcer |
| Starting date | July 2012 |
| Contact information | William Tettelbach, Intermountain Medical Center, Myrray, Utah, USA |
| Notes | Recruiting |
NCT02070835.
| Trial name or title | Study of ReCell® treating for diabetic foot ulcers |
| Methods | Randomised controlled trial |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | ReCell® with skin graft (experiment group) versus skin graft (control group) |
| Outcomes | Healing rate by week 4, recurrent rate at 6 months, complication rate at week 4 |
| Starting date | March 2013 |
| Contact information | Hu Zhicheng, First Affiliated Hospital, Sun Yat‐Sen University |
| Notes | Recruiting |
NCT02120755.
| Trial name or title | A randomized comparison of AmnioClear™ human allograft amniotic membrane versus moist wound dressing in the treatment of diabetic wounds |
| Methods | Randomised controlled trial |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | AmnioClear™ human allograft amniotic membrane versus standard moist wound dressing |
| Outcomes | Reduction in wound size at week 12 |
| Starting date | May 2014 |
| Contact information | Cameron Howes, Duke University |
| Notes | Not yet recruiting |
NCT02331147.
| Trial name or title | Allogenic dermis versus standard care in the management of diabetic foot ulcers |
| Methods | Randomised controlled trial |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Application of human allogenic dermis with dressing application |
| Outcomes | Proportion of ulcers completely healed ulcers at 6 weeks |
| Starting date | December 2014 |
| Contact information | Charles M Zelen, Professional Education and Research Institute, Roanoke, Viginia, USA |
| Notes | Recruiting |
NCT02399826.
| Trial name or title | Study of amniotic membrane graft in the management of diabetic foot ulcers |
| Methods | Randomised controlled trial |
| Participants | Inclusion Criteria:
Exclusion Criteria:
|
| Interventions | Amniotic membrane/amnioband |
| Outcomes | Proportion healed wounds at 4 and 12 weeks. Mean time to healing and cost‐effectiveness |
| Starting date | March 2015 |
| Contact information | Lawrence Didomenico, Lower Extremity Institute of Research and Therapy, Canfield, Ohio, USA |
| Notes | Recruiting |
ABI: ankle brachial index
ADA: American Diabetes Association UT: University of Texas Diabetic Wound Classification
Contributions of authors
Katrien Santema: conceived, designed and co‐ordinated the review; extracted data and checked quality of data extraction; undertook and checked quality assessment; interpreted and analysed data; performed statistical analysis and checked quality of statistical analysis; wrote and edited the review, including writing the first draft; approved the final review prior to submission; and wrote to study authors/experts/companies.
Paul Poyck: extracted data and checked quality of data extraction; undertook and checked quality assessment; interpreted and analysed data; performed statistical analysis and checked quality of statistical analysis; performed part of writing and editing the review; and approved the final review prior to submission.
Dirk Ubbink: extracted data and checked quality of data extraction; undertook and checked quality assessment; interpreted and analysed data; performed statistical analysis and checked quality of statistical analysis; performed part of writing and editing the review; and approved the final review prior to submission.
Contributions of editorial base
Joan Webster (Editor): edited the protocol and review, advised on methodology, interpretation and content and approved the final protocol prior to submission.
Sally Bell‐Syer and Gill Rizzello (Managing Editors): co‐ordinated the editorial process; advised on interpretation and content; edited the protocol and review.
Amanda Briant: designed the search strategy and edited the search methods section. Rocio Rodriguez ran the searches.
Sources of support
Internal sources
-
Academic Medical Centre at the University of Amsterdam, Amsterdam, Netherlands.
Salary
External sources
This project was supported by the National Institute for Health Research (NIHR) via Cochrane Infrastructure funding to Cochrane Wounds. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health, UK.
Declarations of interest
Katrien Santema: none known.
Paul Poyck: none known.
Dirk Ubbink: none known.
