Abstract
Background
Previous randomised trials and meta‐analyses have shown that nasal continuous positive airway pressure (NCPAP) is a useful method for providing respiratory support after extubation. However, this treatment sometimes 'fails' in infants, and they may require endotracheal re‐intubation with its attendant risks and expense. Nasal intermittent positive pressure ventilation (NIPPV) can augment NCPAP by delivering ventilator breaths via nasal prongs. Older children and adults with chronic respiratory failure benefit from NIPPV, and the technique has been applied to neonates. However, serious side effects including gastric perforation have been reported with older methods of providing NIPPV.
Objectives
Primary objective
To compare effects of management with NIPPV versus NCPAP on the need for additional ventilatory support in preterm infants whose endotracheal tube was removed after a period of intermittent positive pressure ventilation.
Secondary objectives
To compare rates of gastric distension, gastrointestinal perforation, necrotising enterocolitis and chronic lung disease; duration of hospitalisation; and rates of apnoea, air leak and mortality for NIPPV and NCPAP.
Search methods
We used the standard search strategy of the Cochrane Neonatal Review Group to search the Cochrane Central Register of Controlled Trials (CENTRAL; 2015, Issue 9), MEDLINE via PubMed (1966 to 28 September 2015), Embase (1980 to 28 September 2015) and the Cumulative Index to Nursing and Allied Health Literature (CINAHL; 1982 to 28 September 2015). We also searched clinical trials databases, conference proceedings and reference lists of retrieved articles for randomised controlled trials and quasi‐randomised trials.
Selection criteria
We included randomised and quasi‐randomised trials comparing use of NIPPV versus NCPAP in extubated preterm infants. NIPPV included non‐invasive support delivered by a mechanical ventilator or a bilevel device in a synchronised or non‐synchronised way. Participants included ventilated preterm infants who were ready to be extubated to non‐invasive respiratory support. Interventions compared were NIPPV, delivered by short nasal prongs or nasopharyngeal tube, and NCPAP, delivered by the same methods.
Types of outcomes measures included failure of therapy (respiratory failure, rates of endotracheal re‐intubation); gastrointestinal complications (i.e. abdominal distension requiring cessation of feeds, gastrointestinal perforation or necrotising enterocolitis); pulmonary air leak; chronic lung disease (oxygen requirement at 36 weeks' postmenstrual age) and mortality.
Data collection and analysis
Three review authors independently extracted data regarding clinical outcomes including extubation failure; endotracheal re‐intubation; rates of apnoea, gastrointestinal perforation, feeding intolerance, necrotising enterocolitis, chronic lung disease and air leak; and duration of hospital stay. We analysed trials using risk ratio (RR), risk difference (RD) and the number needed to treat for an additional beneficial outcome (NNTB) or an additional harmful outcome (NNTH) for dichotomous outcomes, and mean difference (MD) for continuous outcomes. We used the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach to assess the quality of evidence.
Main results
Through the search, we identified 10 trials enrolling a total of 1431 infants and comparing extubation of infants to NIPPV or NCPAP. Three trials had methodological limitations and possible selection bias.
Five trials used the synchronised form of NIPPV, four used the non‐synchronised form and one used both methods. Eight studies used NIPPV delivered by a ventilator, one used a bilevel device and one used both methods. When all studies were included, meta‐analysis demonstrated a statistically and clinically significant reduction in the risk of meeting extubation failure criteria (typical RR 0.70, 95% CI 0.60 to 0.80; typical RD ‐0.13, 95% CI ‐0.17 to ‐0.08; NNTB 8, 95% CI 6 to 13; 10 trials, 1431 infants) and needing re‐intubation (typical RR 0.76, 95% CI 0.65 to 0.88; typical RD ‐0.10, 95% CI ‐0.15 to ‐0.05; NNTB 10, 95% CI 7 to 20; 10 trials, 1431 infants). We graded evidence for these outcomes as moderate, as all trial interventions were unblinded. Although methods of synchronisation varied (Graseby capsule or pneumotachograph/flow‐trigger), the five trials that synchronised NIPPV showed a statistically significant benefit for infants extubated to NIPPV in terms of prevention of extubation failure up to one week after extubation.
Unsynchronised NIPPV also reduced extubation failure. NIPPV provided via a ventilator is more beneficial than that provided by bilevel devices in reducing extubation failure during the first week. When comparing interventions, investigators found no significant reduction in rates of chronic lung disease (typical RR 0.94, 95% CI 0.80 to 1.10; typical RD ‐0.02, 95% CI ‐0.08 to 0.03) or death, and no difference in the incidence of necrotising enterocolitis. Air leaks were reduced in infants randomised to NIPPV (typical RR 0.48, 95% CI 0.28 to 0.82; typical RD ‐0.03, 95% CI ‐0.05 to ‐0.01; NNTB 33, 95% CI 20 to 100). We graded evidence quality as moderate (unblinded studies) or low (imprecision) for secondary outcomes.
Authors' conclusions
Implications for practice
NIPPV reduces the incidence of extubation failure and the need for re‐intubation within 48 hours to one week more effectively than NCPAP; however, it has no effect on chronic lung disease nor on mortality. Synchronisation may be important in delivering effective NIPPV. The device used to deliver NIPPV may be important; however, data are insufficient to support strong conclusions. NIPPV does not appear to be associated with increased gastrointestinal side effects.
Implications for research
Large trials should establish the impact of synchronisation of NIPPV on safety and efficacy of the technique and should compare the efficacy of bilevel devices versus a ventilator for providing NIPPV.
Plain language summary
Nasal intermittent positive pressure ventilation (NIPPV) versus nasal continuous positive airway pressure (NCPAP) for preterm neonates after extubation
Review question
Does nasal intermittent positive pressure ventilation (NIPPV) confer short‐term and long‐term benefits without causing harm to premature infants coming off a ventilator? How does it compare with nasal continuous positive airway pressure (NCPAP)?
Background
Evidence suggests that NIPPV increases the effectiveness of NCPAP in preterm babies who no longer need an endotracheal tube (tube in the windpipe). Preterm babies with breathing problems often require help from a machine (ventilator) that provides regular breaths through a tube in the windpipe. The process of extubation or removal of this tube does not always go smoothly, and the tube may need to be re‐inserted if the baby cannot manage without assistance. NCPAP and NIPPV are ways of supporting babies' breathing in a minimally invasive way ‐ the tubes are short and reach only to the back of the nose, thus causing minimal damage to the lungs. NCPAP and NIPPV may be used after extubation to reduce the number of babies who need re‐insertion of the endotracheal tube. NCPAP provides steady pressure to the back of the nose that is transmitted to the lungs, helping the baby breathe more comfortably. NIPPV provides the same support along with some breaths via the ventilator.
Study characteristics
We searched scientific databases for studies comparing NCPAP versus NIPPV in preterm infants (born before 37 completed weeks of pregnancy) who no longer need an endotracheal tube. We looked at breathing problems, the need for the endotracheal tube to be re‐instated and side effects. Evidence is current to September 2015.
Key results
We found ten trials comparing NCPAP versus NIPPV. Six of ten studies that compared NCPAP and NIPPV showed that NIPPV reduced the need for re‐insertion of the endotracheal tube. Future studies must determine how NIPPV can best be delivered to infants.
Quality of the evidence
In clinical trials, clinicians and investigators were aware of the intervention received by each infant (NIPPV or NCPAP). Therefore, we graded the quality of evidence for the primary outcome (breathing problems and need for re‐insertion of the endotracheal tube) as moderate.
Summary of findings
Summary of findings for the main comparison. NIPPV versus NCPAP.
| NIPPV versus NCPAP | ||||||
| Patient or population: preterm neonates after extubation Setting: neonatal intensive care unit Intervention: NIPPV Comparison: NCPAP | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with NCPAP | Risk with NIPPV | |||||
| Respiratory failure post extubation | Study population | RR 0.70 (0.60 to 0.80) | 1431 (10 studies) | Moderatea | Risk of bias: intervention unblinded OIS 554 |
|
| 413 per 1000 | 289 per 1000 (248 to 330) | |||||
| Endotracheal re‐intubation during the week post extubation | Study population | RR 0.76 (0.65 to 0.88) | 1301 (8 studies) |
Moderatea | Risk of bias: intervention unblinded OIS 724 |
|
| 396 per 1000 | 301 per 1000 (257 to 348) | |||||
| Abdominal distension requiring cessation of feeds | Study population | RR 1.27 (0.64 to 2.53) | 199 (4 studies) |
Lowa,b | Risk of bias: intervention unblinded Imprecision: wide confidence intervals | |
| 112 per 1000 | 143 per 1000 (72 to 284) | |||||
| Gastrointestinal perforation | Study population | RR 0.94 (0.60 to 1.48) | 1066 (5 studies) |
Moderatea | Risk of bias: intervention unblinded | |
| 66 per 1000 | 62 per 1000 (40 to 98) | |||||
| Necrotising enterocolitis | Study population | RR 0.87 (0.64 to 1.19) | 1214 (6 studies) | Moderatea | Risk of bias: intervention unblinded | |
| 127 per 1000 | 110 per 1000 (81 to 151) | |||||
| Chronic lung disease (oxygen supplementation at 36 weeks) | Study population | RR 0.94 (0.80 to 1.10) | 1140 (6 studies) |
Moderatea | Risk of bias: intervention unblinded | |
| 355 per 1000 | 334 per 1000 (284 to 391) | |||||
| Pulmonary air leak | Study population | RR 0.48 (0.28 to 0.82) | 1229 (6 studies) | Moderatea | Risk of bias: intervention unblinded OIS 749 |
|
| 61 per 1000 | 29 per 1000 (17 to 50) | |||||
| Duration of hospitalisation (days) | Mean duration of hospitalisation (days) was 0 | Mean duration of hospitalisation (days) in the intervention group was 2.77 higher (0.04 to 5.51 higher) | 238 (4 studies) | Lowa,b | Risk of bias: intervention unblinded Imprecision: wide confidence intervals | |
| Death before discharge | Study population | RR 0.69 (0.48 to 0.99) | 1237 (6 studies) | Moderatea | Risk of bias: intervention unblinded Imprecision OIS 1844 |
|
| 104 per 1000 | 72 per 1000 (50 to 103) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence. High quality: We are very confident that the true effect lies close to the estimate of effect. Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of effect but may be substantially different. Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of effect. Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. | ||||||
aIntervention unblinded.
bImprecise estimate (wide confidence intervals).
Background
Description of the condition
Preterm infants may experience difficulty with spontaneous, unassisted breathing for a variety of reasons, including lung immaturity, chest wall instability, upper airway obstruction and poor central respiratory drive. Historically, the primary method of support for these infants has consisted of endotracheal intubation and intermittent positive pressure ventilation. Although this method is effective, it is accompanied by complications (upper airway damage, bronchopulmonary dysplasia, sepsis) and is associated with considerable economic cost. Minimising the duration of endotracheal intubation or avoiding it completely has been a goal of neonatal intensive care. Nasal continuous positive airway pressure (NCPAP) is a less invasive way of providing respiratory support for neonates at risk of, or actually experiencing, respiratory failure. One systematic review of trials comparing NCPAP versus treatment with oxyhood concluded that NCPAP begun immediately after a period of endotracheal intubation reduced the rate of adverse events (apnoea, respiratory acidosis and increased oxygen requirements) leading to re‐intubation (Bancalari 2013; Davis 2003b). In this systematic review, approximately 25% of all preterm infants allocated to NCPAP failed extubation; therefore, the need exists to improve outcomes further for infants thought to no longer require an endotracheal tube.
Adults and older children with acute or chronic ventilatory failure of various origins, including chronic obstructive pulmonary disease (Bott 1993), severe kyphoscoliosis (Ellis 1988) and pre‐lung transplantation cystic fibrosis (Piper 1992), have been treated with intermittent positive pressure ventilation delivered via a nasal interface. Clinicians have reported improvements in respiratory function.
Description of the intervention
Nasal intermittent positive pressure ventilation (NIPPV) is a simple, effective mode of respiratory support. NIPPV augments continuous positive airway pressure (CPAP) with superimposed inflations to a set peak pressure (typically 15 to 22 cmH2O). NIPPV may be delivered by nasal mask or prongs, which may be short or long, single or binasal. Some devices attempt to synchronise inflations with the infant's respiratory efforts (Owen 2007). Synchronisation can occur via pneumatic capsules detecting abdominal wall movements or by airway‐derived flow signals. Trigger delays occur, and trigger response is less consistent at high spontaneous breathing rates (Owen 2016).
How the intervention might work
NIPPV has been used in neonates for a variety of indications. About 53% of Canadian tertiary care nurseries reported using NIPPV in the mid‐1980s (Ryan 1989). More recently, 44 of 91 (48%) neonatal units in the UK reported using NIPPV (Owen 2008). The physiological benefits of this technique have been evaluated. NIPPV reduces asynchronous thoracoabdominal motion, perhaps by reducing tube resistance or providing better stabilisation of the chest wall, or both (Kiciman 1998). Its use improves tidal and minute volumes and decreases the inspiratory effort required by neonates compared with NCPAP (Moretti 1999). NIPPV has not been provided without problems in neonates; Garland 1985 reported an association between use of ventilation via nasal prongs and increased risk of gastrointestinal perforation. In the past, lack of high‐quality evidence has led to variability in practice between neonatal intensive care units with respect to NIPPV.
