Abstract
Background
Necrotizing enterocolitis (NEC) is the most common emergency involving the gastrointestinal tract occurring in the neonatal period. There have been published reports that suggest that oral immunoglobulins (Ig)A and IgG produce an immunoprotective effect in the gastrointestinal mucosa.
Objectives
To determine the effect of oral immunoglobulin on the incidence of necrotizing enterocolitis and other complications in preterm or low birth weight (or both) neonates.
Search methods
We used the standard search strategy of the Cochrane Neonatal Group. We searched the Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library 2016, Issue 1), PubMed (1966 to January 2016), CINAHL (1982 to January 2016) and EMBASE (1980 to January 2016) and conference proceedings.
Selection criteria
All randomized or quasi‐randomised controlled trials where oral immunoglobulins were used as prophylaxis against NEC in preterm (less than 37 weeks' gestation) or low birth weight (less than 2500 gram), or both, neonates.
Data collection and analysis
We performed data collection and analysis in accordance with the standard methods of the Cochrane Neonatal Review Group.
Main results
The search identified five studies on oral immunoglobulin for the prevention of NEC of which three met the inclusion criteria. In this review of the three eligible trials (including 2095 neonates), the oral administration of IgG or an IgG/IgA combination did not result in a significant reduction in the incidence of definite NEC (typical risk ratio (RR) 0.84, 95% confidence interval (CI) 0.57 to 1.25; typical risk difference (RD) ‐0.01, 95% CI ‐0.03 to 0.01; 3 studies, 1840 infants), suspected NEC (RR 0.84, 95% CI 0.49 to 1.46; RD ‐0.01, 95% CI ‐0.02 to 0.01; 1 study, 1529 infants), need for surgery (typical RR 0.21, 95% CI 0.02 to 1.75; typical RD ‐0.03, 95% CI ‐0.06 to 0.00; 2 studies, 311 infants) or death from NEC (typical RR 1.10, 95% CI 0.47 to 2.59; typical RD 0.00, 95% CI ‐0.01 to 0.01; 3 studies, 1840 infants).
Authors' conclusions
Based on the available trials, the evidence does not support the administration of oral immunoglobulin for the prevention of NEC. There are no randomized controlled trials of oral IgA alone for the prevention of NEC.
Plain language summary
Oral immunoglobulin for preventing necrotizing enterocolitis in preterm and low birth weight neonates
Review question: Does the use of oral immunoglobulin reduce the incidence of necrotizing enterocolitis and other complications in preterm or low birth weight (or both) neonates?
Background: Immunoglobulin given orally for preventing emergency intestinal problems (necrotizing enterocolitis) in premature and low birth weight newborn infants. Destructive inflammation of the intestine (called necrotizing enterocolitis, NEC) is caused by gas‐producing bacteria that ferment milk. It is a potential problem for newborn preterm (born before their due date) and low birth weight (born at less than 2500 grams) infants. Even after leaving hospital, affected infants may need frequent and prolonged hospitalisation because of continuing nutritional problems. This makes it difficult for parents both emotionally and financially. Immunoglobulins are proteins found in the blood that give the body immunity to disease. Immunoglobulins (types IgA and IgG) taken by mouth (orally) may protect susceptible infants from developing NEC.
Study characteristics: We searched the medical literature through January 2016 and found three randomized controlled trials (clinical studies where people are randomly put into one of two or more treatment groups) (with 2095 newborn infants). Treatment was started either in the first 24 hours following birth (two small studies) or following commencement of oral feeding (enteral) (one large well‐controlled study). In this large study, infants generally received breast milk, whereas they received formula milk in the other two studies.
Results: Giving immunoglobulin (IgG alone or IgG plus IgA combination) did not reduce the incidence of NEC, need for surgery related to NEC or death from NEC, either during or after the study period. Immunoglobulins could possibly cause breakdown of red blood cells (called haemolysis) (red blood cells are common cells in the blood that delivery oxygen to organs), but no clinically important haemolysis was apparent. There were no other reported side effects.
Quality of the evidence: There was low‐very low evidence for all the major outcomes. The major factor that affected the quality of evidence was the lack of precision in the result estimates, as the calculated plausible range of the effects (the 95% confidence intervals) were wide.
Summary of findings
Summary of findings for the main comparison. Summary of findings table for oral immunoglobulin versus control.
| Patient or population: preventing necrotizing enterocolitis in preterm and low birth weight neonates Setting: Neonatal Intensive Care Intervention: Oral immunoglobulin Comparison: control | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with control | Risk with Oral immunoglobulin | |||||
| Definite necrotizing enterocolitis (NEC) during study period | Study population | RR 0.84 (0.57 to 1.25) | 1840 (3 RCTs) | Low (1, 2, 3) | Incomplete outcome data (Eibl 1988) High rate of non‐compliance (Lawrence 2001) Unclear allocation concealment |
|
| 55 per 1000 | 47 per 1000 (32 to 69) | |||||
| Moderate | ||||||
| 60 per 1000 | 50 per 1000 (34 to 75) | |||||
| Definite necrotizing enterocolitis (NEC) during study period ‐ IgA/IgG | Study population | RR 0.08 (0.00 to 1.39) | 179 (1 RCT) | Very low (1, 3, 4) | Incomplete outcome data (Eibl 1988) Unclear allocation concealment. Imprecision: broad confidence interval |
|
| 66 per 1000 | 5 per 1000 (0 to 92) | |||||
| Moderate | ||||||
| 66 per 1000 | 5 per 1000 (0 to 92) | |||||
| Definite necrotizing enterocolitis (NEC) during study period ‐ IgG | Study population | RR 0.95 (0.63 to 1.42) | 1661 (2 RCTs) | Low (2, 3, 4) | High rate of non‐compliance (Lawrence 2001) Unclear allocation concealment. Imprecision: Broad confidence intervals |
|
| 54 per 1000 | 52 per 1000 (34 to 77) | |||||
| Moderate | ||||||
| 57 per 1000 | 54 per 1000 (36 to 81) | |||||
| Definite NEC after study period | Study population | RR 1.30 (0.47 to 3.60) | 1661 (2 RCTs) | Very low (2, 3, 4) | High rate of non‐compliance (Lawrence 2001) Unclear allocation concealment. Imprecision: Broad confidence intervals |
|
| 7 per 1000 | 9 per 1000 (3 to 26) | |||||
| Moderate | ||||||
| 4 per 1000 | 5 per 1000 (2 to 14) | |||||
| NEC‐related surgery during study period | Study population | RR 0.21 (0.02 to 1.75) | 311 (2 RCTs) | Very Low (1, 3, 4) | Incomplete outcome data (Eibl 1988) Unclear allocation concealment. Imprecision: Broad confidence intervals |
|
| 25 per 1000 | 5 per 1000 (1 to 44) | |||||
| Moderate | ||||||
| 24 per 1000 | 5 per 1000 (0 to 42) | |||||
| NEC‐related deaths during study period | Study population | RR 1.10 (0.47 to 2.59) | 1840 (3 RCTs) | Very low (1, 2, 3, 4) | Incomplete outcome data (Eibl 1988) High rate of non‐compliance (Lawrence 2001) Unclear allocation concealment |
|
| 10 per 1000 | 11 per 1000 (5 to 25) | |||||
| Moderate | ||||||
| 15 per 1000 | 16 per 1000 (7 to 39) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Incomplete outcome data (Eibl 1988)
2 High rate of non‐compliance (Lawrence 2001)
3 Unclear allocation concealment
4 Imprecision: broad confidence intervals
Background
Description of the condition
Necrotizing enterocolitis (NEC) is the most common emergency involving the gastrointestinal tract occurring in the neonatal period (Henry 2009; Lin 2006). NEC is characterized by acute onset of intestinal inflammatory necrosis that exhibits as abdominal distension, gastrointestinal bleeding and pneumatosis intestinalis on abdominal X‐ray (Tudehope 2005). The origin of the intramural gas has been presumed to be from bacterial fermentation from gas‐producing bacteria and a substrate (milk) (Willoughby 1994). NEC is a disease of the newborn, which indicates that the pathogenesis is somehow linked to physiological characteristics unique to the newborn intestine (Edelstone 1982). The majority of neonates with NEC are premature or low birth weight (Cikrit 1984; Yee 2012), and there is an inverse relationship between gestational age, birth weight and onset of NEC (Yee 2012). The incidence of NEC is between 5% and 15% (Luig 2005; Stoll 2010). NEC characteristically presents between seven and 14 days of life, although, increasingly NEC can also present several weeks after birth, particularly in very low birth weight infants (Yee 2012).
