Abstract
Background
Topical local anaesthetics provide effective analgesia for patients undergoing numerous superficial procedures, including repair of dermal lacerations. The need for cocaine in topical anaesthetic formulations has been questioned because of concern about adverse effects, thus novel preparations of cocaine‐free anaesthetics have been developed. This review was originally published in 2011 and has been updated in 2017.
Objectives
To assess whether benefits of non‐invasive topical anaesthetic application occur at the expense of decreased analgesic efficacy. To compare the efficacy of various single‐component or multi‐component topical anaesthetic agents for repair of dermal lacerations. To determine the clinical necessity for topical application of the ester anaesthetic, cocaine.
Search methods
For this updated review, we searched the following databases: Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 11), Cumulative Index to Nursing and Allied Health Literature (CINAHL; 2010 to December 2016), Embase (2010 to December 2016) and MEDLINE (2010 to December 2016). We did not limit this search by language or format of publication. We contacted manufacturers, international scientific societies and researchers in the field. Weemailed selected journalsand reviewed meta‐registers of ongoing trials. For the previous version of this review, we searched these databases to November 2010.
Selection criteria
We included randomized controlled trials (RCTs) that evaluated the efficacy and safety of topical anaesthetics for repair of dermal laceration in adult and paediatric participants.
Data collection and analysis
Two review authors independently assessed trial quality and extracted data. We contacted study authors for additional information when needed. We collected adverse event information from trial reports. We assessed methodological risk of bias for each included study and employed the GRADE approach to assess the overall quality of the evidence.
Main results
The present updated review included 25 RCTs involving 3278 participants. The small number of trials in each comparison group and the heterogeneity of outcome measures precluded quantitative analysis of data for all but one outcome: pain intensity. In two pooled studies, the mean self‐reported visual analogue scale (VAS; 0 to 100 mm) score for topical prilocaine‐phenylephrine (PP) was higher than the mean self‐reported VAS (0 to 100 mm) score for topical tetracaine‐epinephrine‐cocaine (TAC) by 5.59 points (95% confidence interval (CI) 2.16 to 13.35). Most trials that compared infiltrated and topical anaesthetics were at high risk of bias, which is likely to have affected their results. Researchers found that several cocaine‐free topical anaesthetics provided effective analgesic efficacy. However, data regarding the efficacy of each topical agent are based mostly on single comparisons in trials with unclear or high risk of bias. Mild, self‐limited erythematous skin induration occurred in one of 1042 participants who had undergone application of TAC. Investigators reported no serious complications among any of the participants treated with cocaine‐based or cocaine‐free topical anaesthetics. The overall quality of the evidence according to the GRADE system is low owing to limitations in design and implementation, imprecision of results and high probability of publication bias (selective reporting of data). Additional well‐designed RCTs with low risk of bias are necessary before definitive conclusions can be reached.
Authors' conclusions
We have found two new studies published since the last version of this review was prepared. We have added these studies to those previously included and have conducted an updated analysis, which resulted in the same review conclusions as were presented previously.
Mostly descriptive analysis indicates that topical anaesthetics may offer an efficacious, non‐invasive means of providing analgesia before suturing of dermal lacerations. Use of cocaine‐based topical anaesthetics might be hard to justify, given the availability of other effective topical anaesthetics without cocaine. However, the overall quality of the evidence according to the GRADE system is low owing to limitations in design and implementation, imprecision of results and high probability of publication bias (selective reporting of data). Additional well‐designed RCTs with low risk of bias are necessary before definitive conclusions can be reached.
Plain language summary
Local anaesthesia (numbing medicine) that is directly applied to the skin can provide pain control for repair of skin lacerations
Background: Pain control during suturing of torn skin is generally achieved by injecting medication into the skin (infiltration) to numb the area. This injection itself may cause pain, but topical anaesthetics are applied directly to the skin and are painless to administer. Cocaine was one of the first anaesthetics to be successfully applied topically. Concerns over adverse effects of cocaine, its potential misuse and the administrative burden of dispensing a controlled substance led to the development of cocaine‐free topical anaesthetics. Multiple cocaine‐free topical anaesthetics have been found to provide effective anaesthesia for repair of dermal lacerations.
Study characteristics: The evidence is current to December 2016. We included in this review 25 randomized controlled trials involving 3278 participants. Studies included both adults and children. Fifteen of the included trials used self‐reporting of pain intensity by trial participants to determine the effectiveness of local anaesthetics.
Key results: Study results suggest that directly applying local anaesthetics to the skin is an effective, non‐invasive way of providing pain control during suturing or stapling of skin lacerations. Study findings on the efficacy of individual topical anaesthetics were limited by study design, and data on the efficacy of each topical agent were obtained mostly from single trials. Researchers reported no serious side effects following the use of cocaine‐containing or cocaine‐free topical anaesthetics. The overall broadly comparable effectiveness of cocaine‐free topical anaesthetics for skin laceration repair brings into question the necessity to include cocaine as a component of local anaesthetic solutions. The small number of trials in each comparison group and the range of outcome measures assessed prevented pooling and quantitative analysis of data for all but the single outcome of pain intensity.
Additional studies are necessary to directly compare the effectiveness of different formulations of topical anaesthetics. Our review was limited to pain control for repair of superficial lacerations, and our results might not be generalizable to deeper lacerations or more complex procedures performed on intact skin. Further research is needed to strengthen the evidence and to overcome the weakness of the included studies.
Quality of the evidence: The overall quality of the evidence was low owing to limitations in study design, ways that studies were carried out (implementation), imprecision of results and high probability of selective data reporting. Most of the trials that compared infiltrated and topical anaesthetics were at high risk of bias, and this was likely to influence measured effects.
Summary of findings
Summary of findings for the main comparison. Primary outcome: topical local anaesthetics compared with infiltrated local anaesthetics or other topical agents for repair of dermal lacerations.
| Pain control using topical local anaesthetics compared with infiltrated local anaesthetics or other topical agents for pain control during repair of dermal lacerations | ||||||
|
Patient or population: adults and paediatric patients with dermal laceration Settings: any medical setting Intervention: topical local anaesthetics for pain control during repair of dermal laceration Comparison: infiltrated local anaesthetics or other topical agents for pain control during repair of dermal lacerations | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| (Infiltrated local anaesthetics or other topical agents) | (Topical local anaesthetics) | |||||
| Pain intensity measures Cocaine‐containing topical anaesthetics vs infiltrated local anaesthetics |
See comment | See comment | Not estimable | 1006 (6 studies) | ⊕⊕⊝⊝ Lowa | Unable to mathematically combine results because of heterogeneity of outcome measures |
| Pain intensity measures Comparisons between different cocaine‐containing topical anaesthetics |
See comment | See comment | Not estimable | 530 (4 studies) | ⊕⊕⊝⊝ Lowb | Unable to mathematically combine results because each topical anaesthetic comparison was limited to a single study |
| Pain intensity measures Cocaine‐free topical anaesthetics compared with infiltrated local anaesthetics |
See comment | See comment | Not estimable | 543 (6 studies) | ⊕⊕⊝⊝ Lowc | Unable to mathematically combine results because of heterogeneity of outcome measures |
| Pain intensity measures Cocaine‐fee topical anaesthetics compared with cocaine‐containing topical anaesthetics |
See comment | See comment | Not estimable | 1231 (11 studies) | ⊕⊕⊝⊝ Lowd | Two of the 11 trials studied a common topical anaesthetic and could be mathematically combined. |
| Pain intensity measures Comparisons between different cocaine‐free topical anaesthetics |
See comment | See comment | Not estimable | 656 (5 studies) | ⊕⊕⊝⊝ Lowe | Trials could not be mathematically combined because each study compared a different cocaine‐free topical anaesthetic. |
| Anaesthetic‐related adverse effects | Study population |
RR 0 (0 to 0) |
1686 (11 studies) | |||
| 1 per 1000 | 0 per 1000 (0 to 0) | |||||
| Medium‐risk population | ||||||
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
aEach of the trials had high risk of bias in multiple domains or unclear risk of bias in three domains.
bTwo of the four trials had at least one domain that was at high risk of bias.
cTwo of the trials had unclear risk of bias in multiple domains, and the other two studies had high risk of bias in two domains.
dSix of the studies had high risk of bias for at least one domain, and the other five studies had unclear risk of bias for one or more domains.
eEach of the five trials had unclear risk of bias in one or more domains. However, no trials contained any domains that were clearly at high risk
Summary of findings 2. Primary outcome subanalysis: pain intensity measures of topical prilocaine‐phenylephrine (PP) and topical tetracaine‐epinephrine‐cocaine (TAC).
| Primary outcome subanalysis: pain intensity measures of topical prilocaine‐phenylephrine (PP) and topical tetracaine‐epinephrine‐cocaine (TAC) | ||||||
|
Patient or population: treatment repair of dermal laceration Setting: any medical setting Intervention: topical prilocaine‐phenylephrine (PP) Comparison: topical tetracaine‐epinephrine‐cocaine (TAC) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with topical tetracaine‐epinephrine‐cocaine (TAC) | Risk with topical prilocaine‐phenylephrine (PP) | |||||
| Participant self‐reported VAS (0‐100 mm) pain scores | Mean participant self‐reported VAS (0‐100 mm) pain score was 0. | Mean participant self‐reported VAS (0‐100 mm) pain scores in the intervention group was 5.59. | ‐ | 240 (2 studies) | Lowa | 5.59 (95% CI for effect estimate, 2.16 to 13.35) |
| *Risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence. High quality: We are very confident that the true effect lies close to the estimate of effect. Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of effect but may be substantially different. Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of effect. Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. | ||||||
aEach of the trials had unclear risk of bias in one or more domains. However, no trials included any domains that were clearly at high risk.
Background
Local anaesthetic efficacy (capacity for producing desired anaesthetic effect) during procedures such as wound repair is assessed by the patient's self‐report of pain intensity during the intervention. Acceptable tools for quantifying pain intensity include the visual analogue scale (VAS), the numerical rating scale, the verbal rating scale, the Faces scale and other validated descriptors of pain intensity or relief. Studies have shown non‐concordance between participants' and practitioners' assessments of procedure‐related pain intensity (Benzon 2011; Castarlenas 2016; Choiniere 1990; Hjermstad 2011; Singer 1999; Stephenson 1994).
Description of the condition
Pain caused by repair of torn skin may be an unpleasant experience for patients. Analgesia or pain control is conventionally achieved through local anaesthetic infiltration. Local anaesthetics make up a class of drugs that interrupt the transmission of electrical impulses along sensory nerves by inactivating sodium channels (Stoelting 1999). However, infiltration of local anaesthetics, which involves injecting medication into the skin, may itself cause significant pain (Kundu 2002). Many patients, especially children, fear or dislike needles. Topical anaesthetics are not injected. Rather, agents are directly applied to a local area of the skin. Therefore, topical anaesthesia may be preferable to infiltration anaesthesia for pain control during skin laceration repair. Topical anaesthetics are available in several forms, including solutions, gels, creams, ointments and skin patches. Adverse reactions to topical local anaesthetics include local responses (rash, stinging) and systemic allergic reactions (diffuse swelling, difficulty breathing, anaphylaxis) (Drug Facts and Comparisons 2015). An overdose of topical local anaesthetics may adversely affect the cardiovascular or central nervous system (Drug Facts and Comparisons 2015). Untoward effects resulting from high systemic levels of local anaesthetics include hypotension, cardiac arrhythmias (bradycardia, ventricular fibrillation, asystole), light‐headedness, double vision, a metallic taste, drowsiness and seizures (Stoelting 1999). In 1980, Pryor et al published the first report on successful use of topical anaesthesia for repair of torn skin (Pryor 1980). The initial formulation, tetracaine‐adrenaline‐cocaine (TAC), gained widespread acceptance in North America and has largely supplanted infiltration anaesthesia for this purpose (the term 'epinephrine' rather than 'adrenaline' is used in the USA) (Grant 1992). However, the necessity to include cocaine in topical anaesthetic formulations has been questioned owing to concern over possible adverse effects (Bush 2002; Grant 1992). Although application of TAC to skin lacerations results in undetectable or low systemic cocaine levels (Terndrup 1992; Vinci 1999), inadvertent mucosal application or overdose may cause significant cocaine absorption, resulting in serious consequences such as seizures (Dailey 1988; Daya 1988; Tipton 1988; Wehner 1984). Moreover, administrative and financial burdens accompany dispensing of a controlled substance that is widely abused in the community. Accordingly, over the past decade, novel preparations of cocaine‐free topical anaesthetics have been developed. Analysis of the efficacy and safety of established and recently developed topical anaesthetics is needed.
Pain caused by repair of dermal lacerations may be an unpleasant experience for patients. Analgesia or pain control is conventionally achieved through local anaesthetic infiltration (i.e. injection). However, injection of local anaesthetics into the skin may itself cause significant pain (Kundu 2002). Many patients, especially children, fear or dislike needles. Topical anaesthetics are not injected. Rather, agents are directly applied to the locally traumatized area or to adjoining skin. Therefore, topical anaesthesia may be preferable to infiltration anaesthesia for pain control during skin laceration repair.
Description of the intervention
Repair of superficial dermal laceration is usually a minor procedure that is done in an outpatient setting. Wound repair could be done with surgical sutures or by non‐invasive approaches such as skin adhesive or glue; in any case, pain control is required. Traditionally, this is accomplished by infiltrating the wound with local anaesthetics, possibly supplemented with systemic analgesia or sedation.
Local anaesthetics constitute a class of drugs that interrupt the transmission of electrical impulses along nerves by inactivating sodium channels (Brunton 2011; Stoelting 1999). Adverse reactions to topical local anaesthetics include local responses (rash, stinging) and systemic allergic reactions (diffuse swelling, difficulty breathing, anaphylaxis) (Dickerson 2014; Drug Facts and Comparisons 2015). An overdose of topical local anaesthetics may adversely affect the cardiovascular or central nervous system (Drug Facts and Comparisons 2015). Untoward effects from high systemic levels of local anaesthetics include hypotension, cardiac arrhythmias (bradycardia, ventricular fibrillation, asystole), light‐headedness, double vision, a metallic taste, drowsiness and seizures (Brunton 2011; Stoelting 1999).
Tradiltionally, local anaesthetics were injected locally, but recently, newer preparations have allowed local anaesthetics to be applied topically without the discomfort or anxiety that frequently accompanies needle injections. We aimed to compare the application of topical anaesthetics versus traditional infiltration for pain control during wound repair.
We included in this review only trials that evaluated the efficacy of topical local anaesthetics for repair of dermal (skin) lacerations. We included comparisons between:
1. infiltrated local anaesthetic agents and topically applied local anaesthetic agents; and
2. various topical local anaesthetic formulations versus a control formulation.
How the intervention might work
Local anaesthetics make up a class of drugs that interrupt the transmission of electrical impulses along nerves by inactivating sodium channels (Brunton 2011; Stoelting 1999). Topical anaesthetics are available in several different forms, including solutions, gels, creams, ointments and skin patches.
Why it is important to do this review
In 1980, Pryor et al published the first report of successful use of topical anaesthesia for repair of torn skin (Pryor 1980). The initial formulation, tetracaine‐adrenaline‐cocaine (TAC), gained widespread acceptance in North America, largely supplanting infiltration anaesthesia for this purpose (the word 'epinephrine' rather than 'adrenaline' is used in the USA) (Grant 1992). However, the necessity to include cocaine in topical anaesthetic formulations has been questioned owing to concern over possible adverse effects (Bush 2002; Grant 1992). Although application of TAC to skin lacerations results in undetectable or low systemic cocaine levels (Terndrup 1992; Vinci 1999), inadvertent mucosal application or overdose may cause significant cocaine absorption, resulting in serious consequences such as seizures (Dailey 1988; Daya 1988; Tipton 1988; Wehner 1984). Moreover, administrative and financial burdens accompany dispensing of a controlled substance that is widely abused in the community. Accordingly, novel preparations of cocaine‐free topical anaesthetics have been developed. Analysis of the efficacy and safety of established and recently developed topical anaesthetics is needed.
Objectives
To assess whether benefits of non‐invasive topical anaesthetic application occur at the expense of decreased analgesic efficacy. To compare the efficacy of various single‐component or multi‐component topical anaesthetic agents for repair of dermal lacerations. To determine the clinical necessity for topical application of the ester anaesthetic, cocaine.
Methods
Criteria for considering studies for this review
Types of studies
We included only randomized controlled trials (RCTs) and quasi‐randomized trials. Blinding was not an exclusion criterion. We included relevant trials that were published in abstract format or were presented at national or international society meetings. We attempted to locate unpublished studies by contacting relevant manufacturers and investigators. We did not consider data from review articles, case reports or letters to the editor.
Types of participants
We included adult and paediatric participants of either sex. We did not set a minimum age threshold so that we could identify as many relevant studies as possible.
Types of interventions
We included only trials that evaluated the efficacy of topical local anaesthetics for pain control during repair of dermal (skin) lacerations. We included comparisons between:
infiltrated local anaesthetic agents and topically applied local anaesthetic agents; and
different topical local anaesthetic formulations.
We defined topical anaesthetics as agents that are directly applied to the skin to produce numbness. We included both amide and ester local anaesthetics. We accepted topical preparations that contain more than one local anaesthetic. We also included multi‐component topical anaesthetics that contain vasoconstrictors (i.e. cocaine, adrenaline). Acceptable formulations of topical local anaesthetics have included solution, gel, cream, ointment, lotion, jelly, balm, and aerosol spray. We excluded studies that administered local anaesthetics via iontophoresis (a mild electrical current). We excluded studies in which investigators applied topical anaesthetics to mucous membranes (moist linings of the mouth, nose and eyes). To ensure that procedures evaluated involved approximately equivalent intensity and quality of pain, we limited the technique of skin closure to instrumentation involving suture placement or stapling. We excluded studies that examined less invasive approaches to repair of lacerations, such as application of tape or tissue adhesives. We included only studies in which participants had superficial injuries involving the epidermis or dermal layers. We did not consider deeper wounds involving the fascia or non‐skin structures. We set no limitations on the dimensions of the laceration, but we excluded procedures on infected wounds. We excluded studies in which study personnel administered systemic analgesics or sedatives that may influence the participants' perceived or reported pain intensity.
Types of outcome measures
Both primary and secondary outcomes are the same as those described in the 2011 review (Eidelman 2011); we have slightly rewritten them to improve clarity.
Primary outcomes
Our primary outcome was participant‐reported pain intensity during wound repair. We included any type of pain intensity scale that was described clearly by study authors. Although we attempted to apply statistical methods to normalize the data and perform a meta‐analysis, we could not do this because of the small number of trials in each comparison group and their heterogeneous outcomes.
Secondary outcomes
Indirect predictors of pain intensity during wound repair, including incidence of topical anaesthetic failure necessitating systemic sedation or analgesia; requirement for supplemental local anaesthetic dosing; participants' acceptance of anaesthesia; participants' behavioural responses; and observer (clinician or family) assessment of pain intensity during wound repair.
Topical anaesthesia‐related acute toxicity (reported shortly after application, e.g. neurological and cardiovascular toxicity) and other adverse effects (e.g. allergic reaction).
Search methods for identification of studies
Electronic searches
For this updated review, we searched the following databases: Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 11), Cumulative Index to Nursing and Allied Health Literature (CINAHL; 2010 to December 2016), Embase (2010 to December 2016) and MEDLINE (2010 to December 2016). We did not limit our search by language or format of publication. We contacted manufacturers, scientific societies and researchers in the field. (For the previously published version of this review, we searched to November 2010 (Eidelman 2011).)
We sought unpublished studies by directly contacting primary investigators for the included trials. We searched for additional papers by reviewing the references of each retrieved study.
We searched MEDLINE, CENTRAL and CINAHL by using the search strategy described in Appendix 1, Appendix 2 and Appendix 3. We combined the MEDLINE search with the first two levels of the optimal trial search (Higgins 2011). We searched Embase by using the search strategy found in Appendix 4.
We searched meta‐registers of ongoing trials (http://www.controlled‐trials.com/; clinicaltrials.gov). We identified one ongoing study (Ridderikhof 2015) but excluded it because it did not meet our inclusion criteria: It was not an RCT but rather was an observational case series. We identified no studies awaiting classification.
We limited included trials to human studies. We applied no language restrictions during the literature search.
Searching other resources
We manually searched the following journals (1980 through 2009), or we searched them electronically (by searching via different search engines and/or inquiring by email to the appropriate department of a journal publisher (2010 through 2015)).
Academic Emergency Medicine.
Annals of Emergency Medicine.
Emergency Medicine Clinics of North America.
Journal of Emergency Medicine.
Emergency Medicine Australasia (formerly known as Emergency Medicine).
Elsevier B.V. (email inquiry 2015).
We reviewed abstracts presented at the following national or international society meetings (before 2010), and in 2015, we emailed the following societies to ask about relevant new abstracts.
American Academy of Pain Medicine (AAPM).
American Pain Society (APS).
American College of Emergency Physicians (ACEP).
American Society of Anesthesiologists (ASA).
American Society of Regional Anesthesia and Pain Medicine (ASRA).
European Society of Regional Anaesthesia and Pain Therapy (ESRA).
Society for Academic Emergency Medicine (SAEM).
We contacted the following manufacturers of topical anaesthetics to inquire about ongoing or unpublished trials.
AstraZeneca.
Endo Pharmaceuticals.
Ferndale Laboratories.
New England Compounding Center.
Smith & Nephew.
Topicaine.NET.
Novocol.
Henry Schein, Inc.
Ferndale Pharma Group, Inc.
We contacted study authors and searched articles from the reference lists of retrieved articles. We also searched the US National Institutes of Health electronic website (ClinicalTrials.gov).
Data collection and analysis
Selection of studies
Two review authors (BT and CE, AE, DC or EM) independently reviewed study titles and abstracts identified by the search strategy. We obtained the full publication if at least one review author decided that the study potentially met inclusion criteria. Two review authors (BT and AE, CE or EM) independently examined the full articles retrieved and selected trials that met the inclusion criteria. In the event of disagreement, we consulted another review author (DC).
Data extraction and management
For the latest version of this review, two review authors independently extracted data using the uniform data extraction sheet (Appendix 5). We compared information retrieved by each pair of review authors to verify accuracy, and we resolved disagreements by consensus.
For this update, we have identified two new articles that met the inclusion criteria (Jenkins 2014; Lee 2013); both provided descriptive data. We updated the data collection form (Appendix 5) so it reflects interim changes in assessment of selective reporting and sample size biases. Two review authors (BT and AE, CE, DC or EM) independently extracted data from each article and re‐extracted data from previously included articles to assess selective reporting and potential bias as judged from sample size. In cases of disagreement, we consulted a third review author to resolve the issue.
Assessment of risk of bias in included studies
Two review authors independently assessed each study for risk of bias. In cases of disagreement, we consulted a third review author. We applied the Higgins 2001 (Version 5.1.0, Chapter 8) 'Risk of bias’ tool to both earlier and newly included studies. In addition, we included the sample size risk of bias: We considered studies with 200 or more participants per group to be at low risk, studies with 50 to 200 participants per group to have unknown risk and studies with fewer than 50 participants to be at high risk (Mcnicol 2015).
Measures of treatment effect
Dichotomous data
We planned to analyse dichotomous data using Review Manager (RevMan 5.3). Specifically, we would have computed the relative risk. However, owing to lack of relevant data in the included studies, we did not analyse dichotomous data. The small number of trials in each comparison group and the heterogeneity of outcome measures precluded meta‐analysis for most comparisons. Therefore, we performed a mostly descriptive analysis. For the comparison of topical prilocaine‐phenylephrine (PP) and topical tetracaine‐epinephrine‐cocaine (TAC), reported outcomes (pain intensity measures) could be statistically combined, thus we pooled the data. We performed statistical calculations by using Review Manager (RevMan 5.3).
Continuous data
We pooled participant self‐reported VAS scores (which are continuous outcomes) using means and standard deviations (SDs) to derive mean differences (MDs) as well as 95% confidence intervals (CIs).
Unit of analysis issues
All included trials included parallel arms with different interventions. Investigators randomized participants to one of the arms and reported and analysed results for each individual. We identified no issues with double assignment or reporting.
Dealing with missing data
For prior updates, if necessary, we sent email or a letter by postal mail to the contact author to request missing information. We sought additional data from eight trials, but we were able to successfully obtain additional information from only one study (Smith 1997a). Furthermore, we contacted by email and received responses from two primary authors ‐ Drs Amy Ernst and Gary Smith ‐ regarding whether they may have included any of the participants' data in more than one of their studies (Ernst 1990; Ernst 1995a; Ernst 1995b; Ernst 1997; Smith 1996; Smith 1997a; Smith 1997b; Smith 1998a). We did not need to request missing data for the two new included studies (Jenkins 2014; Lee 2013).
Assessment of heterogeneity
We computed Chi2 values to test for heterogeneity. We noted heterogeneity in the single comparison that could be statistically combined, thus we used a random‐effects model for meta‐analysis.
Assessment of reporting biases
We followed instructions from Higgins 2011 (Version 5.1.0) regarding assessment of risk of reporting bias at the study level.
Data synthesis
The small number of trials in each comparison group and the heterogeneity of outcome measures precluded meta‐analysis for most comparisons. Therefore, we performed a mostly descriptive analysis.
In the prior version of this review, reported outcomes (pain intensity measures) for the comparison of topical PP and topical TAC could be statistically combined, thus we pooled the data (Eidelman 2011).
We performed statistical calculations by using Review Manager (RevMan 5.3).
Subgroup analysis and investigation of heterogeneity
We intended to perform a subgroup analysis to determine whether results were different between adult and paediatric participants. We considered participants younger than 18 years old to be paediatric participants and those aged 18 years or older to be adults. However, subgroup analysis by age was not possible because of the small number of studies in each comparison group. Also, many trials included only paediatric or only adult participants. Moreover, studies that included both adult and paediatric participants did not separately report outcomes for the different age groups.
Sensitivity analysis
We performed sensitivity analyses for inclusion or exclusion during data collection by producing a table that reflected prespecified inclusion and exclusion criteria.
'Summary of findings' table and GRADE
In adherence with Higgins 2011 (Version 5.1.0), we populated a 'Summary of findings' table for the primary outcome ‐ pain control during laceration repair. We used the GRADE system to assess the overall quality of evidence (GRADEpro GDT 2015). Owing to limitations in the number and design of retrieved studies, our analysis was mostly descriptive and limited (Table 1). However, we were successful in pooling data for a comparison of topical PP and topical TAC (Table 2) and for the primary outcome ‐ pain control during laceration repair.
The GRADE system categorizes level of quality as follows.
High = randomized trials; or double‐upgraded observational studies.
Moderate = downgraded randomized trials; or upgraded observational studies.
Low = double‐downgraded randomized trials; or observational studies.
Very low = triple‐downgraded randomized trials; or downgraded observational studies; or case series/case reports.
We decreased the grade by one point for each of the following.
Limitations in the design and implementation of available studies suggesting high likelihood of bias.
Indirectness of evidence (indirect population, intervention, control, outcomes).
Unexplained heterogeneity or inconsistency of results (including problems with subgroup analyses).
Imprecision of results (wide confidence intervals).
High probability of publication bias.
We increased the grade by one point for each of the following.
