Abstract
Background
Breast cancer continues to be the most commonly diagnosed cancer in women globally. Early detection, diagnosis and treatment of breast cancer are key to better outcomes. Since many women will discover a breast cancer symptom themselves, it is important that they are breast cancer aware i.e. have the knowledge, skills and confidence to detect breast changes and present promptly to a healthcare professional.
Objectives
To assess the effectiveness of interventions for raising breast cancer awareness in women.
Search methods
We searched the Cochrane Breast Cancer Group's Specialised Register (searched 25 January 2016), Cochrane Central Register of Controlled Trials (CENTRAL; 2015, Issue 12) in the Cochrane Library (searched 27 January 2016), MEDLINE OvidSP (2008 to 27 January 2016), Embase (Embase.com, 2008 to 27 January 2016), the World Health Organization’s International Clinical Trials Registry Platform (ICTRP) search portal and ClinicalTrials.gov (searched 27 Feburary 2016). We also searched the reference lists of identified articles and reviews and the grey literature for conference proceedings and published abstracts. No language restriction was applied.
Selection criteria
Randomised controlled trials (RCTs) focusing on interventions for raising women’s breast cancer awareness i.e. knowledge of potential breast cancer symptoms/changes and the confidence to look at and feel their breasts, using any means of delivery, i.e. one‐to‐one/group/mass media campaign(s).
Data collection and analysis
Two authors selected studies, independently extracted data and assessed risk of bias. We reported the odds ratio (OR) and 95% confidence intervals (CIs) for dichotomous outcomes and mean difference (MD) and standard deviation (SD) for continuous outcomes. Since it was not possible to combine data from included studies due to their heterogeneity, we present a narrative synthesis. We assessed the quality of evidence using GRADE methods.
Main results
We included two RCTs involving 997 women: one RCT (867 women) randomised women to receive either a written booklet and usual care (intervention group 1), a written booklet and usual care plus a verbal interaction with a radiographer or research psychologist (intervention group 2) or usual care (control group); and the second RCT (130 women) randomised women to either an educational programme (three sessions of 60 to 90 minutes) or no intervention (control group).
Knowledge of breast cancer symptoms
In the first study, knowledge of non‐lump symptoms increased in intervention group 1 compared to the control group at two years postintervention, but not significantly (OR 1.1, 95% CI 0.7 to 1.6; P = 0.66; 449 women; moderate‐quality evidence). Similarly, at two years postintervention, knowledge of symptoms increased in the intervention group 2 compared to the control group but not significantly (OR 1.4, 95% CI 0.9 to 2.1; P = 0.11; 434 women; moderate‐quality evidence). In the second study, women’s awareness of breast cancer symptoms had increased one month post intervention in the educational group (MD 3.45, SD 5.11; 65 women; low‐quality evidence) compared to the control group (MD −0.68, SD 5.93; 65 women; P < 0.001), where there was a decrease in awareness.
Knowledge of age‐related risk
In the first study, women’s knowledge of age‐related risk of breast cancer increased, but not significantly, in intervention group 1 compared to control at two years postintervention (OR 1.8; 95% CI 0.9 to 3.5; P < 0.08; 447 women; moderate‐quality evidence). Women's knowledge of risk increased significantly in intervention group 2 compared to control at two years postintervention (OR 4.8, 95% CI 2.6 to 9.0; P < 0.001; 431 women; moderate‐quality evidence). In the second study, women’s perceived susceptibility (how at risk they considered themselves) to breast cancer had increased significantly one month post intervention in the educational group (MD 1.31, SD 3.57; 65 women; low‐quality evidence) compared to the control group (MD −0.55, SD 3.31; 65 women; P = 0.005), where a decrease in perceived susceptibility was noted.
Frequency of Breast Checking
In the first study, no significant change was noted for intervention group 1 compared to control at two years postintervention (OR 1.1, 95% CI 0.8 to 1.6; P = 0.54; 457 women; moderate‐quality evidence). Monthly breast checking increased, but not significantly, in intervention group 2 compared to control at two years postintervention (OR 1.3, 95% CI 0.9 to 1.9; P = 0.14; 445 women; moderate‐quality evidence). In the second study, women’s breast cancer preventive behaviours increased significantly one month post intervention in the educational group (MD 1.21, SD 2.54; 65 women; low‐quality evidence) compared to the control group (MD 0.15, SD 2.94; 65 women; P < 0.045).
Breast Cancer Awareness
Women’s overall breast cancer awareness did not change in intervention group 1 compared to control at two years postintervention (OR 1.8, 95% CI 0.6 to 5.30; P = 0.32; 435 women; moderate‐quality evidence) while overall awareness increased in the intervention group 2 compared to control at two years postintervention (OR 8.1, 95% CI 2.7 to 25.0; P < 0.001; 420 women; moderate‐quality evidence). In the second study, there was a significant increase in scores on the Health Belief Model (that included the constructs of awareness and perceived susceptibility) at one month postintervention in the educational group (mean 1.21, SD 2.54; 65 women) compared to the control group (mean 0.15, SD 2.94; 65 women; P = 0.045).
Neither study reported outcomes relating to motivation to check their breasts, confidence to seek help, time from breast symptom discovery to presentation to a healthcare professional, intentions to seek help, quality of life, adverse effects of the interventions, stages of breast cancer, survival estimates or breast cancer mortality rates.
Authors' conclusions
Based on the results of two RCTs, a brief intervention has the potential to increase women’s breast cancer awareness. However, findings of this review should be interpreted with caution, as GRADE assessment identified moderate‐quality evidence in only one of the two studies reviewed. In addition, the included trials were heterogeneous in terms of the interventions, population studied and outcomes measured. Therefore, current evidence cannot be generalised to the wider context. Further studies including larger samples, validated outcome measures and longitudinal approaches are warranted.
Plain language summary
Interventions for raising breast cancer awareness in women
Review question We reviewed the evidence about the effect of different interventions for raising breast cancer awareness in women. We found two randomised controlled trials, the highest quality of research evidence.
Background Breast cancer is the most commonly diagnosed cancer in women. Early detection, diagnosis and treatment of breast cancer are key to better outcomes. Since many women will discover a breast symptom themselves, it is important that they are breast cancer aware i.e. that they have the knowledge, skills and confidence to notice any breast changes and visit their doctor promptly.
Study characteristics A search for trials investigating interventions on breast cancer awareness in women was run in January 2016. We found two trials with a total of 997 women.
The Promoting Early Presentation (PEP) study, funded by Breast Cancer UK, involved randomising 867 women to receive one of three interventions: (1) a written booklet and usual care, (2) a written booklet and usual care plus one‐to‐one discussion with a healthcare professional or (3) usual care only. Women were aged between 67 to 70 years and recruited into the study at breast cancer screening units in the UK.
The Zahedan University of Medical Sciences (ZUMS) study involved randomising 130 women into two groups that received either: (1) an educational programme using written and oral materials that focused on "breast cancer preventive behaviours" (e.g. having a healthy diet and positive beliefs towards breast self‐examining behaviour) or (2) no intervention. Women were employed at ZUMS and aged between 35 and 39 years.
Key outcomes Study outcomes were measured differently in the two studies. The PEP study assessed outcomes at one month, one year and two years after the intervention. The ZUMS study measured outcomes at one month after the intervention. Since the studies were very different in terms of the participants' age, interventions, outcomes and time points measured, the results are reported separately.
Knowledge of breast cancer symptoms In PEP: women's knowledge of breast cancer symptoms seemed to somewhat improve after receiving either the written booklet or written booklet plus verbal interaction. These results improved when compared to usual care at 2 years postintervention. In ZUMS: women’s awareness of breast cancer symptoms increased one month after the educational programme. Knowledge of age‐related risk of breast cancer In PEP: knowledge of age‐related risk increased for women who had received a written booklet and interacted with a healthcare professional compared to usual care at 2 years postintervention. For women who only received the booklet, there was less of a comparable increase in knowledge. In ZUMS: this study only measured if women perceived themselves to be at risk of getting breast cancer. This self‐perception of risk did increase at one month following the intervention.
Self‐reported breast checking In PEP: women's reported monthly breast checking increased, but not significantly, at 2 years postintervention compared to usual care. In ZUMS: women's reported "breast cancer preventive behaviours" increased one month after the intervention. Specifically, this refers to their positive beliefs towards breast self‐examining behaviour.
Overall breast cancer awareness In PEP: women's breast cancer awareness overall did not change after receiving a booklet alone compared to usual care at 2 years after the intervention. However, breast cancer awareness increased in women who had received a written booklet and interacted with a healthcare professional. This behaviour change was in comparison to usual care at 2 years postintervention. In ZUMS: women's "breast cancer preventive behaviours" were reported to increase at one month.
None of the studies reported on other parts of breast awareness, the intention to seek help, quality of life, adverse effects of the interventions, or breast cancer‐related outcomes. Quality of the evidence The evidence was considered to be moderate quality in the PEP study and low quality in the ZUMS study. Neither study clearly defined ‘breast cancer awareness’. The lack of high quality studies limited our ability to draw conclusions. However, the PEP study results suggest that combining written information and a one‐to‐one discussion had a long‐term effect on increasing women’s breast cancer awareness. In the future, studies should use larger samples and follow the women for a longer time.
Background
Description of the condition
Although death rates are declining (Siegal 2013), breast cancer continues to be the most commonly diagnosed cancer in women globally (Bray 2012). According to World Health Organization (WHO) Global Health Estimates, worldwide over 508,000 women are estimated to have died in 2011 due to breast cancer (WHO 2014). Early diagnosis of breast cancer is linked to more favourable outcomes and longer survival (Richards 1999a; Richards 1999b). While screening mammography is effective in detecting some breast cancers, accuracy is dependent on breast density (Carney 2003). Additionally, many breast tumours are initially detected by women themselves (not by mammographic screening) (Cancer Research UK 2014). However, some women postpone presenting to a healthcare professional (HCP) on finding a breast symptoms for a variety of reasons, including fear of a cancer diagnosis (O'Mahony 2011). In an attempt to develop consistency in definitions and approaches used in early cancer‐diagnosis research, Weller 2012 developed the 'Aarhus checklist' as a resource for researchers. In their paper outlining this checklist, the "patient interval" is described as (i) the "time taken to interpret bodily changes/symptoms" i.e. "appraisal interval" and (ii) the "time taken to act upon those interpretations and seek help" i.e. the "help‐seeking interval" (Weller 2012). To date, in relation to breast symptoms, this "patient interval" varies from one month to three months or even more (Arndt 2002; Meechan 2002; O'Mahony 2009; Jones 2010; O'Mahony 2011; Forbes 2012; O'Mahony 2013). Postponement of help‐seeking (previously referred to as 'delay') is associated in particular with women's lack of knowledge of non‐lump breast symptoms (e.g. nipple changes; O'Mahony 2013). This is a worrying situation given the increased emphasis on prompt presentation of breast symptoms and the associated link with better healthcare outcomes for women who are diagnosed with breast cancer earlier in the disease trajectory.
Breast screening may be carried out in a number of ways including breast self‐examination (BSE), clinical breast examination and screening mammography (MacBride 2012). Screening mammography is described as "the most widely used and best available tool for detecting breast cancer" (MacBride 2012). However, the efficacy of screening programmes is dependent on women's participation (Chan 2007). Since many women will present with a palpable breast mass (MacBride 2012), it is important that they have the knowledge, skills and confidence necessary to detect and seek help for breast cancer symptoms.
BSE is described as "a regular, repetitive monthly palpation to a rigorous set method performed by the woman at the same time each month" (Thornton 2008), using a method that has been formally taught to women (MacBride 2012). While BSE continues to be advocated for early detection of breast symptoms (American Cancer Society 2014), a Cochrane Review reported lack of evidence to support the use of breast screening by BSE or clinical examination of breasts by a HCP in improving breast cancer mortality rates (Kösters 2003). Nonetheless, the review highlighted the need for women to be able to identify breast changes and seek prompt medical advice should they discover changes that may be breast cancer. This is further reiterated by the WHO 2014 report, which recommends BSE as a means of "raising awareness amongst women at risk" of breast cancer. Additionally, the practice of BSE is incorporated into some breast health awareness interventions (Chan 2007; Kharboush 2011), and into breast health‐promoting strategies (Byrne 2009). Alternatively, the term 'breast checking' has been used as part of a measure of breast cancer awareness where women were asked to report on how frequently they checked their breasts (Linsell 2009; Forbes 2011a; Forbes 2012).
Currently the term 'breast awareness' is cited in relation to breast cancer screening and early detection of breast cancer (National Comprehensive Cancer Network 2013; NICE 2013; American Cancer Society 2014; IARC 2014). Furthermore, a recent editorial in The Breast Journal highlighted "a paradigm shift" from BSE to breast awareness, advocating that breast awareness become part of general breast health education (MacBride 2012). Breast awareness involves women having confidence to 'look at and feel' their breasts so that they know what is normal for their own body and what changes to look and feel for (Irish Cancer Society 2013; National Comprehensive Cancer Network 2013; NHS Breast Cancer Screening Programme 2013). In addition, breast awareness requires that women have an understanding of the implications of breast changes and consult with their healthcare provider promptly (MacBride 2012). Therefore, the concepts of 'breast awareness' and 'breast cancer awareness' are inextricably linked. In addition BSE and, more recently, breast checking are referred to as the behavioural components of each.
Whilst breast awareness is frequently advocated, evidence suggests that women generally are not breast aware (Kharboush 2011). Furthermore, it is suggested that public education about cancer symptoms and the value of early detection could enhance early presentation and improve cancer outcomes (Robb 2009). Thus, the need for HCPs to increase awareness of breast cancer symptoms in women is crucial. In the United Kingdom, the Promoting Early Presentation (PEP) intervention was developed providing older women (who are at much higher risk of developing breast cancer) with knowledge, skills, confidence and motivation to present early with breast cancer symptoms (Linsell 2009; Forbes 2011a; Forbes 2012). However, due to the earlier median age of diagnosis for breast cancer, compared with other major cancers, it is suggested that women have a slightly higher probability of developing cancer before age 60 years (Siegal 2011). Currently, in Ireland 50% of women who are newly diagnosed with breast cancer are under 60 years of age (mean age of diagnosis is 59.6 years; National Cancer Registry Ireland 2012). A recent American study concluded that while age is not an independent predictor of delayed diagnosis of breast cancer, further research is necessary to enhance symptom recognition amongst women and healthcare providers (Partridge 2012). Thus, more women of all ages, in particular young women, might present with breast cancer at an earlier stage. In addition, the need to reduce the health and economic burden of a breast cancer diagnosis in younger women (aged 20 to 49 years) in the United States of America has recently been highlighted (Ekwueme 2014). Thus, increasing breast awareness in women is necessary if these targets are to be met. A systematic review of interventions to promote cancer awareness and early presentation found limited evidence of the effectiveness of such interventions (Austoker 2009). In addition, there is lack of evidence of the impact of increased breast cancer awareness on early detection of breast cancer. Therefore, a review to assess the effectiveness of interventions for raising breast cancer awareness in women is warranted.
