Abstract
Background
Indacaterol is an inhaled long‐acting beta2‐agonist that is administered once daily and has been investigated as a treatment for chronic obstructive pulmonary disease (COPD). Four different doses have been investigated (75 mcg, 150 mcg, 300 mcg and 600 mcg). The relative effects of different doses of once‐daily indacaterol in the management of patients with COPD are uncertain.
Objectives
To compare the efficacy and safety of indacaterol versus placebo and alternative twice‐daily long‐acting beta2‐agonists for the treatment of patients with stable COPD.
Search methods
We identified trials from the Cochrane Airways Group Specialised Register of trials (CAGR), handsearched respiratory journals and meeting abstracts and searched the Novartis trials registry and ClinicalTrials.gov. The date of the most recent search was 8 November 2014.
Selection criteria
We included all randomised controlled trials comparing indacaterol at any dose versus placebo or alternative long‐acting beta2‐agonists. Trials were required to be of at least 12 weeks' duration and had to include adults older than 18 years with a confirmed spirometric diagnosis of COPD.
Data collection and analysis
Two review authors (JBG, EJD) independently assessed for possible inclusion all citations identified as a result of the search. Disagreements were resolved through discussion or, if required, through resolution by a third review author (RWB). One review author (JBG) extracted data from trials identified by the search and entered these data into Review Manager 5.1 for statistical analysis. Data entry was cross‐checked by a second review author (EJD, CJC).
Main results
A total of 13 trials with 9961 participants were included in the review. Ten trials with a total of 8562 participants involved an indacaterol versus placebo comparison. Five trials with a total of 4133 participants involved an indacaterol versus twice‐daily beta2‐agonist comparison. The comparator beta2‐agonists were salmeterol, formoterol and eformoterol. One of these trials, with a total of 90 participants, provided no data that could be used in this review. Two trials included both indacaterol versus placebo and indacaterol versus twice‐daily beta2‐agonist comparisons. Trials were between 12 weeks and 52 weeks in duration. Overall the quality of the evidence was strong, and risk of significant bias was minimal in most of the included studies. Enrolled participants had stable COPD across a range of spirometric severities. Forced expiratory volume in 1 second (FEV1) was generally between 30% and 80% predicted, and a mean FEV1 of approximately 50% was predicted in most studies. Patients with concurrent respiratory disease, including asthma, were excluded. Concomitant use of inhaled corticosteroids was permitted.
The primary objectives were to compare trough FEV1 at the end of dosing, exacerbation rates and quality of life. Significant adverse events, mortality and dyspnoea were included as secondary outcomes. Compared with placebo, a significant and clinically relevant improvement in trough FEV1 was noted with indacaterol (mean difference (MD) 149.11, 95% confidence interval (CI) 137.09 to 161.12). In addition, compared with placebo, a significant improvement in mean St George Respiratory Questionaire (SGRQ) score (MD ‐3.60, 95% CI ‐4.36 to ‐2.83) was reported, and the proportion of participants experiencing clinically relevant improvement in SGRQ score was significantly greater (odds ratio (OR) 1.64, 95% CI 1.46 to 1.845. Compared with twice‐daily beta2‐agonists, a small but statistically significant increase in trough FEV1 was seen with indacaterol (MD 61.71 mL, 95% CI 41.24 to 82.17). Differences between indacaterol and twice‐daily beta2‐agonists in mean SGRQ scores (MD ‐0.81, 95% CI ‐2.28 to 0.66) and in the proportions of participants achieving clinically relevant improvements in SGRQ scores (OR 1.07, 95% CI 0.87 to 1.32) were not statistically significant, but the confidence intervals are too wide to permit the conclusion that the treatments were equivalent.
Authors' conclusions
For patients with stable COPD, use of indacaterol versus placebo results in statistically significant and clinically meaningful improvements in lung function and quality of life. The clinical benefit for lung function is at least as good as that seen with twice‐daily long‐acting beta2‐agonists, but the comparative effect on quality of life remains uncertain, as important differences cannot be excluded.
Plain language summary
Indacaterol for the treatment of people with stable COPD
Review question
1. What is the effect of treatment with indacaterol versus no treatment on stable COPD?
2. What is the effect of treatment with indacaterol versus twice‐daily beta2‐agonists on stable COPD?
Background
Chronic obstructive pulmonary disease (COPD) is a progressive lung disease that causes shortness of breath and impairs quality of life. In addition, sudden worsening of symptoms (acute exacerbations) may require additional treatment or hospitalisation and may result in further impairment in quality of life.
Several different medicines can be used to treat patients with COPD; inhaled long‐acting beta2‐agonists are one example. Until recently, inhaled long‐acting beta2‐agonists required twice‐daily dosing. Indacaterol is an inhaled beta2‐agonist that requires once‐daily dosing.
We aimed to assess the following.
1. The effect of indacaterol in the treatment of participants with stable COPD.
2. How indacaterol compares with available alternative twice‐daily long‐acting beta2‐agonists.
Study characteristics
13 trials with a total of 9961 participants were included in this review. Ten trials with a total of 8562 participants involved an indacaterol versus placebo comparison. Five trials with a total of 4133 participants involved an indacaterol versus twice‐daily beta2‐agonist comparison. Two trials included both indacaterol versus placebo and indacaterol versus twice‐daily beta2‐agonist comparisons. Trials were between 12 and 52 weeks duration and compared doses between 75 mcg and 600 mcg. In most trials, mean forced expiratory volume in 1 second (FEV1) was approximately 50% predicted.
Key results
1. Indacaterol is an effective medication for the treatment of patients with stable COPD. It results in improved lung function and quality of life.
2. Indacaterol led to improvements in lung function that were clinically similar to those seen with twice‐daily long‐acting beta2‐agonists.
3. No measurable difference was noted between indacaterol and twice‐daily long‐acting beta2‐agonists with respect to quality of life, but important differences cannot be excluded.
4. No significant difference was observed in the number of participants suffering a serious adverse event or mortality, but the confidence intervals were too wide because very few events could be used to rule out important differences.
Quality of the evidence
Overall the quality of the evidence was judged to be high.
Summary
Indacaterol is an effective treatment for patients with stable COPD; it offers benefits that are clinically similar to those of existing twice‐daily preparations within the same class of medication but provides the possible advantage of once‐daily dosing.
Summary of findings
Summary of findings for the main comparison. Indacaterol versus placebo.
| Indacaterol versus placebo | ||||||
|
Patient or population: people with COPD
Settings: community
Intervention: indacaterol Comparator: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Indacaterol | |||||
| End‐of‐study trough FEV1 mL Follow‐up: 12 to 52 weeks | Mean end‐of‐study trough FEV1 in control groups was 1170 to 1360 mL | Mean end‐of‐study trough FEV1 in the intervention groups was 149.11 mL higher (137.09 to 161.12 higher) | 5001 (10 studies) | ⊕⊕⊕⊕ High | This value is greater than the minimum clinically important difference of 100 mL (Donahue 2005) | |
| Number of participants with a clinically significant improvement in QOL SGRQ Follow‐up: 12 to 52 weeks | 425 per 1000 | 548 per 1000 (519 to 578)a | OR 1.64 (1.46 to 1.845 | 4906 (9 studies) | ⊕⊕⊕⊕ High | |
| Number of participants with clinically significant improvement in dyspnoea TDI Follow‐up: 12 to 52 weeks | 440 per 1000 | 607 per 1000 (576 to 636)a | OR 1.96 (1.73 to 2.22) | 4577 (8 studies) | ⊕⊕⊕⊕ High | |
| Number of participants experiencing 1 or more exacerbations Follow‐up: 12 to 52 weeks | 222 per 1000 | 188 per 1000 (167 to 212) | OR 0.81 (0.7 to 0.94) | 4807 (7 studies) | ⊕⊕⊕⊕ High | |
| Serious adverse events Follow‐up: 12 to 52 weeks | 72 per 1000 | 72 per 1000 (60 to 87) | OR 1.00 (0.82 to 1.23) | 6065 (9 studies) | ⊕⊕⊕⊝ Moderateb | |
| Mortality Follow‐up: 12 to 52 weeks | 4 per 1000 | 2 per 1000 (1 to 4) | OR 0.42 (0.16 to 1.08) | 5694 (9 studies) | ⊕⊕⊕⊝ Moderateb | |
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio. | ||||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
aBaseline risk calculated from raw responder numbers in placebo arm at end of treatment. Absolute benefit and 95% CIs calculated from www.nntonline.net/visualrx/. b95% CIs around the point estimate of effect include both appreciable benefit and no difference.
Summary of findings 2. Indacaterol versus twice‐daily long‐acting beta2‐agonists for chronic obstructive pulmonary disease.
| Indacaterol versus twice‐daily long‐acting beta2‐agonists for chronic obstructive pulmonary disease | ||||||
| Patient or population: patients with chronic obstructive pulmonary disease Settings: community Intervention: indacaterol Comparison: twice‐daily long‐acting beta2‐agonists | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Twice‐daily long‐acting beta2‐agonists | Indacaterol | |||||
| End‐of‐study trough FEV1 mL Follow‐up: 12 to 52 weeks | Mean end‐of‐study trough FEV1 in the control groups was 1310 to 1390 mL | Mean end‐of‐study trough FEV1 in the intervention groups was 73.76 mL higher (57.33 to 90.19 higher) | 2708 (4 studies) | ⊕⊕⊕⊕ High | This value is less than the minimum clinically important difference of 100 mLd (Donahue 2005) | |
| Number of participants with a clinically significant improvement in quality of life SGRQ Follow‐up: 26 to 52 weeks | 498 per 1000 | 515 per 1000 (464 to 567)a | OR 1.07 (0.87 to 1.32) | 1520 (2 studies) | ⊕⊕⊕⊝ Moderateb | |
| Number of participants with a clinically significant improvement in dyspnoea TDI Follow‐up: 12 to 52 weeks | 581 per 1000 | 606 per 1000 (566 to 647)a | OR 1.11 (0.94 to 1.32) | 2536 (3 studies) | ⊕⊕⊕⊝ Moderateb | |
| Number of participants experiencing at least 1 exacerbation Exacerbations Follow‐up: 26 to 52 weeks | 241 per 1000 | 254 per 1000 (215 to 297) | OR 1.07 (0.86 to 1.33) | 1869 (2 studies) | ⊕⊕⊕⊝ Moderateb | |
| Serious adverse events Adverse events Follow‐up: 12 to 52 weeks | 78 per 1000 | 80 per 1000 (63 to 101) | OR 1.02 (0.79 to 1.32) | 3266 (4 studies) | ⊕⊕⊕⊝ Moderateb | |
| Mortality Deaths Follow‐up: 12 to 52 weeks | 2 per 1000 | 2 per 1000 (1 to 7) | OR 1.00 (0.31 to 3.28) | 3266 (4 studies) | ⊕⊕⊕⊝ Moderatec | |
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio. | ||||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
aBaseline risk taken from raw responder numbers at the end of treatment. Absolute risk and 95% CIs calculated from www.nntonline.net/visualrx. b95% CIs around the point estimate of effect include both no difference and appreciable benefit. c95% CIs around the point estimate of effect include both significant benefit and significant harm.
dMinimum clinically important difference.
Background
Chronic obstructive pulmonary disease (COPD) is a leading cause of morbidity and mortality globally. Several pharmacotherapeutic interventions have demonstrated efficacy in modifying a variety of long‐term clinical outcomes associated with the disease. These include inhaled corticosteroids, inhaled long‐acting muscarinic antagonists and inhaled long‐acting beta2‐agonists. The latter class, used alone or in combination, has an established role in the treatment of COPD, particularly with respect to reducing exacerbations and improving quality of life. Until recently, although these agents have been classed as 'long‐acting,' their pharmacokinetic profile has required twice‐daily dosing. Indacaterol is a new beta2‐agonist that is administered once daily and has recently been approved by several regulatory authorities around the world for the treatment of patients with stable COPD. As it requires only once‐daily dosing, indacaterol offers possible benefits for adherence over previously available agents.
Description of the condition
Chronic obstructive pulmonary disease was the fifth leading cause of death worldwide in 2002, and is projected to become the third leading cause by 2030 (WHO 2008). It presents a considerable financial and social burden for both societies and individuals (Buist 2007; Gershon 2010). This chronic, usually progressive disease, which is characterised by airflow limitation that is not fully reversible, occurs as a consequence of exposure to noxious particles or gases (GOLD 2014). Exposure to cigarette smoke is the most important risk factor for development of the disease in high‐income countries. In low‐income countries, exposure to smoke from the burning of biomass fuels indoors has been identified as an additional important cause. Although patients may be asymptomatic in early stages of disease, its clinical course is characterised by progressive dyspnoea, often associated with chronic cough and sputum production. This course is often punctuated by 'exacerbations,' defined as acute deterioration in symptoms of dyspnoea, cough or sputum beyond day‐to‐day fluctuations in the disease. Such exacerbations have a major impact on quality of life and in developed countries account for the greatest burden on healthcare systems (GOLD 2014).
Description of the intervention
Indacaterol is an inhaled once‐daily beta2‐agonist that results in smooth muscle relaxation and bronchodilation. It has been investigated for the treatment of patients with COPD, predominantly those with moderate to severe spirometric deficits. It was approved in 2009 by the European Medicines Agency (EMA) for the treatment of patients with COPD and in 2011 by the Food and Drug Administration (FDA) in the United States.
How the intervention might work
Similar to other beta2‐agonists, indacaterol is thought to work through stimulation of beta2‐adrenergic receptors within respiratory smooth muscle, resulting in bronchodilation. This in turn improves respiratory mechanics, resulting in improved dyspnoea.
Why it is important to do this review
Chronic obstructive pulmonary disease is a common disorder that is associated with significant morbidity and mortality. Given the irreversible effects of the disease, available pharmacological options for its treatment are relatively limited. As it was recently approved across several healthcare jurisdictions, including Europe and the United States, prescription of this medication is likely to escalate in the future. Therefore it is important that potential prescribers have a keen understanding of the efficacy and safety of this drug, both in its own right and compared with other available treatments for the disease, in particular, twice‐daily long‐acting beta2‐agonists.
Objectives
To compare the efficacy and safety of indacaterol versus placebo and alternative twice‐daily long‐acting beta2‐agonists for the treatment of patients with stable COPD.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials of at least 12 weeks' duration. We did not exclude trials on the basis of blinding. Trials using additional bronchodilators that were not part of the comparison were excluded because of the possibility that they might introduce bias.
Types of participants
Adults older than 18 years with a confirmed spirometric diagnosis of COPD.
Types of interventions
Experimental intervention: once‐daily indacaterol at any dose.
-
Comparator interventions:
Placebo.
Twice‐daily long‐acting beta2‐agonists.
Types of outcome measures
Outcome measures did not form part of the eligibility criteria for inclusion of studies in this review.
Primary outcomes
Trough forced expiratory volume in one second (FEV1).
Mean difference in quality of life.
Number of participants with a clinically significant improvement in quality of life.
Secondary outcomes
Peak FEV1.
Mean difference in dyspnoea.
Number of participants experiencing a clinically significant improvement in dyspnoea.
Serious adverse events.
Mortality.
Number of participants experiencing at least one protocol‐defined exacerbation.
Search methods for identification of studies
Electronic searches
We identified trials from the Cochrane Airways Group Specialised Register of trials (CAGR), which is maintained by the Information Specialist for the group. The register is derived from systematic searches of bibliographic databases, including the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, the Cumulative Index to Nursing and Allied Health Literature (CINAHL), the Allied and Complementary Medicine Database (AMED) and PsycINFO, and handsearching of respiratory journals and meeting abstracts (please see Appendix 1 for further details). The TSC searched all records in the CAGR coded as 'COPD' using the following terms:
(indacaterol or OnBrez or Breezhaler or Arcapta or ultra‐long* or "ultra long*").
This search was carried out in August 2014. We also conducted a search of ClinicalTrials.gov (www.clinicaltrials.gov) and of the Novartis clinical trials registry (www.novctrd.com). We searched all databases from their inception to the present and imposed no restriction on language of publication.
Searching other resources
We searched reference lists of all primary studies and review articles for additional references. We contacted authors of identified trials and asked them to identify other published and unpublished studies. We also contacted manufacturers and experts in the field.
Data collection and analysis
Selection of studies
Two review authors (JBG, EJD) independently assessed for potential inclusion all citations that were identified as a result of the search. Disagreement was resolved through discussion. Abstracts and full‐text papers were assessed for inclusion, and disagreements were resolved through discussion or, if required, through resolution by a third review author (RWB).
Data extraction and management
One review author (JBG) extracted data from trials identified by the search and entered these data into Review Manager 5.1 (RevMan 2011) for statistical analysis. Data were cross‐checked by a second review author (EJD, CJC).
Assessment of risk of bias in included studies
Two review authors (JBG, EJD) independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Disagreements were resolved by discussion and through consultation with a third review author (RWB). We assessed risk of bias according to the following domains.
Allocation sequence generation.
Concealment of allocation.
Blinding of participants and investigators.
Incomplete outcome data.
Selective outcome reporting.
Each source of bias was graded as having low, high or unclear risk.
Measures of treatment effect
We analysed dichotomous data as odds ratios (ORs) using the Mantel‐Haenszel method. We analysed continuous data using mean differences (MDs).
Unit of analysis issues
Dichotomous data were analysed using participants rather than events as the unit of analysis. For repeated observations, the longest follow‐up from each study was selected. When an estimate of an effect measure was presented (rather than summary data for the intervention group) and a P value or a confidence interval (CI) was provided, the standard error (SE) was estimated as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Dealing with missing data
Investigators and study sponsors were contacted to verify key study characteristics and to provide missing numerical outcome data when possible. Data were analysed on an intention‐to‐treat basis, except in some instances where the study sponsor was required to provide outcome data where there were small numerical differences between the participants randomised and participants analysed.
Assessment of heterogeneity
We used the I2 statistic to measure heterogeneity among the trials included in each analysis. We considered I2 > 50% to be significant (see protocol), and when this was the case, potential causes of heterogeneity were explored. We postulated a priori that potential sources of heterogeneity would be due to the following.
Differences in methodological quality and risk of bias.
Differences in usage of concomitant inhaled and systemic medications.
Differences in doses of indacaterol or comparator long‐acting beta2‐agonists.
Assessment of reporting biases
We contacted study authors and manufacturers to obtain missing outcome data. We identified additional trials by searching the manufacturers' trial registers, by contacting the manufacturers directly and by searching ClinicalTrials.gov (http://clinicaltrials.gov/).
Data synthesis
We used adjusted analysis of covariance (ANCOVA) as the primary method of synthesising study results when these were available, and we combined them using the generic inverse variance method in RevMan. This method was not specified in the protocol but offers the advantage of taking into account participant characteristics (including baseline values). When such data were not available, we used raw end‐of‐study data instead. Types of outcome data used for FEV1, quality of life and dyspnoea are included in Table 3 and Table 4.
1. Data extraction for indacaterol versus placebo.
| Study | FEV1 | Mean SGRQ | SGRQ responders | Mean TDI | Mean TDI responders |
| Feldman 2010 | Adjusted | Raw | Raw | Not assessed | Not assessed |
| Dahl 2010 | Adjusted | Adjusted | Raw | Adjusted | Raw |
| Donohue 2010 | Adjusted | Adjusted | Adjusteda | Adjusted | Adjusted |
| Kornmann 2011 | Adjusted | Adjusted | Adjusted | Raw | Adjusted |
| Kinoshita 2012 | Adjusted | Adjusted | Raw | Adjusted | Raw |
| Mroz 2013 | Raw | Raw | Not assessed | Not assessed | Not assessed |
| Bateman 2013 | Adjusted | Adjusted | Raw | Adjusted | Raw |
| Yao 2014 | Adjusted | Adjusted | Raw | Adjusted | Raw |
| Kerwin 2011 Study 1 | Adjusted | Adjusted | Raw | Adjusted | Raw |
| Kerwin 2011 Study 2 | Adjusted | Adjusted | Raw | Adjusted | Raw |
aRaw data used for analysis by trial duration (12‐week data).
2. Data extraction for indacaterol versus alternative twice‐daily long‐acting beta2‐agonists.
| Study | FEV1 | Mean SGRQ | SGRQ responders | Mean TDI | Mean TDI responders |
| Dahl 2010 | Adjusted | Adjusted | Raw | Raw | Raw |
| Korn 2011 | Adjusted | Not analysed | Not analysed | Adjusted | Adjusted |
| Kornmann 2011 | Adjusted | Adjusted | Adjusted | Adjusted | Rawa |
| To 2011 | Adjusted | Not analysed | Not analysed | Not analysed | Not analysed |
a12‐Week raw data.
A 'Summary of findings' table for six key outcomes in each comparison was created using GRADEpro software, in keeping with methods described in Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Additional results are detailed in the body of this report.
Subgroup analysis and investigation of heterogeneity
We planned to carry out the following subgroup analyses.
Global Initiative on Obstructive Lung Disease (GOLD) class 2, GOLD class 3 and GOLD class 4 for both placebo and long‐acting beta2‐agonist (LABA) comparisons.
Salmeterol versus formoterol/eformoterol for LABA comparison.
Trials of between 12 and 24 weeks and trials ≥ 24 weeks.
We used only primary outcomes for these subgroup analyses. We performed subgroup analyses according to indacaterol dose on both primary and secondary outcomes as post hoc analyses.
When we identified substantial heterogeneity, we explored this by performing a sensitivity analysis; we systematically excluded studies from the overall analysis on the basis of potential sources of heterogeneity as mentioned above.
Sensitivity analysis
We investigated studies at high risk of bias by removing these studies as part of a sensitivity analysis.
Results
Description of studies
See Characteristics of included studies and Characteristics of excluded studies.
Results of the search
The search retrieved 194 references. A total of 117 records were screened after duplicates were removed. Twelve were ultimately included for quantitative analysis (Figure 1).
1.

Study flow diagram.
