Abstract
Background
Between 4% and 25% of school‐aged children at some stage complain of recurrent abdominal pain (RAP) of sufficient severity to interfere with their daily lives. When no clear organic cause is found, the children are managed with reassurance and simple measures; a large range of pharmacological interventions have been recommended for use in these children.
Objectives
To determine the effectiveness of pharmacological interventions for RAP in children of school age.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL), Ovid MEDLINE, Embase, and eight other electronic databases up to June 2016. We also searched two trials registers and contacted researchers of published studies.
Selection criteria
Randomised controlled trials involving children aged five to 18 years old with RAP or an abdominal pain‐related functional gastrointestinal disorder, as defined by the Rome III criteria (Rasquin 2006). The interventions were any pharmacological intervention compared to placebo, no treatment, waiting list, or standard care. The primary outcome measures were pain intensity, pain duration or pain frequency, and improvement in pain. The secondary outcome measures were school performance, social or psychological functioning, and quality of daily life.
Data collection and analysis
Two review authors independently screened titles, abstracts, and potentially relevant full‐text reports for eligible studies. Two review authors extracted data and performed a 'Risk of bias' assessment. We used the GRADE approach to rate the overall quality of the evidence. We deemed a meta‐analysis to be not appropriate as the studies were significantly heterogeneous. We have consequently provided a narrative summary of the results.
Main results
This review included 16 studies with a total of 1024 participants aged between five and 18 years, all of whom were recruited from paediatric outpatient clinics. Studies were conducted in seven countries: seven in the USA, four in Iran, and one each in the UK, Switzerland, Turkey, Sri Lanka, and India. Follow‐up ranged from two weeks to four months. The studies examined the following interventions to treat RAP: tricyclic antidepressants, antibiotics, 5‐HT4 receptor agonists, antispasmodics, antihistamines, H2 receptor antagonists, serotonin antagonists, selective serotonin re‐uptake inhibitors, a dopamine receptor antagonist, and a hormone. Although some single studies reported that treatments were effective, all of these studies were either small or had key methodological weaknesses with a substantial risk of bias. None of these 'positive' results have been reproduced in subsequent studies. We judged the evidence of effectiveness to be of low quality. No adverse effects were reported in these studies.
Authors' conclusions
There is currently no convincing evidence to support the use of drugs to treat RAP in children. Well‐conducted clinical trials are needed to evaluate any possible benefits and risks of pharmacological interventions. In practice, if a clinician chooses to use a drug as a 'therapeutic trial', they and the patient need to be aware that RAP is a fluctuating condition and any 'response' may reflect the natural history of the condition or a placebo effect, rather than drug efficacy.
Plain language summary
Drug treatment of recurrent abdominal pain in children
Review question
Do medications improve the pain or other symptoms experienced by children with recurrent abdominal pain (RAP)?
Background
Recurrent abdominal pain in childhood is a term used to describe unexplained episodes of tummy pain for which no cause can be found. The pain is often accompanied by other symptoms, such as diarrhoea or facial pallor. Some researchers have therefore classified different syndromes of unexplained pain according to these other associated symptoms. Recurrent abdominal pain is common in children. It is likely that the underlying cause or trigger differs among children.
Study characteristics
We searched the scientific literature worldwide up to June 2016 for research studies of drug treatments for children with RAP. We found 16 studies that met our criteria, examining antidepressants, antibiotics, antihistamines, antispasmodics, a dopamine receptor antagonist, and a hormone treatment. Fourteen studies compared drug treatments to a placebo, and two to usual medical care. The trials were carried out in seven countries: seven in the USA, four in Iran, one in the UK, one in Switzerland, one in Turkey, one in Sri Lanka, and one in India. The studies included a total of 1024 children aged between five and 18 years. All children were recruited from outpatient clinics. Follow‐up lasted between two weeks and four months.
Key results
This review suggests there is no evidence for the use of medications to improve symptoms or the child's quality of life. Consequently, if medications are prescribed, this should be done within a well‐conducted clinical trial. If a medication is prescribed to a child with RAP, it must be remembered that RAP varies with time, and therefore any improvement or worsening may due to the natural history of the condition rather than a medication response.
Quality of evidence
Many of the studies had some weaknesses in their design and how they were reported, therefore the overall quality of the evidence for medications in RAP is low. The studies with better methods included few children and have not been reproduced by other researchers since.
Summary of findings
Summary of findings for the main comparison. Antispasmodics compared to placebo for recurrent abdominal pain.
| Antispasmodics compared to placebo for recurrent abdominal pain | ||||||
|
Patient or population: school‐aged children (5 to 18 years of age) with recurrent abdominal pain Settings: hospital paediatric outpatient clinics Intervention: antispasmodic drugs Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (SD) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Intervention | |||||
| Pain duration (mean pain duration, assessed at 4 weeks) | The mean duration of pain in the control group was 6.17 (± 11.61). | The mean duration of pain in the intervention group was51.6 (± 23.74). | MD ‐25.4 (‐35.5 to ‐15.3) | 120 (1) | ⊕⊝⊝⊝ Very low1 |
Asgarshirazi 2015 No evidence of efficacy |
| Pain improvement (clinician judged, assessed at 2 weeks) | 9 of 21 children in the control group had an improvement in pain. | 15 of 21 children in the intervention group had an improvement in pain. | OR 3.33 (0.93 to 12.01) | 42 (1) | ⊕⊝⊝⊝ Very low2 |
Kline 2001 No evidence of efficacy |
| Pain frequency (episodes of pain in 4 weeks, assessed after 4 weeks) | The mean number of episodes of pain in the control group was 21.6 (32.4). | The mean number of episodes of pain in the intervention group was10.3 (14). | MD 11.3 (2.4 to 20.1) | 132 (1) | ⊕⊝⊝⊝ Very low3 |
Narang 2015 No evidence of efficacy |
| Pain improvement (self reported response to treatment, assessed at 4 weeks) | The response to treatment in the control group was 30.3%. | The response to treatment in the intervention group was 40.6%. | OR 1.6 (0.7 to 3.4) | 115 (1) | ⊕⊕⊝⊝ Low4 |
Pourmoghaddas 2014 No evidence of efficacy |
| *The basis for the assumed risk (e.g., the median control group risk across studies) is provided in footnotes. The corresponding risk is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; OR: odds ratio; SD: standard deviation | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Downgraded for low methodological quality due to single, small study; risk of bias from incomplete outcome data and differential loss of participants between groups. The placebo differed in preparation and dose timing compared to the intervention drug. 2Downgraded for low methodological quality due to single, small study; risk of bias from selective outcome reporting and short follow‐up. 3Downgraded for low methodological quality due to single, small study; risk of bias from selective outcome reporting and the method of altering the drug doses. 4Downgraded for single, small study. Not duplicated.
Background
Description of the condition
This review is an update of a previously published review in the Cochrane Library on 'Pharmacological interventions for recurrent abdominal pain (RAP) and irritable bowel syndrome (IBS) in childhood' (Huertas‐Ceballos 2008a). Recurrent abdominal pain (RAP) is a common problem in paediatric practice. It has been suggested that 4% to 25% of school‐aged children at some stage suffer from RAP that interferes with their activities of daily living (Abu‐Arafeh 1995; Apley 1958; Faull 1986; Williams 1996; Øster 1972). Recurrent abdominal pain is often regarded as a relatively benign condition, but it is important to note the associated morbidity and anxiety it causes for children and carers. The condition is associated with school absences, hospital admissions, and, on occasion, unnecessary surgical intervention (Scharff 1997; Stickler 1979; Størdal 2005; Walker 1998). Symptoms sometimes continue into adulthood (Apley 1975). The abdominal pain is commonly associated with other symptoms, including headaches, recurrent limb pains, pallor, and vomiting (Abu‐Arafeh 1995; Apley 1958; Faull 1986; Hyams 1995; Stickler 1979; Stone 1970; Øster 1972).
It is generally accepted that RAP in children represents a group of functional gastrointestinal disorders with an unclear aetiology. Children suffer either chronic or recurrent gastrointestinal symptoms that are not explained by a structural, biochemical, or inflammatory process. Apley first sought to define the condition in the 1950s, suggesting that the diagnostic label should be based on the presence of at least three episodes of severe abdominal pain (often, but not necessarily, with associated systemic symptoms) over three months, with no established organic cause (Apley 1958). More recently, an international consensus definition with a symptom‐based classification system with specific categories for paediatric presentations has been produced, known as the Rome III criteria (Rasquin 2006). We have used RAP throughout this review as an umbrella term to refer to the five categories included within this classification, which are: functional dyspepsia, irritable bowel syndrome (IBS), abdominal migraine, functional abdominal pain, and functional abdominal pain syndrome. It should be noted that the pain classification for each of the Rome III diagnoses is defined by at least one episode per week for at least two months; this varies from Apley's original definition of RAP (Apley 1958). The Rome classification is not based on known pathophysiological differences between the conditions, but rather on the constellation of clinical features. It remains unclear the extent to which separating children into these categories defines groups that are distinct clinical entities likely to respond differently to treatment. Nonetheless, this classification has been welcomed following the historical use of diverse terms, some implying causation. These include abdominal migraine (Bain 1974; Farquar 1956; Hockaday 1992; Symon 1986), abdominal epilepsy (Stowens 1970), the irritable bowel syndrome in childhood (Stone 1970), allergic‐tension‐fatigue syndrome (Sandberg 1973; Speer 1954), neurovegetative dystonia (Peltonen 1970; Rubin 1967), functional gastrointestinal disorder (Drossman 1995), and the irritated colon syndrome (Harvey 1973; Painter 1964).
There is no consensus about which of the numerous proposed causal pathways result in the heterogeneous presentations of chronic abdominal pain, although it has been suggested that physical, emotional, and environmental factors may contribute to the manifestation of unexplained abdominal pain. When considering the diverse proposed mechanisms, it is unsurprising that a variety of treatments have been suggested. The treatment approaches can be grouped into pharmacological, dietary, or psychosocial (psychological, behavioural, or both). Reviews of the effectiveness of psychosocial and dietary interventions for RAP (Huertas‐Ceballos 2008b; Huertas‐Ceballos 2009), published in 2008 and 2009, have been updated as companions to this updated review (Abbott 2017; Newlove‐Delgado in press, respectively).
Description of the intervention
A range of pharmacological treatments have been tried and tested for RAP in childhood: analgesics, dicyclomine (Edwards 1994), pizotifen (Christensen 1995; Symon 1995), herbal extracts (Zhang 1991), and many other drugs (Bain 1974; Worawattanakul 1999). A number of randomised controlled trials have reported on the use of peppermint oil for IBS in adults (Grigoleit 2005), the results of which have been interpreted as suggesting it to be a beneficial intervention. However, an earlier review reached no clear conclusion on efficacy due to poor methodological quality of the included studies (Pittler 1998). Other possible causal factors have been postulated, including food allergies (Poley 1973), reaction to food additives (Anonymous 1984), infectious agents like Helicobacter pylori, and parasitic infestation (Heldenberg 1995; Primelles 1990; Wardhan 1993).
How the intervention might work
The aetiology of abdominal pain‐related functional gastrointestinal disorders is unclear. It has been suggested that visceral hypersensitivity (Di Lorenzo 2001; Van Ginkel 2001), autonomic dysfunction (Good 1995), and gut dysmotility may contribute, which may be initiated by an inflammatory, infective, traumatic, or allergic trigger (Mayer 2002; Milla 2001).
Conventional analgesics have been proposed to work by interrupting these abnormal physiological pain responses, which become pathological. Antispamodics have been proposed to alter gut dysmotility, including peppermint oil, which has antispasmodic actions (Hills 1991). Serotonin (5‐hydroxytryptamine) agonists may relieve symptoms by causing vasoconstriction and stimulation of the release of other vasoactive substances, thus inhibiting neurogenic inflammation; this has been found in migraine headaches (Goadsby 2000). Antidepressants treat the associated symptoms, and there is evidence of effectiveness in treating IBS in adults (Ruepert 2011).
Why it is important to do this review
Recurrent abdominal pain in children is very common, and in daily clinical practice there is no consensus on which treatments to offer patients. The approach is therefore inconsistent. It was important to do this review to establish if there is evidence for the effectiveness of pharmacological interventions in children with RAP. This review updates a previous review published in 2008 (Huertas‐Ceballos 2008a). Companion reviews addressing the effectiveness of psychosocial, Huertas‐Ceballos 2008b, and dietary interventions for RAP, Huertas‐Ceballos 2009, are also being updated (Abbott 2017; Newlove‐Delgado in press, respectively), so together they can guide clinicians, patients, and their families in treatment decisions.
Objectives
To determine the effectiveness of pharmacological interventions for RAP in children of school age.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs).
Types of participants
Children aged five to 18 years with RAP or an abdominal pain‐related functional gastrointestinal disorder, as defined by the Rome III criteria (Rasquin 2006).
Recurrent abdominal pain is defined as at least three episodes of pain interfering with normal activities within a three‐month period. The Rome III criteria recognises five abdominal pain‐related categories: abdominal migraine, irritable bowel syndrome (IBS), functional dyspepsia, functional abdominal pain, and functional abdominal pain syndrome (Rasquin 2006).
Types of interventions
Any pharmacological intervention compared to placebo, no treatment, waiting list, or standard care.
Types of outcome measures
Primary outcomes
Pain intensity
Pain duration or pain frequency
Improvement in pain
As there is no standard method for measuring pain in this condition, studies could have used any validated measurement of pain such as a Likert scale, visual analogue scale, or questionnaire such as the Abdominal Pain Index (Walker 1997), which exists in various versions and formats. The trials could also have used 'the proportion of participants with a significant improvement in pain' as an outcome, as defined by the trial author.
Secondary outcomes
The following were secondary outcomes, as measured by a validated tool.
School performance
Social or psychological functioning
Quality of daily life
Search methods for identification of studies
Electronic searches
We ran the first literature searches in April 2013 and updated them in April 2014, March 2015, and again in June 2016. We searched the electronic databases and trial registers listed below.
Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 5) in the Cochrane Library and which includes the Cochrane Developmental, Psychosocial and Learning Problems Specialised Register (searched 10 June 2016).
Ovid MEDLINE In‐Process & Other Non‐Indexed Citations and Ovid MEDLINE (1946 to current; searched 9 June 2016).
Embase Ovid (1974 to current; searched 9 June 2016).
CINAHL Healthcare Databases Advanced Search (Cumulative Index to Nursing and Allied Health Literature; 1981 to current; searched 9 June 2016).
PsycINFO Ovid (1806 to current; searched 9 June 2016).
ERIC ProQuest (Educational Resources Information Center; 1966 to current; searched 9 June 2016).
BEI ProQuest (British Education Index; 1975 to current; searched 9 June 2016).
ASSIA ProQuest (Applied Social Sciences Index and Abstracts; 1987 to current; searched 9 June 2016).
AMED Healthcare Databases Advanced Search (Allied and Complementary Medicine; 1985 to current; searched 9 June 2016).
LILACS (Latin American and Caribbean Literature in Health Sciences; lilacs.bvsalud.org/en; searched 9 June 2016).
OpenGrey (opengrey.eu; searched 9 June 2016).
ClinicalTrials.gov (clinicaltrials.gov; searched 9 June 2016).
World Health Organization International Clinical Trials Registry Platform (WHO ICTRP; apps.who.int/trialsearch; searched 9 June 2016).
We revised the search terms from the original Cochrane RAP reviews (Huertas‐Ceballos 2008; Huertas‐Ceballos 2009a; Huertas‐Ceballos 2009b); we consequently ran the searches for all available years. We used RCT filters where appropriate and imposed no language limits. We translated any non‐English language studies identified so that they could be screened and considered for inclusion. The search strategies for each database are reported in Appendix 1.
Searching other resources
We used the Science Citation Index (Web of Science) for forward citation searching to identify papers in which the included reports had been cited, and we checked the reference lists of the included reports to identify any additional studies, including any ongoing or unpublished work. We also contacted researchers who have published studies in this field to ask for details of any relevant trials.
Data collection and analysis
Selection of studies
Two review authors (RAA, AB, TVND, or AEM) independently screened the titles and abstracts of studies identified by the search for relevance. We obtained the full‐text reports of any paper that we judged to be potentially suitable for inclusion and then identified studies for inclusion against the Criteria for considering studies for this review. Any disagreements were resolved through discussion with a third review author (JTC).
Data extraction and management
Two review authors (RAA, AB, JTC, TVND, or AEM) extracted data and entered the data into Cochrane's statistical software Review Manager 5 (Review Manager 2014). All review authors used the same data extraction form. We collected the following data.
Study characteristics: number of participating children, inclusion and exclusion criteria, type of intervention and comparison, intervention characteristics (duration, frequency, setting), and number of withdrawals.
Participant characteristics: sex, age, and diagnosis (e.g. RAP or a syndrome defined by the Rome III criteria) (Rasquin 2006).
Outcome measures: measurement of pain and any secondary outcomes measured.
Assessment of risk of bias in included studies
We considered the following domains when assessing the risk of bias of included studies:
selection bias (random sequence generation and allocation concealment);
performance bias (blinding of participants and personnel);
detection bias (blinding of outcome assessment);
attrition bias (incomplete outcome data);
reporting bias (selective outcome reporting); and
other sources of bias. We assessed all included studies for other sources of bias that may have altered the estimate of treatment effect, such as differential loss to follow‐up, whether the data collection tools were valid, whether there was sufficient power in terms of appropriate sample size, whether baseline parameters were similar, and whether data analyses were appropriate.
Two review authors (RAA, AB, JTC, TVND, or AEM) independently assessed each study. We classified the risk of bias as low, high, or unclear based on the methods detailed in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). See Table 2. We considered a trial as having an overall low risk of bias if most of the above domains were assessed as at low risk of bias. We considered a trial as having an overall high risk of bias if several of the above domains were assessed as at high risk of bias or unclear risk of bias.
1. Assessment of risk of bias in included studies.
| Domain | 'Risk of bias' judgement | ||
| Low | High | Unclear | |
| Selection bias | |||
| Random sequence generation | If the study details any of the following methods: (1) simple randomisation (such as coin‐tossing, throwing dice, or dealing previously shuffled cards, a list of random numbers, or computer‐generated random numbers); or (2) restricted randomisation such as blocked, ideally with varying block sizes or stratified groups, provided that within‐group randomisation is not affected | If the study details no randomisation or an inadequate method such as alternation, assignment based on date of birth, case record number, and date of presentation. These latter methods may be referred to as ‘quasi‐random’. | If there is insufficient detail to judge the risk of bias |
| Allocation concealment | If the study details concealed allocation sequence in sufficient detail to determine that allocations could not have been foreseen in advance of, or during, enrolment | If the study details a method where the allocation is known prior to assignment | If there is insufficient detail to judge the risk of bias |
| Performance bias | |||
| Blinding of participants and personnel | If the study details a method of blinding participants and personnel. Detail would need to be sufficient to show that participants and personnel were unable to identify the therapeutic intervention from the control intervention. | If the methods detail that the participants or study personnel were not blinded to the study medication or placebo | If there is insufficient detail to judge the risk of bias |
| Detection bias | |||
| Blinding of outcome assessment | If the study details a blinded outcome assessment. This may only be possible for outcomes that are externally assessed. | If the outcome assessment is not blinded. We expect this may be unavoidable for self rated outcomes of unblinded interventions. | If there is insufficient detail to judge the risk of bias |
| Attrition bias | |||
| Incomplete outcome data | If the study reports attrition and exclusions, including the numbers in each intervention group (compared with total randomised participants), reasons for attrition or exclusions, and any re‐inclusions; the impact of missing data is not believed to have altered the conclusions; and reasons for the missing data are acceptable | We may judge the risk of attrition bias to be high due to the amount, nature, or handling (such as per‐protocol analysis) of incomplete outcome data. | If there is insufficient detail to judge the risk of bias, e.g. if the number of children randomised to each treatment is not reported |
| Reporting bias | |||
| Selective reporting | If there is complete reporting of all outcome data. This will be determined based on comparison of the protocol and published study, if available. | If the reporting is selective so that some outcome data are not reported | If there is insufficient detail to judge the risk of bias, e.g. protocols are unavailable |
| Other sources of bias | |||
| Other bias | If the study is judged to be at low of risk of other potential sources of bias, such as no differential loss to follow‐up or an adequate washout period in cross‐over trials | If there are other sources of bias, such as differential loss to follow‐up or an inadequate washout period in cross‐over trials | If there is insufficient detail to judge the risk of bias |
Measures of treatment effect
Continuous data
For continuous data (e.g. number of days of pain), we analysed means and standard deviations (SDs), if these were available or could be calculated and if there was no clear evidence of skewness in the distribution, and presented these with 95% confidence intervals (CIs).