Newer devices now on the market, which are not ventilators, provide bilevel respiratory support (higher positive pressure followed by lower pressure); however, they have limited peak pressure capabilities (i.e. they generally cannot provide more than 10 cmH2O of peak positive pressure). Some devices have the ability to synchronise higher pressure with the infant's own breaths by using a trigger biphasic mode. Whether these devices are comparable with more conventional NIPPV delivered via a ventilator is unclear, as they cannot attain peak inspiratory pressure (PIP) similar to that achieved by NIPPV delivered via a ventilator. Whether synchronisation adds clinical benefit remains unclear (Davis 2009). For the purposes of this review, we will use the term 'NIPPV' to describe all forms of non‐invasive ventilation that intermittently provide supportive breaths or higher pressure.
Why it is important to do this review
Avoiding or reducing time spent on invasive ventilation is a goal of treatment for all premature infants. Many published studies have reported use of various forms of NIPPV or bilevel support, often including a small number of infants or a single centre. We are conducting this review to provide bedside clinicians with the best available evidence regarding their choice of non‐invasive support.
Objectives
Primary objective
To determine effects of management with NIPPV versus NCPAP on the need for additional ventilatory support in preterm infants whose endotracheal tube was removed after a period of intermittent positive pressure ventilation.
Secondary objectives
To compare rates of gastric distension, gastrointestinal perforation, necrotising enterocolitis and chronic lung disease; duration of hospitalisation; and rates of apnoea, air leak and mortality for NIPPV and NCPAP.
Methods
Criteria for considering studies for this review
Types of studies
Randomised and quasi‐randomised trials.
Types of participants
Preterm infants (i.e. those born before 37 completed weeks' gestation) extubated after a period of endotracheal intubation.
Types of interventions
Intermittent positive pressure ventilation provided by a ventilator or a bilevel device and administered via the nasal route by short nasal prongs or nasopharyngeal tubes versus NCPAP delivered by the same methods. NIPPV included non‐invasive support delivered by a mechanical ventilator or a bilevel device in a synchronised or non‐synchronised way.
Types of outcome measures
Primary outcomes
Respiratory failure defined by respiratory acidosis, increased oxygen requirement or apnoea that was frequent or severe, leading to additional ventilatory support during the week post extubation
Secondary outcomes
Endotracheal re‐intubation during the week post extubation
Rates of abdominal distension requiring cessation of feeds
Rates of gastrointestinal perforation diagnosed radiologically or at operation
Rates of necrotising enterocolitis (NEC), defined according to modified Bell's criteria (stage 2 to 3)
Rates of chronic lung disease (CLD), defined as requirement for supplemental oxygen at 28 days of life or at 36 weeks' postmenstrual age
Duration of hospitalisation
Rates of apnoea and bradycardia expressed as events per hour
Rates of pulmonary air leak
Mortality
Neurodevelopmental status at 18 to 24 months (post hoc, 2015 update)
Search methods for identification of studies
We used the criteria and standard methods of Cochrane and the Cochrane Neonatal Review Group (see the Cochrane Neonatal Group search strategy for specialized register).
We conducted a comprehensive search including the Cochrane Central Register of Controlled Trials (CENTRAL; 2015, Issue 9) in the Cochrane Library; MEDLINE via PubMed (1966 to 28 September 2015); Embase (1980 to 28 September 2015); and the Cumulative Index to Nursing and Allied Health Literature (CINAHL; 1982 to 28 September 2015) using the following search terms: (nasal continuous positive airway pressure OR NCPAP OR nasal intermittent positive pressure ventilation OR NIPPV OR nasal intermittent mandatory ventilation OR NIMV OR nasal distending pressure OR nasal positive pressure OR nasal ventilation OR non‐invasive positive pressure ventilation OR synchronized intermittent mandatory ventilation OR SIMV OR nasopharyngeal synchronized intermittent mandatory ventilation OR bilevel CPAP OR BiCPAP OR BiPAP OR SiPAP), plus database‐specific limiters for randomised controlled trials (RCTs) and neonates (see Appendix 1 for the full search strategies used for each database). We applied no language restrictions.
We searched clinical trials registries for ongoing and recently completed trials (clinicaltrials.gov; the World Health Organization International Trials Registry and Platform (www.whoint/ictrp/search/en/) and the ISRCTN Registry).
Data collection and analysis
Three review authors (BL, PGD and HK) used standard methods of Cochrane and the Cochrane Neonatal Review Group to assess the methodological quality of trials.
Selection of studies
We included all randomised and quasi‐randomised controlled trials of NIPPV compared with NCPAP in ventilated preterm infants ready for extubation. Three review authors (BL, PGD and HK) independently reviewed results of the updated search and selected studies for inclusion. We resolved disagreements by discussion.
Data extraction and management
Three review authors (BL, PGD and HK) independently extracted data, compared entries and resolved differences by discussion. For the 2015 update, one review author (BL) and the Cochrane Neonatal Review Group Editor extracted data, compared entries and resolved differences.
Assessment of risk of bias in included studies
Three review authors (BL, PGD and HK) independently assessed the quality of studies using the following criteria: blinding of randomisation, blinding of intervention, completeness of follow‐up and blinding of outcome measurement. We sought additional information from study authors when required.
For the update, we assessed risk of bias using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) and presented this information in the 'Risk of bias tables'.
We assessed the methodological quality of studies by using the following criteria.
-
Sequence generation (checking for possible selection bias): For each included study, we categorised the method used to generate the allocation sequence as:
low risk (any truly random process, e.g. random number table; computer random number generator);
high risk (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number); or
unclear risk.
-
Allocation concealment (checking for possible selection bias): For each included study, we categorised the method used to conceal the allocation sequence as:
low risk (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth); or
unclear risk.
-
Blinding (checking for possible performance bias): For each included study, we categorised the methods used to blind study participants and personnel from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes. We categorised methods as:
low risk for participants, personnel and outcome assessors;
high risk for participants, personnel and outcome assessors; or
unclear risk for participants, personnel and outcome assessors.
-
Incomplete outcome data (checking for possible attrition bias through withdrawals, drop‐outs, protocol deviations): For each included study and for each outcome, we described completeness of data including attrition and exclusions from the analysis. We noted whether attrition and exclusions were reported, numbers included in the analysis at each stage (compared with the total number of randomised participants), reasons for attrition or exclusion when reported and whether missing data were balanced across groups or were related to outcomes. When sufficient information was reported or supplied by trial authors, we re‐included missing data in the analyses. We categorised methods as:
low risk (less than 20% missing data);
high risk (20% or more missing data); or
unclear risk.
-
Selective reporting bias: For each included study, we described how we investigated the possibility of selective outcome reporting bias and what we found. We assessed methods as:
low risk (when it was clear that all of the study's prespecified outcomes and all expected outcomes of interest to the review had been reported);
high risk (when not all of the study's prespecified outcomes had been reported; one or more reported primary outcomes were not prespecified; outcomes of interest were reported incompletely and so could not be used; or study did not include results of a key outcome that would have been expected to have been reported); or
unclear risk.
-
Other sources of bias: For each included study, we described any important concerns that we had about other possible sources of bias (e.g. whether a potential source of bias was related to the specific study design, whether the trial was stopped early owing to some data‐dependent process). We assessed whether each study was free of other problems that could put it at risk of bias as:
low risk;
high risk; or
unclear risk.
We made explicit judgements regarding whether studies were at high risk of bias, according to the criteria given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We assessed the likely magnitude and direction of the bias, and whether we considered it likely to impact findings. If needed, we planned to explore the impact of the level of bias by undertaking sensitivity analyses (see Sensitivity analysis).
Quality of evidence
We assessed the quality of evidence for the main comparison at the outcome level using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach (Guyatt 2011a). This methodological approach considers evidence from RCTs as high quality that may be downgraded on the basis of consideration of any of five areas: design (risk of bias), consistency across studies, directness of the evidence, precision of estimates and presence of publication bias (Guyatt 2011a). The GRADE approach results in an assessment of the quality of a body of evidence according to one of the following four grades. High: We are very confident that the true effect lies close to that of the estimate of effect. Moderate: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of effect but may be substantially different. Low: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of effect. Very low: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect (Schünemann 2013). Review authors independently assessed the quality of the evidence found for outcomes identified as critical or important for clinical decision making. These outcomes included:
Respiratory failure (defined by respiratory acidosis, increased oxygen requirement or apnoea that was frequent or severe, leading to additional ventilatory support during the week post extubation);
Endotracheal re‐intubation during the week post extubation;
Rates of CLD, defined as requirement for supplemental oxygen at 36 weeks' postmenstrual age;
Rates of pulmonary air leak;
Rates of NEC, defined according to modified Bell's criteria (stage 2 to 3);
Mortality; and
Neurodevelopmental outcomes at 18 to 24 months.
When we considered risk of bias arising from inadequate concealment of allocation, randomised assignment, complete follow‐up or blinded outcome assessment to reduce our confidence in effect estimates, we downgraded the quality of evidence accordingly (Guyatt 2011b). We evaluated consistency by examining similarity of point estimates, extent of overlap of confidence intervals and statistical criteria including measurement of heterogeneity (I2). We downgraded the quality of evidence when large and unexplained inconsistency across study results was present (i.e. some studies suggest important benefit and others no effect or harm without a clinical explanation) (Guyatt 2011d). We assessed precision on the basis of the width of the 95% confidence interval (CI) and calculation of the optimal information size (OIS). If the total number of participants included in the pooled effect estimation was less than the number of participants generated by a conventional sample size calculation for a single adequately powered trial, we considered rating down for imprecision (Guyatt 2011c). When trials were conducted in populations other than the target population, we downgraded the quality of evidence because of indirectness (Guyatt 2011e). We entered data (i.e. pooled estimates of effects and corresponding 95% confidence Intervals) and explicit judgements for each of the above aspects assessed into the Guideline Development Tool ‐ the software used to create ‘Summary of findings’ tables (GRADEpro 2008). We explained in footnotes or comments in the ‘Summary of findings’ table all judgements involving assessment of the study characteristics described above.
Measures of treatment effect
We analysed categorical data (proportion requiring re‐intubation) using risk ratio (RR), risk difference (RD) and number needed to treat for an additional beneficial outcome (NNTB) or an additional harmful outcome (NNTH). We reported 95% confidence intervals (CIs) on all estimates. We analysed continuous data (frequency of apnoea) using mean difference (MD). We applied the fixed‐effect model.
Assessment of heterogeneity
We examined heterogeneity between trials by inspecting forest plots and quantifying the impact of heterogeneity using the I2 statistic. If noted, we planned to explore possible causes of statistical heterogeneity by performing prespecified subgroup analyses (e.g. differences in study quality, participants, intervention regimens or outcome assessments).
Data synthesis
We used the Mantel‐Haenszel method for estimates of typical RR and RD. We analysed continuous outcomes using the inverse variance method. We applied the fixed‐effect model for all meta‐analyses.
Subgroup analysis and investigation of heterogeneity
We planned to perform subgroup analyses using the same methods to determine whether responses differed according to methods of NIPPV delivery and whether or not methylxanthines were used concurrently. We planned subgroup analyses on the basis of characteristics of participants, including birth weight (e.g. infants weighing less than 1000 grams) and corrected age at the time of intervention (e.g. infants less than 28 weeks' gestation).
Sensitivity analysis
We planned sensitivity analyses for situations for which this might affect interpretation of significant results (e.g. when risk of bias was associated with the quality of some included trials or missing outcome data). We judged none to be necessary in this review.
Results
Description of studies
Included studies
We identified ten trials that met the inclusion criteria of this review (1431 infants) (Figure 1) (Barrington 2001; Friedlich 1999; Gao 2010; Jasani 2016; Kahramaner 2014; Khalaf 2001; Khorana 2008; Kirpalani 2013; Moretti 2008; O'Brien 2012). We provided details in the Characteristics of included studies table.
1.
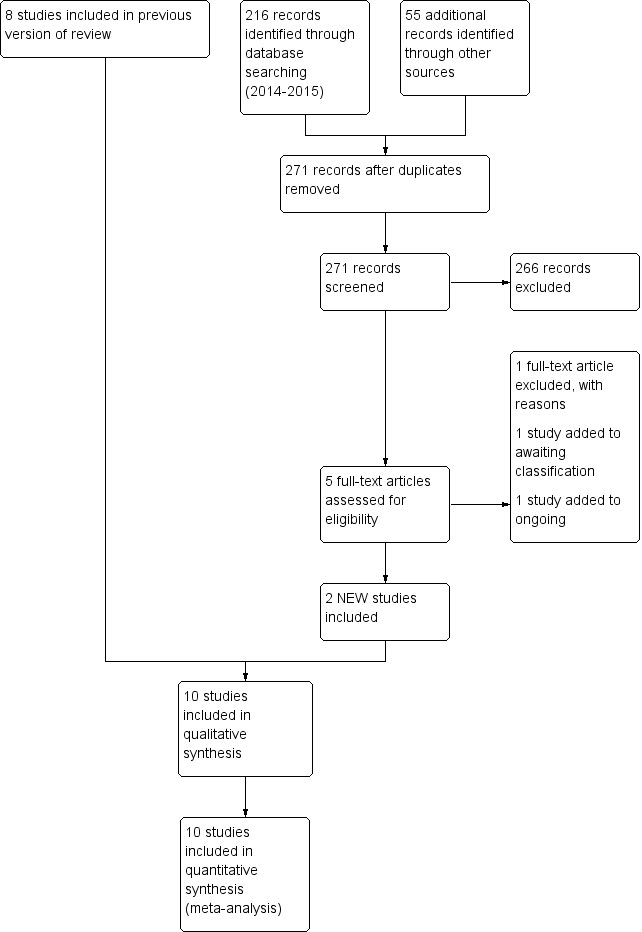
Study flow diagram: review update.
