Abstract
Background
Safe interventions to enhance cognitive function in cognitively healthy people would be very valuable for several reasons, including a better quality of life and professional success. While L‐carnitine has been reported to enhance cognitive function in some conditions, its efficacy is disputed. The evidence of its efficacy for cognitively healthy people has not previously been systematically reviewed.
Objectives
To assess the efficacy and safety of L‐carnitine for the enhancement of cognitive function in people without cognitive impairment.
Search methods
We searched ALOIS, the Cochrane Dementia and Cognitive Improvement Group's Specialized Register, on 4 November 2016. We used the search terms 'L‐carnitine' or 'acetyl‐L‐carnitine' or 'propionyl‐L‐carnitine' or 'ALC' or 'PLC' or 'ALCAR' or 'ALPAR'. We ran additional separate searches in several other sources to ensure that we retrieved the most up‐to‐date results. We also reviewed the bibliographies of the randomised controlled trials identified and contacted the authors and known experts in the field and pharmaceutical companies to identify additional published or unpublished data.
Selection criteria
Eligible trials were randomised controlled trials (RCTs) or quasi‐RCTs, parallel‐group or cross‐over, that compared L‐carnitine or its derivatives, acetyl‐L‐carnitine or propionyl‐L‐carnitine, at any dose and for any length of treatment, with placebo or no treatment in cognitively healthy people of any age and either gender.
Data collection and analysis
We used standard methodological procedures expected by Cochrane. Two review authors independently selected trials and evaluated the methodological quality, then extracted and analysed data from the included trials.
Main results
Only two RCTs were eligible. One was a cross‐over trial with 18 participants. The other randomised 400 participants to one of four treatments, of which two (L‐carnitine and placebo) were relevant to this review, but the exact numbers of participants in these two treatment groups was not reported. All participants were young adults. Methodological details were poorly reported, and we considered the risk of bias in both studies to be unclear. The trials assessed different cognitive outcomes. We could extract cognitive data on approximately 200 participants from one trial. We found no evidence that L‐carnitine has any effect on reaction time, vigilance, immediate memory, or delayed recall after three days of treatment. This trial report stated that there was a small number of adverse effects, none of which were serious. The small cross‐over trial also reported no effect of L‐carnitine on cognition, but did not provide data; no information was provided on adverse effects. We considered the available evidence to be of very low quality for all reported outcomes.
Authors' conclusions
Due to the limited number of included trials, short‐term treatment, and inadequate reporting, we were unable to draw any conclusions about the efficacy or safety of L‐carnitine for cognitive enhancement in healthy adults. Well‐designed, randomised, placebo‐controlled trials of L‐carnitine for cognition enhancement in cognitively healthy people, with large samples and relatively long‐term follow‐up, are still needed.
Keywords: Humans; Young Adult; Attention; Attention/drug effects; Carnitine; Carnitine/therapeutic use; Cognition; Cognition/drug effects; Memory; Memory/drug effects; Memory, Short‐Term; Memory, Short‐Term/drug effects; Nootropic Agents; Nootropic Agents/therapeutic use; Publication Bias; Randomized Controlled Trials as Topic; Reaction Time; Reaction Time/drug effects
Plain language summary
L‐carnitine for cognitive enhancement in people without cognitive impairment
Background
Cognition (or cognitive function) is a term used to describe thinking skills, including attention, memory, and reasoning. Supplements and drugs are sometimes used by healthy people to try to improve cognitive function and perform better at work or while studying. These supplements and drugs are known as cognitive enhancers. L‐carnitine, which is found naturally in the diet, especially in meat, but can also be produced in the body, has been suggested as a possible cognitive enhancer. It is sold on its own as a dietary supplement and is found in some mixed supplements or 'energy drinks'. In this review, we searched for clinical trials in which healthy people taking L‐carnitine were compared with similar people taking a dummy pill (placebo). We hoped to learn whether or not L‐carnitine improves cognitive function and whether it is associated with side effects.
Results
We found only two trials to include in the review. One trial treated approximately 200 participants with L‐carnitine or placebo for three days; the other trial treated only 18 participants with only a single dose of L‐carnitine. Both trials included healthy young people with an average age of about 21. The trials measured different aspects of cognition using different tests. The smaller trial was only reported as an abstract, and there was no usable data, although the authors said that they found no evidence of an effect of L‐carnitine on cognitive function. Important information was missing from the paper describing the other trial, but we found no evidence that L‐carnitine had any effect on any of the aspects of cognition that were measured. Only the report of the larger trial mentioned adverse effects of treatment, which were all described as minor and occurring equally among those receiving L‐carnitine and those receiving placebo.
Quality of the evidence
It was difficult to properly assess the quality of the included trials because of poor reporting. We considered there to be a serious risk of bias due to poor study methods, and further uncertainty about the results because the studies were so small. We also considered the studies to be too short to adequately address our research question. Due to these factors, we considered the quality of the evidence to be very low.
Conclusions
Given the limited amount of evidence of very low quality, we were not able to draw any conclusions about the effect of L‐carnitine on cognitive function or its safety in healthy people. Larger, better‐quality studies conducted over a longer period of time are needed to answer our review question.
Summary of findings
Summary of findings for the main comparison. L‐carnitine compared to placebo for cognitive enhancement in people without cognitive impairment.
| L‐carnitine compared to placebo for cognitive enhancement in people without cognitive impairment | |||||
| Patient or population: healthy young women Setting: community Intervention: L‐carnitine Comparison: placebo | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with L‐carnitine | ||||
| Reaction time (8 lamps) Assessed with: reaction time task with 8 potentially illuminated lamps Follow‐up: mean 3 days | The mean reaction time (8 lamps) was 416 milliseconds | MD 5 milliseconds fewer (23.18 fewer to 13.18 more) | 200 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 2 3 | The number of participants was an approximation, since the report did not specify the actual numbers of participants in each study group. |
| Vigilance Assessed with: rapid information‐processing task Follow‐up: mean 3 days | The mean vigilance was 23.5 digits | MD 1.2 digits fewer (3.06 fewer to 0.66 more) | 200 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 2 3 | |
| Immediate memory Assessed with: recall of word list Scale from: 0 to 30 Follow‐up: mean 3 days | The mean immediate memory was 11.6 words | MD 1 words fewer (2.07 fewer to 0.07 more) | 200 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 2 3 | |
| Delayed memory Assessed with: recall of word list Scale from: 0 to 30 Follow‐up: mean 3 days | The mean delayed memory was 8.9 words | MD 0.9 words fewer (1.97 fewer to 0.17 more) | 200 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 2 3 | |
| Adverse effects ‐ not reported | It stated that 92% of participants reported no side effects and that the numbers of participants who had some minor reaction were similar among the experimental groups, but the actual number of participants reporting any adverse effect in each group is unknown. | — | ⊕⊝⊝⊝ VERY LOW 1 2 3 | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RCT: randomised controlled trial | |||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. | |||||
1Downgraded one level due to study limitations. Most of the 'Risk of bias' judgements were 'unclear', so we considered there to be a serious concern about risk of bias. 2Downgraded one level due to indirectness. The follow‐up period to observe the change in cognitive function in people without cognitive impairment was very short. 3Downgraded one level due to imprecision. The results came from a single, small study.
Background
Description of the condition
Cognitive functions, such as attention, perception, memory, and language, are crucial for human functioning. These capacities are used to process information, including acquiring, selecting, representing, and retaining relevant data, and to guide behaviours (Sandberg 2006). Evidence from long‐term follow‐up studies and systematic reviews suggests an inverse association between early‐life cognitive function and adult risk of unintentional injury and mortality (Calvin 2011; Osler 2007). With the continuous development of society, better cognition is also considered to mean stronger social adaptability and higher quality of life (Clark 2014; Eickenhorst 2012; Franke 2011). The maintenance and improvement of cognitive function has therefore been gradually gaining attention, with interest in dietary supplements or drugs that might enhance cognition (Clark 2014; Deline 2014; Eickenhorst 2012).
