Abstract
Background
Oral nonsteroidal anti‐inflammatory drugs (NSAIDs) are widely used in the treatment of pain in fibromyalgia, despite being considered not to be effective.
Objectives
To assess the analgesic efficacy, tolerability (drop‐out due to adverse events), and safety (serious adverse events) of oral nonsteroidal anti‐inflammatory drugs for fibromyalgia in adults.
Search methods
We searched CENTRAL, MEDLINE, and Embase for randomised controlled trials from inception to January 2017. We also searched the reference lists of retrieved studies and reviews, and online clinical trial registries.
Selection criteria
We included randomised, double‐blind trials of two weeks' duration or longer, comparing any oral NSAID with placebo or another active treatment for relief of pain in fibromyalgia, with subjective pain assessment by the participant.
Data collection and analysis
Two review authors independently extracted data and assessed trial quality and potential bias. Primary outcomes were participants with substantial pain relief (at least 50% pain relief over baseline or very much improved on Patient Global Impression of Change scale (PGIC)) or moderate pain relief (at least 30% pain relief over baseline or much or very much improved on PGIC), serious adverse events, and withdrawals due to adverse events; secondary outcomes were adverse events, withdrawals due to lack of efficacy, and outcomes relating to sleep, fatigue, and quality of life. Where pooled analysis was possible, we used dichotomous data to calculate risk difference (RD) and number needed to treat for an additional beneficial outcome (NNT), using standard methods. We assessed the quality of the evidence using GRADE and created a 'Summary of findings' table.
Main results
Our searches identified six randomised, double‐blind studies involving 292 participants in suitably characterised fibromyalgia. The mean age of participants was between 39 and 50 years, and 89% to 100% were women. The initial pain intensity was around 7/10 on a 0 to 10 pain scale, indicating severe pain. NSAIDs tested were etoricoxib 90 mg daily, ibuprofen 2400 mg daily, naproxen 1000 mg daily, and tenoxicam 20 mg daily; 146 participants received NSAID and 146 placebo. The duration of treatment in the double‐blind phase varied between three and eight weeks.
Not all studies reported all the outcomes of interest. Analyses consistently showed no significant difference between NSAID and placebo: substantial benefit (at least 50% pain intensity reduction) (risk difference (RD) ‐0.07 (95% confidence interval (CI) ‐0.18 to 0.04) 2 studies, 146 participants; moderate benefit (at least 30% pain intensity reduction) (RD ‐0.04 (95% CI ‐0.16 to 0.08) 3 studies, 192 participants; withdrawals due to adverse events (RD 0.04 (95% CI ‐0.02 to 0.09) 4 studies, 230 participants; participants experiencing any adverse event (RD 0.08 (95% CI ‐0.03 to 0.19) 4 studies, 230 participants; all‐cause withdrawals (RD 0.03 (95% CI ‐0.07 to 0.14) 3 studies, 192 participants. There were no serious adverse events or deaths. Although most studies had some measures of health‐related quality of life, fibromyalgia impact, or other outcomes, none reported the outcomes beyond saying that there was no or little difference between the treatment groups.
We downgraded evidence on all outcomes to very low quality, meaning that this research does not provide a reliable indication of the likely effect. The likelihood that the effect could be substantially different is very high. This is based on the small numbers of studies, participants, and events, as well as other deficiencies of reporting study quality allowing possible risks of bias.
Authors' conclusions
There is only a modest amount of very low‐quality evidence about the use of NSAIDs in fibromyalgia, and that comes from small, largely inadequate studies with potential risk of bias. That bias would normally be to increase the apparent benefits of NSAIDs, but no such benefits were seen. Consequently, NSAIDs cannot be regarded as useful for treating fibromyalgia.
Plain language summary
Oral NSAIDs for treating fibromyalgia pain in adults
Bottom line
We found very low‐quality evidence that oral non‐steroidal anti‐inflammatory drugs (NSAIDs) have no effect on pain or other symptoms in people with moderate or severe pain from fibromyalgia. Ibuprofen and diclofenac are common NSAIDs.
Background
Fibromyalgia is characterised by persistent, widespread pain, sleep problems, and fatigue. NSAIDs are drugs with analgesic (pain‐killing), antipyretic (fever‐reducing) effects, and also with anti‐inflammatory effects at higher doses. NSAIDs are frequently used to treat rheumatic diseases.
Our definition of a good result was someone who had a high level of pain relief and was able to keep taking the medicine without side effects that made them want to stop.
Study characteristics
We searched for clinical trials in which NSAIDs were used to treat symptoms of fibromyalgia in adults. The latest search was in January 2017. Six studies satisfied the inclusion criteria, randomising 292 participants to treatment with NSAID or placebo. NSAIDs tested were etoricoxib 90 mg daily, ibuprofen 2400 mg daily, naproxen 1000 mg daily, and tenoxicam 20 mg daily; 146 participants received NSAID and 146 placebo. Study duration was between three and eight weeks. Not all studies reported the outcomes of interest.
Key results
We found no difference between NSAID or placebo for a range of outcomes. Pain reduction by half or better was experienced by 1 in 10 with NSAID and 2 in 10 with placebo. Pain reduction by a third or better was experienced by about 2 in 10 with both NSAID and placebo. Side effects were experienced by 3 in 10 with NSAID and 2 in 10 with placebo.
Quality of the evidence
The evidence was of very low quality. This means that the research does not provide a reliable indication of the likely effect. The chance that the real effect of NSAIDs could be substantially different is very high. Small studies like those in this review tend to overestimate results of treatment compared to the effects found in larger, better studies. The very low‐quality evidence and the lack of any obvious benefit mean that NSAIDs cannot be regarded as useful for the management of fibromyalgia.
Summary of findings
Summary of findings for the main comparison. NSAID compared with placebo for fibromyalgia.
| NSAID compared with placebo for fibromyalgia | ||||||
|
Patient or population: adults with fibromyalgia Settings: community Intervention: any NSAID Comparison: placebo | ||||||
|
Outcomes (at trial end) |
Probable outcome with NSAID | Probable outcome with placebo | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments |
| Substantial pain relief: at least 50% reduction in pain, or PGIC much improved |
110 per 1000 | 180 per 1000 | RD ‐0.07 (95% CI ‐0.18 to 0.04) | 2 studies 146 participants 21 events |
Very low quality | Downgraded three levels due to small number of studies, participants, and events |
| Moderate pain relief: at least 30% reduction in pain, or PGIC much or very much improved |
220 per 1000 | 260 per 1000 | RD ‐0.04 (95% CI ‐0.16 to 0.08) | 3 studies 192 participants 46 events |
Very low quality | Downgraded three levels due to small number of studies, participants, and events |
| Serious adverse events | None reported | None reported | Not calculated | No data | Very low quality | No events |
| Adverse event withdrawal | 50 per 1000 | 20 per 1000 | RD 0.04 (95% CI ‐0.02 to 0.09) | 4 studies 230 participants 8 events |
Very low quality | Downgraded three levels due to small number of studies, participants, and events |
| Participants experiencing any adverse event | 310 per 1000 | 220 per 1000 | RD 0.08 (95% CI ‐0.03 to 0.19) | 4 studies 230 participants 61 events |
Very low quality | Downgraded three levels due to small number of studies, participants, and events |
| All cause withdrawal | 230 per 1000 | 200 per 1000 | RD 0.03 (‐0.07 to 0.14) | 3 studies 192 participants 41 events |
Very low quality | Downgraded three levels due to small number of studies, participants, and events |
| Death | No data | No data | Not calculated | No data | Very low quality | No events |
| CI: Confidence interval; PGIC: Patient Global Impression of Change; RD: Risk difference | ||||||
| Descriptors for levels of evidence (EPOC 2015):
High quality: this research provides a very good indication of the likely effect. The likelihood that the effect will be substantially differenta is low.
Moderate quality: this research provides a good indication of the likely effect. The likelihood that the effect will be substantially differenta is moderate.
Low quality: this research provides some indication of the likely effect. However, the likelihood that it will be substantially differenta is high.
Very low quality: this research does not provide a reliable indication of the likely effect. The likelihood that the effect will be substantially differenta is very high. aSubstantially different: a large enough difference that it might affect a decision. | ||||||
Background
This review is based on a template for reviews of drugs used to relieve fibromyalgia. The aim is for all reviews to use the same methods, based on new criteria for what constitutes reliable evidence in chronic pain (Moore 2010a; Appendix 1) and fibromyalgia (Mease 2009).
Description of the condition
Fibromyalgia symptoms can be assessed by self report of the person using the fibromyalgia criteria and severity scales for clinical and epidemiological studies, a modification of the American College of Rheumatology (ACR) Preliminary Diagnostic Criteria for Fibromyalgia (so‐called Fibromyalgia Symptom Questionnaire) (Wolfe 2011). Fibromyalgia was previously defined by the American College of Rheumatology (ACR) 1990 classification criteria as widespread pain lasting for longer than three months with tenderness on palpation at 11 or more of 18 specified tender points (Wolfe 1990). For a clinical diagnosis, the ACR 1990 classification criteria (Wolfe 1990), and the ACR 2010 Preliminary Diagnostic Criteria (Wolfe 2010), can both be used. Lacking a specific laboratory test, diagnosis is established by a history of the key symptoms and the exclusion of somatic diseases sufficiently explaining the key symptoms (Wolfe 2010). The indexing of fibromyalgia within the International Classification of Diseases is under debate. While some rheumatologists have thought of it as a specific pain disorder and central sensitivity syndrome (Clauw 2014; Yunus 2008), research points at small fibre pathology in a subgroup of people with fibromyalgia that may be of pathophysiological (functional changes associated with or resulting from disease) importance (Oaklander 2013; Üçeyler 2013a). In psychiatry and psychosomatic medicine, fibromyalgia symptoms are categorised as a functional somatic syndrome, a bodily distress syndrome, a somatic physical symptom disorder, or a somatoform disorder (Häuser 2014a).
Fibromyalgia is a heterogeneous condition. The definite aetiology (causes) of this syndrome remains unknown. A model of interacting biological and psychosocial variables in the predisposition, triggering, and development of the chronicity of fibromyalgia symptoms has been suggested (Sommer 2012a). Depression (Forseth 1999), genetics (Arnold 2013; Lee 2012), obesity combined with physical inactivity (Mork 2010), physical and sexual abuse in childhood (Häuser 2011), sleep problems (Mork 2012), and smoking predict future development of fibromyalgia (Choi 2010). Psychosocial stress (working place and family conflicts) and physical stress (infections, surgery, accidents) might trigger the onset of chronic widespread pain and fatigue (Clauw 2014; Sommer 2012a). Depression and post‐traumatic stress disorder worsen fibromyalgia symptoms (Häuser 2013a; Lange 2010).
Several factors are associated with the pathophysiology of fibromyalgia, but the relationship is unclear. The functional changes include alteration of sensory processing in the brain, reduced reactivity of the hypothalamus‐pituitary‐adrenal axis to stress, increased pro‐inflammatory and reduced anti‐inflammatory cytokine profiles (produced by cells involved in inflammation), disturbances in neurotransmitters such as dopamine and serotonin, and small fibre pathology (Oaklander 2013; Sommer 2012a; Üçeyler 2013a). Prolonged exposure to stress, as outlined above, may contribute to these functional changes in predisposed individuals (Bradley 2009).
People with fibromyalgia often report high disability levels and poor quality of life along with extensive use of medical care (Häuser 2015). Many people with fibromyalgia are significantly disabled, and experience moderate or severe pain for many years (Bennett 2007). Chronic painful conditions comprised five of the 11 top‐ranking conditions for years lived with disability in 2010 (Vos 2012), and are responsible for considerable loss of quality of life, employment, and increased health costs (Moore 2014a).
Fibromyalgia is common. One component of fibromyalgia, chronic widespread pain, is not only associated with other symptoms such as poor sleep, fatigue, and depression (Wolfe 2014a), but also estimated to affect 11% of the general population (Mansfield 2016). Numerous studies have investigated prevalence of fibromyalgia in different settings and countries. A review gave a global mean prevalence of potential cases of fibromyalgia of 2.7% (range 0.4% to 9.3%), with a mean in the Americas of 3.1%, in Europe of 2.5%, and in Asia of 1.7% (Queiroz 2013). Changes in diagnostic criteria do not appear to have significantly affected estimates of prevalence (Wolfe 2013). A survey using a modification of the 2010 ACR criteria found a prevalence of 1.8% in a large US survey, but 73% of these reported being given a different diagnosis by their physician (Walitt 2015). Estimates of prevalence in specific populations vary greatly, but have been reported to be as high as 9% in female textile workers in Turkey and 10% in metalworkers in Brazil (59% in those with repetitive strain injury; Queiroz 2013). When the 1990 ACR criteria are used for clinical surveys, women are more frequently diagnosed with the disorder. Using these criteria, the women to men ratio has ranged from 8:1 to 30:1 in people who were studied in clinical institutions and surveys. However, with criteria that do not use tender point examination, the sex ratio can be close to equal. The sex ratio has ranged from 4:1 to 1:1 in studies that were conducted in the general population using the research criteria for fibromyalgia (Häuser 2015; Queiroz 2013).
Fibromyalgia pain is known to be difficult to treat effectively, with only a minority of individuals experiencing a clinically relevant benefit from any intervention. A multidisciplinary approach is recommended by evidence‐based guidelines, with pharmacological treatment being combined with physical or cognitive training, or both. Interventions aim to reduce the key symptoms of fibromyalgia (pain, sleep problems, fatigue) and the associated symptoms (depression, disability) and to improve daily functioning (Eich 2012; Fitzcharles 2013). Conventional analgesics are usually not effective. Treatment is often offered with antidepressants such as serotonin and noradrenaline reuptake inhibitors (Häuser 2013b; Lunn 2014), tricyclic agents such as amitriptyline (Moore 2012a), or anticonvulsants such as gabapentin or pregabalin (Moore 2011a; Üçeyler 2013b; Wiffen 2013). The proportion of people who achieve worthwhile pain relief (typically at least 50% reduction in pain intensity) is small (Moore 2013a), and generally reaches only 10% to 15% more than with placebo, with numbers needed to treat for an additional beneficial outcome (NNTs) of about 7 to 10 (Kalso 2013; Wiffen 2013). With some treatments, people who experience a good level of pain relief also report substantial reductions in other symptoms, such as fatigue, sleep problems, depression and anxiety, and also experience significant improvement in quality of life, function and ability to work (Moore 2010b; Straube 2011). Fibromyalgia is not particularly different from other chronic pain with regard to a small proportion of trial participants having a good response to analgesic treatment (Moore 2013b).
