Abstract
Background
Clostridium difficile (C. difficile) is recognized as a frequent cause of antibiotic‐associated diarrhoea and colitis. This review is an update of a previously published Cochrane review.
Objectives
The aim of this review is to investigate the efficacy and safety of antibiotic therapy for C. difficile‐associated diarrhoea (CDAD), or C. difficile infection (CDI), being synonymous terms.
Search methods
We searched MEDLINE, EMBASE, CENTRAL and the Cochrane IBD Group Specialized Trials Register from inception to 26 January 2017. We also searched clinicaltrials.gov and clinicaltrialsregister.eu for ongoing trials.
Selection criteria
Only randomised controlled trials assessing antibiotic treatment for CDI were included in the review.
Data collection and analysis
Three authors independently assessed abstracts and full text articles for inclusion and extracted data. The risk of bias was independently rated by two authors. For dichotomous outcomes, we calculated the risk ratio (RR) and corresponding 95% confidence interval (95% CI). We pooled data using a fixed‐effect model, except where significant heterogeneity was detected, at which time a random‐effects model was used. The following outcomes were sought: sustained symptomatic cure (defined as initial symptomatic response and no recurrence of CDI), sustained bacteriologic cure, adverse reactions to the intervention, death and cost.
Main results
Twenty‐two studies (3215 participants) were included. The majority of studies enrolled patients with mild to moderate CDI who could tolerate oral antibiotics. Sixteen of the included studies excluded patients with severe CDI and few patients with severe CDI were included in the other six studies. Twelve different antibiotics were investigated: vancomycin, metronidazole, fusidic acid, nitazoxanide, teicoplanin, rifampin, rifaximin, bacitracin, cadazolid, LFF517, surotomycin and fidaxomicin. Most of the studies were active comparator studies comparing vancomycin with other antibiotics. One small study compared vancomycin to placebo. There were no other studies that compared antibiotic treatment to a placebo or a 'no treatment' control group. The risk of bias was rated as high for 17 of 22 included studies. Vancomycin was found to be more effective than metronidazole for achieving symptomatic cure. Seventy‐two per cent (318/444) of metronidazole patients achieved symptomatic cure compared to 79% (339/428) of vancomycin patients (RR 0.90, 95% CI 0.84 to 0.97; moderate quality evidence). Fidaxomicin was found to be more effective than vancomycin for achieving symptomatic cure. Seventy‐one per cent (407/572) of fidaxomicin patients achieved symptomatic cure compared to 61% (361/592) of vancomycin patients (RR 1.17, 95% CI 1.04 to 1.31; moderate quality evidence). Teicoplanin may be more effective than vancomycin for achieving a symptomatic cure. Eightly‐seven per cent (48/55) of teicoplanin patients achieved symptomatic cure compared to 73% (40/55) of vancomycin patients (RR 1.21, 95% CI 1.00 to 1.46; very low quality evidence). For other comparisons including the one placebo‐controlled study the quality of evidence was low or very low due to imprecision and in many cases high risk of bias because of attrition and lack of blinding. One hundred and forty deaths were reported in the studies, all of which were attributed by study authors to the co‐morbidities of the participants that lead to acquiring CDI. Although many other adverse events were reported during therapy, these were attributed to the participants' co‐morbidities. The only adverse events directly attributed to study medication were rare nausea and transient elevation of liver enzymes. Recent cost data (July 2016) for a 10 day course of treatment shows that metronidazole 500 mg is the least expensive antibiotic with a cost of USD 13 (Health Warehouse). Vancomycin 125 mg costs USD 1779 (Walgreens for 56 tablets) compared to fidaxomicin 200 mg at USD 3453.83 or more (Optimer Pharmaceuticals) and teicoplanin at approximately USD 83.67 (GBP 71.40, British National Formulary).
Authors' conclusions
No firm conclusions can be drawn regarding the efficacy of antibiotic treatment in severe CDI as most studies excluded patients with severe disease. The lack of any 'no treatment' control studies does not allow for any conclusions regarding the need for antibiotic treatment in patients with mild CDI beyond withdrawal of the initiating antibiotic. Nonetheless, moderate quality evidence suggests that vancomycin is superior to metronidazole and fidaxomicin is superior to vancomycin. The differences in effectiveness between these antibiotics were not too large and the advantage of metronidazole is its far lower cost compared to the other two antibiotics. The quality of evidence for teicoplanin is very low. Adequately powered studies are needed to determine if teicoplanin performs as well as the other antibiotics. A trial comparing the two cheapest antibiotics, metronidazole and teicoplanin, would be of interest.
Plain language summary
Antibiotic therapy for Clostridium difficile‐associated diarrhoea in adults
Background
Clostridium difficile (C. difficile) is a bacterium that can live harmlessly in the colon, but when an individual takes an antibiotic for another condition, the C. difficile can grow and replace most of the normal bacterial flora that live in the colon. This overgrowth causes C. difficile‐associated diarrhoea (also known as C. difficile infection ‐ CDI). The symptoms of CDI include diarrhoea, fever and pain. CDI may be only mild but in many cases is very serious and, if untreated, can be fatal. There are many proposed treatments for CDI, but the most common are withdrawing the antibiotic that caused the CDI and prescribing an antibiotic that kills the bacterium. Many antibiotics have been tested in clinical trials for effectiveness and this review studies the comparisons of these antibiotics. This review is an update of a previously published Cochrane review.
Methods
We searched the medical literature up to 26 January 2017. All randomised trials that compare two different antibiotics, or variations in dosing of a single antibiotic for treatment of CDI were included. Trials comparing antibiotic to placebo (e.g. a sugar pill) or no treatment were sought but, save for one poor quality placebo‐controlled trial, none were found. Trials that compared antibiotics to a non‐antibiotic treatment were not included.
Results
Twenty‐two studies (total 3215 participants) were included. The majority of studies enrolled participants with mild to moderate CDI who could tolerate oral antibiotics. Sixteen of the included studies excluded participants with severe CDI and few participants with severe CDI were included in the other studies. Twelve different antibiotics were assessed. Most of the studies compared vancomycin or metronidazole with other antibiotics. One small study compared vancomycin to placebo (e.g. sugar pill). There were no other studies that compared antibiotic treatment to a placebo or a no treatment control group. Seventeen of the 22 included studies had quality issues. In four studies, vancomycin was found to be superior to metronidazole for achieving sustained symptomatic cure (defined as resolution of diarrhoea and no recurrence of CDI). We rated the quality of the evidence supporting this finding as moderate. A new antibiotic, fidaxomicin, was, in two large studies, found to be superior to vancomycin. The quality of the evidence supporting this finding was moderate. It should be noted that the differences in effectiveness between these antibiotics were not too great and that metronidazole is far less expensive than either vancomycin and fidaxomicin. A pooled analysis of two small studies suggests that teicoplanin may be more effective than vancomycin for achieving symptomatic cure. The quality of the evidence supporting this finding was very low. The quality of the evidence for the other seven antibiotics in this review was very poor because the studies were very small, and many patients dropped out of these studies before completion. One hundred and forty deaths were reported in the studies, all of which were attributed to participants preexisting health problems. The only side effects attributed to antibiotics were rare nausea and temporary elevation of liver enzymes. Recent cost data (July 2016) for a 10 day course of treatment shows that metronidazole 500 mg is the least expensive antibiotic with a cost of USD 13. Vancomycin 125 mg costs USD 1779 compared to fidaxomicin 200 mg at USD 3453.83 or more and teicoplanin at approximately USD 83.67.
Conclusion
No firm conclusions can be drawn regarding the effectiveness of antibiotic treatment in severe CDI as most studies excluded these patients. The lack of any 'no treatment' control studies does not allow for any conclusions regarding the need for antibiotic treatment in patients with mild CDI beyond withdrawal of the antibiotic that caused CDI. Nonetheless, moderate quality evidence suggests that vancomycin is superior to metronidazole and fidaxomicin is superior to vancomycin. The differences in effectiveness between these antibiotics were not too large and the advantage of metronidazole is its far lower cost compared to the other antibiotics. The quality of evidence for teicoplanin is very low. Larger studies are needed to determine if teicoplanin performs as well as the other antibiotics. A trial comparing the two cheapest antibiotics, metronidazole and teicoplanin would be of interest.
Summary of findings
Summary of findings for the main comparison. Metronidazole versus vancomycin for Clostridium difficile‐associated diarrhoea in adults.
| Metronidazole versus Vancomycin for Clostridium difficile‐associated diarrhoea in adults | ||||||
| Patient or population: patients with Clostridium difficile‐associated diarrhoea in adults Settings: Intervention: Metronidazole versus Vancomycin | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Metronidazole versus Vancomycin | |||||
| Symptomatic cure with all exclusions treated as failures Follow‐up: mean 4 weeks | 792 per 10001 | 713 per 1000 (665 to 768) | RR 0.9 (0.84 to 0.97) | 872 (4 studies) | ⊕⊕⊕⊝ moderate2 | |
| Bacteriologic cure Follow‐up: mean 4 weeks | 529 per 10001 | 449 per 1000 (328 to 619) | RR 0.85 (0.62 to 1.17) | 163 (2 studies) | ⊕⊝⊝⊝ very low2,3 | |
| Cure (combined symptomatic and bacteriologic cure) ‐ mild disease Follow‐up: mean 4 weeks | 841 per 10001 | 740 per 1000 (597 to 917) | RR 0.88 (0.71 to 1.09) | 90 (1 study) | ⊕⊝⊝⊝ very low3,4,5 | |
| Cure (combined symptomatic and bacteriologic cure) ‐ severe disease | 711 per 10001 | 526 per 1000 (369 to 739) | RR 0.74 (0.52 to 1.04) | 82 (1 study) | ⊕⊝⊝⊝ very low3,4,5 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Control group risk comes from control arm of meta‐analysis, based on included trials. 2 High risk of bias. 3 Serious imprecision. 4 Unclear risk of bias. 5 Unclear if stratification by severity was post hoc.
Summary of findings 2. Teicoplanin versus vancomycin for Clostridium difficile‐associated diarrhoea in adults.
| Teicoplanin versus Vancomycin for Clostridium difficile‐associated diarrhoea in adults | ||||||
| Patient or population: patients with Clostridium difficile‐associated diarrhoea in adults Settings: Intervention: Teicoplanin versus Vancomycin | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Teicoplanin versus Vancomycin | |||||
| Symptomatic Cure Follow‐up: mean 4 weeks | 727 per 10001 | 880 per 1000 (727 to 1000) | RR 1.21 (1 to 1.46) | 110 (2 studies) | ⊕⊝⊝⊝ very low2,3 | |
| Bacteriologic Cure Follow‐up: mean 4 weeks | 452 per 10001 | 822 per 1000 (537 to 1000) | RR 1.82 (1.19 to 2.78) | 59 (1 study) | ⊕⊝⊝⊝ very low2,4 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Control group risk comes from control arm of meta‐analysis, based on included trials. 2 High risk of bias. Blinding not done in either study and other pathogens not excluded in Wenisch 3 Serious imprecision. Two very small studies 4 Serious imprecision. Just a single small study
Summary of findings 3. Fidaxomicin compared to vancomycin for Clostridium difficile‐associated diarrhoea in adults.
| Fidaxomicin compared to Vancomycin for Clostridium difficile‐associated diarrhoea in adults | ||||||
| Patient or population: patients with Clostridium difficile‐associated diarrhoea in adults Settings: Intervention: Fidaxomicin Comparison: Vancomycin | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Vancomycin | Fidaxomicin | |||||
| Symptomatic Cure Follow‐up: mean 4 weeks | 610 per 10001 | 713 per 1000 (652 to 774) | RR 1.17 (1.07 to 1.27) | 1164 (2 studies) | ⊕⊕⊕⊝ moderate2 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Control group risk comes from control arm of meta‐analysis, based on included trials. 2 High risk of bias in both studies.
Summary of findings 4. Bacitracin versus vancomycin for Clostridium difficile‐associated diarrhoea in adults.
| Bacitracin versus Vancomycin for Clostridium difficile‐associated diarrhoea in adults | ||||||
| Patient or population: patients with Clostridium difficile‐associated diarrhoea in adults Settings: Intervention: Bacitracin versus Vancomycin | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Bacitracin versus Vancomycin | |||||
| Symptomatic Cure Follow‐up: mean 4 weeks | 462 per 10001 | 268 per 1000 (157 to 457) | RR 0.58 (0.34 to 0.99) | 104 (2 studies) | ⊕⊝⊝⊝ very low2,3 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Control group risk comes from control arm of meta‐analysis, based on included trials. 2 High risk of bias. Attrition and blinding issues in one trial. 2 Very serious imprecision. Two very small studies with few events.
Background
Description of the condition
The administration of antibiotics is known to be associated with the development of diarrhoea in patients. Erythromycin and the clavulanate in amoxicillin‐clavulanate cause diarrhoea by accelerating gastrointestinal motility (Bartlett 2002). Still other antibiotics are thought to cause diarrhoea by reducing the number of fecal anaerobes, thereby decreasing carbohydrate digestion and absorption, leading to an osmotic diarrhoea (Bartlett 2002). Nearly 15% of hospitalised patients receiving beta‐lactam antibiotics develop diarrhoea (McFarland 1995). Clostridium difficile (C. difficile) infection of the colon (CDI) is another cause of antibiotic‐associated diarrhoea that is thought to be caused by a presumed overgrowth of native or newly acquired C. difficile (Fekety 1993). C. difficile is implicated in 20 to 30% of patients with antibiotic‐associated diarrhoea, in 50 to 70% of those with antibiotic‐associated colitis and in more than 90% of those with antibiotic‐associated pseudomembranous colitis (Bartlett 1980; Bartlett 1990; George 1982; Kelly 1994). Approximately 5% of healthy adults asymptomatically carry low concentrations of C. difficile in their colon, and the growth of these bacteria has been shown in vitro to be held in check by normal gut flora (Fekety 1993). The exact mechanism by which C. difficile overgrowth occurs is still unclear, but it can occur with the administration of oral, parenteral or topical antibiotics (Fekety 1993:Thomas 2003).
It is important to explain the terms used in conjunction with C. difficile infection, as the studies analysed in this review involve various descriptions of C. difficile disease. Clostridium difficile‐associated diarrhoea (CDAD) occurs in a patient with diarrhoea that has tested positive for C. difficile toxin or a positive stool culture for C. difficile. C. difficile colitis involves a stool test positive for the organism and signs of mucosal inflammation seen on endoscopy. Pseudomembranous colitis refers to the actual presence of pseudomembranes seen on endoscopy. CDI is a term used in an increasing number of publications but by no means to the exclusion of CDAD. Both terms connote a patient with symptomatic diarrhoea and laboratory evidence of C. difficile as the cause of that diarrhoea. Pseudomembranous colitis is meant to connote the same disease but is not quite synonymous, as many patients with antibiotic‐associated diarrhoea do not undergo the endoscopy and biopsy necessary for the diagnosis of colitis or the endoscopy and visualization of pseudomembranes. This systematic review will use the term CDI for all forms of symptomatic C. difficile infection for its analyses. This review is an update of a previously published Cochrane review (Bricker 2005; Nelson 2007; Nelson 2011), and includes six new trials assessing three new antibiotics.
Description of the intervention
The standard treatment for primary infection caused by C. difficile is an antibiotic. This is usually done in conjunction with with the cessation of the antibiotic that caused the alteration of the gut flora in the first place (when feasible, considering the patient's clinical condition) with subsequent overgrowth of Clostridium difficile.
How the intervention might work
By treatment with an antibiotic that is specifically effective against Clostridium difficile, toxin production is reduced and repopulation of the gut with normal gut flora is possible.
Why it is important to do this review
During the early part of the 21st century, much higher mortality rates from CDI than previous reports were reported in Quebec (Pepin 2005). This was related to the appearance of a new variant of C. difficile, which is capable of secreting much higher amounts of toxin A and B and is more resistant to standard antibiotic therapy (Louie 2005). This new variant resulted in a higher incidence of CDI among hospitalised patients (McDonald 2005), a greater need for urgent colectomy for toxic colitis and a high mortality rate (e.g. 37%; Pepin 2005). This variant, ribotype 027, has spread from Quebec to the USA, and Europe (Louie 2005; Laurence 2006). In the United States hospitalizations for CDI doubled between 2000 and 2010 and was expected to continue to increase in 2011 and 2012. CDI is the leading cause of gastrointestinal‐related death in the USA. CDI is estimated to have caused 14,000 deaths in the USA in 2007 and is the most common healthcare‐associated infection. The estimated cost of CDI is USD 4,800 million per year for acute care facilities alone in the USA (Lessa 2015). Unlike the UK, where both incidence and mortality have declined in recent years (HPA 2016), the Agency for Healthcare Research and Quality has shown a consistent increase in CDI from the turn of the century through 2015 (AHRQ 2016; Steiner 2015).
The emergence of this highly virulent bacterium adds urgency to the identification of effective therapy.
Objectives
The aim of this review was to investigate the efficacy and safety of antibiotic therapy for CDI, to identify the most effective antibiotic treatment for CDAD in adults and to determine the need for stopping the causative antibiotic during therapy.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) assessing antibiotic treatment for CDI were considered for inclusion.