The pathogenesis of NEC appears to be multifactorial, with any unifying hypothesis of its cause and prevention remaining unconfirmed (Patole 2007; Stoll 1994a). NEC is reported to be due to contributory factors such as mucosal injury caused by ischaemia, infection and intraluminal injury with subsequent circulatory, immunological and inflammatory host responses to the injury (Maheshwari 2011; Stoll 1994b). It is now well established that an exaggerated release of mediators of inflammation induced by microbial factors such as bacterial endotoxin, plays an important role in the development of noxious sequelae that follow infection of the host with pathogenic micro‐organisms (Wolf 1994). An exaggerated release of mediators of inflammation has also been implicated in the pathogenesis of NEC (Claud 2009). Tumour necrosis factor alpha (TNF‐α) and platelet activating factor (PAF) are considered to play a synergistic and central role in the inflammatory cascade that leads to NEC (Hseuh 2003). The reported mortality rate for NEC is between 20% and 30% (Fitzgibbons 2009; Stoll 1994a), and has not changed appreciably since the 1990s (Petrosyan 2009), because of the increasing survival of smaller infants (Neu 2011). Infants that require surgery have the greatest mortality (Neu 2012). Long‐term outcome is less certain. At discharge, many of the neonates remain at significant risk of frequent and prolonged hospitalizations due to nutritional compromise or stricture as a consequence of NEC (Petrosyan 2009). Approximately 20% to 40% of neonates who develop NEC eventually require surgical intervention (Hseuh 2003; Petrosyan 2009). This leads to increased resource utilization and possibly impaired growth and developmental outcome (Hintz 2005). The risk of neurodevelopmental dysfunction is increased in children who require surgery (Martin 2010). Additional morbidity arises from parental emotional grief and financial costs (Neu 2011; Simon 1994).
Various preventive interventions have been tried to reduce the risk and severity of NEC (Lin 2005; Lucas 1990). NEC has been reported to be six to 10 times less common with exclusive breastfeeding compared to infants who were exclusively formula fed (Lucas 1990). The prophylactic administration of oral gentamicin in selected babies at high risk for NEC has resulted in significant decrease in the incidence of NEC (Grylack 1978); but the use of oral gentamicin in preterm infants is not recommended because of the concerns of the development of antibiotic‐resistant organisms. The prolonged use of initial empirical therapy in early postnatal days may be associated with increased risk of NEC or death (Cotten 2009). Therefore, the use of antibiotics in preterm infants should be monitored carefully and empirical use should be avoided. Human milk oligosaccharides appear to be one of the promising component to prevent NEC. In neonatal rats, a specific isomer of HMO was identified to be protective against NEC (Jantscher‐Krenn 2012). The administration of prophylactic enteral probiotics in preterm infants have been reported to reduce the incidence of severe NEC as well as mortality (Jacobs 2013; Lin 2005). The arginine supplementation in preterm infants appeared to be protective in decreasing the rate of NEC but no significant impact on neurodevelopment outcome at 36 months of corrected age (Mitchell 2014). In addition, it has been proposed that oral immunoglobulins may be an effective preventive intervention for NEC (Wolf 1994).
Description of the intervention
Immunoglobulins play an essential role in the body's immune system. Immunoglobulins are large glycoproteins that are secreted by plasma cells and function as antibodies in the immune response by binding with specific antigens. They attach to foreign substances such as bacteria, and assist in destroying them. There are five classes of immunoglobulins: IgA, IgD, IgE, IgG and IgM (Attaelmannan 2000). Oral immunoglobulin may provide a prophylactic effect against NEC because of its immunoprotective effect, or its heterologous antibodies against infection of the gastrointestinal tract (Wolf 1994).
How the intervention might work
It has been proposed that orally administered antibodies bind to the antigen at the level of the gastrointestinal mucosa, which leads to intra‐luminal agglutination of potentially infectious pathogens and, thus, interfere with colonization of the mucosal surface by infectious pathogens, and neutralizes bacterial toxic factors or viral particles (Wolf 1994). IgA, being a secretory immunoglobulin, might be expected to be more efficacious in protecting the neonatal gastrointestinal tract than the more readily available IgG. Bauer 1992 reviewed three trials of prophylactic intravenous immunoglobulin administration and reported a borderline statistically significant reduction of NEC.
Why it is important to do this review
Prevention and treatment of NEC has become an area of priority for research due to the increasing number of preterm survivors at risk (Patole 2007). There have also been reports of the effectiveness of using oral immunoglobulins as prophylaxis against NEC in premature and low birth weight neonates (Wolf 1994). It has been proposed that oral immunoglobulins produce an immunoprotective effect in the gastrointestinal mucosa. However, there are concerns regarding the strength of the evidence of the effectiveness of the use of oral immunoglobulins. The authors have been unable to identify any previous systematic reviews on the use of oral immunoglobulin for the prevention of NEC.
Objectives
To determine the effect of oral immunoglobulin on the incidence of necrotizing enterocolitis and other complications in preterm or low birth weight (or both) neonates.
Methods
Criteria for considering studies for this review
Types of studies
Randomized or quasi‐randomised controlled trials.
Types of participants
Preterm (less than 37 weeks' gestation) or low birth weight (less than 2500 grams), or both neonates.
Types of interventions
Immunoglobulin administered orally as prophylaxis against NEC versus placebo or no treatment.
Types of outcome measures
Primary outcomes
Diagnosis of definite NEC during the study period, defined as clinical evidence of gastrointestinal and systemic illness, confirmed by pneumatosis intestinalis, pneumoperitoneum, portal venous gas, surgery or postmortem.
Secondary outcomes
Suspected NEC during the study period.
Surgery for NEC during the study period.
NEC‐related death; by 28 days post‐delivery, by discharge and by one year (late or post‐discharge).
Length of stay in hospital (days).
Hospital re‐admissions within the first year of life.
Days receiving total parenteral nutrition.
Growth and development in childhood.
Parental emotional and financial costs.
Adverse effects of treatment.
Search methods for identification of studies
Electronic searches
We searched the following electronic databases: Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library 2016, Issue 1), PubMed, CINAHL and EMBASE from 1966 or as available to January 31, 2016 using the text words 'necrotising enterocolitis OR necrotizing enterocolitis' AND 'immunoglobulin' OR 'IgA', OR 'IgG' with constraints 'neonate OR infant' (Appendix 1).
Searching other resources
We examined the references in all studies identified as potentially relevant. We searched the abstracts from the annual meetings of the Pediatric Academic Societies (1993 to 2014), the European Society for Paediatric Research (1995 to 2014), the UK Royal College of Paediatrics and Child Health (2000 to 2014) and the Perinatal Society of Australia and New Zealand (2000 to 2014). We identified no new trials for this update. We also searched clinical trials registries for ongoing or recently completed trials (clinicaltrials.gov, controlled‐trials.com and who.int/ictrp) to January 31, 2016.
