Abstract
Background
Non‐alcohol related fatty liver disease (commonly called non‐alcoholic fatty liver disease (NAFLD)) is liver steatosis in the absence of significant alcohol consumption, use of hepatotoxic medication, or other disorders affecting the liver such as hepatitis C virus infection, Wilson's disease, and starvation. NAFLD embraces the full spectrum of disease from pure steatosis (i.e. uncomplicated fatty liver) to non‐alcoholic steatohepatitis (NASH), via NASH‐cirrhosis to cirrhosis. The optimal pharmacological treatment for people with NAFLD remains uncertain.
Objectives
To assess the comparative benefits and harms of different pharmacological interventions in the treatment of NAFLD through a network meta‐analysis and to generate rankings of the available pharmacological treatments according to their safety and efficacy. However, it was not possible to assess whether the potential effect modifiers were similar across different comparisons. Therefore, we did not perform the network meta‐analysis, and instead, assessed the comparative benefits and harms of different interventions using standard Cochrane methodology.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, Science Citation Index Expanded, the World Health Organization International Clinical Trials Registry Platform, and ClinicalTrials.com to August 2016.
Selection criteria
We included only randomised clinical trials (irrespective of language, blinding, or publication status) in participants with NAFLD. We excluded trials which included participants who had previously undergone liver transplantation. We considered any of the various pharmacological interventions compared with each other or with placebo or no intervention.
Data collection and analysis
We calculated the odds ratio (OR) and rate ratio with 95% confidence intervals (CI) using both fixed‐effect and random‐effects models based on an available participant analysis with Review Manager. We assessed risk of bias according to the Cochrane risk of bias tool, controlled risk of random errors with Trial Sequential Analysis, and assessed the quality of the evidence using GRADE.
Main results
We identified 77 trials including 6287 participants that met the inclusion criteria of this review. Forty‐one trials (3829 participants) provided information for one or more outcomes. Only one trial was at low risk of bias in all domains. All other trials were at high risk of bias in one or more domains. Overall, all the evidence was very low quality. Thirty‐five trials included only participants with non‐alcohol related steatohepatitis (NASH) (based on biopsy confirmation). Five trials included only participants with diabetes mellitus; 14 trials included only participants without diabetes mellitus. The follow‐up in the trials ranged from one month to 24 months.
We present here only the comparisons of active intervention versus no intervention in which two or more trials reported at least one of the following outcomes: mortality at maximal follow‐up, serious adverse events, and health‐related quality of life, the outcomes that determine whether a treatment should be used.
Antioxidants versus no intervention
There was no mortality in either group (87 participants; 1 trial; very low quality evidence). None of the participants developed serious adverse events in the trial which reported the proportion of people with serious adverse events (87 participants; 1 trial; very low quality evidence). There was no evidence of difference in the number of serious adverse events between antioxidants and no intervention (rate ratio 0.89, 95% CI 0.36 to 2.19; 254 participants; 2 trials; very low quality evidence). None of the trials reported health‐related quality of life.
Bile acids versus no intervention
There was no evidence of difference in mortality at maximal follow‐up (OR 5.11, 95% CI 0.24 to 107.34; 659 participants; 4 trials; very low quality evidence), proportion of people with serious adverse events (OR 1.56, 95% CI 0.84 to 2.88; 404 participants; 3 trials; very low quality evidence), or the number of serious adverse events (rate ratio 1.01, 95% CI 0.66 to 1.54; 404 participants; 3 trials; very low quality evidence) between bile acids and no intervention. None of the trials reported health‐related quality of life.
Thiazolidinediones versus no intervention
There was no mortality in either group (74 participants; 1 trial; very low quality evidence). None of the participants developed serious adverse events in the two trials which reported the proportion of people with serious adverse events (194 participants; 2 trials; very low quality evidence). There was no evidence of difference in the number of serious adverse events between thiazolidinediones and no intervention (rate ratio 0.25, 95% CI 0.06 to 1.05; 357 participants; 3 trials; very low quality evidence). None of the trials reported health‐related quality of life.
Source of funding
Twenty‐six trials were partially‐ or fully‐funded by pharmaceutical companies that would benefit, based on the results of the trial. Twelve trials did not receive any additional funding or were funded by parties with no vested interest in the results. The source of funding was not provided in 39 trials.
Authors' conclusions
Due to the very low quality evidence, we are very uncertain about the effectiveness of pharmacological treatments for people with NAFLD including those with steatohepatitis. Further well‐designed randomised clinical trials with sufficiently large sample sizes are necessary.
Plain language summary
Medical treatment for people with non‐alcohol related fatty liver disease
Review question
We aimed to assess different medications to treat people with non‐alcohol related fatty liver disease.
Background
Non‐alcoholic fatty liver disease (NAFLD) is an accumulation of fat in the liver in people who have no history of significant alcohol consumption, use of medicines, diseases such as hepatitis C virus infection, or other conditions such as starvation that can damage the liver. Fatty liver can lead to liver damage resulting in inflammation (non‐alcohol related steatohepatitis or NASH) or liver scarring (liver cirrhosis). The best way to treat people with NAFLD is not clear. We sought to resolve this issue by searching for existing trials on the topic.
Selection criteria and date of search We included all randomised clinical trials (clinical studies where people are randomly put into one of two or more intervention groups) reported to August 2016.
Study characteristics
We included 77 randomised clinical trials that involved a total of 6287 participants. Of these, 41 trials (3829 participants) provided information for one or more outcomes for this review. Thirty‐five trials only included participants with NASH; five included only people with diabetes mellitus; and 14 included only people who did not have diabetes mellitus. The average follow‐up period in the trials ranged from one month to two years in the trials that reported this information. We excluded trials in which participants with NAFLD had undergone liver transplantation before the trial. As well as conducting standard Cochrane analysis, we also planned to conduct network meta‐analysis (a technique that enables comparison of different treatments that are not directly compared to each other in the trials). However, the nature of available information meant we could not determine if the network meta‐analysis results were reliable.
Specific outcomes we looked for were numbers of deaths, adverse events, and assessment of health‐related quality of life.
Study funding sources
Twelve trials did not receive any additional funding or were funded by sources with no vested interest in the results; 26 were funded by drug companies that could potentially benefit from trial results; and the funding source was not available from 39 trials.
Key results
Included trials compared drug treatments such as bile acids, antioxidants, phosphodiesterase type 4 inhibitor, glucocorticosteroid inhibitor, anti‐cholesterol drugs and anti‐diabetes drugs with a fake treatment (placebo) or no treatment.
Antioxidants versus no intervention
There were no deaths in either group (87 participants, 1 trial). None of the participants developed serious adverse events in the trial which reported the percentage of people with serious adverse events (87 participants, 1 trial). There was no evidence of difference in the number of serious adverse events between antioxidants and no intervention (254 participants, 2 trials).
Bile acids versus no intervention
There was no evidence of difference in deaths at maximal follow‐up (659 participants, 4 trials), percentage of people with serious adverse events (404 participants, 3 trials), or the number of serious adverse events (404 participants, 3 trials) between bile acids and no intervention. None of the trials reported health‐related quality of life.
Thiazolidinediones versus no intervention
There were no deaths in either group (74 participants, 1 trial). None of the participants developed serious adverse events in the two trials which reported the percentage of people with serious adverse events (194 participants, 2 trials). There was no evidence of difference in the number of serious adverse events between thiazolidinediones and no intervention (357 participants, 3 trials). None of the trials reported health‐related quality of life.
We found no evidence of benefit from any of the compared interventions in people with fatty liver disease. There is significant uncertainty in this issue, and we need further high quality randomised clinical trials with sufficiently large group of participants.
Quality of evidence
Evidence quality was very low overall, and there was a high risk of bias. This means there is a possibility of making conclusions that wrongly interpret benefits or harms of treatments because of the ways the studies were conducted.
Summary of findings
Summary of findings for the main comparison. Antioxidants versus no intervention for non‐alcohol related fatty liver disease.
| Antioxidants versus no intervention for non‐alcohol related fatty liver disease | |||||
| Patient or population: participants with non‐alcohol related fatty liver disease (NAFLD) Settings: secondary or tertiary care Intervention: antioxidants Control: no intervention | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (trials) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| No intervention | Antioxidants | ||||
|
Mortality Follow‐up: 12 months |
There were no events in either group | 87 (1 trial) |
⊕⊝⊝⊝ very low1,2,3 | ||
|
Serious adverse events (proportion) Follow‐up: 12 months |
There were no events in either group | 87 (1 trial) |
⊕⊝⊝⊝ very low1,2,3 | ||
|
Serious adverse events (number of events) Follow‐up: 12 months to 22 months |
101 per 1000 | 90 per 1000 (36 to 221) | rate ratio 0.89 (0.36 to 2.19) | 254 (2 trials) | ⊕⊝⊝⊝ very low1,2,4 |
| Health‐related quality of life | None of the trials reported this outcome | ||||
| *The basis for the assumed risk is the mean control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval. | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1 Downgraded one level for risk of bias because of the high risk of bias in the trial(s). 2 Downgraded one level for imprecision because of sample size.
3 Downgraded one level for imprecision because of lack of events. 4 Downgraded one level for imprecision because of wide confidence intervals.
Summary of findings 2. Bile acids versus no intervention for non‐alcohol related fatty liver disease.
| Bile acids versus no intervention for non‐alcohol related fatty liver disease | |||||
| Patient or population: participants with non‐alcohol related fatty liver disease (NAFLD) Settings: secondary or tertiary care Intervention: bile acids Control: no intervention | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (trials) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| No intervention | Bile acids | ||||
|
Mortality at maximal follow‐up Follow‐up: 1 to 18 months |
10 per 1000 | 49 per 1000 (2 to 520) |
OR 5.11 (0.24 to 107.34) |
659 (4 trials) |
⊕⊝⊝⊝ very low1,2,3 |
|
Serious adverse events (proportion) Follow‐up: 1 to 17 months |
64 per 1000 | 96 per 1000 (54 to 165) | OR 1.56 (0.84 to 2.88) | 404 (3 trials) | ⊕⊝⊝⊝ very low1,2,3 |
|
Serious adverse events (number of events) Follow‐up: 1 to 17 months |
101 per 1000 | 102 per 1000 (67 to 156) | Rate ratio 1.01 (0.66 to 1.54) | 404 (3 trials) | ⊕⊝⊝⊝ very low1,2,3 |
| Health‐related quality of life | None of the trials reported this outcome. | ||||
| *The basis for the assumed risk is the mean control group risk across studies, except for mortality at maximal follow‐up where there were no deaths; a control group proportion of 1% was used for mortality at maximal follow‐up. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio. | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1 Downgraded one level for risk of bias because of the high risk of bias in the trial(s). 2 Downgraded one level for imprecision because of sample size. 3 Downgraded one level for imprecision because of wide confidence intervals.
Summary of findings 3. Thiazolidinediones versus no intervention for non‐alcohol related fatty liver disease.
| Thiazolidinediones versus no intervention for non‐alcohol related fatty liver disease | |||||
| Patient or population: participants with non‐alcohol related fatty liver disease (NAFLD) Settings: secondary or tertiary care Intervention: thiazolidinediones Control: no intervention | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (trials) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| No intervention | Thiazolidinediones | ||||
|
Mortality at maximal follow‐up Follow‐up: 12 months |
There were no events in either group |
74 (1 trial) |
⊕⊝⊝⊝ very low1,2,3 | ||
|
Serious adverse events (proportion) Follow‐up: 6 to 12 months |
There were no events in either group | 194 (2 trials) | ⊕⊝⊝⊝ very low1,2,3 | ||
|
Serious adverse events (number of events) Follow‐up: 6 to 12 months |
101 per 1000 | 25 per 1000 (6 to 106) | rate ratio 0.25 (0.06 to 1.05) | 357 (3 trials) | ⊕⊝⊝⊝ very low1,2,4 |
| Health‐related quality of life | None of the trials reported this outcome | ||||
| *The basis for the assumed risk is the mean control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval. | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1 Downgraded one level for risk of bias because of the high risk of bias in the trial(s). 2 Downgraded one level for imprecision because of sample size. 3 Downgraded one level for imprecision because of lack of events. 4 Downgraded one level for imprecision because of wide confidence intervals.
Background
Description of the condition
Fatty liver disease is steatosis (accumulation of fat ‐ usually triglycerides) in the liver parenchymal cells (NCBI 2014). Non‐alcohol related fatty liver disease (also called non‐alcoholic fatty liver disease (NAFLD)) is liver steatosis in the absence of significant alcohol consumption; use of medications such as methotrexate, tamoxifen, or steroids; or other disorders such as hepatitis C virus infection, Wilson's disease, starvation, and lecithin cholesterol acyltransferase (LCAT) deficiency that result in fat accumulation (Chalasani 2012). Fatty liver disease includes a spectrum of disorders ranging from simple steatosis or non‐alcoholic fatty liver (NAFL) (fat accumulation without evidence of liver parenchymal cell injury), non‐alcoholic steatohepatitis (NASH) (fat accumulation with liver parenchymal injury but without cirrhosis), to NASH cirrhosis (advanced liver fibrosis with current or previous NAFL or NASH) to cirrhosis (Chalasani 2012; Rinella 2015).
The prevalence of NAFLD varies between 19% and 33% in different populations, depending upon ethnicity, region of origin (also among people of similar ethnicity), being overweight or obese, and having other disorders such as diabetes mellitus or hypertension (Bedogni 2005; Park 2006; Dassanayake 2009; Koehler 2012; Lazo 2013; Fleischman 2014; Li 2014; Shen 2014; Nishioji 2015). The major risk factors associated with increased prevalence of NAFLD are being male, increasing age, ethnicity (e.g. Mexican‐Americans have higher prevalence of fatty liver than other ethnic groups), hypertension, hypercholesterolaemia, diabetes mellitus, lower socio‐economic level, lower level educational attainment, and lower physical activity (Bedogni 2005; Park 2006; Dassanayake 2009; Koehler 2012; Lazo 2013; Fleischman 2014; Shen 2014; Lonardo 2015).
The mean age of people with NAFLD varies between 40 years and 60 years (Bedogni 2005; Dassanayake 2009; Shen 2014). In studies with long‐term follow‐up, the mean age of people with NAFLD ranged between 45 years and 50 years (Adams 2005; Bedogni 2007; Soderberg 2010; Onnerhag 2014). After a mean follow‐up period of 8 years to 28 years, the presence of NAFLD increased overall long‐term mortality compared to the general population without NAFLD (Adams 2005; Bedogni 2007; Ong 2008; Soderberg 2010; Onnerhag 2014).
People with NAFLD are at risk of dying before reaching the mean life expectancy at birth (Adams 2005; Bedogni 2007; Ong 2008; Soderberg 2010; Onnerhag 2014). It is widely believed that people with simple steatosis rarely progress to advanced liver disease but people with NASH may develop cirrhosis (Chalasani 2012). It has been reported that in people with NAFLD, liver fibrosis was the only histological feature associated with increased mortality and requirement for liver transplantation (Angulo 2015). In a trial that followed people with simple steatosis and NASH for a mean of 28 years, similar rates of mortality were observed between participants in the intervention and control groups (Soderberg 2010). However, mortality was higher than the general population mortality rate. It is noteworthy that NAFLD is associated with metabolic syndrome (presence of three of the following factors: hypertension, raised triglycerides, lowered high‐density lipoprotein cholesterol, raised fasting glucose, and central obesity; Alberti 2009) (Ballestri 2016). Therefore, increased mortality in people with NAFLD may be related to metabolic syndrome rather than NAFLD per se.
Fat accumulates within the liver cells when there is an imbalance between the mechanisms that reduce fat in cells (such as oxidation of fatty acids or secretion of lipoproteins) and mechanisms that increase fat in cells (such as increased uptake of fat and increased production of fat). The accumulation of fat leading to NAFLD is believed to be mediated by insulin resistance because insulin resistance increases the breakdown of peripheral adipose tissue with resultant increased influx of free fatty acids (FFA), promotes the synthesis of new triglycerides within the liver, and decreases the oxidation of FFAs (Abdelmalek 2007). The accumulation of fat in the liver causes injury due to pro‐inflammatory cytokines (Riley 2007). However, the mechanism by which only a proportion of people develop advanced liver fibrosis or primary liver cancer (hepatocellular cancer or HCC) is unclear (Abdelmalek 2007).
Ultrasound is a widely used method for screening the general population for NAFLD; however, it is operator‐dependent (Hernaez 2011), and may miss 15 people with fatty liver disease out of every 100 people screened (Hernaez 2011). It may also yield false‐positive results in 7 out of 100 people without fatty liver disease (Hernaez 2011).
Description of the intervention
Various interventions have been tried in the treatment of people with NAFLD. These include lifestyle modifications such as dietary changes and increased exercise (not included in this review) and a wide range of agents, such as those that decrease: weight (e.g. orlistat); insulin resistance (insulin‐sensitising agents; such as metformin and thiazolidinediones (e.g. pioglitazone, rosiglitazone)); and oxidative stress (e.g. vitamin E, herbal preparations such as milk thistle (silymarin or Silybum marianum extract) and S‐adenosylmethionine); agents such as statins (e.g. simvastatin, atorvastatin); secondary bile acids or analogues such as ursodeoxycholic acid or obeticholic acid; omega‐3 fatty acids that play a role in fat metabolism; angiotensin‐converting enzyme (ACE) inhibitors such as ramipril or angiotensin II receptor antagonists such as losartan; and weight reduction surgery (bariatric surgery) (not included in this review) in obese people with NAFLD (Adorini 2012; Anstee 2012; Chalasani 2012; Paschos 2012; Abenavoli 2013a).
How the intervention might work
Lifestyle modifications such as diet and increased exercise, agents (e.g. orlistat) and surgeries resulting in weight loss (not included in this review), and insulin‐sensitising agents such as metformin or thiazolidinediones are aimed at decreasing insulin resistance (Chalasani 2012; Thoma 2012). Milk thistle, vitamin E, and S‐adenosylmethionine decrease oxidative damage to liver cells (Anstee 2012; Chalasani 2012; Abenavoli 2013a). Bile acids play a role in fat metabolism and have anti‐inflammatory and anti‐fibrotic properties (Adorini 2012). Statins and omega‐3 fatty acids decrease circulating cholesterol levels and hence may decrease fatty liver (Chalasani 2012). ACE inhibitors and angiotensin II receptor antagonists inhibit the production or action of angiotensin II and therefore may decrease liver fibrosis, which may be mediated by the renin‐angiotensin‐aldosterone axis (Paschos 2012).
Why it is important to do this review
The optimal pharmacological treatment of people with NAFLD is unknown. Currently, no pharmacological treatment is recommended routinely in the treatment for all people with NAFLD. In people who do not have diabetes mellitus but who have biopsy‐confirmed NASH, vitamin E has been recommended as the first‐line treatment (Chalasani 2012). Pioglitazone may also be considered for people with biopsy‐confirmed NASH (Chalasani 2012). Screening for NAFLD is not recommended because of the uncertainties surrounding the effectiveness of diagnostic tests and treatment options (Chalasani 2012).
Network meta‐analysis enables direct and indirect evidence to be combined and to rank different interventions in terms of different outcomes (Salanti 2011; Salanti 2012). There has been no previous Cochrane Review on this topic. This Cochrane Review and attempted network meta‐analysis aimed to provide the best evidence for the role of different pharmacological interventions in the treatment of people NAFLD.
Objectives
To assess the comparative benefits and harms of different pharmacological interventions in the treatment of NAFLD through a network meta‐analysis and to generate rankings of the available pharmacological interventions according to their safety and efficacy. However, it was not possible to assess whether the potential effect modifiers were similar across different comparisons. Therefore, we did not perform the network meta‐analysis, and instead, assessed the comparative benefits and harms of different interventions using standard Cochrane methodology.
When more trials become available with adequate description of potential effect modifiers, we will attempt to conduct network meta‐analysis to generate rankings of the available interventions according to their safety and efficacy. This is why we retained the planned methodology for network meta‐analysis in our Appendix 1. Once data appear allowing for the conduct of network meta‐analysis, we will move back Appendix 1 into the Methods section.
Methods
Criteria for considering studies for this review
Types of studies
We considered only randomised clinical trials for this meta‐analysis irrespective of language, publication status, or date of publication. We excluded other designs because of the risk of bias. However, sSuch exclusions are understood to shift the focus more to potential benefits at the risk of not fully assessing the risks of adverse events and serious adverse events.
Types of participants
We included randomised clinical trials with participants with non‐alcoholic fatty liver disease (NAFLD) irrespective of the method of diagnosis, diabetic status of participants, or presence of non‐alcoholic steatohepatitis (NASH). We excluded randomised clinical trials in which participants had undergone liver transplantation previously.
Types of interventions
We considered any of the following pharmacological interventions for people with NAFLD, either alone or in combination and could be compared versus each other or versus placebo or no intervention.
The interventions that we considered a priori were:
orlistat;
metformin;
thiazolidinediones (e.g. pioglitazone, rosiglitazone);
other anti‐diabetes drugs;
vitamin E or other antioxidants;
milk thistle (silymarin or Silybum marianum extract);
S‐adenosylmethionine;
statins (e.g. simvastatin, atorvastatin);
secondary bile acids or derivatives (ursodeoxycholic acid, obeticholic acid); and
angiotensin‐converting enzyme (ACE) inhibitors or angiotensin II receptor antagonists.
This above list of interventions was not an exhaustive list. If we identified any other pharmacological interventions that we were not aware of, we considered them as eligible and included them in the review if they were used primarily for the treatment of people with NAFLD.
Types of outcome measures
We planned to assess the comparative benefits and harms of the available pharmacological interventions aimed at treating people with NAFLD for the following outcomes.
Primary outcomes
Mortality at maximal follow‐up.
-
Mortality:
short‐term mortality (up to one year);
medium‐term mortality (one to five years).
-
Adverse events (within three months after cessation of treatment). Depending on the availability of data, we attempted to classify adverse events as serious or non‐serious. We defined a non‐serious adverse event as any untoward medical occurrence not necessarily having a causal relationship with the treatment but resulting in a dose reduction or discontinuation of treatment (at any time after commencement of treatment) (ICH‐GCP 1997). We defined a serious adverse event as any that could increase mortality; is life threatening; requires hospitalisation; results in persistent or significant disability; was a congenital anomaly or birth defect; or any important medical event that might have jeopardised the person or required intervention for its prevention. We used definitions applied by study authors for non‐serious and serious adverse events:
proportion of participants with serious adverse events;
number of serious adverse events;
proportion of participants with any type of adverse event; and
number of any type of adverse event.
-
Health‐related quality of life as defined in the included trials using a validated scale such as EQ‐5D or 36‐item Short Form (SF‐36) (EuroQol 2014; Ware 2014):
short‐term (up to one year);
medium‐term (one to five years); and
long‐term (beyond five years).
We considered long‐term quality of life to be more important than short‐ or medium‐term quality of life, although short‐ and medium‐term quality of life are also important primary outcomes.
Secondary outcomes
-
Liver transplantation (maximal follow‐up):
proportion of participants with liver transplantation; and
time to liver transplantation.
-
Decompensated liver disease (maximal follow‐up):
proportion of participants with decompensated liver disease; and
time to liver decompensation.
-
Cirrhosis (maximal follow‐up):
proportion of participants with cirrhosis; and
time to cirrhosis.
Resolution of fatty liver disease (maximal follow‐up).
Unvalidated surrogate outcomes
We included two additional histological outcomes as potential surrogate outcomes (fibrosis score and NAFLD activity score) post hoc (Gluud 2007). This was applied for exploratory purposes because these outcomes are now accepted by regulatory agencies to expedite drug approval processes for NAFLD treatment via an accelerated approval pathway (Sanyal 2016). We did not make any inferences based on observations for these outcomes.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) to August 2016;, MEDLINE (OvidSP) (from January 1947 to August 2016), Embase (OvidSP) (from January 1974 to August 2016), and Science Citation Index Expanded (Web of Knowledge) (Royle 2003) (from January 1945 to August 2016). We did not apply language restrictions. We also searched the World Health Organization International Clinical Trials Registry Platform Search Portal (apps.who.int/trialsearch/), which searches various trial registers, including ISRCTN and ClinicalTrials.gov/ up to August 2016 (Appendix 2).
Searching other resources
We also searched the references of the included trials and Cochrane reviews on NAFLD.
Data collection and analysis
Selection of studies
Two review authors (SO or RL) independently identified trials for inclusion by screening the titles and abstracts. We sought full‐text articles for any references that at least one review author identified for potential inclusion. We selected trials for inclusion based on full‐text articles.
Data extraction and management
Two review authors (SO or RL or KG) independently extracted the following data.
-
Outcome data (for each outcome and for each treatment arm whenever applicable):
number of participants randomised;
number of participants included for the analysis;
number of participants with events for binary outcomes, mean and standard deviation for continuous outcomes, number of events for count outcomes, and number of participants with events and mean follow‐up period for time‐to‐event outcomes; and
definition of outcomes or scale used if appropriate.
-
Data on potential effect modifiers:
participant characteristics such as age, sex, comorbidities, and proportion of participants with NASH;
details of the intervention and control (including dose, frequency, and duration); and
risk of bias (assessment of risk of bias in included studies).
-
Other data:
year and language of publication;
country in which the participants were recruited;
year(s) in which the trial was conducted;
inclusion and exclusion criteria; and
follow‐up time points of the outcome.
We planned to obtain data separately for people with NASH and people without NASH if available. We planned to seek unclear or missing information by contacting the trial authors. If there was any doubt about if trials completely or partially reported the same participant data, (by identifying common authors and centres), we attempted to contact the trial authors to clarify if data were duplicated. We resolved any differences in opinion through discussion.
Assessment of risk of bias in included studies
We followed guidance from the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) and described in the Cochrane Hepato‐Biliary Group Module (Gluud 2016) to assess the risk of bias in included studies using the following methods (Schulz 1995; Moher 1998; Kjaergard 2001; Wood 2008; Savović 2012a; Savović 2012b; Lundh 2017).
Allocation sequence generation
Low risk of bias: the study authors performed sequence generation using computer random number generation or a random number table. Drawing lots, tossing a coin, shuffling cards, and throwing dice were adequate if an independent person not otherwise involved in the study performed them.
Unclear risk of bias: the study authors did not specify the method of sequence generation.
High risk of bias: the sequence generation method was not random. We planned to only include such studies for assessment of harms.
Allocation concealment
Low risk of bias: the participant allocations could not have been foreseen in advance of, or during, enrolment. A central and independent randomisation unit controlled allocation. The investigators were unaware of the allocation sequence (e.g. if the allocation sequence was hidden in sequentially numbered, opaque, and sealed envelopes).
Unclear risk of bias: the study authors did not describe the method used to conceal the allocation so the intervention allocations may have been foreseen before, or during, enrolment.
High risk of bias: it is likely that the investigators who assigned the participants knew the allocation sequence. We planned only to include such studies for assessment of harms.
Blinding of participants and personnel
Low risk of bias: any of the following: no blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding; or blinding of participants and key study personnel ensured, and it is unlikely that the blinding could have been broken.
Unclear risk of bias: any of the following: insufficient information to permit judgement of ‘low risk’ or ‘high risk’; or the trial did not address this outcome.
High risk of bias: any of the following: no blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding; or blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding.
Blinded outcome assessment
Low risk of bias: any of the following: no blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding; or blinding of outcome assessment ensured, and unlikely that the blinding could have been broken.
Unclear risk of bias: any of the following: insufficient information to permit judgement of ‘low risk’ or ‘high risk’; or the trial did not address this outcome.
High risk of bias: any of the following: no blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding; or blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding.
Incomplete outcome data
Low risk of bias: missing data were unlikely to make treatment effects depart from plausible values. The study used sufficient methods, such as multiple imputation, to handle missing data.
Unclear risk of bias: there was insufficient information to assess whether missing data in combination with the method used to handle missing data were likely to induce bias on the results.
High risk of bias: the results were likely to be biased due to missing data.
Selective outcome reporting
Low risk of bias: the trial reported the following pre‐defined outcomes: mortality, decompensated liver disease, requirement for transplantation, or treatment‐related adverse events. If the original trial protocol was available, the outcomes should be those called for in that protocol. If the trial protocol was obtained from a trial registry (e.g. www.clinicaltrials.gov), the outcomes sought should be those enumerated in the original protocol if the trial protocol was registered before or at the time that the trial was begun. If the trial protocol was registered after the trial was begun, those outcomes will not be considered to be reliable.
Unclear risk of bias: not all pre‐defined, or clinically relevant and reasonably expected, outcomes were reported fully, or it was unclear whether data on these outcomes were recorded or not.
High risk of bias: one or more pre‐defined or clinically relevant and reasonably expected outcomes were not reported, despite the fact that data on these outcomes should have been available and even recorded.
For‐profit bias
Low risk of bias: the trial appeared to be free of industry sponsorship or other type of for‐profit support that could manipulate the trial design, conduct, or results of the trial.
Unclear risk of bias: the trial may or may not be free of for‐profit bias as no information on clinical trial support or sponsorship was provided.
High risk of bias: the trial was sponsored by industry or received other type of for‐profit support.
Other bias
Low risk of bias: the trial appeared to be free of other components (e.g. inappropriate control or dose or administration of control, baseline differences, early stopping) that could put it at risk of bias.
Unclear risk of bias: the trial may or may not be free of other components that could put it at risk of bias.
High risk of bias: there were other factors in the trial that could put it at risk of bias (e.g. inappropriate control or dose or administration of control, baseline differences, early stopping).
We considered a trial at low risk of bias if we assessed the trial to be at low risk of bias across all domains. Otherwise, we considered trials to be at unclear risk of bias or at high risk of bias regarding one or more domains as at high risk of bias.
Measures of treatment effect
For dichotomous variables (e.g. short‐ and medium‐term mortality or liver transplantation, proportion of participants with adverse events, decompensated liver disease, cirrhosis, or hepatocellular carcinoma), we calculated the odds ratio (OR) with 95% confidence intervals (CI). For continuous variables (e.g. health‐related quality of life reported on the same scale), we planned to calculate the mean difference with 95% CI. We planned to use standardised mean difference (SMD) values with 95% CI for health‐related quality of life if included trials used different scales. For count outcomes (e.g. number of adverse events), we calculated the rate ratio with 95% CI. For time‐to‐event data (e.g. mortality at maximal follow‐up or requirement for liver transplantation, time to liver decompensation, and time to cirrhosis), we planned to use the hazard ratio (HR) with 95% confidence intervals. We also calculated Trial Sequential Analysis‐adjusted CI to control random errors (Thorlund 2011; Wetterslev 2017).
Unit of analysis issues
The unit of analysis was people with NAFLD according to the intervention group to which they were randomly assigned.
Cluster randomised clinical trials
We did not anticipate to find cluster randomised clinical trials. However, if they were found, they were to be included, provided that the effect estimate adjusted for cluster correlation was available.
Cross‐over randomised clinical trials
We planned to include outcomes after the first treatment period only from cross‐over randomised clinical trials. NAFLD is a chronic disease and treatment could potentially have residual effects.
Trials with multiple treatment groups
We planned to collect data for all trial treatment groups that met the inclusion criteria.
Dealing with missing data
We performed intention‐to‐treat analyses where possible (Newell 1992). Otherwise, we used available data (e.g. trials may report only per‐protocol analysis results). As such per‐protocol analyses may be biased, we planned to conduct best‐worst case scenario analysis (good outcome in intervention group and bad outcome in control group) and worst‐best case scenario analysis (bad outcome in intervention group and good outcome in control group) as sensitivity analyses whenever possible.
We planned to impute the standard deviation from P values for continuous outcomes (Higgins 2011). If data were distributed normally, we planned to use the median for meta‐analysis when the mean was not available. If it was not possible to calculate the standard deviation from the P value or the confidence intervals, we planned to impute this using the largest standard deviation from other trials for that outcome. This imputation technique may decrease the weight of the study for calculation of mean differences and may bias the effect estimate to no effect for calculation of standardised mean differences (Higgins 2011).
Assessment of heterogeneity
We assessed clinical and methodological heterogeneity by carefully examining the characteristics and design of included trials. We assessed the presence of clinical heterogeneity by comparing effect estimates in the presence or absence of symptoms, the presence or absence of NASH, the diabetes status of participants, and drug doses. Different trial designs and risk of bias may contribute to methodological heterogeneity. We used the I² test and Chi² test for heterogeneity, and overlapping of CIs to assess heterogeneity.
Assessment of reporting biases
We planned to assess visual asymmetry on a funnel plot to explore reporting bias in the presence of at least 10 trials that could be included for a direct comparison (Egger 1997; Macaskill 2001). In the presence of heterogeneity that could be explained by subgroup analysis, we planned to produce a funnel plot for each eligible subgroup in the presence of an adequate number of trials (at least 10 trials). We planned to use the linear regression approach described by Egger 1997 to determine funnel plot asymmetry.
We also considered selective reporting as evidence of reporting bias.
Data synthesis
We conducted the meta‐analyses according to Cochrane methods and recommendations (Higgins 2011) using Review Manager 5 (RevMan 2014). We used both random‐effects (DerSimonian 1986) and fixed‐effect models (DeMets 1987). In the case of a discrepancy between the models, we reported both results; otherwise, we reported only the fixed‐effect model results.
Calculation of required information size and Trial Sequential Analysis
Details of the sample size calculation is presented in Appendix 3. We performed Trial Sequential Analysis to control the risk of random errors (Wetterslev 2008; Thorlund 2011; TSA 2011) when there were at least two trials included in the meta‐analysis. We used an alpha error as per Jakobsen 2014, 90% power (10% beta error), 20% relative risk reduction, control group proportion observed in the trials, and the diversity observed in the meta‐analysis.
Subgroup analysis and investigation of heterogeneity
We planned to assess differences in effect estimates among the following subgroups.
Trials with low risk of bias compared to trials at high risk of bias.
Participants with NASH compared to participants with NAFLD but without NASH.
Participants with diabetes mellitus compared to participants without diabetes mellitus.
Different doses of pharmacological interventions.
We planned to use the Chi² test for subgroup differences.
Sensitivity analysis
If a trial reported only per‐protocol analysis results, we planned to re‐analyse the results using the best‐worst scenario and worst‐best case scenario as sensitivity analyses whenever possible.
GRADE and 'Summary of findings' tables
We created 'Summary of findings' tables using the following outcomes: mortality, serious adverse events, and health‐related quality of life (Table 1; Table 2; Table 3). We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess evidence quality relating to trials that contribute data to the meta‐analyses for the specified outcomes. We used methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We justified all decisions to downgrade the quality of evidence in footnotes and comments to aid understanding of the review where necessary.
Results
Description of studies
Results of the search
We identified 2851 references through electronic searches of CENTRAL (n = 361), MEDLINE (n = 816), Embase (n = 461), Science Citation Index Expanded (n = 793), World Health Organization International Clinical Trials Registry Platform (n = 227) and ClinicalTrials.gov (n = 193). After the removal of 1209 duplicates we obtained 1642 references. We then excluded 1496 clearly irrelevant references from screening titles and reading abstracts. We retrieved 146 references for further assessment. No references were identified from scanning reference lists of randomised trials. We excluded 33 studies (34 reports) (see Characteristics of excluded studies). In total, 77 randomised clinical trials (112 reports) met the inclusion criteria (Figure 1).
1.
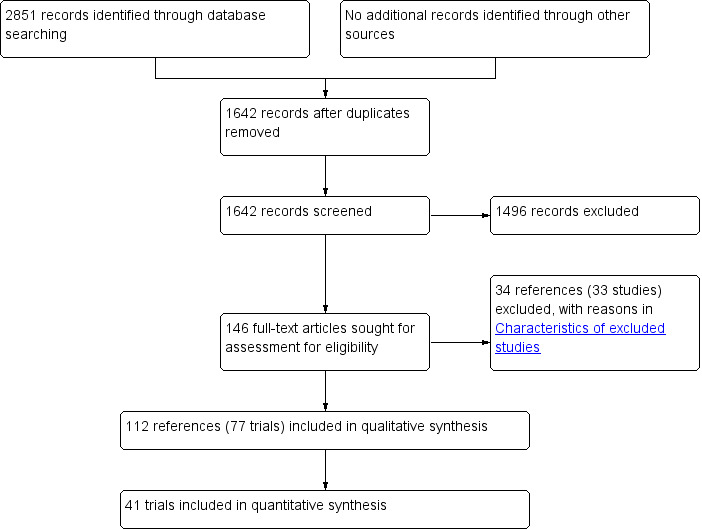
Study flow diagram.
Included studies
We included 77 trials that met the inclusion criteria for this review that involved 6287 participants. However, 36 trials did not contribute any data for this review (Kugelmas 2003; Santos 2003; Mendez‐Sanchez 2004; Sanyal 2004; Uygun 2004; Bugianesi 2005; Morita 2005; Cui 2006; Lewis 2006; Hajaghamohammadi 2008; Copaci 2009; Gastaldelli 2009; Harrison 2009; Hashemi 2009; Yaginuma 2009; Foster 2011; Sofer 2011; Fogari 2012; Hajiaghamohammadi 2012; Razavizadeh 2012; Askarimoghadam 2013; Basu 2013; Cusi 2013; Kakazu 2013; Taghvaei 2013; Kedarisetty 2014; Solhi 2014; Song 2014; Stilidi 2014; Baranova 2015; Bonfrate 2015; Klyarytskaya 2015; Shiffman 2015; Siddique 2015; Sunny 2015; Wang 2015).
We included data from a total of 3829 participants in one or more analyses in the review. The mean or median age of the participants ranged from 33 years to 62 years in the trials that reported this information. The proportion of females ranged from 6.7% to 85.2% in the trials that reported this information. Thirty‐five trials included participants with non‐alcohol related steatohepatitis (NASH) only (Harrison 2003; Kugelmas 2003; Merat 2003; Lindor 2004; Uygun 2004; Morita 2005; Belfort 2006; Dufour 2006; Aithal 2008; Ratziu 2008; Copaci 2009; Gastaldelli 2009; Gomez 2009; Harrison 2009; Hashemi 2009; Nelson 2009; Shields 2009; Leuschner 2010; Omer 2010; Sanyal 2010; Ratziu 2011; Torres 2011; Van Wagner 2011; Sharma 2012; Cusi 2013; Kakazu 2013; Kedarisetty 2014; Ratziu 2014; Chan 2015; Loomba 2015; Neuschwander‐Tetri 2015; Sunny 2015; Alam 2016; Armstrong 2016; Ratziu 2016). The remainder did not report the proportion of participants with non‐alcoholic fatty liver disease (NAFLD) and NASH or did not report data separately for those with and without NASH.
Five trials included only participants with diabetes mellitus (Morita 2005; Nar 2009; Mudaliar 2013; Song 2014; Wang 2015); and 14 trials included only those who did not have diabetes mellitus (Uygun 2004; Bugianesi 2005; Athyros 2006; Belfort 2006; Aithal 2008; Hajaghamohammadi 2008; Sanyal 2010; Fogari 2012; Hajiaghamohammadi 2012; Basu 2013; Gianturco 2013; Basu 2014; Solhi 2014; Aller 2015). The remainder did not report proportions of people with diabetes mellitus or did not report data separately for those with and without diabetes mellitus.
The interventions, controls, number of participants included in each trial, and follow‐up periods, are reported in Table 4. Overall, the mean or median follow‐up was from 1 month to 18 months.
1. Characteristics of included studies (by comparison).
| Study name (total participants randomised) | Intervention(s) | Control | Total after post‐randomisation drop‐outs (number who dropped out) | NASH | NASH only | Diabetes mellitus | People with diabetes only | People without diabetes mellitus only | Average follow‐up period (months) |
| Fogari 2012 (150) | Renin‐angiotensin‐aldosterone system inhibitor | Antihypertensives | 141 (9) | Not stated | Not stated | 0/141 (0.0%) | No | Yes | 12 |
| Ersoz 2005 (57) | Bile acids | Antioxidants | 56 (1) | 6/56 (10.7%) | No | 14/56 (25.0%) | No | No | 6 |
| Harrison 2009 (50) | Orlistat plus antioxidants | Antioxidants | 41 (9) | 41/41 (100.0%) | Yes | 4/41 (9.8%) | No | No | 9 |
| Kedarisetty 2014 (116) | Pentoxifylline plus antioxidants | Antioxidants | 116 (0) | 116/116 (100.0%) | Yes | Not stated | Not stated | Not stated | 12 |
| Polyzos 2011 (31) | Renin‐angiotensin‐aldosterone system inhibitor plus antioxidants | Antioxidants | 31 (not stated) | 16/31 (51.6%) | No | 5/31 (16.1%) | No | No | 2 |
| Bugianesi 2005 (57) | Sulphonylureas | Antioxidants | 57 (not stated) | Not stated | Not stated | 0/57 (0.0%) | No | Yes | 12 |
| Sanyal 2010 (247) | Thiazolidinediones | Antioxidants | 247 (0) | 247/247 (100.0%) | Yes | 0/247 (0.0%) | No | Yes | 22 |
| Basu 2013 (80) | Thiazolidinediones | Antioxidants | 80 (not stated) | Not stated | Not stated | 0/80 (0.0%) | No | Yes | 12 |
| Sanyal 2004 (20) | Thiazolidinediones plus antioxidants | Antioxidants | 20 (0) | Not stated | Not stated | Not stated | Not stated | Not stated | 6 |
| Parikh 2016 (250) | Antioxidants | Bile acids | 233 (17) | 35/233 (15.0%) | No | Not stated | Not stated | Not stated | 12 |
| Copaci 2009 (94) | Intervention 1: Pentoxifylline Intervention 2: Pentoxifylline plus bile acids | Bile acids | 94 (not stated) | 94/94 (100.0%) | Yes | Not stated | Not stated | Not stated | 12 |
| Stilidi 2014 (58) | Renin‐angiotensin‐aldosteronesystem inhibitor plus bile acids | Bile acids | 58 (not stated) | Not stated | Not stated | Not stated | Not stated | Not stated | 6 |
| Shiffman 2015 (38) | Anti‐caspase | No intervention | 38 (not stated) | Not stated | Not stated | Not stated | Not stated | Not stated | 1 |
| Ratziu 2016 (276) | Anti‐fibrotic | No intervention | 274 (2) | 274/274 (100.0%) | Yes | 107/274 (39.1%) | No | No | 12 |
| Harrison 2003 (49) | Antioxidants | No intervention | 45 (4) | 45/45 (100.0%) | Yes | 19/45 (42.2%) | No | No | 6 |
| Kugelmas 2003 (16) | Antioxidants | No intervention | 16 (not stated) | 16/16 (100.0%) | Yes | Not stated | Not stated | Not stated | 3 |
| Gomez 2009 (60) | Antioxidants | No intervention | 60 (0) | 60/60 (100.0%) | Yes | Not stated | Not stated | Not stated | 6 |
| Magosso 2013 (87) | Antioxidants | No intervention | 87 (0) | Not stated | Not stated | Not stated | Not stated | Not stated | 12 |
| Basu 2014 (155) | Antioxidants | No intervention | 155 (0) | Not stated | Not stated | 0/155 (0.0%) | No | Yes | 6 |
| Santos 2003 (30) | Bile acids | No intervention | 30 (not stated) | Not stated | Not stated | Not stated | Not stated | Not stated | 3 |
| Lindor 2004 (174) | Bile acids | No intervention | 166 (8) | 166/166 (100.0%) | Yes | Not stated | Not stated | Not stated | 24 |
| Mendez‐Sanchez 2004 (27) | Bile acids | No intervention | 23 (4) | Not stated | Not stated | Not stated | Not stated | Not stated | 1 |
| Leuschner 2010 (186) | Bile acids | No intervention | 186 (0) | 186/186 (100.0%) | Yes | 21/186 (11.3%) | No | No | 18 |
| Ratziu 2011 (126) | Bile acids | No intervention | 126 (0) | 126/126 (100.0%) | Yes | 40/126 (31.7%) | No | No | 12 |
| Mudaliar 2013 (64) | Bile acids | No intervention | 64 (0) | Not stated | Not stated | 64/64 (100.0%) | Yes | No | 1 |
| Safadi 2014 (60) | Bile acids | No intervention | 57 (3) | 6/57 (10.5%) | No | Not stated | Not stated | Not stated | 4 |
| Neuschwander‐Tetri 2015 (283) | Bile acids | No intervention | 283 (not stated) | 283/283 (100.0%) | Yes | 149/283 (52.7%) | No | No | 17 |
| Gianturco 2013 (200) | Intervention 1: Bile acids plus antioxidants Intervention 2: Antioxidants Intervention 3: Bile acids | No intervention | 196 (4) | Not stated | Not stated | 0/196 (0.0%) | No | Yes | 12 |
| Dufour 2006 (48) | Intervention 1: Bile acids plus antioxidants Intervention 2: Bile acids | No intervention | 40 (8) | 40/40 (100.0%) | Yes | Not stated | Not stated | Not stated | 24 |
| Stefan 2014 (82) | Glucocorticosteroid inhibitor | No intervention | 80 (2) | Not stated | Not stated | Not stated | Not stated | Not stated | 3 |
| Morita 2005 (10) | Other anti‐diabetes medication | No intervention | 10 (not stated) | 10/10 (100.0%) | Yes | 10/10 (100.0%) | Yes | No | 5 |
| Armstrong 2016 (52) | Other anti‐diabetes medication | No intervention | 52 (0) | 52/52 (100.0%) | Yes | 17/52 (32.7%) | No | No | 17 |
| Wang 2015 (68) | Intervention 1: Other anti‐diabetes medication Intervention 1: Sulphonylureas plus other anti‐diabetes medication Sulphonylureas | No intervention | 68 (not stated) | Not stated | Not stated | 68/68 (100.0%) | Yes | No | 6 |
| Merat 2003 (30) | Other cholesterol‐lowering agents | No intervention | 27 (3) | 27/27 (100.0%) | Yes | Not stated | Not stated | Not stated | 6 |
| Loomba 2015 (50) | Other cholesterol‐lowering agents | No intervention | 50 (not stated) | 50/50 (100.0%) | Yes | 14/50 (28.0%) | No | No | 6 |
| Van Wagner 2011 (30) | Pentoxifylline | No intervention | 26 (4) | 26/26 (100.0%) | Yes | Not stated | Not stated | Not stated | 12 |
| Ratziu 2014 (99) | Phosphodiesterase type 4 inhibitor | No intervention | 96 (3) | 96/96 (100.0%) | Yes | Not stated | Not stated | Not stated | 3 |
| Alam 2016 (50) | Renin‐angiotensin‐aldosterone system inhibitor | No intervention | 30 (20) | 30/30 (100.0%) | Yes | 8/30 (26.7%) | No | No | 12 |
| Hajaghamohammadi 2008 (50) | Silymarin | No intervention | 50 (not stated) | Not stated | Not stated | 0/50 (0.0%) | No | Yes | 2 |
| Hashemi 2009 (100) | Silymarin | No intervention | 100 (not stated) | 100/100 (100.0%) | Yes | Not stated | Not stated | Not stated | 6 |
| Taghvaei 2013 (41) | Silymarin | No intervention | 41 (not stated) | Not stated | Not stated | Not stated | Not stated | Not stated | 6 |
| Solhi 2014 (80) | Silymarin | No intervention | 64 (16) | Not stated | Not stated | 0/64 (0.0%) | No | Yes | 2 |
| Chan 2015 (64) | Silymarin | No intervention | 64 (not stated) | 64/64 (100.0%) | Yes | Not stated | Not stated | Not stated | 11 |
| Aller 2015 (36) | Silymarin plus antioxidants | No intervention | 36 (not stated) | 15/36 (41.7%) | No | 0/36 (0.0%) | No | Yes | 3 |
| Bonfrate 2015 (40) | Silymarin plus antioxidants | No intervention | 40 (not stated) | Not stated | Not stated | Not stated | Not stated | Not stated | 6 |
| Lewis 2006 (175) | Statins | No intervention | 175 (not stated) | Not stated | Not stated | Not stated | Not stated | Not stated | 9 |
| Nelson 2009 (16) | Statins | No intervention | 16 (0) | 16/16 (100.0%) | Yes | 7/16 (43.8%) | No | No | 12 |
| Baranova 2015 (20) | Statins | No intervention | 20 (not stated) | Not stated | Not stated | Not stated | Not stated | Not stated | 6 |
|
Foster 2011 (80) |
Statins plus antioxidants | No intervention | 80 (not stated) | Not stated | Not stated | Not stated | Not stated | Not stated | Not stated |
| Uygun 2004 (36) | Sulphonylureas | No intervention | 34 (2) | 34/34 (100.0%) | Yes | 0/34 (0.0%) | No | Yes | 6 |
| Haukeland 2009 (48) | Sulphonylureas | No intervention | 44 (4) | Not stated | Not stated | 12/44 (27.3%) | No | No | 6 |
| Nar 2009 (34) | Sulphonylureas | No intervention | 34 (not stated) | Not stated | Not stated | 34/34 (100.0%) | Yes | No | 6 |
| Shields 2009 (19) | Sulphonylureas | No intervention | 19 (not stated) | 19/19 (100.0%) | Yes | Not stated | Not stated | Not stated | 12 |
| Garinis 2010 (50) | Sulphonylureas | No intervention | 45 (5) | Not stated | Not stated | Not stated | Not stated | Not stated | 6 |
| Sofer 2011 (63) | Sulphonylureas | No intervention | 63 (not stated) | Not stated | Not stated | Not stated | Not stated | Not stated | 4 |
| Belfort 2006 (55) | Thiazolidinediones | No intervention | 47 (8) | 47/47 (100.0%) | Yes | 0/47 (0.0%) | No | Yes | 6 |
| Cui 2006 (124) | Thiazolidinediones | No intervention | 124 (not stated) | Not stated | Not stated | Not stated | Not stated | Not stated | 6 |
| Aithal 2008 (74) | Thiazolidinediones | No intervention | 74 (0) | 74/74 (100.0%) | Yes | 0/74 (0.0%) | No | Yes | 12 |
| Ratziu 2008 (64) | Thiazolidinediones | No intervention | 63 (1) | 63/63 (100.0%) | Yes | 20/63 (31.7%) | No | No | 16 |
| Gastaldelli 2009 (48) | Thiazolidinediones | No intervention | 48 (not stated) | 48/48 (100.0%) | Yes | Not stated | Not stated | Not stated | 6 |
| Yaginuma 2009 (20) | Thiazolidinediones | No intervention | 20 (not stated) | Not stated | Not stated | Not stated | Not stated | Not stated | 12 |
| Jin 2010 (120) | Thiazolidinediones | No intervention | 120 (not stated) | Not stated | Not stated | Not stated | Not stated | Not stated | 6 |
| Cusi 2013 (101) | Thiazolidinediones | No intervention | 101 (not stated) | 101/101 (100.0%) | Yes | 52/101 (51.5%) | No | No | 18 |
| Kakazu 2013 (25) | Thiazolidinediones | No intervention | 24 (1) | 24/24 (100.0%) | Yes | Not stated | Not stated | Not stated | 24 |
| Sunny 2015 (50) | Thiazolidinediones | No intervention | 50 (not stated) | 50/50 (100.0%) | Yes | Not stated | Not stated | Not stated | 18 |
| Yan 2015 (122) | Thiazolidinediones | No intervention | 122 (not stated) | Not stated | Not stated | Not stated | Not stated | Not stated | 4 |
| Athyros 2006 (186) | Intervention 1: Statins Intervention 2: Statins plus other cholesterol‐lowering agents | Other cholesterol‐lowering agents | 186 (0) | Not stated | Not stated | 0/186 (0.0%) | No | Yes | 12 |
| Razavizadeh 2012 (100) | Antioxidants | Silymarin | 100 (not stated) | Not stated | Not stated | Not stated | Not stated | Not stated | 2 |
| Hajiaghamohammadi 2012 (66) | Intervention 1: Thiazolidinediones Intervention 2: Sulphonylureas | Silymarin | 66 (not stated) | Not stated | Not stated | 0/66 (0.0%) | No | Yes | 2 |
| Siddique 2015 (67) | Thiazolidinediones | Statins | 67 (not stated) | Not stated | Not stated | Not stated | Not stated | Not stated | 6 |
| Klyarytskaya 2015 (51) | Renin‐angiotensin‐aldosterone system inhibitor plus statins plus antioxidants | Statins plus antioxidants | 51 (not stated) | Not stated | Not stated | Not stated | Not stated | Not stated | 12 |
| Askarimoghadam 2013 (93) | Sulphonylureas plus antioxidants | Sulphonylureas | 93 (not stated) | Not stated | Not stated | Not stated | Not stated | Not stated | 6 |
| Song 2014 (70) | Sulphonylureas plus other anti‐diabetes medication | Sulphonylureas | 67 (3) | Not stated | Not stated | 67/67 (100.0%) | Yes | No | 4 |
| Sharma 2012 (60) | Pentoxifylline | Thiazolidinediones | 59 (1) | 59/59 (100.0%) | Yes | Not stated | Not stated | Not stated | 6 |
| Omer 2010 (64) | Sulphonylureas | Thiazolidinediones | 64 (not stated) | 64/64 (100.0%) | Yes | Not stated | Not stated | Not stated | 12 |
| Razavizade 2013 (80) | Sulphonylureas | Thiazolidinediones | 80 (0) | Not stated | Not stated | 6/80 (7.5%) | No | No | 4 |
| Torres 2011 (135) | Intervention 1: Thiazolidinediones plus renin‐angiotensin‐aldosterone system inhibitor Intervention 2: Thiazolidinediones plus sulphonylureas | Thiazolidinediones | 89 (46) | 89/89 (100.0%) | Yes | 18/89 (20.2%) | No | No | 11 |
NASH: non‐alcoholic steatohepatitis.
