ABSTRACT
Hox temporal collinearity (TC) is a mysterious feature of embryogenesis. This article is opportune because of a recent challenge to TC’s existence This challenge is examined and the evidence that TC does exist is presented. Its function is discussed. Temporal collinearity is thought to be important because it lays the basis for Hox spatial collinearity and the vertebrate A-P axial pattern. The time-space translation mechanism whereby this occurs is examined.
KEYWORDS: Hox genes, anterior-posterior (A-P) patterning, developmental timing
Introduction
Hox Collinearity – the ordered temporal and spatial expression of Hox genes, in sequences matching their genomic order in the Hox clusters, in early vertebrate embryos, is a problem that has fascinated and intrigued biologists since it was discovered. This phenomenon is thought to be central for the embryogenesis of the human bodyplan (as with the bodyplans of all other vertebrates). In particular, Hox temporal collinearity is thought to play a crucial role. Hox collinearity is also therefore potentially extremely important for emergent areas in contemporary medicine – targeted destruction of specific cancers, and also particularly targeted stem cell therapy and in vitro culture of specific organoids that all depend on precisely identifying and manipulating the time-space addresses of cells in the developmental trajectory [1–4]. Elucidating the mechanisms regulating Hox collinearity is thus of prime importance. In this article I discuss the questions whether temporal collinearity actually exists and why it is important. The surprising conclusions help clarify the mechanism of development. This review is opportune because a recent publication has questioned whether vertebrate temporal collinearity actually exists. In fact, this relevant and provocative paper motivated the present review.
Does temporal collinearity exist?
In an interesting recent paper, Kondo et al [5] questioned whether vertebrate Hox temporal collinearity actually exists. Using normalized Hox gene expression profiles in Xenopus laevis, they defined 8 time slots for reaching half maximal expression. Group 1 (first expressed) contains only Hox1 paralogues. Group 8 (last expressed) contains only paralogue groups 11–13 (Figure1.5). The other groups contain considerable mixtures of Hox genes from different paralogue groups. They questioned that temporal collinearity exists in Xenopus laevis and stated that “though the complete set of hox genes are identified in many animals, no comprehensive analysis of temporal expression during development has been reported”. The implication is that there is currently not yet evidence for vertebrate temporal collinearity.
Figure 1.
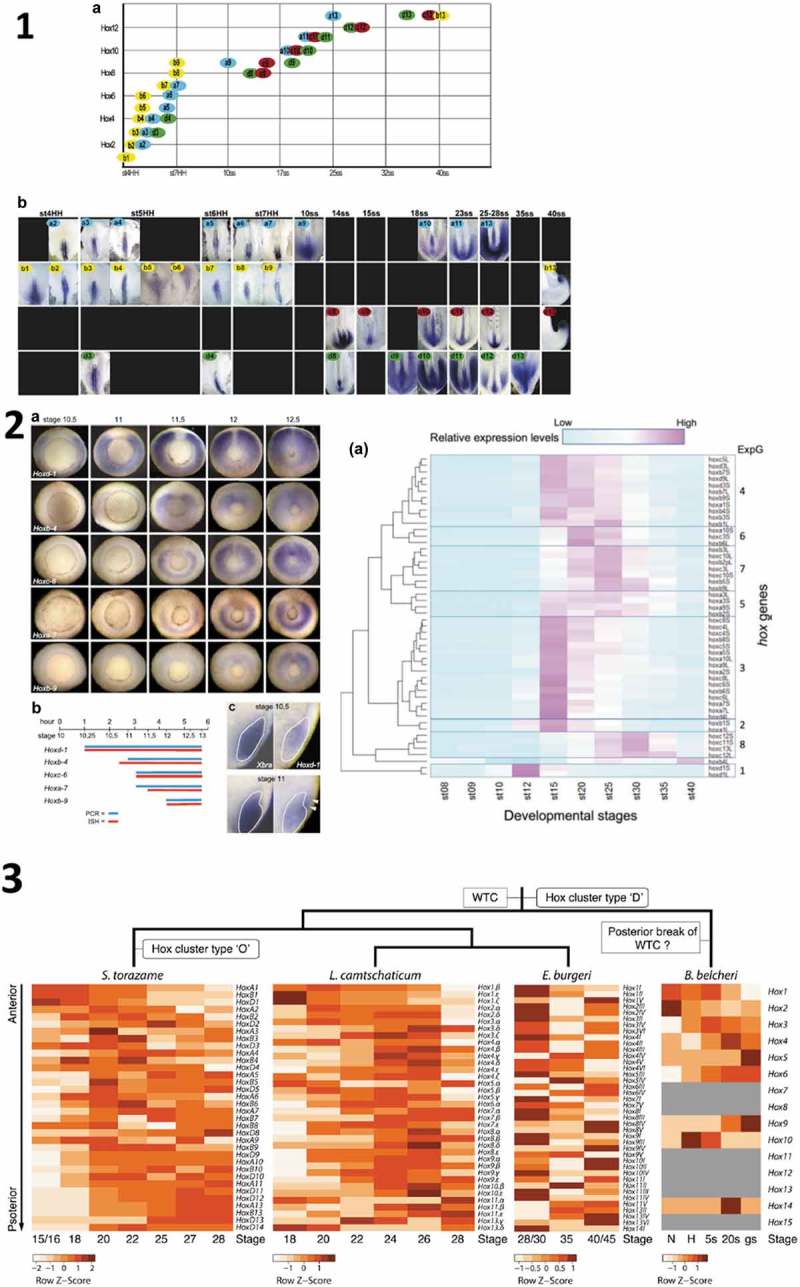
1 Temporal collinearity (TC) in chicken [12]. 1. 2 left: TC In Xenopus laevis [8]. 1. 2 right: Apparent Lack of TC in RNA Seq. data from X. laevis [5]. 1.3: WTC in Catshark, Lamprey, and STC in Cephalochordate (Brachyostoma) [13]. The chicken data in 1.1 [12] show perfect early TC but at different rates in the B,D, A clusters.