Types of participants
The types of participants included: (I) patients with diarrhoea: various definitions exist usually describing the consistency of stool, number of bowel movements per day, duration of symptoms, and rarely stool volume; (ii) patients with C. difficile in stool identified by stool culture positive for C. difficile and or by stool positive for C. difficile cytotoxin; (iii) patients who had received prior antibiotic therapy for an infection other than C. difficile; and (iv) patients 18 years of age of older.
Patients were excluded if they did not have diarrhoea or if there was no evidence of C. difficile infection.
Types of interventions
Only studies involving antibiotic therapy for C. difficile‐associated diarrhoea were included. These studies may include comparisons among different antibiotics, between different timing or doses of the same antibiotic, or between antibiotic therapy and placebo. Studies were excluded if they compared antibiotic to non‐antibiotic therapy (such as resins, vaccines, probiotics or biologicals) or if, in conjunction with antibiotic treatment, the intervention being tested was of a non‐antibiotic such as those listed above. In addition interventions used for the prevention of CDI are not included in this review.
Types of outcome measures
The following outcomes were sought:
Sustained symptomatic cure: An initial resolution of the diarrhoea and no evidence of recurrence of diarrhoea due to CDI.
Bacteriologic cure: Conversion from a stool positive for any CDI assay to negative and no recurrence of CDI.
No specific minimum time limit was set for either of these determinations of recurrence.
Death.
Drug‐related adverse events (DRAE) of the antibiotic treatments.
Cost of each of the major antibiotics.
Search methods for identification of studies
Electronic searches
We searched MEDLINE, EMBASE , CENTRAL and the Cochrane IBD Group Specialized Register from inception to 26 January 2017. The search strategies are reported in Appendix 1. Studies published only as abstracts were not explicitly excluded.
Searching other resources
In addition, trials were sought in the Clinicaltrials.gov and Clinicaltrialsregister.eu registers using the search term 'Clostridium difficile'.
Data collection and analysis
Selection of studies
At least two authors (three in this update) examined all the citations and abstracts derived from the electronic search strategy and independently selected trials to be included. Full reports of potentially relevant trials were retrieved to assess eligibility. Reviewers were not blind to the names of trials' authors, institutions or journals. Any disagreements about trial inclusion were resolved by group discussion.
Data extraction and management
Data extraction was performed independently by at least two authors (three in this update). Results were compared between reviewers and all studies were presented for group discussion. Where data may have been collected but not reported, further information was sought from the individual trial authors. When dropouts occurred and were not included in the analyses, and the randomisation group was known, these dropouts were treated as treatment failures in this meta‐analysis.
Assessment of risk of bias in included studies
Two review authors independently assessed the included studies using the Cochrane Collaboration tool for assessing risk of bias (Higgins 2011). Criteria for quality assessment includes:
(1) Selection bias: We rated random sequence generation as 'low risk' if the investigators described a random component in the sequence generation process. We rated as 'high risk' if the investigators described a non‐random component in the sequence generation process. We rated as 'unclear risk' if insufficient information about the sequence generation process was provided.
(2) Performance bias: We rated allocation sequence concealment as 'low risk' if participants and investigators enrolling participants could not foresee treatment assignment. We rated as 'high risk' if participants or investigators enrolling participants could possibly foresee assignments and thus introduce selection bias. We rated as 'unclear risk' if the method of concealment was not described or not described in sufficient detail to allow a definite judgement.
(3) Detection bias: We rated blinding of participants and personnel as 'low risk' if blinding of participants and personnel was ensured, and it was unlikely that the blinding could have been broken. We rated as 'high risk' if there was no blinding or incomplete blinding, and the outcome was likely to be influenced by a lack of blinding or blinding of participants was attempted, but it was likely that the blinding could have been broken, and the outcome was likely to be influenced by lack of blinding. We rated as 'unclear risk' if insufficient information was provided to allow a definite judgement. We rated binding of outcome assessment as 'low risk' if blinding of outcome assessors was ensured, and it was unlikely that the blinding could have been broken. However, due to the nature of the intervention, for several outcome items blinding of outcome assessors was not possible and may be a potential source of bias. We rated as 'high risk' if if there was no blinding of outcome assessors, and the outcome was likely to be influenced by a lack of blinding or blinding of outcome assessors was attempted, but it was likely that the blinding could have been broken, and the outcome was likely to be influenced by a lack of blinding. We rated as 'unclear risk' if insufficient information was provided to allow a definite judgement.
(4) Attrition bias: We rated incomplete outcome data as 'low risk' if there were no missing outcome data, or if there were fewer than 10% post‐randomisation dropouts or withdrawals. We rated as 'high risk' if the reason for missing outcome data was likely to be related to the true outcome. We rated as 'unclear risk' if there was insufficient reporting of attrition and exclusions to permit judgement.
We rated selective reporting as 'low risk' if the study protocol was available and all of the pre‐specified primary and secondary outcomes that were of interest to the review were reported in the pre‐specified manor or the study protocol was not available but it was clear that the published reports included all expected outcomes, including those that were pre‐specified. We rated as 'high risk' if the pre‐specified primary outcomes were not all reported, or one or more outcomes of interest for the review were reported incompletely so that the data could not be used for meta‐analysis, or the study report failed to include results for a key outcome that would be expected to have been reported for such a study. We rated as 'unclear risk' if insufficient information was provided to allow a definite judgement.
(5) Selective Reporting
Selective reporting was focused in this review on whether recurrence data were collected and presented for each study. This is a necessary element in the determination of sustained clinical response.
(6) Other bias We rated other bias as 'low risk' if the study appears to be free of other sources of bias. We rated as 'high risk' if there was at least one other important risk of bias. We rated as 'unclear risk' if there may have been a risk of bias, but there was either insufficient information to assess whether an important risk of bias exists or insufficient rationale or evidence that an identified problem introduced bias.
Measures of treatment effect
For dichotomous outcomes, we calculated the risk ratio (RR) with corresponding 95% confidence interval (95% CI).
Unit of analysis issues
The unit of analysis was the individual patient.
Dealing with missing data
We contacted study authors for missing data. Dropouts were considered to be treatment failures for the analyses.
Assessment of heterogeneity
Clinical heterogeneity was assessed by examining the patient population, interventions and outcome definition and assessment. Methodological heterogeneity was assessed by looking at the components of the risk of bias assessment. Statistical heterogeneity was assessed by calculating the Chi2 and I2 statistics. We considered heterogeneity to be significant if the the P value for Chi2 was < 0.10 or I2 was > 60%.
Assessment of reporting biases
We assessed selective reporting as described above. If any of the pooled analyses included 10 or more studies, we planned to assess potential publication bias by constructing a funnel plot.
Data synthesis
Data were entered and analysed in Revman 5.3. For dichotomous outcomes we calculated the pooled RR and corresponding 95% CI. Due to the small number of studies in each analysis and the lack of statistical heterogeneity in those few analyses with more than one study, a fixed‐effect model was used for comparisons with more than one included study. When considerable clinical heterogeneity was encountered between comparisons, as in Analysis 1.1, both fixed‐effect and random‐effects models were calculated.
1.1. Analysis.

Comparison 1 Metronidazole vs Vancomycin, Outcome 1 Sustained Symptomatic Cure with all exclusions treated as failures.
Subgroup analysis and investigation of heterogeneity
For this systematic review, we assessed specific antibiotics individually. No further subgroup analyses were planned.
Sensitivity analysis
We planned for sensitivity analyses based on study quality. Howveer, given the poor overall quality of the studies in this systematic review no sensitivity analyses are reported in this update.
Summary of Findings
All included studies are RCTs. We used the Grades of Recommendation, Assessment, Development and Evaluation (GRADE) approach to assess the overall quality of the evidence supporting the primary outcome and selected secondary outcomes (Schünemann 2011). The overall quality of evidence can be graded into four levels:
1. High: Further research is very unlikely to change our confidence in the estimate of effect;
2. Moderate: Further research is likely to have an impact on our confidence in the estimate of effect and may change the estimate;
3. Low: Further research is very likely to have an important impact on our confidence on the estimate of effect and is likely to change the estimate; or
4. Very low: Any estimate of effect is very uncertain.
The overall quality of evidence can be downgraded by one (serious concern) or two levels (very serious concern) for the following reasons: risk of bias, inconsistency (e.g. unexplained heterogeneity, inconsistency of results), indirectness (e.g. indirect population, intervention, control, outcomes), publication bias, and imprecision (e.g. wide confidence intervals, single trial). Imprecision was determined principally by a small number of studies for a comparison (two or fewer studies) and fewer than 100 events for that comparison. We used the GRADEproGDT software to produce the Summary of Findings Tables.
Cost
Cost estimates were determined by obtaining information from American retail pharmacies (i.e. GoodRx which is a compendium of six pharmacies and Walgreens/Boots) for a 10 day course of therapy, except for Teicoplann which as obtained from the British National Formulary.
Results
Description of studies
Results of the search
A literature search conducted on 26 January 2017 identified 3842 studies. After duplicates were removed a total of 2372 studies remained for review of titles and abstracts. Two authors independently reviewed the titles and abstracts of these studies and 43 studies were selected for full text review (See Figure 1). Twenty‐one studies were subsequently excluded for reasons cited in the Characteristics of excluded studies table. In addition, two phase III trials of a new drug, cadazolid, were identified by searching trial registries (NCT01983683; NCT01987895).
1.
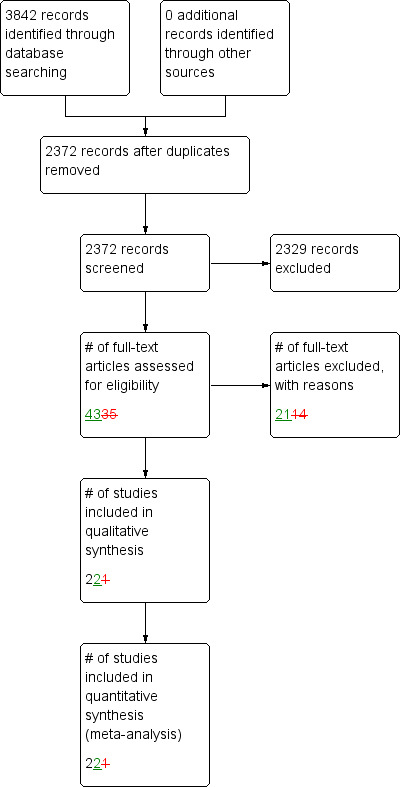
Study flow diagram.
Included studies
In the original review there were nine included studies. The first update included three additional studies (Lagrotteria 2006; Musher 2006; Wullt 2004). The second update included three additional studies (Louie 2009; Musher 2009; Zar 2007). In this third update seven more studies were added (Cornley 2012; Garey 2011; Johnson 2014; Lee 2016a; Louie 2011; Louie 2015; Mullane 2015).
The 22 included studies (total 3215 participants) involve patients with diarrhoea who have recently received antibiotics for an infection other than C. difficile. The definition of diarrhoea ranged from at least two loose stools per day with an associated symptom such as rectal temperature > 38 ºC (Anonymous 1994), to at least six loose stools in 36 hours (Teasley 1983; Louie 2009). All of the studies included a 'loose' or 'liquid' description of the stool in their definitions of diarrhoea. In terms of exclusion criteria, patients with HIV were only explicitly excluded from one study (Anonymous 1994). Pregnant women were excluded from two studies (Anonymous 1994; Zar 2007). All other studies had varying and vague exclusion criteria ranging from "other obvious cause of diarrhoea" (Teasley 1983) to "anatomic abnormality" (Young 1985). Both Louie 2009 and Musher 2009 excluded patients deemed clinically unstable and ''not expected to survive the study period''. Zar 2007 and most other authors excluded patients with suspected or proven life‐threatening intra‐abdominal complications. Johnson 2014 accepted a positive endoscopy at enrolment as long as a positive toxin assay followed. Mullane 2015 excluded patients if they had 12 or more stools per day. Among the seven new studies only two specifically excluded patients with Crohn's disease or ulcerative colitis (Lee 2016a; Louie 2011). In fact patients in all 22 included studies were well enough to tolerate oral medication. None of the prescribed antibiotics were given intravenously.
The studies used different methods for detecting CDI. Teasley 1983 defined C. difficile disease as stool being positive for C. difficile by culture or cytotoxin assay, or by the presence of pseudomembranes on endoscopy. Stool was tested for other enteric pathogens, but the authors did not comment on the presence or absence of these pathogens. Young 1985 and Zar 2007 defined CDI in the same manner as Teasley 1983. Young 1985 tested for the presence of other enteric pathogens and excluded patients if their stool tested positive for any other pathogen. Dudley 1986 and Fekety 1989 and Louie 2009 defined C. difficile disease by both culture or cytotoxin assay. Dudley 1986 and Fekety 1989 did not test for the presence of other enteric pathogens. De Lalla 1992 defined C. difficile disease as stool being positive for cytotoxin, culture and evidence of colitis on endoscopy. De Lalla 1992 excluded patients if their stool tested positive for any other pathogen. Wenisch 1996 defined C. difficile disease as stool positive for cytotoxin or endoscopic evidence of colitis and fecal leukocytes. Wenisch 1996 did not perform stool culture on any of the study patients. The Swedish CDI study defined C. difficile disease as being positive for cytotoxin or culture or both and excluded patients if their stool tested positive for any other pathogen (Anonymous 1994). Eight studies used toxin assay and symptomatic diarrhoea to define C. difficile disease (Cornley 2012; Garey 2011; Johnson 2014; Lagrotteria 2006; Louie 2011; Musher 2006; Musher 2009; Wullt 2004). Endoscopic evidence of pseudomembranous colitis was used in Louie 2011, Cornley 2012 and Johnson 2014 for initial enrolment, but subsequent positive toxin assay was also required.
Eighteen of the 22 included studies compared different antibiotics for the treatment of C. difficile‐associated diarrhoea. Teasley 1983 compared oral vancomycin 500 mg four times a day for 10 days with oral metronidazole 250 mg four times a day for 10 days. Young 1985 compared oral vancomycin 125 mg four times a day for 7 days with oral bacitracin 20,000 units four times a day for 7 days. Dudley 1986 compared oral vancomycin 500 mg four times a day for 10 days with oral bacitracin 25,000 units four times a day for 10 days. De Lalla 1992 compared oral vancomycin 500 mg four times a day for 10 days with oral teicoplanin 100 mg two times a day for 10 days. Wenisch 1996 compared four antibiotics: oral vancomycin 500 mg three times a day for 10 days with oral metronidazole 500 mg three times a day for 10 days with oral fusidic acid 500 mg three times a day for 10 days with oral teicoplanin 400 mg two times a day for 10 days. Wullt 2004 compared 250 mg of fusidic acid three times per day to 400 mg of metronidazole three times per day for 7 days each. Musher 2006 compared 250 mg of metronidazole four times per day for 10 days to 500 mg of nitazoxanide twice a day given for either 7 or 10 days. Musher 2009 compared 125mg of vancomycin four times per day for 10 days to 500 mg of nitazoxanide twice a day for 10 days. Zar 2007 compared 125 mg of vancomycin four times per day with 250 mg of metronidazole four times per day for 10 days. Cornley 2012 and Louie 2011 published studies of identical design in two large diverse populations comparing an oral 10 day course of fidaxomicin 200 mg every 12 hours to vancomycin 125 mg every 6 hours. Johnson 2014 was a three armed study, whose principal objective was to assess the efficacy of Tolevamer, a resin that binds Clostridium difficile toxin. The other two arms of this multi‐institutional, multinational study compared the efficacy of vancomycin and metronidazole to Tolevamer and thus also to each other. Due to its size, this study added significantly to the data for the comparison of metronidazole to vancomycin. The Tolevamer arm of this study was not included in this review. Two other new antibiotics were reported by Louie 2015 (Cadazolid) and Mullane 2015 (LFF571). Neither of these recent studies stratified by disease severity before or after randomisation. The Cadazolid study reported three different dose groups separately but since the results were similar for the outcome assessment these groups were combined for the comparison to vancomycin. Lee 2016a compared two doses of a new antibiotic, surotomycin, to vancomycin in a non‐inferiority trial, similar to Cornley 2012 and Louie 2011).
Garey 2011 studied the effect of rifaximin 400 mg three times daily for 20 days compared to placebo in a group who had had CDI successfully treated with either metronidazole or vancomycin (the choice of these antibiotics being physician choice and non‐random) in order to prevent a highly likely occurrence, relapse of CDI. Strictly speaking this is not an eligible trial, in that it assesses a prophylactic intervention. However its goal was to define an antibiotic combination the best achieves a long term cure of CDI , perhaps more comparable in that regard to Johnson 1992.
The Swedish CDI study was a dose finding study which compared oral teicoplanin given at a constant daily dose but in differing dose schedules throughout the day (Anonymous 1994, also cited in some sources as Wistrom 1994). Fekety 1989 performed a dosage‐finding study for oral vancomycin by comparing 125 mg four times a day with 500 mg four times a day for an average of 10 days. Louie 2009 compared fidaxomicin (OPT‐80) given at either 50 mg, 100 mg or 200 mg doses twice daily for ten days. Only one study was placebo‐controlled and compared vancomycin to placebo (Keighley 1978).
