Abstract
Introduction:
Talactoferrin Alfa (TLF) is a unique recombinant form of human lactoferrin. The hypothesized mechanism of action involves TLF binding to the intestinal endothelium, which induces dendritic cell maturation and cytokine release leading to infiltration of tumor with monocytes and T-lymphocytes and inhibition of tumor growth. Based on promising phase II trial results, this correlative study was undertaken to further examine immune mechanism of action of TLF in metastatic NSCLC patients.
Methods:
Talactoferrin was administered orally at 1.5 gm bid weeks 1–12 with 2 weeks off on a 14-week cycle. Enrolled patients had a pathologic diagnosis of NSCLC and were previously treated with at least 2 lines of systemic treatment. Patients had a CT guided core biopsy of tumor before initiation of talactoferrin and at week 7 on TLF. Flow cytometry was performed and quantitative immunohistochemistry for immune correlates was performed on the biopsied specimens.
Results:
Four patients with metastatic NSCLC were enrolled. The trial was halted prematurely in light of the negative phase III trial results with the compound as a single agent in NSCLC. For the 2 patients who had repeat on-treatment tumor biopsies, a consistent increase in monocytes as a percentage of total immune cells was observed. Otherwise, no clear trend of increase or decrease was observed in any other immune cell parameters compared to matched patient pre-treatment biopsies.
Conclusion:
Repeat biopsies for immune correlates by flow cytometry and quantitative immunohistochemistry in NSCLC patients are feasible. In the few patients sampled before trial closure, increased monocytes as a total percentage of the immune cell population within tumor was observed in response to TLF.
Introduction:
Lung cancer is the leading cause of cancer deaths worldwide and over 220,000 new diagnoses of lung cancer are estimated in 2013 in the United States alone1. When metastatic NSCLC patients relapse or are refractory to platinum based chemotherapy, the prognosis is often poor with survival often on the order of months, with limited 2nd and 3rd line treatment options. 2–4. Several recent immunotherapeutics including anti-CTLA and anti-PD1 antibodies have had promising clinical trial results, perhaps harkening to new treatment options so desperately needed for this group of patients5, 6.
Lactoferrin is an important endogenous immunomodulatory protein with anti-infective and anticancer activity in animal models1. Talactoferrin Alfa (TLF) is a unique recombinant form of human lactoferrin structurally identical to native human lactoferrin, except in its glycosylation2 and is not systemically absorbed. The hypothesized mechanism of action involves TLF binding to the intestinal endothelium, which induces dendritic cell maturation and cytokine release leading to infiltration of monocytes and T-lymphocytes into the tumor microenvironment and inhibition of tumor growth3.
In preclinical studies, following oral administration, TLF is transported into the small intestinal Peyer’s patches, where it theoretically recruits circulating immature dendritic cells bearing tumor antigens to the GALT and induces their maturation7. This induces a strong systemic innate and adaptive immune response mediated by anti-cancer Natural Killer (NK) cells, CD8+ lymphocytes and NK-T cells, activation of tumor-draining lymph nodes, cellular infiltration of distant tumors and tumor-cell death8. Oral TLF has been shown to inhibit the growth of implanted tumors at distant sites and potentiates the anti-tumor activity of conventional chemotherapy in mice9. TLF administered to immunocompetent mice implanted with HNSCC cells in the floor of the mouth resulted in tumor growth inhibition that was T-cell dependent with tumor specimens infiltrated with increased CD4+ and CD8+ T-cells 10. Since TLF is not systemically absorbed, little is known about its mechanism of action in humans--in particular changes in immune cell populations within the tumor microenvironment that occur in response to TLF.
A phase II trial of TLF in relapsed/refractory NSCLC showed a 2.4-month improvement in overall survival compared to placebo4. Another phase II clinical trial that combined talactoferrin with carboplatin and paclitaxel in frontline treatment of metastatic NSCLC showed a significant increase in response rate11 compared to carboplatin and paclitaxel alone. In the setting of these two positive randomized phase II trials, we initiated this correlative study to further examine immune mechanism of action of TLF in metastatic NSCLC patients. These promising phase II trial results also prompted two randomized, phase III trials including a trial of single agent talactoferrin versus placebo in relapsed/refractory NSCLC patients and a trial of carboplatin/paclitaxel/talactoferrin versus carboplatin/paclitaxel alone as frontline therapy12. Both trials were unfortunately negative for overall survival and also for progression free survival as well as all pertinent subset analyses. These negative results prompted us to stop enrollment on this correlative study early. Despite the negative results of these phase III trials, it is important to examine whether this non-systemically absorbed immunotherapy had any on-target immune effects in the tumor microenvironment of patients we enrolled on trial.