Large magnitude of effect.
All plausible confounding reducing a demonstrated effect or suggesting a spurious effect when results show no effect.
Dose‐response gradient.
Results
Description of studies
Results of the search
Flow of studies
For this update, we identified two studies that met criteria for inclusion (Jenkins 2014; Lee 2013). A total of 25 RCTs met the inclusion criteria for this updated review. None of the 25 included trials were industry sponsored. We have provided detailed descriptions of each trial in the Characteristics of included studies table. We have presented detailed search results in Figure 1.
1.
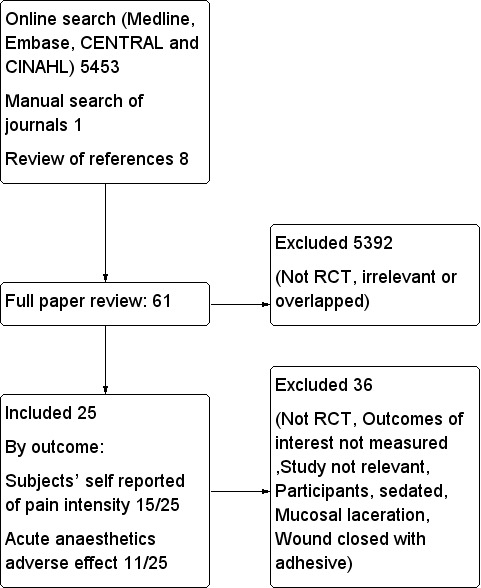
Flow diagram.
Details
In the previous version (Eidelman 2011), two review authors' independent review of abstracts and titles identified by electronic database searches (total 2820 articles before 2010) yielded 39 potentially relevant studies. We obtained each of these 39 trials in full and examined them for possible inclusion in the review. Sixteen of the 39 retrieved trials did not meet the inclusion criteria. Furthermore, we identified eight additional potentially relevant papers through review of obtained study references (Bass 1990; Bonadio 1988a; Bonadio 1988b; Chipont 2001; Liebelt 1997; Peirluisi 1989; Yamamoto 1997) or by manual searches of journals (Bonadio 1992). However, none of the eight papers met the inclusion criteria for this review. We have provided a detailed description of each of these 24 studies in the Characteristics of excluded studies table.
From studies that presented results in bar graph format (Anderson 1990; Ernst 1990; Smith 1996; Smith 1997a; Smith 1997b; Smith 1998a), two review authors (AE, IE) independently extracted numerical data by measuring graphs with a ruler. We then calculated the average of their two measurements. For one RCT, we calculated the standard deviation (SD) for the mean pain score of each experimental group by multiplying the standard error of the mean (SEM) by the square root of the sample size (Smith 1997b). For three studies, we calculated mean pain scores and SDs from individual participant data (Anderson 1990; Ernst 1990; Gaufberg 2007). White and associates reported their results in separate groups according to characteristics of the laceration (length and location) (White 1986). We pooled pain scores for each anaesthetic group and reported the results collectively. Furthermore, to facilitate statistical comparisons, we converted VAS pain scores reported on a 10‐cm scale to a 100‐mm scale by multiplying scores by 10 (Adler 1998; Kuhn 1996; Zempsky 1997).
In the present update, independent review by two review authors of abstracts and titles identified by electronic database searches (total 2633 articles published in 2010 to 2016) yielded 13 potentially relevant studies. We obtained each of the 13 new trials in full and examined them for possible inclusion in the review, in addition to the 39 previously included studies. Eleven of the 13 retrieved trials did not meet the inclusion criteria. We were unable to locate any unpublished studies that qualified for the present review, despite direct communication with pertinent manufacturers and investigators.
Included studies
We included 25 RCTs involving 3278 participants. The small number of trials in each comparison group and the heterogeneity of outcome measures precluded quantitative analysis of data for all but one outcome: pain intensity assessed on a visual analogue scale. Most trials that compared infiltrated and topical anaesthetics were at high risk of blinding, allocation concealment and/or sample size bias, which is likely to affect interpretation of results. Several cocaine‐free topical anaesthetics were found to provide effective analgesic efficacy. However, data regarding the efficacy of each topical agent are based mostly on single comparisons in trials with unclear or high risk of bias. Mild, self‐limited erythematous skin induration occurred in one case out of a total of 1042 participants who underwent application of topical TAC. Researchers reported no serious complications for any of the participants treated with cocaine‐based or cocaine‐free topical anaesthetics.
Participants
Trials included a total of 3278 adult and paediatric participants. Four trials included only adult participants (Ernst 1995b; Gaufberg 2007; Jenkins 2014; White 1986). One trial enrolled only paediatric participants who were 10 years of age or younger (Schaffer 1985). Another trial was limited to children, but investigators did not specify the upper age limit (Bonadio 1990). The remaining 19 studies enrolled both adult and paediatric participants according to the definition provided above. Inclusion criteria applied in 10 of the retrieved trials potentially allowed children younger than three years old to be enrolled (Anderson 1990; Blackburn 1995; Hegenbarth 1990; Pryor 1980; Schaffer 1985; Schilling 1995; Smith 1996; Smith 1997a; Smith 1997b; Smith 1998a). The trials by Ernst and Smith included no duplicate participant data (Ernst 1990; Ernst 1995a; Ernst 1995b; Ernst 1997; Smith 1996; Smith 1997a; Smith 1997b; Smith 1998a).
Interventions
Wound closure
Investigators in 23 studies performed wound closure solely with sutures. In one study, researchers repaired lacerations using both sutures and staples (Krief 2002). In another trial, clinicians repaired lacerations by using skin staples in a minority (7%) of participants (Hegenbarth 1990). Researchers reported no alternative techniques of wound repair. Lacerations were located in four anatomical regions: face, scalp, extremities and, less commonly, the trunk. All lacerations were superficial, and dermal injuries ranged from less than 1.0 cm to 10.0 cm in length.
Topical anaesthetics
The 25 included RCTs studied different topical anaesthetics (listed in Appendix 6). Four studies included multiple arms that compared more than two different anaesthetic agents (Smith 1996; Smith 1997a; Smith 1997b; Smith 1998a). Smith 1996 included six different groups, including five different topical anaesthetics and an infiltrated local anaesthetic arm. Smith 1997a evaluated two topical anaesthetics and infiltrated local anaesthetic. Smith 1997b compared four different topical anaesthetics, and Smith 1998a studied three different topical agents.
Seventeen of the 25 studies compared different forms of topical anaesthetics, and only a minority of trials contained arms with infiltrated local anaesthetic groups. Therefore, the main comparison involved different topical preparations.
We performed no subgroup analysis (or meta‐regression) owing to the small number of trials in each comparison group.
Outcomes
Our primary outcome measure was analgesic efficacy, as reflected in participants' self‐reports of pain intensity during repair of the wound. Fifteen of the included trials determined anaesthetic efficacy through the participants' self‐reports of pain intensity (Blackburn 1995; Ernst 1995a; Ernst 1995b; Ernst 1997; Gaufberg 2007; Jenkins 2014; Kendall 1996; Krief 2002; Kuhn 1996; Lee 2013; Smith 1996; Smith 1997b; Smith 1998a; White 1986; Zempsky 1997). Unless otherwise specified, investigators assessed discomfort during suturing or stapling and used multiple tools for participant self‐report of pain intensity. Twelve studies used VAS pain scale scores (Ernst 1995b; Ernst 1997; Gaufberg 2007; Jenkins 2014; Kendall 1996; Krief 2002; Kuhn 1996; Lee 2013; Smith 1996; Smith 1997b; Smith 1998a; Zempsky 1997). Three RCTs used a Faces pain scale (Blackburn 1995; Kendall 1996; Kuhn 1996), and two trials used verbal numerical pain ratings (0 to 10) (Ernst 1995a; White 1986).
We extracted secondary outcome measures from the RCTs. Nine trials provided observer‐reported VAS pain intensity scores (Ernst 1995b; Ernst 1997; Kendall 1996; Krief 2002; Kuhn 1996; Smith 1996; Smith 1997a; Smith 1998a; Zempsky 1997). Three studies used observer‐rated Likert scores for pain intensity (Smith 1996; Smith 1997a; Smith 1997b). Two RCTs used observer‐reported Faces pain scales (Blackburn 1995; Kuhn 1996), and one used an observer‐rated multi‐dimensional pain intensity scale (Ernst 1995a). Four trials calculated the percentage or absolute number of sutures eliciting pain (Bonadio 1990; Ernst 1995a; Ernst 1995b; Ernst 1997), and 11 studies reported the requirement for supplemental lidocaine infiltration (Anderson 1990; Blackburn 1995; Ernst 1995a; Ernst 1997; Hegenbarth 1990; Jenkins 2014; Krief 2002; Schaffer 1985; Vinci 1996; White 1986; Zempsky 1997). Eight RCTs assessed the effectiveness of anaesthesia by probing the laceration with a needle (Anderson 1990; Ernst 1990; Ernst 1997; Hegenbarth 1990; Jenkins 2014; Kuhn 1996; Resch 1998; Schilling 1995), and seven included a verbal categorical scale to describe anaesthetic effectiveness (Pryor 1980; Resch 1998; Schaffer 1985; Schilling 1995; Smith 1996; Smith 1997b; Vinci 1996). Two studies employed an observer‐reported compliance rating (Anderson 1990; Smith 1996), and two RCTs used observer‐rated acceptability of wound repair (Kendall 1996; Pryor 1980). Two studies reported the total number of topical anaesthetic doses (Gaufberg 2007; Vinci 1996). Each of the following secondary outcome measures was used by a single trial: the Childrens Hospital of Eastern Ontario Pain Scale (CHEOPS) (Kuhn 1996), observer numerical rating of anaesthetic effectiveness (Ernst 1990), the Restrained Infants, Children Distress Rating Scale (RICDRS) (Smith 1996) and the amount of local anaesthetic used (Gaufberg 2007).
Adverse effects
Thirteen trials explicitly assessed and reported the nature and incidence of topical local anaesthetic‐related acute adverse effects (Blackburn 1995; Bonadio 1990; Ernst 1990; Ernst 1995a; Hegenbarth 1990; Jenkins 2014; Kendall 1996; Kuhn 1996; Lee 2013; Resch 1998; Schaffer 1985; Schilling 1995; Vinci 1996).
Excluded studies
We excluded 36 studies for one of the following reasons: not an RCT, outcomes of interest not measured, irrelevant study (i.e. study involved use of local anaesthetics for other than skin laceration purposes), participants sedated, mucosal laceration or wound closed with adhesive. Further information can be found in the Characteristics of excluded studies section and in Figure 1.
Studies awaiting classification
We identified no studies awaiting classification.
Ongoing studies
We identified one ongoing study but excluded it, as it did not meet our inclusion criteria (Ridderikhof 2015); this study was an observational case series ‐ not an RCT.
Risk of bias in included studies
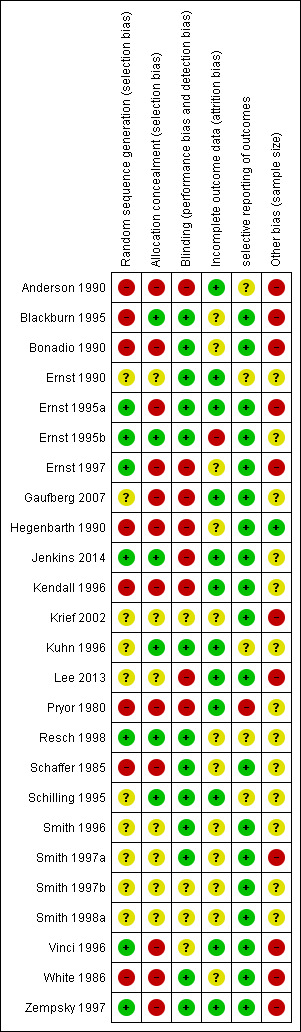
For this updated review, we analysed risk of bias in the 25 included trials by assessing randomization (sequence generation), blinding, allocation concealment, incomplete outcome data, selective reporting and sample size. Further information regarding risk of bias can be found in the 'Risk of bias' graph (Figure 2), summary (Figure 3) and tables (Characteristics of included studies).
2.
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.
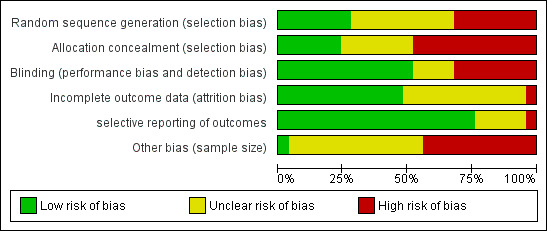
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Allocation was adequately concealed in six of the 25 studies (24%) (Blackburn 1995; Ernst 1995b; Jenkins 2014; Kuhn 1996; Resch 1998; Schilling 1995) and was unclear in seven other studies (28%) (Ernst 1990; Krief 2002; Smith 1996; Smith 1997a; Smith 1997b; Smith 1998a; Lee 2013).
Random sequence generation was adequate in seven of the 25 trials (28%) (Ernst 1995a; Ernst 1995b; Ernst 1997; Jenkins 2014; Resch 1998; Vinci 1996; Zempsky 1997), and information was insufficient to allow a judgement in 10 studies (40%) (Ernst 1990; Gaufberg 2007; Krief 2002; Kuhn 1996; Lee 2013; Schilling 1995; Smith 1996; Smith 1997a; Smith 1997b; Smith 1998a).
Blinding
Thirteen of 25 studies (52%) adequately blinded participants and personnel to the identity of the anaesthetic (Blackburn 1995; Bonadio 1990; Ernst 1990; Ernst 1995a; Ernst 1995b; Kuhn 1996; Resch 1998; Schaffer 1985; Schilling 1995; Smith 1996; Smith 1997a; White 1986; Zempsky 1997). Information was insufficient in four papers (17%) to confirm adequate blinding (Krief 2002; Smith 1997b; Smith 1998a; Vinci 1996). However, 13 of 17 studies (76%) that compared different forms of topical anaesthetics were appropriately blinded. Nine of the 10 trials that compared topical anaesthetic versus infiltrated anaesthetic were not blinded (Anderson 1990; Ernst 1997; Gaufberg 2007; Hegenbarth 1990; Jenkins 2014; Kendall 1996; Lee 2013; Pryor 1980; Smith 1996). One trial (Smith 1997a) was adequately blinded because after the topical or local anaesthetic was administered, investigators videotaped suturing procedures. An observer who was completely blinded to which form of anaesthetic the participant had received later reviewed these videotapes.
Incomplete outcome data
Twelve trials (48%) appropriately addressed incomplete outcome data (Anderson 1990; Ernst 1990; Ernst 1995a; Gaufberg 2007; Jenkins 2014; Kendall 1996; Kuhn 1996; Lee 2013; Pryor 1980; Schilling 1995; Vinci 1996; Zempsky 1997). Researchers did so because they noted a balance in the number of excluded participants between different groups (reasons for exclusion are unlikely to be related to pain scores during the trial), or because they reported no drop‐outs or exclusions. Attrition bias was unclear in 12 studies (48%) (Blackburn 1995; Bonadio 1990; Ernst 1997; Hegenbarth 1990; Krief 2002; Resch 1998; Schaffer 1985; Smith 1996; Smith 1997a; Smith 1997b; Smith 1998a; White 1986).
Selective reporting
We concluded that 19 (76%) articles described all outcomes in the Methods section and adequately reported study results (Blackburn 1995; Bonadio 1990; Ernst 1995a; Ernst 1995b; Ernst 1997; Gaufberg 2007; Hegenbarth 1990; Jenkins 2014; Kendall 1996; Krief 2002; Lee 2013; Schaffer 1985; Smith 1996; Smith 1997a; Smith 1997b; Smith 1998a; Vinci 1996; White 1986; Zempsky 1997). We found unclear selective reporting bias in five articles (20%) (Anderson 1990; Ernst 1990; Kuhn 1996; Resch 1998; Schilling 1995) (e.g. subgroup analysis based on laceration location, sex or age not prespecified).
Other potential sources of bias
Sample size bias
Thirteen (52%) studies had unclear sample size risk, defined as 50 to 200 participants per treatment arm (Ernst 1990; Ernst 1995b; Gaufberg 2007; Jenkins 2014; Kendall 1996; Kuhn 1996; Pryor 1980; Resch 1998; Schaffer 1985; Schilling 1995; Smith 1996; Smith 1997b; Smith 1998a); most of these included 60 to 70 participants per treatment arm. We found only one study with low risk, defined as more than 200 participants per treatment arm (Hegenbarth 1990); one arm included 262 participants, and the other included 205.
Effects of interventions
We first present the evidence regarding cocaine‐containing topical anaesthetics. We included comparisons between cocaine‐based topical anaesthetics and each of the following: (1) infiltrated local anaesthetics; and (2) different formulations of cocaine‐based topical agents. Next, we summarize the evidence evaluating cocaine‐free topical anaesthetics. We compared cocaine‐free topical agents with each of the following: (1) infiltrated local anaesthetics; (2) formulations of cocaine‐containing topical agents; and (3) different formulations of cocaine‐free topical anaesthetics; both of the newly included studies (Jenkins 2014; Lee 2013) belong to category “2a”.
We also report the data on acute anaesthetic‐related adverse effects. We have provided a detailed and inclusive description of each of the 25 trials in the Characteristics of included studies table.
Intervention 1. Evaluation of cocaine‐containing topical anaesthetics
1a. Cocaine‐containing topical anaesthetics versus local anaesthetic infiltration (six studies)
Six studies compared a topical cocaine‐based agent versus infiltrated local anaesthetic (see Table 3 for detailed study information). Five studies compared topical TAC versus infiltrated local anaesthetic. We could not mathematically combine outcomes because of the diversity of measures used to assess anaesthetic efficacy (Anderson 1990; Hegenbarth 1990; Pryor 1980; Smith 1996; Smith 1997a); these five studies enrolled a total of 1194 participants.
1. Cocaine‐containing topical anaesthetics versus infiltrated local anaesthetics.
| Study | Anaesthetics | Participant self‐reported pain scores | Secondary outcome measures | Incidence of anaesthetic toxicity |
| Anderson 1990 | Topical tetracaine‐epinephrine‐cocaine (TAC) vs infiltrated lidocaine | None | 1) Adequate initial anaesthesia (TAC = 89% vs infiltrated local anaesthetic = 79%; P = non‐significant) 2) Physician compliance scale(1 = complete compliance to 4 = continuous resistance) (mean score ± SD: TAC = 1.25 ± 0.57 vs infiltrated local anaesthetic = 1.94 ± 1.12; P < 0.002) 3) Requirement for supplemental lidocaine infiltration (topical TAC = 18% vs infiltrated local anaesthetic = 23%; P = non‐significant) | Not reported |
| Hegenbarth 1990 | Topical TAC vs infiltrated lidocaine | None | 1) Adequate initial anaesthesia for facial and scalp lacerations (topical TAC = 81% vs infiltrated local anaesthetic = 87%; P = 0.005). Adequate initial anaesthesia for extremity and trunk wounds (topical TAC = 43% vs infiltrated local anaesthetic = 89%; P < 0.0001) | 0/467 |
| Pryor 1980 | Topical TAC vs infiltrated lidocaine | None | 1) Verbal rating of anaesthetic efficacy (complete: TAC = 84% vs infiltrated local anaesthetic = 88%; P = not reported) 2) Anaesthetic acceptability: Participants 17 years or younger preferred topical TAC (P < 0.005); results showed no differences between the 2 anaesthetic groups among participants older than 17 years of age | Not reported |
| Smith 1996 | Topical TAC vs infiltrated lidocaine | Patient‐reported VAS (100 mm) pain scores (mean scores: topical TAC = 12.0 vs infiltrated local anaesthetic = 26.3; P = NS) | 1) Observer‐reported VAS pain scores 2) Observer‐reported Likert pain scores 3) Oberver‐rated Restrained Infants and Children Disress Rating Scale 4). Suture technician‐rated anaesthetic effectiveness | Not reported |
| Smith 1997a | Topical TAC vs infiltrated lidocaine | None | 1) Observer‐reported VAS pain scores (suture technicians, research assistants, videotape reviewers) 2) Observer‐reported Lickert (1‐7) pain scores (parents, suture technicians) 3) Requirement for supplemental lidocaine infiltration (See Characteristics of included studies for data.) |
Not reported |
| Kendall 1996 | Topical (epinephrine‐cocaine) AC vs infiltrated lidocaine | The study pooled patient‐reported VAS and Wong‐Baker Faces pain scores (mean score: topical AC = 4.50 vs infiltrated local anaesthetic = 4.40; P = NS) | 1) Physician‐rated VAS pain scores 2) Parent‐rated VAS scores 3) Parents' rating of overall acceptability of procedure | 0/107 |
AC: epinephrine (adrenaline) and cocaine; BN: bupivacaine‐noradrenaline; BP: blood pressure; cm: centimetre; c/w:compared with; ED: emergency department; EMLA: Eutectic Mixture of Local Anesthetics (lidocaine and prilocaine); EN: etidocaine‐noradrenaline; LAT: lidocaine, epinephrine and tetracaine (same as LET); LE: lidocaine and epinephrine; LET: same as LAT; LG: local gel; LI: local infiltration; MAC: bupivacaine 0.5%, epinephrine 1:2000, cocaine 10.0%; mm: milli‐metre; MN: mepivacaine‐noradrenaline; PN: prilocaine‐noradrenaline; N: number; NS: not significant; P = P value; PP: prilocaine, phenylephrine; RCT: randomized controlled trial; RICDRS: Restrained Infants and Children Distress Rating Scale; SD: standard deviation; SE: standard error; TA: tetracaine and epinephrine; TAC: tetracaine, epinephrine and cocaine; TLP: tetracaine, lidocaine and phenylephrine; TP: tetracaine and phenylephrine; VAS: visual analogue scale; vs: versus; w/w: weight per weight.
Primary outcome: participant report of pain intensity during wound repair
Anaesthetic efficacy measures for topical TAC were inconsistent in efficacy reporting. One study found that topical adrenaline‐cocaine (AC) provided analgesia equivalent to that of local anaesthetic infiltration (Kendall 1996).
Secondary outcomes: indirect predictors of pain intensity during wound repair
Adequate initial anaesthesia and/or requirement for supplemental lidocaine: Anderson 1990 and Hegenbarth 1990 found minimal differences between comparison groups. However, Smith 1997a found that fewer participants in the TAC group than in the Mepivanor group needed supplemental lidocaine rescue owing to inadequate anaesthesia as assessed by suture technicians (2, or 8.3%, vs 9, or 37.5%, respectively; P = 0.04).
Participant compliance during suturing: Anderson 1990 found that participant compliance during suturing for TAC was significantly better than for lidocaine or placebo (P < 0.002).
Participant preference: Hegenbarth 1990 reported that 92% of parents of participants who received TAC for facial or scalp laceration repair preferred it for the future compared with 57% of parents whose children received lidocaine (P < 0.0001). The difference in parent preference was not statistically significant for other body areas. Pryor 1980 reported that parents of children between one and five years of age preferred topically applied TAC over infiltrated lidocaine (P < 0.005), and that participants five to 17 years old self‐reported an even more significant preference for TAC (P < 0.0001).
Duration of procedure: Pryor 1980 found that the duration of the suturing procedure was significantly shorter for topical TAC than for infiltrated lidocaine in the one‐ to five‐year‐old age group (P < 0.005). For participants 11 to 17 years old, results similarly suggested that the procedure for the TAC group had a shorter duration, but this finding was not statistically significant. Data showed no duration difference between other age groups studied. Smith 1996 reported no difference in the duration of suturing between TAC and lidocaine infiltration in all age groups studied (two to 17 years old; P = 0.15).
Observer VAS ratings: Smith 1996 found that VAS ratings by observers (suture technicians and research assistants) and participants showed that, compared with lidocaine infiltration, Bupivanor had a small but statistically significantly superior performance for face and scalp lacerations. In the same study, Bupivanor outperformed TAC for repair of face and scalp lacerations, but this finding did not reach statistical significance. Smith 1997a showed statistically significantly higher VAS scores (i.e. poorer pain control), as observed by research assistants or technicians, with topical Mepivanor solution than with TAC or lidocaine.
Failed anaesthesia: Kendall 1996 found a higher incidence of failed anaesthetics in the lidocaine group than in the AC group (24% vs 10%; P < 0.01).
Acute adverse effects and toxicity: Please see "Intervention 3. Anaesthetic‐related acute adverse effects" subsection below.
Evidence quality for primary and secondary outcomes
The following trials had high risk of bias in multiple domains (Anderson 1990; Hegenbarth 1990; Pryor 1980) or unclear risk of bias in three domains (Smith 1996; Smith 1997a). One study found that topical AC provided equivalent analgesia to local anaesthetic infiltration (Kendall 1996). However, this study was not blinded and risk of bias was high for both sequence generation and allocation concealment. In conclusion, although the trials mentioned were RCTs, we downgraded the overall GRADE score for each measured outcome to low owing to limitations in design and implementation, imprecision of results and high probability of publication bias (selective reporting of data) (see Characteristics of included studies).
1b. Comparisons between different cocaine‐containing topical anaesthetics (four studies)
Four studies with 530 participants in total compared topical TAC versus another cocaine‐based topical anaesthetic (Table 4).
2. Comparisons between different cocaine‐containing topical anaesthetics.
| Study | Topical Anaesthetics | Patient self‐reported pain scores | Secondary outcome measures | Incidence anaesthetic toxicity |
| Kuhn 1996 | Bupivacaine‐adrenaline‐cocaine (MAC) vs tetracaine‐epinephrine‐cocaine (TAC) | 1) In children < 12 years of age: Wong‐Baker Faces (1‐9) Scale (mean score ± SD: topical MAC = 2.35 ± .50 vs topical TAC = 2.46 ± 2.34; P = 0.96) 2) Participants 12 years of age or older: VAS (100 mm) pain scale (mean score ± SD: topical MAC = 6.9 ± 10.9 vs topical TAC = 12.0 ± 14.5; P = 0.16) |
1) Adequacy of initial anaesthesia 2) Participant preference for topical anaesthesia in the future |
0/180 |
| Bonadio 1990 | TAC vs adrenaline‐cocaine (AC) | None | 1) Physician calculated total number of 'sutures eliciting pain' (topical AC = 7/103 (4%) vs topical TAC = 7/151 (7%); P = not reported) | 0/55 |
| Ernst 1990 | TAC vs cocaine (C) | None | 1) Incidence of 'poor anaesthesia' (topical cocaine = 20% vs topical TAC = 12%; P = not reported) 2) Physician numerical rating of anaesthetic effectiveness (0 = least effective to 10 = most effective) (mean scores ± SD: topical cocaine = 6.44 ± 3.48 vs topical TAC = 7.74 ± 3.03; P = 0.005) |
0/139 |
| Vinci 1996 | TAC (two different strengths) vs tetracaine‐cocaine (TC) | None |
Topical TAC 1 vs topical TC:
1) Complete anaesthesia (TAC 1 = 73% vs TC = 28%; P < 0.001) 2) Requirement for second dose of topical anaesthetic (TAC 1 = 30% vs topical TC = 66%; P < 0.003) 3) Requirement for supplemental lidocaine infiltration (TAC 1 = 6% vs topical TC = 9%; P = not reported) Topical TAC 2 vs topical TC: 1) Complete anaesthesia (TAC 2 = 63% vs TC = 28%; P < 0.001) 2) Requirement for second dose of topical anaesthetic (TAC 2 = 46% vs TC = 66%; P < 0.003) 3) Requirement for supplemental lidocaine infiltration (TAC 2 = 2% vs TC = 9%; P = not reported) |
1/156 (erythematous rash 1 day after application of standard topical TAC) |
Primary outcome: participant report of pain intensity during wound repair
Anaesthetic efficacy did not differ between TAC and either topical bupivacaine‐adrenaline‐cocaine (Marcain (Astra)‐adrenaline‐cocaine (MAC) (Kuhn 1996) or adrenaline‐cocaine (AC) (Bonadio 1990)). Neither cocaine (C) (Ernst 1990) nor tetracaine‐cocaine (TC) (Vinci 1996) was found to be an effective topical anaesthetic.
