Abstract
BACKGROUND
Since routine immunization could change the epidemiological profile of hepatitis A virus (HAV) infection in the future, it is important to determine the baseline immunity to HAV across Turkey.
OBJECTIVE
The aim of this study was to determine the seroprevalence of hepatitis A among individuals 6 years of age and older in Yozgat, Turkey.
DESIGN
Cross-sectional.
SETTING
Community in central region of Turkey.
PATIENTS AND METHODS
Questionnaires and blood specimens were collected and the presence of hepatitis A IgG antibodies against hepatitis A virus was determined quantitatively by ELISA.
MAIN OUTCOME MEASURES
The rates of hepatitis A immunity by age group.
SAMPLE SIZE
1862.
RESULTS
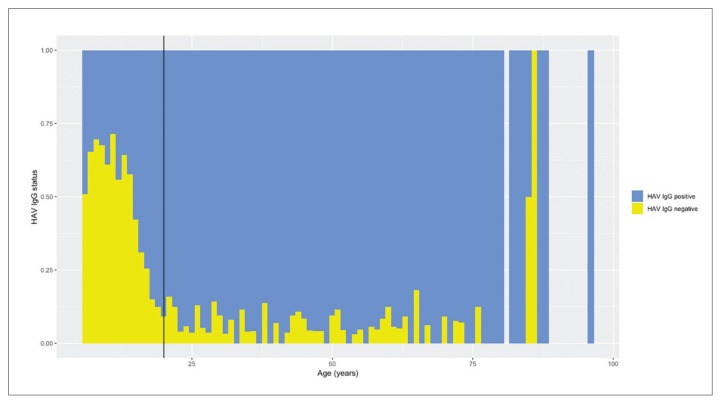
Immunity to hepatitis A was 79.1% (n=1473). The mean (SD) age was 17.1 (14.7) years in the nonimmune group and 37.8 (19.5) years in the immune group (P<.001), and immunity increased with age. No significant difference in immunity rate was detected between genders in children and adults. The seropositivity rate for subjects ages 6–19 years was lower than in subjects aged 20–96 years (52.2% versus 93.9%; P<.001).
CONCLUSION
A catch-up vaccination program is needed for persons aged 6–19 years in Yozgat.
LIMITATIONS
Single region data which can not be generalized. For this reason, a multi-centered study that can reflect the whole country is recommended.
Hepatitis A virus (HAV), the causative agent of hepatitis A, is a nonenveloped, single-stranded positive-sense RNA virion that belongs to the family of Picornaviridae and genus Hepatovirus. HAV is transmitted by the fecal-oral route, which involves direct contact with an infected individual or consumption of contaminated food or water. Humans are the only known reservoir. Children younger than the age 6 years usually develop asymptomatic HAV infection; symptoms are usually seen among older children and adults.1 Hepatitis A infection is a self-limited disease which does not progress chronically. Less than 1 percent of hepatitis A patients develop fulminant hepatic failure. Infection produces lifelong immunity.2 Hepatitis A has been a vaccine preventable infection since 1992, but the vaccine is only recommended for areas where a high proportion of the population is at risk of developing symptomatic infection.3
The disease usually occurs during early childhood and after infection lifelong immunity is gained. Improvement of hygienic conditions in recent decades has changed the usual age of HAV infection from early childhood to adolescence or adulthood.4 In 2006, the Centers for Disease Control and Prevention recommended HAV vaccine to all children between 12–23 months of age.5 Since October 2012, the Ministry of Health of Turkey has ordered mandatory vaccination of children.6 As hepatitis A infection is usually asymptomatic, most epidemiological studies depend on investigation of past infection (anti-HAV IgG) rather than investigation of acute infection (anti-HAV IgM). Investigation of anti-HAV IgG in various age groups gives information about both current and past epidemiological patterns.7
The age-distribution of HAV seroprevalence varies according to geographical, environmental and socioeconomic conditions, and reflects current and recent hepatitis A epidemiology in particular countries and regions. The endemicity of this infection varies depending on sanitary and hygienic conditions and socioeconomic differences between countries and in various regions of the same country.3 Turkey has intermediate endemicity in the western and central regions,3 and high endemicity in eastern and southern regions.8 Yozgat is located in the central region of Turkey. The aim of the present study was to determine the age-specific pattern of immunity of hepatitis A among unvaccinated individuals in Yozgat to provide a basis for the development and implementation of immunization policies for the prevention of hepatitis A infection.