If different scales were used to measure the same clinical outcome, we combined the standardised mean differences (SMDs) across the studies, and presented these with 95% CIs.
Dichotomous data
For dichotomous data (e.g. pain improved, yes or no), we analysed data using odds ratios (ORs) and presented these with 95% CIs.
Unit of analysis issues
No methods were used as we did not perform a meta‐analysis. For methods archived for future updates of this review, please see Appendix 2 and our protocol (Martin 2014a).
Dealing with missing data
We first contacted the original investigators to request any missing data, but we received no responses. We did not impute any values. We did not feel it was relevant to carry out a sensitivity analysis, with and without the missing data, as it was not possible to conduct a meta‐analysis. For methods archived for future updates of this review, please see Appendix 2 and our protocol (Martin 2014a).
We collected the proportions of participants for whom no outcome data were obtained and reported them in the assessment of Risk of bias in included studies. We have also provided this information for each study in the 'Risk of bias' tables, beneath the Characteristics of included studies tables. We explored the potential impact of missing data on the findings of the review in the Discussion section.
Assessment of heterogeneity
We anticipated finding considerable heterogeneity between included studies. We assessed clinical heterogeneity by examining the distribution of relevant participant characteristics (e.g. age, definition of RAP) and study differences (e.g. concealment of randomisation, blinding of outcome assessors, interventions or outcome measures).
We did not use our prescribed methods for assessing statistical heterogeneity as we did not perform a meta‐analysis. For methods archived for future updates of this review, please see Appendix 2 and our protocol (Martin 2014a).
Assessment of reporting biases
We examined the report of each study to assess for selective outcome reporting. We assessed the study as adequate if it met the following criteria:
the study protocol was available and all of the study's prespecified (primary and secondary) outcomes that were of interest to the review were reported in the prespecified way; or
the study protocol was not available, but it was clear that the published reports included all expected outcomes, including those that were prespecified.
Data synthesis
Given the heterogeneity of drug classes and pain measurements used in the included studies, it was not possible to carry out a meta‐analysis (DerSimonian 1986). We have therefore provided a narrative description of the results. For methods archived for future updates of this review, please see Appendix 2 and our protocol (Martin 2014a).
Summary of findings
We used the GRADE approach to assess the overall quality of the body of evidence for a specific outcome (Grade Working Group 2013). We used GRADEpro to assess and present the findings in a 'Summary of findings' table (GRADEpro GDT 2015). There were 11 comparisons in total, as we found 11 classes of drugs in the studies. We chose to produce a 'Summary of findings' table for antispasmodic drugs versus placebo (Table 1), as this was the most commonly investigated drug class in the studies, with a total of four studies. We presented pain, the primary outcome for this review, in the 'Summary of findings' table. The measurement of pain varied between studies, as explained in the Types of outcome measures section.
We judged the studies included for each outcome using five criteria: risk of bias, indirectness, inconsistency, imprecision, and publication bias. We used limitations in the design and implementation to assess the overall risk of bias of included studies for each outcome; we downgraded an outcome if the majority of studies had unclear or high risk of bias. We assessed indirectness if a population, intervention, or outcome was not of direct interest to the review. Inconsistency was determined by the heterogeneity of results. If an outcome had a heterogeneity outcome of greater than 70%, we downgraded the quality of the outcome. Imprecision was assessed by the number of participants included in an outcome and by CIs. We downgraded an outcome when only a small number of participants could be included in the analysis or the analysis had wide CIs. Finally, we downgraded for publication bias if studies failed to report outcomes in the published manuscript or if there was a suspicion that null findings had not been published or reported (Schünemann 2011, section 12.2.2).
We gave each outcome a quality marking ranging from 'very low' to 'high'.
High quality: "further research is unlikely to change our estimate of effect".
Moderate quality: "further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate".
Low quality: "further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate".
Very low quality: "we are very uncertain about the estimate" (Balshem 2011).
Subgroup analysis and investigation of heterogeneity
We used no methods as we did not perform a meta‐analysis. For methods archived for future updates of this review, please see Appendix 2 and our protocol (Martin 2014a).
Sensitivity analysis
We used no methods as we did not perform a meta‐analysis. For methods archived for future updates of this review, please see Appendix 2 and our protocol (Martin 2014a).
Results
Description of studies
For a full description of the main characteristics of the studies, including details on participants and setting, intervention aspects, and outcome measures, see Characteristics of included studies. See also Characteristics of excluded studies.
Results of the search
For this updated review, we chose to redesign the search strategy in order to include the recognised terms for different types of RAP, as defined by the Rome criteria (Rasquin 2006). Consequently, we ran our searches without date restrictions on each database. The results of the searching and screening process are shown in the PRISMA flow chart (Figure 1). We screened a total of 9649 titles and abstracts, 230 of which we carried forward for further screening at full‐text. We excluded 214 reports and included 16 studies.
1.
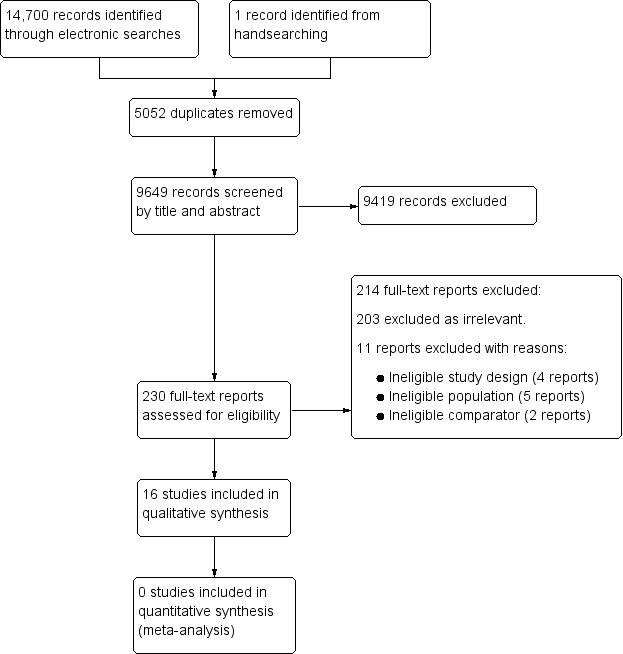
Study flow diagram.
Included studies
For details, please see Characteristics of included studies.
We included 16 studies in the review (Asgarshirazi 2015; Bahar 2008; Collins 2011; Heyland 2012; Karabulut 2013; Karunanayake 2015; Khoshoo 2006; Kline 2001; Narang 2015; Pourmoghaddas 2014; Roohafza 2014; Sadeghian 2008; Saps 2009; See 2001; Symon 1995; Zybach 2016), three of which we included in the previous version of this review (Kline 2001; See 2001; Symon 1995).
Participants
The number of participants per study ranged from 12, in Zybach 2016, to 115 (Pourmoghaddas 2014; Roohafza 2014). Eight studies had more than 50 participants (Asgarshirazi 2015; Collins 2011; Karabulut 2013; Karunanayake 2015; Narang 2015; Pourmoghaddas 2014; Roohafza 2014; Saps 2009).
Location
Seven studies were based in the USA (Bahar 2008; Collins 2011; Khoshoo 2006; Kline 2001; Saps 2009; See 2001; Zybach 2016), four in Iran (Asgarshirazi 2015; Pourmoghaddas 2014; Roohafza 2014; Sadeghian 2008), one in the UK (Symon 1995), one in Switzerland (Heyland 2012), one in Turkey (Karabulut 2013), one in Sri Lanka (Karunanayake 2015), and one in India (Narang 2015).
Settings
All 16 studies were conducted in hospital paediatric outpatient clinics.
Study duration
The study duration ranged from two weeks (Collins 2011; Kline 2001; Sadeghian 2008) to four months (Symon 1995). See Characteristics of included studies for details of each study.
Interventions
Pharmacological interventions, drug classes used; tricyclic antidepressants (two studies) (Bahar 2008; Saps 2009), antibiotics (two studies) (Collins 2011; Heyland 2012), antimuscarinics (one study) (Karabulut 2013), 5‐HT4 receptor agonists (one study) (Khoshoo 2006), antispasmodics (four studies) (Asgarshirazi 2015; Kline 2001; Narang 2015; Pourmoghaddas 2014), selective serotonin re‐uptake inhibitors (one study) (Roohafza 2014), antihistamines (one study) (Sadeghian 2008), H2 receptor antagonists (one study) (See 2001), serotonin antagonist (one study) (Symon 1995), dopamine receptor antagonist (one study) (Karunanayake 2015), and a hormone (one study) (Zybach 2016).
Study design
Three studies were cross‐over trials (See 2001; Symon 1995; Zybach 2016). One study had two intervention arms (Asgarshirazi 2015); the second intervention is reported in the accompanying review of dietary interventions (Newlove‐Delgado in press). The remaining 12 studies were single‐intervention, placebo‐controlled trials (Bahar 2008; Collins 2011; Heyland 2012; Karabulut 2013; Karunanayake 2015; Khoshoo 2006; Kline 2001; Narang 2015; Pourmoghaddas 2014; Roohafza 2014; Sadeghian 2008; Saps 2009). All studies were randomised.
Outcomes
Primary outcome
All 16 included trials measured pain; the method of measurement varied. Tools included: visual analogue scale (VAS; 0 to 10), mean pain score and number of days of pain. Some trials used the parental or child report of adequate pain relief as the outcome measure.
Secondary outcomes
One study measured school attendance (Narang 2015). One study measured social and psychological functioning (Roohafza 2014); the authors assessed self rated "depression, anxiety, and somatization" scores before and after treatment. Two studies measured quality of life (Bahar 2008; Karunanayake 2015). Other outcomes included: gastrointestinal symptoms scale and global assessment of well‐being. See Characteristics of included studies for details of outcome measures used in studies.
Excluded studies
We examined 230 full‐text reports and excluded 214. Of these, 203 reports were clearly irrelevant. Of the 11 remaining full‐text reports, we excluded four due to ineligible study design (Christensen 1995; Cucchiara 1992; Dehghani 2011; Kaminski 2009), five due to ineligible populations (Di Nardo 2011; Everitt 2010; Lloyd‐Still 1990; Van Outryve 2005; Yadav 1989), and two because of ineligible comparators (Grillage 1990; Xiao 2013). Please see Characteristics of excluded studies.
Risk of bias in included studies
For a summary, see Figure 2 and Figure 3.
2.
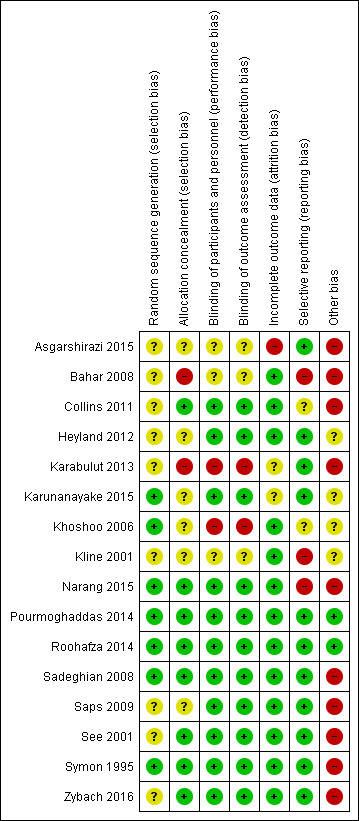
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.
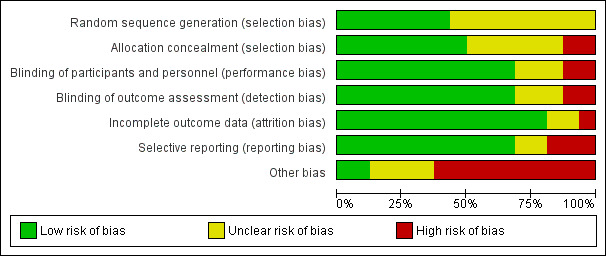
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Of the 16 included studies, we judged seven to be at low risk of bias for random sequence generation (Karunanayake 2015; Khoshoo 2006; Narang 2015; Pourmoghaddas 2014; Roohafza 2014; Sadeghian 2008; Symon 1995), and nine to be at unclear risk, largely because the authors did not explain their method of randomisation (Asgarshirazi 2015; Bahar 2008; Collins 2011; Heyland 2012; Karabulut 2013; Kline 2001; Saps 2009; See 2001; Zybach 2016).
We rated eight studies at low risk of bias because allocation concealment had been achieved (Collins 2011; Narang 2015; Pourmoghaddas 2014; Roohafza 2014; Sadeghian 2008; See 2001; Symon 1995; Zybach 2016), six studies at unclear risk of bias because the authors did not mention allocation concealment (Asgarshirazi 2015; Heyland 2012; Karunanayake 2015; Khoshoo 2006; Kline 2001; Saps 2009), and two studies at high risk of bias because the methods implied that allocation concealment had not been achieved (Bahar 2008; Karabulut 2013).
Blinding
We judged 11 studies with clear blinding of participants to be at low risk of bias (Collins 2011; Heyland 2012; Karunanayake 2015; Narang 2015; Pourmoghaddas 2014; Roohafza 2014; Sadeghian 2008; Saps 2009; See 2001; Symon 1995; Zybach 2016). We judged the risk of performance bias as unclear in three studies (Asgarshirazi 2015; Bahar 2008; Kline 2001), and high in two studies where blinding was not achieved (Karabulut 2013; Khoshoo 2006). These judgements were mirrored in the risk of detection bias, as the primary outcomes were all self or parent reported and therefore relied on blinding.
Incomplete outcome data
Outcome data were largely complete, and we judged 13 of the 16 included studies to have a low risk of attrition bias (Bahar 2008; Collins 2011; Heyland 2012; Khoshoo 2006; Kline 2001; Narang 2015; Pourmoghaddas 2014; Roohafza 2014; Sadeghian 2008; Saps 2009; See 2001; Symon 1995; Zybach 2016). We judged one study to have an unclear risk of attrition bias, as the authors did not report the numerical outcome data or the number of participants completing the study, but rather reported results as a percentage without a total (Karabulut 2013). We judged one further study to have an unclear risk of attrition bias due to insufficient information in the conference abstract and no further information from personal communication with the study authors (Karunanayake 2015). We judged one study to be at a high risk of attrition bias, as there were differential losses to follow‐up between the groups (Asgarshirazi 2015).
Selective reporting
We considered the risk of reporting bias to be unclear in two studies (Collins 2011; Khoshoo 2006), and high in three studies (Bahar 2008; Kline 2001; Narang 2015), because the authors did not report results for all the outcomes mentioned in the methods section. We rated the risk of reporting bias as low in 11 studies (Asgarshirazi 2015; Heyland 2012; Karabulut 2013; Karunanayake 2015; Pourmoghaddas 2014; Roohafza 2014; Sadeghian 2008; Saps 2009; See 2001; Symon 1995; Zybach 2016).
Other potential sources of bias
We judged the risk of other potential sources of bias as high in 10 studies (Asgarshirazi 2015; Bahar 2008; Collins 2011; Karabulut 2013; Narang 2015; Sadeghian 2008; Saps 2009; See 2001; Symon 1995; Zybach 2016), and unclear in four studies (Heyland 2012; Karunanayake 2015; Khoshoo 2006; Kline 2001), due to lack of details on baseline characteristics, lack of power calculations, lack of details on how outcomes were measured, and insufficient washout periods in cross‐over trials. We rated two studies at low risk of other potential sources of bias (Pourmoghaddas 2014; Roohafza 2014).
For each domain above, where there was insufficient information to make a judgement of high or low risk of bias, we wrote to all trial authors for clarification (Abbott 2014a [pers comm]; Abbott 2014b [pers comm]; Martin 2014b [pers comm]; Martin 2014c [pers comm]; Martin 2016a [pers comm]; Martin 2016b [pers comm]; Martin 2016c [pers comm]; Martin 2016d [pers comm]; Martin 2016e [pers comm]; Martin 2016f [pers comm]; Martin 2016g [pers comm]). As we received limited response, we assigned ratings of unclear risk of bias for these studies in these domains.
Effects of interventions
See: Table 1
Comparison 1. Tricyclic antidepressants compared to placebo
Two studies, Bahar 2008 and Saps 2009, evaluated amitriptyline compared to placebo in 123 children with functional gastrointestinal disorders as defined by the Rome II criteria (Rasquin‐Weber 1999).
Bahar 2008 assessed multiple outcomes measuring pain (using a pain‐rating scale and VAS), quality of life, and IBS symptoms for up to 13 weeks. The quality of life tool had been validated in adults (Patrick 1997). The authors reported no significant difference in pain between the two groups, but provided no data to support this. The mean quality of life scores were 109.4 and 127.5 at baseline for the intervention and control groups, respectively. At 13 weeks' follow‐up, they were 126.2 and 129.8 for the intervention and control groups, respectively. The authors suggested that a higher proportion of participants in the intervention group showed a 15% increase in quality of life at 13 weeks compared to the control group (P = 0.002). The clinical significance of this is unclear. Of note, the two groups had a 14% difference of mean quality of life scores at baseline. This may have been a post‐hoc analysis, as it is not mentioned in the methods of the study. The study authors did not provide the standard deviations for these data. We wrote to the study authors to request further information but received no response (Abbott 2014b [pers comm]).
Saps 2009 used author‐defined outcomes to evaluate improvement in pain from amitriptyline compared to placebo at four weeks' follow‐up. They found no difference between the intervention and control groups when assessing the self reported outcomes of "how well did the medication relieve your pain" and "overall how do you feel your problem is". Out of the 46 children in the amitriptyline group, 23 experienced an improvement in pain, compared to 22 out of 44 in the control group (OR 1.05, 95% CI 0.45 to 2.45).
We were unable to perform a meta‐analysis due to the use of different outcome measures and lack of numerical data. The GRADE quality rating for this outcome was very low, due to the small number of studies with methodological flaws.
Comparison 2. Antibiotics compared to placebo
Two studies, Collins 2011 and Heyland 2012, evaluated antibiotics compared to placebo in 112 children with Rome II criteria for IBS, functional dyspepsia, functional abdominal pain, or abdominal migraine (Rasquin‐Weber 1999), and RAP (Apley 1958).
Collins 2011 assessed the effectiveness of rifaximin compared to placebo. The authors used 10 self reported outcomes on a VAS and a pain questionnaire to evaluate improvement in pain. The 10 self reported outcomes included: incomplete evacuation, abdominal pain, diarrhoea, constipation, urgency to pass stool, passage of mucus, straining, and faecal soiling. The authors stated that there were no differences between the intervention and control groups for any of the outcomes. The authors did not present their data to support this conclusion. We contacted the authors but received no response (Abbott 2014a [pers comm]).
Heyland 2012 also reported the use of antibiotics to treat RAP. They compared co‐trimoxazole to placebo on the mean pain index. This was a 10‐point VAS measuring pain severity for each group. The mean pain index changed from 6.9 pre‐treatment to 4.1 post‐treatment in the intervention group, a mean change of ‐2.9. In the placebo group, the mean pain index changed from 7.4 pre‐treatment to 3.0 post‐treatment, a mean change of ‐4.4. The authors found no difference in scores on the mean change of pain index between the two groups. No raw data were given. We contacted the authors but received no response (Martin 2016c [pers comm]).
