Abstract
Background
The long chain polyunsaturated fatty acids (LCPUFA) docosahexaenoic acid (DHA) and arachidonic acid (AA) are considered essential for maturation of the developing brain, retina and other organs in newborn infants. Standard infant milk formulae are not supplemented with LCPUFA; they contain only alpha‐linolenic acid and linoleic acid, from which formula‐fed infants must synthesise their own DHA and AA, respectively. Over the past few years, some manufacturers have added LCPUFA to formula milk and have marketed these products as providing an advantage for the overall development of full‐term infants.
Objectives
To assess whether supplementation of formula milk with LCPUFA is both safe and beneficial for full‐term infants, while focusing on effects on visual function, neurodevelopment and physical growth.
Search methods
Two review authors independently searched the Cochrane Central Register of Controlled Trials (CENTRAL; December 2016), MEDLINE (Ovid, 1966 to December 2016), Embase (Ovid, 1980 to December 2016), the Cumulative Index to Nursing and Allied Health Literature (CINAHL; 1980 to December 2016) and abstracts of the Pediatric Academic Societies (2000 to 2016). We applied no language restrictions.
Selection criteria
We reviewed all randomised controlled trials (RCTs) evaluating effects of LCPUFA supplemented versus non‐supplemented formula milk on visual function, neurodevelopment and physical growth. We did not include trials reporting only biochemical outcomes.
Data collection and analysis
Two review authors extracted data independently. We assessed risk of bias of included studies using the guidelines of the Cochrane Neonatal Review Group. When appropriate, we conducted meta‐analysis to determine a pooled estimate of effect.
Main results
We identified 31 RCTs and included 15 of these in the review (N = 1889).
Nine studies assessed visual acuity, six of which used visual evoked potentials (VEP), two Teller cards and one both. Four studies reported beneficial effects, and the remaining five did not. Meta‐analysis of three RCTs showed significant benefit for sweep VEP acuity at 12 months (log of the minimum angle of resolution (logMAR)) (mean difference (MD) ‐0.15, 95% confidence interval (CI) ‐0.17 to ‐0.13; I2 = 0; three trials; N = 244), but meta‐analysis of three other RCTs showed no benefit for visual acuity measured with Teller cards at 12 months (cycles/degree) (MD ‐0.01, 95% CI ‐0.12 to 0.11; I2 = 0; three trials; N = 256). GRADE analysis for the outcome of visual acuity indicated that the overall quality of evidence was low.
Eleven studies measured neurodevelopmental outcomes at or before two years. Nine studies used Bayley Scales of Infant Development, version II (BSID‐II), and only two of these studies reported beneficial effects. Meta‐analysis revealed no significant differences between LCPUFA and placebo groups in BSID Mental Developmental Index (MDI) scores at 18 months (MD 0.06, 95% CI ‐2.01 to 2.14; I2 = 75%; four trials; N = 661) and no significant differences in BSID Psychomotor Development Index (PDI) scores at 18 months (MD 0.69, 95% CI ‐0.78 to 2.16; I2 = 61%; four trials; N = 661). Results showed no significant differences between the two groups in BSID‐II scores at one year and two years of age. One study reported better novelty preference measured by the Fagan Infant Test at nine months. Another study reported better problem solving at 10 months. One study used the Brunet and Lezine test to assess the developmental quotient and found no beneficial effects. Follow‐up of some infants in different studies at three, six and nine years of age revealed no beneficial effects of supplementation. GRADE analysis of these outcomes indicated that the overall quality of evidence was low.
Thirteen studies measured physical growth; none found beneficial or harmful effects of supplementation. Meta‐analysis of five RCTs showed that the supplemented group had lower weight (z scores) at one year of age (MD ‐0.23, 95% CI ‐0.40 to ‐0.06; I2 = 83%; N = 521) and that the two groups showed no significant differences with respect to length and head circumference (z scores). Meta‐analysis at 18 months and at two years revealed no significant differences between the two groups with respect to weight (kg), length (cm) and head circumference (cm). GRADE analysis of these outcomes indicated that the overall quality of evidence was low.
Authors' conclusions
Most of the included RCTs reported no beneficial effects or harms of LCPUFA supplementation on neurodevelopmental outcomes of formula‐fed full‐term infants and no consistent beneficial effects on visual acuity. Routine supplementation of full‐term infant milk formula with LCPUFA cannot be recommended at this time.
Keywords: Humans; Infant; Infant, Newborn; Child Development; Dietary Supplements; Infant Nutritional Physiological Phenomena; Arachidonic Acid; Arachidonic Acid/administration & dosage; Body Weight; Cephalometry; Docosahexaenoic Acids; Docosahexaenoic Acids/administration & dosage; Evoked Potentials, Visual; Fatty Acids, Unsaturated; Fatty Acids, Unsaturated/administration & dosage; Growth; Infant Formula; Infant Formula/chemistry; Randomized Controlled Trials as Topic; Term Birth; Visual Acuity; Visual Acuity/physiology
Plain language summary
Long chain polyunsaturated fatty acid supplementation in infants born at term
Review question: Does feeding full‐term babies with formula milk enriched with long chain polyunsaturated fatty acids (LCPUFA) result in improved vision and overall neurodevelopment compared with feeding formula milk not enriched with LCPUFA?
Background: LCPUFA is a type of fat that is essential for the development of brain and vision in newborn babies. Breast milk contains adequate amounts of LCPUFA and hence is considered better than formula milk. Some milk formulae with added LCPUFA are commercially available.
Study characteristics: This review analysed studies that compared outcomes of full‐term babies (born at ≥ 37 weeks of pregnancy) who were given formula milk enriched with LCPUFA versus outcomes of full‐term babies fed formula milk without enrichment with LCPUFA.
Key results: Review authors found that full‐term babies fed formula milk supplemented with LCPUFA did not have better outcomes than were reported for full‐term babies fed formula milk without LCPUFA.
Quality of evidence: We considered the overall quality of evidence to be low.
Summary of findings
Summary of findings for the main comparison. LCPUFA supplemented formula compared with control formula for term infants.
| LCPUFA supplemented formula compared with control formula for term infants for clinical outcomes (visual function, neurodevelopment and physical growth) | ||||||
| Patient or population: term infants Settings: hospital and community Intervention: LCPUFA supplemented formula Comparison: control formula | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control formula | LCPUFA supplemented formula | |||||
| Visual acuity/Teller cards at 12 months (cycles/degree) ‐ DHA and AA vs normal term formula | Mean visual acuity (cycles/degree) ranged across control groups from 3.31 to 10 | Mean visual acuity (cycles/degree) ranged across intervention groups from 3.28 to 9.77 | MD ‐0.01 (95% CI ‐0.12 to 0.11) | 256 (3 studies) | ⊕⊕⊝⊝ low | Downgraded 2 levels Reasons: small sample size, high rate of attrition |
| Sweep VEP acuity at 12 months (LogMAR) ‐ DHA and AA vs normal term formula | Mean sweep VEP acuity (LogMAR) ranged across control groups from 0.31 to 0.339 | Mean sweep VEP acuity (LogMAR) ranged across intervention groups from 0.14 to 0.2 | MD ‐0.15 (95% CI ‐0.17 to ‐0.13) | 244 (3 studies) | ⊕⊕⊝⊝ low | Downgraded 2 levels Reasons: small sample size, high rate of attrition in 2 RCTs |
| MDI scores (Bayley) at 18 months ‐ DHA and AA vs normal term formula | Mean MDI ranged across control groups from 98.3 to 105.4 | Mean MDI ranged across intervention groups from 94.5 to 105.6 | MD 0.06 (95% CI ‐ 2.01 to 2.14) | 661 (4 studies) | ⊕⊕⊝⊝ low | Downgraded 2 levels Reasons: small sample size, high rate of attrition in 2 RCTs, high statistical heterogeneity (I² = 75%) |
| PDI scores (Bayley) at 18 months ‐ DHA and AA vs normal term formula | Mean PDI ranged across control groups from 96.4 to 102 | Mean PDI ranged across intervention groups from 95.9 to 105.8 | MD 0.69 (95% CI ‐0.78 to 2.16) | 661 (4 studies) | ⊕⊕⊝⊝ low | Downgraded 2 levels Reasons: small sample size, high rate of attrition in 2 RCTs, high statistical heterogeneity (I² = 61%) |
| Weight at 12 months (z scores) ‐ DHA and AA vs normal term formula | Mean z scores for weight ranged across control groups from ‐0.21 to 0.35 | Mean z scores for weight ranged across intervention groups from ‐0.9 to 0.4 | MD ‐0.23 (95% CI ‐0.40 to ‐0.06) | 521 (5 studies) | ⊕⊕⊝⊝ low | Downgraded 2 levels Reasons: small sample size, high rate of attrition in 3 RCTs, unclear allocation concealment in 2 RCTs, high statistical heterogeneity (I² = 83%) |
| Length at 12 months (z scores) ‐ DHA and AA vs normal term formula | Mean z scores for length ranged across control groups from ‐0.11 to 0.34 | Mean z scores for length ranged across control groups from ‐0.04 to 0.16 | MD ‐0.04 (95% CI ‐0.19 to 0.11) | 521 (5 studies) | ⊕⊕⊝⊝ low | Downgraded 2 levels Reasons: small sample size, high rate of attrition in 3 RCTs, unclear allocation concealment in 2 RCTs |
| Head circumference at 12 months (z scores) ‐ DHA and AA vs normal term formula | Mean z scores for head circumference ranged across control groups from 0.18 to 0.94 | Mean z scores for head circumference ranged across control groups from 0.01 to 0.93 | MD ‐0.13 (95% CI ‐0.32 to 0.05) | 464 (4 studies) | ⊕⊕⊝⊝ low | Downgraded 2 levels Reasons: small sample size, high rate of attrition in 3 RCTs |
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: confidence interval; MD, mean difference | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: We are very uncertain about the estimate | ||||||
Background
The perinatal omega‐3 long chain polyunsaturated fatty acid docosahexaenoic acid (DHA) is considered essential for cortical circuit maturation in the developing brain (McNamara 2015). Strong evidence based on animal and human studies indicates that the n‐6 long chain polyunsaturated fatty acid arachidonic acid (AA) is also critical for infant growth, brain development and health (Hadley 2016). Evidence suggesting that breast‐fed infants have a long‐term developmental advantage over formula‐fed infants has been available for many years (Anderson 1999; Isaacs 2010; Kramer 2008; Lucas 1992; Morrow‐Tlucak 1988; Oddy 2011; Rogers 1978; Temboury 1994). Although most of these studies did not relate their findings to fatty acid supply, some reports suggest that low levels of LCPUFA found in formula‐fed infants may contribute to lower IQ scores (Bjerve 1992; Neuringer 1986; Rogers 1978).
Description of the condition
Dietary fat is fundamental during infancy for providing energy, fat‐soluble vitamins and essential fatty acids. Interest has recently focused on the importance of long chain polyunsaturated fatty acids (LCPUFA) such as DHA and AA in infant nutrition. These fatty acids are found in high proportions in the structural lipids of cell membranes, particularly those of the central nervous system and retina (Fleith 2005). Their accretion occurs primarily during the last trimester of pregnancy and the first year of life (Clandinin 1980).
Description of the intervention
LCPUFA are supplied via placental transfer during pregnancy and through breast milk after birth. Standard infant formulae contain only the precursor essential fatty acids (EFA) alpha‐linolenic acid (ALA, the omega‐3 precursor) and linoleic acid (LA, the omega‐6 precursor), from which formula‐fed infants must synthesise their own DHA and AA, respectively. The absence of LCPUFA in formula may be exacerbated by inhibited incorporation of endogenously produced LCPUFA by high concentrations of LA in some formulae.
How the intervention might work
Biochemical studies in term and preterm infants indicate that infants fed formula not supplemented with LCPUFA have significantly less DHA and AA in their erythrocytes relative to those fed breast milk (Clark 1992). Studies have also demonstrated that infants fed formula milk have lower levels of LCPUFA in the cerebral cortex compared with breast‐fed infants (Farquharson 1995), suggesting that infant formulae containing only LA and ALA may not be effective in meeting the full EFA requirements of infants. Hence supplementing formula milk with DHA and AA may improve the outcomes of formula‐fed infants.
Why it is important to do this review
In a non‐randomised study, investigators reported that term infants fed breast milk had better visual evoked potential (VEP) acuities and higher DHA levels than those receiving formula, and that visual function correlated with DHA status (Makrides 1993). Over the past few years, many manufacturers have added LCPUFA to milk formulae for term infants and have frequently marketed these products as providing an advantage for infant development. The cost of supplemented formulae is generally higher than that of non‐supplemented formulae. A systematic review of randomised and non‐randomised trials in term infants concluded that use of term formula supplemented with DHA can improve visual acuity at two months and probably at four months of age (SanGiovanni 2000). Another review of both animal and human studies (McCann 2005) concluded that animals with experimentally induced severe DHA deficiency benefit from DHA supplementation in their diet but that effects on cognitive outcomes in human studies are inconclusive. Meta‐analysis (Makrides 2005) and previous versions of this Cochrane review (Simmer 2001; Simmer 2008; Simmer 2011) found neither benefit nor harm for term infants supplemented with DHA alone or with both DHA and AA.
We conducted this review to update existing evidence on the effect of LCPUFA supplementation on formula‐fed full‐term infants.
Objectives
To assess whether supplementation of formula milk with LCPUFA is both safe and beneficial for full‐term infants, while focusing on effects on visual function, neurodevelopment and physical growth.
Methods
Criteria for considering studies for this review
Types of studies
Only randomised and quasi‐randomised clinical trials were eligible for inclusion. We defined a trial as quasi‐random if the method used to allocate study infants to study milk formula groups was not statistically random or was not clearly stated.
Types of participants
Healthy infants ≥ 37 weeks' gestation at birth.
Types of interventions
Milk formula enriched with DHA plus AA or with DHA alone compared with standard milk formula. LCPUFA supplements could be derived from any source including fish oil, egg triglycerides or fungal oils.
To be eligible for inclusion, the trial should have met all of the following criteria.
Study formula was commenced within two weeks after birth.
Study formula was the only source of milk from the time of randomisation until at least eight weeks of age.
Follow‐up data on clinical outcomes of interest were available for a minimum of three months.
The following trials were not eligible for inclusion.
Trials using breast milk in addition to study formula during the first eight weeks of life.
Trials reporting only biochemical outcomes.
Types of outcome measures
Visual acuity: measured with Teller acuity cards or VEP.
Neurodevelopmental outcomes: assessed as general quotient (GQ), intelligence quotient (IQ) and other measures of cognitive function.
Physical growth: weight, length and head circumference.
Biochemical outcomes: not reported in this review.
Search methods for identification of studies
We used the standard search strategy of the Cochrane Neonatal Review Group, which included electronic searches of MEDLINE (1946 to December 2016), Embase (1980 to December 2016), the Cumulative Index to Nursing and Allied Health Literature (CINAHL; 1982 to December 2016) and the Cochrane Central Register of Controlled Trials (CENTRAL; December 2016). We also searched e‐abstracts of Paediatric Academic Societies meetings (2000 to 2016) and searched MEDLINE and EMBASE for relevant articles by using the following MeSH terms or text words: [Polyunsaturated fatty acids OR Arachidonic Acid OR Docosahexaenoic acid OR Omega‐3 Fatty acids OR Omega‐6 fatty acids OR N‐3 Fatty Acid OR N‐6 Fatty Acid] AND [Infant, Newborn OR Infant OR Infant Formula]. We restricted final citations to Clinical Trial OR Randomised Controlled Trial OR Pragmatic Clinical Trial. We reviewed the reference lists of published narrative and systematic reviews to identify potential RCTs. We applied no language restrictions. Three review authors (SR, BJ and SP) independently searched various databases to identify trials that would be eligible for inclusion. We contacted study authors to ask that they clarify reported data or provide additional data including details of study methods. We sent study authors a standardised table and asked them to provide missing data not included in their published article.
We also searched clinical trials registries for ongoing and recently completed trials (clinicaltrials.gov; controlled‐trials.com; who.int/ictrp).
Data collection and analysis
Selection of studies
We included all randomised and quasi‐randomised controlled trials that fulfilled the selection criteria. SR, BJ and SP screened the titles and abstracts of all identified studies and obtained full‐text articles for all potentially relevant trials. SR, BJ and SP assessed independently the full text of these reports to assess their eligibility for inclusion in the review. We resolved disagreements by discussion among all review authors and by consensus.
Data extraction and management
SR and BJ separately extracted, assessed and coded all data for each study using a form that was designed specifically for this review. SR contacted trial authors to clarify methods and to obtain additional information. For each study, SR entered final data into RevMan and SP checked the data as entered. We resolved disagreements by discussion and by consensus.
Assessment of risk of bias in included studies
Two review authors (SR and BJ) independently assessed the risk of bias (low, high or unclear) of all included trials using the Cochrane ‘Risk of bias’ tool (Higgins 2011) for the following domains: sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias) and any other bias. We resolved all disagreements by discussion and by consensus. See Appendix 5 for a detailed description of risk of bias for each domain.
Measures of treatment effect
We used the standard methods of the Cochrane Neonatal Review Group. For continuous data, we used the mean difference (MD) and its 95% confidence interval (CI). We included no categorical outcomes data in the review.
Unit of analysis issues
If available, we planned to combine results from cluster trials with results from other trials by using generic inverse variance methods.
Dealing with missing data
If participant drop‐out led to missing data, we planned to conduct intention‐to‐treat analyses. We endeavoured to obtain missing data by contacting trial authors.
Assessment of heterogeneity
We estimated treatment effects of individual trials and examined heterogeneity between trials by inspecting forest plots and by quantifying the impact of heterogeneity using the I² statistic. If we detected statistical heterogeneity, we planned to explore possible causes (e.g. differences in study quality, participants, intervention regimens, outcome assessments).
Assessment of reporting biases
If we included at least 10 studies in the meta‐analysis, we planned to assess publication bias by using the funnel plot (Egger 1997).
Data synthesis
When the participant population and the intervention were almost similar, we considered it appropriate to pool the data. Some studies randomised infants into three groups: DHA alone, DHA plus AA and control formula. We entered outcome data from each of these studies into RevMan as if each consisted of two separate studies (i.e. DHA plus AA vs control and DHA vs control). However, in performing the meta‐analysis, we did not pool the data for DHA plus AA versus control and DHA versus control because control group infants were the same for both DHA alone and DHA plus AA groups of infants. We used RevMan 5.3 and applied the fixed‐effect model in completing the meta‐analysis.
Quality of the evidence
We used the GRADE approach, as outlined in the GRADE Handbook (Schünemann 2013), to assess the quality of evidence for the following (clinically relevant) outcomes at one year of age: visual acuity (based on VEP); physical growth (weight, length and head circumference); and neurodevelopmental outcomes (Bayley Scales of Infant Development‐II).
Two review authors (BJ and SR) independently assessed the quality of the evidence for each of the outcomes above. We considered evidence from RCTs as high quality but downgraded the evidence one level for serious (or two levels for very serious) limitations on the basis of the following: design (risk of bias), consistency across studies, directness of the evidence, precision of estimates and presence of publication bias. We used the GRADEpro Guideline Development Tool (GRADEpro GDT) to create a ‘Summary of findings’ table to report evidence quality.
The GRADE approach results in an assessment of the quality of a body of evidence according to one of four grades.
High: We are very confident that the true effect lies close to the estimate of effect.
Moderate: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of effect but may be substantially different.
Low: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of effect.
Very low: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect.
Subgroup analysis
We conducted planned subgroup analyses based on the type of LCPUFA supplementation provided (DHA alone and DHA plus AA).
Results
Description of studies
We identified 31 studies as potentially eligible, of which we included 15 and excluded 16. Figure 1 provides details of the study selection process. The Characteristics of included studies table summarises details of participants and study methods. All trials enrolled infants of ≥ 37 weeks' gestation at birth. The source of LCPUFA was egg yolk phospholipids in Agostini 1995,Auestad 1997, Carlson 1996 and Lucas 1999.Birch 1998,Birch 2005,Birch 2010,Makrides 1995 and Makrides 1999 derived LCPUFA from fish oil and evening primrose oil. Morris 2000 used single‐cell oils as the source of LCPUFA. Bouwstra 2005 used LCPUFA derived from egg yolk, tuna oil and single‐cell oil produced by the soil fungus, Mortierella alpina. Willats 1998 used LCPUFA derived from egg lipids, milk fat and vegetable oils. Auestad 2001 used fish and fungus oil in one study group and egg yolk triglyceride‐derived LCPUFA in the other study group. Lapillonne 2000 used LCPUFA derived from fish oil, and the source of LCPUFA in Ben 2004 was not clear.
1.
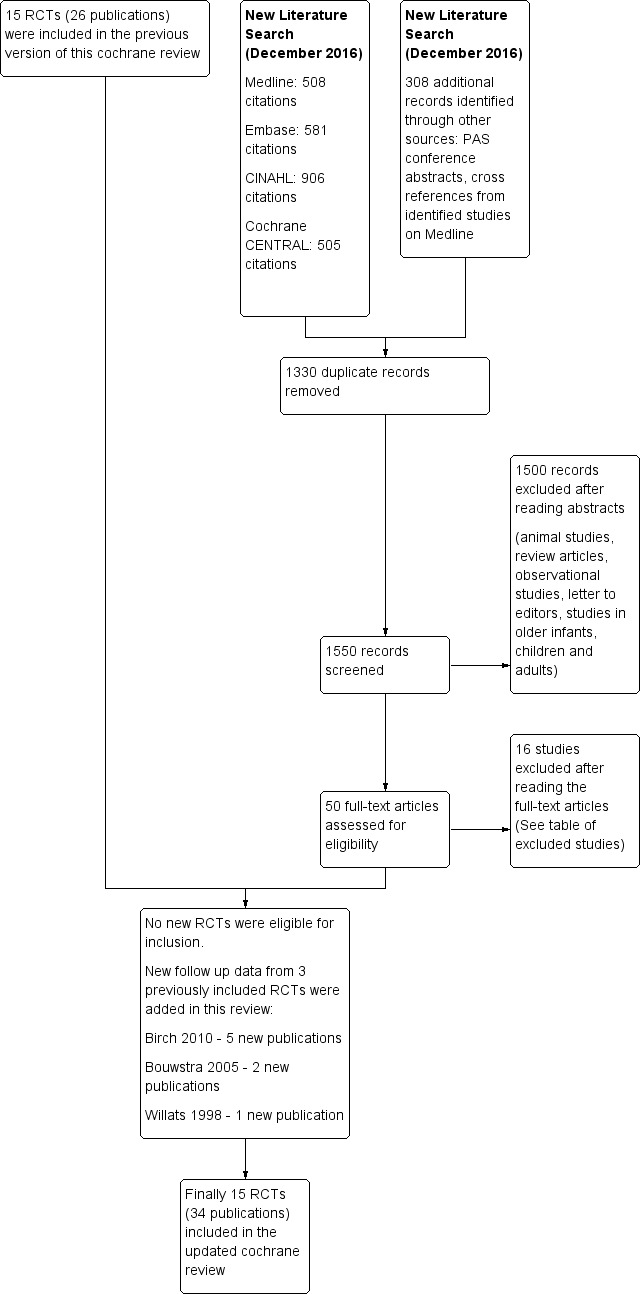
Study flow diagram.