Cognitive enhancement has been defined as the amplification or extension of one or more core capacities of the mind, thus improving a person's information processing systems and allowing them to achieve better performance in their daily life (McKendrick 2014; Sandberg 2006). Safe interventions that enhance cognitive function would be very valuable.
Description of the intervention
L‐carnitine (the enantiomeric form of carnitine) is a quaternary ammonium compound synthesised from the amino acids lysine or methionine. It is an important contributor to cellular energy metabolism. Although the substance was discovered in 1905, its crucial role in metabolism was not elucidated until 1955, and its deficiency was not described until 1972 (Bhattacharyya 1995; Kelly 1998). The most significant source of L‐carnitine in the human diet is meat; humans also can synthesise L‐carnitine from dietary amino acids. L‐carnitine is produced in the liver and kidneys and stored mainly in the most active metabolic tissue such as the skeletal muscles, heart, brain, and sperm. The plasma concentration of L‐carnitine varies with age, increasing until a person is nearly 70 years old, then tending to diminish in parallel with the reduction in body mass index and muscle mass (Malaguarnera 1999). Acetyl‐L‐carnitine (ALC) and propionyl‐L‐carnitine (PLC) are the most important naturally occurring carnitine derivatives. The body can convert L‐carnitine to ALC or PLC, and vice versa. L‐carnitine and its natural derivatives may therefore theoretically play similar roles in the body, but due to their chemical structures they appear to be present in different strengths in some tissues (Malaguarnera 2012; Mingorance 2011).
Although L‐carnitine is supplied exogenously and can be synthesised endogenously, both primary and secondary deficiencies do occur. Carnitine deficiency can be acquired or result from inborn errors of metabolism (Stanley 2004). Preterm infants are at risk for carnitine deficiency due to impaired synthesis and insufficient renal tubular resorption (Evangeliou 2003). Secondary carnitine deficiency is more common and is usually associated with dialysis in chronic renal failure, although it can also be induced by intestinal resection, severe infection, and liver disease.
Besides being found in certain foods (e.g. meats, dairy products, and some plants), L‐carnitine is also available in the form of supplements or included in some mixed products, such as energy drinks and vitamin mixtures. At least four formulations of L‐carnitine supplements are currently available, that is liquid, tablet, capsule, and powder, and it is sometimes supplied as one component in a multicomponent preparation or a dietary intervention (L‐Carnitine 2015). Oral supplementation of L‐carnitine in individual dosages greater than 2 g appears to offer no advantage, since the mucosal absorption of carnitine appears to be saturated at about a 2 g dose (Harper 1988). Maximum blood concentration is reached approximately 3.5 hours after an oral dose and slowly decreases, with a half‐life of about 15 hours (Bach 1983). Elimination of carnitine occurs primarily through the kidneys (Bach 1983).
Reported side effects of L‐carnitine include agitation, headache, diarrhoea, nausea, vomiting, anorexia, and abdominal discomfort, mostly of mild or moderate severity (Hudson 2003; Montgomery 2003; Wang 2014).
L‐carnitine and its derivatives have been proposed as a treatment, or as an adjunct to conventional medicine, for many conditions, including stable angina, intermittent claudication, diabetic neuropathy, kidney disease and dialysis, hyperthyroidism, male infertility, erectile dysfunction, chronic fatigue syndrome, Alzheimer's disease, and memory impairment (Hudson 2003; Montgomery 2003; Ribas 2014; Shang 2014; Wang 2014). Acetyl‐L‐carnitine is thought to traverse the blood‐brain barrier more readily and may have a preferential effect on the central nervous system, whereas PLC may have better bioavailability and specifically target skeletal and cardiac muscle (Malaguarnera 2012; Mingorance 2011).
How the intervention might work
L‐carnitine is involved in energy production
L‐carnitine is a cofactor required for transformation of free long‐chain fatty acids into acylcarnitines, and for their subsequent transport into the mitochondrial matrix, where they are oxidised to produce energy. Increasing L‐carnitine content might increase the rate of fatty acid oxidation, permitting a reduction of glucose use, preserving muscle glycogen content, and ensuring maximal rates of oxidative adenosine triphosphate (ATP) production (Brass 1994a; Brass 1994b; Brass 1994c).
Improvement of cognitive function may be connected to processes influenced by the brain's energy production
In brain tissue, the L‐carnitine shuttle mediates translocation of the acetyl moiety from mitochondria into the cytosol and thus contributes to the synthesis of acetylcholine and acetylcarnitine (Montgomery 2003; Nalecz 1996). Acetyl‐L‐carnitine can traverse the blood‐brain barrier and modulate phospholipid metabolism, synaptic morphology, and synaptic transmission, and can enhance the synthesis and release of macromolecules such as neurotrophic factors, neurohormones, and multiple neurotransmitters (Benton 2004; Pettegrew 2000; Virmani 2004). These actions have stimulated interest in its effects on cognition.
Why it is important to do this review
People are interested in maintaining and improving cognitive function for several reasons, including a better quality of life and professional success. L‐carnitine has been assessed as a cognitive‐enhancing agent that can be supplied as a nutritional aid. Its use in cognitively impaired people has been reviewed (Hudson 2003; Montgomery 2003; Spagnoli 1991). This systematic review of efficacy and safety aimed to carefully examine the evidence and establish whether it is acceptable for use in people without cognitive impairment for cognitive enhancement.
Objectives
To assess the efficacy and safety of L‐carnitine for the enhancement of cognitive function in people without cognitive impairment.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) or quasi‐RCTs in which L‐carnitine or its derivatives (acetyl‐L‐carnitine (ALC) or propionyl‐L‐carnitine (PLC)) were given with the intention of enhancing cognitive function and were compared with a placebo or no‐treatment control group. Trials could have a parallel‐group or cross‐over design.
Types of participants
People with intact cognition of any age and either gender were eligible. All participants had no self reported history or evidence of neurologic and psychiatric disorders, and they achieved normal scores when assessed by any acceptable cognitive tests.
We excluded people with a diagnosis of mild cognitive impairment or dementia caused by any kind of disorder (e.g. Alzheimer's disease) or other significant illnesses associated with cognitive impairment (such as schizophrenia, depression, or generalised anxiety disorder). People with known carnitine deficiency were also excluded. However, people with illnesses or disorders of other systems (e.g. diabetes mellitus, hypertension) but without existing cognitive impairment were included.
Types of interventions
Active intervention: L‐carnitine and its derivative, ALC or PLC, irrespective of formulation, dose, or duration of treatment. We did not include trials assessing a multicomponent preparation or dietary supplement containing L‐carnitine or its derivatives.
Control intervention: placebo or no treatment.
Types of outcome measures
Primary outcomes
Cognitive function as measured by psychometric tests. These should be validated for use in healthy populations and may include comprehensive neuropsychological test batteries or tests of individual cognitive domains (e.g. memory functions including verbal memory, visual memory, immediate or delayed recall, executive function, attention, etc.).
Incidence and severity of adverse effects.
Search methods for identification of studies
We searched for all RCTs and quasi‐RCTs of L‐carnitine for cognitive enhancement, without language restrictions.
Electronic searches
We searched ALOIS (www.medicine.ox.ac.uk/alois), the Cochrane Dementia and Cognitive Improvement Group's Specialized Register. We used the search terms 'L‐carnitine' or 'acetyl‐L‐carnitine' or 'propionyl‐L‐carnitine' or 'ALC' or 'PLC' or 'ALCAR' or 'ALPAR'.
ALOIS is maintained by the Information Specialists of the Cochrane Dementia and Cognitive Improvement Group and contains dementia and cognitive improvement studies identified from:
monthly searches of a number of major healthcare databases: MEDLINE, Embase, CINAHL, PsycINFO, and LILACS;
monthly searches of a number of trial registers: metaRegister of Controlled Trials, UMIN Japan Trial Register, World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (which covers ClinicalTrials.gov, ISRCTN, Chinese Clinical Trials Register, German Clinical Trials Register, Iranian Registry of Clinical Trials, and the Netherlands National Trials Register, as well as others);
quarterly search of the Cochrane Central Register of Controlled Trials (CENTRAL);
six‐monthly searches of a number of grey literature sources: ISI Web of Knowledge Conference Proceedings, Index to Theses, Australasian Digital Theses.
