Abstract
Background
Respiratory morbidity including respiratory distress syndrome (RDS) is a serious complication of preterm birth and the primary cause of early neonatal mortality and disability. While researching the effects of the steroid dexamethasone on premature parturition in fetal sheep in 1969, Liggins found that there was some inflation of the lungs of lambs born at gestations at which the lungs would be expected to be airless. Liggins and Howie published the first randomised controlled trial in humans in 1972 and many others followed.
Objectives
To assess the effects of administering a course of corticosteroids to the mother prior to anticipated preterm birth on fetal and neonatal morbidity and mortality, maternal mortality and morbidity, and on the child in later life.
Search methods
We searched Cochrane Pregnancy and Childbirth's Trials Register (17 February 2016) and reference lists of retrieved studies.
Selection criteria
We considered all randomised controlled comparisons of antenatal corticosteroid administration (betamethasone, dexamethasone, or hydrocortisone) with placebo, or with no treatment, given to women with a singleton or multiple pregnancy, prior to anticipated preterm delivery (elective, or following spontaneous labour), regardless of other co‐morbidity, for inclusion in this review. Most women in this review received a single course of steroids; however, nine of the included trials allowed for women to have weekly repeats.
Data collection and analysis
Two review authors independently assessed trials for inclusion and risk of bias, extracted data and checked them for accuracy. The quality of the evidence was assessed using the GRADE approach.
Main results
This update includes 30 studies (7774 women and 8158 infants). Most studies are of low or unclear risk for most bias domains. An assessment of high risk usually meant a trial had potential for performance bias due to lack of blinding. Two trials had low risks of bias for all risk of bias domains.
Treatment with antenatal corticosteroids (compared with placebo or no treatment) is associated with a reduction in the most serious adverse outcomes related to prematurity, including: perinatal death (average risk ratio (RR) 0.72, 95% confidence interval (CI) 0.58 to 0.89; participants = 6729; studies = 15; Tau² = 0.05, I² = 34%; moderate‐quality); neonatal death (RR 0.69, 95% CI 0.59 to 0.81; participants = 7188; studies = 22), RDS (average RR 0.66, 95% CI 0.56 to 0.77; participants = 7764; studies = 28; Tau² = 0.06, I² = 48%; moderate‐quality); moderate/severe RDS (average RR 0.59, 95% CI 0.38 to 0.91; participants = 1686; studies = 6; Tau² = 0.14, I² = 52%); intraventricular haemorrhage (IVH) (average RR 0.55, 95% CI 0.40 to 0.76; participants = 6093; studies = 16; Tau² = 0.10, I² = 33%; moderate‐quality), necrotising enterocolitis (RR 0.50, 95% CI 0.32 to 0.78; participants = 4702; studies = 10); need for mechanical ventilation (RR 0.68, 95% CI 0.56 to 0.84; participants = 1368; studies = 9); and systemic infections in the first 48 hours of life (RR 0.60, 95% CI 0.41 to 0.88; participants = 1753; studies = 8).
There was no obvious benefit for: chronic lung disease (average RR 0.86, 95% CI 0.42 to 1.79; participants = 818; studies = 6; Tau² = 0.38 I² = 65%); mean birthweight (g) (MD ‐18.47, 95% CI ‐40.83 to 3.90; participants = 6182; studies = 16; moderate‐quality); death in childhood (RR 0.68, 95% CI 0.36 to 1.27; participants = 1010; studies = 4); neurodevelopment delay in childhood (RR 0.64, 95% CI 0.14 to 2.98; participants = 82; studies = 1); or death into adulthood (RR 1.00, 95% CI 0.56 to 1.81; participants = 988; studies = 1).
Treatment with antenatal corticosteroids does not increase the risk of chorioamnionitis (RR 0.83, 95% CI 0.66 to 1.06; participants = 5546; studies = 15; moderate‐quality evidence) or endometritis (RR 1.20, 95% CI 0.87 to 1.63; participants = 4030; studies = 10; Tau² = 0.11, I² = 28%; moderate‐quality). No increased risk in maternal death was observed. However, the data on maternal death is based on data from a single trial with two deaths; four other trials reporting maternal death had zero events (participants = 3392; studies = 5; moderate‐quality).
There is no definitive evidence to suggest that antenatal corticosteroids work differently in any pre‐specified subgroups (singleton versus multiple pregnancy; membrane status; presence of hypertension) or for different study protocols (type of corticosteroid; single course or weekly repeats).
GRADE outcomes were downgraded to moderate‐quality. Downgrading decisions (for perinatal death, RDS, IVH, and mean birthweight) were due to limitations in study design or concerns regarding precision (chorioamnionitis, endometritis). Maternal death was downgraded for imprecision due to few events.
Authors' conclusions
Evidence from this update supports the continued use of a single course of antenatal corticosteroids to accelerate fetal lung maturation in women at risk of preterm birth. A single course of antenatal corticosteroids could be considered routine for preterm delivery. It is important to note that most of the evidence comes from high income countries and hospital settings; therefore, the results may not be applicable to low‐resource settings with high rates of infections.
There is little need for further trials of a single course of antenatal corticosteroids versus placebo in singleton pregnancies in higher income countries and hospital settings. However, data are sparse in lower income settings. There are also few data regarding risks and benefits of antenatal corticosteroids in multiple pregnancies and other high‐risk obstetric groups. Further information is also required concerning the optimal dose‐to‐delivery interval, and the optimal corticosteroid to use.
We encourage authors of previous studies to provide further information, which may answer any remaining questions about the use of antenatal corticosteroids in such pregnancies without the need for further randomised controlled trials. Individual patient data meta‐analysis from published trials is likely to answer some of the evidence gaps. Follow‐up studies into childhood and adulthood, particularly in the late preterm gestation and repeat courses groups, are needed. We have not examined the possible harmful effects of antenatal corticosteroids in low‐resource settings in this review. It would be particularly relevant to explore this finding in adequately powered prospective trials.
Keywords: Female; Humans; Infant, Newborn; Pregnancy; Premature Birth; Adrenal Cortex Hormones; Adrenal Cortex Hormones/administration & dosage; Betamethasone; Betamethasone/administration & dosage; Dexamethasone; Dexamethasone/administration & dosage; Fetal Organ Maturity; Fetal Organ Maturity/drug effects; Hydrocortisone; Hydrocortisone/administration & dosage; Lung; Lung/drug effects; Lung/embryology; Maternal Death; Perinatal Death; Prenatal Care; Prenatal Care/methods; Respiratory Distress Syndrome, Newborn; Respiratory Distress Syndrome, Newborn/prevention & control
Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth
What is the issue?
Babies born very early, or very preterm, are at risk of having breathing difficulties and other serious health problems at birth, as a child and later in life. Some babies born very early do not survive these difficulties. Some babies have health problems that prevent them from developing as they should and can lead to problems with movement or learning. Corticosteroids are medicines given to women in early labour to help the babies' lungs to mature more quickly and so reduce the number of babies who die or suffer breathing problems at birth.
Why is this important?
Breathing problems are the main cause of death and serious health problems for babies born very early. Pregnant women who have ruptured membranes or spontaneous preterm labour can take corticosteroids to help mature the baby's lungs. In this review, we compared women and babies who had these medicines to women and babies who did not.
What evidence did we find?
We searched Cochrane Pregnancy and Childbirth's Trials Register (17 February 2016).
We looked at 30 trials where corticosteroids were given to women at risk of preterm birth (7774 women and 8158 infants). The trials were all carried out in hospitals in high‐income countries. Our review shows that a single course of a corticosteroids, given to the mother in preterm labour and before the baby is born, helps to develop the baby's lungs and reduces complications such as breathing problems. Furthermore, this treatment results in fewer babies dying at birth, and fewer babies having other serious health problems that commonly affect babies born very early (such as bleeding in the brain or damage to the baby's intestines).
For the mother, having a single course of corticosteroids did not appear to impact on the number of women who had infections of the womb (chorioamnionitis or endometritis). There were too few data available to fully assess the outcome of maternal death.
The quality of the trial evidence was moderate, which means that we can be reasonably confident that future studies of corticosteroids in similar hospital settings will come to the same conclusions about the benefits and safety of treatment for women and babies.
What does this mean?
Most pregnant women who are at risk of giving birth very early or very preterm will benefit from having a corticosteroid medicine. These medicines appear to be safe for pregnant women and babies when given in hospital settings in high‐income countries, and they improve the chance that the preterm baby will survive and avoid immediate health problems. We have less information about the impact of steroids on women with multiple pregnancy and on women with other problems during pregnancy such as high blood pressure or ruptured membranes. We are uncertain whether a specific steroid or dosage is best for women and babies.
Evidence in this review comes from high‐income countries and hospital settings; therefore, the results may not be applicable to low‐resource settings with high rates of infections.
Summary of findings
Summary of findings for the main comparison.
Corticosteroids versus placebo or no treatment
| Corticosteroids versus placebo or no treatment | ||||||
| Patient or population: pregnant women at high risk of preterm birth receiving a corticosteroid or placebo/no treatment; women with singleton and multiple pregnancy and intact and ruptured membranes Setting: hospital settings in high‐income countries. For example, data for RDS come from 28 trials in 15 different countries, but only one of these countries is of lower income (Tunisia) Intervention: corticosteroids (dexamethasone or betamethasone) according to various doses and regimens; some trials with weekly repeats Comparison: placebo (usually normal saline) or no treatment | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo or no treatment | Risk with corticosteroids | |||||
| Maternal death | Study population | RR 0.98 (0.06 to 15.50) | 3392 (5 RCTs) | ⊕⊕⊕⊝ Moderate1 | RR based on 2 deaths in a single trial (1 death in each group). Four trials reported zero events | |
| 1 per 1000 | 1 per 1000 (0 to 9) | |||||
| Chorioamnionitis | Study population | RR 0.83 (0.66 to 1.06) | 5546 (15 RCTs) | ⊕⊕⊕⊝ Moderate2 | ||
| 48 per 1000 | 40 per 1000 (32 to 51) | |||||
| Endometritis (infections) | Study population | RR 1.20 (0.87 to 1.63) | 4030 (10 RCTs) | ⊕⊕⊕⊝ Moderate2,3 | 7 of 10 trials reported endometritis; the remaining trials report 'infections' | |
| 33 per 1000 | 39 per 1000 (27 to 59) | |||||
| Perinatal deaths | Study population | average RR 0.72 (0.58 to 0.89) | 6729 (15 RCTs) | ⊕⊕⊕ Moderate4 | ||
| 102 per 1000 | 73 per 1000 (59 to 91) | |||||
| Respiratory distress syndrome | Study population | average RR 0.66 (0.56 to 0.77) | 7764 (28 RCTs) | ⊕⊕⊕ Moderate5 | ||
| 176 per 1000 | 116 per 1000 (98 to 135) | |||||
| Intraventricular haemorrhage | Study population | average RR 0.55 (0.40 to 0.76) | 6093 (16 RCTs) | ⊕⊕⊕ Moderate6 | ||
| 51 per 1000 | 28 per 1000 (20 to 39) | |||||
| Mean birthweight (grams) (less is worse) |
Absolute risks not calculated | The mean birthweight was 18.47g less (40.83g less to 3.90g more) | 6182 (16 RCTs) | ⊕⊕⊕ Moderate7 | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
1Few events and wide confidence interval led to a downgrade for imprecision (‐1). Because maternal death is a rare event and the total population is over 3000 women, we have opted for (‐1) rather than (‐2). 2Wide confidence interval crossing the line of no effect (‐1). 3Value of I2 = 34% with random‐effects model. We have not downgraded evidence for heterogeneity. 4Value of I2 = 37% with random‐effects model. We have not downgraded for heterogeneity. Result downgraded once for risks of bias in included trials (‐1). 5Value of I2 = 47% with random‐effects model. We have not downgraded for heterogeneity. Result downgraded once for risks of bias in included trials (‐1). 6Value of I2 = 33% with random‐effects model. We have not downgraded for heterogeneity. Result downgraded once for risks of bias in included trials (‐1). 7The confidence interval showed a difference at most on average of 40 g in weight; because this is less than 10% of the lightest average for babies in any trial, we have not downgraded evidence for imprecision. We have downgraded the result for risks of bias concerns in included trials (‐1).
Background
Description of the condition
Respiratory distress syndrome (RDS) is a serious complication of preterm birth and the primary cause of early neonatal death and disability (Rodriguez 2002). It affects up to half of babies born before 28 weeks and a third of babies born before 32 weeks. Approximately 42% of extremely low birthweight babies have RDS (less than 1500 g) (Hintz 2007).
Respiratory failure in these infants occurs as a result of surfactant deficiency, poor lung anatomical development and immaturity in other organs. Neonatal survival after preterm birth improves with gestation (Doyle 2001a), reflecting improved maturity of organ systems. However, those who survive early neonatal care are at increased risk of long‐term neurological disability (Doyle 2001b).
Some understanding of fetal lung development may be useful in understanding why RDS occurs and why corticosteroids work. Fetal lung development can be divided into five stages: embryonic, pseudoglandular, canalicular, terminal sac and alveolar. The lung first appears as an outgrowth of the primitive foregut at 22 to 26 days after conception. By 34 days, the outgrowth has divided into left and right sides and further to form the major units of the lung. Mature lungs contain more than 40 different cell types derived from this early tissue. From eight to 16 weeks' gestation, the major bronchial airways and associated respiratory units of the lung are progressively formed. At this time the lung blood vessels also begin to grow in parallel. From 17 to 25 weeks' gestation, the airways grow, widen and lengthen (canalisation). Terminal bronchioles with enlargements that subsequently give rise to terminal sacs (the primitive alveoli) are formed. These are the functional units of the lung (respiratory lobules). It is at this stage that the increasing proximity of blood capillaries begins the air‐blood interface, required for effective air exchange. This can only take place at the terminal bronchioles. At the end of the canalicular stage, type I and II pneumocytes can be seen in the alveoli. From 28 to 35 weeks' gestation, the alveoli can be counted and with increasing age they become more mature. Lung volume increases four‐fold between 29 weeks and term. Alveolar number shows a curvilinear increase with age but a linear relationship with bodyweight. At birth there are an average of 150 million alveoli (half the expected adult number). The alveoli produce surfactant. The alveolar stage continues for one to two years after birth. In the preterm infant, low alveolar numbers probably contribute to respiratory dysfunction.
The fetal lung also matures biochemically with increasing gestation. Lamellar bodies, which store surfactant, appear at 22 to 24 weeks. Surfactant is a complex mixture of lipids and apoproteins, the main constituents of which are dipalmitoylphosphatidyl choline, phosphatidylglycerol and apoproteins A, B, C and D. Surfactant is needed to maintain stability when breathing out, to prevent collapse of the alveoli. Premature infants have a qualitative and quantitative deficiency of surfactant, which predisposes to RDS. At the low lung volume associated with expiration, surface tension becomes very high, leading to atelectasis with subsequent intrapulmonary shunting, ventilation perfusion inequalities, and ultimately respiratory failure. Capillary leakage allows inhibitors from plasma to reach alveoli and inactivate any surfactant that may be present. Hypoxia, acidosis and hypothermia (common problems in the very preterm infant) can reduce surfactant synthesis required to replenish surfactant lost from the system. The pulmonary antioxidant system develops in parallel to the surfactant system and deficiency in this also puts the preterm infant at risk of chronic lung disease.
Description of the intervention
While researching the effects of the steroid dexamethasone on premature parturition in fetal sheep in 1969, Liggins found that there was some inflation of the lungs of lambs born at gestations at which the lungs would be expected to be airless (Liggins 1969). Liggins and Howie performed the first randomised controlled trial in humans of betamethasone for the prevention of RDS in 1972 (Liggins 1972a).
Several clinical trials have been performed on the effects of corticosteroids before preterm birth since the original Liggins study. The first structured review on corticosteroids in preterm birth was published in 1990 (Crowley 1990). This review showed that corticosteroids given prior to preterm birth (as a result of either preterm labour or planned preterm delivery) are effective in preventing RDS and neonatal mortality. Corticosteroid treatment was also associated with a significant reduction in the risk of intraventricular haemorrhage (IVH). Corticosteroids appear to exert major vasoconstrictive effects on fetal cerebral blood flow, protecting the fetus against IVH at rest and when challenged by conditions causing vasodilatation such as hypercapnia (Schwab 2000). Crowley found no effect on necrotising enterocolitis or chronic lung disease from antenatal corticosteroid administration. The influence of the results of the original trial and Crowley's review was the subject of a Wellcome Witness Seminar (Wellcome 2005) held in 2004.
Corticosteroids have become the mainstay of prophylactic treatment in preterm birth, as a result of these findings and subsequent work. However, there have remained a number of outstanding issues regarding the use of antenatal corticosteroids. The original trial by Liggins suggested an increased rate of stillbirth in women with hypertension syndromes (Liggins 1976). There is concern about using corticosteroids in women with premature rupture of membranes due to the possible increased risk of neonatal and maternal infection (Imseis 1996; NIH 1994). The efficacy of this treatment in multiple births has only been addressed retrospectively (Turrentine 1996). From the time of the original Liggins paper, debate has continued around whether the treatment is effective at lower gestations and at differing treatment‐to‐delivery intervals. Recently, debate has also centred around whether treatment is effective at latter gestations, up to and including term delivery (Sotiriadis 2009). These issues will be addressed in this review in subgroup analyses. The effectiveness and safety of repeat doses of corticosteroids for women who remain undelivered, but at increased risk of preterm birth after an initial course of treatment, is addressed in a separate Cochrane Review (Crowther 2015).
Recent epidemiological evidence and animal work suggests that there may be adverse long‐term consequences of antenatal exposure to corticosteroids (Seckl 2000). Exposure to excess corticosteroids before birth is hypothesised to be a key mechanism underlying the fetal origins of adult disease hypothesis (Barker 1998; Benediktsson 1993). This hypothesis postulates a link between impaired fetal growth, and cardiovascular disease and type 2 diabetes in later life along with their risk factors of impaired glucose tolerance, dyslipidaemia, and hypertension (Barker 1998). A large body of animal experimental work has documented impaired glucose tolerance and increased blood pressure in adult animals after antenatal exposure to corticosteroids (Clark 1998; Dodic 1999; Edwards 2001). Thus, this review has considered blood pressure, glucose intolerance, dyslipidaemia, and hypothalamo‐pituitary‐adrenal axis function in childhood and adulthood.
Experimental animal studies have also shown decreased brain growth in preterm and term infants exposed to single courses of corticosteroid (Huang 1999; Jobe 1998). This review has therefore also addressed long‐term neurodevelopment and other childhood and adult outcomes after antenatal corticosteroid exposure.
How the intervention might work
Liggins 1972a theorised that dexamethasone might have accelerated the appearance of pulmonary surfactant. The hypothesis is that corticosteroids act to trigger the synthesis of ribonucleic acid that codes for particular proteins involved in the biosynthesis of phospholipids or in the breakdown of glycogen. Subsequent work has suggested that, in animal models, corticosteroids mature a number of organ systems (Padbury 1996; Vyas 1997).
Why it is important to do this review
There was a need for an updated systematic review of the effects of prophylactic corticosteroids for preterm birth, as a result of current interest and due to further published trials. In the previous review we were able to re‐analyse the Auckland Steroid Study by intention‐to‐treat. This study contributes 15% of the participants to the review so this was an important development for the review. This update is needed because it has been some time since the previous version was published, review methodology for Cochrane Reviews has changed, and we attempted to standardise the review with the Cochrane Review on 'Repeat doses of prenatal corticosteroids for women at risk of preterm birth for improving neonatal health outcomes' (Crowther 2015).
Objectives
To assess the effects of administering a course of corticosteroids to the mother prior to anticipated preterm birth on fetal and neonatal morbidity and mortality, maternal mortality and morbidity, and on the child in later life.
Methods
Criteria for considering studies for this review
Types of studies
We considered all randomised controlled comparisons of antenatal corticosteroid administration (betamethasone, dexamethasone, or hydrocortisone) with placebo, or with no treatment, given to women prior to anticipated preterm delivery (elective, or following spontaneous labour), regardless of other co‐morbidity, for inclusion in this review. Quasi‐randomised (e.g. allocation by date of birth or record number), cross‐over and cluster‐randomised trials were not eligible for inclusion. We included trials where the method of randomisation was not specified in detail in the expectation that their inclusion in this review would encourage the study authors to make available further information on the method of randomisation. We excluded trials where non‐randomised cohorts were amalgamated with randomised participants if the results of the randomised participants could not be separated out. We also excluded trials that tested the effect of corticosteroids along with other co‐interventions. We included trials in which placebo was not used in the control group. We also included published, unpublished and ongoing randomised trials with reported data.
Types of participants
Women, with a singleton or multiple pregnancy, expected to deliver preterm as a result of either spontaneous preterm labour, preterm prelabour rupture of the membranes or planned preterm delivery.
Types of interventions
Trials tested a corticosteroid capable of crossing the placenta (betamethasone, dexamethasone, hydrocortisone) compared with placebo or with no treatment. Most trials tested a single course of steroid, though some included trials allowed for weekly repeats. We discarded data from trials involving the use of methyl‐prednisolone (Block 1977; Schmidt 1984), as this corticosteroid has not been shown to induce maturation in animal models and is known to have altered placental transfer (Block 1977). We planned predefined subgroups to separately examine primary outcomes in women and infants depending on the specific drug used. Single versus multiple doses of corticosteroids is the subject of another Cochrane Review (Crowther 2015).
Types of outcome measures
Primary outcomes chosen were those which were thought to be the most clinically valuable in assessing effectiveness and safety of the treatment for the woman and her offspring. Secondary outcomes included possible complications and other measures of effectiveness.
Primary outcomes
For the woman:
death;
chorioamnionitis (however defined by study authors);
endometritis (however defined by study authors and including infections).
For the fetus/neonate:
perinatal death;
neonatal deaths;
fetal deaths;
RDS;
moderate/severe RDS;
chronic lung disease (need for continuous supplemental oxygen at 28 days postnatal age or 36 weeks' postmenstrual age, whichever was later);
intraventricular haemorrhage (IVH) (diagnosed by ultrasound, diagnosed by autopsy);
mean birthweight (g).
For the child:
death;
neurodevelopmental disability at follow‐up (blindness, deafness, moderate/severe cerebral palsy (however defined by study authors), or development delay/intellectual impairment (defined as developmental quotient or intelligence quotient less than ‐2 standard deviation below population mean)).
For the child as adult:
death;
neurodevelopmental disability at follow‐up (blindness, deafness, moderate/severe cerebral palsy (however defined by study authors), or development delay/intellectual impairment (defined as developmental quotient or intelligence quotient less than ‐2 standard deviation below population mean)).
Secondary outcomes
For the woman:
fever after trial entry requiring the use of antibiotics;
intrapartum fever requiring the use of antibiotics;
postnatal fever;
admission to intensive care unit;
side effects of therapy;
glucose intolerance (however defined by study authors);
hypertension (however defined by study authors).
For the fetus/neonate:
Apgar score less than seven at five minutes;
interval between trial entry and birth;
mean length at birth (height);
mean head circumference at birth;
mean skin fold thickness at birth;
small‐for‐gestational age (however defined by study authors);
mean placental weight;
neonatal blood pressure;
admission to neonatal intensive care unit (NICU);
need for inotropic support;
mean duration of inotropic support (days);
need for mechanical ventilation/continuous positive airways pressure;
mean duration of mechanical ventilation/continuous positive airways pressure (days);
air leak syndrome;
duration of oxygen supplementation (days);
surfactant use;
systemic infection in first 48 hours of life;
proven infection while in the NICU)
necrotising enterocolitis;
hypothalamo‐pituitary‐adrenal (HPA) axis function (however defined by study authors).
For the child:
mean weight;
mean head circumference;
mean height;
mean skin fold thickness;
abnormal lung function (however defined by study authors);
mean blood pressure;
glucose intolerance (however defined by study authors);
HPA axis function (however defined by study authors);
dyslipidaemia (however defined by study authors);
visual impairment (however defined by study authors);
hearing impairment (however defined by study authors);
developmental delay (defined as developmental quotient less than ‐2 standard deviation below population mean);
intellectual impairment (defined as intelligence quotient less than ‐2 standard deviation below population mean);
cerebral palsy (however defined by study authors);
behavioural/learning difficulties (however defined by study authors).
For the child as adult:
mean weight;
mean head circumference;
mean height;
mean skin fold thickness;
abnormal lung function (however defined by study authors);
mean blood pressure;
glucose intolerance (however defined by study authors);
HPA axis function (however defined by study authors);
dyslipidaemia (however defined by study authors);
mean age at puberty;
bone density (however defined by study authors);
educational achievement (completion of high school, or however defined by study authors);
visual impairment (however defined by study authors);
hearing impairment (however defined by study authors);
intellectual impairment (defined as intelligence quotient less than ‐2 standard deviation below population mean).
For health services:
mean length of antenatal hospitalisation for women (days);
mean length of postnatal hospitalisation for women (days);
mean length of neonatal hospitalisation (days);
cost of maternal care (in 10s of 1000s of USD);
cost of neonatal care (in 10s of 1000s of USD).
Search methods for identification of studies
The following methods section of this review is based on a standard template used by Cochrane Pregnancy and Childbirth.
Electronic searches
We searched Cochrane Pregnancy and Childbirth’s Trials Register by contacting their Information Specialist (17 February 2016).
The Register is a database containing over 22,000 reports of controlled trials in the field of pregnancy and childbirth. For full search methods used to populate Pregnancy and Childbirth’s Trials Register including the detailed search strategies for CENTRAL, MEDLINE, Embase and CINAHL; the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service, please follow this link to the editorial information about Cochrane Pregnancy and Childbirth in the Cochrane Library and select the ‘Specialized Register ’ section from the options on the left side of the screen.
Briefly, Cochrane Pregnancy and Childbirth’s Trials Register is maintained by their Information Specialist and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE (Ovid);
weekly searches of Embase (Ovid);
monthly searches of CINAHL (EBSCO);
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Search results are screened by two people and the full text of all relevant trial reports identified through the searching activities described above is reviewed. Based on the intervention described, each trial report is assigned a number that corresponds to a specific Pregnancy and Childbirth review topic (or topics), and is then added to the Register. The Information Specialist searches the Register for each review using this topic number rather than keywords. This results in a more specific search set which has been fully accounted for in the relevant review sections (Included studies; Excluded studies).
Searching other resources
We searched the reference lists of retrieved studies.
We did not apply any language or date restrictions.
Data collection and analysis
For methods used in the previous version of this review, see Roberts 2006.
For this update, we used the following methods to assess the new reports that were identified as a result of the updated search.
The following methods section of this review is based on a standard template used by Cochrane Pregnancy and Childbirth.
Selection of studies
Two review authors assessed the trials for eligibility and methodological quality without consideration of the results. Reasons for excluding any trial are detailed in the Characteristics of excluded studies table. Trials were not assessed blind, as we knew the author's name, institution and the source of publication. We resolved any disagreement by discussion until we reached consensus.
Data extraction and management
Two review authors extracted the data, checked them for discrepancies and processed them as described in Higgins 2011a. We contacted authors of each included trial for further information, if we thought this to be necessary.
Assessment of risk of bias in included studies
Two review authors independently assessed the risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b). We resolved any disagreement by discussion.
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the methods used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the methods as:
low risk of bias (any truly random process, e.g. random number table; computer random‐number generator; tossing a coin, minimisation);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number; quasi‐randomised studies were excluded from the review);
unclear risk of bias (unclear description or no description of randomisation sequence generation).
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal the allocation sequence in sufficient detail and determine whether intervention allocation could have been foreseen in advance of, or during, recruitment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study all the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We also provided any information relating to whether the intended blinding was effective. Where blinding was not possible, we assessed whether the lack of blinding was likely to have introduced bias.
We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel;
low, high or unclear risk of bias for outcome assessors
where low risk of bias was when there was blinding or where we assessed that the outcome or the outcome measurement was not likely to have been influenced by lack of blinding.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We have assessed methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations)
We described for each included study the completeness of outcome data for each main outcome, including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported, the numbers included in the analyses at each stage (compared with the total randomised participants), reasons for attrition/exclusion where reported, and any re‐inclusions in analyses undertaken.
We assessed the methods as:
low risk of bias (e.g. where there were no missing data or where reasons for missing data were balanced across groups);
high risk of bias (e.g. where missing data were likely to be related to outcomes or were not balanced across groups);
unclear risk of bias (e.g. where there was insufficient reporting of attrition or exclusions to permit a judgement to be made).
(5) Selective reporting bias
We described for each included study how we examined the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it was clear that all of the study's pre‐specified outcomes and all expected outcomes of interest to the review were reported);
high risk of bias (where not all the study's pre‐specified outcomes were reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest were reported incompletely and so could not be used; study failed to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other sources of bias
We described for each included study any important concerns we had about other possible sources of bias. For example, was there a potential source of bias related to the specific study design? Was the trial stopped early due to some data‐dependent process? Was there extreme baseline imbalance? Had the study been claimed to be fraudulent?
We assessed whether each study was free of other problems that could put it at risk of bias:
low risk of bias;
high risk of bias;
unclear.
(7) Overall risk of bias
We made explicit judgements about risk of bias for important outcomes both within and across studies. With reference to (1) to (6) above we assessed the likely magnitude and direction of the bias and whether we considered it likely to impact on the findings.
Assessment of the quality of the evidence using GRADE
For this update the quality of the evidence was assessed using the GRADE approach as outlined in the GRADE Handbook in order to assess the quality of the body of evidence relating to the following outcomes for the main comparison, corticosteroids versus placebo or no treatment.
Maternal death
Chorioamnionitis (however defined by study authors)
Endometritis (however defined by study authors and including infections)
Perinatal death
Respiratory distress syndrome
Intraventricular haemorrhage (IVH) (diagnosed by ultrasound, diagnosed by autopsy)
Mean birthweight (g)
We used the GRADEpro Guideline Development Tool to import data from Review Manager 5 (RevMan 5) (RevMan 2014) in order to create 'Summary of findings' tables. A summary of the intervention effect and a measure of quality for each of the above outcomes was produced using the GRADE approach. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. The evidence can be downgraded from 'high quality' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Measures of treatment effect
Dichotomous data
In the original review, a weighted estimate of the typical treatment effect across studies was performed using the 'Peto method' (i.e. 'the typical odds ratio': the odds of an unfavourable outcome among treatment‐allocated participants to the corresponding odds among controls). For this update, we have calculated risk ratios (RR) and 95% confidence intervals (CI) for dichotomous data. Although odds ratios have been commonly used in meta‐analysis, there is potential for them to be interpreted incorrectly, and current advice is that risk ratios should be used wherever possible (Deeks 2011). We analysed outcomes on an intention‐to‐treat basis.
Continuous data
For continuous data, we used the mean difference (MD) with 95% CI where outcomes were measured using the same instrument. Where different instruments were used we planned to use the standardised mean difference with 95% CI.
Unit of analysis issues
Cluster‐randomised trials
Cluster‐randomised trials were not considered eligible for inclusion in this review.
Cross‐over trials
Cross‐over trials were not considered eligible for inclusion in this review.
Other unit of analysis issues
Where possible for multiple pregnancies, the number of babies was used as the denominator for fetal and neonatal outcomes.
Dealing with missing data
In cases where trial data were missing, we first sought information from the original trial investigators. Details of trial authors contacted and the questions asked of them are contained in Characteristics of included studies. In addition, and where possible, we performed analyses on all outcomes on an intention‐to‐treat basis. It was our intention to include in the analyses all women randomly assigned to each group and to analyse all women in the group to which they were allocated, regardless of whether or not they received the allocated intervention.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the Tau², I² (Higgins 2003) and Chi² statistics. We regarded heterogeneity as substantial if an I² was greater than 30% and either the Tau² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity. Where we found substantial heterogeneity we used a random‐effects model to conduct the analysis and attempted to explain possible sources of heterogeneity (Deeks 2011).
Assessment of reporting biases
If there were 10 or more studies in the meta‐analysis we investigated reporting biases (such as publication bias) using funnel plots (Sterne 2011). We assessed funnel plot asymmetry visually. If asymmetry was suggested by a visual assessment, we performed exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using the RevMan 5 software (RevMan 2014). We used fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect: that is, where trials were examining the same intervention, and the trials’ populations and methods were judged sufficiently similar.
If there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or if substantial statistical heterogeneity was detected, we used random‐effects meta‐analysis to produce an overall summary if an average treatment effect across trials was considered clinically meaningful. The random‐effects summary was treated as the average of the range of possible treatment effects and we have discussed the clinical implications of treatment effects differing between trials. If the average treatment effect was not clinically meaningful, we planned not to combine trials. If we used random‐effects analyses, we presented the results as the average treatment effect with 95% CIs, and the estimates of Tau² and I².
Subgroup analysis and investigation of heterogeneity
We performed analysis of clinical groups for primary outcomes only (where data were available).
We analysed the following clinical groups:
singleton versus multiple pregnancy;
intact membranes versus ruptured membranes at first dose;
pregnancy‐induced hypertension syndromes;
type of glucocorticoid (betamethasone, dexamethasone, hydrocortisone);
decade of trial (post‐hoc, i.e.not pre‐specified in the protocol);
protocol with weekly repeats (post hoc, i.e. not pre‐specified in the protocol);
gestational age at trial entry (post hoc, i.e. not pre‐specified in the protocol).
All covariates were proposed after deliberation with clinical experts. We planned to explore potential differences in the effect of corticosteroids in distinct clinical populations, such as pregnant women with ruptured membranes or multiple pregnancy, and in different types of trials.
For the main analysis we did not adjust data for multiple pregnancies to take account of non‐independence of outcomes for babies from the same pregnancy. For some outcomes there will be a higher correlation between babies from the same pregnancy than between babies from different pregnancies. The degree of non‐independence of outcomes for babies from multiple pregnancies will vary considerably depending on the outcome and the type of multiple pregnancy. For some outcomes the risk of an adverse event will be highly correlated in babies from the same pregnancy (e.g. preterm birth); while for others the degree of correlation will be lower (e.g. fetal death) but still higher than for babies from different pregnancies. In view of this non‐independence, subgroup analysis examining fetal and neonatal outcomes in singleton versus multiple pregnancies must be interpreted with particular caution.
We found that some trials included in this review had a protocol of weekly repeat doses of corticosteroid if the mother remained undelivered. None of the trials that allowed weekly repeat doses reported outcomes separately for those exposed to repeat doses. We performed a post hoc analysis for primary outcomes of trials where a single course was used versus those where weekly repeat doses were allowed in the protocol to determine if the inclusion of such trials biased our results. Single versus multiple doses of corticosteroids is the subject of another Cochrane Review (Crowther 2015). The analysis in this update will differ from that of the single versus multiple doses review, because the latter review includes only those studies where the women were randomised to either single or multiple doses.
Because the case‐fatality rate for RDS has decreased with improvements in neonatal care, we postulated that the effect of corticosteroids may not be as apparent in more recent trials. This hypothesis was tested in a post‐hoc subgroup analysis with trials grouped by the main decade of recruitment or publication of results.
Many trials did not report outcome data split according to the listed clinical characteristics (covariates). Due to this missing information, the total number of events/participants in subgroup analysis for some outcomes does not match the overall analysis. We have indicated in footnotes on the forest plots where the data are discrepant between the main analysis and the clinical subgroups.
All analyses by the covariates listed above should be considered hypothesis‐generating.
Finally, it should be noted that we did not conduct subgroup analysis where there were too few trials reporting data to conduct meaningful analyses.
Sensitivity analysis
We have not conducted any formal sensitivity analysis based on risks of bias in included trials. We conducted sensitivity analysis to determine whether conclusions were robust to decisions made during the review process ‐ for example, regarding missing data, the definitions of subgroups or the impact of single trials.
We conducted sensitivity analyses for the following specific cases: where we found heterogeneity greater than 50% for primary outcomes (see Comparison 1); where we found small amounts of missing data reported for subgroups compared with the numbers reported in the main analyses (see Comparison 3); where specific trials fitted into multiple potential subgroups for our analysis of gestational age at trial entry (see Comparison 8); and for analysis of results according to the decade of the trial (see Analysis 6.6).
Analysis 6.6.
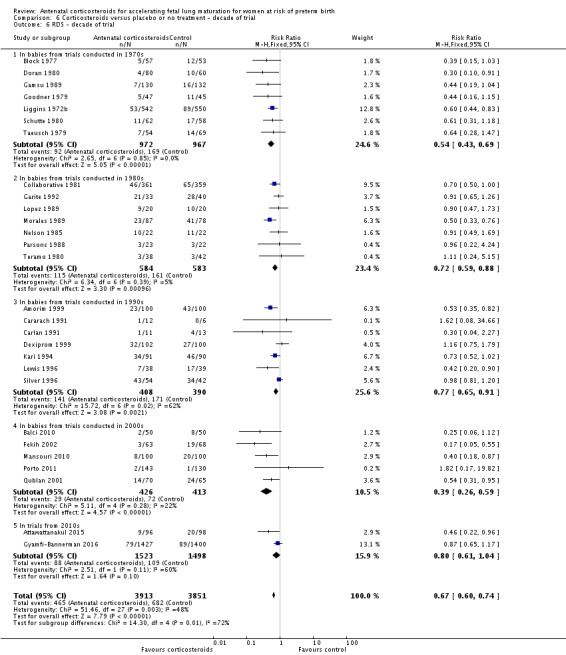
Comparison 6 Corticosteroids versus placebo or no treatment ‐ decade of trial, Outcome 6 RDS ‐ decade of trial.
Results
Description of studies
Results of the search
A total of 48 studies were identified and 30 met the inclusion criteria. Twenty‐eight were excluded. One study report previously in ongoing studies was included at this update with the full trial report (Gyamfi‐Bannerman 2016).
Included studies
Thirty studies met our inclusion criteria, with data available for 7774 women and 8158 infants. The included studies were conducted over a wide range of gestational ages, including those of extreme prematurity and late prematurity. Obstetric indications for recruitment to trials were premature rupture of membranes, spontaneous preterm labour and planned preterm delivery. Please also refer to the Characteristics of included studies tables.
The included studies came from a range of healthcare systems and treatment eras. Thirteen of the studies were conducted in the USA (Block 1977; Carlan 1991; Collaborative 1981; Garite 1992; Goodner 1979; Gyamfi‐Bannerman 2016; Lewis 1996; Morales 1989; Nelson 1985; Parsons 1988; Shanks 2010; Silver 1996; Taeusch 1979), two studies each were conducted in Finland (Kari 1994; Teramo 1980), Iran (Khazardoust 2012; Mansouri 2010), and Brazil (Amorim 1999; Porto 2011), and one study from each of the following countries, Colombia (Lopez 1989), Spain (Cararach 1991), South Africa (Dexiprom 1999), Turkey (Balci 2010), Canada (Doran 1980),Tunisia (Fekih 2002), United Kingdom (Gamsu 1989), New Zealand (Liggins 1972b), Jordan (Qublan 2001), Thailand (Attawattanakul 2015) and the Netherlands (Schutte 1980). In this update, nine recent trials since 2000 contribute approximately 51% of the data available for analysis (Attawattanakul 2015; Balci 2010; Fekih 2002; Gyamfi‐Bannerman 2016; Khazardoust 2012; Mansouri 2010; Porto 2011; Qublan 2001; Shanks 2010).
It should be noted that Khazardoust 2012 contributes no outcome data to the review.
Multiple pregnancy
The majority of trials recruited only women with singleton pregnancy. Twelve trials Collaborative 1981, Dexiprom 1999, Doran 1980, Fekih 2002, Gamsu 1989, Garite 1992, Kari 1994, Liggins 1972b, Schutte 1980, Silver 1996, Taeusch 1979 and Teramo 1980 recruited women with singleton or multiple pregnancy. Of these, only Collaborative 1981, Gamsu 1989, Liggins 1972b and Silver 1996 reported outcome data separately for included women with multiple pregnancy. For two trials recruitment was unclear, and we analysed available data with the mixed population clinical group (Goodner 1979 and Lopez 1989).
Membrane status
Several trials specifically excluded women with premature rupture of membranes: Amorim 1999, Attawattanakul 2015, Balci 2010, Garite 1992, Kari 1994 and Shanks 2010. Twelve trials reported outcome data for women with premature rupture of membranes (Cararach 1991; Carlan 1991; Dexiprom 1999; Fekih 2002; Lewis 1996; Liggins 1972b; Lopez 1989; Morales 1989; Nelson 1985; Parsons 1988; Qublan 2001; Schutte 1980). The remaining included trials reported data for a mixed population or the membrane status of included women was unclear. Only Liggins 1972b reported outcome data separately for women with intact or ruptured membranes.
Type of Steroid
Seven of the included studies used dexamethasone as the corticosteroid in the treatment arm (1585 women and 1708 infants), while 21 studies used betamethasone (6133 women and 6314 infants). One study did not specify the corticosteroid used (Cararach 1991; 18 women and infants), and one study used either betamethasone or dexamethasone (Shanks 2010; 32 women and infants).
Decade of trial
Four included trials were published during the 1970s; nine during the 1980s; eight during the 1990s; five during the 2000s, and four during the 2010s. The largest trial contributing the most data to the review is the recent ALPS study (n = 2831; Gyamfi‐Bannerman 2016). Please see the Included studies tables for details.
Gestational age at trial entry
We have attached a table stating the gestational parameters for trials included in the review (Table 10). For the analysis of clinical subgroups for this update, we have compared trials recruiting women at gestational age of less than and including 35 weeks + 0 days with trials recruiting women 34 weeks + 0 days' gestation or greater for the review's primary outcomes. Most trials fall on either side of this division, with the exception of four studies; Block 1977, Collaborative 1981, Liggins 1972b, and Teramo 1980. Data from Liggins 1972b was available for women receiving their first dose at less than 35 weeks + 0 days and from between 35 weeks + 0 days and 37 weeks + 0 days, footnotes detailing this have been added to the appropriate forest plots. The majority of women in the remaining three studies (Block 1977; Collaborative 1981; Teramo 1980) received their first dose prior to 34 weeks + 0 days, therefore we included these studies in the younger gestational age grouping for the analysis (women less than, and including, 35 weeks and 0 days), but undertook a sensitivity analysis with the studies' data removed.
Table 1.
Gestational age parameters for included trials
| Trial | Year | Minimum (weeks+days) |
Maximum (weeks+days) |
| Amorim 1999 | 1999 | 28+0 | 34+6 |
| Attawattanakul 2015 | 2015 | 34+0 | 36+6 |
| Balci 2010 | 2010 | 34+0 | 36+6 |
| Block 1977 | 1976 | Not reported | 36+6 |
| Carlan 1991 | 1991 | 24+0 | 34+6 |
| Cararach 1991 | 1994 | 28+0 | 30+6 |
| Collaborative 1981 | 1981 | 26+0 | 37+0 |
| Dexiprom 1999 | 1999 | 28+0 | 34+6 |
| Doran 1980 | 1980 | 24+0 | 34+6 |
| Fekih 2002 | 2002 | 26+0 | 34+6 |
| Gamsu 1989 | 1989 | Not reported | 34+6 |
| Garite 1992 | 1992 | 24+0 | 27+6 |
| Goodner 1979 | 1979 | Not reported | 33+6 |
| Gyamfi‐Bannerman 2016 | 2016 | 34+0 | 36+6 |
| Kari 1994 | 1994 | 24+0 | 31+6 |
|
Khazardoust 2012 (no outcome data) |
2012 | 34+0 | 37+0 |
| Lewis 1996 | 1996 | 24+0 | 34+6 |
| Liggins 1972b | 1972 | 24+0 | 36+6 |
| Lopez 1989 | 1989 | 27+0 | 35+0 |
| Mansouri 2010 | 2010 | 35+0 | 36+6 |
| Morales 1989 | 1989 | 26+0 | 34+6 |
| Nelson 1985 | 1985 | 28+0 | 34+6 |
| Parsons 1988 | 1988 | 25+0 | 32+6 |
| Porto 2011 | 2011 | 34+0 | 36+6 |
| Qublan 2001 | 2001 | 27+0 | 34+6 |
| Schutte 1980 | 1980 | 26+0 | 32+6 |
| Shanks 2010 | 2010 | 34+0 | 36+6 |
| Silver 1996 | 1996 | 24+0 | 29+6 |
| Taeusch 1979 | 1979 | Not reported | 33+6 |
| Teramo 1980 | 1980 | 28+0 | 35+6 |
Weekly repeats
Most trials included in this review tested a single course of corticosteroid. Nine of the included studies allowed weekly repeat courses of study medication in their study protocols (Amorim 1999; Carlan 1991; Fekih 2002; Garite 1992; Lewis 1996; Morales 1989; Parsons 1988; Qublan 2001; Silver 1996) (932 women and 946 infants). We conducted post hoc analysis of primary outcomes comparing studies testing a single course of study medication with studies allowing weekly repeat courses.
Excluded studies
We excluded 28 studies. Reasons for exclusion included the following.
The study did not compare a corticosteroid with placebo or no treatment (Abuhamad 1999; Althabe 2015; Dola 1997; Egerman 1998; Garite 1981; Iams 1985; Koivisto 2007; Magee 1997; Minoui 1998; Mulder 1997; Rotmensch 1999; Whitt 1976).
The study was not a randomised controlled trial (Grgic 2003; Halac 1990; Maksic 2008).
The study was a quasi‐randomised trial (Asnafei 2004; Liu 2006; Morales 1986; Morrison 1978; Simpson 1985).
Study participants were combined with a non‐randomised cohort and results were not presented separately (Butterfill 1979; Kuhn 1982).
Two studies were excluded from this update because of greater than 20% post‐randomisation exclusions (Papageorgiou 1979; Schmidt 1984).
Several studies compared repeat‐dose corticosteroids and are eligible for inclusion in the Crowther 2015 review (Khandelwal 2012; Koivisto 2007; Kurtzman 2008; McEvoy 2010).
Refer to Characteristics of excluded studies table.
Risk of bias in included studies
Three studies that were included in the previous review have been excluded. Two (Papageorgiou 1979; Schmidt 1984) were excluded because of greater than 20% post‐randomisation exclusions. The third (Morales 1986) was excluded as it was quasi‐randomised.
Figure 1 and Figure 2 illustrate the risks of bias which are explained in more detail below.
Figure 1.
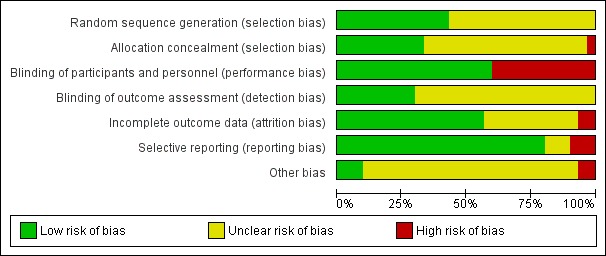
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
Figure 2.