Investigators in all studies commenced the trial formula within the first two weeks of life. The duration of use of the study formula was two months in Bouwstra 2005; three months in Morris 2000; four months in Agostini 1995,Birch 1998,Lapillonne 2000 and Willats 1998; six months in Ben 2004 and Lucas 1999; seven months in Makrides 1995; and one year in Auestad 1997, Auestad 2001, Birch 2005, Birch 2010, Carlson 1996 and Makrides 1999.
Lapillonne 2000 and Makrides 1995 compared DHA‐enriched versus normal term formula. Auestad 1997,Birch 1998 and Makrides 1999 randomised infants into three groups: DHA alone, DHA plus AA and control formula. All other studies compared formula enriched with DHA plus AA versus the control formula.
Auestad 2001 examined effects of LCPUFA from two different sources (egg yolk triglyceride and fish/fungus oil) versus control formula and reported outcomes separately. Given that the aim of our review was to compare LCPUFA (irrespective of the source) versus standard formula, we asked study authors to provide combined outcome data for infants given LCPUFA from both sources. The study authors kindly obliged and provided the combined outcome data. Birch 2010 studied different concentrations of DHA (0.32%, 0.64%, 0.96%) versus control formula. For this review, we chose the 0.32% DHA group as the intervention arm because this level is similar to that used in other included studies.
Birch 2010 provided additional study information for the updated review in 2011 (Simmer 2011). For the previous version of this review (Simmer 2008), the authors of Agostini 1995,Auestad 1997,Auestad 2001,Ben 2004,Birch 2005, Bouwstra 2005, Lapillonne 2000,Makrides 1995,Makrides 1999,Morris 2000 and Willats 1998 provided additional information; we did not contact the authors of Carlson 1996 and Lucas 1999 because all of the required information was available in the published literature; and Clausen 1996 and Decsi 1995 acknowledged the request but did not provide the requested information.
Agostini 1995,Auestad 2001,Birch 1998,Birch 2005,Birch 2010, Bouwstra 2005, Carlson 1996,Lucas 1999, Makrides 1995,Makrides 1999 and Willats 1998 described sample size and power calculations. Auestad 1997,Ben 2004, Lapillonne 2000 and Morris 2000 did not provide clear information on this.
We excluded 16 studies: Jorgenson 1996 because investigators did not commence supplements until infants were three to four weeks of age; Birch 2002 because researchers randomised infants to receive the study formula at six weeks of age; Voigt 2002 because study authors compared milk formulae versus different amounts of alpha linolenic acid; and Decsi 1995 and Clausen 1996 because study methods were not clear, and required data on outcomes of interest were not available. Study authors acknowledged our letter but did not provide the requested information. We excluded Carlson 1999 because trial authors expressed concern about the possibility of significant methodological issues in their study; Agostoni 2009 because DHA/placebo supplementation was given to breast‐fed babies; Gibson 2009 because investigators supplemented the study milk formula with a probiotic (Bifidobacterium lactis) in addition to LCPUFA but the control formula included neither; Field 2008 and Field 2010 because researchers did not assess clinical outcomes of interest but instead assessed laboratory markers of immune function; Fleddermann 2014 because the intervention formula contained reduced protein alpha lactalbumin in addition to LCPUFA; Meldrum 2012 because term infants enrolled were not solely formula fed; NCT02092857 because the outcome of interest was immunological (number of antigen‐presenting B cells); Lapillonne 2014 because this was not an RCT; Patterson 2016 because study authors compared formula milk supplemented with two different sources of DHA (algal‐derived DHA single cell oil (DHASCO) vs marine algae‐derived single cell oil (DHASCO‐B)); and Visentin 2016 because trial authors reported on red blood cell membrane fatty acid composition.
Risk of bias in included studies
We considered Agostini 1995,Auestad 1997,Auestad 2001,Birch 1998,Birch 2005,Birch 2010, Bouwstra 2005, Carlson 1996,Lapillonne 2000,Lucas 1999,Makrides 1995,Makrides 1999,Morris 2000 and Willats 1998 to have low risk of bias for most of the domains assessed. Follow‐up rates ranged from 60% to 90% among studies for various outcomes. The follow‐up rate in Ben 2004 was very low, with only 33% of study infants followed up at six months for the primary outcome. We provide details of assessment in the 'Risk of bias' table and in Figure 2.
2.
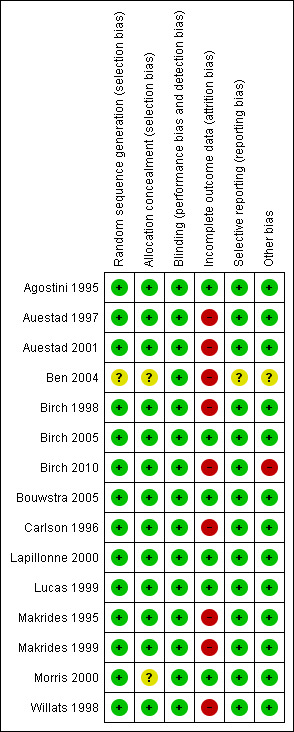
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Effects of interventions
See: Table 1
Visual acuity assessment methods
Visual acuity is a measure of the smallest element that can be resolved and can be assessed in infants with the use of gratings, which consist of black and white stripes or checkerboard patterns. Researchers can measure grating acuity by using behavioural or VEP methods. Each pairing of a black and white stripe is referred to as a cycle, and the spatial frequency of a grating is defined by the number of cycles per degree of viewing angle. As grating spatial frequency increases, the stripes become finer and are more difficult to discriminate, eventually appearing as an even grey to the observer. Grating acuity is the highest spatial frequency at which the stripes can be resolved.
The VEP refers to electrical activity of the brain that is generated in response to a reversing contrast checkerboard or grating. The VEP is recorded from an electrode that is placed over the occipital pole and is classified as transient, steady state or sweep. A transient VEP is elicited by checkerboard reversing from one to three times/s, and a steady‐state VEP is elicited by checkerboard reversing from six to 20 times/s. For a sweep VEP, black and white striped grating is used. The amplitude of the VEP increases linearly with spatial frequency near the visual acuity threshold. Linear regression is used to fit a straight line through the linear portion of the VEP amplitude versus the spatial frequency curve, and visual acuity is determined at the intercept of the regression line with the spatial frequency axis. VEP are reported as logMAR (minimum angle of resolution), which corresponds to the smallest black and white check pattern that the infant can discriminate from a grey background (the smaller the value, the better the acuity) or as cycles/degree (the larger the value, the better the acuity).
Behavioural methods for assessing visual acuity rely on the strong preference shown by infants for patterned stimuli over non‐patterned stimuli. Both the acuity card procedure (ACP) and the forced preferential looking (FPL) procedure have been used in conjunction with Teller acuity cards to measure the development of visual acuity in infants. The FPL procedure tests binocular grating acuity; the tester views the infant through a peephole, without knowledge of spatial frequency gratings on the cards, and makes a forced‐choice judgement about which card the infant prefers. Individual acuities are converted to cycles/degree, and standard deviations (SD) in octaves are determined by dividing one log SD by 0.3.
LCPUFA supplemented versus control formula
Visual acuity
Visual acuity at four months of age: steady state VEP, logMAR (Analysis 1.1)
Studies using DHA plus AA: Makrides 1999 reported on this outcome. Investigators found no statistically significant differences between LCPUFA and control (0.74 ± 0.09 vs 0.73 ± 0.12, respectively).
Studies using DHA alone: Makrides 1995 and Makrides 1999 reported this outcome. Makrides 1995 reported statistically significant differences between LCPUFA and control groups. Infants in the LCPUFA group had better visual acuity at four months than controls. Makrides 1999 showed no statistically significant differences. Pooled meta‐analysis of the two trials revealed no statistically significant differences between LCPUFA and control (MD ‐0.03, 95% CI ‐0.10 to 0.03).
Visual acuity at four months of age: sweep VEP (logMAR) (Analysis 1.2)
Studies using both DHA and AA: Birch 1998, Birch 2005 and Birch 2010 reported this outcome. All three studies showed statistically significant differences between LCPUFA and control. Infants in the LCPUFA group had better visual acuity than those in the control group. Pooled meta‐analysis of all three studies showed statistically significant benefit of LCPUFA for visual acuity (MD ‐0.08, 95% CI ‐0.10 to ‐0.05).
Studies using DHA alone: Birch 1998 reported this outcome. Results showed statistically significant benefit for visual acuity among infants in the LCPUFA group compared with those in the control group (0.46 ± 0.08 vs 0.54 ± 0.13).
Visual acuity at four months of age: sweep VEP, cycles/degree (Analysis 1.3)
Studies using DHA plus AA: Auestad 1997 reported on this outcome. Results showed no statistically significant differences between LCPUFA and control (6.61 ± 1.21 vs 7.08 ± 1.35).
Studies using DHA alone: Auestad 1997 reported on this outcome and provided values in graphs. Results showed no statistically significant differences between LCPUFA and control groups.
Visual acuity at four months of age: Teller cards (cycles/degree) (Analysis 1.4)
Studies using DHA plus AA: Auestad 1997, Auestad 2001 and Carlson 1996 reported on this outcome. None of these studies showed statistically significant differences between LCPUFA and control groups. Pooled meta‐analysis of data from these studies showed no statistically significant differences between LCPUFA and control (MD ‐0.11, 95% CI ‐0.24 to 0.02).
Studies using DHA alone: Auestad 1997 reported on this outcome and described no statistically significant differences between LCPUFA and control groups. Study authors presented results in graphs.
Visual acuity at six months of age: sweep VEP (cycles/degree) (Analysis 1.5)
Studies using DHA and AA: Auestad 1997 reported this outcome and found no statistically significant differences between LCPUFA and control groups (13.18 ± 1.38 vs 13.49 ± 1.35).
Studies using DHA alone: Auestad 1997 reported this outcome and described no statistically significant differences between LCPUFA and control groups. Study authors presented results in graphs.
Visual acuity at six months of age: Teller cards (cycles/degree) (Analysis 1.6)
Studies using DHA plus AA: Auestad 1997, Auestad 2001 and Carlson 1996 reported this outcome. None of these studies reported statistically significant differences between LCPUFA and control groups. Pooled meta‐analysis of data from these studies revealed no statistically significant differences between LCPUFA and control groups (MD 0.02, 95% CI ‐0.11 to 0.15).
Studies using DHA alone: Auestad 1997 reported this outcome. Study authors found no statistically significant differences between LCPUFA and control groups. They presented these results in graphs.
Visual acuity at seven to eight months of age: steady state VEP (logMAR) (Analysis 1.7)
Studies using DHA plus AA: Makrides 1999 reported on this outcome. Researchers found no statistically significant differences between LCPUFA and control groups (0.39 ± 0.17 vs 0.39 ± 0.19).
Studies using DHA alone: Makrides 1995 and Makrides 1999 reported this outcome. Makrides 1995 reported statistically significant benefit of LCPUFA supplementation for visual acuity. Makrides 1999 described no statistically significant differences between LCPUFA and control. Pooled meta‐analyses of both studies revealed no statistically significant differences between LCPUFA and control (MD ‐0.02, 95% CI ‐0.14 to 0.10).
Visual acuity at 12 months of age: sweep VEP (logMAR) (Analysis 1.8)
Studies using DHA plus AA: Birch 1998, Birch 2005 and Birch 2010 reported this outcome. All three studies showed statistically significant differences between LCPUFA and control groups. Infants in the LCPUFA group had better visual acuity than those in the control group. Pooled meta‐analysis of all three studies showed statistically significant differences between LCPUFA and control groups (MD ‐0.15, 95% CI ‐0.17 to ‐0.13).
Studies using DHA alone: Birch 1998 reported on this outcome. Study authors found statistically significant benefit for visual acuity in the LCPUFA group compared with the control group (0.19 ± 0.12 vs 0.33 ± 0.10) (MD ‐0.14, 95% CI ‐0.21 to ‐0.07).
Visual acuity at 12 months of age: sweep VEP (cycles/degree) (Analysis 1.9)
Studies using DHA and AA: Auestad 1997 reported this outcome. Results showed no statistically significant differences between LCPUFA and control groups (15.48 ± 1.32 vs 15.48 ± 1.32).
Studies using DHA alone: Auestad 1997 reported this outcome. Researchers found no statistically significant differences between LCPUFA and control groups. They provided results in graphs.
Visual acuity at 12 months of age: Teller cards (cycles/degree) (Analysis 1.10)
Studies using DHA plus AA: Auestad 1997, Auestad 2001 and Carlson 1996 reported this outcome. None of these studies reported statistically significant differences between LCPUFA and control groups. Pooled meta‐analysis of data provided by these studies showed no statistically significant differences between LCPUFA and control groups (MD ‐0.01, 95% CI ‐0.12 to 0.11). Figure 3
3.
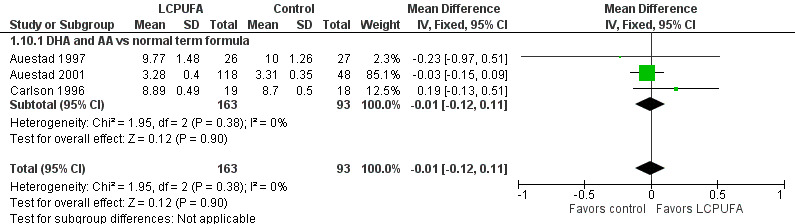
Forest plot of comparison: 1 LCPUFA supplemented vs control formula, outcome: 1.10 Visual acuity/Teller cards at 12 m (cycles/degree).
Studies using DHA alone: Auestad 1997 reported this outcome. Study authors found no statistically significant differences between LCPUFA and control groups and provided study results in graphs.
Visual acuity at three years of age: Teller cards (cycles/degree) (Analysis 1.11)
Studies using DHA plus AA: Auestad 1997 reported on this outcome. Study authors reported that they found no statistically significant differences between LCPUFA and control groups (28.2 ± 0.6 vs 30.3 ± 0.7; P = 0.74). However, statistical analysis of the same data on RevMan suggested better visual acuity among controls (MD ‐2.10, 95% CI ‐2.41 to ‐1.79; P < 0.00001).
Studies using DHA alone: Auestad 1997 reported on this outcome. Study authors reported that they found no statistically significant differences between LCPUFA and control groups (27.5 ± 0.6 vs 30.3 ± 0.7; P = 0.74). However, statistical analysis of the same data through RevMan suggested better visual acuity among controls (MD ‐2.80, 95% CI ‐3.11 to ‐2.49; P < 0.00001).
Neurodevelopmental outcomes
Bayley Scales of Infant Development
Auestad 1997,Auestad 2001,Ben 2004,Birch 1998,Birch 2010, Bouwstra 2005, Lucas 1999,Makrides 1995 and Makrides 1999assessed neurodevelopmental outcomes at various ages using the Bayley Scales of Infant Development.
Bayley assessment at three months of age: MDI (Analysis 1.12)
Studies using DHA plus AA: Ben 2004 reported on this outcome. Researchers found no statistically significant differences in MDI scores between LCPUFA and control groups (107.88 ± 7.91 vs 105.4 ± 9.2, respectively).
Studies using DHA alone: none.
Bayley assessment at three months of age: PDI (Analysis 1.13)
Studies using DHA plus AA: Ben 2004 reported this outcome. Study authors reported that they found no statistically significant differences in PDI scores between LCPUFA and control groups (110.06 ± 6.17 vs 106.4 ± 6.37, respectively). However, statistical analysis of the same data through RevMan suggested better PDI scores in the LCPUFA group (MD 3.66, 95% CI 0.43 to 6.89; P = 0.03).
Studies using DHA alone: none.
Bayley assessment at six months of age: MDI (Analysis 1.14)
Studies using DHA plus AA: Auestad 2001 and Ben 2004 reported this outcome. Both studies showed no statistically significant differences in MDI scores between LCPUFA and control groups. Pooled meta‐analysis of data from these two studies showed no statistically significant differences in MDI scores between LCPUFA and control groups (MD ‐0.59, 95% CI ‐2.26 to 1.07).
Studies using DHA alone: none.
Bayley assessment at six months: PDI (Analysis 1.15)
Studies using DHA plus AA: Auestad 2001 and Ben 2004 reported this outcome. Both studies reported no statistically significant differences in PDI scores between LCPUFA and control groups. Pooled meta‐analysis of data from these two studies showed no statistically significant differences in PDI scores between LCPUFA and control groups (MD 0.23, 95% CI ‐2.47 to 2.94).
Studies using DHA alone: none,
Bayley assessment at one year: MDI (Analysis 1.16)
Studies using DHA plus AA: Auestad 1997, Auestad 2001 and Makrides 1999 reported this outcome. None of these studies showed statistically significant differences in MDI scores between LCPUFA and control groups. Pooled analysis of data from these three trials revealed no statistically significant differences in MDI scores between LCPUFA and control groups (MD ‐0.95, 95% CI ‐3.38 to 1.49).
Studies using DHA alone: Auestad 1997, Makrides 1995 and Makrides 1999; reported this outcome. None of these studies showed statistically significant differences in MDI scores between LCPUFA and control groups. Pooled meta‐analysis of data from these three trials revealed no statistically significant differences in MDI scores between LCPUFA and control groups (MD ‐0.27, 95% CI ‐4.36 to 3.83).
Bayley assessment at one year: PDI (Analysis 1.17)
Studies using DHA plus AA: Auestad 1997, Auestad 2001 and Makrides 1999 reported this outcome. None of these studies showed statistically significant differences in PDI scores between LCPUFA and control groups. Pooled meta‐analysis of data from these three trials revealed no statistically significant differences in PDI scores between LCPUFA and control groups (MD ‐2.48, 95% CI ‐5.83 to 0.86).
Studies using DHA alone: Auestad 1997, Makrides 1995 and Makrides 1999 reported this outcome. None of these studies reported statistically significant differences in PDI scores between LCPUFA and control groups. Pooled meta‐analysis of data from these three trials revealed no statistically significant differences in PDI scores between LCPUFA and control groups (MD ‐1.70, 95% CI ‐6.62 to 3.22).
Bayley assessment at 18 months: MDI (Analysis 1.18)
Studies using DHA plus AA: Birch 1998, Bouwstra 2005 and Lucas 1999 reported this outcome. Birch 2010 reported this outcome only for participants from the Dallas centre and provided no information on study participants from the Kansas centre (Drover 2011, additional reporting of Birch 2010). Birch 1998 and Birch 2010 (i.e. Drover 2011) showed statistically significant improvement in MDI scores at 18 months in the LCPUFA supplemented group. Bouwstra 2005 and Lucas 1999 described no statistically significant differences in MDI scores at 18 months. Pooled meta‐analysis of data from all four trials revealed no statistically significant differences in MDI scores between LCPUFA and control groups (MD 0.06, 95% CI ‐2.01 to 2.14). Figure 4
4.
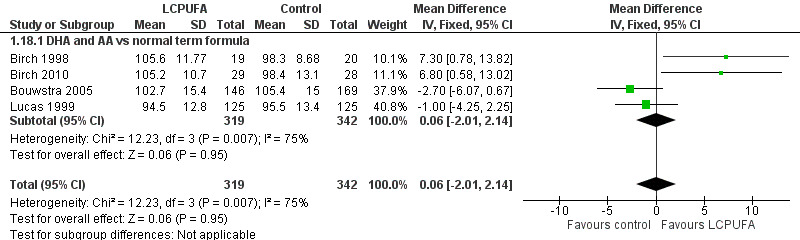
Forest plot of comparison: 1 LCPUFA supplemented vs control formula, outcome: 1.18 MDI (Bayley Scale score) at 18 m.
Studies using DHA alone: none.
Bayley assessment at 18 months: PDI (Analysis 1.19)
Studies using DHA plus AA: Birch 1998,Bouwstra 2005 and Lucas 1999 reported this outcome. Birch 2010 reported this outcome only for participants from the Dallas centre and provided no information for study participants from the Kansas centre (Drover 2011). None of these studies showed statistically significant differences in PDI scales between LCPUFA and control groups. Pooled meta‐analysis of data from all four trials revealed no statistically significant differences in PDI scores between LCPUFA and control groups (MD 0.69, 95% CI ‐0.78 to 2.16). Figure 5
5.
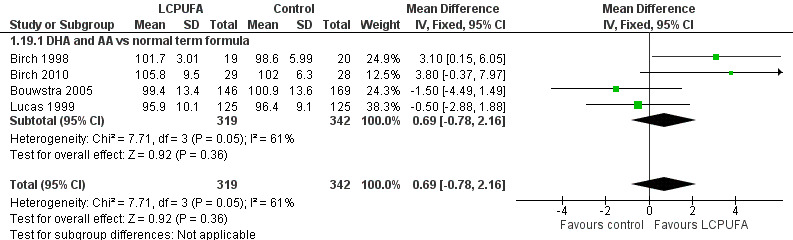
Forest plot of comparison: 1 LCPUFA supplemented vs control formula, outcome: 1.19 PDI (Bayley Scale score) at 18 m.
Studies using DHA alone: none.
Bayley assessment at two years: MDI (Analysis 1.20)
Studies using DHA plus AA: Makrides 1999 reported this outcome. Results showed no statistically significant differences in MDI scores between LCPUFA and control groups (102.00 ± 23.00 vs 104.00 ± 13.00).