Included studies
A total of 13 trials were identified for inclusion. Two trials compared indacaterol versus both placebo and an alternative long‐acting beta2‐agonist.(Dahl 2010; Kornmann 2011). Ten trials with a total of 8562 participants involved a placebo comparison (Bateman 2013; Dahl 2010; Donohue 2010; Feldman 2010; Kerwin 2011 Study 1; Kerwin 2011 Study 2; Kinoshita 2012; Kornmann 2011; Mroz 2013; Yao 2014). Five trials with a total of 4133 participants involved a long‐acting beta2‐agonist comparison (Dahl 2010; Izbicki 2014; Korn 2011; Kornmann 2011; To 2011); formoterol was the long‐acting beta2‐agonist in Dahl 2010, and salmeterol was the long‐acting beta2‐agonist in Korn 2011, Kornmann 2011 and To 2011. Izbicki 2014 did not provide data that could be used in this review. One trial did not perform a direct comparison of indacaterol versus placebo or a twice‐daily beta2‐agonist but included indacaterol and placebo arms (Bateman 2013). For the 75 mcg indacaterol analysis, data were derived from two 12‐week trials with identical methodology (Kerwin 2011 Study 1; Kerwin 2011 Study 2). All studies other than Mroz 2013 were sponsored by Novartis, and at least one author of all published papers was an employee of Novartis. All trials were between 12 and 52 weeks in duration. Participants were recruited across a wide range of centres, predominantly in the United States, Canada, Europe and Asia. Inclusion criteria were similar across all trials. Participants were 40 years of age or older with confirmed COPD, as defined by GOLD criteria; had an FEV1 of between 30% and 80% predicted; and had at least a 10‐pack‐year smoking history. In all studies other than Mroz 2013, participant characteristics were well matched between intervention and control arms. In most trials, mean FEV1 was approximately 50% to 55% predicted. Yao 2014 deliberately enrolled participants with more severe disease, and mean FEV1 in this trial was approximately 35% predicted in active and control arms. In Mroz 2013, Izbicki 2014 and To 2011, the mean FEV1 was not explicitly stated. Participants were required to have been on stable doses of maintenance therapy in the six to eight weeks before study commencement. Inhaled corticosteroids were continued at fixed doses. Except when a specific comparison was performed, alternative long‐acting bronchodilators were ceased. Participants with asthma were excluded. Individuals with unstable COPD and those whose condition had recently exacerbated were also generally excluded. Outcomes assessed included a variety of spirometric outcomes, quality of life as measured by St George Respiratory Questionnaire (SGRQ), dyspnoea as measured by the Transitional Dyspnoea Index, adverse events, mortality and exacerbations. The definition of an exacerbation was not standardised across trials, and definitions of exacerbations were not universally reported. In two trials (Dahl 2010; Donohue 2010), an exacerbation was defined as the onset of worsening of one or more respiratory symptom (dyspnoea, cough, sputum purulence or volume or wheeze) for three or more consecutive days requiring an escalation in treatment (administration of systemic steroids, antibiotics or oxygen) and/or a hospital admission or emergency department visit. In two trials (Kerwin 2011 Study 1; Kerwin 2011 Study 2), the definition was worsening of two or more major symptoms (dyspnoea, sputum volume or purulence) or worsening of one major and one minor symptom (sore throat, cold, fever without other cause, increased cough or increased wheeze) for at least two consecutive days and requiring treatment with antibiotics and/or steroids. In two trials (Kinoshita 2012; Kornmann 2011), exacerbations were not included as prespecified outcomes and definitions were not available. However data were supplied upon request by study authors and by Novartis. In Feldman 2010, exacerbations were included in a global assessment of adverse events, and data were unavailable for this outcome. In most studies, mixed‐model statistical analyses were performed, with treatment, smoking status and, when relevant, country as fixed effects, and baseline FEV1 and reversibility as co‐variates. Missing data were generally imputed using last observation carried forward.
Excluded studies
Of 23 full‐text articles reviewed, 11 were excluded (see Characteristics of excluded studies). Five were excluded because study duration was less than 12 weeks (Barnes 2010; Beeh 2011; Khindri 2011; Magnussen 2010; Van de Maele 2010). Two studies were excluded because open‐label tiotropium was administered in both indacaterol and placebo arms (Mahler 2012 Study 1; Mahler 2012 Study 2). One study was a meta‐analysis of three trials already included in the review (Jones 2011). Another study was not a randomised controlled trial (Hataji 2013). One study did not compare indacaterol versus placebo or another long‐acting beta2‐agonist (Buhl 2011). Another was a 26‐week continuation study of Donohue 2010, in which participants randomly assigned in the original study were asked to consent to continuation, and this was therefore no longer a comparison of participants as randomly assigned (Chapman 2011). The final analysis for this trial included data over the entire 52‐week period encompassed by Donohue 2010 and Chapman 2011; therefore to avoid double counting of participants, the two trials could not be combined in the same meta‐analysis.
Risk of bias in included studies
Overall risk of bias was judged to be low (see Figure 2).
2.
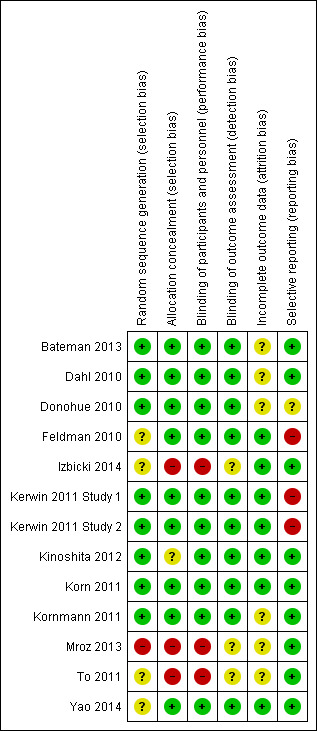
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Randomisation was generally adequate and automated systems were used in most studies. In Yao 2014, To 2011 and Feldman 2010, the method of randomisation was unclear. Mroz 2013 was judged to be at high risk of selection bias in view of baseline imbalances in this study.
Blinding
Blinding was generally sufficient to protect against significant performance and detection bias. To 2011 was an open‐label trial, and it is possible that this may have introduced bias. Blinding in Mroz 2013 was uncertain, as no clear report a placebo inhaler device was provided.
Incomplete outcome data
Outcome reporting was generally adequate, although in some studies handling and reporting of incomplete outcome data were not clear. Rates of dropout were fairly similar across experimental and control arms—generally between 10% and 20% across different studies—with a tendency toward slightly greater loss of participants from placebo arms. It seems unlikely that this has led to significant systematic bias.
Selective reporting
Risk of selective reporting bias was generally low. However in Kerwin 2011 Study 1 and Kerwin 2011 Study 2, a variety of secondary outcomes were incompletely reported and risk of reporting bias was judged to be high. In Feldman 2010 SGRQ score was not a prespecified outcome. However SGRQ scores were supplied by Novartis upon request.
Other potential sources of bias
None identified.
Effects of interventions
Indacaterol versus placebo
Trough FEV1 at the end of the dosing interval
Higher scores measured using spirometry indicate improvement in lung function, and 100 mL represents a clinically important difference in FEV1 (Donahue 2005). Ten trials contributed data on this outcome from 5001 participants. Compared with placebo, the mean trough FEV1 was significantly greater with indacaterol (MD 149.11, 95% CI 137.09 to 161.12) (Analysis 1.1). The trough FEV1 was significantly greater for indacaterol than for placebo for 75 mcg (MD 130.00 mL, 95% CI 101.72 to 158.28), 150 mcg (MD 146.52 mL, 95% CI 129.94 to 163.11), 300 mcg (MD 169.27 mL, 95% CI 144.52 to 194.02) and 600 mcg doses (MD 150.00 mL, 95% CI 100.62 to 199.38). Significant heterogeneity was identified in the 300 mcg analysis (I2 = 53%). This was largely a consequence of results from Mroz 2013. This study was a much smaller study than the Novartis‐sponsored trials and was at higher risk of bias (Figure 2). In addition only raw end‐of‐study data were available, and these showed significantly overestimates of treatment effect in this study due to poorly matched experimental and placebo arms at trial commencement (baseline FEV1 was 1.22 L in the placebo group and 1.78 L in the indacaterol group at study commencement). Sensitivity analysis was performed by excluding Mroz 2013 from the 300 mcg analysis. No significant change in the estimate of treatment effect was noted for the 300 mcg dose of indacaterol compared with placebo (MD 167.78 mL, 95% CI 142.98 to 192.57). Exclusion of Mroz 2013 from the entire analysis similarly had no significant impact on the overall estimate of treatment effect of indacaterol compared with placebo (MD 148.74 mL, 95% CI 136.72 to 160.76). Subgroup analysis of trials of less than 24 weeks (MD 148.99 mL, 95% CI 129.11 to 168.86) and 24 weeks or longer (MD 149.26 mL, 95% CI 134.01 to 164.51) demonstrated significant increases in trough FEV1 with indacaterol compared with placebo (Analysis 1.2). Heterogeneity in subgroup analysis of trials of less than 24‐weeks was significant (I2 = 66). Statistical heterogeneity was largely explained by the results reported by Mroz 2013, which as discussed above was a small study with less robust methodology.The estimate of treatment effect in Kinoshita 2012 was also slightly greater than in the remaining three studies.The reason for this is unclear, as aside from Mroz 2013, all studies had similar trial methodologies and statistical analyses, and were judged to be generally at low risk of bias.
1.1. Analysis.
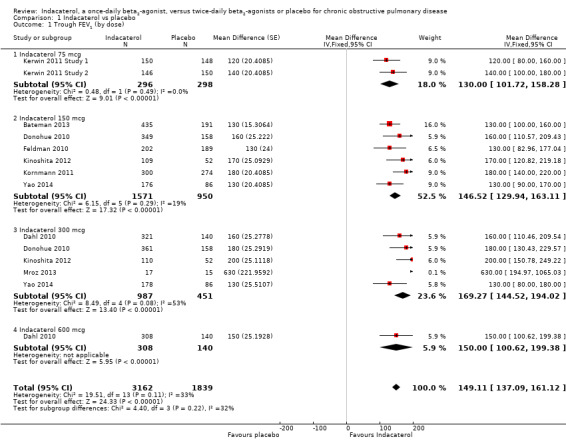
Comparison 1 Indacaterol vs placebo, Outcome 1 Trough FEV1 (by dose).
1.2. Analysis.
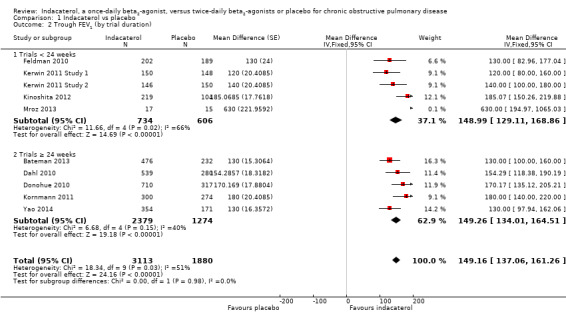
Comparison 1 Indacaterol vs placebo, Outcome 2 Trough FEV1 (by trial duration).
Data were insufficient for planned subgroup analysis by GOLD class severity.
Quality of life
Lower scores measured using the SGRQ indicate improvement in quality of life; four units represents a clinically important difference (Jones 2002). Ten trials contributed data from 4938 participants for this outcome. Compared with placebo, the mean SGRQ score was significantly lower with indacaterol (MD ‐3.60, 95% CI ‐4.36 to ‐2.83) (Analysis 1.3). Mean SGRQ scores were significantly lower with indacaterol than with placebo for 75 mcg (MD ‐3.70, 95% CI ‐5.66 to ‐1.74), 150 mcg (MD ‐3.43, 95% CI ‐4.53 to ‐2.32), 300 mcg (MD ‐3.49, 95% CI ‐4.94 to ‐2.03) and 600 mcg doses (MD ‐4.60, 95% CI ‐7.07 to ‐2.13). No significant statistical heterogeneity was noted. Sensitivity analysis was performed by removing Mroz 2013 because of concerns over methodological quality. This did not significantly alter the estimate of effect for indacaterol compared with placebo overall, nor for the 300 mcg subgroup analysis. Planned subgroup analysis by trial duration demonstrated slightly greater improvement in mean SGRQ for trials of less than 24 weeks (MD ‐4.11, 95% CI ‐5.60 to ‐2.62) than for trials 24 weeks or longer in duration (MD ‐3.15, 95% CI ‐4.12 to ‐2.19), but the difference between subgroups was not statistically significant (test for subgroup differences: Chi² = 1.11, df = 1 (P value 0.25), I² = 10.3%) (Analysis 1.4).
1.3. Analysis.
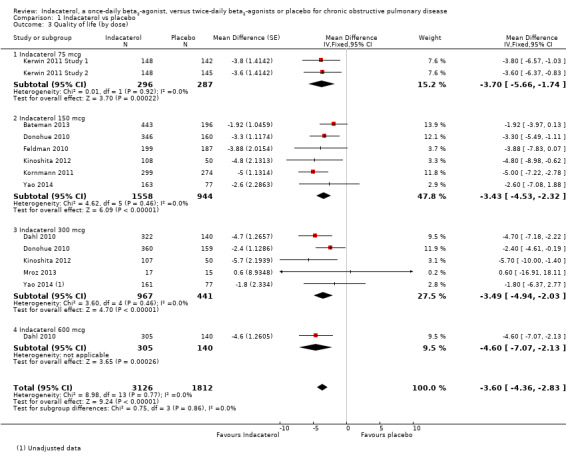
Comparison 1 Indacaterol vs placebo, Outcome 3 Quality of life (by dose).
1.4. Analysis.
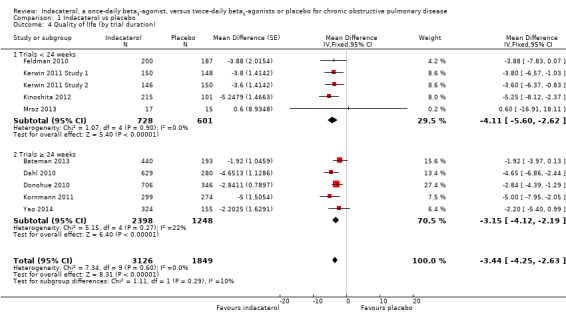
Comparison 1 Indacaterol vs placebo, Outcome 4 Quality of lIfe (by trial duration).
Data were insufficient for planned subgroup analysis by GOLD class severity.
Number of participants with a clinically significant improvement in quality of life
Compared with placebo, the odds of achieving an improvement in SGRQ score of at least four points overall were significantly greater with indacaterol (OR 1.64, 95% CI 1.46 to 1.85) (Analysis 1.5). We estimate that for 1000 participants with stable COPD treated for 12 to 52 weeks, 121 more participants (95% CI 94 to 151) would experience a clinically significant improvement in quality of life with indacaterol than with placebo (as shown in the Cates plot in Figure 3).
1.5. Analysis.
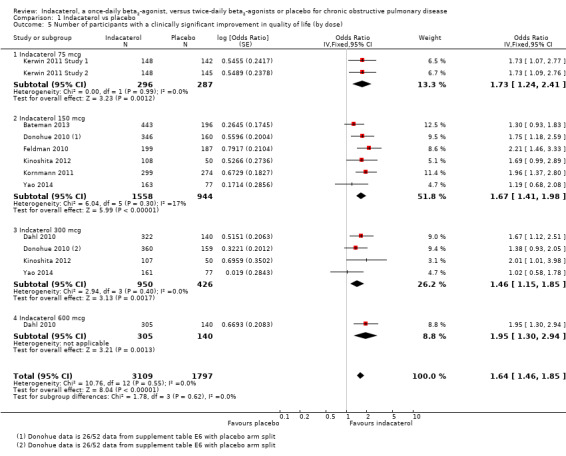
Comparison 1 Indacaterol vs placebo, Outcome 5 Number of participants with a clinically significant improvement in quality of life (by dose).
3.
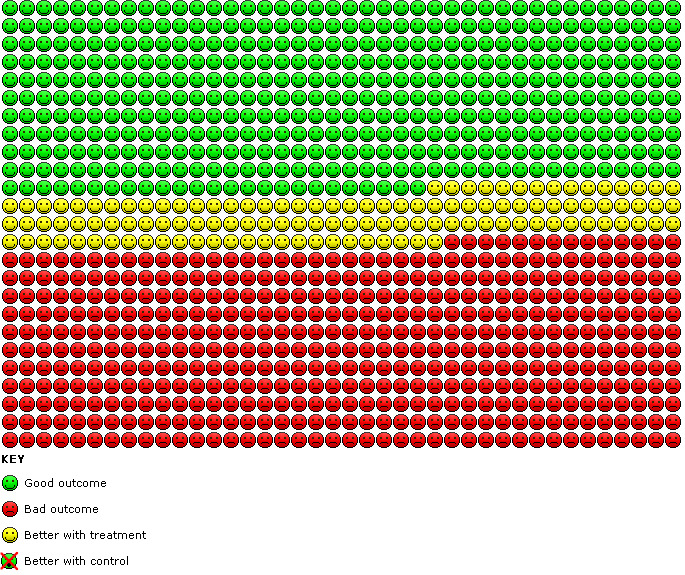
Cates plot. Participants with a clinically significant improvement in quality of life with indacaterol compared with placebo.
Compared with placebo, the odds of achieving an improvement in SGRQ score of at least four points were significantly greater for 75 mcg indacaterol (OR 1.73, 95% CI 1.24 to 2.41), 150 mcg indacaterol (OR 1.67, 95% CI 1.41 to 1.98), 300 mcg indacaterol (OR 1.46, 95% CI 1.15 to 1.85) and 600 mcg indacaterol doses (OR 1.95, 95% CI 1.30 to 2.94). No significant difference between subgroups was noted (test for subgroup differences: Chi² = 1.78, df = 3 (P value 0.62), I² = 0%). Planned subgroup analysis by trial duration demonstrated slightly increased odds of achieving an improvement in SGRQ of at least four points in trials of less than 24 weeks (OR 1.90, 95% CI 1.51 to 2.38) compared with trials 24 weeks or longer (OR 1.45, 95% CI 1.26 to 1.67), and the difference between subgroups was significant (test for subgroup differences: Chi² = 3.86, df = 1 (P value 0.02), I² = 74.1%) (Analysis 1.6).
1.6. Analysis.
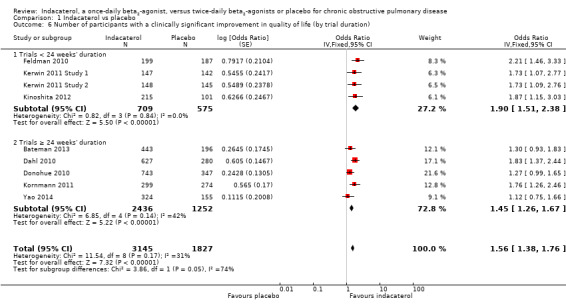
Comparison 1 Indacaterol vs placebo, Outcome 6 Number of participants with a clinically significant improvement in quality of life (by trial duration).
Data were insufficient for planned subgroup analysis by GOLD class severity.
Peak FEV1
Six trials contributed data on this outcome from 1657 participants. Overall peak FEV1 was significantly greater with indacaterol than with placebo (MD 181.21 mL, 95% CI 129.10 to 233.32) (Analysis 1.9). Peak FEV1 was significantly greater for indacaterol than for placebo for 75 mcg (MD 196.56 mL, 95% CI 107.15 to 285.98), 150 mcg (MD 200.91 mL, 95% CI 111.71 to 290.12) and 300 mcg doses (MD 173.50 mL, 95% CI 69.92 to 277.09). No statistically significant difference in peak FEV1 was noted with indacaterol compared with placebo for the 600 mcg dose (MD 30.00 mL, 95% CI ‐172.77 to 232.77). Data for the 600 mcg comparison were derived from one 52‐week trial (Dahl 2010), whereas data for the other comparisons came from trials of between 12 weeks' and 26 weeks' duration. Overall no significant statistical heterogeneity was noted (I2 = 0%).
1.9. Analysis.
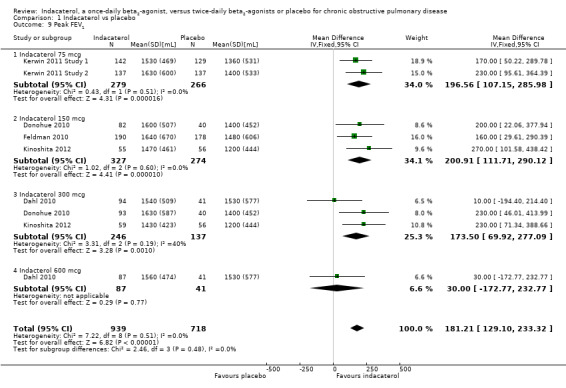
Comparison 1 Indacaterol vs placebo, Outcome 9 Peak FEV1.
Dyspnoea
Higher scores on the Transitional Dyspnoea Index (TDI) indicate improvement in breathlessness; one unit represents a clinically important difference (Witek 2003). Eight trials contributed data from 4722 participants for this outcome. Compared with placebo, overall mean TDI score was significantly higher with indacaterol (MD 1.00, 95% CI 0.82 to 1.17) (Analysis 1.7). Mean TDI scores were significantly greater with indacaterol than with placebo for 75 mcg (MD 0.77, 95% CI 0.27 to 1.27), 150 mcg (MD 0.96, 95% CI 0.70 to 1.22), 300 mcg (MD 1.13, 95% CI 0.83 to 1.43) and 600 mcg doses (MD 0.98, 95% CI 0.51 to 1.45). Statistical heterogeneity was significant in the 75 mcg subgroup analysis (I2 = 54%), which included two trials of identical methodology and participants with similar demographics. However overall statistical heterogeneity was not significant.
1.7. Analysis.
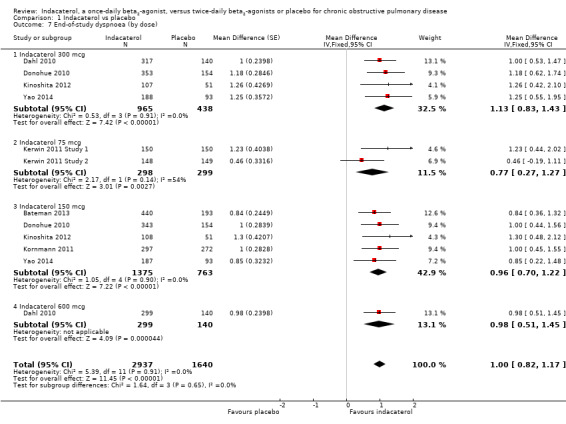
Comparison 1 Indacaterol vs placebo, Outcome 7 End‐of‐study dyspnoea (by dose).
Number of participants experiencing a clinically significant improvement in dyspnoea
Compared with placebo, the odds of achieving an improvement in TDI score greater than or equal to 1 overall were significantly greater with indacaterol (OR 1.96, 95% CI 1.73 to 2.22) (Analysis 1.8). We estimate that for 1000 participants with stable COPD treated for 12 to 52 weeks, 166 more participants (95% CI 136 to 196) would have a clinically significant improvement in dyspnoea with indacaterol than without (Figure 4).
