Abstract
Background
Restless legs syndrome (RLS) is a common disease affecting about 5% to 15% of the population. Symptoms of RLS can be severe in a minority of and can have a major impact on sleep, mostly sleep initiation, and quality of life. Benzodiazepines are drugs that can induce and maintain sleep and, hence, intuitively are thought to be beneficial to people with RLS. Altough benzodiazepines, particularly clonazepam, are used to treat RLS symptoms, a systematic review done by the American Academy of Sleep Medicine stated that benzodiazepines should not be used as a first‐line treatment, although could be used as a coadjuvant therapy.
Objectives
To evaluate the efficacy and safety of benzodiazepine compared to placebo or other treatment for idiopathic RLS, including unconfounded trials comparing benzodiazepines versus open control.
Search methods
In March 2016 we searched CENTRAL, MEDLINE, Embase and LILACS We checked the references of each study and contacted study authors to identify any additional studies. We considered studies published in any language.
Selection criteria
Randomised clinical trials of benzodiazepine treatment in idiopathic RLS.
Data collection and analysis
We did not perform data collection and analysis, since we did not include any studies,
Main results
We did not identify any studies that met the inclusion criteria of the review. Two cross‐over studies are awaiting classification because the cross‐over trials did not give data at the end of the first cross‐over period.
Authors' conclusions
The effectiveness of benzodiazepines for RLS treatment is currently unknown.
Plain language summary
Benzodiazepines for restless legs syndrome
Background
Restless legs syndrome (RLS) is a common and distressing disease affecting 5% to 15% of the population. Many physicians are still unaware of the disease and do not recognize its symptoms. This disease can affect sleep significantly, impairing quality of life. There is a need for safe and effective treatment for RLS. Current treatments include dopamine agonists, anticonvulsants, and opioids.
Question
Are benzodiazepines effective and safe for people with RLS?
Methods
We searched the literature for studies in any language, published or not, that considered benzodiazepines for the treatment of RLS.
Results
No studies were included in the review.
Discussion
Benzodiazepines have been used for a long time in people with RLS, because these drugs help sleep initiation and maintenance. As there was no properly conducted systematic review on the effectiveness of benzodiazepines, we performed one on this topic.
Conclusion
This systematic review shows that there is no good data to support or refute the use of benzodiazepines to treat symptoms of RLS.
Background
Description of the condition
Restless Legs Syndrome (RLS) is a sensory motor disorder characterized by a distressing urge to move the legs and, sometimes, other parts of the body as well, usually accompanied by a marked sense of discomfort or pain in the legs or other affected body parts (Allen 2003).
The prevalence of RLS is estimated at 5% to 15% in adults, and many physicians do not recognize its symptoms (Carlos 2015). It is more common in women, and can affect children as well (Picchietti 2005; Picchietti 2007; Yeh 2012). When frequency or severity of symptoms, or both, are added to the diagnostic criteria, the prevalence of RLS ranges from 2.2% to 7.9%. If the diagnosis is based in a clinical interview, taking into account all possible differential diagnosis, the prevalence declines to between 1.9% and 4.6% (Ohayon 2012; Picchietti 2005).
Other features commonly found in adults with RLS include sleep disturbance, daytime fatigue, and decreased quality of life ratings (Picchietti 2005), mostly in people who also have iron deficiency anaemia (Allen 2013; Picchietti 2005).
The physical examination is typically normal. RLS may be either idiopathic (primary RLS, which often has a familial component) or secondary (occurring in conjunction with other medical conditions, particularly iron deficiency anaemia, pregnancy, or end‐stage renal disease). Secondary RLS tends to remit without evidence of reoccurrence when the secondary condition is resolved ‐ for example, after renal transplantation in people with end‐stage renal disease, and postpartum in women with RLS occurring in pregnancy (Lee 2001; Hübner 2013; Winkelmann 2002).
Periodic leg movements in sleep (PLMS) are characterized by brief (0.5‐ to 5.0‐second) lower‐extremity movements during sleep, which typically occur at 20‐ to 90‐second intervals, most commonly during the first three hours of sleep (AASM 2005). The affected individual is usually not aware of the movements or of the associated transient partial arousals (Picchietti 2005; Trenkwalder 2005). Recently some data have suggested that PLMS are associated with adverse cardiovascular outcome and cardiac hypertrophy (Mirza 2013). Eighty to ninety per cent of individuals with RLS have PLMS, but PLMS is not specific to RLS (Rye 2012).
Description of the intervention
The treatment of RLS includes pharmacological and non‐pharmacological therapies. The most common pharmacological agents used in clinical practice for the treatment of RLS are levodopa, dopamine agonists, opioids, benzodiazepines, and anticonvulsants (Garcia‐Borreguero 2013; Trenkwalder 2005). The treatment of isolated PLMS is still controversial (Hornyak 2006).
Benzodiazepines have been used for treatment or prophylaxis of seizure, psychiatric disorders, and pain syndromes, although no evidence supports the use of clonazepam, a frequently used benzodiazepine drug to treat RLS, in chronic neuropathic pain (Corrigan 2012).
The central nervous system activity of benzodiazepines occurs mainly by potentiation of gamma‐aminobutyric acid (GABA), an inhibitory neurotransmitter. Other mechanisms, such as an increase in central synthesis of serotonin, may also be responsible for this activity but the mechanism by which benzodiazepines improve RLS is not clear (Joy 1997).