To view a list of all sources searched for ALOIS, see 'About ALOIS' on the ALOIS website.
We ran additional separate searches in many of the above sources to ensure that we identified studies involving participants without cognitive impairment and that we retrieved the most up‐to‐date results. The search strategies can be found in Appendix 1.
Searching other resources
We checked the references of published studies to identify additional trials. We also reviewed the bibliographies of the RCTs identified and contacted the authors to identify additional published or unpublished data where possible.
Data collection and analysis
Selection of studies
Two review authors (MZ and NC) independently evaluated the titles and abstracts identified from the register search. The full text of all potentially relevant studies were retrieved for assessment, and the same two review authors independently determined which trials fit the inclusion criteria. Any disagreements were resolved by discussion or by consulting a third review author (LH) if necessary.
Data extraction and management
Two review authors (MZ and NC) independently extracted data from the trials onto a data extraction form. The form included the study name, type of design, study population size, duration, number of participant withdrawals, participants analysed in the different treatment groups, inclusion and exclusion criteria, intervention (route and dosage), and outcomes. One review author (MZ) entered the data into Review Manager 5 software, and a second review author (NC) checked the data entry (RevMan 5).
Assessment of risk of bias in included studies
We assessed the risk of bias in included studies considering the method of random sequence generation, allocation concealment, blinding, completeness of outcome data, selective outcome reporting, and any other potential sources of bias. Two review authors (NC and MZ) performed this assessment independently using the Cochrane 'Risk of bias' tool (Higgins 2011a). We then judged all trials on each domain and categorised them as follows:
low risk of bias for all key domains: low risk of bias;
unclear risk of bias for one or more key domains: unclear risk of bias;
high risk of bias for one or more key domains: high risk of bias.
We evaluated the following items for included cross‐over trials according to the method of assessing risk of bias in cross‐over trials (Higgins 2011a):
whether the cross‐over design was suitable;
whether there was a carry‐over effect (the washout period should be no shorter than five half‐life durations of each intervention);
whether only first‐period data were available;
incorrect analysis; and
comparability of results with those from parallel‐group trials.
Measures of treatment effect
We analysed the data using Review Manager 5 software (RevMan 5). We expressed the results as risk ratios (RRs) with 95% confidence intervals (CIs) for dichotomous outcomes and as mean differences (MDs) or standardised mean differences (SMDs) for continuous outcomes.
Unit of analysis issues
We included only studies randomising individuals. We included cross‐over trials as long as either first‐period data or data taking account of the cross‐over design were available. If a study included repeated measures of the same outcome, then we defined several outcomes based on different periods of follow‐up and performed separate analyses. If studies compared more than two intervention groups, we selected the most relevant pair of intervention groups to include in the analyses (Higgins 2011b).
Dealing with missing data
We contacted the original investigators to request missing data whenever possible.
We recorded the amount of missing data and reasons given.
We addressed the potential impact of missing data on the findings of the review in the Discussion section.
Assessment of heterogeneity
We planned to assess heterogeneity among trials by using the Chi2 test, and to use I2 values to quantify inconsistency across studies (Deeks 2011; Higgins 2002; Higgins 2003), however heterogeneity was not assessed as no meta‐analysis was performed.
Assessment of reporting biases
We assessed the within‐study risk of bias from selective outcome reporting for each included trial according to the standard described in the Assessment of risk of bias in included studies section. We tried to obtain the study protocol, and then compared outcomes in the protocol and published report. When the protocol was unavailable, we compared outcome measures listed in the methods section with those reported in the results section. We planned to use funnel plots to investigate the possibility of between‐study publication bias if there were sufficient numbers of included studies (at least 10 included in a meta‐analysis) (Sterne 2011), but as we included only two trials, we did not use a funnel plot.
Data synthesis
We described the included trials but did not perform any meta‐analysis due to insufficient data and differences between the included trials.
Subgroup analysis and investigation of heterogeneity
No subgroup analyses were performed due to insufficient data.
Sensitivity analysis
No sensitivity analyses were performed due to insufficient data.
Presentation of results and 'Summary of findings' table
We rated our confidence in the evidence for each outcome based on the GRADE criteria of risk of bias, imprecision, inconsistency between studies, indirectness, and publication bias (Schünemann 2011). We presented the results for reaction time, vigilance, immediate memory, delayed memory, and adverse effects in a 'Summary of findings' table, based on the methods described in Chapter 11 of the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2011).
Results
Description of studies
Results of the search
After a software de‐duplication and first assessment by the Cochrane Dementia and Cognitive Improvement Group Information Specialist, 191 results were passed to the review authors for further assessment. We identified 22 potentially eligible records by evaluating the titles and abstracts, but excluded 20 references for 18 studies (see Characteristics of excluded studies) after screening their full text. Only two trials fulfilled the inclusion criteria (see Characteristics of included studies) (Figure 1) (Benton 2004; Muñiz‐Pumares 2011).
1.

Study flow diagram.
Included studies
The Benton 2004 study aimed to assess the effects of carnitine, lecithin, or their combination on cognition in healthy adult women, while the Muñiz‐Pumares 2011 study evaluated the effects of acute supplementation with L‐carnitine on exercise performance, cognitive function, and cortisol in healthy adults. The total number of participants in the two included trials was 418. Benton 2004 had a parallel‐group design and enrolled 400 participants, but there were four intervention groups, of which only two were relevant to this review, and the actual numbers of participants randomly allocated to each group were unknown (although we can assume it was approximately 100). Muñiz‐Pumares 2011 was a cross‐over trial that enrolled 18 participants; it was published only as a conference abstract.
The mean age of participants in both trials was around 21 years, indicating that most of them were adolescents or young adults. The Benton 2004 study recruited only women, while the Muñiz‐Pumares 2011 study enrolled participants of both sexes. The demographic characteristics were not compared between groups at baseline in either study, but both trials included healthy volunteers only (Benton 2004; Muñiz‐Pumares 2011).
Neither trial clearly described inclusion and exclusion criteria: the Benton 2004 trial recruited female undergraduates by posters, from which we could speculate that they included healthy young people. Muñiz‐Pumares and colleagues performed their study among 18 healthy, active men and women, but as this study was published only in abstract form, detailed inclusion and exclusion criteria were not specified (Muñiz‐Pumares 2011).
L‐carnitine was administered orally in both included trials. The Benton 2004 trial had four comparison arms, treated with lecithin, carnitine, lecithin plus carnitine, or placebo, respectively. We extracted data from the carnitine and placebo groups for the present review. Muñiz‐Pumares 2011 compared rhodiola rosea beverage with and without L‐carnitine. The Muñiz‐Pumares 2011 trial gave participants a single dose of 500 mg of L‐carnitine, while Benton and colleagues gave their participants 500 mg of carnitine a day, in two divided doses, for three continuous days (Benton 2004). Neither trial report mentioned participant withdrawal or loss to follow‐up.
Our primary efficacy outcome was cognitive function. Both included studies evaluated cognitive function, but the instruments used to evaluate it varied. The Benton 2004 study used recall of word list, reaction times, and an information‐processing task. The Muñiz‐Pumares 2011 study assessed cognition using Rapid Visual Information Processing (RVP), Visual Recognition Memory (VRM), and the Stroop Colour‐Word Test.
Only one of the included trials referred to side effects associated with L‐carnitine (Benton 2004).
Excluded studies
We excluded 18 potentially relevant studies for the following reasons: not an RCT or a quasi‐RCT according to the full text (Salvioli 1994; Vecchi 1990); enrolled participants with cognitive dysfunction or illnesses associated with cognitive impairment (Bonavita 1986; Cipolli 1990; Malaguarnera 2007; Malaguarnera 2008; Malaguarnera 2014; Mantero 1989; Martinotti 2011; Pueschel 2006; Vecchi 1990; Warner 1997); included participants with known carnitine deficiency (Cruciani 2009); used interventions other than L‐carnitine or its derivatives alone (Chan 2010; Hakkou 1990; Hoffman 2010; Yonei 2008); did not evaluate any of the outcome measures specified in our protocol (Cavallini 2004; Martinotti 2011; Sugino 2007).