It has been reported by many other authors that TC actually does exist in vertebrates. This has been reported during early A-P axis formation in different early vertebrate embryos; both in studies predating Kondo et al. [6–12], and in later studies [13,14] (Figure1:1–4). TC is thought to be important [7,14–16]. Admittedly, TC was detected in some of the earlier studies using smaller numbers of Hox genes, in less extensive studies than the full genome study by Kondo et al. Contrary to what Kondo et al. say, these genes were never “selected” as far as we know. They were simply the Hox genes that were then cloned and available. Some studies focussed on a single Hox gene cluster. Others used Hox genes from multiple clusters. These other proposals and findings apparently conflict with Kondo et al’s postulate. What is the solution to this conflict?
The reports of temporal collinearity in vertebrates and cephalochordates are actually very comprehensive and cumulatively very impressive (12 Hox genes examined in mouse, 9 Hox genes in Xenopus, 34 Hox genes in chicken, 34 Hox genes in catshark, 34 in lamprey, 34 in hagfish and 12 in branchiostoma (cephalochordate)) (Figure 1). Four of these investigations, like Kondo’s study, are nearly whole genome studies. Cumulatively, they leave essentially no doubt at all that whole Hox cluster temporal collinearity (WTC: 13) does exist and is a general rule in vertebrates. Invertebrates, including cephalochordates, sometimes show partial temporal collinearity (sub cluster collinearity: STC) [13,17]. The only question that remains is whether Xenopus is a rare exception to the vertebrate WTC rule. We address this question (already unlikely because of previous findings [8]) and other important aspects below.
Hox expression time courses during development are complex. There are common features but there are also qualitative differences between different Hox gene expression profiles. Some Hox genes are also much less expressed than others. The complexity of Hox expression time courses evidently reflects the well documented fact that the same Hox genes have different functions at different stages in development [18,19]. Considering this, a normalized half maximal value on a total Hox expression time course (as used by Kondo et al. [5],) is perhaps not the ideal measure to assess temporal collinearity.
It has become evident to us that the key time when Hox temporal collinearity is or could be important is in very early developmental (gastrula-neurula-tailbud) stages of Hox expression, when the bodyplan of the A-P axis is laid down and before the detailed structure of the body is determined. Considering this fact, temporal collinearity can best be assessed using only initial expression in the very early parts of Hox expression profiles. At this point in time, Hox expression levels are low (>10,000–100,000 transcripts per embryo). And the early Hox expression domains contain of order 1000 cells (in the ~100,000 cell embryo). This means that each expressing cell will contain~10–100 Hox transcripts: enough to mediate a Hox function [20]. We think that at this time, temporal collinearity reflects the fact that Hox genes mediate specific early functions related to setting up the body’s axial pattern. See below.
