Abstract
Background
Individuals with pulmonary hypertension (PH) have reduced exercise capacity and quality of life. Despite initial concerns that exercise training may worsen symptoms in this group, several studies have reported improvements in functional capacity and well‐being following exercise‐based rehabilitation in PH.
Objectives
To assess the efficacy and safety of exercise‐based rehabilitation for people with PH. Primary outcomes were exercise capacity, adverse events during the intervention period and health‐related quality of life (HRQoL). Secondary outcomes included cardiopulmonary haemodynamics, functional class, clinical worsening during follow‐up, mortality and changes in B‐type natriuretic peptide.
Search methods
We searched the Cochrane Airways Specialised Register of Trials up to August 2016, which is based on regular searches of CINAHL, AMED, Embase, PubMed, MEDLINE, PsycINFO and registries of clinical trials. In addition we searched CENTRAL and the PEDro database up to August 2016 and handsearched relevant journals.
Selection criteria
All randomised controlled trials (RCTs) focusing on exercise‐based rehabilitation programmes for PH.
Data collection and analysis
Two reviewers extracted data independently. For binary outcomes, we calculated odds ratios and their 95% confidence interval (CI), on an intention‐to‐treat basis. For continuous data, we estimated the mean difference (MD) between groups and its 95% CI. We employed a random‐effects model for analyses. We assessed risk of bias for included studies and created 'Summary of findings' tables using GRADE.
Main results
We included six RCTs and were able to extract data from five studies. The total number of included participants was 206. The majority of participants were Group I pulmonary artery hypertension (PAH). Study duration ranged from three to 15 weeks. Exercise programmes included both inpatient‐ and outpatient‐based rehabilitation that incorporated both upper and lower limb exercise. The mean six‐minute walk distance following exercise training was 60.12 metres higher than control (30.17 to 90.07 metres, n = 165, 5 RCTs, low‐quality evidence; minimal important difference was 30 metres), the mean peak oxygen uptake was 2.4 ml/kg/minute higher (1.4 to 3.4 ml/kg/min, n = 145, 4 RCTs, low‐quality evidence) and the mean peak power in the intervention groups was 16.4 W higher (10.9 to 22.0 higher, n = 145, 4 RCTs, low‐quality evidence). The mean change in HRQoL for the SF‐36 physical component score was 4.63 points higher (0.80 to 8.47 points, n = 33, 2 RCTs, low‐quality evidence) and for the SF‐36 mental component score was 4.17 points higher (0.01 to 8.34 points; n = 33; 2 RCTs, low‐quality evidence). One study reported a single adverse event, where a participant stopped exercise training due to lightheadedness.
Authors' conclusions
In people with PH, exercise‐based rehabilitation results in clinically relevant improvements in exercise capacity. Exercise training was not associated with any serious adverse events. Whilst most studies reported improvements in HRQoL, these may not be clinically important. Overall, we assessed the quality of the evidence to be low. The small number of studies and lack of information on participant selection makes it difficult to generalise these results across the spectrum of people with PH.
Plain language summary
Exercise‐based rehabilitation in pulmonary hypertension
What is pulmonary hypertension? Pulmonary hypertension is a condition in which the blood pressure in the arteries that carry blood from the heart to the lungs is elevated well above normal. Often with a gradual onset, it affects individuals of all ages, significantly reduces quality of life and results in premature death.
Bottom Line. We reviewed randomised controlled trials to determine whether exercise training improved short‐ and long‐term patient outcomes in people with pulmonary hypertension. The number of participants in randomised controlled trials of exercise‐based rehabilitation for pulmonary hypertension was relatively small. These studies all reported large increases in exercise capacity as evaluated by six‐minute walk distance, maximal oxygen consumption and peak power. Health‐related quality of life was also improved, but to a lesser extent. Serious adverse events were rare with only one report of a participant being required to stop exercise training due to feeling lightheaded. There were no reports of death or other adverse events with exercise training.
What evidence did we find and how good was it? The review included six studies on 206 people with pulmonary hypertension and we could combine data from five of these studies. We could only use data for 165 participants, however not all of these data could be included in the analysis for all outcome measures. The majority of studies implemented an inpatient exercise rehabilitation programme with only a small number of studies examining an outpatient programme. The methods used to conduct these trials were of low quality. Given this low‐quality evidence, it was not possible to generalise the results of this review across the spectrum of people with pulmonary hypertension.
Summary of findings
Summary of findings for the main comparison. Exercise compared to control for pulmonary hypertension.
| Exercise compared to control for pulmonary hypertension | |||||
| Patient or population: people with pulmonary hypertension Settings: inpatient or outpatient rehabilitation, or both Intervention: exercise training Comparison: control: people that had usual care and did not undertake exercise training programme | |||||
| Outcomes | Illustrative comparative effects* (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Response on control | Treatment effect | ||||
| Control | Exercise | ||||
| Change in functional exercise capacity (6MWD) Distance, metres Follow‐up median 12 weeks | Median change = 5 m | The mean exercise capacity 6MWD in the intervention groups was 60.12 higher (30.17 to 90.07 higher) | 165 (5 studies) | ⊕⊕⊝⊝ low1,2 | Subgroup PAH: (2 studies, n = 36), mean 6MWD for intervention group was 33.84 m higher (0.95 to 66.73 higher); these studies used outpatient exercise rehabilitation whilst other studies contributing to meta‐analysis had an inpatient training component Minimal important difference was 30 metres |
|
Exercise capacity: VO2peak Oxygen uptake, ml/kg/min Follow‐up median 13.5 weeks |
Median change = ‐0.25 ml/kg/min | The mean VO2peak in the intervention groups was 2.41 ml/kg/min higher (1.38 to 3.44 higher) | 145 (4 studies) | ⊕⊕⊝⊝ low1,2 | Subgroup PAH (2 studies, n = 36), the mean VO2peak in the intervention groups was 1.28 ml/kg/min higher (‐0.19 to 2.75 higher); these two studies used outpatient exercise rehabilitation whilst other studies contributing to meta‐analysis had an inpatient training component |
|
Exercise capacity: peak power watts Follow‐up median 13.5 weeks |
Median change = 1 watt | The mean exercise capacity: peak power in the intervention groups was 16.44 W higher (10.90 to 21.99 higher) | 145 (4 studies) | ⊕⊕⊝⊝ low1,2 | Subgroup PAH (2 studies, n = 36), the mean peak power in the intervention groups was 14.24 watts higher (5.78 to 22.70 higher); these two studies used outpatient exercise rehabilitation whilst other studies contributing to meta‐analysis had an inpatient training component |
|
HRQoL SF‐36: PCS units Follow‐up median 11 weeks |
Median change = ‐0.49 units | The mean HRQoL SF‐36: PCS in the intervention groups was 4.63 higher (0.80 to 8.47 higher) | 33 (2 studies) | ⊕⊕⊝⊝ low2,3 | Both studies were only PAH |
|
HRQoL SF‐36: MCS units Follow‐up median 11 weeks |
Median change = ‐0.31 units | The mean HRQoL SF‐36: MCS in the intervention groups was 4.17 higher (0.01 to 8.34 higher) | 33 (2 studies) | ⊕⊕⊝⊝ low2,3 | Both studies were only PAH |
| *The basis for the response on control is the median control group response across studies | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1 Two studies did not report random sequence generation, no studies reported allocation concealment 2 Indirectness: 2 studies did not report number of people assessed to achieve sample size; trial participants may represent a highly selected subgroup of people with PH
3 Imprecision (2 small studies of 33 participants) and neither reported allocation concealment
Background
Description of the condition
Pulmonary hypertension (PH) is a progressive vasculopathy characterised by extensive remodelling of the pulmonary vasculature resulting in a narrowing of the arterial lumen (Casserly 2009). There is a marked increase in pulmonary vascular resistance resulting in right ventricular remodelling and eventual failure, which, in the majority of cases, results in patient death (Tuder 2013). Confirmatory diagnosis of PH is made via right heart catheterisation in which the patient has a resting mean pulmonary artery pressure of greater than 25 mmHg (Hoeper 2013). PH may arise in association with a broad range of disease states (over 40) of both known and unknown cause. International guidelines classified PH into the following five clinical groups (Simonneau 2013).
Group 1: pulmonary arterial hypertension (PAH)
Group 2: PH due to left heart disease
Group 3: PH due to lung diseases or hypoxia, or both
Group 4: chronic thromboembolic PH (CTEPH)
Group 5: PH with unclear multifactorial mechanisms.
Given the evolving definition of PH, the incidence and prevalence of the disease is difficult to define (Strange 2012). One recent study suggested that this varies markedly between the five clinical groups. In an observational cohort study of over 10,000 individuals from Armadale and the surrounding region in Western Australia, Strange 2012 reported the minimum indicative prevalence for all groups of PH was 326/100,000, with left heart disease associated with Group 2 being the most prevalent. Registries of prevalent and incident cases from around the world have now been published (McGoon 2013), suggesting an increased global awareness of the disease.
Regardless of aetiology, PH is characterised by limited exercise capacity and a progressive increase in breathlessness. Until recently, treatment options for PH remained limited and patient prognosis poor. One early registry of people with PH reported a median survival time of 2.8 years post diagnosis (D'Alonzo 1991). The development of PH‐specific drug therapies, targeted at the pulmonary vasculature, has significantly improved prognosis. This improved survival has been reflected in several of the more recently published registries (McGoon 2013). For example, the United States Registry to Evaluate Early and Long‐Term PAH Disease Management (REVEAL) registry of over 3500 prevalent and incident cases recorded between 2006 and 2009, reported five‐year survival rates for PAH at 57% (Benza 2010).
Advances in PH‐specific therapies have improved survival and slowed disease progression. As a result, other treatment options aimed at improving outcomes such as exercise capacity and quality of life have been explored. In people with other chronic heart and lung diseases, there is strong evidence that exercise training improves functional capacity, quality of life and even long‐term survival (Spruit 2013). However, until very recently, exercise rehabilitation has been actively discouraged in people with PH for fear it would worsen symptoms and negatively impact on cardiac function (Galie 2013). Whilst guidelines released in December 2013 recommend exercise training, the guideline authors acknowledge that gaps in the knowledge exist including knowledge of the optimal training dose, characteristics of supervision, mechanisms of adaptation and the impact of exercise training on long‐term survival (Galie 2013).
Description of the intervention
Exercise‐based rehabilitation programmes include aerobic and strength training elements designed to improve both aerobic capacity and muscle strength. Aerobic training involves the activation of a large skeletal muscle mass through an extended period of cycling or walking exercise that is between 20 and 40 minutes in duration. Strength training programmes involve upper and lower body muscle groups with the participant completing a number of sets of exercises at a fixed percentage of a repetition maximum (RM) Spruit 2013. Programmes are typically offered in an outpatient or inpatient setting, involving two to three sessions per week typically over at least a four‐week period.
How the intervention might work
In healthy young and older patients, exercise training results in improved oxygen transport and uptake at peak exercise through both central and peripheral adaptations. Central adaptations include an increase in maximal cardiac output, through an increase in stroke volume (Ogawa 1992). Central adaptations are the result of volume overload mediated cardiac remodelling that leads to improved cardiac function at rest and during exercise (Ogawa 1992; Pluim 2000). In the periphery, greater skeletal muscle oxidative capacity occurs with an increase in enzymes associated with cellular respiration, in particular those involved in the citric acid cycle (the Krebs cycle) and oxidative phosphorylation (Gollnick 1973; Coggan 1992). In addition, there is an increase in the capillary density per myofibril (Gollnick 1973; Coggan 1992). As a result of these central and peripheral adaptations, there is not only an increased delivery of oxygen to the exercising myofibril, there is also increased capacity to metabolise oxygen for the production of adenosine triphosphate. Transition between myofibril types typically occurs with an increase in the fast twitch oxidative and a decrease in fast twitch glycolytic fibres following exercise training (Gollnick 1973; Coggan 1992; Ennion 1995). Moreover, there is an increase in the cross sectional area of slow twitch (Type I) and Type IIa fibres in trained individuals (Gollnick 1973; Coggan 1992).
In PH, the factors which contribute to exercise limitation are complex (Fowler 2012; Panagiotou 2015; Babu 2016b). The changes in the pulmonary vasculature associated with PH results in a significant increase in pulmonary artery pressure and right ventricular afterload during exercise (Riley 2000; Provencher 2008). Right ventricular contractility is decreased and there is a reduced capacity for stroke volume and therefore for cardiac output to increase during exercise (Fowler 2012). Moreover, people with PH have a reduced heart rate response to exercise (chronotropic incompetence), which further decreases the ability for cardiac output to increase during exercise (Provencher 2006). As a result, people with PH have a blunted increase in cardiac output during exercise that significantly reduces peak oxygen transport. In the periphery, people with PH appear to have marked skeletal muscle dysfunction consistent with a reduced oxidative capacity (Mainguy 2010a). Compared to controls, people with PH had a lower percentage of Type I fibres and increased concentrations of enzymes associated with glycolytic (anaerobic) metabolism (Mainguy 2010a). These central and peripheral changes would result in a substantial reduction in the ability to transport and utilise oxygen during exercise.
In people with chronic lung disease, lower limb exercise training and strength training have both been demonstrated to increase exercise capacity and quality of life (Spruit 2013). The primary site of adaptation appears to be the skeletal muscle, with little change in cardiac function following exercise training in people with chronic heart and lung disease (Vogiatzis 2013). For example, in people with chronic obstructive pulmonary disease there is evidence that exercise training results in improved skeletal muscle structure and function with little change in cardiac function (Whittom 1998; Vogiatzis 2013). Whilst preliminary evidence in a small number of people suggests that there is some improvement in skeletal muscle function following exercise training in PH (de Man 2009; Mainguy 2010b), it remains unclear if these changes result in improved exercise capacity or if they relate to improved long‐term outcomes. Currently there is limited evidence for any central changes following exercise training in PH.
Why it is important to do this review
The objective of this review was to assess the efficacy and safety of exercise‐based rehabilitation for people with PH. In other chronic lung disease populations, for example chronic obstructive pulmonary disease, this form of rehabilitation is safe and has demonstrable benefits in terms of improvement in exercise capacity, lower limb muscle strength and quality of life (Spruit 2013). Until recently there had been a reluctance to recommend exercise‐based rehabilitation for PH due to the fact that it may worsen the patient's long‐term health outcomes (Galie 2009). Given international guidelines recommending exercise training in PH (Galie 2013, Galie 2015), it is important that the current state of the evidence regarding the efficacy and safety of exercise‐based rehabilitation is established. The results of this review will provide essential information to clinicians who may consider referring people with PH for exercise‐based rehabilitation, and help guide decisions on which PH patients may be suitable.