Description of the intervention
For this Cochrane Review, we considered any intervention designed to raise awareness of breast cancer. We included the interventions of information or education, or both, specific to: (i) breast cancer (potential breast cancer symptoms/changes); and (ii) breast awareness (i.e. women having the confidence to look at and feel (palpate) their breasts so that they know what is normal for their own body and what changes to look and feel for). We extracted details relating to: (i) the format (written, verbal, online); (ii) timing (number of sessions; time between sessions and duration of follow‐up period); (iii) method of delivery (one‐to‐one/group/mass media campaign(s); (iv) content; and (v) theoretical underpinnings of each intervention.
How the intervention might work
The development of complex health‐related interventions requires a clear theoretical basis (Campbell 2007; Craig 2008). Understanding of the theoretical perspectives underpinning such interventions is critical if their effectiveness and usefulness in practice are to be evaluated (Michie 2012). Therefore, clarity surrounding the factors linked to breast cancer awareness and initiation of behavioural change is critical in terms of designing interventions to raise breast cancer awareness in women.
Currently, information and education relating to the promotion of breast cancer awareness are either specifically targeted at 'high risk' individuals who have a greater risk of developing breast cancer or they may be directed towards women in general. However, there is a need to develop more innovative strategies to promote breast health awareness and early detection of breast cancer in women (Byrne 2009). It is suggested that early diagnosis of breast cancer (enhanced by raising women's breast cancer awareness) could lead to decreased morbidity or mortality rates, or both (Richards 2009).
Why it is important to do this review
Increasing women's awareness of breast cancer symptoms aims to increase the number of women who present early to a HCP with symptoms. Early presentation to a HCP has the potential to increase early detection of breast cancer resulting in early treatment and enhanced survival rates for women (Richards 2009). Conversely, as highlighted by Kösters 2003, increased BSE may lead to unnecessary anxiety, medical consultations and costly follow‐up screening procedures for women. It could be argued that increasing breast cancer awareness could have similar effects. However, the benefits of early detection and prompt presentation of symptoms could outweigh these.
It is apparent that there has been no systematic review undertaken on the effects of educational interventions for raising breast cancer awareness in women. Such a review would provide clarity in relation to these interventions and on outcomes for women who are subsequently diagnosed with breast cancer. Thus, a systematic review to determine the impact of interventions for raising breast cancer awareness in women would benefit HCPs globally in their efforts to reduce the breast cancer burden through its early detection (Bray 2012), diagnosis and treatment. Data from the review will determine the impact of increased breast cancer awareness on earlier detection, stage of cancer at diagnosis and survival outcomes. This knowledge could help to direct future strategies around the promotion of breast cancer awareness and contribute to the global effort to reduce mortality and morbidity due to breast cancer.
Objectives
To assess the effectiveness of interventions for raising breast cancer awareness in women.
Methods
Criteria for considering studies for this review
Types of studies
We searched for randomised controlled trials (RCTs) and cluster RCTs of interventions for raising breast cancer awareness in women. In addition, we considered non‐randomised studies provided they had (i) a control group and (ii) pre‐ and post‐test estimate of the effectiveness of the intervention. Attention was given to the description of levels of potential confounders (e.g. age, socioeconomic status, education level, ethnicity, family history of breast cancer, previous benign disease) in the intervention and non‐intervention group within studies and whether or not researchers adjusted for these.
Types of participants
Women (with or without a previous breast cancer diagnosis) specifically recruited to receive an intervention to raise breast cancer awareness were eligible for inclusion. We excluded interventions aimed at raising breast cancer awareness amongst HCPs.
Types of interventions
Group or individual educational interventions specifically focusing on raising breast cancer awareness in women were eligible for inclusion. Interventions promoting breast cancer awareness for women through the provision of information on (i) breast cancer symptoms and (ii) breast awareness (i.e. women having the confidence to look at and feel (palpate) their breasts so that they know what is normal for their own body and what changes to look and feel for) were sought (Linsell 2009; Forbes 2011a; Forbes 2012).
Intervention in any setting (i.e. clinical/online/community) and involving one single session or a number of sessions were considered. Interventions provided in a combination of various formats including written brochures, video/audio tape, online or media campaigns, were sought.
We excluded studies in which the intervention formed part of a multi‐component intervention in order to avoid confounding effects (i.e. interventions associated with knowledge of other cancers, other chronic illnesses and general lifestyle behaviours). We excluded interventions promoting uptake of breast cancer screening or BSE, exclusively.
Standard care or no intervention was eligible as the comparator.
Types of outcome measures
Primary outcomes
Women's knowledge of breast cancer symptoms. This was based on patient‐reported knowledge and awareness scores from validated scales e.g. Breast Cancer Awareness Measure: BCAM (Linsell 2010). In addition, we used other measures of knowledge or awareness where reported e.g. knowledge of age‐related risk.
Measure of confidence to check breasts i.e. engagement in self‐care behaviours relating to breast awareness (i.e. women looking at and feeling/palpating their breasts so that they know what is normal for their own body and what changes to look and feel for). This was apparent in data reported by women relating to their engagement in these self‐care behaviours i.e. frequency of breast checking at specific time frames.
Measure of breast cancer awareness overall (where available).
Measures of women's motivation to check their breasts, as outlined above (where available).
Measures of confidence to seek help when breast cancer symptoms were noticed (where available).
Measure of time from breast cancer symptom being noticed to presentation to a HCP (time‐to‐event data) indicating postponed or prompt help‐seeking behaviour (where available).
Measure of women's intentions to seek help in the event of noticing a breast cancer symptom and their perceptions of barriers to help‐seeking (where available).
Secondary outcomes
Quality of life (classified by scales used by the trial authors) or any measure of health status (i.e. physical, psychological, social, spiritual, existential).
Adverse effects of receiving the intervention on breast cancer awareness or adverse outcomes related to false positive findings of symptoms (such as increased anxiety) assessed by any validated self‐report scale, or both.
Stage of breast cancer at diagnosis i.e. tumour, node, metastases status (where reported).
Survival estimates: i.e. measured either from time of intervention or time of breast cancer diagnosis (where reported).
Breast cancer‐specific mortality and all‐cause mortality (where reported).
Main outcomes for assessing the quality of the evidence
Two review authors (MOM and JH) independently assessed the quality of the evidence using the GRADE approach as outlined in Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2011), and on the GRADE web‐site (http://www.gradeworkinggroup.org/). We assigned ratings of 'high', 'moderate', 'low' or 'very low' to each of the following primary outcomes: knowledge of breast cancer symptoms, knowledge of age‐related risk, frequency of breast checking and breast cancer awareness. The quality of the evidence for adverse events or secondary outcomes was not assessed as these were not reported in the studies reviewed.
Search methods for identification of studies
Electronic searches
We searched the following databases:
The Cochrane Breast Cancer Group's (CBCG's) Specialised Register (searched 25 January 2016). Details of search strategies used by the CBCG for the identification of studies and procedures to code references are outlined in the CBCG's module (http://onlinelibrary.wiley.com/o/cochrane/clabout/articles/BREASTCA/frame.html). We extracted studies coded with the key words "breast cancer", "breast cancer awareness", "breast awareness", "health education", "health promotion", "educational program", and "educational intervention" and considered those studies for inclusion.
Cochrane Central Register of Controlled Trials (CENTRAL; 2015, Issue 12) in the Cochrane Library (searched 27 January 2016). See Appendix 1.
MEDLINE OvidSP (2008 to 27 January 2016). See Appendix 2.
Embase (Embase.com, 2008 to 27 January 2006). See Appendix 3 and Appendix 4.
The WHO International Clinical Trials Registry Platform (ICTRP) search portal (apps.who.int/trialsearch/Default.aspx) for all prospectively registered and ongoing trials (searched 27 February 2016). See Appendix 5.
Clinicaltrials.gov (ClinicalTrials.gov) (searched 27 February 2016). See Appendix 6.
Searching other resources
Bibliographic searching
We sought further studies from reference lists of identified relevant studies or reviews. A copy of the full article for each reference reporting a potentially eligible study was obtained. Where this was not possible, we attempted to contact trial authors for additional information. We also performed a forwards citation search as appropriate.
Grey literature
We checked relevant grey literature (i.e. unpublished data such as reports, conference proceedings/abstracts, and doctoral theses) to enable us to retrieve as much information as possible and minimise the effects of publication bias (up to 22 March 2016).
Data collection and analysis
Selection of studies
Two review authors (MOM and JH) independently assessed the titles and abstracts of each identified study for inclusion in the review and examined compliance of identified studies with the eligibility criteria. Following this initial evaluation, we retrieved the full‐text articles of all potentially relevant publications. We resolved any disagreements regarding eligibility of studies by consulting a third author (MAC). Excluded studies were recorded in the 'Characteristics of excluded studies' section. We translated studies reported in a language other than English, if necessary.
Data extraction and management
Two review authors (MOM and JH) extracted data from all relevant studies using a data extraction form.
Information recorded included:
study details: author, date, country of origin;
participants: socio‐demographics (age, socio‐economic status, education level, ethnicity); breast cancer history (previous or family history, or both); number receiving intervention, number receiving usual care;
methods: study aim, design, total study duration;
intervention details: content, format, timing (already outlined), method of delivery and theoretical underpinnings (if any);
outcomes: extraction of all relevant findings related to primary and secondary outcomes as specified previously;
withdrawals, length and method of follow–up and the number of participants followed up;
miscellaneous issues.
Non‐randomised studies were not identified. If identified in the review update, we intend to record the following information.
Methods used to control for confounders.
Adjusted and unadjusted outcome measures.
For RCTs, we resolved any disagreements regarding extraction of quantitative data by consulting a third author (MAC). Where necessary, we sought additional data or information from the original trial authors. Where we retrieved studies with more than one publication, our decisions regarding which version to include depended on its match with the inclusion criteria and relevance to the overall aim of the review. One additional report was included (Forbes 2011a relating to PEP).
Assessment of risk of bias in included studies
Two review authors (MOM and JH) independently assessed the risk of bias of included studies using the Cochrane 'Risk of bias' tool (Chapter 8 of Higgins 2011), the Cochrane EPOC Group's 'Risk of bias' criteria (Cochrane EPOC Group 2013) and Norris 2013. As the review did not include non‐randomised studies, it was not necessary to use guidance from Chapter 13 of Higgins 2011.
For assessing the risk of bias in RCTs, we considered the six bias domains of: selection bias; performance bias; detection bias; attrition bias; reporting bias; and other potential sources of bias. We assigned each risk of bias domain a judgement of 'high', 'low' or 'unclear' risk of bias. In the event of not reaching consensus, the option of consulting a third review author (MAC) was available. Where necessary, we contacted the original trial authors to seek further clarification of methods used. We summarised the results using both a 'Risk of bias' graph and a 'Risk of bias' summary. Key questions addressing each bias criterion are outlined below.
Selection bias
Was the allocation sequence adequately generated?
We scored:
'low risk' if a random component in the sequence generation process is described (e.g. referring to a random number table);
'high risk' when a non‐random method is used (e.g. performed by date of admission);
'unclear risk' if not specified in the paper (Cochrane EPOC Group 2013).
Was the allocation adequately concealed?
We scored:
'low risk' if participants and investigators enrolling participants could not foresee assignment (e.g. a centralised randomisation scheme, an on‐site computer system or sealed opaque envelopes were used) (Higgins 2011, Section 8.10; Cochrane EPOC Group 2013);
'high risk' if participants and investigators enrolling participants could foresee assignment. Also non‐randomised studies should be scored 'high risk';
'unclear risk' if not specified in the paper (Higgins 2011, Section 8.10; Cochrane EPOC Group 2013).
For future updates, if non‐randomised studies are included, we would consider the following questions:
Were baseline outcome measurements similar?
We will score:
'low risk' if performance or patient outcomes were measured prior to the intervention, and no important differences were present across study groups;
'high risk' if important differences were present and not adjusted for in the analysis;
'unclear risk' if not specified in the paper (Cochrane EPOC Group 2013).
Were baseline characteristics similar?
'Low risk' if baseline characteristics of the experimental and control groups are reported and are similar.
'Unclear risk' if it is not clear in the paper (e.g. characteristics were mentioned in text but no data were presented).
'High risk' if there is no report of characteristics in text or tables or if there are statistically significant differences between control and intervention providers (Cochrane EPOC Group 2013).
Was there adequate adjusting for confounding?
'Low risk' if appropriate methods were used to adjust for confounding.
'Unclear risk' if the methods used to adjust for confounding were not reported.
'High risk' if potential confounding from the following variables were not addressed: age, socio‐economic status, education level, ethnicity, family history of breast cancer, previous benign disease (Higgins 2011, Section 13.5; Norris 2013).
Performance/detection bias
Was knowledge of the allocated interventions adequately prevented during the study?
We scored:
'low risk' if the trial authors state explicitly that the primary outcome variables were assessed blindly or the outcomes are objective, e.g. time‐to‐event data (prompt or delayed help‐seeking behaviour, i.e. presentation of breast symptoms to HCP);
'high risk' if the outcomes were not assessed blindly;
'unclear risk' if not specified in the paper (Cochrane EPOC Group 2013).
Attrition bias
Were incomplete outcome data adequately addressed?
We scored:
'low risk' if missing outcome measures were unlikely to bias the results (e.g. reasons for missing outcome data are unlikely to be related to true outcome, missing outcomes data are balanced in numbers across intervention groups with similar reasons for missing data across groups or missing data were imputed using appropriate methods) (Higgins 2011, Section 8.13);
'high risk' if missing outcome data was likely to bias the results;
'unclear risk' if it is not specified in the paper (Cochrane EPOC Group 2013).
Reporting bias
Were reports of the study free from selective outcome reporting?
We scored:
'low risk' if there is no evidence that outcomes were selectively reported (e.g. pre–specified outcomes are available in the study protocol or all relevant outcomes in the methods section are reported in the Results section);
'high risk' if some pre‐specified outcomes were subsequently omitted from the results;
'unclear risk' if not specified in the paper (Cochrane EPOC Group 2013; Higgins 2011, Section 8.14).
Was the study free from selective analysis reporting?
We scored:
'low risk' for each outcome if there is no evidence that analyses were selectively reported (e.g. analyses were outlined in the Methods section of the protocol or paper);
'high risk' if there is evidence of selective analysis reporting (e.g. multiple adjusted analyses have been carried out and only one reported or 'extreme' cut points have been used for creating categorical outcomes);
'unclear' if not specified in the paper (Cochrane EPOC Group 2013; Norris 2013).
Measures of treatment effect
We intended using Review Manager 2014 to perform all analyses. Final decisions regarding if and how to combine outcomes depended on how data were reported in the included studies. We consulted Section 9.2 'Types of data and effect measures' of Higgins 2011 to guide our decisions.