Secondary outcome: indirect predictors of pain intensity during wound repair
Acute adverse effects and toxicity: Please see "Intervention 3. Anaesthetic‐related acute adverse effects" subsection below.
Evidence quality
Kuhn 1996 had unclear risk of bias for sequence generation but low risk of bias for the other three key domains. Bonadio 1990 did not use a formal pain scoring scale to assess the efficacy of AC and had high risk of bias for both sequence generation and allocation concealment.
Although the trials mentioned were RCTs, we downgraded the overall GRADE score for each measured outcome to low owing to limitations in design and implementation, imprecision of results and high probability of publication bias (selective reporting of data) (see Characteristics of included studies).
Intervention 2. Evaluation of cocaine‐free topical anaesthetics
2a. Cocaine‐free topical anaesthetics versus infiltrated local anaesthetic (six studies)
Six RCTs with 627 total participants compared five different cocaine‐free topical anaesthetics versus infiltrated local anaesthetic (Table 5). We could not mathematically combine the two studies of topical mepivacaine‐noradrenaline (MN) because of heterogeneity in outcome measures, and Smith 1996 did not report SDs for pain scores.
3. Cocaine‐free topical anaesthetics versus infiltrated local anaesthetics.
| Study | Anaesthetics | Participant self‐reported pain scores | Secondary outcome measures | Incidence of anaesthetic toxicity |
| Ernst 1997 | Topical lidocaine‐epinephrine‐tetracaine (LAT) vs infiltrated lidocaine | VAS (100 mm) pain scores (median values: topical LAT = 0 vs infiltrated local anaesthetic = 0; P = 0.48) | 1) Physician‐rated VAS pain scores 2) Requirement for supplemental lidocaine infiltration 3) Percentage of painful sutures |
Not reported |
| Gaufberg 2007 | Topical lidocaine‐epinephrine (LE) vs infiltrated lidocaine | VAS (100 mm) pain scores (mean score ± SD: topical TLE = 0.16 ± 0.46 vs infiltrated lidocaine = 0.20 ± 0.49; P = 0.59) | 1) Amount of lidocaine required (mg) 2) Total number of topical anaesthetic applications |
Not reported |
| Smith 1996 | Topical bupivacaine‐norepinephrine (BN), topical etidocaine‐norepinephrine (EN), topical mepivacaine‐norepinephrine (MN) and topical prilocaine‐norepinephrine (PN) vs infiltrated lidocaine | VAS (100 mm) pain scores (mean scores: BN = 18.3, EN = 46.5, MN = 27.0, PN = 36.0 vs infiltrated anaesthetic = 26.3, standard deviations not reported) (no significant difference between any of the cocaine‐free topical agents and infiltrated lidocaine) |
1) Observer‐reported VAS pain scores 2) Observer‐reported Likert pain scores 3) Oberver‐rated Restrained Infants and Children Disress Rating Scale 4) Suture technician‐rated anaesthetic effectiveness |
Not reported |
| Smith 1997a | Topical mepivacaine‐norepinephrine (MN) vs infiltrated lidocaine | None | 1) Observer‐reported VAS pain scale scores 2) Observer‐reported Lickert pain scores 3) Requirement for supplemental lidocaine infiltration (See characteristics of included studies for data) |
Not reported |
| Jenkins 2014 | Topical anaesthetic putty (containing 4.94% w/w lidocaine hydrochloride, equivalent to 4% w/w lidocaine base) vs lidocaine infiltration (1% w/v) | Mean pain score was 0.78 + 1.12 (SD) after lidocaine infiltration, 1.49 + 1.76 after topical anaesthetic putty. | 1) Need for rescue anaesthesia 2) Wound evaluation score 7‐10 days after treatment 3) Wound infection 4) Wound dehiscence 5) Adverse effects (inflamed wound or resuturing). |
No anaesthetic toxicity reported |
| Lee 2013 | Topical anaesthetic lidocaine, adrenaline and tetracaine (LAT) (4% lidocaine, 1:2 000 adrenaline, 1% tetracaine) vs lidocaine infiltration. Dosage of neither group was reported. | LAT gel group reported mean (± SE) pain intensity of 2.5 (0.52) vs 2.6 (0.58) for the lidocaine infiltration group. Pain during LAT application was 1.5 (0.40) vs 2.6 (0.58) during lidocaine infiltration (P ≤ 0.01). | 1) Pain score by parents or clinicians (intended to be gathered for children < 10 years old but such data were not reported) 2) Wound complications (infection, dehiscence, missing sutures) |
None reported |
AC: epinephrine (adrenaline) and cocaine; BN: bupivacaine‐noradrenaline; BP: blood pressure; cm: centimetre; c/w: compared with; ED: emergency department; EMLA: Eutectic Mixture of Local Anesthetics (lidocaine and prilocaine); EN: etidocaine‐noradrenaline; LAT: lidocaine, epinephrine and tetracaine (same as LET); LE: lidocaine and epinephrine; LET: same as LAT; LG: local gel; LI: local infiltration; MAC: bupivacaine 0.5%, epinephrine 1:2000, cocaine 10.0%; mm: milli‐metre; MN: mepivacaine‐noradrenaline; PN: prilocaine‐noradrenaline; N: number; NS: not significant; P = P value; PP: prilocaine, phenylephrine; RCT: randomized controlled trial; RICDRS: Restrained Infants and Children Distress Rating Scale; SD: standard deviation; SE: standard error; TA: tetracaine and epinephrine; TAC: tetracaine, epinephrine and cocaine; TLP: tetracaine, lidocaine and phenylephrine; TP: tetracaine and phenylephrine; VAS: visual analogue scale; vs: versus; w/w: weight per weight.
Primary outcome: participant report of pain intensity during wound repair
Smith 1996 found no significant differences in VAS pain scores between infiltrated lidocaine and four different noradrenaline‐containing topical anaesthetics, including bupivacaine‐noradrenaline (BN), etidocaine‐noradrenaline (EN), mepivacaine‐noradrenaline (MN) and prilocaine‐noradrenaline (PN). Smith 1997a also compared topical MN with infiltrated lidocaine and found that the latter provided better analgesia. Researchers found no significant differences between infiltrated local anaesthetic and either topical lidocaine‐adrenaline‐tetracaine (LAT) (Ernst 1997) or topical lidocaine‐epinephrine (TLE) (Gaufberg 2007).
Jenkins 2014 compared topical anaesthetic putty (4.94% lidocaine HCl, equivalent to 4% lidocaine base) to a maximum of 10 grams versus infiltrated lidocaine 1% for pain during suturing in 54 and 56 participants, respectively. Mean pain score during suturing was 0.78 ± 1.12 (SD) on a 0 to 10 VAS after lidocaine infiltration versus 1.49 ± 1.76 after topical anaesthetic putty. Both one‐sided 95% confidence interval (CI) limits plus (owing to their non‐normal distribution) non‐parametric comparisons of median scores showed non‐inferiority of topical anaesthetic putty compared with infiltrated lidocaine.
Lee 2013 compared topical anaesthetic gel comprising LAT (4% lidocaine, 1:2000 adrenaline, 1% tetracaine) versus lidocaine 1% infiltration in 23 and 17 participants, respectively, for pain during suturing. Investigators reported the dosage for neither group. The LAT gel group reported a mean (± standard error (SE)) pain intensity of 2.5 (0.52) versus 2.6 (0.58) for lidocaine infiltration. Pain during LAT application was 1.5 (0.40) versus 2.6 (0.58) during lidocaine infiltration (P ≤ 0.01). Researchers concluded that LAT gel for repair of minor lacerations was as efficacious as infiltrated lidocaine in terms of participant comfort.
Secondary outcome: indirect predictors of pain intensity during wound repair
Jenkins 2014 reported that:
the number of participants requiring rescue anaesthesia was three of 56 (5.3%) in the lidocaine infiltration group and four of 54 (7.4%) in the topical anaesthetic putty group; and
the “wound evaluation score” obtained seven to 10 days after treatment showed that 12 of 54 (22.22%) in the topical anaesthetic putty group had less than perfect scores versus five of 56 (8.9%) in the infiltration group.
Ernst 1997 found no difference in effectiveness of LAT compared with injected lidocaine as reported by physicians (P = 0.83). The number of sutures causing pain was not statistically significantly different (P = 0.28).
Gaufberg 2007 found that 95% of participants given TLE rated their experience as "excellent," compared with 5% of participants in the control group (P < 0.001). Anaesthesia lasted significantly longer for LTE than for control (P < 0.001) and the amount of lidocaine in the TLE application was comparable with that in the control (P ∼ 0.90).
Smith 1996 found that observers rated Bupivanor as being as effective as TAC and 1% lidocaine infiltration. Smith 1997a showed statistically significantly higher VAS scores (i.e. worse pain control) assessed by observers for Mepivanor than for TAC or lidocaine.
For reported acute adverse effects and toxicity, see the "Intervention 3. Anaesthetic‐related acute adverse effects" subsection below.
Evidence quality for primary and secondary outcomes
Both of the trials by Smith and associates had unclear risk of bias in at least three key domains. Also, in Smith 1996, comparisons of infiltrated lidocaine and topical anaesthetics were not blinded. Moreover, Smith 1997a did not employ participant self‐reported pain scoring scales but instead relied on observer estimates of pain. None of these trials were blinded: Ernst 1997; Gaufberg 2007; Jenkins 2014; Lee 2013. Ernst 1997; Gaufberg 2007; andLee 2013did not properly perform or describe allocation concealment. Again, although the trials mentioned were RCTs, we downgraded the overall GRADE score for each measured outcome to low owing to limitations in design and implementation, imprecision of results and a high probability of publication bias (selective reporting of data) (see Characteristics of included studies).
2b. Cocaine‐free topical anaesthetics versus cocaine‐containing topical anaesthetics (11 studies)
Eleven trials with a total of 1314 participants compared 13 different cocaine‐free topical anaesthetics versus topical TAC (Table 6).Each of these studies employed TAC as the cocaine‐containing topical preparation.
4. Cocaine‐free topical anaesthetics versus cocaine‐containing topical anaesthetics.
| Study | Topical anaesthetics | Participant self‐reported pain scores | Secondary outcome measures | Incidence of anaesthetic toxicity |
| Smith 1996 | Bupivacaine‐norepinephrine (BN), etidocaine‐norepinephrine (EN), mepivacaine‐norepinephrine (MN) and prilocaine‐norepinephrine (PN) vs tetracaine‐epinephrine‐cocaine (TAC) | Participant‐reported VAS (100 mm) pain scores (mean scores: BN = 18.3, EN = 46.5, MN, PN = 36.0 vs TAC = 12.0, standard deviations not reported) (TAC significantly outperformed EN; no significant differences between any other groups) |
1) Observer‐reported VAS and Likert pain scale scores 2) Observer‐rated Restrained Infants and Children Disress Rating Scale 3) Suture technician‐rated anaesthetic effectiveness |
Not reported |
| Smith 1997a | Mepivacaine‐norepinephrine (MN) vs TAC | None | 1) Observer‐reported VAS pain scores (suture technicians, research assistants, videotape reviewers) 2) Observer‐reported Lickert (1‐7) pain scores (parents, suture technicians) 3) Requirement for supplemental lidocaine infiltration (See Characteristics of included studies for data.) |
Not reported |
| Smith 1997b | Prilocaine‐phenylephrine (PP), tetracaine‐phenylephrine (TP) and tetracaine‐lidocaine‐phenylephrine (TLP) vs TAC | VAS (100 mm) pain scores (mean score ± SD: PP = 29.0 ± 43.4, TP = 24.2 ± 37.2, TLP = 30.6 ± 40.3 vs TAC = 17.6 ± 34.1 (no significant differences between groups; P = 0.5) | 1) Oberver‐reported VAS (100 mm) pain scores 2) Oberver‐reported Likert (1‐7) pain scores 3) Suture technicians‐rated anaesthetic effectiveness |
Not reported |
| Smith 1998a | Prilocaine‐phenylephrine (PP) and bupivacaine‐phenylephrine (BP) vs TAC | VAS (100 mm) pain scores (mean score ± SD: PP = 21.0 ± 28.0 and BP = 41.0 ± 35.0 vs TAC = 18.0 ± 24.0) (no differences reported between groups; P = 0.07) | Observer‐reported VAS pain scores (suture technicians, research assistants and parents) | Not reported |
| Ernst 1995a | LAT vs TAC | Modified multi‐dimensional pain scale (0‐10) (mean ranked sum: LAT = 49.0 vs TAC = 46.9; P = 0.71) | 1) Physician‐rated modified multi‐dimensional pain scale (0‐10) 2) Percentage of sutures causing pain 3) Requirement for supplemental lidocaine infiltration |
0/95 |
| Ernst 1995b | LAT vs TAC | VAS (100 mm) pain scores (mean ranked sum: LET = 45.3 vs TAC = 50.8; P = 0.27) | 1) Physician‐reported VAS scores 2) Percentage of sutures causing pain |
Not reported |
| Schilling 1995 | LAT vs TAC | None | 1) Adequacy of initial anaesthesia (LAT = 74.4% vs TAC = 79.5%; P = 0.46) 2) Anaesthetic effectiveness (complete anaesthesia: LAT = 82.4% vs topical TAC = 75.9%; P = 0.18) |
0/151 |
| Zempsky 1997 | Lidocaine‐prilocaine (EMLA) vs TAC | VAS (100 mm) pain scores (mean score ± SD: EMLA = 46.0 ± 26.0 vs TAC = 40.0 ± 25.0; P = 0.50) | 1) Observer‐rated VAS pain scores 2) Requirement for supplemental lidocaine infiltration |
Not reported |
| Blackburn 1995 | Lidocaine‐epinephrine (LE) vs TAC | Faces pain scale (1‐9) scores (mean score ± SD: LE = 3.29 ± 1.92 vs TAC = 2.66 ± 1.78; P = 0.33) | Requirement for supplemental lidocaine infiltration | 0/35 |
| Schaffer 1985 | Tetracaine‐epinephrine (TA) vs TAC | None | 1) Physician‐rating of anaesthetic effectiveness (complete anaesthesia: TA = 47.1% vs TAC = 75%' P < 0.05) 2) Requirement for rescue lidocaine infiltration (TA = 27.5% vs TAC = 8.9%; P = 0.01) |
0/107 |
| White 1986 | Tetracaine (T) vs TAC | Numerical pain scale (0‐10) score (mean scores: tetracaine = 5.6 vs TAC = 3.53; P < 0.05; standard deviations not reported) | Requirement for supplemental lidocaine infiltration | Not reported |
AC: epinephrine (adrenaline) and cocaine; BN: bupivacaine‐noradrenaline; BP: blood pressure; cm: centimetre; c/w: compared with; ED: emergency department; EMLA: Eutectic Mixture of Local Anesthetics (lidocaine and prilocaine); EN: etidocaine‐noradrenaline; LAT: lidocaine, epinephrine and tetracaine (same as LET); LE: lidocaine and epinephrine; LET: same as LAT; LG: local gel; LI: local infiltration; MAC: bupivacaine 0.5%, epinephrine 1:2000, cocaine 10.0%; mm: milli‐metre; MN: mepivacaine‐noradrenaline; PN: prilocaine‐noradrenaline; N: number; NS: not significant; P = P value; PP: prilocaine, phenylephrine; RCT: randomized controlled trial; RICDRS: Restrained Infants and Children Distress Rating Scale; SD: standard deviation; SE: standard error; TA: tetracaine and epinephrine; TAC: tetracaine, epinephrine and cocaine; TLP: tetracaine, lidocaine and phenylephrine; TP: tetracaine and phenylephrine; VAS: visual analogue scale; vs: versus; w/w: weight per weight
Primary outcome: participant report of pain intensity during wound repair
Smith and associates published four papers relevant to this comparison (Smith 1996; Smith 1997a; Smith 1997b; Smith 1998a). In comparisons confined to a single application, Smith and associates found similar analgesic efficacy between topical TAC and each of the following topical agents: bupivacaine‐noradrenaline (BN), prilocaine‐noradrenaline (PN), tetracaine‐lidocaine‐phenylephrine (TLP) and tetracaine‐phenylephrine (TP) (Smith 1996; Smith 1997b). Two papers compared topical prilocaine‐phenylephrine (PP) versus topical TAC (Smith 1997b; Smith 1998a). In Analysis 1.1, we pooled participant‐reported VAS (100 mm) pain scores and found no differences between topical PP and topical TAC (weighted mean difference (WMD) 5.56, 95% CI ‐2.20 to 13.32).
1.1. Analysis.
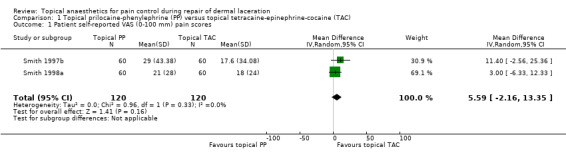
Comparison 1 Topical prilocaine‐phenylephrine (PP) versus topical tetracaine‐epinephrine‐cocaine (TAC), Outcome 1 Patient self‐reported VAS (0‐100 mm) pain scores.
Two studies presented conflicting conclusions regarding the efficacy of topical MN (Smith 1996; Smith 1997a). We could not statistically combine these trials because investigators used different pain intensity scales to determine anaesthetic efficacy, and Smith 1996 did not report standard deviations for study outcomes. Three studies found similar efficacy between topical LAT and TAC (Ernst 1995a; Ernst 1995b; Schilling 1995). One RCT found no difference in pain scores among children anaesthetized with EMLA cream (lidocaine 2.5% and prilocaine 2.5%) or topical TAC (Zempsky 1997). Blackburn 1995 found no difference in the efficacy of topical lidocaine‐adrenaline (LE) versus topical TAC. Topical TAC was superior to etidocaine‐noradrenaline (Smith 1996), topical bupivacaine‐phenylephrine (Smith 1998a), topical tetracaine‐adrenaline (Schaffer 1985) and topical tetracaine (White 1986).
Secondary outcome: indirect predictors of pain intensity during wound repair
Ernst 1995b reported that physicians found that LAT was more effective than TAC during suturing (P = 0.0093). Smith 1996 found that observers rated Bupivanor as being as effective as TAC and 1% lidocaine infiltration. Smith 1997a showed statistically significantly higher observer‐reported VAS scores (i.e. more intense pain) for Mepivanor than for TAC or lidocaine. Smith 1997b reported statistically significant inferiority of Prilophen versus TAC using Likert scale scores provided by suture technicians and research assistants, but not by parents. Schilling 1995 found a statistically significant difference between TAC and LET in duration of anaesthesia on the cheek or chin area (X2; P = 0.04). Smith 1998a reported no statistically significant differences between the effectiveness of prilocaine‐phenylephrine and TAC for any of the observer groups. Schaffer 1985 found drowsiness or excitability following use of TAC in 10.7% versus 7.8% in the tetracaine and adrenaline groups, respectively ‐ a statistically insignificant difference. For acute adverse effects and toxicity, please see the effects subsection "Intervention 3. Anaesthetic‐related acute adverse effects" below.
Evidence quality for primary and secondary outcomes
Each of the four trials by Smith and associates had unclear risk of bias for three or more key domains. Zempsky 1997 did not conceal allocation appropriately. Blackburn 1995 seems not to have employed random sequence generation: "The TAC and TLE solutions were arbitrarily assigned to single‐dose (10 mL), sequentially numbered vials by the pharmacist”. It was unclear whether Schilling 1995 used appropriate sequence generation but risk of bias was low for the other domains. The two trials by Ernst and associates (Ernst 1995a; Ernst 1995b) had high risk of bias for one key domain. We could not merge results because we found heterogeneity in outcome measures.
Again, although the trials mentioned were RCTs, we downgraded the overall GRADE score for each measured outcome to low owing to limitations in design and implementation, imprecision of results and high probability of publication bias (selective reporting of data) (see Characteristics of included studies).
2c. Comparisons between different cocaine‐free topical anaesthetics (five studies)
Five RCTs with 895 total participants evaluated different cocaine‐free topical anaesthetics (Table 7).
5. Comparisons between different cocaine‐free topical anaesthetics.
| Study | Topical anaesthetics | Participant self‐reported pain scores | Secondary outcome measures | Incidence of anaesthetic toxicity |
| Smith 1996 | Bupivacaine‐norepinephrine (BN) vs etidocaine‐norepinephrine (EN) vs mepivacaine‐norepinephrine (MN) vs prilocaine‐norepinephrine (PN) | Patient‐reported VAS (100 mm) pain scores (mean scores: topical BN = 18.3 vs topical EN = 46.5 vs topical MN = 27.0 vs topical PN = 36.0) (no significant differences between any cocaine‐free topical groups) | 1) Observer‐reported VAS and Likert pain scale scores 2) Observer‐rated Restrained Infants and Children Disress Rating Scale 3) Suture technician‐rated anaesthetic effectiveness |
Not reported |
| Smith 1997b | Prilocaine‐phenylephrine (PP) vs tetracaine‐phenylephrine (TP) vs tetracaine‐lidocaine‐phenylephrine (TLP) | VAS (100 mm) pain scores (mean score ± SD: PP = 29.0 ± 43.4 vs TP = 24.2 ± 37.2 vs TLP = 30.6 ± 40.3) (no significant differences between groups; P = 0.5) | 1) Oberver‐reported VAS (100 mm) pain scores 2) Oberver‐reported Likert (1‐7) pain scores 3) Suture technicians rated anaesthetic effectiveness |
Not reported |
| Smith 1998a | Prilocaine‐phenylephrine (PP) vs bupivacaine‐phenylephrine (BP) | VAS (100 mm) pain scores (mean score ± SD: topical PP = 21.0 ± 28.0 vs topical BP = 41.0 ± 35.0; P = 0.07) | Observer‐reported VAS pain scores (suture technicians, research assistants and parents) | Not reported |
| Krief 2002 | Lidocaine‐prilocaine (EMLA) vs lidocaine‐epinephrine‐tetracaine (LAT) | VAS (100 mm) pain scores were not significantly different between the 2 groups (mean pain scores not provided; P > 0.05). | 1) Observer‐reported VAS pain scores (legal guardian and physician) 2) Requirement for supplemental lidocaine infiltration |
Not reported |
| Resch 1998 | Topical LAT gel vs LAT solution | None | 1) Adequacy of initial anaesthesia (adequate anaesthesia: LAT solution = 84% vs LAT gel = 82%; P > 0.05) 2) Effectiveness of anaesthesia (complete anaesthesia: LAT solution = 76% vs LAT gel = 85%; P = 0.007) |
0/194 |
AC: epinephrine (adrenaline) and cocaine; BN: bupivacaine‐noradrenaline; BP: blood pressure; cm: centimetre; c/w: compared with; ED: emergency department; EMLA: Eutectic Mixture of Local Anesthetics (lidocaine and prilocaine); EN: etidocaine‐noradrenaline; LAT: lidocaine, epinephrine and tetracaine (same as LET); LE: lidocaine and epinephrine; LET: same as LAT; LG: local gel; LI: local infiltration; MAC: bupivacaine 0.5%, epinephrine 1:2000, cocaine 10.0%; mm: milli‐metre; MN: mepivacaine‐noradrenaline; PN: prilocaine‐noradrenaline; N: number; NS: not significant; P = P value; PP: prilocaine, phenylephrine; RCT: randomized controlled trial; RICDRS: Restrained Infants and Children Distress Rating Scale; SD: standard deviation; SE: standard error; TA: tetracaine and epinephrine; TAC: tetracaine, epinephrine and cocaine; TLP: tetracaine, lidocaine and phenylephrine; TP: tetracaine and phenylephrine; VAS: visual analogue scale; vs: versus; w/w: weight per weight
Primary outcome: participant report of pain intensity during wound repair
Smith 1996 found no significant differences in anaesthetic efficacy between four different noradrenaline‐containing topical anaesthetics, including bupivacaine‐noradrenaline (BN), etidocaine‐noradrenaline (EN), mepivacaine‐noradrenaline (MN) and prilocaine‐noradrenaline (PN). Another multi‐arm RCT (Smith 1997b) demonstrated no significant differences between three different topical formulations that contained the vasoconstrictor phenylephrine, including prilocaine‐phenylephrine (PP), tetracaine‐phenylephrine (TP) and tetracaine‐lidocaine‐phenylephrine (TLP). A third trial by the same primary author concluded that topical PP and bupivacaine‐phenylephrine (BP) had similar efficacy (Smith 1998a). Krief 2002 found no significant differences between pain scores among participants treated with topical EMLA or LAT. Resch 1998 concluded that the solution and gel formulations of LAT provided comparable analgesic efficacy.
Secondary outcome: indirect predictors of pain intensity during wound repair
Krief 2002 presented higher physician‐reported VAS scores (i.e. poorer pain control) when using EMLA compared with LAT. For acute adverse effects and toxicity, please see the effects subsection "Intervention 3. Anaesthetic‐related acute adverse effects" below.
Evidence quality for primary and secondary outcomes
Each of the papers by Smith and associates, as well as theKrief 2002 study, had unclear risk of bias in at least three domains. Resch 1998 showed unclear management of incomplete data but otherwise was at low risk of bias.
Again, although the trials mentioned were RCTs, we downgraded the overall GRADE score for each measured outcome to low owing to limitations in design and implementation, imprecision of results and high probability of publication bias (selective reporting of data) (see Characteristics of included studies).
Intervention 3. Anaesthetic‐related acute adverse effects
Approximately half of the included trials (12/25 enrolling 1713 participants) reported data regarding the incidence of potential anaesthetic‐related acute adverse effects. We have displayed details in Table 1. Studies reported only one episode of a local anaesthetic‐related complication (acute toxicity or subacute adverse effects). In Vinci 1996, a single paediatric participant developed a large indurated, erythematous reaction one day after application of topical TAC. The skin reaction completely resolved with antihistamine treatment and warm compresses, and investigators described no other incidents of local anaesthetic‐induced reactions or toxicity. Schaffer 1985 reported that after discharge home, 10.7% of children treated with TAC and 7.8% who received topical AC became drowsy or excitable. However, none of these symptoms occurred in the emergency department, and no evidence suggested that symptoms were causally related to the topical anaesthetic. Two trials that included an infiltrated local anaesthetic group reported data on acute side effects (Hegenbarth 1990; Kendall 1996). None of the combined 256 participants administered local anaesthesia via infiltration in these two studies reported any adverse effects.
