ABSTRACT
Glomerular endothelial cell injury plays an important role in the development and progression of diabetic nephropathy (DN). The expression and function of klotho in glomerular endothelial cells remain unclear. Thus, this study aimed to investigate the expression and the functional role of klotho in DN progression in mice and in high glucose (HG)-induced cell injury of human renal glomerular endothelial cells (HRGECs) and the underlying mechanism. In this study, HRGECs were cultured with media containing HG to induce endothelial cell injury and db/db mice were used as DN model mice. Klotho was overexpressed or knocked down in HRECs to evaluate its role in HG-induced HRGECs injury. klotho-overexpressing adenovirus (rAAV-klotho) was injected into db/db mice via the tail vein to further validate the protective effect of klotho in DN. Decreased klotho expression was observed in DN patients, DN mice, and HG-exposed HRGECs. Furthermore, klotho overexpression significantly abolished the HG-induced HRGECs injury and activation of Wnt/β-catenin pathway and RAAS. In contrast, klotho knockdown exerted the opposite effects. Moreover, klotho attenuated diabetic nephropathy in db/db mice, which was also associated with inhibition of the Wnt/β-catenin pathway and RAAS. In conclusion, klotho attenuates DN in db/db mice and ameliorates HG-induced injury of HRGECs.
KEYWORDS: Diabetic nephropathy, klotho, HRGECs, Wnt/β-catenin, RAAS
Introduction
Diabetic nephropathy (DN) is a serious microvascular complication of diabetes and is currently the leading cause of end-stage renal disease globally [1,2]. Hyperglycemia activates various pathways and induces the release of pro-inflammatory cytokines and generation of reactive oxygen species (ROS), leading to dysfunction of renal endothelial cells, which, in turn, may further contribute to the progression of DN [2]. Increasing studies have suggested that glomerular endothelial cell injury plays an important role in the development and progression of DN [3–5]. However, at present, the exact pathogenesis of DN has not yet been completely elucidated.
The klotho gene was originally identified as a potent suppressor of aging in mice [6]. Klotho is a 1012-amino-acid single-pass transmembrane protein that is primarily expressed in the kidney [7]. Klotho acts as an endocrine substance and plays multiple roles in antioxidation, lifespan extension, insulin sensitivity regulation, and stem cell preservation [7,8]. In recent years, the roles of klotho in diabetes and renal diseases have attracted increasing attention [8–11]. Decreased klotho expression was observed in distal convoluted tubules in both humans and animals with DN [12]. The decline in plasma klotho has also been shown to predict progression of DN in patients with type 2 diabetes [13]. Up to date, studies concerning klotho in DN mainly focus on renal tubular epithelial cells; however, and the expression and function of klotho in glomerular endothelial cells remain unclear.
Klotho is an endogenous antagonist of Wnt/β-catenin signaling [14]. Wnt/β-catenin signaling pathway plays a prominent role in cell survival and apoptosis and is implicated in organ development, tumorigenesis, and tissue fibrosis [15,16]. Activation of Wnt/β-catenin signaling protects the cells against cellular damage; however, evidence has also shown that aberrant activation of this pathway may contribute to the development of DN, which indicated the dual role of Wnt/β-catenin signaling under DN conditions [15]. The renin-angiotensin-aldosterone system (RAAS) is a hormone system that regulates blood pressure and fluid balance. Inhibition of RAAS plays a pivotal role in the treatment of chronic kidney diseases (CKD) [17]. It has been shown that blockade of Wnt/β-catenin signaling can inhibit RAAS genes, thereby reversing kidney injury [18].
Based on the aforementioned studies, we hypothesized that klotho can inhibit oxidative stress and inflammatory response and inhibit high glucose (HG)-induced glomerular endothelial cell injury by inhibiting Wnt/β-catenin signaling pathway and RAAS.
Results
Decreased klotho expression in DN patients
Compared with the normal renal tissues around the tumor from patients with kidney carcinoma without diabetes, DN renal tissues showed significantly decreased expression of klotho, both at mRNA (Figure 1(a)) and protein (Figure 1(b)) level. The data of IHC analysis further validated the results of RT-qPCR and western blot (Figure 1(c)). Taken together, these results indicated that downregulation of klotho was correlated with DN pathogenesis.
Figure 1.
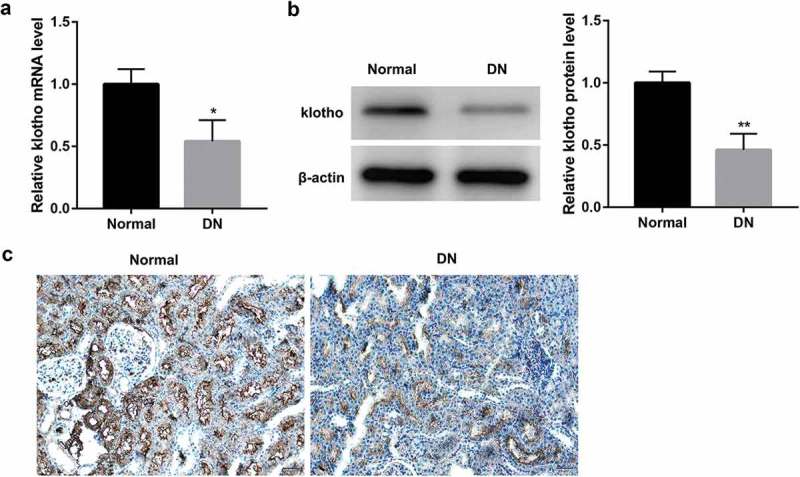
Decreased klotho expression in DN patients.
The mRNA level of klotho measured by RT-qPCR (a), the protein level of klotho measured by western blot (b), and representative images of immunohistochemical staining for klotho in the renal tissues from patients with diabetic nephropathy (DN, n = 10) compared with the normal tissues around the tumor from patients with kidney carcinoma without diabetes (Normal, n = 10) (c). *p < 0.05, **p < 0.01 vs. the normal group.