In addition to treating CDI with antibiotics, the above studies also dealt with the initial, offending antibiotic in different ways. Only three studies explicitly reported that all initial antibiotics were stopped (Wenisch 1996; Young 1985; Zar 2007). Three of the other studies made attempts to stop the initial antibiotics, but these antibiotics were continued if deemed essential to the patient's clinical treatment (Anonymous 1994; Dudley 1986; Teasley 1983). Louie 2009 excluded any patients requiring concurrent antibiotic therapy, but made no reference to the initial antibiotics used in the included participants. The remaining studies either stated that the initial antibiotic was either stopped or changed (Fekety 1989) or did not make any reference to the initial antibiotic at all (Cornley 2012; De Lalla 1992; Garey 2011; Johnson 2014; Lagrotteria 2006; Louie 2011; Musher 2006; Musher 2009; Wullt 2004; Louie 2011).
Zar 2007 defined cure as a combination of resolution of diarrhoea and conversion of stool to toxin negative, whereas other authors reported these outcomes separately, or did not report bacteriologic cure at all. Zar 2007 is the first study to stratify patients (perhaps after randomisation) by disease severity when assessing the relative efficacy of metronidazole and vancomycin. This is of importance because this general concept of efficacy assessment has been adopted in subsequent studies (Cornley 2012; Johnson 2014; Louie 2011; Musher 2009), and three major guideline publications including the UK Health Protection Agency (HPA 2008), the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) (Bauer 2009), and the combined Infectious Diseases Society of America (IDSA), and the Society for Healthcare Epidemiology of America (SHEA), which is reported on the Centers for Disease Control (CDC) website (Cohen 2010). No study since Zar 2007 has specified in its methods that patients' randomisation was stratified by disease severity, although four more studies reported response to treatment by severity classes in post hoc subgroup analyses (Cornley 2012; Johnson 2014; Louie 2011; Musher 2009). None of the included studies stratified the randomisation of patients based on cessation or continuation of the antibiotic that caused the CDI.
Excluded studies
Excluded studies and the reasons for exclusion can be found in the Characteristics of excluded studies table.
Risk of bias in included studies
The results of the risk of bias analysis are presented in Figure 2 and Figure 3. Quality concerns include unclear allocation concealment, unclear generation of the allocation sequence and blinding of researchers and participants. Only 11 of the 22 studies reported adequate allocation concealment (Cornley 2012; Dudley 1986; Garey 2011; Keighley 1978; Lagrotteria 2006; Lee 2016a; Louie 2011; Musher 2009; Wullt 2004; Zar 2007). Twelve studies adequately explained the method used for random sequence generation (Cornley 2012; Dudley 1986; Fekety 1989; Garey 2011; Lagrotteria 2006; Louie 2009; Louie 2011; Louie 2015; Mullane 2015; Wenisch 1996; Wullt 2004; Zar 2007). Eleven studies clearly stated that the researchers and the subjects were both blinded (Cornley 2012; Garey 2011; Johnson 2014; Lee 2016a; Louie 2011; Louie 2015; Mullane 2015; Musher 2006; Musher 2009; Wullt 2004; Zar 2007). Two studies stated that patients were blinded, but it was unclear if outcome assessors were blinded (Dudley 1986; Young 1985). Outcome assessors were not blinded in the Fekety 1989 study. Patients were not blinded in the Lagrotteria 2006 study. Louie 2009 was a dose finding study and was not blinded. The Wenisch 1996, De Lalla 1992 and Boero 1990 studies were not blinded. Attempts were made to contact the authors to clarify these issues and as of the submission of this review correspondence was not established.
2.

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
3.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
The included studies have additional quality issues. One diagnostic concern is that 17 of the 22 included studies used C. difficile cytotoxin stool assay as the means of identifying infected patients. Two other studies required stool positive for cytotoxin and growth of C. difficile on culture to establish the presence of CDI (De Lalla 1992; Fekety 1989). Although it was not specifically reported in the text how C. difficile was identified as the cause of CDI in the Lee 2016a study, the strain of C. difficile in each patients was reported in the demographics. Keighley 1978 separated patients who had positive cytotoxin from those patients who had positive cultures. However, the most important quality issue encountered in this review was that no study in which there were dropouts included those dropouts in their analyses, so effectively all participants were analysed as treated. In some studies this had a major impact, since randomisation occurred before disease confirmation, and participants were eliminated after randomisation when they were found not to have CDI. In some cases this was as low as 2% of those randomised (Musher 2009), however, in several studies the dropout rate was as high as 50% due to exclusions after randomisation (Anonymous 1994; Dudley 1986; Keighley 1978). For all studies in this review dropouts were considered to be treatment failures.
For the initial diagnosis of CDI, most included studies did not exclude the presence of other pathogens in the stool as the cause of diarrhoea. It is possible that a patient could have been infected with both C. difficile and another enteric pathogen. The studies do not consistently state whether the initial, offending antibiotic was stopped and if it was stopped, whether a different antibiotic was started in its place. Although Zar 2007 screened stool samples for Shigella, Salmonella and Campylobacter, all patients were negative for these microbes in this study. Additionally, only Young 1985 controlled for patients whose diarrhoea rapidly resolved on its own with the removal of the initial antibiotic by including only those patients with persistent symptoms. For these patients the diarrhoea may not have been caused by C. difficile, although it was present in the stool, or the bacterium was successfully purged by the patient. Recent studies relied on positive toxin assay and diarrhoea alone to make the diagnosis. Some studies included endoscopic confirmation of pseudomembranes, although this was not uniformly done even within these studies. Zar 2007 did include pre‐existing pseudomembrane checks, although no patients had pre‐existing pseudomembranous colitis in this study.
Each specific trial also had its own unique quality issues as well. In the Teasley 1983 study which compared vancomycin to metronidazole, the offending antibiotics believed to have brought about the CDI were said to be discontinued unless they were "essential for treatment." This was also true and quantified by Cornley 2012, Louie 2011 and Johnson 2014 but not allocated to such treatment in a random manner. Teasley 1983 did not indicate how many patients did not have their offending antibiotic discontinued. However these studies and others may confound the interpretation of results as the original antibiotic may have caused diarrhoea by a different mechanism. Dudley 1986 and Zar 2007 and Johnson 2014 (although positive endoscopy at recruitment was required for inclusion) did not have test results for C. difficile in the stool until after randomisation. As a result, all of the patients with diarrhoea that were C. difficile negative were dropped from these studies. Wenisch 1996 had a total of seven dropouts from all of the treatment arms combined. However, Wenisch 1996 did not indicate the treatment allocation of these patients, therefore precluding an accurate intention‐to‐treat analysis of the results. In the Swedish CDI study there was an intermediate measure of cure called 'improvement,' but the criteria for improvement were never described (Anonymous 1994). For the purposes of this systematic review, a conservative approach was taken and the 'improved' patients were not considered to have their clinical symptoms resolved. Fekety 1989 had a dropout rate of 18% and the duration of the antibiotic therapy was determined by each individual physician treating patients. The average duration of therapy was 10 days with a range of 5 to 15 days (Fekety 1989). Dropouts were 13% and 23% respectively in the Wullt 2004 and Musher 2006 studies, making an intention‐to‐treat analysis difficult. However, both studies reported dropouts by allocation group. There were no dropouts in the smaller Lagrotteria 2006 study. Zar 2007 had a dropout rate of 12.8% (22/172), however, the study still achieved predetermined power. Zar 2007 also excluded "suspected or proven life‐threatening intra‐abdominal complications" as well as prescription of either vancomycin or metronidazole in the 14 days prior to enrolment. Zar 2007 retested stool for recurrence at 21 days after cure (defined as symptom‐free and toxin‐free stool at day 10). This protocol was not based on evidence, and it should be noted that current guidelines for management of asymptomatic carriers of C. difficile are conservative. Although Zar 2007 screened for prior antibiotic use, no data were reported on which antibiotics were used. Although Musher 2009 was a multi‐centre randomised trial, 37 of 50 patients came from the principal investigator's hospital. The study excluded patients undergoing enteral tube feeding. Another exclusion criterion was use of "any drug with anti‐C. difficile activity ‐ with the exception of ≤3 oral doses of metronidazole or vancomycin ‐ within 1 week" of entry. It should be noted that Musher 2009 had a dropout rate of 18.4% (9/49) and was terminated early due to "slower‐than‐expected recruitment". It also fell far short of its predetermined sample size of 350.
Selective reporting bias was detected in only two studies (Boero 1990,Fekety 1989). None of the other studies reported very long follow up after innitial description of clinical response, varying from 14 (Musher 2009) to 40 (Wullt 2004) days.
Louie 2009 was a phase IIa trial and was an open label study for mild to moderate C. difficile infection and excluded all patients with severe disease and those who were "not expected to survive the study period". While patients kept a symptom diary, they were only formally observed at study entry and at the end of treatment or withdrawal.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
Metronidazole versus vancomycin Symptomatic cure was improved with vancomycin over metronidazole with the addition of the large study by Johnson 2014. Seventy‐two per cent (318/444) of metronidazole patients achieved symptomatic cure compared to 79% (339/428) of vancomycin patients (RR 0.90, 95% CI 0.84 to 0.97; Analysis 1.1; fixed‐effect; insignificant heterogeneity). A random‐effects model showed a non‐significant benefit for vancomycin (RR 0.92, 95% CI 0.82 to 1.04). We rated the strength of the evidence supporting the symptomatic cure outcome as moderate due to high risk of bias and due to the non‐reporting of additional pathogens that may have caused diarrhoea. Only two studies reported on bacteriologic cure (Teasley 1983; Wenisch 1996), and the strength of evidence for this outcome was very low due to high risk of bias (i.e. lack of blinding) and very serious imprecision (Analysis 1.2). Looking at the severity stratification reported by Zar 2007 (Analysis 1.3), the evidence was also very low due to high risk of bias and very serious imprecision (See Table 1).
1.2. Analysis.

Comparison 1 Metronidazole vs Vancomycin, Outcome 2 Bacteriologic Cure.
1.3. Analysis.

Comparison 1 Metronidazole vs Vancomycin, Outcome 3 Sustained Cure (Combined symptomatic and bacteriologic cure).
One study presented an outcome not used in the other included studies (Zar 2007) which was a combination of symptomatic and bacteriologic cure (Zar 2007). Subsequent studies have only assessed bacteriologic evidence of CDI in patients with persistent or recurrent symptoms and therefore could not report bacteriologic cure (Cornley 2012; Johnson 2014; Louie 2011). Zar 2007 was also the first study to report differential efficacy of the two antibiotics based on disease severity.
Bacitracin versus vancomycin Vancomycin was also superior to bacitracin for achieving symptomatic cure. Twenty‐seven per cent (14/52) of bacitracin patients achieved symptomatic cure compared to 46% (24/52) vancomycin patients (RR 0.58, 95% CI 0.34 to 0.99; Analysis 2.1; no heterogeneity). We rated the strength of the evidence for this outcome as very low due to a high risk of bias (i.e. lack of blinding in one study) and serious imprecision (See Table 4).
2.1. Analysis.

Comparison 2 Bacitracin vs Vancomycin, Outcome 1 Symptomatic Cure.
Teicoplanin versus vancomycin Sustained symptomatic cure was improved with teicoplanin compared to vancomycin, 87% (48/55) of patients who received teicoplanin achieved symptomatic cure compared to 73% (40/55) of patients who received vancomycin (RR 1.21, 95% CI 1.00 to 1.46; no heterogeneity; Analysis 3.1). Bacteriologic cure was reported by one small study. The quality of the evidence was very low in both cases due to a high risk of bias (i.e. lack of blinding) and serious imprecision due to small study sizes (See Table 3).
3.1. Analysis.

Comparison 3 Teicoplanin vs Vancomycin, Outcome 1 Symptomatic Cure.
Fidaxomicin versus Vancomycin Fidaxomicin was more effective than vancomycin for achieving symptomatic cure in two large studies. Seventy‐one per cent (407/572) of fidaxomicin patients achieved symptomatic cure compared to 61% (361/592) of vancomycin patients (RR 1.17, 1.07 to 1.27, Analysis 5.1; no heterogeneity). The quality of the evidence was rated as moderate due to high risk of bias (Table 3)
5.1. Analysis.

Comparison 5 Fidaxomicin vs Vancomycin, Outcome 1 Symptomatic Cure.
Interventions for which there was only a single, usually very small, included study, or in some cases two studies, are reported below. All of these comparisons are subject to very serious imprecision due to the small number of participants and events within the study population and in some cases high risk of bias or inconsistency issues as well, so the quality of evidence for these comparisons was either low or very low.
Vancomycin versus placebo In a very small study (n = 44) vancomycin was found to be superior to placebo for symptomatic cure (RR 9.0, 95% 1.24 to 65.16) and for bacteriologic cure (RR 10.0, 95% CI 1.4 to 71.62). We rated the overall quality of the evidence supporting these outcomes as very low due to serious imprecision and high risk of bias (Keighley 1978).
Rifaximin versus Vancomycin In a very small study of 20 patients symptomatic cure was not found to be significantly different in those treated with Rifaximin compared to vancomycin (RR 0.9, 95% CI 0.69 to 1.18). We rated the quality of the evidence as very low due to high risk of bias and serious imprecision (Boero 1990).
Fusidic acid versus vancomycin No difference in symptomatic cure was found in a single small study comparing vancomycin to fusidic acid (Wenisch 1996). Seventy‐seven per cent (24/31) of patients who received vancomycin achieved symptomatic cure compared to 66% (19/29) of patients who received fusidic acid (RR 0.85, 95% CI 0.61 to 1.17). There was also in no difference in bacteriologic cure (RR 0.68, 95% CI 0.44 to 1.06). The quality of the evidence was rated as very low due to high risk of bias and serious imprecision). Wullt 2004 also studied this comparison in 131 patients but reported no recurrence data. Addition of Wullt 2004 to the analysis did not greatly change the results (RR 0.94, 95% CI 0.83 to 1.08) or the GRADE rating.
Nitazoxanide versus vancomycin Symptomatic cure was achieved in one small study (n = 49) almost equally with vancomycin and nitazoxanide. Sixty‐seven per cent (18/27) of vancomycin patients were cured compared to 73% (16/22) of patients who received nitazoxanide (RR 1.09, 95% CI 0.75 to 1.58). The quality of evidence was low due to serious imprecision (Musher 2009).
Metronidazole versus Nitazoxanide A single small study found little difference in the efficacy of metronidazole and nitazoxanide in achieving symptomatic cure (RR 0.97, 95% CI 0.70 to 1.34). The quality of the evidence was very low due to high risk of bias (i.e. attrition) and serious imprecision (Musher 2006).
Metronidazole versus Metronidazole and Rifampin In a single small study (n = 39) there was little difference between metronidazole alone and metronidazole combined with rifampin in achieving symptomatic cure (RR 1.09, 95% CI 0.49 to 2.41). The quality of the evidence was very low due to high risk of bias and serious imprecision (Lagrotteria 2006).
Metronidazole versus Teicoplanin In one small study (n = 59) teicoplanin was not significantly more effective than metronidazole in achieving symptomatic cure (RR 0.87, 95% CI 0.69 to 1.09). The quality of the evidence was very low due to high risk of bias (i.e. lack of blinding and failure to exclude other pathogens) and serious imprecision (Wenisch 1996).
Metronidazole versus Fusidic Acid Metronidazole was no more effective than fusidic acid for achieving symptomatic cure in two small studies (RR 1.00, 95% CI 0.78 to 1.30). Similar results were seen in these two small studies for bacteriologic cure (RR 1.02, 95% CI 0.83 to 1.25; no heterogeneity). The quality of the evidence was very low due to high risk of bias (i.e. lack of blinding and attrition) and serious imprecision (Wenisch 1996; Wullt 2004).
Teicoplanin versus Fusidic Acid In one small study (n = 57) teicoplanin was more effective then fusidic acid for achieving symptomatic cure (RR 1.36, 95% CI 1.02 to 1.83), and bacteriologic cure (RR 1.73, 95% CI 1.19 to 2.51). The quality of evidence was very low due to high risk of bias (i.e. no blinding and failure to rule out other enteric pathogens) and serious imprecision (Wenisch 1996).
Dose Finding One small study (n = 56, Fekety 1989), found no difference in symptomatic cure between high and low dose regimens of vancomycin (RR 0.95, 95% CI 0.65 to 1.38) in one small study. For fidaxomicin, also known as Opt‐80 (n = 48, Louie 2009), it appeared that the higher (200 mg) dose was more effective than low dose for symptomatic cure (RR 1.26, 95% CI 1.03 to 1.54). Both studies were very small and also had a high risk of bias (i.e. attrition, lack of blinding), and serious imprecision. The quality of the evidence was very low for both comparisons.