Sources of funding
We found that 12 trials did not report receiving any additional funding or were supported by parties without vested interest in the results (Kugelmas 2003; Merat 2003; Morita 2005; Nelson 2009; Polyzos 2011; Fogari 2012; Hajiaghamohammadi 2012; Kakazu 2013; Razavizade 2013; Yan 2015; Alam 2016; Parikh 2016). Twenty‐six trials were funded by commercial pharmaceutical companies which would benefit from the results of the trial (Santos 2003; Lindor 2004; Athyros 2006; Belfort 2006; Dufour 2006; Aithal 2008; Ratziu 2008; Gomez 2009; Haukeland 2009; Leuschner 2010; Sanyal 2010; Ratziu 2011; Torres 2011; Cusi 2013; Magosso 2013; Mudaliar 2013; Basu 2014; Ratziu 2014; Safadi 2014; Stefan 2014; Loomba 2015; Neuschwander‐Tetri 2015; Shiffman 2015; Sunny 2015; Armstrong 2016; Ratziu 2016). The source of funding was not reported in 39 trials.
Excluded studies
We presented the reasons for the 33 excluded studies in Characteristics of excluded studies.
Risk of bias in included studies
Risk of bias is summarised in Figure 2, Figure 3, and Table 5. Only one small trial was assessed at low risk of bias in all domains (Razavizade 2013). All other included trials were assessed at unclear or high risk of bias for one or more domains.
2.
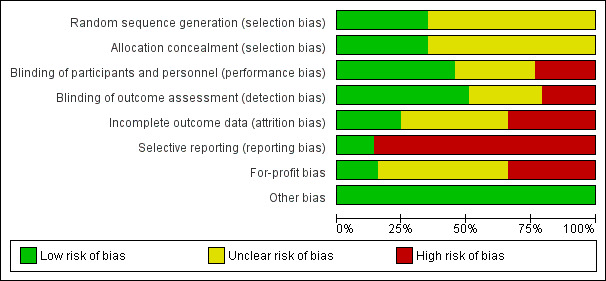
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
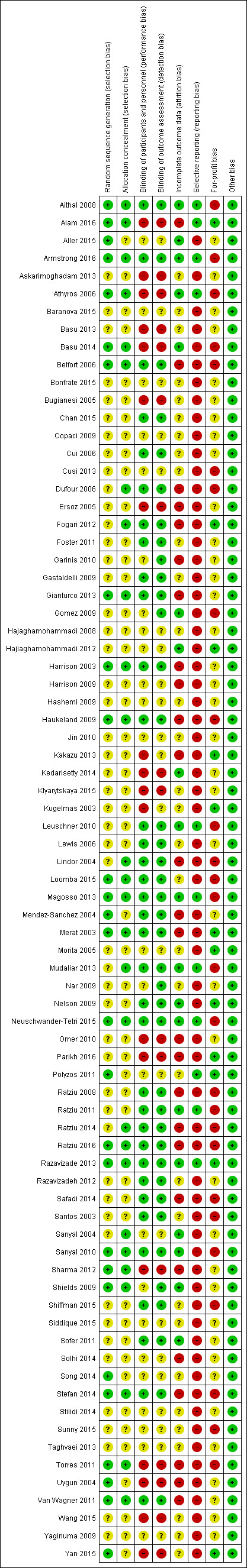
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
2. Risk of bias (by comparison).
| Study name | Intervention(s) and controls | Random sequence generation | Allocation concealment | Blinding of participants and personnel | Blinding of outcom assessment | Incomplete outcome data | Selective reporting | For‐profit bias | Other bias |
| Fogari 2012 | Renin‐angiotensin‐aldosterone system inhibitor Control: Antihypertensives | Unclear | Low | Low | Low | High | High | Low | Low |
| Ersoz 2005 | Bile acids Control: Antioxidants | Unclear | Unclear | High | High | High | High | Unclear | Low |
| Harrison 2009 | Orlistat plus antioxidants Control: Antioxidants | Unclear | Unclear | Unclear | Unclear | High | High | Unclear | Low |
| Kedarisetty 2014 | Pentoxifylline plus antioxidants Control: Antioxidants | Unclear | Unclear | High | High | Low | High | Unclear | Low |
| Polyzos 2011 | Renin‐angiotensin‐aldosterone system inhibitor plus antioxidants Control: Antioxidants | Low | Unclear | Unclear | Unclear | Unclear | Low | Low | Low |
| Bugianesi 2005 | Sulphonylureas Control: Antioxidants | Unclear | Unclear | High | High | Unclear | High | Unclear | Low |
| Sanyal 2010 | Thiazolidinediones Control: Antioxidants | Low | Low | Low | Low | Low | Low | High | Low |
| Basu 2013 | Thiazolidinediones Control: Antioxidants | Unclear | Unclear | High | High | Unclear | High | Unclear | Low |
| Sanyal 2004 | Thiazolidinediones plus antioxidants Control: Antioxidants | Unclear | low | Unclear | Unclear | low | High | Unclear | Low |
| Parikh 2016 | Antioxidants Control: Bile acids | Unclear | Unclear | High | High | High | High | Low | Low |
| Copaci 2009 | Intervention 1: Pentoxifylline Intervention 2: Pentoxifylline plus bile acids Control: Bile acids | Unclear | Unclear | Unclear | Unclear | Unclear | High | Unclear | Low |
| Stilidi 2014 | Renin‐angiotensin‐aldosterone system inhibitor plus bile acids Control: Bile acids | Unclear | Unclear | Unclear | Unclear | Unclear | High | Unclear | Low |
| Shiffman 2015 | Anti‐caspase Control: No intervention | Unclear | Unclear | Low | Low | Unclear | High | High | Low |
| Ratziu 2016 | Anti‐fibrotic Control: No intervention | Low | Low | Low | Low | High | High | High | Low |
| Harrison 2003 | Antioxidants Control: No intervention | Low | Low | Low | Low | High | High | Unclear | Low |
| Kugelmas 2003 | Antioxidants Control: No intervention | Unclear | Unclear | High | Unclear | Unclear | High | Low | Low |
| Gomez 2009 | Antioxidants Control: No intervention | Unclear | Unclear | Unclear | Low | Low | High | High | Low |
| Magosso 2013 | Antioxidants Control: No intervention | Low | Low | Low | Low | Low | Low | High | Low |
| Basu 2014 | Antioxidants Control: No intervention | Low | Low | High | High | Low | High | High | Low |
| Santos 2003 | Bile acids Control: No intervention | Unclear | Unclear | Low | Low | Unclear | High | High | Low |
| Lindor 2004 | Bile acids Control: No intervention | Unclear | Low | Low | Low | High | High | High | Low |
| Mendez‐Sanchez 2004 | Bile acids Control: No intervention | Low | Unclear | Low | Low | High | High | Unclear | Low |
| Leuschner 2010 | Bile acids Control: No intervention | Unclear | Unclear | Low | Low | Low | Low | High | Low |
| Ratziu 2011 | Bile acids Control: No intervention | Unclear | Unclear | Low | Low | Low | Low | High | Low |
| Mudaliar 2013 | Bile acids Control: No intervention | Unclear | Low | Low | Low | Low | Low | High | Low |
| Safadi 2014 | Bile acids Control: No intervention | Unclear | Unclear | Low | Low | High | High | High | Low |
| Neuschwander‐Tetri 2015 | Bile acids Control: No intervention | Low | Low | Low | Low | Low | High | High | Low |
| Gianturco 2013 | Intervention 1: Bile acids plus antioxidants Intervention 2: Antioxidants Intervention 3: Bile acids Control: No intervention | Low | Low | Low | Low | High | High | Unclear | Low |
| Dufour 2006 | Intervention 1: Bile acids plus antioxidants Intervention 2: Bile acids Control: No intervention | Unclear | Low | Low | Low | High | High | High | Low |
| Stefan 2014 | Glucocorticosteroid inhibitor Control: No intervention | Low | Low | Low | Low | High | High | High | Low |
| Morita 2005 | Other anti‐diabetes medication Control: No intervention | Unclear | Unclear | Unclear | Unclear | Unclear | High | Low | Low |
| Armstrong 2016 | Other anti‐diabetes medication Control: No intervention | Low | Low | Low | Low | Low | Low | High | Low |
| Wang 2015 | Intervention 1: Other anti‐diabetes medication Intervention 2: Sulphonylureas plus other anti‐diabetes medication Intervention 3: Sulphonylureas Control: No intervention | Unclear | Unclear | High | High | Unclear | High | Unclear | Low |
| Merat 2003 | Other cholesterol‐lowering agents Control: No intervention | Low | Low | Low | Low | High | High | Low | Low |
| Loomba 2015 | Other cholesterol‐lowering agents Control: No intervention | Low | Low | Low | Low | Unclear | High | High | Low |
| Van Wagner 2011 | Pentoxifylline Control: No intervention | Low | Low | Low | Low | High | High | Unclear | Low |
| Ratziu 2014 | Phosphodiesterase type 4 inhibitor Control: No intervention | Unclear | Low | Low | Low | High | High | High | Low |
| Alam 2016 | Renin‐angiotensin‐aldosterone system inhibitor Control: No intervention | Low | Low | High | High | High | Low | Low | Low |
| Hajaghamohammadi 2008 | Silymarin Control: No intervention | Unclear | Unclear | Unclear | Unclear | Unclear | High | Unclear | Low |
| Hashemi 2009 | Silymarin Control: No intervention | Unclear | Unclear | Unclear | Unclear | Unclear | High | Unclear | Low |
| Taghvaei 2013 | Silymarin Control: No intervention | Unclear | Unclear | Unclear | Unclear | Unclear | High | Unclear | Low |
| Solhi 2014 | Silymarin Control: No intervention | Unclear | Unclear | Unclear | Unclear | High | High | Unclear | Low |
| Chan 2015 | Silymarin Control: No intervention | Unclear | Unclear | Low | Low | Unclear | High | Unclear | Low |
| Aller 2015 | Silymarin plus antioxidants Control: No intervention | Low | Unclear | Unclear | Unclear | Low | High | Unclear | Low |
| Bonfrate 2015 | Silymarin plus antioxidants Control: No intervention | Unclear | Unclear | Unclear | Unclear | Unclear | High | Unclear | Low |
| Lewis 2006 | Statins Control: No intervention | Unclear | Unclear | Low | Low | Unclear | High | Unclear | Low |
| Nelson 2009 | Statins Control: No intervention | Unclear | Unclear | Low | Low | Low | High | Low | Low |
| Baranova 2015 | Statins Control: No intervention | Unclear | Unclear | Unclear | Unclear | Unclear | High | Unclear | Low |
| Foster 2011 | Statins plus antioxidants Control: No intervention | Unclear | Unclear | Low | Low | Unclear | High | Unclear | Low |
| Uygun 2004 | Sulphonylureas Control: No intervention | Low | Unclear | High | High | High | High | Unclear | Low |
| Haukeland 2009 | Sulphonylureas Control: No intervention | Low | Low | Low | Low | High | High | High | Low |
| Nar 2009 | Sulphonylureas Control: No intervention | Unclear | Unclear | Unclear | Low | Unclear | High | Unclear | Low |
| Shields 2009 | Sulphonylureas Control: No intervention | Low | Low | Unclear | Low | Low | High | Unclear | Low |
| Garinis 2010 | Sulphonylureas Control: No intervention | Unclear | Unclear | Unclear | Low | High | High | Unclear | Low |
| Sofer 2011 | Sulphonylureas Control: No intervention | Unclear | Unclear | Low | Low | Low | High | Unclear | Low |
| Belfort 2006 | Thiazolidinediones Control: No intervention | Low | Low | Low | Low | High | High | High | Low |
| Cui 2006 | Thiazolidinediones Control: No intervention | Unclear | Unclear | Low | Low | Unclear | High | Unclear | Low |
| Aithal 2008 | Thiazolidinediones Control: No intervention | Low | Low | Low | Low | Low | Low | High | Low |
| Ratziu 2008 | Thiazolidinediones Control: No intervention | Unclear | Unclear | Low | Low | High | High | High | Low |
| Gastaldelli 2009 | Thiazolidinediones Control: No intervention | Unclear | Unclear | Low | Low | Unclear | High | Unclear | Low |
| Yaginuma 2009 | Thiazolidinediones Control: No intervention | Unclear | Unclear | Unclear | Unclear | Unclear | High | Unclear | Low |
| Jin 2010 | Thiazolidinediones Control: No intervention | Unclear | Unclear | Unclear | Unclear | Unclear | High | Unclear | Low |
| Cusi 2013 | Thiazolidinediones Control: No intervention | Unclear | Unclear | Unclear | Unclear | Unclear | High | High | Low |
| Kakazu 2013 | Thiazolidinediones Control: No intervention | Unclear | Unclear | High | Unclear | High | High | Low | Low |
| Sunny 2015 | Thiazolidinediones Control: No intervention | Unclear | Unclear | Unclear | Unclear | Unclear | High | High | Low |
| Yan 2015 | Thiazolidinediones Control: No intervention | Low | Unclear | High | High | Unclear | High | Low | Low |
| Athyros 2006 | Intervention 1: Statins Intervention 2: Statins plus other cholesterol‐lowering agents Control: Other cholesterol‐lowering agents | Low | Low | High | High | Low | Low | High | Low |
| Razavizadeh 2012 | Antioxidants Control: Silymarin | Unclear | Unclear | Low | Low | Unclear | High | Unclear | Low |
| Hajiaghamohammadi 2012 | Thiazolidinediones Sulphonylureas Control: Silymarin | Unclear | Unclear | Unclear | Unclear | Low | High | Low | Low |
| Siddique 2015 | Thiazolidinediones Control: Statins | Unclear | Unclear | Unclear | Unclear | Unclear | High | Unclear | Low |
| Klyarytskaya 2015 | Renin‐angiotensin‐aldosterone system inhibitor plus statins plus antioxidants Control: Statins plus antioxidants | Unclear | Unclear | High | High | Unclear | High | Unclear | Low |
| Askarimoghadam 2013 | Sulphonylureas plus antioxidants Control: Sulphonylureas | Unclear | Unclear | High | High | Unclear | High | Unclear | Low |
| Song 2014 | Sulphonylureas plus other anti‐diabetes medication Control: Sulphonylureas | Low | Unclear | Unclear | Unclear | Unclear | High | Unclear | Low |
| Sharma 2012 | Pentoxifylline Control: Thiazolidinediones | Low | Low | High | High | High | High | Unclear | Low |
| Omer 2010 | Sulphonylureas Control: Thiazolidinediones | Unclear | Unclear | High | High | High | High | Unclear | Low |
| Razavizade 2013 | Sulphonylureas Control: Thiazolidinediones | Low | Low | Low | Low | Low | Low | Low | Low |
| Torres 2011 | Intervention 1: Thiazolidinediones plus renin‐angiotensin‐aldosterone system inhibitor Intervention 2: Thiazolidinediones plus sulphonylureas Control: Thiazolidinediones | Low | Low | High | High | High | High | High | Low |
Allocation
We assessed 27 trials at low risk of bias due to adequate reporting and application of random sequence generation (Harrison 2003; Merat 2003; Mendez‐Sanchez 2004; Uygun 2004; Athyros 2006; Belfort 2006; Aithal 2008; Haukeland 2009; Shields 2009; Sanyal 2010; Polyzos 2011; Torres 2011; Van Wagner 2011; Sharma 2012; Gianturco 2013; Magosso 2013; Razavizade 2013; Basu 2014; Song 2014; Stefan 2014; Aller 2015; Loomba 2015; Neuschwander‐Tetri 2015; Yan 2015; Alam 2016; Armstrong 2016; Ratziu 2016). The remainder were assessed at unclear risk of bias.
We assessed 26 trials at low risk of bias due to allocation concealment (Harrison 2003; Merat 2003; Lindor 2004; Athyros 2006; Belfort 2006; Dufour 2006; Aithal 2008; Haukeland 2009; Shields 2009; Sanyal 2010; Torres 2011; Van Wagner 2011; Fogari 2012; Sharma 2012; Gianturco 2013; Magosso 2013; Mudaliar 2013; Razavizade 2013; Basu 2014; Ratziu 2014; Stefan 2014; Loomba 2015; Neuschwander‐Tetri 2015; Alam 2016; Armstrong 2016; Ratziu 2016). The remainder were assessed at unclear risk of bias.
We found that 21 trials were at low risk of bias due to random sequence generation and allocation concealment (Harrison 2003; Merat 2003; Athyros 2006; Belfort 2006; Aithal 2008; Haukeland 2009; Shields 2009; Sanyal 2010; Torres 2011; Van Wagner 2011; Sharma 2012; Gianturco 2013; Magosso 2013; Razavizade 2013; Basu 2014; Stefan 2014; Loomba 2015; Neuschwander‐Tetri 2015; Alam 2016; Armstrong 2016; Ratziu 2016).
Blinding
We assessed 35 trials at low risk of performance bias: both participants and healthcare providers were blinded (Santos 2003; Harrison 2003; Merat 2003; Lindor 2004; Mendez‐Sanchez 2004; Belfort 2006; Cui 2006; Dufour 2006; Lewis 2006; Aithal 2008; Ratziu 2008; Gastaldelli 2009; Haukeland 2009; Nelson 2009; Leuschner 2010; Sanyal 2010; Foster 2011; Ratziu 2011; Sofer 2011; Van Wagner 2011; Fogari 2012; Razavizadeh 2012; Gianturco 2013; Magosso 2013; Mudaliar 2013; Razavizade 2013; Ratziu 2014; Safadi 2014; Stefan 2014; Chan 2015; Loomba 2015; Neuschwander‐Tetri 2015; Shiffman 2015; Armstrong 2016; Ratziu 2016). We found that 18 trials were at high risk of performance bias (Kugelmas 2003; Uygun 2004; Bugianesi 2005; Ersoz 2005; Athyros 2006; Omer 2010; Torres 2011; Sharma 2012; Askarimoghadam 2013; Basu 2013; Kakazu 2013; Basu 2014; Kedarisetty 2014; Klyarytskaya 2015; Wang 2015; Yan 2015; Alam 2016; Parikh 2016). The remainder were at unclear risk of bias.
Our assessment found that 39 trials were at low risk of detection bias (Santos 2003; Harrison 2003; Merat 2003; Lindor 2004; Mendez‐Sanchez 2004; Belfort 2006; Cui 2006; Dufour 2006; Lewis 2006; Aithal 2008; Ratziu 2008; Gastaldelli 2009; Gomez 2009; Haukeland 2009; Nar 2009; Nelson 2009; Shields 2009; Garinis 2010; Leuschner 2010; Sanyal 2010; Foster 2011; Ratziu 2011; Sofer 2011; Van Wagner 2011; Fogari 2012; Razavizadeh 2012; Gianturco 2013; Magosso 2013; Mudaliar 2013; Razavizade 2013; Ratziu 2014; Safadi 2014; Stefan 2014; Chan 2015; Loomba 2015; Neuschwander‐Tetri 2015; Shiffman 2015; Armstrong 2016; Ratziu 2016). We found that 16 trials were at high risk of detection bias (Uygun 2004; Bugianesi 2005; Ersoz 2005; Athyros 2006; Omer 2010; Torres 2011; Sharma 2012; Askarimoghadam 2013; Basu 2013; Basu 2014; Kedarisetty 2014; Klyarytskaya 2015; Wang 2015; Yan 2015; Alam 2016; Parikh 2016). The remainder were at unclear risk of bias.
Thirty‐five trials were assessed at low risk of performance and detection bias (Santos 2003; Harrison 2003; Merat 2003; Lindor 2004; Mendez‐Sanchez 2004; Belfort 2006; Cui 2006; Dufour 2006; Lewis 2006; Aithal 2008; Ratziu 2008; Gastaldelli 2009; Haukeland 2009; Nelson 2009; Leuschner 2010; Sanyal 2010; Foster 2011; Ratziu 2011; Sofer 2011; Van Wagner 2011; Fogari 2012; Razavizadeh 2012; Gianturco 2013; Magosso 2013; Mudaliar 2013; Razavizade 2013; Ratziu 2014; Safadi 2014; Stefan 2014; Chan 2015; Loomba 2015; Neuschwander‐Tetri 2015; Shiffman 2015; Armstrong 2016; Ratziu 2016). The remainder were at unclear or high risk of performance and detection bias.
Incomplete outcome data
Eighteen trials were at low risk of bias due to missing outcome and hence attrition bias (Athyros 2006; Aithal 2008; Gomez 2009; Nelson 2009; Shields 2009; Leuschner 2010; Sanyal 2010; Ratziu 2011; Sofer 2011; Hajiaghamohammadi 2012; Magosso 2013; Mudaliar 2013; Razavizade 2013; Basu 2014; Kedarisetty 2014; Aller 2015; Neuschwander‐Tetri 2015; Armstrong 2016). We found that 26 trials were at high risk of bias due to missing outcome data (Harrison 2003; Merat 2003; Lindor 2004; Mendez‐Sanchez 2004; Uygun 2004; Ersoz 2005; Belfort 2006; Dufour 2006; Ratziu 2008; Harrison 2009; Haukeland 2009; Garinis 2010; Omer 2010; Torres 2011; Van Wagner 2011; Fogari 2012; Sharma 2012; Gianturco 2013; Kakazu 2013; Ratziu 2014; Safadi 2014; Solhi 2014; Stefan 2014; Alam 2016; Parikh 2016; Ratziu 2016). The remainder were at unclear risk of bias.
Selective reporting
Published protocols were not available for any of the included trials. We assessed that 11 trials were at low risk of bias due to selecting outcome reporting bias (Athyros 2006; Aithal 2008; Leuschner 2010; Polyzos 2011; Ratziu 2011; Magosso 2013; Mudaliar 2013; Razavizade 2013; Neuschwander‐Tetri 2015; Alam 2016; Armstrong 2016). The remainder were at high risk of selective outcome reporting bias.
Other potential sources of bias
Twelve trials reported not receiving any additional funding or support from parties with vested interest in the results and were considered to be at low risk of for‐profit bias (Kugelmas 2003; Merat 2003; Morita 2005; Nelson 2009; Polyzos 2011; Fogari 2012; Hajiaghamohammadi 2012; Kakazu 2013; Razavizade 2013; Yan 2015; Alam 2016; Parikh 2016). Twenty‐six trials were partly‐ or fully‐funded by pharmaceutical companies that would benefit from trial results (Santos 2003; Lindor 2004; Athyros 2006; Belfort 2006; Dufour 2006; Aithal 2008; Ratziu 2008; Gomez 2009; Haukeland 2009; Leuschner 2010; Sanyal 2010; Ratziu 2011; Torres 2011; Cusi 2013; Magosso 2013; Mudaliar 2013; Basu 2014; Ratziu 2014; Safadi 2014; Stefan 2014; Loomba 2015; Neuschwander‐Tetri 2015; Shiffman 2015; Sunny 2015; Armstrong 2016; Ratziu 2016). Sources of funding was not reported in 39 trials.
No trials were at risk of bias due to other factors such as baseline differences, stopping trials early, or inappropriate controls.
Effects of interventions
See: Table 1; Table 2; Table 3
Primary outcomes
Mortality at maximal follow up
A total of 11 trials including 1222 participants reported deaths after follow‐up periods from 1 month to 18 months (Athyros 2006; Aithal 2008; Leuschner 2010; Polyzos 2011; Ratziu 2011; Magosso 2013; Mudaliar 2013; Razavizade 2013; Neuschwander‐Tetri 2015; Alam 2016; Armstrong 2016). There were only two deaths in participants who received bile acid (2/141 = 1.4%). These deaths were reported in the trial which followed‐up participants for about 17 months (Neuschwander‐Tetri 2015). There were no deaths in any other trials or interventions (Analysis 1.1). Since there were few events, we have not presented the short‐term mortality (up to 1 year) and medium‐term mortality (1 to 5 years) separately.
1.1. Analysis.
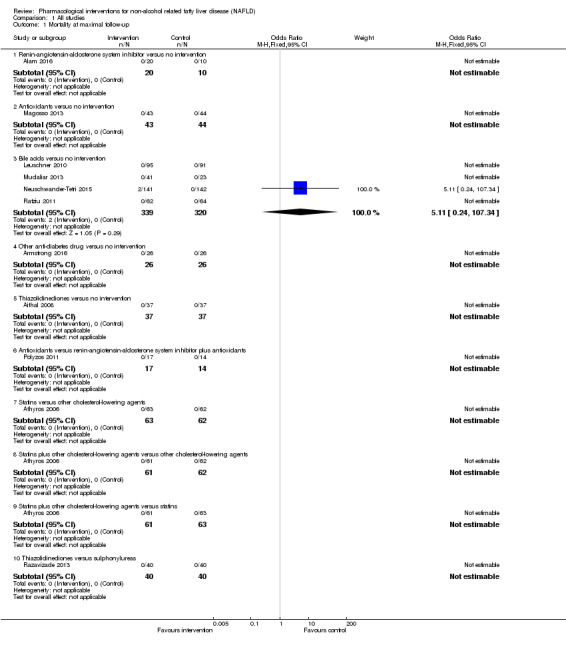
Comparison 1 All studies, Outcome 1 Mortality at maximal follow‐up.
Proportion of participants with serious adverse events
A total of 19 trials including 1748 participants reported proportions of serious adverse events (Merat 2003; Athyros 2006; Aithal 2008; Hashemi 2009; Nelson 2009; Jin 2010; Polyzos 2011; Van Wagner 2011; Magosso 2013; Mudaliar 2013; Razavizade 2013; Ratziu 2014; Safadi 2014; Stefan 2014; Aller 2015; Neuschwander‐Tetri 2015; Alam 2016; Armstrong 2016; Ratziu 2016). The proportion of people with serious adverse events seemed lower in people who received phosphodiesterase type 4 inhibitor (1/66 (1.5%)) versus no intervention (4/30 (13.3%)) (OR 0.10, 95% CI 0.01 to 0.94; 96 participants; 1 trial). There was no evidence of differences in other comparisons (Analysis 1.2).
1.2. Analysis.
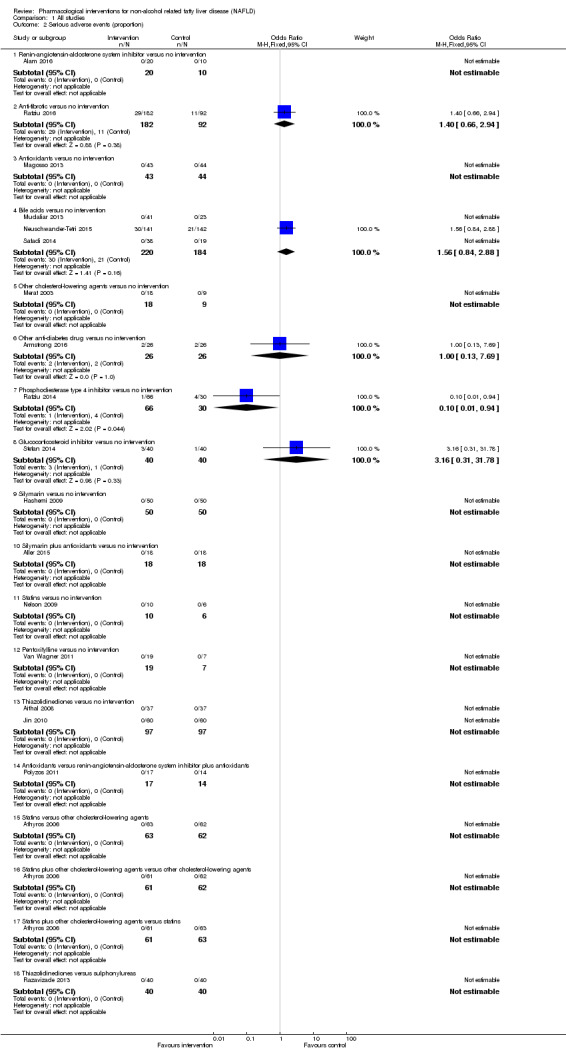
Comparison 1 All studies, Outcome 2 Serious adverse events (proportion).
Number of serious adverse events
A total of 18 trials including 1693 participants reported numbers of serious adverse events (Merat 2003;Athyros 2006; Aithal 2008; Hashemi 2009; Nelson 2009; Jin 2010; Sanyal 2010; Polyzos 2011; Magosso 2013; Mudaliar 2013; Razavizade 2013; Ratziu 2014; Safadi 2014; Stefan 2014; Aller 2015; Neuschwander‐Tetri 2015; Alam 2016; Armstrong 2016). There was no evidence of difference in other comparisons (Analysis 1.3).
1.3. Analysis.
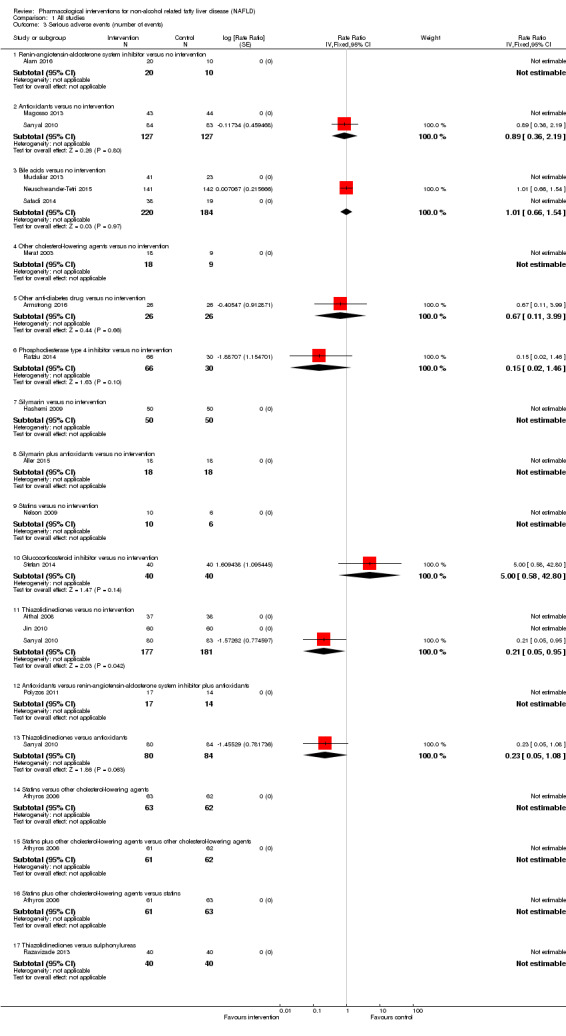
Comparison 1 All studies, Outcome 3 Serious adverse events (number of events).
Proportion of participants with any type of adverse event
A total of 17 trials including 1606 participants reported proportions of adverse events (Merat 2003; Lindor 2004; Ersoz 2005; Athyros 2006; Aithal 2008; Nelson 2009; Jin 2010; Sharma 2012; Magosso 2013; Mudaliar 2013; Razavizade 2013; Basu 2014; Ratziu 2014; Stefan 2014; Aller 2015; Armstrong 2016; Parikh 2016). The proportion of people who experienced adverse events was higher in the phosphodiesterase type 4 inhibitor group (54/66 (81.8%)) versus no intervention (18/30 (60.0%)) (OR 3.00, 95% CI 1.15 to 7.85; 96 participants; 1 trial). There was no evidence of differences in other comparisons (Analysis 1.4).
1.4. Analysis.
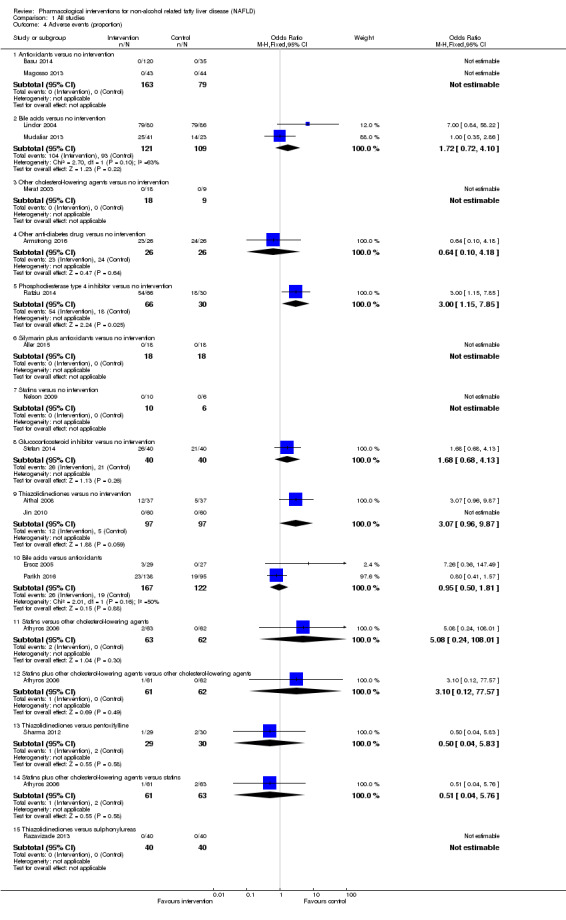
Comparison 1 All studies, Outcome 4 Adverse events (proportion).
Number of any type of adverse event
A total of 22 trials including 2319 participants reported numbers of adverse events (Merat 2003; Lindor 2004; Ersoz 2005; Athyros 2006; Aithal 2008; Nelson 2009; Jin 2010; Leuschner 2010; Sanyal 2010; Ratziu 2011; Van Wagner 2011; Sharma 2012; Magosso 2013; Mudaliar 2013; Razavizade 2013; Basu 2014; Stefan 2014; Aller 2015; Loomba 2015; Neuschwander‐Tetri 2015; Yan 2015; Armstrong 2016). The rate of adverse events was higher in participants who received bile acid (rate ratio 1.19, 95% CI 1.06 to 1.33; 825 participants; 5 trials; I² = 51%) and glucocorticosteroid inhibitor (rate ratio 1.56, 95% CI 1.05 to 2.31; 80 participants; 1 trial) versus no intervention. There was no evidence of differences in any other comparisons (Analysis 1.5).
1.5. Analysis.
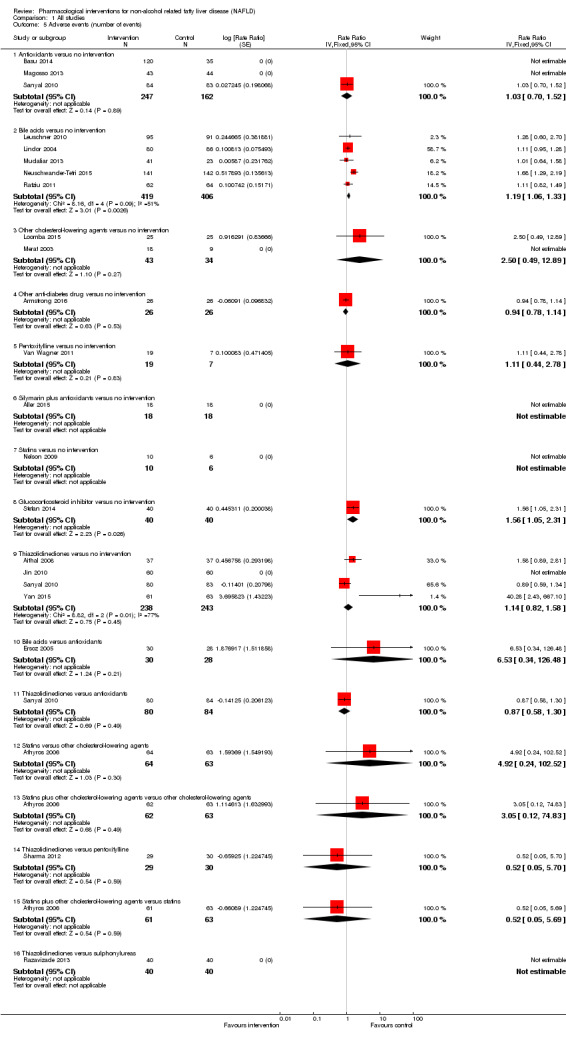
Comparison 1 All studies, Outcome 5 Adverse events (number of events).
Health‐related quality of life
No included trial reported on quality of life.
Secondary outcomes
Liver transplantation
A total of nine trials including 639 participants reported proportions of people who underwent liver transplantation (Athyros 2006; Belfort 2006; Aithal 2008; Polyzos 2011; Magosso 2013; Razavizade 2013; Stefan 2014; Alam 2016; Armstrong 2016). No trial participants required liver transplantation during the follow‐up period. Therefore, the outcome 'time‐to‐liver transplantation' was not applicable in these trials. None of the remaining trials reported time‐to‐liver transplantation.
Decompensated liver disease
A total of nine trials including 765 participants reported decompensated liver disease (Athyros 2006; Aithal 2008; Polyzos 2011; Ratziu 2011; Magosso 2013; Razavizade 2013; Stefan 2014; Alam 2016; Armstrong 2016). No trial participants developed decompensated liver disease during the follow‐up period. Therefore, the outcome 'time‐to‐decompensated liver disease' was not applicable in these trials. None of the remaining trials reported time‐to‐decompensated liver disease.
Cirrhosis
A total of 11 trials including 798 participants reported proportions of people who developed cirrhosis (Athyros 2006; Belfort 2006; Aithal 2008; Haukeland 2009; Polyzos 2011; Magosso 2013; Razavizade 2013; Stefan 2014; Chan 2015; Alam 2016; Armstrong 2016). Overall 4/236 (1.7%) participants in the no intervention group developed cirrhosis. There was no evidence of difference in other comparisons (Analysis 1.6). None of the trials reported time‐to‐cirrhosis.
1.6. Analysis.
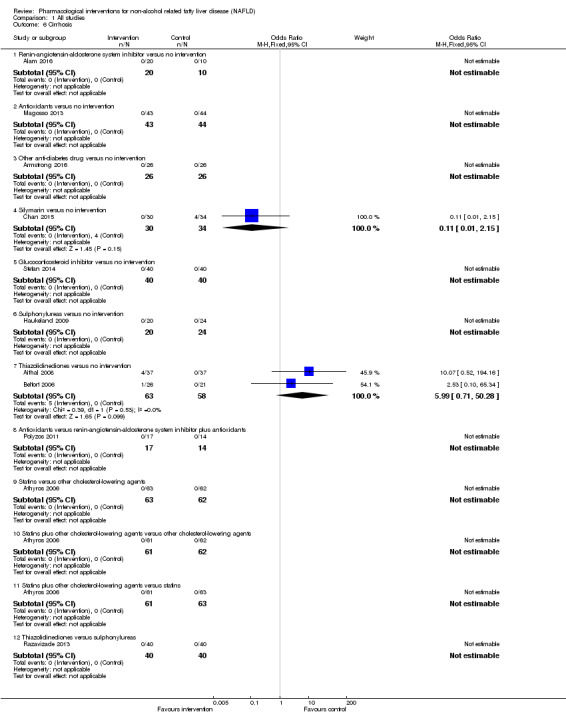
Comparison 1 All studies, Outcome 6 Cirrhosis.
Resolution of fatty liver disease
A total of 16 trials including 1343 participants reported proportions of people whose fatty liver disease resolved (Harrison 2003; Ersoz 2005; Athyros 2006; Belfort 2006; Aithal 2008; Haukeland 2009; Nar 2009; Garinis 2010; Sanyal 2010; Torres 2011; Magosso 2013; Chan 2015; Loomba 2015; Neuschwander‐Tetri 2015; Alam 2016; Armstrong 2016).
Resolution rates were higher in participants who received antioxidants (adjusted proportion: 32.0%) versus no intervention (26/149 (17.4%)) (OR 2.23, 95% CI 1.28 to 3.87; 299 participants; 3 trials; I² = 0%). Resolution of fatty liver disease also seemed higher in participants who received other anti‐diabetes medications (9/23 (39.1%)) versus no intervention (2/22 (9.1%)) (OR 6.43, 95% CI 1.20 to 34.41; 45 participants; 1 trial).
The proportion of people among whom resolution of fatty liver disease seemed higher in those who received statins (42/63 (66.7%)) versus other cholesterol‐lowering agents (26/62 (41.9%)) (OR 2.77, 95% CI 1.34 to 5.73; 125 participants; 1 trial). This effect also seemed higher in participants who received statins plus other cholesterol‐lowering agents (43/61 (70.5%)) versus other cholesterol‐lowering agents (26/62 (41.9%)) (OR 3.31, 95% CI 1.57 to 6.98; 123 participants; 1 trial).
There was no evidence of differences in any other comparisons.
Unvalidated surrogate outcomes
We could not perform a meta‐analysis because many trials that reported fibrosis scores and NAFLD activity score did not provide mean or standard deviation or both. A summary of differences between fibrosis scores and NAFLD Activity Scores (NAS) are presented (Appendix 4; Appendix 5). None of the interventions were consistently associated with decreased scores.
Subgroup analyses
Because of the paucity of data, we did not use the tests for subgroup differences. However, we presented analyses of the subsets for participants with non‐alcohol related steatohepatitis only, those with diabetes mellitus only, and those who did not have diabetes mellitus only.
Non‐alcohol related steatohepatitis
See Analysis 2.1; Analysis 2.2; Analysis 2.3; Analysis 2.4; Analysis 2.5; Analysis 2.6.
2.1. Analysis.
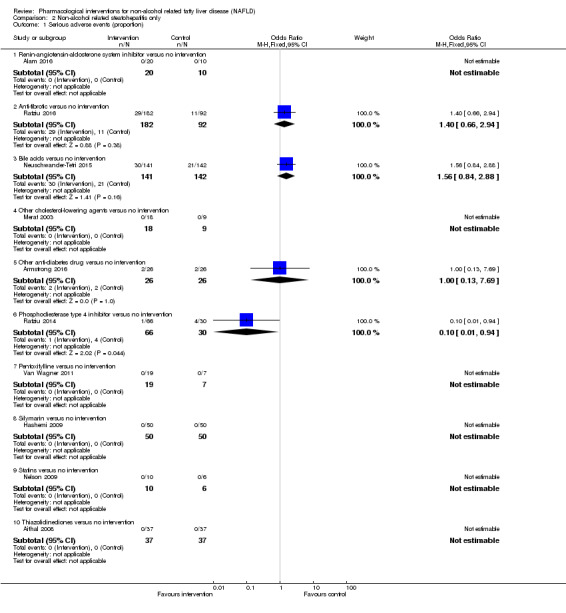
Comparison 2 Non‐alcohol related steatohepatitis only, Outcome 1 Serious adverse events (proportion).