Ten different RCTs that studied cocaine‐based topical anaesthetics explicitly reported information about acute adverse effects (Blackburn 1995; Bonadio 1990; Ernst 1990; Ernst 1995a; Hegenbarth 1990; Kendall 1996; Kuhn 1996; Schaffer 1985; Schilling 1995; Vinci 1996). Pooled data on 1042 participants from these 10 trials showed only a single acute adverse reaction (incidence 0.096%). This complication (local induration in a paediatric participant) was not serious and is described above. A total of five RCTs that used cocaine‐free topical agents reported data on anaesthetic‐related toxicity or side effects (Blackburn 1995; Ernst 1995a; Resch 1998; Schaffer 1985; Schilling 1995). None of the 358 participants in these five RCTs experienced any acute adverse reactions. Lee 2013 reported wound complications as a secondary outcome. Participants assigned to receive LAT gel developed infection (five participants), dehiscence (one participant) and missing sutures (one participant). Corresponding outcomes in the lidocaine infiltration group included infection in two of 14 participants, dehiscence in none and lost sutures in none. Again, studies found that LAT and infiltrated lidocaine have comparable side effect profiles. Jenkins 2014 reported wound infection (four cases in the infiltration group vs two in the topical anaesthetic putty group); wound dehiscence (two cases in the topical anaesthetic putty group); and adverse effects (one inflamed wound in the topical anaesthetic putty group and one wound requiring resuturing in both groups).
Discussion
The topic of the present review is limited to repair of dermal lacerations. Therefore, the results may not be generalizable to repair of wounds located on mucosal surfaces. Also, the dermis provides a barrier to penetration of topical anaesthetic, and so our findings may not be applicable to instrumentation of intact skin.
Summary of main results
The present review consists of a descriptive analysis. Two predominant limitations precluded meta‐analysis. First, most of the comparisons between specific anaesthetic agents were accomplished in single trials. Only in a few instances were agents compared across multiple studies. Moreover, trials employed numerous measures of anaesthetic efficacy. In fact, only 15 of the 25 included studies used a validated pain scale. The primary outcome measure was analgesic efficacy, reflected in the participant's self‐report of pain intensity during repair of the wound. We extracted surrogate pain scores provided by observers; however, participants' and practitioners' assessments of procedure‐related pain reveal non‐concordance (Choiniere 1990; Singer 1999; Stephenson 1994). Therefore, during analysis, we considered surrogate pain scores only when participant‐reported pain scales were not available.
Overall completeness and applicability of evidence
Our systematic review addressed four principal questions regarding topically applied local anaesthetics for dermal laceration repair.
First, we assessed whether benefits of non‐invasive, topical anaesthetic application occur at the expense of decreased analgesic efficacy. We obtained data from a single study that had unclear risk of blinding bias(Smith 1997a); the remainder of the trials were at high risk of blinding bias (Anderson 1990; Ernst 1997; Gaufberg 2007; Hegenbarth 1990; Jenkins 2014; Kendall 1996; Lee 2013, Pryor 1980; Smith 1996). Smith 1997a did not use participant self‐reported pain scores to determine anaesthetic efficacy but instead used observer‐estimated pain scores. Therefore, we found a paucity of high‐quality studies with low risk of bias on which we could base definitive conclusions regarding efficacy of topical anaesthetics versus infiltrated local anaesthesia.
Our second objective was to compare the efficacy of various single‐component or multi‐component topical anaesthetic agents for repair of dermal lacerations. We obtained data from studies that had unclear risk of bias (Ernst 1990; Kuhn 1996; Schilling 1995; Smith 1996; Smith 1997a; Smith 1997b; Smith 1998a) or high risk of bias (Blackburn 1995; Bonadio 1990; Ernst 1995a; Ernst 1995b; Jenkins 2014; Lee 2013; Schaffer 1985; Vinci 1996; White 2004; Zempsky 1997). We have summarized the findings of individual trials in Table 4, Table 5, Table 6 and Table 7. However, the evidence reflects bias that may cause some doubt about the findings, or may even significantly weaken the results.
The third objective was to determine the clinical necessity for topical application of the ester anaesthetic, cocaine. We included in the review 13 randomised controlled trials (RCTs), which assessed the effectiveness of cocaine‐free topical anaesthetics. None of these studies were at low risk of bias. We mathematically combined data from two studies and found that topical prilocaine‐phenylephrine (PP) provided effective analgesia (Smith 1997b; Smith 1998a). However, both of these studies had unclear risk of bias for each key domain, leading to some uncertainty about the results. A single RCT assessed each of the additional formulations of topical cocaine‐free anaesthetics. Results from studies with unclear risk of bias show that the following agents may provide effective topical analgesia: lidocaine‐adrenaline‐tetracaine (LAT) (Schilling 1995), bupivacaine‐noradrenaline (BN) (Smith 1996), prilocaine‐noradrenaline (PN) (Smith 1996), tetracaine‐lidocaine‐phenylephrine (TLP) (Smith 1997b), tetracaine‐phenylephrine (TP) (Smith 1997b) and lidocaine‐prilocaine (EMLA) (Krief 2002). Topical LAT, which exploits the rapid onset of lidocaine and the long duration of tetracaine (Altman 1985), has been the most widely studied cocaine‐free formulation. However, before definitive conclusions can be reached, additional investigation is warranted through trials that are well designed and are conducted to assess anaesthetic efficacy by using validated patient self‐reported pain scoring scales.
Finally, we evaluated the safety of both cocaine‐containing and cocaine‐free topical anaesthetics. Many of the included trials (14 of 25) reported data regarding the incidence of anaesthetic‐related acute adverse effects. Only one study reported a topical local anaesthetic‐related side effect (Vinci 1996). The reaction consisted of a large indurated, erythematous reaction that occurred after topical application of tetracaine‐epinephrine‐cocaine (TAC). No trials reported serious complications, such as seizures or anaphylactic reactions. Although reported data are insufficient to reveal the exact incidence of complications, if topical anaesthetics are applied as directed and appropriately dosed, serious adverse effects are probably infrequent. Combined observations from 10 trials that administered cocaine‐based agents and explicitly reported data on side effects revealed one adverse reaction among 1042 total participants (incidence 0.096%). Ten studies that administered cocaine‐free anaesthetic agents reported data on toxicity, and none of the participants in these groups experienced acute adverse reactions.
Quality of the evidence
The present review consists of a descriptive analysis. Two predominant limitations precluded meta‐analysis. First, most of the comparisons between each of the specific anaesthetic agents were accomplished in one trial. Only in a few instances were similar agents compared in multiple studies. Moreover, trials employed diverse outcome measures to determine anaesthetic efficacy. In fact, only 15 of the 25 included studies used a validated pain scale. The primary outcome measure was analgesic efficacy, reflected in participants' self‐report of pain intensity during repair of the wound. We extracted surrogate pain scores provided by observers and found that participants' and practitioners' assessments of procedure‐related pain showed non‐concordance (Choiniere 1990; Singer 1999; Stephenson 1994). Therefore, our analysis employed surrogate pain scores only when participant‐reported pain scores were not available.
In conclusion, although the trials mentioned were RCTs, we downgraded the overall GRADE score for each measured outcome to low owing to limitations in design and implementation, imprecision of results and high probability of publication bias (selective reporting of data) (see Characteristics of included studies).
Potential biases in the review process
Cochrane support staff conducted the search for this review to ensure comprehensiveness and inclusion of all possible studies. We have assessed all types of bias required by the 2011 version of the Higgins Cochrane Handbook for Systematic Reviews of Interventions (version 5.1.0). Two independent review authors judged inclusion or exclusion of articles to strengthen the decision‐making process. We included all reported participants without exclusion due to gender, age or comorbid health issues. We included all types of RCTs and quasi‐randomized trials without exclusion due to language, sample size, local aesthetics used or treatment setting. A potential weakness of our review is the exclusion of studies including participants with deep traumatic wounds or therapeutic incisions, or comparisons with other non‐invasive treatments such as glue, but our focus was intended to decrease heterogeneity in the review population.
Two independent review authors extracted and entered data, which were sent to all participating review authors for confirmation. We were unable to perform a meta‐analysis and we reported most data descriptively, which is a weakness of our review.
The present review has other sources of potential bias as well. The primary outcome was participants' self‐report of pain intensity during repair of the wound via validated pain scales. However, a significant number of included trials used observer‐reported pain scores or other surrogate outcomes to determine anaesthetic efficacy. Results show non‐concordance between participants' pain scores and ratings by physician, parents and other proxies (Choiniere 1990; Singer 1999; Stephenson 1994). Moreover, 21 of the 25 included RCTs enrolled paediatric participants, and evaluation of pain in children can be challenging. Researchers have used several pain scales, including the visual analogue scale (VAS) and the Faces scale, in a reliable and validated manner among children as young as five years (Berde 1991; Lander 1993; Zeltzer 1991). Also, evidence supports the validity of tools for measuring acute pain in children as young as three years old (Tyler 1993). However, the youngest age at which children can credibly quantify pain intensity is controversial (Tyler 1993), and behavioural pain scales for early verbal and preverbal children remain to be validated (Crellin 2007). Therefore, we cannot exclude the possibility that pain assessment in younger paediatric participants may not be accurate. Eight studies were not blinded, and four used unclear blinding strategies.
Agreements and disagreements with other studies or reviews
We found no disagreement in final study results between any of the included studies, nor with other previously published studies or review articles, with the exception of conflicting conclusions about efficacy from two studies of topical mepivacaine‐noradrenaline (MN) (Smith 1996; Smith 1997a). We could not combine these two trials because investigators used different pain intensity scales to determine anaesthetic efficacy, and Smith 1996 did not report the standard deviations of outcomes. However, all other trials concluded that topical local anaesthetics are at least as effective as infiltrated ones in laceration repair and provide the advantage of decreasing the pain of application. An earlier review (Grant 1992) found that TAC is as effective as lidocaine infiltration in dermal laceration repair; however, the minimum effective dose remains to be established to avoid side effects. Throughout subsequent years, multiple RCTs have reported the same results (see Results section). With the development of new local anaesthetics, the use of cocaine has been questioned and might be nowadays unjustifiable by many, as has been found in the included studies (see Results section).
Our updated version of this review confirms the results of the previous version (Eidelman 2011).
Authors' conclusions
Implications for practice.
Injection of anaesthetics per se induces discomfort and may worsen 'needle anxiety' among paediatric participants while distorting the wound site (Kundu 2002). Therefore, topical anaesthetics are preferable if they do in fact provide similar analgesia to injected local anaesthetics. Individual studies have suggested that some topical formulations may have similar efficacy to conventional local anaesthetics. However, because of methodological heterogeneity and lack of high‐quality trials, definitive conclusions for clinical practice cannot be reached at this time.
If cocaine‐free topical anaesthetics have similar effectiveness as cocaine‐containing agents, then the latter can no longer be justified in light of their high cost and potential adverse effects. Topical lidocaine‐adrenaline‐tetracaine (LAT), which exploits the rapid onset of lidocaine and the long duration of tetracaine, has been the most widely studied cocaine‐free formulation. However, additional studies with sound methodological design are necessary before definitive conclusions for clinical practice can be drawn.
Researchers have reported no serious complications among any participants treated with cocaine‐based or cocaine‐free topical anaesthetics. One mild, self‐limiting skin reaction did occur in one case after application of topical TAC. Nevertheless, clinicians should exhibit caution and apply topical formulations only as directed, while avoiding mucous membrane contact and following appropriate dosing regimens.
We have found two new studies published since the time of the last version of this review. We have added these studies to those previously included and have updated the analysis. This new analysis yielded the same conclusions as were previously presented.
In conclusion, based mostly on descriptive analysis, we believe that topical anaesthetics may in fact be an efficacious, non‐invasive means of providing analgesia before suturing of dermal lacerations. However, data regarding the efficacy of each topical anaesthetic are based mostly on single comparisons in trials that have unclear or high risk of bias. Before definitive conclusions can be drawn, additional methodologically well‐designed studies with low risk of bias are necessary. Future research should focus on the efficacy of cocaine‐free anaesthetics in light of the burden of dispensing cocaine ‐ a controlled substance that is widely abused.
Implications for research.
More investigation is warranted to compare topical lidocaine‐adrenaline‐tetracaine (LAT) versus other potentially efficacious, cocaine‐free topical anaesthetics such as bupivacaine‐noradrenaline (BN), prilocaine‐phenylephrine (PP) and tetracaine‐lidocaine‐phenylephrine (TLP). Also, future research could evaluate additional clinically useful topical local anaesthetics or combinations. Recent clinical application of novel formulations of existing local anaesthetics such as microsomal encapsulated bupivacaine (Chahar 2012) or those with an intrinsically long duration of action such as saxotoxin (Lobo 2015) may expand the range of available topical local anaesthetics.
Furthermore, additional methodologically sound studies that are less likely to be flawed by bias or confounding variables are needed. Many of the included trials did not determine analgesic efficacy by using validated, participant self‐reported pain scales but instead used observer‐reported pain scores or other elementary surrogate measures. Future trials should adopt uniform outcomes that reflect participants' own assessments of procedure‐related pain intensity. Young children and developmentally impaired adults may benefit most from non‐invasive, effective topical anaesthesia before laceration repair. Therefore, validated behavioural pain and distress scales for preverbal or early verbal children, and for cognitively impaired adults, will facilitate determination of the efficacy and safety of topical anaesthetics for these patient subgroups.
What's new
| Date | Event | Description |
|---|---|---|
| 1 December 2016 | New citation required but conclusions have not changed |
|
| 1 December 2016 | New search has been performed | New search date: December 2016. New studies: 2 included/36 excluded/0 ongoing/0 awaiting classification Other review updates.
|
History
Protocol first published: Issue 3, 2005 Review first published: Issue 6, 2011
| Date | Event | Description |
|---|---|---|
| 18 May 2015 | Amended | Updated declaration of interest statement. |
| 31 May 2012 | Amended | Updated contact details. |
| 17 April 2012 | Amended | Updated contact details. |
| 18 January 2012 | Amended | Updated contact details. |
| 1 September 2008 | Amended | Converted review to new review format. |
Acknowledgements
We would like to thank Jane Cracknell, Managing Editor of the Cochrane Anaesthesia, Critical and Emergency Care Collaborative Review Group, for help and editorial advice provided during preparation of this update of the systematic review. We also acknowledge the efforts of Karen Hovhannisyan in assisting with the literature search .
We would like to thank Mathew Zacharias, Marialena Trivella, Ronan O’Sullivan, Stephen Priestley, Sujesh Bansal and Nete Villebro for their help and editorial advice in the past, Dr Joseph Lau for providing advice on statistics, and Dr Mukhtar Zaidi for providing pharmacological consultation.
Appendices
Appendix 1. Search strategy for CENTRAL, the Cochrane Library
#1 MeSH descriptor Lacerations, this term only #2 MeSH descriptor Wounds and Injuries, this term only #3 MeSH descriptor Facial Injuries explode all trees #4 MeSH descriptor Finger Injuries explode all trees #5 MeSH descriptor Wounds, Penetrating explode all trees #6 MeSH descriptor Hand Injuries explode all trees #7 MeSH descriptor Sutures explode all trees #8 MeSH descriptor Surgical Stapling explode all trees #9 (laceration* or wound* or suture or stapling or repair*):ti,ab #10 ((facial or dermal or cutaneous or finger or hand or eyelid) near injur*):ti,ab #11 (penetrat* near wound*) #12 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11) #13 (topical near (an?esthe* or lidocain* or lignocain* or tetracain* or amethocain* or benzocain* or butamben or dibucain* or pramoxin* or prilocain* or etidocian)) #14 emla* or (eutectic mixture of local an?esthe*) or (tetracaine?adrenaline?cocain*) or (tetracaine?epinephrine?cocain*) or (lidocaine?adrenaline?tetracain*) or (lidocaine?epinephrine?tetracain*) or (spray or ointment or gel or cream or lotion or jelly or balm):ti,ab #15 MeSH descriptor Administration, Topical, this term only #16 MeSH descriptor Ointments, this term only #17 MeSH descriptor Gels, this term only #18 (#13 OR #14 OR #15 OR #16 OR #17) #19 (#12 AND #18)
Appendix 2. Search strategy for MEDLINE (Ovid SP)
1. laceration.mp. or exp lacerations/ or exp facial Injuries/ or exp finger injuries/ or exp wounds, penetrating/ or exp hand injuries/ or exp sutures/ or exp surgical stapling/ or ((wounds.mp. or exp wounds/) and injuries/) or (injury adj3 (hand or eyelid or finger or facial or dermal)).mp. or cutaneous.mp. or staple.mp. or repair.mp. 2. (topical adj3 (an?esthe* or lidocaine or lignocaine or lidoderm or tetracaine or amethocaine or benzocaine or butamben or pramoxine or prilocaine or topical)).mp. or exp administration, topical/ or topical.ti,ab.or emla.mp.or eutectic mixture of local an?esthe*.mp. or tetracaine‐adrenaline‐cocaine.mp. or tetracaine‐epinephrine‐cocaine.mp. or lidocaine‐adrenaline‐tetracaine.mp. or lidocaine‐epinephrine‐tetracaine.mp. or spray.ti,ab. or ointment.mp. or exp ointments/ or gel.mp. or exp gels/ or cream.mp. or lotion.mp. or jelly.mp. or balm.mp. 3. 1 and 2 4. ((randomized controlled trial or controlled clinical trial).pt. or randomized.ab. or placebo.ab. or clinical trials as topic.sh. or randomly.ab. or trial.ti.) not (animals not (humans and animals)).sh. 5. 3 and 5
Appendix 3. Search strategy for CINAHL (EBSCO host)
S28 S20 and S27 S27 S21 or S22 or S23 or S24 or S25 or S26 S26 AB random* or controlled trial* or mulicenter or placebo* S25 (MM 'Multicenter Studies') S24 (MH 'Prospective Studies+') S23 (MM 'Double‐Blind Studies') or (MM 'Single‐Blind Studies') or (MM 'Triple‐Blind Studies') S22 (MM 'Placebos') S21 (MM 'Random Assignment') or (MH 'Clinical Trials+') S20 S12 and S19 S19 S13 or S14 or S15 or S16 or S17 or S18 S18 AB emla* or (eutectic mixture of local an?esthe*) or (tetracaine?adrenaline?cocain*) or (tetracaine?epinephrine?cocain*) or (lidocaine?adrenaline?tetracain*) or (lidocaine?epinephrine?tetracain*) S17 AB spray or ointment or gel or cream or lotion or jelly or balm S16 AB ( an?esthe* or lidocain* or lignocain* or tetracain* or amethocain* or benzocain* or butamben or dibucain* or pramoxin* or prilocain* or etidocian* ) and topical S15 (MH 'Gels') S14 (MH 'Ointments') S13 (MH 'Administration, Topical') S12 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 S11 AB laceration* or wound* or injur* or stapl* or repairor or suture* S10 AB penetrat* and AB wound* S9 AB ( facial or dermal or cutaneous or finger or hand or eyelid ) and AB injur* S8 (MH 'Surgical Stapling') S7 (MH 'Sutures') S6 (MH 'Hand Injuries') S5 (MH 'Finger Injuries') S4 (MH 'Arm Injuries') S3 (MH 'Facial Injuries') S2 (MH 'Wounds and Injuries+') or (MH 'Wounds, Penetrating+') S1 (MH 'Tears and Lacerations')
Appendix 4. Search strategy for Embase (Ovid SP)
1. exp laceration/ or injury/ or exp face‐injury/ or exp finger‐injury/ or exp hand‐injury/ or exp suture/ (123072) 2. (laceration* or wound* or injur* or ((facial or dermal or cutaneous or finger or hand or eyelid) adj3 injur*)).ti,ab. or (penetrat* adj3 wound*).mp. or (stapl* or repairor or suture*).ti,ab. 3. 1 or 2 4. (topical adj3 (an?esthe* or lidocain* or lignocain* or tetracain* or amethocain* or benzocain* or butamben or dibucain* or pramoxin* or prilocain* or etidocian)).mp. 5. (emla* or eutectic mixture of local an?esthe* or tetracaine?adrenaline?cocain* or tetracaine?epinephrine?cocain* or lidocaine?adrenaline?tetracain* or lidocaine?epinephrine?tetracain*).mp. 6. (spray or ointment or gel or cream or lotion or jelly or balm).ti,ab. 7. topical‐drug‐administration/ or ointment/ or gel/ 8. 6 or 4 or 7 or 5 9. 8 and 3 10. (placebo.sh. or controlled study.ab. or random*.ti,ab. or trial*.ti,ab.) not (animals not (humans and animals)).sh. 11. 10 and 9
Appendix 5. Data extraction form
| First author | Journal/Conference proceedings, etc. | Year |
| |
| Dates study conducted | Not reported |
| Country and setting (centres and departments) | |
| Source of funding | |
| Conflicts of interest overall | |
| Declaration of interests for each researcher |
| Trial characteristics | |
| Further details | |
| RCT/Quasi | |
| Study size (number of participants) | |
| Setting of study (single‐centre vs multi‐centre, inpatient vs outpatient) | |
| Participant characteristics | |
| Further details | |
| Age (mean, median, range, etc.) | |
| Sex of participants (numbers, percentages) | |
| Wound characteristics (length, location of laceration, etc.) | |
| Anaesthetic characteristics | |
| Further details | |
| Infiltrated anaesthetic (agent, dosage) | |
| Topical anaesthetic (agent, dosage, duration of application) | |
| Were systemic analgesics or sedatives given? | |
| Outcomes | |
| Further details | |
| Primary measure of pain intensity (patient self‐report using pain scale such as VAS, numerical rating, etc.) | |
| Secondary measure of pain intensity (incidence of topical anaesthetic failure, requirement of supplemental anaesthesia, participant behavioural responses, etc.) | |
| Anaesthetic‐related toxicity or acute adverse events | |
Methodological quality
| Sequence generation | |
| State here method used to generate sequence and reasons for grading | Grade (circle) |
| |
Low risk |
| High risk | |
| Unclear risk | |
| Allocation concealment | |
| State here method used to conceal allocation and reasons for grading | Grade (circle) |
| |
Low risk |
| High risk | |
| Unclear risk | |
| Blinding | |
| State here method used to blind study and reasons for grading | Grade (circle) |
| |
Low risk |
| High risk | |
| Unclear risk | |
| Description of withdrawals and drop‐outs | |
| State here method used to address incomplete outcome data | Grade (circle) |
| |
Low risk |
| High risk | |
| Unclear risk | |
| Selection bias (selective outcome reporting) | |
| State here method used for selective reporting of outcomes, time points, subgroups or analyses. | Grade (circle) |
| Low risk | |
| High risk | |
| Unclear risk | |
|
Sample size (per arm) Low risk ≥ 200 participants enrolled Unclear risk 50 to 199 participants High risk < 50 |
Grade (circle) High risk Low risk Unclear risk |
Appendix 6. Local anaesthetics and vasoconstrictors including alternative names
Cocaine‐containing topical anaesthetics
AC = Epinephrine‐cocaine or adrenaline‐cocaine
C = Cocaine
MAC = Bupivacaine‐epinephrine‐cocaine or bupivacaine‐adrenaline‐cocaine
TAC = Tetracaine‐epinephrine‐cocaine or tetracaine adrenaline‐cocaine
TC = Tetracaine‐cocaine
Cocaine‐Free Topical Anaesthetics
Anaesthetic putty (containing 4.94% w/w lidocaine hydrochloride, equivalent to 4% w/w lidocaine base)
BN = Bupivacaine‐norepinephrine
EMLA = Eutectic mixture of local anaesthetics = lidocaine‐prilocaine
EN = Etidocaine‐norepinephrine
LAT = LET = Lidocaine‐epinephrine‐tetracaine or lidocaine‐adrenaline‐tetracaine
LE = Lidocaine‐epinephrine orlidocaine‐adrenaline
MN = Mepivacaine‐norepinephrine
PN = Prilocaine‐norepinephrine
PP = Prilocaine‐phenylephrine
T = Tetracaine
TE = Tetracaine‐epinephrine or tetracaine‐adrenaline TLP = Tetracaine‐lidocaine‐phenylephrine
TP = Tetracaine‐phenylephrine
Alternative names for local anaesthetics and vasoconstrictors
Epinephrine is the same as adrenaline
Bupivacaine is also called marcaine or sensoricaine
Lidocaine is also called xylocaine.
Data and analyses
Comparison 1. Topical prilocaine‐phenylephrine (PP) versus topical tetracaine‐epinephrine‐cocaine (TAC).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Patient self‐reported VAS (0‐100 mm) pain scores | 2 | 240 | Mean Difference (IV, Random, 95% CI) | 5.59 [‐2.16, 13.35] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Anderson 1990.
| Methods | Single‐centre RCT, paediatric emergency department, United States | |
| Participants | 151 patients younger than 18 years old with lacerations on the scalp (n = 31), face (n = 79) or extremity (n = 41) | |
| Interventions | 1. Topical TAC solution (tetracaine 0.5%, epinephrine 1:2000, cocaine 11.8%), applied for 5 to 10 minutes (n = 56) 2. Intradermal infiltration with lidocaine 1% (n = 53) 3. Topical placebo solution, applied for 5 to 10 minutes (n = 42) | |
| Outcomes | 1. Before laceration repair, the physician probed the wound with a 25‐gauge needle to determine adequacy of initial anaesthesia.
2. The physician graded participant compliance during the suturing process on a 4‐point scale (1 ‐ complete compliance, 2 ‐ occasional resistance, 3 ‐ frequent resistance, 4 ‐ continuous resistance).
3. Supplemental lidocaine infiltration was required. Results of topical TAC versus topical placebo include the following. 1. Adequate initial anaesthesia (topical TAC = 89% vs topical placebo = 17%; P < 0.0001) 2. Physician compliance scale (1‐4) ratings (complete compliance to continuous resistance) (mean score ± SD: topical TAC = 1.25 ± 0.57 vs topical placebo = 1.93 ± 0.96; P < 0.002) 3. Requirement of supplemental lidocaine infiltration (topical TAC = 18% vs topical placebo = 83%; P < 0.0001) Results of topical TAC versus infiltrated local anaesthetic include the following. 1. Adequate initial anaesthesia (topical TAC = 89% vs infiltrated local anaesthetic = 79%; P = non‐significant) 2. Physician compliance scale (1‐4) ratings (complete compliance to continuous resistance) (mean score ± SD: topical TAC = 1.25 ± 0.57 vs infiltrated local anaesthetic = 1.94 ± 1.12; P < 0.002) 3. Requirement of supplemental lidocaine infiltration (topical TAC = 18% vs infiltrated local anaesthetic = 23%; P = non‐significant) |
|
| Intervention dates | August 1986 to May 1987 | |
| Declaration of interest | Not reported | |
| Notes | Funding not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "The last digit of the patient's medical record number was used to enter patients into either the intradermal or topical group". Comment: probably not done |
| Allocation concealment (selection bias) | High risk | Quote: "The last digit of the patient's medical record number was used to enter patients into either the intradermal or topical group". Comment: probably not done |
| Blinding (performance bias and detection bias) All outcomes | High risk | Quote: "Individual study vials containing 5ml of TAC or placebo were prepared in the pharmacy of University of Massachusetts Medical Center following a standard protocol and assigned numbers"; "The ED staff member evaluating and suturing the patient were blind to the solution contained in the vials". Comment: Comparisons of topical TAC and topical placebo were probably blinded. However, comparisons between lidocaine infiltration and topical TAC were probably unblinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 153 eligible patients, 2 refused to participate. 151 randomized, no missing outcome data |
| selective reporting of outcomes All outcomes | Unclear risk | All outcomes discussed in Methods section reported in Results. Subgroup analysis based on location of laceration was not prespecified. |
| Other bias (sample size) | High risk | 56 TAC: 53 lidocaine 42 placebo |
Blackburn 1995.
| Methods | Single‐centre RCT, emergency department, community‐based teaching hospital, United States | |
| Participants | 35 adult and paediatric patients (minimum age of 2 years) with facial and scalp lacerations, ≤ 6 cm in length | |
| Interventions | 1. Topical TAC solution (tetracaine 0.5%, epinephrine 1:2000, cocaine 10.4%), applied for 20 minutes (n = 18) 2. Topical TLE solution (lidocaine 5% and epinephrine 1:2000), applied for 20 minutes (n = 17) | |
| Outcomes | 1. The participant reported discomfort using a facial effective pain scale (1‐9), which consisted of 9 faces with various emotional expressions. However, in a few cases, the participant was too young to use the pain scale, so the physician estimated the participant's pain using the same Faces scale. The study combined self‐reported and surrogate Faces pain scale scores in the final results.