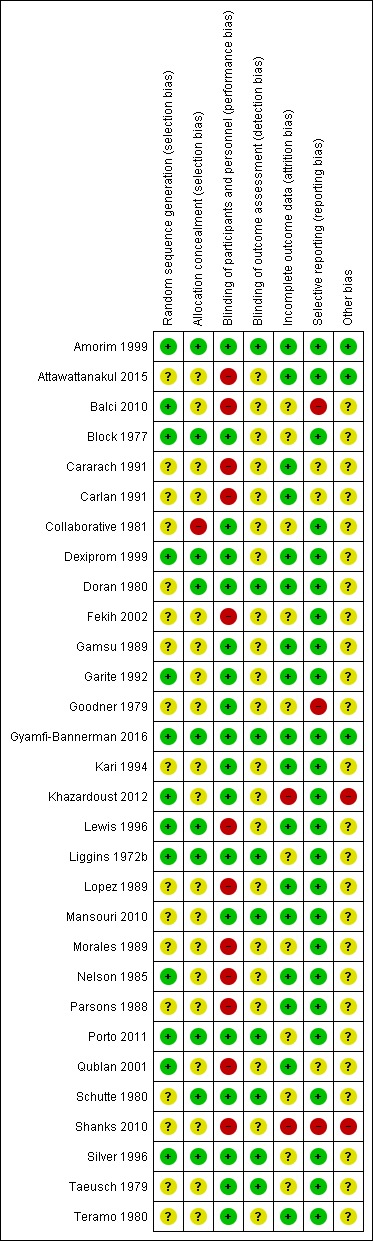
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study
Allocation
Sequence generation
We have summarised the methods of randomisation used in the included studies in the Characteristics of included studies table. Thirteen studies used computer‐generated or random number‐generated randomisation sequences (Amorim 1999; Balci 2010; Block 1977; Dexiprom 1999; Garite 1992; Gyamfi‐Bannerman 2016; Khazardoust 2012; Lewis 1996; Liggins 1972b; Nelson 1985; Porto 2011; Qublan 2001; Silver 1996). We considered these studies at low risk of bias from sequence generation. The 17 remaining studies did not describe the method of sequence generation in sufficient detail to enable a judgement of low risk.
Allocation concealment
Thirteen studies used coded drug boxes/vials in order to conceal the randomisation sequence or study treatment. We assessed one of these studies as having a high risk of bias due to a sealed envelope containing the identity of the contents being attached to each vial "to be opened in emergency only in case of an emergency"; the manuscripts do not state how often these were opened (Collaborative 1981). We assessed a further two studies as unclear risk due to insufficient information provided to confirm the boxes were sequentially numbered (Taeusch 1979; Teramo 1980).
Six studies used sealed envelopes (Garite 1992; Khazardoust 2012; Lewis 1996; Morales 1989; Nelson 1985; Shanks 2010), only one of which was described as opaque (Lewis 1996). The remaining studies did not specify if the envelopes were opaque and we therefore assessed them as having an unclear risk of bias.
Eleven studies did not include any description of the method of allocation concealment and we also assessed them as having an unclear risk of bias (Attawattanakul 2015; Cararach 1991; Carlan 1991; Fekih 2002; Gamsu 1989; Goodner 1979; Kari 1994; Lopez 1989; Mansouri 2010; Parsons 1988; Qublan 2001).
Blinding
Eighteen of the included trials were placebo controlled with the majority of these studies using normal saline, or the vehicle of the corticosteroid preparation, as the placebo (Amorim 1999; Block 1977; Collaborative 1981; Dexiprom 1999; Doran 1980; Gamsu 1989; Garite 1992; Goodner 1979; Gyamfi‐Bannerman 2016; Kari 1994; Khazardoust 2012; Liggins 1972b; Mansouri 2010; Porto 2011; Schutte 1980; Silver 1996; Taeusch 1979; Teramo 1980). The remainder of the included trials were not blinded as they used expectant management in the control arm (Attawattanakul 2015; Balci 2010; Cararach 1991; Carlan 1991; Fekih 2002; Lewis 1996; Lopez 1989; Morales 1989; Nelson 1985; Parsons 1988; Qublan 2001; Shanks 2010).
Blinding of outcome assessors was reported in nine of the 30 trials (Amorim 1999; Doran 1980; Gyamfi‐Bannerman 2016; Liggins 1972b; Mansouri 2010; Porto 2011; Schutte 1980; Silver 1996; Taeusch 1979).
Incomplete outcome data
Nine of the 30 studies reported no losses to follow‐up at birth, which was their only time point for measuring outcome (Attawattanakul 2015; Cararach 1991; Doran 1980; Gamsu 1989; Mansouri 2010; Nelson 1985; Parsons 1988; Qublan 2001; Teramo 1980). In the remaining studies, losses to follow‐up were generally small and less than 5%. There was no evidence to suggest that these exclusions occurred preferentially in one arm or the other of the studies, and we assessed all of them as low risk of bias. We assessed 11 trials as unclear risk of bias due to lack of information or unknown impact of stated exclusions. We assessed two trials as high risk of bias due to loss of over 20% (Shanks 2010) or unclear exclusion (Khazardoust 2012); neither of these trials conducted intention‐to‐treat analysis.
The four studies (Collaborative 1981; Kari 1994; Liggins 1972b; Schutte 1980) that reported long‐term follow‐up after the neonatal period had their follow‐up data included regardless of the follow‐up rate unless there was evidence of bias in follow‐up rates between the treatment and control groups; this was not found to be the case. The Collaborative 1981 trial reported 37% loss to follow‐up at three years of age and we judged it to be at unclear risk of bias. Kari 1994 reported 11% loss to follow‐up at two years of age and we judged it as low risk of bias. Liggins 1972b reported 18% loss to follow‐up at four to six years and 44% losses at the 30‐year follow‐up, we judged risk of bias as unclear. Schutte 1980 reported 12% loss to follow‐up at age 10 to 14 years and 21% at the 20‐year follow‐up, we judged risk of bias as unclear.
Selective reporting
Pre‐specified outcomes appear to have been reported on in 24 of the trials; we assessed these trials as low risk of bias (Amorim 1999; Attawattanakul 2015; Block 1977; Collaborative 1981; Dexiprom 1999; Doran 1980; Fekih 2002; Gamsu 1989; Garite 1992; Gyamfi‐Bannerman 2016; Kari 1994; Khazardoust 2012; Lewis 1996; Liggins 1972b; Lopez 1989; Mansouri 2010; Morales 1989; Nelson 1985; Parsons 1988; Porto 2011; Schutte 1980; Silver 1996; Taeusch 1979; Teramo 1980). Three studies were only available in abstract form and were not published as full‐text articles (Cararach 1991; Carlan 1991; Goodner 1979); we assessed these trials as unclear risk of bias. One trial reported on maternal outcomes that were not pre‐specified (Balci 2010) and one trial pre‐specified RDS as an outcome but did not report the data (Shanks 2010). Shanks 2010 also only reported on maternal outcomes. A third trial (Goodner 1979) only reported on RDS and no other maternal or neonatal outcomes; we assessed these three trials as high risk of bias.
Other potential sources of bias
We assessed Shanks 2010 as high risk of other bias because the trial was stopped early due to problems with recruitment.
In only ten studies was evidence available to suggest that sample‐size calculations had been performed prospectively (Attawattanakul 2015; Amorim 1999; Collaborative 1981; Dexiprom 1999; Gyamfi‐Bannerman 2016; Kari 1994; Porto 2011; Shanks 2010; Silver 1996; Taeusch 1979).
In most trials there was insufficient information to asses if other sources of bias existed. There were no other potential sources of bias identified in one trial Amorim 1999. We assessed one other trial (Khazardoust 2012) as being at high risk of bias for the following reason: "data was analysed for 35 women in the intervention arm versus 40 in the control arm because two delivered before cytokine sampling after the second dose of betamethasone, one opted out of the study and two developed high blood pressure".
We were unclear if further translation of Mansouri 2010 would clarify trial methods and consequent risk of bias domains.
Effects of interventions
See: Table 1
1. Antenatal corticosteroids versus placebo or no treatment (all included studies)
Primary outcomes
Data were not available for all primary outcomes from all included studies.
For the mother
We found similar rates of maternal death in treatment arms, but the calculated risk ratio (RR) is based on just two events from a single trial (one death in each arm); four trials report zero events in both treatment arms limiting our confidence in this finding (RR 0.98, 95% CI 0.06 to 15.50; participants = 3392; studies = 5; moderate‐quality evidence) (Analysis 1.1). There were similar rates of maternal infection: chorioamnionitis (RR 0.83, 95% CI 0.66 to 1.06; participants = 5546; studies = 15; moderate‐quality evidence) (Analysis 1.2) and endometritis (RR 1.20, 95% CI 0.87 to 1.63; participants = 4030; studies = 10; I² = 28%; moderate quality evidence) (Analysis 1.3).
Analysis 1.1.
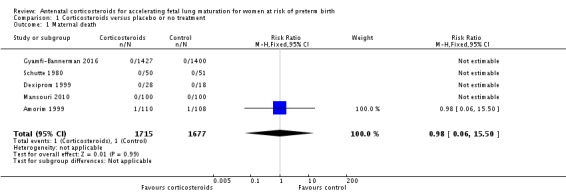
Comparison 1 Corticosteroids versus placebo or no treatment, Outcome 1 Maternal death.
Analysis 1.2.
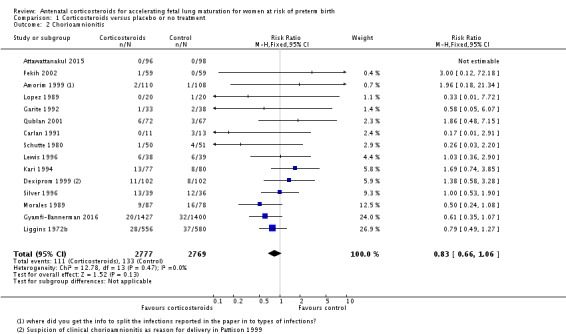
Comparison 1 Corticosteroids versus placebo or no treatment, Outcome 2 Chorioamnionitis.
Analysis 1.3.
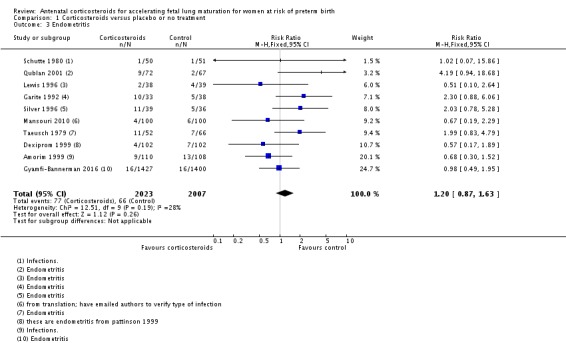
Comparison 1 Corticosteroids versus placebo or no treatment, Outcome 3 Endometritis.
For the fetus or neonate
Treatment with antenatal corticosteroids was associated with an overall average reduction in perinatal death of 28% (average RR 0.72, 95% CI 0.58 to 0.89; participants = 6729; studies = 15; I² = 34%; Tau² = 0.05; moderate‐quality evidence) (Analysis 1.4). This reduction is mainly due to a reduction in neonatal death of 31% (RR 0.69, 95% CI 0.59 to 0.81; participants = 7188; studies = 22) (Analysis 1.5), rather than an impact on fetal death (RR 0.98, 95% CI 0.74 to 1.30; participants = 6729; studies = 15) (Analysis 1.6) where results are inconclusive.
Analysis 1.4.
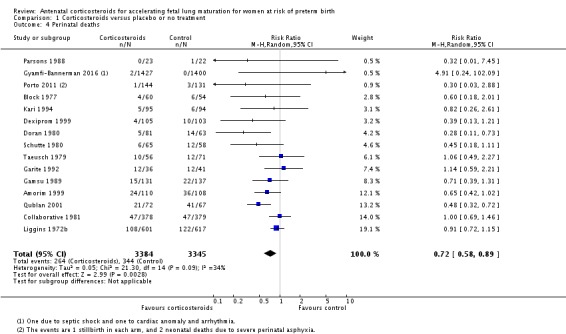
Comparison 1 Corticosteroids versus placebo or no treatment, Outcome 4 Perinatal deaths.
Analysis 1.5.
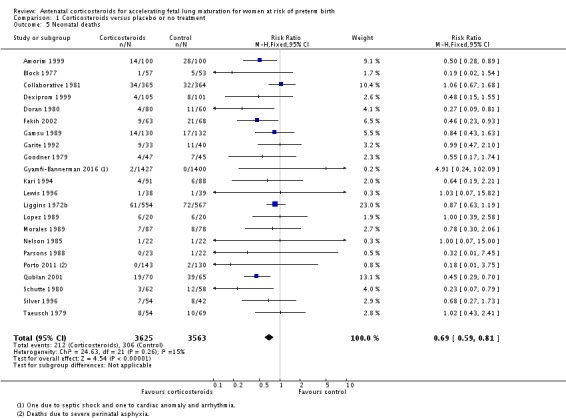
Comparison 1 Corticosteroids versus placebo or no treatment, Outcome 5 Neonatal deaths.
Analysis 1.6.
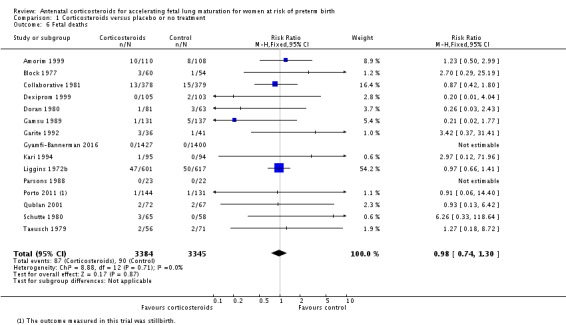
Comparison 1 Corticosteroids versus placebo or no treatment, Outcome 6 Fetal deaths.
Treatment with antenatal corticosteroids was associated with an overall average reduction in RDS of 34% (average RR 0.66, 95% CI 0.56 to 0.77; participants = 7764; studies = 28; I² = 48%; Tau² = 0.06; moderate‐quality evidence) (Analysis 1.7). Moderate to severe RDS was reduced by 41% compared with no exposure to antenatal corticosteroids (average RR 0.59, 95% CI 0.38 to 0.91; participants = 1686; studies = 6; I² = 52%; Tau² = 0.14) (Analysis 1.8). The impact of corticosteroids on chronic lung disease was inconclusive (average RR 0.86, 95% CI 0.42 to 1.79; participants = 818; studies = 6; I² = 65%; Tau² = 0.38) (Analysis 1.9).
Analysis 1.7.
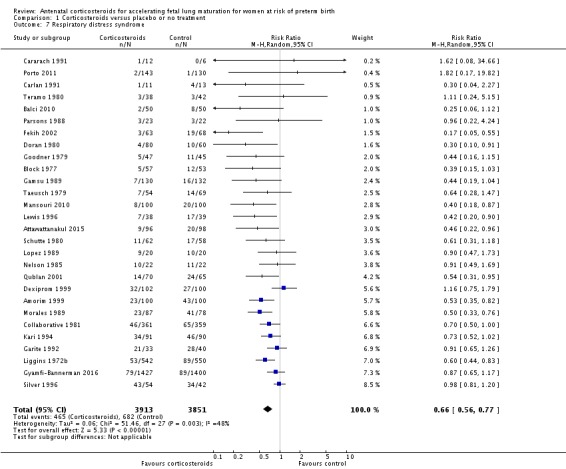
Comparison 1 Corticosteroids versus placebo or no treatment, Outcome 7 Respiratory distress syndrome.
Analysis 1.8.
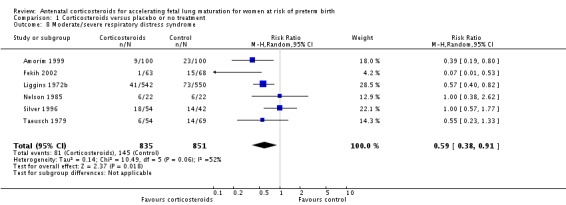
Comparison 1 Corticosteroids versus placebo or no treatment, Outcome 8 Moderate/severe respiratory distress syndrome.
Analysis 1.9.
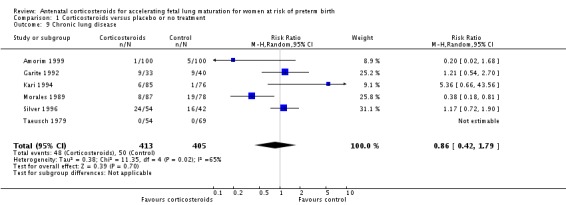
Comparison 1 Corticosteroids versus placebo or no treatment, Outcome 9 Chronic lung disease.
Sensitivity analysis
Moderate/severe RDS and chronic lung disease both had heterogeneity greater than 50%. For moderate/severe RDS (I² = 52%) when we removed one trial (Fekih 2002) with dramatic results favouring steroid use the heterogeneity reduced to 28% for a partial explanation of heterogeneity. Fekih 2002 took place in Tunisia and tested two doses of IM betamethasone 24 hours apart against no treatment (with weekly treatment repeats); the trial was reported in French, and there was limited information to assess several risk of bias domains. The meta‐analysis for chronic lung disease also had heterogeneity over 50%. All included trials were relatively small; three tested betamethasone and three dexamethasone, but the drug used did not explain heterogeneity; neither did the fact that four trials had weekly repeats and two did not (analyses not shown). None of our covariates (membrane status, multiple pregnancy, or decade of trial) explained the heterogeneity found.
Treatment with antenatal corticosteroids was associated with an overall average reduction in IVH of 45% (average RR 0.55, 95% CI 0.40 to 0.76; participants = 6093; studies = 16; I² = 33%; Tau² = 0.10; moderate‐quality evidence) (Analysis 1.10). A reduction was also seen for infants with severe IVH (Grades 3 and 4) (RR 0.26, 95% CI 0.11 to 0.60; participants = 3438; studies = 6; analysis not shown).
Analysis 1.10.
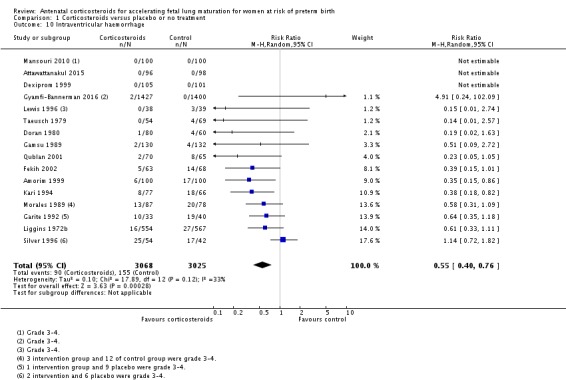
Comparison 1 Corticosteroids versus placebo or no treatment, Outcome 10 Intraventricular haemorrhage.
Babies in both treatment groups had similar mean birthweight (mean difference (MD) ‐18.47, 95% CI ‐40.83 to 3.90; participants = 6182; studies = 16; I² = 5%; moderate‐quality evidence) (Analysis 1.11).
Analysis 1.11.
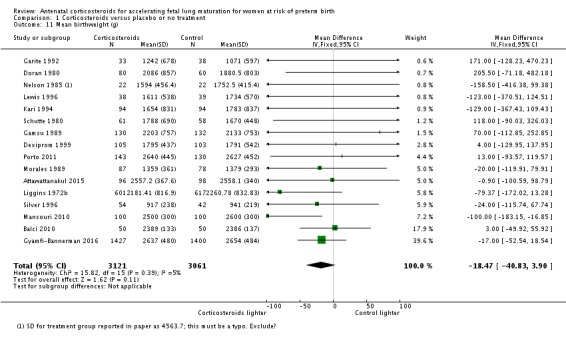
Comparison 1 Corticosteroids versus placebo or no treatment, Outcome 11 Mean birthweight (g).
For the child
The impact of corticosteroid exposure on death in childhood was inconclusive (RR 0.68, 95% CI 0.36 to 1.27; participants = 1010; studies = 4) (Analysis 1.12), with a similar result for neurodevelopmental delay (RR 0.64, 95% CI 0.14 to 2.98; participants = 82; studies = 1) (Analysis 1.13).
Analysis 1.12.
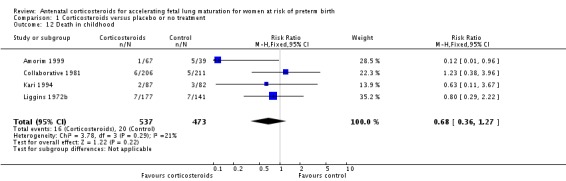
Comparison 1 Corticosteroids versus placebo or no treatment, Outcome 12 Death in childhood.
Analysis 1.13.
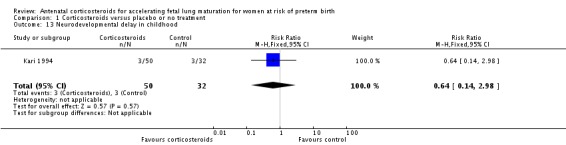
Comparison 1 Corticosteroids versus placebo or no treatment, Outcome 13 Neurodevelopmental delay in childhood.
For the child as adult
The impact of corticosteroid exposure on death into adulthood was also inconclusive (RR 1.00, 95% CI 0.56 to 1.81; participants = 988; studies = 1) (Analysis 1.14).
Analysis 1.14.
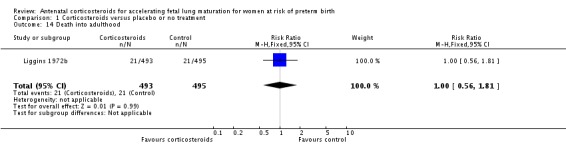
Comparison 1 Corticosteroids versus placebo or no treatment, Outcome 14 Death into adulthood.
Secondary outcomes
Data were available for several of the secondary outcomes that related to the mother, fetus or neonate, child, adult and health services.
For the mother
Women in both treatment groups had similar rates of: fever after trial entry requiring the use of antibiotics (average RR 0.95, 95% CI 0.43 to 2.06; participants = 481; studies = 4; I² = 61%, Tau² = 0.35) (Analysis 1.15), intrapartum fever requiring the use of antibiotics (average RR 0.66, 95% CI 0.09 to 4.89; participants = 319; studies = 2; I² = 36%, Tau² = 0.74) (Analysis 1.16), postnatal fever (RR 0.92, 95% CI 0.64 to 1.33; participants = 1323; studies = 5) (Analysis 1.20), admission to adult intensive care unit (RR 0.74, 95% CI 0.26 to 2.05; participants = 319; studies = 2) (Analysis 1.18), and hypertension (RR 1.00, 95% CI 0.36 to 2.76; participants = 220; studies = 1) (Analysis 1.19).
Analysis 1.15.
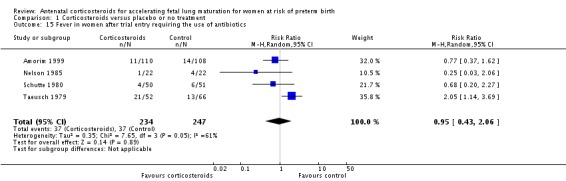
Comparison 1 Corticosteroids versus placebo or no treatment, Outcome 15 Fever in women after trial entry requiring the use of antibiotics.
Analysis 1.16.
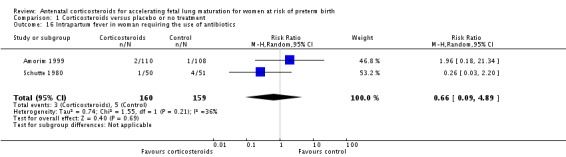
Comparison 1 Corticosteroids versus placebo or no treatment, Outcome 16 Intrapartum fever in woman requiring the use of antibiotics.
Analysis 1.20.
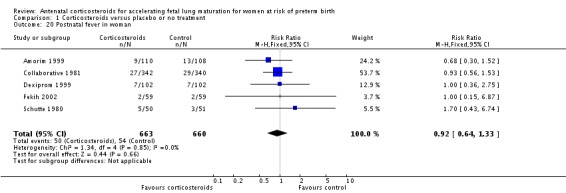
Comparison 1 Corticosteroids versus placebo or no treatment, Outcome 20 Postnatal fever in woman.
Analysis 1.18.
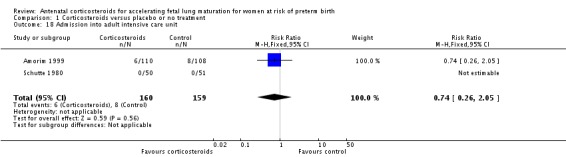
Comparison 1 Corticosteroids versus placebo or no treatment, Outcome 18 Admission into adult intensive care unit.
Analysis 1.19.
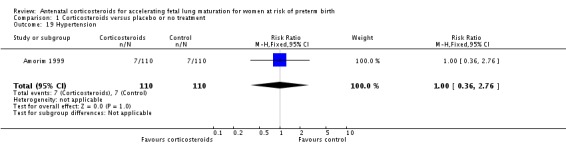
Comparison 1 Corticosteroids versus placebo or no treatment, Outcome 19 Hypertension.
Five trials reported no side effects for women in any arm. In a sixth trial more women receiving antenatal corticosteroids reported side effects of treatment (RR 0.69, 95% CI 0.59 to 0.82; participants = 3572; studies = 6; all events from a single trial; Analysis 1.17). Most side effects were pain or bruising at the injection site (close to 80% of reported side effects in both arms); other side effects were local reactions at the injection site, gastrointestinal upset, headache and other.
Analysis 1.17.
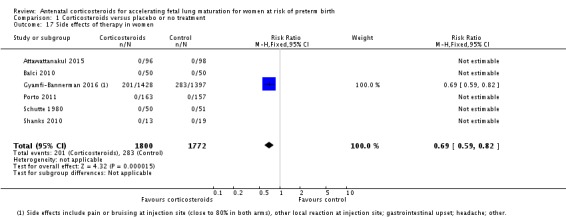
Comparison 1 Corticosteroids versus placebo or no treatment, Outcome 17 Side effects of therapy in women.
One small study (Amorim 1999), reported that women in the corticosteroid arm were more likely to have glucose intolerance than in the control arm (RR 2.71, 95% CI 1.14 to 6.46; participants = 123; studies = 1; Analysis 1.21). This study used a treatment regimen that included weekly repeat doses of corticosteroids if the infant remained undelivered.
Analysis 1.21.
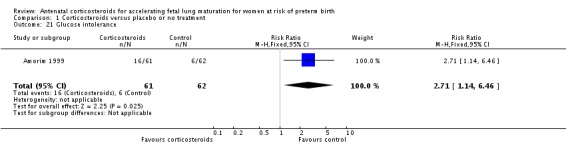
Comparison 1 Corticosteroids versus placebo or no treatment, Outcome 21 Glucose intolerance.
For the fetus or neonate
Treatment with antenatal corticosteroids was associated with a reduction in the incidence of necrotising enterocolitis (RR 0.50, 95% CI 0.32 to 0.78; participants = 4702; studies = 10) (Analysis 1.22). Treatment with antenatal corticosteroids was also associated with fewer infants having systemic infection in the first 48 hours after birth (RR 0.60, 95% CI 0.41 to 0.88; participants = 1753; studies = 8) (Analysis 1.23); however, infants in both treatment arms had similar rates of proven infection while in the NICU (average RR 0.77, 95% CI 0.55 to 1.08; participants = 5707; studies = 13; I² = 34%; Tau² = 0.09) (Analysis 1.24).
Analysis 1.22.
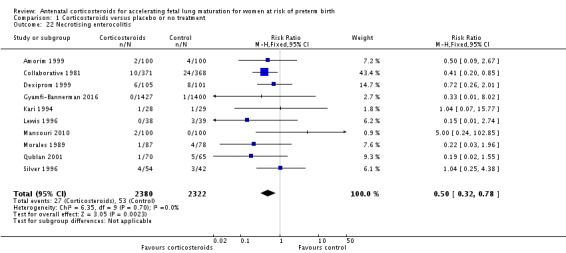
Comparison 1 Corticosteroids versus placebo or no treatment, Outcome 22 Necrotising enterocolitis.
Analysis 1.23.
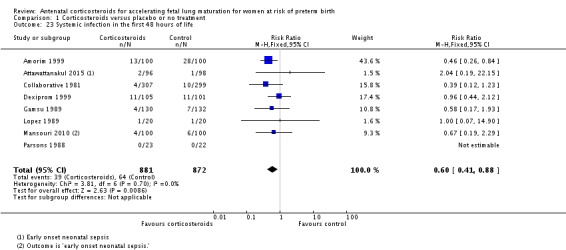
Comparison 1 Corticosteroids versus placebo or no treatment, Outcome 23 Systemic infection in the first 48 hours of life.
Analysis 1.24.
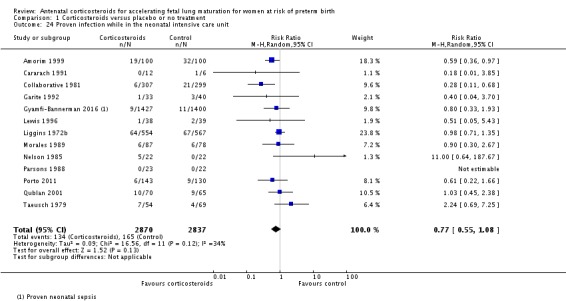
Comparison 1 Corticosteroids versus placebo or no treatment, Outcome 24 Proven infection while in the neonatal intensive care unit.
Treatment with antenatal corticosteroids was associated with less need for neonatal respiratory support, with a reduction in the need for mechanical ventilation/CPAP (RR 0.68, 95% CI 0.56 to 0.84; participants = 1368; studies = 9) (Analysis 1.25). Infants receiving corticosteroids also required less oxygen supplementation (MD ‐2.86 days, 95% CI ‐5.51 to ‐0.21 days; one study, 73 infants) (Analysis 1.27), and fewer infants receiving corticosteroids needed surfactant (RR 0.68, 95% CI 0.51 to 0.90; participants = 3556; studies = 5) (Analysis 1.28).
Analysis 1.25.
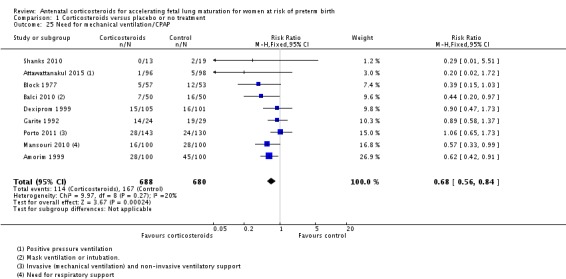
Comparison 1 Corticosteroids versus placebo or no treatment, Outcome 25 Need for mechanical ventilation/CPAP.
Analysis 1.27.
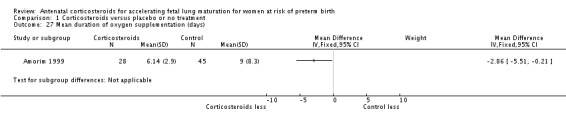
Comparison 1 Corticosteroids versus placebo or no treatment, Outcome 27 Mean duration of oxygen supplementation (days).
Analysis 1.28.
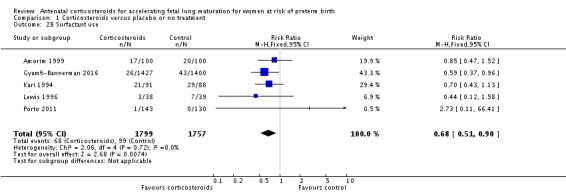
Comparison 1 Corticosteroids versus placebo or no treatment, Outcome 28 Surfactant use.
Infants in treatment and control groups had similar results for several outcomes: time requiring mechanical ventilation/CPAP (MD ‐1.91 days, 95% CI ‐4.59 to 0.76 days; participants = 471; studies = 3; I² = 77%; Tau² = 3.28) (Analysis 1.26), air leak syndrome (RR 0.76, 95% CI 0.32 to 1.80; participants = 2965; studies = 2) (Analysis 1.29), interval between trial entry and delivery (MD 0.23 days, 95% CI ‐1.86 to 2.32 days; participants = 1513; studies = 3) (Analysis 1.31), incidence of small‐for‐gestational‐age infants (RR 1.11, 95% CI 0.96 to 1.28; participants = 3478; studies = 5) (Analysis 1.32), or HPA axis function (cortisol MD 3.94 , 95% CI ‐3.12 to 11.00 log units; participants = 27; studies = 1) (Analysis 1.33).
Analysis 1.26.
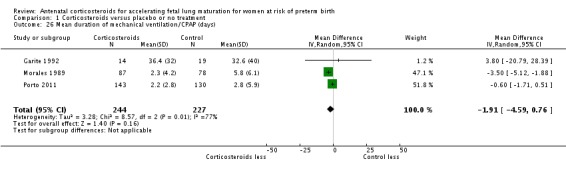
Comparison 1 Corticosteroids versus placebo or no treatment, Outcome 26 Mean duration of mechanical ventilation/CPAP (days).
Analysis 1.29.
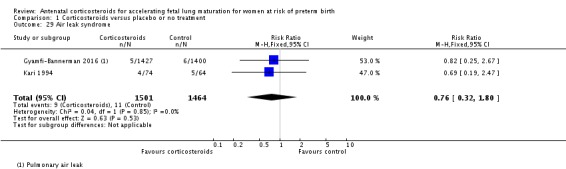
Comparison 1 Corticosteroids versus placebo or no treatment, Outcome 29 Air leak syndrome.
Analysis 1.31.
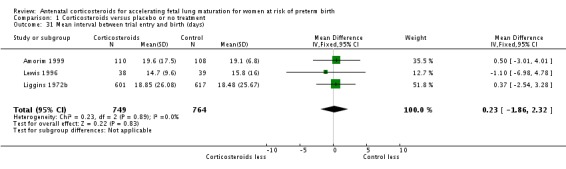
Comparison 1 Corticosteroids versus placebo or no treatment, Outcome 31 Mean interval between trial entry and birth (days).
Analysis 1.32.
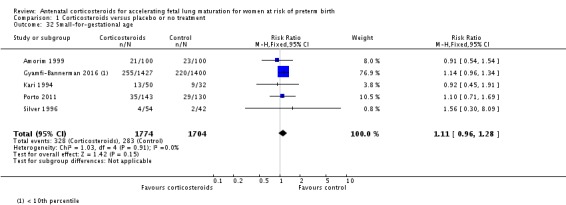
Comparison 1 Corticosteroids versus placebo or no treatment, Outcome 32 Small‐for‐gestational age.
Analysis 1.33.
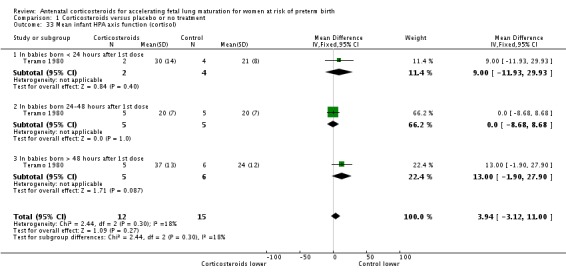
Comparison 1 Corticosteroids versus placebo or no treatment, Outcome 33 Mean infant HPA axis function (cortisol).
Fewer infants exposed to antenatal corticosteroids had an Apgar score less than seven at five minutes of age (RR 0.81, 95% CI 0.67 to 0.98; participants = 2419; studies = 10) (Analysis 1.30), or required admission into a NICU (RR 0.90, 95% CI 0.84 to 0.97; participants = 3803; studies = 7) (Analysis 1.34).
Analysis 1.30.
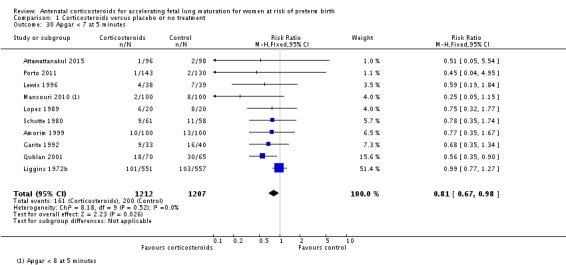
Comparison 1 Corticosteroids versus placebo or no treatment, Outcome 30 Apgar < 7 at 5 minutes.
Analysis 1.34.
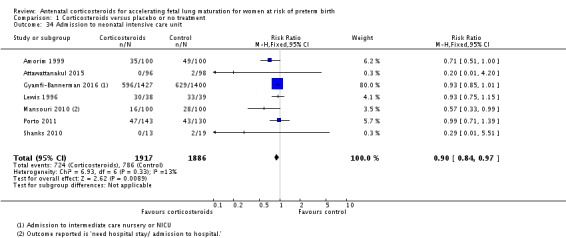
Comparison 1 Corticosteroids versus placebo or no treatment, Outcome 34 Admission to neonatal intensive care unit.
For the child
Treatment with corticosteroids was associated with less developmental delay in childhood (RR 0.49, 95% CI 0.24 to 1.00; participants = 518; studies = 2; age at follow‐up three years in one study and unknown in one study) (Analysis 1.35), but results for cerebral palsy less conclusive (RR 0.60, 95% CI 0.34 to 1.03; P = 0.86; participants = 904; studies = 5, age at follow‐up was two to six years in four studies, and unknown in one study) (Analysis 1.36).
Analysis 1.35.
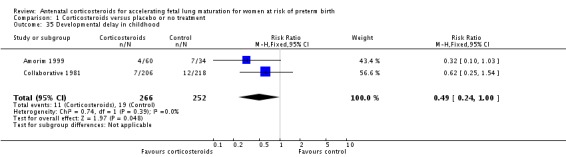
Comparison 1 Corticosteroids versus placebo or no treatment, Outcome 35 Developmental delay in childhood.
Analysis 1.36.
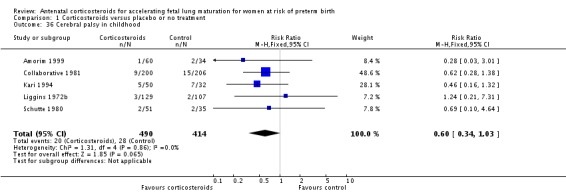
Comparison 1 Corticosteroids versus placebo or no treatment, Outcome 36 Cerebral palsy in childhood.
Children with and without treatment had similar results for: childhood weight (MD 0.30 kg, 95% CI ‐0.39 to 1.00 kg; participants = 333; studies = 2) (Analysis 1.37), height (MD 1.02 cm, 95% CI ‐0.26 to 2.29 cm; participants = 334; studies = 2) (Analysis 1.38), head circumference (MD 0.27 cm, 95% CI ‐0.08 to 0.63 cm; participants = 328; studies = 2) (Analysis 1.39), lung function (vital capacity (VC) MD ‐1.68 % predicted, 95% CI ‐5.12 to 1.75 % predicted; participants = 150; studies = 2) (Analysis 1.40), forced expiratory volume in one second (FEV1) (MD ‐4.73 % predicted, 95% CI ‐10.13 to 0.67 % predicted; participants = 75; studies = 1) (Analysis 1.41); FEV1/VC (MD ‐0.94, 95% CI ‐3.63 to 1.76; participants = 150; studies = 2; I² = 31%; Tau² = 1.78) (Analysis 1.42), systolic blood pressure (MD ‐1.60 mmHg, 95% CI ‐4.06 to 0.86 mmHg; participants = 223; studies = 1) (Analysis 1.43), visual impairment (RR 0.55, 95% CI 0.24 to 1.23; participants = 166; studies = 2) (Analysis 1.44), hearing impairment (RR 0.64, 95% CI 0.04 to 9.87; participants = 166; studies = 2) (Analysis 1.45), behavioural/learning difficulties (RR 0.86, 95% CI 0.35 to 2.09; participants = 90; studies = 1) (Analysis 1.47) or intellectual impairment (RR 0.86, 95% CI 0.44 to 1.69; participants = 778; studies = 3) (Analysis 1.46).
Analysis 1.37.
Comparison 1 Corticosteroids versus placebo or no treatment, Outcome 37 Mean childhood weight (kg).
Analysis 1.38.
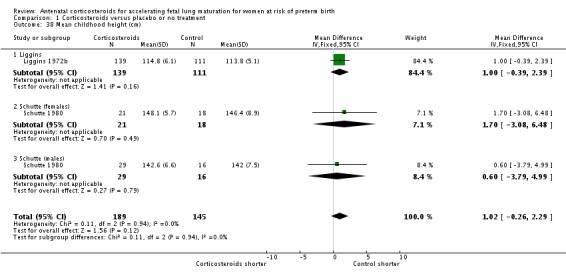
Comparison 1 Corticosteroids versus placebo or no treatment, Outcome 38 Mean childhood height (cm).
Analysis 1.39.
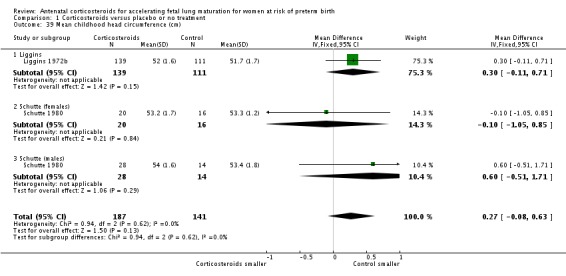
Comparison 1 Corticosteroids versus placebo or no treatment, Outcome 39 Mean childhood head circumference (cm).
Analysis 1.40.
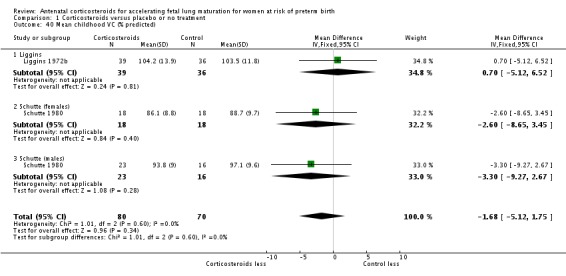
Comparison 1 Corticosteroids versus placebo or no treatment, Outcome 40 Mean childhood VC (% predicted).
Analysis 1.41.
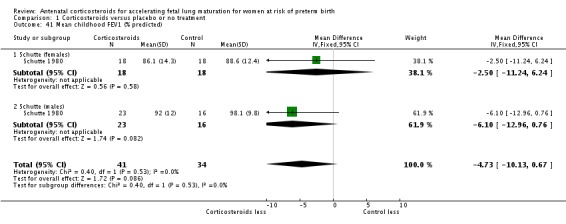
Comparison 1 Corticosteroids versus placebo or no treatment, Outcome 41 Mean childhood FEV1 (% predicted).
Analysis 1.42.
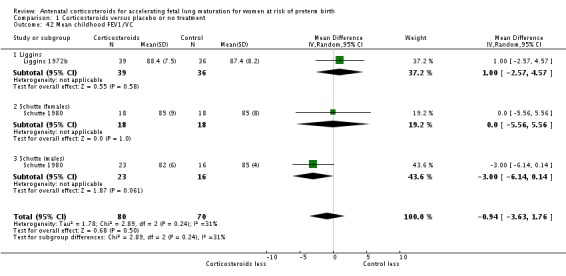
Comparison 1 Corticosteroids versus placebo or no treatment, Outcome 42 Mean childhood FEV1/VC.
Analysis 1.43.
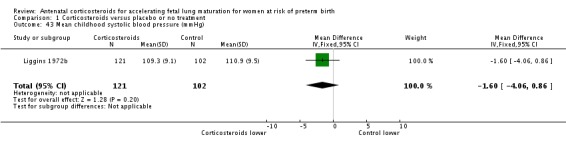
Comparison 1 Corticosteroids versus placebo or no treatment, Outcome 43 Mean childhood systolic blood pressure (mmHg).
Analysis 1.44.
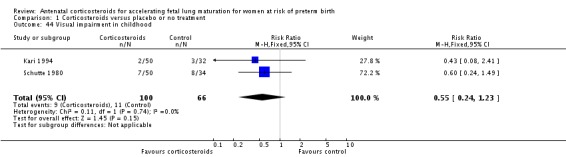
Comparison 1 Corticosteroids versus placebo or no treatment, Outcome 44 Visual impairment in childhood.
Analysis 1.45.
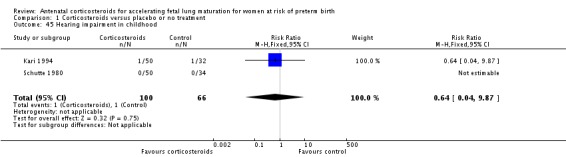
Comparison 1 Corticosteroids versus placebo or no treatment, Outcome 45 Hearing impairment in childhood.
Analysis 1.47.
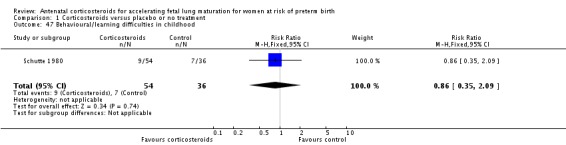
Comparison 1 Corticosteroids versus placebo or no treatment, Outcome 47 Behavioural/learning difficulties in childhood.
Analysis 1.46.
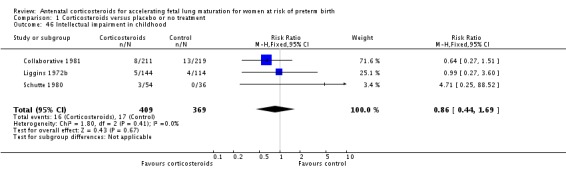
Comparison 1 Corticosteroids versus placebo or no treatment, Outcome 46 Intellectual impairment in childhood.
For the child as adult
Long‐term follow‐up in one study (Liggins 1972b) showed increased insulin release 30 minutes following a fasting 75 g oral glucose tolerance test (MD 0.16 log insulin units, 95% CI 0.04 to 0.28 log insulin units; participants = 412; studies = 1) in 30‐year‐olds who had been exposed to antenatal corticosteroid. Results were inconclusive for fasting glucose concentrations (MD 0.01 mmol/L, 95% CI ‐0.09 to 0.11 mmol/L; participants = 432; studies = 1), or 30 minutes following a 75 g oral glucose tolerance test (MD 0.19 mmol/L, 95% CI ‐0.14 to 0.52 mmol/L; participants = 413; studies = 1). At 120 minutes following a 75 g oral glucose tolerance test, exposure to antenatal corticosteroids was associated with a reduction in glucose concentration (MD ‐0.27 mmol/L; 95% CI ‐0.52 to ‐0.02 mmol/L; P = 0.04; participants = 410; studies = 1) (Analysis 1.48; Analysis 1.49). However, the study reported no difference between those exposed to antenatal corticosteroids and those not exposed in the prevalence of diabetes (results not shown).
Analysis 1.48.