Materials and Methods:
Enrollment:
Patients with biopsy proven NSCLC with metastatic disease by AJCC v7.0 criteria who had progressive disease through at least 2 lines of treatment were enrolled at Stanford University School of Medicine (SUMC) on a protocol approved by the SUMC Institutional Review Board. All patients were administered TLF 1.5 gm in 15 mL phosphate buffer twice a day for 12 weeks on with 2 weeks off TLF per 14-week cycle. In this single-arm phase Ib correlative study, adverse events were graded by CTCAE v4.0 criteria and response was measured by RECIST 1.1 guidelines.(REF) Imaging to assess progression was obtained every 8 weeks on trial. Patients were removed from trial upon disease progression, intolerable toxicity or withdrawal of consent.
Patients were consented for core biopsy before starting treatment with talactoferrin and at week-7 on treatment. A Stanford pathologist confirmed presence of tumor in the biopsied specimen. Tumor was assayed for changes in pertinent immune cell subsets in response to TLF by flow cytometry and quantitative immunohistochemistry.
Tissue processing and Flow Cytometry:
Fresh core biopsies of tumor/non-tumor tissue were transported on ice in M199 medium. The tissue cores were briefly rinsed in cold PBS in petri dishes. Some of the cores were placed in 10% formalin overnight at room temperature for paraffin embedding and subsequent immunohistochemistry. The remaining cores were mechanically dissociated into a single cell suspension by mashing on a 70um cell strainer. Cells were pelleted by centrifugation at 1200rpm and washed with 1% BSA in PBS prior to staining for flow cytometry analysis.
Cells from the core biopsies were resuspended in 1% BSA in PBS (FACS buffer). After incubation with Fc blocking antibody for 10 min at 4 degree Celsius (1:70), cells were stained with the appropriate fluorescently conjugated antibodies and Live/Dead Blue (life technologies). They were then washed with FACS buffer and immediately analyzed. The antibodies used were EpCAM (Biolegend, San Diego, CA, USA) for epithelial cells, and CD45 (BD Pharmingen, San Diego, CA, USA) for immune cells, CD3 (Biolegend, San Diego, CA, USA), CD4 (Life technologies, Carlsbad, CA, USA) and CD8 (BD Biosciences, San Jose, CA, USA) for T cells, CD14 (eBiosciences, San Diego, CA, USA) for monocytes, CD56 (BD Biosciences, San Jose, CA, USA) and CD16 (BD Biosciences, San Jose, CA, USA) for NK cells, CD3 CD56 and CD16 for NK-T cells, CD19 (BD Pharmingen, San Diego, CA, USA) for B cells, CD11c (BD Biosciences, San Jose, CA, USA) and HLA-DR (BD Horizon, San Jose, CA, USA) for myeloid dendritic cells and HLA-DR and CD304 (Miltenyi Biotec, Bergisch Gladbach, Germany) for plasmacytoid dendritic cells. Please refer to Supplementary Figure 1 for the gating strategy used for flow cytometry (demonstrated on healthy peripheral blood mononuclear cells).
Quantitative Immunohistochemistry:
Multiplexed IHC was performed on paraffin embedded tissue using CD4 (Biocare, Concord, CA, USA), Foxp3 (Abcam, Cambridge, MA, USA), CD8 (Biocare), and CD56 (Epitomics, Burlingame, CA, USA) primary antibodies as antigen targets and IgG AP or IgG HRP as secondary antibodies. Antigen-antibody reactions were revealed with DAB (Biocare), Perma Blue (Diagnostics Biosystems, Pleasanton, CA, USA), Vulcan Fast Red (Biocare), or Vina Green (Biocare) substrates.
Slides were batch scanned using an automated Vectra™ Imaging System (CalperLS/Perkin Elmer, Hopkinton, MA, USA). Analysis algorithms were created and used in Nuance™ and Inform™ quantitative analysis software (CalperLS/Perkin Elmer, Hopkinton, MA, USA) to enumerate target cell populations. Over two thousand 200x HPF images were generated and analyzed.
