Abstract
Background
The goal of fetal monitoring in labour is the early detection of a hypoxic baby. There are a variety of tools and methods available for intermittent auscultation (IA) of the fetal heart rate (FHR). Low‐ and middle‐income countries usually have only access to a Pinard/Laënnec or the use of a hand‐held Doppler device. Currently, there is no robust evidence to guide clinical practice on the most effective IA tool to use, timing intervals and length of listening to the fetal heart for women during established labour.
Objectives
To evaluate the effectiveness of different tools for IA of the fetal heart rate during labour including frequency and duration of auscultation.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (19 September 2016), contacted experts and searched reference lists of retrieved articles.
Selection criteria
All published and unpublished randomised controlled trials (RCTs) or cluster‐RCTs comparing different tools and methods used for intermittent fetal auscultation during labour for fetal and maternal well‐being. Quasi‐RCTs, and cross‐over designs were not eligible for inclusion.
Data collection and analysis
All review authors independently assessed eligibility, extracted data and assessed risk of bias for each trial. Data were checked for accuracy.
Main results
We included three studies (6241 women and 6241 babies), but only two studies are included in the meta‐analyses (3242 women and 3242 babies). Both were judged as high risk for performance bias due to the inability to blind the participants and healthcare providers to the interventions. Evidence was graded as moderate to very low quality; the main reasons for downgrading were study design limitations and imprecision of effect estimates.
Intermittent Electronic Fetal Monitoring (EFM) using Cardiotocography (CTG) with routine Pinard (one trial)
There was no clear difference between groups in low Apgar scores at five minutes (reported as < six at five minutes after birth) (risk ratio (RR) 0.66, 95% confidence interval (CI) 0.24 to 1.83, 633 babies, very low‐quality evidence). There were no clear differences for perinatal mortality (RR 0.88, 95% CI 0.34 to 2.25; 633 infants, very low‐quality evidence). Neonatal seizures were reduced in the EFM group (RR 0.05, 95% CI 0.00 to 0.89; 633 infants, very low‐quality evidence). Other important infant outcomes were not reported: mortality or serious morbidity (composite outcome), cerebral palsy or neurosensory disability. For maternal outcomes, women allocated to intermittent electronic fetal monitoring (EFM) (CTG) had higher rates of caesarean section for fetal distress (RR 2.92, 95% CI 1.78 to 4.80, 633 women, moderate‐quality evidence) compared with women allocated to routine Pinard. There was no clear difference between groups in instrumental vaginal births (RR 1.46, 95% CI 0.86 to 2.49, low‐quality evidence). Other outcomes were not reported (maternal mortality, instrumental vaginal birth for fetal distress and or acidosis, analgesia in labour, mobility or restriction during labour, and postnatal depression).
Doppler ultrasonography with routine Pinard (two trials)
There was no clear difference between groups in Apgar scores < seven at five minutes after birth (reported as < six in one of the trials) (average RR 0.76, 95% CI 0.20 to 2.87; two trials, 2598 babies, I2 = 72%, very low‐quality evidence); there was high heterogeneity for this outcome. There was no clear difference between groups for perinatal mortality (RR 0.69, 95% CI 0.09 to 5.40; 2597 infants, two studies, very low‐quality evidence), or neonatal seizures (RR 0.05, 95% CI 0.00 to 0.91; 627 infants, one study, very low‐quality evidence). Other important infant outcomes were not reported (cord blood acidosis, composite of mortality and serious morbidity, cerebral palsy, neurosensory disability). Only one study reported maternal outcomes. Women allocated to Doppler ultrasonography had higher rates of caesarean section for fetal distress compared with those allocated to routine Pinard (RR 2.71, 95% CI 1.64 to 4.48, 627 women, moderate‐quality evidence). There was no clear difference in instrumental vaginal births between groups (RR 1.35, 95% CI 0.78 to 2.32, 627 women, low‐quality evidence). Other maternal outcomes were not reported.
Intensive Pinard versus routine Pinard (one trial)
One trial compared intensive Pinard (a research midwife following the protocol in a one‐to‐one care situation) with routine Pinard (as per protocol but midwife may be caring for more than one woman in labour). There was no clear difference between groups in low Apgar score (reported as < six this trial) (RR 0.90, 95% CI 0.35 to 2.31, 625 babies, very low‐quality evidence). There were also no clear differences identified for perinatal mortality (RR 0.56, 95% CI 0.19 to 1.67; 625 infants, very low‐quality evidence), or neonatal seizures (RR 0.68, 95% CI 0.24 to 1.88, 625 infants, very low‐quality evidence)). Other infant outcomes were not reported. For maternal outcomes, there were no clear differences between groups for caesarean section or instrumental delivery (RR 0.70, 95% CI 0.35 to 1.38, and RR 1.21, 95% CI 0.69 to 2.11, respectively, 625 women, both low‐quality evidence)) Other outcomes were not reported.
Authors' conclusions
Using a hand‐held (battery and wind‐up) Doppler and intermittent CTG with an abdominal transducer without paper tracing for IA in labour was associated with an increase in caesarean sections due to fetal distress. There was no clear difference in neonatal outcomes (low Apgar scores at five minutes after birth, neonatal seizures or perinatal mortality). Long‐term outcomes for the baby (including neurodevelopmental disability and cerebral palsy) were not reported. The quality of the evidence was assessed as moderate to very low and several important outcomes were not reported which means that uncertainty remains regarding the use of IA of FHR in labour.
As intermittent CTG and Doppler were associated with higher rates of caesarean sections compared with routine Pinard monitoring, women, health practitioners and policy makers need to consider these results in the absence of evidence of short‐ and long‐term benefits for the mother or baby.
Large high‐quality randomised trials, particularly in low‐income settings, are needed. Trials should assess both short‐ and long‐term health outcomes, comparing different monitoring tools and timing for IA.
Keywords: Female; Humans; Infant, Newborn; Pregnancy; Heart Rate, Fetal; Labor, Obstetric; Auscultation; Auscultation/instrumentation; Auscultation/methods; Cardiotocography; Cardiotocography/instrumentation; Cardiotocography/methods; Cesarean Section; Fetal Distress; Fetal Distress/diagnosis; Infant, Newborn, Diseases; Infant, Newborn, Diseases/diagnosis; Perinatal Mortality; Seizures; Seizures/diagnosis; Ultrasonography, Doppler
Plain language summary
What is the most effective way to listen intermittently to the baby’s heart in labour to improve the baby’s well‐being?
What is the issue?
One method of monitoring a baby's well‐being is to listen to the fetal heart rate and its pattern intermittently during labour (intermittent auscultation). There are several ways that the baby's heart rate can be measured. Some tools for listening to the baby's heart are made from wood, plastic or aluminium (Pinard, Laennec and fetoscope), and there are also electronic tools of varying sophistication, including hand‐held (battery or wind‐up operated) Doppler ultrasound (Doppler) and cardiotocogram (CTG), which is sometimes referred to as electronic fetal monitoring (EFM).
Why is this important?
The aim of monitoring is so that babies in difficulty can be accurately identified and interventions (such as caesarean section or instrumental vaginal birth) can be used to improve outcomes for the baby.
What evidence did we find?
We wanted to look at which types listening tools and timing for intermittent auscultation are most effective. We considered hand held listening devices, e.g. hand‐held Dopplers and various Pinard stethoscopes. We searched for studies (19 September 2016) and found three randomised controlled studies from Africa, involving 6241 women in established labour. Data from one of the studies were inconsistent and we were unable to include them in the results. This means 3242 women and their babies were included in the analyses. The results of the studies may have been biased as it was not possible to blind women and staff, and the overall the quality of the evidence was judged to be of moderate to very low quality.
One study compared intermittent EFM with routine Pinard and showed no clear difference between groups in low baby Apgar scores at five minutes after the birth (very low‐quality evidence) or in perinatal mortality (low‐quality evidence) although neonatal seizures were reduced in the EFM group (low‐quality evidence). Other important infant outcomes (such as cerebral palsy) were not reported. Women who had intermittent EFM had higher rates of caesarean section for fetal distress (moderate‐quality evidence), but there was no clear difference between groups in instrumental vaginal births (low‐quality evidence). Other important outcomes for women were not reported (maternal mortality, analgesia in labour, mobility or restriction during labour, and postnatal depression).
Two studies compared Doppler ultrasonography with routine Pinard. There was no clear difference between groups in low Apgar scores at five minutes after birth (very low‐quality evidence) or for perinatal mortality (very low‐quality evidence), or neonatal seizures (very low‐quality evidence). Other important infant outcomes were not reported. Only one study reported outcomes for women. Those that had Doppler ultrasonography had higher rates of caesarean section for fetal distress compared with routine Pinard (moderate‐quality evidence). There was no clear difference in instrumental vaginal births between groups (low‐quality evidence). Other maternal outcomes were not reported.
One trial compared intensive Pinard (a research midwife in a one‐to‐one care situation) with routine Pinard (the midwife may have been caring for more than one woman in labour). There was no clear difference between groups in low Apgar scores (very low‐quality evidence), perinatal mortality (very low‐quality evidence), or neonatal seizures (very low‐quality evidence)). Other infant outcomes were not reported. For women, there were no clear differences between groups for caesarean section or instrumental delivery (both low‐quality evidence)) Other outcomes were not reported.
What does this mean?
As intermittent EFM and Doppler were associated with higher rates of caesarean sections compared with routine Pinard monitoring, women, health practitioners and policy makers need to consider these results in the absence of evidence of short‐ and long‐term benefits for the mother or baby.
Large high‐quality studies comparing different monitoring tools and timing for intermittent auscultation are needed. Studies should assess both short‐ and long‐term health outcomes, and should collect information on women's views.
Summary of findings
Summary of findings for the main comparison. Intermittent ausculation of fetal heart rate in labour for fetal well‐being ‐ Intermittent electronic fetal monitoring (CTG) versus routine Pinard (outcomes for the baby).
| Intermittent ausculation of fetal heart rate in labour for fetal well‐being ‐ Intermittent electronic fetal monitoring (CTG) (inconsistent/ opportunistic paper tracing) versus routine Pinard (outcomes for the baby). | ||||||
| Patient or population: women in established labour and their babies. Setting: all studies were conducted in Africa (Zimbabwe and Uganda). Intervention: electronic fetal monitoring (CTG) without paper tracing. Comparison: routine Pinard. | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with routine Pinard | Risk with Intermittent electronic fetal monitoring | |||||
| Apgar < 7 at 5 minutes | 29 per 1000 | 19 per 1000 (7 to 52) | RR 0.66 (0.24 to 1.83) | 633 (1 RCT) | ⊕⊕⊝⊝ VERY LOW 1,2 | Low event rate. Study reported Apgar score < 6 at 5 minutes. |
| Cord blood acidosis | 0 per 1000 | 0 per 1000 (0 to 0) | not estimable | (0 studies) | No data reported for cord blood acidosis in the included studies. | |
| Neonatal seizures | 29 per 1000 | 1 per 1000 (0 to 25) | RR 0.05 (0.00 to 0.89) | 633 (1 RCT) | ⊕⊕⊝⊝ LOW 1,3 |
Low event rates. Routine Pinard group (9/315) compared to the intermittent EFM (CTG) group (0/318). |
| Perinatal mortality | 29 per 1000 | 25 per 1000 (10 to 64) | RR 0.88 (0.34 to 2.25) | 633 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1,2 | Neonatal deaths included, unable to separate out from reported data. Low event rates 8/318 for intermittent EFM (CTG) group and 9/315 for routine Pinard group. |
| Composite of mortality and serious morbidity | 0 per 1000 | 0 per 1000 (0 to 0) | not estimable | (0 studies) | No data reported for a composite of mortality and serious morbidity in the included studies. | |
| Cerebral palsy | 0 per 1000 | 0 per 1000 (0 to 0) | not estimable | (0 studies) | No data reported for cerebral palsy in the included studies. | |
| Neurosensory disability | 0 per 1000 | 0 per 1000 (0 to 0) | not estimable | (0 studies) | No data reported for neurosensory disability in the included studies at either 6 months or 1 year. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: we are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: we have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Blinding of participants and health professionals not possible; high risk of performance bias and it is unclear if outcome assessors were blinded. Downgraded one level.
2 Evidence of imprecision; single trial with low event rate and wide 95% CI crossing the line of no effect. Downgraded two levels.
3 Evidence of imprecision, evidence based on a single trial with low event rates. Downgraded one level.
Summary of findings 2. Intermittent ausculation of fetal heart rate in labour for fetal well‐being ‐ Intermittent electronic fetal monitoring (CTG) versus routine Pinard (outcomes for the mother).
| Intermittent ausculation of fetal heart rate in labour for fetal well‐being ‐ Intermittent electronic fetal monitoring (CTG) (inconsistent/ opportunistic paper tracing) versus Routine Pinard (outcomes for the mother). | ||||||
| Patient or population: women in established labour and their babies. Setting: all studies were conducted in Africa (Zimbawe and Uganda). Intervention: electronic fetal monitoring (CTG) without paper tracing. Comparison: routine Pinard. | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with routine Pinard | Risk with Intermittent electronic fetal monitoring intensive Pinard | |||||
| Caesarean section for fetal distress and/or fetal acidosis | 60 per 1000 | 176 per 1000 (107 to 290) | RR 2.92 (1.78 to 4.80) | 633 (1 RCT) | ⊕⊕⊕⊝ MODERATE 1, | |
| Instrumental vaginal birth | 67 per 1000 | 97 per 1000 (57 to 166) | RR 1.46 (0.86 to 2.49) | 633 (1 RCT) | ⊕⊕⊝⊝ LOW 1,2, | |
| Maternal mortality | 0 per 1000 | 0 per 1000 (0 to 0) | not estimable | (0 studies) | No data reported for maternal mortality in the included studies. | |
| Any pharmacological or non‐pharmacological analgesia use excluding epidural | 0 per 1000 | 0 per 1000 (0 to 0) | not estimable | (0 studies) | No data reported for any pharmacological or non‐ pharmacological analgesia use excluding epidural in the included studies. | |
| Epidural anaesthesia for pain relief excluding for caesarean section | 0 per 1000 | 0 per 1000 (0 to 0) | not estimable | (0 studies) | No data reported for epidural anaesthesia for pain relief excluding for caesarean section in the included studies. However, 1 trial reported that no epidural analgesia was available in the labour ward. | |
| Mobility or restriction during labour | 0 per 1000 | 0 per 1000 (0 to 0) | not estimable | (0 studies) | No data reported for mobility or restriction during labour in the included studies. | |
| Postnatal depression | 0 per 1000 | 0 per 1000 (0 to 0) | not estimable | (0 studies) | No data reported for postnatal depression in the included studies. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: we are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: we have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Blinding of participants and health professionals not possible; high risk of performance bias and it is unclear if outcome assessors were blinded. Downgraded one level.
2 Evidence of imprecision with wide confidence intervals. Downgraded one level.
Summary of findings 3. Intermittent ausculation of fetal heart rate in labour for fetal well‐being ‐ Doppler versus routine Pinard (outcomes for the baby).
| Intermittent ausculation of fetal heart rate in labour for fetal well‐being ‐ Doppler versus Routine Pinard (outcomes for the baby) | ||||||
| Patient or population: women in established labour and their babies. Setting: all studies were conducted in Africa (Zimbabwe and Uganda). Intervention: Doppler. Comparison: routine Pinard. | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with Routine Pinard | Risk with Doppler | |||||
| Apgar < 7 at 5 minutes | 20 per 1000 | 15 per 1000 (4 to 58) | RR 0.76 (0.20 to 2.87) | 2598 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 1,2, 3 | One of the studies contributing data reported Apgar score < 6. |
| Cord blood acidosis | 0 per 1000 | 0 per 1000 (0 to 0) | not estimable | (0 studies) | No data reported for cord blood acidosis in the included studies. | |
| Seizures in the neonatal period | 29 per 1000 | 1 per 1000 (0 to 26) | RR 0.05 (0.00 to 0.91) | 627 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1, 4 | Event rates are low 0/312 for Doppler and 9/315 for routine Pinard. |
| Perinatal mortality | 12 per 1000 | 8 per 1000 (1 to 63) | RR 0.69 (0.09 to 5.40) | 2597 (2 RCTs) | ⊕⊕⊝⊝ VERY LOW 1, 2, 5 | Event rates 13/1304 for Doppler and 15/1293 for routine Pinard. Neonatal deaths included, unable to separate out from reported data. |
| Composite of mortality and serious morbidity | 0 per 1000 | 0 per 1000 (0 to 0) | not estimable | (0 studies) | No data reported for a composite of mortality and serious morbidity in the included studies. | |
| Cerebral palsy | 0 per 1000 | 0 per 1000 (0 to 0) | not estimable | (0 studies) | No data reported for cerebral palsy in the included studies. | |
| Neurosensory disability | 0 per 1000 | 0 per 1000 (0 to 0) | not estimable | (0 studies) | No data reported for neurosensory disability in the included studies. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: we are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: we have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Blinding of participants and health professionals not possible; high risk of performance bias and it is unclear if outcome assessors were blinded. Downgraded one level.