Objectives
To assess the efficacy and safety of exercise‐based rehabilitation for people with PH.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs). We included studies reported in full or abstract form as well as any relevant, unpublished data.
Types of participants
We included adults with a diagnosis of PH. We included all five clinical groups of PH (Simonneau 2013), independent of whether the patients were stable on therapy (i.e. change of therapy over the past three months).
Types of interventions
We included trials comparing exercise‐based rehabilitation with usual care or no exercise‐based rehabilitation. Exercise‐based rehabilitation of any frequency and duration was eligible for inclusion, including inpatient, outpatient or home‐based settings. We included exercise programmes of any length; however, we only included trials in which exercise training was supervised. We excluded exercise programmes that only provided exercise advice. We included exercise‐based programmes prescribing aerobic or strength training, or both.
We planned to analyse exercise‐rehabilitation that only included a strength‐training programme separately, however no such trials were found. The control group included individuals randomised to a programme of education which had no specific exercise prescription component.
Types of outcome measures
Primary outcomes
-
Exercise capacity
Measures of exercise capacity included but were not confined to outcomes such as the six‐minute walk distance (6MWD), peak exercise capacity (VO2peak), peak power (Wpeak) and measures derived during the assessment of exercise capacity such as breathing efficiency (VE/VCO2 slope) and anaerobic threshold
-
Serious adverse events during the intervention period
We used this measure to assess the short‐term safety of exercise training in PH. We defined adverse events as:
mortality;
disease progression, defined according to the investigators' definition;
symptoms precluding training, such as illness, lightheadedness, syncope or presyncope; and
discontinuation of the study
Health‐related quality of life measured by any validated generic or disease‐specific quality‐of‐life measure
Secondary outcomes
-
Cardiopulmonary haemodynamics
These included measures made using echocardiographic, right heart catheter or magnetic resonance imaging techniques
Outcome measures included, but were not confined to indices such as mean pulmonary artery pressure (mPAP), mean pulmonary vascular resistance, right ventricular systolic pressure, tricuspid annular plane systolic excursion, ventricular ejection fraction, ventricular end diastolic volume and ventricular end systolic volume
Functional Class measured by the New York Heart Association (NYHA) Classification/ (NYHA 1994) World Health Organisation (WHO) Functional Classification Rubin 2004
-
Clinical worsening during the follow‐up period.
The impact of exercise training on clinical worsening was assessed using the investigators definition
Typically clinical worsening is defined using a combination of outcomes including survival, hospitalisation due to PH, transplantation, requirement for additional pharmacological therapy, a reduction in functional class and or a reduction in the six‐minute walk test (Frost 2013)
For the purpose of this study, we treated mortality during the follow‐up period as a separate secondary outcome measure
-
Mortality during the follow‐up period
We recorded all deaths reported following the exercise intervention
We treated these deaths separately to those that occurred during the exercise training period, which were recorded by the primary outcome measure, serious adverse events
-
B‐type natriuretic peptide
A commonly used marker of right ventricular dysfunction in PH that is correlated with survival (Casserly 2009)
We examined changes in B‐type natriuretic peptide following exercise‐based rehabilitation
Reporting one of more of the outcomes listed here was not an inclusion criterion for the review.
Search methods for identification of studies
Electronic searches
We identified trials from searches of the following databases.
The Cochrane Airways Register of Trials: all years to 23 August 2016
Cochrane Central Register of Controlled Trials (CENTRAL; 2016, issue 8) (via the Cochrane Register of Studies (CRS‐Web): searched 23 August 2016
MEDLINE (Ovid): 1950 to August week 1 2016
Embase (Ovid): 1974 to week 33 2016
Physiotherapy Evidence Database (PEDro): all years to 23 August 2016
The database search strategies are listed in Appendix 1. We searched all databases from their inception to August 2016, with no restriction on language or type of publication. We identified handsearched conference abstracts and grey literature from the CENTRAL database. We also conducted a search of ClinicalTrials.gov (clinicaltrials.gov/) and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) search portal (apps.who.int/trialsearch/).
Searching other resources
We checked reference lists of all primary studies and review articles for additional references. We searched for errata or retractions from included studies published in full‐text on PubMed on 16 August 2016.
Data collection and analysis
Selection of studies
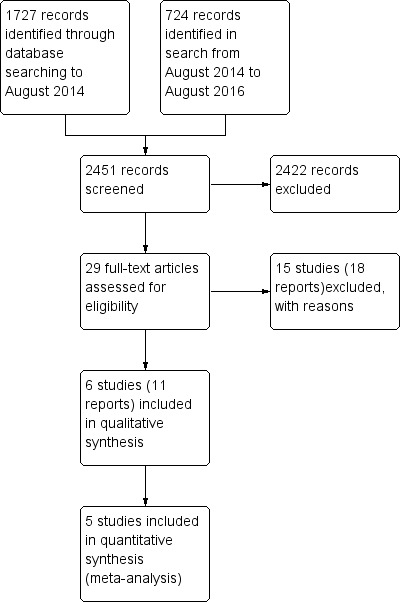
Two review authors (NM and AH) independently screened titles and abstracts for inclusion and coded them as 'retrieve' (eligible or potentially eligible/unclear) or 'do not retrieve'. We retrieved the full‐text study reports/publication, and two review authors (NM and AH) independently screened the full‐text and identified studies for inclusion, and identified and recorded reasons for exclusion of the ineligible studies. We resolved any disagreement through discussion. We identified and excluded duplicates and collated multiple reports of the same study so that each study rather than each report was the unit of interest in the review. We used Covidence (Covidence 2016) to manage the selection process. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram (Figure 1) and Characteristics of excluded studies table.
1.
Study flow diagram
Data extraction and management
We used a data collection form for study characteristics and outcome data which was piloted on one study in the review. Two review authors (NM and AH) extracted study characteristics from included studies in Covidence.
We extracted the following study characteristics.
Methods: study design, total duration of study, details of any 'run in' period, number of study centres and location, study setting, withdrawals, and date of study.
Participants: number enrolled, mean age, age range, gender, severity of condition, diagnostic criteria, baseline echocardiography and right heart catheter data, baseline lung function, inclusion criteria, and exclusion criteria.
Interventions: intervention, training dose (intensity, frequency and duration of exercise training), comparison, concomitant medications, and excluded medications.
Outcomes: primary and secondary outcomes specified and collected, and time points reported.
Notes: funding for trial, and notable conflicts of interest of trial authors.
We noted in the Characteristics of included studies table if outcome data were not reported in a usable way. We resolved disagreements by consensus. One review author (NM) transferred data into the Cochrane Collaboration's statistical software, Review Manager (RevMan) (RevMan 2014). We double‐checked that data were entered correctly by comparing the data presented in the systematic review with the study reports. A second review author (AH) spot‐checked study characteristics for accuracy against the trial report.
Assessment of risk of bias in included studies
Two review authors (NM and AH) independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). We resolved any disagreements by discussion.
We assessed the risk of bias according to the following domains:
random sequence generation;
allocation concealment;
blinding of participants and personnel;
blinding of outcome assessment;
incomplete outcome data;
selective outcome reporting;
other bias.
We graded each potential source of bias as high, low or unclear and provided a quote from the study report together with a justification for our judgment in a 'Risk of bias' table. We summarised the risk of bias judgements across different studies for each of the domains listed. We considered blinding separately for different key outcomes where necessary (e.g. for unblinded outcome assessment, risk of bias for all‐cause mortality may be very different than for a HRQoL scale).
When considering treatment effects, we took into account the risk of bias for the studies that contributed to that outcome.
Assessment of bias in conducting the systematic review
We conducted the review according to the published protocol and reported any deviations from it in thDifferences between protocol and review section of the systematic review.
Measures of treatment effect
We analysed dichotomous data as odds ratios (ORs). For continuous data, we used mean differences (MDs) or standardised mean differences (SMDs). Where it was reported, we used the change from baseline. Where the change from baseline was not reported, we used the adjusted results or final score. We did not combine data expressed as change from baseline with that reported as other metrics. We entered data presented as a scale with a consistent direction of effect.
We undertook meta‐analyses only where this was meaningful, that is, if the treatments, participants and the underlying clinical question were similar enough for pooling to make sense.
We narratively described skewed data reported as medians and interquartile ranges.
Where multiple trial arms were reported in a single trial, we planned to include only the relevant arms, however no trials of this nature were identified.
Unit of analysis issues
Where studies randomly allocated the participants to either the exercise‐based rehabilitation or control, we considered the participant as the unit of analysis. We excluded cross‐over trials due to the potential carry‐over effects of exercise training.
Dealing with missing data
We contacted trial investigators or study sponsors in order to verify key study characteristics and obtain missing numerical outcome data where possible (e.g. when a study was identified as abstract only). Where this was not possible, and the missing data were thought to introduce serious bias, we explored the impact of including such studies in the overall assessment of results by a sensitivity analysis.
Assessment of heterogeneity
We used the I2 statistic to measure heterogeneity among the trials in each analysis (Higgins 2003). If we identified substantial heterogeneity, we explored possible causes by prespecified subgroup analysis (Deeks 2011).
Assessment of reporting biases
If we were able to pool more than 10 trials, we planned to create and examine a funnel plot to explore possible small study and publication biases, however insufficient numbers of trials were identified.
Data synthesis
We performed a pooled quantitative synthesis where the trials were clinically homogeneous. We pooled data using a random‐effects model to incorporate between‐study heterogeneity into the meta‐analysis. Data from an intention‐to‐treat analysis were used where available. Where the trials were clinically heterogeneous, we performed a narrative synthesis. We used RevMan HAL, developed by the Cochrane Schizophrenia Group (http://szg.cochrane.org/revman‐hal), to construct a first draft of the results section.
'Summary of findings' table
We created a 'Summary of findings' table using the following outcomes: exercise capacity, serious adverse events, cardiopulmonary haemodynamics, quality of life, functional class, mortality and clinical worsening during follow‐up. We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of a body of evidence as it relates to the studies which contribute data to the meta‐analyses for the prespecified outcomes. We used methods and recommendations described in the Cochrane Handbook for Systematic Reviews of Interventions(Schünemann 2011) using GRADEpro software (GRADEpro GDT 2015). We justified all decisions to downgrade or upgrade the quality of studies in the Footnotes section of Table 1, and we made comments to aid readers' understanding of the review where necessary.
Subgroup analysis and investigation of heterogeneity
We planned to carry out the following subgroup analyses.
-
Type of PH:
we analysed data separately for people with PAH only (Group 1).
-
Severity of PH:
we planned to compare the outcomes of less severe disease classification (NYHA Class I/II) with those with more severe disease classification (NYHA Class III/IV), however insufficient data were available.
We used the following outcomes in subgroup analyses:
exercise capacity;
serious adverse events;
health‐related quality of life.
We used the formal test for subgroup interactions in RevMan 2014.
Sensitivity analysis
We performed sensitivity analyses to examine the effects of methodological quality on the pooled estimate by removing studies that were at high or unclear risk of bias for the domains of blinding and incomplete outcome data.
Results
Description of studies
See Characteristics of included studies, Characteristics of excluded studies and Characteristics of studies awaiting classification for complete details.
Results of the search
The PRISMA table shows results of our searchFigure 1
In the original search to August 2014, we found 1727 papers that were potentially relevant. We conducted an additional search from August 2014 to August 2016 and identified an additional 724 papers. After removing duplicates and the clearly irrelevant material we selected 29 full‐text papers to be further assessed for inclusion. Of these, we excluded 15 studies (18 reports) because they did not meet our inclusion criteria. Finally, after careful scrutiny, we were left with six studies (11 reports).
Included studies
Refer to Characteristics of included studies. The total number of participants from included participants was 206. Sample sizes ranged from 10 to 87 participants. Most participants had PAH (Group 1 PH) or chronic thromboembolic PH. The mean age of participants ranged from 47 to 56 years, and the mPAP on right heart catheterisation ranged from 40 to 52 mmHg. All participants were stable on medical therapy.
Excluded studies
From the 29 full‐text papers reviewed, we excluded 15 studies (18 reports). Reasons for exclusion were that studies were not randomised controlled trials (RCTs, n = 8), did not include exercise training (n = 3), was a review (n = 1), included the wrong population (n = 1) or used the wrong intervention (n = 2). Full details of the reasons for exclusion are included in the Characteristics of excluded studies section.
Risk of bias in included studies
Details on our judgements on the potential risks of bias are summarised in Figure 2 and Figure 3, with full details in the Characteristics of included studies table.
2.
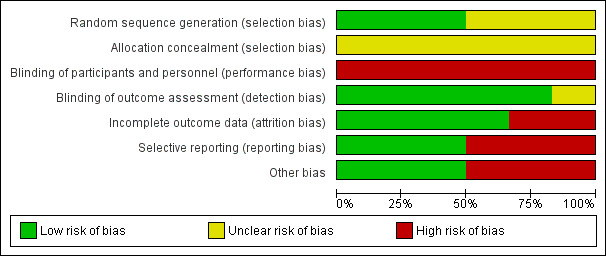
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
3.
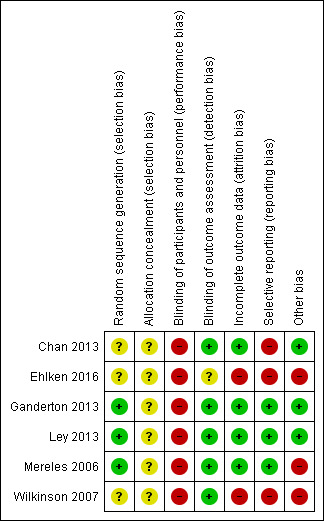
Risk of bias summary: review authors' judgements about each risk of bias item for each included study
Allocation
None of the studies provided details on how allocation was concealed and we therefore judged them to be at unclear risk of bias in this domain. Three of the studies (Mereles 2006; Ganderton 2013; Ley 2013) provided details on how the randomisation sequence was generated and we judged them to be at low risk. For the remaining studies we were unable to ascertain details of random sequence generation.
Blinding
We rated all six of the studies as having a high risk of bias for blinding of participants and personnel. Given the nature of the intervention (exercise training) it was not possible to blind participants or personnel to the intervention. Five out of six studies reported blinding of outcome assessors.
Incomplete outcome data
Based on our review, we rated four of the studies as low risk with regards to attrition bias (Mereles 2006; Chan 2013; Ganderton 2013; Ley 2013) with each of these studies reporting no or very small numbers of dropouts. We rated the largest study, Ehlken 2016, as high risk as there was a differential rate of attrition with 17% dropout in the intervention group as opposed to 0% dropout in the control.