2.2. Analysis.
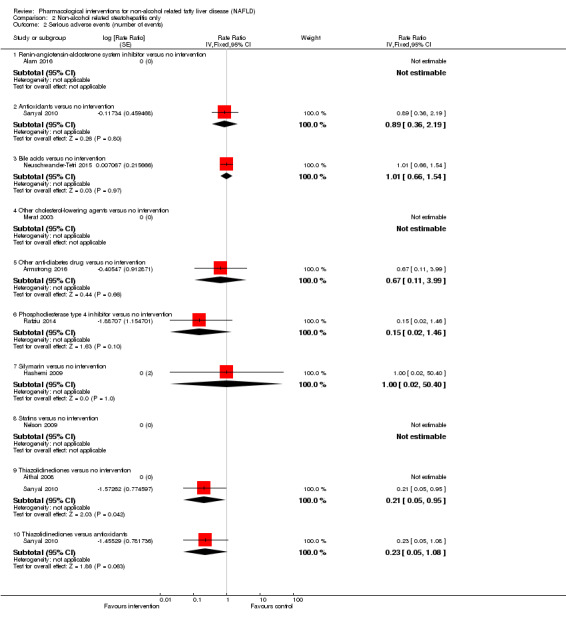
Comparison 2 Non‐alcohol related steatohepatitis only, Outcome 2 Serious adverse events (number of events).
2.3. Analysis.
Comparison 2 Non‐alcohol related steatohepatitis only, Outcome 3 Adverse events (proportion).
2.4. Analysis.
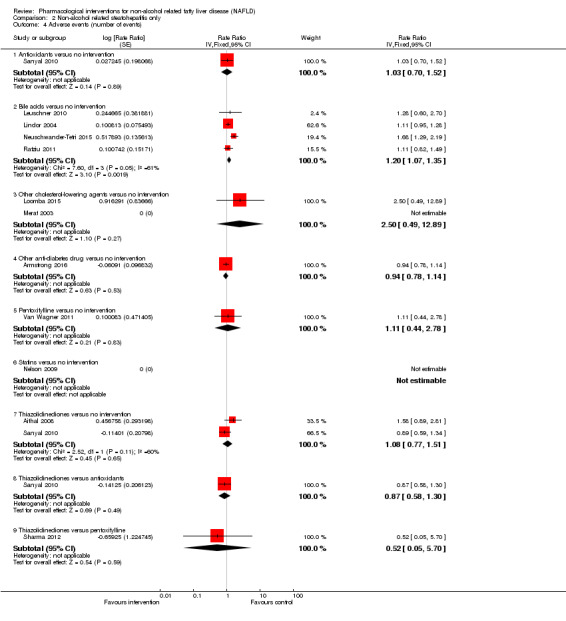
Comparison 2 Non‐alcohol related steatohepatitis only, Outcome 4 Adverse events (number of events).
2.5. Analysis.
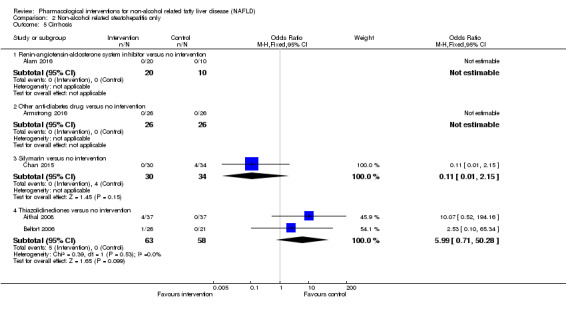
Comparison 2 Non‐alcohol related steatohepatitis only, Outcome 5 Cirrhosis.
2.6. Analysis.
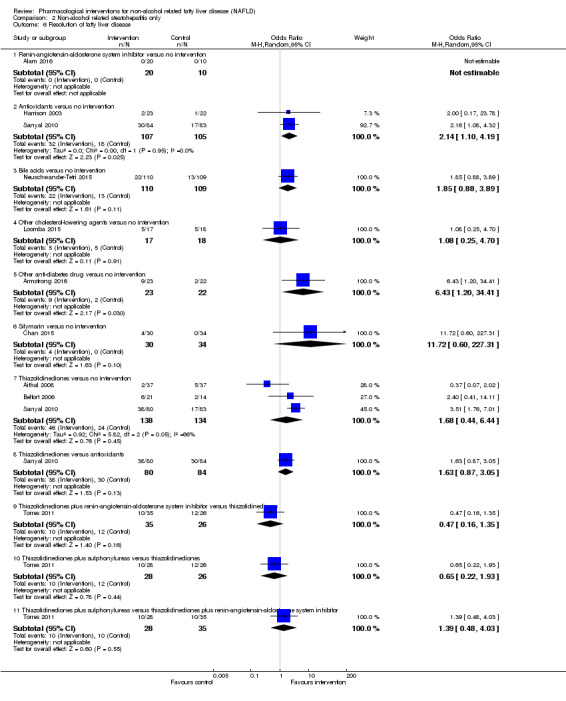
Comparison 2 Non‐alcohol related steatohepatitis only, Outcome 6 Resolution of fatty liver disease.
There was evidence of a difference between participants who received phosphodiesterase type 4 inhibitor and those who received no intervention for proportion of serious adverse events. There were fewer adverse events in participants who received phosphodiesterase type 4 inhibitor (rate ratio 0.21, 95% CI 0.05 to 0.95; 239 participants; 2 trials; I² not assessable ‐ only 1 trial contributed data to the analysis).
There was evidence of a difference between participants who received phosphodiesterase type 4 inhibitor group and no intervention for proportion of any adverse events. There were more adverse events in participants who received phosphodiesterase type 4 inhibitor (OR 3.00, 95% CI 1.15 to 7.85; 96 participants; 1 trial). There was evidence of a difference between those who received bile acids and no intervention for proportion of any adverse events; there were more adverse events in participants who received bile acids (rate ratio 1.20, 95% CI 1.07 to 1.35; 761 participants; 4 trials; I² = 61%).
There was evidence of a difference between participants who received antioxidants and no intervention for proportion of people with resolution of fatty liver disease. Results were in favour of the antioxidants (OR 2.14, 95% CI 1.10 to 4.19; 212 participants; 2 trials; I² = 0%). There was also evidence of a difference between participants who received other anti‐diabetes medications and no intervention favouring other anti‐diabetes medications (OR 6.43, 95% CI 1.20 to 34.41; 45 participants; 1 trial). There was no evidence of differences in other comparisons.
Participants with diabetes mellitus
See Analysis 3.1; Analysis 3.2; Analysis 3.3; Analysis 3.4.
3.1. Analysis.
Comparison 3 People with diabetes mellitus only, Outcome 1 Serious adverse events (proportion).
3.2. Analysis.
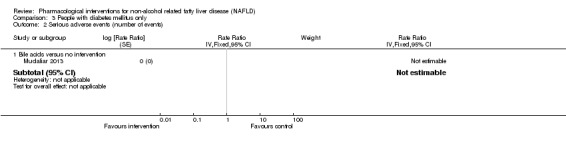
Comparison 3 People with diabetes mellitus only, Outcome 2 Serious adverse events (number of events).
3.3. Analysis.
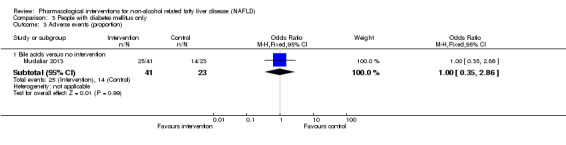
Comparison 3 People with diabetes mellitus only, Outcome 3 Adverse events (proportion).
3.4. Analysis.
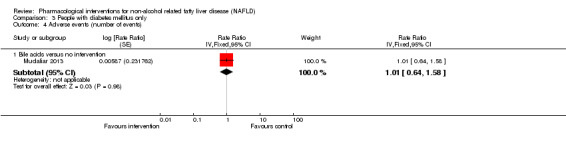
Comparison 3 People with diabetes mellitus only, Outcome 4 Adverse events (number of events).
There was no evidence of differences in any of the comparisons.
Participants without diabetes mellitus
See Analysis 4.1; Analysis 4.2; Analysis 4.3; Analysis 4.4; Analysis 4.5; Analysis 4.6.
4.1. Analysis.
Comparison 4 People without diabetes mellitus only, Outcome 1 Serious adverse events (proportion).
4.2. Analysis.
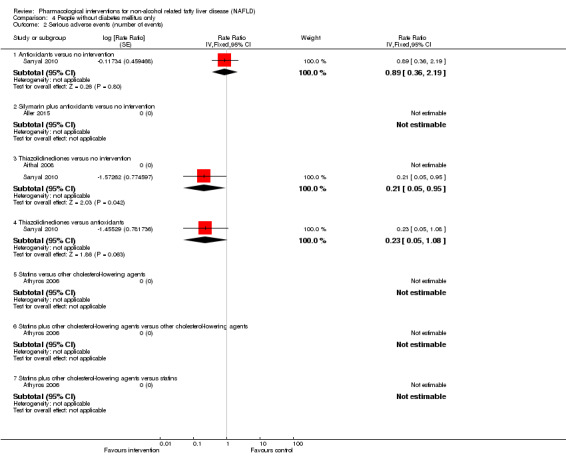
Comparison 4 People without diabetes mellitus only, Outcome 2 Serious adverse events (number of events).
4.3. Analysis.
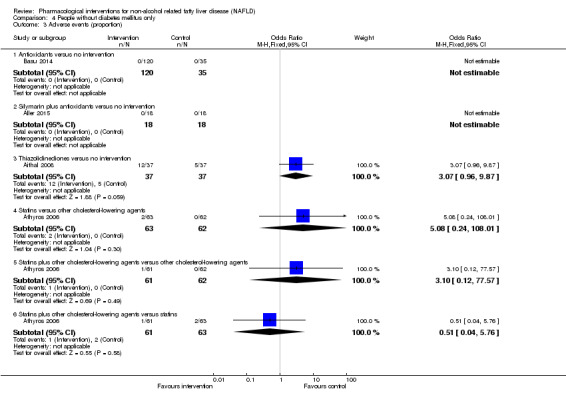
Comparison 4 People without diabetes mellitus only, Outcome 3 Adverse events (proportion).
4.4. Analysis.
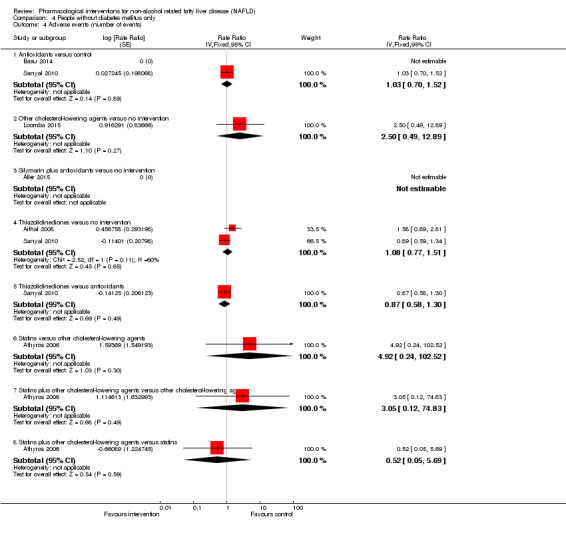
Comparison 4 People without diabetes mellitus only, Outcome 4 Adverse events (number of events).
4.5. Analysis.
Comparison 4 People without diabetes mellitus only, Outcome 5 Cirrhosis.
4.6. Analysis.
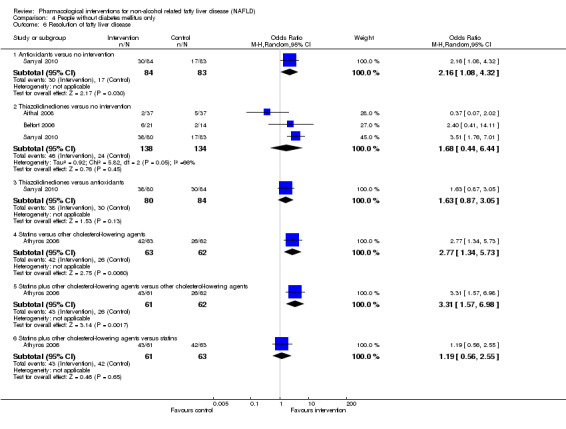
Comparison 4 People without diabetes mellitus only, Outcome 6 Resolution of fatty liver disease.
There was evidence of a difference between participants who received thiazolidinediones and no intervention for number of serious adverse events. There were fewer adverse events in those who received thiazolidinediones (rate ratio 0.21, 95% CI 0.05 to 0.95; 239 participants; 2 trials; I² not assessable ‐ only one trial contributed to the analysis). There was evidence of a difference between those who received antioxidants and no intervention for proportion of people with resolution of fatty liver disease favouring antioxidants (OR 2.16, 95% CI 1.08 to 4.32; 167 participants; 1 trial).
There was evidence of a difference between participants who received statins and other cholesterol‐lowering agents for proportion of people with resolution of fatty liver disease, which favoured statins (OR 2.77, 95% CI 1.34 to 5.73; 125 participants; 1 trial). There was also evidence of a difference between those who received statins plus other cholesterol‐lowering agents and other cholesterol‐lowering agents alone for proportion of people with resolution of fatty liver disease favouring statins plus other cholesterol‐lowering agents (OR 3.31, 95% CI 1.57 to 6.98; 123 participants; 1 trial).
Sensitivity analysis
We did not perform a sensitivity analysis based on different scenarios of imputation because there were too few data to inform analyses. We did not impute standard deviation; therefore, we did not perform a sensitivity analysis to assess the impact of imputing the standard deviation.
Reporting bias
We did not assess reporting bias by creating a funnel plot because there were too few trials in each comparison.
Fixed‐effect versus random‐effects models
The interpretation of results was not altered based on the model used for analysis except for thiazolidinediones versus no intervention; there was no evidence of difference according to the random‐effects model analysis (OR 1.68, 95% CI 0.44 to 6.44; participants = 272; trials = 3; I² = 66%). However, the proportion of people with higher rates of resolution of fatty liver disease seemed higher in people who received thiazolidinediones compared with no intervention (OR 2.41, 95% CI 1.36 to 4.28; participants = 272; trials = 3; I² = 66%).
Required information size calculations and Trial Sequential Analysis
The required information size for identifying a 20% relative risk reduction in the different outcomes based on an alpha error of 5%, a beta error of 20%, and the control group proportion observed in trials were as follows.
Mortality at maximal follow‐up (control group proportion: 0%): not estimable.
Serious adverse events (proportion) (control group proportion: 6.4%): 10,402 participants.
Adverse events (proportion) (control group proportion: 38.9%): 1178 participants.
Liver transplantation (control group proportion: 0%): not estimable.
Decompensated liver disease (control group proportion: 0%): not estimable.
Cirrhosis (control group proportion: 1.7%): 40,922 participants.
Resolution of fatty liver disease (control group proportion: 12.9%): 4838 participants.
These sample sizes were uncorrected for heterogeneity. In the presence of heterogeneity, for example, in the presence of a heterogeneity of 25%, the required information size for adverse events (proportion) is 1178/(1‐0.25) = 1571 participants.
Very few of the required sample sizes were reached in the comparisons in which there was no evidence of difference. Therefore, beta error could not be excluded in these comparisons.
Two or more trials contributed to the analyses of the following outcomes.
Adverse events (proportion): bile acids versus no intervention.
Adverse events (proportion): bile acids versus antioxidants.
Cirrhosis: thiazolidinediones versus no intervention.
Resolution of fatty liver disease: antioxidants versus no intervention.
Resolution of fatty liver disease: sulphonylureas versus no intervention.
Resolution of fatty liver disease: thiazolidinediones versus no intervention.
The accrued sample size was too small to draw trial sequential monitoring boundaries (Figure 4; Figure 5). The cumulative Z‐curve did not cross the conventional boundaries, except for resolution of fatty liver disease when antioxidants were compared with no intervention. The Trial Sequential Analysis‐adjusted confidence intervals could not be calculated because of the small accrued sample sizes.
4.
Trial Sequential Analysis (TSA) for adverse events (proportion) and cirrhosis for different comparisons. TSA was performed using an alpha error of 2.5% for adverse events (proportion) and 2% for cirrhosis, power of 90% (10% beta error), 20% relative risk reduction (RRR), control group proportion (Pc) observed in the trials, and the diversity observed in the meta‐analysis. The trial sequential monitoring boundaries were not drawn because the accrued sample sizes (adverse events (proportion): bile acids versus no intervention = 230 participants; adverse events (proportion): bile acids versus antioxidants = 289 participants; cirrhosis: thiazolidinediones versus no intervention = 121 participants) were only fractions of the diversity‐adjusted required information size (DARIS) (adverse events (proportion): bile acids versus no intervention = 52,522 participants; adverse events (proportion): bile acids versus no intervention = 6141 participants; cirrhosis: thiazolidinediones versus no intervention = 67,859 participants). The cumulative Z‐curve (blue line) does not cross the conventional P boundary (dotted green lines). There was a high risk of random error in all comparisons.
5.
Trial Sequential Analysis (TSA) for adverse events (proportion) and cirrhosis for different comparisons. TSA was performed using an alpha error of 2%, 90% power (10% beta error), 20% relative risk reduction (RRR), control group proportion (Pc = 12.9%) observed in the trials, and the diversity‐observed in the meta‐analysis. The trial sequential monitoring boundaries were not drawn because the accrued sample sizes (antioxidants versus no intervention = 299 participants; sulphonylureas versus no intervention = 123 participants; thiazolidinediones versus no intervention = 272 participants) were only fractions of the diversity adjusted required information size (DARIS) (antioxidants versus no intervention = 8028 participants; sulphonylureas versus no intervention = 11,394 participants; thiazolidinediones versus no intervention = 39,680 participants). The cumulative Z‐curve (blue line) does not cross the conventional P boundary (dotted green lines). There was a high risk of random error in all comparisons.
Quality of evidence
The overall quality of evidence was very low for all outcomes (Table 1). The quality of evidence was downgraded because of high risk of bias (downgraded by one level), small sample sizes for all outcomes with wide confidence intervals or lack of events (downgraded by two levels for imprecision), and heterogeneity (downgraded by one level for inconsistency) for some outcomes.
Discussion
Summary of main results
We included 76 trials (6207 participants); data from 3829 participants in 41 trials were included in one or more analyses of review outcomes. Although we intended to perform a network meta‐analysis, we did not report results because there was only one closed loop (i.e. comparisons for which there were estimates from direct comparisons and indirect comparisons) for only one outcome (number of adverse events) and there was evidence of inconsistency. Therefore, we reported results of direct pair‐wise comparisons and frequentist meta‐analysis.
There was no evidence of any reduction in mortality or any of the known complications of non‐alcoholic fatty liver disease (NAFLD), that is, cirrhosis, decompensated cirrhosis, or requirement for liver transplantation. The follow‐up period in trials ranged from 1 month to 24 months, and most trials had follow‐up periods of less than 12 months, which is not enough time for NAFLD or non‐alcoholic steatohepatitis (NASH) complications to develop. As a result, the proportion of people who developed complications was very low, regardless of whether or not they received an intervention. Furthermore, the duration of follow‐up was the same as the treatment period in most trials. It is not clear how long the interventions should continue to provide clinical improvement.
The Federal Drug Agency (FDA) in the US consented to the use of the two unvalidated surrogate outcomes 'resolution of steatohepatitis without worsening of fibrosis' or 'improvement in the fibrosis score without worsening of the steatohepatitis' or both at the time of approval of drugs, through an accelerated access pathway, with sponsor obligation to conduct a post‐market trial to demonstrate that their improvement translated into a clinically meaningful benefit to patients (Sanyal 2016). However, there is no evidence that this is a good surrogate outcome (Gluud 2007). We explored evidence of differences in histological outcomes, but we did not find any consistent pattern of improvement in histological outcomes as shown in Appendix 4 and Appendix 5.
Future randomised clinical trials ought to be adequately powered to measure differences in clinically important outcomes such as mortality, health‐related quality of life, cirrhosis, decompensated cirrhosis, and liver transplantation.
Overall completeness and applicability of evidence
The trials included people with and without NASH and those with and without diabetes mellitus but most excluded people with advanced liver cirrhosis and those with other liver diseases. Therefore, findings from this review are applicable to people with NAFLD who do not have advanced liver cirrhosis or those without other co‐existing liver diseases.
Quality of the evidence
The overall quality of evidence was assessed as very low for all outcomes. Major reasons for downgrading evidence quality were high risk of bias, especially excluding participants from analyses after randomisation; small sample sizes, and gross imprecision. Overall, there were serious concerns about whether the effect estimates observed were accurate.
Potential biases in the review process
We applied standard Cochrane methods to conduct this review and performed thorough searches of the literature. However, the period searched included the pre‐mandatory trial registration era and it is possible that some trials on interventions that were not effective or were harmful were not reported. Publication bias added to the imprecision of our findings with greater risk of overestimating benefits and underestimating harms.
We planned to perform a network meta‐analysis. However, we found insufficient information, and it was not possible to assess if potential effect modifiers were similar across different comparisons. There were also differences in potential effect modifiers when information was available, and we were therefore unable to conduct a network meta‐analysis. There was evidence of inconsistency and differences in effect estimates obtained from direct comparisons and network meta‐analysis results. Results from the network meta‐analysis were not reported because they may not be reliable.
A limitation of the review was the high risk of bias in the included trials resulting in assessment of low or very low quality of evidence. The review was further limited by a paucity of data. There were few trials included in each comparison, many of which included only one trial. This made assessment of whether effect estimates were reproducible difficult. and also makes the assessment of inconsistency underpowered in those comparisons with more than one trial. Lack of evidence of inconsistency should not be considered the same as lack of inconsistency. This paucity of data decreases the confidence in the results.
We excluded studies that compared variations in the included interventions, and hence, this review does not provide information on whether particular variations of interventions are better than others.
Moreover, we only included randomised clinical trials that were known to focus mostly on benefits and did not collect and report harms in a detailed manner. Accordingly, we may have missed a large number of studies that address reporting of harms. As a result, this review was biased toward reporting and analysing benefits. We did not search for interventions and trials registered with regulatory authorities (e.g. the USA FDA and the European Medicines Agency, etc). This approach may have missed trials (many of which are likely to be unpublished) to possibly influence making comparisons appear more advantageous. However, this is principally of academic interest only; we found no evidence of benefit for any intervention in people with primary biliary cholangitis, that is, there is no reason to suggest that any interventions should be used in routine clinical practice regardless of adverse event profiles.
In our results, we give some indication of how heterogeneity may further drive up the required information size of the meta‐analyses to make them robust to reject or accept plausible null hypothesis (Jakobsen 2014; Wetterslev 2017). Furthermore, we also totally and naïvely ignored the increased family‐wise error rate by using alpha of 5% or 2.5% in spite of our primary and secondary outcomes as well as plans on assessing outcomes at many time points, running substantial risks for committing type I error risks (Jakobsen 2014; Wetterslev 2017). In the future, we will consider these risks before we embark on the update and conduct analyses. However, revising the alpha level when there are only one or two outcomes that determine the use of treatment, particularly when they were not reported is contentious and is of academic interest only since the imprecision in GRADE and Trial Sequential Analyses using an alpha error of 5% already indicate high risk of random error.
We planned to perform a network meta‐analysis. However, it was not possible to assess whether the potential effect modifiers were similar across different comparisons. Performing a network meta‐analysis in this scenario can be misleading. Therefore, we did not perform the network meta‐analysis, and assessed the comparative benefits and harms of different interventions using standard Cochrane methodology.
Only a fraction of the required sample size was reached for all comparisons. There was insufficient information to determine effects of interventions unequivocally. Some interventions were found to have better resolution of fatty liver disease. The different studies reported resolution of fatty liver disease difference. There was also evidence of heterogeneity in the results in some of the comparisons (thiazolidinediones versus control). For the only comparison in which there was evidence of differences in the proportion of people with resolution of fatty liver disease and in which more than one trial was included, there was no evidence of heterogeneity despite the differences in the way that resolution of fatty liver disease was assessed. Although there was evidence of difference in the resolution of fatty liver disease, the Trial Sequential Analysis showed that the trial sequential monitoring boundaries were not crossed (Figure 4), indicating the high risks of random errors, in addition to the systematic errors in the trials included in the analysis. Therefore, there is a lot of uncertainty over these findings. In addition, there was no consistent evidence that one of the interventions improved the unvalidated surrogate outcomes such as fibrosis scores or NAFLD activity scores in histology, adding more uncertainty to the effectiveness of the interventions.
Agreements and disagreements with other studies or reviews
We identified two network meta‐analyses on this topic (Singh 2015; Sawangjit 2016) and several systematic reviews on the interventions included in this Cochrane Review (Lirussi 2007; Orlando 2007; Li 2011; Mahady 2011; Li 2013; Xiang 2013; Ji 2014). We agree with the finding reported by the authors of many of these reviews that further well‐designed randomised clinical trials are needed on this topic. We disagree with Singh 2015 which concluded that future trials of combination therapies targeting distinct histological features are warranted. There is no evidence that any of the histological features are valid surrogate outcomes (Gluud 2007).
We also disagree with Dongiovanni 2015 who suggested that statins may have a protective effect for people with NAFLD and Zhou 2016 who suggested that statins may prevent hepatocellular carcinoma in people who are at high risk of developing this disease. However, these suggestions are based on observational evidence and Dongiovanni 2015 used unvalidated surrogate histological markers to arrive at their conclusion.
Bile acids have not been shown to be harmful to treat other conditions (Gurung 2013; Saffioti 2017a; Saffioti 2017b) apart from alcoholic hepatitis (Buzetti 2017). We found that bile acids can increase rates of adverse events. The differences observed among reviews may be due to random error; observations were made in only a few participants. It is also possible that the harms of bile acids may differ among groups of patients. This is only of academic interest because there was no evidence that bile acids are beneficial for people with NAFLD.
Authors' conclusions
Implications for practice.
Due to the very low quality evidence, we are very uncertain about the effectiveness of pharmacological interventions for non‐alcohol related fatty liver disease including participants with steatohepatitis.
Implications for research.
Randomised clinical trials need to be conducted and reported according to the SPIRIT (Standard Protocol Items: Recommendations for Interventional Trials) statement (Chan 2013) and reported according to the CONSORT (Consolidated Standards for Reporting of Trials) statement (Schulz 2010). Future randomised clinical trials should be adequately powered, involve people who are generally seen in clinics rather than in highly selected participants, employ blinding, avoid post‐randomisation drop‐outs or planned cross‐overs. Future trials should be planned to investigate clinically important outcomes such as mortality, health‐related quality of life, cirrhosis, decompensated cirrhosis, and liver transplantation. NAFLD is a slowly progressing disease and expected liver‐related outcomes may be identified only on long‐term follow‐up or in very large cohorts. It may be difficult to design trials with sufficiently long follow‐up periods to identify the effects of pharmacological interventions on NAFLD.
What's new
| Date | Event | Description |
|---|---|---|
| 12 April 2017 | Amended | The Cochrane Central Editorial Unit requested removal of the 'attempted network meta‐analysis' phrase from the end of the review title, as this further description of the review might create confusion in the reader. Although we followed the planned methodology for network meta‐analysis, it was not possible to assess whether the potential effect modifiers were similar across different comparisons. Therefore, we did not perform the network meta‐analysis, and instead, assessed the comparative benefits and harms of different interventions using standard Cochrane methodology. |
Notes
Considerable overlap is evident between the 'Methods' sections of this review and those of other reviews written by the same group of authors.
Acknowledgements
We thank the Cochrane Comparing of Multiple Interventions Methods Group and the Cochrane Hepato‐Biliary Group for their support and advice.
Cochrane Review Group funding acknowledgement: The Danish State is the largest single funder of the Cochrane Hepato‐Biliary Group through its investment in The Copenhagen Trial Unit, Centre for Clinical Intervention Research, Rigshospitalet, Copenhagen University Hospital, Denmark. Disclaimer: The views and opinions expressed in this review are those of the authors and do not necessarily reflect those of the Danish State or The Copenhagen Trial Unit.
Peer reviewers: Andrea Cipriani; UK; Amedeo Lonardo, Italy; Richard Parker, UK. Contact editor: Christian Gluud, Denmark. Sign‐off editor: Christian Gluud, Denmark.
Appendices
Appendix 1. Methods for a network meta‐analysis
Measures of treatment effect
Relative treatment effects
For dichotomous variables (e.g. proportion of participants with serious adverse events or any adverse events), we will calculate the odds ratio with 95% credible interval (or Bayesian confidence interval) (Severini 1993). For continuous variables (e.g. quality of life reported on the same scale), we will calculate the mean difference with 95% credible interval. We will use standardised mean difference values with 95% credible interval for quality of life if included trials use different scales. For count outcomes (e.g. number of adverse events and serious adverse events), we will calculate the rate ratio with 95% credible interval. For time‐to‐event data (e.g. mortality at maximal follow‐up), we will calculate hazard ratio with 95% credible interval.
Relative ranking
We will estimate the ranking probabilities for all treatments of being at each possible rank for each intervention. Then, we will obtain the surface under the cumulative ranking curve (SUCRA) (cumulative probability) and rankogram (Salanti 2011; Chaimani 2013).
Unit of analysis issues
We will collect data for all trial treatment groups that meet the inclusion criteria. The codes for analysis, that we will use, accounts for the correlation between the effect sizes from from trials with more than two groups.
Assessment of heterogeneity
We will assess clinical and methodological heterogeneity by carefully examining the characteristics and design of included trials. We will assess the presence of clinical heterogeneity by comparing effect estimates under different categories of potential effect modifiers. Different study designs and risk of bias may contribute to methodological heterogeneity.
We will assess the statistical heterogeneity by comparing the results of the fixed‐effect model meta‐analysis and the random‐effects model meta‐analysis, between‐study standard deviation (tau² and comparing this with values reported in the study of the distribution of between‐study heterogeneity (Turner 2012)), and by calculating I² (using Stata/SE 14.2). If we identify substantial heterogeneity, clinical, methodological, or statistical, we will explore and address heterogeneity in a subgroup analysis (see ‘Subgroup analysis and investigation of heterogeneity for network meta‐analysis’ section).
Assessment of transitivity across treatment comparisons
We will evaluate the plausibility of transitivity assumption (the assumption that the participants included in the different studies with different immunosuppressive regimens can be considered to be a part of a multi‐arm randomised clinical trial and could potentially have been randomised to any of the treatments) (Salanti 2012). In other words, any participant that meets the inclusion criteria is, in principle, equally likely to be randomised to any of the above eligible interventions. If there is any concern that the clinical safety and effectiveness are dependent upon the effect modifiers, we will continue to do traditional Cochrane pair‐wise comparisons and we will not perform a network meta‐analysis on all participant subgroups.
Assessment of reporting biases
For the network meta‐analysis, we will judge the reporting bias by the completeness of the search (i.e. searching various databases and including conference abstracts), as we do not currently find any meaningful order to perform a comparison‐adjusted funnel plot as suggested by Chaimani 2012. However, if we find any meaningful order, for example, the control group used depended upon the year of conduct of the trial, we will use comparison‐adjusted funnel plot as suggested by Chaimani 2012.
Data synthesis
Methods for indirect and mixed comparisons
We will conduct network meta‐analyses to compare multiple interventions simultaneously for each of the primary and secondary outcomes. Network meta‐analysis combines direct evidence within trials and indirect evidence across trials (Mills 2012). We will obtain a network plot to ensure that the trials were connected by treatments using Stata/SE 14.2 (Chaimani 2013). The network plot for mortality at maximal follow‐up based on current data is presented in Figure 29. We will exclude any trials that were not connected to the network. We will conduct a Bayesian network meta‐analysis using the Markov chain Monte Carlo method in OpenBUGS 3.2.3 as per the guidance from the National Institute for Health and Care Excellence (NICE) Decision Support Unit (DSU) documents (Dias 2014a). We will model the treatment contrast (i.e. log odds ratio for binary outcomes, mean difference or standardised mean difference for continuous outcomes, log rate ratio for count outcomes, and log hazard ratio for time‐to‐event outcomes) for any two interventions ('functional parameters') as a function of comparisons between each individual intervention and an arbitrarily selected reference group ('basic parameters') (Lu 2006) using appropriate likelihood functions and links. We will use binomial likelihood and logit link for binary outcomes, Poisson likelihood and log link for count outcomes, binomial likelihood and complementary log‐log link for time‐to‐event outcomes, and normal likelihood and identity link for continuous outcomes. We will perform a fixed‐effect model and random‐effects model for the network meta‐analysis. We will report both models for comparison with the reference group in a forest plot. For pairwise comparison, we will report the fixed‐effect model if the two models reported similar results; otherwise, we will report the more conservative model.
6.
The network plots showing the comparisons in mortality at maximal follow‐up. The size of the node (circle) provides a measure of the number of trials in which the particular treatment was included as one of the arms. The thickness of the line provides a measure of the number of direct comparisons between two nodes (treatments).
Abbreviation: ACE inhibitors = angiotensin‐converting enzyme inhibitors
We will use a hierarchical Bayesian model using three different initial values using codes provided by NICE DSU (Dias 2014a). We will use a normal distribution with large variance (10,000) for treatment effect priors (vague or flat priors). For the random‐effects model, we will use a prior distributed uniformly (limits: 0 to 5) for between‐trial standard deviation but assumed similar between‐trial standard deviation across treatment comparisons (Dias 2014a). We will use a 'burn‐in' of 5000 simulations, check for convergence visually, and run the models for another 10,000 simulations to obtain effect estimates. If we did not obtain convergence, we will increase the number of simulations for 'burn‐in'. If we do not obtain convergence still, we will use alternate initial values and priors using methods suggested by Van Valkenhoef 2012. We will also estimate the probability that each intervention ranks at one of the possible positions using the NICE DSU codes (Dias 2014a).
Assessment of inconsistency
We will assess inconsistency (statistical evidence of the violation of transitivity assumption) by fitting both an inconsistency model and a consistency model. We will use the inconsistency models used in the NICE DSU manual, as we plan to use a common between‐study deviation for the comparisons (Dias 2014b). In addition, we will use the design‐by‐treatment full interaction model (Higgins 2012) and IF (inconsistency factor) plots (Chaimani 2013) to assess inconsistency. In the presence of inconsistency, we will assess whether the inconsistency is because of clinical or methodological heterogeneity by performing separate analyses for each of the different subgroups mentioned in the ‘Subgroup analysis and investigation of heterogeneity for network meta‐analysis’ section below.
If there is evidence of inconsistency, we will identify areas in the network where substantial inconsistency might be present in terms of clinical and methodological diversities between trials and, when appropriate, limit network meta‐analysis to a more compatible subset of trials.
Direct comparison
We will perform the direct comparisons using the same codes and the same technical details.
Sample size calculations
To control for the risk of random errors, we will interpret the information with caution when the accrued sample size in the network meta‐analysis (i.e. across all treatment comparisons) was less than the required sample size (required information size). For calculation of the required information size, see Appendix 3.
Subgroup analysis and investigation of heterogeneity for network meta‐analysis
We will assess the differences in the effect estimates between the subgroups listed in Subgroup analysis and investigation of heterogeneity using meta‐regression with the help of the OpenBUGS code (Dias 2012a) if we include a sufficient number of trials. We will use the potential modifiers as study level co‐variates for meta‐regression. We will calculate a single common interaction term (Dias 2012a). If the 95% credible intervals of the interaction term do not overlap zero, we will consider this as evidence of difference in subgroups.
Presentation of results
We will present the effect estimates with 95% CrI for each pairwise comparisons calculated from the direct comparisons and network meta‐analysis. We will also present the cumulative probability of the treatment ranks (i.e. the probability that the treatment is within the top two, the probability that the treatment is within the top three, etc.) in graphs (surface under the cumulative ranking curve or SUCRA) (Salanti 2011). We will also plot the probability that each treatment is best, second best, third best etc for each of the different outcomes (rankograms), which are generally considered more informative (Salanti 2011; Dias 2012b).
We will present the 'Summary of findings' tables for mortality. In the 'Table 1', we will follow the approach suggested by Puhan et al. (Puhan 2014). First, we will calculate the direct and indirect effect estimates and 95% credible intervals using the node‐splitting approach (Dias 2010), i.e. calculate the direct estimate for each comparison by including only trials in which there was direct comparison of treatments and the indirect estimate for each comparison by excluding the trials in which there was direct comparison of treatments. Then we will rate the quality of direct and indirect effect estimates using GRADE which takes into account the risk of bias, inconsistency, directness of evidence, imprecision, and publication bias (Guyatt 2011). Then, we will present the estimates of the network meta‐analysis and rate the quality of network meta‐analysis effect estimates as the best quality of evidence between the direct and indirect estimates (Puhan 2014). In addition, in the same table, we will present illustrations and information on the number of trials and participants as per the standard 'Summary of Findings' Table.
Appendix 2. Search strategies
| Database | Time span | Search strategy |
| Cochrane Central Register of Controlled Trials (CENTRAL) | Issue 8, 2016 | #1 MeSH descriptor: [Fatty Liver] explode all trees #2 (liver and (fatty or steatosis or steatoses)) #3 NAFLD #4 #1 or #2 or #3 #5 orlistat #6 MeSH descriptor: [Metformin] explode all trees #7 (dimethylbiguanidine or dimethylguanylguanidine or glucophage or metformin) #8 MeSH descriptor: [Thiazolidinediones] explode all trees #9 (pioglitazone or rosiglitazone or glitazone) #10 MeSH descriptor: [Vitamin E] explode all trees #11 (Tocopherol or (vitamin adj E)) #12 MeSH descriptor: [Milk Thistle] explode all trees #13 ((milk adj thistle) or Carduus or Silybum or silymarin) #14 MeSH descriptor: [S‐Adenosylmethionine] explode all trees #15 Adenosylmethionine #16 MeSH descriptor: [Hydroxymethylglutaryl‐CoA Reductase Inhibitors] explode all trees #17 (simvastatin or lovastatin or pravastatin or atorvastatin) #18 (cholic adj acid) or UDCA #19 MeSH descriptor: [Angiotensin‐Converting Enzyme Inhibitors] explode all trees #20 ((ACE or "Angiotensin converting enzyme") adj (inhibitors or antagonists)) or ramipril or lisinopril #21 MeSH descriptor: [Angiotensin Receptor Antagonists] explode all trees #22 ((angiotensin receptor adj (blockers or antagonists or inhibitors)) or losartan or candesartan or telmisartan or valsartan) #23 #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 #24 #4 and #23 |
| MEDLINE (OvidSP) | January 1947 to August 2016 | 1. randomized controlled trial.pt. 2. controlled clinical trial.pt. 3. randomized.ab. 4. placebo.ab. 5. drug therapy.fs. 6. randomly.ab. 7. trial.ab. 8. groups.ab. 9. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 10. exp animals/ not humans.sh. 11. 9 not 10 12. exp Fatty Liver/ 13. (liver and (fatty or steatosis or steatoses)).ti,ab. 14. NAFLD.ti,ab. 15. 12 or 13 or 14 16. orlistat.ti,ab. 17. exp Metformin/ 18. (dimethylbiguanidine or dimethylguanylguanidine or glucophage or metformin).ti,ab. 19. exp Thiazolidinediones/ 20. (pioglitazone or rosiglitazone or glitazone).ti,ab. 21. exp Vitamin E/ 22. (Tocopherol or (vitamin adj E)).ti,ab. 23. exp Milk Thistle/ 24. ((milk adj thistle) or Carduus or Silybum or silymarin).ti,ab. 25. exp S‐Adenosylmethionine/ 26. Adenosylmethionine.ti,ab. 27. exp Hydroxymethylglutaryl‐CoA Reductase Inhibitors/ 28. (simvastatin or lovastatin or pravastatin or atorvastatin).ti,ab. 29. exp Cholic Acid/ 30. ((cholic adj acid) or UDCA).ti,ab. 31. exp Angiotensin‐Converting Enzyme Inhibitors/ 32. (((ACE or "Angiotensin converting enzyme") adj (inhibitors or antagonists)) or ramipril or lisinopril).ti,ab. 33. exp Angiotensin Receptor Antagonists/ 34. ((angiotensin receptor adj (blockers or antagonists or inhibitors)) or losartan or candesartan or telmisartan or valsartan).ti,ab. 35. or/16‐34 36. 11 and 15 and 35 |
| Embase (OvidSP) | January 1974 to August 2016 | 1. exp crossover‐procedure/ or exp double‐blind procedure/ or exp randomized controlled trial/ or single‐blind procedure/ 2. (((((random* or factorial* or crossover* or cross over* or cross‐over* or placebo* or double*) adj blind*) or single*) adj blind*) or assign* or allocat* or volunteer*).af. 3. 1 or 2 4. exp fatty liver/ 5. (liver and (fatty or steatosis or steatoses)).ti,ab. 6. NAFLD.ti,ab. 7. 4 or 5 or 6 8. exp tetrahydrolipstatin/ 9. orlistat.ti,ab. 10. exp metformin/ 11. (dimethylbiguanidine or dimethylguanylguanidine or glucophage or metformin).ti,ab. 12. exp 2,4 thiazolidinedione derivative/ 13. (pioglitazone or rosiglitazone or glitazone).ti,ab. 14. exp alpha tocopherol/ 15. (Tocopherol or (vitamin adj E)).ti,ab. 16. exp Silybum marianum/ 17. ((milk adj thistle) or Carduus or Silybum or silymarin).ti,ab. 18. exp s adenosylmethionine/ 19. Adenosylmethionine.ti,ab. 20. exp hydroxymethylglutaryl coenzyme A reductase inhibitor/ 21. (simvastatin or lovastatin or pravastatin or atorvastatin).ti,ab. 22. exp bile acid/ 23. ((cholic adj acid) or UDCA).ti,ab. 24. exp dipeptidyl carboxypeptidase inhibitor/ 25. (((ACE or "Angiotensin converting enzyme") adj (inhibitors or antagonists)) or ramipril or lisinopril).ti,ab. 26. exp angiotensin receptor antagonist/ 27. ((angiotensin receptor adj (blockers or antagonists or inhibitors)) or losartan or candesartan or telmisartan or valsartan).ti,ab. 28. or/8‐27 29. 3 and 7 and 28 |
| Science Citation Index Expanded (Web of Knowledge) | January 1945 to August 2016 | #1 TS = ((liver and (fatty or steatosis or steatoses)) or NAFLD) #2 TS = (orlistat or dimethylbiguanidine or dimethylguanylguanidine or glucophage or metformin or pioglitazone or rosiglitazone or glitazone or Tocopherol or ("vitamin E") or ("milk thistle") or Carduus or Silybum or silymarin or Adenosylmethionine or simvastatin or lovastatin or pravastatin or atorvastatin or ("cholic acid") or UDCA or ((ACE or "Angiotensin converting enzyme") and (inhibitors or antagonists)) or ramipril or lisinopril or (angiotensin receptor and (blockers or antagonists or inhibitors)) or losartan or candesartan or telmisartan or valsartan) #3 TS=(random* OR rct* OR crossover OR masked OR blind* OR placebo* OR meta‐analysis OR systematic review* OR meta‐analys*) #4 #1 AND #2 AND #3 |
| World Health Organization International Clinical Trials Registry Platform Search Portal (apps.who.int/trialsearch/Default.aspx) | August 2016 | Condition: fatty liver |
| ClinicalTrials.gov | August 2016 | Interventional Studies | "non‐alcoholic fatty liver disease" | Phase 2, 3, 4 |
Appendix 3. Sample size calculation
The five‐year mortality in people with non‐alcohol related fatty liver disease is about 20% (Adams 2005). The required information size based on a control group proportion of 20%, a relative risk reduction of 20% in the experimental group, type I error of 5%, and type II error of 20% is 2894 participants. Network analyses are more prone to the risk of random errors than direct comparisons (Del Re 2013). Accordingly, a greater sample size is required in indirect comparisons than direct comparisons (Thorlund 2012). The power and precision in indirect comparisons depends upon various factors, such as the number of participants included under each comparison and the heterogeneity between the trials (Thorlund 2012). If there is no heterogeneity across the trials, the sample size in indirect comparisons would be equivalent to the sample size in direct comparisons. The effective indirect sample size can be calculated using the number of participants included in each direct comparison (Thorlund 2012). For example, a sample size of 2500 participants in the direct comparison A versus C (nAC) and a sample size of 7500 participants in the direct comparison B versus C (nBC) results in an effective indirect sample size of 1876 participants. However, in the presence of heterogeneity within the comparisons, the sample size required is higher. In the above scenario, for an I² statistic for each of the comparisons A versus C (IAC2) and B versus C (IBC2) of 25%, the effective indirect sample size is 1407 participants. For an I² statistic for each of the comparisons A versus C and B versus C of 50%, the effective indirect sample size is 938 participants (Thorlund 2012). If there were only three groups and the sample size in the trials is more than the required information size, we will calculate the effective indirect sample size using the following generic formula (Thorlund 2012):
((nAC x (1 ‐ IAC2)) x (nBC x (1 ‐ IBC2))/((nAC x (1 ‐ IAC2)) + (nBC x (1 ‐ IBC2)).
There is currently no method to calculate the effective indirect sample size for a network analysis involving more than three intervention groups.