2. Rescue lidocaine infiltration was required.
3. The study reported any acute adverse reactions directly related to the anaesthetic. Results included the following. 1. Faces pain scale (1‐9) scores (mean score ± SD: topical TLE = 3.29 ± 1.92 vs topical TAC = 2.66 ± 1.78; P = 0.33) 2. Requirement for supplemental lidocaine infiltration (topical TLE = 6% vs topical TAC = 6%; P = not reported) 3. No acute anaesthetic‐related adverse effects |
|
| Intervention dates | May to August 1992 | |
| Declaration of interest | Not reported | |
| Notes | Funding not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "The TAC and TLE solutions were arbitrarily assigned to a single‐dose (10ml), sequentially numbered vials by the pharmacist. The vials, with the specific contents unknown to the emergency physician, were forwarded to the ED as requested". Comment: probably not done |
| Allocation concealment (selection bias) | Low risk | Quote: "The solutions were made visibly identical by adding methylene blue to the TLE solution so that it matched the intrinsic blue colour of TAC". "The vials, with the specific contents unknown to the emergency physician, were forwarded to the ED as requested". Comment: probably done |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "The solutions were made visibly identical by adding methylene blue to the TLE solution so that it matched the intrinsic blue colour of TAC". Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 35 participants in study but reporting of attrition or exclusions insufficient to permit judgement |
| selective reporting of outcomes All outcomes | Low risk | All outcomes described in Methods were fully reported in Results section. Adverse events noted |
| Other bias (sample size) | High risk | Total N = 35: 17 participants in the TLE group; 18 in the TAC group |
Bonadio 1990.
| Methods | Single‐centre RCT, Department of Emergency Medicine, Children’s Hospital Wisconsin, Milwaukee, Wisconsin, United States | |
| Participants | 55 paediatric patients with facial lacerations | |
| Interventions | 1. Topical TAC solution (tetracaine 0.5%, epinephrine 1:2000, cocaine 11.8%), applied for 10 to 15 minutes (n = 24) 2. Topical AC solution (epinephrine 1:2000, cocaine 11.8%), applied for 10 to 15 minutes (n = 31) | |
| Outcomes | 1. The physician calculated the total number of 'sutures eliciting pain' using the following system. Each suture placed involved 2 points; an entrance and an exit piercing of the wound tissue with the needle. A painful response consisted of a verbal participant experiencing a painful sensation or a non‐verbal participant beginning to cry, or crying with greater intensity. The total number of 'sutures placed eliciting pain' was calculated by dividing the total number of painful responses by 2.
2. The study reported any acute adverse effects due to the anaesthetic. Results included the following. 1. The physician calculated the total number of 'sutures eliciting pain' (topical AC = 7/103 (4%) vs topical TAC = 7/151 (7%); P = not‐reported). 2. No acute anaesthetic‐related adverse effects were noted. |
|
| Intervention dates | Not reported | |
| Declaration of interest | No explicit documentation regarding conflicts of interest. | |
| Notes | Source of funding: general academic paediatric development fellowship from The Robert Wood Johnson Foundation; and Grant 10066 from The Robert Wood Foundation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "...as in each case an assistant randomly selected one of the two solutions for physician application..." Comment: probably not done |
| Allocation concealment (selection bias) | High risk | Quote: "an assistant randomly selected one of the two solutions for physician application..." Comment: probably not done |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "The managing physician was 'blind' to which preparation was being administered...the physician was informed of the solution composition only after the suturing procedure and pain scoring were completed". Comment: probably done, assuming the 2 solutions were visually identical |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 55 participants in study but reporting of attrition or exclusions insufficient to permit judgement |
| selective reporting of outcomes All outcomes | Low risk | The study protocol is available, and all of the study’s prespecified outcomes have been reported in the prespecified way. |
| Other bias (sample size) | High risk | 55 paediatric participants: 1. Topical TAC solution, n = 24 2. Topical AC solution, n = 31 |
Ernst 1990.
| Methods | RCT, single centre, emergency department, United States | |
| Participants | 139 adult and paediatric patients older than 5 years of age, with laceration of the face (n = 53), scalp (n = 33), extremity (n = 52) or trunk (n = 1), measuring < 5 cm in length | |
| Interventions | 1. Topical TAC solution (tetracaine 0.5%, epinephrine 1:2000, cocaine 11.8%), applied for 5 to 10 minutes (n = 69) 2. Topical cocaine solution 11.8%, applied for 5 to 10 minutes (n = 70) | |
| Outcomes | 1. The physician assessed the adequacy of initial anaesthesia by pricking the wound with a pin. If pain was elicited with pinprick, then 1% lidocaine was infiltrated, and the participant was assigned to the 'poor anaesthesia' group.
2. Among participants who did not require infiltrated lidocaine, the physician rated the effectiveness of anaesthesia during suturing on a numerical scale (0‐10). 3. Investigators reported acute adverse reactions directly related to the anaesthetic. Results include the following. 1. Incidence of 'poor anaesthesia' (topical cocaine = 20% vs topical TAC = 12%; P = not reported) 2. Physician rating of anaesthetic effectiveness on a numerical scale (0‐10; least effective to most effective) (mean scores ± SD: topical cocaine = 6.44 ± 3.48 vs topical TAC = 7.74 ± 3.03; P = 0.005) 3. No acute anaesthetic‐related adverse effects |
|
| Intervention dates | Not reported | |
| Declaration of interest | Not reported | |
| Notes | Source of funding: Saint Francis Hospital and Medical Center Study author contacted for additional information but did not reply |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "TAC and cocaine solutions were randomly distributed with only a number from 1‐150 appearing on each vial". Comment: unclear; exact mechanism of randomization not described |
| Allocation concealment (selection bias) | Unclear risk | Quote: "TAC and cocaine solutions were randomly distributed with only a number from 1‐150 appearing on each vial". "The investigator was blinded as to the identity of the agent. The code was kept in the pharmacy and was available to the investigators only in case of emergency". Comment: unclear; allocation concealment possible if a pharmacy‐controlled randomization process was used. However, this is not explicitly reported, so we decided upon unclear risk. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "The investigator was blinded as to the identity of the agent. The code was kept in the pharmacy and was available to the investigators only in case of emergency". Comment: probably done, assuming local anaesthetic solutions are identical in colour |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 148 participants were enrolled and 9 were excluded from the study before unblinding and analysis (4 improper application, 4 participant younger than 5 years and one with laceration too large). We concluded low risk of bias because the number of excluded participants was balanced between the 2 interventions, and reasons for exclusion are unlikely to be related to pain scores during suturing. |
| selective reporting of outcomes All outcomes | Unclear risk | All outcomes described in Methods section were reported in Results. Subgroup analyses by site and age were not prespecified. |
| Other bias (sample size) | Unclear risk | Total N = 139: 70 in the cocaine‐treated group 69 in the TAC‐treated group |
Ernst 1995a.
| Methods | Single‐centre RCT, Department of Medicine, Section of Emergency Medicine, Louisiana State University, New Orleans, Louisiana, United States | |
| Participants | 95 patients age 5 to 17 years with lacerations on the face (n = 64) or scalp (n = 31), ≤ 7 cm in length | |
| Interventions | 1. Topical LAT gel (lidocaine 4%, epinephrine 1:2000, tetracaine 0.5%), applied for 10 to 30 minutes (n = 48) 2. Topical TAC gel (tetracaine 0.5%, epinephrine 1:2000, cocaine 11.8%), applied for 10 to 30 minutes (n = 47) | |
| Outcomes | 1. Participant‐rated modified multi‐dimensional pain scale (0‐10)
2. Physician‐rated modified multi‐dimensional pain scale (0‐10)
3. Percentage of sutures causing pain
4. Requirement of supplemental lidocaine infiltration
5. Acute adverse reactions directly related to the anaesthetic reported by investigators Results include the following. 1. Participant‐reported modified multi‐dimensional pain scale (0‐10) scores (mean ranked sum: topical LAT = 49.0 vs topical TAC = 46.9; P = 0.71) 2. Physician‐assigned multi‐dimensional pain scale (0‐10) scores (mean ranked sum: topical LAT = 48.7 vs topical TAC = 47.3; P = 0.80) 3. Percentage of sutures placed causing pain (mean ranked sum: topical LET = 49.57 vs topical TAC = 46.39; P = 0.51) 4. Requirement of supplemental lidocaine infiltration (topical LET = 4%, topical TAC = 6%; P = not reported) 5. No acute anaesthetic‐related adverse effects |
|
| Intervention dates | Not reported | |
| Declaration of interest | Not reported | |
| Notes | Funding not reported ‐Study author contacted for additional information but did not reply |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Gels were randomized according to a random numbers table". Comment: probably done |
| Allocation concealment (selection bias) | High risk | Quote: "randomized according to a random numbers table" Comment: probably not done |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Patients and physicians performing suturing were blinded to which gels were being used. Only the numbers 1‐100 appeared on the capped syringes". Comment: probably done, assuming the 2 gels were visually identical |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 100 participants entered into the trial, but 5 were excluded before statistical analysis because topical anaesthesia was inadequate and lidocaine infiltration was required. Two participants in the LAT group and 3 in the TAC group were excluded. We judged low risk of bias because the number of excluded participants was balanced between the 2 interventions. |
| selective reporting of outcomes All outcomes | Low risk | All prespecified primary outcomes were reported: Physicians and participants or parents rated anaesthesia effectiveness during suturing utilizing a modified multi‐dimensional pain scale. Prespecified secondary outcomes were also reported: Participants or parents reported the number of sutures causing pain, which was analysed as percent of total sutures placed. Quote: “Both physician and patient or parent rated the anaesthesia effectiveness during suturing utilizing a modified multidimensional pain scale…. Patients or parents reported the number of sutures causing pain, which was analysed as percent of total sutures placed”. Table 1 lists demographics (age, sex), wound size, location, amount of anaesthetic used and number of sutures placed. Table 2 reports percent of sutures causing pain in each topical anaesthesia group. Table 3 reports physician vs participant rating for pain scores for each topical anaesthesia group. |
| Other bias (sample size) | High risk | LAT GEL = 48 participants TAC gel = 47 participants |
Ernst 1995b.
| Methods | Single‐centre RCT, Department of Medicine, Section of Emergency Medicine, Louisiana State University, New Orleans, Louisiana, United States | |
| Participants | 95 adult patients with laceration of the face (n = 81) or scalp (n = 13) ≤ 7 cm in length | |
| Interventions | 1. Topical LAT solution (lidocaine 4%, epinephrine 1:2000, tetracaine 0.5%), applied for 10‐30 minutes (n = 48) 2. Topical TAC solution (tetracaine 0.5%, epinephrine 1:2000, cocaine 11.8%), applied for 10‐30 minutes (n = 47) | |
| Outcomes | 1. Participant‐rated VAS (100 mm) pain score
2. Physician‐rated VAS (100 mm) pain score
3. Percentage of sutures eliciting pain Results include the following. 1. Participant‐reported VAS (100 mm) pain scores (mean ranked sum: topical LET = 45.3 vs topical TAC = 50.8; P = 0.27) 2. Physician‐reported VAS scores (mean ranked sum: topical LAT = 41.6 vs topical TAC = 54.6; P = 0.01) 3. Percentage of sutures causing pain (mean ranked sum: topical LET = 42.8% vs topical TAC = 53.3%; P = 0.36) |
|
| Intervention dates | ||
| Declaration of interest | Not reported | |
| Notes | Funding resource: supported by a grant from the Louisiana State University Emergency Medicine Residency Grant Fund. Study author contacted for additional information but did not reply |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Solutions were randomized according to a random numbers table". Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote: "The solutions were prepared by a pharmacist and were available in coded sterile, capped 3ml syringes". "Both TAC and LAT were clear solutions..." "Patients and physicians performing wound closure were blinded". Comment: probably done |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "The solutions were prepared by a pharmacist and were available in coded sterile, capped 3ml syringes with a cotton ball for application". "Both TAC and LAT were clear solutions mixed from powders". "Patients and physicians performing wound closure were blinded". Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 100 total participants enrolled but only 95 were included in final data analysis. Four participants were excluded because they required additional injected lidocaine (1 LAT group, 3 in TAC group), and 1 because of improper data collection. We judged 'no' (high risk of bias) because requirement of additional lidocaine is directly related to pain intensity during laceration repair. |
| selective reporting of outcomes All outcomes | Low risk | All outcomes described in Methods reported fully in Results. Adverse events reported |
| Other bias (sample size) | Unclear risk | 47 receiving TAC and 48 receiving LAT. Total N = 95 |
Ernst 1997.
| Methods | Single‐centre RCT, urban emergency department, United States | |
| Participants | 66 paediatric and adult patients, older than 5 years of age with laceration on the face (n = 30), scalp (n = 10) or extremity (n = 24), 1.5 to 10 cm in length | |
| Interventions | 1. Topical LAT gel (lidocaine 4%, epinephrine 1:2000, tetracaine 0.5%), applied for 10 to 20 minutes (n = 33) 2. Intradermal infiltration with lidocaine 1%, epinephrine, buffered with 8.4% NaHCO3 (n = 33) | |
| Outcomes | 1. Participant‐rated VAS (100 mm) pain scale scores
2. Physician‐rated VAS (100 mm) pain scale scores
3. Requirement of supplemental lidocaine infiltration
4. Percentage of sutures placed eliciting pain Results included the following. 1. Participant self‐reported VAS (100 mm) pain scores (median values (interquartile range): topical LAT = 0 (0‐1.35) vs infiltrated local anaesthetic = 0 (0‐0.6); P = 0.48, standard deviations not reported) 2. Physician‐reported VAS (100 mm) pain scores (median values (interquartile range): topical LAT = 0 (0‐0.55) vs infiltrated local anaesthetic = 0 (0‐0.35); P = 0.83, standard deviations not reported) 3. Percentage of sutures causing pain (topical LAT = 13% vs infiltrated local anaesthetic = 6%; P = 0.28) 4. Requirement of supplemental infiltrated anaesthesia (LAT = 6% vs infiltrated anaesthetic = 0%; P = not reported) |
|
| Intervention dates | Not reported | |
| Declaration of interest | Not reported | |
| Notes | Funding not reported Study author contacted for additional information but did not reply |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The doses of anaesthetic were numbered 1‐66 according to a computer generated random table of numbers prepared before the study". Comment: probably done |
| Allocation concealment (selection bias) | High risk | Quote: "physicians and patients were not blinded to the form of anaesthesia". Comment: probably not done |
| Blinding (performance bias and detection bias) All outcomes | High risk | Quote: "Because of the obvious differences in form and application, physicians and patients were not blinded to the form of anaesthesia". Comment: probably not done |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 66 participants included in study but reporting of attrition or exclusions insufficient to permit judgement |
| selective reporting of outcomes All outcomes | Low risk | All prespecified primary outcomes were reported: Participant and physician ranked pain of suturing with validated linear visual analogue scale. Prespecified secondary outcomes were also reported: Necessity for additional lidocaine and treatment success or failure were recorded at the time of the procedure. Quote: “The primary endpoints were patient and physician perception of application or injection pain and anaesthesia effectiveness…. Patients and physicians ranked the pain of injection or application and the pain of suturing using a previously validated linear visual analog scale so that each laceration had four associated measurements of pain”. Quote: “The length of the laceration, location, length of time anaesthesia lasted, amount of anaesthesia used, necessity for additional lidocaine, and treatment success or failure were recorded at the time of the procedure, along with any complications”. Table 1 lists demographics (age, sex), wound size, initial amount of anaesthesia, need for more anaesthesia and location. Table 2 reports physician and participant ratings of pain of local and topical anaesthetic application (VAS) ‐ effectiveness. Table 3 reports physician vs participant rating for pain scores of suturing (VAS). Table 4 reports percent of sutures causing pain per participant. |
| Other bias (sample size) | High risk | Quote: “66 subjects were entered in the study. Topical LAT = 33, infiltrated lidocaine = 33”. |
Gaufberg 2007.
| Methods | Single‐centre RCT, community teaching hospital emergency department, United States | |
| Participants | 100 adult patients older than 18 years of age with lacerations involving scalp (n = 15), face (n = 15), lower extremity (n = 13), upper extremity (n = 15) or hands (n = 42) Laceration length ranged from < 1 cm to > 5 cm. | |
| Interventions | 1. Topical LE solution (lidocaine 5%, epinephrine 0.025%), applied for 10 to 15 minutes for 1 to 4 sequential layered applications (n = 50) 2. Intradermal infiltration with lidocaine (n = 50) |
|
| Outcomes | 1. Participant‐rated VAS (100 mm) pain scale scores 2. Amount of lidocaine required (mg) 3. Number of applications of topical anaesthetic 4. Difficulty with wound healing or infection Results included the following. 1. Participant‐reported VAS (100 mm) pain scores (mean score ± SD: topical TLE = 0.16 ± 0.46 vs infiltrated lidocaine = 0.20 ± 0.49; P = 0.59) 2. Amount of lidocaine required (mean score: TLE = 135 mg vs infiltrated lidocaine = 124 mg; P = 0.90, SD not reported) 3. Number of anaesthetic applications of TLE (mean score = 2.7; 2 participants (4%) required 1 layer, 17 (34%) required 2 layers, 26 (52%) required 3 layers, 5 (10%) required 4 layers) 4. No participants had poor wound healing or infection. |
|
| Intervention dates | Not reported | |
| Declaration of interest | Not reported | |
| Notes | Funding not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "We performed a prospective, randomized controlled trial.." Comment: unclear; study reported to be randomized but method of sequence generation not described |
| Allocation concealment (selection bias) | High risk | Comment: probably not done. Interventions of topical anaesthesia vs infiltrated anaesthesia are visually different. No mechanism used to conceal the intervention from participants or study personnel was described. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Quote: "100 patient[s] were enrolled in a randomized controlled trial..." Comment: probably not done, as study did not report blinding and compared topical vs infiltrated forms of anaesthesia |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 100 enrolled participants in study, no missing outcome data or exclusions |
| selective reporting of outcomes All outcomes | Low risk | All prespecified primary outcomes were reported: patient‐reported VAS pain scores Prespecified secondary outcomes were also reported: amount of lidocaine required, number of applications of topical anaesthetic and difficulty with wound healing. Quote: “The effectiveness of anaesthesia was assessed by the patient immediately after the procedure using a 1‐10 visual analog pain scale administered by a third‐party. The subject was instructed to assess the pain from application or anaesthesia, and the pain from suturing the wound”. Table 2. Application of anaesthesia Table 3. Pain during application of anaesthetic Table 4. Effectiveness of anaesthesia during wound repair Table 5. Follow‐up interview after wound repair for 79 participants |
| Other bias (sample size) | Unclear risk | Infiltrated lidocaine = 50 participants Topical TLE = 50 participants |
Hegenbarth 1990.
| Methods | Two‐centre RCT, emergency departments, Uunited States | |
| Participants | 467 patients, 18 years of age or younger, with dermal lacerations on the face, scalp, extremity and trunk | |
| Interventions | 1. TAC solution (tetracaine 0.5%, epinephrine 1:2000, cocaine 11.8%),
applied for 30 minutes (n = 262) 2. Intradermal infiltration with lidocaine 1% (n = 205) |
|
| Outcomes | Pain during the suturing process was not directly assessed.
1. Before laceration repair, the physician probed the wound with a 26‐gauge needle to determine adequacy of initial anaesthesia (adequate, inadequate or unable to access). The physician administered infiltrated anaesthetic to participants in the TAC group with 'inadequate' anaesthesia.
2. Investigators reported any acute adverse reactions to the anaesthetic. Results include the following. 1. Adequate initial anaesthesia for facial and scalp lacerations (topical TAC = 81% vs infiltrated local anaesthetic = 87%; P = 0.005). Adequate initial anaesthesia for the extremity and trunk wound group (topical TAC = 43% vs infiltrated local anaesthetic = 89%; P < 0.0001) 2. No acute anaesthetic‐related adverse effects |
|
| Intervention dates | December 1986 to November 1987 | |
| Declaration of interest | Not reported | |
| Notes | Funding not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "Randomization of anaesthetic treatment was determined by the final digit of the patients medical record number, with odd numbers receiving lidocaine and even numbers receiving TAC". Comment: probably not done |
| Allocation concealment (selection bias) | High risk | Quote: "Randomization of anaesthetic treatment was determined by the final digit of the patient's medical record number". "unblinded study" Comment: probably not done |
| Blinding (performance bias and detection bias) All outcomes | High risk | Quote: "We conducted a prospective, randomized, unblinded study..." Comment: probably not done |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 467 participants included in the study but reporting of attrition or exclusions insufficient to permit judgement |
| selective reporting of outcomes All outcomes | Low risk | All outcomes described in Methods section were fully reported in Results, including subgroup analyses by area of laceration. |
| Other bias (sample size) | Low risk | 262 children received TAC (218 facial or scalp and 44 extremity or trunk wounds), and 205 received lidocaine (158 facial or scalp and 47 extremity or trunk wounds). |
Jenkins 2014.
| Methods | RCT, single‐centre, hospital emergency department, Northern Ireland | |
| Participants | 110 (54 topical anaesthetic putty, 56 lidocaine infiltration), median age (range): infiltration 35 (18‐84), topical anaesthetic putty 35 (20‐81) Male: 94 (85.5%), female: 16 (14.5%). Topical anaesthetic putty group had 10 F, 44 M; lidocaine infiltration group had 6 F, 50 M. Wounds: < 8 cm long and needing suturing or stapling |
|
| Interventions | 1. Topical anaesthetic putty (containing 4.94% w/w lidocaine hydrochloride,
equivalent to 4% w/w lidocaine base) 2. Lidocaine infiltration (1% w/v) |
|
| Outcomes |
Primary outcomes: Participant‐reported 0‐10 VAS during sensory testing with a 21‐gauge needle “directly after treatment”. Mean pain score was 0.78 + 1.12 (SD) after lidocaine infiltration, 1.49 + 1.76 after topical anaesthetic putty. Overlapping 1‐sided 95% CI limits plus (because data were not normally distributed) non‐parametric contrasting of median scores; both showed non‐inferiority of topical anaesthetic putty c/w infiltrated lidocaine Secondary outcomes: Need for rescue anaesthesia (required by 3 in infiltration group and 4 in topical anaesthetic putty group), “wound evaluation score” obtained 7‐10 days after treatment (12 in topical anaesthetic putty group had less than perfect scores vs 5 in infiltration group), presence of wound infection (4 in infiltration group vs 2 in topical anaesthetic putty group), dehiscence (2 in topical anaesthetic putty group) and adverse effects (1 inflamed wound in topical anaesthetic putty group, 1 required resuturing in each group) No anaesthetic toxicity reported |
|
| Intervention dates | Not reported | |
| Declaration of interest | The wound putty used in this study was not a proprietary product and was not produced commercially. The putty was manufactured by 2 of the study authors ‐ Drs. Murphy and McCarron. After the success of this trial, Drs. Jenkins and McCarron sought to protect certain aspects of the putty formulation in both the United States and Europe. This patent application was pending at the time of publication and was related to a certain aspect of the formulation that enables lidocaine to be included. The authors of this study received no funding from commercial sources to support the study. Funding for this study was obtained through a peer‐reviewed competitive process from the Public Health Agency in Northern Ireland. Drs. Jenkins and McCarron were pursuing sources of capital to commercialise the putty but had not yet secured this funding. |
|
| Notes | Sourse of funding: supported by the Research and Development Office (Northern Ireland) Trauma and Rehabilitation Recognised Research Group (RRG 8.46 RRG/3273/06) Rescue medication: no systemic anaesthesia or analgesia mentioned. However, “The decision to offer or use rescue anaesthesia rested with the treating investigator”. Rescue = wound margin infiltration with a further dose of 1% lidocaine for the 7 (4 in the topical anaesthetic putty group, 3 in the lidocaine infiltration group) who received it |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization sequence generated by Microsoft Excel version 14.3.9 through a permuted block randomization technique, with a block size of 8 |
| Allocation concealment (selection bias) | Low risk | Randomization sequence provided in opaque, serially numbered envelopes |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open label Quote: “Because of the nature of the treatment, it was not feasible to blind either the participants or the investigators to the treatment received". [Extractor’s note: not necessarily true, could have used placebo infiltration and placebo topical putty] |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the first, acute part of the study; 19 did not complete the follow‐up wound assessment. |
| selective reporting of outcomes All outcomes | Low risk | All outcome‐related data collected during the acute phase were complete. |
| Other bias (sample size) | Unclear risk | 54 topical anaesthetic putty 56 lidocaine infiltration |
Kendall 1996.
| Methods | Single‐centre RCT, Accident and Emergency Department of Gloucestershire Royal Hospital, United Kingdom | |
| Participants | 107 paediatric patients, 3‐16 years old, with lacerations < 4 cm in length, located anywhere on the body except mucous membranes or digits | |
| Interventions | 1. Topical AC solution (epinephrine 1:2000, cocaine 4.7%), applied for 10‐15 minutes (n = 51) 2. Intradermal infiltration with lidocaine 1% (n = 51) | |
| Outcomes | 1. Children younger than 10 years of age rated pain during both laceration repair and anaesthetic application using the Wong‐Baker Faces Scale. Patients 10 years of age or older used a VAS (10 cm) score to rate pain during suturing and anaesthetic administration.