1.8. Analysis.
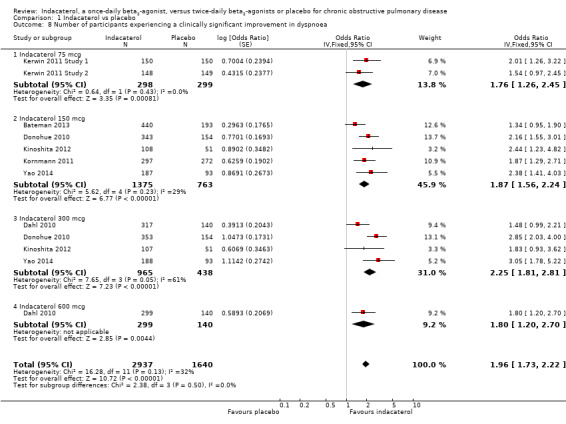
Comparison 1 Indacaterol vs placebo, Outcome 8 Number of participants experiencing a clinically significant improvement in dyspnoea.
4.
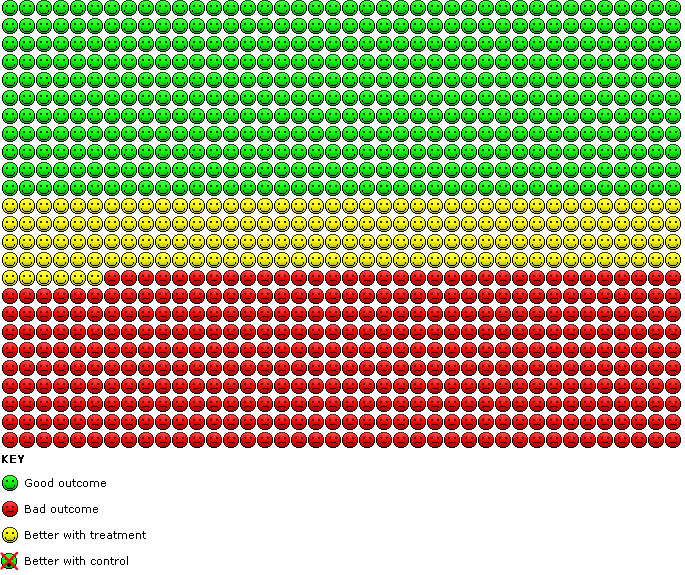
Cates plot. Participants with a clinically significant improvement in dyspnoea with indacaterol compared with placebo.
Compared with placebo, the odds of achieving an improvement in TDI score of at least one point were significantly greater for 75 mcg indacaterol (OR 1.76, 95% CI 1.26 to 2.45), 150 mcg indacaterol (OR 1.87, 95% CI 1.56 to 2.24), 300 mcg indacaterol (OR 2.25, 95% CI 1.81 to 2.81) and 600 mcg doses (OR 1.80, 95% CI 1.20 to 2.70). Overall no significant statistical heterogeneity was observed. Heterogeneity was significant in the 300 mcg comparison, which included four trials (Dahl 2010; Donohue 2010; Kinoshita 2012; Yao 2014). The odds of achieving a significant improvement in TDI were lower in Dahl 2010 and Kinoshita 2012 than in Donohue 2010 and Yao 2014. The reasons for this are unclear. Trial participants had similar degrees of airflow limitation (mean FEV1 was generally 50% predicted), trial methodology was similar, all were judged to be at relatively low risk for significant systematic bias and all used similar mixed‐models statistical approaches to data analysis. The effect of statistical heterogeneity was explored by performing a random‐effects analysis, which did not result in a significantly different point estimate of effect for this outcome.
Serious adverse events
Nine trials contributed data on serious adverse events from 6065 participants. Overall no statistically significant difference in the odds of experiencing a serious adverse event was noted for indacaterol compared with placebo (OR 1.00,95% CI 0.82 to 1.23). Subgroup analysis by dose did not demonstrate significant differences between placebo and indacaterol 75 mcg (OR 0.60, 95% CI 0.24 to 1.46), indacaterol 150 mcg (OR 1.01, 95% CI 0.75 to 1.37), indacaterol 300 mcg (OR 1.04, 95% CI 0.74 to 1.45) and indacaterol 600 mcg (OR 1.09, 95% CI 0.65 to 1.83) (test for subgroup differences: Chi² = 3.07, df = 3 (P value 0.38), I² = 2.2%) (Analysis 1.10). The confidence intervals are too wide to rule out important differences in serious adverse events between indacaterol and placebo.
1.10. Analysis.
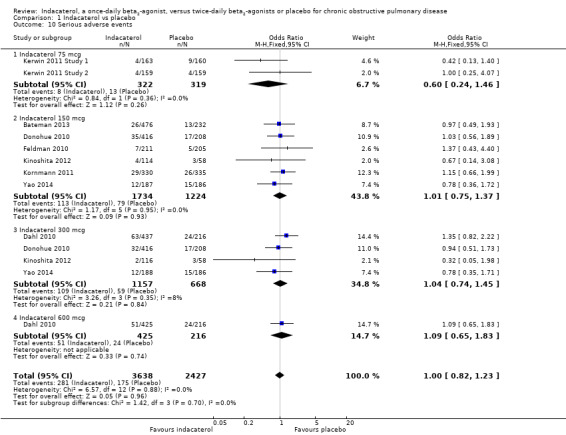
Comparison 1 Indacaterol vs placebo, Outcome 10 Serious adverse events.
Mortality
Nine trials contributed data on mortality from 5694 participants. Overall no significant difference was observed in the odds of mortality with indacaterol compared with placebo (OR 0.42, 95% CI 0.16 to 1.08). Subgroup analysis by dose did not demonstrate significant differences between placebo and indacaterol 75 mcg (OR 0.19, 95% CI 0.01 to 4.07), indacaterol 150 mcg (OR 0.86, 95% CI 0.23 to 3.16), indacaterol 300 mcg (OR 0.25, 95% CI 0.02 to 2.72) and indacaterol 600 mcg (OR 0.10, 95% CI 0.00 to 2.11) (test for subgroup differences: Chi² = 2.40, df = 3 (P value 0.49), I² = 0%) (Analysis 1.11). The confidence intervals are too wide to rule out important differences in mortality between indacaterol and twice‐daily beta2‐agonists.
1.11. Analysis.
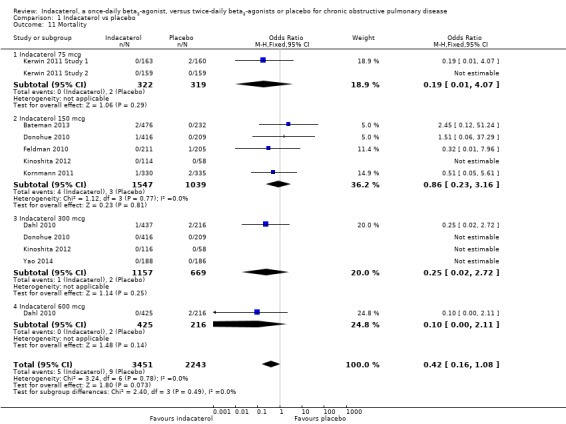
Comparison 1 Indacaterol vs placebo, Outcome 11 Mortality.
Number of participants experiencing at least one protocol‐defined exacerbation
Compared with placebo, the overall odds of experiencing at least one exacerbation were significantly less with indacaterol (OR 0.81, 95% CI 0.70 to 0.94). Subgroup analysis by dose did not demonstrate significant differences between indacaterol 75 mcg (OR 0.85, 95% CI 0.49 to 1.45), 150 mcg (OR 0.82, 95% CI 0.66 to 1.02), 300 mcg (OR 0.84, 95% CI 0.65 to 1.09) and 600 mcg (OR 0.74, 95% CI 0.51 to 1.06) (test for subgroup differences: Chi² = 0.37, df = 3 (P value 0.95), I² = 0%) (Analysis 1.12).
1.12. Analysis.
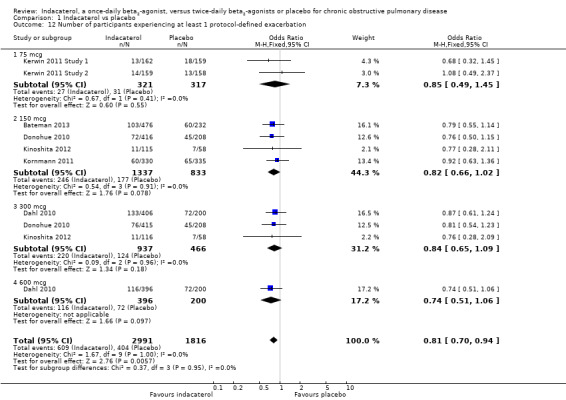
Comparison 1 Indacaterol vs placebo, Outcome 12 Number of participants experiencing at least 1 protocol‐defined exacerbation.
Indacaterol versus alternative twice‐daily beta2‐agonists
Trough FEV1 at the end of the dosing interval
Higher scores measured using spirometry indicate an improvement in lung function, and 100 mL represents a clinically important difference in FEV1 (Donahue 2005). Four trials contributed data on this outcome from 4708 participants. Formoterol and salmeterol were compared with 150 mcg, 300 mcg and 600 mcg doses of indacaterol. Compared with alternative twice‐daily beta2‐agonists, the mean trough FEV1 was significantly greater with indacaterol (MD 73.76 mL, 95% CI 57.33 to 90.19) (Analysis 2.1). The trough FEV1 was significantly greater for indacaterol compared with twice‐daily beta2‐agonists for 150 mcg (MD 62.56 mL, 95% CI 42.71 to 82.40), 300 mcg (MD 97.17 mL, 95% CI 60.51 to 133.83) and 600 mcg doses (MD 100.00 mL, 95% CI 51.21 to 148.79). Trough FEV1 was slightly greater for the 300 mcg and 600 mcg doses than for the 150 mcg dose, but this finding was not statistically significant (test for subgroup differences: Chi² = 3.90, df = 2 (P value 0.14), I² = 48.7%). Overall no significant heterogeneity was observed (I2 = 15%).. Subgroup analysis by type of twice‐daily beta2‐agonist demonstrated a significant increase in FEV1 for indacaterol compared with salmeterol (MD 64.50 mL, 95% CI 45.79 to 83.20). Data for the formoterol comparison were derived from one trial only (Dahl 2010), which compared 600 mcg indacaterol versus placebo. This study demonstrated a significant improvement in trough FEV1 with indacaterol compared with formoterol (MD 98.19 mL, 95% CI 68.88 to 127.50). Subgroup analysis by trial duration demonstrated a significantly increased FEV1 with indacaterol compared with alternative twice‐daily beta2‐agonists for trials 24 weeks or longer (MD 122.98 mL, 95% CI 102.37 to 143.59) and for trials of less than 24 weeks (MD 60.00 mL, 95% CI 37.00 to 83.00) (Analysis 2.2). Heterogeneity in analysis of trials 24 weeks or longer was significant (I2 = 73%). The estimate of effect from Dahl 2010 was significantly greater than in the other three studies. The reasons for this are unclear, although it is possible that the modified intention‐to‐treat analysis used in this study (participants from six sites were excluded for non‐conformance with good clinical practice) may have contributed. Only one study (Korn 2011) was less than 24 weeks in duration.
2.1. Analysis.
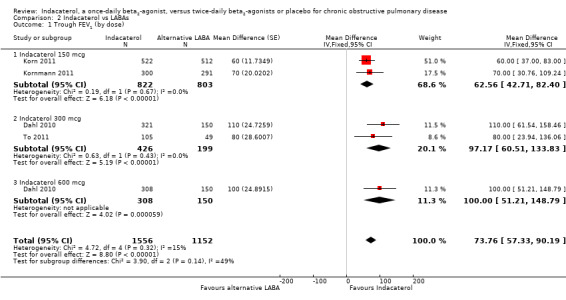
Comparison 2 Indacaterol vs LABAs, Outcome 1 Trough FEV1 (by dose).
2.2. Analysis.
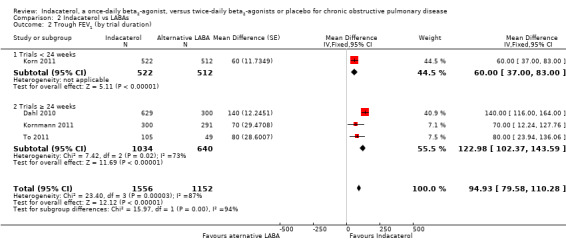
Comparison 2 Indacaterol vs LABAs, Outcome 2 Trough FEV1 (by trial duration).
Data were insufficient for planned subgroup analysis by GOLD class severity.
Quality of life
Lower scores measured using the SGRQ indicate improvement in quality of life; four units represents a clinically important difference (Jones 2002). Two trials contributed data on this outcome from 1523 participants. Formoterol and salmeterol were compared with 150 mcg, 300 mcg and 600 mcg doses of indacaterol. Overall no statistically significant differences in mean SGRQ scores were noted between indacaterol and twice‐daily beta2‐agonists (MD ‐0.81, 95% CI ‐2.28 to 0.66) (Analysis 2.3). Kornmann 2011 was the only trial that contributed data to the 150 mcg comparison, whilst Dahl 2010 was the only trial that performed 300 mcg and 600 mcg comparisons. No significant differences were noted between indacaterol and twice‐daily beta2‐agonists for the 150 mcg (MD ‐1.20, 95% CI ‐3.42 to 1.02), 300 mcg (MD ‐0.50, 95% CI ‐3.27 to 2.27) and 600 mcg doses (MD ‐0.50, 95% CI ‐3.27 to 2.27). Both trials were 24 weeks or longer in duration. Therefore subgroup analysis by trial duration was not performed. Subgroup analysis by type of twice‐daily beta2‐agonist demonstrated no significant differences between indacaterol and salmeterol (MD ‐1.20, 95% CI ‐3.42 to 1.02) or between indacaterol and formoterol (MD 0.42, 95% CI ‐1.21 to 2.05).
2.3. Analysis.
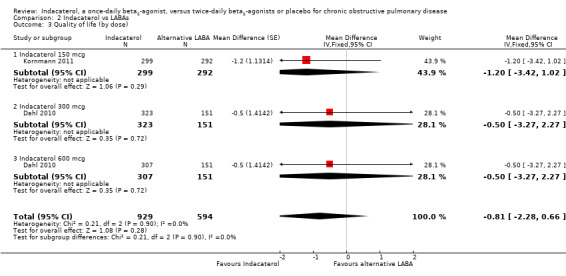
Comparison 2 Indacaterol vs LABAs, Outcome 3 Quality of life (by dose).
Data were insufficient for planned subgroup analysis by GOLD class severity.
Number of participants with a clinically significant improvement in quality of life
Two trials contributed data on this outcome from 1520 participants. Formoterol and salmeterol were compared with 150 mcg, 300 mcg and 600 mcg doses of indacaterol. Overall no significant difference was observed between indacaterol and twice‐daily beta2‐agonists in the odds of achieving a clinically significant improvement in SGRQ (OR 1.07, 95% CI 0.87 to 1.32) (Analysis 2.5). No significant differences were noted between indacaterol and alternative twice‐daily beta2‐agonists in the odds of achieving a clinically significant improvement in quality of life with 150 mcg (OR 1.17, 95% CI 0.85 to 1.61), 300 mcg (OR 0.93, 95% CI 0.63 to 1.37) and 600 mcg doses (OR 1.09, 95% CI 0.74 to 1.61). Both trials were 24 weeks or longer in duration (Analysis 2.6). Therefore subgroup analysis by trial duration was not performed.
2.5. Analysis.
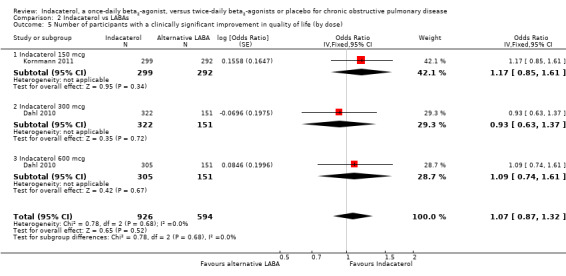
Comparison 2 Indacaterol vs LABAs, Outcome 5 Number of participants with a clinically significant improvement in quality of life (by dose).
2.6. Analysis.
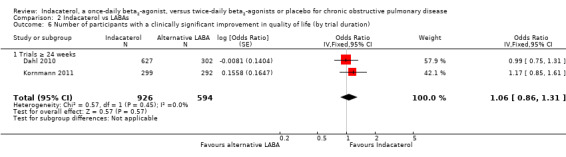
Comparison 2 Indacaterol vs LABAs, Outcome 6 Number of participants with a clinically significant improvement in quality of life (by trial duration).
Data were insufficient for planned subgroup analysis by GOLD class severity.
Peak FEV1
Two trials contributed data on this outcome from 491 participants. Formoterol and salmeterol were compared with 150 mcg, 300 mcg and 600 mcg doses of indacaterol. No significant difference was observed between indacaterol and alternative twice‐daily beta2‐agonists with respect to peak FEV1 (MD 4.68, 95% CI ‐93.79 to 103.16) (Analysis 2.9). Subgroup analysis by dose demonstrated no significant differences between indacaterol and alternative twice‐daily beta2‐agonists for the 150 mcg (MD 40.00, 95% CI ‐113.72 to 193.72), 300 mcg (MD ‐30.00, 95% CI ‐212.29 to 152.29) and 600 mcg doses (MD ‐10.00, 95% CI ‐190.45 to 170.45). No significant heterogeneity was observed (I2 = 0%).
2.9. Analysis.
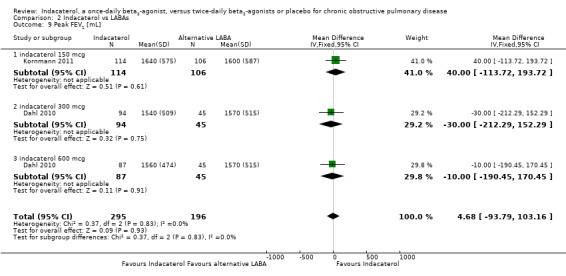
Comparison 2 Indacaterol vs LABAs, Outcome 9 Peak FEV1 [mL].
Dyspnoea
Higher scores on the TDI indicate improvement in breathlessness; one unit represents a clinically important difference (Witek 2003). Three trials contributed data on this outcome from 2404 participants. Formoterol and salmeterol were compared with 150 mcg, 300 mcg and 600 mcg doses of indacaterol. Compared with twice‐daily long‐acting beta2‐agonists, overall mean TDI score was significantly greater with indacaterol (MD 0.54, 95% CI 0.30 to 0.79) (Analysis 2.7). Subgroup analysis by dose demonstrated a significant increase in TDI with indacaterol 150 mcg compared with twice‐daily long‐acting beta2‐agonists (MD 0.66, 95% CI 0.37 to 0.95). No significant differences were observed between indacaterol and twice‐daily long‐acting beta2‐agonists for 300 mcg (MD 0.19, 95% CI ‐0.46 to 0.84) and 600 mcg doses (MD 0.30, 95% CI ‐0.35 to 0.95). The 150 mcg comparison involved two trials (Korn 2011; Kornmann 2011) of 12 weeks' and 26 weeks' duration, respectively; both used salmeterol as the active comparator. Data on the 300 mcg and 600 mcg doses were received from only one 52‐week trial (Dahl 2010), which used formoterol as the active comparator.
2.7. Analysis.
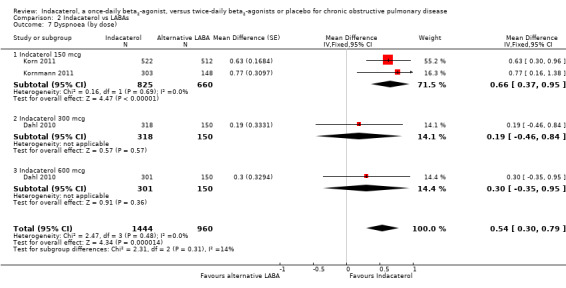
Comparison 2 Indacaterol vs LABAs, Outcome 7 Dyspnoea (by dose).
Number of participants experiencing a clinically significant improvement in dyspnoea
Three trials contributed data on this outcome from 2536 participants. Formoterol and salmeterol were compared with 150 mcg, 300 mcg and 600 mcg doses of indacaterol. Overall, no significant differences were observed in the odds of experiencing an improvement in TDI greater than or equal to one point with indacaterol compared with twice‐daily long‐acting beta2‐agonists (OR 1.11, 95% CI 0.94 to 1.32) (Analysis 2.8). Subgroup analysis by dose demonstrated no significant differences between indacaterol and alternative twice‐daily beta2‐agonists for the 150 mcg (OR 1.21, 95% CI 0.98 to 1.50), 300 mcg (OR 0.87, 95% CI 0.59 to 1.29) and 600 mcg doses (OR 1.06, 95% CI 0.72 to 1.58). Heterogeneity in the 150 mcg comparison was significant (I2 = 65%). Korn 2011 demonstrated significant improvement in the odds of achieving an improvement in TDI greater than or equal to one point, whereas Kornmann 2011 and Dahl 2010 did not. The reason for the difference between Korn 2011 and Kornmann 2011 for this particular outcome is unclear, especially given the similar improvements in mean TDI noted in these two studies. Both were large trials recruiting more than 1000 participants; both used salmeterol as the active comparator, had similar trial methodologies and statistical analyses, recruited participants from generally similar geographic locations and enrolled participants with similar baseline characteristics. Both trials were judged to be at generally low risk of bias.
2.8. Analysis.
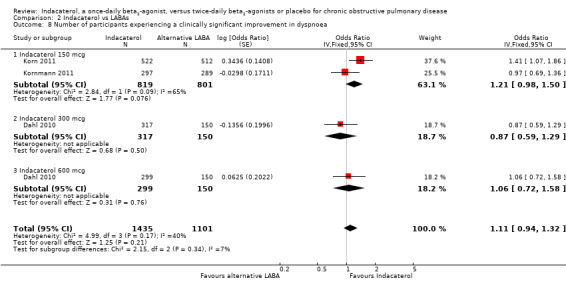
Comparison 2 Indacaterol vs LABAs, Outcome 8 Number of participants experiencing a clinically significant improvement in dyspnoea.