The complexity of Hox gene expression profiles owes partly to the fact that Hox genes are expressed sequentially in different tissues. Some of the most informative studies above (in Xenopus and chicken) showed Hox temporal collinearity using in situ hybridisation. This revealed that the first expression of Hox genes occurs in NOM (non organizer mesoderm) or presomitic mesoderm (depending on the timing of Hox gene initiation) in Xenopus and in primitive streak or presomitic mesoderm in chicken [7–9,11,12] respectively and that a second phase of expression occurs in neurectoderm in both. These studies showed convincing temporal collinearity in the first tissue where the Hox genes are expressed: gastrula NOM mesoderm and presomitic mesoderm in Xenopus (9 Hox genes) [8], primitive streak -ingressing mesoderm and presomitic mesoderm in chicken (34 Hox genes) [7,9,11,12]. See Figure 1: 1–3. These studies avoided an artefact that could not be avoided by Kondo et al. [5], who used RNA seq. Namely that if the initial mesodermal phase of expression of a Hox gene is very weak and subsequent phases are strong, the initial phase may be masked, causing this Hox gene to be misplaced out of the TC sequence.
The present conflict in the literature is likely to be resolved definitively by attention to the aspects above.
What is the importance of temporal collinearity?
Vertebrate Hox temporal collinearity is potentially important because there is interest in the idea that this leads to spatial collinearity: ie. to the spatial Hox A-P axial pattern. This concept is formulated and argued in detail in the Time-Space Translation (TST) Hypothesis [8,21]. It is now the general consensus that this time-space transformation occurs [7,8,14,22]. The TST hypothesis is illustrated in Figure 2 and the correlation between the temporal and spatial sequences of Hox gene expression in spatial and temporal collinearity is shown in Table 1. Two observations pointing to TST are that the temporal sequence of Hox genes in TC and their spatial sequence in spatial collinearity (SC) are very closely correlated (Table 1) and that at a certain point in developmental time, the temporal TC pattern (travelling waves of Hox gene expression) becomes static and generates the initial spatial pattern [7,8,14]. A third indication is that the TC-SC temporal- spatial transition has been shown to be mediated by organizer action, dorsal-ventral differences, and BMP- anti BMP interactions [8]. The Xenopus temporally collinear Hox sequence starts in ventrolateral, BMP rich NOM. It is converted to a dorsal spatial pattern, after mesodermal convergence extension movements, by anti-BMP signals from the Speman organizer. These findings were originally made in Xenopus (Wacker et al., 2004) but the same mechanism is now indicated by observations in the congruent tissues in chicken and zebrafish [23,24].
Figure 2.
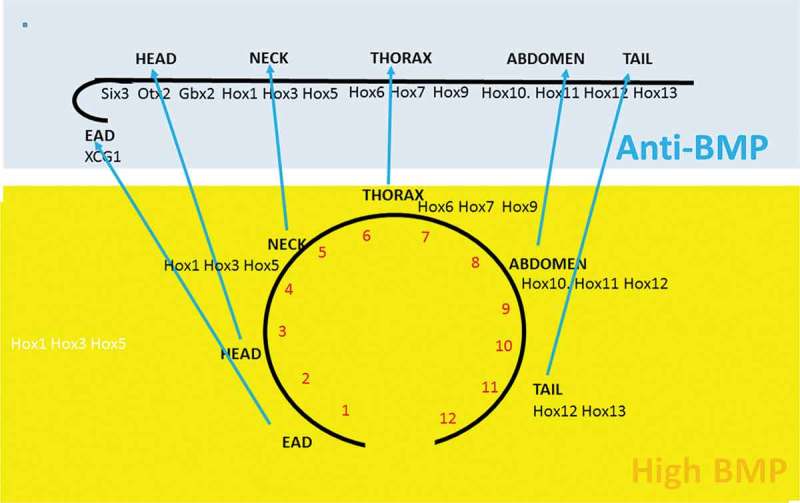
Time-Space Translation [8] A BMP dependent timer (clock) running in a high BMP tissue (Xenopus NOM mesoderm) determines potential A-P axial values as a function of time (A early-P late). The clock is stoppped sequentially by anti- BMP organizer signals as NOM cells migrate to the dorsal side of the embryo in an early to late sequence to yield an A-P sequence of stable A-P identities. The sequence is extended forward from the postereior Hox expressing trunk-tail prt of the axis to imclude the anterior head nd (most anterior) EAD [37].