We were unable to perform a meta‐analysis due to lack of numerical data. These papers offer no evidence of the effectiveness of antibiotics to treat functional gastrointestinal disorders. The GRADE quality rating for this outcome was very low, due to lack of reliable outcome data to assess precision of treatment effect, and small and single studies for any drug intervention.
Comparison 3. Antimuscarinic drugs compared to usual care
Karabulut 2013 compared trimebutine to usual care in 78 children with IBS as defined by Rome III criteria (Rasquin 2006). The authors reported a self defined outcome of pain improvement, "adequate relief", as assessed by the parents. There was an improvement in 37 out of 39 children treated with trimebutine and in eight out of 39 children treated with usual care (P < 0.0001; OR 71.7, 95% CI 14.2 to 362.7). However, it is important to note that the intervention was not blinded and that the outcome measure was parental assessment, therefore performance and detection bias alone could explain the results. Consequently, the GRADE quality rating was very low, and the results should be interpreted with caution.
Comparison 4. 5‐HT4 agonists compared to usual care
Khoshoo 2006 compared tegaserod with usual care in 48 children with constipation‐predominant IBS. The authors reported 14 out of 21 children with good pain reduction in the treatment group compared with 5 out of 27 children in the control group (P < 0.05 (exact P value not reported in paper); OR 8.8, 95% CI 2.3 to 33.2). This suggests that tegaserod may be effective in relieving abdominal pain in constipation‐predominant IBS. Due to the small number of children participating in the study and the risk of performance and detection bias, this result should be interpreted with caution. Importantly, it is not clear if this was a post‐hoc analysis. The GRADE quality rating for this comparison was therefore very low.
Comparison 5. Antispasmodics compared to placebo
Four trials compared antispasmodics to placebo in 377 children with functional abdominal pain (Asgarshirazi 2015; Kline 2001; Narang 2015; Pourmoghaddas 2014). For a summary of antispasmodics, see Table 1.
Asgarshirazi 2015 compared peppermint oil, placebo and a synbiotic Lactol (containing a probiotic and fructo‐oligosaccharide) in a three‐arm RCT. One hundred and twenty children with functional gastrointestinal disorders based on Rome III criteria were included in the study, but those with abdominal migraine were excluded. Changes in severity, duration, and frequency of pain and any adverse effects were reported at four weeks. The results for the synbiotic intervention are reported in an accompanying dietary review (Newlove‐Delgado in press). Thirty‐four children completed the study and were analysed in the peppermint oil intervention group and 25 in the placebo group. The authors reported the intervention group compared to placebo: mean difference (MD) in pain duration ‐25.4 minutes/day (95% CI ‐35.5 to ‐15.3), MD in frequency of pain ‐1.4 episodes/week (95% CI ‐2.0 to ‐0.8), and MD in severity of pain ‐1.1 on a numerical rating scale (95% CI ‐1.8 to ‐0.4). Notably, this trial was at risk of bias from incomplete outcome data and differential loss of children between groups (38% drop out in the placebo group and 15% in the intervention group). The placebo was different in preparation and dose timing compared to the intervention drug.
Kline 2001 also compared peppermint oil capsules with placebo in 42 children with IBS as defined by the Rome criteria (Rasquin‐Weber 1999). The authors reported that, at two weeks, 15 out of 21 children in the peppermint group had a clinician‐judged improvement in pain compared to 9 out of 21 in the placebo group (OR 3.33, 95% CI 0.93 to 12.01). The authors reported the 15‐item Gastrointestinal Symptom Rating Scale (Svedulend 1988), which measures frequency, duration, and impact on daily life, as showing no difference between groups, but do not provide the data in the study. We contacted the trial authors but received no response (Martin 2016f [pers comm]). The authors reported that daily diaries completed by children showed significantly lower mean pain severity in the peppermint oil group. The authors provided neither data nor explanation of how the analysis of the daily diaries was carried out, although the P value for this comparison was reported to be less than 0.03. No side effects were reported in either group. This study therefore provides insufficient evidence to support the use of peppermint oil in the treatment of RAP.
Narang 2015 compared drotaverine to placebo in a RCT with 132 children with RAP as defined by Apley (Apley 1958). They assessed children's pain severity and frequency, school attendance, and parental judgement of well‐being (Likert scale) after four weeks. The authors provided no results for pain severity, only pain frequency, reporting the mean number of episodes of pain in four weeks and number of pain‐free days in four weeks. They found a mean of 10.3 (SD = 14) episodes of pain in the 64 children receiving drotaverine and a mean of 21.6 episodes (SD = 32.4) in the 60 children receiving placebo. The MD between the groups in episodes of pain was 11.3 (95% CI 2.4 to 20.1). The mean number of pain‐free days was 17.4 (SD = 8.2) in the intervention group and 15.6 (SD = 8.7) in the placebo group; MD 1.8, 95% CI ‐1.2 to 4.8. The authors reported the mean number of school days missed as 0.24 (SD = 0.85) in the drotaverine group and 0.71 (SD = 1.59) in the placebo group. The MD between these two groups was 0.46 (95% CI 0.01 to 0.91).
Pourmoghaddas 2014 randomised 115 children to mebeverine versus placebo, and found no effectiveness of mebeverine in treating functional abdominal pain in children. Self reported treatment response rates in the mebeverine and placebo groups were 40.6% and 30.3%, respectively at four weeks' postintervention (P = 0.469; OR 1.6, 95% CI 0.7 to 3.4) and 54.2% and 41.0%, respectively at 12 weeks' postintervention (P = 0.416; OR 1.7, 95% CI 0.8 to 3.6). This was an intention‐to‐treat analysis; the authors used last observed carried forward to substitute for missing data. The authors found no difference between the groups in scores on the physician‐rated change of the Clinical Global Improvement‐Severity scale, at four or 12 weeks' postintervention (NIMH 1985).
A meta‐analysis of studies evaluating antispasmodic drugs was not possible due to the heterogeneity of the interventions and variation in outcome measures. The overall GRADE quality rating for evidence evaluating this comparison was very low.
Comparison 6. Selective serotonin re‐uptake inhibitors (SSRIs) compared to placebo
Roohafza 2014 compared citalopram and placebo in 115 children with functional abdominal pain as defined by Rome III criteria (Rasquin 2006). The authors found no difference in treatment response rate between the groups at four weeks' postintervention (40.6% citalopram, 30.3% placebo; P = 0.169; OR 1.6, 95% CI 0.7 to 3.4) or at 12 weeks' postintervention (52.5% citalopram, 41.0% placebo; P = 0.148; OR 1.6, 95% CI 0.8 to 3.3). This was an intention‐to‐treat analysis. The authors used last observed carried forward to substitute for missing data. In addition, there were no differences between the two groups on scores for the secondary outcomes of self assessed change in severity of depression, as assessed using the Children's Depression Inventory (Kovacs 1985); anxiety, as assessed using the Revised Children's Manifest Anxiety Scale (Reynolds 1979); or somatization, as assessed using the Children's Somatization Inventory (Walker 2009), at four or 12 weeks' postintervention. The citalopram group experienced more drowsiness (37.2% citalopram, 16.2% placebo; P = 0.025) and dry mouth (44.1% citalopram, 23.2% placebo; P = 0.034) compared to the placebo group. The overall GRADE quality rating for evidence evaluating this comparison was very low.
Comparison 7. Antihistamines compared to placebo
Sadeghian 2008 randomised 29 children with functional abdominal pain as defined by Rome II criteria to cyproheptadine or placebo (Rasquin‐Weber 1999). They reassessed the outcome measures of pain intensity, pain frequency, and global self judgement of improvement in symptoms at two weeks. Pain intensity and pain frequency improved in 9 out of 15 children in the treatment group and 2 out of 14 children in the placebo group (OR 9.0, 95% CI 1.46 to 55.48). While this result suggests effectiveness, it should be interpreted with caution due to the very low GRADE quality rating of the evidence. The study was at risk of bias from the small sample size (imprecision of treatment effect), used non‐validated measurement tools, and the findings have not been reproduced in other studies.
Comparison 8. H2 receptor antagonists compared to placebo
One study, See 2001, compared famotidine versus placebo in a randomised cross‐over trial of 25 children with RAP, as defined by Apley 1958. The authors provided results from the first period of the trial during which 12 children received famotidine and 13 children received placebo. They reported that 8 out of 12 children receiving famotidine in the first period improved compared to 2 out of 13 children receiving placebo in the first period (OR 11.0, 95% CI 1.6 to 75.5). They also reported scores on an "abdominal pain score" (APS), which included three components: pain frequency, pain severity, and a peptic index score, which evaluates a number of symptoms, including nausea, vomiting, and nocturnal waking. This appears not to be a validated tool used in other studies. The authors reported no statistically significant difference in scores between famotidine and placebo, considering all the data from both periods. They reported finding an improvement in APS on famotidine (mean 3.37 (SD ± 3.53)) and on placebo (mean 1.66 (SD ± 2.7)). The MD in improvement on APS is 1.71. We were not able to provide confidence intervals around this difference due to insufficient data from the study authors. This is a cross‐over trial, and we did not impute values for the correlation coefficient. The authors of the previous review were unsuccessful in contacting the trial authors (Huertas‐Ceballos 2008a). The GRADE quality rating was very low due to the lack of primary data to confirm findings, no washout period between cross‐over of interventions, and the use of an invalidated tool.
Comparison 9. Serotonin antagonists compared to placebo
Symon 1995 reported the effects of pizotifen versus placebo in a restricted subgroup of 16 children with RAP. Although the children satisfied the standard definition of RAP (Apley 1958), they also had to report associated facial pallor, and either one first‐degree relative or two second‐degree relatives with a history of migraine or throbbing headache to be included in the study. This was a cross‐over study comparing the MD in the number of days on which children reported abdominal pain while taking pizotifen and placebo. The children reported a MD of 8.21 (95% CI 2.93 to 13.48) fewer days of pain while taking the intervention drug. The authors also reported the MD on the "Index of Severity", which was ‐16.21 (95% CI ‐26.51 to ‐5.90), and the MD on the "Index of Misery", which was ‐56.07 (95% CI ‐94.07 to ‐18.07). These appear not to be validated tools, but were judged by the trial authors to measure improvement. The study authors reported P values of 0.005 and 0.007 for these two comparisons, respectively. The authors reported that the trial was stopped early as a result of an interim analysis conducted when their initial supplies of drug preparations reached their expiry dates. They did not provide details of the size of the sample they had originally planned to include in the study. These findings have not been replicated in other studies. The GRADE rating was very low for this comparison.
Comparison 10. Dopamine receptor antagonist compared to placebo
Karunanayake 2015 compared domperidone to placebo in 89 children aged five to 12 years with abdominal pain‐predominant functional gastrointestinal disorders as defined by Rome III criteria (Rasquin 2006). Two primary outcomes were specified: cure and improvement. Cure was abdominal pain less than 25 mm on the VAS and no impact on daily activity. Improvement was pain relief and sense of improvement recorded on the Global Assessment Scale. No further information on how these primary outcomes were defined, when they were measured, or who assessed them was provided. The study is available as a conference abstract, and despite having written to the authors twice (Martin 2016a [pers comm]; Martin 2016d [pers comm]), we have no further published or unpublished information. The authors state that there was no difference in "cure" between the two groups, but provided no data to support this. They state that 37 out of 47 (78.7%) of the children treated with domperidone had "significant improvement" and 25 out of 42 (59.5%) in the placebo group had "significant improvement". This gives an OR for improvement of 2.52 (95% CI 0.99 to 6.39). Due to the lack of information about this outcome measurement and the limited possible 'Risk of bias' assessment, this result should be interpreted with caution. Regarding secondary outcomes, the domperidone group reported a significant reduction in abdominal pain severity (70.84% versus 48.18%) and improvement on the motility index (29.3% versus 8.6%) after intervention. No such difference was seen in improvement of quality of life and family impact. It was also unclear how these secondary outcomes were defined, measured, or who assessed them. The GRADE rating was very low for this comparison.
Comparison 11. Hormone treatment compared to placebo
Zybach 2016 reported the therapeutic effect of melatonin compared to placebo in 12 children aged 11 to 16 years with functional abdominal pain as defined by Rome III criteria (Rasquin 2006). This was a cross‐over study with no washout period. No power calculation was performed, but due to the low number of children, the study may have been underpowered. The authors found no difference in pain response reported by those treated with melatonin compared to placebo: OR 0.71 (95% CI 0.14 to 3.58). The authors also reported no change in mean sleep duration: melatonin group 9.9 (SD ± 3.53) hours; placebo group 9.41 (SD ± 2.7) hours. The GRADE rating for this comparison was very low.
Discussion
Summary of main results
Recurrent abdominal pain is an extremely common condition in childhood, and survey data in the USA suggest that many paediatricians use drug treatment for RAP (Edwards 1994). The most striking result of this review is the paucity of good‐quality, placebo‐controlled trials for all of the drugs that have been recommended for use in children with RAP.
In 2006, the paediatric Rome III criteria were devised to classify paediatric functional gastrointestinal disorders (Rasquin 2006). Diagnoses included within this classification comprised five categories defined on the basis of symptom profiles: IBS, functional dyspepsia, functional abdominal pain, abdominal migraine, and functional abdominal pain syndrome. The consistent symptom in each of these profiles is unexplained abdominal pain, which, prior to the development of this classification, was the complaint used to identify patients. It remains unclear the extent to which separating children into these subgroups defines patients who have different psychological or pathophysiological mechanisms underlying their symptoms or predicting their treatment response.
The studies with positive results are either small, single studies that have not been replicated or are larger studies with methodological flaws. Therefore, there is insufficient evidence to recommend any drug treatment.
There was no evidence from Bahar 2008 or Saps 2009 to suggest that amitriptyline is effective in treating RAP. Similarly, there was no evidence that antibiotics have a role in the treatment of RAP (Collins 2011; Heyland 2012). A single study reported that trimebutine (an antimuscarinic drug) was extremely effective in treating RAP (Karabulut 2013), but this was based on reports of symptoms by parents who were aware of whether their children were either receiving active treatment or not. A single, small study with a high risk of bias evaluated tegaserod (a 5‐HT4 agonist) (Khoshoo 2006). The findings of this study suggest that tegaserod may be effective, but further evidence is required before it can be recommended. We found two trials of peppermint oil: one trial showed no clear efficacy, but the small numbers may mean it was underpowered (Kline 2001), and the second trial had key methodological flaws requiring that it be interpreted with caution (Asgarshirazi 2015). There is no current evidence to recommend peppermint oil. Narang 2015 suggested benefit from drotaverine in some of the outcomes measured; others were either unreported or the children received no benefit from the intervention. Pourmoghaddas 2014 found no effectiveness of mebeverine in treating functional abdominal pain in children. Similarly, Roohafza 2014 compared citalopram and placebo and found no difference in treatment response rate between the groups. Sadeghian 2008 and See 2001 suggested that antihistamines and H2 receptor antagonists respectively may be effective in treating RAP. However, these results should be interpreted with caution due to risk of bias in the studies, small sample numbers, and therefore imprecision of estimates. In addition, the results of these single studies have not been reproduced. Symon 1995 reported the effects of pizotifen versus placebo in a subgroup of children with RAP fulfilling the definition of abdominal migraine. In the 14 children studied, the mean number of days of pain was less in the pizotifen group. The results of this small study, which was stopped early as this interim analysis was conducted when the drug supply had expired, has not been replicated in the last 20 years. Karunanayake 2015 published in abstract form a study of domperidone versus placebo; there was insufficient information on the outcomes measured and the quality of the study to conclude if domperidone may be effective in treating RAP. Zybach 2016 investigated melatonin compared to placebo in a small number of children in a cross‐over trial and found no efficacy. For a summary of these results, please see Table 1.
Overall completeness and applicability of evidence
This review highlights some issues concerning the overall completeness and applicability of the evidence of benefits and harms of pharmacological interventions for children and adolescents with RAP: the lack of trials conducted in specific subgroups of RAP as defined by the Rome III criteria (Rasquin 2006); the lack of trials assessing the same class of drug; and the lack of sustained intervention and follow‐up beyond the period of intervention.
The majority of studies included children within the broad diagnosis of RAP, which meant that children could be presenting with a variety of RAP classifications such as IBS, functional abdominal pain, or functional dyspepsia. This meant that it was not possible to investigate whether particular classes of drugs benefited particular subgroups of RAP more than other subgroups.
Lastly, most of the interventions were relatively short in duration (two to six weeks), and very few had medium‐ or long‐term follow‐up, which limits the ability to assess whether any benefits are sustained in the long term.
Quality of the evidence
The overall quality of this evidence was low.
Potential biases in the review process
The present systematic review has many strengths. We developed a protocol for this review according to guidance provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b). We published our protocol before we embarked on the review itself (Martin 2014a). We conducted extensive searches of relevant databases and checked forward and backward citations of all included studies. We also contacted authors of included studies for additional data when the presented data were insufficient or data were missing, to maximise our ability to pool data on comparable outcomes within comparable intervention types. Two review authors, working independently, selected trials for inclusion and extracted data. Disagreements were resolved by discussion between team members. We assessed the risk of bias in all trials according to the recommendations provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a).
We did not include studies that had a mix of children, adolescents, and young adults when it was not possible to separate the data for children younger than 18 years of age. Likewise, we did not include studies that did not specify recruiting children or adolescents, and which presented mean ages of the population sample exceeding 20 years of age. Both these issues raise the possibility of bias in our review process, as we did not write to these authors asking whether or not they collected data for children younger than 18 years of age. However, we believe this potential bias is not likely to have changed our conclusions.
Agreements and disagreements with other studies or reviews
The previous version of this review, Huertas‐Ceballos 2008a, included only three studies (Kline 2001; See 2001; Symon 1995). This update includes 16 studies and reached a similar conclusion: there is no evidence to support the use of drugs to treat RAP or functional gastrointestinal disorders in children. Another Cochrane review evaluating the effectiveness of antidepressants in pain‐related functional abdominal disorders in children also agrees with this conclusion (Kaminski 2009).
Authors' conclusions
Implications for practice.
Overall, this review provides only extremely weak evidence for the efficacy of some pharmacological agents in children with RAP. The lack of clear evidence of effectiveness for any drug suggests that there is little reason for their use outside of well‐conducted clinical trials. Clinicians may choose to prescribe drugs to children whose symptoms are severe and who have not responded to simple management. However, when using drugs as a 'therapeutic trial', clinicians need to be aware that RAP is a fluctuating condition and any 'response' may reflect the natural history of the condition or a placebo effect, rather than drug efficacy.
Implications for research.
The pathogenesis of RAP in children remains unclear (Hyams 1998). There is an obvious need for further studies to be conducted to elucidate this aetiology. It may be that the complaint of pain is a unifying manifestation for a wide variety of causal pathways and triggers relating to psychological and physical processes. It is unlikely that RAP is a single disease entity. Further trials are therefore needed not only to guide the management of children with RAP, but also to validate the usefulness of suggested classifications (Rasquin 2006).
What's new
| Date | Event | Description |
|---|---|---|
| 10 June 2016 | New citation required and conclusions have changed | Following a new search in April 2013 and updated searches in April 2014, March 2015, and June 2016, we added 13 new studies. |
| 31 January 2015 | New search has been performed | The review supersedes the previous version, following a new protocol and new search up to June 2016 (see Published notes). |
Notes
This is a new review, which supersedes a previously published review (Huertas‐Ceballos 2008a).
Acknowledgements
We acknowledge the work of the original review authors: Angela Huertas‐Ceballos, Stuart Logan, Cathy Bennett, Sarah See, Colin Macarthur, and Morris Zwi. We would also like to acknowledge the work of the Cochrane Developmental, Psychosocial and Learning Problems Review Group, the editors, statistician, and peer reviewers who commented on early drafts of this review.