Selective reporting
We found three studies to have low risk of reporting bias (Mereles 2006; Ganderton 2013; Ley 2013). Two studies did not provide complete results when compared to those provided on the trial registry (Chan 2013; Ehlken 2016). The final study of Wilkinson 2007 was only in abstract form, making it difficult to ascertain if the data reported were complete.
Other potential sources of bias
We found three of the studies to be of low risk with regards to other sources of bias (Chan 2013; Ganderton 2013; Ley 2013). We were unable to rule out some selection bias in the three other studies (Mereles 2006; Wilkinson 2007; Ehlken 2016). Neither of the studies by Mereles 2006 and Wilkinson 2007 provided a CONSORT diagram and hence there is no detail how many participants they screened to achieve the enrolment target (Schulz 2010). The study by Ehlken 2016 , whilst providing a CONSORT diagram, did not provide any detail on how many patients were screened and how they applied the inclusion/exclusion criteria to achieve the target enrolment of 95 participants.
Effects of interventions
See: Table 1
Studies in this review compared exercise‐based rehabilitation to no intervention, education alone or usual care. In total there were six relevant randomised studies. We extracted data for meta‐analyses from five of the studies (Mereles 2006; Chan 2013; Ganderton 2013; Ley 2013; Ehlken 2016), allowing for comparison between exercise‐based intervention and control. We were unable to obtain data for analysis from the study by Wilkinson 2007 (published only as an abstract) despite several attempts to contact the study authors. See Table 1 for the main comparisons between the intervention and control groups. In total there were 21 outcomes evaluated including primary outcomes of exercise capacity, adverse events and health‐related quality of life.
Primary outcomes
Exercise capacity
Five studies (n = 165 PH participants) reported changes in the 6MWD (Mereles 2006; Chan 2013; Ganderton 2013; Ley 2013; Ehlken 2016) or changes in exercise capacity derived from an incremental exercise test (Mereles 2006; Chan 2013; Ganderton 2013; Ehlken 2016). The mean increase in 6MWD of 60.12 m (MD 30.17 to 90.07 higher, Analysis 1.1, Figure 4) was well in excess of the minimal important difference of 30 metres (Mathai 2012; Holland 2014). However there was marked heterogeneity across studies (I2= 64%).
1.1. Analysis.
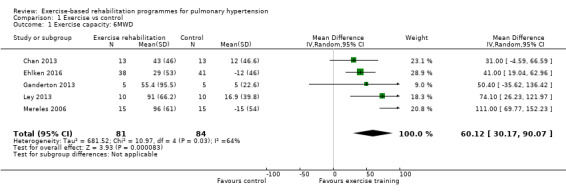
Comparison 1 Exercise vs control, Outcome 1 Exercise capacity: 6MWD.
4.
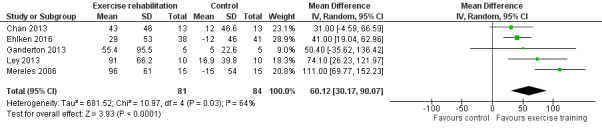
Forest plot of comparison: 1 Exercise vs control, outcome: 1.1 Exercise capacity: 6MWD
Four studies reported the impact of exercise‐based rehabilitation on peak exercise capacity determined from a cardiopulmonary exercise testing (CPET) (Mereles 2006; Chan 2013; Ganderton 2013; Ehlken 2016). There were significant increases in both VO2peak with exercise‐based rehabilitation compared to control (MD 2.4 ml/kg/min, 95% CI 1.4 to 3.4, Analysis 1.2) with no significant heterogeneity across studies (I2= 37%). Similarly, increases in peak power favoured exercise rehabilitation (MD 16.4 W, 95% CI 10.9 to 22.0, Analysis 1.3) with no significant heterogeneity (I2= 0%). Three studies reported changes in the anaerobic threshold, one of which was reported as time to anaerobic threshold (Chan 2013), whilst the other two reported this in ml/min (Mereles 2006; Ganderton 2013). Pooled analysis showed an increase in the standardised mean difference favouring the exercise rehabilitation group (SMD 1.05, 95% CI 0.53 to 1.58, I2= 0%, Analysis 1.4). Whilst there is no reported minimal important difference (MID) for CPET‐derived measures of exercise capacity in PH, the increase in peak power is in excess of the MID reported for chronic obstructive pulmonary disease of 5 to 10 W (Sutherland 2005).
1.2. Analysis.
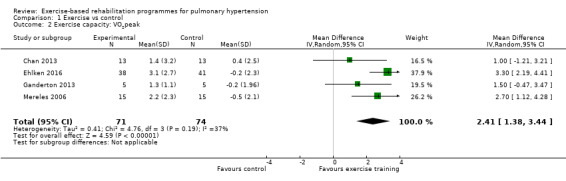
Comparison 1 Exercise vs control, Outcome 2 Exercise capacity: VO2peak.
1.3. Analysis.
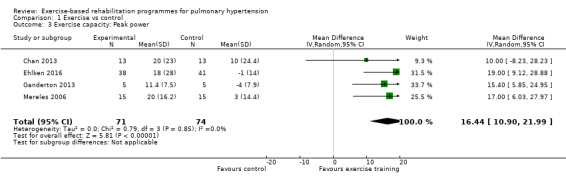
Comparison 1 Exercise vs control, Outcome 3 Exercise capacity: Peak power.
1.4. Analysis.
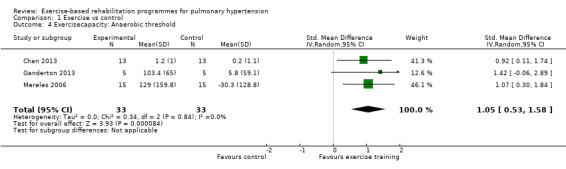
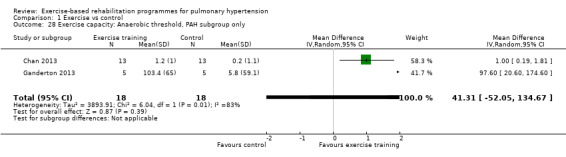
Comparison 1 Exercise vs control, Outcome 4 Exercisecapacity: Anaerobic threshold.
To date few studies have examined possible mechanisms for improved exercise capacity following exercise training in PH. In their study Ley 2013 reported improved pulmonary perfusion using magnetic resonance imaging (MRI). These authors suggested that exercise training may improve perfusion of the lungs or contractile function, or both. However it was noted that none of the changes in cardiac function correlated with changes in 6MWD. In their study Ehlken 2016 completed a right heart catheterisation (RHC) in a subgroup of exercise and control subjects. They reported improved pulmonary haemodynamics with a lowering of mean pulmonary artery pressure in the exercise group compared to the control group. For the exercise group there was an improvement in submaximal and maximal cardiac output. The authors hypothesised that exercise training may improve right ventricular (RV) function, however RV function was not directly measured.
Apart from these central changes there is some evidence that exercise training improves skeletal muscle oxidative capacity, similar to what is seen with exercise training in other chronic lung disease populations. Small observational studies by Mainguy 2010b (n = 5) and de Man 2009 (n = 19) both reported improvements in skeletal muscle oxidative capacity and capillary density. These preliminary results would suggest that the mechanism for adaptation to exercise training may be the result of improved skeletal muscle oxidative capacity and capillarisation and potentially improved oxygen delivery through improved cardiac function.
Overall the quality of evidence for changes in exercise capacity was rated as low due to imprecision and selective reporting. For details see Table 1.
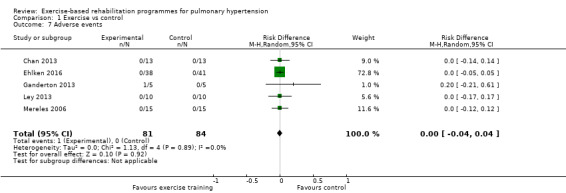
Serious adverse events
Only one study reported any adverse event that precluded a participant from training in a single session (Ganderton 2013). In this study one subject was reported to have stopped training for a single session due to extreme lightheadedness. No other studies reported any serious adverse events as we defined them in the protocol, that is, mortality, disease progression, symptoms that precluded training or discontinuation of the study.
Health‐related quality of life
Quality of life was reported using either the Short‐Form 36 (SF‐36) questionnaire (Mereles 2006; Chan 2013; Ganderton 2013; Ehlken 2016) or using the Cambridge Pulmonary Hypertension Outcome Review (CAMPHOR), a PH‐specific questionnaire (Chan 2013; Ganderton 2013).
We have reported the changes in the physical component scores (PCS) and mental component score (MCS) of the SF‐36 in Table 1 and Analysis 1.5 and Analysis 1.6 as these provide us with a summary of the global improvement in both physical and emotional aspects of quality of life. Changes in PCS and MCS were reported in two of the smaller studies (Chan 2013; Ganderton 2013). Analysis showed that exercise‐based interventions favoured improved outcomes for PCS (MD 4.63, 95% CI 0.80 to 8.47, Analysis 1.5 ) and MCS (MD 4.17, 95% CI 0.01 to 8.34, Analysis 1.6) with no significant heterogeneity between studies. Both of these studies also examined changes in health‐related quality of life using the CAMPHOR and reported greater improvement in the exercise‐based rehabilitation group in each of the subscores for activities (MD‐1.33, 95% CI ‐3.56 to 0.90, Analysis 1.16), symptoms (MD ‐3.08, 95% CI ‐7.78 to 1.62, Analysis 1.17) and overall quality of life (MD ‐5.42, 95% CI ‐8.03 to ‐2.81, Analysis 1.18), although there was marked heterogeneity for the activities and symptoms domains (I2 = 67% and 88% respectively).
1.5. Analysis.
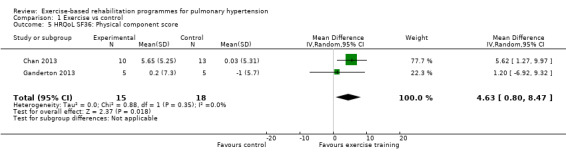
Comparison 1 Exercise vs control, Outcome 5 HRQoL SF36: Physical component score.
1.6. Analysis.
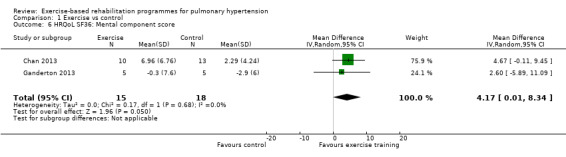
Comparison 1 Exercise vs control, Outcome 6 HRQoL SF36: Mental component score.
1.16. Analysis.
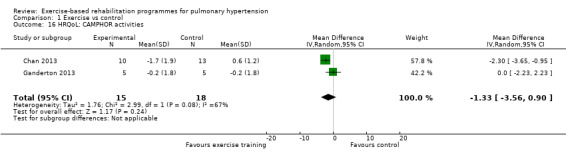
Comparison 1 Exercise vs control, Outcome 16 HRQoL: CAMPHOR activities.
1.17. Analysis.
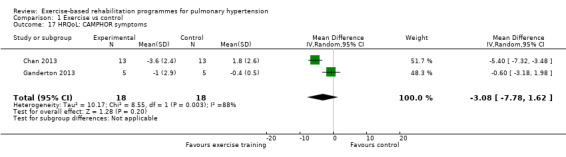
Comparison 1 Exercise vs control, Outcome 17 HRQoL: CAMPHOR symptoms.
1.18. Analysis.
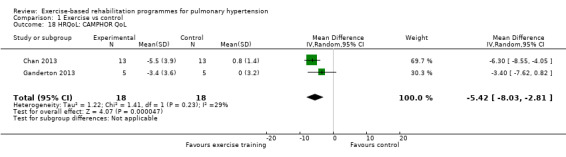
Comparison 1 Exercise vs control, Outcome 18 HRQoL: CAMPHOR QoL.
Four of the studies (n = 118 randomised) reported changes in quality of life using the domains of the Short‐Form 36 (SF‐36) questionnaire (Mereles 2006; Chan 2013; Ganderton 2013; Ehlken 2016). Exercise‐based rehabilitation resulted in a substantial improvement in outcome scores for 'Role physical' (MD 21.8, 95% CI 14.40 to 29.23, Analysis 1.9), 'Vitality' (MD 13.47, 95% CI 7.55 to 19.40, Analysis 1.14), and 'Social function' (MD 14.01, 95% CI 9.82 to 18.21, Analysis 1.15). Pooled analysis found there was no improvement in 'Physical function' (MD 6.13, 95% CI ‐3.73 to 16.00, Analysis 1.8), 'Bodily pain' (MD 5.64, 95% CI ‐3.09 to 14.36, Analysis 1.10,) 'General health' (MD 5.76, 95% CI ‐0.80 to 12.32, Analysis 1.11), 'Mental health' (MD 6.21, 95% CI ‐1.85 to 14.27, Analysis 1.12) and 'Role emotional' (MD 2.79, 95% CI ‐7.43 to 13.01, Analysis 1.13).
1.9. Analysis.
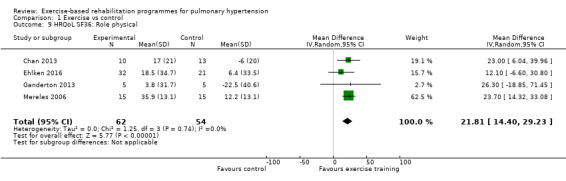
Comparison 1 Exercise vs control, Outcome 9 HRQoL SF36: Role physical.
1.14. Analysis.
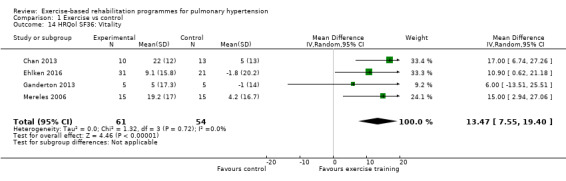
Comparison 1 Exercise vs control, Outcome 14 HRQol SF36: Vitality.
1.15. Analysis.
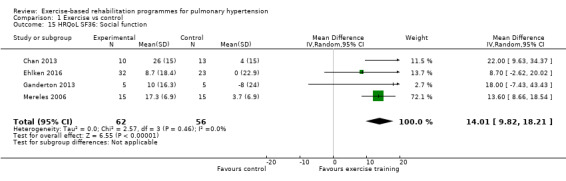
Comparison 1 Exercise vs control, Outcome 15 HRQoL SF36: Social function.