Table 1.
The temporal sequence of Hox genes in temporal collinearity closely correlates with the later spatial sequence in spatial collinearity 20/23 in chicken, 9/9 in Xenopus. Red symbols indicate Hox genes out of sequence. There is turbulence in the chicken TC and SC sequences due to the different rates of TC in different Hox clusters but most of these aberrations translate from TC to SC.
| Comparison of temporal and spatial collinearities | ||||
|---|---|---|---|---|
| Denans et al., 2015Gouveia et al. 2015, Iimura and Pourquie. 2006, Gaunt and Strachan, 1996 | Burke et al. 2005 | Gouveia et al. 2015, Gaunt and Strachan. 1996 | Wacker et al. 2004 | McNulty et al. 2005 Godsave et al., 1998, Wacker et al. 2004, In der Rieden et al. 2011. Jansen et al. 2007 |
| TC chicken | SC chicken | SC chicken | TC Xenopus | SC Xenopus |
| B1 | B1 B1 | D1, A1, B1 | D1 B1, A1 | |
| B2 | B2 | |||
| B3 | B3 B3 | |||
| B4 | B4 | B4 | B4 | B4 |
| B5 | D4 | B5 | ||
| B6=A2 | B6 | C6 | C6 | |
| A3 | ||||
| A4 | A4 | |||
| D3 | ||||
| B7 | B7=A7 | B7=A7 | ||
| A5 | ||||
| D4 | ||||
| A6 | ||||
| B8 | B8 | |||
| B9 | B9 | B9 | ||
| A7 | ||||
| A9 | A9 | |||
| D8 | ||||
| C8 | C8 | B9 | B9 | |
| C9 | C9 | |||
| A10 | D9 | |||
| D9 | A10 | |||
| C10 | C10=D10 | |||
| A11 | A11 | |||
| D10 | ||||
| C11 | C11 | |||
| D11 | D11 | |||
| A13 | ||||
| C12 | ||||
| D12 | D12 | |||
| D13 | ||||
| C13 | D13 | D13 | ||
| B13 | ||||
| 34 | 12+, 3- | 8+ | 9+ | |
| 20+, 3- | 9+ | |||
| 20/23 | 9/9 | |||
The mechanism of hox collinearity depends on hox functioning
Collinearity involves and requires Hox functioning. Hox gain and loss of function experiments (Figure 3) show this unambiguously. Notably, inactivating the whole Xenopus Hox1 paralogue group downregulates or deletes expression of all later expressed, more 5ʹ posterior hox genes examined as well as all Hox1 paralogues. And this also upregulates expression of the closely neighbouring more anterior regulatory gene, Gbx2 [25]. Similarly, downregulating expression of Xenopus laevis Hoxc6 downregulates expression of all later expressed, more 5ʹposterior Hox genes tested and upregulates expression of the close anterior neighbours Hoxc4 and Hoxc5 [26]. Similarly, Hoxb1 or Hoxb3 loss of function in human teratocarcinoma cells blocks expression of all paralogues and of all more 5ʹ posterior Hox genes but not of more 3ʹanterior Hox genes [27]. In addition, partial body axes can be set up in Xenopus hyperdorsalised noggin embryos, which normally show no Hox expression and make essentially no axis, by ectopically expressing a Hox gene. If the Hox gene is Hoxd1, the axis is a near complete posterior axis, running backward from the Hoxd1 position. If Hoxb4, Hoxc6, Hoxa7 or Hoxb9 are ectopically expressed, less complete axes form, running backward in each case from the appropriate homologous Hox position. The Hoxb9 axis is thus the least complete, consisting only of abdomen and tail (Hox9-13) [28]. It appears that the Hox functions involved are collinear Hox-Hox interactions: inter- paralogue autoregulation (where a Hox gene induces itself and its paralogues: A), posterior induction (induction of more 5ʹposterior Hox genes. Possibly only posterior nearest neighbours: PI) and posterior dominance (Inhibition of function and or of expression of more 3ʹanterior Hox genes: PD). Evidence for these is in [28]. Response elements and enhancers that could possibly mediate the A and PI interactions or interctions like them have been identified in some Hox genes [29–34]. The PD interaction includes but is more extensive than an earlier proposed interaction: posterior prevalence [35]. Apart from the above indications of a role for Hox functionality (macrocollinearity) (Durston 2018), there are indications that some aspects of collinearity are confined to individual Hox clusters. Different clusters had different timing for early TC in the Denans study [12]. These observations indicate involvement of a cluster-limited aspect of collinearity control; nanocollinearity: possibly collinear chromatin opening [10,36].