Risk of bias in included studies
Both trials reported little or no information to allow accurate assessment of the risk of bias (see Characteristics of included studies; Figure 2) (Higgins 2011a).
2.
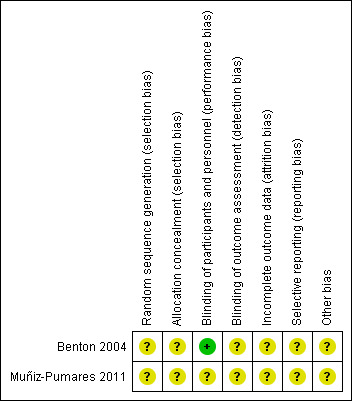
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Benton 2004 had a parallel‐group design and stated that participants were allocated randomly to one of the four intervention groups. Muñiz‐Pumares 2011 had a cross‐over design and stated that the interventions were given to each participant in random order. However, neither study provided sufficient information about the methods used for random sequence generation or allocation concealment.
Blinding
Both trials stated they were double‐blind, but we could not judge who was blinded in practice (participants, personnel involved in treatment, or efficacy evaluators) according to the study reports. We could only speculate that the participants and treatment implementers may have been blinded to the interventions administered to participants in the Benton 2004 trial, because placebos or active supplements were contained in similar capsules, and all participants were asked to guess what kind of ingredients had been consumed at the end of the study; whether the efficacy evaluators were also blinded was not specified. The Muñiz‐Pumares 2011 study only briefly referred to blinding in broad terms as "double‐blind", with no other relevant information available specifying who was blinded.
Incomplete outcome data
In both trials the study duration was very short (one day in Muñiz‐Pumares 2011 and three days in Benton 2004). Such a short study duration is likely to imply small numbers of participants lost to follow‐up. However, the reports did not mention missing data or the use of intention‐to‐treat analysis. We considered that there was insufficient information to judge the risk of attrition bias due to incomplete outcome data.
Selective reporting
Protocols were not available for either trial. Both trials used multiple measures of cognitive function, but neither specified primary and secondary outcomes. We considered the risk of bias due to selective reporting to be unclear for both studies. We did not perform funnel plots to investigate the possibility of publication bias because only two trials were included.
Other potential sources of bias
We considered another potential source of bias for both trials, that is participants' demographic characteristics and their cognitive functional state were not compared at baseline, therefore we were unsure about the comparability between groups. In the cross‐over study (Muñiz‐Pumares 2011), there was no discussion of an appropriate washout period or of possible carry‐over effects.
Effects of interventions
See: Table 1
Primary outcomes
Cognitive function
Both included trials assessed cognitive function as one of the primary efficacy outcomes, but the instruments used and the types of data differed between trials. Benton and colleagues evaluated several domains of cognitive function, including reaction times, attention, and short‐term memory, using a battery of tests. Means and standard deviations for each outcome measure were listed, but the report did not specify the numbers of participants in each experimental group, and our attempt to obtain this information from the trial authors failed (although we can assume the number of participants receiving L‐carnitine or placebo was approximately 200). From the reported data, we calculated the MD and 95% CI for each efficacy outcome. We found no evidence that three days of carnitine (500 mg daily) administration had any effect on the above cognitive functions compared to placebo (reaction time task with one, two, four, or eight potentially illuminated lamps, respectively (Benton 2004; Jensen 1987): MD ‐6.00, 95% CI ‐18.00 to 6.00, P = 0.33; MD ‐2.00, 95% CI ‐13.02 to 9.02, P = 0.72; MD 0.00, 95% CI ‐11.95 to 11.95, P = 1.00; MD ‐5.00, 95% CI ‐23.18 to 13.18, P = 0.59; vigilance test: MD ‐1.20, 95% CI ‐3.06 to 0.66, P = 0.21; immediate memory: MD ‐1.00, 95% CI ‐2.07 to 0.07, P = 0.07; delayed memory: MD ‐0.90, 95% CI ‐1.97 to 0.17, P = 0.10) (Table 1) (Benton 2004).
The other trial was only reported as an abstract (Muñiz‐Pumares 2011). Its authors stated that they found no significant differences in the cognitive variables they measured between L‐carnitine and placebo treatment periods (P > 0.05), but the actual data were unavailable.
As most of our judgements about risk of bias were 'unclear', we considered that there was serious concern about risk of bias in these studies. In addition, because the results came from single small studies, we also considered that there was serious concern about the precision of the results. Furthermore, both trials had very short follow‐up periods to observe change in cognitive function in people without cognitive impairment, therefore we also had serious doubts about the directness of the evidence to answer the healthcare questions of the present review. Finally, as only two trials were included, both of which were published in English, we were unable to exclude the possibility of publication bias. Hence, we judged all of the available evidence to be of very low quality according to GRADE criteria (Table 1) (Atkins 2004; Schünemann 2011), meaning that we are unable to draw any conclusions about efficacy from this evidence.
Incidence and severity of adverse effects
We planned to categorise adverse events into 'serious' or 'not serious' subtypes. Serious adverse events were those leading to death; were life‐threatening; required inpatient hospitalisation or prolongation of existing hospitalisation; resulted in persistent or significant disability; or any important medical event that might have jeopardised the participant or required intervention to prevent it. We considered all other adverse events to be non‐serious (ICHEWG 1997). Only one of the included trials assessed adverse effects of L‐carnitine administration (Benton 2004). Benton 2004 stated that 92% of participants reported no side effects and that the numbers of participants who had some minor reaction (e.g. tiredness, hunger, headache, or stomachache) were similar amongst the experimental groups. The other trial did not provide any relevant information (Muñiz‐Pumares 2011).
We also judged this evidence to be of very low quality, based on several GRADE considerations, as mentioned above: risk of bias, imprecision, and indirectness (Table 1), therefore we can draw no conclusions about adverse effects of L‐carnitine when used in cognitively healthy people.
Discussion
L‐carnitine seemed to be a promising intervention for treating or preventing cognitive impairment based on its pharmacological actions, but its efficacy as a cognitive enhancer for healthy people was uncertain, thus prompting the present systematic review. We have done our best to collect and extract all possible data to conduct a more complete systematic analysis, however relevant evidence was lacking.
Summary of main results
We included only two trials in the present systematic review, both of which administered L‐carnitine to cognitively healthy people orally and assessed cognitive function after treatment.
Both trials reported no significant difference between L‐carnitine and placebo for a variety of cognitive test scores, but no meta‐analysis could be performed due to obviously different designs, methods, study periods, inconsistent outcome measures, and unavailable data.
One trial reported a small number of adverse effects, none of which was serious. The incidence of adverse effects was stated to be similar between intervention groups, but detailed data were not provided.
Overall completeness and applicability of evidence
Both studies included healthy young people. Treatment durations were very short ‐ either a single dose, in Muñiz‐Pumares 2011, or a continuous three‐day treatment period, in Benton 2004 ‐ and no follow‐up after the end of treatment was performed. The studies were therefore unable to address the question of lasting cognitive effects.
Quality of the evidence
Due to inadequate reporting, we judged the risk of bias in all or almost all domains in both studies to be unclear. Overall, we considered the available evidence to be of very low quality due to potential risk of bias, imprecision, and indirectness.
Potential biases in the review process
We were unable to exclude the possibility of publication bias.
Agreements and disagreements with other studies or reviews
The purpose of treatment and the methods needed to evaluate cognitive effects in people without cognitive impairment are different from those of studies aiming to treat people who already have, or are at high risk of, cognitive impairment, but the mechanisms of the effects of L‐carnitine on cognitive function may be to some extent similar. Several studies have reported that L‐carnitine can improve cognitive function or reduce the rate of cognitive deterioration in people with Alzheimer's disease/mild cognitive impairment (Montgomery 2003; Spagnoli 1991; Thal 1996), Parkinson's disease (Puca 1990), hepatic encephalopathy (Cecere 2002; Malaguarnera 2013), and chronic alcoholism (Tempesta 1990). However, other good‐quality trials or meta‐analyses did not find any efficacy of L‐carnitine in treating people with Alzheimer's disease/mild cognitive impairment (Hudson 2003; Thal 2000).