Klotho overexpression significantly abolished the HG-induced HRGECs injury
We next assessed the effect of high glucose on klotho expression. Data revealed that HG treatment significantly decreased the mRNA (Figure 2(a)) and protein (Figure 2(c)) expression of klotho in HRGECs, as well as level of klotho (Figure 2(b)) secreted from HRGECs.
Figure 2.
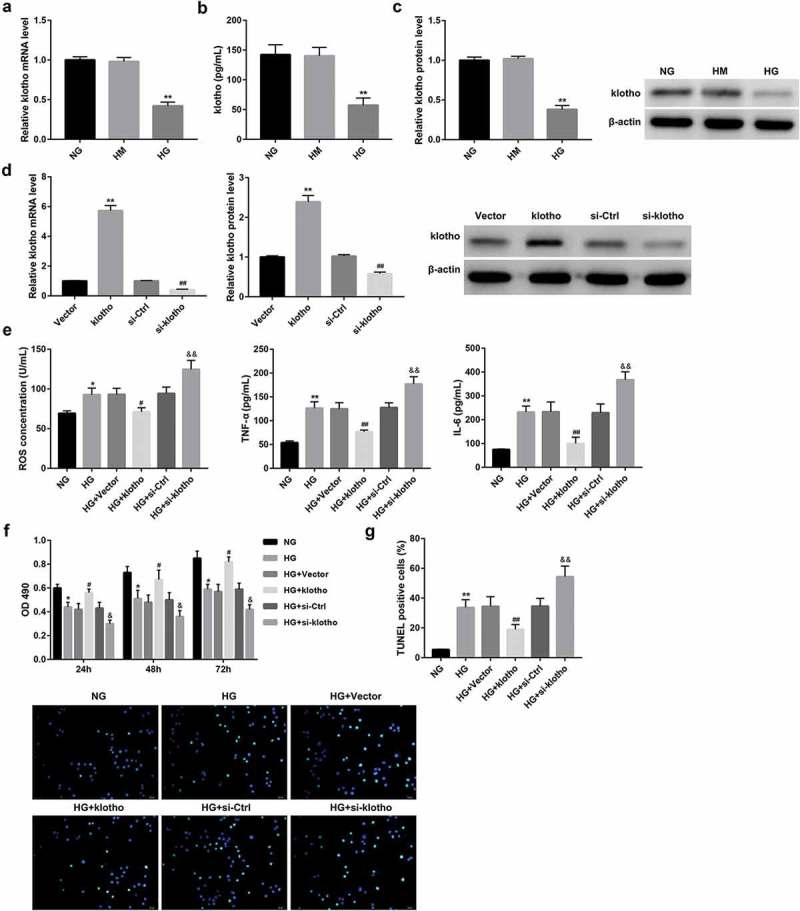
Klotho overexpression significantly abolished the HG-induced HRGECs injury.
The mRNA (a) and protein (c) expression of klotho in HRGECs, as well as secretion of klotho (b) from HRGECs which were cultured with NG (5.6 mmol/L D-glucose), HM (5.6 mmol/L D-glucose plus 14.4 mmol/L mannitol), and HG (20 mmol/L D-glucose) for 24 h, were examined by RT-qPCR, western blot, and ELISA, respectively. **p < 0.01 vs. the HM group. In another experiment, HRGECs were transfected with pcDNA3.1-klotho, empty pcDNA3.1 vector, si-klotho, or si-Ctrl followed by HG stimulation for 24 h. (d) The overexpression and knockdown efficiency of klotho in HRGECs 48 h post-transfection was confirmed by RT-qPCR and western blot. **p < 0.01 vs. the vector group, ##p < 0.01 vs. the si-Ctrl group. The effect of klotho expression on levels of ROS, TNF-α, and IL-6 (e) in HRGECs, plus HRGECs proliferation (f) and apoptosis (g) was examined using ELISA, MTT, and TUNEL staining method, respectively. Data are presented as the means ± SD (n = 3). *p < 0.05, **p < 0.01 vs. the NG group, #p < 0.05, ##p < 0.01 vs. the HG+vector group, &p < 0.05, &&p < 0.01 vs. the HG+si-Ctrl group.
We next verified the potential role of klotho expression on HG-induced HRGECs injury. To address this, we transfected HRGECs with pcDNA3.1-klotho, si-klotho, or their corresponding controls. The overexpression and knockdown efficiency of klotho were confirmed by both RT-qPCR and western blot (Figure 2(d)). The results also showed that HG treatment induced cell injury in HRGECs, as evidenced by a notable increase in levels of ROS, TNF-α, and IL-6 in HRGECs (Figure 2(e)) and apoptosis of HRGECs (Figure 2(g)), but a decrease in proliferation of HRGECs (Figure 2(f)). Importantly, klotho overexpression significantly abolished, whereas klotho knockdown enhanced the HG-induced HRGECs injury (Figure 2(e-g)).
Klotho overexpression abrogated the HG-induced activation of Wnt/β-catenin and RAAS
Western blot analyses revealed that protein levels of the Wnt/β-catenin pathway-related proteins (Wnt1, β-catenin, p-GSK3β, cyclin D1, and c-myc) and RAAS-related proteins (Ang-II, AT1R, ET-1, ETAR, and MR) in HREGCs were significantly upregulated by HG stimulation (Figure 3(a,b)). Furthermore, HG stimulation notably enhanced secretion of Ang-II from HRGECs (Figure 3(c)). Of note, the activation of Wnt/β-catenin pathway and RAAS was significantly abrogated by klotho overexpression but enhanced by klotho knockdown. Collectively, these data indicated the potential involvement of Wnt/β-catenin pathway and RAAS in klotho-mediated attenuation of HRGECs injury under HG stimulation.
Figure 3.

Klotho overexpression abrogated the HG-induced activation of Wnt/β-catenin and RAAS.