Description of the intervention
Nonsteroidal anti‐inflammatory drugs (NSAIDs) are the most commonly used analgesics (Laine 2001). NSAIDs act by inhibiting the cyclooxygenases (COXs), which synthesise prostaglandins that are involved in inflammation and pain. The analgesic and anti‐inflammatory actions of NSAIDs are attributed to the inhibition of cyclooxygenase‐2 (COX‐2), while their adverse gastrointestinal effects are attributed to the inhibition of cyclooxygenase‐1 (COX‐1). Traditional NSAIDs such as ibuprofen are non‐selective. COX‐2‐selective NSAIDs were thus developed to reduce adverse gastrointestinal effects, but were later considered to increase the risk of myocardial infarction and stroke (Bhala 2014), and some drugs were withdrawn (EMEA 2005; FDA 2004). Whether available drugs increase the risk of cardiovascular effects is a matter of dispute, with the randomised trial evidence pointing to some increased risk for many (Bhala 2014), while large‐scale observational studies can point to no increased risk or even a reduced risk of serious harm (Mangoni 2010). The balance of benefits and risks is fine (Moore 2014b).
How the intervention might work
One current hypothesis is that damage to the peripheral nerves is followed by an inflammatory reaction that relates to increased production of prostaglandins, amplifying sodium currents and calcium influx in peripheral nociceptive neurons, and enhancing neurotransmitter release in the central nervous system (CNS) and depolarisation of second‐order nociceptive neurons (Vo 2009). Preclinical data suggest an immune pathogenesis of neuropathic pain, but clinical evidence of a central role of the immune system is less clear (Calvo 2012). NSAIDs inhibit the production of prostaglandins, and thus could lessen the peripheral and central sensory hypersensitivity that occurs with nerve injury‐associated inflammation. NSAIDs have been shown to reduce sensory hypersensitivity in animal models (Hasnie 2007; Kawakami 2002). These putative mechanisms may also have relevance to fibromyalgia because of possible CNS inflammation in people with fibromyalgia (Geiss 2012; Kadetoff 2012), and because people with neuropathic pain and fibromyalgia can experience very similar sensory phenomena (Koroschetz 2011).
Why it is important to do this review
NSAIDs are widely used in the treatment of fibromyalgia, despite weak evidence of efficacy in this condition. A telephone survey of women with fibromyalgia found that about 30% were taking NSAIDs, compared with only 8% of women without fibromyalgia (Shaver 2009). Over 3000 US adults with fibromyalgia participated in an 11‐year longitudinal study of fibromyalgia outcomes, and while NSAID use fell over the period, it was still 44% in 2010 (Wolfe 2014b), and analgesics including NSAIDs are frequently used in Germany (Häuser 2012). This is despite the recommendations of the European League against Rheumatism (EULAR), the American Pain Society (APS), and the Association of Medical Societies of Germany (AWMF) recommending that NSAIDs are not used to treat fibromyalgia (Häuser 2010). Of course, NSAIDs may be used to treat people with fibromyalgia who have concomitant musculoskeletal conditions for which NSAID use is appropriate.
We are unaware of any systematic review specifically relating to the efficacy of NSAIDs in fibromyalgia, though broader reviews have identified a general lack of evidence (Häuser 2014b), or not addressed NSAIDs (Nüesch 2013).
In addition, the standards used to assess evidence in chronic pain trials have changed substantially, with particular attention being paid to trial duration, withdrawals, and statistical imputation following withdrawal, all of which can substantially alter estimates of efficacy. The most important change is the move from using average pain scores, or average change in pain scores, to the number of people who have a large decrease in pain (by at least 50%) and who continue in treatment, ideally in trials of eight to 12 weeks or longer. Pain intensity reduction of 50% or more has been shown to correlate with improvements in comorbid symptoms, function, and health‐related quality of life generally for acute and chronic pain (Conaghan 2015; Moore 2013a; Peloso 2016), and specifically for fibromyalgia (Moore 2010c; Straube 2011). These standards are set out in the reference guide for pain studies (PaPaS 2012).
This Cochrane Review will assess evidence using methods that make both statistical and clinical sense, and will use developing criteria for what constitutes reliable evidence in chronic pain (Moore 2010a). The trials included and analysed will need to meet a minimum of reporting quality (blinding, randomisation), validity (duration, dose and timing, diagnosis, outcomes, etc), and size (ideally at least 500 participants in a comparison in which the NNT is 4 or above; Moore 1998). This approach sets high standards and marks a departure from how reviews were conducted previously. The use of unbiased trials reduces the chances of overestimating treatment effects (Mills 2015).
Objectives
To assess the analgesic efficacy, tolerability (drop‐out due to adverse events), and safety (serious adverse events) of oral nonsteroidal anti‐inflammatory drugs for fibromyalgia in adults.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) with double‐blind assessment of participant outcomes following two weeks of treatment or longer, although the emphasis of the review was on studies with a duration of eight weeks or longer. We required full journal publication, with the exception of online clinical trial results, summaries of otherwise unpublished clinical trials, and abstracts with sufficient data for analysis. We did not include short abstracts (usually meeting reports). We excluded studies that were not randomised, studies of experimental pain, case reports, and clinical observations.
Trials needed at least 10 participants per treatment arm. The protocol required a minimum of 20 participants per arm because of growing evidence of bias in small studies (Dechartres 2013; Dechartres 2014; Moore 1998). We amended this in order to review all available information where there was so little.
Types of participants
Studies included adults aged 18 years and above, diagnosed with fibromyalgia using the ACR 1990 classification criteria (Wolfe 1990), the ACR 2010 Preliminary Diagnostic Criteria (Wolfe 2010), or the modified ACR 2010 preliminary diagnostic criteria (research criteria) (Moore 2014c; Wolfe 2011). We also considered other diagnostic criteria for older studies.
Types of interventions
Oral NSAID, at any dose, administered for the relief of fibromyalgia pain, and compared to placebo or any active comparator.
Types of outcome measures
We anticipated that studies would use a variety of outcome measures, with the majority using standard subjective scales (numerical rating scale or visual analogue scale) for pain intensity or pain relief, or both. We were particularly interested in the Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT) definitions for moderate and substantial benefit in chronic pain studies (Dworkin 2008). These are defined as at least 30% pain relief over baseline (moderate), at least 50% pain relief over baseline (substantial), much or very much improved on Patient Global Impression of Change (PGIC) (moderate), and very much improved on PGIC (substantial). These dichotomous outcomes should be used where pain responses do not follow a normal (Gaussian) distribution. People with chronic pain desire high levels of pain relief, ideally more than 50%, and with pain not worse than mild (Moore 2013a; O'Brien 2010).
Primary outcomes
Participant‐reported pain relief of 50% or greater or PGIC very much improved (substantial improvement)
Participant‐reported pain relief of 30% or greater or PGIC much or very much improved (moderate improvement)
Safety: participants experiencing any serious adverse event. Serious adverse events typically include any untoward medical occurrence or effect that at any dose results in death, is life‐threatening, requires hospitalisation or prolongation of existing hospitalisation, results in persistent or significant disability or incapacity, is a congenital anomaly or birth defect, is an 'important medical event' that may jeopardise the person, or may require an intervention to prevent one of the above characteristics or consequences.
Tolerability: withdrawals due to adverse events
Secondary outcomes
Participants experiencing any adverse event
Withdrawals due to lack of efficacy and for any cause
Participant‐reported improvement of health‐related quality of life in the Fibromyalgia Impact Questionnaire (FIQ) of 14% or greater (Bennett 2009)
Specific adverse events using the Medical Dictionary for Regulatory Activities (MedDRA) classification, where reported (www.meddra.org)
Outcomes (expected to be continuous variables) relating to sleep problems, depression, anxiety, and fatigue
Search methods for identification of studies
Electronic searches
We searched the following databases, without language restrictions.
The Cochrane Central Register of Controlled Trials (CENTRAL) (via Cochrane Register of Studies Online) (6 January 2017) (Appendix 2)
MEDLINE (via Ovid) (1946 to 6 January 2017) (Appendix 3)
Embase (via Ovid) (1974 to 6 January 2017) (Appendix 4)
Oxford Pain Relief Database (Jadad 1996)
Searching other resources
We examined the bibliographies of any RCTs identified and review articles, and searched clinical trials databases (ClinicalTrials.gov (ClinicalTrials.gov) and World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (apps.who.int/trialsearch/)) to identify additional published or unpublished data. We did not routinely contact investigators or study sponsors.
Data collection and analysis
Selection of studies
We determined eligibility by reading the abstract of each study identified by the search. We eliminated studies that clearly did not satisfy the inclusion criteria, and obtained full copies of the remaining studies. Two review authors made the decisions. At least two review authors read these studies independently and reached agreement by discussion if necessary. We did not anonymise the studies before assessment. A PRISMA flow chart shows the study flow (Moher 2009).
Data extraction and management
Two review authors extracted data independently using a standard form and checked for agreement before entry into Review Manager 5 (RevMan 2014), or any other analysis tool. We included information about the pain condition and number of participants treated, drug and dosing regimen, control intervention, study design, study duration and follow‐up, analgesic outcome measures and results, withdrawals, and adverse events (participants experiencing any adverse event, specific adverse events, or a serious adverse event).
Assessment of risk of bias in included studies
We used the Oxford Quality Score as the basis for inclusion (Jadad 1996), limiting inclusion to studies that were randomised and double‐blind as a minimum.
Two review authors independently assessed risk of bias for each study, using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a), and adapted from those used by Cochrane Pregnancy and Childbirth, with any disagreements resolved by discussion.
We assessed the following for each study.
Random sequence generation (checking for possible selection bias). We assessed the method used to generate the allocation sequence as: low risk of bias (any truly random process, random number table; computer random‐number generator); unclear risk of bias (when the method used to generate the sequence was not clearly stated). We excluded studies at a high risk of bias that used a non‐random process (odd or even date of birth; hospital or clinic record number).
Allocation concealment (checking for possible selection bias). The method used to conceal allocation to interventions prior to assignment determines whether intervention allocation could have been foreseen in advance of, or during, recruitment, or changed after assignment. We assessed the methods as: low risk of bias (telephone or central randomisation; consecutively‐numbered, sealed, opaque envelopes); unclear risk of bias (when method not clearly stated). We excluded studies that did not conceal allocation and were therefore at a high risk of bias (open list).
Blinding of participants and personnel (checking for possible performance bias). We assessed the methods used to blind study participants and personnel from knowledge of which intervention a participant received. We assessed methods as: low risk of bias (study stated identical tablets; matched in appearance and smell); unclear risk of bias (study stated that it was blinded but did not provide an adequate description of how it was achieved). We excluded studies that were not double‐blind.
Blinding of outcome assessment (checking for possible detection bias). We only accepted studies where assessment of pain and symptoms such as fatigue or sleep problems was self‐reported by participants, and assessed the methods used to blind study participants and any outcome assessors from knowledge of which intervention a participant received. We assessed the methods as: low risk of bias (study had a clear statement that outcome assessors were unaware of treatment allocation, and ideally described how this was achieved); unclear risk of bias (study stated that outcome assessors were blind to treatment allocation but lacked a clear statement on how it was achieved). We excluded studies where outcome assessment was not blinded.
Incomplete outcome data (checking for possible attrition bias due to the amount, nature, and handling of incomplete outcome data). We assessed the methods used to deal with incomplete data as: low risk of bias (fewer than 10% of participants did not complete the study or used 'baseline observation carried forward' analysis, or both); unclear risk of bias (used 'last observation carried forward' (LOCF) analysis); or high risk of bias (used 'completer' analysis).
Size of study (checking for possible biases confounded by small size). We assessed studies as being at low risk of bias (200 participants or more per treatment arm); unclear risk of bias (50 to 199 participants per treatment arm); or high risk of bias (fewer than 50 participants per treatment arm).
Reporting bias due to selective outcome reporting: we checked if an a priori study protocol was available and if all outcomes of the study protocol were reported in the publications of the study. We assigned a low risk of reporting bias if the study protocol was available and all of the study's prespecified (primary and secondary) outcomes that were of interest in the review had been reported in the prespecified way, or if the study protocol was not available but it was clear that the published reports contained all expected outcomes, including those that were prespecified (convincing text of this nature may be uncommon). We assigned a high risk of reporting bias if not all of the study's prespecified primary outcomes had been reported; one or more primary outcomes was reported using measurements, analysis methods, or subsets of the data (subscales) that were not prespecified; one or more reported primary outcomes were not prespecified (unless clear justification for their reporting was provided, such as an unexpected adverse event); one or more outcomes of interest in the review was reported incompletely so that it could not be entered in a meta‐analysis; the study report did not include results for a key outcome that would be expected to have been reported for such a study.
Group similarity at baseline (selection bias): we assessed similarity of the study groups at baseline for the most important prognostic clinical and demographic indicators. We assigned a low risk of bias if groups were similar at baseline for demographic factors, value of main outcome measure(s), baseline symptoms relevant to main outcomes, and important prognostic factors. We assigned a high risk of bias if groups were not similar at baseline for demographic factors, value of main outcome measure(s), and important prognostic factors.
Measures of treatment effect
We used dichotomous data to calculate risk difference (RD) with 95% confidence intervals (CI) using a fixed‐effect model. We calculated NNTs as the reciprocal of the absolute risk difference (McQuay 1998). For unwanted effects, the number needed to treat for an additional beneficial outcome becomes the number needed to treat for an additional harmful outcome (NNH) and is calculated in the same manner.
We used the following terms to describe adverse outcomes in terms of harm or prevention of harm.
When significantly fewer adverse outcomes occurred with treatment than with control (placebo or active), we used the term the'number needed to treat to prevent one event' (NNTp).
When significantly more adverse outcomes occurred with treatment compared with control (placebo or active), we used the term the 'number needed to treat for an additional harmful outcome or to cause one event' (NNH).
For continuous data, we calculated standardised mean differences (SMDs) with 95% CIs using a random‐effects model because we anticipated clinical heterogeneity. We planned to use Cohen's categories to evaluate the magnitude of the effect size, calculated by SMD, with Hedges' g of 0.2 = small, 0.5 = medium, and 0.8 = large (Cohen 1988). We would regard g less than 0.2 to be a 'not substantial' effect size. We assumed a minimally important difference if Hedges' g was 0.2 or greater (Fayers 2014).
The threshold for 'clinically relevant benefit' or 'clinically relevant harm' was set for categorical variables by an absolute risk reduction or increase of 10% or greater corresponding an NNT or NNH of 10 or less (Moore 2008). The threshold 'clinically relevant benefit' was set for continuous variables by an effect size more than 0.2 (Fayers 2014).
Unit of analysis issues
The unit of analysis was the individual participant. If two active treatment arms were separately compared with a single control arm in an analysis, we would split the total number in the control arm between the active arms to avoid double counting.
Dealing with missing data
We used an intention‐to‐treat (ITT) analysis where the ITT population consisted of participants who were randomised, took at least one dose of the assigned study medication, and provided at least one post‐baseline assessment. We would assign missing participants zero improvement.
Assessment of heterogeneity
We dealt with clinical heterogeneity by combining studies that examined similar diagnostic criteria for fibromyalgia. We planned to assess statistical heterogeneity visually (L'Abbé 1987), and with the I2 statistic (Higgins 2003). When the I2 value was greater than 50%, we would consider possible reasons for this.