We predicted that the data could be:
dichotomous (yes/no) (i.e. increased knowledge/awareness of breast cancer symptoms; increased confidence to engage in self‐care behaviours relating to breast cancer awareness, such as increased confidence or motivation to palpate breasts or frequency of breast checking; increased breast cancer awareness overall; increased confidence to seek help; increased intention to seek help/time‐to‐event, such as early presentation of potential breast cancer symptoms; mortality and presence of adverse effect of the intervention);
continuous (e.g. changes in anxiety scales as a result of the intervention and any of the pertinent outcomes, where reported as such);
ordinal (e.g. tumour classification i.e. stage/size; categories on a quality of life scale such as 'mild', 'moderate' or 'severe');
nominal: perceptions of barriers to seeking help;
time‐to‐event: breast cancer‐specific mortality and survival estimates.
We estimated the effect measurement for dichotomous outcomes using the odds ratio as the summary statistic. We planned to analyse ordinal data either as continuous data (tumour classification i.e. stage/size) or dichotomous data (cancer type: in situ yes/no or invasive yes/no). In the case of quality of life, we planned to analyse data as continuous or dichotomous depending on how trial authors report data in the included studies. In this review, data on tumour classification or quality‐of‐life data were not reported in the included studies.
If appropriate, we planned to determine the effect measurement for continuous data (changes in anxiety levels/decreased quality of life/economic factors, and any of the pertinent outcomes, where reported as such) using the mean difference (MD) and standard deviation SD (when outcomes are measured using the same scales). In the event of anxiety and quality of life being measured using different scales, we planned to calculate the standardised mean difference (SMD), where appropriate. In this review version, information related to anxiety, quality of life and economic factors were not reported in the included studies.
Time‐to‐event data were not reported in the included studies. Therefore, in future review updates, if such data are available, we plan to use hazard ratios as the summary statistic. We used 95% confidence intervals (CIs) throughout the review.
Unit of analysis issues
Unit of analysis regarding women's breast cancer awareness over specific time frames have been categorised as short, medium and long term i.e. six months, 12 months (PEP), and two years respectively (Forbes 2011a). As far as possible, we identified periods of follow‐up (postintervention) in a similar fashion to reflect the effects of interventions on raising breast cancer awareness within short‐term (≤ one‐month), medium‐term (≤ one‐year) and long‐term (≤ two‐year) time intervals.
Dealing with missing data
We sought information on missing data by contacting the trial authors. One of the PEP trialists confirmed that missing data were not imputed but a repeated measure analysis, which included all women with a questionnaire from at least one time point, was performed. Women with missing data at a particular time point did not contribute to the point estimate at that time point. Attempts to contact the ZUMS trialists were unsuccessful. Therefore, in this review we presented the data narratively and discussed it in the main text of the review.
Assessment of heterogeneity
Due to the diversity of outcome measures and associated reporting in the included studies, an assessment of heterogeneity was not performed. In future review updates, when reviewing comparable outcome measures, we plan to assess inconsistency in study results i.e. statistical heterogeneity (due to clinical or methodological diversity) by reviewing forest plots that display the study‐specific estimates of our outcome measures along with the 95% CIs where poor overlap of results indicates the presence of statistical heterogeneity (Higgins 2011). We plan to quantify inconsistency across studies using the I² statistic which describes "the percentage of variability in effect estimates that is due to heterogeneity rather than sampling error (chance)" (Higgins 2011). It is suggested that a result greater than 50% indicates substantial heterogeneity (Higgins 2011).
Assessment of reporting biases
As there were fewer than 10 included studies, we were unable to formally assess reporting bias using funnel plots. In future review updates, we will attempt to contact the trial authors to provide information on missing data. Where this is not possible and we suspect bias, we plan to determine the impact of including such studies by testing for funnel plot asymmetry (as recommended in Section 10.4.3.1 of Higgins 2011). We will include outcomes and implications of results in the Discussion section, where appropriate.
Data synthesis
As data were not combined in this review, in future review updates we plan to present the data using Review Manager 2014. In the current review version, three review authors (MOM, JH and TF) extracted data, and reported the findings (as outlined in Higgins 2011).
In future review updates, we plan to use a random‐effects model. However, if we detect homogeneity across studies, we intend to use a fixed‐effect model for meta‐analysis (Higgins 2011). Random‐effects pooled estimates are proposed as follows. For dichotomous outcomes, we plan to pool odds ratios using the DerSimonian and Laird random‐effects method (DerSimonian 1986; Higgins 2011). Regarding continuous outcomes, we plan to pool data using the inverse variance random‐effects method. We plan to obtain pooled estimates of time‐to‐event outcomes using the random‐effects inverse variance method. We propose that fixed‐effect pooled estimates will be obtained using the Mantel‐Haenszel method for dichotomous outcomes and inverse‐variance method for continuous outcomes. Regarding time‐to‐event data, we plan to use the inverse variance method to pool estimates of the log(hazard ratio) (Higgins 2011).
The GRADE approach (outlined in Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions,Schünemann 2011, on the GRADE web‐site (http://www.gradeworkinggroup.org/) and in Ryan 2016) was used to assess the quality of the evidence. We assigned ratings of 'high', 'moderate', 'low' or 'very low' to each of the following primary outcomes: breast cancer knowledge, age‐related risk, breast‐checking behaviour and breast cancer awareness. This involved two review authors (MOM and JH) independently assessing the evidence for each outcome using five domains. These domains relate to: (i) risk of bias of the included studies (ii) inconsistency (i.e. heterogeneity) (iii) indirectness (relevance to the review question) (iv) imprecision (i.e. wide confidence intervals) and (v) publication bias. As it was not reasonable to pool estimates, we provided a narrative account of the results of individual included studies. We were unable to prepare a 'Summary of findings' table using GRADEpro software (as initially planned and as recommended in Chapter 1 of Higgins 2011).
Subgroup analysis and investigation of heterogeneity
We proposed to perform subgroup analysis as necessary. It was considered that this would involve analysis of subsets of participants (determined by age groups; socio‐economic factors; cultural issues; previous history of breast cancer); study details (geographical location); mode of delivery of intervention (face‐to‐face/online/one‐to‐one or group session); and time point at which the outcome was assessed (i.e. ≤ one month or ≤ one year or ≤ two years) postintervention. Due to a lack of included studies, small sample size of one study (ZUMS) and homogeneity of samples in both studies, subgroup analysis and investigation of heterogeneity were not performed in this review.
Sensitivity analysis
We proposed to perform sensitivity analysis to assess the robustness of the review. Also, we planned to repeat the analysis excluding studies of low methodological quality. The results of sensitivity analyses were to be reported in a summary table. However owing to the small number of studies included in this review, a sensitivity analysis was not possible.
Results
Description of studies
Results of the search
A total of 15,966 records were retrieved through a search of databases in the original search. One additional study, Zeinomar 2013, was located using the 'forward citation' function in Google in which another potentially eligible study, Calderon 2010, was cited. Another cross‐reference, Williams 2013, was located within one record reviewed (Ford 2014). Seven records were located from the grey literature search. In total 10 additional records were reviewed; Figure 1.
1.
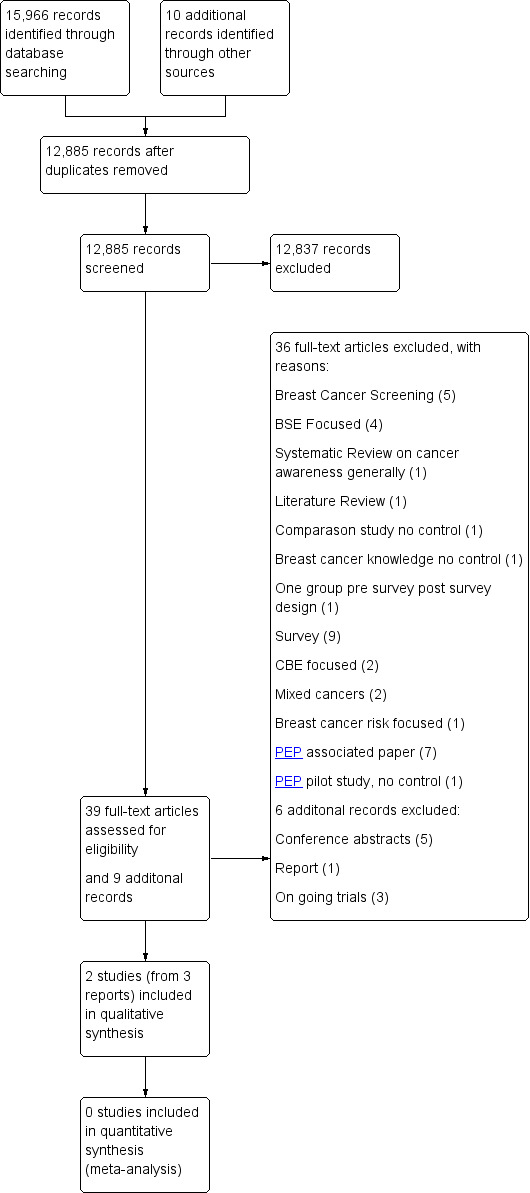
Study flow diagram.
After exclusion of duplicates (n = 3099) and screening of remaining records (12,885) by title or abstract, 39 full‐text papers and nine additional records were retrieved for evaluation. Two trials in three publications (PEP: Linsell 2009 and Forbes 2011a; ZUMS: Eskandari‐Torbaghan 2014) met the inclusion criteria as they related specifically to the promotion of breast cancer awareness and three additional trials were deemed to be 'ongoing studies' (NCT02205736; NCT01299623; IRCT201305068742N2).
Included studies
Two studies, PEP and ZUMS met the inclusion criteria as they related specifically to the promotion of breast cancer awareness. PEP was reported in two publications: Linsell 2009 and Forbes 2011a.
The Promoting Early Presentation (PEP) of breast cancer symptoms study investigated the efficacy of an intervention to equip older women with the knowledge, skills, confidence and motivation to detect breast cancer symptoms and seek help promptly at one‐month, one‐year (Linsell 2009), and two‐year time‐points (Forbes 2011a). The Zahedan University of Medical Sciences (ZUMS) study sought to determine the effects of an educational intervention on "breast cancer preventive behaviors" among female university medical staff, at one month postintervention.
Design
A total of 997 women were enrolled in the two RCTs (PEP; ZUMS). PEP enrolled women from August 2007 to May 2008 and ZUMS enrolled female university medical staff in 2013.
Sample Size
PEP enrolled 867 women aged 67 to 70 years and ZUMS enrolled 130 younger women aged mean (standard deviation (SD)) 35.38 years (8.01) (intervention group) and mean (SD) 34.39 years (8.98) (control group).
Setting
Women were recruited from seven breast screening units in London and Surrey (PEP) and from among employees at Zahedan University of Medical Sciences (ZUMS), in Iran.
Participants
PEP included women attending their final routine appointments on the National Health Service (NHS) Breast Screening Programme in the United Kingdom. ZUMS included a community of women employed at Zahedan University of Medical Sciences.
Interventions
The PEP study used two types of interventions: (i) intervention 1 comprised a booklet conveying key breast cancer awareness messages, given by a radiographer to each woman who had received her final routine mammogram, in addition to the usual care; (ii) intervention 2 comprised a 10‐minute one‐to‐one verbal interaction with a radiographer or research psychologist plus booklet plus usual care. The messages in the booklet were delivered in a positive, collaborative and motivational style, the booklet was referred to throughout and given to the woman to take home. Photographs of breast cancer signs were shown to the woman and breast checking was demonstrated and rehearsed using a silicone breast. The radiographer tailored the key messages by checking the woman’s understanding and answering any questions.
The control group received the usual care i.e. the screening unit receptionist informed each woman who had received her final routine mammogram that she was no longer eligible for routine screening but advised her that she might continue to be screened every 3 years on request. The receptionist provided a card with contact details and a suggested date for contact (PEP).
The ZUMS study examined the effects of an educational training programme on the breast cancer preventive behaviours of female employees of Zahedan University of Medical Sciences (ZUMS) in Iran. The educational programme was based on the Health Belief Model (i.e. perceived susceptibility, seriousness, benefits, barriers and self‐efficacy) and delivered by the researchers through lectures, questions and answers, PowerPoint presentations, videos, an educational booklet and compact disc (CD), over three 60‐ to 90‐minute sessions. The aim of the sessions was to increase women’s awareness of breast cancer symptoms, their knowledge regarding right time for mammography and to improve their practice of "breast cancer preventive behaviours" (including physical activity, healthy diet and positive beliefs towards breast self‐examining behaviour, clinical examination and mammography).
The control group received no intervention.
Time points
Outcomes were measured at baseline, one month and one year (Linsell 2009), and two years (Forbes 2011a); and pre‐ and one‐month post intervention (ZUMS). Different tests were used as outcome measures in each study.
Both studies are described in further details in the 'Characteristics of included studies' table.
Ongoing trials
Three ongoing trials were identified from searches conducted on the WHO ICTRP. One trial is almost complete as confirmed with the director of the study (NCT02205736). Another is ongoing and recruitment is complete (IRCT201305068742N2), and recruitment status of a third is unknown (NCT01299623). Attempts to contact the principal investigators of the preceding three trials were unsuccessful.
Excluded studies
We excluded 36 full‐text publications (Reimer 2002; Pavic 2007; Burgess 2008; Austoker 2009; Burgess 2009; Abd El Aziz 2009; Calderon 2010; Forbes 2010a; Forbes 2010b; Forbes 2011b; Kharboush 2011; Parsa 2011; Forbes 2012; Abuidris 2013; Williams 2013; Zeinomar 2013; Anakwenze 2014; Ford 2014; Forster 2014; Ginsburg 2014; Keating 2014; Khalili 2014; Liu 2014; Mena 2014; Wu 2014; Acikgoz 2015; Alipour 2015; Al Khasawaneh 2015; Campbell 2015; Dey 2015; Duman 2015; Gupta 2015; Hassan 2015; Livaudais‐Toman 2015; Miller 2015; Özmen 2015).
Reasons for exclusion were interventions focused on breast cancer screening (Reimer 2002; Pavic 2007; Abuidris 2013; Khalili 2014; Alipour 2015); one cross‐sectional study on breast self‐examination (BSE) (Parsa 2011); two interventions on BSE (Kharboush 2011; Miller 2015); one intervention study on BSE with no control group (Abd El Aziz 2009); a systematic review on interventions for general cancer awareness (Austoker 2009); a literature review on breast cancer awareness in Indian women (Gupta 2015); a comparison study with no control group (Calderon 2010); an educational intervention for breast cancer knowledge with no control group (Zeinomar 2013); a one‐group pre‐survey and postsurvey design study on breast cancer awareness and screening (Wu 2014); nine cross‐sectional surveys (Anakwenze 2014; Keating 2014; Liu 2014; Mena 2014; Acikgoz 2015; Al Khasawaneh 2015; Dey 2015; Duman 2015; Özmen 2015); one trial focusing on clinical breast examination (CBE) and BSE (Hassan 2015); one RCT focusing on CBE (Ginsburg 2014); two studies with a mixed cancer focus (Williams 2013; Ford 2014); and one intervention focusing on awareness of personal risk of breast cancer (Livaudais‐Toman 2015).