Benzodiazepines are given in RLS to improve the quality of sleep and reduce PLMS (Saletu 2001) although the American Academy of Sleep Medicine Practice Guideline does not recommend benzodiazepines as first‐line treatment (Aurora 2012). They may also ameliorate the waking symptoms of RLS, but this finding is less well established, and the use of these drugs in the daytime is limited by risks of sedation. The inappropriate use of benzodiazepines also can lead to psychological dependence and physiological tolerance. Known adverse effects are increased risk of falling, sedation, drowsiness, ataxia, muscular inco‐ordination, hypotonia, and dysarthria. Behavioral disturbances such as aggressiveness, irritability, agitation, and hyperkinesis have occurred principally in children (Glass 2005). Benzodiazepines can also worsen obstructive sleep apnoea syndrome (Guilleminault 1990; Horguchi 1992).
The essentially normal presynaptic dopaminergic binding studies using F‐dopa PET or beta‐CIT‐SPECT (imaging diagnostic tools) in people with RLS lend support to the hypothesis that dopaminergic neurons and spinal pathways could be more involved in the pathophysiological mechanisms of the syndrome than the nigrostriatal system (Wetter 2004).
Although dopamine can be increased in the synaptic cleft of people with RLS, the strongest evidence for a dopaminergic role in the pathophysiology of RLS comes from the pharmacological response to medications that increase dopamine function (Allen 2004; Earley 2013). Functional imaging studies have shown reduced fluorodopa uptake or reduced D2 receptor binding in the corpus striatum (Wetter 2004).
Iron is a cofactor in dopamine production and there is a brain iron deficiency in people with RLS. Studies with iron‐deprived rats indicate that low CSF ferritin and high transferrin can be expected to occur with reduced brain iron (Allen 2004; Rizzo 2013).
How the intervention might work
Benzodiazepines may be useful in the treatment of RLS because they act as hypnotic drugs, reducing sleep latency, prolonging total sleep time, reducing waking after sleep onset, and diminishing the arousal threshold (Bostwick 2012; Howard 2014 ). Furthermore, these drugs reduce anxiety which is frequently associated with RLS (Brand 2013). Although some features of these drugs may benefit people with RLS, others might act in the opposite way: for instance this class of drugs may act as opioid antagonists attenuating opioid anti nociception (Daghero 1987; Gear 1997).
Why it is important to do this review
RLS is a frequent sleep disorder affecting 5% to 15% of the general population, impacting negatively on their quality of life. Dopaminergic agonists, L‐Dopa, opioids, anticonvulsants are frequently‐used medications to alleviate the symptoms of RLS, but some people do not respond to these drugs or have side effects that prevent their use. Whilst the AASM (Aurora 2012) Clinical Practice Guideline advised against the use of benzodiazepines as first‐line agents for RLS, it was not constructed with sufficient scientific and methodological rigour to permit definitive conclusions. At any rate, the Guideline does state that clonazepam can be used as an adjunctive medication, and benzodiazepines, mostly clonazepam, have been used to reduce the symptoms of RLS for many years.
Objectives
To evaluate the efficacy and safety of benzodiazepine compared to placebo or other treatment for idiopathic RLS, including unconfounded trials comparing benzodiazepines versus open control.
Methods
Criteria for considering studies for this review
Types of studies
All randomized controlled trials (RCTs) and quasi‐RCTs (quasi‐RCTs are defined as trials using inadequate allocation assignment such as date of birth, day of the week or month of the year, person's medical record number, or just allocating every alternate person). We considered either studies with a parallel‐group or a cross‐over design.
Types of participants
Inclusion criteria
We considered children and adults who met any clinical criteria for idiopathic RLS (ASDA 1990; DCSAD 1979; ICSD 2014; Walters 1995). The clinical diagnostic criteria lists four essential components (Allen 2003).
An urge to move the legs, usually accompanied or caused by uncomfortable and unpleasant sensations in the legs (sometimes the urge to move is present without the uncomfortable sensations, and sometimes the arms or other body parts are involved in addition to the legs).
The urge to move or unpleasant sensations begin or worsen during periods of rest or inactivity, such as lying or sitting.
The urge to move or unpleasant sensations are partially or totally relieved by movement, such as walking or stretching, at least as long as the activity continues.
The urge to move or unpleasant sensations are worse in the evening or night than during the day, or only occur in the evening or night (when symptoms are very severe, the worsening at night may not be noticeable but must have been previously present).
We excluded studies exclusively examining people with PLMD (periodic movement limb disorder), without symptoms of RLS.
Exclusion criteria
We excluded studies that included people with secondary forms of RLS, such as metabolic, neuropathic, or renal disease.
Types of interventions
We included unconfounded trials comparing benzodiazepine drugs to placebo or no treatment (open control) as well as trials comparing benzodiazepines to other treatments, pharmacological or not.
Types of outcome measures
We included short‐term and long‐term studies.
Primary outcomes
Improvement of RLS symptoms, as assessed by a validated scale (Allen 2001; IRLSSG 2003).
Secondary outcomes
Subjective sleep quality (any description about sleep quality, i.e. well‐being, improvement of fatigue)
Sleep quality, as measured by overnight polysomnography (sleep efficiency, total sleep time, arousal index, PLMS index)
Quality of life, as measured by a validated scale, such as SF‐36
-
Adverse events described in terms of:
number of withdrawals due to adverse events;
number of participants with any adverse events associated with the interventions.