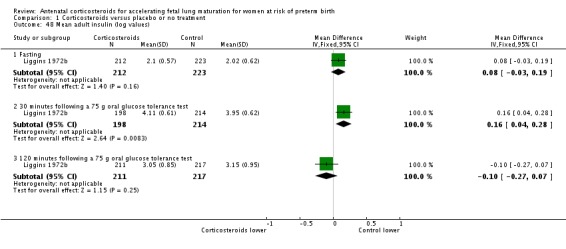
Comparison 1 Corticosteroids versus placebo or no treatment, Outcome 48 Mean adult insulin (log values).
Analysis 1.49.
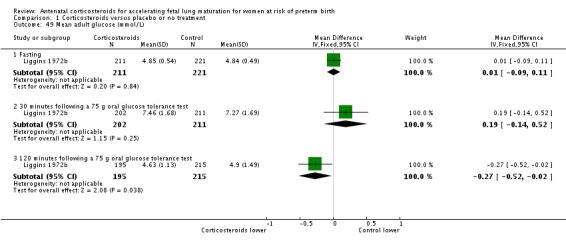
Comparison 1 Corticosteroids versus placebo or no treatment, Outcome 49 Mean adult glucose (mmol/L).
The impact of corticosteroids on the following was inconclusive: weight (MD ‐0.83 kg, 95% CI ‐6.41 to 4.76 kg; participants = 538; studies = 2; I² = 60%; Tau² = 14.50) (Analysis 1.50), height (MD 0.91 cm, 95% CI ‐0.28 to 2.10 cm; participants = 537; studies = 2) (Analysis 1.51), head circumference (MD 0.03 cm, 95% CI ‐0.33 to 0.38 cm; participants = 537; studies = 2) (Analysis 1.52), skin fold thickness (triceps MD ‐0.02 log units, 95% CI ‐0.11 to 0.07 log units; participants = 456; studies = 1) (Analysis 1.53), systolic blood pressure (MD ‐1.53 mmHg, 95% CI ‐4.50 to 1.44 mmHg; participants = 545; studies = 2; I² = 47%; Tau² = 3.29) (Analysis 1.54), HPA axis function (cortisol MD 0.06 log units, 95% CI ‐0.02 to 0.14 log units; participants = 444; studies = 1) (Analysis 1.55), cholesterol (MD ‐0.11 mmol/L, 95% CI ‐0.28 to 0.06 mmol/L; participants = 445; studies = 1) (Analysis 1.56), age at puberty (MD for girls 0 years, 95% CI ‐0.94 to 0.94 years; participants = 38; studies = 1) (Analysis 1.57), educational achievement (RR 0.94, 95% CI 0.80 to 1.10; participants = 534; studies = 1) (Analysis 1.58), visual impairment (RR 0.91, 95% CI 0.53 to 1.55; participants = 192; studies = 1) (Analysis 1.59), hearing impairment (RR 0.24, 95% CI 0.03 to 2.03; participants = 192; studies = 1) (Analysis 1.60) or intellectual impairment (RR 0.24, 95% CI 0.01 to 4.95; participants = 273; studies = 2) (Analysis 1.61). There was no difference between those exposed to antenatal corticosteroids and those not exposed for lung function or bone density at age 30 years in participants followed from one study (Liggins 1972b).
Analysis 1.50.
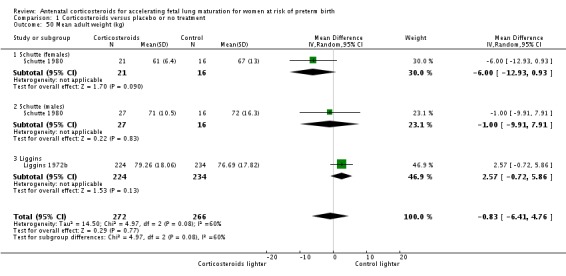
Comparison 1 Corticosteroids versus placebo or no treatment, Outcome 50 Mean adult weight (kg).
Analysis 1.51.
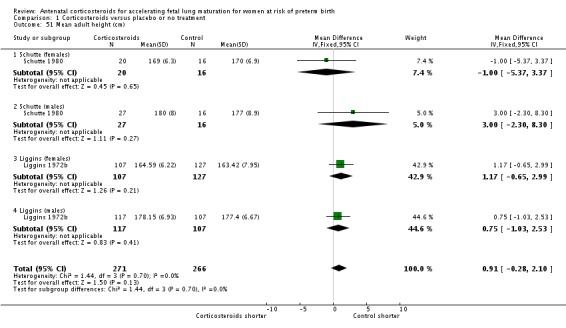
Comparison 1 Corticosteroids versus placebo or no treatment, Outcome 51 Mean adult height (cm).
Analysis 1.52.
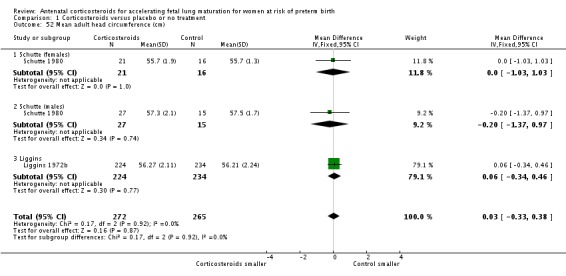
Comparison 1 Corticosteroids versus placebo or no treatment, Outcome 52 Mean adult head circumference (cm).
Analysis 1.53.
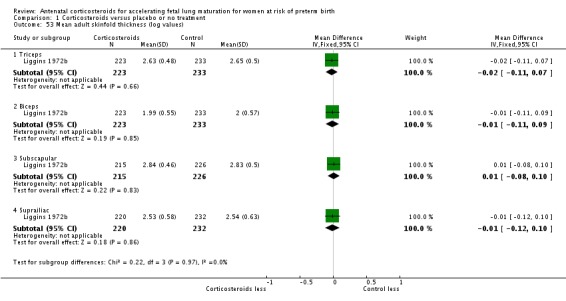
Comparison 1 Corticosteroids versus placebo or no treatment, Outcome 53 Mean adult skinfold thickness (log values).
Analysis 1.54.
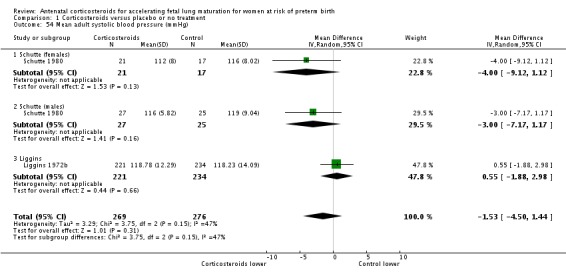
Comparison 1 Corticosteroids versus placebo or no treatment, Outcome 54 Mean adult systolic blood pressure (mmHg).
Analysis 1.55.
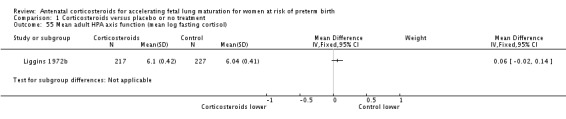
Comparison 1 Corticosteroids versus placebo or no treatment, Outcome 55 Mean adult HPA axis function (mean log fasting cortisol).
Analysis 1.56.
Comparison 1 Corticosteroids versus placebo or no treatment, Outcome 56 Mean cholesterol in adulthood (mmol/L).
Analysis 1.57.
Comparison 1 Corticosteroids versus placebo or no treatment, Outcome 57 Mean age at puberty (years).
Analysis 1.58.
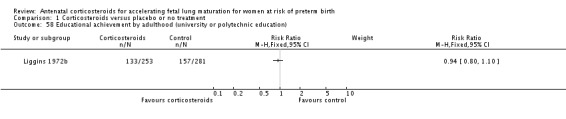
Comparison 1 Corticosteroids versus placebo or no treatment, Outcome 58 Educational achievement by adulthood (university or polytechnic education).
Analysis 1.59.
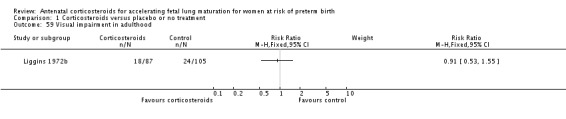
Comparison 1 Corticosteroids versus placebo or no treatment, Outcome 59 Visual impairment in adulthood.
Analysis 1.60.
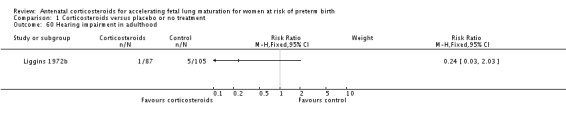
Comparison 1 Corticosteroids versus placebo or no treatment, Outcome 60 Hearing impairment in adulthood.
Analysis 1.61.
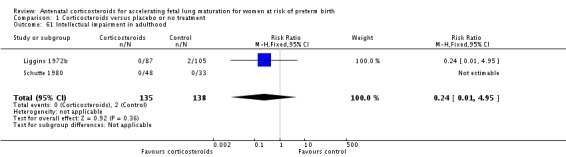
Comparison 1 Corticosteroids versus placebo or no treatment, Outcome 61 Intellectual impairment in adulthood.
Results were similar for treatment groups for all of the other child‐as‐an‐adult outcomes examined (Analysis 1.62; Analysis 1.63; Analysis 1.64; Analysis 1.65; Analysis 1.66; Analysis 1.67; Analysis 1.68; Analysis 1.69; Analysis 1.70; Analysis 1.71; Analysis 1.72; Analysis 1.73; Analysis 1.74; Analysis 1.75; Analysis 1.76; Analysis 1.77; Analysis 1.78; Analysis 1.79; Analysis 1.80; Analysis 1.81; Analysis 1.82; Analysis 1.83).
Analysis 1.62.
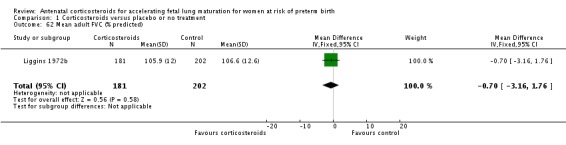
Comparison 1 Corticosteroids versus placebo or no treatment, Outcome 62 Mean adult FVC (% predicted).
Analysis 1.63.
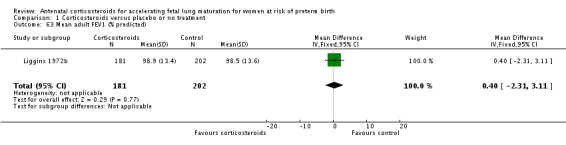
Comparison 1 Corticosteroids versus placebo or no treatment, Outcome 63 Mean adult FEV1 (% predicted).
Analysis 1.64.
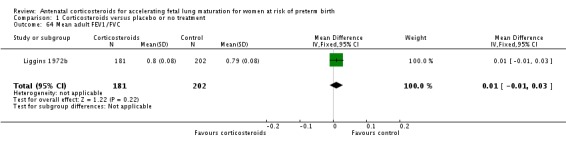
Comparison 1 Corticosteroids versus placebo or no treatment, Outcome 64 Mean adult FEV1/FVC.
Analysis 1.65.
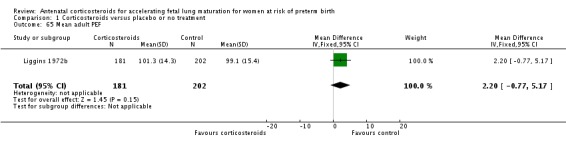
Comparison 1 Corticosteroids versus placebo or no treatment, Outcome 65 Mean adult PEF.
Analysis 1.66.
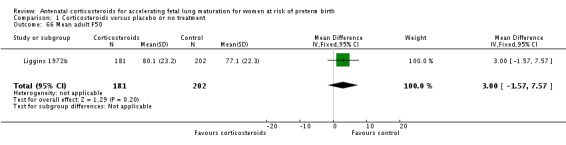
Comparison 1 Corticosteroids versus placebo or no treatment, Outcome 66 Mean adult F50.
Analysis 1.67.
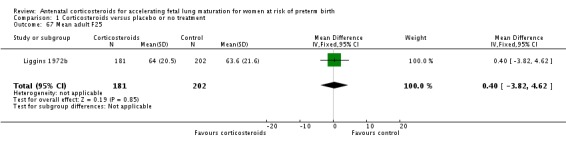
Comparison 1 Corticosteroids versus placebo or no treatment, Outcome 67 Mean adult F25.
Analysis 1.68.
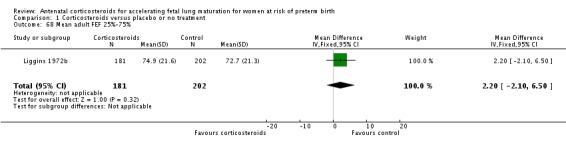
Comparison 1 Corticosteroids versus placebo or no treatment, Outcome 68 Mean adult FEF 25%‐75%.
Analysis 1.69.
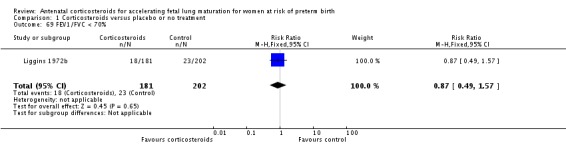
Comparison 1 Corticosteroids versus placebo or no treatment, Outcome 69 FEV1/FVC < 70%.
Analysis 1.70.
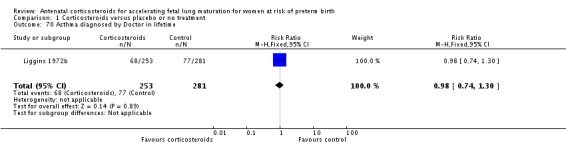
Comparison 1 Corticosteroids versus placebo or no treatment, Outcome 70 Asthma diagnosed by Doctor in lifetime.
Analysis 1.71.
Comparison 1 Corticosteroids versus placebo or no treatment, Outcome 71 Wheezing in last 12 months.
Analysis 1.72.
Comparison 1 Corticosteroids versus placebo or no treatment, Outcome 72 Current Asthma.
Analysis 1.73.
Comparison 1 Corticosteroids versus placebo or no treatment, Outcome 73 Further respiratory diagnosis (includes pneumonia, upper airway conditions and bronchitis).
Analysis 1.74.
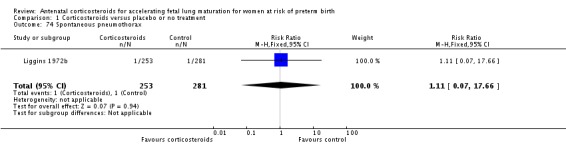
Comparison 1 Corticosteroids versus placebo or no treatment, Outcome 74 Spontaneous pneumothorax.
Analysis 1.75.
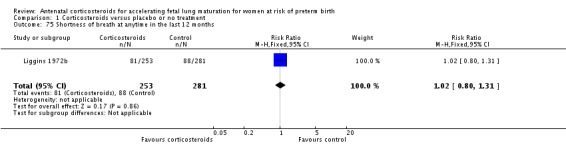
Comparison 1 Corticosteroids versus placebo or no treatment, Outcome 75 Shortness of breath at anytime in the last 12 months.
Analysis 1.76.
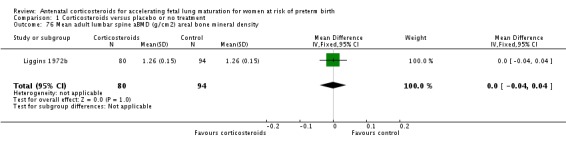
Comparison 1 Corticosteroids versus placebo or no treatment, Outcome 76 Mean adult lumbar spine aBMD (g/cm2) areal bone mineral density.
Analysis 1.77.
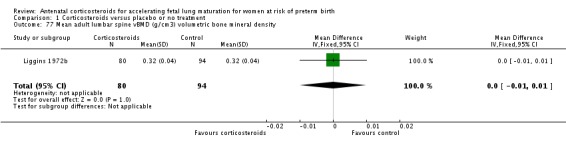
Comparison 1 Corticosteroids versus placebo or no treatment, Outcome 77 Mean adult lumbar spine vBMD (g/cm3) volumetric bone mineral density.
Analysis 1.78.
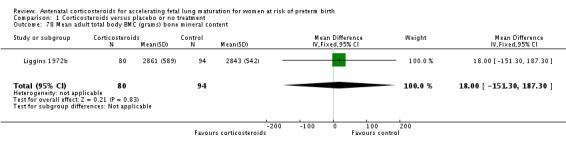
Comparison 1 Corticosteroids versus placebo or no treatment, Outcome 78 Mean adult total body BMC (grams) bone mineral content.
Analysis 1.79.
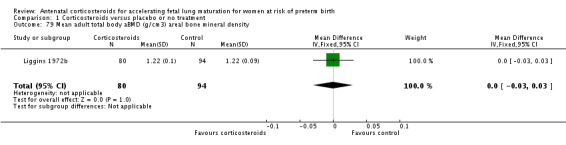
Comparison 1 Corticosteroids versus placebo or no treatment, Outcome 79 Mean adult total body aBMD (g/cm3) areal bone mineral density.
Analysis 1.80.
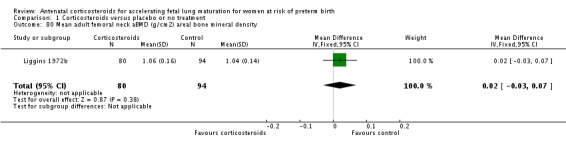
Comparison 1 Corticosteroids versus placebo or no treatment, Outcome 80 Mean adult femoral neck aBMD (g/cm2) areal bone mineral density.
Analysis 1.81.
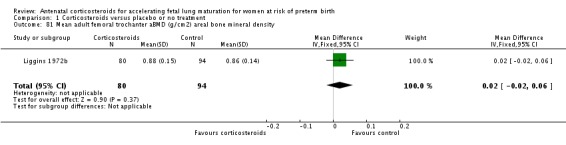
Comparison 1 Corticosteroids versus placebo or no treatment, Outcome 81 Mean adult femoral trochanter aBMD (g/cm2) areal bone mineral density.
Analysis 1.82.
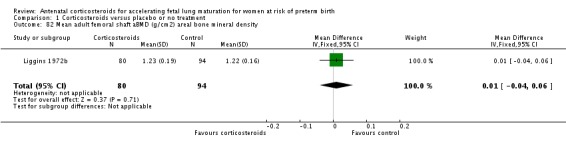
Comparison 1 Corticosteroids versus placebo or no treatment, Outcome 82 Mean adult femoral shaft aBMD (g/cm2) areal bone mineral density.
Analysis 1.83.
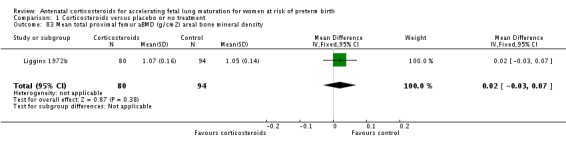
Comparison 1 Corticosteroids versus placebo or no treatment, Outcome 83 Mean total proximal femur aBMD (g/cm2) areal bone mineral density.
For the health services
Use of corticosteroids did not appear to shorten antenatal hospitalisation in women in a single small trial (MD 0.50 days, 95% CI ‐1.40 to 2.40 days; participants = 218; studies = 1) (Analysis 1.84); results were also inconclusive for postnatal hospitalisation in women (MD 0.00 days, 95% CI ‐1.72 to 1.72 days; participants = 218; studies = 1) (Analysis 1.85).
Analysis 1.84.
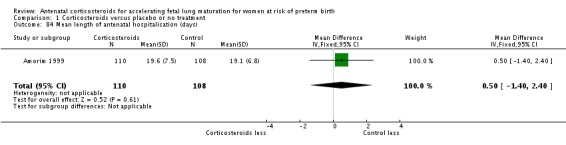
Comparison 1 Corticosteroids versus placebo or no treatment, Outcome 84 Mean length of antenatal hospitalisation (days).
Analysis 1.85.
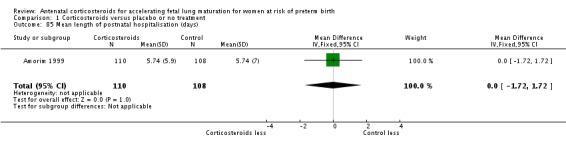
Comparison 1 Corticosteroids versus placebo or no treatment, Outcome 85 Mean length of postnatal hospitalisation (days).
Mansouri 2010 (Iran) reported equal numbers of women in each group requiring a hospital stay of more than three days (12/100 corticosteroid and 12/100 placebo); Attawattanakul 2015 (Thailand) reported a similar overall maternal length of stay for both treatment groups (corticosteroid mean 3.57 (SD 0.87), n = 96; control mean 3.58 (SD 0.75), n = 98); and Gyamfi‐Bannerman 2016 (USA; n = 2827) reported a median maternal length of hospital stay of three days (IQR 3 to 5 days) for both treatment groups.
Infants with and without corticosteroids required similar stays in hospital (MD 0.18 days, 95% CI ‐0.51 to 0.87 days; participants = 788; studies = 5) (Analysis 1.86). Gyamfi‐Bannerman 2016 (USA) reported a median neonatal hospitalisation of seven days (IQR 4 to 12 days) in the corticosteroid group (n = 1427) and a median of eight days (IQR 4 to 13 days) for the controls (n = 1400).
Analysis 1.86.
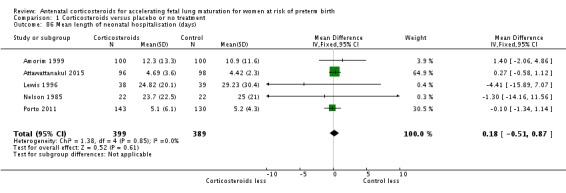
Comparison 1 Corticosteroids versus placebo or no treatment, Outcome 86 Mean length of neonatal hospitalisation (days).
Investigation of publication bias
Where more than 10 studies contributed data to the analyses, we inspected funnel plots for evidence of asymmetry and possible publication bias (Figure 3; Figure 4; Figure 5; Figure 6; Figure 7; Figure 8; Figure 9). Funnel plots for two outcomes 1.4 Perinatal deaths (Figure 4) and 1.11 IVH (Figure 8) both showed asymmetry. For perinatal deaths, two studies with very low event rates were the funnel plot outliers; one small study (Parsons 1988) had no events in the corticosteroid arm and one death in the control group, and one large study (Gyamfi‐Bannerman 2016) had no events in the control arm and two deaths in the corticosteroid group. For IVH, the funnel plot mapped 13 of the 16 studies due to no events in both arms of three studies (Attawattanakul 2015; Dexiprom 1999; Mansouri 2010). Two small studies (Lewis 1996; Taeusch 1979) had considerably larger effect sizes than the rest (with no events in the corticosteroid arm), one large study (Gyamfi‐Bannerman 2016) had no events in the control arm, and these studies contributed to funnel plot asymmetry. Publication bias could not be excluded as some of the asymmetry for both of these outcomes appeared attributable to small studies with positive results.
Figure 3.
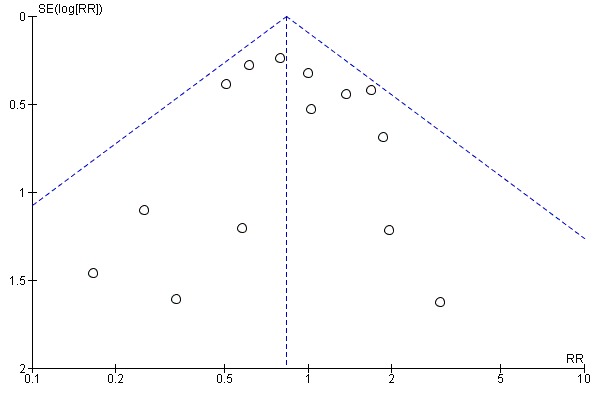
Funnel plot of comparison: 1 Corticosteroids versus placebo or no treatment, outcome: 1.2 Chorioamnionitis
Figure 4.
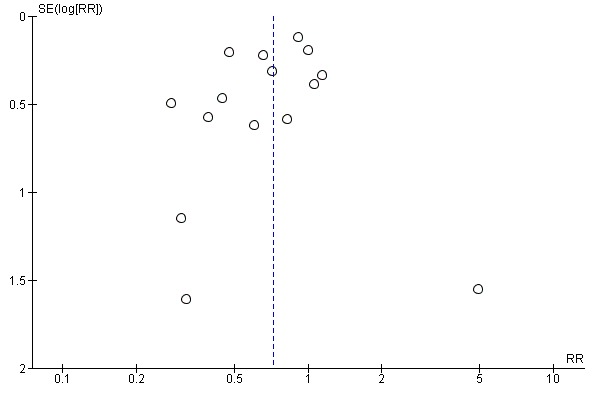
Funnel plot of comparison: 1 Corticosteroids versus placebo or no treatment, outcome: 1.4 Perinatal deaths
Figure 5.
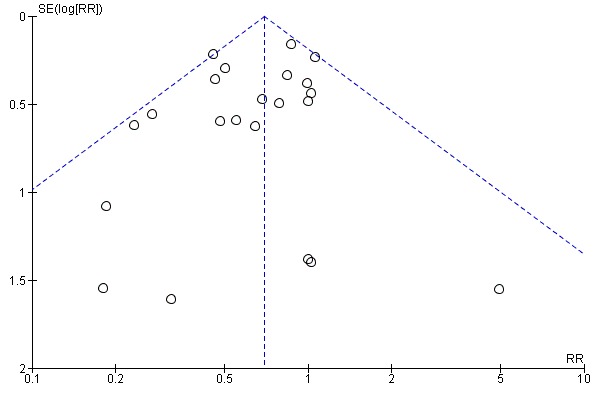
Funnel plot of comparison: 1 Corticosteroids versus placebo or no treatment, outcome: 1.5 Neonatal deaths
Figure 6.
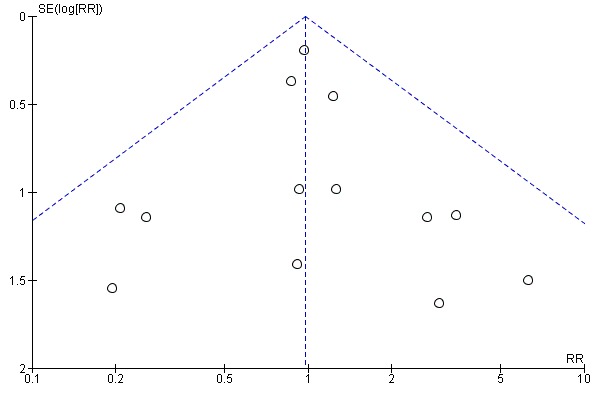
Funnel plot of comparison: 1 Corticosteroids versus placebo or no treatment, outcome: 1.6 Fetal deaths
Figure 7.
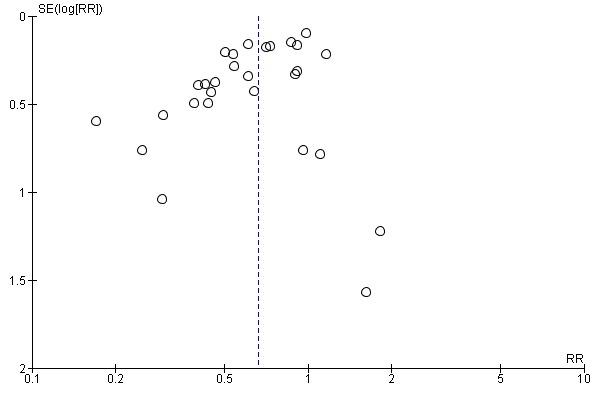
Funnel plot of comparison: 1 Corticosteroids versus placebo or no treatment, outcome: 1.7 Respiratory distress syndrome
Figure 8.
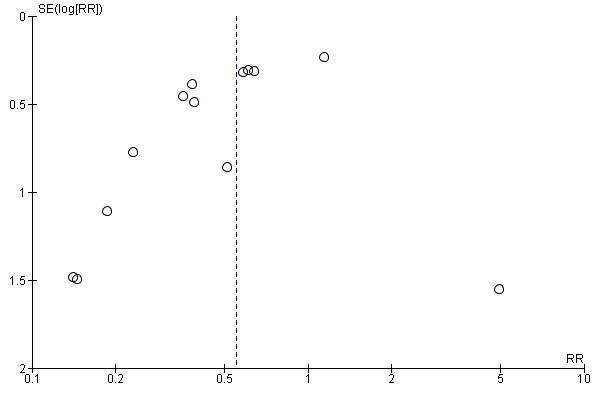
Funnel plot of comparison: 1 Corticosteroids versus placebo or no treatment, outcome: 1.10 Intraventricular haemorrhage
Figure 9.
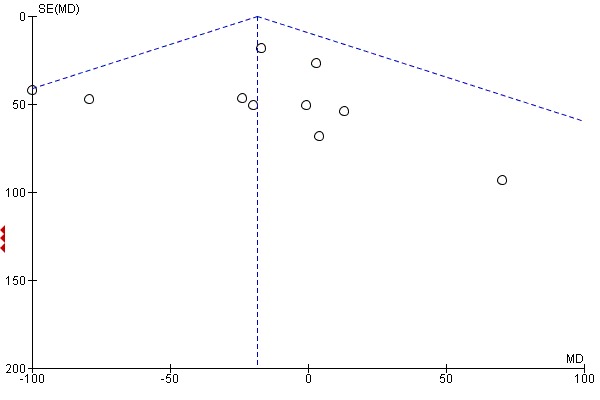
Funnel plot of comparison: 1 Corticosteroids versus placebo or no treatment, outcome: 1.11 Mean birthweight (g)
In addition, we inspected funnel plots for evidence of asymmetry and possible publication bias in further analyses where exactly 10 trials contributed data: necrotising enterocolitis, need for mechanical ventilation/CPAP and Apgar less than seven at five minutes. For 1.26 need for mechanical ventilation there was asymmetry (Figure 10); two studies (Attawattanakul 2015; Shanks 2010) with sparse events and wide confidence intervals were the outliers. For 1.31 Apgar less than seven at five minutes of age (Figure 11), all studies apart from one (Gyamfi‐Bannerman 2016) favoured corticosteroids, creating the asymmetry. Due to the limited number of studies contributing to the funnel plots, and the impact of small studies and sparse events, we could not confirm or exclude possible publication bias for these outcomes.
Figure 10.
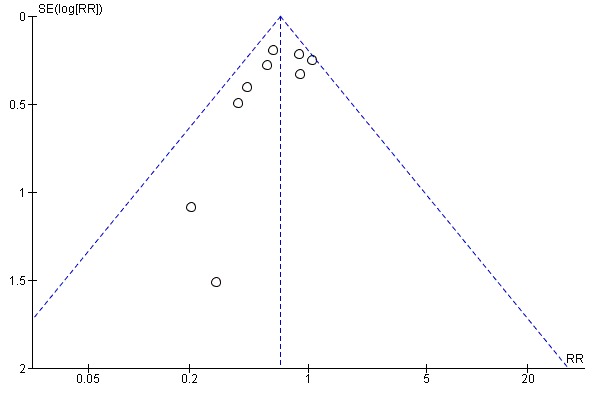
Funnel plot of comparison: 1 Corticosteroids versus placebo or no treatment, outcome: 1.25 Need for mechanical ventilation/CPAP
Figure 11.
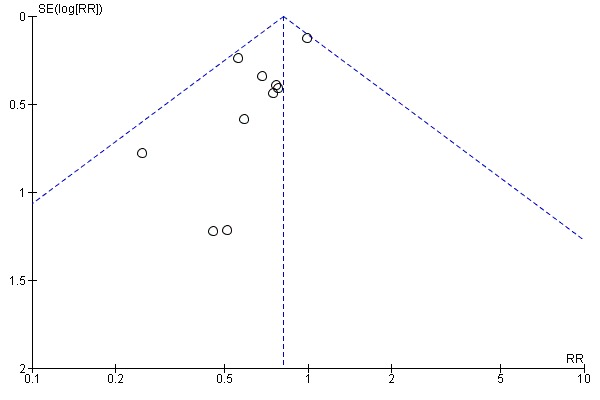
Funnel plot of comparison: 1 Corticosteroids versus placebo or no treatment, outcome: 1.30 Apgar < 7 at 5 minutes
Clinical subgroups
We have analysed the results for prespecified clinical subgroups (covariates) in the comparisons 2, 3 and 4, and added further post hoc analyses to explore the possible impact of change in practice over time (comparison 6), protocols with weekly steroid administration (comparison 7), and gestational age at randomisation (comparison 8). Where there were a sufficient number of trials reporting data for meaningful analyses, we have explored the evidence for the review primary outcomes for women and neonates. These analyses are hypothesis‐generating only and should not be interpreted as conclusive.
2. Antenatal corticosteroids versus placebo or no treatment (singleton and women with multiple pregnancies)
Discrete outcome data for those women delivering multiple pregnancies were available from only four studies (Collaborative 1981; Gamsu 1989; Liggins 1972b; Silver 1996), with the remainder of the studies including only singleton pregnancies, or reporting data from combined singleton and multiple pregnancies. We have been unable to confirm whether the Mansouri 2010 trial included only singleton pregnancy, but this is suggested by the equal numbers of women and infants reported. We have included data from this study in the singleton subgroup.
For the mother
There is no evidence that singleton or multiple pregnancy led to different rates of chorioamnionitis (Analysis 2.1) in women exposed to corticosteroids. Maternal death and endometritis were not reported separately by multiple pregnancy in any study, and so we did not conduct these subgroup analysis.
Analysis 2.1.
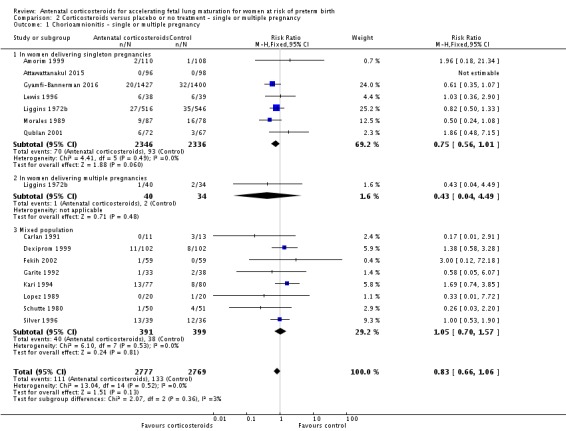
Comparison 2 Corticosteroids versus placebo or no treatment ‐ single or multiple pregnancy, Outcome 1 Chorioamnionitis ‐ single or multiple pregnancy.
For the child
There is no evidence that singleton or multiple pregnancy led to different rates of death (perinatal (Analysis 2.2); neonatal (Analysis 2.3); or fetal (Analysis 2.4)), RDS (Analysis 2.5), IVH (Analysis 2.6) or birthweight (Analysis 2.7) in infants exposed to corticosteroids.
Analysis 2.2.
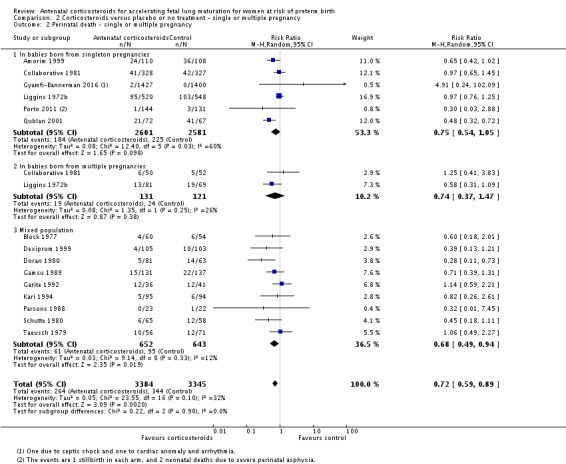
Comparison 2 Corticosteroids versus placebo or no treatment ‐ single or multiple pregnancy, Outcome 2 Perinatal death ‐ single or multiple pregnancy.
Analysis 2.3.
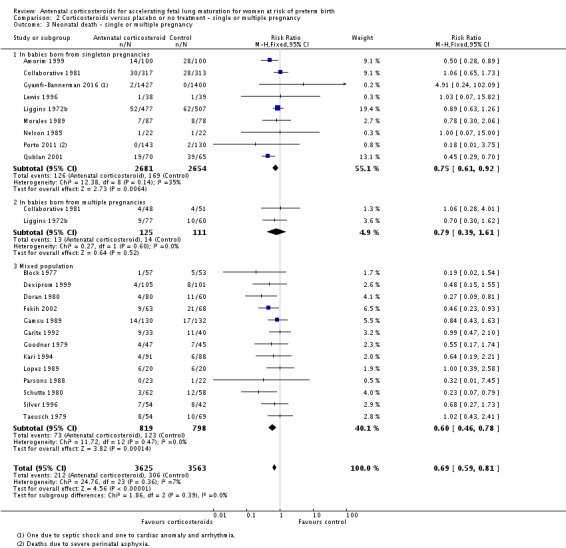
Comparison 2 Corticosteroids versus placebo or no treatment ‐ single or multiple pregnancy, Outcome 3 Neonatal death ‐ single or multiple pregnancy.
Analysis 2.4.
Comparison 2 Corticosteroids versus placebo or no treatment ‐ single or multiple pregnancy, Outcome 4 Fetal death ‐ single or multiple pregnancy.
Analysis 2.5.
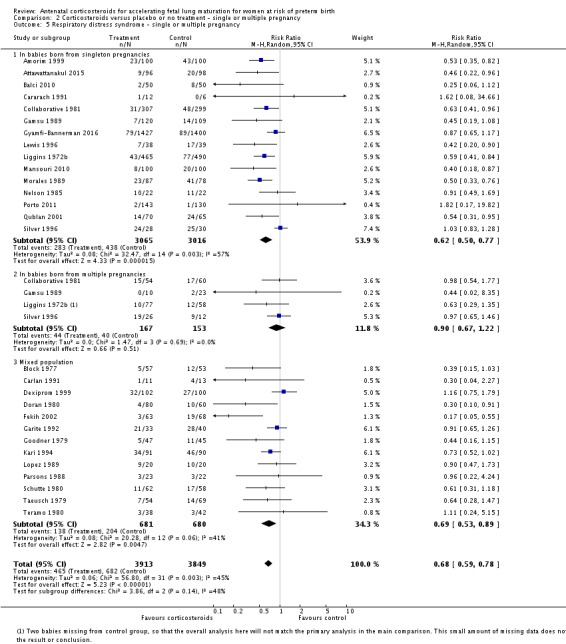
Comparison 2 Corticosteroids versus placebo or no treatment ‐ single or multiple pregnancy, Outcome 5 Respiratory distress syndrome ‐ single or multiple pregnancy.
Analysis 2.6.
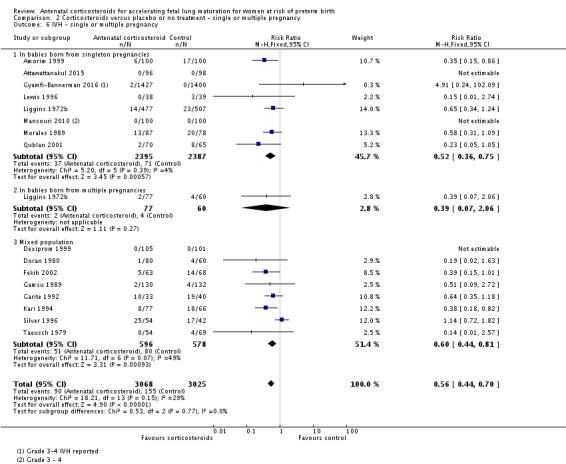
Comparison 2 Corticosteroids versus placebo or no treatment ‐ single or multiple pregnancy, Outcome 6 IVH ‐ single or multiple pregnancy.
Analysis 2.7.
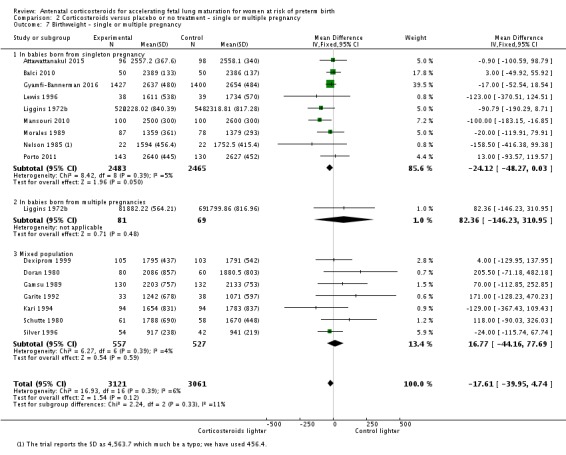
Comparison 2 Corticosteroids versus placebo or no treatment ‐ single or multiple pregnancy, Outcome 7 Birthweight ‐ single or multiple pregnancy.
Chronic lung disease and moderate/severe RDS were not reported separately by multiple pregnancy in any study, and so we did not conduct these subgroup analysis.
3. Antenatal corticosteroids versus placebo or no treatment (by presence or absence of ruptured membranes)
Discrete outcome data from women with intact membranes at the first dose of study medication were available from six studies (Amorim 1999; Attawattanakul 2015; Collaborative 1981; Garite 1992; Kari 1994; Liggins 1972b), discrete outcome data from women with ruptured membranes at the first dose of study medication were available from 12 studies (Cararach 1991; Carlan 1991; Dexiprom 1999; Fekih 2002; Lewis 1996; Liggins 1972b; Lopez 1989; Morales 1989; Nelson 1985; Parsons 1988; Qublan 2001; Schutte 1980), with the remainder of the studies not reporting rupture of membrane status or reporting combined data from women with intact and ruptured membranes.
Relevant subgroups compared below are: 1. pregnant women with intact membranes, 2. with ruptured membranes, and 3. pregnant women for whom membrane status was not reported separately or mixed populations. Analyses with small amounts of data missing are the following: 3.2. Endometritis (Schutte 1980); 3.3 Perinatal death (Liggins 1972b); 3.4. Neonatal death (Liggins 1972b); 3.5. Fetal death (Liggins 1972b; Schutte 1980); 3.6. RDS (Liggins 1972b); 3.7. IVH (Liggins 1972b); and 3.8. Birthweight (Liggins 1972b). Overall totals for these outcomes will not match our main analyses in Comparison 1 due to missing data. We have conducted sensitivity analysis with imputed data and found no difference in results for any outcome (analyses not shown). We have added footnotes to the forest plots where data used for specific trials do not match those in the main analyses due to missing data. We were unable to investigate the impact of missing data for the continuous outcome of birthweight.
For the mother
Women with rupture of membranes who were exposed to corticosteroids did not show different rates of chorioamnionitis (Analysis 3.1) or endometritis (Analysis 3.2) from women without rupture of membranes. Maternal death was zero in the treatment and control arms in relevant studies of women with ruptured membranes, thus this subgroup analysis was not undertaken.
Analysis 3.1.
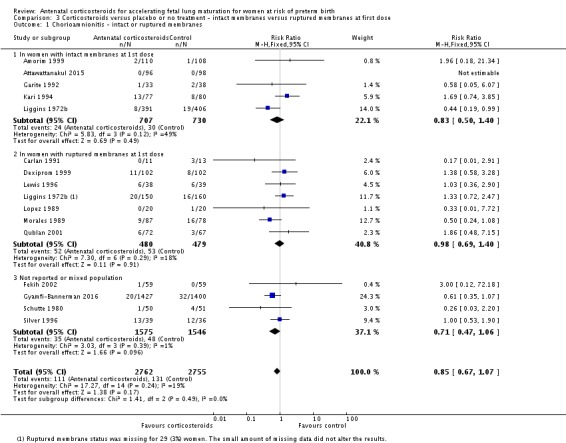
Comparison 3 Corticosteroids versus placebo or no treatment ‐ intact membranes versus ruptured membranes at first dose, Outcome 1 Chorioamnionitis ‐ intact or ruptured membranes.
Analysis 3.2.
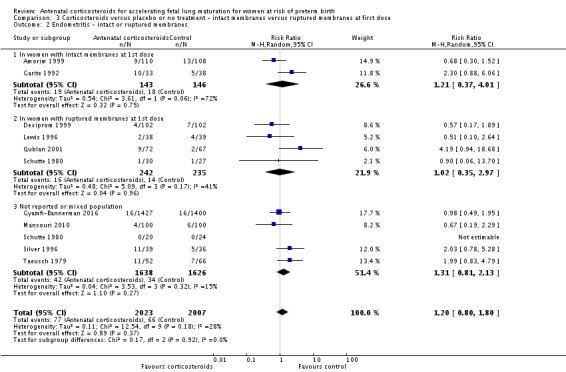
Comparison 3 Corticosteroids versus placebo or no treatment ‐ intact membranes versus ruptured membranes at first dose, Outcome 2 Endometritis ‐ intact or ruptured membranes.
For the child
There is no evidence that rupture of membrane status led to different rates of death (perinatal (Analysis 3.3); neonatal (Analysis 3.4); or fetal (Analysis 3.5)), RDS (Analysis 3.6), IVH Analysis 3.7() or birthweight (Analysis 3.8) in infants exposed to corticosteroids. Chronic lung disease and moderate/severe RDS were not reported separately by rupture of membrane status in any study, and so we did not conduct these subgroup analyses.
Analysis 3.3.
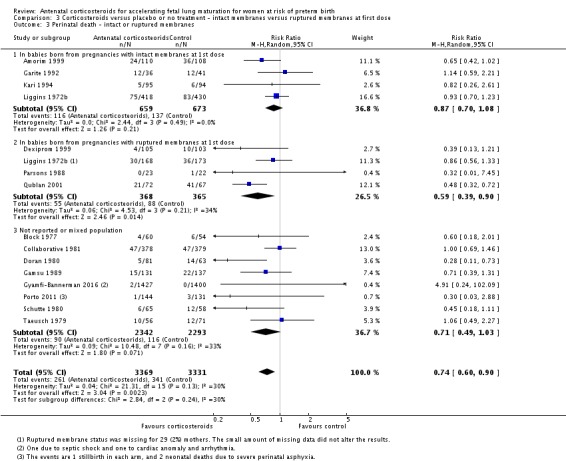
Comparison 3 Corticosteroids versus placebo or no treatment ‐ intact membranes versus ruptured membranes at first dose, Outcome 3 Perinatal death ‐ intact or ruptured membranes.
Analysis 3.4.