HRGECs were transfected with pcDNA3.1-klotho, empty pcDNA3.1 vector, si-klotho, or si-Ctrl followed by HG stimulation (20 mmol/L D-glucose) for 24 h. (a-b) Western blot was performed to examine protein expression of the Wnt1/β-catenin pathway-related proteins (Wnt1, β-catenin, p-GSK3β, cyclin D1, and c-myc) and RAAS-related proteins (Ang-II, AT1R, ET-1, ETAR, and MR). (c) ELISA was performed to evaluate the level of Ang-II secreted from HRGECs. Data are presented as the means ± SD (n = 3). *p < 0.05, **p < 0.01 vs. the NG group, #p < 0.05, ##p < 0.01 vs. the HG+vector group, &p < 0.05, &&p < 0.01 vs. the HG+si-Ctrl group.
Klotho attenuated HG-induced HRGECs injury through inhibiting Wnt/β-catenin pathway and RAAS
To further validate whether klotho attenuated HG-induced HRGECs injury through inhibiting Wnt/β-catenin and RAAS, SKL2001 (an activator of Wnt signaling), atrasentan (a selective ETAR antagonist) or finerenone (an MR antagonist) were added into the klotho-overexpressing HRGECs 1 h before HG stimulation for 24 h. Data revealed that SKL2001 significantly restored the klotho-mediated inhibition of levels of ROS, iNOS, TNF-α, and IL-6 (Figure 4(a)) and apoptosis of HRGECs (Figure 4(c)), whereas inhibited and the klotho-mediated promotion of HRGECs proliferation (Figure 4(b)). These results indicated that SKL2001 reversed the klotho-mediated attenuation of HRGECs injury under HG stimulation. Furthermore, both atrasentan and finerenone effectively enhanced the klotho-mediated attenuation of HRGECs injury under HG stimulation (Figure 4(a-c)).
Figure 4.
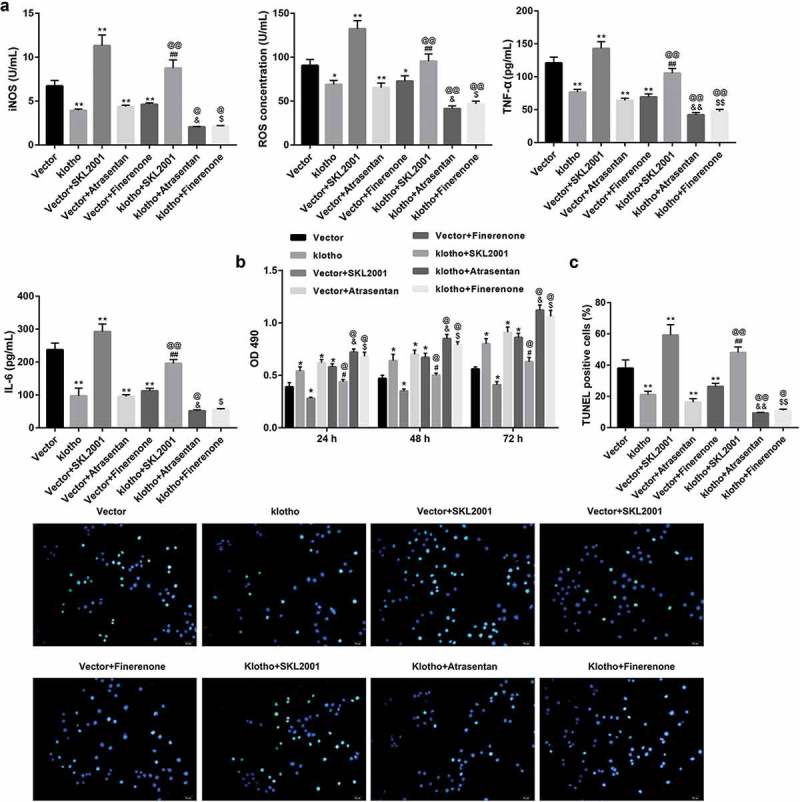
Klotho attenuated HG-induced HRGECs injury through inhibiting Wnt/β-catenin pathway and RAAS.
HRGECs were transfected with pcDNA3.1-klotho and empty pcDNA3.1 vector; and SKL2001 (an activator of Wnt signaling), atrasentan (a selective ETAR antagonist) or finerenone (a MR antagonist) were added into the transfected HRGECs 1 h before HG stimulation. After 24 h of incubation in HG medium, concentrations of ROS, iNOS, TNF-α, and IL-6 in HRGECs were examined using ELISA method (a), cell proliferation (b) and apoptosis (c) of HRGECs were assessed by MTT and TUNEL staining method, respectively. Data are presented as the means ± SD (n = 3). *p < 0.05, **p < 0.01 vs. the vector group, @p <0.05, @@p < 0.01 vs. the klotho group, #p < 0.05, ##p < 0.01 vs. the vector+SKL2001 group, &p < 0.05, &&p < 0.01 vs. the vector+atrasentan group, $p < 0.05, $$p < 0.01 vs. the vector+ finerenone group.
Klotho attenuates diabetic nephropathy in db/db mice
To further in vivo validate the protective effects of klotho in DN, we injected rAAV-klotho into db/db mice (DN model mice) via the tail vein. Compared with the control db/m mice, DN mice exhibited higher levels of 24-h urine protein, KW, and KHI (Figure 5(a)), concentrations of ROS and MDA in renal tissues (Figure 5(b)), Ang-II, ET-1, TNF-α, and IL-6 in sera (Figure 5(c)). However, the BW (Figure 5(a)), renal SOD concentration (Figure 5(b)), and serum klotho (Figure 5(c)) were significantly lower in the DN group than the control group. Furthermore, renal tissues in the DN group showed notable glomerular swelling, renal tubular vacuolar degeneration (Figure 6(a)) and increased renal collagen deposition compared with the control group (Figure 6(b)). Of note, the injection of rAAV-klotho into db/db mice significantly ameliorated the DN-associated oxidative stress, inflammation, and pathological alterations. Moreover, the renal tissues from the DN mice showed notably lower protein level of klotho, but higher protein levels of the Wnt1/β-catenin pathway-related proteins and RAAS-related proteins when compared with the control mice (Figure 6(c)). The results also showed that rAAV-klotho injection significantly inhibited protein levels of the Wnt1/β-catenin pathway-related proteins and RAAS-related proteins. The effect of atrasentan and finerenone was similar with rAAV-klotho (Figures 5 and 6).