We excluded seven records associated with the PEP study on (i) the development of the psycho‐educational intervention (Burgess 2008), (ii) a qualitative study on the involvement of users in the design of the PEP intervention (Forbes 2010a), (iii) a conference abstract of a paper describing the PEP intervention (Forbes 2010b), (iv) a conference abstract of a paper on training the trainers (Forbes 2011b), (v) a first report of a national pilot of the PEP (Forbes 2012), (vi) an evaluation of further development of the PEP intervention with no control group (Forster 2014), and (vii) promotion of early presentation in older women in general practice with no control group (Campbell 2015). Burgess 2009, a before‐and‐after pilot study associated with the PEP study with no control group, was also excluded (see Characteristics of excluded studies).
Five additional conference abstracts were identified and excluded from the review as follows: Park 2011 (screening focused); Latha 2013 (no control group); Ersek 2014 (survey); Pakai 2014 (cross‐sectional survey); and Ferguson 2015 (mixed cancer focus). Also, one report identified through the grey literature search, Eadie 2008, was excluded as it referred to an exploratory study to Guide the Development of a Pilot Breast Education and Awareness Campaign.
Risk of bias in included studies
As this review included RCTs, two review authors (MOM and JH) independently assessed the risk of bias of included studies using the Cochrane 'Risk of bias' tool (Chapter 8 of Higgins 2011) and the Cochrane EPOC Group's 'Risk of bias' criteria (Cochrane EPOC Group 2013).The risk of bias in included studies has been summarised in Figure 2.
2.
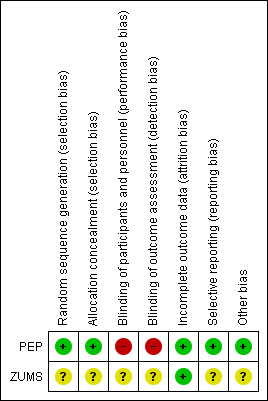
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Both studies used randomisation in assigning participants to the intervention and control arms; however the methods used varied. The PEP study was at low risk of bias for sequence generation and allocation concealment. Sequence generation involved stratified block randomisation (block sizes of three, six and nine) by the radiographer and assignments were enclosed in sequentially numbered, opaque, sealed envelopes and stored by the trial coordinator before randomisation to ensure allocation concealment. The risk of bias for random sequence generation and allocation concealment in the ZUMS study was unclear as insufficient information was provided in the publication and attempts to contact the authors were unsuccessful.
Blinding
Blinding of participants and of personnel (performance bias) was not addressed in the publications. However, this would have been difficult to achieve due to the nature of the interventions in both studies. Blinding of participants and personnel was rated as unclear in the ZUMS study as insufficient information was available to make a valid judgement.
In the PEP study lack of blinding of participants and personnel (radiographers) was confirmed with the authors, therefore the potential for performance bias was rated as high.
Blinding of outcome assessment (detection bias) for the ZUMS study was not addressed in the publication, therefore it was rated as unclear. Communication with the authors of the PEP study confirmed lack of blinding of outcome assessors, thus we rated detection bias as high.
Incomplete outcome data
In PEP, women excluded from the targeted population and women lost to follow‐up were detailed with reasons and no attrition was reported. Thus, attrition bias was low. In ZUMS, no women were excluded, lost to follow‐up and no attrition was reported. Therefore this study was at low risk of attrition bias.
Selective reporting
All relevant outcomes detailed in the methods sections were adequately reported in the results section of PEP, therefore the study was rated at low risk of reporting bias. However, detail on the meaning of "breast cancer preventive behaviours" and how the construct was measured were lacking in ZUMS, therefore it was rated at unclear risk of bias for this domain.
Other potential sources of bias
Lack of clarity around the constructs of the Health Belief Model in ZUMS puts it at unclear risk of bias. In addition, training of the interventionists (i.e. the main investigators) and assessment of adherence to the intervention protocol was not addressed. This augmented the risk of bias in ZUMS.
Effects of interventions
Women's knowledge of breast cancer symptoms
PEP study
PEP assessed women’s knowledge of the warning signs of breast cancer from a list (n = 11: two lump and nine non‐lump) of possible symptoms. To score one point, the woman had to identify at least five non‐lump symptoms, i.e. over half.
At one month postintervention, there appeared to be no difference in the proportion of women who were able to correctly identify five or more non‐lump symptoms in intervention group 1 compared to the control group (OR 1.1, 95% CI 0.8 to 1.5; P = 0.61; 496 women). After one month post‐intervention, there was an increase in the proportion of women who were correctly able to identify five or more non‐lump symptoms in intervention group 2 compared to the control group (OR 2.5, 95% CI 1.7 to 3.6; P < 0.001; 488 women).
At one year postintervention, there was an increase in the proportion of women who were correctly able to identify five or more non‐lump symptoms in intervention group 2 compared to the control group (OR 1.7, 95% CI 1.1 to 2.4; P = 0.01; 463 women). No difference was found at one year for intervention group 1 (OR 1.3, 95% CI 0.9 to 1.9; P = 0.23; 469 women).
At two years postintervention, knowledge of non‐lump symptoms increased in intervention group 1 compared to the control group, but not significantly (OR 1.1, 95% CI 0.7 to 1.6; P = 0.66; 449 women). At two years postintervention, knowledge of non‐lump symptoms also increased in the intervention group 2 compared to the control group but not significantly (OR 1.4, 95% CI 0.9 to 2.1; P = 0.11; 434 women).
Given that events are common for knowledge of breast cancer symptoms PEP, misinterpretation of the OR as RR will overestimate the intervention effect, as outlined in section 9.2.2.3. of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
ZUMS study
Awareness of breast cancer symptoms was measured using a 16 item‐questionnaire and calculated results based on scores of 2 (correct answer), 1 (no comment) or 0 (incorrect answer) pre‐ and one month post intervention ZUMS. Details of the questionnaire were not provided and we were unable to contact the primary author to clarify further. Regarding knowledge of breast cancer symptoms, the construct of awareness was reported. An increase in women’s awareness of breast cancer symptoms was detected one month post intervention (mean difference (SD) 3.45 (5.11) in the experimental group (65 women), compared to the control group, where there was a decrease in awareness (mean difference (SD) −0.68 (5.93) (65 women), P < 0.001).
Given the different measures for this outcome, a meta‐analysis was not performed. In summary, in the PEP study women’s knowledge of breast cancer symptoms increased significantly in the intervention 2 group at one month and one year postintervention. This effect was not sustained at two years. In the ZUMS study, an increase in women’s awareness of breast cancer symptoms was detected one month post intervention.
We downgraded the quality of the evidence for this outcome to moderate in the PEP study (due to lack of blinding of participants, personnel and outcome assessors) and limited sample (i.e. older age group of women exiting the NHS screening programme in London and South East England) and to low in the ZUMS study, due to unclear risk of bias, limited sample (female employees in one University of Medical Sciences in Iran) and small number of events.
Women's knowledge of age‐related risk
PEP study
Women were questioned on their knowledge of age‐related risk of getting breast cancer (i.e. that a 70‐year‐old woman was more likely to get breast cancer compared to a 30‐year‐old or a 50‐year‐old woman) and scored one point if they responded correctly.
At one month postintervention, the proportion of women who knew that a 70‐year‐old woman was at most risk of breast cancer increased in intervention group 1 compared to the control group (OR 3.2, 95% CI 1.8 to 5.8; P < 0.001; 503 women). At one month postintervention, the proportion of women who knew that a 70‐year‐old woman was at most risk of breast cancer increased in intervention group 2 compared to the control group (OR 9.5, 95% CI 5.1 to 17.6; P < 0.001; 499 women).
At one year postintervention, the proportion of women who knew that a 70‐year‐old woman was at most risk of breast cancer increased in intervention group 1 compared to the control group (OR 3.4, 95% CI 1.8 to 6.7, P < 0.001; 471 women) and in intervention group 2 compared to the control group (OR 7.4, 95% CI 3.7 to 14.7; P < 0.001; 468 women).
At two years postintervention, the proportion of women who knew that a 70‐year‐old woman was at most risk of breast cancer increased in intervention 1 group (OR 1.8, 95% CI 0.9 to 3.5; P < 0.08; 447 women) though not significantly compared to the control groups. The proportion of women who knew that a 70‐year‐old woman was at most risk of breast cancer increased significantly in intervention group 2 (OR 4.8, 95% CI 2.6 to 9.0; P < 0.001; 431 women) compared to the control group.
ZUMS study
Age‐related risk as such was not investigated in the ZUMS study. Women’s attitude to their perceived susceptibility (i.e. women’s considerations around their risk) of getting breast cancer was determined by their responses to a six‐item, five‐option Likert scale with scores for each item ranging from 4 (totally agree) to 0 (totally disagree).
An increase in women’s perceptions of their susceptibility to breast cancer was detected one month post intervention (mean difference (SD) 1.31 (3.57) in the experimental group (65 women) compared to the control group, where a decrease was noted (mean difference (SD) −0.55 (3.31), 65 women), P = 0.005).
Given the different measures for this outcome, a meta‐analysis was not performed. In summary, in the PEP study, women’s knowledge of breast cancer risk increased significantly in both intervention 1 and 2 groups, at one month and one year. However, this increase was only sustained at two years for intervention 2. In the ZUMS study, women’s perceived susceptibility to breast cancer increased significantly one month after the intervention.
We downgraded the quality of the evidence for this outcome to moderate in the PEP study (due to lack of blinding of participants, personnel and outcome assessors) and limited sample (i.e. older age group of women exiting the NHS screening programme in London and South East England) and to low in the ZUMS study, due to unclear risk of bias, limited sample (female employees in one University of Medical Sciences in Iran) and small number of events.
Frequency of breast checking
PEP study
Women's frequency of breast checking was measured in categories ranging from “rarely or never”, “at least every 6 months”, “at least once a month" to “at least once a week”. A score of one point was allocated if the woman reported checking her breasts either at least once a month or at least once a week (PEP).
At baseline, almost half of the women reported checking their breasts at least once a month. At one month postintervention, there was no effect on the breast checking behaviour of intervention group 1 (booklet and usual care) compared to the control group (OR 1.2, 95% CI 0.9 to 1.6; P = 0.25; 517 women). At one month postintervention, the proportion of women checking their breasts at least monthly increased in intervention group 2 (interaction plus booklet and usual care) compared to the control group (OR 2.0, 95% CI 1.4 to 2.8; P < 0.001; 508 women).
At one year postintervention, the effects on intervention group 1 (OR 1.1, 95% CI 0.8 to 1.6; 482 women) and intervention group 2 (OR 1.3, 95% CI 0.9 to 1.8; 473 women) compared to the control group were no longer significant (P = 0.47 and P = 0.23), respectively.
At two years postintervention, the proportion of women checking their breasts at least monthly increased in intervention group 1 compared to the control group (OR 1.1, 95% CI 0.8 to 1.6; 457 women), but not significantly (P = 0.54). At two years postintervention, the proportion of women checking their breasts at least monthly also increased in intervention group 2 compared to the control group (OR 1.3, 95% CI 0.9 to 1.9; 445 women), but not significantly (P = 0.14). An increase in breast checking from 53.3% at baseline (285 women) to 68.6% (229 women) following the usual care was also reported in the control groups after two years (Forbes 2011a).
Given that events are common for frequency of checking breasts in the PEP study, misinterpretation of the OR as RR will overestimate the intervention effect, as outlined in section 9.2.2.3. of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
ZUMS study
Women’s "breast cancer preventive behaviours" (which included their positive beliefs towards breast self‐examining behaviour) were measured using a five‐item, four‐point Likert scale with scores ranging from 3 (always) to 0 (never) (ZUMS).
One month post intervention, women’s breast cancer preventive behaviours increased in the experimental group (mean difference (SD) 1.21 (2.54); 65 women) compared to the control group (mean difference (SD) 0.15 (2.94); 65 women; P = 0.045).
Meta‐analysis was not done for this outcome due to heterogeneity of outcomes and outcome measures. In summary, in the PEP study women’s frequency of breast checking increased significantly after one month for intervention 2. In the ZUMS study women’s breast cancer preventive behaviours increased significantly one month post intervention.
We downgraded the quality of the evidence for this outcome to moderate in the PEP study (due to lack of blinding of participants, personnel and outcome assessors) and limited sample (i.e. older age group of women exiting the NHS screening programme in London and South East England) and to very low in the ZUMS study, due to unclear risk of bias, limited sample (female employees in one University of Medical Sciences in Iran), small number of events and lack of clarity in the outcome being measured.
Breast cancer awareness
PEP study
The primary outcome of interest in the PEP study was the proportion of women achieving breast cancer awareness at one, six and 12 months following intervention 1 and intervention 2 (Forbes 2011a). The breast cancer awareness score was a combination of responses to the three questions from the trial‐specific questionnaire, relating to: knowledge of symptoms, knowledge of age‐related risk and reported breast checking.
At one month postintervention, the proportion of women who were breast cancer aware increased in intervention group 1 compared to the control group (OR 4.4, 95% CI 1.6 to 2.0; P = 0.004; 481 women). At one month postintervention, the proportion of women who were breast cancer aware increased in intervention group 2 compared to the control group (OR 24.0, 95% CI 7.7 to 73.7; P < 0.001; 473 women).
At one year postintervention, the proportion of women who were breast cancer aware increased in intervention group 1 compared to the control group (OR 3.5, 95% CI 1.2 to 10.5; P = 0.025; 456 women). At one year postintervention, the proportion of women who were breast cancer aware increased in intervention group 2 compared to the control group (OR 15.2, 95% CI 4.8 to 47.8; P < 0.001; 454 women).
At two years postintervention, the effects were not sustained in intervention group 1 compared with the control group (OR 1.8, 95% CI 0.6 to 5.30; P = 0.32; 435 women). At two years postintervention, the proportion of women who were breast cancer aware increased in intervention group 2 compared to the control group (OR 8.1, 95% CI 2.7 to 25.0; P < 0.001; 420 women; Forbes 2011a).
ZUMS study
Breast cancer awareness per se was not reported in ZUMS.
Meta‐analysis was not done due to variability of measurements used. In summary, in the PEP study women’s breast cancer awareness increased at one month and one year following interventions 1 and 2. At two years, only the effects of intervention 2 were sustained.
We downgraded the quality of the evidence for this outcome to moderate in the PEP study (due to lack of blinding of participants, personnel and outcome assessors) and limited sample (i.e. older age group of women exiting the NHS screening programme in London and South East England).
Women's motivation to check their breasts
This outcome was not assessed in either study.
Confidence to seek help when breast cancer symptoms are noticed
This outcome was not assessed in either study.