Other trials have examined the effect of ingestion of nutritional supplements containing L‐carnitine and other ingredients, and have concluded that the supplements could maintain cognitive function in healthy participants compared to placebo, but the efficacy of L‐carnitine alone was not evaluated, and relevant data could not be extracted from those trials (Chan 2010; Hoffman 2010). It is therefore not possible to draw any conclusions from these studies about L‐carnitine specifically.
L‐carnitine has also been used as an alternative or additional treatment for some conditions besides cognitive impairment, such as heart disease, kidney disease, mood disorders in the elderly, and peripheral neuropathies. Its efficacy for these conditions is also not well supported by evidence and is controversial, but most studies have found that its use in humans is relatively well tolerated without serious adverse effects (Hudson 2003; Malaguarnera 2012; Montgomery 2003; Wang 2014).
Authors' conclusions
Implications for practice.
Due to the limited number of included trials, short‐term treatment, and inadequate reporting, we were unable to draw any conclusions about the efficay or safety of L‐carnitine for cognitive enhancement in healthy adults.
Implications for research.
Well‐designed, randomised, placebo‐controlled trials of L‐carnitine with longer‐term follow‐up are still needed to evaluate the efficacy and determine the long‐term safety of L‐carnitine for cognitive enhancement in cognitively healthy people.
Notes
The protocol was first published in Issue 11, 2011 of the Cochrane Library (Yang 2011), but the title and types of participants have been changed in the updated protocol and review to make the objectives of the review clearer.
Acknowledgements
We are grateful for the assistance of Sue Marcus, Managing Editor; Anna Noel‐Storr, Information Specialist; and other editors of the Cochrane Dementia and Cognitive Improvement Group.
Appendices
Appendix 1. Sources searched and search strategies
| Source | Search strategy | Hits retrieved |
| 1. ALOIS (www.medicine.ox.ac.uk/alois/) [Last searched: 4 November 2016] |
l‐carnitine or "acetyl‐L‐carnitine" or "propionyl‐L‐carnitine" or "ALC" or "PLC" or "ALCAR" or ''ALPAR". | 4 |
| 2. MEDLINE In‐process and other non‐indexed citations and MEDLINE 1946‐present (Ovid SP) [Last searched: 4 November 2016] |
1. L‐carnitine.ti,ab. 2. Carnitine/ 3. (ALC or PLC or ALCAR or ALPAR).ti,ab. 4. "acetyl‐l‐carnitine".ti,ab. 5. "propionyl‐l‐carnitine".mp. 6. or/1‐5 7. randomized controlled trial.pt. 8. controlled clinical trial.pt. 9. randomi?ed.ab. 10. placebo.ab. 11. drug therapy.fs. 12. randomly.ab. 13. trial.ab. 14. groups.ab. 15. or/7‐14 16. (animals not (humans and animals)).sh. 17. 15 not 16 18. 6 and 17 19. (cognit* or memory or mental or brain).ti,ab. 20. dement*.ti,ab. 21. alzheimer*.ti,ab. 22. exp Dementia/ 23. (healthy or older or elder* or aged).ti,ab. 24. Aged/ 25. seniors.ti,ab. 26. or/19‐25 27. 18 and 26 |
1106 |
| 3. Embase 1974‐2016 December 30 (Ovid SP) [Last searched: 4 November 2016] |
1. L‐carnitine.ti,ab. 2. carnitine/ 3. (ALC or PLC or ALCAR or ALPAR).ti,ab. 4. "acetyl‐l‐carnitine".ti,ab. 5. "propionyl‐l‐carnitine".mp. 6. or/1‐5 7. randomized controlled trial/ 8. controlled clinical trial/ 9. randomi?ed.ab. 10. placebo.ab. 11. randomly.ab. 12. trial.ab. 13. groups.ab. 14. "double‐blind*".ti,ab. 15. or/7‐14 16. 6 and 15 17. (cognit* or memory or mental or brain).ti,ab. 18. dement*.ti,ab. 19. alzheimer*.ti,ab. 20. exp dementia/ 21. (healthy or older or elder* or aged).ti,ab. 22. aged/ 23. seniors.ti,ab. 24. or/17‐23 25. 16 and 24 |
1322 |
| 4. PsycINFO 1806‐December week 4 2015 (Ovid SP) [Last searched: 4 November 2016] |
1. L‐carnitine.ti,ab. 2. carnitine.mp. 3. (ALC or PLC or ALCAR or ALPAR).ti,ab. 4. "acetyl‐l‐carnitine".ti,ab. 5. "propionyl‐l‐carnitine".mp. 6. or/1‐5 7. Clinical Trials/ 8. randomi?ed.ab. 9. placebo.ab. 10. randomly.ab. 11. trial.ab. 12. groups.ab. 13. (RCT or CCT).ti,ab. 14. "double‐blind*".ti,ab. 15. or/7‐14 16. 6 and 15 |
320 |
| 5. CINAHL (EBSCOhost) [Last searched: 4 November 2016] |
1. TX L‐carnitine 2. (MH "Carnitine") 3. TX ALC OR PLC OR ALCAR OR ALPAR 4. TX "acetyl‐l‐carnitine" 5. TX "propionyl‐l‐carnitine" 6. S1 or S2 or S3 or S4 or S5 7. TX random* 8. TX placebo* 9. TX (RCT OR CCT) 10. (MH "Clinical Trials") OR (MH "Randomized Controlled Trials") 11. TX "double‐blind*" 12. TX "single‐blind*" 13. AB groups 14. AB trial 15. S7 or S8 or S9 or S10 or S11 or S12 or S13 or S14 16. S6 and S15 17. TX (cognit* OR memory OR mental OR brain) 18. TX dement* 19. TX alzheimer* 20. (MH "Dementia+") 21. TX (healthy OR older OR elder* OR aged) 22. S17 or S18 or S19 or S20 or S21 23. S16 and S22 |
266 |
| 6. Web of Science and conference proceedings [Last searched: 4 November 2016] |
Topic: (("l‐carnitine" OR carnitine) AND (random* OR trial OR placebo OR "double blind*" OR "blinded" OR "single blind*" OR "control group*") AND cognit*) Timespan: All years. Search language=Auto |
127 |
| 7. LILACS (BIREME) [Last searched: 4 November 2016] |
"l‐carnitine" OR carnitine [Words] and cognition OR cognitive [Words] | 3 |
| 8. CENTRAL (the Cochrane Library) (Issue 4 of 4, 2015) [Last searched: 4 November 2016] |
#1 ALC or PLC or ALCAR or ALPAR #2 "acetyl‐l‐carnitine" #3 "propionyl‐l‐carnitine" #4 L‐carnitine #5 Carnitine #6 MeSH descriptor: [Carnitine] explode all trees #7 #1 or #2 or #3 or #4 or #5 or #6 in Trials #8 cognit* or memory or mental or brain #9 healthy or older or elder* or aged #10 dement* or Alzheimer* #11 #8 or #9 or #10 in Trials #12 #7 and #11 in Trials |
730 |
| 9. ClinicalTrials.gov (www.clinicaltrials.gov) [Last searched: 4 November 2016] |
Advanced search: Condition: cognition OR cognitive AND Intervention: L‐carnitine | 2 |
| 10. WHO ICTRP Search Portal (apps.who.int/trialsearch) [includes: Australian New Zealand Clinical Trials Registry; ClinicalTrials.gov; ISRCTN; Chinese Clinical Trial Registry; Clinical Trials Registry – India; Clinical Research Information Service – Republic of Korea; German Clinical Trials Register; Iranian Registry of Clinical Trials; Japan Primary Registries Network; Pan African Clinical Trial Registry; Sri Lanka Clinical Trials Registry; The Netherlands National Trial Register] [Last searched: 4 November 2016] |
Advanced search: Condition: cognition OR cognitive AND Intervention: L‐carnitine | 0 |
| TOTAL before de‐duplication and first assess | 3880 | |
| TOTAL after de‐duplication and first assess | 181 | |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Benton 2004.