Dose Timing The dose of teicoplanin was maintained constant but administered in varying intervals in this small single study (n = 92). The dose was administered either twice or four times a day. There was no difference in symptomatic cure between doses administered twice or four times daily (RR 0.57, 95% CI 0.27 to 1.20). The study was terminated prematurely due to a higher than expected failure rate in the twice daily group. The quality of evidence in the study was very low due to high risk of bias (i.e. attrition) and serious imprecision (Anonymous 1994).
Rifaximin to diminish relapse risk After primary therapy of CDI with either metronidazole or vancomycin, rifaximin was found in one study to be insignificantly more effective than placebo for avoiding relapse (RR 0.61, 95% CI 0.36 to 1.02). The quality of evidence was low due to high risk of bias and imprecision (Garey 2011).
Cadazolid versus Vancomycin In this small single study , three different doses of a new antibiotic, cadazolid, were compared to vancomycin in 82 patients. The results for all three doses were very similar and so we combined the dose groups as a single exposure to vancomycin, also with very similar effect (RR 1.11, 95% CI 0.50 to 2.00). The quality of the evidence was moderate due to imprecision (Louie 2015).
LFF517 versus vancomycin This new antibiotic, as yet without a name, was compared to vancomycin in 76 randomised patients of whom 32 did not finish the study (RR 0.87, 95% CI 0.49 to 1.57). The quality of the evidence was very low due to high risk of bias attrition and serious imprecision (Mullane 2015).
Surotomycin versus vancomycin One study (n = 209) compared two doses of surotomycin, a new antibiotic, to vancomycin (Lee 2016a). The results of the two doses were almost identical so these groups were combined in comparison to vancomycin. Surotomycin was marginally more effective than vancomycin for maintaining a sustained clinical cure (RR 1.22, 95% CI 0.95 to 1.56). The quality of evidence was moderate due to imprecision.
Older guidelines discuss treatment of unresponsive disease with an increased dose of vancomycin 500 mg four times daily, intravenous metronidazole 500 mg three times daily and rifampicin 300 mg twice daily. However, this regimen was shown to have no clinical benefit in primary CDI (Garey 2011; Lagrotteria 2006). The criteria defining severity stratification have differed across the studies included in this review (Bauer 2009; Cohen 2010; HPA 2016Musher 2009; Zar 2007). Lactate > 5 is perhaps the most valid marker of extreme disease severity, but it is associated with a 75% operative mortality. Therefore an earlier marker and proven therapy would be of more use (Cohen 2010). No randomised studies address this issue.
The patients with relapse of diarrhoea after treatment were re‐treated in various ways. Dudley 1986 re‐treated with the same antibiotic and Young 1985 re‐treated with the antibiotic from the other arm of the study. De Lalla 1992 stated that the patients with relapse were re‐treated, but analysed the results in aggregate, making it impossible to compare the antibiotics for efficacy. The Fekety 1989 vancomycin dose‐finding study re‐treated all nine relapses with vancomycin and subsequently cured these patients. Wenisch 1996 treated recurrent disease with teicoplanin with a reported a 100% cure rate. Louie 2009 re‐treated with either metronidazole or vancomycin, but did not report on the success of this treatment. The remaining studies did not report on the re‐treatment of relapses. As in the previous paragraph, the focus of randomised trials has been the prevention of recurrence and not its treatment. Johnson 2014 did enroll patients with recurrent disease as well as primary disease, but there is no mention in the paper that the recurrent patients were randomised separately so that the reporting of outcome of primary versus recurrent patients is once again a post hoc subgroup analysis (Assmann 2000).
Secondary outcome: Death The secondary outcome measured in this review, death, occurred infrequently in all of the studies. There were 140 deaths among the patients in the included studies. All of the reported deaths were described by the study authors as being due to the underlying morbidity that preceded CDI rather than the antibiotics used to treat CDI (Table 5). Although most of these patients had severe diseases prior to the diagnosis of CDI, it seems plausible that the CDI or antibiotic therapy could have contributed to their deaths. Dudley 1986 reported one death and one partial colectomy after failed treatment of CDI with bacitracin. Both patients were crossed‐over to vancomycin before their adverse outcome, with the first patient improving prior to his death, and the second patient continuing to have CDI. Fekety 1989 reported one death in each of the two vancomycin treatment arms. The Swedish CDI study reported one death in the two times per day teicoplanin group, but the authors did not specify if it was part of the CDI sub‐section of the study or part of the general dosage‐finding portion of the study (Anonymous 1994). Teasley 1983 had three deaths in the whole study, including two deaths in the vancomycin treatment arm. The authors attributed all three deaths to underlying illness and not to CDAD or the antibiotic therapy, a common claim in other studies as well. De Lalla 1992 reported two deaths and Musher 2009 reported one death. Both studies did not specify the treatment group and attributed the deaths to the patients' underlying disease. Zar 2007 reported eight deaths, four in each treatment group, but did not comment on the cause of mortality. Cornley 2012 and Louie 2011 reported 74 deaths in their two large studies, all felt to be due to the patient's primary disease and not associated with either antibiotic being investigated. Johnson 2014 reported 23 and 16 deaths in the metronidazole and vancomycin groups respectively again attributed to the patients' underlying comorbidity. There were two deaths in Louie 2015. Lee 2016a reported four deaths, two in each of the two treatment groups, all attributed to underlying disease.
1. Summary study.
| Study | n=Total | Deaths |
Harms due to Intervention |
Attrition% |
Stratified by Severity |
| Anonymous 1994 | 49 | 1 | joint pain | <10% | |
| Boero 1990 | 20 | 0 | 0 | 0 | |
| Cornley 2012 | 535 | 20 Fid 17 Van |
none | 4.9% | Yes post randomisation |
| De Lalla 1992 | 51 | 2 | NR | 10% | |
| Dudley 1986 | 62 | 0 | NR | 52% | |
| Fekety 1989 | 46 | 0 | NR | 18% | |
| Garey 2011 | 79 | skin rash | 14% | ||
| Johnson 2014 | 1118 | 23 Met 16 Van |
none | 1.8% | Yes post randonisation |
| Lagrotteria 2006 | 39 | 2 | 0 | ||
| Lee 2016a | 209 | 4 | 5 | 7% | |
| Louie 2009 | 49 | 0 | 4% | ||
| Louie 2011 | 629 | 16 Fid 21 Van |
elevated liver enzymes |
5.2% | Yes post randomisation |
| Louie 2015 | 82 | 2 | 7.3% | ||
| Mullane 2015 | 72 | 0 | 44% | ||
| Musher 2006 | 142 | 4 | 23% | ||
| Musher 2009 | 50 | 0 | 2% | Yes post randomisation |
|
| Teasley 1983 | 101 | 2 | NR | 7% | |
| Wenisch 1996 | 126 | 3 | 5.5% | ||
| Wullt 2004 | 131 | 0 | 26% | ||
| Young 1985 | 42 | 0 | NR | 0 | |
| Zar 2007 | 172 | 7 | 13% | Yes but uncertain when |
|
| TOTAL | 140 |
NR: None reported
Drug‐related adverse events (DRAE) DRAEs were reported very differently in the included studies. It was reported that there were none (or not reported) in six studies (De Lalla 1992; Dudley 1986;Fekety 1989;Teasley 1983;Young 1985;Zar 2007). At the opposite end of the spectrum, quite remarkably high incidences of what was described as therapy emergent adverse events (TEAE) were reported by Cornley 2012 (75% with fidaxomicin and 71.5% for vancomycin, 26.5% and 22.3% described as serious), Louie 2011 (62.3%, 25% serious for fidaxomicin, 60.4%, 24.1% serious for vancomycin), and Johnson 2014 (35.3% vancomycin, 31.2% metronidazole). All three studies reported that around 1% of these TEAEs were due to study medications. All the other studies, attributed adverse events to the many serious co‐morbidities these patients had that gave rise to the CDI. Anecdotally reported DRAEs included pruritis, skin rash (Garey 2011), joint pain (Anonymous 1994), nausea (Zar 2007) and vomiting (Wenisch 1996), and elevation of liver enzymes (Louie 2011). Zar 2007 reported two adverse events that were clearly drug‐related, one patient in each treatment group had nausea and were switched to the other drug and completed therapy (as a crossover). None of the TEAEs are presented in a way that allows quantification of DRAE across studies in a summary of findings table. Most of the adverse events in Lee 2016a were indistinguishable from symptoms of CDI. Five patients ceased taking the medications due to unspecified adverse events (Lee 2016a).
Cost Two studies quantified the cost of treatment (Musher 2009; Wenisch 1996). Wenisch 1996 compared four different antibiotics. The cost data calculated at the time of the initial publication of this review for a course of treatment were: Metronidazole USD 35; Fusidic acid USD 287; Vancomycin USD 2030; and Teicoplanin USD 3430 (Bricker 2005). Musher 2009 compared two antibiotics. The cost data were as follows: Vancomycin USD 618 and Nitazoxanide USD 316 (Musher 2009).
More recent cost data (July 2016) for a 10 day course of treatment includes Metronidazole #20 500 mg USD 13 (Health Warehouse); Vancomycin 125 mg USD 1779 (Walgreens for 56 tablets); Fidaxomicin #20 200 mg: USD 3453.83 or more (Optimer Pharmaceuticals) and Teicoplanin approximately USD 83.67 (GBP 71.40, British National Formulary).
Discussion
Perhaps the biggest change for this update when compared to previous versions of this review is the change in outcome assessment. Recurrence rates have been reported to be high after initial resolution of CDI symptoms and assays, topping 20% (Vardakas 2012). There has been increasing emphasis on addressing the risk of recurrence of CDI as part of the primary therapy, with the desired outcome being a sustained symptomatic cure and bacteriologic cure (Cornley 2012; Garey 2011; Johnson 2012; Louie 2011). The previous set of outcomes for this systematic review have been condensed to: sustained symptomatic and bacteriologic cure, along with adverse events of the intervention and death. This should not just instruct how best to treat CDI patients but also provide the optimal protection for other hospitalised patients. In addition the number of outcomes assessed compared to previous versions is decreased by more than 50%, thus addressing the issue of multiplicity (Sedgwick 2014).
Summary of main results
The main results of this update have changed considerably since 2011. Moderate quality evidence suggests that vancomycin is more effective than metronidazole for achieving symptomatic cure, but the evidence is less supportive for bacteriologic cure (very low quality evidence; See Analysis 1.1, Analysis 1.2, Analysis 1.3, Table 1). Moderate quality evidence suggests that fidaxomicin is superior to vancomycin for achieving symptomatic cure with no direct data on bacteriologic cure (See Analysis 5.1, Table 3). Very low quality evidence from older smaller studies suggests that teicoplanin is a potentially effective drug compared to vancomycin or metronidazole (See Analysis 3.1, Table 2). Surotomycin was marginally superior to vancomycin (Analysis 6.1) based on a single study (n = 209). The use of the remaining antibiotics for treatment of CDI including bacitracin, fusidic acid, nitazoxanide, cadazolid, LFF571, rifaximin and rifampin was not supported by the evidence. The evidence supporting a specific therapy for bacteriologic cure is less conclusive, principally because the more recent and largest studies have not reported data that would allow assessment of bacteriologic cure (Cornley 2012; Johnson 2014; Louie 2011). Rifaximin given after a course of vancomycin of metronidazole may diminish the likelihood of recurrent CDI (Garey 2011).
Overall completeness and applicability of evidence
The available evidence applies directly to the goals of this review: to assess the efficacy of antibiotic therapy for CDI. The results of this review are applicable to patients with mild CDI who can tolerate oral antibiotics. All of the included studies excluded patients with severe CDI.
Quality of the evidence
The only placebo‐controlled study of primary CDI was Keighley 1978. This is important since it could have provided evidence on how likely it is that CDI would spontaneously resolve. Keighley 1978 assessed the efficacy of oral vancomycin 125 mg taken four times a day for five days compared to placebo for the treatment of pseudomembranous colitis. All patients were recruited from a surgical ward and blinded during the five day treatment period. It is unclear from the study report whether the outcome assessor was blinded or not. Broad inclusion criteria were used. Any patient with postoperative diarrhoea was recruited. This led to the randomisation of patients with postoperative diarrhoea not related to C. difficile. The only exclusion criterion was a previous history of pseudomembranous colitis. The study not only divided patients by their treatment regimen, but also divided patients into three groups based on stool analysis results. Group one had stool that was culture positive for C. difficile and positive for C. difficile cytotoxin. Group two had stool that was culture positive for C. difficile only. Group three had stool that was culture negative and negative for cytotoxin. Keighley 1978 reported that only 21 of 44 randomised patients had some evidence of C. difficile infection. Of these patients 16 were toxin positive and 5 were culture positive. Twelve of these patients were in the vancomycin group and nine were in the placebo group. Keighley 1978 defined clinical response measured by stool frequency as resolution. Clinical response was higher in patients who received vancomycin in groups one and two, but response was not affected by treatment in group three. Keighley 1978 describes the numbers of patients taking vancomycin or placebo in whom stool converted to negative for C. difficile strain, negative for fecal cytotoxin and negative for histological evidence of pseudomembranous colitis. There are several major bias concerns with the Keighley 1978 study. The number of patients was small, and follow‐up was inadequate. During the convalescence period, there were specimens that were either not taken or mislaid. Two‐thirds of the patients in the vancomycin group fell into this category. Follow‐up conclusions were based on only four patients from the total of 12 patients with evidence of C. difficile infection that received vancomycin.
As mentioned above, Zar 2007 has had a great influence because of its stratification of outcome assessment by disease severity. Because of this, potential issues bear special attention. Despite the publication date, recruitment for this study ended in 2002, before the 027 strain of C. difficile reached the Chicago area, where the Zar 2007 study was conducted, and the severity of disease seen in this mutation had not yet been witnessed. Yet, in spite of excluding the sickest patients with CDI from the study, eight participants died within five days of the onset of therapy and were excluded from further analysis rather than being regarded as treatment failures. Randomisation also occurred before the diagnosis of C. difficile was established, and 14 randomised subjects were subsequently found to be C. difficile negative and excluded from analysis. If randomisation, was done prior to severity stratification, this may not sufficiently validate this concept of severity stratification in antibiotic choice as described by Zar 2007.
Two very large and for the most part well conducted studies of identical design compared fidaxomicin and vancomycin (Cornley 2012; Louie 2011). The quality issues in these studies are fairly minor compared to the above studies. The dropout rate was only 5%. These studies utilized a non‐inferiority design. The necessity of doing this rather than a standard analysis was not discussed, although the design itself has been a frequent topic of discussion elsewhere (Garattini 2007; Gotzsche 2006; Soonawala 2010). An intention‐to‐treat analysis (ITT) was not reported in either publication, rather a 'modified intention‐to‐treat' which excluded a number of dropouts and a per protocol analysis, excluding even more participants were reported. Again the purpose of this was not discussed, and curious insofar as including the dropouts as treatment failures in a standard ITT analysis demonstrates the superiority of fadixamicin over vancomycin (Analysis 5.1). The studies were both funded by Optimer Incorporated and the Louie 2011 manuscript was written by a part time employee of the company.
The most recent added studies (Louie 2015; Mullane 2015) are small and, like the previous two studies, theses studies also do not present results as a pure ITT analysis but a modified ITT or per protocol fashion. In this review, we conducted all analyses using the original number of patients randomised to each group as the denominator.
Potential biases in the review process
We believe that our changes in outcome assessment reduce the risk of bias in the review process. The results of this review are now more clinically realistic, and are now less subject to multiplicity (Sedgwick 2014).
Agreements and disagreements with other studies or reviews
Bishara 2007 found no statistically significant difference in symptomatic cure between metronidazole and vancomycin in a pooled analysis of three studies (RR 0.93; 95% CI 0.80 to 1.09) treating exclusions, deaths and relapses as treatment failures. This result is similar to the pooled analysis reported in this review prior to the inclusion of Johnson 2014. In a very heterogenous systematic review that included retrospective studies and prospective cohorts as well as RCTs, and non‐antibiotic therapies as well, Vardakas 2012 also found very similar results when comparing metronidazole to vancomycin for both initial symptomatic response and recurrence risk. Drekonja 2011 published a systematic review of antibiotic therapy for CDI that was limited to RCTs. Eleven studies were included in this review, all of which are also included in the present review and it also was published before the six studies that were added to this update. The outcomes assessed are similar to those presented in the last update of this review. The outcomes assessed in this update have been reduced to symptomatic cure (initial clinical response minus recurrence), bacteriologic cure (initial clearance of C. difficile from the stool, minus recurrence), adverse events related to the intervention, and death. Di 2015 reports a systematic review comparing metronidazole to vancomycin with four RCTs (all included in this review) combined with two cohort studies. The results are largely similar to this review. Bagdasarian 2015 presents an broadly based review that deals extensively with diagnostic techniques and non‐antibiotic therapy as well as antibiotic treatment. It cites the major published treatment guidelines including those shown in Table 6 as well as the American College of Gastroenterology guidelines. No reductive meta‐analysis was done for this review. Stewart 2013 was a systematic review of surgical mortality in critically ill patients with CDI and as such is the first review of this particular spectrum of CDI, an area where there are no RCTs. Surgery was found to be superior to medical therapy once patients had reached the threshold for severity (OR 0.703, 95% CI 0.497 to 0.994). AHRQ 2016 updated their systematic review and guidelines for diagnosis, prevention and therapy for CDI. This review was not limited to RCTs or antibiotic therapy.