Assessment of reporting biases
The aim of this review was to use dichotomous pain outcomes of known utility and of value to people with fibromyalgia (Hoffman 2010; Moore 2010b; Moore 2010c; Moore 2010d; Moore 2013a). The review did not depend on what the authors of the original studies chose to report or not, though clearly difficulties arose in studies that did not report any dichotomous results. We extracted and used continuous data for pain, which probably reflect efficacy and utility poorly, but which may be useful for illustrative purposes.
We planned to assess publication bias using a method designed to detect the amount of unpublished data with a null effect required to make any result for pain clinically irrelevant (usually taken to mean an NNT of 10 or higher; Moore 2008).
Data synthesis
We planned to use a fixed‐effect model for meta‐analysis because we did not anticipate considerable clinical homogeneity. We would use a random‐effects model for meta‐analysis if there was significant clinical heterogeneity and it was considered appropriate to combine studies.
Quality of the evidence
We used the GRADE approach to assess the quality of evidence related to each of the key outcomes, and report our judgement on the quality of the evidence in Table 1 (Schünemann 2011a; Appendix 5).
We paid particular attention to inconsistency, where point estimates varied widely across studies or confidence intervals of studies showed minimal or no overlap (Guyatt 2011), and potential for publication bias, based on the amount of unpublished data required to make the result clinically irrelevant (Moore 2008).
In addition, there may be circumstances where the overall rating for a particular outcome needs to be adjusted as recommended by GRADE guidelines (Guyatt 2013a). For example, if there were so few data that the results were highly susceptible to the random play of chance, or if a study used LOCF imputation in circumstances where there were substantial differences in adverse event withdrawals, one would have no confidence in the result, and would need to downgrade the quality of the evidence by 3 levels, to very low quality. In circumstances where there were no data reported for an outcome, we would report the level of evidence as very low quality (Guyatt 2013b).
'Summary of findings' table
We have included a 'Summary of findings' table as set out in the PaPaS author guide (PaPaS 2012), and recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2011b). The table includes, where possible, outcomes equivalent to moderate or substantial benefit of at least 30% and at least 50% pain intensity reduction, PGIC (possibly at least substantial improvement and at least moderate improvement) (Dworkin 2008), withdrawals due to lack of efficacy, withdrawals due to adverse events, serious adverse events, and death (a particular serious adverse event).
For the 'Summary of findings' table we used the following descriptors for levels of evidence (EPOC 2015):
High: this research provides a very good indication of the likely effect. The likelihood that the effect will be substantially differenta is low.
Moderate: this research provides a good indication of the likely effect. The likelihood that the effect will be substantially differenta is moderate.
Low: this research provides some indication of the likely effect. However, the likelihood that it will be substantially differenta is high.
Very low: tThis research does not provide a reliable indication of the likely effect. The likelihood that the effect will be substantially differenta is very high.
aSubstantially different: a large enough difference that it might affect a decision.
Subgroup analysis and investigation of heterogeneity
Possible issues for subgroup analysis were drug, dose, and formulation. A minimum of two studies and 200 participants would have to have been available for any subgroup analysis.
We also planned to consider a subgroup analysis according to the inclusion and exclusion criteria of included studies, because a major limitation of most drug trials in fibromyalgia is the exclusion of people with mental disorders and relevant medical diseases.
Sensitivity analysis
We planned no sensitivity analysis because the evidence base was known to be too small to allow reliable analysis.
Results
Description of studies
Results of the search
We found 10 studies in our electronic searches for which we obtained full‐text articles for detailed assessment. Of these, we included six and excluded four (Figure 1).
1.
Study flow diagram
Included studies
We included six studies in our review as randomised, double‐blind studies in suitably characterised fibromyalgia (Goldenberg 1986; Kravitz 1994; Mahagna 2016; Quijada‐Carrera 1996; Russell 1991; Yunus 1989). In these studies the mean age of participants was between 39 and 50 years, and 89% to 100% were women. The initial pain intensity was recorded as between 60% and 75% of the maximum on the scale (equivalent to 6.0 to 7.5 on a 0 to 10 numerical rating scale (NRS)).
Participants excluded from the studies were those typical of chronic pain studies with NSAIDs ‐ pregnancy, breast feeding, previous peptic ulcer or bleeding, sensitivity or allergy, or serious medical conditions or (in Kravitz 1994) major psychiatric disorder. Of note, four studies explicitly excluded participants with inflammatory rheumatic diseases (Kravitz 1994; Mahagna 2016; Quijada‐Carrera 1996; Russell 1991) and one other study (Yunus 1989) probably did so. Most probably, four studies excluded participants with osteoarthritis (Mahagna 2016; Quijada‐Carrera 1996; Russell 1991; Yunus 1989).
Diagnostic criteria used were those of Yunus 1981 (Goldenberg 1986; Kravitz 1994; Yunus 1989), ACR 1990 (Wolfe 1990) (Mahagna 2016; Quijada‐Carrera 1996), and Russell 1986 (Russell 1991); Russell 1991 noted that almost all included participants also met the diagnostic criteria of ACR 1990 and Yunus 1981. Studies were all conducted in an outpatient setting, in Israel, Spain, and the USA. All the studies reported some degree of industry funding.
NSAIDs tested were etoricoxib 90 mg daily (Mahagna 2016), ibuprofen 2400 mg daily (Kravitz 1994; Russell 1991; Yunus 1989), naproxen 1000 mg daily (Goldenberg 1986), and tenoxicam 20 mg daily (Quijada‐Carrera 1996). In these parallel‐group studies, 146 participants received NSAID and 146 placebo. The duration of treatment in the double‐blind phase varied; three weeks (Yunus 1989), five weeks (Kravitz 1994), six weeks (Goldenberg 1986; Mahagna 2016; Russell 1991), and eight weeks (Quijada‐Carrera 1996). All NSAIDs and analgesics were discontinued between three days and three weeks before the initial visit.
Data extracted from the six included studies are in Appendix 6.
Excluded studies
We excluded four full‐text articles because they were not double‐blind (Fossaluzza 1992), or because the condition studied was not fibromyalgia (Donald 1980; Le Gallez 1988; Schorn 1986).
Risk of bias in included studies
Oxford Quality Scores were 3/5 for four studies and 4/5 for two studies. Results for risk of bias are shown in Figure 2.
2.
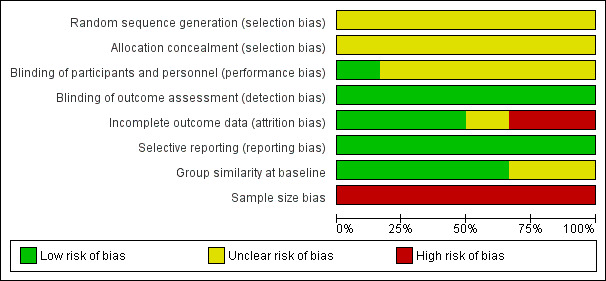
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
Allocation
No study adequately described the method of allocation or allocation concealment.
Blinding
Only one study gave details to indicate that placebo and active drugs were matched (Yunus 1989). Participants reported their own pain scores in all studies, and these were consequently assessed as low risk of bias for efficacy, but their assessment of adverse events was generally unclear.
Incomplete outcome data
For three studies we considered risk of bias low, because data on all participants seemed sensibly reported (Goldenberg 1986; Kravitz 1994; Yunus 1989). For one, the use of LOCF imputation meant that we judged this unclear (Quijada‐Carrera 1996), and for two we judged the risk of bias high because most secondary outcomes were not reported or because the results reported were of a completer analysis (Mahagna 2016; Russell 1991).
Selective reporting
We judged selective reporting as low risk, as our interest was not what was reported, but what outcomes are important to participants with chronic pain, including fibromyalgia (Moore 2013a).
Other potential sources of bias
Groups were generally similar at baseline, or this was not reported in sufficient detail. In each study the group size was below 50 participants in each treatment arm, leading to a judgement of high risk of bias in all studies.
Effects of interventions
See: Table 1
No study reported any significant efficacy difference between NSAID and placebo on any measure. Few reported the measures of interest. Table 1 summarises the results.
Pain relief of 50% or greater or PGIC very much improved (substantial improvement)
This was reported by two studies (Mahagna 2016; Quijada‐Carrera 1996), though for one we chose to interpret a reported clinically significant improvement as substantial improvement, as the study also reported 25% pain reduction in a larger number of participants (Quijada‐Carrera 1996).
The proportion of participants with substantial improvement at study end with NSAID was 11% (8/73).
The proportion of participants with substantial improvement at study end with placebo was 18% (13/73).
The RD for NSAID compared with placebo was ‐0.07 (95% CI ‐0.18 to 0.04) (Analysis 1.1).
1.1. Analysis.
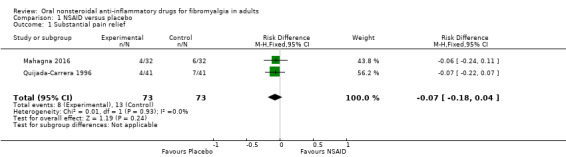
Comparison 1 NSAID versus placebo, Outcome 1 Substantial pain relief.
We downgraded the evidence for this outcome by three levels to very low quality because of the small size of studies and pooled data set, and because there were only 21 actual events in the analysis.
Pain relief of 30% or greater or PGIC much or very much improved (moderate improvement)
This was reported by three studies (Mahagna 2016; Quijada‐Carrera 1996; Yunus 1989), and we chose to interpret as moderate improvement a 25% pain reduction (Quijada‐Carrera 1996).
The proportion of participants with moderate improvement at study end with NSAID was 22% (21/95).
The proportion of participants with moderate improvement at study end with placebo was 26% (25/97).
The RD for NSAID compared with placebo was ‐0.04 (95% CI ‐0.16 to 0.08) (Analysis 1.2).
1.2. Analysis.
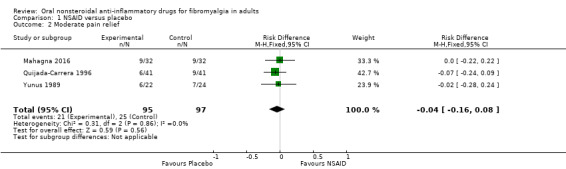
Comparison 1 NSAID versus placebo, Outcome 2 Moderate pain relief.
We downgraded the evidence for this outcome by three levels to very low quality because of the small size of studies and pooled data set, and because there were only 46 actual events in the analysis.
Serious adverse events
No serious adverse events were reported in any study, apart from a single case of sedation, memory problems, and impaired mentation (Kravitz 1994). Whether this should be classified as serious is unclear from the report. It was considered a typical benzodiazepine adverse event in a study where benzodiazepines were also being used. This participant was not taking benzodiazepines, but the response diminished when the placebo benzodiazepine dose was reduced.
No trial mentioned any deaths occurring.
We assessed the quality of the evidence as very low quality because of the very small number of events, or no events in the case of death.
Withdrawals due to adverse events
Adverse event withdrawals were reported in all studies. Two did not report withdrawals by treatment group (Kravitz 1994; Russell 1991), but four did (Goldenberg 1986; Mahagna 2016; Quijada‐Carrera 1996; Yunus 1989).
The proportion of participants withdrawing because of an adverse event with NSAID was 5% (6/114).
The proportion of participants withdrawing because of an adverse event with placebo was 2% (2/116).
The RD for NSAID compared with placebo was 0.04 (95% CI ‐0.02 to 0.09) (Analysis 1.3).
1.3. Analysis.
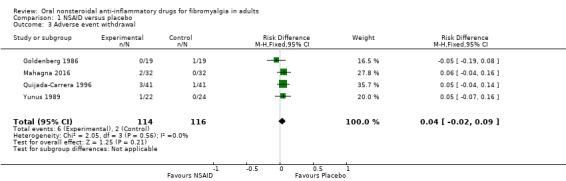
Comparison 1 NSAID versus placebo, Outcome 3 Adverse event withdrawal.
We downgraded the evidence for this outcome by three levels to very low quality because of the small size of studies and pooled data set, and because there were only eight actual events in the analysis.
Participants experiencing any adverse event
This outcome was reported by four studies (Goldenberg 1986; Mahagna 2016; Quijada‐Carrera 1996; Yunus 1989).
The proportion of participants with at least one adverse event with NSAID was 31% (35/114); range 11% to 44%.
The proportion of participants with at least one adverse event with placebo was 22% (26/116); range 11% to 41%.
The RD for NSAID compared with placebo was 0.08 (95% CI ‐0.03 to 0.19) (Analysis 1.4).
1.4. Analysis.
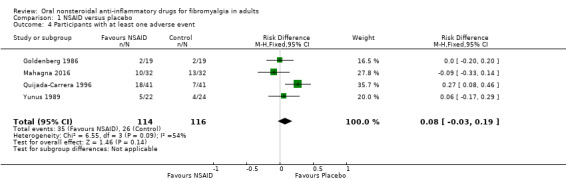
Comparison 1 NSAID versus placebo, Outcome 4 Participants with at least one adverse event.
We downgraded the evidence for this outcome by three levels to very low quality because of the small size of studies and pooled data set, and because there were only 61 actual events in the analysis.
Withdrawals due to lack of efficacy and for any cause
Lack of efficacy withdrawal was reported by treatment group in only two studies (Mahagna 2016; Quijada‐Carrera 1996). In the former there were no lack of efficacy withdrawals in either group, and in the latter there were 3/41 with tenoxicam and 3/41 with placebo.
Withdrawal for any cause was reported by treatment group in three studies (Mahagna 2016; Quijada‐Carrera 1996; Yunus 1989).
The proportion of participants withdrawing for any cause with NSAID was 23% (22/95); range 9% to 41%.
The proportion of participants withdrawing for any cause with placebo was 20% (19/97); range 6% to 37%.
The RD for NSAID compared with placebo was 0.03 (95% CI ‐0.07 to 0.14) (Analysis 1.5).
1.5. Analysis.
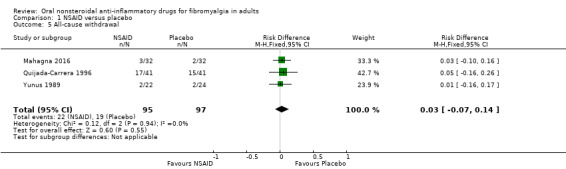
Comparison 1 NSAID versus placebo, Outcome 5 All‐cause withdrawal.
We downgraded the evidence for this outcome by three levels to very low quality because of the small size of studies and pooled data set, and because there were only 41 actual events in the analysis. There was also inconsistency between studies, with the bulk of the withdrawals in one of the three studies (32/41 withdrawals), despite there being no statistical heterogeneity (I2 = 0).
Participant‐reported improvement of health‐related quality of life
Although most of the studies had some measures of health‐related quality of life, fibromyalgia impact, or other outcomes, none reported the outcomes beyond saying that there was no or little difference between the treatment groups.
Specific adverse events
These were not reported in sufficient detail or consistency to be amenable to analysis.