METHODS
This cross-sectional epidemiologic study was planned to determine the HAV antibody levels among individuals 6 years of age and older in Yozgat. Children younger than 6 years of age were excluded because Turkey added HAV vaccine to routine immunization schedule in 2012. Based on the assumption that 80% of the individuals would have immunity and a population of 421 041 in Yozgat, the required sample size was calculated as 1750 at a confidence interval of 99% and with a deviation of 2.5%. Data was collected for patients who gave blood samples in the hospital for any reason between January 2017 and June 2017. Age and sex were taken into account in selecting patients for inclusion. Five mL of venous blood was collected from each individual, and serum samples were stored at −20°C until tested. Anti-HAV IgG status was determined quantitatively by immunosorbent enzyme- linked assay (ELISA) kits (DIA.PRO, Milano, Italy). The cut-off values were established according to the kits’ manufacturer guidelines.
All volunteers completed a structured questionnaire that assessed demographic and socioeconomic characteristics. The questionnaire included information on age, gender, education level, number of siblings, economical status and vaccination history. The study was approved by the Bozok University clinical research ethics committee (Registration number: 04.05.2016 – 11/03). The aim and methods of the study were described to each participant. After giving information about the study, the participants were requested to sign a written informed consent form for enrollment. Confidentiality was kept during the study. There was no potential risk for enrollment, and participants were notified that they could quit at any time.
Potential risk factors were analyzed by univariate and multivariate analysis. Continuous variables were expressed as mean and standard deviation (SD) and categorical variables were expressed as percentage. Proportions were compared by the chi-square test. The independent-groups t test was used to compare continuous variables if normality assumptions were met; otherwise, groups were compared by the Wilcoxon rank sum test. To test the independence of the risk factors for hepatitis A immunity the significant variables (P<.05) in the univariate analyses were entered into a multivariate logistic regression model with backward selection of independent variables. Statistics were run with Software package STATA 11.0 (College Station, Texas, USA).
RESULTS
We enrolled 1862 subjects in the study, which included 858 subjects from urban areas and 1004 from rural areas (Table 1). The mean (SD) age of the subjects was 33.5 (20.4) years (range 6–96 years). A total of 905 (48.6%) subjects were male and 957 (51.4%) female. The overall immunity against hepatitis A was 79.1% (n=1473). The mean (SD) age was 17.1 (14.7) years in the nonimmune group and 37.8 (19.5) years in the immune group (P<.001). No significant differences in immunity rates were detected between genders for all age groups. None of the subjects had a vaccination history for HAV. The seropositivity rate was lower in subjects younger than age 20 years of age (52.2% versus 93.9%; P<.001) (Figure 1). Immunity rates in rural areas were higher than in urban areas (P<.001). In the multivariate analysis, older age (odds ratio 1.084; 95% CI 1.072–1.095; P<.001) and residence in rural area (odds ratio 1.455; 95% CI 1.130–1.874; P=.004) were independently associated with hepatitis A immunity (Table 2).
Table 1.
Immunity to hepatitis A infection by age group and residential area.
| Age groups (years) | All participants by residential area | Immunity by residential area | Immunity by age group | Total | |||
|---|---|---|---|---|---|---|---|
| Urban | Rural | Urban | Rural | İmmune cases | Nonimmune cases | ||
|
| |||||||
| 6–9 | 123 (61.5) | 77 (38.5) | 46 (62.2) | 28 (37.8) | 74 (37) | 126 (63) | 200 |
| 10–14 | 113 (59.5) | 77 (40.5) | 40 (55.6) | 32 (44.4) | 72 (37.9) | 118 (62.1) | 190 |
| 15–19 | 128 (47.6) | 141 (52.4) | 88 (44.4) | 110 (55.6) | 198 (73.6) | 71 (26.4) | 269 |
| 20–29 | 94 (36.3) | 165 (63.7) | 83 (35) | 154 (65.0) | 237 (91.4) | 22 (8.6) | 259 |
| 30–39 | 110 (43.5) | 143 (56.5) | 104 (43.5) | 135 (56.5) | 239 (94.5) | 14 (5.5) | 253 |
| 40–49 | 94 (39.7) | 143 (60.3) | 87 (38.8) | 137 (61.2) | 224 (94.5) | 13 (5.5) | 237 |
| 50–59 | 89 (42.2) | 122 (57.8) | 86 (43) | 114 (57) | 200 (94.8) | 11 (5.2) | 211 |
| >60 | 107 (44) | 136 (56) | 100 (43.7) | 129 (56.3) | 229 (94.2) | 14 (5.8) | 243 |
| All | 858 (46.1) | 1004 (53.9) | 634 (43) | 839 (57) | 1473 (79.1) | 389 (20.9) | 1862 |
Data are number (percentage).