2. Published Guidelines for Antibiotic Treatment of CDAD.
| HPA1 | ESCMID2 | SHEA & IDSA3 | |
| MILD CDAD | Stop inciting antibiotic and observe, or oral metronidazole 500 mg three times daily Alternate dosing also recommended and change to vancomycin if no better in 4 days |
Stop inciting antibiotic and observe, or oral metronidazole 500 mg three times daily | Stop inciting antibiotic, or oral metronidazole 500 mg three times daily for 10 days |
| SEVERE CDAD | Oral vancomycin 500 mg four times daily with tapering | Oral vancomycin 125 mg four times daily for 10 days | Oral vancomycin 125 mg four times daily for 10 days |
| SEVERE AND CANNOT TOLERATE ORAL MEDS | Intravenous metronidazole and vancomycin via nasogastric tube or enemas four times daily | Same | Same |
| SURGERY | For toxic megacolon or lactate > 5 | For perforation, toxic megacolon, Ileus, lactate > 5 | |
| RECURRENCE | First as Primary 2nd: oral vancomycin with taper |
Same |
Of interest is one of the excluded studies (Johnson 1992). Johnson 1992 was a randomised, placebo‐controlled, non‐blinded trial of oral vancomycin 125 mg four times a day for 10 days versus oral metronidazole 500 mg two times a day for 10 days versus placebo for the treatment of asymptomatic carriers of C. difficile. This is in fact the only well conducted placebo‐controlled trial related to CDI. Keighley 1978, also a placebo‐controlled trial, suffers from excessive attrition, small size, selection bias and lost specimens. In Keighley 1978 asymptomatic C. difficile excretors were identified by rectal swab during infection control surveillance of medical and surgical inpatients at a US Veterans Affairs hospital. Patients were included if they did not have diarrhoea or abdominal symptoms and were not allergic to vancomycin or metronidazole. C. difficile infection was identified by stool culture and cytotoxin assay. The main outcome was presence or absence of C. difficile in the stool during treatment and two months after treatment. A total of 30 patients were equally divided into the three treatment arms. C. difficile organisms were not detected during and immediately after treatment in 9/10 patients treated with vancomycin compared with 3/10 patients treated with metronidazole and 2/10 patients in the placebo group. In contrast, by the end of follow‐up ranging from 40 to over 90 days, C. difficile organisms were not detected in 3/10 patients treated with vancomycin compared with 5/10 patients treated with metronidazole and 8/10 patients in the placebo group. The authors concluded that asymptomatic fecal excretion of C. difficile was transient in most patients and treatment with metronidazole was not effective for eliminating C. difficile from stool. Although treatment with vancomycin was temporarily effective, it was associated with a significantly higher rate of C. difficile carriage two months after treatment and therefore was not recommended for asymptomatic carriers of C. difficile. Although these results were not statistically significant, they are of concern and have stimulated further investigation of strategies to prevent the long‐term carrier state of C. difficile, especially in hospitalised patients (Cornley 2012; Garey 2011; Louie 2011).
Johnson 1992 suggests that the best means of accomplishing the second goal of treatment ‐ elimination of the C. difficile bacterium from the stool of hospitalised patients to prevent spread of infection, is best accomplished with no antibiotic at all. The study by Garey 2011 would suggest otherwise but like many of the other smaller studies, this study was plagued by a high number of dropouts. While vancomycin has high in vitro activity against C. difficile, it is still not certain that CDI is best treated with this or any other antibiotic (Bartlett 1980), especially in mild disease. Antibiotic synergy has only been investigated in one study (Lagrotteria 2006), and no clinical benefit was seen by adding rifampin to metronidazole.
A propos of this last point, if one does decide to treat CDI, it should be with two goals: improvement of the patient's clinical condition and prevention of spread of C. difficile infection to other patients. Given these two considerations, one should choose the antibiotic that brings both symptomatic cure and bacteriologic cure. In this regard (Johnson 2012), teicoplanin might be a good choice, because it was significantly better than vancomycin for bacteriologic cure and has borderline superior effectiveness in terms of symptomatic cure. Teicoplanin is not available in the United States for unknown reasons, which must be taken into account (Hopkins 2009). Fidaxomicin also presents a new good option, although cost issues have been raised. Now that fidaxomicin has been approved for use in CDI, post marketing surveys should be informative. Most treatment guidelines still recommend metronidazole or no treatment for mild disease and vancomycin for more severe or recurrent illness (Table 6).
Wenisch 1996 is an important study because it directly compares several antibiotics. This avoids making unrealistic assumptions about relative efficacy made across several randomised trials (Baker 2002). The virulence of C. difficile underlines the need to consider new treatments. Data on four new antibiotics including fidaxomicin, cadazolid, LFF571 and surotomycin are included in this update (Cornley 2012; Louie 2011; Louie 2015; Mullane 2015). Several other antibiotics are in development (Locher 2014; Miesel 2014; NCT01983683; NCT01987895). The concept of toxin binding has been investigated and deserves further attention (Louie 2006). A C. difficile vaccine has also been developed and may provide another alternative to antibiotic treatment (Sougioultzis 2005). Novel therapies, some long forgotten, such as fecal repopulation are reappearing (Bowden 1981; Khoruts 2010; Van Nood 2013).
The severity scale designed by Zar 2007 is important as, for the first time, it attempts to stratify those affected by CDI into mild versus severe disease. Such a scale would be valuable, not only for the standardisation of future studies, but for use in clinical practice, particularly as the study by Johnson 1992, for example, suggests that mild disease may be successfully treated without antibiotics (Table 6). The severity scale designed by Zar 2007 uses any two of the following to indicate severe disease: pyrexia (≥38.3°C), serum albumin of < 2.5 mg/dl, leukocytosis of 15,000 cells/mm3 and age (≥60 years). There is some discrepancy between subsequent studies as to the appropriate cut‐off points used. For example, Louie 2009 was specifically designed to include patients with mild or moderate disease, and did not exclude patients as 'severe' unless they had a leukocytosis of ≥30,000 cells/mm.3 The concept of disease severity stratification for specific therapeutic choice has been widely accepted as shown in most guideline publications (Table 6).
These recommendations should be treated with caution because there is poor evidence supporting recommendations for the treatment of severe disease in Table 6 due to the exclusion of such patients in the included studies. Patients in all included studies in this review were well enough to be treated with oral antibiotics alone. In addition, the criteria for defining the most severely ill patients in the Zar 2007 study were not at that time clearly associated with prognosis. Most importantly the stratification may have occurred after randomisation and after a number of exclusions, constituting a post hoc subgroup analysis by disease severity. The results of such analyses have been described as best used for exploratory purposes and should not affect trial conclusions (Assmann 2000). No statistical tests of interaction were performed. Yet five subsequent trials (Cornley 2012; Johnson 2014; Louie 2009;Louie 2011; Musher 2009), none of which report stratification for severity prior to randomisation, and three recent published guidelines (Bauer 2009; Cohen 2010; HPA 2016), have adopted this principal of stratification. These guidelines endorse the efficacy of vancomycin for severe disease (Table 6).
The best means of identifying C. difficile is somewhat controversial (Thomas 2003). Gerding 1995 in their position paper for the Society for Healthcare Epidemiology of America recommend either a combination of stool culture for C. difficile and cytotoxin assay or stool cytotoxin testing alone. Gerding 1995 states that most strains of C. difficile produce either both toxin A and toxin B, or neither. Additionally, treatment of patients with diarrhoea who are toxin negative, but culture positive is controversial in that non toxigenic strains of C. difficile are not considered pathogenic (Gerding 1995). The American College of Gastroenterology practice parameters on the diagnosis and treatment of CDI state that C. difficile can be identified by either positive culture or toxin assay and that if both tests are negative and the diarrhoea persists, then additional stool studies with the same or different tests should be ordered (Fekety 1997). In light of these two practice guidelines, papers identifying C. difficile by either culture or toxin assay were included in our analysis. The recent CDC paper (Lessa 2015), a prevalence study in the USA, uses a new stool test, the Nucleic Acid Amplification Test and not symptoms for the diagnosis of CDI. This is a marked change from all the studies included in this review. The current use of polymerase chain reaction (PCR) is still controversial, risking potential over diagnosis and treatment (Bagdasarian 2015).
The most recent data UK show a more than 50% drop in CDI mortality through 2013, after a rapid rise in mortality up to 2007 (HPA 2016; Karas 2010; Redelings 2007). This may be due to a greater than 50% drop in incidence of CDI in the UK. This may be the result of effective prevention and isolation measures (HPA 2016), and improving awareness of causative antibiotics (Privitera 1991; Thomas 2003; Novell 2010). This is a marked difference from the recently presented prevalence data from the CDC in the USA, in which no decline is documented in the USA, rather a continuing rise (Lessa 2015). This may in part be due to the increased sensitivity of newer diagnostic test for CDI.
Cost was investigated in this update. Previous versions of this review had data that are now far out of date. In short, the cost of teicoplanin has decreased (but not vancomycin) and for now at least the cost of a course of fidaxomicin is in excess of USD 3400.
Authors' conclusions
Implications for practice.
Although antibiotic therapy for CDI is universally prescribed, the evidence specifically for any antibiotic therapy for CDI is not supported by randomised trials, as the only placebo‐controlled study is inadequate. The same can be said for many surgical treatments of this potentially fatal disease, and one cannot envision those RCTs ever being done. No firm conclusions can be drawn regarding the efficacy of antibiotic treatment in severe CDI as most studies excluded patients with severe disease. But for an asymptomatic carrier of C. difficile or one with mild symptoms, the indication for treatment is more doubtful. The lack of any 'no treatment' control studies does not allow for any conclusions regarding the need for antibiotic treatment in patients with mild CDI beyond withdrawal of the initiating antibiotic. This review also cannot define the efficacy or timing of cessation of the initial, offending antibiotic, because none of the included studies applied a strict or consistent protocol in regard to its continuation. As a corollary, this review cannot establish the proper amount of time between cessation of the initial antibiotic and beginning antibiotic therapy for CDI, to see if the offending antibiotic might be causing diarrhoea by a mechanism other than C. difficile overgrowth, as none of the above studies addressed this research question. When an antibiotic for CDI is chosen, moderate quality evidence suggests that vancomycin is superior to metronidazole and fidaxomicin is superior to vancomycin. The differences in effectiveness between these antibiotics were not too large and the advantage of metronidazole is its far lower cost compared to the vancomycin and fidaxomicin. Current guidelines generally recommend metronidazole or vancomycin depending on the clinical situation. Teicoplanin may be a more effective alternative to vancomycin but the quality of the evidence is very low.
Implications for research.
Given the uncertainty involved in antibiotic therapy for CDI, adequately powered, placebo‐controlled, randomised trials that assess (1) cessation of the initial antibiotic and (2) timing and duration of CDI therapy are recommended. This is really important since many antibiotics can cause diarrhoea separate from CDI and by a different mechanism. In such cases, withdrawal of that antibiotic cures the diarrhoea. All included studies treated the antibiotic that gave rise to CDI in a haphazard way, in many cases treating doctors feeling that the primary disease needed ongoing treatment. The rigorousness of CDI diagnosis suffers in this situation. Vancomycin or metronidazole should be considered for these studies. Teicoplanin should be considered for further investigation because it may be superior to both of these antibiotics. A trial comparing the two cheapest antibiotics, metronidazole and teicoplanin, would be of interest. Such trials would require the randomisation of a large number of patients. These comparisons may help to determine which, if any, antibiotic is most suitable for treating CDI. There should be strict criteria for the diagnosis of CDI: prior exposure to antibiotics, persistent diarrhoea that does not respond to removal of the offending antibiotic and supportive therapy. The primary outcomes should be sustained symptomatic cure, defined as cessation of symptoms during the time of treatment and for 30 to 60 days after, and evidence of sustained bacteriologic cure, defined as symptomatic cure above. Secondary outcomes should include adverse events related to the intervention, surgery and death. If disease severity is to be a factor in antibiotic choice, severity should be assessed prior to randomisation.
It would be useful to develop a universal system for grading the severity of CDI. The increasing severity of the disease also highlights the need to investigate antibiotic treatment in severely ill patients with CDI. Data for this group are currently lacking. Other issues that need to be addressed in future trials include: the treatment of recurrent symptoms, the use of intravenous compared to oral antibiotics, antibiotic resistance, synergistic antibiotic combinations and other types of interventions (e.g. the use resins combined with antibiotics, vaccines, or fecal repopulation).
The most recent guidelines used a new assay that has increased sensitivity, with the implication from the CDC that this PCR test is now the gold standard. However the sensitivity of this method has resulted in the possible over diagnosis of CDI (Bagdasarian 2015), which may be in part be a result of the increasing incidence of CDI (Lessa 2015). Despite the growing popularity of PCR, none of the included studies used PCR for the diagnosis of CDI.
What's new
| Date | Event | Description |
|---|---|---|
| 26 January 2017 | New search has been performed | A new search was ran on 26 January 2017. Seven new studies were added. |
| 26 January 2017 | New citation required and conclusions have changed | Updated review with some new conclusions and new authors. There are three changes for this update of a review last published in 2011. First is a change in the protocol, and specifically in the outcomes being assessed. It has become apparent that the most valid outcomes to assess the efficacy of treatment for Clostridium difficile infection (CDI) is sustained cure (defined as initial resolution of symptoms and no recurrence of CDI) and bacterial cure (defined as conversion from a stool positive for any CDI assay to negative and no recurrence of CDI).These outcomes, plus harms of the interventions and cost of a usual course of therapy, have become the primary outcomes reported in this review to diminish multiplicity. |
Acknowledgements
Partial funding for the Cochrane IBD Group (April 1, 2016 ‐ March 31, 2018) has been provided by Crohn's and Colitis Canada (CCC).
Appendices
Appendix 1. Search strategies
MEDLINE (Ovid)
[mp=title, abstract, original title, name of substance word, subject heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier]
1. exp Anti‐Bacterial Agents/
2. exp Aminoglycosides/
3. exp Cephalosporins/
4. (Vancomycin or Metronidazole or Fusidic acid or Nitazoxanide or Teicoplanin or Rifampicin or Rifaximin or Bacitracin or Fidaxomicin or Amoxicillin or Azithromycin or Cephalosporin* or Cephalexin or Ciprofloxacin or Clarithromycin or Clindamycin or Doxycycline or Erythromycin or Flouroquinolone* or Levofloxacin or Macrolide* or Nitrofurantoin or Penicillin or Tetracycline or Trimethoprim or antibiotic* or anti‐bacterial* or anti bacterial* or antibacterial* or bacteriocid* or bactericid* or antimicrobial* or anti‐microbial*).mp.
5. 1 or 2 or 3 or 4
6. exp Clostridium difficile/
7. exp Clostridium Infections/
8. exp Diarrhea/dt [Drug Therapy]
9. exp Enterocolitis, Pseudomembranous/dt [Drug Therapy]
10. (Clostridium difficile or difficile or pseudomembranous enterocolitis).mp.
11. 6 or 7 or 8 or 9 or 10
12. 5 and 11
13. randomised controlled trial.pt.
14. controlled clinical trial.pt.
15. randomized.ab.
16. placebo.ab.
17. clinical trial.sh.
18. randomly.ab.
19. trial.ti.
20. 13 or 14 or 15 or 16 or 17 or 18 or 19
21. humans.sh.
22. 20 and 21
23. 12 and 22
EMBASE (Ovid)
[mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword]
1. *antiinfective agent/
2. (Vancomycin or Metronidazole or Fusidic acid or Nitazoxanide or Teicoplanin or Rifampicin or Rifaximin or Bacitracin or Fidaxomicin or Amoxicillin or Azithromycin or Cephalosporin* or Cephalexin or Ciprofloxacin or Clarithromycin or Clindamycin or Doxycycline or Erythromycin or Flouroquinolone* or Levofloxacin or Macrolide* or Nitrofurantoin or Penicillin or Tetracycline or Trimethoprim or antibiotic* or anti‐bacterial* or anti bacterial* or antibacterial* or bacteriocid* or bactericid* or antimicrobial* or anti‐microbial*).m_titl.
3. 1 or 2
4. *Clostridium difficile/
5. *Clostridium infection/
6. *diarrhoea/dt [Drug Therapy]
7. *pseudomembranous colitis/dt [Drug Therapy]
8. (Clostridium difficile or difficile or pseudomembranous enterocolitis).mp.
9. 4 or 5 or 6 or 7 or 8
10. 3 and 9
11. randomised controlled trial/
12. randomization/
13. controlled study/
14. multicenter study/
15. phase 3 clinical trial/
16. phase 4 clinical trial/
17. double blind procedure/
18. single blind procedure/
19. ((singl* or doubl* or trebl* or tripl*) adj (blind* or mask*)).ti,ab.
20. (random* or cross* over* or factorial* or placebo* or volunteer*).ti,ab.