Appendices
Appendix 1. Search strategies
Cochrane Central Register of Controlled Studies (CENTRAL; 2016, Issue 5) in the Cochrane Library
Search dates: 19 April 2013 (990 records); 11 April 2014 (1271 records); 26 March 2015 (49 records); 10 June 2016 (81 records).
#1 Pain*:ti,ab #2 Ache*:ti,ab #3 Sore*:ti,ab #4 Discomfort*:ti,ab #5 Distress*:ti,ab #6 Cramp*:ti,ab #7 Disorder:ti,ab #8 Disorders:ti,ab #9 Symptom:ti,ab #10 Symptoms:ti,ab #11 Migraine:ti,ab #12 Migraines:ti,ab #13 Epilep*:ti,ab #14 Colic*:ti,ab #15 Syndrome:ti,ab #16 Syndromes:ti,ab #17 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 #18 Stomach*:ti,ab #19 Abdom*:ti,ab #20 Intestin*:ti,ab #21 Viscera*:ti,ab #22 Tummy:ti,ab #23 Bowel*:ti,ab #24 Belly:ti,ab #25 Gastrointestinal:ti,ab #26 GI:ti,ab #27 Gastric:ti,ab #28 #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 #29 #17 and #28 #30 Colonic disease*:ti,ab #31 Irritable bowel:ti,ab #32 IBS:ti,ab #33 Functional dyspepsia:ti,ab #34 MeSH descriptor: [Irritable Bowel Syndrome] explode all trees #35 MeSH descriptor: [Colonic Diseases, Functional] explode all trees #36 MeSH descriptor: [Abdominal Pain] explode all trees #37 MeSH descriptor: [Dyspepsia] explode all trees #38 MeSH descriptor: [Colic] explode all trees #39 MeSH descriptor: [Abdomen, Acute] explode all trees #40 #29 or #30 or #31 or #32 or #33 or #34 or #35 or #36 or #37 or #38 or #39 #41 Recurr*:ti,ab #42 Chronic*:ti,ab #43 Intermittent*:ti,ab #44 Episode*:ti,ab #45 Bout:ti,ab #46 Bouts:ti,ab #47 Spasm*:ti,ab #48 Transitory:ti,ab #49 Transient:ti,ab #50 Functional:ti,ab #51 Continu*:ti,ab #52 Paroxysmal:ti,ab #53 Persistent:ti,ab #54 Idiopathic:ti,ab #55 Unspecifi*:ti,ab #56 Non specifi*:ti,ab #57 Nonspecific*:ti,ab #58 Motility:ti,ab #59 MeSH descriptor: [Recurrence] explode all trees #60 #41 or #42 or #43 or #44 or #45 or #46 or #47 or #48 or #49 or #50 or #51 or #52 or #53 or #54 or #55 or #56 or #57 or #58 or #59 #61 #40 and #60 #62 irritable bowel syndrome*:ti,ab #63 #61 or #62 #64 Child*:ti,ab #65 Adolescen*:ti,ab #66 Boy*:ti,ab #67 Girl*:ti,ab #68 teen*:ti,ab #69 Schoolchild*:ti,ab #70 Young adult*:ti,ab #71 Youth*:ti,ab #72 Pediatric*:ti,ab #73 Paediatric*:ti,ab #74 Student*:ti,ab #75 Pupil*:ti,ab #76 Juvenile*:ti,ab #77 Young person*:ti,ab #78 MeSH descriptor: [Child] explode all trees #79 MeSH descriptor: [Adolescent] explode all trees #80 MeSH descriptor: [Young Adult] explode all trees #81 MeSH descriptor: [Students] explode all trees #82 #64 or #65 or #66 or #67 or #68 or #69 or #70 or #71 or #72 or #73 or #74 or #75 or #76 or #77 or #78 or #79 or #80 or #81 #83 #63 and #82
Ovid MEDLINE In‐Process & Other Non‐Indexed Citations and Ovid Medline (1946 to present)
Search dates: 11 April 2013 (6238 records); 11 April 2014 (5957 records); 25 March 2015 (223 records); 9 June 2016 (300 records).
1 stomach*.tw. 2 abdom*.tw. 3 intestin*.tw. 4 viscera*.tw. 5 tummy.tw. 6 bowel*.tw. 7 belly.tw. 8 gastrointestinal.tw. 9 gi.tw. 10 gastric.tw. 11 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 12 pain*.tw. 13 Ache*.tw. 14 Sore*.tw. 15 Discomfort*.tw. 16 Distress*.tw. 17 Cramp*.tw. 18 Disorder$1.tw. 19 Symptom$1.tw. 20 Migraine$1.tw. 21 Epilep*.tw. 22 syndrome$1.tw. 23 colic*.tw. 24 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 25 irritable bowel$.tw. 26 ibs.tw. 27 functional dyspepsia.tw. 28 25 or 26 or 27 29 ((stomach* or abdom* or intestin* or viscera* or tummy or bowel* or belly or gastrointestinal or gi or gastric) adj3 (pain* or Ache* or Sore* or Discomfort* or Distress* or Cramp* or Disorder$1 or Symptom$1 or Migraine$1 or Epilep* or syndrome$1 or colic*)).tw. 30 exp Irritable Bowel Syndrome/ 31 exp Colonic Diseases/ 32 exp Abdominal Pain/ 33 exp Dyspepsia/ 34 exp Colic/ 35 exp Abdomen, Acute/ 36 30 or 31 or 32 or 33 or 34 or 35 37 28 or 29 or 36 38 Recurr*.tw. 39 Chronic*.tw. 40 Intermittent*.tw. 41 Bout$1.tw. 42 spasm*.tw. 43 Transitory.tw. 44 Transient.tw. 45 Functional.tw. 46 Continu*.tw. 47 Paroxysmal.tw. 48 Persistent.tw. 49 Idiopathic.tw. 50 unspecifi*.tw. 51 Non specifi*.tw. 52 nonspecifi*.tw. 53 motility.tw. 54 episod*.tw. 55 38 or 39 or 40 or 41 or 42 or 43 or 44 or 45 or 46 or 47 or 48 or 49 or 50 or 51 or 52 or 53 or 54 56 exp Recurrence/ 57 55 or 56 58 37 and 57 59 irritable bowel syndrome*.tw. 60 58 or 59 61 randomized controlled trial.pt. 62 controlled clinical trial.pt. 63 randomi#ed.ab. 64 placebo$.ab. 65 randomly.ab. 66 trial.ab. 67 groups.ab. 68 exp animals/ not humans.sh. 69 or/61‐67 70 69 not 68 71 60 and 70 72 exp Child/ 73 exp Adolescent/ 74 exp Young Adult/ 75 exp Students/ 76 Child*.tw. 77 Adolescen*.tw. 78 Young person*.tw. 79 Boy*.tw. 80 Girl*.tw. 81 teen*.tw. 82 Schoolchild*.tw. 83 Young adult*.tw. 84 Youth*.tw. 85 P*ediatric*.tw. 86 Student*.tw. 87 Pupil*.tw. 88 Juvenile*.tw. 89 72 or 73 or 74 or 75 or 76 or 77 or 78 or 79 or 80 or 81 or 82 or 83 or 84 or 85 or 86 or 87 or 88 90 71 and 89
Embase Ovid (1974 to present)
Search dates: 11 April 2013 (2272 records); 11 April 2014 (2523 records); 25 March 2015 (250 records); 9 June 2016 (345 records).
1 recurr*.tw. 2 chronic*.tw. 3 intermittent*.tw. 4 bout$1.tw. 5 spasm*.tw. 6 transitory.tw. 7 transient.tw. 8 functional.tw. 9 continu*.tw. 10 paroxysmal.tw. 11 persistent.tw. 12 idiopathic.tw. 13 unspecifi*.tw. 14 non specifi*.tw. 15 nonspecifi*.tw. 16 motility.tw. 17 episod*.tw. 18 or/1‐17 19 exp recurrent disease/ 20 18 or 19 21 stomach*.tw. 22 abdom*.tw. 23 intestin*.tw. 24 viscera*.tw. 25 tummy.tw. 26 bowel*.tw. 27 belly.tw. 28 gastrointestinal.tw. 29 gi.tw. 30 gastric.tw. 31 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 32 pain*.tw. 33 Ache*.tw. 34 Sore*.tw. 35 Discomfort*.tw. 36 Distress*.tw. 37 Cramp*.tw. 38 Disorder$1.tw. 39 Symptom$1.tw. 40 Migraine$1.tw. 41 Epilep*.tw. 42 syndrome$1.tw. 43 colic*.tw. 44 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 or 43 45 irritable bowel$.tw. 46 ibs.tw. 47 functional dyspepsia.tw. 48 45 or 46 or 47 49 ((stomach* or abdom* or intestin* or viscera* or tummy or bowel* or belly or gastrointestinal or gi or gastric) adj3 (pain* or Ache* or Sore* or Discomfort* or Distress* or Cramp* or Disorder$1 or Symptom$1 or Migraine$1 or Epilep* or syndrome$1 or colic*)).tw. 50 48 or 49 51 exp colic/ 52 exp irritable colon/ 53 exp abdominal pain/ 54 exp dyspepsia/ 55 colon disease/ 56 50 or 51 or 52 or 53 or 54 or 55 57 20 and 56 58 irritable bowel syndrome*.tw. 59 57 or 58 60 Clinical trial/ 61 Randomized controlled trial/ 62 Randomization/ 63 Single blind procedure/ 64 Double blind procedure/ 65 Crossover procedure/ 66 Placebo/ 67 Randomi?ed controlled trial$.tw. 68 Rct.tw. 69 Random allocation.tw. 70 Randomly allocated.tw. 71 Allocated randomly.tw. 72 (allocated adj2 random).tw. 73 Single blind$.tw. 74 Double blind$.tw. 75 ((treble or triple) adj blind$).tw. 76 Placebo$.tw. 77 Prospective study/ 78 or/60‐77 79 Case study/ 80 Case report.tw. 81 Abstract report/ or letter/ 82 or/79‐81 83 78 not 82 84 59 and 83 85 exp Child/ 86 exp Adolescent/ 87 exp Young Adult/ 88 exp Students/ 89 Child*.tw. 90 Adolescen*.tw. 91 Young person*.tw. 92 Boy*.tw. 93 Girl*.tw. 94 teen*.tw. 95 Schoolchild*.tw. 96 Young adult*.tw. 97 Youth*.tw. 98 P*ediatric*.tw. 99 Student*.tw. 100 Pupil*.tw. 101 Juvenile*.tw. 102 85 or 86 or 87 or 88 or 89 or 90 or 91 or 92 or 93 or 94 or 95 or 96 or 97 or 98 or 99 or 100 or 101 103 84 and 102
CINAHL Healthcare Databases Advanced Search (Cumulative Index to Nursing and Allied Health Literature; 1981 to present)
Search dates: 18 April 2013 (175 records); 11 April 2014 (195 records); 26 March 2015 (21 records); 9 June 2016 (11 records).
1. CINAHL; recurr*.ti,ab; 2. CINAHL; chronic*.ti,ab; 3. CINAHL; intermittent*.ti,ab; 4. CINAHL; (bout OR bouts).ti,ab; 5. CINAHL; spasm*.ti,ab; 6. CINAHL; transitory.ti,ab; 7. CINAHL; transient.ti,ab; 8. CINAHL; functional.ti,ab; 9. CINAHL; continu*.ti,ab; 10. CINAHL; paroxysmal.ti,ab; 11. CINAHL; persistent.ti,ab; 12. CINAHL; idiopathic.ti,ab; 13. CINAHL; unspecifi*.ti,ab; 14. CINAHL; "non specifi*".ti,ab; 15. CINAHL; nonspecifi*.ti,ab; 16. CINAHL; motility.ti,ab; 17. CINAHL; episod*.ti,ab; 18. CINAHL; exp RECURRENCE/; 19. CINAHL; 1 OR 2 OR 3 OR 4 OR 5 OR 6 OR 7 OR 8 OR 9 OR 10 OR 11 OR 12 OR 13 OR 14 OR 15 OR 16 OR 17 OR 18; 20. CINAHL; stomach*.ti,ab; 21. CINAHL; abdom*.ti,ab; 22. CINAHL; intestin*.ti,ab; 23. CINAHL; viscera*.ti,ab; 24. CINAHL; tummy.ti,ab; 25. CINAHL; bowel*.ti,ab; 26. CINAHL; belly.ti,ab; 27. CINAHL; gastrointestinal.ti,ab; 28. CINAHL; gi.ti,ab; 29. CINAHL; gastric.ti,ab; 30. CINAHL; 20 OR 21 OR 22 OR 23 OR 24 OR 25 OR 26 OR 27 OR 28 OR 29; 31. CINAHL; pain*.ti,ab; 32. CINAHL; Ache*.ti,ab; 33. CINAHL; Sore*.ti,ab; 34. CINAHL; Discomfort*.ti,ab; 35. CINAHL; Distress*.ti,ab; 36. CINAHL; Cramp*.ti,ab; 37. CINAHL; (Disorder OR Disorders).ti,ab; 38. CINAHL; (Symptom OR Symptoms).ti,ab; 39. CINAHL; (Migraine OR Migraines).ti,ab;. 40. CINAHL; Epilep*.ti,ab; 41. CINAHL; (syndrome OR syndromes).ti,ab; 42. CINAHL; colic*.ti,ab; 43. CINAHL; 31 OR 32 OR 33 OR 34 OR 35 OR 36 OR 37 OR 38 OR 39 OR 40 OR 41 OR 42; 44. CINAHL; 30 AND 43; 45. CINAHL; "irritable bowel*".ti,ab; 46. CINAHL; ibs.ti,ab; 47. CINAHL; "functional dyspepsia".ti,ab; 48. CINAHL; exp COLIC/; 49. CINAHL; exp IRRITABLE BOWEL SYNDROME/; 50. CINAHL; exp COLONIC DISEASES, FUNCTIONAL/; 51. CINAHL; exp ABDOMINAL PAIN/; 52. CINAHL; exp DYSPEPSIA/; 53. CINAHL; 45 OR 46 OR 47 OR 48 OR 49 OR 50 OR 51 OR 52; 54. CINAHL; 44 OR 53; 55. CINAHL; 19 AND 54; 56. CINAHL; (irritable AND bowel AND syndrome*).ti,ab; 57. CINAHL; 55 OR 56; 58. CINAHL; Child*.ti,ab; 59. CINAHL; Adolescen*.ti,ab; 60. CINAHL; "Young person*".ti,ab; 61. CINAHL; Boy*.ti,ab; 62. CINAHL; Girl*.ti,ab; 63. CINAHL; teen*.ti,ab; 64. CINAHL; Schoolchild*.ti,ab; 65. CINAHL; "Young adult*".ti,ab; 66. CINAHL; Youth*.ti,ab; 67. CINAHL; Student*.ti,ab; 68. CINAHL; Pupil*.ti,ab; 69. CINAHL; Juvenile*.ti,ab; 70. CINAHL; exp CHILD/; 71. CINAHL; exp STUDENTS/; 72. CINAHL; 70 OR 71; 73. CINAHL; Pediatric*.ti,ab; 74. CINAHL; Paediatric*.ti,ab; 75. CINAHL; 67 OR 68 OR 69 OR 72 OR 73 OR 74; 76. CINAHL; 63 OR 64 OR 65 OR 66; 77. CINAHL; 58 OR 59 OR 60 OR 61 OR 62; 78. CINAHL; 70 OR 73 OR 74 OR 75; 79. CINAHL; 57 AND 78; 80. CINAHL; exp RANDOMIZED CONTROLLED TRIALS/; 81. CINAHL; random*.ti,ab; 82. CINAHL; "clin* trial*".ti,ab; 83. CINAHL; (singl* OR doubl* OR tripl* OR trebl*).ti,ab; 84. CINAHL; (mask* OR blind*).ti,ab; 85. CINAHL; 83 AND 84; 86. CINAHL; "random* allocate*".ti,ab; 87. CINAHL; "random assign*".ti,ab; 88. CINAHL; exp RANDOM ASSIGNMENT/; 89. CINAHL; exp CLINICAL TRIALS/; 90. CINAHL; exp META ANALYSIS/; 91. CINAHL; 88 OR 89 OR 90; 92. CINAHL; 80 OR 81 OR 82 OR 85 OR 86 OR 87; 93. CINAHL; 91 OR 92; 94. CINAHL; 79 AND 93;
PsycINFO Ovid (1806 to present)
Search dates: 18 April 2013 (238 records); 11 April 2014 (757 records); 25 March 2015 (47 records); 9 June 2016 (87 records).
1 stomach*.tw. 2 abdom*.tw. 3 intestin*.tw. 4 viscera*.tw. 5 tummy.tw. 6 bowel*.tw. 7 belly.tw. 8 gastrointestinal.tw. 9 gi.tw. 10 gastric.tw. 11 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 12 pain*.tw. 13 Ache*.tw. 14 Sore*.tw. 15 Discomfort*.tw. 16 Distress*.tw. 17 Cramp*.tw. 18 Disorder$1.tw. 19 Symptom$1.tw. 20 Migraine$1.tw. 21 Epilep*.tw. 22 syndrome$1.tw. 23 colic*.tw. 24 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 25 irritable bowel$.tw. 26 ibs.tw. 27 functional dyspepsia.tw. 28 25 or 26 or 27 29 ((stomach* or abdom* or intestin* or viscera* or tummy or bowel* or belly or gastrointestinal or gi or gastric) adj3 (pain* or Ache* or Sore* or Discomfort* or Distress* or Cramp* or Disorder$1 or Symptom$1 or Migraine$1 or Epilep* or syndrome$1 or colic*)).tw. 30 exp Irritable Bowel Syndrome/ 31 exp Dyspepsia/ 32 recurr*.tw. 33 chronic*.tw. 34 intermittent*.tw. 35 bout$1.tw. 36 spasm*.tw. 37 transitory.tw. 38 transient.tw. 39 functional.tw. 40 continu*.tw. 41 paroxysmal.tw. 42 persistent.tw. 43 idiopathic.tw. 44 unspecifi*.tw. 45 non specifi*.tw. 46 nonspecifi*.tw. 47 motility.tw. 48 episod*.tw. 49 or/32‐48 50 irritable bowel syndrome*.tw. 51 exp Students/ 52 Child*.tw. 53 Adolescen*.tw. 54 Young person*.tw. 55 Boy*.tw. 56 Girl*.tw. 57 teen*.tw. 58 Schoolchild*.tw. 59 Young adult*.tw. 60 Youth*.tw. 61 P*ediatric*.tw. 62 Student*.tw. 63 Pupil*.tw. 64 Juvenile*.tw. 65 28 or 29 or 30 or 31 66 49 and 65 67 50 or 66 68 51 or 52 or 53 or 54 or 55 or 56 or 57 or 58 or 59 or 60 or 61 or 62 or 63 or 64 69 67 and 68
ERIC ProQuest (Education Resources Information Center; 1966 to present)
Search dates: 19 April 2013 (276 records); 11 April 2014 (294 records); 26 March 2015 (0 records); 9 June 2016 (2 records).
(ab,ti(Pain*) OR ab,ti(Ache*) OR ab,ti(Sore*) OR ab,ti(Discomfort*) OR ab,ti(Distress*) OR ab,ti(Cramp*) OR ab,ti(Disorder) OR ab,ti(Disorders) OR ab,ti(Symptom*) OR ab,ti(Migraine*) OR ab,ti(Epilep*) OR ab,ti(Colic*) OR ab,ti(Syndrome*))
AND
(Ab,ti(Recurr*) OR ab,ti(Chronic*) OR ab,ti(Intermittent*) OR ab,ti(Episode*) OR ab,ti(Bout) OR ab,ti(Bouts) OR ab,ti((Spasm*) OR ab,ti(Transitory) OR ab,ti(Transient) OR ab,ti(Functional) OR ab,ti(Continu*) OR ab,ti(paroxysmal) OR ab,ti(Persistent) OR ab,ti (Idiopathic) OR ab,ti(Unspecifi*) OR ab,ti(Non specifi*) OR ab,ti(motility) OR SU.EXACT.EXPLODE("Recurrence"))
AND
(Ab,ti(Stomach*) OR ab,ti(Abdom*) OR ab,ti(Sore*) OR ab,ti(Intestin*) OR ab,ti(Viscera*) OR ab,ti(Tummy) OR ab,ti(Bowel*) OR ab,ti(Belly) OR ab,ti(Gastrointestinal) OR ab,ti(GI) OR ab,ti(Epilep*) OR ab,ti(Gastric))
OR
(Ab,ti(irritable bowel*) OR ab,ti(ibs) OR ab,ti(colonic disease*) OR ab,ti(functional dyspepsia))
British Education Index ProQuest (1975 to present)
Search dates: 19 April 2013 (46 records); 11 April 2014 (48 records); 26 March 2015 (0 records); 9 June 2016 (5 records).