Figure 3.
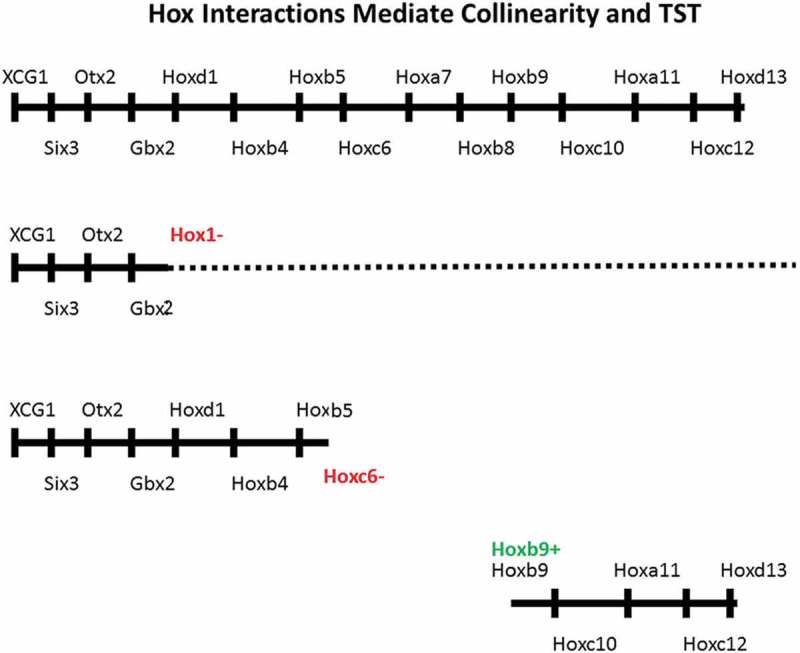
Hox Interactions mediate collinearity and TST. Above: the sequence of genes in the whole Xenopus A-P axis: Starting anteriorly with the EAD gene XAG1, going on to head genes and finishing, posteriorly, with Hox pralogue groups 1–13. Next down: Hox1 LOF: The whole posterior axis, including Hox1 is deleted or compromised. Gbx2 is upregulated (not shown) Next down: Hoxc6 LOF: The posterior axis from and including Hox6 is deleted. Hoxc4 and Hoxc5 are upregulated (not shown) Next: Hoxb9 GOF in a noggin embryo. A partial axis is generated, starting at Hox9 [5,26,28].
The data reviewed above support the idea that vertebrates show temporal collinearity. And that temporal collinearity leads to the spatial SC pattern.
Conclusion
The findings above clarify some of the confusion about Hox temporal collinearity. They contribute to a deeper understanding of Hox collinearity’s nature and why we can observe it and its role in axial patterning. Hox temporal collinearity does exist generally in early vertebrate development and it has an essential function. Details remain to be filled in but the bones of the Hox collinearity mechanism are becoming clear. The unfolding Hox collinearity mystery is clearly approaching its denouement . These insights translate into molecular genetic, time-space instructions for making different body parts in the developing embryo. Understanding the underlying mechanisms for A-P patterning enables culture in vitro of different major body regions. This is clear from gain and loss of function expts (GOF, LOF) for Hox genes and other axial regulators. Eg. LOF for Xenopus Hoxc6 cuts off the posterior axis at the neck-thorax boundary [26]: the whole embryo becomes EAD-head-neck. LOF for the Hox1 paralogue group cuts off the posterior axis at the Head/neck boundary [25]. GOF for Hoxb9 in Hoxless embryos initiates a partial posterior axis, running posteriorly from the thorax-abdomen boundary to the tip of the tail [28].These insights will be key to future stem cell and organoid culture applications because different organs form at specific A-P levels in the body. For example, the forebrain is anterior to all Hox genes, the pectoral girdle and heart are in the thorax, kidneys are in the abdomen and the sacrum is at posterior abdomen level. The findings above enable initiating development of different body regions by applying the correct instructions in vitro. They will enable initiation of simultaneous development of the different cell types characteristic for particular body regions. This represents the start of a new discipline, where detailed knowledge of the pathways regulating spatial development will enable in vitro culture of authentic organs.