Search methods for identification of studies
Electronic searches
Cochrane Central Register of Controlled Trials (CENTRAL) 2016, Issue 4, in the Cochrane Library (accessed March 2016; Appendix 1).
MEDLINE (1966 to March 2016), using the optimally sensitive search strategy developed for the Cochrane Collaboration for the identification of randomized controlled trials in MEDLINE (Lefebvre 2011) (Appendix 2).
Embase (1980 to March 2016), using a search strategy adapted from that developed for the Cochrane Collaboration for the identification of randomized controlled clinical trials (Appendix 3).
LILACS (1982 to March 2016), using a search strategy adapted from that developed for the Cochrane Collaboration for the identification of randomized controlled clinical trials (Appendix 4).
Searching other resources
We assessed references from original papers and abstracts, reviews, systematic reviews, meta‐analyses, and ongoing and unpublished trials registers (World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP); ClinicalTrials.gov) were assessed to identify any additional studies. We contacted some researchers in the field of RLS to ask if they knew of any relevant unpublished material.
Data collection and analysis
We planned to use the GRADE approach to assess the quality of the evidence for each outcome across any included trials.
Selection of studies
We used the search strategies described above to obtain titles and abstracts of relevant studies, which were independently screened by LC and KC.
They initially retained review articles that might include relevant data or information on trials. The review authors independently assessed the retrieved abstracts, and if necessary, the full texts of these studies, to determine which studies met the inclusion criteria.
Data extraction and management
Since we did not include any study, there was no data extraction performed.
Assessment of risk of bias in included studies
We planned to assess, independently, the risk of bias of any included studies without blinding to authorship or journal, resolving any disagreement by discussion. We would have incorporated the 'Risk of bias' assessments into the 'Risk of bias' tables, as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We planned to consider: adequate sequence generation, allocation concealment, blinding of participants, personnel, and outcome assessment, incomplete outcome data, selective outcome reporting, and other biases and to assign 'Low risk', 'High risk', or 'Unclear risk' judgements to each criterion. 'Unclear risk' would indicate that there was insufficient information to permit a clear judgement.
Measures of treatment effect
For dichotomous variables we planned to calculate the risk ratio (RR) with 95% confidence interval (CI), using the random‐effects model. For continuous outcomes we planned to calculate mean differences (MD) or standardized mean differences (SMD) with 95% CI, using the random‐effects model. We would have used SMD to measure the same outcomes on different scales. We planned to analyse both change and endpoint data.
Unit of analysis issues
In future updates, we planned to include cross‐over trials if they report data in sufficient detail to enable us to incorporate this in to the review according to the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011). We plan to evaluate two cross‐over periods with a paired analysis, and assess the impact of a carry‐over effect as part of the 'Risk of bias' assessment.
Dealing with missing data
In suspected cases of missing data, we planned to contact the primary investigator of the study. In addition, we would perform a worst case scenario analysis, considering participants with missing data as treatment failures.
Assessment of heterogeneity
We planned to assess heterogeneity with the Chi2 test, which we would have assumed to be present when the significance level (P value) was lower than 0.10, and with the I2 test, assuming substantial heterogeneity when I2 was greater than 50% (Higgins 2003). If significant heterogeneity were present, we would attempt to explain the differences based on clinical characteristics of any included studies.
Assessment of reporting biases
To reduce reporting bias, we contacted as many authors involved in RLS research as possible, and asked about any unpublished trials they might be aware of. We also searched in the International Committee of Medical Journal Editors for trials that had been registered and not published. We had planned to use a funnel plot (trial effect versus trial size) to explore the possibility of publication bias, if we found sufficient studies (10 or more) for any of the primary analyses.
Data synthesis
For clinically homogeneous studies, we intended to pool the data in a meta‐analysis, using the fixed‐effect model as a default, and the inverse variance method.
GRADE and 'Summary of findings' table
We would have used the GRADE approach to assess the quality of the evidence for each outcome across any included trials, and to present the results in a 'Summary of findings' table. We planned to categorize the primary outcomes at the highest level, and downgrade them due to the following study limitations: limitations in design, inconsistency of results, indirectness of evidence, imprecision of results, and publication bias. We would have decreased the quality of the evidence by one point in case of serious problems with the risk of bias criteria, or two points if there were very serious problems.
Subgroup analysis and investigation of heterogeneity
Had sufficient data been available, we would have carried out subgroup analyses according to subcategory to explore possible sources of heterogeneity as follows.
Age (children and adults)
Severity (mild, moderate and severe)
Gender
Length of treatment
Ethnic group
-
Type of medication
clonazepam
temazepam
flurazepam
quazepam
estazolam
triazolam
alprazolam
diazepam
clobazam
lorazepam
oxazepam
zolpidem
zaleplon
zopiclone
eszopiclone
Sensitivity analysis
We planned to perform sensitivity analysis through assessment of the methodological characteristics of the included studies, as well as of the clinical characteristics of their participants, using the following strategies.