21. 16 or 13 or 17 or 19 or 12 or 18 or 14 or 11 or 20 or 15
22. "human*".ti,ab.
23. (animal* or nonhuman*).ti,ab.
24. 23 and 22
25. 23 not 24
26. 21 not 25
27. 10 and 26
Cochrane Library (CENTRAL)
#1 MeSH descriptor Anti‐Bacterial Agents explode all trees
#2 MeSH descriptor Aminoglycosides explode all trees
#3 MeSH descriptor Cephalosporins explode all trees
#4 (Vancomycin or Metronidazole or Fusidic acid or Nitazoxanide or Teicoplanin or Rifampicin or Rifaximin or Bacitracin or Fidaxomicin or Amoxicillin or Azithromycin or Cephalosporin* or Cephalexin or Ciprofloxacin or Clarithromycin or Clindamycin or Doxycycline or Erythromycin or Flouroquinolone* or Levofloxacin or Macrolide* or Nitrofurantoin or Penicillin or Tetracycline or Trimethoprim or antibiotic* or anti‐bacterial* or anti bacterial* or antibacterial* or bacteriocid* or bactericid* or antimicrobial* or anti‐microbial*):ti,ab,kw
#5 (#1 OR #2 OR #3 OR #4)
#6 MeSH descriptor Clostridium difficile explode all trees
#7 MeSH descriptor Clostridium Infections explode all trees
#8 MeSH descriptor Diarrhea explode all trees with qualifier: DT
#9 MeSH descriptor Enterocolitis, Pseudomembranous explode all trees with qualifier: DT
#10 (Clostridium difficile or difficile or pseudomembranous enterocolitis):ti,ab,kw
#11 (#6 OR #7 OR #8 OR #9 OR #10)
#12 (#5 AND #11)
Data and analyses
Comparison 1. Metronidazole vs Vancomycin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Sustained Symptomatic Cure with all exclusions treated as failures | 4 | 872 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.84, 0.97] |
| 2 Bacteriologic Cure | 2 | 163 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.62, 1.17] |
| 3 Sustained Cure (Combined symptomatic and bacteriologic cure) | 1 | 172 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.68, 0.99] |
| 3.1 Mild disease | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.71, 1.09] |
| 3.2 Severe disease | 1 | 82 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.52, 1.04] |
Comparison 2. Bacitracin vs Vancomycin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Symptomatic Cure | 2 | 104 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.34, 0.99] |
Comparison 3. Teicoplanin vs Vancomycin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Symptomatic Cure | 2 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [1.00, 1.46] |
| 2 Bacteriologic Cure | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.82 [1.19, 2.78] |
3.2. Analysis.

Comparison 3 Teicoplanin vs Vancomycin, Outcome 2 Bacteriologic Cure.
Comparison 4. Metronidazole vs Fusidic Acid.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Symptomiatic Cure | 2 | 191 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.88, 1.41] |
| 2 Bacteriologic Cure | 2 | 191 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.84, 1.36] |
4.1. Analysis.

Comparison 4 Metronidazole vs Fusidic Acid, Outcome 1 Symptomiatic Cure.
4.2. Analysis.

Comparison 4 Metronidazole vs Fusidic Acid, Outcome 2 Bacteriologic Cure.
Comparison 5. Fidaxomicin vs Vancomycin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Symptomatic Cure | 2 | 1164 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [1.07, 1.27] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Anonymous 1994.
| Methods | RCT | |
| Participants | CDAD | |
| Interventions | Teicoplanin dose study 100 mg twice daily (n = 49) versus 50 mg fours times a day (n = 43) | |
| Outcomes | Cure Bacteriologic resolution Relapse | |
| Notes | Also cited as Wistrom et al | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Abstract reports randomised, but not how |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Abstract reports double blind |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 47% dropouts, 20/43 in 100 mg twice daily group and 25/49 in 50 mg four times daily group |
| Selective reporting (reporting bias) | Low risk | Expected outcomes were reported |
| Other bias | Unclear risk | Criteria for 'improved' outcome not described |
Boero 1990.
| Methods | RCT | |
| Participants | CDI | |
| Interventions | Rifamixin (n=10) versus vancomycin (n=10) | |
| Outcomes | Combined symptomatic and bacteriologic resolution | |
| Notes | Recurrence not assessed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts |
| Selective reporting (reporting bias) | High risk | No recurrence data |
| Other bias | High risk | Did not exclude other pathogens in the stool as causes of diarrhoea Unclear inclusion criteria and unclear cure criteria |
Cornley 2012.
| Methods | RCT Multicenter USA and Europe |
|
| Participants | Adults with diarrhoea and stool positive for C. difficile toxin A or B or both n = 535 | |
| Interventions | Fidaxomicin 200 mg (n = 270) every 12 hours or vancomycin 125 mg (n = 265) every 6 hours by mouth for 10 days | |
| Outcomes | < 3 bowel movements in 24 hours and toxin negative 20 other outcomes measured, the most important being symptomatic recurrence |
|
| Notes | Non‐inferiority trial with intention‐to‐treat analysis not presented Identical design to (Louie 2011) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation schedule |
| Allocation concealment (selection bias) | Low risk | Participants and investigators were masked to treatment allocation and masking was maintained through database lock |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind: study drugs were over‐encapsulated and identical in appearance |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts less than 10% |
| Selective reporting (reporting bias) | Low risk | Expected outcomes were reported |
| Other bias | High risk | Subgroup analyses by severity and other factors without prior stratification of the randomisation |
De Lalla 1992.
| Methods | RCT | |
| Participants | CDI | |
| Interventions | Vancomycin (n = 24) versus teicoplanin (n = 27) | |
| Outcomes | Cure Bacteriologic resolution Relapse | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | No mention of blinding of patients or outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5/51 dropouts; 4/24 in vancomycin group and 1/27 in teicoplanin group |
| Selective reporting (reporting bias) | Low risk | Expected outcomes were reported |
| Other bias | Unclear risk | Not described |
Dudley 1986.
| Methods | RCT | |
| Participants | CDI | |
| Interventions | Vancomycin (n = 31) versus bacitracin (n = 31) | |
| Outcomes | Cure Bacteriologic resolution Relapse | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Unclear whether assessor was blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 52% dropouts, groups not specified |
| Selective reporting (reporting bias) | Low risk | Expected outcomes were reported |
| Other bias | High risk | Did not exclude other pathogens in the stool as causes of diarrhoea Good randomisation technique, but did not test for C. difficile in stool until after randomisation |
Fekety 1989.
| Methods | RCT | |
| Participants | CDI | |
| Interventions | Vancomycin dose study 125 mg (n = 28) versus 500 mg (n = 28) both groups were dosed four times daily | |
| Outcomes | Cure Bacteriologic resolution Relapse | |
| Notes | High dropout rate | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers table |
| Allocation concealment (selection bias) | High risk | Not used |
| Blinding (performance bias and detection bias) All outcomes | High risk | Outcome assessor not blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 18% dropouts, groups not specified |
| Selective reporting (reporting bias) | High risk | No recurrence data |
| Other bias | High risk | Did not exclude other pathogens in the stool as causes of diarrhoea Wide range of treatment duration |
Garey 2011.
| Methods | RCT single centre | |
| Participants | Adults with > 3 unformed bowel movements in 24 hours and stool positive for C. difficile toxin (type unspecified) N = 79 |
|
| Interventions | Following a standard course of either metronidazole or vancomycin for C. dif for 10‐14 days (physician choice), patients were randomised to placebo (n = 40) or rifaximin 400 mg (n = 39) by mouth for 20 days | |
| Outcomes | Recurrent diarrhoea and stool positive for C. dif after initial resolution of the illness | |
| Notes | Recurrence prophylaxis study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | By study pharmacist |
| Allocation concealment (selection bias) | Low risk | Centralised randomisation by pharmacist |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind specified Study medication and matching placebo were dispensed with a specific study number to ensure blinding of investigators and patients |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 14% dropouts |
| Selective reporting (reporting bias) | Low risk | Expected outcomes were reported |
| Other bias | Unclear risk | Non‐random distribution of primary antibiotic therapy |
Johnson 2014.
| Methods | RCT | |
| Participants | 1118 patients from 200 centers in Europe (109) and the United States (91) with symptomatic primary or recurrent Clostridium difficile infection characterized by >3 bowel movements per day and a positive stool assay for CDI toxin | |
| Interventions | Tolevamer 3 g three times daily x 14 days (n = 563), oral metronidazole 375 mg per day x 10 days (n = 289) or oral vancomycin 125 mg per day x 10 days (n = 266) | |
| Outcomes | Symptomatic resolution of the diarrhoea to < 2 solid bowel movements per day Bacteriologic (toxin) confirmation was not sought unless symptoms failed to resolve or recurred |
|
| Notes | Tolevamer is a resin that binds C. difficile toxin The results of the tolevamer arm of this study were not included in this review |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blinding specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 6 dropouts in the vancomycin group and 4 in the metronidazole group |
| Selective reporting (reporting bias) | Low risk | Expected outcomes were reported |
| Other bias | High risk | Subgroup analyses by severity and other factors without prior stratification of the randomisation |
Keighley 1978.
| Methods | RCT | |
| Participants | CDI | |
| Interventions | Vancomycin (n = 22) versus placebo (n = 22) | |
| Outcomes | Cure and bacteriologic resolution | |
| Notes | Recurrence not assessed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | Centralized randomisation by pharmacy |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Patients were blinded but it is unclear whether the outcome observer was blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 52% dropouts; 12/22 in vancomycin group and 13/22 in placebo group and additional lost data |
| Selective reporting (reporting bias) | Low risk | Expected outcomes were reported |
| Other bias | High risk | Other pathogens not excluded Poor follow up procedures |
Lagrotteria 2006.
| Methods | RCT | |
| Participants | CDI | |
| Interventions | Metronidazole (n = 20) versus metronidazole plus rifampin (n = 19) 39 randomised | |
| Outcomes | Resolution of diarrhoea within 10 days and relapse within 40 days | |
| Notes | No dropouts | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was computer generated, and blinded study staff enrolled patients using numbered packages" |
| Allocation concealment (selection bias) | Low risk | Quote: "The sequence of randomization numbers was concealed until the end of the study" Comment: As study staff were blinded before enrolment, there does not seem to be a source of bias |
| Blinding (performance bias and detection bias) All outcomes | High risk | Single‐blinded Quote: "A placebo was not used" Comment: This could have been placebo controlled although good justification was given" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts |
| Selective reporting (reporting bias) | Low risk | Expected outcomes were reported |
| Other bias | High risk | Did not exclude other pathogens in the stool as causes of diarrhoea |
Lee 2016a.
| Methods | RCT ‐ multicenter | |
| Participants | Adult patients (> 18 years of age) with CDI | |
| Interventions | Surotomycin 125 mg once daily (n = 68) or 250 mg once daily (n = 71) or vancomycin 125 four times daily (n = 70) all by mouth for 10 days | |
| Outcomes | Cure (resolution of diarrhoea) and recurrence of diarrhoea | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Pills identical Outcome assessors did not know allocation group |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A bit problematic: Apparently 11/209 but PRISMA states 18/209 |
| Selective reporting (reporting bias) | Low risk | Expected outcomes were reported |
| Other bias | Low risk | There did not appear to be any other sources of bias |
Louie 2009.
| Methods | RCT | |
| Participants | CDI | |
| Interventions | OPT 80 at 3 doses (100 mg, 200 mg and 400 mg) n = 16 in each group OPT‐80 became Fidaxomicin |
|
| Outcomes | Resolution of diarrhoea and abdominal discomfort within the 10 day treatment period without requiring any additional therapy and relapse within 6 weeks of end of treatment | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The trial used "interactive voice randomization system" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | "the study was a dose‐finding, randomized, open‐label study" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4% dropouts not included in analysis |
| Selective reporting (reporting bias) | Low risk | Expected outcomes were reported |
| Other bias | Unclear risk | Patients with severe disease were excluded and only patients with a primary episode or first relapse were included Patients were excluded if they had > 24 hrs antibiotic (metronidazole or vancomycin) prior to enrolment |
Louie 2011.
| Methods | RCT multicenter (USA and Canada) | |
| Participants | Adults with diarrhoea and stool positive for C. difficile toxin A and/or B (N = 629) | |
| Interventions | Fidaxomicin 200 mg (n = 302) every 12 hours or vancomycin 125 mg (n = 327) every 6 hours by mouth | |
| Outcomes |
< 3 bowel movements in 24 hours and toxin negative 20 other outcomes measured, the most important being symptomatic recurrence |
|
| Notes | Non‐inferiority trial with intention‐to‐treat analysis not presented Article written by part time Optimer Pharmaceuticals employee |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Low risk | Interactive voice response system |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | < 10% dropouts |
| Selective reporting (reporting bias) | Low risk | Expected outcomes were reported |
| Other bias | High risk | Subgroup analyses by severity and other factors without prior stratification of the randomisation |
Louie 2015.
| Methods | RCT | |
| Participants | CDI (N = 84) | |
| Interventions | Cadazolid in 3 doses ‐ 250 mg twice daily (n = 20), 500 mg twice daily (n = 22) and 1000 mg twice daily (n = 20) verus vancomycin 125 mg four times daily (n = 22) | |
| Outcomes | Sustained clinical response | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Low risk | Interactive voice response system |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind, double dummy |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 6 dropouts ‐ 7% |
| Selective reporting (reporting bias) | Low risk | Expected outcomes were reported |
| Other bias | Unclear risk | Not reported |
Mullane 2015.
| Methods | RCT | |
| Participants | CDI (N = 72) | |
| Interventions | LFF571 (n = 46) versus vancomycin (n = 26) | |
| Outcomes | Sustained clinical resolution | |
| Notes | Initally 1:1 randomization, switched to 5:1 halfway through | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Multicenter with randomization cards drawn at each center |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Evaluator blind" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 17 dropouts ‐ 23.6% |
| Selective reporting (reporting bias) | Low risk | Expected outcomes were reported |
| Other bias | Unclear risk | 8 (LFF) and 5 (V) serious adverse events |
Musher 2006.
| Methods | RCT | |
| Participants | CDI | |
| Interventions | Nitazoxanide in two doses (n = 44) versus metronidazole (n = 98) | |
| Outcomes | Resolution of diarrhoea at 7 and 31 days and time to resolution | |
| Notes | 32 dropouts | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were randomised to 1 of 3 groups, in double‐blinded fashion" Comment: No mention is made of how randomisation was established |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind: tablets made to look identical |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 23% dropouts, groups not specified |
| Selective reporting (reporting bias) | Low risk | Expected outcomes were reported |
| Other bias | High risk | Did not exclude other pathogens in the stool as causes of diarrhoea |
Musher 2009.
| Methods | RCT | |
| Participants | CDI (N = 49) | |
| Interventions | Vancomycin 125 mg 6 hourly (n = 27) versus nitazoxanide 500 mg 12 hourly (n = 22) | |
| Outcomes | Complete resolution of symptoms and signs attributable to C difficile within 3 days after completion of therapy Relapse |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | Centralized randomisation (randomisation codes held by study sponsor) |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind, double‐dummy: all patients had active and placebo tablets for the duration of the study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2% dropout not included in analysis |
| Selective reporting (reporting bias) | Low risk | Expected outcomes were reported Unclear if recurrence separated from primary healing but follow‐up equal to most studies measuring recurrence |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Teasley 1983.
| Methods | RCT | |
| Participants | CDI (N = 101) | |
| Interventions | Vancomycin (n = 56) versus metronidazole (n = 45) | |
| Outcomes | Cure Bacteriologic resolution | |
| Notes | No relapse data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 7% dropouts; 4 from vancomycin group and 3 from metronidazole group |
| Selective reporting (reporting bias) | Low risk | Expected outcomes were reported |
| Other bias | High risk | Did not exclude other pathogens in the stool as causes of diarrhoea Also unclear as to which patients had original antibiotic removed |
Wenisch 1996.
| Methods | RCT | |
| Participants | CDI (N = 126) | |
| Interventions | Vancomycin (n = 31) versus metronidazole (n = 31) versus teicoplanin (n = 28) versus fusidic acid (n = 29) | |
| Outcomes | Cure Bacteriologic resolution Relapse | |
| Notes | Cost data presented N for each group a bit unclear as 126 randomised and 7 dropped out, leaving the specified numbers for analysis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were randomised according to a table of random numbers" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | No mention of blinding of patients or outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5.5% dropouts, groups not specified |
| Selective reporting (reporting bias) | Low risk | Expected outcomes were reported |
| Other bias | High risk | Did not exclude other pathogens in the stool as causes of diarrhoea Did exclude patients with no WBCs in stool sample "to insure inclusion of patients with significant disease due to C. difficile" Did however explicitly report that original offending antibiotic had been stopped |
Wullt 2004.