| Methods | Randomised: methods of randomisation and allocation concealment were not described Double‐blind Placebo and positive controlled Duration: 3 days |
|
| Participants | Country: UK Number of participants: 400 females Age: average 21.8 years Inclusion: people in good health and not taking any supplements similar to experiment interventions Exclusion: not specified |
|
| Interventions | There were 4 treatment groups: carnitine 500 mg per day; lecithin 1.6 g per day; carnitine 500 mg and lecithin 1.6 g per day; placebo. 4 capsules containing 1 of the above agents were given each day, 2 in the morning and 2 in the evening, for 3 days. | |
| Outcomes |
Each participant took the battery of cognitive tests 3 times: before and after 3‐day treatment, and 30 minutes after they consumed either a placebo or glucose drink after the treatment. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation was not described. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | It was stated as double‐blind; identical capsules were used for placebo and active supplement, and at end of study all participants were asked to guess what kind of ingredients had been consumed. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It was stated as double‐blind, but details were not provided. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The experimental duration was short, but the number of participants lost to the study was not specified. |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is unavailable, so there was insufficient information to permit a clear judgement. |
| Other bias | Unclear risk | The inclusion and exclusion criteria were not clearly described, and the baseline conditions were not compared between groups, therefore we are unsure about the comparability of the participants. |
Muñiz‐Pumares 2011.
| Methods | Randomised cross‐over design: methods of randomisation and allocation concealment were not described Double‐blind Placebo controlled Duration: only a single dose was given, and the outcome measures were evaluated 45 minutes after the medical beverage ingestion and immediately after exercise |
|
| Participants | Number of participants: 18 healthy volunteers with an average age as 21 ± 6 years Inclusion: healthy, active men and women |
|
| Interventions | Experimental group: L‐carnitine 500 mg + rhodiola rosea beverage (250 mg, 3% Rosavin) Control group: placebo + rhodiola rosea beverage (250 mg, 3% Rosavin) 250 mL medical beverage was given to each participant 45 min before exercise. |
|
| Outcomes |
|
|
| Notes | We found only a conference (International Sports Science + Sports Medicine Conference) abstract. We attempted to obtain detailed information but our efforts were unsuccessful. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | We were unable to obtain detailed methods. |
| Allocation concealment (selection bias) | Unclear risk | We were unable to obtain detailed methods. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | We were unable to obtain detailed methods. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | We were unable to obtain detailed methods. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | We were unable to obtain details. |
| Selective reporting (reporting bias) | Unclear risk | We were unable to obtain details. |
| Other bias | Unclear risk | We were unable to obtain details. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bonavita 1986 | The study included people with senile brain symptoms, which was not a specific neurological disorder but often referred to a number of neuropsychic signs and/or symptoms. According to baseline assessment of cognitive function, at least some of the participants were with cognitive dysfunction at enrolment. |
| Cavallini 2004 | The study mainly evaluated the efficacy of carnitine and androgen for sexual dysfunction, depressed mood, and fatigue. Our evaluation of the full text found no data relevant to the cognition outcomes studied in the present review. |
| Chan 2010 | The experimental intervention was a nutriceutical formulation containing acetyl‐L‐carnitine as well as other nutritional components, but the efficacy of acetyl‐L‐carnitine was not evaluated alone, and relevant data could not be extracted from this trial. |
| Cipolli 1990 | The study enrolled mentally impaired elderly, although the severity was mild. |
| Cruciani 2009 | Participants were with known carnitine deficiency. |
| Hakkou 1990 | The intervention studied in this trial was DL‐carnitine, which is not L‐carnitine or its derivative, as required in our protocol. |
| Hoffman 2010 | The experimental intervention was a multicomponent supplement containing acetyl‐L‐carnitine. |
| Malaguarnera 2007 | The inclusion criteria did not refer to the level of cognitive function, but the results showed that the average Mini–Mental State Examination of participants was only about 16 points at baseline, which indicated that at least some of the participants were with obvious cognitive dysfunction. |
| Malaguarnera 2008 | The study enrolled elderly people with a diagnosis of chronic fatigue syndrome, the criteria of which included cognitive complaints such as impaired memory or concentration. |
| Malaguarnera 2014 | Participants were with hepatitis C virus infection, which was associated with cognitive impairment and mood disturbance. |
| Mantero 1989 | Participants were geriatric patients with mental deterioration. |
| Martinotti 2011 | The study was designed to evaluate the effects of acetyl‐L‐carnitine for alcohol‐dependent participants, but the main outcome measures were anhedonia, melancholic, and negative symptoms; cognitive function was not measured. Furthermore, alcohol dependence is associated with cognitive impairment. |
| Pueschel 2006 | The study evaluated people with Down syndrome, who had an increased risk of developing cognitive impairment. |
| Salvioli 1994 | The study is not an RCT or a quasi‐RCT according to the full text. |
| Sugino 2007 | This trial aimed to evaluate the effects of citric acid and L‐carnitine for physical fatigue; there was no mention of evaluation of any types of cognitive functions. |
| Vecchi 1990 | The study is not an RCT or a quasi‐RCT according to the description, and the participants were with mild mental impairment. |
| Warner 1997 | All participants had a neurologic disorder, i.e. epilepsy. |
| Yonei 2008 | The experimental intervention was a multicomponent dietary supplement containing L‐carnitine. |
RCT: randomised controlled trial
Differences between protocol and review
We planned to calculate a weighted treatment effect using a fixed‐effect model or a random‐effects model across trials, but no treatment effect across trials could be calculated because there was not enough relevant data.
For missing data, we planned to use an intention‐to‐treat method or include per protocol data, but neither was done because there was insufficient information.
We planned to conduct sensitivity analyses, heterogeneity assessment, and subgroup analyses, but these were not done because only two trials were included and no meta‐analysis could be performed.
We planned to construct a 'Summary of findings' table and to include the results for global cognitive function, memory, executive function, and adverse effects. However, according to the available data from a single study (Benton 2004), we presented the results for reaction time, vigilance, immediate memory, delayed memory, and adverse effects in the 'Summary of findings' table.
Contributions of authors
Ning Chen and Mi Yang: wrote the draft of the review.
Muke Zhou, Ning Chen, Jian Guo, Jing Xiao: performed data collection, methodological quality assessment, and analyses.
Li He: the corresponding author, developed the proposal, offered expert advice, reviewed and revised the draft, and is responsible for developing and updating the review.
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Institute for Health Research (NIHR), UK.
This review was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to the Cochrane Dementia and Cognitive Improvement Group. The views and opinions expressed herein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service (NHS), or Department of Health.
Declarations of interest
Ning Chen: None known. Mi Yang: None known. Muke Zhou: None known. Jing Xiao: None known. Jian Guo: None known. Li He: None known.