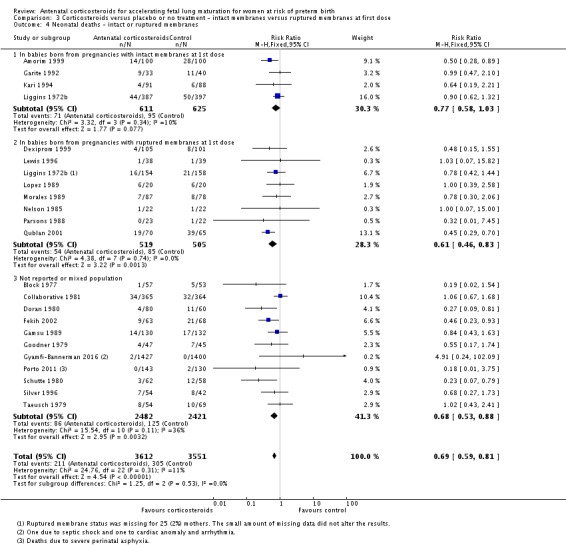
Comparison 3 Corticosteroids versus placebo or no treatment ‐ intact membranes versus ruptured membranes at first dose, Outcome 4 Neonatal deaths ‐ intact or ruptured membranes.
Analysis 3.5.
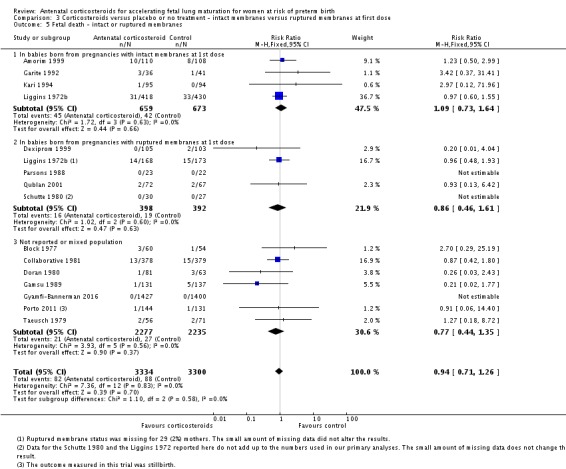
Comparison 3 Corticosteroids versus placebo or no treatment ‐ intact membranes versus ruptured membranes at first dose, Outcome 5 Fetal death ‐ intact or ruptured membranes.
Analysis 3.6.
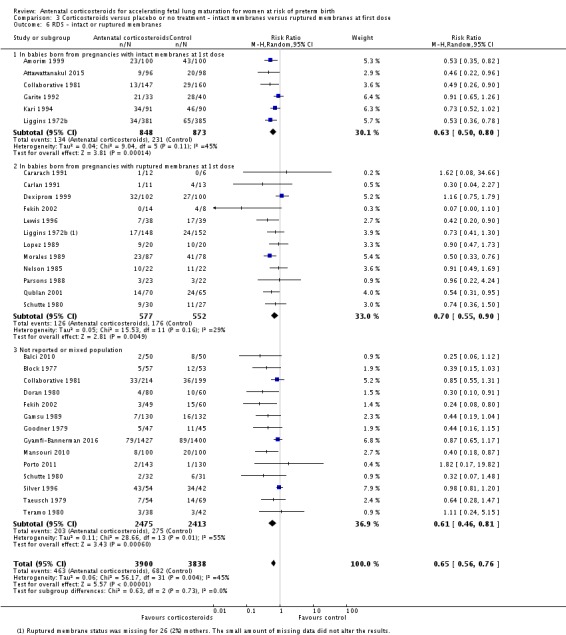
Comparison 3 Corticosteroids versus placebo or no treatment ‐ intact membranes versus ruptured membranes at first dose, Outcome 6 RDS ‐ intact or ruptured membranes.
Analysis 3.7.
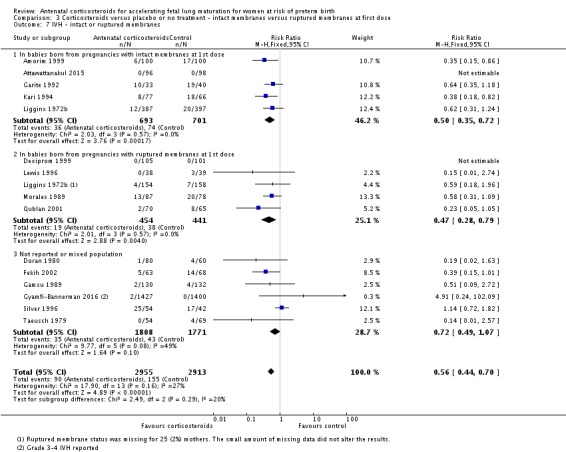
Comparison 3 Corticosteroids versus placebo or no treatment ‐ intact membranes versus ruptured membranes at first dose, Outcome 7 IVH ‐ intact or ruptured membranes.
Analysis 3.8.
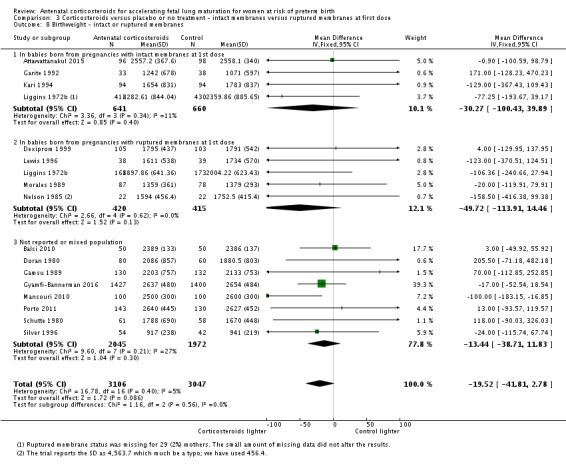
Comparison 3 Corticosteroids versus placebo or no treatment ‐ intact membranes versus ruptured membranes at first dose, Outcome 8 Birthweight ‐ intact or ruptured membranes.
4. Antenatal corticosteroids versus placebo or no treatment (for women with hypertension syndrome)
Meaningful analysis was not possible for several primary outcomes due to the small number of trials reporting results by presence or absence of hypertension syndromes.
For the mother
For maternal death, only one trial with events was eligible for the hypertension group (Amorim 1999); no study that was eligible for the 'not reported' or 'no hypertension or hypertension syndromes excluded' subgroups had any events, and we did not conduct analysis.
For chorioamnionitis and endometritis, there were too few trials reporting on hypertension to produce meaningful subgroup analyses. For chorioamnionitis there were two trials; for endometritis there was one trial.
For the child
There was no evidence that maternal hypertension status led to different rates of death (perinatal (Analysis 4.2); fetal (Analysis 4.3); or neonatal (Analysis 4.4)) in infants exposed to corticosteroids.
Analysis 4.2.
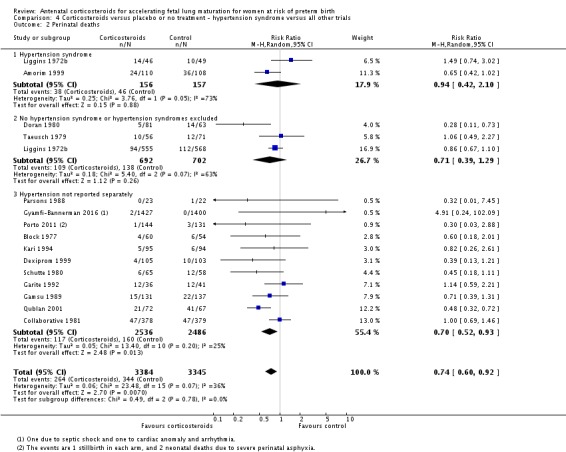
Comparison 4 Corticosteroids versus placebo or no treatment ‐ hypertension syndrome versus all other trials, Outcome 2 Perinatal deaths.
Analysis 4.3.
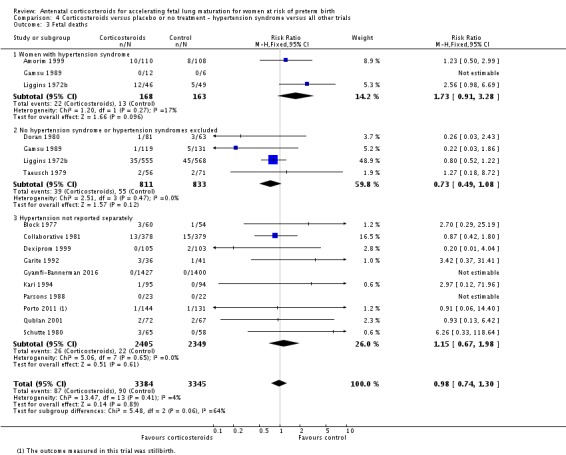
Comparison 4 Corticosteroids versus placebo or no treatment ‐ hypertension syndrome versus all other trials, Outcome 3 Fetal deaths.
Analysis 4.4.
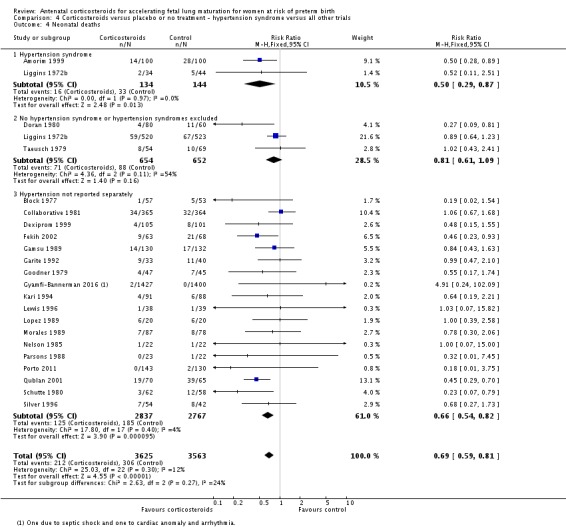
Comparison 4 Corticosteroids versus placebo or no treatment ‐ hypertension syndrome versus all other trials, Outcome 4 Neonatal deaths.
Corticosteroids were effective at preventing RDS in infants of women with and without hypertension syndromes (Analysis 4.1).
Analysis 4.1.
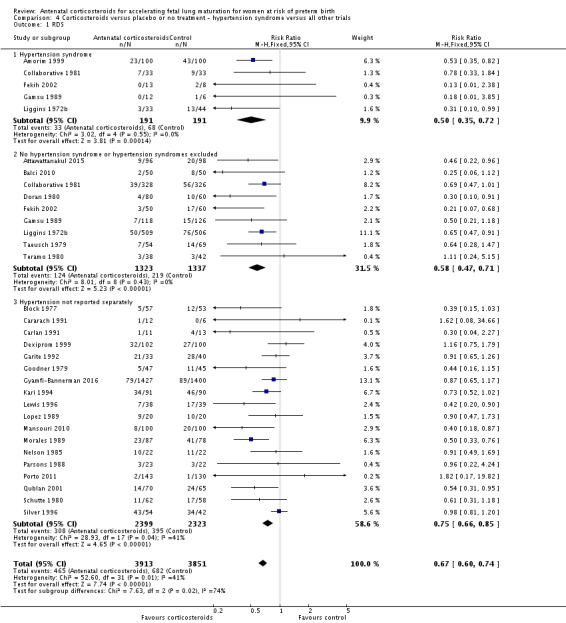
Comparison 4 Corticosteroids versus placebo or no treatment ‐ hypertension syndrome versus all other trials, Outcome 1 RDS.
There were too few trials eligible for the hypertension syndrome subgroup (one study) to conduct subgroup analysis for the outcome of chronic lung disease.
5. Antenatal corticosteroids versus placebo or no treatment (by type of corticosteroid)
Seven (Attawattanakul 2015; Collaborative 1981; Dexiprom 1999; Kari 1994; Qublan 2001; Silver 1996; Taeusch 1979) of the included studies used dexamethasone as the corticosteroid in the treatment arm (1585 women and 1708 infants), while 21 (Amorim 1999; Balci 2010; Block 1977; Carlan 1991; Doran 1980; Fekih 2002; Gamsu 1989; Garite 1992; Goodner 1979; Gyamfi‐Bannerman 2016; Khazardoust 2012; Lewis 1996; Liggins 1972b; Lopez 1989; Mansouri 2010; Morales 1989; Nelson 1985; Parsons 1988; Porto 2011; Schutte 1980; Teramo 1980) studies used betamethasone (6133 women and 6314 infants). One study did not specify the corticosteroid used (Cararach 1991; 18 women and infants), and one study used either betamethasone or dexamethasone (Shanks 2010; 32 women and infants).
For the mother
For maternal death, there was insufficient event data to conduct subgroup analysis by type of corticosteroid.
There was no evidence that type of corticosteroid used led to different rates of endometritis (Analysis 5.2). Betamethasone appeared to result in less maternal chorioamnionitis (RR 0.67, 95% CI 0.50 to 0.90; participants = 4777; studies = 10) than dexamethasone (RR 1.35, 95% CI 0.89 to 2.05; participants = 769; studies = 5) (Test for subgroup differences: Chi² = 7.16, df = 1 (P = 0.007), I² = 86.0%; Analysis 5.1).
Analysis 5.2.
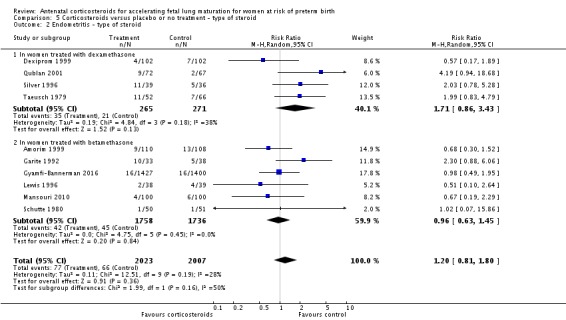
Comparison 5 Corticosteroids versus placebo or no treatment ‐ type of steroid, Outcome 2 Endometritis ‐ type of steroid.
Analysis 5.1.
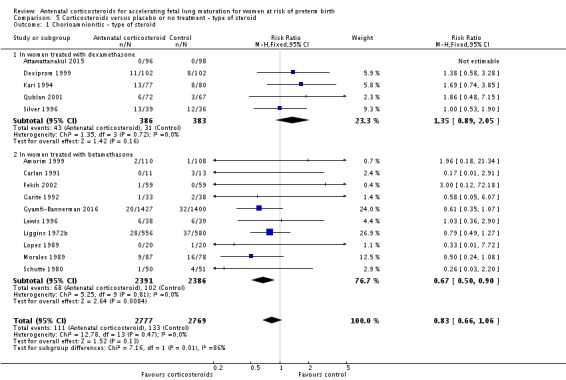
Comparison 5 Corticosteroids versus placebo or no treatment ‐ type of steroid, Outcome 1 Chorioamnionitis ‐ type of steroid.
For the child
There was no evidence that type of corticosteroid used led to different rates of death (perinatal (Analysis 5.3); neonatal (Analysis 5.4); or fetal (Analysis 5.5)), RDS (Analysis 5.6), IVH (Analysis 5.7), birthweight (Analysis 5.8), moderate/severe RDS (Analysis 5.9), or chronic lung disease (Analysis 5.10).
Analysis 5.3.
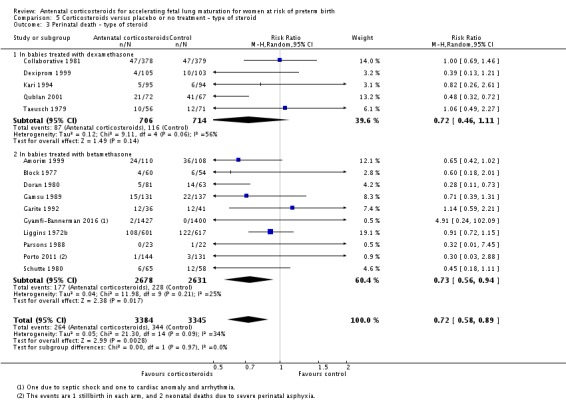
Comparison 5 Corticosteroids versus placebo or no treatment ‐ type of steroid, Outcome 3 Perinatal death ‐ type of steroid.
Analysis 5.4.
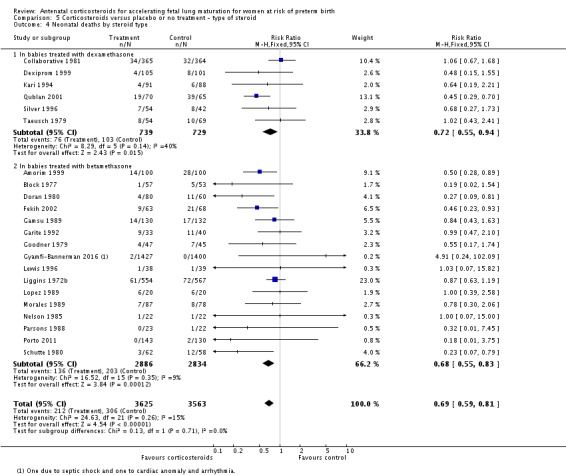
Comparison 5 Corticosteroids versus placebo or no treatment ‐ type of steroid, Outcome 4 Neonatal deaths by steroid type.
Analysis 5.5.
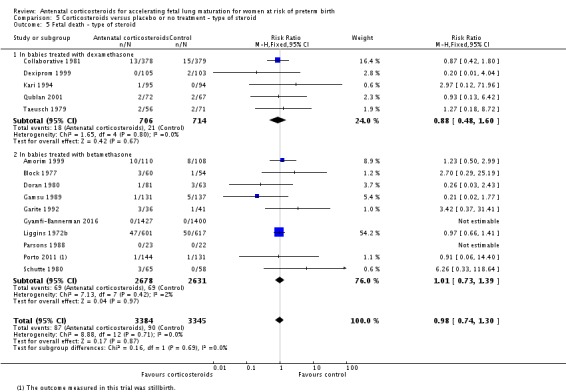
Comparison 5 Corticosteroids versus placebo or no treatment ‐ type of steroid, Outcome 5 Fetal death ‐ type of steroid.
Analysis 5.6.
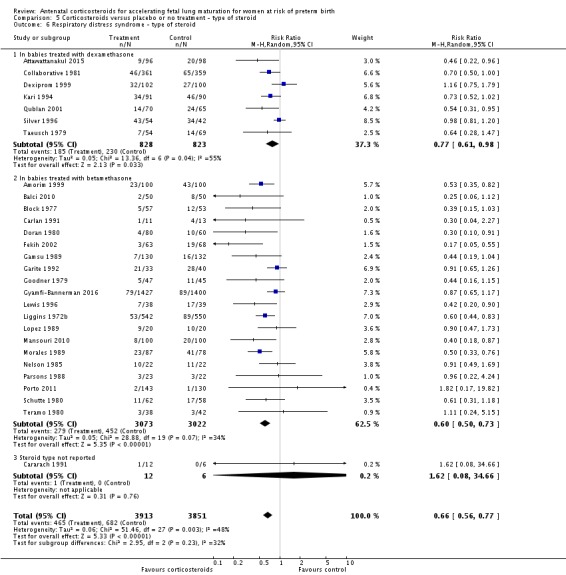
Comparison 5 Corticosteroids versus placebo or no treatment ‐ type of steroid, Outcome 6 Respiratory distress syndrome ‐ type of steroid.
Analysis 5.7.
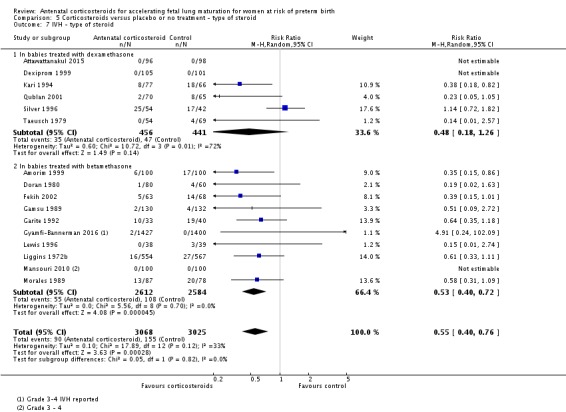
Comparison 5 Corticosteroids versus placebo or no treatment ‐ type of steroid, Outcome 7 IVH ‐ type of steroid.
Analysis 5.8.
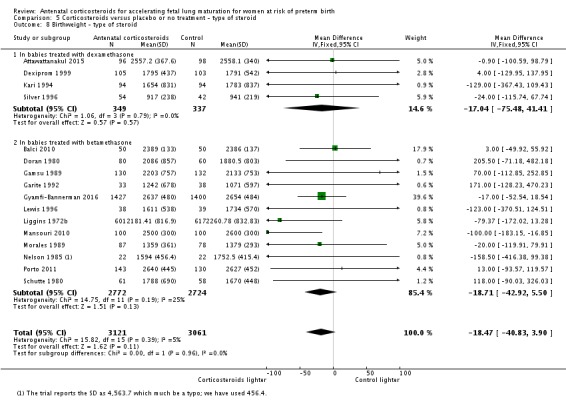
Comparison 5 Corticosteroids versus placebo or no treatment ‐ type of steroid, Outcome 8 Birthweight ‐ type of steroid.
Analysis 5.9.
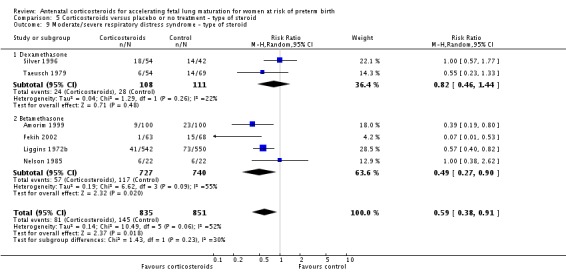
Comparison 5 Corticosteroids versus placebo or no treatment ‐ type of steroid, Outcome 9 Moderate/severe respiratory distress syndrome ‐ type of steroid.
Analysis 5.10.
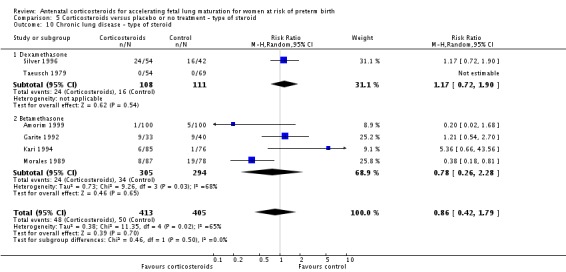
Comparison 5 Corticosteroids versus placebo or no treatment ‐ type of steroid, Outcome 10 Chronic lung disease ‐ type of steroid.
6. Antenatal corticosteroids versus placebo or no treatment (by decade of trial)
The subgroup tests in RevMan 5 are not ideal to test whether or not there were trends across decades; the test can only indicate if decades differ. We advise caution when interpreting the findings below, especially regarding survival across decades. We also wonder if the trials from the 1980s that stand out for having worse findings are actually first‐wave trials with less impressive results that were published later.
For the mother
There was no evidence that the decade of study enrolment led to different rates of chorioamnionitis (Analysis 6.1) or endometritis (Analysis 6.2) in women exposed to corticosteroids. Maternal death was zero in the treatment and control arms of all but one relevant study, thus we did not undertake this subgroup analysis.
Analysis 6.1.
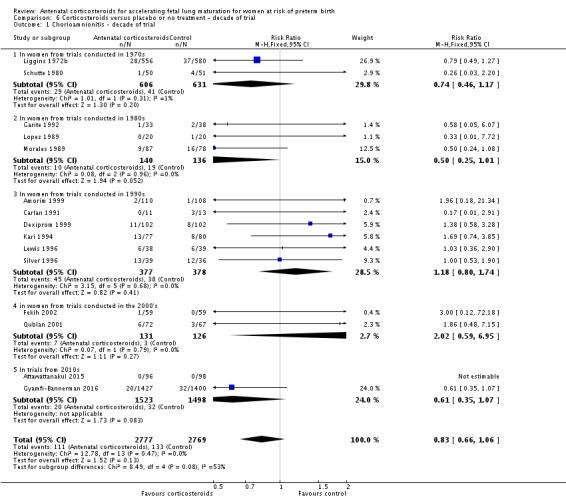
Comparison 6 Corticosteroids versus placebo or no treatment ‐ decade of trial, Outcome 1 Chorioamnionitis ‐ decade of trial.
Analysis 6.2.
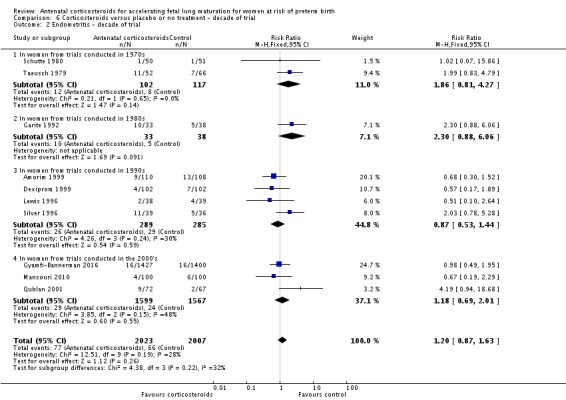
Comparison 6 Corticosteroids versus placebo or no treatment ‐ decade of trial, Outcome 2 Endometritis ‐ decade of trial.
For the child
There was no evidence that the decade of study enrolment led to different rates of IVH (Analysis 6.7) or birthweight (Analysis 6.8) in infants exposed to corticosteroids.
Analysis 6.7.
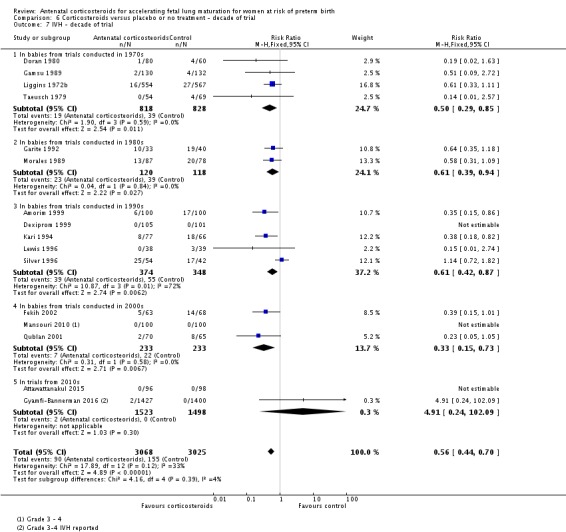
Comparison 6 Corticosteroids versus placebo or no treatment ‐ decade of trial, Outcome 7 IVH ‐ decade of trial.
Analysis 6.8.
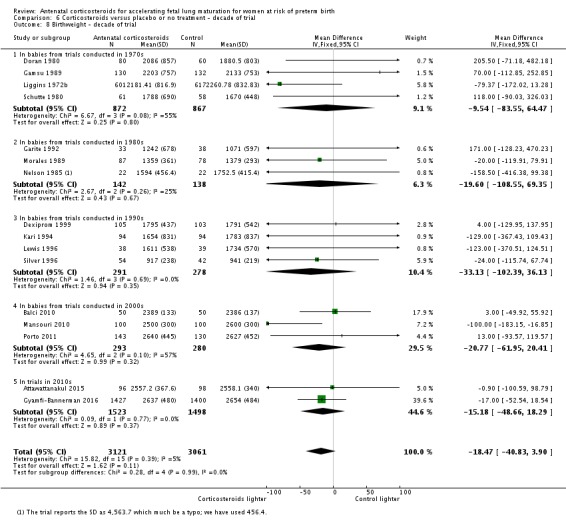
Comparison 6 Corticosteroids versus placebo or no treatment ‐ decade of trial, Outcome 8 Birthweight ‐ decade of trial.
There was evidence that the decade of study enrolment may have led to different rates of perinatal death (perinatal death, with random‐effects model: test for subgroup differences: Chi² = 10.73, df = 4 (P = 0.03), I² = 62.7%) (Analysis 6.3). This was predominantly due to differences in neonatal deaths (neonatal death: test for subgroup differences: Chi² = 12.40, df = 4 (P = 0.01), I² = 67.8%) (Analysis 6.4) rather than fetal deaths (Analysis 6.5). Neonatal deaths were reduced in infants exposed to corticosteroids in studies conducted in the 1970s, 1990s and 2000s, but not the 1980s or 2010s (Analysis 6.4). Only one study (Gyamfi‐Bannerman 2016), the largest in the review, contributed data to the decade of the 2010s. This study was conducted solely in infants born after 33 weeks, when neonatal deaths are rare, with the control arm having zero events in 1400 participants (two deaths in the treatment arm).
Analysis 6.3.
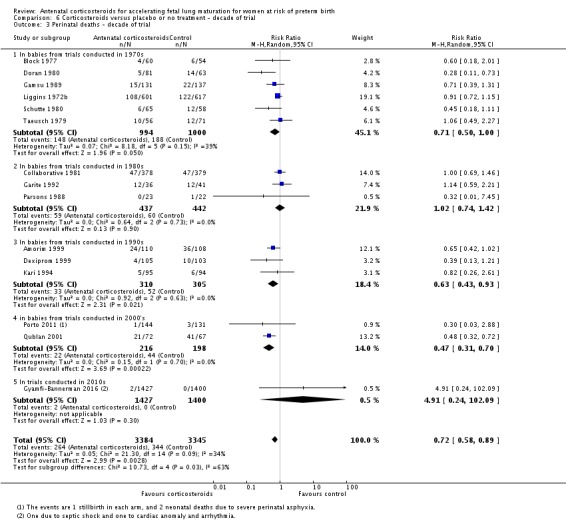
Comparison 6 Corticosteroids versus placebo or no treatment ‐ decade of trial, Outcome 3 Perinatal deaths ‐ decade of trial.
Analysis 6.4.
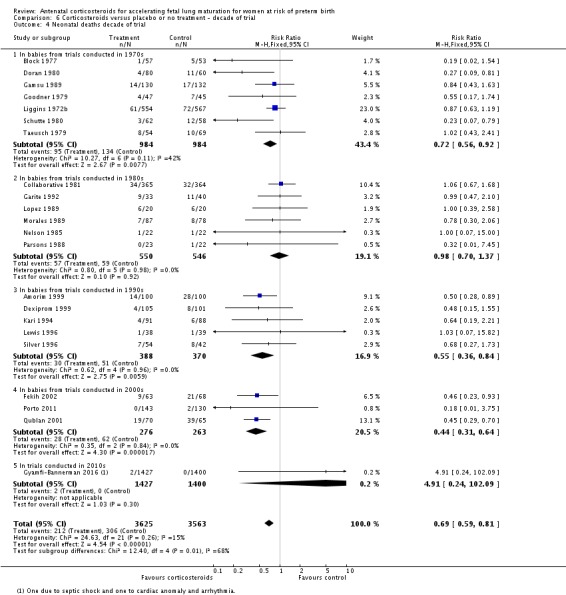
Comparison 6 Corticosteroids versus placebo or no treatment ‐ decade of trial, Outcome 4 Neonatal deaths decade of trial.
Analysis 6.5.
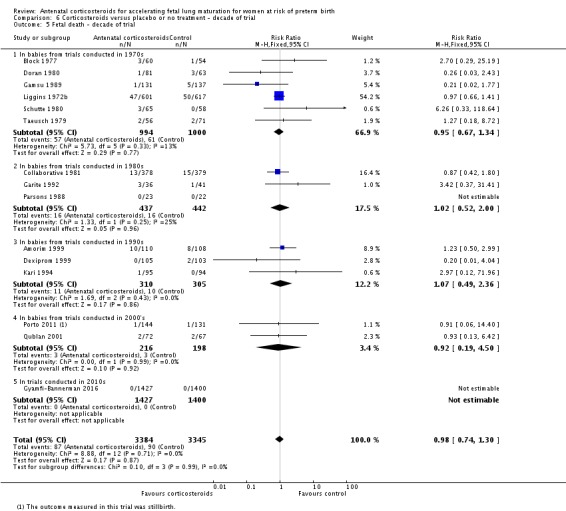
Comparison 6 Corticosteroids versus placebo or no treatment ‐ decade of trial, Outcome 5 Fetal death ‐ decade of trial.
There was evidence that the decade of study enrolment may have led to different rates of RDS in infants exposed to corticosteroids (RDS: test for subgroup differences: Chi² = 14.30, df = 4 (P = 0.006), I² = 72.0%) (Analysis 6.6). We carried out a sensitivity analysis removing each decade from the overall analysis set and repeating the test for subgroup differences without the decade removed. Removal of all decades apart from the 2000s resulted in significant subgroup differences, suggesting that data from studies conducted in the 2000s contributed significantly to the finding that the decade of study enrolment led to different rates of RDS in infants exposed to corticosteroids. Studies conducted during the 2000s had the greatest efficacy in reducing RDS in infants exposed to corticosteroids (RR 0.39, 95% CI 0.26 to 0.59; participants = 839; studies = 5; I2 = 22%) (Analysis 6.6). RDS was reduced in infants exposed to corticosteroids in studies conducted in the 1970s, 1980s, and 1990s, but not in the 2010s (Analysis 6.6).
There was no evidence of a difference in rates of moderate/severe RDS or chronic lung disease across decades; there were single trials in many subgroups and therefore we have not shown this analysis.
7. Antenatal corticosteroids versus placebo or no treatment (by presence or absence in protocol of weekly repeat doses of corticosteroid)
Nine of the included studies allowed weekly repeat courses of study medication in their study protocols (Amorim 1999; Carlan 1991; Fekih 2002; Garite 1992; Lewis 1996; Morales 1989; Parsons 1988; Qublan 2001; Silver 1996) (932 women and 946 infants).
For the mother
There was no evidence that protocols that allowed weekly repeat doses of corticosteroids led to different rates of chorioamnionitis (Analysis 7.1) or endometritis (Analysis 7.2) in women exposed to corticosteroids. Maternal death was zero in the treatment and control arms of all but one relevant study, thus we did not undertake this subgroup analysis.
Analysis 7.1.
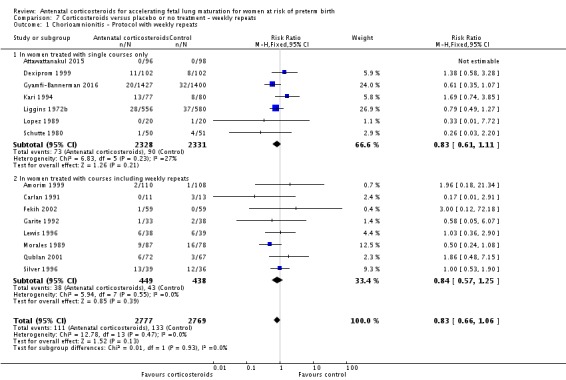
Comparison 7 Corticosteroids versus placebo or no treatment ‐ weekly repeats, Outcome 1 Chorioamnionitis ‐ Protocol with weekly repeats.
Analysis 7.2.
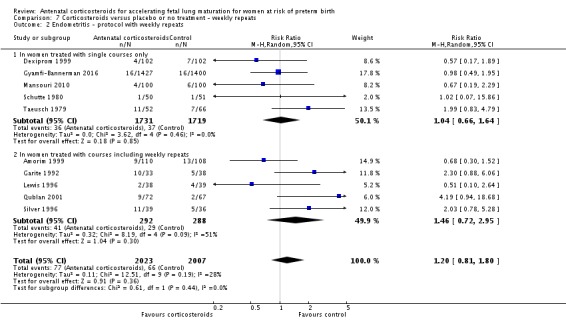
Comparison 7 Corticosteroids versus placebo or no treatment ‐ weekly repeats, Outcome 2 Endometritis ‐ protocol with weekly repeats.
For the child
There was no evidence that protocols that allowed weekly repeat doses of corticosteroids led to different rates of death (perinatal (Analysis 7.3); neonatal (Analysis 7.4); fetal (Analysis 7.5)), RDS (Analysis 7.6), IVH (Analysis 7.7) or birthweight (Analysis 7.8) in infants exposed to corticosteroids. For chronic lung disease, only one trial contributed data to the single‐course subgroup and we did not conduct analysis.
Analysis 7.3.
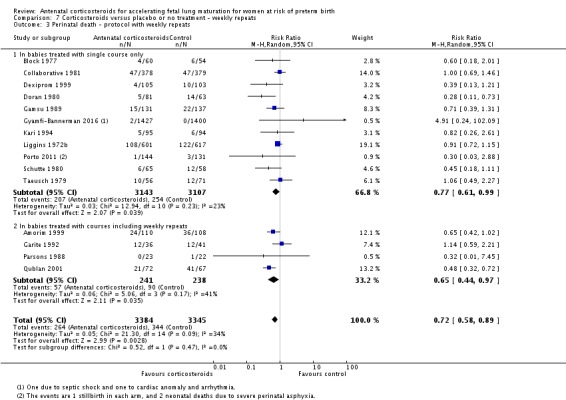
Comparison 7 Corticosteroids versus placebo or no treatment ‐ weekly repeats, Outcome 3 Perinatal death ‐ protocol with weekly repeats.
Analysis 7.4.
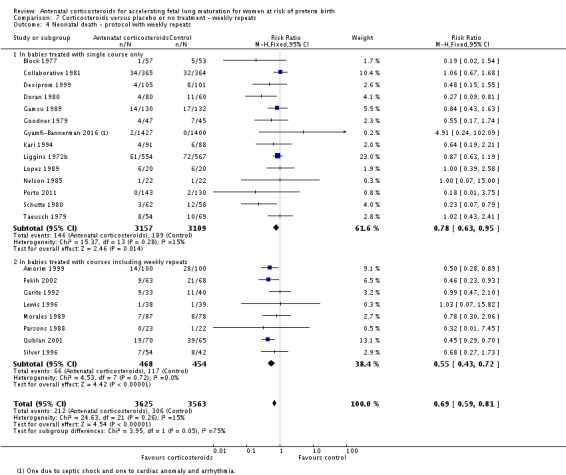
Comparison 7 Corticosteroids versus placebo or no treatment ‐ weekly repeats, Outcome 4 Neonatal death ‐ protocol with weekly repeats.
Analysis 7.5.
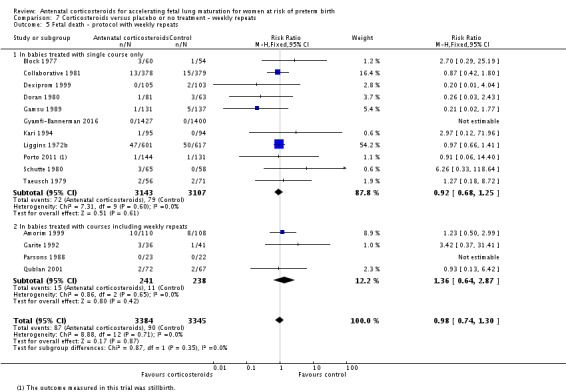
Comparison 7 Corticosteroids versus placebo or no treatment ‐ weekly repeats, Outcome 5 Fetal death ‐ protocol with weekly repeats.
Analysis 7.6.
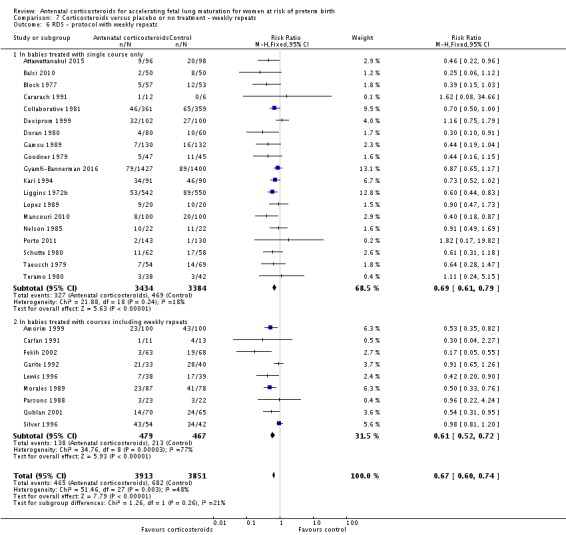
Comparison 7 Corticosteroids versus placebo or no treatment ‐ weekly repeats, Outcome 6 RDS ‐ protocol with weekly repeats.
Analysis 7.7.
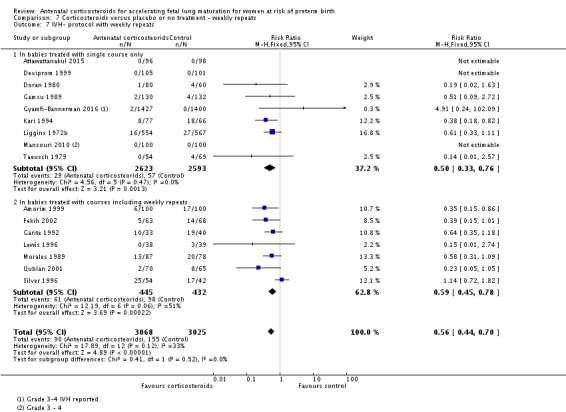
Comparison 7 Corticosteroids versus placebo or no treatment ‐ weekly repeats, Outcome 7 IVH‐ protocol with weekly repeats.
Analysis 7.8.
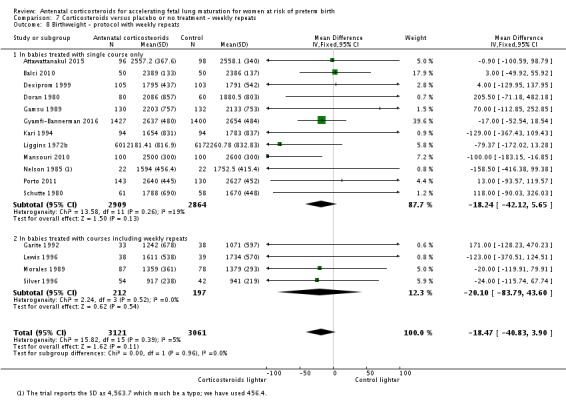
Comparison 7 Corticosteroids versus placebo or no treatment ‐ weekly repeats, Outcome 8 Birthweight ‐ protocol with weekly repeats.
8. Gestational age at trial entry (less than or equal to 35 weeks + 0 days; greater than or equal to 34 weeks + 0 days)
We have split studies according to the gestational age at which pregnant women entered trials to receive their first dose of corticosteroids and have considered two, slightly overlapping subgroups: 1) women less than, and including, 35 weeks and 0 days and 2) women greater than, and including, 34 weeks and 0 days. Twenty studies (Amorim 1999; Cararach 1991; Carlan 1991; Dexiprom 1999; Doran 1980; Fekih 2002; Gamsu 1989; Garite 1992; Goodner 1979; Kari 1994; Khazardoust 2012; Lewis 1996; Lopez 1989; Morales 1989; Nelson 1985; Parsons 1988; Qublan 2001; Schutte 1980; Silver 1996; Taeusch 1979) contributed data to the younger gestational age group and six studies (Attawattanakul 2015; Balci 2010; Gyamfi‐Bannerman 2016; Mansouri 2010; Porto 2011; Shanks 2010) contributed data to the older gestational age group. Of these 26 studies, 17 (Amorim 1999; Attawattanakul 2015; Balci 2010; Carlan 1991; Dexiprom 1999; Doran 1980; Fekih 2002; Gamsu 1989; Gyamfi‐Bannerman 2016; Lewis 1996; Khazardoust 2012; Lopez 1989; Morales 1989; Nelson 1985; Porto 2011; Qublan 2001; Shanks 2010) have data in the overlapping 34 weeks + 0 days to 34 weeks + 6 days gestational age group between the two groups. A further four studies could be analysed in either group (Block 1977; Collaborative 1981; Liggins 1972b; Teramo 1980). We addressed these issues as follows: data from Liggins 1972b were available for women entering trials at less than 35 weeks + 0 days and from between 35 weeks + 0 days and 37 weeks + 0 days. The majority of women in the remaining three studies (Block 1977; Collaborative 1981; Teramo 1980) were of less than 34 weeks + 0 days gestation, therefore we included these studies in the younger‐gestational‐age grouping for the analysis (women less than and including 35 weeks and 0 days), but we undertook a sensitivity analysis with the studies' data removed.
For the mother
There was no evidence that gestational age at trial entry led to different rates of chorioamnionitis (Analysis 8.1) in women exposed to corticosteroids. There were insufficient studies in the later gestational age group to evaluate endometritis. Maternal death was zero in the treatment and control arms of all but one relevant study, thus this subgroup analysis was not undertaken.
Analysis 8.1.
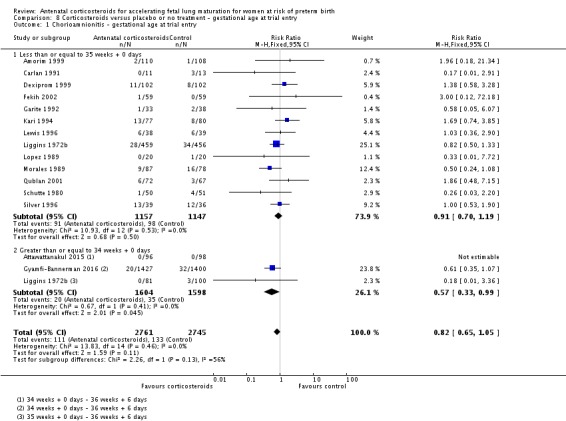
Comparison 8 Corticosteroids versus placebo or no treatment ‐ gestational age at trial entry, Outcome 1 Chorioamnionitis ‐ gestational age at trial entry.
For the infant
There was no evidence that gestational age at trial entry led to different rates of death (perinatal (Analysis 8.2); neonatal (Analysis 8.3); fetal (Analysis 8.4)), RDS (Analysis 8.5), IVH (Analysis 8.6) or birthweight (Analysis 8.7) in infants exposed to corticosteroids.
Analysis 8.2.
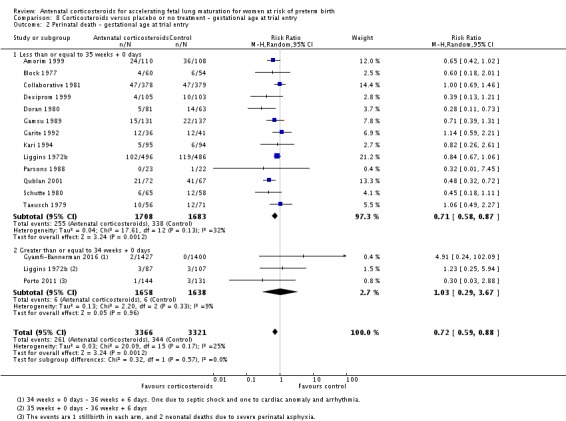
Comparison 8 Corticosteroids versus placebo or no treatment ‐ gestational age at trial entry, Outcome 2 Perinatal death ‐ gestational age at trial entry.
Analysis 8.3.
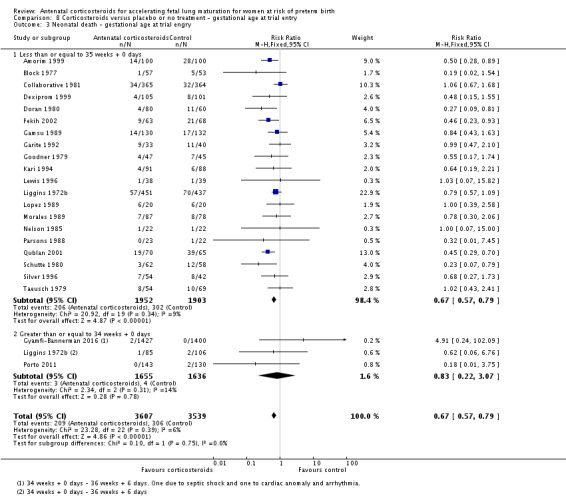
Comparison 8 Corticosteroids versus placebo or no treatment ‐ gestational age at trial entry, Outcome 3 Neonatal death ‐ gestational age at trial engry.