Edited (no change to conclusions), comment added to review
References
References to studies included in this review
Brigido 2006 {published data only}
- Brigido SA. The use of an acellular dermal regenerative tissue matrix in the treatment of lower extremity wounds: a prospective 16‐week pilot study. International Wound Journal 2006;3(3):181‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Caravaggi 2003 {published data only}
- Caravaggi C, Giglio R, Pritelli C, Sommaria M, Dalla Noce S, et al. HYAFF 11‐based autologous dermal and epidermal grafts in the treatment of noninfected diabetic plantar and dorsal foot ulcers: a prospective, multicenter, controlled, randomized clinical trial. Diabetes Care 2003;26(10):2853‐9. [DOI] [PubMed] [Google Scholar]
DiDomenico 2011 {published data only}
- DiDomenico L, Emch KJ, Landsman AR, Landsman A. A prospective comparison of diabetic foot ulcers treated with either a cryopreserved skin allograft or a bioengineered skin substitute. Wounds 2011;23(7):184‐9. [PubMed] [Google Scholar]
Edmonds 2009 {published data only}
- Edmonds M, the European and Australian Apligraf Diabetic Foot Ulcer Study Group. Apligraf in the treatment of neuropathic diabetic foot ulcers. The International Journal of Lower Extremity Wounds 2009;8(1):11‐8. [DOI] [PubMed] [Google Scholar]
Gentzkow 1996 {published data only}
- Gentzkow GD, Iwasaki SD, Hershon KS, Mengel M, Prendergast JJ, Ricotta JJ, et al. Use of dermagraft, a cultured human dermis, to treat diabetic foot ulcers. Diabetes Care 1996;19(4):350‐4. [DOI] [PubMed] [Google Scholar]
Landsman 2008 {published data only}
- Gilligan AM, Waycaster CR, Landsman AL. Wound closure in patients with DFU: a cost‐effectiveness analysis of two cellular/tissue‐derived products. Journal of Wound Care 2015;23(3):149‐56. [DOI] [PubMed] [Google Scholar]
- Landsman A, Roukis TS, DeFronzo DJ, Agnew P, Petranto RD, Suprenant M. Living cells or collegen matrix: which is more beneficial in the treatment of diabetic foot ulcers. Wounds 2008;20(5):111‐6. [PubMed] [Google Scholar]
Lipkin 2003 {published data only}
- Lipkin S, Chaikof E, Isseroff Z, Silverstein P. Effectiveness of bilayered cellular matrix in healing of neuropathic diabetic foot ulcers. Wounds 2003;15(7):230‐6. [Google Scholar]
Marston 2003 {published data only}
- Frykberg RG, Marston WA, Cardinal M. The incidence of lower‐extremity amputation and bone resection in diabetic foot ulcer patients treated with a human fibroblast‐derived dermal substitute. Advances in Skin & Wound Care 2015;28:17‐20. [DOI] [PubMed] [Google Scholar]
- Marston WA, Hanft J, Norwood P, Pollak R. The efficacy and safety of Dermagraft in improving the healing of chronic diabetic foot ulcers: results of a prospective randomized trial. Diabetes Care 2003;26(6):1701‐5. [DOI] [PubMed] [Google Scholar]
Naughton 1997 {published data only}
- Naughton G, Mansbridge J, Gentzkow G. A metabolically active human dermal replacement for the treatment of diabetic foot ulcer. Artificial Organs 1997;21(11):1203‐10. [DOI] [PubMed] [Google Scholar]
- Pollak RA, Edington H, Jensen JL, Kroeker RO, Gentzkow GD. A human dermal replacement for the treatment of diabetic foot ulcers. Wounds 1997;9:175‐83. [Google Scholar]
Puttirutvong 2004 {published data only}
- Puttirutvong P. Meshed skin graft versus split thickness skin graft in diabetic ulcer coverage. Journal of the Medical Association of Thailand 2004;87(1):66‐72. [PubMed] [Google Scholar]
Reyzelman 2009 {published data only}
- Reyzelman A, Crews RT, Moore JC, Moore L, Mukker JS, Offutt S, et al. Clinical effectiveness of an acellular dermal regenerative tissue matrix compared to standard wound management in healing diabetic foot ulcers: a prospective, randomised, multicentre study. International Wound Journal 2009;6(3):196‐208. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sanders 2014 {published data only}