Appendix 4. Difference in fibrosis scores
| Study name | Intervention(s) | Control | Mean or median change score of final score in intervention(s) | Mean or median change score of final score in control | Difference in mean or median |
| Sanyal 2010 | Thiazolidinediones | Antioxidants | ‐0.4 ‐0.3 | ‐0.1 | ‐0.3 ‐0.2 |
| Gomez 2009 | Antioxidants | No intervention | ‐0.72 | ‐0.55 | ‐0.17 |
| Harrison 2003 | Antioxidants | No intervention | ‐0.67 | ‐0.3 | ‐0.37 |
| Leuschner 2010 | Bile acids | No intervention | 0 | ‐0.08 | 0.08 |
| Lindor 2004 | Bile acids | No intervention | 0 | ‐0.1 | 0.1 |
| Neuschwander‐Tetri 2015 | Bile acids | No intervention | ‐0.2 | 0.1 | ‐0.3* |
| Dufour 2006 | Bile acids plus antioxidants Bile acids | No intervention | 0.44 0.32 | 0.56 | ‐0.10 ‐0.24 |
| Armstrong 2016 | Other anti‐diabetes medication | No intervention | ‐0.2 | ‐0.2 | 0 |
| Loomba 2015 | Other cholesterol‐lowering agents | No intervention | 1 | 1 | 0 |
| Van Wagner 2011 | Pentoxifylline | No intervention | ‐0.2 | 0.4 | ‐0.6 |
| Alam 2016 | Renin‐angiotensin‐aldosterone system inhibitor | No intervention | ‐0.65 | 0.3 | ‐0.95* |
| Chan 2015 | Silymarin | No intervention | ‐0.367 | 0.147 | ‐0.514 |
| Shields 2009 | Sulphonylureas | No intervention | 1.56 | 1.9 | ‐0.34 |
| Uygun 2004 | Sulphonylureas | No intervention | 0.92 | 1.12 | ‐0.2 |
| Belfort 2006 | Thiazolidinediones | No intervention | ‐0.9 | ‐0.63 | ‐0.27 |
| Ratziu 2008 | Thiazolidinediones | No intervention | 0.03 | ‐0.18 | 0.21 |
| Sharma 2012 | Pentoxifylline | Thiazolidinediones | 0.91 | 0.9 | 0.01 |
| Torres 2011 | Thiazolidinediones plus renin‐angiotensin‐aldosterone system inhibitor Thiazolidinediones plus sulphonylureas | Thiazolidinediones | ‐0.32 ‐0.59 | ‐0.7 | 0.38 0.11 |
|
Footnotes Lower indicates better * = statistically significant difference | |||||
Appendix 5. Difference in non‐alcohol related fatty liver disease activity score
| Study name | Intervention(s) | Control | Mean or median change score of final score in intervention(s) | Mean or median change score of final score in control | Difference in mean or median |
| Sanyal 2010 | Thiazolidinediones | Antioxidants | ‐1.9 ‐1.9 | ‐0.5 | ‐1.4 ‐1.4 |
| Gomez 2009 | Antioxidants | No intervention | ‐3.64 | ‐2.25 | ‐1.39* |
| Leuschner 2010 | Bile acids | No intervention | ‐1.22 | ‐1.03 | ‐0.19 |
| Neuschwander‐Tetri 2015 | Bile acids | No intervention | ‐1.7 | ‐0.7 | ‐1* |
| Gianturco 2013 | Bile acids plus antioxidants Antioxidants Bile acids | No intervention | 0.68 1 1.14 | 1.02 | ‐0.34* ‐0.02 0.12* |
| Armstrong 2016 | Other anti‐diabetes medication | No intervention | ‐1.3 | ‐0.8 | ‐0.5 |
| Loomba 2015 | Other cholesterol‐lowering agents | No intervention | 4 | 5 | ‐1 |
| Van Wagner 2011 | Pentoxifylline | No intervention | ‐1.4 | ‐0.3 | ‐1.1 |
| Alam 2016 | Renin‐angiotensin‐aldosterone system inhibitor | No intervention | ‐2.15 | ‐1.1 | ‐1.05* |
| Chan 2015 | Silymarin | No intervention | ‐0.7333 | ‐0.706 | ‐0.0273 |
| Haukeland 2009 | Sulphonylureas | No intervention | 3.1 | 3.25 | ‐0.15 |
| Shields 2009 | Sulphonylureas | No intervention | 3.8 | 3.4 | 0.4 |
| Uygun 2004 | Sulphonylureas | No intervention | 1.15 | 1.3 | ‐0.15 |
| Ratziu 2008 | Thiazolidinediones | No intervention | ‐1 | 0 | ‐1 |
| Omer 2010 | Sulphonylureas | Thiazolidinediones | 10 9.6 | 10.3 | ‐0.3 ‐0.7 |
| Torres 2011 | Thiazolidinediones plus renin‐angiotensin‐aldosterone system inhibitor Thiazolidinediones plus sulphonylureas | Thiazolidinediones | ‐1.37 ‐1.32 | ‐1.77 | ‐0.40 ‐0.45 |
|
Footnotes Lower indicates better * = statistically significant difference | |||||
Data and analyses
Comparison 1. All studies.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality at maximal follow‐up | 11 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Renin‐angiotensin‐aldosterone system inhibitor versus no intervention | 1 | 30 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Antioxidants versus no intervention | 1 | 87 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.3 Bile acids versus no intervention | 4 | 659 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.11 [0.24, 107.34] |
| 1.4 Other anti‐diabetes drug versus no intervention | 1 | 52 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.5 Thiazolidinediones versus no intervention | 1 | 74 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.6 Antioxidants versus renin‐angiotensin‐aldosterone system inhibitor plus antioxidants | 1 | 31 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.7 Statins versus other cholesterol‐lowering agents | 1 | 125 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.8 Statins plus other cholesterol‐lowering agents versus other cholesterol‐lowering agents | 1 | 123 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.9 Statins plus other cholesterol‐lowering agents versus statins | 1 | 124 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.10 Thiazolidinediones versus sulphonylureas | 1 | 80 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Serious adverse events (proportion) | 19 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Renin‐angiotensin‐aldosterone system inhibitor versus no intervention | 1 | 30 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Anti‐fibrotic versus no intervention | 1 | 274 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.40 [0.66, 2.94] |
| 2.3 Antioxidants versus no intervention | 1 | 87 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.4 Bile acids versus no intervention | 3 | 404 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.56 [0.84, 2.88] |
| 2.5 Other cholesterol‐lowering agents versus no intervention | 1 | 27 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.6 Other anti‐diabetes drug versus no intervention | 1 | 52 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.13, 7.69] |
| 2.7 Phosphodiesterase type 4 inhibitor versus no intervention | 1 | 96 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.1 [0.01, 0.94] |
| 2.8 Glucocorticosteroid inhibitor versus no intervention | 1 | 80 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.16 [0.31, 31.78] |
| 2.9 Silymarin versus no intervention | 1 | 100 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.10 Silymarin plus antioxidants versus no intervention | 1 | 36 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.11 Statins versus no intervention | 1 | 16 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.12 Pentoxifylline versus no intervention | 1 | 26 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.13 Thiazolidinediones versus no intervention | 2 | 194 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.14 Antioxidants versus renin‐angiotensin‐aldosterone system inhibitor plus antioxidants | 1 | 31 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.15 Statins versus other cholesterol‐lowering agents | 1 | 125 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.16 Statins plus other cholesterol‐lowering agents versus other cholesterol‐lowering agents | 1 | 123 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.17 Statins plus other cholesterol‐lowering agents versus statins | 1 | 124 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.18 Thiazolidinediones versus sulphonylureas | 1 | 80 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Serious adverse events (number of events) | 18 | Rate Ratio (Fixed, 95% CI) | Subtotals only | |
| 3.1 Renin‐angiotensin‐aldosterone system inhibitor versus no intervention | 1 | 30 | Rate Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Antioxidants versus no intervention | 2 | 254 | Rate Ratio (Fixed, 95% CI) | 0.89 [0.36, 2.19] |
| 3.3 Bile acids versus no intervention | 3 | 404 | Rate Ratio (Fixed, 95% CI) | 1.01 [0.66, 1.54] |
| 3.4 Other cholesterol‐lowering agents versus no intervention | 1 | 27 | Rate Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.5 Other anti‐diabetes drug versus no intervention | 1 | 52 | Rate Ratio (Fixed, 95% CI) | 0.67 [0.11, 3.99] |
| 3.6 Phosphodiesterase type 4 inhibitor versus no intervention | 1 | 96 | Rate Ratio (Fixed, 95% CI) | 0.15 [0.02, 1.46] |
| 3.7 Silymarin versus no intervention | 1 | 100 | Rate Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.8 Silymarin plus antioxidants versus no intervention | 1 | 36 | Rate Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.9 Statins versus no intervention | 1 | 16 | Rate Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.10 Glucocorticosteroid inhibitor versus no intervention | 1 | 80 | Rate Ratio (Fixed, 95% CI) | 5.00 [0.58, 42.80] |
| 3.11 Thiazolidinediones versus no intervention | 3 | 358 | Rate Ratio (Fixed, 95% CI) | 0.21 [0.05, 0.95] |
| 3.12 Antioxidants versus renin‐angiotensin‐aldosterone system inhibitor plus antioxidants | 1 | 31 | Rate Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.13 Thiazolidinediones versus antioxidants | 1 | 164 | Rate Ratio (Fixed, 95% CI) | 0.23 [0.05, 1.08] |
| 3.14 Statins versus other cholesterol‐lowering agents | 1 | 125 | Rate Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.15 Statins plus other cholesterol‐lowering agents versus other cholesterol‐lowering agents | 1 | 123 | Rate Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.16 Statins plus other cholesterol‐lowering agents versus statins | 1 | 124 | Rate Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.17 Thiazolidinediones versus sulphonylureas | 1 | 80 | Rate Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Adverse events (proportion) | 17 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Antioxidants versus no intervention | 2 | 242 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Bile acids versus no intervention | 2 | 230 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.72 [0.72, 4.10] |
| 4.3 Other cholesterol‐lowering agents versus no intervention | 1 | 27 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.4 Other anti‐diabetes drug versus no intervention | 1 | 52 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.10, 4.18] |
| 4.5 Phosphodiesterase type 4 inhibitor versus no intervention | 1 | 96 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.0 [1.15, 7.85] |
| 4.6 Silymarin plus antioxidants versus no intervention | 1 | 36 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.7 Statins versus no intervention | 1 | 16 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.8 Glucocorticosteroid inhibitor versus no intervention | 1 | 80 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.68 [0.68, 4.13] |
| 4.9 Thiazolidinediones versus no intervention | 2 | 194 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.07 [0.96, 9.87] |
| 4.10 Bile acids versus antioxidants | 2 | 289 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.50, 1.81] |
| 4.11 Statins versus other cholesterol‐lowering agents | 1 | 125 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.08 [0.24, 108.01] |
| 4.12 Statins plus other cholesterol‐lowering agents versus other cholesterol‐lowering agents | 1 | 123 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.10 [0.12, 77.57] |
| 4.13 Thiazolidinediones versus pentoxifylline | 1 | 59 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.04, 5.83] |
| 4.14 Statins plus other cholesterol‐lowering agents versus statins | 1 | 124 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.04, 5.76] |
| 4.15 Thiazolidinediones versus sulphonylureas | 1 | 80 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Adverse events (number of events) | 22 | Rate Ratio (Fixed, 95% CI) | Subtotals only | |
| 5.1 Antioxidants versus no intervention | 3 | 409 | Rate Ratio (Fixed, 95% CI) | 1.03 [0.70, 1.52] |
| 5.2 Bile acids versus no intervention | 5 | 825 | Rate Ratio (Fixed, 95% CI) | 1.19 [1.06, 1.33] |
| 5.3 Other cholesterol‐lowering agents versus no intervention | 2 | 77 | Rate Ratio (Fixed, 95% CI) | 2.50 [0.49, 12.89] |
| 5.4 Other anti‐diabetes drug versus no intervention | 1 | 52 | Rate Ratio (Fixed, 95% CI) | 0.94 [0.78, 1.14] |
| 5.5 Pentoxifylline versus no intervention | 1 | 26 | Rate Ratio (Fixed, 95% CI) | 1.11 [0.44, 2.78] |
| 5.6 Silymarin plus antioxidants versus no intervention | 1 | 36 | Rate Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.7 Statins versus no intervention | 1 | 16 | Rate Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.8 Glucocorticosteroid inhibitor versus no intervention | 1 | 80 | Rate Ratio (Fixed, 95% CI) | 1.56 [1.05, 2.31] |
| 5.9 Thiazolidinediones versus no intervention | 4 | 481 | Rate Ratio (Fixed, 95% CI) | 1.14 [0.82, 1.58] |
| 5.10 Bile acids versus antioxidants | 1 | 58 | Rate Ratio (Fixed, 95% CI) | 6.53 [0.34, 126.48] |
| 5.11 Thiazolidinediones versus antioxidants | 1 | 164 | Rate Ratio (Fixed, 95% CI) | 0.87 [0.58, 1.30] |
| 5.12 Statins versus other cholesterol‐lowering agents | 1 | 127 | Rate Ratio (Fixed, 95% CI) | 4.92 [0.24, 102.52] |
| 5.13 Statins plus other cholesterol‐lowering agents versus other cholesterol‐lowering agents | 1 | 125 | Rate Ratio (Fixed, 95% CI) | 3.05 [0.12, 74.83] |
| 5.14 Thiazolidinediones versus pentoxifylline | 1 | 59 | Rate Ratio (Fixed, 95% CI) | 0.52 [0.05, 5.70] |
| 5.15 Statins plus other cholesterol‐lowering agents versus statins | 1 | 124 | Rate Ratio (Fixed, 95% CI) | 0.52 [0.05, 5.69] |
| 5.16 Thiazolidinediones versus sulphonylureas | 1 | 80 | Rate Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Cirrhosis | 11 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Renin‐angiotensin‐aldosterone system inhibitor versus no intervention | 1 | 30 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Antioxidants versus no intervention | 1 | 87 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.3 Other anti‐diabetes drug versus no intervention | 1 | 52 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.4 Silymarin versus no intervention | 1 | 64 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 2.15] |
| 6.5 Glucocorticosteroid inhibitor versus no intervention | 1 | 80 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.6 Sulphonylureas versus no intervention | 1 | 44 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.7 Thiazolidinediones versus no intervention | 2 | 121 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.99 [0.71, 50.28] |
| 6.8 Antioxidants versus renin‐angiotensin‐aldosterone system inhibitor plus antioxidants | 1 | 31 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.9 Statins versus other cholesterol‐lowering agents | 1 | 125 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.10 Statins plus other cholesterol‐lowering agents versus other cholesterol‐lowering agents | 1 | 123 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.11 Statins plus other cholesterol‐lowering agents versus statins | 1 | 124 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.12 Thiazolidinediones versus sulphonylureas | 1 | 80 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Resolution of fatty liver disease | 16 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Renin‐angiotensin‐aldosterone system inhibitor versus no intervention | 1 | 30 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 Antioxidants versus no intervention | 3 | 299 | Odds Ratio (M‐H, Random, 95% CI) | 2.23 [1.28, 3.87] |
| 7.3 Bile acids versus no intervention | 1 | 219 | Odds Ratio (M‐H, Random, 95% CI) | 1.85 [0.88, 3.89] |
| 7.4 Other cholesterol‐lowering agents versus no intervention | 1 | 35 | Odds Ratio (M‐H, Random, 95% CI) | 1.08 [0.25, 4.70] |
| 7.5 Other anti‐diabetes drug versus no intervention | 1 | 45 | Odds Ratio (M‐H, Random, 95% CI) | 6.43 [1.20, 34.41] |
| 7.6 Silymarin versus no intervention | 1 | 64 | Odds Ratio (M‐H, Random, 95% CI) | 11.72 [0.60, 227.31] |
| 7.7 Sulphonylureas versus no intervention | 3 | 123 | Odds Ratio (M‐H, Random, 95% CI) | 1.02 [0.23, 4.41] |
| 7.8 Thiazolidinediones versus no intervention | 3 | 272 | Odds Ratio (M‐H, Random, 95% CI) | 1.68 [0.44, 6.44] |
| 7.9 Bile acids versus antioxidants | 1 | 56 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.10 Thiazolidinediones versus antioxidants | 1 | 164 | Odds Ratio (M‐H, Random, 95% CI) | 1.63 [0.87, 3.05] |
| 7.11 Statins versus other cholesterol‐lowering agents | 1 | 125 | Odds Ratio (M‐H, Random, 95% CI) | 2.77 [1.34, 5.73] |
| 7.12 Statins plus other cholesterol‐lowering agents versus other cholesterol‐lowering agents | 1 | 123 | Odds Ratio (M‐H, Random, 95% CI) | 3.31 [1.57, 6.98] |
| 7.13 Statins plus other cholesterol‐lowering agents versus statins | 1 | 124 | Odds Ratio (M‐H, Random, 95% CI) | 1.19 [0.56, 2.55] |
| 7.14 Thiazolidinediones plus renin‐angiotensin‐aldosterone system inhibitor versus thiazolidinediones | 1 | 61 | Odds Ratio (M‐H, Random, 95% CI) | 0.47 [0.16, 1.35] |
| 7.15 Thiazolidinediones plus sulphonylureas versus thiazolidinediones | 1 | 54 | Odds Ratio (M‐H, Random, 95% CI) | 0.65 [0.22, 1.93] |
| 7.16 Thiazolidinediones plus sulphonylureas versus thiazolidinediones plus renin‐angiotensin‐aldosterone system inhibitor | 1 | 63 | Odds Ratio (M‐H, Random, 95% CI) | 1.39 [0.48, 4.03] |
1.7. Analysis.
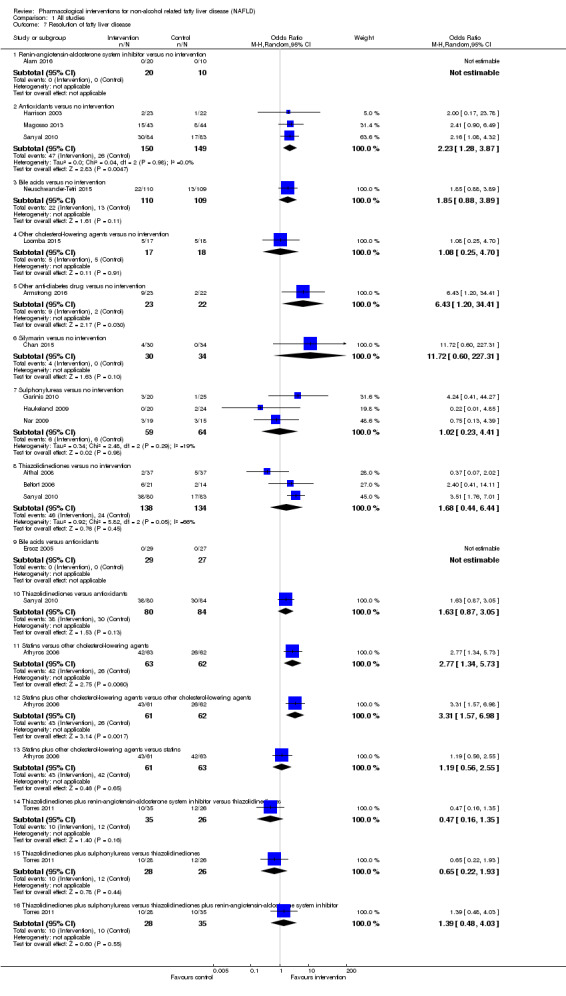
Comparison 1 All studies, Outcome 7 Resolution of fatty liver disease.
Comparison 2. Non‐alcohol related steatohepatitis only.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Serious adverse events (proportion) | 10 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Renin‐angiotensin‐aldosterone system inhibitor versus no intervention | 1 | 30 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Anti‐fibrotic versus no intervention | 1 | 274 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.40 [0.66, 2.94] |
| 1.3 Bile acids versus no intervention | 1 | 283 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.56 [0.84, 2.88] |
| 1.4 Other cholesterol‐lowering agents versus no intervention | 1 | 27 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.5 Other anti‐diabetes drug versus no intervention | 1 | 52 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.13, 7.69] |
| 1.6 Phosphodiesterase type 4 inhibitor versus no intervention | 1 | 96 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.1 [0.01, 0.94] |
| 1.7 Pentoxifylline versus no intervention | 1 | 26 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.8 Silymarin versus no intervention | 1 | 100 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.9 Statins versus no intervention | 1 | 16 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.10 Thiazolidinediones versus no intervention | 1 | 74 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Serious adverse events (number of events) | 9 | Rate Ratio (Fixed, 95% CI) | Subtotals only | |
| 2.1 Renin‐angiotensin‐aldosterone system inhibitor versus no intervention | 1 | Rate Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Antioxidants versus no intervention | 1 | Rate Ratio (Fixed, 95% CI) | 0.89 [0.36, 2.19] | |
| 2.3 Bile acids versus no intervention | 1 | Rate Ratio (Fixed, 95% CI) | 1.01 [0.66, 1.54] | |
| 2.4 Other cholesterol‐lowering agents versus no intervention | 1 | Rate Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.5 Other anti‐diabetes drug versus no intervention | 1 | Rate Ratio (Fixed, 95% CI) | 0.67 [0.11, 3.99] | |
| 2.6 Phosphodiesterase type 4 inhibitor versus no intervention | 1 | Rate Ratio (Fixed, 95% CI) | 0.15 [0.02, 1.46] | |
| 2.7 Silymarin versus no intervention | 1 | Rate Ratio (Fixed, 95% CI) | 1.0 [0.02, 50.40] | |
| 2.8 Statins versus no intervention | 1 | Rate Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.9 Thiazolidinediones versus no intervention | 2 | Rate Ratio (Fixed, 95% CI) | 0.21 [0.05, 0.95] | |
| 2.10 Thiazolidinediones versus antioxidants | 1 | Rate Ratio (Fixed, 95% CI) | 0.23 [0.05, 1.08] | |
| 3 Adverse events (proportion) | 8 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Bile acids versus no intervention | 1 | 166 | Odds Ratio (M‐H, Fixed, 95% CI) | 7.0 [0.84, 58.22] |
| 3.2 Other cholesterol‐lowering agents versus no intervention | 1 | 27 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.3 Other anti‐diabetes drug versus no intervention | 1 | 52 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.10, 4.18] |
| 3.4 Phosphodiesterase type 4 inhibitor versus no intervention | 1 | 96 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.0 [1.15, 7.85] |
| 3.5 Statins versus no intervention | 1 | 16 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.6 Thiazolidinediones versus no intervention | 1 | 74 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.07 [0.96, 9.87] |
| 3.7 Bile acids versus antioxidants | 1 | 56 | Odds Ratio (M‐H, Fixed, 95% CI) | 7.26 [0.36, 147.49] |
| 3.8 Thiazolidinediones versus pentoxifylline | 1 | 59 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.04, 5.83] |
| 4 Adverse events (number of events) | 12 | Rate Ratio (Fixed, 95% CI) | Subtotals only | |
| 4.1 Antioxidants versus no intervention | 1 | Rate Ratio (Fixed, 95% CI) | 1.03 [0.70, 1.52] | |
| 4.2 Bile acids versus no intervention | 4 | Rate Ratio (Fixed, 95% CI) | 1.20 [1.07, 1.35] | |
| 4.3 Other cholesterol‐lowering agents versus no intervention | 2 | Rate Ratio (Fixed, 95% CI) | 2.50 [0.49, 12.89] | |
| 4.4 Other anti‐diabetes drug versus no intervention | 1 | Rate Ratio (Fixed, 95% CI) | 0.94 [0.78, 1.14] | |
| 4.5 Pentoxifylline versus no intervention | 1 | Rate Ratio (Fixed, 95% CI) | 1.11 [0.44, 2.78] | |
| 4.6 Statins versus no intervention | 1 | Rate Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.7 Thiazolidinediones versus no intervention | 2 | Rate Ratio (Fixed, 95% CI) | 1.08 [0.77, 1.51] | |
| 4.8 Thiazolidinediones versus antioxidants | 1 | Rate Ratio (Fixed, 95% CI) | 0.87 [0.58, 1.30] | |
| 4.9 Thiazolidinediones versus pentoxifylline | 1 | Rate Ratio (Fixed, 95% CI) | 0.52 [0.05, 5.70] | |
| 5 Cirrhosis | 5 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Renin‐angiotensin‐aldosterone system inhibitor versus no intervention | 1 | 30 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Other anti‐diabetes drug versus no intervention | 1 | 52 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.3 Silymarin versus no intervention | 1 | 64 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 2.15] |
| 5.4 Thiazolidinediones versus no intervention | 2 | 121 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.99 [0.71, 50.28] |
| 6 Resolution of fatty liver disease | 10 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Renin‐angiotensin‐aldosterone system inhibitor versus no intervention | 1 | 30 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Antioxidants versus no intervention | 2 | 212 | Odds Ratio (M‐H, Random, 95% CI) | 2.14 [1.10, 4.19] |
| 6.3 Bile acids versus no intervention | 1 | 219 | Odds Ratio (M‐H, Random, 95% CI) | 1.85 [0.88, 3.89] |
| 6.4 Other cholesterol‐lowering agents versus no intervention | 1 | 35 | Odds Ratio (M‐H, Random, 95% CI) | 1.08 [0.25, 4.70] |
| 6.5 Other anti‐diabetes drug versus no intervention | 1 | 45 | Odds Ratio (M‐H, Random, 95% CI) | 6.43 [1.20, 34.41] |
| 6.6 Silymarin versus no intervention | 1 | 64 | Odds Ratio (M‐H, Random, 95% CI) | 11.72 [0.60, 227.31] |
| 6.7 Thiazolidinediones versus no intervention | 3 | 272 | Odds Ratio (M‐H, Random, 95% CI) | 1.68 [0.44, 6.44] |
| 6.8 Thiazolidinediones versus antioxidants | 1 | 164 | Odds Ratio (M‐H, Random, 95% CI) | 1.63 [0.87, 3.05] |
| 6.9 Thiazolidinediones plus renin‐angiotensin‐aldosterone system inhibitor versus thiazolidinediones | 1 | 61 | Odds Ratio (M‐H, Random, 95% CI) | 0.47 [0.16, 1.35] |
| 6.10 Thiazolidinediones plus sulphonylureas versus thiazolidinediones | 1 | 54 | Odds Ratio (M‐H, Random, 95% CI) | 0.65 [0.22, 1.93] |
| 6.11 Thiazolidinediones plus sulphonylureas versus thiazolidinediones plus renin‐angiotensin‐aldosterone system inhibitor | 1 | 63 | Odds Ratio (M‐H, Random, 95% CI) | 1.39 [0.48, 4.03] |
Comparison 3. People with diabetes mellitus only.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Serious adverse events (proportion) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Bile acids versus no intervention | 1 | 64 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Serious adverse events (number of events) | 1 | Rate Ratio (Fixed, 95% CI) | Subtotals only | |
| 2.1 Bile acids versus no intervention | 1 | Rate Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Adverse events (proportion) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Bile acids versus no intervention | 1 | 64 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.35, 2.86] |
| 4 Adverse events (number of events) | 1 | Rate Ratio (Fixed, 95% CI) | Subtotals only | |
| 4.1 Bile acids versus no intervention | 1 | Rate Ratio (Fixed, 95% CI) | 1.01 [0.64, 1.58] |
Comparison 4. People without diabetes mellitus only.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Serious adverse events (proportion) | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Silymarin plus antioxidants versus no intervention | 1 | 36 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Thiazolidinediones versus no intervention | 1 | 74 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.3 Statins versus other cholesterol‐lowering agents | 1 | 125 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.4 Statins plus other cholesterol‐lowering agents versus other cholesterol‐lowering agents | 1 | 123 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.5 Statins plus other cholesterol‐lowering agents versus statins | 1 | 124 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Serious adverse events (number of events) | 4 | Rate Ratio (Fixed, 95% CI) | Subtotals only | |
| 2.1 Antioxidants versus no intervention | 1 | Rate Ratio (Fixed, 95% CI) | 0.89 [0.36, 2.19] | |
| 2.2 Silymarin plus antioxidants versus no intervention | 1 | Rate Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Thiazolidinediones versus no intervention | 2 | Rate Ratio (Fixed, 95% CI) | 0.21 [0.05, 0.95] | |
| 2.4 Thiazolidinediones versus antioxidants | 1 | Rate Ratio (Fixed, 95% CI) | 0.23 [0.05, 1.08] | |
| 2.5 Statins versus other cholesterol‐lowering agents | 1 | Rate Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.6 Statins plus other cholesterol‐lowering agents versus other cholesterol‐lowering agents | 1 | Rate Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.7 Statins plus other cholesterol‐lowering agents versus statins | 1 | Rate Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Adverse events (proportion) | 4 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Antioxidants versus no intervention | 1 | 155 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Silymarin plus antioxidants versus no intervention | 1 | 36 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.3 Thiazolidinediones versus no intervention | 1 | 74 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.07 [0.96, 9.87] |
| 3.4 Statins versus other cholesterol‐lowering agents | 1 | 125 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.08 [0.24, 108.01] |
| 3.5 Statins plus other cholesterol‐lowering agents versus other cholesterol‐lowering agents | 1 | 123 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.10 [0.12, 77.57] |
| 3.6 Statins plus other cholesterol‐lowering agents versus statins | 1 | 124 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.04, 5.76] |
| 4 Adverse events (number of events) | 6 | Rate Ratio (Fixed, 95% CI) | Subtotals only | |
| 4.1 Antioxidants versus control | 2 | Rate Ratio (Fixed, 95% CI) | 1.03 [0.70, 1.52] | |
| 4.2 Other cholesterol‐lowering agents versus no intervention | 1 | Rate Ratio (Fixed, 95% CI) | 2.50 [0.49, 12.89] | |
| 4.3 Silymarin plus antioxidants versus no intervention | 1 | Rate Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.4 Thiazolidinediones versus no intervention | 2 | Rate Ratio (Fixed, 95% CI) | 1.08 [0.77, 1.51] | |
| 4.5 Thiazolidinediones versus antioxidants | 1 | Rate Ratio (Fixed, 95% CI) | 0.87 [0.58, 1.30] | |
| 4.6 Statins versus other cholesterol‐lowering agents | 1 | Rate Ratio (Fixed, 95% CI) | 4.92 [0.24, 102.52] | |
| 4.7 Statins plus other cholesterol‐lowering agents versus other cholesterol‐lowering agents | 1 | Rate Ratio (Fixed, 95% CI) | 3.05 [0.12, 74.83] | |
| 4.8 Statins plus other cholesterol‐lowering agents versus statins | 1 | Rate Ratio (Fixed, 95% CI) | 0.52 [0.05, 5.69] | |
| 5 Cirrhosis | 4 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Sulphonylureas versus no intervention | 1 | 44 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Thiazolidinediones versus no intervention | 2 | 121 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.99 [0.71, 50.28] |
| 5.3 Statins versus other cholesterol‐lowering agents | 1 | 125 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.4 Statins plus other cholesterol‐lowering agents versus other cholesterol‐lowering agents | 1 | 123 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.5 Statins plus other cholesterol‐lowering agents versus statins | 1 | 124 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Resolution of fatty liver disease | 4 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Antioxidants versus no intervention | 1 | 167 | Odds Ratio (M‐H, Random, 95% CI) | 2.16 [1.08, 4.32] |
| 6.2 Thiazolidinediones versus no intervention | 3 | 272 | Odds Ratio (M‐H, Random, 95% CI) | 1.68 [0.44, 6.44] |
| 6.3 Thiazolidinediones versus antioxidants | 1 | 164 | Odds Ratio (M‐H, Random, 95% CI) | 1.63 [0.87, 3.05] |
| 6.4 Statins versus other cholesterol‐lowering agents | 1 | 125 | Odds Ratio (M‐H, Random, 95% CI) | 2.77 [1.34, 5.73] |
| 6.5 Statins plus other cholesterol‐lowering agents versus other cholesterol‐lowering agents | 1 | 123 | Odds Ratio (M‐H, Random, 95% CI) | 3.31 [1.57, 6.98] |
| 6.6 Statins plus other cholesterol‐lowering agents versus statins | 1 | 124 | Odds Ratio (M‐H, Random, 95% CI) | 1.19 [0.56, 2.55] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aithal 2008.
| Methods | Randomised clinical trial | |
| Participants | Country: United Kingdom. Number randomised: 74. Post‐randomisation drop‐outs: 0 (0%). Revised sample size: 74. Average age: 54 years. Females: 29 (39,2%). NASH: 74 (100%). Diabetics: 0 (0%). Average follow‐up period in months: 12. Inclusion criteria 1. Age 18 to 70 years of age. 2. Biopsy proven NASH. 3. If under lipid lowering treatment, stable dosage in the previous 3 months before the run‐in period. Exclusion criteria 1. History of alcohol excess more than 210 g per week for men and more than 140 g per week for women. 2. Liver diseases other than NAFLD. 3. Treatment with drugs associated with fatty liver. 4. Diabetes. 5. Only simple steatosis at biopsy. 6. Treatment with weight‐reduction medications. 7. Pregnancy or lactation. 8. Current or previous heart failure. 9. Renal impairment. | |
| Interventions | Participants were randomly assigned to two groups. Group 1: pioglitazone (N = 37). Further details: pioglitazone (30 mg/day). Group 2: control (N = 37). Further details: control: placebo. Duration of treatment: 12 months. All people also underwent diet and lifestyle modification. | |
| Outcomes | Outcomes reported: 1. Deaths 2. Adverse events 3. Decompensated liver disease 4. Liver transplantation 5. Cirrhosis. | |
| Notes | Authors provided additional information in February 2016. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed via the arand computer program (Pharmacy department, University Hospitals NHS Trust, Nottingham, UK) in blocks of 4 ". |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomisation was done in research pharmacy and study nurse provided tablets to the patients. " Comment: Replies by authors. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "randomized, double‐blind, placebo controlled trial". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "randomized, double‐blind, placebo controlled trial". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no post‐randomisation drop‐outs. |
| Selective reporting (reporting bias) | Low risk | Comment: mortality and adverse events were reported. |
| For‐profit bias | High risk | Quote: "Takeda Pharmaceuticals UK provided the pioglitazone and placebo tablets for this investigator‐initiated study". |
| Other bias | Low risk | Comment: no other risk of bias. |
Alam 2016.
| Methods | Randomised clinical trial | |
| Participants | Country: Bangladesh. Number randomised: 50. Post‐randomisation drop‐outs: 20 (40%). Revised sample size: 30. Average age: 42 years. Females: 23 (76,7%). NASH: 30 (100%). Diabetics: 8 (26,7%). Average follow‐up period in months: 12. Inclusion criteria 1. Patients aged 18 to 65 years in whom NAFLD activity score ≥ 5 in liver histology. Exclusion criteria 1. Alcohol intake > 20 g/day. 2. Presence of comorbid conditions such as chronic hepatitis of other causes, chronic obstructive pulmonary disease, chronic kidney disease, congestive cardiac failure, history of recent myocardial infarction, hypothyroidism. 3. Decompensated cirrhosis of liver. 4. Alanine aminotransferase (ALT) > five times upper normal limit. 5. History of taking angiotensin receptor blocker or angiotensin converting enzyme inhibitors. | |
| Interventions | Participants were randomly assigned to two groups. Group 1: telmisartan (N = 20). Further details: telmisartan 40 mg OD. Group 2: control (N = 10). Further details: control: no intervention. Duration of treatment: 12 months. All people also underwent lifestyle modification. | |
| Outcomes | Outcomes reported: 1. Mortality 2. Adverse events 3. Decompensated liver disease 4. Liver transplantation 5. Cirrhosis 6. Change in fibrosis score 7. Change in NAS score 8. Resolution of fatty liver disease. | |
| Notes | Authors provided additional information in September 2016 Reasons for post‐randomisation drop‐outs: lack of interest in undergoing liver biopsy. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Random sequence was generated by lottery. Each subject was requested to pick up one among folded papers on which their destined group name was inscribed". Comment: author replies. |
| Allocation concealment (selection bias) | Low risk | Quote: "Random sequence was generated by lottery. Each subject was requested to pick up one among folded papers on which their destined group name was inscribed". Comment: author replies. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "This was an open‐label RCT". |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "This was an open‐label RCT". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: there were post‐randomisation drop‐outs, which may be related to the treatment that the participants received. |
| Selective reporting (reporting bias) | Low risk | Comment: mortality and adverse events were reported. |
| For‐profit bias | Low risk | Quote: "Alam S, Kabir J, Mustafa G, Gupa UD, Hasan SKMN and Alam KAK declare that there is no financial relation with any person or organization for this study". |
| Other bias | Low risk | Comment: no other risk of bias. |
Aller 2015.
| Methods | Randomised clinical trial | |
| Participants | Country: Spain. Number randomised: 36. Post‐randomisation drop‐outs: not stated. Revised sample size: 36. Average age: 47 years. Females: 14 (38,9%). NASH: 15 (41.7%). Diabetics: 0 (0%). Average follow‐up period in months: 3. Inclusion criteria 1. Patients with biopsy proven NAFLD. Exclusion criteria 1. Hepatitis B or C, Cytomegalovirus, Epstein Barr infections. 2. Non organ‐specific autoantibodies. 3. Alcohol consumption. 4. Diabetes mellitus. 5. Impaired glucose tolerance. 6. Medication (blood‐pressure lowering medication and statins). 7. Hereditary defects (iron and copper storage diseases and alpha 1‐antitrypsin deficiency). | |
| Interventions | Participants were randomly assigned to two groups. Group 1: silymarin plus antioxidants (N = 18). Further details: silymarin 2 tablets per day plus antioxidants: vitamin E 36 mg per day. Group 2: control (N = 18). Further details: control: no intervention. Duration of treatment: 3 months. All people also underwent lifestyle modification which included hypocalorific diet and exercise program. | |
| Outcomes | Outcomes reported: 1. Adverse events. | |
| Notes | Reasons for post‐randomisation drop‐outs: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "All patients were randomized (table of numbers)". |
| Allocation concealment (selection bias) | Unclear risk | Quote: "All patients were randomized (table of numbers)". |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no post‐randomisation drop‐outs. |
| Selective reporting (reporting bias) | High risk | Comment: protocol was not available; mortality was not reported. |
| For‐profit bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other risk of bias. |
Armstrong 2016.
| Methods | Randomised clinical trial | |
| Participants | Country: UK. Number randomised: 52. Post‐randomisation drop‐outs: 0 (0%). Revised sample size: 52. Average age: 51 years. Females: 21 (40.4%). NASH: 52 (100%). Diabetics: 17 (32.7%). Average follow‐up period in months: 17. Inclusion criteria 1. Patients with biopsy confirmed NASH (within 6 months prior to recruitment). 2. 18 to 70 years of age. 3. Body‐mass index (BMI) of 25 kg/m² at screening. Exclusion criteria 1. Substantial alcohol consumption. 2. Poor glycaemic control. 3. Child‐Pugh B/C cirrhosis. 4. Other causes of liver disease. 5. Confounding concomitant drug use (including insulin, incretin mimetics, thiazolidinediones, vitamin E). 6. Disorders such as a medical history of pancreatitis and pancreatic or thyroid carcinoma. | |
| Interventions | Participants were randomly assigned to two groups. Group 1: liraglutide (N = 26). Further details: liraglutide started at 0.6 mg/day to reach a maximum dose of 1.8 mg/day. Group 2: control (N = 26). Further details: control: placebo. Duration of treatment: 11 months. All patients received advice on lifestyle modification | |
| Outcomes | Outcomes reported: 1. Mortality. 2. Adverse events 3. Decompensated liver disease 4. Liver transplantation 5. Cirrhosis 6. Change in fibrosis score. 7. Change in NAS score. 8. Resolution of fatty liver. | |
| Notes | Authors provided additional information in September 2016 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Centre‐delegated staff telephoned randomisation officers at the Cancer Research UK Clinical Trials Unit (Birmingham, UK), who used a computer‐generated, centrally administered procedure to randomly assign eligible patients ". |
| Allocation concealment (selection bias) | Low risk | Quote: "Centre‐delegated staff telephoned randomisation officers at the Cancer Research UK Clinical Trials Unit (Birmingham, UK), who used a computer‐generated, centrally administered procedure to randomly assign eligible patients ". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double‐blind, randomised, placebo‐controlled phase 2 study". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double‐blind, randomised, placebo‐controlled phase 2 study". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: this was low for adverse events but high for change in fibrosis score, NAS score, and resolution of NAFLD as 7 patients were excluded from these analysis. |
| Selective reporting (reporting bias) | Low risk | Comment: mortality and adverse events were reported. |
| For‐profit bias | High risk | Quote: "Wellcome Trust, National Institute of Health Research, and Novo Nordisk". |
| Other bias | Low risk | Comment: no other risk of bias. |
Askarimoghadam 2013.
| Methods | Randomised clinical trial | |
| Participants | Country: Iran. Number randomised: 93. Post‐randomisation drop‐outs: not stated. Revised sample size: 93. Average age: not stated. Females: not stated. NASH: not stated. Diabetics: not stated. Average follow‐up period in months: 6. Inclusion criteria 1. Diagnosis of NAFLD based on ultrasound. 2. Age 18 to 65 years. | |
| Interventions | Participants were randomly assigned to two groups. Group 1: metformin plus antioxidants (N = 40). Further details: metformin 1500 mg/day plus antioxidants: vitamin E 400 IU/day. Group 2: metformin (N = 53). Further details: metformin 1500 mg/day. Duration of treatment: 6 months. Overweight people in both groups received weight loss advice. | |
| Outcomes | None of the outcomes of interest for this review were reported. | |
| Notes | Reasons for post‐randomisation drop‐outs: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "A randomized clinical trial". |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "open label". |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "open label". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: protocol was not available; neither mortality nor adverse events were reported. |
| For‐profit bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other risk of bias. |
Athyros 2006.
| Methods | Randomised clinical trial | |
| Participants | Country: Greece. Number randomised: 186. Post‐randomisation drop‐outs: 0 (0%). Revised sample size: 186. Average age: 60 years. Females: 66 (35.5%). NASH: not stated Diabetics: 0 (0%). Average follow‐up period in months: 12. Inclusion criteria 1. Metabolic syndrome. 2. Low density lipoprotein cholesterol (LDL) > 3.4 mmol/L (130 mg/dL) 3. Ultrasonographic evidence of fatty liver. 4. Elevated AST or ALT activity. Exclusion criteria 1. Diabetes. 2. Cardiovascular disease. 3. History of excessive alcohol ingestion (> 20 g/day). 4. Other liver diseases. 5. Impaired renal function (serum creatinine > 115 µmol/L; 1.5 mg/dL). 6. Aminotransferase > 3 times the upper limit of normality. 7. Creatine kinase activity > 5 times the upper limit of normal (ULN). | |
| Interventions | Participants were randomly assigned to three groups. Group 1: atorvastatin (N = 63). Further details: atorvastatin (20 mg/day). Group 2: fenofibrate (N = 62). Further details: fenofibrate (200 mg/day). Group 3: atorvastatin plus fenofibrate (N = 61). Further details: atorvastatin (20 mg/day) plus fenofibrate (200 mg/day). Duration of treatment: 12 months. All people also underwent diet and lifestyle modification. | |
| Outcomes | Outcomes reported: 1. Mortality 2. Adverse events 3. Liver cirrhosis 4. Decompensated liver disease 5. Liver transplantation. | |
| Notes | Authors provided additional information in February 2016. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Random Number Generation Computer Program". |
| Allocation concealment (selection bias) | Low risk | Quote: "The randomisation sequence was generated by AAP, the enrolment was performed by OIG, OIK and KG and the random allocation was performed by VGA, who was blinded to hypolipidaemic drug treatment". Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "prospective, open‐label, randomized". |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "prospective, open‐label, randomized". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no post‐randomisation drop‐outs. |
| Selective reporting (reporting bias) | Low risk | Comment: mortality and adverse events were reported. |
| For‐profit bias | High risk | Quote: " Some of the authors have given talks, attended conferences and participated in trials and advisory boards sponsored by various pharmaceutical companies". |
| Other bias | Low risk | Comment: no other risk of bias. |
Baranova 2015.
| Methods | Randomised clinical trial | |
| Participants | Country: Russia. Number randomised: 20. Post‐randomisation drop‐outs: not stated. Revised sample size: 20. Average age: 52 years. Females: 12 (60%). NASH: not stated. Diabetics: not stated. Average follow‐up period in months: 6. Inclusion criteria 1. Patients with metabolic syndrome (high blood pressure, dyslipidaemia, and NAFLD) Exclusion criteria 1. Patients with severe chronic diseases, heart disease, chronic heart failure, atrial fibrillation, myocardial infarction, stroke, unstable angina 2. Inability to accept ACE inhibitors. | |
| Interventions | Participants were randomly assigned to two groups. Group 1: rosuvastatin (N = not stated). Further details: rosuvastatin (dose not stated). Group 2: control (N = not stated). Further details: control: no intervention. Duration of treatment: 6 months. Number of participants in each group was not stated. All received advice on lifestyle changes. | |
| Outcomes | None of the outcomes of interest were reported in this trial. | |
| Notes | Reasons for post‐randomisation drop‐outs: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "6 months randomised study". |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: protocol was not available; neither mortality nor adverse events were reported. |
| For‐profit bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other risk of bias. |
Basu 2013.
| Methods | Randomised clinical trial | |
| Participants | Country: USA. Number randomised: 80. Post‐randomisation drop‐outs: not stated. Revised sample size: 80. Average age: not stated Females: not stated NASH: not stated Diabetics: 0 (0%). Average follow‐up period in months: 12. Inclusion criteria 1. Diagnosis of NAFLD. Exclusion criteria 1. Alcohol consumption > 30 g/day. 2. HIV. 3. Steatosis inducing medications like herbal supplementations. 4. Lipodystrophy. 5. Overt diabetes. 6. Pregnancy. 7. Hypersensitivity to study medications. | |
| Interventions | Participants were randomly assigned to two groups. Group 1: pioglitazone (N = not stated). Further details: pioglitazone 15 mg (frequency not stated). Group 2: antioxidants (N = not stated). Further details: antioxidants: vitamin E (dose and frequency not stated). Duration of treatment: 12 months. In both groups, half of patients received curcumin which was chosen at random. | |
| Outcomes | None of the outcomes of interest for this review were reported. | |
| Notes | Reasons for post‐randomisation drop‐outs: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "a randomized open label placebo controlled clinical prospective trial ". |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "open label". |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "open label". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: protocol was not available; neither mortality nor adverse events were reported. |
| For‐profit bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other risk of bias. |
Basu 2014.
| Methods | Randomised clinical trial | |
| Participants | Country: USA. Number randomised: 155. Post‐randomisation drop‐outs: 0 (0%). Revised sample size: 155. Average age: 36 years. Females: 102 (65.8%). NASH: not stated Diabetes: 0 (0%). Average follow‐up period in months: 6. Inclusion criteria 1. BMI > 28. 2. Diagnosis of NAFLD/NASH. Exclusion criteria 1. Diabetes. 2. BMI > 33. 3. Alcohol intake > 30 g/day. 4. Hepatitis B or C. 5. Hypothyroidism. 6. Medications including herbs and supplements. | |
| Interventions | Participants were randomly assigned to two groups. Group 1: antioxidants (N = 120). Further details: antioxidants: vitamin E (700 IU/day) and/or alfa lipoic acid (300 mg/day). Group 2: control (N = 35). Further details: control: placebo. Duration of treatment: 6 months. | |
| Outcomes | Outcomes reported: 1. Adverse events. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The envelopes were used for concealment but the randomization was based on random numbers generated by a computer". |
| Allocation concealment (selection bias) | Low risk | Quote: "The envelopes were used for concealment but the randomization was based on random numbers generated by a computer. People uninvolved with the study were tasked with the randomization process". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "open label". |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "open label". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no post‐randomisation drop‐outs. |
| Selective reporting (reporting bias) | High risk | Comment: protocol was not available; mortality was not reported. |
| For‐profit bias | High risk | Quote: "Editorial assistance was provided under the direction of the authors by Med Think SciCom with support from Salix Pharmaceuticals, Inc". |
| Other bias | Low risk | Comment: no other risk of bias. |
Belfort 2006.
| Methods | Randomised clinical trial | |
| Participants | Country: USA, Italy. Number randomised: 55. Post‐randomisation drop‐outs: 8 (14,5%). Revised sample size: 47. Average age: 51 years. Females: 26 (55.3%). NASH: 47 (100%). Diabetics: 0 (0%). Average follow‐up period in months: 6. Inclusion criteria 1. Biopsy‐proven NASH. 2. Impaired glucose tolerance or type 2 diabetes mellitus (DM). Exclusion criteria 1. AST and ALT elevated ≥ to 2.5 times the upper limit of normal. 2. History of alcohol use (> 1 drink per day). 3. Fasting glucose more or equal to 240 mg/dL. 4. Type 1 diabetes. 5. Heart disease. 6. Hepatic (other than NASH) disease. 7. Renal disease. 8. Metformin, thiazolidinediones or insulin use. | |
| Interventions | Participants were randomly assigned to two groups. Group 1: pioglitazone (N = 26). Further details: pioglitazone (30 mg/day increased to 45 mg/day after 2 months). Group 2: control (N = 21). Further details: control: placebo. Duration of treatment: 6 months. All people also underwent dietary advice. | |
| Outcomes | Outcomes reported: 1. Cirrhosis 2. Resolution of fatty liver disease 3. Change in fibrosis score. | |
| Notes | Reasons for post‐randomisation drop‐outs: discontinued treatment, withdrew from study, developed medical complications. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was computer‐generated by the research pharmacy". |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization was computer‐generated by the research pharmacy, and the investigators were unaware of the treatment assignments". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "randomized, double‐blind, placebo controlled trial". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "randomized, double‐blind, placebo controlled trial". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: there were post‐randomisation drop‐outs, which may be related to the treatment that the participants received. |
| Selective reporting (reporting bias) | High risk | Comment: protocol was not available; neither mortality nor adverse events were reported. |
| For‐profit bias | High risk | Quote: "Supported by grants from the National Center for Research Resources (MO1‐RR‐01346, to the Frederic C. Bartter General Clinical Research Center and its Imaging Core), Takeda Pharmaceuticals, and the Veterans Affairs Medical Research Fund". |
| Other bias | Low risk | Comment: no other risk of bias. |
Bonfrate 2015.