1.8. Analysis.
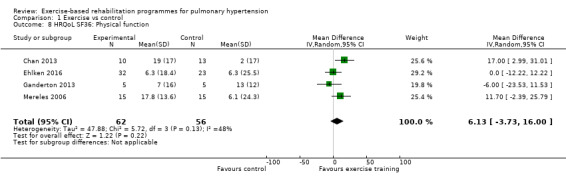
Comparison 1 Exercise vs control, Outcome 8 HRQoL SF36: Physical function.
1.10. Analysis.
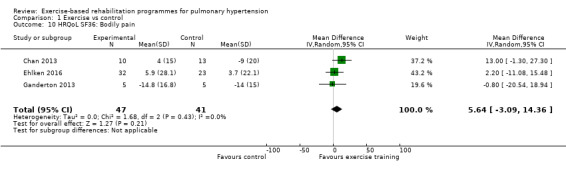
Comparison 1 Exercise vs control, Outcome 10 HRQoL SF36: Bodily pain.
1.11. Analysis.
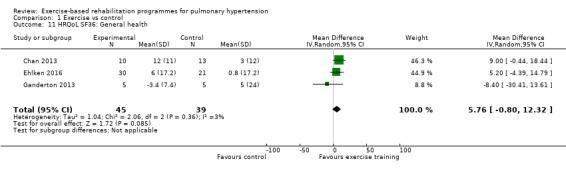
Comparison 1 Exercise vs control, Outcome 11 HRQoL SF36: General health.
1.12. Analysis.
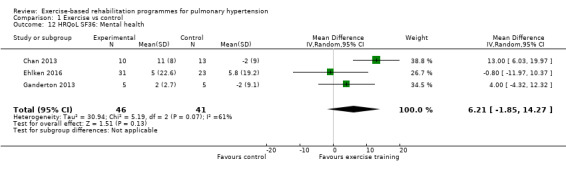
Comparison 1 Exercise vs control, Outcome 12 HRQoL SF36: Mental health.
1.13. Analysis.
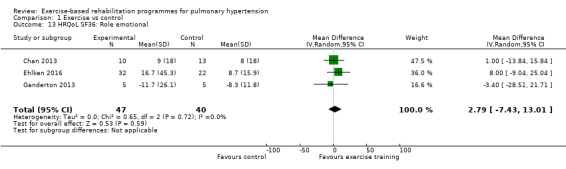
Comparison 1 Exercise vs control, Outcome 13 HRQoL SF36: Role emotional.
Secondary outcomes
Cardiopulmonary haemodynamics
Only the study by Ehlken 2016 reported changes in cardiopulmonary haemodynamics measured using (RHC) following exercise‐based rehabilitation. In a subset of the study participants (31 exercise and 28 control) the authors reported a significant decrease (P < 0.01) in mPAP following exercise training (MD‐9.00, 95% CI ‐13.60 to ‐4.40, Analysis 1.19).
1.19. Analysis.
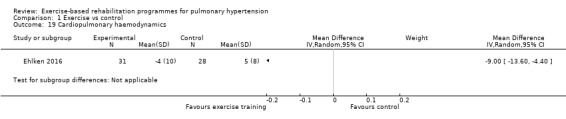
Comparison 1 Exercise vs control, Outcome 19 Cardiopulmonary haemodynamics.
Functional class
Only two studies reported changes in functional class for exercise and control groups Mereles 2006; Ganderton 2013. Improvement in functional class favoured exercise rehabilitation (MD ‐0.60, 95% CI ‐0.85 to ‐0.35, Analysis 1.20).
1.20. Analysis.
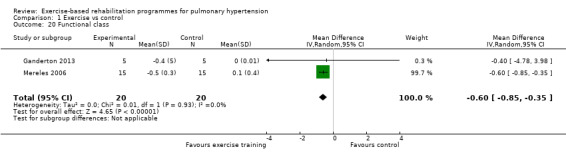
Comparison 1 Exercise vs control, Outcome 20 Functional class.
Clinical worsening during follow‐up period
No data were available for analysis.
Mortality during follow‐up period
No data were available for analysis.
B‐type natriuretic peptide
Only the study of Ehlken 2016 reported changes in B‐type natriuretic peptide for exercise and control groups. These authors reported a non significant (P = 0.36) improvement in B‐type natriuretic peptide with exercise rehabilitation (MD ‐236.00, 95% CI ‐744.48 to 272.48, Analysis 1.21).
1.21. Analysis.
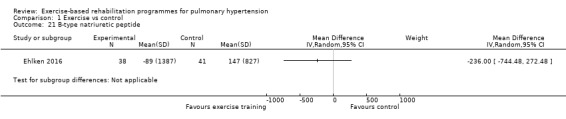
Comparison 1 Exercise vs control, Outcome 21 B‐type natriuretic peptide.
Sensitivity analysis
For our sensitivity analysis we removed studies that did not specify blinding of outcome measurements or had incomplete outcome data (attrition bias). As a result two studies were removed from the analysis of exercise outcomes (Wilkinson 2007; Ehlken 2016). We did not undertake sensitivity analysis for changes in health‐related quality of life as the studies of Wilkinson 2007 and Ehlken 2016 were not included in the original HRQoL analysis. Sensitivity analysis did not change the pattern of findings, with the exercise group showing improvements in 6MWD (MD 67.91 metres, 95% CI 27.12 to 108.69, Analysis 1.22), VO2peak (MD 1.94 ml/kg/m2, 95% CI 0.86 to 3.01, Analysis 1.23), and peak power (MD 15.27 Watts, 95% CI 8.57 to 21.97, Analysis 1.24) compared to control.
1.22. Analysis.
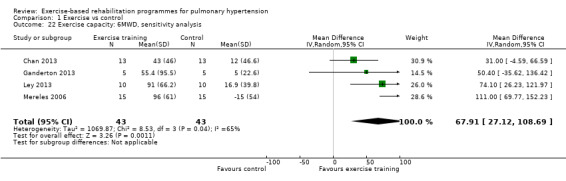
Comparison 1 Exercise vs control, Outcome 22 Exercise capacity: 6MWD, sensitivity analysis.
1.23. Analysis.
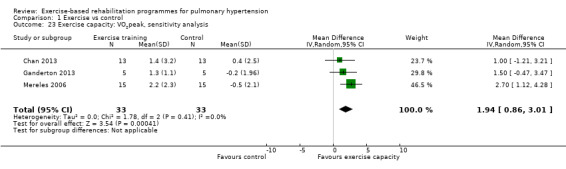
Comparison 1 Exercise vs control, Outcome 23 Exercise capacity: VO2peak, sensitivity analysis.
1.24. Analysis.
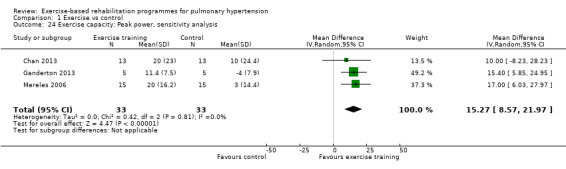
Comparison 1 Exercise vs control, Outcome 24 Exercise capacity: Peak power, sensitivity analysis.
Subgroup analysis
Type of PH
We compared the outcomes for different subgroups of PH using the classification outlined by Hoeper 2013. Three of the studies included a mixed group of PH participants, including both those with PAH (i.e. those from Group I, Hoeper 2013) and chronic thromboembolic pulmonary hypertension (i.e. Group 4, CTEPH, Hoeper 2013) (Mereles 2006; Ley 2013; Ehlken 2016). We were unable to extract data separately for the subgroups in these studies. We performed a subgroup analysis for the two studies that only included PAH (Group 1) participants (Chan 2013; Ganderton 2013). The increase in 6MWD, whilst much lower than the group as a whole, still exceeded the MID (MD 33.84 metres, 95% CI 0.95 to 66.73, Analysis 1.25). Likewise the increases in VO2peak (MD 1.28 ml/kg/min, 95% CI ‐0.19 to 2.75, Analysis 1.26) and peak power (MD 14.24 Watts, 95% CI 5.78 to 22.70, Analysis 1.27) were lower in the subgroup of participants with PAH. However these studies also differed in the setting and nature of the exercise rehabilitation programme delivered (see 'Setting of exercise rehabilitation programme' below) and it is therefore not possible to attribute these differences solely to diagnosis.
1.25. Analysis.
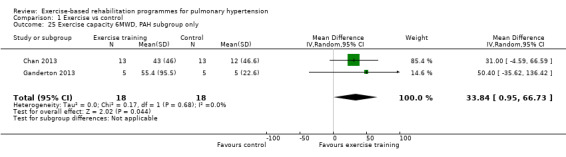
Comparison 1 Exercise vs control, Outcome 25 Exercise capacity 6MWD, PAH subgroup only.
1.26. Analysis.
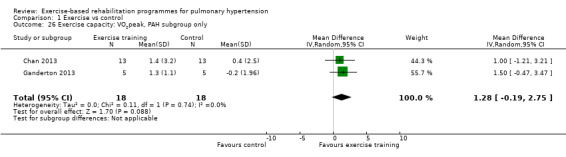
Comparison 1 Exercise vs control, Outcome 26 Exercise capacity: VO2peak, PAH subgroup only.
1.27. Analysis.
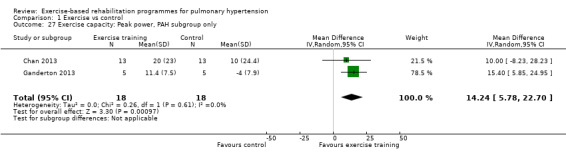
Comparison 1 Exercise vs control, Outcome 27 Exercise capacity: Peak power, PAH subgroup only.
Severity of PH
Insufficient data were available to perform subgroup analysis according to disease severity.
Setting of exercise rehabilitation programme
We identified an additional source of potential heterogeneity whilst exploring the heterogeneity in 6MWD responses (Analysis 1.1). Three studies used inpatient programmes of three weeks' duration (training seven days per week) (Mereles 2006; Ley 2013; Ehlken 2016), in some cases followed by a 12‐week, home‐based programme (Mereles 2006; Ehlken 2016), whilst the remaining studies used outpatient training programmes. Because of the observed heterogeneity we chose to examine results for programmes that included inpatient training components in the exercise‐based rehabilitation intervention separately to those that only included outpatient programmes, as inpatient programmes may allow closer supervision and greater intensity of exercise prescription. Note for the studies of Mereles 2006 and Ehlken 2016 we reported outcomes following the three‐week inpatient plus 12‐week home‐based programme (i.e. 15 weeks) whereas for Ley 2013 we have reported outcomes following the three‐week inpatient programme.
Studies that incorporated an inpatient model of exercise rehabilitation (Mereles 2006; Ley 2013; Ehlken 2016) reported very large improvements in 6MWD, however marked heterogeneity was still present across these three studies (mean improvement 72.79 metres, 95% CI 28.09 to 117.49, I2 = 78%). The studies that relied totally on outpatient‐based exercise programmes (Chan 2013; Ganderton 2013) randomised only 36 people with PH (24% of total subject sample) and reported a smaller mean difference in 6MWD favouring the exercise group of 33.84 metres (0.95 to 66.73 metres higher) but with no evidence of statistical heterogeneity (I2 = 0%). The test for subgroup differences was not significant (P = 0.17, Analysis 1.29). It should be noted that both these studies only included participants with PAH, so these subgroup analyses by setting give rise to the same results as those for the subgroup analysis according to type of PH.
1.29. Analysis.
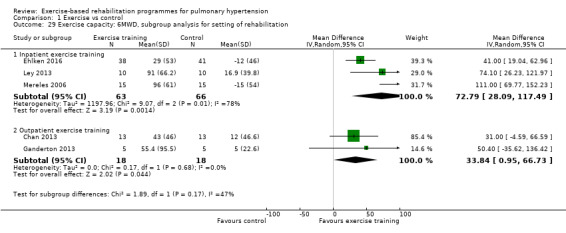
Comparison 1 Exercise vs control, Outcome 29 Exercise capacity: 6MWD, subgroup analysis for setting of rehabilitation.
Discussion
Summary of main results
The aim of this review was to examine the efficacy of exercise‐based rehabilitation in people with PH. The included studies reported large and clinically significant improvements in exercise capacity, measured using both the 6MWD and CPET. However there was marked heterogeneity across trials for 6MWD; we were unable to determine whether this was due to differences in study populations (PAH versus other), settings (inpatient versus outpatient) or the severity of the disease (where there was insufficient evidence to assess). There were also improvements in quality of life, measured using both PH‐specific and non‐specific tools, although the magnitude of these changes may not be clinically important. There was only a single reportable adverse event. These results are based on a relatively small number of participants (there were 206 participants in the trials, but data from only 165 in the forest plot with the most data) from only five RCTs. It was not possible to determine the impact of exercise rehabilitation on the secondary outcomes of cardiopulmonary haemodynamics, functional class or B‐type natriuretic peptide due to insufficient data. No studies reported on the effects of rehabilitation, time to clinical worsening or mortality. The quality of evidence was generally low, with no studies reporting allocation concealment, and the potential for selection bias, as there were few details provided regarding screening of potential artificialness. All outcomes were short term, measured immediately following the rehabilitation period, so the longer‐term effects of exercise rehabilitation remain unknown.
Overall completeness and applicability of evidence
Most participants in the studies had a diagnosis of PAH, so our results should be applied primarily in that group. There was a small number of participants with CTEPH, however their results could not be extracted separately, so it is difficult to be confident regarding the effects of exercise rehabilitation in this group. Of the studies completed to date, none have included groups of participants who had PH associated with connective tissue disease or congenital heart disease, PH due to left heart disease, or PH due to lung disease, so our results cannot be applied to these groups. Few participants in functional class IV were included, so the impact of exercise rehabilitation in those with the most severe disease remains unclear. Importantly, all studies only included participants who were stable on medical therapy (including no recent syncope), so it is in this group that exercise rehabilitation can be applied.
Three of the six studies used an inpatient rehabilitation programme of at least three weeks in duration, with exercise training taking place seven days per week (Mereles 2006; Ley 2013; Ehlken 2016). The magnitude of improvement in exercise outcomes appeared to be greater following these programmes compared to those who used an outpatient exercise‐based rehabilitation model, where supervised training took place only two to three times per week (Chan 2013; Ganderton 2013). However we were unable to determine whether the underlying diagnosis of participants also affected the outcomes. The inpatient exercise rehabilitation programmes delivered closer supervision, more sophisticated monitoring and a higher frequency of training than the outpatient programmes, which may contribute to better exercise outcomes. Such inpatient cardiorespiratory rehabilitation programmes are common in some parts of Europe, but are virtually non‐existent in other parts of the world such as the UK, Australia and the USA. Such differences in health system organisation may affect the type of exercise rehabilitation model that can be applied in PH. However it should be noted that improvements following outpatient training, although smaller in magnitude, were clinically important.