((ab,ti(Stomach*) OR ab,ti(Abdom*) OR ab,ti(Intestin*) OR ab,ti(Viscera*) OR ab,ti(Tummy) OR ab,ti(Bowel*) OR ab,ti(Belly) OR ab,ti(Gastrointestinal) OR ab,ti(GI) OR ab,ti(Gastric))
AND
((ab,ti(Pain*) OR ab,ti(Ache*) OR ab,ti(Sore*) OR ab,ti(Discomfort*) OR ab,ti(Distress*) OR ab,ti(Cramp*) OR ab,ti(Disorder) OR ab,ti(Disorders) OR ab,ti(Symptom) OR OR ab,ti(Symptoms) OR ab,ti(Migraine) OR ab,ti(Migraines) OR ab,ti(Epilep*) OR ab,ti(Colic*) OR ab,ti(Syndrome) OR ab,ti(Syndromes))
OR
(Ab,ti(irritable bowel*) OR ab,ti(ibs) OR ab,ti(Functional dyspepsia))
Applied Social Sciences Index and Abstracts ProQuest (ASSIA; 1987 to present)
Search dates: 19 April 2013 (179 records); 11 April 2014 (545 records); 26 March 2015 (27 records); 9 June 2016 (48 records).
((ab,ti(Stomach*) OR ab,ti(Abdom*) OR ab,ti(Intestin*) OR ab,ti(Viscera*) OR ab,ti(Tummy) OR ab,ti(Bowel*) OR ab,ti(Belly) OR ab,ti(Gastrointestinal) OR ab,ti(GI) OR ab,ti(gastric)
AND
(ab,ti(Pain*) OR ab,ti(Ache*) OR ab,ti(Sore*) OR ab,ti(Discomfort*) OR ab,ti(Distress*) OR ab,ti(Cramp*) OR ab,ti(Disorder) OR ab,ti(Disorders) OR ab,ti(Symptom*) OR ab,ti(Symptoms) OR ab,ti(Migraine*) OR ab,ti(Epilep*) OR ab,ti(Syndrome) OR ab,ti(Syndromes) OR ab,ti(colic*)
AND
(ab,ti(Recurr*) OR ab,ti(Chronic*) OR ab,ti(Intermittent*) OR ab,ti(Episode*) OR ab,ti(Bout) OR ab,ti(bouts) OR ab,ti(Spasm*) OR ab,ti(Transitory) OR ab,ti(Transient) OR ab,ti(Functional) OR ab,ti(Continu*) OR ab,ti(Paroxysmal) OR ab,ti(Persistent) OR ab,ti(Idiopathic) OR ab,ti(Unspecifi*) OR ab,ti(Non specifi*) OR ab,ti(motility))
OR
(ab,ti(irritable bowel) OR ab,ti(ibs) OR ab,ti(functional dyspepsia))
Allied and Complementary Medicine Healthcare Databases Advanced Search (AMED; 1985 to present)
Search dates: 18 April 2013 (63 records); 11 April 2014 (74 records); 25 March 2015 (1 record); 9 June 2016 (1 record).
1. AMED; Recurr*.ti,ab; 2. AMED; Chronic*.ti,ab; 3. AMED; Intermittent*.ti,ab; 4. AMED; Episod*.ti,ab; 5. AMED; (Bout OR Bouts).ti,ab; 6. AMED; Spasm*.ti,ab; 7. AMED; Transitory.ti,ab; 8. AMED; Transient.ti,ab; 9. AMED; Functional.ti,ab; 10. AMED; Continu*.ti,ab; 11. AMED; Paroxysmal.ti,ab; 12. AMED; Persistent.ti,ab; 13. AMED; Idiopathic.ti,ab; 14. AMED; Unspecifi*.ti,ab; 15. AMED; "Non specifi*".ti,ab; 16. AMED; Nonspecific*.ti,ab; 17. AMED; Motility.ti,ab; 18. AMED; exp RECURRENCE/; 19. AMED; 1 OR 2 OR 3 OR 4 OR 5 OR 6 OR 7 OR 8 OR 9 OR 10 OR 11 OR 12 OR 13 OR 14 OR 15 OR 16 OR 17 OR 18;. 20. AMED; Pain*.ti,ab; 21. AMED; Ache*.ti,ab; 22. AMED; Sore*.ti,ab; 23. AMED; Discomfort*.ti,ab; 24. AMED; Distress*.ti,ab; 25. AMED; Cramp*.ti,ab; 26. AMED; (Disorder OR Disorders).ti,ab; 27. AMED; (Symptom OR Symptoms).ti,ab; 28. AMED; (Migraine OR Migraines).ti,ab; 29. AMED; Epilep*.ti,ab; 30. AMED; Colic*.ti,ab; 31. AMED; (Syndrome OR Syndromes).ti,ab; 32. AMED; 20 OR 21 OR 22 OR 23 OR 24 OR 25 OR 26 OR 27 OR 28 OR 29 OR 30 OR 31; 33. AMED; Stomach*.ti,ab; 34. AMED; Abdom*.ti,ab; 35. AMED; Intestin*.ti,ab; 36. AMED; Viscera*.ti,ab; 37. AMED; Tummy.ti,ab; 38. AMED; Bowel*.ti,ab; 39. AMED; Belly.ti,ab; 40. AMED; Gastrointestinal.ti,ab; 41. AMED; GI.ti,ab; 42. AMED; Gastric.ti,ab; 43. AMED; 33 OR 34 OR 35 OR 36 OR 37 OR 38 OR 39 OR 40 OR 41 OR 42; 44. AMED; 32 AND 43; 45. AMED; "Colonic disease*".ti,ab; 46. AMED; "Irritable bowel".ti,ab; 47. AMED; IBS.ti,ab; 86 48. AMED; "Functional dyspepsia".ti,ab; 49. AMED; exp IRRITABLE BOWEL SYNDROME/; 50. AMED; exp COLONIC DISEASE/; 51. AMED; exp ABDOMINAL PAIN/; 52. AMED; exp DYSPEPSIA/; 53. AMED; 45 OR 46 OR 47 OR 48 OR 49 OR 50 OR 51 OR 52; 54. AMED; 44 OR 53; 55. AMED; 19 AND 54; 56. AMED; (irritable AND bowel AND syndrome*).ti,ab; 57. AMED; Child*.ti,ab; 58. AMED; Adolescen*.ti,ab; 59. AMED; Boy*.ti,ab; 60. AMED; Girl*.ti,ab; 61. AMED; teen*.ti,ab; 62. AMED; Schoolchild*.ti,ab; 63. AMED; "Young adult*".ti,ab; 64. AMED; Youth*.ti,ab; 767 results. 65. AMED; (Pediatric* OR Paediatric*).ti,ab; 66. AMED; Student*.ti,ab; 67. AMED; Pupil*.ti,ab; 68. AMED; Juvenile*.ti,ab; 69. AMED; "Young person*".ti,ab; 70. AMED; exp CHILD/; 71. AMED; exp ADOLESCENT/; 72. AMED; exp STUDENTS/; 73. AMED; 57 OR 58 OR 59 OR 60 OR 61 OR 62 OR 63 OR 64 OR 65 OR 66 OR 67 OR 68 OR 69 OR 70 OR 71 OR 72; 74 AMED; 55 OR 56; 75. AMED; 74 AND 73;
LILACS (Latin American and Caribbean Health Science Information database; lilacs.bvsalud.org/en; all available years)
Search dates: 19 April 2013 (11 records); 11 April 2014 (13 records); 26 March 2015 (0 records); 9 June 2016 (0 records).
((Pt randomized controlled trial OR Pt controlled clinical trial OR Mh randomized controlled trials OR Mh random allocation OR Mh double‐blind method OR Mh single‐blind method) AND NOT (Ct animal AND NOT (Ct human and Ct animal)) OR (Pt clinical trial OR Ex E05.318.760.535$ OR (Tw clin$ AND (Tw trial$ OR Tw ensa$ OR Tw estud$ OR Tw experim$ OR Tw investiga$)) OR ((Tw singl$ OR Tw simple$ OR Tw doubl$ OR Tw doble$ OR Tw duplo$ OR Tw trebl$ OR Tw trip$) (trial$ OR ensa$ OR estud$ OR experim$ OR investiga$ OR singl$ OR simple$ OR Tw doubl$ OR Tw doble$ OR Tw duplo$ OR Tw trebl$ OR Tw trip$) AND (Tw blind$ OR Tw cego$ OR Tw ciego$ OR Tw mask$ OR Tw mascar$)) OR Mh placebos OR Tw placebo$ OR (Tw random$ OR Tw randon$ OR Tw casual$ OR Tw acaso$ OR Tw azar OR Tw aleator$) OR Mh research design) AND NOT (Ct animal AND NOT (Ct human and Ct animal)) OR (Ct comparative study OR Ex E05.337$ OR Mh follow‐up studies OR Mh prospective studies OR Tw control$ OR Tw prospectiv$ OR Tw volunt$ OR Tw volunteer$) AND NOT (Ct animal AND NOT (Ct human and Ct animal))) [Palavras]
and ((recurr$ or chronic$ or intermittent$ or bout or bouts or spasm$ or transitory or transient or functional or continu$ or Paroxysmal or Persistent or Idiopathic or unspecifi$ or Non specifi$ or nonspecific$ or motility or episode$) [Palavras] and (pain$ or ache$ or sore$ or discomfort$ or distress$ cramp$ or colic$ or disorder or disorders or symptom or symptoms or Migraine$ or Epilep* or syndrome$) and (stomach$ or abdom$ or intestin$ or viscera$ or tummy$ or bowel$ or belly or gastrointestinal or gi or gastric)) [Palavras]
OpenGrey (www.opengrey.eu; 1980 to present)
Search dates : 19 April 2013 (1 record); 11 April 2014 (1 record); 26 March 2015 (0 records); 9 June 2016 (0 records).
Irritable bowel syndrom* Ibs functional dyspepsia Chronic* AND (abdom* OR stomach* OR intestin* OR viscera* OR tummy OR bowel* OR belly or gastrointestinal OR gi OR gastric)) Recurr* AND (abdom* OR stomach* OR intestin* OR viscera* OR tummy OR bowel* OR belly or gastrointestinal OR gi OR gastric)) Intermittent* AND (abdom* OR stomach* OR intestin* OR viscera* OR tummy OR bowel* OR belly or gastrointestinal OR gi OR gastric)) Bout* AND (abdom* OR stomach* OR intestin* OR viscera* OR tummy OR bowel* OR belly or gastrointestinal OR gi OR gastric)) spasm* AND (abdom* OR stomach* OR intestin* OR viscera* OR tummy OR bowel* OR belly or gastrointestinal OR gi OR gastric)) Transitory AND (abdom* OR stomach* OR intestin* OR viscera* OR tummy OR bowel* OR belly or gastrointestinal OR gi OR gastric)) Transient AND (abdom* OR stomach* OR intestin* OR viscera* OR tummy OR bowel* OR belly or gastrointestinal OR gi OR gastric)) Functional AND (abdom* OR stomach* OR intestin* OR viscera* OR tummy OR bowel* OR belly or gastrointestinal OR gi OR gastric)) Continu* AND (abdom* OR stomach* OR intestin* OR viscera* OR tummy OR bowel* OR belly or gastrointestinal OR gi OR gastric)) Paroxysmal AND (abdom* OR stomach* OR intestin* OR viscera* OR tummy OR bowel* OR belly or gastrointestinal OR gi OR gastric)) Persistent AND (abdom* OR stomach* OR intestin* OR viscera* OR tummy OR bowel* OR belly or gastrointestinal OR gi OR gastric)) Idiopathic AND (abdom* OR stomach* OR intestin* OR viscera* OR tummy OR bowel* OR belly or gastrointestinal OR gi OR gastric)) unspecifi* AND (abdom* OR stomach* OR intestin* OR viscera* OR tummy OR bowel* OR belly or gastrointestinal OR gi OR gastric)) Non specifi* AND (abdom* OR stomach* OR intestin* OR viscera* OR tummy OR bowel* OR belly or gastrointestinal OR gi OR gastric)) nonspecifi* AND (abdom* OR stomach* OR intestin* OR viscera* OR tummy OR bowel* OR belly or gastrointestinal OR gi OR gastric)) motility AND (abdom* OR stomach* OR intestin* OR viscera* OR tummy OR bowel* OR belly or gastrointestinal OR gi OR gastric)) episod* AND (abdom* OR stomach* OR intestin* OR viscera* OR tummy OR bowel* OR belly or gastrointestinal OR gi OR gastric))
ClinicalTrials.gov (clinicaltrials.gov; 2007 to present)
Search dates: 11 April 2014 (69 records); 26 March 2015 (35 records); 9 June 2016 (62 records).
“irritable bowel” OR “abdominal pain” in the condition field. Limited to children.
World Health Organization International Clinical Trials Registry Platform (WHO ICTRP; apps.who.int/trialsearch; 1999 to present)
Search dates: 11 April 2014 (106 records); 26 March 2015 (4 records); 9 June 2016 (32 records).
“irritable bowel” OR “abdominal pain” in the condition field. Limited to children and interventional studies.
Appendix 2. Additional methods
We detailed methods for each of the topics below in our protocol (Martin 2014a), but did not use them in the review because we had insufficient data to perform a meta‐analysis.
Types of outcome measures.
Unit of analysis issues.
Dealing with missing data.
Assessment of heterogeneity.
Assessment of reporting bias.
Data synthesis.
Subgroup analysis and investigation of heterogeneity.
Sensitivity analysis.
We describe these methods, which have been archived for use in future updates of this review, in the table below.
| Types of outcome measures |
| We expect studies to vary in their duration of postintervention follow‐up. We will therefore group studies according to duration of follow‐up: immediate outcome measurement, short term (less than three months), medium term (three to 12 months), and long term (greater than 12 months). |
| Unit of analysis issues |
| If we find the following three types of trials, we will consider their results as per guidance in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011c, section 9.3). Cross‐over trials For cross‐over trials with random allocation to period and an appropriate washout period, we will include the relevant effect estimate within the meta‐analysis, using the generic inverse variance method in Review Manager 5 (Review Manager 2014). An appropriate washout period may vary with the interventions (including drug pharmacokinetics) and outcome measurements. Considering that RAP can be a stable and chronic condition, a washout period of several weeks may be sufficient. Cluster‐RCTs Cluster‐randomised trials randomise groups of people rather than individuals. For each cluster‐randomised trial, we will first determine whether or not the data incorporate sufficient controls for clustering (such as robust standard errors or hierarchical linear models). If data do not have proper controls, then we will attempt to obtain an appropriate estimate of the data's intracluster correlation coefficient. If we cannot find an estimate in the trial report, then we will request an estimate from the trial report authors. If the authors do not provide an estimate, if possible, we will obtain one from a similar study and conduct a sensitivity analysis to determine if the results are robust when different values are imputed. We will do this according to procedures described in Higgins 2011c (section 9.3). This will prevent the meta‐analysis from being based on clustered data that has not been properly controlled. Trials with multiple intervention groups This is a common scenario. To avoid any unit of analysis errors in the meta‐analysis, we will use the following approach for a study that could contribute multiple comparisons.
We will not perform a multiple‐treatment meta‐analysis, as the clinical heterogeneity would mean the results have little clinical meaning. |
| Dealing with missing data |
| We may carry out a sensitivity analysis to establish if the inclusion of studies with high levels of missing data significantly alters the findings of the review. |
| Assessment of reporting bias |
|
Publication bias If we identify sufficient trials (at least 10), we will use the outcome data to produce a funnel plot to investigate the likelihood of overt publication bias (Sutton 2000). Any asymmetry of the funnel plot may indicate possible publication bias. We will explore other reasons for asymmetry such as poor methodological quality or heterogeneity. We will look for publication bias by comparing the results of the published and unpublished data. |
| Assessment of heterogeneity |
| We will describe statistical heterogeneity (observed variability in study results that is greater than that expected to occur by chance) by calculating I² (Higgins 2003). I² describes approximately the proportion of variation in point estimates due to heterogeneity rather than sampling error. An I² more than 50% may indicate significant heterogeneity. We will use a Chi² test to further assess the role of heterogeneity on the strength of the evidence. We will regard any result with a P value lower than 0.10 as indicative of heterogeneity. We will interpret this cautiously and use it to help quantify the impact of heterogeneity on the results of the meta‐analysis (Higgins 2003). |
| Data synthesis |
| We will use Review Manager 5 for statistical analysis (Review Manager 2014). Two review authors (RAA, AB, JTC, TVND, or AEM) will independently enter data into Review Manager 5. We will report summary statistics for continuous data as mean differences or standardised mean differences using a random‐effects model. For dichotomous data, we will report odds ratios using a random‐effects model. We intend to use a a random‐effects model as we anticipate significant statistical and clinical heterogeneity. |
| Subgroup analysis and investigation for heterogeneity |
If sufficient trials are available, we will examine the following subgroups to explore clinical heterogeneity:
Subgroup analysis can be misleading because the studies may not be designed and powered to show difference within subgroups. We will therefore undertake subgroup analyses with caution. |
| Sensitivity analysis |
We will conduct primary analyses based on available data on the outcomes of interest. Following this, we will use a sensitivity analysis to assess the robustness of conclusions in relation to two aspects of study design:
We will also conduct a sensitivity analysis to establish the effect of missing data on the estimate of treatment effect, by performing the analysis with and without the studies with significant missing data to determine if this alters the conclusions. |
Footnotes
RAP: recurrent abdominal pain RCT: randomised controlled trial
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Asgarshirazi 2015.
| Methods |
Design: 3‐arm randomised placebo‐controlled trial Duration: 4 weeks |
|
| Participants |
Country: Iran Setting: paediatric gastroenterology outpatient clinic Sample size: 120 children (40 synbiotic, 40 peppermint oil, 40 control) Gender: peppermint oil group: 15 girls and 19 boys; control group: 17 girls and 8 boys Mean age (SD): 7.06 (+/‐2.38) years peppermint oil group; 7.42(+/‐2.49) years control group Dropouts/withdrawals: 32 withdrawals, number analysed = 88 Diagnosis: functional gastrointestinal disorders using Rome III (Rasquin 2006) |
|
| Interventions |
Intervention: Colpermin (peppermint oil 187 mg capsule), 1 capsule if < 45 kg and 2 capsules if > 45 kg), 3 x day, 30 minutes before each meal Control: folic acid tablet (1 mg), 1 tablet, once daily, 30 minutes before breakfast or lunch 3rd arm: synbiotic Lactol tablet (containing a probiotic and fructo‐oligosaccharide) (see dietary review Newlove‐Delgado in press for this comparison) |
|
| Outcomes |
|
|
| Notes |
Study dates: September 2012 to August 2014 Funding: not stated Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The authors state that participants were randomised in blocks, but give no details of the method of random sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | The authors do not acknowledge this concept in the paper. In personal correspondence, the authors reported that it was not important (Asgarshirazi 2016 [pers comm]). Therefore, on balance, we judged this to be at unclear of risk of bias. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The study authors do not specifically state that participants were blinded, although they do say that the nurse that carried out the questionnaire was blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | As above, note also that the placebo regimen of folic acid tablets was once daily and the intervention regimen of peppermint oil capsules was 3 times daily. These different preparations and timings could have introduced bias, as the outcome was self reported and therefore influenced by the participants experience. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 32 participants were excluded or withdrawn after randomisation. Attrition differed between the groups, 15% of the intervention group and 38% of the placebo group. |
| Selective reporting (reporting bias) | Low risk | All outcomes in the methods appear to be reported. |
| Other bias | High risk | No power calculation. The intervention and placebo groups differed at baseline: duration of pain in the intervention group was 67.05 mean minutes/day (SD +/‐ 36.97) and the placebo group was 53.4 mean minutes/day (SD +/‐ 16.81). |
Bahar 2008.