Time from breast cancer symptom being noticed to presentation to a HCP
This outcome was not assessed in either study.
Women's intentions to seek help in the event of noticing a breast cancer symptom and their perceptions of barriers to help
This outcome was not assessed in either study.
Quality of life
This outcome was not assessed in either study.
Adverse effects of receiving the intervention on breast cancer awareness or adverse outcomes related to false positive findings of symptoms
This outcome was not assessed in either study.
Stage of breast cancer at diagnosis
This outcome was not assessed in either study.
Survival estimates
This outcome was not assessed in either study.
Breast cancer‐specific mortality and all‐cause mortality
This outcome was not assessed in the either study.
The quality of the evidence for adverse events or secondary outcomes was not assessed as these were not reported in the included studies.
Discussion
Summary of main results
We included two RCTs (PEP and ZUMS) involving 997 women that investigated the effects of interventions on women’s breast cancer awareness. A meta‐analysis was not conducted as these two studies used different measures for the primary outcomes of women’s knowledge of breast cancer symptoms and age‐related risk and confidence to check their breasts. The PEP study aimed at increasing women’s breast cancer awareness (i.e. knowledge of breast cancer symptoms, knowledge of age‐related risk and confidence to check breasts) at one‐month, one‐year and two‐year time points. The researchers based their intervention on a theoretical framework for delayed presentation of breast symptoms (Bish 2005). ZUMS sought to improve women’s "breast cancer preventive behaviours" (including physical activity, healthy diet and positive beliefs towards breast self‐examination, clinical examination and mammography) one month post intervention, based on the Health Belief Model.
Knowledge of breast cancer symptoms
In one study, PEP, women’s knowledge of breast cancer symptoms increased significantly at the one‐month and one‐year time points, following a combination of usual care, booklet and one‐to‐one verbal interaction (intervention 2). In ZUMS, an increase in women’s awareness of breast cancer symptoms was detected one month post a group training programme intervention.
Women's knowledge of age‐related risk
Usual care and booklet (intervention 1) and a combination of usual care, booklet and one‐to‐one verbal interaction (intervention 2) both caused a significant increase in women’s knowledge of breast cancer risk at one month and one year in the PEP study. This increase was only sustained at two years for intervention 2. In ZUMS, women’s perceived susceptibility to breast cancer increased significantly one month after a group training programme.
Frequency of breast checking
Women’s reported monthly breast checking increased significantly following intervention 2 at one month in PEP. In ZUMS, women’s "breast cancer preventive behaviours" (which included their positive beliefs towards breast self‐examining behaviour) had increased significantly at one month postintervention.
Breast cancer awareness
The PEP study reported that women’s breast cancer awareness increased at one month and one year following interventions 1 and 2. At two years, only the effects of intervention 2 were sustained. Breast cancer awareness was not reported in the ZUMS study.
The two trials provide evidence that educating women about breast cancer using written material or a combination of written material and a 10‐minute interaction in one‐to‐one format or an intervention of three 60‐ to 90‐minute sessions, has potential to increase women’s knowledge of breast cancer symptoms, knowledge of age‐related risks and frequency of breast checking. In one study, a combination of these outcome measurements indicated increased breast cancer awareness which was present at one year following both types of intervention but was sustained at two years only following the combination of booklet and one‐to‐one interaction (PEP). Sustainment of outcomes (knowledge of breast cancer symptoms, breast cancer susceptibility and breast cancer preventive behaviours generally) for longer than one month was not measured in the ZUMS study, which utilised three 60‐ to 90‐minute group presentations only.
The evidence provided from the review suggests that an educational intervention on breast cancer knowledge, using a combination of written material and verbal interaction in one‐to‐one format, has potential to increase women’s breast cancer awareness, over time. However, due to the limited number of studies, small sample size in one study and heterogeneous nature of interventions and outcomes measured, no conclusions can be drawn from the review regarding the effects of interventions on breast cancer awareness. In addition, the term “breast cancer preventive behaviours” as used in the ZUMS study needs to be interpreted with caution as although the practice of breast self‐examination, clinical examination and clinical mammography aim to detect early breast cancer, they can never prevent breast cancer. Therefore, current evidence cannot be generalised to the wider context.
Overall completeness and applicability of evidence
The overall applicability of evidence is limited and incomplete. This review identified only two RCTs that investigated the effectiveness of two educational interventions on breast cancer awareness. The overall outcome of breast cancer awareness i.e. women’s knowledge of breast cancer symptoms/changes and confidence to look and feel their breasts (i.e. breast checking and breast self‐examining behaviour) to identify what is normal and know what changes to look for, together with knowledge of breast cancer risk factors (or perceived susceptibility to breast cancer) were evident in both studies. However, the PEP study measured the outcome of breast cancer awareness specifically whereas the ZUMS study measured women’s "breast cancer preventive behaviours". Neither study addressed time from noticing a breast cancer symptom to presentation to a HCP; women’s intentions to seek help; quality of life; adverse effect of the intervention; breast cancer‐specific mortality; and all‐cause mortality. However, one preliminary evaluative study, excluded from the current review due to absence of control group data, reported no significant increase in cancer worry one month post intervention to promote breast cancer awareness (Burgess 2009). Finally, interventions in the current review were partly dependent on participants' ability to comprehend the written word. This limits transferability to individuals with lower literacy levels, visual impairment, learning or intellectual disabilities.
Quality of the evidence
Since the outcomes of interest were defined, measured and presented in different ways, it was not possible to present the quality of the evidence for each outcome in a 'Summary of findings' table. A narrative account of the evidence within studies for each outcome was presented. Overall, we rated the evidence in the PEP study to be moderate quality with low risk of bias. We rated the ZUMS study to be low quality with unclear risk of bias. Both studies are heterogeneous in terms of the population, type of intervention, content, timing and outcome measurements. Nonetheless, we are moderately confident that a brief intervention can have a sustained positive effect on some of the elements of breast cancer awareness in older women. However, further trials with larger samples focusing on the key outcome measures associated with breast cancer awareness are warranted. While it must be acknowledged that blinding would have been difficult to achieve for the type of intervention under review, clear definition of the concept of interest is necessary to guide future high‐quality trials. Please refer to Figure 2 for a graphic representation of the methodological quality of studies included.
Potential biases in the review process
The review was conducted in line with the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We performed a comprehensive, up‐to‐date search in the most relevant databases. A methodological filter was not used, to ensure that no studies would be missed. Nor did we use a language restriction. The studies identified measured the first two primary outcomes of interest to the review and findings were positive in both studies in terms of increasing breast cancer awareness and breast cancer preventive behaviours, respectively. Although the number of studies per outcome and intervention was too small to allow performance of meta‐analysis, a narrative account and assessment of the quality of the findings was presented. We corresponded with the authors of both studies to clarify issues; these data were obtained for PEP, which adds to the completeness of the review. On the other hand, some additional information on the outcome measures in the ZUMS study would have enhanced the review process.
An important limitation to the review is that it focused specifically on the concept of breast cancer awareness, a concept that lacks clarity in the literature. Initially much debate ensued over the topic of interest i.e. breast awareness or breast cancer awareness, both terms being interconnected as breast cancer awareness incorporates breast awareness. We used part of the operational definition of breast cancer awareness as outlined in the PEP study i.e. women’s knowledge of breast cancer symptoms and confidence to check breasts — their reported breast checking. Additionally, measurement of age‐related risk was also an aspect of breast cancer awareness measured within the PEP study, which we did not include in the protocol in order to broaden the focus of our search. However, it is reported as an outcome in the review as it was specifically reported in PEP and is inherent in the concept of susceptibility in ZUMS. Similarly, the overall outcome of breast cancer awareness is reported in the PEP study, and thus was considered important to report in this review, although this was not outlined in the protocol. Furthermore, due to our focus on the broader construct of breast cancer awareness, studies that reported knowledge of breast cancer only or breast screening in isolation were excluded from the review. However, this facilitated decisions around our inclusion and exclusion of studies which was helpful given the number of studies yielded from our searches of the databases. Subsequently, more precision around types of studies to be included in the search would enhance the review process.
Agreements and disagreements with other studies or reviews
This review is the first systematic review on interventions for breast cancer awareness in women. Only two trials, one large (n = 567) UK‐based study and one small (n = 130) Iranian study met the inclusion criteria. A Cochrane Review on breast self‐examination included two large population‐based studies (388,535 women) from Russia (1999) and Shanghai (2002) (Kösters 2003, updated in 2007). The first searched the Cochrane Breast Cancer Group Specialised Register, the Cochrane Library and MEDLINE (October 2002). The updated review repeated this search and updated the searches in the Cochrane Library and MEDLINE (PubMed) in October 2007. This search yielded three RCTs, one of which was excluded from analysis due to early termination of the study (Philippines 2006). The final review found lack of evidence to support the use of breast self‐examination as a screening tool for improving breast cancer mortality rates. Therefore, the promotion of breast self‐examination as a single screening method cannot be recommended. However, the review emphasised the need for women to be able to identify breast changes and seek medical advice promptly in the event of noticing a breast change. Subsequently, there has been a paradigm shift from the concept of breast self‐examination to the broader concept of breast awareness (MacBride 2012; National Comprehensive Cancer Network 2013; NICE 2013; American Cancer Society 2014; IARC 2014) and breast cancer awareness (PEP), hence the focus of our review. While our search identified a plethora of studies on interventions promoting screening and breast self‐examination, there is a paucity of studies on interventions specifically for breast cancer awareness.
A systematic review was conducted in 2009 by Austoker and colleagues, on the effectiveness of interventions to increase cancer awareness or promote early presentation (Austoker 2009). The search strategy (the Cochrane Library, MEDLINE, Embase and PsycINFO from 2000 to 2008) yielded five RCTs carried out in the UK, United States and the Netherlands. One of the US studies, excluded from our review due to its focus on breast cancer screening in women (n = 1091: aged 42 to 57 years), involved an intensive intervention of tailored written information on breast cancer risk, reinforced with a newsletter at 12 months and two telephone counselling sessions (Reimer 2002). This intervention increased the proportion of women who correctly answered a question on age‐related risk of breast cancer. While important, this study focuses on only one aspect of knowledge related to breast cancer awareness. Furthermore, the main aim of the intervention was to promote breast cancer screening as opposed to breast cancer awareness. The reviewers found limited evidence that interventions lead to greater cancer awareness and earlier presentation in the short term and concluded that further research is warranted on the optimum interventions for long‐term cancer awareness, which was the focus of our review in relation to breast cancer (Austoker 2009).
A recent structured literature review (using Cochrane methodology) on breast cancer awareness amongst women (n = 70: aged 15 to 70 years) in India, reported a search of the MEDLINE, Cochrane Library, CINAHL and SCOPUS databases up to 2014, yielding 13 eligible studies (Gupta 2015). The review reported that awareness levels of risk factors for breast cancer were low amongst Indian women (within 10 studies) and HCPs i.e. nurses and students nurses (within three studies). The reviewers recommend more intensive health‐promoting intervention programmes focusing on risk factors, prevention screening and management of breast cancer. While this review highlights the importance of women being knowledgeable about breast cancer risk, again it is limited in that it focuses on only one aspect of breast cancer awareness as opposed to a broader, more holistic definition of the concept, as outlined by PEP.
Heterogeneity of terminology and outcomes being assessed in these reviews makes it difficult to compare and contrast findings. Nonetheless, what can be said is that researchers need to be clear on the definitions of the concepts and outcomes being measured in studies and reviews. To date, there appears to be much confusion around the terms breast awareness, breast cancer awareness and breast screening, all of which require clarification.
Authors' conclusions
Implications for practice.
The aim of this review was to investigate the effectiveness of educational interventions on breast cancer awareness in women. However, the moderate quality of the evidence prevents any clear conclusions on the best design and content of an educational intervention to increase breast cancer awareness in women. Nonetheless, the evidence from one study indicates that an educational intervention on breast cancer symptoms, age‐related risk and breast checking behaviour, using a combination of written material and a brief one‐to‐one interaction, has the potential to increase breast cancer awareness in older women, over a sustained period of time. Furthermore, the review highlights lack of clarity around terms used in the literature in relation to breast awareness/breast cancer awareness. If early detection and treatment of breast cancer is to become a reality for women, clarity around these key concepts is necessary. This is important for clinicians and policy makers who are involved in the development and implementation of strategies to promote breast cancer awareness.
Implications for research.
In view of the low number of studies identified, heterogeneity of interventions used, inconsistency in outcomes measured and the overall moderate quality of the evidence available, further research is necessary. Initial clarity around the concept of breast cancer awareness would facilitate the development of future interventions. Future high‐quality trials are necessary to provide more robust evidence on the effectiveness of such interventions for increasing breast cancer awareness for women. Detailed description of interventions, mode of delivery, measurement tools, time to follow‐up, outcome assessment and sustainability of the effect need to be provided. Additionally, attention to the adverse effects of the intervention will help identify whether further support might be needed for women. Increased focus on the long‐term effects of interventions on women's help‐seeking, stage of diagnosis (if relevant), quality of life and morbidity and mortality rates need to be assessed in long‐term RCTs. Broadening the scope of studies to access the wider population of women is crucial. Literacy levels and cost effectiveness will need to be considered also.
Acknowledgements
The authors would like to thank: Ava Grace Tan‐Koay and Slavica Berber, Information Specialists of the Cochrane Breast Cancer Group (CBCG), for their support with the searches; Bita Mesgarpour (Assistant Professor in Pharmacoepidemiology and Drug Safety, Director of National and International Affairs National Institute for Medical Research Development (NIMAD), Ministry of Health and Medical Education in Iran), for assisting with translation of one of the papers; authors of included and excluded studies who were helpful in clarifying detail and providing additional data; and the Cochrane Breast Cancer Group, in particular Melina Willson, for support throughout the review process.