Data collection and analysis
We followed the procedures of the Cochrane Neonatal Review Group (CNRG) throughout.
Selection of studies
Two review authors screened the title and abstract of all studies identified by the above search strategy. We re‐assessed the full text of any potentially eligible reports and excluded those studies that did not meet all of the inclusion criteria. We discussed any disagreements until we achieved consensus.
Data extraction and management
We used a data collection form to aid extraction of relevant information from each included study. Two review authors extracted the data separately and independently assessed the trials for their methodological quality and subsequent inclusion in the review. We resolved any disagreements by discussion until we achieved consensus. If data from the trial reports were insufficient, we contacted the investigators for further information.
Assessment of risk of bias in included studies
Review authors independently assessed risk of bias for the included studies using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreements successfully by discussion. Therefore, it was not necessary to involve a review arbiter. We completed the 'Risk of bias' table addressing the following methodological issues.
Random sequence generation (checking for possible selection bias)
For each included study, we described the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups. We assessed the risk of bias methods as:
low risk (any truly random process, e.g. random number table; computer random number generator);
high risk (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk.
Allocation concealment (checking for possible selection bias)
For each included study, we described the method used to conceal the allocation sequence in sufficient detail and determine whether intervention allocation could have been foreseen in advance of, or during, recruitment, or changed after assignment. We assessed the risk of bias methods as:
low risk (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk (open random allocation; unsealed or non‐opaque envelopes; alternation; date of birth);
unclear risk.
Blinding (checking for possible performance bias)
For each included study, we described the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We judged the study to be at low risk of bias if it was blinded, or if we judged that the lack of blinding could not have affected the results. We assessed blinding separately for different outcomes and classes of outcomes. We assessed the risk of bias methods as:
adequate, inadequate or unclear for participants;
adequate, inadequate or unclear for personnel;
adequate, inadequate or unclear for outcome assessors.
Incomplete outcome data (checking for possible attrition bias through withdrawals, drop‐outs and protocol deviations)
For each included study and for each outcome or class of outcome, we described the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we planned to re‐include missing data in the analyses. We assessed the risk of bias methods as:
adequate (less than 20% missing data);
inadequate;
unclear.
Selective reporting bias
For each included study, we described how we investigated the possibility of selective outcome reporting bias and what we found. We assessed the risk of bias methods as:
low risk (where it was clear that all of the study's pre‐specified outcomes and all expected outcomes of interest to the review were reported);
high risk (where not all of the study's pre‐specified outcomes had been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest were reported incompletely and so could not be used; study did not include results of a key outcome that would have been expected to have been reported);
unclear risk.
Other sources of bias
For each included study, we described any important concerns that we had about other possible sources of bias (e.g. early termination of trial due to data‐dependant process, extreme baseline imbalance, etc.). We assessed whether each study was free of other problems that could put it at risk of bias. We assessed other sources of bias as:
low risk;
high risk;
unclear.
Overall risk of bias
We made judgements as to whether studies were at high risk of bias, according to the criteria given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). With reference to overall risk of bias, we assessed the likely magnitude and direction of the bias and whether we considered it likely to impact on the findings.
Measures of treatment effect
All the studies only reported continuous data. We calculated risk ratio (RR), risk difference (RD) and NNTB, NNTH for dichotomous data with 95% confidence intervals (CI).
Unit of analysis issues
The unit of analysis was the participating infant in individually randomized trials.
Assessment of heterogeneity
If more than one trial was included in a meta‐analysis, we examined the treatment effects of individual trials and heterogeneity between trial results by inspecting the forest plots. We calculated the I2 statistic for each analysis to quantify inconsistency across studies and describe the percentage of variability in effect estimates that may be due to heterogeneity rather than sampling error. If substantial heterogeneity was detected (I2 greater than 50%), we intended to explore the possible causes (e.g. differences in study design, participants, interventions or completeness of outcome assessments) in sensitivity analyses.
Data synthesis
We used the fixed‐effect model in Review Manager 5 for meta‐analysis (RevMan 2012).
Quality of Evidence
We assessed the quality of evidence for the main comparison at the outcome level using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach (Guyatt 2011a). This methodological approach considers evidence from randomised controlled trials as high quality that may be downgraded based on consideration of any of five areas: design (risk of bias), consistency across studies, directness of the evidence, precision of estimates and presence of publication bias. (Guyatt 2011a). The GRADE approach results in an assessment of the quality of a body of evidence in one of four grades: 1) High: We are very confident that the true effect lies close to that of the estimate of the effect; 2) Moderate: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different; 3) Low: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect; 4) Very Low: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect (Schunemann 2013). The review authors independently assessed the quality of the evidence found for outcomes identified as critical or important for clinical decision making. These outcomes include: diagnosis of definite NEC during the study period, defined as clinical evidence of gastrointestinal and systemic illness, confirmed by pneumatosis intestinalis, pneumoperitoneum, portal venous gas, surgery or postmortem; surgery for NEC during the study period; NEC‐related death; by 28 days post‐delivery, by discharge and by one year (late or post‐discharge); overall mortality; duration of hospitalisation. In cases where we considered the risk of bias arising from inadequate concealment of allocation, randomised assignment, complete follow‐up or blinded outcome assessment to reduce our confidence in the effect estimates, we downgraded the quality of evidence accordingly (Guyatt 2011b). We evaluated consistency by similarity of point estimates, extent of overlap of confidence intervals and statistical criteria including measurement of heterogeneity (I2). We downgraded the quality of evidence when large and unexplained inconsistency across studies results was present (i.e. some studies suggest important benefit and others no effect or harm without a clinical explanation) (Guyatt 2011d). Precision was assessed based on the width of the 95% confidence interval (CI) and by calculating the optimal information size (OIS). If the total number of patients included in the pooled effect estimation was less than the number of patients generated by a conventional sample size calculation for a single adequately powered trial, we considered rating down for imprecision (Guyatt 2011c). When trials were conducted in populations other than the target population, we downgraded the quality of evidence because of indirectness (Guyatt 2011e). We entered data (i.e. pooled estimates of the effects and corresponding 95% confidence Interval) and explicit judgments for each of the above aspects assessed into the Guideline Development Tool, the software used to create ‘Summary of findings’ tables (GRADEpro 2008). We explained all judgements involving the assessment of the study characteristics described above in footnotes or comments in the ‘Summary of findings’ table.
Subgroup analysis and investigation of heterogeneity
We planned subgroup analysis for the following pre‐specified subcategories:
dose of oral immunoglobulin; timing of administration of oral immunoglobulin (early versus late);
type of oral immunoglobulin (IgG or IgA);
gestation of participants (less than 28 weeks; 28 to 32 weeks; 33 to 36 weeks);
birth weight of participants (less than 1000 g; 1000 to 1500 g; greater than 1500 g to less than 2500 g).
Results
Description of studies
See: tables Characteristics of included studies; Characteristics of excluded studies tables.
Results of the search
The search identified five studies on oral immunoglobulin for the prevention of NEC of which three met the inclusion criteria. The three studies were published. See Characteristics of included studies; Characteristics of excluded studies tables. For the review update we identified 31 records through database searching (2011‐2016). We performed additional searches and identified 43 additional records through other sources. After removing duplicates, there were 74 records. We evaluated the abstracts or full‐text of the articles and found no new relevant studies (Figure 1).
1.