| Methods | Randomised clinical trial | |
| Participants | Country: Italy. Number randomised: 40. Post‐randomisation drop‐outs: not stated. Revised sample size: 40. Average age: not stated. Females: not stated. NASH: not stated. Diabetics: not stated. Average follow‐up period in months: 6. Inclusion criteria 1. Patients with NAFLD and metabolic disorders. | |
| Interventions | Participants were randomly assigned to two groups. Group 1: silymarin plus antioxidants (N = not stated). Further details: silymarin plus antioxidants: vitamin E (Eurosil). Group 2: control (N = not stated). Further details: control: no intervention. Duration of treatment: 6 months. Number of participants in each group was not stated. | |
| Outcomes | None of the outcomes of interest were reported in this trial. | |
| Notes | Reasons for post‐randomisation drop‐outs: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Subjects were randomized 1:1 into Eurosil 85‐vit. E complex or placebo for six months". |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: protocol was not available; neither mortality nor adverse events were reported. |
| For‐profit bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other risk of bias. |
Bugianesi 2005.
| Methods | Randomised clinical trial | |
| Participants | Country: Italy. Number randomised: 57. Post‐randomisation drop‐outs: not stated. Revised sample size: 57. Average age: 41 years. Females: 7 (12.3%). NASH: not stated. Diabetics: 0 (0%). Average follow‐up period in months: 12. Inclusion criteria 1. Increased ALT values: > 1.5 times the upper limit of normal. 2. NAFLD. Exclusion criteria 1. Alcohol consumption > 20 g/day. 2. Positive screening for B or C viral hepatitis. 3. Autoimmune hepatitis or coeliac disease. 4. Gene markers of familiar haemochromatosis. 5. Diabetes. 6. BMI more or equal than 35 kg/m². | |
| Interventions | Participants were randomly assigned to two groups. Group 1: metformin (N = 29). Further details: metformin 2 g/day. Group 2: antioxidants (N = 28). Further details: antioxidants: vitamin E 400 mg twice daily. Duration of treatment: 12 months. All people also underwent exercise and dietary advice. Another group which involved prescriptive low calorie diet as the other groups did not receive this intervention. | |
| Outcomes | None of the outcomes of interest were reported in this trial. | |
| Notes | Reasons for post‐randomisation drop‐outs: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The randomization procedure was centralized in Bologna, and based on a random sequence". Comment: Further details were not available. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Sealed envelopes". Comment: Further information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "open label, randomized trial". |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "open label, randomized trial". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "Authors used an intention‐to‐treat analysis based on last observation carried forward technique". |
| Selective reporting (reporting bias) | High risk | Comment: protocol was not available; neither mortality nor adverse events were reported. |
| For‐profit bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other risk of bias. |
Chan 2015.
| Methods | Randomised clinical trial | |
| Participants | Country: Malaysia. Number randomised: 64. Post‐randomisation drop‐outs: not stated. Revised sample size: 64. Average age: 50 years. Females: 36 (56.3%). NASH: 64 (100%). Diabetics: not stated. Average follow‐up period in months: 11. Inclusion criteria 1. Patients with NASH. | |
| Interventions | Participants were randomly assigned to two groups. Group 1: silymarin (N = 30). Further details: silymarin 700 mg thrice daily. Group 2: control (N = 34). Further details: control: placebo. Duration of treatment: 11 months. | |
| Outcomes | Outcomes reported: 1. Cirrhosis. 2. Change in fibrosis score. 3. Change in NAS score. 4. Resolution of NASH. | |
| Notes | Reasons for post‐randomisation drop‐outs: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "This is a randomized, double‐blind, placebo‐controlled study of silymarin". |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double‐blind". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double‐blind". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: protocol was not available; neither mortality nor adverse events were reported. |
| For‐profit bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other risk of bias. |
Copaci 2009.
| Methods | Randomised clinical trial | |
| Participants | Country: Romania. Number randomised: 94. Post‐randomisation drop‐outs: not stated. Revised sample size: 94. Average age: 49 years. Females: 44 (46.8%). NASH: 94 (100%). Diabetics: not stated. Average follow‐up period in months: 12. Inclusion criteria 1. Biopsy proven NASH. Exclusion criteria 1. Liver diseases other than NAFLD. 2. Insulin treatment. 3. Renal failure. | |
| Interventions | Participants were randomly assigned to three groups. Group 1: pentoxifylline (N = 32). Further details: pentoxifylline 1200 mg/day. Group 2: UDCA (N = 30). Further details: UDCA 13 mg/kg/day. Group 3: pentoxifylline plus UDCA (N = 32). Further details: pentoxifylline 1200 mg/day and UDCA 13 mg/kg/day. Duration of treatment: 12 months. All people also underwent lifestyle modification (diet and regular exercise). | |
| Outcomes | None of the outcomes of interest were reported. | |
| Notes | Reasons for post‐randomisation drop‐outs: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly assigned to three groups:" |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: protocol was not available; neither mortality nor adverse events were reported. |
| For‐profit bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other risk of bias. |
Cui 2006.
| Methods | Randomised clinical trial | |
| Participants | Country: China. Number randomised: 124. Post‐randomisation drop‐outs: not stated. Revised sample size: 124. Average age: 45 years. Females: 60 (48.4%). NASH: not stated. Diabetics: not stated. Average follow‐up period in months: 6. Inclusion criteria 1. Alcohol consumption less than 40 g/week. 2. Elevated transaminases. 3. US proven NAFLD. 4. Histologically proven steatosis. Exclusion criteria 1. Viral hepatitis. 2. Total parenteral nutrition. 3. Other causes of fatty liver disease. 4. Lipid lowering drug. 5. Cirrhosis. | |
| Interventions | Participants were randomly assigned to two groups. Group 1: rosiglitazone (N = 63). Further details: rosiglitazone 4 mg twice daily. Group 2: control (N = 60). Further details: control: placebo. Duration of treatment: 6 months. Both groups received dietary and exercise advice. | |
| Outcomes | None of the outcomes of interest were reported. | |
| Notes | Reasons for post‐randomisation drop‐outs: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomized, double‐blind treatment group". |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "randomized, double‐blind treatment group". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "randomized, double‐blind treatment group". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: protocol was not available; neither mortality nor adverse events were reported. |
| For‐profit bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other risk of bias. |
Cusi 2013.
| Methods | Randomised clinical trial. | |
| Participants | Country: USA. Number randomised: 101. Post‐randomisation drop‐outs: not stated. Revised sample size: 101. Average age: 51 years. Females: not stated. NASH: 101 (100%). Diabetics: 52 (51.5%). Average follow‐up period in months: 18. Inclusion criteria 1. Biopsy proven NASH. 2. Prediabetes or type 2 diabetes. | |
| Interventions | Participants were randomly assigned to two groups. Group 1: pioglitazone (N = not stated). Further details: pioglitazone (dose and duration not stated). Group 2: control (N = not stated). Further details: control: placebo. Duration of treatment: 18 months. | |
| Outcomes | None of the outcomes of interest were reported. | |
| Notes | Reasons for post‐randomisation drop‐outs: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "We randomized 101 patients with biopsy‐proven NASH ". |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: protocol was not available; neither mortality nor adverse events were reported. |
| For‐profit bias | High risk | Quote: "Grant/Research Support: Takeda, Novartis, Mannkind". |
| Other bias | Low risk | Comment: no other risk of bias. |
Dufour 2006.
| Methods | Randomised clinical trial | |
| Participants | Country: Switzerland. Number randomised: 48. Post‐randomisation drop‐outs: 8 (16.7%). Revised sample size: 40. Average age: not stated. Females: not stated. NASH: 40 (100%). Diabetics: not stated. Average follow‐up period in months: 24. Inclusion criteria 1. Aged between 18 and 75 years. 2. Biopsy proven NASH performed within 6 months from inclusion. 3. Persistent elevation of ALT levels of at least 1.5 times the upper limit of normal. 4. Weekly alcohol consumption < 40 g. Exclusion criteria 1. Positive screening for B or C viral hepatitis. 2. Abnormal transferrin saturation. 3. ANA title more than 1:80. 4. Histologic findings suggestive of other liver diseases. 5. Decompensated cirrhosis. 6. Serious diseases limiting life expectancy. 7. Pregnancy or lactation. 8. Treatment with NASH‐inducing drugs (amiodarone, calcium channel blockers, tamoxifen) or oral anticoagulant. | |
| Interventions | Participants were randomly assigned to three groups. Group 1: UDCA plus antioxidants (N = 12). Further details: UDCA 12 ‐ 15 mg/kg/day plus antioxidants: vitamin E 400 IU twice daily. Group 2: UDCA (N = 15). Further details: UDCA 12 to 15 mg/kg/day. Group 3: control (N = 13). Further details: control: placebo. Duration of treatment: 6 months. All people also underwent dietary advice. | |
| Outcomes | Outcomes reported: 1. Change in fibrosis scores. | |
| Notes | Reasons for post‐randomisation drop‐outs: did not have paired biopsy. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The pharmacy established before the start of the study a list randomly assigning each patient to 1 of the 3 arms of the study". Comment: Further details were not available. |
| Allocation concealment (selection bias) | Low risk | Quote: "The pharmacy established before the start of the study a list randomly assigning each patient to 1 of the 3 arms of the study". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The patients as well as their physicians were blinded to the treatment until completion of the whole study". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The patients as well as their physicians were blinded to the treatment until completion of the whole study". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: there were post‐randomisation drop‐outs, which may be related to the treatment that the participants received. |
| Selective reporting (reporting bias) | High risk | Comment: protocol was not available; neither mortality nor adverse events were reported. |
| For‐profit bias | High risk | Quote: "Capsules containing UDCA 250 mg and placebo capsules were provided by Falk Pharma GmH (Freiburg, Germany). Tablets containing vitamin E (natural d‐tocopherol) 400 IU and placebo tablets were provided by Antistress AG (Rapperswil, Switzerland)". |
| Other bias | Low risk | Comment: no other risk of bias. |
Ersoz 2005.
| Methods | Randomised clinical trial | |
| Participants | Country: Turkey. Number randomised: 57. Post‐randomisation drop‐outs: 1 (1.8%). Revised sample size: 56. Average age: 47 years. Females: 23 (41.1%). NASH: 6 (10.7%). Diabetics: 14 (25%). Average follow‐up period in months: 6. Inclusion criteria 1. ALT levels at least 1.2 times the upper limit of normal despite a three‐month weight reducing diet. 2. Biopsy proven NAFLD. Exclusion criteria 1. Alcohol intake > 20 g/day. 2. Viral hepatitis B and C. 3. Other hepatic diseases including auto‐immune hepatitis, Wilson's disease, haemochromatosis and alpha‐1 antitrypsin deficiency. 4. Severe cardiac, pulmonary, renal or psychological problems. | |
| Interventions | Participants were randomly assigned to two groups. Group 1: UDCA (N = 29). Further details: UDCA 10 mg/kg/day. Group 2: antioxidants (N = 27). Further details: antioxidants: vitamin E 600 IU/day and vitamin C 500 mg/day. Duration of treatment: 6 months. | |
| Outcomes | Outcomes reported: 1. Adverse events 2. Resolution of fatty liver disease. | |
| Notes | Reasons for post‐randomisation drop‐outs: discontinued participation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "prospective, open‐label, randomized". |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "prospective, open‐label, randomized". |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "prospective, open‐label, randomized". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: there were post‐randomisation drop‐outs, which may be related to the treatment that the participants received. |
| Selective reporting (reporting bias) | High risk | Comment: protocol was not available; neither mortality nor adverse events were reported. |
| For‐profit bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other risk of bias. |
Fogari 2012.
| Methods | Randomised clinical trial | |
| Participants | Country: Italy. Number randomised: 150. Post‐randomisation drop‐outs: 9 (6%). Revised sample size: 141. Average age: not stated. Females: not stated. NASH: not stated. Diabetics: 0 (0%). Average follow‐up period in months: 12. Inclusion criteria 1. Mild‐moderate hypertension (> 140/90 mmHg). 2. Normal cholesterol (LDL < 160 mg/dL). 3. Overweight or obesity (BMI between 25 and 34.9 kg/m²). 4. US proven hepatic steatosis. Exclusion criteria 1. Malignant or secondary hypertension. 2. Impaired kidney function. 3. Muscle toxicity. 4. CPK > 2 times upper limit of normal. 5. Hb less than 8 g/dL. 6. Diabetes mellitus. 7. Valvular heart disease. 8. Hypertensive retinopathy of III‐IV grade. 9. Unstable cardiovascular condition in the previous 6 months (congestive heart failure NYHA class 1 to 4 or history of myocardial infarction or stroke). 10. Pregancy or lactation. 11. Contra‐indication or intolerance to angiotensin 1 receptor blockers, calcium channel blockers or HMG‐CoA inhibitors. | |
| Interventions | Participants were randomly assigned to two groups. Group 1: losartan (N = 71). Further details: losartan 50 mg/day increased to 100 mg/day after one month. Group 2: amlodipine (N = 70). Further details: amlodipine 5 mg/day increased to 10 mg/day after one month. Duration of treatment: 6 months. After this simvastatin was added to both groups for another 6 months. | |
| Outcomes | None of the outcomes of interest were reported in this trial. | |
| Notes | Reasons for post‐randomisation drop‐outs: lost‐to‐follow up, side effects. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomization was done using a drawing of envelopes containing randomization codes prepared by a statistician". Comment: Further details were not available. |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization was done using a drawing of envelopes containing randomization codes prepared by a statistician". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "randomized, double‐blind, parallel study". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "randomized, double‐blind, parallel study". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: there were post‐randomisation drop‐outs, which may be related to the treatment that the participants received. |
| Selective reporting (reporting bias) | High risk | Comment: protocol was not available; neither mortality nor adverse events were reported. |
| For‐profit bias | Low risk | Quote: "The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties". |
| Other bias | Low risk | Comment: no other risk of bias. |
Foster 2011.
| Methods | Randomised clinical trial | |
| Participants | Country: USA. Number randomised: 80. Post‐randomisation drop‐outs: not stated. Revised sample size: 80. Average age: not stated. Females: not stated. NASH: not stated. Diabetics: not stated. Average follow‐up period in months: not stated. Inclusion criteria 1. CT proven hepatic steatosis. Exclusion criteria 1. Coronary artery disease. 2. Insulin‐dependent diabetes. 3. Bleeding diathesis. 4. Severe anaemia. 5. Cancer within past 5 years. | |
| Interventions | Participants were randomly assigned to two groups. Group 1: atorvastatin plus antioxidants (N = 44). Further details: atorvastatin 20 mg/day plus vitamin C 1 g/day plus vitamin E 1,000 U/day. Group 2: control (N = 36). Further details: control: placebo. Duration of treatment: not reported. | |
| Outcomes | None of the outcomes of interest were reported in this trial. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double‐blind, placebo‐controlled". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double‐blind, placebo‐controlled". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: protocol was not available; neither mortality nor adverse events were reported. |
| For‐profit bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other risk of bias. |
Garinis 2010.
| Methods | Randomised clinical trial | |
| Participants | Country: Italy. Number randomised: 50. Post‐randomisation drop‐outs: 5 (10%). Revised sample size: 45. Average age: 44 years. Females: 38 (84.4%). NASH: not stated. Diabetics: not stated. Average follow‐up period in months: 6. Inclusion criteria 1. Ultrasound proven NAFLD. 2. BMI more than 25 kg/m². Exclusion criteria 1. Heart disease. 2. Renal failure. 3. Smoking habits. 4. Alcohol intake > 20 g/day. 5. Viral, autoimmune, metabolic or genetic liver diseases. 6. Drugs known for inducing liver steatosis. | |
| Interventions | Participants were randomly assigned to two groups. Group 1: metformin (N = 20). Further details: metformin 1 g per day. Group 2: control (N = 25). Further details: control: no intervention. Duration of treatment: 6 months. Both groups received hypocaloric diet. | |
| Outcomes | Outcomes reported: Resolution of fatty liver. | |
| Notes | Reasons for post‐randomisation drop‐outs: non‐compliance to treatment. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomized into two groups". |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All patients underwent US liver evaluation by a single experienced operator (M.D.S.), blinded to the clinical data". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: there were post‐randomisation drop‐outs, which may be related to the treatment that the participants received. |
| Selective reporting (reporting bias) | High risk | Comment: protocol was not available; neither mortality nor adverse events were reported. |
| For‐profit bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other risk of bias. |
Gastaldelli 2009.
| Methods | Randomised clinical trial | |
| Participants | Country: USA. Number randomised: 48. Post‐randomisation drop‐outs: not stated. Revised sample size: 48. Average age: not stated. Females: not stated. NASH: 48 (100%). Diabetics: not stated. Average follow‐up period in months: 6. Inclusion criteria 1. Biopsy proven NASH. | |
| Interventions | Participants were randomly assigned to two groups. Group 1: pioglitazone (N = not stated). Further details: pioglitazone 45 mg/day. Group 2: control (N = not stated). Further details: control: placebo. Duration of treatment: 6 months. Both groups received hypocaloric diet. | |
| Outcomes | None of the outcomes of interest were reported. | |
| Notes | Reasons for post‐randomisation drop‐outs: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients received a hypocaloric diet and were randomized (double‐blind) to PIO (45 mg/d) or placebo (Placebo) for 6 months". |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double‐blind". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double‐blind". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: protocol was not available; neither mortality nor adverse events were reported. |
| For‐profit bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other risk of bias. |
Gianturco 2013.
| Methods | Randomised clinical trial | |
| Participants | Country: Italy. Number randomised: 200. Post‐randomisation drop‐outs: 4 (2%). Revised sample size: 196. Average age: 62 years. Females: 92 (46.9%). NASH: not stated. Diabetics: 0 (0%). Average follow‐up period in months: 12. Inclusion criteria 1. Biopsy proven NAFLD. Exclusion criteria 1. History of HBV or HCV infection. 2. Gallstones. 3. Alcohol consumption. 4. Renal failure. 5. Diabetes. | |
| Interventions | Participants were randomly assigned to four groups. Group 1: UDCA plus antioxidants (N = 52). Further details: UDCA (300 mg/day) plus antioxidants: alpha lipoic acid (400 mg/ day). Group 2: antioxidants (N = 52). Further details: antioxidants: alpha lipoic acid (400 mg/ day). Group 3: UDCA (N = 46). Further details: UDCA (300 mg/day). Group 4: control (N = 46). Further details: control: no intervention. Duration of treatment: 12 months. All four groups received hypocaloric diet. | |
| Outcomes | Outcomes reported: 1. NAFLD activity score. | |
| Notes | Reasons for post‐randomisation drop‐outs: onset of diabetes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computerized random numbers". |
| Allocation concealment (selection bias) | Low risk | Quote: "The ALA and UDCA were in capsule forms and were identical in appearance. They were prepared in bottles and consecutively numbered for each patient, according to the randomization schedule". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double‐blind, randomized clinical trial ". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double‐blind, randomized clinical trial ". Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: there were post‐randomisation drop‐outs, which may be related to the treatment that the participants received. |
| Selective reporting (reporting bias) | High risk | Comment: protocol was not available; neither mortality nor adverse events were reported. |
| For‐profit bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other risk of bias. |
Gomez 2009.
| Methods | Randomised clinical trial | |
| Participants | Country: Cuba. Number randomised: 60. Post‐randomisation drop‐outs: 0 (0%). Revised sample size: 60. Average age: 47 years. Females: 26 (43.3%). NASH: 60 (100%). Diabetics: not stated. Average follow‐up period in months: 6. Inclusion criteria 1. Biopsy proven NASH. 2. Age between 18 and 70 year old. 3. Weekly alcohol consumption < 20 g. Exclusion criteria 1. Any other liver disease. 2. HBV or HCV positivity. 3. Pregnancy or lactation. 4. Decompensated cirrhosis. 5. Drug related NAFLD gastrointestinal by‐pass. 6. Treatment with UDCA, vitamin E, pioglitazone, betaine, rosiglitazone, metformin, pentoxyphilline or gemfibrozil. 7. Use of statin within the 6 month period before enrolment. 8. Fasting glucose level less than 250 mg/dL. 9. Contraindication to liver biopsy. 10. BMI more or equal to 35 kg/m². 11. Concomitant disease with reduced life expectancy. 12. Severe psychiatric conditions and drug dependence. | |
| Interventions | Participants were randomly assigned to two groups. Group 1: antioxidants (N = 22). Further details: antioxidants: visuid 50 g/day (antioxidant). Group 2: control (N = 20). Further details: control: no intervention. Duration of treatment: 6 months. Both groups received hypocaloric diet and exercise advice. | |
| Outcomes | Outcomes reported: 1. Change in fibrosis score. 2. Change in NAFLD activity score. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomization was conducted by blocks of 4". |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Biopsy specimens were examined by a single pathologist who was unaware of the patients’ clinical and biochemical data, treatment assignment and liver biopsy sequence". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no post‐randomisation drop‐outs.. |
| Selective reporting (reporting bias) | High risk | Comment: protocol was not available; neither mortality nor adverse events were reported. |
| For‐profit bias | High risk | Quote: "Supported in part by a grant from Catalysis Laboratories, Spain". |
| Other bias | Low risk | Comment: no other risk of bias. |
Hajaghamohammadi 2008.
| Methods | Randomised clinical trial | |
| Participants | Country: Iran. Number randomised: 50. Post‐randomisation drop‐outs: not stated. Revised sample size: 50. Average age: 40 years. Females: 18 (36%). NASH: not stated. Diabetics: 0 (0%). Average follow‐up period in months: 2. Inclusion criteria 1. Ultrasound proven NAFLD. 2. Elevated AST and ALT. Exclusion criteria 1. Diabetes. 2. Alcohol abuse. 3. Positive markers for autoimmune or viral hepatitis. | |
| Interventions | Participants were randomly assigned to two groups. Group 1: silymarin (N = 25). Further details: silymarin 140 mg/day. Group 2: control (N = 25). Further details: control: placebo. Duration of treatment: 2 months. | |
| Outcomes | None of the outcomes of interest were reported in this trial. | |
| Notes | Reasons for post‐randomisation drop‐outs: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly assigned to each group". |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "Placebo was completely similar to the active drug respecting the shape, color and package and all its ingredients were identical to the main drug except for silymarin active extract which did not exist in the placebo ". Comment: an identical placebo was used; however, there was no mention about blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Placebo was completely similar to the active drug respecting the shape, color and package and all its ingredients were identical to the main drug except for silymarin active extract which did not exist in the placebo ". Comment: an identical placebo was used; however, there was no mention about blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: protocol was not available; neither mortality nor adverse events were reported. |
| For‐profit bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other risk of bias. |
Hajiaghamohammadi 2012.
| Methods | Randomised clinical trial | |
| Participants | Country: Iran. Number randomised: 66. Post‐randomisation drop‐outs: 0 (0%). Revised sample size: 66. Average age: 33 years. Females: 24 (36.4%). NASH: not stated. Diabetics: 0 (0%). Average follow‐up period in months: 2. Inclusion criteria 1. Ultrasound proven NAFLD. 2. Elevated AST and ALT. Exclusion criteria 1. Diabetes. 2. Alcohol consumption. 3. Positive markers for autoimmune or viral hepatitis. 4. Use of drugs like statins, NSAID and fibrate. 5. Chronic liver disease. | |
| Interventions | Participants were randomly assigned to three groups. Group 1: pioglitazone (N = 22). Further details: pioglitazone 15 mg once daily. Group 2: metformin (N = 22). Further details: metformin 500 mg/day. Group 3: silymarin 140 mg/day (N = 22). Further details: silymarin 140 mg/day. Duration of treatment: 2 months. | |
| Outcomes | None of the outcomes of interest were reported in this trial. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly allocated patients into three intervention groups". |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no post‐randomisation drop‐outs. |
| Selective reporting (reporting bias) | High risk | Comment: protocol was not available; neither mortality nor adverse events were reported. |
| For‐profit bias | Low risk | Quote: "Funding/Support: None declared". |
| Other bias | Low risk | Comment: no other risk of bias. |
Harrison 2003.
| Methods | Randomised clinical trial | |
| Participants | Country: U.S.A. Number randomised: 49. Post‐randomisation drop‐outs: 4 (8.2%). Revised sample size: 45. Average age: 51 years. Females: 25 (55.6%). NASH: 45 (100%). Diabetics: 19 (42.2%). Average follow‐up period in months: 6. Inclusion criteria 1. Biopsy proven NASH performed within 6 months before the study. 2. Age more than 18 years old. 3. Elevation of transaminases. Exclusion criteria 1. Other causes for chronic liver disease like hepatitis B and C, hereditary haemochromatosis, alpha1 antitrypsin deficiency, Wilson’s disease, or autoimmune liver disease. 2. Use of drugs associated with steatohepatitis, such as tamoxifen, steroids, chloroquine, or amiodarone. 3. Prior surgical procedures, such as gastroplasty, jejunoileal or jejunocolic bypass. 4. Evidence of decompensated liver disease, such as a history of or the presence of ascites, bleeding varices, or hepatic encephalopathy 5. Pregnancy. 6. Total parenteral nutrition within the past 6 months. 7. History of organ transplant. 8. Other conditions that have been known to cause NASH or worsen the disease. 9. History of alcohol consumption > 10 g per day. | |
| Interventions | Participants were randomly assigned to two groups. Group 1: antioxidants (N = 23). Further details: antioxidants: vitamin E 1000 IU/day and vitamin C 1000 mg/day. Group 2: control (N = 22). Further details: control: placebo. Duration of treatment: 6 months. | |
| Outcomes | Outcomes reported: 1. Change in fibrosis score. 2. Resolution of NAFLD. | |
| Notes | Reasons for post‐randomisation drop‐outs: Did not complete the study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer‐generated randomization table". |
| Allocation concealment (selection bias) | Low risk | Quote: "This randomization table was kept by the pharmacy where the vitamins or placebo were to be obtained by the patient. The patients were assigned to either the vitamin group or the placebo group, based on the coded randomization table, so that only the pharmacist knew which intervention the patient was receiving". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "so that only the pharmacist knew which intervention the patient was receiving…..Both the principal investigator and pathologist were blinded as to the patient’s intervention". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "so that only the pharmacist knew which intervention the patient was receiving…..Both the principal investigator and pathologist were blinded as to the patient’s intervention". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: there were post‐randomisation drop‐outs, which may be related to the treatment that the participants received. |
| Selective reporting (reporting bias) | High risk | Comment: protocol was not available; neither mortality nor adverse events were reported. |
| For‐profit bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other risk of bias. |
Harrison 2009.
| Methods | Randomised clinical trial | |
| Participants | Country: USA. Number randomised: 50. Post‐randomisation drop‐outs: 9 (18%). Revised sample size: 41. Average age: 47 years. Females: 28 (68.3%). NASH: 41 (100%). Diabetics: 4 (9.8%). Average follow‐up period in months: 9. Inclusion criteria 1. Biopsy proven NASH within 24 months before enrolment. 2. Ages > 18 years. Exclusion criteria 1. Other liver disease than NASH. 2. Decompensated liver disease. 3. History of alcohol consumption > 20 g/day in the past 2 years. 3. Prior surgical weight loss procedures within the past 6 months. 4. Use of UDCA, rosiglitazone, pioglitazone, metformin in the previous 6 months. | |
| Interventions | Participants were randomly assigned to two groups. Group 1: orlistat plus antioxidants (N = 23). Further details: orlistat 120 mg thrice daily plus antioxidants: vitamin E 800 IU once daily. Group 2: antioxidants (N = 18). Further details: antioxidants: vitamin E 800 IU once daily. Duration of treatment: 9 months. Both groups received hypocaloric diet and exercise advice. | |
| Outcomes | None of the outcomes of interest were reported in this trial. | |
| Notes | Reasons for post‐randomisation drop‐outs: Withdrew consent, lost‐to follow‐up, unable to obtain pre‐treatment trichrome value. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "prospective, parallel, randomized treatment trial". |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Liver histology (H&E stain and Masson’s trichrome stain) was evaluated in a blinded fashion at study completion by an expert hepatopathologist (E.B.)". Comment: It was not clear whether the remaining outcomes were assessed by blinded assessors. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: there were post‐randomisation drop‐outs, which may be related to the treatment that the participants received. |
| Selective reporting (reporting bias) | High risk | Comment: protocol was not available; neither mortality nor adverse events were reported. |
| For‐profit bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other risk of bias. |
Hashemi 2009.
| Methods | Randomised clinical trial | |
| Participants | Country: Iran. Number randomised: 100. Post‐randomisation drop‐outs: not stated. Revised sample size: 100. Average age: 39 years. Females: 43 (43%). NASH: 100 (100%). Diabetics: not stated. Average follow‐up period in months: 6. Inclusion criteria 1. Sonographic evidence of fatty liver. 2. Elevated ALT > 1.2 times of the normal. 3. Suggestive histological evidence of NASH. 4. Presence of strong risk factors such as type 2 diabetes or obesity (BMI > 30 kg/m²). Exclusion criteria 1. Intake of ethanol > 20 g per day. 2. Use of drugs known to produce fatty liver disease (steroids, oestrogens, amiodarone, tamoxifen, or other chemotherapeutic agents ) in the previous 6 months. 3. Viral hepatitis B and C, auto‐immune hepatitis, Wilson’s disease, haemochromatosis, and alpha‐1 antitrypsin deficiency. 4. Severe comorbid medical conditions (cardiac, pulmonary, renal, or psychological problems). | |
| Interventions | Participants were randomly assigned to two groups. Group 1: silymarin (N = 50). Further details: silymarin 280 mg/day. Group 2: control (N = 50). Further details: control: placebo. Duration of treatment: 6 months. | |
| Outcomes | None of the outcomes of interest were reported in this trial. | |
| Notes | Reasons for post‐randomisation drop‐outs: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomized controlled trial". |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: although an identical placebo was used, there was no mention of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: although an identical placebo was used, there was no mention of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: protocol was not available; neither mortality nor adverse events were reported. |
| For‐profit bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other risk of bias. |
Haukeland 2009.
| Methods | Randomised clinical trial | |
| Participants | Country: Norway. Number randomised: 48. Post‐randomisation drop‐outs: 4 (8.3%). Revised sample size: 44. Average age: 47 years. Females: 12 (27.3%). NASH: not stated. Diabetics: 12 (27.3%). Average follow‐up period in months: 6. Inclusion criteria 1. Histologically proven NAFLD by biopsy performed within 18 months prior to enrolment. 2. ALT and AST elevated (> upper limit of normal) 3. Impaired glucose tolerance or type 2 diabetes. Exclusion criteria 1. Loss > 5 kg since the time of biopsy. 2. Previous or ongoing treatment with insulin, metformin, thiazolinediones. 3. Kidney failure, pharmacologically‐treated heart failure, significant coronary heart disease (NYHA 3 or 4), moderate to severe chronic obstructive lung disease. 4. Liver cirrhosis. 5. Liver disease other than NAFLD. 6. Alcohol consumption > 24 g per day. | |
| Interventions | Participants were randomly assigned to two groups. Group 1: metformin (N = 20). Further details: metformin 2500 mg to 3000 mg/day (escalating dose from 500 mg/day). Group 2: control (N = 24). Further details: control: placebo. Duration of treatment: 6 months. Both groups received lifestyle modification advice (dietary and physical activity) | |
| Outcomes | Outcomes reported: 1. NAFLD activity score 2. Cirrhosis. 3. Resolution of fatty liver disease. | |
| Notes | Reasons for post‐randomisation drop‐outs: Did not complete the study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer‐assisted process of minimalization". |
| Allocation concealment (selection bias) | Low risk | Quote: "The allocation code was blinded to patients and investigators until all patients had completed the trial". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The allocation code was blinded to patients and investigators until all patients had completed the trial". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The allocation code was blinded to patients and investigators until all patients had completed the trial". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: there were post‐randomisation drop‐outs, which may be related to the treatment that the participants received. |
| Selective reporting (reporting bias) | High risk | Comment: protocol was not available; neither mortality nor adverse events were reported. |
| For‐profit bias | High risk | Quote: "This work was supported by Eastern Norway Regional Health Authority (grant) and Merck Sante´ (delivery of study medication) ". |
| Other bias | Low risk | Comment: no other risk of bias. |
Jin 2010.
| Methods | Randomised clinical trial | |
| Participants | Country: China. Number randomised: 120. Post‐randomisation drop‐outs: not stated. Revised sample size: 120. Average age: 52 years. Females: 55 (45.8%). NASH: not stated. Diabetics: not stated. Average follow‐up period in months: 6. Inclusion criteria 1. NASH. Exclusion criteria 1. ALT and AST > 100 IU/L. 2. Hepatitis B or C antigen or antibody. | |
| Interventions | Participants were randomly assigned to two groups. Group 1: pioglitazone (N = 60). Further details: pioglitazone 30 mg once daily. Group 2: control (N = 60). Further details: control: no intervention. Duration of treatment: 6 months. | |
| Outcomes | Outcomes reported: 1. Adverse events. | |
| Notes | Reasons for post‐randomisation drop‐outs: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly divided". |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: protocol was not available; mortality was not reported. |
| For‐profit bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other risk of bias. |
Kakazu 2013.
| Methods | Randomised clinical trial | |
| Participants | Country: Japan. Number randomised: 25. Post‐randomisation drop‐outs: 1 (4%). Revised sample size: 24. Average age: 57 years. Females: 18 (75%). NASH: 24 (100%). Diabetics: not stated. Average follow‐up period in months: 24. Inclusion criteria 1. Biopsy proven NASH. 2. Impaired glucose tolerance or diabetes. Exclusion criteria 1. Liver disease other than NAFLD. 2. Decompensated liver disease. 3. Alcohol consumption > 20 g per day. 4. Use of drugs associated with fatty liver. | |
| Interventions | Participants were randomly assigned to two groups. Group 1: pioglitazone (N = 12). Further details: pioglitazone 15 mg/day. Group 2: control (N = 12). Further details: control: no intervention. Duration of treatment: 24 months. Both groups received dietary and physical activity advice. | |
| Outcomes | None of the outcomes of interest were reported in this trial. | |
| Notes | Reasons for post‐randomisation drop‐outs: lost to follow‐up. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The subjects were randomly assigned to either a diet only group or diet plus pioglitazone". |
| Allocation concealment (selection bias) | Unclear risk | Quote: "a sealed envelope technique". Comment: Further details were not available. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "No placebo was given to patients not given pioglitazone supplementation". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: although an identical placebo was used, there was no mention of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: there were post‐randomisation drop‐outs, which may be related to the treatment that the participants received. |
| Selective reporting (reporting bias) | High risk | Comment: protocol was not available; neither mortality nor adverse events were reported. |
| For‐profit bias | Low risk | Quote: "This work was supported by Grant‐in‐Aid for Young Scientists (B), no. 23790762, from the Ministry of Education, Culture, Sports, Science and Technology of Japan, and grants from Ministry of Health, Labor, and Welfare of Japan". |
| Other bias | Low risk | Comment: no other risk of bias. |
Kedarisetty 2014.
| Methods | Randomised clinical trial | |
| Participants | Country: India. Number randomised: 116. Post‐randomisation drop‐outs: 0 (0%). Revised sample size: 116. Average age: not stated. Females: not stated. NASH: 116 (100%). Diabetics: not stated. Average follow‐up period in months: 12. Inclusion criteria 1. Biopsy‐proven NASH. | |
| Interventions | Participants were randomly assigned to two groups. Group 1: pentoxifylline and Antioxidants (N = 58). Further details: pentoxifylline 400 mg thrice daily plus antioxidants: vitamin E 400 IU twice daily. Group 2: antioxidants (N = 58). Further details: antioxidants: vitamin E 400 IU twice daily. Duration of treatment: 12 months. All people also underwent diet and lifestyle modification. | |
| Outcomes | None of the outcomes of interest were reported. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Consecutive histologically proven patients with NASH were randomized to either". |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "This is the first randomized open label trial". |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "This is the first randomized open label trial". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no post‐randomisation drop‐outs. |
| Selective reporting (reporting bias) | High risk | Comment: protocol was not available; neither mortality nor adverse events were reported. |
| For‐profit bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other risk of bias. |
Klyarytskaya 2015.
| Methods | Randomised clinical trial | |
| Participants | Country: Russia. Number randomised: 51. Post‐randomisation drop‐outs: not stated. Revised sample size: 51. Average age: 45 years. Females: 31 (60.8%). NASH: not stated. Diabetics: not stated. Average follow‐up period in months: 12. Inclusion criteria 1. Patients with NASH. 2. Adult patients (aged ≥18 years). 3. Increased ALT and/or alkaline phosphatase (AP) > 2 times compared to the normal. 4. No hereditary liver diseases (Wilson’s disease, haemochromatosis, and antitrypsin deficiency). 5. A negative result of enzyme immunoassay (ELISA) for blood markers of viral Hepatitis B, C and D. 6. A negative result ELISA blood for markers of auto‐immune hepatitis. 7. Avoidance of use of hepatotoxic drugs. Exclusion criteria 1. Alcohol consumption > 30 g/day for men, > 20 g/day for women. 2. History of acute viral hepatitis over the past 12 months. 3. The presence of concomitant decompensated diseases. 4. Pregnancy, lactation period. | |
| Interventions | Participants were randomly assigned to two groups. Group 1: losartan plus atorvastatin plus antioxidants (N = 26). Further details: losartan 50 mg/day and atorvastatin 20 mg/day plus antioxidants: vitamin E 800 IU/day. Group 2: atorvastatin plus antioxidants (N = 25). Further details: atorvastatin 20 mg/day plus antioxidants: vitamin E 800 IU/day. Duration of treatment: 12 months. All people also underwent lifestyle modification. | |
| Outcomes | None of the outcomes of interest were reported in this trial. | |
| Notes | Reasons for post‐randomisation drop‐outs: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "An open randomised prospective comparative study". |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "An open randomised prospective comparative study". |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "An open randomised prospective comparative study". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: protocol was not available; neither mortality nor adverse events were reported. |
| For‐profit bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other risk of bias. |
Kugelmas 2003.
| Methods | Randomised clinical trial | |
| Participants | Country: USA. Number randomised: 16. Post‐randomisation drop‐outs: not stated. Revised sample size: 16. Average age: 47 years. Females: 9 (56.3%). NASH: 16 (100%). Diabetics: not stated. Average follow‐up period in months: 3. Inclusion criteria 1. Aged 18 to 65 years. 2. Biopsy proven NASH. 3. Negative serologic markers for known chronic liver diseases. Exclusion criteria 1. Decompensated liver disease. 2. Other chronic liver diseases. 3. Ongoing total parenteral nutrition. 4. Jejunal ileal bypass. 5. HIV infection. 6. Vitamin E replacement within 3 months before enrolment. 7. History of alcohol abuse or consumption of an average > 1 drink per week in the past 6 months. | |
| Interventions | Participants were randomly assigned to two groups. Group 1: antioxidants (N = 9). Further details: antioxidants: vitamin E 800 IU/day. Group 2: control (N = 7). Further details: control: no intervention. Duration of treatment: 3 months. Both groups received dietary and physical activity advice. | |
| Outcomes | No outcomes of interest were reported in this trial. | |
| Notes | Reasons for post‐randomisation drop‐outs: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "In a single‐blinded fashion (principal investigator was blinded), patients were randomized to receive 800 IU of vitamin E daily". |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "In a single‐blinded fashion (principal investigator was blinded), patients were randomized to receive 800 IU of vitamin E daily". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "In a single‐blinded fashion (principal investigator was blinded), patients were randomized to receive 800 IU of vitamin E daily". Comment: It was not clear whether all the assessments were made by the principal investigator. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: protocol was not available; neither mortality nor adverse events were reported. |
| For‐profit bias | Low risk | Quote: "Supported by National Institutes of Health grants MO1 RR02602, AA00297 (to D.B.H.), AA014185 (D.B.H.), AA01762 (to C.J.M.), and AA10496 (to C.J.M.); Kentucky Science and Engineering Foundation grant; and the Department of Veterans Affairs". |
| Other bias | Low risk | Comment: no other risk of bias. |
Leuschner 2010.
| Methods | Randomised clinical trial | |
| Participants | Country: Multicentre, international. Number randomised: 186. Post‐randomisation drop‐outs: 0 (0%). Revised sample size: 186. Average age: 43 years. Females: 60 (32.3%). NASH: 186 (100%). Diabetics: 21 (11.3%). Average follow‐up period in months: 18. Inclusion criteria 1. Aged > 18 years. 2. Biopsy proven NASH within 1 month prior or after the first visit (NAS score > 6). 3. ALT level at least 1.5 times the upper limit of normal. 4. Metabolic syndrome. 5. Type II diabetes or hypertriglyceridemia or BMI more than 25 kg/m². 8. Alcohol consumption < 70 g/week. Exclusion criteria 1. Liver cirrhosis. 2. Hepatitis B or C markers. 3. Antinuclear antibody/smooth muscle antibody titers > 1:160. 4. Cholestatic liver diseases. 5. Wilson disease. 6. Haemochromatosis. 7. Alpha1‐antitripsin deficiency. 8. History of HIV. 9. Recent intake of potential liver‐toxic drugs or drug interacting with ursodeoxycholic acid (UDCA). 10. Treatment with UDCA, metformin, glitazones, vitamin E, angiotensin receptor antagonists in the last 3 months prior to the study entry. 11 Alcohol consumption > 70 g/week. 12. Mean corpuscolar volume more than 101 fl. 13. Pregnancy or lactation or insufficient contraception in fertile women. 14. Patients unreliable or not compliant. | |
| Interventions | Participants were randomly assigned to two groups. Group 1: UDCA (N = 95). Further details: UDCA 23 to 28 mg/kg/day. Group 2: control (N = 91). Further details: control: placebo. Duration of treatment: 18 months. | |
| Outcomes | Outcomes reported: 1. Mortality 2. Adverse events 3. Change in fibrosis score 4. Change in NAFLD activity score. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Double‐Blind, Randomized, Placebo‐Controlled Trial". |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Double‐Blind, Randomized, Placebo‐Controlled Trial". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Double‐Blind, Randomized, Placebo‐Controlled Trial". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: all patients were included for adverse events; patients were excluded for histological analysis. |
| Selective reporting (reporting bias) | Low risk | Comment: mortality and adverse events were reported. |
| For‐profit bias | High risk | Quote: "This study was supported by Dr. Falk Pharma GmbH (Freiburg, Germany)". |
| Other bias | Low risk | Comment: no other risk of bias. |
Lewis 2006.
| Methods | Randomised clinical trial | |
| Participants | Country: USA. Number randomised: 175. Post‐randomisation drop‐outs: not stated. Revised sample size: 175. Average age: 50 years. Females: not stated. NASH: not stated. Diabetics: not stated. Average follow‐up period in months: 9. Inclusion criteria 1. Diagnosis of NAFLD. 2. Total cholesterol > 160 mg/dL. 3. LDL > 100 mg/dL. 4. Triglycerides < 400 mg/dL. | |
| Interventions | Participants were randomly assigned to two groups. Group 1: pravastatin (N = 90). Further details: pravastatin 80 mg/day. Group 2: control (N = 85). Further details: control: placebo. Duration of treatment: 9 months. | |
| Outcomes | No outcomes of interest for this review were reported. | |
| Notes | Reasons for post‐randomisation drop‐outs: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "with 90 randomized to Prava and 85 to PBO". |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double‐blind". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double‐blind". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: protocol was not available; neither mortality nor adverse events were reported. |
| For‐profit bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other risk of bias. |
Lindor 2004.
| Methods | Randomised clinical trial | |
| Participants | Country: Multicentre, international. Number randomised: 174. Post‐randomisation drop‐outs: 8 (4.6%). Revised sample size: 166. Average age: 47 years. Females: 93 (56%). NASH: 166 (100%). Diabetics: not stated. Average follow‐up period in months: 24. Inclusion criteria 1. Aged 18 to 75 years. 2. Biopsy proven NASH (at least 10% steatosis ) within 6 months before the enrolment. 3. Persistent elevation of serum alanine aminotransferase (ALT) or aspartate aminotransferase (AST) at least 1.5 times the upper limit of normal for at least 3 months. 4. Weekly ethanol consumption < 40 g. Exclusion criteria 1. Treatment with ursodeoxycholic acid or chenodeoxycholic acid in the 3 months prior to the study. 2. Anticipated need for liver transplantation within 1 year or recurrent variceal bleeding, spontaneous portosystemic encephalopathy, diuretic resistant ascites, or bacterial peritonitis. 3. Pregnancy or lactation. 4. Treatment with any drugs associated with steatohepatitis (e.g. corticosteroids, high‐dose estrogens, methotrexate, amiodarone, calcium channel blockers, spironolactone, sulfasalazine, naproxen, or oxacillin) in the 6 months prior to the study. 5. Laboratory or histologic findings highly suggestive of liver disease of another etiology, such as chronic viral hepatitis, autoimmune hepatitis, primary biliary cirrhosis, biliary obstruction, or genetic liver diseases such as haemochromatosis, alpha‐1‐antitrypsin deficiency, or Wilson's disease. | |
| Interventions | Participants were randomly assigned to two groups. Group 1: UDCA (N = 80). Further details: UDCA 13 to 15 mg/kg/day. Group 2: control (N = 86). Further details: control: placebo. Duration of treatment: 24 months. | |
| Outcomes | Outcomes reported: 1. Adverse events 2. Change in fibrosis score | |
| Notes | Reasons for post‐randomisation drop‐outs: protocol violations. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "prospective, randomized, double‐blind, placebo controlled trial". |
| Allocation concealment (selection bias) | Low risk | Quote: "The patients brought their entry forms to the pharmacy. Each patient’s name and clinic or medical record number was recorded, and each patient was assigned a study number. Patients were then given their study drug based on the previously randomized list". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The investigators, study coordinators, and patients were blinded as to the treatment administered". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The investigators, study coordinators, and patients were blinded as to the treatment administered". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: there were post‐randomisation drop‐outs, which may be related to the treatment that the participants received.. |
| Selective reporting (reporting bias) | High risk | Comment: protocol was not available; mortality was not reported. |
| For‐profit bias | High risk | Quote: "Supported, in part, by Axcan Pharma, Inc., Quebec, Canada". |
| Other bias | Low risk | Comment: no other risk of bias. |
Loomba 2015.
| Methods | Randomised clinical trial | |
| Participants | Country: USA. Number randomised: 50. Post‐randomisation drop‐outs: not stated. Revised sample size: 50. Average age: 49 years. Females: 31 (62%). NASH: 50 (100%). Diabetics: 14 (28%). Average follow‐up period in months: 6. Inclusion criteria 1. Patients with biopsy proven NASH. 2. Aged ≥ 18 years. 3. ALT > upper limit of normal (19 U/L for women and 30 U/L for men). 4. Presence of hepatic steatosis as defined by ≥ 5% on MRI. Exclusion criteria 1. Evidence of other forms of liver disease shown by the presence of serum hepatitis B surface antigen, hepatitis C viral RNA, positive auto‐immune serologies with biopsy consistent with autoimmune hepatitis, haemochromatosis by 3+ or 4+ stainable iron on biopsy and homozygosity/heterozygosity on genetic analysis, low ceruloplasmin levels with biopsy suggestive of Wilson's disease, or low alpha‐1‐antitrypsin levels with biopsy suggestive of alpha‐1‐antitrypsin disease. 2. Alcohol intake > 30 g/day in the previous 10 years or > 10 g/day in the previous year. 3. Decompensated cirrhosis with Child‐Pugh score > 7 points. 4. Active substance abuse. 5. Significant systemic illnesses. 6. Renal insufficiency. 7. Positive human immunodeficiency virus test. 8. Pregnancy. 9. Evidence of hepatocellular carcinoma. 10. Ingestion of drugs known to cause hepatic steatosis. 11. Ingestion of drugs known to improve NASH such as vitamin E or pioglitazone. 12. Contraindications to liver biopsy or inability to undergo MRI. | |
| Interventions | Participants were randomly assigned to two groups. Group 1: ezetimibe (N = 25). Further details: ezetimibe 10 mg once daily. Group 2: control (N = 25). Further details: control: placebo. Duration of treatment: 6 months. | |
| Outcomes | Outcomes reported: 1. Adverse events 2. Fibrosis score 3.NAS score 4. Resolution of fatty liver disease | |
| Notes | Reasons for post‐randomisation drop‐outs: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer‐generated numbers". |
| Allocation concealment (selection bias) | Low risk | Quote: "Independent investigational drug services pharmacists dispensed either active or placebo treatment pills, which were identical in appearance". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double‐blind". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double‐blind". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: 4 patients did not compelete the treatment. It was not clear whether these patients were included in the results for adverse events. For histological assessment only 17 patients and 18 patients were included in the analysis. |
| Selective reporting (reporting bias) | High risk | Comment: protocol was not available; mortality was not reported. |
| For‐profit bias | High risk | Quote: "Supported by an investigator‐initiated study grant to R.L. by Merck.". |
| Other bias | Low risk | Comment: no other risk of bias. |
Magosso 2013.