2. Physician‐rated VAS (10 cm) pain scale scores
3. Parent‐rated VAS (10 cm) pain scale scores
4. Parent rated overall acceptability of the procedure.
5. Study reported any acute adverse effects to the anaesthetic. Results include the following. (standard deviations not reported) 1. Participant‐rated pain scores (pooled VAS and Wong‐Baker Faces scores) (mean score: topical AC = 4.50 vs infiltrated local anaesthetic = 4.40; P = NS) 2. Physician‐rated VAS (10 cm) pain scale scores (mean score: topical AC = 2.60 vs infiltrated local anaesthetic = 3.60; P = NS) 3. Parent‐rated VAS (10 cm) pain scale scores (mean score: topical AC = 3.10 vs infiltrated local anaesthetic = 3.80; P = NS) 4. Parent rating of overall acceptability of the procedure (topical AC = 14.5% unacceptable vs infiltrated local anaesthetic = 39% unacceptable; P < 0.01) 5. No acute anaesthetic‐related adverse effects |
|
| Intervention dates | January to November 1994 | |
| Declaration of interest | No explicit documentation regarding conflicts of interest | |
| Notes | No sources of funding mentioned | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "Children presenting with an appropriate laceration were consecutively assigned to receive either conventional intradermal lignocaine or topical AC preparation". Comment: probably not done |
| Allocation concealment (selection bias) | High risk | Quote: "consecutively assigned to receive either conventional intradermal lignocaine or topical AC preparation" "Groups could not be blinded". Comment: probably not done |
| Blinding (performance bias and detection bias) All outcomes | High risk | Quote: "The nature of the trial meant that the two groups could not be blinded". Comment: probably not done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 120 participants were enrolled but 13 were excluded before data analysis (incomplete data collection for 8, 2 received Steristrips and not sutures, 3 did not attend follow‐up). We concluded low risk of bias because reasons for exclusion were unlikely to be related to pain scores during laceration repair. |
| selective reporting of outcomes All outcomes | Low risk | The study protocol is available, and all of the study’s prespecified outcomes have been reported in the prespecified way. |
| Other bias (sample size) | Unclear risk | 1. Topical AC solution, n = 56 2. Intradermal infiltration with lidocaine, n = 51 |
Krief 2002.
| Methods | RCT (unclear if single centre or multi‐centre) | |
| Participants | 41 adult and paediatric patients, 5 to 23 years of age, with simple lacerations < 5 cm in length | |
| Interventions | 1. Topical LET gel (lidocaine, epinephrine, tetracaine), applied for 60 minutes (n = 22) 2. EMLA cream (lidocaine 2.5%, prilocaine 2.5%), applied for 60 minutes (n = 19) |
|
| Outcomes | 1. Participant‐rated VAS (100 mm) pain scale scores
2. Legal guardian‐rated VAS (100 mm) pain scale scores (when applicable) 3. Physician‐rated VAS (100 mm) pain scale scores 4. Requirement of supplemental lidocaine infiltration Pain scores were obtained at 4 points in time: after irrigation, first suture or staple placement, last suture or staple placement and during supplemental infiltration of lidocaine (if applicable). Results include the following. 1. Participant self‐reported VAS (100 mm) pain scores were not significantly different between the 2 anaesthetic groups (mean pain scores not provided; P > 0.05). 2. Legal guardian‐reported VAS (100 mm) pain scores were not significantly different between the 2 groups (mean pain scores not provided; P > 0.05). 3. Physician‐reported VAS (100 mm) pain scores were greater in the EMLA group during irrigation (mean VAS EMLA = 21.4 mm vs LET gel = 10.1 mm; P = 0.3) and during first suture/staple placement (mean VAS EMLA = 41.7 mm vs LET gel = 14.0 mm; P = 0.004). 4. Requirement of supplemental infiltrated anaesthesia: 13/19 participants in the EMLA group required infiltrated lidocaine (68%) compared with 5/22 in the LET group (23%) (P = 0.005%) |
|
| Intervention dates | Not reported | |
| Declaration of interest | Not reported | |
| Notes | Trial published as an abstract only. Source of funding not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "We conducted a double‐blind, randomized trial...". Comment: unclear, as method of sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "We conducted a double‐blind, randomized trial..." Comment: unclear, as reported to be double‐blind but no details provided |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 41 participants included in the study but reporting of attrition or exclusions insufficient to permit judgement |
| selective reporting of outcomes All outcomes | Low risk | The study protocol is available, and all of the study’s prespecified outcomes have been reported. |
| Other bias (sample size) | High risk | 41 participants: 1. Topical LET gel, n = 22 2. EMLA cream, n = 19 |
Kuhn 1996.
| Methods | Single‐centre (2 hospitals) RCT, emergency departments of 2 tertiary referral hospitals (1 paediatric), Adelaide South Australia | |
| Participants | 180 adult and paediatric patients, 6 years of age or older, with lacerations 3‐7 cm in length, located on the head (n = 114) or extremity (n = 66) | |
| Interventions | 1. Topical MAC solution (bupivacaine 0.5%, epinephrine 1:2000, cocaine 10.0%), applied for at least 10 to 15 minutes for head lacerations and for 30 minutes for extremity wounds (n = 92) 2. Topical TAC solution (tetracaine 0.5%, epinephrine 1:2000, cocaine 10.0%), applied for at least 10 to 15 minutes for head lacerations and for 30 minutes for extremity wounds (n = 88) | |
| Outcomes | 1. Children younger than 12 years of age rated pain during laceration repair using the Wong‐Baker Faces scale.
2. Participants 12 years of age or older used a VAS (10 cm) score to rate pain during suturing.
3. The physician assessed the effectiveness of initial anaesthesia using pinprick.
4. Participants noted their preference for topical anaesthesia in the future.
5. Investigators reported any acute adverse effects to the anaesthetic. Results include the following. 1. Children younger than 12 years of age used the Wong‐Baker Faces Scale (1‐9) (mean score ± SD: topical MAC = 2.35 ± .50 vs topical TAC = 2.46 ± 2.34; P = 0.96). 2. Participants 12 years of age or older used the VAS (100 mm) pain scale (mean score ± SD: topical MAC = 6.9 ± 10.9 vs topical TAC = 12.0 ± 14.5; P = 0.16). 3. Adequacy of initial anaesthesia (topical MAC = 73% vs topical TAC = 74%; P = 0.87) 4. Participants' preference for topical anaesthesia in the future (topical MAC = 77% vs topical TAC = 81%; P = 0.42) 5. No acute anaesthetic‐related adverse effects |
|
| Intervention dates | Feburary 1992 to April 1994 | |
| Declaration of interest | No explicit documentation regarding conflicts of interest | |
| Notes | Source of funding: grant from Society of Hospital Pharmacists of Australia | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The study was a double‐blinded, randomized, prospective trial.." Comment: unclear, as study reported to be randomized but method of sequence generation was not described |
| Allocation concealment (selection bias) | Low risk | Quote: "Solutions of MAC and modified TAC were prepared and placed in syringes marked A or B by a pharmacist not involved in study. All study participants remained blinded throughout the trial". Comment: probably done, assuming solutions were visually identical |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Solutions of MAC and modified TAC were prepared and placed in syringes marked A or B by a pharmacist not involved in study. All study participants remained blinded throughout the trial". Comment: probably done, assuming solutions were visually identical |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 191 participants were enrolled but 10 were excluded before data analysis (5 younger than 6 years of age, 2 had wounds greater than 5 mm deep, 2 were not sutured, 1 had a digital laceration). We concluded low risk of bias because reasons for exclusion were unlikely to be related to pain scores during laceration repair. |
| selective reporting of outcomes All outcomes | Unclear risk | The study protocol did not describe prespecified outcomes. |
| Other bias (sample size) | Unclear risk | 180 participants: 1. Topical MAC solution, n = 92 2. Topical TAC solution, n = 88 |
Lee 2013.
| Methods | Single‐centre RCT, Department of Emergency Medicine, Singapore General Hospital | |
| Participants | n = 40, > 1 year to 70 years (only 1 patient > 10 years old was included in the study), 29 males (72.5%), 11 females (27.5%). Length of the wounds was 3.1 cm for the LG group and 3.5 cm for the LI group. Depth of the wounds was 0.5 cm and 0.57 cm, respectively. | |
| Interventions | 1. LAT gel (n = 23): mean length of wound/cm (SE) 3.1 cm (SE 0.31). Mean depth of wound/cm (SE) 0.5 (0.07). Location of wound: head 17/23 (74.0%), trunk 0/23 (0%) and limb 6/23 (26%) 2. Infiltrated lidocaine (n = 17): mean length of wound/cm (SE) 3.5 cm (SE 0.36). Mean depth of wound/cm (SE) 0.57(0.08). Location of wound: head 11/17 (64.7%), trunk 0/17 (0%) and limb 6/17 (35.3%) |
|
| Outcomes | 1. LAT gel: a. Efficacy: 10 cm VAS pain score by participant (mean ± SE) = 2.5 (0.52) b. Pain during application (mean ± SE): 1.5 (0.40) Pain score by parents, clinician or participants younger than 10 years old; results not provided 2. Lignocaine infiltration: a. Efficacy: 10 cm VAS pain score by participant (mean ± SE) = 2.6 (0.58) b. Pain during application (mean ± SE): 3.5 (0.46) Pain score by parents, clinician or participants younger than 10 years old; results not provided Complications: 1. No acute anaesthetic complications in either group 2. One week later, assessed for wound complications 1. LAT gel (study lists 25 but probably typographical error because only 23 participants in this treatment arm) a. Wound Infection, 5/25 (5/23?) b. Wound dehiscence = 1/25 (1/23?) c. Stitches lost = 1/25 (1/23?) 2. Lignocaine infiltration a. Wound Infection, 2/14 b. Wound dehiscence, 0/14 c. Stitches lost, 0/14 |
|
| Intervention dates | Janurary to April 2003 | |
| Declaration of interest | None. | |
| Notes | Souce of funding: none | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Suitable participants were assigned to 2 arms of treatment via sealed envelopes. However, precise method of sequence generation was not described. |
| Allocation concealment (selection bias) | Unclear risk | Use of assigned envelopes described but information proved insufficient to allow a decision between low risk and high risk |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not blinded and outcome could be affected by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 40 patients recruited and no drop‐outs mentioned |
| selective reporting of outcomes All outcomes | Low risk | All prespecified primary outcomes were reported. |
| Other bias (sample size) | High risk | LAT gel = 23 participants Infiltrated lidocaine = 17 participants |
Pryor 1980.
| Methods | Single‐centre RCT, Army Medical Center emergency department, United States | |
| Participants | 158 adult and paediatric patients, range 10 months to 53 years old (mean = 9 years old) | |
| Interventions | 1. Topical TAC solution (tetracaine 0.5%, epinephrine 1:2000, cocaine 11.8%), applied for minimum of 10 minutes (n = 82) 2. Intradermal infiltration with lidocaine (n = 76) | |
| Outcomes | 1. Participants 10 years of age or older rated anaesthetic efficacy (complete, partial or none) depending on whether they experienced pain during laceration repair.
2. Also, after completion of wound repair, participant or parent rated anaesthetic acceptability (excellent, good or poor). Results include the following. 1. Verbal rating (complete, partial or none) of anaesthetic efficacy (complete: topical TAC = 84% vs infiltrated local anaesthetic = 88%; P = not reported) 2. Anaesthetic acceptability: Participants 17 years of age and younger preferred topical TAC (P < 0.005); no difference between the 2 anaesthetic groups among participants older than 17 years of age. |
|
| Intervention dates | October to December 1979 | |
| Declaration of interest | Not reported | |
| Notes | Funding not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "A prospective study of topical TAC and lidocaine infiltration was taken with the last digit of the patients military sponsor's social security number used as the selection variable, odd numbered patients were anaesthetised with topical TAC; even numbered patients were anaesthetised with lidocaine". Comment: probably not done |
| Allocation concealment (selection bias) | High risk | Quote: "the last digit of the patient's military sponsor's social security number used as the selection variable" Comment: probably not done. Anaesthetic agents visually different, and no mention of safeguards to prevent concealment of identity |
| Blinding (performance bias and detection bias) All outcomes | High risk | Quote: none Comment: probably not blinded, as the paper did not state whether participants or clinicians were blinded between topical and infiltrated anaesthetics |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A total of 158 participants enrolled with no drop‐outs or exclusions. |
| selective reporting of outcomes All outcomes | High risk | All outcomes described in Methods section were reported in Results, but method of assessing anaesthetic adequacy appears inconsistent between Methods and Results sections. Subgroup analysis by age was described in Methods, but results were not presented for all subgroups for each outcome. Wound complications were measured at 3 time points, but results were presented only for overall rate. No adverse events due to anaesthetic administration were reported. Some results are presented only graphically. |
| Other bias (sample size) | Unclear risk | 82 received topical TAC and 76 received lidocaine infiltration for anaesthesia. |
Resch 1998.
| Methods | Single‐centre RCT, emergency department, University of Minnesota‐affiliated Children’s Hospital, Minneapolis, Minnesota, United States | |
| Participants | 194 paediatric patients with lacerations of the face and scalp | |
| Interventions | 1. Topical LAT solution (lidocaine 4%, epinephrine 1:2000, tetracaine 0.5%), applied for 20 minutes (n = 103) 2. Topical LAT gel (lidocaine 4%, epinephrine 1:2000, tetracaine 0.5%), applied for 20 minutes (n = 91) | |
| Outcomes | 1. The physician assessed the adequacy of initial anaesthesia by probing the wound with a 27‐gauge needle. If any pain was elicited with probing, the anaesthetic was considered 'inadequate' and infiltrated lidocaine was given.
2. At the conclusion of laceration repair, the physician rated anaesthetic effectiveness (complete, partial or incomplete) based on painful responses during suturing.
3. The study reported acute adverse reactions directly related to the anaesthetic. Results include the following. 1. Adequacy of initial anaesthesia (adequate anaesthesia: LET solution = 84% vs LET gel = 82%; P > 0.05) 2. Effectiveness of anaesthesia (complete anaesthesia: LET solution = 76% vs LET gel = 85%; P = 0.007) 3. No acute anaesthetic‐related adverse effects |
|
| Intervention dates | March 1995 to March 1996 | |
| Declaration of interest | Not reported | |
| Notes | Funding not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A computer‐generated random number table was used by a hospital pharmacy personnel to label standard amber vials from 1 to 200". Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote: :hospital pharmacy personnel to label standard amber vials from 1 to 200" "it was required that the study medication be applied by a nurse not involved in the suturing" Comment: probably done |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "To ensure blinding of suture personnel, in the trial, it was required that the study medication be applied by a nurse not involved in the suturing" Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 200 participants enrolled and 3 withdrawn before test of initial anaesthesia because participants were unco‐operative or complicated laceration did not meet inclusion criteria. Of the 197 available for analysis, 3 data sheets were inadvertently lost. We concluded low risk of bias because plausible effect size among missing outcomes was not enough to have a clinically relevant impact on observed effect size. |
| selective reporting of outcomes All outcomes | Unclear risk | All prespecified primary and secondary outcomes were reported: physician determination of adequacy of anaesthetic before repair and anaesthetic effectiveness during repair. Adverse effects also reported Quote: “Pain assessment was a 2‐stage process that evaluated adequacy of anaesthesia before suturing and effectiveness of anaesthesia during suturing”. “Effectiveness of anaesthesia during suturing was divided into 3 categories: complete, partial, and incomplete”. “Complications assessed were redness, drainage, fever, tenderness, swelling, or contact with medical personnel for wound‐related issues other than suture removal”. Quote: “Of the 194 patients, 162 (83.5%) obtained adequate anaesthesia as determined by the 27‐gague needle test”. Table 3. Efficacy of LET solution versus LET gel for topical anaesthesia of face and scalp (includes information on complete, partial and Incomplete effectiveness) Complications: “No adverse effects were noted in the 194 patients during the procedure. 13 patients who were not able to be contacted… one patient in each study arm sought medical care for a wound infection". |
| Other bias (sample size) | Unclear risk | Quote: “LET solution = 103 subjects, LET gel = 91 subjects” |
Schaffer 1985.
| Methods | Single‐centre RCT, Spokane Minor Emergency Centers, Spokane, Washington, United States | |
| Participants | 107 paediatric patients 10 years of age or younger, with laceration on the face (n = 84) or scalp (n = 23) | |
| Interventions | 1. Topical TAC solution (tetracaine 0.5%, epinephrine 1:2000, cocaine 11.8%), applied for 10 minutes (n = 56) 2. Topical TA solution (tetracaine 0.5%, epinephrine 1:2000), applied for 10 minutes (n = 51) | |
| Outcomes | 1. The physician rated anaesthetic effectiveness (complete, partial or inadequate) according to the ability of the participant to tolerate manipulation of the wound during repair. The anaesthesia was 'complete' if the participant did not cry, complain or wince during suturing. The anaesthesia was 'partial' if the participant had some discomfort but did have an avoidance reaction. 'Inadequate' anaesthesia was defined as obvious discomfort with minimal manipulation of the wound.
2. Rescue lidocaine infiltration was required.
3. The study reported any acute adverse reactions to the anaesthetic. Results include the following. 1. Physician rating (complete, partial or inadequate) of anaesthetic effectiveness (complete anaesthesia: topical TA = 47.1% vs topical TAC = 75%; P < 0.05) 2. Requirement of rescue lidocaine infiltration (topical TA = 27.5% vs topical TAC = 8.9%; P = 0.01) 3. No acute anaesthetic‐related adverse effects. However, after returning home from the emergency department, 10.7% of children treated with TAC and 7.8% who received topical AC became drowsy or excitable. No evidence suggested that symptoms were causally related to the topical anaesthetic, and the study author concluded that these were not anaesthetic‐induced adverse effects. |
|
| Intervention dates | January to July 1983 | |
| Declaration of interest | Not reported | |
| Notes | Funding not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "Patients who received topical anaesthesia were randomized by alternating between A and B solutions". Comment: probably not done |
| Allocation concealment (selection bias) | High risk | Quote: "...randomized by alternating between A and B solutions" Comment: probably not done |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Neither patients nor treating physicians were informed of the composition of the anaesthetic solutions". Comment: probably done, assuming topical TAC and TA were visually identical |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 107 participants included in study but reporting of attrition or exclusions insufficient to permit judgement |
| selective reporting of outcomes All outcomes | Low risk | All prespecified primary outcomes were reported: Treating physician rated anaesthetic effectiveness on the basis of participant tolerance of manipulation of wound during suturing (complete, partial, inadequate). The only prespecified secondary outcome was wound infection, which was reported. Quote: “The relative effectiveness of anaesthesia was assessed subjectively by treating physician based on ability of patient to tolerate manipulation of would during repair”. Table 1. Anesthesia effectiveness (treatment) Table 2. Wound location (initial examination) Table 3. Signs of wound infection (follow‐up visits) |
| Other bias (sample size) | Unclear risk | Quote: “Topical TAC = 56 patients, topical TA = 51 patients” |
Schilling 1995.
| Methods | Single‐centre RCT, emergency department of a university‐affiliated private children's hospital, United States | |
| Participants | 151 patients, age 1 to 17 years, with facial (69.6%) and scalp (30.4%) lacerations | |
| Interventions | 1. Topical TAC solution (tetracaine 0.5%, epinephrine 1:2000, cocaine 11.8%), applied for 15 minutes (n = 73) 2. Topical LET solution (lidocaine 4%, epinephrine 0.1%, tetracaine 0.5%), applied for 15 minutes (n = 78) | |
| Outcomes | 1. The physician assessed the adequacy of initial anaesthesia by probing the wound with a 27‐gauge needle.
2. After laceration repair, the physician rated anaesthetic effectiveness (complete, partial or incomplete). Anaesthesia was 'complete' if the participant did not have a painful response to suturing. Anaesthesia was 'partial' if the participant had a painful response to suturing, between 15 and 30 minutes after removal of topical solution. Anaesthesia was considered 'incomplete' if the participant had a painful response within 15 minutes after removal of the topical agent.
3. Investigators reported any acute adverse reactions directly related to the anaesthetic. Results include the following. 1. Adequacy of initial anaesthesia (topical LET = 74.4% vs topical TAC = 79.5%; P = 0.46) 2. Physician‐rated anaesthetic effectiveness (complete, partial, incomplete) (complete anaesthesia: topical LAT = 82.4% vs topical TAC = 75.9%; P = 0.18) 3. No acute anaesthetic‐related adverse effects |
|
| Intervention dates | June 1992 to May 1993 | |
| Declaration of interest | Not reported | |
| Notes | Source of funding: financial support provided by the FA Bean Education and Research Fund, Minneapolis Children's Medical Center | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "...vials of the anaesthetic solutions were assigned random numbers.." Comment: unclear, as study was reported to be randomized, but method of sequence generation was not described |
| Allocation concealment (selection bias) | Low risk | Quote: "Both TAC and LET solutions are aqueous and have the same blue tint and viscosity". "labelled to ensure appropriate blindness of suture personnel" "A double blind topical application using 3ml of the test solutions was performed [at] study entry". Comment: probably done |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Both TAC and LET solutions are aqueous and have the same blue tint and viscosity. Unit‐dose, amber vials of the anaesthetic solutions were assigned random numbers; labelled to ensure appropriate blindness of suture personnel; and stored under refrigeration in the ED. A double blind topical application using 3ml of the test solutions was performed [at] study entry". Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 171 participants were initially enrolled, but data analysis was performed for only 151 participants. Five participants were excluded after consent was obtained (1 sedated before anaesthetic administration, 2 topical anaesthetics applied for inappropriate duration, 2 data sheets lost). 15 additional participants were withdrawn before evaluation of anaesthetic effectiveness because participants were unco‐operative or because it was discovered that the wound involved deeper tissue layers than inclusion criteria permitted. We concluded low risk of bias because reasons for exclusion were unlikely to be related to pain scores during laceration repair |
| selective reporting of outcomes All outcomes | Unclear risk | All outcomes described in Methods were fully reported in Results section, but subgroup analyses (area of face, age of participant) were not prespecified. Adverse events were reported |
| Other bias (sample size) | Unclear risk | 73 participants were treated with TAC; 78 participants received LET |
Smith 1996.
| Methods | Single‐centre RCT, emergency department, Children’s Hospital, Columbus, Ohio, United States | |
| Participants | 240 patients, 2 to 17 years old, with lacerations ≤ 5 cm located on the face (n = 134), scalp (n = 57) or extremity (n = 49) | |
| Interventions | 1. Bupivanor (BN) solution (0.48% bupivacaine with 1:26,000 norepinephrine), applied for 20 minutes (n = 30) 2. Etidonor (EN) solution (0.95% etidocaine with 1:26,000 norepinephrine), applied for 20 minutes (n = 30) 3. Mepivanor (MN) solution (1.90% mepivacaine with 1:26,000 norepinephrine), applied for 20 minutes (n = 30) 4. Prilonor (PN) solution (3.81% prilocaine with 1:26,000 norepinephrine), applied for 20 minutes (n = 30) 5. TAC solution (tetracaine 1.00%, epinephrine 1:4000, cocaine 4.0%), applied for 20 minutes (n = 60) 6. Infiltrated lidocaine 1% (n = 60) | |
| Outcomes | 1. Participants 5 years of age or older, with reported discomfort on the VAS (100 mm) pain scale
2. Observer‐reported VAS (100 mm) pain scale scores (suture technicians and research assistants)
3. Observer‐reported Likert (1‐7) pain scale scores (parents and suture technicians).
4. Observer‐rated (RICDRS) Restrained Infants and Children Disress Rating Scale (0‐8) (research assistant and suture technician)
5. Suture technician‐rated anaesthetic effectiveness scale Results (topical BN vs topical EN vs topical MN vs topical PN vs topical TAC vs infiltrated local anaesthetic) include the following. (standard deviations not reported for any outcomes) 1. Participant‐reported VAS (100 mm) pain scores (mean scores: topical BN = 18.3 vs topical EN = 46.5 vs topical MN = 27.0 vs topical PN = 36.0 vs topical TAC = 12.0 vs infiltrated local anaesthetic = 26.3) (TAC significantly outperformed EN; P < 0.05; no significant differences between any other groups) 2a. Suture technician‐reported VAS (100 mm) pain scores (mean scores: topical BN = 2.0 vs topical EN =6.3 vs topical MN = 4.8 vs topical PN = 6.2 vs topical TAC = 2.8 vs infiltrated local anaesthetic = 2.0 (EN significantly outperformed by BN, TAC and infiltrated anaesthetic; P < 0.05; no significant differences between any other groups) 2b. Research assistant‐reported VAS (100 mm) pain scores (mean scores: topical BN =3.3 vs topical EN =7.7 vs topical MN = 4.9 vs topical PN = 8.9 vs topical TAC = 2.9 vs infiltrated local anaesthetic = 1.9) (TAC outperformed both EN and PN; P < 0.05; infiltrated anaesthetic outperformed both EN and PN; P < 0.05; no significant differences between any other groups) 3a. Suture technician‐reported Likert (1‐7) pain scores (mean scores: topical BN = 2.05 vs topical EN = 2.6 vs topical MN = 2.4 vs topical PN = 2.1 vs topical TAC = 1.55 vs infiltrated local anaesthetic = 1.6 (TAC outperformed both EN and MN; P < 0.05; infiltrated anaesthetic outperformed both EN and MN; P < 0.05; no significant differences between any other groups) 3b. Parent‐reported Likert (1‐7) pain scores (mean scores: topical BN = 2.8 vs topical EN = 3.5 vs topical MN = 3.3 vs topical PN = 3.6 vs topical TAC = 2.11 vs infiltrated local anaesthetic = 2.33 (TAC outperformed EN, MN and PN; P < 0.05; infiltrated anaesthetic outperformed EN and PN; P < 0.05; no significant differences between any other groups) 4a. Suture technician‐reported RICDRS (0‐8) (mean scores: topical BN = 2.5 vs topical EN = 3.6 vs topical MN = 2.3 vs topical PN = 2.5 vs topical TAC = 1.4 vs infiltrated local anaesthetic = 1.63 (TAC outperformed EN; P < 0.05; infiltrated anaesthetic outperformed EN; P < 0.05; no significant differences between any other groups) 4b. Research assistant‐reported RICDRS (0‐8) (mean scores: topical BN = 2.4 vs topical EN = 3.1 vs topical MN = 2.7 vs topical PN = 2.9 vs topical TAC = 1.6 vs infiltrated local anaesthetic = 1.8 (TAC outperformed both EN and PN; P < 0.05; infiltrated anaesthetic outperformed EN; P < 0.05; no significant differences between any other groups) 5. Anaesthetic effectiveness scale (scores not reported) (TAC outperformed EN and MN; P < 0.05; infiltrated anaesthetic outperformed BN, EN, MN, PN; P < 0.05; no significant differences between any other groups) |
|
| Intervention dates | July to December 1992 | |
| Declaration of interest | No explicit documentation regarding conflicts of interest | |
| Notes | Source of funding: Ohio State University Seed Grant Program, Bremer Research Foundation, Ohio State University and Samuel J. Roessler Memorial Scholarship Fund Study author contacted to request additional study data; study author replied but unable to provide the missing information. High risk of bias for local anaesthetic vs topical anaesthetic, as this comparison was not blinded. However,unclear risk of bias in 3 domains for comparisons of different topical anaesthetics because of appropriate blinding |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Study patients were assigned to one of six anaesthetic treatment groups using block randomization". Comment: unclear, as exact method of selecting the blocks was not reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: unclear |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Comparisons among the five topical preparations were double blinded. Because lidocaine was given as an injection, its identity was not blinded"; "Anesthetics were prepared in advance by Children's Hospital pharmacy, sealed in envelopes labelled with a study identification number, and stored in a locked cabinet in the emergency department". Comment: probably blinded between comparisons of different topical agents, but probably not blinded between comparisons of infiltrated lidocaine and topical anaesthetic |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 240 participants included in the study but reporting of attrition or exclusions insufficient to permit judgement |
| selective reporting of outcomes All outcomes | Low risk | The study protocol is available, and all of the study’s prespecified outcomes have been reported in the prespecified way. |
| Other bias (sample size) | Unclear risk | 240 participants enrolled: 1. Bupivanor (BN) solution, n = 30 2. Etidonor (EN) solution, n = 30 3. Mepivanor (MN), n = 30 4. Prilonor (PN) solution, n = 30 5. TAC solution, n = 60 6. Infiltrated lidocaine, n = 60 |
Smith 1997a.