- Sanders L, Landsman AD, Landsman A, Keller N, Cook J, Hopson M. A prospective, multicenter, randomized, controlled clinical trial comparing a bioengineered skin substitute to a human skin allograft. Ostomy Wound Management 2014;60(8):26‐38. [PubMed] [Google Scholar]
Uccioli 2011 {published data only}
- Uccioli L, Giurato L, Ruotolo V, Ciavarella A, Grimaldi MS, Piaggesi A, et al. Two‐step autologous grafting using HYAFF scaffolds in treating difficult diabetic foot ulcers: results of a multicenter, randomized controlled clinical trial with long‐term follow‐up. The International Journal of Lower Extremity Wounds 2011;10(2):80‐5. [DOI] [PubMed] [Google Scholar]
Veves 2001 {published data only}
- Veves A, Falanga V, Armstrong DG, Sabolinski ML. Graftskin, a human skin equivalent, is effective in the management of noninfected neuropathic diabetic foot ulcers: a prospective randomized multicenter clinical trial. Diabetes Care 2001;24(2):290‐5. [DOI] [PubMed] [Google Scholar]
You 2012 {published data only}
- You HJ, Han SK, Lee, JW, Chang H. Treatment of diabetic foot ulcers using cultured allogeneic keratinocytes‐‐a pilot study. Wound Repair and Regeneration 2012;20(4):491‐9. [DOI] [PubMed] [Google Scholar]
You 2014 {published data only}
- You HJ, Han SK, Rhie JW. Randomised controlled clinical trial for autologous fibroblast‐hyaluronic acid complex in treating diabetic foot ulcers. Journal of Wound Care 2014;23(11):521‐30. [DOI] [PubMed] [Google Scholar]
Zelen 2013 {published data only}
- Zelen CM, Serena TE, Denoziere G, Fetterolf DE. A prospective, randomised comparative parallel study amniotic membrane wound graft in the management of diabetic foot ulcers. International Wound Journal 2013;10(5):502‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Hanft 2002 {published data only}
- Hanft JR, Suprenant MS. Healing of chronic foot ulcer in diabetic patients treated with a human fibroblast‐derived dermis. The Journal of Foot & Ankle Surgery 2002;41(5):291‐9. [DOI] [PubMed] [Google Scholar]
Moustafa 2007 {published data only}
- Moustafa M, Bullock AJ, Creagh FM, Heller S, Jeffcoate W, Game F, et al. Randomized, controlled, single‐blind study on use of autologous keratinocytes on a transfer dressing to treat nonhealing diabetic ulcers. Regenerative medicine 2007;2(6):887‐902. [DOI] [PubMed] [Google Scholar]
Niezgoda 2005 {published data only}
- Niezgoda JA, Gils CC, Frykberg RG, Hodde JP. Randomized clinical trial comparing OASIS Wound Matrix to Regranex Gel for diabetic ulcers. Advances in Skin & Wound Care 2005;18(5 Pt 1):258‐66. [DOI] [PubMed] [Google Scholar]
Pham 1999 {published data only}
- Pham HT, Rosenblum BI, Lyons TE, Giueini JM, Charzan JS, Habershaw GM, et al. Evaluation of a human skin equivalent for the treatment of diabetic foot ulcers in a prospective, randomized, clinical trial. Wounds 1999;11(4):1044‐6. [Google Scholar]
Sams 2002 {published data only}
- Sams HH, Chen J, King LE. Graftskin treatment of difficult to heal diabetic foot ulcers: one center's experience. Dermatologic Surgery 2002;28:698‐703. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Lavery 2014 {published data only}
- Lavery LA, Fulmer J, Shebetka KA, Regulski M, Vayser D, Fried D, et al. The Grafix Diabetic Foot Ulcer Study Group. The efficacy and safety of Grafix® for the treatment of chronic diabetic foot ulcers: results of a multi‐centre, controlled, randomised, blinded, clinical trial. International Wound Journal 2014;11:554‐60. [DOI: 10.1111/iwj.12329] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
NCT01693133 {published data only}
- NCT01693133. Trial of Dehydrated Human Amnion/Chorion Membrane (dHACM) In the Management of Diabetic Foot Ulcers. https://www.clinicaltrials.gov/ct2/show/NCT01693133 (accessed 14 August 2015).
NCT02070835 {published data only}
- NCT02070835. Study of ReCell® Treating for Diabetic Foot Ulcers. https://clinicaltrials.gov/ct2/show/NCT02070835 (accessed 14 August 2015).