Appendices
Appendix 1. CENTRAL
#1 MeSH descriptor: [Breast Neoplasms] explode all trees
#2 breast near cancer*
#3 breast near neoplasm*
#4 breast near carcinoma*
#5 breast near tumour*
#6 breast near tumor*
#7 #1 or #2 or #3 or #4 or #5 or #6
#8 breast cancer awareness or breast awareness
#9 breast near aware*
#10 MeSH descriptor: [Health Knowledge, Attitudes, Practice] explode all trees
#11 MeSH descriptor: [Health Education] explode all trees
#12 MeSH descriptor: [Health Promotion] explode all trees
#13 MeSH descriptor: [Awareness] explode all trees
#14 awareness or education or knowledge
#15 (promot* or educat* or knowledge or intervention* or inform* or aware*)
#16 educat* near intervention*
#17 #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16
#18 #7 and #17
Appendix 2. MEDLINE
| 1 | Case‐Control Studies/ |
| 2 | Control Groups/ |
| 3 | Matched‐Pair Analysis/ |
| 4 | Retrospective Studies/ |
| 5 | ((case* adj5 control*) or (case adj3 comparison*) or control group*).ti,ab. |
| 6 | or/1‐5 |
| 7 | Cohort Studies/ |
| 8 | Longitudinal Studies/ |
| 9 | Follow‐Up Studies/ |
| 10 | Prospective Studies/ |
| 11 | Retrospective Studies/ |
| 12 | cohort.ti,ab. |
| 13 | longitudinal.ti,ab. |
| 14 | prospective.ti,ab. |
| 15 | retrospective.ti,ab. |
| 16 | or/7‐15 |
| 17 | randomized controlled trial.pt. |
| 18 | controlled clinical trial.pt. |
| 19 | randomized.ab. |
| 20 | placebo.ab. |
| 21 | Clinical Trials as Topic/ |
| 22 | randomly.ab. |
| 23 | trial.ti. |
| 24 | (crossover or cross‐over).tw. |
| 25 | Pragmatic Clinical Trials as Topic/ |
| 26 | pragmatic clinical trial.pt. |
| 27 | or/17‐26 |
| 28 | exp Breast Neoplasms/ |
| 29 | (breast adj6 cancer$).tw. |
| 30 | (breast adj6 neoplasm$).tw. |
| 31 | (breast adj6 carcinoma$).tw. |
| 32 | (breast adj6 tumo?r$).tw. |
| 33 | or/28‐32 |
| 34 | breast cancer awareness.tw. |
| 35 | breast awareness.tw. |
| 36 | (breast adj5 aware*).tw. |
| 37 | awareness.tw. |
| 38 | Awareness/ |
| 39 | Health Knowledge, Attitudes, Practice/ |
| 40 | exp Health Education/ |
| 41 | exp Health Promotion/ |
| 42 | education.tw. |
| 43 | knowledge.tw. |
| 44 | (promot* or educat* or knowledge or intervention* or inform* or aware*).tw. |
| 45 | (education* adj5 intervention*).tw. |
| 46 | Health Communication/ |
| 47 | Patient Education as Topic/ |
| 48 | or/34‐47 |
| 49 | and/33,48 |
| 50 | animals/ not (humans/ and animals/) |
| 51 | 49 not 50 |
| 52 | and/6,51 |
| 53 | and/16,51 |
| 54 | and/27,51 |
| 55 | limit 54 to yr="2008 ‐Current" |
Appendix 3. Embase
| #1 | 'case control study'/syn OR 'case control study' OR ('case control' OR 'case base' OR 'case matched' OR retrospective) NEXT/3 (analys* OR design* OR evaluation* OR research OR stud* OR survey* OR trial*) |
| #2 | (cohort OR concurrent OR incidence OR longitudinal OR followup OR 'follow up' OR prospective OR retrospective) NEXT/1 (analys* OR design* OR evaluation* OR research OR stud* OR survey* OR trial*) OR 'prospective method'/exp OR 'retrospective study'/syn |
| #3 | random* OR factorial* OR crossover* OR cross NEXT/1 over* OR placebo* OR (doubl* AND blind*) OR (singl* AND blind*) OR assign* OR allocat* OR volunteer* OR 'crossover procedure'/exp OR 'crossover procedure' OR 'double blind procedure'/exp OR 'double blind procedure' OR 'randomized controlled trial'/exp OR 'randomized controlled trial' OR 'single blind procedure'/exp OR 'single blind procedure' |
| #4 | 'breast'/exp OR 'breast disease'/exp AND 'neoplasm'/exp OR 'breast tumor'/exp OR (breast* NEAR/5 neoplas*):ab,ti OR (breast* NEAR/5 cancer*):ab,ti OR (breast* NEAR/5 carcin*):ab,ti OR (breast* NEAR/5 tumo*):ab,ti OR (breast* NEAR/5 metasta*):ab,ti OR (breast* NEAR/5 malig*):ab,ti |
| #5 | 'breast cancer'/exp OR 'breast cancer' OR 'breast neoplasm' OR 'breast carcinoma'/exp OR 'breast carcinoma' OR 'breast tumour' OR 'breast tumor'/exp OR 'breast tumor' |
| #6 | #4 OR #5 |
| #7 | 'breast cancer awareness' |
| #8 | 'breast awareness' |
| #9 | breast NEAR/5 aware* |
| #10 | aware* |
| #11 | 'health education'/exp OR 'health education' OR health NEAR/5 education |
| #12 | 'health promotion'/exp OR 'health promotion' OR health NEAR/5 promotion |
| #13 | 'awareness'/exp OR awareness |
| #14 | 'education'/exp OR education |
| #15 | 'knowledge'/exp OR knowledge |
| #16 | (promot* OR inform* OR aware* OR educat* OR knowledge OR intervention*) |
| #17 | educat* NEAR/5 intervention* |
| #18 | #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 |
| #19 | #6 AND #18 |
| #20 | #19 AND [humans]/lim AND [embase]/lim |
| #21 | #1 AND #20 |
| #22 | #2 AND #20 |
| #23 | #3 AND #20 |
| #24 | #23 AND [2008‐2014]/py |
Appendix 4. Embase (via Ovid SP)
| 1 | Randomized controlled trial/ |
| 2 | Controlled clinical study/ |
| 3 | Random$.ti,ab. |
| 4 | randomization/ |
| 5 | intermethod comparison/ |
| 6 | placebo.ti,ab. |
| 7 | (compare or compared or comparison).ti. |
| 8 | ((evaluated or evaluate or evaluating or assessed or assess) and (compare or compared or comparing or comparison)).ab. |
| 9 | (open adj label).ti,ab. |
| 10 | ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab. |
| 11 | double blind procedure/ |
| 12 | parallel group$1.ti,ab. |
| 13 | (crossover or cross over).ti,ab. |
| 14 | ((assign$ or match or matched or allocation) adj5 (alternate or group$1 or intervention$1 or patient$1 or subject$1 or participant$1)).ti,ab. |
| 15 | (assigned or allocated).ti,ab. |
| 16 | (controlled adj7 (study or design or trial)).ti,ab. |
| 17 | (volunteer or volunteers).ti,ab. |
| 18 | human experiment/ |
| 19 | trial.ti. |
| 20 | or/1‐19 |
| 21 | exp case control study/ |
| 22 | case control study.ti,ab. |
| 23 | ((case control or case base or case matched or retrospective) adj1 (analys* or design* or evaulation* or research or stud* or survey* or trial*)).ti,ab. |
| 24 | 21 or 22 or 23 |
| 25 | exp retrospective study/ |
| 26 | exp prospective study/ |
| 27 | ((cohort or concurrent or incidence or longitudinal or followup or 'follow up' or prospective or retrospective) adj1 (analys* or design* or evaluation* or research or stud* or survey* or trial*)).ti,ab. |
| 28 | 25 or 26 or 27 |
| 29 | exp breast/ |
| 30 | exp breast disease/ |
| 31 | (29 or 30) and exp neoplasm/ |
| 32 | exp breast tumor/ |
| 33 | exp breast cancer/ |
| 34 | exp breast carcinoma/ |
| 35 | (breast$ adj5 (neoplas$ or cancer$ or carcin$ or tumo$ or metasta$ or malig$)).ti,ab. |
| 36 | 29 or 30 or 31 or 32 or 33 or 34 or 35 |
| 37 | breast cancer awareness.tw. |
| 38 | breast awareness.tw. |
| 39 | (breast adj5 aware$).tw. |
| 40 | aware$.tw. |
| 41 | exp health education/ |
| 42 | (health adj5 education).tw. |
| 43 | exp health promotion/ |
| 44 | (health adj5 promotion).tw. |
| 45 | exp awareness/ or awareness.tw. |
| 46 | exp education/ or education.tw. |
| 47 | exp knowledge/ or knowledge.tw. |
| 48 | (prmot$ or inform$ or aware$ or educat$ or knowledge or intervention$).tw. |
| 49 | (educat$ adj5 intervention$).tw. |
| 50 | or/37‐49 |
| 51 | 36 and 50 |
| 52 | limit 51 to (human and embase and yr="2014 ‐Current") |
| 53 | 20 and 52 |
| 54 | 24 and 52 |
| 55 | 28 and 52 |
Appendix 5. WHO ICTRP search portal
Basic searches:
Interventions for breast cancer awareness in women
breast cancer awareness AND intervention*
breast cancer AND education
breast cancer AND knowledge
breast cancer AND information
Advanced searches:
1. Title: Interventions for breast cancer awareness in women;
Recruitment Status: ALL
2. Title: breast cancer awareness;
Recruitment Status: ALL
3. Condition: breast cancer or breast cancer awareness;
Intervention: health promotion or health education or educational program* or educational intervention*;
Recruitment Status: ALL
Appendix 6. Clinicaltrials.gov
Basic searches:
Interventions for breast cancer awareness in women
breast cancer awareness AND intervention*
breast cancer AND education
breast cancer AND knowledge
breast cancer AND information
Advanced searches:
1. Title: Interventions for breast cancer awareness in women
Recruitment: ALL
Study results: All studies
Study type: All studies
Gender: Studies with female participants
2. Title: breast cancer awareness
Recruitment: ALL
Study results: All studies
Study type: All studies
Gender: Studies with female participants
3. Condition: breast cancer or breast cancer awareness
Intervention: health promotion OR health education OR educational program* OR educational intervention*
Recruitment: ALL
Study results: All studies
Study type: All studies
Gender: Studies with female participants
Characteristics of studies
Characteristics of included studies [ordered by study ID]
PEP.
| Methods | Accrual: August 2007 to May 2008 Muticentre (breast screening units n = 7); UK‐based Parallel group randomised controlled trial using stratified block randomisation (block sizes of three, six and nine). Follow‐up: one month; one year |
|
| Participants | Women aged 67 to 70 attending for final routine appointment on the UK NHS Breast Screening programme. | |
| Interventions |
Intervention group 1 (n = 287): received a booklet conveying key breast cancer awareness messages, given by a radiographer to each woman who had received her final routine mammogram, in addition to the usual care. Intervention group 2 (n = 294): received a 10‐min one‐to‐one verbal interaction with a radiographer or research psychologist plus booklet plus usual care. Control group (n = 286): received the usual care (women were informed that they were no longer eligible for routine screening, advised to continue to be screened every 3 years on request and given a card with contact details and a suggested date for contact). |
|
| Outcomes |
Primary Outcomes Knowledge of breast cancer symptoms, knowledge of age‐related risk, confidence to check breasts and overall breast cancer awareness. Breast cancer awareness Women’s breast cancer awareness measured using a breast cancer awareness score (a combination of responses to the three questions from the trial‐specific questionnaire on: knowledge of symptoms, knowledge of age‐related risk and reported breast checking). |
|
| Notes | Two‐year follow‐up reported by Forbes 2011a. Study funded by Cancer Research UK. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified block randomisation described. |
| Allocation concealment (selection bias) | Low risk | Detail on randomisation provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Lack of blinding of personnel & participants. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Lack of blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Detail on attrition provided. |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes detailed in Methods section were reported in Results section. |
| Other bias | Low risk | No other potential sources of bias were identified. |
ZUMS.
| Methods | RCT Accrual not specified Convenient sample randomised into intervention or control group; randomisation method not specified Follow‐up: one month |
|
| Participants | Female employees of Zahedan University of Medical Sciences (ZUMS) Mean (SD) age: Intervention group 35.38 (8.01); Control group: 34.39 (8.98) |
|
| Interventions |
Intervention group (n = 65) An educational programme of three 60‐ to 90‐min sessions delivered by the researchers through lectures, questions and answers, PowerPoint presentations, videos, an educational booklet and compact disc (CD) to increase women’s breast cancer awareness. Effects were measured one month later. Control group (n = 65) No intervention. |
|
| Outcomes |
Primary Outcomes Women’s awareness of breast cancer symptoms and attitudes towards breast cancer (perceived susceptibility, perceived seriousness, perceived benefits and barriers and self‐efficacy). Secondary Outcomes Breast cancer preventive behaviours (i.e. breast self‐examining behaviour, clinical breast examination, mammography and healthy diet). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information provided to make a valid judgement. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided to make a valid judgement. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information provided to make a valid judgement. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information provided to make a valid judgement. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No women were excluded, lost to follow‐up and no attrition was reported. |
| Selective reporting (reporting bias) | Unclear risk | Lack of information on the meaning of breast cancer preventive behaviours and how the construct was measured. |
| Other bias | Unclear risk | (i) Lack of clarity around the constructs of the Health Belief Model (ii) training of interventionists and (iii) adherence to intervention protocol. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abd El Aziz 2009 | Intervention study on BSE with no control group. |
| Abuidris 2013 | A pilot study of an intervention focused on breast cancer screening. |
| Acikgoz 2015 | Cross‐sectional study with no control group. |
| Al Khasawaneh 2015 | Cross‐sectional survey. |
| Alipour 2015 | Breast cancer screening focused. |
| Anakwenze 2014 | Cross‐sectional survey. |
| Austoker 2009 | A systematic review on interventions for general cancer awareness. |
| Burgess 2008 | PEP associated paper: describing the development of the psycho‐educational intervention. |
| Burgess 2009 | PEP‐associated paper: reports on a preliminary evaluation before and after study with no control group. |
| Calderon 2010 | Comparison study with no control group. |
| Campbell 2015 | PEP‐associated paper, promoting early presentation in older women in general practice, no control group. |
| Dey 2015 | Cross‐sectional survey. |
| Duman 2015 | Cross‐sectional survey. |
| Eadie 2008 | Exploratory qualitative study. |
| Ersek 2014 | Conference abstract: survey. |
| Ferguson 2015 | Conference abstract: mixed cancer focus. |
| Forbes 2010a | PEP‐associated paper: qualitative study on the involvement of users in the design of the PEP intervention. |
| Forbes 2010b | PEP‐associated paper: promoting early symptomatic presentation in older women with breast cancer in the NHS breast screening programme – conference abstract. |
| Forbes 2011b | PEP‐associated paper: a report on training the trainers – conference abstract. |
| Forbes 2012 | PEP‐associated paper: a first report of a National pilot of the PEP study. |
| Ford 2014 | Mixed cancer focus: breast and cervical screening education. |
| Forster 2014 | PEP‐associated paper: evaluation of further development of PEP, no control group. |
| Ginsburg 2014 | RCT focusing on adherence to clinical breast examination (CBE). |
| Gupta 2015 | Literature review on breast cancer awareness in Indian women. |
| Hassan 2015 | RCT focusing on BSE and CBE. |
| Keating 2014 | Cross‐sectional survey. |
| Khalili 2014 | Breast cancer screening focused. |
| Kharboush 2011 | Intervention to increase practice of BSE. |
| Latha 2013 | Quasi‐experimental study, no control group – conference abstract. |
| Liu 2014 | Cross‐sectional survey. |
| Livaudais‐Toman 2015 | Intervention focusing on awareness of risk of breast cancer. |
| Mena 2014 | Cross‐sectional survey. |
| Miller 2015 | Cross‐sectional study on BSE. |
| Pakai 2014 | Cross‐sectional survey – conference abstract. |
| Park 2011 | Conference abstract: based on screening mammography. |
| Parsa 2011 | A cross‐sectional study on knowledge related to BSE. |
| Pavic 2007 | Intervention focused on breast cancer screening. |
| Reimer 2002 | RCT on breast cancer screening. |
| Williams 2013 | Mixed cancer focus: cervical and breast cancer. |
| Wu 2014 | One‐group pre‐survey and post‐survey design. |
| Zeinomar 2013 | An educational intervention for breast cancer knowledge and screening behaviours with no control group. |
| Özmen 2015 | Cross‐sectional survey on screening and breast cancer awareness. |
Characteristics of ongoing studies [ordered by study ID]
IRCT201305068742N2.