Studies using DHA alone: Makrides 1999 reported on this outcome. Study results showed no statistically significant differences in MDI scores between LCPUFA and control groups (108 ± 16 vs 104 ± 13).
Bayley assessment at two years: PDI (Analysis 1.21)
Studies using DHA plus AA: Makrides 1999 reported this outcome and described no statistically significant differences in PDI scores between LCPUFA and control groups (96.00 ± 21.00 vs 97.00 ± 15.00).
Studies using DHA alone: Makrides 1999 reported on this outcome and described no statistically significant differences in PDI scores between LCPUFA and control groups (104.00 ± 17.00 vs 97.00 ± 15.00).
Other tests of cognitive function
Agostini 1995 assessed the developmental quotient (DQ) using the Brunet and Lezine developmental test. Investigators reported higher DQ at four months of age for LCPUFA infants compared with control infants. However, repeat assessments at 12 and 24 months with the same assessment tool revealed no difference in DQ between LCPUFA and control groups.
DQ at four months: 105.3 ± 9.4 versus 96.5 ± 10.9 in LCPUFA versus control groups, respectively (P = 0.009).
DQ at 12 months: 101.5 ± 9.2 versus 101.2 ± 8.0 in LCPUFA versus control groups, respectively (P = 0.4).
DQ at 24 months: 101 ± 10.3 versus 99.1 ± 7.1 in LCPUFA versus control groups, respectively (P = 0.89).
Auestad 1997 assessed the IQ of study infants at 3.25 years of age using Stanford‐Binet scales. Results showed no statistically significant differences in IQ scores between DHA, DHA plus AA and control groups (DHA: 99 ± 12; DHA and AA: 101 ± 13; control: 103 ± 15; ANOVA P = 0.14).
Auestad 2001 used the Fagan Infant Test of Development, which measures novelty preference on the basis of the observation that after habituation to a familiar stimulus has occurred, an infant will show a preference for a different (novel) stimulus if both familiar and novel stimuli are presented together. A novelty preference score is derived for the average percentage of total time spent viewing the novel stimuli on 10 discrete paired comparison tests. Infants with average scores > 57% are said to have a significant novelty preference (i.e. time spent looking at novel stimuli compared with familiar stimuli is greater than by chance alone). Novelty preference has been interpreted as an early measure of information processing capacity (Fagan 1970; Fagan 1983). Auestad 2001 observed no statistically significant differences between LCPUFA and control groups in novelty preference (57.8 ± 6.7 vs 57.1 ± 5.3, respectively).
Birch 2010 performed assessments using the Behaviour Rating Scale (BRS), which evaluated relevant aspects of behaviour during test taking, such as emotional regulation, quality of movement and orientation/engagement, and found no significant diet group differences.
Colombo 2011 tested study participants from the original Birch 2010 study at four, six and nine months of age on a visual habituation protocol that yielded both behavioural and psychophysiological indices of attention. Infants in all DHA + AA supplemented conditions had lower heart rates than those in the non‐supplemented condition and showed no dose response for this effect. The distribution of time that infants spent in different phases of attention (a cognitive index derived from the convergence of behavioural and cardiac responses) varied as a function of dosage. Infants supplemented at the two lower DHA doses spent proportionately more time engaged in active stimulus processing than infants fed non‐supplemented formula, whereas infants fed the highest dose were intermediate and did not differ from any other group.
Drover 2012 assessed effects of different dietary concentrations of DHA provided during the first 12 months of life on language development and school readiness among participants from the original Birch 2010 study. Dietary DHA during the first year of life did not enhance school readiness nor language development. Children who consumed infant formula with 0.32% and 0.96% DHA showed lower receptive vocabulary scores than control infants at two but not at 3.5 years of age.
Colombo 2013 re‐enrolled infants from Birch 2010 at 18 months and followed them every six months until six years using age‐appropriate standardised and specific cognitive tests. LCPUFA supplementation did not influence performance on standardised tests of language and performance at 18 months; however, results showed significant positive effects on rule learning and inhibition tasks from three to five years, on the Peabody Picture Vocabulary Test at five years and on the Wechsler Primary Preschool Scales of Intelligence at six years. Results showed no beneficial effects of LCPUFA on tasks of spatial memory, simple inhibition or advanced problem solving.
de Jong 2010 assessed school age (nine years) outcomes of participants from the original Bouwstra 2005 study and found no significant differences in Neurological Optimality Scale score, minor neurological dysfunction (MND) and cognitive function between supplemented and non‐supplemented groups, and no consistent beneficial effects of postnatal LCPUFA supplementation on cognitive function. Results revealed a beneficial role of LCPUFA in the subgroup of children exposed to maternal smoking during pregnancy.
Lucas 1999 assessed development using Knobloch, Passamanik and Sherrards Development Screening Inventory at nine months. Results showed no statistically significant differences between LCPUFA and control infants (103.8 ± 8.3 vs 104.4 ± 8.7 in LCPUFA vs control groups, respectively).
Willats 1998 assessed infant cognitive behaviour at 10 months of age using problem‐solving assessment. Results showed statistically significant benefit of LCPUFA supplementation. Infants who received LCPUFA supplemented formula had significantly more intentional solutions than infants who received the control formula (median 2.0 vs 0; P = 0.021). Intention scores were also higher in the LCPUFA group (14.0 (11.8 to 17.1) vs 11.5 (10.0 to 13.3); P = 0.035). IQ scores of children who were fed a formula containing LCPUFA or no LCPUFA did not differ at the age of six years. However, children who received LCPUFA processed information faster than children who received non‐supplemented formula.
Physical growth
Weight at four months (Analysis 1.22)
Studies using DHA plus AA: Auestad 1997, Auestad 2001 and Makrides 1999 reported this outcome. Auestad 2001 reported outcomes as figures. Auestad 1997 reported outcomes as z scores. None of these studies found statistically significant differences between LCPUFA and control groups. Meta‐analysis was not possible because only Makrides 1999 provided data as means and standard deviations.
Studies using DHA alone: Auestad 1997, Lapillonne 2000 and Makrides 1999 reported this outcome. Auestad 1997 reported this outcome as z scores. None of these studies reported statistically significant differences between LCPUFA and control groups. Meta‐analysis of data from Lapillonne 2000 and Makrides 1999 revealed no statistically significant differences between LCPUFA and control groups (MD ‐0.03, 95% CI ‐0.33 to 0.27).
Length at four months (Analysis 1.23)
Studies using DHA plus AA: Auestad 1997, Auestad 2001 and Makrides 1999 reported this outcome. Auestad 2001 reported outcomes in graphs. Auestad 1997 reported outcomes as z scores. None of these studies reported statistically significant differences between LCPUFA and control groups. Meta‐analysis was not possible because only Makrides 1999 provided data as means and standard deviations.
Studies using DHA alone: Auestad 1997, Lapillonne 2000 and Makrides 1999 reported this outcome. Auestad 1997 reported this outcome as z scores. Meta‐analysis of pooled data from Lapillonne 2000 and Makrides 1999 revealed no statistically significant differences between LCPUFA and control groups (MD 0.03, 95% CI ‐1.00 to 1.06).
Head circumference at four months (Analysis 1.24)
Studies using DHA plus AA: Auestad 1997, Auestad 2001 and Makrides 1999 reported this outcome. Auestad 2001 reported outcomes as figures. Auestad 1997 reported outcomes as z scores. None of these studies reported statistically significant differences between LCPUFA and control groups. Meta‐analysis was not possible because only Makrides 1999 provided data as means and standard deviations.
Studies using DHA alone: Auestad 1997, Lapillonne 2000 and Makrides 1999 reported this outcome. Auestad 1997 reported outcomes as z scores. Meta‐analysis of pooled data from Lapillonne 2000 and Makrides 1999 revealed no statistically significant differences between LCPUFA and control groups (MD ‐0.01, 95% CI ‐0.53 to 0.51).
Weight at six months (kg) (Analysis 1.25)
Studies using DHA plus AA: Auestad 1997,Auestad 2001,Ben 2004,Birch 1998,Bouwstra 2005, Lucas 1999 and Morris 2000 reported this outcome. Auestad 1997 and Birch 1998 reported outcomes as z scores. Ben 2004 reported outcomes as rates of growth per week. None of these studies reported statistically significant differences between LCPUFA and control groups. Data from Auestad 2001,Bouwstra 2005, Lucas 1999 and Morris 2000 were available in a format for meta‐analysis. Pooled meta‐analysis of data from these three studies revealed no statistically significant differences between LCPUFA and control groups (MD 0.01, 95% CI ‐0.11 to 0.13).
Studies using DHA alone: none.
Length at six months (cm) (Analysis 1.26)
Studies using DHA plus AA: Auestad 1997,Auestad 2001,Ben 2004, Birch 1998,Bouwstra 2005, Lucas 1999 and Morris 2000 reported this outcome. Auestad 1997 and Birch 1998 reported outcomes as z scores. Ben 2004 reported outcomes as rates of growth. None of these studies reported statistically significant differences between LCPUFA and control groups. Data from Auestad 2001, Bouwstra 2005, Lucas 1999 and Morris 2000 were available in a format for meta‐analysis. Pooled meta‐analysis of data from these three studies revealed no statistically significant differences between LCPUFA and control groups (MD ‐0.13, 95% CI ‐0.47 to 0.21).
Studies using DHA alone: none.
Head circumference at six months (cm) (Analysis 1.27)
Studies using DHA plus AA: Auestad 1997,Auestad 2001, Ben 2004,Birch 1998,Bouwstra 2005, Lucas 1999 and Morris 2000 reported this outcome. Auestad 1997 and Birch 1998 reported outcomes as z scores. Ben 2004 reported outcomes as rates of growth. None of these studies reported statistically significant differences between LCPUFA and control groups. Data from Auestad 2001, Bouwstra 2005,Lucas 1999 and Morris 2000 were available in a format for meta‐analysis. Pooled meta‐analysis of these three studies revealed no statistically significant differences between LCPUFA and control groups (MD ‐0.06, 95% CI ‐0.25 to 0.13).
Studies using DHA alone: none.
Weight at one year (kg) (Analysis 1.28)
Studies using DHA plus AA: Agostini 1995,Auestad 1997,Auestad 2001,Birch 1998,Birch 2005, Bouwstra 2005, Makrides 1999 and Morris 2000 reported this outcome. Birch 1998 reported data in graphs. None of these studies reported statistically significant differences between LCPUFA and control groups. Pooled meta‐analysis of data from Agostini 1995,Auestad 2001, Bouwstra 2005, Makrides 1999 and Morris 2000; revealed no statistically significant differences between LCPUFA and control groups (MD ‐0.11, 95% CI ‐0.28 to 0.05).
Studies using DHA alone: Auestad 1997, Makrides 1995 and Makrides 1999 reported this outcome. None of these studies found statistically significant differences between LCPUFA and control groups. Pooled meta‐analysis of data from Makrides 1995 and Makrides 1999 revealed no statistically significant differences between LCPUFA and control groups (MD ‐0.43, 95% CI ‐0.96 to 0.09).
Weight at one year (z scores) (Analysis 1.29)
Studies using DHA plus AA: Agostini 1995,Auestad 1997,Birch 1998,Birch 2005 and Birch 2010 reported this outcome. Birch 1998 reported data in graphs. None of these studies reported statistically significant differences between LCPUFA and control groups. However, pooled meta‐analysis of z scores from Agostini 1995,Auestad 1997,Auestad 2001,Birch 2005 and Birch 2010 revealed statistically significantly lower weight in the LCPUFA group compared with the control group (MD ‐0.23, 95% CI ‐0.4 to ‐0.06). Figure 6
6.
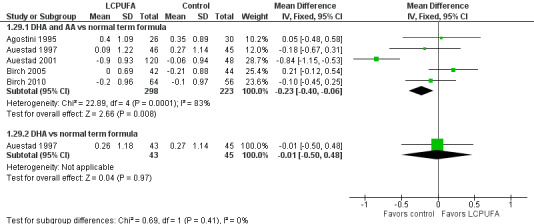
Forest plot of comparison: 1 LCPUFA supplemented vs control formula, outcome: 1.29 Weight at 12 m, z score.
Studies using DHA alone: Auestad 1997 reported this outcome. Investigators found no statistically significant differences between LCPUFA and control groups.
Length at one year (cm) (Analysis 1.30)
Studies using DHA plus AA: Agostini 1995,Auestad 2001, Bouwstra 2005, Makrides 1999 and Morris 2000 reported this outcome. None of these individual studies found statistically significant differences between LCPUFA and control groups. Pooled meta‐analysis of data from Agostini 1995,Auestad 2001,Bouwstra 2005, Makrides 1999 and Morris 2000 revealed no statistically significant differences between LCPUFA and control groups (MD ‐0.15, 95% CI ‐0.57 to 0.28).
Studies using DHA alone: Makrides 1995 and Makrides 1999 reported this outcome. Neither of these studies reported statistically significant differences between LCPUFA and control groups. Pooled meta‐analysis of data from Makrides 1995 and Makrides 1999 revealed no statistically significant differences between LCPUFA and control groups (MD ‐0.95, 95% CI ‐2.05 to 0.15).
Length at one year (z scores) (Analysis 1.31)
Studies using DHA plus AA: Agostini 1995,Auestad 1997,Auestad 2001,Birch 1998,Birch 2005 and Birch 2010 reported these outcomes. Birch 1998 reported data in figures. None of these individual studies found statistically significant differences between LCPUFA and control groups. Pooled meta‐analysis of z scores from Agostini 1995,Auestad 1997,Auestad 2001 and Birch 2005 revealed no statistically significant differences between LCPUFA and control groups (MD ‐0.04, 95% CI ‐0.19 to 0.11). Figure 7
7.
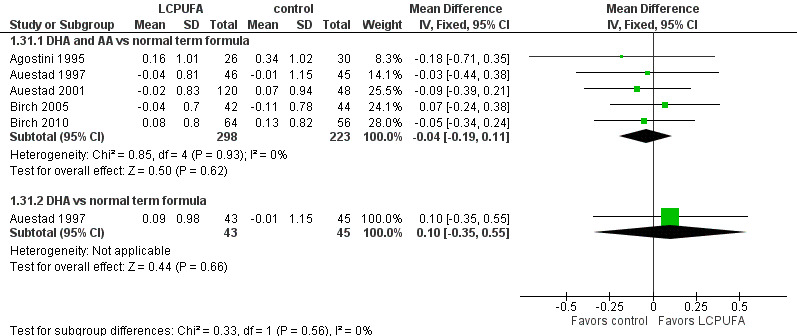
Forest plot of comparison: 1 LCPUFA supplemented vs control formula, outcome: 1.31 Length at 12 m, z score.
Studies using DHA alone: Auestad 1997 reported this outcome and found no statistically significant differences between LCPUFA and control groups (0.09 ± 0.98 vs ‐0.01 ± 1.15).
Head circumference at one year (cm) (Analysis 1.32)
Studies using DHA plus AA: Auestad 2001,Bouwstra 2005, Makrides 1999 and Morris 2000 reported this outcome. None of these individual studies reported statistically significant differences between LCPUFA and control groups. Pooled meta‐analysis of data from these studies revealed no statistically significant differences between LCPUFA and control groups (MD ‐0.13, 95% CI ‐0.36 to 0.11).
Studies using DHA alone: Makrides 1995 and Makrides 1999 reported this outcome. Neither of these studies reported statistically significant differences between LCPUFA and control groups. Pooled meta‐analysis of data from these studies revealed no statistically significant differences between LCPUFA and control groups (MD ‐0.22, 95% CI ‐0.80 to 0.37).
Head circumference at one year (z scores) (Analysis 1.33)
Studies using DHA plus AA: Auestad 1997,Auestad 2001,Birch 1998,Birch 2005 and Birch 2010 reported this outcome. Birch 1998 provided data in figures. None of these studies reported statistically significant differences between LCPUFA and control groups. Pooled meta‐analysis of z scores from Auestad 1997,Auestad 2001,Birch 2005 and Birch 2010 revealed no statistically significant differences between LCPUFA and control groups (MD ‐0.13, 95% CI ‐0.32 to 0.05). Figure 8
8.
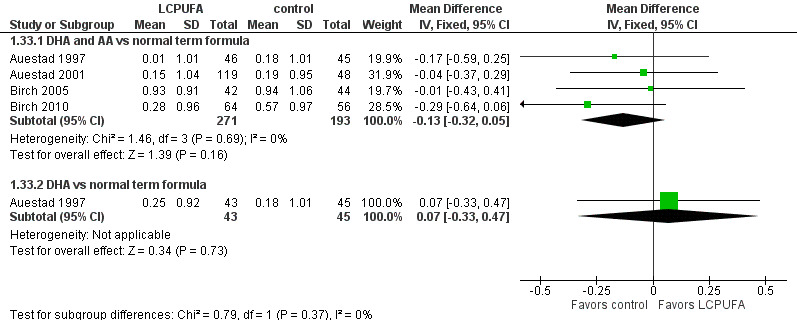
Forest plot of comparison: 1 LCPUFA supplemented vs control formula, outcome: 1.33 Head circumference at 12 m, z score.
Studies using DHA alone: Auestad 1997 reported this outcome and found no statistically significant differences between LCPUFA and control groups (0.25 ± 0.92 vs 0.18 ± 1.01).
Weight at 18 months (kg) (Analysis 1.34)
Studies using DHA plus AA: Bouwstra 2005 and Lucas 1999 reported this outcome. Investigators found no statistically significant differences between the two groups. Pooled meta‐analysis of data from both trials revealed no statistically significant differences between LCPUFA and control groups (MD ‐0.04, 95% CI ‐0.25 to 0.17).
Studies using DHA alone: none.
Length at 18 months (cm) (Analysis 1.35)
Studies using DHA plus AA: Bouwstra 2005 and Lucas 1999 reported this outcome. Researchers found no statistically significant differences between the two groups. Pooled meta‐analysis of data from both trials revealed no statistically significant differences between LCPUFA and control groups (MD ‐0.19, 95% CI ‐0.71 to 0.34).
Studies using DHA alone: none.
Head circumference at 18 months (cm) (Analysis 1.36)
Studies using DHA plus AA: Bouwstra 2005 and Lucas 1999 reported this outcome. Investigators found no statistically significant differences between the two groups. Pooled meta‐analysis of data from both trials revealed no statistically significant differences between LCPUFA and control groups (MD ‐0.07, 95% CI ‐0.32 to 0.19).
Studies using DHA alone: none.
Weight at two years (kg) (Analysis 1.37)
Studies using DHA plus AA: Makrides 1999 reported this outcome and found no statistically significant differences between LCPUFA and control formulae (12.78 ± 1.53 vs 13.54 ± 1.40).
Studies using DHA alone: Makrides 1999 reported this outcome and found no statistically significant differences between LCPUFA and control groups (12.75 ± 1.47 vs 13.54 ± 1.39).
Length at two years (cm) (Analysis 1.38)
Studies using DHA plus AA: Makrides 1999 reported this outcome and found no statistically significant differences between LCPUFA and control formulae.
Studies using DHA alone: Makrides 1999 reported this outcome and found no statistically significant differences between LCPUFA and control groups.
Head circumference at two years (cm) (Analysis 1.39)
Studies using DHA plus AA: Makrides 1999 reported this outcome and found no statistically significant differences between LCPUFA and control formulae.
Studies using DHA alone: Makrides 1999 reported this outcome and found no statistically significant differences between LCPUFA and control groups.
Physical growth at three years
Studies using DHA plus AA: Auestad 1997 described outcomes separately for boys and girls at 3.25 years of age. Researchers found no statistically significant differences between LCPUFA and control groups in both sexes for length, weight and head circumference at 3.25 years of age.
Studies using DHA alone: Auestad 1997 described outcomes separately for boys and girls at 3.25 years of age. Investigators found no statistically significant differences between LCPUFA and control groups in both sexes for length, weight and head circumference at 3.25 years of age.
Physical growth at six years
Birch 2010 described anthropometric outcomes at six years of age in a new publication (Currie 2015). Study authors found that LCPUFA supplementation during infancy predicted greater length in infancy and higher weight and stature‐for‐age percentiles from two to six years of age but no increase in body mass index (BMI) or BMI‐for‐age percentile.
Physical growth at nine years
de Jong 2011 described anthropometric outcomes at nine years of age among participants from the original study of Bouwstra 2005. Researchers found no statistically significant differences between LCPUFA and control groups for length, weight and head circumference at nine years.
Other outcomes
Foiles 2016 reported that LCPUFA supplemented infants from the original Birch 2010 study had reduced risk of skin and respiratory allergic disease during childhood until four years of age.
de Jong 2011 measured blood pressure and heart rate of enrolled infants from the original study of Bouwstra 2005 at nine years of age. Study authors concluded that short‐term LCPUFA supplementation does not influence cardiovascular development at nine years of age.
Discussion
Data from 1889 term infants included in 15 randomised controlled trials (RCTs) do not demonstrate clear or consistent benefit of supplementing formula with long chain polyunsaturated fatty acids (LCPUFA) for visual acuity, neurodevelopmental outcomes and physical growth in term infants. Our ability to pool study data was limited because of significant conceptual heterogeneity between some included studies. We noted variation among studies regarding type, concentration and duration of supplementation of LCPUFA, as well as in outcomes assessed and methods used to assess outcomes. Some studies measured visual acuity at 1.5, four, six, nine and twelve months and at three years; tested visual acuity by using sweep visual evoked potentials (VEP), steady‐state VEP and Teller cards; and assessed neurodevelopmental outcomes at three, four, six, 12 and 18 months, and at two, three, six and nine years. Most studies assessed neurodevelopmental outcomes by using Bayley scores. Some assessed physical growth at four, six and 12 months and at two and three years. Some studies used standard physical measurements like weight (kg), length (cm) and head circumference (cm). Others described z scores for physical measurements.
Birch 1998 (and follow‐up report Birch 2007), Birch 2005, Birch 2010 and Makrides 1995 reported beneficial effects of LCPUFA supplementation for visual acuity. Other RCTs such as Auestad 1997, Auestad 2001, Carlson 1996 and Makrides 1999 have not replicated these effects.