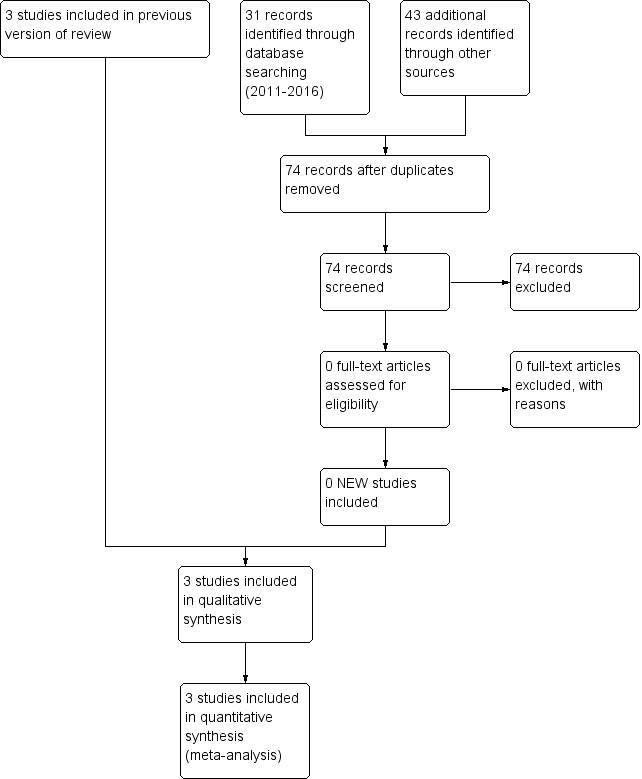
Study flow diagram: review update
Included studies
A total of 2095 neonates participated in the three trials. Eibl 1988 studied neonates weighing between 800 and 2000 g. Rubaltelli 1991 studied neonates weighing less than 1500 g or 34 weeks' gestation or less. Lawrence 2001 studied neonates weighing 1500 g or less. The Eibl 1988 and Rubaltelli 1991 studies did not use a placebo and the Lawrence 2001 study used a placebo (albumin). The studies used varying doses and combinations of IgG/IgA. Lawrence 2001 used only IgG, Rubaltelli 1991 used IgG with a trace of IgM and IgA, and Eibl 1988 used an IgA‐IgG preparation. There were no studies that investigated the use of only IgA. Treatment was started in the first 24 hours following birth in the Eibl 1988 and Rubaltelli 1991 studies and following initiation of enteral feeding in the Lawrence 2001 study.
Excluded studies
We excluded two studies. The Fast 1994 study had no placebo arm (oral gentamicin versus an oral IgG/IgA mixture) and the Richter 1998 study was an historical cohort study and not a randomised or quasi‐randomised trial.
Risk of bias in included studies
Details of the methodological quality assessments are given in the Characteristics of included studies table. We completed a 'Risk of bias' table for each eligible study and present our overall assessment of risk of bias using a 'Risk of bias' graph (Figure 2) and 'Risk of bias' summary (Figure 3).
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
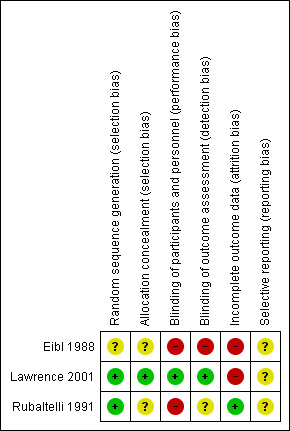
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
All of the studies used formal randomisation. Allocation was adequately concealed in all of the studies.
Blinding
Only one study, Lawrence 2001, reported that the assessment of the primary outcome of NEC was blinded.
Incomplete outcome data
In the Lawrence 2001 study, 10 out of 43 cases of definite NEC in the treatment group and 12 out of 41 cases of definite NEC in the control group did not receive any of the trial solutions prior to their illness. The rate of exclusion of neonates after randomisation was high (59%) in the study by Eibl 1988.
Effects of interventions
See: Table 1
Although the trials reported outcomes such as definite NEC, suspected NEC, death and need for surgery, none of the studies reported the pre‐specified outcomes length of stay in hospital, hospital re‐admission, total parenteral nutrition administration, growth and development in childhood, and parental emotional and financial costs. Death was reported as 'during and after the study period', but not reported as 28 days post‐delivery, discharge or by one year (as listed in the pre‐specified outcome measures). It was not anticipated that several outcome measures would be reported 'after the study period'. The decision to report these outcomes was made post‐hoc. No data were available for subgroup analysis other than with regard to class of immunoglobulin, IgG/IgA.
In this review of three trials (including 2095 neonates), two of the studies investigated the use of IgG (nil or trace IgA) (Lawrence 2001; Rubaltelli 1991), and one study investigated the use of an IgG/IgA combination (Eibl 1988). The administration of oral immunoglobulin did not reduce the incidence of definite NEC, suspected NEC, surgery‐related NEC or death from NEC, either during or after the study period.
Definite necrotizing enterocolitis during study period (Outcome 1.1)
Three trials reported the incidence of definite NEC during the study period and there was no reduction in any trial or overall (typical RR 0.84, 95% CI 0.57 to 1.25; typical RD ‐0.01, 95% CI ‐0.03 to 0.01; 3 studies, 1840 infants; Analysis 1.1).
1.1. Analysis.
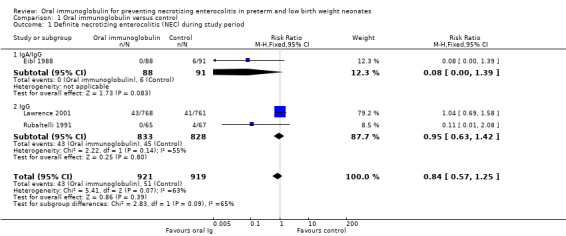
Comparison 1 Oral immunoglobulin versus control, Outcome 1 Definite necrotizing enterocolitis (NEC) during study period.
Definite necrotizing enterocolitis after the study period (Outcome 1.2)
Two trials reported the incidence of definite NEC after the study period and there was no reduction in either trial or overall (typical RR 1.30, 95% CI 0.47 to 3.60; typical RD 0.00, 95% CI ‐0.01 to 0.01; Analysis 1.2).
1.2. Analysis.
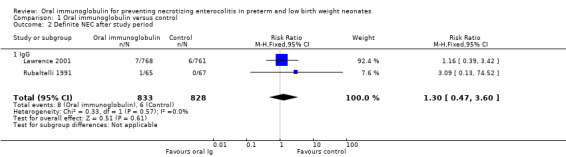
Comparison 1 Oral immunoglobulin versus control, Outcome 2 Definite NEC after study period.
Suspected necrotizing enterocolitis during the study period (Outcome 1.3)
One trial reported the incidence of suspected NEC during the study period and there was no statistically significant reduction (RR 0.84, 95% CI 0.49 to 1.46; RD ‐0.01, 95% CI ‐0.02 to 0.01; 1 study, 1529 infants; Analysis 1.3).
1.3. Analysis.
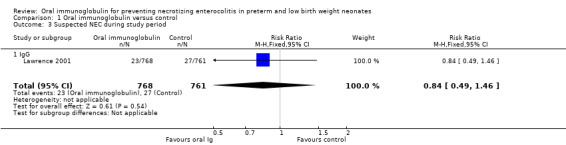
Comparison 1 Oral immunoglobulin versus control, Outcome 3 Suspected NEC during study period.
Necrotizing enterocolitis‐related surgery during the study period (Outcome 1.4)
The two small trials reported the number of neonates requiring surgery during the study period. There was no statistically significant reduction in either trial or overall (typical RR 0.21, 95% CI 0.02 to 1.75; typical RD ‐0.03, 95% CI ‐0.06 to 0.00; 2 studies, 311 infants; Analysis 1.4).