Figure 5.
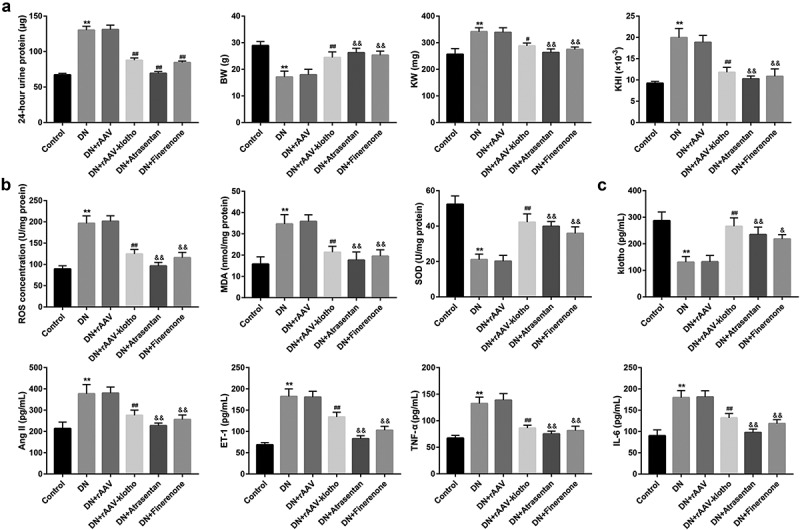
Klotho attenuated the DN-associated oxidative stress and inflammation in db/db mice.
The db/db mice were randomly divided into the five groups (n = 6/each group): the DN model group (db/db mice), DN+rAAV group, DN+rAAV-klotho group, DN+atrasentan group, and DN+finerenone group. The db/m mice served as the control group. (a) The 24-h urine protein, the body weight (BW), the bilateral kidney weight (KW), and the kidney hypertrophy index (KHI; KHI = KW/BW) were shown. The concentrations of ROS, MDA, and SOD in kidney tissues (b), and klotho, Ang-II, ET-1, TNF-α, and IL-6 in mouse sera (c) were measured by ELISA. **p < 0.01 vs. the control group, #p < 0.05, ##p < 0.01 vs. the DN+rAAV group, &p < 0.05, &&p < 0.01 vs. the DN group.
Figure 6.
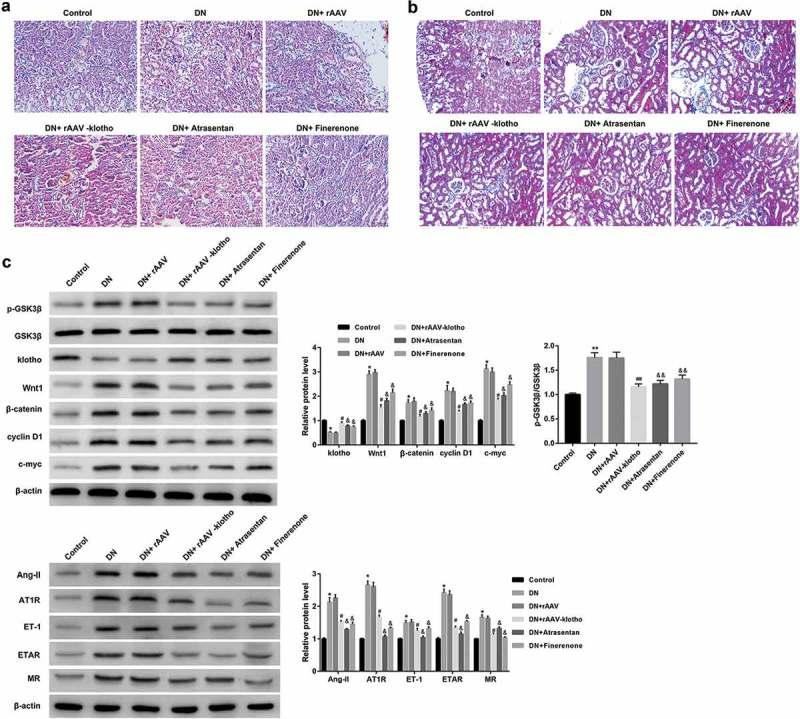
Klotho attenuated the DN-associated pathological alterations and inhibited Wnt1/β-catenin pathway and RAAS in db/db mice.
Representative images of hematoxylin and eosin (HE) staining (a) and Masson trichrome staining (b) of mouse renal tissues were shown. (c) The protein levels of klotho, the Wnt1/β-catenin pathway-related proteins (Wnt1, β-catenin, p-GSK3β, cyclin D1, and c-myc) and RAAS-related proteins (Ang-II, AT1R, ET-1, ETAR, and MR) were examined by western blot. *p < 0.05, **p < 0.01 vs. the control group, #p < 0.05, ##p < 0.01 vs. the DN+rAAV group; &p < 0.05, &&p < 0.01 vs. the DN group.