| Trial name or title | The impact of education on preventive behavior of breast cancer |
| Methods | Parallel‐group, triple‐blind, randomised controlled trial |
| Participants | Women over age 30 who are not diagnosed with breast cancer |
| Interventions | Intervention 1: intervention groups: face‐to‐face interviews and training (6 sessions) based on preventive behaviour of breast cancer. Intervention 2: Control: face‐to‐face training (for moral reasons) will be provided in 6 sessions on preventive behavior of breast cancer, post intervention 1. |
| Outcomes | Promotion of preventive breast cancer behaviour and awareness of breast cancer prevention; time frame: 3 months postintervention |
| Starting date | 12 June 2013 |
| Contact information | khazaie_m@yahoo.com khazaee@razi.tums.ac.ir |
| Notes | IRCT201305068742N2 |
NCT01299623.
| Trial name or title | Disseminating Breast Cancer Prevention to African American Women |
| Methods | Parallel‐group, double‐blind, randomised controlled trial, 3‐ and 6‐month follow‐up, N = 200 |
| Participants | African American women, aged 35 to 70. |
| Interventions | Active Comparator: Evidential Group Participants received education about breast cancer prevention and specific information about African American women and breast cancer risk. Active Comparator: Non‐Evidential Group Participants in this group received general information about breast cancer prevention without anything specific to African American women. |
| Outcomes | Primary Outcome: women's knowledge about breast cancer prevention; communication about breast cancer prevention with HCP; the extent to which participants distrust medical research. Secondary Outcomes: assessment of the relationship between participants' education and primary outcomes; assessment of the relationship between participants' family history and the primary outcomes; assessment of the relationship between participants' beliefs about research and the primary outcomes |
| Starting date | 17 February 2011 |
| Contact information | Benita L Weathers, MPH weathers@mail.med.upenn.edu |
| Notes | NCT01299623 |
NCT02205736.
| Trial name or title | A Cross Sectional Study of a Breast Health Education Program for Under‐served Latino Women in Union and Mecklenburg County. |
| Methods | Intervention Model: Single Group Assignment, N = 250 approximately. |
| Participants | Latino women 18 years and over. |
| Interventions | Small group breast health educational sessions for Latino women in home setting. |
| Outcomes | Change in baseline of breast cancer health literacy; time frame: one year. |
| Starting date | Registered on 20 July 2014 |
| Contact information | Daniel Carrizosa, MD, Carolinas Healthcare System, Levine Cancer Institute. |
| Notes | NCT02205736 |
Differences between protocol and review
Knowledge of age‐related risk was added as a primary outcome as (i) it was a considered to be an important dimension of knowledge on breast cancer and breast cancer awareness overall and (ii) was specifically reported in the PEP study as an outcome measure for breast cancer awareness.
Confidence to check breasts was reported as frequency of breast checking in the PEP study, and as such within the current review.
Breast cancer awareness was added as (i) we had included the key dimensions of BCA i.e. knowledge of symptoms and confidence to check breasts as outcomes to include in the review (ii) it was considered a central construct to the review and (iii) it was reported as the primary outcome in the PEP study.
Other studies measuring breast cancer awareness would have been included provided they met the specific criteria for types of studies to include in the review. Where studies were non‐randomised, inclusion was dependent on (i) a control group and (ii) pre‐ and post‐test estimates of the effectiveness of the intervention.
Contribution of authors: MGF added to drafting of protocol.
Mark Corrigan details change to: Mark A Corrigan, Cork Breast Research Centre, Cork University Hospital, Cork, Ireland and Royal College of Surgeons, Dublin, Ireland. He now reads as MAC in text.
Search for Embase (via OvidSP) added as access to Embase.com platform no longer available.
Contributions of authors
Drafted the protocol: MOM, JH, MGF
Study selection: MOM, JH
Extracted data from studies: MOM, JH
Entered data into RevMan: MOM
Carried out the analysis: MOM, JH, TF
Interpreted the analysis: MOM, JH, HC, TF, MAC, EAG
Drafted the final review: MOM, JH
Disagreement resolution: MAC
Updated the review: MOM
Sources of support
Internal sources
-
Professor Eileen Savage PhD Med BNS RGN RCN RM, Ireland.
Chair in Nursing & Head of School, and Colleagues in the Catherine McAuley School of Nursing and Midwifery, Brookfield Health Sciences Complex, University College Cork, Ireland for their support in the completion of this review.
External sources
-
Health Research Board, Ireland.
This review is funded by a Cochrane Fellowship awarded by the Health Research Board, Ireland
Declarations of interest
MOM: none known. HC: none known. TF: none known. MAC: none known. EF: none known. EAG: none known. MGF: none known. JH: none known.
New
References
References to studies included in this review
PEP {published data only}
- Forbes LJ, Linsell L, Atkins L, Burgess C, Tucker L, Omar L, et al. A promoting early presentation intervention increases breast cancer awareness in older women after 2 years: a randomised controlled trial. British Journal of Cancer 2011;105(1):18‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsell L, Forbes LJ, Kapari M, Burgess C, Omar L, Tucker L, et al. A randomised controlled trial of an intervention to promote early presentation of breast cancer in older women: effect on breast cancer awareness. British Journal of Cancer 2009;101(Suppl 2):S40‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
ZUMS {published data only}
- Eskandari‐Torbaghan A, Kalan‐Farmanfarma K, Ansari‐Moghaddam A, Zarei Z. Improving breast cancer preventive behavior among female medical staff: The use of educational intervention based on health belief model. Malaysian Journal of Medical Sciences 2014;21(5):44‐50. [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Abd El Aziz 2009 {published data only}
- Abd El Aziz HM, Akl OA, Ibrahim HK. Impact of A Health Education Intervention Program about Breast Cancer among Women in a Semi‐urban Area in Alexandria, Egypt. The Journal of the Egyptian Public Health Association 2009;84(1‐2):219‐43. [PubMed] [Google Scholar]
Abuidris 2013 {published data only}
- Abuidris DO, Elsheikh A, Ali M, Musa H, Elgaili E, Ahmed AO, et al. Breast‐cancer screening with trained volunteers in a rural area of Sudan: A pilot study. Lancet Oncology 2013;14(4):363‐70. [DOI] [PubMed] [Google Scholar]
Acikgoz 2015 {published data only}
- Acikgoz A, Cehreli R, Ellidokuz H. Determination of knowledge and behavior of women working at a hospital on breast cancer early detection methods, and investigation of efficiency of planned education. [Turkish]. Meme Sagligi Dergisi / Journal of Breast Health 2015;11(1):31‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Alipour 2015 {published data only}
- Alipour S, Jannat F, Hosseini L. Teaching breast cancer screening via text messages as part of continuing education for working nurses: a case‐control study. Asian Pacific Journal of Cancer Prevention 2014;15(14):5607‐9. [DOI] [PubMed] [Google Scholar]
Al Khasawaneh 2015 {published data only}
- Al Khasawaneh EM, Siddiqui ST, Seshan V, Al Ajmi A, Al Farsi Y. Breast cancer awareness and early detection practices among Omani women: A pilot study. Breast 2015;24:S75. [Google Scholar]
Anakwenze 2014 {published data only}
- Anakwenze CP, Coronado‐Interis E, Aung M, Jolly PE. A theory‐based intervention to improve breast cancer awareness and screening in Jamaica. Prevention Science 2014;16(4):578‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Austoker 2009 {published data only}
- Austoker J, Bankhead C, Forbes LJ, Atkins L, Martin F, Robb K, et al. Interventions to promote cancer awareness and early presentation: Systematic review. British Journal of Cancer 2009;101(Suppl 2):S31‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Burgess 2008 {published data only}
- Burgess CC, Bish AM, Hunter HS, Salkovskis P, Michell M, Whelehan P, et al. Promoting early presentation of breast cancer: Development of a psycho‐educational intervention. Chronic Illness 2008;4(1):13‐7. [DOI] [PubMed] [Google Scholar]
Burgess 2009 {published data only}
- Burgess CC, Linsell L, Kapari M, Omar L, Michell M, Whelehan P, et al. Promoting early presentation of breast cancer by older women: A preliminary evaluation of a one‐to‐one health professional‐delivered intervention. Journal of Psychosomatic Research 2009;67(5):377‐87. [DOI] [PubMed] [Google Scholar]
Calderon 2010 {published data only}
- Calderon JL, Bazargan M, Sangasubana N, Hays RD, Hardigan P, Baker RS. A comparison of two educational methods on immigrant Latinas breast cancer knowledge and screening behaviors. Journal of Health Care for the Poor and Underserved 2010;21(3 Supplement):76–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Campbell 2015 {published data only}
- Campbell J, Pyer M, Rogers S, Jones J, Ramirez AJ, Forbes LJ. Promoting early presentation of breast cancer in women over 70 years old in general practice. Journal of Public Health 2016;38(3):591‐8. [DOI] [PubMed] [Google Scholar]
Dey 2015 {published data only}
- Dey S, Mishra A, Govil J, Dhillon PK. Breast cancer awareness at the community level among women in Delhi, India. Asian Pacific Journal of Cancer Prevention 2015;16(13):5243‐51. [DOI] [PubMed] [Google Scholar]
Duman 2015 {published data only}
- Duman NB, Yilmazel G, Pinar G, Buyukgonenc L. The risk level of breast cancer and breast cancer awareness among the Turkish women aged 65 years and older. Uluslararasi Hematoloji‐Onkoloji Dergisi 2015;25(1):60‐9. [Google Scholar]
Eadie 2008 {published data only}
- Eadie D, Freeman J. Exploratory Research to Guide the Development of a Pilot Breast Education and Awareness Campaign Phase 1: Main Findings Prepared for Breakthrough Breast Cancer. Cancer Research UK 2008.
Ersek 2014 {published data only}
Ferguson 2015 {published data only}
- Ferguson M, Schapira MM, Wileyto EP. Targeted newsletters to promote cancer control in African Americans. Journal of General Internal Medicine. 2015; Vol. 30:S263‐4.
Forbes 2010a {published data only}
- Forbes LJ, Nicholls CM, Linsell L, Graham J, Tompkins C, Ramirez AJ. Involving users in the design of a randomised controlled trial of an intervention to promote early presentation in breast cancer: qualitative study. BMC Medical Research Methodology 2010;10(1):110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Forbes 2010b {published data only}
- Forbes LJL, Atkins L, Sellars S, Patnick J, Tucker L, Ramirez AJ. Promoting early symptomatic presentation in older women with breast cancer in the NHS breast screening programme. Breast Cancer Research. 2010; Vol. 12 (Supplement 3):1.
Forbes 2011b {published data only}
- Forbes L, Atkins L, Jupp D, Green D, Scanlon K, Ramirez A. Evaluation of an intervention to promote breast cancer awareness and early presentation: the train the trainers breast health promotion course. Psycho‐Oncology. 2011; Vol. 20 (Supplement 2) 105–300:273.
Forbes 2012 {published data only}
- Forbes LJ, Forster AS, Dodd RH, Tucker L, Laming R, Sellars S, et al. Promoting early presentation of breast cancer in older women: implementing an evidence‐based intervention in routine clinical practice. Journal of Cancer Epidemiology 2012;2012:Article ID 835167. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ford 2014 {published data only}
- Ford S, Meghea C, Estes T, Hamade H, Lockett M, Williams KP. Assessing the fidelity of the Kin KeeperSM prevention intervention in African American, Latina and Arab women. Health Education Research 2014;29(1):158‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Forster 2014 {published data only}
- Forster AS, Forbes LJ, Abraham C, Warburton FG, Douglas E, Ramirez AJ. Promoting early presentation of breast cancer: A preliminary evaluation of a written intervention. Chronic Illness 2014;10(1):18‐30. [DOI] [PubMed] [Google Scholar]
Ginsburg 2014 {published data only}
- Ginsburg OM, Chowdhury M, Wu W, Chowdhury MT, Pal BC, Hasan R. An mHealth model to increase clinic attendance for breast symptoms in rural Bangladesh: can bridging the digital divide help close the cancer divide?. Oncologist 2014;19(2):177‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gupta 2015 {published data only}
- Gupta A, Shridhar K, Dhillon PK. A review of breast cancer awareness among women in India: cancer literate or awareness deficit. European Journal of Cancer 2015;51(14):2058‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hassan 2015 {published data only}
- Hassan LM, Mahmoud N, Miller AB, Iraj H, Mohsen M, Majid J, et al. Evaluation of effect of self‐examination and physical examination on breast cancer. Breast 2015;24(4):487‐90. [DOI] [PubMed] [Google Scholar]
Keating 2014 {published data only}
- Keating NL, Kouri EM, Ornelas HA, Méndez O, Valladares LM, Knaul FM. Evaluation of breast cancer knowledge among health promoters in Mexico before and after focused training. Oncologist 2014;19(10):1091‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Khalili 2014 {published data only}
- Khalili S, Shojaiezadeh D, Azam K, Kheirkhah Rahimabad K, Kharghani Moghadam M, Khazir Z. The effectiveness of education on the health beliefs and practices related to breast cancer screening among women referred to Shahid Behtash Clinic, Lavizan Area, Tehran, using Health Belief Model. Journal of Health and Hygiene 2014;5(1):45‐58. [Google Scholar]
Kharboush 2011 {published data only}
- Kharboush IF, Ismail HM, Kandil AA, Mamdouh HM, Muhammad YY, Sharkawy OG, et al. Raising the breast health awareness amongst women in an urban slum area in Alexandria, Egypt. Breast Care 2011;6(5):375‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Latha 2013 {published data only}
- Latha S. Effectiveness of structured teaching programme on breast cancer among the at risk women of a selected hospital at mangalore. Nepal Journal of Epidemiology. Conference: International Conference on Research Methodology and Scientific Writing. 2013; Vol. 4:2.