1.4. Analysis.
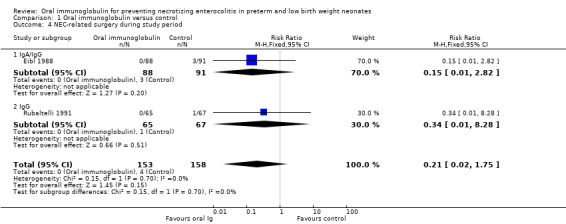
Comparison 1 Oral immunoglobulin versus control, Outcome 4 NEC‐related surgery during study period.
Necrotizing enterocolitis‐related deaths during the study period (Outcome 1.5)
Three trials reported the incidence of NEC‐related deaths during the study period and there was no reduction in any trial or overall (typical RR 1.10, 95% CI 0.47 to 2.59; typical RD 0.00, 95% CI ‐0.01 to 0.01; 3 studies, 1840 infants; Analysis 1.5).
1.5. Analysis.
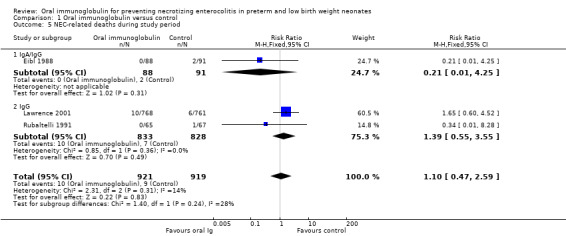
Comparison 1 Oral immunoglobulin versus control, Outcome 5 NEC‐related deaths during study period.
Necrotizing enterocolitis‐related deaths after the study period (Outcome 1.6)
One trial reported the incidence of NEC‐related deaths after the study period and there was no reduction (RR 1.98, 95% CI 0.18 to 21.81; RD 0.00, 95% CI 0.00 to 0.01; 1 study, 1529 infants; Analysis 1.6).
1.6. Analysis.

Comparison 1 Oral immunoglobulin versus control, Outcome 6 NEC‐related deaths after study period.
Subgroup analysis
Two studies investigated the use of oral IgG (nil or trace IgA) (Lawrence 2001; Rubaltelli 1991). The Lawrence 2001 study was large compared to the Rubaltelli 1991 study and thus dominated the results. Oral IgG did not reduce the incidence of definite NEC during the study period (typical RR 0.95, 95% CI 0.63 to 1.42; typical RD 0.00, 95% CI ‐0.02 to 0.02), suspected NEC (RR 0.84, 95% CI 0.49 to 1.46; RD ‐0.01, 95% CI ‐0.02 to 0.01), need for surgery (RR 0.34, 95% CI 0.01 to 8.28; RD ‐0.01, 95% CI ‐0.06 to 0.03), definite NEC after the study period (typical RR 1.30, 95% CI 0.47 to 3.60; typical RD 0.00, 95% CI ‐0.01 to 0.01) or death due to NEC during the study period (typical RR 1.39, 95% CI 0.55 to 3.55; typical RD 0.00, 95% CI ‐0.01 to 0.01).
One study investigated the use of an oral IgA/IgG combination (73% IgA, 26% IgG) (Eibl 1988). There were trends for this combination to reduce the incidence of definite NEC during the study period (RR 0.08, 95% CI 0.00 to 1.39; RD ‐0.07, 95% CI ‐0.12 to ‐0.01; number needed to treat for an additional beneficial outcome (NNTB) 14, 95% CI 8 to 100), death due to NEC during study period (RR 0.21, 95% CI 0.01 to 4.25; RD ‐0.02, 95% CI ‐0.06 to 0.02) and need for surgery (RR 0.15, 95% CI 0.01 to 2.82; RD ‐0.03, 95% CI ‐0.08 to 0.01). None of the results were statistically significant.
One of the studies reported an increased incidence of Heinz bodies in the intervention group receiving oral immunoglobulin (Lawrence 2001). However, the proportion of neonates given blood transfusions was similar in the intervention and control groups (62.2% with intervention versus 69.7% with control) suggesting that clinically important haemolysis did not occur. There were no other reported adverse effects from the administration of oral immunoglobulin.
Discussion
Summary of main results
We included three trials in the review. The randomised trials by Eibl 1988 and Rubaltelli 1991 were small and outcome assessment was not blinded. The Eibl 1988 study also had a large number of post randomisation exclusions in both the intervention and control groups (59%). The study by Lawrence 2001 was a large randomised, placebo‐controlled, double‐blind study. Rubaltelli 1991 and Eibl 1988 excluded neonates that received breast milk whereas, in the Lawrence 2001 trial, 90% of neonates received breast milk. Breast milk has previously been reported to have a protective effect against NEC (Lucas 1990), and this was acknowledged by Lawrence 2001. Eibl 1988 and Rubaltelli 1991 used similar doses of oral immunoglobulin (600 mg/day in Eibl 1988 and 500 mg/day in Rubaltelli 1991). Lawrence 2001 used a higher dose of 1200 mg/kg/day. The larger dose of oral immunoglobulin does not appear to have produced a greater response.
Eibl 1988 and Rubaltelli 1991 administered the oral immunoglobulin within the first 24 hours following birth. However, Lawrence 2001 did not administer the oral immunoglobulin until after the initiation of enteral feeding. Thus, in the Lawrence 2001 study, 31% of neonates did not start the treatment until the fifth day or later. The effect of the timing of the administration of immunoglobulin on the incidence of NEC is unknown. However, in clinical practice it would be difficult to administer oral immunoglobulins to neonates who were unable to tolerate fluids orally.
The trials by Lawrence 2001 and Rubaltelli 1991 used predominately IgG. The study by Eibl 1988 used an immunoglobulin mixture containing 73% IgA and 26% IgG. To date, there is no randomised trial of IgA alone in the prevention of NEC, and the question of whether IgA has a protective effect against NEC is unanswered.
Eibl 1988 studied neonates weighing between 800 and 2000 g. Rubaltelli 1991 studied neonates weighing less than 1500 g or 34 weeks' gestation or less and Lawrence 2001 similarly studied neonates weighing 1500 g or less. The association between prematurity or low birth weight and NEC are well known. Despite the increasing survival rate of extremely low birth weight neonates, there are no published randomised studies exclusively targeting extremely low birth weight neonates. It would clearly be important to stratify the groups at risk by gestational age and weight.
Overall completeness and applicability of evidence
In the Lawrence 2001 trial, 10 out of 43 cases of definite NEC in the treatment group and 12 out of 41 cases of definite NEC in the control group did not receive any of the trial solutions prior to their illness. In the Eibl 1988 trial, the rate of exclusion of neonates after randomisation was high (59%). Only one of the trials performed sample size estimation (Lawrence 2001).
Quality of the evidence
There was low or very low evidence for all the major outcomes. The major factor that affected the quality of evidence was the lack of precision in the result estimates, as the calculated plausible range of the effects (the 95% confidence intervals) were wide (Table 1).
Authors' conclusions
Implications for practice.
Based on the available trials, the evidence does not support the administration of oral immunoglobulin for the prevention of necrotizing enterocolitis (NEC). There are no randomised controlled trials of oral IgA alone in the prevention of NEC.
Implications for research.
Future trials should examine the effects of oral IgA in extremely low birth weight neonates (less than 1000 grams). In addition to examining effect on NEC, consideration should be given to reporting outcomes such as length of stay in hospital, hospital re‐admissions, need for total parenteral nutrition administration, growth and development in childhood, and parenteral emotional and financial costs in any future studies. Given an incidence of NEC in this population of 8%, 1000 neonates would be required to show a 50% reduction in NEC at the 5% level (two tailed).