Acknowledgements
I thank: Elife and O.Pourquie for permission to reproduce Fig. 1.1 from Denans et al., Elife DOI: 10.7554/eLife.04379 (2015), Elsevier for permission to reproduce 1.2 (left) from Wacker et al., Dev. Biol. 268 (2004), and Wiley for permission to reproduce 1.2 (right) from Kondo et al, Dev. Growth. Differ. 59 (2017). I thank Nature Springer for permission to reproduce Fig. 1.3, from Pascual-Anaya et al., Nat Ecol. Evol. 2 (2018)
Disclosure statement
No potential conflict of interest was reported by the author.
References
- [1].Hasty P, Ramirezsolis R, Krumlauf R, et al. Introduction of a subtle mutation into the Hox-2.6 locus in embryonic stem-cells. Nature. 1991;350(6315):243–246. [DOI] [PubMed] [Google Scholar]
- [2].Howell JC, Wells JM.. Generating intestinal tissue from stem cells: potential for research and therapy. Regen Med. 2011;6(6):743–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Lippmann ES, Williams CE, Ruhl DA, et al. Deterministic HOX patterning in human pluripotent stem cell-derived neuroectoderm. Stem Cell Reports. 2015;4(4):632–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Shah N, Sukumar S. The Hox genes and their roles in oncogenesis. Nat Rev Cancer. 2010;10(5):361–371. [DOI] [PubMed] [Google Scholar]
- [5].Kondo M, Yamamoto T, Takahashi S, et al. Comprehensive analyses of hox gene expression in Xenopus laevis embryos and adult tissues. Dev Growth Differ. 2017;59:526–539. [DOI] [PubMed] [Google Scholar]
- [6].Izpisúa-Belmonte JC, Falkenstein H, Dollé P, et al. Murine genes related to the Drosophila AbdB homeotic genes are sequentially expressed during development of the posterior part of the body. Embo J. 1991;10(8):2279–2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Gaunt S, Strachan L. Temporal colinearity in expression of anterior Hox genes in developing chick embryos. Dev Dyn. 1996;207(3):270–280. [DOI] [PubMed] [Google Scholar]
- [8].Wacker S, Jansen H, McNulty C, et al. Timed interactions between the Hox expressing non-organiser mesoderm and the Spemann organiser generate positional information during vertebrate gastrulation. Dev Biol. 2004;268(1):207–219. [DOI] [PubMed] [Google Scholar]
- [9].Iimura T, Pourquie O. Collinear activation of Hoxb genes during gastrulation is linked to mesoderm cell ingression. Nature. 2006;442:568–571. [DOI] [PubMed] [Google Scholar]
- [10].Soshnikova N, Duboule D. Epigenetic temporal control of mouse Hox genes in Vivo. Science. 2009;324:1320–1323. [DOI] [PubMed] [Google Scholar]
- [11].Gouveia A, Marcelino HM, Gonçalves L, et al. Patterning in time and space: hoxB cluster gene expression in the developing chick embryo. Cell Cycle. 2015;14(1):135–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Denans N, Iimura T, Pourquie O. Hox genes control vertebrate body elongation by collinear Wnt repression. eLife. 2015;4:e04379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Pascual-Anaya J, Sato I, Sugahara F, et al. Hagfish and lamprey Hox genes reveal conservation of temporal colinearity in vertebrates. Nat Ecol Evol. 2018;2(5):859–866. [DOI] [PubMed] [Google Scholar]
- [14].Deschamps J, Duboule D. Embryonic timing, axial stem cells, chromatin dynamics, and the Hox clock. Genes Dev. 2018;31:1406–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Monteiro AS, Ferrier DEK. Hox genes are not always Colinear. Int J Biol Sci. 2006;2(3):95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Duboule D. The rise and fall of Hox gene clusters. Development. 2007;134:2549–2560. [DOI] [PubMed] [Google Scholar]
