Abstract
Therapies for KRas cancers remain a major clinical need. In the current issue of Cancer Cell, Sanclemente and coworkers in Mariano Barbacid’s group validate c-Raf as a prime target for these cancers. c-Raf ablation caused regression of advanced KRasG12V/Trp53 tumors, without obvious systemic toxicity and without affecting MAPK signaling.
c-Raf was shown to be downstream of Ras more than 30 years ago (Smith et al., 1986). The discovery that Raf proteins bind directly to Ras, and the discovery of the importance of the Raf/MAPK pathway in cancer cells, encouraged efforts to develop Raf kinase inhibitors to treat Ras-driven cancers. At that time (the early 1990s), no RAF mutations had been found in human cancer, but Raf’s intimate relationship with Ras made it attractive, even though kinases had not yet been widely endorsed as drug targets. Indeed, the first c-Raf inhibitor to enter clinical trials, sorafenib, failed to make an impact on the Ras-driven cancers for which it was intended. Later, vemurafenib produced dramatic responses in B-Raf-driven melanoma but also failed in Ras mutant cancers (reviewed in Karoulia et al., 2017). Most unexpectedly, these inhibitors, along with other, more potent and selective, Raf inhibitors, were shown to induce rather than inhibit Raf kinase activity, both in Ras-driven cancers and, to a lesser extent, in normal cells. While this renders them ineffective in Ras mutant cancers, they are ideally suited to target B-Raf-driven tumors, especially those driven by V600E B-Raf. In these tumors, they inhibit B-Raf kinase without provoking paradoxical activation, but, as Neil Rosen first pointed out, their ability to activate Raf kinase in normal cells provides an unprecedented therapeutic window, allowing the high doses needed for clinical efficacy.
Drug-induced paradoxical Raf activation is the result of inactivated, drug-bound Raf binding to drug-free Raf and provoking its kinase activity. Likewise, kinase-impaired B-Raf mutants that cause human cancer bind and activate wild-type Raf (Heidorn et al., 2010). Furthermore, KSR proteins, members of the Raf superfamily, were long thought to be kinase-inactive, allosteric regulators of Raf kinase activity. However, crystal structures of KSR2-MEK1 complexes revealed that KSR proteins possess kinase activity after all, but they depend on allosteric activation by B-Raf to promote MEK phosphorylation (Brennan et al., 2011). Ironically, B-Raf provides the allosteric activation function, and KSR provides the kinase activity.
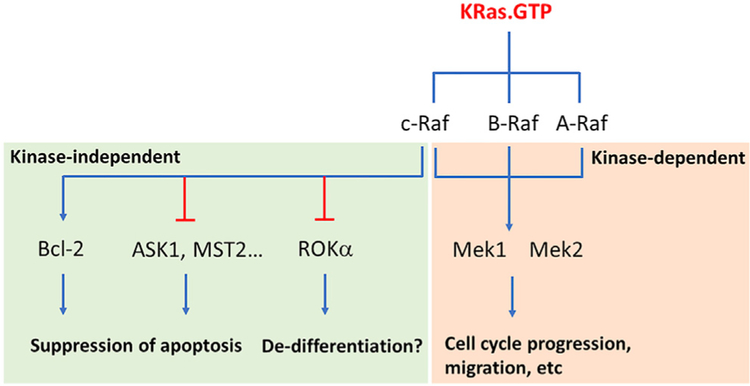
With these mechanisms in mind, it may not be surprising that lethal effects of knocking out c-Raf in mice can be rescued by a kinase-dead Raf allele (Hüser et al., 2001). These alleles could promote allosteric activation of wild-type Raf proteins and thus restore MAPK activity. However, analysis of the properties of kinase-dead Raf proteins revealed another complication of c-Raf biology: c-Raf can bind and regulate proteins involved in apoptosis through mechanisms that do not involve kinase activity or the MAPK pathway at all. These properties include interaction with Bcl-2 and with pro-apoptotic kinases ASK1 and MST2 (Matallanas et al., 2011). c-Raf ablation also activates ROKα, again through kinase-independent mechanisms (Ehrenreiter et al., 2009) (Figure 1).
Figure 1. c-Raf Activates Signaling through Kinase-Independent Pathways.
Loss of c-Raf activates apoptosis through several possible effector pathways and activates ROK1 activity, potentially affecting cell differentiation. Loss of c-Raf in vivo has little, if any, effect on MEK activity, either because of homeostatic feedback mechanisms that compensate following loss of Raf kinase or because c-Raf is a minor contributor to MAPK signaling in KRas cancer cells.
These issues re-surface in the new work from Sanclemente and colleagues in this issue of Cancer Cell (Sanclemente et al., 2018). They analyzed established KRas/p53 mutant tumors from mice in which c-Raf had been ablated. They focused on c-Raf because previous studies from their own group, and from David Tuveson’s group, implicated c-Raf as the most important Raf isoform in KRas tumor initiation (Blasco et al., 2011; Karreth et al., 2011). In this study, c-Raf ablation was performed in established tumors to phenocopy effects of drug treatment in a clinical setting. Most tumors in which c-Raf ablated showed striking tumor regression. Ablation of B-Raf had little effect on its own or in combination with c-Raf, consistent with earlier studies on tumor initiation, but it is an important result nonetheless. Tumors that regressed showed evidence of apoptosis, again consistent with previous work. The more surprising result was that tumors regressed with no reduction in MAPK signaling in normal or tumor tissue.