Birch 1998 and Birch 2010 showed benefits of LCPUFA supplementation for Mental Development Index (MDI) scores at 18 months. Willats 1998 demonstrated that LCPUFA supplementation resulted in better problem‐solving skills at 10 months of age. Other RCTs such as Agostini 1995,Auestad 1997,Auestad 2001,Ben 2004, Bouwstra 2005, Lucas 1999,Makrides 1995 and Makrides 1999 have not replicated these beneficial effects on neurodevelopmental outcomes. Few trials have reported long‐term follow‐up data. Follow‐up data from Bouwstra 2005 showed no beneficial effect of LCPUFA supplementation on neurological function, cognitive development and cardiovascular and anthropometric development at nine years of age (de Jong 2010; de Jong 2012). Follow‐up data from Birch 2010 revealed higher height and weight‐for‐age percentiles but not body mass index (BMI) percentiles from birth to six years of age in the LCPUFA supplemented group (Currie 2015). In addition, Birch 2010 showed that LCPUFA supplementation in infancy improved performance on executive function and verbal measures tested at five and six years of age, respectively (Colombo 2013, additional reporting of Birch 2010). The follow‐up study to Willats 1998 showed that IQ scores of children fed a formula containing LCPUFA or no LCPUFA did not differ at the age of six years (Willatts 2013).
Various theories have been suggested as reasons for such inconsistent results. Lauritzen 2001 proposed that a higher dose of docosahexaenoic acid (DHA) may be necessary to achieve beneficial effects. Uauy 2003 proposed that both higher dose and longer duration of LCPUFA supplementation are needed to achieve better outcomes. However, studies that used LCPUFA supplementation until one year of age (Auestad 1997; Auestad 2001; Carlson 1996; Makrides 1999) failed to demonstrate beneficial effects of LCPUFA supplementation. Birch 2010 found beneficial effects of supplementation (0.32% DHA and 0.64% arachidonic acid (AA)) and reported that higher doses of DHA (0.64%, 0.96%) did not confer additional benefit for visual acuity.
In a recent review (Meldrum 2011), review authors suggested that sample size, genetic polymorphisms, gender, source of supplement, dose, timing of supplementation, duration of supplementation, compliance with treatment and selection of the test for assessment of neurodevelopmental outcomes may be the factors responsible for inconsistent results.
The Dallas/Kansas group that used LCPUFA derived from single‐cell microalgae (Crypthecodinium cohnii: DHA) and fungi (Mortierella alpina: AA) at a DHA concentration of at least 0.32% for one year has shown consistently beneficial effects (Birch 2005; Birch 2010). Future RCTs may be needed to consider this approach.
None of the included studies showed significant effects of LCPUFA supplementation on weight, length and head circumference until nine years of age. Results were the same irrespective of type, concentration and duration of LCPUFA supplementation. Even though meta‐analysis of pooled data revealed marginally lower weight z scores at one year of age in the LCPUFA supplemented group, these differences were small and are unlikely to be of clinical significance. Greater weight gain during this crucial period may mean higher risk of metabolic syndrome later in life but may be associated with improved neurocognitive outcomes.
The main limitations of our Cochrane review are related to limitations of the included studies such as small sample size, use of variable tools for assessment of visual function and neurodevelopment, different ages of participants at assessment and high attrition rates.
In recent years, various systematic reviews and meta‐analyses have evaluated the effect of LCPUFA in term neonates. We provide details below.
Makrides 2005 conducted a meta‐analysis of 14 RCTs (n = 1846) to examine the effect of LCPUFA supplementation on the growth of term infants. Results showed no significant effect of LCPUFA supplementation on infant weight, length or head circumference at any assessment age.
A meta‐analysis of individual patient data in Rosenfeld 2009, which included 624 infants from two full‐term RCTs (Bouwstra 2005; Lucas 1999) and 439 infants from two preterm trials, found lack of any effect of LCPUFA supplementation on children's physical growth at 18 months of age.
Beyerlein 2010 conducted an individual patient data (IPD) meta‐analysis of 870 infants from four large RCTs (two preterm RCTs and two term RCTs) of LCPUFA supplementation in formula. For term infants, investigators reported no significant differences in Bayley Scales of Infant Development (BSID) scores at 18 months of age between LCPUFA and control groups (N = 529, mean difference for MDI scores ‐2.2, 95% CI ‐4.8 to 0.4; mean difference for PDI scores ‐1.2, 95% CI ‐3.3 to 0.9). Study authors concluded that LCPUFA supplementation of infant formula does not have a clinically meaningful effect on neurodevelopment as assessed by Bayley scores at 18 months.
Qawasmi 2012 conducted a meta‐analysis of 12 RCTs (six term and six preterm) to examine the efficacy of LCPUFA supplementation of infant formula for early cognitive development. Researchers found no significant association between LCPUFA supplementation of infant formula and cognitive development at ∼one year of age in term infants.
Qawasmi 2013 conducted a meta‐analysis of 19 RCTs (12 term and seven preterm) and concluded that LCPUFA supplementation improves visual acuity up to 12 months of age among term infants. Study authors extracted the mean visual acuity and standard deviation (SD) values from figures if not reported in text or tables, and this may not have been a reliable approach. Other limitations of this meta‐analysis have been described in the Database of Abstracts of Reviews of Effects (DARE), prepared by the Centre for Reviews and Dissemination (https://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0053686/; accessed 20 December 2016).
Jiao 2014 evaluated the role of DHA in cognitive function among infants, children and adults by conducting an extensive systematic review. Study authors reported that 'the age of participants throughout all of the trials ranged from birth to 86 years, which covers nearly the entire scale of the human life span'. They found that DHA supplements improved Psychomotor Development Index (PDI) and Mental Development Index (MDI) scores among infants, but included studies (n = 7) differed in their inclusion criteria, with ages of enrolled infants ranging from birth to nine months.
Sun 2015 evaluated the validity and reliability of neurocognitive endpoints used in DHA and AA infant formula supplementation trials. Study authors included RCTs from both preterm and full‐term infant populations. They concluded that available data are currently inadequate to conclude that DHA/AA supplementation has a clinically meaningful beneficial effect on neurological development. They brought into sharp focus the limitations of tools used currently for neurocognitive assessment and stressed the need for development and use of well‐defined, valid and reliable outcome measures for use in future clinical trials.
Quin 2016 conducted a systematic review and meta‐analysis of RCTs and semi‐RCTs of omega‐3 fatty acid supplementation during prenatal and postnatal periods. Review authors reported that n‐3 PUFA supplementation for infants delivered maternally or directly through formula does not improve visual acuity, language development or cognition. They also reported that n‐3 PUFA supplements affect infant immune development and reduce pro‐inflammatory responses among supplemented breast‐fed and fortified formula‐fed/directly supplemented infants. They concluded that overall, evidence does not support continued supplementation of infant formula with long chain n‐3 PUFA, in light of its negative impact on development of immune responses.
Overall, the results of the other systematic reviews on this topic are similar to those of our Cochrane review, which found no significant benefits of LCPUFA supplementation of formula milk for term infants.
However, recommendations/opinions as to whether infant formula should be supplemented with LCPUFA have varied. The European Food Safety Authority (EFSA; EFSA 2014) recently recommended that "there is no necessity to add ARA to infant and follow‐on formulae". This group recommended that "DHA should be added to infant formulae and follow‐on formulae, even though there is currently no conclusive evidence for any effects beyond infancy of addition of DHA to infant formula on any of the health outcomes studied". The opinion of the EFSA is shared by some experts in the field (Lauritzen 2015), but other experts have warned that the EFSA recommendation for not providing AA supplementation for infant formula puts infants at risk and should be revised (Crawford 2015; Forsyth 2015).
In effect, the same currently available evidence has yielded three different opinions/recommendations: One group believes that infant formula needs to be supplemented with DHA but not with AA (EFSA 2014; Lauritzen 2015); another group believes that it is 'dangerous not to add ARA, especially in the presence of DHA' (Crawford 2015; Forsyth 2015); and many systematic reviews along with this Cochrane review have found no consistent benefit of DHA or AA supplementation for term infants, although the overall quality of evidence was low. This means that the controversial issue of LCPUFA supplementation of formula milk for term infants has not yet been resolved. Well‐conducted RCTs with adequate sample size and reliable and consistent endpoints are essential to address this issue definitively. Until this is done, routine and compulsory LCPUFA supplementation for formula‐fed term infants cannot be recommended.
Authors' conclusions
Implications for practice.
Data from RCTs do not support the need for routine supplementation of formula for term infants with LCPUFA to improve visual acuity, neurodevelopment or physical growth.
Implications for research.
Further research assessing the influence of LCPUFA supplementation could consider use of high‐dose DHA (at least 0.32%) and long duration of supplementation (at least one year). Sources of LCPUFA that may be preferred for future research include single‐cell microalgae (DHA) and fungi (AA). Adequate sample sizes are required to evaluate complex intellectual outcomes and to identify gender differences and any effect of different polymorphisms known to influence the metabolism of fatty acids.
What's new
| Date | Event | Description |
|---|---|---|
| 30 December 2016 | New citation required but conclusions have not changed | Conclusions of the review remain unchanged |
| 30 December 2016 | New search has been performed | This updates the review titled "Longchain polyunsaturated fatty acid supplementation in infants born at term" (Simmer 2011) We completed the literature search in April 2016 and repeated it in December 2016. We added 1 new review author |
History
Protocol first published: Issue 4, 1998 Review first published: Issue 4, 1998
| Date | Event | Description |
|---|---|---|
| 10 July 2011 | New citation required but conclusions have not changed | We added 1 new study to the updated review Conclusions of the review remain unchanged |
| 10 July 2011 | New search has been performed | This updates the review titled "Longchain polyunsaturated fatty acid supplementation in infants born at term" (Simmer 2008) We updated the search in April 2011 |
| 10 June 2008 | Amended | We converted the review to new review format |
| 2 September 2007 | New citation required but conclusions have not changed | We made substantive amendments |
| 2 September 2007 | New search has been performed | This review updates the existing review titled "Longchain polyunsaturated fatty acid supplementation of infants born at term", published in the Cochrane Library, Issue 4, 2001 (Simmer 2001) We added 6 new randomised trials to this review update. We performed subgroup analysis based on type of LCPUFA supplementation provided (DHA plus AA vs DHA alone) |
Acknowledgements
Maria Makrides, Nancy Auestad, Xiaoming Ben, Eileen Birch, Susan Carlson, Carlo Agostoni, Geraint Morris, Mijna Hadders‐Algra, Dennis Hoffman, Alexandre Lapillone and Peter Willatts for clarifying existing data, clarifying methods and providing additional information from their studies.
Appendices
Appendix 1. Appendix 1: MEDLINE search strategy
Ovid MEDLINE(R) 1946 to present with daily update, Ovid MEDLINE(R) daily epub ahead of print, in‐process & other non‐indexed citations (28 December 2016)
1. Polyunsaturated fatty acid.mp. or Fatty Acids, Unsaturated/: 24403 citations
2. Fish Oils/ or Docosahexaenoic Acids/ or docosahexanoic acid.mp. or Fatty Acids, Omega‐3/: 24019 citations
3. n3 fatty acid.mp. : 35 citations
4. n6 fatty acid.mp: 10 citations
5. Arachidonic acid.mp. or arachidonic acid/: 44933 citations
6. 1 or 2 or 3 or 4 or 5: 82726 citations
7. Infant/ or Infant, Newborn/ or Infant Formula/: 1132619 citations
8. 6 and 7: 2304 Citations
9. limit 8 to (clinical trial or controlled clinical trial or pragmatic clinical trial or randomised controlled trial): 508 citations
Appendix 2. Appendix 2: Embase search strategy
| Embase (Ovid) 1980 to 28 December 2016 |
1. Polyunsaturated fatty acid.mp. or Fatty Acids, Unsaturated/: 33673 citations
2. Fish Oils/ or Docosahexaenoic Acids/ or docosahexanoic acid.mp. or Fatty Acids, Omega‐3/: 41081 citations
3. n3 fatty acid.mp. : 41 citations
4. n6 fatty acid.mp: 8 citations
5. Arachidonic acid.mp. or arachidonic acid/: 55542 citations
6. 1 or 2 or 3 or 4 or 5: 111096 citations
7. Infant/ or Infant, Newborn/ or Infant Formula/: 945827 citations
8. 6 and 7: 3782 Citations
9. limit 7 to (clinical trial or controlled clinical trial or pragmatic clinical trial or randomised controlled trial): 581 citations
Appendix 3. Appendix 3: CINAHL search strategy
| S1 | docosahexanoic acid: 2583 citations |
| S2 | omega‐3: 6657 citations |
| S3 | Omega‐6: 997 citations |
| S4 | arachidonic acid: 1711 |
| S5 | poly unsaturated fatty acid:16 citations |
| S6 | polyunsaturated fatty acids: 2192 citations |
| S7 | fish oil: 3059 citations |
| S8 | n‐3 fatty acid: 784 citations |
| S9 | n‐3 fatty acids: 784 citations |
| S10 | n‐6 fatty acids:119 citations |
| S11 | infant: 208105 citations |
| S12 | newborn infant:1851 citations |
| S13 | infant formula:3235 citations |
| S14 | S11 OR S12 OR S13: 208105 citations |
| S15 | S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10: 11103 citations |
| S16 | S14 AND S15: 906 citations |
Appendix 4. Appendix 4: Cochrane CENTRAL search strategy
#1. "long chain polyunsaturated fatty acid"(Word variations have been searched): 346 Citations
#2. Arachidonic Acid: 1378 Citations
#3. Docosahexanoic acid: 73 Citations
#4. Omega 3: Citations: 3734 Citations
#5. Omega 6: 2337 Citations
#6. Omega‐3: 3339 Citations
#7. Omega‐6: 475 Citations
#8. LCPUFA: 162 Citations
#9. #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8: 5054 Citations
#10. Infant: 39347 Citations
#11. Neonate: 1322 Citations
#12. Newborn Infant: 17586 Citations
#13. Milk Formula: 1599 Citations
#14. Formula Milk: 1599 Citations
#15. #10 OR #11 or #12 or #13 or #14:39948 Citations
#16. #9 AND #15: 505 Citations
Appendix 5. Appendix 5: Risk of bias tool
Risk of bias of studies was assessed by two review authors (SR and BJ). We resolved disagreements by discussion among all four review authors and by consensus. We entered information into the Risk of bias table using the following criteria.
1. Was there adequate sequence generation (checking for possible selection bias): The method used to generate the allocation sequence in each included study was described as low risk (any truly random process, e.g. random number table, computer random number generator); high risk (any non‐random process, e.g. odd or even date of birth, hospital or clinic record number); unclear risk.
2. Was there adequate allocation concealment (checking for possible selection bias): The method used to conceal the allocation sequence in each included study was described as: low risk (e.g. telephone or central randomisation, consecutively numbered sealed opaque envelopes); high risk (open random allocation, unsealed or non‐opaque envelopes, alternation, date of birth); unclear risk.
3. Was there adequate blinding (checking for possible performance bias): The methods used to blind personnel from knowledge of which intervention participants received. Was knowledge of the allocated intervention adequately prevented during the study? At the time of outcome assessment? Categorised as low risk, high risk or unclear risk.
4. Were incomplete outcome data addressed (checking for possible attrition bias through withdrawals, dropouts, protocol deviations): If attrition and exclusion were reported, numbers included in the analysis at each stage (compared with total randomised participants), reasons for attrition or exclusion when reported and whether missing data were balanced across groups or were related to outcomes were reported. We assessed methods as low risk (< 20% missing data); high risk (≥ 20% missing data); unclear risk.
5. Was there selective reporting bias: The possibility of selective outcome reporting bias was investigated. We assessed methods as low risk (when it was clear that all of the study's prespecified outcomes were reported); high risk (when not all of the study's prespecified outcomes were reported; one or more reported primary outcomes were not prespecified; outcomes of interest were reported incompletely; study failed to include results of a key outcome that would have been expected to have been reported); unclear risk.
6. Were there any other sources of potential bias: Any important concerns about other potential sources of bias (e.g. whether a potential source of bias was related to the specific study design, whether the trial was stopped early owing to some data‐dependent process) were described. We assessed whether each study was free of other problems that could put it at risk of bias as low risk; high risk; unclear risk.
Data and analyses
Comparison 1. LCPUFA supplemented vs control formula.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 VEP acuity at 4 m (logMAR, steady state) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 DHA and AA vs normal term formula | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.07, 0.09] |
| 1.2 DHA vs normal term formula | 2 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.10, 0.03] |
| 2 Sweep VEP acuity at 4 m (logMAR) | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 DHA and AA vs normal term formula | 3 | 266 | Mean Difference (IV, Fixed, 95% CI) | ‐0.08 [‐0.10, ‐0.05] |
| 2.2 DHA vs normal term formula | 1 | 41 | Mean Difference (IV, Fixed, 95% CI) | ‐0.08 [‐0.15, ‐0.01] |
| 3 Sweep VEP acuity at 4 m (cycles/degree) | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | ‐0.47 [‐1.16, 0.22] |
| 3.1 DHA and AA vs normal term formula | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | ‐0.47 [‐1.16, 0.22] |
| 4 Visual acuity/Teller cards at 4 m (cycles/degree) | 3 | 264 | Mean Difference (IV, Fixed, 95% CI) | ‐0.11 [‐0.24, 0.02] |
| 4.1 DHA and AA vs normal term formula | 3 | 264 | Mean Difference (IV, Fixed, 95% CI) | ‐0.11 [‐0.24, 0.02] |
| 5 Sweep VEP acuity at 6 m (cycles/degree) | 1 | 53 | Mean Difference (IV, Fixed, 95% CI) | ‐0.31 [‐1.04, 0.42] |
| 5.1 DHA and AA vs normal term formula | 1 | 53 | Mean Difference (IV, Fixed, 95% CI) | ‐0.31 [‐1.04, 0.42] |
| 6 Visual acuity/Teller cards at 6 m (cycles/degree) | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 DHA and AA vs normal term formula | 3 | 256 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.11, 0.15] |
| 7 VEP acuity at 7‐8 m (logMAR, steady state) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 DHA and AA vs normal term formula | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.13, 0.13] |
| 7.2 DHA vs normal term formula | 2 | 52 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.14, 0.10] |
| 8 Sweep VEP acuity at 12 months (logMAR) | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.1 DHA and AA vs normal term formula | 3 | 244 | Mean Difference (IV, Fixed, 95% CI) | ‐0.15 [‐0.17, ‐0.13] |
| 8.2 DHA vs normal term formula | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | ‐0.14 [‐0.21, ‐0.07] |
| 9 Sweep VEP acuity at 12 m (cycles/degree) | 1 | 53 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.71, 0.71] |
| 9.1 DHA and AA vs normal term formula | 1 | 53 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.71, 0.71] |
| 10 Visual acuity/Teller cards at 12 m (cycles/degree) | 3 | 256 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.12, 0.11] |
| 10.1 DHA and AA vs normal term formula | 3 | 256 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.12, 0.11] |
| 11 Visual acuity at 3 years (Teller acuity cards; cycles/degree) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 11.1 DHA and AA vs normal term formula | 1 | 68 | Mean Difference (IV, Fixed, 95% CI) | ‐2.10 [‐2.41, ‐1.79] |
| 11.2 DHA vs normal term formula | 1 | 68 | Mean Difference (IV, Fixed, 95% CI) | ‐2.80 [‐3.11, ‐2.49] |
| 12 MDI (Bayley) score at 3 m | 1 | 58 | Mean Difference (IV, Fixed, 95% CI) | 2.48 [‐1.90, 6.86] |
| 12.1 DHA and AA vs normal term formula | 1 | 58 | Mean Difference (IV, Fixed, 95% CI) | 2.48 [‐1.90, 6.86] |
| 13 PDI (Bayley) score at 3 m | 1 | 58 | Mean Difference (IV, Fixed, 95% CI) | 3.66 [0.43, 6.89] |
| 13.1 DHA and AA vs normal term formula | 1 | 58 | Mean Difference (IV, Fixed, 95% CI) | 3.66 [0.43, 6.89] |
| 14 MDI (Bayley) score at 6 m | 2 | 207 | Mean Difference (IV, Fixed, 95% CI) | ‐0.59 [‐2.26, 1.07] |
| 14.1 DHA and AA vs normal term formula | 2 | 207 | Mean Difference (IV, Fixed, 95% CI) | ‐0.59 [‐2.26, 1.07] |
| 15 PDI (Bayley) score at 6 m | 2 | 206 | Mean Difference (IV, Fixed, 95% CI) | 0.23 [‐2.47, 2.94] |
| 15.1 DHA and AA vs normal term formula | 2 | 206 | Mean Difference (IV, Fixed, 95% CI) | 0.23 [‐2.47, 2.94] |
| 16 MDI (Bayley score) at 1 year | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 16.1 DHA and AA vs normal term formula | 3 | 298 | Mean Difference (IV, Fixed, 95% CI) | ‐0.95 [‐3.38, 1.49] |
| 16.2 DHA vs normal term formula | 3 | 160 | Mean Difference (IV, Fixed, 95% CI) | ‐0.27 [‐4.36, 3.83] |
| 17 PDI (Bayley score) at 1 year | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 17.1 DHA and AA vs normal term formula | 3 | 298 | Mean Difference (IV, Fixed, 95% CI) | ‐2.48 [‐5.83, 0.86] |
| 17.2 DHA vs normal term formula | 3 | 160 | Mean Difference (IV, Fixed, 95% CI) | ‐1.70 [‐6.62, 3.22] |
| 18 MDI (Bayley score) at 18 m | 4 | 661 | Mean Difference (IV, Fixed, 95% CI) | 0.06 [‐2.01, 2.14] |
| 18.1 DHA and AA vs normal term formula | 4 | 661 | Mean Difference (IV, Fixed, 95% CI) | 0.06 [‐2.01, 2.14] |
| 19 PDI (Bayley score) at 18 m | 4 | 661 | Mean Difference (IV, Fixed, 95% CI) | 0.69 [‐0.78, 2.16] |
| 19.1 DHA and AA vs normal term formula | 4 | 661 | Mean Difference (IV, Fixed, 95% CI) | 0.69 [‐0.78, 2.16] |
| 20 MDI (Bayley score) at 2 years | 1 | 79 | Mean Difference (IV, Fixed, 95% CI) | 1.85 [‐5.26, 8.96] |
| 20.1 DHA and AA vs normal term formula | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | ‐2.0 [‐13.88, 9.88] |
| 20.2 DHA vs normal term formula | 1 | 41 | Mean Difference (IV, Fixed, 95% CI) | 4.0 [‐4.88, 12.88] |
| 21 PDI (Bayley score) at 2 years | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 21.1 DHA and AA vs normal term formula | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐12.71, 10.71] |
| 21.2 DHA vs normal term formula | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | 7.00 [‐3.32, 17.32] |
| 22 Weight at 4 months | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 22.1 DHA and AA vs normal term formula | 1 | 46 | Mean Difference (IV, Fixed, 95% CI) | 0.15 [‐0.22, 0.52] |
| 22.2 DHA vs normal term formula | 2 | 71 | Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.33, 0.27] |
| 23 Length at 4 months | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 23.1 DHA and AA vs normal term formula | 1 | 46 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐1.45, 1.45] |
| 23.2 DHA vs normal term formula | 2 | 71 | Mean Difference (IV, Fixed, 95% CI) | 0.03 [1.00, 1.06] |
| 24 Head circumference at 4 months | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 24.1 DHA and AA vs normal term formula | 1 | 46 | Mean Difference (IV, Fixed, 95% CI) | 0.5 [‐0.26, 1.26] |
| 24.2 DHA vs normal term formula | 2 | 71 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.53, 0.51] |
| 25 Weight at 6 m (kg) | 4 | 830 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.11, 0.13] |
| 26 Length at 6 m (cm) | 4 | 830 | Mean Difference (IV, Fixed, 95% CI) | ‐0.13 [‐0.47, 0.21] |
| 27 Head circumference at 6 m (cm) | 4 | 830 | Mean Difference (IV, Fixed, 95% CI) | ‐0.06 [‐0.25, 0.13] |
| 28 Weight at 12 m (kg) | 6 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 28.1 DHA and AA vs normal term formula | 5 | 689 | Mean Difference (IV, Fixed, 95% CI) | ‐0.11 [‐0.28, 0.05] |
| 28.2 DHA vs normal term formula | 2 | 75 | Mean Difference (IV, Fixed, 95% CI) | ‐0.43 [‐0.96, 0.09] |
| 29 Weight at 12 m, z score | 5 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 29.1 DHA and AA vs normal term formula | 5 | 521 | Mean Difference (IV, Fixed, 95% CI) | ‐0.23 [‐0.40, ‐0.06] |
| 29.2 DHA vs normal term formula | 1 | 88 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.50, 0.48] |
| 30 Length at 12 m (cm) | 6 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 30.1 DHA and AA vs normal term formula | 5 | 689 | Mean Difference (IV, Fixed, 95% CI) | ‐0.15 [‐0.57, 0.28] |
| 30.2 DHA vs normal term formula | 2 | 75 | Mean Difference (IV, Fixed, 95% CI) | ‐0.95 [‐2.05, 0.15] |
| 31 Length at 12 m, z score | 5 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 31.1 DHA and AA vs normal term formula | 5 | 521 | Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.19, 0.11] |
| 31.2 DHA vs normal term formula | 1 | 88 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.35, 0.55] |
| 32 Head circumference at 12 m (cm) | 5 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 32.1 DHA and AA vs normal term formula | 4 | 633 | Mean Difference (IV, Fixed, 95% CI) | ‐0.13 [‐0.36, 0.11] |
| 32.2 DHA vs normal term formula | 2 | 75 | Mean Difference (IV, Fixed, 95% CI) | ‐0.22 [‐0.80, 0.37] |
| 33 Head circumference at 12 m, z score | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 33.1 DHA and AA vs normal term formula | 4 | 464 | Mean Difference (IV, Fixed, 95% CI) | ‐0.13 [‐0.32, 0.05] |
| 33.2 DHA vs normal term formula | 1 | 88 | Mean Difference (IV, Fixed, 95% CI) | 0.07 [‐0.33, 0.47] |
| 34 Weight at 18 m (kg) | 2 | 563 | Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.25, 0.17] |
| 34.1 DHA and AA vs normal term formula | 2 | 563 | Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.25, 0.17] |
| 35 Length at 18 m (cm) | 2 | 565 | Mean Difference (IV, Fixed, 95% CI) | ‐0.19 [‐0.71, 0.34] |
| 35.1 DHA and AA vs normal term formula | 2 | 565 | Mean Difference (IV, Fixed, 95% CI) | ‐0.19 [‐0.71, 0.34] |
| 36 Head circumference at 18 m (cm) | 2 | 565 | Mean Difference (IV, Fixed, 95% CI) | ‐0.07 [‐0.32, 0.19] |
| 36.1 DHA and AA vs normal term formula | 2 | 565 | Mean Difference (IV, Fixed, 95% CI) | ‐0.07 [‐0.32, 0.19] |
| 37 Weight at 2 years (kg) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 37.1 DHA and AA vs normal term formula | 1 | 39 | Mean Difference (IV, Fixed, 95% CI) | ‐0.76 [‐1.68, 0.16] |
| 37.2 DHA vs normal term formula | 1 | 43 | Mean Difference (IV, Fixed, 95% CI) | ‐0.79 [‐1.65, 0.07] |
| 38 Height at 2 years (cm) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 38.1 DHA and AA vs normal term formula | 1 | 39 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐2.07, 2.07] |
| 38.2 DHA vs normal term formula | 1 | 43 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐2.09, 1.49] |
| 39 Head circumference at 2 years (cm) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 39.1 DHA and AA vs normal term formula | 1 | 39 | Mean Difference (IV, Fixed, 95% CI) | 0.5 [‐0.47, 1.47] |
| 39.2 DHA vs normal term formula | 1 | 43 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.68, 0.88] |