- [17].Wang S, Zhang J, Jiao W, et al. Scallop genome provides insights into evolution of bilaterian karyotype and development. Nat Ecol Evol 2017;1:article number: 0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Weatherbee SD, Halder G, Kim J, et al. Ultrabithorax regulates genes at several levels of the wing-patterning hierarchy to shape the development of the Drosophila haltere. Genes Dev. 1998;12:1474–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Pavlopoulos A, Akam M. Hox gene Ultrabithorax regulates distinct sets of target genes at successive stages of Drosophila haltere morphogenesis. Proc Natl Acad Sci U S A. 2011;108(7):2855–2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Pare AD, Kosman D, Beaver W, et al. Transcriptional analysis of the Hox gene Scr at single molecule resolution yields evidence for transcriptional bursting during Drosophila embryogenesis. Curr Biol. 2009;19(23):2037–2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Durston AJ, Zhu K. A time space translation hypothesis for vertebrate axial patterning. Semin Cell Dev Biol. 2015;42:86–93. [DOI] [PubMed] [Google Scholar]
- [22].Duboule D. Temporal colinearity and the phylotypic progression: a basis for the stability of a vertebrate Bauplan and the evolution of morphologies through heterochrony. Dev Suppl. 1994;135–142. [PubMed] [Google Scholar]
- [23].Dias AS, de Almeida I, Belmonte JM, et al. Somites without a clock. Science. 2014. DOI: 10.1126/science.1247575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Tucker JA, Mintzer KA, Mullins MC. The BMP signaling gradient patterns dorsoventral tissues in a temporally progressive manner along the anteroposterior axis. Dev Cell. 2008;14(1):108–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].McNulty C, Peres J, van den Akker W, et al. Knockdown of the complete Hox paralogous group 1 leads to dramatic hindbrain and neural crest defects. Development. 2005;132(12):2861–2871. [DOI] [PubMed] [Google Scholar]
- [26].Zhu K, Spaink HP, Durston AJ. Hoxc6 loss of function truncates the main body axis in xenopus. Cell Cycle. 2017a;16: 1136–1138. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Faiella A, Zappavigna V, Mavilio F, et al. Inhibition of retinoic acid-induced activation of 3ʹ human HOXB genes by antisense oligonucleotides affects sequential activation of genes located upstream in the four HOX clusters. Proc Natl Acad Sci USA. 1994;91:5335–5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Zhu K, Spaink HP, Durston AJ. Collinear Hox- Hox interactions are involved in patterning the vertebrate anteroposterior (A-P) axis. PLoS ONE. 2017;12(4):e0175287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Popperl H, Bienz M, Studer M, et al. Segmental expressoion of Hoxb1 is controlled by a highly conserved utoregulatory loop dependent upon exd/pbx. Cell. 1995;81:1031–1045. [DOI] [PubMed] [Google Scholar]
- [30].Maconochie M, Nonchev S, Studer M, et al. Cross regulation in the mouse Hoxb complex: expression of Hoxb2 is regulated by Hoxb1. Genes Dev. 1997. DOI: 10.1101/gad.11.14.1885 [DOI] [PubMed] [Google Scholar]
- [31].Ferretti E, Marshall H, Pöpperl H, et al. Segmental expression of Hoxb2 in r4 requires two separate sites that integrate cooperative interactions between Prep1, Pbx and Hox proteins. Development. 2000;127:155–166. [DOI] [PubMed] [Google Scholar]
- [32].Manzanares `M, Bel-Vialar S, Ariza-McNaughton L, et al. Independent regulation of initiation and maintenance phases of Hoxa3 expression in the vertebrate hindbrain involve auto- and cross-regulatory mechanisms. Development. 2001;128:3595–3607. [DOI] [PubMed] [Google Scholar]
- [33].Kwan CT, Tsang *SL, Krumlauf R, et al. Regulatory analysis of the mouse Hoxb3 gene: multiple elements work in concert to direct temporal and spatial patterns of expression. Dev Biol. 2001;232:176–190. [DOI] [PubMed] [Google Scholar]
- [34].Tümpel S, Cambronero F, Ferretti E, et al. Expression of Hoxa2 in rhombomere 4 is regulated by a conserved cross-regulatory mechanism dependent upon Hoxb1. Dev Biol. 2007;302:646–660. [DOI] [PubMed] [Google Scholar]
- [35].Duboule D. Patterning in the vertebrate limb. Curr Opin Genet Dev. 1991;1:211–216. [DOI] [PubMed] [Google Scholar]
- [36].Noordermeer D, Leleu M, Schorderet P, et al. Temporal dynamics and developmental memory of 3D chromatin architecture at Hox gene loci. ELife. 2014;3e02557. DOI: 10.7554/eLife20557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Durston AJ. Time, space and the vertebrate body axis. Semin Cell Dev Biol. 2015;42:66–77. [DOI] [PubMed] [Google Scholar]