Discussion
In recent years, the roles of klotho in renal diseases have attracted increasing attention [8–11]. Decreased renal klotho expression has been observed in various experimental mouse models of kidney diseases and humans with chronic kidney diseases, including DN [12,19,20]. Furthermore, the decline in plasma klotho has also been shown to predict progression of DN in patients with type 2 diabetes [13]. Prior studies have shown that klotho deficiency correlates with progression and complications in chronic kidney disease including cardiac hypertrophy, vascular calcification, and secondary hyperparathyroidism [21,22]. In vitro and in vivo experiments have demonstrated that Klotho can attenuate glomerular hypertrophy and glomerular injury [23,24] in DN. For example, Lin et al. [19] have demonstrated that deficiency exacerbates early DN in STZ-induced diabetic mice. Kang et al. [8] have also shown that the increased serum klotho that was mediated by miR-199b-5p is a possible mechanism by which atrasentan prevents renal tubular injury in DN.
Despite these observations as described above, the functional role of klotho in glomerular endothelial cells remains to be elucidated. Increasing studies have suggested that glomerular endothelial cell injury contributes to the progression of DN [3–5]. The findings in this study demonstrate that klotho overexpression significantly abolished the HG-induced HRGECs injury and attenuated DN progression in db/db mice. Our study further supports the protective role of klotho in kidney function in DN.
Klotho protein exists in two forms: membrane klotho and secreted klotho. Membrane klotho functions as an obligate co-receptor for fibroblast growth factor-23 (FGF23), whereas secreted klotho functions as a humoral factor independently of FGF23 [25]. Secreted Klotho is a known multifunctional protein that inhibits signaling pathways of TGF-β1, Wnt, and insulin/insulin-like growth factor-1 (IGF-1). [25–28]. klotho acts as an inhibitor of the Wnt/beta-catenin pathway [29], and loss of klotho contributes to kidney injury by derepression of Wnt/β-catenin signaling [14]. Another study also indicated that transplantation of adipose-derived mesenchymal stem cells might attenuate renal injury in streptozotocin-induced DN via activating klotho and thus inhibiting the Wnt/β-catenin pathway [30]. In accordance with these observations, the results in this study revealed that klotho overexpression significantly abolished the HG-induced activation of Wnt/β-catenin pathway, as confirmed by reduced expression of Wnt1, β-catenin, p-GSK3β, and two known target genes of the Wnt/β-catenin pathway c-myc and cyclin D1.
Evidence has shown that aberrant activation of this pathway may contribute to the development of DN [15]. Wnt/β-catenin signaling is reactivated after renal injury in various kidney disorders including DN [15,31]. Furthermore, klotho expression is negatively correlated with the expression of Wnt/β-catenin signaling [14]. Moreover, HG can activate Wnt/β-catenin signaling in tubular epithelial cells [32]. The results in this study showed that Wnt/β-catenin pathway is activated in renal tissues of DN mice and in HG-exposed HRGECs.
RAAS play pivotal roles in the regulation of blood pressure, electrolytes, and fluid homeostasis. The RAAS genes consist of renin, angiotensinogen (AGT), angiotensin converting enzyme (ACE), angiotensin I (Ang I), angiotensin II (Ang-II), angiotensin II type 1 receptor (AT1R), and angiotensin II type 2 receptor (AT2R) [33,34]. Inhibition of RAAS plays a pivotal role in the treatment of kidney diseases [17]. In this study, the results showed that RAAS was activated in renal tissues of DN mice and in HG-induced HRGECs, which was inhibited by klotho overexpression. It has been shown that overexpression of either β-catenin or different Wnt ligands induces the expression of all RAAS genes [18]. Meanwhile, blockade of Wnt/β-catenin signaling can inhibit RAAS genes, thereby reversing kidney injury [18]. Thus, the protective role of klotho in DN may be due to its inhibition of Wnt/β-catenin signaling and RAAS.
In conclusion, the present study demonstrates that klotho overexpression significantly abolishes the HG-induced HRGECs injury and attenuates diabetic nephropathy in db/db mice. To a certain extent, these results may be due to the klotho-mediated inhibition of Wnt/β-catenin pathway and RAAS. The findings provide a further understanding of the role of klotho in DN progression.
Materials and methods
Collection of human renal tissues
Human renal tissues were collected from patients in the Second Affiliated Hospital of Nanchang University. Human experimental procedures were conducted in accordance with the protocol approved by the Clinical Research Ethics Committee of the Second Affiliated Hospital of Nanchang University. Written informed consent was obtained from all patients.
DN renal tissues were collected from patients with DN which were diagnosed depending on the presence of diabetes, massive proteinuria, and other histological changes typical of DN (n = 10). Normal tissues around the tumor from patients with kidney carcinoma without diabetes served as the normal control (n = 10). The renal tissues in each group were prepared for detection of klotho expression using real-time quantitative reverse transcription PCR (RT-qPCR), western blot, and immunohistochemical analyses.
Immunohistochemistry
Immunohistochemistry (IHC) was performed to assess klotho expression in renal tissues in each group as previously described [9]. The 2-μm thickness sections were deparaffinized in xylene and hydrated using an ethanol-deionized water series, followed by 15 min of treatment with 3% H2O2 in methanol to block endogenous peroxidase activity. After being washed three times, sections were stained with the anti-klotho antibody (1: 500; MAB1819; R&D Systems, USA). Slides were counterstained with hematoxylin.
Animals
Specific pathogen-free (SPF) BKS.Cg-m+/+Leprdb/J (db/db) mice (n = 30) and their matched heterozygotes (db/m) mice (n = 6) were purchased from Model Animal Research Center of Nanjing University (Nanjing, China). All mice were kept under constant temperature and humidity with 12 h light-dark cycles, and had free access to food and water at a temperature of 25°C ± 1°C and humidity of 50%. The animal experiment was approved by Ethics Committee of the Second Affiliated Hospital of Nanchang University.