Liu 2014 {published data only}
- Liu MX, Li J, Geng YL, Wang YC, Li J, Chen YJ, et al. Correlation study of knowledge and behavior regarding breast care among female undergraduate students in China. Asian Pacific Journal of Cancer Prevention 2014;15(24):10943‐7. [DOI] [PubMed] [Google Scholar]
Livaudais‐Toman 2015 {published data only}
- Livaudais‐Toman J, Karliner LS, Tice JA, Kerlikowske K, Gregorich S, Pérez‐Stable EJ, et al. Impact of a primary care based intervention on breast cancer knowledge, risk perception and concern: A randomized, controlled trial. Breast 2015;24(6):6758‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mena 2014 {published data only}
- Mena M, Wiafe‐Addai B, Sauvaget C, Ali IA, Wiafe SA, Dabis F, et al. Evaluation of the impact of a breast cancer awareness program in rural Ghana: A cross‐sectional survey. International Journal of Cancer 2014;134(4):913‐24. [DOI] [PubMed] [Google Scholar]
Miller 2015 {published data only}
- Miller AB, Harirchi I, Lotfi MH, Noori M, Mirzaei M, Jafarizadea M, et al. Yazd breast cancer project profile; a community based trial for the evaluation of self‐examination and physical examination of the breast cancer disease. Iranian Journal of Medical Sciences 2015;40(6):531‐6. [PMC free article] [PubMed] [Google Scholar]
Özmen 2015 {published data only}
- Özmen T, Yüce S, Güler T, Ulun C, Özaydın N, Pruthi S, et al. Barriers against mammographic screening in a socioeconomically underdeveloped population: a population‐based, cross‐sectional study. Journal of Breast Health 2016;12(2):72‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pakai 2014 {published data only}
Park 2011 {published data only}
- Park K, Hong WH, Kye SY, Jung E, Kim MH, Park HG. Community‐based intervention to promote breast cancer awareness and screening: The Korean experience. BMC Public Health 2011;11(1):1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Parsa 2011 {published data only}
- Parsa P, Kandiah M, Parsa N. Factors associated with breast self‐examination among Malaysian women teachers. Eastern Mediterranean Health Journal 2011;17(6):509‐16. [PubMed] [Google Scholar]
Pavic 2007 {published data only}
- Pavic D, Schnell M, Dancel R, Sultana S, Lin L, Sejpal S, et al. Comparison of three methods to increase knowledge about breast cancer and breast cancer screening in screening mammography patients. Academic Radiology 2007;14(5):553‐60. [DOI] [PubMed] [Google Scholar]
Reimer 2002 {published data only}
- Rimer B, Halabi S, Sugg Skinner C, Lipkus I, Strigo T, Kaplan E, et al. Effects of a mammography decision‐making intervention at 12 and 24 months. American Journal of Preventive Medicine 2002;22(4):247‐57. [DOI] [PubMed] [Google Scholar]
Williams 2013 {published data only}
- Williams KP, Roman L, Meghea CI, Penner L, Hammad A, Gardiner J. Kin KeeperSM: design and baseline characteristics of a community‐based randomized controlled trial promoting cancer screening in Black, Latina, and Arab women. Contemporary Clinical Trials 2013;34(2):312‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wu 2014 {published data only}
- Wu TY, Lin C, Chen SL, Jung Y. A Community‐Based Intervention to Promote Breast Cancer Awareness and Screening among Asian American Women. International Quarterly of Community Health Education 2014;34(2):171‐85. [DOI] [PubMed] [Google Scholar]
Zeinomar 2013 {published data only}
- Zeinomar N, Moslehi R. The effectiveness of a community‐based breast cancer education intervention in the New York state capital region. Journal of Cancer Education 2013;28(3):466‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
IRCT201305068742N2 {unpublished data only}
- IRCT201305068742N2. The Impact of education on preventive behavior of breast cancer [Evaluation of the effectiveness of the educational intervention on promotion of preventive behavior of breast cancer on at risk women in Tehran ‐]. http://apps.who.int/trialsearch/Trial2.aspx?TrialID=IRCT201305068742N2 Date first received: 12 June 2013.
NCT01299623 {unpublished data only}
- NCT01299623. Breast Cancer Prevention Education [Disseminating Breast Cancer Prevention to African American Women]. https://clinicaltrials.gov/ct2/show/NCT01299623 Date first received: 17 February 2011.
NCT02205736 {unpublished data only}
- NCT02205736. Breast Health Education for Underserved Latino Women. https://clinicaltrials.gov/ct2/show/NCT02205736 Date first received: 29 July 2014.
Additional references
American Cancer Society 2014
- American Cancer Society. Breast cancer early detection breast awareness and self‐exam. http://www.cancer.org/cancer/breastcancer/moreinformation/breastcancerearlydetection/breast‐cancer‐early‐detection‐acs‐recs‐bse (accessed 31 January 2014).
Arndt 2002
- Arndt V, Stürmer T, Stegmaier C, Ziegler H, Dhom G, Brenner H. Patient delay and stage of diagnosis among breast cancer patients in Germany ‐ a population based study. British Journal of Cancer 2002;86(7):1034‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bish 2005
- Bish A, Ramirez A, Burgess C, Hunter M. Understanding why women delay in seeking help for breast cancer symptoms. Journal of Psychosomatic Research 2005;58(4):321‐6. [DOI] [PubMed] [Google Scholar]
Bray 2012
- Bray F, Jemal A, Grey N, Ferlay J, Forman D. Global cancer transitions according to the Human Development Index (2008–2030): a population‐based study. Lancet Oncology 2012;13(8):790‐801. [DOI] [PubMed] [Google Scholar]
Byrne 2009
- Byrne S, Robles‐Rodriguez E. Educational parties as a strategy to promote breast health awareness and screening in underserved female populations. Oncology Nursing Forum 2009;36(2):145‐8. [DOI] [PubMed] [Google Scholar]
Campbell 2007
- Campbell NC, Murray E, Darbyshire J, Emery J, Farmer A, Griffiths F, et al. Designing and evaluating complex interventions to improve health care. BMJ 2007;334(7591):455‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cancer Research UK 2014
- Cancer Research UK. Finding breast cancer early. http://www.cancerresearchuk.org/about‐cancer/type/breast‐cancer/about/finding‐breast‐cancer‐early (accessed 04 July 2014).
Carney 2003
- Carney PA, Miglioretti DL, Yankaskas BC, Kerlikowske K, Rosenberg R, Rutter CM, et al. Individual and combined effects of age, breast density, and hormone replacement therapy use on the accuracy of screening mammography. Annals of Internal Medicine 2003;138(3):168‐75. [DOI] [PubMed] [Google Scholar]
Chan 2007
- Chan SS, Chow DM, Loh EK, Wong DC, Cheng KK, Fung WY, et al. Using a community‐based outreach program to improve breast health awareness among women in Hong Kong. Public Health Nursing 2007;24(3):265‐73. [DOI] [PubMed] [Google Scholar]
Cochrane EPOC Group 2013
- Cochrane Effective Practice and Orgainisation of Care (EPOC) Group. Suggested risk of bias criteria for EPOC reviews. http://epocoslo.cochrane.org/epoc‐specific‐resources‐review‐authors (accessed 03 April 2014).
Craig 2008
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M, Medical Research Council Guidance. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008;337:a1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. [DOI] [PubMed] [Google Scholar]
Ekwueme 2014
- Ekwueme DU, Guy GP Jr, Rim SH, White A, Hall IJ, Fairley TL, et al. Health and economic impact of breast cancer mortality in young women, 1970–2008. American Journal of Preventive Medicine 2014;46(1):71‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Eskandari‐Torbaghan 2014
- Eskandari‐Torbaghan A, Kalan‐Farmanfarma K, Ansari‐Moghaddam A, Zarei Z. Improving breast cancer preventive behavior among female medical staff: The use of educational intervention based on health belief model. Malaysian Journal of Medical Sciences 2014;21(5):44‐50. [PMC free article] [PubMed] [Google Scholar]
Forbes 2011a
- Forbes LJ, Linsell L, Atkins L, Burgess C, Tucker L, Omar L, et al. A promoting early presentation intervention increases breast cancer awareness in older women after 2 years: a randomised controlled trial. British Journal of Cancer 2011;105(1):18‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro [Computer program]
- McMaster University. GRADEpro. McMaster University, 2014.
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
IARC 2014
- International Agency for Research on Cancer (IARC). IARC Screening Group. http://screening.iarc.fr/breastindex.php (accessed 31 January 2014).
Irish Cancer Society 2013
- Irish Cancer Society. Preventing breast cancer: Becoming breast aware. http//www.cancer.ie/cancer‐information/breast‐cancer/prevention (accessed 17 February 2014).
Jones 2010
- Jones SC, Gregory P, Nehill C, Barrie L, Luxford K, Nelson A, et al. Australian women’s awareness of breast cancer symptoms and responses to potential symptoms. Cancer Causes Control 2010;21(6):945‐58. [DOI] [PubMed] [Google Scholar]
Kösters 2003
- Kösters JP, Gøtzsche PC. Regular self‐examination or clinical examination for early detection of breast cancer. Cochrane Database of Systematic Reviews 2003, Issue 2. [DOI: 10.1002/14651858.CD003373] [DOI] [PMC free article] [PubMed] [Google Scholar]
Linsell 2009
- Linsell L, Forbes LJ, Kapari M, Burgess C, Omar L, Tucker L, et al. A randomised controlled trial of an intervention to promote early presentation of breast cancer in older women: effect on breast cancer awareness. British Journal of Cancer 2009;101(Suppl 2):S40‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Linsell 2010
- Linsell L, Forbes LJ, Burgess C, Kapari M, Thurnham A, Ramirez AJ. Validation of a measurement tool to assess awareness of breast cancer. European Journal of Cancer 2010;46(8):1374‐81. [DOI] [PubMed] [Google Scholar]
MacBride 2012
- MacBride MB, Pruthi S, Bevers T. The evolution of breast self‐examination to breast awareness. The Breast Jounal 2012;18(6):641‐3. [DOI] [PubMed] [Google Scholar]
Meechan 2002
- Meechan G, Collins J, Petrie K. Delay in seeking medical care for self‐detected breast symptoms in New Zealand women. New Zealand Medical Journal 2002;115(1166):U257. [PubMed] [Google Scholar]
Michie 2012
- Michie S, Johnston M. Theories and techniques of behaviour change: Developing a cumulative science of behaviour change. Health Psychology Review 2012;6(1):1‐6. [DOI: 10.1080/17437199.2012.654964] [DOI] [PubMed] [Google Scholar]
National Cancer Registry Ireland 2012
- National Cancer Registry Ireland. Breast cancer incidence, mortality, treatment and survival in Ireland: 1994‐2009. Cork, Ireland: National Cancer Registry, 2012. [Google Scholar]
National Comprehensive Cancer Network 2013
- National Comprehensive Cancer Network (NCCN). National Comprehensive Cancer Network (NCCN) Guidelines 2013. www.nccn.org/professionals/physician_gls/pdf/breast‐screening.pdf (accessed 31 January 2014).
NHS Breast Cancer Screening Programme 2013
- National Office of NHS Cancer Screening Programmes, Public Health England. NHS Breast Cancer Screening Programme 2013. www.cancerscreening.nhs.uk/breastscreen/breastawareness.html (accessed 02 January 2014).
NICE 2013
- National Institute for Health and Clinical Excellence (NICE). Familial Breast Cancer: Full Guideline. http://publications.nice.org.uk/familial‐breast‐cancer‐cg41/guidance#breast‐awareness‐and‐examination (accessed 31 January 2014).
Norris 2013
- Norris SL, Moher D, Reeves BC, Shea B, Loke Y, Garner S, et al. Issues relating to selective reporting when including non‐randomised studies in systematic reviews on the effects of healthcare interventions. Research Synthesis Methods 2013;4(1):36‐47. [DOI] [PubMed] [Google Scholar]
O'Mahony 2009
- O'Mahony M, Hegarty J. Factors influencing women in seeking help from a Health Care Professional on self discovery of a breast symptom, in an Irish context. Journal of Clinical Nursing 2009;18(14):2020‐9. [DOI] [PubMed] [Google Scholar]
O'Mahony 2011
- O'Mahony M, Hegarty J, McCarthy G. Women's help seeking behaviour for self discovered breast cancer symptoms. European Journal of Oncology Nursing 2011;15(5):410‐8. [DOI] [PubMed] [Google Scholar]
O'Mahony 2013
- O'Mahony M, McCarthy G, Corcoran P, Hegarty J. Shedding light on women’s help seeking behaviour for self discovered breast symptoms. European Journal of Oncology Nursing 2013;17(5):632‐9. [DOI] [PubMed] [Google Scholar]
Partridge 2012
- Partridge AH, Hughes ME, Ottesen RA, Wong YN, Edge SB, Theriault RL, et al. The effect of age on delay in diagnosis and stage of breast cancer. The Oncologist 2012;17(6):775‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Philippines 2006
- Pisani, P, Parkin, D.M, Ngelangel, C, Esteban, D, Gibson, L, Munson, M, Reyes, M.G, Laudico. A. Outcome of screening by clinical examination of the breast in a trial in the Philippines. International Journal of Cancer 2006;118(1):149‐154. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Richards 1999a
- Richards MA, Smith P, Ramirez AJ, Fentiman IS, Rubens RD. The influence on survival of delay in the presentation and treatment of symptomatic breast cancer. British Journal of Cancer 1999;79(5‐6):858‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Richards 1999b
- Richards MA, Westcombe AM, Love SB, Littlejohns P, Ramirez AJ. Influence of delay on survival in patients with breast cancer: a systematic review. Lancet 1999;353(9159):1119‐26. [DOI] [PubMed] [Google Scholar]
Richards 2009
- Richards MA. The National Awareness and Early Diagnosis Initiative in England: assembling the evidence. British Journal of Cancer 2009;101(Suppl 2):S1‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Robb 2009
- Robb K, Stubbings S, Ramirez A, Macleod U, Austoker J, Waller J, et al. Public awareness of cancer in Britain: a population‐based survey of adults. British Journal of Cancer 2009;101(Suppl 2):S18‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ryan 2016
- Ryan R, Hill S. Cochrane Consumers and Communication Group: How to GRADE the quality of the evidence. cccrg.cochrane.org/author‐resources. Version 1.0 June 2016 (accessed August 2016).
Schünemann 2011
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, Guyatt GH. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org, [updated March 2011]. [Google Scholar]
Siegal 2011
- Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA: A Cancer Journal for Clinicians 2011;61(4):212‐36. [DOI] [PubMed] [Google Scholar]
Siegal 2013
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA: A Cancer Journal for Clinicians 2013;63(1):11‐30. [DOI] [PubMed] [Google Scholar]
Thornton 2008
- Thornton H, Pillarisetti RR. 'Breast awareness' and 'breast self‐examination' are not the same. What do these terms mean? Why are they confused? What can we do?". European Journal of Cancer 2008;44(15):2118‐21. [DOI] [PubMed] [Google Scholar]
Weller 2012
- Weller D, Vedsted P, Rubin G, Walter FM, Emery J, Scott S, et al. The Aarhus statement: improving design and reporting of studies on early cancer diagnosis. British Journal of Cancer 2012;106(7):1262‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2014
- World Health Organization. Breast cancer :prevention and control. http://www.who.int/cancer/detection/breastcancer/en/ (accessed 31 January 2014).