Serious adverse events
Four trials contributed data on serious adverse events from 3266 participants. Formoterol and salmeterol were compared with 150 mcg, 300 mcg and 600 mcg doses of indacaterol. Compared with twice‐daily long‐acting beta2‐agonists, no statistically significant difference in serious adverse events was reported with indacaterol (OR 1.02, 95% CI 0.79 to 1.32) (Analysis 2.10). No significant differences were observed between twice‐daily beta2‐agonists and indacaterol 150 mcg (OR 1.44, 95% CI 0.92 to 2.25), indacaterol 300 mcg (OR 1.00, 95% CI 0.66 to 1.52) and indacaterol 600 mcg (OR 0.71, 95% CI 0.45 to 1.13). The confidence intervals are too wide to rule out important differences in serious adverse events between indacaterol and twice‐daily beta2‐agonists.
2.10. Analysis.
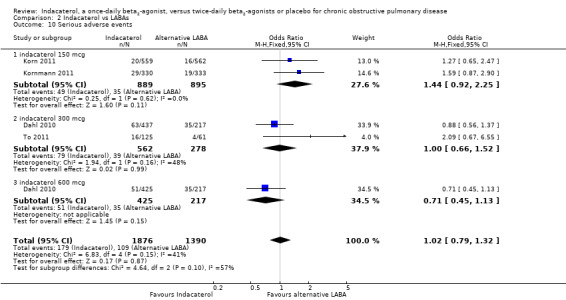
Comparison 2 Indacaterol vs LABAs, Outcome 10 Serious adverse events.
Mortality
Four trials contributed data on mortality from 3266 participants. Formoterol and salmeterol were compared with 150 mcg, 300 mcg and 600 mcg doses of indacaterol. Compared with twice‐daily long‐acting beta2‐agonists, no significant differences in mortality were noted with indacaterol (OR 1.00, 95% CI 0.31 to 3.28) (Analysis 2.11). No significant differences were reported between twice‐daily beta2‐agonists and indacaterol 150 mcg (OR 2.35, 95% CI 0.35 to 15.98), indacaterol 300 mcg (OR 0.82, 95% CI 0.11 to 6.27) and indacaterol 600 mcg (OR 0.17, 95% CI 0.01 to 4.18). The confidence intervals are too wide to rule out important differences in mortality between indacaterol and twice‐daily beta2‐agonists.
2.11. Analysis.
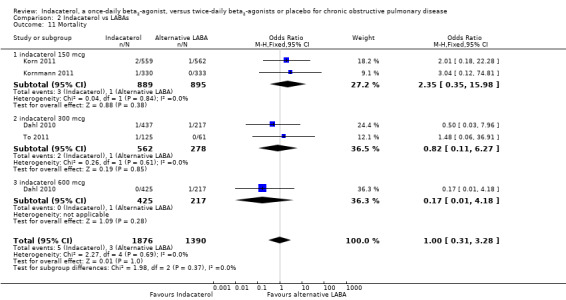
Comparison 2 Indacaterol vs LABAs, Outcome 11 Mortality.
Number of participants experiencing at least one protocol‐defined exacerbation
Two trials contributed data on this outcome from 1869 participants. Formoterol and salmeterol were compared with 150 mcg, 300 mcg and 600 mcg doses of indacaterol. Compared with twice‐daily long‐acting beta2‐agonists, no significant differences were observed in the odds of experiencing at least one exacerbation with indacaterol (OR 1.04, 95% CI 0.84 to1.29) (Analysis 2.12). Compared with twice‐daily beta2‐agonists, no significant differences were observed in the odds of experiencing at least one exacerbation with indacaterol 150 mcg (OR 1.21, 95% CI 0.80 to 1.82), indacaterol 300 mcg (OR 1.06, 95% CI 0.74 to 1.53) and indacaterol 600 mcg (OR 0.90, 95% CI 0.62 to 1.30).
2.12. Analysis.
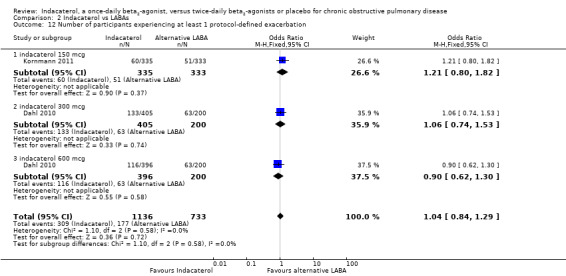
Comparison 2 Indacaterol vs LABAs, Outcome 12 Number of participants experiencing at least 1 protocol‐defined exacerbation.
Discussion
Summary of main results
Indacaterol versus placebo
Compared with placebo, once‐daily dosing with indacaterol results in statistically significant and clinically relevant increases in trough forced expiratory volume in one second (FEV1) (Analysis 1.1). Subgroup analysis by dose demonstrated similar improvements in trough FEV1 across 75 mcg, 150 mcg, 300 mcg and 600 mcg doses. All exceeded the minimum clinically relevant difference of 100 mL (Donahue 2005). Planned subgroup analysis by trial duration demonstrated a sustained response to indacaterol over 52 weeks, with similar improvements in trough FEV1 reported in trials of less than 24 weeks and in 24 weeks or longer in duration (Analysis 1.2).
Quality of life was improved for a significant number of participants with indacaterol compared with placebo. The overall mean St George Respiratory Questionnaire (SGRQ) score was lower with indacaterol (Analysis 1.3). Similar responses were noted across 75 mcg, 150 mcg, 300 mcg and 600 mcg doses. Although the overall point estimate of effect did not reach the accepted four unit minimum clinically important difference for an improvement in quality of life (Jones 2002), the odds of achieving an SGRQ score improvement of four or more points were significantly greater with indacaterol than with placebo over 12 to 52 weeks. We estimate that for 1000 participants with stable chronic obstructive pulmonary disease (COPD), 121 more participants would experience a clinically significant improvement in quality of life with indacaterol than without (Figure 3). The odds of a clinically significant improvement in quality of life were similar with all four doses of indacaterol.
Other planned primary outcome analyses were exacerbation rates and proportions of people with a clinically significant deterioration in quality of life. Data were insufficient to include these outcomes in a meta‐analysis.
Secondary outcomes included mean dyspnoea scores, proportions of participants with a clinically significant improvement in dyspnoea, peak FEV1, serious adverse events and mortality. As data were insufficient for a comparison of exacerbation rates, we also compared the number of participants experiencing at least one exacerbation as a further post hoc secondary analysis.
Overall mean dyspnoea scores (Analysis 1.7), odds of achieving a clinically significant improvement in dyspnoea (Witek 2003) (Analysis 1.8) and peak FEV1 (Analysis 1.9) were all significantly improved with indacaterol compared with placebo. We estimate that for 1000 participants with stable COPD, 166 more participants would have a clinically significant improvement in dyspnoea with indacaterol than with placebo over 12 to 52 weeks (Figure 4).
The overall odds of experiencing at least one exacerbation were significantly less with indacaterol than with placebo (Analysis 1.12). Many trials were of short duration, and the definition of exacerbation was not standardised across trials. Furthermore, it was not possible to measure exacerbation rates as had been planned because data were insufficient. Finally, for each tested dose, a statistically significant reduction in the number of participants experiencing at least one exacerbation was not demonstrated. Therefore, whilst inspection of the forest plot does not suggest a significant dose‐response effect, it is difficult to be confident about the true effect of indacaterol on exacerbations for any individual dose. Nonetheless, we estimate that overall, for 1000 participants treated with indacaterol for stable COPD, 34 fewer participants would experience at least one exacerbation over a treatment period of 12 to 52 weeks compared with untreated participants.
No significant difference was noted between indacaterol and placebo in the number of participants suffering a serious adverse event (Analysis 1.10) or mortality (Analysis 1.11), but the confidence intervals are too wide to rule out important differences.
Other planned secondary outcomes were 24‐hour area under the curve FEV1, peak forced vital capacity (FVC) and number of participants experiencing clinically significant deterioration in dyspnoea. Data were insufficient for analysis of these outcomes.
Indacaterol versus twice‐daily long‐acting beta2‐agonists (LABAs)
Fewer data were available for the comparison of indacaterol versus alternative long‐acting beta2‐agonists, and only four trials overall contributed data (Dahl 2010; Korn 2011; Kornmann 2011; To 2011).
Compared with twice‐daily beta2‐agonists, trough FEV1 was numerically greater with indacaterol (Analysis 2.1), although this did not exceed the generally accepted minimal clinically important difference (Donahue 2005). Similar improvements were seen with indacaterol at 150 mcg, 300 mcg and 600 mcg doses. A sustained response to indacaterol was once again demonstrated, with improvements in trough FEV1 reported with indacaterol in trials greater than and less than 24 weeks in duration (Analysis 2.2). Subgroup analysis by type of twice‐daily beta2‐agonist demonstrated small and probably clinically irrelevant improvements in trough FEV1 in the formoterol comparison than in the salmeterol comparison, although only Dahl 2010 involved a formoterol comparison.
Overall, quality of life was not significantly different with indacaterol compared with twice‐daily beta2‐agonists, and no significant differences were demonstrated in terms of mean SGRQ scores (Analysis 2.3) or in the proportion of participants achieving a clinically significant improvement in SGRQ (Analysis 2.5) (Jones 2002). Only two trials contributed quality of life data (Dahl 2010; Kornmann 2011); therefore meaningful subgroup analysis is not possible.
Other planned primary outcome analyses included exacerbation rates and proportions of people with a clinically significant deterioration in quality of life. Data were insufficient for inclusion of these outcomes in a meta‐analysis.
Secondary outcomes included mean dyspnoea scores, proportions of participants with a clinically significant improvement in dyspnoea, peak FEV1, serious adverse events and mortality. As in the placebo comparison, data were insufficient for a comparison of exacerbation rates, and so the number of participants experiencing at least one exacerbation was examined as a further post hoc secondary analysis.
The overall mean dyspnoea score was significantly greater with indacaterol than with twice‐daily beta2‐agonists, but this finding did not exceed the minimum clinically important difference (Witek 2003) (Analysis 2.7). The odds of achieving a clinically significant improvement in dyspnoea were not statistically significantly different with indacaterol than with twice‐daily beta2‐agonists (Analysis 2.8). In the 150 mcg comparison (Korn 2011; Kornmann 2011), a statistically significant improvement in mean Transitional Dyspnoea Index (TDI) was seen with indacaterol compared with salmeterol, although this was likely to have been clinically irrelevant. In these two trials, the odds of achieving a clinically significant improvement in dyspnoea were increased only in Korn 2011. The reason for the discrepancy in this outcome between Korn 2011 and Kornmann 2011 is unclear. Both trials used similar methodology, enrolled participants with similar degrees of airflow limitation and were believed to be at overall relatively low risk of bias. Only one study examined 300 mcg and 600 mcg dose comparisons (Dahl 2010). No significant difference in mean TDI or in the odds of achieving a clinically significant improvement in dyspnoea was noted at 300 mcg or 600 mcg doses. The major methodological difference between the 150 mcg comparison and the 300 mcg and 600 mcg comparisons was the use of formoterol in Dahl 2010 and salmeterol in Korn 2011 and Kornmann 2011. In addition the former was a 52‐week study, whereas the latter two were trials of 6 months' duration or less. Given the limited number of trials available, significant caution should be applied in drawing any conclusions from subgroup analyses for these outcomes.
No significant differences were noted in the odds of experiencing at least one exacerbation, the odds of a serious adverse event or mortality between indacaterol and twice‐daily beta2‐agonists, either overall or within any of the subgroups by dose, although again the confidence intervals are too wide to rule out important differences.
Other planned secondary outcomes were 24‐hour area under the curve FEV1, peak FVC and number of participants experiencing a clinically significant deterioration in dyspnoea. Data were insufficient for analysis of these outcomes.
Overall completeness and applicability of evidence
Indacaterol versus placebo
A strong body of evidence is based on a total of 9961 participants overall. All trials aside from Mroz 2013 were sponsored by the manufacturer, and inclusion and exclusion criteria were similar. All trials assessed participants with stable COPD across a range of spirometric severities; mean FEV1 was approximately 50% predicted in most trials. One trial specifically enrolled participants with severe disease, and mean FEV1 was 35% predicted (Yao 2014). Broad international recruitment was seen across these studies, with participants enrolled predominantly from the United States, Canada, Europe, India, Asia and China. It is therefore likely that the results could be generalised to most symptomatic patients with stable COPD and a postbronchodilator FEV1 of between 30% and 80% predicted. Patients with a diagnosis of asthma, those requiring long‐term oxygen therapy and those with concomitant pulmonary disease were generally excluded from trials, and results should be extrapolated to such patients with caution. In addition patients with diabetes, active malignancy, history of long QT syndrome or prolonged QTc were generally excluded. Four doses were compared with placebo: 75 mcg, 150 mcg, 300 mcg and 600 mcg. Most of the data have been derived from trials assessing 150 mcg and 300 mcg doses of indacaterol. For 75 mcg, two 12‐week trials of identical design were conducted, and for the 600 mcg comparison, one 52‐week trial was completed. Therefore relatively fewer data have been reported for these doses.
Fewer data were also found for comparisons with alternative long‐acting beta2‐agonists, with only four trials contributing to final analyses. Results for these outcomes therefore should also be interpreted with some caution.
Quality of the evidence
The evidence was generally of good quality. All included data were reported by randomised controlled trials, with generally limited potential for significant bias. Trials demonstrated similar estimates of treatment effects in the same direction for primary outcomes. When significant heterogeneity was identified, this was often explained by differences in the methodological quality of included trials. In a few select instances, statistical heterogeneity was difficult to explain, with relevant trials having similar inclusion and exclusion criteria, enrolling participants with similar severity of disease and using similar methodology and statistical approaches. Mroz 2013 was a small study that was judged to be of lower methodological quality. The method used for random sequence generation, allocation concealment and blinding of participants and personnel was not specified. In Kerwin 2011 Study 1 and Kerwin 2011 Study 2, some predefined secondary endpoints were not published, although outcomes of interest were made available by the manufacturer. To 2011 was an open‐label trial with significant potential for bias. Finally, it is possible that in Dahl 2010, exclusion of participants from six investigator sites for non‐conformance with good clinical practice may have introduced bias.
Potential biases in the review process
Bias in the review process was minimised by the use of comprehensive search terms across six separate medical bibliographic databases including the Cochrane Central Register of Controlled Trials. In addition, the manufacturers' registers of trials were manually searched and respiratory journals were handsearched for additional references. All references were cross‐checked against clincicaltrials.gov, and an additional search of this database was performed. Two review authors independently determined inclusion and exclusion of trials, extracted data and judged risk of bias to minimise error.
For continuous outcomes, in most cases mean adjusted data were extracted from published ANCOVA analyses. However in some instances only raw end‐of‐study data were available, and when this was the case, we combined adjusted and raw data (Table 3 and Table 4). It is possible that this approach may have introduced some bias into the results, although the overall effect of this is likely to be very low. One open‐label study comparing indacaterol versus alternative long‐acting beta2‐agonists reported quality of life data that could not be used in this review; this may have introduced bias (Izbicki 2014). However only 90 participants were included in this study and the overall impact is likely to be very low.
Most reported data were obtained from methodologically robust randomised controlled trials, and the potential for introduction of significant systematic biases within these trials generally is believed to be low. Rates of attrition were generally between 10% and 20%, often with slightly greater loss of participants from placebo than from experimental arms. Loss to follow‐up was most commonly due to unsatisfactory therapeutic effect or to adverse events. It is possible therefore that unmatched attrition between placebo and experimental arms may have introduced some bias, and such bias would most likely lead to underestimation of the treatment effects of indacaterol. However, again the overall impact of such bias is likely to be low.
Agreements and disagreements with other studies or reviews
Han 2013 performed a systematic review comparing the odds of a clinically significant improvement in dyspnoea with indacaterol versus placebo, and their results are consistent with the findings of this review. As in this review, investigators demonstrated increased odds of achieving a TDI improvement greater than or equal to one point with 75 mcg, 150 mcg and 300 mcg doses of indacaterol compared with placebo, with similar estimates of effect noted across all doses.
Rodrigo 2012 performed a systematic review that included a comparison of any dose of indacaterol versus tiotropium and alternative long‐acting beta2‐agonists, and assessed trough FEV1 and the odds of clinically significant improvements in dyspnoea and quality of life. This study demonstrated a similar small improvement in trough FEV1 with indacaterol at any dose compared with twice‐daily beta2‐agonists. However, in contrast to this review, the odds of achieving a clinically significant improvement in quality of life were greater with indacaterol than with alternative long‐acting beta2‐agonists. This finding was due to a greater estimate of effect in pooled results from Dahl 2010. The reason for this difference is unclear, although fewer participants were analysed in our 52‐week analysis for this outcome than were reported in Rodrigo 2012. Data were insufficient for this outcome, and unadjusted 52‐week data were supplied for this review by the manufacturer upon request. Therefore in our review, this outcome will not include imputed data from participants who dropped out of the study. In addition we performed a fixed‐effect generic inverse variance analysis, whereas these review authors performed a Mantel‐Haenszel meta‐analysis using a random‐effects model to account for differences in participant demographics and trial methodologies. Rodrigo 2012 also demonstrated increased odds of a clinically significant improvement in dyspnoea for indacaterol compared with twice‐daily long‐acting beta2‐agonists, whereas we found no significant differences between the two interventions. The point estimate of effect for Kornmann 2011 was greater than in our review for this outcome. This review used generic inverse variance to include published 26‐week data, whereas these review authors again used a random‐effects Mantel‐Haenszel meta‐analysis.
Decramer 2012 pooled data from Donohue 2010, Kornmann 2011 and Dahl 2010 and analysed a maintenance treatment‐naive subgroup. Review authors demonstrated clinically significant improvements in trough FEV1, dyspnoea and quality of life for indacaterol 150 mcg and 300 mcg compared with placebo. No significant difference in the hazard ratio was noted for time to first exacerbation, although review authors did report a reduction in risk of exacerbation for participants receiving maintenance treatment. No significant increase in serious adverse events was reported. These results are consistent with the findings of this review and suggest that findings can be extended to patients not previously given alternative maintenance therapy.
Chung 2013 performed a systematic review comparing indacaterol versus placebo and alternative twice‐daily beta2‐agonists. This review judged the evidence to be generally of lower quality caused by potential bias associated with unclear sequence generation. The authors of this review believed that risk of bias due to inadequate sequence generation was low across most studies. Compared with placebo, these review authors found a similar clinically relevant improvement in trough FEV1. They did not pool results for quality of life or dyspnoea. Compared with twice‐daily long‐acting beta2‐agonists, review authors found a similar small improvement in trough FEV1. They did not pool results for quality of life or dyspnoea but noted that no significant difference was reported for these outcomes in any of the included trials.
Jiang 2013 also performed a systematic review comparing indacaterol versus placebo and alternative bronchodilators (including tiotropium). Compared with placebo, review authors demonstrated similar clinically significant improvements in trough FEV1 and mean dyspnoea scores. They included fewer trials and tiotropium analyses in their alternative bronchodilator comparisons; therefore these results cannot be directly compared with our own.
Finally, model‐based approaches have suggested a dose‐response relationship below 150 mcg (Renard 2011) and individual trials have suggested that 300 mcg of indacaterol conveyed incremental benefits above 150 mcg with respect to symptom control, as evidenced by lower dyspnoea scores and less requirement for rescue short‐acting bronchodilator use (Ribeiro 2012). No clear dose‐response effect was seen across the range of outcomes and analyses included in our review, although the incremental response with 300 mcg versus 150 mcg indacaterol has been reported in participants with more severe COPD (Donohue 2010), and we were unable to perform subgroup analysis by severity of COPD.
Authors' conclusions
Implications for practice.
Indacaterol provides clinically meaningful improvements in lung function that are associated with improvements in quality of life and dyspnoea across all doses between 75 mcg and 600 mcg. In addition, indacaterol reduces the chance of experiencing an exacerbation. Indacaterol is therefore an appropriate treatment for patients with confirmed symptomatic stable COPD who do not have concurrent respiratory disease including asthma.
Indacaterol offers an alternative to twice‐daily beta2‐agonists and results in clinically similar improvements in lung function, with the possible advantage of once‐daily dosing. Some uncertainty remains regarding its effect on quality of life, however the effects of indacaterol and twice‐daily beta2‐agonists for this outcome are likely comparable.
Evidence is currently insufficient to confirm the effects of indacaterol on serious adverse events and mortality.
Implications for research.
Further long‐term data would be useful for defining the impact of indacaterol on exacerbations, serious adverse events and mortality.
Further data would be useful for defining potential differences in efficacy between indacaterol and alternative long‐acting beta2‐agonists, particularly with respect to quality of life and dyspnoea.
Further data examining potential dose‐response curves would be useful, particularly with respect to severity of underlying COPD.
Feedback
Interpretation of the review data, 27 January 2016
Summary
Comment: Written by: Marlys LeBras and Aaron Tejani
We read with interest the review of the newly approved long acting beta2‐agonist (LABA), indacaterol, by Geake et al. (1). Indacaterol is an alternative to twice‐daily LABA currently marketed for the management of chronic obstructive pulmonary disease (COPD) based on efficacy and safety data summarized in this review (1). We agree that confidence intervals were too wide to exclude important differences in regards to serious adverse events (SAE) or mortality although no significant differences were observed between groups (1). We were happy to learn there was no clear dose‐response effect observed across the range of outcome and analyses included in the review and understand the inability of authors to perform subgroup analysis by severity of COPD (1).
We were interested in truly understanding the information provided in the review and the data from the trials assessing indacaterol. We approached this by assessing trials of indacaterol compared to placebo that contributed the most weight to outcomes we considered clinically important in the management of COPD, namely: quality of life (QOL), exacerbations, SAE, and mortality. The following are some concerns we have identified with the trials included in review that may affect reader’s certainty in the results of trials and conclusions drawn.
1a) The same issue presented itself for both analysis 1.3: QOL (by dose) and analysis 1.12: number of patients experiences at least 1 exacerbation, of which Dahl et al. and Donohue et al. trials contributed substantial weight (QOL: 19%, 24%; exacerbations: 33.7%, 25.1% respectively) to pooled estimates (2,3). Regarding QOL, we found inconsistent reporting throughout the review. For example, the abstract states “indacaterol versus placebo results in statistically significant and clinically meaningful improvements in lung function and quality of life” and the “overall quality of the evidence was strong”, yet the implication for practice section states, “some uncertainty remains regarding relation to quality of life”(1). Furthermore, the discussion states, "the overall point estimate of effect did not reach the accepted four unit minimum clinically important difference for an improvement in quality of life, the odds of achieving a St. George Respiratory Questionnaire (SGRQ) score improvement of four or more points were significantly greater with indacaterol than with placebo” (1).