2 Evidence of imprecision with wide 95% CI crossing the line of no effect. Downgraded one level.
3 There was high heterogeneity for this outcome.
4 Evidence of imprecision, with wide 95% CI crossing the line of no effect and low event rate. Downgraded 2 levels.
5 There was high heterogeneity for this outcome. Downgraded one level.
Summary of findings 4. Intermittent ausculation of fetal heart rate in labour for fetal well‐being ‐ Doppler versus routine Pinard (outcomes for the mother).
| Intermittent ausculation of fetal heart rate in labour for fetal well‐being ‐ Doppler versus Routine Pinard (outcomes for the mother) | ||||||
| Patient or population: women in established labour and their babies. Setting: all studies were conducted in Africa (Zimbabwe and Uganda). Intervention: Doppler. Comparison: routine Pinard. | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with routine Pinard | Risk with Doppler | |||||
| Caesarean section for fetal distress and/or fetal acidosis | 60 per 1000 | 163 per 1000 (99 to 270) | RR 2.71 (1.64 to 4.48) | 627 (1 RCT) | ⊕⊕⊕⊝ MODERATE 1, | |
| Instrumental vaginal birth | 67 per 1000 | 90 per 1000 (52 to 155) | RR 1.35 (0.78 to 2.32) | 627 (1 RCT) | ⊕⊕⊝⊝ LOW 1,2 | |
| Maternal mortality | 0 per 1000 | 0 per 1000 (0 to 0) | not estimable | (0 studies) | No data reported for maternal mortality in the included studies. | |
| Any pharmacological or non‐pharmacological analgesia use excluding epidural | 0 per 1000 | 0 per 1000 (0 to 0) | not estimable | (0 studies) | No data reported for any pharmacological or non‐pharmacological use excluding epidural in the included studies. | |
| Epidural anaesthesia for pain relief excluding for caesarean section | 0 per 1000 | 0 per 1000 (0 to 0) | not estimable | (0 studies) | No data reported for epidural anaesthesia for pain relief excluding for caesarean section in the included studies. However, 1 trial reported that no epidural analgesia was available in the labour ward. | |
| Mobility or restriction during labour | 0 per 1000 | 0 per 1000 (0 to 0) | not estimable | (0 studies) | No data reported for mobility or restriction during labour in the included studies. | |
| Postnatal depression | 0 per 1000 | 0 per 1000 (0 to 0) | not estimable | (0 studies) | No data reported for postnatal depression in the included studies. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: we are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: we have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Blinding of participants and health professionals not possible; high risk of performance bias and it is unclear if outcome assessors were blinded. Downgraded one level.
2 Wide confidence interval. Downgraded one level.
Summary of findings 5. Intermittent ausculation of fetal heart rate in labour for fetal well‐being ‐ Intensive Pinard versus routine Pinard (outcomes for the baby).
| Intermittent ausculation of fetal heart rate in labour for fetal well‐being ‐ Intensive Pinard versus Routine Pinard (outcomes for the baby) | ||||||
| Patient or population: women in established labour and their babies. Setting: all studies were conducted in Africa (Zimbabwe and Uganda). Intervention: intensive Pinard. Comparison: routine Pinard. | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with routine Pinard | Risk with Intensive Pinard | |||||
| Apgar < 7 at 5 minutes | 29 per 1000 | 26 per 1000 (10 to 66) | RR 0.90 (0.35 to 2.31) | 625 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1,2 | Study reported Apgar score < 6 at 5 minutes. |
| Cord blood acidosis | 0 per 1000 | 0 per 1000 (0 to 0) | not estimable | (0 studies) | No data reported for cord blood acidosis in the included studies. | |
| Neonatal seizures | 29 per 1000 | 19 per 1000 (7 to 54) | RR 0.68 (0.24 to 1.88) | 625 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1,2 | |
| Perinatal mortality | 29 per 1000 | 16 per 1000 (5 to 48) | RR 0.56 (0.19 to 1.67) | 625 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1,2 | Neonatal deaths included, unable to separate out from reported data. |
| Composite of mortality and serious morbidity | 0 per 1000 | 0 per 1000 (0 to 0) | not estimable | (0 studies) | No data reported for a composite of mortality and serious morbidity in the included studies. | |
| Cerebral palsy | 0 per 1000 | 0 per 1000 (0 to 0) | not estimable | (0 studies) | No data reported for cerebral palsy in the included studies. | |
| Neurosensory disability | 0 per 1000 | 0 per 1000 (0 to 0) | not estimable | (0 studies) | No data reported for neurosensory disability in the included trial. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: we are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: we have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Blinding of participants and health professionals not possible; high risk of performance bias and it is unclear if outcome assessors were blinded. Downgraded 1 level.
2 Evidence was imprecise; wide 95% CI crossing the line of no effect and low event rate. Downgraded 2 levels.
Summary of findings 6. Intermittent ausculation of fetal heart rate in labour for fetal well‐being ‐ Intensive Pinard versus routine Pinard (outcomes for the mother).
| Intermittent ausculation of fetal heart rate in labour for fetal well‐being ‐ Intensive Pinard versus Routine Pinard (outcomes for the mother) | ||||||
| Patient or population: women in established labour and their babies. Setting: all studies were conducted in Africa (Zimbawe and Uganda). Intervention: intensive Pinard. Comparison: routine Pinard. | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with routine Pinard | Risk with Intensive Pinard | |||||
| Caesarean section for fetal distress and/or fetal acidosis | 60 per 1000 | 42 per 1000 (21 to 83) | RR 0.70 (0.35 to 1.38) | 625 (1 RCT) | ⊕⊕⊝⊝ LOW 1,2 | |
| Instrumental vaginal birth | 67 per 1000 | 81 per 1000 (46 to 141) | RR 1.21 (0.69 to 2.11) | 625 (1 RCT) | ⊕⊕⊝⊝ LOW 1,2 | |
| Maternal morbidity | 0 per 1000 | 0 per 1000 (0 to 0) | not estimable | (0 studies) | No data reported for maternal morbidity in the included studies. | |
| Any pharmacological or non‐pharmacological analgesia use excluding epidural | 0 per 1000 | 0 per 1000 (0 to 0) | not estimable | (0 studies) | No reported data for any pharmacological or non‐pharmacological analgesia use excluding epidural. | |
| Epidural anaesthesia for pain relief excluding caesarean section | 0 per 1000 | 0 per 1000 (0 to 0) | not estimable | (0 studies) | No data reported for epidural anaesthesia for pain relief excluding caesarean section in the included studies. However, 1 trial reported that no epidural analgesia was available in the labour ward. | |
| Mobility or restriction during labour | 0 per 1000 | 0 per 1000 (0 to 0) | not estimable | (0 studies) | No data reported for mobility or restriction during labour in the included studies. | |
| Postnatal depression | 0 per 1000 | 0 per 1000 (0 to 0) | not estimable | (0 studies) | No data reported for post natal depression in the included studies. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: we are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: we have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Blinding of participants and health professionals not possible; high risk of performance bias and it is unclear if outcome assessors were blinded. Downgraded one level.
2 Some imprecision with wide CI crossing the line of no effect. Downgraded one level.
Background
Description of the condition
Auscultation of the fetal heart during labour became a universal 'standard of care' during the first half of the 20th century to monitor fetal well‐being (Schmidt 2000; Hindley 2005). The fetal heart rate (FHR) and patterns of the FHR are used in the assessment of fetal well‐being in an effort to identify those fetuses who might have, or be at risk of, developing hypoxaemia. There are currently two main methods of monitoring the FHR in labour: continuous and intermittent. Continuous FHR monitoring consists of the continuous and simultaneous monitoring of the FHR and maternal uterine contractions onto a paper tracing called a cardiotocograph (CTG). Intermittent auscultation (IA) involves listening to the fetal heart beat periodically with a Pinard or fetal stethoscope or hand‐held Doppler device and recording a single measure of the heart rate at that time. IA during labour can mean listening to the fetal heart for anywhere from five‐ to 30‐minute intervals before and/or during and/or immediately after a contraction.
In low‐ and middle‐income countries, IA is commonly practiced with a Pinard or fetal stethoscope (also known as an obstetric stethoscope or fetoscope, Pinard or Laënnec stethoscope), or sometimes with an ordinary stethoscope if no other auscultation tool is available. Hand‐held Doppler devices are also used to auscultate the fetal heart, particularly in high‐income countries. However, as with other electronic fetal monitoring (EFM) devices, such as CTG, the disadvantages of hand‐held Doppler devices are considerable for low‐ and middle‐income countries because of cost, access to reliable source of electricity or batteries, and maintenance issues (Irwig 1998; Arasaratnam 2013). Recently in Uganda, a prototype of a wind‐up Doppler ultrasound FHR monitor (or wind‐up fetal Doppler) has been tested by PowerFree Education Technology, a South Africa based not‐for‐profit organisation, in collaboration with Philips. A wind‐up fetal Doppler is not reliant on battery or electricity and may make this device more accessible to low‐ and middle‐income countries (PowerFree Education Technology 2014).
EFM devices, in particular CTG machines with or without access to fetal scalp blood and cord blood testing are not commonly available in low‐ and middle‐income countries. Continuous EFM use in labour, usually with a CTG, has been the subject of two other Cochrane reviews (Alfirevic 2013; Neilson 2015). The results from these reviews indicated that continuous CTG monitoring during labour is associated with a reduction in neonatal seizures, but there are no significant differences in cerebral palsy or infant mortality or other neonatal assessments (e.g. Apgar score). Continuous CTG was associated with an increase in caesarean sections and instrumental vaginal births. It is difficult how to interpret these results. With the limited evidence of the effectiveness of continuous CTG, there is also a renewed interest worldwide in IA during normal labour, not only for low‐ and middle‐income countries (Goodwin 2000, Albers 2001; Sholapurkar 2015). This review evaluates the effectiveness of different tools for intermittent ausculation of the FHR during labour including frequency and duration of auscultation.
Historical context
Fetal heart sounds were first described in the 17th century in poetry (Freeman 1991). Direct FHR auscultation for fetal well‐being has been practiced for many centuries, mainly with the practitioner's ear placed on the mother's abdomen (Gultekin‐Zootzmann 1975). Mayor, a Swiss surgeon, first reported direct FHR auscultation in 1818 (Solt 2005). The first medical texts to describe and discuss auscultation were written in 1819 by Laennec and in 1822 by Keregaredec (Solt 2005; Maude 2010), followed by DeLee‐Hills in 1922 (Chez 2000), and by Pinard in the late 1880s (Mainstone 2004). Their auscultation tools are currently still in use and have undergone little change since their early development.
Description of the intervention
IA is the auditory technique for sampling and counting the FHR at particular intervals with the human ear. It uses bone conduction to assist in hearing the opening and closing of the valves of the fetal heart and is often practised by listening and counting the fetal heart sounds through the mother's abdominal wall for one minute (ACNM 2007). The FHR is best heard over the fetal back/anterior shoulder and therefore it is good practice to perform auscultation of the FHR as part of a comprehensive abdominal examination, which should include inspection, palpation and auscultation (RANZCOG 2006; NCCWCH 2008; NICE 2014). Clinical practice guidelines vary in their recommendations of IA and with recommendations for duration of auscultation of the fetal heart rate ranging from 15 to 30 to 60 seconds, after a contraction, in the first stage of labour and from auscultating after every contraction to every five minutes in the second stage of labour (ACOG 1995; Liston 2002; NZCOM 2005; RANZCOG 2006; NICE 2014). The FHR is recorded as a single number.
In 1893, von Winckel, a German physician, identified clinical criteria for identification of the potentially compromised fetus, based on work also done by Schatz in 1885 and Kehrer in 1867 (Goodlin 1979). Von Winckel identified the normal range of FHR as 120 to 160 beats per minute (bpm) and identified fetal bradycardia as less than 120 bpm and fetal tachycardia greater than 160 bpm (Goodlin 1979). Seitz in 1903 (Gultekin‐Zootzmann 1975), and later Cox in 1950 (Schmidt 2000), identified further possible indicators of fetal distress by describing and differentiating between three types of FHR: early decelerations, late decelerations and decelerations during uterine contractions. Early decelerations refer to the transient decrease of the FHR with the onset of the deceleration at the onset of the contraction, and late decelerations of the transient decrease of the FHR beginning at or after the peak of the contraction phase. Accelerations of the FHR that are transient are regarded as a reassuring sign of fetal well‐being (Freeman 2012; Saxena 2013). It is recognised that during labour natural alterations of the FHR pattern can occur. For example, FHR patterns may differ depending on whether the fetus is undergoing a period of rest or activity or a period of stress induced by maternal uterine activity. Maternal opioids, in particular pethidine and meptazinol have been reported to impact on the FHR pattern causing fetal acidosis (Anderson 2011; Kranke 2013; Goodson 2014). Most clinical guidelines today indicate the normal baseline FHR range as 110 to 160 bpm (RANZCOG 2006; NICE 2014).
How the intervention might work
Auscultation tools have been used since the 1800s and how they work is described in detail below. These individual tools were introduced on the premise that they would facilitate early detection of abnormal FHR patterns thought to be associated with hypoxia and therefore identify the babies who may require more intensive heart rate monitoring through, for example, continuous CTG monitoring or require to be delivered, usually by emergency caesarean section. There are several auscultation tools in use including the following.
Laënnec
In 1818, Rene‐Theophile‐Hyacinthe Laënnec, a French physician, devised a straight wooden tube for listening to breathing sounds of the human chest through his ear after having observed two children playing with sticks listening to the ends of a long piece of wood that transmitted the sounds of pin scratches. He built a 25 cm by 2.5 cm hollow wooden 'stick'. This was the first mono‐aural device built and he called it the stethoscope (stetho (Greek), meaning chest) (Laënnec 1819). While it was initially used for listening to adult heart and chest sounds, JA Le Jumeau and Viscount de Kergaradec soon described auscultation of the fetal heart with a Laënnec stethoscope (Solt 2005). The Laënnec used in obstetrics today is still about 25 cm long with a 2.5 cm hollow diameter with a small flat bell curve opening at one end. The bell curve is placed onto the mother's abdomen and the end with the smaller diameter into the practitioner's ear (Chez 2000).
Pinard
In the late 1880s, A. Pinard, a French physician, developed a mono‐aural fetoscope to listen to fetal heart sounds. It became known as the Pinard or Pinard horn. It is shaped like a trumpet and its flat end is placed on the practitioner's ear while the horn part is placed on the pregnant mother's abdomen to listen to the FHR. The horn/bell shaped end is produced with a variety of diameters from 12.5 cm to 16.5 cm, depending on the choice of the individual Pinard maker (Gapultos 2008). The early Pinard was produced out of wood. More recently, it is also produced in plastic and aluminium, with the wooden option still preferred by many practitioners. While Laënnec and Pinard are inexpensive and readily available in many countries, they may be difficult to be used in certain labouring and birthing position (Lewis 2015).
Fetal stethoscope or fetoscope or DeLee‐Hillis
In 1917, D Hillis, an American physician (Hillis 1917), and in 1922 JB DeLee (Goodlin 1979), also an American physician, both developed and described an identical fetoscope for obtaining better fetal heart sounds. This simultaneous development caused some controversy, which was eventually resolved by naming the instrument DeLee‐Hillis. It uses the practitioner's forehead to conduct sound and is made from metal and plastic. Shaped much like the stethoscope, two tubes extend out of the main long plastic tube; these are inserted into the practitioner's ears. Similar to the Pinard, on the other end, the fetoscope fans out into a bell/horn shape that is placed on the mother's abdomen. The diameter of the bell/horn is smaller than that of a Pinard. It is still in use today, mainly in the UK and USA (Chez 2000). Some DeLee‐Hillis also come with a head strap, which enables the practitioner to have both hands free. Similarly to Pinard and Laënnec, DeLee is inexpensive but may be difficult to be used in certain labouring and birthing position (Lewis 2015).
Stethoscope
The stethoscope is a common tool used in clinical practice today. In 1852, G Cammann, an American physician, invented the binaural stethoscope, building on Laënnec's earlier invention. It consisted of a Y‐shaped tube, with the longer section fitted with interchangable wide and narrow ends intended for lung and heart examination. While initially made from wood it quickly changed to plastic, rubber and metal. The stethoscope can also be used for fetal auscultation and is often used in the absence of any other tool to ascertain the FHR (Bishop 1980), with the diaphragm or small bell placed onto the mother's abdomen. While relatively inexpensive, using a stethoscope requires access to spare parts for replacements of the ear pieces and diaphragm, which may not be available in all countries.
Hand‐held fetal Doppler/Sonic Aid device
A hand‐held Doppler device uses ultrasound technology to bounce sound waves off the fetal heart valves or walls and converts it into sound. This is then heard and/or displayed as a representation of the fetal cardiac cycle/fetal heart beat (Goodwin 2000). The device comes in a range of portable sizes as well as waterproof options. It requires the use of conducting gel, similar to the practice when using other ultrasound technology. Older models require the practitioner to count the FHR, as there is no display unit. When the FHR is read from a display unit during IA, it needs to be clearly stated that this is the case. Listening with a hand‐held Doppler is more comfortable for the woman as she can be in any labouring and birthing positions. The FHR is audible for everyone present during the fetal auscultation. This device is considerably more expensive than other IA tools and requires access to spare parts, as well as batteries or access to reliable electricity for rechargeable batteries (Lewis 2015). With the recent development of a wind‐up fetal Doppler this may reduce costs, as no batteries are needed (PowerFree Education Technology 2014).