Inclusion criteria varied somewhat between trials; seven trials enrolled very low birth weight (VLBW) infants (i.e. infants at moderate risk of requiring endotracheal re‐intubation), one trial exclusively enrolled extremely low birth weight infants (ELBW) and two trials enrolled premature infants at less than 35 or 36 weeks' gestation. In six trials, use of methylxanthines was mandatory (Barrington 2001; Jasani 2016; Kahramaner 2014; Khalaf 2001; Khorana 2008; Moretti 2008), and in three other trials, it was extensively prescribed (Friedlich 1999; Kirpalani 2013; O'Brien 2012). Infants were extubated from generally low levels of ventilator support (ventilator rates less than 25 breaths per minute and oxygen concentrations less than 40%). Differences in settings between studies were small. An interesting variation in ventilatory strategies was noted between centres, in spite of little variation in enrolment criteria. Barrington 2001,Gao 2010,Jasani 2016, Kahramaner 2014,Khalaf 2001,Moretti 2008 and O'Brien 2012 extubated infants at a median age of less than one week, whereas Friedlich 1999 and Khorana 2008 extubated infants later (median age of 18.5 days and 21 days in the two groups in Friedlich 1999; mean age of 12.9 days and 6.9 days in the two groups in Khorana 2008).
Five trials synchronised NIPPV delivery; three trials used the Infant Star ventilator with Star Synch abdominal capsule (Barrington 2001; Friedlich 1999; Khalaf 2001), and two used more recent ventilators (Gao 2010; Moretti 2008). One trial used bilevel devices (SiPAP) to deliver non‐synchronised intermittent positive pressure ventilation (O'Brien 2012), two trials used ventilators to deliver non‐synchronised NIPPV (Jasani 2016; Kahramaner 2014) and another permitted use of ventilator‐driven NIPPV (synchronised or not) or bilevel devices (synchronised or not) (Kirpalani 2013).
Ventilator settings applied after extubation varied between studies. Intermittent mechanical ventilation rates varied between 10 per minute and 40 per minute, and PIP from that used before extubation to 2 cmH2O to 4 cmH2O above that used before extubation. Levels used in NCPAP groups also varied between studies (between 3 cmH2O and 8 cmH2O). No attempt was made to match NIPPV and NCPAP groups with respect to mean airway pressure delivered. Different prongs were used to deliver NCPAP and NIPPV; three studies used nasopharyngeal prongs (single or binasal) (Friedlich 1999; Kahramaner 2014; Khorana 2008), and the others used short nasal prongs. Barrington 2001, Jasani 2016, Khalaf 2001 and Moretti 2008 assessed the primary outcome (respiratory failure) over 72 hours post extubation, Friedlich 1999 and Kahramaner 2014 over 48 hours and Khorana 2008,Kirpalani 2013 and O'Brien 2012 over seven days. The assessment period was unclear in Gao 2010.
Three studies (Barrington 2001; Friedlich 1999; Khalaf 2001) permitted rescue NIPPV for infants not responding to NCPAP but analysed the primary outcome on an intention‐to‐treat basis. Criteria for offering rescue treatment varied; some trials proposed more stringent guidelines for extubation, and duration of follow‐up to endpoints for re‐intubation differed from 48 hours to discharge from the neonatal intensive care unit. Therefore, the term 'need for endotracheal re‐intubation', although available for each of the trials, assumed a clinically different meaning for each.
Excluded studies
We excluded 14 studies (see Characteristics of excluded studies table) for the following reasons.
Enrolled non‐intubated or very shortly intubated (< 6 hours) infants with respiratory distress (Bhandari 2007; Bisceglia 2007; Kishore 2009; Kugelman 2007; Meneses 2011; Ramanathan 2012).
Enrolled infants with apnoea of prematurity (Lin 1998; Pantalitschka 2009; Ryan 1989).
Compared NIPPV versus oxygen via head box after extubation (Kumar 2011).
Was not listed on Clinicaltrials.gov (DeSimone 2010).
Included term infants and infants with respiratory distress and post extubation (Shi 2010).
Reported none of the clinical outcomes listed in the inclusion criteria of this review (Ali 2007; Moretti 1999).
Ongoing studies and studies awaiting clarification include El‐Farash 2013,Estay 2013,Shi 2013,Silveira 2015 and Victor 2011.
Risk of bias in included studies
We assessed methodological quality using the criteria of the Cochrane Neonatal Review Group (Figure 2).
2.
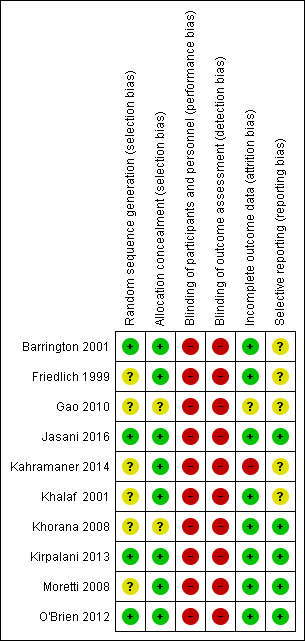
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Random sequence generation: Eight of the included trials met this criterion. This was unclear in two trials (Gao 2010; Kahramaner 2014).
Allocation concealment: This was adequate in eight trials and unclear in two (Gao 2010; Khorana 2008).
Blinding of participants and personnel: No study attempted to blind participants and personnel.
Blinding of outcome assessments: No study attempted this.
Incomplete outcome data: Risk was low in eight trials and unclear in two (Gao 2010; Kahramaner 2014).
Selective reporting: Five trials did not have clearly specified outcomes (Barrington 2001; Friedlich 1999; Gao 2010; Kahramaner 2014; Khalaf 2001).
Other sources of bias: We found no major sources of other bias in the included trials.
Effects of interventions
See: Table 1
Discussion between review authors resulted in no disagreement regarding quality assessment and data extraction from the ten identified trials.
Nasal intermittent positive pressure ventilation versus nasal continuous positive airway pressure to prevent extubation failure
Primary outcome
Respiratory failure post extubation (outcomes 1.1, 1.2 and 1.3)
Six of the ten trials showed statistically significant benefit in terms of respiratory failure 48 hours to seven days post extubation for infants extubated to NIPPV: typical risk ratio (RR) 0.70, 95% confidence interval (CI) 0.60 to 0.80; typical risk difference (RD) ‐0.13, 95% CI ‐0.17 to ‐0.08, with eight infants (95% CI 6 to 13) needing to be treated with NIPPV to prevent one extubation failure (Analysis 1.1). Only one study followed the number of re‐intubations to discharge (Kirpalani 2013) and found no differences between groups. We graded the quality of evidence for this outcome as moderate (unblinded intervention).
1.1. Analysis.
Comparison 1 NIPPV versus NCPAP to prevent extubation failure, Outcome 1 Respiratory failure post extubation.
Seven trials used short binasal prongs (Barrington 2001; Gao 2010; Jasani 2016; Khalaf 2001; Kirpalani 2013; Moretti 2008; O'Brien 2012), two used bi‐nasopharyngeal prongs (Friedlich 1999; Khorana 2008) and one used a shortened endotracheal tube in one nostril (Kahramaner 2014). Both prongs and tube were effective (post hoc analysis).
Owing to methodological limitations (risk of selection bias) in three studies (Gao 2010; Kahramaner 2014; Khorana 2008), we performed a post hoc analysis for the outcome respiratory failure post extubation, including only higher‐quality studies in the analysis (Analysis 1.3). Results were largely unchanged: typical RR 0.73, 95% CI 0.63 to 0.85; typical RD ‐0.11, 95% CI ‐0.16 to ‐0.06.
1.3. Analysis.
Comparison 1 NIPPV versus NCPAP to prevent extubation failure, Outcome 3 Post hoc analysis (high‐quality studies): respiratory failure post extubation.
Secondary outcomes
Endotracheal re‐intubation (outcome 1.2)
Not all NCPAP infants reaching extubation failure criteria were re‐intubated, because a varying proportion of infants in each trial were offered rescue therapy with NIPPV. The pooled estimate of rates of endotracheal re‐intubation favoured NIPPV (typical RR 0.76, 95% CI 0.65 to 0.88; typical RD ‐0.10, 95% CI ‐0.15 to ‐0.05; number needed to treat for an additional beneficial outcome (NNTB) 10, 95% CI 7 to 20; Analysis 1.2). We graded the quality of evidence for this outcome as moderate (unblinded intervention).
1.2. Analysis.
Comparison 1 NIPPV versus NCPAP to prevent extubation failure, Outcome 2 Endotracheal re‐intubation.
Gastrointestinal side effects (outcomes 2.1, 2.2 and 2.3)
Friedlich 1999, Barrington 2001 and Jasani 2016 reported rates of feeding cessation, and Khalaf 2001 provided unpublished data for this outcome. Other studies reported feeding intolerance. Investigators reported no significant differences between groups (typical RR 1.27, 95% CI 0.64 to 2.53; typical RD 0.03, 95% CI ‐0.06 to 0.12; Analysis 2.1). We graded the quality of evidence for this outcome as low (unblinded intervention and imprecision of results).
2.1. Analysis.
Comparison 2 NIPPV versus NCPAP and gastrointestinal complications, Outcome 1 Abdominal distension leading to cessation of feeds.
Results showed no significant differences between groups in the incidence of gastrointestinal perforation (typical RR 0.94, 95% CI 0.60 to 1.48; Analysis 2.2). Six trials reported rates of NEC (Friedlich 1999; Kahramaner 2014; Khorana 2008; Kirpalani 2013; Moretti 2008; O'Brien 2012) and revealed no differences between groups (typical RR 0.87, 95% CI 0.64 to 1.19; Analysis 2.3). We graded the quality of evidence for these outcomes as moderate (unblinded intervention).
2.2. Analysis.
Comparison 2 NIPPV versus NCPAP and gastrointestinal complications, Outcome 2 Gastrointestinal perforation.
2.3. Analysis.
Comparison 2 NIPPV versus NCPAP and gastrointestinal complications, Outcome 3 Necrotising enterocolitis.
Pulmonary outcomes (outcomes 3.1, 3.2 and 3.3)
Six trials reported oxygen need at 36 weeks' corrected gestational age (Barrington 2001; Jasani 2016; Khalaf 2001; Kirpalani 2013; Moretti 2008; O'Brien 2012). Infants randomised to NIPPV did not have significantly lower rates of CLD compared with infants randomised to NCPAP (typical RR 0.94, 95% CI 0.80 to 1.10; typical RD ‐0.02, 95% CI ‐0.08 to 0.03; Analysis 3.1). We graded the quality of evidence for this outcome as moderate (unblinded intervention). Heterogeneity between trials was also moderate (I2= 51%).
3.1. Analysis.
Comparison 3 NIPPV versus NCPAP to improve pulmonary outcomes, Outcome 1 Chronic lung disease (oxygen supplementation at 36 weeks).
In view of new trials added to the review since its first publication, we added the outcome of air leaks to the review. Six trials reported this outcome (Gao 2010; Jasani 2016; Kahramaner 2014; Kirpalani 2013; Moretti 2008; O'Brien 2012) and described reduced air leaks among infants randomised to NIPPV (typical RR 0.48, 95% CI 0.28 to 0.82; typical RD ‐0.03, 95% CI ‐0.05 to ‐0.01; NNTB 33, 95% CI 20 to 100; Analysis 3.2). We rated the quality of evidence for this outcome as moderate (unblinded intervention).
3.2. Analysis.
Comparison 3 NIPPV versus NCPAP to improve pulmonary outcomes, Outcome 2 Air leaks.
Mortality (outcome 4.1)
Six trials (1237 infants) reported mortality (Jasani 2016; Kahramaner 2014; Khorana 2008; Kirpalani 2013; Moretti 2008; O'Brien 2012). Khorana 2008 reported no deaths, and meta‐analysis revealed a small difference between treatment groups (typical RR 0.69, 95% CI 0.48 to 0.99; typical RD ‐0.03, 95% CI ‐0.06 to 0.00; Analysis 4.1). We graded evidence for this outcome as moderate (unblinded intervention).
4.1. Analysis.
Comparison 4 NIPPV versus NCPAP and mortality, Outcome 1 Death before discharge.