1b) We, and perhaps other readers, would find it useful for authors to comment on why the analysis of the data one way demonstrated a non‐clinically meaningful difference in QOL while another did increase the odds of achieving a clinically significant outcome and the implication to practice.
1c) Both Dahl et al. and Donohue et al. methodology included last observation carried forward (LOCF) for dealing with missing data (2,3). LOCF is a form of analysis that may introduces bias as it assumes missing values are random and participant’s response is stable from the point of dropout to trial completion, which is especially a complex issue for chronic, progressive diseases such as COPD (4). For example, Dahl et al. reports 68% (placebo) to 77% (indacaterol 300 mcg) of patients completing the trial (2). We were unable to find the denominator (N) for QOL outcome in the article, supplementary, United States Food and Drug Administration Approved Drug Products (Drugs@FDA) or European Medicines Agency (EMEA) report (5‐7). Dahl et al. reports for indacaterol 300 mcg N = 437 randomized to treatment, N = 338 completed the trial and N = 405 were analyzed for efficacy and for indacaterol 600 mcg N = 428 randomized to treatment, N = 326 completed the trial and N = 396 analyzed for efficacy (2). In the review by Geake et al., the N used for indacaterol 300 mcg is 323 and indacaterol 600 mcg is 307 (1). Where did these numbers come from?
1d) Furthermore, we were unable to find the odds ratio of the number of participants with a clinically significant improvement in QOL reported in by Geake et al. in analysis 1.5 and note the N reported for indacaterol 300 mcg is 322 and indacaterol 600 mcg is 305 which is different from analysis 1.3 (1).
1e) Analysis was not intention‐to‐treat (ITT) as the reported N for QOL outcome does not match those originally randomized and this can generate false results, specifically an exaggeration of effect size (4, 8).
1f) Regarding exacerbations, we again found inconsistent reporting throughout the review. For example, the abstract states, “data was insufficient for analysis of differences in exacerbation rates for both placebo and twice daily beta2‐agonist comparisons”, yet implications for practice states, "indacaterol reduces the chance of experiencing an exacerbation” (1).
1g) As noted above, both Dahl et al. and Donohue et al. utilized LOCF for missing data (2,3). For example, Donohue et al. reports 69% (placebo) to 82% (indacaterol 300 mcg) of patients randomized completing the trial (3). In addition, there is uncertainty in the reported numbers in Donohue et al. as the FDA reported numbers are different for the number of patients randomized in comparison to the published article (3, 6). Furthermore, sensitivity analysis with imputed data for patients who discontinued prematurely almost doubled rates of exacerbation per year from 0.50, 0.53, 0.53, and 0.72 to 0.95, 0.86, 0.93, and 1.33 respectively for indacaterol 150 mg, 300 mg, tiotropium, and placebo which demonstrates a wide range of variability (3). Please see concerns regarding potential bias introduced by use of LOCF and compromised ITT analysis stated above.
2a) We are concerned that not all studies included in the review contributed data to SAE and mortality outcomes. Geake et al. found ten studies that compared indacaterol to placebo, yet only nine studies contributed data on SAE or mortality pooled estimates (1). Although Mroz et al. was the smallest trial included in the review and SAE or mortality was not a pre‐specified outcome we feel that it would be very unlikely that data on these outcomes does not exist and there was no documentation of contact with authors (1,9).
2b) In addition, the missing data for these outcomes was not documented in the selective reporting section of the risk of bias tool, the results section or the discussion section.
2c) As the confidence intervals for these outcomes are wide it would be helpful to include all information available to improve the precision of the estimate.
3) The primary outcomes of the review should include exacerbations instead of the surrogate trough forced expiratory volume in one second (FEV1) in addition to QOL outcomes since exacerbation is a more clinically meaningful outcome compared to FEV1. In addition, section 5.4.1 in the Cochrane Handbook states, “surrogate outcome measures… are potentially misleading and should be avoided or interpreted with caution because they may not predict clinically important outcomes accurately” (10). Other COPD reviews, such as the Cochrane review of LABAs for COPD listed QOL and exacerbations as primary outcomes and trough FEV1 as a secondary outcome (11). Additionally, we, and perhaps other readers, would find it useful for authors to comment on the relationship between improvement in FEV1 to improvement in clinical outcomes. We were unsure of this relationship and largely found that although most COPD medications increase FEV1 they have not resulted in decreased mortality (1, 11‐13) and impact on QOL and exacerbations is difficult to discern due to attrition and analysis including LCOF and incomplete ITT as described by Stabler et al. for example (14).
4) The authors should consider searching national and international trial registers in addition to ClinicalTrials.gov for unpublished studies. We find both EMEA and FDA trial regulatory documents related to drug licensing useful as manufactures submit all published and unpublished data when requesting licensing in a that specific country. These documents are comprehensive as evidenced by Hart et al., which identified 299 unpublished outcomes when comparing FDA documents to published trial reports (15). While searching these regulatory documents, we found inconsistent reported numbers with the Donohue et al. trial as noted above which should be documented in the selective bias section of the risk of bias tool (3). The effect of including unpublished data must be measured for each outcome as important differences may be found for some outcomes but not others and the direction and magnitude of the effect cannot be predicted (15).
5) We, and perhaps other readers, would find it useful for authors to document which trial investigators or sponsors were contacted, for which questions, and the investigator or sponsors response as data available in the review, as noted above for Dahl et al., was not available in published article or supplementary (2). Section 6.6.2.2 of the Cochrane Handbook recommends to list individuals or organizations contacted (10).
6) We found inconsistencies in the assessment of selective reporting bias in the review. In the text section of the risk of bias in included studies only Kerwin et al. was noted as having a secondary outcomes incompletely reported and scored as “high risk"; yet, in the Donohue et al risk of bias tool stated that “days of poor control” was not reported yet scored as “unclear risk” (1). Why was this trial not treated the same as the Kerwin et al. trial? Furthermore, the Cochrane Handbook recommends interpreting unclear risk as high risk and should be included in the text section of the risk of bias in included trials (10).
7a) We, and perhaps other readers, would find it useful for authors to include information regarding dose‐response in the abstract of the review. The abstract states in the background section that, “Four different doses have been investigated (75 mcg, 150 mcg, 300 mcg and 600 mcg) (1). The relative effects of different doses of once‐daily indacaterol in the management of patients with COPD are uncertain”, but no information is provided in the main results or author’s conclusion section of what was found (1).
7b) Furthermore, it would be useful for authors to reference the following statement from the discussion, “although the incremental response with 300 mcg versus 150 mcg indacaterol has been reported in participants with more severe COPD” so that reader’s can access more information if they so wish (1).
8) We, and perhaps other readers, were confused with the author’s meaning of “durable” as this terminology is used in summarizing main results and in implications for practice for the sub‐group analysis of FEV1 and QOL outcomes but not defined in the methods (1). As noted above, we are uncertain of the clinical utility of FEV1 and the relevance of this analysis. However, we would suggest that when describing these sub‐group analyses to minimize confusion authors simply state the results and do not use the use the term “durable”.
9a) Regarding implications of practice, as per the Cochrane Handbook, we believe that making specific recommendations for an action goes beyond a systematic review. If authors wish to state, “indacaterol is therefore an appropriate treatment for patients with confirmed symptomatic stable COPD” they should refer to section 12.7.2 and first describe the quality of evidence, benefits versus risk tradeoff and patient’s values and preferences (1,10).
9b) Furthermore, “symptomatic” should not be used to describe the population that these results are generalizable to as this was not an explicit criterion for inclusion into the trial.
In summary, incorporation of the above recommendations will help authors provide reader’s with a more critical assessment of included studies and conclusions drawn.
References:
Geake JB, Dabscheck EJ, Wood‐Baker R, Cates CJ. Indacaterol, a once‐daily beta2‐agonist, versus twice‐daily beta2‐agonists or placebo for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2015, Issue 1. Art. No.: CD010139. DOI: 10.1002/14651858.CD010139.pub2.
Dahl R, Chung KF, Buhl R, Magnussen H, Nonikov V, Jack D, et al. Efficacy of a new once‐daily long‐acting inhaled beta2‐agonist indacaterol versus twice‐daily formoterol in COPD. Thorax 2010;65:473–9.
Donahue JF, Fogarty C, Lotvall J, Mahler D, Worth H, Yorgancioglu A, et al. Once‐daily bronchodilators for chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2010;182(2):155–62.
Molnar FJ, Hutton B, Fergusson D. Does analysis using “last observation carried forward” introduce bias in dementia research? CMAJ 2008;179(8):751‐3.
Dahl R, Chung KF, Buhl R, Magnussen H, Nonikov V, Jack D, et al. Efficacy of a new once‐daily long‐acting inhaled beta2‐agonist indacaterol versus twice‐daily formoterol in COPD: Online data repository. Thorax 2010;65:473–9.
Center for Drug Evaluation and Research. Clinical review: Arcapta Neohaler. FDA. February 14, 2011.
European Medicines Agency: Evaluation of Medicines for Human Use. Assessment report for Onbrez Breezhaler. London. December 17, 2009.
Abraha I, Cherubini A, Cozzolino F, De Florio R, Luchetta ML, Rimland JM, et al. Deviations from intention to treat analysis in randomized trials and treatment effect estimates: Meta‐epidemiological study. BMJ 2015;350:h2445.
Mroz R.M, Minarowski L, Chyczewska E. Indacaterol add‐ on therapy improves lung function, exercise capacity and life quality of COPD patients. Advances in Experimental Medicine and Biology 2013;756:23–8.
Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Kew KM, Mavergames C, Walters JAE. Long‐acting beta2‐agonists for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2013, Issue 10. Art. No.: CD010177. DOI: 10.1002/14651858.CD010177.pub2.
Nannini LJ, Lasserson TJ, Poole P. Combined corticosteroid and long‐acting beta(2)‐agonist in one inhaler versus long‐acting beta(2)‐agonists for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2012, Issue12. Art. No.: CD006829. doi: 10.1002/14651858.CD006829.pub2.
Karner C, Chong J, Poole P. Tiotropium versus placebo for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2014, Issue 7. Art. No.: CD009285. DOI: 10.1002/14651858.CD009285.pub3.
Stabler S, Tejani AM, Bruchet N. Not quite a breath of fresh air: Use of combination inhalers in COPD. Can Fam Physician 2012;58:149‐50.
Hart B, Lundh A, Bero L. Effect of reporting bias on meta‐analyses of drug trials: Reanalysis of meta‐analyses. BMJ 2011;344:d7202.
I agree with the conflict of interest statement below:
I certify that I have no affiliations with or involvement in any organization or entity with a financial interest in the subject matter of my feedback.
Reply
The review authors are grateful for the careful and considered critique and insightful comments contained within this commentary. We provide the following responses, including suggested changes to the manuscript as below.
1a) The sentence referred to in the implications for practice section relates to the paragraph discussing the twice‐daily beta agonist comparison (not the placebo comparison), and is therefore referenced specifically to this comparison. For clarification, we have reworded the abstract as follows: “For patients with stable COPD, use of indacaterol versus placebo results in statistically significant and clinically meaningful improvements in lung function and quality of life. The clinical benefit for lung function is at least as good as that seen with twice‐daily long‐acting beta2‐agonists, but the comparative effect on quality of life remains uncertain, as important differences cannot be excluded” and we have changed accordingly.
1b) We acknowledge the slight difference in outcomes between these two different analyses might cause some confusion. We believe that differences in overall quality of life might not have reached statistical significance across all populations studied. However, it is likely that a subgroup of patients will respond in a clinically meaningful way with regards to QOL with the intervention, and are more likely to do so than without. A more detailed discussion is available in the following reference: Cates C, Karner C. Clinical importance cannot be ruled out using mean difference alone. BMJ (Clinical research ed 2015;351(nov20 4):h5496‐h96 doi: 10.1136/bmj.h5496
1c) The denominator was provided by the manufacturer. The numbers analysed are slightly different to the entire population randomised as data were only carried forward a maximum of 12 weeks. This data can be provided upon request.
1d) This data was supplied by the manufacturer. We felt that small differences in participant numbers were unlikely to have substantial impact on the overall results.
1e) We agree with the reviewers. In the characteristics of included studies table we note that Dahl used a modified intention to treat analysis. Overall we felt the impact of this approach was unlikely to have had substantial impact on the outcome in question.
1f) We agree and have removed the statement on exacerbations from the abstract.
1g) The review authors understand that this sensitivity analysis uses the assumption that all those who dropped out of treatment did badly and all those who dropped out of placebo did well, which we do not regard as plausible. We do acknowledge there are issues with the data, and point again to the information contained within the characteristics of included studies table, as well as the risk of bias summary, which also highlights potential issues with the outcome data from these studies. However, these studies contributed significantly to the total numbers of participants available, and we feel that excluding them on the basis of the outcome data provided could potentially lead to greater bias in the outcomes.
2a) We did make contact with the study authors, and they did not provide any SAE or mortality data. We did not find this surprising as the numbers were very low and therefore the failure to observe any SAEs/mortality would not have been unexpected, and in any case they had not planned a priori to collect this data.
2b) We did not regard this as selective reporting for the reasons mentioned above.
2c) The precision of this estimate will ultimately be very low irrespective of any further information/clarification as participant numbers are extremely low.
3) We agree with your comments regarding the use of surrogate measures as primary outcomes. We had planned to measure exacerbation rates and include this as a primary outcome, for exactly the reasons you outlined. Unfortunately, as we reported in our review, there were insufficient data to measure exacerbation rates. Therefore the number of patients experiencing at least one protocol defined exacerbation was included as a post‐hoc analysis instead. Given this was a post‐hoc analysis, we felt it most appropriate to include as a secondary outcome. We retained trough FEV1 as a primary outcome to maintain fidelity with our protocol, and because, correctly or incorrectly, this outcome remains an important consideration for most pharmaceutical regulatory bodies around the world. Therefore, it is likely to be of interest to an audience that includes persons within the health care policy and public health domains. We agree that whilst an increase of 100ml in trough FEV1 is widely considered to represent a minimum clinically important difference, as stated in our review, the correlation between improvements in FEV1 and clinical outcomes is relatively modest.
4) As we reported in the review “We also conducted a search of ClinicalTrials.gov (www.clinicaltrials.gov) and of the Novartis clinical trials registry (www.novctrd.com)”. We acknowledge that manufacturers may submit additional data in licensing applications to that published in the medical literature, but note that this does not necessarily improve the clarity of the data.
5) We state that study sponsors were contacted to provide missing data, but can specify that Novartis and the authors of the Mroz study provided missing data. This has now been specified in the review.
6) It was unclear to the authors as to whether this would have introduced significant bias specifically to the review, as “days of poor control” was not an outcome that we had ever planned to analyse.
7a) We provide statements identifying that there were similar results seen across all doses of indacaterol studied. These can be found in the summary of main results section, as well as in the implications for practice section. Additionally forest plots allow for visual inspection of the same, and the similar numerical changes are provided in the results section. We did not consider this information to be of sufficient importance to be included in the abstract.
7b) We have slightly amended this text and provided references to assist the reader in accessing further relevant information in this area.
8) The review authors agree and have removed or replaced the term ‘durable’ with “sustained” where relevant.
9a) The review authors disagree that measured statements regarding the potential use of a pharmaceutical is beyond the remit of a systematic review that is specifically setting out to assess efficacy and safety of that pharmaceutical, and would argue that clinicians considering whether to prescribe indacaterol are furnished with enough information within the review to assist them in making a clinical decision (that always involves a risk‐benefit analysis) for any individual patient.
9b) We agree with the premise of this statement. However, given a significant component of the overall goal of treatment with indacaterol relies upon symptomatic improvement (dyspnoea, and by proxy quality of life) we felt it important to emphasize that treatment should be directed to those patients in whom there were symptoms to ameliorate, and not anyone with spirometrically confirmed COPD. Further, we feel it very likely that patients with COPD exacerbations are very likely to be symptomatic between exacerbations.
In summary, and as stated previously, we are grateful for this detailed commentary, and believe the changes made as a result will help strengthen the review.
Contributors
Marlys LeBras and Aaron Tejani, email contact: marlys.lebras@gmail.com
James B Geake, first author of the review
Discrepancies between my view of the data, 28 January 2017
Summary
Comment: Written by: Vijaya Musini
Cochrane reviews need to be transparent and readers should be able to verify data independently from published literature.
a) For QoL score: analysis 1.3 (N = 2505 at 150 mcg dose) and analysis 1.5 (N = 2,661) ‐ How can 156 more patients achieve improvement when SGRQ score is not available for these patients? Similar error at 300 mcg (N=1438 versus 1536, respectively); 98 more patients evaluated. Data entry for Donohue 2010 should be N = 173 for MD and 319 for four point difference in SGRQ score.
b) Similar error at 600 mg for analysis 1.7 (N=582) and for analysis 1.8 (N=439). Why are 143 patients missing and why is their data not accounted for?
c) Why were analyses 1.3 and 1.4 conducted using mean difference with SEM yet analyses 1.5 and 1.6 conducted using log odds ratio?
d) Data on number of patients with ≥ 1 exacerbation: it is not clear how patients who withdrew from studies were accounted for in the analysis.
e) The clinically important outcome of 'patients with ≥ 1 moderate to severe exacerbation' is not reported in your review.
f) I disagree with your risk of bias assessments: I have judged high risk of: selection bias; performance bias, as there is a significant increase in total withdrawals and differential loss of patients in the placebo group due to lack of therapeutic effect leading to possible loss of blinding which was not tested or reported at the end of the study; attrition bias, as accounting of data in patients who withdrew is not reported; selective outcome reporting, as the manufacturer does not provide available data in published literature; and funding bias as all trials were conducted by the manufacturer and employees of the company are involved in the writing of the manuscript.
g) Data entry errors: mortality data (Feldman 2010) incidence decreased from 211 to 104 in the 150 mcg group and from 205 to 96 in the placebo group.
h) SoF table 1: Serious adverse events outcomes (N = 8122) is an error. These exceed total randomized patients in all included studies.
I have completed a systematic review of indacaterol versus placebo that includes the risk of bias figures, forest plots and summary of findings table for your reference (http://www.ti.ubc.ca/letter102).
I agree with the conflict of interest statement below:
I certify that I have no affiliations with or involvement in any organization or entity with a financial interest in the subject matter of my feedback.
Reply
In response to this feedback we have made the following changes and comments. The data have been carefully reviewed by the entire author group and small numerical errors in data entry have been corrected. The changes have not resulted in any statistically significant, nor any clinically meaningful changes to any of the results.
a) Thank you for pointing this out. This discrepancy was the result of incorrect data entry which has now been corrected. Happily it has not resulted in any numerically or clinically significant changes to the final results. b) Thank you for pointing out this small error which reflected incorrect data entry. This has been corrected and results in no clinically or statistically significant changes to the conclusions of the review.
c) We believe the statistical analyses used to measure these outcomes are appropriate and in keeping with the Cochrane methodology. Analyses 1.3 and 1.4 compare mean differences in outcomes, whereas 1.5 and 1.6 compare relative rates of a discrete clinical event. d) The methods of analysis have been described in characteristics of studies table, which were for the majority of studies intention to treat or modified intention to treat analyses. Patients were not censored from this analysis if they withdrew. e) We agree that this would be an interesting analysis. We did not set out a priori to assess this and therefore have not performed the analysis in our own review. As is specifically outlined in the "Differences between protocol and review" section we did aim to analyse rates of exacerbations and exacerbations requiring hospitalisation as an important primary outcome (which we believe would be a more appropriate statistical analysis for this outcome), but there were insufficient data to do so. Therfore we analysed the number of patients experiencing at least one exacerbation and this was relegated to a secondary outcome.
Having said all this the definition of an exacerbation varies, as does the grading of severity. Often it is the baseline physiological fragility rather than the degree of homeostatic disturbance that defines a patient's presentation to acute tertiary care and therefore the severity grading. It is clear that exacerbations treated on an ambulatory basis are also associated with, and likely at least in part responsible for decrements in quality of life and lung function. Therefore we believe that an overall measurement of exacerbations is a clinically relevant outcome. f) There is of course an element of subjectivity in grading bias, even when done within a formal framework. We have addressed particular biases that you describe in the risk of bias tables and in the "Potential biases in the review process" section, and we believe our overall assessments of risk of bias are satisfactory. Please note also with particular respect to funding bias that we are operating within the guidance of the current Cochrane methodology and recommendations which do not recognise this as a core bias. g) Thank you for pointing this out. This discrepancy was the result of incorrect data entry which has now been corrected. Happily it has not resulted in any numerically or clinically significant changes to the final results. h) Thank you ‐ this has been corrected.
Contributors
Dr. Vijaya Musini
Email Address: vijaya.musini@ti.ubc.ca
Assistant Professor, Department of Anesthesiology Pharmacology & Therapeutics, University of British Columbia, Vancouver BC, Canada.
What's new
| Date | Event | Description |
|---|---|---|
| 1 March 2017 | Feedback has been incorporated | Feedback has been received regarding some numerical discrepancies in participant numbers within the analyses of some trials. The data have been carefully reviewed by the entire author group and small numerical errors in data entry have been corrected. The changes have not resulted in any statistically significant, nor any clinically meaningful changes to any of the results. |
History
Protocol first published: Issue 10, 2012 Review first published: Issue 1, 2015
| Date | Event | Description |
|---|---|---|
| 27 April 2016 | Feedback has been incorporated | A Feedback comment and response from the review authors have been added to the Feedback section of this review. Edits have been made in response to the comments on the review including minor edits to the abstract and Plain Language Summary, 'Dealing with missing data', Results section, Discussion section, Conclusions, Characteristics of included studies and Data and analyses sections. None of these changes affects the original conclusions of the review. |
Acknowledgements
We are very grateful to Ms Liz Stovold, Trials Search Co‐ordinator/Information Specialist, for designing the search strategy.
We are very grateful to Dr Emma Welsh, Managing Editor of the Cochrane Airways Group, for advice provided regarding preparation of the manuscript.
We are very grateful to Ms Robyn Dearsley for assistance provided with manuscript style and editing.
Milo Puhan was the editor of this review and commented critically on the review and assisted with signing off the review for publication.