Electronic fetal monitoring (EFM) with a cardiotocograph (CTG)
Sometimes the external transducer of an EFM device (most commonly from a CTG) alone is used for IA. However, this is not recommended as such practice results in intermittent CTG not IA (Feinstein 2000; Goodwin 2000). Most CTG machines use autocorrelation where the FHR is frequently averaged and displayed as digital interface and printed on a CTG paper trace. This is not the same as counting the fetal heart for a minute or other recommended periods of time. Therefore, intermittent CTG should not be confused with the counting technique of IA or incorrectly labelled as such (Feinstein 2000; Goodwin 2000).
Why it is important to do this review
Current evidence from a Cochrane review (Alfirevic 2013) on the use of continuous CTG as a form of EFM showed that compared to IA, continuous CTG does not significantly reduce perinatal death but is associated with a reduction in neonatal seizures. There is, however, a significant increase in caesarean sections and assisted vaginal births associated with continuous CTG . There were no differences between the two groups in relation to Apgar scores, neonatal admission or hypoxic ischaemic encephalopathy.
Many clinical practice guidelines recommend continuous CTG for high‐risk women with access to fetal scalp blood sampling (Walsh 2007; Walsh 2008; NICE 2014). This indicates an increased need for the practice of IA of the FHR during labour for pregnant women identified as 'low risk' of intrapartum fetal hypoxia. However, the optimal tool, timing and methods of performing IA is unknown.
Apart from Alfirevic's review (Alfirevic 2013), there are several other Cochrane reviews that relate to assessing fetal well‐being during labour including a review of fetal electrocardiogram (ECG) for fetal monitoring during labour (Neilson 2015), fetal pulse oximetry for fetal assessment in labour (East 2014) and vibroacoustic stimulation for fetal assessment in labour in the presence of a non‐reassuring FHR trace (East 2013). Devane assessed CTG versus IA of fetal heart on admission to the labour ward for assessment of fetal well‐being (Devane 2012) and found a probability that admission CTG increases the caesarean section rate by approximately 20% when monitoring normal labour without detecting important differences in perinatal mortality. However, Devane 2012 also comments that the data lacked power to identify these differences significantly. While some of these reviews compare intermittent with continuous monitoring, none address the issue of optimal tools and timing for IA. With the explosion of digital computer technology referred to often as artificial intelligence, expert systems (ESs) have been promoted to be useful in labour rooms. ESs can assist in complex clinical decision‐making and potentially serve as a mechanism to improve interpretation of FHR tracings. The Lutomski 2015 review concluded that no recommendations for clinical practice can be made for the use of ES for FHR interpretation for intermittent and continuous fetal auscultation as there is currently no strong evidence available and high‐quality trials are needed.
Low‐income countries have limited access to any electronic auscultation tool that can be used for ausculting the FHR continuously for women with high risk in labour or when concerns for the FHR arises. In all maternity settings clinicians want to know the evidence for the use of IA tools and timing for intermittently monitoring the FHR in labour.
Objectives
To evaluate the effectiveness of different tools for intermittent ausculation (IA) of the fetal heart rate (FHR) during labour including frequency and duration of auscultation.
Methods
Criteria for considering studies for this review
Types of studies
All published and unpublished randomised controlled trials (RCTs) or cluster‐RCTs comparing different tools and methods used for intermittent fetal auscultation for fetal and maternal well‐being. Quasi‐RCTs, and cross‐over designs were not eligible for inclusion.
Types of participants
Pregnant women in labour and their babies.
Types of interventions
Different methods of intermittent fetal heart auscultation during labour in which one method was compared to another, or different timings of the same method were compared.
Types of outcome measures
Main outcomes for the baby
Primary outcome
Apgar < seven at five minutes.
Infant mortality or serious morbidity (composite outcome).
Secondary outcomes
Perinatal mortality (stillbirth, and neonatal deaths excluding lethal congenital anomalies).
Meconium liquor.
Fetal bradycardia (as specified by trial authors).
Fetal tachycardia (as specified by trial authors).
Early decelerations (as specified by trial authors).
Decelerations with contractions (as specified by trial authors).
Late decelerations (as specified by trial authors).
Apgar < seven at 10 minutes.
Cord blood acidosis (as specified by the trial authors; but most likely a cord blood pH ≤ 7.10).
Neonatal infections.
Breastfeeding.
Admissions to the neonatal intensive care unit (NICU) or neonatal unit (NNU).
Length of stay in NICU or NNU.
Seizures in the neonatal period, either clinically apparent or detected by electro‐encephalographic (EEG) recordings.
Hypoxic ischaemic encephalopathy.
Cerebal palsy.
Neurodevelopmental disability assessed at six months of age.
Neurodevelopmental disability assessed at 12 months of age.
Main outcomes for the mother
Primary outcomes
Caesarean section for fetal distress and/or fetal acidosis.
Instrumental vaginal birth for fetal distress and/or fetal acidosis.
Secondary outcomes
Death.
Caesarean section.
Instrumental vaginal birth.
Vaginal birth.
Length of labour.
Use of pain relief (pharmacological or non‐pharmacological).
Use of epidural analgesia.
Amniotomy ‐ artificial rupture of membranes.
Duration of ruptured membranes.
Group B streptococcus status of the mother.
Maternal temperature during labour.
Mobility or restriction during labour.
Episiotomy.
Perineal trauma requiring repair.
Maternal acidosis.
Maternal satisfaction with auscultation tool during labour.
Maternal satisfaction with timing intervals of auscultation in labour.
Overall satisfaction with the labour.
Skin‐to‐skin contact.
Postpartum depression.
Search methods for identification of studies
The following methods section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting their Information Specialist (19 September 2016).
The Register is a database containing over 22,000 reports of controlled trials in the field of pregnancy and childbirth. For full search methods used to populate the Pregnancy and Childbirth Group’s Trials Register including the detailed search strategies for CENTRAL, MEDLINE, Embase and CINAHL; the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service, please follow this link to the editorial information about the Cochrane Pregnancy and Childbirth Group in the Cochrane Library and select the ‘Specialized Register ’ section from the options on the left side of the screen.
Briefly, the Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by their Information Specialist and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE (Ovid);
weekly searches of Embase (Ovid);
monthly searches of CINAHL (EBSCO);
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Search results are screened by two people and the full text of all relevant trial reports identified through the searching activities described above is reviewed. Based on the intervention described, each trial report is assigned a number that corresponds to a specific Pregnancy and Childbirth Group review topic (or topics), and is then added to the Register. The Information Specialist searches the Register for each review using this topic number rather than keywords. This results in a more specific search set which has been fully accounted for in the relevant review sections (Included studies; Excluded studies).
Searching other resources
We searched the reference lists of relevant papers. We contacted the author of Mahomed 1994 for additional information about clarifying the timing intervals for the intervention, which we received. We also contacted Mdoe 2015 about randomisation, inclusion criteria, intervention description of 'free play' and inconsistency with published outcome data. Only clarifying information about randomisation and inclusion criteria was received.
We did not apply any language or date restrictions.
Data collection and analysis
The methodology for data collection and analysis is based on the Cochrane Handbook of Systematic Reveiws of Intervention (Higgins 2011).
Selection of studies
Four authors, Ruth Martis (RM), Detty Nurdiati (DN), Ova Emilia (OE) and Julie Brown (JB), independently examined the results of the search to ascertain if the studies met the inclusion criteria. The reason for excluded studies have been set out in the Characteristics of excluded studies table. We did not encounter any disagreements.
Data extraction and management
RM, DN and OE independently extracted data using a purposefully designed data extraction form. We resolved any discrepancies through discussion. Data were entered into Review Manager software (RevMan 2014) and checked for accuracy by RM and DN. JB and RM extracted data independently for the Byaruhanga 2015 and Mdoe 2015 trials which were added after the most recent literature search. No disagreements occurred.
Mahomed 1994, the author of one of the included studies was contacted to clarify discrepancies between the two publications of the trial. The author confirmed that the trial methods were correctly reported in the 1994 publication. In addition, the author also confirmed that the duration and frequency of auscultation was for one minute during and immediately after a contraction in every 10‐minute period.
Clarification was also sought and obtained from Mdoe 2015 on randomisation and on inclusion criteria. Further clarification was requested for the description of the intervention 'free play' and discrepancies in the published outcome data as it did not correspond with the total number of participants identified. The author suggested waiting until the trial results would be published in approximately six months. This meant that the inconsistent numbers from Mdoe 2015 could not contribute outcome data for this review.
Assessment of risk of bias in included studies
Four review authors (RM, DN, OE, JB) independently assessed the risk of bias using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) and contained in RevMan (RevMan 2014). We did not have any disagreement.
(1) Sequence generation (checking for possible selection bias)
We described for each included trial the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included trial the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the method as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for the included studies the method used to blind trial participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding would be unlikely to affect results. We were aware that blinding women and health professionals in which different auscultation tools were used was not possible.
We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
For the included studies we described the methods used for blinding of outcome assessment to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes. We assessed methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations)
We described for the included studies the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported; the numbers included in the analysis at each stage (compared with the total randomised participants); reasons for attrition or exclusion where reported; and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we re‐included missing data in the analyses which we undertook.
We assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
(5) Selective reporting bias
We investigated the possibility of selective reporting bias by identifying all outcomes reported in the methods section of the results publication and cross‐checking to see if these were reported in the results section of the trial publications.
We assessed the risk of bias for selective reporting as:
low risk of bias (where it was clear that all of the trial’s prespecified outcomes and all expected outcomes of interest to the review had been reported);
high risk of bias (where not all the trial’s pre‐specified outcomes had been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest were reported incompletely and so could not be used; trial failed to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for the included studies any important concerns we had about other possible sources of bias. We assessed whether each trial was free of other problems that could put it at risk of bias:
low risk of other bias;
high risk of other bias;
unclear whether there was risk of other bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Handbook (Higgins 2011). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we consider it was likely to impact on the findings. We explored the impact of the level of bias through undertaking sensitivity analyses ‐ see Sensitivity analysis below.
Assessment of the quality of the evidence using the GRADE approach
The quality of the evidence of the included trial was assessed using the GRADE approach as outlined in the GRADE Handbook (chapter 5). We produced 'Summary of findings' tables using the GRADEpro Guideline Development Tool. A summary of the intervention effect and a measure of quality for each of the outcomes was produced using the GRADE approach. GRADEpro uses five criteria (trial limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. We chose seven maternal and seven infant outcomes (seven is the maximum number of outcomes permitted with the GRADEpro software), as listed below. The comparisons were different methods/tools of intermittent fetal heart auscultation during labour in which one method/tool was compared to another, or different timings of the same method were compared.
For the included studies the infant and maternal outcomes assessed for quality using the GRADE approach were Apgar < seven at five minutes, neonatal seizures, perinatal mortality, composite of mortality and serious morbidity, caesarean section for fetal distress and/or fetal acidosis and instrumental vaginal birth for fetal distress and/or fetal acidosis. No other data were available for the other listed outcomes. Table 1; Table 2; Table 3; Table 4; Table 5; Table 6.
Outcomes for the baby
Apgar < seven at five minutes.
Cord blood acidosis.
Neonatal seizures.
Perinatal mortality.
Infant mortality or serious morbidity (composite outcome).
Cerebral palsy.
Neurosensory disability.
Outcomes for the mother
Caesarean section for fetal distress and/or fetal acidosis.
Instrumental vaginal birth for fetal distress and/or fetal acidosis*.
Maternal mortality.
Any pharmacological or non‐pharmacological analgesia use excluding epidural.
Epidural anaesthesia for pain relief excluding for caesarean section.
Mobility or restriction during labour.
Postnatal depression.
(*Reasons for instrumental vaginal births were not reported in the trials contributing data for this outcome; therefore in the 'Summary of findings' tables, we have reported results for all instrumental vaginal births which is one of our secondary outcomes.)
Measures of treatment effect
Dichotomous data
Dichotomous data were presented as a summary risk ratio with 95% confidence intervals.
Continuous data
For continuous data, we used the mean difference with 95% confidence intervals.
Unit of analysis issues
Cluster‐randomised trials
There were no cluster‐randomised trials included for analysis for this review. In future updates of this review, if we include cluster‐randomised trials in the analyses, we will adjust their sample sizes using the methods described in the Handbook using an estimate of the intra‐cluster correlation co‐efficient (ICC) derived from the trial (if possible), or from another source. If ICCs from other sources are used, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster‐randomised trials and individually‐randomised trials, then we plan to synthesise the relevant information. We will consider it reasonable to combine the results from both if there is little clinical and statistical heterogeneity between the trial designs and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely.
Multi‐arm‐randomised trials
For the one multi‐arm trial (Mahomed 1994), we made pair‐wise comparisons to avoid double counting data.
Cross‐over trials
Cross‐over trials were not included for analysis as this would not be an appropriate trial design for this intervention.
Dealing with missing data
We noted the levels of attrition for included studies. We explored, using sensitivity analysis, the impact of including studies with high levels of missing data in the overall assessment of treatment effect.
Intention‐to‐treat analysis (ITT)
For all outcomes, we analysed the data, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes are known to be missing. We analysed data on all participants with available data in the group to which they are allocated, regardless of whether or not they received the allocated intervention. If in the original reports participants were not analysed in the group to which they were randomised, and there was sufficient information in the trial report, we attempted to restore them to the correct group.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the Tau², I² and Chi² statistics. We regarded heterogeneity as substantial where the Tau² was greater than zero and either an I² was greater than 30% or there was a low P value (less than 0.10) in the Chi² test for heterogeneity.
Assessment of reporting biases
This review included three studies. In future updates we will investigate reporting biases (such as publication bias) using funnel plots for outcomes with 10 or more studies in the meta‐analysis and plan to assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
We used a fixed‐effect meta‐analysis for combining data where we considered it was reasonable to assume that the studies were estimating a similar underlying treatment effect such that the trials were examining the same intervention and that the population under investigation and the research methods were similar. We used the Review Manager software (RevMan 2014) to perform these meta‐analyses.
However, where there was clinical or methodological heterogeneity between studies sufficient to suggest that treatment effects might differ between trials, we used random‐effects meta‐analysis representing the results as the average treatment effect with 95% confidence intervals, and the estimates of Tau² and I².
Subgroup analysis and investigation of heterogeneity
We were unable to carry out our prespecified subgroup analyses because of insufficient data. In future updates, if more data become available, we will carry out the following subgroup analysis, using interaction tests as described by Deeks 2001.
Low‐risk versus high‐risk pregnancy.
Gestation at least 37 weeks versus less than 36+6 weeks.
First stage versus second stage of labour.
Singleton pregnancy with cephalic presentation versus singleton pregnancy with breech presentation versus twin pregnancy.
Primiparity versus multiparity
Subgroup analyses will be restricted to this review's primary outcomes.
In future updates, we will assess potential subgroup differences by subgroup interaction tests available within RevMan (RevMan 2014). We will report the results of subgroup analyses quoting the Chi2 statistic and P value, and the interaction test I² value.
Sensitivity analysis
We carried out sensitivity analyses to explore the effect of trial quality for the primary outcomes in the review. Where there was risk of bias associated with a particular aspect of trial quality (e.g. inadequate allocation concealment), this was explored by sensitivity analyses. In future updates, we will carry out sensitivity analysis to investigate the effect of the randomisation unit if we combine data from cluster‐RCTS along with data with individually‐randomised trials.
Results
Description of studies
See: Characteristics of included studies.
Results of the search
See: Figure 1.
1.
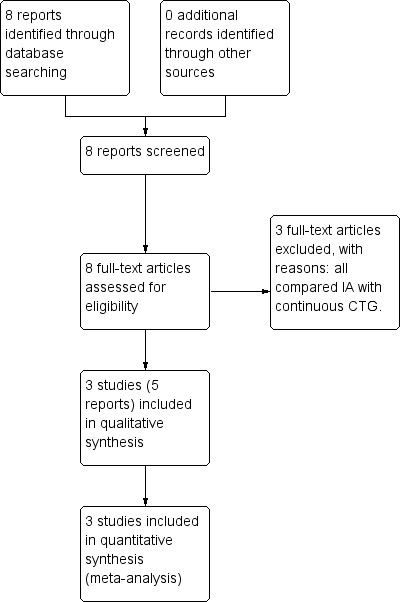
Study flow diagram.
We identified eight reports of six trials from our search (Haverkamp 1979; Wood 1981; MacDonald 1985; Mahomed 1994, Byaruhanga 2015, Mdoe 2015). Some information regarding trial methods, interventions and results were reported differently in Mahomed's two publications. Review author R Martis contacted the author who confirmed that the 1994 publication reported these correctly. Of the seven publications, three studies were eligible for inclusion (Mahomed 1994; Byaruhanga 2015; Mdoe 2015) (see Included studies) and three studies were excluded (Haverkamp 1979; Wood 1981; MacDonald 1985) (see Excluded studies).
Included studies
Three randomised controlled trials involving a total of 6241 women and 6241 babies were included into this review (Mahomed 1994; Byaruhanga 2015; Mdoe 2015). Two trials were included for meta‐analysis (Mahomed 1994; Byaruhanga 2015) with a total of 3242 women and their babies. Mdoe 2015 had numerical inconsistencies in its publication and could not be used for meta‐analysis. Mdoe clarified that results would be published at the end of 2016. It is anticipated the results will be included once they are published in the next update of this review.