Results:
The trial was closed before completion of expected accrual after the results of the Phase III FORTIS-M trial showed no overall survival (OS) or progression-free survival (PFS) benefit of TLF compared with placebo in relapsed/refractory, metastatic NSCLC12. Four NSCLC patients were enrolled at the time the trial was closed to accrual (Table 1). One patient had a partial response and one patient had prolonged stable disease on TLF.
Table 1:
Summary of Patients
| Patient | Sex | Age | NSCLC Histology | Prior Lines of Treatment | Best Response to TLF | PFS (weeks) |
|---|---|---|---|---|---|---|
| 1001 | F | 50 | Adenocarcinoma | 5 | PR | 34 |
| 1002 | M | 52 | Adenosquamous | 2 | SD | 24 |
| 1003 | M | 62 | Adenocarcinoma | 3 | PD | 7 |
| 1004 | F | 75 | Adenocarcinoma | 2 | PD | 7 |
All patients had CT-guided core biopsy of tumor and 50% of patients (2/4) had on treatment repeat biopsies at week-7. One patient (1001) also had biopsy of adjacent liver tissue without tumor. One patient experienced progression of disease before the biopsy and was taken off trial. Another patient declined repeat biopsy after being informed of the negative results of the phase IIII trial.
Of the two patients who had pre-treatment and on-treatment biopsies, we were able to detect and compare immune cell populations by quantitative IHC and flow cytometry before treatment and on talactoferrin (Figures 1–3). In the two patients with repeat samples before trial closure, increased monocytes as a total percentage of the immune cell population by flow cytometry within tumor was observed in response to TLF–a 1.7 fold increase in patient 1001 and a 5 fold increase in patient 1002 (Figure 3). No consistent changes either by flow cytometry or qIHC in CD4 or CD8 T-cells, NK cells or NK-T cells were observed. Myeloid and plasmacytoid dendritic cells were undetectable owing to the low numbers of immune cells in the core biopsies.
Figure 1:
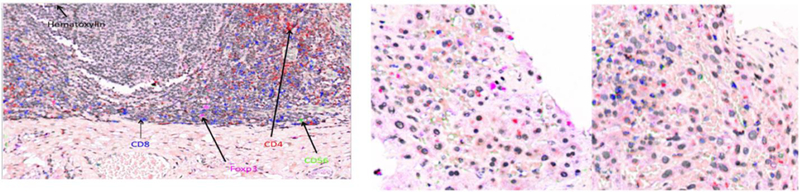
Quantitative Immunohistochemistry of Liver Metastasis for Patient 1001 before TLF and Week 7 on Treatment (Right-Center, Right). Tonsillar Epithelium Positive Control (Left). CD8+ T-cells blue. CD4+ T-Cells Red. FoxP3+ Treg-Cells Magenta. CD56+ Cells Green.
Figure 3:
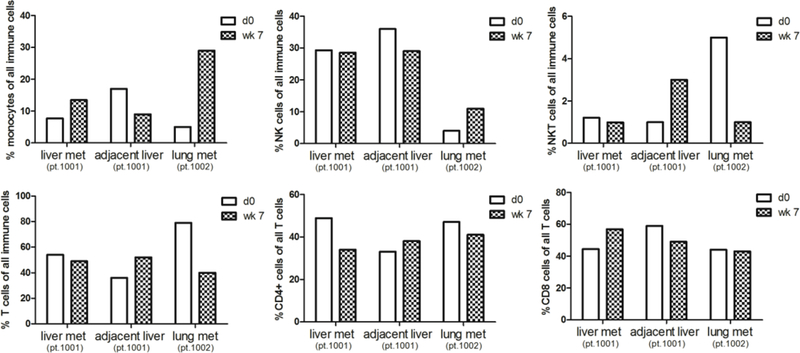
Flow Cytometry of Immune Cell Subsets Pretreatment and Week 7 on TLF. Core biopsies were obtained from patients 1001 and 1002 pre-treatment and week 7 on talactoferrin. The biopsy was taken either from a tumor-bearing site (1001-T and 1002-T) or a control biopsy was taken from an adjacent non-tumor containing site (1001-NT). Represented are percentages of different immune cell subsets detected by flow cytometry out of total immune cells or total T cells (for CD4+ and CD8+ T cell subsets). CD14 used as monocytes. CD56 and CD16 used for NK cells and CD56, CD16 and CD3 used for NK/T cells.