What's new
| Date | Event | Description |
|---|---|---|
| 20 March 2017 | Amended | Author affiliations updated |
History
Protocol first published: Issue 4, 1999 Review first published: Issue 3, 2001
| Date | Event | Description |
|---|---|---|
| 9 May 2016 | Amended | Author affiliations updated |
| 31 January 2016 | New search has been performed | This updates the review "Oral immunoglobulin for preventing necrotizing enterocolitis in preterm and low birth weight neonates" (Foster 2004). |
| 31 January 2016 | New citation required but conclusions have not changed | Review updated‐no change to conclusions |
| 14 November 2013 | New search has been performed | Review updated‐no change to conclusions |
| 20 January 2013 | Amended | Contact details updated. |
| 26 March 2011 | New search has been performed | This updates the review "Oral immunoglobulin for preventing necrotizing enterocolitis in preterm and low birth weight neonates" (Foster 2004). Updated search in March 2011 did not identify any new studies. Conclusions remain the same. |
| 26 March 2011 | New search has been performed | This is an update of "Oral immunoglobulin for preventing necrotizing enterocolitis in preterm and low birth weight neonates" published in The Cochrane Library, Issue 3 2001. No new eligible trials were found. There is no change to the conclusion that there is not enough evidence to support the administration of oral immunoglobulin for the prevention of NEC. |
| 15 February 2011 | Amended | Contact details updated. |
| 18 September 2008 | Amended | Converted to new review format. |
| 26 October 2003 | New search has been performed | This review updates the existing review "Oral immunoglobulin for preventing necrotizing enterocolitis in preterm and low birth weight neonates" which was published in The Cochrane Library, Issue 3, 2001 (Foster 2001). An unpublished study (Lawrence 1996) included in the preceding version of the review has been published (Lawrence 2001) and new information from the published version of the study has been added to the review. No new trials have been identified. |
| 26 October 2003 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We would like to acknowledge the contribution of Prof. David Henderson‐Smart for his valuable advice and supervision and the Australasian Satellite of the Neonatal Review Group.
Editorial support of the Cochrane Neonatal Review Group has been funded with Federal funds from the Eunice Kennedy Shriver National Institute of Child Health and Human Development National Institutes of Health, Department of Health and Human Services, USA, under Contract No. HHSN267200603418C.
Appendices
Appendix 1. Search Strategy
Search Terms: (necrotising enterocolitis OR necrotizing enterocolitis) AND (immunoglobulin OR IgA OR IgG)
Plus the following database‐specific terms:
PubMed: ((infant, newborn[MeSH] OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or infan* or neonat*) AND (randomised controlled trial [pt] OR controlled clinical trial [pt] OR Clinical Trial[ptyp] OR randomised [tiab] OR placebo [tiab] OR clinical trials as topic [mesh: noexp] OR randomly [tiab] OR trial [ti]) NOT (animals [mh] NOT humans [mh]))
Embase: (infant, newborn or newborn or neonate or neonatal or premature or very low birth weight or low birth weight or VLBW or LBW or Newborn or infan* or neonat*) AND (human not animal) AND (randomised controlled trial or controlled clinical trial or randomised or placebo or clinical trials as topic or randomly or trial or clinical trial)
CINAHL: (infant, newborn OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or Newborn or infan* or neonat*) AND (randomised controlled trial OR controlled clinical trial OR randomised OR placebo OR clinical trials as topic OR randomly OR trial OR PT clinical trial)
Cochrane Library: (infant or newborn or neonate or neonatal or premature or very low birth weight or low birth weight or VLBW or LBW)
Trials databases:
Clinicaltrials.gov: (necrotising enterocolitis OR necrotizing enterocolitis) AND (immunoglobulin OR IgA OR IgG) AND infant
Controlled‐trials.com: (necrotising enterocolitis OR necrotizing enterocolitis) AND (immunoglobulin OR IgA OR IgG) AND infant
WHO ICTRP: (necrotising enterocolitis OR necrotizing enterocolitis) AND (infant OR neonate)
Data and analyses
Comparison 1. Oral immunoglobulin versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Definite necrotizing enterocolitis (NEC) during study period | 3 | 1840 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.57, 1.25] |
| 1.1 IgA/IgG | 1 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.08 [0.00, 1.39] |
| 1.2 IgG | 2 | 1661 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.63, 1.42] |
| 2 Definite NEC after study period | 2 | 1661 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.47, 3.60] |
| 2.1 IgG | 2 | 1661 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.47, 3.60] |
| 3 Suspected NEC during study period | 1 | 1529 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.49, 1.46] |
| 3.1 IgG | 1 | 1529 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.49, 1.46] |
| 4 NEC‐related surgery during study period | 2 | 311 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.02, 1.75] |
| 4.1 IgA/IgG | 1 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.01, 2.82] |
| 4.2 IgG | 1 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.28] |
| 5 NEC‐related deaths during study period | 3 | 1840 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.47, 2.59] |
| 5.1 IgA/IgG | 1 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.01, 4.25] |
| 5.2 IgG | 2 | 1661 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.39 [0.55, 3.55] |
| 6 NEC‐related deaths after study period | 1 | 1529 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.98 [0.18, 21.81] |
| 6.1 IgG | 1 | 1529 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.98 [0.18, 21.81] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Eibl 1988.
| Methods | Parallel randomised controlled trial | |
| Participants | 434 low birth weight neonates: 800‐2000 grams. Ineligible if breast fed, or had severe congenital malformations, cardiac malformations and haemorrhage | |
| Interventions | Treatment commenced within 24 hours following birth Intervention group: oral IgG/IgA 600 mg divided into ≥ 3 doses for 28 days. 211 neonates Control group: no placebo used. Infants in control group were fed only infant formula or infant formula plus pasturized human milk. 223 neonates | |
| Outcomes | NEC assessed by pneumatosis intestinalis (no definition given) or free gas in peritoneum or portal venous tract or by histopathological examination of tissue obtained during surgery or autopsy | |
| Notes | No sample size estimation. Study was finished at a not prospectively defined point. 59% post randomisation exclusion rate in intervention and control groups | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomly allocated" to group A or group B. No other information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding (no placebo) |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 59% loss post‐randomisation: 123 infants in the intervention group and 111 in the control group were withdrawn from the study within the first week because breast milk from their mothers became available. Infants withdrawn from the study were followed until the end of the 4th week of life for the possible development of NEC. 21 infants, all assigned to the control group, were excluded from evaluation because of incomplete protocols for the daily feeding regimen or mistakes in the protocols (e.g. a control child receiving IgA for a few days) |
| Selective reporting (reporting bias) | Unclear risk | We were unable to obtain the study protocol |
Lawrence 2001.
| Methods | Parallel randomised controlled trial | |
| Participants | 1529 preterm neonates: birth weight ≤ 1500 g. Ineligible if enterally fed for > 24 hours prior to enrolment. Eligible if breast fed | |
| Interventions | Treatment commenced when enteral feeds commenced Intervention group: oral IgG 1200 mg/kg/day only divided into at least 4 doses for 28 days. 768 neonates Control group: placebo of oral albumin 2400 mg/kg/day, coloured to look identical to treatment. Albumin was used to simulate the viscosity of the IgG solution. 31% of neonates did not receive the study solution until after the 5th day of life due to a delay in tolerating oral feeds. 761 neonates | |
| Outcomes | NEC assessed by clinical criteria without radiological or pathological confirmation were:
|
|
| Notes | Sample size estimation done 10 neonates out of 43 cases of definite NEC in the treatment group and 12 out of 41 cases in control group did not receive any trial solution prior to diagnosis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation using a list of random numbers with 5 IgG and placebo neonates in each block of 10 envelopes |
| Allocation concealment (selection bias) | Low risk | The hospital pharmacists provided ampoules of trial solution identified only by name |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of participants and personnel. The trial solutions were packaged in identical glass snap‐top vials |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The X‐rays of all infants diagnosed with NEC were reviewed independently by 2 radiologists who were unaware of the treatment group or identity of the infants. X‐rays of infants in which there was diagnostic discordance between the 2 radiologists were jointly reviewed and a consensus reached |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Intolerance to feeds, consent withdrawal and protocol errors delayed study treatment. Thus, 26% of neonates who subsequently developed NEC had not received study medication |
| Selective reporting (reporting bias) | Unclear risk | We were unable to obtain the study protocol |
Rubaltelli 1991.