Analysis 8.4.
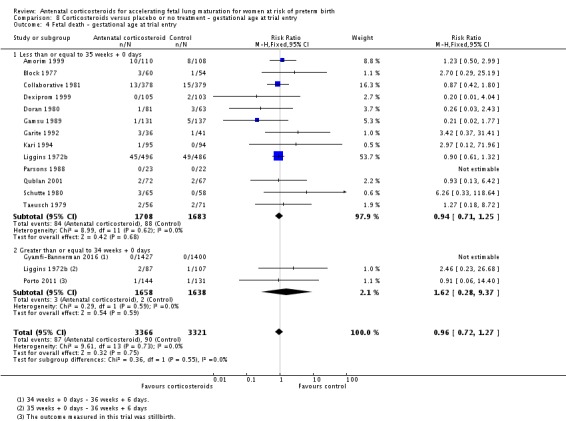
Comparison 8 Corticosteroids versus placebo or no treatment ‐ gestational age at trial entry, Outcome 4 Fetal death ‐ gestational age at trial entry.
Analysis 8.5.
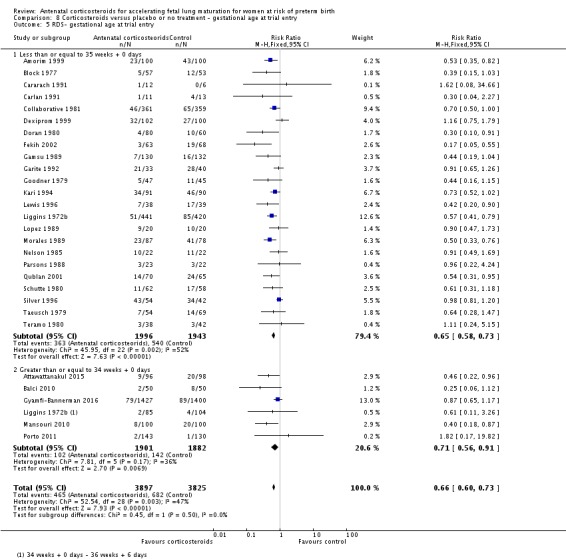
Comparison 8 Corticosteroids versus placebo or no treatment ‐ gestational age at trial entry, Outcome 5 RDS‐ gestational age at trial entry.
Analysis 8.6.
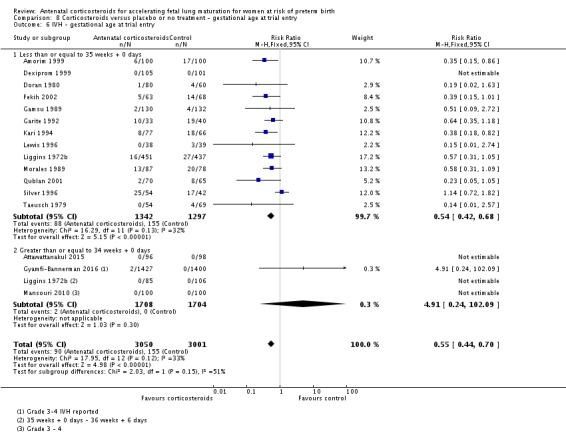
Comparison 8 Corticosteroids versus placebo or no treatment ‐ gestational age at trial entry, Outcome 6 IVH ‐ gestational age at trial entry.
Analysis 8.7.
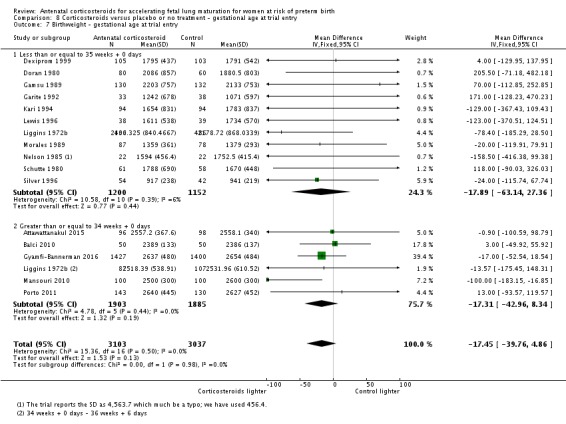
Comparison 8 Corticosteroids versus placebo or no treatment ‐ gestational age at trial entry, Outcome 7 Birthweight ‐ gestational age at trial entry.
Chronic lung disease and moderate/severe RDS were not reported by studies occurring in later gestations, and so we did not conduct these subgroup analyses.
Discussion
Summary of main results
The results of the 30 studies included in this updated review support the conclusion of the previous review (Roberts 2006), that treatment with antenatal corticosteroids reduces perinatal death, neonatal death, RDS, and IVH in preterm infants. Treatment with antenatal corticosteroids is not associated with changes in the rates of maternal death, maternal endometritis or chorioamnionitis, fetal death, neonatal chronic lung disease, or birthweight. Treatment with antenatal corticosteroids is associated with a reduction in the incidence of neonatal necrotising enterocolitis and systemic infections in the first 48 hours of life, as well as a reduction in the need for respiratory support and NICU admission.
Whether antenatal corticosteroids are beneficial in the current era of advanced neonatal practice has been questioned on the basis that previous conclusions concerning their benefits drew on data from the 1970s. In this update, we have included nine trials published since 2000 (Attawattanakul 2015; Balci 2010; Fekih 2002; Gyamfi‐Bannerman 2016; Khazardoust 2012; Mansouri 2010; Porto 2011; Qublan 2001; Shanks 2010), as well as analyses for the previous decades. These more recent trials contributed 51% of the overall data to the review. Overall, the results show consistent benefits of steroid use, without any strong evidence that antenatal corticosteroids are not beneficial in the current era of advanced neonatal practice. In subgroup analysis two decades suggested heterogeneity of the results between decades for only one each of the eight primary outcomes analysed; studies conducted in the decade of the 2000s appeared to show that corticosteroids had a greater effect on reducing RDS, the opposite result to that expected if antenatal corticosteroids are not beneficial in the current era of advanced neonatal practice. Studies conducted in the 1980s appeared to show that corticosteroids had no effect in reducing neonatal death (removal of this group in sensitivity analysis explained subgroup heterogeneity), with no evidence of effect also seen in the most recent decade (2010s). However, as the sole study (Gyamfi‐Bannerman 2016) conducted in the 2010s was conducted in near term infants who have very low rates of neonatal death, and with studies conducted in the decades of the 1990s and 2000s showing a clear statistical and clinical benefit in terms of neonatal death, RDS and IVH, we conclude that antenatal corticosteroids continue to have a strong role in supporting current methods of obstetric and neonatal practice.
The gestational age range at which antenatal corticosteroids provide benefit has been subject to debate. Previous reviews have had difficulty demonstrating benefit at gestations less than 26 weeks and beyond 36 weeks (Roberts 2006). Once again it was not possible to test this adequately using trial level data; ideally this question should be investigated with individual patient data analysis using a priori agreed gestational age cut‐offs. In this update, we examined outcomes based on gestational age divisions of up to, and including, 35 weeks + 0 days and greater than, and including, 34 weeks + 0 days. Although this post hoc analysis is exploratory, and 17 studies have data in the overlapping 34 weeks + 0 days to 35 weeks + 0 days gestational age group, we found no evidence of a difference in effect of antenatal corticosteroids in the two gestational age groups for the seven primary outcomes analysed. The most recent study (Gyamfi‐Bannerman 2016) included in this review enrolled 2831 women from 34 weeks + 0 days until 36 weeks + 5 days, and found a clinical benefit in terms of a primary outcome of requirement for respiratory support in the first 72 hours of life (11.6% versus 14.4%), but with increased neonatal hypoglycaemia (24% versus 15%) for which the long‐term effects remain unknown. Consistent with this we have demonstrated a clear statistical and clinical benefit of corticosteroids on RDS in six studies providing data from 34 weeks + 0 days gestation, but not with other primary outcomes. Thus in very late preterm gestation women (from 35 weeks + 0 days) the use of antenatal corticosteroids needs to be considered in light of the balance of risks and benefits.
The relationship between the time interval from first dose to delivery and outcome, and how this is influenced by factors such as whether corticosteroids were given and how many doses a women received, can only be determined by an individual patient data analysis. We were not able to do this in this update. We were able to undertake an analysis comparing outcomes in mothers and children exposed to studies allowing only a single course versus study protocols allowing weekly repeats if infants remained undelivered. We found no differences between these two study protocol groups. The effect of repeated doses of antenatal corticosteroids is the subject of a separate Cochrane Review (Crowther 2015), which found that although repeated doses reduced the severity of neonatal lung disease, there were insufficient data to exclude other beneficial or harmful effects to the mother or infant. The Crowther review awaits the outcome of trials looking at the long‐term effects of repeated courses of antenatal corticosteroids.
We did not find any evidence that the effect of antenatal corticosteroids was different in the subgroups of women with multiple pregnancies, premature rupture of membranes and hypertension syndromes. However as discrete RDS data from infants of women with multiple pregnancies contributed to 4.1% of the total RDS data, and discrete data from infants of women with hypertension syndromes and premature rupture of membranes contributed to 4.9% and 14.5% of the total RDS data respectively, there needs to be caution in the interpretation of these findings. Further caution is required due to the number of studies in which subgroup classification data were not available.
In this update, we have included a comparison of studies using dexamethasone as a trial protocol with studies using betamethasone. We found no evidence of a difference in efficacy between the two types of corticosteroids, apart from less maternal chorioamnionitis occurring with betamethasone. Our analysis is subject to bias as allocation to one type of corticosteroid or the other was not subject to randomisation. However, consistent with our results a review by Brownfoot 2013 and colleagues (10 studies; 1089 women and 1161 infants) compared different corticosteroid regimens and found insufficient evidence to support the use of one corticosteroid over the other.
There are insufficient data from follow‐up studies into childhood (Collaborative 1981; Kari 1994; Liggins 1972b; Schutte 1980) and into adulthood (Liggins 1972b; Schutte 1980) included in this review. Just one small study reported neurodevelopmental delay in childhood (Kari 1994; n = 82). There are also limited data for developmental delay in childhood (two trials; n = 518). Five trials report potential improvement in rates of cerebral palsy in childhood, though confidence intervals are wide and cross the line of no effect. Just two included studies followed up into adulthood (Liggins 1972b; Schutte 1980) and found no differences in intellectual impairment or educational achievement between those exposed to a single course of antenatal corticosteroids and those exposed to placebo. This has largely contributed to dispelling previous concerns regarding decreased brain growth after antenatal corticosteroid exposure from animal studies (Huang 1999; Jobe 1998).
Exposure to excess corticosteroids before birth is a postulated mechanism underlying the fetal origins of adult disease hypothesis (Barker 1998; Benediktsson 1993). Increased insulin release has been found 30 minutes following a 75 g oral glucose tolerance test in one follow‐up study conducted at age 30 (Liggins 1972b). However, the same study found no difference in blood pressure, fasting lipids, body size, hypothalamo‐pituitary‐adrenal axis function or the prevalence of diabetes or cardiovascular disease. Thus, while the finding of increased insulin resistance in adulthood provides support for excess corticosteroids as a mechanism underlying the fetal origins of adult disease hypothesis, it should not be seen as a reason to withhold antenatal corticosteroids given the large and clinically substantial benefits seen in the neonatal period.
Overall completeness and applicability of evidence
We have attempted to identify all available published and unpublished randomised trial data for the use of antenatal corticosteroids for women at risk of preterm birth. Additional data have been obtained and included where possible. We feel that the data are comprehensive and relevant to women at risk of preterm birth. Comparisons of repeat antenatal corticosteroid regimens, of different antenatal corticosteroids and of the use of antenatal corticosteroids at term before elective birth are described in other Cochrane Reviews (Brownfoot 2013; Crowther 2015; Sotiriadis 2009).
The evidence here is applicable to most hospital settings in mid‐ or high‐income countries. More evidence from low‐income settings would help support the overall applicability of the data. For example, data in this review for RDS come from 15 different countries, but only one of these is a low‐income country (Tunisia). The issue of generalisability of the current evidence has also been highlighted in the recent cluster‐randomised trial (Althabe 2014). This trial suggested harms from better compliance with antenatal corticosteroid administration in women at risk of delivering preterm in communities of low‐resource settings (Althabe 2014).
Quality of the evidence
The evidence described in this review is based on 30 randomised controlled trials comparing antenatal corticosteroids with no antenatal corticosteroids. Overall the evidence is consistent. There are some limitations in 13 of the trials where there was no blinding of the intervention, and there was insufficient information in 14 trials to enable the review authors to make judgements on the processes of randomisation or allocation concealment. The lack of information is most likely due to the era in which the trials were conducted, when this information was not a requirement for publication.
We assessed seven outcomes with GRADE methodology, as shown in the 'Summary of findings' table (Table 1). For pregnant women, evidence was graded as of moderate quality for three outcomes: maternal death, chorioamnionitis and endometritis. Downgrading in each case was for imprecision due to wide confidence intervals crossing the line of no effect. There were very few data for maternal death.
For infants, evidence for four outcomes was also graded to be of moderate quality. We downgraded evidence for perinatal death, RDS, IVH and birthweight for risks of bias in the included trials. A grade of moderate quality suggests we have some reservations about the available data and its quality due specifically to unclear risks of bias for allocation concealment or randomisation and unclear or high risks of bias for lack of blinding or incomplete outcome data in some trials included in the meta‐analyses.
Potential biases in the review process
We believe we have made sufficient attempts to reduce the potential bias of the review process. We have attempted to identify all relevant trials and two or more researchers have independently appraised the trial and extracted the data required. Where data were missing, we have contacted the original trialists and some additional data have been provided that enhances the content of this review.
Agreements and disagreements with other studies or reviews
Current systematic reviews of antenatal corticosteroids including the World Health Organization have used earlier versions of this review on which to base their recommendations (Hofmeyr 2009).
A systematic review conducted for a bi‐national clinical practice guideline for Australia and New Zealand in 2015 reported on the same maternal and neonatal benefits as the primary outcomes of this systematic review (Antenatal Corticosteroid CPG Panel 2015).
A recent systematic review of observational studies has analysed the use of antenatal corticosteroids in specific populations of pregnant women at risk of impending preterm birth (with gestational age 34 to 37 weeks); the authors considered evidence for pregnant women with gestational diabetes mellitus; pregnant women undergoing elective caesarean section (34 to 37 weeks' gestation; pregnant women with chorioamnionitis; and pregnant women with growth‐restricted fetuses) (Amiya 2016). There was no available evidence (randomised or observational) for women with gestational diabetes or for pregnant women undergoing elective caesarean section. For pregnant women with chorioamnionitis, pooled evidence from eight studies (1424 women) showed a benefit of steroid use for the outcomes of neonatal death, RDS, IVH and severe IVH; consistent with the conclusions of this review. There were no data available from these studies for maternal outcomes for women with chorioamnionitis. For pregnant women with growth‐restricted fetuses, the results were inconclusive. There were no clear benefits for growth‐restricted babies for outcomes measuring neonatal mortality or morbidity, including RDS, and the authors called for further research in this population. Using GRADE methods, review authors assessed all evidence for individual outcomes in the review as of low or very low quality, due to observational study design and, most often, imprecision due to wide confidence intervals (Amiya 2016).
As mentioned above, additional evidence is required to better understand the potential for adverse effects with steroid use in low‐resource settings (Althabe 2014; Azad 2014).
Authors' conclusions
The evidence from this review update supports the continued use of a single course of antenatal corticosteroids in women at risk of preterm birth. Treatment with antenatal corticosteroids reduces the risk of perinatal death, neonatal death, RDS, IVH, necrotising enterocolitis, need for respiratory support and NICU admission, even in the current era of advanced neonatal care.
Antenatal corticosteroids can continue to be used in women at high risk of preterm birth. Further information is required regarding the optimal dose‐to‐delivery interval, the optimal steroid, the effects in multiple pregnancy and long‐term effects into adulthood. We advise that birth should not be delayed to administer antenatal corticosteroids when there are serious concerns about maternal condition that will be alleviated by expedited delivery.
It is important to note that most of the evidence in this review comes from high‐income countries and hospital settings; therefore, the results may not be applicable to low‐resource settings with high rates of infections.
There is little need for further trials of a single course of antenatal corticosteroids versus placebo in singleton pregnancies in high‐income countries and hospital settings. However, data are sparse in lower‐income settings. There are also few data regarding risks and benefits of antenatal corticosteroids in multiple pregnancies and other high‐risk obstetric groups. We encourage authors of previous studies to provide further information, which may answer any remaining questions about the use of antenatal corticosteroids in such pregnancies without the need for further randomised controlled trials. Individual patient data meta‐analysis from published trials is likely to answer some of the evidence gaps. Follow‐up studies into childhood and adulthood, particularly in the late‐preterm‐gestation and repeat‐courses groups are needed. The possible harmful effects of antenatal corticosteroids in low‐resource settings were not examined in this review. It would be particularly relevant to explore this finding in adequately powered prospective trials.
Feedback
Nachum, September 2002
Summary
Are there enough data to indicate the efficacy of antenatal steroids in twins?
(Summary of comment received from Zohar Nachum, September 2002)
Reply
Only two small trials report outcome following a multiple pregnancy. Therefore there is currently not enough evidence to support the use of corticosteroids in multiple pregnancy. Nevertheless, in view of the strength of the overall evidence, it would seem sensible to offer a single course of steroids to women with a multiple pregnancy at risk of preterm birth.
(Summary of response from Devender Roberts and Stuart Dalziel, May 2006)
Contributors
Devender Roberts Stuart Dalziel
Preston, August 2002
Summary
It is unclear whether quasi‐randomised trials should be included. The abstract states they are included, types of studies says they are excluded, and a quasi‐randomised study has been included (Morales 1986).
Also some data appear to be missing from the meta‐analysis. Silver 1995 does not contribute any information to the outcome neonatal death, yet the data are reported in the abstract you reference (7/54 deaths on dexamethasone, 8/42 deaths on placebo).
(Summary of comments received from Carol Preston, August 2002)
Reply
The protocol for the updated review excluded quasi‐randomised studies, and Morales 1986 has therefore been excluded. The data for neonatal deaths in Silver 1995 are now included in the meta‐analysis.
(Summary of response from Devender Roberts and Stuart Dalziel, May 2006)
Contributors
Devender Roberts Stuart Dalziel
Liabsuetrakul, September 2003
Summary
The results, and reviewer's conclusions, are that administering corticosteroids (24 mg betamethasone, or 24 mg dexamethasone) to women who are expected to give birth at 28‐34 weeks' gestation reduces neonatal morbidity and mortality. However, there is no clarification of how this should be prescribed. Standard regimens are for 48 hours treatment, using either 12 mg betamethasone IM every 24 hours, or 6 mg dexamethasone IM every 12 hours. But data in this review show the maximum benefit for corticosteroids is after 24 hours of treatment.
I have some questions about how to maximise the benefit in clinical practice.
1) For a woman in preterm labor who is being given tocolytic treatment to facilitate steroid administration, how long should tocolytics be continued, 24 hours or 48 hours?
2) Would the benefit of steroids be the same for a modified regimen over 24 hours, for example 8 mg dexamethasone IM every 8 hours for 3 doses, or 12 mg dexamethasone IM every 12 hours? Will this affect adrenal suppression and fetal growth like repeated doses?
3) Do we need a review comparing the benefits and adverse events between different regimens of prophylactic corticosteroids?
(Summary of comments from Tippawan Liabsuetrakul, September 2003)
Reply
These questions have all been addressed by sub‐group analyses in the updated review.
(Summary of response from Devender Roberts and Stuart Dalziel, May 2006)
Contributors
Devender Roberts Stuart Dalziel
Selinger, December 2005
Summary
Why do the corticosteroids need to be administered by intramuscular injection? Is there any evidence that this is preferable to oral administration?
(Summary of comment from Mark Selinger, December 2005)
Reply
Presumably the original sheep studies were done with parenteral steroids, so perhaps the initial extrapolation to humans was intramuscular use. We are not aware of evidence about the effects of oral administration.
(Summary of response from Devender Roberts and Stuart Dalziel, May 2006)
Contributors
Devender Roberts Stuart Dalziel
Hutchon, May 2006
Summary
There have been two recent reports(1,2) of 30‐year follow‐up of people recruited whilst in utero to Liggins 1972a. Both used intention‐to‐treat analysis, as does this review. One of these reports (1) stated " that there were similar numbers of neonatal survivors with much the same perinatal morbidity in both treatment and control groups". Clearly this means that Liggins 1972a showed no overall benefit in terms of survival or morbidity, which to me seem the most important end points.
Liggins 1972a forms a major part of this Cochrane review, yet the data from the follow‐up reports differ from those in the review. This new evidence therefore raises questions about the validity of the Cochrane meta‐analysis. There are also discrepancies between this version of the review, and its earlier published versions, for some of the other trials. The version published in Effective care in Pregnancy and Childbirth (3) contained 12 trials reporting the effect of corticosteroids on early neonatal death (0‐7 days). Some of these 12 are in the analysis presented here of corticosteroids versus placebo for the outcome neonatal death (0‐28 days). However, for Liggins 1972a, Block 1977, Gamsu 1989, and Morales 1989 the data remain unchanged between the two reviews. Does this mean there were no deaths from 8‐28 days? We now know this is not true for Liggins 1972a. There is also something peculiar about the randomisation in Schmidt 1984. Between appearing in Effective Care in Pregnancy and Childbirth and inclusion in the Cochrane review 15 women were added to this study, all in the treatment group and with no change in the number of deaths.
I understand an update of the review is in preparation. However, since the early nineties it would have been considered unethical to carry out a randomised trial of steroids versus placebo and so I do not expect any new trials to have become available since the last Cochrane review in 2002.
(Summary of feedback from David Hutchon, May 2006)
References
1. Dalziel SR, Walker NK, Parag V, Mantell C, Rea HH, Rodgers A et al. Cardiovascular risk factors after exposure to antenatal betamethasone: 30‐year follow‐up of a randomised controlled trial. Lancet 2005;365:1856‐62.
2. Dalziel SR, Lim VK, Lambert A, McCarthy D, Parag V, Rodgers A et al. Antenatal exposure to betamethasone: psychological functioning and health related quality of life 31 years after inclusion in a randomised controlled trial. BMJ 2005;331:665‐8.
3. Table 45.12 In: Chalmers I, Enkin M, Keirse MJNC, eds. Effective care in pregnancy and childbirth. Oxford: Oxford University Press, 1989:754.
Reply
Since Effective Care in Pregnancy and Childbirth appeared, nine randomised controlled trials of antenatal corticosteroids have been published. These trials are now included in the updated Cochrane review. This updated review shows the contribution of each study to the outcome measures, and describes the methodological quality of each included trial.
For Liggins 1972a, the previous Cochrane review (Crowley 1996) included data that were published at that time Hence, data for perinatal death (stillbirth or death in the first week of life) were included. However, the updated Cochrane review includes an intention‐to‐treat analysis of the original data from Liggins 1972a. These data were not available for the previous review (Crowley 1996). This updated review therefore now includes data for neonatal death (death in the first 28 days of life) in Liggins 1972a.
Data reported for Schmidt 1984 included a third arm of women and infants who had been excluded from randomisation. This study is now excluded from the review.
(Suumary of response from Devender Roberts and Stuart Dalziel, May 2006)
Contributors
Devender Roberts Stuart Dalziel
Hutchon, January 2007
Summary
It is good to see the updated review has incorporated intention to treat analysis for all the trials. In the paragraph entitled "Effects of antenatal corticosteroids for preterm birth" the third sentence referring to the 1990 review by Crowley et al (1) is not strictly correct. "This review showed that corticosteroids ... are effective in preventing respiratory distress syndrome and neonatal mortality." In fact that analysis was for early neonatal deaths (deaths in the first seven days) only. Subsequently the Cochrane review used neonatal deaths (deaths in the first 28 days) and, as I pointed out in my feedback on the last update, data from some of the trials (Liggins 1972a, Block 1977, Gamsu 1989, and Morales 1989) are still the same as the previous data reported as early neonatal deaths. Therefore, to be correct, the above sentence should end "...preventing respiratory distress and early neonatal mortality."
Confusion remains regarding the results of three trials. Differences in the data for neonatal death between this update and the previous version (Table 1) are unexplained. For Block 1977 and Gamsu 1989 the differences are minor, but for Morales 1986 they are larger. These changes merit some comment.
Table 1 Differences in the data for neonatal mortality:
Block 1977 Previous update: Treatment (n/N) = 1/69; Control (n/N) = 5/61 This update: Treatment (n/N) = 1/57; Control (n/N) = 5/53
Gamsu 1989 Previous update: Treatment (n/N) = 14/131; Control (n/N) = 20/137 This update: Treatment (n/N) = 14/130; Control (n/N) = 17/132
Morales 1986 Previous update: Treatment (n/N) = 7/121; Control (n/N) = 13/124 This update: Treatment (n/N) = 7/87; Control (n/N) = 8/78
Finally, data from Liggins 1972a has been adjusted and is now presented as an intention to treat analysis. Precise details about the cause of death are not available. Data for Block 1977, Gamsu 1989 and Morales 1986 are not quite as old as that for Liggins 1972a, nevertheless, it is surprising that secure reanalysis of these studies was available after all these years.
1.Crowley P, Chalmers I, Keirse MJNC. The effects of corticosteroid administration before preterm delivery: an overview of the evidence from controlled trials. British Journal of Obstetrics and Gynaecology 1990; 97:11‐25
(Summary of feedback from David Hutchon, January 2007)
Reply
A reply from the authors will be published as soon as it is available.
Contributors
David Hutchon
Vlassov, 15 March 2008
Summary
The title of the review is misleading; the objectives of the review, as well as the outcomes evaluated, are NOT about fetal lung maturation only.
(Summary of feedback from Vasiliy Vlassov, March 2008)
Reply
The results of the review do include data for outcomes other than fetal lung maturity. For the update, we did not want to significantly alter the title of the review. The intention of the original review was to assess the effect on fetal lung maturation. We felt it would be too radical a change for this first update to have a completely different title. We will consider this comment for future updates.
(Reply from Devender Roberts, June 2008)
Contributors
Devender Roberts
Berghella, 23 January 2013
Summary
This review is one of the best and most comprehensive I have seen. However, I would suggest though adding ‘neonatal hypoglycemia’ as an outcome.
(Comment submitted by Vincenzo Berghella, January 2013)
Reply
Thank you for your comments. We will consider this for the next update.
Contributors
Devender Roberts, August 2016.
Acknowledgements
P Crowley's first, unstructured review of antenatal corticosteroids was conducted at the suggestion of Professor Dennis Hawkins in 1980. Dr Anne Anderson encouraged her to use it as a basis for an early meta‐analysis in 1981. Her work at the National Perinatal Epidemiology Unit in 1980 to 1981 was funded by the National Maternity Hospital, Dublin at the suggestion of the then Master, Dr Dermot MacDonald. This review was first published in structured form on the Oxford Database of Perinatal Trials in 1989. The preparation and continued updating of the original review would have been impossible without the help of Iain and Jan Chalmers, Marc Keirse, Jini Hetherington, Sonja Henderson and Professor Zarko Alfirevic.
Acknowledgements to Professor James Neilson and Professor Jane Harding for their help with the previous update. Many thanks to Sonja Henderson for sound advice at all times. Acknowledgements also to all the study authors who provided us with additional data.
Thanks to Almira Opardija for translating Grgic 2003. Thanks also to Bita Mesgarpour for translating Mansouri 2010.
In the 2016 update we would like to thank Tineke Crawford for her contribution to editing the review and updating some of the evidence. We would also like to thank Leanne Jones and Therese Dowswell for their contribution to editing the review.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
N Medley's work on this project was supported by the University of Liverpool's Harris‐Wellbeing of Women Preterm Birth Centre research award.
S Dalziel's time was supported by the Health Research Council of New Zealand (HRC13/556).
As part of the pre‐publication editorial process, this review has been commented on by three peers (an editor and two referees who are external to the editorial team), a member of the Pregnancy and Childbirth Group's international panel of consumers and the Group's Statistical Adviser.
Data and analyses
Comparison 1.
Corticosteroids versus placebo or no treatment
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Maternal death | 5 | 3392 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.06, 15.50] |
| 2 Chorioamnionitis | 15 | 5546 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.66, 1.06] |
| 3 Endometritis | 10 | 4030 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.87, 1.63] |
| 4 Perinatal deaths | 15 | 6729 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.58, 0.89] |
| 5 Neonatal deaths | 22 | 7188 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.59, 0.81] |
| 6 Fetal deaths | 15 | 6729 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.74, 1.30] |
| 7 Respiratory distress syndrome | 28 | 7764 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.56, 0.77] |
| 8 Moderate/severe respiratory distress syndrome | 6 | 1686 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.38, 0.91] |
| 9 Chronic lung disease | 6 | 818 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.42, 1.79] |
| 10 Intraventricular haemorrhage | 16 | 6093 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.40, 0.76] |
| 11 Mean birthweight (g) | 16 | 6182 | Mean Difference (IV, Fixed, 95% CI) | ‐18.47 [‐40.83, 3.90] |
| 12 Death in childhood | 4 | 1010 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.36, 1.27] |
| 13 Neurodevelopmental delay in childhood | 1 | 82 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.14, 2.98] |
| 14 Death into adulthood | 1 | 988 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.56, 1.81] |
| 15 Fever in women after trial entry requiring the use of antibiotics | 4 | 481 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.43, 2.06] |
| 16 Intrapartum fever in woman requiring the use of antibiotics | 2 | 319 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.09, 4.89] |
| 17 Side effects of therapy in women | 6 | 3572 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.59, 0.82] |
| 18 Admission into adult intensive care unit | 2 | 319 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.26, 2.05] |
| 19 Hypertension | 1 | 220 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.36, 2.76] |
| 20 Postnatal fever in woman | 5 | 1323 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.64, 1.33] |
| 21 Glucose intolerance | 1 | 123 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.71 [1.14, 6.46] |
| 22 Necrotising enterocolitis | 10 | 4702 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.32, 0.78] |
| 23 Systemic infection in the first 48 hours of life | 8 | 1753 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.41, 0.88] |
| 24 Proven infection while in the neonatal intensive care unit | 13 | 5707 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.55, 1.08] |
| 25 Need for mechanical ventilation/CPAP | 9 | 1368 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.56, 0.84] |
| 26 Mean duration of mechanical ventilation/CPAP (days) | 3 | 471 | Mean Difference (IV, Random, 95% CI) | ‐1.91 [‐4.59, 0.76] |
| 27 Mean duration of oxygen supplementation (days) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 28 Surfactant use | 5 | 3556 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.51, 0.90] |
| 29 Air leak syndrome | 2 | 2965 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.32, 1.80] |
| 30 Apgar < 7 at 5 minutes | 10 | 2419 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.67, 0.98] |
| 31 Mean interval between trial entry and birth (days) | 3 | 1513 | Mean Difference (IV, Fixed, 95% CI) | 0.23 [‐1.86, 2.32] |
| 32 Small‐for‐gestational age | 5 | 3478 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.96, 1.28] |
| 33 Mean infant HPA axis function (cortisol) | 1 | 27 | Mean Difference (IV, Fixed, 95% CI) | 3.94 [‐3.12, 11.00] |
| 33.1 In babies born < 24 hours after 1st dose | 1 | 6 | Mean Difference (IV, Fixed, 95% CI) | 9.0 [‐11.93, 29.93] |
| 33.2 In babies born 24‐48 hours after 1st dose | 1 | 10 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐8.68, 8.68] |
| 33.3 In babies born > 48 hours after 1st dose | 1 | 11 | Mean Difference (IV, Fixed, 95% CI) | 13.0 [‐1.90, 27.90] |
| 34 Admission to neonatal intensive care unit | 7 | 3803 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.84, 0.97] |
| 35 Developmental delay in childhood | 2 | 518 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.24, 1.00] |
| 36 Cerebral palsy in childhood | 5 | 904 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.34, 1.03] |
| 37 Mean childhood weight (kg) | 2 | 333 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐0.39, 1.00] |
| 37.1 Liggins | 1 | 250 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [‐0.32, 1.12] |
| 37.2 Schutte (females) | 1 | 39 | Mean Difference (IV, Fixed, 95% CI) | ‐2.40 [‐6.55, 1.75] |
| 37.3 Schutte (males) | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐3.88, 3.68] |
| 38 Mean childhood height (cm) | 2 | 334 | Mean Difference (IV, Fixed, 95% CI) | 1.02 [‐0.26, 2.29] |
| 38.1 Liggins | 1 | 250 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [‐0.39, 2.39] |
| 38.2 Schutte (females) | 1 | 39 | Mean Difference (IV, Fixed, 95% CI) | 1.70 [‐3.08, 6.48] |
| 38.3 Schutte (males) | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [‐3.79, 4.99] |
| 39 Mean childhood head circumference (cm) | 2 | 328 | Mean Difference (IV, Fixed, 95% CI) | 0.27 [‐0.08, 0.63] |
| 39.1 Liggins | 1 | 250 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐0.11, 0.71] |
| 39.2 Schutte (females) | 1 | 36 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐1.05, 0.85] |
| 39.3 Schutte (males) | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [‐0.51, 1.71] |
| 40 Mean childhood VC (% predicted) | 2 | 150 | Mean Difference (IV, Fixed, 95% CI) | ‐1.68 [‐5.12, 1.75] |
| 40.1 Liggins | 1 | 75 | Mean Difference (IV, Fixed, 95% CI) | 0.70 [‐5.12, 6.52] |
| 40.2 Schutte (females) | 1 | 36 | Mean Difference (IV, Fixed, 95% CI) | ‐2.60 [‐8.65, 3.45] |
| 40.3 Schutte (males) | 1 | 39 | Mean Difference (IV, Fixed, 95% CI) | ‐3.30 [‐9.27, 2.67] |
| 41 Mean childhood FEV1 (% predicted) | 1 | 75 | Mean Difference (IV, Fixed, 95% CI) | ‐4.73 [‐10.13, 0.67] |
| 41.1 Schutte (females) | 1 | 36 | Mean Difference (IV, Fixed, 95% CI) | ‐2.5 [‐11.24, 6.24] |
| 41.2 Schutte (males) | 1 | 39 | Mean Difference (IV, Fixed, 95% CI) | ‐6.10 [‐12.96, 0.76] |
| 42 Mean childhood FEV1/VC | 2 | 150 | Mean Difference (IV, Random, 95% CI) | ‐0.94 [‐3.63, 1.76] |
| 42.1 Liggins | 1 | 75 | Mean Difference (IV, Random, 95% CI) | 1.0 [‐2.57, 4.57] |
| 42.2 Schutte (females) | 1 | 36 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐5.56, 5.56] |
| 42.3 Schutte (males) | 1 | 39 | Mean Difference (IV, Random, 95% CI) | ‐3.0 [‐6.14, 0.14] |
| 43 Mean childhood systolic blood pressure (mmHg) | 1 | 223 | Mean Difference (IV, Fixed, 95% CI) | ‐1.60 [‐4.06, 0.86] |
| 44 Visual impairment in childhood | 2 | 166 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.24, 1.23] |
| 45 Hearing impairment in childhood | 2 | 166 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.04, 9.87] |
| 46 Intellectual impairment in childhood | 3 | 778 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.44, 1.69] |
| 47 Behavioural/learning difficulties in childhood | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.35, 2.09] |
| 48 Mean adult insulin (log values) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 48.1 Fasting | 1 | 435 | Mean Difference (IV, Fixed, 95% CI) | 0.08 [‐0.03, 0.19] |
| 48.2 30 minutes following a 75 g oral glucose tolerance test | 1 | 412 | Mean Difference (IV, Fixed, 95% CI) | 0.16 [0.04, 0.28] |
| 48.3 120 minutes following a 75 g oral glucose tolerance test | 1 | 428 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.27, 0.07] |
| 49 Mean adult glucose (mmol/L) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 49.1 Fasting | 1 | 432 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.09, 0.11] |
| 49.2 30 minutes following a 75 g oral glucose tolerance test | 1 | 413 | Mean Difference (IV, Fixed, 95% CI) | 0.19 [‐0.14, 0.52] |
| 49.3 120 minutes following a 75 g oral glucose tolerance test | 1 | 410 | Mean Difference (IV, Fixed, 95% CI) | ‐0.27 [‐0.52, ‐0.02] |
| 50 Mean adult weight (kg) | 2 | 538 | Mean Difference (IV, Random, 95% CI) | ‐0.83 [‐6.41, 4.76] |
| 50.1 Schutte (females) | 1 | 37 | Mean Difference (IV, Random, 95% CI) | ‐6.0 [‐12.93, 0.93] |
| 50.2 Schutte (males) | 1 | 43 | Mean Difference (IV, Random, 95% CI) | ‐1.0 [‐9.91, 7.91] |
| 50.3 Liggins | 1 | 458 | Mean Difference (IV, Random, 95% CI) | 2.57 [‐0.72, 5.86] |
| 51 Mean adult height (cm) | 2 | 537 | Mean Difference (IV, Fixed, 95% CI) | 0.91 [‐0.28, 2.10] |
| 51.1 Schutte (females) | 1 | 36 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐5.37, 3.37] |
| 51.2 Schutte (males) | 1 | 43 | Mean Difference (IV, Fixed, 95% CI) | 3.0 [‐2.30, 8.30] |
| 51.3 Liggins (females) | 1 | 234 | Mean Difference (IV, Fixed, 95% CI) | 1.17 [‐0.65, 2.99] |
| 51.4 Liggins (males) | 1 | 224 | Mean Difference (IV, Fixed, 95% CI) | 0.75 [‐1.03, 2.53] |
| 52 Mean adult head circumference (cm) | 2 | 537 | Mean Difference (IV, Fixed, 95% CI) | 0.03 [‐0.33, 0.38] |
| 52.1 Schutte (females) | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐1.03, 1.03] |
| 52.2 Schutte (males) | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐1.37, 0.97] |
| 52.3 Liggins | 1 | 458 | Mean Difference (IV, Fixed, 95% CI) | 0.06 [‐0.34, 0.46] |
| 53 Mean adult skinfold thickness (log values) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 53.1 Triceps | 1 | 456 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.11, 0.07] |
| 53.2 Biceps | 1 | 456 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.11, 0.09] |
| 53.3 Subscapular | 1 | 441 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.08, 0.10] |
| 53.4 Suprailiac | 1 | 452 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.12, 0.10] |
| 54 Mean adult systolic blood pressure (mmHg) | 2 | 545 | Mean Difference (IV, Random, 95% CI) | ‐1.53 [‐4.50, 1.44] |
| 54.1 Schutte (females) | 1 | 38 | Mean Difference (IV, Random, 95% CI) | ‐4.0 [‐9.12, 1.12] |
| 54.2 Schutte (males) | 1 | 52 | Mean Difference (IV, Random, 95% CI) | ‐3.0 [‐7.17, 1.17] |
| 54.3 Liggins | 1 | 455 | Mean Difference (IV, Random, 95% CI) | 0.55 [‐1.88, 2.98] |
| 55 Mean adult HPA axis function (mean log fasting cortisol) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 56 Mean cholesterol in adulthood (mmol/L) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 57 Mean age at puberty (years) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 57.1 Schutte (females) | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.94, 0.94] |
| 58 Educational achievement by adulthood (university or polytechnic education) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 59 Visual impairment in adulthood | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 60 Hearing impairment in adulthood | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 61 Intellectual impairment in adulthood | 2 | 273 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.01, 4.95] |
| 62 Mean adult FVC (% predicted) | 1 | 383 | Mean Difference (IV, Fixed, 95% CI) | ‐0.70 [‐3.16, 1.76] |
| 63 Mean adult FEV1 (% predicted) | 1 | 383 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [‐2.31, 3.11] |
| 64 Mean adult FEV1/FVC | 1 | 383 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.01, 0.03] |
| 65 Mean adult PEF | 1 | 383 | Mean Difference (IV, Fixed, 95% CI) | 2.20 [‐0.77, 5.17] |
| 66 Mean adult F50 | 1 | 383 | Mean Difference (IV, Fixed, 95% CI) | 3.0 [‐1.57, 7.57] |
| 67 Mean adult F25 | 1 | 383 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [‐3.82, 4.62] |
| 68 Mean adult FEF 25%‐75% | 1 | 383 | Mean Difference (IV, Fixed, 95% CI) | 2.20 [‐2.10, 6.50] |
| 69 FEV1/FVC < 70% | 1 | 383 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.49, 1.57] |
| 70 Asthma diagnosed by Doctor in lifetime | 1 | 534 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.74, 1.30] |
| 71 Wheezing in last 12 months | 1 | 534 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.84, 1.35] |
| 72 Current Asthma | 1 | 534 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.74, 1.48] |
| 73 Further respiratory diagnosis (includes pneumonia, upper airway conditions and bronchitis) | 1 | 534 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.69, 2.59] |
| 74 Spontaneous pneumothorax | 1 | 534 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.07, 17.66] |
| 75 Shortness of breath at anytime in the last 12 months | 1 | 534 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.80, 1.31] |
| 76 Mean adult lumbar spine aBMD (g/cm2) areal bone mineral density | 1 | 174 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.04, 0.04] |
| 77 Mean adult lumbar spine vBMD (g/cm3) volumetric bone mineral density | 1 | 174 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.01, 0.01] |
| 78 Mean adult total body BMC (grams) bone mineral content | 1 | 174 | Mean Difference (IV, Fixed, 95% CI) | 18.0 [‐151.30, 187.30] |
| 79 Mean adult total body aBMD (g/cm3) areal bone mineral density | 1 | 174 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.03, 0.03] |
| 80 Mean adult femoral neck aBMD (g/cm2) areal bone mineral density | 1 | 174 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.03, 0.07] |
| 81 Mean adult femoral trochanter aBMD (g/cm2) areal bone mineral density | 1 | 174 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.02, 0.06] |
| 82 Mean adult femoral shaft aBMD (g/cm2) areal bone mineral density | 1 | 174 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.04, 0.06] |
| 83 Mean total proximal femur aBMD (g/cm2) areal bone mineral density | 1 | 174 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.03, 0.07] |
| 84 Mean length of antenatal hospitalisation (days) | 1 | 218 | Mean Difference (IV, Fixed, 95% CI) | 0.5 [‐1.40, 2.40] |
| 85 Mean length of postnatal hospitalisation (days) | 1 | 218 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐1.72, 1.72] |
| 86 Mean length of neonatal hospitalisation (days) | 5 | 788 | Mean Difference (IV, Fixed, 95% CI) | 0.18 [‐0.51, 0.87] |
Comparison 2.
Corticosteroids versus placebo or no treatment ‐ single or multiple pregnancy
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Chorioamnionitis ‐ single or multiple pregnancy | 15 | 5546 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.66, 1.06] |
| 1.1 In women delivering singleton pregnancies | 7 | 4682 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.56, 1.01] |
| 1.2 In women delivering multiple pregnancies | 1 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.04, 4.49] |
| 1.3 Mixed population | 8 | 790 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.70, 1.57] |
| 2 Perinatal death ‐ single or multiple pregnancy | 15 | 6729 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.59, 0.89] |
| 2.1 In babies born from singleton pregnancies | 6 | 5182 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.54, 1.05] |
| 2.2 In babies born from multiple pregnancies | 2 | 252 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.37, 1.47] |
| 2.3 Mixed population | 9 | 1295 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.49, 0.94] |
| 3 Neonatal death ‐ single or multiple pregnancy | 22 | 7188 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.59, 0.81] |
| 3.1 In babies born from singleton pregnancies | 9 | 5335 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.61, 0.92] |
| 3.2 In babies born from multiple pregnancies | 2 | 236 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.39, 1.61] |
| 3.3 Mixed population | 13 | 1617 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.46, 0.78] |
| 4 Fetal death ‐ single or multiple pregnancy | 15 | 6729 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.73, 1.29] |
| 4.1 In babies born from singleton pregnancies | 6 | 5182 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.75, 1.45] |
| 4.2 In babies born from multiple pregnancies | 2 | 252 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.20, 1.40] |
| 4.3 Mixed population | 9 | 1295 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.50, 1.99] |
| 5 Respiratory distress syndrome ‐ single or multiple pregnancy | 28 | 7762 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.59, 0.78] |
| 5.1 In babies born from singleton pregnancies | 15 | 6081 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.50, 0.77] |
| 5.2 In babies born from multiple pregnancies | 4 | 320 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.67, 1.22] |
| 5.3 Mixed population | 13 | 1361 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.53, 0.89] |
| 6 IVH ‐ single or multiple pregnancy | 16 | 6093 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.44, 0.70] |
| 6.1 In babies born from singleton pregnancies | 8 | 4782 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.36, 0.75] |
| 6.2 In babies born from multiple pregnancies | 1 | 137 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.07, 2.06] |
| 6.3 Mixed population | 8 | 1174 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.44, 0.81] |
| 7 Birthweight ‐ single or multiple pregnancy | 16 | 6182 | Mean Difference (IV, Fixed, 95% CI) | ‐17.61 [‐39.95, 4.74] |
| 7.1 In babies born from singleton pregnancy | 9 | 4948 | Mean Difference (IV, Fixed, 95% CI) | ‐24.12 [‐48.27, 0.03] |
| 7.2 In babies born from multiple pregnancies | 1 | 150 | Mean Difference (IV, Fixed, 95% CI) | 82.36 [‐146.23, 310.95] |
| 7.3 Mixed population | 7 | 1084 | Mean Difference (IV, Fixed, 95% CI) | 16.77 [‐44.16, 77.69] |
Comparison 3.