Outcomes (expected to be continuous variables)
Outcomes (expected to be continuous variables) relating to sleep problems, depression, anxiety, and fatigue were not reported.
Discussion
Summary of main results
Participants in these studies typically had moderate or severe pain of fibromyalgia, often long‐lasting, and with an initial average pain score of about 7/10 at the start of the studies. A pain score of 7/10 would be regarded as severe pain. The primary pain outcomes of this review were 'substantial' pain relief, ideally a reduction in pain intensity by 50% or more, and 'moderate' pain relief, a reduction by 30% or more, which was sustained over the duration of the trial, typically three months. These outcomes are judged as desirable by people with pain (Moore 2013a).
Some, but not all, of the six small studies reported these pain outcomes or something very similar to them. Although the number of participants providing information for these and other outcomes barely amounted to 200 in total, we performed analyses for a number of the outcomes. NSAIDs proved no better than placebo in producing pain relief. While there was no difference in a number of adverse event measures and withdrawals, the small amount of information available in these studies was quite inadequate to examine rare but serious adverse events of NSAIDs.
The bottom line is that there is no evidence that NSAIDs are beneficial for pain reduction or any other outcome in fibromyalgia, despite being used commonly to treat the condition (Häuser 2012; Shaver 2009; Wolfe 2014b).
Small studies of modest quality tend to overestimate effects of treatment ((Dechartres 2013; Dechartres 2014; Moore 1998; Nüesch 2013), and to have a positive bias towards the experimental intervention. No such benefits were seen. Consequently, NSAIDs cannot be regarded as useful for treating fibromyalgia.
Overall completeness and applicability of evidence
The demographic data of the study participants were typical of people with fibromyalgia, being mainly women in their 50s, with a reliable diagnosis of fibromyalgia, with moderate or severe pain and functional disability. However, participants with inflammatory rheumatic diseases and mental disorders, which are frequently associated with fibromyalgia, were excluded by the studies. The study results cannot therefore be applied to many people with fibromyalgia in routine clinical care.
Quality of the evidence
All of the studies in the review were described as randomised and double‐blind, had Oxford Quality Scores of 3/5 or above, and were at high risk of bias only for their small size. A number of quality measures were inadequately described, however, and some risk of bias cannot be excluded. Studies generally lasted for four weeks or longer, and while the longest duration was eight weeks, the studies would all be considered relatively short term for a chronic condition. Diagnostic criteria for inclusion were reasonable, using appropriate definitions and duration of pain. Participants had to have had initial pain of at least moderate intensity, meaning that studies would be sensitive enough to measure any analgesic effect.
Sample sizes were small, increasing the risk of random chance effects and small study bias. About half of the studies reported clinically useful outcomes, so that pooled analyses were typically on only about 200 participants, where chance effects are possible (Moore 1998). In view of the small sample sizes, as well as uncertainties for other possible risks of bias, we chose to downgrade the quality of the evidence by three levels, to very low quality. Very low quality means that this research does not provide a reliable indication of the likely effect. The likelihood that the effect could be substantially different is very high.
Potential biases in the review process
We know of no potential biases in the review process. We had planned to calculate the number of participants who would need to be in trials with zero effect (risk ratio of 1.0) needed for the point estimate of the NNT to increase beyond a clinically useful level (Moore 2008), but this method is not applicable with low effect sizes.
Agreements and disagreements with other studies or reviews
Previous systematic reviews have examined the efficacy of NSAIDs in fibromyalgia. Four of the six studies in this review were included in a previous broad review and guidance, with a negative recommendation (Sommer 2012b). Other reviews have identified a general lack of evidence (Häuser 2014b), or have not addressed NSAIDs (Nüesch 2013). NSAIDs are either ignored or not recommended by guidelines for treating fibromyalgia (Häuser 2012; Macfarlane 2017; Sommer 2012b).
A separate Cochrane Review is investigating the use of combination pharmacotherapy in fibromyalgia (Gilron 2013). It is likely to support a negative view of NSAIDs in fibromyalgia, based on results of trials included in this review (Goldenberg 1986; Russell 1991).
Authors' conclusions
Implications for practice.
For people with pain in fibromyalgia
There is no evidence to support the suggestion that nonsteroidal anti‐inflammatory drugs (NSAIDs) have any efficacy in relieving pain or other symptoms in people with fibromyalgia. There is limited evidence to indicate that NSAIDs are without any effect. Some people with fibromyalgia may have other painful conditions such inflammatory rheumatic diseases or osteoarthritis for which an NSAID can be useful.
For clinicians
There is no evidence to support the suggestion that NSAIDs have any efficacy in relieving pain in people with fibromyalgia. There is limited evidence to indicate that NSAIDs are without any effect. Any biases in the small studies we identified would be expected to work to increase estimates of efficacy, and the fact that no efficacy was found strengthens the conclusions that NSAIDs are ineffective. Some people with fibromyalgia may have other painful conditions for which an NSAID is indicated.
For policy makers
There is no evidence to support the suggestion that NSAIDs have any efficacy in relieving pain in people with fibromyalgia. There is limited evidence to indicate that NSAIDs are without any effect. Any biases in the small studies we have would be expected to work to increase estimates of efficacy, and the fact that no efficacy was found strengthens the conclusions that NSAIDs are ineffective. Some people with fibromyalgia may have other painful conditions for which an NSAID can be useful, such as concomitant inflammatory rheumatic diseases or osteoarthritis.
For funders
There is no evidence to support the suggestion that NSAIDs have any efficacy in relieving pain in people with fibromyalgia. There is limited evidence to indicate that NSAIDs are without any effect. In the absence of any additional supporting evidence, NSAIDs should probably not be recommended, except at the discretion of a pain specialist with particular expertise in fibromyalgia.
Implications for research.
General
Although the amount of clinical trial evidence in this review is limited, the absence of any signal for efficacy calls into question the ethics and value of any new study for people with fibromyalgia without concomitant inflammatory rheumatic disease osteoarthritis. The design of studies in fibromyalgia, and the outcomes, are well understood; but as any NSAID effect is likely to be small, an enriched‐enrolment randomised‐withdrawal (EERW) design might provide the highest sensitivity to detect a signal (Moore 2015).
Design
The design of trials is generally adequate, but reporting of clinically relevant outcomes using appropriate imputation for withdrawal would improve the relevance of the findings for clinical practice. The use of EERW designs for comparison with classic trial designs indicates that good quality EERW designs of long duration may be appropriate for fibromyalgia.
Measurement (endpoints)
Assessment of fibromyalgia symptoms should be based on dichotomous participant‐reported outcomes of proven clinical utility; that usually means pain intensity reduction of at least 50% (or possibly 30%) without withdrawal from treatment, typically at 12 weeks after dosing stabilisation. The end point most usefully used in EERW trials, of maintenance of therapeutic response without withdrawal, might be used as a primary outcome in future trials with that design.
Comparison between active treatments
Studies involving other treatments including non‐pharmacological interventions may be valuable in the context of fibromyalgia. A multi‐component approach reflects current practice.
What's new
| Date | Event | Description |
|---|---|---|
| 18 February 2020 | Amended | Clarification added to Declarations of interest. |
| 28 March 2017 | Review declared as stable | See Published notes. |
History
Protocol first published: Issue 8, 2016 Review first published: Issue 3, 2017
| Date | Event | Description |
|---|---|---|
| 28 May 2019 | Amended | Contact details updated. |
Notes
A new search within two years is not likely to identify any potentially relevant studies likely to change the conclusions. Therefore, following discussion with the authors and editors, this review has now been stabilised until 2022, at which point we will assess the review for updating. If appropriate, we will update the review before this date if new evidence likely to change the conclusions is published, or if standards change substantially which necessitate major revisions.
Acknowledgements
We developed the protocol in collaboration with Cochrane Musculoskeletal, Cochrane Neuromuscular and Cochrane Pain, Palliative and Supportive Care, and followed an agreed template for fibromyalgia. The editorial process was managed by Cochrane Pain, Palliative and Supportive Care.
Institutional support was provided by the Oxford Pain Relief Trust.
The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Pain, Palliative and Supportive Care Review Group. Disclaimer: the views and opinions expressed herein are those of the review authors and do not necessarily reflect those of the NIHR, National Health Service (NHS), or the Department of Health.
Appendices
Appendix 1. Methodological considerations for chronic pain
There have been several changes in how the efficacy of conventional and unconventional treatments is assessed in chronic painful conditions. The outcomes are now better defined, particularly with new criteria for what constitutes moderate or substantial benefit (Dworkin 2008); older trials may only report participants with 'any improvement'. Newer trials tend to be larger, avoiding problems from the random play of chance. Newer trials also tend to be of longer duration, up to 12 weeks, and longer trials provide a more rigorous and valid assessment of efficacy in chronic conditions. New standards have evolved for assessing efficacy in neuropathic pain, and we are now applying stricter criteria for the inclusion of trials and assessment of outcomes, and are more aware of problems that may affect our overall assessment. To summarise some of the recent insights that must be considered in this new review:
Pain results tend to have a U‐shaped distribution rather than a bell‐shaped distribution. This is true in acute pain (Moore 2011b; Moore 2011c), back pain (Moore 2010d), and arthritis (Moore 2010b), as well as in fibromyalgia (Straube 2010); in all cases average results usually describe the experience of almost no‐one in the trial. Data expressed as averages are potentially misleading, unless they can be proven to be suitable.
As a consequence, we have to depend on dichotomous results (the individual either has or does not have the outcome) usually from pain changes or patient global assessments. The Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT) group has helped with their definitions of minimal, moderate, and substantial improvement (Dworkin 2008). In arthritis, trials of less than 12 weeks duration, and especially those shorter than eight weeks, overestimate the effect of treatment (Moore 2010b); the effect is particularly strong for less effective analgesics, and this may also be relevant in neuropathic‐type pain.
The proportion of patients with at least moderate benefit can be small, even with an effective medicine, falling from 60% with an effective medicine in arthritis to 30% in fibromyalgia (Moore 2009; Moore 2010b; Moore 2013b; Moore 2014c; Straube 2008; Sultan 2008). A Cochrane review of pregabalin in neuropathic pain and fibromyalgia demonstrated different response rates for different types of chronic pain (higher in diabetic neuropathy and postherpetic neuralgia and lower in central pain and fibromyalgia) (Moore 2009). This indicates that different neuropathic pain conditions should be treated separately from one another, and that pooling should not be done unless there are good grounds for doing so.
Individual patient analyses indicate that patients who get good pain relief (moderate or better) have major benefits in many other outcomes, affecting quality of life in a significant way (Moore 2010c; Moore 2014a).
Imputation methods such as last observation carried forward (LOCF), used when participants withdraw from clinical trials, can overstate drug efficacy especially when adverse event withdrawals with drug are greater than those with placebo (Moore 2012b).
Appendix 2. Search strategy for CENTRAL (via Cochrane Register of Studies Online)
MESH DESCRIPTOR Fibromyalgia EXPLODE ALL TREES (622)
(fibromyalgi* or fibrosti* or FM or FMS):TI,AB,KY (2258)
1 OR 2 (2258)
MESH DESCRIPTOR Anti‐Inflammatory Agents, Non‐Steroidal EXPLODE ALL TREES (14842)
("non‐steroidal anti‐inflammatory drug*"):TI,AB,KY (1304)
("nonsteroidal anti‐inflammatory drug*"):TI,AB,KY (1392)
("nonsteroidal antiinflammatory drug*"):TI,AB,KY (520)
aceclofenac:TI,AB,KY (111)
acemetacin:TI,AB,KY (92)
MESH DESCRIPTOR Apazone (29)
azapropazone:TI,AB,KY (48)
celecoxib:TI,AB,KY (1023)
MESH DESCRIPTOR Ketoprofen (416)
ketoprofen:TI,AB,KY (901)
dexketoprofen:TI,AB,KY (131)
MESH DESCRIPTOR Diclofenac (1422)
diclofenac:TI,AB,KY (3552)
MESH DESCRIPTOR Etodolac (83)
etodolac:TI,AB,KY (189)
fenbufen:TI,AB,KY (63)
MESH DESCRIPTOR Fenoprofen (36)
fenoprofen:TI,AB,KY (86)
MESH DESCRIPTOR Flurbiprofen (364)
flurbiprofen:TI,AB,KY (683)
MESH DESCRIPTOR Ibuprofen (1195)
ibuprofen:TI,AB,KY (2690)
MESH DESCRIPTOR Indomethacin (1391)
indomet?acin:TI,AB,KY (2660)
MESH DESCRIPTOR Mefenamic Acid (111)
(mefenamic acid):TI,AB,KY (256)
meloxicam:TI,AB,KY (289)
nabumetone:TI,AB,KY (150)
MESH DESCRIPTOR Naproxen (830)
naproxen:TI,AB,KY (1631)
MESH DESCRIPTOR Piroxicam (579)
piroxicam:TI,AB,KY (1048)
MESH DESCRIPTOR Sulindac (139)
sulindac:TI,AB,KY (283)
tenoxicam:TI,AB,KY (355)
(tiaprofenic acid):TI,AB,KY (122)
4 OR 5 OR 6 OR 7 OR 8 OR 9 OR 10 OR 11 OR 12 OR 13 OR 14 OR 15 OR 16 OR 17 OR 18 OR 19 OR 20 OR 21 OR 22 OR 23 OR 24 OR 25 OR 26 OR 27 OR 28 OR 29 OR 30 OR 31 OR 32 OR 33 OR 34 OR 35 OR 36 OR 37 OR 38 OR 39 OR 40 (22510)
3 AND 41 (42)
Appendix 3. Search strategy for MEDLINE (via Ovid)
Fibromyalgia/ (7186)
exp Somatosensory disorders/ (18558)
(fibromyalgi* or fibrosti* or FM or FMS).mp. (23212)
1 or 2 or 3 or 4 (41566)
exp Anti‐Inflammatory Agents, Non‐Steroidal/ (177035)
"non‐steroidal anti‐inflammatory drug*".tw. (10458)
"nonsteroidal anti‐inflammatory drug*".tw. (11140)
"nonsteroidal antiinflammatory drug*".tw. (3497)
aceclofenac.tw. (270)
acemetacin.tw. (116)
Apazone/ (168)
azapropazone.tw. (212)
celecoxib.tw. (4506)
Ketoprofen/ (2434)
ketoprofen.tw. (3062)
dexketoprofen.tw. (152)
Diclofenac/ (6650)
diclofenac.tw. (8328)
Etodolac/ (445)
etodolac.tw. (551)
etoricoxib.tw. (491)
fenbufen.tw. (251)
Fenoprofen/ (279)
fenoprofen.tw. (383)
Flurbiprofen/ (1728)
flurbiprofen.tw. (2108)
Ibuprofen/ (7447)
Ibuprofen.tw. (9841)
Indomethacin/ (27581)
Indomet?acin.tw. (33785)
Mefenamic Acid/ (981)
mefenamic acid.tw. (1062)
meloxicam.tw. (1453)
nabumetone.tw. (394)
Naproxen/ (3667)
naproxen.tw. (4872)
Piroxicam/ (2620)
piroxicam.tw. (2610)
Sulindac/ (1485)
sulindac.tw. (1857)
tenoxicam.tw. (523)
tiaprofenic acid.tw. (317)
5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 (198876)
randomized controlled trial.pt. (430183)
controlled clinical trial.pt. (88793)
randomized.ab. (293432)
random*.tw (767209)
double‐blind*.ab. (111761)
44 or 45 or 46 or 47 or 48 (940492)
4 and 43 and 49 (125)
Appendix 4. Search strategy for Embase (via Ovid)
Fibromyalgia/ (15958)
exp Somatosensory disorders/ (76765)
(fibromyalgi* or fibrosti* or FM or FMS).mp. (36691)
1 or 2 or 3 or 4 (112986)
exp Anti‐Inflammatory Agents, Non‐Steroidal/ (498480)
"non‐steroidal anti‐inflammatory drug*".tw. (15394)
"nonsteroidal anti‐inflammatory drug*".tw. (14281)
"nonsteroidal antiinflammatory drug*".tw. (4445)
aceclofenac.tw. (806)
acemetacin.tw. (174)
Apazone/ (1164)
azapropazone.tw. (279)
celecoxib.tw. (6965)
Ketoprofen/ (11546)
ketoprofen.tw. (4612)
dexketoprofen.tw. (329)
Diclofenac/ (33484)
diclofenac.tw. (13906)
Etodolac/ (2451)
etodolac.tw. (840)
etoricoxib.tw. (937)
fenbufen.tw. (353)
Fenoprofen/ (2593)
fenoprofen.tw. (523)
Flurbiprofen/ (6992)
flurbiprofen.tw. (2676)
Ibuprofen/ (41477)
Ibuprofen.tw. (15030)
Indomethacin/ (73968)
Indomet?acin.tw. (40751)
Mefenamic Acid/ (5267)
mefenamic acid.tw. (1432)
meloxicam.tw. (2192)
nabumetone.tw. (553)
Naproxen/ (23300)
naproxen.tw. (7342)
Piroxicam/ (10565)
piroxicam.tw. (3878)
Sulindac/ (6432)
sulindac.tw. (2166)
tenoxicam.tw. (809)
tiaprofenic acid.tw. (477)
5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 (509009)
Randomized Controlled Trial/ (419274)
Double‐blind procedure/ (133950)
Random allocation/ (63934)
(random* or factorial* or placebo* or (doubl* adj blind*)).tw. (1255783)
44 or 45 or 46 or 47 (1361319)
4 and 43 and 48 (1030)
Appendix 5. GRADE: criteria for assigning grade of evidence
The GRADE system uses the following criteria for assigning a quality level to a body of evidence (Chapter 12, Schünemann 2011a).