Figure 1.
Hepatitis A serostatus proportions by age (only 2 and 1 subjects for ages 85 and 86 years, respectively). Vertical line at 20 years.
Table 2.
Multiple logistic regression analysis for hepatitis A immunity.
| Independent risk factors | Odds ratio | 95% Confidence interval (CI) | P |
|---|---|---|---|
|
| |||
| Age | 1.084 | 1.072–1.095 | <.001 |
| Residental area | 1.455 | 1.130–1.874 | .004 |
Log likelihood=−744.40003, Pseudo R2=0.2200
DISCUSSION
Levels of anti-HAV antibodies in different age groups is an important indicator of age-specific incidence rates of HAV infection. This is critical to estimate the risk of HAV acquisition by age.1 Our study demonstrated that the HAV seropositivity rate increased with age, with 93.9% of our study participants 20–96 years of age being positive for anti-HAV antibodies. There was a characteristic distribution of the hepatitis A immunity by increasing age in our study and the seropositivity rate for subjects aged 6–19 years was lower than the other age groups. The difference was statistically significant. We also observed significant differences in the immunity rate between rural and urban area. The immunity rates in rural areas were higher than in urban areas. Previous data from Turkey have indicated a gradual increase in anti-HAV seroprevalence with age, with 11%, 34%, 33%, 68%, 93%, 96%, 92%, and 92% of subjects aged 0–4, 5–9, 10–14, 15–24, 25–44, 45–59, 60–70 and 70–92 years respectively, being positive for anti-HAV.7,9–11
An expanded program on immunization for children, including hepatitis A infection, has been implemented in Turkey since October 2012 that is funded by the Turkish government. Hepatitis A vaccine is administered to children in two doses at 18 months and 24 months of age.6 Children younger than 6 years of age were excluded from our study, as vaccination was introduced into the routine childhood vaccination schedule in Turkey in 2012. None of the subjects included in the study had a vaccination history, so seropositivity can be attributed to natural infection. This is the first report on anti-HAV seroprevalence rates among children and adults reported by age group in Yozgat, Turkey.
The data in our study also indicates an alteration in the epidemiology of HAV infection with time; the younger age groups, especially age 6–19 years, are more susceptible to HAV infection in Yozgat as compared to older age groups. Countries like Turkey have undergone important socioeconomic improvement in recent years. Usually, the seroepidemiology of the disease varies from a high incidence of asymptomatic infections in early childhood to development of outbreaks resulting in symptomatic cases in adults. Universal childhood vaccination is cost-effective in these countries.
HAV vaccine was added to routine immunization schedule in 2012 in Turkey. Since routine immunization could change the epidemiological profile of the country in the future, it is important to determine the baseline immunity to HAV across Turkey.7 Several years ago, epidemiological changes in age-specific HAV infection happened in most of the developed countries and in some countries with increasing economic growth such as Spain and Korea. Some countries in the Middle East and North Africa (MENA) region, such as Saudi Arabia, Kuwait, United Arab Emirates, Turkey and others, have reported similar changes in the last 5 years.
In a systematic review on the worldwide prevalence of HAV infection, the World Health Organization (WHO, 2009) reported that anti-HAV positivity was in intermediate ranges in MENA countries.12 Despite variability among MENA countries and within the countries themselves, positive HAV immunity is estimated to be present in more than 80% of the adult population and more than 50% of children. Although seroprevalence levels of anti- HAV among adults are high in most countries in the MENA region, especially in Saudi Arabia, Egypt, Jordan, Iran, and Turkey, the WHO study showed a sharp decline among children. The 2009 WHO report indicates that children and young adults in Iran, Iraq, Saudi Arabia, and Turkey have a lower anti-HAV seroprevalence. Our results show the age-related HAV epidemiological shift with a relatively low anti-HAV seroprevalence detected in young adults. Our results demonstrate the increased susceptibility of younger age groups and need for a catch-up vaccination against HAV in older children. Our data shows similarities to those reported from other MENA countries. Recently, WHO (2012) recommended that vaccination against hepatitis A should be placed into the national immunization schedule for children younger than one year of age.13
Universal childhood vaccination against hepatitis A is advised for regions with intermediate endemicity, while targeted vaccination is recommended in low endemicity regions. Turkey’s current policy of recommending hepatitis A immunization for all children seems to be the best choice at least for the next decade or two. A catch-up vaccination program is required for age 6–19 years in Yozgat. Our seroprevalence data shows transmission risk of HAV infection in children is greater than in adults aged 20 and older. Increased susceptibility to HAV infection at younger ages can result from HAV outbreaks among unvaccinated children and teenagers.