NCT02120755 {published data only}
- NCT02120755. A Randomized Comparison of AmnioClear™ Human Allograft Amniotic Membrane vs. Moist Wound Dressing in the Treatment of Diabetic Wounds. https://clinicaltrials.gov/ct2/show/NCT02120755 (accessed 14 August 2015).
NCT02331147 {published data only}
- NCT02331147. Allogenic Dermis Versus Standard Care in the Management of Diabetic Foot Ulcers. https://clinicaltrials.gov/ct2/show/NCT02331147 (accessed 14 August 2015).
NCT02399826 {published data only}
- NCT02399826. Study of Amniotic Membrane Graft in the Management of Diabetic Foot Ulcers. https://clinicaltrials.gov/ct2/show/NCT02399826 (accessed 14 August 2015).
Additional references
Abbot 2005
- Abbott CA, Garrow AP, Carrington AL, Morris J, Ross ER, Boulton AJ. Foot ulcer risk is lower in South Asian and African‐Caribbean compared to European diabetic patients in the UK: the North‐West Diabetes Footcare Study. Diabetes Care 2005;28(8):1869–75. [DOI] [PubMed] [Google Scholar]
Apelqvist 1995
- Apelqvist J, Ragnarson‐Tennvall G, Larsson J, Persson U. Long‐term costs for foot ulcers in diabetic patients in a multidisciplinary setting. Foot & Ankle International 1995;16(7):388‐94. [DOI] [PubMed] [Google Scholar]
Apelqvist 1999
- Apelqvist J, Larsson J. What is the most effective way to reduce incidence of amputation in the diabetic foot?. Diabetes/Metabolism Research and Reviews 1999;16 Suppl 1:75‐83. [DOI] [PubMed] [Google Scholar]
Bergin 2006
- Bergin S, Wraight P. Silver based wound dressings and topical agents for treating diabetic foot ulcers. Cochrane Database of Systematic Reviews 2006, Issue 1. [DOI: 10.1002/14651858.CD005082.pub2] [DOI] [PubMed] [Google Scholar]
Blozik 2008
- Blozik E, Scherer M. Skin replacement therapies for diabetic foot ulcers: systematic review and meta‐analysis. Diabetes Care 2008;41(4):693‐4. [DOI] [PubMed] [Google Scholar]
Boulton 2008
- Boulton AJ. The diabetic foot: grand overview, epidemiology and pathogenesis. Diabetes/Metabolism Research and Reviews 2008;24 Suppl 1:3‐6. [DOI] [PubMed] [Google Scholar]
Cruciani 2013
- Cruciani M, Lipsky BA, Mengoli C, Lalla F. Granulocyte‐colony stimulating factors as adjunctive therapy for diabetic foot infections. Cochrane Database of Systematic Reviews 2013, Issue 8. [DOI: 10.1002/14651858.CD006810.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Danaei 2011
- Danaei G, Finucane MM, Lu Y, Singh GM, Cowan MJ, Paciorek CJ, et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country‐years and 2·7 million participants. The Lancet 2011;378(9785):31‐40. [DOI] [PubMed] [Google Scholar]
Dumville 2013a
- Dumville JC, O'Meara S, Deshpande S, Speak K. Alginate dressings for healing diabetic foot ulcers. Cochrane Database of Systematic Reviews 2013, Issue 6. [DOI: 10.1002/14651858.CD009110.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dumville 2013b
- Dumville JC, Deshpande S, O'Meara S, Speak K. Foam dressings for healing diabetic foot ulcers. Cochrane Database of Systematic Reviews 2013, Issue 6. [DOI: 10.1002/14651858.CD009111.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dumville 2013c
- Dumville JC, O'Meara S, Deshpande S, Speak K. Hydrogel dressings for healing diabetic foot ulcers. Cochrane Database of Systematic Reviews 2013, Issue 7. [DOI: 10.1002/14651858.CD009101.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dumville 2013d
- Dumville JC, Deshpande S, O'Meara S, Speak K. Hydrocolloid dressings for healing diabetic foot ulcers. Cochrane Database of Systematic Reviews 2013, Issue 8. [DOI: 10.1002/14651858.CD009099.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Edwards 2010
- Edwards J, Stapley S. Debridement of diabetic foot ulcers. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD003556.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Falanga 1998