Design
Mahomed 1994 is a parallel four‐armed randomised controlled trial. Both Byaruhanga 2015 and Mdoe 2015 studies are parallel randomised controlled trials with two arms each.
Sample size
Of the three included studies, Mdoe 2015 was the largest trial with 2999 women enrolled. Byaruhanga 2015 recruited 1987 women and Mahomed 1994 included 1255 women.
Setting
All three studies were conducted in Africa. Mahomed 1994 was conducted in Zimbabwe (Harare) at a referral maternity hospital. Byaruhanga 2015 was conducted at the Nsambya teaching hospital in Uganda (Kampala) and Mdoe 2015's trial involved two settings in the United Republic of Tanzania i.e. an urban setting at the Muhimbili National hospital and a rural setting at the Haydom Lutheran Hospital. In all settings, fetal blood gas and cord blood sampling or epidural analgesia were not available.
Participants
All studies included women in active labour with a singleton pregnancy, cephalic presentation, ≥ 37 weeks' gestation, with a cervical dilatation ≤ 7 cm and normal fetal heart rate (FHR) at admission (120 to 160 bpm) recorded by the health professional attending the admission. Byaruhanga 2015 and Mdoe 2015 only included women identified with low‐risk factors. Mahomed 1994 included women with obstetric and medial risk factors, but with normal FHR on admission to the hospital. In all trials, women were excluded if they arrived at the trial sites in the second stage of labour. Additionally, Byaruhanga 2015 excluded women with antepartum haemorrhage, intrauterine fetal death and women having an elective caesarean. Mahomed 1994 reported that the only high‐risk women excluded from the trial were those presenting with placental abruption or eclampsia. Mdoe 2015 did not publish any additional exclusion criteria other than excluding women who arrived in second stage.
Mahomed 1994 and Byaruhanga 2015 described exclusion and inclusion criteria for participants and identified the maternal characteristics clearly in their publications. Mdoe 2015's publication did not provide clear descriptions of these. See Characteristics of included studies.
Interventions
We did not anticipate that one trial would include electronic fetal monitoring (EFM) with a cardiotocograph (CTG) as an intermittent auscultation (IA) intervention (Mahomed 1994). After discussion, we decided to include this arm of the intervention as well, as it appears that only the FHR was auscultated with the CTG transducer and used for clinical management decisions.
Mahomed 1994 compared intermittent electronic fetal monitoring using a CTG abdominal transducer by a research midwife or doctor, Doppler ultrasonography by a research midwife, intensive Pinard by a research midwife with routine use of Pinard by the midwife on‐duty. The intervention protocol outlined listening intermittently for one minute (60 seconds) to the FHR during and immediately after a contraction occurring in the last 10 minutes of every half hour for all groups. If the fetal heart pattern was found to be abnormal, this changed to listening for one minute (60 seconds) during and immediately after a contraction occurring in the last 10 minutes of every 20 minutes and a doctor was informed.
Byaruhanga 2015 auscultated the FHR intermittently for one minute (60 seconds) with a hand‐held wind‐up Doppler immediately after a contraction every 30 minutes in the first stage of labour, every 15 minutes in the second stage before pushing and every five minutes in the second stage when pushing.
Mdoe 2015 auscultated intermittently FHR with a hand‐held Doppler in urban and rural settings. Frequency of IA and length of listening of FHR was not described but stated as 'free play'. It is unclear what this means.
Comparisons
In the Mahomed 1994 trial, women in the control group were randomised to receive IA of the FHR with a Pinard as was the routine at the hospital, which expected the FHR to be auscultated for one minute during the last 10 minutes of every half hour during and immediately after a contraction by the midwife on‐duty, who may care for other labouring women at the same time and would endeavour to follow the hospital recommendation for routine auscultation as much as possible. This did mean that some women did not receive one‐to‐one care, as indicated for the intervention groups. It is not described how many labouring women were in this situation.
Byaruhanga 2015 compared intermittent FHR auscultation for one minute (60 seconds) using a Pinard immediately after a contraction every 30 minutes in the first stage of labour, every 15 minutes in the second stage before pushing and every five minutes in the second stage when pushing.
Mdoe 2015 also compared its intervention to intermittent FHR auscultation using a Pinard and Doppler, but did not describe the frequency and length of listening to the FHR.
Outcomes
Mahomed 1994 reported data for immediate maternal, fetal and neonatal outcomes. Our prespecified outcomes included Apgar score < seven at five minutes after the birth. However, in the Mahomed 1994 trial the outcome reported was Apgar < six at five minutes. As only two studies contributed data to the review, we decided that although this was not the same as out pre‐specified cut‐off, we would include it as it provided some indication of the condition of the infants soon after the birth.
Mahomed 1994 reported "stillbirth or neonatal death" as a single outcome but did not clearly report the number of stillbirths and neonatal deaths separately for each arm of the trial.
Byaruhanga 2015 and Mdoe 2015 reported clinical measures focusing primarily on neonatal outcomes and both reported data for caesarean section but did not provide the indications for these. Byaruhanga 2015 reported "intrapartum stillbirth" and "neonatal death prior to discharge" and we combined these outcomes for our perinatal mortality outcome.
Mdoe 2015 listed a further three neonatal outcomes (admission to neonatal unit (NNU), stillbirth and neonatal deaths) in the publication, but did not provide outcome data.
All included studies documented the FHR as a single rate.
None of the included studies reported long‐term outcomes for the mother and the baby.
It was not possible from the available outcome data to separate IA for first and second stage of labour or to perform subgroup analyses according to risk status or parity.
Excluded studies
Three studies comparing IA with continuous electronic FHR monitoring were excluded, as CTG monitoring was not one of the included comparisons for this review (Haverkamp 1979; Wood 1981; MacDonald 1985). See the Characteristics of excluded studies table.
Risk of bias in included studies
See 'Risk of bias' table for the included studies in Characteristics of included studies, Figure 2 and Figure 3.
2.
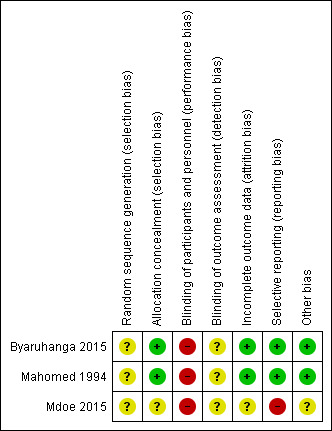
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included trial.
3.
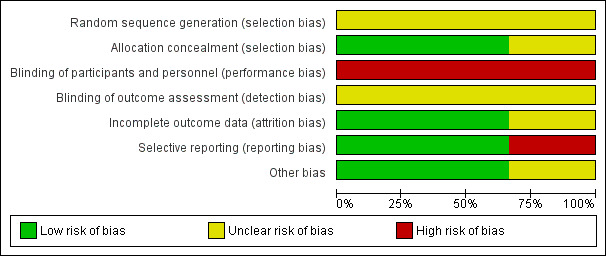
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Random sequence generation ‐ none of the included studies stated how their randomisation was generated. Therefore, all studies identified as having an unclear risk of bias for sequence generation.
Allocation concealment ‐ Mahomed 1994 and Byaruhanga 2015 both reported using sequentially numbered, sealed, opaque envelopes, the risk of bias for allocation concealment was judged to be low. Further information on methods of allocation used in the Mdoe 2015 trial was provided by trial authors through email correspondence as it was not clear from the published abstract. Authors stated that the trial was "one to one open label" and was judged to be of unclear risk of bias for allocation concealment.
Blinding
Performance bias ‐ Mahomed 1994 and Byaruhanga 2015 did not report on blinding of participants and clinicians. Mdoe 2015 identified through email correspondence that open‐label randomisation was used in their trial i.e. participants and clinicians knew which group women had been randomised to. It is highly unlikely that blinding of the intervention for clinicians and participants was achieved in the Mahomed 1994 and Byaruhanga 2015 trials as the intervention tool used is impossible to conceal. Performance bias was therefore judged to be of high risk for all three studies.
Detection bias ‐ Mahomed 1994, Byaruhanga 2015 and Mdoe 2015 did not report on blinding of outcome assessors. Therefore detection bias was judged to be unclear. Additionally, blinding may also be important for the assessment of the outcomes. Although some outcomes were objectively measured and unlikely to be affected by knowledge of group assignment, some outcomes were judged to have some degree of subjectivity and may possibly be at high risk of detection bias. These would include Apgar scores, early and late FHR decelerations and neonatal infections.
Incomplete outcome data
Mahomed 1994's trial reported there were no losses to follow‐up and no drop out. Eighteen of the women allocated to the EFM (CTG) group did not receive the intervention, as birth was either too rapid or was prevented by technical problems with the machine. For a further 24 women in the EFM (CTG) group, traces (the printed record produced by the CTG) were of poor quality, usually because of frequent loss of contact, machine failure, or poor marking on the paper. However, outcomes for all women were measured as planned, and included in the statistical analysis on an intention‐to‐treat basis. We judged this trial to be at a low risk for attrition bias. In the Byaruhanga 2015 trial, eight women in the Pinard group were excluded from the analysis (one woman was lost to follow‐up, one woman birthed before the partogram was started, two women had undiagnosed breech births and four women had undiagnosed multiple births), and equally data from eight women for the Doppler group were not analysed (three women birthed before their partograms were started, three women had undiagnosed breech births and two women had undiagnosed multiple births). Both studies Mahomed 1994and Byaruhanga 2015 were judged to be of low risk for attrition. Mdoe 2015 did not report how incomplete outcome data were addressed but the numerical data published are inconsistent and pending clarification on publication of main trial results. Attrition bias for this trial was judged to be at unclear risk of attrition bias.
Selective reporting
All intended outcomes appear to have been reported for Mahomed 1994 and Byaruhanga 2015. Both studies Mahomed 1994 and Byaruhanga 2015 were judged to be of low risk for selective reporting. In the Mdoe 2015 trial the pre‐specified outcomes (admission to neonatal unit, five‐minute Apgar score less than seven, death and fresh stillbirth) did not match the reported outcomes except for Apgar score less than seven. We judged this trial of high risk for reporting bias. No trial protocols of any trials were available.
Other potential sources of bias
No other potential sources of bias were identified for Mahomed 1994 and Byaruhanga 2015. Mdoe 2015 was assessed as 'unclear risk' of other bias ‐ this trial report was only an abstract, and it remains unclear if the groups were balanced at baseline.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6
We included three studies (Mahomed 1994;Byaruhanga 2015; Mdoe 2015) with usable data available from two studies (Mahomed 1994;Byaruhanga 2015) involving 3242 women and 3242 babies.
Mdoe 2015's trial had numerical inconsistencies in its publication and clarification is pending. Therefore it could not be used for analysis.
1.0 Intermittent electronic fetal monitoring (EFM) cardiotocograph (CTG) versus routine Pinard
We did not anticipate that one trial would include EFM with a CTG as an IA intervention (Mahomed 1994). After discussion, we decided to include this arm of the intervention as well, as it appears that only the FHR was auscultated with the CTG transducer and used for clinical management decisions.
One trial compared intermittent EFM (CTG) with routine Pinard (Mahomed 1994).Table 2
Neonatal primary outcome
1.1 There was no clear difference in Apgar scores < seven at five minutes after birth (reported as six at five minutes after birth in the Mahomed 1994 trial) between the intermittent EFM (CTG) and the routine Pinard groups (risk ratio (RR) 0.66, 95% CI 0.24 to 1.83, one trial, 633 babies, very low‐quality evidence,Analysis 1.1). The evidence was downgraded due to lack of blinding and imprecision (evidence being based on a single trial with low event rates). (Table 1). Infant mortality or serious morbidity (composite outcome) was not reported).
1.1. Analysis.
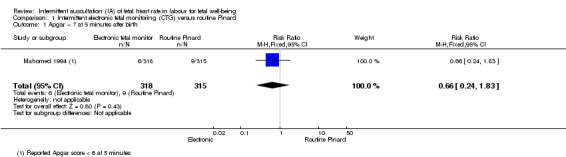
Comparison 1 Intermittent electronic fetal monitoring (CTG) versus routine Pinard, Outcome 1 Apgar < 7 at 5 minutes after birth.
Maternal primary outcomes
1.2 Women allocated to intermittent EFM (CTG) had significantly higher rates of caesarean section for fetal distress (RR 2.92, 95% CI 1.78 to 4.80, one trial, 633 women, heterogeneity not applicable, moderate‐quality evidence,Analysis 1.2) compared to women allocated to routine Pinard. Evidence was downgraded due to study design limitations (Table 2).
1.2. Analysis.
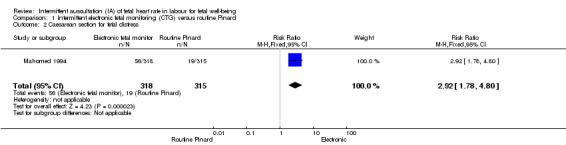
Comparison 1 Intermittent electronic fetal monitoring (CTG) versus routine Pinard, Outcome 2 Caesarean section for fetal distress.
Instrumental vaginal birth for fetal distress and/or acidosis was not reported.
Neonatal secondary outcomes
1.3 The results for perinatal mortality (stillbirth and neonatal death) showed no clear difference between the intermittent EFM (CTG) group compared to the routine Pinard group (RR 0.88, 95% CI 0.34 to 2.25, one trial, 633 babies, very low‐quality evidence, Analysis 1.3). Evidence was downgraded due to imprecision (wide 95% CIs and low event rates), and study design limitations (Table 1).
1.3. Analysis.
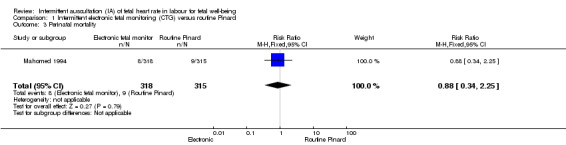
Comparison 1 Intermittent electronic fetal monitoring (CTG) versus routine Pinard, Outcome 3 Perinatal mortality.
1.4 Intermitent EFM (CTG) was associated with increased likelihood of detecting abnormal FHR patterns (bradycardia and tachycardia) compared with the routine Pinard (RR 6.08, 95% CI 4.21 to 8.79, one trial, 633 babies, Analysis 1.4). Evidence should be interpreted with caution due to wide 95% CIs.
1.4. Analysis.
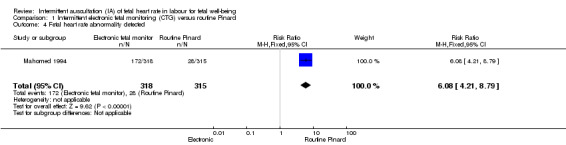
Comparison 1 Intermittent electronic fetal monitoring (CTG) versus routine Pinard, Outcome 4 Fetal heart rate abnormality detected.
1.5 Intermitent EFM (CTG) was associated with increased detection of early and late decelerations compared to routine Pinard (RR 2.84, 95% CI 1.82 to 4.45, one trial, 633 babies, Analysis 1.5). Evidence should be interpreted with caution due to wide 95% CIs.
1.5. Analysis.
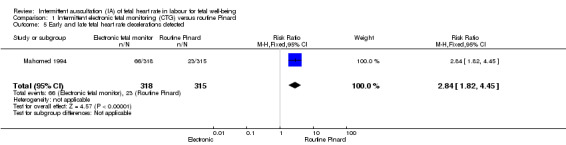
Comparison 1 Intermittent electronic fetal monitoring (CTG) versus routine Pinard, Outcome 5 Early and late fetal heart rate decelerations detected.
1.6 There was no difference in neonatal intensive care unit/neonatal unit (NICU/NNU) admission rates between routine Pinard (control) and EFM (CTG) (RR 0.89, 95% CI 0.63 to 1.25, one trial, 633 babies, Analysis 1.6).
1.6. Analysis.
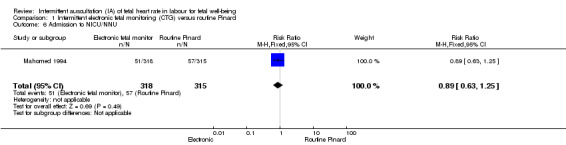
Comparison 1 Intermittent electronic fetal monitoring (CTG) versus routine Pinard, Outcome 6 Admission to NICU/NNU.
1.7 There were no seizures in the neonatal period in the intermittent EFM (CTG) group (0/318) compared with nine in the routine Pinard group (9/315) (RR 0.05, 95% CI 0.00 to 0.89, one trial, 633 babies, very low‐quality evidence, Analysis 1.7). Evidence was downgraded due to imprecision (wide CIs and low event rates), and bias associated with study design.
1.7. Analysis.
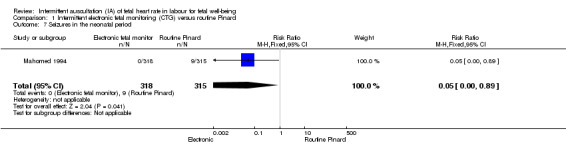
Comparison 1 Intermittent electronic fetal monitoring (CTG) versus routine Pinard, Outcome 7 Seizures in the neonatal period.
1.8 The incidence of hypoxic ischaemic encephalopathy was lower in the intermittent EFM (CTG) group (2/318) compared to the routine Pinard group (10/315) (RR 0.20, 95% CI 0.04 to 0.90, one trial, 633 babies, Analysis 1.8). Evidence should be interpreted with caution due to wide 95% CIs and low event rates.