1.1. Analysis.
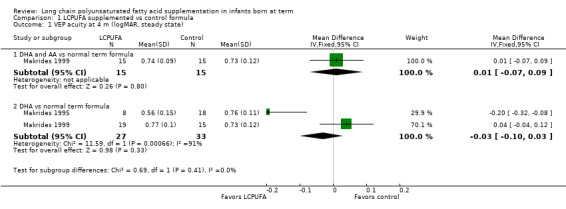
Comparison 1 LCPUFA supplemented vs control formula, Outcome 1 VEP acuity at 4 m (logMAR, steady state).
1.2. Analysis.
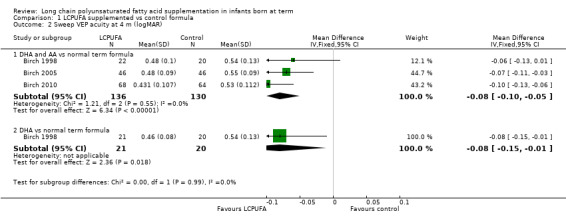
Comparison 1 LCPUFA supplemented vs control formula, Outcome 2 Sweep VEP acuity at 4 m (logMAR).
1.3. Analysis.
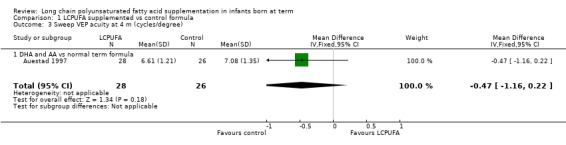
Comparison 1 LCPUFA supplemented vs control formula, Outcome 3 Sweep VEP acuity at 4 m (cycles/degree).
1.4. Analysis.
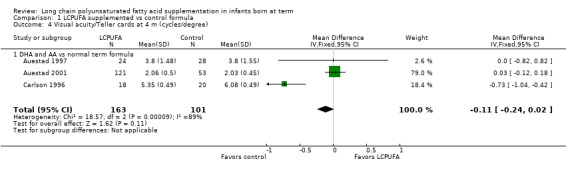
Comparison 1 LCPUFA supplemented vs control formula, Outcome 4 Visual acuity/Teller cards at 4 m (cycles/degree).
1.5. Analysis.
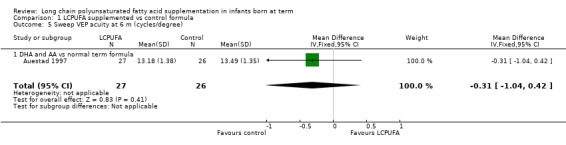
Comparison 1 LCPUFA supplemented vs control formula, Outcome 5 Sweep VEP acuity at 6 m (cycles/degree).
1.6. Analysis.
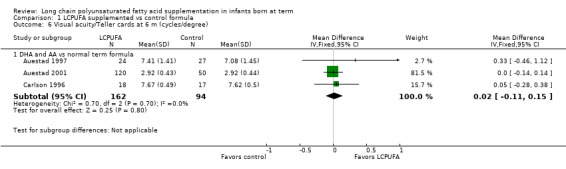
Comparison 1 LCPUFA supplemented vs control formula, Outcome 6 Visual acuity/Teller cards at 6 m (cycles/degree).
1.7. Analysis.
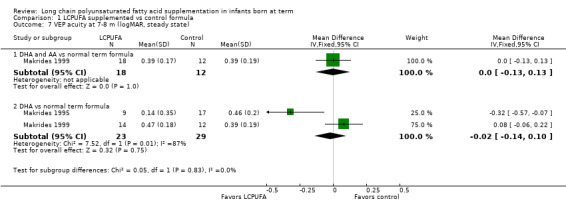
Comparison 1 LCPUFA supplemented vs control formula, Outcome 7 VEP acuity at 7‐8 m (logMAR, steady state).
1.8. Analysis.
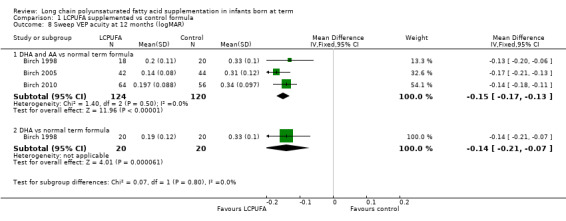
Comparison 1 LCPUFA supplemented vs control formula, Outcome 8 Sweep VEP acuity at 12 months (logMAR).
1.9. Analysis.
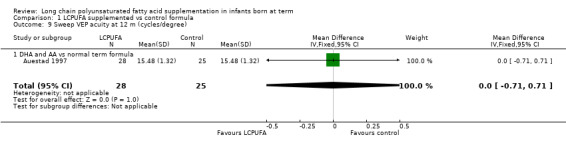
Comparison 1 LCPUFA supplemented vs control formula, Outcome 9 Sweep VEP acuity at 12 m (cycles/degree).
1.10. Analysis.
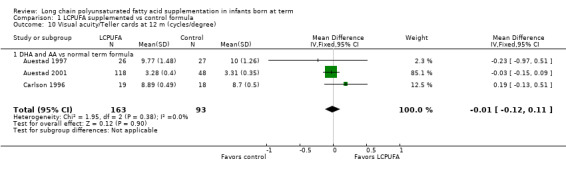
Comparison 1 LCPUFA supplemented vs control formula, Outcome 10 Visual acuity/Teller cards at 12 m (cycles/degree).
1.11. Analysis.
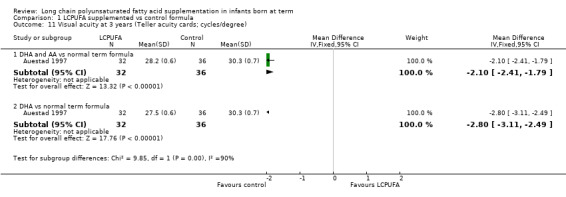
Comparison 1 LCPUFA supplemented vs control formula, Outcome 11 Visual acuity at 3 years (Teller acuity cards; cycles/degree).
1.12. Analysis.
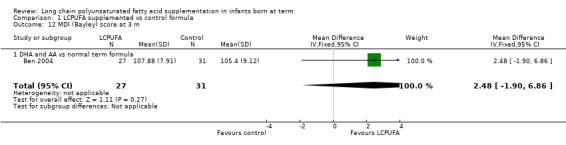
Comparison 1 LCPUFA supplemented vs control formula, Outcome 12 MDI (Bayley) score at 3 m.
1.13. Analysis.
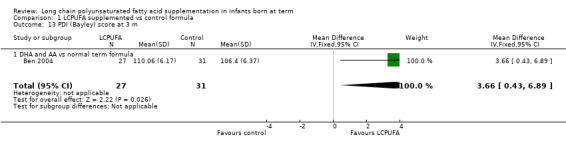
Comparison 1 LCPUFA supplemented vs control formula, Outcome 13 PDI (Bayley) score at 3 m.
1.14. Analysis.
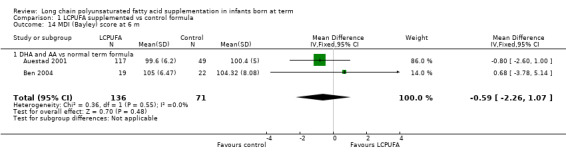
Comparison 1 LCPUFA supplemented vs control formula, Outcome 14 MDI (Bayley) score at 6 m.
1.15. Analysis.
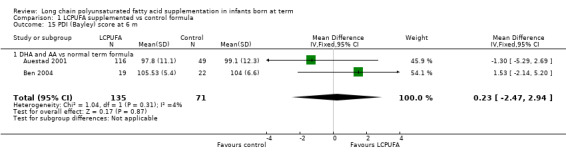
Comparison 1 LCPUFA supplemented vs control formula, Outcome 15 PDI (Bayley) score at 6 m.
1.16. Analysis.
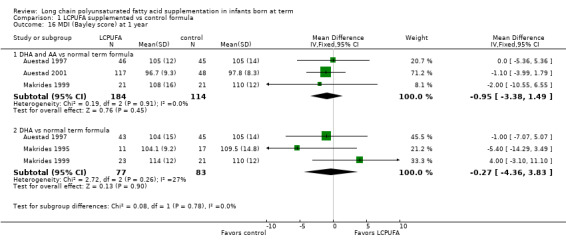
Comparison 1 LCPUFA supplemented vs control formula, Outcome 16 MDI (Bayley score) at 1 year.
1.17. Analysis.
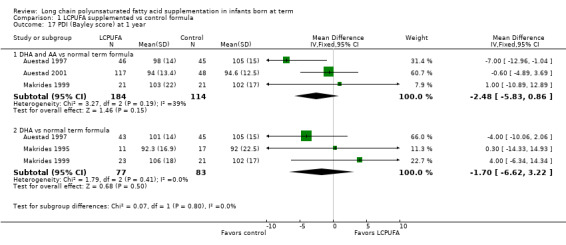
Comparison 1 LCPUFA supplemented vs control formula, Outcome 17 PDI (Bayley score) at 1 year.
1.18. Analysis.
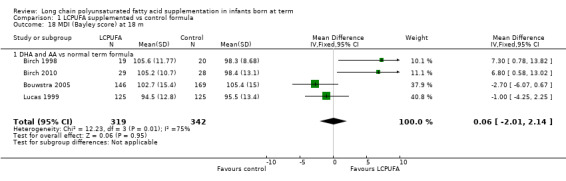
Comparison 1 LCPUFA supplemented vs control formula, Outcome 18 MDI (Bayley score) at 18 m.
1.19. Analysis.
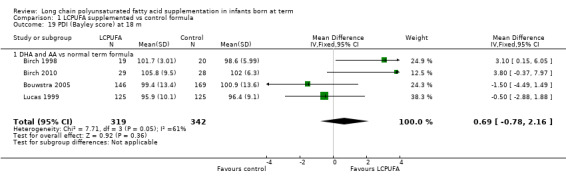
Comparison 1 LCPUFA supplemented vs control formula, Outcome 19 PDI (Bayley score) at 18 m.
1.20. Analysis.
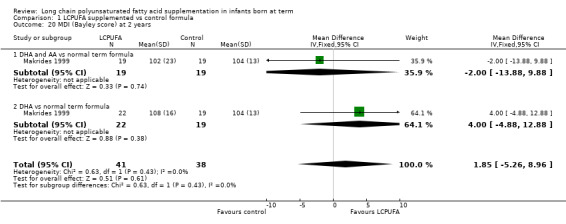
Comparison 1 LCPUFA supplemented vs control formula, Outcome 20 MDI (Bayley score) at 2 years.
1.21. Analysis.
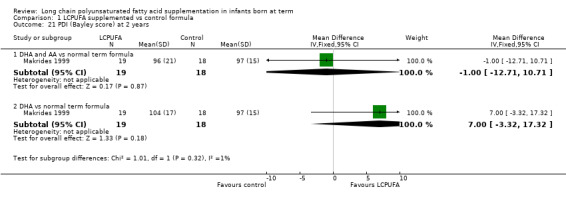
Comparison 1 LCPUFA supplemented vs control formula, Outcome 21 PDI (Bayley score) at 2 years.
1.22. Analysis.
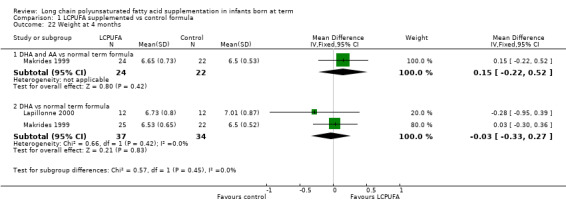
Comparison 1 LCPUFA supplemented vs control formula, Outcome 22 Weight at 4 months.
1.23. Analysis.
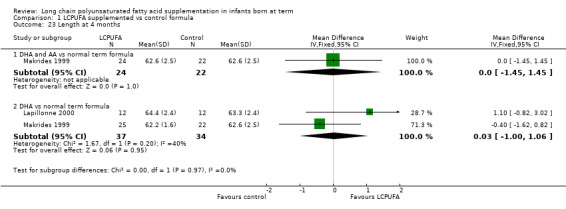
Comparison 1 LCPUFA supplemented vs control formula, Outcome 23 Length at 4 months.
1.24. Analysis.
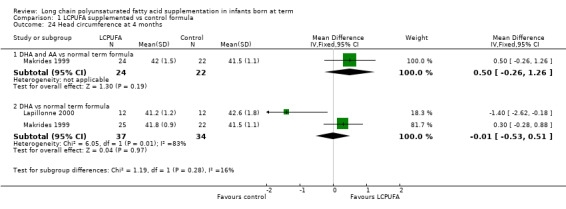
Comparison 1 LCPUFA supplemented vs control formula, Outcome 24 Head circumference at 4 months.
1.25. Analysis.
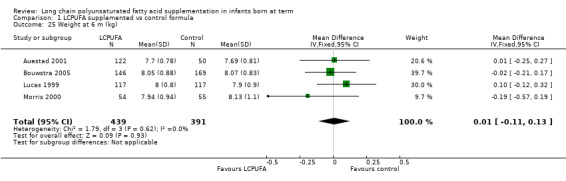
Comparison 1 LCPUFA supplemented vs control formula, Outcome 25 Weight at 6 m (kg).
1.26. Analysis.
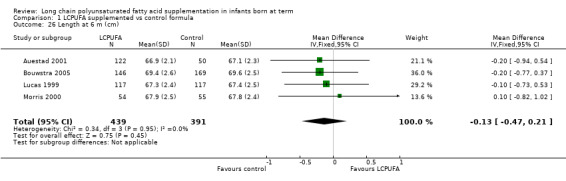
Comparison 1 LCPUFA supplemented vs control formula, Outcome 26 Length at 6 m (cm).
1.27. Analysis.
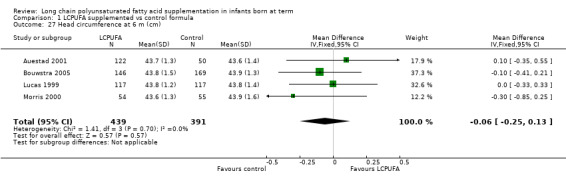
Comparison 1 LCPUFA supplemented vs control formula, Outcome 27 Head circumference at 6 m (cm).
1.28. Analysis.
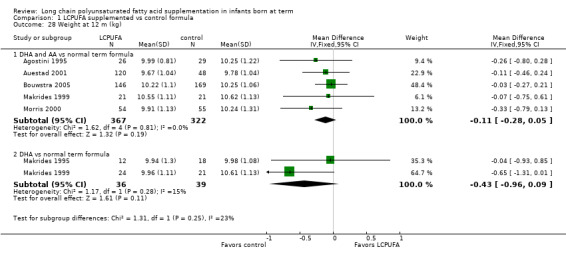
Comparison 1 LCPUFA supplemented vs control formula, Outcome 28 Weight at 12 m (kg).
1.29. Analysis.
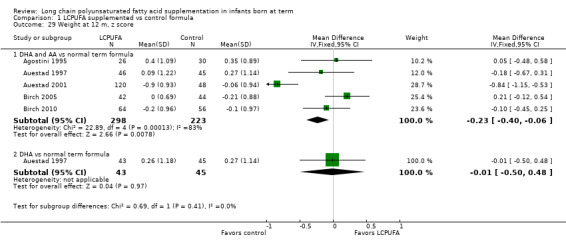
Comparison 1 LCPUFA supplemented vs control formula, Outcome 29 Weight at 12 m, z score.
1.30. Analysis.
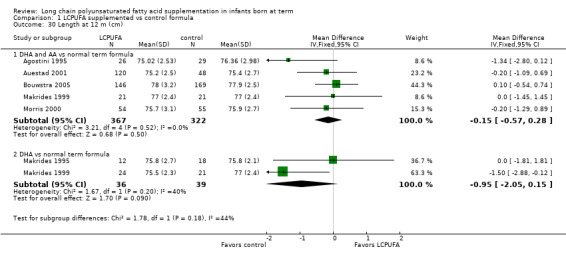
Comparison 1 LCPUFA supplemented vs control formula, Outcome 30 Length at 12 m (cm).
1.31. Analysis.
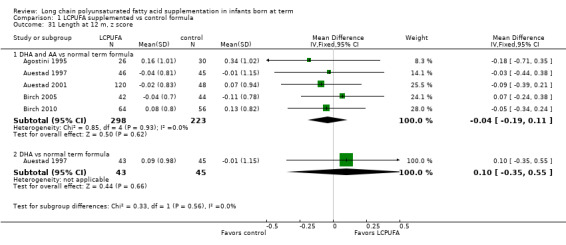
Comparison 1 LCPUFA supplemented vs control formula, Outcome 31 Length at 12 m, z score.