| Methods |
Design: double‐blinded, placebo‐controlled, randomised trial Duration: 13 weeks |
|
| Participants |
Country: USA Setting: private paediatric gastroenterology outpatient clinic Sample size: 33 children (intervention 16, control 16) Gender: 9 boys, 24 girls Mean age: intervention 15.3 years; control 14.2 years. Overall range 12 to 18 years Dropouts/withdrawals: 0 Diagnosis: meet the Rome II criteria for IBS (Rasquin‐Weber 1999) |
|
| Interventions |
Intervention: 7‐week course of oral amitriptyline (10 mg if 30 to 50 kg, 20 mg if 50 to 80 kg, 30 mg if > 80 kg), taken at night Control: placebo |
|
| Outcomes |
Timing of outcome assessment: measured at 6, 10, and 13 weeks |
|
| Notes |
Study dates: 2002 to 2005 Funding: James and Diane Brooks Medical Research Foundation and AstraZeneca Conflicts of interest: not mentioned |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The authors state that participants were randomised, but give no details of the method of randomisation. |
| Allocation concealment (selection bias) | High risk | The authors do not mention this concept in the paper. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | This is mentioned in the title but no details are provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The outcome measurement is self assessed, therefore if the participant is blinded so too is the outcome assessment. No details of the blinding are provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The authors state that no participants dropped out after starting treatment. |
| Selective reporting (reporting bias) | High risk | The authors state in their methods that improvement in overall quality of life score is the primary outcome but provide no details of a threshold regarded as improvement. At baseline, the quality of life score for the intervention group was 14% lower than that of the placebo group, 109.4 and 127.5 respectively. In the analysis, at 10 and 13 weeks' follow‐up, the quality of life score was 128.0 and 126.2 respectively for the intervention group and 129.4 and 129.8 respectively for the placebo group. The authors conclude that the number of participants with a greater than 15% improvement is significantly higher in the intervention group; this could be wholly explained by the difference at baseline. This may also be a post‐hoc analysis, as the authors do not mention this in the methods. The authors also selected some symptoms from the functional abdominal pain score and reported these as improved, e.g. periumbilical and right lower quadrant pain, but abdominal pain in other areas showed no improvement with the intervention. |
| Other bias | High risk | There is no power calculation. Baseline data of the participants are not provided. Compliance with the intervention is mentioned. There is no raw data to verify the statistics quoted. We wrote to the authors to request these data but received no response. The validity of the outcome assessment tool or clinical relevance of the reported outcomes are not mentioned. For example, is a 15% improvement in the quality of life scale at 13 weeks, but not at 10 weeks, clinically important? The number of participants is small and the follow‐up is short. |
Collins 2011.
| Methods |
Design: double‐blinded, placebo‐controlled, randomised trial Duration: 2 weeks |
|
| Participants |
Country: USA Setting: paediatric gastroenterology clinic at a children's hospital and an outreach clinic Sample size: 75 children (intervention 49, control 26) Gender: intervention 15 boys, 34 girls; control 6 boys, 20 girls Mean age: intervention 12.5 years (SD 3.01); control 13.16 years (SD 2.97) Dropouts/withdrawals: 5: intervention 3, control 2 Diagnosis: Rome II criteria for IBS, functional dyspepsia, functional abdominal pain, or abdominal migraine (Rasquin‐Weber 1999) |
|
| Interventions |
Intervention: 550 mg rifaximin 3 times daily for 10 days Control: placebo |
|
| Outcomes |
Timing of outcome assessment: 2 weeks |
|
| Notes | 10 symptoms = bloating, excess gas, incomplete evacuation, abdominal pain, diarrhoea, constipation, urgency, passage of mucus, straining, faecal soiling Study dates: 2010 Funding: the study was funded by Salix Pharmaceuticals, Saban Research Institute, and Children's Hospital of Los Angeles Conflicts of interest: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The authors state that participants were randomised, but provide no details of the method of randomisation. |
| Allocation concealment (selection bias) | Low risk | The authors state that randomisation was performed by personnel not associated with the study and that the study personnel were blinded. This implies allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The authors state that the participants and personnel were blinded and that a matching placebo was used. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The outcomes were measured by self report in a questionnaire and the participants were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The authors state that outcome assessment is missing for 5 participants, 3 from the intervention group and 2 from the placebo group. This is a low number of loss to follow‐up and within what would be expected for any study. There was no differential loss to follow‐up between the groups. |
| Selective reporting (reporting bias) | Unclear risk | The authors do not present the raw data, i.e. from the 10‐point visual analogue scale of GI symptoms or the overall improvement score. They provide only a statement that none of the differences were significant. We wrote to the authors to provide the raw data to verify this but received no response. We are therefore unable to comment on the completeness of the outcome data or the degree of selective reporting. |
| Other bias | High risk | The power calculation is not based on a primary outcome but a laboratory test ‐ the detection of small intestinal bacteria overgrowth ‐ and is therefore not valid. The outcome is measured by self report for 10 symptoms, but the validity of this method is not mentioned. The number of participants is small and the follow‐up is short. |
Heyland 2012.
| Methods |
Design: double‐blinded, placebo‐controlled, randomised trial Duration: 3 weeks |
|
| Participants |
Country: Switzerland Setting: patients referred to the Department of Paediatric Gastroenterology and Nutrition at the University Children's Hospital, Zurich Sample size: 37 children (intervention 20, control 17) Gender: intervention 9 boys, 11 girls; control 7 boys, 10 girls Mean age: intervention 10.6 years (SD not reported; range not reported); control 11.4 years (SD not reported; range not reported) Dropouts/withdrawals: 3, not known from which group Diagnosis: Apley's criteria for RAP (Apley 1958) |
|
| Interventions |
Intervention: 6 mg/kg/day trimethoprim and 30 mg/kg/day sulfamethoxazole in 2 divided doses for 7 days Control: placebo twice daily for 7 days |
|
| Outcomes |
Timing of outcome assessment: 2 weeks |
|
| Notes |
Study dates: 2004 to 2008 Funding: not stated in the paper Conflicts of interest: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The authors state that participants were randomised, but provide no details of the method of randomisation. |
| Allocation concealment (selection bias) | Unclear risk | The authors state that randomisation occurred after obtaining written, informed consent, but no additional information is provided. It is therefore unclear if there was allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The authors state that the intervention was blinded and a placebo was used. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome was self reported and the participants were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The authors state that 3 participants withdrew from the study after inclusion, but this is unlikely to have significantly altered the results. |
| Selective reporting (reporting bias) | Low risk | The primary outcome, Pain Index, was reported for all participants. |
| Other bias | Unclear risk | The power calculation is inadequate for this outcome measurement, as it is based on the breath test findings and not the primary outcome. The baseline data were presented and showed no imbalances. The number of participants is small and the follow‐up is short. The funding is not stated. The authors report no conflicts of interest. |
Karabulut 2013.
| Methods |
Design: randomised controlled trial comparing trimebutine with usual care Duration: 3 weeks |
|
| Participants |
Country: Turkey Setting: patients attending the general paediatric clinic at the Istanbul University Hospital, Department of Paediatric Gastroenterology Sample size: 78 children (intervention 39, control 39) Gender: 31 boys, 47 girls. Distribution between groups not given. Mean age: 9.79 years (SD 3.45). Distribution between groups not given. Dropouts/withdrawals: 0 Diagnosis: IBS according to the Rome III criteria (Rasquin 2006) |
|
| Interventions |
Intervention: 3 mg/kg/day trimebutine maleate, in 3 divided doses for 3 weeks Control: usual care |
|
| Outcomes |
Timing of outcome assessment: 3 weeks |
|
| Notes |
Study dates: 2007 to 2008 Funding: none Conflicts of interest: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The authors state that participants were randomised, but give no details of the method of randomisation. |
| Allocation concealment (selection bias) | High risk | This concept is not mentioned in the paper. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The participants and study personnel could not have been blinded as there was no placebo for the control group, who received standard care instead. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The parents were asked to report the outcome, and they would have known whether the child was taking trimebutine or not. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The authors do not mention this; the raw data is not reported, only the percentage of children who improved. We therefore cannot be sure about the loss to follow‐up or how the missing data were handled. |
| Selective reporting (reporting bias) | Low risk | The authors state that parental assessment of pain relief was their only outcome. |
| Other bias | High risk | No power calculation was performed and no baseline data are given. Compliance with the intervention is not mentioned. The outcome measurement is not validated. The number of participants is small and the follow‐up is short. We wrote to the authors to request details regarding the above issues, but received no response. |
Karunanayake 2015.
| Methods |
Design: randomised, placebo‐controlled trial Duration: insufficient detail. The intervention was given for 8 weeks but when the outcomes were measured is not stated. |
|
| Participants |
Country: Sri Lanka Setting: outpatient clinic of the university paediatric unit Sample size: 100 children Gender: no details about the children at recruitment. In the 89 children that completed the study, 33/47 in the intervention group were girls, and 22/42 in the placebo group were girls. Mean age: no details. The authors state that the study was designed to recruit children aged 5 to 12 years. Dropouts/withdrawals: 11 Diagnosis: abdominal pain‐predominant functional gastrointestinal diseases fulfilling Rome III criteria (Rasquin 2006) |
|
| Interventions |
Intervention: domperidone 10 mg, 3 times per day, before meals for 8 weeks Control: placebo |
|
| Outcomes | Primary outcomes:
Secondary outcomes were significant improvement in:
Timing of outcome assessment: no details |
|
| Notes |
Study dates: no details Funding: no details Conflicts of interest: no details This study is published as a conference abstract. We wrote to the authors and received no further information (Martin 2016a [pers comm]; Martin 2016d [pers comm]). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The authors state that computer‐generated random numbers were used. |
| Allocation concealment (selection bias) | Unclear risk | The authors do not mention this. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The authors state that participants were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The authors state that participants and investigators who assessed outcome measures were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | There is insufficient detail in the published information to assess this. |
| Selective reporting (reporting bias) | Low risk | All stated outcomes appear to be reported, although with very limited data. |
| Other bias | Unclear risk | There is insufficient detail in the published information to assess this. |
Khoshoo 2006.
| Methods |
Design: randomised controlled trial comparing tegaserod plus laxative with laxative alone Duration: 4 weeks |
|
| Participants |
Country: USA Setting: medical centre in North America (no further detail given by authors) Sample size: 48 children (intervention 21, control 27) Gender: intervention 8 boys, 13 girls; control 11 boys, 16 girls Mean age: intervention 15.1 years (SD not reported); control 15.37 years (SD not reported) Dropouts/withdrawals: 0 Diagnosis: constipation‐predominant IBS according to Rome II criteria (Rasquin‐Weber 1999) |
|
| Interventions |
Intervention: 6 mg tegaserod once daily, oral, on an empty stomach Control: usual care |
|
| Outcomes |
Timing of outcome assessment: 4 weeks |
|
| Notes |
Study dates: 2006 Funding: authors state no external financial support was received Conflicts of interest: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Authors state that they used a simple random numbers table to allocate the treatment groups. |
| Allocation concealment (selection bias) | Unclear risk | This concept is not mentioned in the paper. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The control group was not given a placebo, therefore if informed consent was obtained before inclusion, participants would have been aware of the implications of their group allocation. The authors state in the discussion that the participants were unaware of the different groups. This is concerning. We asked the authors about this but have not received a reply. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Self reported outcome and therefore not blinded, as explained in performance bias section, see above |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There appears to be no loss to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | The authors report all the outcomes they stated in the methods, but it is unclear from the methods if the definition of a good pain reduction was a prespecified outcome; it may be post‐hoc analysis. |
| Other bias | Unclear risk | The 2 groups are similar at baseline. A power calculation is performed. The eligibility criteria are stated. It is unclear if the pain‐rating tool is validated as the authors state "standard pain rating scale" but give no further details. |
Kline 2001.
| Methods |
Design: double‐blinded, placebo‐controlled, randomised trial Duration: 2 weeks |
|
| Participants |
Country: USA Setting: 3 specialist paediatric gastroenterology departments Sample size: 42 children (intervention 21, control 21) Gender: 17 boys, 25 girls. Distribution between groups not given. Mean age: 12 years (range 8 to 17 years). Distribution between groups not given. Dropouts/withdrawals: 8 (4 from intervention, 4 from control) Diagnosis: RAP, defined by Apley (Apley 1958) |
|
| Interventions |
Intervention: peppermint oil capsule taken 3 times daily Control: placebo (arachis oil) capsule |
|
| Outcomes |
Timing of outcome assessment: 2 weeks |
|
| Notes |
Study dates: 1999 Funding: Tillotts Pharma AG Switzerland Conflicts of interest: none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The authors state this was a randomised controlled trial, but give no details of method of randomisation. |
| Allocation concealment (selection bias) | Unclear risk | The authors did not mention this concept. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The authors state that the trial was double‐blinded and describe that the same company produced identical intervention and placebo tablets. However, it is unclear who, if any, of the study personnel were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | There is insufficient information in the paper to judge this because the outcome was based on clinician judgement, parent report, and child report, and the blinding of all these outcome assessors was not explained. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The authors state that 8 participants withdrew from the study. Reasons for withdrawal (4 from each group) are clearly stated in the paper and appear not to be related to treatment effects (2 because of long travel distances to the clinic; 2 because they were prescribed antibiotics for other reasons; 4 because they were unable to swallow pills). |
| Selective reporting (reporting bias) | High risk | The authors do not report all 5 outcomes mentioned in the methods section. |
| Other bias | Unclear risk | There was no power calculation. The eligibility criteria were clearly stated. The authors state that the groups were similar at baseline, but the details are not reported. Compliance with the intervention is unclear. The follow‐up is short. |
Narang 2015.
| Methods |
Design: double‐blinded, placebo‐controlled, randomised trial Duration: 4 weeks |
|
| Participants |
Country: India Setting: tertiary paediatric gastroenterology clinic Sample size: 132 children (intervention 66, control 66) Gender: intervention group: 33 boys, 33 girls; control group: 39 boys, 27 girls Mean age: intervention group: 7.1 (+/‐ 2.1); control group: 7.4 (+/‐ 2.6) Dropouts/withdrawals: 8 (4 from intervention, 4 from control) Diagnosis: RAP, defined by Apley (Apley 1958) |
|
| Interventions |
Intervention: drotaverine 20 mg (10 mL) liquid 3 x daily for children 4 to 6 years, 40 mg tablet 3 x daily for children > 6 years Control: placebo Both groups could have extra doses if pain was experienced. |
|
| Outcomes |
Timing of outcome assessment: 4 weeks |
|
| Notes |
Study dates: September 2012 to September 2013 Funding: Walter Bushnell Pvt. Ltd. The company supplied a grant and medication for the study. They had no role in trial design, data collection, or manuscript preparation. Conflicts of interest: none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Authors state that they used computer‐generated block randomisation with variable block sizes. Stratified for age (4 to 6 and > 6 years). |
| Allocation concealment (selection bias) | Low risk | Clearly explained by authors |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Clearly explained by authors |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The outcomes were self and parental reported, therefore participant blinding meant the outcome assessment was also blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low loss to follow‐up: 6 from placebo group and 2 from intervention group |
| Selective reporting (reporting bias) | High risk | The outcomes explained in the methods were not all reported, e.g. results for pain severity are not given. In addition, the outcome 'number of school days missed in 4 weeks' reported in the results was not mentioned in the methods. |
| Other bias | High risk | We were concerned that the method of giving further drug doses if pain is reported could alter the trial outcome reporting, as if the drug works the child may ask for it more frequently. Therefore, administration of the intervention and outcome assessment are not independent. |
Pourmoghaddas 2014.
| Methods |
Design: double‐blinded, placebo‐controlled, randomised trial of mebeverine versus placebo Duration: 12 weeks |
|
| Participants |
Country: Iran Setting: paediatric gastroenterology outpatient clinic Sample size: 115 children (intervention 59, control 56) Gender: 39 boys, 48 girls (children who completed follow‐up) Mean age: 8.5 years (SD 2.1 years) for all children who completed follow‐up. Distribution between treatment groups not given. Dropouts/withdrawals: intervention 19, control 17 Diagnosis: functional abdominal pain, defined by Rome III criteria (Rasquin 2006) |
|
| Interventions |
Intervention: mebeverine tablets, 135 mg twice daily, for a duration of 4 weeks Control: placebo |
|
| Outcomes |
Primary outcome: 2‐point reduction in FACES Pain Scale (scale 1 to 6) or report of "no pain" Secondary outcome: physician‐rated global severity and improvement using the Clinical Global Impression Severity and Improvement Scales (scale 1 to 7) Timing of outcome assessment: 12 weeks |
|
| Notes |
Study dates: 2013 Funding: The Isfahan University of Medical Sciences Conflicts of interest: none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by computer‐generated random numbers in 4 blocks |
| Allocation concealment (selection bias) | Low risk | Authors state allocation was concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Authors state that the doctors, study participants, and study personnel assessing the outcomes were blinded. The drug codes for labelling the bottles were known only by the pharmacist. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The primary outcome was self reported and therefore blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The loss to follow‐up was equal between the 2 groups and was 28/115 (24%) of randomised participants, shown in flow diagram. |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in the methods are reported. |
| Other bias | Low risk | 2 groups similar at baseline. Power calculation performed. No conflict of interest. Funded by an academic institution |
Roohafza 2014.
| Methods |
Design: double‐blinded, placebo‐controlled, randomised trial of citalopram versus placebo Duration: 12 weeks |
|
| Participants |
Country: Iran Setting: paediatric gastroenterology outpatient clinic Sample size: 115 children (intervention 59, control 56) Gender: intervention group: 11 males, 32 females; control group: 19 males, 24 females (children who completed follow‐up) Mean age: intervention 10.4 years (SD 1.9 years); control 8.5 years (SD 2.2 years) for children who completed follow‐up Dropouts/withdrawals: 29 children (intervention 16, control 13) Diagnosis: functional abdominal pain, defined by Rome III criteria (Rasquin 2006) |
|
| Interventions |
Intervention: citalopram tablets 10 mg/day for the 1st week and then 20 mg/day for a total duration of 4 weeks Control: placebo |
|
| Outcomes |
Primary outcome: 2‐point reduction in FACES Pain Scale (scale 1 to 6) or report of "no pain" Secondary outcome: self rated depression, anxiety, and somatization scores Timing of outcome assessment: 12 weeks |
|
| Notes |
Study dates: 2013 Funding: The Isfahan University of Medical Sciences Conflicts of interest: none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by computer‐generated random numbers in 4 blocks |
| Allocation concealment (selection bias) | Low risk | Authors state that allocation was concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Authors state that the doctors, study participants, and study personnel assessing the outcomes were blinded. The drug codes for labelling the bottles were known only by the pharmacist. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The primary outcome was self reported and therefore blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The loss to follow‐up was equal between the 2 groups and was 29/115 (25%) of randomised participants, shown in flow diagram. |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in the methods are reported. |
| Other bias | Low risk | 2 groups similar at baseline, except for slight difference in age. Power calculation performed. No conflict of interest. Funded by an academic institution |
Sadeghian 2008.