These discoveries raise the question of which aspects of c-Raf biology can indeed be attributed to its kinase activity. c-Raf biological activity is evident when its kinase activity is perturbed through activation or ablation. Activated c-Raf causes Noonan syndrome, for example, a disease of elevated MAPK signaling. In this situation, Raf kinase activity is most likely the driver. However, c-Raf functions inferred from depletion point toward non-kinase c-Raf functions, because depletion does not affect MAPK signaling, as shown in the current study, and because kinase-defective mutants of c-Raf are able to rescue the defects, as shown previously. Each of these interpretations has caveats: sustained MAPK output could be the result of feedback or homeostasis following ablation, and kinase-defective mutants could affect Raf kinase signaling though allosteric interactions with wild-type Raf proteins or could retain cryptic kinase activity, like KSR proteins, in addition to their non-kinase functions.
Although these issues need to be further clarified, the major rationale for the study of Sanclemente and colleagues (Sanclemente et al., 2018) was to evaluate c-Raf as a drug target, and this is the main importance of the paper. They conclude that c-Raf is an excellent target for KRas mutant cancers but that blocking kinase activity is unlikely to be effective. This is at odds with ongoing efforts to develop compounds that block c-Raf without invoking paradoxical activation. The authors suggest that strategies aimed at degrading c-Raf would be more effective. Although this may be true for most targets, the rationale for c-Raf is more compelling. This is an exciting prospect, although drugs that promote degradation through subversion of E3 ligases, for example, are still at very early stages of development. Likewise, ablation of c-Raf by small interfering RNA sounds attractive, but this approach still faces technical challenges. Another strategy might involve selective targeting of pathways that c-Raf regulates through non-kinase mechanisms. Unfortunately, these are pathways that c-Raf appears to suppress rather than activate, which obviously complicates this approach considerably and they may only be activated as a stress response to c-Raf ablation. Furthermore, activation of ROKα, ASK1, and MST2 may be hard to phenocopy with small molecules. In the effort to harness c-Raf biology to target KRas mutant cancers, the goal posts seemed to have moved again.
REFERENCES
- Blasco RB, Francoz S, Santamaría D, Caña-mero M, Dubus P, Charron J, Baccarini M, and Barbacid M (2011). c-Raf, but not B-Raf, is essential for development of K-Ras oncogene-driven non-small cell lung carcinoma. Cancer Cell 19, 652–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan DF, Dar AC, Hertz NT, Chao WC, Burlingame AL, Shokat KM, and Barford D (2011). A Raf-induced allosteric transition of KSR stimulates phosphorylation of MEK. Nature 472, 366–369. [DOI] [PubMed] [Google Scholar]
- Ehrenreiter K, Kern F, Velamoor V, Meissl K, Galabova-Kovacs G, Sibilia M, and Baccarini M (2009). Raf-1 addiction in Ras-induced skin carcinogenesis. Cancer Cell 16, 149–160. [DOI] [PubMed] [Google Scholar]
- Heidorn SJ, Milagre C, Whittaker S, Nourry A, Niculescu-Duvas I, Dhomen N, Hussain J, Reis-Filho JS, Springer CJ, Pritchard C, and Marais R (2010). Kinase-dead BRAF and oncogenic RAS cooperate to drive tumor progression through CRAF. Cell 140, 209–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hüser M, Luckett J, Chiloeches A, Mercer K, Iwobi M, Giblett S, Sun XM, Brown J, Marais R, and Pritchard C (2001). MEK kinase activity is not necessary for Raf-1 function. EMBO J 20, 1940–1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karoulia Z, Gavathiotis E, and Poulikakos PI (2017). New perspectives for targeting RAF kinase in human cancer. Nat. Rev. Cancer 17, 676–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karreth FA, Frese KK, DeNicola GM, Baccarini M, and Tuveson DA (2011). C-Raf is required for the initiation of lung cancer by K-Ras(G12D). Cancer Discov 1, 128–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matallanas D, Birtwistle M, Romano D, Zebisch A, Rauch J, von Kriegsheim A, and Kolch W (2011). Raf family kinases: old dogs have learned new tricks. Genes Cancer 2, 232–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanclemente M, Francoz S, Estaban-Burgos L, Bousquet-Mur E, Djurec M, Lopez-Casas PP, Hidalgo M, Guerra M, Musteanu M, and Barbacid M (2018). c-Raf ablation induces regression of advanced KRas/Trp53 mutant lung adenocarcinomas by a mechanism independent of MAPK signaling. Cancer Cell 33, this issue, 217–228. [DOI] [PubMed] [Google Scholar]
- Smith MR, DeGudicibus SJ, and Stacey DW (1986). Requirement for c-ras proteins during viral oncogene transformation. Nature 320, 540–543. [DOI] [PMC free article] [PubMed] [Google Scholar]