| Methods | RCT | |
| Participants | CDI (N = 131) | |
| Interventions | Fusidic acid (n = 67) versus metronidazole (n = 64) | |
| Outcomes | Cessation of diarrhoea and conversion to toxin negative | |
| Notes | 17 dropouts | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "An independent statistician provided a computer‐generated list of random set numbers" |
| Allocation concealment (selection bias) | Low risk | Quote: "the investigator teams were unaware of the treatment allocation" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | All medication packs were coded and contained identical‐looking pills |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 26% dropouts; 20/67 in fusidic acid group and 14/64 from metronidazole group |
| Selective reporting (reporting bias) | Low risk | Expected outcomes were reported |
| Other bias | High risk | Did not exclude other pathogens in the stool as causes of diarrhoea |
Young 1985.
| Methods | RCT | |
| Participants | CDI (N = 42) | |
| Interventions | Vancomycin versus bacitracin; 21 in each group | |
| Outcomes | Cure Bacteriologic resolution Relapse | |
| Notes | Cost data presented | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Patients blinded but unclear whether assessors were too ‐ although the abstract reports it was double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts |
| Selective reporting (reporting bias) | Low risk | Expected outcomes were reported |
| Other bias | Low risk | Controls for diarrhoea resolution on removal of offending antibiotic |
Zar 2007.
| Methods | RCT | |
| Participants | CDI (N = 172) | |
| Interventions | Vancomycin (n = 82) versus metronidazole (n = 90) | |
| Outcomes | Cessation of diarrhoea Conversion to C. difficile toxin A negative stool Relapse at 21 days post cure |
|
| Notes | 12.5% dropout, but achieved power | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "a member of pharmacy staff randomised participants by selecting a card from a sealed envelope..." |
| Allocation concealment (selection bias) | Low risk | Centralized randomisation by pharmacy using sealed envelope |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "patients received either vancomycin liquid and a placebo tablet that was similar in appearance to metronidazole or a metronidazole tablet and an unpleasantly‐flavoured placebo liquid" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 13% dropouts, missing outcome data balanced in numbers across intervention groups with similar reasons for missing data across groups |
| Selective reporting (reporting bias) | Low risk | Expected outcomes were reported |
| Other bias | Unclear risk | No patients with suspected or life‐threatening intraabdominal complications (perforation or obstruction) were included No patients in ITU or with pseudomembranous colitis were included Unclear whether prior antibiotics had been stopped The timing of randomisation for the stratification of severity of CDI was unclear No mention of stratification before randomisation was made in the text but the authors stated so by e‐mail correspondence |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Barker 2015 | Probiotic protocol |
| Basu 2011 | Abstract only |
| Brumley 2016 | Non‐randomised study |
| Cammarota 2015 | Fecal microbiota transplant |
| De Bruyn 2016 | C. difficile vaccine |
| Forster 2016 | Non‐randomised study |
| Gerding 2016 | Duplicate data to (Louie 2015) |
| Hossam 2016 | Non‐randomised study |
| Johnson 1992 | Participants were asymptomatic carriers of Clostridium difficile without diarrhoea |
| Kaki 2016 | Non‐randomised study |
| Lee 2016b | Fecal microbiota transplant |
| Lee 2016c | Pooled analysis of 2 trials |
| Louie 2006 | RCT with a non antibiotic arm (tolevamer) |
| Lowy 2010 | Does not compare two antibiotics and focuses on the recurrence of Clostridium difficile rather than treatment of the existing infection |
| Mattila 2008 | RCT with a non antibiotic arm (Clostridium difficile immune whey) |
| McFarland 2002 | Non‐randomised study |
| Mullane 2016 | RCT ‐ prophylaxis of C. difficile |
| Noren 2006 | Publication of antibiotic resistance development from a group previously reported by Wullt 2004. |
| Numan 2007 | Assessment of immune whey efficacy |
| Thabit 2015 | Non‐randomised study |
| Welch 2016 | Non‐randomised study |
Characteristics of studies awaiting assessment [ordered by study ID]
Pardi 2012.
| Methods | RCT |
| Participants | CDAD (N = 232) |
| Interventions | Rifaximin (n = 117) versus vancomycin (n = 115) |
| Outcomes | Sustained clinical response |
| Notes | Abstract only published 2014 |
Shyh 2014.
| Methods | RCT |
| Participants | CDAD n = 20 |
| Interventions | Oral vancomycin + intravenous metronidazole verus oral vancomycin alone |
| Outcomes | Sustained clinical response |
| Notes | Abstract only published 2014 |
Characteristics of ongoing studies [ordered by study ID]
NCT01983683.
| Trial name or title | Phase 3 study with cadazolid in CDAD (AC061A302) |
| Methods | RCT |
| Participants | CDI |
| Interventions | Cadazolid and vancomycin |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | Recruiting |
NCT01987895.
| Trial name or title | Phase 3 study with cadazolid in CDAD |
| Methods | RCT |
| Participants | CDI |
| Interventions | Cadazolid and vancomycin |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | Recruiting |
Differences between protocol and review
Decrease in the number of outcomes assessed to only sustained symptomatic cure, bacteriologic cure (i.e. in each case initial resolution minus recurrences), adverse events due to the interventions and death. The inclusion criteria were expanded to include studies with no treatment control groups.
Contributions of authors
E Bricker, R Garg, R Nelson, J Hansen, A Loza, and T Novak all contributed to the original version of the review published in Issue 1, 2005 of the Cochrane Library.
Dr R Nelson prepared the update for Issue 3, 2007.
The 2011 update was prepared by R.Nelson with Kelsey P, Leeman H, Meardon N, Patel H, Paul K, Rees R, Taylor B, Wood E, Malakun R , students at the Unverisity of Sheffield School of Medicine as part of the Master Class Symposium.
The 2017 update was prepared by R. Nelson with Katie Suda and Charlesnika Evans
Sources of support
Internal sources
none, UK.
External sources
none, UK.
Declarations of interest
None known.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Anonymous 1994 {published data only}
- Anonymous. Treatment of Clostridium difficile‐associated diarrhea and colitis with an oral preparation of teicoplanin; a dose finding study. The Swedish CDAD Study Group. Scandinavian Journal of Infectious Diseases 1994;26(3):309‐16. [DOI] [PubMed] [Google Scholar]
Boero 1990 {published data only}
- Boero M, Berti E, Morgando A, Verme G. Treatment of pseudomembranous colitis: a randomized controlled trial with rifaximin vs vancomycin [Terapia della colite da Clostridium difficile: risultati di uno studio randomizzato aperto rifaximina vs. vancomicina]. Microbiologia Medica 1990;5(2):74‐7. [Google Scholar]
Cornley 2012 {published data only}
- Cornely OA, Crook DW, Esposito R, Poirier A, Somero MS, Weiss K, et al. Fidaxomicin versus vancomycin for infection with Clostridium difficile in Europe, Canada, and the USA: a double‐blind, non‐inferiority, randomised controlled trial. Lancet Infectious Diseases 2012;12(4):281‐9. [DOI] [PubMed] [Google Scholar]
De Lalla 1992 {published data only}
- Lalla F, Nicolin R, Rinaldi E, Scarpellini P, Rigoli R, Manfrin V, et al. Prospective study of oral teicoplanin versus oral vancomycin for therapy of pseudomembranous colitis and Clostridium difficile‐associated diarrhea. Antimicrobial Agents and Chemotherapy 1992;36(10):2192‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dudley 1986 {published data only}
- Dudley MN, McLaughlin JC, Carrington G, Frick J, Nightingale CH, Quintiliani R. Oral bacitracin vs vancomycin therapy for Clostridium difficile‐induced diarrhea. A randomized double‐blind trial. Archives of Internal Medicine 1986;146(6):1101‐4. [PubMed] [Google Scholar]
Fekety 1989 {published data only}
- Fekety R, Silva J, Kauffman C, Buggy B, Deery HG. Treatment of antibiotic‐associated Clostridium difficile colitis with oral vancomycin: comparison of two dosage regimens. American Journal of Medicine 1989;86(1):15‐9. [DOI] [PubMed] [Google Scholar]
Garey 2011 {published data only}
- Garey KW, Ghantoji SS, Shah DN, Habib M, Arora V, Jiang ZD, et al. A randomized, double‐blind, placebo‐controlled pilot study to assess the ability of rifaximin to prevent recurrent diarrhoea in patients with Clostridium difficile infection. Journal of Antimicrobial Chemotherapy 2011;66(12):2850‐5. [DOI] [PubMed] [Google Scholar]
Johnson 2014 {published data only}
- Johnson S, Louie TJ, Gerding DN, Cornely OA, Chasan‐Taber S, Fitts D, et al. Vancomycin, metronidazole, or tolevamer for Clostridium difficile infection: results from two multinational, randomized, controlled trials. Clinical Infectious Diseases 2014;59(3):345‐54. [DOI] [PubMed] [Google Scholar]
Keighley 1978 {published data only}
- Keighley MR, Burdon DW, Arabi Y, Williams JA, Thompson H, Youngs D, et al. Randomised controlled trial of vancomycin for pseudomembranous colitis and postoperative diarrhoea. British Medical Journal 1978;2(6153):1667‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lagrotteria 2006 {published data only}
- Lagrottereria D, Holmes S, Smieja M, Smaill F, Lee C. Prospective, randomized inpatient study of oral metronidazole versus oral metronidazole and rifampin for treatment of primary episode of Clostridium difficile‐associated diarrhea. Clinical Infectious Diseases 2006;43(5):547‐52. [DOI] [PubMed] [Google Scholar]
Lee 2016a {published data only}
- Lee CH, Patino H, Stevens C, Rege S, Chesnel L, Louie T, et al. Surotomycin versus vancomycin for Clostridium difficile infection: Phase 2, randomized, controlled, double‐blind, non‐inferiority, multicentre trial. Journal of Antimicrobial Chemotherapy 2016;71(10):2964‐71. [DOI] [PubMed] [Google Scholar]
Louie 2009 {published data only}
- Louie T, Miller M, Donskey C, Mullane K, Goldstein EJ. Clinical outcomes, safety and pharmacokinetics of OPT‐80 in a phase 2 trial with patients with Clostridium difficile infection. Antimicrobial Agents and Chemotherapy 2009;53(1):223‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Louie 2011 {published data only}
- Louie TJ, Miller MA, Mullane KM, Weiss K, Lentnek A, Golan Y, et al. Fidaxomicin versus vancomycin for Clostridium difficile infection. New England Journal of Medicine 2011;364(5):422‐31. [DOI] [PubMed] [Google Scholar]
Louie 2015 {published data only}
- Louie T, Nord CE, Talbot GH, Wilcox M, Gerding DN, Buitrago M, et al. Multicenter, double‐blind, randomized, phase 2 study evaluating the novel antibiotic cadazolid in patients with Clostridium difficile infection. Antimicrobial Agents and Chemotherapy 2015;59(10):6266‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mullane 2015 {published data only}
- Mullane K, Lee C, Bressler A, Buitrago M, Weiss K, Dabovic K, et al. Multicenter, randomized clinical trial to compare the safety and efficacy of LFF571 and vancomycin for Clostridium difficile infections. Antimicrobial Agents and Chemotherapy 2015;59(3):1435‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Musher 2006 {published data only}
- Musher DM, Logan N, Hamill RJ, Dupont HL, Lentnek A, Gupta A, et al. Nitazoxanide for the treatment of Clostridium difficile colitis. Clinical Infectious Diseases 2006;43(4):421‐7. [DOI] [PubMed] [Google Scholar]
Musher 2009 {published data only}
- Musher DM, Logan N, Bressler AM, Johnson DP, Rossignol JF. Nitazoxanide versus vancomycin in Clostridium difficile infection: a randomized, double‐blind study. Clinical Infectious Diseases 2009;48(4):41‐6. [DOI] [PubMed] [Google Scholar]
Teasley 1983 {published data only}
- Teasley DG, Gerding DN, Olson MM, Peterson LR, Gebhard RL, Schwartz MJ, et al. Prospective randomised trial of metronidazole versus vancomycin for Clostridium difficile‐associated diarrhoea and colitis. Lancet 1983;2(8358):1043‐6. [DOI] [PubMed] [Google Scholar]
Wenisch 1996 {published data only}
- Wenisch C, Parschalk B, Hasenhundl M, Hirschl AM, Graninger W. Comparison of vancomycin, teicoplanin, metronidazole, and fusidic acid for the treatment of Clostridium difficile‐associated diarrhea. Clinical Infectious Diseases 1996;22(5):813‐8. [DOI] [PubMed] [Google Scholar]
Wullt 2004 {published data only}
- Wullt M, Odenholt I. A double‐blind randomized controlled trial of fusidic acid and metronidazole for treatment of an initial episode of Clostridium difficile‐associated diarrhoea. Journal of Antimicrobial Chemotherapy 2004;54(1):211‐6. [DOI] [PubMed] [Google Scholar]
Young 1985 {published data only}
- Young GP, Ward PB, Bayley N, Gordon D, Higgins G, Trapani JA, et al. Antibiotic‐associated colitis due to Clostridium difficile: double‐blind comparison of vancomycin with bacitracin. Gastroenterology 1985;89(5):1038‐45. [DOI] [PubMed] [Google Scholar]
Zar 2007 {published and unpublished data}
- Zar FA, Bakkanagari SR, Moorthi KM, Davis MB. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile‐associated diarrhea, stratified by disease severity. Clinical Infectious Diseases 2007;45(3):302‐7. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Barker 2015 {published data only}
- Barker A, Duster M, Valentine S, Archbald‐Pannone L, Guerrant R, Safdar N. Probiotics for Clostridium difficile infection in adults (PICO): Study protocol for a double‐blind, randomized controlled trial. Contemporary Clinical Trials 2015;44:26‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Basu 2011 {published data only}
- Basu P, Krishnaswamy N, Shah J, Tang C. Comparison of nitazoxanide and vancomycin taper regimens as a salvage therapy for patients with recurrent mild to moderate Clostridium difficile infection (CDI). Gastroenteology 2011;140(5):s325. [Google Scholar]
Brumley 2016 {published data only}
- Brumley PE, Malani AN, Kabara JJ, Pisani J, Collins CD. Effect of an antimicrobial stewardship bundle for patients with Clostridium difficile infection. Journal of Antimicrobial Chemotherapy 2016;71(3):836‐40. [DOI] [PubMed] [Google Scholar]
Cammarota 2015 {published data only}
- Cammarota G, Masucci L, Ianiro G, Bibbo S, Dinoi G, Costamagna G, et al. Randomised clinical trial: Faecal microbiota transplantation by colonoscopy vs. vancomycin for the treatment of recurrent Clostridium difficile infection. Alimentary Pharmacology and Therapeutics 2015;41(9):835‐43. [DOI] [PubMed] [Google Scholar]
De Bruyn 2016 {published data only}
- Bruyn G, Saleh J, Workman D, Pollak R, Elinoff V, Fraser NJ, et al. Defining the optimal formulation and schedule of a candidate toxoid vaccine against Clostridium difficile infection: A randomized phase 2 clinical trial. Vaccine 2016;34(19):2170‐8. [DOI] [PubMed] [Google Scholar]
Forster 2016 {published data only}
Gerding 2016 {published data only}
- Gerding DN, Hecht DW, Louie T, Nord CE, Talbot GH, Cornely OA, et al. Susceptibility of Clostridium difficile isolates from a phase 2 clinical trial of cadazolid and vancomycin in C. difficile infection. Journal of Antimicrobial Chemotherapy 2016;71(1):213‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hossam 2016 {published data only}
- Hossam M, Elsisi G. Cost‐effectiveness analysis of fidaxomicin versus oral vancomycin for the treatment of Clostridium difficile infection in Egypt. Value in Health 2016;19(7):A513. [DOI] [PubMed] [Google Scholar]
Johnson 1992 {published data only}
- Johnson S, Homann SR, Bettin KM, Quick JN, Clabots CR, Peterson LR, et al. Treatment of asymptomatic Clostridium difficile carriers (fecal excretors) with vancomycin or metronidazole. A randomized, placebo‐controlled trial. Annals of Internal Medicine 1992;117(4):297‐302. [DOI] [PubMed] [Google Scholar]
Kaki 2016 {published data only}
- Kaki R, Brooks A, Main C, Jayaratne P, Mertz D. Does extending Clostridium difficile treatment in patients who are receiving concomitant antibiotics reduce the rate of relapse?. Internet Journal of Infectious Diseases 2016;15(1):1‐5. [Google Scholar]
Lee 2016b {published data only}
- Lee CH, Steiner T, Petrof EO, Smieja M, Roscoe D, Nematallah A, et al. Frozen vs fresh fecal microbiota transplantation and clinical resolution of diarrhea in patients with recurrent Clostridium difficile infection: A randomized clinical trial. JAMA 2016;315(2):142‐9. [DOI] [PubMed] [Google Scholar]
Lee 2016c {published data only}
- Lee C, Louie TJ, Weiss K, Valiquette L, Gerson M, Arnott W, et al. Fidaxomicin versus vancomycin in the treatment of Clostridium difficile infection: Canadian outcomes. Canadian Journal of Infectious Diseases and Medical Microbiology 2016;2016:Article Number: 8048757. [DOI] [PMC free article] [PubMed] [Google Scholar]
Louie 2006 {published data only}
- Louie TJ, Peppe J, Watt CK, Johnson D, Mohammed R, Dow G, et al. Tolevamer, a novel nonantibiotic polymer, compared with vancomycin in the treatment of mild to moderately severe Clostridium difficile‐associated diarrhea. Clinical Infectious Diseases 2006;43(4):411‐20. [DOI] [PubMed] [Google Scholar]
Lowy 2010 {published data only}
- Lowy I, Molrine DC, Leav BA, Blair BM, Baxter R, Gerding DN, et al. Treatment with monoclonal antibodies against Clostridium difficile toxins. New England Journal of Medicine 2010;362(3):197‐205. [DOI] [PubMed] [Google Scholar]
Mattila 2008 {published data only}
- Mattila E, Anttila VJ, Broas M, Marttila H, Poukka P, Kuusisto K, et al. A randomized, double‐blind study comparing Clostridium difficile immune whey and metronidazole for recurrent Clostridium difficile‐associated diarrhoea: Efficacy and safety of a prematurely interrupted trial. Scandinavian Journal of Infectious Diseases 2008;40(9):702‐8. [DOI] [PubMed] [Google Scholar]
McFarland 2002 {published data only}
- McFarland LV, Elmer GW, Surawicz CM. Breaking the cycle: treatment strategies for 163 cases of recurrent Clostridium difficile disease. American Journal of Gastroenterology 2002;97(7):1769‐75. [DOI] [PubMed] [Google Scholar]
Mullane 2016 {published data only}
- Mullane KM, Adachi J, Dubberke E, Alexander B, Broyde N, Sears P. Outcomes of deflect‐1: A multicenter, blinded, randomized clinical trial of fidaxomicin (FDX) vs. placebo (PLC) for prophylaxis of Clostridium difficile‐associated diarrhea (CDAD) in subjects undergoing hematopoietic stem cell transplantation (HSCT). Biology of Blood and Marrow Transplantation 2016;22(3 Suppl 1):S171. [Google Scholar]
Noren 2006 {published data only}
- Noren T, Wullt M, Akerlund T, Back E, Odenholt I, Burman LG. Frequent emergence of resistance in Clostridium difficile during treatment of C. difficile‐associated diarrhea with fusidic acid. Antimicrobial Agents and Chemotherapy 2006;50(9):3028‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Numan 2007 {published data only}
- Numan SC, Veldkamp P, Kuijper EJ, Berg RJ, Dissel JT. Clostridium difficile‐associated diarrhoea: bovine anti‐clostridium difficile whey protein to help aid the prevention of relapses. Gut 2007;56(6):888‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thabit 2015 {published data only}
- Thabit AK, Nicolau DP. Impact of vancomycin faecal concentrations on clinical and microbiological outcomes in Clostridium difficile infection. International Journal of Antimicrobial Agents 2015;46(2):205‐8. [DOI] [PubMed] [Google Scholar]
Welch 2016 {published data only}
- Welch HK, Nagel JL, Patel TS, Gandhi TN, Chen B, Leon J, et al. Effect of an antimicrobial stewardship intervention on outcomes for patients with Clostridium difficile infection. American Journal of Infection Control 2016;44(12):1539‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
Pardi 2012 {published data only (unpublished sought but not used)}
- Pardi DS, Brennan R, Spinnell M, Gareca MG, Greenberg E, Tian W, et al. The efficacy and safety of rifaximin vs. vancomycin in the treatment of mild to moderate C. difficile infection: A randomized double‐blind active comparator trial. Gastroenterology 2012;142(5 Suppl 1):S599. [Google Scholar]
Shyh 2014 {published data only (unpublished sought but not used)}
- Shyh G, Todorov D, Cohen H, Mukhtarzad R, Brooks S. Oral vancomycin plus intravenous metronidazole versus oral vancomycin in severe Clostridium difficile‐associated diarrhea: a single center study. Pharmacotherapy 2014;34(10):259‐60. [Google Scholar]
References to ongoing studies
NCT01983683 {unpublished data only}
- NCT01983683. A multi‐center, randomized, double‐blind study to compare the efficacy and safety of cadazolid versus vancomycin in subjects with Clostridium difficile‐associated diarrhea (CDAD). clinicaltrials.gov/ct2/show/NCT01983683 (accessed 7 November 2013).