Separating RCTs published as abstracts
Separating RCTs of high risk of bias as assessed by allocation concealment
Separating RCTs without an intention‐to‐treat analysis
Separating cross‐over studies from the analysis
Age (children and adults)
Severity (mild, moderate, severe)
Gender
Length of treatment
Ethnic group
Medication
Results
Description of studies
Results of the search
Our search of electronic databases yielded a total of 1518 publications (Figure 1): 246 records in CENTRAL, 280 in MEDLINE, 989 in Embase, and 3 in LILACS, with 118 duplicate records. After analysing titles and summaries, we selected three of these publications full‐text analysis (Boghen 1986; Manconi 2012; Montagna 1984). Two small cross‐over studies are awaiting classification (Boghen 1986; Montagna 1984). Both of these studies had short washout periods for clonazepam and there was a lack of analysis based on the paired‐results. Uncertainty over how far the results of these studies are affected by possible carry‐over effects, together with the age of the studies, led us to list them as awaiting classification. (Characteristics of studies awaiting classification).
1.
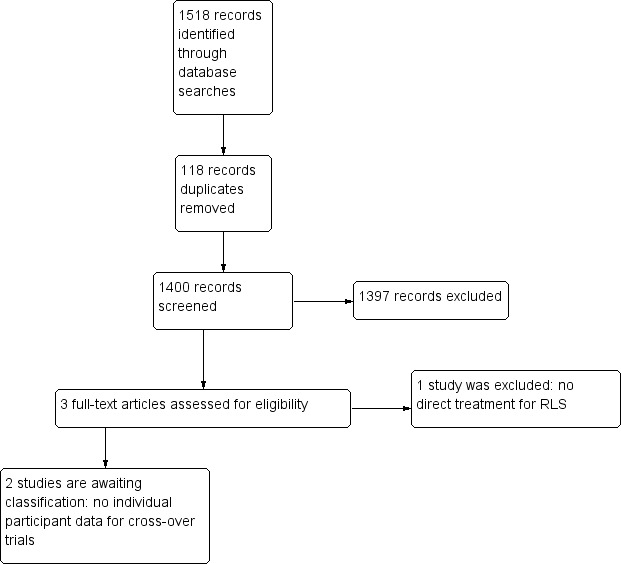
Study flow diagram
Included studies
No studies were included.
Excluded studies
Manconi 2012 was a one‐night‐only, single‐blind, placebo‐controlled study where the intervention was mostly designed to evaluate neurophysiological variables in this particular group of people and not to treat RLS.
Risk of bias in included studies
Not applicable
Allocation
Not applicable
Blinding
Not applicable
Incomplete outcome data
Not applicable
Selective reporting
Not applicable
Other potential sources of bias
Not applicable
Effects of interventions
Not applicable
Discussion
Summary of main results
This review does not provide evidence to assess the effects of benzodiazepines in the treatment of RLS. Two studies from three that were selected to be read in full are awaiting classification (Boghen 1986; Montagna 1984). Lack of detailed information prevented us from using data.
Given our lack of knowledge about the role of clonazepam or other benzodiazepines in the treatment of RLS, only well‐designed clinical trials will answer this question. This is of relevance, since clonazepam is suggested to be of benefit in the control of augmentation related to the treatment of RLS, an emergent clinical problem associated with the use of dopaminergic agonists for RLS (Kurlan 2013).
Overall completeness and applicability of evidence
It was not possible to include any studies in this review, so our research question was not answered, and the value of benzodiazepines in the treatment of RLS is still unknown.
Quality of the evidence
There is no evidence about the effect or absence of effect of benzodiazepines for RLS.
Potential biases in the review process
It is always possible that some unpublished studies (usually those showing negative results) exist but were not identified by our searches. It is also possible that the excluded trials could bring some relevant information if the individual data were assessed.
Agreements and disagreements with other studies or reviews
Although this review did not find any scientific evidence regarding the value of benzodiazepines for RLS, two case series suggest that benzodiazepines can be of some help for people with RLS (Saletu 2001; Schenck 1996), as they may improve the effectiveness and quality of sleep, and suggest that clonazepam in particular may be effective in inducing sleep in these people; however, neither of these case series provide specific data on RLS symptoms, and their conclusions are based on indirect improvement of RLS as measured by improvement in sleep quality. A 1999 AASM review (Hening 1999) suggested benzodiazepines as an option for the treatment of RLS. The 2012 update of this review, however (Aurora 2012), did not recommend use of this pharmacological class, although both versions were based on the same primary studies (Boghen 1986; Montagna 1984), which are awaiting assessment for inclusion in our review and that we found not to support any of the suggested recommendations.
Authors' conclusions
Implications for practice.
The literature lacks robust randomized clinical trials addressing the effect of benzodiazepines for restless legs syndrome (RLS). The effectiveness of benzodiazepines for RLS treatment is currently unknown.
Implications for research.
It is highly recommended that new trials address the effectiveness of treating RLS with benzodiazepines. At present we do not know if this class of drugs, especially clonazepam that has been used for this purpose for decades, is beneficial for people with RLS. Trials should be designed carefully and should preferably be parallel‐group design to avoid problems with a long carry‐over effect of some benzodiazepines like clonazepam. Since RLS is a prevalent disease, studies should include appropriate sample sizes to ensure sufficient statistical power to detect clinically meaningful effects. Outcomes including improvement of RLS symptoms measured in valid scale, improvement of sleep quality, reduction of periodic leg movements in sleep (PLMS) index, and quality of life as a whole require evaluation. Since the impact of RLS may be different in other populations and cultures across the world, a comprehensive clinical trial would ideally be multinational. The absence of ongoing studies may reflect the lack of interest of clinical researchers in this class of drugs for RLS.