| Methods | Randomised clinical trial | |
| Participants | Country: Malaysia. Number randomised: 87. Post‐randomisation drop‐outs: 0 (0%). Revised sample size: 87. Average age: 50 years. Females: 53 (60.9%). NASH: not stated. Diabetics: not stated. Average follow‐up period in months: 12. Inclusion criteria 1. Aged ≥ 35 years. 2. Mild untreated hypercholesterolaemia (total cholesterol 200 to 240 mg/dL or LDL 100‐161 mg/dL). 3. Ultrasound proven NAFLD. 4. AST, ALT and GGT < 3 times the respective upper limit value of 53 IU/l, 40 IU/l and 49 IU/l for males or 32 IU/l for females. Exclusion criteria 1. Anti‐hyperlipidaemic treatment or vitamin E within 3 months before enrolment. 2. Alcohol consumption > 20 g/day. 3. Previous cardiovascular event. 4. Previous hepatitis. | |
| Interventions | Participants were randomly assigned to two groups. Group 1: antioxidants (N = 43). Further details: antioxidants: tocotrienols 200 mg twice daily. Group 2: control (N = 44). Further details: control: placebo. Duration of treatment: 12 months. | |
| Outcomes | Outcomes reported: 1. Mortality 2. Adverse events 3. Liver cirrhosis 4. Decompensated liver disease 5. Liver transplantation 6. Resolution of fatty liver disease. | |
| Notes | Authors provided additional information in February 2016. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Participants were randomised using a computer generated random allocation sequence". |
| Allocation concealment (selection bias) | Low risk | Quote: "The researcher (WJW) who generated the random allocation sequence and assigned participants was blinded to subjects’ clinical data and was independent from the persons who enrolled participants". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Researchers and volunteers were blinded to the assigned treatment". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Researchers and volunteers were blinded to the assigned treatment". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no post‐randomisation drop‐outs. |
| Selective reporting (reporting bias) | Low risk | Comment: mortality and adverse events were reported. |
| For‐profit bias | High risk | Quote: "The authors acknowledge the Malaysian Palm Oil Board (MPOB) for providing the supporting research grant". |
| Other bias | Low risk | Comment: no other risk of bias. |
Mendez‐Sanchez 2004.
| Methods | Randomised clinical trial | |
| Participants | Country: Mexico. Number randomised: 27. Post‐randomisation drop‐outs: 4 (14.8%). Revised sample size: 23. Average age: 39 years. Females: 23 (100%). NASH: not stated. Diabetics: not stated. Average follow‐up period in months: 1. Inclusion criteria 1. BMI more than 30. 2. Ages 20 to 60 years. 3. Willing to join a diet plan for 6 weeks. 4. Normal serum potassium and calcium levels. 5. Abnormal serum transaminases not related to other causes of liver disease (viral or auto‐immune hepatitis, haemochromatosis, alcohol). 6. Ultrasound evidence of hepatic steatosis. 7. Negative pregnancy test. Exclusion criteria 1. History of hypothyroidism or Cushing syndrome. 2. Eating disorder or psychological problems. 3. Use of oral bile acid preparations, aluminium‐based antacids of lithium. 4. Long‐term use of nonsteroidal anti‐inflammatory agents (including aspirin) or antihyperlipidemic agents within 2 weeks of entering the trial. | |
| Interventions | Participants were randomly assigned to two groups. Group 1: UDCA (N = 12). Further details: UDCA 1200 mg/day. Group 2: control (N = 11). Further details: control: placebo. Duration of treatment: 1 month. Both groups received hypocaloric diet | |
| Outcomes | No outcomes of interest were reported in this trial. | |
| Notes | Reasons for post‐randomisation drop‐outs: withdrew prematurely, became pregnant. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "according to a table of random numbers". |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "A double‐blind placebo‐controlled trial". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "A double‐blind placebo‐controlled trial". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: there were post‐randomisation drop‐outs, which may be related to the treatment that the participants received. |
| Selective reporting (reporting bias) | High risk | Comment: protocol was not available; neither mortality nor adverse events were reported. |
| For‐profit bias | Unclear risk | Quote: "This work was partly supported by Medica Sur Clinic & Foundation". Comment: the source of remaining funds was not reported. |
| Other bias | Low risk | Comment: no other risk of bias. |
Merat 2003.
| Methods | Randomised clinical trial | |
| Participants | Country: Iran. Number randomised: 30. Post‐randomisation drop‐outs: 3 (10%). Revised sample size: 27. Average age: 36 years. Females: 6 (22.2%). NASH: 27 (100%). Diabetics: not stated. Average follow‐up period in months: 6. Inclusion criteria 1. Biopsy proven NASH. 2. Aged 15 to 60 years. 3. Liver function test alteration lasted for at least three months (AST and ALT > 1.2 times upper limit of normal). Exclusion criteria 1. Alcohol consumption. 2. Viral hepatitis B or C. 3. Auto‐immune hepatitis. 4. Wilson's disease. 5. Haemochromatosis. 6. Alpha1‐antitrypsin deficiency. 7. Pregnancy, lactation or women who wished to have children in the following years. 8. Severe comorbidities (cardiac, pulmonary, renal or psychological). | |
| Interventions | Participants were randomly assigned to two groups. Group 1: probucol (N = 18). Further details: probucol 500 mg once daily. Group 2: control (N = 9). Further details: control: placebo. Duration of treatment: 6 months. | |
| Outcomes | Outcomes reported: 1. Adverse events. | |
| Notes | Reasons for post‐randomisation drop‐outs: withdrew from study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed by a computer generated random list of the containers’ numbers". |
| Allocation concealment (selection bias) | Low risk | Quote: "Patients found eligible for the study in any of the three study centers were referred to a single investigator who assigned new cases sequentially to the next available container on the list". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "a double‐blind randomized controlled study". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "a double‐blind randomized controlled study". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: there were post‐randomisation drop‐outs, which may be related to the treatment that the participants received.. |
| Selective reporting (reporting bias) | High risk | Comment: protocol was not available; mortality was not reported. |
| For‐profit bias | Low risk | Quote: "This work was funded by the Digestive Disease Research Center of Tehran University of Medical Sciences". |
| Other bias | Low risk | Comment: no other risk of bias. |
Morita 2005.
| Methods | Randomised clinical trial | |
| Participants | Country: Japan. Number randomised: 10. Post‐randomisation drop‐outs: not stated. Revised sample size: 10. Average age: 50 years. Females: 7 (70%). NASH: 10 (100%). Diabetics: 10 (100%). Average follow‐up period in months: 5. Inclusion criteria 1. Biopsy proven NASH. 2. ALT > 30 UI/l. 3. Diabetes. 4. Ultrasound or CT proven liver steatosis. Exclusion criteria 1. Alcohol intake > 20 g/day. 2. Hepatitis B or C. 3. ANA or AMA positivity. | |
| Interventions | Participants were randomly assigned to two groups. Group 1: nateglinide (N = 5). Further details: nateglinide 270 mg/day. Group 2: control (N = 5). Further details: control: no intervention. Duration of treatment: 5 months. Both groups received dietary and physical activity advice | |
| Outcomes | No outcomes of interest were reported in this trial. | |
| Notes | Reasons for post‐randomisation drop‐outs: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The subjects were randomly divided into two groups". |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: protocol was not available; neither mortality nor adverse events were reported. |
| For‐profit bias | Low risk | Quote: "This study was supported by the grant 16590150 from the Ministry of Education, Science, Sports, and Culture of Japan and part of a project for establishing new high technology research center supported by the Ministry of Education, Science, Sports, and Culture of Japan". |
| Other bias | Low risk | Comment: no other risk of bias. |
Mudaliar 2013.
| Methods | Randomised clinical trial | |
| Participants | Country: USA. Number randomised: 64. Post‐randomisation drop‐outs: 0 (0%). Revised sample size: 64. Average age: 52 years. Females: 31 (48.4%). NASH: not stated. Diabetics: 64 (100%). Average follow‐up period in months: 1. Inclusion criteria 1. Type 2 diabetes. 2. NAFLD defined by one or more of the following criteria: ALT ≥ 47 IU/l for females and 56 for males; AST ≥ 47 IU/l for females and 60 IU/l for males; enlarged liver (on ultrasound or other imaging technique) and diagnostic histologic findings shown on prior biopsy (in the prior 5 years). Exclusion criteria 1. Viral hepatitis B or C. 2. Primary biliary cirrhosis. 3. Primary sclerosing cirrhosis. 4. AST > 155 IU/l for females and 200 for males and ALT > 155 IU/l for females and 185 IU/l for males. 5. Bilirubin level > 2 times upper limit of normal. 6. Use of anti‐diabetes drugs except for metformin and sulphonylureas. 7. Alcohol consumption > 210 mL/week or other substance abuse in the previous 2 years. 8. Significant heart or renal disease. | |
| Interventions | Participants were randomly assigned to two groups. Group 1: obeticholic acid (N = 41). Further details: obeticholic acid 25 mg or 50 mg once daily (dose decided by randomisation). Group 2: control (N = 23). Further details: control: placebo. Duration of treatment: 1 month. | |
| Outcomes | Outcomes reported: 1. Mortality 2. Adverse events. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "This number was preprinted on the patient drug kit according to the master randomization schedule". Comment: Details on how this randomization schedule was drawn were not available. |
| Allocation concealment (selection bias) | Low risk | Quote: "Eligible patients were assigned a 3‐digit patient randomization number. This number was preprinted on the patient drug kit according to the master randomization schedule. The drug kit was dispensed by the site pharmacists". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double‐blind, placebo controlled". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double‐blind, placebo controlled". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no post‐randomisation drop‐outs. |
| Selective reporting (reporting bias) | Low risk | Comment: mortality and adverse events were reported. |
| For‐profit bias | High risk | Quote: "Supported by a research grant from Intercept Pharmaceuticals, Inc.". |
| Other bias | Low risk | Comment: no other risk of bias. |
Nar 2009.
| Methods | Randomised clinical trial | |
| Participants | Country: Turkey. Number randomised: 34. Post‐randomisation drop‐outs: not stated. Revised sample size: 34. Average age: 47 years. Females: 25 (73.5%). NASH: not stated. Diabetics: 34 (100%). Average follow‐up period in months: 6. Inclusion criteria 1. Type 2 diabetes. 2. Ultrasound proven NAFLD. Exclusion criteria 1. Anti‐diabetes medication. 2. Acute or chronic viral hepatitis. 3. Autoimmune hepatitis. 4. Excessive alcohol consumption. 5. History of malignancy, renal impairment, haemodynamic instability, diseases of pituitary adrenal glands and pancreas. 6. Prolonged use of corticosteroids or sexual hormones. 7. Use of antihyperlipidaemic agents and anti‐obesity medications. | |
| Interventions | Participants were randomly assigned to two groups. Group 1: metformin (N = 19). Further details: metformin 1700 mg/day. Group 2: control (N = 15). Further details: control: no intervention. Duration of treatment: 6 months. Both groups received dietary and physical activity advice | |
| Outcomes | Outcomes reported: 1. Resolution of fatty liver disease. | |
| Notes | Reasons for post‐randomisation drop‐outs: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were randomly assigned into two study groups". |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Same operator performed all US procedures and was blind to the randomization of the patients". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: protocol was not available; neither mortality nor adverse events were reported. |
| For‐profit bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other risk of bias. |
Nelson 2009.
| Methods | Randomised clinical trial | |
| Participants | Country: USA. Number randomised: 16. Post‐randomisation drop‐outs: 0 (0%). Revised sample size: 16. Average age: 53 years. Females: 5 (31.3%). NASH: 16 (100%). Diabetics: 7 (43.8%). Average follow‐up period in months: 12. Inclusion criteria 1. Aged ≥ 18 years. 2. Biopsy proven NASH within 6 months before enrolment. 3. Compensated liver disease. 4. Serum creatinine < 1.4 mg/dL. 5. Total cholesterol > 200 mg/dL or triglycerides > 200 mg/dL or LDL > 130 mg/dL. Exclusion criteria 1. Any other cause of liver disease. 2. Alcohol consumption > 1 drink/day. 3. Prior gastroplasty, jejuno‐ileal or jejuno‐colic bypass. 4. Prior exposure to organic solvent. 5. Total parenteral nutrition in the previous 6 months. 6. Prior organ transplantation. 7. Use of statin in the previous 12 months. 8. Use of tamoxifen, prednisone, chloroquine, methotrexate, highly active antiretroviral therapy, amiodarone, or any other hepatotoxic medications. 9. Serum transaminases level > 3 times the upper limit of normal. | |
| Interventions | Participants were randomly assigned to two groups. Group 1: Simvastatin (N = 10). Further details: Simvastatin 40 mg/day. Group 2: control (N = 6). Further details: control: placebo. Duration of treatment: 12 months. | |
| Outcomes | Outcomes reported: 1. Adverse events. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomized to receive either simvastatin 40 mg or placebo once daily for 12 months". |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double‐blind randomized placebo‐controlled trial ". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double‐blind randomized placebo‐controlled trial ". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no post‐randomisation drop‐outs.. |
| Selective reporting (reporting bias) | High risk | Comment: protocol was not available; mortality was not reported. |
| For‐profit bias | Low risk | Quote: "Funding: None". |
| Other bias | Low risk | Comment: no other risk of bias. |
Neuschwander‐Tetri 2015.
| Methods | Randomised clinical trial | |
| Participants | Country: USA. Number randomised: 283. Post‐randomisation drop‐outs: not stated. Revised sample size: 283. Average age: 51 years. Females: 187 (66.1%). NASH: 283 (100%). Diabetics: 149 (52.7%). Average follow‐up period in months: 17. Inclusion criteria 1. Aged ≥ 18 years at the time of screening. 2. Histological evidence of definite or borderline non‐alcoholic steatohepatitis based upon a liver biopsy obtained 90 days or less before randomisation. 3. NAS score ≥ 4 with a score ≥ 1 in each component of the score. Exclusion criteria 1. Presence of cirrhosis. 2. Other causes of liver disease. 3. Substantial alcohol consumption (> 20 g/day for women or > 30 g/day for men). 4. Other confounding conditions. | |
| Interventions | Participants were randomly assigned to two groups. Group 1: obeticholic acid (N = 141). Further details: obeticholic acid 25 mg OD. Group 2: control (N = 142). Further details: control: placebo. Duration of treatment: 17 months. | |
| Outcomes | Outcomes reported: 1. Mortality 2. Adverse events 3. Change in fibrosis score 4. Change in NAS score. 5. Resolution of fatty liver disease. | |
| Notes | Reasons for post‐randomisation drop‐outs: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer‐generated, centrally administered procedure". |
| Allocation concealment (selection bias) | Low risk | Quote: "computer‐generated, centrally administered procedure.Treatment was assigned centrally using a web‐based application". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Patients, investigators, clinical site staff, and pathologists were masked to treatment assignment". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Patients, investigators, clinical site staff, and pathologists were masked to treatment assignment". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: all participants were included for safety issues and non‐histological outcomes but 64 participants were excluded for histological outcomes. |
| Selective reporting (reporting bias) | Low risk | Comment: mortality and adverse events were reported. |
| For‐profit bias | High risk | Quote: "National Institute of Diabetes and Digestive and Kidney Diseases, Intercept Pharmaceuticals". |
| Other bias | Low risk | Comment: no other risk of bias. |
Omer 2010.
| Methods | Randomised clinical trial | |
| Participants | Country: Turkey. Number randomised: 64. Post‐randomisation drop‐outs: not stated. Revised sample size: 64. Average age: 49 years. Females: 29 (45.3%). NASH: 64 (100%). Diabetics: not stated. Average follow‐up period in months: 12. Inclusion criteria 1. Impaired glucose metabolism (type 2 diabetes or impaired glucose tolerance). 2. Elevated ALT for at least 6 months before enrolment. 3. NAFLD activity score at least of 5 in liver biopsy performed within 6 months before enrolment. 4. Diet and exercise program for at least 12 weeks before enrolment. Exclusion criteria 1. Alcohol consumption > 20 g/day. 2. Use of oral anti‐diabetes, insulin or a chemotherapeutic agent. 3. Presence of other chronic liver diseases, such as metabolic liver diseases, auto‐immune liver diseases, and chronic viral hepatitis B or C. 4. HIV infection. 5. Pregnancy or lactation. 6. Candidate for organ transplantation. 7. Malignancy. 8. Renal function impairment (serum creatinine more than 1.5 mg/dL in men and 1.4 mg/dL in women). 9. Clinically significant systemic illness. | |
| Interventions | Participants were randomly assigned to two groups. Group 1: metformin (N = 22). Further details: metformin 1700 mg/day. Group 2: rosiglitazone (N = 20). Further details: rosiglitazone 4 mg/day. Duration of treatment: 12 months. Both groups received dietary and physical activity advice | |
| Outcomes | Outcomes reported: 1. NAFLD activity score. | |
| Notes | Reasons for post‐randomisation drop‐outs: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "open‐label, randomized, preliminary and single‐center study". |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "open‐label, randomized, preliminary and single‐center study". |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "open‐label, randomized, preliminary and single‐center study". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: Biopsy was performed and reported in only a proportion of the randomised population. |
| Selective reporting (reporting bias) | High risk | Comment: protocol was not available; neither mortality nor adverse events were reported. |
| For‐profit bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other risk of bias. |
Parikh 2016.
| Methods | Randomised clinical trial | |
| Participants | Country: India. Number randomised: 250. Post‐randomisation drop‐outs: 17 (6.8%). Revised sample size: 233. Average age: 42 years. Females: not stated. NASH: 35 (15%). Diabetics: not stated. Average follow‐up period in months: 12. Inclusion criteria 1. Patients with NAFLD with abnormal ALT or AST. 2. Aged 18 to 80 years. Exclusion criteria 1. History of alcohol intake > 20 g per day (during previous 5 years). 2. Hepatitis B antigen (HBsAg) reactive. 3. Presence of antibody against hepatitis C (anti‐HCV) human immunodeficiency virus (HIV) reactive. 4. Active hepatitis. 5. Biliary obstruction on ultrasound. 6. Diagnosed as cirrhosis at any time in the past. 7. Tuberculosis. 8. Malabsorption. 9. Chronic drug use. 10. Pregnancy. 11. Any cardiorespiratory comorbid conditions. | |
| Interventions | Participants were randomly assigned to two groups. Group 1: antioxidants (N = 95). Further details: antioxidants: vitamin E 400 IU twice daily. Group 2: UDCA (N = 138). Further details: UDCA 300 mg twice daily. Duration of treatment: 12 months. All people also underwent lifestyle modification (diet and regular exercise). | |
| Outcomes | Outcomes reported: 1. Adverse events. | |
| Notes | Reasons for post‐randomisation drop‐outs: lost‐to‐follow up. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "prospective, single center, open‐labeled, RCT". |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "prospective, single center, open‐labeled, RCT". |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "prospective, single center, open‐labeled, RCT". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: there were post‐randomisation drop‐outs, which may be related to the treatment that the participants received. |
| Selective reporting (reporting bias) | High risk | Comment: protocol was not available; neither mortality nor adverse events were reported. |
| For‐profit bias | Low risk | Quote: "Financial support and sponsorship: Nil". |
| Other bias | Low risk | Comment: no other risk of bias. |
Polyzos 2011.
| Methods | Randomised clinical trial | |
| Participants | Country: Greece. Number randomised: 31. Post‐randomisation drop‐outs: not stated. Revised sample size: 31. Average age: not stated. Females: not stated. NASH: 16 (51.6%). Diabetics: 5 (16.1%). Average follow‐up period in months: 2. Inclusion criteria 1. Aged ≥ 18 years. 2. Ultrasound liver brightness and increased liver function tests for at least 6 months before liver biopsy. 3. Biopsy proven NAFLD. Exclusion criteria 1. Known intolerance to spironolactone. 2. Ethanol consumption > 20 g/day. 3. History of liver disease (chronic viral hepatitis, auto‐immune hepatitis, drug‐induced liver disease, primary biliary cirrhosis, haemochromatosis, Wilson's disease, a1‐antitrypsin deficiency). 4. Exposure to hepatotoxic drugs or evidence of liver cirrhosis. | |
| Interventions | Participants were randomly assigned to two groups. Group 1: spironolactone plus antioxidants (N = 14). Further details: spironolactone 25 mg/day plus antioxidants: vitamin E 400 IU/day. Group 2: antioxidants (N = 17). Further details: antioxidants: vitamin E 400 IU/day. Duration of treatment: planned 12 months. The report includes only 2 months of treatment. | |
| Outcomes | Outcomes reported: 1. Mortality. 2. Adverse events. 3. Cirrhosis. 4. Decompensated liver disease. 5. Liver transplantation. | |
| Notes | Authors provided additional information in February 2016 Reasons for post‐randomisation drop‐outs: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation was accomplished by a computer program before the screening of the first patient". |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | Low risk | Comment: mortality and adverse events were reported. |
| For‐profit bias | Low risk | Quote: "This research received no specific grant from any funding agency in the public, commercial, or not‐for‐profit sectors". |
| Other bias | Low risk | Comment: no other risk of bias. |
Ratziu 2008.
| Methods | Randomised clinical trial | |
| Participants | Country: France. Number randomised: 64. Post‐randomisation drop‐outs: 1 (1,6%). Revised sample size: 63. Average age: 54 years. Females: 26 (41.3%). NASH: 63 (100%). Diabetics: 20 (31.7%). Average follow‐up period in months: 16. Inclusion criteria 1. Aged 18 to 75 years. 2. Biopsy proven NASH (and steatosis > 20%). 3. Elevated ALT (> 28 UI/L for women and > 35 UI/L for men at baseline and at least 2 abnormal values in the last 6 months). Exclusion criteria 1 .Presence of bland steatosis on liver biopsy or steatosis with no specific inflammation. 2. Daily alcohol consumption > 30 g in men and 20 g in women whether current or in the past. 3. Any cause of liver disease other than NASH, including suspicion of drug‐induced liver injury (introduction of a new drug in the past 6 months without prior documentation of elevated ALT level). 4. Treatment with insulin for diabetes or with ursodeoxycholic acid. 5. Cardiac insufficiency (NYHA class I). 6. Current or past treatment with drugs that can induce steatohepatitis. 7. Neoplastic disease. 8. Child B or C cirrhosis. 9. Pregnancy. 10. Organ transplantation. 11. Haemoglobin level < 10 g/dL. 12. Polymorphonuclear count < 750/mm³. 13. Platelet count < 50,000/mm³ | |
| Interventions | Participants were randomly assigned to two groups. Group 1: rosiglitazone (N = 32). Further details: rosiglitazone 4 mg/day for 1 month increased to 8 mg/day thereafter. Group 2: control (N = 31). Further details: control: placebo. Duration of treatment: 12 months. Both groups received dietary and physical activity advice | |
| Outcomes | Outcomes reported: 1. Change in fibrosis score 2. Change in NAFLD activity score. | |
| Notes | Reasons for post‐randomisation drop‐outs: withdrew consent. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomization (presealed envelopes) was conducted by blocks of 4 and stratified on metformin use". |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Randomization (presealed envelopes) was conducted by blocks of 4 and stratified on metformin use". Comment: Further details were not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "randomized, double‐blind, placebo controlled trial ". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "randomized, double‐blind, placebo controlled trial ". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: there were post‐randomisation drop‐outs, which may be related to the treatment that the participants received. |
| Selective reporting (reporting bias) | High risk | Comment: protocol was not available; neither mortality nor adverse events were reported. |
| For‐profit bias | High risk | Quote: "GlaxoSmithKline provided rosiglitazone and placebo for this trial and partly funded the trial". |
| Other bias | Low risk | Comment: no other risk of bias. |
Ratziu 2011.
| Methods | Randomised clinical trial | |
| Participants | Country: France. Number randomised: 126. Post‐randomisation drop‐outs: 0 (0%). Revised sample size: 126. Average age: 50 years. Females: 31 (24.6%). NASH: 126 (100%). Diabetics: 40 (31.7%). Average follow‐up period in months: 12. Inclusion criteria 1. Aged > 18 years. 2. Increased ALT (≥ 50 UI/L) in at least three determinations within the past 12 months. 3. Biopsy proven NASH. Exclusion criteria 1. > one normal ALT level within the 12 months before screening. 2. No inflammation on liver biopsy which excluded the diagnosis of NASH. 3. Alcohol consumption > 30 g/day for men and 20 g/day for women. 4. Liver diseases other than NAFLD. 5. Child B or C cirrhosis. 6. Secondary NASH. 7. Treatment with UDCA in the previous 12 months, with vitamin E in the previous 6 months or with glitazone in the previous 3 months. 8. Newly instituted antihyperglycaemic therapy in the previous 4 months. 9. Loss ≥ 15% body weight since liver biopsy. 10. Hepatocellular carcinoma. 11. Pregnancy or breastfeeding. | |
| Interventions | Participants were randomly assigned to two groups. Group 1: UDCA (N = 62). Further details: UDCA 28 to 35 mg/kg/day. Group 2: control (N = 64). Further details: control: placebo. Duration of treatment: 12 months. Both groups received dietary and physical activity advice. | |
| Outcomes | Outcomes reported: 1. Mortality. 2. Adverse events. 3. Decompensated cirrhosis. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "This multicenter, randomized, double‐blind, parallel arm, placebo‐controlled phase II study of HD‐UDCA was conducted in 15 centers in France". |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "This multicenter, randomized, double‐blind, parallel arm, placebo‐controlled phase II study of HD‐UDCA was conducted in 15 centers in France". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "This multicenter, randomized, double‐blind, parallel arm, placebo‐controlled phase II study of HD‐UDCA was conducted in 15 centers in France". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no post‐randomisation drop‐outs. |
| Selective reporting (reporting bias) | Low risk | Comment: mortality and adverse events were reported. |
| For‐profit bias | High risk | Quote: "This trial was sponsored and funded by Axcan Pharma S.A. V. Ratziu is a consultant to Astellas Pharma, Axcan Pharma, Gilead Sciences, Genentech, Intercept Pharmaceuticals, and Sanofi‐Aventis". |
| Other bias | Low risk | Comment: no other risk of bias. |
Ratziu 2014.
| Methods | Randomised clinical trial | |
| Participants | Country: Multicentre, international. Number randomised: 99. Post‐randomisation drop‐outs: 3 (3%). Revised sample size: 96. Average age: 45 years. Females: 26 (27.1%). NASH: 96 (100%). Diabetics: not stated. Average follow‐up period in months: 3. Inclusion criteria 1. Patients with NASH without cirrhosis. 2. Aged ≥ 18 years. 3. ALT > 1.5 times normal limit or > 60 U/L on more than 1 occasion. Exclusion criteria 1. Uncontrolled diabetes. 2. Hepatic cirrhosis. 3. Liver disease other than NASH. 4. Excessive alcohol use (20 g/day for women and 30 g/day for men). 5. Weight change > 5% in the prior 6 months. | |
| Interventions | Participants were randomly assigned to two groups. Group 1: ASP9832 (N = 66). Further details: ASP9832 50 mg and 100 mg (random). Group 2: control (N = 30). Further details: control: placebo. Duration of treatment: 3 months. | |
| Outcomes | Outcomes reported: 1. Adverse events. | |
| Notes | Reasons for post‐randomisation drop‐outs: discontinued treatment. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The randomization codes were created by an external organization". |
| Allocation concealment (selection bias) | Low risk | Quote: "The randomization codes were created by an external organization and had been concealed until the end of the study". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The participants were blinded to the received treatment; in addition, neither the investigator nor the pharmacist, nor the sponsor was aware of the treatment group". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The participants were blinded to the received treatment; in addition, neither the investigator nor the pharmacist, nor the sponsor was aware of the treatment group". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: there were post‐randomisation drop‐outs, which may be related to the treatment that the participants received. |
| Selective reporting (reporting bias) | High risk | Comment: protocol was not available; mortality was not reported. |
| For‐profit bias | High risk | Quote: "All clinical trials were sponsored by Astellas Pharma Europe BV". |
| Other bias | Low risk | Comment: no other risk of bias. |
Ratziu 2016.
| Methods | Randomised clinical trial | |
| Participants | Country: Multicentre, international. Number randomised: 276. Post‐randomisation drop‐outs: 2 (0.7%). Revised sample size: 274. Average age: 53 years. Females: 123 (44.9%). NASH: 274 (100%). Diabetics: 107 (39.1%). Average follow‐up period in months: 12. Inclusion criteria 1. Patients with NASH without cirrhosis. 2. Aged 18 to 75 years. Exclusion criteria 1. Daily alcohol consumption > 2 drink units/d (equivalent to 20 g) in women and 3 drink units/d (30 g) in men. 2. Steatohepatitis was due to secondary causes. 3. Any other chronic liver disease was identified. | |
| Interventions | Participants were randomly assigned to two groups. Group 1: elafibranor (N = 182). Further details: elafibranor (80 mg or 120 mg: trial started initally at 80 mg but the dose was changed to 120 mg later). Group 2: control (N = 92). Further details: control: placebo. Duration of treatment: 12 months. | |
| Outcomes | Outcomes reported: 1. Adverse events. | |
| Notes | Reasons for post‐randomisation drop‐outs: not treated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer‐generated coding list". |
| Allocation concealment (selection bias) | Low risk | Quote: "allocation was performed centrally for all sites through a web system". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: " Patients, investigators, clinical site staff, and the pathologist were masked to treatment assignment". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: " Patients, investigators, clinical site staff, and the pathologist were masked to treatment assignment". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: there were post‐randomisation drop‐outs, which may be related to the treatment that the participants received. |
| Selective reporting (reporting bias) | High risk | Comment: since there was no significant difference between the groups in the primary outcome, the definition was revised and according to the new definition, there was statistically significant difference. |
| For‐profit bias | High risk | Quote: "The study was funded by Genfit SA.". |
| Other bias | Low risk | Comment: no other risk of bias. |
Razavizade 2013.
| Methods | Randomised clinical trial | |
| Participants | Country: Iran. Number randomised: 80. Post‐randomisation drop‐outs: 0 (0%). Revised sample size: 80. Average age: 35 years. Females: 12 (15%). NASH: not stated. Diabetics: 6 (7.5%). Average follow‐up period in months: 4. Inclusion criteria 1. Aged ≥ 18 years. 2. Ultrasound proven NAFLD. 3. Persistently elevated transaminases (≥ 40 UI/L). 4. NAFLD liver fat score > ‐0.64. Exclusion criteria 1. Alcohol consumption > 20 g/day for men and 10 g/day for women. 2. Type 1 diabetes. 3. Heart disease. 4. Liver diseases (viral hepatitis, auto‐immune hepatitis, Wilson disease, haemochromatosis, liver mass lesion). 5. Renal disease (creatinine > 1.5 mg/dL). 6. Severe systemic comorbidities. 7. Neoplasm. 8. Any medication in the previous 3 months. 9. Previous treatment with thiazolinediones, biguanides or insulin. 10. Pregnancy or breastfeeding. | |
| Interventions | Participants were randomly assigned to two groups. Group 1: metformin (N = 40). Further details: metformin 1 g per day. Group 2: pioglitazone (N = 40). Further details: pioglitazone 30 mg/day. Duration of treatment: 4 months. Both groups received hypocaloric diet | |
| Outcomes | Outcomes reported: 1. Mortality. 2. Adverse events. 3. Cirrhosis. 4. Decompensated liver disease. 5. Liver transplantation. | |
| Notes | Authors provided additional information in February 2016. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "predefined computer‐generated block randomization table". |
| Allocation concealment (selection bias) | Low risk | Quote: "An investigator who was not involved in data collection and treatment, performed the enrolment patients and their assignments into treatment groups". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "randomized double blind clinical trial". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "randomized double blind clinical trial". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no post‐randomisation drop‐outs. |
| Selective reporting (reporting bias) | Low risk | Comment: mortality and adverse events were reported. |
| For‐profit bias | Low risk | Quote: "This study was supported by the research funds of Kashan University of Medical Sciences (No: 29‐5‐1‐2851)". |
| Other bias | Low risk | Comment: no other risk of bias. |
Razavizadeh 2012.
| Methods | Randomised clinical trial | |
| Participants | Country: Iran. Number randomised: 100. Post‐randomisation drop‐outs: not stated. Revised sample size: 100. Average age: 38 years. Females: 24 (24%). NASH: not stated. Diabetics: not stated. Average follow‐up period in months: 2. Inclusion criteria 1. US fatty liver. 2. Persistently elevated ALT. Exclusion criteria 1. Causes of liver disease other than NAFLD. | |
| Interventions | Participants were randomly assigned to two groups. Group 1: antioxidants (N = not stated). Further details: antioxidants: vitamin E 400 IU/day. Group 2: silymarin (N = not stated). Further details: silymarin 140 mg/day. Duration of treatment: 2 months. | |
| Outcomes | None of the outcomes of interest were reported. | |
| Notes | Reasons for post‐randomisation drop‐outs: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "They were randomly assigned to take…". |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double‐blind". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double‐blind". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: protocol was not available; neither mortality nor adverse events were reported. |
| For‐profit bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other risk of bias. |
Safadi 2014.
| Methods | Randomised clinical trial | |
| Participants | Country: Israel. Number randomised: 60. Post‐randomisation drop‐outs: 3 (5%). Revised sample size: 57. Average age: 40 years. Females: 16 (28.1%). NASH: 6 (10.5%). Diabetics: not stated. Average follow‐up period in months: 4. Inclusion criteria 1. Histologically proven NAFLD or NASH by biopsy performed within 18 months prior to enrolment. 2. Aged 18 to 75 years. | |
| Interventions | Participants were randomly assigned to two groups. Group 1: aramchol (N = 38). Further details: aramchol (100 mg once daily or 300 mg once daily: randomly allocated to the two doses). Group 2: control (N = 19). Further details: control: placebo. Duration of treatment: 3 months. | |
| Outcomes | Outcomes reported: 1. Adverse events. | |
| Notes | Reasons for post‐randomisation drop‐outs: withdrew consent; major protocol violation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomized, double‐blind, placebo‐controlled trial". |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double‐blind". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double‐blind". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: there were post‐randomisation drop‐outs, which may be related to the treatment that the participants received. |
| Selective reporting (reporting bias) | High risk | Comment: protocol was not available; mortality was not reported. |
| For‐profit bias | High risk | Quote: "Supported by Galmed Medical Research, Ltd.". |
| Other bias | Low risk | Comment: no other risk of bias. |
Santos 2003.
| Methods | Randomised clinical trial | |
| Participants | Country: Brasil. Number randomised: 30. Post‐randomisation drop‐outs: not stated. Revised sample size: 30. Average age: 38 years. Females: 2 (6.7%). NASH: not stated. Diabetics: not stated. Average follow‐up period in months: 3. Inclusion criteria 1. BMI ≥ 25. 2. ALT, AST or GGT ≥ 1.5 times the upper limit of normal for more than six months. 3. Ultrasound proven liver steatosis. Exclusion criteria 1. Alcohol consumption > 40 g/week. 2. Decompensated diabetes. 3. Total cholesterol or triglycerides more than 300 mg/dL. 4. Intake of hepatotoxic medications. 5. HBV or HCV infection. 6. Other concomitant hepatic or systemic diseases. | |
| Interventions | Participants were randomly assigned to two groups. Group 1: UDCA (N = 15). Further details: UDCA 10 mg/kg/day. Group 2: control (N = 15). Further details: control: placebo. Duration of treatment: 3 months. | |
| Outcomes | No outcomes of interest were reported in this trial. | |
| Notes | Reasons for post‐randomisation drop‐outs: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomized double‐blind study". |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "randomized double‐blind study". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "randomized double‐blind study". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: protocol was not available; neither mortality nor adverse events were reported. |
| For‐profit bias | High risk | Quote: "Ursodeoxycholic acid was kindly provided by Zambon Laboratories, São Paulo, SP, Brazil". |
| Other bias | Low risk | Comment: no other risk of bias. |
Sanyal 2004.
| Methods | Randomised clinical trial | |
| Participants | Country: USA. Number randomised: 20. Post‐randomisation drop‐outs: 0 (0%). Revised sample size: 20. Average age: 47 years. Females: 10 (50%). NASH: not stated. Diabetics: not stated. Average follow‐up period in months: 6. Inclusion criteria 1. Patients with biopsy proven NASH. Exclusion criteria 1. Age < 18 years. 2. Diabetes mellitus. 3. Cirrhosis. 4. Weight gain or loss > 5 pounds in the month preceding entry. 5. Severe comorbid conditions limiting life expectancy to < 1 year. 6. Pregnancy. 7. Symptomatic gallstone disease. 8. Those being considered for or who had bariatric surgery. 9. Iatrogenic NASH. 10. Concomitant presence of other causes of liver disease (eg. hepatitis C). 11. Refusal to give informed consent or have a liver biopsy examination performed . | |
| Interventions | Participants were randomly assigned to two groups. Group 1: pioglitazone plus antioxidants (N = 10). Further details: pioglitazone 30 mg once daily plus antioxidants: vitamin E 400 IU once daily. Group 2: antioxidants (N = 10). Further details: antioxidants: vitamin E 400 IU once daily. Duration of treatment: 6 months. All people also had low‐calorie diet. | |
| Outcomes | None of the outcomes of interest were reported. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "A randomized prospective trial". |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization was performed by an independent statistician in the General Clinical Research Center ". |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no post‐randomisation drop‐outs. |
| Selective reporting (reporting bias) | High risk | Comment: protocol was not available; neither mortality nor adverse events were reported. |
| For‐profit bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other risk of bias. |
Sanyal 2010.
| Methods | Randomised clinical trial | |
| Participants | Country: USA. Number randomised: 247. Post‐randomisation drop‐outs: 0 (0%). Revised sample size: 247. Average age: 46 years. Females: 147 (59.5%). NASH: 247 (100%). Diabetics: 0 (0%). Average follow‐up period in months: 22. Inclusion criteria 1. Biopsy proven NASH (definite or possible) with NAFLD activity score of at least 4 and ballooning score of at least 1, performed within 6 months before randomization. Exclusion criteria 1. Diabetes. 2. Alcohol consumption > 20 g/day for women and 30 g/day for men for at least 3 consecutive months in the previous 5 years. 3. Cirrhosis. 4. Hepatitis C or other liver diseases. 5. Heart failure NYHA (New York Heart Association) II‐IV. 6. Drugs known for inducing steatohepatitis. | |
| Interventions | Participants were randomly assigned to three groups. Group 1: pioglitazone (N = 80). Further details: pioglitazone 30 mg once daily. Group 2: antioxidants (N = 84). Further details: antioxidants: vitamin E 800 IU once daily. Group 3: control (N = 83). Further details: control: placebo. Duration of treatment: 22 months. | |
| Outcomes | Outcomes reported: 1. Adverse events. 3. Resolution of fatty liver disease. 4. Change in fibrosis score. 5. Change in NAFLD activity score. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomization plan was prepared and administered centrally by the Data Coordinating Center (DCC). Requests for randomizations were made by the clinical staff using a web‐based application". |
| Allocation concealment (selection bias) | Low risk | Quote: "The randomization plan was prepared and administered centrally by the Data Coordinating Center (DCC). Requests for randomizations were made by the clinical staff using a web‐based application". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "randomized, multicenter, double‐masked, placebo‐controlled trial". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "randomized, multicenter, double‐masked, placebo‐controlled trial". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no post‐randomisation drop‐outs for main clinical outcomes but high for histological outcomes. |
| Selective reporting (reporting bias) | High risk | Comment: protocol was not available; mortality was not reported. |
| For‐profit bias | High risk | Quote: "Additional funding was provided by Takeda Pharmaceuticals North America through a Cooperative Research and Development Agreement with the NIH". |
| Other bias | Low risk | Comment: no other risk of bias. |
Sharma 2012.
| Methods | Randomised clinical trial | |
| Participants | Country: India. Number randomised: 60. Post‐randomisation drop‐outs: 1 (1.7%). Revised sample size: 59. Average age: 39 years. Females: 24 (40.7%). NASH: 59 (100%). Diabetics: not stated. Average follow‐up period in months: 6. Inclusion criteria 1. Aged 18 to 70 years. 2. ALT > 1.2 time the upper limit of normal on three occasions at least 1 month apart in the last 6 months. 3. Ultrasound proven fatty liver. 4. Liver biopsy showing steatosis, necro‐inflammation activity, ballooning and/or fibrosis. Exclusion criteria 1. Alcohol intake > 20 g/day. 2. Viral or auto‐immune hepatitis. 3. Primary biliary cirrhosis. 4. Wilson's disease. 5. Haemochromatosis. 6. Biliary obstruction. 7. Decompensated cirrhosis. 8. Drugs ingestion for > 4 weeks during past 6 months (amiodarone, methotrexate, perhexiline, glucocorticoids, estrogens, tamoxifen, nifedipine, diltiazem). 9. Pregnancy. 10. Insulin treated diabetes. | |
| Interventions | Participants were randomly assigned to two groups. Group 1: pentoxifylline (N = 30). Further details: pentoxifylline 400 mg thrice daily. Group 2: pioglitazone (N = 29). Further details: pioglitazone 30 mg once daily. Duration of treatment: 6 months. Both groups received hypocaloric diet and exercise advice. | |
| Outcomes | Outcomes reported: 1. Adverse events. 2. Fibrosis score. | |
| Notes | Reasons for post‐randomisation drop‐outs: lost to follow‐up. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed by computer program". |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization was performed by computer program". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "open label randomized controlled trial". |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "open label randomized controlled trial". Comment: Low for histological assessment as the histologist was blind to the treatment groups. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: there were post‐randomisation drop‐outs, which may be related to the treatment that the participants received.. |
| Selective reporting (reporting bias) | High risk | Comment: protocol was not available; mortality was not reported. |
| For‐profit bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other risk of bias. |
Shields 2009.
| Methods | Randomised clinical trial | |
| Participants | Country: USA. Number randomised: 19. Post‐randomisation drop‐outs: not stated. Revised sample size: 19. Average age: 47 years. Females: 6 (31.6%). NASH: 19 (100%). Diabetics: not stated. Average follow‐up period in months: 12. Inclusion criteria 1. Biopsy proven NASH within 18 months of enrolment. 2. BMI > 27. 3. Fasting blood sugar between 110 and 125 mg/dL. 4. Diagnosis of polycystic ovarian syndrome or the metabolic syndrome. 5. Aged > 17 years. 6. Geographical stability for 1 year from study inclusion. 7. Unremarkable serology for other chronic liver diseases. Exclusion criteria 1. Known diabetes mellitus type 1 or 2. 2. Fasting blood sugar > 125 mg/dL. 3. Prior history of alcoholic liver disease. 4. Any other known chronic liver disease. 5. Renal insufficiency defined as a serum creatinine > 1.2 mg/dL. 6. Known allergic reaction to metformin. 7. Prior use of an insulin‐sensitisers agents such as metformin or thiazolidinedione. 8. Gastric bypass within 2 years. 9. Untreated thyroid disease. 10. Coagulopathy. 11.Chronic thrombocytopenia. 12. Significant alcohol use defined as a consumption > 20 g/day or 80 g/week during the 2 years prior to study enrolment. | |
| Interventions | Participants were randomly assigned to two groups. Group 1: metformin (N = 9). Further details: metformin 500 mg/day increased to 1000 mg/day after 3 months if there was no improvement of serum transaminases. Group 2: control (N = 10). Further details: control: placebo. Duration of treatment: 12 months. Both groups received dietary and exercise advice. | |
| Outcomes | Outcomes reported: 1. Fibrosis score. 2. NAFLD activity score. | |
| Notes | Reasons for post‐randomisation drop‐outs: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Subjects who met eligibility requirements were randomized to group A or B by the pharmacy using a computer‐generated program". |
| Allocation concealment (selection bias) | Low risk | Quote: "Subjects who met eligibility requirements were randomized to group A or B by the pharmacy using a computer‐generated program". |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All biopsies were evaluated separately in a blinded fashion by two study pathologists who scored the histology using the scoring system proposed by Brunt et al". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "The analysis was carried out on an intention‐to‐treat basis". |
| Selective reporting (reporting bias) | High risk | Comment: protocol was not available; neither mortality nor adverse events were reported. |
| For‐profit bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other risk of bias. |
Shiffman 2015.
| Methods | Randomised clinical trial | |
| Participants | Country: USA. Number randomised: 38. Post‐randomisation drop‐outs: not stated. Revised sample size: 38. Average age: not stated. Females: not stated. NASH: not stated. Diabetics: not stated. Average follow‐up period in months: 1. Inclusion criteria 1. Patients with proven NAFLD or NASH with elevated AST at least 1.5 times > normal limits on 2 occasions. 2. Stable dose of statins, fibrates, sulphonylureas, metformin. | |
| Interventions | Participants were randomly assigned to two groups. Group 1: emricasan (N = not stated). Further details: emricasan 25 mg twice daily. Group 2: control (N = not stated). Further details: control: placebo. Duration of treatment: 1 month. | |
| Outcomes | None of the outcomes of interest were reported in this trial. | |
| Notes | Reasons for post‐randomisation drop‐outs: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly assigned to receive". |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double‐blind". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double‐blind". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: protocol was not available; neither mortality nor adverse events were reported. |
| For‐profit bias | High risk | Quote: "Conatus Pharmaceuticals". |
| Other bias | Low risk | Comment: no other risk of bias. |
Siddique 2015.
| Methods | Randomised clinical trial | |
| Participants | Country: India. Number randomised: 67. Post‐randomisation drop‐outs: not stated. Revised sample size: 67. Average age: not stated. Females: not stated. NASH: not stated. Diabetics: not stated. Average follow‐up period in months: 6. Inclusion criteria 1. Patients with NAFLD. | |
| Interventions | Participants were randomly assigned to two groups. Group 1: pioglitazone (N = 34). Further details: pioglitazone (dose not stated). Group 2: rosuvastatin (N = 33). Further details: rosuvastatin (dose not stated). Duration of treatment: 6 months. All people also underwent dietary lifestyle modification. | |
| Outcomes | None of the outcomes of interest were reported in this trial. | |
| Notes | Reasons for post‐randomisation drop‐outs: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "a randomized trial with nested control study". |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: protocol was not available; neither mortality nor adverse events were reported. |
| For‐profit bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other risk of bias. |
Sofer 2011.
| Methods | Randomised clinical trial | |
| Participants | Country: Israel. Number randomised: 63. Post‐randomisation drop‐outs: not stated. Revised sample size: 63. Average age: 54 years. Females: 32 (50.8%). NASH: not stated. Diabetics: not stated. Average follow‐up period in months: 4. Inclusion criteria 1. Ultrasound proven NAFLD. 2. Exclusion of viral, auto‐immune or drug induced liver diseases. 3. Exclusion of alcohol intake > 20 g/day. Exclusion criteria 1. History of unstable angina. 2. Myocardial infarction. 3. Cerebrovascular accident. 4. Major surgery within the 6 months preceding the entrance to the study. 5. Creatinine > 1.5 mg/dL. 6. Electrolyte abnormalities. | |
| Interventions | Participants were randomly assigned to two groups. Group 1: metformin (N = 32). Further details: metformin 850 mg to 1700 mg/day. Group 2: control (N = 31). Further details: control: placebo. Duration of treatment: 4 months. | |
| Outcomes | None of the outcomes of interest were reported. | |
| Notes | Reasons for post‐randomisation drop‐outs: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly assigned to 1 of 2 groups". |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "This was a randomized, placebo‐controlled, double‐blinded study". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "This was a randomized, placebo‐controlled, double‐blinded study". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "An intention‐to‐treat analysis". |
| Selective reporting (reporting bias) | High risk | Comment: protocol was not available; neither mortality nor adverse events were reported. |
| For‐profit bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other risk of bias. |
Solhi 2014.