NCT01987895 {unpublished data only}
- NCT01987895. A multi‐center, randomized, double‐blind study to compare the efficacy and safety of cadazolid versus vancomycin in subjects with Clostridium difficile‐associated diarrhea (CDAD). clinicaltrials.gov/ct2/show/NCT01987895 (accessed 7 November 2013).
Additional references
AHRQ 2016
- Minnesota Evidence‐based Practice Center. Early diagnosis, prevention and treatment of Clostridium difficile: Update. AHRQ Publication No. 16‐EHC012‐EF March, 2016; Vol. #172:1‐37.
Assmann 2000
- Assmann SF, Pocock SJ, Enos LE, Kasten LE. Subgroup analysis and other (mis)uses of baseline data in clinical trials. Lancet 2000;355(9209):1064‐9. [DOI] [PubMed] [Google Scholar]
Bagdasarian 2015
- Bagdasarian N, Rao K, Malani PN. Diagnosis and treatment of Clostridium difficile in adults: a systematic review. JAMA 2015;313(4):398‐408. [DOI] [PMC free article] [PubMed] [Google Scholar]
Baker 2002
- Baker SG, Kramer BS. The transitive fallacy for randomized trials: if A bests B and B bests C in separate trials, is A better than C?. BMC Medical Research Methodology 2002;2:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bartlett 1980
- Bartlett JG, Taylor NS, Chang T, Dzink J. Clinical and laboratory observations in Clostridium difficile colitis. American Journal of Clinical Nutrition 1980;33(11 Suppl):2521‐6. [DOI] [PubMed] [Google Scholar]
Bartlett 1990
- Bartlett JG. Clostridium difficile: clinical considerations. Reviews of Infectious Diseases 1990;12 Suppl 2:S243‐51. [DOI] [PubMed] [Google Scholar]
Bartlett 2002
- Bartlett JG. Clinical practice. Antibiotic‐associated diarrhea. New England Journal of Medicine 2002;346(5):334‐9. [DOI] [PubMed] [Google Scholar]
Bauer 2009
- Bauer MP, Kuijper EJ, Dissel JT. European society of clinical microbiology and infectious diseases (ESCMID): treatment guidance for Clostridium difficile infection (CDI). Clinical Microbiology and Infection 2009;15(12):1067‐79. [DOI] [PubMed] [Google Scholar]
Bishara 2007
- Bishara J, Wattad M, Paul M. Vancomycin and metronidazole for the treatment of Clostridium difficile‐associated diarrhea. Clinical Infectious Diseases 2007;45(12):1646‐7. [DOI] [PubMed] [Google Scholar]
Bowden 1981
- Bowden TA Jr, Mansberger AR Jr, Lykins LE. Pseudomembranous enterocolitis: mechanism for restoring floral homeostasis. American Surgeon 1981;47(4):178‐83. [PubMed] [Google Scholar]
Cohen 2010
- Cohen SH, Gerding DN, Johnson S, Kelly CP, Loo VG, McDonald LC, et al. Clinical practice guidelines for clostridium difficile infection in adults: 2010 update for the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infection Control and Hospital Epidemiology 2010;31(5):431‐55. [DOI] [PubMed] [Google Scholar]
Di 2015
- Di X, Bai N, Zhang X, Liu B, Ni W, Wang J, et al. A meta‐analysis of metronidazole and vancomycin for the treatment of Clostridium difficile infection, stratified by disease severity. Brazilian Journal of Infectious Diseases 2015;19(4):339‐49. [PUBMED: 26001980] [DOI] [PMC free article] [PubMed] [Google Scholar]
Drekonja 2011
- Drekonja DM, Butler M, MacDonald R, Bliss D, Filice GA, Rector TS, et al. Comparative effectiveness of Clostridium difficile treatments: a systematic review. Annals of Internal Medicine 2011;155(12):839‐47. [DOI] [PubMed] [Google Scholar]
Fekety 1993
- Fekety R, Shah AB. Diagnosis and treatment of Clostridium difficile colitis. JAMA 1993;269(1):71‐5. [PubMed] [Google Scholar]
Fekety 1997
- Fekety R. Guidelines for the diagnosis and management of Clostridium difficile‐associated diarrhea and colitis. American Journal of Gastroenterology 1997;92(5):739‐50. [PubMed] [Google Scholar]
Garattini 2007
- Garattini S, Bertele V. Non‐inferiority trials are unethical because they disregard patients' interests. Lancet 2007;370:1875‐6. [DOI] [PubMed] [Google Scholar]
George 1982
- George WL, Rolfe RD, Finegold SM. Clostridium difficile and its cytotoxin in feces of patients with antimicrobial agent‐associated diarrhea and miscellaneous conditions. Journal of Clinical Microbiology 1982;15(6):1049‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gerding 1995
- Gerding DN, Johnson S, Peterson LR, Mulligan ME, Silva J Jr. Clostridium difficile‐associated diarrhea and colitis. Infection Control and Hospital Epidemiology 1995;16(8):459‐77. [DOI] [PubMed] [Google Scholar]
Gotzsche 2006
- Gotzsche P. Lessons from and cautions about noninferiority and equivalence randomized trials. JAMA 2006;295(10):1172‐4. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. [Google Scholar]
Hopkins 2009
- Johns Hopkins. Teicoplanin. www.hopkins‐abxguide.org/antibiotics/antibacterial/glycopeptide/teicoplanin.html?contentInstanceId=254836 2009.
HPA 2008
- Health Protection Agency of the Department of Health, UK. Clostridium difficile infection: How to deal with the problem. www.gov.uk/government/uploads/system/uploads/attachment_data/file/340851/Clostridium_difficile_infection_how_to_deal_with_the_problem.pdf (accessed 26 January 2017).
HPA 2016
- Health Protection Agency. Deaths involving Clostridium difficile or MRSA, Wales: 2015. www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/bulletins/deathsinvolvingclostridiumdifficilewales/2015 (accessed 26 January 2017).
Johnson 2012
- Johnson S, Gerding DN, Louie TJ, Ruiz NM, Gorbach SL. Sustained clinical response as an endpoint in treatment trials of Clostridium difficile‐associated diarrhea. Antimicrobial Agents and Chemotherapy 2012;56(8):4043‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Karas 2010
- Karas JA, Enoch DA, Aliyu SH. A review of mortality due to Clostridium difficile infection. Journal of Infection 2010;61(1):1‐8. [DOI] [PubMed] [Google Scholar]
Kelly 1994
- Kelly CP, Pothoulakis C, LaMont JT. Clostridium difficile colitis. New England Journal of Medicine 1994;330(4):257‐62. [DOI] [PubMed] [Google Scholar]
Khoruts 2010
- Khoruts A, Dicksved J, Jansson JK, Sadowsky MJ. Changes in the composition of the human fecal microbiome after bacteriotherapy for recurrent Clostridium difficile‐associated diarrhea. Journal of Clinical Gastroenterology 2010;44(5):354‐60. [DOI] [PubMed] [Google Scholar]
Laurence 2006
- Laurence J. Deaths from "dirty hospital bug" double in five years. The Independant 26 May, 2006:11.
Lessa 2015
- Lessa FC, Mu Y, Bamberg WM, Beldavs ZG, Dumyati GK, Dunn JR, et al. Burden of Clostridium difficile infection in the United States. New England Journal of Medicine 2015;372(9):825‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Locher 2014
- Locher HH, Caspers P, Bruyere T, Schroeder S, Pfaff P, Knezevic A, et al. Investigations of the mode of action and resistance development of cadazolid, a new antibiotic for treatment of Clostridium difficile infections. Antimicrobial Agents and Chemotherapy 2014;58(2):901‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Louie 2005
- Louie TJ. How should we respond to the highly toxogenic NAP1/ribotype 027 strain of Clostridium difficile?. Canadian Medical Association Journal 2005;173(9):1049‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
McDonald 2005
- McDonald LC, Killgore GE, Thompson A, Owens RC Jr, Kazakova SV, Sambol SP, et al. An epidemic, toxin gene‐variant strain of Clostridium difficile. New England Journal of Medicine 2005;353(23):2433‐41. [DOI] [PubMed] [Google Scholar]
McFarland 1995
- McFarland LV, Surawicz CM, Greenberg RN, Elmer GW, Moyer KA, Melcher SA, et al. Prevention of beta‐lactam‐associated diarrhea by Saccharomyces boulardii compared with placebo. American Journal of Gastroenterology 1995;90(3):439‐48. [PubMed] [Google Scholar]
Miesel 2014
- Miesel L, Hecht DW, Osmolski JR, Gerding D, Flattery A, Li F, et al. Kibdelomycin is a potent and selective agent against toxigenic Clostridium difficile. Antimicrobial Agents and Chemotherapy 2014;58(4):2387‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Novell 2010
- Novell MJ, Morreale CA. The relationship between inpatient fluoroquinolone use and Clostridium difficile‐associated diarrhea. Annals of Pharmacotherapy 2010;44(5):826‐31. [DOI] [PubMed] [Google Scholar]
Pepin 2005
- Pepin J, Valiquette L, Cossette B. Mortality attributable to nosocomial Clostridium difficile‐associated disease during an epidemic caused by a hypervirulent strain in Quebec. Canadian Medical Association Journal 2005;173(9):1037‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Privitera 1991
- Privitera G, Scarpellini P, Ortisi G, Nicastro G, Nicolin R, Lalla F. Prosepctive study of Clostridium difficile intestinal colonization and disease following single‐dose antibiotic prophylaxis in surgery. Antimicrobial Agents and Chemotherapy 1991;35(1):208‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Redelings 2007
- Redelings MD, Sorvillo F, Mascola L. Increase in Clostridium difficile‐related mortality rates, United States, 1999‐2004. Emerging Infectious Diseases 2007;13(9):1417‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, Available from www.cochrane‐handbook.org, 2011. [Google Scholar]
Sedgwick 2014
- Sedgwick P. Multiple hypothesis testing and Bonferroni's correction. BMJ (Clinical research ed.) 2014;349:g6284. [DOI] [PubMed] [Google Scholar]
Soonawala 2010
- Soonawala D, Middelberg RA, Egger M, Vandenbroucke JP, Dekkers OM. Efficacy of experimental treatments compared with standard treatments in non‐inferiority trials: a meta‐analysis of randomized controlled trials.. International Journal of Epidemiology 2010;39(6):1567‐81. [DOI] [PubMed] [Google Scholar]
Sougioultzis 2005
- Sougioultzis S, Kyne L, Drudy D, Keates S, Maroo S, Pothoulakis C, et al. Clostridium difficile toxoid vaccine in recurrent C. difficile‐associated diarrhea. Gastroenterology 2005;128(3):764‐70. [DOI] [PubMed] [Google Scholar]
Steiner 2015
- Steiner C, Barrett M, Sun Y. HCUP projections: Clostridium difficile hospitalizations. HCUP Projections Report 2015;02:1‐12. [Google Scholar]
Stewart 2013
- Stewart DB, Hollenbeak CS, Wilson MZ. Is colectomy for fulminant Clostridium difficile colitis life saving? A systematic review. Colorectal Disease 2013;15(7):798‐804. [DOI] [PubMed] [Google Scholar]
Thomas 2003
- Thomas C, Stevenson M, Riley TV. Antibiotics and hospital‐acquired Clostridium difficile‐associated diarrhoea: a systematic review. Journal of Antimicrobial Chemotherapy 2003;51(6):1339‐50. [DOI] [PubMed] [Google Scholar]
Van Nood 2013
- Nood E, Vrieze A, Nieuwdorp M, Fuentes S, Zoetendal E, Vos W, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. New England Journal of Medicine 2013;368(5):407‐15. [DOI] [PubMed] [Google Scholar]
Vardakas 2012
- Vardakas KZ, Polyzos KA, Patouni K, Rafailidis PI, Samonis G, Falagas ME. Treatment failure and recurrence of Clostridium difficile infection following treatment with vancomycin or metronidazole: a systematic review of the evidence. International Journal of Antimicrobial Agents 2012;40(1):1‐8. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Bricker 2005
- Bricker E, Garg R, Nelson R, Loza A, Novak T, Hansen J. Antibiotic treatment for Clostridium difficile‐associated diarrhea in adults. Cochrane Database of Systematic Reviews 2005, Issue 1. [DOI: 10.1002/14651858.CD004610.pub2] [DOI] [PubMed] [Google Scholar]
Nelson 2007
- Nelson R. Antibiotic treatment for Clostridium difficile‐associated diarrhea in adults. Cochrane Database of Systematic Reviews 2007, Issue 3. [DOI: 10.1002/14651858.CD004610.pub3] [DOI] [PubMed] [Google Scholar]
Nelson 2011
- Nelson RL, Kelsey P, Leeman H, Meardon N, Patel H, Paul K, et al. Antibiotic treatment for Clostridium difficile‐associated diarrhea in adults. Cochrane Database of Systematic Reviews 2011, Issue 9. [DOI: 10.1002/14651858.CD004610.pub4] [DOI] [PubMed] [Google Scholar]