What's new
| Date | Event | Description |
|---|---|---|
| 6 November 2008 | Amended | Converted to new review format. |
Acknowledgements
We are thankful for the immense help provided by the following people or institution: Mrs. Ema Roque; Mrs. Denise Mitchell; the editors of the Cochrane Movement Disorders Group; Escola Paulista de Medicina from Universidade Federal de São Paulo (UNIFESP); Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal Nível Superior (CAPES), Cochrane Editorial Unit (CEU) pre‐publication, and all people involved in this review.
Appendices
Appendix 1. CENTRAL search strategy
"#1 RESTLESS LEGS SYNDROME "#2 (restless next leg*) "#3 (ekbom* next syndrome) "#4 (periodic next leg next movements*) "#5 (periodic next limb next movements*) "#6 (PLM or PLMS or PLMD) "#7 (#1 or #2 or #3 or #4 or #5 or #6)
Appendix 2. MEDLINE search strategy
"MEDLINE search strategy (1966 to most recent): "#1 restless legs syndrome [Text Word] "#2 restless legs syndrome [All Fields] "#3 ekbom syndrome [All Fields] "#4 (periodic [All Fields] OR movement [All Fields]) AND (leg OR legs) "#5 (periodic [All Fields] OR movement [All Fields]) AND (limb OR limbs) "#6 (periodic [All Fields] OR movement [All Fields]) AND extremities [All Fields] "#7 nocturnal myoclonus syndrome [All Fields] "#8 periodic limb movement disorder "#9 periodic limb movement disorders "#10 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 "#11 "clonazepam" [All Fields] "#12 "temazepam" [All Fields] "#13 "flurazepam" [All Fields] "#14 "quazepam" [All Fields] "#15 "estazolam" [All Fields] "#16 "triazolam" [All Fields] "#17 "alprazolam" [All Fields] "#18 "diazepam" [All Fields] "#19 "clobazam" [All Fields] "#20 "lorazepam" [All Fields] "#21 "oxazepam" [All Fields] "#22 "zolpidem" [All Fields] "#23 "zaleplon" [All Fields] "#24 "zopiclone" [All Fields] "#25 "eszopiclone" [All Fields] "#26 #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 "#27 #10 AND #26
Appendix 3. Embase search strategy
"EMBASE search strategy (1980 to most recent): "#1 Restless Legs Syndrome/ "#2 restless leg$.tw "#3 Ekbom$ syndrome.tw "#4 periodic leg movement$.tw "#5 periodic limb movement$.tw "#6 (PLM or PLMS or PLMD).tw "#7 #1 OR #2 OR #3 OR #4 OR #5 OR #6 "#8 "clonazepam.tw "#9 "temazepam .tw "#10 "flurazepam.tw "#11 "quazepam .tw "#12 "estazolam .tw "#13 "triazolam.tw "#14 "alprazolam .tw "#15 "diazepam.tw "#16 "clobazam .tw "#17 "lorazepam.tw "#18 "oxazepam.tw "#19 "zolpidem.tw "#20 "zaleplon.tw "#21 "zopiclone.tw "#22 "eszopiclone .tw "#23 #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 "#24 #7 AND #23
Appendix 4. LILACS search strategy
"LILACS search strategy (1982 to most recent): "#1 síndrome das pernas inquietas [Palavras]". "#2 (tw syndrome or tw sindrome) and (leg or legs or pierna$ or perna$ or limb$) [Palavras] and inquieta$ or restless "#3 (movement$ and periodic) [Palavras] "#4 #1 OR #2 OR #3 "#5 "clonazepam" [Palavras] "#6 "temazepam" [Palavras] "#7 "flurazepam" [Palavras] "#8 "quazepam" [Palavras] "#9 "estazolam" [Palavras] "#10 "triazolam" [Palavras] "#11 "alprazolam" [Palavras] "#12 "diazepam" [Palavras] "#13 "clobazam" [Palavras] "#14 "lorazepam" [Palavras] "#15 "oxazepam" [Palavras] "#16 "zolpidem" [Palavras] "#17 "zaleplon" [Palavras] "#18 "zopiclone" [Palavras] "#19 "eszopiclone" [Palavras] "#20 #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 "#21 #4 AND #20
Characteristics of studies
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Manconi 2012 | Primary goal was PLMS treatment. Just one night of intervention. Different scales were used before and after intervention. |
PLMS: periodic leg movements in sleep
Characteristics of studies awaiting assessment [ordered by study ID]
Boghen 1986.
| Methods | Analysis: clonazepam vs placebo Treatment duration: 4 weeks Follow‐up: not reported Center: University of Montreal, Canada Design: randomized clinical trial, double‐blind, cross‐over, placebo‐controlled |
| Participants | N = 6 Drop out: none Diagnosis: clinically diagnosed Exclusions: pregnant women, and people with systemic illness, such as diabetes and liver or renal diseases Gender: 3 women, 3 men Race: not reported Age (years): 31–61 (mean 46) Setting: University clinic |
| Interventions | Group 1: (n = 3) clonazepam (4 weeks) followed by placebo (4 weeks) Group 2: (n = 3) placebo (4 weeks) followed by clonazepam (4 weeks) Clonazepam was started at 0.5mg in the 1st week and progressively increased until the 4th week as follows: 1st week 1/2 h before bedtime; 2nd week 18:00 h, 3rd week at 12:00 h, and in the 4th at 8:00 h Placebo = identical appearing Washout = not reported |
| Outcomes | Self‐rating system in which participants assigned a score daily to the degree of discomfort experienced in the previous 24 h (0‐4) |
| Notes | Study authors seemed to be aware of the carry‐over effect but they did not state if they allowed for it in the study. We had no success in contacting the study authors for information about the cross‐over results. |
Montagna 1984.