New
References
References to studies included in this review
Benton 2004 {published data only}
- Benton D, Donohoe RT. The influence on cognition of the interactions between lecithin, carnitine and carbohydrate. Psychopharmacology (Berl) 2004;175:84‐91. [DOI] [PubMed] [Google Scholar]
Muñiz‐Pumares 2011 {published data only}
- Muñiz‐Pumares, Lage‐Guede A, Firth‐Clark A, Allgrove J. Effects of acute supplementation with Rhodiola rosea and L‐carnitine on exercise performance, cognitive function and cortisol in healthy active volunteers. British Journal of Sports Medicine 2011;45:A1. [Google Scholar]
References to studies excluded from this review
Bonavita 1986 {published data only}
- Bonavita E. Study of the efficacy and tolerability of L‐acetylcarnitine therapy in the senile brain. International Journal of Clinical Pharmacology, Therapy, and Toxicology 1986;24(9):511‐6. [PubMed] [Google Scholar]
Cavallini 2004 {published data only}
- Cavallini G, Caracciolo S, Vitali G, Modenini F, Biagiotti G. Carnitine versus androgen administration in the treatment of sexual dysfunction, depressed mood, and fatigue associated with male aging. Urology 2004;63(4):641‐6. [DOI] [PubMed] [Google Scholar]
Chan 2010 {published data only}
- Chan A, Remington R, Kotyla E, Lepore A, Zemianek J, Shea TB. A vitamin/nutriceutical formulation improves memory and cognitive performance in community‐dwelling adults without dementia. Journal of Nutrition, Health & Aging 2010;14(3):224‐30. [DOI] [PubMed] [Google Scholar]
Cipolli 1990 {published data only}
- Cipolli C, Chiari G. Effects of L‐acetylcarnitine on mental deterioration in the aged: initial results. Clinical Therapeutics 1990;132(6 Suppl):479‐510. [PubMed] [Google Scholar]
Cruciani 2009 {published data only}
- Cruciani RA, Dvorkin E, Homel P, Culliney B, Malamud S, Lapin J, et al. L‐carnitine supplementation in patients with advanced cancer and carnitine deficiency: a double‐blind, placebo‐controlled study. Journal of Pain and Symptom Management 2009;37(4):622‐31. [DOI] [PubMed] [Google Scholar]
Hakkou 1990 {published data only}
- Hakkou F, Jaouen C, Iraki L. A comparative study of cyproheptadine and DL carnitine on psychomotor performance and memory in healthy volunteers. Fundamental & Clinical Pharmacology 1990;4(2):191‐200. [DOI] [PubMed] [Google Scholar]
Hoffman 2010 {published data only}
- Hoffman JR, Ratamess NA, Gonzalez A, Beller NA, Hoffman MW, Olson M, et al. The effects of acute and prolonged CRAM supplementation on reaction time and subjective measures of focus and alertness in healthy college students. Journal of the International Society of Sports Nutrition 2010;15(7):39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Malaguarnera 2007 {published data only}
- Malaguarnera M, Cammalleri L, Gargante MP, Vacante M, Colonna V, Motta M. L‐Carnitine treatment reduces severity of physical and mental fatigue and increases cognitive functions in centenarians: a randomized and controlled clinical trial. American Journal of Clinical Nutrition 2007;86:1738‐44. [DOI] [PubMed] [Google Scholar]
Malaguarnera 2008 {published data only}
- Malaguarnera M, Gargante MP, Cristaldi E, Colonna V, Messano M, Koverech A, et al. Acetyl L‐carnitine (ALC) treatment in elderly patients with fatigue. Archives of Gerontology and Geriatrics 2008;46(2):181‐90. [DOI] [PubMed] [Google Scholar]
Malaguarnera 2014 {published data only}
- Malaguarnera G, Pennisi M, Gagliano C, Vacante M, Malaguarnera M, Salomone S, et al. Acetyl‐L‐carnitine supplementation during HCV therapy with pegylated interferon‐α 2b plus ribavirin: effect on work performance; a randomized clinical trial. Hepatitis Monthly 2014;14(5):e11608. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mantero 1989 {published data only}
- Mantero MA, Barbero M, Giannini R, Grosso VG, Tomasina C, Iannuccelli M. Acetyl‐L‐carnitine as a therapeutic agent for mental deterioration in geriatric patients. (Double‐blind placebo controlled study). New Trends in Clinical Neuro Pharmacology 1989;3(1):17‐24. [Google Scholar]
Martinotti 2011 {published data only}
- Martinotti G, Andreoli S, Reina D. Acetyl‐l‐Carnitine in the treatment of anhedonia, melancholic and negative symptoms in alcohol dependent subjects. Progress in Neuro‐Psychopharmacology & Biological Psychiatry 2011;35(4):953‐8. [DOI] [PubMed] [Google Scholar]
Pueschel 2006 {published data only}
- Pueschel SM. The effect of acetyl‐L‐carnitine administration on persons with Down syndrome. Research in Developmental Disabilities 2006;27(6):599‐604. [DOI] [PubMed] [Google Scholar]
Salvioli 1994 {published data only}
- Salvioli G, Neri M. L‐acetylcarnitine treatment of mental decline in the elderly. Drugs Under Experimental and Clinical Research 1994;20(4):169‐76. [PubMed] [Google Scholar]
Sugino 2007 {published data only}
- Sugino T, Aoyagi S, Shirai T, Kajimoto Y, Kajimoto O. Effects of citric acid and l‐carnitine on physical fatigue. Journal of Clinical Biochemistry and Nutrition 2007;41(3):224‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vecchi 1990 {published data only}
- Neri M, Cortelloni C, Vreese L. Methodology of a clinical controlled study of L‐acetylcarnitine. Clinica Terapeutica 1990;132(6 Suppl):457‐68. [PubMed] [Google Scholar]
- Vecchi GP, Chiari G, Cipolli C, Cortelloni C, Vreese L, Neri M. Acetyl‐l‐carnitine treatment of mental impairment in the elderly: Evidence from a multicentre study. Archives of Gerontology and Geriatrics 1991;Suppl 2:159‐68. [Google Scholar]
- Vecchi GP, Neri M, Cortelloni C, Vreese L, Sirotti C, Cipolli C, et al. Methodology of a controlled clinical study for cerebral aging evaluation. International Journal of Clinical Pharmacology Research 1990;10(1‐2):145‐52. [PubMed] [Google Scholar]
Warner 1997 {published data only}
- Warner MH, Anderson GD, McCarty JP, Farwell JR. Effect of carnitine on measures of energy levels, mood, cognition, and sleep in adolescents with epilepsy treated with valproate. Journal of Epilepsy 1997;10:126‐30. [Google Scholar]
Yonei 2008 {published data only}
- Yonei Y, Takahashi Y, Hibino S, Watanabe M, Yoshioka T. Effects on the human body of a dietary supplement containing L‐carnitine and garcinia cambogia extract: a study using double‐blind tests. Journal of Clinical Biochemistry and Nutrition 2008;42(2):89‐103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Atkins 2004
- Atkins D, Best D, Briss PA, Eccles M, Falck‐Ytter Y, Flottorp S, et al. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bach 1983
- Bach AC, Schirardin H, Sihr MO, Storck D. Free and total carnitine in human serum after oral ingestion of L‐carnitine. Diabetes & Metabolism 1983;9:121‐4. [PubMed] [Google Scholar]
Bhattacharyya 1995
- Bhattacharyya PK, Friedman S, Fraenkel G. The effect of some derivatives and structural analogs of carnitine on the nutrition of Tenebrio molitor. Archives of Biochemistry and Biophysics 1955;54(2):424‐31. [DOI] [PubMed] [Google Scholar]
Brass 1994a
- Brass EP, Hoppel CL, Hiatt WR. Effect of intravenous L‐carnitine on carnitine homeostasis and fuel metabolism during exercise in humans. Clinical Pharmacology & Therapeutics 1994;55:681‐92. [DOI] [PubMed] [Google Scholar]
Brass 1994b
- Brass EP. Overview of coenzyme A metabolism and its role in cellular toxicity. Chemico‐Biological Interactions 1994;90:203‐14. [DOI] [PubMed] [Google Scholar]
Brass 1994c
- Brass EP, Hiatt WR. Carnitine metabolism during exercise. Life Sciences 1994;54:1383‐93. [DOI] [PubMed] [Google Scholar]
Calvin 2011
- Calvin CM, Deary IJ, Fenton C, Roberts BA, Der G, Leckenby N, et al. Intelligence in youth and all‐cause‐mortality: systematic review with meta‐analysis. International Journal of Epidemiology 2011 Jun;40(3):626‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cecere 2002