| Methods | Single‐centre RCT, emergency department or a large children’s hospital, United States | |
| Participants | 71 patients, 2‐16 years old, with lacerations ≤ 5 cm in length located on the face (n = 43) or scalp (n = 28) | |
| Interventions | 1. Mepivanor (MN) solution (mepivacaine 2%, norepinephrine 1:100,000), applied for 20 minutes (n = 24) 2. TAC solution (tetracaine 1.0%, epinephrine 1:4000, cocaine 4.0%), applied for 20 minutes (n = 24) 3. Intradermal infiltration with lidocaine 1% (n = 23) | |
| Outcomes | 1. Observer‐reported VAS (100 mm) pain scale scores (suture technicians, research assistants and videotape reviewers)
2. Observer‐reported Lickert (1‐7) pain scale scores (parents, suture technicians)
3. Requirement for supplemental lidocaine infiltration Results (topical MN vs topical TAC vs infiltrated local anaesthetic) include the following. 1a. Suture technician‐reported VAS (100 mm) pain scores (mean score ± SD: topical MN = 7.1 ± 12.5 vs topical TAC = 2.0 ± 2.7 vs infiltrated anaesthetic = 1.8 ± 4.0) (Both topical TAC and infiltrated anaesthetic outperformed topical MN; P = 0.003.) 1b. Research assistant‐reported VAS (100 mm) pain scores (mean score ± SD: topical MN = 14.8 ± 19.5 vs topical TAC = 4.7 ± 8.5 vs infiltrated anaesthetic = 3.0 ± 4.0). (Both topical TAC and infiltrated anaesthetic outperformed topical MN; P = 0.0003.) 1c. Videotape reviewer‐reported VAS (100 mm) pain scores (mean score ± SD: topical MN = 5.0 ± 12.5 vs topical TAC = 5.25 ± 16.42 vs infiltrated anaesthetic = 2.0 ± 5.9) (no reported differences between groups; P > 0.05) 2a. Suture technician‐reported Likert (1‐7) pain scores (mean score ± SD: topical MN = 2.2 ± 1.4 vs topical TAC = 1.7 ± 0.9 vs infiltrated anaesthetic = 1.6 ± 1.0) (no reported differences between groups; P = 0.18) 2b. Parent‐reported Likert (1‐7) pain scores (mean score ± SD: topical MN = 3.7 ± 1.9 vs topical TAC = 2.4 ± 1.8 vs infiltrated anaesthetic = 2.4 ± 1.6) (Both topical TAC and infiltrated anaesthetic outperformed topical MN; P = 0.02.) 3. Requirement for supplemental lidocaine infiltration (topical MN = 37.5% vs topical TAC = 8.3%; P = 0.04) |
|
| Intervention dates | Not reported | |
| Declaration of interest | Not reported | |
| Notes | Source of funding: Support was provided by a grant from the Children’s Hopsital Research Foundation, Columbus, Ohio (Grant #020‐876). Obtained additional study data by directly contacting study author |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Enrolled patients were assigned to receive one of three anaesthetic preparations by block randomization". Comment: unclear, as exact method of selecting the blocks not described in the study |
| Allocation concealment (selection bias) | Unclear risk | Comment: unclear |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Comparions between topical Mepivanor and TAC were blinded to all observers. Since lidocaine was given as an injection, its identity was not blinded to those present for the procedure. However, after the anaesthetic was administered, suturing procedures were videotaped. These videotapes were later reviewed by an observer who was completely blinded to which local anaesthetic the patient had received". Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 71 participants included in the study but reporting of attrition or exclusions insufficient to permit judgement |
| selective reporting of outcomes All outcomes | Low risk | All prespecified primary outcomes were reported: observer‐reported VAS pain score by suture technicians, research assistants ascertained at the end of the suturing procedure. Also, Lickert pain scale scores (participant, suture technician) Prespecified secondary outcomes were also reported: pain during application of anaesthesia and requirement for supplemental lidocaine infiltration. Quote: “Pain perceptions of suture technicians, research assistants were ascertained at the end of the suturing procedure by means of the visual analogue scale (VAS)… Pain perceptions of the parents and suture technicians were also measured using a seven‐point Likert scale…Observers were instructed to base their pain scores on the pain experienced as the needle pierced the skin in order to measure actual anaesthetic performance”. Figure 1. Mean VAS pain score by anaesthetic treatment group for suture technicians compared with research assistants compared with videotape reviewer. Figure 2. Mean Likert scale to rate the amount of pain they thought the child experienced during suturing by each anaesthetic treatment group for suture technicians compared with parents for all laceration types of repair. Additional reporting: “Suture technicians were instructed to give additional lidocaine by infiltration.. if they felt that the child had inadequate wound anaesthesia. Two patients received lidocaine rescue in the TAC group compared to 9 patients in the Mepivanor group”. “..Sixty six patients returned within 48 hours for a wound check. All wounds were healing without complication at that time, except for one patient…. There was one additional complication reported at the 2‐week follow up for a patient”. |
| Other bias (sample size) | High risk | Quote: “Seventy‐one patients were enrolled in the study. 23 received lidocaine, 24 received TAC, 24 were given Mepivanor”. |
Smith 1997b.
| Methods | Single‐centre RCT, emergency department, Children’s Hospital, Columbus, Ohio, United States | |
| Participants | 240 patients, 1 to 18 years of age, with lacerations ≤ 5 cm in length, located on the face (51%), scalp (30%), extremity (18%) or other site (1%) | |
| Interventions | 1. Prilophen (PP) solution (prilocaine 3.56%, phenylephrine 0.99%), applied for 20 minutes (n = 60) 2. Tetraphen (TP) solution (tetracaine 1.0%, phenylephrine 5.0%), applied for 20 minutes (n = 60) 3. Tetralidophen (TLP) solution (tetracaine 1.0%, lidocaine 1.0%, phenylephrine 2.5%), applied for 20 minutes (n = 60) 4. TAC solution (tetracaine 1.0%, epinephrine 1:4000, cocaine 4.0%), applied for 20 minutes (n = 60) | |
| Outcomes | 1. Participants 5 years of age or older reported VAS (100 mm) pain scale scores.
2. Observer‐reported VAS (100 mm) pain scale scores (suture technicians, research assistants and parents)
3. Observer‐reported Likert (1‐7) pain scale scores (suture technicians, research assistants and parents)
4. Suture technicians rated anaesthetic effectiveness (complete, partial or no anaesthesia) Results (topical PP vs topical TP vs topical TLP vs topical TAC) include the following. 1. Participant self‐reported VAS (100 mm) pain scores (mean score ± SD: topical PP = 29.0 ± 43.4 vs topical TP = 24.2 ± 37.2 vs topical TLP = 30.6 ± 40.3 vs topical TAC = 17.6 ± 34.1) (no reported differences between groups; P = 0.5) 2a. Suture technician‐rated VAS (100 mm) pain scores (mean score ± SD: topical PP = 7.4 ± 16.0 vs topical TP = 5.1 ± 12.6 vs topical TLP = 6.0 ± 13.5 vs topical TAC = 3.5 ± 11.8) (Topical TAC performed significantly better then topical PP; reported P = 0.04.) 2b. Research assistant‐rated VAS (100 mm) pain scores (mean score ± SD: topical PP = 1.6 ± 2.6 vs topical TP = 1.9 ± 4.2 vs topical TLP = 1.3 ± 1.7 vs topical TAC = 0.9 ± 1.7) (no reported differences between groups; P = 0.09) 2c. Parent‐rated VAS (100 mm) pain scores (mean score ± SD: topical PP = 20.0 ± 21.7 vs topical TP = 20.2 ± 21.7 vs topical TLP = 18.2 ± 18.6 vs topical TAC = 14.0 ± 18.6) (no reported differences between groups; P = 0.09) 3a. Suture technician‐reported Likert (1‐7) pain scores (median score: topical PP = 2.0 vs topical TP = 1.0 vs topical TLP = 2.0 vs topical TAC = 1.0) (Topical TAC performed significantly better than topical PP or topical TLP; P = 0.01.) 3b. Research assistant‐reported Likert (1‐7) pain scores (median score: topical PP = 2.0 vs topical TP = 1.0 vs topical TLP = 2.0 vs topical TAC = 1.0) (Topical TAC performed significantly better than topical PP or topical TLP; P = 0.03.) 3c. Parent‐reported Likert (1‐7) pain scores (median score: topical PP = 2.0 vs topical TP = 2.0 vs topical TLP = 2.0 vs topical TAC = 2.0) (mo reported differences between any of the groups; P = 0.06) 4. Anaesthetic effectiveness (complete anaesthesia: topical PP = 63% vs topical TP = 67% vs topical TLP = 65% vs topical TAC = 80%) (mo reported differences between any of the groups; P =.18) |
|
| Intervention dates | June to September 1994 | |
| Declaration of interest | No explicit documentation regarding conflicts of interest | |
| Notes | Source of funding: Grant 020‐898 from Children’s Hospital Research Foundation and Samuel J. Roessler Memorial Scholarship Fund Study author contacted to request additional study data; study author replied but unable to provide missing information |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly assigned to one of four anaesthetic treatment groups.." Comment: unclear, as study was reported to be randomized but method of sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "using a prospective, randomized, double‐blind design..." "Anesthetic agents were sealed in envelopes labelled with a study identification number and stored in a locked cabinet in the emergency department". Comment: probably done, assuming topical solutions visually identical |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 240 participants included in the study but reporting of attrition or exclusions insufficient to permit judgement |
| selective reporting of outcomes All outcomes | Low risk | The study protocol is available, and all of the study’s prespecified outcomes have been reported in the prespecified way. |
| Other bias (sample size) | Unclear risk | 240 children enrolled: 1. Prilophen (PP) solution, n = 60 2. Tetraphen (TP) solution, n = 60 3. Tetralidophen (TLP) solution, n = 60 4. TAC solution, n = 60 |
Smith 1998a.
| Methods | Single‐centre RCT, emergency department or a large children’s hospital, United States | |
| Participants | 180 patients, 1 to 18 years old, with lacerations ≤ 5 cm, located on the face (n = 76), scalp (n = 59), extremity (n = 43) or other (n = 2) | |
| Interventions | 1. Prilophen (PP) solution (3.56% prilocaine, 0.10% phenylephrine), applied for 20 minutes (n = 60) 2. Bupivaphen (BP) solution (0.67% bupivacaine, 0.10% phenylephrine), applied for 20 minutes (n = 60) 3. TAC solution (tetracaine 1.0%, epinephrine 1:4000, cocaine 4.0%), applied for 20 minutes (n = 60) | |
| Outcomes | 1. Participants 5 years of age and older self‐reported pain using a VAS (100 mm) scale.
2. Observer‐reported VAS (100 mm) pain scale scores (suture technicians, research assistants and parents) Results (topical PP vs topical BP vs topical TAC) included the following. 1. Participant self‐reported VAS (100 mm) pain scores (mean score ± SD: topical PP = 21.0 ± 28.0 vs topical BP = 41.0 ± 35.0 vs topical TAC = 18.0 ± 24.0) (no differences reported between groups; P = 0.07) 2a. Suture technician‐rated VAS (100 mm) pain scores (mean score ± SD: topical PP = 3.8 ± 8.5 vs topical BP = 5.0 ± 9.0 vs topical TAC = 1.5 ± 3.0) (Topical TAC outperformed topical BP; P = 0.006; no differences between TAC and PP; no differences between BP and PP) 2b. Research assistant‐rated VAS (100 mm) pain scores (mean score ± SD: topical PP = 3.0 ± 6.0 vs topical BP = 3.8 ± 4.9 vs topical TAC = 1.4 ± 2.1) (Topical TAC outperformed topical BP; P = 0.002; no differences between TAC and PP; no differences between BP and PP) 2c. Parent‐rated VAS (100 mm) pain scores (mean score ± SD: topical PP = 24.0 ± 24.5 vs topical BP = 29.0 ± 28.0 vs topical TAC = 17.0 ± 20.5) (TAC outperformed BP; P = 0.03; no differences between TAC and PP; no differences between BP and PP) |
|
| Intervention dates | Not reported | |
| Declaration of interest | Not reported | |
| Notes | Funding source: supported by Grant 020‐898 from the Children’s Hospital Research Foundation, Columbus, Ohio. Stipend support for medical students was provided by the Samuel L. Roessler Memorial Medical Scholarship Fund. Study author contacted to request additional study data; study author replied but unable to provide missing information |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "68 patients were assigned to each of the three anaesthetic treatment groups using block randomization". Comment: unclear, as exact method of selecting the blocks not reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "using a prospective, randomized, double‐blind design..." "Anesthetics were sealed in envelopes labelled with a study identification number and stored in a locked cabinet in the ED". Comment: probably done, assuming solutions visually identical |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 180 participants included in the study but reporting of attrition or exclusions insufficient to permit judgement |
| selective reporting of outcomes All outcomes | Low risk | All prespecified primary and secondary outcomes were reported: VAS pain scores during suturing by participants and observers (suture technicians, research assistants, parents) Quote: “Pain perceptions of suture technicians, research assistants, parents and patients 5 years of age and older were ascertained using a visual analogue scale (VAS)… Observers based pain scores on the pain experienced as the needle pierced the skin in order to measure actual anaesthetic performance”. Figure 1. Mean VAS pain score by anaesthetic treatment group for suture technicians compared with research assistants for all types of laceration of repair. Figure 2. Mean VAS pain score by anaesthetic treatment group for participants compared with parents for all types of laceration repair. Figure 3. Mean VAS pain score by anaesthetic treatment group for suture technicians compared with research assistants for only face and scalp laceration repairs. Figure 4. Mean VAS pain score by anaesthetic treatment group for participants compared with parents for face and scalp lacerations only. Additional reporting: 1. Complications at follow‐up were listed as “2 wound infections, 1 case of wound drainage that resolved without antibiotics, 3 cases of lost stitches, and 3 cases of wound dehiscence”. |
| Other bias (sample size) | Unclear risk | Quote: “Participants were 180 children. Three groups each of 60 subjects each: TAC vs Prilophen vs Bupivaphen". |
Vinci 1996.
| Methods | Single‐centre RCT, urban paediatric emergency department, Boston, Massachusetts, United States | |
| Participants | 156 patients, 3 to 18 years old, with lacerations on the face/scalp (n = 102), extremity (n = 47) or trunk (n = 7) | |
| Interventions | 1. TAC 1 solution (tetracaine 0.5%, epinephrine 1:2000, cocaine 11.8%), applied for 15 to 30 minutes (n = 49) 2. TAC 2 solution (tetracaine 1.0%, epinephrine 1:2000, cocaine 4.0%), applied for 15 to 30 minutes (n = 49) 3. TAC 3 solution (tetracaine 1.0%, cocaine 4.0%), applied for 15 to 30 minutes (n = 58) | |
| Outcomes | 1. Physician rating of anaesthetic effectiveness (complete, partial or no anaesthesia). Anaesthesia was 'complete' if the participant did not move, flinch or grimace during repair. Anaesthesia was 'partial' if the participant complained of pain, moved or grimaced. If supplemental lidocaine infiltration was required, then 'no anaesthesia' was given.
2. Requirement for a second application of topical anaesthetic
3. Requirement for supplemental lidocaine infiltration
4. The study reported acute adverse effects directly due to the anaesthetic. Results for TAC 1 (standard formulation) vs TAC 3 (tetracaine‐cocaine) include the following. 1. Incidence of complete anaesthesia (topical TAC 1 = 73% vs topical TAC 3 = 28%; P < 0.001) 2. Requirement for a second dose of topical anaesthetic (topical TAC 1 = 30% vs topical TAC 3 = 66%; P < 0.003) 3. Requirement for supplemental lidocaine infiltration (topical TAC 1 = 6% vs topical TAC 3 = 9%; P = not reported) Results for TAC 2 (higher concentration tetracaine, lower concentration cocaine) vs TAC 3 (tetracaine‐cocaine) include the following. 1. Incidence of complete anaesthesia (topical TAC 2 = 63% vs topical TAC 3 = 28%; P < 0.001) 2. Requirement for a second dose of topical anaesthetic (topical TAC 2 = 46% vs topical TAC 3 = 66%; P < 0.003) 3. Requirement for supplemental lidocaine infiltration (topical TAC 2 = 2% vs topical TAC 3 = 9%; P = not reported) 4. A single paediatric participant developed an erythematous rash 1 day after application of standard topical TAC. |
|
| Intervention dates | Not reported | |
| Declaration of interest | No explicit documentation regarding conflicts of interest | |
| Notes | Source of funding: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The solutions were batched in lots of 10 doses to limit expiration of the study drugs. The order of batching was generated using a standard table of random numbers". Comment: probably done |
| Allocation concealment (selection bias) | High risk | Quote: "The order of batching was generated using a standard table of random numbers". Comment: probably not done |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "...we conducted a randomized, prospective, double‐blind, clinical trial comparing three different formulations of cocaine‐containing topical anaesthetics". Unclear: In the Introduction section, reported to be a double‐blind study, but no details provided in Methods or any other sections |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A total of 165 participants were randomized in the study, and no missing outcome data or exclusions |
| selective reporting of outcomes All outcomes | Low risk | The study protocol is available, and all of the study’s prespecified outcomes have been reported in the prespecified way. |
| Other bias (sample size) | High risk | 165 participants: 1. TAC 1 solution, n = 49 2. TAC 2 solution, n = 49 3. TAC 3 solution, n = 58 |
White 1986.
| Methods | Single‐centre RCT, emergency department at Arizona Health Sciences Center, Arizona, United States | |
| Participants | 68 adult patients, older than 18 years of age, with lacerations < 5 cm in length, located on the face (n = 22) or non‐facial (n = 46) | |
| Interventions | 1. TAC solution (tetracaine 0.5%, epinephrine 1:2000, cocaine 10.0%), applied for 5 to 10 minutes (n = 36) 2. Tetracaine solution (tetracaine 0.5%), applied for 5 to 10 minutes (n = 32) | |
| Outcomes | 1. Participant‐rated numerical pain scale score (0‐10)
2. Requirement of supplemental lidocaine infiltration Results include the following. 1. Participant‐rated numerical pain scale (0‐10) score (mean pain scores: topical tetracaine = 5.6 vs topical TAC = 3.53; P < 0.05; standard deviations not reported) 2. Requirement for rescue lidocaine infiltration (topical tetracaine = 59% vs topical TAC = 36%; P = not reported) |
|
| Intervention dates | Not reported | |
| Declaration of interest | No explicit documentation regarding conflicts of interest | |
| Notes | Source of funding: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "Prior to delivery to the emergency department, the TAC and tetracaine solutions were assigned odd or even numbers"; "Randomization was achieved by matching the vials to the odd or even numbers at the end of the hospital number". Comment: probably not done |
| Allocation concealment (selection bias) | High risk | Quote: "Randomization was achieved by matching the vials to the odd or even numbers at the end of the hospital number". Comment: probably not done |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Only the pharmacist preparing the solutions knew which vials contained tetracaine and which contained TAC". Comment: probably done, assuming visually identical solutions |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 68 patients participated in the study. It is not clear whether the same number were randomized, or whether any were withdrawn. |
| selective reporting of outcomes All outcomes | Low risk | The study protocol is available, and all of the study’s prespecified outcomes have been reported in the prespecified way. |
| Other bias (sample size) | High risk | Total N = 68: 1. TAC solution, n = 36 2. Tetracaine solution, n = 32 |
Zempsky 1997.
| Methods | Single‐centre RCT, emergency department of Children’s Hospital of Pittsburgh, Pittsburgh, Pennsylvania, United States | |
| Participants | 32 patients, 5 to 18 years old, with lacerations < 5 cm long, located on the extremity (n = 32) | |
| Interventions | 1. EMLA cream (lidocaine 2.5%, prilocaine 2.5%), applied for maximum of 60 minutes (n = 16) 2. TAC solution (formulation not reported by study), applied for maximum of 30 minutes (n = 16). | |
| Outcomes | 1. Participant‐rated VAS (100 mm) pain scores
2. Observer‐rated VAS (100 mm) pain scores by suturing physician and parent
3. Requirement for supplemental lidocaine infiltration Results included the following. 1. Participant‐rated VAS (100 mm) pain scores (mean score ± SD: EMLA = 46.0 ± 26.0 vs topical TAC = 40.0 ± 25.0; P = 0.50) 2. Parent‐rated VAS (100 mm) pain scores (mean score ± SD: EMLA = 42.0 ± 15.0 vs topical TAC = 43.0 ± 25.0; P = 1.0) and physician‐rated VAS (100 mm) pain scores (mean score ± SD: EMLA = 30.0 ± 16.0 vs topical TAC = 26.0 ± 14.0; P = 0.45) 3. Requirement for supplemental lidocaine infiltration (EMLA = 15% vs topical TAC = 55%; P = 0.03) |
|
| Intervention dates | April to December 1994 | |
| Declaration of interest | Not reported | |
| Notes | Funding source: supported by Grant 5M01 RR00084 from the General Clinical Research Center, Children’s Hospital of Pittsburgh | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...the patient was randomized into one of the two study groups by a table of random numbers" Comment: probably done |
| Allocation concealment (selection bias) | High risk | Quote: "...the patient was randomized into one of the two study groups by a table of random numbers" Comment: probably not done |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "The suturers, who were blinded to the patients' assignments, were not investigators in the study and were not allowed to see the patient until the anaesthetic had been removed and the wound irrigated" Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A total of 32 participants enrolled with no drop‐outs or exclusions |
| selective reporting of outcomes All outcomes | Low risk | All prespecified primary outcomes were reported: observer‐ or participant‐reported VAS pain scores during suturing One prespecified secondary outcome was also reported: need for supplemental infiltrated lidocaine Quote: “Assessment of pain associated with the entire procedure was conducted independently by the suturing physician, the patient, and the parent or guardian on the 10‐cm visual analogue scale (VAS)” Table. Pain scores on a 10‐cm VAS contains participant, parent and physician VAS scores Figure. Efficacy of EMLA and TAC demonstrates efficacy adequacy of anaesthesia after the procedure began Additional reporting: Complications were listed with “one case of wound dehiscence before suture removal in each group and no wound infections were seen in either group" |
| Other bias (sample size) | High risk | Quote: “a convenience sample of 32 patients were enrolled in our study group: EMLA cream 16 subjects and TAC solution 16 patients” |
AC: epinephrine (adrenaline) and cocaine; BN: bupivacaine‐noradrenaline; BP:blood pressure; CI: confidence interval; cm: centimetre; c/w: compared with; ED: emergency department; EMLA: Eutectic Mixture of Local Anesthetics (lidocaine and prilocaine); EN: etidocaine‐noradrenaline; LAT: lidocaine, epinephrine and tetracaine (same as LET); LE: lidocaine and epinephrine; LET: same as LAT; LG: local gel; LI: local infiltration; MAC: bupivacaine 0.5%, epinephrine 1:2000, cocaine 10.0%; mm: milli‐metre; MN: mepivacaine‐noradrenaline; PN: prilocaine‐noradrenaline; N: number; NS: not significant; P = P value; PP: prilocaine, phenylephrine; RCT: randomized controlled trial; RICDRS: Restrained Infants and Children Distress Rating Scale; SD: standard deviation; SE: standard error; TA: tetracaine and epinephrine; TAC: tetracaine, epinephrine and cocaine; TLE: topical lidocaine and epinephrine; TLP: tetracaine, lidocaine and phenylephrine; TP; tetracaine and phenylephrine; VAS: visual analogue scale; vs: versus; w/w: weight per weight.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adler 1998 | Study compared topical lidocaine‐epinephrine‐tetracaine (LET) only vs placebo. No comparison with infiltrated local anaesthetics or other topical anaesthetics |
| Adriansson 2004 | Topical xylocaine was not the primary anaesthetic for repair of the dermal injury. Instead the topical anaesthetic was only pretreatment given before infiltration with local anaesthetic. |
| Akan 2012 | Stimulus was breast surgery, not laceration repair. Also, deep tissue may be involved. |
| Alster 2013 | Stimulus was a cosmetic procedure, not dermal laceration repair. |
| Anderson 2012 | Review article, not a trial |
| Bartfield 1995 | Topical agent was not the primary anaesthetic for repair of the dermal injury. Topical agent was only pretreatment given before infiltration with local anaesthetic. |
| Bartfield 1996 | Topical agent was not the primary anaesthetic for repair of the dermal injury. Topical agent was only pretreatment given before infiltration with local anaesthetic. |
| Bass 1990 | Not a randomized controlled trial. No controls, and all participants received topical lignocaine‐adrenaline‐cocaine |
| Beg 2010 | Procedure is minimally invasive genealogical procedure, not dermal laceration repair. |
| Bonadio 1988a | Not a randomized controlled trial. No controls, and all participants received topical TAC |
| Bonadio 1988b | Not a randomized controlled trial. No controls, and all participants received topical TAC |
| Bonadio 1992 | Not a randomized controlled trial. No controls, and all participants received TAC gel |
| Bonadio 1996 | Study evaluated participants with lacerations located on mucous membranes. |
| Chale 2006 | Compared local anaesthetic vs digital anaesthesia. All lacerations were pretreated with topical anaesthetic, but this was done only to reduce pain from local anaesthetic infiltration. Topical anaesthesia was not used to reduce pain from repair of lacerations. |
| Chipont 2001 | Not a randomized controlled trial. No controls, and all participants received topical LAT |
| Christensen, 2013 | Procedure is wound VAC change, not laceration repair. Also, local anaesthetic was injected into the wound VAC sponge rather than into the skin. |
| Gyftopoulos 2011 | Stimulus was minor surgery on adult penis, not laceration repair. |
| Liebelt 1997 | Not a randomized controlled trial. Instead, this is a review article. |
| Little 2004 | Outcomes of interest not measured; some lacerations repaired by non‐invasive procedures with additional analgesia/anaesthesia administrated to some participants. |
| Lupo 2010 | Not a study on repair of lacerations |
| Park 2015 | Topical anaesthetic was not the primary anaesthetic. Study compares topical local anaesthetics plus infiltration vs infiltration only. |
| Peirluisi 1989 | Not a randomized controlled trial; this is a retrospective study. Also, outcomes were not relevant to this review. |
| Priestley 2003 | Outcomes of interest were not measured. |
| Ridderikhof 2015 | Not an RCT |
| Saariniemi 2013 | Intervention was blepharoplasty rather then laceration repair. |
| Singer 2000 | Topical anaesthetic was only a pretreatment given before infiltration with local anaesthetic. Also, some wound closures were performed with adhesives. |
| Singer 2001 | Topical anaesthetic was only a pretreatment given before infiltration with local anaesthetic. Also, some wound closures were performed with adhesives. |
| Smith 1990 | Some participants (12) were sedated with chloral hydrate. |
| Smith 1998b | Study evaluated participants with lacerations located on mucous membranes. |
| Smith 1998c | Study evaluated patients with lacerations located on mucous membranes. |
| Sobanko 2012 | This is a review article. |
| Spillman 2012 | This is a review article, not a trial. |
| Spivey 1987 | Outcomes of interest were not measured. |
| Stewart 1998 | Topical agent was not the primary anaesthetic for repair of the dermal injury. Topical agent was only a pretreatment given before lidocaine infiltration. |
| White 2004 | Not a randomized controlled trial. No controls, and all participants received LAT gel |
| Yamamoto 1997 | Not a randomized controlled trial |
LAT: lidocaine, adrenaline, and tetracaine; LET: lidocaine‐epinephrine‐tetracaine; TAC: tetracaine‐adrenaline‐cocaine; VAC: vacuum.