| Methods | Analysis: clonazepam vs placebo vs vibration Treatment duration: 1 week Follow‐up: not reported Center: University of Bologna, Italy Design: randomized clinical trial, double‐blind, cross‐over, placebo‐controlled |
| Participants | N = 6 Drop out: none Diagnosis: clinical Exclusions: not reported Gender: 3 women, 3 men Race: not reported Age (years): mean 54.3 (range 44‐64) Setting: University Clinic |
| Interventions | Group 1: (n = 3) clonazepam Group 2: (n = 3) placebo Clonazepan 1 mg or placebo were taken 1/2 h before bedtime Washout: 3 days between each treatment |
| Outcomes | 4‐point subjective scale to evaluate sleep quality, leg jerking, leg dysaesthesia |
| Notes | Study authors also applied a 15‐min vibration over one of the sural regions after the 1st phase protocol. That intervention and outcome was not blinded. Although the study authors compared drug and placebo intervention with vibration we did not consider their results in this review for the reason already stated, and because it was not stated in our protocol. We had no success in contacting the study authors for information about the cross‐over results. |
Differences between protocol and review
Cochrane methods
Review updated with most recent Cochrane methodology: 'Risk of bias' assessments and GRADE system adopted for assessment of the quality of the evidence. This was necessary because Cochrane methodology has been improved since the protocol was written in 2008.
Dealing with missing data
Added: our attempt to contact study authors as a means of obtaining such data
Subgroup analysis
Added: the variables gender, length of treatment, and ethnic group. RLS is more prevalent in women, and the risk factors and associated factors are also different. As it is a chronic disease, length of treatment should always be a variable of interest, both in terms of assessing adverse events and in terms of persistence of therapeutic effect, tolerance, and dependence, all of which are expected concerns with the benzodiazepine class. Some ethnic groups have a lower prevalence of RLS, and one may suppose their responses to interventions may differ as well.
Sensitivity analysis
Added: the variable length of treatment, as the duration of intervention varies widely across studies:
age (children and adults);
severity (mild, moderate, severe);
gender;
length of treatment;
ethnic group;
medication.
These variables also play an important role in ensuring the robustness of the collected data and explaining the presence of heterogeneity.
In the Allocation item, substituted "lower quality" with "high risk of bias", the term used after implementation of the GRADE system.
Methods not implemented
We did not include any study, so we did not implemented the following items from the protocol: sensitivity analysis, heterogeneity analysis, subgroup analysis, all the applicable measures for continuous variables (mean difference (MD) and 95% Confidence Intervals (IC), or the standardised mean difference (SMD) and 95% CI, and dichotomous variables (risk ratio (RR) with 95% CI).
Contributions of authors
Karla Carlos: literature searching, study selection, data extraction, statistical analysis, drafting of written submissions, development of final review.
Luciane Carvalho: literature searching, study selection, data extraction, statistical analysis, drafting of written submissions, development of final review.
Lucila Prado: literature searching, study selection, data extraction, statistical analysis, drafting of written submissions, development of final review.
Cristiane Fiquene Conti: protocol development, literature searching.
Marcio Moyses de Oliveira: protocol development, literature searching.
Gilmar Fernandes do Prado: protocol development, literature searching, study selection, data extraction, statistical analysis, drafting of written submissions, development of final review.
Sources of support
Internal sources
No sources of support supplied
External sources
CAPES ‐ Programa Demanda Social, Brazil.
CNPq ‐ Process # 300646/2013‐5, Brazil.