Discussion:
We were able to detect immune cell subsets in core biopsy NSCLC specimens by both flow cytometry and quantitative IHC in patients before and on treatment with talactoferrin. In both patients biopsied there was an increase in monocytes as a total percentage of immune cells by flow cytometry, which may reflect increased myeloid dendritic cells consistent with the proposed mechanism of action of TLF. However, given the small sample size, this evidence should be considered anecdotal.
With our small sample size, it is unclear whether talactoferrin, which is not systemically absorbed, is having any on-target immune effect in the tumor microenvironment. This study does highlight that core biopsy to assess immune correlates by quantitative IHC and flow cytometry is feasible. Though clinical trials with talactoferrin in NSCLC do not appear to improve patient outcomes, other clinical trials in NSCLC employing immunotherapy hold great promise. Biopsy for immune correlates may elucidate eventual mechanisms of resistance to treatment or biomarkers of clinical benefit, similar to the approach taken for examining targeted therapeutics of oncogenic drivers in NSCLC.
Trials employing repeat biopsy in NSCLC are becoming increasingly common—particularly in detecting oncogenic driver mutations and mechanisms of resistance to targeted therapy. Here we show the feasibility of repeat biopsy for immunohistochemistry and flow cytometry for immune correlates before and during treatment with immunotherapy, which often requires more tissue to obtain adequate numbers of immune cells by flow cytometry and other immune cell detection methods. As with analysis of oncogenic driver mutations, repeat biopsy will likely become increasingly important to study mechanisms of resistance and also be important in determining true disease progression from pseudoprogression in light of emerging clinical trial data with initial apparent disease progression by standard RECIST criteria with certain immunotherapies (followed by eventual clinical benefit) caused by infiltration of immune cells rather than tumor growth6.
One patient had a partial response (35% shrinkage of target lesions) that was unexpected, since the response rate of talactoferrin alone in NSCLC in the phase II trial of talactoferrin alone was 4%15. This patient had a relatively low burden of disease and received stereotactic radiotherapy to liver lesions prior to starting talactoferrin. It is possible that the continued tumor shrinkage resulted from continued anti-tumor effect or post-radiation changes after stereotactic radiotherapy. Abscopal effect where local radiotherapy is associated with regression of cancer at other non-radiated sites has been described in a patient with metastatic melanoma treated with radiotherapy and ipilumumab16, but we have no clear evidence of on-target effects of talactoferrin, which is not systemically absorbed when taken orally.
This correlative study was begun after the randomized phase III clinical trial comparing TLF to placebo in relapsed/refractory patients completed enrollment. The negative results of overall survival, progression free survival and all pertinent subset analysis in this phase III trial despite promising phase II trial results highlight the ideal use of immune correlative studies in conjunction with early phase drug development to help elucidate mechanism of action and on-target effects of therapy early on in drug development and enhance the co-development of correlative biomarkers.
Supplementary Material
Figure 2:
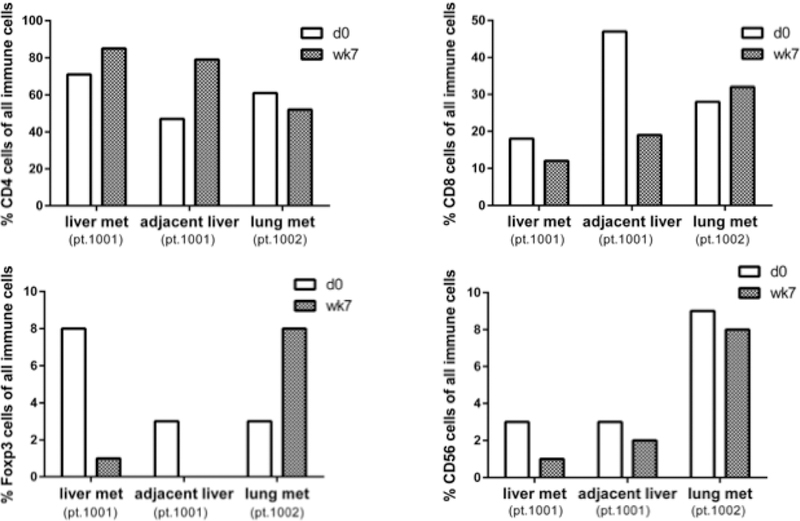
Quantitative Immunohistochemistry Results of Repeat Biopsies Before and on TLF of Pertinent Immune Cell Populations of pt. 1001 Liver metastasis and benign adjacent liver tissue and pt. 1002 lung tumor.