1.32. Analysis.
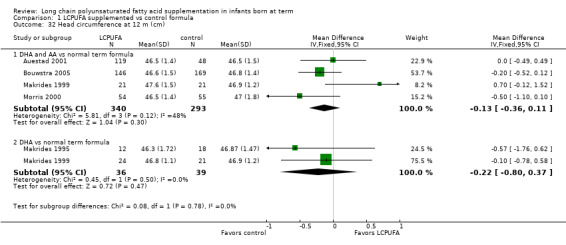
Comparison 1 LCPUFA supplemented vs control formula, Outcome 32 Head circumference at 12 m (cm).
1.33. Analysis.
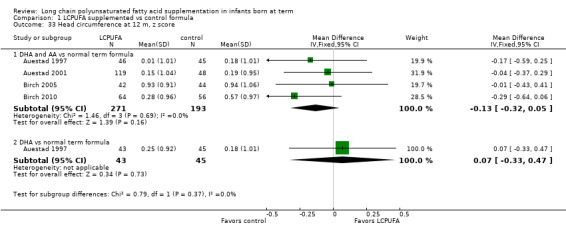
Comparison 1 LCPUFA supplemented vs control formula, Outcome 33 Head circumference at 12 m, z score.
1.34. Analysis.
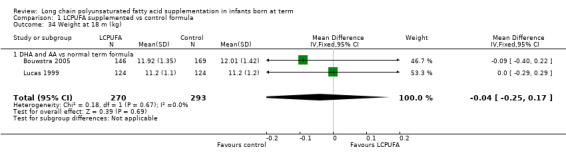
Comparison 1 LCPUFA supplemented vs control formula, Outcome 34 Weight at 18 m (kg).
1.35. Analysis.
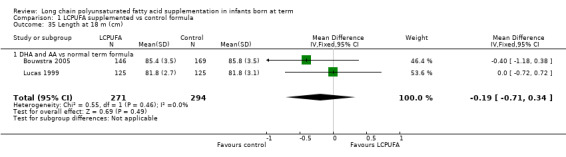
Comparison 1 LCPUFA supplemented vs control formula, Outcome 35 Length at 18 m (cm).
1.36. Analysis.
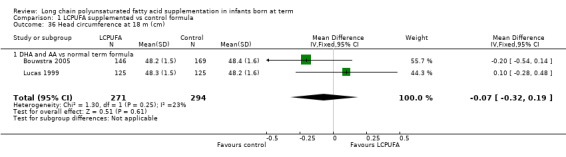
Comparison 1 LCPUFA supplemented vs control formula, Outcome 36 Head circumference at 18 m (cm).
1.37. Analysis.
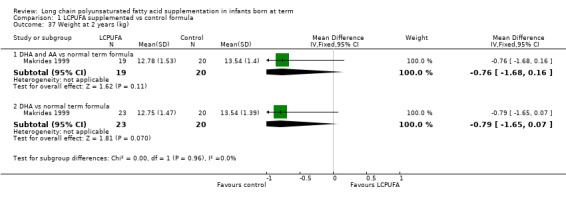
Comparison 1 LCPUFA supplemented vs control formula, Outcome 37 Weight at 2 years (kg).
1.38. Analysis.
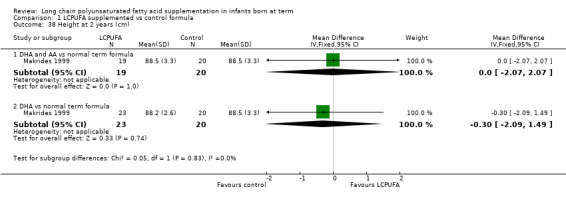
Comparison 1 LCPUFA supplemented vs control formula, Outcome 38 Height at 2 years (cm).
1.39. Analysis.
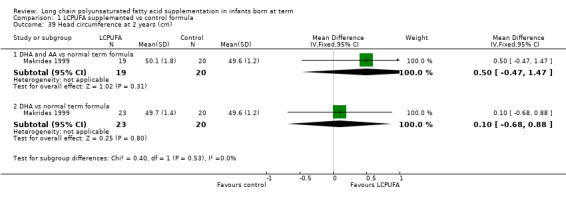
Comparison 1 LCPUFA supplemented vs control formula, Outcome 39 Head circumference at 2 years (cm).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Agostini 1995.
| Methods | Single‐centre study in Milan, Italy | |
| Participants | N = 60. Inclusion criteria: term infants (37 to 42 weeks), 5 minute Apgar score > 7, absence of disease. Exclusion criteria: not mentioned LCPUFA formula: N = 29 (GA 39.0 ± 1.3 weeks, BW 3.168 ± 0.448 kg) Control formula: N = 31 (GA 39.4 ± 1.4 weeks, BW 3.299 ± 0.453 kg) | |
| Interventions | Supplemented formula contained DHA (0.3%) and AA (0.44%). Control formula did not contain DHA nor AA. Study milk formulae were fed from within third day of life until 4 months. Source of LCPUFA was egg yolk phospholipids | |
| Outcomes | Brunet‐Lezine test of global neurodevelopment at 4, 12 and 24 months, Plasma and RBC phospholipid DHA and AA at 4 months and 24 months, physical growth at 1 year | |
| Notes | 30 infants in the breast‐fed reference group. Study authors responded by providing additional information regarding study methods | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Use of a time balanced randomisation table |
| Allocation concealment (selection bias) | Low risk | Use of sealed envelopes |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Both investigators and family members were blinded to the intervention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Follow‐up rate > 90% |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported |
| Other bias | Low risk | Appears to be free of other biases |
Auestad 1997.
| Methods | Three‐centre RCT in Kansas, Portland and Seattle | |
| Participants | N = 134. Inclusion criteria: term infants ≥ 37 weeks' gestation, AGA. Exclusion criteria: Apgar score < 7 at 5 minutes, physical or metabolic defects, received IV lipid infusion or blood transfusion, mothers with diabetes, hyperlipidaemia or perinatal infection LCPUFA (DHA and AA) group: N = 46 (GA 39.3 ± 1.3 weeks, BW 3.50 ± 0.46 kg) LCPUFA (DHA alone) group: N = 43 (GA 39.7 ± 1.2 weeks, BW 3.54 ± 0.46 kg) Control group: N = 45 (GA 39.8 ± 1.1 weeks, BW 3.600 ± 0.47 kg) | |
| Interventions | DHA plus AA formula was enriched with DHA (0.13%) and AA (0.45%). DHA alone formula was enriched with DHA (0.2%). Control formula was standard milk without addition of DHA and AA. Infants were randomised within 9 days after birth. Study formulae were fed ad libitum as the sole source of nutrition for first 4 months and as exclusive milk beverage up to 12 months of age. Source of LCPUFA was egg yolk phospholipids | |
| Outcomes | RBC fatty acid levels at 2, 4, 6 and 12 months. Growth measured at 1, 2, 4, 6, 9 and 12 months. Visual acuity at 2, 4, 6, 9, 12 and 39 months. Visual acuity measured by the Teller acuity card procedure or sweep spatial frequency VEP. Global development assessed at 1 year (BSID) and at 3 years (Stanford Binet IQ). Language development assessed at 14 months (McArthur Communicative Development Inventory) and at 3 years (Peabody Picture Vocabulary Test) | |
| Notes | Breast‐fed reference group: n = 63. Study authors had provided additional information for the previous version of this review. We contacted them to request more information for this update. Study authors acknowledged receipt of our letter but did not provide requested information | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centralised randomisation |
| Allocation concealment (selection bias) | Low risk | Centralised randomisation |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Assessors of developmental outcomes were unaware of infants' group assignment and medical history |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Outcomes of only infants who completed the study were reported. Less than 80% follow‐up for visual acuity outcomes at different ages |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported |
| Other bias | Low risk | Appears to be free of other biases |
Auestad 2001.
| Methods | RCT in 4 centres (Missouri, Arkansas, Pennsylvania and Arizona) | |
| Participants | N = 404 (initially enrolled). Inclusion criteria: term infants between 37 and 42 weeks' gestation, ≤ 9 days, birth weight ≥ 2500 grams, 5 minute Apgar score ≥ 7, ability to tolerate milk‐based formula or breast milk. Exclusion criteria: significant cardiac, ophthalmological, gastrointestinal or hematological or metabolic disease, milk protein allergy, maternal medical history known to have adverse effects on the foetus, tuberculosis, HIV, perinatal infection, substance abuse LCPUFA (DHA and AA) supplemented formula derived from egg triglyceride: N = 80 (GA 39 ± 1.3 weeks, BW 3.39 ± 0.47 kg) LCPUFA (DHA and AA) supplemented formula derived from fish and fungus oil: N = 82 (GA 39.3 ± 1.2 weeks, BW 3.41 ± 0.41 kg) Control formula: N = 7 (GA 39.4 ± 1.2 weeks, BW 3.45 ± 0.44 kg) | |
| Interventions | Study formula was milk formula supplemented with DHA (0.13%) and AA (0.45%). Control formula was standard milk without DHA and AA added. Infants were randomised within 9 days of birth. Study formulae were fed ad libitum as the sole source of nutrition for first 4 months and as exclusive milk beverage up to 12 months of age. Source of LCPUFA was fish and fungus oil in one group and egg yolk triglyceride in the other | |
| Outcomes | Fatty acid profiles in red cell lipids, physical growth at 1, 2, 4, 6, 9 and 12 months. Visual acuity measured by Teller acuity card procedure at 2, 4, 6 and 12 months, Fagan test of infant intelligence at 6 and 9 months, Bayley Scales of Infant Development at 6 and 12 months, language assessment with McArthur's communicative developmental inventories at 9 and 14 months, parental reporting of infant temperament at 6 and 12 months | |
| Notes | Study authors reported outcome data separately for milk formula enriched with LCPUFA derived from fish/fungus oil and milk formula enriched with LCPUFA derived from egg triglyceride. Our outcome of interest was the effect of LCPUFA rather than the source of LCPUFA, so we asked study authors to provide combined outcome data. Study authors provided the requested data . Breast‐fed control group: N = 82 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated with a random permuted blocks algorithm |
| Allocation concealment (selection bias) | Low risk | Centralised randomisation |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Measures of growth, visual acuity, information processing, general development, language and temperament were assessed by masked clinical tests |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 239 out of 404 enrolled infants completed the study, and only those results were reported. Less than 80% of enrolled infants completed the study |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported |
| Other bias | Low risk | Appears to be free of other biases |
Ben 2004.
| Methods | Single‐centre RCT in China | |
| Participants | N = 121. Included: infants of gestational age 37 to 40 weeks. Exclusion criteria: infants with congenital anomalies LCPUFA supplemented formula: N = 69 Control formula: N = 52 Gestational age and birth weight details not available | |
| Interventions | LCPUFA group was given milk formula enriched with DHA and AA. LCPUFA content of the formula was not clear. Control group was fed with standard milk formula without DHA and AA added. Infants were randomly assigned to the study formula before 2 weeks of life. Assigned diets were fed from day of enrolment to 6 months of age. Source of LCPUFA was not clear | |
| Outcomes | Fatty acid profiles in red cell lipids, physical growth and neurodevelopmental outcomes at 3 and 6 months of age | |
| Notes | Study authors published a short version of the article in a Chinese medical journal. The full article with raw data was provided by study authors on request. Study authors also clarified study methods. Breast‐fed reference group = 26 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used to generate random sequence was unclear |
| Allocation concealment (selection bias) | Unclear risk | Method used for allocation concealment was unclear |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blinding of intervention and outcome assessors was performed |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Follow‐up rate was 48% at 3 months and 33% at 6 months |
| Selective reporting (reporting bias) | Unclear risk | Details not available |
| Other bias | Unclear risk | Details not available |
Birch 1998.
| Methods | Single‐centre RCT conducted in Dallas, Texas, USA | |
| Participants | N = 79. Inclusion criteria: infants of gestational age 37 to 40 weeks, singleton births and appropriate for gestational age. Exclusion criteria: family history of milk protein allergy or genetic or familial eye disease, maternal vegetarian or vegan dietary pattern, maternal metabolic disease, anaemia or infection, congenital malformation or infection, jaundice, perinatal asphyxia, meconium aspiration syndrome, admission to NICU LCPUFA (DHA and AA) supplemented formula: N = 27 LCPUFA (DHA alone) supplemented formula: N = 26 Control formula: N = 26 Mean gestational age and birth weight: not given | |
| Interventions | One group was fed with formula milk enriched with DHA (0.36%) and AA (0.72%). Another group was fed formula milk enriched with DHA alone (0.36%). Control group was fed standard milk formula without DHA and AA added. Infants were randomly assigned to the study formula between 1 and 5 days of life. Assigned diets were fed from within 5 days of birth until 17 weeks of age. Source of LCPUFA was single‐cell oil | |
| Outcomes | Blood lipids were measured at 17 and 52 weeks. Growth, sweep VEP and forced preferential looking were measured at 6, 17, 26 and 52 weeks. Bayley Scales of Infant Development were measured at 18 months | |
| Notes | Breast‐fed reference: n = 29 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Use of block randomisation schedule |
| Allocation concealment (selection bias) | Low risk | Use of sealed envelopes |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Investigators conducting blood lipid analysis and visual function testing were masked to type of formula provided to infants |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 70% to 86% follow up for different outcomes |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported |
| Other bias | Low risk | Appears to be free of other biases |
Birch 2005.
| Methods | Single‐centre RCT conducted in Dallas, Texas, USA | |
| Participants | N = 103. Included: infants of gestational age 37 to 40 weeks, singleton births, appropriate for gestational age. Exclusion criteria: family history of milk protein allergy or genetic or familial eye disease, maternal vegetarian or vegan dietary pattern, maternal metabolic disease, anaemia or infection, congenital malformation or infection, jaundice, perinatal asphyxia, meconium aspiration syndrome, admission to NICU LCPUFA supplemented formula: N = 51 Control formula: N = 52 Mean gestational age and birth weight not given | |
| Interventions | 'LCPUFA' group was given milk formula enriched with DHA (0.36%) and AA (0.72%). Control group was fed standard milk formula without DHA and AA added. Infants were randomly assigned to study formula between 1 and 5 days of life. Assigned diets were fed from day of enrolment to 52 weeks of age. Source of DHA was single‐cell algal oil (Crypthecodinium cohnii); source of AA was fungal oil (Mortierella alpina) | |
| Outcomes | Fatty acid profiles in red cell lipids, physical growth, visual outcomes: sweep VEP acuity, random dot stereo acuity | |
| Notes | Study authors clarified method details and provided additional information on outcome data. No breast‐fed control group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Use of single randomisation schedule at a central location. Randomisation schedule had random‐length blocks (block length varied from 6 to 12) |
| Allocation concealment (selection bias) | Low risk | Use of sealed envelopes |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Each diet was masked by 2 colour codes and 2 number codes, for a total of 4 possible diet assignments. Study authors informed that outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 83% to 92% follow‐up rates for different outcomes at different stages |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported |
| Other bias | Low risk | Appears to be free of other biases |
Birch 2010.
| Methods | Randomised controlled trial conducted in Dallas (5 hospitals) and Kansas (2 hospitals) | |
| Participants | N = 170. Included: healthy, full‐term (37 to 42 weeks) formula‐fed infants. Excluded: infants who had received human milk within 24 hours of randomisation, with disease or congenital anomaly likely to affect visual development and neurodevelopment, poor formula intake, known or suspected intolerance to cow's milk formula. Also excluded were infants born to mothers with chronic illnesses such as HIV, renal or hepatic disease, diabetes, alcoholism or substance abuse LCPUFA supplemented formula: N = 84 Control formula: N = 86 | |
| Interventions | 'LCPUFA' group was given milk formula enriched with DHA (0.32%) and AA (0.64%). Control group was fed standard milk formula without DHA and AA added. Infants were randomly assigned to study formula between 1 and 9 days of life. Assigned diets were fed from day of enrolment to 1 year of age. Assigned formula was the sole source of nutrition until ≈4 months of age. Source of DHA was single‐cell algal oil (Crypthecodinium cohnii); source of AA was fungal oil (Mortierella alpina) | |
| Outcomes | Sweep VEP acuity, fatty acid profiles in red cell lipids, anthropometry, formula intake and tolerance at 1.5, 4, 6, 9 and 12 months of age. VEP visual acuity at 12 months of age was the primary outcome of interest. Quality of attention, heart rate, age‐appropriate standardised and specific cognitive tests (18 months to 6 years every 6‐monthly), growth until 6 years of age, school readiness and receptive vocabulary were other long‐term outcomes of interest | |
| Notes | The study included 4 groups: control (0% DHA), 0.32% DHA, 0.64% DHA, 0.96% DHA. For this review, we used the 0.32% DHA group as the intervention arm. DHA supplemented formulae also provided 0.64% arachidonic acid. Study authors clarified a few method issues and provided requested information. Standard errors of means were provided by study authors, and Cochrane review authors converted them into standard deviations | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer programme with a random number generation function was used to create the randomisation sequence |
| Allocation concealment (selection bias) | Low risk | Use of sealed envelopes |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Each infant's study formula group allocation was masked until all infants had reached 12 months of age and data collection had been completed, validated and locked |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Only 120/170 enrolled infants (70.5%) completed the study |
| Selective reporting (reporting bias) | Low risk | For the primary outcome, reporting was free of selective reporting bias |
| Other bias | High risk | Bayley Scale scores at 18 months of age were reported only for study participants from the Dallas centre. Results for study participants at the Kansas centre were not reported. Study authors mention in the manuscript that this occurred because this phase 2 trial was done separately by study centres using different protocols and data collection and analysis |
Bouwstra 2005.
| Methods | Single‐centre RCT conducted in Netherlands | |
| Participants | N = 315. Included: infants of gestational age 37 to 42 weeks. Exclusion criteria: congenital anomalies, infants from multiple births, mothers with significant disability, mothers with insufficient mastery of Dutch language, adopted or foster infants and formula‐fed infants who had received human milk for more than 5 days LCPUFA supplemented formula: N = 146 Control formula: N = 169 | |
| Interventions | 'LCPUFA' group was given milk formula enriched with DHA (0.3%) and AA (0.45%). Control group was fed standard milk formula without DHA and AA added. Infants were randomly assigned to the study formula between 1 and 5 days of life. Assigned diets were fed from day of enrolment for 2 months. Source of LCPUFA was egg yolk, tuna oil and single‐cell oil produced by the soil fungus, Mortierella alpina | |
| Outcomes | Neurodevelopmental assessment using Hempel and Bayley Scales. Hempel assessment is a standardised technique designed for detection of minor signs of neurological dysfunction and physical growth. Anthropometric, cardiovascular, cognitive and behavioural assessments were done at nine years of age | |
| Notes | Study authors provided additional information regarding various outcomes. Breast‐fed reference group: 160 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Use of central computerized randomisation with blocked design |
| Allocation concealment (selection bias) | Low risk | Central computerised randomisation |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Parents and examiners were unaware of the type of formula feeding that infants received |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Follow‐up was 92% |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported |
| Other bias | Low risk | Appears to be free of other biases |
Carlson 1996.
| Methods | Single‐centre RCT conducted in Memphis, Tennessee, USA | |
| Participants | N = 39. Inclusion criteria: infants born at term (37 to 43 weeks' PMA) without IUGR and with no medical problems likely to influence long‐term growth and development. Exclusion criteria: not mentioned LCPUFA: N = 19 (GA 39.8 ± 1.2 weeks, BW 3.285 ± 0.448 kg) Control formula: N = 20 (GA 40.3 ± 0.9 weeks, BW 3.327 ± 0.331 kg) | |
| Interventions | Supplemented formula was enriched with DHA (0.10%) and AA (0.43%). Control formula did not include DHA and AA. Infants were randomised within 24 hours of birth to receive study milk formula. Study formula was fed for 1 year. Source of LCPUFA was egg yolk phospholipids | |
| Outcomes | RBC and plasma fatty acid levels at 2, 4, 6 and 12 months Visual acuity (Teller acuity cards) at 2, 4, 6, 9 and 12 months | |
| Notes | Breast‐fed reference group: N = 19 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Two investigators were unaware of infants' dietary treatments and results of earlier acuity tests |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 58 of the initially recruited 90 completed the study (LCPUFA: 20; control formula: 19; breast‐fed reference group: 19). 36 were lost to follow‐up (LCPUFA: 9; control formula: 11; breast‐fed reference group: 16) |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported |
| Other bias | Low risk | Appears to be free of other biases |
Lapillonne 2000.
| Methods | Single‐centre RCT conducted in France | |
| Participants | N = 24. Inclusion criteria: healthy term appropriate for gestational age infants. Exclusion criteria: infants of mothers who had history of cocaine or alcohol abuse, hyperlipidaemia, diabetes, strict vegetarian or vegan diets LCPUFA supplemented formula: N = 12 (GA 39.3 ± 1.1 weeks, BW 3.378 ± 0.426 kg) Control formula: N = 12 (GA 40.1 ± 1.2 weeks, BW 3.311 ± 0.0448 kg) | |
| Interventions | 'LCPUFA' group was given milk formula enriched with DHA (0.31%). Control group was fed standard milk formula without DHA added. Assigned diets were fed from day 3 of life until 4 months of age. Source of LCPUFA was fish oil | |
| Outcomes | Fatty acid levels in RBCs at 4 months; weight, length and head circumference at 2 and 4 months of age | |
| Notes | Study authors responded by providing additional information regarding study methods | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Use of computer‐generated random allocation list |
| Allocation concealment (selection bias) | Low risk | Use of sealed opaque envelopes |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Caregivers, parents and outcome assessors were blinded to the intervention group |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Follow‐up > 80% |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported |
| Other bias | Low risk | Appears to be free of other biases |
Lucas 1999.