Declarations of interest
Karla Carlos: none known
Luciane Carvalho: none known
Lucila Prado: none known
Cristiane Fiquene Conti: none known
Marcio Moyses de Oliveira: none known
Gilmar Fernandes do Prado: none known
New
References
References to studies excluded from this review
Manconi 2012 {published data only}
- Manconi M, Ferri R, Zucconi M, Bassetti CL, Fulda S, Arico D, et al. Dissociation of periodic leg movements from arousals in restless legs syndrome. Annals of Neurology 2012;71:834‐44. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Boghen 1986 {published data only}
- Boghen D, Lamothe L, Elie R, Godbout R, Montplaisir J. The treatment of the restless legs syndrome with clonazepam: a prospective controlled study. Canadian Journal of Neurological Sciences 1986;13(3):245‐7. [DOI] [PubMed] [Google Scholar]
Montagna 1984 {published data only}
- Montagna P, Sassoli de Bianchi L, Zucconi M, Cirignotta F, Lugaresi E. Clonazepam and vibration in restless legs syndrome. Acta Neurologica Scandinavica 1984;69(6):428‐30. [DOI] [PubMed] [Google Scholar]
Additional references
AASM 2005
- American Academy of Sleep Medicine. International Classification of Sleep Disorders. Diagnostic and Coding Manual. 2nd Edition. Westchester: AASM, 2005. [Google Scholar]
Allen 2001
- Allen RP, Earley CJ. Validation of Johns Hopkins restless legs severity scale. Sleep Medicine 2001;2(3):239‐42. [DOI] [PubMed] [Google Scholar]
Allen 2003
- Allen RP, Picchietti D, Hening WA, Trenkwalder C, Walters AS, Montplaisi J, et al. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Medicine 2003;4(2):101‐9. [DOI] [PubMed] [Google Scholar]
Allen 2004
- Allen RP. Dopamine and iron in the pathophysiology of restless legs syndrome. Sleep Medicine 2004;(5):385‐91. [DOI] [PubMed] [Google Scholar]
Allen 2013
- Allen RP, Auerbach S, Bahrain H, Auerbach M, Earley CJ. The prevalence and impact of restless legs syndrome on patients with iron deficiency anaemia. American Journal of Hematology 2013;88(4):261–4. [DOI] [PubMed] [Google Scholar]
ASDA 1990
- American Sleep Disorders Association. Diagnostic Cassification Steering Committee. The International Classification of Sleep Disorders: Diagnostic and Coding Manual. Rochester: MN:ASDA, 1990. [Google Scholar]
Aurora 2012
- Aurora RN, Kristo DA, Bista SR, Rowley JA, Zak RS, Casey KR, et al. Update to the AASM Clinical Practice Guideline: “The Treatment of Restless Legs Syndrome and Periodic Limb Movement Disorder in Adults—An Update for 2012: Practice Parameters with an Evidence‐Based Systematic Review and Meta‐Analyses”. Sleep 2012;35(8):1039‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bostwick 2012
- Bostwick JR, Casher MI, Shinji Y. Benzodiazepines: a versatile clinical tool; evidence supports their use for alcohol withdrawal, insomnia, anxiety disorders, and other conditions. Current Psychiatry 2012;11:54‐64. [Google Scholar]
Brand 2013
- Brand S, Beck J, Hatzinger M, Holsboer‐Trachsler E. Patients suffering from restless leg syndrome have low internal locus of control and poor psychological functioning compared to healthy controls. Neuropsychobiology 2013;68:51‐58. [DOI] [PubMed] [Google Scholar]
Carlos 2015
- Carlos K, Prado LBF, Carvalho LBC, Prado GF. Willis‐Ekbom Disease or restless legs syndrome?. Sleep Medicine 2015;16:1156‐9. [DOI] [PubMed] [Google Scholar]
Corrigan 2012
- Corrigan R, Derry S, Wiffen PJ, Moore RA. Clonazepam for neuropathic pain and fibromyalgia in adults. Cochrane Database of Systematic Reviews 2012, Issue 5. [DOI: 10.1002/14651858.CD009486.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Daghero 1987
- Daghero AM, Bradley EL, Kissin I. Midazolam antagonizes the analgesic effect of morphine in rats. Anesthesia & Analgesia 1987;66:944‐7. [PubMed] [Google Scholar]
DCSAD 1979
- Association of Sleep Disorders Centers and the Association for the Psychophysiological Study of Sleep. Diagnostic classification of sleep and arousal disorders 1979 first edition . Sleep 1979;2:1‐154. [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Earley 2013
- Earley CJ, Kuwabara H, Wong DF, Gamaldo C, Salas RE, Brašic JR, et al. Increased synaptic dopamine in the putamen in restless legs syndrome . Sleep 2013;36(1):51‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Garcia‐Borreguero 2013
- Garcia‐Borreguero D, Kohnen R, Silber MH, Winkelman JW, Earley CJ, Högl B, et al. The long‐term treatment of restless legs syndrome/Willis–Ekbom Disease: evidence‐based guidelines and clinical consensus best practice guidance: a report from the International Restless Legs Syndrome Study Group. Sleep Medicine 2013;14(7):675–84. [DOI] [PubMed] [Google Scholar]
Gear 1997
- Gear RW, Miaskowski C, Heller PH, Paul SM, Gordon NC, Levine JD. Benzoadizepine mediated antagonism of opioid analgesia. Pain 1997;71:25‐9. [DOI] [PubMed] [Google Scholar]
Glass 2005
- Glass J, Lanctôt KL, Herrmann N, Sproule BA, Busto UE. Sedative hypnotics in older people with insomnia: meta‐analysis of risks and benefits. BMJ 2005;331:1169. [DOI: 10.1136/bmj.38623.768588.47] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guilleminault 1990
- Guilleminault C. Benzodiazepines, breathing, and sleep. The American Journal of Medicine 1990;88(3):S25‐8. [DOI] [PubMed] [Google Scholar]