Animal experiments
The db/db mice were used as DN model mice, and the db/m mice were as the normal control mice. When urinary microalbumin levels in db/db mice were significantly higher than those in db/m mice, db/db mice were randomly grouped into the following five groups (n = 6/each group): the DN model group (db/db mice), DN+rAAV group (rAAV vectors were injected into db/db mice via tail vein at a dose of 3 × 108 particles/mouse), DN+rAAV-klotho group (rAAV-klotho was injected into db/db mice via tail vein at a dose of 3 × 108 particles/mouse), DN+atrasentan group (ETAR antagonist atrasentan was administered into db/db mice by oral gavage at a dose of 5 mg/kg/d), DN+finerenone group (MR antagonist finerenone was administered into db/db mice by oral gavage a dose of 10 mg/kg/d). The recombinant adeno-associated virus (rAAV) vector containing the mouse klotho gene (rAAV-klotho) was constructed as previously described [10].
The 24-h urine samples were collected from the mice in metabolic cages, and the 24-h urine protein was detected using an automated biochemical analyzer (DADE Xpand, USA). After 8 wk of injection, the mice were sacrificed under anesthesia.
Blood was collected and centrifuged at 2,500 rpm for 20 min. The resulting upper layer of serum was collected and stored at −80°C until ELISA analysis. The renal tissue samples of these mice were collected and prepared for the subsequent experiments. The bilateral kidney weight (KW) and the body weight (BW) were weighed using an electronic balance, and the kidney hypertrophy index (KHI) was calculated according to the formula: KHI = KW/BW. A portion of kidneys was cut into sections and fixed in 4% buffered paraformaldehyde for hematoxylin and eosin (HE) staining and Masson staining. The remaining kidneys were stored at −80°C before being used for western blot and ELISA.
Kidney histology
HE staining and Masson trichrome staining were performed to evaluate the renal pathological changes, glomerular morphological changes and renal fibrosis in the mouse kidneys. Kidney sections were fixed in 4% buffered paraformaldehyde, embedded in paraffin, and then sectioned at 4 μm thickness. The resulting sections were prepared for HE and Masson staining according to standard protocols. All sections were evaluated using a light microscope (Olympus BH-2; Olympus Corporation, Japan).
Cell culture
HRGECs were purchased from ScienCell Research Laboratories (USA). HRGECs were maintained in Dulbecco’s Modified Eagle’s medium (DMEM)/F12 (Gibco, USA) containing 10% fetal bovine serum (FBS; Gibco), 100 μg/ml streptomycin, and 100 IU/ml penicillin, at 37°C in a humidified air with 5% CO2. Cells were cultured in serum-free media for 24 h before each experiment.
In vitro experimental protocol
To assess the effect of glucose on klotho expression, after 24 h of incubation, the culture medium of HRGECs was replaced with medium containing normal glucose (NG group; 5.6 mmol/L D-glucose), high mannitol (HM, osmotic control group; 5.6 mmol/L D-glucose plus 14.4 mmol/L mannitol) or high glucose (HG group; 20 mmol/L D-glucose). After 24 h of incubation, the mRNA and protein expression of klotho in HRGECs, as well as secretion of klotho from HRGECs was examined by RT-qPCR, western blot, and ELISA, respectively.
In another experiment, to determine the effect of klotho expression on HG-induced HRGECs injury, Wnt/β-catenin pathway, and RAAS, HRGECs were transfected with pcDNA3.1-klotho, si-klotho, or their corresponding controls. After 24 h of incubation in HG medium, cells were subjected to the following experiments: determination of ROS concentration, measurement of levels of pro-inflammatory cytokines TNF-α and IL-6, cell proliferation assay, cell apoptosis assay, and determination of expression of the Wnt/β-catenin pathway-related proteins and RAAS-related molecules.
In another experiment, to investigate whether Wnt/β-catenin pathway and RAAS were involved in klotho-mediated abolishment of HREGCs injury under HG stimulation, we transfected HRGECs with pcDNA3.1-klotho and empty pcDNA3.1 vector; and SKL2001 (an activator of Wnt signaling; 40 μM in DMSO; Selleck, USA), atrasentan (also known as ABT-627, a selective ETAR antagonist; 1 μM in DMSO; Sigma-Aldrich, USA) or finerenone (an MR antagonist; 10 nM in DMSO; Bayer Pharma AG, Germany) were added into the transfected cells 1 h before HG stimulation. After 24 h of incubation in HG medium, cells were subjected to the following experiments: determination of concentrations of ROS and iNOS, measurement of levels of pro-inflammatory cytokines TNF-α and IL-6, cell proliferation assay, and cell apoptosis assay.
Plasmid construction and cell transfection
To overexpress klotho, the klotho cDNA fragments were cloned into the pcDNA 3.1 vector (Invitrogen, USA), generating pcDNA3.1-klotho. An empty pcDNA3.1 vector served as the control. To knockdown klotho, small interfering RNA (siRNA)-klotho (si-klotho) and a scramble control siRNA (si-Ctrl) were designed and synthesized by GenePharma (Shanghai, China). When HRGECs reached 75%-80% confluence, cells were transfected with these vectors using LipofectamineTM 3000 (Invitrogen) and siRNAs using Lipofectamine™ RNAiMAX Transfection Reagent (Invitrogen), respectively, according to the manufacturer’s instructions. After 48 h of transfection, cells were harvested for qRT-PCR and western blot analysis to examine the overexpression or knockdown efficiency.
RT-qPCR
Total RNA was extracted from human renal tissues or HRGECs using a TRIzol reagent (Invitrogen) following the manufacturer’s instructions. Subsequently, RNA was reverse transcribed into cDNAs using the iScript cDNA synthesis kit (Bio-Rad, USA). The cDNAs were amplified through RT-qPCR by SYBR Green Real-time PCR Master Mix (TOYOBO, Japan). The specific primers were as follows: klotho forward: 5ʹ-AACTACATTCAAGTAAGTCAGC-3ʹ, klotho reverse: 5ʹ-CAGAGTGGTATCTACTAGTG-3ʹ. β-actin forward, 5ʹ-GTGGACATCCGCAAAGAC-3ʹ, β-actin reverse, 5ʹ-AAAGGGTGTAACGCAACTA-3ʹ. The relative expression of candidate genes was calculated by the 2−ΔΔCt method and normalized to the internal control β-actin.