| Methods | Two‐centre RCTs conducted in Nottingham and Leicester, England | |
| Participants | N = 309. Inclusion criteria: term infants ≥ 37 weeks' gestation and appropriate for gestational age singletons. Exclusion criteria: presence of congenital anomalies LCPUFA: 154 (GA 40.0 ± 1.29 weeks and BW 3542 ± 409 grams) Control formula: 155 (GA 40.1 ± 1.30 weeks and BW 3648 ± 459 grams) | |
| Interventions | 'LCPUFA' group was given milk formula enriched with DHA (0.32%) and AA (0.30%). Control group was fed standard milk formula without DHA and AA added. Infants were assigned to study formula within first week of life. Study formula was continued for 6 months. Source of LCPUFA was egg yolk phospholipids | |
| Outcomes | Primary endpoint was development at 18 months assessed by Bayley Scales of Infant Development (MDI and PDI). Secondary endpoint was development at 9 months assessed by Knobloch, Passamanick and Sherrards tests. Growth and gastrointestinal tolerance were also assessed at 6, 9 and 18 months. Incidences of atopy, eczema, wheeze and infection were documented | |
| Notes | Infants who were breast‐fed for at least 6 weeks were a reference group (n = 138). Study authors published a correction to outcomes reported in 2002, stating that they inadvertently reversed the 2 diet codes. Hence the outcomes of standard formula were those of infants fed LCPUFA formula, and vice versa. We have entered the correct data into this review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random permuted block design stratified by centre and by sex was used to generate the allocation schedule |
| Allocation concealment (selection bias) | Low risk | Use of sealed opaque envelopes |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Mothers and study personnel were unaware of the dietary allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Follow‐up rates of 81% |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported |
| Other bias | Low risk | Appears to be free of other biases |
Makrides 1995.
| Methods | Single‐centre RCT conducted in Adelaide Sample size calculation: yes Concealment of allocation: yes Blinding to intervention: yes Blinding to outcome assessment: yes Completeness of follow‐up: no (60% to 81% for various primary outcomes) |
|
| Participants | N = 32. Inclusion criteria: healthy term appropriate for gestational age infants born at 37 to 42 weeks. Exclusion criteria: infants of mothers who had history of lipid metabolism disorders, diabetes, drug or alcohol abuse LCPUFA supplemented formula: N = 13 (GA 39.1 ± 1.7 weeks, BW 3.288 ± 0.525 kg) Control formula: N = 19 (GA 39.6 ± 1.2 weeks, BW 3.650 ± 0.0416 kg) | |
| Interventions | 'LCPUFA' group was given milk formula enriched with DHA (0.35%). In addition, formula was enriched with EPA and GLA. Control group was fed standard milk formula without DHA and AA added. Assigned diets were fed from birth to 30 weeks of life. Source of LCPUFA was fish oil and evening primrose oil | |
| Outcomes | Plasma and red blood cell fatty acid levels at 6, 16 and 30 weeks; visual evoked potential acuity at 16 and 30 weeks; Bayley Scales of Infant Development at 1 year | |
| Notes | Breast‐fed reference group, n = 28 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Use of central computerised randomisation |
| Allocation concealment (selection bias) | Low risk | Adequate; use of sealed opaque envelopes |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Mothers were unaware of formula type |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 60% to 81% follow‐up for various primary outcomes |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported |
| Other bias | Low risk | Appears to be free of other biases |
Makrides 1999.
| Methods | Single‐centre RCT conducted in Adelaide | |
| Participants | N = 83. Inclusion criteria: healthy term infants. Exclusion criteria: small for gestational age, congenital disease, infants of insulin‐dependent diabetic mothers, history of drug or alcohol abuse in the mother LCPUFA (DHA and AA) supplemented formula: N = 28 (GA 39.8 ± 1.3 weeks; BW 3549 ± 521 grams) LCPUFA (DHA alone) supplemented formula: N = 27 (GA 39.6 ± 1.1 weeks, BW 3378 ± 431 grams) Control formula: N = 28 (GA 39.6 ± 1.5 weeks, BW 3549 ± 497 grams) | |
| Interventions | 'LCPUFA' group was given milk formula enriched with DHA (0.34%) and AA (0.34%). Another LCPUFA group received milk formula enriched with DHA alone (0.34%). Control group was fed standard milk formula without DHA and AA added. Infants were randomly assigned to study formula within 7 days of life. Assigned milk formula was sole source of nutrition for 4 months. Subsequently, study formula was the only source of milk until 1 year of age. Source of LCPUFA was egg yolk phospholipids (DHA + AA group) and tuna oil (DHA group) | |
| Outcomes | Plasma and RBC fatty acid levels at 6, 16 and 34 weeks and 1 year of age. Physical growth at 6, 16 and 34 weeks and at 1 and 2 years of age. VEP at 16 and 34 weeks. Bayley Scales of Infant Development at 1 and 2 years | |
| Notes | Breast‐fed reference group, n = 63 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers |
| Allocation concealment (selection bias) | Low risk | Adequate; use of sealed opaque envelopes |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Investigators and families were blinded to randomisation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 60% to 85% for various outcomes |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported |
| Other bias | Low risk | Appears to be free of other biases |
Morris 2000.
| Methods | Single‐centre RCT in Wales | |
| Participants | N = 109. Included: infants at full‐term gestation with birth weight 2.5 to 4.5 kg. Exclusion criteria: congenital anomalies, infants from multiple births LCPUFA supplemented formula: N = 54 (BW 3.31 ± 0.48 kg) Control formula: N = 55 (BW 3.35 ± 0.46 kg) Mean gestational age of study infants was not given | |
| Interventions | LCPUFA formula was enriched with DHA (0.2%) and AA (0.4%). Control formula was not enriched with DHA and AA. Study formula was started within 72 hours of birth and was given for first 12 weeks. Source of LCPUFA was single‐cell oils | |
| Outcomes | Physical growth at 6 weeks, 3 months, 6 months and 1 year; general health of infants | |
| Notes | Study authors replied with clarification regarding study methods. No breast‐fed control group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "block randomisation in double blind fashion" |
| Allocation concealment (selection bias) | Unclear risk | Details were not available |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Parents, caregivers and professionals were blinded to milk type |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Follow‐up 78% |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported |
| Other bias | Low risk | Appears to be free of other biases |
Willats 1998.
| Methods | Single‐centre RCT in Scotland | |
| Participants | N = 72. Included: term infants (37 to 42 weeks) with birth weight between 2.5 and 4 kg. Exclusion criteria: not mentioned LCPUFA supplemented formula: N = 34 Control formula: N = 38 Mean birth weight and gestational age data of study infants not given | |
| Interventions | LCPUFA formula was enriched with DHA (0.15% to 0.25%) and AA (0.3% to 0.4%). Control formula did not contain DHA or AA. Study milk formula was given from birth until 4 months of age. Source of LCPUFA was egg lipids, milk fat and vegetable oils | |
| Outcomes | Infant cognition measured by a means‐end problem‐solving test at 10 months. Assessments of intelligence quotient (IQ), attention control (Day‐Night Test) and speed of processing on Matching Familiar Figures Test (MFFT) was done at 6 years for enrolled infants | |
| Notes | Results are given as medians and quartiles and therefore are provided in text, not in tables. No breast‐fed control group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Use of computer‐generated randomisation table. Randomisation was stratified to ensure sex matching |
| Allocation concealment (selection bias) | Low risk | Pharmacy coded formulae administered to babies |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Mothers and investigators were unaware of group assignments |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Completeness of follow‐up: 44 of 72 (61%) infants completed study outcome assessment |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported |
| Other bias | Low risk | Appears to be free of other biases |
AA: arachidonic acid AGA: appropriate for gestational age BSID: Bayley Scales of Infant Development BW: body weight DHA: docosahexaenoic acid EPA: eicosapentaenoic acid GA: gestational age GLA: gamma‐linolenic acid HIV: human immunodeficiency virus IUGR: intrauterine growth rate LCPUFA: long chain polyunsaturated fatty acids MDI: Mental Developmental Index NICU: neonatal intensive care unit PDI: Psychomotor Developmental Index PMA: postmenstrual age RBC: red blood cells RCT: randomised controlled trial VEP: visual evoke potentials
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Agostoni 2009 | RCT. DHA/placebo supplementation was given to breast‐fed infants |
| Birch 2002 | Late age (6 weeks) at which study formula was started |
| Carlson 1999 | Methods not clear. Outcomes of interest not available. Study authors expressed concern about methodological issues of their study |
| Clausen 1996 | Methods not clear. Time of start of study formula and duration of supplementation not clear |
| Decsi 1995 | Methods not clear. Outcomes of interest not available |
| Field 2008 | Study assessed effect of LCPUFA supplemented formula milk on laboratory markers of immune function |
| Field 2010 | Outcomes of interest were lab markers: phenotype and cytokine levels such as (interleukin [IL]‐2, IL‐4, IL‐6, IL‐10, IL‐12, interferon [IFN]‐gamma, tumour necrosis factor [TNF]‐alpha, TGF‐beta) after incubation with phytohemagglutinin (PHA), beta‐lactoglobulin or soy protein |
| Fleddermann 2014 | Single‐centre RCT. Intervention formula contained reduced protein and added alpha lactalbumin, in addition to LCPUFA |
| Gibson 2009 | Study milk formula was supplemented with probiotic (Bifidobacterium lactis) in addition to LCPUFA; control formula had neither |
| Jorgenson 1996 | Late age at which supplementation was commenced |
| Lapillonne 2014 | Multi‐centre, prospective, observational, open‐label study that evaluated respiratory outcomes with LCPUFA supplementation during first year |
| Meldrum 2012 | Randomised, double‐blind, placebo‐controlled trial. Healthy term infants (breast/formula fed) were assigned to receive a DHA‐enriched FO supplement (containing at least 250 mg DHA/d and 60 mg EPA/d) or placebo (olive oil) from birth to 6 months. Study infants were not solely formula fed |
| NCT02092857 | RCT comparing LCPUFA vs placebo in formula milk, but outcome of interest was number of antigen‐presenting B cells |
| Patterson 2016 | Multi‐centre RCT. Compared formula milk supplemented with 2 different sources of DHA (algal‐derived DHA single‐cell oil (DHASCO) vs marine algae‐derived single‐cell oil (DHASCO‐B) |
| Visentin 2016 | RCT that compared red cell membrane fatty acid levels of 24 infants who received standard term formula vs 25 control infants who received the same formula supplemented with higher DHA and AA content for at least 4 months before the age of 6 months. Clinical outcomes were not reported |
| Voigt 2002 | Milk formulae with different amounts of alpha linolenic acid were compared |
DHA: docosahexaenoic acid DHASCO: algal‐derived DHA single‐cell oil DHASCO‐B: marine algae‐derived DHA single‐cell oil EPA: eicosapentaenoic acid FO: fish oil IFN: interferon IL: interleukin LCPUFA: long chain polyunsaturated fatty acids PHA: phytohemagglutinin RCT: randomised controlled trial TNF: tumour necrosis factor
Contributions of authors
2016 review update:
BJ: literature search, assessment of eligibility, data extraction, assessment of risk of bias of included RCTs, writing and reviewing of the manuscript.
SR: literature search, assessment of eligibility, data extraction, assessment of risk of bias of included RCTs, entry of data into RevMan and data analysis, contact with study authors for additional information, reviewing of the manuscript.
SP: literature search, assessment of eligibility, verification of assessment of risk of bias of included RCTs, verification of data entered into RevMan by SR, reviewing of the manuscript.
KS: reviewing of the manuscript, overall supervision for update of the meta‐analysis.
2011 review update:
SR: literature search, assessment of eligibility and quality of studies, data extraction, entry of data into RevMan and data analysis, contact with study authors for additional information, writing of the manuscript.
SP: literature search, assessment of eligibility and quality of studies, verification of data entered into RevMan by SR, reviewing of the manuscript.
KS: assessment of eligibility of studies for inclusion, reviewing of the manuscript, guidance and supervision for update of the meta‐analysis.
2008 review update:
SR: literature search, assessment of eligibility and quality of studies, contact with authors of original trials, data extraction, entry of data into RevMan, writing of the manuscript.
SP: literature search, assessment of eligibility and quality of studies, verification of data entered into RevMan by SR, reviewing of the manuscript.
KS: referee author, checking and editing of the manuscript.
2001:
KS: literature search, assessment of eligibility and quality of studies, data extraction and data analysis, writing of the manuscript.
2000:
Original review: KS: design and preparation of protocol, literature search, assessment of eligibility and quality of studies, data extraction and data analysis, writing of the manuscript.
Sources of support
Internal sources
King Edward Memorial Hospital for Women, Perth, Australia.
Princess Margaret Hospital for Childern, Perth, Australia.
External sources
-
Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services, USA.
Editorial support of the Cochrane Neonatal Review Group has been funded with Federal funds from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services, USA, under Contract No. HHSN275201600005C
-
National Institute for Health Research, UK.
UK Editorial support for Cochrane Neonatal has been funded with funds from a UK National Institute of Health Research Grant (NIHR) Cochrane Programme Grant (13/89/12). The views expressed in this publication are those of the review authors and are not necessarily those of the NHS, the NIHR or the UK Department of Health
Declarations of interest
None.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Agostini 1995 {published data only}
- Agostini C, Trojan S, Bellu R, Riva E, Bruzzese MG, Giovannini M. Developmental quotient at 24 months and fatty acid composition of diet in early infancy: a follow up study. Archives of Diseases in Childhood 1997;76(5):421‐4. [PUBMED: 9196357 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agostini C, Trojan S, Bellu R, Riva E, Giovannini M. Neurodevelopmental quotient of healthy term infants at 4 months and feeding practice: the role of long‐chain polyunsaturated fatty acids. Pediatric Research 1995;38(2):262‐6. [DOI: 10.1203/00006450-199508000-00021] [DOI] [PubMed] [Google Scholar]
- Agostoni C, Riva E, Scaglioni S, Marangoni F, Radaelli G, Giovannini M. Dietary fats and cholesterol in Italian infants and children. American Journal of Clinical Nutrition 2000;72(5 Suppl):1384S‐91S. [PUBMED: 11063482 ] [DOI] [PubMed] [Google Scholar]
Auestad 1997 {published and unpublished data}
- Auestad N, Montalto MB, Hall RT, Fitzgerald KM, Wheeler RE, Connor WE, et al. Visual acuity, erythrocyte fatty acid composition, and growth in term infants fed formulas with long chain polyunsaturated fatty acids for one year. Pediatric Research 1997;41(1):1‐10. [DOI: 10.1203/00006450-199701000-00001] [DOI] [PubMed] [Google Scholar]
- Auestad N, Scott DT, Janowsky JS, Jacobsen C, Carroll RE, Montalto MB, et al. Visual, cognitive, and language assessments at 39 months: a follow‐up study of children fed formulas containing long‐chain polyunsaturated fatty acids to 1 year of age. Pediatrics 2003;112(3 pt 1):e177‐83. [PUBMED: 12949309 ] [DOI] [PubMed] [Google Scholar]
- Scott DT, Janowsky JS, Carroll RE, Taylor JA, Auestad N, Montalto MB. Formula supplementation with long‐chain polyunsaturated fatty acids: are there developmental benefits?. Pediatrics 1998;102(5):E59. [PUBMED: 9794989 ] [DOI] [PubMed] [Google Scholar]
Auestad 2001 {published data only}
- Auestad N, Halter R, Hall RT, Blatter M, Bogle ML, Burks W, et al. Growth and development in term infants fed long‐chain polyunsaturated fatty acids: a double‐masked, randomized, parallel, prospective, multivariate study. Pediatrics 2001;108(2):372‐81. [PUBMED: 11483802 ] [DOI] [PubMed] [Google Scholar]
Ben 2004 {published data only}
- Ben XM, Zhou XY, Zhao WH, Yu WL, Pan W, Zhang WL, et al. Growth and development of term infants fed with milk with long‐chain polyunsaturated fatty acid supplementation. Chinese Medical Journal 2004;117(8):1268‐70. [PUBMED: 15361309 ] [PubMed] [Google Scholar]
Birch 1998 {published data only}
- Birch EE, Garfield S, Castañeda Y, Hughbanks‐Wheaton D, Uauy R, Hoffman D. Visual acuity and cognitive outcomes at 4 years of age in a double‐blind, randomized trial of long‐chain polyunsaturated fatty acid‐supplemented infant formula. Early Human Development 2007;83(5):279‐84. [DOI: 10.1016/j.earlhumdev.2006.11.003] [DOI] [PubMed] [Google Scholar]
- Birch EE, Garfield S, Hoffman DR, Uauy R, Birch DG. A randomized controlled trial of early dietary supply of long‐chain polyunsaturated fatty acids and mental development in term infants. Developmental Medicine and Child Neurolology 2000;42(3):174‐81. [PUBMED: 10755457 ] [DOI] [PubMed] [Google Scholar]
- Birch EE, Hoffman DR, Uauy R, Birch DG, Prestidge C. Visual acuity and the essentiality of docosahexaenoic acid and arachidonic acid in the diet of term infants. Pediatric Research 1998;44(2):201‐9. [DOI: 10.1203/00006450-199808000-00011] [DOI] [PubMed] [Google Scholar]
- Hoffman DR, Birch EE, Birch DG, Uauy R, Castaneda YS, Lapus MG, et al. Impact of early dietary intake and blood lipid composition of long‐chain polyunsaturated fatty acids on later visual development. Journal of Pediatric Gastroenterology and Nutrition 2000;31(5):540‐53. [PUBMED: 11144440 ] [DOI] [PubMed] [Google Scholar]
Birch 2005 {published data only}
- Birch EE, Castaneda YS, Wheaton DH, Birch DG, Uauy RD, Hoffman DR. Visual maturation of term infants fed long‐chain polyunsaturated fatty acid‐supplemented or control formula for 12 mo. American Journal of Clinical Nutrition 2005;81(4):871‐9. [PUBMED: 15817866 ] [DOI] [PubMed] [Google Scholar]
Birch 2010 {published data only}
- Birch EE, Carlson SE, Hoffman DR, Fitzgerald‐Gustafson KM, Fu VL, Drover JR, et al. The DIAMOND (DHA Intake And Measurement Of Neural Development) Study: a double‐masked, randomized controlled clinical trial of the maturation of infant visual acuity as a function of the dietary level of docosahexaenoic acid. American Journal of Clinical Nutrition 2010;91(4):848‐59. [DOI: 10.3945/ajcn.2009.28557] [DOI] [PubMed] [Google Scholar]
- Colombo J, Carlson SE, Cheatham CL, Fitzgerald‐Gustafson KM, Kepler A, Doty T. Long‐chain polyunsaturated fatty acid supplementation in infancy reduces heart rate and positively affects distribution of attention. Pediatric Research 2011;70(4):406‐10. [DOI: 10.1203/PDR.0b013e31822a59f5] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo J, Carlson SE, Cheatham CL, Shaddy DJ, Kerling EH, Thodosoff JM, et al. Long‐term effects of LCPUFA supplementation on childhood cognitive outcomes. American Journal of Clinical Nutrition 2013;98(2):403‐12. [DOI: 10.3945/ajcn.112.040766] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie LM, Tolley EA, Thodosoff JM, Kerling EH, Sullivan DK, Colombo J, et al. Long chain polyunsaturated fatty acid supplementation in infancy increases length‐ and weight‐for‐age but not BMI to 6 years when controlling for effects of maternal smoking. Prostaglandins, Leukotrienes, and Essential Fatty Acids 2015;98:1‐6. [DOI: 10.1016/j.plefa.2015.04.001] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drover JR, Felius J, Hoffman DR, Castañeda YS, Garfield S, Wheaton DH, et al. A randomized trial of DHA intake during infancy: school readiness and receptive vocabulary at 2‐3.5 years of age. Early Human Development 2012;88(11):885‐91. [DOI: 10.1016/j.earlhumdev.2012.07.007] [DOI] [PubMed] [Google Scholar]
- Drover JR, Hoffman DR, Castañeda YS, Morale SE, Garfield S, Wheaton DH, et al. Cognitive function in 18‐month‐old term infants of the DIAMOND study: a randomized, controlled clinical trial with multiple dietary levels of docosahexaenoic acid. Early Human Development 2011;87(3):223‐30. [DOI: 10.1016/j.earlhumdev.2010.12.047] [DOI] [PubMed] [Google Scholar]
- Foiles AM, Kerling EH, Wick JA, Scalabrin DM, Colombo J, Carlson SE. Formula with long‐chain polyunsaturated fatty acids reduces incidence of allergy in early childhood. Pediatric Allergy and Immunology 2016;27(2):156‐61. [DOI: 10.1111/pai.12515] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bouwstra 2005 {published data only}
- Bouwstra H, Dijck‐Brouwer DA, Boehm G, Boersma ER, Muskiet FA, Hadders‐Algra M. Long‐chain polyunsaturated fatty acids and neurological developmental outcome at 18 months in healthy term infants. Acta Paediatrica 2005;94(1):26‐32. [PUBMED: 15858956] [DOI] [PubMed] [Google Scholar]
- Jong C, Boehm G, Kikkert HK, Hadders‐Algra M. The Groningen LCPUFA study: no effect of short‐term postnatal long‐chain polyunsaturated fatty acids in healthy term infants on cardiovascular and anthropometric development at 9 years. Pediatric Research 2011;70(4):411‐6. [DOI: 10.1203/PDR.0b013e31822a5ee0] [DOI] [PubMed] [Google Scholar]
- Jong C, Kikkert HK, Fidler V, Hadders‐Algra M. Effects of long‐chain polyunsaturated fatty acid supplementation of infant formula on cognition and behaviour at 9 years of age. Developmental Medicine and Child Neurology 2012;54(12):1102‐8. [DOI: 10.1111/j.1469-8749.2012.04444.x] [DOI] [PubMed] [Google Scholar]
- Jong C, Kikkert HK, Fidler V, Hadders‐Algra M. The Groningen LCPUFA study: no effect of postnatal long‐chain polyunsaturated fatty acids in healthy term infants on neurological condition at 9 years. The British Journal of Nutrition 2010;104(4):566‐72. [DOI: 10.1017/S0007114510000863] [DOI] [PubMed] [Google Scholar]
Carlson 1996 {published data only}
- Carlson SE, Ford AJ, Werkman SH, Peeples JM, Koo WWK. Visual acuity and fatty acid status of term infants fed human milk and formulas with and without docosahexaenoate and arachidonate from egg yolk lecithin. Pediatric Research 1996;39(5):882‐8. [DOI: 10.1203/00006450-199605000-00024] [DOI] [PubMed] [Google Scholar]
Lapillonne 2000 {published data only}
- Lapillonne A, Brosssard N, Claris O, Reygrobellet B, Salle BL. Erythrocyte fatty acid composition in term infants fed human milk or a formula enriched with a low eicosapentanoic acid fish oil for 4 months. European Journal of Pediatrics 2000;159(1‐2):49‐53. [PUBMED: 10653329 ] [DOI] [PubMed] [Google Scholar]
Lucas 1999 {published data only}
- Lucas A, Morley R, Stephenson T, Elias‐Jones A. Long‐chain polyunsaturated fatty acids and infant formula. Lancet 2002;360(9340):1178. [DOI: 10.1016/S0140-6736(02)11228-1] [DOI] [PubMed] [Google Scholar]
- Lucas A, Stafford M, Morley R, Abbott R, Stephenson T, Macfadyen U, et al. Efficacy and safety of long‐chain polyunsaturated fatty acid supplementation of infant‐formula milk: a randomised trial. Lancet 1999;354(9194):1948‐54. [DOI: 10.1016/S0140-6736(99)02314-4] [DOI] [PubMed] [Google Scholar]
Makrides 1995 {published and unpublished data}
- Makrides M, Neumann M, Simmer K, Pater J, Gibson R. Are long‐chain polyunsaturated fatty acids essential nutrients in infancy?. Lancet 1995;345(8963):1463‐8. [PUBMED: 7769900 ] [DOI] [PubMed] [Google Scholar]
Makrides 1999 {published and unpublished data}
- Makrides M, Neumann MA, Simmer K, Gibson RA. A critical appraisal of the role of long‐chain polyunsaturated fatty acids on neural indices of term infants: a randomised controlled trial. Pediatrics 2000;105(1 pt 1):32‐8. [PUBMED: 10617701 ] [DOI] [PubMed] [Google Scholar]
- Makrides M, Neumann MA, Simmer K, Gibson RA. Dietary long‐chain polyunsaturated fatty acids do not influence growth of term infants: a randomised clinical trial. Pediatrics 1999;104(3):468‐75. [DOI] [PubMed] [Google Scholar]
Morris 2000 {published data only}
- Morris G, Moorcraft J, Mountjoy A, Wells JC. A novel infant formula milk with added long‐chain polyunsaturated fatty acids from single‐cell sources: a study of growth, satisfaction and health. European Journal of Clinical Nutrition 2000;54(12):883‐6. [PUBMED: 11114686 ] [DOI] [PubMed] [Google Scholar]
Willats 1998 {published data only}
- Willatts P, Forsyth JS, DiModugno MK, Varma S, Colvin M. Effect of long‐chain polyunsaturated fatty acids in infant formula on problem solving at 10 months of age. Lancet 1998;352(9129):688‐91. [PUBMED: 9728984 ] [DOI] [PubMed] [Google Scholar]
- Willatts P, Forsyth S, Agostoni C, Casaer P, Riva E, Boehm G. Effects of long‐chain PUFA supplementation in infant formula on cognitive function in later childhood. American Journal of Clinical Nutrition 2013;98(2):536S‐42S. [DOI: 10.3945/ajcn.112.038612] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Agostoni 2009 {published data only}
- Agostoni C, Zuccotti GV, Radaelli G, Besana R, Podestà A, Sterpa A, et al. Docosahexaenoic acid supplementation and time at achievement of gross motor milestones in healthy infants: a randomized, prospective, double‐blind, placebo‐controlled trial. The American Journal of Clinical Nutrition 2009;89(1):64‐70. [DOI: 10.3945/ajcn.2008.26590] [DOI] [PubMed] [Google Scholar]
Birch 2002 {published data only}
- Birch EE, Hoffman DR, Castaneda YS, Fawcett SL, Birch DG, Uauy RD. A randomized controlled trial of long‐chain polyunsaturated fatty acid supplementation of formula in term infants after weaning at 6 wk of age. American Journal of Clinical Nutrition 2002;75(3):570‐80. [PUBMED: 11864865 ] [DOI] [PubMed] [Google Scholar]
Carlson 1999 {published data only}
- Carlson SE, Mehra S, Kagey WJ, Merkel KL, Diersen‐Schade DA, Harris CL, et al. Growth and development of term infants fed formulas with docosahexaenoic acid (DHA) from algal oil or fish oil and arachidonic acid from fungal oil. Pediatric Research 1999;45:278A. [10.1203/00006450‐199904020‐01656] [Google Scholar]
Clausen 1996 {published data only}
- Clausen V, Damli A, Schenck UV, Koletzko B. Influence of long‐chain polyunsaturated fatty acids (LCPUFA) on early visual acuity and mental development of term infants. Proceedings of the American Oil Chemists' Society. Barcelona, 1996.