Corticosteroids versus placebo or no treatment ‐ intact membranes versus ruptured membranes at first dose
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Chorioamnionitis ‐ intact or ruptured membranes | 15 | 5517 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.67, 1.07] |
| 1.1 In women with intact membranes at 1st dose | 5 | 1437 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.50, 1.40] |
| 1.2 In women with ruptured membranes at 1st dose | 7 | 959 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.69, 1.40] |
| 1.3 Not reported or mixed population | 4 | 3121 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.47, 1.06] |
| 2 Endometritis ‐ intact or ruptured membranes | 10 | 4030 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.80, 1.80] |
| 2.1 In women with intact membranes at 1st dose | 2 | 289 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.37, 4.01] |
| 2.2 In women with ruptured membranes at 1st dose | 4 | 477 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.35, 2.97] |
| 2.3 Not reported or mixed population | 5 | 3264 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [0.81, 2.13] |
| 3 Perinatal death ‐ intact or ruptured membranes | 15 | 6700 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.60, 0.90] |
| 3.1 In babies born from pregnancies with intact membranes at 1st dose | 4 | 1332 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.70, 1.08] |
| 3.2 In babies born from pregnancies with ruptured membranes at 1st dose | 4 | 733 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.39, 0.90] |
| 3.3 Not reported or mixed population | 8 | 4635 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.49, 1.03] |
| 4 Neonatal deaths ‐ intact or ruptured membranes | 22 | 7163 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.59, 0.81] |
| 4.1 In babies born from pregnancies with intact membranes at 1st dose | 4 | 1236 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.58, 1.03] |
| 4.2 In babies born from pregnancies with ruptured membranes at 1st dose | 8 | 1024 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.46, 0.83] |
| 4.3 Not reported or mixed population | 11 | 4903 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.53, 0.88] |
| 5 Fetal death ‐ intact or ruptured membranes | 15 | 6634 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.71, 1.26] |
| 5.1 In babies born from pregnancies with intact membranes at 1st dose | 4 | 1332 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.73, 1.64] |
| 5.2 In babies born from pregnancies with ruptured membranes at 1st dose | 5 | 790 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.46, 1.61] |
| 5.3 Not reported or mixed population | 7 | 4512 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.44, 1.35] |
| 6 RDS ‐ intact or ruptured membranes | 28 | 7738 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.56, 0.76] |
| 6.1 In babies born from pregnancies with intact membranes at 1st dose | 6 | 1721 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.50, 0.80] |
| 6.2 In babies born from pregnancies with ruptured membranes at 1st dose | 12 | 1129 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.55, 0.90] |
| 6.3 Not reported or mixed population | 14 | 4888 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.46, 0.81] |
| 7 IVH ‐ intact or ruptured membranes | 15 | 5868 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.44, 0.70] |
| 7.1 In babies born from pregnancies with intact membranes at 1st dose | 5 | 1394 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.35, 0.72] |
| 7.2 In babies born from pregnancies with ruptured membranes at 1st dose | 5 | 895 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.28, 0.79] |
| 7.3 Not reported or mixed population | 6 | 3579 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.49, 1.07] |
| 8 Birthweight ‐ intact or ruptured membranes | 16 | 6153 | Mean Difference (IV, Fixed, 95% CI) | ‐19.52 [‐41.81, 2.78] |
| 8.1 In babies born from pregnancies with intact membranes at 1st dose | 4 | 1301 | Mean Difference (IV, Fixed, 95% CI) | ‐30.27 [‐100.43, 39.89] |
| 8.2 In babies born from pregnancies with ruptured membranes at 1st dose | 5 | 835 | Mean Difference (IV, Fixed, 95% CI) | ‐49.72 [‐113.91, 14.46] |
| 8.3 Not reported or mixed population | 8 | 4017 | Mean Difference (IV, Fixed, 95% CI) | ‐13.44 [‐38.71, 11.83] |
Comparison 4.
Corticosteroids versus placebo or no treatment ‐ hypertension syndrome versus all other trials
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 RDS | 28 | 7764 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.60, 0.74] |
| 1.1 Hypertension syndrome | 5 | 382 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.35, 0.72] |
| 1.2 No hypertension syndrome or hypertension syndromes excluded | 9 | 2660 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.47, 0.71] |
| 1.3 Hypertension not reported separately | 18 | 4722 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.66, 0.85] |
| 2 Perinatal deaths | 15 | 6729 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.60, 0.92] |
| 2.1 Hypertension syndrome | 2 | 313 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.42, 2.10] |
| 2.2 No hypertension syndrome or hypertension syndromes excluded | 3 | 1394 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.39, 1.29] |
| 2.3 Hypertension not reported separately | 11 | 5022 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.52, 0.93] |
| 3 Fetal deaths | 15 | 6729 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.74, 1.30] |
| 3.1 Women with hypertension syndrome | 3 | 331 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.73 [0.91, 3.28] |
| 3.2 No hypertension syndrome or hypertension syndromes excluded | 4 | 1644 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.49, 1.08] |
| 3.3 Hypertension not reported separately | 10 | 4754 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.67, 1.98] |
| 4 Neonatal deaths | 22 | 7188 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.59, 0.81] |
| 4.1 Hypertension syndrome | 2 | 278 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.29, 0.87] |
| 4.2 No hypertension syndrome or hypertension syndromes excluded | 3 | 1306 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.61, 1.09] |
| 4.3 Hypertension not reported separately | 18 | 5604 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.54, 0.82] |
Comparison 5.
Corticosteroids versus placebo or no treatment ‐ type of steroid
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Chorioamnionitis ‐ type of steroid | 15 | 5546 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.66, 1.06] |
| 1.1 In women treated with dexamethasone | 5 | 769 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.89, 2.05] |
| 1.2 In women treated with betamethasone | 10 | 4777 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.50, 0.90] |
| 2 Endometritis ‐ type of steroid | 10 | 4030 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.81, 1.80] |
| 2.1 In women treated with dexamethasone | 4 | 536 | Risk Ratio (M‐H, Random, 95% CI) | 1.71 [0.86, 3.43] |
| 2.2 In women treated with betamethasone | 6 | 3494 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.63, 1.45] |
| 3 Perinatal death ‐ type of steroid | 15 | 6729 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.58, 0.89] |
| 3.1 In babies treated with dexamethasone | 5 | 1420 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.46, 1.11] |
| 3.2 In babies treated with betamethasone | 10 | 5309 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.56, 0.94] |
| 4 Neonatal deaths by steroid type | 22 | 7188 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.59, 0.81] |
| 4.1 In babies treated with dexamethasone | 6 | 1468 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.55, 0.94] |
| 4.2 In babies treated with betamethasone | 16 | 5720 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.55, 0.83] |
| 5 Fetal death ‐ type of steroid | 15 | 6729 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.74, 1.30] |
| 5.1 In babies treated with dexamethasone | 5 | 1420 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.48, 1.60] |
| 5.2 In babies treated with betamethasone | 10 | 5309 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.73, 1.39] |
| 6 Respiratory distress syndrome ‐ type of steroid | 28 | 7764 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.56, 0.77] |
| 6.1 In babies treated with dexamethasone | 7 | 1651 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.61, 0.98] |
| 6.2 In babies treated with betamethasone | 20 | 6095 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.50, 0.73] |
| 6.3 Steroid type not reported | 1 | 18 | Risk Ratio (M‐H, Random, 95% CI) | 1.62 [0.08, 34.66] |
| 7 IVH ‐ type of steroid | 16 | 6093 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.40, 0.76] |
| 7.1 In babies treated with dexamethasone | 6 | 897 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.18, 1.26] |
| 7.2 In babies treated with betamethasone | 10 | 5196 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.40, 0.72] |
| 8 Birthweight ‐ type of steroid | 16 | 6182 | Mean Difference (IV, Fixed, 95% CI) | ‐18.47 [‐40.83, 3.90] |
| 8.1 In babies treated with dexamethasone | 4 | 686 | Mean Difference (IV, Fixed, 95% CI) | ‐17.04 [‐75.48, 41.41] |
| 8.2 In babies treated with betamethasone | 12 | 5496 | Mean Difference (IV, Fixed, 95% CI) | ‐18.71 [‐42.92, 5.50] |
| 9 Moderate/severe respiratory distress syndrome ‐ type of steroid | 6 | 1686 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.38, 0.91] |
| 9.1 Dexamethasone | 2 | 219 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.46, 1.44] |
| 9.2 Betamethasone | 4 | 1467 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.27, 0.90] |
| 10 Chronic lung disease ‐ type of steroid | 6 | 818 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.42, 1.79] |
| 10.1 Dexamethasone | 2 | 219 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.72, 1.90] |
| 10.2 Betamethasone | 4 | 599 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.26, 2.28] |
Comparison 6.
Corticosteroids versus placebo or no treatment ‐ decade of trial
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Chorioamnionitis ‐ decade of trial | 15 | 5546 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.66, 1.06] |
| 1.1 In women from trials conducted in 1970s | 2 | 1237 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.46, 1.17] |
| 1.2 In women from trials conducted in 1980s | 3 | 276 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.25, 1.01] |
| 1.3 In women from trials conducted in 1990s | 6 | 755 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.80, 1.74] |
| 1.4 in women from trials conducted in the 2000's | 2 | 257 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.02 [0.59, 6.95] |
| 1.5 In trials from 2010s | 2 | 3021 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.35, 1.07] |
| 2 Endometritis ‐ decade of trial | 10 | 4030 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.87, 1.63] |
| 2.1 In women from trials conducted in 1970s | 2 | 219 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.86 [0.81, 4.27] |
| 2.2 In women from trials conducted in 1980s | 1 | 71 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.30 [0.88, 6.06] |
| 2.3 In women from trials conducted in 1990s | 4 | 574 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.53, 1.44] |
| 2.4 In women from trials conducted in the 2000's | 3 | 3166 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.69, 2.01] |
| 3 Perinatal deaths ‐ decade of trial | 15 | 6729 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.58, 0.89] |
| 3.1 In babies from trials conducted in 1970s | 6 | 1994 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.50, 1.00] |
| 3.2 In babies from trials conducted in 1980s | 3 | 879 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.74, 1.42] |
| 3.3 In babies from trials conducted in 1990s | 3 | 615 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.43, 0.93] |
| 3.4 in babies from trials conducted in 2000's | 2 | 414 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.31, 0.70] |
| 3.5 In trials conducted in 2010s | 1 | 2827 | Risk Ratio (M‐H, Random, 95% CI) | 4.91 [0.24, 102.09] |
| 4 Neonatal deaths decade of trial | 22 | 7188 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.59, 0.81] |
| 4.1 In babies from trials conducted in 1970s | 7 | 1968 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.56, 0.92] |
| 4.2 In babies from trials conducted in 1980s | 6 | 1096 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.70, 1.37] |
| 4.3 In babies from trials conducted in 1990s | 5 | 758 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.36, 0.84] |
| 4.4 In babies from trials conducted in 2000s | 3 | 539 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.31, 0.64] |
| 4.5 In trials conducted in 2010s | 1 | 2827 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.91 [0.24, 102.09] |
| 5 Fetal death ‐ decade of trial | 15 | 6729 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.74, 1.30] |
| 5.1 In babies from trials conducted in 1970s | 6 | 1994 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.67, 1.34] |
| 5.2 In babies from trials conducted in 1980s | 3 | 879 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.52, 2.00] |
| 5.3 In babies from trials conducted in 1990s | 3 | 615 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.49, 2.36] |
| 5.4 In babies from trials conducted in 2000's | 2 | 414 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.19, 4.50] |
| 5.5 In trials conducted in 2010s | 1 | 2827 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 RDS ‐ decade of trial | 28 | 7764 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.60, 0.74] |
| 6.1 In babies from trials conducted in 1970s | 7 | 1939 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.43, 0.69] |
| 6.2 In babies from trials conducted in 1980s | 7 | 1167 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.59, 0.88] |
| 6.3 In babies from trials conducted in 1990s | 7 | 798 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.65, 0.91] |
| 6.4 In babies from trials conducted in 2000s | 5 | 839 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.26, 0.59] |
| 6.5 In trials from 2010s | 2 | 3021 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.61, 1.04] |
| 7 IVH ‐ decade of trial | 16 | 6093 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.44, 0.70] |
| 7.1 In babies from trials conducted in 1970s | 4 | 1646 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.29, 0.85] |
| 7.2 In babies from trials conducted in 1980s | 2 | 238 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.39, 0.94] |
| 7.3 In babies from trials conducted in 1990s | 5 | 722 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.42, 0.87] |
| 7.4 In babies from trials conducted in 2000s | 3 | 466 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.15, 0.73] |
| 7.5 In trials from 2010s | 2 | 3021 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.91 [0.24, 102.09] |
| 8 Birthweight ‐ decade of trial | 16 | 6182 | Mean Difference (IV, Fixed, 95% CI) | ‐18.47 [‐40.83, 3.90] |
| 8.1 In babies from trials conducted in 1970s | 4 | 1739 | Mean Difference (IV, Fixed, 95% CI) | ‐9.54 [‐83.55, 64.47] |
| 8.2 In babies from trials conducted in 1980s | 3 | 280 | Mean Difference (IV, Fixed, 95% CI) | ‐19.60 [‐108.55, 69.35] |
| 8.3 In babies from trials conducted in 1990s | 4 | 569 | Mean Difference (IV, Fixed, 95% CI) | ‐33.13 [‐102.39, 36.13] |
| 8.4 In babies from trials conducted in 2000s | 3 | 573 | Mean Difference (IV, Fixed, 95% CI) | ‐20.77 [‐61.95, 20.41] |
| 8.5 In trials in 2010s | 2 | 3021 | Mean Difference (IV, Fixed, 95% CI) | ‐15.18 [‐48.66, 18.29] |
Comparison 7.
Corticosteroids versus placebo or no treatment ‐ weekly repeats
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Chorioamnionitis ‐ Protocol with weekly repeats | 15 | 5546 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.66, 1.06] |
| 1.1 In women treated with single courses only | 7 | 4659 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.61, 1.11] |
| 1.2 In women treated with courses including weekly repeats | 8 | 887 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.57, 1.25] |
| 2 Endometritis ‐ protocol with weekly repeats | 10 | 4030 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.81, 1.80] |
| 2.1 In women treated with single courses only | 5 | 3450 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.66, 1.64] |
| 2.2 In women treated with courses including weekly repeats | 5 | 580 | Risk Ratio (M‐H, Random, 95% CI) | 1.46 [0.72, 2.95] |
| 3 Perinatal death ‐ protocol with weekly repeats | 15 | 6729 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.58, 0.89] |
| 3.1 In babies treated with single course only | 11 | 6250 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.61, 0.99] |
| 3.2 In babies treated with courses including weekly repeats | 4 | 479 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.44, 0.97] |
| 4 Neonatal death ‐ protocol with weekly repeats | 22 | 7188 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.59, 0.81] |
| 4.1 In babies treated with single course only | 14 | 6266 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.63, 0.95] |
| 4.2 In babies treated with courses including weekly repeats | 8 | 922 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.43, 0.72] |
| 5 Fetal death ‐ protocol with weekly repeats | 15 | 6729 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.74, 1.30] |
| 5.1 In babies treated with single course only | 11 | 6250 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.68, 1.25] |
| 5.2 In babies treated with courses including weekly repeats | 4 | 479 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.64, 2.87] |
| 6 RDS ‐ protocol with weekly repeats | 28 | 7764 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.60, 0.74] |
| 6.1 In babies treated with single course only | 19 | 6818 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.61, 0.79] |
| 6.2 In babies treated with courses including weekly repeats | 9 | 946 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.52, 0.72] |
| 7 IVH‐ protocol with weekly repeats | 16 | 6093 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.44, 0.70] |
| 7.1 In babies treated with single course only | 9 | 5216 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.33, 0.76] |
| 7.2 In babies treated with courses including weekly repeats | 7 | 877 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.45, 0.78] |
| 8 Birthweight ‐ protocol with weekly repeats | 16 | 6182 | Mean Difference (IV, Fixed, 95% CI) | ‐18.47 [‐40.83, 3.90] |
| 8.1 In babies treated with single course only | 12 | 5773 | Mean Difference (IV, Fixed, 95% CI) | ‐18.24 [‐42.12, 5.65] |
| 8.2 In babies treated with courses including weekly repeats | 4 | 409 | Mean Difference (IV, Fixed, 95% CI) | ‐20.10 [‐83.79, 43.60] |
| 9 Moderate/severe respiratory distress syndrome | 6 | 1686 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.38, 0.91] |
| 9.1 Single course | 3 | 1259 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.44, 0.83] |
| 9.2 Weekly repeats | 3 | 427 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.13, 1.32] |
Analysis 7.9.
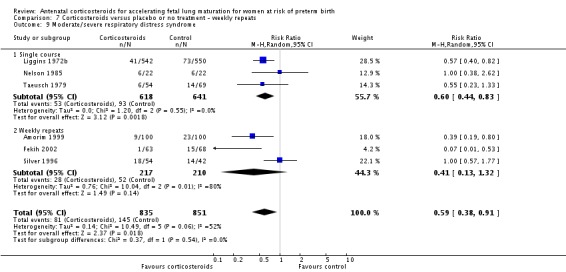
Comparison 7 Corticosteroids versus placebo or no treatment ‐ weekly repeats, Outcome 9 Moderate/severe respiratory distress syndrome.
Comparison 8.
Corticosteroids versus placebo or no treatment ‐ gestational age at trial entry
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Chorioamnionitis ‐ gestational age at trial entry | 15 | 5506 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.65, 1.05] |
| 1.1 Less than or equal to 35 weeks + 0 days | 13 | 2304 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.70, 1.19] |
| 1.2 Greater than or equal to 34 weeks + 0 days | 3 | 3202 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.33, 0.99] |
| 2 Perinatal death ‐ gestational age at trial entry | 15 | 6687 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.59, 0.88] |
| 2.1 Less than or equal to 35 weeks + 0 days | 13 | 3391 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.58, 0.87] |
| 2.2 Greater than or equal to 34 weeks + 0 days | 3 | 3296 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.29, 3.67] |
| 3 Neonatal death ‐ gestational age at trial engry | 22 | 7146 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.57, 0.79] |
| 3.1 Less than or equal to 35 weeks + 0 days | 20 | 3855 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.57, 0.79] |
| 3.2 Greater than or equal to 34 weeks + 0 days | 3 | 3291 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.22, 3.07] |
| 4 Fetal death ‐ gestational age at trial entry | 15 | 6687 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.72, 1.27] |
| 4.1 Less than or equal to 35 weeks + 0 days | 13 | 3391 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.71, 1.25] |
| 4.2 Greater than or equal to 34 weeks + 0 days | 3 | 3296 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.62 [0.28, 9.37] |
| 5 RDS‐ gestational age at trial entry | 28 | 7722 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.60, 0.73] |
| 5.1 Less than or equal to 35 weeks + 0 days | 23 | 3939 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.58, 0.73] |
| 5.2 Greater than or equal to 34 weeks + 0 days | 6 | 3783 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.56, 0.91] |
| 6 IVH ‐ gestational age at trial entry | 16 | 6051 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.44, 0.70] |
| 6.1 Less than or equal to 35 weeks + 0 days | 13 | 2639 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.42, 0.68] |
| 6.2 Greater than or equal to 34 weeks + 0 days | 4 | 3412 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.91 [0.24, 102.09] |
| 7 Birthweight ‐ gestational age at trial entry | 16 | 6140 | Mean Difference (IV, Fixed, 95% CI) | ‐17.45 [‐39.76, 4.86] |
| 7.1 Less than or equal to 35 weeks + 0 days | 11 | 2352 | Mean Difference (IV, Fixed, 95% CI) | ‐17.89 [‐63.14, 27.36] |
| 7.2 Greater than or equal to 34 weeks + 0 days | 6 | 3788 | Mean Difference (IV, Fixed, 95% CI) | ‐17.31 [‐42.96, 8.34] |
What's new
| Date | Event | Description |
|---|---|---|
| 17 February 2016 | New search has been performed | Search updated. The methods updated and the analyses have been restructured. 'Summary of findings' table has been incorporated. |
| 17 February 2016 | New citation required but conclusions have not changed | Nine new studies added for this update (Attawattanakul 2015; Balci 2010; Goodner 1979; Gyamfi‐Bannerman 2016; Khazardoust 2012; Lopez 1989; Mansouri 2010; Porto 2011; Shanks 2010). The review now includes a total of 30 studies. The conclusions remain unchanged. |
History
| Date | Event | Description |
|---|---|---|
| 23 January 2013 | Feedback has been incorporated | Feedback 8 received from Vincenzo Berghella. |
| 30 April 2010 | Amended | Search updated. Fourteen reports added to Studies awaiting classification. |
| 25 June 2008 | Feedback has been incorporated | Feedback from Vasiliy Vlassov added with a reply from the review author. |
| 23 June 2008 | Amended | Converted to new review format. |
| 14 March 2007 | Feedback has been incorporated | Feedback from David Hutchon added. |
| 30 October 2005 | New search has been performed | The review substantially updates the Crowley 2006 review due to new Cochrane guidelines for inclusion and exclusion of studies and the need for the review to be standardised with the repeat courses of prenatal corticosteroids review. Six new trials have been included (Amorim 1999; Dexiprom 1999; Fekih 2002; Lewis 1996; Nelson 1985; Qublan 2001). Three studies that were included in the previous review have been excluded. The results are now presented as relative risks. Results from recent follow‐up studies have been included. Individual participant data were available from the Liggins and Howie study and these were analysed completely by intention‐to‐treat analysis for the first time. These data contribute nearly a third of the data to the review. This represents an important development. The review also provides new information on corticosteroid use in the presence of rupture of membranes, hypertension syndromes, in multiple pregnancies and according to gestational age at first corticosteroid dose. |
Differences between protocol and review
The methods have been updated to current standard methods text for the Cochrane Pregnancy and Childbirth Group.
The following subgroups were not pre‐specified in the protocol:
decade of trial;
gestational age at trial entry;
protocol with weekly repeats.
In the 2016 update, comparison one has been re‐structured to include only the main analysis, with all clinical groups moved to subsequent comparisons. We have also deleted subgroups from previous versions of the review related to post‐randomisation variables (gestational age to delivery and ruptured membranes at specific time points). A 'Summary of findings' table has been incorporated in this update (2016).
We clarified the primary outcome of deaths (fetal/neonatal) to perinatal deaths. Neonatal deaths and fetal deaths are still presented separately as primary outcomes.
We renamed outcomes of mean length for children and adults as mean height.
In response to referee feedback we changed the name of the primary outcome 'puerperal sepsis' to 'endometritis (including infections)'. Most trials (7/10) in this analysis specifically reported endometritis.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Type of study: RCT Method of treatment allocation: computer‐generated randomisation sequence with randomisation code kept by the chief pharmacist. The pharmacy provided coded drug boxes. Stratification: none stated Placebo: yes, same volume of similar appearing vehicle Sample size calculation: yes Intention‐to‐treat analyses: no Losses to follow‐up: yes, 2 (1%) women in the placebo group dropped out after randomisation Funding: Instituto Materno‐Infantil de Pernambuco, Brazil | |
| Participants | Location: Instituto Materno‐Infantil de Pernambuco, Recife, state of Pernambuco, Brazil Timeframe: April 1997‐June 1998 Eligibility criteria: women with severe pre‐eclampsia, singleton pregnancy with a live fetus and gestational age between 26‐34 weeks. Likely minimal interval of 24 h between drug administration and delivery. Lung immaturity was confirmed by the foam test in fetuses of 30‐34 weeks. Gestational age range: 26‐34 weeks Exclusion criteria: indication for immediate delivery, diabetes, PROM, maternal disease, congenital malformations, perinatal haemolytic disease, Group B streptococcal infection Total recruited: 220 women and infants. 110 women and infants in each arm | |
| Interventions | 12 mg betamethasone IM, repeated after 24 h and weekly thereafter if delivery had not occurred. Control group received identical placebo. Delivery was at 34 weeks or in the presence of maternal or fetal compromise in both groups. | |
| Outcomes | Maternal outcomes (death, chorioamnionitis, maternal infection, fever after trial entry requiring antibiotics, intrapartum fever requiring antibiotics, postnatal fever, admission to ICU, glucose intolerance, hypertension), fetal/neonatal outcomes (fetal death, neonatal death, RDS, chronic lung disease, IVH, birthweight, Apgar score < 7, interval between trial entry and delivery, small‐for‐gestational age, admission to NICU, need for mechanical ventilation/CPAP, duration of oxygen supplementation, surfactant use, systemic infection in the first 48 h of life, proven infection while in the NICU, necrotising enterocolitis), childhood outcomes (death, developmental delay, cerebral palsy) and health service outcomes reported (length of antenatal hospitalisation for women, length of postnatal hospitalisation for women, length of neonatal hospitalisation) | |
| Notes | Further information obtained from the study authors, including substantial unpublished data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer‐generated randomisation sequence." |
| Allocation concealment (selection bias) | Low risk | "Randomisation code kept by the chief pharmacist." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and investigators were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome assessors was not described, but it is likely as the authors state, "the data analysis was carried out without knowledge of which of the treatments corresponded to corticosteroid and which to placebo". The code was fully broken only after the analysis was completed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 women (1%) in the placebo group voluntarily dropped out after randomisation. |
| Selective reporting (reporting bias) | Low risk | Study protocol was not available, but study appears to report on all pre‐specified outcomes |
| Other bias | Low risk | The groups were comparable at baseline. The study appears free of other sources of bias. |
| Methods | Type of study: open label RCT Method of treatment allocation: method of randomisation not stated. Block randomisation used Stratification: none stated Placebo: no, comparison was no treatment Sample size calculation: "Sample size was calculated to have type one error of 5 percent and 80 percent power to detect a reduction of 50 percent in rate of respiratory distress. Rate of respiratory distress in late preterm infant was assumed to be 28.9 percent based on Wang ML, et al. Accordingly, the number of study population was at least 95 pregnant women in each group." Intention‐to‐treat analyses: yes Losses to follow‐up: no Funding: not stated, though authors declare no competing interests |
|
| Participants | Location: Chonburi Hospital, Thailand Timeframe: March 2013‐March 2014 Eligibility criteria: all pregnant women with singleton pregnancy admitted in labour (defined as "regular uterine contraction at least 4 times in 20 minutes or 8 times in 60 minutes and cervical dilatation more than 1 cm and cervical effacement at least 80 percent") with a gestational age of 34 weeks + 0 d to 36 weeks + 6 d Gestational age range: 34 weeks + 0 d‐36 weeks + 6 d Exclusion criteria: "Participants who had history of corticosteroid administration in current pregnancy, history of dexamethasone allergy, systemic infection, multifetal pregnancy, complicated pregnancy including overt diabetes mellitus, gestational diabetes mellitus (GDM), pregnancy induced hypertension (PIH), placenta previa and abruptio placentae, positive or unknown sexual transmitted disease serology, PROM, evidence of fetal amniotic membrane leakage confirmed by two of the following test; pooling, nitrazine test, fern test or cough test, known fetal intrauterine restriction, oligohydramnios, non‐reassuring fetal heart rate tracing, fetal death, fetal anomaly, suspicious of chorioamnionitis (fetal tachycardia >160/min, maternal fever > 37.8°C, uterine tenderness, foul smelling amniotic fluid), cervical dilatation more than 7 cm, were excluded from our study." Total recruited: 194 women and infants; 96 women and infants in the treatment arm and 98 women and infants in the control arm. |
|
| Interventions | The treatment group received 6 mg dexamethasone IM, up to 4 doses 12 h apart. The control group received no treatment. |
|
| Outcomes | Maternal outcomes (chorioamnionitis, side effects of therapy in women) Fetal/neonatal outcomes (RDS, IVH, birthweight, necrotising enterocolitis, systemic infection in the first 48 h of life, need for mechanical ventilation/CPAP, Apgar score < 7, admission to NICU) |
|
| Notes | Labour augmentation performed if needed even if women had not received full course of steroids. 6 (6%) women in the intervention group received a full course of steroids; most women (75/96 (78%)) in the intervention arm received just 1 dose of dexamethasone. Data for 'maternal local or systemic adverse reactions to treatment' have been included in the review under our outcome of maternal side effects. Data from the trial are available for the following outcomes: low birthweight (not defined); hypoglycaemia in infant; need for respiratory support in infant (6/96 treatment and 14/98 control; (these data are in addition to the need for 'positive pressure ventilation' included in the review outcome 'need for mechanical ventilation'); and maternal length of stay (not separated into intrapartum and postpartum) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. Method reported as block randomisation only |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open label, participants would have been aware of allocation. Delivery nurse not blinded but all other hospital staff delivering care were blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The data were retrieved from chart review and hospital staff were blinded apart from delivery room nurses. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5 women in the dexamethasone delivered after 1 week and were included in ITT analysis |
| Selective reporting (reporting bias) | Low risk | Relevant outcome data reported |
| Other bias | Low risk | The groups were comparable at baseline. |
| Methods | Type of study: RCT Method of treatment allocation: computer‐generated random number table, sequential sealed envelopes, not stated if opaque Stratification: none stated Placebo: no, comparison was no treatment Sample size calculation: not stated Intention‐to‐treat analyses: yes Losses to follow‐up: 30 infants with fetal distress, meconium‐stained liquor and who delivered within less than 24 h were excluded from the study (14 in control group, 16 in steroid group) Funding: not stated | |
| Participants | Location: Dept of Obstetrics and Gynecology, Hospital of Meram, Faculty of Medicine, Selcuk University, Konya, Turkey Timeframe: January 2007 and May 2009. Eligibility criteria: 34‐36 weeks' gestation based on LMP. If unsure dates, fetal biometric measurements of 33‐36 weeks on abdominal ultrasonography (done on admission). The mother had had at least 2 contractions lasting more than 30 seconds in 10 min on cardiotocography, and cervical dilatation > 3 cm with 80% effacement Gestational age range: 34 + 0‐36 + 0 weeks Exclusion criteria: obstetric complications (severe IUGR, pre‐eclampsia, placental abruption, placenta praevia), multiple pregnancies, those who had already received antenatal corticosteroid therapy, PROM, or suspicion of chorioamnionitis, fetal anomaly, fetal distress, severe systemic disease (heart disease, hyperthyroidism, hypothyroidism, renal disease, diabetes mellitus) Total recruited: 100 (50 women and babies in each group) |
|
| Interventions | The treatment group received a single dose of 12 mg betamethasone IM. The control group received no treatment. Women who delivered at least 24 h after betamethasone administration were included in the study. |
|
| Outcomes | Apgar score at 1 and 5 minutes, need for resuscitation, development of RDS | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Generated by a computer" |
| Allocation concealment (selection bias) | Unclear risk | "Sequential sealed envelopes" not stated if opaque or not |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to comparison group receiving no treatment and treatment group receiving corticosteroids, blinding of participants and personnel would not have been possible. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessors is not described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 30 infants with fetal distress, meconium‐stained liquor and who delivered within less than 24 h were excluded from the study (14 in control group, 16 in steroid group). Intention‐to‐treat analysis was used |
| Selective reporting (reporting bias) | High risk | Maternal complications were not pre‐specified but were reported |
| Other bias | Unclear risk | Insufficient information to asses if other sources of bias exist. |
| Methods | Type of study: RCT Method of treatment allocation: computer‐generated randomisation sequence. Coded drug boxes were provided. Stratification: none stated Placebo: yes, normal saline Sample size calculation: no Intention‐to‐treat analyses: no Losses to follow‐up: yes, 14 (10%) women delivered elsewhere and were lost to follow‐up. 6 (4%) women were excluded from analyses as they failed to complete the protocol. Funding: Schering Corporation, Kenilworth, New Jersey, USA; and The Upjohn Company, Kalamazoo, Michigan, USA | |
| Participants | Location: Department of Gynecology and Obstetrics at the University of Oklahoma College of Medicine, Oklahoma City, Oklahoma, USA Timeframe: not stated in manuscript, the study is coded as 1970s for the review Eligibility criteria: women with preterm labour and PROM Gestational age range: not stated Exclusion criteria: not stated Total recruited: the number randomised to each group not stated. Data are available on 114 infants; 60 infants in the treatment arm and 54 infants in the control arm | |
| Interventions | 12 mg betamethasone IM repeated after 24 h if delivery had not occurred Control group received 1 mL normal saline IM repeated after 24 h if delivery had not occurred. If there was evidence of progressive cervical dilatation an alcohol infusion was given in order to attempt to delay delivery for at least 48 h. In women with PROM delivery was induced if serial white blood cell counts or temperatures became elevated regardless of time elapsed since drug administration. |
|
| Outcomes | Fetal/neonatal outcomes reported (fetal death, neonatal death, RDS, need for mechanical ventilation/CPAP) | |
| Notes | This study included a third arm (125 mg methylprednisolone IM repeated after 24 h if delivery had not occurred). The data for the review report the betamethasone and control arms only. Overall data were available for 150 living infants, of whom 128 were preterm. Further information was requested from the study authors but there was no reply. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer generated randomisation sequence." |
| Allocation concealment (selection bias) | Low risk | "Consecutively numbered boxes containing randomly selected study drug or placebo." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Clinicians were never aware of the contents of the coded box. Placeob was saline so it is likely that participants were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessors was not described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 14 (10%) women delivered elsewhere and were lost to follow‐up. 6 (4%) women were excluded from analyses as they failed to complete the protocol (1 in the betamethasone group, 2 in the methylprednisolone group, and 3 in the control group). |
| Selective reporting (reporting bias) | Low risk | Study protocol not available, but appears to report on all pre‐specified outcomes. |
| Other bias | Unclear risk | Insufficient information to assess if other sources of bias exist. |
| Methods | Type of study: RCT Method of treatment allocation: method of randomisation not stated. Stratification: none stated Placebo: no Sample size calculation: no Intention‐to‐treat analyses: yes Losses to follow‐up: no Funding: FIS; Perinatal Section of SEGO | |
| Participants | Location: Hospital Clinic, University of Barcelona, Spain Timeframe: 1987‐1990 Eligibility criteria: women with PROM Gestational age range: 28‐30 weeks Exclusion criteria: none stated Total recruited: 18 women and infants; 12 women and infants in the treatment arm and 6 women and infants in the control arm | |
| Interventions | Type and dose of corticosteroid used in the treatment group is not stated Control group received expectant management | |
| Outcomes | Fetal/neonatal outcome reported (RDS) | |
| Notes | Study only available as an abstract. Further information was requested from the study authors but there was no reply | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not stated |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants and personnel not stated, although unlikely as placebo was not used |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessment was not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Study only available as an abstract |
| Other bias | Unclear risk | Study was only available as an abstract. Further information was requested from the study authors, but there was no reply |
| Methods | Type of study: RCT Method of treatment allocation: method of randomisation not stated. Stratification: none stated Placebo: no Sample size calculation: no Intention‐to‐treat analyses: no Losses to follow‐up: yes, 2 (8%) infants with documented pulmonary maturity and 5 (17%) women with subsequent sealed membranes were not analysed Funding: not stated | |
| Participants | Location: University of South Florida Medical School, Tampa, Florida, USA Timeframe: not stated in manuscript, the study is coded as 1990s for the review Eligibility criteria: women with PROM Gestational age range: 24‐34 weeks Exclusion criteria: not stated Total recruited: the number randomised to each group is not stated. Data are available on 24 women and infants; 13 women and infants in the treatment arm and 11 women and infants in the control arm | |
| Interventions | 12 mg betamethasone IM repeated after 24 h and weekly thereafter until delivery or 34 weeks. Control group received expectant management. | |
| Outcomes | Maternal outcome (chorioamnionitis), fetal/neonatal outcomes (RDS, birthweight, days of mechanical ventilation/CPAP) and health service outcomes reported (days in NICU, neonatal days in hospital, neonatal hospital cost). However due to lack of SD data only chorioamnionitis and RDS data were included in the review. | |
| Notes | This study included a third arm (12 mg betamethasone IM 24‐hourly for 2 doses and 400 mcg methylprednisolone IV 8‐hourly for 6 doses, repeated weekly until delivery or 34 weeks. The data for the review report the betamethasone and control arms only. Further information was requested from the study authors but there was no reply. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not stated |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants and personnel not stated, although unlikely as placebo was not used |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 (8%) infants with documented pulmonary maturity and 5 (17%) women with subsequent sealed membranes were not analysed |
| Selective reporting (reporting bias) | Unclear risk | SD data not stated so a number of outcomes are not able to be included in the review |
| Other bias | Unclear risk | Only available as an abstract. Full paper not published |
| Methods | Type of study: RCT Method of treatment allocation: method of randomisation not stated. Coded drug boxes with sequentially‐numbered vials containing study drug were used. Sealed envelope containing the identity of the contents of was attached to each vial "to be opened in emergency only in case of an emergency". The manuscripts do not state how often these were opened. Stratification: yes, within each hospital Placebo: yes, identical appearance Sample size calculation: yes Intention‐to‐treat analyses: no Losses to follow‐up: yes, 2 (0%) infants in the control arm were lost to RDS follow‐up as neonates and 240 (37%) children were lost to follow‐up at age 3 (124 in the treatment arm and 116 in the control arm) Funding: National Institutes of Health, USA | |
| Participants | Location: 5 university hospitals in the USA Timeframe: March 1977‐March 1980 Eligibility criteria: women at high risk of preterm delivery. L/S ratio < 2.0 in cases of uncertain gestation, hyperthyroidism, hypertension, placental insufficiency, drug addiction, methadone use or gestational age > 34 weeks Gestational age range: 26‐37 weeks Exclusion criteria: > 5 cm of cervical dilatation, anticipated delivery < 24 h or > 7 d, intrauterine infection, previous glucocorticoid treatment, history of peptic ulcer disease, active tuberculosis, viral keratitis, severe fetal Rhesus sensitisation, infant unlikely to be available for follow‐up Total recruited: 696 women and 757 infants; 349 women and 378 infants in the treatment arm and 347 women and 379 infants in the control arm | |
| Interventions | 4 doses of 5 mg dexamethasone phosphate IM 12 h apart Control group received placebo | |
| Outcomes | Maternal outcomes (postnatal fever), fetal/neonatal outcomes (fetal death, neonatal death, RDS, birthweight, interval between trial entry and delivery, systemic infection in the first 48 h of life, proven infection while in the NICU, necrotising enterocolitis), childhood outcomes (death, lung function, developmental delay, intellectual impairment, cerebral palsy) and health service outcomes were reported (length of neonatal hospitalisation) | |
| Notes | Further information was requested from the authors but there was no reply | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not stated |
| Allocation concealment (selection bias) | High risk | Sealed envelope containing the identity of the contents of was attached to each vial "to be opened in emergency only in case of an emergency". The manuscripts do not state how often these were opened. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Both placebo and steroid were dispensed as 10 ml clear, colourless solutions which differed only in that one contained the steroid". It is likely that participants were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessors was not described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 2 (0.27%) infants in the control arm were lost to RDS follow‐up as neonates. At age 3, 240 (37%) children were lost to follow‐up (124 in the treatment arm and 116 in the control arm), or had died (47 in the treatment arm and 46 in the control arm). |
| Selective reporting (reporting bias) | Low risk | Study protocol not available, but appears to report on all pre‐specified outcomes |
| Other bias | Unclear risk | Insufficient information to asses if other sources of bias exist. |
| Methods | Type of study: RCT Method of treatment allocation: computer‐generated randomisation. Sequentially‐numbered drug boxes were used. Stratification: yes, by hospital Placebo: yes, normal saline Sample size calculation: yes Intention‐to‐treat analyses: no Losses to follow‐up: yes, 7 (3%) women and infants were excluded from analysis (3 women did not have PROM, 2 women were < 26 weeks at randomisation, 1 woman received off‐protocol corticosteroid, a neonatal bed was not available in 1 case) Funding: Medical Research Council, South Africa; Donmed Pharmaceuticals, South Africa | |
| Participants | Location: 6 hospitals in South Africa Timeframe: not stated in the manuscripts, the study is coded as 1990s for the review Eligibility criteria: women with PROM between 28‐34 weeks or with an estimated fetal weights of 1000 g‐2000 g if the gestational age was unknown Gestational age range: 28‐34 weeks Exclusion criteria: cervical dilatation > 4 cm, evidence of infection, evidence of antepartum haemorrhage, < 19 years old Total recruited: 204 women and 208 infants; 102 women and 105 infants in the treatment arm and 102 women and 103 infants in the control arm | |
| Interventions | 2 doses of 12 mg dexamethasone IM 24 h apart Control group received placebo All women also received ampicillin, metronidazole and hexoprenaline if contractions present in < 24 h |
|
| Outcomes | Maternal outcomes (maternal death, chorioamnionitis, endometritis, postnatal fever), fetal/neonatal outcomes reported (fetal death, neonatal death, RDS, IVH, birthweight, need for mechanical ventilation/CPAP, systemic infection in the first 48 h of life, necrotising enterocolitis) | |
| Notes | Study authors supplied additional data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Central allocation. Sequentially‐numbered drug boxes were used |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of participants likely as identical looking placebo was used. Blinding of study personnel was not described, other than "double blind". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessors was not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 7 (3%) women and infants were excluded from analysis (3 women did not have PROM, 2 women were < 26 weeks at randomisation, 1 woman received off‐protocol corticosteroid, a neonatal bed was not available in 1 case) |
| Selective reporting (reporting bias) | Low risk | Study protocol not available, but appears to report on all pre‐specified outcomes |
| Other bias | Unclear risk | Study was discontinued before target sample size was reached due to increasing body of evidence of the use of corticosteroids in women with PPROM being advantageous to the infants, and it was felt unnecessary to conduct further trials of antenatal corticosteroids in women with PPROM |
| Methods | Type of study: RCT Method of treatment allocation: method of randomisation not stated. Coded drug boxes were provided. Randomisation code was kept on file at the Pharmacy Department of Toronto General Hospital. Stratification: yes, by gestational age into 2 subgroups; 24‐32 weeks and 33‐34 weeks Placebo: yes, vehicle of steroid preparation consisting of 0.2 mg benzalkonium chloride and 0.1 mg disodium edentate per mL Sample size calculation: no Intention‐to‐treat analyses: yes Losses to follow‐up: no Funding: The Hospital for Sick Children Foundation, Canada; Schering Corporation, Canada; Ontario Ministry of Health Provincial Research Grant PR 279, Canada | |
| Participants | Location: 6 teaching hospitals in Toronto, Canada Timeframe: January 1975‐June 1978 Eligibility criteria: women with PROM, spontaneous preterm labour or planned preterm delivery Gestational age range: 24 and 34 weeks. Exclusion criteria: women with pre‐eclampsia or in whom steroids were contraindicated on medical grounds. Total recruited: 137 women and 144 infants; 75 women and 81 infants in the treatment arm and 62 women and 63 infants in the control arm | |
| Interventions | 4 doses of 3 mg betamethasone acetate and 3 mg betamethasone sodium phosphate IM 12 h apart Control group received 4 doses of identical placebo | |
| Outcomes | Fetal/neonatal outcomes were reported (fetal death, neonatal death, RDS, IVH, birthweight, days of mechanical ventilation) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not stated |
| Allocation concealment (selection bias) | Low risk | Coded drug boxes were provided. Randomisation code was kept on file at the Pharmacy Department of Toronto General Hospital. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | It is likely that participants were blinded as both placebo and corticosteroid solutions were identical. Blinding of study personnel was not described other than to state "double blind". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome assessors was not described, but is likely as the authors state "The key to the code was not broken until the whole study was completed". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up |
| Selective reporting (reporting bias) | Low risk | Study protocol not available, but appears to report on all pre‐specified outcomes |
| Other bias | Unclear risk | Insufficient information to asses if other sources of bias exist |
| Methods | Type of study: RCT Method of treatment allocation: method of randomisation not stated. Stratification: none stated Placebo: no Sample size calculation: no Intention‐to‐treat analyses: no Losses to follow‐up: yes, number of post‐randomisation exclusions not stated Funding: not stated | |
| Participants | Location: CHU Farhat Hached, Sousse, Tunisia Timeframe: January 1998‐June 1999 Eligibility criteria: women in preterm labour Gestational age range: 26‐34 weeks Exclusion criteria: gestational diabetes, > 4 cm cervical dilatation, fetal abnormalities, contraindication to corticosteroids, delivery elsewhere or after 34 weeks (post‐randomisation exclusions) Total recruited: 118 women and 131 infants; 59 women and 63 infants in the treatment arm and 59 women and 68 infants in the control arm | |
| Interventions | Abstract and full report state slightly different protocols for the intervention arm. The abstract stated that 24 mg betamethasone was given as two 12 mg IM doses at 24 h apart. The full text states that this regimen was repeated weekly. Women had two doses of 12 mg given 24 h apart, and this regimen was repeated weekly. Control group received expectant management | |
| Outcomes | Maternal outcomes (chorioamnionitis, postnatal fever) and fetal/neonatal outcomes reported (neonatal death, RDS, IVH) | |
| Notes | Article in French, abstract in English. Article translated by review authors (La Tunisie Medicale, 2002, Vol 80; No. 5: 260‐265). Further information was requested from the study authors but there was no reply | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not stated |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding is unlikely as placebo was not used |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessors was not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number of post‐randomisation exclusions not stated |
| Selective reporting (reporting bias) | Low risk | Study protocol not available, but appears to report on all pre‐specified outcomes |
| Other bias | Unclear risk | Insufficient information to asses if other sources of bias exist |
| Methods | Type of study: RCT Method of treatment allocation: method of randomisation not stated. Stratification: yes, by hospital Placebo: yes, vehicle of betamethasone preparation Sample size calculation: no Intention‐to‐treat analyses: yes Losses to follow‐up: no Funding: Glaxo Group Research Ltd, Greenford, Middlesex, UK | |
| Participants | Location: 11 hospitals in the UK Timeframe: mid 1975‐February 1978 Eligibility criteria: women with spontaneous or planned preterm delivery Gestational age range: < 34 weeks Exclusion criteria: contraindication to corticosteroids, contraindications to postponing delivery, diabetes, suspected intrauterine infection Total recruited: 251 women and 268 infants; 126 women and 131 infants in the treatment arm and 125 women and 137 infants in the control arm | |
| Interventions | 6 doses of 4 mg betamethasone phosphate IM 8 h apart Control group received 6 doses of placebo All women with spontaneous labour received IV salbutamol |
|
| Outcomes | Fetal/neonatal outcomes reported (fetal death, neonatal death, RDS, IVH, birthweight, systemic infection in the first 48 h of life) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not stated |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | It is likely that participants were blinded as placebo was used. Blinding of study personnel was not described other than "double‐blind". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessors was not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up reported |
| Selective reporting (reporting bias) | Low risk | Study protocol not available, but appears to report on all pre‐specified outcomes |
| Other bias | Unclear risk | Insufficient information to asses if other sources of bias exist |
| Methods | Type of study: RCT Method of treatment allocation: random‐number table generated randomisation sequence by pharmacy. The pharmacy provided consecutive sealed envelopes. Stratification: none stated Placebo: yes, normal saline Sample size calculation: no Intention‐to‐treat analyses: no Losses to follow‐up: yes, 5 (7%) women delivered elsewhere and were lost to follow‐up (4 in treatment arm and 1 in control arm) Funding: Long Beach Memorial Foundation, USA | |
| Participants | Location: Long Beach Memorial Women's Hospital, California, USA Timeframe: December 1984‐May 1990 Eligibility criteria: women likely to deliver between 24 h and 7 d with spontaneous preterm labour or planned preterm delivery Gestational age range: 24‐27 + 6 weeks Exclusion criteria: PROM, clinical or laboratory evidence of infection, contraindication to or previously given corticosteroids, diabetes Total recruited: 76 women and 82 infants; 37 women and 40 infants in the treatment arm and 39 women and 42 infants in the control arm | |
| Interventions | 2 doses of 6 mg betamethasone acetate and 6 mg betamethasone phosphate IM 24 h apart, repeated weekly if still < 28 weeks and thought likely to deliver within the next week Control group received 2 doses of placebo. Women undelivered after 28 weeks and 1 week post their last dose of study medication were allowed glucocorticoids at the discretion of their physicians. | |
| Outcomes | Maternal outcomes (chorioamnionitis, endometritis), fetal/neonatal outcomes reported (fetal death, neonatal death, RDS, chronic lung disease, IVH, birthweight, Apgar < 7, need for mechanical ventilation/CPAP, duration of mechanical ventilation/CPAP, proven neonatal infection while in NICU) | |
| Notes | It is not stated how many women received corticosteroids off protocol. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random‐number table generated randomisation sequence by pharmacy |