Western blot
Western blot was performed to examine protein expression of klotho, the Wnt/β-catenin pathway-related proteins, and RAAS-related proteins. Briefly, human or mouse renal tissues or cultured HRGECs in vitro were extracted in RIPA lysis buffer (Beyotime, Shanghai, China). Then, equal protein from cell lysates was separated by 10% SDS-PAGE gels and transferred onto PVDF membrane (Millipore, USA). Afterwards, the membrane was blocked with 5% non-fat milk and then incubated at 4°C overnight with the following primary antibodies against: Klotho (R&D Systems), Wnt (Abcam, USA), β-catenin (Cell Signaling Technology, USA), phosphorylated glycogen synthase kinase-3β (p-GSK3β, Cell Signaling Technology), cyclin D1 (Cell Signaling Technology), c-myc (Santa Cruz Biotechnology, USA), Angiopoietin-II (Ang-II; Santa Cruz Biotechnology), angiotensin II type 1 receptor (AT1R; Abcam), endothelin 1 (ET-1; Abcam), endothelin A receptor (ETAR; Abcam), and mineralocorticoid receptor (MR; Abcam), followed by horseradish peroxidase (HRP)-labeled secondary antibodies (Beyotime). The protein analysis was visualized by Quantity One software (Bio-Rad, USA). β-actin served as the loading control.
Enzyme-linked immunosorbent assay (ELISA)
The levels of klotho (LifeSpan BioSciences, USA), TNF-α (R&D Systems), IL-6 (R&D Systems), Ang-II (LifeSpan BioSciences), iNOS (LifeSpan BioSciences), and ROS (LifeSpan BioSciences) in cell culture fluid of HRGECs were measured using their commercial ELISA kits according to the manufacturer’s instructions.
The levels of SOD, malondialdehyde (MDA), and ROS in renal tissues from mice were measured using their commercial ELISA kits (MyBioSource, USA) according to the manufacturer’s instructions.
The serum levels of klotho (LifeSpan BioSciences), Ang-II (LifeSpan BioSciences), ET-1 (LifeSpan BioSciences), TNF-α (R&D Systems), and IL-6 (R&D Systems) in mice were measured using their commercial ELISA kits according to the manufacturer’s instructions.
Cell proliferation assay
The HRGCs proliferation was assessed by an MTT assay kit (Beyotime) according to the manufacturer’s instructions. Briefly, the treated cells (at a density of 5 × 103 cells/well) were seeded into 96-well plates and cultured for 24 h. Afterward, MTT (20 μL; 5 mg/mL) was added into each well for another 4 h of incubation at 37°C. Then, the culture medium was replaced with DMSO (150 μL) to solubilize the crystals for 10 min. Cellular viability was determined by measuring the optical density (OD) at 490 nm by a spectrophotometer (Multiskan MK3, Thermo, USA). Cellular viability was normalized to control well.
Cell apoptosis assay
The HRGECs apoptosis was evaluated by In Situ Cell Death Detection Kit, Fluorescein Kit (Roche, USA) based on TUNEL technology according to the manufacturer’s instructions. The images of the FITC-labeled TUNEL-positive cells were captured using fluorescent microscopy (Nikon Corporation, Japan). The nick-ends were labeled in green indicating the apoptotic cells, and the cell nucleus was labeled in blue by DAPI (Invitrogen). A merge between the nick-ends (green) and nucleus (blue) labeling showed up as purple.
Statistical analysis
All statistical analyses were performed using SPSS 16.0 for Windows (SPSS, Inc., USA). The data are presented as the mean ± standard deviation (SD) from three independent experiments. The unpaired Student’s t-test was used to analyze differences between the two groups. One-way analysis of variance (ANOVA) was used to analyze differences among three or more groups. p < 0.05 was considered statistically significant.
Funding Statement
This work was supported by the Supporting Project for the Foregoers of Main Disciplines of Jiangxi Province (No. 20162BCB22023), the “5511” Innovative Drivers for Talent Teams of Jiangxi Province (No. 20165BCB18018), and the Nature Science Foundation of Jiangxi Province (No. 20181BAB205016).