High: randomised trials; or double‐upgraded observational studies
Moderate: downgraded randomised trials; or upgraded observational studies
Low: double‐downgraded randomised trials; or observational studies
Very low: triple‐downgraded randomised trials; or downgraded observational studies; or case series/case reports
Factors that may decrease the quality level of a body of evidence are:
limitations in the design and implementation of available studies suggesting high likelihood of bias;
indirectness of evidence (indirect population, intervention, control, outcomes);
unexplained heterogeneity or inconsistency of results (including problems with subgroup analyses);
imprecision of results (wide confidence intervals);
high probability of publication bias.
Factors that may increase the quality level of a body of evidence are:
large magnitude of effect;
all plausible confounding would reduce a demonstrated effect or suggest a spurious effect when results show no effect;
dose‐response gradient.
Appendix 6. Data extracted from individual studies
| Study | Efficacy | Participants with at least one adverse event | Serious adverse events | Withdrawals |
| Goldenberg 1986 USA | No significant difference between naproxen and placebo on any measure | Naproxen 2/19 Placebo 2/19 Each had 1 dyspepsia, and 1 diarrhoea | None reported | 1 naproxen (not returned) 1 placebo (epigastric distress) |
| Kravitz 1994 USA | No significant difference between naproxen and placebo on any measure | AE data not reported in straightforward way | 1 ibuprofen sedation, memory problems, impaired mentation | 1 ibuprofen due to pre‐existing skin problem 14 did not complete all five weeks due to lack of efficacy, but data not given by treatment |
| Mahagna 2016 NCT00755521 Israel | 50% pain intensity reduction etoricoxib 4/32 placebo 6/32 30% pain intensity reduction etoricoxib 9/32 placebo 9/32 No significant difference FIQ No significant difference in SF‐36 No significant difference in HARS or HADS scales | Total AE etoricoxib 10/32 placebo 13/32 | None | All cause etoricoxib 3/32 placebo 2/32 AE withdrawal etoricoxib 2/32 placebo 0/32 LoE withdrawal etoricoxib 0/32 placebo 0/32 |
| Quijada‐Carrera 1996 Spain | Clinically significant improvement tenoxicam 4/41 placebo 7/41 25% reduction in pain tenoxicam 6/41 placebo 9/41 No difference in number of tender points, sleep quality, or morning stiffness | At least 1 AE tenoxicam 18/41 placebo 7/41 | None reported | All cause tenoxicam 17/41 placebo 15/41 AE withdrawal tenoxicam 3/41 placebo 1/41 LoE withdrawal tenoxicam 4/41 placebo 3/41 |
| Russell 1991 USA | No significant difference reported between ibuprofen and placebo for any outcome | No data | None reported | AE withdrawals ibuprofen 0 placebo 4 |
| Yunus 1989 USA | Mean pain score 2.4 in both groups at 3 weeks No significant difference for sleep, morning fatigue, stiffness, or other symptoms Moderate or complete pain relief at 3 weeks ibuprofen 6/22 placebo 7/24 | At least one AE ibuprofen 5/22 placebo 4/24 | None reported | All cause withdrawal by week 3 ibuprofen 2/22 placebo 2/24 AE withdrawal ibuprofen 1/22 placebo 0/24 |
AE: adverse event; FIQ: Fibromyalgia Impact Questionnaire; HADS: Hospital Anxietry and Depression (Scale); HARS: Hamilton Anxiety Rating Scale; LoE: lack of efficacy
Data and analyses
Comparison 1. NSAID versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Substantial pain relief | 2 | 146 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.07 [‐0.18, 0.04] |
| 2 Moderate pain relief | 3 | 192 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.04 [‐0.16, 0.08] |
| 3 Adverse event withdrawal | 4 | 230 | Risk Difference (M‐H, Fixed, 95% CI) | 0.04 [‐0.02, 0.09] |
| 4 Participants with at least one adverse event | 4 | 230 | Risk Difference (M‐H, Fixed, 95% CI) | 0.08 [‐0.03, 0.19] |
| 5 All‐cause withdrawal | 3 | 192 | Risk Difference (M‐H, Fixed, 95% CI) | 0.03 [‐0.07, 0.14] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Goldenberg 1986.
| Methods | Randomised, double‐blind, placebo‐controlled, parallel‐group Probably single centre in USA Duration of screening and washout not reported, 6‐week parallel group phase No obvious imputation for withdrawals if participants completed 2 visits No NSAID or other drug for 3 days before initial visit Minimum pain intensity 4/10 or higher |
|
| Participants |
Naproxen: N = 19 Placebo: N = 19 No demographic information for separate groups. Overall mean age 44 years (range 21‐69), 95% women, 87% white Pain baseline (extracted from figure): 7.6 Mean years of chronic pain 3.5 (0.5‐20) years Inclusion criteria: modified Yunus 1981 Exclusion criteria: history of peptic ulcer disease or cardiac arrhythmias, or if they were taking medications that could not be stopped |
|
| Interventions |
|
|
| Outcomes |
Pain: 0‐10 cm PGIC much or very much improved: 0‐10 cm Fatigue: 0‐10 cm Sleep problems: 0‐10 cm Adverse events (AEs): no information provided Health‐related quality of life: not assessed Psychological distress: not assessed |
|
| Notes | Oxford Quality Score R = 1 DB = 1 W = 1 Total = 3/5 No conflicts of interest reported Funding source ‐ Arthritis Foundation, and Syntex |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details reported |
| Allocation concealment (selection bias) | Unclear risk | No details reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details reported, though both "blinded" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participant reported, or all participants evaluated in a blinded manner by one assessor |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Over 90% of participants reported data, and no imputation mentioned |
| Selective reporting (reporting bias) | Low risk | All important outcomes reported |
| Group similarity at baseline | Unclear risk | No information |
| Sample size bias | High risk | Fewer than 50 participants per treatment arm |
Kravitz 1994.
| Methods | Randomised, double‐blind, placebo‐controlled, parallel‐group Probably single centre in USA Diagnostic criteria of Yunus 1981 1 week screening and washout, 1 week parallel‐group phase Single missing data point imputation, but all were completers Placebo ibuprofen for 1 week before baseline visit No minimum pain intensity |
|
| Participants |
Ibuprofen: N = 15 Placebo: N = 16 No important demographic differences noted. Overall mean age 48 years (SD 11), 92% women, 90% White Inclusion criteria: modified Yunus 1981 Exclusion criteria: pregnant or child‐bearing potential, nursing, allergy or sensitivity to study drugs, peptic ulcer or bleeding, alcohol or drug abuse, major depression, suicidal ideation, psychosis or schizophrenia, fibromyalgia of secondary cause. Previous psychiatric illness not an exclusion if participant not currently ill |
|
| Interventions | Ibuprofen 600 mg four times a day Placebo |
|
| Outcomes |
Pain: 0‐100 mm PGIC much or very much improved: 7‐point scale Fatigue: not assessed Sleep problems: not assessed Adverse events (AEs): no information provided Health‐related quality of life: not assessed Psychological distress: HRDS, BDI, HARS |
|
| Notes | Oxford Quality Score R = 1 DB = 1 W = 1 Total = 3/5 No conflicts of interest reported Funding source ‐ Arthritis Foundation, and Syntex |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details reported |
| Allocation concealment (selection bias) | Unclear risk | No details reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details reported. Not mentioned that placebo identical |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participant‐reported for major outcome. Not mentioned if other measures evaluated in a blinded manner by one assessor |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All important outcomes reported |
| Selective reporting (reporting bias) | Low risk | All important outcomes reported |
| Group similarity at baseline | Low risk | No important demographic differences between groups |
| Sample size bias | High risk | Fewer than 50 participants per treatment arm |
Mahagna 2016.
| Methods | Randomised, double‐blind, placebo‐controlled, parallel‐group 2 medical centres in Israel Duration of screening and washout not reported, 6 weeks parallel group phase LOCF imputation for withdrawals No NSAID or coxib for 2 weeks before enrolment No stated minimum pain intensity |
|
| Participants |
Etoricoxib: N = 32; 100% female; race not reported; mean age 49.8 (SD 13.2) years; pain baseline 6.4 ± 1.7 on BPI; years since diagnosis 3.5 (SD 6.2) years Placebo: N = 32; 100% female; race not reported; mean age 51.0 (SD 9.7) years; pain baseline 6.4 ± 1.8 on BPI; years since diagnosis 5.2 (SD 6.6) years Inclusion criteria: ACR 1990 criteria Exclusion criteria: confirmed pregnancy or breast feeding, with active or previous coronary artery disease, congestive heart failure, coexisting neoplastic conditions (not including basal cell carcinoma), coexisting rheumatic conditions, active or previous gastrointestinal bleeding, renal failure, comorbid conditions causing significant disability and those with uncontrolled hypertension |
|
| Interventions | Etoricoxib 90 mg fixed dose Placebo |
|
| Outcomes |
Pain: Brief Pain inventory NRS 0‐10; 30% and 50% and more pain relief rates reported PGIC much or very much improved: not assessed Fatigue: FIQ VAS 0‐10 subscale scores not reported Sleep problems: FIQ VAS 0‐10 subscale scores not reported Adverse events (AEs): "Assessment of adverse effects was conducted by actively addressing this issue with each patient at each encounter." Health‐related quality of life: FIQ total score 0‐100 Psychological distress: SF‐36 mental health summary score (50‐0) |
|
| Notes | Oxford Quality Score R = 1 DB = 1 W = 1 Total = 3/5 No conflicts of interest reported Funding source ‐ MSD |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details reported |
| Allocation concealment (selection bias) | Unclear risk | No details reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participant‐reported outcomes; participants were adequately blinded to intervention. Blinding of outcome assessors of safety not adequately described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Most secondary outcomes not reported |
| Selective reporting (reporting bias) | Low risk | Pain baseline scores reported |
| Group similarity at baseline | Low risk | Similar at baseline |
| Sample size bias | High risk | Fewer than 50 participants per treatment arm |
Quijada‐Carrera 1996.
| Methods | Randomised, double‐blind, placebo‐controlled, parallel‐group Single centre (ambulatory rheumatology clinic), Spain Duration screening and washout not reported, 8 weeks parallel‐group phase Analyses based on at least 3 weeks in study, and separately for ITT population. No imputation described Analgesics and NSAIDs discontinued 3 weeks before enrolment No stated minimum pain intensity |
|
| Participants |
Tenoxicam: N = 32; pain baseline (0‐100) 65.9 (SD 27); disease duration 7.7 (SD 5) years Placebo: N = 32; pain baseline (0‐100) 66.5 (SD 19); disease duration 11.6 (SD 9.5) years Bromazepam: N = 33; pain baseline (0‐100) 63 (SD 19); disease duration 10.5 (SD 8.7) years Tenoxicam plus bromazepam: N = 37; pain baseline (0‐100) 58.6 (SD 20); disease duration 12.6 (SD 9.5) years The 4 study groups did not differ significantly with respect to age, sex, weight, height, education level, or work type. Except for duration of fibromyalgia symptoms, the clinical characteristics of participants at baseline were similar in the 4 treatment groups. The mean duration of disease symptoms was lower in the tenoxicam group than in the other 3 treatment groups Inclusion criteria: ACR 1990 criteria Exclusion criteria: "The following laboratory tests should be within normal limits: erythrocyte sedimentation rate, complete blood count, glucose, urea, uric acid, creatinine, creatinine phosphokinase, aldolase, lactic dehydrogenase, serum glutamic oxalate, serum glutamic pyruvic transaminase, alkaline phosphatase, rheumatoid factor, antinuclear antibodies, and thyroid‐stimulating hormone. Roentgenograms of cervical and lumbar spine, hands, knees and hips were also taken. For the purposes of the study fibromyalgia patients should not have more than two locations with degenerative radiographic signs. pregnant or lactating, had a previous history of hypersensitivity to NSAIDs or benzodiazepines, suffered from peptic ulceration, inflammatory joint diseases, connective tissue diseases, hematologic, muscular, neurologic, renal or infectious disorders" |
|
| Interventions |
Rescue and/or allowed medication: no information on rescue medication given; psychotropic drugs except bromazepam were not allowed |
|
| Outcomes |
Pain: Pain (time period not reported) 0‐10 cm; only rates of 25% and more pain relief reported PGIC much or very much improved: global assessment of fibromyalgia; rates of markedly improved or asymptomatic reported Fatigue: not assessed Sleep problems: rates of marked improvement of sleep quality reported Adverse events (AEs): no information provided Health‐related Quality of life: not assessed Psychological distress: not assessed |
|
| Notes | Oxford Quality Score R = 1 DB = 1 W = 1 Total = 3/5 No conflicts of interest reported Funding source ‐ not reported; Roche Spain provided the study medication |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details reported |
| Allocation concealment (selection bias) | Unclear risk | No details reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participant‐reported outcomes; participants were adequately blinded to intervention. Blinding of outcome assessors of safety not adequately described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Imputation using LOCF for efficacy data. ITT analysis |
| Selective reporting (reporting bias) | Low risk | Most secondary outcomes reported |
| Group similarity at baseline | Low risk | Similar at baseline |
| Sample size bias | High risk | Fewer than 50 participants per treatment arm |
Russell 1991.