| Methods | Parallel randomised controlled trial | |
| Participants | 132 neonates < 1500 grams or ≤ 34 weeks' gestation, or both. Ineligible if breast fed during first 15 days, known cardiopathy, congenital malformation or haemorrhagic syndromes. No exclusions before or after randomisation | |
| Interventions | Treatment commenced within 24 hours following birth Intervention group: 500 mg oral IgG/trace IgA for 15 days post‐delivery divided into 5 doses. 65 neonates Control group: no placebo used. 67 neonates | |
| Outcomes | NEC assessed by abdominal distention, vomitus or biliary gastric residues, and gastrointestinal bleeding. Clinical suspicion confirmed by presence of intramural gas or gas in portal systems or pneumoperitoneum or histological examination of biopsy specimen obtained during surgery or autopsy, or a combination of these | |
| Notes | No sample size estimation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by sealed envelopes |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding (no placebo used) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcome data reported |
| Selective reporting (reporting bias) | Unclear risk | We were unable to obtain a protocol |
NEC: necrotizing enterocolitis.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Fast 1994 | No placebo arm. Oral gentamicin vs. an oral IgG/IgA mixture. |
| Richter 1998 | Not a randomized or quasi‐randomised trial. This is an historical cohort study. |
Differences between protocol and review
We added the methodology and plan for Summary of findings tables and GRADE recommendations, which were not included in the original protocol or the originally published version of the review.
Contributions of authors
JF and MC independently assessed studies for inclusion in the original review. JF and RS updated the review and MC commented on the review update.
Sources of support
Internal sources
Westmead Hospital, Sydney, Australia.
External sources
-
Eunice Kennedy Shriver National Institute of Child Health and Human Development National Institutes of Health, Department of Health and Human Services, USA.
Editorial support of the Cochrane Neonatal Review Group has been funded with Federal funds from the Eunice Kennedy Shriver National Institute of Child Health and Human Development National Institutes of Health, Department of Health and Human Services, USA, under Contract No. HHSN275201100016C
Declarations of interest
None.
Edited (no change to conclusions)
References
References to studies included in this review
Eibl 1988 {published data only}
- Eibl MM, Wolf HM, Furnkranz H, Rosenkranz A. Prevention of necrotizing enterocolitis in low‐birth‐weight infants by IgA‐IgG feeding. New England Journal of Medicine 1988;319(1):1‐7. [PUBMED: 3288866] [DOI] [PubMed] [Google Scholar]
Lawrence 2001 {published data only}
- Lawrence G, Tudehope D, Baumann K, Jeffery H, Gill A, Cole M, et al. Enteral human IgG for prevention of necrotising enterocolitis: a placebo‐controlled, randomised trial. Lancet 2001;357(9274):2090‐4. [PUBMED: 11445103] [DOI] [PubMed] [Google Scholar]
- Lawrence GW, The Australian NEC Study Group, Baumann K, Swanson C. Controlled double blind trial of oral human IgG in preventing neonatal enterocolitis (NEC). Proceedings of the Australian New Zealand Perinatal Society. 1996:A80.
Rubaltelli 1991 {published data only}
- Rubaltelli FF, Benini F, Sala M. Prevention of necrotizing enterocolitis in neonates at risk by oral administration of monomeric IgG. Developmental Pharmacology and Therapeutics 1991;17(3‐4):138‐43. [PUBMED: 1841829] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Fast 1994 {published data only}
- Fast C, Rosegger H. Necrotizing enterocolitis prophylaxis: oral antibiotics and lyophilized enterobacteria vs oral immunoglobulins. Acta Paediatrica. Supplement 1994;396:86‐90. [PUBMED: 8086694] [DOI] [PubMed] [Google Scholar]
Richter 1998 {published data only}
- Richter D, Bartmann P, Pohlandt F. Prevention of necrotizing enterocolitis in extremely low birth weight infants by IgG feeding?. European Journal of Pediatrics 1998;157(11):924‐5. [PUBMED: 9835438] [DOI] [PubMed] [Google Scholar]
Additional references
Attaelmannan 2000
- Attaelmannan M, Levinson SS. Understanding and identifying monoclonal gammopathies. Clinical Chemistry 2000;46(8 Pt 2):1230‐8. [PUBMED: 10926917] [PubMed] [Google Scholar]
Bauer 1992
- Bauer CR. Necrotizing enterocolitis. In: Sinclair JC, Bracken MB editor(s). Effective Care of the Newborn Infant. Oxford: Oxford University Press, 1992. [Google Scholar]
Cikrit 1984
- Cikrit D, Mastandrea J, West KW, Schreiner RL, Grosfeld JL. Necrotizing enterocolitis: factors affecting mortality in 101 surgical cases. Surgery 1984;96(4):648‐55. [PUBMED: 6484808] [PubMed] [Google Scholar]
Claud 2009
- Claud EC. Neonatal necrotizing enterocolitis ‐ inflammation and intestinal immaturity. Anti‐Inflammatory & Anti‐Allergy Agents in Medicinal Chemistry 2009;8(3):248‐59. [PUBMED: 20498729] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cotten 2009
- Cotten CM, Taylor S, Stoll B, Goldberg RN, Hansen NI, Sánchez PJ, et al. Prolonged duration of initial empirical antibiotic treatment is associated with increased rates of necrotizing enterocolitis and death for extremely low birth weight infants. Pediatrics 2009;123(1):58‐66. [PUBMED: 19117861] [DOI] [PMC free article] [PubMed] [Google Scholar]
Edelstone 1982
- Edelstone DI, Holzman IR. Fetal intestinal oxygen consumption at various levels of oxygenation. American Journal of Physiology 1982;242(1):H50‐4. [PUBMED: 7058913] [DOI] [PubMed] [Google Scholar]
Fitzgibbons 2009
- Fitzgibbons SC, Ching Y, Yu D, Carpenter J, Kenny M, Weldon C, et al. Mortality of necrotizing enterocolitis expressed by birth weight categories. Journal of Pediatric Surgery 2009;44(6):1072‐5. [PUBMED: 19524719] [DOI] [PubMed] [Google Scholar]
GRADEpro 2008 [Computer program]
- Brozek J, Oxman A, Schünemann H. GRADEpro [Version 3.2 for Windows]. The GRADE Working Group, 2008.