Differences between protocol and review
We made the following changes to the published protocol (Eidelman 2005a).
We have changed the title from "Topical anaesthetics for repair of torn skin" to "Topical anaesthetics for pain control during repair of dermal laceration" for clarity.
The name of co‐review author Cristy L Baldwin has been changed to Cristy L Eidelman.
We have rephrased review objectives for clarity and simplification.
We have rephrased review outcomes for clarity and simplification.
We have updated methods and data collection on the basis of updated Cochrane standards.
Contributions of authors
Conceiving the review: Daniel Carr (DC). Co‐ordinating the original review and the 2011 update review: Anthony Eidelman(AE) and DC.
Co‐ordinating the current 2016 updated review: DC and Baraa Tayeb (BT). Undertaking manual searches: BT. Screening search results: BT, DC, AE, Cristy Eidelman (CE) and Ewan McNicol (EM). Organizing retrieval of papers: BT. Screening retrieved papers against inclusion criteria: BT, AE, CE and EM. Appraising the quality of papers: DC, AE, CE, BT and EM. Abstracting data from papers: DC, AE, CE, BT and EM. Writing to authors of papers for additional information: AE and BT. Providing additional data about papers: BT and AE. Obtaining and screening data from unpublished studies: AE. Managing data for the review: BT and EM. Entering data into Review Manager and reviewing entered data (RevMan 5.3): BT and CE. Analysing RevMan 5.3 statistical data: BT. Performing other statistical analyses not using RevMan 5.3: BT, AE, CE and EM. Interpreting data: AE, CE, EM, BT and DC. Performing statistical analysis: BT and EM. Writing the review: BT, EM, CE, AE and DC. Securing funding for the original review: DC. Performing previous work that served as the foundation of the present study: EM, CE, AE and DC. Serving as guarantor for the review (one review author): BT. Taking responsibility for reading and checking the review before submission: BT, EM, DC and CE.
Sources of support
Internal sources
None, Other.
External sources
No sources of support supplied
Declarations of interest
Baraa O Tayeb: none known.
Anthony Eidelman: none known.
Cristy L Eidelman: none known.
Ewan D McNicol: none known.
Daniel B Carr has served as an officer, committee member and lecturer for various professional organizations and community medical centres. None of these activities involved topical application of local anaesthetics. He had patents issued (2012 to 2016) that reflected his work before joining Javelin/Hospira. These patents relate to multi‐valent (e.g. opioid‐tachykinin) peptides. None of them relate to topical local anaesthetics applied for any purpose, nor does Dr. Carr have any financial interest in these or any other patents.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Anderson 1990 {published data only}
- Andersen AB, Colecchi C, Baronoski R, DeWitt TG. Local anesthesia in pediatric patients: topical TAC versus lidocaine. Annals of Emergency Medicine 1990;19(5):519‐22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Blackburn 1995 {published data only}
- Blackburn PA, Butler KH, Hughes MJ, Clark MR, Riker RL. Comparison of tetracaine‐adrenaline‐cocaine (TAC) with topical lidocaine‐epinephrine (TLE): efficacy and cost. American Journal of Emergency Medicine 1995;13(3):315‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bonadio 1990 {published data only}
- Bonadio WA, Wagner V. Efficacy of tetracaine‐adrenaline‐cocaine topical anesthetic without tetracaine for facial laceration repair in children. Pediatrics 1990;86(6):856‐7. [MEDLINE: ] [PubMed] [Google Scholar]
Ernst 1990 {published data only}
- Ernst AA, Crabbe LH, Winsemius DK, Bragdon R, Link R. Comparison of tetracaine, adrenaline, and cocaine with cocaine alone for topical anesthesia. Annals of Emergency Medicine 1990;19(1):51‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ernst 1995a {published data only}
- Ernst AA, Marvez E, Nick TG, Chin E, Wood E, Gonzaba WT. Lidocaine adrenaline tetracaine gel versus tetracaine adrenaline cocaine gel for topical anesthesia in linear scalp and facial lacerations in children aged 5 to 17 years. Pediatrics 1995;95(2):255‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Ernst 1995b {published data only}
- Ernst AA, Marvez‐Valls E, Nick TG, Weiss SJ. LAT (lidocaine‐adrenaline‐tetracaine) versus TAC (tetracaine‐adrenaline‐cocaine) for topical anesthesia in face and scalp lacerations. American Journal of Emergency Medicine 1995;13(2):151‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ernst 1997 {published data only}
- Ernst AA, Marvez‐Valls E, Nick TG, Mills T, Minvielle L, Houry D. Topical lidocaine adrenaline tetracaine (LAT gel) versus injectable buffered lidocaine for local anesthesia in laceration repair. Western Journal of Medicine 1997;167(2):79‐81. [MEDLINE: ] [PMC free article] [PubMed] [Google Scholar]
Gaufberg 2007 {published data only}
- Gaufberg SV, Walta MJ, Workman TP. Expanding the use of topical anesthesia in wound management: sequential layered application of topical lidocaine with epinephrine. The American Journal of Emergency Medicine 2007;25(4):379‐84. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hegenbarth 1990 {published data only}
- Hegenbarth MA, Altieri MF, Hawk WH, Greene A, Ochsenschlager DW, O'Donnell R. Comparison of topical tetracaine, adrenaline, and cocaine anesthesia with lidocaine infiltration for repair of lacerations in children. Annals of Emergency Medicine 1990;12(1):63‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Jenkins 2014 {published data only}
- Jenkins M, Murphy D, Little C, McDonald J, McCarron P. A non‐inferiority randomized controlled trial comparing the clinical effectiveness of anesthesia obtained by application of a novel topical anesthetic putty with the infiltration of lidocaine for the treatment of lacerations in the emergency department. Annals of Emergency Medicine 2014;63(6):704‐10. [PUBMED: 24439713] [DOI] [PubMed] [Google Scholar]
Kendall 1996 {published data only}
- Kendall JM, Charters A, McCabe SE. Topical anaesthesia for children's lacerations: an acceptable approach?. Journal of Accident and Emergency Medicine 1997;14(1):119‐22. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Krief 2002 {published data only}
- Krief W, Sadock V, Tunik M, Mojica M, Manikian A. EMLA vs LET for topical anesthesia in wound repair. Academic Emergency Medicine 2002;9(5):398. [Google Scholar]
Kuhn 1996 {published data only}
- Kuhn M, Rossi SO, Plummer JL, Raftos J. Topical anaesthesia for minor lacerations: MAC versus TAC. Medical Journal of Australia 1996;164(5):277‐80. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lee 2013 {published data only}
- Lee J, Laxmikantha N, Ong M, Wong E, Wee J. Comparing lignocaine‐adrenaline‐tetracaine gel with lignocaine infiltration for anesthesia during repair of lacerations: a randomized trial. World Journal of Emergency Medicine 2013;4(4):281–4. [PUBMED: 25215133] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pryor 1980 {published data only}
- Pryor GJ, Kilpatrick WR, Opp DR. Local anesthesia in minor lacerations: topical TAC vs lidocaine infiltration. Annals of Emergency Medicine 1980;9(11):568‐71. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Resch 1998 {published data only}
- Resch K, Schilling C, Borchert BD, Klatzko M, Uden D. Topical anesthesia for pediatric lacerations: a randomized trial of lidocaine‐epinephrine‐tetracaine solution versus gel. Annals of Emergency Medicine 1998;32(6):693‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schaffer 1985 {published data only}
- Schaffer DJ. Clinical comparison of TAC anesthetic solutions with and without cocaine. Annals of Emergency Medicine 1985;14(11):1077‐80. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schilling 1995 {published data only}
- Schilling CG, Bank DE, Borchert BA, Klatzko MD, Uden DL. Tetracaine, epinephrine (adrenalin), and cocaine (TAC) versus lidocaine, epinephrine, and tetracaine (LET) for anesthesia of lacerations in children. Annals of Emergency Medicine 1995;25(2):203‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Smith 1996 {published data only}
- Smith GA, Strausbaugh SD, Harbeck‐Weber C, Shields BJ, Powers JD, Hackenberg D. Comparison of topical anesthetics without cocaine to tetracaine‐adrenaline‐cocaine and lidocaine infiltration during repair of lacerations: bupivacaine‐norepinephrine is an effective new topical anesthetic agent. Pediatrics 1996;97(3):301‐7. [MEDLINE: ] [PubMed] [Google Scholar]
Smith 1997a {published data only}
- Smith GA, Strausbaugh SD, Harbeck‐Weber C, Shields BJ, Powers JD. Comparison of topical anesthetics with lidocaine infiltration during laceration repair in children. Clinical Pediatrics 1997;36(1):17‐23. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Smith 1997b {published data only}
- Smith GA, Strausbaugh SD, Harbeck‐Weber C, Cohen DM, Shields BJ, Powers JD. New non‐cocaine‐containing topical anesthetics compared with tetracaine‐adrenaline‐cocaine during repair of lacerations. Pediatrics 1997;100(5):825‐30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Smith 1998a {published data only}
- Smith GA, Strausbaugh SD, Harbeck‐Weber C, Cohen DM, Shields BJ, Powers JD, et al. Prilocaine‐phenylephrine and bupivacaine‐phenylephrine topical anesthetics compared with tetracaine‐adrenaline‐cocaine during repair of lacerations. American Journal of Emergency Medicine 1998;16(2):121‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Vinci 1996 {published data only}
- Vinci RJ, Fish S. Efficacy of topical anesthesia in children. Archives of Pediatrics and Adolescent Medicine 1996;150(5):466‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
White 1986 {published data only}
- White WB, Iserson KV, Criss E. Topical anesthesia for laceration repair: tetracaine versus TAC (tetracaine, adrenaline, and cocaine). Journal of Emergency Medicine 1986;4(4):319‐22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Zempsky 1997 {published data only}
- Zempsky WT, Karasic RB. EMLA versus TAC for topical anesthesia of extremity wounds in children. Annals of Emergency Medicine 1997;30(2):163‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Adler 1998 {published data only}
- Adler AJ, Dubinisky I, Eisen J. Does the use of topical lidocaine, epinephrine, and tetracaine solution provide sufficient anesthesia for laceration repair?. Academic Emergency Medicine 1998;5(2):108‐12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Adriansson 2004 {published data only}
- Adriansson C, Suserud BO, Bergbom I. The use of topical anaesthesia at children's minor lacerations: an experimental study. Accident and Emergency Nursing 2004;12(2):74‐84. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Akan 2012 {published data only}
- Akan A, Eryavuz Y, Kamali S, Simşek S, Hot S, Bademci R. A randomized, placebo controlled study: EMLA in minor breast surgery. Minerva Chirurgica 2012;67(2):181‐5. [PUBMED: 22487920] [PubMed] [Google Scholar]
Alster 2013 {published data only}
- Alster T, Stewart D, Cohen J, Taylor M, Sadick N. Clinical overview on the efficacy and safety of a self‐occlusive topical anesthetic cream containing lidocaine and tetracaine. Journal of the American Academy of Dermatology 2013;68(4):AB22. [DOI: ] [Google Scholar]
Anderson 2012 {published data only}
- Anderson K. Towards evidence‐based emergency medicine: best BETs from the Manchester Royal Infirmary. Emergency Medicine Journal 2012;29(4):339‐40. [PUBMED: 22420000 ] [DOI] [PubMed] [Google Scholar]
Bartfield 1995 {published data only}
- Bartfield JM, Raccio‐Robak N, Salluzzo RF. Does topical lidocaine attenuate the pain of infiltration of buffered lidocaine?. Academic Emergency Medicine 1995;2(2):104‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bartfield 1996 {published data only}
- Bartfield JM, Lee FS, Raccio‐Robak N, Salluzzo RF, Asher SL. Topical tetracaine attenuates the pain of infiltration of buffered lidocaine. Academic Emergency Medicine 1996;3(11):1001‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bass 1990 {published data only}
- Bass DH, Wormald PJ, McNally J, Rode H. Topical anaesthesia for repair of minor lacerations. Archives of Disease in Childhood 1990;65(11):1272‐3. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Beg 2010 {published data only}
- Beg N, Vancaillie T, Hewitt A, Eggermont J, Armstrong G. Lignocaine gel in minimally invasive surgery – a pilot cohort study. Australia New Zealand Journal of Obstetrics and Gynaecology 2010;50(4):382‐4. [DOI] [PubMed] [Google Scholar]
Bonadio 1988a {published data only}
- Bonadio WA, Waner V. Half‐strength TAC topical anesthetic. Clinical Pediatrics (Philadelphia) 1988;27(10):495‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bonadio 1988b {published data only}
- Bonadio WA, Wagner V. Efficacy of TAC topical anesthetic for repair of pediatric lacerations. American Journal of Diseases of Children 1988;142(2):203‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bonadio 1992 {published data only}
- Bonadio WA, Wagner VR. Adrenaline‐cocaine gel topical anesthetic for dermal laceration repair in children. Annals of Emergency Medicine 1992;21(12):1435‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bonadio 1996 {published data only}
- Bonadio WA. Safe and effective method for application of tetracaine, adrenaline, and cocaine to oral lacerations. Annals of Emergency Medicine 1996;28(4):396‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Chale 2006 {published data only}
- Chale S, Singer AJ, Marchini S, McBride MJ, Kennedy D. Digital versus local anesthesia for finger lacerations: a randomized controlled trial. Academic Emergency Medicine 2006;13(10):1046‐50. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Chipont 2001 {published data only}
- Chipont Benabent E, Garcia‐Hermosa P, Alio Y, Sanz JL. Benabent sutura de laceraciones cutaneas con gel LAT (lidocaina, adrenalina, tetracaina). Archivos de la Sociedad Espanola de Oftalmologia 2001;76:505‐8. [PubMed] [Google Scholar]
Christensen, 2013 {published data only}
- Christensen T, Thorum T, Kubiak E. Lidocaine analgesia for removal of wound vacuum‐assisted closure dressings: a randomized double‐blinded placebo‐controlled trial. Journal of Orthopaedic Trauma 2013;27(2):107‐12. [DOI] [PubMed] [Google Scholar]
Gyftopoulos 2011 {published data only}
- Gyftopoulos K. The efficacy and safety of topical EMLA cream application for minor surgery of the adult penis. Urology Annals 2012;4(3):145‐9. [PUBMED: 23248519 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Liebelt 1997 {published data only}
- Liebelt EL. Current concepts in laceration repair. Current Opinion in Pediatrics 1997;9(5):459‐64. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Little 2004 {published data only}
- Little S, Brosnahan J. Topical anaesthetic applied at triage reduced treatment time in children presenting to the emergency department with minor lacerations. Evidence‐Based Nursing 2004;7(1):9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lupo 2010 {published data only}
- Lupo M, Swetman G, Waller W. The effect of lidocaine when mixed with large gel particle hyaluronic acid filler tolerability and longevity: a six‐month trial. Journal of Drugs in Dermatology 2010;9(9):1097‐100. [PUBMED: 20865841] [PubMed] [Google Scholar]
Park 2015 {published data only}
- Park SW, Oh TS, Choi JW, Eom JS, Hong JP, Koh KS, et al. Topical EMLA cream as a pretreatment for facial lacerations. Archives of Plastic Surgery 2015;42(1):28‐33. [PUBMED: 25606486] [DOI] [PMC free article] [PubMed] [Google Scholar]
Peirluisi 1989 {published data only}
- Pierluisi GJ, Terndrup TE. Influence of topical anesthesia on the sedation of pediatric emergency department patients with lacerations. Pediatric Emergency Care 1989;5(4):211‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Priestley 2003 {published data only}
- Priestley S, Kelly AM, Chow L, Powell C, Williams A. Application of topical local anesthetic at triage reduces treatment time for children with lacerations: a randomized controlled trial. Annals of Emergency Medicine 2003;42(1):34‐40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ridderikhof 2015 {unpublished data only}
- Ridderikhof. The use of local anesthetics in wounds. ISRCTN14408476 DOI 10.1186/ISRCTN14408476.
Saariniemi 2013 {published data only}
- Saariniemi KM, Salmi AM, Kuokkanen HO. Can topical EMLA cream be used safely in upper blepharoplasty?. European Journal of Plastic Surgery 2013;36:485–8. [DOI: 10.1007/s00238-013-0822-7] [DOI] [Google Scholar]
Singer 2000 {published data only}
- Singer A, Stark M. Pretreatment of lacerations with lidocaine, epinephrine and tetracaine at triage: a randomized double‐blinded trial. Academic Emergency Medicine 2000;7(7):751‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Singer 2001 {published data only}
- Singer A, Stark M. LET versus EMLA for pretreating lacerations: a randomized trial. Academic Emergency Medicine 2001;8(3):223‐30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Smith 1990 {published data only}
- Smith SM, Barry RC. A comparison of three formulations of TAC (tetracaine, adrenalin, cocaine) for anesthesia of minor lacerations in children. Pediatric Emergency Care 1990;6(4):166‐70. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Smith 1998b {published data only}
- Smith G, Strausbaugh S, Harbeck‐Weber C, Cohen D, Shields B, Powers J. Prilocaine‐phenylephrine topical anesthesia for repair of mucous membrane lacerations. Pediatric Emergency Care 1998;14(5):324‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Smith 1998c {published data only}
- Smith G, Strausbaugh S, Harbeck‐Weber C, Cohen D, Shields B, Powers J. Tetracaine‐lidocaine‐phenylephrine topical anesthesia compared with lidocaine infiltration during repair of mucous membrane lacerations in children. Clinical Pediatrics (Philadelphia) 1998;37(7):405‐12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sobanko 2012 {published data only}
- Sobanko J, Miller C, Alster T. Topical anesthetics for dermatologic procedures: a review. Dermatologic Surgery 2012;38(5):709‐21. [22243434 ] [DOI] [PubMed] [Google Scholar]
Spillman 2012 {published data only}
- Spillman N. A synthetical view of pediatrics, lidocaine, and procedural pain relief. Plastic Surgical Nursing 2012;32(2):54‐8. [DOI] [PubMed] [Google Scholar]
Spivey 1987 {published data only}
- Spivey WH, McNamara RM, Mackenzie RS, Bhat S, Burdick WP. A clinical comparison of lidocaine and bupivacaine. Annals of Emergency Medicine 1987;16(7):752‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Stewart 1998 {published data only}
- Stewart G, Simpson P, Rosenberg N. Use of topical lidocaine in pediatric laceration repair: a review of topical anesthesia. Pediatric Emergency Care 1998;4(6):419‐23. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
White 2004 {published data only}
- White NJ, Kim MK, Brousseau DC, Bergholte J, Hennes H. The anesthetic effectiveness of lidocaine‐adrenaline‐tetracaine gel on finger lacerations. Pediatric Emergency Care 2004;20(12):812‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Yamamoto 1997 {published data only}
- Yamamoto LG, Young LL, Roberts JL. Informed consent and parental choice of anesthesia and sedation for the repair of small lacerations in children. American Journal of Emergency Medicine 1997;15(3):285‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Additional references
Altman 1985
- Altman RS, Smith‐Coggins R, Ampel LL. Local anesthetics. Annals of Emergency Medicine 1985;14(12):1209‐17. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Benzon 2011
- Benzon H, Raja SN, Fishman SM, Liu S, Cohen SP. Essentials of Pain Medicine. St Louis, MO: Elsevier Health Science, 2011. [Google Scholar]
Berde 1991
- Berde CB, Lehn BM, Yee JD, Sethna NF, Russo D. Patient‐controlled analgesia in children and adolescents: a randomized, prospective comparison with intramuscular administration of morphine for postoperative analgesia. Journal of Pediatrics 1991;118(3):460‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Brunton 2011
- Brunton L, Chabner B, Knollmann B. Goodman and Gilman's The Pharmacological Basis of Therapeutics, Local Anesthetics. New York: McGraw‐Hill, 2011. [Google Scholar]
Bush 2002
- Bush S. Is cocaine needed in topical anesthesia?. Emergency Medicine Journal 2002;19(5):418‐22. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Castarlenas 2016
- Castarlenas E, Vega R, Jensen MP, Miró J. Self‐report measures of hand pain intensity: current evidence and recommendations. Hand Clinics 2016;32(1):11‐9. [PUBMED: 26611384] [DOI] [PubMed] [Google Scholar]
Chahar 2012
- Chahar P, Cummings KC III. Liposomal bupivacaine: a review of a new bupivacaine formulation. Journal of Pain Research 2012;5:257‐64. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Choiniere 1990
- Choiniere M, Melzack R, Girard N, Rondeau J, Paquin MJ. Comparisons between patients' and nurses' assessment of pain and medication efficacy in severe burn injuries. Pain 1990;40(2):143‐52. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Crellin 2007
- Crellin D, Sullivan TP, Babl FE, O'Sullivan R, Hutchinson A. Analysis of the validation of existing behavioral pain and distress scales for use in the procedural setting. Paediatric Anaesthesia 2007;17(8):720‐33. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Dailey 1988
- Dailey RH. Fatality secondary to misuse of TAC solution. Annals of Emergency Medicine 1988;17(2):159‐60. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Daya 1988
- Daya MR, Burton BT, Schleiss MR, DiLiberti JH. Recurrent seizures following mucosal application of TAC. Annals of Emergency Medicine 1988;17(6):646‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Dickerson 2014
- Dickerson DM, Apfelbaum JL. Local anesthetic systemic toxicity. Aesthetic Surgery Journal 2014;34(7):1111‐9. [PUBMED: 25028740 ] [DOI] [PubMed] [Google Scholar]
Drug Facts and Comparisons 2015
- Facts, Comparisons Corporate. Drug Facts and Comparisons 2015. Facts and Comparisons, 2015. [Google Scholar]
Grant 1992
- Grant S, Hoffman R. Use of tetracaine, epinephrine, and cocaine as a topical anesthetic in the emergency department. Annals of Emergency Medicine 1992;21(8):987‐97. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Highly sensitive search strategies for identifying reports of randomized controlled trials in MEDLINE; Chapter 6.3.2.1. www.cochrane‐handbook.org. Chichester, UK.
Hjermstad 2011
- Hjermstad MJ, Fayers PM, Haugen DF, Caraceni A, Hanks GW, Loge JH, et al. Studies comparing numerical rating scales, verbal rating scales, and visual analogue scales for assessment of pain intensity in adults: a systematic literature review. Journal of Pain and Symptom Management 2011;41(6):1079‐93. [PUBMED: 21621130] [DOI] [PubMed] [Google Scholar]
Kundu 2002
- Kundu S, Achar S. Principles of office anesthesia. Part II. Topical anesthesia. American Family Physician 2002;66(1):99‐102. [MEDLINE: ] [PubMed] [Google Scholar]
Lander 1993
- Lander J, Fowler‐Kerry S. TENS for children's procedural pain. Pain 1993;52(2):209‐16. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lobo 2015
- Lobo K, Donado C, Comelissen L, Kim J, Ortiz R, Peake RW, et al. A phase 1, dose‐escalation, double‐blind, block‐randomized, controlled trial of safety and efficacy of neosaxitoxin alone and in combination with 0.2% bupivacaine, with and without epinephrine, for cutaneous anesthesia. Anesthesiology 2015;123(4):873‐85. [PUBMED: 26372144] [DOI] [PubMed] [Google Scholar]
Mcnicol 2015
- McNicol ED, Ferguson MC, Hudcova J. Patient controlled opioid analgesia versus non‐patient controlled opioid analgesia for postoperative pain. Cochrane Database of Systematic Reviews 2015,;Issue 6.:Art. No.: CD003348. [DOI: 10.1002/14651858] [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 5.3 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Singer 1999
- Singer A, Richman P, Kowalska A, Thode H. Comparison of patient and practitioner assessments of pain from commonly performed emergency department procedures. Annals of Emergency Medicine 1999;33(6):652‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Stephenson 1994
- Stephenson NL. A comparison of nurse and patient: perceptions of postsurgical pain. Journal of Intravenous Nursing 1994;17(5):235‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Stoelting 1999
- Stoelting R. Pharmacology And Physiology In Anesthetic Practice. Pharmacology and Physiology in Anesthetic Practice. Baltimore, MD: Lippincott Williams and Wilkins, 1999. [Google Scholar]
Terndrup 1992
- Terndrup TE, Walls HC, Mariani PJ, Gavula DP, Madden CM, Cantor RM. Plasma cocaine and tetracaine levels following application of topical anesthesia in children. Annals of Emergency Medicine 1992;21(2):162‐6. [1739203] [DOI] [PubMed] [Google Scholar]
Tipton 1988
- Tipton G, Dewitt G, Eisenstein S. Topical TAC solution for local anesthesia in children, prescribing inconsistency and acute toxicity. Southern Medical Journal 1988;82(11):1344‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tyler 1993
- Tyler D, Tu A, Douthit J, Chapman C. Toward validation of pain measurement tools for children: a pilot study. Pain 1993;52(3):301‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Vinci 1999
- Vinci RJ, Fish S, Mirochnick M. Cocaine absorption after application of a viscous cocaine‐containing TAC solution. Annals of Emergency Medicine 1999;34(4 Pt 1):498‐502. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wehner 1984
- Wehner D, Hamilton G. Seizures following topical application of local anesthetics to burn patients. Annals of Emergency Medicine 1984;13(6):456‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Zeltzer 1991
- Zeltzer L, Regalado M, Nichter LS, Barton D, Jennings S, Pitt L. Iontophoresis versus subcutaneous injection: a comparison of two methods of local anesthesia delivery in children. Pain 1991;44(1):73‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Eidelman 2005a
- Eidelman A, Weiss J, Enu IK, Lau J, McNicol ED, Carr DB. Topical anaesthetics for repair of dermal laceration. Cochrane Database of Systematic Reviews 2005, Issue 3. [DOI: 10.1002/14651858.CD005364] [DOI] [PubMed] [Google Scholar]
Eidelman 2005b
- Eidelman A, Weiss J, Enu I, Lau J, Carr D. Comparative efficacy and costs of various topical anesthetics for repair of dermal lacerations: a systematic review of randomized, controlled trials. Journal of Clinical Anesthesia 2005;17(2):106‐16. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Eidelman 2011
- Eidelman A, Weiss JM, Baldwin CL, Enu IK, McNicol ED, Carr DB. Topical anaesthetics for repair of dermal laceration. Cochrane Database of Systematic Reviews 2011, Issue 6. [DOI: 10.1002/14651858.CD005364.pub2] [DOI] [PubMed] [Google Scholar]