Duration of hospitalisation (outcome 5.1)
Meta‐analysis of the four studies that reported this outcome showed that duration of hospitalisation was longer in infants who were randomised to NIPPV (Analysis 5.1). However, these results should be viewed with caution because liberal use of rescue NIPPV for infants not responding to NCPAP within the first days of extubation makes a difference, and longer‐term outcomes, should they exist, are more difficult to establish. We graded the quality of evidence for this outcome as low (unblinded intervention and imprecision of results).
5.1. Analysis.
Comparison 5 NIPPV versus NCPAP and duration of hospital admission, Outcome 1 Duration of hospital admission (days).
Rates of apnoea (outcome 6.1)
Barrington 2001 used continuous multi‐channel recording to detect apnoeic events and noted a trend towards fewer apnoeic episodes per day in the NIPPV group, which did not reach statistical significance (mean difference (MD) ‐3.10, 95% CI ‐7.92 to 1.72; Analysis 6.1).
6.1. Analysis.
Comparison 6 NIPPV versus NCPAP and apnoea, Outcome 1 Rates of apnoea (episodes/24 h).
Subgroup analyses
We planned to perform subgroup analyses to determine whether responses differed according to the modality used to deliver NIPPV (synchronised or not, bilevel devices or NIPPV provided by a ventilator).
Synchronised versus non‐synchronised NIPPV
Five studies used synchronised NIPPV (Barrington 2001; Friedlich 1999; Gao 2010; Khalaf 2001; Moretti 2008), and four did not (Jasani 2016; Kahramaner 2014; Khorana 2008; O'Brien 2012); one study did not prescribe use of either synchronised or non‐synchronised NIPPV (Kirpalani 2013). Both synchronised (typical RR 0.25, 95% CI 0.15 to 0.41) and non‐synchronised studies (typical RR 0.65, 95% CI 0.46 to 0.93) showed overall benefit, and the trial using both methods (typical RR 0.86, 95% CI 0.72 to 1.01) showed no benefit of NIPPV for preventing extubation failure (Analysis 7.1). Heterogeneity was high in this subgroup analysis.
7.1. Analysis.
Comparison 7 NIPPV versus NCPAP (synchronised vs non‐synchronised), Outcome 1 Respiratory failure post extubation.
When CLD was examined by method of delivery of NIPPV, synchronised NIPPV was associated with a reduction in CLD in three trials (181 infants) that could be pooled for this analysis (typical RR 0.64, 95% CI 0.44 to 0.95; typical RD ‐0.15, 95% CI ‐0.28 to 0.02; Analysis 7.6). Air leaks were also reduced in the two trials that used synchronised NIPPV and reported this outcome (typical RR 0.35, 95% CI 0.14 to 0.90; Analysis 7.7).
7.6. Analysis.
Comparison 7 NIPPV versus NCPAP (synchronised vs non‐synchronised), Outcome 6 Chronic lung disease (oxygen supplementation at 36 weeks).
7.7. Analysis.
Comparison 7 NIPPV versus NCPAP (synchronised vs non‐synchronised), Outcome 7 Pulmonary air leak.
NIPPV provided by a ventilator versus bilevel devices
Eight trials used NIPPV delivered via a ventilator, one used bilevel devices (O'Brien 2012) and another used both a ventilator and bilevel devices (Kirpalani 2013). Six of the eight trials using a ventilator to generate NIPPV showed benefit of NIPPV in preventing respiratory failure post extubation (typical RR 0.32, 95% CI 0.22 to 0.47); the two trials that used bilevel (typical RR 0.78, 95% CI 0.50 to 1.21) or both ventilator and bilevel (typical RR 0.86, 95% CI 0.72 to 1.01) showed no benefit (Analysis 8.1). Heterogeneity was high in this subgroup analysis.
8.1. Analysis.
Comparison 8 NIPPV versus NCPAP (ventilator‐generated NIPPV vs bilevel NIPPV), Outcome 1 Respiratory failure post extubation.
When CLD was examined by device delivering NIPPV, NIPPV delivered by a ventilator was associated with a reduction in CLD in the five trials (298 infants) that could be pooled for this analysis (typical RR 0.69, 95% CI 0.50 to 0.95; typical RD ‐0.12, 95% CI ‐0.22 to ‐0.02; Analysis 8.6). Air leaks were also reduced in the four trials that reported this outcome (typical RR 0.35, 95% CI 0.16 to 0.79; Analysis 8.7).
8.6. Analysis.
Comparison 8 NIPPV versus NCPAP (ventilator‐generated NIPPV vs bilevel NIPPV), Outcome 6 Chronic lung disease (oxygen supplementation at 36 weeks).
8.7. Analysis.
Comparison 8 NIPPV versus NCPAP (ventilator‐generated NIPPV vs bilevel NIPPV), Outcome 7 Pulmonary air leak.
We had planned to perform subgroup analysis to determine whether use of methylxanthines alters response. Almost all infants received methylxanthines before extubation, so we did not perform the planned subgroup analysis. We could not perform planned subgroup analyses based on birth weight and age at randomisation, as trial authors did not present data in ways that would allow us to ascertain this information.
Discussion
Meta‐analysis performed for this updated review showed a strong effect of non‐invasive positive pressure ventilation (NIPPV) on extubation failure but no overall effect on chronic lung disease (CLD). Asymmetrical funnel plots for the primary outcome suggest possible small study bias. When outcomes were examined by subgroups, synchronised NIPPV and NIPPV delivered by a ventilator demonstrated short‐term benefit for extubation failure and long‐term pulmonary effects for CLD and pulmonary air leaks. These subgroup analyses included a smaller number of infants and high heterogeneity; thus results should be interpreted with caution. Individual neonatal intensive care units may interpret these results differently.
Most trials included in this review were small, single‐centre studies, and some used devices no longer available on the market. Additional data from one large pragmatic study increased heterogeneity in the meta‐analysis, likely as the result of broader inclusion criteria, multi‐centre status and examination of many devices providing NIPPV. Generalisation of these results to many currently available devices may not be appropriate.
Six of the ten trials identified in this review had no major methodological limitations. Three trials had potential selection bias (Gao 2010; Kahramaner 2014; Khorana 2008), and one was terminated early owing to enrolment difficulties (O'Brien 2012). Because of the nature of the interventions, it has been impossible to blind carers, and bias may have arisen through uneven use of co‐interventions. Investigators dealt with potential confounders, such as methylxanthine usage and weaning strategies, by having management protocols in place; use of objective failure criteria in these extubation trials enhances our confidence in their findings.
Provision of synchronised NIPPV requires a ventilator capable of delivering this mode of support. Few such ventilators are available on the market. Further research should define whether this modality is definitely superior to non‐synchronised NIPPV, and which type of synchronisation is optimal. Similarly, the effect of bilevel or bilevel positive airway pressure devices deserves more study. In preparing this review, we could not determine benefits for a subgroup of infants (smaller and more immature). A newly available method (neurally adjusted ventilatory assist, or NAVA) has shown promise in small case series (Lee 2015; Stein 2012), but further assessment is needed.
Authors' conclusions
Implications for practice.
Use of nasal intermittent positive pressure ventilation (NIPPV) and nasal continuous positive airway pressure (NCPAP) after extubation reduces the incidence of extubation failure within 48 hours to seven days. Studies using synchronised NIPPV and delivering NIPPV to infants by a ventilator observed benefits more consistently. Investigators noted no overall reduction in chronic lung disease among infants randomised to NIPPV and reported a reassuring absence of the gastrointestinal side effects that had been reported in previous case series.
Implications for research.
Future trials should enrol sufficient infants to detect differences in important outcomes such as death or chronic lung disease and should compare different categories of devices. These trials should establish the impact of synchronisation of NIPPV on safety and efficacy of the technique as well as the best combination of settings for NIPPV (rate, peak pressure and positive end expiratory pressure).
What's new
| Date | Event | Description |
|---|---|---|
| 28 September 2015 | New search has been performed | Two new studies were added to the meta‐analysis. |
| 28 September 2015 | New citation required but conclusions have not changed | Search was updated 28 September 2015. |
History
Protocol first published: Issue 4, 1999 Review first published: Issue 3, 2001
| Date | Event | Description |
|---|---|---|
| 23 June 2008 | New search has been performed | This review updated the previous version of "Nasal intermittent positive pressure ventilation (NIPPV) versus nasal continuous positive airway pressure NCPAP) for preterm infants after extubation," which was last updated in the Cochrane Library, Issue 3, 2003 (Davis 2003). A repeat literature search found no new trials eligible for inclusion. No substantive changes have been made to the review. One potentially eligible trial (Yllescas 2004) was presented at APS and may be included in a future update. |
| 14 April 2008 | Amended | The review was converted to new review format. |
| 14 April 2003 | New search has been performed | A repeat literature search showed no new trials eligible for inclusion; no substantive changes were made to the review. |
| 9 May 2001 | New citation required and conclusions have changed | This was a new review. |
Acknowledgements
The review authors acknowledge the generosity of Drs Friedlich, Barrington and Bhandari, who supplied 'preprints' of their manuscripts and additional information for this review. Review authors also acknowledge the generosity of the steering committee of Kirpalani 2013, which provided further data for analysis.
Appendices
Appendix 1. Standard search methods
PubMed: ((infant, newborn[MeSH] OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or infan* or neonat*) AND (randomized controlled trial [pt] OR controlled clinical trial [pt] OR Clinical Trial[ptyp] OR randomized [tiab] OR placebo [tiab] OR clinical trials as topic [mesh: noexp] OR randomly [tiab] OR trial [ti]) NOT (animals [mh] NOT humans [mh])) Embase: (infant, newborn or newborn or neonate or neonatal or premature or very low birth weight or low birth weight or VLBW or LBW or Newborn or infan* or neonat*) AND (human not animal) AND (randomized controlled trial or controlled clinical trial or randomized or placebo or clinical trials as topic or randomly or trial or clinical trial) CINAHL: (infant, newborn OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or Newborn or infan* or neonat*) AND (randomized controlled trial OR controlled clinical trial OR randomized OR placebo OR clinical trials as topic OR randomly OR trial OR PT clinical trial) Cochrane Library: (infant or newborn or neonate or neonatal or premature or very low birth weight or low birth weight or VLBW or LBW)
Data and analyses
Comparison 1. NIPPV versus NCPAP to prevent extubation failure.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Respiratory failure post extubation | 10 | 1431 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.60, 0.80] |
| 1.1 Short (nasal) prongs | 7 | 1275 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.63, 0.84] |
| 1.2 Long (nasopharyngeal) prongs | 3 | 156 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.14, 0.65] |
| 2 Endotracheal re‐intubation | 8 | 1301 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.65, 0.88] |
| 3 Post hoc analysis (high‐quality studies): respiratory failure post extubation | 7 | 1266 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.63, 0.85] |
Comparison 2. NIPPV versus NCPAP and gastrointestinal complications.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Abdominal distension leading to cessation of feeds | 4 | 199 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.64, 2.53] |
| 2 Gastrointestinal perforation | 5 | 1066 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.60, 1.48] |
| 3 Necrotising enterocolitis | 6 | 1214 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.64, 1.19] |
Comparison 3. NIPPV versus NCPAP to improve pulmonary outcomes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Chronic lung disease (oxygen supplementation at 36 weeks) | 6 | 1140 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.80, 1.10] |
| 2 Air leaks | 6 | 1229 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.28, 0.82] |
Comparison 4. NIPPV versus NCPAP and mortality.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death before discharge | 6 | 1237 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.48, 0.99] |
Comparison 5. NIPPV versus NCPAP and duration of hospital admission.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Duration of hospital admission (days) | 4 | 238 | Mean Difference (IV, Fixed, 95% CI) | 2.77 [0.04, 5.51] |
Comparison 6. NIPPV versus NCPAP and apnoea.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Rates of apnoea (episodes/24 h) | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | ‐3.10 [‐7.92, 1.72] |
Comparison 7. NIPPV versus NCPAP (synchronised vs non‐synchronised).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Respiratory failure post extubation | 10 | 1431 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.60, 0.80] |
| 1.1 Synchronised NIPPV | 5 | 272 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.15, 0.41] |
| 1.2 Non‐synchronised NIPPV | 4 | 314 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.46, 0.93] |
| 1.3 Mixed NIPPV devices | 1 | 845 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.72, 1.01] |
| 2 Endotracheal re‐intubation during the week post extubation | 10 | 1431 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.64, 0.85] |
| 2.1 Synchronised NIPPV | 5 | 272 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.19, 0.57] |
| 2.2 Non‐synchronised NIPPV | 4 | 314 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.46, 0.93] |
| 2.3 Mixed NIPPV devices | 1 | 845 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.72, 1.01] |
| 3 Abdominal distension requiring cessation of feeds | 3 | 136 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.76 [0.77, 4.05] |
| 3.1 Synchronised NIPPV | 3 | 136 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.76 [0.77, 4.05] |
| 3.2 Non‐synchronised NIPPV | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.3 Mixed NIPPV devices | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Gastrointestinal perforation | 5 | 1052 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.72, 1.01] |
| 4.1 Synchronised NIPPV | 3 | 159 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Non‐synchronised NIPPV | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 Mixed NIPPV devices | 1 | 845 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.72, 1.01] |
| 5 Necrotising enterocolitis | 6 | 1214 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.64, 1.19] |
| 5.1 Synchronised NIPPV | 5 | 1147 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.64, 1.20] |
| 5.2 Non‐synchronised NIPPV | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.11, 4.79] |
| 5.3 Mixed NIPPV devices | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Chronic lung disease (oxygen supplementation at 36 weeks) | 6 | 1108 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.79, 1.10] |
| 6.1 Synchronised NIPPV | 3 | 181 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.44, 0.95] |
| 6.2 Non‐synchronised NIPPV | 2 | 185 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.47, 1.16] |
| 6.3 Mixed NIPPV devices | 1 | 742 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.88, 1.30] |
| 7 Pulmonary air leak | 6 | 1222 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.44, 1.02] |
| 7.1 Synchronised NIPPV | 2 | 113 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.14, 0.90] |
| 7.2 Non‐synchronised NIPPV | 3 | 259 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.58, 2.08] |
| 7.3 Mixed NIPPV devices | 1 | 850 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.29, 1.28] |
| 8 Rates of apnoea (episodes/24 h) | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | ‐3.10 [‐7.92, 1.72] |
| 8.1 Synchronised NIPPV | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | ‐3.10 [‐7.92, 1.72] |
| 8.2 Non‐synchronised NIPPV | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.3 Mixed NIPPV devices | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Duration of hospitalisation (days) | 4 | 244 | Mean Difference (IV, Fixed, 95% CI) | 2.72 [‐0.01, 5.44] |
| 9.1 Synchronised NIPPV | 3 | 181 | Mean Difference (IV, Fixed, 95% CI) | 3.39 [0.52, 6.25] |
| 9.2 Non‐synchronised NIPPV | 1 | 63 | Mean Difference (IV, Fixed, 95% CI) | ‐3.48 [‐12.20, 5.24] |
| 9.3 Mixed NIPPV devices | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Death before discharge | 6 | 1237 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.48, 0.99] |
| 10.1 Synchronised NIPPV | 2 | 111 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.21, 4.44] |
| 10.2 Non‐synchronised NIPPV | 3 | 266 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.16, 0.75] |
| 10.3 Mixed NIPPV devices | 1 | 860 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.55, 1.31] |