The exercise rehabilitation protocols tested included lower limb endurance training (walking or cycling), usually with resistance exercises for the upper and lower limbs. These protocols are similar to those recommended for standard pulmonary (Spruit 2013) and cardiac (Piepoli 2014) rehabilitation programmes. Additional components in some studies included stretching, breathing techniques such as pursed lip breathing, body perception, yoga, and strengthening of respiratory muscles (Mereles 2006). Further data is required to identify the contribution of these additional components to rehabilitation outcomes. The similarity of the core rehabilitation components to those delivered in pulmonary and cardiac rehabilitation programmes (lower limb endurance training, upper and lower limb resistance training) suggests that people with PH could receive their rehabilitation within these existing services, which could improve uptake into practice. However some studies in this review used specialised exercise prescription and monitoring practices that may not occur routinely in existing cardiopulmonary rehabilitation programmes (e.g. low‐intensity interval training, continuous monitoring of oxyhaemoglobin saturation and heart rate, restriction of exercise heart rate to less than 120 beats per minute) (Mereles 2006; Ley 2013; Ehlken 2016). Whilst no significant adverse events were documented during supervised exercise training in the studies included in this review, it is clear that exercise is not entirely without risk in PH (Morris 2015) and international guidelines currently suggest that exercise rehabilitation should be undertaken "...by centres experienced in both PH patient care and rehabilitation of compromised patients" (Galie 2015).
Quality of the evidence
It was encouraging that five out of six included studies reported blinding of outcome assessors, which is important for rehabilitation studies where many of the important outcomes (exercise capacity, HRQoL) could be affected by knowledge of group assignment. Random sequence generation and allocation concealment were generally not well reported. However the major source of potential bias related to reporting of participant selection. For three of the six studies it was not clear how many people had been assessed in order to achieve the required sample size. Pulmonary hypertension comprises a diverse group of patients with wide variation in disease severity. In contrast the participants in the included trials were predominantly from Group 1 and tended to have mild to moderate disease. It remains possible that the participants in these studies were a highly selected group who responded well to exercise training. Future studies should carefully report their screening and selection procedures in accordance with CONSORT requirements (Schulz 2010).
Potential biases in the review process
All data were extracted independently by two review authors using Covidence and discrepancies were resolved through discussion (Covidence 2016). Risk of bias ratings were also completed independently by two review authors. We included studies that were published only in abstract form, to ensure that all available trials were included. However, despite attempts to contact the authors of one abstract, additional data were not available (Wilkinson 2007). This may have influenced assessment of trial quality and some estimates of effect. We included an additional subgroup analysis (inpatient versus outpatient rehabilitation setting) that was not included in our protocol. This was because the marked heterogeneity in exercise outcomes prompted us to further explore the differences between studies, but we acknowledge that it is difficult to draw firm conclusions from this analysis due the post hoc nature of the approach.
Agreements and disagreements with other studies or reviews
Currently there are four published systematic reviews on exercise training in PH (Buys 2015; Pandey 2015; Yuan 2015; Babu 2016a), however the included studies, methods of analysis and assessment of study quality differed within these reviews. Like the current review, the systematic review of Buys 2015 examined only controlled trials up to December 2013, not all of which were randomised. The authors extracted five studies, three of which (Mereles 2006; Chan 2013; Ley 2013) were included in our analyses and used an adapted PEDro scale to rate the quality of these studies. This review also included the studies by Fox 2011 and Martinez‐Quintana 2010 both of which were excluded from our analysis as subjects were non‐randomly allocated to exercise or control groups. Overall this review generated similar results as the current review with a large increase in 6MWD (5 studies, MD for exercise group 72.5 m, 95% CI: 46.0 to 99.1) and VO2peak (3 studies, MD for exercise group 2.2 ml/kg‐1/min‐1, 95% CI 46.0 to 99.1). The other three systematic reviews (Pandey 2015; Yuan 2015;Babu 2016a) included both randomised controlled trials and observational studies and hence analysed a larger number of studies. Babu 2016a reported that exercise training resulted in large changes in exercise capacity, health‐related quality of life and very few adverse events in 15 included studies, four of which were classified as randomised controlled trials. These authors did not undertake a meta‐analysis of the studies. Yuan 2015 did undertake a meta‐analysis, reporting large increases in exercise capacity (6MWD and peak exercise capacity), health‐related quality of life (measured using the SF‐36) and few adverse events in the 12 studies they classified as being either randomised (n = 2), observational‐control (n = 4) or observational (n = 6). The authors undertook a subgroup analysis of randomised trials and whilst producing similar results to our study for exercise capacity (MD for exercise group 62 m, 95% CI: 45.6 to 78.8), these authors included data from Weinstein 2013, which we considered to be a duplicate report of one of the studies included in our review (Chan 2013). Moreover Yuan 2015 included the study by Fox 2011 as an RCT, a study excluded from our analysis. Pandey 2015 included 16 studies, with a subgroup of six parallel‐group studies. Similar to Yuan 2015 these authors included the study of Fox 2011 in this analysis. Pandey 2015 also included the study of Martinez‐Quintana 2010, in their parallel‐group analysis, a study again excluded from our analysis. Like other reviews, Pandey reported large increases in exercise capacity measured using the 6MWD and quality of life. Whilst these systematic reviews overall reported treatment effects of a similar magnitude to the current review, there were differences in the rating of the quality of evidence. Using the Downs and Black Quality Index (Downs and Black 1998), Babu 2016a rated the four included RCTs as providing good‐quality evidence (Chan 2013; Weinstein 2013; Ley 2013; Mereles 2006), however issues of possible selection bias were not identified. Pandey 2015 used the Cochrane risk of bias assessment tool to evaluate the quality of the extracted controlled intervention trials. Similar to our findings the authors reported that the majority of studies used random sequence generation and blinded assessment. The authors did not however recognise the potential for selection bias in their analysis. There does not appear to have been any attempt to report on the quality of evidence in the meta‐analysis conducted by Yuan 2015.
Authors' conclusions
Implications for practice.
This review suggests that supervised exercise‐based rehabilitation is likely to be safe for people with pulmonary hypertension (PH) who are stable on medical therapy and can lead to meaningful improvements in exercise capacity. Clinical importance of improvements in health‐related quality of life (HRQoL) is less clear. Although it is possible that programmes with an inpatient component may confer a greater magnitude of benefits, it must be acknowledged that these are not available in many parts of the world, and clinically meaningful benefits are still achieved with outpatient programmes. It is possible that people with PH could safely undertake rehabilitation in standard pulmonary or heart failure rehabilitation programmes, although different exercise prescription and monitoring practices appear necessary. These results apply primarily to people with moderate PH (New York Heart Association (NYHA)/World Health Organization (WHO) Functional Class, class II and III); the impact of rehabilitation in class IV is unknown. The duration of benefits for exercise‐based rehabilitation in PH is also unknown.
Implications for research.
Future randomised controlled trials are needed to inform the application of exercise‐based rehabilitation across the spectrum of people with PH, including diagnostic subgroups such as chronic thromboembolic PH, and those with more severe disease. It is essential that future trials provide clarity around participant selection in a CONSORT diagram, so that it is clear to which participants the results can be applied. Additional studies are needed to determine the optimal exercise training strategy for people with PH, including modality and intensity of training, length of programme, degree of supervision and the optimal setting for delivery of exercise training (e.g. inpatient versus outpatient). Longer‐term studies are required to assess the durability of benefits, and to determine the effect of exercise rehabilitation on critical outcomes such as time to clinical worsening and survival.
What's new
| Date | Event | Description |
|---|---|---|
| 1 February 2017 | Amended | Added the total number of participants |
Acknowledgements
Rebecca Normansell was the Editor for this review and commented critically on the review.
The Background and Methods sections of this review are based on a standard template used by Cochrane Airways.
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to the Cochrane Airways Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS, or the Department of Health.
Appendices
Appendix 1. Database search strategies
Cochrane Airways Register of Trials
#1 PULM:MISC1 #2 MeSH DESCRIPTOR Hypertension, Pulmonary Explode All #3 MeSH DESCRIPTOR Pulmonary Heart Disease #4 "pulmonary vascular disease":TI,AB #5 #1 or #2 or #3 or #4 #6 MeSH DESCRIPTOR exercise Explode All #7 MeSH DESCRIPTOR Exercise Therapy Explode All #8 MeSH DESCRIPTOR Exercise Tolerance #9 MeSH DESCRIPTOR Physical Fitness #10 MeSH DESCRIPTOR Physical Exertion #11 MeSH DESCRIPTOR Ergometry #12 MeSH DESCRIPTOR Bicycling #13 MeSH DESCRIPTOR Weight Lifting #14 MeSH DESCRIPTOR Muscle Strength #15 exercis*:TI,AB #16 conditioning or ergometry or treadmill or endurance or "upper limb" or "lower limb":TI,AB #17 walk* or swim* or cycle* or cycling or bicycl* or jog*:TI,AB #18 ((strength* or resistance* or weight*) NEAR3 train*):TI,AB #19 aerobic*:TI,AB #20 rehabilitat*:TI,AB #21 #6 OR #7 OR #8 or #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 #22 #5 and #21
CENTRAL (CRSO)
#1MESH DESCRIPTOR Hypertension, Pulmonary EXPLODE ALL TREES #2MESH DESCRIPTOR Pulmonary Heart Disease #3(pulmonary* NEAR3 hypertens*):TI,AB,KY #4("pulmonary vascular disease*"):TI,AB,KY #5#1 OR #2 OR #3 OR #4 #6MESH DESCRIPTOR Exercise EXPLODE ALL TREES #7MESH DESCRIPTOR EXERCISE THERAPY EXPLODE ALL TREES #8MESH DESCRIPTOR Exercise Tolerance #9MESH DESCRIPTOR Physical Fitness #10MESH DESCRIPTOR Physical Exertion #11MESH DESCRIPTOR Ergometry EXPLODE ALL TREES #12MESH DESCRIPTOR Bicycling #13MESH DESCRIPTOR Weight Lifting #14MESH DESCRIPTOR Muscle Strength EXPLODE ALL TREES #15exercis*:TI,AB,KY #16(conditioning or ergometry or treadmill or endurance or "upper limb" or "lower limb"):TI,AB,KY #17(walk* or swim* or cycle* or cycling or bicycl* or jog*):TI,AB,KY #18((strength* or resistance* or weight*) NEAR3 train*):TI,AB,KY #19aerobic*:TI,AB,KY #20rehabilitat*:TI,AB,KY #21#6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 #22#5 AND #21
MEDLINE (Ovid)
1. exp Hypertension, Pulmonary/
2. Pulmonary Heart Disease/
3. (pulmonary adj3 hypertens$).ti,ab.
4. pulmonary vascular disease.ti,ab.
5. or/1‐4
6. exp Exercise/
7. exp Exercise Therapy/
8. Exercise Tolerance/
9. Physical Fitness/
10. Physical Exertion/
11. exp Ergometry/
12. Bicycling/
13. Weight Lifting/
14. Muscle Strength/
15. exercis$.ti,ab.
16. (conditioning or ergometry or treadmill or endurance or "upper limb" or "lower limb").ti,ab.
17. (walk$ or swim$ or cycle$ or cycling or bicycl$ or jog$).ti,ab.
18. ((strength$ or resistance$ or weight$) adj3 train$).ti,ab.
19. aerobic$.ti,ab.
20. rehabilitat$.ti,ab.
21. or/6‐20
22. 5 and 21
23. (controlled clinical trial or randomized controlled trial).pt.
24. (randomized or randomised).ab,ti.
25. placebo.ab,ti.
26. randomly.ab,ti.
27. trial.ab,ti.
28. groups.ab,ti.