Appendices
Appendix 1. Sources and search methods for the Cochrane Airways Group Specialised Register (CAGR)
Electronic searches: core databases
| Database | Frequency of search |
| CENTRAL | Monthly |
| MEDLINE (Ovid) | Weekly |
| EMBASE (Ovid) | Weekly |
| PsycINFO (Ovid) | Monthly |
| CINAHL (EBSCO) | Monthly |
| AMED (EBSCO) | Monthly |
Handsearches: core respiratory conference abstracts
| Conference | Years searched |
| American Academy of Allergy, Asthma and Immunology (AAAAI) | 2001 onwards |
| American Thoracic Society (ATS) | 2001 onwards |
| Asia Pacific Society of Respirology (APSR) | 2004 onwards |
| British Thoracic Society Winter Meeting (BTS) | 2000 onwards |
| Chest Meeting | 2003 onwards |
| European Respiratory Society (ERS) | 1992, 1994, 2000 onwards |
| International Primary Care Respiratory Group Congress (IPCRG) | 2002 onwards |
| Thoracic Society of Australia and New Zealand (TSANZ) | 1999 onwards |
MEDLINE search strategy used to identify trials for the CAGR
COPD search
1. Lung Diseases, Obstructive/
2. exp Pulmonary Disease, Chronic Obstructive/
3. emphysema$.mp.
4. (chronic$ adj3 bronchiti$).mp.
5. (obstruct$ adj3 (pulmonary or lung$ or airway$ or airflow$ or bronch$ or respirat$)).mp.
6. COPD.mp.
7. COAD.mp.
8. COBD.mp.
9. AECB.mp.
10. or/1‐9
Filter to identify RCTs
1. exp "clinical trial [publication type]"/
2. (randomised or randomised).ab,ti.
3. placebo.ab,ti.
4. dt.fs.
5. randomly.ab,ti.
6. trial.ab,ti.
7. groups.ab,ti.
8. or/1‐7
9. Animals/
10. Humans/
11. 9 not (9 and 10)
12. 8 not 11
The MEDLINE strategy and the RCT filter are adapted to identify trials in other electronic databases.
Data and analyses
Comparison 1. Indacaterol vs placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Trough FEV1 (by dose) | 10 | 5001 | Mean Difference (Fixed, 95% CI) | 149.11 [137.09, 161.12] |
| 1.1 Indacaterol 75 mcg | 2 | 594 | Mean Difference (Fixed, 95% CI) | 130.0 [101.72, 158.28] |
| 1.2 Indacaterol 150 mcg | 6 | 2521 | Mean Difference (Fixed, 95% CI) | 146.52 [129.94, 163.11] |
| 1.3 Indacaterol 300 mcg | 5 | 1438 | Mean Difference (Fixed, 95% CI) | 169.27 [144.52, 194.02] |
| 1.4 Indacaterol 600 mcg | 1 | 448 | Mean Difference (Fixed, 95% CI) | 150.0 [100.62, 199.38] |
| 2 Trough FEV1 (by trial duration) | 10 | 4993 | Mean Difference (Fixed, 95% CI) | 149.16 [137.06, 161.26] |
| 2.1 Trials < 24 weeks | 5 | 1340 | Mean Difference (Fixed, 95% CI) | 148.99 [129.11, 168.86] |
| 2.2 Trials ≥ 24 weeks | 5 | 3653 | Mean Difference (Fixed, 95% CI) | 149.26 [134.01, 164.51] |
| 3 Quality of life (by dose) | 10 | 4938 | Mean Difference (Fixed, 95% CI) | ‐3.60 [‐4.36, ‐2.83] |
| 3.1 Indacaterol 75 mcg | 2 | 583 | Mean Difference (Fixed, 95% CI) | ‐3.70 [‐5.66, ‐1.74] |
| 3.2 Indacaterol 150 mcg | 6 | 2502 | Mean Difference (Fixed, 95% CI) | ‐3.43 [‐4.53, ‐2.32] |
| 3.3 Indacaterol 300 mcg | 5 | 1408 | Mean Difference (Fixed, 95% CI) | ‐3.49 [‐4.94, ‐2.03] |
| 3.4 Indacaterol 600 mcg | 1 | 445 | Mean Difference (Fixed, 95% CI) | ‐4.6 [‐7.07, ‐2.13] |
| 4 Quality of lIfe (by trial duration) | 10 | 4975 | Mean Difference (Fixed, 95% CI) | ‐3.44 [‐4.25, ‐2.63] |
| 4.1 Trials < 24 weeks | 5 | 1329 | Mean Difference (Fixed, 95% CI) | ‐4.11 [‐5.60, ‐2.62] |
| 4.2 Trials ≥ 24 weeks | 5 | 3646 | Mean Difference (Fixed, 95% CI) | ‐3.15 [‐4.12, ‐2.19] |
| 5 Number of participants with a clinically significant improvement in quality of life (by dose) | 9 | 4906 | Odds Ratio (Fixed, 95% CI) | 1.64 [1.46, 1.85] |
| 5.1 Indacaterol 75 mcg | 2 | 583 | Odds Ratio (Fixed, 95% CI) | 1.73 [1.24, 2.41] |
| 5.2 Indacaterol 150 mcg | 6 | 2502 | Odds Ratio (Fixed, 95% CI) | 1.67 [1.41, 1.98] |
| 5.3 Indcaterol 300 mcg | 4 | 1376 | Odds Ratio (Fixed, 95% CI) | 1.46 [1.15, 1.85] |
| 5.4 Indacaterol 600 mcg | 1 | 445 | Odds Ratio (Fixed, 95% CI) | 1.95 [1.30, 2.94] |
| 6 Number of participants with a clinically significant improvement in quality of life (by trial duration) | 9 | 4972 | Odds Ratio (Fixed, 95% CI) | 1.56 [1.38, 1.76] |
| 6.1 Trials < 24 weeks' duration | 4 | 1284 | Odds Ratio (Fixed, 95% CI) | 1.90 [1.51, 2.38] |
| 6.2 Trials ≥ 24 weeks' duration | 5 | 3688 | Odds Ratio (Fixed, 95% CI) | 1.45 [1.26, 1.67] |
| 7 End‐of‐study dyspnoea (by dose) | 8 | 4577 | Mean Difference (Fixed, 95% CI) | 1.00 [0.82, 1.17] |
| 7.1 Indacaterol 300 mcg | 4 | 1403 | Mean Difference (Fixed, 95% CI) | 1.13 [0.83, 1.43] |
| 7.2 Indacaterol 75 mcg | 2 | 597 | Mean Difference (Fixed, 95% CI) | 0.77 [0.27, 1.27] |
| 7.3 Indacaterol 150 mcg | 5 | 2138 | Mean Difference (Fixed, 95% CI) | 0.96 [0.70, 1.22] |
| 7.4 Indacaterol 600 mcg | 1 | 439 | Mean Difference (Fixed, 95% CI) | 0.98 [0.51, 1.45] |
| 8 Number of participants experiencing a clinically significant improvement in dyspnoea | 8 | 4577 | Odds Ratio (Fixed, 95% CI) | 1.96 [1.73, 2.22] |
| 8.1 Indacaterol 75 mcg | 2 | 597 | Odds Ratio (Fixed, 95% CI) | 1.76 [1.26, 2.45] |
| 8.2 Indacaterol 150 mcg | 5 | 2138 | Odds Ratio (Fixed, 95% CI) | 1.87 [1.56, 2.24] |
| 8.3 Indacaterol 300 mcg | 4 | 1403 | Odds Ratio (Fixed, 95% CI) | 2.25 [1.81, 2.81] |
| 8.4 Indacaterol 600 mcg | 1 | 439 | Odds Ratio (Fixed, 95% CI) | 1.80 [1.20, 2.70] |
| 9 Peak FEV1 | 6 | 1657 | Mean Difference (IV, Fixed, 95% CI) | 181.21 [129.10, 233.32] |
| 9.1 Indacaterol 75 mcg | 2 | 545 | Mean Difference (IV, Fixed, 95% CI) | 196.56 [107.15, 285.98] |
| 9.2 Indacaterol 150 mcg | 3 | 601 | Mean Difference (IV, Fixed, 95% CI) | 200.91 [111.71, 290.12] |
| 9.3 Indacaterol 300 mcg | 3 | 383 | Mean Difference (IV, Fixed, 95% CI) | 173.50 [69.92, 277.09] |
| 9.4 Indacterol 600 mcg | 1 | 128 | Mean Difference (IV, Fixed, 95% CI) | 30.0 [‐172.77, 232.77] |
| 10 Serious adverse events | 9 | 6065 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.82, 1.23] |
| 10.1 Indacaterol 75 mcg | 2 | 641 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.24, 1.46] |
| 10.2 Indacaterol 150 mcg | 6 | 2958 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.75, 1.37] |
| 10.3 Indacaterol 300 mcg | 4 | 1825 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.74, 1.45] |
| 10.4 Indacaterol 600 mcg | 1 | 641 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.65, 1.83] |
| 11 Mortality | 9 | 5694 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.16, 1.08] |
| 11.1 Indacaterol 75 mcg | 2 | 641 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.01, 4.07] |
| 11.2 Indacaterol 150 mcg | 5 | 2586 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.23, 3.16] |
| 11.3 Indacaterol 300 mcg | 4 | 1826 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.02, 2.72] |
| 11.4 Indacaterol 600 mcg | 1 | 641 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.00, 2.11] |
| 12 Number of participants experiencing at least 1 protocol‐defined exacerbation | 7 | 4807 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.70, 0.94] |
| 12.1 75 mcg | 2 | 638 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.49, 1.45] |
| 12.2 150 mcg | 4 | 2170 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.66, 1.02] |
| 12.3 300 mcg | 3 | 1403 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.65, 1.09] |
| 12.4 600 mcg | 1 | 596 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.51, 1.06] |
Comparison 2. Indacaterol vs LABAs.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Trough FEV1 (by dose) | 4 | 2708 | Mean Difference (Fixed, 95% CI) | 73.76 [57.33, 90.19] |
| 1.1 Indacaterol 150 mcg | 2 | 1625 | Mean Difference (Fixed, 95% CI) | 62.56 [42.71, 82.40] |
| 1.2 Indcaterol 300 mcg | 2 | 625 | Mean Difference (Fixed, 95% CI) | 97.17 [60.51, 133.83] |
| 1.3 Indacaterol 600 mcg | 1 | 458 | Mean Difference (Fixed, 95% CI) | 100.0 [51.21, 148.79] |
| 2 Trough FEV1 (by trial duration) | 4 | 2708 | Mean Difference (Fixed, 95% CI) | 94.93 [79.58, 110.28] |
| 2.1 Trials < 24 weeks | 1 | 1034 | Mean Difference (Fixed, 95% CI) | 60.0 [37.00, 83.00] |
| 2.2 Trials ≥ 24 weeks | 3 | 1674 | Mean Difference (Fixed, 95% CI) | 122.98 [102.37, 143.59] |
| 3 Quality of life (by dose) | 2 | 1523 | Mean Difference (Fixed, 95% CI) | ‐0.81 [‐2.28, 0.66] |
| 3.1 Indacaterol 150 mcg | 1 | 591 | Mean Difference (Fixed, 95% CI) | ‐1.2 [‐3.42, 1.02] |
| 3.2 Indacaterol 300 mcg | 1 | 474 | Mean Difference (Fixed, 95% CI) | ‐0.5 [‐3.27, 2.27] |
| 3.3 Indacaterol 600 mcg | 1 | 458 | Mean Difference (Fixed, 95% CI) | ‐0.5 [‐3.27, 2.27] |
| 4 Quality of lIfe (by trial duration) | 2 | 1523 | Mean Difference (Fixed, 95% CI) | 0.42 [‐1.21, 2.05] |
| 4.1 Trials ≥ 24 weeks | 2 | 1523 | Mean Difference (Fixed, 95% CI) | 0.42 [‐1.21, 2.05] |
| 5 Number of participants with a clinically significant improvement in quality of life (by dose) | 2 | 1520 | Odds Ratio (Fixed, 95% CI) | 1.07 [0.87, 1.32] |
| 5.1 Indacaterol 150 mcg | 1 | 591 | Odds Ratio (Fixed, 95% CI) | 1.17 [0.85, 1.61] |
| 5.2 Indacaterol 300 mcg | 1 | 473 | Odds Ratio (Fixed, 95% CI) | 0.93 [0.63, 1.37] |
| 5.3 Indacaterol 600 mcg | 1 | 456 | Odds Ratio (Fixed, 95% CI) | 1.09 [0.74, 1.61] |
| 6 Number of participants with a clinically significant improvement in quality of life (by trial duration) | 2 | 1520 | Odds Ratio (Fixed, 95% CI) | 1.06 [0.86, 1.31] |
| 6.1 Trials ≥ 24 weeks | 2 | 1520 | Odds Ratio (Fixed, 95% CI) | 1.06 [0.86, 1.31] |
| 7 Dyspnoea (by dose) | 3 | 2404 | Mean Difference (Fixed, 95% CI) | 0.54 [0.30, 0.79] |
| 7.1 Indcaterol 150 mcg | 2 | 1485 | Mean Difference (Fixed, 95% CI) | 0.66 [0.37, 0.95] |
| 7.2 Indacaterol 300 mcg | 1 | 468 | Mean Difference (Fixed, 95% CI) | 0.19 [‐0.46, 0.84] |
| 7.3 Indacaterol 600 mcg | 1 | 451 | Mean Difference (Fixed, 95% CI) | 0.3 [‐0.35, 0.95] |
| 8 Number of participants experiencing a clinically significant improvement in dyspnoea | 3 | 2536 | Odds Ratio (Fixed, 95% CI) | 1.11 [0.94, 1.32] |
| 8.1 Indacaterol 150 mcg | 2 | 1620 | Odds Ratio (Fixed, 95% CI) | 1.21 [0.98, 1.50] |
| 8.2 Indacaterol 300 mcg | 1 | 467 | Odds Ratio (Fixed, 95% CI) | 0.87 [0.59, 1.29] |
| 8.3 Indacaterol 600 mcg | 1 | 449 | Odds Ratio (Fixed, 95% CI) | 1.06 [0.72, 1.58] |
| 9 Peak FEV1 [mL] | 2 | 491 | Mean Difference (IV, Fixed, 95% CI) | 4.68 [‐93.79, 103.16] |
| 9.1 indacaterol 150 mcg | 1 | 220 | Mean Difference (IV, Fixed, 95% CI) | 40.0 [‐113.72, 193.72] |
| 9.2 indacaterol 300 mcg | 1 | 139 | Mean Difference (IV, Fixed, 95% CI) | ‐30.0 [‐212.29, 152.29] |
| 9.3 indacaterol 600 mcg | 1 | 132 | Mean Difference (IV, Fixed, 95% CI) | ‐10.0 [‐190.45, 170.45] |
| 10 Serious adverse events | 4 | 3266 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.79, 1.32] |
| 10.1 indacaterol 150 mcg | 2 | 1784 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.92, 2.25] |
| 10.2 indacaterol 300 mcg | 2 | 840 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.66, 1.52] |
| 10.3 indacaterol 600 mcg | 1 | 642 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.45, 1.13] |
| 11 Mortality | 4 | 3266 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.31, 3.28] |
| 11.1 indacaterol 150 mcg | 2 | 1784 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.35 [0.35, 15.98] |
| 11.2 indacaterol 300 mcg | 2 | 840 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.11, 6.27] |
| 11.3 indacaterol 600 mcg | 1 | 642 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.01, 4.18] |
| 12 Number of participants experiencing at least 1 protocol‐defined exacerbation | 2 | 1869 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.84, 1.29] |
| 12.1 indacaterol 150 mcg | 1 | 668 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.80, 1.82] |
| 12.2 indacaterol 300 mcg | 1 | 605 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.74, 1.53] |
| 12.3 indacaterol 600 mcg | 1 | 596 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.62, 1.30] |
2.4. Analysis.
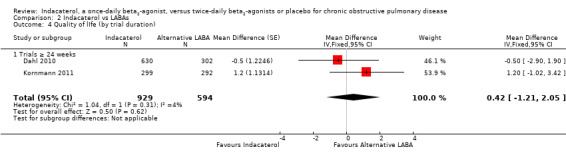
Comparison 2 Indacaterol vs LABAs, Outcome 4 Quality of lIfe (by trial duration).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bateman 2013.
| Methods |
Design: multi‐centre, randomised, double‐blind, parallel‐group, placebo‐controlled trial. 26‐week duration. Additional bronchodilators other than albuterol were discontinued. Inhaled corticosteroids were continued at the same dose. Efficacy outcomes were analysed on an intention‐to‐treat basis. Safety outcomes were analysed according to the treatment received. Patients were recruited from research centres in Europe, North America, South America, Asia, Australia, China, Taiwan and South Africa Run‐in:14 days |
|
| Participants |
Population: 2144 participants with stable moderate to severe COPD by GOLD criteria were randomly assigned (QVA149 110/50 (glycopyrronium/indacaterol) 475, indacaterol 477, glycopyrronium 475, tiotropium 483, placebo 234). Predominantly male population (75.4%). Mean age of 64 years. Predominantly Caucasian and Asian population. Most participants had moderate COPD by GOLD criteria. QVA149, indacaterol, glycopyrronium, tiotropium and placebo arms (57.8%, 56.5%, 57.9%, 58.8% and 57.8%) were taking concomitant inhaled corticosteroids respectively. Mean FEV1 was 54% to 55% predicted across all experimental arms Inclusion criteria: adults > 39 years with stable GOLD stage 2 or 3 (by 2008 criteria) COPD and at least a 10‐pack‐year smoking history, postbronchodilator FEV1/FVC < 70% (400 mcg salbutamol), FEV1 < 80% but >29% Exclusion criteria: pregnant women or women of childbearing potential, history of medication intolerance to any of the classes of trial medications, history of long QT syndrome or QTc > 450 seconds or other clinically significant ECG abnormalities, uncontrolled diabetes, narrow‐angle glaucoma, prostatic hyperplasia, bladder neck obstruction or moderate to severe chronic kidney disease, malignancy within previous 5 years, requirement for long‐term oxygen therapy, exacerbation within the previous 6 weeks or lower respiratory tract infection within 4 weeks, previous lung surgery, history of asthma, active participation in pulmonary rehabilitation |
|
| Interventions | 1. Indacaterol/Glycopyrronium (QVA149) 110/50 mcg 2. Indacaterol 150 mcg 3. Glycopyrronium 50 mcg 4. Open‐label tiotropium 18 mcg 5. Placebo |
|
| Outcomes | Primary outcome: trough FEV1 at 26 weeks for QVA 149 vs its mono components Secondary outcomes: QVA149, indacaterol and glycopyrronium versus placebo, 26‐week TDI and SGRQ scores, rescue medication use, health status, participant symptoms, safety and tolerability, cardiovascular safety, other lung function endpoints |
|
| Notes | Study funded by Novartis and Novartis employees contributed to manuscript preparation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation via interactive response technology (IRT) |
| Allocation concealment (selection bias) | Low risk | IRT linked the participant to a treatment arm with a unique medication number for the study drug. Randomisation data remained strictly confidential and inaccessible to anyone involved in the study until the time of unbinding |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identity of treatments was concealed by identical packaging, labelling, schedule of administration, appearance, taste and colour. Tiotropium was open‐label. Bioanalysts of pharmacokinetic samples were unblinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were blinded to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Rates and reasons for dropouts were clearly reported. Higher rate of dropout from the placebo arm than from other treatment arms was due to protocol deviation, consent withdrawal and unsatisfactory therapeutic effect |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcomes were reported |
Dahl 2010.
| Methods |
Design: double‐blind, parallel, randomised, controlled trial. 52 weeks' duration. Additional inhaled bronchodilators other than albuterol discontinued. Modified intention‐to‐treat analysis performed. Origin of participants not stated Run‐in: 2 weeks |
|
| Participants |
Population: 1732 participants with a diagnosis of moderate to severe COPD were randomly assigned. Mean age was 63 years. Recruitment was predominantly from Europe, Russia and the UK. In the indacaterol 300 mcg, indacaterol 600 mcg, formoterol, and placebo arms, 55.6%, 53.2%, 50.9% and 51.9% of participants were taking concomitant ICS, respectively. Mean FEV1 was between 50% and 52% predicted in all arms of the study Inclusion criteria: age > 40, smoking history > 20 pack‐years, FEV1/FVC < 0.7, postbronchodilator FEV1 30% to 80% predicted Exclusion criteria: respiratory tract infection or hospitalisation in previous 6 weeks, oral corticosteroids or change in ICS in previous month, diagnosis of asthma |
|
| Interventions | 1. Indacaterol 300 mcg 2. Indacaterol 600 mcg 3. Formoterol 12 mg 4. Placebo |
|
| Outcomes |
Primary endpoint: 24‐hour postdose trough FEV1 after 12 weeks, active medication compared with placebo. Other outcomes: Transitional Dyspnoea Index (TDI), use of as needed salbutamol, St George Respiratory Questionnaire (SGRQ), BODE index (body mass index, obstruction, dyspnoea, exercise), safety and tolerability Follow‐up on days 1, 2, 15, 29, 84, 85, 113, 168, 197, 253, 364 and 365 Values reported at baseline and at weeks 12 and 52 |
|
| Notes | Study was supported by Novartis, and some authors were Novartis employees. Novartis directly supplied data for: Indacaterol versus placebo (trough FEV1, quality of life, dyspnoea, peak FEV1, number of participants experiencing at least one exacerbation, and mortality); Indacaterol versus LABA (trough FEV1, quality of life, dyspnoea, peak FEV1, number of participants experiencing at least one exacerbation). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly allocated to treatment using an automated interactive system |
| Allocation concealment (selection bias) | Low risk | Allocation via automated interactive system, with both participants and investigators blinded to allocation; double‐dummy technique |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded trial |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were blinded to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Reasons for dropout across treatment and control arms reported. Higher dropout rate in placebo arm. Efficacy data from 6 sites excluded on the basis of "non‐conformance with good clinical practice" |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported, not just statistically significant outcomes |
Donohue 2010.