Decsi 1995 {published data only}
- Decsi T, Koletzko B. Growth, fatty acid composition of plasma lipid classes, and plasma retinol and alpha‐tocopherol concentrations in full‐term infants fed formula enriched with omega‐6 and omega‐3 long‐chain polyunsaturated fatty acids. Acta Paediatrica 1995;84(7):725‐32. [PUBMED: 7549287 ] [DOI] [PubMed] [Google Scholar]
Field 2008 {published data only}
- Field CJ, Aerde JE, Robinson LE, Clandinin MT. Effect of providing a formula supplemented with long‐chain polyunsaturated fatty acids on immunity in full‐term neonates. The British Journal of Nutrition 2008;99(1):91‐9. [DOI: 10.1017/S0007114507791845] [DOI] [PubMed] [Google Scholar]
Field 2010 {published data only}
- Field CJ, Van Aerde JE, Goruk S, Clandinin MT. Effect of feeding a formula supplemented with long‐chain polyunsaturated fatty acids for 14 weeks improves the ex vivo response to a mitogen and reduces the response to a soy protein in infants at low risk for allergy. Journal of Pediatric Gastroenterology and Nutrition 2010;50(6):661‐9. [DOI: 10.1097/MPG.0b013e3181b99cd5] [DOI] [PubMed] [Google Scholar]
Fleddermann 2014 {published data only}
- Fleddermann M, Demmelmair H, Grote V, Nikolic T, Trisic B, Koletzko B. Infant formula composition affects energetic efficiency for growth: the BeMIM study, a randomized controlled trial. Clinical Nutrition 2014;33(4):588‐95. [DOI: 10.1016/j.clnu.2013.12.007] [DOI] [PubMed] [Google Scholar]
Gibson 2009 {published data only}
- Gibson RA, Barclay D, Marshall H, Moulin J, Maire JC, Makrides M. Safety of supplementing infant formula with long‐chain polyunsaturated fatty acids and Bifidobacterium lactis in term infants: a randomised controlled trial. British Journal of Nutrition 2009;101(11):1706‐13. [DOI: 10.1017/S0007114508084080] [DOI] [PubMed] [Google Scholar]
Jorgenson 1996 {published data only}
- Horby Jorgensen M, Holmer G, Lund P, Hernell O, Michaelsen KF. Effect of formula supplemented with docosahexaenoic acid and gamma‐linolenic acid on fatty acid status and visual acuity in term infants. Journal of Pediatric Gastroenterology and Nutrition 1998;26(4):412‐21. [PUBMED: 9552137 ] [DOI] [PubMed] [Google Scholar]
- Jorgenson MH, Hernell O, Lund P, Holmer G, Michaelson KF. Visual acuity of 4 month term infants in relation to docosahexaenoic acid intake; a randomised study. Journal of Pediatric Gastroenterology and Nutrition 1996;22(4):436. [Google Scholar]
Lapillonne 2014 {published data only}
- Lapillonne A, Pastor N, Zhuang W, Scalabrin DM. Infants fed formula with added long chain polyunsaturated fatty acids have reduced incidence of respiratory illnesses and diarrhea during the first year of life. BMC Pediatrics 2014;14:168. [DOI: 10.1186/1471-2431-14-168] [DOI] [PMC free article] [PubMed] [Google Scholar]
Meldrum 2012 {published data only}
- Meldrum SJ, D'Vaz N, Simmer K, Dunstan JA, Hird K, Prescott SL. Effects of high‐dose fish oil supplementation during early infancy on neurodevelopment and language: a randomised controlled trial. British Journal of Nutrition 2012;108(8):1443‐54. [DOI: 10.1017/S0007114511006878] [DOI] [PubMed] [Google Scholar]
NCT02092857 {published data only}
- NCT02092857. Assessment of Arachidonic Acid Supplementation in Infant Formula on the Immune Response of Infants. https://clinicaltrials.gov/ct2/show/NCT02092857?term=NCT02092857&rank=1 (first received 18 March 2014).
Patterson 2016 {published data only}
- Patterson AC, Maditz KH, Harris C, Wampler J, Kirchoff A, Zissman E. Growth and tolerance of a routine infant formula with an alternative DHA source fed to term infants. FASEB Journal 2016;30(1 Suppl):671.1. [Google Scholar]
Visentin 2016 {published data only}
- Visentin S, Vicentin D, Magrini G, Santandreu F, Disalvo L, Sala M, et al. Red blood cell membrane fatty acid composition in infants fed formulas with different lipid profiles. Early Human Development 2016;100:11‐5. [DOI: 10.1016/j.earlhumdev.2016.05.018; CN‐01177612] [DOI] [PubMed] [Google Scholar]
Voigt 2002 {published data only}
- Voigt RG, Jensen CL, Fraley JK, Rozelle JC, Brown FR 3rd, Heird WC. Relationship between omega3 long‐chain polyunsaturated fatty acid status during early infancy and neurodevelopmental status at 1 year of age. Journal of Human Nutrition and Diet 2002;15(2):111‐20. [PUBMED: 11972740 ] [DOI] [PubMed] [Google Scholar]
Additional references
Beyerlein 2010
- Beyerlein A, Hadders‐Algra M, Kennedy K, Fewtrell M, Singhal A, Rosenfeld E, et al. Infant formula supplementation with long‐chain polyunsaturated fatty acids has no effect on Bayley developmental scores at 18 months of age ‐ IPD meta‐analysis of 4 large clinical trials. Journal of Pediatric Gastroenterology and Nutrition 2010;50(1):79‐84. [DOI: 10.1097/MPG.0b013e3181acae7d] [DOI] [PubMed] [Google Scholar]
Bjerve 1992
- Bjerve KS, Bredde OL, Bonaa K, Johnson H, Vatten L, Vik T. Clinical and epidemiological studies with alpha linolenic acid and longchain n‐3 fatty acids. Essential Fatty Acids and Eicosanoids. Third International Conference on Essential Fatty Acids and Eicosanoids. Champaign (IL): American Oli Chemists' Society, 1992:173.
Clandinin 1980
- Clandinin MT, Chapell JE, Leong S, Heim T, Swyer PR, Chance GW. Intrauterine fatty acid accretion rates in human brain: implications for fatty acid requirements. Early Human Development 1980;4(2):121‐9. [PUBMED: 7408742 ] [DOI] [PubMed] [Google Scholar]
Clark 1992
- Clark KJ, Makrides M, Neumann MA, Gibson RA. Determination of the optimal ratio of linoleic acid to alpha linolenic acid in infant formulas. Journal of Pediatrics 1992;120(4 pt 2):S151‐8. [PUBMED: 1348533 ] [DOI] [PubMed] [Google Scholar]
Crawford 2015
- Crawford MA, Wang Y, Forsyth S, Brenna JT. The European Food Safety Authority recommendation for polyunsaturated fatty acid composition of infant formula overrules breast milk, puts infants at risk, and should be revised. Prostaglandins, Leukotrienes, and Essential Fatty Acids 2015;102‐103:1‐3. [DOI: 10.1016/j.plefa.2015.07.005; PUBMED: 26432509] [DOI] [PubMed] [Google Scholar]
Currie 2015
- Currie LM, Tolley EA, Thodosoff JM, Kerling EH, Sullivan DK, Colombo J, et al. Long chain polyunsaturated fatty acid supplementation in infancy increases length‐ and weight‐for‐age but not BMI to 6 years when controlling for effects of maternal smoking. Prostaglandins, Leukotrienes, and Essential Fatty Acids 2015;98:1‐6. [DOI: 10.1016/j.plefa.2015.04.001] [DOI] [PMC free article] [PubMed] [Google Scholar]
EFSA 2014
- European Food Safety Authority. Scientific opinion on the essential composition of infant and follow‐on formulae. EFSA Journal 2014;12(7):3760. [DOI: 10.2903/j.efsa.2014.3760] [DOI] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315(7109):629‐34. [PUBMED: 9310563 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fagan 1970
- Fagan JF 3rd. Memory in the infant. Journal of Experimental Child Psychology 1970;9(2):217‐26. [PUBMED: 5452116 ] [DOI] [PubMed] [Google Scholar]
Fagan 1983
- Fagan JF, Singer LT. Infant recognition memory as a measure of intelligence. In: Lipsitt LP editor(s). Advances in Infancy Research. Vol. 2, New York, NY: Ablex, 1983:31‐78. [Google Scholar]
Farquharson 1995
- Farquharson J, Jamieson EC, Abbasi KA, Patrick WJ, Logan RW, Cockburn F. Effect of diet on the fatty acid composition of the major phospholipids of infant cerebral cortex. Archives of Disease in Childhood 1995;72(3):198‐203. [PUBMED: 7741563 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fleith 2005
- Fleith M, Clandinin MT. Dietary PUFA for preterm and term infants: review of clinical studies. Critical Reviews in Food Science and Nutrition 2005;45(3):205‐29. [PUBMED: 16048149 ] [DOI] [PubMed] [Google Scholar]
Forsyth 2015
GRADEpro GDT [Computer program]
- GRADE Working Group, McMaster University. GRADEpro GDT. Version accessed 30 December 2016. Hamilton (ON): GRADE Working Group, McMaster University, 2014.
Hadley 2016
- Hadley KB, Ryan AS, Forsyth S, Gautier S, Salem N Jr. The essentiality of arachidonic acid in infant development. Nutrients 2016;8(4):216. [DOI: 10.3390/nu8040216] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. handbook.cochrane.org.
Isaacs 2010
- Isaacs EB, Fischl BR, Quinn BT, Chong WK, Gadian DG, Lucas A. Impact of breast milk on intelligence quotient, brain size, and white matter development. Pediatric Research 2010;67(4):357‐62. [DOI: 10.1203/PDR.0b013e3181d026da] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jiao 2014
Kramer 2008
- Kramer MS, Aboud F, Mironova E, Vanilovich I, Platt RW, Matush L, et al. Breastfeeding and child cognitive development: new evidence from a large randomized trial. Archives of General Psychiatry 2008;65(5):578‐84. [DOI: 10.1001/archpsyc.65.5.578] [DOI] [PubMed] [Google Scholar]
Lauritzen 2001
- Lauritzen L, Hansen HS, Jorgensen MH, Michaelsen KF. The essentiality of long chain n‐3 fatty acids in relation to development and function of the brain and retina. Progress in Lipid Research 2001;40(1‐2):1‐94. [PUBMED: 11137568 ] [DOI] [PubMed] [Google Scholar]
Lauritzen 2015
- Lauritzen L, Fewtrell M, Agostoni C. Dietary arachidonic acid in perinatal nutrition: a commentary. Pediatric Research 2015;77(1‐2):263‐9. [DOI: 10.1038/pr.2014.166; PUBMED: 25314584] [DOI] [PubMed] [Google Scholar]
Lucas 1992
- Lucas A, Morley R, Cole TJ, Lister G, Leeson‐Payne C. Breastmilk and subsequent intelligence quotient in children born preterm. Lancet 1992;339(8788):261‐4. [PUBMED: 1346280 ] [DOI] [PubMed] [Google Scholar]
Makrides 1993
- Makrides M, Simmer K, Goggin M, Gibson RA. Erythrocyte docosahexaenoic acid correlates with the visual response of the healthy, term infant. Pediatric Research 1993;33(4 pt 1):425‐7. [DOI: 10.1203/00006450-199304000-00021] [DOI] [PubMed] [Google Scholar]
Makrides 2005
- Makrides M, Gibson RA, Udell T, Ried K, International LCPUFA Investigators. Supplementation of infant formula with long‐chain polyunsaturated fatty acids does not influence the growth of term infants. American Journal of Clinical Nutrition 2005;81(5):1094‐101. [DOI] [PubMed] [Google Scholar]
McCann 2005
- McCann JC, Ames BN. Is docosahexaenoic acid, an n‐3 long‐chain polyunsaturated fatty acid, required for development of normal brain function? An overview of evidence from cognitive and behavioral tests in humans and animals. American Journal of Clinical Nutrition 2005;82(2):281‐95. [PUBMED: 16087970 ] [DOI] [PubMed] [Google Scholar]
McNamara 2015
- McNamara RK, Vannest JJ, Valentine CJ. Role of perinatal long‐chain omega‐3 fatty acids in cortical circuit maturation: Mechanisms and implications for psychopathology. World Journal of Psychiatry 2015;5(1):15‐34. [DOI: 10.5498/wjp.v5.i1.15] [DOI] [PMC free article] [PubMed] [Google Scholar]
Meldrum 2011
- Meldrum SJ, Smith MA, Prescott SL, Hird K, Simmer K. Achieving definitive results in long‐chain polyunsaturated fatty acid supplementation trials of term infants: factors for consideration. Nutrition Reviews 2011;69(4):205‐14. [DOI: 10.1111/j.1753-4887.2011.00381.x] [DOI] [PubMed] [Google Scholar]
Morrow‐Tlucak 1988
- Morrow‐Tlucak M, Haude RH, Ernhart CB. Breastfeeding and cognitive development in the first two years of life. Social Science & Medicine 1988;26(6):635‐9. [PUBMED: 3363405 ] [DOI] [PubMed] [Google Scholar]
Neuringer 1986
- Neuringer M, Connor WE, Lin DS, Barstad L, Luck S. Biochemical and functional effects of prenatal and postnatal n‐3 fatty acids on retina and brain in rhesus monkeys. Proceedings of the National Academy of Sciences of the United States of America 1986;83(11):4021‐5. [PUBMED: 3459166 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Oddy 2011
- Oddy WH, Li J, Whitehouse AJ, Zubrick SR, Malacova E. Breastfeeding duration and academic achievement at 10 years. Pediatrics 2011;127(1):e137‐45. [DOI: 10.1542/peds.2009-3489] [DOI] [PubMed] [Google Scholar]
Qawasmi 2012
- Qawasmi A, Landeros‐Weisenberger A, Leckman JF, Bloch MH. Meta‐analysis of long‐chain polyunsaturated fatty acid supplementation of formula and infant cognition. Pediatrics 2012;129(6):1141‐9. [DOI: 10.1542/peds.2011-2127] [DOI] [PMC free article] [PubMed] [Google Scholar]
Qawasmi 2013
- Qawasmi A, Landeros‐Weisenberger A, Bloch MH. Meta‐analysis of LCPUFA supplementation of infant formula and visual acuity. Pediatrics 2013;131(1):e262‐72. [DOI: 10.1542/peds.2012-0517] [DOI] [PMC free article] [PubMed] [Google Scholar]
Quin 2016
- Quin C, Erland BM, Loeppky JL, Gibson DL. Omega‐3 polyunsaturated fatty acid supplementation during the pre and post‐natal period: a meta‐analysis and systematic review of randomized and semi‐randomized controlled trials. Journal of Nutrition and Intermediary Metabolism 2016;5:34‐54. [Google Scholar]
Rogers 1978
- Rogers B. Feeding in infancy and later ability and attainment; a longitudinal study. Developmental Medicine and Child Neurology 1978;20(4):241‐6. [DOI: ] [DOI] [PubMed] [Google Scholar]
Rosenfeld 2009
- Rosenfeld E, Beyerlein A, Hadders‐Algra M, Kennedy K, Singhal A, Fewtrell M, et al. IPD meta‐analysis shows no effect of LC ‐PUFA supplementation on infant growth at 18 months. Acta Paediatrica 2009;98:91‐7. [DOI] [PubMed] [Google Scholar]
SanGiovanni 2000
- SanGiovanni JP, Berkey CS, Dwyer JT, Colditz GA. Dietary essential fatty acids, long‐chain polyunsaturated fatty acids, and visual resolution acuity in healthy full term infants: a systematic review. Early Human Development 2000;57(3):165‐88. [PUBMED: 10742608 ] [DOI] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editors. GRADE Working Group. GRADE Handbook for Grading Quality of Evidence and Strength of Recommendations. https://gdt.gradepro.org/app/handbook/handbook.html. Updated October 2013.
Sun 2015
- Sun H, Como PG, Downey LC, Murphy D, Ariagno RL, Rodriguez W. Infant formula and neurocognitive outcomes: impact of study end‐point selection. Journal of Perinatology 2015;35(10):867‐74. [DOI: 10.1038/jp.2015.87; PUBMED: 26248129] [DOI] [PubMed] [Google Scholar]
Temboury 1994
- Temboury MC, Otero A, Ploanco I, Arribas E. Influence of breastfeeding on the infant's intellectual performance. Journal of Pediatric Gastroenterology and Nutrition 1994;18(1):32‐6. [PUBMED: 8126615 ] [DOI] [PubMed] [Google Scholar]
Uauy 2003
- Uauy R, Hoffman DR, Mena P, Llanos A, Birch EE. Term infant studies of DHA and ARA supplementation on neurodevelopment: results of randomized controlled trials. Journal of Pediatrics 2003;143(4 suppl):S17‐25. [PUBMED: 14597910 ] [DOI] [PubMed] [Google Scholar]
Willatts 2013
- Willatts P, Forsyth S, Agostoni C, Casaer P, Riva E, Boehm G. Effects of long‐chain PUFA supplementation in infant formula on cognitive function in later childhood. American Journal of Clinical Nutrition 2013;98(2):536S‐42S. [DOI: 10.3945/ajcn.112.038612] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Simmer 1998
- Simmer K. Longchain polyunsaturated fatty acid supplementation of infants born at term. Cochrane Database of Systematic Reviews 1998, Issue 4. [DOI: 10.1002/14651858.CD000376] [DOI] [PubMed] [Google Scholar]
Simmer 2001
- Simmer K. Longchain polyunsaturated fatty acid supplementation of infants born at term. Cochrane Database of Systematic Reviews 2001, Issue 4. [DOI: 10.1002/14651858.CD000376] [DOI] [PubMed] [Google Scholar]
Simmer 2008
- Simmer K, Rao S, Patole S. Longchain polyunsaturated fatty acid supplementation for infants born at term. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD000376.pub2] [DOI] [PubMed] [Google Scholar]
Simmer 2011
- Simmer K, Patole SK, Rao SC. Long‐chain polyunsaturated fatty acid supplementation in infants born at term. Cochrane Database of Systematic Reviews 2011, Issue 4. [DOI: 10.1002/14651858.CD000376.pub3] [DOI] [PubMed] [Google Scholar]