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].Magee C, Grieve DJ, Watson CJ, et al. Diabetic nephropathy: a tangled web to unweave. Cardiovasc Drugs Ther. 2017;31:579–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Cheng H, Harris RC.. Renal endothelial dysfunction in diabetic nephropathy. Cardiovasc Hematol Disord Drug Targets. 2014;14:22–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Fu J, Lee K, Chuang PY, et al. Glomerular endothelial cell injury and cross talk in diabetic kidney disease. Am J Physiol Renal Physiol. 2015;308:F287–F297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Eleftheriadis T, Pissas G, Antoniadi G, et al. Allopurinol protects human glomerular endothelial cells from high glucose-induced reactive oxygen species generation, p53 overexpression and endothelial dysfunction. Int Urol Nephrol. 2018;50:179–186. [DOI] [PubMed] [Google Scholar]
- [5].Eleftheriadis T, Tsogka K, Pissas G, et al. Activation of general control nonderepressible 2 kinase protects human glomerular endothelial cells from harmful high-glucose-induced molecular pathways. Int Urol Nephrol. 2016;48:1731–1739. [DOI] [PubMed] [Google Scholar]
- [6].Kuro-O M. Klotho in health and disease. Curr Opin Nephrol Hypertens. 2012;21:362–368. [DOI] [PubMed] [Google Scholar]
- [7].Hu MC, Moe OW. Klotho as a potential biomarker and therapy for acute kidney injury. Nat Rev Nephrol. 2012;8:423–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kang WL, Xu GS. Atrasentan increased the expression of klotho by mediating miR-199b-5p and prevented renal tubular injury in diabetic nephropathy. Sci Rep. 2016;6:19979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Cho NJ, Han DJ, Lee JH, et al. Soluble klotho as a marker of renal fibrosis and podocyte injuries in human kidneys. PloS one. 2018;13:e0194617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Deng M, Luo Y, Li Y, et al. Klotho gene delivery ameliorates renal hypertrophy and fibrosis in streptozotocin-induced diabetic rats by suppressing the Rho-associated coiled-coil kinase signaling pathway. Mol Med Rep. 2015;12:45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Neyra JA, Hu MC. Potential application of klotho in human chronic kidney disease. Bone. 2017;100:41–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Asai O, Nakatani K, Tanaka T, et al. Decreased renal alpha-Klotho expression in early diabetic nephropathy in humans and mice and its possible role in urinary calcium excretion. Kidney Int. 2012;81:539–547. [DOI] [PubMed] [Google Scholar]
- [13].Kim SS, Song SH, Kim IJ, et al. Decreased plasma alpha-Klotho predict progression of nephropathy with type 2 diabetic patients. J Diabetes Complications. 2016;30:887–892. [DOI] [PubMed] [Google Scholar]
- [14].Zhou L, Li Y, Zhou D, et al. Loss of Klotho contributes to kidney injury by derepression of Wnt/beta-catenin signaling. J Am Soc Nephrol. 2013;24:771–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Xiao L, Wang M, Yang S, et al. A glimpse of the pathogenetic mechanisms of Wnt/beta-catenin signaling in diabetic nephropathy. Biomed Res Int. 2013;2013:987064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Liu P, Chen B, Gu Y, et al. PNMA1, regulated by miR-33a-5p, promotes proliferation and EMT in hepatocellular carcinoma by activating the Wnt/beta-catenin pathway. Biomed Pharmacothe. 2018;108:492–499. [DOI] [PubMed] [Google Scholar]
- [17].Komers R, Plotkin H. Dual inhibition of renin-angiotensin-aldosterone system and endothelin-1 in treatment of chronic kidney disease. Am J Physiol Regul Integr Comp Physiol. 2016;310:R877–R884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Zhou L, Li Y, Hao S, et al. Multiple genes of the renin-angiotensin system are novel targets of Wnt/beta-catenin signaling. J Am Soc Nephrol. 2015;26:107–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Lin Y, Kuro-O M, Sun Z. Genetic deficiency of anti-aging gene klotho exacerbates early nephropathy in STZ-induced diabetes in male mice. Endocrinology. 2013;154:3855–3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Shimamura Y, Hamada K, Inoue K, et al. Serum levels of soluble secreted α-Klotho are decreased in the early stages of chronic kidney disease, making it a probable novel biomarker for early diagnosis. Clin Exp Nephrol. 2012;16:722–729. [DOI] [PubMed] [Google Scholar]
- [21].Irifuku T, Doi S, Sasaki K, et al. Inhibition of H3K9 histone methyltransferase G9a attenuates renal fibrosis and retains klotho expression. Kidney Int. 2016;89:147–157. [DOI] [PubMed] [Google Scholar]
- [22].Hu MC, Kuro-O M, Moe OW. Secreted klotho and chronic kidney disease. Adv Exp Med Biol. 2012;728:126–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Oh HJ, Nam BY, Wu M, et al. Klotho plays a protective role against glomerular hypertrophy in a cell cycle-dependent manner in diabetic nephropathy. Am J Physiol Renal Physiol. 2018;315:F791–f805. [DOI] [PubMed] [Google Scholar]
- [24].Kadoya H, Satoh M, Haruna Y, et al. Klotho attenuates renal hypertrophy and glomerular injury in Ins2Akita diabetic mice. Clin Exp Nephrol. 2016;20:671–678. [DOI] [PubMed] [Google Scholar]
- [25].Doi S, Zou Y, Togao O, et al. Klotho inhibits transforming growth factor-beta1 (TGF-beta1) signaling and suppresses renal fibrosis and cancer metastasis in mice. J Biol Chem. 2011;286:8655–8665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Liu H, Fergusson MM, Castilho RM, et al. Augmented Wnt signaling in a mammalian model of accelerated aging. Science (New York, NY). 2007;317:803–806. [DOI] [PubMed] [Google Scholar]
- [27].Wolf I, Levanon-Cohen S, Bose S, et al. Klotho: a tumor suppressor and a modulator of the IGF-1 and FGF pathways in human breast cancer. Oncogene. 2008;27:7094–7105. [DOI] [PubMed] [Google Scholar]
- [28].Kurosu H, Yamamoto M, Clark JD, et al. Suppression of aging in mice by the hormone Klotho. Science (New York, NY). 2005;309:1829–1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Tang X, Wang Y, Fan Z, et al. Klotho: a tumor suppressor and modulator of the Wnt/beta-catenin pathway in human hepatocellular carcinoma. Lab Invest. 2016;96:197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ni W, Fang Y, Xie L, et al. Adipose-derived mesenchymal stem cells transplantation alleviates renal injury in streptozotocin-induced diabetic nephropathy. J Histochem Cytochem. 2015;63:842–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].He W, Kang YS, Dai C, et al. Blockade of Wnt/beta-catenin signaling by paricalcitol ameliorates proteinuria and kidney injury. J Am Soc Nephrol. 2011;22:90–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Zhou T, He X, Cheng R, et al. Implication of dysregulation of the canonical wingless-type MMTV integration site (WNT) pathway in diabetic nephropathy. Diabetologia. 2012;55:255–266. [DOI] [PubMed] [Google Scholar]
- [33].Rahimi Z. The role of renin angiotensin aldosterone system genes in diabetic nephropathy. Can J Diabetes. 2016;40:178–183. [DOI] [PubMed] [Google Scholar]
- [34].Yang J, Shang J, Zhang S, et al. The role of the renin-angiotensin-aldosterone system in preeclampsia: genetic polymorphisms and microRNA. J Mol Endocrinol. 2013;50:R53–R66. [DOI] [PubMed] [Google Scholar]