| Methods |
Design: double‐blinded, placebo‐controlled, randomised trial Duration: 2 weeks |
|
| Participants |
Country: Iran Setting: paediatric gastroenterology clinic in Tehran Sample size: 36 children (included after dropouts 29 children: intervention 15, control 14) Gender: intervention: 7 boys, 8 girls; control: 5 boys, 9 girls Mean age: intervention 7.2 years (range 4.5 to 11 years); control 7.7 years (range 5 to 12 years) Dropouts/withdrawals: 7 (unclear from which group) Diagnosis: functional abdominal pain, defined by Rome II criteria (Rasquin‐Weber 1999) |
|
| Interventions |
Intervention: 0.25 to 0.5 mg/kg/day cyproheptadine in 2 divided doses for 2 weeks Control: placebo |
|
| Outcomes |
Timing of outcome assessment: 2 weeks |
|
| Notes |
Study dates: 2006 to 2007 Funding: none declared Conflicts of interest: none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The authors state that the random allocation was performed using a randomised, quadruple order of A and B. |
| Allocation concealment (selection bias) | Low risk | The authors explain that the study personnel (research nurse and study physician) were blinded. This implies allocation concealment, as they did not know the group to which participants were allocated. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The authors state that the placebo was similar. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The participants were blinded and the outcome was self reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 36 participants were randomised. The authors state that 7 participants were excluded from the final analysis (5 for poor compliance and 2 for incomplete outcome data). We assume that they came equally from each group, as the included numbers of participants were 15 and 14 for the intervention and control groups, respectively (total 29). |
| Selective reporting (reporting bias) | Low risk | The primary outcome was reported. |
| Other bias | High risk | No power calculation was performed. The outcome was not measured by a validated tool. The sample size was small and the outcome was measured over a short time duration. There was no intention‐to‐treat analysis as the non‐compliant participants were excluded. |
Saps 2009.
| Methods |
Design: double‐blinded, placebo‐controlled, randomised trial Duration: 4 weeks |
|
| Participants |
Country: USA Setting: 6 paediatric tertiary care hospitals, gastroenterology clinics Sample size: 90 children (intervention 46, control 44) Gender: 18 boys, 72 girls. Distribution between groups not given. Mean age: 12.7 years (range 8 to 17 years) Dropouts/withdrawals: 7 (3 from intervention group, 4 from control group) Diagnosis: functional abdominal pain, functional dyspepsia, or IBS, defined by Rome II criteria (Rasquin‐Weber 1999) |
|
| Interventions |
Intervention: 10 mg/day amitriptyline if weight < 35 kg, 20 mg/day amitriptyline if weight > 35 kg, for 4 weeks Control: placebo |
|
| Outcomes |
Timing of outcome assessment: 4 weeks |
|
| Notes |
Study dates: 2003 to 2006 Funding: grant from American College of Gastroenterology and National Center for Research Resources, National Institutes of Health Conflicts of interest: none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The authors state that participants were randomised, but give no details of the method of randomisation. The 2 groups look similar at baseline, which suggests the method of randomisation was adequate. |
| Allocation concealment (selection bias) | Unclear risk | This concept is not mentioned in the paper. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The authors state that participants and personnel were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The outcomes are self and parent reported. The participants were blinded, and therefore so too was the outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 90 participants were included, of whom 83 completed the study, giving a 7.8% loss to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All outcomes detailed in the methods were reported. |
| Other bias | High risk | The study was under‐recruited, as the power calculation stated that 120 participants were required, but only 90 were recruited. |
See 2001.
| Methods |
Design: double‐blinded, placebo‐controlled, randomised, cross‐over trial Duration 6 weeks |
|
| Participants |
Country: USA Setting: tertiary paediatric hospital clinic at Mount Sinai Medical Center Sample size: 25 children (cross‐over trial so all participants received both interventions) Gender: 13 boys, 12 girls Mean age: 10.5 years (range 5.5 to 16.77 years) Dropouts/withdrawals: 1 participant, excluded after enrolment (as Giardia detected in stool) Diagnosis: RAP, defined by Apley's criteria (Apley 1958) |
|
| Interventions |
Intervention: 1 mg/kg/day famotidine in 2 divided doses for 3 weeks Control: placebo |
|
| Outcomes |
Timing of outcome assessment: 3 weeks |
|
| Notes |
Study dates: 1998 Funding: none declared Conflicts of interest: none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The authors state that participants were randomised, but give no details of the method of randomisation. |
| Allocation concealment (selection bias) | Low risk | The authors do not mention allocation concealment, but they state that the study personnel were blinded, only the pharmacy was aware of allocation. We can therefore assume that at the point of randomisation, the study personnel did not know the treatment group to which each participant would be allocated. Outcomes were reported by children and parents, who were blinded to allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The authors state that both groups were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The outcomes were self reported, and the participants were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The authors report that of the 26 children originally enrolled, 1 was excluded due to detection of Giardia. There were no further drops outs and therefore data were presented for 25 children. |
| Selective reporting (reporting bias) | Low risk | All outcomes mentioned in the methods were reported. |
| Other bias | High risk | No power calculation, small sample size, baseline data presented and the groups appear similar. The cross‐over trial had no washout period. The cross‐over trial varied according to response to the first treatment, which introduces bias as it is recognised that RAP is a condition that fluctuates over time. The raw data are not given, and we received no response from the authors to our request for this information. |
Symon 1995.
| Methods |
Design: double‐blinded, placebo‐controlled, randomised trial Duration 4 months |
|
| Participants |
Country: UK Setting: hospital general paediatric clinic Sample size: 14 children Gender: not stated by authors Mean age: not reported (range 5 to 13 years) Dropouts/withdrawals: 2 Diagnosis: abdominal migraine = RAP (Apley 1958), with episodes associated with facial pallor, first‐degree relative with headaches |
|
| Interventions |
Intervention: 0.5 mg pizotifen syrup (0.25 mg/5 mL) in 2 divided doses per day. After 1 month the dose was increased to 0.75 mg/day if there had been no improvement. Control: placebo |
|
| Outcomes |
Timing of outcome assessment: 4 months |
|
| Notes |
Study dates: 1995 Funding: none declared Conflicts of interest: none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | From correspondence with the authors, it was established that the order for treatment allocation was both blinded and randomised. This was done in the hospital pharmacy, therefore the study personnel were also blinded. |
| Allocation concealment (selection bias) | Low risk | From correspondence with the authors, it was established that the randomisation was performed by the hospital pharmacy, and therefore the study personnel were also blinded. This implies there was allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | As explained above |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The outcomes were reported by parent and child, who were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 participants (14%) were excluded, as they had no outcome data. |
| Selective reporting (reporting bias) | Low risk | All outcomes mentioned in the methods were reported. |
| Other bias | High risk | No baseline data, no power calculation, no washout period for a cross‐over trial. The intervention was changed according to response; the authors stated that the medication was doubled if there was no response. The trial stopped early due to lack of drug supply. |
Zybach 2016.
| Methods |
Design: cross‐over, placebo‐controlled, randomised trial Duration: 5 weeks |
|
| Participants |
Country: USA Setting: hospital general paediatric clinic Sample size: 14 children Gender: intervention group (girls 58%; boys 5, girls 7), placebo group (girls 58%; boys 5, girls 7) Mean age: intervention group: 13.8 (+/‐ 1.6 years), placebo group: 13.8 (+/‐ 1.6 years) Dropouts/withdrawals: 2 Diagnosis: functional abdominal pain, according to Rome III criteria |
|
| Interventions |
Intervention: melatonin 5 mg (20 mL) at night, once daily for 14 days Control: placebo |
|
| Outcomes |
Timing of outcome assessment: 2 weeks |
|
| Notes |
Study dates: not stated by authors, published in 2016 Funding: none declared Conflicts of interest: none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The authors state that participants were randomised, but give no details of the method of randomisation. |
| Allocation concealment (selection bias) | Low risk | Authors clearly state that parents were consented for the trial, and participant’s assent was obtained prior to study procedures, which implies that allocation concealment was achieved. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding explained, complete for participant and study team, the drug and placebo were labelled in pharmacy. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The outcome assessment was self reported pain by the child, who was interviewed by the study team. Both the child and study team were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 14 enrolled, 12 completed the study; 14% dropout rate |
| Selective reporting (reporting bias) | Low risk | No evidence from the paper of outcomes that were not reported. |
| Other bias | High risk | Small study with no power calculation. This is a cross‐over study with no washout period. |
GI: gastrointestinal IBS: irritable bowel syndrome PedsQL: Pediatric Quality of Life Inventory RAP: recurrent abdominal pain SD: standard deviation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Christensen 1995 | Ineligible study design |
| Cucchiara 1992 | Ineligible study design |
| Dehghani 2011 | Ineligible study design |
| Di Nardo 2011 | Ineligible population |
| Everitt 2010 | Ineligible population |
| Grillage 1990 | Ineligible comparator |
| Kaminski 2009 | Ineligible study design: literature review |
| Lloyd‐Still 1990 | Ineligible population |
| Van Outryve 2005 | Ineligible population |
| Xiao 2013 | Ineligible comparator |
| Yadav 1989 | Ineligible population |
Differences between protocol and review
The differences between the protocol and the review are detailed in the Additional Methods Table in Appendix 2.
Rebecca Whear joined the team following the publication of the protocol.
Contributions of authors
Review design: AEM, SL
Review co‐ordination: AEM
-
Data collection:
Search strategy design: AEM, AB
Searches undertaken: AEM, AB
Search results screened: AEM, TVND, RAA, AB, JTC, RW
Retrieval of papers: AEM, AB
Paper screening and appraisal, and extraction of data: AEM, TVND, RAA, AB, JTC, RW
Writing to authors for additional information: AEM, AB, RAA, TVND
Entering the data into Review Manager 5: AEM, TVND, RAA, AB, JTC
Analysis of the data: AEM, TVND, RAA, AB, JTC, SL
-
Interpretation of the data:
Methodological perspective: AEM, TVND, RAA, AB, JTC
Clinical perspective: AEM, TVND, SL
Sources of support
Internal sources
-
National Institute for Health Research Collaboration for Leadership in Applied Health Research and Care South West Peninsula (PenCLAHRC), UK.
Funds the work of the evidence synthesis team.
External sources
None, Other.
Declarations of interest
The work of the evidence synthesis team is funded by the National Institute for Health Research Collaboration for Leadership in Applied Health Research and Care South West Peninsula (PenCLAHRC). The Funder has had no role in the review itself.
Alice E Martin: none known. Tamsin V Newlove‐Delgado: none known. Rebecca A Abbott: none known. Alison Bethel: none known. Joanna Thompson‐Coon: none known. Rebecca Whear: none known Stuart Logan: none known.
The authors who practice clinical paediatrics are Alice E Martin and Stuart Logan. Alice is a Paediatric Trainee and works under the guidance of various consultant paediatricians. Stuart is a Consultant Paediatrician and treats children according to current best evidence, in light of their preference. There are therefore no conflicts of interest with this review.
New
References
References to studies included in this review
Asgarshirazi 2015 {published and unpublished data}
- Asgarshirazi M, Shariat M, Dalili H. Comparison of the effects of pH‐dependent peppermint oil and synbiotic lactol (Bacillus coagulans + Fructooligosaccharides) on childhood functional abdominal pain: a randomized placebo‐controlled study. Iran Red Cresent Medical Journal 2015;17(4):e23844. [DOI: 10.5812/ircmj.17(4)2015.23844; PMC4443394; PUBMED: 26023339] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bahar 2008 {published data only}
- Bahar RJ, Collins BS, Steinmetz B, Ament ME. Double‐blind placebo‐controlled trial of amitriptyline for the treatment of irritable bowel syndrome in adolescents. Journal of Pediatrics 2008;152(5):685‐9. [DOI: 10.1016/j.jpeds.2007.10.012; PUBMED: 18410774] [DOI] [PubMed] [Google Scholar]
Collins 2011 {published data only}
- Collins BS, Lin HC. Double‐blind, placebo‐controlled antibiotics treatment study of small intestinal bacterial overgrowth in children with chronic abdominal pain. Journal of Pediatric Gastroenterology and Nutrition 2011;52(4):382‐6. [DOI: 10.1097/MPG.0b013e3181effa3b; PUBMED: 21240023] [DOI] [PubMed] [Google Scholar]
Heyland 2012 {published data only}
- Heyland K, Friedt M, Buehr P, Braegger CP. No advantage for antibiotic treatment over placebo in Blastocystis hominis‐positive children with recurrent abdominal pain. Journal of Pediatric Gastroenterology and Nutrition 2012;54(5):677‐9. [DOI: 10.1097/MPG.0b013e31823a29a7; PUBMED: 22002479] [DOI] [PubMed] [Google Scholar]
Karabulut 2013 {published data only}
- Karabulut GS, Beşer OF, Erginöz E, Kutlu T, Cokuğraş FÇ, Erkan T. The incidence of irritable bowel syndrome in children using the Rome III criteria and the effect of trimebutine treatment. Journal of Neurogastroenterology and Motility 2013;19(1):90‐3. [DOI: 10.5056/jnm.2013.19.1.90; PMC3548133; PUBMED: 23350053] [DOI] [PMC free article] [PubMed] [Google Scholar]
Karunanayake 2015 {published data only}
- Karunanayake A, Devanarayana NM, Rajindrajith S, Silva A. OP‐7 Therapeutic effects of domperidone on abdominal pain‐predominant functional gastrointestinal disorders: randomised, double‐blind, placebo‐controlled trial. Journal of Pediatric Gastroenterology and Nutrition 2015;61(4):511‐2. [DOI: 10.1097/01.mpg.0000472211.46517.33; PUBMED: 26439548] [DOI] [Google Scholar]
Khoshoo 2006 {published data only}
- Khoshoo V, Armstead C, Landry L. Effect of a laxative with and without tegaserod in adolescents with constipation predominant irritable bowel syndrome. Alimentary Pharmacology and Therapeutics 2006;23(1):191‐6. [DOI: 10.1111/j.1365-2036.2006.02705.x; PUBMED: 16393297] [DOI] [PubMed] [Google Scholar]
Kline 2001 {published data only}
- Kline RM, Kline JJ, Palma J, Barbero GJ. Enteric‐coated, pH‐dependent peppermint oil capsules for the treatment of irritable bowel syndrome in children. Journal of Pediatrics 2001;138(1):125‐8. [PUBMED: 11148527] [DOI] [PubMed] [Google Scholar]
Narang 2015 {published data only}
- Narang M, Shah D, Akhtar H. Efficacy and safety of drotaverine hydrochloride in children with recurrent abdominal pain: a randomized placebo controlled trial. Indian Pediatrics 2015;52(10):847‐51. [PUBMED: 26499007] [DOI] [PubMed] [Google Scholar]
Pourmoghaddas 2014 {published data only}
- Pourmoghaddas Z, Saneian H, Roohafza H, Gholamrezaei A. Mebeverine for pediatric functional abdominal pain: a randomized, placebo‐controlled trial. BioMed Research International 2014;191026:Epub 2014 Jun 25. [DOI: 10.1155/2014/191026; PMC4095832; PUBMED: 25089264] [DOI] [PMC free article] [PubMed] [Google Scholar]
Roohafza 2014 {published data only}
- Roohafza H, Pourmoghaddas Z, Saneian H, Gholamrezaei A. Citalopram for pediatric functional abdominal pain: a randomized, placebo‐controlled trial. Neurogastroenterology & Motility 2014;26(11):1642‐50. [DOI: 10.1111/nmo.12444] [DOI] [PubMed] [Google Scholar]
Sadeghian 2008 {published data only}
- Sadeghian M, Farahmand F, Fallahi GH, Abbasi A. Cyproheptadine for the treatment of functional abdominal pain in childhood: a double‐blinded randomized placebo‐controlled trial. Minerva Pediatrica 2008;60(6):1367‐74. [PUBMED: 18971897] [PubMed] [Google Scholar]
Saps 2009 {published data only}
- Saps M, Youssef N, Miranda A, Nurko S, Hyman P, Cocjin J, et al. Multicenter, randomised, placebo‐controlled trial of amitriptyline in children with functional gastrointestinal disorders. Gastroenterology 2009;137(4):1261‐9. [DOI: 10.1053/j.gastro.2009.06.060] [DOI] [PMC free article] [PubMed] [Google Scholar]
See 2001 {published data only}
- See MC, Birnbaum AH, Schechter CB, Goldenberg MM, Benkov KJ. Double‐blind, placebo‐controlled trial of famotidine in children with abdominal pain and dyspepsia: global and quantitative assessment. Digestive Diseases and Sciences 2001;46(5):985‐92. [PUBMED: 11341669] [DOI] [PubMed] [Google Scholar]
Symon 1995 {published data only}
- Symon DN, Russell G. Double blind placebo controlled trial of pizotifen syrup in the treatment of abdominal migraine. Archives of Disease in Childhood 1995;72(1):48‐50. [PMC1510964; PUBMED: 7717738] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zybach 2016 {published data only}
- Zybach K, Friesen CA, Schurman JV. Therapeutic effect of melatonin on pediatric functional dyspepsia: a pilot study. World Journal of Gastrointestinal Pharmacology and Therapeutics 2016;7(1):156‐61. [DOI: 10.4292/wjgpt.v7.i1.156; PMC4734949; PUBMED: 26855822] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Christensen 1995 {published data only}
- Christensen MF. Letter: Double blind placebo controlled trial of pizotifen syrup in the treatment of abdominal migraine. Archives of Disease in Childhood 1995;73(2):183. [DOI: 10.1136/adc.73.2.183-a] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cucchiara 1992 {published data only}
- Cucchiara S, Bortolotti M, Minella R, Manzi G, Vitiello G, Iervolino C, et al. Children with functional gastrointestinal symptoms: effects of cisapride on fasting antroduodenal motility, gastric emptying of liquids and symptoms. European Journal of Gastroenterology and Hepatology 1992;4(8):627‐33. [Google Scholar]
Dehghani 2011 {published data only}
- Dehghani SM, Imanieh MH, Oboodi R, Haghighat M. The comparative study of the effectiveness of cimetidine, ranitidine, famotidine, and omeprazole in treatment of children with dyspepsia. ISRN Pediatrics 2011;219287:Epub 2011 Apr 5. [DOI: 10.5402/2011/219287; PMC3263570; PUBMED: 22389770] [DOI] [PMC free article] [PubMed] [Google Scholar]
Di Nardo 2011 {published data only}
- Nardo G, Barbara G, Oliva S, Cremon C, Aloi M, Mallardo S, et al. Efficacy and tolerability of alpha‐galactosidase on gas related symptoms in pediatric irritable bowel syndrome. A randomized, double‐blind, placebo controlled trial. Digestive and Liver Disease 2011;43(Suppl 5):S412. [DOI: 10.1016/S1590-8658(11)60645-2] [DOI] [Google Scholar]
Everitt 2010 {published data only}
- Everitt HA, Moss‐Morris RE, Sibelli A, Tapp L, Coleman N, Yardley LS, et al. Management of irritable bowel syndrome in primary care: feasibility randomised controlled trial of mebeverine, methylcellulose, placebo and a patient self‐management cognitive behavioural therapy website. (MIBS trial). BMC Gastroenterology 2010;10:136. [DOI: 10.1186/1471-230X-10-136; PMC2998449; PUBMED: 21087463] [DOI] [PMC free article] [PubMed] [Google Scholar]
Grillage 1990 {published data only}
- Grillage MG, Nankani JN, Atkinson SN, Prescott P. A randomised, double‐blind study of mebeverine versus dicyclomine in the treatment of functional abdominal pain in young adults. British Journal of Clinical Practice 1990;44(5):176‐9. [PUBMED: 2202405] [PubMed] [Google Scholar]
Kaminski 2009 {published data only}
- Kaminski A, Kamper A, Thaler K, Chapman A, Gartlehner G. Antidepressants for the treatment of abdominal pain‐related functional gastrointestinal disorders in children and adolescents. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD008013] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lloyd‐Still 1990 {published data only}
- Lloyd‐Still JD. Azathioprine and the treatment of chronic inflammatory bowel disease. Journal of Pediatrics 1990;117(5):732‐5. [DOI: 10.1016/S0022-3476(05)83329-3] [DOI] [PubMed] [Google Scholar]
Van Outryve 2005 {published data only}
- Outryve S, Outryve M, Pelckmans P. Tegaserod: the ultimate treatment for constipation‐predominant IBS? [Tegaserod bij het obstipatie‐predominante prikkelbaredarmsyndroom: de ultieme behandeling?]. Tijdschrift voor Geneeskunde 2005;61(16):1110‐7. [DOI: 10.2143/TVG.61.16.5002245] [DOI] [Google Scholar]
Xiao 2013 {published data only}
- Xiao M, Qiu X, Yue D, Cai Y, Mo Q. Influence of hippophae rhamnoides on two appetite factors, gastric emptying and metabolic parameters, in children with functional dyspepsia. Hellenic Journal of Nuclear Medicine 2013;16(1):38‐43. [DOI: 10.1967/s002449910070; PUBMED: 23529392] [DOI] [PubMed] [Google Scholar]
Yadav 1989 {published data only}
- Yadav SK, Jain AK, Tripathi SN, Gupta JP. Irritable bowel syndrome: therapeutic evaluation of indigenous drugs. Indian Journal of Medical Research 1989;90:496‐503. [PUBMED: 2697693] [PubMed] [Google Scholar]
Additional references
Abbott 2014a [pers comm]
- Abbott R. Study information [personal communication]. Email to: B Collins 14 March 2014.