Hening 1999
- Hening W, Allen R, Earley C, Kushida C, Picchietti D, Silber M. The treatment of restless legs syndrome and periodic limb movement disorder: an American Academy of Sleep Medicine Review . Sleep 1999;22:970‐99. [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Horguchi 1992
- Horguchi J, Inami Y, Sasaki A, Nishimatsu O, Sukegawa T Nishimatsu O, et al. Periodic leg movements in sleep with restless legs syndrome: effect of clonazepam treatment. Japanese Journal of Psychiatry and Neurology 1992;19(3):180‐1. [DOI] [PubMed] [Google Scholar]
Hornyak 2006
- Hornyak M, Feige B, Riemann D, Voderholzer U. Periodic leg movements in sleep and periodic limb movement disorder: prevalence, clinical significance and treatment. Sleep Medicine Reviews 2006;10:169–77. [DOI] [PubMed] [Google Scholar]
Howard 2014
- Howard P, Twycross R, Shuster J, Mihalyo M. Benzodiazepine. Journal of Pain and Symptom Management 2014;47:955‐64. [DOI] [PubMed] [Google Scholar]
Hübner 2013
- Hübner A, Krafft A, Gadient S, Werth E, Zimmermann R, Bassetti CL. Characteristics and determinants of restless legs syndrome in pregnancy. A prospective study. Neurology 2013;80(8):738‐42. [DOI] [PubMed] [Google Scholar]
ICSD 2014
- American Academy of Sleep Medicine. The International Classification of Sleep Disorders ‐ Third Edition (ICSD‐3). 3rd Edition. Darien: American Academy of Sleep Medicine, 2014. [Google Scholar]
IRLSSG 2003
- The International Restless Legs Syndrome Study Group. Validation of The International Restless Legs Syndrome Study Group rating scale for restless legs syndrome. Sleep Medicine 2003;4:121‐32. [DOI] [PubMed] [Google Scholar]
Joy 1997
- Joy MS. Clonazepam: therapy for restless legs syndrome. ANNA 1997;24:686‐9. [PubMed] [Google Scholar]
Kurlan 2013
- Kurlan R, Rabin M. Augmentation in restless legs syndrome: poor response to sudden withdrawal of dopaminergic therapy. Journal of Parkinsonism and Restless Legs Syndrome 2013;3:49‐52. [Google Scholar]
Lee 2001
- Lee KA, Zaffke ME, Baratte B. Restless legs syndrome and sleep disturbance during pregnancy: the role of folate and iron. Journal of Women's Health and Gender‐Based Medicine 2001;10(4):335‐41. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Mirza 2013
- Mirza M, Shen Win‐Kuang, Sofi A, Mori N, Tajik AJ, Jahangir A. Frequent periodic leg movement during sleep is associated with left ventricular hypertrophy and adverse cardiovascular outcomes. Journal of the American Society of Echocardiography 2013;26(7):783‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ohayon 2012
- Ohayon MM, O'Hara R, Vitiello MV. Epidemiology of restless legs syndrome: a synthesis of the literature. Sleep Medicine Reviews 2012;16(4):283–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Picchietti 2005
- Picchietti D, Winkelman JW. Restless legs syndrome, periodic limb movements in sleep, and depression. Sleep 2005;28(7):891‐8. [PubMed] [Google Scholar]
Picchietti 2007
- Picchietti D, Allen RP, Walters AS, Davidson JE, Myers A, Ferini‐Strambi L. Restless legs syndrome: prevalence and impact in children and adolescents‐the Peds REST study. Pediatrics 2007;120(2):253‐66. [DOI] [PubMed] [Google Scholar]
Rizzo 2013
- Rizzo G, Manners D, Testa C, Tonon C, Vetrugno R, Marconi S, et al. Low brain iron content in idiopathic restless legs syndrome patients detected by phase imaging. Movement Disorders 2013;13:1886‐90. [DOI: 10.1002/mds.25576] [DOI] [PubMed] [Google Scholar]
Rye 2012
- Rye DB, Trotti LM. Restless legs syndrome and periodic leg movements of sleep. Neurologic Clinics 2012;30(4):1137–66. [DOI] [PubMed] [Google Scholar]
Saletu 2001
- Saletu M, Anderer P, Saletu‐Zyhlarz G, Prause W, Semler B, Zoghlamia A, et al. Restless legs syndrome (RLS) and periodic limb movement disorder (PLMD): acute placebo‐controlled sleep laboratory studies with clonazepam. European Neuropsychopharmacology 2011;11:153‐61. [DOI] [PubMed] [Google Scholar]
Schenck 1996
- Schenck CH, Mahowald MW. Long‐term, nightly benzodiazepine treatment of injurious parasomnias and other disorders of disrupted nocturnal sleep in 170 adults. The American Journal of Medicine 1996;100:333‐7. [DOI] [PubMed] [Google Scholar]
Trenkwalder 2005
- Trenkwalder C, Paulus W, Walters AS. The restless legs syndrome review. Lancet Neurology 2005;4(8):465‐75. [DOI] [PubMed] [Google Scholar]
Walters 1995
- Walters A, Aldrich M, Allen RP, Anconi‐Israel S, Bucholz D, Chokroverty S, et al. Towards a better definition of the restless legs syndrome. Movement Disorders 1995;10:634‐42. [DOI] [PubMed] [Google Scholar]
Wetter 2004
- Wetter TC, Eisenensehr I, Trenkwalder C. Functional neuroimaging studies in restless legs syndrome. Sleep Medicine 2004;5:401‐6. [DOI] [PubMed] [Google Scholar]
Winkelmann 2002
- Winkelmann J, Stautner A, Samtleben W, Trankwalder CW. Long‐term course of restless legs syndrome in dialysis patients after kidney transplantation. Movement Disorders 2002;17(5):1072‐76. [DOI] [PubMed] [Google Scholar]
Yeh 2012
- Yeh P, Walters AS, Tsuang JW. Restless legs syndrome: a comprehensive overview on its epidemiology, risk factors, and treatment. Sleep and Breathing 2012;16:987‐1007. [DOI] [PubMed] [Google Scholar]