Grylack 1978
- Grylack LJ, Scanlon JW. Oral gentamicin therapy in the prevention of neonatal necrotising enterocolitis. A controlled double blind trial. American Journal of Diseases in Childhood 1978;132(12):1192‐4. [PUBMED: 362900] [DOI] [PubMed] [Google Scholar]
Guyatt 2011a
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction‐GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [PUBMED: 21195583] [DOI] [PubMed] [Google Scholar]
Guyatt 2011b
- Guyatt GH, Oxman AD, Vist G, Kunz R, Brozek J, Alonso‐Coello P, et al. GRADE guidelines: 4. Rating the quality of evidence‐‐study limitations (risk of bias). Journal of Clinical Epidemiology 2011;64(4):407‐15. [PUBMED: 21247734] [DOI] [PubMed] [Google Scholar]
Guyatt 2011c
- Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso‐Coello P, Rind D, et al. GRADE guidelines 6. Rating the quality of evidence‐‐imprecision. Journal of Clinical Epidemiology 2011;64(12):1283‐93. [PUBMED: 21839614] [DOI] [PubMed] [Google Scholar]
Guyatt 2011d
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 7. Rating the quality of evidence‐‐inconsistency. Journal of Clinical Epidemiology 2011;64(12):1294‐302. [PUBMED: 21803546] [DOI] [PubMed] [Google Scholar]
Guyatt 2011e
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 8. Rating the quality of evidence‐‐indirectness. Journal of Clinical Epidemiology 2011;64(12):1303‐10. [PUBMED: 21802903] [DOI] [PubMed] [Google Scholar]
Henry 2009
- Henry MC, Moss RL. Necrotizing enterocolitis. Annual Review of Medicine 2009;60:111‐24. [PUBMED: 18817461] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hintz 2005
- Hintz SR, Kendrick DE, Stoll BJ, Vohr BR, Fanaroff AA, Donovan EF, et al. Neurodevelopmental and growth outcomes of extremely low birth weight infants after necrotizing enterocolitis. Pediatrics 2005;115(3):696‐703. [PUBMED: 15741374] [DOI] [PubMed] [Google Scholar]
Hseuh 2003
- Hsueh W, Caplan MS, Qu XW, Tan XD, Plaen IG, Gonzalez‐Crussi F. Neonatal necrotizing enterocolitis: clinical considerations and pathogenetic concepts. Pediatric and Developmental Pathology 2003;6(1):6‐23. [PUBMED: 12424605] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jacobs 2013
- Jacobs SE, Tobin JM, Opie GF, Donath S, Tabrizi SN, Pirotta M, et al. ProPrems Study Group. Probiotic effects on late‐onset sepsis in very preterm infants: a randomized controlled trial. Pediatrics 2013;132(6):1055‐62. [PUBMED: 24249817] [DOI] [PubMed] [Google Scholar]
Jantscher‐Krenn 2012
- Jantscher‐Krenn E, Zherebtsov M, Nissan C, Goth K, Guner YS, Naidu N, et al. The human milk oligosaccharide disialyllacto‐N‐tetraose prevents necrotising enterocolitis in neonatal rats. Gut 2012;61(10):1417‐25. [PUBMED: 22138535] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lin 2005
- Lin HC, Su BH, Chen AC, Lin TW, Tsai CH, Yeh TF, et al. Oral probiotics reduce the incidence and severity of necrotising enterocolitis in very low birth weight infants. Pediatrics 2005;115(1):1‐4. [PUBMED: 15629973] [DOI] [PubMed] [Google Scholar]
Lin 2006
- Lin PW, Stoll BJ. Necrotising enterocolitis. Lancet 2006;368(9543):1271‐83. [PUBMED: 17027734] [DOI] [PubMed] [Google Scholar]
Lucas 1990
- Lucas A, Cole TJ. Breast milk and neonatal necrotising enterocolitis. Lancet 1990;336(8730):1519‐23. [PUBMED: 1979363] [DOI] [PubMed] [Google Scholar]
Luig 2005
- Luig M, Lui K, NSW & ACT NICUS Group. Epidemiology of necrotizing enterocolitis ‐ part I: changing regional trends in extremely preterm infants over 14 years. Journal of Paediatrics and Child Health 2005;41(4):169‐73. [PUBMED: 15813869] [DOI] [PubMed] [Google Scholar]
Maheshwari 2011
- Maheshwari A, Corbin LL, Schelonka RL. Neonatal necrotizing enterocolitis. Research and Reports in Neonatology 2011;1:39‐53. [Google Scholar]
Martin 2010
- Martin CR, Dammann O, Allred EN, Patel S, O'Shea TM, Kuban KCK, et al. Neurodevelopment of extremely preterm infants who had necrotizing enterocolitis with or without late bacteremia. Journal of Paediatrics 2010;157(5):751‐6. [PUBMED: 20598317] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mitchell 2014
- Mitchell K, Lyttle A, Amin H, Robertson HL, Lodha AK. Arginine supplementation in prevention of necrotising enterocolitis in the premature infant: an updated systematic review. BMC Pediatrics 2014;14:226. [PUBMED: 25205007] [DOI] [PMC free article] [PubMed] [Google Scholar]
Neu 2011
- Neu J, Walker WA. Necrotizing enterocolitis. New England Journal of Medicine 2011;364(3):255‐64. [PUBMED: 21247316] [DOI] [PMC free article] [PubMed] [Google Scholar]
Neu 2012
- Neu J, Mihatsch W. Recent developments in necrotizing enterocolitis. Journal of Parenteral and Enteral Nutrition 2012;36(1 Suppl):30S‐5S. [PUBMED: 22237874] [DOI] [PubMed] [Google Scholar]
Patole 2007
- Patole S. Prevention and treatment of necrotising enterocolitis in preterm neonates. Early Human Development 2007;83(10):635‐42. [PUBMED: 17826009] [DOI] [PubMed] [Google Scholar]
Petrosyan 2009
- Petrosyan M, Guner YS, Williams M, Grishin A, Ford HR. Current concepts regarding the pathogenesis of necrotizing enterocolitis. Pediatric Surgery International 2009;25(4):309‐18. [PUBMED: 19301015] [DOI] [PubMed] [Google Scholar]
RevMan 2012 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Schunemann 2013
- Schunemann H, Brozek J, Guyatt G, Oxman A, editors. GRADE Working Group. GRADE handbook for grading quality of evidence and strength of recommendations. Available from www.guidelinedevelopment.org/handbook Updated October 2013.
Simon 1994
- Simon NP. Follow‐up for infants with necrotizing enterocolitis. Clinics in Perinatology 1994;21(2):411‐24. [PUBMED: 8070234] [PubMed] [Google Scholar]
Stoll 1994a
- Stoll BJ. Epidemiology of necrotizing enterocolitis. Clinics in Perinatology 1994;21(2):205‐18. [PUBMED: 8070222] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stoll 1994b
- Stoll BJ, Kliegman RM. Necrotizing enterocolitis. Clinics in Perinatology 1994;21:xi. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stoll 2010
- Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics 2010;126(3):443‐56. [PUBMED: 20732945] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tudehope 2005
- Tudehope DI. The epidemiology and pathogenesis of neonatal necrotizing enterocolitis. Journal of Paediatric and Child Health 2005;41(4):167‐8. [PUBMED: 15813868] [DOI] [PubMed] [Google Scholar]
Willoughby 1994
- Willoughby RE, Pickering LK. Necrotizing enterocolitis and infection. Clinics in Perinatology 1994;21(2):307‐15. [PUBMED: 8070228] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wolf 1994
- Wolf HM, Eibl MM. The anti‐inflammatory effect of an immunoglobulin (IgA‐IgG) preparation and its possible relevance for the prevention of necrotizing enterocolitis. Acta Paediatrica 1994;396 Suppl:37‐40. [PUBMED: 8086680] [DOI] [PubMed] [Google Scholar]
Yee 2012
- Yee WH, Soraisham AS, Shah VS, Aziz K, Yoon W, Lee SK, Canadian Neonatal Network. Incidence and timing of presentation of necrotizing enterocolitis in preterm infants. Pediatrics 2012;129(2):e298. [PUBMED: 22271701] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Foster 2001
- Foster J, Cole M. Oral immunoglobulin for preventing necrotizing enterocolitis in preterm and low birth‐weight neonates. Cochrane Database of Systematic Reviews 2001, Issue 3. [DOI: 10.1002/14651858.CD001816] [DOI] [PubMed] [Google Scholar]
Foster 2004
- Foster J, Cole M. Oral immunoglobulin for preventing necrotizing enterocolitis in preterm and low birth‐weight neonates. Cochrane Database of Systematic Reviews 2004, Issue 1. [DOI: 10.1002/14651858.CD001816.pub2] [DOI] [PubMed] [Google Scholar]