29. or/23‐28
30. Animals/
31. Humans/
32. 30 not (30 and 31)
33. 29 not 32
34. 22 and 33
Embase (Ovid)
1. exp pulmonary hypertension/ 2. (pulmonary adj3 hypertens$).ti,ab. 3. pulmonary vascular disease.ti,ab. 4. or/1‐3 5. exp exercise/ 6. exp kinesiotherapy/ 7. exp ergometry/ 8. exp bicycle/ 9. exp weight lifting/ 10. muscle strength/ 11. exercis$.ti,ab. 12. (conditioning or ergometry or treadmill or endurance or "upper limb" or "lower limb").ti,ab. 13. (walk$ or swim$ or cycle$ or cycling or bicycl$ or jog$).ti,ab. 14. ((strength$ or resistance$ or weight$) adj3 train$).ti,ab. 15. aerobic$.ti,ab. 16. rehabilitat$.ti,ab. 17. or/5‐16 18. 4 and 17 19. Randomized Controlled Trial/ 20. randomization/ 21. controlled clinical trial/ 22. Double Blind Procedure/ 23. Single Blind Procedure/ 24. Crossover Procedure/ 25. (clinica$ adj3 trial$).tw. 26. ((singl$ or doubl$ or trebl$ or tripl$) adj3 (mask$ or blind$ or method$)).tw. 27. exp Placebo/ 28. placebo$.ti,ab. 29. random$.ti,ab. 30. ((control$ or prospectiv$) adj3 (trial$ or method$ or stud$)).tw. 31. (crossover$ or cross‐over$).ti,ab. 32. or/19‐31 33. exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/ 34. human/ or normal human/ or human cell/ 35. 33 and 34 36. 33 not 35 37. 32 not 36 38. 18 and 37
PEDro
| Field | Search term |
| Abstract & Title | pulmonary hypertension |
| Method | clinical trial |
ClinicalTrials.gov
| search field | search term |
| Study type | Interventional |
| Condition | Pulmonary hypertension |
| intervention | Exercise |
Data and analyses
Comparison 1. Exercise vs control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Exercise capacity: 6MWD | 5 | 165 | Mean Difference (IV, Random, 95% CI) | 60.12 [30.17, 90.07] |
| 2 Exercise capacity: VO2peak | 4 | 145 | Mean Difference (IV, Random, 95% CI) | 2.41 [1.38, 3.44] |
| 3 Exercise capacity: Peak power | 4 | 145 | Mean Difference (IV, Random, 95% CI) | 16.44 [10.90, 21.99] |
| 4 Exercisecapacity: Anaerobic threshold | 3 | 66 | Std. Mean Difference (IV, Random, 95% CI) | 1.05 [0.53, 1.58] |
| 5 HRQoL SF36: Physical component score | 2 | 33 | Mean Difference (IV, Random, 95% CI) | 4.63 [0.80, 8.47] |
| 6 HRQoL SF36: Mental component score | 2 | 33 | Mean Difference (IV, Random, 95% CI) | 4.17 [0.01, 8.34] |
| 7 Adverse events | 5 | 165 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.04, 0.04] |
| 8 HRQoL SF36: Physical function | 4 | 118 | Mean Difference (IV, Random, 95% CI) | 6.13 [‐3.73, 16.00] |
| 9 HRQoL SF36: Role physical | 4 | 116 | Mean Difference (IV, Random, 95% CI) | 21.81 [14.40, 29.23] |
| 10 HRQoL SF36: Bodily pain | 3 | 88 | Mean Difference (IV, Random, 95% CI) | 5.64 [‐3.09, 14.36] |
| 11 HRQoL SF36: General health | 3 | 84 | Mean Difference (IV, Random, 95% CI) | 5.76 [‐0.80, 12.32] |
| 12 HRQoL SF36: Mental health | 3 | 87 | Mean Difference (IV, Random, 95% CI) | 6.21 [‐1.85, 14.27] |
| 13 HRQoL SF36: Role emotional | 3 | 87 | Mean Difference (IV, Random, 95% CI) | 2.79 [‐7.43, 13.01] |
| 14 HRQol SF36: Vitality | 4 | 115 | Mean Difference (IV, Random, 95% CI) | 13.47 [7.55, 19.40] |
| 15 HRQoL SF36: Social function | 4 | 118 | Mean Difference (IV, Random, 95% CI) | 14.01 [9.82, 18.21] |
| 16 HRQoL: CAMPHOR activities | 2 | 33 | Mean Difference (IV, Random, 95% CI) | ‐1.33 [‐3.56, 0.90] |
| 17 HRQoL: CAMPHOR symptoms | 2 | 36 | Mean Difference (IV, Random, 95% CI) | ‐3.08 [‐7.78, 1.62] |
| 18 HRQoL: CAMPHOR QoL | 2 | 36 | Mean Difference (IV, Random, 95% CI) | ‐5.42 [‐8.03, ‐2.81] |
| 19 Cardiopulmonary haemodynamics | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 20 Functional class | 2 | 40 | Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐0.85, ‐0.35] |
| 21 B‐type natriuretic peptide | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 22 Exercise capacity: 6MWD, sensitivity analysis | 4 | 86 | Mean Difference (IV, Random, 95% CI) | 67.91 [27.12, 108.69] |
| 23 Exercise capacity: VO2peak, sensitivity analysis | 3 | 66 | Mean Difference (IV, Random, 95% CI) | 1.94 [0.86, 3.01] |
| 24 Exercise capacity: Peak power, sensitivity analysis | 3 | 66 | Mean Difference (IV, Random, 95% CI) | 15.27 [8.57, 21.97] |
| 25 Exercise capacity 6MWD, PAH subgroup only | 2 | 36 | Mean Difference (IV, Random, 95% CI) | 33.84 [0.95, 66.73] |
| 26 Exercise capacity: VO2peak, PAH subgroup only | 2 | 36 | Mean Difference (IV, Random, 95% CI) | 1.28 [‐0.19, 2.75] |
| 27 Exercise capacity: Peak power, PAH subgroup only | 2 | 36 | Mean Difference (IV, Random, 95% CI) | 14.24 [5.78, 22.70] |
| 28 Exercise capacity: Anaerobic threshold, PAH subgroup only | 2 | 36 | Mean Difference (IV, Random, 95% CI) | 41.31 [‐52.05, 134.67] |
| 29 Exercise capacity: 6MWD, subgroup analysis for setting of rehabilitation | 5 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 29.1 Inpatient exercise training | 3 | 129 | Mean Difference (IV, Random, 95% CI) | 72.79 [28.09, 117.49] |
| 29.2 Outpatient exercise training | 2 | 36 | Mean Difference (IV, Random, 95% CI) | 33.84 [0.95, 66.73] |
1.7. Analysis.
Comparison 1 Exercise vs control, Outcome 7 Adverse events.
1.28. Analysis.
Comparison 1 Exercise vs control, Outcome 28 Exercise capacity: Anaerobic threshold, PAH subgroup only.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Chan 2013.
| Methods |
Study design: RCT Study grouping: Parallel group |
|
| Participants |
Baseline characteristics Exercise training
Control
Included criteria: Quote "Patients with World Health Organization (WHO) group 1 PH were recruited from local outpatient clinics and enrolled between September 2009 and October 2011. Men and women were eligible if they were between 21 and 82 years of age, had PH diagnosed by a resting mean pulmonary arterial pressure ≥ 25 mm Hg as measured by right‐sided heart catheterization, were on stable PH therapies for at least 3 months, were sedentary, and had no pulmonary rehabilitation for 6 months prior to enrolment". Excluded criteria: Quote "To avoid “ceiling” or “floor” effects, patients were excluded if they were classified ed as WHO and New York Heart Association (NYHA) functional class I and could walk 400 m during a 6MWT, or classified as functional class IV and could not walk 50 m during a 6MWT. Additional exclusion criteria included FEV1 /FVC ratio ≤ 65%; history of ischaemic heart disease; ejection fraction < 40%; documented pulmonary capillary wedge pressure ≥ 18 mm Hg; significant hepatic, renal, or mitochondrial dysfunctions; severe psychiatric disease; use of medications that may limit exercise capacity or ability to adapt to exercise training; antiretroviral therapies; illicit drugs; tobacco use; or pregnancy". Pretreatment: Control group had worse lung function |
|
| Interventions |
Intervention characteristics Exercise training
Control
|
|
| Outcomes | 6MWD VO2peak Anaerobic threshold HRQoL (SF‐36): Physical functioning HRQoL (SF‐36): Role physical HRQoL (SF36): Bodily pain HRQoL (SF‐36): General health HRQoL (SF‐36): Vitality HRQoL (SF‐36): Social function HRQoL (SF‐36): Role emotional HRQoL (SF‐36): Mental health HRQoL: Physical summary score (SF‐36) HRQoL: Mental summary score (SF‐36) HRQol (CAMPHOR): Symptoms HRQol (CAMPHOR): Activities HRQol (CAMPHOR): QoL NYHA Class |
|
| Identification | This work was supported by the US National Institutes of Health (Intramural Funds 1 Z01 CL060068‐05 CC) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients who enrolled in the protocol were sequentially assigned subject numbers that randomly corresponded to a group receiving concurrent patient education plus aerobic exercise training (EXE) or to a group that received only the patient education portion of the regimen (EDU)." |
| Allocation concealment (selection bias) | Unclear risk | Not specified. Quote " Following the baseline evaluations, patients were informed of the group to which they were randomly assigned" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Study personnel were blind to the randomization of patients during all baseline evaluations." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Investigators administering the CPET, 6MWT, and questionnaires were blind to randomization at baseline." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "criterion (Fig. 1). All 29 of these patients performed base‐ line testing. Based on their test responses, two of these patients were required to obtain additional medical clearance prior to beginning the intervention. One patient declined further participation while the other patient was cleared for participation and subsequently assigned a new subject number upon re‐entry into the protocol. This patient was originally assigned a subject number corresponding to EXE, but at re‐entry the randomization procedure resulted re‐assignment to EDU. As such, 28 patients in total participated in either the EXE or EDU groups (Fig. 1). Of the 14 patients allocated to the EXE group, two patients withdrew due to changes in medication and one withdrew due to low attendance at the exercise sessions. One patient in the EDU group was withdrawn from the study due to medication changes." |
| Selective reporting (reporting bias) | High risk | Comment: Trial protocol at clinicaltrials.gov states that they were also going to collect IPAQ, stages of exercise change, exercise self efficacy, profile of mood states and near infrared spectroscopy |
| Other bias | Low risk | |
Ehlken 2016.
| Methods |
Study design: RCT Study grouping: Parallel group |
|
| Participants |
Baseline characteristics Exercise
Control
Included criteria: participants with PAH and inoperable or persistent CTEPH and chronic right heart failure who were stable on disease‐targeted medication for at least 2 months prior to inclusion were randomly assigned to a control and a training group. Medication remained unchanged during the study period. Excluded criteria: not specified Pretreatment: Nil evident |
|
| Interventions |
Intervention characteristics Exercise
Control
|
|
| Outcomes | 6MWD VO2peak Wpeak (peak power) Morbidity ‐ adverse events Disease Progression Precluded from Training HRQoL (SF‐36): Physical functioning HRQoL (SF‐36): Role physical HRQoL (SF36): Bodily pain HRQoL (SF‐36): General health HRQoL (SF‐36): Vitality HRQoL (SF‐36): Social function HRQoL (SF‐36): Role emotional HRQoL (SF‐36): Mental health Discontinued training Haemodynamics ‐ mPAP (mmHg), PVR (Dynes), cardiac output (L/min) B‐type natriuretic peptide |
|
| Identification | Sponsorship Source: funding to pay the open access publication charges for this article was provided by Centre for Pulmonary Hypertension, Thorax clinic at the University of Heidelberg, Germany Comments Author's contact details Nicola Ehlken University Hospital Heidelberg, nicola.ehlken@med.uni‐heidelberg.de Amalienstrasse 5, Heidelberg D‐69126, Germany |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Does not specify methods of randomisation |
| Allocation concealment (selection bias) | Unclear risk | Does not specify whether allocation was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind participants to intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Assessment of 6MWD, SF‐36 and other efficacy parameter were performed by investigators who were blinded to the clinical data" Not clear whether assessors were blinded to group allocation, especially for primary outcome |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Differential attrition ‐ 17% lost to follow‐up in exercise group, none lost to follow‐up in control group |
| Selective reporting (reporting bias) | High risk | Not all outcomes specified in the trial protocol are reported |
| Other bias | High risk | CONSORT diagram does not report how many people were assessed to arrive at the 95 participants enrolled |
Ganderton 2013.
| Methods |
Study design: RCT Study grouping: Parallel group |
|
| Participants |
Baseline characteristics Exercise
Control
Included criteria: participants were included in the study if they had a confirmed diagnosis of idiopathic PAH, familial PAH or PAH associated with connective tissue disorders, based on elevated pulmonary artery pressures (> 25 mmHg at rest or > 30 mmHg during exercise) measured by right heart catheterisation; were medically stable and had been on PAH‐specific pharmaceutical therapy for 3 months prior to enrolment into the study; were in WHO functional class II or III; and were willing to complete the 12‐week supervised and 12‐week home exercise training programmes. Excluded criteria: participants were excluded if they had:
Pretreatment: nil |
|
| Interventions |
Intervention characteristics Exercise
Control
|
|
| Outcomes | 6MWD VO2peak Wpeak Anaerobic threshold HRQoL (SF‐36): Physical functioning HRQoL (Sf‐36): Role physical HRQoL (SF36): Bodily pain HRQoL (SF‐36): General health HRQoL (SF‐36): Vitality HRQoL (SF‐36): Social function HRQoL (SF‐36): Role emotional HRQoL (SF‐36): Mental health HRQol (CAMPHOR): Symptoms HRQol (CAMPHOR): Activities HRQol (CAMPHOR): QoL Morbidity Disease progression Symptoms precluding training Discontinued training NYHA class HRQoL: Physical summary score (SF‐36) HRQoL: Mental summary score (SF‐36) Assessed at baseline, 12 weeks (post intervention) and 24 weeks (follow‐up) |
|
| Identification |
Sponsorship source: Advanced Lung Disease Unit at Royal Perth Hospital and the Lung Institute of Western Australia Country: Australia Setting: Outpatient, hospital Comments: Author's name: Louise Ganderton Institution: Curtin University Email: louise.ganderton@health.wa.gov.au Address: School of Physiotherapy, Faculty of Health Sciences, The University of Sydney |
|
| Notes | Protocol paper published: Ganderton 2011 Thesis available: http://espace.library.curtin.edu.au:80/R?func=dbin‐jump‐full&local_base=gen01‐era02&object_id=198083 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | From thesis: "Permuted block randomisation with block sizes of four was used to generate a randomisation chart. Fourteen blocks were created in total using a web‐based research randomiser." |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind participants to intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | From thesis: "The primary investigator (LG) carried out all assessments at baseline, 12 weeks and 24 weeks and was blinded to the participants group allocation...The physiotherapists responsible for conducting the exercise training sessions were not involved in any of the formal assessments" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data available on all recruited participants for ITT. However planned to enrol 34 and only recruited 10 |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | |
Ley 2013.
| Methods |
Study design: RCT Study grouping: Parallel group |
|
| Participants |
Baseline characteristics Exercise
Control
Included criteria: adults (≥ 18 years) with confirmed PAH and CTEPH who underwent complete clinical work‐up including RHC. All participants were stable under optimised medical therapy (such as endothelin antagonists, iloprost, sildenafil, calcium channel blockers, anticoagulants, diuretics and supplemental oxygen) for at least 3 months before entering the study. Additional inclusion criteria were WHO functional class II to III Excluded criteria: no recent syncope, and no skeletal or muscle abnormalities prohibiting participation in an exercise training programme Pretreatment: nil |
|
| Interventions |
Intervention characteristics Exercise
Control
|
|
| Outcomes | Morbidity ‐ adverse events Disease progression Precluded from training 6MWD |
|
| Identification |
Sponsorship source: this work was supported by the German National Research Agency (DFG): “Image‐based V/Q analysis” (FOR 474‐2) Country: Germany Setting: inpatient rehabilitation Comments: Author's name: Sebastian Ley Institution: University Hospital Heidelberg Email: ley@gmx.de Address: Department of Diagnostic and Interventional Radiology, University Hospital Heidelberg, Im Neuenheimer Feld 430,69120 Heidelberg, Germany |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were randomly assigned to either a training or a control group using a permuted block randomization procedure." |
| Allocation concealment (selection bias) | Unclear risk | The method of allocation was not specified. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unabel to blind participants or personnel due to the to intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Assessment of 6MWD and MR examination were performed by investigators who were blinded to the clinical data and group assignment of the patients. Evaluation of the MR data was done blinded to the clinical setting and in random order." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised patients were analysed |
| Selective reporting (reporting bias) | Low risk | Unclear whether trial was registered but reporting does not appear selective |
| Other bias | Low risk | |
Mereles 2006.