| Methods | Randomised, double‐blind, placebo‐controlled, parallel‐group Probably single centre, USA 2‐week washout, 6 weeks parallel double‐blind group phase followed by 24‐week open trial No imputation described and some analyses based on 32 participants Analgesics and NSAIDs discontinued 2 weeks before randomisation No stated minimum pain intensity. Measured pain intensity at baseline 6.5/10 |
|
| Participants |
Ibuprofen: N = 17 Placebo: N = 14 No group‐level data. Overall mean age 47 years (SEM 1.2), 89% women; 20% Hispanic; pain baseline 6.2 (SEM 0.2), duration of symptoms or FM diagnosis not reported Inclusion criteria:Russell 1986 (almost all met Yunus 1981, and ACR 1990 criteria) Exclusion criteria: other rheumatic diseases, chronic infections, untreated endocrine disorders, unstable seizure diathesis, psychiatric disorders, or active peptic ulceration |
|
| Interventions | Ibuprofen 600 mg four times a day Placebo |
|
| Outcomes |
Pain: NRS 0‐10 PGIC much or very much improved: not assessed Fatigue: FIQ single item (VAS 0‐10): not assessed Sleep: not assessed Quality of life: HAQ ‐ 24 questions each scored 0‐3 Adverse events (AEs): physical examination, electrocardiograms (ECGs), and laboratory analysis. Further details of assessment of adverse symptoms not reported Depression: HADS and HAS Anxiety: not assessed Disability: not assessed Cognitive disturbances: not assessed Sexual function: not assessed Tenderness: mean tender point threshold (kg/cm²) |
|
| Notes | Oxford Quality Score R = 1 DB = 2 W = 1 Total = 4/5 No conflicts of interest reported Funding source ‐ Upjohn |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details reported |
| Allocation concealment (selection bias) | Unclear risk | No details reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participant‐reported outcomes; participants were adequately blinded to intervention. Blinding of outcome assessors of safety not adequately described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Completer analysis (63/78 ‐ 19% attrition) |
| Selective reporting (reporting bias) | Low risk | Most secondary outcomes reported |
| Group similarity at baseline | Unclear risk | No details reported |
| Sample size bias | High risk | Fewer than 50 participants per treatment arm |
Yunus 1989.
| Methods | Randomised, double‐blind, placebo‐controlled, parallel‐group Single centre (ambulatory rheumatology clinic), USA Duration screening and washout not reported, 3 weeks parallel double‐blind group phase followed by 3 weeks open trial Analyses based on at least 3 weeks in study, and separately for ITT population. No imputation described and some analyses based on 43/46 participants Analgesics and NSAIDs discontinued 1 week before enrolment No stated minimum pain intensity, though 44/46 had moderate or severe pain |
|
| Participants |
Ibuprofen 600 mg four times a day: N = 22; 95% female; race not reported; mean age 38.6 (SD 10.5) years; pain duration 7.3 (SD 6.5 years); pain baseline (1‐4 scale) 2.7 (SD 0.6) Placebo: N = 24; 96% female; race not reported; mean age 39.1 (SD 7.5) years; pain duration 7.7 (SD 7.1 years); pain baseline (1‐4 scale) 2.9 (SD 0.8) Inclusion criteria:Yunus 1981 Exclusion criteria: No underlying condition to explain fibromyalgia symptoms, though this was not a specific exclusion criterion |
|
| Interventions | Ibuprofen 600 mg four times a day Placebo |
|
| Outcomes |
Pain: Average pain severity (NRS 0‐10) last 24 hours; pain relief of 50% or more reported; pain relief 30% and more not reported PGIC much or very much improved: PGIC (1‐7) reported Fatigue: FIQ single item (VAS 0‐10): not reported Sleep: not assessed Quality of life: FIQ total score (0‐80); not reported Adverse events (AEs): physical examination, electrocardiograms (ECGs), and laboratory analysis. Further details of assessment of adverse symptoms not reported Depression: BDI ‐II total score (NRS 0‐63) Anxiety: Beck Anxiety Inventory total score (NRS 0‐63); not reported Disability: FIQ single item (VAS 0‐10): not reported Cognitive disturbances: not assessed Sexual function: not assessed Tenderness: mean tender point threshold (kg/cm²) |
|
| Notes | Oxford Quality Score R = 1 DB = 2 W = 1 Total = 4/5 No conflicts of interest reported Funding source ‐ Wyeth |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details reported |
| Allocation concealment (selection bias) | Unclear risk | No details reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Matched placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participant‐reported, or all participants evaluated in a blinded manner by one assessor |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4/46 withdrew by week 3 |
| Selective reporting (reporting bias) | Low risk | All important outcomes reported |
| Group similarity at baseline | Low risk | Similar at baseline |
| Sample size bias | High risk | Fewer than 50 participants per treatment arm |
ACR: American College of Rheumatology; BDI: Beck Depression Inventory; BPI: British Pain Inventory; DB: double‐blind; FIQ: Fibromyalgia Impact Questionnaire; FM: fibromyalgia; HADS: Hospital Anxietry and Depression Scale; HAQ: Health Assessment Questionnaire; HAS: Health Assessment Scale; HRDS: Hamilton Depression Rating Scale; HARS: Hamilton Anxiety Rating Scale; ITT: Intention to treat; LOCF: last observation carried forward; N: number of participants in study; n: number of participants in treatment arm; NRS: Numerical rating scale; PGIC: Patient Global Impression of Change; R: randomised; SD: standard deviation; SEM: Standard error of the mean; VAS: visual analogue scale; W: withdrawals.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Donald 1980 | Not primarily fibromyalgia |
| Fossaluzza 1992 | Open study |
| Le Gallez 1988 | Soft tissue rheumatism, not fibromyalgia |
| Schorn 1986 | Soft tissue rheumatism, not fibromyalgia |
Differences between protocol and review
The protocol required a minimum of 20 participants per arm because of growing evidence of bias in small studies. We amended this in order to review all available data where there was so little.
We added that we considered other diagnostic criteria for fibromyalgia for older studies that predated the 1990 ACR criteria.
We have updated the wording on GRADE decisions to be in line with the latest version used by Cochrane Effective Practice and organisation of Care (EPOC 2015), being the most recent version of GRADE wording available to us.
Contributions of authors
WH, SD, and RAM drafted the protocol. All authors had input into the protocol development and agreed the final version.
SD and RAM developed and ran the searches. The PaPaS Information Specialist provided support.
WH and RAM selected which studies to include.
WH, SD, and RAM extracted data from studies.
RAM entered data into Review Manager 5 and carried out the analysis. WH and SD checked data entry. All authors interpreted analysis.
Sources of support
Internal sources
-
Oxford Pain Relief Trust, UK.
General institutional support
External sources
-
The National Institute for Health Research (NIHR), UK.
NIHR Cochrane Programme Grant: 13/89/29 ‐ Addressing the unmet need of chronic pain: providing the evidence for treatments of pain
Declarations of interest
SD: none known.
PW: none known.
WH is a specialist in general internal medicine, psychosomatic medicine and pain medicine, who treats patients with fibromyalgia. He is a member of the medical board of the German Fibromyalgia Association. He is the head of the steering committee of the German guideline on fibromyalgia and a member of the steering committee of the European League Against Rheumatism (EULAR) update recommendations on the management of fibromyalgia. He received speaking fees for one educational lecture each from MSD Sharpe & Dohme (2014) and Grünenthal (2015) on pain management.
MM: none known; MM is a specialist physician who treats patients with fibromyalgia.
TRT is a site investigator for the Neuropain project, funded by Pfizer. Since 2014 TRT has consulted with or received lecture fees from pharmaceutical companies related to chronic pain and analgesics: Allergan, Boehringer, Esteve, Hexal, Jannssen‐Cilag, RB.
RB: none known; RB is a retired specialist pain physician who worked with chronic pain patients, including fibromyalgia patients.
RAM has received grant support from Grünenthal relating to individual patient‐level analyses of trial data regarding tapentadol in osteoarthritis and back pain (2015). He has received honoraria for attending boards with Menarini concerning methods of analgesic trial design (2014), with Novartis (2014) about the design of network meta‐analyses, and RB on understanding pharmacokinetics of drug uptake (2015). He has received honoraria from Omega Pharma (2016) and Futura Pharma (2016) for providing advice on trial and data analysis methods.
This review was identified in a 2019 audit as not meeting the current definition of the Cochrane Commercial Sponsorship policy. At the time of its publication it was compliant with the interpretation of the existing policy. As with all reviews, new and updated, at update this review will be revised according to 2020 policy update.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Goldenberg 1986 {published data only}
- Goldenberg DL, Felson DT, Dinerman H. A randomized, controlled trial of amitriptyline and naproxen in the treatment of patients with fibromyalgia. Arthritis and Rheumatism 1986;29(11):1371‐7. [DOI: 10.1002/art.1780291110] [DOI] [PubMed] [Google Scholar]
Kravitz 1994 {published data only}
- Kravitz HM, Katz RS, Helmke N, Jeffriess H, Bukovsky J, Fawcett J. Alprazolam and ibuprofen in the treatment of fibromyalgia‐report of a double‐blind placebo‐controlled study. Journal of Musculoskeletal Pain 1994;2(1):3‐27. [Google Scholar]
Mahagna 2016 {published data only}
- Mahagna H, Amital D, Amital H. A randomised, double‐blinded study comparing giving etoricoxib vs. placebo to female patients with fibromyalgia. International Journal of Clinical Practice 2016;70(2):163‐70. [CTG: NCT00755521; DOI: 10.1111/ijcp.12760] [DOI] [PubMed] [Google Scholar]
Quijada‐Carrera 1996 {published data only}
- Quijada‐Carrera J, Valenzuela‐Castaño A, Povedano‐Gómez J, Fernández‐Rodriguez A, Hernánz‐Mediano W, Gutierrez‐Rubio A, et al. Comparison of tenoxicam and bromazepan in the treatment of fibromyalgia: a randomized, double‐blind, placebo‐controlled trial. Pain 1996;65(2):221‐5. [DOI] [PubMed] [Google Scholar]
Russell 1991 {published data only}
- Russell IJ, Fletcher EM, Michalek JE, McBroom PC, Hester GG. Treatment of primary fibrositis/fibromyalgia syndrome with ibuprofen and alprazolam. A double‐blind, placebo‐controlled study. Arthritis & Rheumatism 1991;34(5):552‐60. [DOI: 10.1002/art.1780340507] [DOI] [PubMed] [Google Scholar]
Yunus 1989 {published data only}
- Yunus MB, Masi AT, Aldag JC. Short term effects of ibuprofen in primary fibromyalgia syndrome: a double blind, placebo controlled trial. Journal of Rheumatology 1989;16(4):527‐32. [PubMed] [Google Scholar]
References to studies excluded from this review
Donald 1980 {published data only}
- Donald JF, Layes Molla A. A comparative double‐blind study of tiaprofenic acid and aspirin in the treatment of muscular rheumatism, fibrositis, sprains and soft tissue injuries in general practice. Journal of International Medical Research 1980;8(6):382‐7. [DOI] [PubMed] [Google Scholar]
Fossaluzza 1992 {published data only}
- Fossaluzza V, Vita S. Combined therapy with cyclobenzaprine and ibuprofen in primary fibromyalgia syndrome. International Journal of Clinical Pharmacology Research 1992;12(2):99‐102. [PubMed] [Google Scholar]
Le Gallez 1988 {published data only}
- Gallez P, Reeve FB, Crawley MA, Bird HA. A double‐blind comparison of ibuprofen, placebo and ibuprofen with meptazinol in soft tissue rheumatism. Current Medical Research and Opinion 1988;10(10):663‐7. [DOI] [PubMed] [Google Scholar]
Schorn 1986 {published data only}
- Schorn D. Tenoxicam in soft‐tissue rheumatism. South African Medical Journal 1986;69(5):301‐3. [PubMed] [Google Scholar]
Additional references
Arnold 2013
- Arnold LM, Fan J, Russell IJ, Yunus MB, Khan MA, Kushner I, et al. The fibromyalgia family study: a genome‐wide linkage scan study. Arthritis and Rheumatism 2013;65:1122‐8. [DOI: 10.1002/art.37842] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bennett 2007
- Bennett RM, Jones J, Turk DC, Russell IJ, Matallana L. An internet survey of 2,596 people with fibromyalgia. BMC Musculoskeletal Disorders 2007;8:27. [DOI: 10.1186/1471-2474-8-27] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bennett 2009
- Bennett RM, Bushmakin AG, Cappelleri JC, Zlateva G, Sadosky AB. Minimal clinically important difference in the fibromyalgia impact questionnaire. Journal of Rheumatology 2009;36:1304‐11. [DOI: 10.3899/jrheum.081090] [DOI] [PubMed] [Google Scholar]
Bhala 2014
- Coxib and traditional NSAID Trialists' (CNT) Collaboration, Bhala N, Emberson J, Merhi A, Abramson S, Arber N, et al. Vascular and upper gastrointestinal effects of non‐steroidal anti‐inflammatory drugs: meta‐analyses of individual participant data from randomised trials. Lancet 2014;382(9894):769‐79. [DOI: 10.1016/S0140-6736(13)60900-9] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bradley 2009
- Bradley LA. Pathophysiology of fibromyalgia. American Journal Medicine 2009;122 (12 Suppl):S22‐30. [DOI: 10.1016/j.amjmed.2009.09.008] [DOI] [PMC free article] [PubMed] [Google Scholar]
Calvo 2012
- Calvo M, Dawes JM, Bennett DL. The role of the immune system in the generation of neuropathic pain. Lancet Neurology 2012;11(7):629‐42. [DOI: 10.1016/S1474-4422(12)70134-5] [DOI] [PubMed] [Google Scholar]
Choi 2010
- Choi CJ, Knutsen R, Oda K, Fraser GE, Knutsen SF. The association between incident self‐reported fibromyalgia and nonpsychiatric factors: 25‐years follow‐up of the Adventist Health Study. Journal of Pain 2010;11:994‐1003. [DOI: 10.1016/j.jpain.2010.01.267] [DOI] [PMC free article] [PubMed] [Google Scholar]
Clauw 2014
- Clauw DJ. Fibromyalgia: a clinical review. JAMA 2014;311(15):1547‐55. [DOI: 10.1001/jama.2014.3266] [DOI] [PubMed] [Google Scholar]
Cohen 1988
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. Abingdon, UK: Lawrence Erlbaum Associates, 1988. [ISBN: 978‐0805802832] [Google Scholar]
Conaghan 2015
- Conaghan PG, Peloso PM, Everett SV, Rajagopalan S, Black CM, Mavros P, et al. Inadequate pain relief and large functional loss among patients with knee osteoarthritis: evidence from a prospective multinational longitudinal study of osteoarthritis real‐world therapies. Rheumatology (Oxford) 2015;54(2):270‐7. [DOI: 10.1093/rheumatology/keu332] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dechartres 2013
- Dechartres A, Trinquart L, Boutron I, Ravaud P. Influence of trial sample size on treatment effect estimates: meta‐epidemiological study. BMJ 2013;f2304:f2304. [DOI: 10.1136/bmj.f2304] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dechartres 2014
- Dechartres A, Altman DG, Trinquart L, Boutron I, Ravaud P. Association between analytic strategy and estimates of treatment outcomes in meta‐analyses. JAMA 2014;312(6):623‐30. [DOI: 10.1001/jama.2014.8166] [DOI] [PubMed] [Google Scholar]
Dworkin 2008
- Dworkin RH, Turk DC, Wyrwich KW, Beaton D, Cleeland CS, Farrar JT, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. Journal of Pain 2008;9(2):105‐21. [DOI: 10.1016/j.jpain.2007.09.005] [DOI] [PubMed] [Google Scholar]
Eich 2012
- Eich W, Häuser W, Arnold B, Bernardy K, Brückle W, Eidmann U, et al. Fibromyalgia syndrome. General principles and coordination of clinical care and patient education. Schmerz 2012;26(3):268‐75. [DOI: 10.1007/s00482-012-1167-z] [DOI] [PubMed] [Google Scholar]
EMEA 2005
- European Medicines Agency. EMEA public statement on the suspension of the marketing authorisation for Bextra (valdecoxib) in the European Union. ema.europa.eu/docs/en_GB/document_library/Public_statement/2009/12/WC500018391.pdf (accessed 23 August 2016) 2005.