- Falanga V, Margolis D, Alvarez O, Auletta M, Maggiacomo F, Altman M, et al. Rapid healing of venous ulcers and lack of clinical rejection with an allogeneic cultured human skin equivalent. Archives of Dermatology 1998;134:293‐300. [DOI] [PubMed] [Google Scholar]
Falanga 2005
- Falanga V. Wound healing and its impairment in the diabetic foot. Lancet 2005;366(9498):1736‐43. [DOI] [PubMed] [Google Scholar]
Fernando 2013
- Fernando ME, Seneviratne RM, Cunningham M, Lazzarini PA, Sangla KS, Mong Tang Y, et al. Intensive versus conventional glycaemic control for treating diabetic foot ulcers. Cochrane Database of Systematic Reviews 2013, Issue 10. [DOI: 10.1002/14651858.CD010764] [DOI] [PMC free article] [PubMed] [Google Scholar]
Frykberg 2015
- Frykberg RG, Marston WA, Cardinal M. The incidence of lower‐extremity amputation and bone resection in diabetic foot ulcer patients treated with a human fibroblast‐derived dermal substitute. Advances in Skin & Wound Care 2015;28(1):17‐20. [DOI] [PubMed] [Google Scholar]
Game 2012
- Game F. Choosing life or limb. Improving survival in the multi‐complex diabetic foot patient. Diabetes/Metabolism Research and Reviews 2012;28 Suppl 1:97‐100. [DOI] [PubMed] [Google Scholar]
Gilligan 2015
- Gilligan AM, Waycaster CR, Landsman AL. Wound closure in patients with DFU: a cost‐effectiveness analysis of two cellular/tissue‐derived products. Journal of Wound Care 2015;24(3):149‐56. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011b
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hinchliffe 2012
- Hinchliffe RJ, Andros G, Apelqvist J, Bakker K, Friederichs S, Lammer J, et al. A systematic review of the effectiveness of revascularization of the ulcerated foot in patients with diabetes and peripheral arterial disease. Diabetes/Metabolism Research and Reviews 2012;28 Suppl 1:179‐217. [DOI] [PubMed] [Google Scholar]
Izumi 2009
- Izumi Y, Satterfield K, Lee S, Harkless LB, Lavery LA. Mortality of first‐time amputees in diabetics: a 10‐year observation. Diabetes Research and Clinical Practice 2009;83:126‐31. [DOI] [PubMed] [Google Scholar]
Jeffcoate 2003
- Jeffcoate WJ, Harding KG. Diabetic foot ulcers. The Lancet 2003;261:1545‐51. [DOI] [PubMed] [Google Scholar]
Jeffcoate 2004
- Jeffcoate WJ, Houtum WH. Amputation as a marker of the quality of foot care in diabetes. Diabetologia 2004;47:2051‐8. [DOI] [PubMed] [Google Scholar]
Jull 2013
- Jull AB, Walker N, Deshpande S. Honey as a topical treatment for wounds. Cochrane Database of Systematic Reviews 2013, Issue 2. [DOI: 10.1002/14651858.CD005083.pub3] [DOI] [PubMed] [Google Scholar]
Kranke 2015
- Kranke P, Bennett MH, Martyn‐St James M, Schnabel A, Debus SE, Weibel S. Hyperbaric oxygen therapy for chronic wounds. Cochrane Database of Systematic Reviews 2015, Issue 6. [DOI: 10.1002/14651858.CD004123.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Langer 2009
- Langer A, Rogowski W. Systematic review of economic evaluations of human cell‐derived wound care products for the treatment of venous leg and diabetic foot ulcers. BMC Health Services Research 2009;9:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Levin 1998
- Levin M. Prevention and treatment of diabetic foot ulcers. Journal of Wound Ostomy and Continence Nursing 1998;21:129‐46. [DOI] [PubMed] [Google Scholar]
Lewis 2013
- Lewis J, Lipp A. Pressure‐relieving interventions for treating diabetic foot ulcers. Cochrane Database of Systematic Reviews 2013, Issue 1. [DOI: 10.1002/14651858.CD002302.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Margolis 1999