Abbott 2014b [pers comm]
- Abbott R. Study information [personal communication]. Email to: R Bahar 25 April 2014.
Abbott 2017
- Abbott RA, Martin AE, Newlove‐Delgado TV, Bethel A, Thompson‐Coon J, Whear R, et al. Psychosocial interventions for recurrent abdominal pain in childhood. Cochrane Database of Systematic Reviews 2017, Issue 1. [DOI: 10.1002/14651858.CD010971.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Abu‐Arafeh 1995
- Abu‐Arafeh I, Russell G. Prevalence and clinical features of abdominal migraine compared with those of migraine headache. Archives of Disease in Childhood 1995;72(5):413‐7. [PMC1511089; PUBMED: 7618907] [DOI] [PMC free article] [PubMed] [Google Scholar]
Anonymous 1984
- Anonymous. Adverse reactions to food additives. Journal of the Royal College of Physicians of London 1984;18(2):115‐6. [PMC free article] [PubMed] [Google Scholar]
Apley 1958
- Apley J, Naish N. Recurrent abdominal pains: a field survey of 1000 school children. Archives of Disease in Childhood 1958;33(168):165‐70. [PMC2012205] [DOI] [PMC free article] [PubMed] [Google Scholar]
Apley 1975
- Apley J. The Child With Abdominal Pains. 2nd Edition. Oxford: Blackwell Scientific Publications, 1975. [Google Scholar]
Asgarshirazi 2016 [pers comm]
- Asgarshirazi M. Re: study information [personal communcation]. Email to: A Martin 1 November 2016.
Bain 1974
- Bain HW. Chronic vague abdominal pain in children. Pediatric Clinics of North America 1974;21(4):991‐1001. [PUBMED: 4427792] [DOI] [PubMed] [Google Scholar]
Balshem 2011
- Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology 2011;64(4):401‐6. [DOI: 10.1016/j.jclinepi.2010.07.015; PUBMED: 21208779] [DOI] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. [PUBMED: 3802833] [DOI] [PubMed] [Google Scholar]
Di Lorenzo 2001
- Lorenzo C, Youssef NN, Sigurdsson L, Scharff L, Griffiths J, Wald A. Visceral hyperalgesia in children with functional abdominal pain. Journal of Pediatrics 2001;139(6):838‐43. [DOI: 10.1067/mpd.2001.118883; PUBMED: 11743510] [DOI] [PubMed] [Google Scholar]
Drossman 1995
- Drossman DA, Creed FH, Fava GA, Olden KW, Patrick DL, Toner BB, et al. Psychosocial aspects of the functional gastrointestinal disorders. Gastroenterology International 1995;8(2):47‐90. [Google Scholar]
Edwards 1994
- Edwards MC, Mullins LL, Johnson J, Bernardy N. Survey of pediatricians' management practices for recurrent abdominal pain. Journal of Pediatric Psychology 1994;19(2):241‐53. [PUBMED: 8051605] [DOI] [PubMed] [Google Scholar]
Farquar 1956
- Farquar HG. Abdominal migraine in children. British Medical Journal 1956;1(4975):1082‐5. [PMC1979859] [DOI] [PMC free article] [PubMed] [Google Scholar]
Faull 1986
- Faull C, Nicol AR. Abdominal pain in six‐year‐olds: an epidemiological study in a new town. Journal of Child Psychology and Psychiatry and Allied Disciplines 1986;27(2):251‐60. [3958079] [PubMed] [Google Scholar]
Goadsby 2000
- Goadsby PJ, Hargreaves RJ. Mechanisms of action of serotonin 5‐HT1B/D agonists: insights into migraine pathophysiology using rizatriptan. Neurology 2000;55(9 Suppl 2):S8‐14. [PUBMED: 11089513] [PubMed] [Google Scholar]
Good 1995
- Good PA. Neurologic investigations of childhood abdominal migraine: a combined electrophysiologic approach to diagnosis. Journal of Pediatric Gastroenterology and Nutrition 1995;21(Suppl 1):S44‐8. [PUBMED: 8708868] [DOI] [PubMed] [Google Scholar]
Grade Working Group 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A. Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach. Updated October 2013. gdt.guidelinedevelopment.org/app/handbook/handbook.html (accessed 22 March 2016).
GRADEpro GDT 2015 [Computer program]
- McMaster University. GRADEpro Guideline Development Tool. Developed by Evidence Prime, Inc., 2015.
Grigoleit 2005
- Grigoleit HG, Grigoleit P. Peppermint oil in irritable bowel syndrome. Phytomedicine 2005;12(8):601‐6. [DOI: 10.1016/j.phymed.2004.10.005; PUBMED: 16121521] [DOI] [PubMed] [Google Scholar]
Harvey 1973
- Harvey RF, Pomare EW, Heaton KW. Effects of increased dietary fibre on intestinal transit. Lancet 1973;301(7815):1278‐80. [DOI: 10.1016/S0140-6736(73)91294-4] [DOI] [PubMed] [Google Scholar]
Heldenberg 1995
- Heldenberg D, Wagner Y, Heldenberg E, Keren S, Auslaender L, Kaufshtein M, et al. The role of Helicobacter pylori in children with recurrent abdominal pain. American Journal of Gastroenterology 1995;90(6):906‐9. [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI: 10.1136/bmj.327.7414.557] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Altman DG, Sterne JAC on behalf of the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011c
- Deeks JJ, Higgins JPT, Altman DG on behalf of the Cochrane Statistical Methods Group. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hills 1991
- Hills JM, Aaronson PI. The mechanism of action of peppermint oil on gastrointestinal smooth muscle. Gastroenterology 1991;101(1):55‐65. [PUBMED: 1646142] [DOI] [PubMed] [Google Scholar]
Hockaday 1992
- Hockaday JM. Is there a place for "abdominal migraine" as a separate entity in the IHS classification? No!. Cephalalgia 1992;12(6):346‐8. [PUBMED: 1473134] [PubMed] [Google Scholar]
Huertas‐Ceballos 2008b
- Huertas‐Ceballos A, Macarthur C, Logan S. Psychosocial interventions for recurrent abdominal pain (RAP) and irritable bowel syndrome (IBS) in childhood. Cochrane Database of Systematic Reviews 2008, Issue 1. [DOI: 10.1002/14651858.CD003014.pub2] [DOI] [PubMed] [Google Scholar]
Huertas‐Ceballos 2009
- Huertas‐Ceballos A, Logan S, Bennett C, Macarthur C. Dietary interventions for recurrent abdominal pain (RAP) and irritable bowel syndrome (IBS) in childhood. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD003019.pub3] [DOI] [PubMed] [Google Scholar]
Hyams 1995
- Hyams JS, Treem WR, Justinich CJ, Davis P, Shoup M, Burke G. Characterization of symptoms in children with recurrent abdominal pain: resemblance to irritable bowel syndrome. Journal of Pediatric Gastroenterology and Nutrition 1995;20(2):209‐14. [PUBMED: 7714688] [DOI] [PubMed] [Google Scholar]
Hyams 1998
- Hyams JS, Hyman PE. Recurrent abdominal pain and the biopsychosocial model of medical practice. Journal of Pediatrics 1998;133(4):473‐8. [PUBMED: 9787683] [DOI] [PubMed] [Google Scholar]
Kovacs 1985
- Kovacs M. The Children's Depression Inventory. Psychopharmacology Bulletin 1985;21(4):995‐8. [PUBMED: 4089116] [PubMed] [Google Scholar]
Martin 2014b [pers comm]
- Martin A. Tegasterod in IBS [personal communication]. Email to: V Khoshoo 16 August 2014.
Martin 2014c [pers comm]
- Martin A. RAP paper in Gastroenterology [personal communication]. Email to: M Saps 6 August 2014.
Martin 2016a [pers comm]
- Martin A. Domperidone in RAP [personal communication]. Email to: A de Silva 6 July 2016.
Martin 2016b [pers comm]
- Martin A. Study information [personal communication]. Email to: C Friesen 29 June 2016.
Martin 2016c [pers comm]
- Martin A. Antibiotics and RAP in children [personal communication]. Email to: C Braegger 7 December 2016.
Martin 2016d [pers comm]
- Martin A. Domperidone in RAP [personal communication]. Email to: A de Silva 27 November 2016.
Martin 2016e [pers comm]
- Martin A. Trimebutine + IBS [personal communication]. Email to: O Beser 7 December 2016.
Martin 2016f [pers comm]
- Martin A. Peppermint oil paper for IBS [personal communication]. Email to: R Kline 7 December 2016.
Martin 2016g [pers comm]
- Martin A. Study information [personal communication]. Email to: M Asgarshirazi 29 September 2016.
Mayer 2002
- Mayer EA, Collins SM. Evolving pathophysiology models of functional gastrointestinal disorders. Gastroenterology 2002;122(7):2032‐48. [PUBMED: 12055608] [DOI] [PubMed] [Google Scholar]
Milla 2001
- Milla PJ. Irritable bowel syndrome in childhood. Gastroenterology 2001;120(1):287‐90. [PUBMED: 11208737] [DOI] [PubMed] [Google Scholar]
Newlove‐Delgado in press
- Newlove‐Delgado T, Martin A, Abbott R, Bethel A, Thompson‐Coon J, Whear R, et al. Pharmacological interventions for recurrent abdominal pain in childhood. Cochrane Database of Systematic Reviews (in press). [DOI] [PMC free article] [PubMed]
NIMH 1985
- National Institute of Mental Health. Rating scales and assessment instruments for use in pediatric psychopharmacology research. Psychopharmacology Bulletin 1985;21(4):714‐1124. [PUBMED: 3911249] [PubMed] [Google Scholar]
Painter 1964
- Painter NS, Truelove SC. Irritable bowel syndrome in childhood. Gut 1964;5:201. [Google Scholar]
Patrick 1997
- Patrick DL, Drossman DA, Ihunnaya OF. A Quality of Life Measure for Persons With Irritable Bowel Syndrome (IBS‐QOL). User's Manual and Scoring Diskette for United States version. Seattle: University of Washington, 1997. [Google Scholar]
Peltonen 1970
- Peltonen T. Recurrent abdominal pain. Pediatrics 1970;46(6):973. [PubMed] [Google Scholar]
Pittler 1998
- Pittler MH, Ernst E. Peppermint oil for irritable bowel syndrome: a critical review and metaanalysis. American Journal of Gastroenterology 1998;93(7):1131‐5. [DOI: 10.1111/j.1572-0241.1998.00343.x; PUBMED: 9672344] [DOI] [PubMed] [Google Scholar]
Poley 1973
- Poley JR, Bhatia M. Recurrent abdominal pain: recurrent controversy. Pediatrics 1973;52(1):144‐5. [PUBMED: 4724433] [PubMed] [Google Scholar]
Primelles 1990
- Primelles D, Grass M, Campoy PE, Estrada R, Carvajal A, Rubino de la Rosa J. Recurrent abdominal pain in childhood: retrospective study of 150 cases [Dolor abdominal recurrente en la infancia: estudio retrospectivo de 150 casos]. Revista Cubana de Pediatria 1990;62(1):18‐27. [Google Scholar]
Rasquin 2006
- Rasquin A, Lorenzo C, Forbes D, Guiraldes E, Hyams JS, Staiano A, et al. Childhood functional gastrointestinal disorders: child/adolescent. Gastroenterology 2006;130(5):1527–37. [DOI: 10.1053/j.gastro.2005.08.063; PUBMED: 16678566] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rasquin‐Weber 1999
- Rasquin‐Weber A, Hyman PE, Cucchiara S, Fleisher DR, Hyams JS, Milla PJ, et al. Childhood functional gastrointestinal disorders. Gut 1999;45(Suppl II):II60–8. [DOI: 10.1136/gut.45.2008.ii60; PMC1766693] [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Reynolds 1979
- Reynolds CR, Richmond BO. Factor structure and construct validity of "what I think and feel": the revised Children's Manifest Anxiety Scale. Journal of Personality Assessment 1979;43(3):281‐3. [DOI] [PubMed] [Google Scholar]
Rubin 1967
- Rubin LS, Barbero GL, Sibinga MS. Pupillary reactivity in children with recurrent abdominal pain. Psychosomatic Medicine 1967;29(2):111‐20. [PUBMED: 6030151] [DOI] [PubMed] [Google Scholar]
Ruepert 2011
- Ruepert L, Quartero AO, Wit NJ, Heijden GJ, Rubin G, Muris JWM. Bulking agents, antispasmodics and antidepressants for the treatment of irritable bowel syndrome. Cochrane Database of Systematic Reviews 2011, Issue 8. [DOI: 10.1002/14651858.CD003460.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sandberg 1973
- Sandberg DH. Additional references on the tension‐fatigue syndrome. Pediatrics 1973;51(2):308‐9. [PUBMED: 4572072] [PubMed] [Google Scholar]
Scharff 1997
- Scharff L. Recurrent abdominal pain in children: a review of psychological factors and treatment. Clinical Psychology Review 1997;17(2):145‐66. [PUBMED: 9140713] [DOI] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Speer 1954
- Speer F. The allergic tension‐fatigue syndrome. Pediatric Clinics of North America 1954;11:1029‐37. [PUBMED: 13204094] [DOI] [PubMed] [Google Scholar]
Stickler 1979
- Stickler GB, Murphy DB. Recurrent abdominal pain. American Journal of Diseases in Children 1979;133(5):486‐9. [PUBMED: 433872] [DOI] [PubMed] [Google Scholar]
Stone 1970
- Stone RT, Barbero GJ. Recurrent abdominal pain in childhood. Pediatrics 1970;45(5):732‐8. [PubMed] [Google Scholar]
Stowens 1970
- Stowens D. Recurrent abdominal pain. Pediatrics 1970;46(6):968‐9. [PubMed] [Google Scholar]
Størdal 2005
- Størdal, K, Nygaard E, Bentsen B. Recurrent abdominal pain: a five‐year follow‐up study. Acta Paediatrica 2005;94(2):234‐6. [PUBMED: 15981760] [DOI] [PubMed] [Google Scholar]
Sutton 2000
- Sutton AJ, Duval SJ, Tweedie RL, Abrams KR, Jones DR. Empirical assessment of effect of publication bias on meta‐analyses. BMJ 2000;320(7249):1574‐7. [DOI: 10.1136/bmj.320.7249.1574] [DOI] [PMC free article] [PubMed] [Google Scholar]
Svedulend 1988
- Svedulend J, Sjodin I, Dotevall G. GSRS ‐ a clinical rating scale for gastrointestinal symptoms in patients with irritable bowel syndrome and peptic ulcer disease. Digestive Diseases and Sciences 1988;33(2):129‐34. [PUBMED: 3123181] [DOI] [PubMed] [Google Scholar]
Symon 1986
- Symon DN, Russell G. Abdominal migraine: a childhood syndrome defined. Cephalalgia 1986;6(4):223‐8. [PUBMED: 3802189] [DOI] [PubMed] [Google Scholar]
Van Ginkel 2001
- Ginkel R, Voskuijl WP, Benninga MA, Taminiau JA, Boeckxstaens GE. Alterations in rectal sensitivity and motility in childhood irritable bowel syndrome. Gastroenterology 2001;120(1):31‐8. [PUBMED: 11208711] [DOI] [PubMed] [Google Scholar]
Walker 1997
- Walker LS, Smith CA, Garber J, Slyke DA. Development and validation of the Pain Response Inventory for children. Psychological Assessment 1997;9(4):392‐405. [Google Scholar]
Walker 1998
- Walker LS, Guite JW, Duke M, Barnard JA, Greene JW. Recurrent abdominal pain: a potential precursor of irritable bowel syndrome in adolescents and young adults. Journal of Pediatrics 1998;132(6):1010‐5. [PUBMED: 9627595] [DOI] [PubMed] [Google Scholar]
Walker 2009
- Walker LS, Beck JE, Garber J, Lambert W. Children's Somatization Inventory: psychometric properties of the revised form (CSI‐24). Journal of Pediatric Psychology 2009;34(4):430‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wardhan 1993
- Wardhan H, Ojha P, Kulshrestha R. Recurrent abdominal pain in children in developing countries frequently has an organic basis. Pediatric Surgery International 1993;8:20‐2. [DOI: 10.1016/0022-3468(93)90195-Q] [DOI] [Google Scholar]
Williams 1996
- Williams K, Chambers M, Logan S, Robinson D. Association of common health symptoms with bullying in primary school children. BMJ 1996;313(7048):17‐9. [PMC2351438] [DOI] [PMC free article] [PubMed] [Google Scholar]
Worawattanakul 1999
- Worawattanakul M, Rhoads JM, Lichtman SN, Ulshen MH. Abdominal migraine: prophylactic treatment and follow up. Journal of Pediatric Gastroenterology and Nutrition 1999;28(1):37‐40. [PUBMED: 9890466] [DOI] [PubMed] [Google Scholar]
Zhang 1991
- Zhang H, Luo LL, Wang Y, Sun M. Diagnosis and treatment for recurrent abdominal pain in infant. Sapparo Medical Journal 1991;60(6):561‐3. [Google Scholar]
Øster 1972
- Øster J. Recurrent abdominal pain, headache and limb pains in children and adolescents. Pediatrics 1972;50(3):429‐36. [PUBMED: 5056416] [PubMed] [Google Scholar]
References to other published versions of this review
Huertas‐Ceballos 2002
- Huertas‐Ceballos A, Macarthur C, Logan S. Pharmacological interventions for recurrent abdominal pain (RAP) in childhood. Cochrane Database of Systematic Reviews 2002, Issue 1. [DOI: 10.1002/14651858.CD003017] [DOI] [PubMed] [Google Scholar]
Huertas‐Ceballos 2008a
- Huertas‐Ceballos AA, Logan S, Bennett C, Macarthur C. Pharmacological interventions for recurrent abdominal pain (RAP) and irritable bowel syndrome (IBS) in childhood. Cochrane Database of Systematic Reviews 2008, Issue 1. [DOI: 10.1002/14651858.CD003017.pub2] [DOI] [PubMed] [Google Scholar]
Martin 2014a
- Martin AE, Newlove‐Delgado TV, Abbott RA, Bethel A, Thompson‐Coon J, Nikolaou V, et al. Pharmacological interventions for recurrent abdominal pain in childhood. Cochrane Database of Systematic Reviews 2014, Issue 2. [DOI: 10.1002/14651858.CD010973] [DOI] [PMC free article] [PubMed] [Google Scholar]