7.2. Analysis.
Comparison 7 NIPPV versus NCPAP (synchronised vs non‐synchronised), Outcome 2 Endotracheal re‐intubation during the week post extubation.
7.3. Analysis.
Comparison 7 NIPPV versus NCPAP (synchronised vs non‐synchronised), Outcome 3 Abdominal distension requiring cessation of feeds.
7.4. Analysis.
Comparison 7 NIPPV versus NCPAP (synchronised vs non‐synchronised), Outcome 4 Gastrointestinal perforation.
7.5. Analysis.
Comparison 7 NIPPV versus NCPAP (synchronised vs non‐synchronised), Outcome 5 Necrotising enterocolitis.
7.8. Analysis.
Comparison 7 NIPPV versus NCPAP (synchronised vs non‐synchronised), Outcome 8 Rates of apnoea (episodes/24 h).
7.9. Analysis.
Comparison 7 NIPPV versus NCPAP (synchronised vs non‐synchronised), Outcome 9 Duration of hospitalisation (days).
7.10. Analysis.
Comparison 7 NIPPV versus NCPAP (synchronised vs non‐synchronised), Outcome 10 Death before discharge.
Comparison 8. NIPPV versus NCPAP (ventilator‐generated NIPPV vs bilevel NIPPV).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Respiratory failure post extubation | 10 | 1431 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.60, 0.80] |
| 1.1 Ventilator‐generated NIPPV | 8 | 450 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.22, 0.47] |
| 1.2 Bilevel NIPPV | 1 | 136 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.50, 1.21] |
| 1.3 Mixed NIPPV devices | 1 | 845 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.72, 1.01] |
| 2 Endotracheal re‐intubation during the week post extubation | 10 | 1431 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.64, 0.85] |
| 2.1 Ventilator‐generated NIPPV | 8 | 450 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.26, 0.59] |
| 2.2 Bilevel NIPPV | 1 | 136 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.50, 1.21] |
| 2.3 Mixed NIPPV devices | 1 | 845 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.72, 1.01] |
| 3 Abdominal distension requiring cessation of feeds | 3 | 136 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.76 [0.77, 4.05] |
| 3.1 Ventilator‐generated NIPPV | 3 | 136 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.76 [0.77, 4.05] |
| 3.2 Bilevel NIPPV | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.3 Mixed NIPPV devices | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Gastrointestinal perforation | 6 | 1133 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.60, 1.48] |
| 4.1 Ventilator‐generated NIPPV | 6 | 1133 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.60, 1.48] |
| 4.2 Bilevel NIPPV | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 Mixed NIPPV devices | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Necrotising enterocolitis | 6 | 1214 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.64, 1.19] |
| 5.1 Ventilator‐generated NIPPV | 4 | 219 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.21, 1.93] |
| 5.2 Bilevel NIPPV | 1 | 136 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.38, 2.78] |
| 5.3 Mixed NIPPV devices | 1 | 859 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.63, 1.24] |
| 6 Chronic lung disease (oxygen supplementation at 36 weeks) | 7 | 1168 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.81, 1.11] |
| 6.1 Ventilator‐generated NIPPV | 5 | 298 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.50, 0.95] |
| 6.2 Bilevel NIPPV | 1 | 128 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.59, 1.55] |
| 6.3 Mixed NIPPV devices | 1 | 742 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.88, 1.30] |
| 7 Pulmonary air leak | 6 | 1229 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.28, 0.82] |
| 7.1 Ventilator‐generated NIPPV | 4 | 243 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.16, 0.79] |
| 7.2 Bilevel NIPPV | 1 | 136 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.3 Mixed NIPPV devices | 1 | 850 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.29, 1.28] |
| 8 Rates of apnoea (episodes/24 h) | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | ‐3.10 [‐7.92, 1.72] |
| 8.1 Ventilator‐generated NIPPV | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | ‐3.10 [‐7.92, 1.72] |
| 8.2 Bilevel NIPPV | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.3 Mixed NIPPV devices | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Duration of hospitalisation (days) | 4 | 244 | Mean Difference (IV, Fixed, 95% CI) | 2.72 [‐0.01, 5.44] |
| 9.1 Ventilator‐generated NIPPV | 4 | 244 | Mean Difference (IV, Fixed, 95% CI) | 2.72 [‐0.01, 5.44] |
| 9.2 Bilevel NIPPV | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.3 Mixed NIPPV devices | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Death before discharge | 6 | 1237 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.48, 0.99] |
| 10.1 Ventilator‐generated NIPPV | 4 | 241 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.18, 0.81] |
| 10.2 Bilevel NIPPV | 1 | 136 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.15, 2.48] |
| 10.3 Mixed NIPPV devices | 1 | 860 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.55, 1.31] |
8.2. Analysis.
Comparison 8 NIPPV versus NCPAP (ventilator‐generated NIPPV vs bilevel NIPPV), Outcome 2 Endotracheal re‐intubation during the week post extubation.
8.3. Analysis.
Comparison 8 NIPPV versus NCPAP (ventilator‐generated NIPPV vs bilevel NIPPV), Outcome 3 Abdominal distension requiring cessation of feeds.
8.4. Analysis.
Comparison 8 NIPPV versus NCPAP (ventilator‐generated NIPPV vs bilevel NIPPV), Outcome 4 Gastrointestinal perforation.
8.5. Analysis.
Comparison 8 NIPPV versus NCPAP (ventilator‐generated NIPPV vs bilevel NIPPV), Outcome 5 Necrotising enterocolitis.
8.8. Analysis.
Comparison 8 NIPPV versus NCPAP (ventilator‐generated NIPPV vs bilevel NIPPV), Outcome 8 Rates of apnoea (episodes/24 h).
8.9. Analysis.
Comparison 8 NIPPV versus NCPAP (ventilator‐generated NIPPV vs bilevel NIPPV), Outcome 9 Duration of hospitalisation (days).
8.10. Analysis.
Comparison 8 NIPPV versus NCPAP (ventilator‐generated NIPPV vs bilevel NIPPV), Outcome 10 Death before discharge.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Barrington 2001.
| Methods | Randomised controlled trial comparing effects of synchronised NIPPV vs NCPAP in preterm infants after extubation | |
| Participants | Infants with birth weight < 1250 grams (mean 831 ± 193 grams), < 6 weeks of age (mean 7.6 ± 9.7 days), requiring < 35% O2 and < 18 breaths/min on SIMV. All infants were loaded with aminophylline before extubation. | |
| Interventions | Experimental group: synchronised NIPPV = nSIMV; ventilator rate 12 breaths/min and PIP 16 cmH2O, PEEP 6 cmH2O, PIP increased to achieve measured pressure ≥ 12 cmH2O. Graseby capsule, Infant Star ventilator and Hudson nasal prongs were used. Control group: NCPAP 6 cmH2O | |
| Outcomes | Primary: failure of extubation by 72 hours because pCO2 > 70 mmHg, oxygen requirement > 70% or apnoea was severe or recurrent (defined) Secondary: rates of re‐intubation, abdominal distension, feeding intolerance and CLD | |
| Notes | Power calculation performed 54 infants enrolled ‐ 27 in each group Most infants not responding to NCPAP were tried on NIPPV before re‐intubation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Table of random numbers |
| Allocation concealment (selection bias) | Low risk | Adequate: sequentially numbered sealed opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unblinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for |
| Selective reporting (reporting bias) | Unclear risk | Secondary outcomes not listed |
Friedlich 1999.
| Methods | Randomised controlled trial comparing effects of synchronised NIPPV vs NCPAP in preterm infants after extubation | |
| Participants | Infants with birth weight 500 to 1500 grams (mean 963 ± 57 grams in experimental group and 944 ± 43 grams in control group) considered ready for extubation (SIMV rate < 12 breaths/min, peak pressure < 23 cmH2O, end expiratory pressure < 6 cmH2O, oxygen requirement < 40%. Aminophylline not mandated but given to about 85% of infants. Extubated at 26.3 ± 6.1 days of life in experimental group and at 19.9 ± 3.8 days of life in control group Excluded were infants with sepsis, NEC, symptomatic PDA, congenital anomalies. | |
| Interventions | Experimental group: nasopharyngeal 3‐Fr gauge tube, Infant Star ventilator, synchronised NIPPV = nSIMV with rate of 10 breaths/min, PIP = that before extubation, PEEP 4‐6 cmH2O, IT 0.6 seconds Control group: nasopharyngeal CPAP to desired level of attending | |
| Outcomes | Primary: failure of extubation by 48 hours because pH < 7.25, pCO2 increased by 25%, oxygen requirement > 60%, SIMV rate > 20 (in NIPPV group), PIP > 26 cmH2O or PEEP > 8 cmH2O in NIPPV group, or apnoea requiring bag and mask ventilation Secondary: endotracheal re‐intubation, abdominal distension, perforation or NEC, feeding delay (not defined) and nasal bleeding | |
| Notes | Power calculation performed. Study was closed early after interim analysis (stopping rule not specified). 41 infants were enrolled: 22 in NIPPV group and 19 in NCPAP group. Most infants not responding to NCPAP were tried on NIPPV before re‐intubation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unspecified |
| Allocation concealment (selection bias) | Low risk | Adequate: sealed randomisation cards |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unblinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All accounted for |
| Selective reporting (reporting bias) | Unclear risk | No outcomes listed, only objectives for the study |
Gao 2010.
| Methods | Randomised controlled trial comparing effects of synchronised NIPPV vs NCPAP in preterm infants after extubation | |
| Participants | Infants at gestational age < 36 weeks (mean 32.3 ± 1.6 weeks in experimental group and 32.6 ± 1.4 weeks in control group) with birth weight < 2000 grams (mean 1264 ± 153 grams in experimental group and 1246 ± 161 grams in control group) with RDS after surfactant Excluded were infants with severe abnormalities, not specified. |
|
| Interventions | Experimental group: synchronised NIPPV via different devices (NEWPORT 150 and 200; Teama; Stephan; Millennium). Rate 40 breaths/min, PIP 20 cmH2O, PEEP 5 cmH2O Control group: NCPAP via Infant Flow Driver (EME); CPAP 4 to 8 cmH2O |
|
| Outcomes | Failed extubation, defined as pCO2 > 70 mmHg or FiO2 > 0.6 to maintain SaO2 > 88% or mean pressure > 8 cmH2O (in NIPPV group) or severe apnoea (in NCPAP group), defined as > 6 episodes in 24 hours or > 2 episodes requiring PPV. Air leak, hypercapnia, hypoxia | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | No study flow diagram; unclear if exclusions; English translation imprecise and unclear |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded intervention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unblinded intervention |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No flow diagram |
| Selective reporting (reporting bias) | Unclear risk | No outcomes listed |
Jasani 2016.