1.8. Analysis.
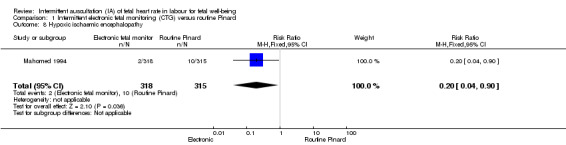
Comparison 1 Intermittent electronic fetal monitoring (CTG) versus routine Pinard, Outcome 8 Hypoxic ischaemic encephalopathy.
The included trial did not report on other listed neonatal secondary review outcomes (meconium liquor, declarations with contractions, Apgar < seven at 10 minutes, cord blood acidosis, neonatal infections, breastfeeding, cerebral palsy, or neurodevelopmental disability at six months or one year).
Maternal secondary outcomes
1.9 Birth by caesarean section (without indications identified) was more likely in the intermittent EFM (CTG) than in the routine Pinard monitoring group (RR 1.92, 95% CI 1.39 to 2.64, one trial, 633 women, Analysis 1.9).
1.9. Analysis.
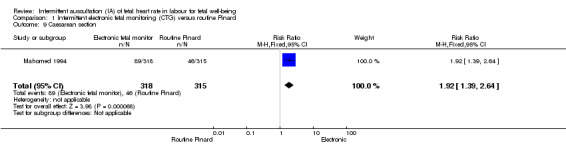
Comparison 1 Intermittent electronic fetal monitoring (CTG) versus routine Pinard, Outcome 9 Caesarean section.
1.10 There was no significant difference in instrumental vaginal births between the intermittent EFM (CTG) group and the routine Pinard group (RR 1.46, 95% CI 0.86 to 2.49, one trial, 633 women, low‐quality evidence, Analysis 1.10). The reasons for instrumental births (e.g. fetal distress) were not reported in the trial contributing data. Evidence was downgraded due to imprecision, and study design limitations (Table 2).
1.10. Analysis.
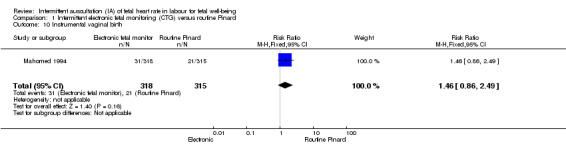
Comparison 1 Intermittent electronic fetal monitoring (CTG) versus routine Pinard, Outcome 10 Instrumental vaginal birth.
1.11 There was no clear difference in the length of labour (in hours) between the intermittent EFM (CTG) and routine Pinard groups (mean difference (MD) 0.90 hours, 95% CI ‐0.05 to 1.85, one trial, 633 women, Analysis 1.11).
1.11. Analysis.
Comparison 1 Intermittent electronic fetal monitoring (CTG) versus routine Pinard, Outcome 11 Length of labour (hours).
The trial did not report on other listed maternal secondary review outcomes (death, vaginal birth, use of pain relief (pharmacological or non‐pharmacological), use of epidural analgesia, amniotomy, duration of ruptured membranes, Group B streptococcus status of the mother, maternal temperature during labour, mobility or restriction during labour, episiotomy, perineal trauma requiring repair, maternal acidosis, maternal satisfaction with auscultation tool during labour, maternal satisfaction with timing intervals of auscultation in labour, overall satisfaction with the labour, skin‐to‐skin contact, postpartum depression).
2.0 Doppler ultrasonography versus routine Pinard
Two studies Mahomed 1994 and Byaruhanga 2015 published usable data for analysis comparing Doppler ultrasonography with routine Pinard use.
Neonatal primary outcomes
2.1 Both Mahomed 1994 and Byaruhanga 2015 identified there was no clear evidence of a difference in Apgar scores < 7 at five minutes after birth (reported as < six in Mahomed 1994's trial) among infants of women in the Doppler ultrasonography group compared to the routine Pinard group (average RR 0.76, 95% CI 0.20 to 2.87; two trials, 2598 babies,Tau2 = 0.69, I2 = 72%, very low‐quality evidenceAnalysis 2.1). The evidence was downgraded for performance bias and imprecision, and I2 results indicate substantial heterogeneity (Table 3). Infant mortality or serious morbidity (composite outcome) was not reported).
2.1. Analysis.
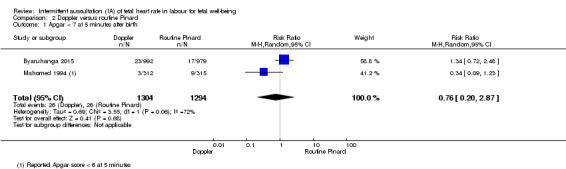
Comparison 2 Doppler versus routine Pinard, Outcome 1 Apgar < 7 at 5 minutes after birth.
Maternal primary outcomes
Only Mahomed 1994 reported on the maternal primary outcomes of this review.
2.2 Women allocated to the Doppler ultrasonography had higher rates of caesarean section for fetal distress than women allocated to routine Pinard monitoring group (RR 2.71, 95% CI 1.64 to 4.48, one trial, 627 women, heterogeneity not applicable, moderate‐quality evidenceAnalysis 2.2). Evidence was downgraded due to performance bias in the trials contributing data (Table 4).
2.2. Analysis.
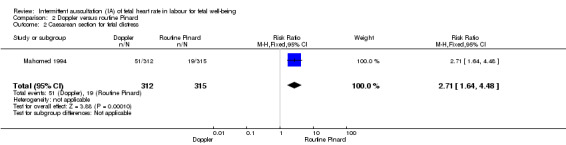
Comparison 2 Doppler versus routine Pinard, Outcome 2 Caesarean section for fetal distress.
Instrumental vaginal birth for fetal distress and/or acidosis was not reported.
Neonatal secondary outcomes
2.3 There was no clear evidence of a difference in perinatal mortality (stillbirth or neonatal death) between Doppler ultrasonography and routine Pinard (average RR 0.69, 95% CI 0.09 to 5.40, two trials, 2597 babies,Tau2 = 1.78; I2 = 81%, very low‐quality evidenceAnalysis 2.3). Evidence was downgraded due to imprecision with wide 95% CI, and study design limitations caused by inability to blind participants and health professionals (Table 3) and I2 indicates substantial heterogeneity.
2.3. Analysis.
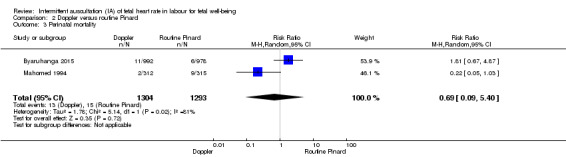
Comparison 2 Doppler versus routine Pinard, Outcome 3 Perinatal mortality.
2.4 Doppler ultrasonography was also associated with being more likely to detect abnormal FHR patterns (bradycardia and tachycardia) compared to routine Pinard (average RR 2.40, 95% CI 1.09 to 5.29; two trials, 2598 babies,Tau2 = 0.29, I2 = 89%, Analysis 2.4). Evidence should be interpreted with caution due to wide 95% CI suggesting imprecision and Í2 indicates substantial heterogeneity.
2.4. Analysis.
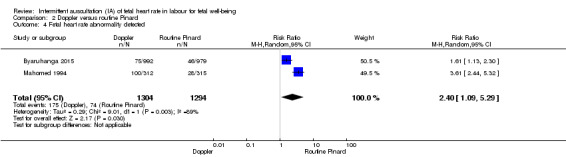
Comparison 2 Doppler versus routine Pinard, Outcome 4 Fetal heart rate abnormality detected.
2.5 Doppler ultrasonography was associated with increased detection of early and late fetal heart rate decelerations compared to the routine Pinard (RR 2.72, 95% CI 1.73 to 4.28, one trial, 627 women, Analysis 2.5). Evidence should be interpreted with caution due to wide 95% CI suggesting imprecision.
2.5. Analysis.
Comparison 2 Doppler versus routine Pinard, Outcome 5 Early and late fetal heart rate decelerations detected.
2.6 There were no clear evidence of a difference with NICU/NNU admissions between the Doppler ultrasonography group and the routine Pinard group (average RR 0.89, 95% CI 0.41 to 1.91; two trials, 2598 babies,Tau2 = 0.26, I2 = 86%, Analysis 2.6).
2.6. Analysis.
Comparison 2 Doppler versus routine Pinard, Outcome 6 Admission to NICU/NNU.
2.7 There was an increased risk of neonatal seizures in the routine Pinard group (9/315) compared to the Doppler ultrasonography group (0/312) (one trial, 627 babies, RR 0.05, 95% CI 0.00 to 0.91, very low‐quality evidence, Analysis 2.7). Evidence from this single trial should be interpreted with caution due to low event rates (Table 3).
2.7. Analysis.
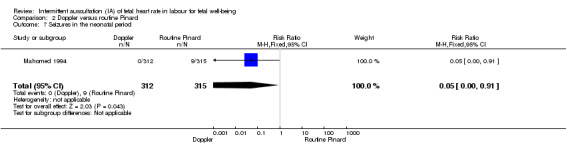
Comparison 2 Doppler versus routine Pinard, Outcome 7 Seizures in the neonatal period.
2.8 The incidence of hypoxic ischaemic encephalopathy was less in the Doppler sonography (1/312) group compared to the routine Pinard group (10/315) (RR 0.10, 95% CI 0.01 to 0.78, one trial, 627 babies, heterogeneity not applicable, Analysis 2.8). Evidence from this single trial should be interpreted with caution due to low event rates. and one trial.
2.8. Analysis.
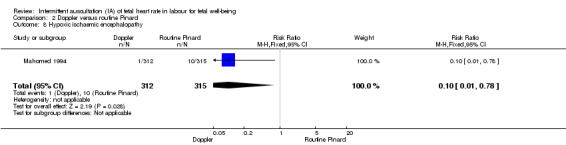
Comparison 2 Doppler versus routine Pinard, Outcome 8 Hypoxic ischaemic encephalopathy.
The studies did not report on other listed neonatal secondary review outcomes (meconium liquor, declarations with contractions, Apgar < seven at 10 minutes, cord blood acidosis, neonatal infections, breastfeeding, cerebral palsy, or neurodevelopmental disability at six months or one year).
Maternal secondary outcomes
2.9 Birth by caesarean section (without indications identified) did not clearly differ between groups (average RR 1.29, 95% CI 0.81 to 2.05; two studies, 2598 women,Tau2 = 0.09, I2 = 83%, Analysis 2.9).
2.9. Analysis.
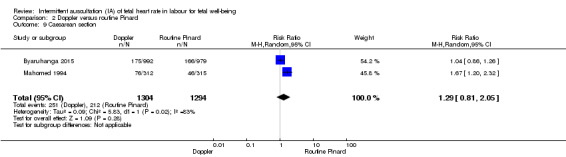
Comparison 2 Doppler versus routine Pinard, Outcome 9 Caesarean section.
2.10 There was no clear evidence of a difference in instrumental vaginal births between women in the Doppler ultrasonography group compared to the routine Pinard auscultation group (RR 1.35, 95% CI 0.78 to 2.32, one trial, 627 women, heterogeneity not applicable, low‐quality evidenceAnalysis 2.10). Reasons for instrumental delivery were not reported. Evidence was downgraded due to imprecision and study design limitations (Table 4).
2.10. Analysis.
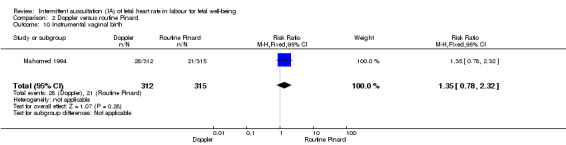
Comparison 2 Doppler versus routine Pinard, Outcome 10 Instrumental vaginal birth.
2.11 There was no evidence of a difference in the length of labour > 18 (in hours) between women in the Doppler ultrasonography and routine Pinard groups (MD 0.00 hours, 95% CI ‐1.07 to 1.07, one trial, 627 women, heterogeneity not applicable, Analysis 2.11).
2.11. Analysis.
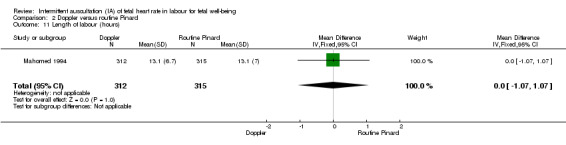
Comparison 2 Doppler versus routine Pinard, Outcome 11 Length of labour (hours).
The studies did not report on other maternal secondary review outcomes as listed in this review (death, vaginal birth, use of pain relief (pharmacological or non‐pharmacological), use of epidural analgesia, amniotomy, duration of ruptured membranes, Group B streptococcus status of the mother, maternal temperature during labour, mobility or restriction during labour, episiotomy, perineal trauma requiring repair, maternal acidosis, maternal satisfaction with auscultation tool during labour, maternal satisfaction with timing intervals of auscultation in labour, overall satisfaction with the labour, skin‐to‐skin contact, postpartum depression).
3.0 Intensive Pinard versus routine Pinard
Only one trial (Mahomed 1994) compared intensive Pinard (a research midwife following the protocol in a one‐to‐one care situation) with routine Pinard (as per protocol but midwife may be caring for more than one women in labour).
Neonatal primary outcomes
3.1 There was no clear evidence of a difference in low Apgar score at five minutes after birth (reported as six in Mahomed 1994's trial) between the intensive Pinard and routine Pinard groups (RR 0.90, 95% CI 0.35 to 2.31, one trial, 625 babies, very low‐quality evidenceAnalysis 3.1). Evidence was downgraded for performance bias and imprecision. Infant mortality or serious morbidity (composite outcome) was not reported).
3.1. Analysis.
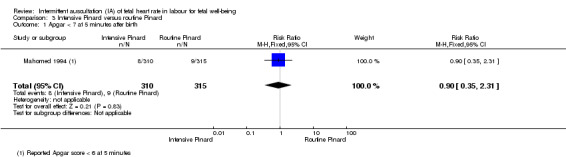
Comparison 3 Intensive Pinard versus routine Pinard, Outcome 1 Apgar < 7 at 5 minutes after birth.
Maternal primary outcomes
3.2 There was no clear evidence of a difference in the incidence of caesarean section for fetal distress between women allocated to the intensive Pinard group and women allocated to the routine Pinard group (RR 0.70, 95% CI 0.35 to 1.38, one trial, 625 women, heterogeneity not applicable, low‐quality evidence, Analysis 3.2). Evidence was downgraded for performance bias and imprecision.
3.2. Analysis.
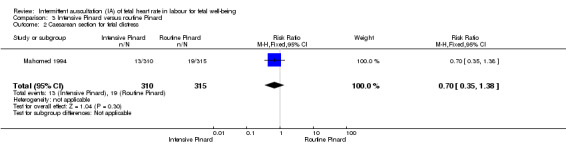
Comparison 3 Intensive Pinard versus routine Pinard, Outcome 2 Caesarean section for fetal distress.
Instrumental vaginal birth for fetal distress and/or acidosis was not reported.
Neonatal secondary outcomes
3.3 There was no clear evidence of a difference in perinatal morality between the intensive Pinard and routine Pinard groups (RR 0.56, 95% CI 0.19 to 1.67, one trial, 625 babies, very low‐quality evidence, Analysis 3.3). Evidence was downgraded for performance bias and imprecision.
3.3. Analysis.
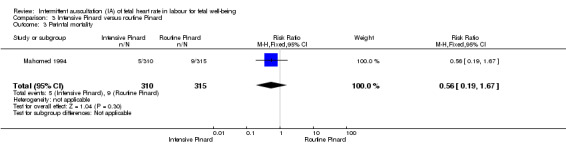
Comparison 3 Intensive Pinard versus routine Pinard, Outcome 3 Perintal mortality.
3.4 The intensive Pinard method was more likely to detect abnormal FHR patterns (bradycardia and tachycardia) than routine Pinard (RR 1.71, 95% CI 1.10 to 2.65, one trial, 625 babies, heterogeneity not applicable, Analysis 3.4).
3.4. Analysis.
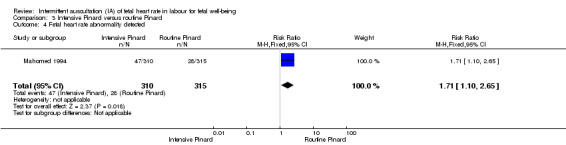
Comparison 3 Intensive Pinard versus routine Pinard, Outcome 4 Fetal heart rate abnormality detected.
3.5 There was no clear evidence of a difference between the intensive Pinard and routine Pinard groups in detection of early and late decelerations (RR 1.33, 95% CI 0.79 to 2.23, one trial, 625 babies, heterogeneity not applicable Analysis 3.5).
3.5. Analysis.
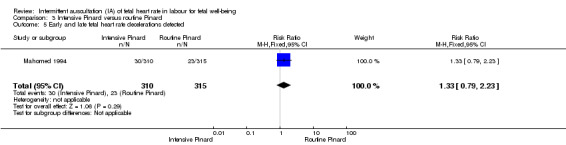
Comparison 3 Intensive Pinard versus routine Pinard, Outcome 5 Early and late fetal heart rate decelerations detected.
3.6 There was no clear evidence of a difference in NICU/NNU admission rates between the intensive Pinard and routine Pinard groups (RR 0.84, 95% CI 0.59 to 1.19, one trial, 625 babies, Analysis 3.6).