- Cecere A, Ciaramella F, Tancredi L, Romano C, Gattoni A. Efficacy of L‐carnitine in reducing hyperammonaemia and improving neuropsychological test performance in patients with hepatic cirrhosis: results of a randomised trial. Clinical Drug Investigation 2002;22(Suppl 1):7‐14. [DOI] [PubMed] [Google Scholar]
Clark 2014
- Clark VP, Parasuraman R. Neuroenhancement: enhancing brain and mind in health and in disease. Neuroimage 2014 Jan 15;85 Pt 3:889‐94. [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Deline 2014
- Deline S, Baggio S, Studer J, N'Goran AN, Dupuis M, Henchoz Y, et al. Use of neuroenhancement drugs: prevalence, frequency and use expectations in Switzerland. International Journal of Environmental Research and Public Health 2014;11(3):3032‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Eickenhorst 2012
- Eickenhorst P, Vitzthum K, Klapp BF, Groneberg D, Mache S. Neuroenhancement among German university students: motives, expectations, and relationship with psychoactive lifestyle drugs. Journal of Psychoactive Drugs 2012;44(5):418‐27. [DOI] [PubMed] [Google Scholar]
Evangeliou 2003
- Evangeliou A, Vlassopoulos D. Carnitine metabolism and deficit ‐ when supplementation is necessary?. Current Pharmaceutical Biotechnology 2003;4:211‐9. [DOI] [PubMed] [Google Scholar]
Franke 2011
- Franke AG, Bonertz C, Christmann M, Engeser S, Lieb K. Attitudes toward cognitive enhancement in users and nonusers of stimulants for cognitive enhancement: a pilot study. AJOB Primary Research 2012;3(1):48‐57. [Google Scholar]
Harper 1988
- Harper P, Elwin CE, Cederblad G. Pharmacokinetics of intravenous and oral bolus doses of L‐carnitine in healthy subjects. European Journal of Clinical Pharmacology 1988;35:555‐62. [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
- Higgins JPT, Deeks JJ (editors). Chapter 7: Selecting studies and collecting data. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hudson 2003
- Hudson SA, Tabet N. Acetyl‐l‐carnitine for dementia. Cochrane Database of Systematic Reviews 2003, Issue 2. [DOI: 10.1002/14651858.CD003158] [DOI] [PMC free article] [PubMed] [Google Scholar]
ICHEWG 1997
- International Conference on Harmonisation Expert Working Group. International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use adopts Consolidated Guideline on Good Clinical Practice in the Conduct of Clinical Trials on Medicinal Products for Human Use. International Digest of Health Legislation 1997;48:231‐4. [PubMed] [Google Scholar]
Jensen 1987
- Jensen AR. Individual differences in the Hick paradigm. Speed of Information‐Processing and Intelligence. Norwood (NJ): Ablex Publishing Corporation, 1987:101–75. [Google Scholar]
Kelly 1998
- Kelly GS. L‐Carnitine: therapeutic applications of a conditionally‐essential amino acid. Alternative Medicine Review 1988;3(5):345‐60. [PubMed] [Google Scholar]
L‐Carnitine 2015
- www.lcarnitine.org. L‐Carnitine. www.lcarnitine.org/ (accessed prior to 16 March 2017).
Malaguarnera 2012
- Malaguarnera M. Carnitine derivatives: clinical usefulness. Current Opinion in Gastroenterology 2012;28(2):166‐76. [DOI] [PubMed] [Google Scholar]
Malaguarnera 2013
- Malaguarnera M. Acetyl‐L‐carnitine in hepatic encephalopathy. Metabolic Brain Disease 2013;28(2):193‐9. [DOI] [PubMed] [Google Scholar]
McKendrick 2014
- McKendrick R, Ayaz H, Olmstead R, Parasuraman R. Enhancing dual‐task performance with verbal and spatial working memory training: continuous monitoring of cerebral hemodynamics with NIRS. NeuroImage 2014 Jan;85 Pt 3:1014‐26. [DOI] [PubMed] [Google Scholar]
Mingorance 2011
- Mingorance C, Rodríguez‐Rodríguez R, Justo ML, Alvarez de Sotomayor M, Herrera MD. Critical update for the clinical use of L‐carnitine analogs in cardiometabolic disorders. Vascular Health and Risk Management 2011;7:169‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Montgomery 2003
- Montgomery SA, Thal LJ, Amrein R. Meta‐analysis of double blind randomised controlled clinical trials acetyl‐l‐carnitine versus placebo in the treatment of mild cognitive impairment and mild Alzheimer's disease. International Clinical Psychopharmacology 2003;18:61‐71. [DOI] [PubMed] [Google Scholar]
Nalecz 1996
- Nalecz KA, Nalecz MJ. Carnitine ‐ a known compound, a novel function in neural cells. Acta Neurobiologiae Experimentalis (Wars) 1996;56:597‐609. [DOI] [PubMed] [Google Scholar]
Osler 2007
- Osler M, Andersen AM, Laursen B, Lawlor DA. Cognitive function in childhood and early adulthood and injuries later in life: the Metropolit 1953 male birth cohort. International Journal of Epidemiology 2007 Feb;36(1):212‐9. [DOI] [PubMed] [Google Scholar]
Pettegrew 2000
- Pettegrew JW, Levine J, McClure RJ. Acetyl‐L‐carnitine physical‐chemical, metabolic, and therapeutic properties: relevance for its mode of action in Alzheimer’s disease and geriatric depression. Molecular Psychiatry 2000;5:616‐32. [DOI] [PubMed] [Google Scholar]
Puca 1990
- Puca FM, Genco S, Specchio LM, et al. Clinical pharmacodynamics of acetyl‐L‐carnitine in patients with Parkinson's disease. International Journal of Clinical Pharmacology Research 1990;10(1‐2):139‐43. [PubMed] [Google Scholar]
RevMan 5 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Ribas 2014
- Ribas GS, Vargas CR, Wajner M. L‐carnitine supplementation as a potential antioxidant therapy for inherited neurometabolic disorders. Gene 2014;533(2):469‐76. [DOI] [PubMed] [Google Scholar]
Sandberg 2006
- Sandberg A, Bostrom N. Converging cognitive enhancements. Annals of the New York Academy of Sciences 2006 Dec;1093:201‐27. [DOI] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings' tables. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Shang 2014
- Shang R, Sun Z, Li H. Effective dosing of L‐carnitine in the secondary prevention of cardiovascular disease: a systematic review and meta‐analysis. BMC Cardiovascular Disorders 2014;14:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
Spagnoli 1991
- Spagnoli A, Lucca U, Menasce G, Bandera L, Cizza G, Forloni G, et al. Long‐term acetyl‐L‐carnitine treatment in Alzheimer's disease. Neurology 1991;41(11):1726‐32. [DOI] [PubMed] [Google Scholar]
Stanley 2004
- Stanley CA. Carnitine deficiency disorders in children. Annals of the New York Academy of Sciences 2004;1033:42‐51. [DOI] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JAC, Egger M, Moher D (editors). Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Tempesta 1990
- Tempesta E, Troncon R, Janiri L, Colusso L, Riscica P, Saraceni G, et al. Role of acetyl‐L‐carnitine in the treatment of cognitive deficit in chronic alcoholism. International Journal of Clinical Pharmacology Research 1990;10:101‐7. [PubMed] [Google Scholar]
Thal 1996
- Thal LJ, Carta A, Clarke WR, Ferris SH, Friedland RP, Petersen RC, et al. A 1‐year multicenter placebo‐controlled study of acetyl‐L‐carnitine in patients with Alzheimer's disease. Neurology 1996;47(3):105‐11. [DOI] [PubMed] [Google Scholar]
Thal 2000
- Thal LJ, Calvani M, Amato A, Carta A. A 1‐year controlled trial of acetyl‐l‐carnitine in early‐onset AD. Neurology 2000;55(6):805‐10. [DOI] [PubMed] [Google Scholar]
Virmani 2004
- Virmani A, Binienda Z. Role of carnitine esters in brain neuropathology. Molecular Aspects of Medicine 2004;25:533‐49. [DOI] [PubMed] [Google Scholar]
Wang 2014
- Wang SM, Han C, Lee SJ, Patkar AA, Masand PS, Pae CU. A review of current evidence for acetyl‐l‐carnitine in the treatment of depression. Journal of Psychiatric Research 2014;53:30‐7. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Yang 2011
- Yang M, Zhou M, Xiao J, Chen N, Guo J, He L. L‐carnitine for cognition in healthy subjects. Cochrane Database of Systematic Reviews 2011, Issue 11. [DOI: 10.1002/14651858.CD009374] [DOI] [Google Scholar]