| Methods | Randomised controlled trial comparing effects of non‐synchronised NIPPV vs NCPAP in preterm infants after extubation | |
| Participants | Preterm infants ≤ 1500 grams at birth (mean 1187 grams at 30.8 weeks in the intervention group; mean 1153 grams at 30.6 weeks in the control group) Excluded were infants with suspected upper airway obstruction, airway anomaly, major cardiopulmonary malformation. |
|
| Interventions | Experimental group: NIPPV provided by Bear Cub ventilator, non‐synchronised. Rate same as pre‐extubation, PIP 4 over pre‐extubation settings, PEEP ≤ 5 Control group: CPAP 5 to 6 cmH2O via Bear Cub or Viasys Infant Flow CPAP |
|
| Outcomes | Failed extubation within 72 hours, defined as meeting 1 or more of the following: pH < 7.26 and pCO2 > 59 mmHg; recurrent apnoea (> 2 episodes per hour); 1 apnoea needing PPV or paO2 < 51 mmHg with FiO2 > .59 Secondary outcomes: duration of non‐invasive ventilation, total duration of ventilation, days on oxygen, air leaks, BPD, PDA, IVH grade 3‐4, NEC, ROP ≥ grade 3, length of stay, mortality, GI perforation |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation software |
| Allocation concealment (selection bias) | Low risk | Opaque sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Intervention could not be blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Despite criteria to define extubation failure, intervention was not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for |
| Selective reporting (reporting bias) | Low risk | |
Kahramaner 2014.
| Methods | Randomised controlled trial comparing effects of non‐synchronised nasal intermittent positive pressure ventilation vs continuous positive airway pressure in preterm infants after extubation | |
| Participants | A total of 67 premature infants at < 35 weeks' gestation with birth weight < 2000 grams receiving mechanical ventilation because of respiratory distress syndrome (RDS) | |
| Interventions | Intervention group: non‐synchronised nasal NIPPV with shortened endotracheal tube via Babylog 8000. Rate 25, PIP 2 cmH2O above pre‐extubation setting, PEEP 6 cmH2O. Control group: NCPAP 6 cmH2O with binasal prongs via Sindi driver | |
| Outcomes | Extubation failure at 48 hours, defined as pH < 7.25 and pCO2 > 60 or severe or frequent apnoea, or FiO2 > 60% to keep SaO2 88% to 93% or frequent desaturations not responding to increasing settings Secondary outcomes: air leak, death, NEC, IVH, severe IVH, ROP, sepsis, nasal injury, BPD |
|
| Notes | Outcomes not specified in the Methods section. No sample size calculation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified in the text |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded intervention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unblinded intervention |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No flow diagram. No sample size calculation |
| Selective reporting (reporting bias) | Unclear risk | No outcomes specified in the Methods section |
Khalaf 2001.
| Methods | Randomised controlled trial comparing effects of synchronised NIPPV vs NCPAP in preterm infants after extubation | |
| Participants | Infants at gestational age < 34 weeks with RDS ventilated by endotracheal tube. Mean birth weight 1088 grams in experimental group and 1032 grams in control group with mean gestational age 28 weeks. Ventilator settings PIP ≤ 16 cmH2O, PEEP ≤ 5 cmH2O, ventilator rate 15 to 25 breaths/min and O2 < 35%. All had a therapeutic blood level of aminophylline and hematocrit > 40%. | |
| Interventions | Experimental group: synchronised NIPPV via Argyle prongs, Infant Star ventilator at PEEP ≤ 5 cmH2O, with ventilator rate 15 to 25 breaths/min and PIP set at 2 to 4 cmH2O above that used pre‐extubation. Gas flow set at 8 to 10 L/min in both groups Control group: NCPAP delivered by Argyle prongs from a Bear Cub or Infant Star ventilator at 4 to 6 cmH2O | |
| Outcomes | Primary: failure of extubation by 72 hours because pH < 7.25 or pCO2 > 60 mmHg, single episode of severe apnoea requiring bag and mask ventilation or frequent apnoea or desaturations (defined) Secondary: included CLD defined as supplemental O2 requirement at 36 weeks' corrected age, days of ventilation and hospitalisation. Data on rates of feeding intolerance provided by study authors | |
| Notes | Power calculation was performed. 64 infants were enrolled: 34 in NIPPV group and 30 in NCPAP group. 2 infants not responding to NCPAP were tried on NIPPV (successfully) before re‐intubation. For the outcome "abdominal distension causing cessation of feeds", denominators were number of infants offered enteral feeds during the 72‐hour study period, i.e. 21 in NIPPV group and 20 in NCPAP group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Low risk | Adequate: sealed randomisation cards |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded intervention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unblinded intervention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for |
| Selective reporting (reporting bias) | Unclear risk | No outcomes stated besides objectives |
Khorana 2008.
| Methods | Randomised controlled trial comparing effects of non‐synchronised NIPPV vs NCPAP in preterm infants after extubation | |
| Participants | Infants ≤ 34 weeks (mean 28 ± 2.6 weeks and 29 ± 2.1 weeks) or birth weight ≤ 1500 grams (mean 984 ± 218 grams and 1185 ± 219 grams) considered ready for extubation (SIMV rate ≤ 15 breaths/min, peak pressure ≤ 15 cmH2O, end expiratory pressure < 6 cmH2O, O2 requirement < 41%. Aminophylline pre‐extubation. Extubated at 12.9 ± 9.9 days of life in experimental group and at 6.9 ± 6.0 days of life in control group Excluded were infants with major congenital malformations, cleft lip or palate, symptomatic PDA, NEC, sepsis, IVH grade 3‐4. | |
| Interventions | Experimental group: non‐synchronised NIPPV via bi‐nasopharyngeal prongs on Bear 750 ventilator; same settings as pre‐extubation on the ventilator Control group: NCPAP delivered by bi‐nasopharyngeal prongs from a Bear 750 ventilator; CPAP level same as pre‐extubation level Aminophylline load before extubation |
|
| Outcomes | Primary: re‐intubation or failure of extubation within 1 week because of:
Secondary: respiratory failure, death, abdominal distension, NEC, gastrointestinal perforation, apnoea, atelectasis and sepsis |
|
| Notes | Imbalance between groups at randomisation (NIPPV group: lower birth weight, fewer boys, higher antenatal steroids) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded intervention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unblinded intervention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for |
| Selective reporting (reporting bias) | Low risk | All specified secondary outcomes were reported. |
Kirpalani 2013.
| Methods | Randomised multi‐centre controlled trial comparing effects of NIPPV vs NCPAP in preterm infants after extubation | |
| Participants | Infants with birth weight < 1000 grams at < 30 weeks 2 subgroups: intubated for > 24 hours and < 28 days at extubation; intubated < 24 hours or never intubated. Infants never intubated were not included in this review. Criteria for extubation and for re‐intubation were provided. |
|
| Interventions | NIPPV (any device) vs NCPAP; guidelines provided for both | |
| Outcomes | Death or moderate to severe BPD according to physiological definition | |
| Notes | 2 subgroups of infants; only subgroup intubated for at least 24 hours and extubated before 28 days included in this review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Web‐based randomisation |
| Allocation concealment (selection bias) | Low risk | Randomisation immediately pre‐extubation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded intervention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unblinded intervention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Flow diagram in full study |
| Selective reporting (reporting bias) | Low risk | |
Moretti 2008.
| Methods | Randomised controlled trial comparing effects of synchronised NIPPV vs NCPAP in preterm infants after extubation | |
| Participants | Infants with birth weight < 1251 grams (mean 908 ± 192 grams in experimental group and 957 ± 213 grams in control group) with RDS requiring ventilation within first 48 hours of life and who met criteria for extubation by day 14 of life. Criteria for extubation: stable or improving clinical condition; receiving assist/control or proportional assist ventilation; low ventilatory setting (FiO2 ≤ 0.35, PIP ≤ 15 cmH2O and ventilator rate ≤ 15 breaths/min or elastance < 1 to maintain pCO2 ≤ 60 mmHg); no clinical or haematological sign of infection; haemoglobin ≥ 10.0 grams/dL Extubated at a median of 4 days (range 1 to 14) and 6 days (range 1 to 14) Excluded were infants with IVH grade 3 to 4, gastrointestinal surgery, congenital malformation and cardiovascular and neuromuscular abnormalities. | |
| Interventions | Experimental group: synchronised NIPPV via short nasal prongs on Giulia ventilator; same settings as pre‐extubation on the ventilator. PEEP 3 to 5 cmH2O, PIP titrated according to infant from 10 to 20 cmH2O, flow rate 6 to 10 L/min
Control group: NCPAP delivered by short nasal prongs from a Giulia ventilator; CPAP level 3 to 5 cmH2O; flow rate 6 to 10 L/min Caffeine load before extubation |
|
| Outcomes | Primary: need for re‐intubation within 72 hours because of:
Secondary: number of days on endotracheal mechanical ventilation, number of days on O2, CLD (O2 at 36 weeks with abnormal chest x‐ray), duration of hospital stay, air leaks, ROP, sepsis, feeding intolerance (presence of 4‐hour gastric aspirate > 25% of feed volume or containing bile) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes to conceal allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded intervention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unblinded intervention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No flow diagram |
| Selective reporting (reporting bias) | Low risk | All outcomes accounted for |
O'Brien 2012.
| Methods | Randomised controlled trial comparing effects of non‐synchronised NIPPV delivered by a bilevel device vs NCPAP in preterm infants after extubation | |
| Participants | Infants with birth weight < 1251 grams (mean 901 ~± 200 grams in experimental group and 896 ± 156 grams in control group) who were intubated at birth for RDS (some were prophylactically intubated to received surfactant) and who met criteria for extubation (no time limit) Criteria for extubation: ventilator rate < 20 breaths/min, PIP ≥ 16 cmH2O and FiO2 ≥ .35. If on high‐frequency oscillatory ventilation: frequency of 9 to 13 Hz, amplitude < 20%, MAP ≤ 8 and FiO2 ≤ 35%. Extubated at a median of 3 days (range 1 to 67) in experimental group and 3 days (range 1 to 62) in control group. All received caffeine during first week of life. Unclear if load pre‐extubation Excluded were infants with congenital anomalies of the upper airway, acquired nasal septum injury and major congenital or chromosomal abnormalities. |
|
| Interventions | Experimental group: NIPPV with SiPAP bilevel device, non‐synchronised. Ventilator rate 20 breaths/min, IT 1.0 second. Predefined upper and lower CPAP based on FiO2: 8 over 5 for FiO2 < 30%; 9 over 6 for FiO2 30% to 50%; and 10 over 7 for FiO2 > 50% Control group: CPAP with SiPAP. Level of CPAP predefined on the basis of FiO2: 5 cmH2O if FiO2 < .30; 6 mH2O if FiO2 .30 to .50; and 7 mH2O if FiO2 > .50 |
|
| Outcomes | Primary: sustained extubation for 7 days. Re‐intubation criteria: severe apnoea (needing PPV), ≥ 4 apnoeic episodes per hour needing moderate stimulation, O2 > 60%, uncompensated respiratory acidosis (pH < 7.25). Re‐intubation also allowed at clinician discretion for other reasons (concerns regarding sepsis) Secondary: adverse events (nasal septal injury or erythema, eyelid oedema, abdominal distension, feeding intolerance, pneumothorax). Feeding intolerance = aspirates ≥ 30% of a feed. Abdominal distension ≥ 10% increase in girth. Also: BPD, PDA treated, NEC, IVH 3 to 4, periventricular leukomalacia, ROP |
|
| Notes | Study authors overestimated successful extubations in their control group. Recruitment was stopped at half the sample size, as clinical practice had changed and babies were no longer being intubated prophylactically. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random list |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded intervention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unblinded intervention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Flow diagram attached |
| Selective reporting (reporting bias) | Low risk | All outcomes accounted for and reported |
BPD: bronchopulmonary dysplasia; CLD: chronic lung disease; CPAP: continuous positive airway pressure; FiO2: fraction of inspired oxygen; IT: inspiratory time; IVH: intraventricular haemorrhage; NCPAP: nasal continuous positive airway pressure; NEC: necrotising enterocolitis; NIPPV: nasal intermittent positive pressure ventilation; nSIMV: nasal synchronised intermittent mechanical ventilation; pO2: partial pressure of oxygen; pCO2: partial pressure of carbon dioxide; PDA: patent ductus arteriosus; PEEP: positive end expiratory pressure; PIP: peak inspiratory pressure; PPV: positive pressure ventilation; RDS: respiratory distress syndrome; ROP: retinopathy of prematurity; SaO2: oxygen saturation measured by blood analysis; SIMV: synchronised intermittent mechanical ventilation; SiPAP: synchronised inspiratory positive airway pressure.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ali 2007 | Study compared a form of NIPPV (non‐invasive pressure support ventilation) vs NCPAP in a randomised cross‐over study. Investigators compared short‐term physiological outcomes (tidal volume, breathing effort, etc.). We excluded this study because investigators reported none of the clinical outcomes listed in the inclusion criteria for this review. |
| Bhandari 2007 | Randomised trial of NIPPV vs conventional ventilation for management of RDS (i.e. different groups compared for a different indication). Timing of extubation was different between groups. |
| Bisceglia 2007 | Randomised trial of NIPPV vs NCPAP for moderate RDS (i.e. different inclusion criteria) |
| DeSimone 2010 | Extubation criteria were different for NIPPV and NCPAP groups, whereas infants randomised to NIPPV were extubated from higher ventilator settings. |
| Kishore 2009 | Infants were enrolled early during acute respiratory illness. Intervention was aimed at treating RDS, not upon extubation. |
| Kugelman 2007 | Infants were enrolled early during acute respiratory illness. Intervention was aimed at treating RDS, not upon extubation. |
| Kumar 2011 | RCT evaluating the role of unsynchronised NIPPV vs head box oxygen for prevention of extubation failure in mechanically ventilated preterm neonates weighing less than 2000 grams |
| Lin 1998 | Infants enrolled had apnoea of prematurity; NIPPV or NCPAP was used for treatment. |
| Meneses 2011 | Infants were enrolled early during acute respiratory illness. Intervention was aimed at treating RDS, not upon extubation. |
| Moretti 1999 | Randomised cross‐over trial Each of 11 infants (mean body weight 1141 grams) received NIPPV and NCPAP in random order for a period of 1 hour. Outcomes included respiratory rates and pulmonary function tests (i.e. not outcome criteria specified in the protocol). |
| Pantalitschka 2009 | Infants enrolled had apnoea of prematurity; NIPPV or NCPAP was used for treatment. |
| Ramanathan 2012 | Infants were randomised after INSURE procedure and extubation was planned within 2 hours of life. We included this study in the review: early NIPPV vs NCPAP for RDS |
| Ryan 1989 | Infants enrolled had apnoea of prematurity; NIPPV or NCPAP was used for treatment. |
| Shi 2010 | RCT comparing NIPPV vs NCPAP. Not all enrolled infants were premature; mean birth weight of enrolled infants was 2380 grams (experimental group) vs 2416 grams (control group). Unclear if intervention was provided to treat RDS, or if RDS occurred after extubation |
INSURE: intubation, surfactant, extubation; NCPAP: nasal continuous positive airway pressure; NIPPV: nasal intermittent positive pressure ventilation; RCT: randomised controlled trial; RDS: respiratory distress syndrome.