3.6. Analysis.
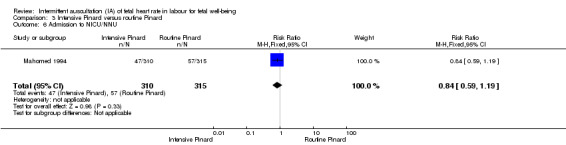
Comparison 3 Intensive Pinard versus routine Pinard, Outcome 6 Admission to NICU/NNU.
3.7 There was no clear evidence of a difference in the incidence of neonatal seizures between the intensive Pinard group the routine Pinard group (RR 0.68, 95% CI 0.24 to 1.88, one trial, 625 babies, very low‐quality evidence, Analysis 3.7). Evidence was downgraded for performance bias and imprecision.
3.7. Analysis.
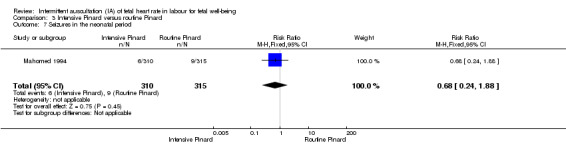
Comparison 3 Intensive Pinard versus routine Pinard, Outcome 7 Seizures in the neonatal period.
3.8 There was no clear difference in the incidence of hypoxic ischaemic encephalopathy between the intensive Pinard and routine Pinard groups (RR 0.71, 95% CI 0.27 to 1.84, one trial, 625 babies, Analysis 3.8).
3.8. Analysis.
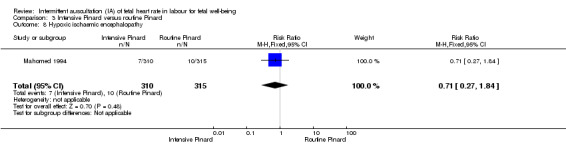
Comparison 3 Intensive Pinard versus routine Pinard, Outcome 8 Hypoxic ischaemic encephalopathy.
The studies did not report on other listed neonatal secondary review outcomes (meconium liquor, declarations with contractions, Apgar ≤ seven at 10 minutes, cord blood acidosis, neonatal infections, breastfeeding, cerebral palsy, or neurodevelopmental disability at six months or one year).
Maternal secondary outcomes
3.9 There was no clear evidence of a difference between the intensive Pinard and routine Pinard groups in the incidence of birth by caesarean section (without indications identified) (RR 0.71, 95% CI 0.46 to 1.08, one trial, 625 women, Analysis 3.9).
3.9. Analysis.
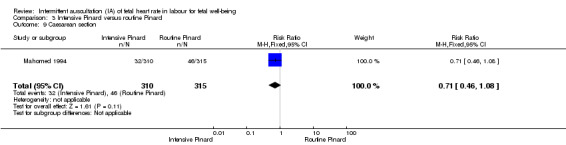
Comparison 3 Intensive Pinard versus routine Pinard, Outcome 9 Caesarean section.
3.10 There was no clear evidence of a difference in instrumental vaginal births between women allocated to the intensive Pinard group and women allocated to the routine Pinard group (RR 1.21, 95% CI 0.69 to 2.11, one trial, 625 women, low‐quality evidence, Analysis 3.10). Reasons for instrumental birth were not reported. Evidence was downgraded for performance and bias and imprecision.
3.10. Analysis.
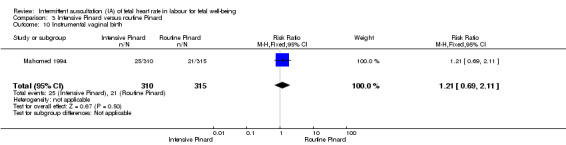
Comparison 3 Intensive Pinard versus routine Pinard, Outcome 10 Instrumental vaginal birth.
3.11 There was no clear difference between the intensive Pinard and routine Pinard groups in the length of labour (in hours) (MD 0.50 hours, 95% CI ‐0.52 to 1.52, heterogeneity not applicable, one trial, 625 women, Analysis 3.11).
3.11. Analysis.
Comparison 3 Intensive Pinard versus routine Pinard, Outcome 11 Length of labour (hours).
The studies did not report on other maternal secondary review outcomes as listed in this review (death, vaginal birth, use of pain relief (pharmacological or non‐pharmacological), use of epidural analgesia, amniotomy, duration of ruptured membranes, Group B streptococcus status of the mother, maternal temperature during labour, mobility or restriction during labour, episiotomy, perineal trauma requiring repair, maternal acidosis, maternal satisfaction with auscultation tool during labour, maternal satisfaction with timing intervals of auscultation in labour, overall satisfaction with the labour, skin‐to‐skin contact, postpartum depression).
Discussion
Summary of main results
The review assessed intermittent auscultation (IA) of the fetal heart rate FHR) in labour for fetal well‐being. We identified three studies (involving 6241 women) that met the inclusion criteria for this systematic review (Mahomed 1994; Byaruhanga 2015; Mdoe 2015). All women were ≥ 37 weeks' gestation, in established labour with ≤ 7 cm dilatation, had a singleton pregnancy and had normal FHR (120 to 160 bpm) on admission to the hospital. All three studies were conducted in Africa and at the participating hospitals fetal scalp blood sampling or cord blood assessment was not available. Byaruhanga 2015 and Mdoe 2015 reported that only women identified with low‐risk factors were included into their studies. Byaruhanga 2015 reported exclusion criteria for participants as women contraindicated for labouring when admitted (e.g. antepartum haemorrhage, intrauterine fetal death on admission, or were having an elective caesarean section) and Mdoe 2015 did not publish any additional exclusion criteria other than excluding women who arrived in second stage. Mahomed 1994 included women with obstetric and medial risk factors and only excluded women with high risks from the trial when they presented with placental abruption or eclampsia. It was not possible from the available data to separate IA for subgroup analysis according to risk status, parity or if women were in first or second stage of labour. Therefore, it is not clear how or if this may have impacted intervention effects.
Intermittent auscultation for FHR was identified as listening every 30 minutes immediately after a contraction for one minute (60 seconds) in first stage of labour in Mahomed 1994 and Byaruhanga 2015 studies, whereas Mdoe 2015 identified intermittent listening as 'free play' in the trial with no further clarification. Byaruhanga 2015 identified that the fetal heart was auscultated every five minutes immediately after a contraction in the second stage of labour with pushing. None of the included studies reported if the FHR was auscultated over the anterior shoulder, the optimum place to hear the FHR.
We examined three comparisons: intermittent EFM (CTG) versus routine Pinard, Doppler ultrasonography versus routine Pinard, and Intensive Pinard versus routine Pinard.
One trial compared intermittent EFM (CTG) with routine Pinard. There was no clear difference between groups in low Apgar scores at five minutes (reported as < six at five minutes after birth) (very low‐quality evidence) or in perinatal mortality (low‐quality evidence). Neonatal seizures were reduced in the EFM group (low‐quality evidence). Other important infant outcomes were not reported: mortality or serious morbidity (composite outcome), cerebral palsy or neurosensory disability. For maternal outcomes, women allocated to intermittent EFM (CTG) had higher rates of caesarean section for fetal distress (moderate‐quality evidence) compared with women allocated to routine Pinard. There was no clear difference between groups in instrumental vaginal births (low‐quality evidence). Other outcomes were not reported (maternal mortality, analgesia in labour, mobility or restriction and postnatal depression).
Two studies compared Doppler ultrasonography with routine Pinard. There was no clear evidence of a difference between groups in Apgar scores < 7 at five minutes after birth (reported as < six in one of the trials) (very low‐quality evidence), in perinatal mortality (very low‐quality evidence), or neonatal seizures (very low‐quality evidence). Other important infant outcomes were not reported: mortality or serious morbidity (composite outcome), cerebral palsy or neurosensory disability. Only one study reported maternal outcomes. Women allocated to the Doppler ultrasonography had higher rates of caesarean section for fetal distress compared with those allocated to routine Pinard (moderate‐quality evidence). There was no clear evidence of a difference in instrumental vaginal births between groups (low‐quality evidence). Other maternal outcomes were not reported (mortality, analgesia in labour, mobility or restriction and postnatal depression).
One trial compared intensive Pinard (a research midwife in a one‐to‐one care situation) with routine Pinard (midwife may be caring for more than one women in labour). There was no evidence of a difference between groups in low Apgar score (reported as < six this trial) (very low‐quality evidence), perinatal mortality (very low‐quality evidence), or neonatal seizures (very low‐quality evidence). Other infant outcomes were not reported. For maternal outcomes, there were no clear differences between groups for caesarean section or instrumental delivery (both low‐quality evidence)) Other outcomes were not reported.
Any ausculation of the FHR during labour attempts to identify those babies at risk of intrapartum hypoxia (Arulkumaran 2000). Apgar scores (< seven) have been used as a surrogate measure for birth asphyxia and possible long‐term adverse neurodevelopmental outcomes. Lie 2010 has published results of a large population‐based cohort trial confirming a strong association between low Apgar score and cerebral palsy in both low and normal birthweight infants. This review found no significant difference in low Apgar scores with different interventions for monitoring FHR. None of the included studies provided data for long‐term follow‐up assessing for neurodevelopmental disability or cerebral palsy.
There were more caesarean sections performed for fetal distress and caesarean sections without indications in the intermittent CTG and Doppler groups compared to the intensive Pinard and routine Pinard groups. This is understandable as the intermittent CTG and Doppler groups also detected significantly higher fetal distress interpreted as early and late fetal heart rate decelerations and brady‐ or tachycardia. Fetal hypoxia/acidosis was not confirmed with fetal scalp blood or umbilical cord blood sampling. It is difficult to interpret these findings in the absence of significant differences for maternal and fetal well‐being.
Furthermore, a Pinard requires the practitioner to have sound hearing ability (no full or partial deafness). It requires the listener to put an ear to one end of the tool and press it into the woman's abdomen. This requires close proximity, as most Pinards are around 15 cm long. Detecting the baby's anterior shoulder for optimal fetal heart sound is often only possible if the woman is reclined or semi‐reclined. This may result in missing significant abnormal fetal heart rate patterns or potentially induce maternal supine hypotension which may cause abnormal fetal heart rate patterns (Thurlow 2002), particularly when the trial protocols required the ausculation to last one minute immediately after a contraction. This would also be difficult to maintain with frequent contractions.
There was no clear evidence of a difference between the groups for NICU/NNU admissions and perinatal death although the caesarean section rate was higher for the CTG (one trial) and Doppler groups (two studies) compared to routine Pinard and the incidence of hypoxic ischaemic encephalopathy significantly higher in the routine Pinard group (Mahomed 1994). It is unclear why the NICU/NNU admission rate does not reflect this. Although the underlying causes for hypoxic ischaemic encephalopathy are not well understood, the condition has often long‐term consequences either physically or neuro‐psychologically and comprehensive long‐term follow‐up of these babies is needed to clarify the association. As noted before, no long‐term follow‐up data are available to assess if these findings are clinically significant and therefore caution needs to be applied when interpreting the findings.
The results of this review demonstrates that IA for FHR in labour with a hand‐held battery or wind‐up Doppler and intermittent CTG transducer (without paper tracing) identifies more FHR abnormalities (tachycardia, bradycardia, early and late decelerations), but leads to an increase in caesarean sections compared to using a Pinard as a listening device without showing significance differences in low Apgar scores at five minutes after the birth, perinatal death and admission to NICU/NNU. There are well‐known risks for the mother having a caesarean section particularly in Africa, the setting of all included studies, (Souza 2010) and these must also be considered when making decisions about methods of monitoring the fetal heart during labour. The findings suggest that intermittent FHR auscultation with a Pinard is comparable to a hand‐held Doppler as an effective listening tool, but this needs to be interpreted with caution as the confidence intervals are wide for FHR abnormality detection and no long‐term follow‐up data for the babies involved are available.
Only one trial Mahomed 1994, reported on neonatal seizures, identified as occurring in the neonatal unit. There were six seizures in the intensive Pinard group, nine in the routine Pinard group and no seizures in the Doppler and intermittent CTG group. These differences did not reach statistical significance and as no long‐term follow‐up outcome data were available it is unclear what these seizures mean in terms of infant well‐being. Mahomed 1994 on page 499, reported "that there were five neonatal deaths from hypoxia caused by delays in the delivery, either because of a delayed decision to operate or because the operating theatre was not available". It is questioned whether this situation could have also contributed to live babies who experienced neonatal seizures in NICU born by delayed emergency caesarean section and as a result of the delay experiencing prolonged fetal hypoxia. However, with the limited data available this could not be confirmed.
While results for perinatal death did not reach statistical significance, most deaths in the Mahomed 1994 trial resulted from a delay in birthing the babies due to either a delay in the decision to operate or the unavailability of the operating theatre, as reported above. This highlights the importance of understanding a trial's context when interpreting the findings and considering applicability across different practice settings.
Overall completeness and applicability of evidence
The inadequate and inconsistent reporting of the data from the Mdoe 2015 trial meant that it could not be included for assessment into this review. In all three included studies, the lack of reporting of a number of secondary outcomes for the mother and the baby, as well as the lack of long‐term follow‐up data for the infant limits the applicability of the evidence.
All trial settings were in Africa, identified as a low‐income country, therefore the results may not be generalisable or transferable to middle‐ or high‐income countries. In low‐income countries, a Pinard or a hand‐held Doppler device might be the only tool available to assess fetal well‐being during labour.
Most of our pre‐specified outcomes were not reported in the included trials. None of the included studies reported on longer‐term outcomes for the baby. In addition, only one of the trials reported on maternal outcomes. One of our important outcomes was instrumental vaginal birth for fetal distress of fetal acidosis, which was not reported. The number of women undergoing instrumental birth was reported in the trial contributing data, the reasons for the intervention were not, and although fetal distress is the most likely reason for assisted delivery, there may have been other reasons such as prolonged labour or maternal exhaustion. In the absence of other data, we included instrumental vaginal birth (for whatever reason) in our 'Summary of findings' tables.
Quality of the evidence
The overall quality of the evidence has been assessed using the GRADE approach as moderate to very low quality and has been summarised in the Table 1; Table 2; Table 3; Table 4; Table 5; Table 6. The main reasons for downgrading evidence were study design limitations (due to lack of blinding) and imprecision (often due to low event rates).
Potential biases in the review process
We have searched the available literature for published and unpublished studies related to IA. There were no restrictions to language or date of publication. We used Cochrane methodology and two review authors independently identified potential studies for inclusion, extracted data and entered data for the review. We believe we have minimised potential biases in the review process.
Agreements and disagreements with other studies or reviews
Given the limited evidence available in this area, we did not identify any major disagreements with other studies or reviews. The NICE 2014 guidelines for intrapartum care recommend IA of the fetal heart during established labour for women without risk factors using either a Pinard stethoscope or Doppler ultrasound immediately after a contraction listening for at least one minute at least every 15 minutes. This recommendation is based on results from reviews and observational studies comparing continuous CTG with IA, not comparing different intermittent listening devices with each other and/or different timing intervals. The recommended timing intervals in the studies included in this review was 30 minutes for the first stage of labour.
Authors' conclusions
Implications for practice.
Intermittent auscultation (IA) with any tool on its own relies on experience of the interpreter and often only pattern recognition is applied to the interpretation rather than fetal physiology and the health context of the mother (Westgate 2007). We found that the use of intermittent electronic fetal monitoring (EFM) cardiotocograph (CTG) with an abdominal transducer, hand‐held Doppler ultrasonography and intensive Pinard led to higher number of abnormal fetal heart rate (FHR) patterns being detected (bradycardia and tachycardia) compared with the normal Pinnard use in practice. Additionally, hand‐held Doppler and intermittent CTG detected more early and late FHR decelerations than any Pinard use.The implications of these results are unclear as low Apgar scores at five minutes after birth showed no clear evidence of a difference and in the settings where these trials were carried out fetal hypoxia/acidosis was unable to be confirmed with fetal blood gas or cord blood sampling (Stein 2006).
There is insufficient evidence to evaluate whether the higher detection rate of abnormal FHR patterns in the intermittent CTG, hand‐held Doppler and intensive Pinard had an impact on clinical outcomes for the baby. Hypoxic ischaemic encephalopathy in the intermittent CTG and Doppler group showed a reduction when compared to the intensive Pinard and routine Pinard group. Event rates are low and it was not possible to separate data for low‐ and high‐risk subgroup analyses and no long‐term follow‐up data for the babies involved are available. It is difficult to interpret the implications of these findings and this means that uncertainty remains regarding the use of IA of FHR in labour.
Women allocated to intermittent CTG and the Doppler group had higher rates of caesarean sections compared to the intensive or routine Pinard monitoring group. Women, health practitioners and policy makers need to consider carefully these results in the absence of short‐ and long‐term benefits for the mother and the baby.
It is important to note that the included trials were conducted in African countries. The usefulness of the findings of this review for high‐income countries will depend on their FHR monitoring practices. In the absence of trial evidence, current clinical practice guidelines for IA in labour for timing and tools are underpinned only by studies comparing continuous fetal auscultation with intermittent auscultations and by the recommendations of international consensus groups.
Implications for research.