| Methods |
Study design: RCT Study grouping: Parallel group |
|
| Participants |
Baseline characteristics Exercise
Control
Included criteria: people with severe chronic PH who were stable and compensated under optimised medical therapy (such as endothelin antagonists, iloprost, sildenafil, calcium channel blockers, anti‐coagulants, diuretics, and supplemental oxygen) for at least 3 months before entering the study were invited to participate. Additional inclusion criteria were age 18‐75 years, WHO functional class II to IV. Excluded criteria: no recent syncope, and no skeletal or muscle abnormalities prohibiting participation in an exercise programme Pretreatment: Nil evident |
|
| Interventions |
Intervention characteristics Exercise
Control
|
|
| Outcomes | 6MWD VO2peak Wpeak Morbidity ‐ adverse events Disease progression Precluded from training Anaerobic threshold HRQoL (SF‐36): Physical functioning HRQoL (SF‐36): Role physical HRQoL (SF36): Bodily pain HRQoL (SF‐36): General health HRQoL (SF‐36): Vitality HRQoL (SF‐36): Social function HRQoL (SF‐36): Role emotional HRQoL (SF‐36): Mental health HRQoL:Physical Summary score (SF36) HRQoL:Mental Summary score (SF36) HRQol (CAMPHOR): QoL NYHA Class Discontinued training |
|
| Identification |
Sponsorship source: this study was funded by a grant from the German Pulmonary Hypertension Group, Pulmonale Hypertonie e.V., Rheinstetten, Germany. Country: Germany Setting: inpatient rehabilitation Comments: Author's name: Derliz Mereles Institution: University Hospital Heidelberg Email: ekkehard_gruenig@med.uni‐heidelberg.de Address: Department of Cardiology and Pneumology, University Hospital Heidelberg, INF 410, D‐69120 Heidelberg |
|
| Notes | Adverse Outcomes Authors report that all participants tolerated training and had no adverse events during training and no progression of the disease as defined by progression of symptoms, PH or right heart failure. Two participants perceived a short episode of dizziness without fainting immediately after bicycle ergometer training. In 1 participant, oxygen saturation dropped from 88% to 74% during exercise, although the training was performed with an oxygen mask. Continuous Outcomes 6MWD is reported as a change from baseline at the post‐inpatient and post‐outpatient time points | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: participants were randomly assigned to either a primary training group or a sedentary control group using a permuted block randomization procedure |
| Allocation concealment (selection bias) | Unclear risk | Comment: there is no comment regarding allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: unable to blind participants and personnel due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The completed questionnaire at baseline was compared with the results after 15 weeks by investigators who were blinded to the patients’ clinical data and group assignment. To avoid bias as far as possible in this study, all measurements and/or offline readings were performed by investigators who were blinded to patient data and group assignment." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts reported |
| Selective reporting (reporting bias) | Low risk | The protocol was not registered or published however the outcome reporting is comprehensive. |
| Other bias | High risk | Comment: No CONSORT diagram so not possible to tell how many people were assessed in order to recruit the sample. |
Wilkinson 2007.
| Methods |
Study design: RCT Study grouping: Parallel group |
|
| Participants |
Baseline characteristics Exercise
Control
Included criteria: "Clinically stable PH patients in a single centre" Excluded criteria: unclear |
|
| Interventions |
Intervention characteristics Exercise
Control
|
|
| Outcomes | Incremental shuttle walk test Endurance shuttle walk test Assessed at baseline and 3 months |
|
| Identification |
Sponsorship source: Country: Setting: Comments: Author's name: Anna Wilkinson Institution: Royal Hallamshire Hospital Email: Address: |
|
| Notes | Reported as two abstracts In the Thorax abstract it does not specify the number in each group, only that 40 were randomised. ERS abstract says 18 in each group. Neither specifies age by allocated group |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Abstract only, does not specify how sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | Abstract only, does not specify |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind participants to intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Blind assessment was undertaken pre intervention and following 3 months" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropouts unclear. 2007 abstract specifies 40 participants and 2008 abstract specifies 36 participants. |
| Selective reporting (reporting bias) | High risk | Abstract only, not all outcomes reported |
| Other bias | High risk | Abstract only |
bpm: beats per minute; CAMPHOR: Cambridge Pulmonary Hypertension Outcome Review; CI: Cardiac Index; CPET: cardiopulmonary exercise test; CTEPH: chronic thromboembolitic pulmonary hypertension; Dual: patients on two pharmacotherapies; FEV1: forced expired volume in one second; FVC: forced vital capacity; HR: heart rate; HRQoL: health‐related quality of life; ITT: intention‐to‐treat; Mono: patients on single pharmacotherapy; mPAP: mean pulmonary artery pressure; NYHA: New York Heart Association; PAH: Pulmonary Artery Hypertension; PASP: Pulmonary Artery Systolic Pressure; PH: Pulmonary Hypertension, PLB: pursed lip breathing; PVR: pulmonary vascular resistance; RCT: randomised controlled trial; SF‐36: Short‐form 36; 6MWD: six minute walk distance; SPO2: oxygen saturation; Triple: patients on 3 pharmacotherapies; QoL: quality of life; VO2peak: peak oxygen uptake; Wpeak: peak power
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Babu 2013 | Review paper |
| Babu 2014 | Not an RCT |
| Barbosa 2011 | No exercise training |
| Becker Grunig 2013 | Not an RCT |
| Bernheim 2007 | Wrong patient population |
| Ehlken 2014 | Not an RCT |
| Fox 2011 | Not an RCT |
| Grunig 2011 | Not an RCT |
| Grunig 2012 | Not an RCT |
| Kabitz 2014 | Not an RCT |
| Kolesnikova 2011a | Wrong intervention |
| Kolesnikova 2011b | Wrong intervention |
| Marvisi 2013 | No exercise training |
| Nagel 2012 | Not an RCT |
| Robalo Cordeiro 2011 | No exercise training |
RCT: randomised controlled trial
Differences between protocol and review
We had intended to perform a subgroup analysis according to severity of PH, but insufficient data were available. We performed an additional subgroup analysis for setting of exercise rehabilitation programme, as there was marked heterogeneity in exercise outcomes that could have been affected by the programme model.
Contributions of authors
NM drafted the protocol with the assistance from AH and FK. NM and AH identified studies for the review, extracted data from the studies and drafted the review. All authors provided critical feedback on the review.
Sources of support
Internal sources
-
Griffith University, Australia.
Salary support, Norman Morris
-
Queensland Health, Australia.
Salary support, Fiona Kermeen
-
Alfred Health and La Trobe University, Australia.
Salary support, Anne Holland
External sources
The authors declare that no such external funding was received for this systematic review, Other.
Declarations of interest
NM: none known
FK: none known
AH: none known
Edited (no change to conclusions)
References
References to studies included in this review
Chan 2013 {published data only}
- Chan L, Chin LM, Kennedy M, Woolstenhulme JG, Nathan SD, Weinstein AA, et al. Benefits of intensive treadmill exercise training on cardiorespiratory function and quality of life in patients with pulmonary hypertension. Chest 2013;143(2):333‐43. [DOI: 10.1378/chest.12-0993] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan L, Kennedy M, Woolstenhulme J, Connors G, Nathan S, Weir N, et al. Improved six‐minute walk distance and cardiorespiratory fitness in patients with pulmonary arterial hypertension following an intensive exercise program. Chest 2011;140(4):854A. [Google Scholar]
- Weinstein AA, Chin LMK, Keyser RE, Kennedy M, Nathan SD, Woolstenhulme JG, et al. Effect of aerobic exercise training on fatigue and physical activity in patients with pulmonary arterial hypertension. Respiratory Medicine 2013;107(5):778‐84. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ehlken 2016 {published data only}
- Ehlken N, Lichtblau M, Klose H, Weidenhammer J, Fischer C, Nechwatal R, et al. Exercise training improves peak oxygen consumption and haemodynamics in patients with severe pulmonary arterial hypertension and inoperable chronic thrombo‐embolic pulmonary hypertension: a prospective, randomized, controlled trial. European Heart Journal 2015;37(1):35‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ganderton 2013 {published data only}
- Ganderton L, Gain K, Fowler R, Lunt D, Winship P, Bostock S, et al. Effects of exercise training on exercise capacity and quality of life in pulmonary arterial hypertension. Respirology 2013;18(Suppl 2):74 (P146). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganderton L, Jenkins S, Gain K, Fowler R, Winship P, Lunt D, et al. Short term effects of exercise training on exercise capacity and quality of life in patients with pulmonary arterial hypertension: protocol for a randomised controlled trial. BMC Pulmonary Medicine 2011;11:25. [DOI: 10.1186/1471-2466-11-25] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ley 2013 {published data only}
- Ley S, Fink C, Risse F, Ehlken N, Fischer C, Ley‐Zaporozhan J, et al. Magnetic resonance imaging to assess the effect of exercise training on pulmonary perfusion and blood flow in patients with pulmonary hypertension. European Radiology 2013;23(2):324‐31. [DOI: ] [DOI] [PubMed] [Google Scholar]
Mereles 2006 {published data only}
- Ehlken N, Mereles D, Kreuscher S, Ghofrani S, Hoeper MM, Halank M, et al. Exercise and respiratory training to improve exercise capacity and quality of life in patients with severe chronic pulmonary hypertension. European Respiratory Journal 2006;28(Suppl 50):372s (2220). [DOI] [PubMed] [Google Scholar]
- Mereles D, Ehlken N, Kreuscher S, Ghofrani S, Hoeper MM, Halank M, et al. Exercise and respiratory training improve exercise capacity and quality of life in patients with severe chronic pulmonary hypertension. Circulation 2006;114(14):1482‐9. [DOI: 10.1161/CIRCULATIONAHA.106.618397] [DOI] [PubMed] [Google Scholar]
Wilkinson 2007 {published data only}
- Wilkinson A, Elliot CA, Condliffe R, Mawson S, Armstrong IJ, Billings C, et al. A randomised‐controlled trial of a physiotherapist‐led rehabilitation programme in patients with pulmonary hypertension. European Respiratory Society 18th Annual Congress; 2008 Oct 3‐7; Berlin. 2008:P1004.
- Wilkinson A, Elliot CA, Mawson S, Armstrong I, Billings C, Kiely DG. A randomised controlled trial to investigate the effects of a physiotherapist‐led rehabilitation programme on exercise capacity and quality of life measures in patients with pulmonary hypertension. Thorax 2007;62(Suppl iii):A16. [Google Scholar]
References to studies excluded from this review
Babu 2013 {published data only}
- Babu AS, Padmakumar R, Maiya AG. A review of ongoing trials in exercise based rehabilitation for pulmonary arterial hypertension. Indian Journal of Medical Research 2013;137(5):900‐6. [PMC free article] [PubMed] [Google Scholar]
Babu 2014 {published data only}
- Babu AS, Padmakumar R, Maiya AG. Effects of the pulmonary hypertension manual (PulHMan) on awareness of exercise among patients with pulmonary hypertension. World Congress of Cardiology Scientific Sessions; 2014 May 4‐7; Melbourne. 2014:e311.
Barbosa 2011 {published data only}
- Barbosa PB, Ferreira EMV, Arakaki JSO, Takara LS, Moura J, Nascimento RB, et al. Kinetics of skeletal muscle O2 delivery and utilization at the onset of heavy‐intensity exercise in pulmonary arterial hypertension. European Journal of Applied Physiology 2011;111(8):1851‐61. [DOI: 10.1007/s00421-010-1799-6] [DOI] [PubMed] [Google Scholar]
Becker Grunig 2013 {published data only}
- Becker‐Gruenig T, Ehlken N, Gorenflo M, Hager A, Halank M, Lichtblau M, et al. Efficacy of exercise training in pulmonary arterial hypertension associated with congenital heart disease. ESC Congress; 2012 25‐28 Aug; Munich 2012;33 suppl 1(264):P1676. [DOI] [PubMed] [Google Scholar]
- Becker‐Grunig T, Klose H, Ehlken N, Lichtblau M, Nagel C, Fischer C, et al. Efficacy of exercise training in pulmonary arterial hypertension associated with congenital heart disease. International Journal of Cardiology 2013;168(1):375‐81. [DOI] [PubMed] [Google Scholar]
Bernheim 2007 {published data only}
- Bernheim AM, Kiencke S, Fischler M, Dorschner L, Debrunner J, Mairbäurl H, et al. Acute changes in pulmonary artery pressures due to exercise and exposure to high altitude do not cause left ventricular diastolic dysfunction. Chest 2007;132(2):380‐7. [DOI: 10.1378/chest.07-0297] [DOI] [PubMed] [Google Scholar]
Ehlken 2014 {published data only}
- Ehlken N, Verduyn C, Tiede H, Staehler G, Karger G, Nechwatal R, et al. Economic evaluation of exercise training in patients with pulmonary hypertension. Lung 2014;192(3):359‐66. [DOI: ] [DOI] [PubMed] [Google Scholar]
Fox 2011 {published data only}
- Fox BD, Kassirer M, Weiss I, Raviv Y, Peled N, Shitrit D, et al. Ambulatory rehabilitation improves exercise capacity in patients with pulmonary hypertension. Journal of Cardiac Failure 2011;17(3):196‐200. [DOI] [PubMed] [Google Scholar]
Grunig 2011 {published data only}
- Grunig E, Ehlken N, Ghofrani A, Staehler G, Meyer FJ, Juenger J, et al. Effect of exercise and respiratory training on clinical progression and survival in patients with severe chronic pulmonary hypertension. Respiration 2011;81(5):394‐401. [DOI] [PubMed] [Google Scholar]
Grunig 2012 {published data only}
- Grunig E, Maier F, Ehlken N, Fischer C, Lichtblau M, Blank N, et al. Exercise training in pulmonary arterial hypertension associated with connective tissue diseases. Arthritis Research and Therapy 2012;14(3):R148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier F, Gruenig E, Ehlken N, Fischer C, Lichtblau M, Blank N, et al. Exercise training in pulmonary arterial hypertension associated with connective tissue diseases. ESC Congress; 2012 25‐29 August; Munich. 2012, issue var.pagings:515. [DOI] [PMC free article] [PubMed]
Kabitz 2014 {published data only}
- Kabitz HJ, Bremer HC, Schwoerer A, Sonntag F, Walterspacher S, Walker DJ, et al. The combination of exercise and respiratory training improves respiratory muscle function in pulmonary hypertension. Lung 2014;192(2):321‐8. [DOI] [PubMed] [Google Scholar]
Kolesnikova 2011a {published data only}
- Kolesnikova E, Arutyunov G, Rylova A, Rylova N. Respiratory muscle trainings started in acute period of complicated myocardial infarction in patients with pulmonary hypertension. Circulation 2011;124(21):suppl 1. [Google Scholar]
Kolesnikova 2011b {published data only}
- Kolesnikova EA, Arutyunov GP, Rylova NV, Rylova AK. Rehabilitation of patients with severe heart failure and pulmonary hypertension. EuroPRevent; 2011 April 14‐16; Geneva. 2011, issue var.pagings:S2.