| Methods |
Design: 26‐week, randomised, double‐blind, placebo‐controlled trial (with open‐label tiotropium). Adaptive seamless extension of 2‐week dose finding study, with 150 mcg and 300 mcg indacaterol doses selected from 4 possible indacaterol doses (75 mcg, 150 mcg, 300 mcg, 600 mcg). Intention‐to‐treat analysis. Spirometry, quality of life and dyspnoea data analysed at 12 weeks Run‐in: no run‐in. Continuation of a 2‐week dose‐finding trial via adaptive seamless design methodology. Participants randomly assigned to indacaterol, tiotropium or placebo continued for a further 26 weeks with additional participants recruited |
|
| Participants |
Population: 1250 participants randomly assigned to indacaterol or placebo (416 to indacaterol 150 mcg, 416 to indacaterol 300 mcg, 418 to placebo). Participants were recruited from the United States, Europe, the Middle East, Asia, India and the UK. Mean age was 63 years. Of the indacaterol 150 mcg, indacaterol 300 mcg and placebo populations, 38.2%, 37.3% and 38.5%, respectively, were receiving concurrent ICS. Mean FEV1 was between 53% and 56% predicted in all arms Inclusion criteria: patients 40 years of age or older with 20‐pack‐year or longer smoking history, FEV1/FVC < 70%, FEV1 < 80% and > 29% Exclusion criteria: asthma, hospitalisation with COPD exacerbation or lower respiratory tract infection within previous 6 weeks, requirement for long‐term oxygen therapy, concomitant pulmonary disease, diabetes, active malignancy, history of long QT syndrome or prolonged QTc, hypersensitivity to study drugs and drugs related to study drugs, recent administration of live attenuated vaccine, history of poor medication adherence, inability to use a dry powder inhaler |
|
| Interventions | 1. Indacaterol 150 mcg 2. Indacaterol 300 mcg 3. Placebo 4. Tiotropium 18 mcg |
|
| Outcomes |
Primary outcome: trough FEV1 at 12 weeks Secondary outcomes: 'days of poor control,' Transitional Dyspnoea Index, SGRQ, non‐inferiority (of at least 1 dose of indacaterol) to tiotropium, adverse events |
|
| Notes | Trial supported by Novartis. Novartis directly supplied data for Indacaterol versus placebo (peak FEV1, number of participants experiencing at least one exacerbation) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Automated interactive voice response system |
| Allocation concealment (selection bias) | Low risk | Allocation via interactive voice system. The only information communicated with sponsor and investigators was the selected doses. Personnel involved in the study remained blinded for the remainder of the study. Tiotropium arm was open label |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Sponsor, investigators and participants remained blinded until the study database was locked. An independent dose selection committee had access to unblinded data at the end of stage 1 but communicated to sponsor and investigators only the chosen doses for stage 2 All participants received medication via single‐dose dry powder inhaler |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Data were analysed by a separate body (Datamap GmbH, funded by Novartis); treatment decodes were received only by programmers and statisticians after stage 2 database was locked |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Completion rates were reported for each arm but reasons for dropout were not further specified. Higher dropout rates were reported from the placebo arm |
| Selective reporting (reporting bias) | Unclear risk | Low risk for primary outcomes Subjective secondary safety outcomes were not prespecified. 'Days of poor control' (a key secondary outcome) was not reported on |
Feldman 2010.
| Methods |
Design: 12‐week, multi‐centre, double‐blind, placebo‐controlled, parallel‐group, randomised controlled trial. Participants recruited from the United States. Additional inhaled bronchodilators other than albuterol discontinued. Run‐in: 14 days |
|
| Participants |
Population: 416 participants. Mean age 63 years. 28.9% and 34.1% of participants were taking concomitant ICS in the indacaterol and placebo arms, respectively, and mean FEV1 was 54.4% and 55.8%, respectively. Recruitment from the United States Inclusion criteria: adults > 40 years with COPD and at least a 20‐pack‐year smoking history Postbronchodilator FEV1/FVC < 70% (400 mcg salbutamol) FEV1 < 80% but > 29% Exclusion criteria: lower respiratory tract infection or hospitalisation with acute exacerbation of COPD within previous 6 weeks, asthma, any alternative significant cardiovascular or respiratory disease, type 1 or poorly controlled type 2 diabetes, history of long QT syndrome or prolonged QTc |
|
| Interventions | 1. Indacaterol 150 mcg 2. Placebo |
|
| Outcomes |
Primary outcome: trough FEV1 at 12 weeks Secondary outcomes: trough FEV1 after 1 dose and at day 29, individual time point FEV1 on day 1 and week 12, peak FEV1 on day 1 and week 12, standardised AUC FEV1 between 5 minutes and 4 hours, 5 minutes and 1 hour, 1 hour and 4 hours at week 12 Other outcomes: diary‐recorded symptoms Unspecified outcomes: SGRQ scores were not a prespecified outcome but were recorded and provided by Novartis |
|
| Notes | Trial sponsored by Novartis, which was also involved in preparation and review of the manuscript. Novartis directly supplied data for: trough FEV1, quality of life, peak FEV1. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation was not explicitly specified: "eligible patients were randomised using validated systems" |
| Allocation concealment (selection bias) | Low risk | Method of allocation concealment was not specified, although all study drugs were identical in appearance and administration schedule |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Excluding participant emergencies, participants, investigators, clinical staff performing assessments and data analysts and sponsors trial team; all were blinded from randomisation to database lock |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Data analysts were blinded until database lock |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Rates and reasons for attrition were clearly reported: similar between groups |
| Selective reporting (reporting bias) | High risk | All prespecified outcomes were reported. SGRQ scores were recorded and provided by Novartis, although they were not a specified outcome in the manuscript |
Izbicki 2014.
| Methods |
Design: 12‐week multi‐centre, randomised, open‐label study comparing indacaterol versus alternative long‐acting beta2‐agonists for patients whose current treatment regimen included a twice‐daily long‐acting beta2‐agonist Run‐in: not stated |
|
| Participants |
Population: 90 participants. Mean age 65 years. Predominantly male participants Inclusion criteria: diagnosis of chronic obstructive pulmonary disease (COPD) (moderate to severe as classified by the Global Initiative for Chronic Obstructive Lung Disease (GOLD) Guidelines, 2007); postbronchodilator forced expiratory volume in 1 second (FEV1) < 80% and ≥ 30% of predicted normal value; postbronchodilator FEV1/FVC (forced vital capacity) < 70%; current COPD bronchodilator treatment includes a LABA bronchodilator or a fixed‐dose combination of LABA and inhaled corticosteroid (ICS) Exclusion criteria: history of asthma; currently receiving treatment for COPD with tiotropium; diabetes Type I or uncontrolled diabetes Type II; history of certain cardiovascular co‐morbid conditions |
|
| Interventions | 1. Indacaterol 150 mcg 2. Alternative twice‐daily beta2‐agonist |
|
| Outcomes | Primary outcome: change in health‐related quality of life as measured by COPD clinical questionnaire | |
| Notes | Trial sponsored by Novartis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Currently available in abstract format only; method of random sequence generation not stated |
| Allocation concealment (selection bias) | High risk | Open‐label study |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Method of blinding of outcome assessment was unclear |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Similar rates of dropout were noted across both study arms |
| Selective reporting (reporting bias) | Low risk | Only one primary outcome has been reported |
Kerwin 2011 Study 1.
| Methods |
Design: 12‐week, double‐blind, randomised, placebo‐controlled trial. 1 arm of 2 identical trials, with analysis performed on combined population of trials. Additional inhaled bronchodilators other than albuterol were discontinued. Run‐in: 2 weeks |
|
| Participants |
Population: 318 participants were randomly assigned. Mean age 61 years. In the indacaterol and placebo arms, 40% and 35% of participants were taking concomitant ICS, respectively, and mean FEV1 was 56% and 54% predicted, respectively Inclusion criteria: > 40 years of age, at least a 10‐pack‐year smoking history FEV1 < 80% and > 29% FEV1/FVC < 70% post 360 mcg albuterol Exclusion criteria: lower respiratory tract infection or hospitalisation with an acute exacerbation of COPD within the previous 6 weeks, asthma, any alternative significant cardiovascular or respiratory disease, type 1 or poorly controlled type 2 diabetes, history of long QT syndrome or prolonged QTc |
|
| Interventions | 1. Indacaterol 75 mcg once daily 2. Placebo |
|
| Outcomes |
Primary outcome: trough FEV1 at 12 weeks Secondary outcomes: other spirometric variables, use of rescue albuterol, quality of life (SGRQ), dyspnoea (TDI), exacerbations, diary card symptom scores |
|
| Notes | Novartis sponsored trial, and Novartis employees were directly involved in preparation and drafting of the manuscript. Novartis directly supplied data for: trough FEV1, quality of life, dyspnoea, peak FEV1, number of participants experiencing at least one exacerbation. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Automated random assignment via active voice response/web system |
| Allocation concealment (selection bias) | Low risk | Participants and investigating staff were blinded to treatment allocation from randomisation to study completion; probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo and Indacaterol were administered via identical inhalers |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Persons performing outcome assessments were blinded to allocations |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Roughly comparable dropout rates, similar reasons for dropouts across groups, rates of dropout slightly higher in the placebo group |
| Selective reporting (reporting bias) | High risk | Dyspnoea and other subjective endpoints were secondary outcomes that were not reported in published data or were reported with minimal detail (although some data are available from Novartis) |
Kerwin 2011 Study 2.
| Methods |
Design: 12‐week, double‐blind, randomised, placebo‐controlled trial. 1 arm of 2 identical trials, with analysis performed on combined population of the trials. Additional inhaled bronchodilators other than albuterol were discontinued. Run‐in: 2 weeks |
|
| Participants |
Population: 323 participants were randomly assigned. Mean age 64 years. In the indacaterol and placebo arms, 43% and 48% of participants were taking concomitant ICS, respectively, and mean FEV1 was 54% and 53%, respectively Inclusion criteria: > 40 years of age, at least a 10‐pack‐year smoking history FEV1 < 80% and > 29% FEV1/FVC < 70% post 360 mcg albuterol Exclusion criteria: lower respiratory tract infection or hospitalisation with an acute exacerbation of COPD within previous 6 weeks, asthma, any alternative significant cardiovascular or respiratory disease, type 1 or poorly controlled type 2 diabetes, history of long QT syndrome or prolonged QTc. Inhaled anticholinergic medications were not permitted |
|
| Interventions | 1. Indacaterol 75 mcg once daily 2. Placebo |
|
| Outcomes |
Primary outcome: trough FEV1 at 12 weeks Secondary outcomes: other spirometric variables, use of rescue albuterol, health status (SGRQ), dyspnoea (TDI), exacerbations, diary card symptom scores |
|
| Notes | Novartis sponsored trial, and Novartis employees were directly involved in preparation and drafting of the manuscript. Novartis directly supplied data for: trough FEV1, quality of life, dyspnoea, peak FEV1, number of participants experiencing at least one exacerbation. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Automated random assignment via active voice response/web system |
| Allocation concealment (selection bias) | Low risk | Participants and investigating staff were blinded to treatment allocation from randomisation to study completion; probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo and indacaterol were administered via identical inhalers |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Persons performing outcome assessments were blinded to allocations |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Roughly comparable dropout rate, similar reasons for dropouts across groups, rates of dropout slightly higher in the placebo group |
| Selective reporting (reporting bias) | High risk | Dyspnoea and other subjective endpoints were secondary outcomes that were not reported in published data or were reported with minimal detail (although some data are available from Novartis) |
Kinoshita 2012.
| Methods |
Design: 12‐week, multi‐centre, randomised, double‐blind, placebo‐controlled, parallel‐group study. Participants were recruited from Asian countries including Japan. Additional inhaled bronchodilators other than albuterol were discontinued. Run‐in: not specified |
|
| Participants |
Population: 347 participants were randomly assigned. Mean age 66.7 years. Mean FEV1 was 53.7%. In the indacaterol 150 mcg, indacaterol 300 mcg and placebo arms, 22%, 22% and 23% of participants were taking concomitant inhaled corticosteroids, respectively Inclusion criteria: adults > 39 years with at least 20‐pack‐year smoking history FEV1 < 80% > 29%, FEV1/FVC < 70% Exclusion criteria: lower respiratory tract infection or hospitalisation with an acute exacerbation of COPD within the previous 6 weeks, requirement for long‐term oxygen therapy, asthma, any alternative significant cardiovascular or respiratory disease, type 1 or poorly controlled type 2 diabetes, history of long QT syndrome or prolonged QTc, history of vaccination with live attenuated vaccines within the previous 30 days or during the run‐in period |
|
| Interventions | 1. Indacaterol 150 mcg 2. Indacaterol 300 mcg 3. Placebo |
|
| Outcomes |
Primary outcome: 12‐week trough FEV1 Secondary outcomes:‐ trough FEV1 at weeks 2, 4, 8, individual time point FEV1 and FVC on day 1, peak FEV1 on day 1 Other outcomes: health status, diary cards, dyspnoea, rescue medication, safety and tolerability |
|
| Notes | Trial was sponsored by Novartis, which assisted in preparation of the manuscript. Novartis directly supplied data for: trough FEV1, dyspnoea, peak FEV1, number of participants experiencing at least one exacerbation. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation using a validated automated system |
| Allocation concealment (selection bias) | Unclear risk | Allocation via an automated system, although the method of allocation concealment was not specified. Matching placebo was used |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participant, caregiver, investigator, outcomes assessor were all blinded, and matching placebo was used |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Higher rate of attrition from placebo arm, primarily due to adverse events |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported |
Korn 2011.
| Methods |
Design: double‐blind, parallel, randomised, controlled trial. 12 weeks' duration. Additional inhaled bronchodilators other than albuterol were discontinued. Intention‐to‐treat analysis was performed. Participants were recruited from the USA, Europe and India Run‐in: 2 weeks |
|
| Participants |
Population: 1123 participants with a diagnosis of moderate to severe COPD were randomly assigned. Mean age was 62.8 years. Mean FEV1 was 51.8% predicted. In the indacaterol and salmeterol arms, 45.8% and 46.1% of participants were taking concomitant inhaled corticosteroids, respectively Inclusion criteria: Adults > 40 years with at least a 10‐pack‐year smoking history FEV1/FVC < 0.7, FEV1 30% to 80% predicted post bronchodilator Exclusion criteria: respiratory tract infection or COPD exacerbation during previous 6 weeks, diagnosis of asthma, concomitant pulmonary disease, long‐term oxygen therapy, type 1 or uncontrolled type 2 diabetes, cancer with less than 5‐year survival, lung cancer, QTc abnormalities, live vaccine in previous 30 days |
|
| Interventions | 1. Indacaterol 150 mcg 2. Salmeterol 50 mcg |
|
| Outcomes |
Primary endpoint:‐ FEV1 standardised area under the curve (AUC) from 5 minutes to 11 hours 45 minutes at week 12 Secondary endpoints: 24‐hour trough FEV1 at week 12, FEV1 and FVC measured over 24 hours, Transitional Dyspnoea Index (TDI) and rescue medication use Follow‐up on days 1, 2, 28, 29, 84, 85 Values were reported at baseline; additional information was obtained from study authors for post‐treatment data |
|
| Notes | Study was supported by Novartis, and some study authors were Novartis employees. Novartis directly supplied data for: trough FEV1, serious adverse events and mortality. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed using an automated interactive voice response system |
| Allocation concealment (selection bias) | Low risk | Allocation was performed via automated interactive voice response system with participants and assessors blinded to allocation; double‐dummy design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Allocated interventions were not known by participants or by personnel during the study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were blinded to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Rates and reasons for dropout were similar across all arms |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported |
Kornmann 2011.
| Methods |
Design: double‐blind, parallel, randomised, controlled trial. 26 weeks' duration. Additional inhaled bronchodilators other than albuterol were discontinued. Patients were recruited from Canada, Europe, South America, India and Taiwan Run‐in: 2 weeks |
|
| Participants |
Population: 1002 participants with a diagnosis of moderate to severe COPD were randomly assigned. Mean age 63 years. In the indacaterol 150 mcg, salmeterol 50 mcg and placebo arms, 45%, 46% and 40% were taking concomitant inhaled corticosteroids, respectively. Mean FEV1 was 54%, 53% and 53%, respectively Inclusion criteria: age > 40, smoking history > 20 pack‐years, FEV1/FVC < 0.7, FEV1 30% to 80% predicted post bronchodilator Exclusion criteria: respiratory tract infection or COPD exacerbation during previous 6 weeks, diagnosis of asthma, concomitant pulmonary disease, long‐term oxygen therapy, type 1 or uncontrolled type 2 diabetes, cancer with less than 5‐year survival, lung cancer, QTc abnormalities, shift workers |
|
| Interventions | 1. Indacaterol 150 mcg 2. Salmeterol 50 mcg 3. Placebo |
|
| Outcomes |
Primary endpoint: 24‐hour postdose trough FEV1 after 12 weeks. Other endpoints: SGRQ, Transitional Dyspnoea Index (TDI), symptom diaries, use of as needed salbutamol Follow‐up at day 2, weeks 4, 8, 12, 26 Values reported at baseline; additional information obtained from study authors for post‐treatment data |
|
| Notes | Study was supported by Novartis, and some authors were Novartis employees. Novartis directly supplied data for: Indacaterol versus placebo (number of participants experiencing at least one exacerbation); Indacaterol versus LABA (dyspnoea, number of participants experiencing at least one exacerbation). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly allocated to treatment using an automated system |
| Allocation concealment (selection bias) | Low risk | Allocation via automated system, double‐dummy design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Allocated interventions were not known by participants or personnel during the study; placebo appears adequate |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were blinded to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Higher dropout rates in placebo arm were largely due to withdrawal of consent or unsatisfactory therapeutic effect |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported, not just statistically significant outcomes |
Mroz 2013.
| Methods |
Design: 12‐week, randomised, controlled trial. Population analysed was not specified. Number of centres was not specified. Alternative inhaled long‐acting beta2‐agonists were ceased. Use of alternative inhaled bronchodilators was not specified Run‐in: not specified |
|
| Participants | Population: 34 predominantly male participants with a diagnosis of COPD were randomly assigned with 17 participants in each arm (spirometric criteria and method of diagnosis of COPD not specified). Mean age 63 years. Other baseline characteristics were not specified | |
| Interventions | 1. Indacaterol 300 mcg 2. Placebo |
|
| Outcomes | Spirometry, lung volumes, diffusing capacity for carbon monoxide, SGRQ, 6‐minute walk distance (6MWD) and 6MWD‐related dyspnoea and fatigue scores and arterial blood oxygen saturation were performed at 4, 8 and 12 weeks | |
| Notes | Authors directly supplied data for: trough FEV1 and quality of life. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Method of randomisation was not specified. Placebo and experimental arms were poorly matched with respect to lung function at the start of the trial, with higher lung function reported in the indacaterol arm, raising the suggestion of inadequate sequence generation |
| Allocation concealment (selection bias) | High risk | Method of allocation was not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Method of blinding was not specified. Unclear whether a placebo inhaler device was used |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear who performed outcome assessments and whether they were blinded to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropouts were not reported |
| Selective reporting (reporting bias) | Low risk | Diffusing capacity of carbon monoxide was not reported. Otherwise all outcomes were reported |
To 2011.
| Methods |
Design: parallel, open‐label, randomised, controlled trial. 52 weeks' duration. Modified intention‐to‐treat analysis. Japanese participants Run‐in: not stated |
|
| Participants |
Population: 186 participants with a diagnosis of moderate to severe COPD were randomly assigned. Mean age was 68 years Inclusion criteria: adults > 40 years with at least a 20‐pack‐year smoking history FEV1/FVC < 0.7, FEV1 30% to 80% predicted post bronchodilator Exclusion criteria: respiratory tract infection or COPD exacerbation during previous 6 weeks, diagnosis of asthma, concomitant pulmonary disease, type 1 or uncontrolled type 2 diabetes, cancer with less than 5‐year survival, lung cancer, certain cardiovascular co‐morbidities |
|
| Interventions | 1. Indacaterol 300 mcg 2. Salmeterol 50 mcg |
|
| Outcomes |
Primary outcome: blood glucose, QTc, serum potassium, blood pressure and pulse rate, other adverse events Secondary outcome: trough FEV1 Follow‐up weeks 4, 8, 12, 24, 36, 44 and 52 |
|
| Notes | Unpublished trial; unable to obtain further data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation was unclear |
| Allocation concealment (selection bias) | High risk | Open‐label trial |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Method of blinding of outcome assessment was unclear |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Similar rates of dropout in both arms for similar reasons |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported |
Yao 2014.
| Methods |
Design: 26‐week, multi‐centre, randomised, double‐blind, placebo‐controlled, parallel‐group trial. Participants were recruited from Austalia, China and India, and were predominantly of Asian ethnicity. Intention‐to‐treat analysis was performed Run‐in: washout of LABAs and LAMAs of 2 days and 7 days, respectively |
|
| Participants |
Population: 563 participants of predominantly Asian ethnicity were randomly assigned from Australia, China and India. Mean age was 65.4 years. Theophylline was allowed to be continued. 34% to 35% of participants were taking concomitant inhaled corticosteroids. Mean FEV1 was 49% to 50% predicted across all groups Mean age: Indacaterol 150 mcg 66.2 years, indacaterol 300 mcg 65.5 years, placebo 64.6 years Inclusion criteria: Adults > 40 years with at least a 10‐pack‐year smoking history FEV1/FVC < 70%, FEV1 < 80% and > 29% Exclusion criteria: lower respiratory tract infection or hospitalisation with an acute exacerbation of COPD within the previous 6 weeks, requirement for long‐term oxygen therapy, asthma, any alternative significant cardiovascular or respiratory disease, type 1 or poorly controlled type 2 diabetes, history of long QT syndrome or prolonged QTc, a history of vaccination with live attenuated vaccines within the previous 30 days or during the run‐in period |
|
| Interventions | 1. Indacaterol 150 mcg 2. Indacaterol 300 mcg 3. Placebo |
|
| Outcomes |
Primary outcome: trough FEV1 at 12 weeks Secondary outcomes: trough FEV1 at other time points, TDI and SGRQ at weeks 8, 12, 26, daily symptoms and rescue medication use |
|
| Notes | Novartis‐sponsored trial | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation was not specified |
| Allocation concealment (selection bias) | Low risk | Not specified, but both interventions and placebo via identical inhalers; probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants, caregivers, investigators all blinded to treatment allocations; probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes assessors blinded to treatment allocations; probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Slightly greater proportion of participants discontinued in placebo arm; main difference was loss to follow‐up and unsatisfactory therapeutic effect in placebo arm |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes reported |
6MWD: 6‐minute walk distance; AUC: area under the curve; BODE: body mass index, obstruction, dyspnoea, and exercise; COPD: chronic obstructive pulmonary disease; FEV1: forced expiratory volume in 1 second; FVC: forced vital capacity; GOLD: Global Initiative for Chronic Obstructive Lung Disease; ICS: inhaled corticosteroid; IRT: interactive response technology; LABA: long‐acting beta2‐agonist; LAMA: long‐acting muscarinic agonist; SGRQ: St George's Respiratory Questionnaire; TDI: Transitional Dyspnoea Index.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Barnes 2010 | An initial dose‐finding 14‐day trial that formed part of a longer trial with an adaptive seamless design |
| Beeh 2011 | Insufficient duration |
| Buhl 2011 | Comparison with tiotropium. No long‐acting beta2‐agonist (LABA) or placebo arm |
| Chapman 2011 | 1. 26‐Week extension of Donohue 2010 for participants consenting to remain in trial. Therefore not truly a randomised controlled trial, and significant potential for the introduction of bias 2. Analysis was performed over the entire 52‐week period; therefore participants would be double‐counted and standard meta‐analysis was not possible |
| Hataji 2013 | Non‐randomised trial |
| Jones 2011 | Meta‐analysis of 3 trials already included in the analysis |
| Khindri 2011 | Healthy participants, length of study insufficient |
| Magnussen 2010 | Insufficient follow‐up |
| Mahler 2012 Study 1 | Open‐label tiotropium was prescribed in both experimental and control arms |
| Mahler 2012 Study 2 | Open‐label tiotropium was prescribed in both experimental and control arms |
| Van de Maele 2010 | Insufficient follow‐up |
Characteristics of ongoing studies [ordered by study ID]
CQVA149A2336.