References:
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin January 2013;63(1):11–30. [DOI] [PubMed] [Google Scholar]
- 2.Hanna N, Shepherd FA, Fossella FV, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol May 1 2004;22(9):1589–1597. [DOI] [PubMed] [Google Scholar]
- 3.Shepherd FA, Dancey J, Ramlau R, et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol May 2000;18(10):2095–2103. [DOI] [PubMed] [Google Scholar]
- 4.Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med July 14 2005;353(2):123–132. [DOI] [PubMed] [Google Scholar]
- 5.Lynch INB TJ, Luft A, Serwatowski P, Barlesi F, Chacko RT, Sebastian M, Siegel J, Cuillerot J, Reck M. Phase II trial of ipilimumab (IPI) and paclitaxel/carboplatin (P/C) in first-line stage IIIb/IV non-small cell lung cancer (NSCLC). J Clin Oncol 2010;28(15s):suppl; abstr 7531. [Google Scholar]
- 6.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med June 28 2012;366(26):2443–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang WP, Iigo M, Sato J, Sekine K, Adachi I, Tsuda H. Activation of intestinal mucosal immunity in tumor-bearing mice by lactoferrin. Jpn J Cancer Res October 2000;91(10):1022–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kelly RJ, Giaccone G. The role of talactoferrin alpha in the treatment of non-small cell lung cancer. Expert Opin Biol Ther September 2010;10(9):1379–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Varadhachary A, Wolf JS, Petrak K, et al. Oral lactoferrin inhibits growth of established tumors and potentiates conventional chemotherapy. Int J Cancer September 1 2004;111(3):398–403. [DOI] [PubMed] [Google Scholar]
- 10.Wolf JS, Li G, Varadhachary A, et al. Oral lactoferrin results in T cell-dependent tumor inhibition of head and neck squamous cell carcinoma in vivo. Clin Cancer Res March 1 2007;13(5):1601–1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Digumarti R, Wang Y, Raman G, et al. A Randomized, Double-Blind, Placebo-Controlled, Phase II Study of Oral Talactoferrin in Combination with Carboplatin and Paclitaxel in Previously Untreated Locally Advanced or Metastatic Non-small Cell Lung Cancer. J Thorac Oncol June 2011;6(6):1098–1103. [DOI] [PubMed] [Google Scholar]
- 12.Ramalingam JC SS, Chang A, Manegold C, Perez-Soler R, Douillard J-Y, Thatcher N, Barlesi F, Owonikoko TK, Wang Y, Pultar P, Zhu J, Malik R, Giaccone G. FORTIS-M, A Randomized, Double-blind, Placebo-controlled Phase 3 Study of Oral Talactoferrin alfa with Best Supportive Care in Patients with Advanced Non-Small Cell Lung Cancer following Two or More Prior Regimens. ESMO 2012:Abstract 2347.
- 13.Anderson KM, Alrefai WA, Bonomi PA, Anderson CA, Dudeja P, Harris JE. A genomic response of H-358 bronchiolar carcinoma cells to MK 886, an inhibitor of 5-lipoxygenase, assessed with a cDNA array. Anticancer Res Jul-Aug 2000;20(4):2433–2439. [PubMed] [Google Scholar]
- 14.Kim ES, Herbst RS, Wistuba I, et al. The BATTLE trial: personalizing therapy for lung cancer. Cancer discovery June 2011;1(1):44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parikh PM, Vaid A, Advani SH, et al. Randomized, Double-Blind, Placebo-Controlled Phase II Study of Single-Agent Oral Talactoferrin n Patients With Locally Advanced or Metastatic Non-Small-Cell Lung Cancer That Progressed After Chemotherapy. J Clin Oncol November 1 2011;29(31):4129–4136. [DOI] [PubMed] [Google Scholar]
- 16.Postow MA, Callahan MK, Barker CA, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med March 8 2012;366(10):925–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