| Methods | Randomised clinical trial | |
| Participants | Country: Iran. Number randomised: 80. Post‐randomisation drop‐outs: 16 (20%). Revised sample size: 64. Average age: 42 years. Females: 29 (45.3%). NASH: not stated. Diabetics: 0 (0%). Average follow‐up period in months: 2. Inclusion criteria 1. Ultrasound proven NASH. 2. Increase in the ALT and AST levels > 1.2 times the upper limit of normal. Exclusion criteria 1. Autoimmune hepatitis. 2. Wilson's disease. 3. Haemochromatosis. 4. Alpha‐1 antitrypsin. 5. Chronic hepatitis B and C. 6. Diabetes. 7. Severe cardiac, pulmonary, renal, or psychological problems. 8. Positive pregnancy test. 9. Daily ethanol consumption > 20 g. 10. Substance abuse. 11. Use of drugs, such as statins, fibrates, NSAIDs, acetaminophen, warfarin, metronidazol, anticonvulsivants, antidepressants, antipsychotics and antihistamines. | |
| Interventions | Participants were randomly assigned to two groups. Group 1: silymarin (N = 33). Further details: silymarin 210 mg/day. Group 2: control (N = 31). Further details: control: placebo. Duration of treatment: 2 months. Both groups received dietary and exercise advice. | |
| Outcomes | None of the outcomes of interest were reported. | |
| Notes | Reasons for post‐randomisation drop‐outs: lost to follow‐up. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The patients were randomly divided into case and control group with random block design method". |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: although a placebo was used, there is no mention about blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: although a placebo was used, there is no mention about blinding. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: there were post‐randomisation drop‐outs, which may be related to the treatment that the participants received. |
| Selective reporting (reporting bias) | High risk | Comment: protocol was not available; neither mortality nor adverse events were reported. |
| For‐profit bias | Unclear risk | Quote: "The project has been performed under financial support of research department of Arak’s university of medical sciences, Arak, Iran". Comment: the pharmaceutical company manufactured the placebo ‐ it was not clear whether it was supplied free for the study. |
| Other bias | Low risk | Comment: no other risk of bias. |
Song 2014.
| Methods | Randomised clinical trial | |
| Participants | Country: China. Number randomised: 70. Post‐randomisation drop‐outs: 3 (4,3%). Revised sample size: 67. Average age: 57 years. Females: 28 (41.8%). NASH: not stated. Diabetics: 67 (100%). Average follow‐up period in months: 4. Inclusion criteria 1. Aged 18 to 77 years. 2. NAFLD. 3. Newly diagnosed type 2 diabetes. Exclusion criteria 1. Auto‐immune hepatitis. 2. Genetic disorders, such as hepatolenticular degeneration, haemochromatosis. 3. Alcohol consumption > 40 g/week. 4. Infectious diseases. 5. Severe liver and kidney dysfunction. 6. Anaemia. 7. Severe thyroid dysfunction. 8. Acute complications of diabetes. | |
| Interventions | Participants were randomly assigned to two groups. Group 1: metformin plus sitagliptin (N = 34). Further details: metformin 500 mg thrice daily plus sitagliptin 100 mg once daily. Group 2: metformin plus glipizide (N = 33). Further details: metformin 500 mg thrice daily plus glipizide 2.5 mg to 5 mg once daily. Duration of treatment: 4 months. Both groups received dietary and exercise advice. | |
| Outcomes | None of the outcomes of interest were reported. | |
| Notes | Reasons for post‐randomisation drop‐outs: Did not complete the study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "random numbers method". |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: protocol was not available; neither mortality nor adverse events were reported. |
| For‐profit bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other risk of bias. |
Stefan 2014.
| Methods | Randomised clinical trial | |
| Participants | Country: Multicentre, international. Number randomised: 82. Post‐randomisation drop‐outs: 2 (2.4%). Revised sample size: 80. Average age: 53 years. Females: 23 (28.8%). NASH: not stated. Diabetics: not stated. Average follow‐up period in months: 3. Inclusion criteria 1. Hepatic steatosis by magnetic resonance spectroscopy. 2. BMI < 27. 3. Aged 35 to 65 years. 4. Insulin resistance. 5. Negative alcohol test and drug screening. 6. Agreement to maintain previous diet and exercise habits. Exclusion criteria 1. History of diabetes. 2. Other liver disease, including chronic viral hepatitis (B or C), alcohol abuse, haemochromatosis, a1‐antitrypsin deficiency, auto‐immune hepatitis, Wilson's disease, primary sclerosing cholangitis or primary biliary cirrhosis, or liver cirrhosis of any cause. 3. Known auto‐immune disease or chronic inflammatory disorder. 4. Myocardial infarction or stroke within 6 months before screening. 5. Use of drugs potentially associated with NAFLD for more than 2 consecutive weeks in the 2 years before screening. 6. Use of anti‐NASH drugs (thiazolidinediones, vitamin E, metformin, ursodeoxycholic acid, S‐adenosylmethionine, betaine, milk thistle, gemfibrozil, anti‐TNF therapies, probiotics) in the 3 months before randomisation. 7. AST or ALT > 2.5 times the upper limit of normal. | |
| Interventions | Participants were randomly assigned to two groups. Group 1: R05093151 (N = 40). Further details: R05093151 (glucocorticosteroid blocker) 200 mg twice daily. Group 2: control (N = 40). Further details: control: placebo. Duration of treatment: 3 months. | |
| Outcomes | Outcomes reported: 1. Adverse events. 2. Cirrhosis. 3. Decompensated liver disease. 4. Liver transplantation. | |
| Notes | Authors provided additional information in March 2016 Reasons for post‐randomisation drop‐outs: withdrew or non‐compliant. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomisation was done via the interactive voice‐response system". |
| Allocation concealment (selection bias) | Low risk | Quote: "randomisation was done via the interactive voice‐response system". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Randomised, double‐blind, placebo‐controlled trial". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Randomised, double‐blind, placebo‐controlled trial". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: there were post‐randomisation drop‐outs, which may be related to the treatment that the participants received. |
| Selective reporting (reporting bias) | High risk | Comment: protocol was not available; mortality was not reported. |
| For‐profit bias | High risk | Quote: "Funding F Hoffman‐La Roche". |
| Other bias | Low risk | Comment: no other risk of bias. |
Stilidi 2014.
| Methods | Randomised clinical trial | |
| Participants | Country: Ukraine. Number randomised: 58. Post‐randomisation drop‐outs: not stated. Revised sample size: 58. Average age: not stated. Females: not stated. NASH: not stated. Diabetics: not stated. Average follow‐up period in months: 6. Inclusion criteria 1. NASH. | |
| Interventions | Participants were randomly assigned to two groups. Group 1: losartan and UDCA (N = 30). Further details: losartan 50 mg/day and UDCA 30 mg/kg/day. Group 2: UDCA (N = 28). Further details: UDCA 30 mg/kg/day. Duration of treatment: 6 months. | |
| Outcomes | None of the outcomes of interest were reported. | |
| Notes | Reasons for post‐randomisation drop‐outs: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "58 NASH patients were randomly assigned ". |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: protocol was not available; neither mortality nor adverse events were reported. |
| For‐profit bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other risk of bias. |
Sunny 2015.
| Methods | Randomised clinical trial | |
| Participants | Country: USA. Number randomised: 50. Post‐randomisation drop‐outs: not stated. Revised sample size: 50. Average age: 54 years. Females: not stated. NASH: 50 (100%). Diabetics: not stated. Average follow‐up period in months: 18. Inclusion criteria 1. Patients with biopsy proven NASH and prediabetes or type 2 diabetes. | |
| Interventions | Participants were randomly assigned to two groups. Group 1: pioglitazone (N = 27). Further details: pioglitazone (dose not stated). Group 2: control (N = 23). Further details: control: placebo. Duration of treatment: 18 months | |
| Outcomes | None of the outcomes of interest were reported in this trial. | |
| Notes | Reasons for post‐randomisation drop‐outs: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were then randomized to pioglitazone (n=27) or placebo (n=23)". |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: protocol was not available; neither mortality nor adverse events were reported. |
| For‐profit bias | High risk | Quote: "American Diabetes Association (1‐08‐CR‐08 to K.C.); Burroughs Wellcome Fund". |
| Other bias | Low risk | Comment: no other risk of bias. |
Taghvaei 2013.
| Methods | Randomised clinical trial | |
| Participants | Country: Iran. Number randomised: 41. Post‐randomisation drop‐outs: not stated. Revised sample size: 41. Average age: not stated. Females: not stated. NASH: not stated. Diabetics: not stated. Average follow‐up period in months: 6. Inclusion criteria 1. Patients with NAFLD. | |
| Interventions | Participants were randomly assigned to two groups. Group 1: silymarin (N = 21). Further details: silymarin 140 mg twice daily. Group 2: control (N = 20). Further details: control: no intervention. Duration of treatment: 6 months. | |
| Outcomes | None of the outcomes of interest were reported. | |
| Notes | Reasons for post‐randomisation drop‐outs: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The patients were randomly divided into case and control groups". |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: protocol was not available; neither mortality nor adverse events were reported. |
| For‐profit bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other risk of bias. |
Torres 2011.
| Methods | Randomised clinical trial | |
| Participants | Country: USA. Number randomised: 135. Post‐randomisation drop‐outs: 46 (34.1%). Revised sample size: 89. Average age: not stated. Females: not stated. NASH: 89 (100%). Diabetics: 18 (20.2%). Average follow‐up period in months: 11. Inclusion criteria 1. Aged 18 to 70 years. 2. Biopsy proven NASH within 6 months before enrolment. Exclusion criteria 1. NYHA class III or IV for heart failure. 2. Insulin‐requiring diabetes. 3. History of thiazolidinediones, metformin, angiotensin receptor blockers use in the 3 months before enrolment. 4. Alcohol consumption > 20 g/day in females and 30/day in males. 5. Serum creatinine on initial screening > 1.4 mg/dL. 6. Known hypersensitivity to a study drug. 7. Known history of diabetic ketoacidosis. 8. Pregnancy or lactation. 9. Evidence of co‐existent chronic liver disease to include viral hepatitis, Wilson's disease, auto‐immune hepatitis, haemochromatosis, primary biliary cirrhosis, or primary sclerosing cholangitis. | |
| Interventions | Participants were randomly assigned to three groups. Group 1: rosiglitazone plus losartan (N = 35). Further details: rosiglitazone 4 mg twice daily plus losartan 50 mg once daily. Group 2: rosiglitazone plus metformin (N = 28). Further details: rosiglitazone 4 mg twice daily plus metformin 500 mg twice daily. Group 3: rosiglitazone 4 mg BD (N = 26). Further details: rosiglitazone 4 mg twice daily. Duration of treatment: 11 months. | |
| Outcomes | Outcomes reported: 1. Change in fibrosis. 2. Change in NAFLD activity score. 3. Resolution of NASH. | |
| Notes | Reasons for post‐randomisation drop‐outs: stopped treatment before end of treatment period (including loss to follow‐up, withdrawal by physician), did not have paired biopsy. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Eligible patients were randomly assigned using a computer‐generated, random‐sequence grid maintained by the principal investigator to one of three treatment arms". |
| Allocation concealment (selection bias) | Low risk | Quote: "Eligible patients were randomly assigned using a computer‐generated, random‐sequence grid maintained by the principal investigator to one of three treatment arms". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Randomized, Prospective, Open Label Trial". |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Randomized, Prospective, Open Label Trial". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: there were post‐randomisation drop‐outs, which may be related to the treatment that the participants received. |
| Selective reporting (reporting bias) | High risk | Comment: protocol was not available; neither mortality nor adverse events were reported. |
| For‐profit bias | High risk | Quote: "Dr. Harrison advises Amylin. He received grants from Mochida and Rottapharm. Dr. Williams is on the speakers' bureau of Vertex and Kadman". |
| Other bias | Low risk | Comment: no other risk of bias. |
Uygun 2004.
| Methods | Randomised clinical trial | |
| Participants | Country: Turkey. Number randomised: 36. Post‐randomisation drop‐outs: 2 (5.6%). Revised sample size: 34. Average age: 41 years. Females: 13 (38.2%). NASH: 34 (100%). Diabetics: 0 (0%). Average follow‐up period in months: 6. Inclusion criteria 1. Biopsy proven NASH. Exclusion criteria 1. Suspected acute or chronic viral hepatitis, auto‐immune hepatitis or any other liver disease. 2. Relative or absolute contra‐indication for metformin. 3. Possible liver disease other than NASH. 4. History of malignant liver disease. 5. Impaired renal function (serum creatinine > 1.5 mg/dL). 6. Heart failure. 7. History of lactic acidosis. 8. Severe infection. 9. Hypoxic status. 10. Serious acute and chronic illnesses. 11. Haemodynamic instability. 12. Aged > 70 years. 13. Diabetes mellitus. 14. Current use of any drugs that may affect the results. 15. GGT levels > 75 IU/l. | |
| Interventions | Participants were randomly assigned to two groups. Group 1: metformin (N = 17). Further details: metformin 850 mg twice daily. Group 2: control (N = 17). Further details: control: no intervention. Duration of treatment: 6 months. Both groups received hypocaloric and low lipid diet. | |
| Outcomes | None of the outcomes of interest were reported. | |
| Notes | Reasons for post‐randomisation drop‐outs: lost to follow‐up; development of autoimmune disease. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "After the selection procedure, patients were randomly assigned into two study groups using random sampling numbers". |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "The small number of patients, the unblind nature of the study and the lack of a placebo group were major drawbacks of this investigation". |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "The small number of patients, the unblind nature of the study and the lack of a placebo group were major drawbacks of this investigation". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: there were post‐randomisation drop‐outs, which may be related to the treatment that the participants received. |
| Selective reporting (reporting bias) | High risk | Comment: protocol was not available; neither mortality nor adverse events were reported. |
| For‐profit bias | Unclear risk | Comment: protocol was not available; neither mortality nor adverse events were reported. |
| Other bias | Low risk | Comment: no other risk of bias. |
Van Wagner 2011.
| Methods | Randomised clinical trial | |
| Participants | Country: USA. Number randomised: 30. Post‐randomisation drop‐outs: 4 (13.3%). Revised sample size: 26. Average age: not stated. Females: not stated. NASH: 26 (100%). Diabetics: not stated. Average follow‐up period in months: 12. Inclusion criteria 1. Aged 18 to 65 years. 2. Biopsy proven NASH within 6 months from enrolment. Exclusion criteria 1. HIV positivity. 2. Ongoing alcohol consumption > 20 g (males) and 10 g (females) daily. 3. Current or past use (in the previous 6 months) of drugs known to cause steatohepatitis (tamoxifen, valproic acid, amiodarone, methotrexate). 4. Current or past history of decompensated liver disease. 5. Renal failure. 6. Evidence of active bleeding. 7. Cerebral or retinal haemorrhage. 8. Concomitant use of thiazolidinediones, weight loss medications, metformin, vitamin E, anti TNF alpha therapy or theophylline. 9. Insulin secretagogues. | |
| Interventions | Participants were randomly assigned to two groups. Group 1: pentoxifylline (N = 19). Further details: pentoxifylline 400 mg thrice daily. Group 2: control (N = 7). Further details: control: placebo. Duration of treatment: 12 months. Both groups received dietary and exercise advice. | |
| Outcomes | Outcomes reported: 1. Adverse events. 2. Change in fibrosis score. 3. Change in NAS score. | |
| Notes | Reasons for post‐randomisation drop‐outs: lost to follow‐up, brain tumour, uncovered alcohol abuse. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A randomization table was generated to distribute groups in a 2:1 ratio". |
| Allocation concealment (selection bias) | Low risk | Quote: "On the morning of the initial visit, subjects were randomized by the Northwestern pharmacy and supplied with corresponding pills PTX or placebo". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Investigators and subjects were blinded to the treatment group". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Investigators and subjects were blinded to the treatment group". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: there were post‐randomisation drop‐outs, which may be related to the treatment that the participants received. |
| Selective reporting (reporting bias) | High risk | Comment: protocol was not available; mortality was not reported. |
| For‐profit bias | Unclear risk | Quote: "This research was supported by investigator initiated funds". Comment: Further information on the source of funding was not available. |
| Other bias | Low risk | Comment: no other risk of bias. |
Wang 2015.
| Methods | Randomised clinical trial | |
| Participants | Country: China. Number randomised: 68. Post‐randomisation drop‐outs: not stated. Revised sample size: 68. Average age: not stated. Females: not stated. NASH: not stated. Diabetics: 68 (100%). Average follow‐up period in months: 6. Inclusion criteria 1. Patients with NAFLD and type 2 diabetes. | |
| Interventions | Participants were randomly assigned to four groups. Group 1: sitagliptin (N = 17). Further details: sitagliptin 100 mg/day. Group 2: metformin (N = 17). Further details: metformin 500 mg thrice daily. Group 3: metformin and sitagliptin (N = 20). Further details: metformin 500 mg thrice daily and sitagliptin 100 mg/day. Group 4: control (N = 14). Further details: control: no intervention. Duration of treatment: 6 months. | |
| Outcomes | None of the outcomes of interest were reported in this trial. | |
| Notes | Reasons for post‐randomisation drop‐outs: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Subjects were randomly divided into 4 groups". |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "open‐label". |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "open‐label". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: protocol was not available; neither mortality nor adverse events were reported. |
| For‐profit bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other risk of bias. |
Yaginuma 2009.
| Methods | Randomised clinical trial | |
| Participants | Country: Japan. Number randomised: 20. Post‐randomisation drop‐outs: not stated. Revised sample size: 20. Average age: not stated. Females: not stated. NASH: not stated. Diabetics: not stated. Average follow‐up period in months: 12. Inclusion criteria 1. NAFLD. 2. Insulin resistance. | |
| Interventions | Participants were randomly assigned to two groups. Group 1: pioglitazone (N = not stated). Further details: pioglitazone 7.5 mg once daily. Group 2: control (N = not stated). Further details: control: no intervention. Duration of treatment: 12 months. | |
| Outcomes | None of the outcomes of interest were reported. | |
| Notes | Reasons for post‐randomisation drop‐outs: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: " randomly assigned for treatment with/without low‐dose pioglitazone". |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: protocol was not available; neither mortality nor adverse events were reported. |
| For‐profit bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other risk of bias. |
Yan 2015.
| Methods | Randomised clinical trial | |
| Participants | Country: China. Number randomised: 122. Post‐randomisation drop‐outs: not stated. Revised sample size: 122. Average age: 52 years. Females: 62 (50.8%). NASH: not stated. Diabetics: not stated. Average follow‐up period in months: 4. Inclusion criteria 1. Patients with NAFLD and impaired glucose tolerance or diabetes. Exclusion criteria 1. Alcohol consumption ≥ 10 g/day for women and 20 g/day for men. 2. Hepatitis B or C, or other liver diseases. 3. Treatment with the following drugs within 4 weeks before enrolment: hypoglycaemic or lipid‐regulating (statins, fibrates) drugs, silybin, ursodeoxycholic acid, bicyclol, phosphatidylcholine and vitamin E and Chinese herbs. 4. Patients with severe metabolic abnormalities and organ dysfunction. | |
| Interventions | Participants were randomly assigned to two groups. Group 1: pioglitazone (N = 60). Further details: pioglitazone 15 mg once daily. Group 2: control (N = 62). Further details: control: no intervention. Duration of treatment: 4 months. All people also underwent lifestyle modification (diet and regular exercise). | |
| Outcomes | Outcomes reported: 1. Adverse events. | |
| Notes | Another group which received a Chinese herb and Pioglitazone was excluded. Reasons for post‐randomisation drop‐outs: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer‐generated random allocation sequence". |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "open‐label clinical trial". |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "open‐label clinical trial". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: It was not clear whether all participants were included in the analysis of adverse events. |
| Selective reporting (reporting bias) | High risk | Comment: protocol was not available; mortality was not reported. |
| For‐profit bias | Low risk | Quote: "Funding was by several Government agencies in China". |
| Other bias | Low risk | Comment: no other risk of bias. |
AST = aspartate transaminase
ALT = alanine transaminase
BMI = Body Mass Index
GGT = gamma glutamyl transferase
HBV = hepatitis B virus
HCV = hepatitis C virus
LDL = low density lipoprotein
MRI = magnetic resonance imaging
NAFLD = non‐alcohol related fatty liver disease
NASH = non‐alcohol related steatohepatitis
NYHA = New York Heart Association
TNF = Tumour Necrosis Factor
UDCA = ursodeoxycholic acid
US = ultrasound
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abenavoli 2013 | Not a pharmacological intervention. |
| Abenavoli 2015 | Not a randomised clinical trial. |
| Acquati 2007 | Comparison of different regimens of same drug class. |
| Athyros 2011 | Comparison of different regimens of same drug class. |
| Carnelutti 2012 | Comparison of pharmacological intervention versus non pharmacological intervention. |
| Corey 2015a | study excluded because on children. |
| Dajani 2015 | Not a pharmacological intervention. |
| Faghihzadeh 2014 | Not a pharmacological intervention |
| Fan 2010 | Not a randomised clinical trial. |
| Fan 2013 | Not a randomised clinical trial. |
| Gastaldelli 2015 | Study on patients without NAFLD. |
| Han 2012 | Comparison of same class of drugs. |
| Han 2014a | Not a pharmacological intervention |
| Idilman 2008 | Not a randomised clinical trial. |
| Jaafari 2012 | Not a randomised clinical trial. |
| Kowdley 2015 | Not a primary study. |
| Kowdley 2015a | Not a primary study. |
| Li 2015 | Not a pharmacological intervention. |
| Lo 2016 | Study on patients without NAFLD. |
| McCormick 2015 | Not a pharmacological intervention. |
| Merat 2015 | Study on patients without NAFLD. |
| Oh 2016 | Study on patients fatigued patients with and without NAFLD. Separate data on people with NAFLD was not reported. |
| Scorletti 2014 | Not a pharmacological intervention. |
| Scorletti 2015 | Not a pharmacological intervention. |
| Shiasi 2014 | Study on children. |
| Sultana 2012 | Not a randomised clinical trial. |
| Talebi 2015 | Not a pharmacological intervention. |
| Tan 2011 | Not a pharmacological intervention. |
| Taniai 2009 | Not a randomised clinical trial. |
| Tsuchiya 2011 | Comparison of two 'other' anti‐diabetes drugs. |
| Vos 2016 | Wrong population. Study on children. |
| Wang 2013 | Not a pharmacological intervention. |
| Zelber‐Sagi 2006 | Not a randomised clinical trial (quasi‐randomised study). |
NAFLD = non‐alcohol related fatty liver disease
Differences between protocol and review
It was not possible to assess if potential effect modifiers were similar across different comparisons. Therefore, we did not perform a network meta‐analysis, and assessed the comparative benefits and harms of different interventions using standard Cochrane methodology. The planned future network meta‐analysis methodology that would be applied in review updates as data permit is presented in Appendix 1.
We performed Trial Sequential Analysis in addition to conventional methods of assessing the risk of random errors using P values.
We included two additional histological outcomes as potential surrogate outcomes (fibrosis score and non‐alcohol related fatty liver disease (NAFLD) activity score) post hoc. We used this only for exploratory purposes because these outcomes are now accepted by regulatory agencies for expediting drug approval in NAFLD through an accelerated approval pathway (Sanyal 2016) and did not make any inferences based on the observations in these outcomes.
Contributions of authors
Simona Onali and Rosa Lombardi identified the studies, extracted data, and completed Characteristics of included studies and Characteristics of excluded studies tables. Kurinchi S Gurusamy extracted data, performed the analysis and wrote the review. Emmanuel Tsochatzis, Brian Davidson, and Douglas Thorburn critically commented on the review.
Sources of support
Internal sources
University College London, UK.
External sources
National Institute for Health Research, UK.
Declarations of interest
This review is independent research funded by the National Institute for Health Research (NIHR Cochrane Programme Grants, 13/89/03 ‐ Evidence‐based diagnosis and management of upper digestive, hepato‐biliary, and pancreatic disorders). The views expressed in this publication are those of the authors and not necessarily those of the National Health Service (NHS), the NIHR, or the Department of Health.
Kurinchi Gurusamy and Brian Davidson have no financial disclosures. Emmanuel Tsochatzis has participated in advisory boards for Astra Zeneca and ViiV Helthcare. Astellas funded Douglas Thorburn for his attendance at the International Liver Transplantation Society meeting in 2014. Douglas Thorburn has also received GBP 25,000 from Boston Scientific to fund a clinical research fellow in 2013. There are no other financial disclosures to report.
Edited (no change to conclusions)
References
References to studies included in this review
Aithal 2008 {published data only}
- Aithal GP, Thomas JA, Kaye PV, Lawson A, Ryder SD, Spendlove I, et al. Randomized, placebo‐controlled trial of pioglitazone in nondiabetic subjects with nonalcoholic steatohepatitis. Gastroenterology 2008;135(4):1176‐84. [DOI] [PubMed] [Google Scholar]
Alam 2016 {published data only}
- Alam S, Kabir J, Das U, Hasan N, Alam AK. Effect of telmisartan on histological activity and fibrosis of non alcoholic steatohepatitis patient a one year randomized control trial. Hepatology International 2015;9(Suppl 1):S116. [Google Scholar]
- Alam S, Kabir J, Mustafa G, Das Gupta U, Hasan S, Alam A. Effect of telmisartan on histological activity and fibrosis of non‐alcoholic steatohepatitis: A 1‐year randomized control trial. Saudi Journal of Gastroenterology 2016;22(1):69‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Aller 2015 {published data only}
- Aller R, Izaola O, Gomez S, Tafur C, Gonzalez G, Berroa E, et al. Effect of silymarin plus vitamin E in patients with non‐alcoholic fatty liver disease. A randomized clinical pilot study. European Review for Medical & Pharmacological Sciences 2015;19(16):3118‐24. [PubMed] [Google Scholar]
Armstrong 2016 {published data only}
- Armstrong MJ, Gaunt P, Aithal GP, Barton D, Hull D, Parker R, et al. Liraglutide safety and efficacy in patients with non‐alcoholic steatohepatitis (lean): A multicentre, double‐blind, randomised, placebo‐controlled phase 2 study. Lancet 2016;387(10019):679‐90. [DOI] [PubMed] [Google Scholar]
Askarimoghadam 2013 {published data only}
- Askarimoghadam F, Arjmandpour A, Adibi P. Effects of metformin plus vitamin E versus metformin alone in treatment of NAFLD: a randomized clinical trial. Journal of Gastroenterology and Hepatology 2013;28:633. [Google Scholar]
Athyros 2006 {published data only}
- Athyros VG, Mikhailidis DP, Didangelos TP, Giouleme OI, Liberopoulos EN, Karagiannis A, et al. Effect of multifactorial treatment on non‐alcoholic fatty liver disease in metabolic syndrome: a randomised study. Current Medical Research & Opinion 2006;22(5):873‐83. [DOI] [PubMed] [Google Scholar]
- Athyros VG, Mikhailidis DP, Papageorgiou AA, Didangelos TP, Peletidou A, Kleta D, et al. Targeting vascular risk in patients with metabolic syndrome but without diabetes. Metabolism: Clinical and Experimental 2005;54(8):1065‐74. [DOI] [PubMed] [Google Scholar]
Baranova 2015 {published data only}
- Baranova EI, Berezina AV, Melioranskaya EI, Polyakova EA. Safety and efficacy of amlodipine, lisinopril and rosuvastatin therapy in patients with metabolic syndrome and nonalcoholic fatty liver disease. Kardiologiya 2015;55(10):68‐75. [DOI] [PubMed] [Google Scholar]
Basu 2013 {published data only}
- Basu P, Mittimani K, Siriki R, Rahaman M, Atluri S, Farhat S, et al. Curcumin, anti‐oxidant, and pioglitazone therapy with inclusion of vitamin E in non‐alcoholic fatty liver disease‐a randomized double blind placebo controlled trial (captive). Hepatology International 2013;7:S73‐4. [Google Scholar]
- Basu P, Shah N, Siriki R, Rahaman M, Farhat S. Curcumin, antioxidant, and pioglitazone therapy with inclusion of vitamin E in non‐alcoholic fatty liver disease: a randomized, open‐label, placebo‐controlled clinical prospective trial (captive). American Journal of Gastroenterology 2013;108:S149‐50. [Google Scholar]
- Basu P, Shah NJ, Farhat S, Siriki R, Mittimanj K, Atluri S, et al. Curcumin, anti‐oxidant, and pioglitazone therapy with inclusion of vitamin E in non alcoholic fatty liver disease‐a randomized open label placebo controlled clinical prospective trial (captive). Gut 2013;62:A23‐4. [Google Scholar]
- Basu P, Shah NJ, Siriki R, Mittimani K, Atluri S, Rahaman A, et al. Curcumin, anti‐oxidant, and pioglitazone therapy with inclusion of vitamin E in non alcoholic fatty liver disease‐a randomized open label placebo controlled clinical prospective trial (captive). Gastroenterology 2013;144(5):S1011‐2. [Google Scholar]
Basu 2014 {published data only}
- Basu P, Shah NJ, Farhat S. Effect of vitamin E and alfa lipoic acid (ala) in non‐alcoholic fatty liver disease: a randomise placebo control open label prospective clinical trial: VAIN trial. Gut 2012;61:A204‐5. [Google Scholar]
- Basu P, Shah NJ, Krishnaswamy N, Farhat S, Nair T. Effect of vitamin E and alfa lipoic acid in non alcoholic fatty liver disease: a randomized clinical trial. Journal of Gastroenterology and Hepatology 2012;27:208. [Google Scholar]
- Basu PP, Krishnaswamy N, Nair T, Shah NJ, Farhat S. Effect of vitamin E and alfa lipoic acid (ala) in non alcoholic fatty liver disease: A randomized placebo control open label prospective clinical trial ‐ VAIN trial. American Journal of Gastroenterology 2011;106:S136‐7. [Google Scholar]
- Basu PP, Krishnaswamy N, Nair TJ, Shah NJ, Farhat S. Effect of vitamin E and alfa lipoic acid (ala) in non alcoholic fatty liver disease: a randomized placebo control open label prospective clinical trial ‐ VAIN trial. Hepatology (Baltimore, Md.) 2011;54:1145A‐6A. [Google Scholar]
- Basu PP, Shah JN, Krishnaswamy NV, Pacana T, Khemani J, Farhat S, et al. Effect of vitamin E and alpha lipoic acid (ala) in non alcoholic fatty liver disease: a randomized placebo‐controlled open‐label prospective clinical trial ‐ VAIN trial. Scandinavian Journal of Gastroenterology 2012;47:S63‐5. [Google Scholar]
- Basu PP, Shah NJ, Aloysius MM, Jr. RSB. Effect of vitamin E and alpha lipoic acid in nonalcoholic fatty liver disease: a randomized, placebo‐controlled, open‐label, prospective clinical trial (VAIN trial). Open Journal of Gastroenterology 2014:199‐207.
Belfort 2006 {published data only}
- Balas B, Belfort R, Harrison SA, Darland C, Finch J, Schenker S, et al. Pioglitazone treatment increases whole body fat but not total body water in patients with non‐alcoholic steatohepatitis. Journal of Hepatology 2007;47(4):565‐70. [DOI] [PubMed] [Google Scholar]
- Belfort R, Harrison SA, Brown K, Darland C, Finch J, Hardies J, et al. A placebo‐controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. New England Journal of Medicine 2006;355(22):2297‐307. [DOI] [PubMed] [Google Scholar]
Bonfrate 2015 {published data only}
- Bonfrate L, Grattagliano I, Portincasa P. The efficacy of eurosil 85‐vit. E complex on metabolic profile in adults with non alcoholic fatty liver disease (NAFLD). A double‐blind randomized placebo‐controlled clinical study. European Journal of Clinical Investigation 2015;45:15. [Google Scholar]
Bugianesi 2005 {published data only}
- Bugianesi E, Gentilcore E, Manini R, Natale S, Vanni E, Villanova N, et al. A randomized controlled trial of metformin versus vitamin E or prescriptive diet in nonalcoholic fatty liver disease. American Journal of Gastroenterology 2005;100(5):1082‐90. [DOI] [PubMed] [Google Scholar]
Chan 2015 {published data only}
- Chan WK, Nik Mustapha NR, Mahadeva S. Silymarin for the treatment of non‐alcoholic steatohepatitis: Interim analysis of a randomized, double‐blind, placebo‐controlled trial. Journal of Hepatology 2015;62:S269. [Google Scholar]
Copaci 2009 {published data only}
- Copaci I, Mindrut E, Micu L, Hortopan M, Voiculescu M. Can disease progression in non‐alcoholic steatohepatitis be stopped?. Journal of Hepatology 2009;50:S358. [Google Scholar]
Cui 2006 {published data only}
- Cui KQ, Zhao XW, Zhang Y, Kang XH, Meng J, Chen XL. Efficacy of rosiglitazone in treatment of nonalcoholic fatty liver disease and its relations with adiponectin. World Chinese Journal of Digestology 2006;14(13):1326‐9. [Google Scholar]
Cusi 2013 {published data only}
- Cusi K, Orsak B, Lomonaco R, Bril F, Ortiz‐Lopez C, Hecht J, et al. Extended treatment with pioglitazone improves liver histology in patients with prediabetes or type 2 diabetes mellitus and NASH. Hepatology (Baltimore, Md.) 2013;58(Suppl S1):248A. [Google Scholar]
- Cusi K, Orsak B, Lomonaco R, Finch J, Ortiz‐Lopez C, Bril F, et al. Safety and efficacy of long‐term pioglitazone treatment for patients with prediabetes or t2dm and NASH. Diabetes 2013;62:A309. [Google Scholar]
Dufour 2006 {published data only}
- Balmer ML, Siegrist K, Zimmermann A, Dufour JF. Effects of ursodeoxycholic acid in combination with vitamin E on adipokines and apoptosis in patients with nonalcoholic steatohepatitis. Liver International 2009;29(8):1184‐8. [DOI] [PubMed] [Google Scholar]
- Dufour J, Oneta CM, Gonvers J, Bihl F, Cerny A, Cereda J, et al. Randomized placebo‐controlled trial of ursodeoxycholic acid with vitamin E in nonalcoholic steatohepatitis. Clinical Gastroenterology and Hepatology 2006;4(12):1537‐43. [DOI] [PubMed] [Google Scholar]
Ersoz 2005 {published data only}
- Ersoz G, Gunsar F, Karasu Z, Akay S, Batur Y, Akarca US. Management of fatty liver disease with vitamin E and C compared to ursodeoxycholic acid treatment. Turkish Journal of Gastroenterology 2005;16(3):124‐8. [PubMed] [Google Scholar]
Fogari 2012 {published data only}
- Fogari R, Maffioli P, Mugellini A, Zoppi A, Lazzari P, Derosa G. Effects of losartan and amlodipine alone or combined with simvastatin in hypertensive patients with nonalcoholic hepatic steatosis. European Journal of Gastroenterology & Hepatology 2012;24(2):164‐71. [DOI] [PubMed] [Google Scholar]
- Fogari R, Mugellini A, Zoppi A, Lazzari P, Maffioli P, Derosa G. Losartan alone or combined with simvastatin improved visceral adipose tissue and inflammation in hypertensive normocholesterolemic patients with non‐alcoholic hepatic steatosis. Journal of Hepatology 2011;54(Suppl 1):S9‐s10. [Google Scholar]
Foster 2011 {published data only}
- Foster T, Budoff MJ, Saab S, Ahmadi N, Gordon C, Guerci AD. Atorvastatin and antioxidants for the treatment of nonalcoholic fatty liver disease: the St Francis heart study randomized clinical trial. American Journal of Gastroenterology 2011;106(1):71‐7. [DOI] [PubMed] [Google Scholar]
Garinis 2010 {published data only}
- Garinis GA, Fruci B, Mazza A, Siena M, Abenavoli S, Gulletta E, et al. Metformin versus dietary treatment in nonalcoholic hepatic steatosis: a randomized study. International Journal of Obesity 2010;34(8):1255‐64. [DOI] [PubMed] [Google Scholar]
Gastaldelli 2009 {published data only}
- Gastaldelli A, Balas B, Belfort R, Harrison S, Finch J, Ciociaro D. Metabolic and anti‐inflammatory beneficial effects of pioglitazone (pio) treatment in patients with non‐alcoholic steatohepatitis (NASH) and their associations with histological improvement. Journal of Hepatology 2009;50(Suppl 1):S24. [Google Scholar]
Gianturco 2013 {published data only}
- Gianturco V, Troisi G, Bellomo A, Bernardini S, D'Ottavio E, Formosa V, et al. Impact of combined therapy with alpha‐lipoic and ursodeoxycolic acid on nonalcoholic fatty liver disease: double‐blind, randomized clinical trial of efficacy and safety. Hepatology International 2013;7(2):570‐6. [DOI] [PubMed] [Google Scholar]
Gomez 2009 {published data only}
- Gomez EV, Miranda AR, Oramas BG, Soler EA, Navarro RL, Bertot LC, et al. Clinical trial: a nutritional supplement viusid, in combination with diet and exercise, in patients with nonalcoholic fatty liver disease. Alimentary Pharmacology & Therapeutics 2009;30(10):999‐1009. [DOI] [PubMed] [Google Scholar]
Hajaghamohammadi 2008 {published data only}
- Hajaghamohammadi AA, Ziaee A, Rafiei R. The efficacy of silymarin in decreasing transaminase activities in non‐alcoholic fatty liver disease: a randomized controlled clinical trial. Hepatitis Monthly 2008;8(3):191‐5. [Google Scholar]
Hajiaghamohammadi 2012 {published data only}
- Hajiaghamohammadi AA, Ziaee A, Oveisi S, Masroor H. Effects of metformin, pioglitazone, and silymarin treatment on non‐alcoholic fatty liver disease: a randomized controlled pilot study. Hepatitis Monthly 2012;12(8):e6099. [DOI] [PMC free article] [PubMed] [Google Scholar]
Harrison 2003 {published data only}
- Harrison SA, Torgerson S, Hayashi P, Ward J, Schenker S. Vitamin E and vitamin C treatment improves fibrosis in patients with nonalcoholic steatohepatitis. American Journal of Gastroenterology 2003;98(11):2485‐90. [DOI] [PubMed] [Google Scholar]
Harrison 2009 {published data only}
- Harrison SA, Fecht W, Brunt EM, Neuschwander‐Tetri BA. Orlistat for overweight subjects with nonalcoholic steatohepatitis: a randomized, prospective trial. Hepatology (Baltimore, Md.) 2009;49(1):80‐6. [DOI] [PubMed] [Google Scholar]
Hashemi 2009 {published data only}
- Hashemi SJ, Hajiani E, Sardabi EH. A placebo‐controlled trial of silymarin in patients with nonalcoholic fatty liver disease. Hepatitis Monthly 2009;9(4):265‐70. [Google Scholar]
Haukeland 2009 {published data only}
- Haukeland JW, Konopski Z, Eggesbø HB, Volkmann HL, Raschpichler G, Bjøro K, et al. Metformin in patients with non‐alcoholic fatty liver disease: a randomized, controlled trial. Scandinavian Journal of Gastroenterology 2009;44(7):853‐60. [DOI] [PubMed] [Google Scholar]
- Haukeland JW, Konopski Z, Loberg EM, Haaland TK, Volkmann HL, Raschpichler G, et al. A randomized, placebo controlled trial with metformin in patients with NAFLD. Hepatology (Baltimore, Md.) 2008;48(4):334A. [Google Scholar]
Jin 2010 {published data only}
- Jin H, Zhou Y, Ming K. Efficacy of pioglitazone in treatment of 60 patients with nonalcoholic steatohepatitis. Pharmaceutical Care and Research 2010;10(3):221‐3. [Google Scholar]
Kakazu 2013 {published data only}
- Kakazu E, Kondo Y, Ninomiya M, Kimura O, Nagasaki F, Ueno Y, et al. The influence of pioglitazone on the plasma amino acid profile in patients with nonalcoholic steatohepatitis (NASH). Hepatology International 2013;7(2):577‐85. [DOI] [PubMed] [Google Scholar]
Kedarisetty 2014 {published data only}
- Kedarisetty CK, Bhardwaj A, Bhadoria AS, Bihari C, Rastogi A, Kanal U, et al. A randomized controlled trial to study the efficacy of combination of pentoxiphylline and vitamin E versus vitamin E in patients with non‐alcoholic steatohepatitis. Journal of Hepatology 2014;60(1 Suppl):S344. [Google Scholar]
Klyarytskaya 2015 {published data only}
- Klyarytskaya IL, Stilidi EI, Maksymova EV. Comparison of different treatment regimens in patients with nonalcoholic fatty liver disease. Eksperimental'Naia i Klinicheskaia Gastroenterologiia 2015;7:12‐7. [PubMed] [Google Scholar]
Kugelmas 2003 {published data only}
- Kugelmas M, Hill DB, Vivian B, Marsano L, McClain CJ. Cytokines and NASH: A pilot study of the effects of lifestyle modification and vitamin E. Hepatology (Baltimore, Md.) 2003;38(2):413‐9. [DOI] [PubMed] [Google Scholar]
Leuschner 2010 {published data only}
- Leuschner UFH, Lindenthal B, Herrmann G, Arnold JC, Rossle M, Cordes HJ, et al. High‐dose ursodeoxycholic acid therapy for nonalcoholic steatohepatitis: a double‐blind, randomized, placebo‐controlled trial. Hepatology (Baltimore, Md.) 2010;52(2):472‐9. [DOI] [PubMed] [Google Scholar]
Lewis 2006 {published data only}
- Lewis JH, Zweig SF, Belder R. Is the lipid reduction seen with high‐dose pravastatin (prava) associated with a fall in alt values in hypercholesterolemic pts with NAFLD? Results from a prospective, randomized double‐blind, placebo (pbo)‐controlled trial. American Journal of Gastroenterology 2006;101(9):S158‐9. [Google Scholar]
Lindor 2004 {published data only}
- Lindor KD, Kowdley KV, Heathcote EJ, Harrison ME, Jorgensen R, Angulo P, et al. Ursodeoxycholic acid for treatment of nonalcoholic steatohepatitis: results of a randomized trial. Hepatology (Baltimore, Md.) 2004;39(3):770‐8. [DOI] [PubMed] [Google Scholar]
Loomba 2015 {published data only}
- Lin SC, Ang B, Hernandez C, Bettencourt R, Jain R, Salotti J, et al. Cardiovascular risk assessment in the treatment of nonalcoholic steatohepatitis: a secondary analysis of the Mozart trial. Therapeutic Advances in Gastroenterology 2016;9(2):152‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomba R, Sirlin CB, Ang B, Bettencourt R, Jain R, Salotti J, et al. Ezetimibe for the treatment of nonalcoholic steatohepatitis: Assessment by novel magnetic resonance imaging and magnetic resonance elastography in a randomized trial (Mozart trial). Hepatology (Baltimore, Md.) 2015;61(4):1239‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Magosso 2013 {published data only}
- Magosso E, Ansari MA, Gopalan Y, Shuaib IL, Khan N, Yuen KH, et al. Tocotrienols and nonalcoholic fatty liver: a clinical experience. Hepatology (Baltimore, Md.) 2010;52:642A. [Google Scholar]
- Magosso E, Ansari MA, Gopalan Y, Shuaib IL, Wong JW, Khan NA, et al. Tocotrienols for normalisation of hepatic echogenic response in nonalcoholic fatty liver: a randomised placebo‐controlled clinical trial. Nutrition Journal 2013;12(1):166. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mendez‐Sanchez 2004 {published data only}
- Mendez‐Sanchez N, Gonzalez V, Chavez‐Tapia N, Ramos MH, Uribe M. Weight reduction and ursodeoxycholic acid in subjects with nonalcoholic fatty liver disease. A double‐blind, placebo‐controlled trial. Annals of Hepatology 2004;3(3):108‐12. [PubMed] [Google Scholar]
Merat 2003 {published data only}
- Merat S, Malekzadeh R, Sohrabi MR, Sotoudeh M, Rakhshani N, Sohrabpour AA, et al. Probucol in the treatment of non‐alcoholic steatohepatitis: a double‐blind randomized controlled study. Journal of Hepatology 2003;38(4):414‐8. [DOI] [PubMed] [Google Scholar]
Morita 2005 {published data only}
- Morita Y, Ueno T, Sasaki N, Tateishi Y, Nagata E, Kage M, et al. Nateglinide is useful for nonalcoholic steatohepatitis (NASH) patients with type 2 diabetes. Hepato‐Gastroenterology 2005;52(65):1338‐43. [PubMed] [Google Scholar]
Mudaliar 2013 {published data only}
- Mudaliar S, Henry RR, Sanyal AJ, Morrow L, Marschall HU, Kipnes M, et al. Efficacy and safety of the farnesoid x receptor agonist obeticholic acid in patients with type 2 diabetes and nonalcoholic fatty liver disease. Gastroenterology 2013;145(3):574‐82.e1. [DOI] [PubMed] [Google Scholar]
Nar 2009 {published data only}
- Nar A, Gedik O. The effect of metformin on leptin in obese patients with type 2 diabetes mellitus and nonalcoholic fatty liver disease. Acta Diabetologica 2009;46(2):113‐8. [DOI] [PubMed] [Google Scholar]
Nelson 2009 {published data only}
- Nelson A, Torres DM, Morgan AE, Fincke C, Harrison SA. A pilot study using simvastatin in the treatment of nonalcoholic steatohepatitis: a randomized placebo‐controlled trial. Journal of Clinical Gastroenterology 2009;43(10):990‐4. [DOI] [PubMed] [Google Scholar]