Characteristics of studies awaiting assessment [ordered by study ID]
Silveira 2015.
| Methods | Randomised, prospective, clinical trial |
| Participants | 80 newborns (gestational age < 37 weeks, birth weight < 2500 grams). Infants could be randomised early (for initial management of respiratory distress syndrome (RDS)) or later, after extubation (mean of day 3). |
| Interventions | 40 infants were treated with nasal continuous positive airway pressure (CPAP) and 40 with nasal intermittent positive‐pressure ventilation (NIPPV) |
| Outcomes | Occurrence of apnoea, progression of respiratory distress, nose bleeding and agitation were defined as ventilation failure. The need for intubation and re‐intubation after failure was observed. |
| Notes | A portion of the study population may be eligible for inclusion in this review. Communication with a study investigator is ongoing. |
Characteristics of ongoing studies [ordered by study ID]
El‐Farash 2013.
| Trial name or title | Nasal Continuous Airway Pressure (n‐CPAP) vs Nasal Bilevel Positive Airway Pressure (n‐BiPAP) for RDS |
| Methods | Randomised controlled trial |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Bilevel positive airway pressure and NCPAP |
| Outcomes | Primary: failure of extubation during the first 48 hours post extubation, defined as uncompensated respiratory acidosis defined as pH < 7.2 and PaCO2 > 60 mmHg (or) major apnoea requiring mask ventilation Secondary: maintenance of successful extubation for 7 days from the hour of extubation |
| Starting date | January 2013 |
| Contact information | Rania A. El‐Farrash, MD |
| Notes |
Estay 2013.
| Trial name or title | Non‐Invasive Ventilation vs Continuous Positive Airway Pressure After Extubation in Very Low Birth Weight Infants |
| Methods | Randomised controlled trial |
| Participants | Infants < 1500 grams and < 34 weeks with RDS and ready for extubation (> 2 hours but < 14 days) Exclusion criteria: major congenital anomalies, presence of cardiovascular instability, intubation < 2 hours, mechanical ventilation > 14 days, using muscle relaxant, airway anomalies, consent not provided or refused |
| Interventions | Non‐synchronised NIPPV and NCPAP |
| Outcomes | Primary: assessment of the need for re‐intubation within the first 72 hours after extubation in the 2 groups Criteria for failure were met by at least 1 of the following: pH < 7.25 and pCO2 > 65 mmHg; more than 2 episodes of recurrent apnoea per hour associated with bradycardia during 4‐hour continuous; 2 episodes of apnoea that required bag and mask ventilation any time during the study; PaO2 < 50 mmHg with FiO2 > 0.6 Secondary: concerning respiratory support: total duration on endotracheal tube ventilation; total duration on NCPAP; total duration on supplemental O2, incidence of pneumothorax, BPD and death. Other outcomes included incidence of patent ductus arteriosus, necrotising enterocolitis, intraventricular haemorrhage grades 3 and 4, retinopathy of prematurity stage 3, time to full feeds and length of hospital stay |
| Starting date | December 2011 |
| Contact information | Alberto Estay, MD |
| Notes | Expected completion date: June 2013 |
NCT02396693.
| Trial name or title | Successful Extubation and Non‐invasive Ventilation in Preterm ≤ 1500 grams |
| Methods | Randomised controlled trial |
| Participants | Infants 26 to 34 weeks, 500 to 1500 grams, with diagnosis of RDS and first elective extubation |
| Interventions | NIPPV (unspecified device) vs bubble CPAP |
| Outcomes | Success rate of extubation, total duration of oxygen use, mechanical ventilation and bronchopulmonary dysplasia (BPD), days of oxygen use, mechanical ventilation |
| Starting date | August 2012 |
| Contact information | Cintia Johnston |
| Notes | Completed. No publication identified |
Shi 2013.
| Trial name or title | Nasal Intermittent Positive Pressure Ventilation in Newborn Infants With Respiratory Distress Syndrome |
| Methods | Randomised controlled trial |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | NIPPV via Bird ventilator or NCPAP (after extubation or as a primary mode of respiratory support) |
| Outcomes | Primary: incidence of mechanical ventilation via endotracheal tube after non‐invasive respiratory support within 7 days Secondary: overall clinical outcomes at 7 days', 28 days' and 36 weeks' postmenstrual age |
| Starting date | January 2008 |
| Contact information | Yuan Shi, MD |
| Notes | Expected completion date: December 2011; information last updated on Clinicaltrials.gov 17 December 2012. Part of the study population could be included in an updated version of this review. |
Victor 2011.
| Trial name or title | A Randomised Controlled Trial of Nasal Biphasic Positive Airway Pressure vs Nasal Continuous Positive Airway Pressure Following Extubation in Infants Less Than 30 Weeks’ Gestation: Study Protocol for a Randomised Controlled Trial |
| Methods | Unblinded multi‐centre randomised trial |
| Participants | Infants born before 30 weeks’ gestation and less than 2 weeks old. Infants with congenital abnormalities and severe intraventricular haemorrhage will be excluded. 540 infants admitted to neonatal centres in England will be randomised at the time of first extubation attempt. |
| Interventions | Unblinded multi‐centre randomised trial comparing NCPAP vs n‐BiPAP |
| Outcomes | Primary aim of this study is to compare rate of extubation failure within 48 hours after first attempt at extubation. Secondary aims are to compare effects of n‐BiPAP and n‐CPAP on the following outcomes. • Maintenance of successful extubation for 7 days post extubation • Oxygen requirement at 28 days of age and at 36 weeks’ postmenstrual age • Total days on ventilator, n‐CPAP/n‐BiPAP • Number of ventilator days after first extubation attempt • pH and partial pressure of carbon dioxide in the first postextubation blood gas • Duration of hospital stay • Rate of abdominal distension requiring cessation of feeds • Rates of apnoea and bradycardia • Age at transfer back to referral centre in days |
| Starting date | |
| Contact information | suresh.victor@manchester.ac.uk 1 Ward 68, 2nd Floor, St Mary’s Hospital for Women and Children, Manchester, UK M13 9WL |
| Notes | Trial registration number: ISRCTN: ISRCTN18921778 http://www.trialsjournal.com/content/12/1/257 TRIALS |
BPD: bronchopulmonary dysplasia; CPAP: continuous positive airway pressure; FiO2: fraction of inspired oxygen; n‐BiPAP: noninvasive cycled respiratory support mechanism; n‐CPAP/NCPAP: nasal continuous positive airway pressure; NIPPV: nasal intermittent positive pressure ventilation; PaCO2: partial pressure of carbon dioxide in arterial blood; pCO2: partial pressure of carbon dioxide; RDS: respiratory distress syndrome.
Differences between protocol and review
We added the methods and plan for 'Summary of findings' tables and GRADE recommendations, which were not included in the original protocol, nor in the previously published review.
Contributions of authors
PGD and BL prepared the protocol for this review.
AGD and HK provided additional material for the Background section.
BL updated the literature search, made independent quality assessments and extracted data before comparing results and resolving differences for this update.
All review authors participated in data analysis and interpretation of results for this updated review.
Sources of support
Internal sources
Royal Women's Hospital, Melbourne, Australia.
Murdoch Children's Research Institute, Melbourne, Australia.
University of Melbourne, Australia.
Royal Hobart Hospital, Australia.
University of Tasmania, Australia.
External sources
National Health and Medical Research Council, Australia.
-
Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services, USA.
Editorial support of the Cochrane Neonatal Review Group has been funded with Federal funds from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services, USA, under Contract No. HHSN275201100016C.
-
National Institute for Health Research, UK.
Editorial support for Cochrane Neonatal has been funded with funds from a UK National Institute of Health Research Grant (NIHR) Cochrane Programme Grant (13/89/12). The views expressed in this publication are those of the authors and not necessarily those of the NHS, the NIHR, or the UK Department of Health.
Declarations of interest
Review authors have declared no conflicts of interest.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Barrington 2001 {published and unpublished data}
- Barrington KJ, Finer NN, Bull D. Randomised controlled trial of nasal synchronized intermittent mandatory ventilation compared with continuous positive airway pressure after extubation of very low birth weight infants. Pediatrics 2001;107(4):638‐41. [DOI] [PubMed] [Google Scholar]
Friedlich 1999 {published data only}
- Friedlich P, Lecart C, Posen R, Ramicone E, Chan L, Ramanathan R. A randomized trial of nasopharyngeal‐synchronised intermittent mandatory ventilation versus nasopharyngeal continuous positive airway pressure in very low birth weight infants following extubation. Journal of Perinatology 1999;19(6 Pt 1):413‐8. [DOI] [PubMed] [Google Scholar]
Gao 2010 {published data only}
- Gao WW, Tan SZ, Chen YB, Zhang Y, Wang Y. Randomized trial of nasal synchronized intermittent mandatory ventilation compared with nasal continuous positive airway pressure in preterm infants with respiratory distress syndrome. Chinese Journal of Contemporary Pediatrics 2010;12(7):524‐6. [PubMed] [Google Scholar]
Jasani 2016 {published data only}
- Jasani B, Nanavati R, Kabra N, Rajdeo S, Bhandari V. Comparison of non‐synchronized nasal intermittent positive pressure ventilation versus nasal continuous positive airway pressure as post‐extubation respiratory support in preterm infants with respiratory distress syndrome: a randomized controlled trial. Journal of Maternal‐Fetal & Neonatal Medicine 2016;29(10):1546‐51. [PUBMED: 26135774] [DOI] [PubMed] [Google Scholar]
Kahramaner 2014 {published data only}
- Kahramaner Z, Erdemir A, Turkoglu E, Cosar H, Sutcuoglu S, Ozer EA. Unsynchronized nasal intermittent positive pressure versus nasal continuous positive airway pressure in preterm infants after extubation. Journal of Maternal‐Fetal & Neonatal Medicine 2014;27(9):926‐9. [DOI: 10.3109/14767058.2013.846316] [DOI] [PubMed] [Google Scholar]
Khalaf 2001 {published data only}
- Khalaf MN, Brodsky N, Hurley J, Bhandari V. A prospective randomised controlled trial comparing synchronized nasal intermittent positive pressure ventilation (SNIPPV) versus nasal continuous positive airway pressure (NCPAP) as mode of extubation. Pediatric Research 1999;45:204a. [DOI] [PubMed] [Google Scholar]
- Khalaf MN, Brodsky N, Hurley J, Bhandari V. A prospective randomized, controlled trial comparing synchronized nasal intermittent positive pressure ventilation versus nasal continuous positive airway pressure as modes of extubation. Pediatrics 2001;108(1):13‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Khorana 2008 {published data only}
- Khorana M, Paradeevisut H, Sangtawesin V, Kanjanapatanakul W, Chotigeat U, Ayutthaya JKN. A randomized trial of non‐synchronized nasopharyngeal intermittent mandatory ventilation (nsNIMV) vs. nasal continuous positive airway pressure (nCPAP) in the prevention of extubation failure in preterm under 1500 grams. Journal of the Medical Association of Thailand 2008;91(3):S136‐42. [PubMed] [Google Scholar]
Kirpalani 2013 {published and unpublished data}
- Kirpalani H, Millar D, Lemyre B, Yoder BA, Chiu A, Roberts RS, NIPPV Study Group. A trial comparing noninvasive ventilation strategies in preterm infants. New England Journal of Medicine 2013;369(7):611‐20. [DOI: 10.1056/NEJMoa1214533] [DOI] [PubMed] [Google Scholar]
Moretti 2008 {published data only}
- Moretti C, Giannini L, Fassi C, Gizzi C, Papoff P, Colarizi P. Nasal flow‐synchronized intermittent positive pressure ventilation to facilitate weaning in very low‐birthweight infants: unmasked randomized controlled trial. Pediatrics International 2008;50:85‐91. [DOI] [PubMed] [Google Scholar]
O'Brien 2012 {published data only}
- O'Brien K, Campbell C, Havlin L, Wenger L, Shah V. Infant flow biphasic nasal continuous positive airway pressure (BP‐nCPAP) vs. infant flow nCPAP for the facilitation of extubation in infants less than or = 1250 grams: a randomized controlled trial. BMC Pediatrics 2012;12:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Ali 2007 {published data only}
- Ali N, Claure N, Alegria X, D'Ugard C, Organero R, Bancalari E. Effects of non‐invasive pressure support ventilation (NI‐PSV) on ventilation and respiratory effort in very low birth weight infants. Pediatric Pulmonology 2007;42:704‐10. [DOI] [PubMed] [Google Scholar]
Bhandari 2007 {published data only}
- Bhandari V, Gavino RG, Nedrelow JH, Pallela P, Salvadore A, Ehrenkranz RA, et al. A randomized controlled trial of synchronized nasal intermittent positive pressure ventilation in RDS. Journal of Perinatology 2007;11:697‐703. [DOI] [PubMed] [Google Scholar]
Bisceglia 2007 {published data only}
- Bisceglia M, Belcastro A, Poerio V, Raimondi F, Mesuraca L, Crugliano C, et al. A comparison of nasal intermittent versus continuous positive pressure delivery for the treatment of moderate respiratory syndrome in preterm infants. Minerva Pediatrica 2007;59:91‐5. [PubMed] [Google Scholar]
DeSimone 2010 {published data only}
- DeSimone OA, Sommers R, Destin K, Mance M, Matook S, Stonestreet B, et al. Nasal intermittent positive pressure ventilation (NIPPV) does not facilitate earlier extubation in infants less than 28 weeks gestation: a pilot study. Pediatric Academic Society. 2010.