Marvisi 2013 {published data only}
- Marvisi M, Herth FJF, Ley S, Poletti V, Chavannes NH, Spruit MA, et al. Selected clinical highlights from the 2012 ERS congress in Vienna. European Respiratory Journal 2013;41(5):1219‐27. [DOI] [PubMed] [Google Scholar]
Nagel 2012 {published data only}
- Nagel C, Prange F, Guth S, Herb J, Ehlken N, Fischer C, et al. Exercise training improves exercise capacity and quality of life in patients with inoperable or residual chronic thromboembolic pulmonary hypertension. PloS one 2012;7(7):e41603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prange F, Guth S, Ehlken N, Reichenberger F, Halank M, Kabitz HJ, et al. Exercise training improved exercise capacity and quality of life in patients with inoperable or residual chronic thromboembolic pulmonary hypertension. ESC Congress; 2012 25‐29 August; Munich. 2012:416. [DOI] [PMC free article] [PubMed]
Robalo Cordeiro 2011 {published data only}
- Robalo Cordeiro C, Singh S, Herth FJ, Ley S, Chavannes NH, Clini E, et al. Selected clinical highlights from the 2010 ERS Congress in Barcelona. European Respiratory Journal 2011;38(1):209‐17. [DOI] [PubMed] [Google Scholar]
Additional references
Babu 2016a
- Babu AS, Padmakumar R, Maiya AG, Mohapatra AK, Kamath RL. Effects of exercise training on exercise capacity in pulmonary arterial hypertension: a systematic review of clinical trials. Heart, Lung and Circulation 2016;25(4):333‐41. [DOI] [PubMed] [Google Scholar]
Babu 2016b
- Babu AS, Arena R, Myers J, Padmakumar R, Maiya AG, Cahalin LP, et al. Exercise intolerance in pulmonary hypertension: mechanism, evaluation and clinical implications. Expert Review of Respiratory Medicine September 2016;10(9):979‐90. [DOI] [PubMed] [Google Scholar]
Benza 2010
- Benza RL, Miller DP, Gomberg‐Maitland M, Frantz RP, Foreman AJ, Coffey CS, et al. Predicting survival in pulmonary arterial hypertension: insights from the registry to evaluate early and long‐term pulmonary arterial hypertension disease management (REVEAL). Circulation 2010;122(2):164‐72. [DOI] [PubMed] [Google Scholar]
Buys 2015
- Buys R, Avila A, Cornelissen VA. Exercise training improves physical fitness in patients with pulmonary arterial hypertension: a systematic review and meta‐analysis of controlled trials. BMC Pulmonary Medicine 2015;15:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Casserly 2009
- Casserly B, Klinger JR. Brain natriuretic peptide in pulmonary arterial hypertension: biomarker and potential therapeutic agent. Drug Design, Development and Therapy 2009;3:269‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
Coggan 1992
- Coggan AR, Spina RJ, King DS, Rogers MA, Brown M, Nemeth PM, et al. Skeletal muscle adaptations to endurance training in 60‐ to 70‐yr‐old men and women. Journal of Applied Physiology 1992;72(5):1780‐6. [DOI] [PubMed] [Google Scholar]
Covidence 2016 [Computer program]
- Veritas Health Innovation. Covidence systematic review software. Melbourne, Australia: Veritas Health Innovation, Accessed 2016.
D'Alonzo 1991
- D'Alonzo GE, Barst RJ, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, et al. Survival in patients with primary pulmonary hypertension: Results from a national prospective registry. Annals of Internal Medicine 1991;115(5):343‐9. [DOI] [PubMed] [Google Scholar]
de Man 2009
- Man FS, Handoko ML, Groepenhoff H, van't Hul AJ, Abbink J, Koppers RJ, et al. Effects of exercise training in patients with idiopathic pulmonary arterial hypertension. European Respiratory Journal 2009;34(3):669‐75. [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
Downs and Black 1998
- Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non‐randomised studies of health care interventions. Journal of Epidemiology and Community Health 1998;52:377‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ennion 1995
- Ennion S, Sant'ana Pereira J, Sargeant AJ, Young A, Goldspink G. Characterization of human skeletal muscle fibres according to the myosin heavy chains they express. Journal of Muscle Research and Cell Motility 1995;16(1):35‐43. [DOI] [PubMed] [Google Scholar]
Fowler 2012
- Fowler RM, Gain KR, Gabbay E. Exercise intolerance in pulmonary arterial hypertension. Pulmonary Medicine 2012;Article ID 359204:10. [DOI: 10.1155/2012/359204] [DOI] [PMC free article] [PubMed] [Google Scholar]
Frost 2013
- Frost AE, Badesch DB, Miller DP, Benza RL, Meltzer LA, McGoon MD. Evaluation of the predictive value of a clinical worsening definition using 2‐year outcomes in patients with pulmonary arterial hypertension: a REVEAL Registry analysis. Chest 2013;144(5):1521‐9. [DOI] [PubMed] [Google Scholar]
Galie 2009
- Galie N, Hoeper MM, Humbert M, Torbicki A, Vachiery JL, Barbera JA, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension. European Respiratory Journal 2009;34(6):1219‐63. [DOI] [PubMed] [Google Scholar]
Galie 2013
- Galie N, Corris PA, Frost A, Girgis RE, Granton J, Jing ZC, et al. Updated treatment algorithm of pulmonary arterial hypertension. Journal of the American College of Cardiology 2013;62(25 Suppl):60‐72. [DOI] [PubMed] [Google Scholar]
Galie 2015
- Galiè N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The joint task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). European Respiratory Journal 2015;46(4):903‐75. [DOI] [PubMed] [Google Scholar]
Gollnick 1973
- Gollnick PD, Armstrong RB, Saltin B, Saubert CW, Sembrowich WL, Shepherd RE. Effect of training on enzyme activity and fiber composition of human skeletal muscle. Journal of Applied Physiology 1973;34(1):107‐11. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- GRADE Working Group, McMaster University. GRADEpro GDT. Version accessed 23 September 2016. Hamilton, Ontario: GRADE Working Group, McMaster University, 2014.
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
Hoeper 2013
- Hoeper MM, Bogaard HJ, Condliffe R, Frantz R, Khanna D, Kurzyna M, et al. Definitions and diagnosis of pulmonary hypertension. Journal of the American College of Cardiology 2013;62(25 Suppl):D42‐50. [DOI] [PubMed] [Google Scholar]
Holland 2014
- Holland AE, Spruit MA, Troosters T, Puhan MA, Pepin V, Saey D, et al. An official European Respiratory Society/American Thoracic Society technical standard: field walking tests in chronic respiratory disease. European Respiratory Journal 2014;44(6):1428‐46. [DOI] [PubMed] [Google Scholar]
Mainguy 2010a
- Mainguy V, Maltais F, Saey D, Gagnon P, Martel S, Simon M, et al. Peripheral muscle dysfunction in idiopathic pulmonary arterial hypertension. Thorax 2010;65(2):113‐7. [DOI] [PubMed] [Google Scholar]
Mainguy 2010b
- Mainguy V, Maltais F, Saey D, Gagnon P, Martel S, Simon M, et al. Effects of a rehabilitation program on skeletal muscle function in idiopathic pulmonary arterial hypertension. Journal of Cardiopulmonary Rehabilitation 2010;30(5):319‐23. [DOI] [PubMed] [Google Scholar]
Martinez‐Quintana 2010
- Martinez‐Quintana E, Miranda‐Calderin G, Ugarte‐Lopetegui A, Rodriguez‐Gonzalez F. Rehabilitation program in adult congenital heart disease patients with pulmonary hypertension. Congenital Heart Disease 2010;5(1):44‐50. [DOI] [PubMed] [Google Scholar]
Mathai 2012
- Mathai SC, Puhan MA, Lam D, Wise RA. The minimal important difference in the6‐minute walk test for patients with pulmonary arterial hypertension. American Journal of Respiratory and Critical Care Medicine September 2012;185(5):428‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
McGoon 2013
- McGoon MD, Benza RL, Escribano‐Subias P, Jiang X, Miller DP, Peacock AJ, et al. Pulmonary arterial hypertension: epidemiology and registries. Journal of the American College of Cardiology 2013;62(25 Suppl):51‐9. [DOI] [PubMed] [Google Scholar]
Morris 2015
- Morris NR, Seale H, Harris J, Hall K, Hopkins P, Kermeen F. Serious adverse events during a 6‐min walk test in patients with pulmonary hypertension. European Respiratory Journal 2015;45(4):1179‐82. [DOI] [PubMed] [Google Scholar]
NYHA 1994
- Criteria Committee of the New York Heart Association. Nomenclature and Criteria for Diagnosis of Diseases of the Heart and Great Vessels. 9. Boston, Mass: Little, Brown & Co, 1994:253‐255. [Google Scholar]
Ogawa 1992
- Ogawa T, Spina RJ, Martin WH 3rd, Kohrt WM, Schechtman KB, Holloszy JO, et al. Effects of aging, sex, and physical training on cardiovascular responses to exercise. Circulation 1992;86(2):494‐503. [DOI] [PubMed] [Google Scholar]
Panagiotou 2015
- Panagiotou M, Peacock AJ, Johnson MK. Respiratory and limb muscle dysfunction in pulmonary arterial hypertension: a role for exercise training?. Pulmonary Circulation 2015;5(3):424‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pandey 2015
- Pandey A, Khunger M, Garg S, Kumbhani DJ, Chin KM, Berry JD. Efficacy and safety of exercise training in chronic pulmonary hypertension: systematic review and meta‐analysis. Circulation Heart Failure 2015;8(6):1032‐43. [DOI] [PubMed] [Google Scholar]
Piepoli 2014
- Piepoli MF, Corrà U, Adamopoulos S, Benzer W, Bjarnason‐Wehrens B, Cupples M, et al. Secondary prevention in the clinical management of patients with cardiovascular diseases. Core components, standards and outcome measures for referral and delivery: a policy statement from the cardiac rehabilitation section of the European Association for Cardiovascular Prevention and Rehabilitation. European Journal Preventative Cardiology 2014;21:664‐81. [DOI] [PubMed] [Google Scholar]
Pluim 2000
- Pluim BM, Zwinderman AH, Laarse A, Wall EE. The athlete's heart. A meta‐analysis of cardiac structure and function. Circulation 2000;101(3):336‐44. [DOI] [PubMed] [Google Scholar]
Provencher 2006
- Provencher S, Chemla D, Herve P, Sitbon O, Humbert M, Simonneau G. Heart rate responses during the 6‐minute walk test in pulmonary arterial hypertension. European Respiratory Journal 2006;27(1):114‐20. [DOI] [PubMed] [Google Scholar]
Provencher 2008
- Provencher S, Herve P, Sitbon O, Humbert M, Simonneau G, Chemla D. Changes in exercise haemodynamics during treatment in pulmonary arterial hypertension. European Respiratory Journal 2008;32(2):393‐8. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Riley 2000
- Riley MS, Porszasz J, Engelen MP, Shapiro SM, Brundage BH, Wasserman K. Responses to constant work rate bicycle ergometry exercise in primary pulmonary hypertension: the effect of inhaled nitric oxide. Journal of the American College of Cardiology 2000;36(2):547‐56. [DOI] [PubMed] [Google Scholar]
Rubin 2004
- Rubin, L. Diagnosis and management of pulmonary arterial hypertension: ACCP evidence‐based clinical practice guidelines.. Chest 2004;126:S7‐S10. [DOI] [PubMed] [Google Scholar]
Schulz 2010
- Schulz KF, Altman DG, Moher D, for the CONSORT Group. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. Annals of Internal Medicine 2010;152(11):726‐32. [DOI] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and ‘Summary of findings' tables. In: Higgins JPT, Green S (editors) Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Simonneau 2013
- Simonneau G, Gatzoulis MA, Adatia I, Celermajer D, Denton C, Ghofrani A, et al. Updated clinical classification of pulmonary hypertension. Journal of the American College of Cardiology 2013;62(25 Suppl):D34‐41. [DOI] [PubMed] [Google Scholar]
Spruit 2013
- Spruit MA, Singh SJ, Garvey C, ZuWallack R, Nici L, Rochester C, et al. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. American Journal of Respiratory and Critical Care Medicine 2013;188(8):e13‐64. [DOI] [PubMed] [Google Scholar]
Strange 2012
- Strange G, Playford D, Stewart S, Deague JA, Nelson H, Kent A, et al. Pulmonary hypertension: prevalence and mortality in the Armadale echocardiography cohort. Heart 2012;98(24):1805‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sutherland 2005
- Sutherland ER, Make BJ. Maximum exercise as an outcome in COPD: minimal clinically important difference. COPD 2005;2(1):137‐41. [DOI] [PubMed] [Google Scholar]
Tuder 2013
- Tuder RM, Archer SL, Dorfmüller P, Erzurum SC, Guignabert C, Michelakis E, et al. Relevant issues in the pathology and pathobiology of pulmonary hypertension. Journal of the American College of Cardiology 2013;62(25 Suppl):D4‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vogiatzis 2013
- Vogiatzis I, Zakynthinos S. The physiological basis of rehabilitation in chronic heart and lung disease. Journal of Applied Physiology 2013;115(1):16‐21. [DOI] [PubMed] [Google Scholar]
Weinstein 2013
- Weinstein AA, Chin LM, Keyser RE, Kennedy M, Nathan SD, Woolstenhulme JG, et al. Effect of aerobic exercise training on fatigue and physical activity in patients with pulmonary arterial hypertension. Respiratory Medicine 2013;107(5):778‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Whittom 1998
- Whittom F, Jobin J, Simard PM, Leblanc P, Simard C, Bernard S, et al. Histochemical and morphological characteristics of the vastus lateralis muscle in patients with chronic obstructive pulmonary disease. Medicine and Science in Sports and Exercise 1998;30(10):1467‐74. [DOI] [PubMed] [Google Scholar]
Yuan 2015
- Yuan P, Yuan XT, Sun XY, Pudasaini B, Liu JM, Hu QH. Exercise training for pulmonary hypertension: a systematic review and meta‐analysis. International Journal of Cardiology 2015;178:142‐6. [DOI] [PubMed] [Google Scholar]