EPOC 2015
- Effective Practice, Organisation of Care (EPOC). EPOC worksheets for preparing a Summary of Findings (SoF) table using GRADE. Resources for review authors. Oslo: Norwegian Knowledge Centre for the Health Services 2015; Vol. Available at: epoc.cochrane.org/epoc‐specific‐resources‐review‐authors (accessed 30 November 2016).
Fayers 2014
- Fayers PM, Hays RD. Don't middle your MIDs: regression to the mean shrinks estimates of minimally important differences. Quality Life Research 2014;23(1):1‐4. [DOI: 10.1007/s11136-013-0443-4] [DOI] [PMC free article] [PubMed] [Google Scholar]
FDA 2004
- Food, Drug Administration. FDA Public Health Advisory: safety of Vioxx. www.fda.gov/drugs/drugSafety/postmarketdrugsafetyinformationforpatientsandproviders/ucm106274.htm (accessed 23 August 2016) 2004.
Fitzcharles 2013
- Fitzcharles MA, Ste‐Marie PA, Goldenberg DL, Pereira JX, Abbey S, Choinière M, et al. 2012 Canadian Guidelines for the diagnosis and management of fibromyalgia syndrome: executive summary. Pain Research Management 2013;18(3):119‐26. [PUBMED: 23748251] [DOI] [PMC free article] [PubMed] [Google Scholar]
Forseth 1999
- Forseth KO, Husby G, Gran JT, Førre O. Prognostic factors for the development of fibromyalgia in women with self‐reported musculoskeletal pain. A prospective study. Journal of Rheumatology 1999;26:2458‐67. [PUBMED: 10555910] [PubMed] [Google Scholar]
Geiss 2012
- Geiss A, Rohleder N, Anton F. Evidence for an association between an enhanced reactivity of interleukin‐6 levels and reduced glucocorticoid sensitivity in patients with fibromyalgia. Psychoneuroendocrinology 2012;37(5):671‐85. [DOI: 10.1016/j.psyneuen.2011.07.021] [DOI] [PubMed] [Google Scholar]
Gilron 2013
- Gilron I, Shum B, Moore RA, Wiffen PJ. Combination pharmacotherapy for the treatment of fibromyalgia. Cochrane Database of Systematic Reviews 2013, Issue 6. [DOI: 10.1002/14651858.CD010585] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 7. Rating the quality of evidence‐‐inconsistency. Journal of Cinical Epidemiology 2011;64(12):1294‐302. [DOI: 10.1016/j.jclinepi.2011.03.017] [DOI] [PubMed] [Google Scholar]
Guyatt 2013a
- Guyatt G, Oxman AD, Sultan S, Brozek J, Glasziou P, Alonso‐Coello P, et al. GRADE guidelines: 11. Making an overall rating of confidence in effect estimates for a single outcome and for all outcomes. Journal of Clinical Epidemiology 2013;66(2):151‐7. [DOI: 10.1016/j.jclinepi.2012.01.006] [DOI] [PubMed] [Google Scholar]
Guyatt 2013b
- Guyatt GH, Oxman AD, Santesso N, Helfand M, Vist G, Kunz R, et al. GRADE guidelines: 12. Preparing summary of findings tables‐binary outcomes. Journal of Clinical Epidemiology 2013;66(2):158‐72. [DOI: 10.1016/j.jclinepi.2012.01.012] [DOI] [PubMed] [Google Scholar]
Hasnie 2007
- Hasnie FS, Breuer J, Parker S, Wallace V, Blackbeard J, Lever I, et al. Further characterization of a rat model of varicella zoster virus‐associated pain: relationship between mechanical hypersensitivity and anxiety‐related behavior, and the influence of analgesic drugs. Neuroscience 2007;144(4):1495‐508. [DOI: 10.1016/j.neuroscience.2006.11.029] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hoffman 2010
- Hoffman DL, Sadosky A, Dukes EM, Alvir J. How do changes in pain severity levels correspond to changes in health status and function in patients with painful diabetic peripheral neuropathy?. Pain 2010;149(2):194‐201. [DOI: 10.1016/j.pain.2009.09.017] [DOI] [PubMed] [Google Scholar]
Häuser 2010
- Häuser W, Thieme K, Turk DC. Guidelines on the management of fibromyalgia syndrome ‐ a systematic review. European Journal of Pain 2010;14(1):5‐10. [DOI: 10.1016/j.ejpain.2009.01.006] [DOI] [PubMed] [Google Scholar]
Häuser 2011
- Häuser W, Kosseva M, Üceyler N, Klose P, Sommer C. Emotional, physical, and sexual abuse in fibromyalgia syndrome: a systematic review with meta‐analysis. Arthritis Care and Research 2011;63:808‐20. [DOI: 10.1002/acr.20328] [DOI] [PubMed] [Google Scholar]
Häuser 2012
- Häuser W, Jung E, Erbslöh‐Möller B, Gesmann M, Kühn‐Becker H, Petermann F, et al. The German fibromyalgia consumer reports ‐ a cross‐sectional survey. BMC Musculoskeletal Disorders 2012;13:74. [DOI: 10.1186/1471-2474-13-74] [DOI] [PMC free article] [PubMed] [Google Scholar]
Häuser 2013a
- Häuser W, Galek A, Erbslöh‐Möller B, Köllner V, Kühn‐Becker H, Langhorst J, et al. Posttraumatic stress disorder in fibromyalgia syndrome: prevalence, temporal relationship between posttraumatic stress and fibromyalgia symptoms, and impact on clinical outcome. Pain 2013;154(8):1216‐23. [DOI: 10.1016/j.pain.2013.03.034] [DOI] [PubMed] [Google Scholar]
Häuser 2013b
- Häuser W, Urrútia G, Tort S, Uçeyler N, Walitt B. Serotonin and noradrenaline reuptake inhibitors (SNRIs) for fibromyalgia syndrome. Cochrane Database of Systematic Reviews 2013, Issue 1. [DOI: 10.1002/14651858.CD010292] [DOI] [PubMed] [Google Scholar]
Häuser 2014a
- Häuser W, Henningsen P. Fibromyalgia syndrome ‐ a somatoform disorder?. European Journal of Pain 2014;18(8):1052‐9. [DOI: 10.1002/j.1532-2149.2014.00453.x] [DOI] [PubMed] [Google Scholar]
Häuser 2014b
- Häuser W, Walitt B, Fitzcharles MA, Sommer C. Review of pharmacological therapies in fibromyalgia syndrome. Arthritis Research and Therapy 2014;16(1):201. [DOI: 10.1186/ar4441] [DOI] [PMC free article] [PubMed] [Google Scholar]
Häuser 2015
- Häuser W, Ablin J, Fitzcharles MA, Littlejon J, Luciano JV, Usui C, et al. Fibromyalgia. Nature Reviews Disease Primers 2015;13(1):15022. [DOI: 10.1038/nrdp.2015.22] [DOI] [PubMed] [Google Scholar]
Jadad 1996
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Controlled Clinical Trials 1996;17(1):1‐12. [DOI: ] [DOI] [PubMed] [Google Scholar]
Kadetoff 2012
- Kadetoff D, Lampa J, Westman M, Andersson M, Kosek E. Evidence of central inflammation in fibromyalgia‐increased cerebrospinal fluid interleukin‐8 levels. Journal of Neuroimmunology 2012;242(1‐2):33‐8. [DOI: 10.1016/j.jneuroim.2011.10.013] [DOI] [PubMed] [Google Scholar]
Kalso 2013
- Kalso E, Aldington DJ, Moore RA. Drugs for neuropathic pain. BMJ 2013;347:f7339. [DOI: 10.1136/bmj.f7339] [DOI] [PubMed] [Google Scholar]
Kawakami 2002
- Kawakami M, Matsumoto T, Hashizume H, Kuribayashi K, Tamaki T. Epidural injection of cyclooxygenase‐2 inhibitor attenuates pain‐related behavior following application of nucleus pulposus to the nerve root in the rat. Journal of Orthopaedic Research 2002;20(2):376‐81. [DOI: 10.1016/S0736-0266(01)00114-0] [DOI] [PubMed] [Google Scholar]
Koroschetz 2011
- Koroschetz J, Rehm SE, Gockel U, Brosz M, Freynhagen R, Tölle TR, et al. Fibromyalgia and neuropathic pain ‐ differences and similarities. A comparison of 3057 patients with diabetic painful neuropathy and fibromyalgia. BMC Neurology 2011;11:55. [DOI: 10.1186/1471-2377-11-55] [DOI] [PMC free article] [PubMed] [Google Scholar]
L'Abbé 1987
- L'Abbé KA, Detsky AS, O'Rourke K. Meta‐analysis in clinical research. Annals of Internal Medicine 1987;107(2):224‐33. [DOI: 10.7326/0003-4819-107-2-224] [DOI] [PubMed] [Google Scholar]
Laine 2001
- Laine L. Approaches to nonsteroidal anti‐inflammatory drug use in the high‐risk patient. Gastroenterology 2001;120(3):594‐606. [DOI: 10.1053/gast.2001.21907] [DOI] [PubMed] [Google Scholar]
Lange 2010
- Lange M, Petermann F. Influence of depression on fibromyalgia: a systematic review [Einfluss von depression auf das fibromyalgiesyndrom: eine systematische literaturanalyse]. Schmerz 2010;24(4):326‐33. [DOI: 10.1007/s00482-010-0937-8] [DOI] [PubMed] [Google Scholar]
Lee 2012
- Lee YH, Choi SJ, Ji JD, Song GG. Candidate gene studies of fibromyalgia: a systematic review and meta‐analysis. Rheumatology International 2012;32(2):417‐26. [DOI: 10.1007/s00296-010-1678-9] [DOI] [PubMed] [Google Scholar]
Lunn 2014
- Lunn MP, Hughes RA, Wiffen PJ. Duloxetine for treating painful neuropathy, chronic pain or fibromyalgia. Cochrane Database of Systematic Reviews 2014, Issue 1. [DOI: 10.1002/14651858.CD007115.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Macfarlane 2017
- Macfarlane GJ, Kronisch C, Dean LE, Atzeni F, Häuser W, Fluß E, et al. EULAR revised recommendations for the management of fibromyalgia. Annals of the Rheumatic Diseases 2017;76(2):318‐28. [DOI: 10.1136/annrheumdis-2016-209724] [DOI] [PubMed] [Google Scholar]
Mangoni 2010
- Mangoni AA, Woodman RJ, Gaganis P, Gilbert AL, Knights KM. Use of non‐steroidal anti‐inflammatory drugs and risk of incident myocardial infarction and heart failure, and all‐cause mortality in the Australian veteran community. British Journal of Clinical Pharmacology 2010;69(6):689‐700. [DOI: 10.1111/j.1365-2125.2010.03627.x] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mansfield 2016
- Mansfield KE, Sim J, Jordan JL, Jordan KP. A systematic review and meta‐analysis of the prevalence of chronic widespread pain in the general population. Pain 2016;157(1):55‐64. [DOI: 10.1097/j.pain.0000000000000314] [DOI] [PMC free article] [PubMed] [Google Scholar]
McQuay 1998
- McQuay H, Moore R. An Evidence‐based Resource for Pain Relief. Oxford: Oxford University Press, 1998. [ISBN: 0‐19‐262718‐X] [Google Scholar]
Mease 2009
- Mease P, Arnold LM, Choy EH, Clauw DJ, Crofford LJ, Glass J, et al. Fibromyalgia syndrome module at OMERACT 9: domain construct. Journal of Rheumatology 2009;36(10):2318‐29. [DOI: 10.3899/jrheum.090367] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mills 2015
- Mills EJ, Ayers D, Chou R, Thorlund K. Are current standards of reporting quality for clinical trials sufficient in addressing important sources of bias?. Contemporary Clinical Trials 2015;45(Pt A):2‐7. [DOI: 10.1016/j.cct.2015.07.019] [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009). Preferred Reporting Items for Systematic Reviews and Meta‐Analyses: The PRISMA Statement. PLoS Medicine 2009;6(7):e1000097. [DOI: 10.1371/journal.pmed1000097] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moore 1998
- Moore RA, Gavaghan D, Tramèr MR, Collins SL, McQuay HJ. Size is everything ‐ large amounts of information are needed to overcome random effects in estimating direction and magnitude of treatment effects. Pain 1998;78(3):209‐16. [DOI: 10.1016/S0304-3959(98)00140-7] [DOI] [PubMed] [Google Scholar]
Moore 2008
- Moore RA, Barden J, Derry S, McQuay HJ. Managing potential publication bias. In: McQuay HJ, Kalso E, Moore RA editor(s). Systematic Reviews in Pain Research: Methodology Refined. Seattle: IASP Press, 2008:15‐24. [ISBN: 978‐0‐931092‐69‐5] [Google Scholar]
Moore 2009
- Moore RA, Straube S, Wiffen PJ, Derry S, McQuay HJ. Pregabalin for acute and chronic pain in adults. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD007076.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moore 2010a
- Moore RA, Eccleston C, Derry S, Wiffen P, Bell RF, Straube S, et al. "Evidence" in chronic pain ‐ establishing best practice in the reporting of systematic reviews. Pain 2010;150(3):386‐9. [DOI: 10.1016/j.pain.2010.05.011] [DOI] [PubMed] [Google Scholar]
Moore 2010b
- Moore RA, Moore OA, Derry S, Peloso PM, Gammaitoni AR, Wang H. Responder analysis for pain relief and numbers needed to treat in a meta‐analysis of etoricoxib osteoarthritis trials: bridging a gap between clinical trials and clinical practice. Annals of the Rheumatic Diseases 2010;69(2):374‐9. [DOI: 10.1136/ard.2009.107805] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moore 2010c
- Moore RA, Straube S, Paine J, Phillips CJ, Derry S, McQuay HJ. Fibromyalgia: moderate and substantial pain intensity reduction predicts improvement in other outcomes and substantial quality of life gain. Pain 2010;149(2):360‐4. [DOI: 10.1016/j.pain.2010.02.039] [DOI] [PubMed] [Google Scholar]
Moore 2010d