| Allocation concealment (selection bias) | Unclear risk | The pharmacy provided consecutive sealed envelopes, not stated if envelopes were opaque |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | It is likely that participants were blinded as placebo was used. Blinding of study personnel was not described other than "double‐blind" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessors was not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5 (7%) women delivered elsewhere and were lost to follow‐up (4 in treatment arm and 1 in control arm). |
| Selective reporting (reporting bias) | Low risk | Study protocol not available, but appears to report on all pre‐specified outcomes |
| Other bias | Unclear risk | It is not stated how many women received corticosteroids off protocol. |
| Methods | Type of study: RCT (abstract) Method of treatment allocation: not described Stratification: not described Placebo: yes, saline Sample size calculation: not stated Intention‐to‐treat analyses: not stated Losses to follow‐up: not stated Funding: not stated |
|
| Participants | Location: Temple University Hospital, Philadelphia, Pennsylvania, USA Timeframe: July 1976‐July 1978 Eligibility criteria: any pregnant woman expected to deliver prior to 34 weeks' gestation between July 1976 and July 1978 at Department of Obs & Gyne at Temple University Hospital Gestational age range: prior to 34 weeks Exclusion criteria: not stated Total recruited: 45 placebo, 47 steroids | |
| Interventions | Treatment group received an IM injection of betamethasone. The control group received an IM injection of saline as placebo. | |
| Outcomes | Neonatal mortality, RDS | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated other than "randomized" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Likely that participants were blinded and possible that study personnel were blinded due to the use of placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessor not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated |
| Selective reporting (reporting bias) | High risk | RDS is the only outcome reported. |
| Other bias | Unclear risk | Only available as an abstract ‐ does not appear to have been published |
| Methods | Type of study: double‐blind, RCT Method of treatment allocation: simple urn method of randomisation Stratification: yes, according to clinical site and gestational age (34‐35 weeks and 36 weeks) Placebo: yes, matching placebo Sample size calculation: yes Intention‐to‐treat analyses: yes Losses to follow‐up: yes, 4 (0.11%) lost to follow‐up; 2 in each treatment group Funding: National Heart, Lung, and Blood Institute, USA; Eunice Kennedy Shriver National Institute of Child Health and Human Development, USA; National Center for Advancing Translational Sciences, National Institutes of Health, USA |
|
| Participants | Location: 17 university‐based clinics in the USA. All centres affiliated with the Maternal–Fetal Medicine Units Network of the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Timeframe: October 2010‐February 2015 Eligibility criteria: women with singleton pregnancy 34 weeks + 0 d‐36 weeks + 5 d gestation at "high probability" of preterm delivery. "High probability was defined as either preterm labor with intact membranes and at least 3 cm dilation or 75% cervical effacement, or spontaneous rupture of the membranes. If neither of these criteria applied, a high probability was defined as expected preterm delivery for any other indication either through induction or cesarean section between 24 h and 7 d after the planned randomisation, as determined by the obstetrical provider." Gestational age range: 34 weeks + 0 d‐36 weeks + 5 d Exclusion criteria: expected delivery < 12 h for any reason, already received antenatal corticosteroids in current pregnancy, chorioamnionitis, 8 cm or more cervical dilation, non‐reassuring fetal status requiring immediate delivery, no gestational age dating by ultrasound before 32 weeks for women with known date for last menstrual period, women without ultrasound dating before 24 weeks' gestation with unknown date of last menstrual period Total recruited: 2831 women and 2831 infants; 1429 women and 1429 infants in the treatment arm and 1402 women and 1402 infants in the control arm | |
| Interventions | Treatment group: (n = 1429 randomised) 2 IM injections of 12 mg betamethasone (equal parts betamethasone sodium phosphate and betamethasone acetate) administered 24 h apart Control group received matching placebo "For those enrolled because of an indication for preterm delivery, labor inductions were expected to start by 36 weeks 5 d, and cesarean deliveries were to be scheduled by 36 weeks 6 days and not before 24 hours after randomization." Control: (n = 1402 randomised) placebo IM injections as above Follow up: to 28 d for oxygen dependency outcome |
|
| Outcomes | Maternal outcome (maternal death, chorioamnionitis, side effects of therapy in women), fetal/neonatal outcomes (perinatal death, fetal death, neonatal death, RDS, IVH, birthweight, necrotising enterocolitis, proven infection while in NICU, need for mechanical ventilation/CPAP, surfactant use, air leak syndrome, Apgar score < 7, small for gestation age, admission to NICU) We asked study authors to clarify the mechanical ventilation/CPAP data presented in Table 2 of the publication; we are unsure if outcome categories are exclusive or not. We have not included data from this trial in the meta‐analysis for 1.25 due to these concerns; data will be included at the next update if confirmed by study authors Data from trial is available for following non‐review outcomes: maternal serious adverse events, infant serious adverse events, hypoglycaemia in infant. Length of stay (maternal and infant) reported as median with IQR only. Randomisation to delivery interval reported as median with IQR only |
|
| Notes | Supplementary appendix published online with data tables and additional information on trial methods relevant to risk of bias. Contact author confirmed no maternal deaths and blinding of researchers abstracting data from maternal and neonatal charts (24.2.2016 by email) ClinicalTrials.gov number, NCT01222247. Ruptured membranes occurred in 22.1% intervention and 21.7% controls
"A total of 860 of 1429 women (60.2%) in the betamethasone group and 826 of 1402 (58.9%) in the placebo group received the prespecified two doses of study medication. Of the 1145 women who did not receive a second dose, 1083 (94.6%) delivered before 24 hours; 6 women did not receive any of the assigned study medication. (In the placebo group, 3 women who consented to participate in the trial subsequently declined the injection, 1 woman delivered after randomization but before the first dose, and 1 received open label betamethasone. In the betamethasone group, 1 woman was in active labor with complete cervical dilation at the time of randomization.)" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Independent data‐coordinating centre with the use of the simple urn method, with stratification according to clinical site and gestational age category (34 to 35 weeks vs. 36 weeks)" |
| Allocation concealment (selection bias) | Low risk | Remote centre performed randomisation and packaged intervention and placebo |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical treatment and placebo packs prepared remotely. Women and staff blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Trained research staff extracted data from maternal and neonatal staff; authors confirmed by email that these researchers were blinded. Charts of babies admitted to special care were reviewed by blinded staff for respiratory outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Two women in each group lost to follow‐up. Data available for 2827 neonates |
| Selective reporting (reporting bias) | Low risk | Supplementary outcome data published online with paper |
| Other bias | Low risk | Few baseline imbalances apart from mean maternal age (28.6 vs. 27.8 years) and Hispanic ethnic background (28.3 vs. 32%) |
| Methods | Type of study: RCT Method of treatment allocation: method of randomisation not stated. Stratification: yes, according to gestational age (24‐27.9 weeks and 28‐31.9 weeks) at each hospital Placebo: yes, normal saline Sample size calculation: yes Intention‐to‐treat analyses: yes Losses to follow‐up: yes, 10 (11%) children in the follow‐up study at age 2 (2 in the treatment arm and 8 in the control arm) Funding: Foundation for Pediatric Research, Finland; Orange County Infant Care Specialists, Finland; The Orion Corporation Research Foundation, Finland; Instrumentarium Corporation Research Foundation, Finland; Arvo and Lea Ylppo Foundation, Finland; Rinnekoti Foundation, Finland; and Organon Company, Oss, The Netherlands | |
| Participants | Location: 5 hospitals in Finland Timeframe: April 1989‐October 1991 Eligibility criteria: women with preterm labour or threatened preterm delivery due to pre‐eclampsia Gestational age range: 24‐31.9 weeks Exclusion criteria: rupture of membranes, chorioamnionitis, congenital abnormalities, proven lung maturity, insulin‐treated diabetes, previously treated with corticosteroids Total recruited: 157 women and 190 infants; 77 women and 95 infants in the treatment arm and 80 women and 95 infants in the control arm | |
| Interventions | 4 doses of 6 mg dexamethasone sodium phosphate IM 12 h apart Control group received 4 doses of placebo. Rescue treatment with exogenous human surfactant was given to infants born 24‐33 weeks, who at 2‐24 h of age required mechanical ventilation with > 40% oxygen for RDS. | |
| Outcomes | Maternal outcome (chorioamnionitis), fetal/neonatal outcomes (fetal death, neonatal death, RDS, chronic lung disease, IVH, birthweight, surfactant use, necrotising enterocolitis, small‐for‐gestational age) and childhood outcomes reported (death, neurodevelopmental delay) | |
| Notes | Efficacy analysis restricted to 91 infants in treatment arm and 88 infants in control arm. 3 infants excluded for protocol violations (1 mother with twins in placebo arm was given corticosteroid, 1 infant in the treatment arm developed RDS but was not given surfactant as it was not available) and 6 infants were excluded because of congenital malformations (2 treatment, 4 placebo) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not stated. "Randomisation in each participating hospital was performed in blocks of 10" |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The investigators and those who provided care were unaware of the treatment allocation". It is likely that participants were blinded as "ampoules containing betamethasone and placebo were identical". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessors was not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 10 (11%) children in the follow‐up study at age 2 (2 in the treatment arm and 8 in the control arm). 1 female placebo‐treated infant born at 27 weeks' gestation died 3 months after the expected date of delivery, 4 infants were lost due to parental refusal, 2 were living overseas, and 3 were in other regions of the country |
| Selective reporting (reporting bias) | Low risk | Study protocol not available, but appears to report on all pre‐specified outcomes |
| Other bias | Unclear risk | Efficacy analysis restricted to 91 infants in treatment arm and 88 infants in control arm. 3 infants excluded for protocol violations (1 mother with twins in placebo arm was given corticosteroid, 1 infant in the treatment arm developed RDS but was not given surfactant as it was not available) and 6 infants were excluded because of congenital malformations (2 treatment, 4 placebo) |
| Methods | Type of study: double‐blind RCT Method of treatment allocation: computer generated Stratification: none stated Placebo: yes, placebo‐controlled Sample size calculation: not described Intention‐to‐treat analyses: no, 5 (13%) of participants in the intervention arm were excluded from analysis post randomisation Losses to follow‐up: yes, as above Funding: "The study was supported by Tehran University of Medial Sciences. The assays were performed At Shahed University of Medical Sciences which we would like to thanks the staff and cooperation of that center in this study." |
|
| Participants | Location: Obstetric emergency department of Vali‐e‐Asr,Hospital, Tehran, Iran Timeframe: June 2006 to July 2010 Eligibility criteria: patients at risk of preterm labor as determined by routine ultrasound examination in the first trimester Gestational age range: 34‐37 weeks Exclusion criteria: only primigravid women with signs of preterm labour were eligible, including "palpable uterine contractions every 5‐8 minutes and Bishop score of 4 and higher associated with cervical dilatation of more than 1 cm and at least 50% of effacement." "Women with systemic diseases, maternal hypertension before or during pregnancy, uterine tenderness, chorioamnionitis signs, symptomatic vaginal infection, rupture of membranes, current use of antibiotics, induced pregnancy, and history of smoking were excluded." Total recruited: 80 women and 80 infants; 40 women and 40 infants in the treatment arm and 40 women and 40 infants in the control arm |
|
| Interventions | The treatment group received 2 doses of 12 mg betamethasone IM. The control group received placebo of saline as per regimen above. |
|
| Outcomes | No outcomes available for the review | |
| Notes | Data are provided on endocervical cytokine levels in women who delivered within and after 1 week but no outcome data available for the review are presented | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation sequence in blocks of 4 |
| Allocation concealment (selection bias) | Unclear risk | Only 1 person (research assistant) had access to the randomisation list. It is unclear whether this person was part of the team performing the study |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo‐controlled trial ‐ saline was used as the placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessors was not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Analysis was not by intention‐to‐treat. 5 participants in the intervention arm were excluded for named reasons and their data were not included |
| Selective reporting (reporting bias) | Low risk | All intended outcomes, i.e. cytokine measurements, were reported |
| Other bias | High risk | Data were analysed for 35 women in the intervention arm versus 40 in the control arm because 2 delivered before cytokine sampling after the second dose of betamethasone, 1 opted out of the study and 2 developed high blood pressure |
| Methods | Type of study: RCT Method of treatment allocation: random‐number table generated randomisation sequence by clinical research nurse uninvolved in clinical care. Sequentially‐numbered sealed opaque envelopes used. Stratification: none stated Placebo: no Sample size calculation: no Intention‐to‐treat analyses: no Losses to follow‐up: yes, 2 (2%) women left hospital after randomisation and were lost to follow‐up (1 woman in each arm) Funding: not stated | |
| Participants | Location: Louisiana State University Medical Center, Shreveport, Louisiana, USA Timeframe: not stated in manuscript, the study is coded as 1990s for the review Eligibility criteria: women with singleton pregnancies with PROM. Women were randomised 12‐24 h after receiving IV ampicillin‐sulbactam Gestational age range: 24‐34 weeks Exclusion criteria: evidence of infection, vaginal examination, cerclage, allergic to penicillin, contraindication to expectant management, lung maturity confirmed by L/S ratio if 32 weeks or more Total recruited: 79 women and infants; 39 women and infants in the treatment arm and 40 women and infants in the control arm | |
| Interventions | The treatment group received 12 mg IM betamethasone repeated at 24 h and weekly if the women had not delivered. The control group received expectant management. | |
| Outcomes | Maternal outcomes (chorioamnionitis, endometritis), fetal/neonatal outcomes (neonatal death, RDS, IVH, birthweight, Apgar < 7, interval between trial entry and delivery, admission to NICU, surfactant use, proven neonatal infection while in NICU, necrotising enterocolitis) and health service outcome reported (length of neonatal hospitalisation) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Clinical research nurse uninvolved in clinical care generated randomisation sequence by using random‐number table, with a random permuted block size of 10 |
| Allocation concealment (selection bias) | Low risk | Sequentially‐numbered sealed opaque envelopes were used |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comparison was "no treatment" so blinding not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessors was not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 (2%) women left hospital against medical advice after randomisation and were lost to follow‐up (1 women in each arm) |
| Selective reporting (reporting bias) | Low risk | Study protocol not available, but appears to report on all pre‐specified outcomes |
| Other bias | Unclear risk | Insufficient information to asses if other sources of bias exist |
| Methods | Type of study: RCT Method of treatment allocation: random‐number table generated randomisation sequence by chief pharmacist. Pharmacy provided coded drug ampoules containing treatment or placebo Stratification: no Placebo: yes, of identical appearance Sample‐size calculation: no Intention‐to‐treat analyses: yes Losses to follow‐up: yes, 54 (18%) children in the follow‐up study at ages 4‐6 years (31 in the treatment arm and 23 in the control arm) and 412 (44%) adults in the follow‐up study at age 30 years (219 in the treatment arm and 193 in the control arm) Funding: Health Research Council of New Zealand, Auckland, New Zealand; Auckland Medical Research Foundation, Auckland, New Zealand; and New Zealand Lottery Grants Board, Wellington, New Zealand |
|
| Participants | Location: National Women's Hospital, Auckland, New Zealand Timeframe: December 1969 and February 1974 Eligibility criteria: women with threatened or planned preterm delivery Gestational age range: 24‐36 weeks Exclusion criteria: imminent delivery, contraindication to corticosteroids Total recruited: 1142 women and 1218 infants; 560 women and 601 infants in the treatment arm and 582 women and 617 infants in the control arm |
|
| Interventions | The treatment group 2 doses of 6 mg betamethasone phosphate and 6 mg betamethasone acetate IM 24 h apart. After the first 717 women had enrolled, the treatment intervention was doubled to 2 doses of 12 mg betamethasone phosphate and 12 mg betamethasone acetate IM 24 h apart. The control group received 6 mg cortisone acetate, which has 1/70th of the corticosteroid potency of the betamethasone. | |
| Outcomes | Maternal outcome (chorioamnionitis), fetal/neonatal outcomes (fetal death, neonatal death, RDS, cerebroventricular haemorrhage, mean birthweight, Apgar score < 7, mean interval between trial entry and delivery, proven infection while in NICU), childhood outcomes (death, mean weight, mean height, mean head circumference, mean lung function, mean blood pressure, intellectual impairment, cerebral palsy) and adulthood outcomes were reported (death, mean weight, mean height, mean head circumference, mean skin fold thickness, mean blood pressure, glucose impairment, HPA axis function, mean cholesterol, educational achievement, visual impairment, hearing impairment, intellectual impairment) | |
| Notes | Review includes new intention‐to‐treat analysis of the complete study and additional data due to the study authors providing individual participant study records | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random‐number table generated randomisation sequence by chief pharmacist |
| Allocation concealment (selection bias) | Low risk | Pharmacy provided coded drug ampoules containing treatment or placebo |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of study personnel was not described. It is likely that participants were blinded as placebo was of identical appearance to the corticosteroid. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | For the diagnosis of RDS, clinical records and chest radiographs were assessed separately and independently, by 1 of the study authors, and by a radiologist. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Incomplete outcome data for 54 (18%) children in the follow‐up study at ages 4‐6 (31 in the treatment arm and 23 in the control arm) and 412 (44%) adults in the follow‐up study at age 30 (219 in the treatment arm and 193 in the control arm) |
| Selective reporting (reporting bias) | Low risk | Study protocol not available, but appears to report on all pre‐specified outcomes |
| Other bias | Unclear risk | Insufficient information to asses if other sources of bias exist. |
| Methods | Type of study: RCT Method of treatment allocation: not described Stratification: not stated Placebo: no. Sample size calculation: not stated Intention‐to‐treat analyses: not stated however, all those randomised were analysed Losses to follow‐up: nil Funding: not stated |
|
| Participants | Location: Department of Obstetrics and Gynecology, Faculty of Medicine, National Univeristy of Colombia Timeframe: August 1983‐December 1985 Eligibility criteria: PROM (confirmed using speculoscopy and ultrasound), no signs of infection, not in labour at time of hospitalisation Gestational age range: 27‐35 weeks' gestation Exclusion criteria: not stated Total recruited: 20 control group, 20 study group | |
| Interventions | The treatment group received 2 doses of 12 mg betamethasone IM, 12 h apart. The control group received no treatment. |
|
| Outcomes | Neonatal mortality, RDS, Apgar score < 7 at 5 min, systemic infection in first 48 h | |
| Notes | Original article in Spanish, translated into English | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated other than "patients were classified randomly into groups" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comparison is "no treatment" so blinding not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessors was not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up reported |
| Selective reporting (reporting bias) | Low risk | Study protocol not available, but appears to report on all pre‐specified outcomes |
| Other bias | Unclear risk | Insufficient information to asses if other sources of bias exist. |
| Methods | Type of study: double‐blind RCT Method of treatment allocation: not described Stratification: not stated Placebo: yes, placebo‐controlled Sample size calculation: not stated Intention‐to‐treat analyses: yes Losses to follow‐up: no Funding: not stated in translation Double‐blind, randomised controlled trial in Kurdistan University of Medical Sciences, Sanandaj, Iran |
|
| Participants | Location: Kurdistan University of Medical Sciences, Sanandaj, Iran Timeframe: "during 2007" stated Eligibility criteria: women at high risk of preterm labour, not described Gestational age range: 35‐36 weeks Exclusion criteria: not stated in our translation Total recruited: 200 women and 200 infants; 100 women and 100 infants in the treatment arm and 100 women and 100 infants in the control arm | |
| Interventions | The treatment group received 2 doses of 12 mg betamethasone, IM. The control group received a placebo of normal saline. |
|
| Outcomes | Maternal outcome (maternal death, maternal infections), fetal/neonatal outcomes reported (RDS, birthweight, necrotising enterocolitis, systemic infection in the first 48 h of life, need for mechanical ventilation/CPAP, Apgar < 7 at 5 min, admission to NICU) | |
| Notes | Original article in Persian; we have obtained a truncated translation for this update. Our translator was unable to translate the definition of respiratory distress syndrome but said that the outcome was based on defined symptoms and confirmed by a paediatrician. Additonal outcome data for this trial are: Maternal length of stay > 3 d (equal numbers in treatment arms) is reported narratively above: mean birthweight and SD in kg has been analysed as g. Data for the trial outcome of 'need for respiratory support' has been included in the review analysis 1.26 'need for mechanical ventilation'. We have been unable to confirm whether the trial included only singleton pregnancy, but this is suggested by the equal numbers of women and infants reported. We have included data from this trial in the singleton subgroup. We had no information about membrane status from our translation, and so this trial has been included in the 'not reported or mixed population subgroup.' Maternal length of stay > 3 d (equal numbers in both arms) is reported narratively. We emailed study investigators for clarification and additional information with no reply (2/2016). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Generation of sequence not stated, but block method specified |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Trial described as double‐blind. Placebo‐controlled trial, and researchers and women were blind to treatment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Neonatal outcomes extracted by blinded paediatrician. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data reported for all women randomised |
| Selective reporting (reporting bias) | Low risk | Relevant outcome data reported |
| Other bias | Unclear risk | We have obtained a basic translation, but future correspondence with authors may clarify some of the risk of bias domains above. |
| Methods | Type of study: RCT Method of treatment allocation: method of randomisation not stated. Sealed envelopes were used. Stratification: none stated Placebo: no Sample size calculation: no Intention‐to‐treat analyses: no Losses to follow‐up: no Funding: not stated | |
| Participants | Location: 3 hospitals in Florida, USA Timeframe: January 1986‐March 1988 Eligibility criteria: women with singleton pregnancies with PROM Gestational age range: 26 and 34 weeks Exclusion criteria: PROM < 12 h before onset of labour, uterine tenderness, foul smelling lochia, fetal tachycardia, allergy to penicillin, congenital abnormalities, L/S ratio 2 or more, unable to obtain an L/S ratio, Dubowitz‐assigned gestational age different from obstetric assessment by 3 weeks (post‐randomisation exclusion) Total recruited: 165 women and infants; 87 women and infants in the treatment arm and 78 women and infants in the control arm | |
| Interventions | 4 treatment arms. Group 1, expectant management. Group 2, expectant management plus 2 doses of 12 mg betamethasone IM 24 h apart, repeated weekly if the women remained undelivered. Group 3, expectant management plus 2 g ampicillin IV every 6 h until cervical cultures were negative. Group 4, combination of group 2 and 3 management. We combined Groups 2 and 4 in the treatment arm for the review, and groups 1 and 3 in the control arm for the review. | |
| Outcomes | Maternal outcome (chorioamnionitis), fetal/neonatal outcomes reported (neonatal death, RDS, chronic lung disease, IVH, birthweight, proven neonatal infection while in NICU, necrotising enterocolitis, duration of mechanical ventilation/CPAP) | |
| Notes | Further information requested from study authors but there was no reply. No information was available on post‐randomisation exclusions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not stated |
| Allocation concealment (selection bias) | Unclear risk | "Sealed envelopes" were used. Not further described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | As comparison was expectant management, blinding of participants and personnel was not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessment was not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No losses to follow‐up noted. No information was available on post‐randomisation exclusions as per exclusion criteria |
| Selective reporting (reporting bias) | Low risk | Study protocol not available, but appears to report on all pre‐specified outcomes |
| Other bias | Unclear risk | Insufficient information to asses if other sources of bias exist |
| Methods | Type of study: RCT Method of treatment allocation: random‐number table generated randomisation sequence with consecutive sealed envelopes used. Stratification: none stated Placebo: no Sample size calculation: no Intention‐to‐treat analyses: yes Losses to follow‐up: no Funding: not stated | |
| Participants | Location: Wake Forest University Medical Center, North Carolina, USA Timeframe: not stated in manuscript, the study is coded as 1980s for the review Eligibility criteria: women with PROM Gestational age range: 28 and 34 weeks Exclusion criteria: fetal distress, active labour, cervical dilatation > 3 cm, sensitivity to tocolytics, PROM > 24 h, existing infection Total recruited: 44 women and infants; 22 women and infants in each arm | |
| Interventions | 3 treatment arms. Group 1, 2 doses of 6 mg or 12 mg betamethasone IM 12 h apart, delivery 24‐48 h after PROM and after 24 h of corticosteroid therapy. Group 2, delivery 24‐48 h after PROM. Group 3, expectant management. We did not include Group 3 in the review. | |
| Outcomes | Fetal/neonatal outcomes (neonatal death, RDS, proven neonatal infection while in NICU) and health service outcome reported (length of neonatal hospitalisation) | |
| Notes | Authors provided further information | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random‐number table generated randomisation sequence |
| Allocation concealment (selection bias) | Unclear risk | Consecutive sealed envelopes were used, not stated if opaque |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants and personnel was not possible due to the nature of the comparison. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessment was not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up or exclusions |
| Selective reporting (reporting bias) | Low risk | Study protocol not available, but appears to report on all pre‐specified outcomes |
| Other bias | Unclear risk | Insufficient information to asses if other sources of bias exist. |
| Methods | Type of study: RCT Method of treatment allocation: method of randomisation not stated. Stratification: none stated Placebo: no Sample size calculation: no Intention‐to‐treat analyses: yes Losses to follow‐up: no Funding: not stated | |
| Participants | Location: University of Illinois, Chicago, USA Timeframe: not stated in manuscript, the study is coded as 1980s for the review Eligibility criteria: women with PROM and < 4 cm of cervical dilatation Gestational age range: 25‐32 weeks Exclusion criteria: infection, fetal distress, fetal anomalies, contraindication to tocolysis Total recruited: 45 women and infants; 23 women and infants in the treatment arm and 22 women and infants in the control arm | |
| Interventions | The treatment group received 2 doses of 12 mg betamethasone IM 12 h apart repeated weekly until 32 weeks. The control group received expectant management. | |
| Outcomes | Fetal/neonatal outcomes reported (fetal death, neonatal death, RDS, systemic infection in the first 48 h of life, proven neonatal infection while in NICU) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not stated |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants and personnel was not possible due to the nature of the comparison. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessment was not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up or exclusions described |
| Selective reporting (reporting bias) | Low risk | Pre‐specified outcomes were reported. |
| Other bias | Unclear risk | Insufficient information to asses if other sources of bias exist. |
| Methods | Type of study: RCT Method of treatment allocation: sealed cardboard boxes numbered according to random number table generated by a statistician not involved in the study Stratification: not stated Placebo: yes, identical to corticosteroid in appearance, volume and colour Sample size calculation: yes Intention‐to‐treat analyses: yes Losses to follow‐up: 43 (13%) women (19 in corticosteroid group and 24 in placebo group) were discharged from hospital still pregnant and were considered post‐randomisation loss to follow‐ups. 2 (1%) women were excluded from the placebo group as they were found to be ineligible after randomisation (multiple pregnancy, and term pregnancy). Two infant stillbirths were also excluded. Funding: supported by the Insitiuto de Medicina Integral Prof Fernando Figueira‐IMIP, a private, not for profit healthcare organisation based in Recife, Brazil. The Institute did not interfere with study design or analysis. |
|
| Participants | Location: Instituto de Medicina Integral Professor Fernando Figueira, Recife, Pernambuco, Brazil Timeframe: April 2008‐June 2010 Eligibility criteria: 34‐36 + 6 weeks' gestation at risk of imminent premature delivery (either spontaneously or if early delivery was recommended as a result of problems with mother or fetus) Gestational age range: 34‐36 + 6 weeks' gestation Exclusion criteria: multiple pregnancy, major congenital malformations, haemorrhage symptoms with active bleeding, clinical evidence of chorioamnionitis, previous use of antenatal corticosteroids, need for immediate resolution of pregnancy for maternal or fetal reasons Total recruited: 320 women and infants; 163 women and infants in the treatment arm and 157 women and infants in the control arm | |
| Interventions | The treatment group received 2 doses of 12 mg IM betamethasone 24 h apart. The control group received IM saline as placebo. |
|
| Outcomes | Maternal outcomes (side effects of therapy in women) and fetal/neonatal outcomes (fetal deaths, neonatal deaths, RDS, birthweight, proven infection while in NICU, need for mechanical ventilation/CPAP, mean duration of mechanical ventilation/CPAP, surfactant use, small for gestational age, admission to NICU) | |
| Notes | For infant outcomes we have used the denominator stated in the published report excluding women who left the trial pregnant. An intention‐to‐treat analysis should have included these women, so for SOF outcomes we carried out a sensitivity analysis to determine if the denominator used made a difference to the overall pooled effect estimate; it did not (data not shown) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table was prepared by a statistician not involved in the study, using random allocation software |
| Allocation concealment (selection bias) | Low risk | The hospital pharmacy prepared sealed cardboard boxes numbered according to the random number table, and containing either betamethasone or placebo, identical in appearance, volume and colour. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Investigators, physicians caring for the women, the women themselves and the statistician were all blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Investigators, physicians caring for the women, the women themselves and the statistician were all blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 43 (13%) women (19 in steroid group and 24 in placebo group) were discharged from hospital still pregnant and were considered post‐randomisation losses to follow‐up. 2 (1%) women were excluded from the placebo group as they were found to be ineligible after randomisation (multiple pregnancy, and term pregnancy). |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes appear to have been reported. |
| Other bias | Unclear risk | States "no significant differences between groups in most baseline characteristics," but does not report where differences exist. |
| Methods | Type of study: RCT Method of treatment allocation: random‐number table generated randomisation sequence Allocation concealment unclear. Stratification: none stated Placebo: no Sample size calculation: no Intention‐to‐treat analyses: yes Losses to follow‐up: no Funding: not stated | |
| Participants | Location: 2 military hospitals in Jordan Timeframe: January 1997‐February 1999 Eligibility criteria: women with singleton pregnancies and PROM Gestational age range: 27‐34 weeks Exclusion criteria: lethal congenital anomaly, fetal death, infection, expected delivery within 12 h Total recruited: 139 women and infants; 72 women and infants in the treatment arm and 67 women and infants in the control arm | |
| Interventions | The treatment group received 4 doses of 6 mg dexamethasone IM 12 h apart, repeated if women had not delivered after 1 week. The control group received expectant management. | |
| Outcomes | Maternal outcomes (chorioamnionitis, endometritis), fetal/neonatal outcomes (fetal death, neonatal death, RDS, IVH, proven neonatal infection while in NICU, necrotising enterocolitis, Apgar < 7) and health service outcome reported (length of neonatal hospitalisation) | |
| Notes | Study authors contacted for further information but no reply. Discrepancy in number of infants with necrotising enterocolitis in manuscript | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random‐number table generated randomisation sequence. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants and personnel was not possible due to the nature of the comparison. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessment was not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up or exclusions stated |
| Selective reporting (reporting bias) | Unclear risk | Discrepancy in number of infants with necrotising enterocolitis in manuscript |
| Other bias | Unclear risk | Funding source not stated |
| Methods | Type of study: RCT Method of treatment allocation: method of randomisation not stated. Coded drug ampoules were provided. Randomisation code was only known to pharmacist. Stratification: none stated Placebo: yes, normal saline Sample size calculation: no Intention‐to‐treat analyses: no Losses to follow‐up: yes, 12 (12%) children in the follow‐up study at ages 10‐12 years (4 in the treatment arm and 8 in the control arm) and 21 (21%) adults in the follow‐up study at age 20 years (10 in the treatment arm and 11 in the control arm) Funding: Dutch Foundation for Research on Prevention (Praeventiefonds Project 28‐1145), the Netherlands | |
| Participants | Location: Department of Obstetrics and Gynaecology and Department of Neonatology, Wilhelmina Gasthuis, University of Amsterdam, Amsterdam, the Netherlands. Timeframe: April 1974‐April 1977 Eligibility criteria: women with preterm labour in whom it was possible to delay delivery by at least 12 h Gestational age range: 26‐32 weeks. Exclusion criteria: no contraindications to the use of corticosteroids or orciprenaline (insulin‐treated diabetes, hyperthyroidism, infection, severe hypertension, cardiac disease, marked fetal growth retardation or fetal distress) Total recruited: 101 women and 123 infants; 50 women and 65 infants in the treatment arm and 51 women and 58 infants in the control arm | |
| Interventions | The treatment group received 8 mg betamethasone phosphate and 6 mg betamethasone acetate IM repeated after 24 h. The control group received an identical placebo. All women received orciprenaline infusion and bed‐rest until 32 weeks. |
|
| Outcomes | Maternal outcomes (death, chorioamnionitis, maternal infections, fever after trial entry requiring antibiotics, intrapartum fever requiring antibiotics, postnatal fever, admission to ICU, side effects of therapy), fetal/neonatal outcomes (fetal death, neonatal death, RDS, IVH, birthweight, Apgar score < 7), childhood outcomes (weight, height, head circumference, lung function, visual impairment, hearing impairment, intellectual impairment, cerebral palsy, behavioural/learning difficulties) and adulthood outcomes were reported (weight, height, head circumference, blood pressure, intellectual impairment, age at puberty) | |
| Notes | Initial study report included a third arm of women (n = 133) and infants (n = 164) who had been excluded from randomisation because they were: 1. already in labour (n = 80) and could not be prolonged for at least 12 h or were already 33 weeks' gestation, or; 2. (n = 53) contra‐indicated for corticosteroids, or; 3. wrongly excluded (n = 5). These women and infants are not included in the review. Two perinatal deaths in the corticosteroid treatment arm were excluded for: 1. intrauterine fetal death due to solutio placentae, and 2. death due to prolapsed umbilical cord. These deaths have been included in the analyses. Infections in infants are listed in Table 6 of the Schutte 1979 original report. There are deaths associated with these infections, and it is not clear when these infections or deaths occurred, or if they have been included in the reported numbers for neonatal or perinatal deaths. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not stated |
| Allocation concealment (selection bias) | Low risk | Coded drug ampoules prepared by pharmacist |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Trial described as double blind, with pharmacist preparing identical treatment and control ampoules |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Staff were blind to treatment group |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 2 perinatal deaths in the corticosteroids group were excluded. Data for infant infections specify additional deaths, and it is unclear whether or not these deaths are counted in the overall total for perinatal deaths. The inclusion of these deaths will not change the overall conclusions of meta‐analysis in favour of corticosteroid use |
| Selective reporting (reporting bias) | Low risk | Primary outcome of the trial was RDS; this and other important outcomes are reported |
| Other bias | Unclear risk | We are unclear as to the impact of exclusions on results, especially for the outcome of perinatal deaths. |
| Methods | Type of study: RCT Method of treatment allocation: not stated other than "randomly assigned" Stratification: not stated Placebo: no Sample size calculation: yes Intention‐to‐treat analyses: no Losses to follow‐up: 7 (22%) women (3 in the study group and 4 in the control group) delivered within 7 d of their initial testing for fetal lung maturity and were excluded from the analysis Funding: supported in part by a Clinical and Translational Science Award, and by a grant from the National Centre for Research Resources, a component of the National Institute of Health and NIH Roadmap for Medical Research |
|
| Participants | Location: Barnes‐Jewish Hospital, St Louis, Missouri, USA Timeframe: May 2003‐May 2008 Eligibility criteria: singleton gestation, between 34 + 0 and 36 + 6 weeks' gestation, immature TDx‐FLM‐II test (< 45 mg/g) (this test measures surfactant to albumin ratio) after clinically indicated amniocentesis to test for fetal lung maturity. Gestational age range: 34 + 0 ‐36 + 6 weeks' gestation Exclusion criteria: multiple gestations, ruptured membranes, uncertain gestational ages, previous steroid treatment in current pregnancy, delivery before completing the steroid course, those unwilling or unable to comply with study protocol Total recruited: 32 women and infants; 13 women and infants in the treatment arm and 19 women and infants in the control arm | |
| Interventions | The treatment group received either 2 doses of betamethasone 12 mg IM 24 h apart, or 4 doses of dexamethasone 6 mg IM 12 h apart. The control group received no treatment. |
|
| Outcomes | Maternal outcomes (side effects of therapy in women) and fetal/neonatal outcomes (need for mechanical ventilation/CPAP, admission to NICU) | |
| Notes | This study was stopped early due to difficulties in participant recruitment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomly assigned" not further described |
| Allocation concealment (selection bias) | Unclear risk | "Sealed envelopes" not further described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Control group received no treatment so blinding of participants and study personnel would not have been possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention is made of blinding of outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 7 (22%) women (3 in the study group and 4 in the control group) delivered within 7 d of their initial testing for fetal lung maturity and were excluded from the analysis. No intention‐to‐treat analysis |
| Selective reporting (reporting bias) | High risk | Hyaline membrane disease is listed as an outcome, but not reported |
| Other bias | High risk | This study was stopped early due to difficulties in patient recruitment |
| Methods | Type of study: RCT Method of treatment allocation: computer‐generated randomisation sequence used Pharmacy provided identical syringes labelled with the woman's study number. Stratification: none stated Placebo: yes, normal saline Sample size calculation: yes Intention‐to‐treat analyses: no Losses to follow‐up: 124 women initially recruited, of whom 49 (40%) remained undelivered after 29 weeks and were not included in the review Funding: not stated | |
| Participants | Location: Northwestern University Medical School, Chicago, Illinois, USA Timeframe: April 1990‐June 1994 Eligibility criteria: women at risk of delivery between 24‐29 weeks Gestational age range: 24‐29 weeks Exclusion criteria: infection, maternal or fetal indications for urgent delivery Total recruited: 75 women and 96 infants; 39 women and 54 infants in the treatment arm and 36 women and 42 infants in the control arm | |
| Interventions | The treatment group received 4 doses of 5 mg dexamethasone IM 12 h apart, repeated weekly if the women remained undelivered. The control group received placebo. All infants born < 30 weeks received prophylactic surfactant at birth. |
|
| Outcomes | Maternal outcomes (chorioamnionitis, endometritis) and fetal/neonatal outcomes reported (neonatal death, RDS, chronic lung disease, IVH, small‐for‐gestational age, birthweight, necrotising enterocolitis) | |
| Notes | Those women undelivered after 29 weeks were eligible for corticosteroid outside the study protocol. These women and their infants are not included in the review as it was not possible to separate out control women who subsequently received corticosteroids | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation sequence used |
| Allocation concealment (selection bias) | Low risk | Pharmacy provided identical syringes labelled with the woman's study number. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Clinical personnel and the patient were effectively blinded to study group assignment" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The severity of RDS, and diagnosis of IVH were "confirmed independently by chart reviews conducted by 1 of the authors blinded to study group assignment" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 49 (40%) of the 124 women initially recruited, remained undelivered after 29 weeks and were not included in the review. |
| Selective reporting (reporting bias) | Low risk | Study protocol not available, but appears to report on all pre‐specified outcomes |
| Other bias | Unclear risk | Insufficient information to asses if other sources of bias exist. |
| Methods | Type of study: RCT Method of treatment allocation: method of randomisation not stated. Coded drug boxes used Stratification: yes, by gestational age at entry Placebo: yes, normal saline Sample size calculation: yes Intention‐to‐treat analyses: no Losses to follow‐up: yes, data not available for maternal outcomes on 4 women (2 in each treatment arm) Funding: not stated |
|
| Participants | Location: 2 hospitals in Boston, USA Timeframe: January 1975‐March 1977 Eligibility criteria: women with preterm labour, PROM or with cervical dilatation < 5 cm at 33 weeks or less and women with an L/S ratio < 2 if > 33 weeks or who had a previous infant with RDS Gestational age range: not stated Exclusion criteria: indication for immediate delivery, obstetrician objection, pre‐eclampsia, previously received corticosteroids Total recruited: 122 women and 127 infants recruited; 39 women and 54 infants randomised to the treatment arm and 36 women and 42 infants randomised to the control arm | |
| Interventions | The treatment group received 6 doses of 4 mg dexamethasone phosphate IM 8 h apart. The control group received placebo. | |
| Outcomes | Maternal outcomes (endometritis, fever after trial entry requiring antibiotics) and fetal/neonatal outcomes reported (fetal death, neonatal death, RDS, chronic lung disease, IVH, proven neonatal infection while in NICU) | |
| Notes | Study authors contacted for further information but there was no reply | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not stated |
| Allocation concealment (selection bias) | Unclear risk | Coded drug boxes were used, but it is not clear how they were coded, e.g. if they were sequentially numbered. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | It is likely that participants were blinded due to the use of an identical looking placebo. Blinding of study personnel was not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Diagnosis of RDS was made prior to breaking the treatment code. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Data not available for maternal outcomes on 4 (3%) women (2 in each treatment arm) with no explanation given. |
| Selective reporting (reporting bias) | Low risk | Study protocol not available, but appears to report on all pre‐specified outcomes |
| Other bias | Unclear risk | Insufficient information to asses if other sources of bias exist. |
| Methods | Type of study: RCT Method of treatment allocation: method of randomisation not stated. Coded drug boxes used. Stratification: none stated Placebo: yes, normal saline Sample size calculation: no Intention‐to‐treat analyses: yes Losses to follow‐up: no Funding: not stated | |
| Participants | Location: University of Helsinki, Finland Timeframe: not stated in manuscript, the study is coded as 1980s for the review Eligibility criteria: women with preterm labour and cervical dilatation < 4 cm without progression of labour upon initial observation of up to 12 h Gestational age range: 28 ‐35 weeks Exclusion criteria: pre‐eclampsia, diabetes Total recruited: 74 women and 80 infants; 36 women and 38 infants in the treatment arm and 38 women and 42 infants in the control arm | |
| Interventions | The treatment group received 2 doses of 12 mg betamethasone IM 24 h apart. The control group received placebo. | |
| Outcomes | Fetal/neonatal outcomes reported (RDS, HPA axis function) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not stated |
| Allocation concealment (selection bias) | Unclear risk | Coded drug boxes were used but it is not clear how they were coded, e.g. if they were sequentially numbered. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | It is likely that participants were blinded due to the use of a placebo "similar in appearance" to the corticosteroid. Blinding of study personnel was not described other than "ampoules were administered to the patients using the double‐blind principle". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessors was not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up or exclusions stated |
| Selective reporting (reporting bias) | Low risk | Study protocol not available, but appears to report on all pre‐specified outcomes |
| Other bias | Unclear risk | The study was discontinued early because the overall incidence of RDS was too low for any meaningful conclusions concerning the efficacy of prevention. |
CPAP: continuous positive airways pressure GDM: gestational diabetes mellitus HPA: hypothalamic‐pituitary‐adrenal ICU: intensive care unit IM: intramuscular IUGR: Iintrauterine growth restriction IV: intravenous IVH: intraventricular haemorrhage LMP: last menstrual period NICU: neonatal intensive care unit PIH: pregnancy induced hypertension PROM: premature rupture of membranes PPROM: prolonged premature rupture of membranes RCT: randomised controlled trial RDS: respiratory distress syndrome Rh: Rhesus SD: standard deviation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abuhamad 1999 | This abstract compares TRH + betamethasone with betamethasone + placebo. |
| Althabe 2015 | This is a trial of strategies to optimise use of corticosteroids. |
| Asnafei 2004 | This study is quasi‐experimental. |
| Butterfill 1979 | Randomised participants are combined with a non‐randomised cohort and cannot be analysed separately. |
| Dola 1997 | This abstract compares TRH + betamethasone with betamethasone + placebo. |
| Egerman 1998 | This trial compares oral vs IM dexamethasone in the prevention of RDS. It does not meet our entry criteria for inclusion of studies for the review. |
| Garite 1981 | This trial compares a policy of corticosteroid therapy followed by elective delivery with a policy of withholding corticosteroids and awaiting delivery so the independent effect of the 2 co‐interventions cannot be evaluated separately. |
| Grgic 2003 | Not a randomised trial. Outcomes for women who received steroids were compared with those that did not. Information obtained from translation sheet. Original article in Bosnian |
| Halac 1990 | Not a randomised trial. Women were allocated to placebo if they were expected to deliver within 24 h and to betamethasone if labour was not expected within 24 h. |
| Iams 1985 | Corticosteroid therapy (hydrocortisone) and co‐intervention of elective delivery was compared to expectant management in PROM. The independent effect of the 2 co‐interventions cannot be evaluated separately. |
| Khandelwal 2012 | Compared different doses of corticosteroid: 12‐hourly vs 24‐hourly. The study includes a repeat dose of corticosteroids and is eligible for inclusion in a different review, 'Repeat doses of prenatal corticosteroids for women at risk of preterm birth for improving neonatal health outcomes' Crowther 2015. |
| Koivisto 2007 | The study includes a repeat dose of corticosteroids and is eligible for inclusion in a different review, 'Repeat doses of prenatal corticosteroids for women at risk of preterm birth for improving neonatal health outcomes' Crowther 2015. |
| Kuhn 1982 | Randomised participants are combined with a non‐randomised cohort and cannot be analysed separately. |
| Kurtzman 2008 | The study includes a repeat dose of corticosteroids and is eligible for inclusion in a different review, 'Repeat doses of prenatal corticosteroids for women at risk of preterm birth for improving neonatal health outcomes' Crowther 2015. |
| Liu 2006 | Quasi‐randomised study that allocated women according to the in‐patient sequence. Compared the effect of dexamethasone combined with vitamin K, dexamethasone alone and no dexamethasone or vitamin K on periventricular/intraventricular haemorrhage. |
| Magee 1997 | This study compares the effects of betamethasone vs dexamethasone on antenatal fetal heart rate. |
| Maksic 2008 | This study appears to be an observational study of 163 premature infants, 80 of whom were exposed to antenatal corticosteroids, and 83 of whom were not. |
| McEvoy 2010 | This trial compares repeat dose corticosteroids and is eligible for inclusion a different review, 'Repeat doses of prenatal corticosteroids for women at risk of preterm birth for improving neonatal health outcomes' Crowther 2015. |
| Minoui 1998 | This study compares the effects of betamethasone vs dexamethasone on antenatal fetal heart rate. |
| Morales 1986 | Quasi‐randomised using medical record number. |
| Morrison 1978 | This study was included in original review. It is excluded from this update because of > 20% post‐randomisation exclusions and the fact that it was possibly quasi‐randomised. |
| Mulder 1997 | This study compares the effects of betamethasone vs dexamethasone on antenatal fetal heart rate. |
| Papageorgiou 1979 | This study was included in original review. It is excluded from this update because of > 20% post‐randomisation exclusions. Of 146 babies included in the study, the paper only reports outcomes for 61. |
| Romejko‐Wolniewicz 2013 | This is a head‐to‐head trial of 2 different regimens and is eligible for the Cochrane review entitled 'Different corticosteroids and regimens for accelerating fetal lung maturation for women at risk of preterm birth' Brownfoot 2013. |
| Rotmensch 1999 | This study compares the effects of betamethasone vs dexamethasone on antenatal fetal heart rate. |
| Schmidt 1984 | This study was included in original review. It is excluded from this update because of > 20% post‐randomisation exclusions. The paper only reports results from 92 of 144 randomised mothers and 97 of 149 randomised babies. |
| Simpson 1985 | Quasi‐randomised study. Randomised participants are combined with a non‐randomised cohort and cannot be analysed separately. |
| Whitt 1976 | This trial compares IM betamethasone with IV cortisol. It does not meet our entry criteria for inclusion of studies for the review. |
IM: intramuscular IV: intravenous PROM: premature rupture of membranes RDS: respiratory distress syndrome TRH: thyrotropin‐releasing hormone vs: versus
Contributions of authors
P Crowley prepared the first version of the Cochrane Review in 1996.