Further large, high‐quality multi‐centred randomised trials, particularly in low‐income settings, are needed. High‐quality trials should assess both short‐term and long‐term health outcomes for women and their babies, comparing different monitoring tools and timing for IA with clear identification of the risk status and parity of the labouring women. Additionally, trial outcomes need to include the labouring positions of women and what model of labouring care was provided, for example, one‐to‐one care, which may be an important contributory factor for effectiveness for IA and fetal well‐being. Trials need to collect information on women’s views and experiences; they also need to examine factors that may influence adherence to monitoring protocols, as well as assessing health service costs. If high‐quality trial results can show a reduction in caesarean sections for example, the reduction in health service costs would be significant.
The IA tools used in the included studies mostly required the labouring woman to lie in a supine position. There have been reports showing an improvement in abnormal FHR patterns if the supine or other at‐risk positions are changed to left lateral (Thurlow 2002). Therefore research with intermittent FHR auscultation needs to address the possible contribution of the supine position on adverse outcomes for the baby, and address the question of whether mobility and different labouring positions (for example, upright position) can reduce abnormal FHR patterns, hypoxia/acidosis, neonatal seizures and improve long‐term psychological outcomes for women.
It seems appropriate to recommend further high‐quality trials evaluating the effectiveness of intermittent fetal auscultation compared to continuous fetal monitoring for women with high‐risk pregnancies in low‐income settings. Women with high‐risk pregnancies are usually the only women that birth in hospitals in low‐income settings, where only a hand‐held Doppler or a Pinard/Laënnec may be available for listening and monitoring the fetal heart rate.
Fetal movements during pregnancy have been identified as one of many important indicators of fetal well‐being (Velazquez 2002; Preston 2010). There is little evidence in the literature as to what fetal movements during labour may mean. Auscultation of the FHR is only a surrogate for fetal hypoxia and fetal movements may add clinical information to help interpret the FHR results. Further research studies on IA may want to consider including fetal movements in labour during and after contractions as a measurable secondary outcome and/or determine if fetal movements as an adjunct clinical measure to intermittent auscultation has merit.
What's new
| Date | Event | Description |
|---|---|---|
| 5 April 2017 | Amended | We have added clarification regarding an intervention to the notes section of the Characteristics of included studies table for the Mahomed 1994 trial, and a brief clarification has also been added to Table 1 and Table 2. |
Acknowledgements
The review authors would like to thank the staff from the Australasian Cochrane Centre for technical support. We also sincerely acknowledge Steve McDonald, Philippa Middleton and Miranda Cumpston for their editorial assistance and support. The Department of Health Science and Allied Health, ARC committee, Christchurch Polytechnic Institute of Technology provided support for Ruth Martis to travel to Australia to finish the review. The Faculty of Medicine, Universitas Gadjah Mada, Yogyakarta, Indonesia, provided support for Ova Emilia and Detty Nurdiati to travel to Australia to finish the review.
We also would like to acknowledge and thank Associate Professor Malinee Laopaiboon, Department of Biostatistics and Demography, Faculty of Public Health, Khon Kaen University, Khon Kaen, Thailand, for her invaluable statistical guidance.
As part of the pre‐publication editorial process, this review has been commented on by four peers (an editor and three referees who are external to the editorial team), a member of the Pregnancy and Childbirth Group's international panel of consumers and the Group's Statistical Adviser.
This project was supported by the National Institute for Health research, via Cochrane Infrastructure funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Data and analyses
Comparison 1. Intermittent electronic fetal monitoring (CTG) versus routine Pinard.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Apgar < 7 at 5 minutes after birth | 1 | 633 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.24, 1.83] |
| 2 Caesarean section for fetal distress | 1 | 633 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.92 [1.78, 4.80] |
| 3 Perinatal mortality | 1 | 633 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.34, 2.25] |
| 4 Fetal heart rate abnormality detected | 1 | 633 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.08 [4.21, 8.79] |
| 5 Early and late fetal heart rate decelerations detected | 1 | 633 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.84 [1.82, 4.45] |
| 6 Admission to NICU/NNU | 1 | 633 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.63, 1.25] |
| 7 Seizures in the neonatal period | 1 | 633 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.05 [0.00, 0.89] |
| 8 Hypoxic ischaemic encephalopathy | 1 | 633 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.04, 0.90] |
| 9 Caesarean section | 1 | 633 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.92 [1.39, 2.64] |
| 10 Instrumental vaginal birth | 1 | 633 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [0.86, 2.49] |
| 11 Length of labour (hours) | 1 | 633 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [‐0.05, 1.85] |
Comparison 2. Doppler versus routine Pinard.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Apgar < 7 at 5 minutes after birth | 2 | 2598 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.20, 2.87] |
| 2 Caesarean section for fetal distress | 1 | 627 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.71 [1.64, 4.48] |
| 3 Perinatal mortality | 2 | 2597 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.09, 5.40] |
| 4 Fetal heart rate abnormality detected | 2 | 2598 | Risk Ratio (M‐H, Random, 95% CI) | 2.40 [1.09, 5.29] |
| 5 Early and late fetal heart rate decelerations detected | 1 | 627 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.72 [1.73, 4.28] |
| 6 Admission to NICU/NNU | 2 | 2598 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.41, 1.91] |
| 7 Seizures in the neonatal period | 1 | 627 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.05 [0.00, 0.91] |
| 8 Hypoxic ischaemic encephalopathy | 1 | 627 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.01, 0.78] |
| 9 Caesarean section | 2 | 2598 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [0.81, 2.05] |
| 10 Instrumental vaginal birth | 1 | 627 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.78, 2.32] |
| 11 Length of labour (hours) | 1 | 627 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐1.07, 1.07] |
Comparison 3. Intensive Pinard versus routine Pinard.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Apgar < 7 at 5 minutes after birth | 1 | 625 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.35, 2.31] |
| 2 Caesarean section for fetal distress | 1 | 625 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.35, 1.38] |
| 3 Perintal mortality | 1 | 625 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.19, 1.67] |
| 4 Fetal heart rate abnormality detected | 1 | 625 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.71 [1.10, 2.65] |
| 5 Early and late fetal heart rate decelerations detected | 1 | 625 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.79, 2.23] |
| 6 Admission to NICU/NNU | 1 | 625 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.59, 1.19] |
| 7 Seizures in the neonatal period | 1 | 625 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.24, 1.88] |
| 8 Hypoxic ischaemic encephalopathy | 1 | 625 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.27, 1.84] |
| 9 Caesarean section | 1 | 625 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.46, 1.08] |
| 10 Instrumental vaginal birth | 1 | 625 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.69, 2.11] |
| 11 Length of labour (hours) | 1 | 625 | Mean Difference (IV, Fixed, 95% CI) | 0.5 [‐0.52, 1.52] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Byaruhanga 2015.
| Methods | Prospective parallel randomised controlled trial. Funding: Grand Challenges Canada provided funding for the trial (grant number CRS1 0018) and Laerdal Foundation for the training module ‘Helping Babies Survive Labour’ (grant number 40038). |
|
| Participants |
Location: Nsambya teaching hospital in Kampala, Uganda. 1 centre. Inclusion criteria: women consented antenatal and reconfirmed in active labour. Women included were in established labour, with singleton pregnancy, cephalic presentation, > 37 weeks' gestation including post term, cervical dilation ≤ 7 cm, normal FHR on admission (120‐160). Exclusion criteria: women in second stage of labour or diagnosed as contraindicated for labouring when admitted (e.g. antepartum haemorrhage), intrauterine fetal death on admission or were having an elective caesarean. 1987 pregnant women were recruited. |
|
| Interventions | Women in established labour received either: Intermittent FHR auscultation with hand‐held wind‐up Doppler for 1 minute (60 seconds) immediately after a contraction:
(n = 1000, 992 included in the analysis) or, Intermittent FHR auscultation with a Pinard for 1 minute (60 seconds) immediately after a contraction for the same time frames as above (n = 978, 979 included in the analysis). |
|
| Outcomes |
Primary outcomes: detection of FHR abnormality in labour (tachycardia > 160 bpm, bradycardia < 110 bpm, atypical variable, late or prolonged decelerations), intrapartum stillbirth and neonatal deaths in first 24 hours. Secondary outcomes: Apgar score < 7 at 5 minutes, admission to special care unit, diagnosis of neonatal encephalopathy (mild moderate or severe) and emergency caesarean section. |
|
| Notes | Emailed A. Montgomery (ann.montgomery@sickkids.ca), identified as corresponding author in the trial about the availability and access to further outcome data of the trial. Responded that she will talk to the team and come back, but this did not happened by date of review submission. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | ‘Randomised’ equally to 1 of 2 groups, no other details. |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, opaque, sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and care providers were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details given. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Less than 1% attrition reported and reasons for losses were provided. 1987 enrolled; 987 to Pinard and 1000 to Doppler. Not analysed for Pinard n = 8 due to loss to follow‐up (n = 1), delivered before monitoring initiated (n = 1), breech birth (n = 2), multiple pregnancy (n = 2). For Doppler n = 8 not analysed; delivered before monitoring initiated (n = 3), breech birth (n = 3), multiple pregnancy (n = 2). |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported. |
| Other bias | Low risk | No other risk of bias identified. Groups appeared balanced at baseline. |
Mahomed 1994.
| Methods | Prospective parallel randomised controlled trial, 1 centre. Funding: this trial was funded by World Health Organization special programme for research, development and research training in human reproduction. |
|
| Participants |
Location: Harare maternity hospital, Zimbabwe, Africa. 1 centre. Inclusion criteria: women consented antenatal. Women included were > 37 weeks' gestation, with obstetric and medical risk factors, in established labour, with singleton baby in cephalic presentation, cervical dilation ≤ 7 cm, normal FHR on admission (120‐160). Exclusion criteria: women excluded from the trial were those presenting with placental abruption or eclampsia. 1255 pregnant women were recruited. |
|
| Interventions |
EFM group (n = 318): The intention was for a CTG with continuous trace via an external abdominal transducer for 10 minutes every half hour if normal tracing and every 20 minutes for 10 minutes if abnormal. Monitoring performed by a trained research midwife and doctor, strictly adhering to the research protocol.(*see notes) Doppler group (n = 312): intermittent auscultation using hand‐held Doppler (ultrasonography), listening for 1 minute, during the last 10 minutes of each half hour, during and immediately after a contraction. Auscultation performed by a research midwife, who had been educated about the trial and its intervention, strictly adhering to the research protocol. Intensive Pinard group (n = 310): intermittent auscultation with a Pinard stethoscope listening for 1 minute during the last 10 minutes of every half hour during and immediately after a contraction. Auscultation performed by a research midwife, who had been educated about the trial and its intervention, strictly adhering to the research protocol. Routine Pinard group (n = 315): intermittent auscultation with a Pinard stethoscope as was routine at the involved hospital, which expected the FHR to be auscultated for 1 minute during last 10 minutes of every half hour during and immediately after a contraction. Auscultation would be performed by the midwife on‐duty, who may care for other labouring women at the same time and would endeavour to follow the hospital recommendation for routine auscultation as much as possible. |
|
| Outcomes |
Baby: Apgar < 6 at 5 minutes of birth, stillbirth or neonatal death, meconium‐stained liquor, admission to NICU/NNU, seizures in neonatal unit, hypoxic ischaemic encephalopathy, prolonged early and late FHR decelerations, abnormal FHR. Mother: caesarean section for fetal distress, total caesarean section, operative (instrumental) vaginal delivery, spontaneous vaginal birth, spontaneous onset of labour, length of labour. The results were recorded as a single rate. |
|
| Notes | * A 10 min tracing was done but not with all participants. In this arm of the study 18 women birthed too quickly and 24 women had an unreadable tracing (frequent loss of contact, paper got stuck). It appears the FH was recorded as one number but late decelerations were noted on the 10 min tracing when they were present assisting with clinical management decisions. There were a number of discrepancies in the way the interventions were described in different trial reports. In the 1992 article on page 460 there was a typo, it reads ' ... every 10 minutes if results were abnormal', should read "..every 20 minutes if..." (clarified via email with main trial author, Mahomed 1994) Furthermore, the 1992 article reported that the nurse in charge applied the intervention of the routine group for fetal auscultation with a Pinard stethoscope. The 1994 article reported that is was the midwife on‐duty (clarified via email with main trial author (Mahomed 1994 that latter statement is correct). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "... Eligible women were randomly allocated to one of four methods of monitoring intrapartum... The randomisation was performed with a random permuted block of 16 numbers..." (p. 498). It is not stated how the random permuted blocks were generated. |
| Allocation concealment (selection bias) | Low risk | Quote: " ...by means of serially numbered sealed opaque envelopes containing the allocation..." (p. 498). |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not stated, however it is unlikely that blinding would be possible with the nature of the interventions, as they were visibly different. Therefore considered as high risk of bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details given. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There seems to be no loss of follow‐up, as numbers remain consistent. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported. |
| Other bias | Low risk | No other risk of bias identified. Groups appeared balanced at baseline. |
Mdoe 2015.
| Methods | Prospective parallel randomised controlled trial. Funding: no details provided. |
|
| Participants |
Location: 2 settings in the United Republic of Tanzania. An urban setting at the Muhimbili National Hospital and rural setting at the Haydom Lutheran Hospital. In all settings fetal blood gas sampling or epidural analgesia were not available. Inclusion criteria: women with low risk, who consented and were in active labour with singleton pregnancies, cephalic presentation, normal FHR (120‐160 bpm) and with a cervical dilatation of ≤ 7 cm. Exclusion criteria: women arriving in second stage, no other exclusion criteria described. It appears 1376 women at Muhimbili National Hospital and 1623 women at Haydom Lutheran Hospital were recruited. A total of 2999 women were randomised. |
|
| Interventions | Intermittent auscultation with hand‐held Doppler (Doppler) comparing urban with rural setting (n = 1521 (not clear)). Frequency of intermittent auscultation and length of listening (timing) not described but stated as 'free play'. Unclear what 'free play' mean. Or intermittent auscultation with Pinard Feteoscope comparing urban with rural setting (n =1475 (not clear)). Frequency of intermittent auscultation and length of listening (timing) not described but stated as 'free play'. Unclear what 'free play' means. |
|
| Outcomes | Outcomes are not reported separately for primary and secondary outcomes. Outcomes reported were caesarean section; abnormal FHR defined as < 120 or > 160 bpm; admission to NNU; 5 minute Apgar score < 7; bag mask ventilation; fresh stillbirth; neonatal death. | |
| Notes | Review author RM emailed Mdoe to seek further details to describe the interventions, in particular what 'free play' was. Clarification was also sought about the published data, as the outcome data presented did not correspond with the total number of participants identified. Mdoe responded stating that It is anticipated that the results of the trial will be published within the next 6 months and suggested to wait for this publication. No further details provided. This trial was only published as an abstract. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | From email correspondence and abstract stated: "randomisation". No further details provided. |
| Allocation concealment (selection bias) | Unclear risk | From email correspondence confirmed '1 to 1 open label'. Allocation concealment not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not stated, however it is unlikely that blinding would be possible with the nature of the interventions, as they were visibly different. Therefore considered as high risk of bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No other details given. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | This is not stated and the numerical data provided had major numerical inconsistencies in the publication and could not be used for meta‐analysis. Unclear which numbers were due to loss of follow‐up. |
| Selective reporting (reporting bias) | High risk | Prespecified outcomes and the outcomes reported do not match. |
| Other bias | Unclear risk | This publication is only an abstract and it remains unclear if the groups were balanced at baseline. |
bpm: beats per minute CTG: cardiotocograph EFM: electronic fetal monitoring FHR: fetal heart rate NICU: neonatal intensive care unit NNU: neonatal unit SD: standard deviation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Haverkamp 1979 | Compared intermittent ausculation with continuous monitoring, which was not one of the included comparisons. |
| MacDonald 1985 | Compared intermittent ausculation with continuous monitoring, which was not one of the included comparisons. |
| Wood 1981 | Compared intermittent ausculation with continuous monitoring, which was not one of the included comparisons. |
Differences between protocol and review
The methods section has been updated to reflect the latest guidance in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) and the Cochrane Pregnancy and Childbirth Group's methodological guidelines. The electronic searches section was updated to include Embase and email alerts.
We did not anticipate that one trial (Mahomed 1994) would include electronic fetal monitoring (EFM) with a cardiotocograph (CTG) as an intermittent auscultation (IA) modality. After discussion, we decided to include this arm of the intervention as well, as it appears that only the fetal heart rate (FHR) was used for clinical management decisions, rather than interpreting the paper tracing. This trial reported Apgar score < six at five minutes as a measure of newborn condition; although our prespecified outcome was Apgar score < seven, we decided to use the data from this trial, as overall only two studies are included in the review and we wanted to use all available data.
Two secondary outcomes for the mother have been removed, as recommended by the peer reviewers. These were: (5) Types of induction of labour, e.g. prostaglandin gel, oxytocin infusion, amniotomy and (6) Spontaneous onset of labour. The reasons provided for this request were that participants are in labour already therefore neither intervention could affect the outcome.
The maternal secondary outcome, 'length of ruptured membranes' was edited to 'duration of ruptured membranes'.
Spontaneous onset of labour versus induction of labour (3) was replaced with first stage versus second stage of labour for subgroup analysis and investigation of heterogeneity.
In this version of the review, we have included a new infant outcome: mortality or serious morbidity, and this outcome is included in our 'Summary of findings' tables.
The GRADE approach to examine the quality of the body of evidence was added to this review.
Contributions of authors
Ruth Martis wrote the protocol with Ova Emilia and Detty Nurdiati providing feedback. Julie Brown joined the review team during the editorial process for this review to assist with data extractions of the newly added studies and finalising the review for publication. All review authors contributed to data extraction, presentation and interpretation of the results, and approved the final version of the review.