Neuschwander‐Tetri 2015 {published data only}
- Chalasani NP, Loomba R, Terrault N, McCullough AJ, Abdelmalek MF, Kowdley KV, et al. Longitudinal changes in fib‐4 and improvement in fibrosis stage with obeticholic acid: a secondary analysis of flint trial. Hepatology (Baltimore, Md.) 2015;62:332A‐3A. [Google Scholar]
- Hameed B, Terrault N, Gill R, Loomba R, Chalasani N, Hoofnagle JH, et al. Separate and combined effects of obeticholic acid and weight loss in nonalcoholic steatohepatitis (NASH). Journal of Hepatology 2015;62:S271. [Google Scholar]
- Hameed B, Terrault N, Gill RM, Loomba R, Chalasani NP, Hoofnagle JH, et al. Clinical and metabolic effects associated with weight loss and obeticholic acid in nonalcoholic steatohepatitis (NASH). Hepatology (Baltimore, Md.) 2015;62:331A. [Google Scholar]
- Kowdley KV, Abdelmalek MF, McCullough A, Loomba R, Hameed B, Chalasani NP, et al. Evaluation of effects of concomitant medications for NASH and associated comorbidities on histological improvements with obeticholic acid. Gastroenterology 2016;150(4 Supple 1):S1144. [Google Scholar]
- Kowdley KV, Abdelmalek MF, McCullough AJ, Loomba R, Hameed B, Chalasani NP, et al. Evaluation of effects of concomitant medications for non‐alcoholic steatohepatitis and associated comorbidities on histological improvements with obeticholic acid. Journal of Hepatology 2016;1:S487‐8. [Google Scholar]
- Neuschwander‐Tetri B, Sanyal A, Loomba R, Chalasani N, Kowdley K, Abdelmalek M, et al. Obeticholic acid for NASH: Benefits in a high risk subgroup and the effects of concomitant statin use. Journal of Hepatology 2015;62:S272. [Google Scholar]
- Neuschwander‐Tetri BA, Loomba R, Sanyal AJ, Lavine JE, Natta ML, Abdelmalek MF, et al. Farnesoid x nuclear receptor ligand obeticholic acid for non‐cirrhotic, non‐alcoholic steatohepatitis (flint): a multicentre, randomised, placebo‐controlled trial. Lancet 2015;385(9972):956‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Omer 2010 {published data only}
- Omer Z, Cetinkalp S, Akyildiz M, Yilmaz F, Batur Y, Yilmaz C, et al. Efficacy of insulin‐sensitizing agents in nonalcoholic fatty liver disease. European Journal of Gastroenterology & Hepatology 2010;22(1):18‐23. [DOI] [PubMed] [Google Scholar]
Parikh 2016 {published data only}
- Parikh P. An open label randomized control study to compare the efficacy of vitamin E versus ursodeoxycholic acid in non‐diabetic Indian NAFLD patients. Clinical Gastroenterology and Hepatology 2015;13(7):E105‐E6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh P, Ingle M, Patel J, Bhate P, Pandey V, Sawant P. An open‐label randomized control study to compare the efficacy of vitamin E versus ursodeoxycholic acid in nondiabetic and noncirrhotic Indian NAFLD patients. Saudi Journal of Gastroenterology 2016;22(3):192‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh P, Sawant P, Ingle M. An open label randomized control study to compare the efficacy of vitamin E versus ursodeoxycholic acid in non diabetic Indian NAFLD patients. Hepatology International 2015;9(Suppl 1):S110‐1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Polyzos 2011 {published data only}
- Polyzos SA, Kountouras J, Zafeiriadou E, Patsiaoura K, Katsiki E, Deretzi G, et al. Effect of spironolactone and vitamin E on serum metabolic parameters and insulin resistance in patients with nonalcoholic fatty liver disease. Journal of the Renin‐Angiotensin‐Aldosterone System 2011;12(4):498‐503. [DOI] [PubMed] [Google Scholar]
Ratziu 2008 {published data only}
- Lemoine M, Serfaty L, Cervera P, Capeau J, Ratziu V. Hepatic molecular effects of rosiglitazone in human non‐alcoholic steatohepatitis suggest long‐term pro‐inflammatory damage. Hepatology Research 2014;44(12):1241‐7. [DOI] [PubMed] [Google Scholar]
- Ratziu V, Giral P, Jacqueminet S, Charlotte F, Hartemann‐Heurtier A, Serfaty L, et al. Rosiglitazone for nonalcoholic steatohepatitis: one‐year results of the randomized placebo‐controlled fatty liver improvement with rosiglitazone therapy (FLIRT) trial. Gastroenterology 2008;135(1):100‐10. [DOI] [PubMed] [Google Scholar]
Ratziu 2011 {published data only}
- Ratziu V, Ledinghen V, Oberti F, Mathurin P, Wartelle‐Bladou C, Renou C, et al. A multicentric, double‐blind, randomised‐controlled trial (RCT) of high dose ursodeoxycholic acid in patients with non‐alcoholic steatohepatitis (NASH). Journal of Hepatology 2009;50:S21. [Google Scholar]
- Ratziu V, Ledinghen V, Oberti F, Mathurin P, Wartelle‐Bladou C, Renou C, et al. A randomized controlled trial of high‐dose ursodesoxycholic acid for nonalcoholic steatohepatitis. Journal of Hepatology 2011;54(5):1011‐9. [DOI] [PubMed] [Google Scholar]
Ratziu 2014 {published data only}
- Ratziu V, Bedossa P, Francque SM, Larrey D, Aithal GP, Serfaty L, et al. Lack of efficacy of an inhibitor of pde4 in phase 1 and 2 trials of patients with nonalcoholic steatohepatitis. Clinical Gastroenterology and Hepatology 2014;12(10):1724‐U202. [DOI] [PubMed] [Google Scholar]
Ratziu 2016 {published data only}
- Ratziu V, Harrison SA, Francque S, Bedossa P, Lehert P, Serfaty L, et al. Elafibranor, an agonist of the peroxisome proliferator‐activated receptor‐alpha and ‐delta, induces resolution of nonalcoholic steatohepatitis without fibrosis worsening. Gastroenterology 2016;150(5):1147‐59. [DOI] [PubMed] [Google Scholar]
Razavizade 2013 {published data only}
- Razavizade M, Jamali R, Arj A, Matini SM, Moraveji A, Taherkhani E. The effect of pioglitazone and metformin on liver function tests, insulin resistance, and liver fat content in nonalcoholic fatty liver disease: a randomized double blinded clinical trial. Hepatitis Monthly 2013;13(5):e9270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razavizadeh M, Arj A. Comparison of the therapeutic effects of pioglitazone and metformin in nonalcoholic steatohepatitis. Journal of Gastroenterology and Hepatology 2012;27:247. [Google Scholar]
Razavizadeh 2012 {published data only}
- Razavizadeh M, Arj A. Comparison of the therapeutic effects of vitamin E and silimarin in nonalcoholic steatohepatitis. Journal of Gastroenterology and Hepatology 2012;27:270. [Google Scholar]
Safadi 2014 {published data only}
- Safadi R, Konikoff FM, Mahamid M, Zelber‐Sagi S, Halpern M, Gilat T, et al. The fatty acid‐bile acid conjugate aramchol reduces liver fat content in patients with nonalcoholic fatty liver disease. Clinical Gastroenterology and Hepatology 2014;12(12):2085‐91. [DOI] [PubMed] [Google Scholar]
Santos 2003 {published data only}
- Santos VN, Lanzoni VP, Szejnfeld J, Shigueoka D, Parise ER. A randomized double‐blind study of the short‐time treatment of obese patients with nonalcoholic fatty liver disease with ursodeoxycholic acid. Brazilian Journal of Medical and Biological Research 2003;36(6):723‐9. [DOI] [PubMed] [Google Scholar]
Sanyal 2004 {published data only}
- Sanyal AJ, Mofrad PS, Contos MJ, Sargeant C, Luketic VA, Sterling RK, et al. A pilot study of vitamin E versus vitamin E and pioglitazone for the treatment of nonalcoholic steatohepatitis. Clinical Gastroenterology and Hepatology 2004;2(12):1107‐15. [DOI] [PubMed] [Google Scholar]
Sanyal 2010 {published data only}
- Corey K, Vuppalanchi R, Wilson L, Cummings O, Chalasani NP. Nash resolution is associated with improvements in HDL and triglycerides but not in LDL or non‐HDL‐C. Hepatology (Baltimore, Md) 2014;60:224A‐5A. [Google Scholar]
- Corey KE, Vuppalanchi R, Vos M, Kohli R, Molleston JP, Wilson L, et al. Improvement in liver histology is associated with reduction in dyslipidemia in children with nonalcoholic fatty liver disease. Journal of Pediatric Gastroenterology and Nutrition 2015;60(3):360‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy CD, Suzuki A, Abdelmalek MF, Burchette JL, Diehl AM. Treatment response in the PIVENS trial is associated with decreased hedgehog pathway activity. Hepatology (Baltimore, Md.) 2015;61(1):98‐107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. New England Journal of Medicine 2010;362(18):1675‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sharma 2012 {published data only}
- Sharma BC, Kumar A, Garg V, Reddy RS, Sakhuja P, Sarin SK. A randomized controlled trial comparing efficacy of pentoxifylline and pioglitazone on metabolic factors and liver histology in patients with non‐alcoholic steatohepatitis. Journal of Clinical and Experimental Hepatology 2012;2(4):333‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shields 2009 {published data only}
- Shields WW, Thompson KE, Grice GA, Harrison SA, Coyle WJ. The effect of metformin and standard therapy versus standard therapy alone in nondiabetic patients with insulin resistance and nonalcoholic steatohepatitis (NASH): A pilot trial. Therapeutic Advances in Gastroenterology 2009;2(3):157‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shiffman 2015 {published data only}
- Shiffman M, Freilich B, Vuppalanchi R, Watt K, Burgess G, Morris M, et al. A placebo‐controlled, multicenter, double‐blind, randomised trial of emricasan in subjects with non‐alcoholic fatty liver disease (NAFLD) and raised transaminases. Journal of Hepatology 2015;62:S282. [Google Scholar]
Siddique 2015 {published data only}
- Siddique KU, Reddy H, Rana H, Singhal S, Parihar A, Pandey A. A comparative study of effect of insulin sensitizers and statins in nonalcoholic fatty liver disease (NAFLD). Diabetes 2015;64:A661. [Google Scholar]
Sofer 2011 {published data only}
- Sofer E, Boaz M, Matas Z, Mashavi M, Shargorodsky M. Treatment with insulin sensitizer metformin improves arterial properties, metabolic parameters, and liver function in patients with nonalcoholic fatty liver disease: a randomized, placebo‐controlled trial. Metabolism: Clinical and Experimental 2011;60(9):1278‐84. [DOI] [PubMed] [Google Scholar]
- Sofer E, Shargorodsky M. Effect of metformin treatment on circulating osteoprotegerin in patients with nonalcoholic fatty liver disease. Hepatology International 2016;10(1):169‐74. [DOI] [PubMed] [Google Scholar]
- Soifer E, Gavish D, Shargorodsky M. Does metformin treatment influence bone formation in patients with nonalcoholic fatty liver disease?. Hormone & Metabolic Research 2015;47(8):556‐9. [DOI] [PubMed] [Google Scholar]
Solhi 2014 {published data only}
- Solhi H, Ghahremani R, Kazemifar AM, Yazdi ZH. Silymarin in treatment of non‐alcoholic steatohepatitis: a randomized clinical trial. Caspian Journal of Internal Medicine 2014;5(1):9‐12. [PMC free article] [PubMed] [Google Scholar]
Song 2014 {published data only}
- Song XX, Jiang T, Kang K, Wen Z. Efficacy of sitagliptin combined with metformin in the initial treatment of type 2 diabetes with non‐alcoholic fatty liver. Chinese Journal of New Drugs 2014;23(2):215‐8. [Google Scholar]
Stefan 2014 {published data only}
- Stefan N, Ramsauer M, Jordan P, Nowotny B, Kantartzis K, Machann J, et al. Inhibition of 11 beta‐hsd1 with ro5093151 for non‐alcoholic fatty liver disease: a multicentre, randomised, double‐blind, placebo‐controlled trial. Lancet Diabetes & Endocrinology 2014;2(5):406‐16. [DOI] [PubMed] [Google Scholar]
Stilidi 2014 {published data only}
- Stilidi EI, Klyaritskay IL. Effect of ursodeoxycholic acid or ursodeoxycholic acid combined with losartan for treatment of non‐alcoholic steatohepatitis. Journal of Hepatology 2014;60(1 Suppl):S334. [Google Scholar]
Sunny 2015 {published data only}
- Sunny N, Bril F, Kalavalapalli S, Sanchez PP, Maximos M, Biernacki D, et al. Pioglitazone therapy improves insulin suppression of branched chain amino acids in patients with prediabetes or T2DM and NAFLD. Diabetes 2015;64:A341. [Google Scholar]
Taghvaei 2013 {published data only}
- Taghvaei T, Bahar A, Hosseini V, Maleki I, Kasrai M. Efficacy of silymarin on treatment of nonalcoholic steatohepatitis. Journal of Mazandaran University of Medical Sciences 2013;23(98):164‐71. [Google Scholar]
Torres 2011 {published data only}
- Torres D, Jones FJ, Shaw J, Williams C, Ward JA, Harrison SA. Open‐label prospective randomized 48 week clinical trial: rosiglitazone versus rosiglitazone and metformin (avandamet) versus rosiglitazone and losartan in the treatment of non‐alcoholic steatohepatitis (NASH). Journal of Hepatology 2011;54:S7‐8. [DOI] [PubMed] [Google Scholar]
- Torres DM, Jones FJ, Shaw JC, Williams CD, Ward JA, Harrison SA. Rosiglitazone versus rosiglitazone and metformin versus rosiglitazone and losartan in the treatment of nonalcoholic steatohepatitis in humans: a 12‐month randomized, prospective, open‐ label trial. Hepatology (Baltimore, Md.) 2011;54(5):1631‐9. [DOI] [PubMed] [Google Scholar]
Uygun 2004 {published data only}
- Uygun A, Kadayifci A, Isik AT, Ozgurtas T, Deveci S, Tuzun A, et al. Metformin in the treatment of patients with non‐alcoholic steatohepatitis. Alimentary Pharmacology & Therapeutics 2004;19(5):537‐44. [DOI] [PubMed] [Google Scholar]
Van Wagner 2011 {published data only}
- Wagner LB, Koppe SWP, Brunt EM, Gottstein J, Gardikiotes K, Green RM, et al. Pentoxifylline for the treatment of non‐alcoholic steatohepatitis: a randomized controlled trial. Annals of Hepatology 2011;10(3):277‐86. [PubMed] [Google Scholar]
Wang 2015 {published data only}
- Wang X, Zhao B, Cheng Y, Bu L, Qu S. Sitagliptin decreases intrahepatic lipid accumulation in diabetic patients with NAFLD. Diabetes 2015;64:A620‐1. [Google Scholar]
Yaginuma 2009 {published data only}
- Yaginuma R, Ikejima K, Kon K, Okumura K, Yamashina S, Suzuki S, et al. Efficacy of low‐dose pioglitazone for the treatment of NAFLD patients in japan. Hepatology (Baltimore, Md.) 2009;50:793A‐4A. [Google Scholar]
Yan 2015 {published data only}
- Yan H, Xia M, Chang X, Bian H, Lin H, Zhang L, et al. Berberine vs. pioglitazone for treatment of nonalcoholic fatty liver disease and its associated impaired glucose metabolism. Diabetes 2014;63:A513‐4. [Google Scholar]
- Yan HM, Xia MF, Wang Y, Chang XX, Yao XZ, Rao SX, et al. Efficacy of berberine in patients with non‐alcoholic fatty liver disease. PLoS ONE 2015;10(8):e0134172. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Abenavoli 2013 {published data only}
- Abenavoli L, Nazionale I, Greco M, Larussa T, Suraci E, Imeneo M, et al. Metabolic effects of diet vs diet and silybin combined with phosphatidylcholine and vitamin E in overweight patients with non‐alcoholic fatty liver disease. Digestive and Liver Disease 2013;45:S165‐6. [Google Scholar]
Abenavoli 2015 {published data only}
- Abenavoli L, Greco M, Nazionale I, Peta V, Milic N, Accattato F, et al. Effects of Mediterranean diet supplemented with silybin‐vitamin E‐phospholipid complex in overweight patients with non‐alcoholic fatty liver disease. Expert Review of Gastroenterology & Hepatology 2015;9(4):519‐27. [DOI] [PubMed] [Google Scholar]
Acquati 2007 {published data only}
- Acquati S, Silvani G, Bondi A, Cicognani R, Bondi G, Gagliardi L, et al. Beyond glycated haemoglobin: effectiveness of rosiglitazone in type‐2 diabetes with hypertension and non‐alcoholic fatty liver. A randomized controlled one‐year study. Atherosclerosis Supplements 2007;8(1):191. [Google Scholar]
Athyros 2011 {published data only}
- Athyros VG, Giouleme O, Ganotakis ES, Elisaf M, Tziomalos K, Vassiliadis T, et al. Safety and impact on cardiovascular events of long‐term multifactorial treatment in patients with metabolic syndrome and abnormal liver function tests: a post hoc analysis of the randomised attempt study. Archives of Medical Science 2011;7(5):796‐805. [DOI] [PMC free article] [PubMed] [Google Scholar]
Carnelutti 2012 {published data only}
- Carnelutti A, Donnini D, Nadalutti G, Luca L, Cappello D, Cugini F, et al. Effect of statin therapy vs diet in hypercholesterolemic patients affected by nonalcoholic steatohepatitis (NASH). Digestive and Liver Disease 2012;44:S25‐6. [Google Scholar]
Corey 2015a {published data only}
- Corey KE, Vuppalanchi R, Wilson LA, Cummings OW, Chalasani N, Nash CRN. Nash resolution is associated with improvements in HDL and triglyceride levels but not improvement in LDL or non‐HDL‐C levels. Alimentary Pharmacology & Therapeutics 2015;41(3):301‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dajani 2015 {published data only}
- Dajani AIM, Abu Hammour AM, Zakaria MA, Al Jaberi MR, Nounou MA, Semrin AIM. Essential phospholipids as a supportive adjunct in the management of patients with NAFLD. Arab Journal of Gastroenterology 2015;16(3‐4):99‐104. [DOI] [PubMed] [Google Scholar]
Faghihzadeh 2014 {published data only}
- Faghihzadeh F, Adibi P, Rafiei R, Hekmatdoost A. Resveratrol supplementation improves inflammatory biomarkers in patients with nonalcoholic fatty liver disease. Nutrition Research 2014;34(10):837‐43. [DOI] [PubMed] [Google Scholar]
Fan 2010 {published data only}
- Fan XF, Deng YQ, Ye L, Li YD, Chen J, Lu WW, et al. Effect of xuezhikang capsule on serum tumor necrosis factor‐alpha and interleukin‐6 in patients with nonalcoholic fatty liver disease and hyperlipidemia. Chinese Journal of Integrative Medicine 2010;16(2):119‐23. [DOI] [PubMed] [Google Scholar]
Fan 2013 {published data only}
- Fan H, Pan Q, Xu Y, Yang X. Exenatide improves type 2 diabetes concomitant with non‐alcoholic fatty liver disease. Arquivos Brasileiros de Endocrinologia e Metabologia 2013;57(9):702‐8. [DOI] [PubMed] [Google Scholar]
Gastaldelli 2015 {published data only}
- Gastaldelli A, Tripathy D, Gaggini M, Musi N, DeFronzo RA. Improvement in hepatic metabolism is associated with reduced conversion to diabetes in IGT subjects treated with pioglitazone (act now study). Diabetologia 2015;58(Suppl 1):S164. [Google Scholar]
Han 2012 {published data only}
- Han KH, Rha SW, Kang HJ, Bae JW, Choi BJ, Choi SY, et al. Evaluation of short‐term safety and efficacy of HMG‐CoA reductase inhibitors in hypercholesterolemic patients with elevated serum alanine transaminase concentrations: PITCH study (PITavastatin versus atorvastatin to evaluate the effect on patients with hypercholesterolemia and mild to moderate hepatic damage). Journal of Clinical Lipidology 2012;6(4):340‐51. [DOI] [PubMed] [Google Scholar]
Han 2014a {published data only}
- Han Y, Shi JP, Ma AL, Xu Y, Ding XD, Fan JG. Randomized, vitamin E‐controlled trial of bicyclol plus metformin in non‐alcoholic fatty liver disease patients with impaired fasting glucose. Clinical Drug Investigation 2014;34(1):1‐7. [DOI] [PubMed] [Google Scholar]
Idilman 2008 {published data only}
- Idilman R, Mizrak D, Corapcioglu D, Bektas M, Doganay B, Sayki M, et al. Clinical trial: Insulin‐sensitizing agents may reduce consequences of insulin resistance in individuals with non‐alcoholic steatohepatitis. Alimentary Pharmacology & Therapeutics 2008;28(2):200‐8. [DOI] [PubMed] [Google Scholar]
Jaafari 2012 {published data only}
- Jaafari Haidarlo A, Rashidbeygi M, Ehsanbakhsh S. Vitamin E, pioglitazone and diet therapy for patients with nonalcoholic fatty liver disease (NAFLD): evaluation of treatment. Journal of Hepatology 2012;56(Suppl 2):S507. [Google Scholar]
Kowdley 2015 {published data only}
- Kowdley KV, Wilson LA, Natta ML, Pai RK, Sanyal AJ. Efficacy and safety of vitamin E for nonalcoholic steatohepatitis: Combined analysis of three controlled trials. Journal of Hepatology 2015;62:S268. [Google Scholar]
Kowdley 2015a {published data only}
- Kowdley KV, Wilson LA, Natta ML, Pai RK, Sanyal AJ. Efficacy and safety of vitamin E in nonalcoholic steatohepatitis patients with and without diabetes: pooled analysis from the PIVENS and FLINT NIDDK NASH CRN trials. Hepatology (Baltimore, Md.) 2015;62:264A. [Google Scholar]
Li 2015 {published data only}
- Li YH, Yang LH, Sha KH, Liu TG, Zhang LG, Liu XX. Efficacy of poly‐unsaturated fatty acid therapy on patients with nonalcoholic steatohepatitis. World Journal of Gastroenterology 2015;21(22):7008‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lo 2016 {published data only}
- Lo J, Lu MT, Kim EA, Nou E, Hallett TR, Park J, et al. Statin effects to reduce hepato steatosis as measured by computed tomography in patients with human immunodeficiency virus. Open Forum Infectious Diseases 2016;3(2):ofw062. [DOI] [PMC free article] [PubMed] [Google Scholar]
McCormick 2015 {published data only}
- McCormick KG, Scorletti E, Bhatia L, Calder PC, Griffin MJ, Clough GF, et al. Impact of high dose n‐3 polyunsaturated fatty acid treatment on measures of microvascular function and vibration perception in non‐alcoholic fatty liver disease: Results from the randomised welcome trial. Diabetologia 2015;58(8):1916‐25. [DOI] [PubMed] [Google Scholar]
Merat 2015 {published data only}
- Merat S, Poustchi H, Hemming K, Jafari E, Radmard AR, Nateghi A, et al. Polypill for prevention of cardiovascular disease in an urban Iranian population with special focus on nonalcoholic steatohepatitis: a pragmatic randomized controlled trial within a cohort (polyiran ‐ liver) ‐ study protocol. Archives of Iranian Medicine 2015;18(8):515‐23. [PubMed] [Google Scholar]
Oh 2016 {published data only}
- Oh B, Choi WS, Park SB, Cho B, Yang YJ, Lee ES, et al. Efficacy and safety of ursodeoxycholic acid composite on fatigued patients with elevated liver function and/or fatty liver: a multi‐centre, randomised, double‐blinded, placebo‐controlled trial. International Journal of Clinical Practice 2016;70(4):302‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Scorletti 2014 {published data only}
- Scorletti E, Bhatia L, McCormick KG, Clough GF, Nash K, Hodson L, et al. Effects of purified eicosapentaenoic and docosahexaenoic acids in nonalcoholic fatty liver disease: results from the Welcome* study. Hepatology (Baltimore, Md.) 2014;60(4):1211‐21. [DOI] [PubMed] [Google Scholar]
Scorletti 2015 {published data only}
- Scorletti E, West AL, Bhatia L, Hoile SP, McCormick KG, Burdge GC, et al. Treating liver fat and serum triglyceride levels in NAFLD, effects of pnpla3 and tm6sf2 genotypes: results from the Welcome trial. Journal of Hepatology 2015;63(6):1476‐83. [DOI] [PubMed] [Google Scholar]
Shiasi 2014 {published data only}
- Shiasi Arani K, Taghavi Ardakani A, Moazami Goudarzi R, Talari HR, Hami K, Akbari H, et al. Effect of vitamin E and metformin on fatty liver disease in obese children‐ randomized clinical trial. Iranian Journal of Public Health 2014;43(10):1417‐23. [PMC free article] [PubMed] [Google Scholar]
Sultana 2012 {published data only}
- Sultana SS, Amin F, Rahman A, Afsana F. A comparative study with metformin and pioglitazone versus metformin alone in nonalcoholic fatty liver disease in newly detected glucose intolerant patients. Diabetologia 2012;55:S500. [Google Scholar]
Talebi 2015 {published data only}
- Talebi Pour B, Jameshorani M, Salmani R, Chiti H. The effect of chlorella vulgaris vs. Artichoke on patients with non‐alcoholic fatty liver disease (NAFLD): a randomized clinical trial. Journal of Zanjan University of Medical Sciences and Health Services 2015;23(100):36‐44. [Google Scholar]
Tan 2011 {published data only}
- Tan HH, Low ASC, Lim KH, Wan WK, Goh BB, Wang YT, et al. A randomized, unblinded pilot trial of essential phospholipids in Chinese subjects with nonalcoholic fatty liver disease (NASH). Journal of Gastroenterology and Hepatology 2011;26:162. [Google Scholar]
Taniai 2009 {published data only}
- Taniai M, Hashimoto E, Tobari M, Yatsuji S, Haruta I, Tokushige K, et al. Treatment of nonalcoholic steatohepatitis with colestimide. Hepatology Research 2009;39(7):685‐93. [DOI] [PubMed] [Google Scholar]
Tsuchiya 2011 {published data only}
- Tsuchiya M. Alogliptin decreases liver fat content in patients with IGT or type Q2 DM: comparing alogliptin and voglibose. Diabetes 2011;60:A595. [Google Scholar]
Vos 2016 {published data only}
- Vos M, Jin R, Welsh J, Konomi J, Karpen SJ, Soler‐Rodriquez D, et al. Losartan improves hepatic inflammation in children with non‐alcoholic fatty liver disease. Gastroenterology 2016;150(4):S1036. [Google Scholar]
Wang 2013 {published data only}
- Wang Q, Lao J, Zou X, Huang Y. Clinical effect of metformin combined with reduced glutathione in non‐alcoholic fatty liver disease. Journal of Gastroenterology and Hepatology 2013;28:186. [Google Scholar]
Zelber‐Sagi 2006 {published data only}
- Zelber‐Sagi S, Kessler A, Brazowsky E, Webb M, Lurie Y, Santo M, et al. A double‐blind randomized placebo‐controlled trial of orlistat for the treatment of nonalcoholic fatty liver disease. Clinical Gastroenterology & Hepatology 2006;4(5):639‐44. [DOI] [PubMed] [Google Scholar]
- Zelber‐Sagi S, Kessler A, Brazowsky E, Webb M, Lurie Y, Santo M, et al. Randomized placebo‐controlled trial of orlistat for the treatment of patients with non alcoholic fatty liver disease (NAFLD). Hepatology (Baltimore, Md) 2004;40(4 Supl 1):237a. [DOI] [PubMed] [Google Scholar]
Additional references
Abdelmalek 2007
- Abdelmalek MF, Diehl AM. Nonalcoholic fatty liver disease as a complication of insulin resistance. Medical Clinics of North America 2007;91(6):1125‐49, ix. [DOI] [PubMed] [Google Scholar]
Abenavoli 2013a
- Abenavoli L, Bellentani S. Milk thistle to treat non‐alcoholic fatty liver disease: dream or reality?. Expert Review of Gastroenterology & Hepatology 2013;7(8):677‐9. [DOI] [PubMed] [Google Scholar]
Adams 2005
- Adams LA, Lymp JF, Sauver J, Sanderson SO, Lindor KD, Feldstein A, et al. The natural history of nonalcoholic fatty liver disease: a population‐based cohort study. Gastroenterology 2005;129(1):113‐21. [DOI] [PubMed] [Google Scholar]
Adorini 2012
- Adorini L, Pruzanski M, Shapiro D. Farnesoid X receptor targeting to treat nonalcoholic steatohepatitis. Drug Discovery Today 2012;17(17‐18):988‐97. [DOI] [PubMed] [Google Scholar]
Alberti 2009
- Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009;120(16):1640‐5. [DOI] [PubMed] [Google Scholar]
Angulo 2015
- Angulo P, Kleiner DE, Dam‐Larsen S, Adams LA, Bjornsson ES, Charatcharoenwitthaya P, et al. Liver fibrosis, but no other histologic features, is associated with long‐term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology 2015;149(2):389‐97.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Anstee 2012
- Anstee QM, Day CP. S‐adenosylmethionine (SAMe) therapy in liver disease: a review of current evidence and clinical utility. Journal of Hepatology 2012;57(5):1097‐109. [DOI] [PubMed] [Google Scholar]
Ballestri 2016
- Ballestri S, Zona S, Targher G, Romagnoli D, Baldelli E, Nascimbeni F, et al. Nonalcoholic fatty liver disease is associated with an almost twofold increased risk of incident type 2 diabetes and metabolic syndrome. Evidence from a systematic review and meta‐analysis. Journal of Gastroenterology and Hepatology 2016;31(5):936‐44. [DOI] [PubMed] [Google Scholar]
Bedogni 2005
- Bedogni G, Miglioli L, Masutti F, Tiribelli C, Marchesini G, Bellentani S. Prevalence of and risk factors for nonalcoholic fatty liver disease: the Dionysos nutrition and liver study. Hepatology (Baltimore, Md.) 2005;42(1):44‐52. [DOI] [PubMed] [Google Scholar]
Bedogni 2007
- Bedogni G, Miglioli L, Masutti F, Castiglione A, Croce LS, Tiribelli C, et al. Incidence and natural course of fatty liver in the general population: the Dionysos study. Hepatology (Baltimore, Md.) 2007;46(5):1387‐91. [DOI] [PubMed] [Google Scholar]
Buzetti 2017
- Buzzetti E, Kalafateli M, Thorburn D, Davidson BR, Thiele M, Gluud LL, et al. Pharmacological interventions for alcoholic liver disease (alcohol‐related liver disease): a network meta‐analysis. Cochrane Database of Systematic Reviews 2017, Issue 3. [DOI: 10.1002/14651858.CD011646.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chaimani 2012
- Chaimani A, Salanti G. Using network meta‐analysis to evaluate the existence of small‐study effects in a network of interventions. Research Synthesis Methods 2012;3(2):161‐76. [DOI] [PubMed] [Google Scholar]
Chaimani 2013
- Chaimani A, Higgins JP, Mavridis D, Spyridonos P, Salanti G. Graphical tools for network meta‐analysis in STATA. PloS One 2013;8(10):e76654. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chalasani 2012
- Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, et al. The diagnosis and management of non‐alcoholic fatty liver disease: practice guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology 2012;55(6):2005‐23. [DOI] [PubMed] [Google Scholar]
Chan 2013
- Chan A‐W, Tetzlaff JM, Altman DG, Laupacis A, Gøtzsche PC, Krleža‐Jerić K, et al. Spirit 2013 statement: Defining standard protocol items for clinical trials. Annals of Internal Medicine 2013;158(3):200‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dassanayake 2009
- Dassanayake AS, Kasturiratne A, Rajindrajith S, Kalubowila U, Chakrawarthi S, Silva AP, et al. Prevalence and risk factors for non‐alcoholic fatty liver disease among adults in an urban Sri Lankan population. Journal of Gastroenterology and Hepatology 2009;24(7):1284‐8. [DOI] [PubMed] [Google Scholar]
Del Re 2013
- Re AC, Spielmans GI, Flückiger C, Wampold BE. Efficacy of new generation antidepressants: differences seem illusory. PLoS One 2013;8(6):e63509. [DOI] [PMC free article] [PubMed] [Google Scholar]
DeMets 1987
- DeMets DL. Methods for combining randomized clinical trials: strengths and limitations. Statistics in Medicine 1987;6(3):341‐50. [DOI] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. [DOI] [PubMed] [Google Scholar]
Dias 2010
- Dias S, Welton NJ, Caldwell DM, Ades AE. Checking consistency in mixed treatment comparison meta‐analysis. Statistics in Medicine 2010;29(7‐8):932‐44. [DOI] [PubMed] [Google Scholar]
Dias 2012a
- Dias S, Welton NJ, Sutton AJ, Ades AE. NICE DSU Technical Support Document 1: introduction to evidence synthesis for decision making, April 2011 (last updated April 2012). www.nicedsu.org.uk/TSD1%20Introduction.final.08.05.12.pdf (accessed 27 March 2014).
Dias 2012b
- Dias S, Sutton AJ, Welton NJ, Ades AE. NICE DSU Technical Support Document 3: heterogeneity: subgroups, meta‐regression, bias and bias‐adjustment, September 2011 (last updated April 2012). www.nicedsu.org.uk/TSD3%20Heterogeneity.final%20report.08.05.12.pdf (accessed 27 March 2014).
Dias 2014a
- Dias S, Welton NJ, Sutton AJ, Caldwell DM, Lu G, Ades AE. NICE DSU Technical Support Document 4: inconsistency in networks of evidence based on randomised controlled trials, May 2011 (last updated April 2014). www.nicedsu.org.uk/TSD4%20Inconsistency.final.15April2014.pdf (accessed 8 October 2014). [PubMed]
Dias 2014b
- Dias S, Welton NJ, Sutton AJ, Ades AE. NICE DSU Technical Support Document 2: a generalised linear modelling framework for pair wise and network meta‐analysis of randomised controlled trials, August 2011 (last updated April 2014). www.nicedsu.org.uk/TSD2%20General%20meta%20analysis%20corrected%2015April2014.pdf (accessed 8 October 2014).
Dongiovanni 2015
- Dongiovanni P, Petta S, Mannisto V, Mancina RM, Pipitone R, Karja V, et al. Statin use and non‐alcoholic steatohepatitis in at risk individuals. Journal of Hepatology 2015;63(3):705‐12. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ (Clinical Research Ed.) 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
EuroQol 2014
- EuroQol. About EQ‐5D, 2014. www.euroqol.org/about‐eq‐5d.html (accessed 8 October 2014).
Fleischman 2014
- Fleischman MW, Budoff M, Zeb I, Li D, Foster T. NAFLD prevalence differs among Hispanic subgroups: the multi‐ethnic study of atherosclerosis. World Journal of Gastroenterology 2014;20(17):4987‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gluud 2007
- Gluud C, Brok J, Gong Y, Koretz RL. Hepatology may have problems with putative surrogate outcome measures. Journal of hepatology 2007;46(4):734‐42. [PUBMED: 17316871] [DOI] [PubMed] [Google Scholar]
Gluud 2016
- Gluud C, Nikolova D, Klingenberg SL. Cochrane Hepato‐Biliary Group. About The Cochrane Collaboration (Cochrane Review Groups (CRGs)) 2016, Issue 10. Art. No.: LIVER.
Gurung 2013
- Gurung V, Stokes M, Middleton P, Milan SJ, Hague W, Thornton JG. Interventions for treating cholestasis in pregnancy. Cochrane Database of Systematic Reviews 2013, Issue 6. [DOI: 10.1002/14651858.CD000493.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction ‐ GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [DOI] [PubMed] [Google Scholar]
Hernaez 2011
- Hernaez R, Lazo M, Bonekamp S, Kamel I, Brancati FL, Guallar E, et al. Diagnostic accuracy and reliability of ultrasonography for the detection of fatty liver: a meta‐analysis. Hepatology (Baltimore, Md.) 2011;54(3):1082‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2012
- Higgins JPT, Jackson D, Barrett JK, Lu G, Ades AE, White IR. Consistency and inconsistency in network meta‐analysis: Concepts and models for multi‐arm studies. Research Synthesis Methods 2012;3(2):98‐110. [DOI] [PMC free article] [PubMed] [Google Scholar]
ICH‐GCP 1997
- International Conference on Harmonisation Expert Working Group. International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. ICH Harmonised Tripartite Guideline. Guideline for Good Clinical Practice CFR & ICH Guidelines. Vol. 1, Philadelphia (PA): Barnett International/PAREXEL, 1997. [Google Scholar]
Jakobsen 2014
- Jakobsen JC, Wetterslev J, Winkel P, Lange T, Gluud C. Thresholds for statistical and clinical significance in systematic reviews with meta‐analytic methods. BMC Medical Research Methodology 2014;14(1):120. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ji 2014
- Ji HF, Sun Y, Shen L. Effect of vitamin E supplementation on aminotransferase levels in patients with NAFLD, NASH, and CHC: results from a meta‐analysis. Nutrition 2014;30(9):986‐91. [DOI] [PubMed] [Google Scholar]
Kjaergard 2001
- Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta‐analyses. Annals of Internal Medicine 2001;135(11):982‐9. [DOI] [PubMed] [Google Scholar]
Koehler 2012
- Koehler EM, Schouten JN, Hansen BE, Rooij FJ, Hofman A, Stricker BH, et al. Prevalence and risk factors of non‐alcoholic fatty liver disease in the elderly: results from the Rotterdam study. Journal of Hepatology 2012;57(6):1305‐11. [DOI] [PubMed] [Google Scholar]
Lazo 2013
- Lazo M, Hernaez R, Eberhardt MS, Bonekamp S, Kamel I, Guallar E, et al. Prevalence of nonalcoholic fatty liver disease in the United States: the Third National Health and Nutrition Examination Survey, 1988‐1994. American Journal of Epidemiology 2013;178(1):38‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Li 2011
- Li W, Zheng L, Sheng C, Cheng X, Qing L, Qu S. Systematic review on the treatment of pentoxifylline in patients with non‐alcoholic fatty liver disease. Lipids in Health & Disease 2011;10:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
Li 2013
- Li Y, Liu L, Wang B, Wang J, Chen D. Metformin in non‐alcoholic fatty liver disease: a systematic review and meta‐analysis. Biomedical Reports 2013;1(1):57‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Li 2014
- Li Z, Xue J, Chen P, Chen L, Yan S, Liu L. Prevalence of nonalcoholic fatty liver disease in mainland of China: a meta‐analysis of published studies. Journal of Gastroenterology and Hepatology 2014;29(1):42‐51. [DOI] [PubMed] [Google Scholar]
Lirussi 2007
- Lirussi F, Azzalini L, Orando S, Orlando R, Angelico F. Antioxidant supplements for non‐alcoholic fatty liver disease and/or steatohepatitis. Cochrane Database of Systematic Reviews 2007, Issue 1. [DOI: 10.1002/14651858.CD004996.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lonardo 2015
- Lonardo A, Bellentani S, Argo CK, Ballestri S, Byrne CD, Caldwell SH, et al. Epidemiological modifiers of non‐alcoholic fatty liver disease: Focus on high‐risk groups. Digestive and Liver Disease 2015;47(12):997‐1006. [DOI] [PubMed] [Google Scholar]
Lu 2006
- Lu G, Ades AE. Assessing evidence inconsistency in mixed treatment comparisons. Journal of the American Statistical Association 2006;101(474):447‐59. [Google Scholar]
Lundh 2017
- Lundh A, Sismondo S, Lexchin J, Busuioc OA, Bero L. Industry sponsorship and research outcome. Cochrane Database of Systematic Reviews 2017, Issue 2. [DOI: 10.1002/14651858.MR000033.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Macaskill 2001
- Macaskill P, Walter SD, Irwig L. A comparison of methods to detect publication bias in meta‐analysis. Statistics in Medicine 2001;20(4):641‐54. [DOI] [PubMed] [Google Scholar]
Mahady 2011
- Mahady SE, Webster AC, Walker S, Sanyal A, George J. The role of thiazolidinediones in non‐alcoholic steatohepatitis ‐ a systematic review and meta analysis. Journal of Hepatology 2011;55(6):1383‐90. [DOI] [PubMed] [Google Scholar]
Mills 2012
- Mills EJ, Ioannidis JP, Thorlund K, Schünemann HJ, Puhan MA, Guyatt GH. How to use an article reporting a multiple treatment comparison meta‐analysis. JAMA 2012;308(12):1246‐53. [DOI] [PubMed] [Google Scholar]
Moher 1998
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. Lancet 1998;352(9128):609‐13. [DOI] [PubMed] [Google Scholar]
NCBI 2014
- National Center for Biotechnology Information (NCBI). Fatty liver, 2014. www.ncbi.nlm.nih.gov/mesh/68005234 (accessed 2 October 2014).
Newell 1992
- Newell DJ. Intention‐to‐treat analysis: implications for quantitative and qualitative research. International Journal of Epidemiology 1992;21(5):837‐41. [DOI] [PubMed] [Google Scholar]
Nishioji 2015
- Nishioji K, Sumida Y, Kamaguchi M, Mochizuki N, Kobayashi M, Nishimura T, et al. Prevalence of and risk factors for non‐alcoholic fatty liver disease in a non‐obese Japanese population, 2011‐2012. Journal of Gastroenterology 2015;50(1):95‐108. [DOI] [PubMed] [Google Scholar]
Ong 2008
- Ong JP, Pitts A, Younossi ZM. Increased overall mortality and liver‐related mortality in non‐alcoholic fatty liver disease. Journal of Hepatology 2008;49(4):608‐12. [DOI] [PubMed] [Google Scholar]
Onnerhag 2014
- Onnerhag K, Nilsson PM, Lindgren S. Increased risk of cirrhosis and hepatocellular cancer during long‐term follow‐up of patients with biopsy‐proven NAFLD. Scandinavian Journal of Gastroenterology 2014;49(9):1111‐8. [DOI] [PubMed] [Google Scholar]
OpenBUGS 3.2.3 [Computer program]
- Members of OpenBUGS Project Management Group. OpenBUGS. Version 3.2.3. Members of OpenBUGS Project Management Group, 2014.
Orlando 2007
- Orlando R, Azzalini L, Orando S, Lirussi F. Bile acids for non‐alcoholic fatty liver disease and/or steatohepatitis. Cochrane Database of Systematic Reviews 2007, Issue 1. [DOI: 10.1002/14651858.CD005160.pub2] [DOI] [PubMed] [Google Scholar]
Park 2006
- Park SH, Jeon WK, Kim SH, Kim HJ, Park DI, Cho YK, et al. Prevalence and risk factors of non‐alcoholic fatty liver disease among Korean adults. Journal of Gastroenterology and Hepatology 2006;21(1 Pt 1):138‐43. [DOI] [PubMed] [Google Scholar]
Paschos 2012
- Paschos P, Tziomalos K. Nonalcoholic fatty liver disease and the renin‐angiotensin system: Implications for treatment. World Journal of Hepatology 2012;4(12):327‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Puhan 2014
- Puhan MA, Schünemann HJ, Murad MH, Li T, Brignardello‐Petersen R, Singh JA, et al. A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta‐analysis. BMJ (Clinical Research Ed.) 2014;349:g5630. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Riley 2007
- Riley P, O'Donohue J, Crook M. A growing burden: the pathogenesis, investigation and management of non‐alcoholic fatty liver disease. Journal of Clinical Pathology 2007;60(12):1384‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rinella 2015
- Rinella ME. Nonalcoholic fatty liver disease: a systematic review. JAMA 2015;313(22):2263‐73. [DOI] [PubMed] [Google Scholar]
Royle 2003
- Royle P, Milne R. Literature searching for randomized controlled trials used in Cochrane reviews: rapid versus exhaustive searches. International Journal of Technology Assessment in Health Care 2003;19(4):591‐603. [DOI] [PubMed] [Google Scholar]
Saffioti 2017a
- Saffioti F, Gurusamy K, Toon CD, Tsochatzis E, Davidson BR, Thorburn D. Pharmacological interventions for primary sclerosing cholangitis: an attempted network meta‐analysis. Cochrane Database of Systematic Reviews 2017, Issue 3. [DOI: 10.1002/14651858.CD011343.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Saffioti 2017b
- Saffioti F, Gurusamy K, Eusebi LH, Tsochatzis E, Davidson BR, Thorburn D. Pharmacological interventions for primary biliary cholangitis: an attempted network meta‐analysis. Cochrane Database of Systematic Reviews 2017, Issue 3. [DOI: 10.1002/14651858.CD011648.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Salanti 2011
- Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple‐treatment meta‐analysis: an overview and tutorial. Journal of Clinical Epidemiology 2011;64(2):163‐71. [DOI] [PubMed] [Google Scholar]
Salanti 2012
- Salanti G. Indirect and mixed‐treatment comparison, network, or multiple‐treatments meta‐analysis: many names, many benefits, many concerns for the next generation evidence synthesis tool. Research Synthesis Methods 2012;3(2):80‐97. [DOI] [PubMed] [Google Scholar]
Sanyal 2016
- Sanyal AJ, Miller V. Regulatory science and drug approval for alcoholic and nonalcoholic steatohepatitis. Gastroenterology 2016;150(8):1723‐7. [PUBMED: 26924092] [DOI] [PMC free article] [PubMed] [Google Scholar]
Savović 2012a
- Savović J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized controlled trials: combined analysis of meta‐epidemiological studies. Health Technology Assessment 2012;16(35):1‐82. [DOI] [PubMed] [Google Scholar]
Savović 2012b
- Savović J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized controlled trials. Annals of Internal Medicine 2012;157(6):429‐38. [DOI] [PubMed] [Google Scholar]
Sawangjit 2016
- Sawangjit R, Chongmelaxme B, Phisalprapa P, Saokaew S, Thakkinstian A, Kowdley KV, et al. Comparative efficacy of interventions on nonalcoholic fatty liver disease (NAFLD): a prisma‐compliant systematic review and network meta‐analysis. Medicine (Baltimore) 2016;95(32):e4529. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. [DOI] [PubMed] [Google Scholar]
Schulz 2010
- Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. PLoS Medicine 2010;7(3):e1000251. [DOI] [PMC free article] [PubMed] [Google Scholar]
Severini 1993
- Severini TA. Bayesian interval estimates which are also confidence intervals. Journal of the Royal Statistical Society. Series B (Methodological) 1993;55(2):533‐40. [Google Scholar]
Shen 2014
- Shen H, Shahzad G, Jawairia M, Bostick RM, Mustacchia P. Association between aspirin use and the prevalence of nonalcoholic fatty liver disease: a cross‐sectional study from the Third National Health and Nutrition Examination Survey. Alimentary Pharmacology and Therapeutics 2014;40(9):1066‐73. [DOI] [PubMed] [Google Scholar]
Singh 2015
- Singh S, Khera R, Allen AM, Murad MH, Loomba R. Comparative effectiveness of pharmacological interventions for nonalcoholic steatohepatitis: a systematic review and network meta‐analysis. Hepatology (Baltimore, Md.) 2015;62(5):1417‐32. [DOI] [PubMed] [Google Scholar]
Soderberg 2010
- Soderberg C, Stal P, Askling J, Glaumann H, Lindberg G, Marmur J, et al. Decreased survival of subjects with elevated liver function tests during a 28‐year follow‐up. Hepatology (Baltimore, Md.) 2010;51(2):595‐602. [DOI] [PubMed] [Google Scholar]
Stata/SE 14.2 [Computer program]
- StataCorp LP. Stata/SE 14.2 for Windows[64‐bit x86‐64]. Version 14. College Station: StataCorp LP, 2017.
Thoma 2012
- Thoma C, Day CP, Trenell MI. Lifestyle interventions for the treatment of non‐alcoholic fatty liver disease in adults: a systematic review. Journal of Hepatology 2012;56(1):255‐66. [DOI] [PubMed] [Google Scholar]
Thorlund 2011
- Thorlund K, Engstrøm J, Wetterslev J, Brok J, Imberger G, Gluud C. User manual for Trial Sequential Analysis (TSA). ctu.dk/tsa/files/tsa_manual.pdf 2011 (accessed 28 November 2016).
Thorlund 2012
- Thorlund K, Mills EJ. Sample size and power considerations in network meta‐analysis. Systematic Reviews 2012;1:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
TSA 2011 [Computer program]
- Copenhagen Trial Unit, Centre for Clinical Intervention Research, Copenhagen. TSA version 0.9. Copenhagen Trial Unit, Centre for Clinical Intervention Research, Copenhagen, 2011.
Turner 2012
- Turner RM, Davey J, Clarke MJ, Thompson SG, Higgins JP. Predicting the extent of heterogeneity in meta‐analysis, using empirical data from the Cochrane Database of Systematic Reviews. International Journal of Epidemiology 2012;41(3):818‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Valkenhoef 2012
- Valkenhoef G, Lu G, Brock B, Hillege H, Ades AE, Welton NJ. Automating network meta‐analysis. Research Synthesis Methods 2012;3(4):285‐99. [DOI] [PubMed] [Google Scholar]
Ware 2014
- Ware JE. SF‐36 health survey update, 2014. www.sf‐36.org/tools/sf36.shtml (accessed 8 October 2014).
Wetterslev 2008
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta‐analysis. Journal of Clinical Epidemiology 2008;61(1):64‐75. [DOI] [PubMed] [Google Scholar]
Wetterslev 2017
- Wetterslev J, Jakobsen JC, Gluud C. Trial Sequential Analysis in systematic reviews with meta‐analysis. BMC Medical Research Methodology 2017;17(1):39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wood 2008
- Wood L, Egger M, Gluud LL, Schulz KF, Jüni P, Altman DG, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta‐epidemiological study. BMJ (Clinical Research Ed.) 2008;336(7644):601‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Xiang 2013
- Xiang Z, Chen YP, Ma KF, Ye YF, Zheng L, Yang YD, et al. The role of ursodeoxycholic acid in non‐alcoholic steatohepatitis: a systematic review. BMC Gastroenterology 2013;13:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhou 2016
- Zhou YY, Zhu GQ, Wang Y, Zheng JN, Ruan LY, Cheng Z, et al. Systematic review with network meta‐analysis: statins and risk of hepatocellular carcinoma. Oncotarget 2016;7(16):21753‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]