S Dalziel and D Roberts revised the protocol for the 2005 update. Both review authors identified included and excluded studies, extracted the data and wrote the discussion. S Dalziel entered the data and re‐analysed data from the New Zealand Trial using intention to treat. D Roberts entered the tables and contacted study authors for additional data.
In 2016 J Brown and N Medley assisted S Dalziel and D Roberts to update the review by entering and re‐analysing the data and drafting text of the review. J Brown created Table 10. N Medley created Table 1.
Sources of support
Internal sources
University of Auckland, New Zealand.
The University of Liverpool, UK.
Liverpool Women's NHS Foundation Trust, UK.
External sources
Harris‐Wellbeing of Women Preterm Birth Centre, UK.
Health Research Council of New Zealand (HRC13/556), New Zealand.
Declarations of interest
Devender Roberts: none known.
Julie Brown: none known.
Nancy Medley: Nancy Medley's work was financially supported by the a grant to University of Liverpool from the Harris‐Wellbeing Preterm Birth Centre.
Stuart R Dalziel: none known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
- Amorim MM, Santos LC, Faundes A. Corticosteroid therapy for prevention of respiratory distress syndrome in severe preeclampsia. American Journal of Obstetrics and Gynecology 1999;180(5):1283‐8. [DOI] [PubMed] [Google Scholar]
- Attawattanakul N, Tansupswatdikul P. Effects of antenatal dexamethasone on respiratory distress in late preterm infant: a randomized controlled trial. Thai Journal of Obstetrics and Gynaecology 2015;23:25‐33. [Google Scholar]
- Balci O, Ozdemir S, Mahmoud AS, Acar A, Colakoglu MC. The effect of antenatal steroids on fetal lung maturation between the 34th and 36th week of pregnancy. Gynecologic and Obstetric Investigation 2010;70(2):95‐9. [DOI] [PubMed] [Google Scholar]
- Block MF, Kling OR, Crosby WM. Antenatal glucocorticoid therapy for the prevention of respiratory distress syndrome in the premature infant. Obstetrics & Gynecology 1977;50:186‐90. [PubMed] [Google Scholar]
- Botet F, Cararach V, Sentis J. Premature rupture of membranes in early pregnancy. Neonatal prognosis. Journal of Perinatal Medicine 1994;22:45‐52. [DOI] [PubMed] [Google Scholar]; Cararach V, Botet F, Sentis J, Carmona F. A multicenter, prospective randomized study in premature rupture of membranes (PROM). Maternal and perinatal complications. International Journal of Gynecology and Obstetrics 1991;36 Suppl:267. [Google Scholar]; Cararach V, Sentis J, Botet F, Rios L. A multicenter, prospective randomized study in premature rupture of membranes (PROM). Respiratory and infectious complications in the newborn. Proceedings of the 12th European Congress of Perinatal Medicine; 1990; Lyon, France. 1990:216. [Google Scholar]
- Carlan SJ, Parsons M, O'Brien WF, Krammer J. Pharmacologic pulmonary maturation in preterm premature rupture of membranes. American Journal of Obstetrics and Gynecology 1991;164:371. [Google Scholar]
- Bauer CR, Morrison JC, Poole WK, Korones SB, Boehm JJ, Rigatto H, et al. A decreased incidence of necrotizing enterocolitis after prenatal glucocorticoid therapy. Pediatrics 1984;73:682‐8. [PubMed] [Google Scholar]; Burkett G, Bauer CR, Morrison JC, Curet LB. Effect of prenatal dexamethasone administration on the prevention of respiratory distress syndrome in twin pregnancies. Journal of Perinatology 1986;6:304‐8. [Google Scholar]; Collaborative Group on Antenatal Steroid Therapy. Amniotic fluid phospholipids after maternal administration of dexamethasone. American Journal of Obstetrics and Gynecology 1983;145:484‐90. [DOI] [PubMed] [Google Scholar]; Collaborative Group on Antenatal Steroid Therapy. Effect of antenatal dexamethasone administration in the infant: long term follow‐up. Journal of Pediatrics 1984;105:259‐67. [DOI] [PubMed] [Google Scholar]; Collaborative Group on Antenatal Steroid Therapy. Effect of antenatal dexamethasone administration on the prevention of respiratory distress syndrome. American Journal of Obstetrics and Gynecology 1981;141:276‐87. [PubMed] [Google Scholar]; Curet LB, Rao AV, Zachman RD, Morrison J, Burkett G, Poole K, et al. Maternal smoking and respiratory distress syndrome. American Journal of Obstetrics and Gynecology 1983;147:446‐50. [DOI] [PubMed] [Google Scholar]; Haning RV, Curet LB, Poole K, Boehnlein LM, Kuzma DL, Meier SM. Effects of fetal sex and dexamethasone on preterm maternal serum concentrations of human chorionic gonadotropin, progesterone, estrone, estradiol, and estriol. American Journal of Obstetrics and Gynecology 1989;161:1549‐53. [DOI] [PubMed] [Google Scholar]; Wiebicke W, Poynter A, Chernick V. Normal lung growth following antenatal dexamethasone treatment for respiratory distress syndrome. Pediatric Pulmonology 1988;5:27‐30. [DOI] [PubMed] [Google Scholar]; Zachman RD. The NIH multicenter study and miscellaneous clinical trials of antenatal corticosteroid administration. In: Farrell PM editor(s). Lung Development: Biological and Clinical Perspectives. Vol. II, London & New York: Academic Press, 1982:275‐96. [Google Scholar]; Zachman RD, Bauer CR, Boehm J, Korones SB, Rigatto H, Rao AV. Effect of antenatal dexamethasone on neonatal leukocyte count. Journal of Perinatology 1988;8:111‐3. [PubMed] [Google Scholar]
- Pattinson RC. A meta‐analysis of the use of corticosteroids in pregnancies complicated by preterm premature rupture of membranes. South African Medical Journal 1999;89(8):870‐3. [PubMed] [Google Scholar]; Pattinson RC, Funk M, Makin JD, Ficki H. The effect of dexamethasone on the immune system of women with preterm premature rupture of membranes: a randomised controlled trial. 15th Conference on Priorities in Perinatal Care in Southern Africa; 1996 March 5‐8; Goudini Spa, South Africa. 1996. [Google Scholar]; Pattinson RC, Makin JD, Funk M, Delport SD, Macdonald AP, Norman K. The use of dexamethasone in women with preterm premature rupture of membranes: a multicentre double blind, placebo controlled randomised trial. South African Medical Journal 1999;89(8):865‐70. [PubMed] [Google Scholar]; The DEXIPROM Study Group. The use of dexamethasone in women with preterm premature rupture of membranes: a multicentre placebo controlled randomised controlled trial. 16th Conference on Priorities in Perinatal Care; 1997; South Africa. 1997:32‐4. [Google Scholar]
- Doran TA, Swyer P, MacMurray B, Mahon W, Enhorning G, Bernstein A, et al. Results of a double blind controlled study on the use of betamethasone in the prevention of respiratory distress syndrome. American Journal of Obstetrics and Gynecology 1980;136:313‐20. [DOI] [PubMed] [Google Scholar]
- Fekih M, Chaieb A, Sboui H, Denguezli W, Hidar S, Khairi H. Value of prenatal corticotherapy in the prevention of hyaline membrane disease in premature infants. Randomized prospective study [Apport de la corticotherapie antenatale dans la prevention de la maladie des membranes hyalines chez le premature. Etude prospective randomisee]. Tunisie Medicale 2002;80(5):260‐5. [PubMed] [Google Scholar]
- Donnai P. UK multicentre trial of betamethasone for the prevention of respiratory distress syndrome. Proceedings of the 6th European Congress of Perinatal Medicine; 1978 Aug 29‐Sept 1; Vienna, Austria. 1978:Abstract no: 81. [Google Scholar]; Gamsu HR, Mullinger BM, Donnai P, Dash CH. Antenatal administration of betamethasone to prevent respiratory distress syndrome in preterm infants: report of a UK multicentre trial. British Journal of Obstetrics and Gynaecology 1989;96:401‐10. [DOI] [PubMed] [Google Scholar]
- Garite TJ, Rumney PJ, Briggs GG. A randomized, placebo‐controlled trial of betamethasone for the prevention of respiratory distress syndrome at 24‐28 weeks gestation. Surgery, Gynecology and Obstetrics 1993;176:37. [DOI] [PubMed] [Google Scholar]; Garite TJ, Rumney PJ, Briggs GG, Harding JA, Nageotte MP, Towers CV, et al. A randomized placebo‐controlled trial of betamethasone for the prevention of respiratory distress syndrome at 24‐28 weeks gestation. American Journal of Obstetrics and Gynecology 1992;166:646‐51. [DOI] [PubMed] [Google Scholar]
- Goodner DM. Antenatal steroids in the treatment of respiratory distress syndrome. 9th World Congress of Gynecology and Obstetrics; 1979 October 26‐31; Tokyo, Japan. 1979:362. [Google Scholar]
- Gyamfi‐Bannerman C. Antenatal late preterm steroids (ALPS): a randomized trial to reduce neonatal respiratory morbidity. American Journal of Obstetrics and Gynecology 2016;214(1 Suppl):S2. [Google Scholar]; Gyamfi‐Bannerman C, Thom EA, Blackwell SC, Tita AT, Reddy UM, Saade GR, et al. Antenatal betamethasone for women at risk for late preterm delivery. New England Journal of Medicine 2016;374(14):1311‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]; NCT01222247. Antenatal late preterm steroids (ALPS): a randomized placebo‐controlled trial. clinicaltrials.gov/ct2/show/NCT01222247 Date first received: 14 October 2010.
- Eronen M, Kari A, Pesonen E, Hallman M. The effect of antenatal dexamethasone administration on the fetal and neonatal ductus arteriosus: a randomised double‐blind study. American Journal of Diseases of Children 1993;147:187‐92. [DOI] [PubMed] [Google Scholar]; Kari MA, Akino T, Hallman M. Prenatal dexamethasone (DEX) treatment before preterm delivery and rescue therapy of exogenous surfactant‐ surfactant components and surface activity in airway specimens (AS). Proceedings of the 14th European Congress of Perinatal Medicine; 1994 June 5‐8; Helsinki, Finland. 1994:Abstract no: 486. [Google Scholar]; Kari MA, Hallman M, Eronen M, Teramo K, Virtanen M, Koivisto M, et al. Prenatal dexamethasone treatment in conjunction with rescue therapy of human surfactant: a randomised placebo‐controlled multicenter study. Pediatrics 1994;93:730‐6. [PubMed] [Google Scholar]; Salokorpi T, Sajaniemi N, Hallback H, Kari A, Rita H, Wendt L. Randomized study of the effect of antenatal dexamethasone on growth and development of premature children at the corrected age of 2 years. Acta Paediatrica 1997;86:294‐8. [DOI] [PubMed] [Google Scholar]
- Hantoushzadeh S, Javadian P, Salmanian B, Ghazanfari T, Kermani A, Abbasalizadeh F, et al. Betamethasone effects on the endocervical inflammatory cytokines in preterm labor: a randomized clinical trial. International Immunopharmacology 2011;11(8):1116‐9. [DOI] [PubMed] [Google Scholar]; Khazardoust S, Javadian P, Salmanian B, Zandevakil F, Abbasalizadeh F, Alimohamadi S, et al. A clinical randomized trial on endocervical inflammatory cytokines and Betamethasone in prime‐gravid pregnant women at risk of preterm labor. Iranian Journal of Immunology 2012;9(3):199‐207. [PubMed] [Google Scholar]
- Lewis D, Brody K, Edwards M, Brouillette RM, Burlison S, London SN. Preterm premature ruptured membranes: a randomized trial of steroids after treatment with antibiotics. Obstetrics & Gynecology 1996;88(5):801‐5. [DOI] [PubMed] [Google Scholar]
- Dalziel SR, Fenwick S, Cundy T, Parag V, Beck TJ, Rodgers A, et al. Peak bone mass after exposure to antenatal betamethasone and prematurity: follow‐up of a randomized controlled trial. Journal of Bone & Mineral Research 2006;21(8):1175‐86. [DOI] [PubMed] [Google Scholar]; Dalziel SR, Liang A, Parag V, Rodgers A, Harding JE. Blood pressure at 6 years of age after prenatal exposure to betamethasone: follow‐up results of a randomized, controlled trial. Pediatrics 2004;114:e373‐e377. [DOI] [PubMed] [Google Scholar]; Dalziel SR, Lim VK, Lambert A, McCarthy D, Parag V, Rodgers A, et al. Antenatal exposure to betamethasone: psychological functioning and health related quality of life 31 years after inclusion in randomised controlled trial. BMJ 2005;331:665. [DOI] [PMC free article] [PubMed] [Google Scholar]; Dalziel SR, Lim VK, Lambert A, McCarthy D, Parag V, Rodgers A, et al. Psychological functioning and health‐related quality of life in adulthood after preterm birth. Developmental Medicine and Child Neurology 2007;49(8):597‐602. [DOI] [PubMed] [Google Scholar]; Dalziel SR, Parag V, Harding JE. Blood pressure at 6 years of age following exposure to antenatal betamethasone. 7th Annual Congress of the Perinatal Society of Australia and New Zealand; 2003 March 9‐12; Tasmania, Australia. 2003:P13. [Google Scholar]; Dalziel SR, Rea HH, Walker NK, Parag V, Mantell C, Rodgers A, et al. Long term effects of antenatal betamethasone on lung function: 30 year follow up of a randomised controlled trial. Thorax 2006;61(8):678‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]; Dalziel SR, Walker NK, Parag V, Mantell C, Rea HH, Rodgers A, et al. Cardiovascular risk factors after exposure to antenatal betamethasone: 30‐year follow‐up of a randomised controlled trial. Lancet 2005;365:1856‐62. [DOI] [PubMed] [Google Scholar]; Dalziel SR, Walker NK, Parag V, Mantell C, Rea HH, Rodgers A, et al. Long‐term effects of antenatal exposure to betamethasone: thirty year follow‐up of a randomised controlled trial [abstract]. Pediatric Research 2004;55 Suppl:101. 14605252 [Google Scholar]; Dalziel SR, Walker NR, Parag V, Mantell C, Rea HH, Rodgers A, et al. Long‐term effects of antenatal exposure to betamethasone: thirty year follow‐up of a randomized controlled trial. Pediatric Academic Societies Annual Meeting; 2004 May 1‐4; San Francisco, USA. 2004. [Google Scholar]; Harding JE, Pang J, Knight DB, Liggins GC. Do antenatal corticosteroids help in the setting of preterm rupture of membranes?. American Journal of Obstetrics and Gynecology 2001;184:131‐9. [DOI] [PubMed] [Google Scholar]; Howie RN. Pharmacological acceleration of lung maturation. In: Villee CA, Villee DB, Zuckerman J editor(s). Respiratory Distress Syndrome. London & New York: Academic Press, 1986:385‐96. [Google Scholar]; Howie RN, Liggins GC. Clinical trial of antepartum betamethasone therapy for prevention of respiratory distress in pre‐term infants. In: Anderson ABM, Beard RW, Brudenell JM, Dunn PM editor(s). Pre‐term Labour. London: RCOG, 1977:281‐9. [Google Scholar]; Howie RN, Liggins GC. Prevention of respiratory distress syndrome in premature infants by antepartum glucocorticoid treatment. In: Villee CA, Villee DB, Zuckerman J editor(s). Respiratory Distress Syndrome. London & New York: Academic Press, 1973:369‐80. [Google Scholar]; Howie RN, Liggins GC. The New Zealand study of antepartum glucocorticoid treatment. In: Farrell PM editor(s). Lung Development: Biological and Clinical Perspectives, II. Academic Press: London & New York, 1982:255‐65. [Google Scholar]; Liggins GC. Prenatal glucocorticoid treatment: prevention of respiratory distress syndrome. Lung maturation and the prevention of hyaline membrane disease, report of 70th Ross Conference on Paediatric Research. Ross Labs, 1976:97‐103. [Google Scholar]; Liggins GC, Howie RN. A controlled trial of antepartum glucocorticoid treatment for prevention of the respiratory distress syndrome in premature infants. Pediatrics 1972;50:515‐25. [PubMed] [Google Scholar]; Liggins GC, Howie RN. Prevention of respiratory distress syndrome by antepartum corticosteroid therapy. Proceedings of Sir Joseph Barcroft Centenary Symposium, Fetal and Neonatal Physiology; 1972; UK. Cambridge University Press: UK, 1973:613‐7. [Google Scholar]; Liggins GC, Howie RN. Prevention of respiratory distress syndrome by maternal steroid therapy. In: Gluck L editor(s). Modern Perinatal Medicine. Chicago: Yearbook Publishers, 1974:415‐24. [Google Scholar]; MacArthur B, Howie RN, DeZoete A, Elkins J. Cognitive and psychosocial development of 4‐year‐old children whose mothers were treated antenatally with betamethasone. Pediatrics 1981;68:638‐43. [PubMed] [Google Scholar]; MacArthur B, Howie RN, DeZoete A, Elkins J. School progress and cognitive development of 6‐year‐old children whose mothers were treated antenatally with betamethasone. Pediatrics 1982;70:99‐105. [PubMed] [Google Scholar]; MacArthur B, Howie RN, DeZoete A, Elkins J, Liang AYL. Long term follow up of children exposed to betamethasone in utero. In: Tejani N editor(s). Obstetrical Events and Developmental Sequelae. CRC Press, 1989:81‐9. [Google Scholar]
- Lopez ALV, Rojas RL, Rodriguez MV, Sanchez AJ. Use of corticoids in preterm pregnancy with premature rupture of membranes [Uso de los corticoides en embarazo pretermino con ruptura prematura de membranas]. Revista Colombiana de Obstetricia y Ginecologia 1989;40:147‐51. [Google Scholar]
- IRCT138901193666N1. Effect of antenatal betamethasone on prevention of respiratory distress syndrome among neonates with gestational age of 35‐36 weeks. www.irct.ir Date first received: 17 May 2010. ; Mansouri M, Seyedolshohadaei F, Company F, Setare S, Mazhari S. Effect of antenatal betamethasone on prevention of respiratory distress syndrome among neonates with gestational age of 35‐36 weeks. Journal of Gorgan University of Medical Sciences 2010;12(3):18‐23. [Google Scholar]
- Morales WJ, Angel JL, O'Brien WF, Knuppel RA. Use of ampicillin and corticosteroids in premature rupture of membranes: a randomized study. Obstetrics & Gynecology 1989;73:721‐6. [PubMed] [Google Scholar]
- Nelson LH, Meis PJ, Hatjis CG, Ernest JM, Dillard R, Schey HM. Premature rupture of membranes: a prospective randomized evaluation of steroids, latent phase and expectant management. Obstetrics & Gynecology 1985;66:55‐8. [PubMed] [Google Scholar]
- Parsons MT, Sobel D, Cummiskey K, Constantine L, Roitman J. Steroid, antibiotic and tocolytic vs no steroid, antibiotic and tocolytic management in patients with preterm PROM at 25‐32 weeks. Proceedings of the 8th Annual Meeting of the Society of Perinatal Obstetricians; 1988 Feb 3‐6; Las Vegas, Nevada. 1988:44. [Google Scholar]; Sobel D, Parsons M, Roitman J, McAlpine L, Cumminsky K. Antenatal antibiotics in PROM prevents congenital bacterial infection. Pediatric Research 1988;23:476A. [Google Scholar]
- Porto AMF, Coutinho IC, Correia JB, Amorim MMR. Effectiveness of antenatal corticosteroids in reducing respiratory disorders in late preterm infants: randomised clinical trial. BMJ 2011;342:d1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qublan H, Malkawi H, Hiasat M, Hindawi IM, Al‐Taani MI, Abu‐Khait SA, et al. The effect of antenatal corticosteroid therapy on pregnancies complicated by premature rupture of membranes. Clinical & Experimental Obstetrics & Gynecology 2001;28(3):183‐6. [PubMed] [Google Scholar]
- Dessens AB, Haas HS, Koppe JG. Twenty year follow up of antenatal corticosteroid treatment. Pediatrics 2000;105(6):1325. [DOI] [PubMed] [Google Scholar]; Dessens AB, Smolders‐de Haas H, Koppe JG. Twenty year follow up in antenatally corticosteroid‐treated subjects. Prenatal and Neonatal Medicine 1998;3 Suppl 1:32. [Google Scholar]; Schmand B, Neuvel J, Smolder‐de Haas H, Hoeks J, Treffers PE, Koppe JG. Psychological development of children who were treated antenatally with corticosteroids to prevent respiratory distress syndrome. Pediatrics 1990;86:58‐64. [PubMed] [Google Scholar]; Schutte MF, Koppe JG, Treffers PE, Breur W. The influence of 'treatment' in premature delivery on incidence of RDS. Proceedings of the 6th European Congress of Perinatal Medicine; 1978 Aug 29‐Sept 1; Vienna, Austria. 1978:Abstract no: 80. [Google Scholar]; Schutte MF, Treffers PE, Koppe JG, Breur W. The influence of betamethasone and orciprenaline on the incidence of respiratory distress syndrome in the newborn after preterm labour. British Journal of Obstetrics and Gynaecology 1980;87:127‐31. [DOI] [PubMed] [Google Scholar]; Schutte MF, Treffers PE, Koppe JG, Breur W, Filedt Kok JC. The clinical use of corticosteroids for the acceleration of fetal lung maturity [Klinische toepassing van corticosteroiden ter bevordering van de foetale long‐rijpheid]. Nederlands Tijdschrift voor Geneeskunde 1979;123:420‐7. [PubMed] [Google Scholar]; Smolders‐de Haas H, Neuvel J, Schmand B, Treffers PE, Koppe JG, Hoeks J. Physical development and medical history of children who were treated antenatally with corticosteroids to prevent respiratory distress syndrome: a 10‐ to 12‐ year follow up. Pediatrics 1990;86(1):65‐70. [PubMed] [Google Scholar]
- Shanks A, Gross G, Shim T, Allsworth J, Moga C, Sadovsky Y, et al. Antenatal steroids for enhancement of fetal lung maturity after 34 weeks: lung maturity and antenatal steroids (LUMAS) study. American Journal of Obstetrics and Gynecology 2008;199(6 Suppl 1):S58. [DOI] [PMC free article] [PubMed] [Google Scholar]; Shanks A, Gross G, Shim T, Allsworth J, Sadovsky Y, Bildirici I. Administration of steroids after 34 weeks of gestation enhances fetal lung maturity profiles. American Journal of Obstetrics and Gynecology 2010;203(1):47.e1‐47.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver RK, Vyskocil CR, Solomon SL, Farrell EE, MacGregor SN, Neerhof MG. Randomized trial of antenatal dexamethasone in surfactant‐treated infants delivered prior to 30 weeks of gestation. American Journal of Obstetrics and Gynecology 1995;172:254. [DOI] [PubMed] [Google Scholar]; Silver RK, Vyskocil CR, Solomon SL, Ragin A, Neerhof MG, Farrell EE. Randomized trial of antenatal dexamethasone in surfactant‐treated infants delivered prior to 30 weeks of gestation. Obstetrics & Gynecology 1996;87:683‐91. [DOI] [PubMed] [Google Scholar]
- Taeusch HW Jr, Frigoletto F, Kitzmiller J, Avery ME, Hehre A, Fromm B, et al. Risk of respiratory distress syndrome after prenatal dexamethasone treatment. Pediatrics 1979;63:64‐72. [PubMed] [Google Scholar]
- Teramo K, Hallman M, Raivio KO. Maternal glucocorticoid in unplanned premature labor. Pediatric Research 1980;14:326‐9. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
- Abuhamad A, Green G, Heyl P, Veciana M. The combined use of corticosteroids and thyrotropin releasing hormone in pregnancies with preterm rupture of membranes: a randomised double blind controlled trial. American Journal of Obstetrics and Gynecology 1999;180(1 Pt 2):S96. [Google Scholar]
- Althabe F, Belizan JM, Mazzoni A, Berrueta M, Hemingway‐Foday J, Koso‐Thomas M, et al. Antenatal corticosteroids trial in preterm births to increase neonatal survival in developing countries: study protocol. Reproductive Health 2012;9(1):22. [DOI] [PMC free article] [PubMed] [Google Scholar]; Althabe F, Belizan JM, McClure EM, Hemingway‐Foday J, Berrueta M, Mazzoni A, et al. A population‐based, multifaceted strategy to implement antenatal corticosteroid treatment versus standard care for the reduction of neonatal mortality due to preterm birth in low‐income and middle‐income countries: the ACT cluster‐randomised trial. Lancet 2014;385(9968):629‐639. [PUBMED: 25458726] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asnafei N, Pourreza R, Miri SM. Pregnancy outcome in premature delivery of between 34‐37 weeks and the effects of corticosteroid on it. Journal of the Gorgan University of Medical Sciences 2004;6(2):57‐60. [Google Scholar]
- Butterfill AM, Harvey DR. Follow‐up study of babies exposed to betamethasone before birth. Archives of Disease in Childhood 1979;54:725. 42361 [Google Scholar]
- Dola C, Nageotte M, Rumney P, Towers C, Asrat T, Freeman R, et al. The effect of antenatal treatment with betamethasone and thyrotropin releasing hormone in patients with preterm premature rupture of membranes. American Journal of Obstetrics and Gynecology 1997;176(1 Pt 2):S49. [Google Scholar]
- Egerman RS, Mercer B, Doss JL, Sibai BM. A randomized controlled trial of oral and intramuscular dexamethasone in the prevention of neonatal respiratory distress syndrome. American Journal of Obstetrics and Gynecology 1998;178(1 Pt 2):S19. [DOI] [PubMed] [Google Scholar]; Egerman RS, Mercer BM, Doss JL, Sibai BM. A randomized, controlled trial of oral and intramuscular dexamethasone in the prevention of neonatal respiratory distress syndrome. American Journal of Obstetrics and Gynecology 1998;179(5):1120‐3. [DOI] [PubMed] [Google Scholar]; Egerman RS, Pierce WF 4th, Andersen RN, Umstot ES, Carr TL, Sibai BM. A comparison of the bioavailability of oral and intramuscular dexamethasone in women in late pregnancy. Obstetrics & Gynecology 1997;89(2):276‐80. [DOI] [PubMed] [Google Scholar]; Egerman RS, Walker RA, Doss JL, Mercer B, Sibai BM, Andersen RN. A comparison between oral and intramuscular dexamethasone in suppressing unconjugated estriol levels during the third trimester. American Journal of Obstetrics and Gynecology 1998;178(1 Pt 2):S182. [DOI] [PubMed] [Google Scholar]; Egerman RS, Walker RA, Mercer BM, Doss JL, Sibai BM, Andersen RA. Comparison between oral and intramuscular dexamethasone in suppressing unconjugated estriol levels during the third trimester. American Journal of Obstetrics and Gynecology 1998;179(5):1234‐6. [DOI] [PubMed] [Google Scholar]
- Garite TJ, Freeman RK, Linzey EM, Braly PS, Dorchester WL. Prospective randomized study of corticosteroids in the management of premature rupture of the membranes and the premature gestation. American Journal of Obstetrics and Gynecology 1981;141:508‐15. [DOI] [PubMed] [Google Scholar]
- Grgic G, Fatusic Z, Bogdanovic G. Stimulation of fetal lung maturation with dexamethasone in unexpected premature labor. Medicinski Arhiv 2003;57(5‐6):291‐4. [PubMed] [Google Scholar]
- Halac E, Halac J, Begue EF, Casanas JM, Idiveri DR, Petit JF, et al. Prenatal and postnatal corticosteroid therapy to prevent neonatal necrotizing enterocolitis: a controlled trial. Journal of Pediatrics 1990;117:132‐8. [DOI] [PubMed] [Google Scholar]
- Iams JD, Talbert ML, Barrows H, Sachs L. Management of preterm prematurely ruptured membranes: a prospective randomized comparison of observation vs use of steroids and timed delivery. American Journal of Obstetrics and Gynecology 1985;151:32‐8. [DOI] [PubMed] [Google Scholar]
- Khandelwal M, Chang E, Hansen C, Hunter K, Milcarek B. Betamethasone dosing interval ‐12 or 24 hours apart?. American Journal of Obstetrics and Gynecology 2012;206(Suppl 1):S10‐S11. [DOI] [PubMed] [Google Scholar]; Khandelwal M, Chang E, Hansen C, Hunter K, Milcarek B. Betamethasone dosing interval: 12 or 24 hours apart? A randomized, noninferiority open trial. American Journal of Obstetrics & Gynecology 2012;206(3):201.e1‐11. [DOI] [PubMed] [Google Scholar]
- Koivisto M, Peltoniemi OM, Saarela T, Tammela O, Jouppila P, Hallman M. Blood glucose level in preterm infants after antenatal exposure to glucocorticoid. Acta Paediatrica 2007;96(5):664‐8. [DOI] [PubMed] [Google Scholar]
- Kuhn RJP, Speirs AL, Pepperell RJ, Eggers TR, Doyle LW, Hutchinson A. Betamethasone, albuterol and threatened premature delivery. Obstetrics & Gynecology 1982;60:403‐8. [PubMed] [Google Scholar]
- Kurtzman J, Garite T, Clark R, Maurel K, The OCRN. Impact of a 'rescue course' of antenatal corticosteroids (ACS): a multi‐center randomized placebo controlled trial. American Journal of Obstetrics and Gynecology 2008;199(6 Suppl 1):S2. [DOI] [PubMed] [Google Scholar]
- Liu J, Wang Q, Zhao JH, Chen YH, Qin GL. The combined antenatal corticosteroids and vitamin K therapy for preventing periventricular‐intraventricular hemorrhage in premature newborns less than 35 weeks gestation. Journal of Tropical Pediatrics 2006;52(5):355‐9. [DOI] [PubMed] [Google Scholar]
- Magee LA, Dawes GS, Moulden M, Redman CW. A randomised controlled comparison of betamethasone with dexamethasone: effects on the antenatal fetal heart rate. British Journal of Obstetrics and Gynaecology 1997;104(11):1233‐8. [DOI] [PubMed] [Google Scholar]
- Maksic H, Hadzagic‐Catibusic F, Heljic S, Dizdarevic J. The effects of antenatal corticosteroid treatment on IVH‐PVH of premature infants. Bosnian Journal of Basic Medical Sciences 2008;8(1):58‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEvoy C, Schilling D, Clay N, Spitale P, Durand M. Neurodevelopmental outcome and growth in infants randomized to a single rescue course of antenatal steroids. Pediatric Academic Societies and Asian Society for Pediatric Research Joint Meeting; 2011 April 30‐May 3; Denver, Colorado, USA. 2011:3829.270. [Google Scholar]; McEvoy C, Schilling D, Peters D, Tillotson C, Spitale P, Wallen L, et al. Respiratory compliance in preterm infants after a single rescue course of antenatal steroids: a randomized controlled trial. American Journal of Obstetrics and Gynecology 2010;202(6):544.e1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]; McEvoy C, Schilling D, Segel S, Spitale P, Wallen L, Bowling S, et al. Improved respiratory compliance in preterm infants after a single rescue course of antenatal steroids: a randomized trial. American Journal of Obstetrics and Gynecology 2008;199(6 Suppl 1):S228. [DOI] [PMC free article] [PubMed] [Google Scholar]; McEvoy C, Schilling D, Spitale P, Gravett M, Durand M. Pulmonary function and respiratory outcomes at 12‐24 months in preterm infants randomized to a single rescue course of antenatal steroids. Pediatric Academic Societies' 2010 Annual Meeting; 2010 May 1‐4; Vancouver, Canada. 2010. [Google Scholar]; McEvoy C, Schilling D, Spitale P, Wallen L, Segel S, Bowling S, et al. Improved respiratory compliance after a single rescue course of antenatal steroids: a randomized controlled trial. Pediatric Academic Societies Annual Meeting; 2007 May 5‐8; Toronto, Canada2007. [DOI] [PMC free article] [PubMed]; McEvoy C, Schilling D, Spitale P, Wallen L, Segel S, Bowling S, et al. Improved respiratory compliance after a single rescue course of antenatal steroids: a randomized controlled trial. Pediatric Academic Societies Annual Meeting; 2008 May 2‐6; Honolulu, Hawaii. 2008. [Google Scholar]; McEvoy C, Schilling D, Spitale P, Wallen P, Segel S, Bowling S, et al. Growth and respiratory outcomes after a single rescue course of antenatal steroids: a randomized trial. Pediatric Academic Societies Annual Meeting; 2009 May 2‐5; Baltimore, USA. 2009. [Google Scholar]; McEvoy CT, Schilling D, Segal S, Spitale P, Wallen L, Bowling S, et al. Improved respiratory compliance in preterm infants <34 weeks after a single rescue course of antenatal steroids. American Journal of Respiratory and Critical Care Medicine 2009;179:A4127 [Poster #423]. [Google Scholar]
- Minoui S, Ville Y, Senat M, Multon O, Fernandez H, Frydman R. Effect of dexamethasone and betamethasone on fetal heart rate variability in preterm labour: a randomized study. British Journal of Obstetrics and Gynaecology 1998;105:749‐55. [DOI] [PubMed] [Google Scholar]; Minoui S, Ville Y, Senat MV, Multon O, Fernandez H, Frydman R. Effect of dexamethasone and betamethasone on fetal heart rate variability in preterm labor a randomized study. Prenatal and Neonatal Medicine 1996;1 Suppl 1:156. [DOI] [PubMed] [Google Scholar]
- Morales WJ, Diebel D, Lazar AJ, Zadrozny D. The effect of antenatal dexamethasone administration on the prevention of respiratory distress syndrome in preterm gestations with premature rupture of membranes. American Journal of Obstetrics and Gynecology 1986;154:591‐5. [DOI] [PubMed] [Google Scholar]
- Morrison JC, Schneider JM, Whybrew WD, Bucovaz ET. Effect of corticosteroids and fetomaternal disorders on the L:S ratio. Obstetrics & Gynecology 1980;56:583‐90. [PubMed] [Google Scholar]; Morrison JC, Schneider JM, Whybrew WD, Bucovaz ET. Effect of corticosteroids and fetomaternal disorders on the L:S ratio. Surgery, Gynecology and Obstetrics 1981;153:464. [PubMed] [Google Scholar]; Morrison JC, Whybrew WD, Bucovaz ET, Scheiner JM. Injection of corticosteroids into mother to prevent neonatal respiratory distress syndrome. American Journal of Obstetrics and Gynecology 1978;131:358‐66. [DOI] [PubMed] [Google Scholar]
- Mulder EJ, Derks JB, Visser GH. Antenatal corticosteroid therapy and fetal behaviour: a randomised evaluation of betamethasone and dexamethasone. British Journal of Obstetrics and Gynaecology 1997;104(11):1239‐47. [DOI] [PubMed] [Google Scholar]
- Papageorgiou AN, Desgranges MF, Masson M, Colle E, Shatz R, Gelfand MM. The antenatal use of betamethasone in the prevention of respiratory distress syndrome: a controlled blind study. Pediatrics 1979;63:73‐9. [PubMed] [Google Scholar]
- Romejko‐Wolniewicz E, Oleszczuk L, Zareba‐Szczudlik J, Czajkowski K. Dosage regimen of antenatal steroids prior to preterm delivery and effects on maternal and neonatal outcomes. Journal of Maternal‐Fetal and Neonatal Medicine 2013;26(3):237‐41. [DOI] [PubMed] [Google Scholar]
- Rotmensch S, Liberati M, Vishne T, Celentano C, Ben‐Rafael Z, Bellati U. The effects of betamethasone versus dexamethasone on computer‐analysed fetal heart rate characteristics: a prospective randomized trial. American Journal of Obstetrics and Gynecology 1998;178(1 Pt 2):S185. [Google Scholar]; Rotmensch S, Liberati M, Vishne TH, Celentano C, Ben‐Rafael Z, Bellati U. The effect of betamethasone and dexamethasone on the fetal heart rate patterns and biophysical activities. A prospective randomized trial. Acta Obstetricia et Gynecologica Scandinavica 1999;78(6):493‐500. [PubMed] [Google Scholar]
- Schmidt PL, Sims ME, Strassner HT, Paul RH, Mueller E, McCart D. Effect of antepartum glucocorticoid administration upon neonatal respiratory distress syndrome and perinatal infection. American Journal of Obstetrics and Gynecology 1984;148:178‐86. [DOI] [PubMed] [Google Scholar]
- Simpson G, Harbert G. Use of beta‐methasone in management of preterm gestation with premature rupture of membranes. Obstetrics & Gynecology 1985;66:168‐75. [PubMed] [Google Scholar]
- Whitt GG, Buster JE, Killam AP, Scragg WH. A comparison of two glucocorticoid regimens for acceleration of fetal lung maturation in premature labor. American Journal of Obstetrics and Gynecology 1976;124:479‐82. [DOI] [PubMed] [Google Scholar]
Additional references
- Althabe F, Belizan J, McClure E, Hemingway‐Foday J, Berrueta M, Mazzoni A, et al. A population‐based, multifaceted strategy to implement antenatal corticosteroid treatment versus standard care for the reduction of neonatal mortality due to preterm birth in low‐income and middle‐income countries: the ACT cluster‐ randomised trial. The Lancet 2014;385(9968):629‐39. [DOI: 10.1016/S0140-6736(14)61651-2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiya RM, Mlunde LB, Ota E, Swa T, Oladapo O, Mori R. Antenatal corticosteroids for reducing adverse maternal and child outcomes in special populations of women at risk of imminent preterm birth: a systematic review and meta‐analysis. PLoS One 2016;11(2):e0147604. [DOI: 10.1371/journal.pone.0147604] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antenatal Corticosteroids Clinical Practice Guidelines Panel. Antenatal corticosteroids given to women prior to birth to improve fetal, infant, child and adult health: Clinical Practice Guidelines. Liggins Institute, The University of Auckland, Auckland, New Zealand2015.
- Azad K, Costello A. Extreme caution is needed before scale‐up of antenatal corticosteroids to reduce preterm deaths in low‐income settings. Lancet 2014;2:e191‐e192. [DOI: 10.1016/S2214-109X(14)70020-8] [DOI] [PubMed] [Google Scholar]
- Barker DJP. Mothers, Babies and Health in Later Life. 2nd Edition. London: Churchill Livingstone, 1998. [Google Scholar]
- Benediktsson R, Lindsay RS, Noble J, Seckl JR, Edwards CR. Glucocorticoid exposure in utero: new model for adult hypertension. Lancet 1993;341(8841):339‐41. [DOI] [PubMed] [Google Scholar]
- Brownfoot FC, Gagliardi DI, Bain E, Middleton P, Crowther CA. Different corticosteroids and regimens for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database of Systematic Reviews 2013, Issue 8. [DOI: 10.1002/14651858.CD006764.pub3] [DOI] [PubMed] [Google Scholar]
- Clark PM. Programming of the hypothalamo‐pituitary‐adrenal axis and the fetal origins of adult disease hypothesis. European Journal of Pediatrics 1998;157(1 Suppl):S7‐S10. [DOI] [PubMed] [Google Scholar]
- Crowley P, Chalmers I, Keirse MJNC. The effects of corticosteroid administration before preterm delivery: an overview of the evidence from controlled trials. British Journal of Obstetrics and Gynaecology 1990;97:11‐25. [DOI] [PubMed] [Google Scholar]
- Crowther CA, McKinlay CJD, Middleton P, Harding JE. Repeat doses of prenatal corticosteroids for women at risk of preterm birth for improving neonatal health outcomes. Cochrane Database of Systematic Reviews 2015, Issue 7. [DOI: 10.1002/14651858.CD003935.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
- Dodic M, Wintour EM, Whitworth JA, Coghlan JP. Effect of steroid hormones on blood pressure. Clinical & Experimental Pharmacology & Physiology 1999;26(7):550‐2. [DOI] [PubMed] [Google Scholar]
- Doyle LW. Victorian Infant Collaborative Study Group. Outcome at 5 years of age of children 23 to 27 weeks' gestation: refining the prognosis. Pediatrics 2001;108(1):134‐41. [DOI] [PubMed] [Google Scholar]
- Doyle LW, Casalaz D. Victorian Infant Collaborative Study Group. Outcome at 14 years of extremely low birthweight infants: a regional study. Archives of Diseases in Childhood: Fetal and Neonatal Edition 2001;85(3):F159‐F164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards LJ, Coulter CL, Symonds ME, McMillen IC. Prenatal undernutrition, glucocorticoids and the programming of adult hypertension. Clinical & Experimental Pharmacology & Physiology 2001;28(11):938‐41. [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JPT, Deeks JJ (editors). Chapter 7: Selecting studies and collecting data. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
- Hintz SR, Meurs KP, Perritt R, Poole WK, Das A, Stevenson DK, et al. Neurodevelopmental outcomes of premature infants with severe respiratory failure enrolled in a randomized controlled trial of inhaled nitric oxide. Journal of Pediatrics 2007;151:e1‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmeyr GJ. Antenatal Corticosteroids For Women At Risk Of Preterm Birth. Geneva: The WHO Reproductive Health Library, World Health Organization, 2 February 2009. [http://apps.who.int/rhl/pregnancy_childbirth/complications/preterm_birth/cd004454_hofmeyrgj_com/en/] [Google Scholar]
- Huang WL, Beazley LD, Quinlivan JA, Evans SF, Nenham JP, Dunlop SA. Effect of corticosteroids on brain growth in fetal sheep. Obstetrics & Gynecology 1999;94(2):213‐8. [DOI] [PubMed] [Google Scholar]
- Imseis HM, Iams JD. Glucocorticoid use in patients with preterm premature rupture of fetal membranes. Seminars in Perinatology 1996;20(5):439‐50. [DOI] [PubMed] [Google Scholar]
- Jobe AH, Wada N, Berry LM, Ikegami M, Ervin MG. Single and repetitive maternal glucocorticoid exposures reduce fetal growth in sheep. American Journal of Obstetrics and Gynecology 1998;178(5):880‐5. [DOI] [PubMed] [Google Scholar]
- Liggins GC. Premature delivery of foetal lambs infused with corticosteroids. Journal of Endocrinology 1969;45:515‐23. [DOI] [PubMed] [Google Scholar]
- Liggins GC, Howie RN. A controlled trial of antepartum glucocorticoid treatment for prevention of the respiratory distress syndrome in premature infants. Pediatrics 1972;50(4):515‐25. [PubMed] [Google Scholar]
- Liggins GC. Prenatal glucocorticoid treatment: prevention of respiratory distress syndrome. Lung maturation and the prevention of hyaline membrane disease, Report of the Seventieth Ross Conference on Pediatric Research, Columbus, Ohio. 1976:97‐103. [Google Scholar]
- National Institutes of Health (NIH) Consensus Development Conference Statement. Effect of corticosteroids for fetal maturation on perinatal outcomes. American Journal of Obstetrics and Gynecology 1994;173:246‐52. [Google Scholar]
- Padbury JF, Ervin MG, Polk DH. Extrapulmonary effects of antenatally administered steroids. Journal of Pediatrics 1996;128(2):167‐72. [DOI] [PubMed] [Google Scholar]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
- Rodriguez RJ, Martin RJ, Fanaroff, AA. Respiratory distress syndrome and its management. In: Fanaroff AA, Martin RJ editor(s). Neonatal‐Perinatal Medicine: Diseases of the Fetus and Infant. St. Louis: Mosby, 2002:1001‐1010. [Google Scholar]
- Schwab M, Roedel M, Akhtar Anwar M, Muler T, Schubert H, Buchwalder LF, et al. Effects of betamethasone administration to the fetal sheep in late gestation on fetal cerebral blood flow. Journal of Physiology 2000;528(3):619‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seckl JR, Cleasby M, Nyirenda MJ. Glucocorticoids, 11beta‐hydroxysteroid dehydrogenase, and fetal programming. Kidney International 2000;57(4):1412‐7. [DOI] [PubMed] [Google Scholar]
- Sotiriadis A, Makrydimas G, Papatheodorou S, Ioannidis JPA. Corticosteroids for preventing neonatal respiratory morbidity after elective caesarean section at term. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD006614.pub2] [DOI] [PubMed] [Google Scholar]
- Sterne JAC, Egger M, Moher D (editors). Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
- Turrentine MA, Wilson PD, Wilkins IA. A retrospective analysis of the effect of antenatal steroid on the incidence of respiratory distress syndrome in preterm twin pregnancies. American Journal of Perinatology 1996;13(6):351‐4. [DOI] [PubMed] [Google Scholar]
- Vyas J, Kotecha S. Effects of antenatal and postnatal corticosteroids on the preterm lung. Archives of Disease in Childhood: Fetal and Neonatal Edition 1997;77(2):F147‐F150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds LA, Tansey EM. Prenatal corticosteroids for reducing morbidity and mortality after preterm birth. The transcript of a Witness Seminar; The Wellcome Trust Centre for History of Medicine at UCL, London2005; Vol. 25.
References to other published versions of this review
- Crowley P. Prophylactic corticosteroids for preterm birth. Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858.CD000065.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts D, Dalziel SR. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858.CD004454.pub2] [DOI] [PubMed] [Google Scholar]