| Trial name or title | A 12 Week Treatment, Multi‐center, Randomized, Double‐blind, Parallel‐group, Placebo and Active Controlled Study to Assess the Efficacy, Safety, and Tolerability of Indacaterol Maleate/Glycopyrronium Bromide in COPD Patients With Moderate to Severe Airflow Limitation. |
| Methods | Randomized, double‐blind, parallel‐group, placebo and active controlled study |
| Participants | Male and female patients ≥ 40 years of age Patients with stable COPD according to GOLD 2011 Patients with a postbronchodilator FEV1 ≥ 30% and < 80% predicted and a postbronchodilator FEV1/FVC < 0.70 Current smokers or ex‐smokers who have a smoking history of at least 10 pack‐years Patients with a modified Medical Research Council (mMRC) grade 2 or greater |
| Interventions | QVA149 Long‐acting muscarinic antagonist (LAMA) Long‐acting beta2‐agonist (LABA) Placebo |
| Outcomes |
Primary outcome: Standardized forced expiratory volume in 1 second Area under the curve following 12 weeks of treatment Secondary outcomes: Total St George Respiratory Questionnaire score Trough forced expiratory volume in 1 second Level of breathlessness experienced by participants using the Transitional Dyspnoea Index following 12 weeks of treatment Rescue medication use (number of puffs) reported by participants using the patient electronic diary following 12 weeks of treatment Daily symptoms reported using the patient electronic diary following 12 weeks of treatment Morning symptoms reported using the patient electronic diary following 12 weeks of treatment Evening symptoms reported using the patient electronic diary following 12 weeks of treatment Forced expiratory volume in 1 second at all time points Forced vital capacity at various time points |
| Starting date | November 2012 |
| Contact information | Novartis Pharmaceuticals |
| Notes |
Novartis CQAB149BIL01.
| Trial name or title | A 12 Week Multi‐centre Randomised Open Label Study Evaluating the Efficacy and Safety of Treatment Regimes That Include ONbrez (Indacaterol) in Patients With Moderate to Severe COPD (MOVE‐ON) |
| Methods | Multi‐centre, randomised, open‐label study |
| Participants | Adults > 39 years with stable COPD and at least a 10‐pack‐year smoking history Postbronchodilator FEV1/FVC < 70% (400 mcg salbutamol) FEV1 < 80% but > 29% Participants already treated with twice‐daily long‐acting beta2‐agonist |
| Interventions | 150 mcg indacaterol vs existing twice‐daily beta2‐agonist |
| Outcomes | Clinical COPD questionnaire score; adverse events |
| Starting date | March 2011 |
| Contact information | Novartis Pharmaceuticals |
| Notes |
Novartis CQVA149A2337.
| Trial name or title | A 12‐Week Treatment, Multi‐center, Randomized, Double‐blind, Parallel‐group, Placebo and Active Controlled Study to Assess the Efficacy, Safety, and Tolerability of Indacaterol Maleate/Glycopyrronium Bromide in COPD Patients With Moderate to Severe Airflow Limitation |
| Methods | Randomized, double‐blind, parallel‐group, placebo and active controlled study |
| Participants | Male and female patients who have signed informed consent and are ≥ 40 years of age Patients with stable chronic obstructive pulmonary disease (COPD) according to GOLD 2011 Patients with a postbronchodilator forced expiratory volume in 1 second (FEV1) ≥ 30% and < 80% predicted and a postbronchodilator FEV1/forced vital capacity (FVC) < 0.70 Current smokers or ex‐smokers who have a smoking history of at least 10 pack‐years Patients with an mMRC grade 2 or greater |
| Interventions | LABA/LAMA Long‐acting muscarinic antagonist (LAMA) Placebo Long‐acting beta2‐agonist (LABA) |
| Outcomes |
Primary outcome: Standardized forced expiratory volume in 1 second (FEV1) Area under the curve (AUC) following 12 weeks of treatment Secondary outcomes: Change in health status based on total score and percentage of participants with clinically significant improvement, as reported by participants using the St George Respiratory Questionnaire (SGRQ) following 12 weeks of treatment Trough forced expiratory volume in 1 second (FEV1) following 12 weeks of treatment Level of breathlessness experienced by participants evaluated using the Transitional Dyspnoea Index (TDI) following 12 weeks of treatment Medication use (number of puffs) reported by participants using the patient electronic diary following 12 weeks of treatment Evaluation of symptoms reported using the patient electronic diary following 12 weeks of treatment Evaluation of forced expiratory volume in 1 second (FEV1) at all time points Evaluation of forced vital capacity (FVC) at all time points |
| Starting date | December 2012 |
| Contact information | Novartis Pharmaceuticals |
| Notes |
AUC: area under the curve; COPD: chronic obstructive pulmonary disease; GOLD: Global Initiative or Chronic Obstructive Lung Disease; FEV1: forced expiratory volume in 1 second ;FVC: forced vital capacity; LABA: long‐acting beta2‐agonist; LAMA: long‐acting muscarinic agonist; mMRC: modified Medical Research Council; SGRQ: St George's Respiratory Questionnaire; TDI: Transitional Dyspnoea Index.
Differences between protocol and review
The method of analysis of exacerbations was changed. We had aimed to transform rate ratios into log rate ratios and to analyse them using fixed‐effect and generic inverse variance (GIV) models in Review Manager 5.1 (RevMan 2011). We had aimed to analyse rates of any exacerbations and rates of exacerbations requiring hospitalisation. However data were insufficient for analysis of these outcomes. Therefore the total number of participants experiencing at least one exacerbation was analysed according to individual study protocols; this was relegated to a secondary outcome.
We planned to assess the number of participants with a clinically significant deterioration in quality of life as a primary outcome. However no data were available for this outcome and it was removed.
We planned to assess the number of participants with a clinically significant deterioration in dyspnoea, 24‐hour area under the curve, FEV1 and peak FVC as secondary outcomes. However no data were available for these outcomes; therefore they were removed.
To address the issue of different doses of indacaterol available through different healthcare jurisdictions internationally, and to assess for possible dose‐response effects, post hoc subgroup analyses by dose were performed. These subgroup analyses were performed on primary and secondary outcomes.
To facilitate inclusion of ANCOVA analyses published in most of the manuscripts, generic inverse variance meta‐analyses were performed for all outcomes other than peak FEV1, exacerbations, adverse events and mortality.
We planned to perform subgroup analyses of different GOLD stage severities for primary outcomes. However this was not possible because data were insufficient.
Trials using additional bronchodilators that were not part of the comparison were excluded because of the potential for introduction of bias.
Contributions of authors
James B Geake (JBG) drafted the manuscript, which was reviewed by Eli J Dabscheck (EJD), Richard Wood‐Baker (RWB) and Christopher J Cates (CJC). JBG extracted data from identified trials identified and entered these data into Review Manager 5.1 (RevMan 2011) for statistical analysis. CJC and EJD cross‐checked extracted data. All review authors reviewed the manuscript before submission for editorial review.
Sources of support
Internal sources
Cochrane Airways Group Scholarship, Australia.
External sources
No sources of support supplied
Declarations of interest
JBG: none known.
EJD: none known.
RWB: none known.
CJC: As CC is the Co‐ordinating Editor of the Cochrane Airways Group, editing and the peer review process for this review were handled by another editor, Milo Puhan.
Edited (no change to conclusions), comment added to review
References
References to studies included in this review
Bateman 2013 {published and unpublished data}
- Bateman ED, Ferguson GT, Barnes N, Gallagher N, Green Y, Henley M, et al. Dual bronchodilation with QVA149 versus single bronchodilator therapy: the SHINE study. European Respiratory Journal 2013 May 30 [Epub ahead of print]. [DOI: 10.1183/09031936.00200212] [DOI] [PMC free article] [PubMed]
Dahl 2010 {published and unpublished data}
- Dahl R, Chung KF, Buhl R, Magnussen H, Nonikov V, Jack D, et al. Efficacy of a new once‐daily long‐acting inhaled beta2‐agonist indacaterol versus twice‐daily formoterol in COPD. Thorax 2010;65:473‐9. [DOI] [PubMed] [Google Scholar]
- Magnussen H, Paggiaro P, Jack D, Owen R, Higgins M, Kramer B. Bronchodilator treatment with indacaterol once‐daily vs formoterol twice‐daily in COPD: a 52‐week study. American Thoracic Society International Conference; 2009 May 15‐20 San Diego. 2009.
- Magnussen H, Paggiaro P, Jack D, Owen R, Higgins M, Kramer B. Indacaterol once‐daily improves health‐related quality of life (HRQOL) in COPD: a 52‐week study. European Respiratory Society 19th Annual Congress; 2009 Sep 12‐15; Vienna. 2009.
Donohue 2010 {published and unpublished data}
- Donahue JF, Fogarty C, Lotvall J, Mahler D, Worth H, Yorgancioglu A, et al. Once‐daily bronchodilators for chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2010;182(2):155‐62. [DOI] [PubMed] [Google Scholar]
Feldman 2010 {published and unpublished data}
- Feldman G, Siler T, Prasad N, Jack D, Piggott S, Owen R, et al. Efficacy and safety of indacaterol 150mcg once‐daily in COPD: a double‐blind randomised 12‐week study. BMC Pulmonary Medicine 2010;10:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Izbicki 2014 {published and unpublished data}
- Izbicki G, Shitrit D, Raz M, Vilayi‐Weiler Z, Fink G, Schwarz Y, et al. Real‐life efficacy of indacaterol and other LABAs in COPD patients in Israel. Chest 2014;145(3):431B. [DOI: 10.1378/chest.1829807] [DOI] [Google Scholar]
Kerwin 2011 Study 1 {published and unpublished data}
- Gotfried M, Kerwin E, Lawrence D, Lassen C, Kramer B. Efficacy of Indacaterol 75mcg once‐daily on dyspnea and health status: results of two double‐blind, placebo‐controlled 12‐week studies. COPD 2012;9(6):629‐36. [DOI] [PubMed] [Google Scholar]
- Kerwin E, Gotfried M, Lawrence D, Lassen C, Kramer B. Efficacy and tolerability of indacaterol 75mcg once daily in patients aged > 40 years with chronic obstructive pulmonary disease: results from 2 double‐blind, placebo‐controlled 12 week studies. Clinical Therapeutics 2011;33(12):1974‐84. [DOI] [PubMed] [Google Scholar]
Kerwin 2011 Study 2 {published and unpublished data}
- Gotfried M, Kerwin E, Lawrence D, Lassen C, Kramer B. Efficacy of Indacaterol 75mcg once‐daily on dyspnea and health status: results of two double‐blind, placebo‐controlled 12‐week studies. COPD 2012;9(6):629‐36. [DOI] [PubMed] [Google Scholar]
- Kerwin E, Gotfried M, Lawrence D, Lassen C, Kramer B. Efficacy and tolerability of indacaterol 75mcg once daily in patients aged > 40 years with chronic obstructive pulmonary disease: results from 2 double‐blind, placebo‐controlled 12 week studies. Clinical Therapeutics 2011;33(12):1974‐84. [DOI] [PubMed] [Google Scholar]
Kinoshita 2012 {published and unpublished data}
- Kinoshita M, Lee SH, Hang LW, Ichinose M, Hosoe M, Okino N, et al. Efficacy and safety of indacaterol 150 and 300 mcg in chronic obstructive pulmonary disease patients from six Asian areas including Japan: a 12‐week placebo controlled study. Respirology 2012;17:379‐89. [DOI] [PubMed] [Google Scholar]
- To Y, Kinoshita M, Lee SH, Hang LW, Ichinose M, Fukuchi Y, et al. Assessing efficacy of indacaterol in moderate and severe COPD patients: a 12‐week study in an Asian population. Respiratory Medicine 2012;106:1715‐21. [DOI] [PubMed] [Google Scholar]
Korn 2011 {published data only}
- Korn S, Kerwin E, Atis S, Amos C, Owen R, Lassen C. Indacaterol once‐daily provides superior efficacy to salmeterol twice‐daily in COPD: a 12‐week study. Respiratory Medicine 2011;105:719‐26. [DOI] [PubMed] [Google Scholar]
Kornmann 2011 {published data only}
- Kornmann O, Dahl R, Centanni S, Dogra A, Owen R, Lassen C, et al. Once‐daily indacaterol versus twice‐daily salmeterol for COPD: a placebo‐controlled comparison. European Respiratory Journal 2011;37:273‐9. [DOI] [PubMed] [Google Scholar]
Mroz 2013 {published and unpublished data}
- Mroz R.M, Minarowski L, Chyczewska E. Indacaterol add‐on therapy improves lung function, exercise capacity and life quality of COPD patients. Advances in Experimental Medicine and Biology 2013;756:23‐8. [DOI: 10.1007/978-94-007-4549-0_4; ISSN: 0065‐2598] [DOI] [PubMed] [Google Scholar]
To 2011 {unpublished data only}
- To Y, Nishimura M, Fukuchi Y, Kitawaki T, Okino N, Lassen C, et al. Long‐term safety and tolerability of indacaterol versus salmeterol in Japanese COPD patients: a 52‐week open‐labeled study. Respirology 2011;16(2):96. [Google Scholar]
Yao 2014 {published and unpublished data}
References to studies excluded from this review
Barnes 2010 {published and unpublished data}
- Barnes PJ, Pocock SJ, Magnussen H, Iqbal A, Kramer B, Higgins M, et al. Integrating indacaterol dose selection in a clinical study in COPD using an adaptive seamless design. Pulmonary Pharmacology and Therapeutics 2010;23(3):165‐71. [DOI] [PubMed] [Google Scholar]
Beeh 2011 {published and unpublished data}
- Beeh KM, Wagner F, Khindri S, Drollmann AF. Effect of indacaterol on dynamic lung hyperinflation and breathlessness in hyperinflated patients with COPD. COPD 2011;8(5):340‐5. [DOI] [PubMed] [Google Scholar]
Buhl 2011 {published and unpublished data}
- Buhl R, Dunn LJ, Disdier C, Lassen C, Amos C, Henley M, et al. Blinded 12‐week comparison of once‐daily indacaterol and tiotropium in COPD. European Respiratory Journal 2011;38(4):797‐803. [DOI] [PubMed] [Google Scholar]
Chapman 2011 {published and unpublished data}
- Chapman KR, Rennard SI, Dogra A, Owen R, Lassen C, Kramer B. Long‐term safety and efficacy of indacaterol, a long‐acting beta2‐agonist, in subjects with COPD: a randomized, placebo‐controlled study. Chest 2011;140(1):68‐75. [DOI] [PubMed] [Google Scholar]
Hataji 2013 {published and unpublished data}
- Hataji O, Naito M, Ito K, Watanabe F, Gabazza E, Taguchi O. Indacaterol improves daily physical activity in patients with chronic obstructive pulmonary disease. International Journal of COPD 2012;8:1‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jones 2011 {published and unpublished data}
- Jones PW, Mahler DA, Gale R, Owen R, Kramer B. Profiling the effects of indacaterol on dyspnoea and health status in patients with COPD. Respiratory Medicine 2011;105(6):892‐9. [DOI] [PubMed] [Google Scholar]
Khindri 2011 {published and unpublished data}
- Beeh KM, Wagner F, Khindri S, Drollmann AF. Effect of indacaterol on dynamic lung hyperinflation and breathlessness in hyperinflated patients with COPD. COPD 2011;8(5):340‐5. [DOI] [PubMed] [Google Scholar]
Magnussen 2010 {published and unpublished data}
- Magnussen H, Verkindre C, Jack D, Jadayel D, Henley M, Woessner R, et al. Indacaterol once‐daily is equally effective dosed in the evening or morning in COPD. Respiratory Medicine 2010;104(12):1869‐76. [DOI] [PubMed] [Google Scholar]
Mahler 2012 Study 1 {published and unpublished data}
- Mahler DA, D'Urzo A, Bateman ED, Ozkan S, White T, Peckitt C, et al. Concurrent use of indacaterol plus tiotropium in patients with COPD provides superior bronchodilation compared with tiotropium alone: a randomised double‐blind comparison. Thorax 2012;67:781‐8. [DOI] [PubMed] [Google Scholar]
Mahler 2012 Study 2 {published and unpublished data}
- Mahler DA, D'Urzo A, Bateman ED, Ozkan S, White T, Peckitt C, et al. Concurrent use of indacaterol plus tiotropium in patients with COPD provides superior bronchodilation compared with tiotropium alone: a randomised double‐blind comparison. Thorax 2012;67:781‐8. [DOI] [PubMed] [Google Scholar]
Van de Maele 2010 {published and unpublished data}
- Maele B, Fabbri LM, Martin C, Horton R, Dolker M, Overend T. Cardiovascular safety of QVA149, a combination of Indacaterol and NVA237, in COPD patients. COPD 2010;7(6):418‐27. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
CQVA149A2336 {published and unpublished data}
- Novartis Pharmaceuticals. A 12‐week treatment, multi‐centre, randomized, double‐blind, parallel‐group, placebo and active controlled study to assess the efficacy, safety, and tolerability of indacaterol maleate/glycopyrronium bromide in COPD patients with moderate to severe airflow limitation. http://www.novctrd.com/ctrdWebApp/clinicaltrialrepository/public/product.jsp?productID=650&diseaseAreaID=9 (accessed 8 February 2014).
Novartis CQAB149BIL01 {published and unpublished data}
- Novartis. A 12‐week multi‐centre randomised open label study evaluating the efficacy and safety of treatment regimens that include onbrez (indacaterol) in patients with moderate to severe COPD (MOVE‐ON). http://www.novctrd.com/ctrdWebApp/clinicaltrialrepository/displayFile.do?trialResult=9624 (accessed 9 May 2013).
Novartis CQVA149A2337 {published and unpublished data}
- Novartis Pharmaceuticals. A 12‐week treatment, multi‐centre, randomised, double‐blind, parallel‐group, placebo and active controlled study to assess the efficacy, safety and tolerability of indacaterol maleate/glycopyrronium bromide in COPD patients with moderate to severe airflow limitation. http://www.clinicaltrials.gov/ct2/show/NCT01712516?term=NCT01712516&rank=1 (accessed on 17 September 2014).
Additional references
Buist 2007
- Buist AS, McBurnie MA, Vollmer WM, Gillespie S, Burney P, Mannino DM, et al. International variation in the prevalence of COPD (the BOLD Study): a population‐based prevalence study. Lancet 2007;370(9589):741‐50. [PUBMED: 17765523] [DOI] [PubMed] [Google Scholar]
Chung 2013
- Chung VCH, Ma PHX, Hui DSC, Tam WWS, Tang JL. Indacaterol for chronic obstructive pulmonary disease: systematic review and meta‐analysis. PLOS ONE August 2013;8(8):1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Decramer 2012
- Decramer M, Rossi A, Lawrence D, McBryan D. Indacaterol therapy in patients with COPD not receiving other maintenance treatment. Respiratory Medicine 2012;106:1706‐14. [DOI] [PubMed] [Google Scholar]
Donahue 2005
- Donahue JF. Minimal clinically important differences in COPD lung function. COPD 2005;2(1):111‐24. [DOI] [PubMed] [Google Scholar]
Gershon 2010
- Gershon AS, Wang C, Wilton AS, Raut R, To T. Trends in chronic obstructive pulmonary disease prevalence, incidence, and mortality in Ontario, Canada, 1996 to 2007: a population‐based study. Archives of Internal Medicine 2010;170(6):560‐5. [PUBMED: 20308643] [DOI] [PubMed] [Google Scholar]
GOLD 2014
- Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. http://www.goldcopd.org/uploads/users/files/GOLD_Report_2014_Jan23.pdf (accessed 12 March 2014).
Han 2013
- Han J, Dai L, Zhong N. Indacaterol on dyspnea in chronic obstructive pulmonary disease: a systematic review and meta‐analysis of randomised placebo‐controlled trials. BMC Pulmonary Medicine 2013;13:26. [DOI: 10.1186/1471-2466-13-26] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Jiang 2013
- Jiang FM, Liang ZA, Zheng QL, Wang RC, Li CT. Safety and efficacy of 12‐week or longer indacaterol treatment in moderate‐to‐severe COPD patients: a systematic review. Lung 2013;191:135‐46. [DOI] [PubMed] [Google Scholar]
Jones 2002
- Jones PW. Interpreting thresholds for a clinically significant change in health status in asthma and COPD. European Respiratory Journal 2002;19(3):398‐404. [DOI] [PubMed] [Google Scholar]
Renard 2011
- Renard D, Looby M, Kramer B, Lawrence D, Morris D, Stanski D. Characterization of the bronchodilatory dose response to indacaterol in patients with chronic obstructive pulmonary disease using model‐based approaches. Respiratory Research 2011;12(1):54. [DOI: 10.1186/1465-9921-12-54] [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Ribeiro 2012
- Ribeiro M, Chapman K. Comparative efficacy of indacaterol in chronic obstructive pulmonary disease. International Journal of COPD 2012;7:145‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rodrigo 2012
- Rodrigo G, Neffen H. Comparison of indacaterol with tiotropium or twice‐daily long‐acting beta agonists for stable COPD. Chest 2012;145(5):1104‐10. [DOI] [PubMed] [Google Scholar]
WHO 2008
- World Health Organization. World Health Statistics. www.who.int/whosis/whostat/2008/en/index.html (accessed 3 September 2012).
Witek 2003
- Witek TJ, Mahler DA. Minimal important difference of the transition dyspnoea index in a multinational clinical trial. European Respiratory Journal 2003;21(2):267‐72. [DOI] [PubMed] [Google Scholar]