Sources of support
Internal sources
-
Christchurch Polytechnic Institute of Technology, CPIT, New Zealand.
The Department of Health Science and Allied Health, ARC committee, provided financial support for Ruth Martis to travel to Australia to finish the review.
-
The Faculty of Medicine, Universitas Gadjah Mada, Yogyakarta, Indonesia.
provided financial support for Ova Emilia and Detty Nurdiati to travel to Australia to finish the review.
Liggins Institute, Univeristy of Auckland, New Zealand.
External sources
-
Australasian Cochrane Centre, Monash University, Melbourne, Australia.
Technical support from the Australasian Cochrane Centre, which included an accommodation grant for OE and DN to stay in Melbourne for a week to complete the review.
UNDP‐UNFPA‐UNICEF‐WHO‐World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP), Department of Reproductive Health and Research (RHR), World Health Organization, Switzerland.
Declarations of interest
Ruth Martis: received support from Cochrane Health Promotion and Public Health Field in the form of a bursary supporting travel to meet with review authors for review progress and data analysis. The bursary was administrated by the SeaOrchid project.
Ova Emilia: received support (for travel and accommodation) from the SeaOrchid Project to be trained in preparing Cochrane Systematic Review in Australia.
Detty S Nurdiati: received support (for travel and accommodation) from the SeaOrchid Project to be trained in preparing Cochrane Systematic Review in Australia. She also received support from the Faculty of Medicine, Universitas Gadjah Mada, Yogyakarta, Indonesia to attend a workshop on completing systematic reviews.
Julie Brown: none known.
Edited (no change to conclusions)
References
References to studies included in this review
Byaruhanga 2015 {published data only}
- Byaruhanga R, Bassani DG, Jagau A, Muwanguzi P, Montgomery AL, Lawn JE. Use of wind‐up fetal Doppler versus Pinard for fetal heart rate intermittent monitoring in labour: a randomised clinical trial. BMJ Open 2015;5(1):e006867. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mahomed 1994 {published data only}
- Mahomed K, Nyoni R, Mlambo T, Jacobus E, Kasule J. Intrapartum foetal heart rate monitoring ‐ continuous electronic vs intermittent doppler ‐ a randomised controlled trial. Central African Journal of Medicine 1992;38:458‐62. [PubMed] [Google Scholar]
- Mahomed K, Nyoni R, Mulambo T, Kasule J, Jacobus E. Randomised controlled trial of intrapartum fetal heart rate monitoring. BMJ 1994;308:497‐500. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mdoe 2015 {published data only}
- Mdoe J, Perlman J, Ersdal HL, Kidanto HL. [3842.13] Randomized controlled study comparing hand held doppler and pinard fetoscope (PF) for fetal heart rate (FHR) monitoring in Tanzania. Pediatric Academic Societies Annual Meeting; 2015 April 25‐28; San Diego, California. 2015.
- Mdoe P, Mduma E, Kidanto H, Moshiro R, Perlman J, Ersdal H. Randomized controlled study comparing hand held doppler and Pinard fetoscope (PF) for fetal heart rate (FHR) monitoring in Tanzania. International Journal of Gynecology and Obstetrics 2015;131(Suppl 5):E121. [Google Scholar]
References to studies excluded from this review
Haverkamp 1979 {published data only}
- Haverkamp AD, Orleans M, Langendoerfer S, McFee J, Murphy J, Thompson HE. A controlled trial of the differential effects of intrapartum fetal monitoring. American Journal of Obstetrics and Gynecology 1979;134(4):399‐412. [DOI] [PubMed] [Google Scholar]
MacDonald 1985 {published data only}
- MacDonald D, Grant A, Sheridean‐Pereira M, Boyland P, Chalmers I. The Dublin randomized controlled trial of intrapartum fetal heart rate monitoring. American Journal of Obstetrics and Gynecology 1985;152(5):524‐39. [DOI] [PubMed] [Google Scholar]
Wood 1981 {published data only}
- Wood C, Renou P, Oats J, Farrell E, Beischer N, Anderson I. A controlled trial of fetal heart rate monitoring in a low‐risk obstetric population. American Journal of Obstetrics and Gynecology 1981;141(5):527‐34. [DOI] [PubMed] [Google Scholar]
Additional references
ACNM 2007
- ACNM. American College of Nurse Midwives. Intermittent auscultation for intrapartum fetal heart rate surveillance. Journal of Midwifery & Women's Health 2007;52(3):314‐9. [DOI] [PubMed] [Google Scholar]
ACOG 1995
- Anonymous. ACOG technical bulletin. Fetal heart rate patterns: monitoring, interpretation, and management. Number 207‐‐July 1995 (replaces No. 132, September 1989). International Journal of Gynaecology and Obstetrics 1995;51(1):65‐74. [PubMed] [Google Scholar]
Albers 2001
- Albers LL. Monitoring the fetus in labour: evidence to support the methods. Journal of Midwifery and Women's Health 2001;46(6):366‐73. [DOI] [PubMed] [Google Scholar]
Alfirevic 2013
- Alfirevic Z, Devane D, Gyte GML. Continuous cardiotocography (CTG) as a form of electronic fetal monitoring (EFM) for fetal assessment during labour. Cochrane Database of Systematic Reviews 2013, Issue 5. [DOI: 10.1002/14651858.CD006066] [DOI] [PubMed] [Google Scholar]
Anderson 2011
- Anderson D. A review of systemic opioids commonly used for labor pain relief. Journal of Midwifery and Women’s Health 2011;56:222‐39. [DOI: 10.1111/j.1542-2011.2011.00061.x] [DOI] [PubMed] [Google Scholar]
Arasaratnam 2013
- Arasaratnam A, Humprheys G. Emerging economies drive frugal innovation. Bulletin of the World Health Organization 2013;91:6‐7. [DOI: 10.2471/BLT.13.020113] [DOI] [PMC free article] [PubMed] [Google Scholar]
Arulkumaran 2000
- Arulkumaran S, Jenkins HML. Perinatal Asphyxia. Chennai, India: Orient Longman Ltd, 2000. [Google Scholar]
Bishop 1980
- Bishop PJ. Evolution of the stethoscope. Journal of the Royal Society of Medicine 1980;73:448‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chez 2000
- Chez BF, Harvey MG, Harvey CJ. Intrapartum fetal monitoring: past, present, and future. Journal of Perinatal and Neonatal Nursing 2000;14(3):1‐18. [DOI] [PubMed] [Google Scholar]
Deeks 2001
- Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta‐analysis. In: Egger M, Davey Smith G, Altman DG editor(s). Systematic Reviews in Health Care: Meta‐analysis in Context. London: BMJ Books, 2001. [Google Scholar]
Devane 2012
- Devane D, Lalor JG, Daly S, McGuire W, Smith V. Cardiotocography versus intermittent auscultation of fetal heart on admission to labour ward for assessment of fetal wellbeing. Cochrane Database of Systematic Reviews 2012, Issue 2. [DOI: 10.1002/14651858.CD005122.pub4] [DOI] [PubMed] [Google Scholar]
East 2013
- East CE, Smyth RMD, Leader LR, Henshall NE, Colditz PB, Lau R, et al. Vibroacoustic stimulation for fetal assessment in labour in the presence of a nonreassuring fetal heart rate trace. Cochrane Database of Systematic Reviews 2013, Issue 1. [DOI: 10.1002/14651858.CD004664.pub3] [DOI] [PubMed] [Google Scholar]
East 2014
- East CE, Begg L, Colditz PB, Lau R. Fetal pulse oximetry for fetal assessment in labour. Cochrane Database of Systematic Reviews 2014, Issue 10. [DOI: 10.1002/14651858.CD004075.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Feinstein 2000
- Feinstein NF. Fetal heart rate auscultation: current and future practice. Journal of Obstetric, Gynecologic, and Neonatal Nursing 2000;29(3):306‐15. [DOI] [PubMed] [Google Scholar]
Freeman 1991
- Freeman T, Garite T, Nageotte M. Fetal Heart Rate Monitoring. 2nd Edition. Baltimore, MD: Williams and Wilkins, 1991. [Google Scholar]
Freeman 2012
- Freeman RK, Garite TJ, Nageotte MP, Miller LA. Fetal Heart Rate Monitoring. 4th Edition. Philadelphia, PA, USA: Wolters Kluwer, Lippincolt, Williams & Wiltkins, 2012. [ISBN: 978‐1‐4511‐1663‐2] [Google Scholar]
Gapultos 2008
- Anonymous. Accoucheur's antique fetal stethoscopes. http://www.fcgapultoscollection.com/scopes.html (accessed July 2008).
Goodlin 1979
- Goodlin RC. History of fetal monitoring. American Journal of Obstetrics and Gynecology 1979;133(3):323‐53. [DOI] [PubMed] [Google Scholar]
Goodson 2014
- Goodson C, Martis R. Pethidine: to prescribe or not to prescribe? A discussion surrounding pethidine's place in midwifery practice and New Zealand prescribing legislation. New Zealand College of Midwives Journal 2014;49:23‐8. [Google Scholar]
Goodwin 2000
- Goodwin L. Intermittent auscultation of the fetal heart rate: a review of general principles. Journal of Perinatal and Neonatal Nursing 2000;14(3):53‐61. [DOI] [PubMed] [Google Scholar]
Gultekin‐Zootzmann 1975
- Gultekin‐Zootzmann B. The history of monitoring the human fetus. Journal of Perinatal Medicine 1975;3:135‐44. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. [updated March 2011]. The Cochrane Collaboration. Available from www.cochrane‐handbook.org 2011.
Hillis 1917
- Hillis DS. Attachment for the stethoscope. JAMA 1917;68:910. [Google Scholar]
Hindley 2005
- HIndley C, Hinsliff SW, Thomson AM. Developing a tool to appraise fetal monitoring guidelines for women at low obstetric risk. Journal of Advanced Nursing 2005;52(3):307‐14. [DOI] [PubMed] [Google Scholar]
Irwig 1998
- Irwig L, Zwarenstein M, Zwi A, Chalmers I. A flow diagram to facilitate selection of interventions and research in health care. Bulletin of the World Health Organization 1998;76(1):17‐24. [PMC free article] [PubMed] [Google Scholar]
Kranke 2013
- Kranke P, Girard T, Lavand’homme P, Melber A, Jokinen J, Muellenbach RM, et al. Must we press on until a young mother dies? Remifentanil patient controlled analgesia in labour may not be suited as a “poor man’s epidural”. BMC Pregnancy and Childbirth 2013;13(139):1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Laënnec 1819
- Laënnec RTH. De Auscultation Mediate ou Trait du Diagnostic des Maladies des Poumon et du Coeur. 1st Edition. Paris: Brosson & Chaudé, 1819. [Google Scholar]
Lewis 2015
- Lewis D, Downe S for the FIGO Intrapartum Fetal Monitoring Expert Consensus Panel. FIGO consensus guidelines on intrapartum fetal monitoring: Intermittent auscultation. International Journal of Gynecology and Obstetrics 2015;131:9‐12. [DOI: ] [DOI] [PubMed] [Google Scholar]
Lie 2010
- Lie KK, Groholt EK, Eskild A. Association of cerebral palsy with Apgar score in low and normal birthweight infants: population based cohort study. BMJ 2010;341:c4990. [DOI: 10.1136/bmj.c4990] [DOI] [PMC free article] [PubMed] [Google Scholar]
Liston 2002
- Liston R, Crane J, Hamilton E, Hughes O, Kuling S, MacKinnon C, et al. Fetal health surveillance in labour. Journal of Obstetrics and Gynaecology Canada: JOGC 2002;24(3):250‐76. [PubMed] [Google Scholar]
Lutomski 2015
- Lutomski JE, Meaney S, Greene RA, Ryan AC, Devane D. Expert systems for fetal assessment in labour. Cochrane Database of Systematic Reviews 2015, Issue 4. [DOI: 10.1002/14651858.CD010708.pub2] [DOI] [Google Scholar]
Mainstone 2004
- Mainstone A. Fetal monitoring advances. British Journal of Midwifery 2004;12(11):704‐7. [Google Scholar]
Maude 2010
- Maude R, Lawson J, Foureur M. Auscultation‐the action of listening. New Zealand College of Midwives Journal 2010;43:13‐18. [Google Scholar]
NCCWCH 2008
- NCCWCH National Collaborating Centre for Women's and Children's Health. Intrapartum care: Care of Healthy Women and their Babies during Childbirth. Revised. London, England: RCOG Press, 2008. [Google Scholar]
Neilson 2015
- Neilson JP. Fetal electrocardiogram (ECG) for fetal monitoring during labour. Cochrane Database of Systematic Reviews 2015, Issue 12. [DOI: 10.1002/14651858.CD000116.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
NICE 2014
- NICE, National Collaborating Centre for Women’s and Children’s Health. Intrapartum Care: Care of Healthy Women and their Babies During Childbirth. Methods, Evidence and Recommendations. NICE Clinical Guideline 190. 2nd Edition. London, UK: NICE, 2014. [Google Scholar]
NZCOM 2005
- New Zealand College of Midwives. Fetal monitoring in labour. Christchurch, New Zealand: New Zealand College of Midwives, 2005. [Google Scholar]
PowerFree Education Technology 2014
- PowerFree Education Technology. Wind‐up heart rate monitor will save babies lives. http://www.southafrica.info/news/fetal‐heart‐rate‐monitor‐061014.htm#.VmC_ReGhfIU (accessed 4 December 2015) 2014.
Preston 2010
- Preston S, Mahomed K, Chadha Y, Flenady V, Gardener G, MacPhail J, et al. Clinical Practice Guideline for the Management of Women who Report Decreased Fetal Movements. Brisbane, Australia: ANZSA, 2010. [Google Scholar]
RANZCOG 2006
- The Royal Australian and New Zealand College of Obstetricians and Gynaecologists. Intrapartum Fetal Surveillance Clinical Guidelines. 2nd Edition. East Melbourne, Victoria, Australia: RANZCOG, 2006. [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Saxena 2013
- Saxena R. Evidence Based Color Atlas of Obstetrics & Gynecology: Diagnosis and Management. New Delhi, India: Jaypee Brothers Medical Publishers Ltd, 2013. [ISBN: 978‐93‐5090‐431‐2] [Google Scholar]
Schmidt 2000
- Schmidt JV, McCartney PR. History and development of fetal heart assessment: a composite. Journal of Obstetric, Gynecologic and Neonatal Nursing 2000;29:295‐305. [DOI] [PubMed] [Google Scholar]
Sholapurkar 2015
- Sholapurkar SL. Intermittent auscultation in labor: Could it be missing many pathological (late) fetal heart rate decelerations? Analytical review and rationale for improvement supported by clinical cases. Journal of Clinical Medicine Research 2015;7(12):919‐25. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Solt 2005
- Solt I, Divon MY. Fetal surveillance tests. In: Blazer S, Zimmer EZ editor(s). The Embryo: Scientific Discovery and Medical Ethics. Basel, Switzerland: Karger, 2005:291‐308. [Google Scholar]
Souza 2010
- Souza JP, Gülmezoglu AM, Lumbiganon P, Laopaiboon M, Carroli G, Fawole B, et al. Caesarean section without medical indications is associated with an increased risk of adverse short term maternal outcomes: the 2004‐2008 WHO Global Survey on Maternal and Perinatal Health. BMC Medicine 2010;8:71. [DOI: 10.1186/1741-7015-8-71] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stein 2006
- Stein W, Hellmeyer L, Misselwitz B, Schmidt S. Impact of fetal blood sampling on vaginal delivery and neonatal outcome in deliveries complicated by pathologic fetal heart rate: a population based cohort study. Journal of Perinatal Medicine 2006;34:479‐83. [DOI: 10.1515/JPM.2006.093] [DOI] [PubMed] [Google Scholar]
Thurlow 2002
- Thurlow JA, Kinsella SM. Intrauterine resuscitation: active management of fetal distress. International Journal of Obstetric Anesthesia 2002;11:105‐16. [DOI] [PubMed] [Google Scholar]
Velazquez 2002
- Velazquez MD, Rayburn WF. Antenatal evaluation of the fetus using fetal movement monitoring. Clinical Obstetrics Gynecology Journal 2002;45:993‐1004. [DOI] [PubMed] [Google Scholar]
Walsh 2007
- Walsh D. Evidence‐Based Care for Normal Labour and Birth. Abingdon, Oxon, UK: Routledge, 2007. [Google Scholar]
Walsh 2008
- Walsh D. CTG use in intrapartum care: assessing the evidence. British Journal of Midwifery 2008;16(6):367‐9. [Google Scholar]
Westgate 2007
- Westgate JA, Wibbens B, Bennet L, Wassink G, Parer JT, Gunn AJ. The intrapartum deceleration in center stage: a physiologic approach to the interpretation of fetal heart rate changes in labor. American Journal of Obstetrics and Gynecology 2007;197(3):236.e1‐236.e11. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Martis 2010
- Martis R, Emilia O, Nurdiati DS. Intermittent auscultation (IA) of fetal heart rate in labour for fetal well‐being. Cochrane Database of Systematic Reviews 2010, Issue 9. [DOI: 10.1002/14651858.CD008680] [DOI] [PMC free article] [PubMed] [Google Scholar]