Kishore 2009 {published data only}
- Kishore MSS, Dutta S, Kumar P. Early nasal intermittent positive pressure ventilation versus continuous positive airway pressure for respiratory distress syndrome. Acta Paediatrica 2009;98:1412‐5. [DOI] [PubMed] [Google Scholar]
Kugelman 2007 {published data only}
- Kugelman A, Feferkorn I, Riskin A, Chistyakov I, Kaufman B, Bader D. Nasal intermittent mandatory ventilation versus nasal continuous positive airway pressure for respiratory distress syndrome: a randomized controlled prospective study. Journal of Pediatrics 2007;150:521‐6. [DOI] [PubMed] [Google Scholar]
Kumar 2011 {published data only}
- Kumar M, Avasthi S, Ahuja S, Malik GK, Singh SN. Unsynchronized nasal intermittent positive pressure ventilation to prevent extubation failure in neonates: a randomized controlled trial. Indian Journal of Pediatrics 2011;78(7):801‐6. [DOI: 10.1007/s12098-010-0357-x; PUBMED: 21287368] [DOI] [PubMed] [Google Scholar]
Lin 1998 {published and unpublished data}
- Lin CH, Wang ST, Lin YJ, Yeh TF. Efficacy of nasal intermittent positive pressure ventilation in treating apnea of prematurity. Pediatric Pulmonology 1998;26(5):349‐53. [DOI] [PubMed] [Google Scholar]
Meneses 2011 {published data only}
- Meneses J, Bhandari V, Guilherme AJ, Herrmann D. Noninvasive ventilation for respiratory distress syndrome: a randomized controlled trial. Pediatrics 2011;127(2):300‐7. [DOI] [PubMed] [Google Scholar]
Moretti 1999 {published data only}
- Moretti C, Gizzi C, Papoff P, Lampariello S, Capoferri M, Calcagnini G, et al. Comparing the effects of nasal synchronized intermittent positive pressure ventilation (nSIPPV) and nasal continuous positive airway pressure (nCPAP) after extubation in very low birth weight infants. Early Human Development 1999;56:166‐77. [DOI] [PubMed] [Google Scholar]
Pantalitschka 2009 {published data only}
- Pantalitschka T, Sievers J, Urschitz MS, Herberts T, Reher C, Poets CF. Randomised crossover trial of four nasal respiratory support systems for apnoea of prematurity in very low birthweight infants. Archives of Disease in Childhood. Fetal and Neonatal Edition 2009;94(4):F245‐8. [DOI] [PubMed] [Google Scholar]
Ramanathan 2012 {published data only}
- Ramanathan R, Sekar KC, Rasmussen M, Bhatia J, Soll RF. Nasal intermittent positive pressure ventilation after surfactant treatment for respiratory distress syndrome in preterm infants under 30 weeks gestation: a randomized, controlled trial. Journal of Perinatology 2012;32(5):336‐43. [DOI] [PubMed] [Google Scholar]
Ryan 1989 {published data only}
- Ryan CA, Finer NN, Peters KL. Nasal intermittent positive‐pressure ventilation offers no advantages over nasal continuous positive airway pressure in apnea of prematurity. American Journal of Diseases in Childhood 1989;143(10):1196‐8. [DOI] [PubMed] [Google Scholar]
Shi 2010 {published data only}
- Shi Y, Tang S, Zhao J, Hu Z, Li T. Efficiency of nasal intermittent positive pressure ventilation vs nasal continuous positive airway pressure on neonatal respiratory distress syndrome: a prospective, randomized, controlled study. Acta Academiae Medicinae Militaris Tertiae 2010;32(18):1991‐4. [Google Scholar]
References to studies awaiting assessment
Silveira 2015 {published data only}
- Silveira CS, Leonardi KM, Melo AP, Zaia JE, Brunherotti MA. Response of preterm infants to 2 noninvasive ventilatory support systems: nasal CPAP and nasal intermittent positive‐pressure ventilation. Respiratory Care 2015;60(12):1772‐6. [26374907] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
El‐Farash 2013 {unpublished data only}
- Nasal Continuous Airway Pressure (n‐CPAP) vs Nasal Bilevel Positive Airway Pressure (n‐BiPAP) for RDS. Ongoing study January 2013.
Estay 2013 {unpublished data only}
- Non‐Invasive Ventilation vs Continuous Positive Airway Pressure After Extubation in Very Low Birth Weight Infants. Ongoing study December 2011.
NCT02396693 {published data only}
- NCT02396693. Successful Extubation and Noninvasive Ventilation in Preterm ≤ 1500g Terms. clinicaltrials.gov/show/NCT02396693 (accessed 7 December 2015).
Shi 2013 {unpublished data only}
- Nasal Intermittent Positive Pressure Ventilation in Newborn Infants With Respiratory Distress Syndrome. Ongoing study January 2008.
Victor 2011 {published data only}
- Victor S, Extubate Trial Group. EXTUBATE: a randomised controlled trial of nasal biphasic positive airway pressure vs. nasal continuous positive airway pressure following extubation in infants less than 30 weeks' gestation: study protocol for a randomised controlled trial. Trials 2011; Vol. 12:257. [DOI] [PMC free article] [PubMed]
Additional references
Bancalari 2013
- Bancalari E, Claure N. The evidence for non‐invasive ventilation in the preterm infant. Archives of Disease in Childhood. Fetal and Neonatal Edition 2013;98(2):F98‐102. [DOI] [PubMed] [Google Scholar]
Bott 1993
- Bott J, Carroll MP, Conway JH, Keilty SE, Ward EM, Brown AM, et al. Randomised controlled trial of nasal ventilation in acute ventilatory failure due to chronic obstructive airways disease. Lancet 1993;341:1555‐7. [DOI] [PubMed] [Google Scholar]
Davis 2003b
- Davis PG, Henderson‐Smart DJ. Nasal continuous positive airways pressure immediately after extubation for preventing morbidity in preterm infants. Cochrane Database of Systematic Reviews 2003, Issue 2. [DOI: 10.1002/14651858.CD000143] [DOI] [PubMed] [Google Scholar]
Davis 2009
- Davis PG, Morley CJ, Owen LS. Non‐invasive respiratory support of preterm neonates with respiratory distress: continuous positive airway pressure and nasal intermittent positive pressure ventilation. Seminars in Fetal and Neonatal Medicine 2009;14:14‐20. [DOI] [PubMed] [Google Scholar]
Ellis 1988
- Ellis ER, Grunstein RR, Chan S, Bye PT, Sullivan CE. Noninvasive ventilatory support during sleep improves respiratory failure in kyphoscoliosis. Chest 1988;94:811‐5. [DOI] [PubMed] [Google Scholar]
Garland 1985
- Garland JS, Nelson DB, Rice T, Neu J. Increased risk of gastrointestinal perforations in neonates mechanically ventilated with either face mask or nasal prongs. Pediatrics 1985;76:406‐10. [PubMed] [Google Scholar]
GRADEpro 2008 [Computer program]
- Brozek J, Oxman A, Schünemann H. GRADEpro. Version 3.2 for Windows. The GRADE Working Group, 2008.
Guyatt 2011a
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction ‐ GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [PUBMED: 21195583] [DOI] [PubMed] [Google Scholar]
Guyatt 2011b
- Guyatt GH, Oxman AD, Vist G, Kunz R, Brozek J, Alonso‐Coello P, et al. GRADE guidelines: 4. Rating the quality of evidence ‐ study limitations (risk of bias). Journal of Clinical Epidemiology 2011;64(4):407‐15. [PUBMED: 21247734] [DOI] [PubMed] [Google Scholar]
Guyatt 2011c
- Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso‐Coello P, Rind D, et al. GRADE guidelines 6. Rating the quality of evidence ‐ imprecision. Journal of Clinical Epidemiology 2011;64(12):1283‐93. [PUBMED: 21839614] [DOI] [PubMed] [Google Scholar]
Guyatt 2011d
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 7. Rating the quality of evidence ‐ inconsistency. Journal of Clinical Epidemiology 2011;64(12):1294‐302. [PUBMED: 21803546] [DOI] [PubMed] [Google Scholar]
Guyatt 2011e
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 8. Rating the quality of evidence ‐ indirectness. Journal of Clinical Epidemiology 2011;64(12):1303‐10. [PUBMED: 21802903] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Kiciman 1998
- Kiciman NM, Andréasson B, Bernstein G, Mannino FL, Rich W, Henderson C, et al. Thoracoabdominal motion in newborns during ventilation delivered by endotracheal tube or nasal prongs. Pediatric Pulmonology 1998;25:175‐81. [DOI] [PubMed] [Google Scholar]
Lee 2015
- Lee J, Kim HS, Jung YH, Shin SH, Choi CW, Kim EK, et al. Non‐invasive neurally adjusted ventilatory assist in preterm infants: a randomised phase II crossover trial. Archives of Disease in Childhood. Fetal and Neonatal Edition 2015;100(6):F507‐13. [DOI] [PubMed] [Google Scholar]
Owen 2007
- Owen LS, Morley CJ, Davis PG. Nasal intermittent positive pressure ventilation: what do we know in 2007?. Archives of Disease in Childhood. Fetal and Neonatal Edition 2007;92(5):F414‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Owen 2008
- Owen LS, Morley CJ, Davis PG. Neonatal nasal intermittent positive pressure ventilation: a survey of practice in England. Archives of Disease in Childhood. Fetal and Neonatal Edition 2008;93(2):F148‐50. [DOI] [PubMed] [Google Scholar]
Owen 2016
- Owen LS, Manley BJ. Nasal intermittent positive pressure ventilation in preterm infants: equipment, evidence and synchronization. Seminars in Fetal and Neonatal Medicine 2016;21(3):146‐153. [DOI] [PubMed] [Google Scholar]
Piper 1992
- Piper AJ, Parker S, Torzillo PJ, Sullivan CE, Bye PT. Nocturnal nasal IPPV stabilizes patients with cystic fibrosis and hypercapnic respiratory failure. Chest 1992;102:846‐50. [DOI] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editors. GWG. GRADE Handbook for Grading Quality of Evidence and Strength of Recommendations. www.guidelinedevelopment.org/handbook. Updated October 2013.
Stein 2012
- Stein D, Howard D. Neurally adjusted ventilatory assist in neonates weighing <1500 grams: a retrospective analysis. Journal of Pediatrics 2012;160(5):786‐9. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Davis 2001
- Davis PG, Lemyre B, Paoli AG. Nasal intermittent positive pressure ventilation (NIPPV) versus nasal continuous positive airway pressure (NCPAP) for preterm neonates after extubation. Cochrane Database of Systematic Reviews 2001, Issue 3. [DOI: 10.1002/14651858.CD003212] [DOI] [PubMed] [Google Scholar]
Davis 2003a
- Davis PG, Lemyre B, Paoli AG. Nasal intermittent positive pressure ventilation (NIPPV) versus nasal continuous positive airway pressure (NCPAP) for preterm neonates after extubation. Cochrane Database of Systematic Reviews 2003, Issue 3. [DOI: 10.1002/14651858.CD003212] [DOI] [PubMed] [Google Scholar]