- Margolis DJ, Kantor J, Berlin JA. Healing of diabetic neuropathic foot ulcers receiving standard treatment. A meta‐analysis. Diabetes Care 1999;22:692‐5. [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred reporting items for systematic reviews and meta‐analyses: The PRISMA Statement. BMJ 2009;339:2535. [PMC free article] [PubMed] [Google Scholar]
Nabuurs‐Franssen 2005
- Nabuurs‐Franssen MH, Huijberts MS, Nieuwenhuijzen Kruseman AC, Willems J, Schaper NC. Health‐related quality of life of diabetic foot ulcer patients and their caregivers. Diabetologia 2005;48(9):1906‐10. [DOI] [PubMed] [Google Scholar]
Pham 2000
- Pham HT, Rich J, Veves A. Wound healing in diabetic foot ulceration. A review and commentary. Wounds 2000;12(4):79‐82. [Google Scholar]
Quattrini 2008
- Quattrini C, Jeziorska M, Boulton AJ, Malik RA. Reduced vascular endothelial growth factor expression and intra‐epidermal nerve fiber loss in human diabetic neuropathy. Diabetes Care 2008;31:140‐5. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schaper 2004
- Schaper NC. Diabetic foot ulcer classification system for research purposes: a progress report on criteria for including patients in research studies. Diabetes/Metabolism Research and Reviews 2004;20 Suppl 1:S90‐5. [DOI] [PubMed] [Google Scholar]
Schulz 2010
- Schultz KF, Altman DG, Moher D, for the CONSORT Group. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340:c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schunemann 2011a
- Schunemann 2011a Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results, 'Summary of findings' tables. In: Higgins JPT. Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Schunemann 2011b
- Schünemann HJ, Oxman AD, Higgins JPT, Deeks JJ, Glasziou P, Guyatt GH. Chapter 12: Interpreting results, drawing conclusions. In: Higgins JPT. Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
SIGN 2014
- Scottish Intercollegiate Guidelines Network (SIGN). Search filters. http://www.sign.ac.uk/methodology/filters.html#random (accessed April 2014).
Singh 2005
- Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA 2005;293:217‐28. [DOI] [PubMed] [Google Scholar]
Skoutas 2009
- Skoutas D, Papanas N, Georgiadis GS, Zervas V, Manes C, Maltezos E, et al. Risk factors for ipsilateral reamputation in patients with diabetic foot lesions. The International Journal of Lower Extremity Wounds 2009;8:69‐74. [DOI] [PubMed] [Google Scholar]
Stockl 2004
- Stockl K, Vanderplas A, Tafesse E, Chang E. Costs of lower‐extremity ulcers among patients with diabetes. Diabetes Care 2004;27:2129–34. [DOI] [PubMed] [Google Scholar]
Stoekenbroek 2014
- Stoekenbroek RM, Santema TB, Legemate DA, Ubbink DT, Brink A, Koelemay MJ. Hyperbaric oxygen for the treatment of diabetic foot ulcers: a systematic review. European Journal of Vascular and Endovascular Surgery 2014;47(6):647‐55. [DOI] [PubMed] [Google Scholar]
Szabo 2009
- Szabo C. Role of nitrosative stress in the pathogenesis of diabetic vascular dysfunction. British Journal of Pharmacology 2009;156:713‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Teng 2010
- Teng YJ, Li YP, Wang JW, Yang KH, Zhang YC, Wang YJ, et al. Bioengineered skin in diabetic foot ulcers. Diabetes, Obesity & Metabolism 2010;12(4):307‐15. [DOI] [PubMed] [Google Scholar]
Valensi 2005
- Valensi P, Girod I, Baron F, Moreau‐Defarges T, Guillon P. Quality of life and clinical correlates in patients with diabetic foot ulcers. Diabetes & Metabolism 2005;31:263‐71. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Santema 2014
- Santema TB, Poyck PPC, Ubbink DT. Skin grafting and tissue replacement for treating foot ulcers in people with diabetes. Cochrane Database of Systematic Reviews 2014, Issue 8. [DOI: 10.1002/14651858.CD011255] [DOI] [PMC free article] [PubMed] [Google Scholar]