Footnotes
Funding: This study was supported by the Bozok University Scientific Research Project Units; Project No: 6602a-TF/16-54.
CONFLICT OF INTEREST: None.
This study was presented as an e-poster at the 28th ECCMID, the European Congress of Clinical Microbiology and Infectious Diseases, which took place in Madrid, Spain, 21–24 April 2018.
REFERENCES
- 1.Melhem NM, Jaffa M, Zaatari M, Awada H, Salibi NE, Ramia S. The changing pattern of hepatitis A in Lebanese adults. Int J Infect Dis. 2015 Jan;30:87–90. doi: 10.1016/j.ijid.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 2.Lai M, Chopra S. Hepatitis A virus infection in adults: Epidemiology, clinical manifestations, and diagnosis. [accessed 2018, 31 July]. Available from: https://www.uptodate.com/contents/hepatitis-a-virus-infection-in-adults-epidemiology-clinical-manifestations-and-diagnosis.
- 3.World Health Organization. WHO position paper on hepatitis A vaccines – June 2012. Wkly Epidemiol Rec. 2012;87:261–76. [Google Scholar]
- 4.Merat S, Rezvan H, Nouraie M, Abolghasemi H, Jamali R, Amini-Kafiabad S, et al. Seroprevalence and risk factors of hepatitis A virus infection in Iran: a population based study. Arch Iran Med. 2010 Mar;13(2):99–104. [PubMed] [Google Scholar]
- 5.Prevention of Hepatitis A Through Active or Passive Immunization. Recommendations of the Advisory Committee on Immunization Practices (ACIP) [accessed 2018 31 July];MMWR Recommendations and Reports. 2006 55:1–23. Available from: https://www.cdc.gov/mmwr/preview/mmwrhtml/rr5507a1.htm. [PubMed] [Google Scholar]
- 6.Saglik Bakanligi TC. Hepatit A asi Uygulamasi Ust Yazisi. [accessed 2018 31 July]. Available from: https://dosyaism.saglik.gov.tr/Eklenti/12472,20121008-1509-hskdan-hepatit-a-asisinin-uygulanmasi-hakkinda-yazipdf.pdf?0.
- 7.Demiray T, Köroglu M, Jacobsen KH, Özbek A, Terzi HA, Altındis M. Hepatitis A virus epidemiology in Turkey as universal childhood vaccination begins: seroprevalence and endemicity by region. Turk J Pediatr. 2016;58(5):480–491. doi: 10.24953/turkjped.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 8.Ceyhan M, Yildirim I, Kurt N, Uysal G, Dikici B, Ecevit C. Differences in hepatitis A seroprevalence among geographical regions in Turkey: a need for regional vaccination recommendations. J Viral Hepat. 2008;15:69–72. doi: 10.1111/j.1365-2893.2008.01034.x. [DOI] [PubMed] [Google Scholar]
- 9.Karsligil T, Eksi F, Balcí, Belgin R. The seroprevalence of hepatitis A and E in our region. Viral Hepat J. 2003;8:155–159. [Google Scholar]
- 10.Erturk A, Copur Cicek A, Cure E, Akdogan RA, Ozturk C. Seroprevalence of hepatitis A in Rize Province and different adult age groups. Viral Hepat J. 2013;19:85–88. [Google Scholar]
- 11.Koroglu M, Demiray T, Terzi HA, Altindis M. Seroprevalence of hepatitis A among different age groups in Sakarya and review of the literature. Viral Hepat J. 2014;20:110–114. [Google Scholar]
- 12.World Health Organization. The global prevalence of hepatitis A virus infection and susceptibility: a systematic review. Geneva, Switzerland: WHO; 2009. [accessed 2018 31 July]. Available from: http://apps.who.int/iris/handle/10665/70180. [Google Scholar]
- 13.WHO position paper on hepatitis A vaccines: June 2012-recommendations. Vaccine. 2013;31:285–6. doi: 10.1016/j.vaccine.2012.10.102. https://doi:10.1016/j.vaccine.2012.10.102. [DOI] [PubMed] [Google Scholar]