- Moore RA, Smugar SS, Wang H, Peloso PM, Gammaitoni A. Numbers‐needed‐to‐treat analyses ‐ do timing, dropouts, and outcome matter? Pooled analysis of two randomized, placebo‐controlled chronic low back pain trials. Pain 2010;151(3):592‐7. [DOI: 10.1016/j.pain.2010.07.013] [DOI] [PubMed] [Google Scholar]
Moore 2011a
- Moore RA, Wiffen PJ, Derry S, McQuay HJ. Gabapentin for chronic neuropathic pain and fibromyalgia in adults. Cochrane Database of Systematic Reviews 2011, Issue 3. [DOI: 10.1002/14651858.CD007938.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moore 2011b
- Moore RA, Straube S, Paine J, Derry S, McQuay HJ. Minimum efficacy criteria for comparisons between treatments using individual patient meta‐analysis of acute pain trials: examples of etoricoxib, paracetamol, ibuprofen, and ibuprofen/paracetamol combinations after third molar extraction. Pain 2011;152(5):982‐9. [DOI: 10.1016/j.pain.2010.11.030] [DOI] [PubMed] [Google Scholar]
Moore 2011c
- Moore RA, Mhuircheartaigh RJ, Derry S, McQuay HJ. Mean analgesic consumption is inappropriate for testing analgesic efficacy in post‐operative pain: analysis and alternative suggestion. European Journal of Anaesthesiology 2011;28(6):427‐32. [DOI: 10.1097/EJA.0b013e328343c569] [DOI] [PubMed] [Google Scholar]
Moore 2012a
- Moore RA, Derry S, Aldington D, Cole P, Wiffen PJ. Amitriptyline for neuropathic pain and fibromyalgia in adults. Cochrane Database of Systematic Reviews 2012, Issue 12. [DOI: 10.1002/14651858.CD008242.pub2] [DOI] [PubMed] [Google Scholar]
Moore 2012b
- Moore RA, Straube S, Eccleston C, Derry S, Aldington D, Wiffen P, et al. Estimate at your peril: imputation methods for patient withdrawal can bias efficacy outcomes in chronic pain trials using responder analyses. Pain 2012;153(2):265‐8. [DOI: 10.1016/j.pain.2011.10.004] [DOI] [PubMed] [Google Scholar]
Moore 2013a
- Moore RA, Straube S, Aldington D. Pain measures and cut‐offs ‐ 'no worse than mild pain' as a simple, universal outcome. Anaesthesia 2013;68(4):400‐12. [DOI: 10.1111/anae.12148] [DOI] [PubMed] [Google Scholar]
Moore 2013b
- Moore A, Derry S, Eccleston C, Kalso E. Expect analgesic failure; pursue analgesic success. BMJ 2013;346:f2690. [DOI: 10.1136/bmj.f2690] [DOI] [PubMed] [Google Scholar]
Moore 2014a
- Moore RA, Derry S, Taylor RS, Straube S, Phillips CJ. The costs and consequences of adequately managed chronic non‐cancer pain and chronic neuropathic pain. Pain Practice 2014;14(1):79‐94. [DOI: 10.1111/papr.12050] [DOI] [PubMed] [Google Scholar]
Moore 2014b
- Moore RA, Derry S, Simon LS, Emery P. Nonsteroidal anti‐inflammatory drugs, gastroprotection, and benefit‐risk. Pain Practice 2014;14(4):378‐95. [DOI: 10.1111/papr.12100] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moore 2014c
- Moore RA, Cai N, Skljarevski V, Tölle TR. Duloxetine use in chronic painful conditions ‐ individual patient data responder analysis. European Journal of Pain 2014;18(1):67‐75. [DOI: 10.1002/j.1532-2149.2013.00341.x] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moore 2015
- Moore RA, Wiffen PJ, Eccleston C, Derry S, Baron R, Bell RF, et al. Systematic review of enriched enrolment, randomised withdrawal trial designs in chronic pain: a new framework for design and reporting. Pain 2015;156(8):1382‐95. [DOI: 10.1097/j.pain.0000000000000088] [DOI] [PubMed] [Google Scholar]
Mork 2010
- Mork PJ, Vasseljen O, Nilsen TI. Association between physical exercise, body mass index, and risk of fibromyalgia: longitudinal data from the Norwegian Nord‐Trøndelag Health Study. Arthritis Care & Research 2010;62:611‐7. [DOI] [PubMed] [Google Scholar]
Mork 2012
- Mork PJ, Nilsen TI. Sleep problems and risk of fibromyalgia: longitudinal data on an adult female population in Norway. Arthritis and Rheumatism 2012;64(1):281‐4. [DOI: 10.1002/art.33346] [DOI] [PubMed] [Google Scholar]
Nüesch 2013
- Nüesch E, Häuser W, Bernardy K, Barth J, Jüni P. Comparative efficacy of pharmacological and non‐pharmacological interventions in fibromyalgia syndrome: network meta‐analysis. Annals of the Rheumatic Diseases 2013;72(6):955‐62. [DOI: 10.1136/annrheumdis-2011-201249] [DOI] [PubMed] [Google Scholar]
O'Brien 2010
- O'Brien EM, Staud RM, Hassinger AD, McCulloch RC, Craggs JG, Atchison JW, et al. Patient‐centered perspective on treatment outcomes in chronic pain. Pain Medicine 2010;11(1):6‐15. [DOI: 10.1111/j.1526-4637.2009.00685.x] [DOI] [PubMed] [Google Scholar]
Oaklander 2013
- Oaklander AL, Herzog ZD, Downs HM, Klein MM. Objective evidence that small‐fiber polyneuropathy underlies some illnesses currently labeled as fibromyalgia. Pain 2013;154(11):2310‐6. [DOI: 10.1016/j.pain.2013.06.001] [DOI] [PMC free article] [PubMed] [Google Scholar]
PaPaS 2012
- Pain, Palliative and Supportive Care Group. PaPaS author and referee guidance. papas.cochrane.org/papas‐documents (accessed 23 August 2016).
Peloso 2016
- Peloso PM, Moore RA, Chen WJ, Lin HY, Gates DF, Straus WL, et al. Osteoarthritis patients with pain improvement are highly likely to also have improved quality of life and functioning. A post hoc analysis of a clinical trial. Scandinavian Journal of Pain 2016;13(10):175‐81. [DOI: 10.1016/j.sjpain.2016.07.002] [DOI] [PubMed] [Google Scholar]
Queiroz 2013
- Queiroz LP. Worldwide epidemiology of fibromyalgia. Current Pain and Headache Reports 2013;17(8):356. [DOI: 10.1007/s11916-013-0356-5] [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Russell 1986
- Russell IJ, Vipraio GA, Morgan WW, Bowden CL. Is there a metabolic basis for the fibrositis syndrome?. American Journal of Medicine 1986;81(Suppl 3A):50‐54. [DOI] [PubMed] [Google Scholar]
Schünemann 2011a
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Schünemann 2011b
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and ‘Summary of findings' tables. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Shaver 2009
- Shaver JL, Wilbur J, Lee H, Robinson FP, Wang E. Self‐reported medication and herb/supplement use by women with and without fibromyalgia. Journal of Womens Health 2009;18(5):709‐16. [DOI: 10.1089/jwh.2008.1194] [DOI] [PubMed] [Google Scholar]
Sommer 2012a
- Sommer C, Häuser W, Burgmer M, Engelhardt R, Gerhold K, Petzke F, et al. Etiology and pathophysiology of fibromyalgia syndrome [Ätiologie und pathophysiologie des fibromyalgiesyndroms]. Schmerz 2012;26(3):259‐67. [DOI: 10.1007/s00482-012-1174-0] [DOI] [PubMed] [Google Scholar]
Sommer 2012b
- Sommer C, Häuser W, Alten R, Petzke F, Späth M, Tölle T, et al. Drug therapy of fibromyalgia syndrome. Systematic review, meta‐analysis and guideline [Arbeitsgemeinschaft der wissenschaftlichen medizinischen fachgesellschaften]. Schmerz 2012;26(3):297‐310. [DOI: 10.1007/s00482-012-1172-2] [DOI] [PubMed] [Google Scholar]
Straube 2008
- Straube S, Derry S, McQuay HJ, Moore RA. Enriched enrollment: definition and effects of enrichment and dose in trials of pregabalin and gabapentin in neuropathic pain. A systematic review. British Journal of Clinical Pharmacology 2008;66(2):266‐75. [DOI: 10.1111/j.1365-2125.2008.03200.x] [DOI] [PMC free article] [PubMed] [Google Scholar]
Straube 2010
- Straube S, Derry S, Moore RA, Paine J, McQuay HJ. Pregabalin in fibromyalgia ‐ responder analysis from individual patient data. BMC Musculoskeletal Disorders 2010;11:150. [DOI: 10.1186/1471-2474-11-150] [DOI] [PMC free article] [PubMed] [Google Scholar]
Straube 2011
- Straube S, Moore RA, Paine J, Derry S, Phillips CJ, Hallier E, et al. Interference with work in fibromyalgia: effect of treatment with pregabalin and relation to pain response. BMC Musculoskeletal Disorders 2011;12:125. [DOI: 10.1186/1471-2474-12-125] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sultan 2008
- Sultan A, Gaskell H, Derry S, Moore RA. Duloxetine for painful diabetic neuropathy and fibromyalgia pain: systematic review of randomised trials. BMC Neurology 2008;8:29. [DOI: 10.1186/1471-2377-8-29] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vo 2009
- Vo T, Rice ASC, Dworkin RH. Non‐steroidal anti‐inflammatory drugs for neuropathic pain: how do we explain continued widespread use?. Pain 2009;143(3):169‐71. [DOI: 10.1016/j.pain.2009.03.013] [DOI] [PubMed] [Google Scholar]
Vos 2012
- Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990‐2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380(9859):2163‐96. [DOI: 10.1016/S0140-6736(12)61729-2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Walitt 2015
- Walitt B, Nahin RL, Katz RS, Bergman MJ, Wolfe F. The prevalence and characteristics of fibromyalgia in the 2012 National Health Interview Survey. PLoS One 2015;10(9):e0138024. [DOI: 10.1371/journal.pone.0138024] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wiffen 2013
- Wiffen PJ, Derry S, Moore RA, Aldington D, Cole P, Rice ASC, et al. Antiepileptic drugs for neuropathic pain and fibromyalgia ‐ an overview of Cochrane reviews. Cochrane Database of Systematic Reviews 2013, Issue 11. [DOI: 10.1002/14651858.CD010567.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wolfe 1990
- Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, et al. The American College of Rheumatology 1990 criteria for the classification of fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis and Rheumatism 1990;33(2):160‐72. [DOI: 10.1002/art.1780330203] [DOI] [PubMed] [Google Scholar]
Wolfe 2010
- Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Katz RS, Mease P, et al. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care and Research 2010;62(5):600‐10. [DOI: 10.1002/acr.20140] [DOI] [PubMed] [Google Scholar]
Wolfe 2011
- Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Häuser W, Katz RS, et al. Fibromyalgia criteria and severity scales for clinical and epidemiological studies: a modification of the ACR Preliminary Diagnostic Criteria for Fibromyalgia. Journal of Rheumatology 2011;38:1113‐22. [DOI: 10.3899/jrheum.100594] [DOI] [PubMed] [Google Scholar]
Wolfe 2013
- Wolfe F, Brähler E, Hinz A, Häuser W. Fibromyalgia prevalence, somatic symptom reporting, and the dimensionality of polysymptomatic distress: results from a survey of the general population. Arthritis Care and Research 2013;645(5):777‐85. [DOI: 10.1002/acr.21931] [DOI] [PubMed] [Google Scholar]
Wolfe 2014a
- Wolfe F, Walitt BT, Häuser W. What is fibromyalgia, how is it diagnosed and what does it really mean?. Arthritis Care and Research 2014;66(7):969‐71. [DOI: 10.1002/acr.22207] [DOI] [PubMed] [Google Scholar]
Wolfe 2014b
- Wolfe F, Walitt BT, Katz RS, Lee YC, Michaud KD, Häuser W. Longitudinal patterns of analgesic and central acting drug use and associated effectiveness in fibromyalgia. European Journal of Pain 2014;17(4):581‐6. [DOI: 10.1002/j.1532-2149.2012.00234.x] [DOI] [PubMed] [Google Scholar]
Yunus 1981
- Yunus M, Masi AT, Calabro JJ, Miller KA, Feigenbaum SL. Primary fibromyalgia (fibrositis): clinical study of 50 patients with matched normal controls. Seminars in Arthritis and Rheumatism 1981;11(1):151‐71. [DOI] [PubMed] [Google Scholar]
Yunus 2008
- Yunus MB. Central sensitivity syndromes: a new paradigm and group nosology for fibromyalgia and overlapping conditions, and the related issue of disease versus illness. Seminars in Arthritis and Rheumatism 2008;37:339‐52. [DOI: 10.1016/j.semarthrit.2007.09.003] [DOI] [PubMed] [Google Scholar]
Üçeyler 2013a
- Üçeyler N, Zeller D, Kahn AK, Kewenig S, Kittel‐Schneider S, Schmid A, et al. Small fibre pathology in patients with fibromyalgia syndrome. Brain 2013;136(6):1857‐67. [DOI: 10.1093/brain/awt053] [DOI] [PubMed] [Google Scholar]
Üçeyler 2013b
- Üçeyler N, Sommer C, Walitt B, Häuser W. Anticonvulsants for fibromyalgia. Cochrane Database of Systematic Reviews 2013, Issue 10. [DOI: 10.1002/14651858.CD010782] [DOI] [PubMed] [Google Scholar]


